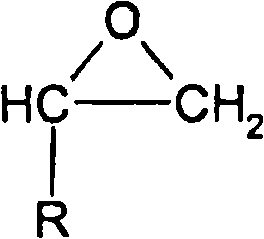
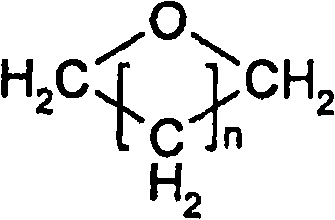
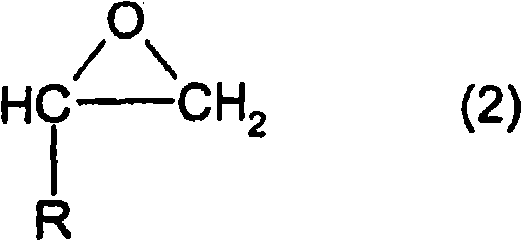
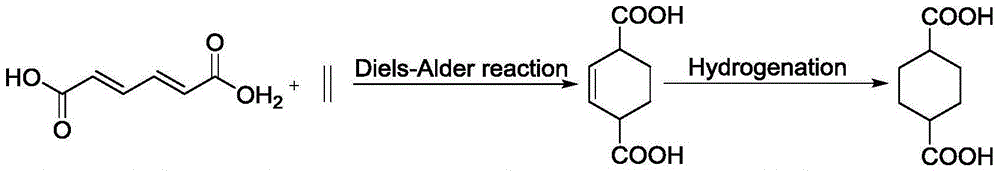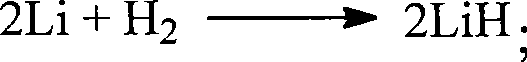Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
110 results about "Diethylene glycol dimethyl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
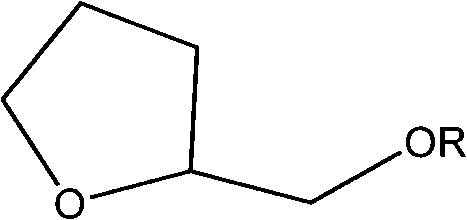
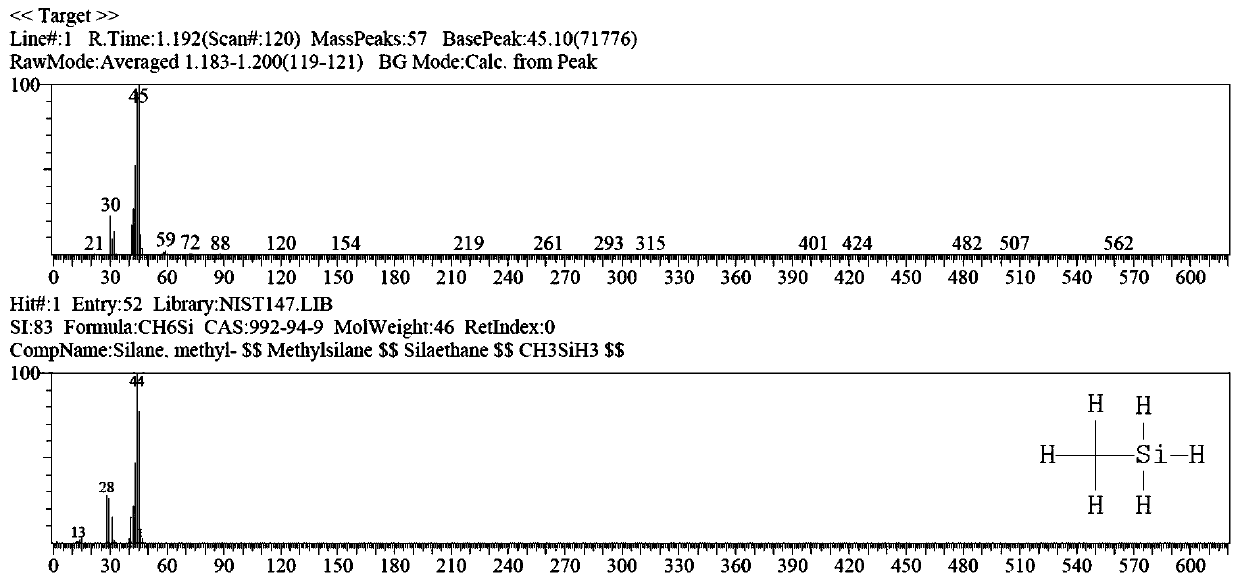
Diglyme, or bis(2-methoxyethyl) ether, is a solvent with a high boiling point. It is an organic compound which is the dimethyl ether of diethylene glycol. (The name "diglyme" is a portmanteau of "diglycol methyl ether.") It is a clear, colorless liquid with a slight ether-like odor.
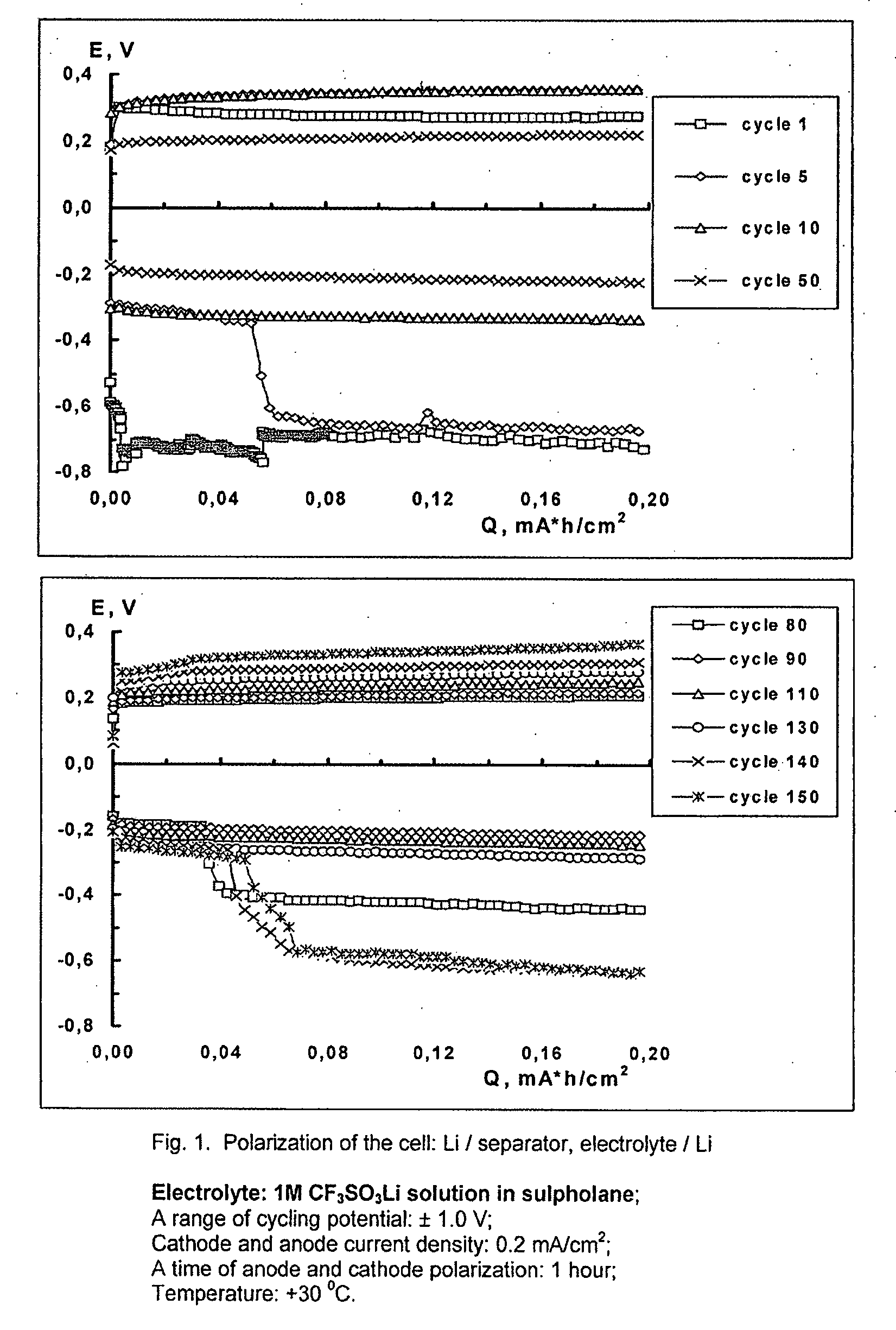
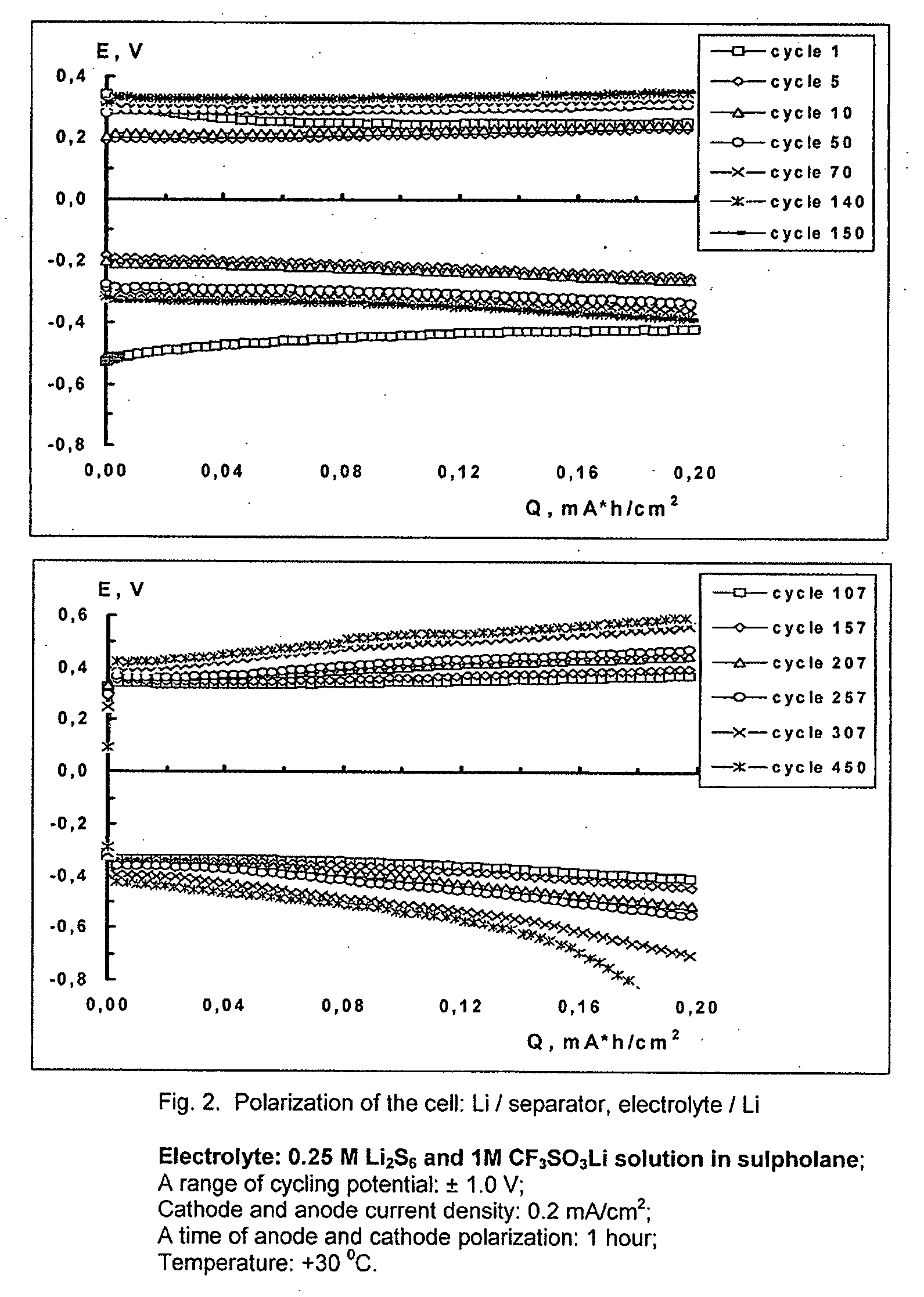
Cell or battery with a metal lithium electrode and electrolytes therefor
InactiveUS20080038645A1Improve cycle lifeOrganic electrolyte cellsLi-accumulatorsSulfolaneMetallic lithium
An electrolyte for rechargeable batteries with a negative electrode of lithium or lithium containing alloys comprising: one or several non-aqueous organic solvents, one or several lithium salts and one or several additives increasing the cycle life of the lithium electrode. The electrolyte solution may comprise one or several solvents selected from the group comprising: tetrahydrofurane, 2-methyltetrahydrofurane, dimethylcarbonate, diethylcarbonate, ethylmethylcarbonate, methylpropylcarbonate, methylpropylpropyonate, ethylpropylpropyonate, methylacetate, ethylacetate, propylacetate, dimetoxyethane, 1,3-dioxalane, diglyme (2-methoxyethil ether), tetraglyme, ethylenecarbonate, propylencarbonate, γ-butyrolactone, and sulfolane. The electrolyte solution may further comprise at least one salt or several salts selected from the group consisting of lithium hexafluorophosphate (LiPF6), lithium hexafluoroarsenate (LiAsF6), lithium perchlorate (LiClO4), lithium sulfonylimid trifluoromethane (LiN(CF3SO2)2)) and lithium trifluorosulfonate (CF3SO3Li) or other lithium salts or salts of another alkali metal or a mixture thereof. Also disclosed is an electrochemical cell or battery with an anode of metallic lithium or a lithium-containing alloy, and such an electrolyte.
Owner:OXIS ENERGY
Diode-addressed color display with lanthanoid phosphors
InactiveUS6165631AImprove quantum efficiencyImprovement factorDischarge tube luminescnet screensCathode ray tubes/electron beam tubesEthylenediamineDisplay device
A diode-addressed color display comprising an UV-diode and a phosphor of the general formula LnL3X2, wherein Ln=Eu3+, Tb3+, Tm3+, Dys3+, Sm3+, L=4-R-4'-benzophenone carboxylic acid, wherein R=phenyl, benzyl, CH3, CF3, C2H5, F, Cl, OCH3, CH3CO; 4-R-4'-benzophenone acetylacetonate, wherein R=phenyl, benzyl, CH3, CF3, C2H5, F, Cl, OCH3, CH3CO; 4-acetophenone carboxylic acid, 4-trifluoroacetophenone carboxylic acid, 4-acetophenone acetylacetonate or 4-trifluoroacetophenone acetylacetonate and X=+E,fra 1 / 2+EE phenanthroline, +E,fra 1 / 2+EE diphenyl phenanthroline, +E,fra 1 / 2+EE 4-Cl-phenanthroline, +E,fra 1 / 2+EE bipyridine, +E,fra 1 / 2+EE ethylenediamine, triphenyl phosphineoxide, trimethyl phosphineoxide, triethyl phosphineoxide, +E,fra 1 / 2+EE diethylene glycol-dimethylether (diglyme) or ethanol.
Owner:US PHILIPS CORP
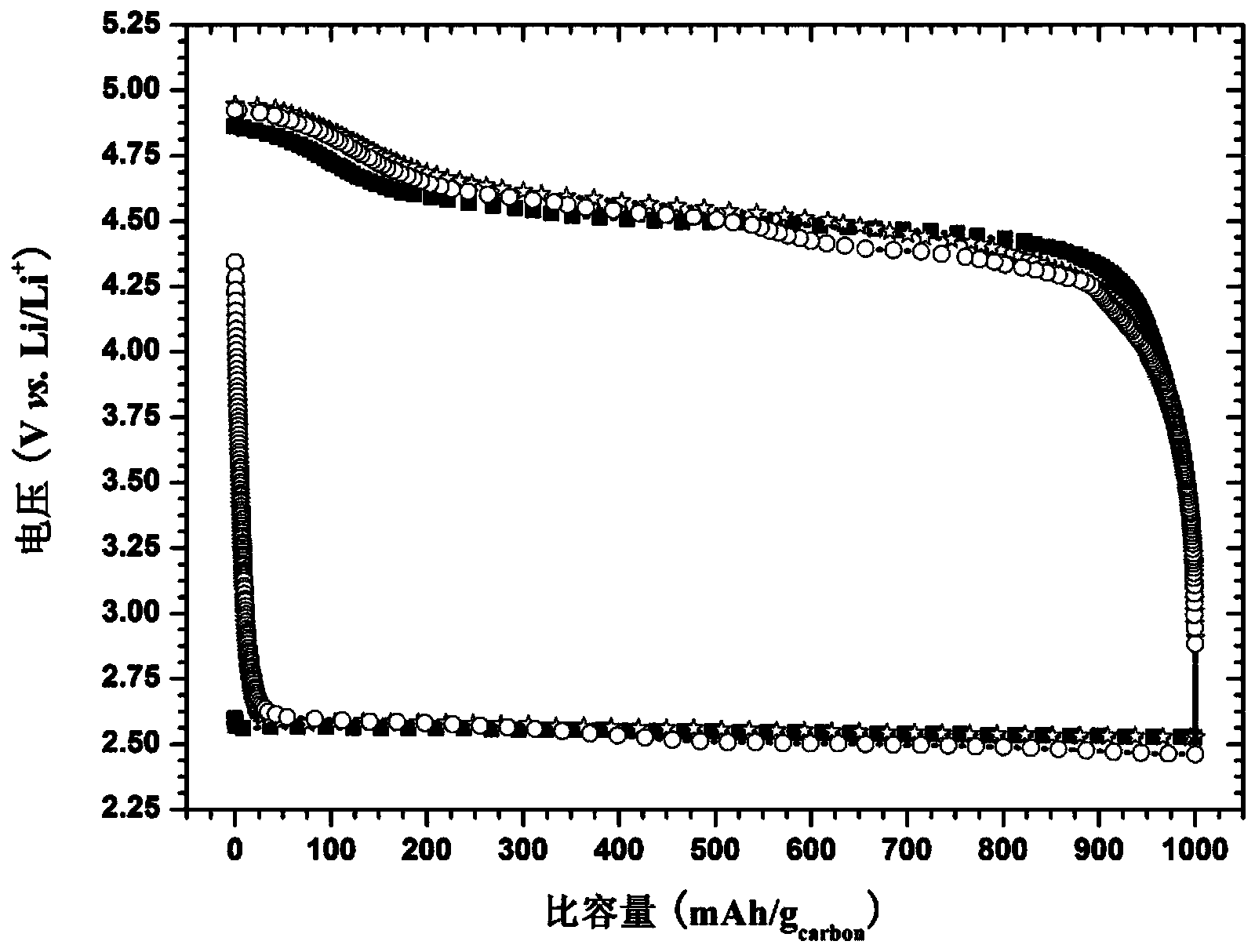
Ether electrolyte and lithium air battery
InactiveCN103996892ANot volatileImprove oxygen solubilityFuel and primary cellsFuel and secondary cellsCharge dischargeOxygen
The invention provides ether electrolyte. The ether electrolyte comprises lithium salt and an organic solvent, wherein the organic solvent comprises one or more of 1,2-dimethoxyethane, diethylene glycol dimethyl ether, triethylene glycol dimethyl ether and tetraethylene glycol dimethyl ether. The ether electrolyte has advantages that the ether electrolyte is unlikely to volatize, the oxygen dissolving degree is high, the electrochemical stable window is wide, and the like, and has excellent chemical stability for a discharging intermediate product O2<-1> or LiO2 and a discharging final product Li2O2. The lithium air battery prepared by utilizing the ethers electrolyte is excellent in long cycling stability. The experimental result shows that a charging-discharging curve is not obviously changed after the lithium air battery assembled by utilizing the ether electrolyte is circularly charged and discharged for 30 times at a limit capacity of 1,000mAh / g under the current density of 100mA / g.
Owner:常州盈华高科储能材料科技有限公司 +1
Preparation method for 1,4-cyclohexane dicarboxylic acid (CHDA) and diester thereof
InactiveCN106467459AReduce dependenceOrganic compound preparationCarboxylic acid esters preparationCyclohexeneN-Butanol
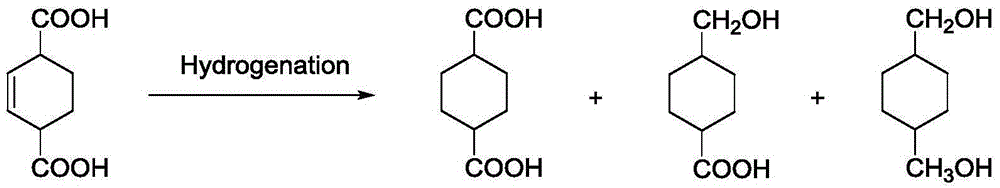
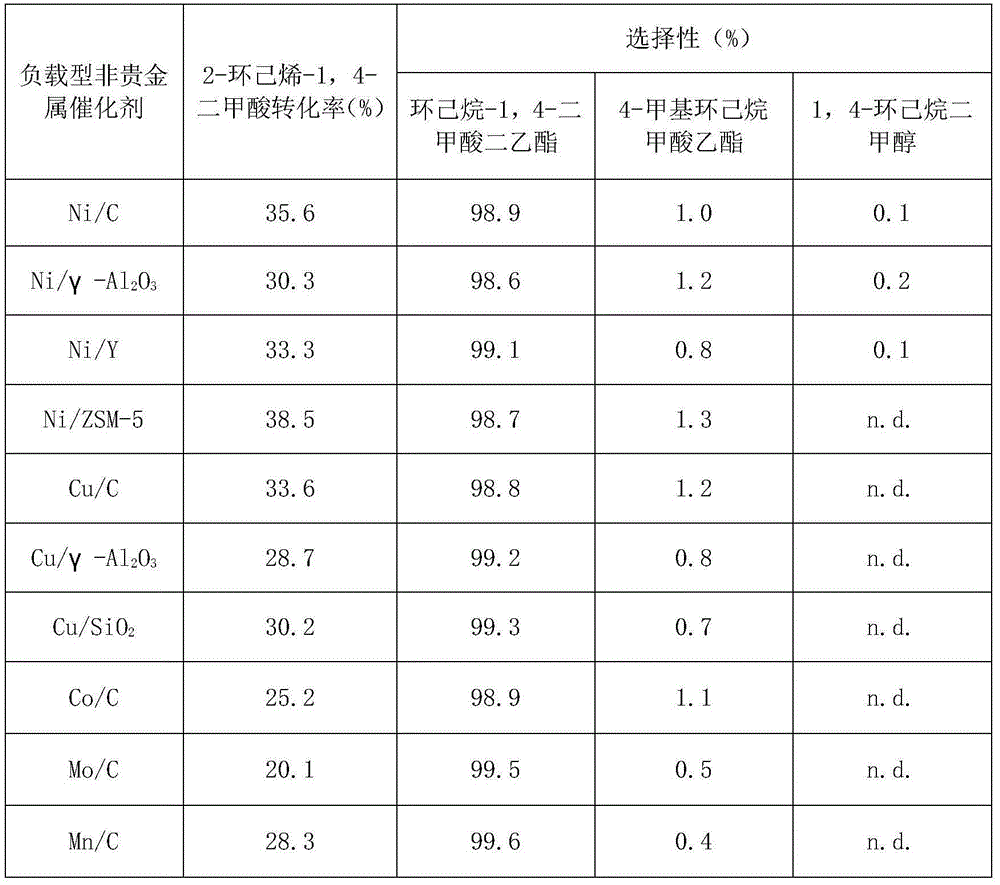
The invention discloses a preparation method for 1,4-cyclohexane diformic acid (CHDA) and diester thereof. In particular, 2-cyclohexene-1,4-dicarboxylic acid is subjected to catalytic hydrogenation to prepare the CHDA or CHDA diester under the effect of a supported metal catalyst with hydrogen under certain pressure fed, a solvent being polar or non-polar, wherein the polar solvent may be water, methanol, ethanol, n-propanol, isopropanol, n-butanol, glycol dimethyl ether and diethylene glycol dimethyl ether, and the non-polar solvent may be one or more than two selected from n-hexane, n-heptane, n-octane, cyclohexane, benzene and toluene. A metal active component of the supported metal catalyst is non-noble metal and / or noble metal. A carrier of the supported metal catalyst may be one or more than two selected from a carbon carrier, a nano metal oxide, a nano non-metal oxide and a molecular sieve. When the conversion rate of the 2-cyclohexene-1,4-diformic acid is more than 98%, the selectivity of the CHDA or CHDA diester can reach 96%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Superlow-temperature lithium ion battery electrolyte and lithium ion battery using same
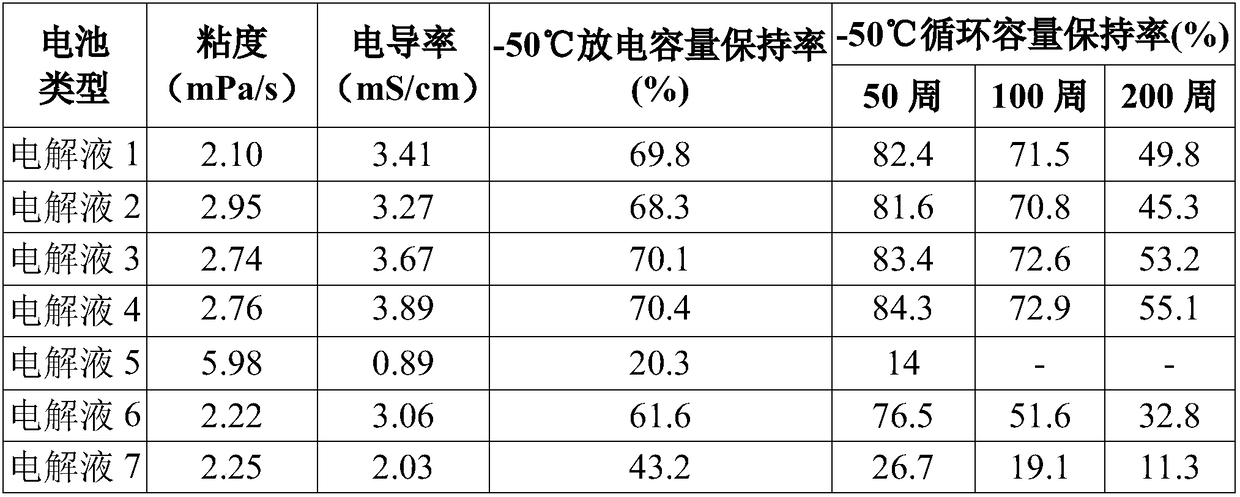
ActiveCN108511800ALow melting pointLow viscositySecondary cellsOrganic electrolytesDifluorophosphateN dimethylformamide
The invention discloses a superlow-temperature lithium ion battery electrolyte. The electrolyte is prepared from the following raw materials of organic solvents, lithium salt and a film forming additive, wherein the organic solvents comprise carbon disulfide, ethyl butyrate, diglyme and N,N-dimethylformamide; the lithium salt is lithium tetrafluoroborate; the film forming additive consists of vinylene carbonate and any one of ethylene sulfate, lithium difluorophosphate and imidodisulfuryl fluoride. Compared with t traditional carbonate solvents, the electrolyte has the advantages that the melting point of the used organic solvent is very low, and the higher ion conductivity is still maintained at superlow temperature of -40 DEG C; the additive forms a film at the surface of a cathode, andcan form a stable SEI (solid electrolyte interface) film with low impedance, so as to quickly intercalate and de-intercalate the lithium ions at low temperature. The invention also discloses the lithium ion battery using the electrolyte. The lithium ion battery can reach excellent low-temperature discharge and cycle properties under the superlow-temperature environment.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Vinyl content method for controlling polybutadiene in elastomer
A method of controlling the vinyl content in polybutadiene of elastomers relates to the polymer synthesis field. Symmetric Lewis base has the following problem in the application that the relatively high content of regulator will reduce the rate of polymerization and impact further polymerization as well as the coupling process after the polymerization. The invention introduces an asymmetric ethers-polar compound in a regulator system to form a composite regulation system with symmetrical ethers or amines compound, wherein, the asymmetric ethers compound is alkylte trahydrofurruryl ethers, and the other polar reagent can be symmetrical ethers or amines compound, such as N,n,n',n',n-Pentamethyldiethylenetriamine (hereinafter referred to as PMEDTA), diethylene glycol dimethyl ether (hereinafter referred to as 2G), triethylene glycol dimethyl ether (hereinafter referred to as 3G). The method of the invention can increase the content of vinyl, without reducing the rate of polymerization; the coupling efficiency of the coupling reaction can reach 90%.
Owner:BEIJING UNIV OF CHEM TECH
Electrolyte used for Li-S battery, Li-S battery and method for preparing electrolyte membrane contained in same
The invention discloses an electrolyte used for a Li-S battery, comprising an electrolyte membrane and electrolyte, wherein the electrolyte membrane takes PVDF (polyvinylidene fluoride) or PVDF-HFP (polyvinylidene fluoride-hexafluoropropylene) as a main chain, the side chain of the main chain comprises one or more than one of lithium sulphonate group, lithium carboxylate group and lithium amide group; and the electrolyte is mixed solution containing at least one of 1,3-dioxolame, dimethoxyethane, diethylene glycol dimethyl ether, triethylene glycol dimethyl ether and sulfolane. The electrolyte, an anode and a cathode form the Li-S battery in the invention. The method for preparing the electrolyte membrane contained in the electrolyte comprises the following steps: firstly dissolving PVDF or PVDF-HFP, heating and stirring, and casting to obtain a membrane; treating the membrane with KOH / ethyl alcohol solution, then transferring the membrane into mixed solution of graft monomer / tetrahydrofuran, and adding formylamine peroxide for grafting; and cleaning the grafted membrane and soaking to obtain the electrolyte membrane. The electrolyte membrane in the invention is simple to prepare,the cost is low, the loss of active substance can be reduced, and the cycle life of the battery can be prolonged.
Owner:NAT UNIV OF DEFENSE TECH
Process for producing silicane
ActiveCN101531367AImprove conversion rateSuitable for industrial productionSilicon hydridesSodium aluminium hydrideSilicon tetrafluoride
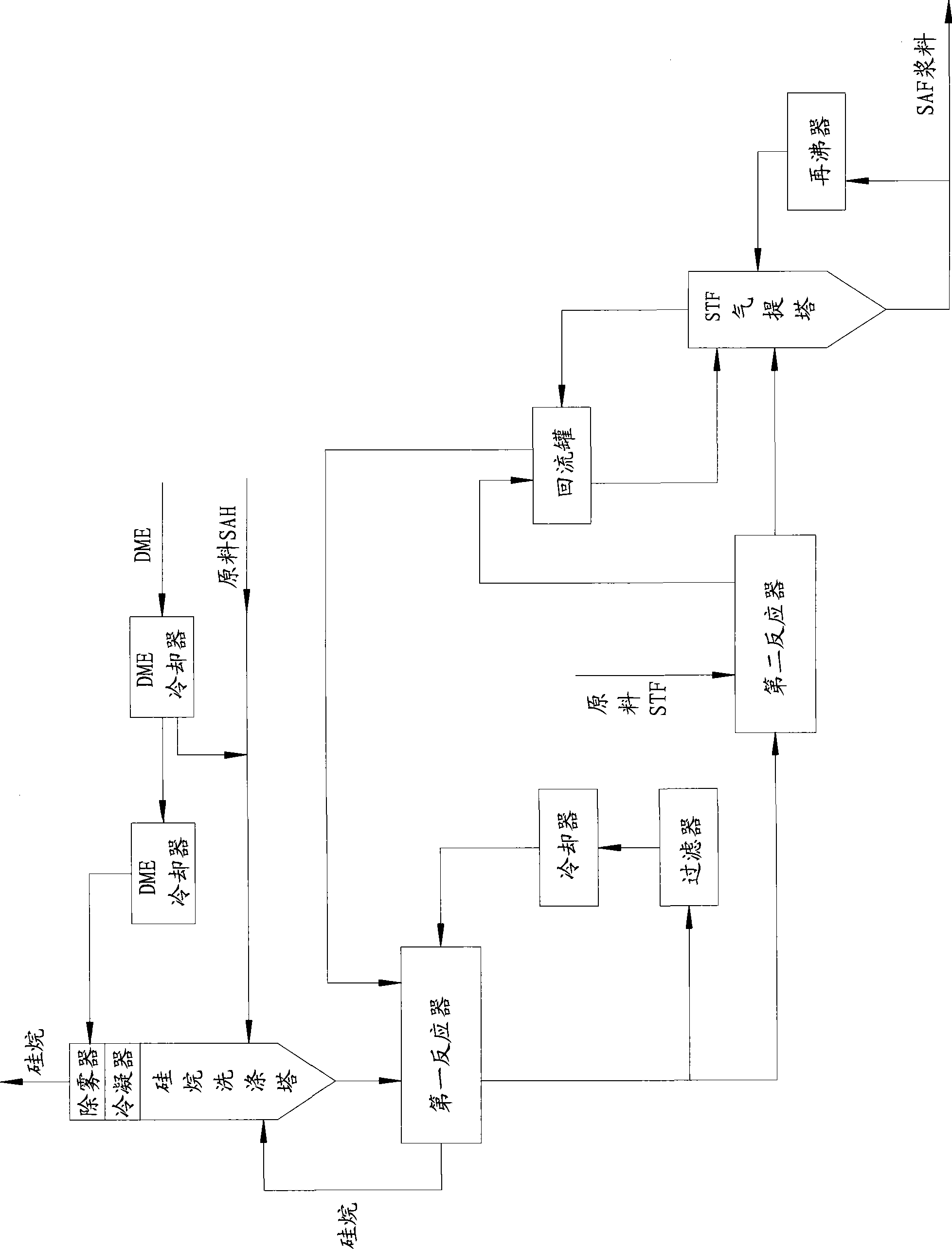
The invention discloses a process for preparing silicane, sodium aluminum hydride solution is passed through a first reactor and a second reactor in order, silicon tetrafluoride gas is passed through the second reactor and the first reactor in order, and the sodium aluminum hydride solution is diethylene glycol dimethyl ether solution of sodium aluminum hydride. The process for preparing silicane provided by the present invention utilizes the direct reaction of the odium aluminum hydride solution and the silicon tetrafluoride to generate silicane, The sodium aluminum hydride solution and the silicon tetrafluoride gas are conversely feeding mutually, in the first reactor, amount of the sodium aluminum hydride is larger than that of the silicon tetrafluoride, so that silicon tetrafluoride can be completely reacted, in the second reactor, amount of the silicon tetrafluoride is larger than that of the sodium aluminum hydride, so that the sodium aluminum hydride can be completely reacted, thereby increasing conversion of the silicane. The process for preparing silicane provided by the present invention is especially suitable for silicane industrialization production.
Owner:YINGLI ENERGY CHINA
Method for producing alkylene glycol diethers
The invention relates to a method for producing alkylene glycol diethers by reacting a linear or cyclic ether with an alkylene oxide in the presence of a Lewis acid. The method is characterized in that the Lewis acid is a mixture of 1 part by weight of HBF4 and / or BF3 and 0.1 to 10 parts by weight of H2SO4, HNO3 and / or H3PO4. The new catalyst system allows to reduce undesired by-products such as e.g. dioxan or dimethyltriethylene glycol, and to increase the quantity of valuable substances such as dimethyl glycol and dimethyl diglycol.
Owner:CLARIANT PROD DEUT GMBH
Production process of waterborne polyurethane coating material having high tension force coating film
ActiveCN104893537AHigh molecular weightEasy to stretchPolyurea/polyurethane coatingsPolyesterPolymer science
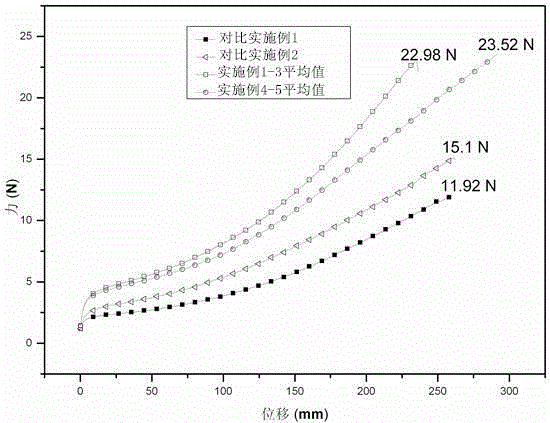
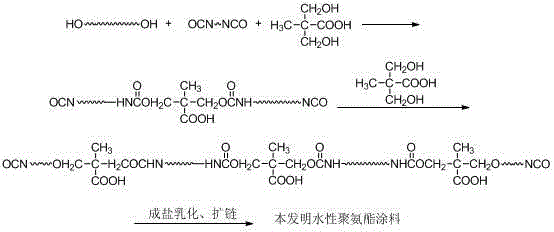
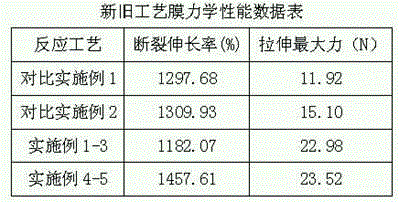
The present invention discloses a production process of a waterborne polyurethane coating material having a high tension force coating film. According to the production process, polyether, polyester polyol and isocyanate are adopted as raw materials, a hydrophilic chain extender is gradually added during the reaction process, an ethylene glycol dimethyl ether-acetone mixed solvent or diethylene glycol dimethyl ether-acetone mixed solvent is added when the reaction achieves the theoretical NCO% value, and finally salification, emulsifying and chain extending are performed to obtain the product. According to the present invention, the hydrophilic groups in the obtained product of the present invention are broadly distributed in the molecular chain compared with the product obtained through the traditional process, wherein the hydrophilic groups are not only distributed in the prepolymer chain end and also distributed in the chain so as to further improve the hydrophilic efficiency of the hydrophilic groups; and according to the structural characteristics of the prepolymer molecular chain, the two solvent systems are selected, such that the elongation and the tension force of the waterborne polyurethane coating after the film forming can be significantly improved with the solvent systems, the compatibility of the solvent system and the aqueous emulsion is good, and the stability is high.
Owner:JIANGSU INSTITUTE OF EDUCATION +1
Methanol gasoline for vehicle and preparation method thereof
InactiveCN104004551AImprove combustion characteristicsIncrease profitLiquid carbonaceous fuelsAlternative fuelsTert butyl
The invention discloses methanol gasoline for a vehicle and a preparation method thereof. The methanol gasoline is prepared from the following raw materials in parts by weight: 60-70 parts of methanol, 15-25 parts of 90# gasoline, 10-15 parts of dipropylene glycol methyl ether acetate, 5-10 parts of tert amyl methyl ether, 5-10 parts of dimethyl carbonate, 4-8 parts of diethylene glycol dimethyl ether, 3-6 parts of 2,2-dimethoxypropane, 1-2 parts of ferrocene, 0.5-1.5 parts of methyl cyclopentadiene tricarbonyl manganese, 0.8-1.4 parts of 2,5-di-tert-butyl hydroquinone, 0.4-0.8 part of glycerol monolaurate, 0.5-1 part of sodium petroleum sulfonate, 0.3-0.6 part of N-Polyoxyethylated-N-tallow-alkylamine, 5-10 parts of wood tar, 3-6 parts of isopropanol, 2.5-4.5 parts of fish meal, 2-3 parts of bone meal, 1-2 parts of stearic acid and 0.5-1 part of urotropine. The methanol gasoline disclosed by the invention is favorable in combustion characteristic, high in utilization ratio, favorable in anti-detonating quality, low in corrosivity, high in safety and reliability, energy-saving and environment-friendly, can be used instead of any of 90#, 93#, 97# and 98# gasoline for a vehicle, is novel environment-friendly alternative fuel, and can reduce pollutant emission and carbon emission; and the combustion emission of the methanol gasoline meets the standards in Europe and America. The methanol gasoline has excellent economic benefits and far-reaching social benefits.
Owner:BEIJING DAMING QIAOHUA ENERGY SCI & TECH
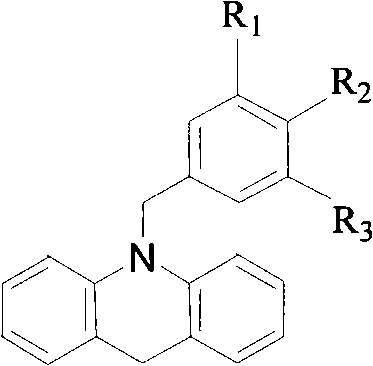
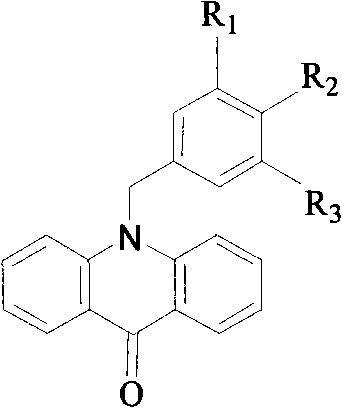
N-benzyl-9, 10- dihydro-acridine compound and preparation method thereof
The invention discloses an N-benzyl-9, 10-dihydro-acridine compound and a preparation method thereof. A structure of the N-benzyl-9, 10-dihydro-acridine compound is shown as a formula I, wherein the R1 is H, OCH2Ph or OCH3; the R2 is H or OCH3; the R3 is H, OCH2Ph or OCH3. The method for preparing the N-benzyl-9, 10-dihydro-acridine compound comprises the steps: under the oxygen isolating condition, adding sodium borohydride and an N-benzyl acridone compound into organic solvent (A) to react for 4-24h at 50-100 DEG C; and then, adding solvent (B) to continuously react for 0.5-1h at 50 -100 DEG C and obtaining the compound shown as the formula I, wherein the organic solvent (A) is diethylene glycol dimethyl ether or tetrahydrofuran, and solvent (B) is methanol or water. The compound has the outstanding advantages of cheap and available materials, simple and convenient synthetic method, higher yield, and the like.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Liquid photosensitive solder-resist resin for LED and preparation method thereof
InactiveCN105061671AWith unsaturatedIncrease photosensitivityPhotosensitive materials for photomechanical apparatusResistPhosphine
The invention discloses a liquid photosensitive solder-resist resin for LED and belongs to the technical field of resin materials. The resin comprises the following raw materials in parts by weight: 30 to 40 parts of methacrylic acid monomer, 1 to 5 parts of polymerization thermal initiator, 8 to 12 parts of acrylic acid, 12 to 15 parts of acid anhydride, 35 to 45 parts of solvent, 0.15 to 0.25 part of catalyst, and 0.01 to 0.02 part of polymerization inhibitor, wherein the solvent comprises one of diethylene glycol dimethyl ether and propylene glycol monomethyl ether; the catalyst is triphenyl phosphine, and the polymerization inhibitor is p-hydroxyanisole. The invention also provides a preparation method of the liquid photosensitive solder-resist resin for LED. The provided liquid photosensitive solder-resist resin has the advantages of simple technology, reasonable formula, and excellent yellowing resistant property and photosensitive performance, the service time of resin is prolonged, the using effect of resin is enhanced, and the resin can meet the production requirements.
Owner:新东方油墨有限公司
Flame-retarding, acid-alkali-resisting wear-resisting fire hose material
InactiveCN106349721AExcellent flame retardantExcellent flame retardant acid and alkali resistanceDecabromobiphenyl etherDecabromodiphenyl ether
The invention relates to a flame-retarding, acid-alkali-resisting wear-resisting fire hose material. The flame-retarding, acid-alkali-resisting wear-resisting fire hose material is prepared from rosin resin, p-tert-octyl phenolformaldehyde resin, amino resin, polyethylene wax emulsion, tall oil fatty acid, butyl acetate, isoamyl propionate, diethylene glycol dimethyl ether, dimethyl fumarate, sodium perborate, imidazoline, ammonium fluosilicate, guanidine phosphate powder, phenyl triethoxy silane, sodium pyrophosphate, chromium oxide, chromium chloride, menthol, barium chromate powder, ferrous orthophosphate, ethylenedioxydiethyl bis, tricresol phosphate ester, decabromodiphenyl ether, nanometer magnesium hydroxide, lignin, morpholine, polyoxyethylene polyoxy propyl alcohol amine ether, alkyl polyglucoside, propylamine, sodium ricinoleic acid. The product has excellent flame retardance, acid-base resistance, anti-mildew and anti-microbial performances, and the product performance is improved.
Owner:袁华
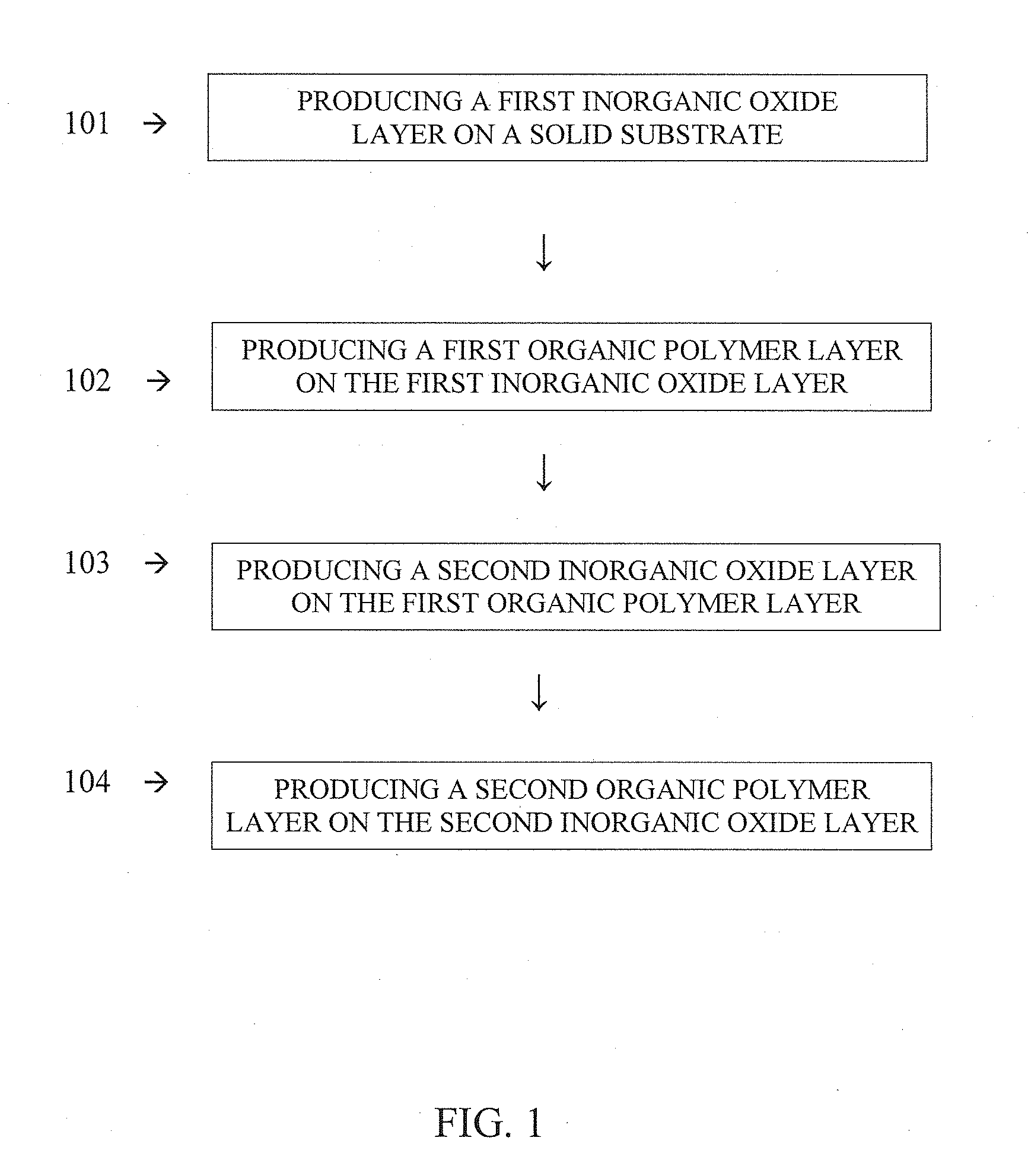
Multilayer heterostructures and their manufacture
ActiveUS20110223433A1Synthetic resin layered productsPretreated surfacesWater vaporOptical transmittance
A method of synthesizing multilayer heterostructures including an inorganic oxide layer residing on a solid substrate is described. Exemplary embodiments include producing an inorganic oxide layer on a solid substrate by a liquid coating process under relatively mild conditions. The relatively mild conditions include temperatures below 225° C. and pressures above 9.4 mb. In an exemplary embodiment, a solution of diethyl aluminum ethoxide in anhydrous diglyme is applied to a flexible solid substrate by slot-die coating at ambient atmospheric pressure, and the diglyme removed by evaporation. An AlOx layer is formed by subjecting material remaining on the solid substrate to a relatively mild oven temperature of approximately 150° C. The resulting AlOx layer exhibits relatively high light transmittance and relatively low vapor transmission rates for water. An exemplary embodiment of a flexible solid substrate is polyethylene napthalate (PEN). The PEN is not substantially adversely affected by exposure to 150° C.
Owner:ALLIANCE FOR SUSTAINABLE ENERGY
Preparation method of soluble low-temperature rapid imidization polyimide film
The invention discloses a preparation method of a soluble and low-temperature rapid imidization polyimide film, and belongs to the technical field of polyimide film preparation. The method comprises the following steps: a polar aprotic organic solvent with a boiling point lower than 200 DEG C is adopted as a solvent, and the polar aprotic organic solvent comprises one or more of diethylene glycol dimethyl ether, diethylene glycol diethyl ether, N,N-dimethyl acetamide, N,N-dimethyl formamide and dimethyl sulfoxide. According to the present invention, the polyimide film can be rapidly prepared through the imidization at the temperature of less than 200 DEG C, and the imidization process does not require the use of any catalyst, such that the important practical significance is provided for the production efficiency improving and the energy consumption reducing.
Owner:富优特(山东)新材料科技有限公司
Application of NiPS3 nanosheet in sodium ion battery and sodium ion battery
InactiveCN111092224AImprove electrochemical performanceFast charge and dischargeMaterial nanotechnologySecondary cellsElectrical batteryElectric capacity
The invention provides an application of a NiPS3 nanosheet in a sodium ion battery and the sodium ion battery. The NiPS3 nanosheet is used as a negative electrode material of the sodium ion battery orone of negative electrode materials, and diethylene glycol dimethyl ether for dissolving NaCF3SO3 is used as electrolyte. The method is characterized in that the high-quality NiPS3 nanosheet is applied to the sodium ion battery to be used as the negative electrode material, and meanwhile, the matched NaCF3SO3 / diethylene glycol dimethyl ether is used as the electrolyte, so formation of a thick SEIfilm in the charging and discharging process can be effectively inhibited, and the sodium ion battery with high electric capacity, high rate capability and quick charging and discharging can be obtained. The sodium ion battery provided can be a button battery, a soft package battery and the like, is green and safe, and has great advantages as a future large-scale energy storage material.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Alkali-soluble solid dispersant resin and preparation method thereof
The invention provides a preparation method of alkali-soluble solid dispersant resin. The preparation method includes uniformly mixing acrylic acid, styrene, alpha-methyl styrene, maleic anhydride, di-tert-butyl peroxide and / or tert-butyl peroxybenzoate at room temperature to obtain mixture A, adding linear alcohol ethoxylates and solvent diethylene glycol dimethyl ether into a reactor with heating to 140-160 DEG C,adding the mixture A under nitrogen atmosphere, then heating up to 170-185 DEG C for polymerization reaction for 20-60 min, and performing reduced pressure distillation after reaction to obtain the alkali-soluble solid dispersant resin. During mass polymerization of styrene and acrylic acid, the hydrophilic-lipophilic amphiphilic component, namely linear alcohol ethoxylates is added, the terminal hydroxyl group of the linear alcohol ethoxylates reacts with maleic anhydride or acrylic acid in the system to form a hydrophilic-lipophilic side chain, the lipophilic group at the terminal of the side chain changes bonding point distribution of dispersant and pigment, bonding stability of dispersant and pigment is improved beneficially, and dispersing performance on pigment is improved.
Owner:河南省中凌煜新材料科技有限公司
Perfluorination method for end group of fluorine-containing polymer
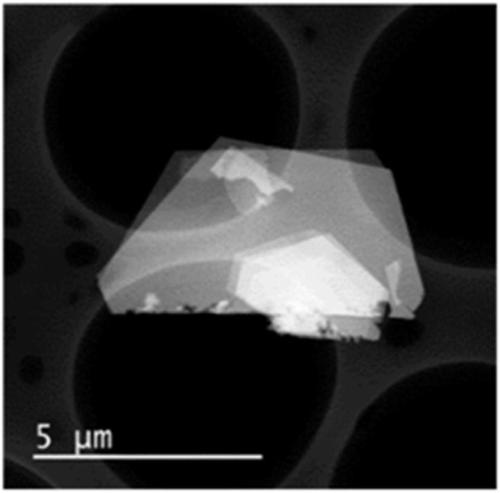
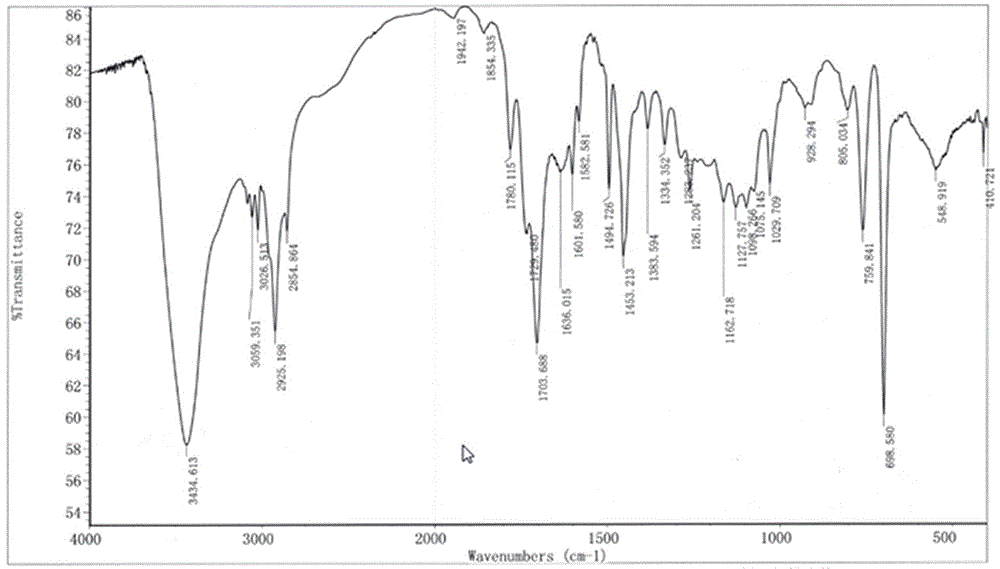
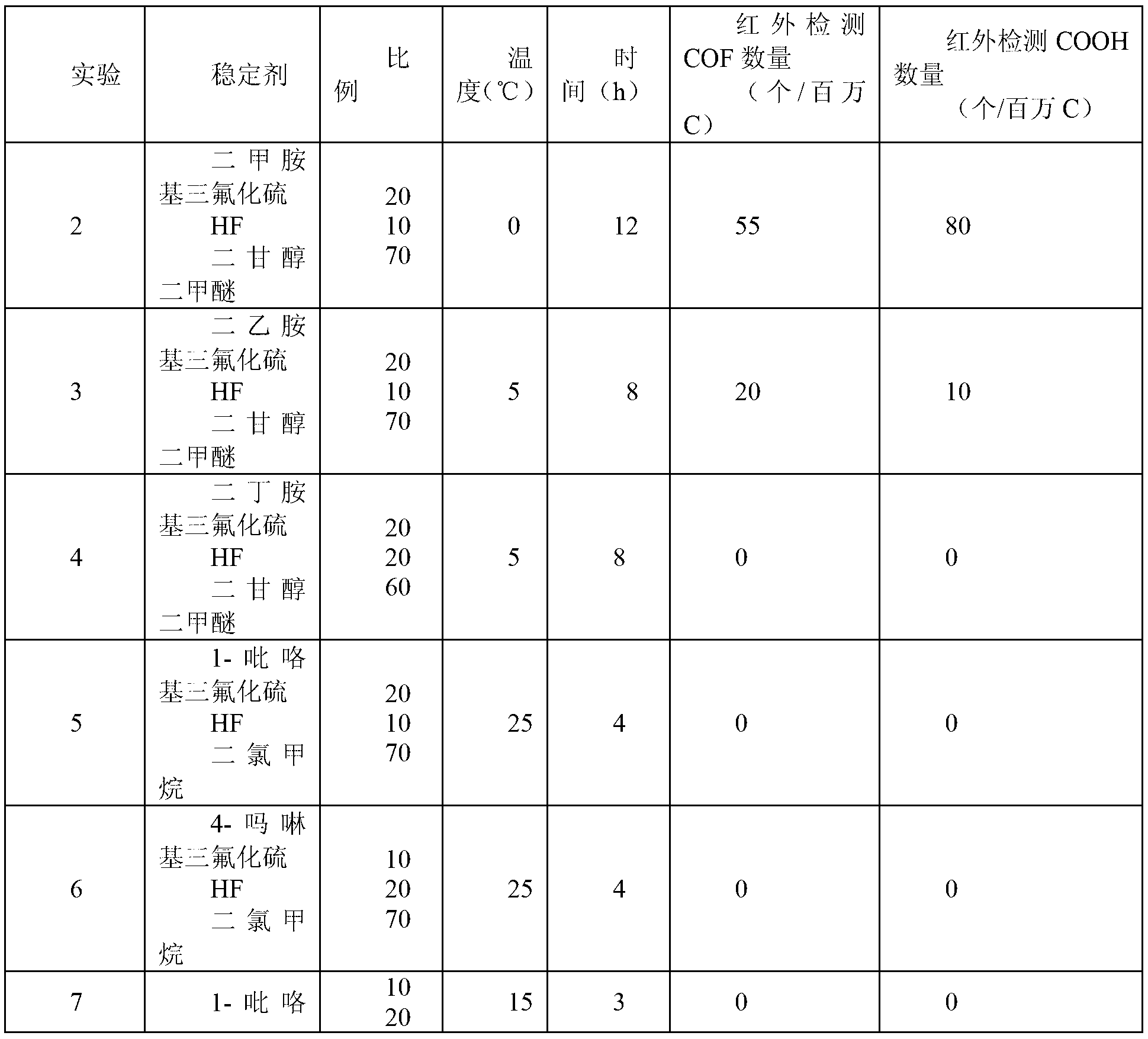
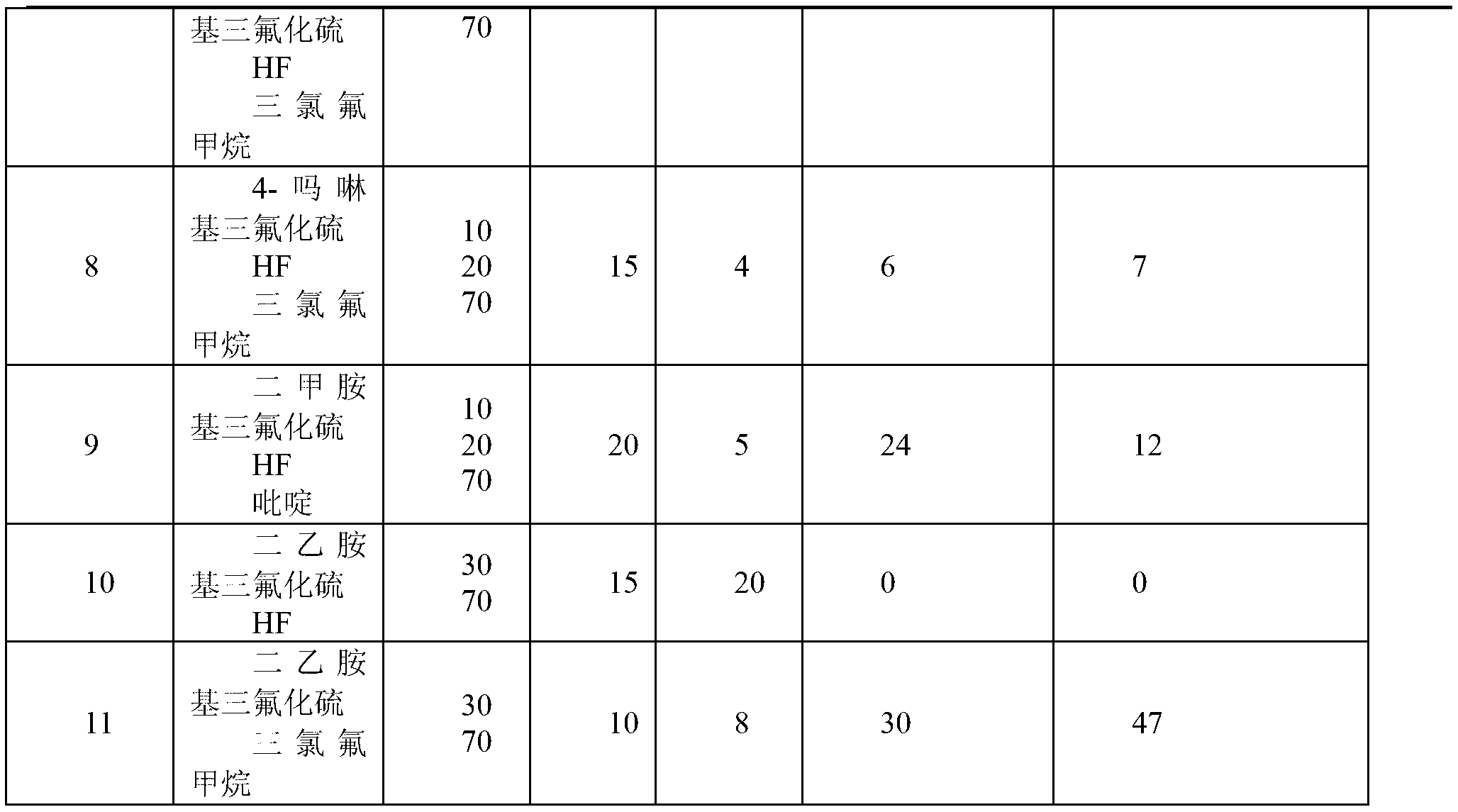
The invention discloses a perfluorination method for end group of fluorine-containing polymer. The method comprises the following steps of: mixing the fluorine polymer with an end group stabilizing reagent, standing or stirring to convert a -COF group and a -COOH group in the polymer into a -CF3 group, wherein the end group stabilizing reagent comprises the following three components: (1) SF3Ra; (2) anhydrous hydrofluoric acid AHF; and (3) one or mixture of more than any two of diethylene glycol dimethyl ether, pyridine, methylene dichloride and trichlorofluoromethane, and the reaction temperature of the perfluorination method for the end group ranges from 0 DEG C TO 100 DEG C. In the method, only specific group in the fluorine polymer is selected to be converted into -CF3, rather than that -CH2- in a polymer molecule chain with the fluoride gas fluorination process is fluorinated into -CF2-.
Owner:ZHEJIANG XINGTENG CHEM
Ether electrolyte as well as preparation method and application thereof
InactiveCN111224166AIncrease profitInhibit shuttleElectrolytesLi-accumulatorsElectrolytic agentLithium sulfur
The invention discloses an ether electrolyte. The ether electrolyte comprises a solute and a solvent; the solute contains lithium salt; the solvent is a mixed solution of diethylene glycol dimethyl ether and 1, 1, 2, 2-tetrafluoroethyl-2, 2, 3, 3-tetrafluoropropyl ether. According to the electrolyte, LiTFSI and LiFSI are mainly used as electrolytes, and G2 and HFE are used as solvents to prepare the novel lithium-sulfur electrolyte. A charge-discharge curve of the lithium-sulfur battery is reduced from two traditional voltage platforms to one, which shows that the electrolyte has low polysulfide dissolving capacity, the shuttle effect of polysulfide can be effectively inhibited, and the lithium-sulfur battery shows excellent cycle performance.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Cleaning agent for clearing laser carving residue from plastic shell of intelligent mobile terminal and cleaning method
InactiveCN107400566AQuality improvementImprove pass rateNon-ionic surface-active compoundsOrganic detergent compounding agentsGlycerolCleansing Agents
The invention relates to a cleaning agent for clearing laser carving residue from a plastic shell of an intelligent mobile terminal. The cleaning agent is prepared from the following ingredients in percentage by mass: 10 to 30 percent of penetrating agents, 10 to 30 percent of corrosion inhibiters, 1 to 10 percent of surfactants, 1 to 20 percent of organic auxiliary agents, 1 to 10 percent of chelating agents and the balance water; the penetrating agents are one kind of substances or a mixture of more than two kinds of substances in the following substances of diethylene glycol monobutyl ether, diethylene glycol dimethyl ether, proprylene glycol monomethyl ether, glycerol, glycol, isopropanol, diglycol and ethanol; the corrosion inhibiters are one kind of substances or a mixture of more than two kinds of substances in the following substances of benzotriazole, sulfydryl phenylpropyl isothiazole, oleic imidamline, citric acid, tartaric acid, oxalic acid, boric acid, dodecyl succinic anhydride, hexanedioic acid, n-caprylic acid, neodecanoic acid, sebacic acid, dodecandioic acid and gluconic acid. The cleaning agent provided by the invention has the advantages that the residue cleaning speed is high; substrates, protection paint and color paint of the plastic shell of the intelligent mobile terminal cannot be corroded and dissolved; safety and environment protection are realized; the mass cleaning is easy.
Owner:SHENZHEN FISHER NEW MATERIALS CO LTD
Portable clothing cleaning paper
InactiveCN102604765ADealing with soiled stains and moreDealing with stain removal etc.Surface-active detergent compositionsDetergent solventsVinyl etherAlcohol
The invention discloses a piece of portable clothing cleaning paper, belonging to the field of detergent. The portable clothing cleaning paper is made by spraying a stain removing solution on a piece of paper or soaking the piece of paper into the stain removing solution. The stain removing solution comprises the following components in percent by weight: 0.5-2.6% of carboxylate derivative AEC-4000 of natural plant oil alcohol polyoxypropylene and polyoxypropylene vinyl ether block copolymer, 3.5-7% of alkyl glycosides, 4-10% of lauryl alcohol polyoxyethylene ether (3) carboxylate symmetric diester succinate, 3.5-7% of natural fatty alcohol ether, 0.1-1% of polyvinylpyrrolidone, 3-7% of diethylene glycol dimethyl ether and the balance of water. The portable clothing cleaning paper is a portable high-efficiency environmentally-friendly product which can be used for cleaning clothing instantly and can solve the problem on the removal of the stain of the clothing contaminated due to unexpected events.
Owner:杜婧玉
Composition for surface cleaning of platinum-osmium alloy
A composition for surface cleaning of a platinum-osmium alloy comprises the following ingredients by weight: 10-30 parts of dihydroxymethyl benzoic acid, 30-50 parts of diethylene glycol dimethyl ether, 5-10 parts of N-methyl pyrrolidone, 50-70 parts of aluminum silicate, 20-30 parts of sodium dodecyl sulfonate, 30-40 parts of 2-ethyl hexanol, 20-30 parts of dimethyl imidazole, 5-10 parts of a surface active agent and 100-200 parts of ionic liquid. Compared with the conventional cleaning agent, the composition for surface cleaning of the platinum-osmium alloy, provided by the invention, has a good cleaning effect on grease, ensures that the residual quantity of a solvent is lower than the conventional technical standard, doesn't produce poisonous and volatile ingredients, and cannot react with metal.
Owner:JIANGSU KANG BAISI MECHANICAL TECH
A kind of method that calcium hydride reduces chlorosilane to prepare hydrosilane
ActiveCN108285467BRelaxed reaction conditionsLow costSilicon organic compoundsSilicon hydridesSilanesEthyl Chloride
Owner:INST OF CHEM CHINESE ACAD OF SCI
Production method of diethylene glycol dimethyl ether
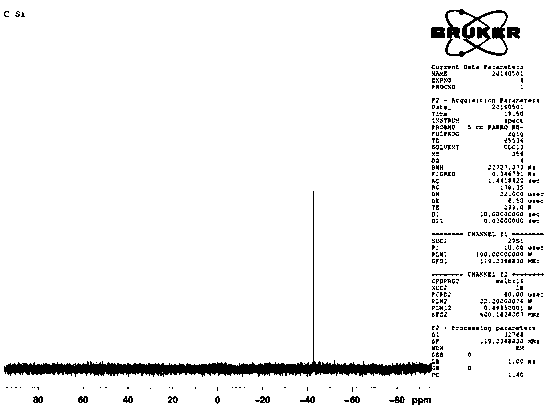
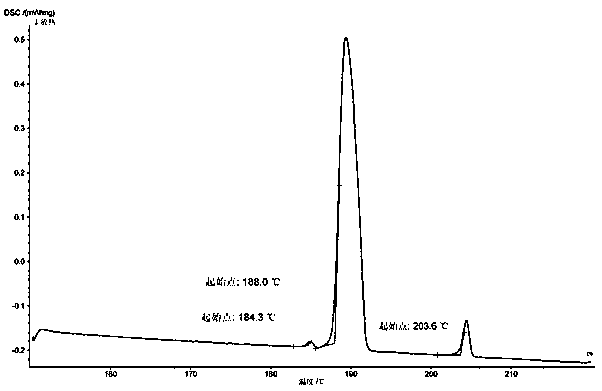
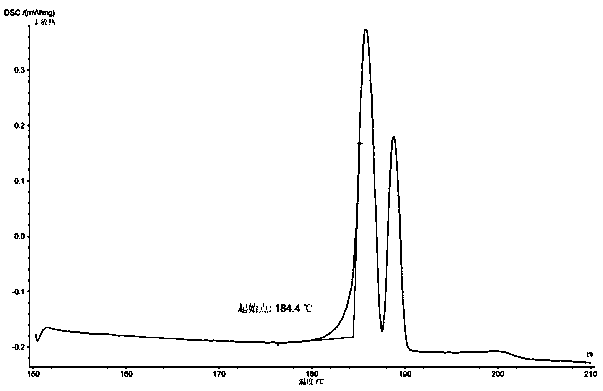
PendingCN112624910AReasonable designGuaranteed production efficiencyOrganic compound preparationChemical/physical/physico-chemical processesDiethylene glycolProcess engineering
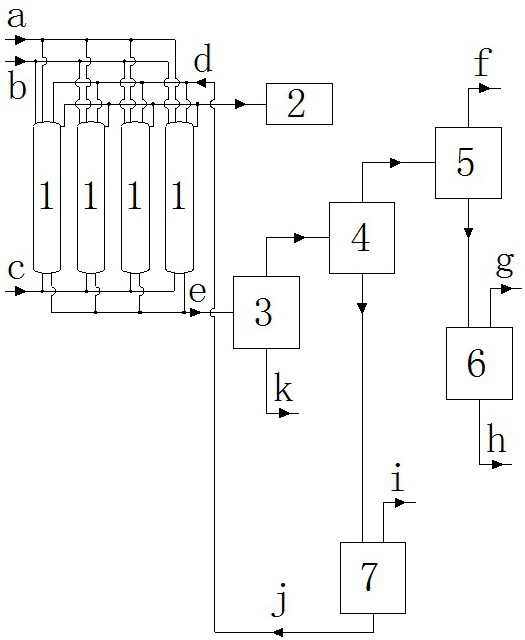
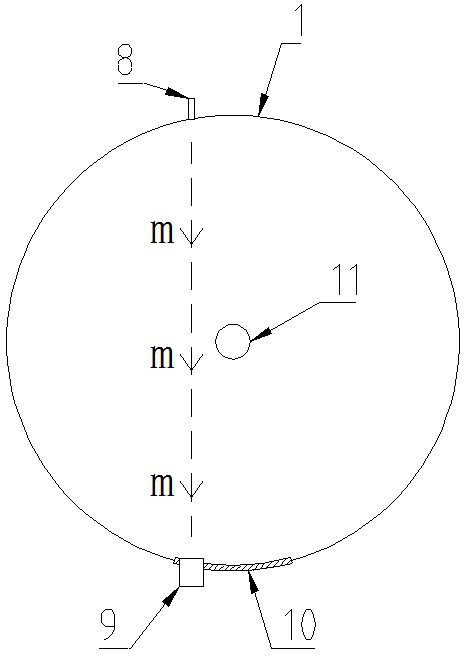
The invention discloses a production method of diethylene glycol dimethyl ether, which comprises four reaction kettles connected in parallel, a settling tank, a pre-separation tower, a dehydration tower, a finished product tower and a solvent recovery tower; the upper end faces of the four reaction kettles are respectively provided with a diethylene glycol methyl ether input passage, a sodium hydroxide input passage and an excessive diethylene glycol methyl ether reuse passage; the lower end faces of the four reaction kettles are respectively provided with a chloromethane input passage and a reaction liquid output passage, all raw materials fully react in the reaction kettles, then enter a settling tank for standing, settling and separating out solid waste sodium chloride, and then are rectified and separated step by step by utilizing a pre-separation tower, a dehydration tower, a finished product tower and a solvent recovery tower according to different boiling points of all components contained in supernatant liquid, thereby finally obtaining a finished product diethylene glycol dimethyl ether, and reusing the added excessive raw material diethylene glycol dimethyl ether in the reaction kettle. The reaction kettle has the advantages of reasonable process route design and high production efficiency, and can quickly and accurately judge whether the reaction in the reaction kettle is finished or not through the spotlight and the photodiode array.
Owner:HENAN HDF CHEM CO LTD
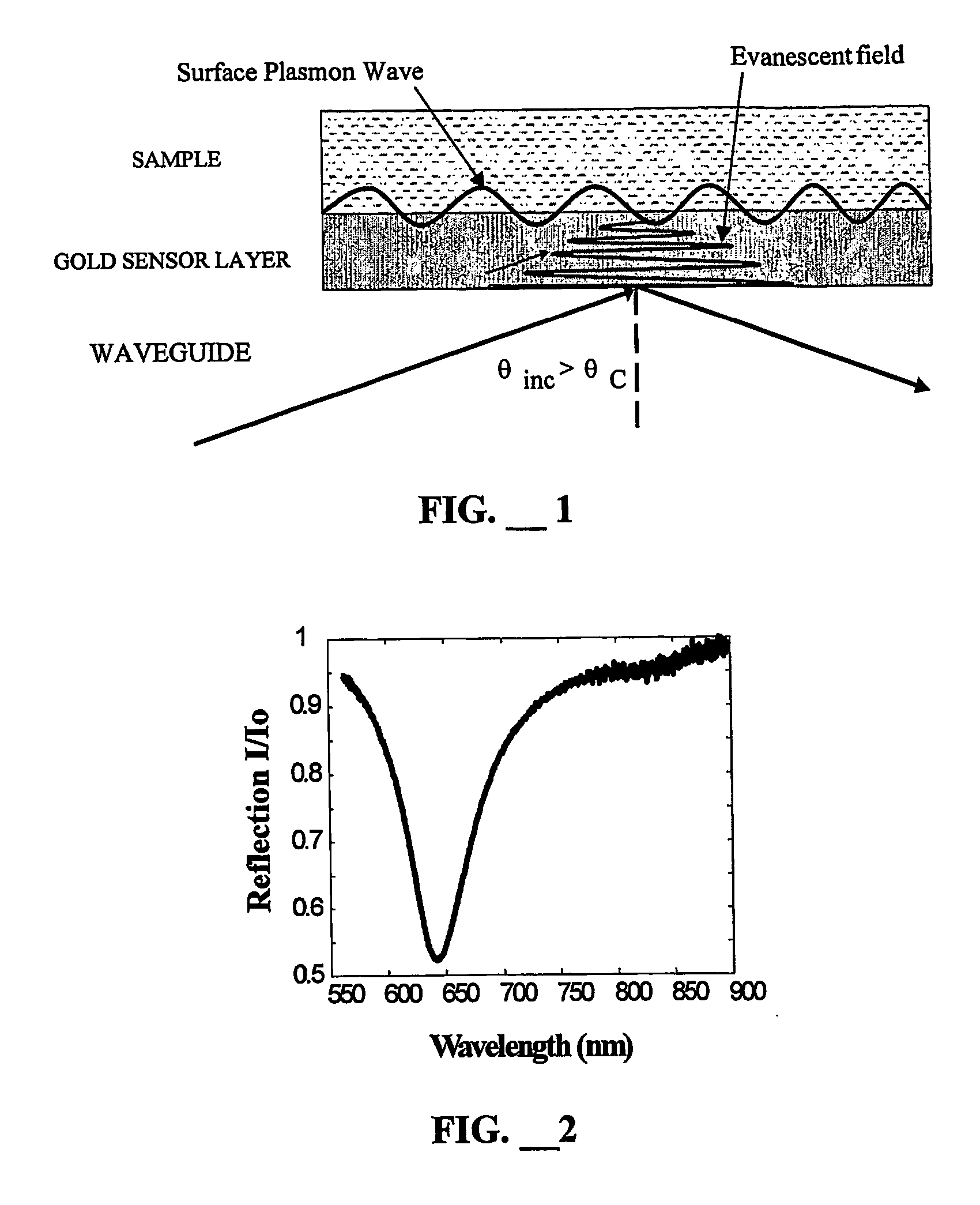
Biocompatible linkers for surface plasmon resonance biosensors
InactiveUS20060258021A1Material nanotechnologyPreparing sample for investigationAnalyteSelf-assembled monolayer
A method of coating an SPR biosensor specific for an analyte to reduce protein fouling, the method has the steps of providing an SPR biosensor, providing a solution of 11-mercaptoundecanol; incubating the SPR biosensor in the 11-mercaptoundecanol solution to form a self-assembling monolayer (SAM); incubating the SPR with SAM in a solution of epichlorohydrin and diglyme; next incubating the SPR in ethanolamine; preparing a solution of EDCNHS and a biocompatible polymer; incubating the SPR from ethanolamine in the EDC / NHS / polymer solution; providing a ligand specific for the analyte in a solution; incubating the polymer-coated SPR in the ligand solution to permit the ligand to react with the polymer-coated SPR; washing the ligand-coated SPR to remove unreacted ligand, thereby providing an SPR capable of reacting with the analyte. Another method replaces the solution for the SAM layer with a solution of MHA or NHS-MHA with HT, and attaches the ligand to the resulting SAM layer.
Owner:ARIZONA STATE UNIVERSITY
Improved wear-resistant ceramic plate
InactiveCN104370531AImprove wear resistanceImprove thermal conductivityAluminium hydroxideSilicon oxide
The invention relates to an improved wear-resistant ceramic plate which is prepared from the following raw materials in parts by weight: 6-11 parts of coal gangue, 9-14 parts of aluminum hydroxide, 7-14 parts of nano aluminum dioxide, 3-8 parts of zircon sand, 4-7 parts of kieselguhr, 5-9 parts of silicon oxide, 5-7 parts of cyclohexane, 5-10 parts of diethylene glycol dimethyl ether, 1-5 parts of SiCl4, 6-8 parts of benzyl dichlorosilane, 3-9 parts of sodium borohydride, 4-8 parts of glazed waste ceramic calcined sand, 3-5 parts of compound stabilizer and 2-7 parts of colored argil. The improved wear-resistant ceramic plate has the advantages of favorable wear resistance, favorable heat conductivity coefficient and long service life.
Owner:QINGDAO TOPLINK INFORMATION TECH
Solvent for treating polystyrene resin and method of treating polystyrene resin with the same
InactiveUS7365151B2Lower the volumeReduce the bulk of the polystyrene resinPlastic recyclingAliphatic hydrocarbonPolystyrene
A solvent for polystyrene resin treatment is used for reducing the volume of a polystyrene resin by contacting it with the solvent; and a method of treating polystyrene resins. The solvent is characterized by comprising 20 to 70 wt. % of diethylene glycol dimethyl ether and 30 to 80 wt. % of C9-13 aliphatic hydrocarbon. The method employs the solvent.
Owner:HAMANO SHIGENOBU
Industrial pollution-free production method of borane ester complex
InactiveCN103880785ASolve processing problemsEffective protectionCalcium/strontium/barium fluoridesSulfide preparationBoron trifluorideWater resources
The invention discloses an industrial pollution-free production method of a borane ester complex. The industrial pollution-free production method comprises the following steps: (1) dropwise adding boron trifluoride diethyl etherate to a reaction kettle; (2) adding sodium borohydride to react with the added boron trifluoride diethyl etherate in the reaction kettle by using diethylene glycol dimethyl ether as a solvent; (3) condensing diborane produced by the reaction in the step (2) by virtue of a condenser, and then transferring into an absorption kettle; (4) enabling the cooled diborane gas obtained in the step (3) to react with tetrahydrofuran or dimethylsulfide in the absorption kettle to obtain a borane complex. By adopting the industrial pollution-free production method of the borane ester complex, the problem of caking during blanking for production or reproduction in later can be solved, and an another problem due to wastewater treatment can also be solved; the industrial pollution-free production method is suitable for industrial production and can be used for effectively protecting the water resource and soil.
Owner:HUABIN BIO TECH
Preparation method of diphenyl cyclosiloxane
PendingCN111072715ASolve problemsHigh yieldSilicon organic compoundsTetramethylammonium hydroxideMeth-
The invention relates to a preparation method of diphenyl cyclosiloxane. In the method, high-purity diphenyl dimethoxysilane free of polychlorinated biphenyl and a small amount of water are adopted asraw materials, diether series compounds such as ethylene glycol diethyl ether and diethylene glycol dimethyl ether with normal-pressure boiling points higher than 100 DEG C are used as solvents, a trace amount of an organic alkali such as tetramethylammonium hydroxide is used as a catalyst, generated methanol is distilled off in a micro-boiling state in a reaction kettle with a rectifying column,and commercial diphenyl cyclosiloxane is obtained in one step. The generated powdery diphenyl cyclosiloxane is extremely easy to separate from the liquid, all solvents are completely recycled and used mechanically, the byproduct methanol can also be rectified and purified for application, and no wastewater is discharged in the whole process.
Owner:仙桃市格瑞化学工业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com