Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
20548 results about "Chemical reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.
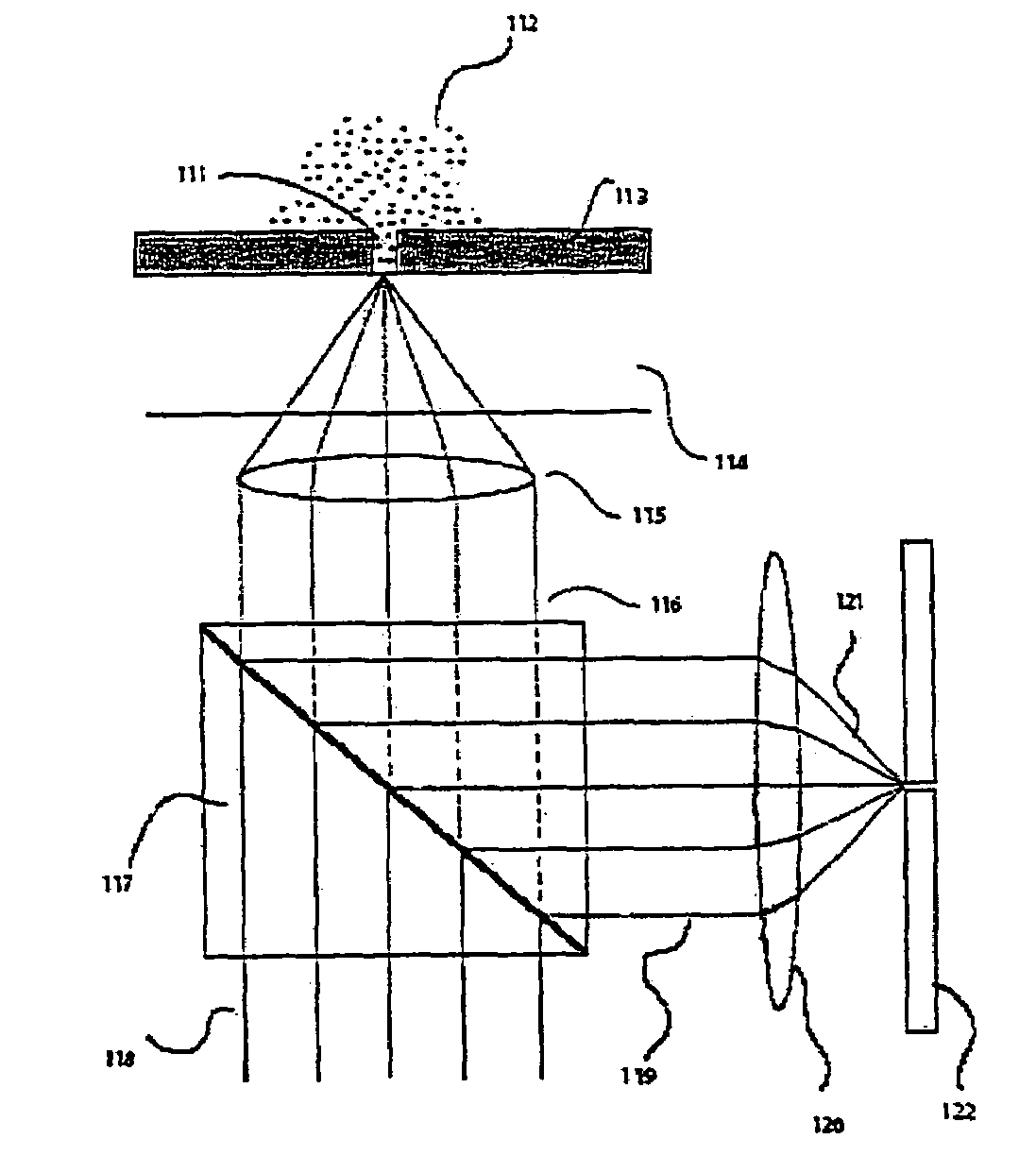
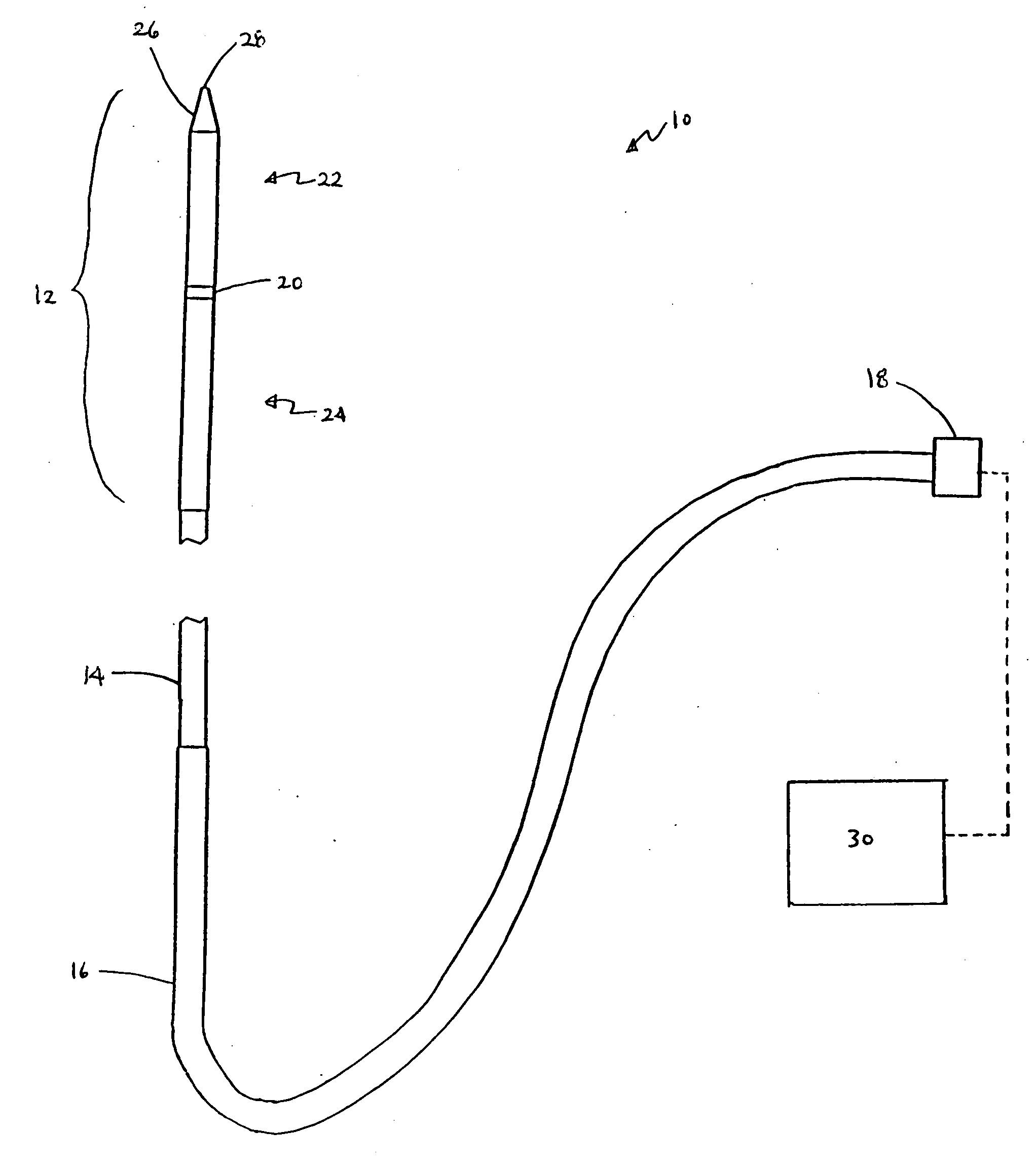
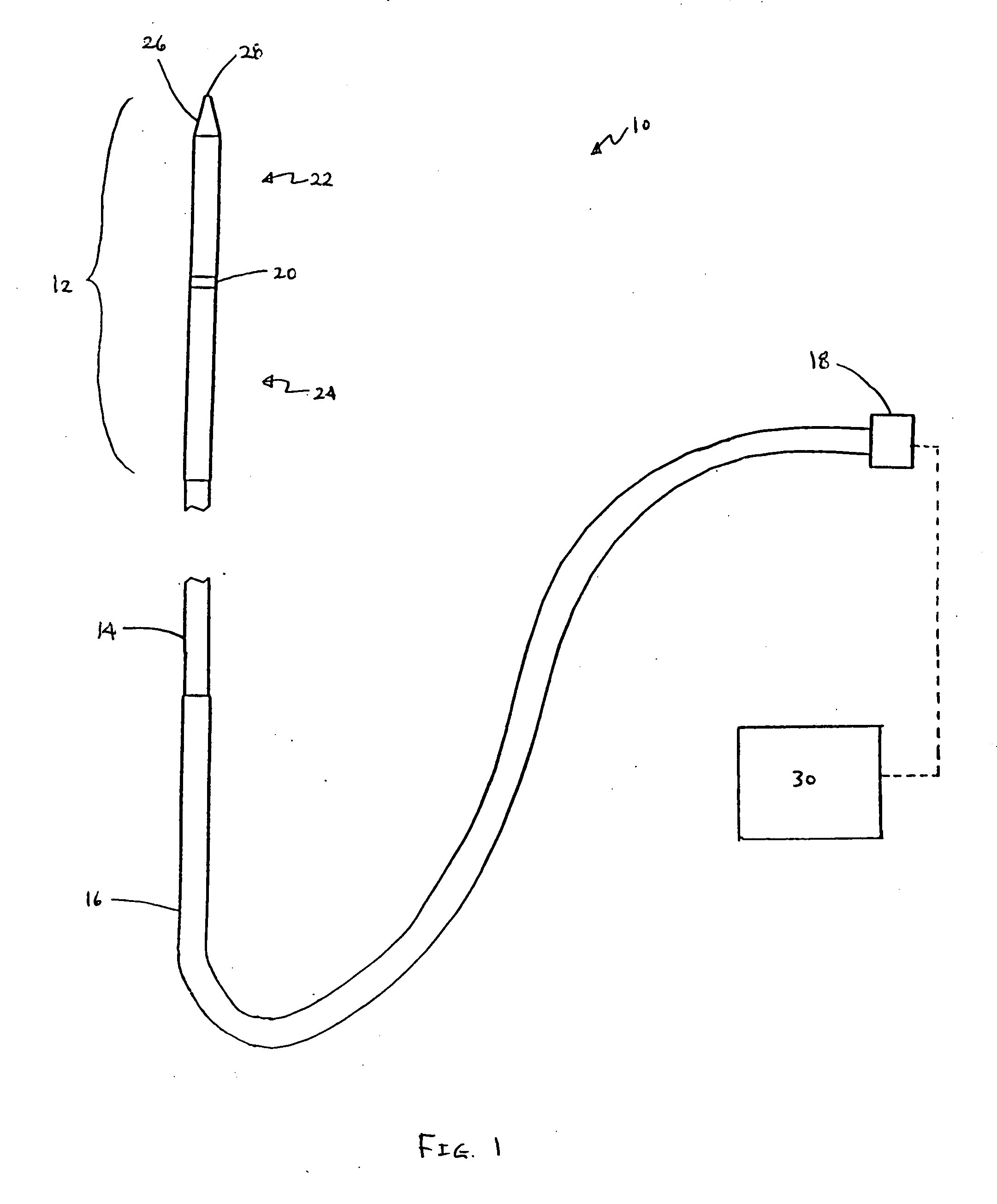
Apparatus and methods for optical analysis of molecules
ActiveUS7170050B2Small volumeEffective volumeRadiation pyrometryLaser detailsMolecular analysisChemical reaction
The present invention relates to optical confinements, methods of preparing and methods of using them for analyzing molecules and / or monitoring chemical reactions. The apparatus and methods embodied in the present invention are particularly useful for high-throughout and low-cost single-molecular analysis.
Owner:NANOFLUIDICS INC +1
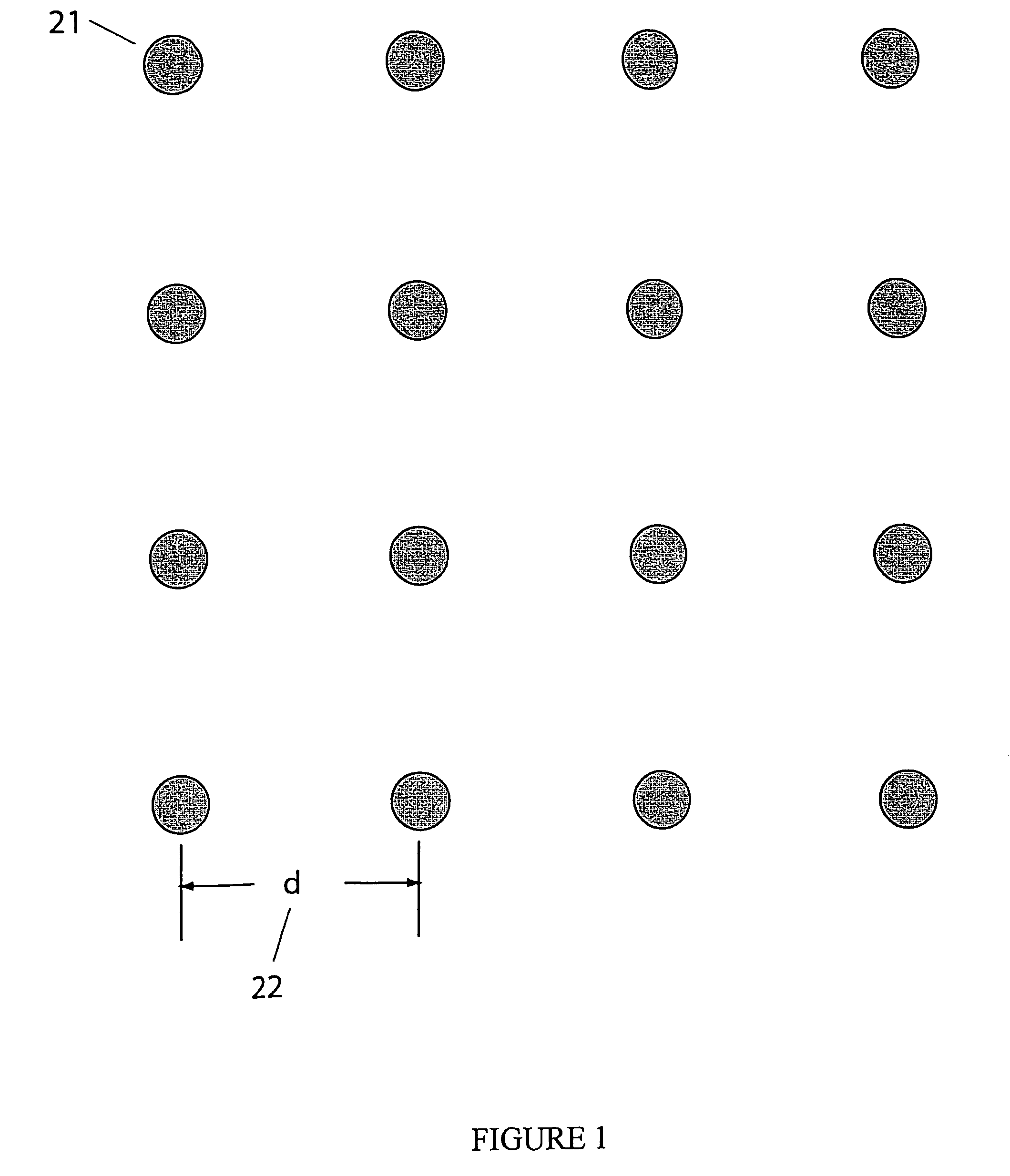
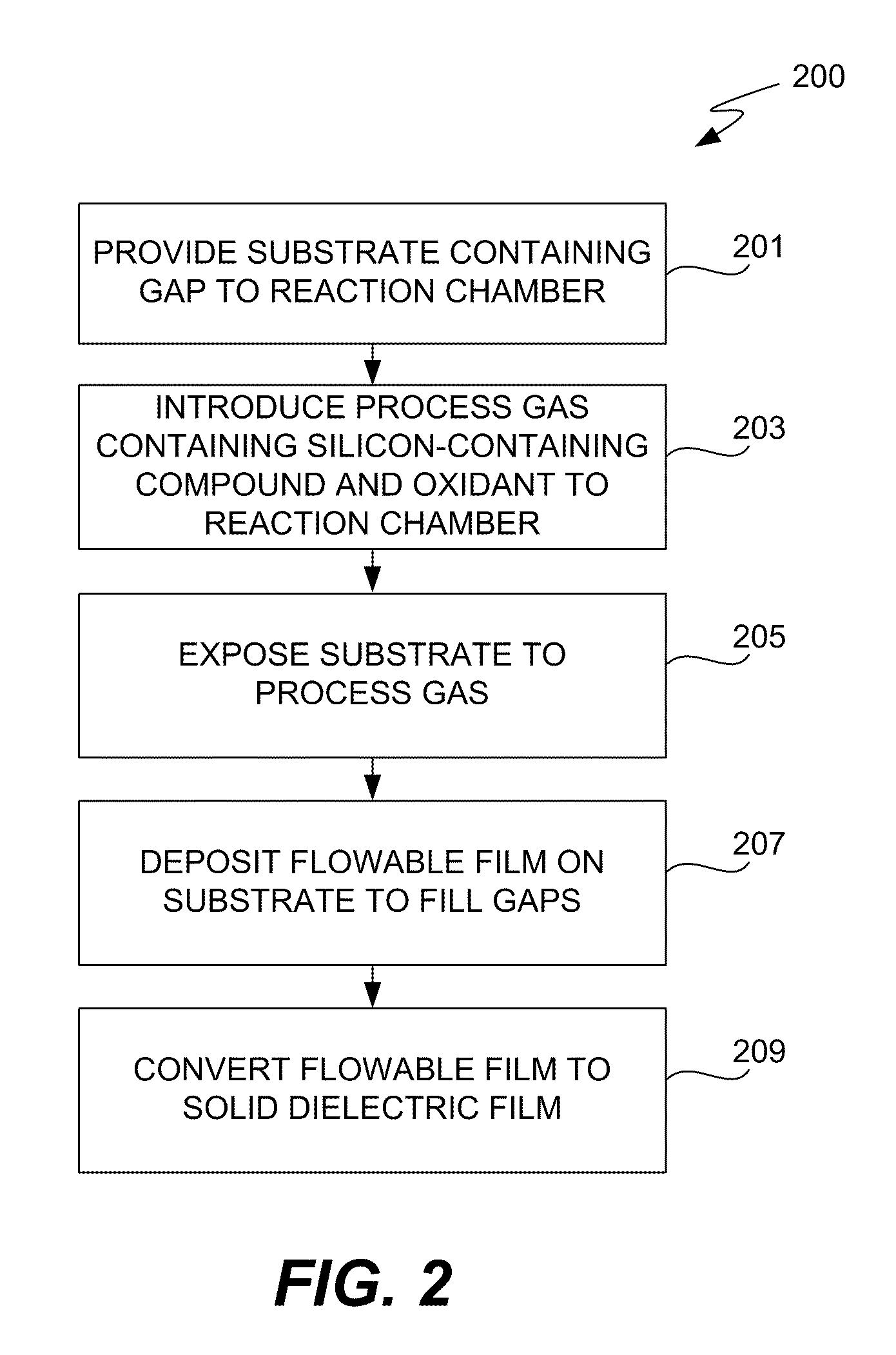
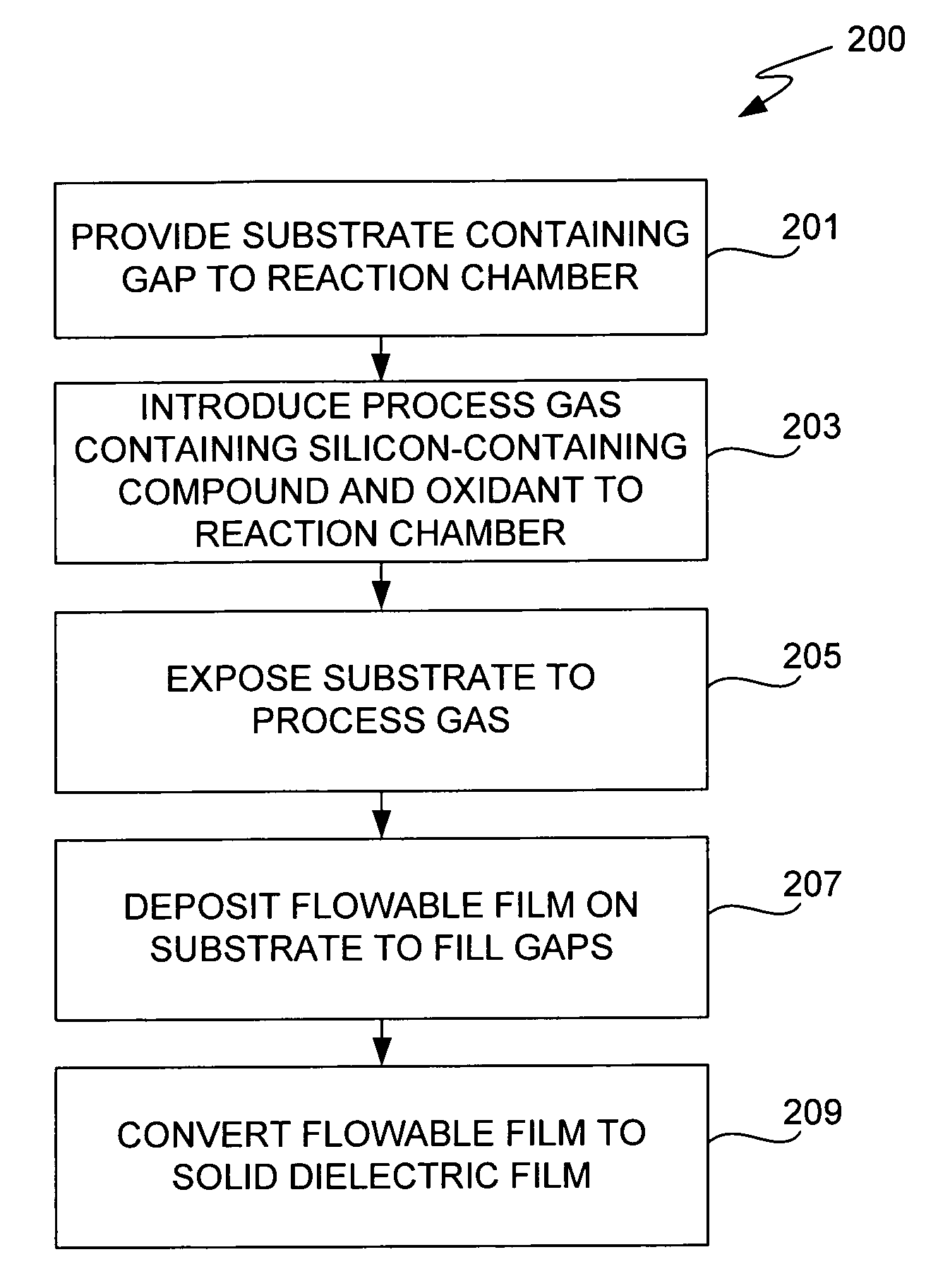
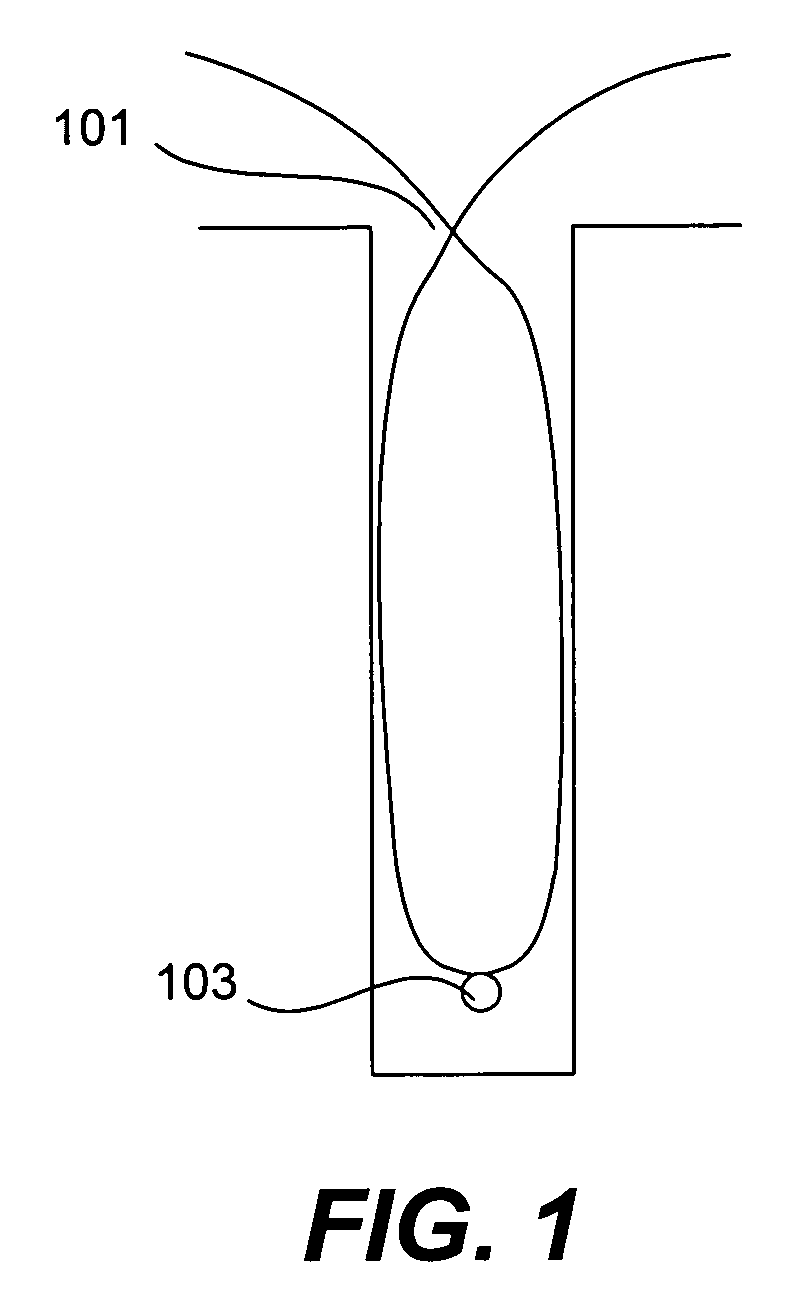
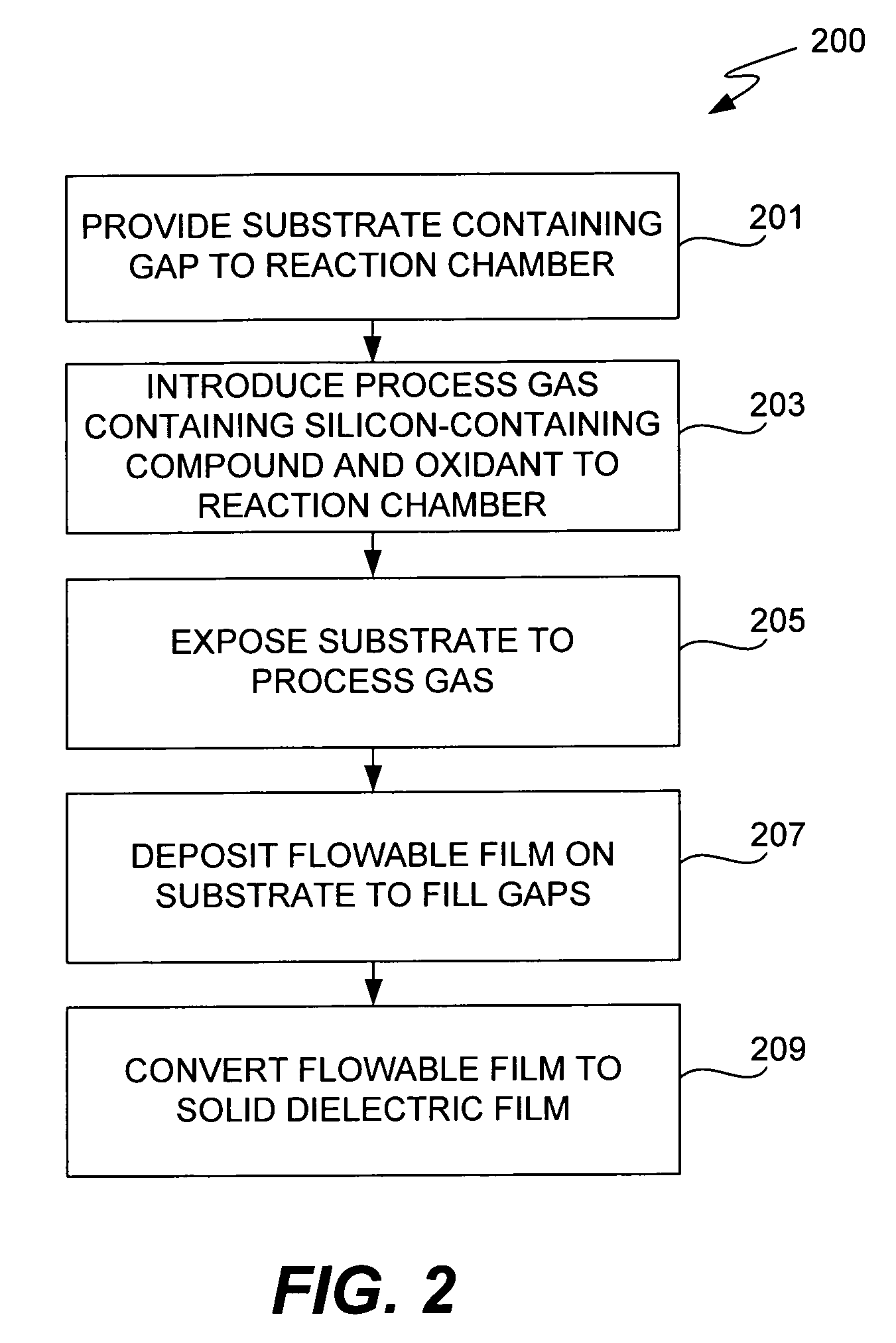
CVD flowable gap fill
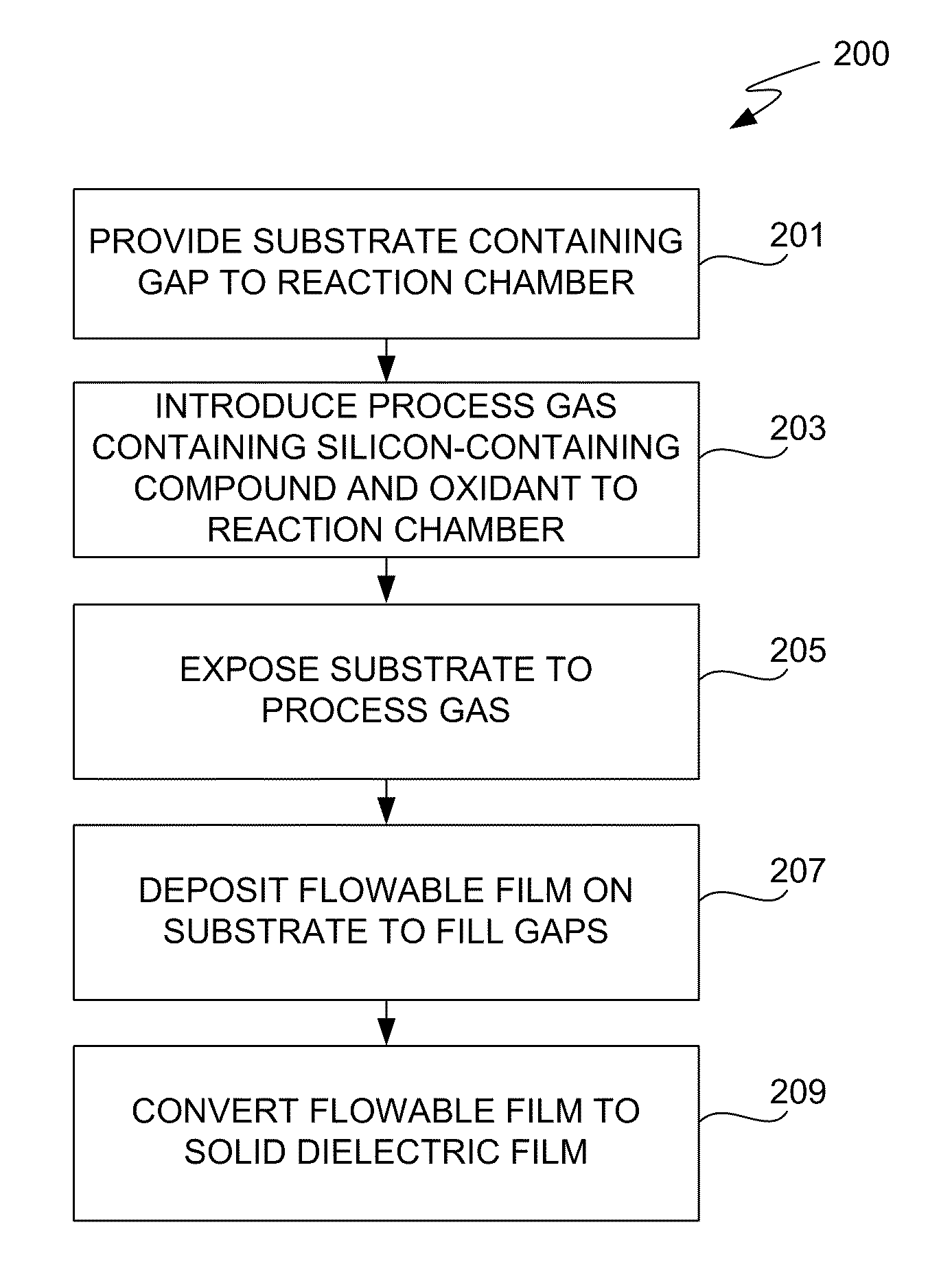
The present invention meets these needs by providing improved methods of filling gaps. In certain embodiments, the methods involve placing a substrate into a reaction chamber and introducing a vapor phase silicon-containing compound and oxidant into the chamber. Reactor conditions are controlled so that the silicon-containing compound and the oxidant are made to react and condense onto the substrate. The chemical reaction causes the formation of a flowable film, in some instances containing Si—OH, Si—H and Si—O bonds. The flowable film fills gaps on the substrates. The flowable film is then converted into a silicon oxide film, for example by plasma or thermal annealing. The methods of this invention may be used to fill high aspect ratio gaps, including gaps having aspect ratios ranging from 3:1 to 10:1.
Owner:NOVELLUS SYSTEMS
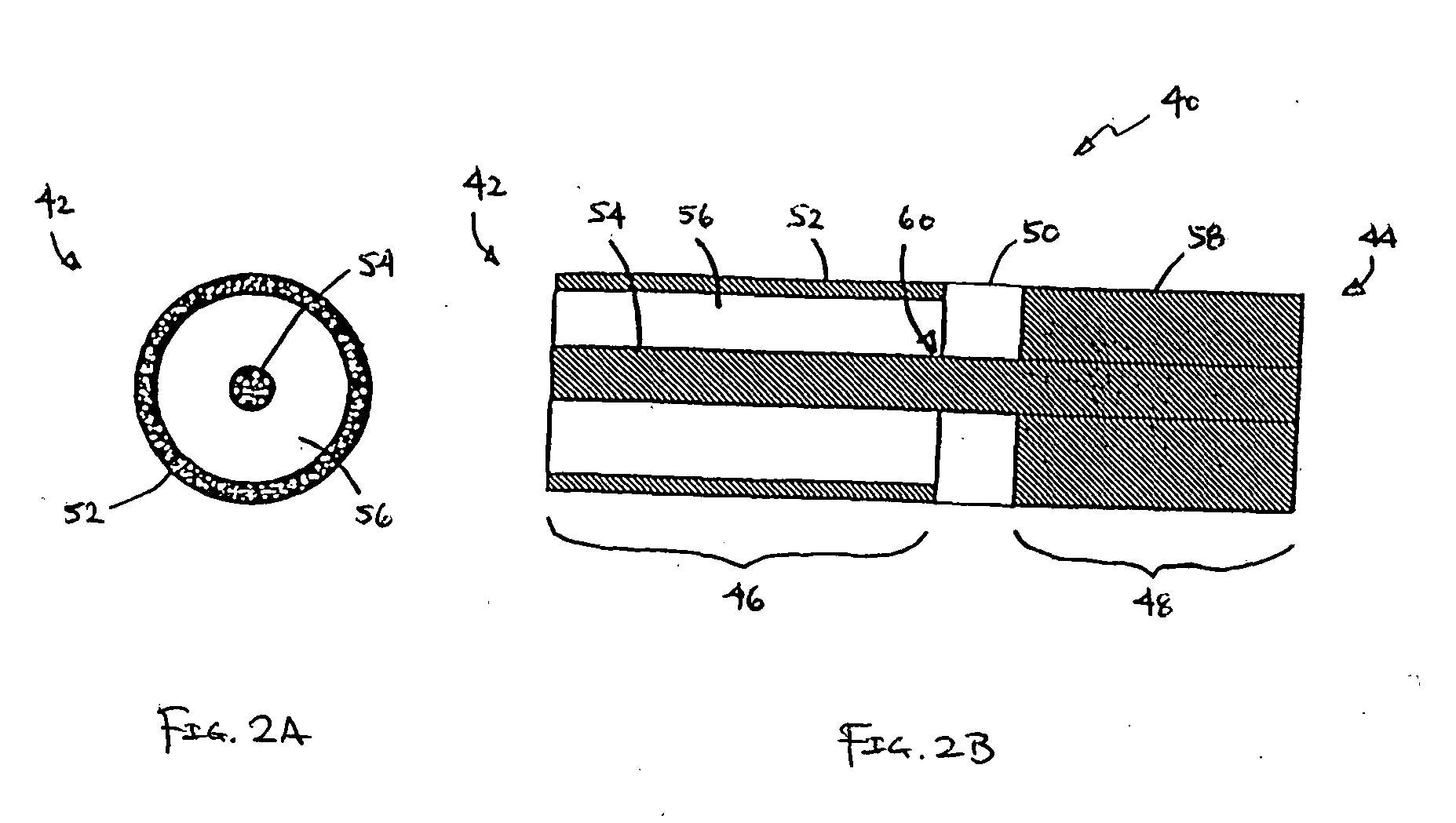
Apparatus and method for analysis of molecules
ActiveUS7302146B2Improve accuracyEasy to implementMaterial nanotechnologyCladded optical fibreMolecular analysisChemical reaction
Owner:PACIFIC BIOSCIENCES
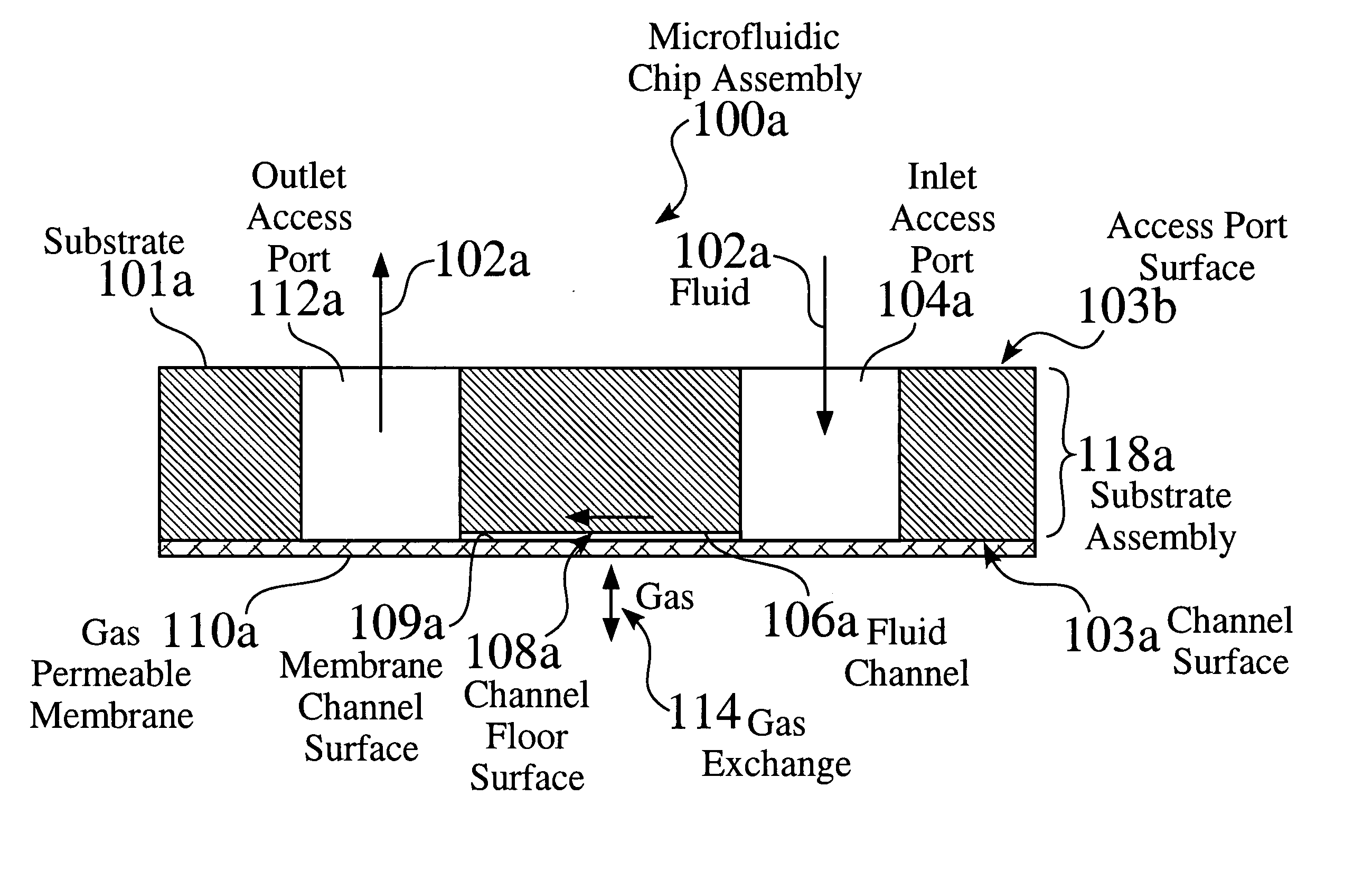
Microfluidic system with integrated permeable membrane
InactiveUS20050266582A1Analysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorChemical reactionCompound (substance)
A microfluidic system for performing chemical reactions or biochemical, biological, or chemical assays utilizing a microfabricated device or “chip.” The system may include, among others, an integrated membrane fabricated from a chemically inert material whose permeability for gases, liquids, cells, and specific molecules, etc. can be selected for optimum results in a desired application.
Owner:CYTODISCOVERY
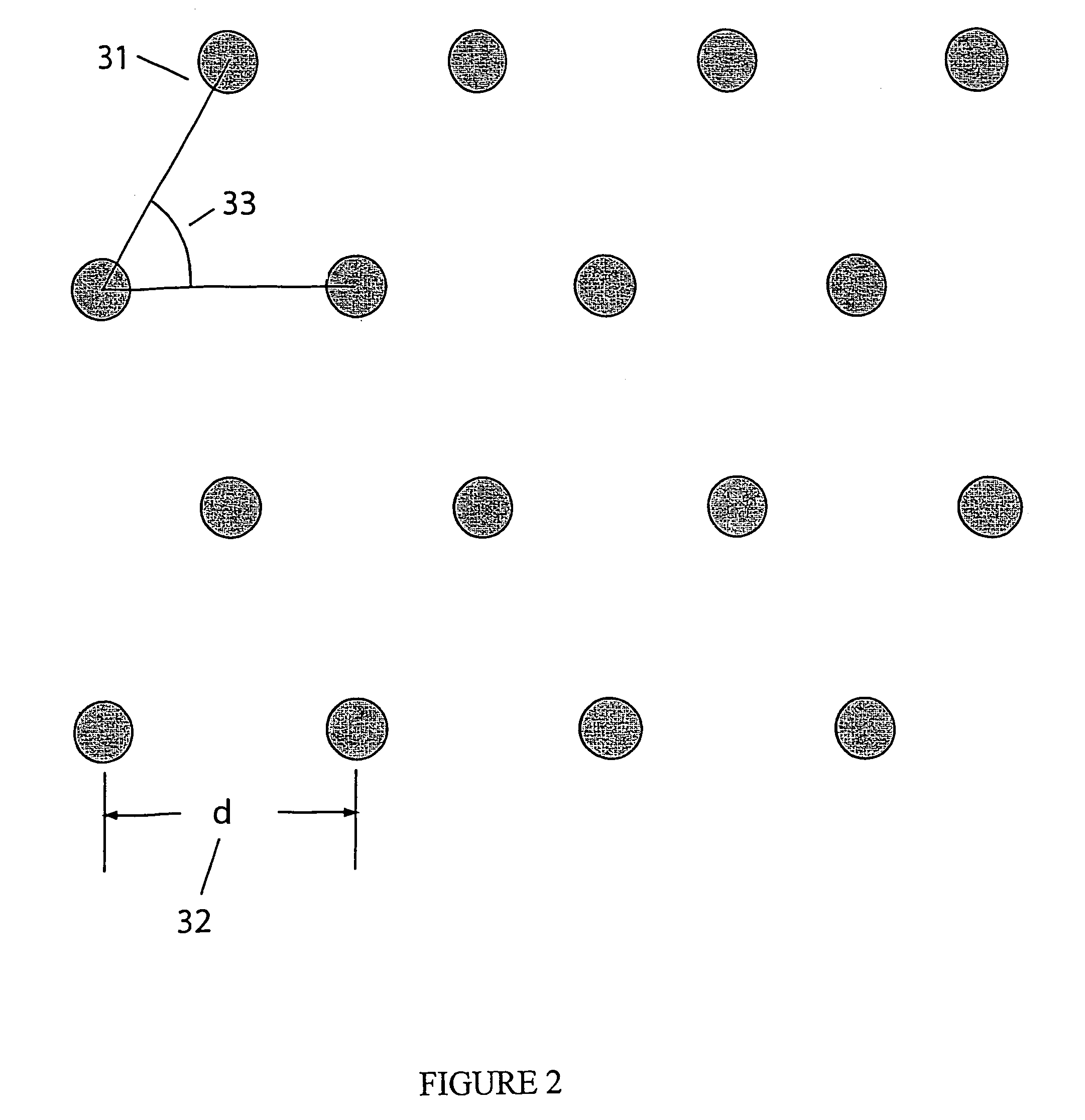
CVD flowable gap fill
The present invention meets these needs by providing improved methods of filling gaps. In certain embodiments, the methods involve placing a substrate into a reaction chamber and introducing a vapor phase silicon-containing compound and oxidant into the chamber. Reactor conditions are controlled so that the silicon-containing compound and the oxidant are made to react and condense onto the substrate. The chemical reaction causes the formation of a flowable film, in some instances containing Si—OH, Si—H and Si—O bonds. The flowable film fills gaps on the substrates. The flowable film is then converted into a silicon oxide film, for example by plasma or thermal annealing. The methods of this invention may be used to fill high aspect ratio gaps, including gaps having aspect ratios ranging from 3:1 to 10:1.
Owner:NOVELLUS SYSTEMS
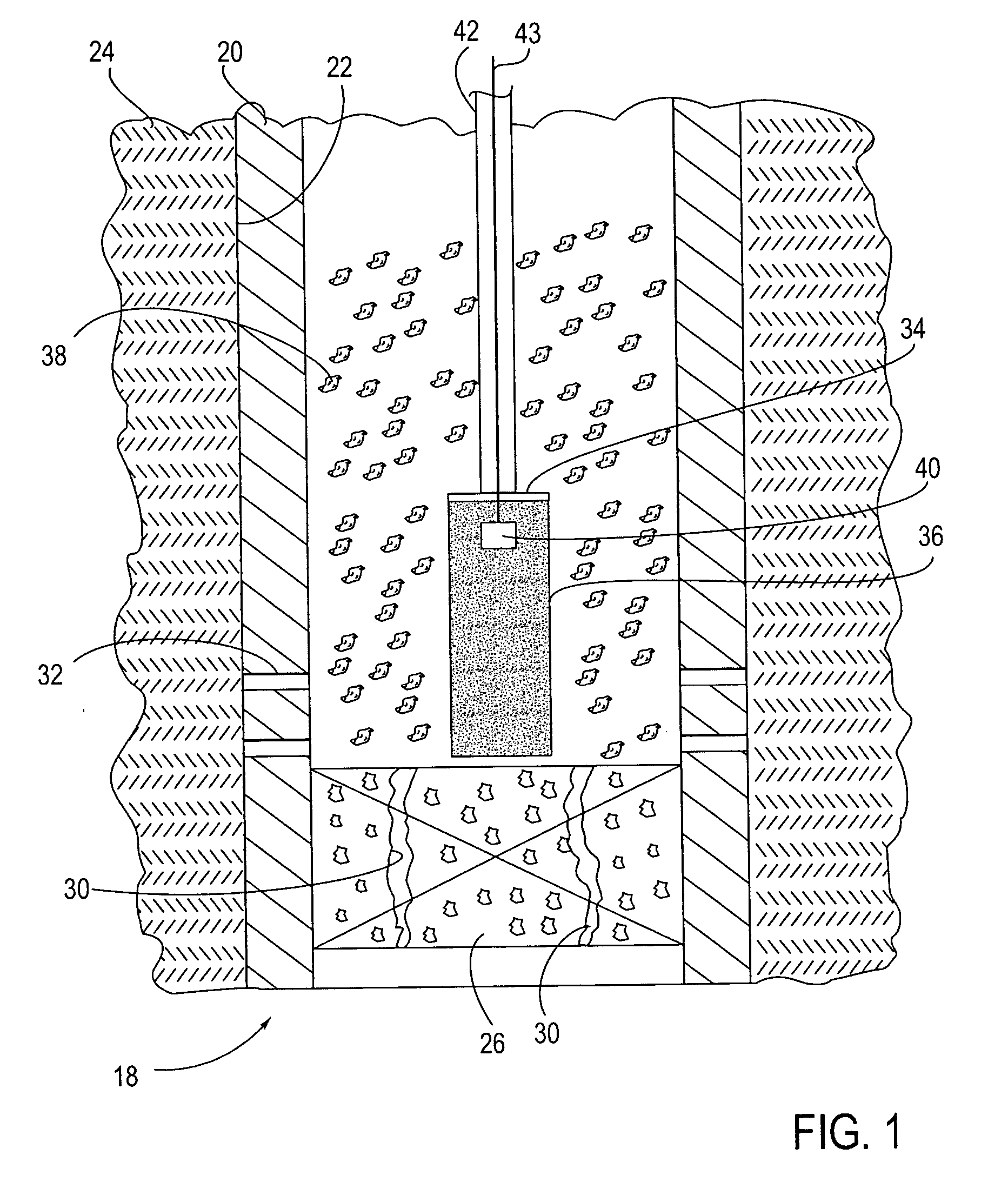
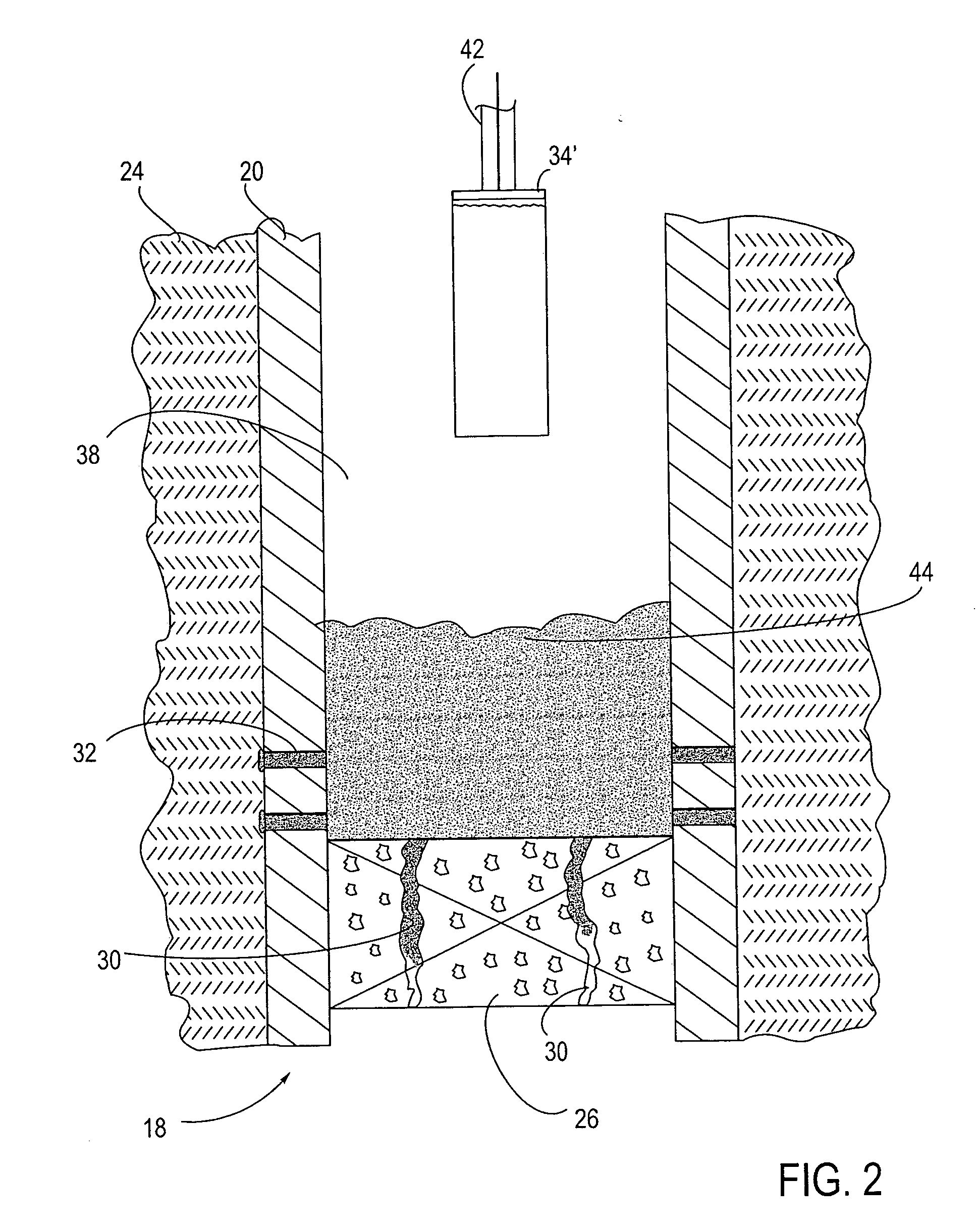
Device and method for wound therapy
A disposable wound-healing device is disclosed that incorporates a housing having a fluid-impermeable material having a cavity and a perimeter that can be sealed in an air-tight manner over a wound region of a patient. The device is capable of producing a negative pressure over the wound region by either removing oxygen from within the cavity, or absorbing fluid into the cavity and then removing the fluid from the cavity. The oxygen may be removed via chemical absorption, by an electrochemical cell or by a chemical reaction that cannibalizes oxygen from the cavity. The fluid may be removed through the use of osmotic or electro-osmotic cells, or through a one-way valve. The negative partial pressure over the wound region promotes healing.
Owner:MICROLIN
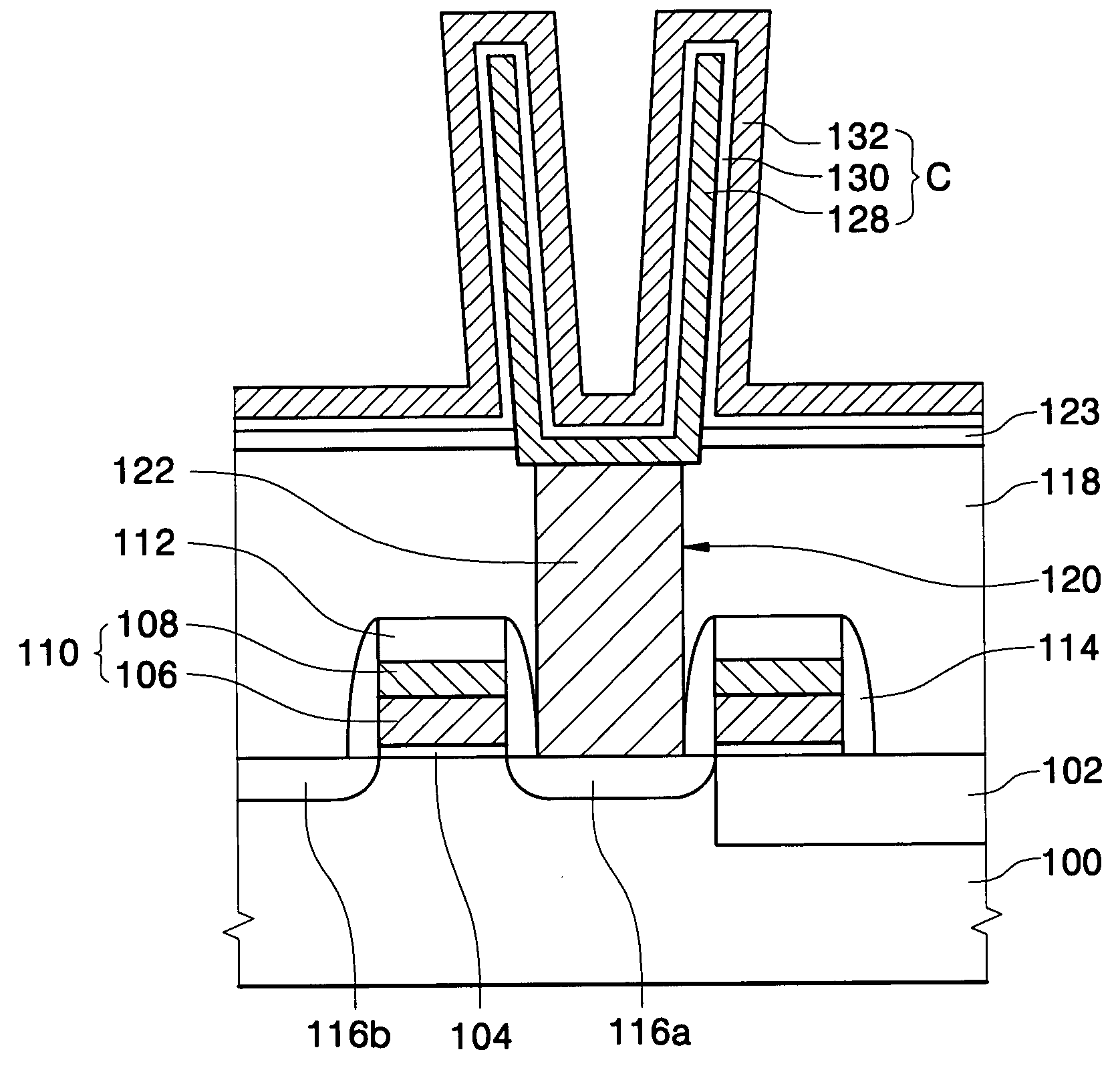
Method of forming a layer and forming a capacitor of a semiconductor device having the same layer
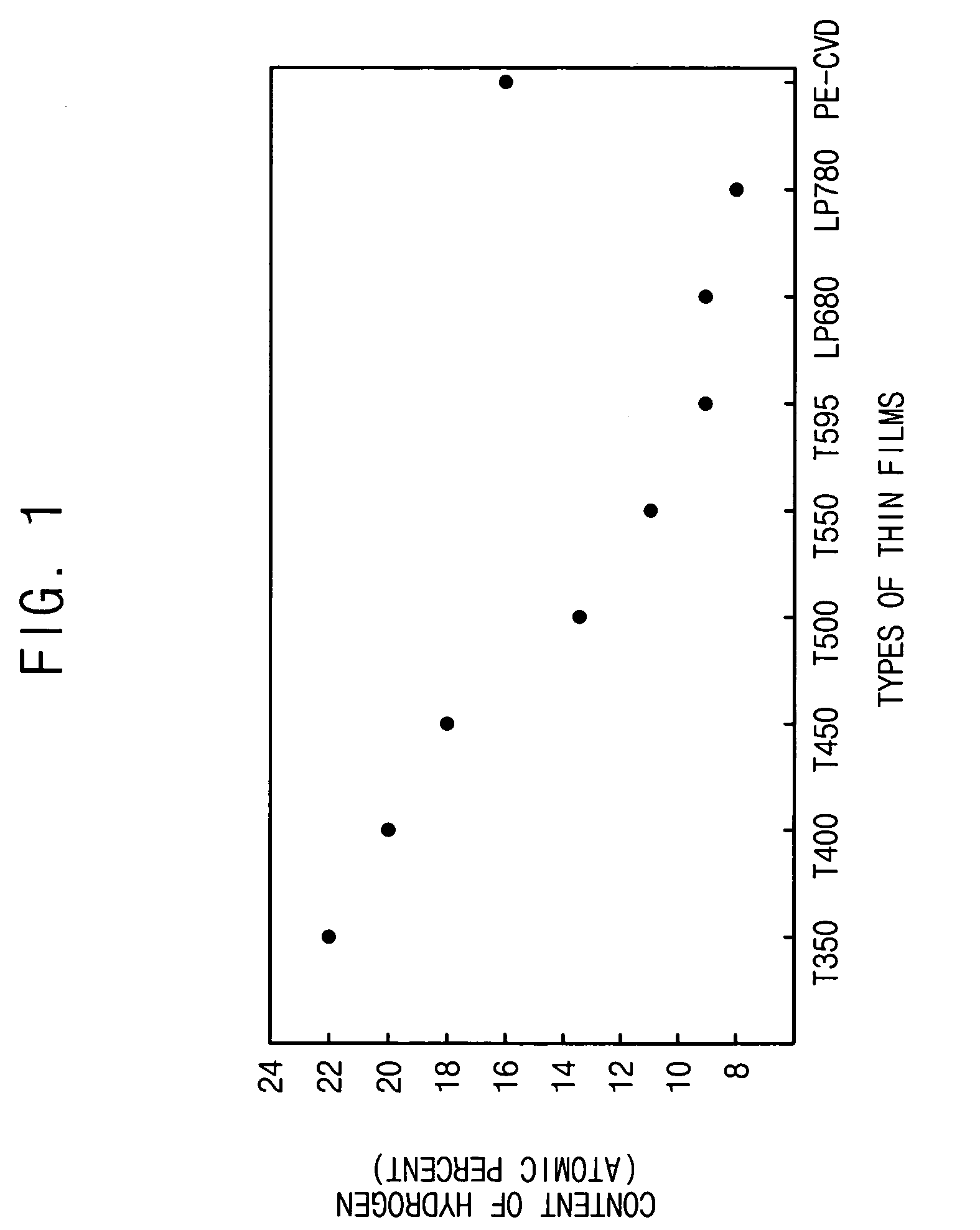
InactiveUS20060014384A1Low hydrogen contentReduce leakage currentSemiconductor/solid-state device manufacturingCapacitorsChemical reactionDevice material
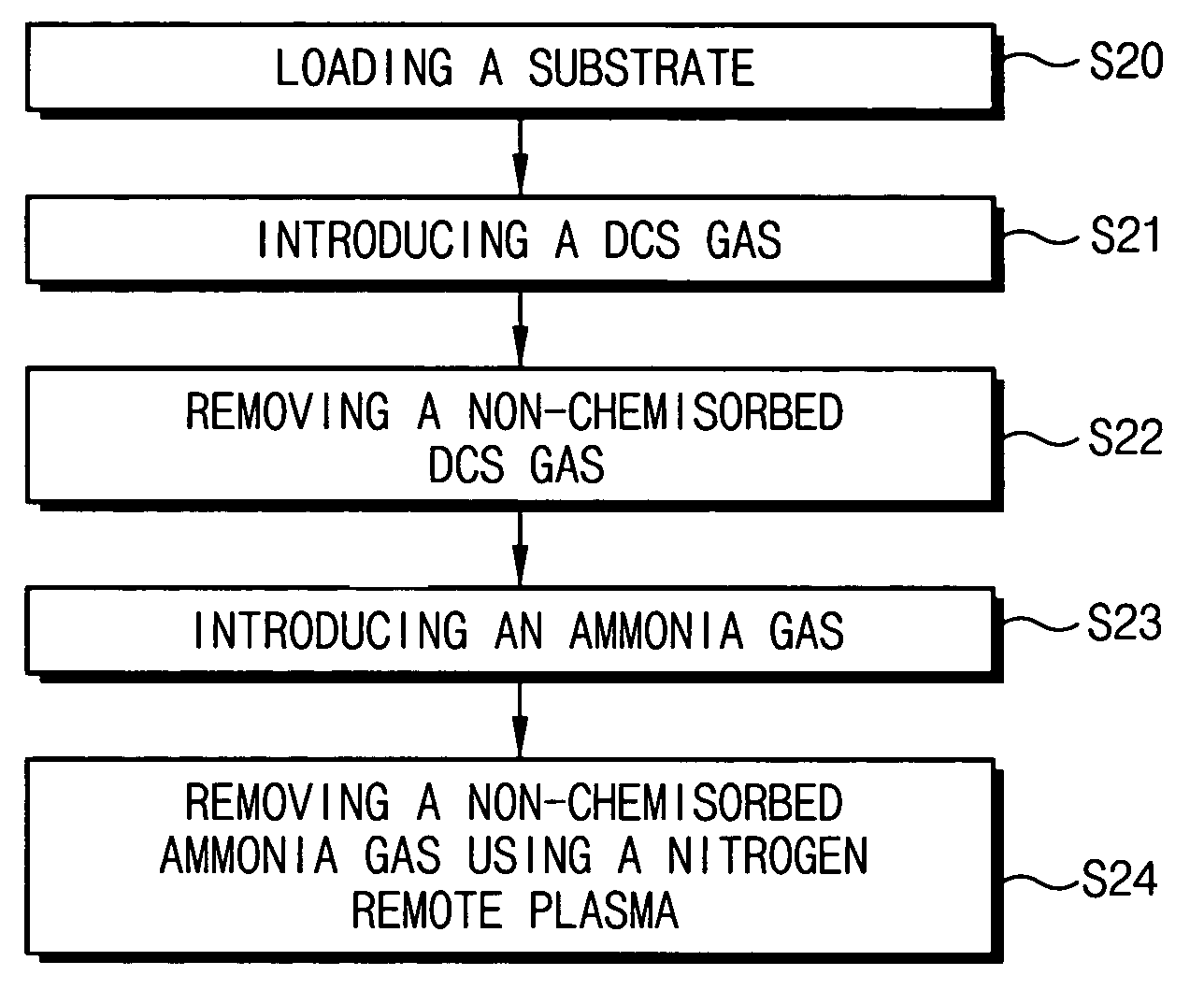
In a method of forming a layer using an atomic layer deposition process, after a substrate is loaded into a chamber, a first reactant is provided onto the substrate. The first reactant is partially chemisorbed on the substrate. A second reactant is introduced into the chamber to form a preliminary layer on the substrate by chemically reacting the second reactant with the chemisorbed first reactant. Impurities in the preliminary layer and unreacted reactants are simultaneously removed using a plasma for removing impurities to thereby form the layer on the substrate. The impurities in the layer may be effectively removed so that the layer may have reduced leakage current.
Owner:SAMSUNG ELECTRONICS CO LTD
Systems and methods of forming particles
InactiveUS20070054119A1Synthetic resin layered productsChemical/physical/physico-chemical microreactorsChemical reactionMicrometer
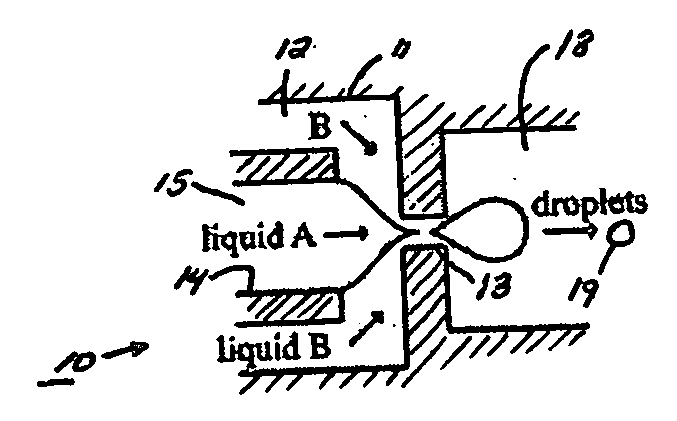
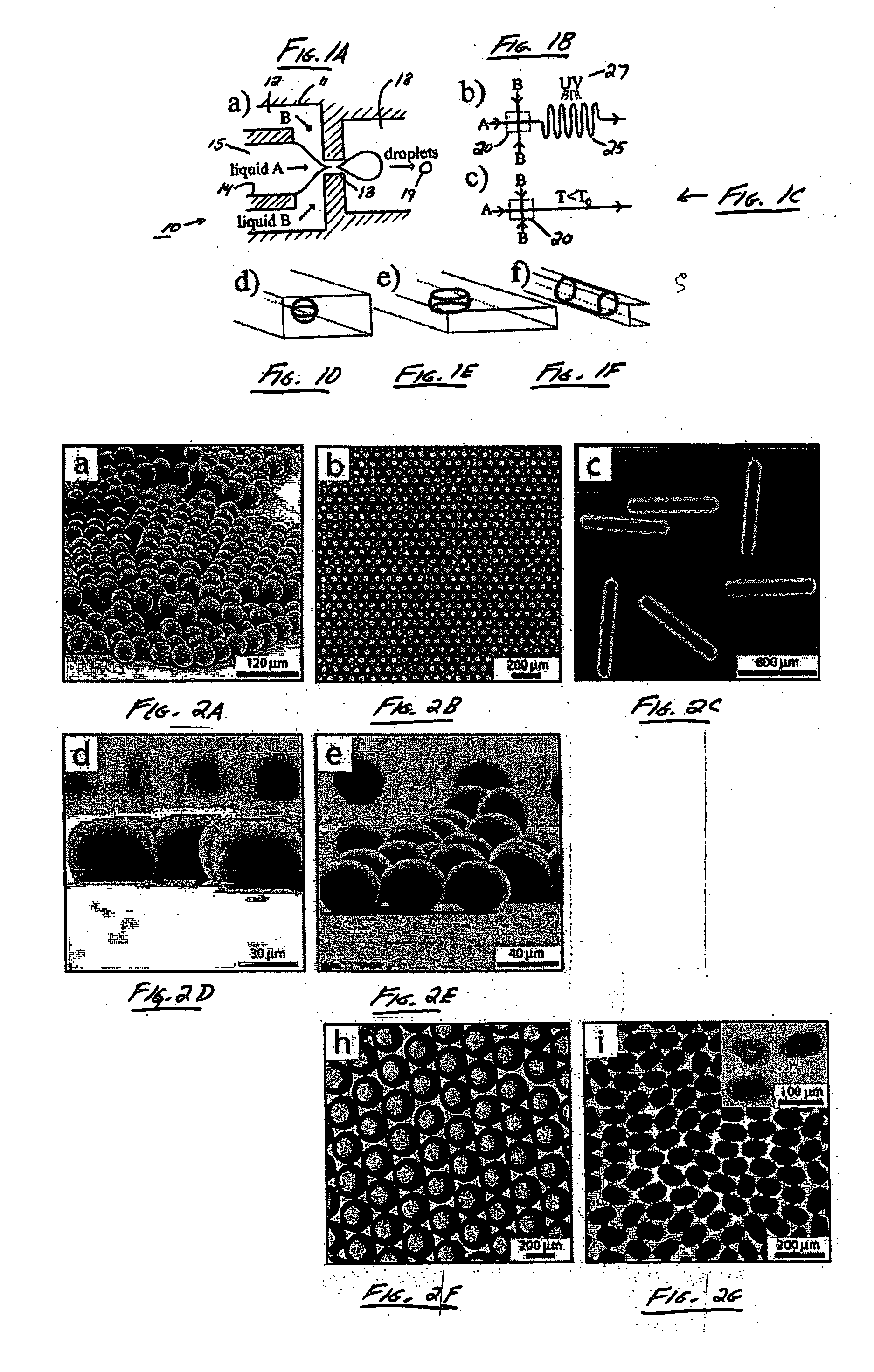
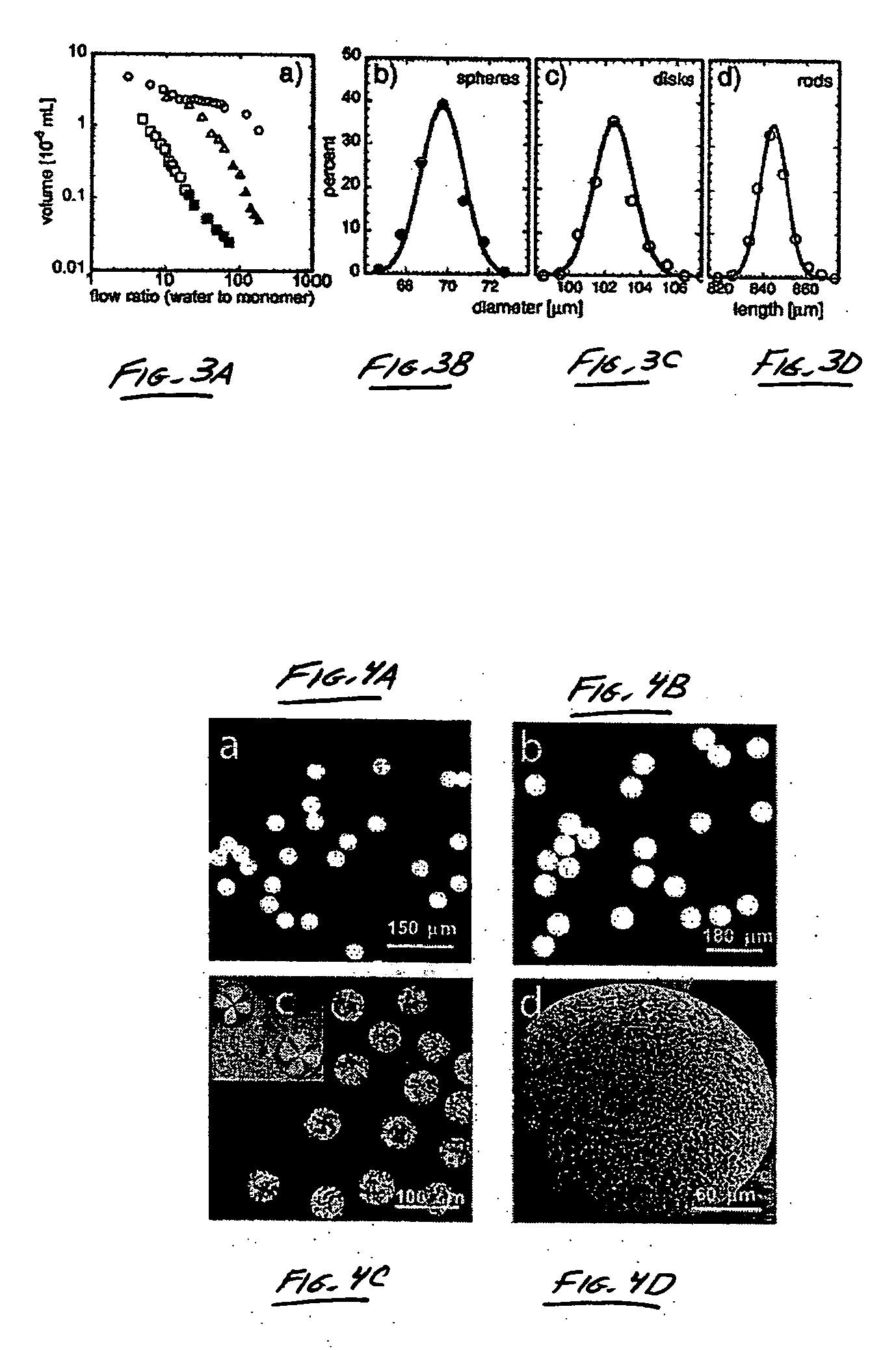
The present invention generally relates to systems and methods of forming particles and, in certain aspects, to systems and methods of forming particles that are substantially monodisperse. Microfluidic systems and techniques for forming such particles are provided, for instance, particles may be formed using gellation, solidification, and / or chemical reactions such as cross-linking, polymerization, and / or interfacial polymerization reactions. In one aspect, the present invention is directed to a plurality of particles having an average dimension of less than about 500 micrometers and a distribution of dimensions such that no more than about 5% of the particles have a dimension greater than about 10% of the average dimension, which can be made via microfluidic systems. In one set of embodiments, at least some of the particles may comprise a metal, and in certain embodiments, at least some of the particles may comprise a magnetizable material. In another set of embodiments, at least some of the particles may be porous. In some embodiments, the invention includes non-spherical particles. Non-spherical particles may be formed, for example, by urging a fluidic droplet into a channel having a smallest dimension that is smaller than the diameter of a perfect mathematical sphere having a volume of the droplet, and solidifying the droplet, and / or by exposing at least a portion of a plurality of particles to an agent able to remove at least a portion of the particles.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
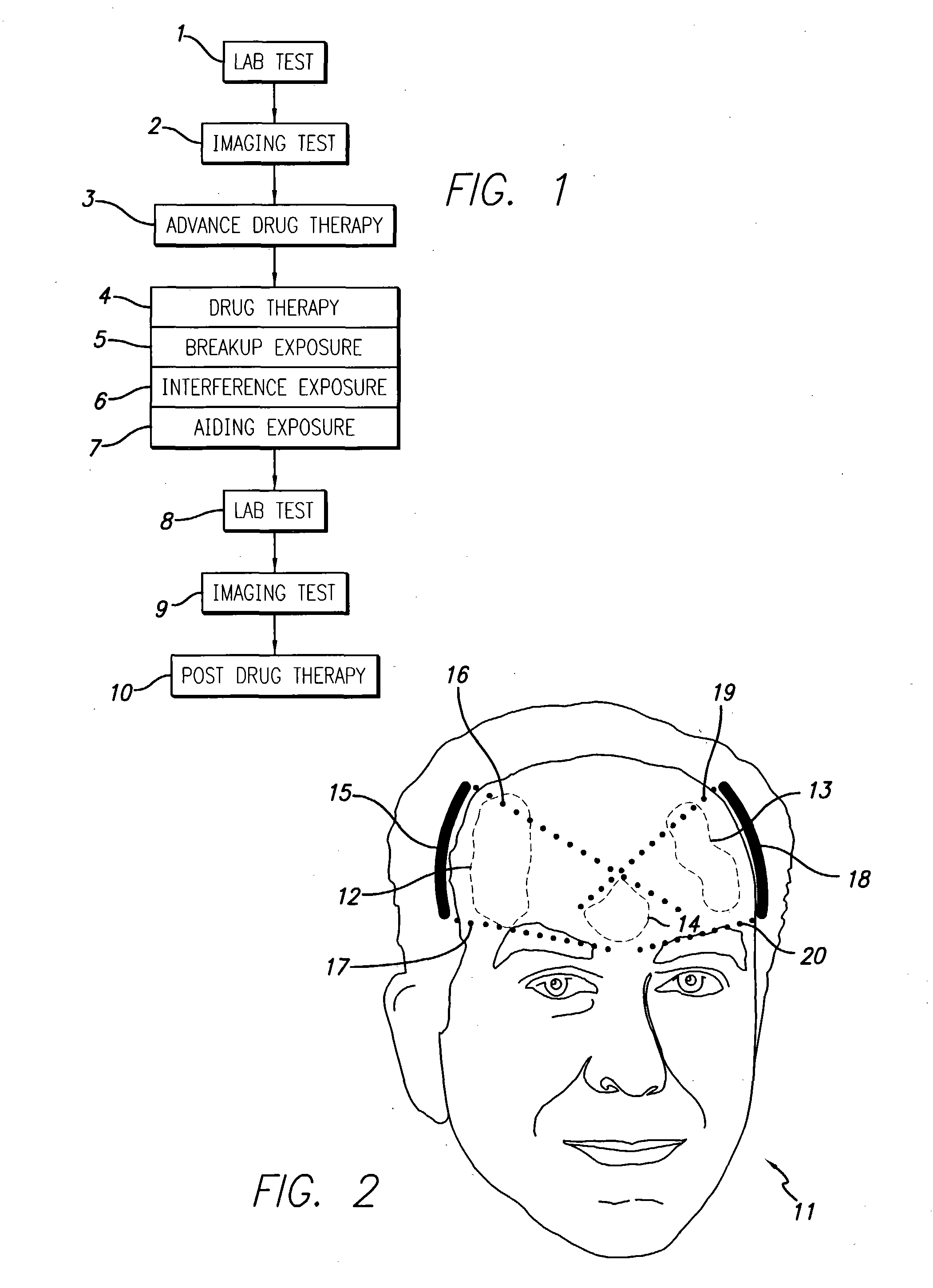
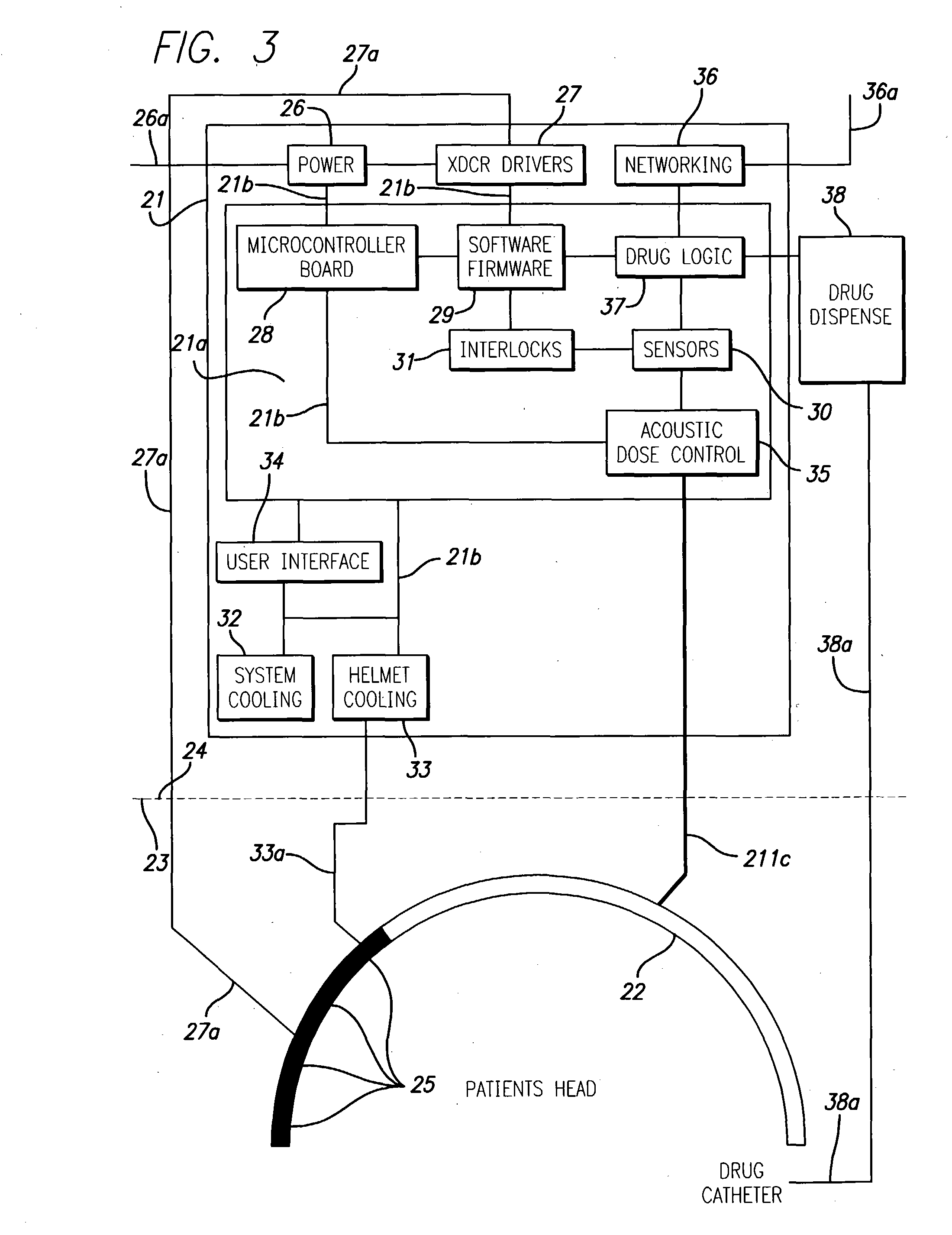
System and methods for treatment of alzheimer's and other deposition-related disorders of the brain
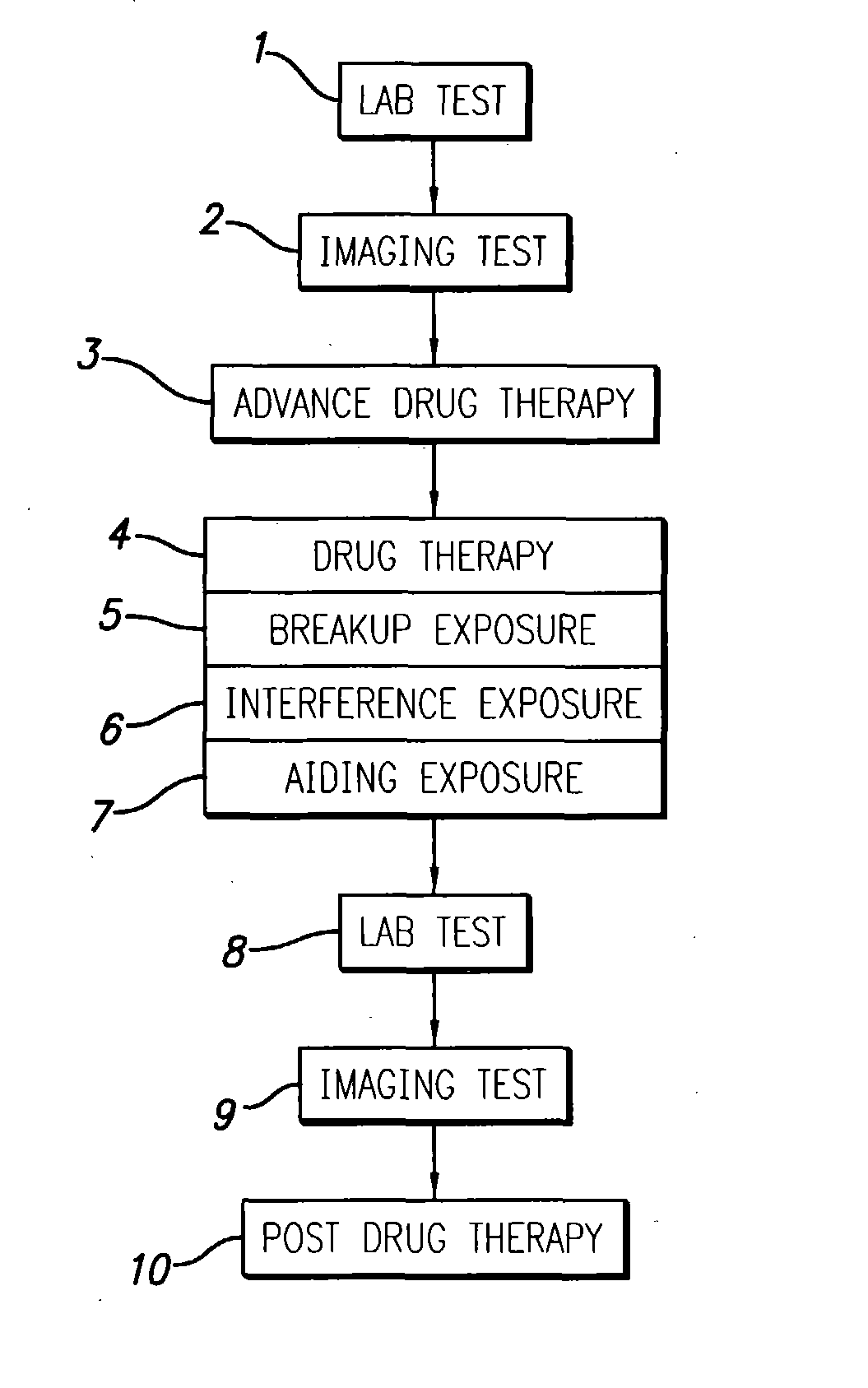
InactiveUS20040049134A1Slowing, stopping or avoiding a patient's cognitive lossesMinimal adverse side effectUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseSide effect
A system and methods are provided for the therapeutic treatment of brain-plaques, fibrils, abnormal-protein related or aggregation-prone protein related deposition-diseases. The system employs acoustic exposure therapy means for delivering therapeutic energy to at least one brain region. The therapy supports at least one of the following processes: (i) physical breakup, erosion, disentanglement, de-aggregation, dissolution, de-agglomeration, de-amalgamation or permeation of the deposits, (ii) interference in at least one deposit formation process, deposition related chemical reaction or biological or genetic pathway contributing to the deposits or deposition-related processes, and (iii) aiding the recovery, growth, regrowth or improved functionality of brain-related cells or functional pathways negatively impacted by, stressed by or disposed to the deposits, deposition-processes or deposition disease state, or supporting the growth of newly transplanted cells anywhere in the brain-related anatomy. The system and methods treat Alzheimer's and other deposition-related disorders of the brain, with minimal adverse side effects to the patient and may be used in cooperation with a drug.
Owner:TOSAYA CAROL A +1
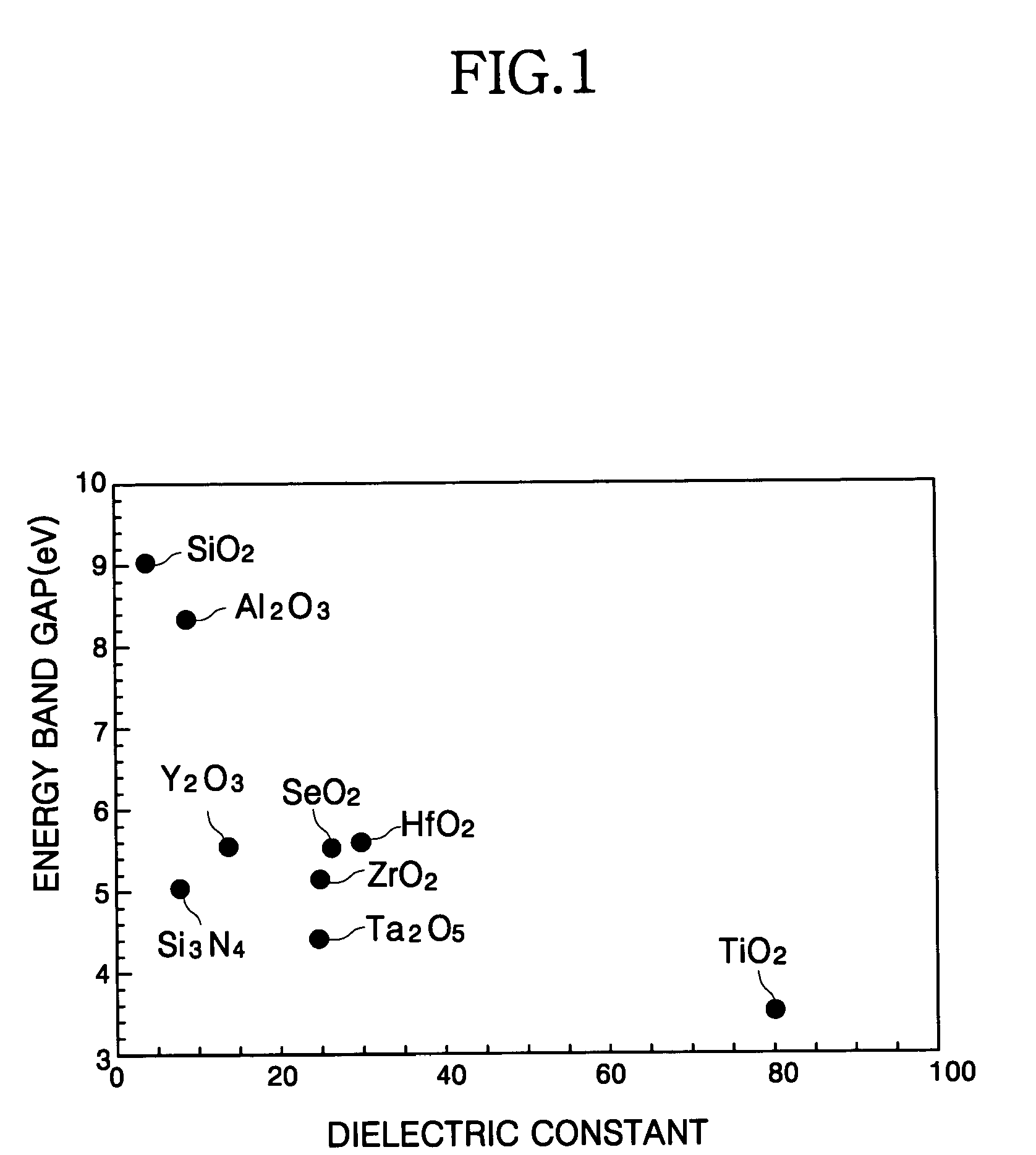
Methods of forming a thin film structure, and a gate structure and a capacitor including the thin film structure
InactiveUS20060013946A1Well formedHigh dielectric constantSemiconductor/solid-state device manufacturingSpecial surfacesChemical reactionSilicon oxide
A thin film structure is formed that includes hafnium silicon oxide using an atomic layer deposition process. A first reactant including tetrakis ethyl methyl amino hafnium (TEMAH) is introduced onto a substrate. A first portion of the first reactant is chemisorbed to the substrate, whereas a second portion of the first reactant is physorbed to the first portion of the first reactant. A first oxidant is provided onto the substrate. A first thin film including hafnium oxide is formed on the substrate by chemically reacting the first oxidant with the first portion of the first reactant. A second reactant including amino propyl tri ethoxy silane (APTES) is introduced onto the first thin film. A first portion of the second reactant is chemisorbed to the first thin film, whereas a second portion of the second reactant is physorbed to the first portion of the second reactant. A second oxidant is provided onto the first thin film. A second thin film including silicon oxide is formed on the first thin film by chemically reacting the second oxidant with the first portion of the second reactant.
Owner:SAMSUNG ELECTRONICS CO LTD
Method for separating analyte from a sample
InactiveUS6893879B2Improve elution efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsSporeChemical reaction
An analyte is separated from a fluid sample by introducing the sample into a cartridge having an extraction chamber containing capture material for capturing the analyte. The sample is forced to flow through the extraction chamber to capture the analyte with the capture material in the extraction chamber. The captured analyte is then eluted from the extraction chamber by forcing an elution fluid to flow through the extraction chamber. The cartridge may optionally include a lysing region for lysing sample components (e.g., cells spores, or microorganisms), a waste chamber for storing waste fluid, and reaction or detection chambers for chemically reacting or detecting the eluted analyte.
Owner:CEPHEID INC
Multiple deposition for integration of spacers in pitch multiplication process
InactiveUS20070049040A1Decorative surface effectsSemiconductor/solid-state device manufacturingChemical reactionGas phase
Pitch multiplication is performed using a two step process to deposit spacer material on mandrels. The precursors of the first step react minimally with the mandrels, forming a barrier layer against chemical reactions for the deposition process of the second step, which uses precursors more reactive with the mandrels. Where the mandrels are formed of amorphous carbon and the spacer material is silicon oxide, the silicon oxide is first deposited by a plasma enhanced deposition process and then by a thermal chemical vapor deposition process. Oxygen gas and plasma-enhanced tetraethylorthosilicate (TEOS) are used as reactants in the plasma enhanced process, while ozone and TEOS are used as reactants in the thermal chemical vapor deposition process. The oxygen gas is less reactive with the amorphous carbon than ozone, thereby minimizing deformation of the mandrels caused by oxidation of the amorphous carbon.
Owner:ROUND ROCK RES LLC
Sequential UV induced chemical vapor deposition
Ion-induced, UV-induced, and electron-induced sequential chemical vapor deposition (CVD) processes are disclosed where an ion flux, a flux of ultra-violet radiation, or an electron flux, respectively, is used to induce the chemical reaction in the process. The process for depositing a thin film on a substrate includes introducing a flow of a first reactant gas in vapor phase into a process chamber where the gas forms an adsorbed saturated layer on the substrate and exposing the substrate to a flux of ions, a flux of ultra-violet radiation, or a flux of electrons for inducing a chemical reaction of the adsorbed layer of the first reactant gas to form the thin film. A second reactant gas can be used to form a compound thin film. The ion-induced, UV-induced, and electron-induced sequential CVD process of the present invention can be repeated to form a thin film of the desired thickness.
Owner:NOVELLUS SYSTEMS
Chemical heat source for use in smoking articles
A non-combustion, chemical heat source includes a heat chamber having a closed end and an open end. The heat chamber includes an apertured heat cartridge disposed at the open end of the chamber, an abutment disposed at the closed end of the chamber, an activating solution, a frangible seal separating the activating solution and the heat cartridge. The heat cartridge includes metallic agents that may come in a variety of configurations. The heat source is activated when the heat cartridge is pushed through and breaks the frangible seal allowing contact between the metallic agents and the activating solution. The heat cartridge includes an aperture in the bottom of the cartridge and absorbent paper surrounding the metallic agents, both of which control the transfer of the activating solution into the metallic agents to cause the chemical reaction. The heat sources may be incorporated into smoking articles and may also be used to heat foods or beverages, in hand warmers, and to heat equipment or materials.
Owner:R J REYNOLDS TOBACCO COMPANY
Apparatuses and methods for deposition of material on surfaces
ActiveUS20060196418A1Adjustable positionFrom chemically reactive gasesChemical vapor deposition coatingChemical reactionCompound (substance)
An apparatus for depositing conformal thin films by sequential self saturating chemical reactions on heated surfaces is disclosed. The apparatus comprises a movable single or dual-lid system that has a substrate holder attached to a reaction chamber lid. In other embodiments, the apparatus comprises an exhaust flow plug, a gas distribution insert, a local heater or a minibatch system. Various methods suitable for ALD (Atomic Layer Deposition) are also enclosed.
Owner:PICOSUN OY
Chemical heat source for use in smoking articles
A non-combustion, chemical heat source includes a heat chamber having a closed end and an open end. The heat chamber includes an apertured heat cartridge disposed at the open end of the chamber, an abutment disposed at the closed end of the chamber, an activating solution, a frangible seal separating the activating solution and the heat cartridge. The heat cartridge includes metallic agents that may come in a variety of configurations. The heat source is activated when the heat cartridge is pushed through and breaks the frangible seal allowing contact between the metallic agents and the activating solution. The heat cartridge includes an aperture in the bottom of the cartridge and absorbent paper surrounding the metallic agents, both of which control the transfer of the activating solution into the metallic agents to cause the chemical reaction. The heat sources may be incorporated into smoking articles and may also be used to heat foods or beverages, in hand warmers, and to heat equipment or materials.
Owner:R J REYNOLDS TOBACCO COMPANY
Devices and methods for cooling microwave antennas
InactiveUS20050015081A1Improve cooling effectElectrotherapySurgical instruments for heatingChemical reactionCompound (substance)
Devices and methods for cooling microwave antennas are disclosed herein. The cooling systems can be used with various types of microwave antennas. One variation generally comprises a handle portion with an elongate outer jacket extending from the handle portion. A microwave antenna is positioned within the handle and outer jacket such that cooling fluid pumped into the handle comes into contact directly along a portion of the length, or a majority of the length, or the entire length of the antenna to allow for direct convective cooling. Other variations include cooling sheaths which form defined cooling channels around a portion of the antenna. Yet another variation includes passively-cooled systems which utilize expandable balloons to urge tissue away from the surface of the microwave antenna as well as cooling sheaths which are cooled through endothermic chemical reactions. Furthermore, the microwave antennas themselves can have cooling lumens integrated directly therethrough.
Owner:TYCO HEALTHCARE GRP LP
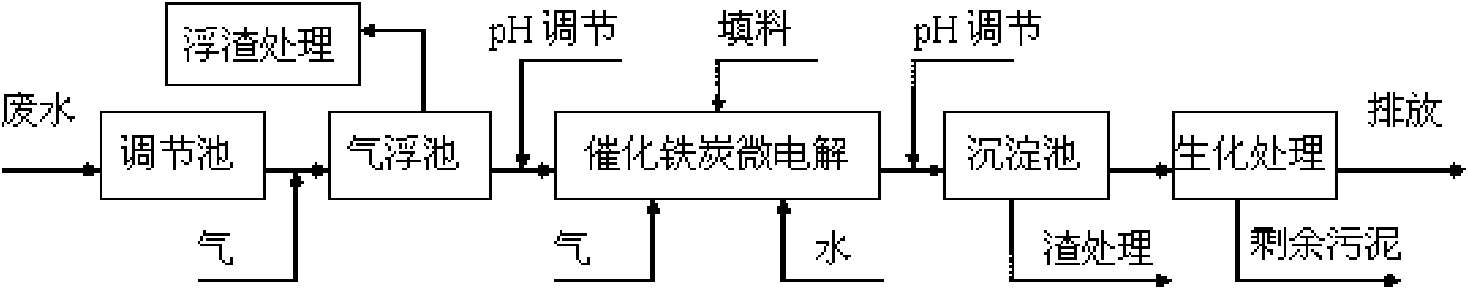
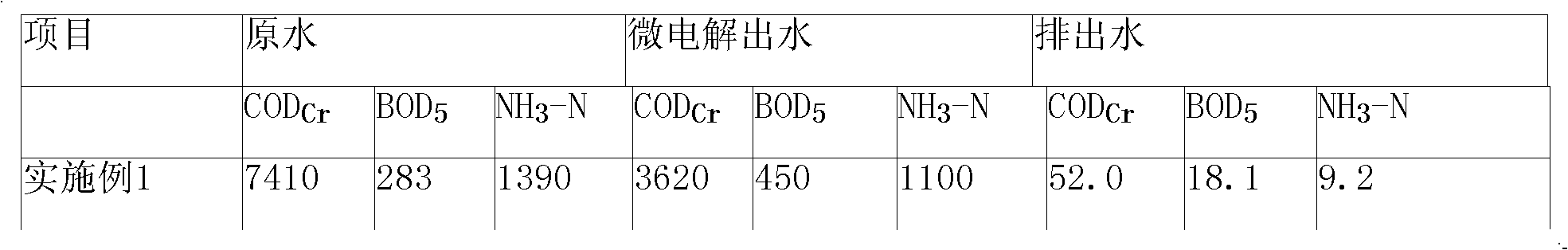
Catalysis and micro-electrolysis combined technology for high-concentration refractory organic wastewater
InactiveCN101665311AReduce processing loadEasy to handleTreatment with aerobic and anaerobic processesMultistage water/sewage treatmentHigh concentrationElectrolysis
The invention relates to a catalysis and micro-electrolysis combined technology for high-concentration refractory organic wastewater; the organic wastewater is collected to an adjusting tank and enters an air floatation tank for air floatation treatment to remove part of the organic matters after the adjustment of water volume and water quality; the scruff is collected or recovered; the wastewatergoes through Ph adjustment and then enters a catalytic iron-carbon and micro-electrolysis unit to improve the biochemical quality; the effluent goes through Ph adjustment and then enters a sedimentation tank; the effluent of the sedimentation tank adopts anoxic-aerobic biochemistry treatment to remove the organic matters and ammonia nitrogen and then is emitted after reaching the standard; and the filler of the catalytic iron-carbon and micro-electrolysis unit comprises iron, carbon and a catalyst, wherein the mass ratio of the iron, carbon and catalyst is 1: (0.3-1.5): (0.01-0.5). The invention can effectively improve the micro-electrolysis electrochemical reaction efficiency and the degrading capability to the organic matters, and reduce the wastewater treatment cost with convenient technological operation.
Owner:CENT SOUTH UNIV
Method of forming oxide layer using atomic layer deposition method and method of forming capacitor of semiconductor device using the same
In a method of forming an oxide layer using an atomic layer deposition and a method of forming a capacitor of a semiconductor device using the same, a precursor including an amino functional group is introduced onto a substrate to chemisorb a portion of the precursor on the substrate. Then, the non-chemisorbed precursor is removed. Thereafter, an oxidant is introduced onto the substrate to chemically react the chemisorbed precursor with the oxidant to form an oxide layer on the substrate. A deposition rate is fast and an oxide layer having a good deposition characteristic may be obtained. Also, a thin oxide film having a good step coverage and a decreased pattern loading rate can be formed.
Owner:SAMSUNG ELECTRONICS CO LTD
Multiple deposition for integration of spacers in pitch multiplication process
InactiveUS20080149593A1Decorative surface effectsSemiconductor/solid-state device manufacturingChemical reactionGas phase
Pitch multiplication is performed using a two step process to deposit spacer material on mandrels. The precursors of the first step react minimally with the mandrels, forming a barrier layer against chemical reactions for the deposition process of the second step, which uses precursors more reactive with the mandrels. Where the mandrels are formed of amorphous carbon and the spacer material is silicon oxide, the silicon oxide is first deposited by a plasma enhanced deposition process and then by a thermal chemical vapor deposition process. Oxygen gas and plasma-enhanced tetraethylorthosilicate (TEOS) are used as reactants in the plasma enhanced process, while ozone and TEOS are used as reactants in the thermal chemical vapor deposition process. The oxygen gas is less reactive with the amorphous carbon than ozone, thereby minimizing deformation of the mandrels caused by oxidation of the amorphous carbon.
Owner:ROUND ROCK RES LLC
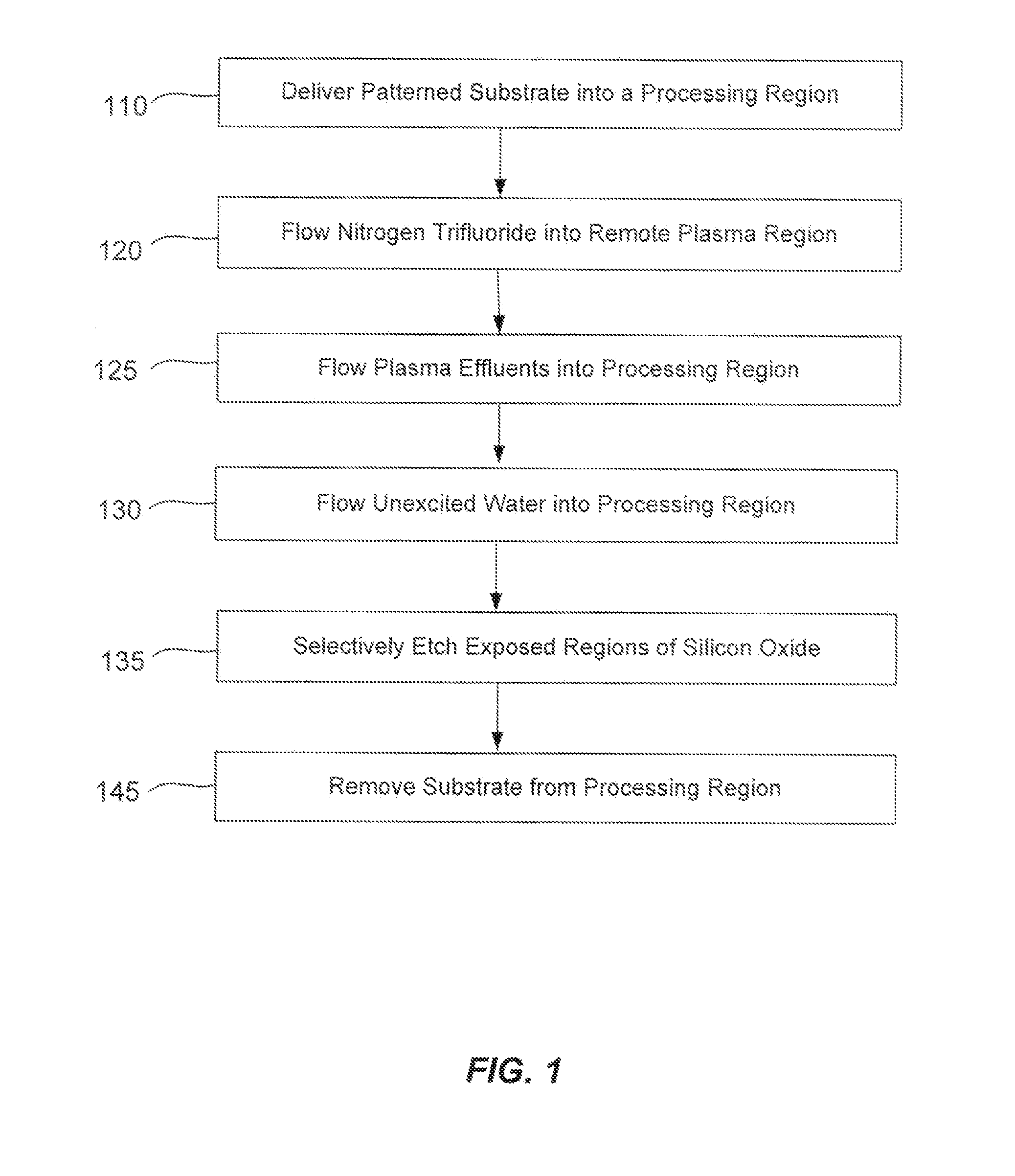
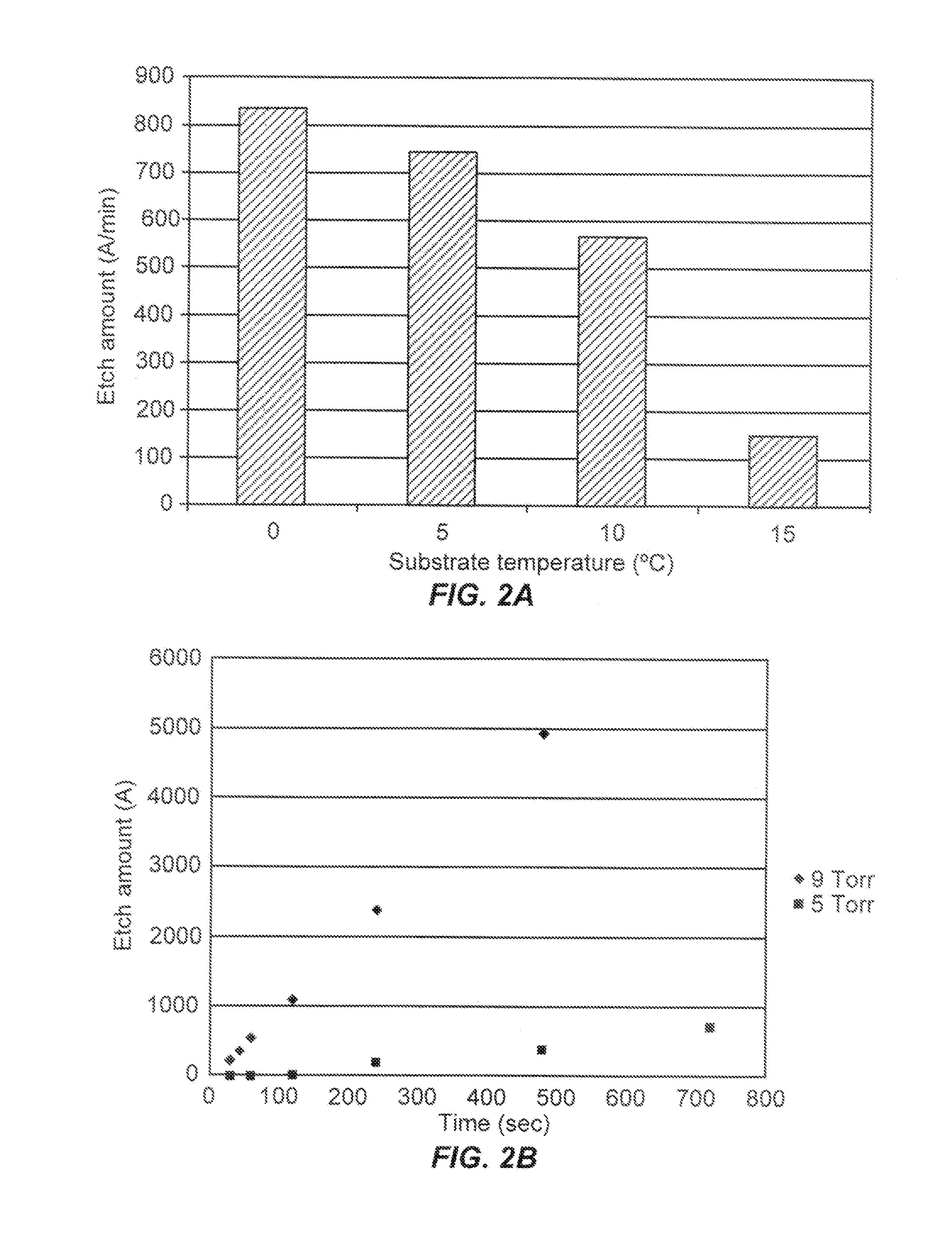
Remotely-excited fluorine and water vapor etch
ActiveUS20120211462A1Small overall deformationElectric discharge tubesDecorative surface effectsChemical reactionRemote plasma
A method of etching exposed silicon oxide on patterned heterogeneous structures is described and includes a remote plasma etch formed from a fluorine-containing precursor. Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents combine with water vapor. The chemical reaction resulting from the combination produces reactants which etch the patterned heterogeneous structures to produce, in embodiments, a thin residual structure exhibiting little deformation. The methods may be used to conformally trim silicon oxide while removing little or no silicon, polysilicon, silicon nitride, titanium or titanium nitride. In an exemplary embodiment, the etch processes described herein have been found to remove mold oxide around a thin cylindrical conducting structure without causing the cylindrical structure to significantly deform.
Owner:APPLIED MATERIALS INC
Reactive chemical containment system
A small scale, but effective, reactive chemical containment system includes apparatus and methods for reaction of process gases exhausted from reaction furnaces with a reactant gas in a non-combustible manner to produce and contain particulate or powder byproducts, thereby removing the process gas from the exhaust gas flow. The apparatus provides process gas inlet, treatment reactive gas diffusion, process gas and treatment reactive gas pre-mixing, primary containment, secondary containment, and outlet zones.
Owner:MKS INSTR INC
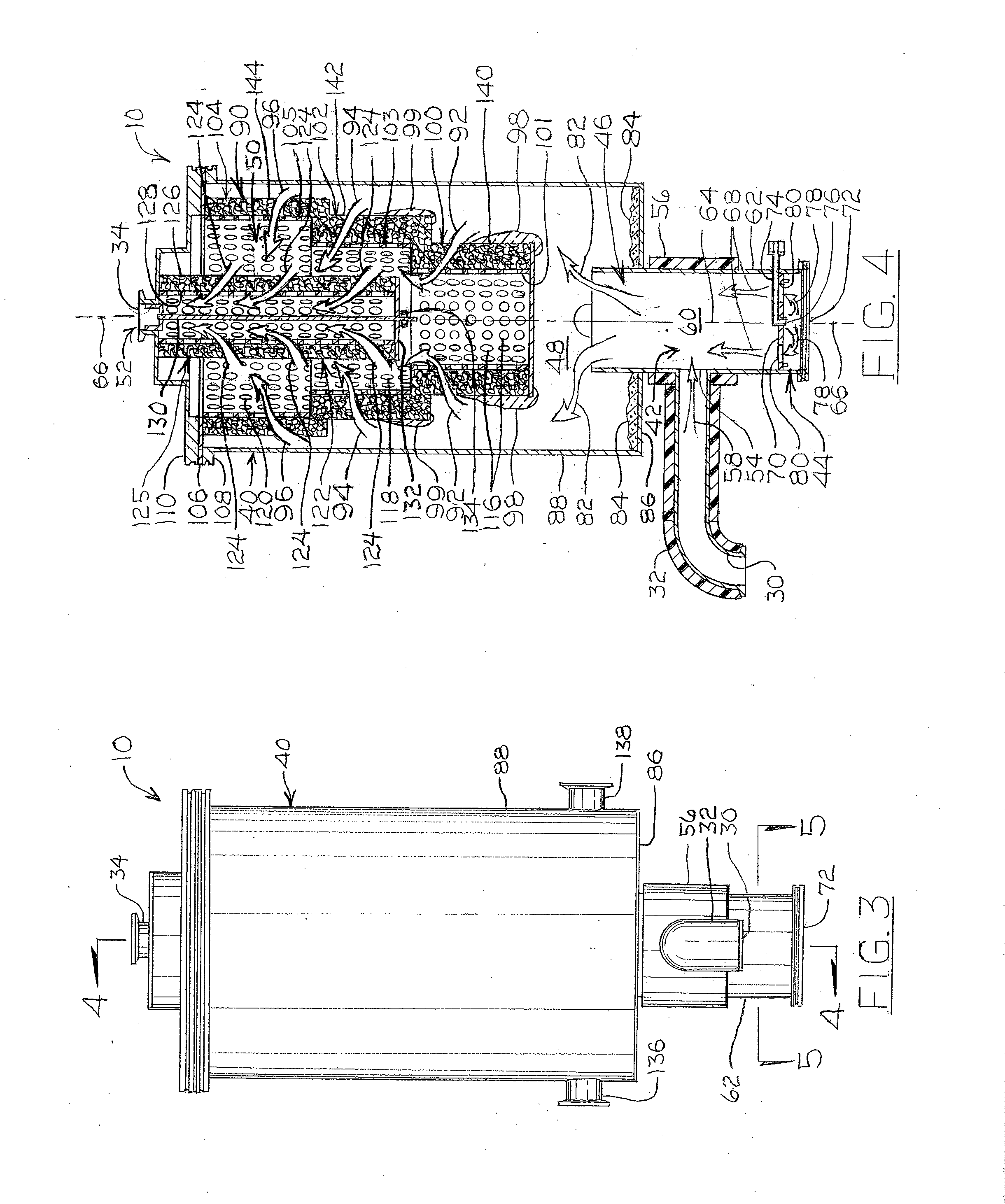
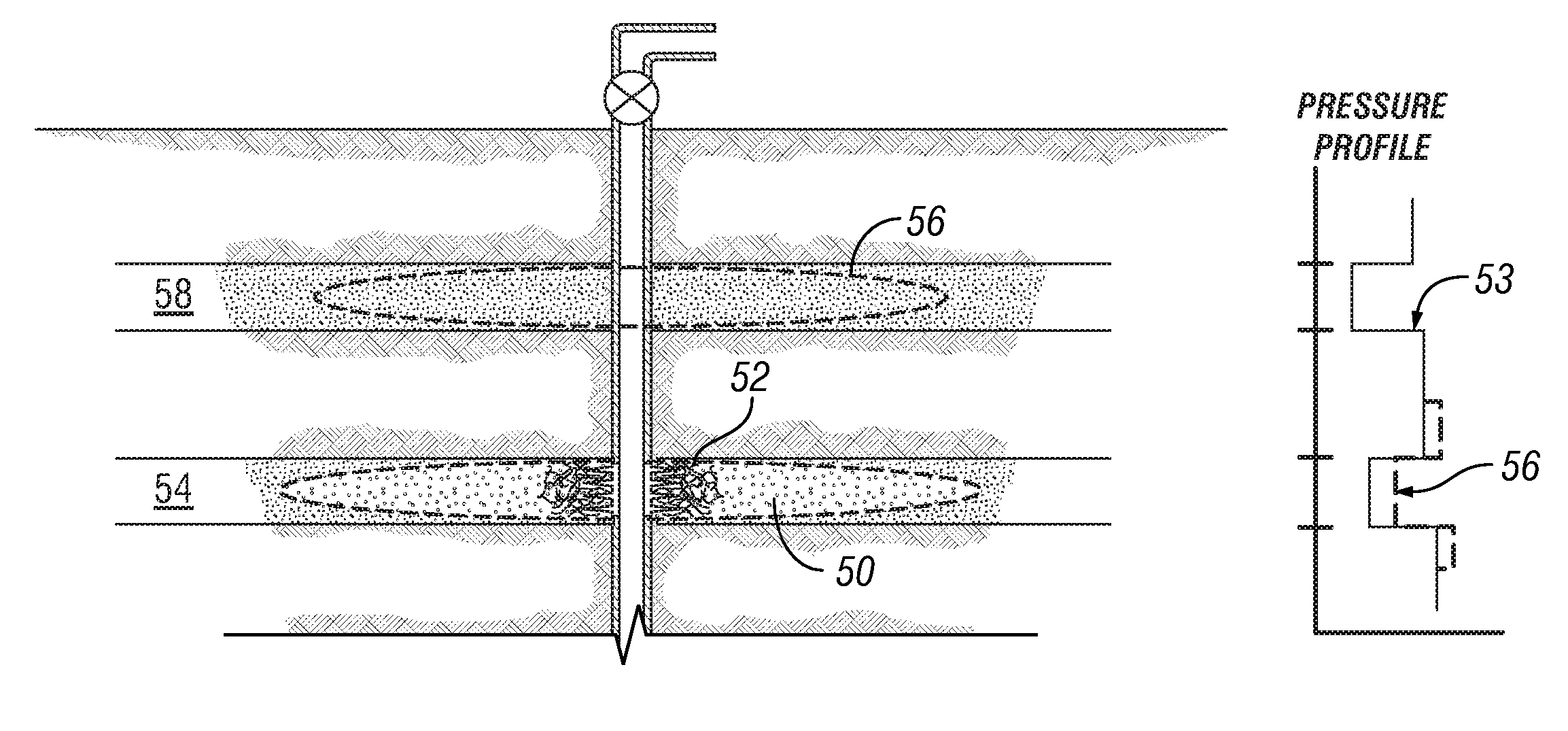
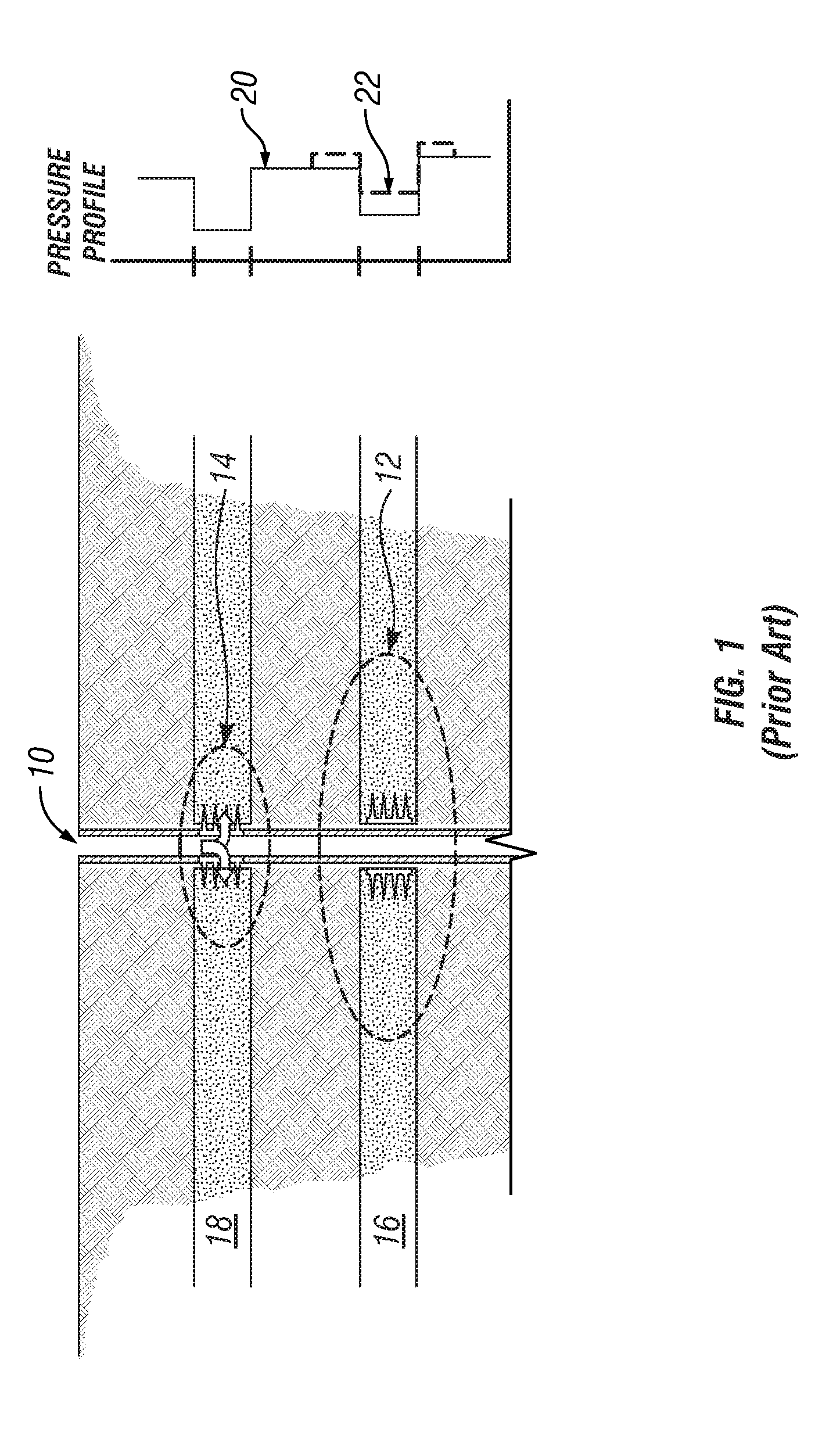
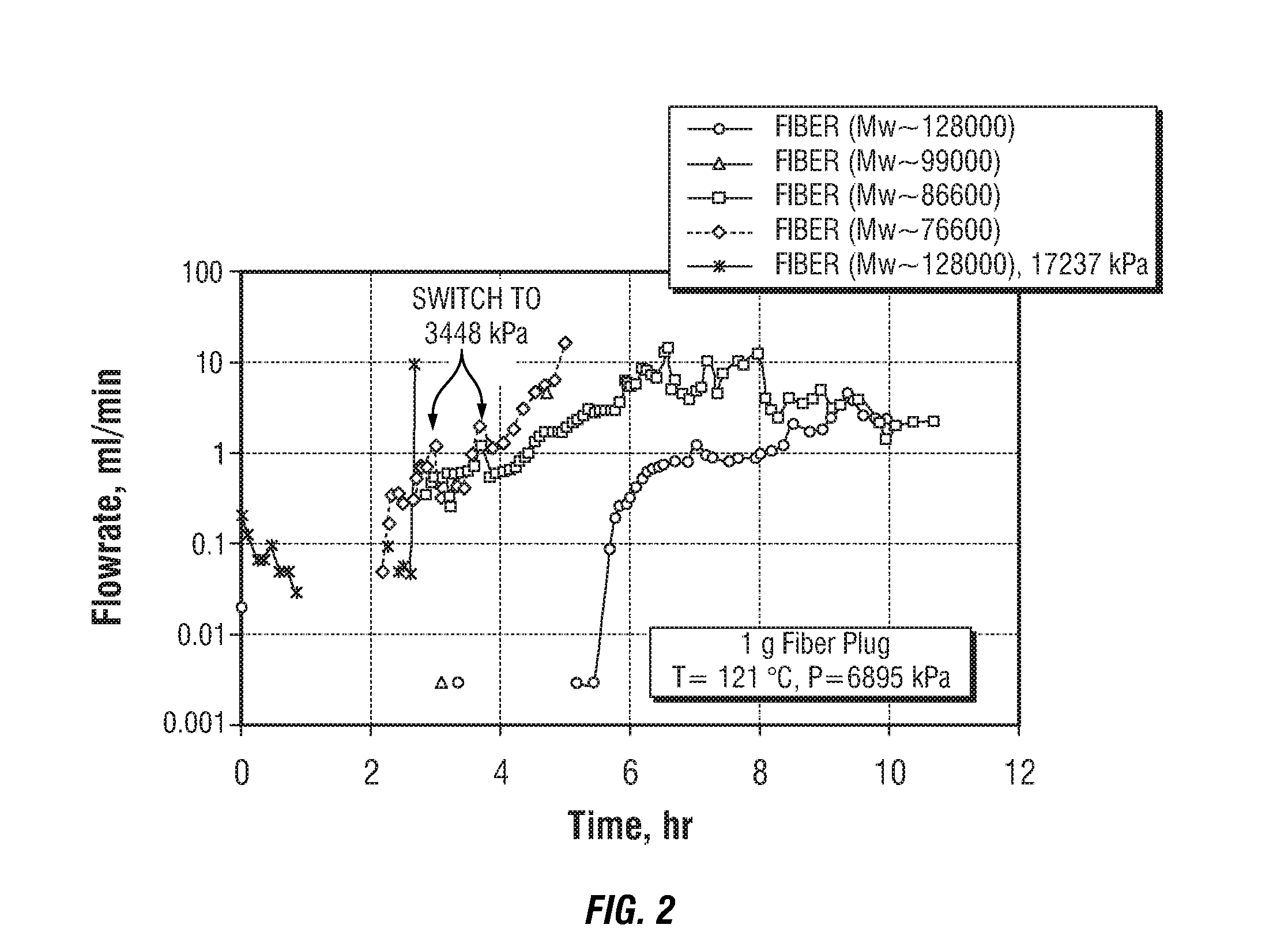
Degradable Material Assisted Diversion
Degradable material assisted diversion (DMAD) methods for well treatment, DMAD treatment fluids, and removable plugs for DMAD in downhole operations. A slurry of solid degradable material is injected into the well, a plug of the degradable material is formed, a downhole operation is performed around the plug diverter, and the plug is then degraded for removal. Degradation triggers can be temperature or chemical reactants, with optional accelerators or retarders to provide the desired timing for plug removal. In multilayer formation DMAD fracturing, the plug isolates a completed fracture while additional layers are sequentially fractured and plugged, and then the plugs are removed for flowback from the fractured layers. In DMAD fluids, an aqueous slurry can have a solids phase including a degradable material and a fluid phase including a viscoelastic surfactant. The solids phase can be a mixture of fibers and a particulate material.
Owner:SCHLUMBERGER TECH CORP
Method of removing oxide layer and semiconductor manufacturing apparatus for removing oxide layer
InactiveUS7488688B2High selectivityEven contactDecorative surface effectsSemiconductor/solid-state device manufacturingReaction layerSusceptor
A method for removing an oxide layer such as a natural oxide layer and a semiconductor manufacturing apparatus which uses the method to remove the oxide layer. A vertically movable susceptor is installed at the lower portion in a processing chamber and a silicon wafer is loaded onto the susceptor when it is at the lower portion of the processing chamber. The air is exhausted from the processing chamber to form a vacuum condition therein. A hydrogen gas in a plasma state and a fluorine-containing gas are supplied into the processing chamber to induce a chemical reaction with the oxide layer on the silicon wafer, resulting in a reaction layer. Then, the susceptor is moved up to the upper portion of the processing chamber, to anneal the silicon wafer on the susceptor with a heater installed at the upper portion of the processing chamber, thus vaporizing the reaction layer. The vaporized reaction layer is exhausted out of the chamber. The oxide layer can be removed with a high selectivity while avoiding damage or contamination of the underlying layer.
Owner:SAMSUNG ELECTRONICS CO LTD
Method and apparatus for repair of wells utilizing meltable repair materials and exothermic reactants as heating agents
A method and apparatus are described for creating a fluid seal in a subterranean well structure having a fluid seal defect. The method comprises introducing a meltable repair material proximate a structure in a subterranean well which has a fluid seal defect or enhanced seal capacity is required or it is desired to temporarily or permanently hydraulically isolate a portion the well or strengthen the structural integrity of well tubulars or tubular hangers. Exothermic reactant materials are located proximate the meltable repair material. The exothermic reactant material is ignited or an exothermic reaction otherwise initiated which supplies heat to and melts the meltable repair material into a molten mass. The molten mass flows and solidifies across the structure and the fluid seal defect to effect a fluid seal in the subterranean well structure or the structural integrity is enhanced. Examples of preferred exothermic reactant materials include thermite, thermate, fusible chemical reactants such as ammonium chloride and sodium nitrate, and oxidizers and accompanying hydrocarbon based fuels. Examples of preferred meltable repair materials include solder or brazing materials and eutectic metals which expand upon cooling and solidifying from a molten state.
Owner:CHEVROU USA INC
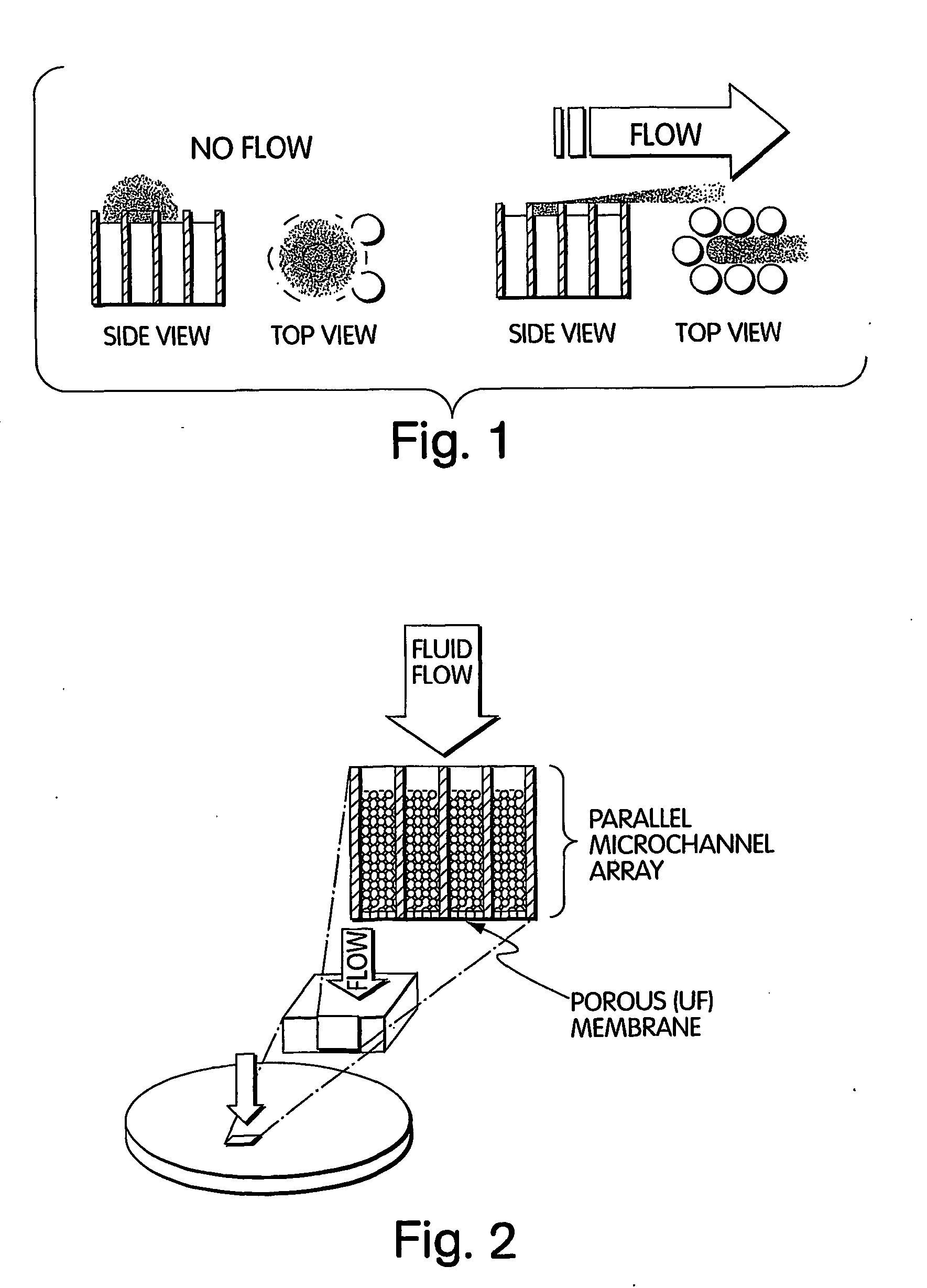
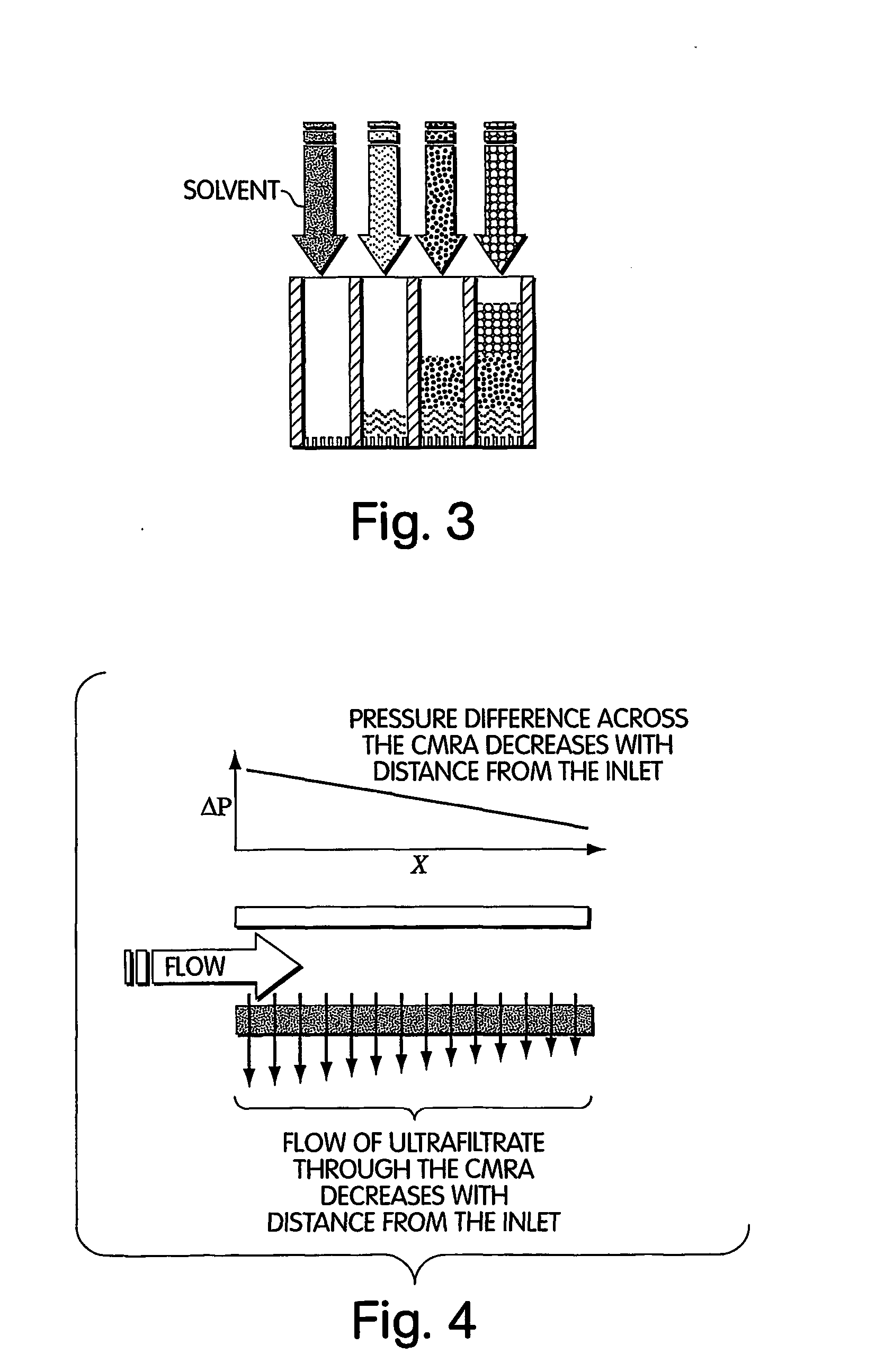
Method for isolation of independent, parallel chemical micro-reactions using a porous filter
InactiveUS20050009022A1Fast deliveryFaster more complete removalBioreactor/fermenter combinationsPeptide librariesChemical reactionCompound (substance)
The present invention relates to the field of fluid dynamics. More specifically, this invention relates to methods and apparatus for conducting densely packed, independent chemical reactions in parallel in a substantially two-dimensional array. Accordingly, this invention also focuses on the use of this array for applications such as DNA sequencing, most preferably pyrosequencing, and DNA amplification.
Owner:WEINER MICHAEL P +4
Systems and methods for managing the development and manufacturing of a drug
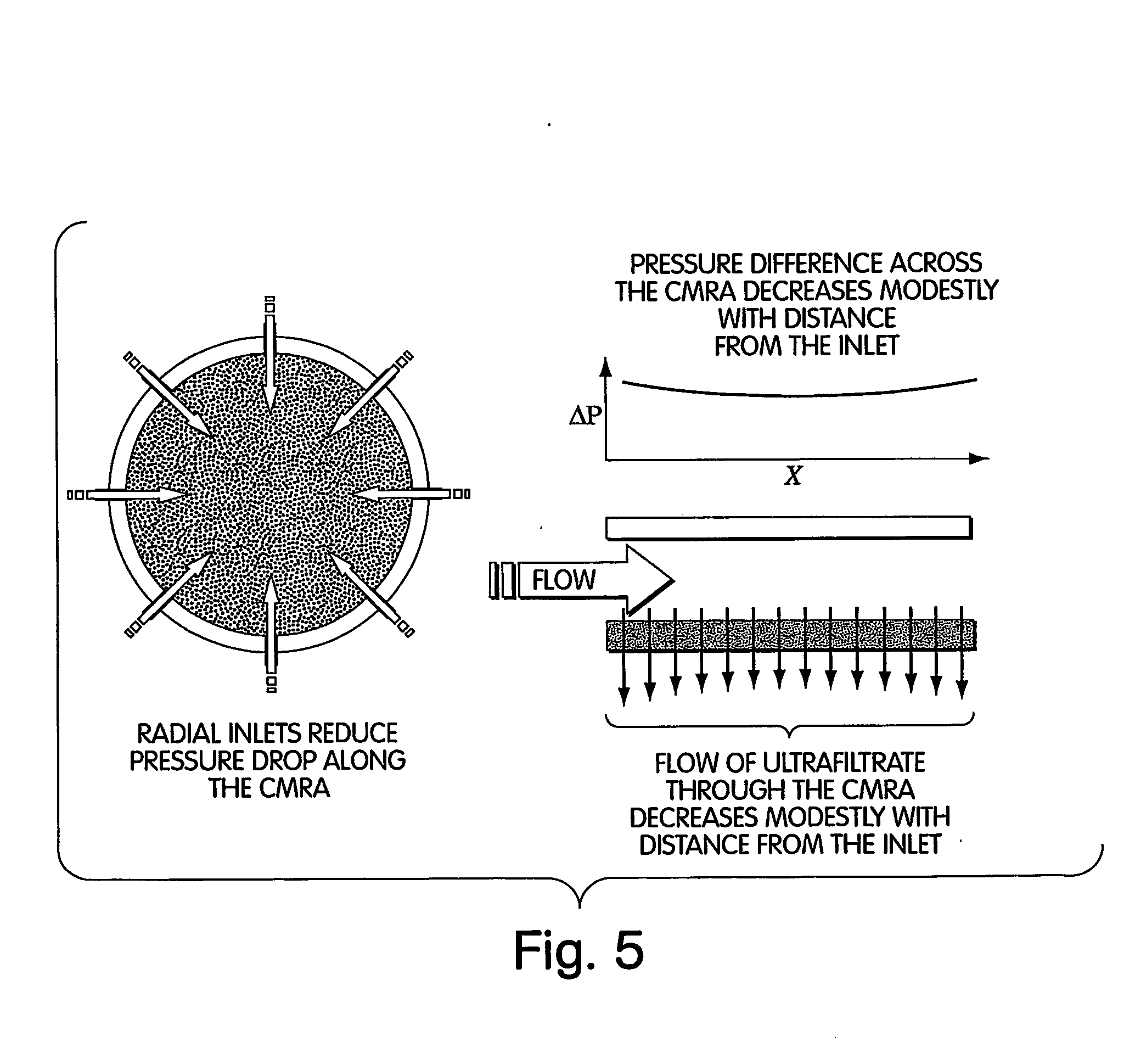
InactiveUS20070192715A1Easy to optimizeManagement complexityComputer-assisted medical data acquisitionTechnology managementGraphicsChemical reaction
Graphical user interfaces, computer readable media, and computer systems for monitoring a chemical process. An administration module sets a plurality of user preferences associated with the chemical process. A people management module defines a user role in the chemical process. An organization module defines an organizational structure of an organization that runs the chemical process. An equipment module defines equipment used in the chemical process. A material module controls a chemical used in the chemical process. A process module defines a chemical reaction in the chemical process.
Owner:ORACLE INT CORP
Encapsulated photovoltaic modules and method of manufacturing same
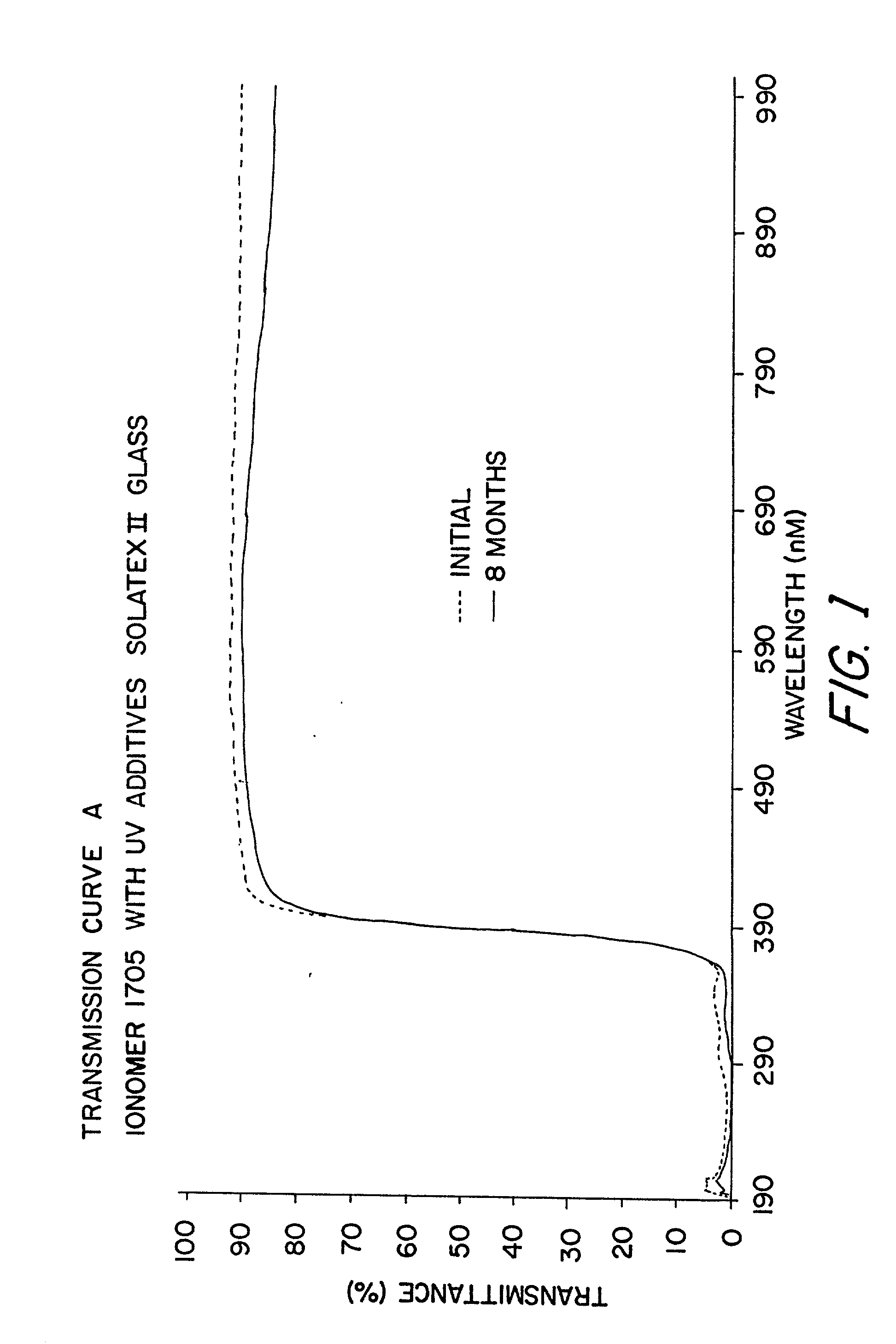
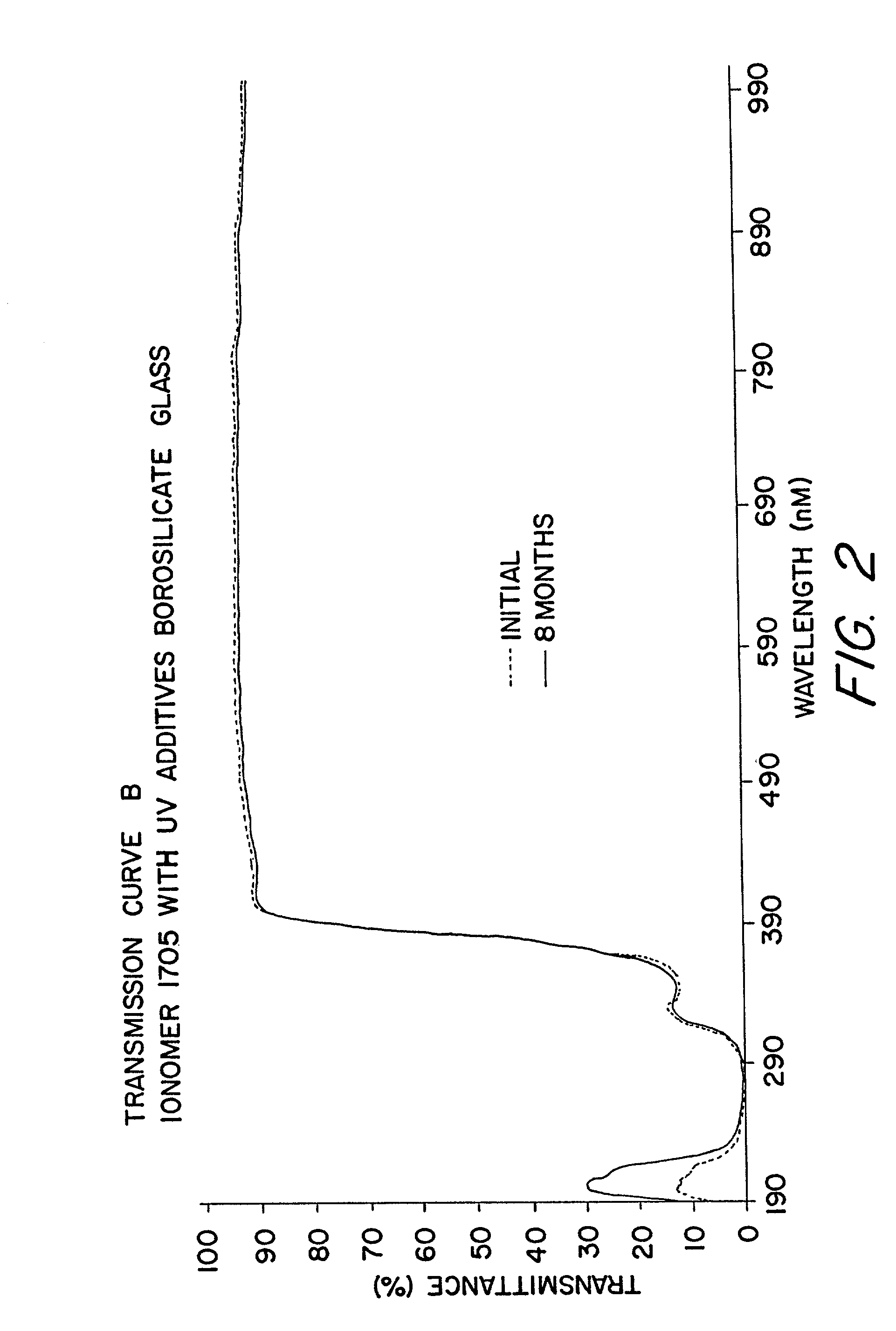
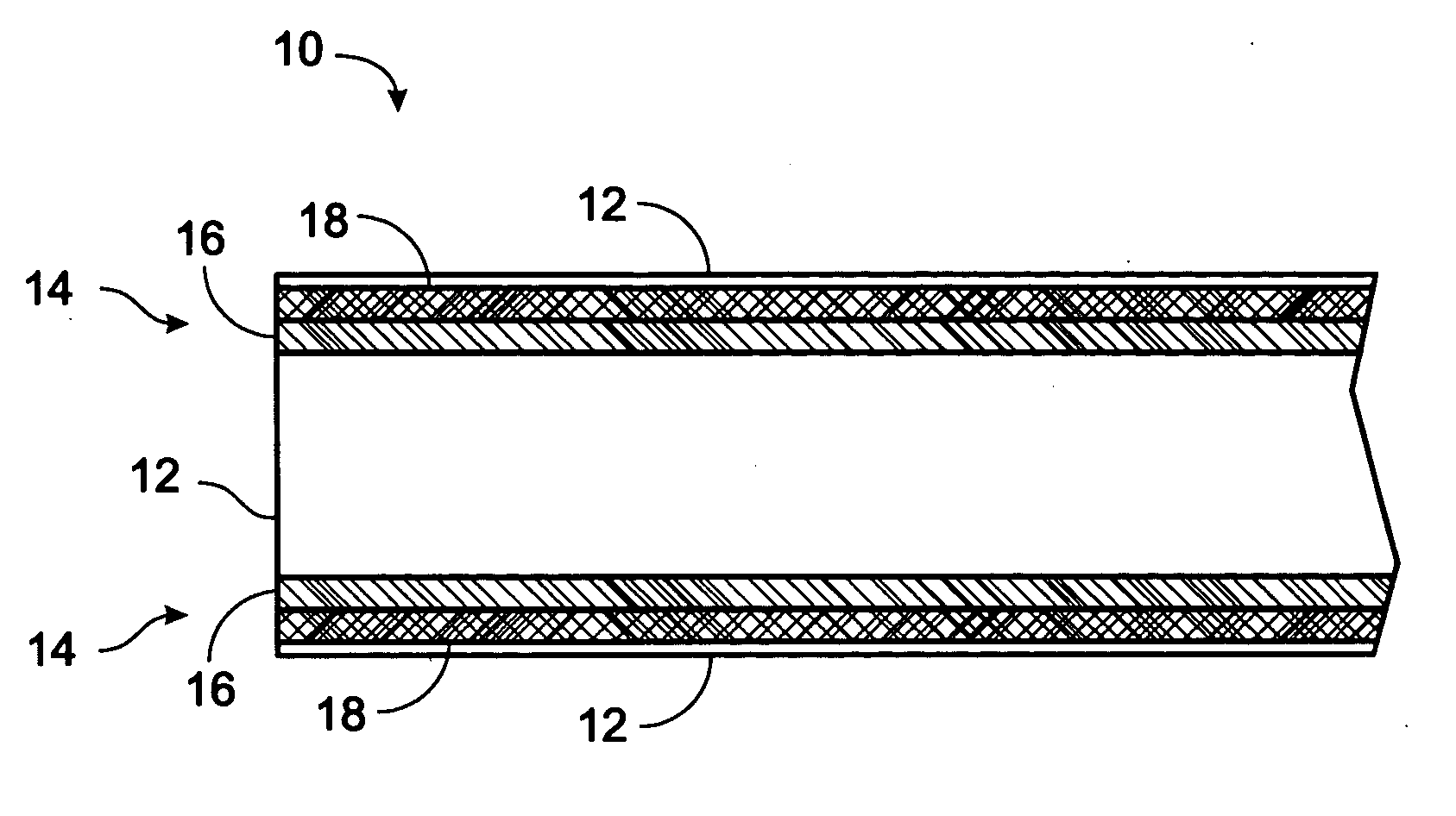
InactiveUS20030000568A1Extended service lifeLong useful lifePV power plantsSemiconductor/solid-state device detailsIonomerChemical reaction
An improved laminated photovoltaic solar cell module and method of manufacture are provided which are characterized by use of a zinc-based ionomer encapsulant to avoid chemical reaction degradation by residues of acidic solder flux at soldered solar cell connections.
Owner:RWE SCHOTT SOLAR
Fire-resistant panel and method of manufacture
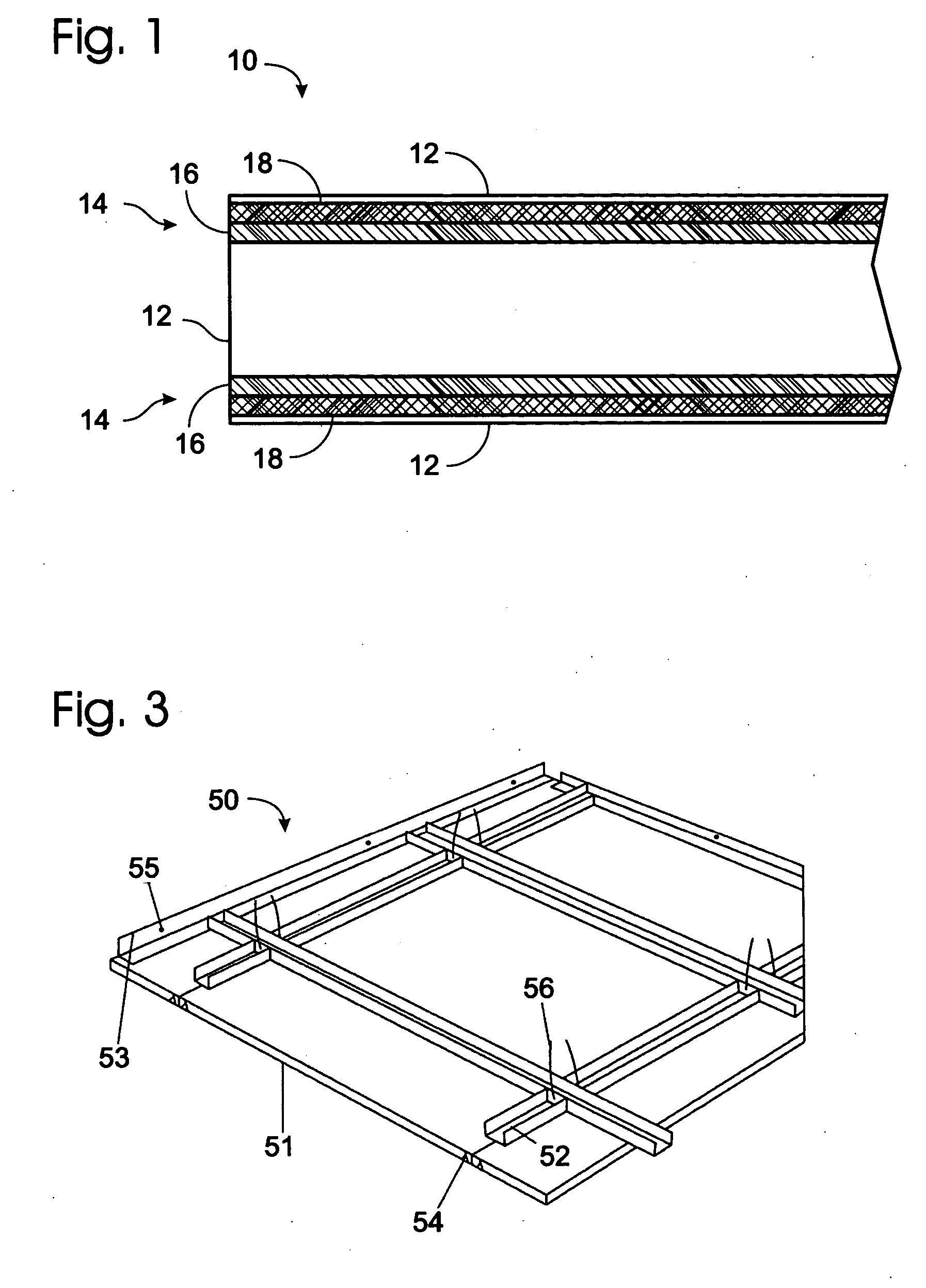
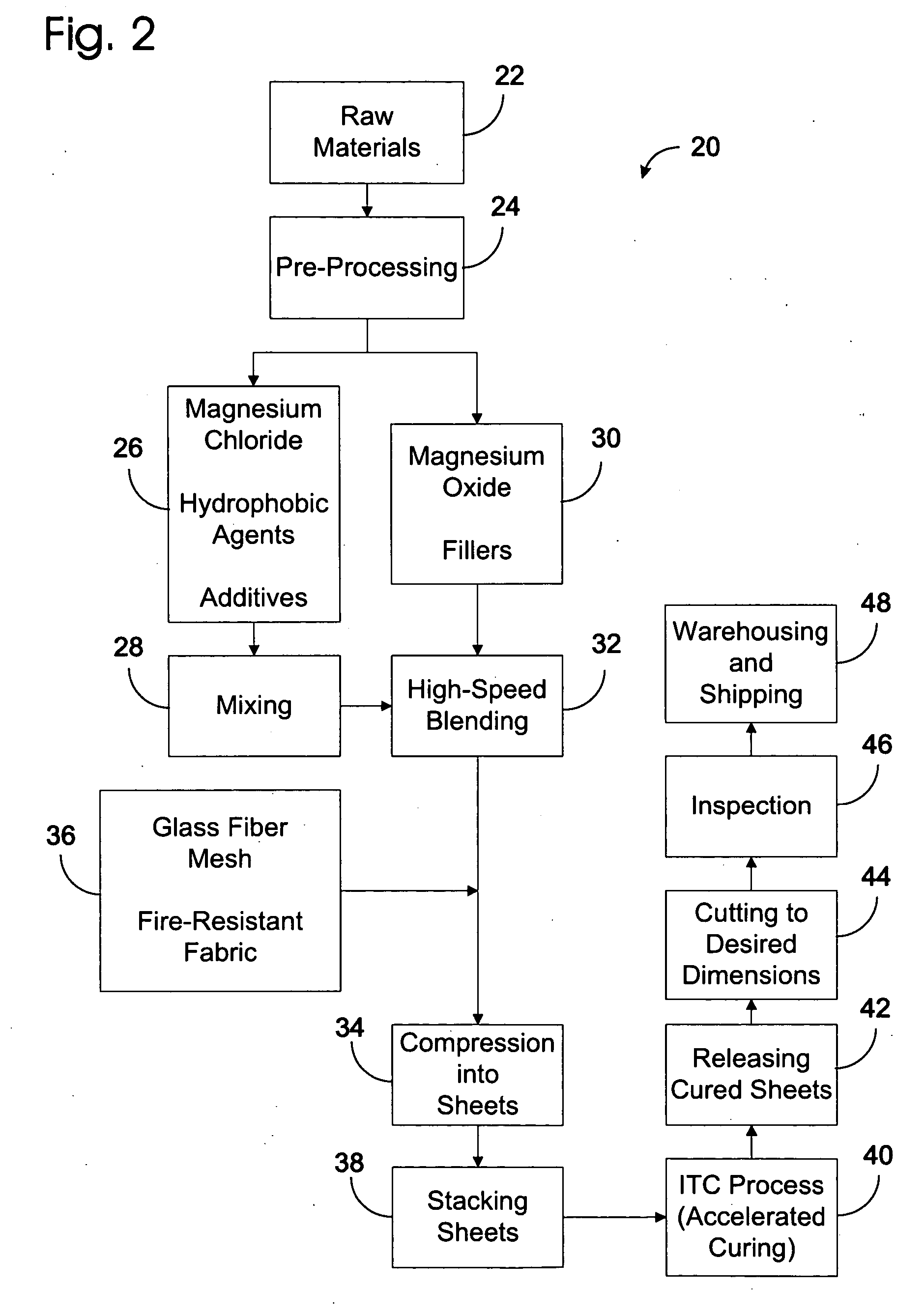
A highly fire-resistant and environmentally-friendly panel of 2 mm to 28 mm may be manufactured by a blending of magnesium compounds, sodium silicate, kaolin, fillers, and additives to form the core materials, reinforced by 4 layers of fire-resistant glass fiber meshes and fabrics. Using a proprietary ITC process that accelerates the chemical reactions of the ingredients to generate sufficient heat without external supply of energy, the panels may be completely cured within 24 hours instead of 10 days. The use of waste materials, energy-saving curing system and no gas emission manufacturing process combined to make this panel an eco-friendly product which offers the world's highest-rated fire resistance of 5 hours, high flexural strength, low density, durability and effective water-resistance.
Owner:REP TECH
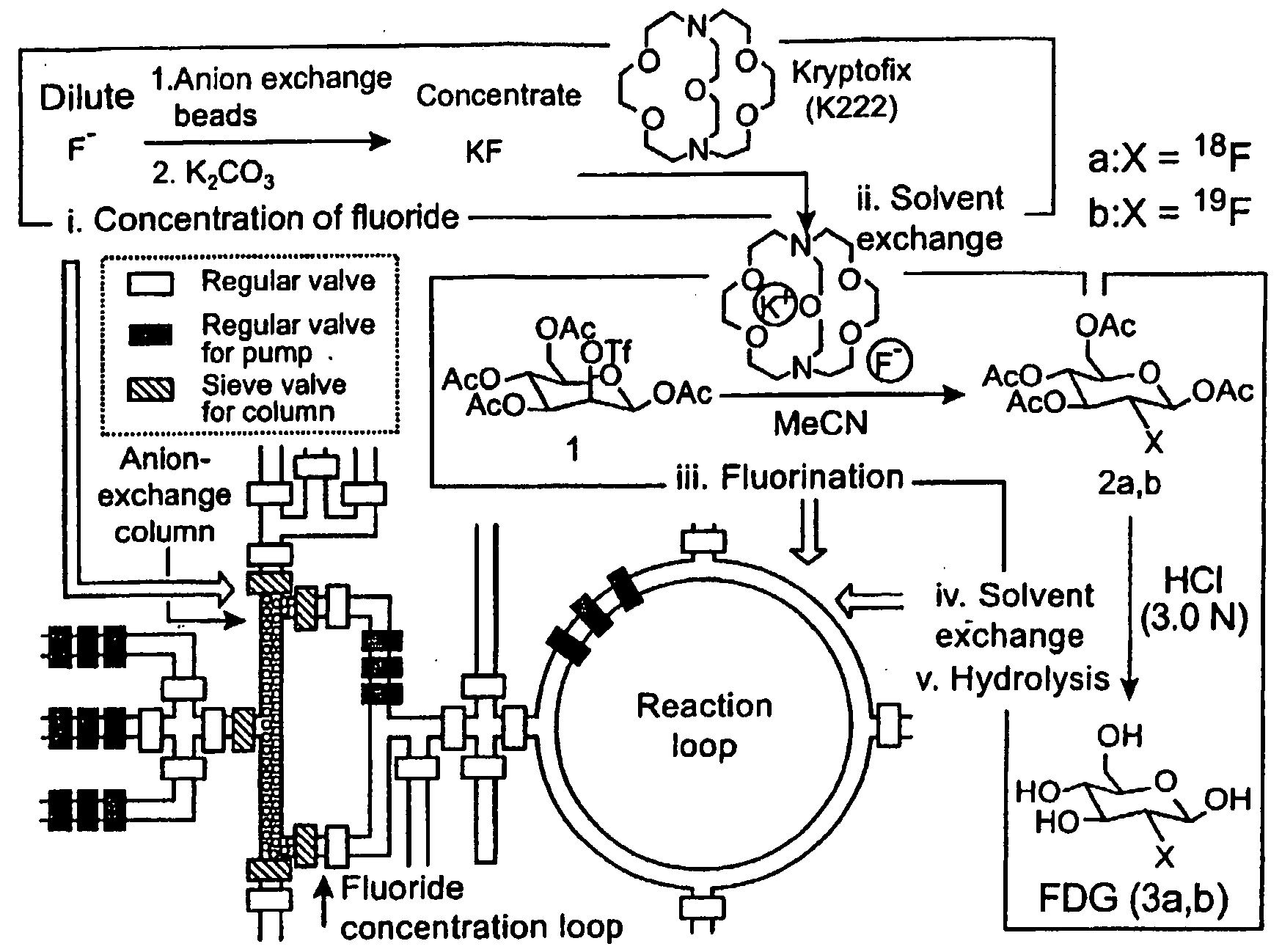
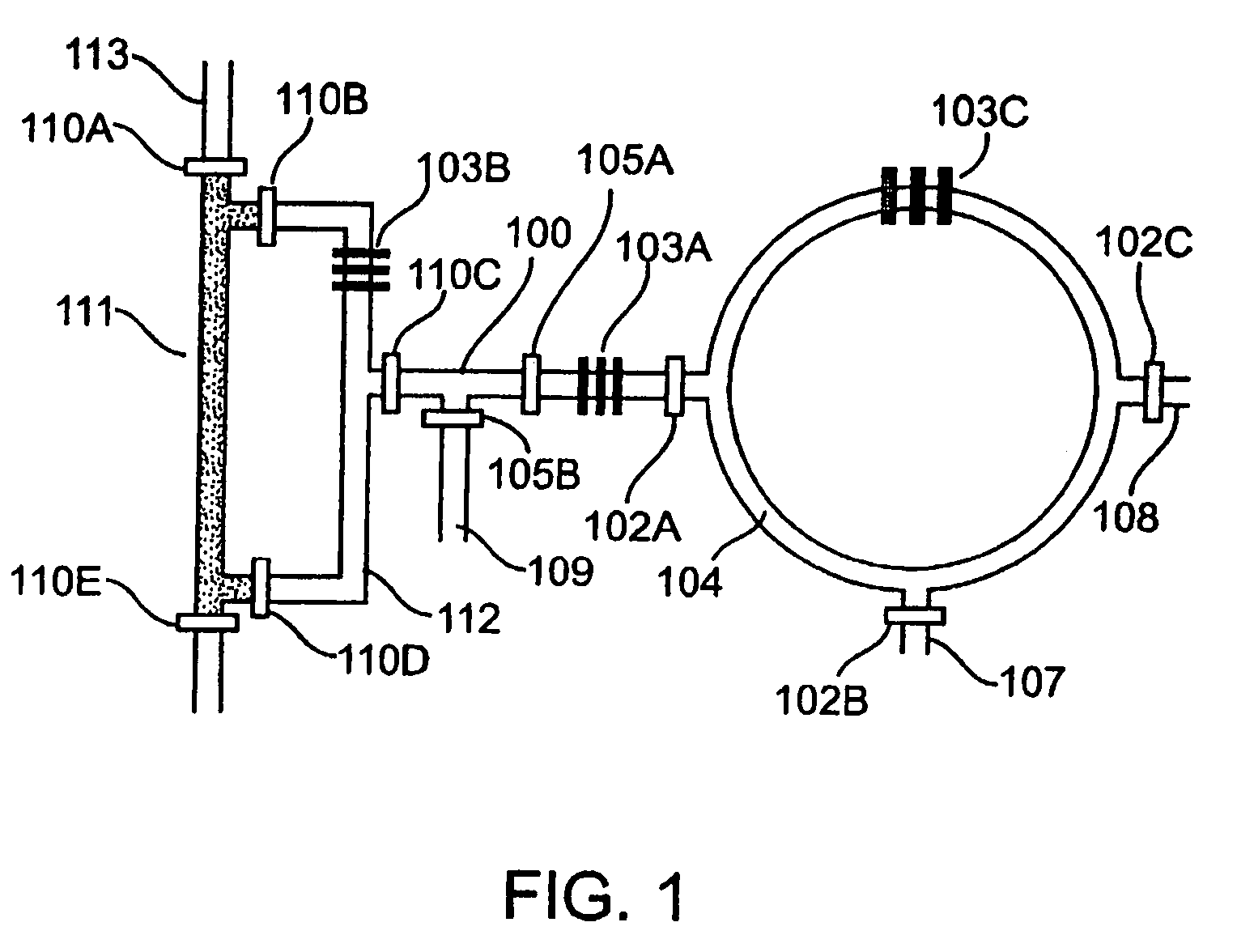
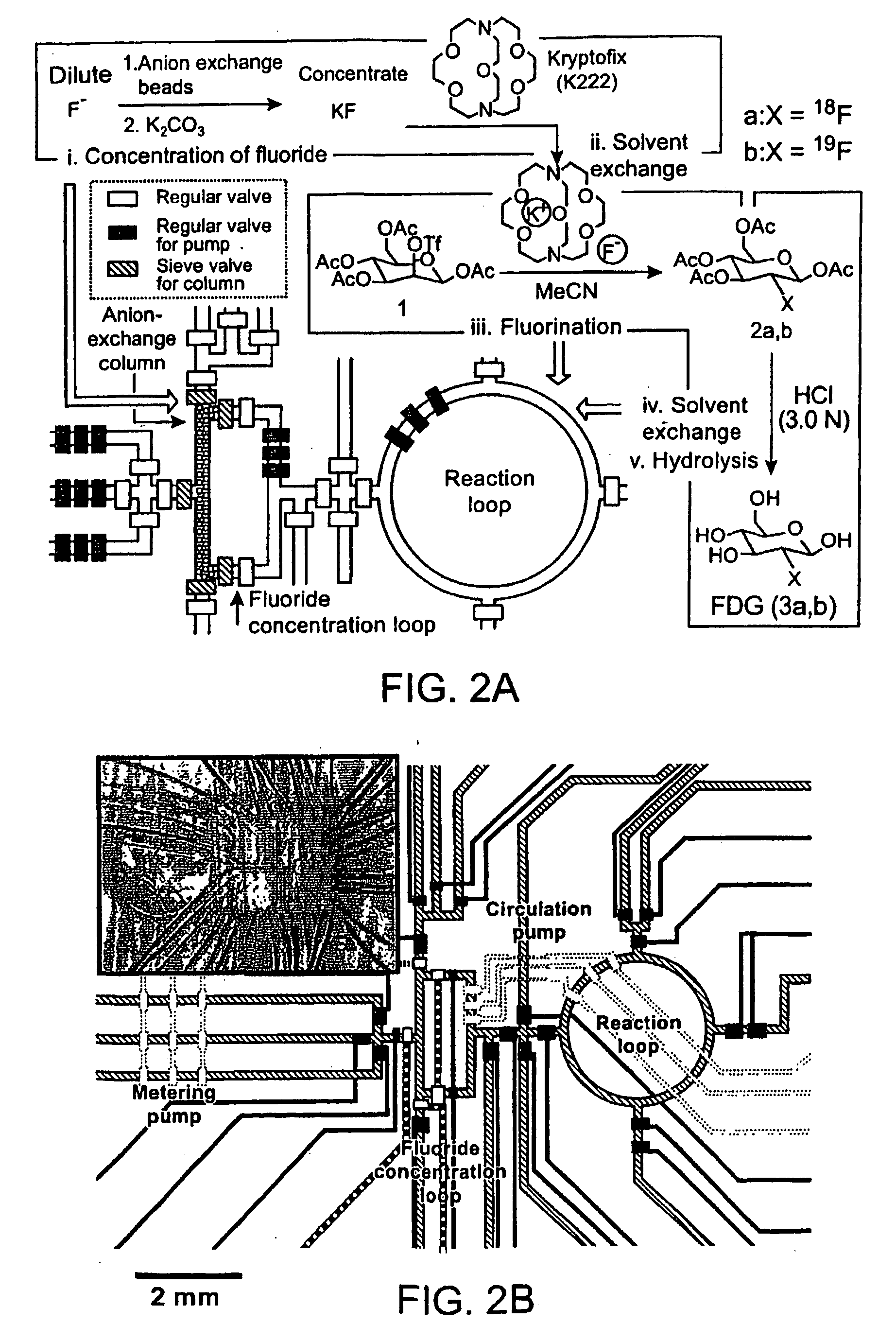
Microfluidic Chemical Reaction Circuits
InactiveUS20080281090A1Shaking/oscillating/vibrating mixersTransportation and packagingChemical reactionCompound (substance)
Owner:CALIFORNIA INST OF TECH +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com