Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
18737 results about "Silicate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
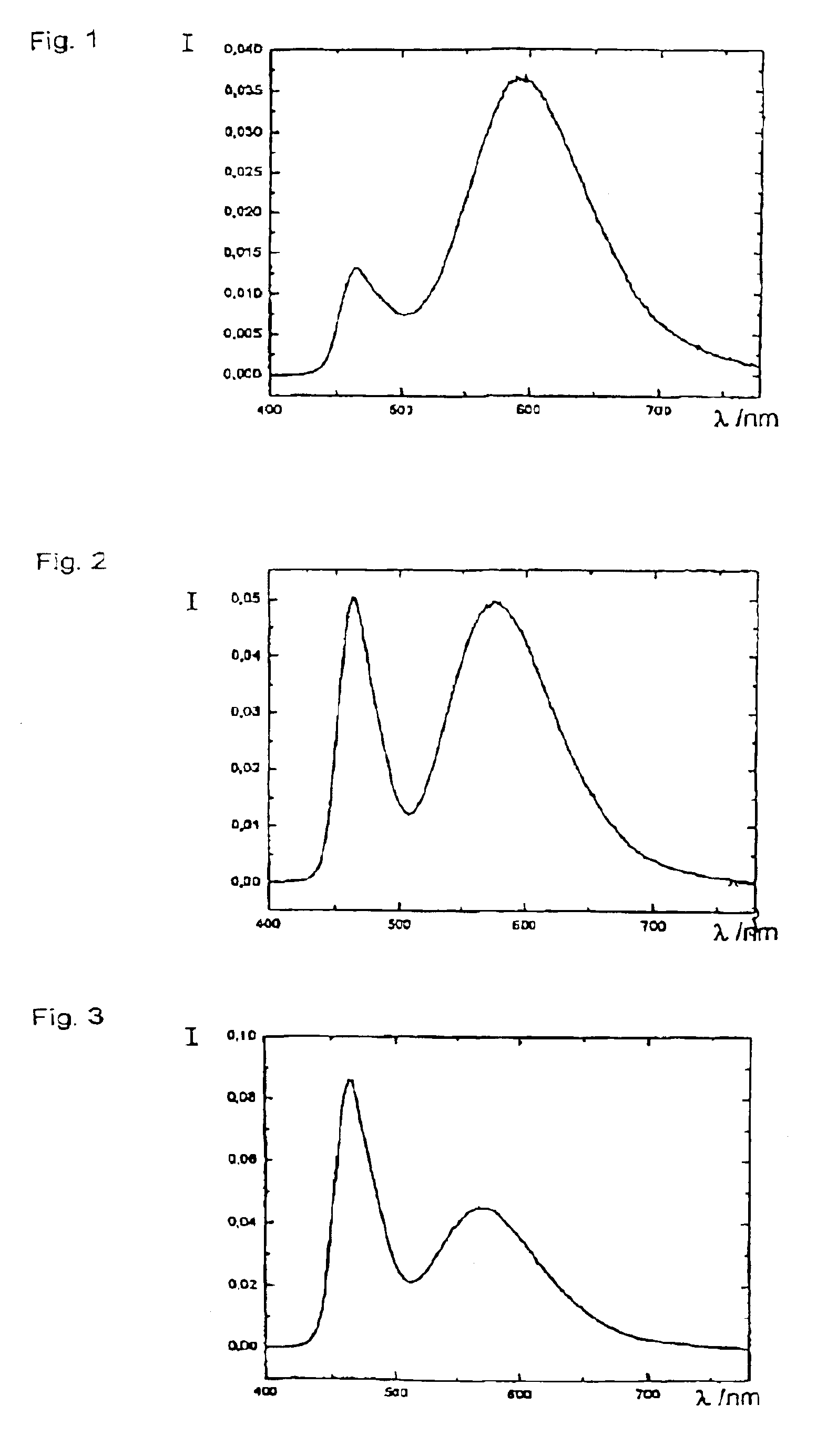
In chemistry, a silicate is any member of a family of anions consisting of silicon and oxygen, usually with the general formula [SiO₄₋ₓ]ₙ, where 0 ≤ x < 2. The family includes orthosilicate SiO⁴⁻₄ (x = 0), metasilicate SiO²⁻₃ (x = 1), and pyrosilicate Si₂O⁶⁻₇ (x = 0.5, n = 2). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate.
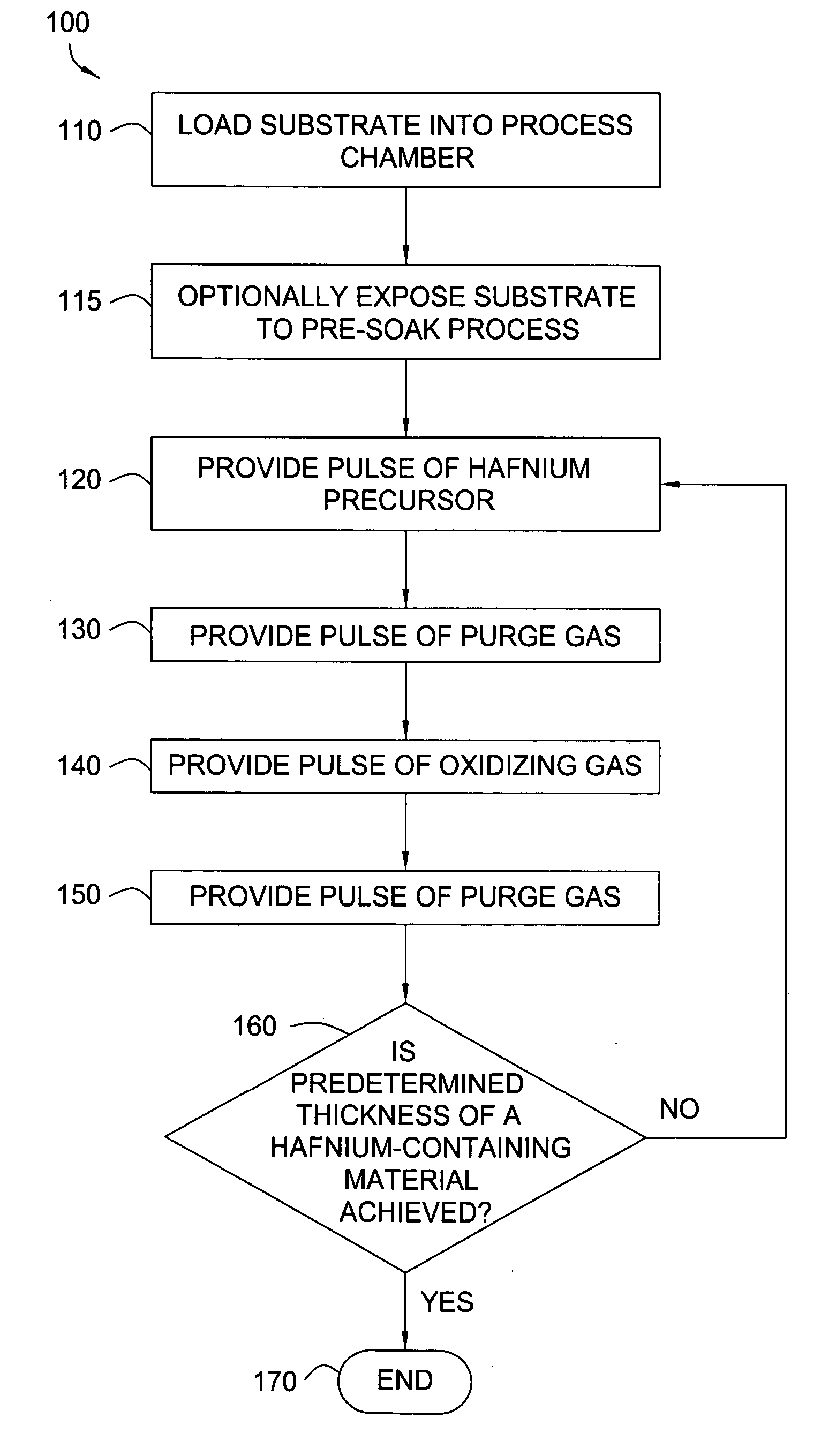
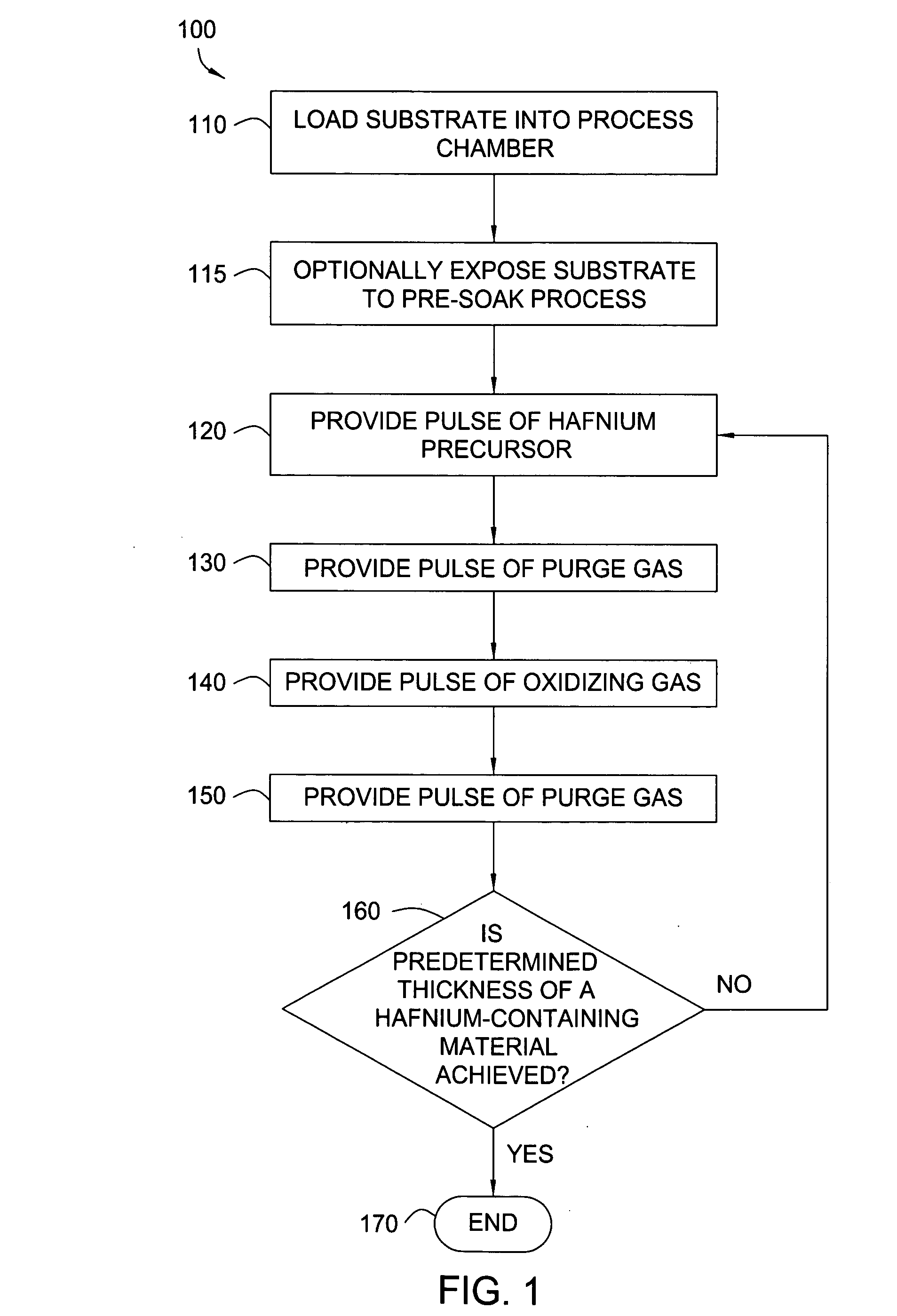
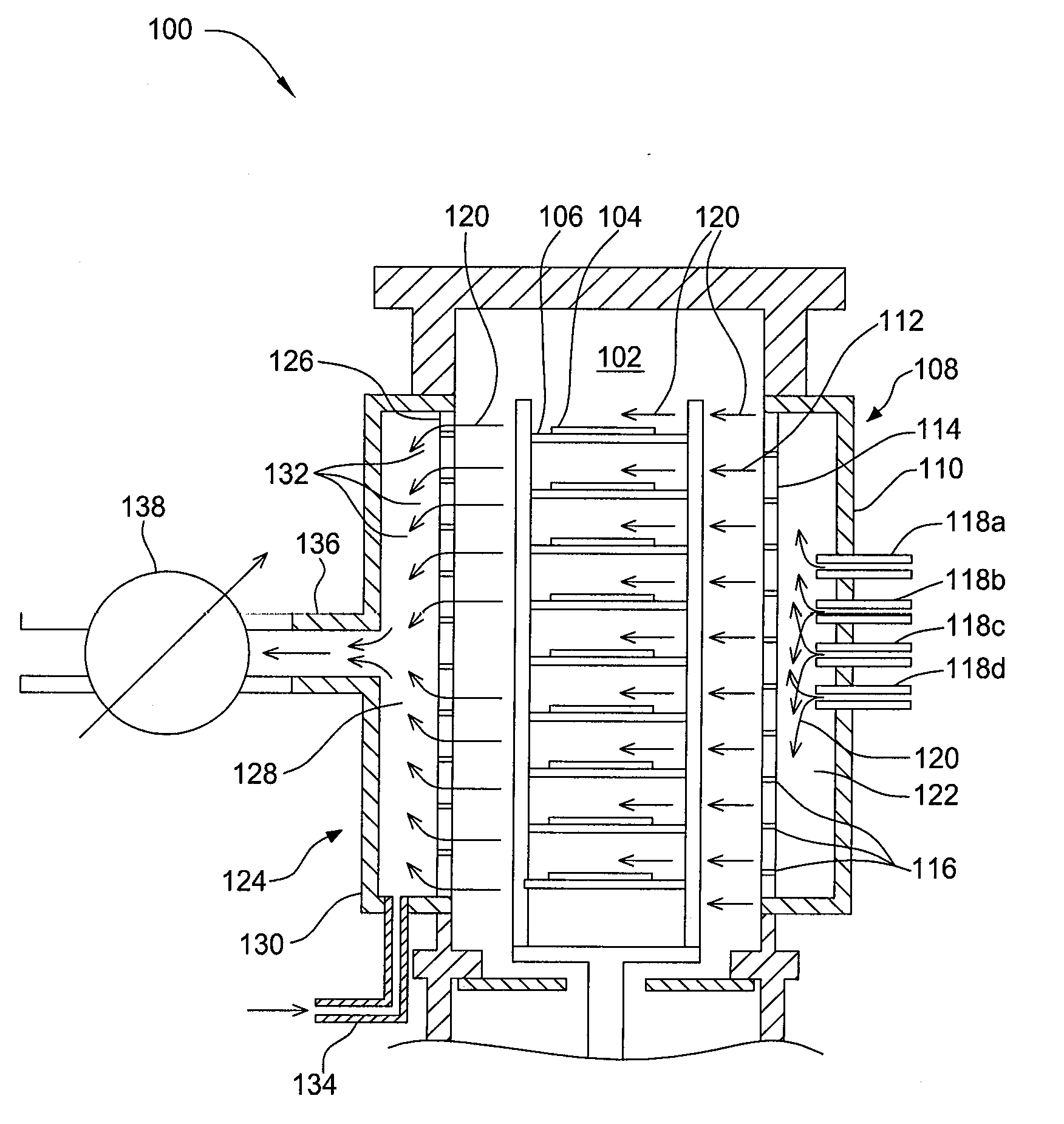
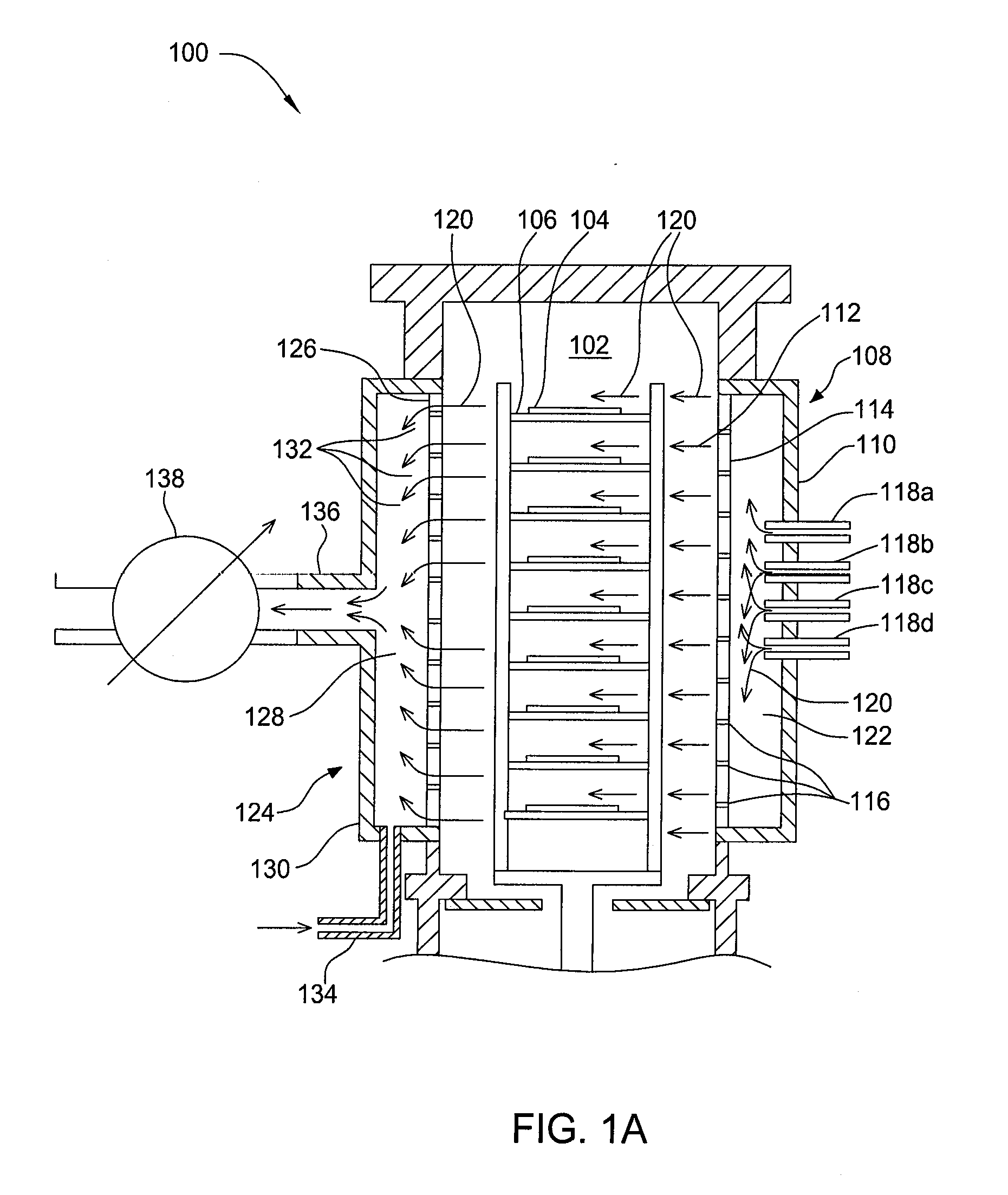
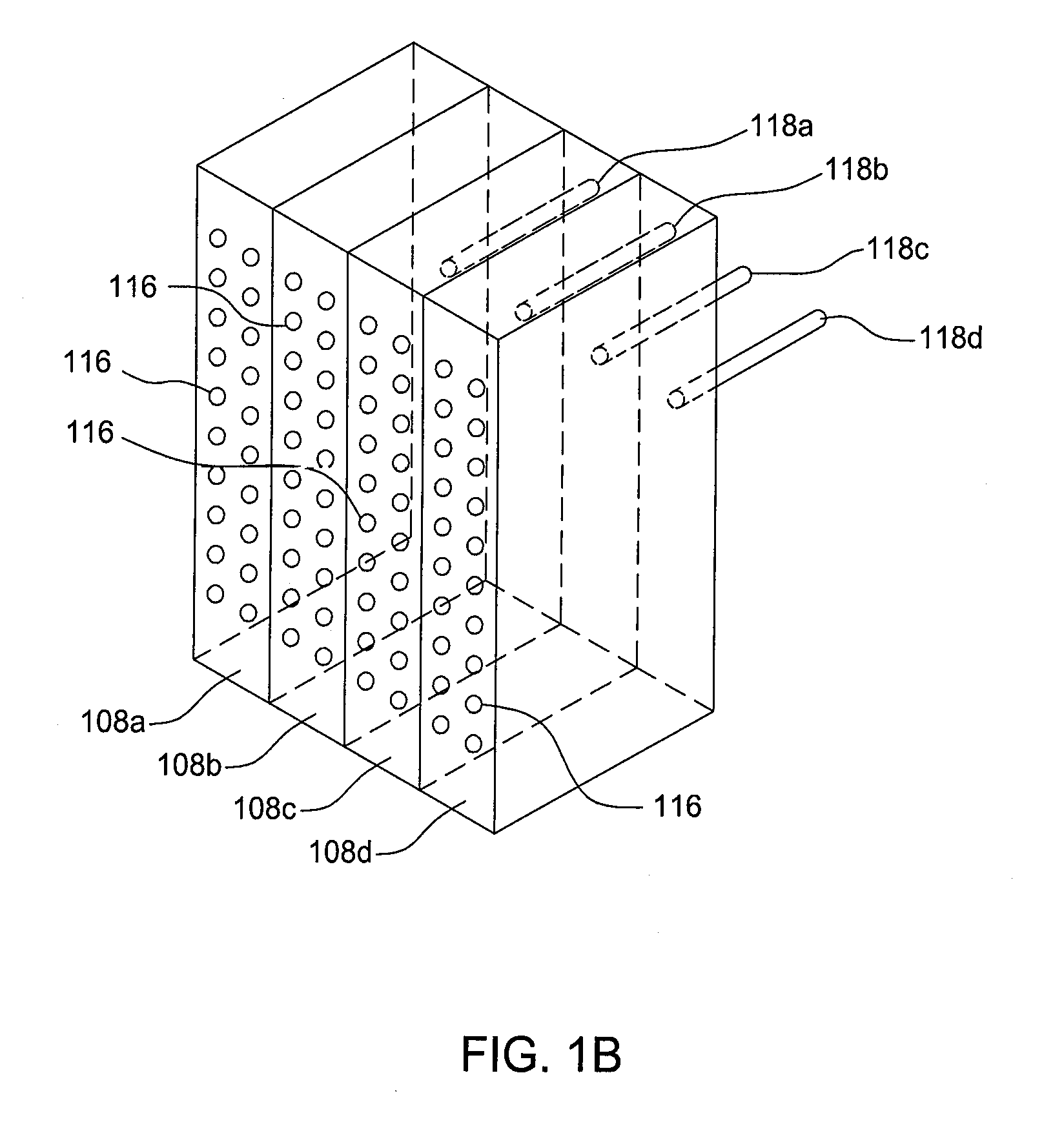
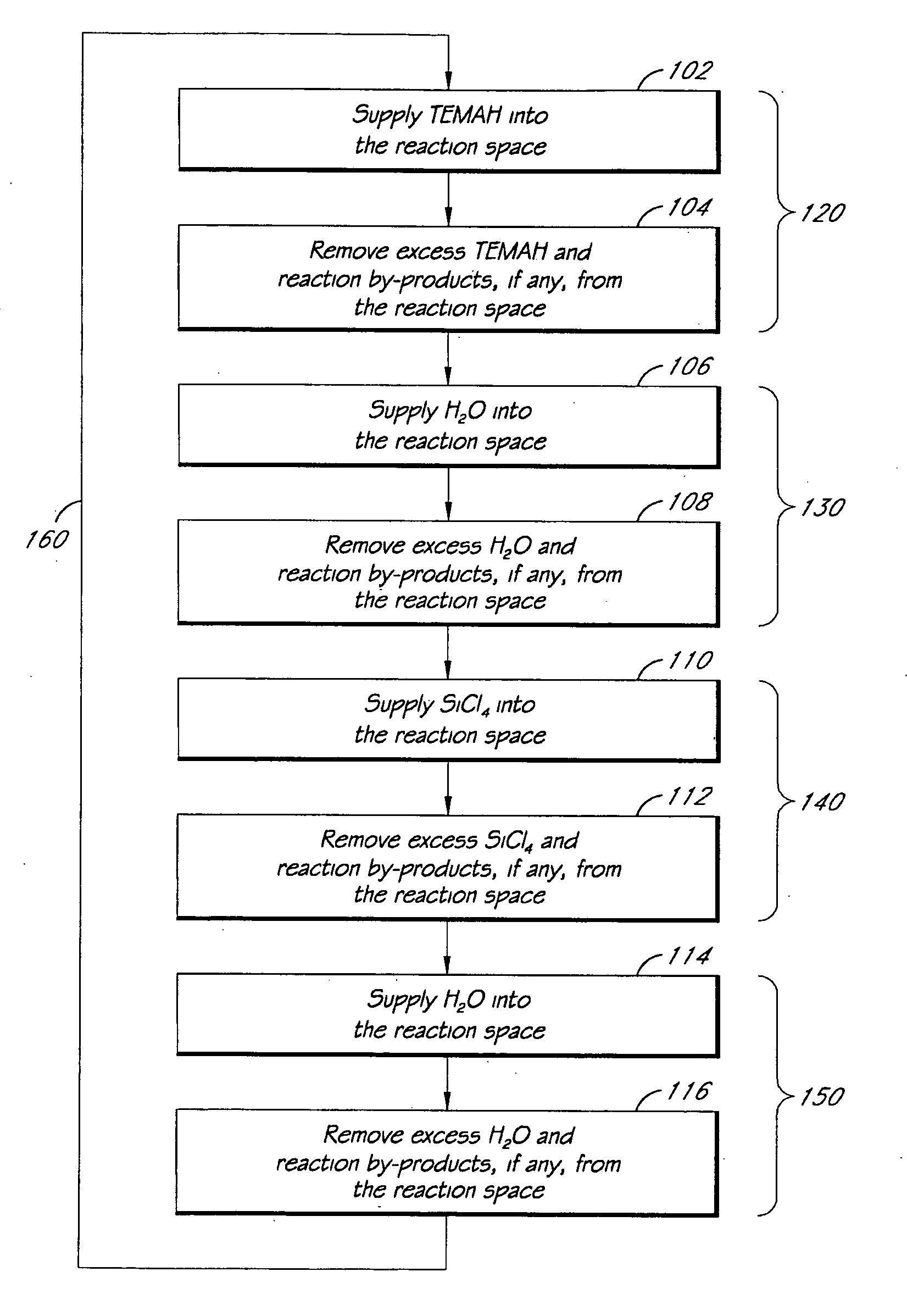
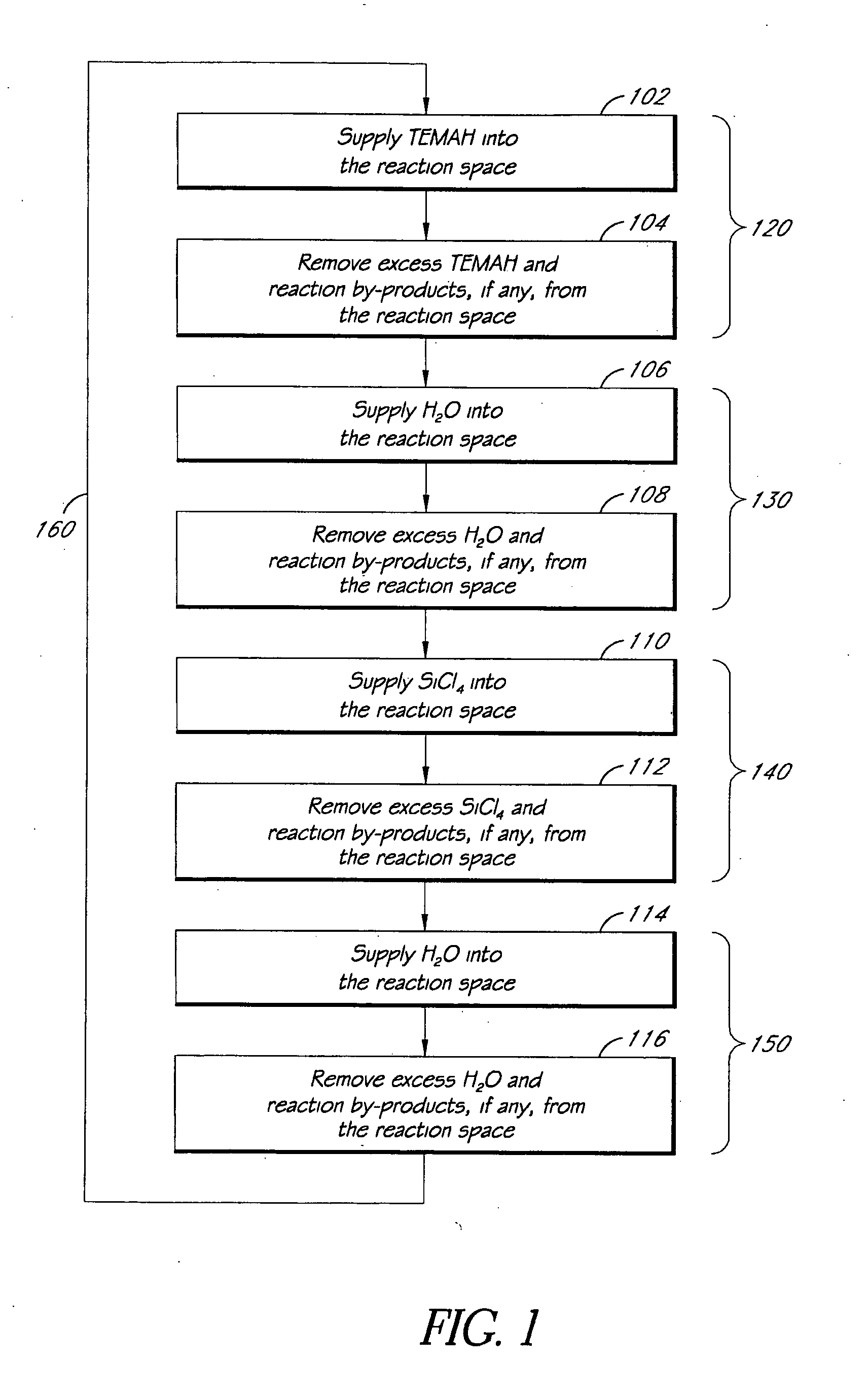
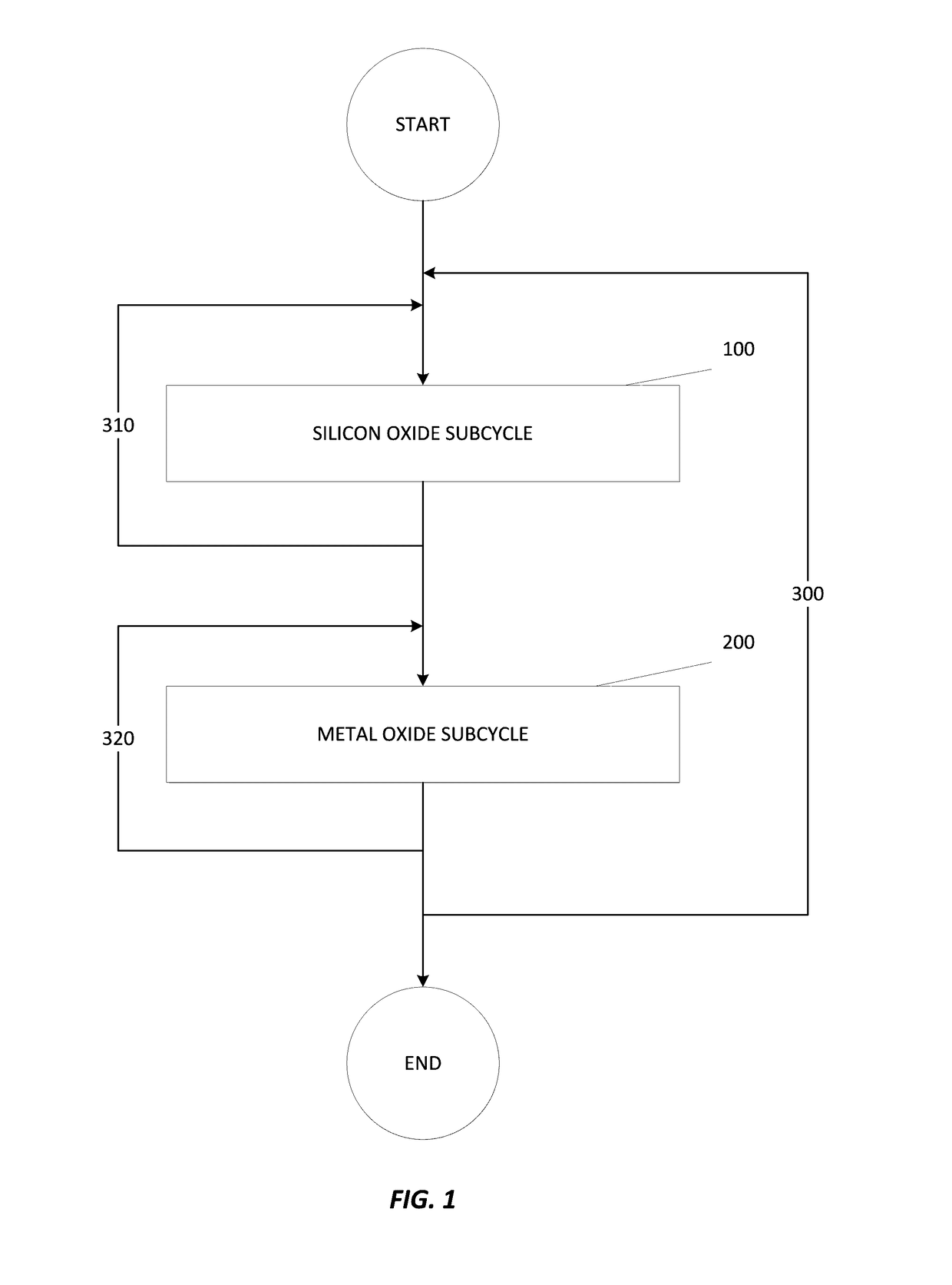
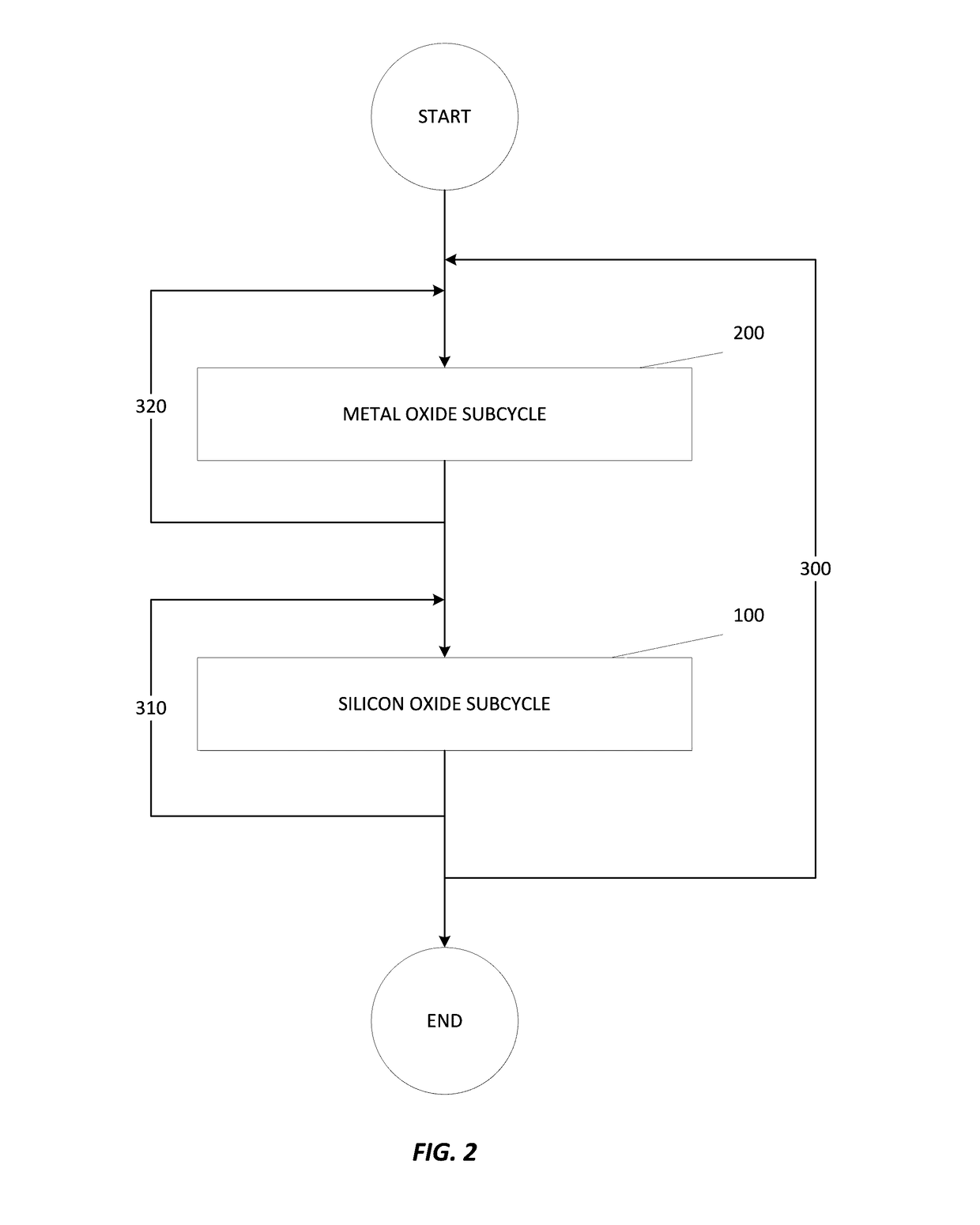
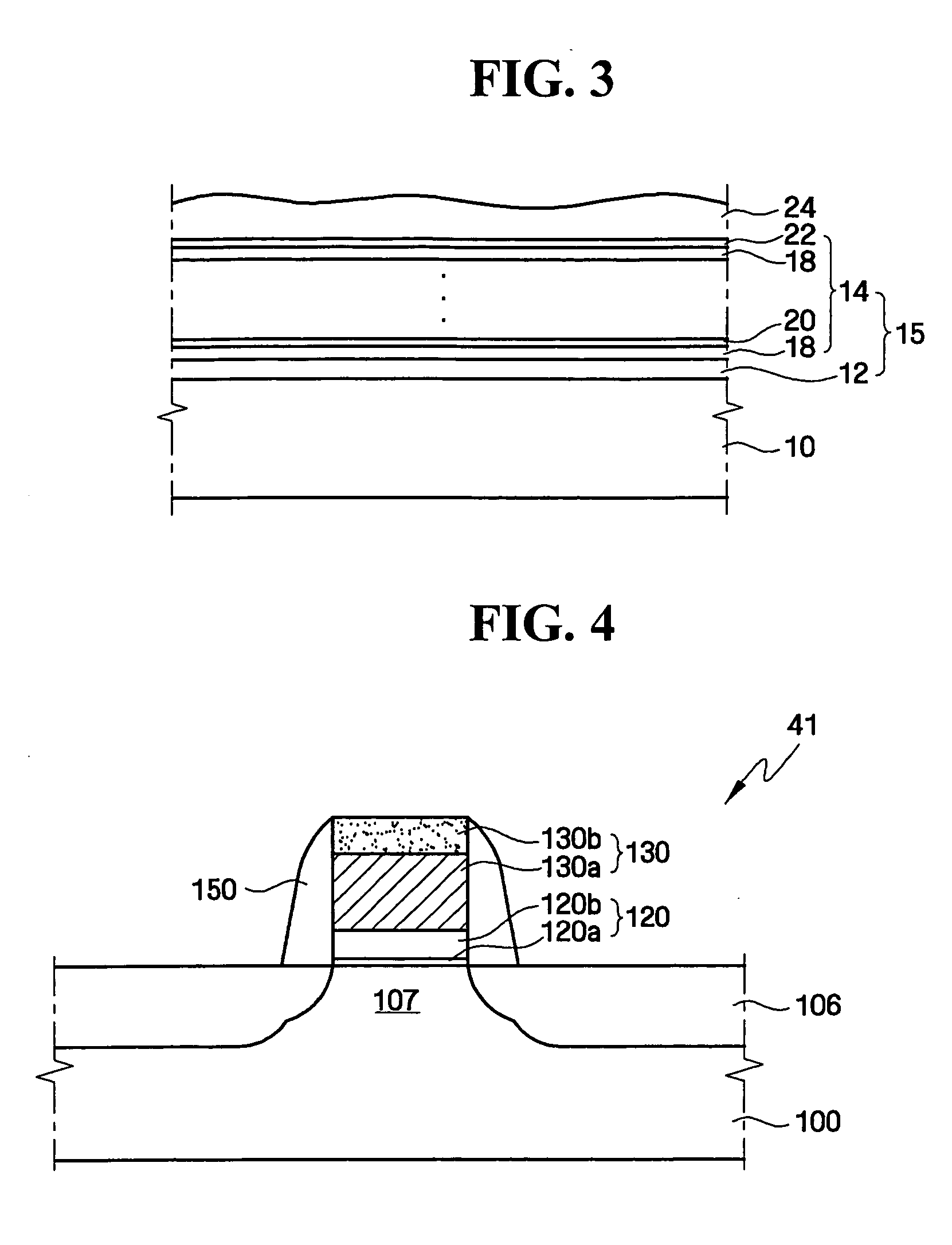
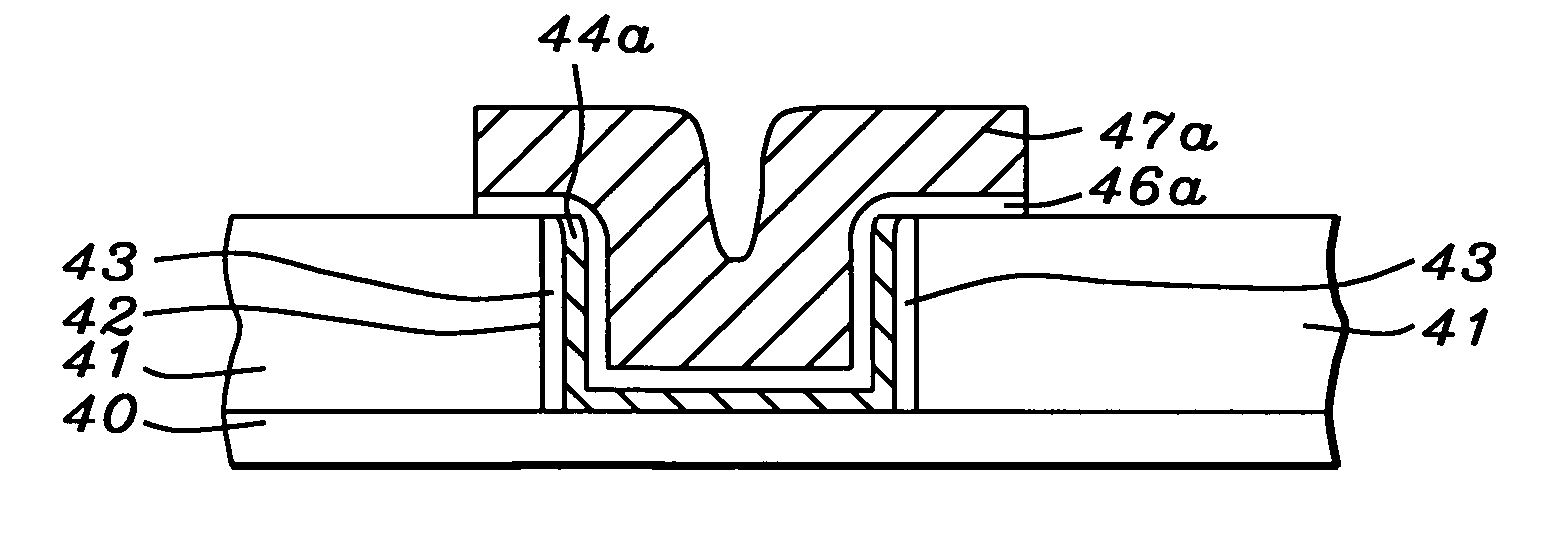
Apparatuses and methods for atomic layer deposition of hafnium-containing high-k dielectric materials
InactiveUS20050271813A1Steam generation heating methodsDecorative surface effectsGas phaseWater vapor
Embodiments of the invention provide methods for depositing dielectric materials on substrates during vapor deposition processes, such as atomic layer deposition (ALD). In one example, a method includes sequentially exposing a substrate to a hafnium precursor and an oxidizing gas to deposit a hafnium oxide material thereon. In another example, a hafnium silicate material is deposited by sequentially exposing a substrate to the oxidizing gas and a process gas containing a hafnium precursor and a silicon precursor. The oxidizing gas usually contains water vapor formed by flowing a hydrogen source gas and an oxygen source gas through a water vapor generator. In another example, a method includes sequentially exposing a substrate to the oxidizing gas and at least one precursor to deposit hafnium oxide, zirconium oxide, lanthanum oxide, tantalum oxide, titanium oxide, aluminum oxide, silicon oxide, aluminates thereof, silicates thereof, derivatives thereof or combinations thereof.
Owner:APPLIED MATERIALS INC
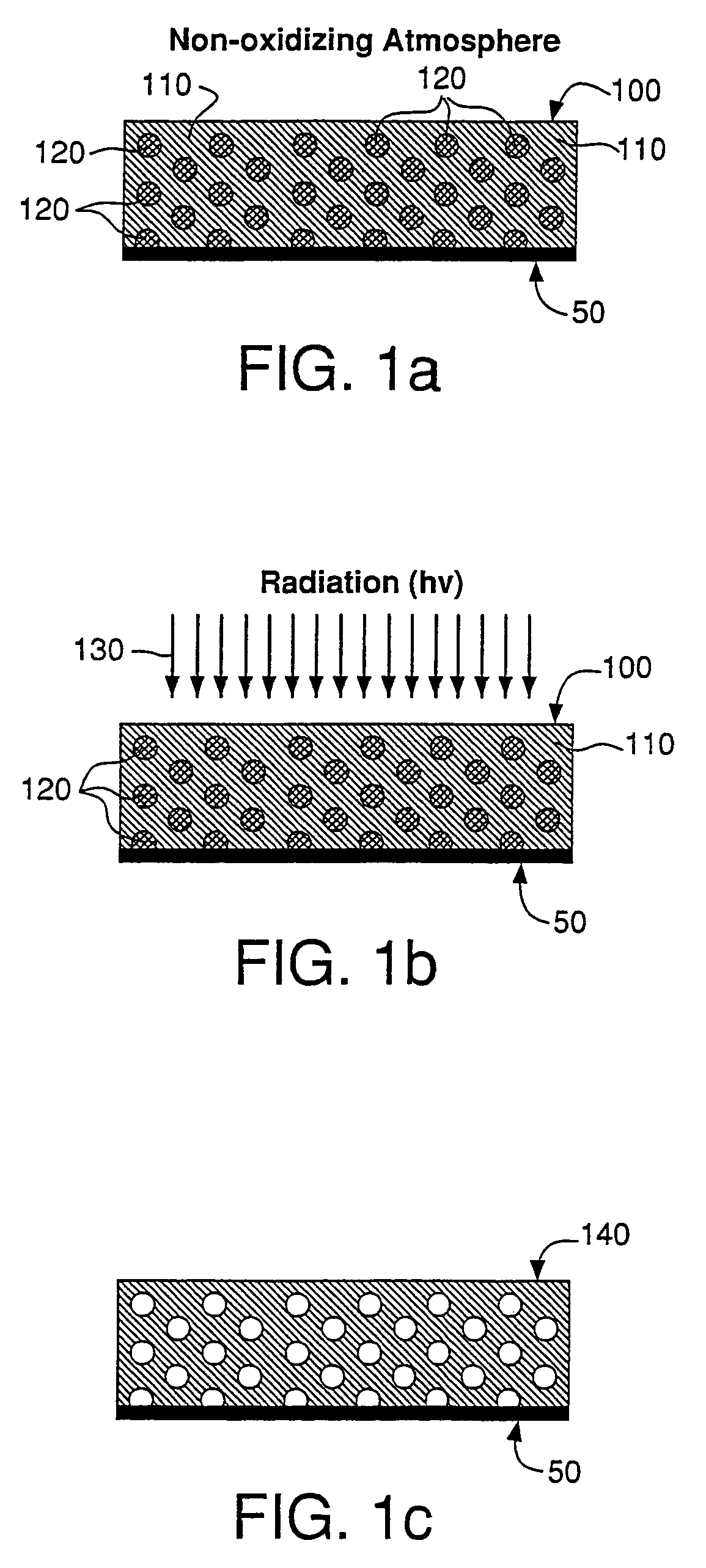
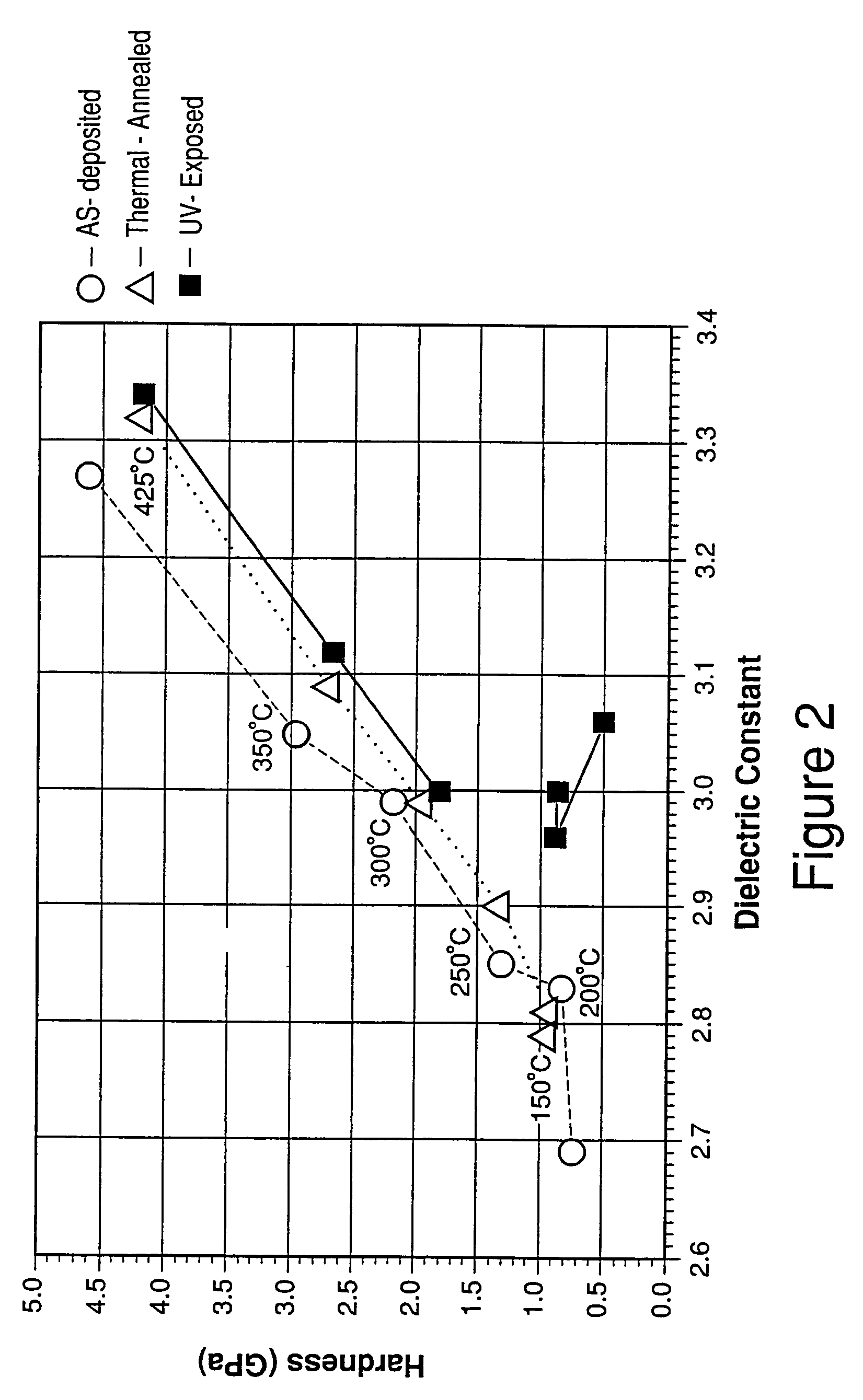
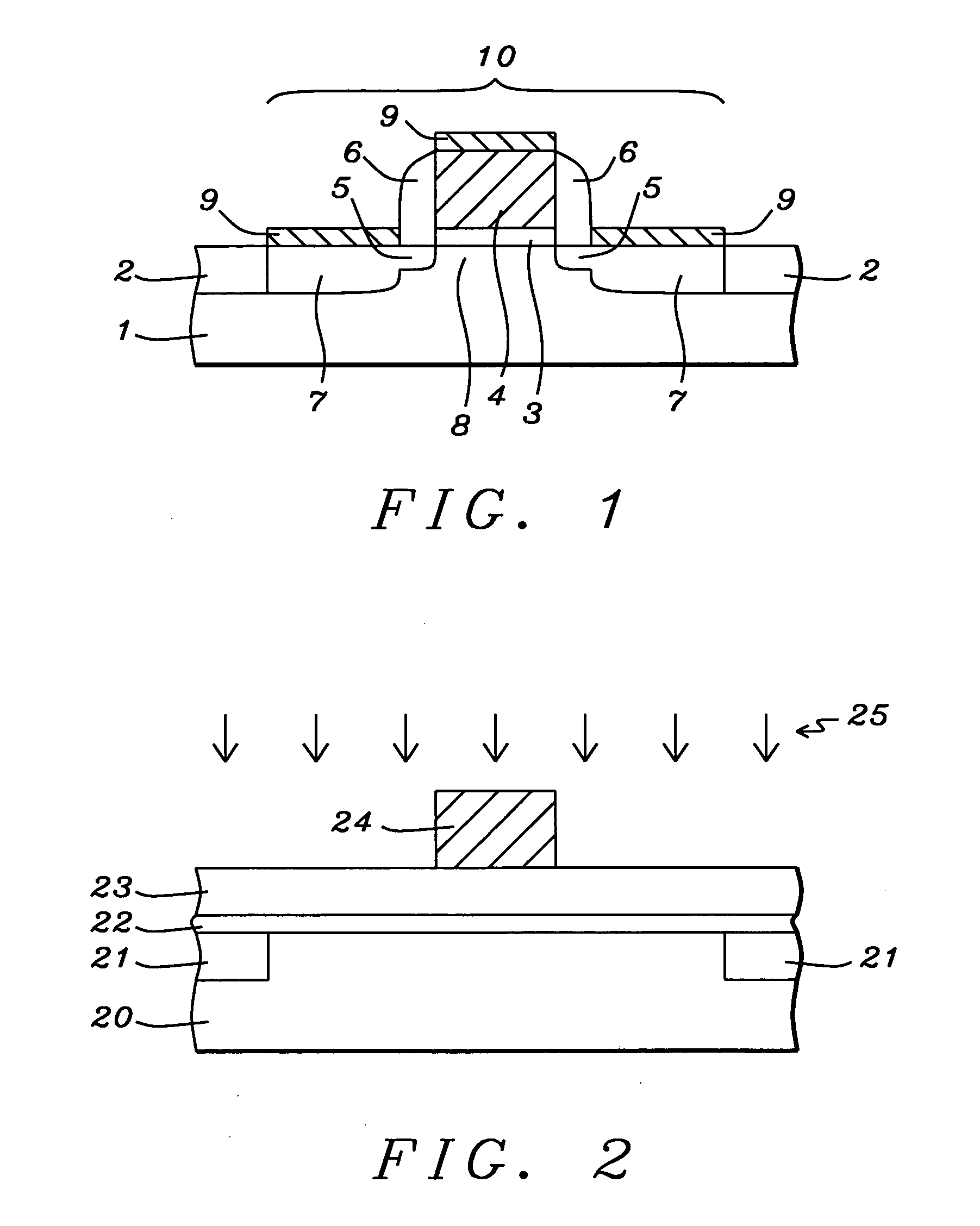
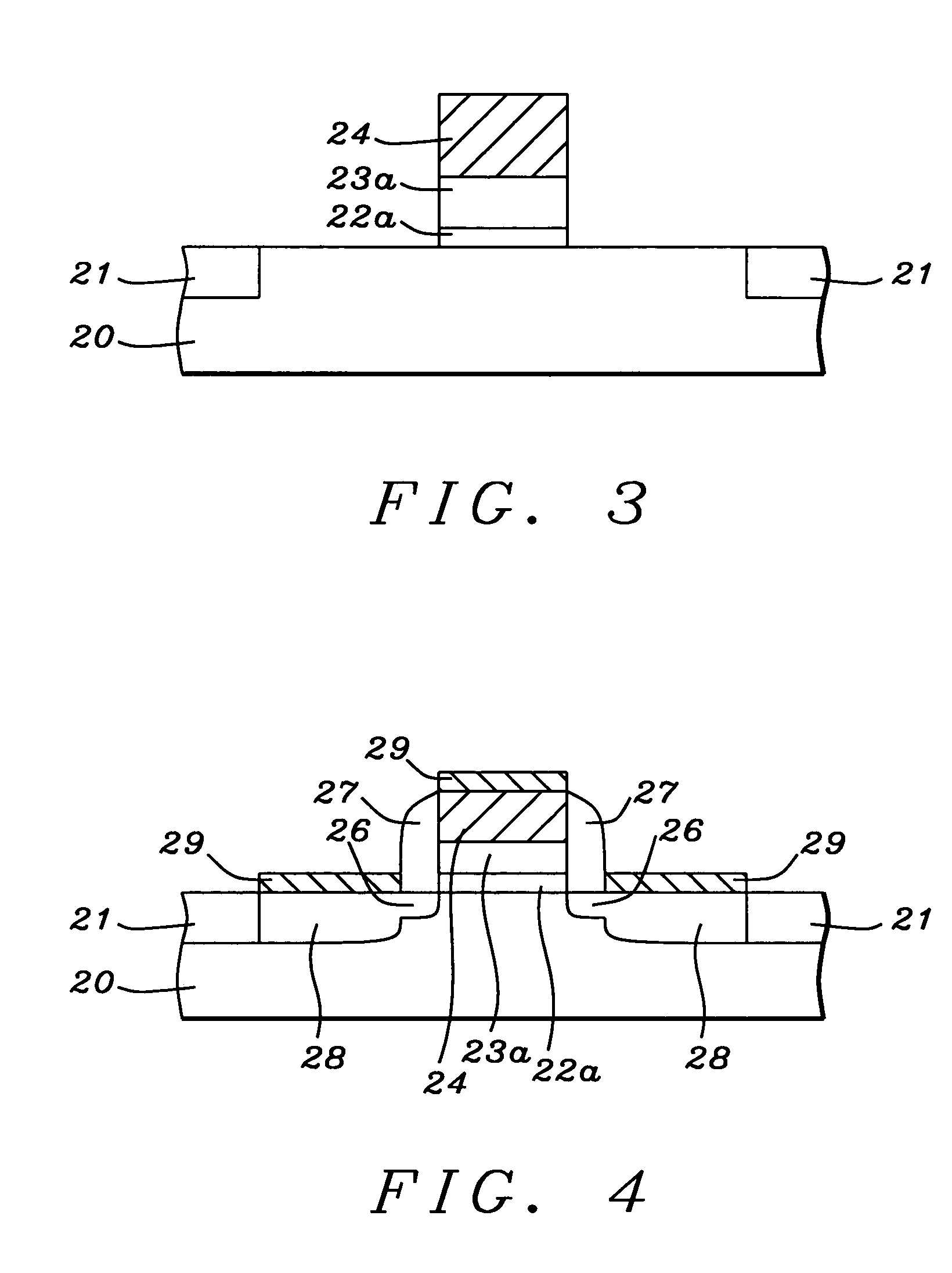
Mechanical enhancement of dense and porous organosilicate materials by UV exposure
InactiveUS7098149B2Low dielectric constantIncrease modulusSemiconductor/solid-state device manufacturingChemical vapor deposition coatingDielectricUltraviolet lights
Low dielectric materials and films comprising same have been identified for improved performance when used as interlevel dielectrics in integrated circuits as well as methods for making same. In one aspect of the present invention, an organosilicate glass film is exposed to an ultraviolet light source wherein the film after exposure has an at least 10% or greater improvement in its mechanical properties (i.e., material hardness and elastic modulus) compared to the as-deposited film.
Owner:VERSUM MATERIALS US LLC
Down-drawable, chemically strengthened glass for cover plate
An alkali aluminosilicate glass that is chemically strengthened and has a down-drawable composition. The glass has a melting temperature less than about 1650° C. and a liquidus viscosity of at least 130 kpoise and, in one embodiment, greater than 250 kpoise. The glass undergoes ion exchange at relatively low temperatures to a depth of at least 30 μm.
Owner:CORNING INC
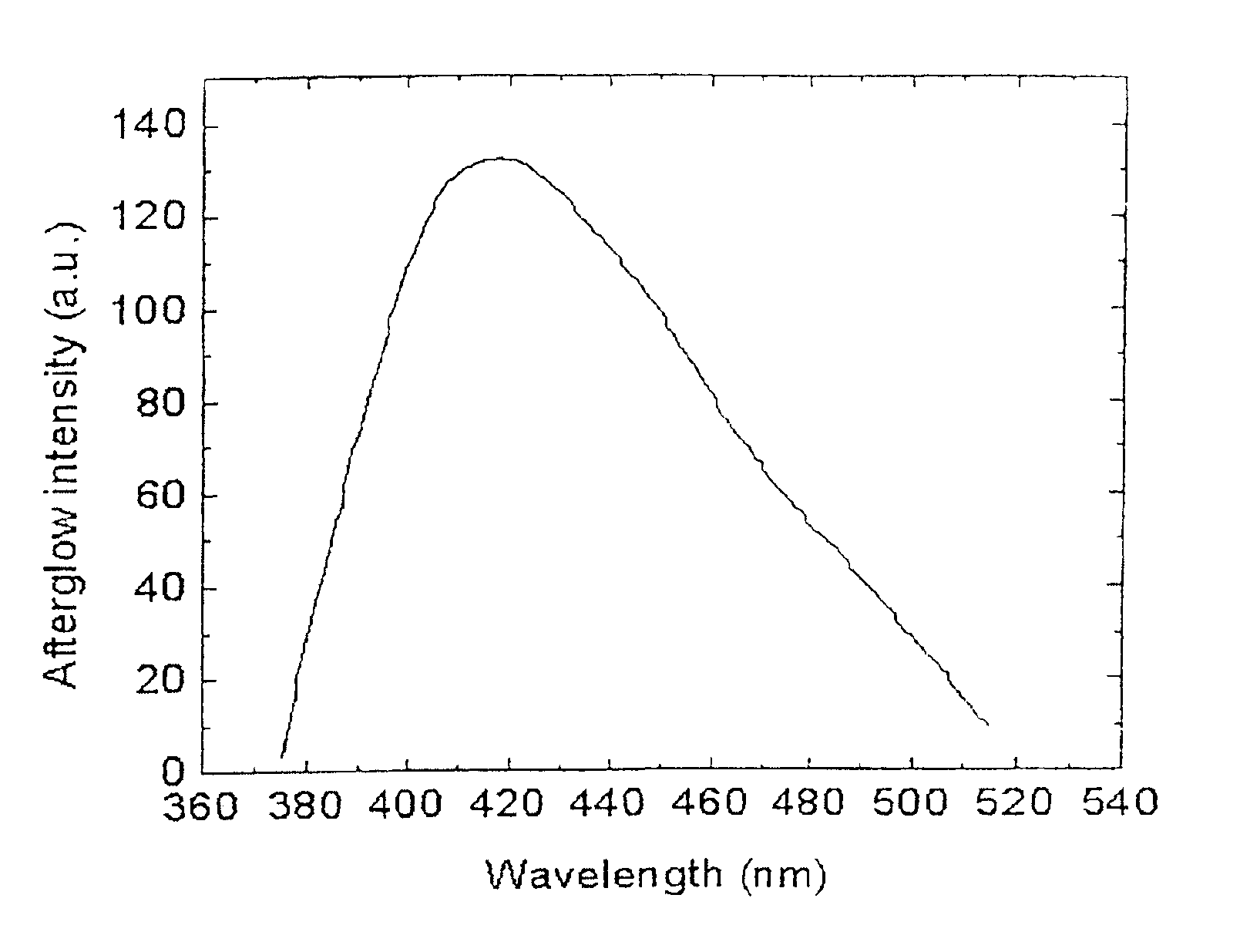
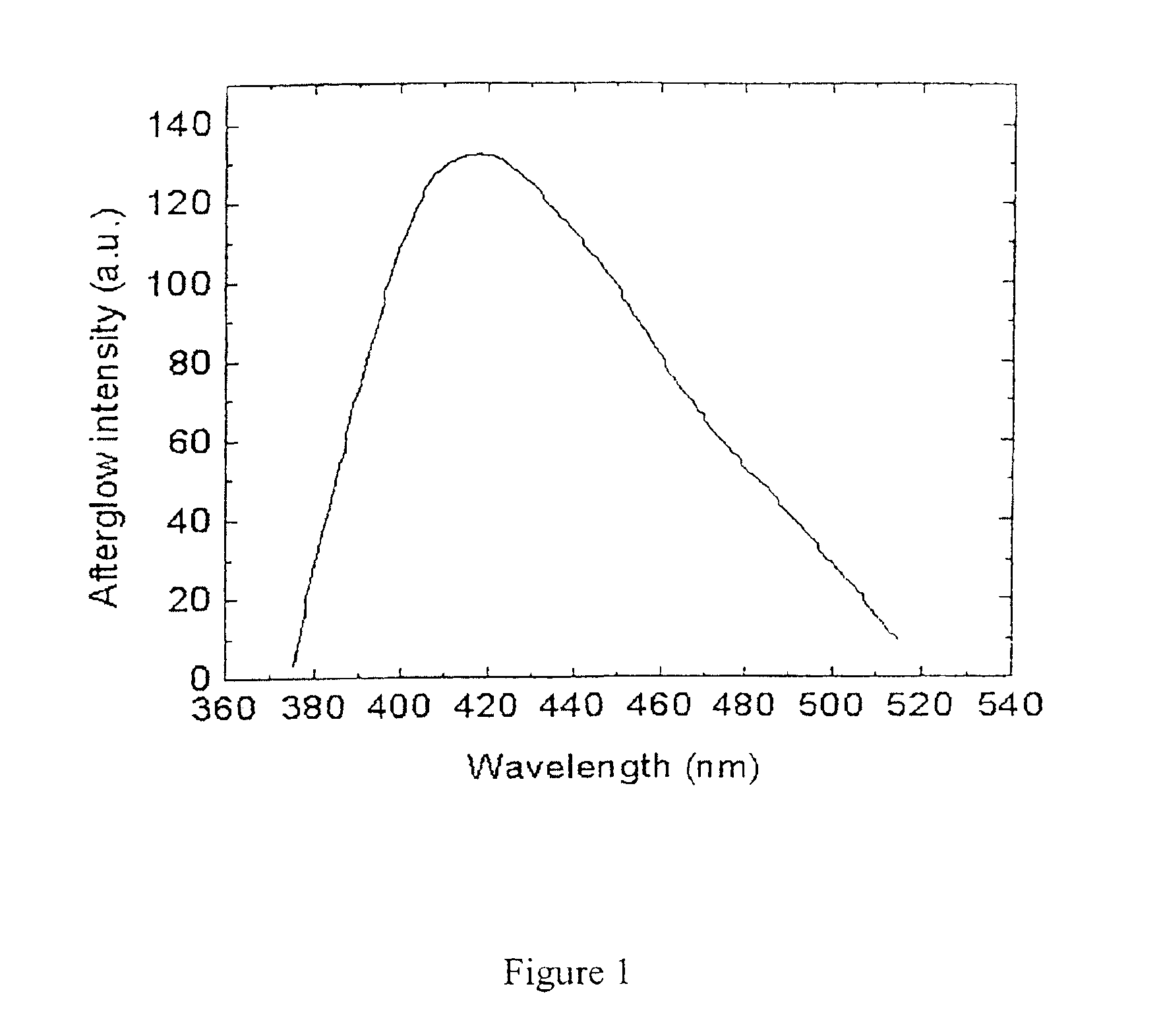
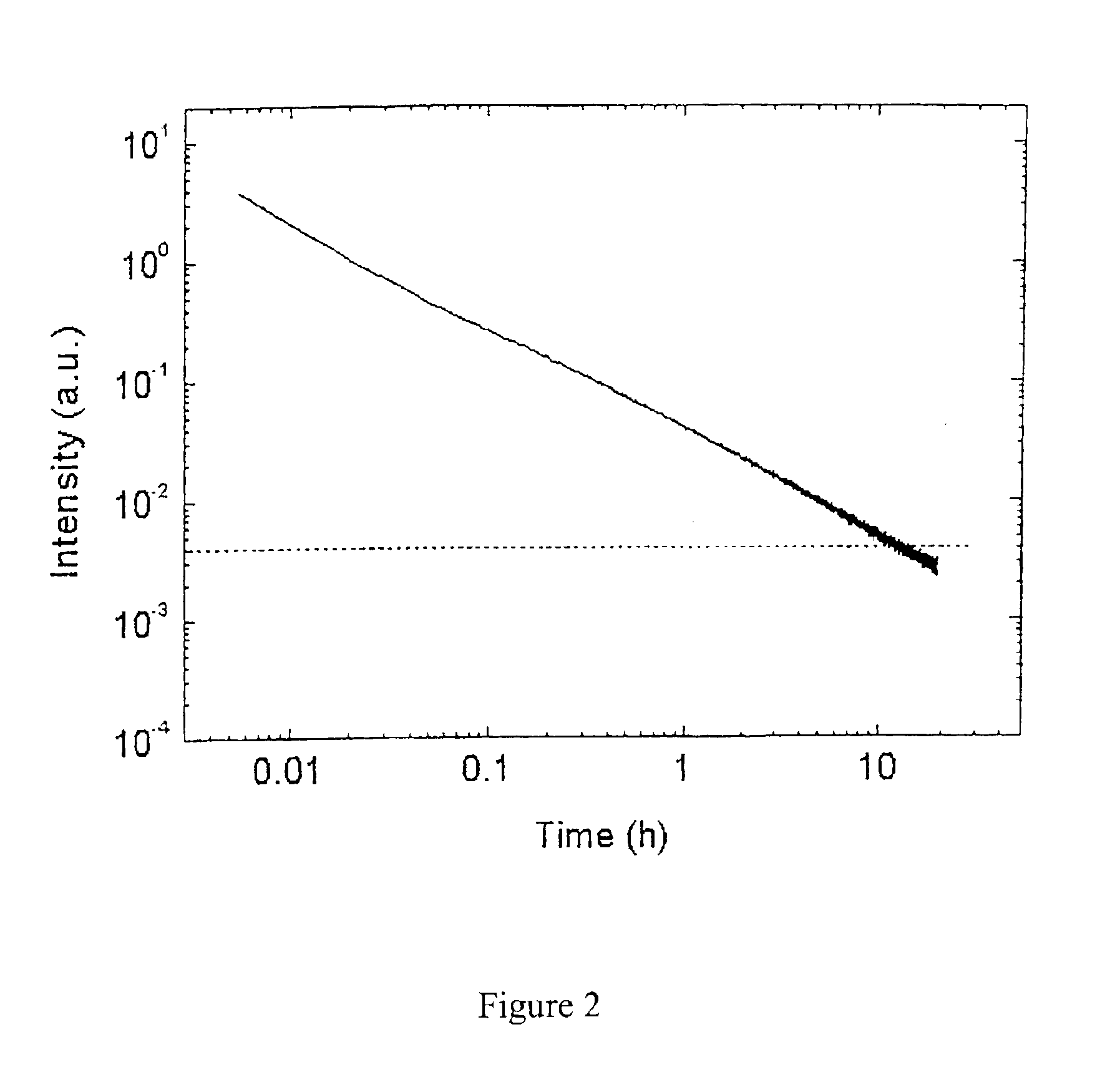
Long persistent phosphors and persistent energy transfer technique
The invention provides long-persistent phosphors, methods for their manufacture and phosphorescent articles. The invention also provides a method for generating a long-persistent phosphorescence at a selected color. The phosphors of the invention may be alkaline earth aluminates, alkaline earth silicates, and alkaline earth aluminosilicates. The phosphors include those activated by cerium. The phosphors also include those in which persistent energy transfer occurs from a donor ion to an acceptor ion, producing persistent emission largely characteristic of the acceptor ion.
Owner:UNIV OF GEORGIA RES FOUND INC +1
Atomic layer deposition of high k metal silicates
InactiveUS20060228888A1Reduce carbon pollutionReduce stepsSolid-state devicesSemiconductor/solid-state device manufacturingHafniumAtomic layer deposition
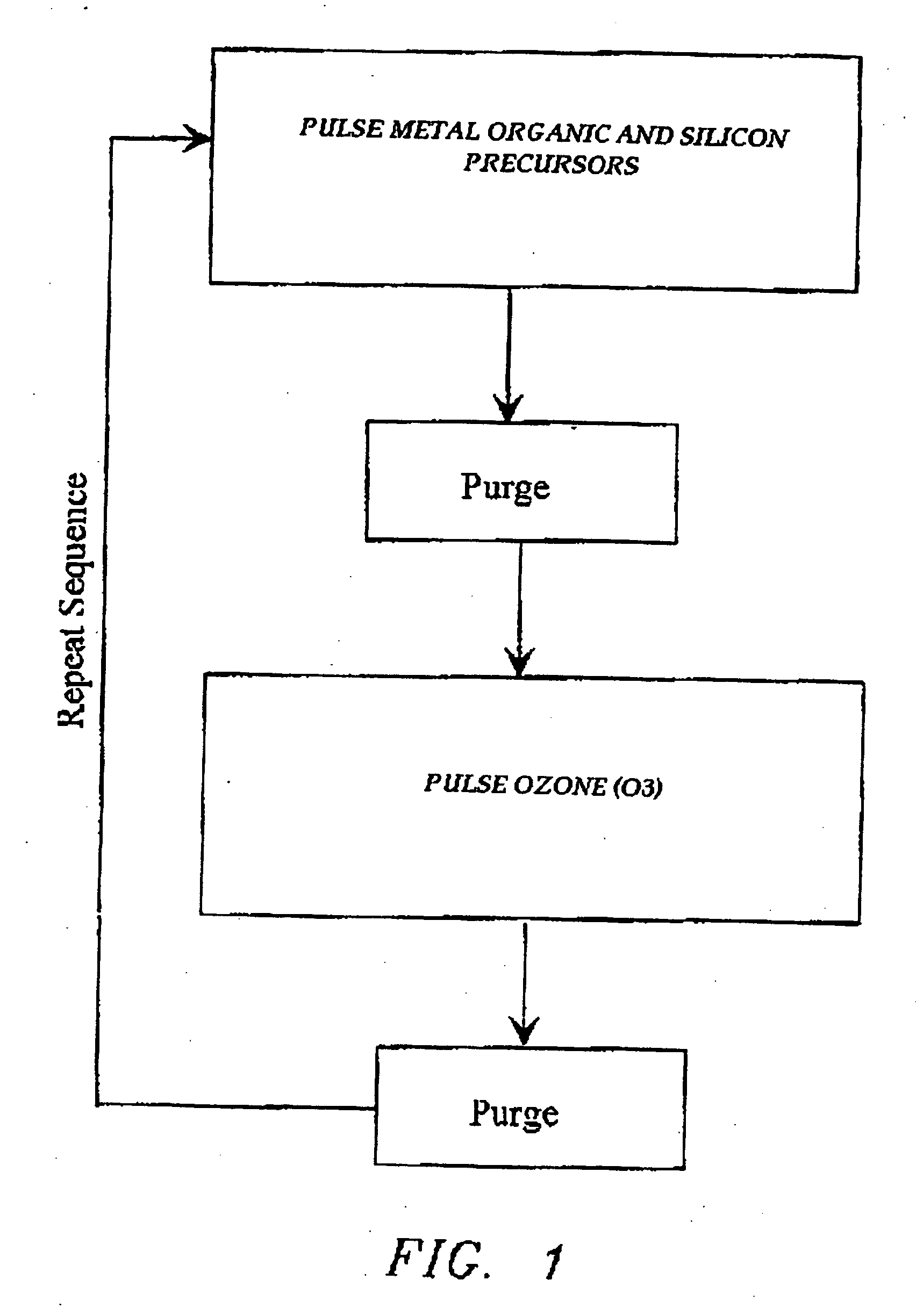
The present invention relates to the atomic layer deposition (“ALD”) of high k dielectric layers of metal silicates, including hafnium silicate. More particularly, the present invention relates to the ALD formation of metal silicates using metal organic precursors, silicon organic precursors and ozone. Preferably, the metal organic precursor is a metal alkyl amide and the silicon organic precursor is a silicon alkyl amide.
Owner:AVIZA TECHNOLOGY INC +1
Method for forming a thin film and method for fabricating a semiconductor device
InactiveUS20050070123A1Improve mobility and reliabilitySemiconductor/solid-state device manufacturingChemical vapor deposition coatingGate dielectricHafnium
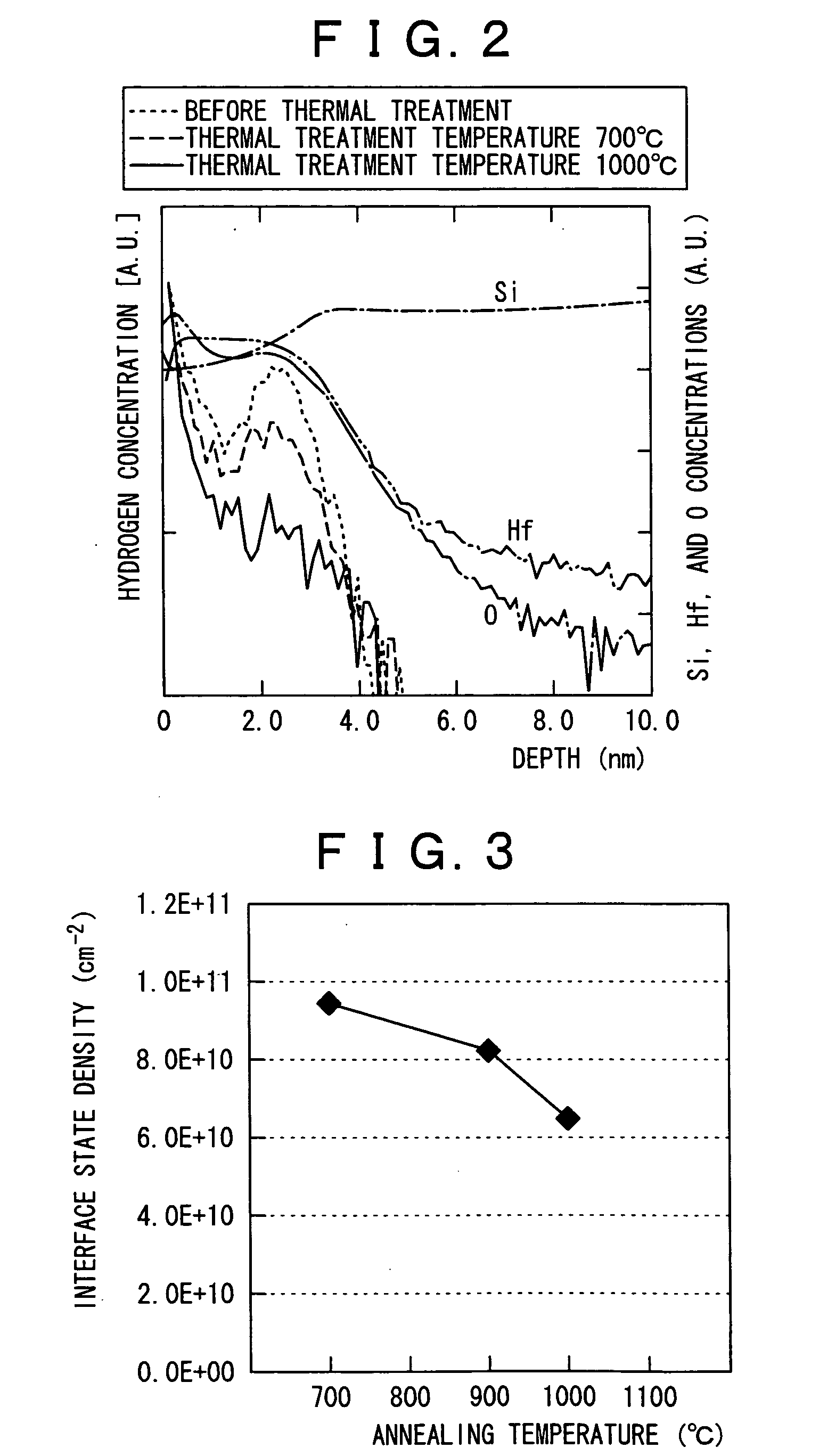
By conducting a high temperature annealing in a nitrogen atmosphere at a temperature at which a hafnium silicate film undergoes no phase separation, hydrogen contained in the film is removed and prevention of boron penetration is made possible. The present invention provides a method for forming a thin film including a step of forming a hafnium silicate film on a substrate by an atomic layer deposition method and a step of carrying out thermal treatment on the hafnium silicate film at a thermal treatment temperature equal to or higher than a temperature at which hydrogen contained in the hafnium silicate film is removed and lower than a temperature at which the hafnium silicate film undergoes no phase separation, and a method for fabricating a semiconductor device for forming a gate dielectric film using the method for forming a thin film.
Owner:SONY CORP
Method of manufacturing semiconductor device
InactiveUS20070066010A1Good step coverageReduce leakage levelTransistorSolid-state devicesDevice materialEvaporation
The invention aims at enabling leakage current characteristics and a step coverage property to be improved by depositing a hafnium silicate film by utilizing an atomic layer evaporation method using a hafnium raw material, a silicon raw material and an oxidizing agent. Disclosed herein is a method of manufacturing a semiconductor device having a trench capacitor including a first electrode formed on an inner surface of a trench, a capacitor insulating film formed on a surface of the first electrode, and a second electrode formed on a surface of the capacitor insulating film. The method includes the step of depositing the capacitor insulating film in a form of a hafnium silicate film by utilizing an atomic layer deposition method using a hafnium raw material, a silicon raw material and an oxidizing agent.
Owner:SONY CORP
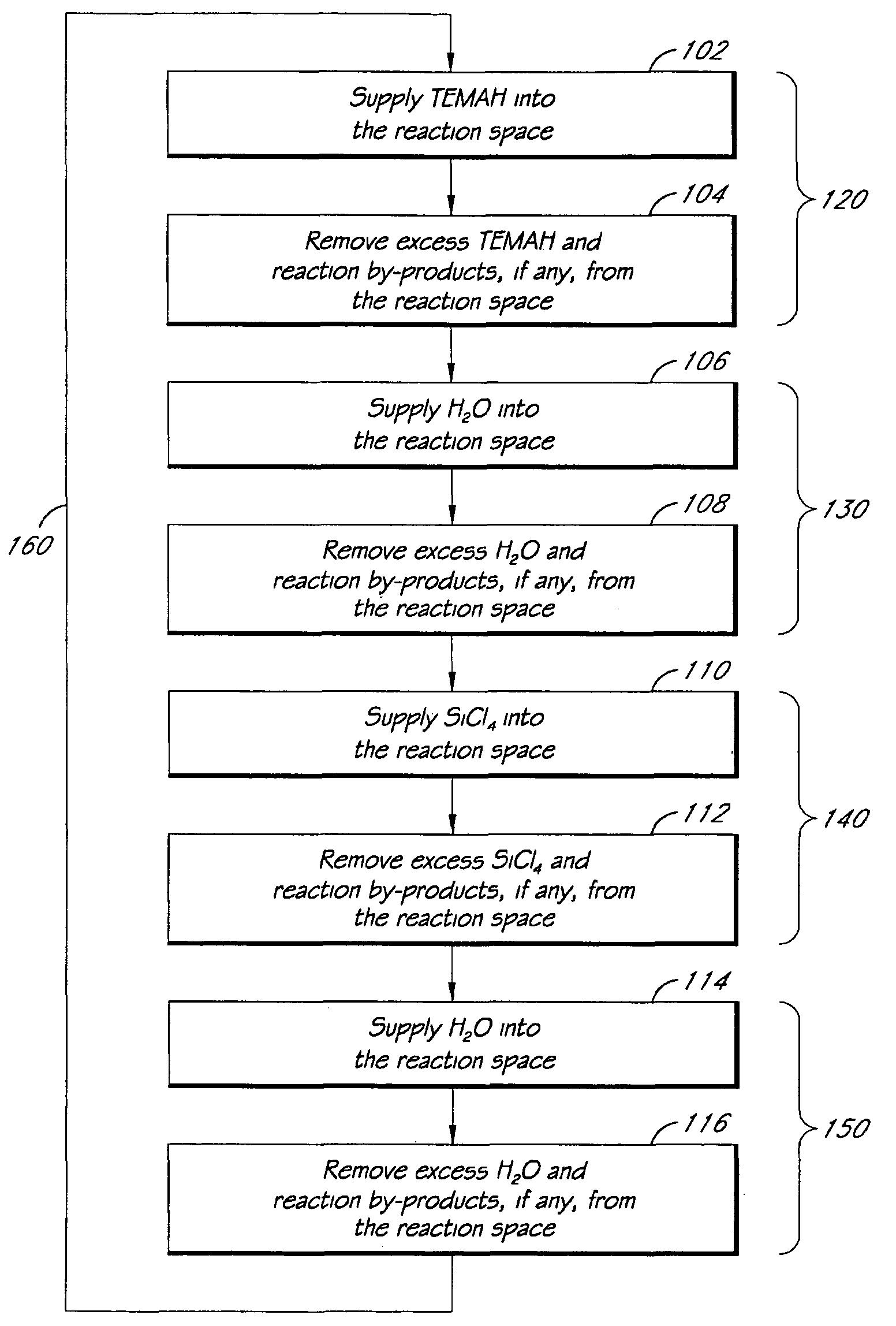
Method of depositing catalyst assisted silicates of high-k materials
InactiveUS20080113096A1Reduce the temperatureSilicon organic compoundsChemical vapor deposition coatingSoaking ProcedureHafnium
A high-k silicate atomic layer deposition method is disclosed. To produce a hafnium silicate layer, a substrate may be exposed to a pulse of a hafnium precursor, a pulse of an oxidizer, a pulse of a silicon precursor, and a pulse of another oxidizer. A catalyst may additionally be co-flowed with one or more reactants into the chamber through a separate inlet. Alternatively, the catalyst may be flowed to the chamber before the reactant is introduced in a soaking procedure. By either co-flowing the catalyst through separate inlets or by performing a catalyst soak, hafnium silicate formation may proceed at a fast rate and / or at a low temperature.
Owner:APPLIED MATERIALS INC
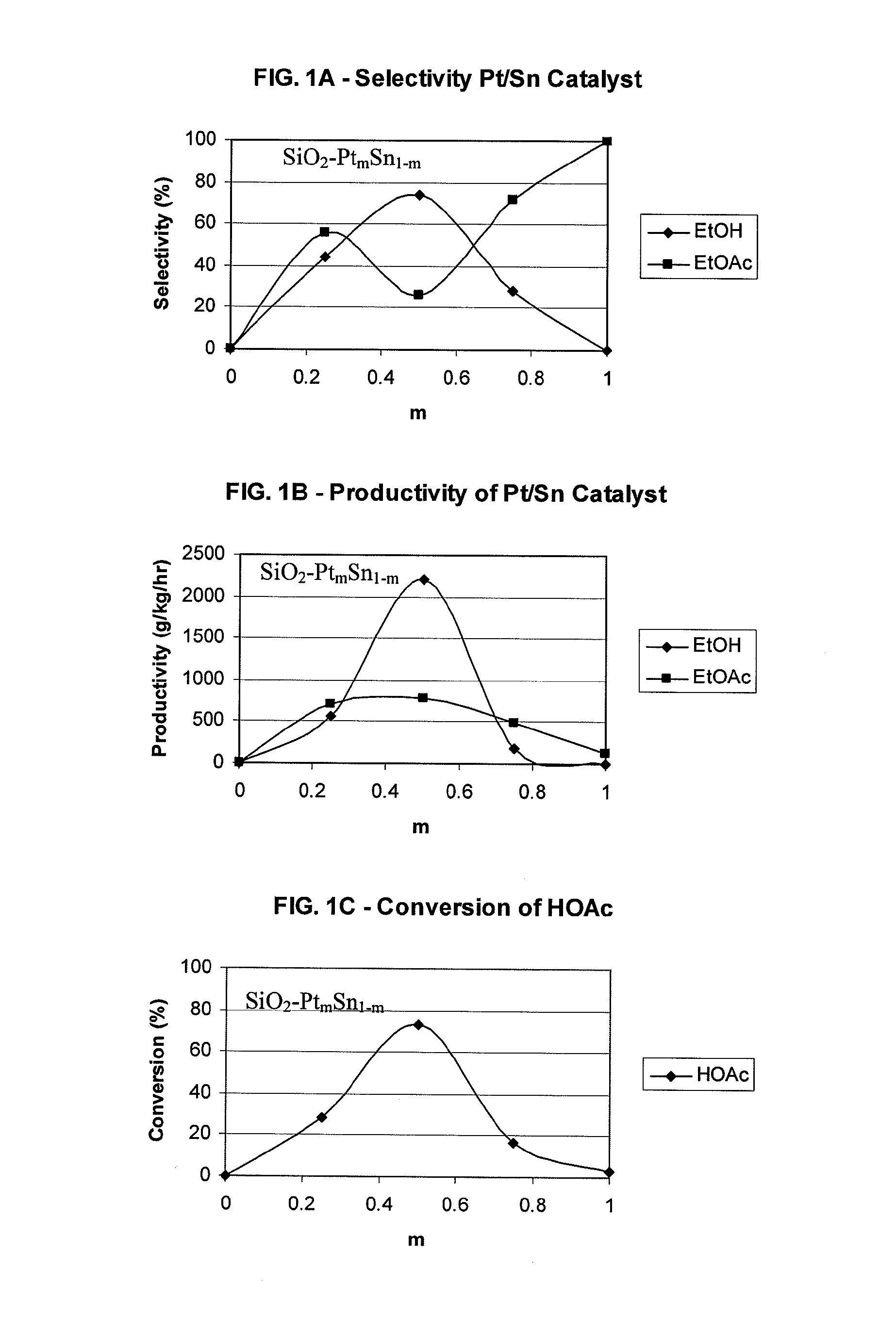
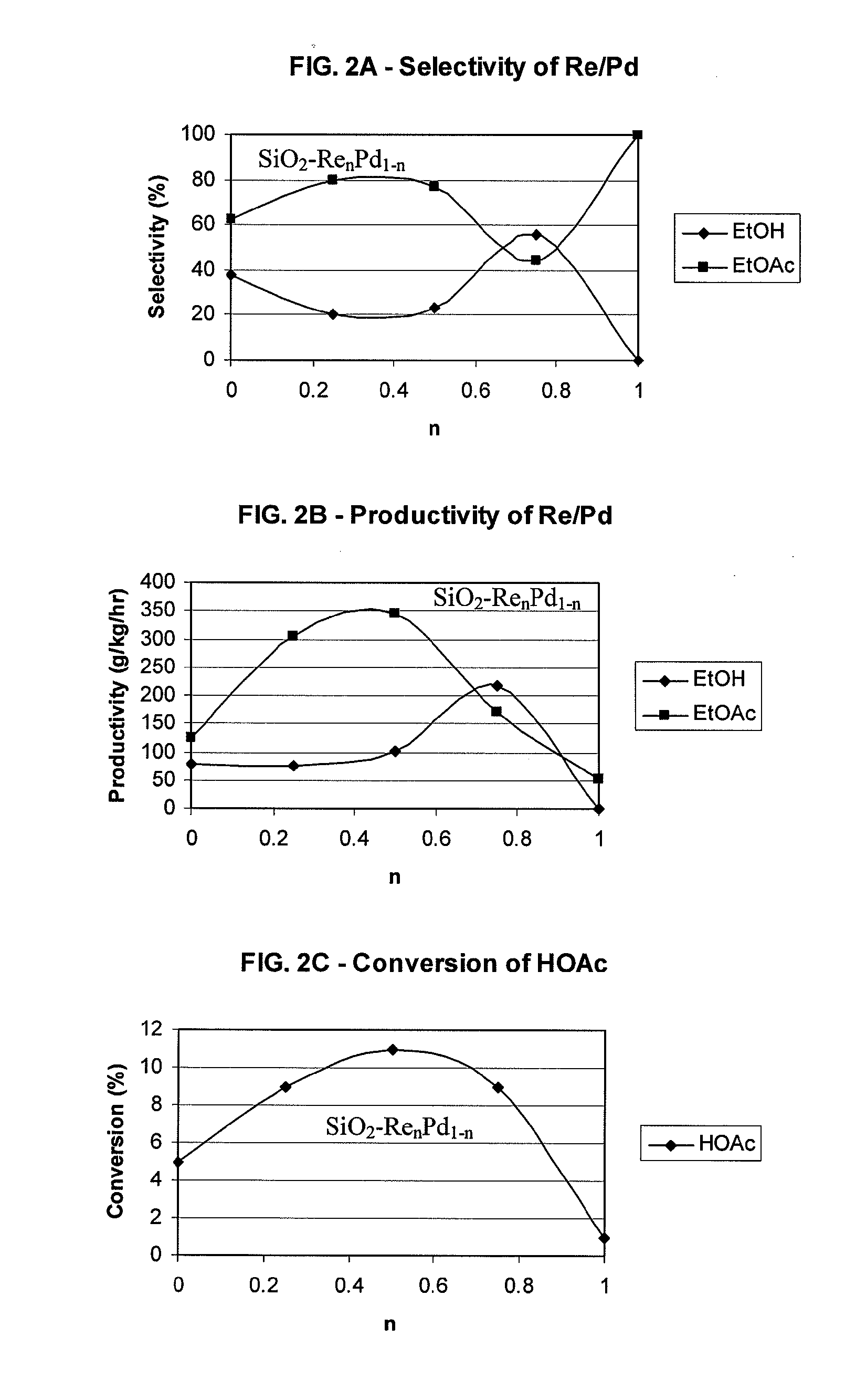
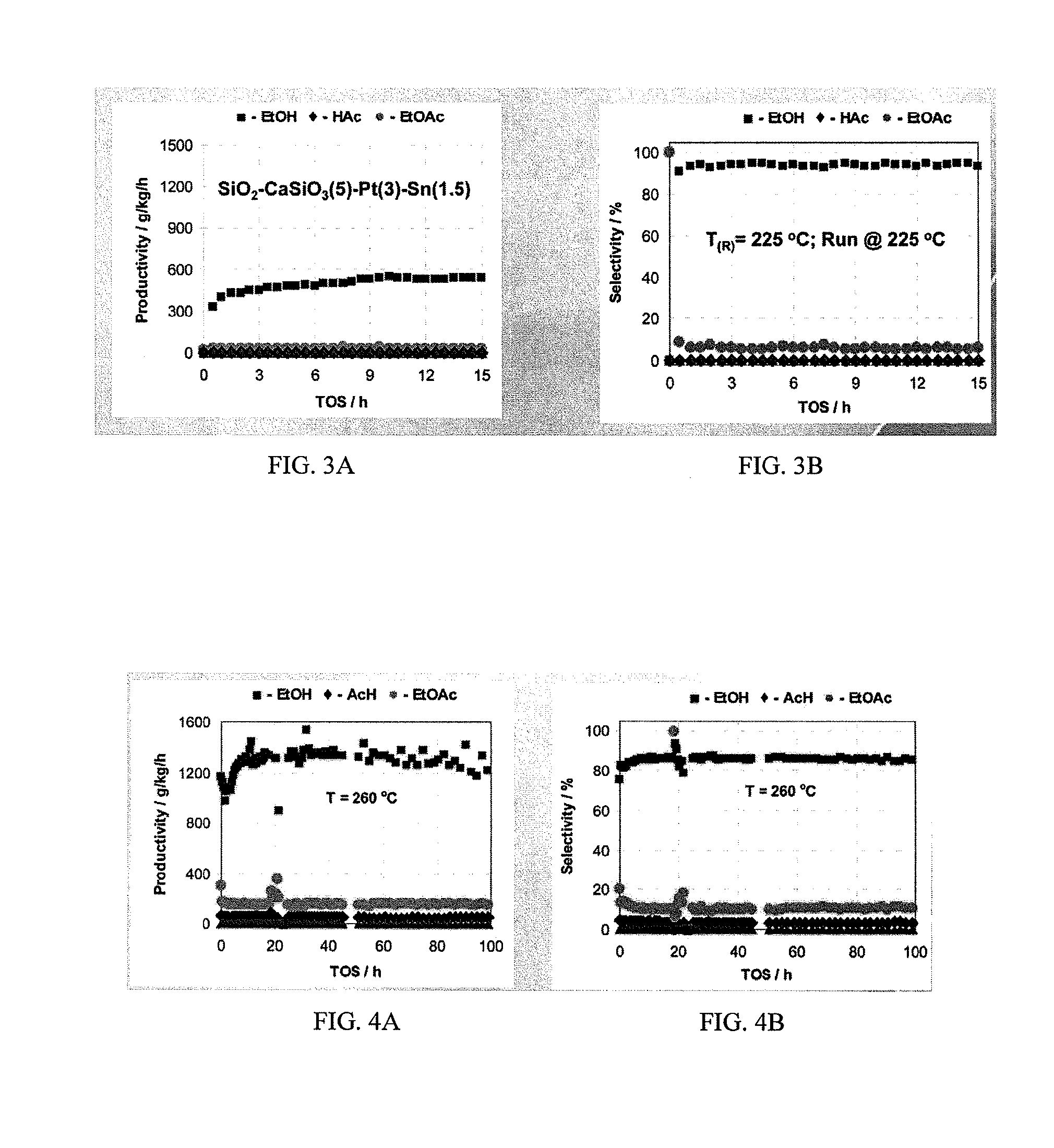
Catalysts for making ethanol from acetic acid
InactiveUS20100197485A1High selectivitySuitable for useMolecular sieve catalystsOrganic compound preparationTungstenIridium
Catalysts and processes for forming catalysts for use in hydrogenating acetic acid to form ethanol. In one embodiment, the catalyst comprises a first metal, a silicaceous support, and at least one metasilicate support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
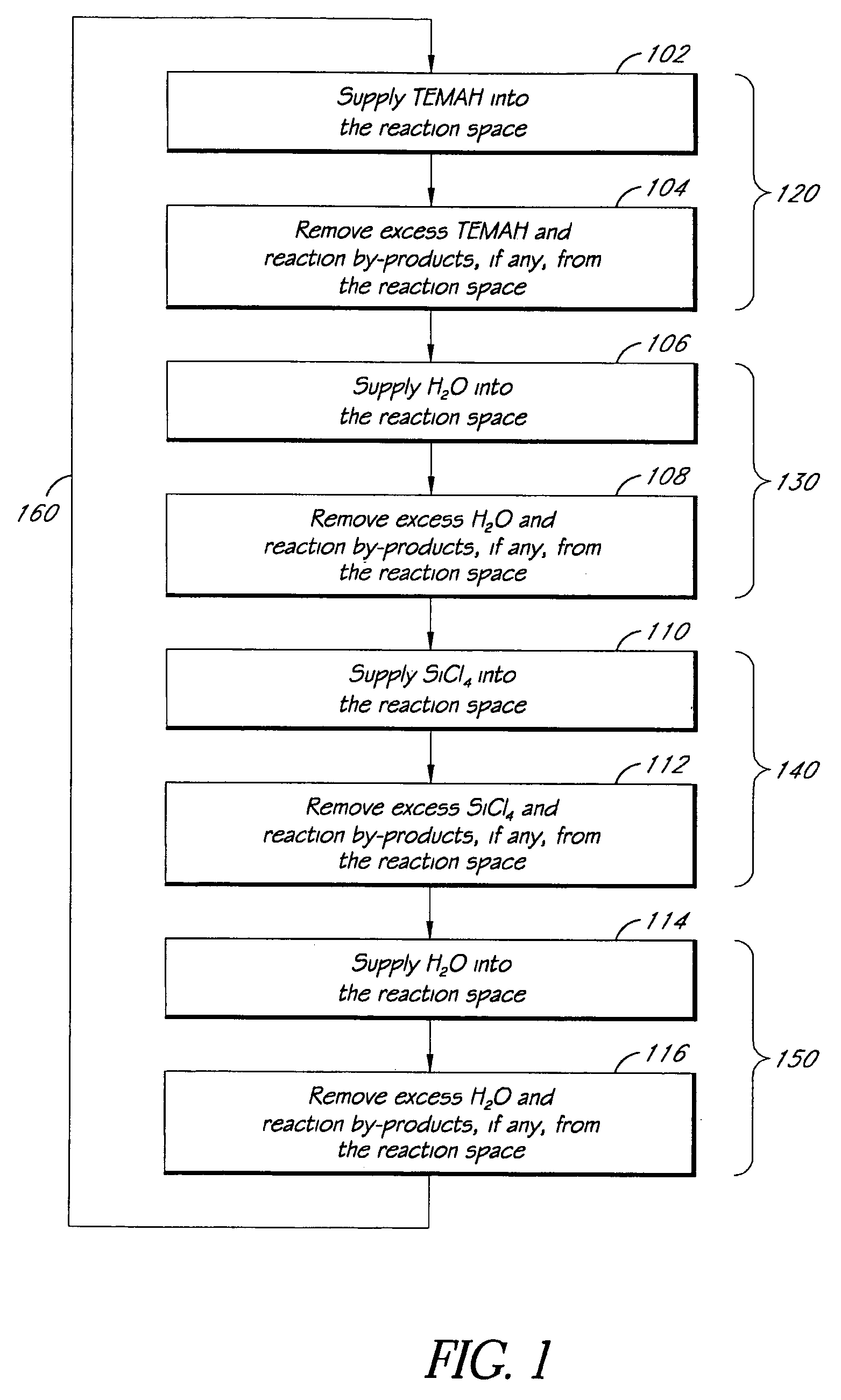
ALD of metal silicate films
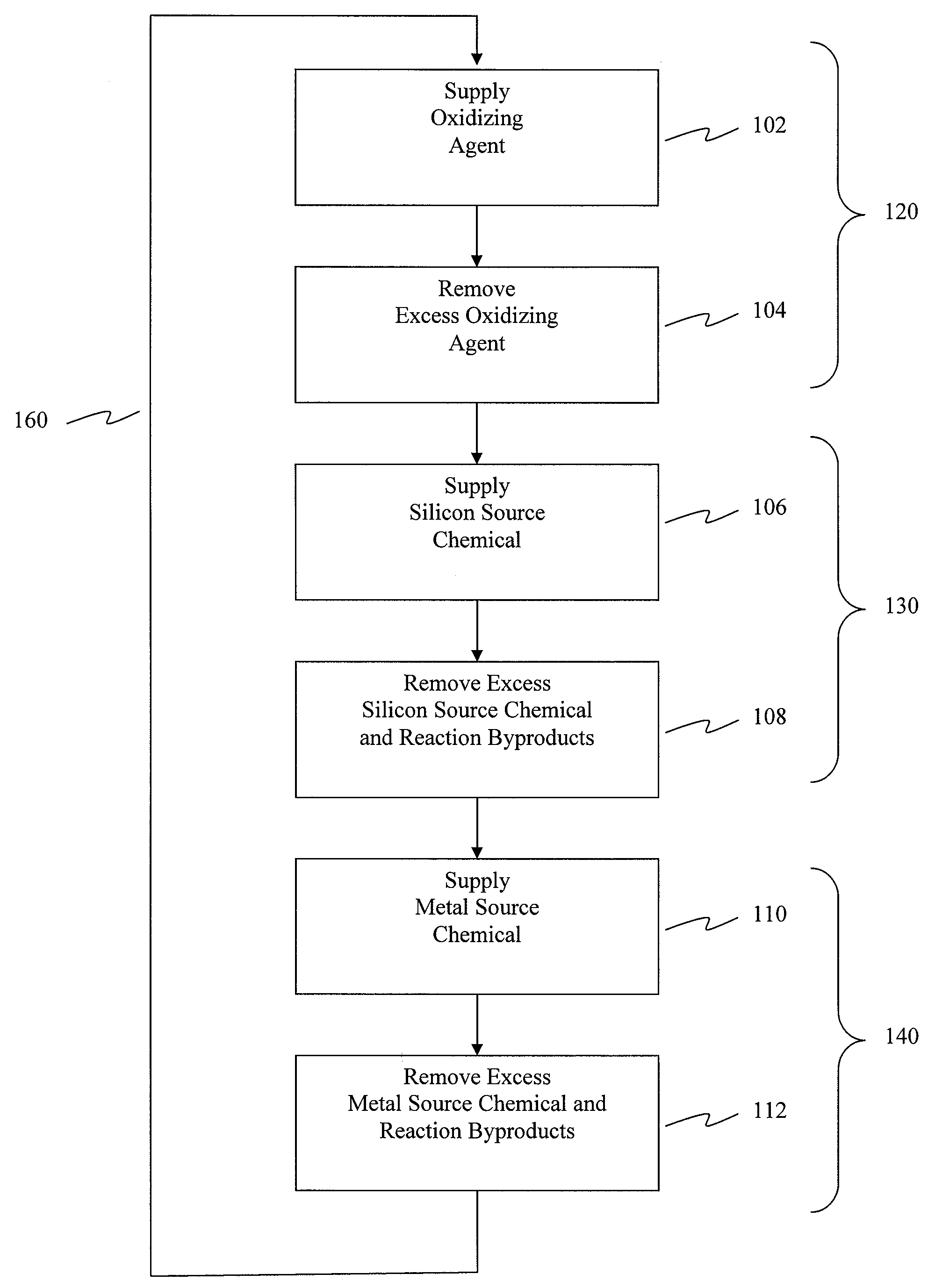
ActiveUS20080020593A1Readily apparentSemiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseHafnium
Methods for forming metal silicate films are provided. The methods comprise contacting a substrate with alternating and sequential vapor phase pulses of a metal source chemical, a silicon source chemical and an oxidizing agent. In preferred embodiments, an alkyl amide metal compound and a silicon halide compound are used. Methods according to preferred embodiments can be used to form hafnium silicate and zirconium silicate films with substantially uniform film coverages on substrate surfaces comprising high aspect ratio features (e.g., vias and / or trenches).
Owner:ASM IP HLDG BV
Implementing atomic layer deposition for gate dielectrics
PendingUS20170110313A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingGate dielectricLanthanum
A method for depositing a thin film onto a substrate is disclosed. In particular, the method forms a transitional metal silicate onto the substrate. The transitional metal silicate may comprise a lanthanum silicate or yttrium silicate, for example. The transitional metal silicate indicates reliability as well as good electrical characteristics for use in a gate dielectric material.
Owner:ASM IP HLDG BV
Recovery of hydrophobicity of low-k and ultra low-k organosilicate films used as inter metal dielectrics
InactiveUS20050106762A1Low costHigh mechanical strengthSolid-state devicesSemiconductor/solid-state device manufacturingChemical treatmentSilylation
Often used to reduce the RC delay in integrated circuits are dielectric films of porous organosilicates which have a silica like backbone with alkyl or aryl groups (to add hydrophobicity to the materials and create free volume) attached directly to the Si atoms in the network. Si—R bonds rarely survive an exposure to plasmas or chemical treatments commonly used in processing; this is especially the case in materials with an open cell pore structure. When Si—R bonds are broken, the materials lose hydrophobicity, due to formation of hydrophilic silanols and low dielectric constant is compromised. A method by which the hydrophobicity of the materials is recovered using a novel class of silylation agents which may have the general formula (R2N)XSiR′Y where X and Y are integers from 1 to 3 and 3 to 1 respectively, and where R and R′ are selected from the group of hydrogen, alkyl, aryl, allyl and a vinyl moiety. Mechanical strength of porous organosilicates is also improved as a result of the silylation treatment.
Owner:GLOBALFOUNDRIES INC
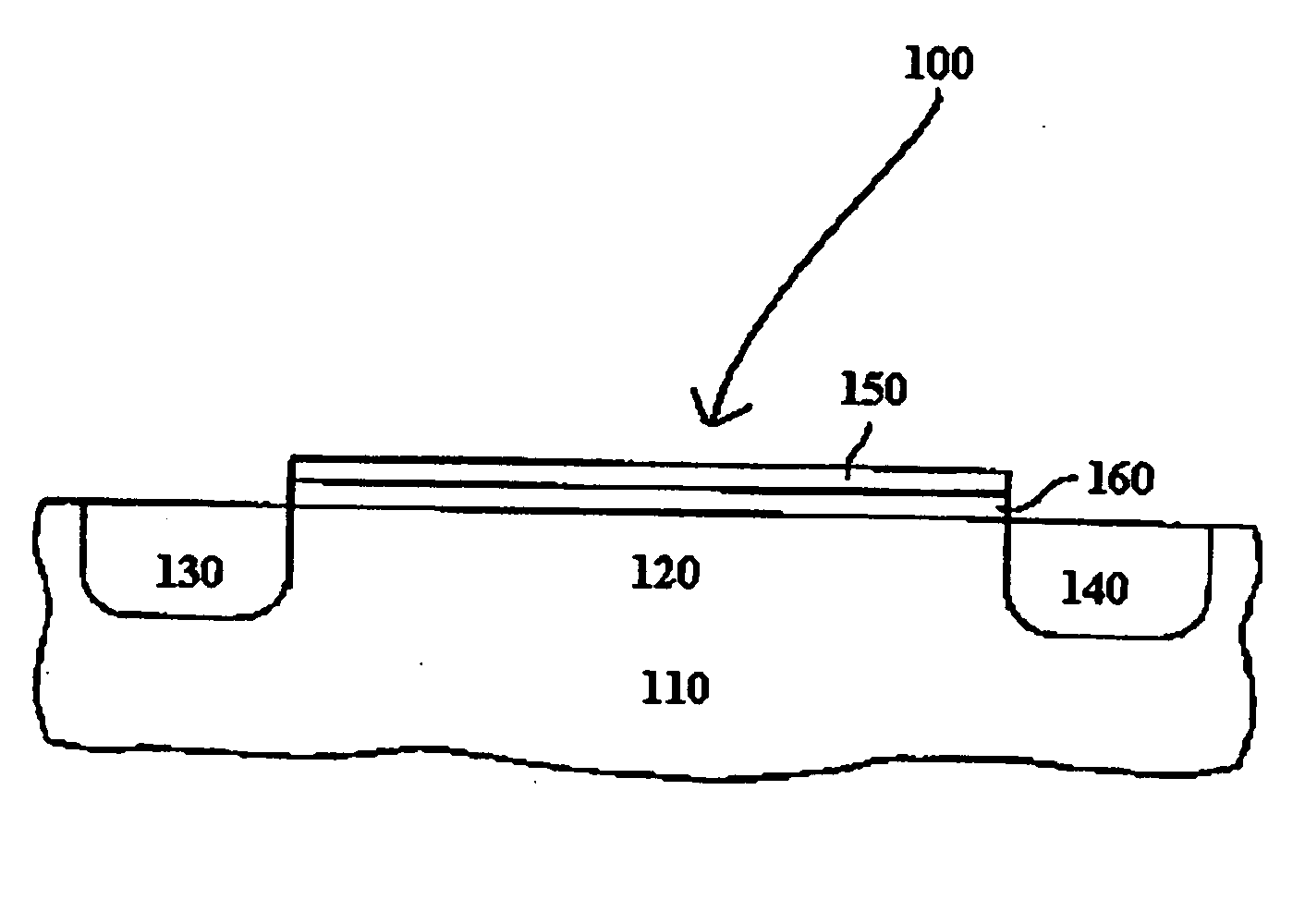
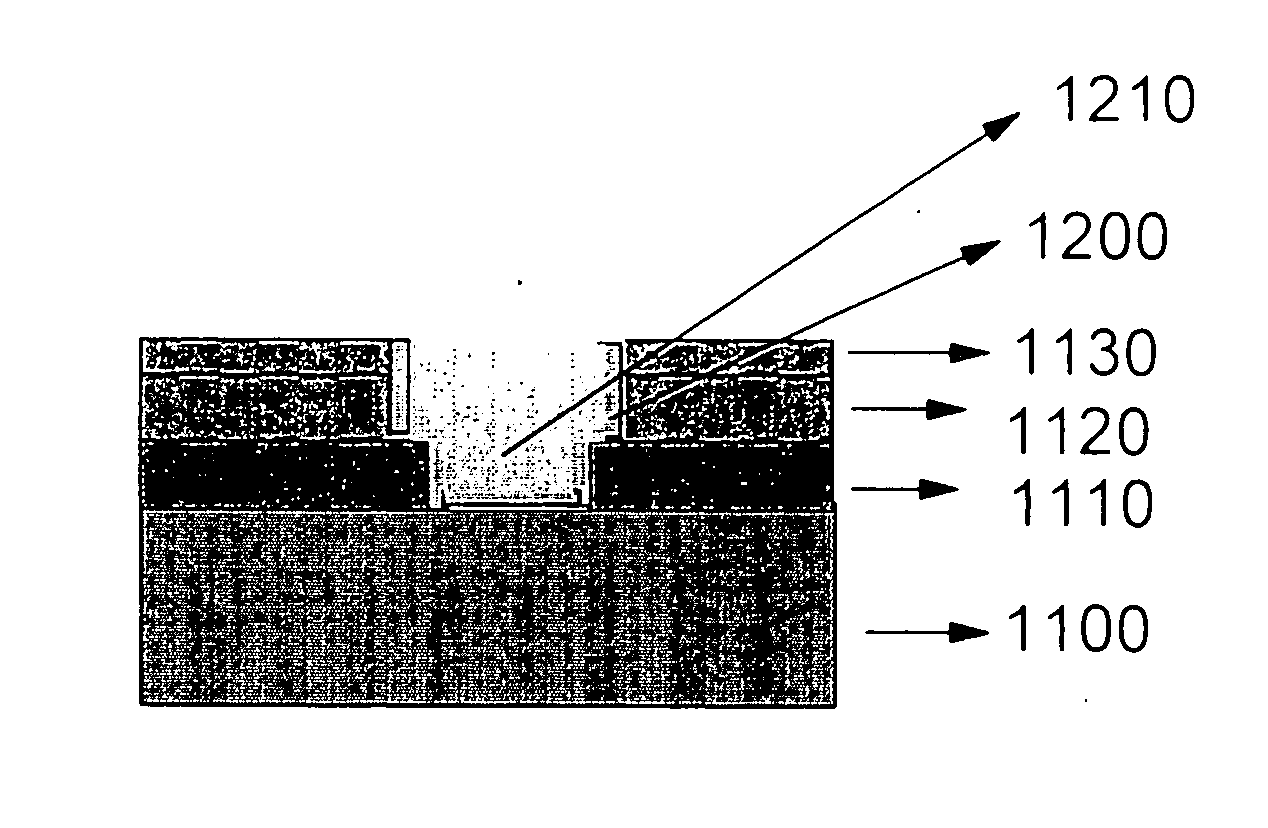
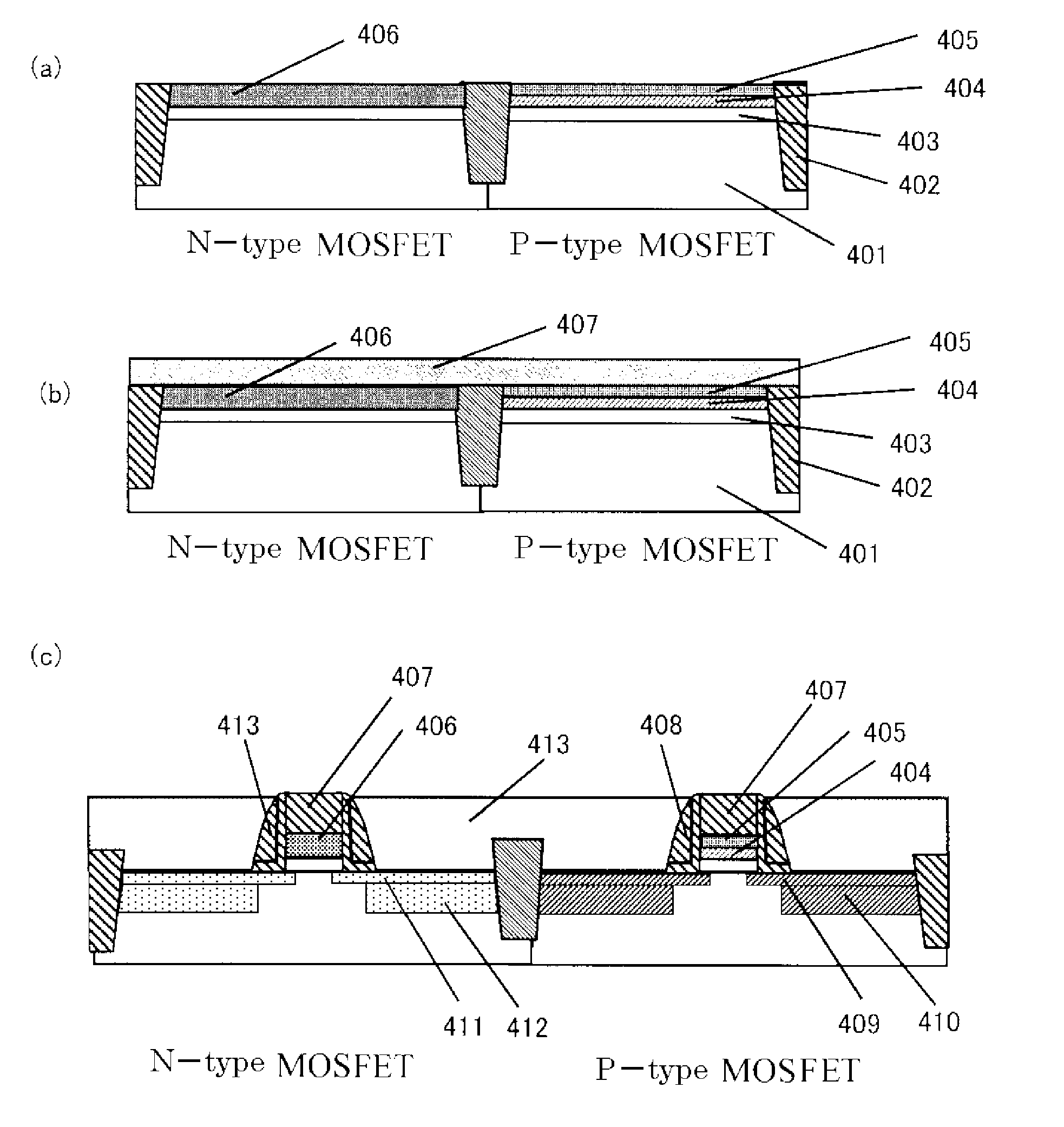
Dielectric layer for semiconductor device and method of manufacturing the same
InactiveUS20050151184A1TransistorSemiconductor/solid-state device manufacturingDevice materialMetal alloy
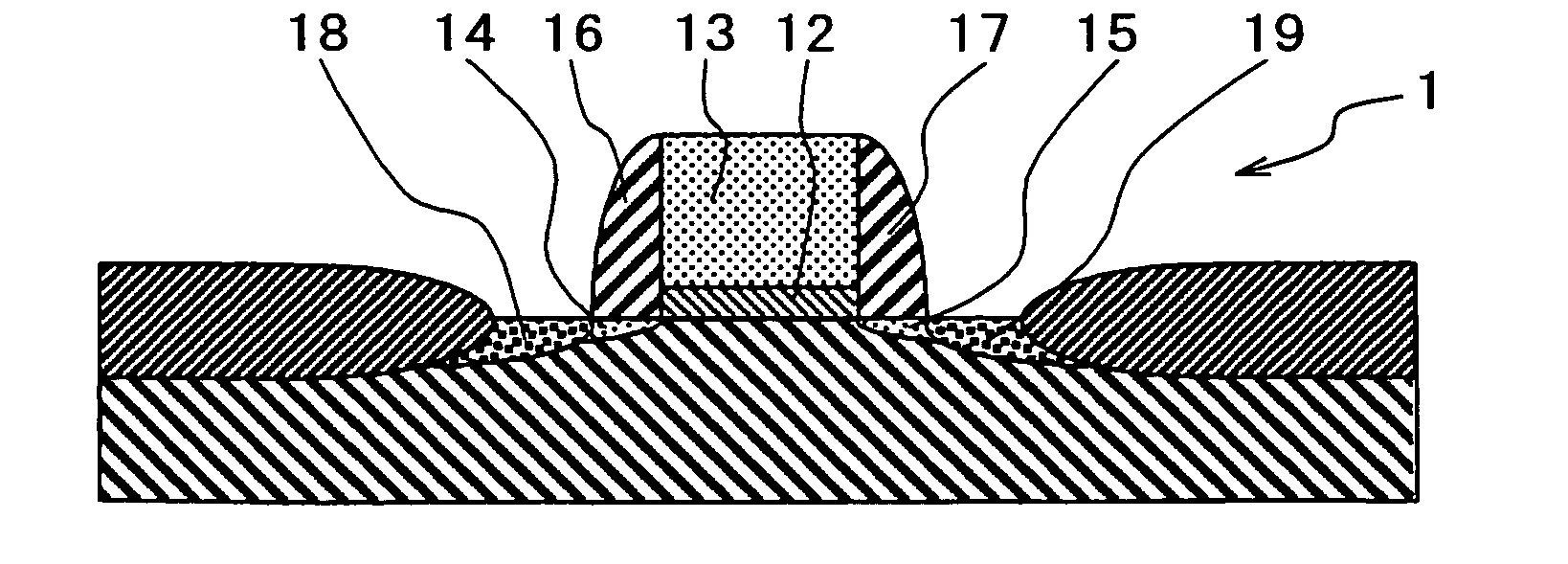
A semiconductor device comprises a silicate interface layer and a high-k dielectric layer overlying the silicate interface layer. The high-k dielectric layer comprises metal alloy oxides.
Owner:SAMSUNG ELECTRONICS CO LTD
ALD of metal silicate films
ActiveUS7795160B2Semiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseHafnium
Owner:ASM IP HLDG BV
Light source comprising a light-emitting element
InactiveUS6809347B2High luminous efficiencyHigh degreePlanar light sourcesPoint-like light sourceLuminophoreManganese
Owner:TOYODA GOSEI CO LTD
Ald of metal silicate films
ActiveUS20080085610A1Semiconductor/solid-state device manufacturingSilicon compoundsGas phaseHafnium
Methods for forming metal silicate films are provided. The methods comprise contacting a substrate with alternating and sequential vapor phase pulses of a silicon source chemical, metal source chemical, and an oxidizing agent, wherein the metal source chemical is the next reactant provided after the silicon source chemical. Methods according to some embodiments can be used to form silicon-rich hafnium silicate and zirconium silicate films with substantially uniform film coverages on substrate surface.
Owner:ASM IP HLDG BV
Zirconium oxide and hafnium oxide etching using halogen containing chemicals
ActiveUS20050164479A1Effective meanSemiconductor/solid-state device manufacturingSemiconductor devicesGate dielectricHafnium
A method is described for selectively etching a high k dielectric layer that is preferably a hafnium or zirconium oxide, silicate, nitride, or oxynitride with a selectivity of greater than 2:1 relative to silicon oxide, polysilicon, or silicon. The plasma etch chemistry is comprised of one or more halogen containing gases such as CF4, CHF3, CH2F2, CH3F, C4F8, C4F6, C5F6, BCl3, Br2, HF, HCl, HBr, HI, and NF3 and leaves no etch residues. An inert gas or an inert gas and oxidant gas may be added to the halogen containing gas. In one embodiment, a high k gate dielectric layer is removed on portions of an active area in a MOS transistor. Alternatively, the high k dielectric layer is used in a capacitor between two conducting layers and is selectively removed from portions of an ILD layer.
Owner:TAIWAN SEMICON MFG CO LTD
Semiconductor device and method of manufacturing the same
ActiveUS20120043617A1Work lessTransistorSemiconductor/solid-state device manufacturingDevice materialPermittivity
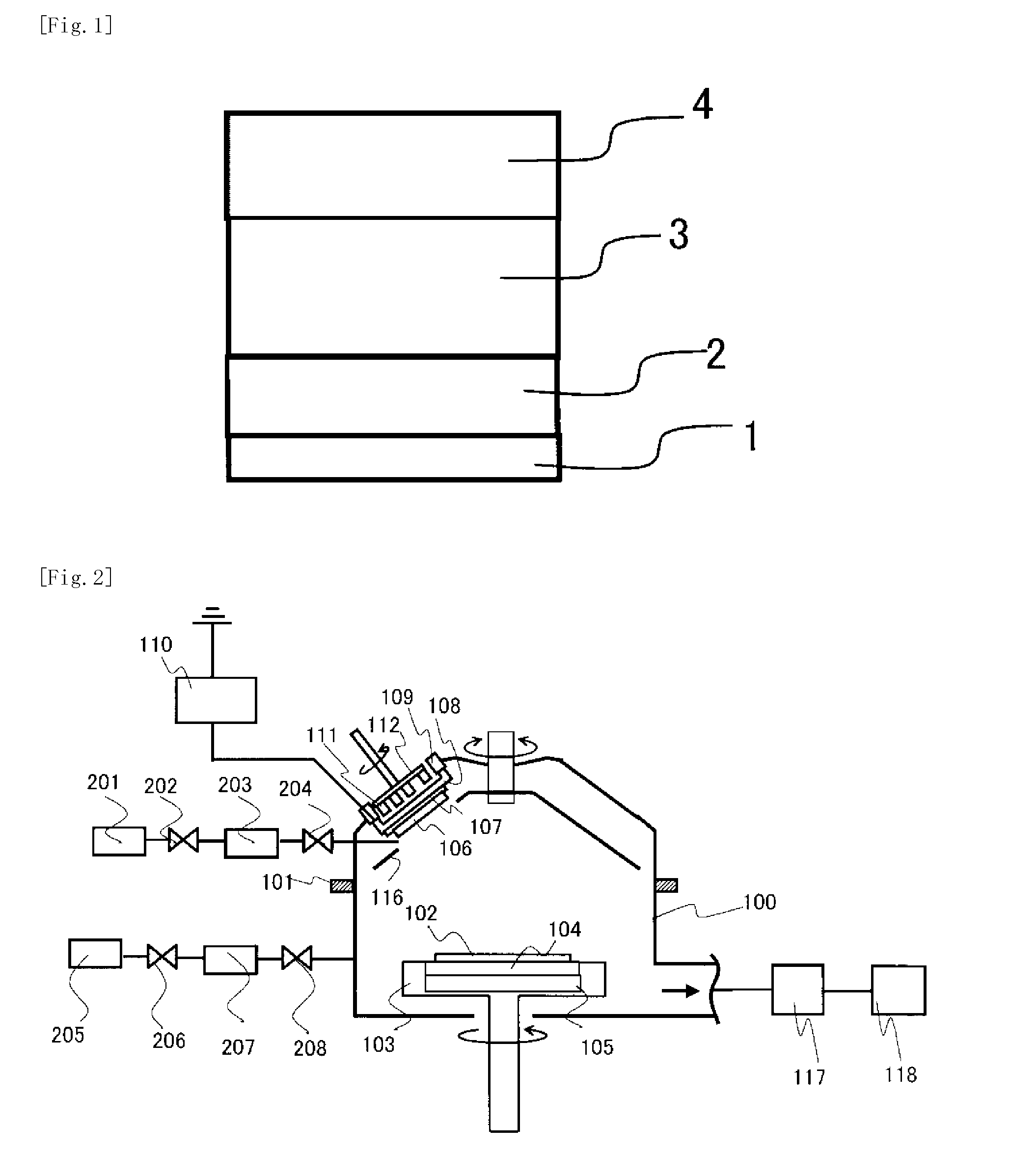
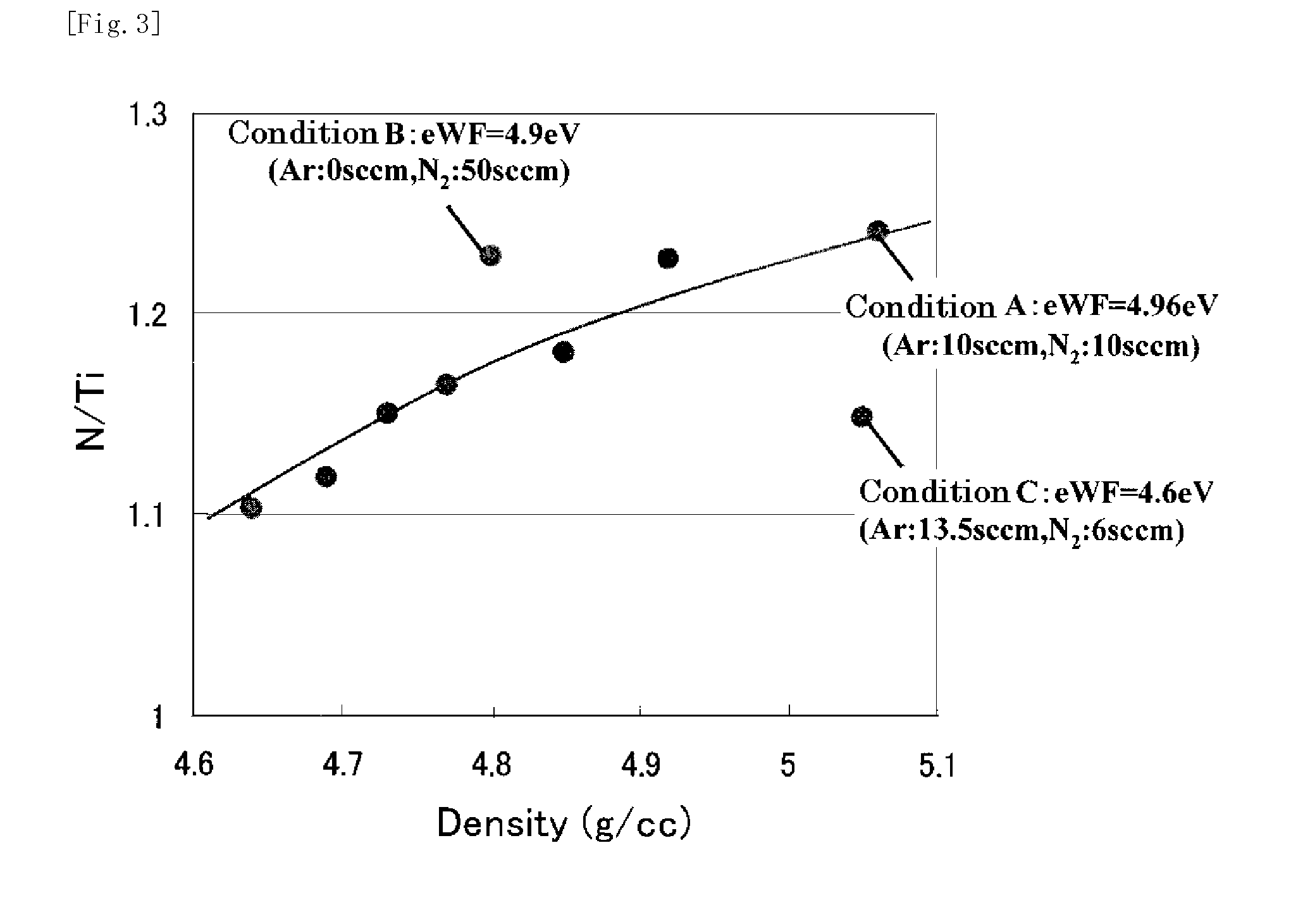
This invention provides a semiconductor device having a field effect transistor comprising agate electrode comprising a metal nitride layer and a polycrystalline silicon layer, and the gate electrode is excellent in thermal stability and realizes a desired work function.In the semiconductor device, a gate insulating film 6 on a silicon substrate 5 has a high-permittivity insulating film formed of a metal oxide, a metal silicate, a metal oxide introduced with nitrogen, or a metal silicate introduced with nitrogen,the gate electrode has a first metal nitride layer 7 provided on the gate insulating film 6 and containing Ti and N, a second metal nitride layer 8 containing Ti and N, and a polycrystalline silicon layer 9, in the first metal nitride layer 7, a molar ratio between Ti and N (N / Ti) is not less than 1.1, and a crystalline orientation X1 is 1.1<X1<1.8, andin the second metal nitride layer 8, the molar ratio between Ti and N (N / Ti) is not less than 1.1, and a crystalline orientation X2 is 1.8≦X2.
Owner:CANON ANELVA CORP
Ceramic material resistant to halogen plasma and member utilizing the same
InactiveUS6916559B2Improve the immunityRecord information storageLight beam reproducingPorosityHalogen
A member used within a plasma processing apparatus and exposed to a plasma of a halogen gas such as BCl3 or Cl2 is formed from a sintered body of metals of Group IIIa of Periodic Table such as Y, La, Ce, Nd and Dy, and Al and / or Si, for example, 3Y2O3.5Al2O3, 2Y2O3.Al2O3, Y2O3.Al2O3 or disilicate or monosilicate, and in particular, in this sintered body, the content of impurity metals of Group IIa of Periodic Table contained in the sintered body is controlled to be 0.15 wt % or more in total. Specifically, for this member, an yttrium-aluminum-garnet sintered body having a porosity of 3% or less and also having a surface roughness of 1 μm or less in center line average roughness Ra is utilized.
Owner:KYOCERA CORP
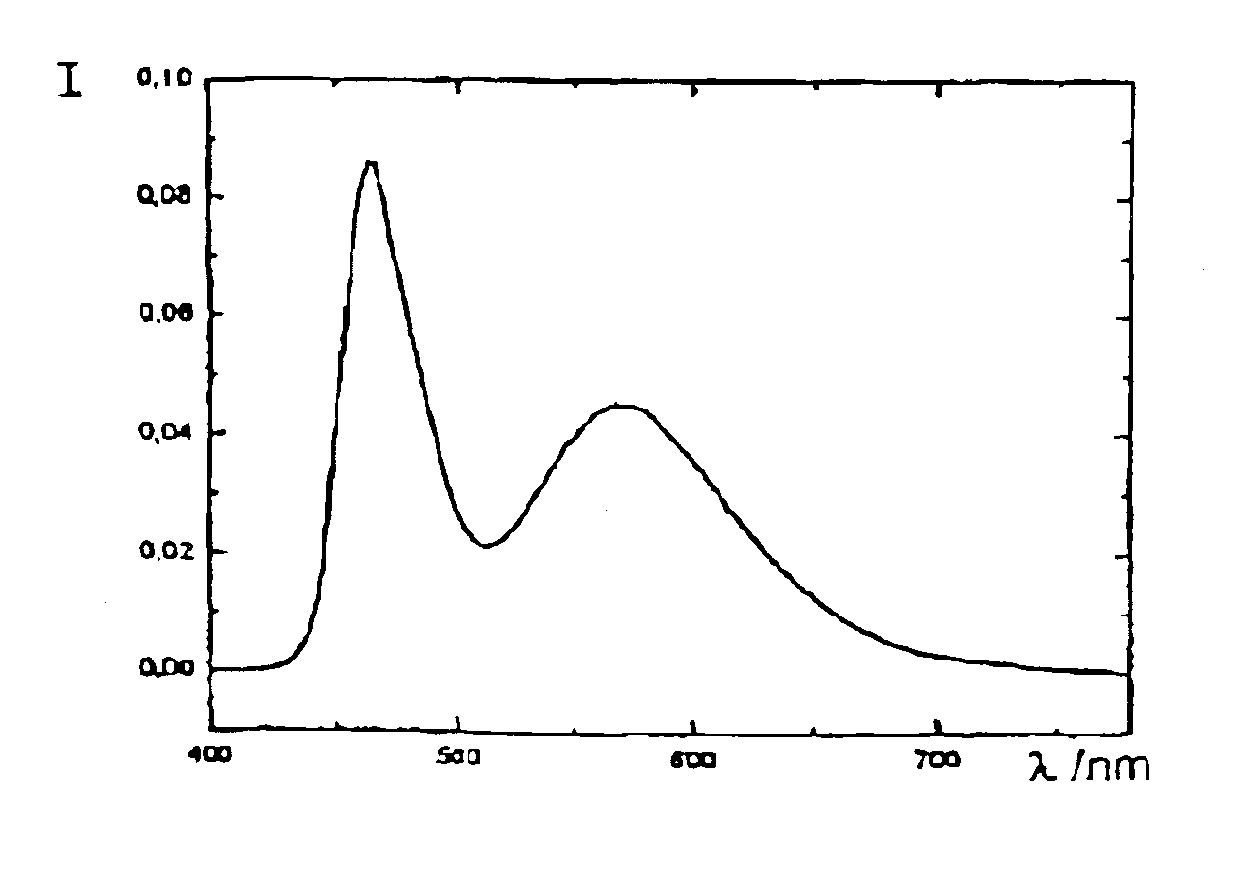
Light-emitting semiconductor device, light-emitting system and method for fabricating light-emitting semiconductor device
InactiveUS7023019B2Suppress color unevennessLittle color unevennessDischarge tube luminescnet screensPoint-like light sourceFluorescenceDevice material
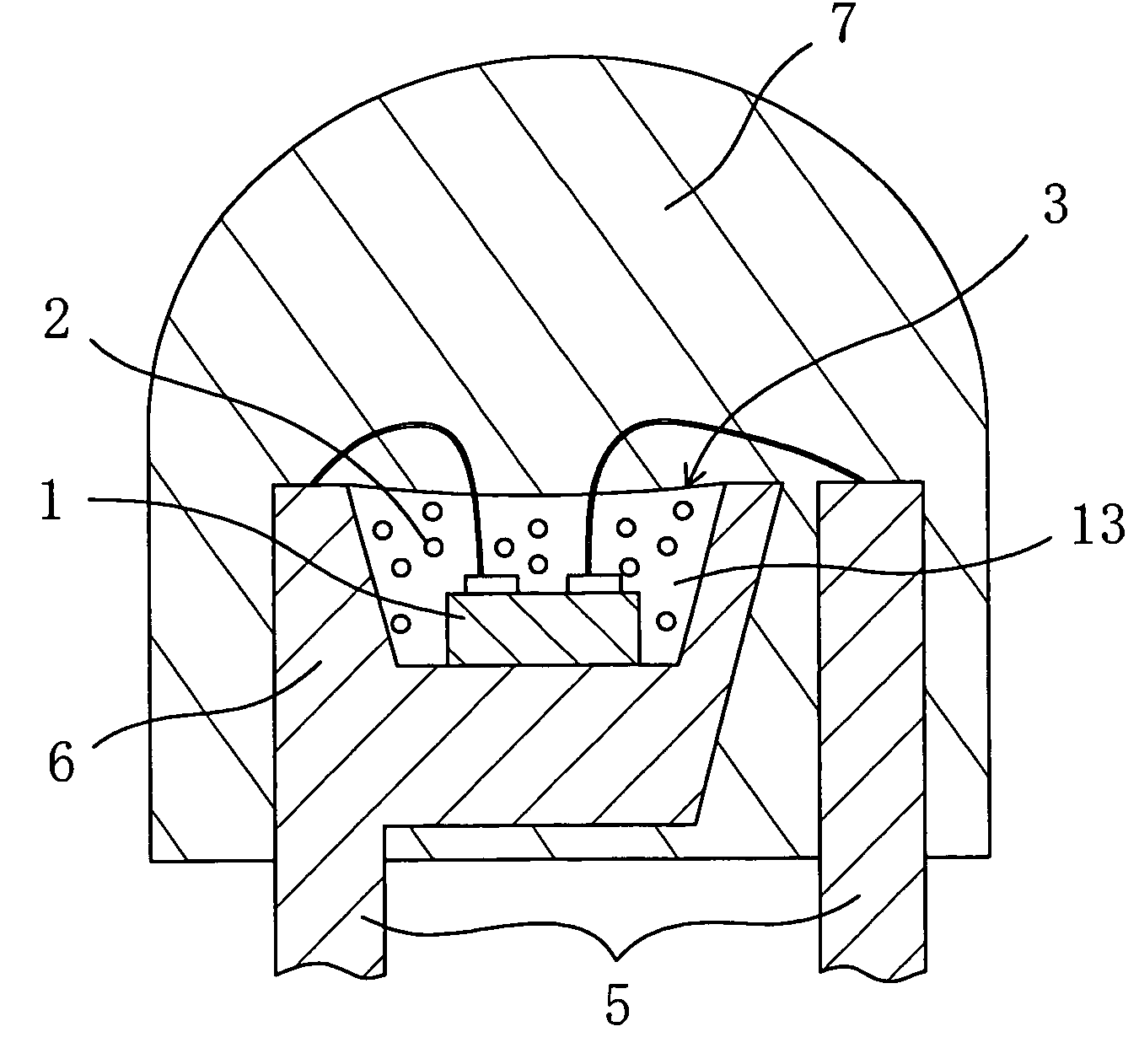
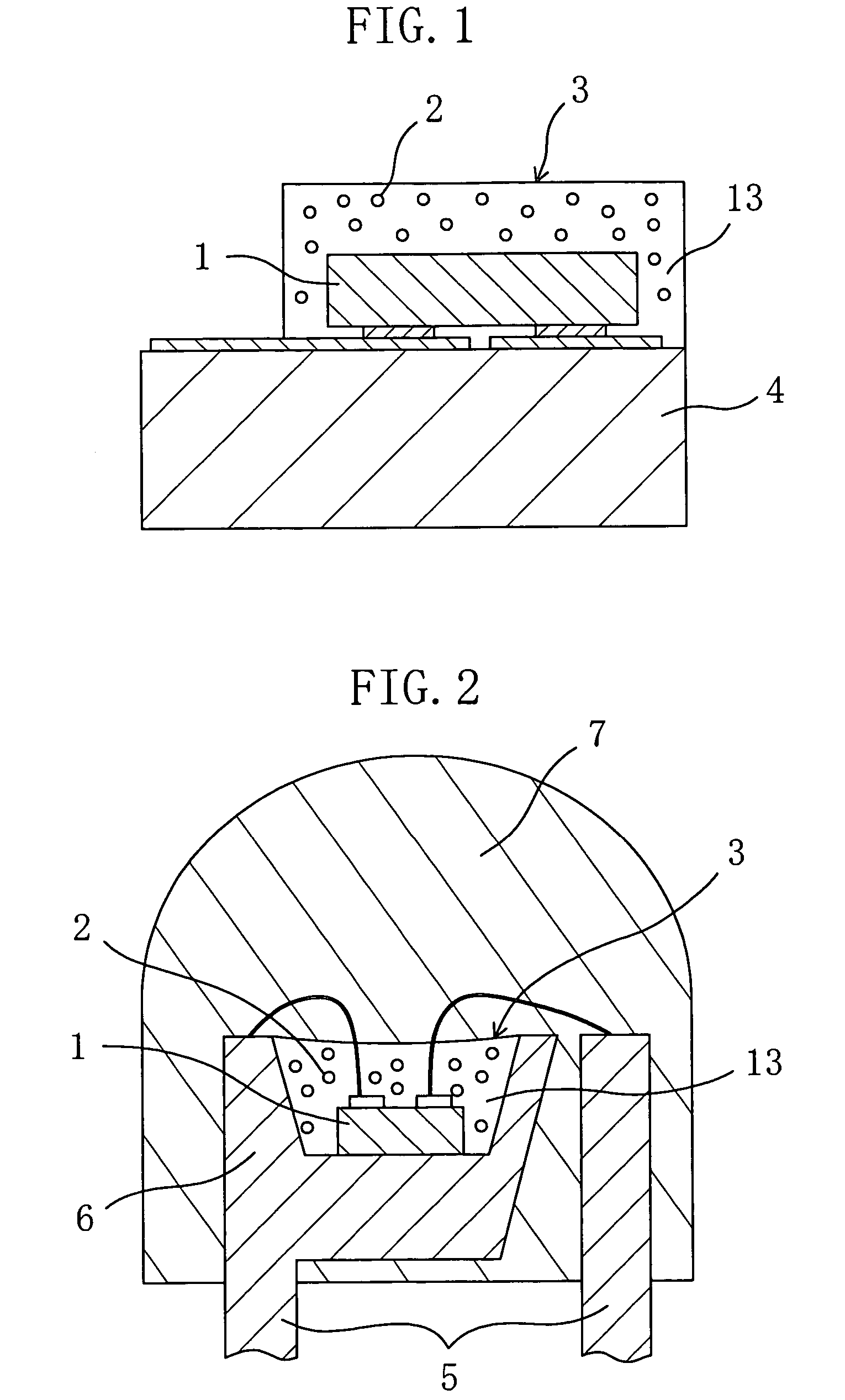
A chip-type light-emitting semiconductor device includes: a substrate 4 ; a blue LED 1 mounted on the substrate 4 ; and a luminescent layer 3 made of a mixture of yellow / yellowish phosphor particles 2 and a base material 13 (translucent resin). The yellow / yellowish phosphor particles 2 is a silicate phosphor which absorbs blue light emitted by the blue LED 1 to emit a fluorescence having a main emission peak in the wavelength range from 550 nm to 600 nm, inclusive, and which contains, as a main component, a compound expressed by the chemical formula: (Sr1-a1-b1-xBaa1Cab1Eux)2SiO4 (0<=a1<=0.3, 0<=b1<=0.8 and 0<x<1). The silicate phosphor particles disperse substantially evenly in the resin easily. As a result, excellent white light is obtained.
Owner:PANASONIC CORP
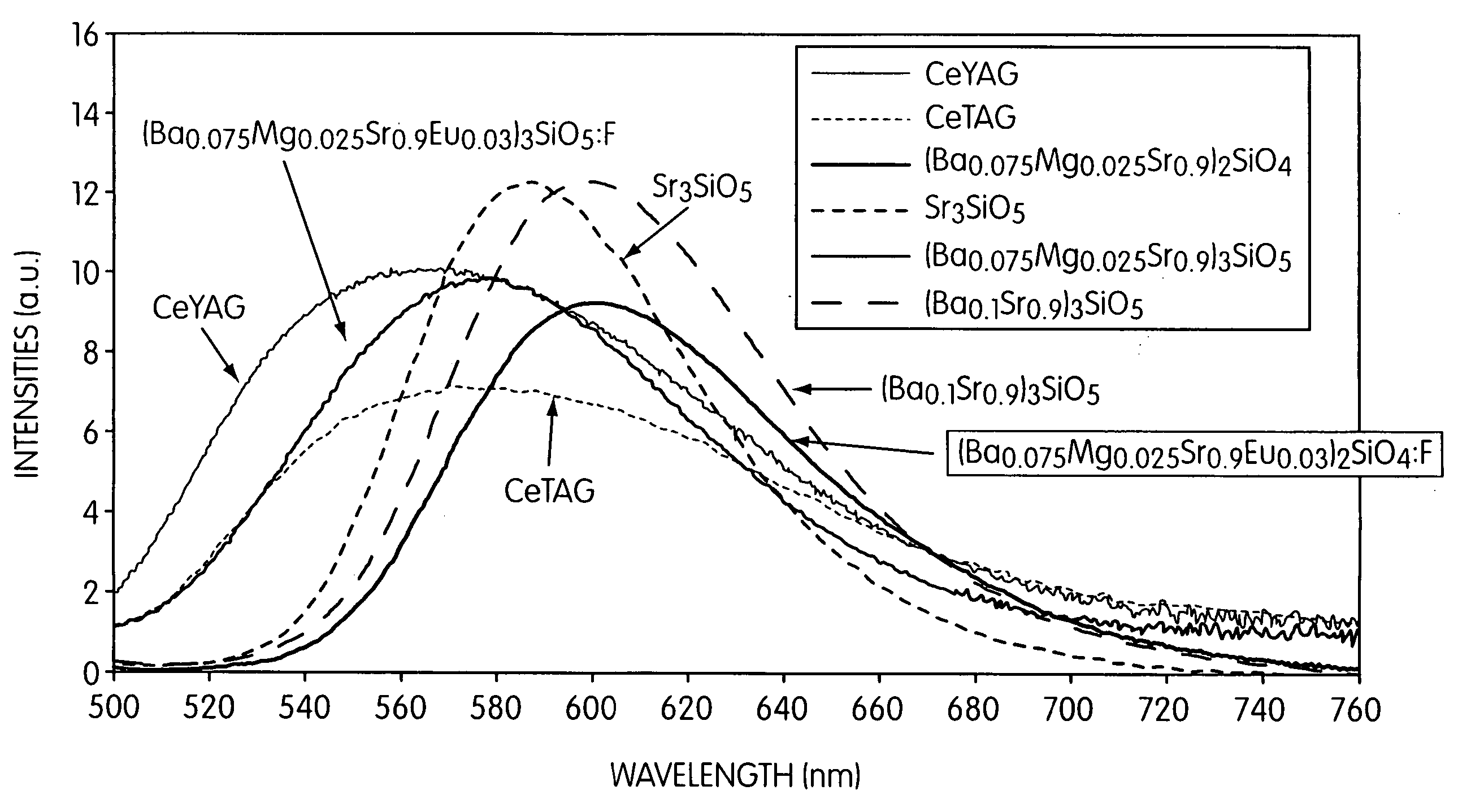
Silicate-based orange phosphors
ActiveUS20070029526A1Discharge tube luminescnet screensDischarge tube solid anodesPhysical chemistryPlasma display
Novel orange phosphors are disclosed having the comprise silicate-based compounds having the formula (Sr,A1)x,(Si,A2)(O,A3)2+x:Eu2+, where A1 is at least one divalent cation (a 2+ ion) including Mg, Ca, Ba, or Zn, or a combination of 1+ and 3+ cations; A2 is a 3+, 4+, or 5+ cation, including at least one of B, Al, Ga, C, Ge, P; A3 is a 1−, 2−, or 3− anion, including F, Cl, and Br; and x is any value between 2.5 and 3.5, inclusive. The formula is written to indicate that the A1 cation replaces Sr; the A2 cation replaces Si, and the A3 anion replaces O. These orange phosphors are configured to emit visible light having a peak emission wavelength greater than about 565 nm. They have applications in white LED illumination systems, plasma display panels, and in orange and other colored LED systems.
Owner:INTEMATIX
Stable solid block metal protecting warewashing detergent composition
InactiveUS6156715AFit tightlyEasy to cleanInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAlkalinityIndustrial setting
The dimensionally stable alkaline solid block warewashing detergent uses an E-form binder forming a solid comprising a sodium carbonate source of alkalinity, a metal corrosion protecting alkali metal silicate composition, a sequestrant, a surfactant package and other optional material. The solid block is dimensionally stable and highly effective in removing soil from the surfaces of dishware in the institutional and industrial environment. The E-form hydrate comprises an organic phosphonate and a hydrated carbonate.
Owner:ECOLAB USA INC
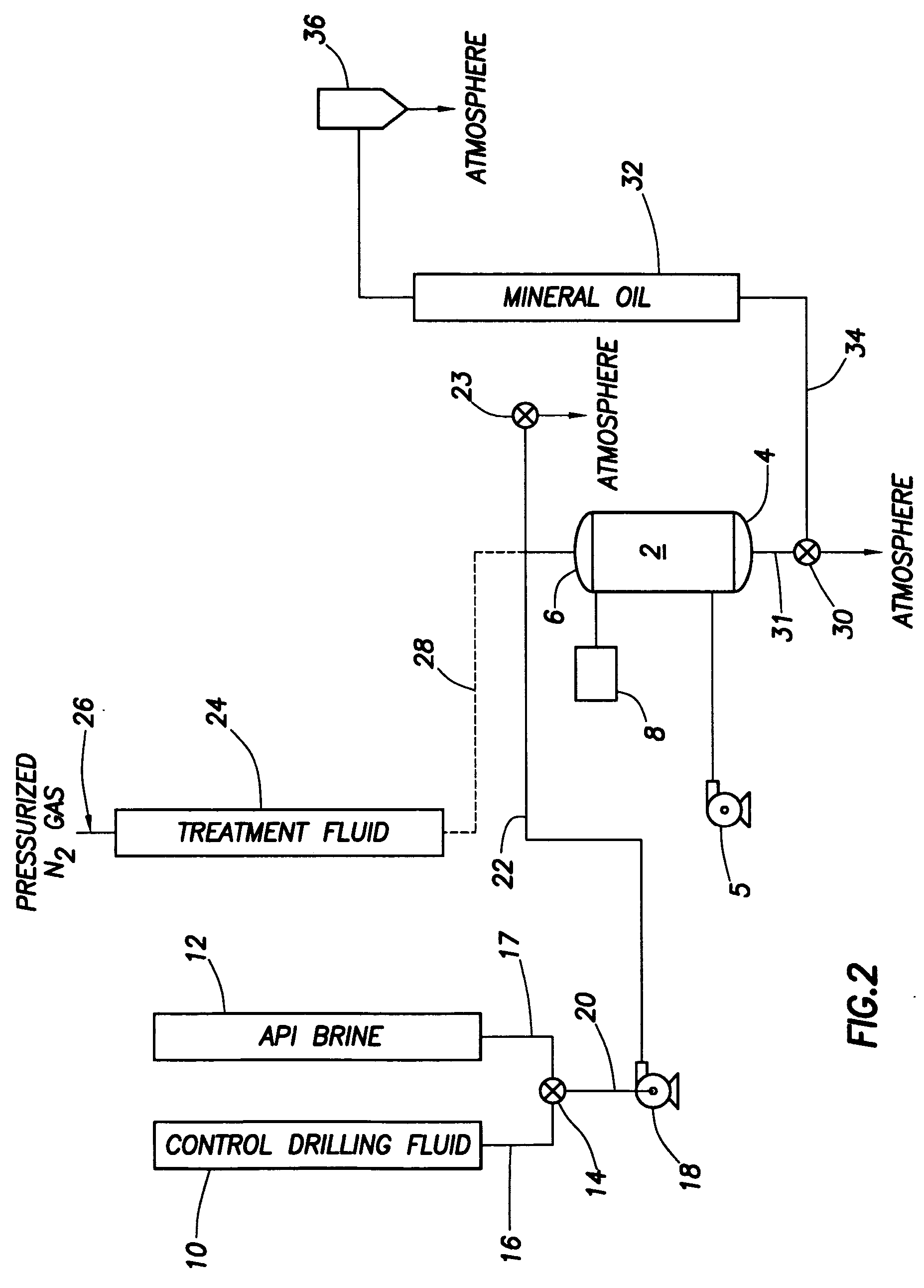
Oilwell sealant compositions comprising alkali swellable latex
Sealant compositions comprising an alkali swellable latex and a pH increasing material and methods of using the same to service a wellbore are provided. In one embodiment, the sealant composition can be used in a wellbore and includes an alkali swellable latex and a pH increasing material. The sealant composition can have a pH of from about 7 to about 14. In other embodiments, the pH increasing material includes a base-producing material. The base-producing material can include alkali and alkali earth metal carbonates, alkali and alkali earth metal bicarbonates, alkali and alkali earth metal hydroxides, alkali and alkali earth metal oxides, alkali and alkali earth metal phosphates, alkali and alkali earth metal hydrogen phosphates, alkali and alkaline earth metal sulphides, alkali and alkaline earth metal salts of silicates, alkali and alkaline earth metal salts of aluminates, water soluble or water dispersible organic amines, polymeric amine, amino alcohols, or combinations thereof.
Owner:HALLIBURTON ENERGY SERVICES INC
Methods And Apparatus For Forming Tantalum Silicate Layers On Germanium Or III-V Semiconductor Devices
InactiveUS20140065842A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingSilicon oxideAtomic layer deposition
Described are apparatus and methods for forming tantalum silicate layers on germanium or III-V materials. Such tantalum silicate layers may have Si / (Ta+Si) atomic ratios from about 0.01 to about 0.15. The tantalum silicate layers may be formed by atomic layer deposition of silicon oxide and tantalum oxide, followed by interdiffusion of the silicon oxide and tantalum oxide layers.
Owner:APPLIED MATERIALS INC
Tunable dielectric compositions including low loss glass
InactiveUS6905989B2Lower sintering temperatureIncrease varietyFixed capacitor dielectricCeramic layered productsBreakdown strengthStrontium titanate
Tunable dielectric materials including an electronically tunable dielectric ceramic and a low loss glass additive are disclosed. The tunable dielectric may comprise a ferroelectric perskovite material such as barium strontium titanate. The glass additive may comprise boron, barium, calcium, lithium, manganese, silicon, zinc and / or aluminum-containing glasses having dielectric losses of less than 0.003 at 2 GHz. The materials may further include other additives such as non-tunable metal oxides and silicates. The low loss glass additive enables the materials to be sintered at relatively low temperatures while providing improved properties such as low microwave losses and high breakdown strengths.
Owner:NXP USA INC
Process for sequestering carbon dioxide and sulfur dioxide
A process for sequestering carbon dioxide, which includes reacting a silicate based material with an acid to form a suspension, and combining the suspension with carbon dioxide to create active carbonation of the silicate-based material, and thereafter producing a metal salt, silica and regenerating the acid in the liquid phase of the suspension.
Owner:PENN STATE RES FOUND
Masonry cement having higher strength and water retention
InactiveCN101182139AHigh strengthSolve the difficult problem of preparing high-strength grade building mortarSolid waste managementCelluloseLower grade
The invention discloses a masonry cement with high strength and water retention. According to the weight percentage, the masonry cement consists of 21-98.99 percent of porland cement clinker, 0-50 percent of blending materials, 1-12 percent of gypsum, 0.01-2 percent of cellulose ether and 0-15 percent of other additives. With the cellulose ether of high water retention material added, the water retention of the masonry cement is greatly improved. At the same time, through breaking through the limitation that the using amount of the blending materials in the original masonry cement is not less than 50 percent, the using amount of the blending materials is lowered to be 50 percent below to improve the strength of novel masonry cement. The strength of the prepared masonry cement is high, and the masonry cement with the strength grades of 12.5, 22.5, 52.5R, 62.5, 62.5R, etc. can be made. The hard problems of using low-grade blending materials to produce masonry cement with high water retention and using low- grade strength masonry cement of 12.5 and 22.5 to produce high-grade strength building mortar are solved.
Owner:SOUTH CHINA UNIV OF TECH
Microbial pesticidal composition
Owner:KUMIAI CHEM IND CO LTD
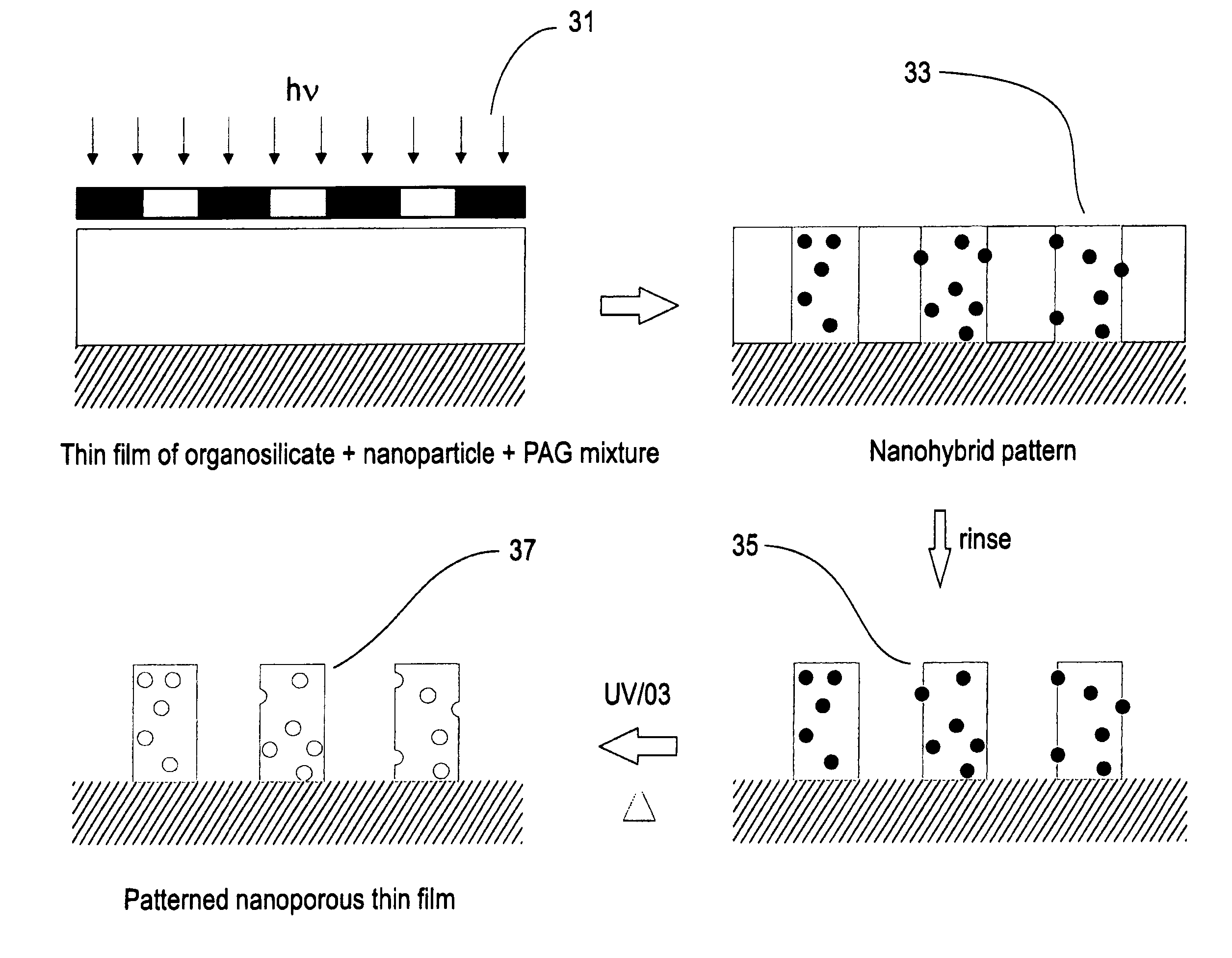
Direct photo-patterning of nanoporous organosilicates, and method of use
InactiveUS7056840B2Simple methodOrganic-compounds/hydrides/coordination-complexes catalystsSemiconductor/solid-state device manufacturingElectromagnetic radiationDielectric permittivity
A low dielectric constant, patterned, nanoporous material and a method of forming the material. The material is formed by depositing a layer onto a substrate, said layer comprising a reactive organosilicate material, a porogen, an initiator, and a solvent; exposing portions of the layer to energy (e.g., thermal energy or electromagnetic radiation) to change the solubility of portions of the organosilicate material with respect to the solvent; selectively removing more soluble portions of the layer to generate a relief pattern; and decomposing the porogen to thereby generate a nanoporous organosilicate layer.
Owner:INT BUSINESS MASCH CORP
Reclamation of a Titanosilicate, and Reconstitution of an Active Oxidation Catalyst
InactiveUS20080064591A1Acceptable product selectivityAcceptable selectivityMolecular sieve catalystsOther chemical processesCatalytic metalTitanium
A method of reclaiming a titanosilicate from a deactivated or spent oxidation catalyst containing a titanosilicate having deposited thereon one or more catalytic metals, such as gold, and optionally, one or more promoter metals, the method involving treating the deactivated catalyst with an oxidant; contacting the oxidant-treated catalyst with acid, preferably aqua regia; washing the titanosilicate to remove residual acid; and optionally drying and / or calcining. A method of reconstituting an active oxidation catalyst from a spent or deactivated oxidation catalyst, the method involving reclaiming the titanosilicate as noted above, and then depositing one or more catalytic metals and, optionally, one or more promoter metals onto the reclaimed titanosilicate.
Owner:DOW GLOBAL TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com