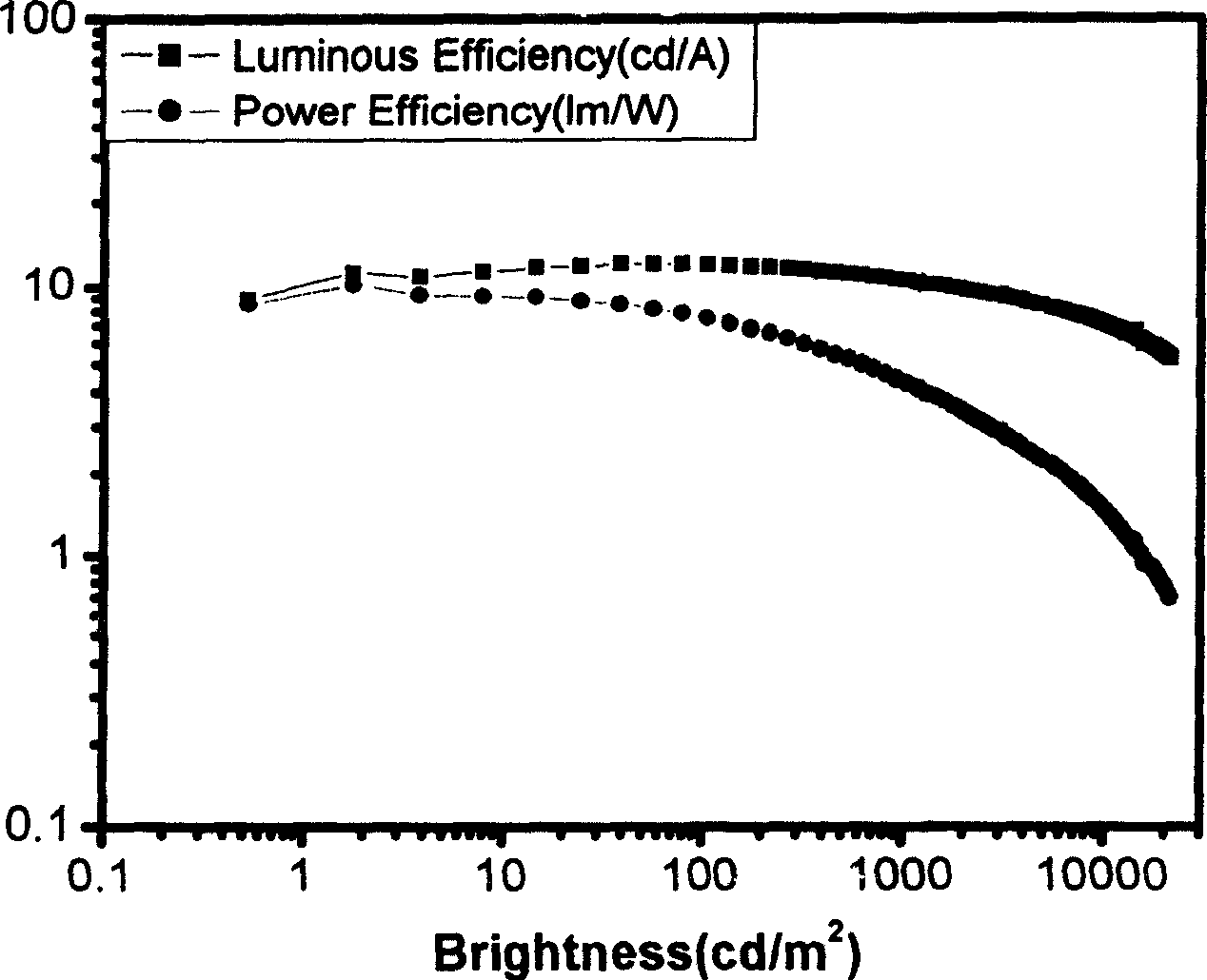
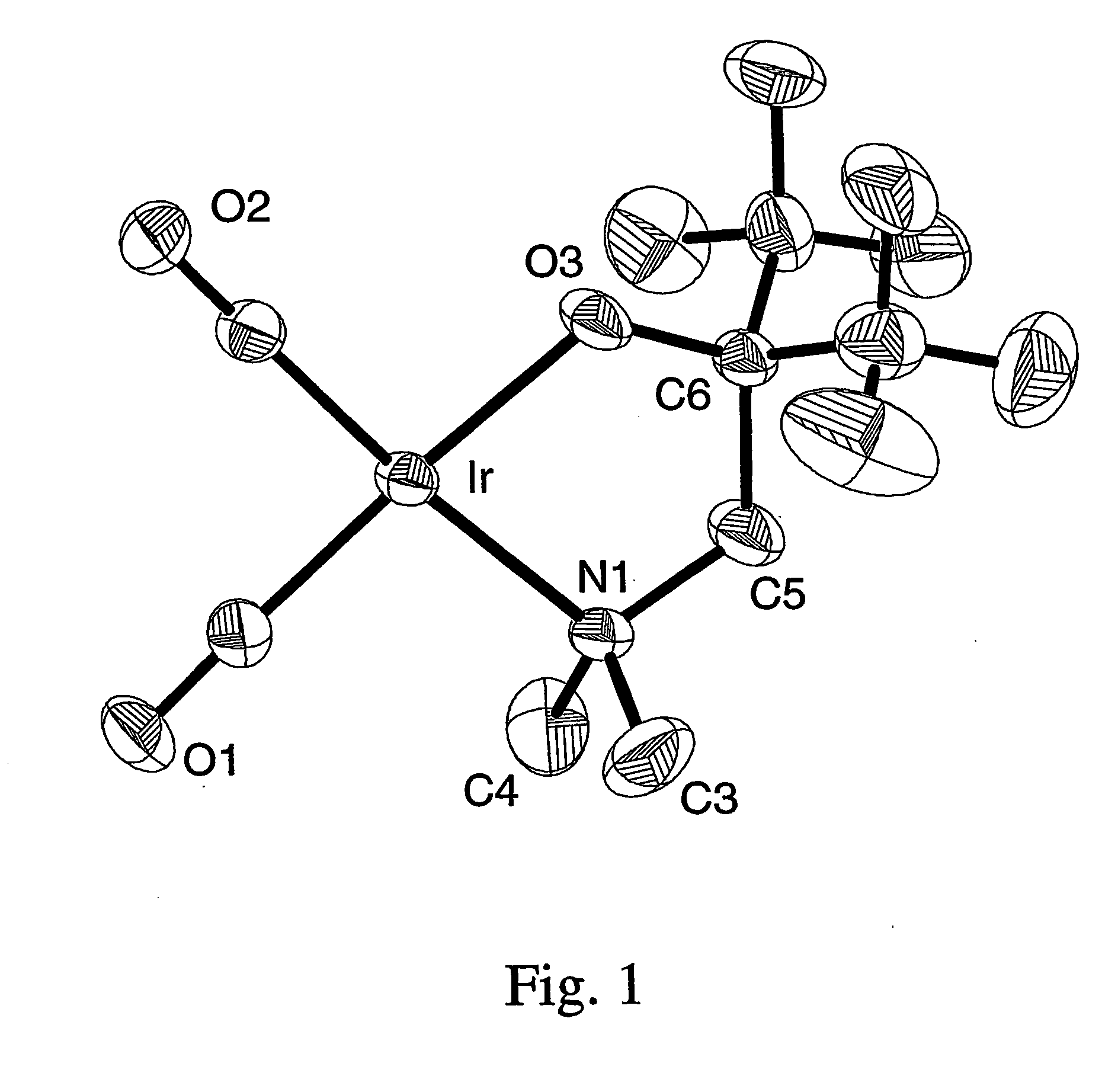
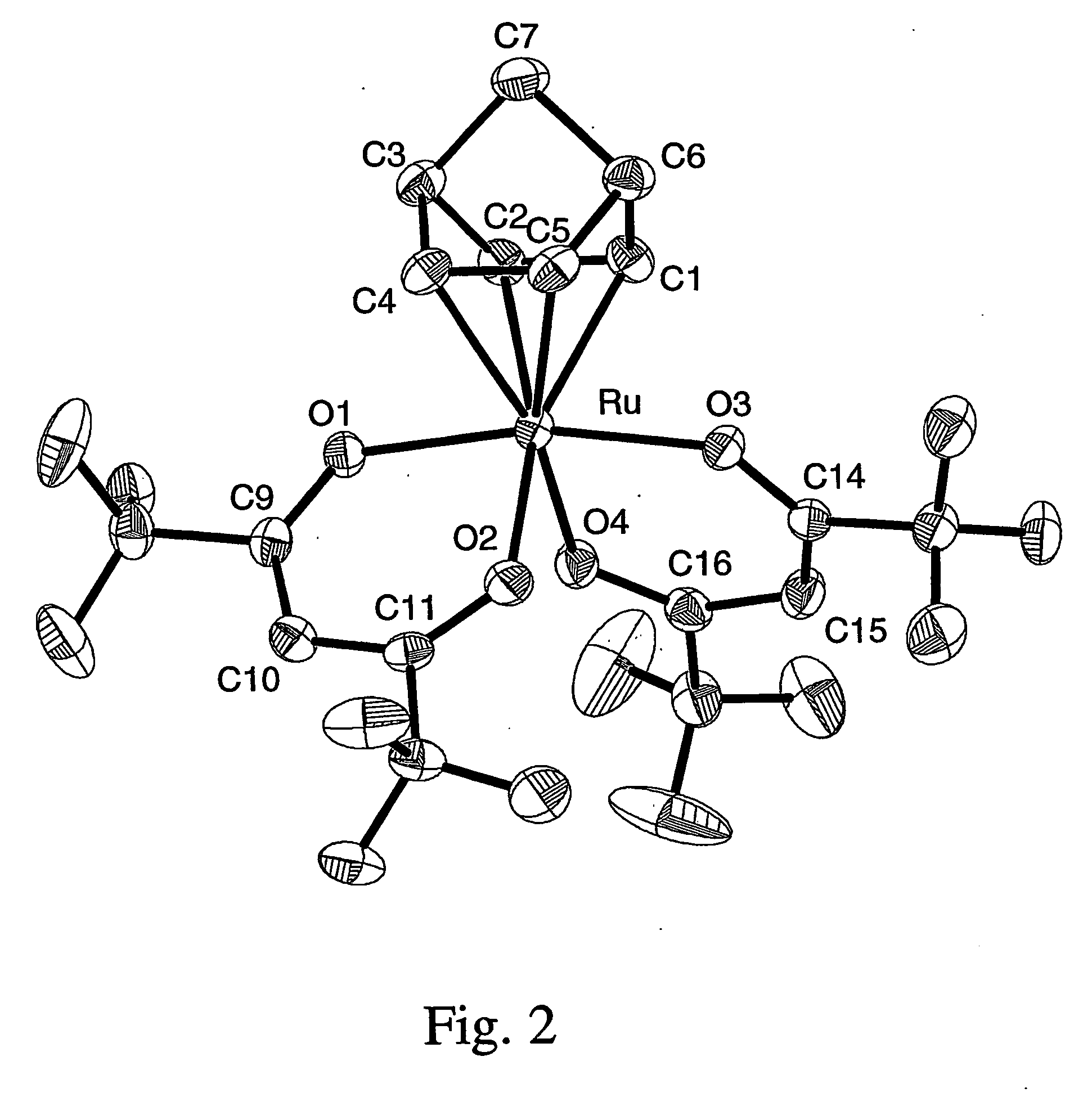
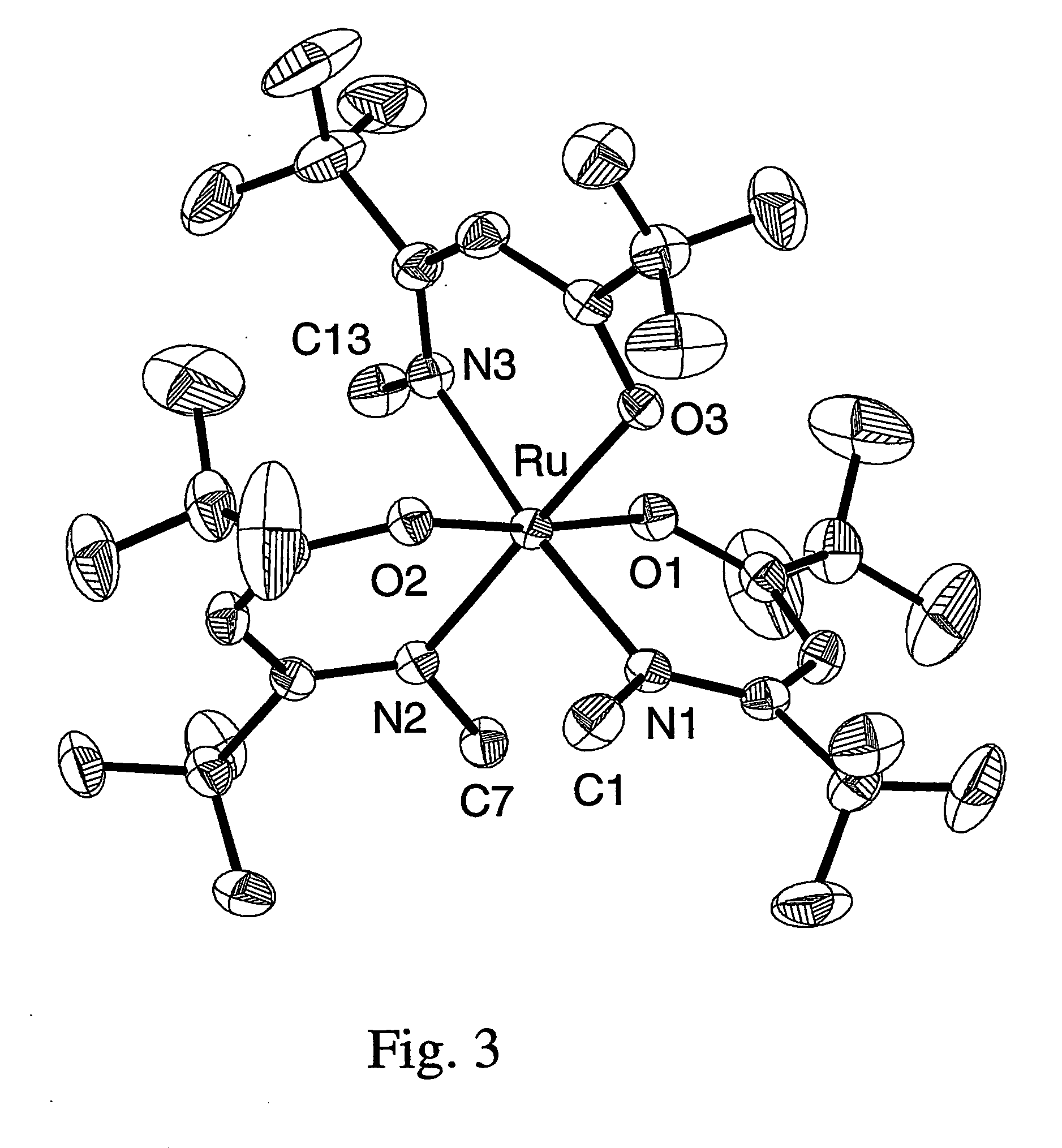
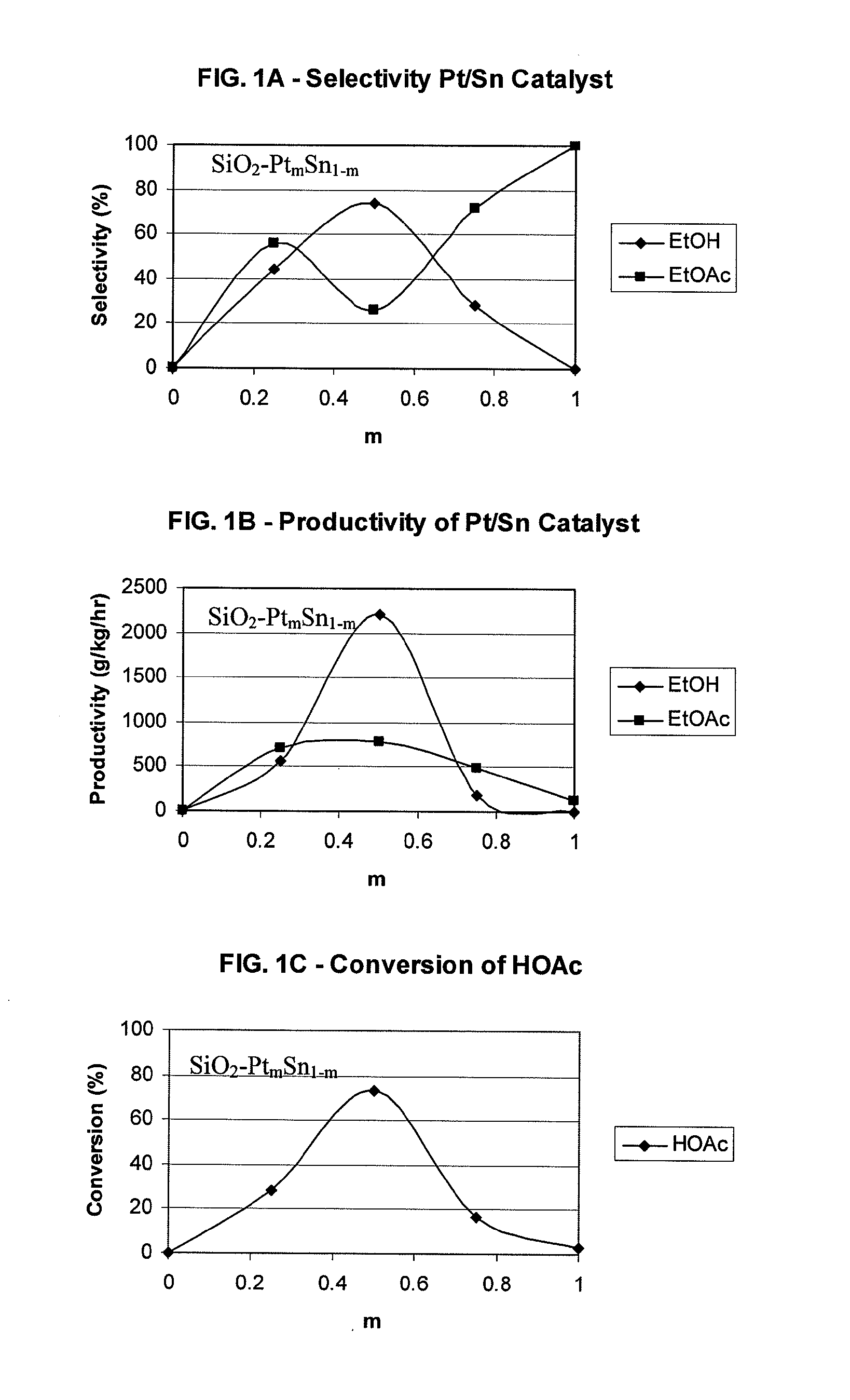
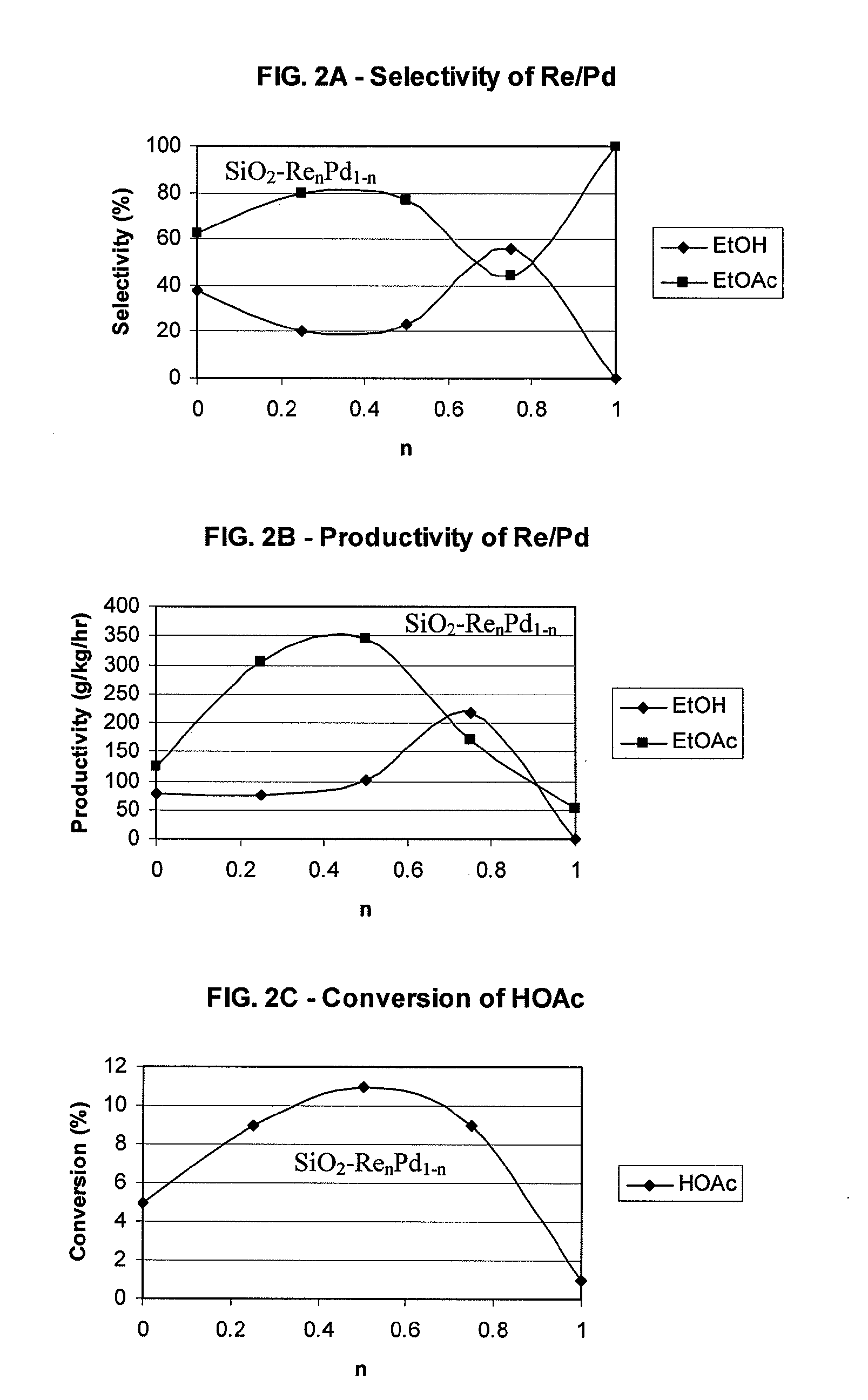
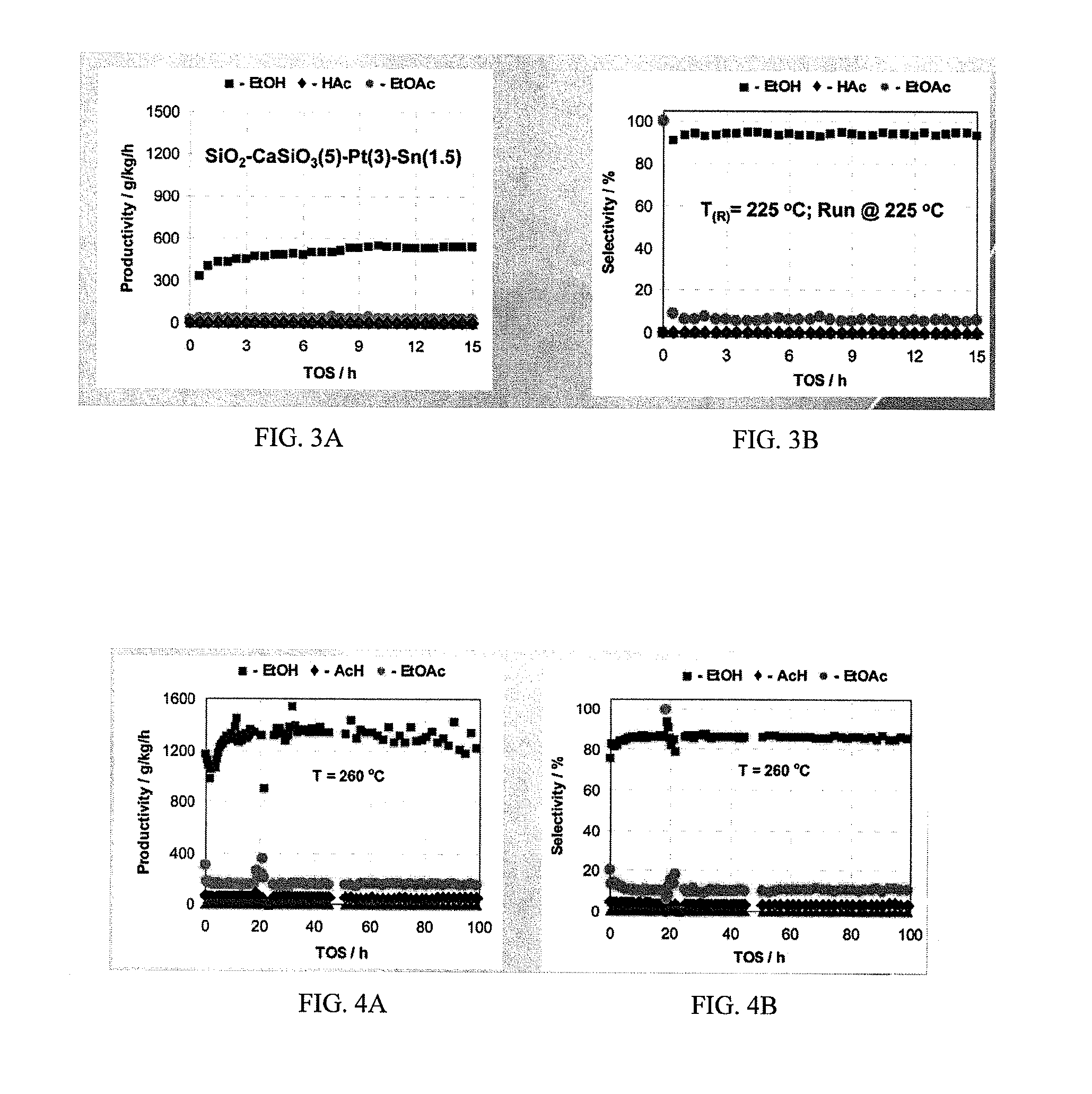
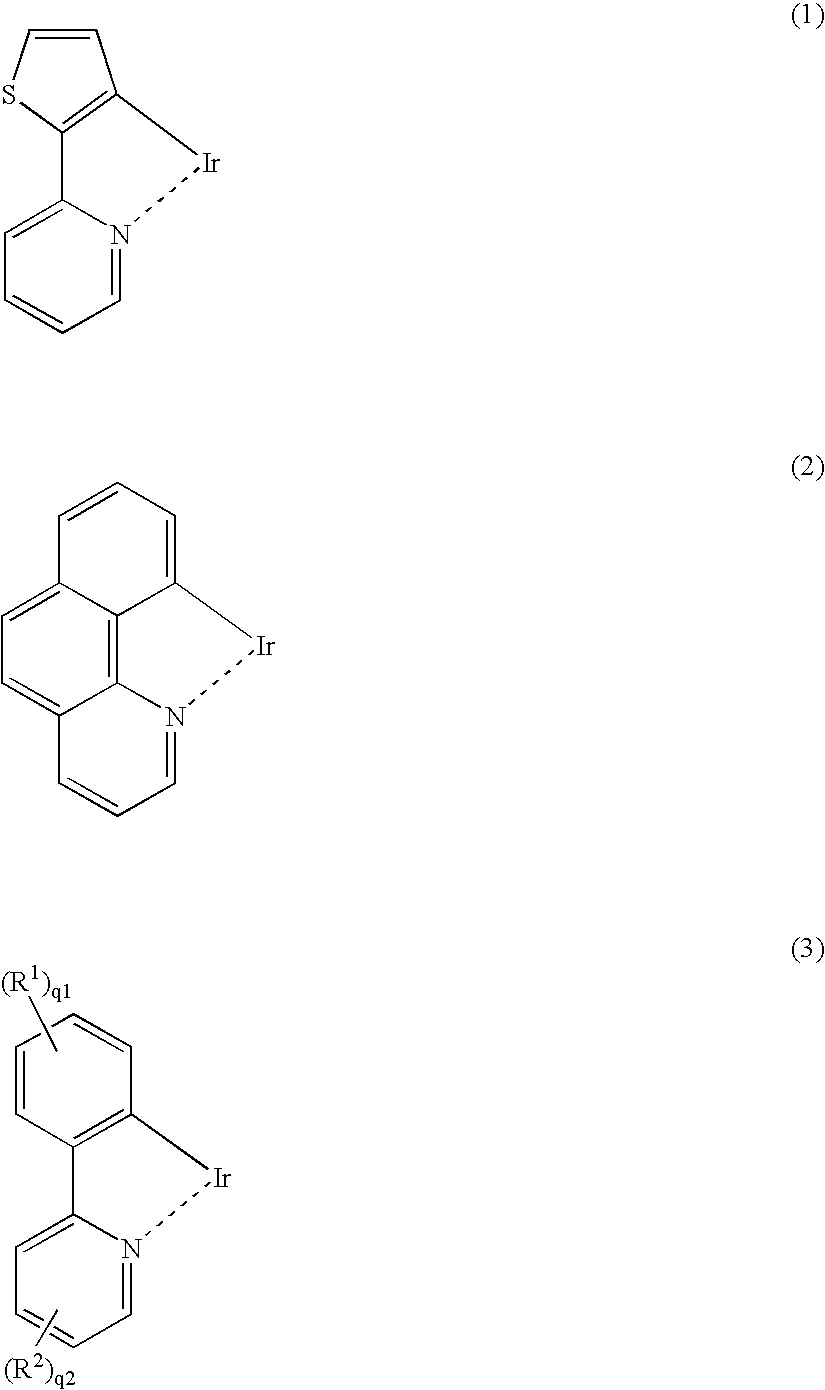
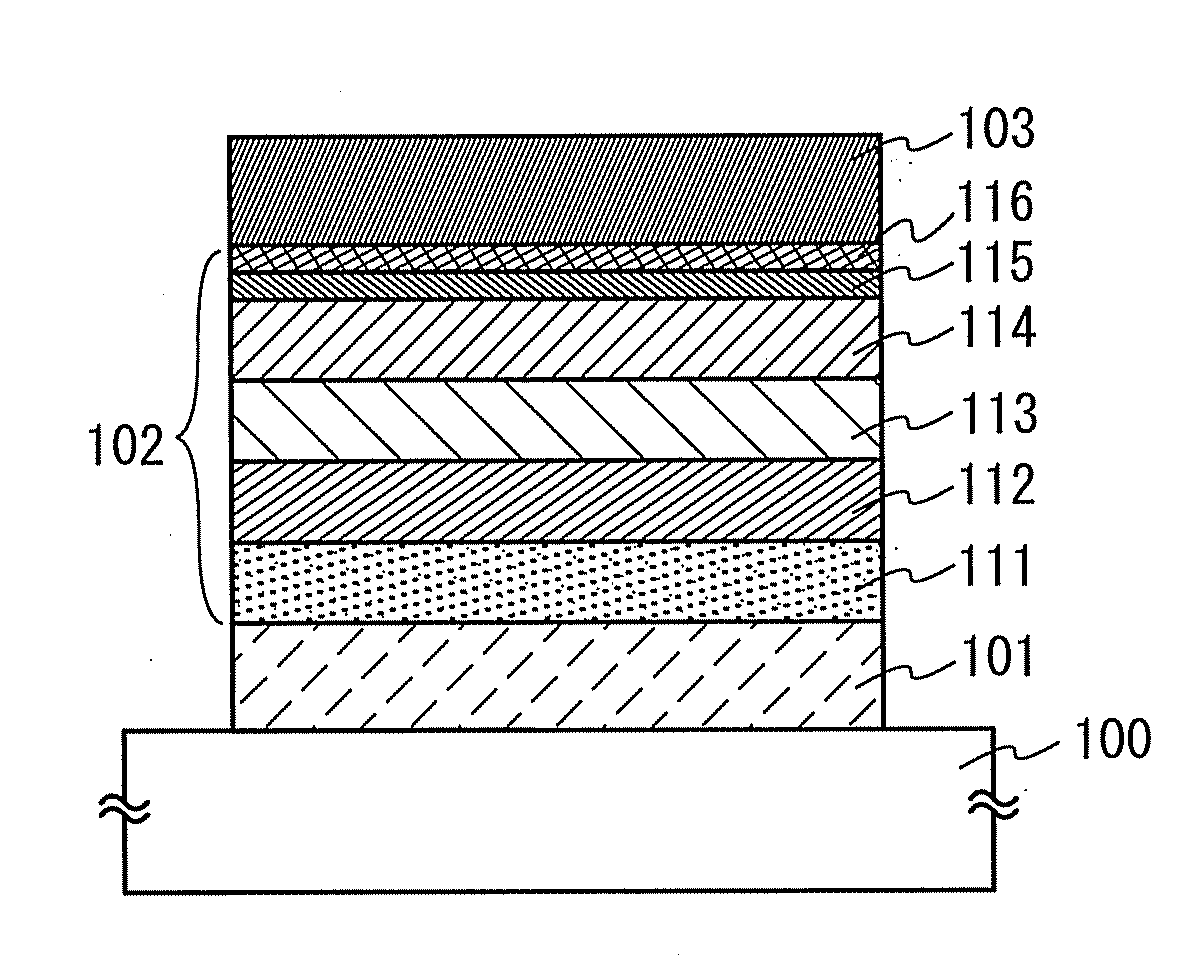
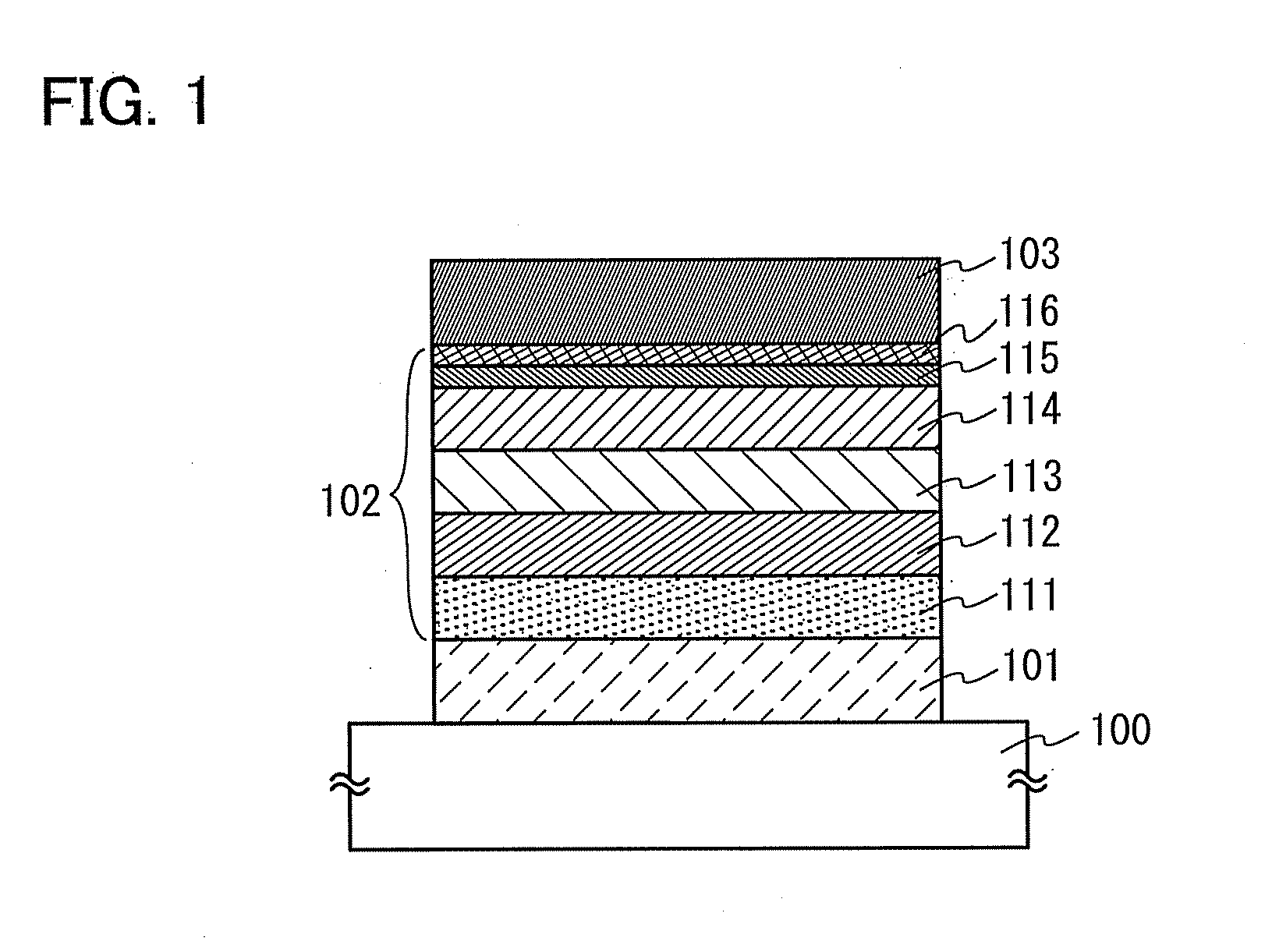
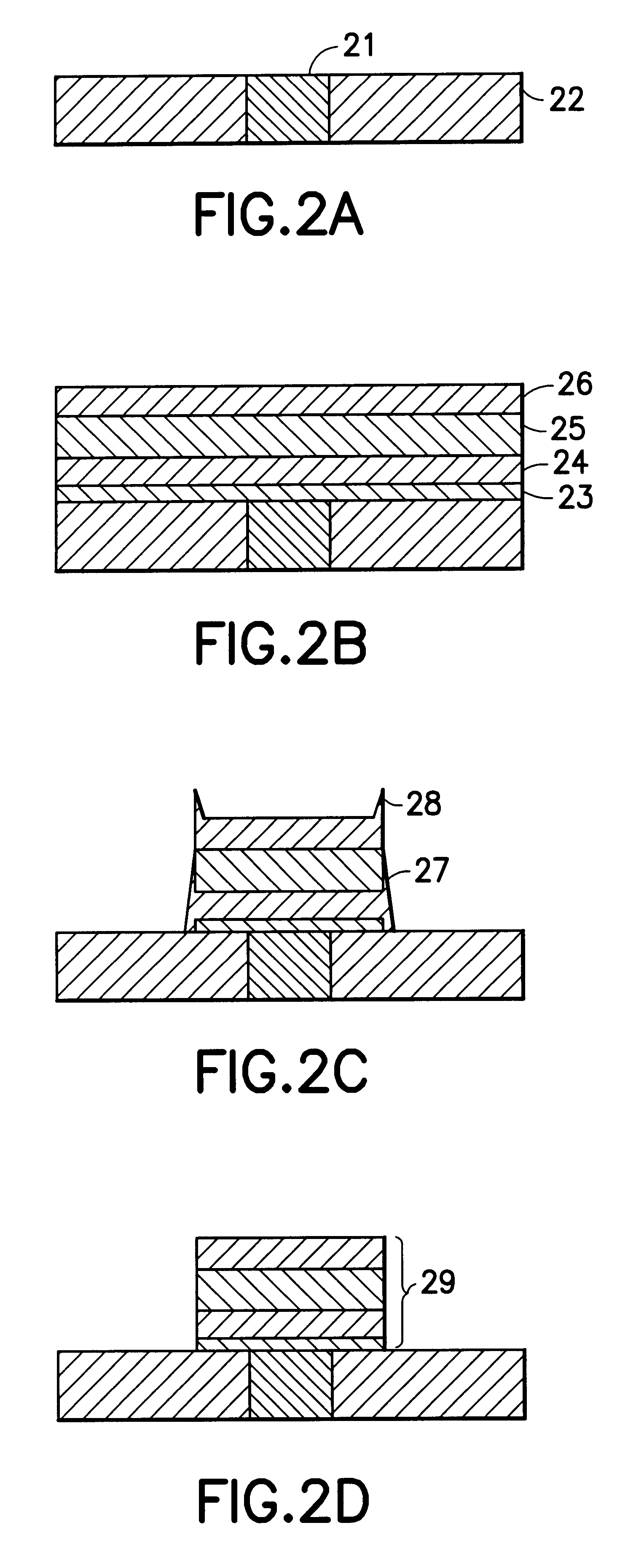
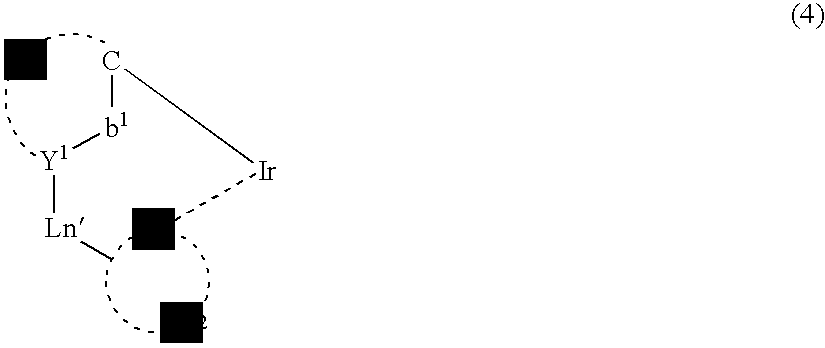Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3607 results about "Iridium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
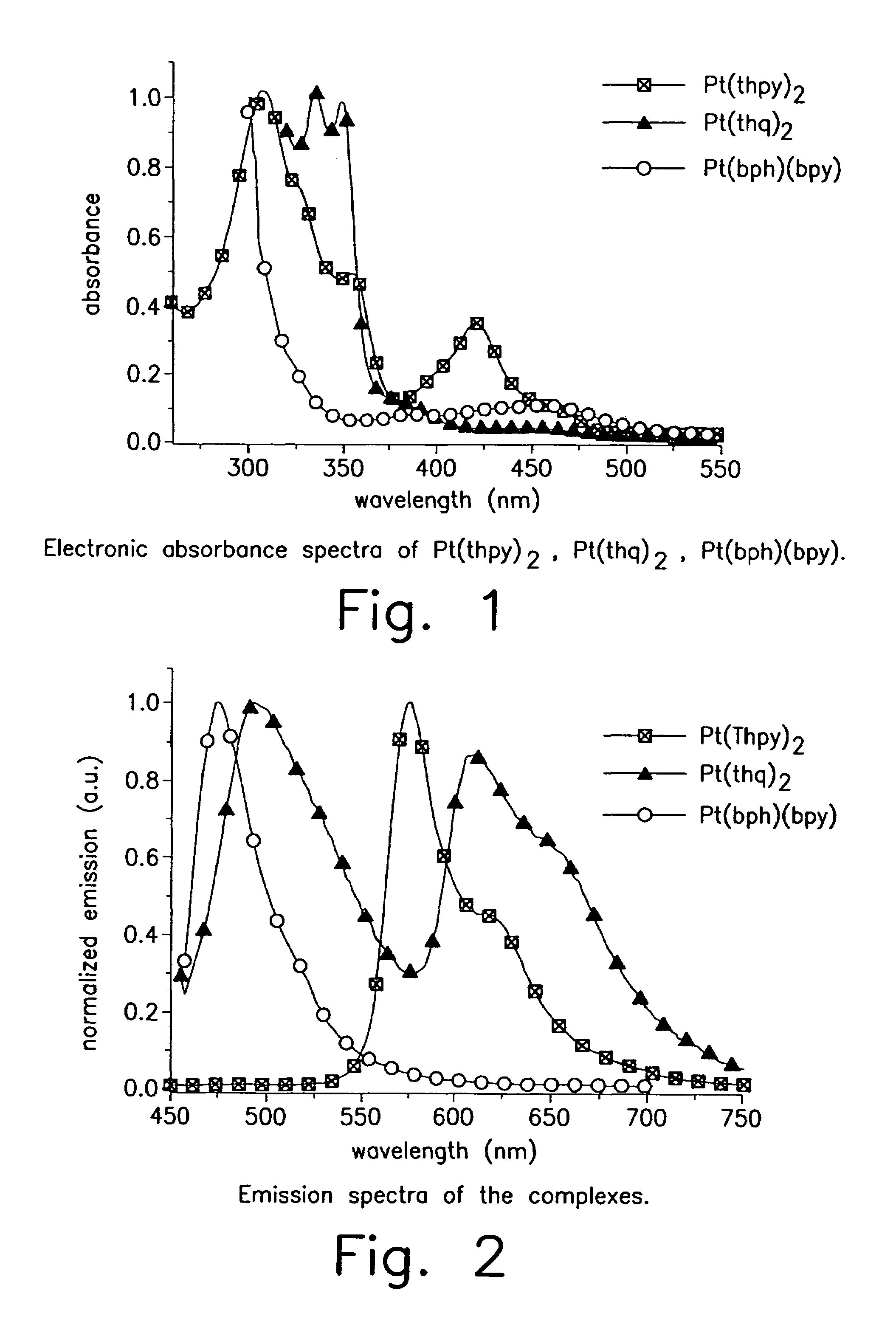
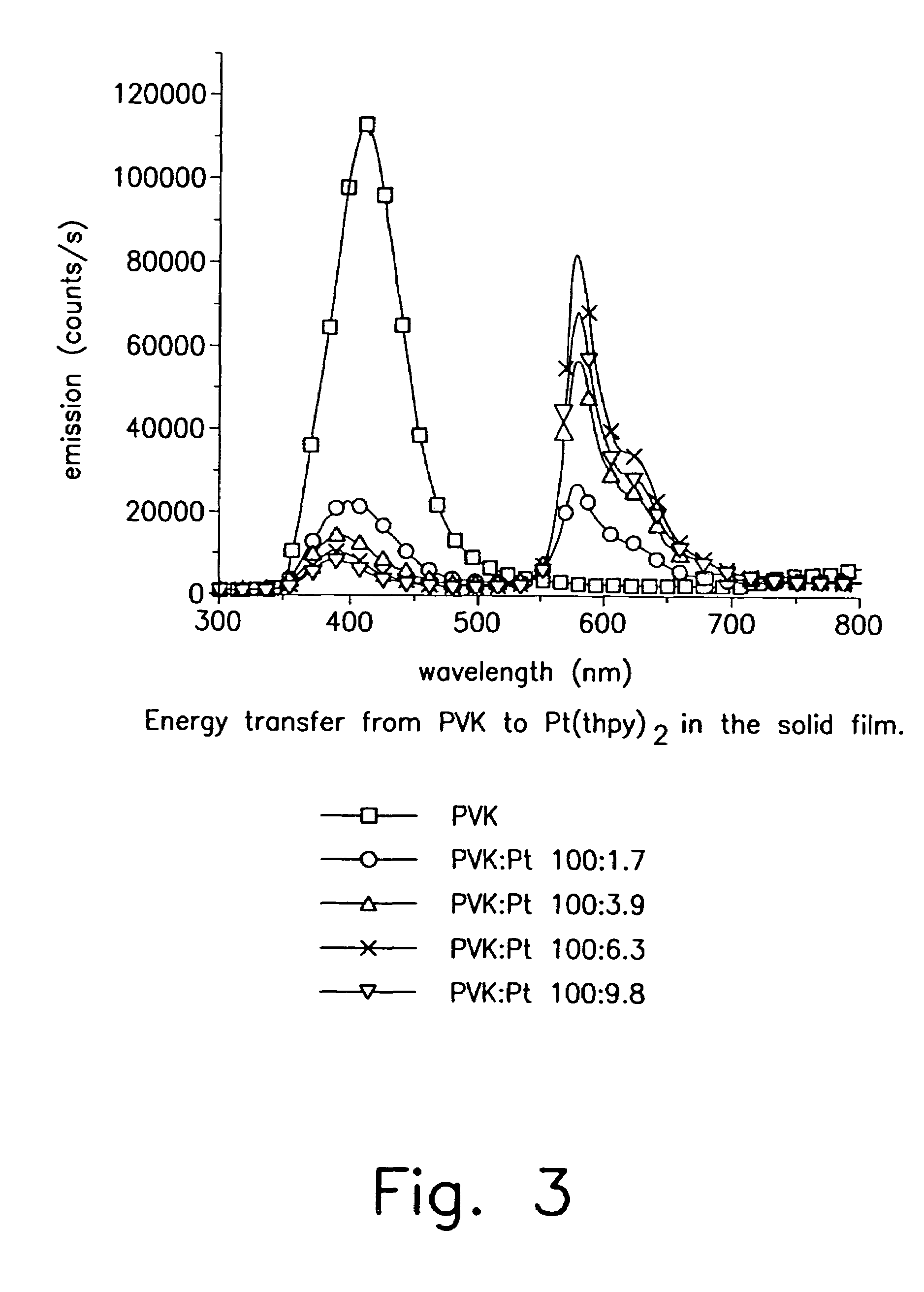
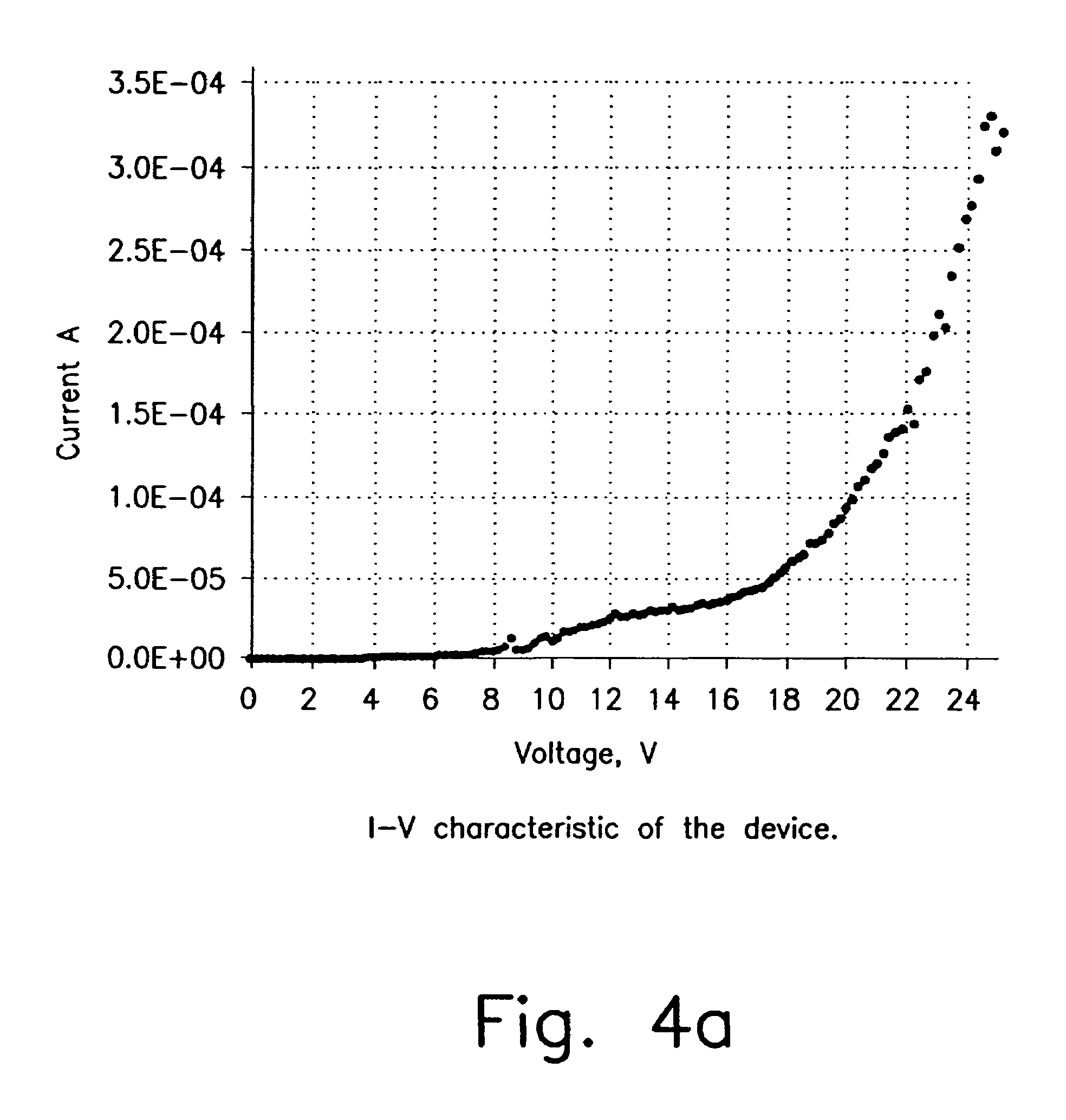
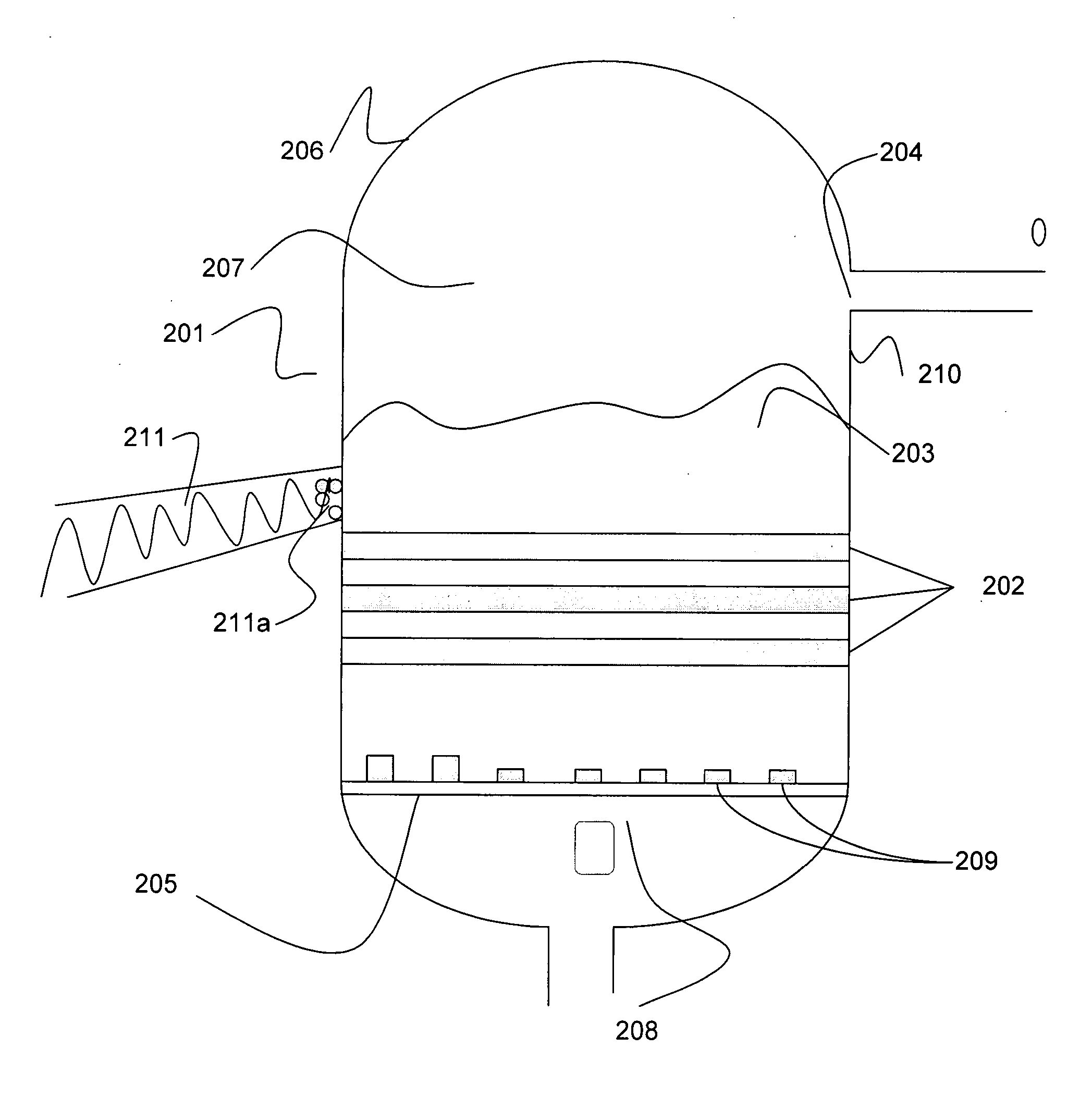
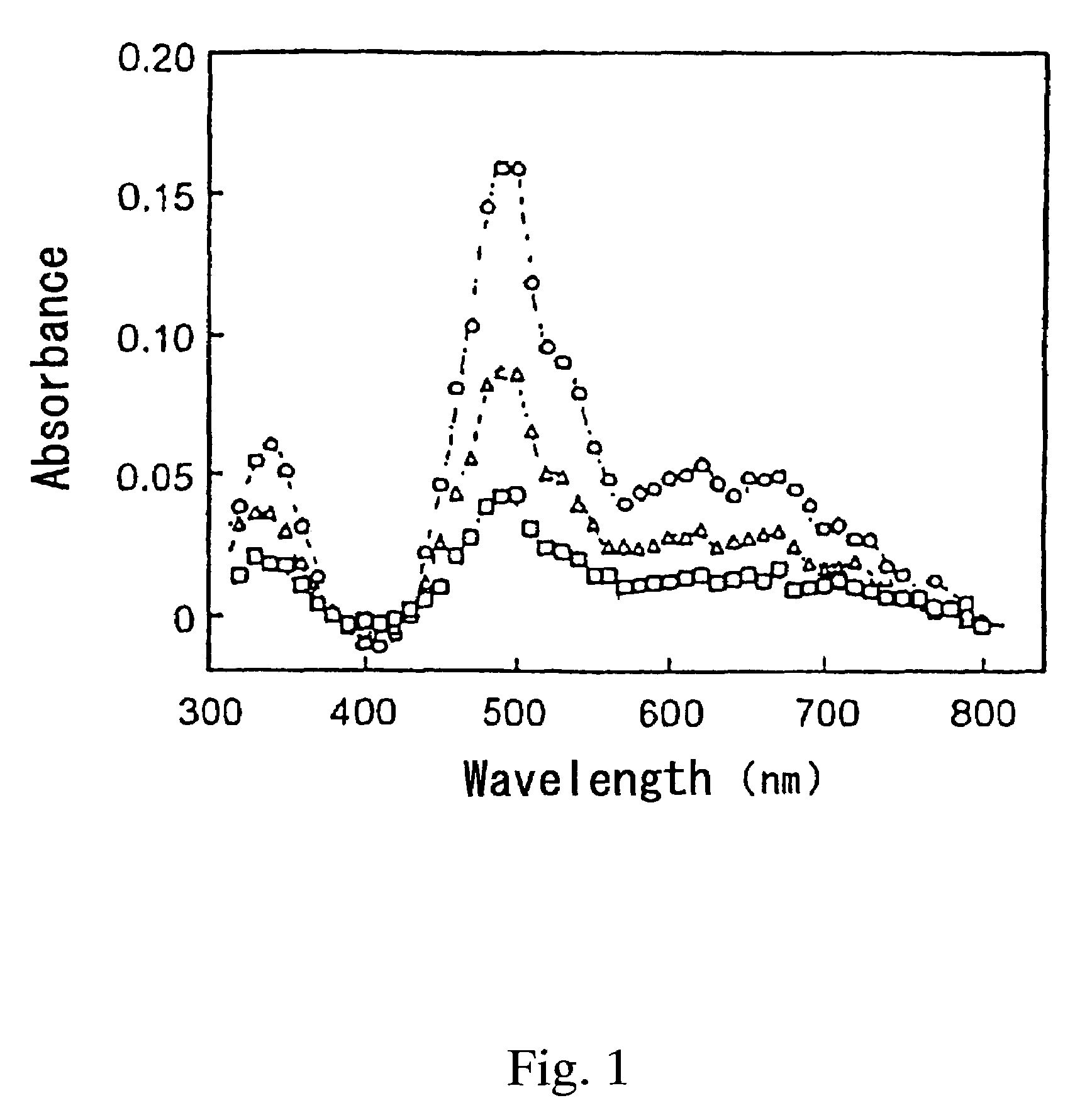
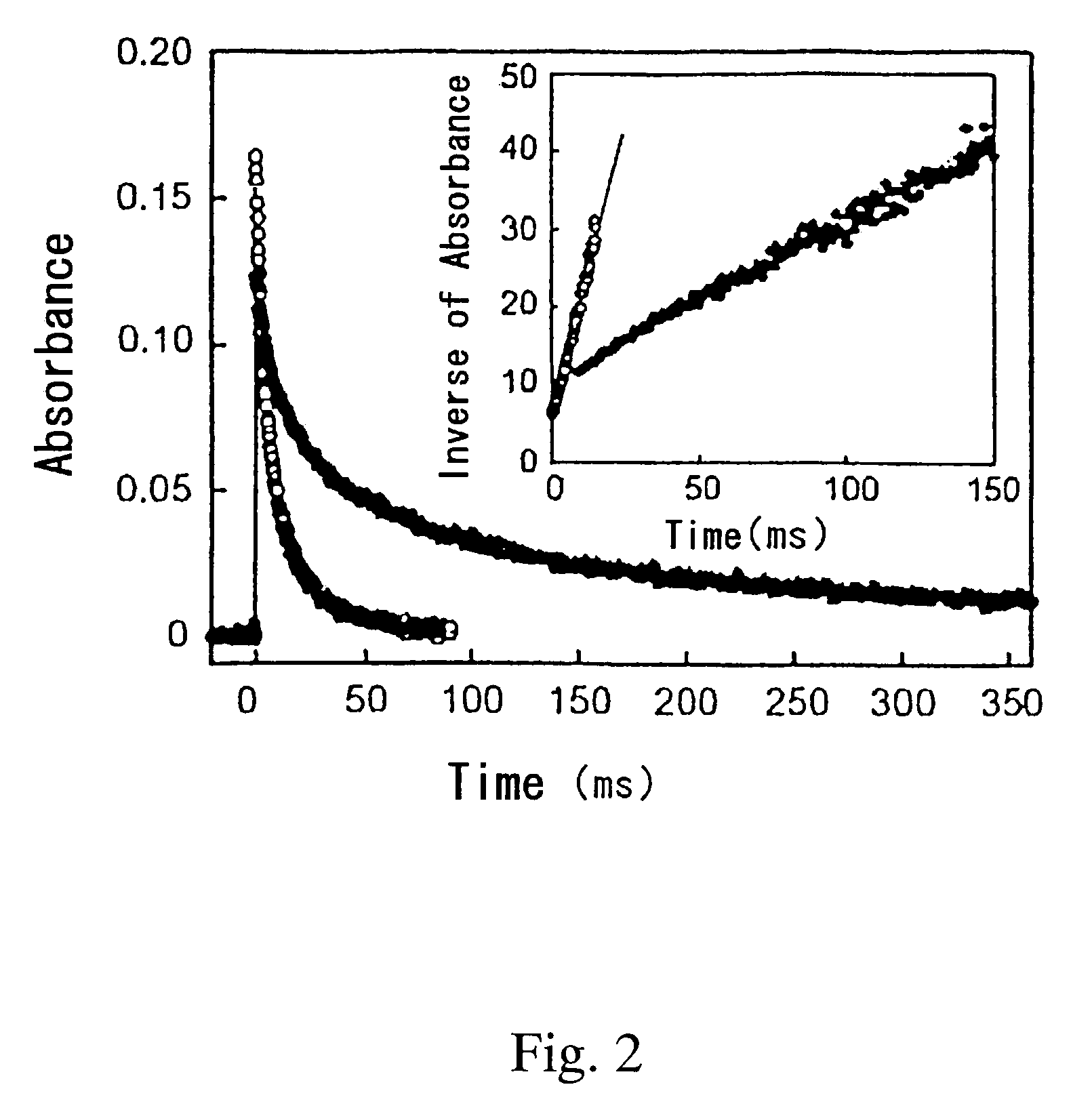
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, iridium is the second-densest metal (after osmium) with a density of 22.56 g/cm³ as defined by experimental X-ray crystallography. At room temperature and standard atmospheric pressure, iridium has a calculated density 0.04 g/cm³ higher than osmium measured the same way. It is the most corrosion-resistant metal, even at temperatures as high as 2000 °C. Although only certain molten salts and halogens are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable.
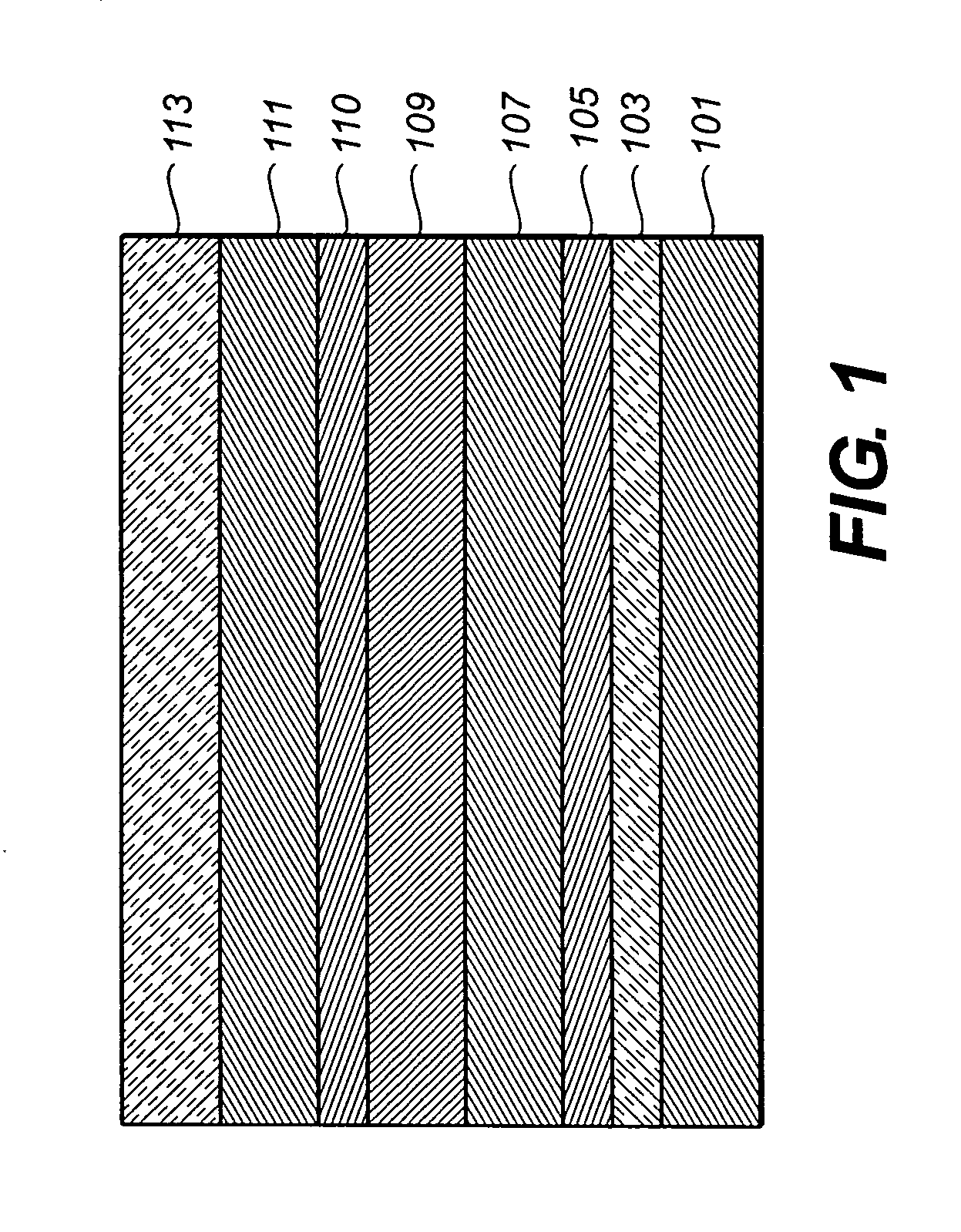
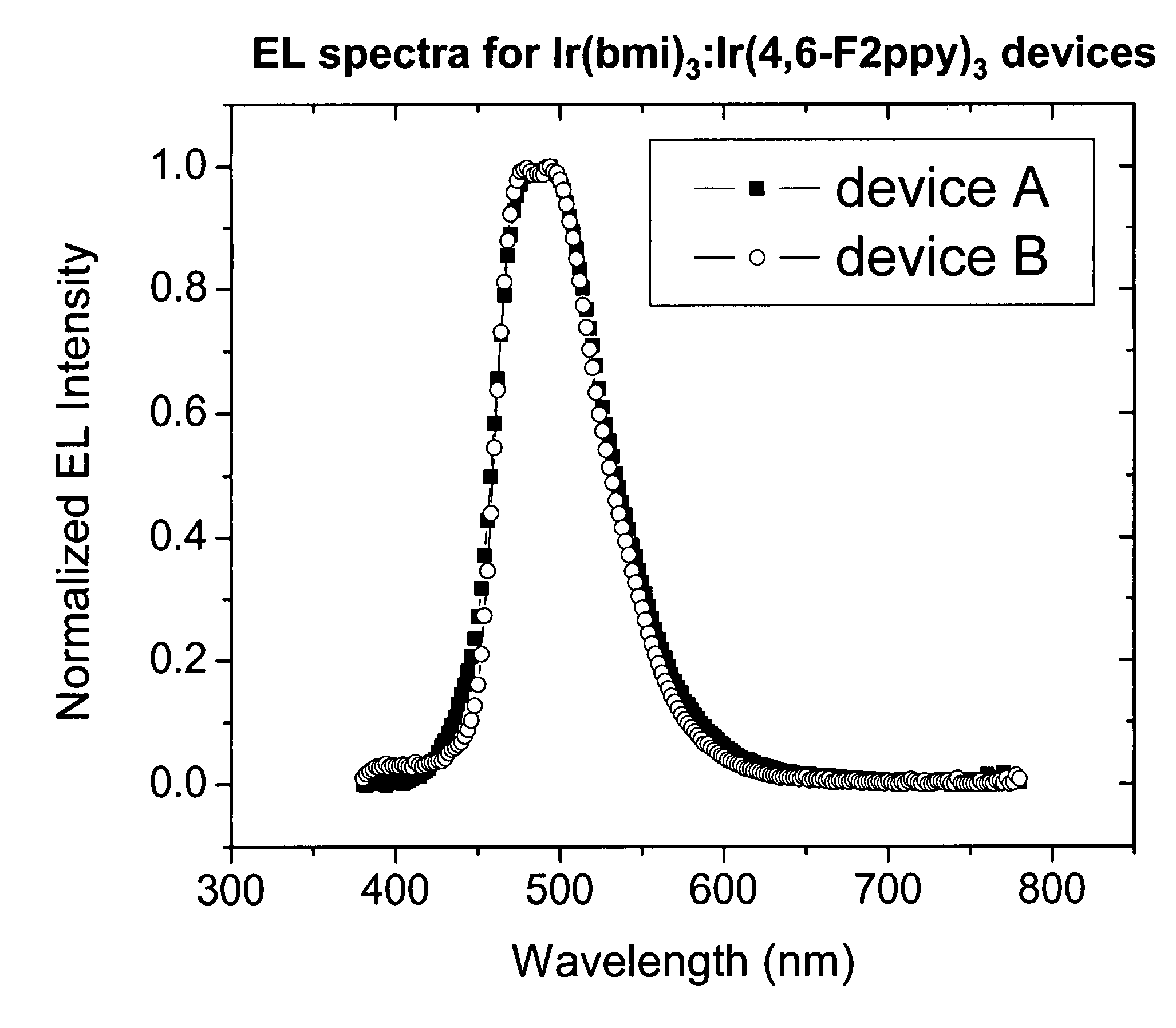
Organic electroluminescent devices
InactiveUS20050123791A1Useful white light emissionSolid-state devicesSemiconductor/solid-state device manufacturingIridiumIsoquinoline
Disclosed is an electroluminescent device comprising a cathode and anode, and therebetween, at least two light-emitting layers wherein the first layer, layer A, comprises a phosphorescent light-emitting organometallic compound comprising iridium and an isoquinoline group and a second layer, layer B, comprising a light-emitting material. Such devices provide useful white light emissions.
Owner:EASTMAN KODAK CO
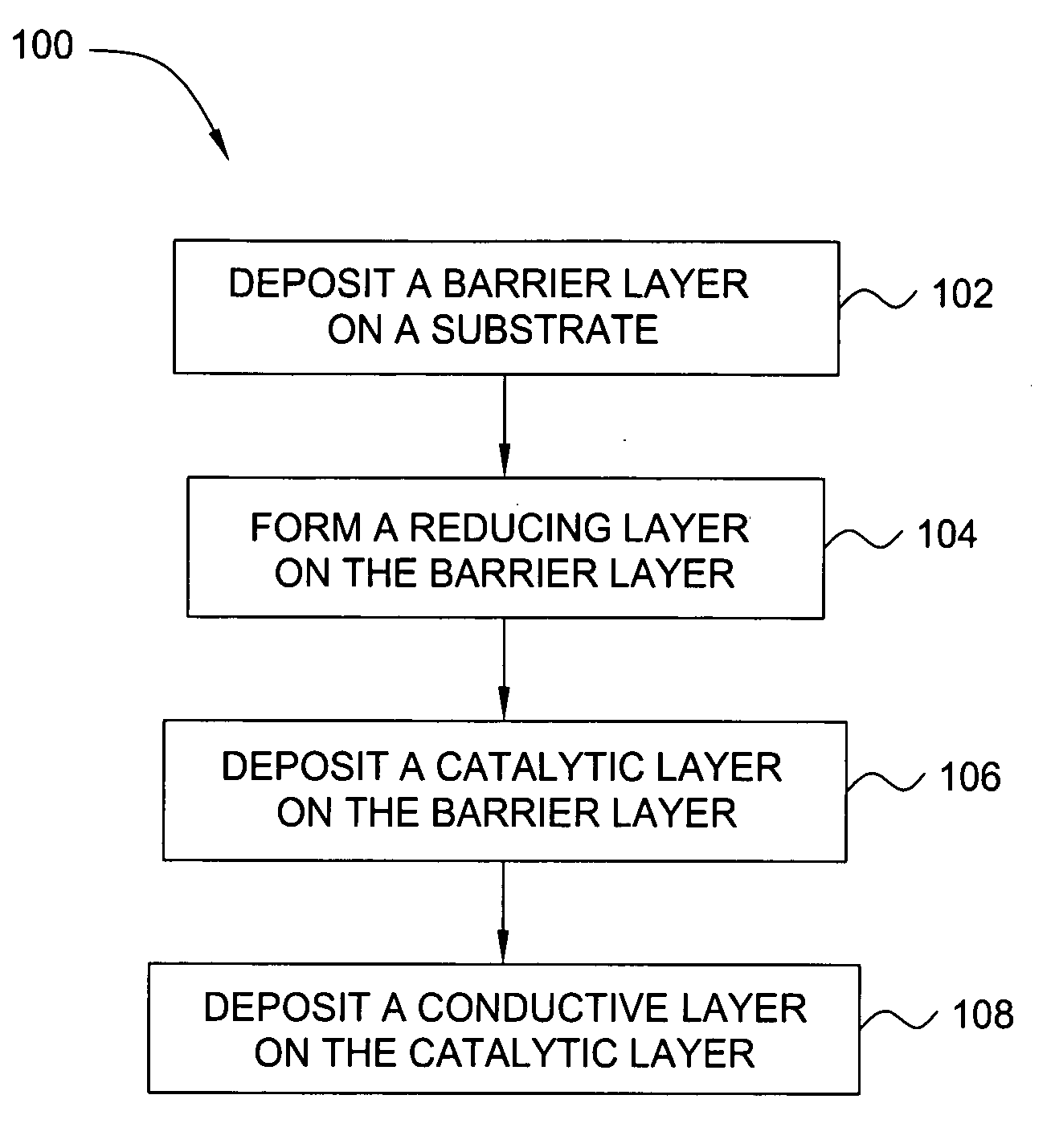
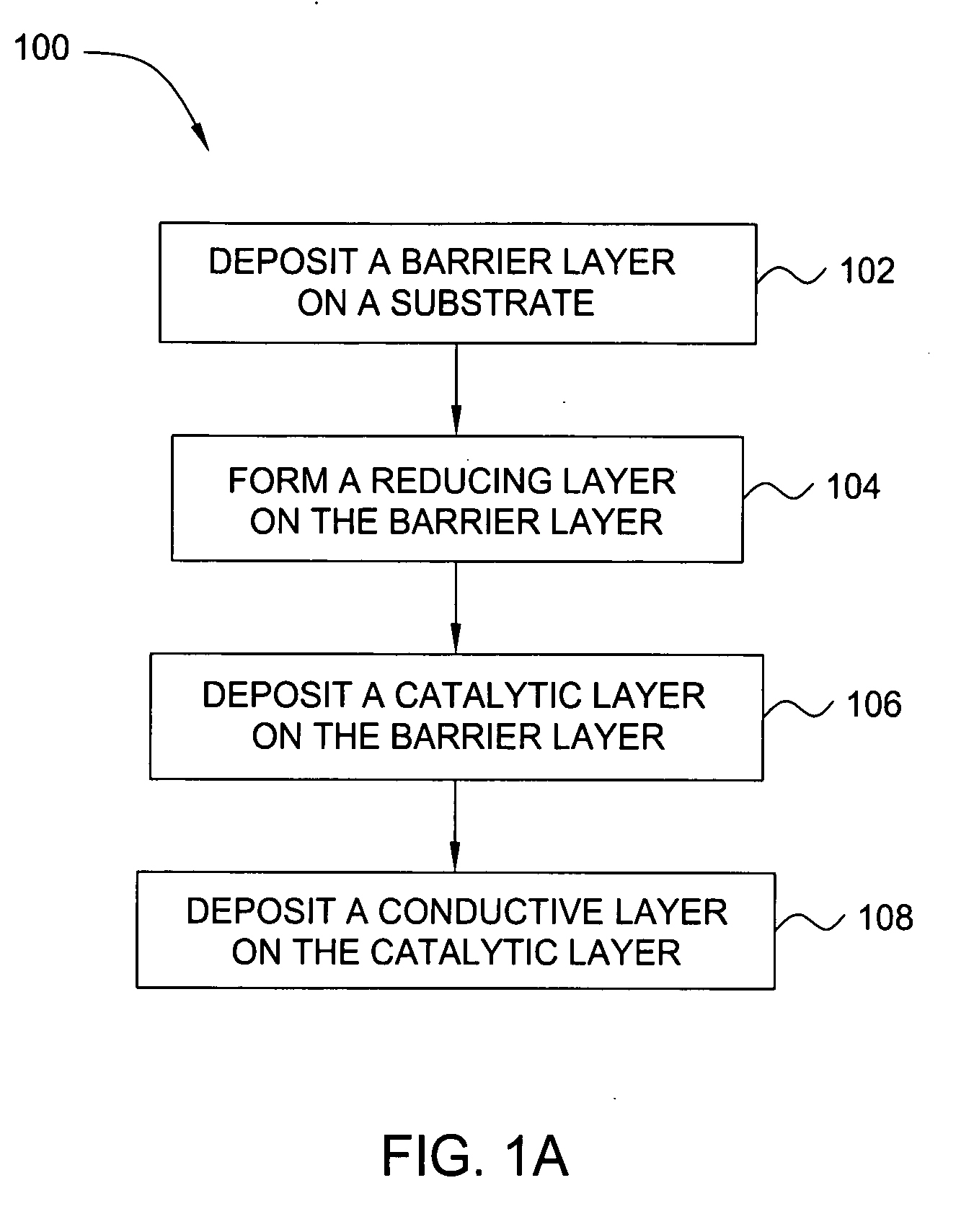
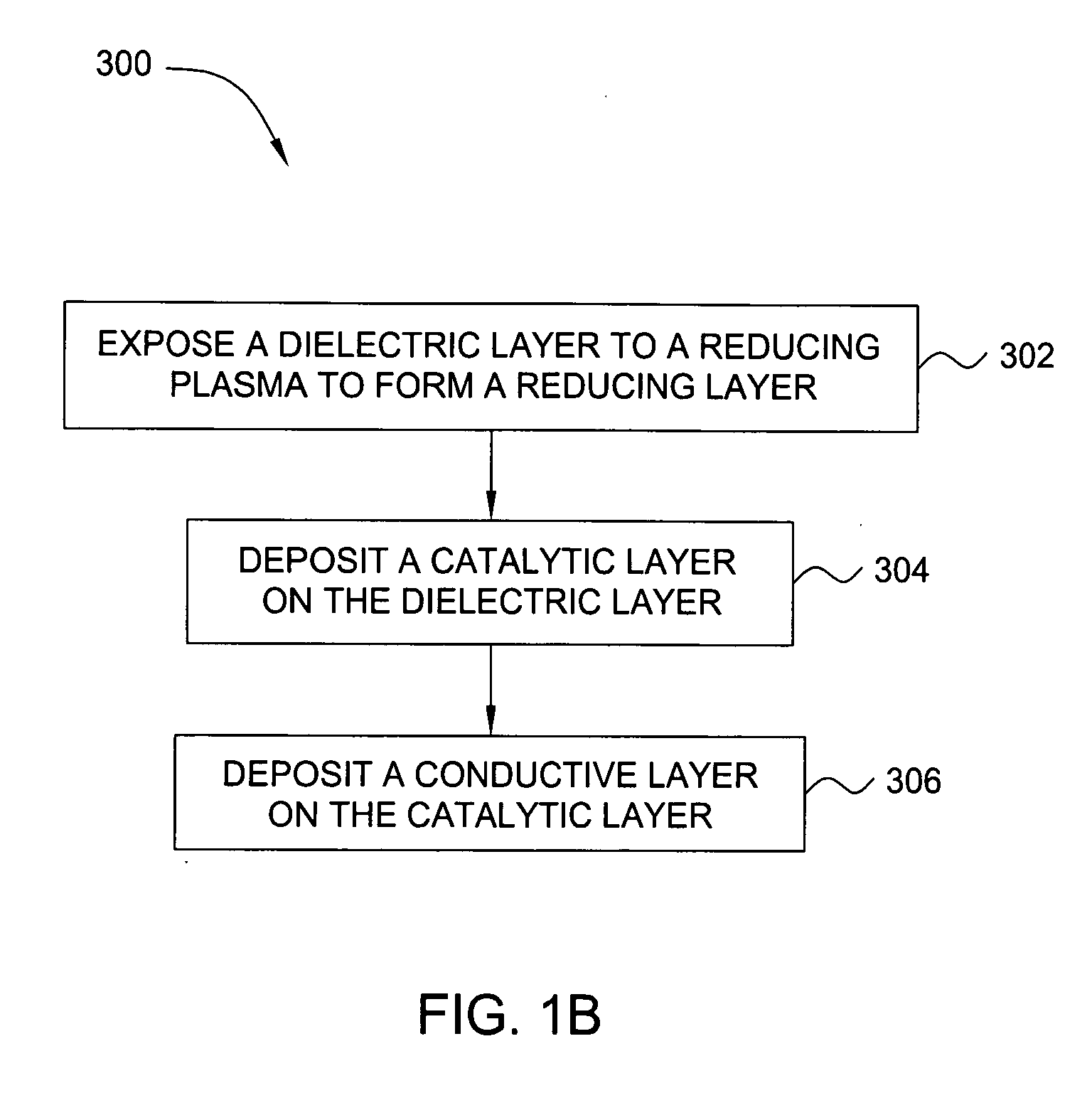
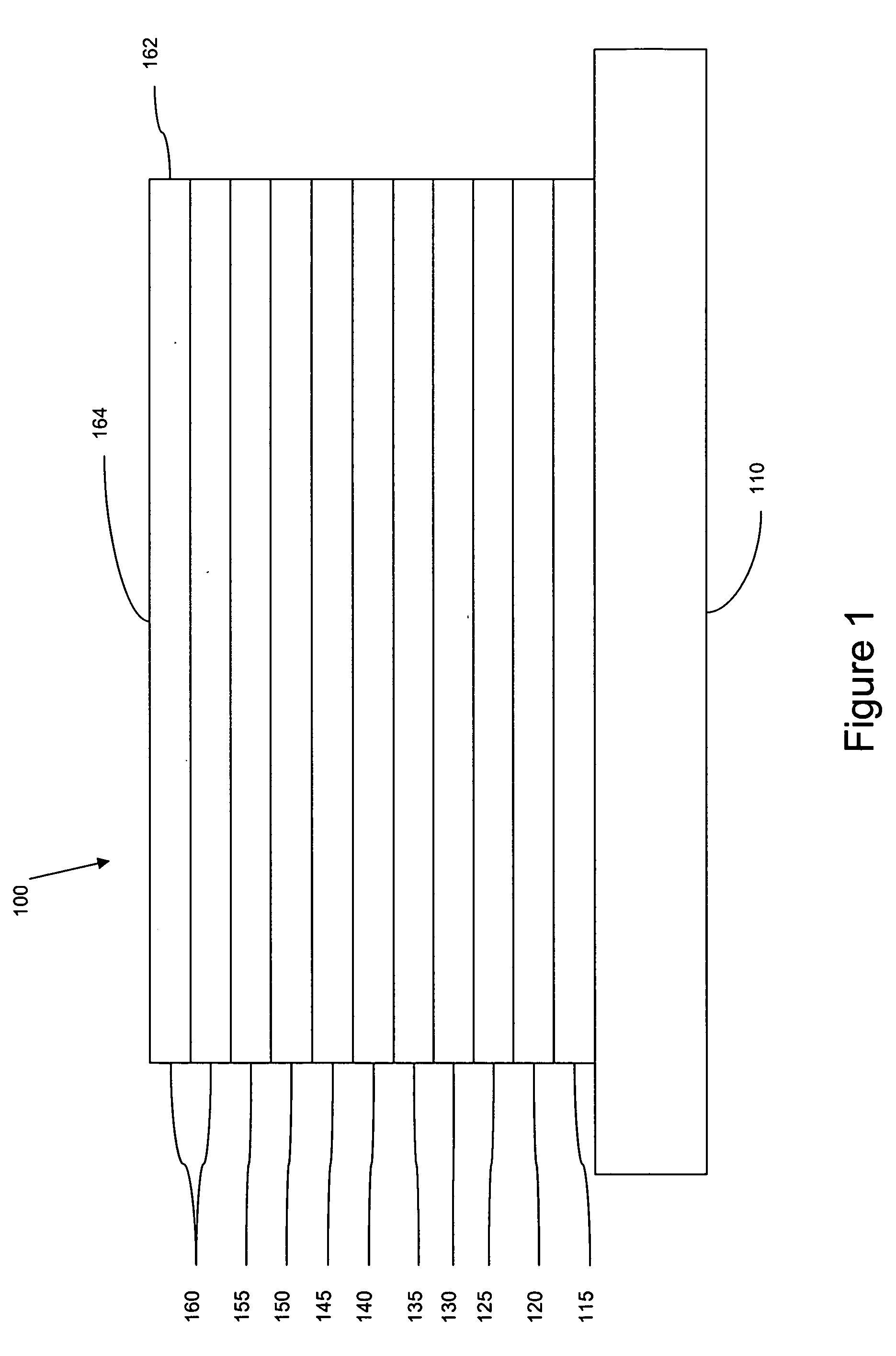
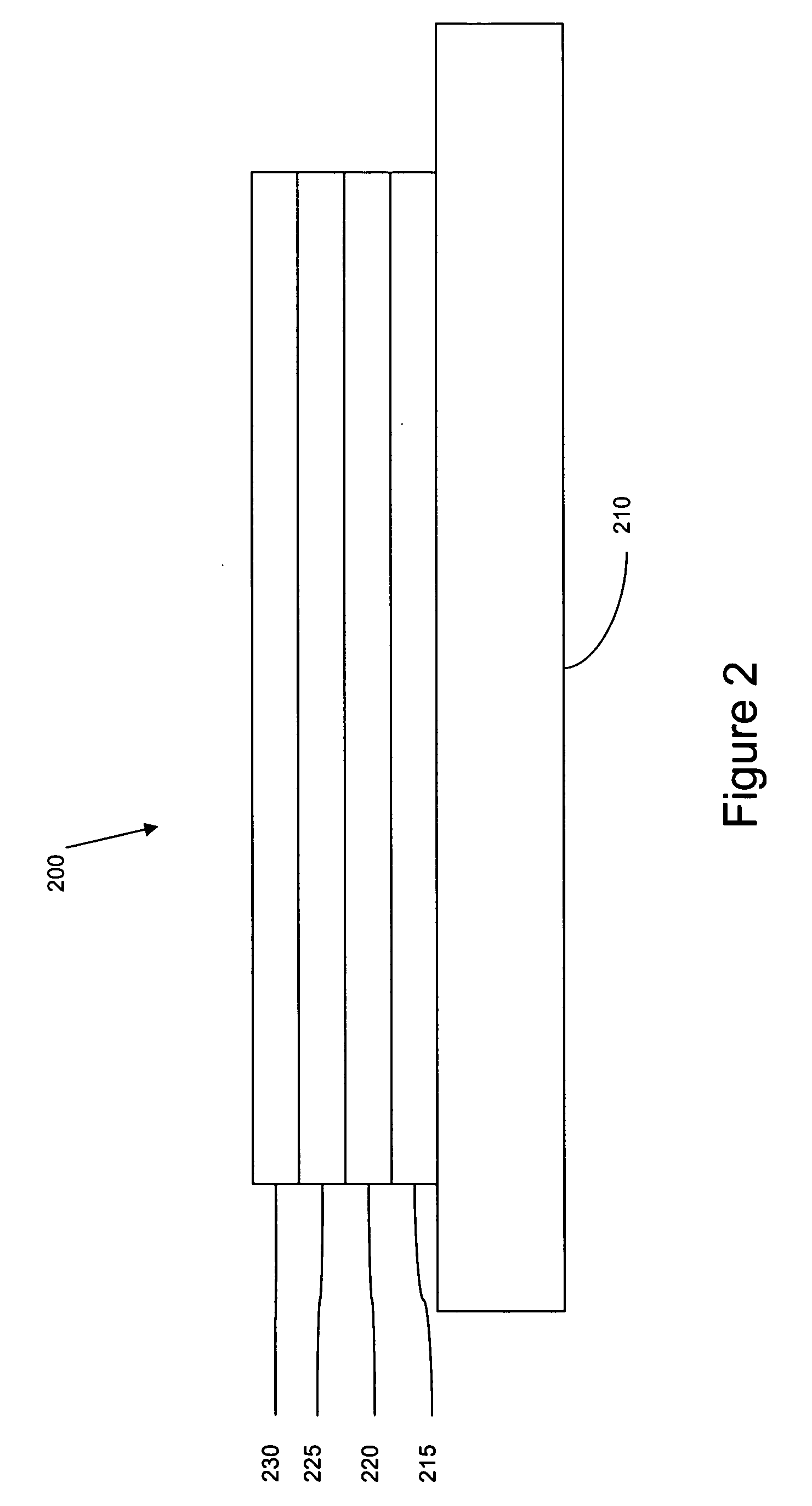
Deposition of an intermediate catalytic layer on a barrier layer for copper metallization
InactiveUS20060240187A1Solid-state devicesSemiconductor/solid-state device manufacturingIridiumSilanes
In one embodiment, a method for depositing a conductive material on a substrate is provided which includes exposing a substrate containing a barrier layer to a volatile reducing precursor to form a reducing layer during a soak process, exposing the reducing layer to a catalytic-metal precursor to deposit a catalytic metal-containing layer on the barrier layer, and depositing a conductive layer (e.g., copper) on the catalytic metal-containing layer. The volatile reducing precursor may include phosphine, diborane, silane, a plasma thereof, or a combination thereof and be exposed to the substrate for a time period within a range from about 1 second to about 30 seconds during the soak process. The catalytic metal-containing layer may contain ruthenium, cobalt, rhodium, iridium, nickel, palladium, platinum, silver, or copper. In one example, the catalytic metal-containing layer is deposited by a vapor deposition process utilizing ruthenium tetroxide formed by an in situ process.
Owner:APPLIED MATERIALS INC
Complexes of red light iridium by using nitrogen heterocycles in quinoline as ligand, and application
ActiveCN1696137AShort lifeImprove efficiencyElectrical apparatusGroup 8/9/10/18 element organic compoundsIridiumNitrogen
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Volatile noble metal organometallic complexes
InactiveUS20050033075A1Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIridiumIodide
A series of noble metal organometallic complexes of the general formula (I): MLaXb(FBC)c, wherein M is a noble metal such as iridium, ruthenium or osmium, and L is a neutral ligand such as carbonyl, alkene or diene; X is an anionic ligand such as chloride, bromide, iodide and trifluoroacetate group; and FBC is a fluorinated bidentate chelate ligand such as beta diketonate, beta-ketoiminate, amino-alcoholate and amino-alcoholate ligand, wherein a is an integer of from zero (0) to three (3), b is an integer of from zero (0) to one (1) and c is an 10 integer of from one (1) to three (3). The resulting noble metal complexes possess enhanced volatility and thermal stability characteristics, and are suitable for chemical vapor deposition(CVD) applications. The corresponding noble metal complex is formed by treatment of the FBC ligand with a less volatile metal halide. Also disclosed are CVD methods for using the noble metal complexes as source reagents for deposition of noble metal-containing films such as Ir, Ru and Os, or even metal oxide film materials IrO2, OsO2 and RuO2.
Owner:NATIONAL TSING HUA UNIVERSITY +1
Organometallic complexes as phosphorescent emitters in organic LEDs
InactiveUS7001536B2Easy to combineDeterioration in emission qualityGroup 8/9/10/18 element organic compoundsElectroluminescent light sourcesIridiumPt element
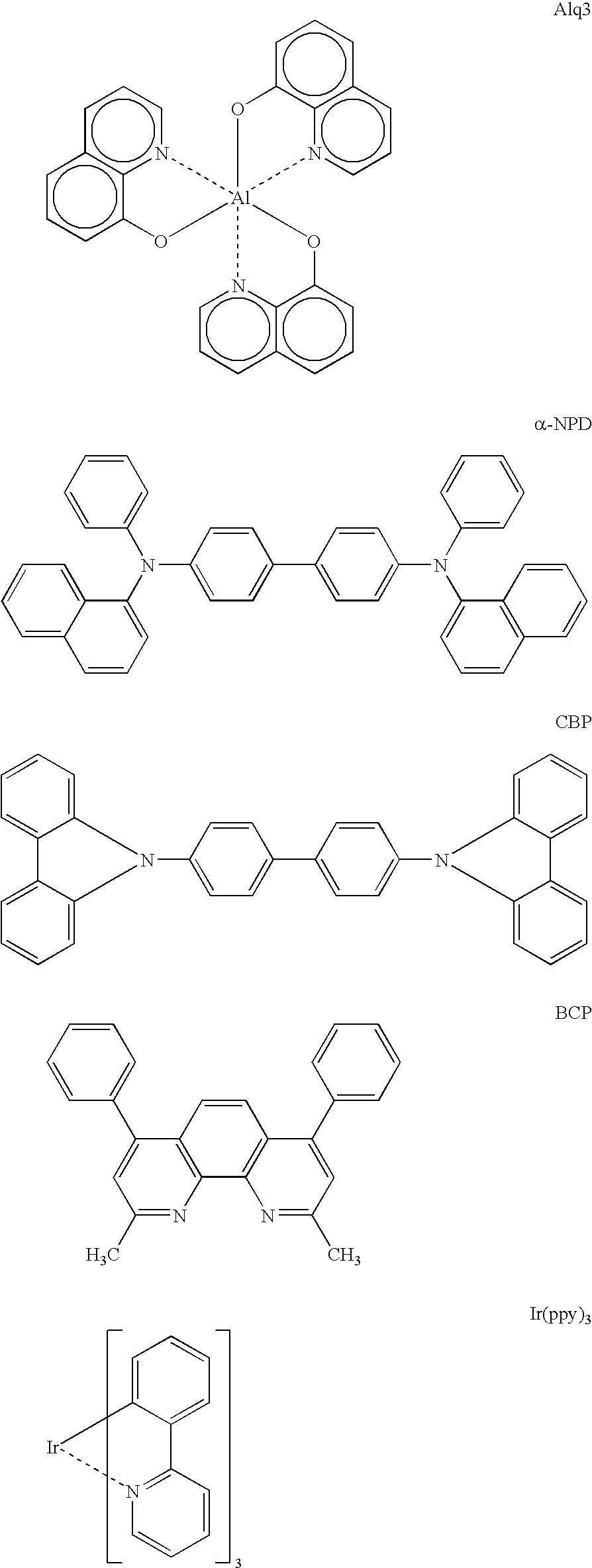
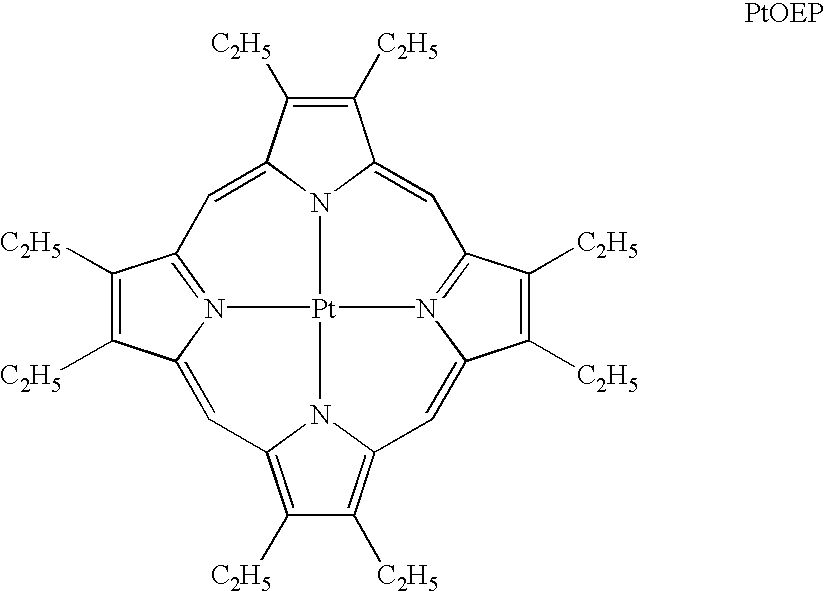
Organic light emitting devices are described wherein the emissive layer comprises a host material containing an emissive molecule, which molecule is adapted to luminesce when a voltage is applied across the heterostructure, and the emissive molecule is selected from the group of phosphorescent organometallic complexes, including cyclometallated platinum, iridium and osmium complexes. The organic light emitting devices optionally contain an exciton blocking layer. Furthermore, improved electroluminescent efficiency in organic light emitting devices is obtained with an emitter layer comprising organometallic complexes of transition metals of formula L2MX, wherein L and X are distinct bidentate ligands. Compounds of this formula can be synthesized more facilely than in previous approaches and synthetic options allow insertion of fluorescent molecules into a phosphorescent complex, ligands to fine tune the color of emission, and ligands to trap carriers.
Owner:THE TRUSTEES FOR PRINCETON UNIV +1
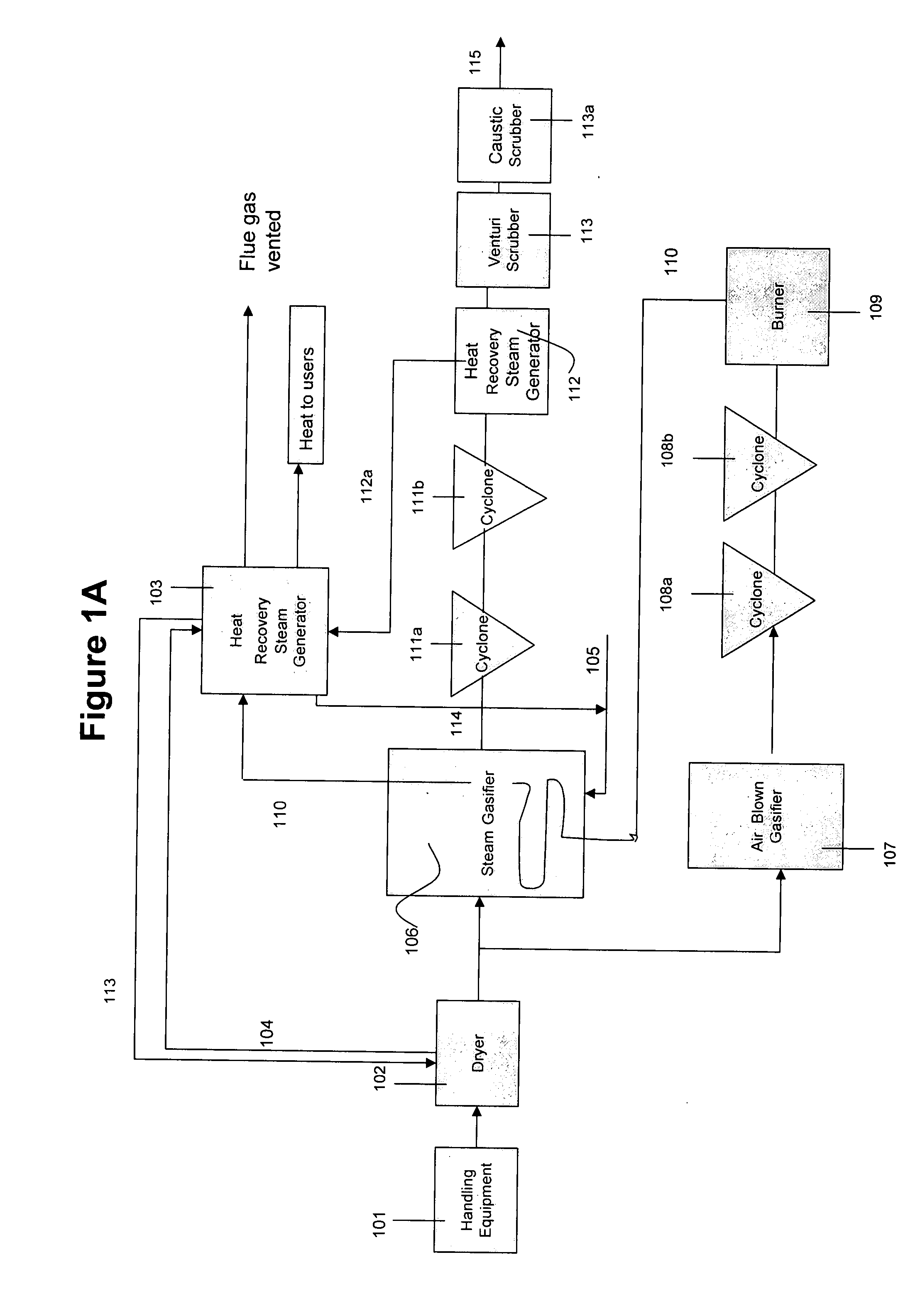
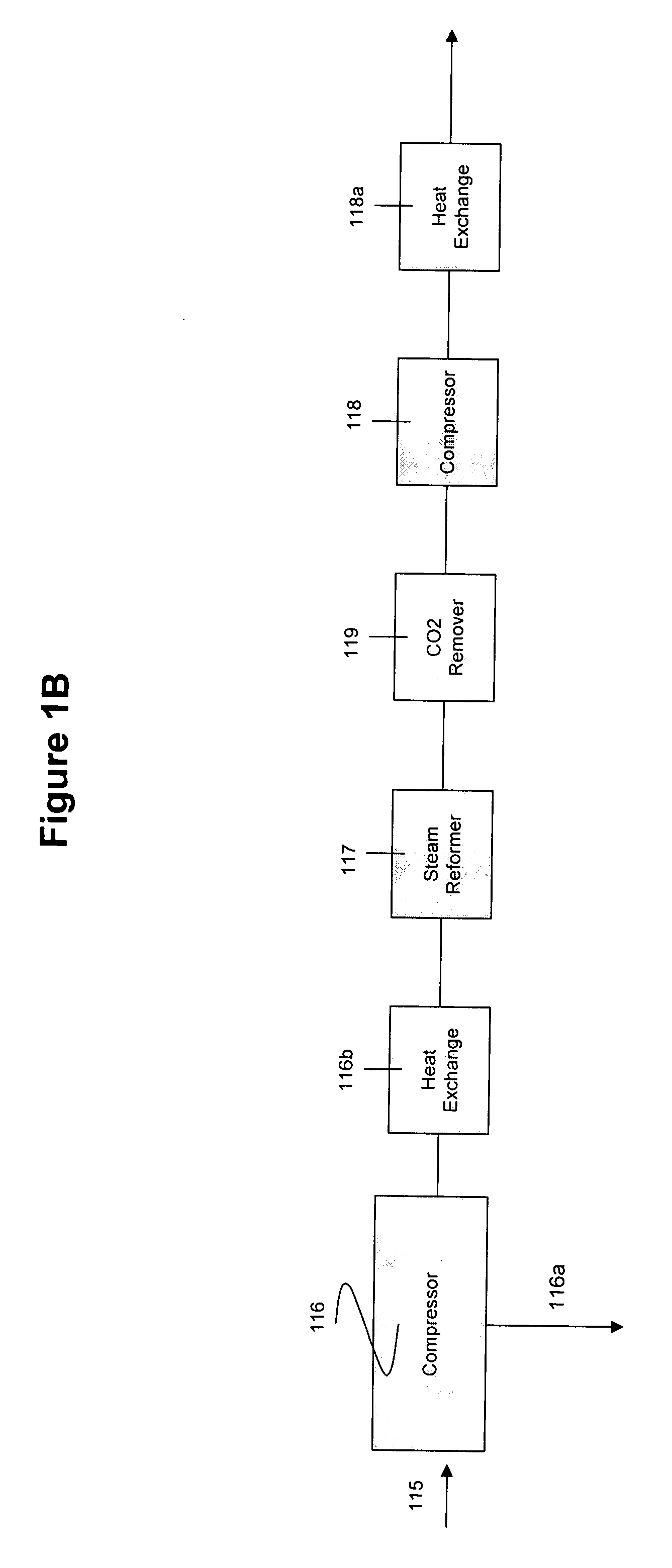
System and method for converting biomass to ethanol via syngas
A method and apparatus for synthesizing ethanol using synthetic routes via synthesis gas are disclosed. A method and apparatus for gasifying biomass, such as biomass, in a steam gasifier that employs a fluidized bed and heating using hot flue gases from the combustion of synthesis gas is described. Methods and apparatus for converting synthesis gas into ethanol are also disclosed, using stepwise catalytic reactions to convert the carbon monoxide and hydrogen into ethanol using catalysts including iridium acetate.
Owner:WOODLAND BIOFUELS
Radiopaque nitinol alloys for medical devices
Owner:ABBOTT CARDIOVASCULAR
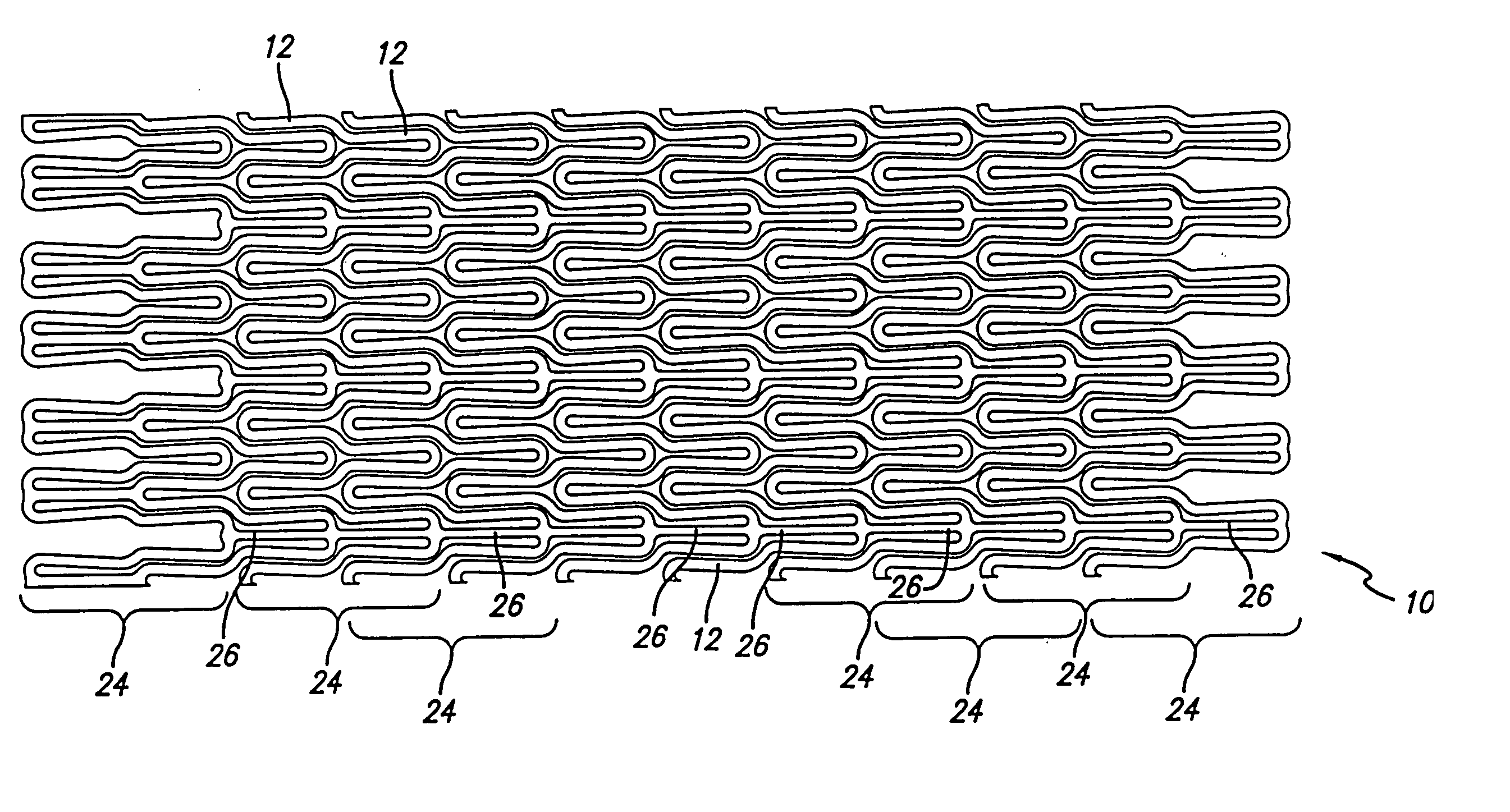
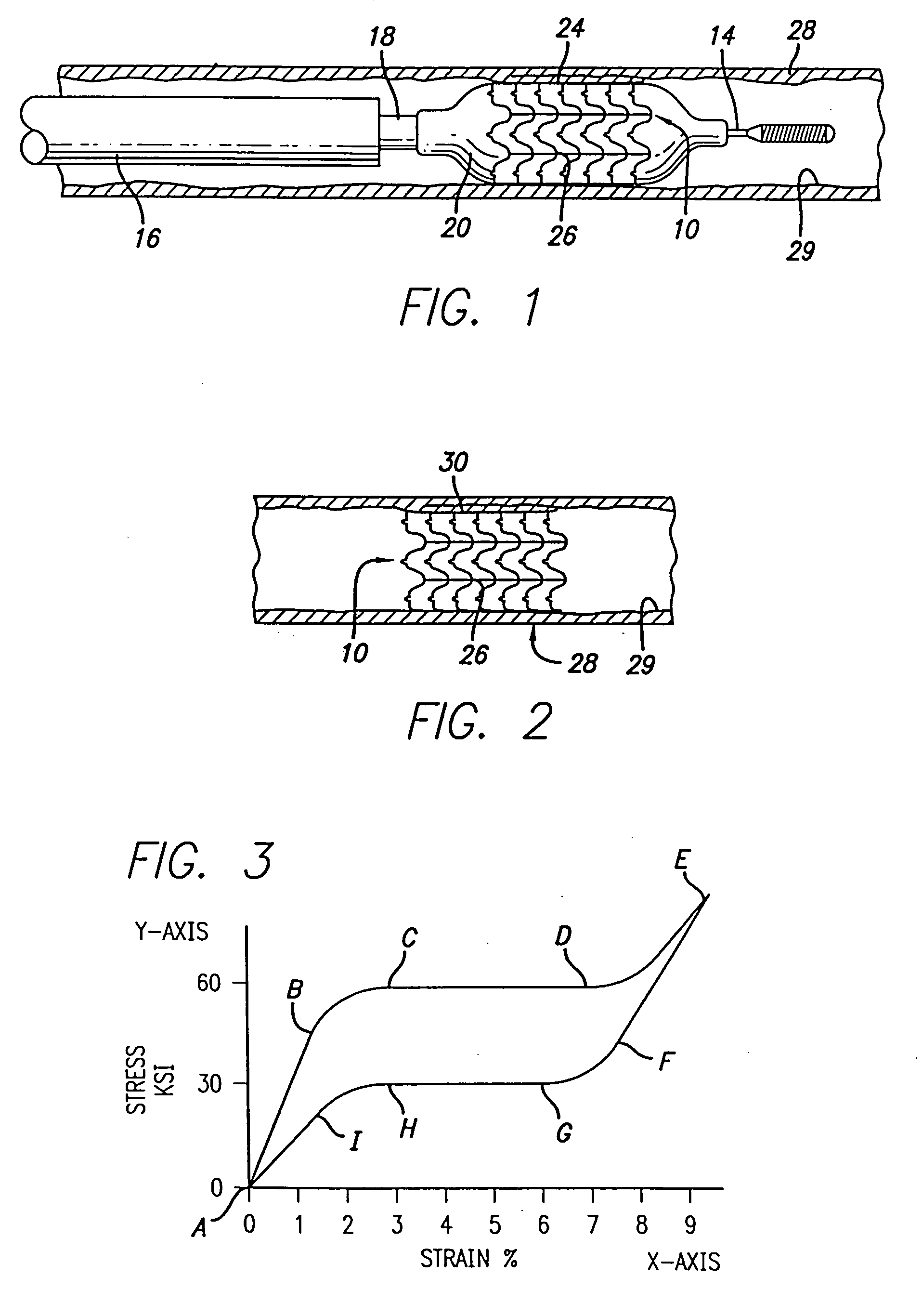
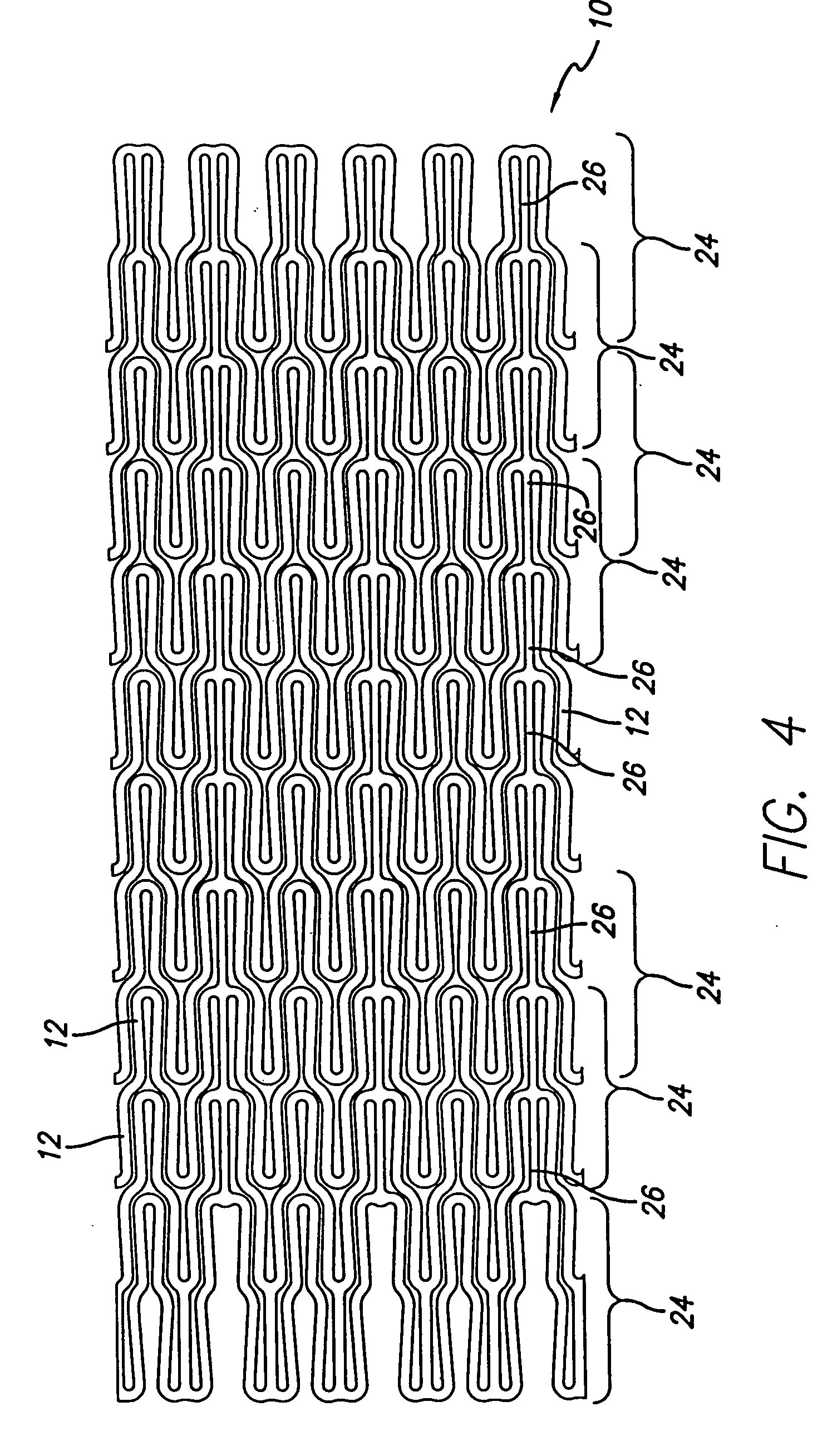
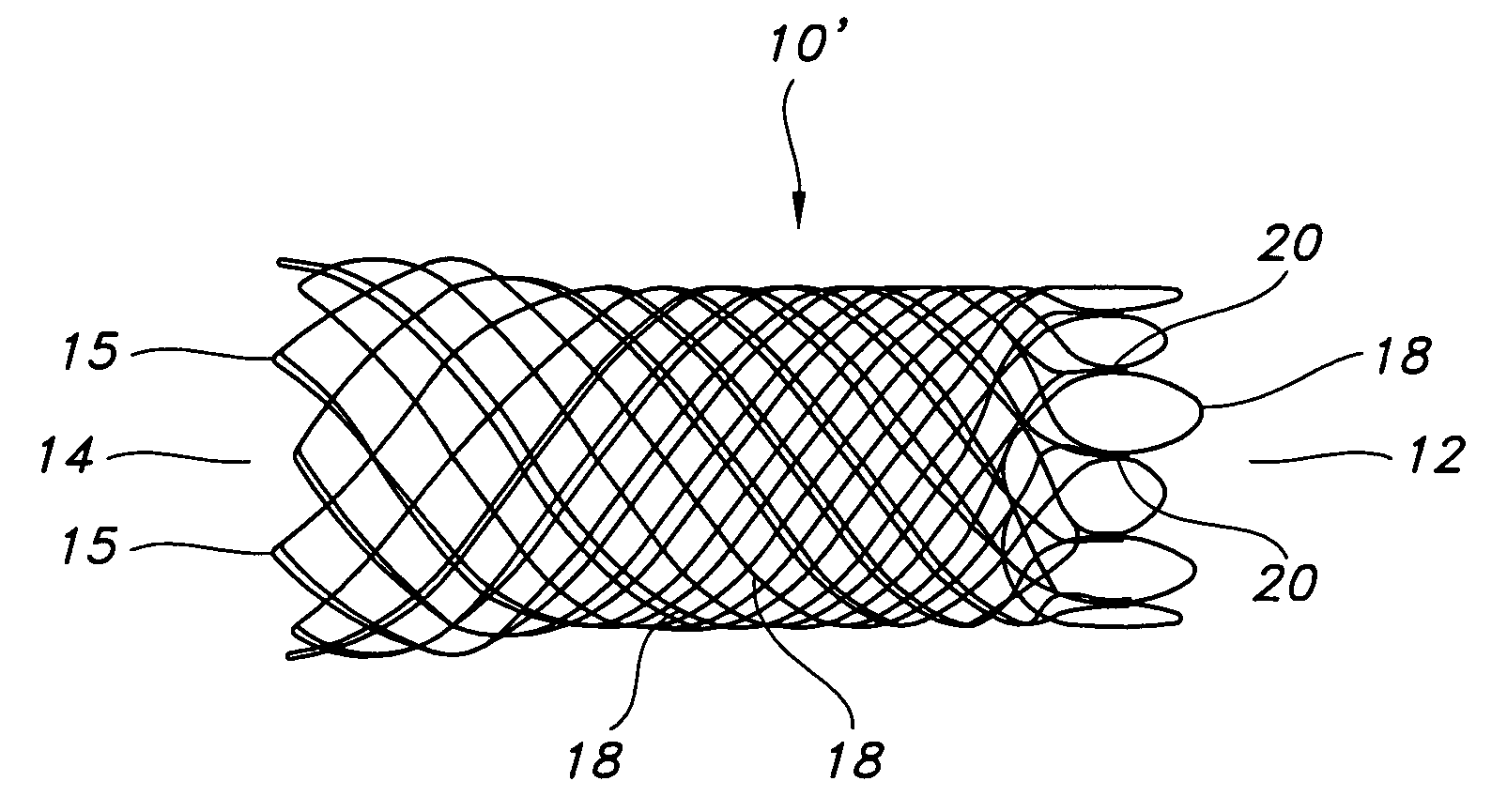
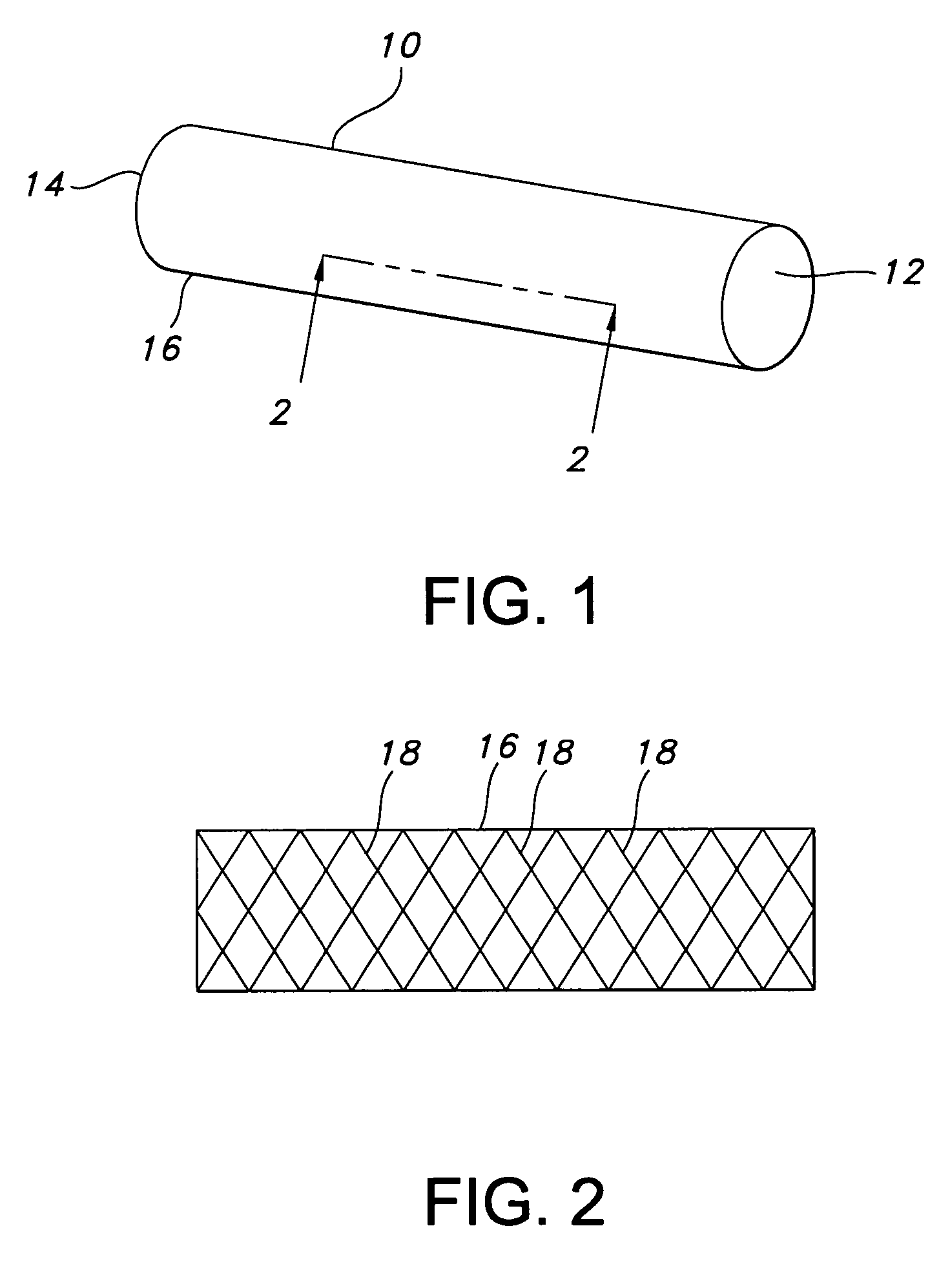
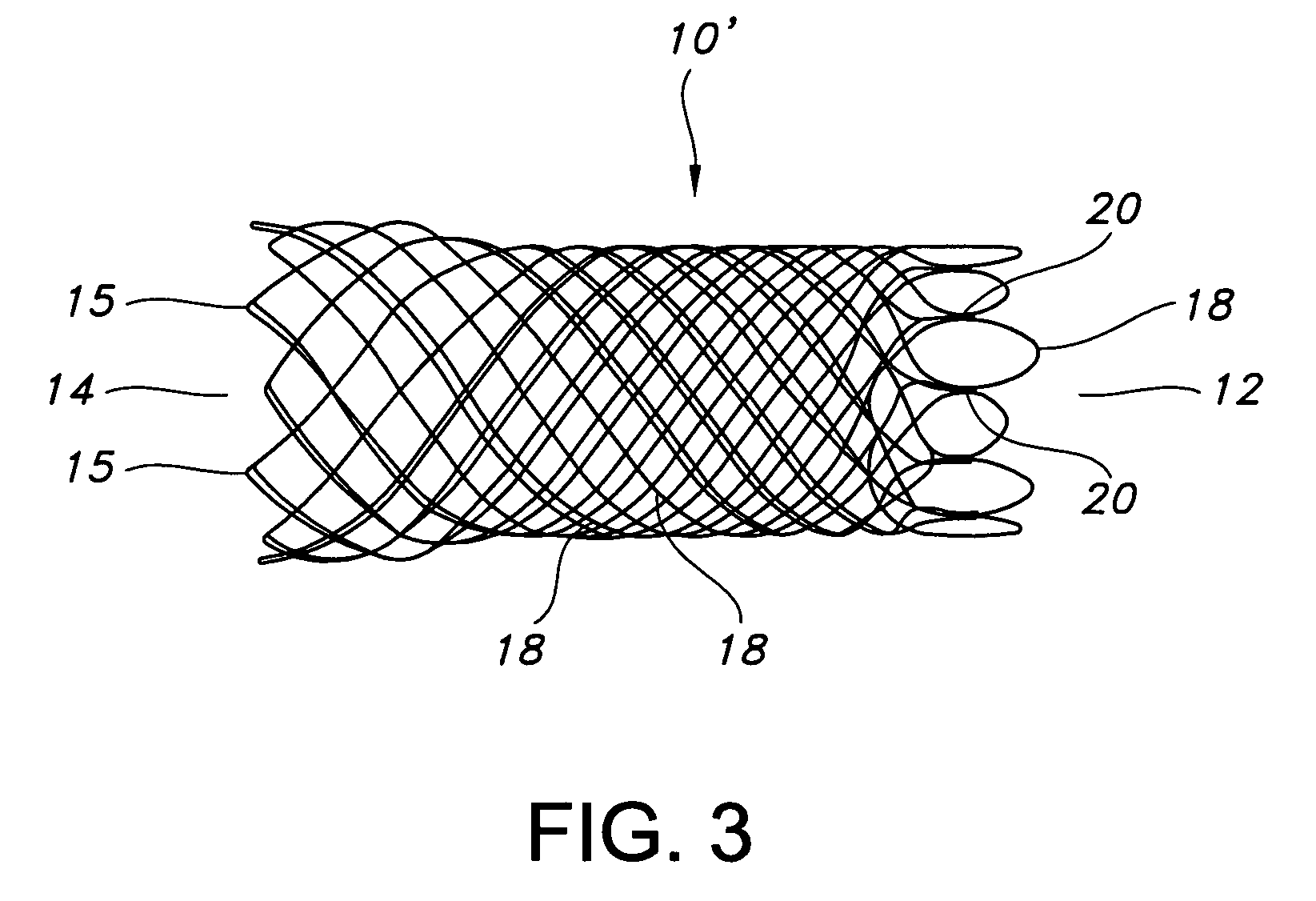
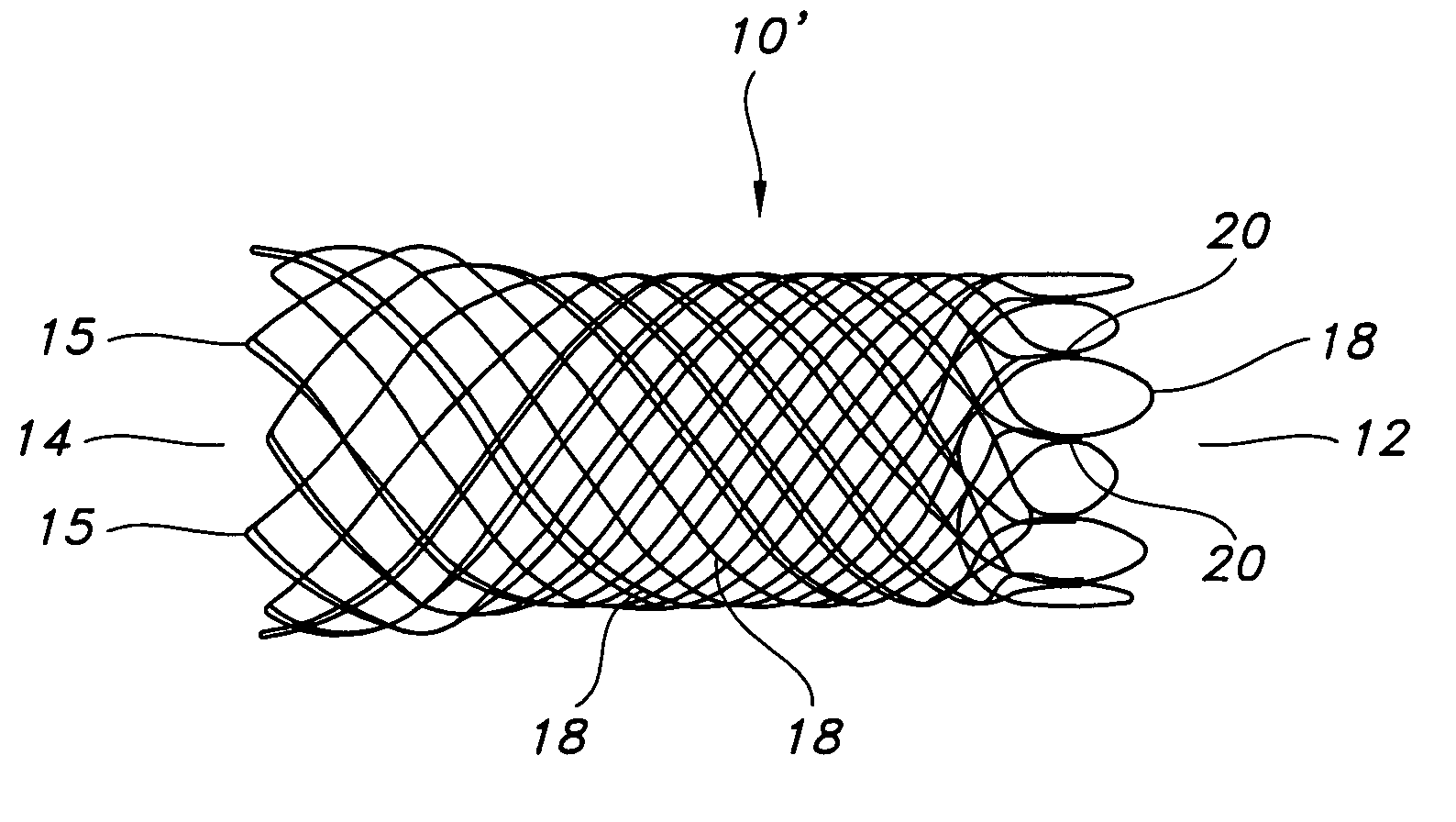
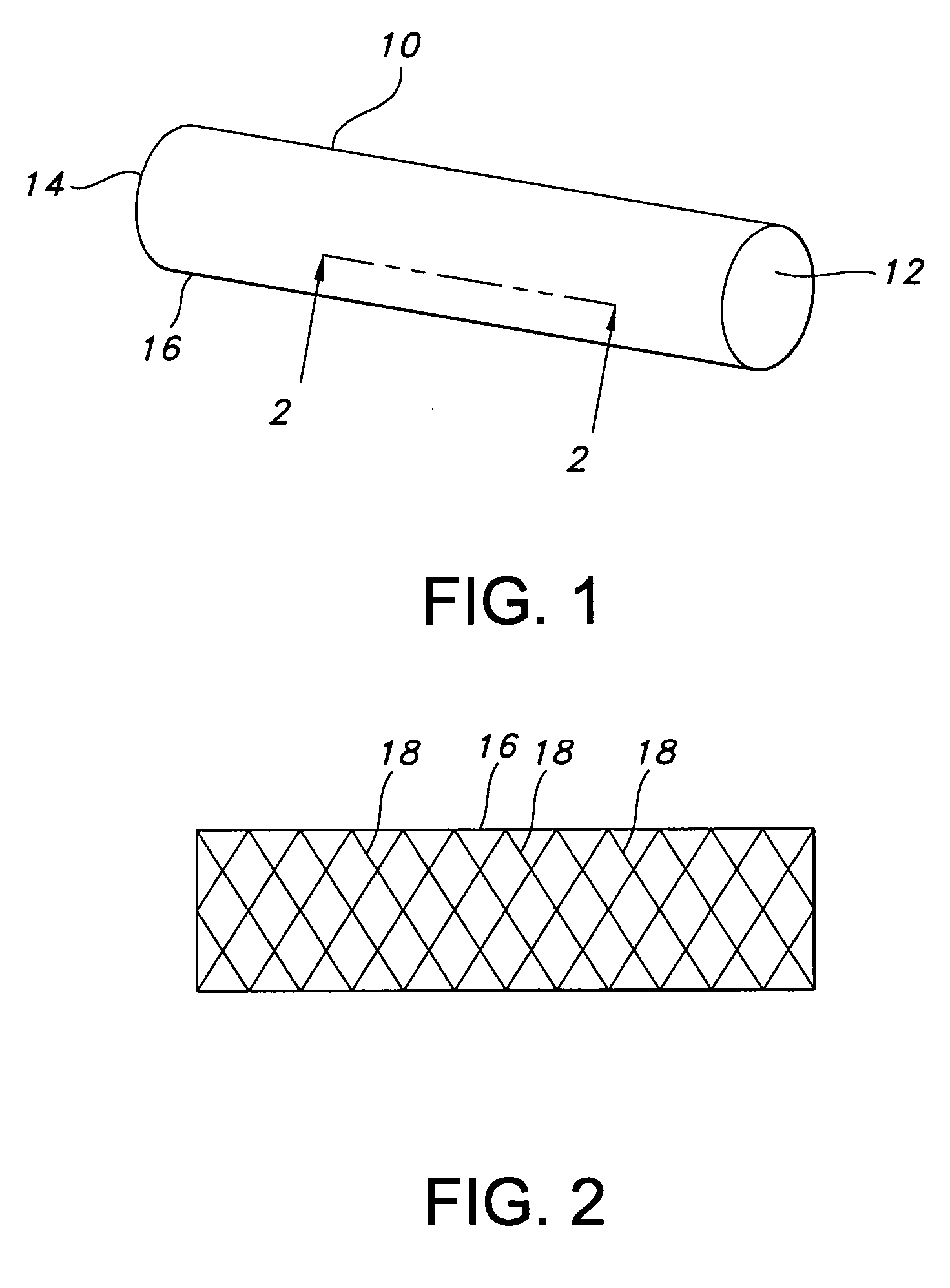
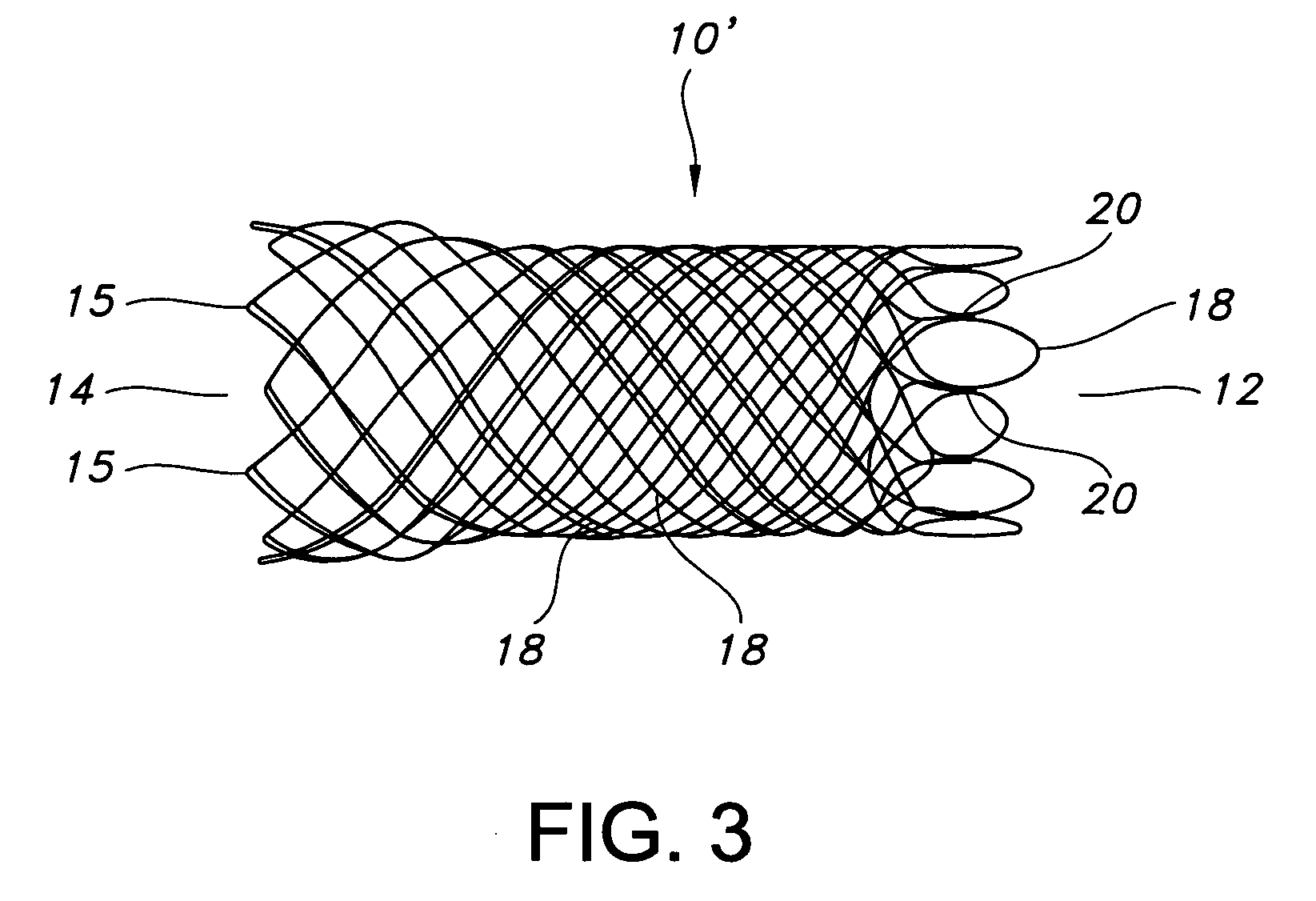
Atraumatic stent with reduced deployment force, method for making the same and method and apparatus for deploying and positioning the stent
An implantable stent includes a plurality of elongate wires braided to form a hollow tubular structure having a tubular wall to define an interior surface and an exterior surface and having opposed open first and second ends, wherein the opposed open first and second ends are atraumatic ends The atraumatic ends of the stent are desirably free of any loose wire ends. The wires include composite wires to enhance visibility of the wires to provide improved external imaging of the wires in the body. The elongate composite wires of the stent may be metallic wires having an outer metallic portion including a first metal, such as nitinol, and an inner metallic core portion including a second metal, which is a radiopaque material, such as gold, barium sulfate, ferritic particles, platinum, platinum-tungsten, palladium, platinum-iridium, rhodium, tantalum or combinations thereof.
Owner:BOSTON SCI SCIMED INC
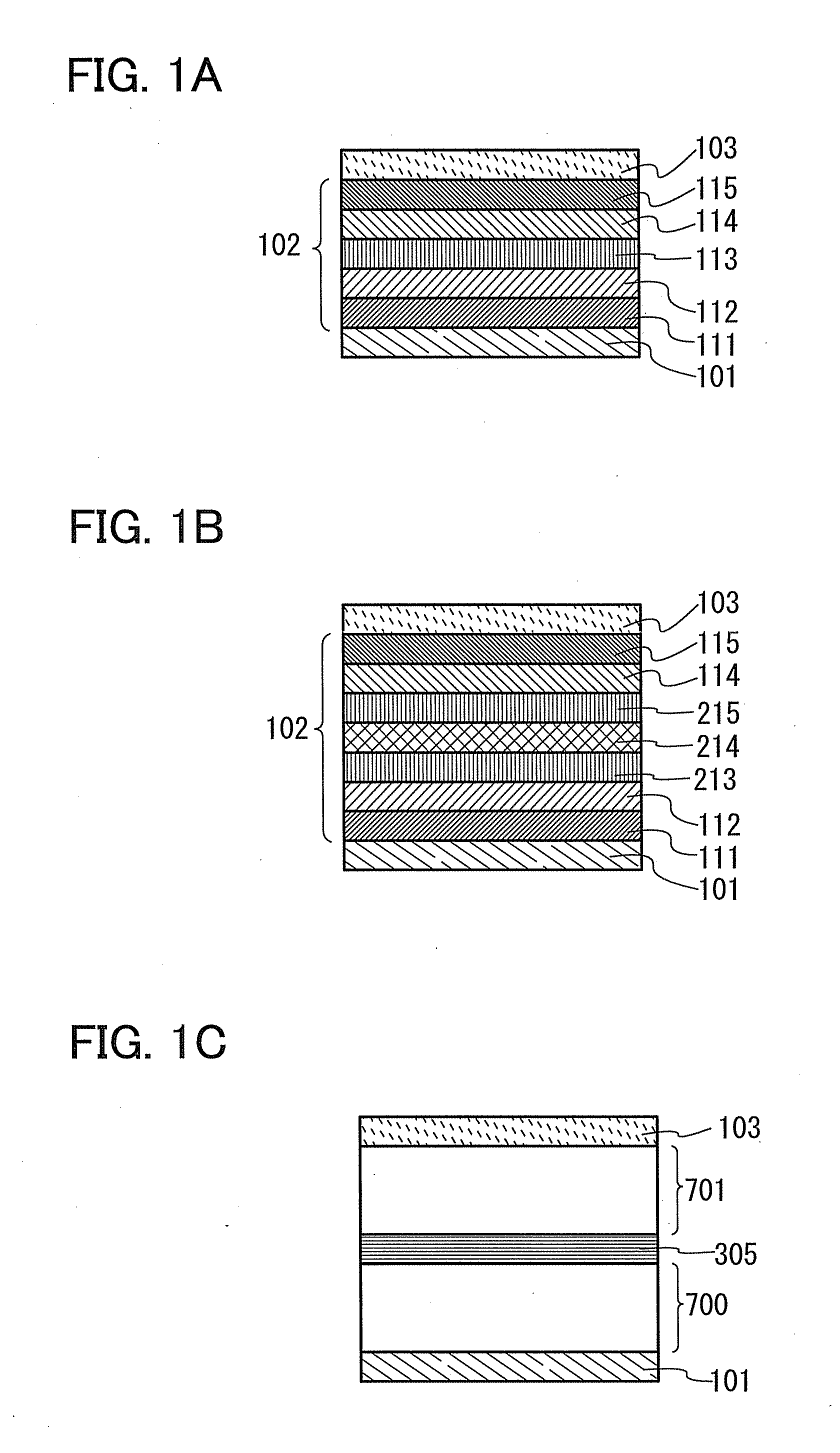
Iridium complex, light-emitting element, display device, electronic device, and lighting device
InactiveUS20160372688A1Improve efficiencyReduce power consumptionIndium organic compoundsSolid-state devicesIridiumSimple Organic Compounds
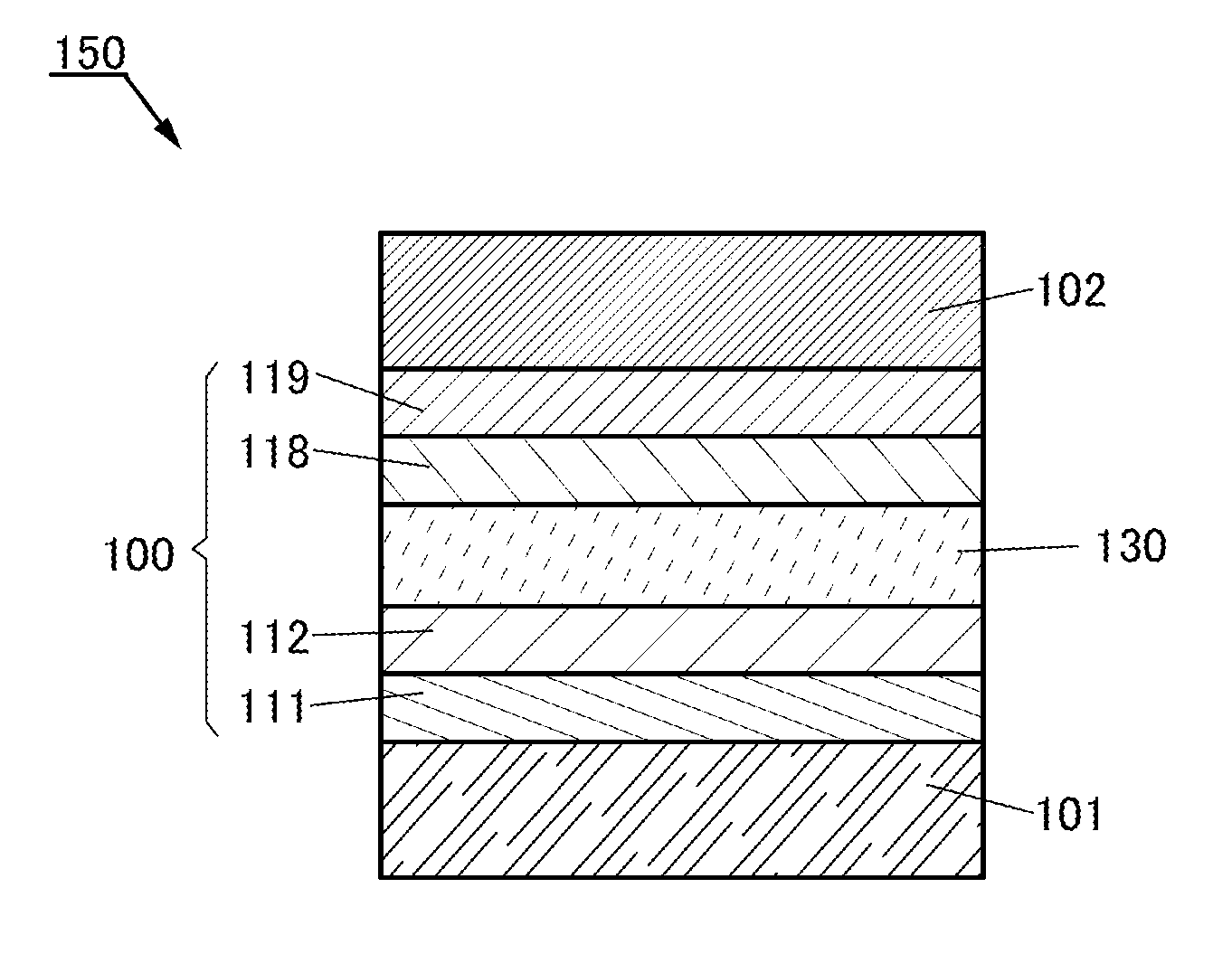
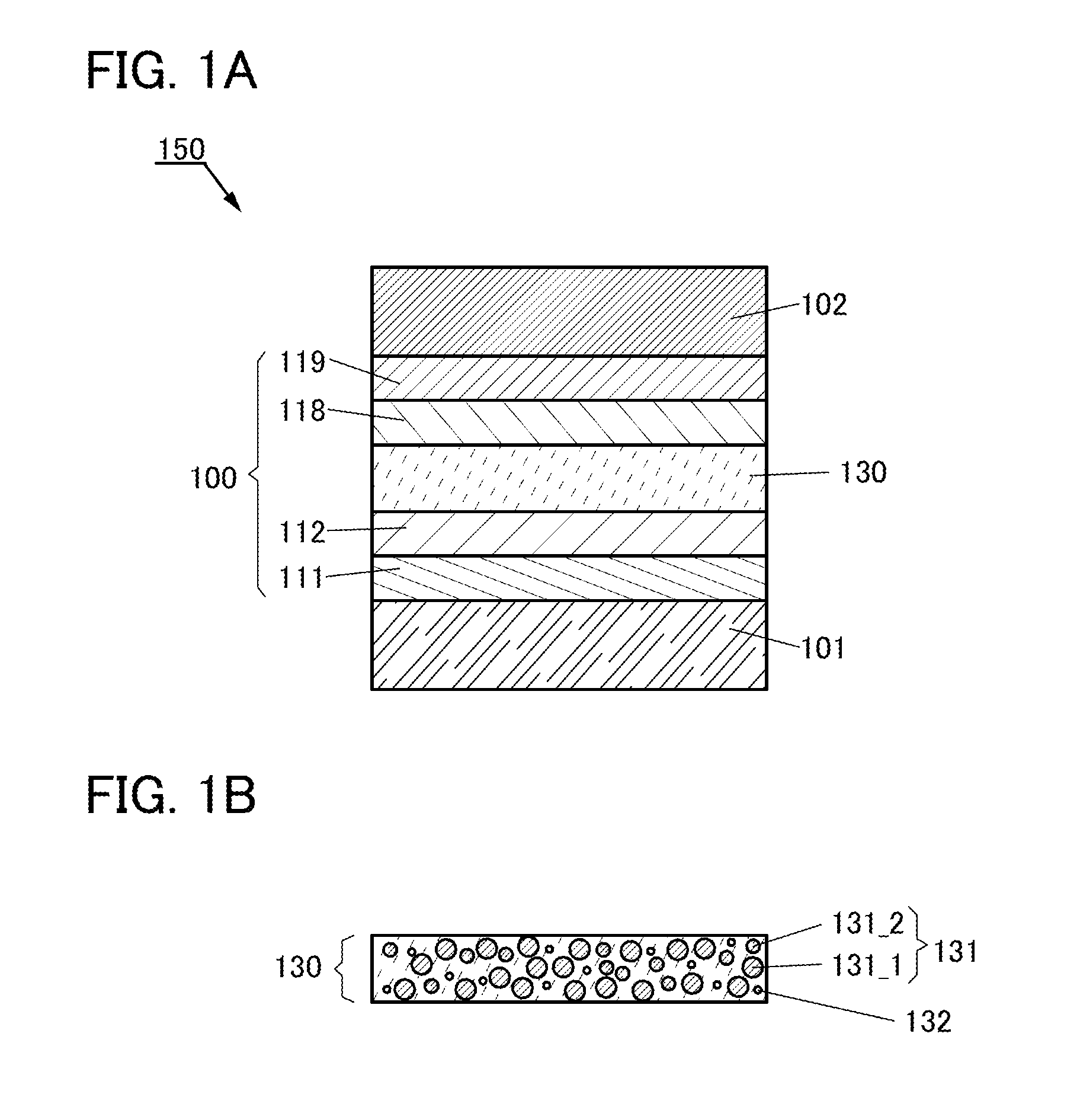
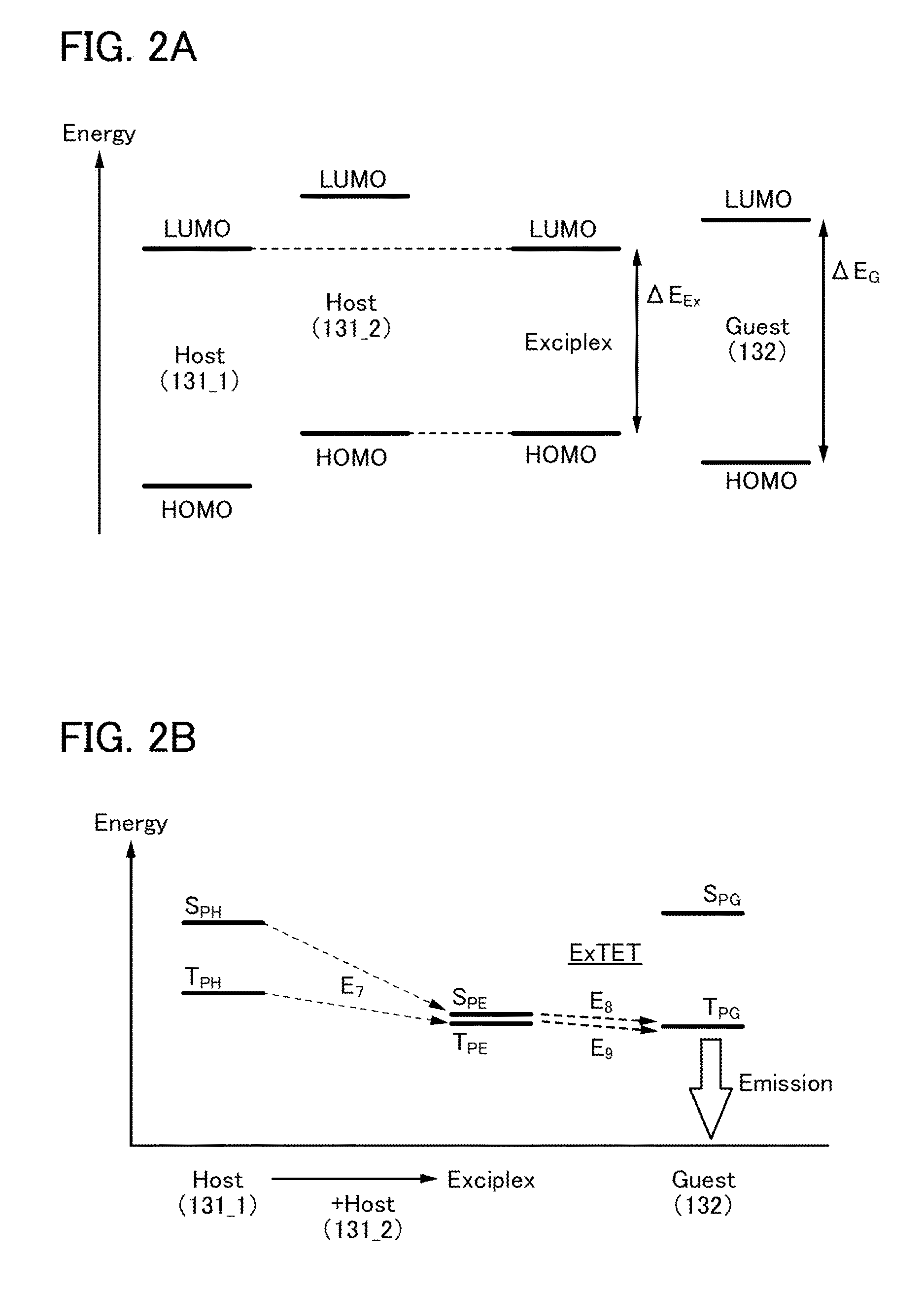
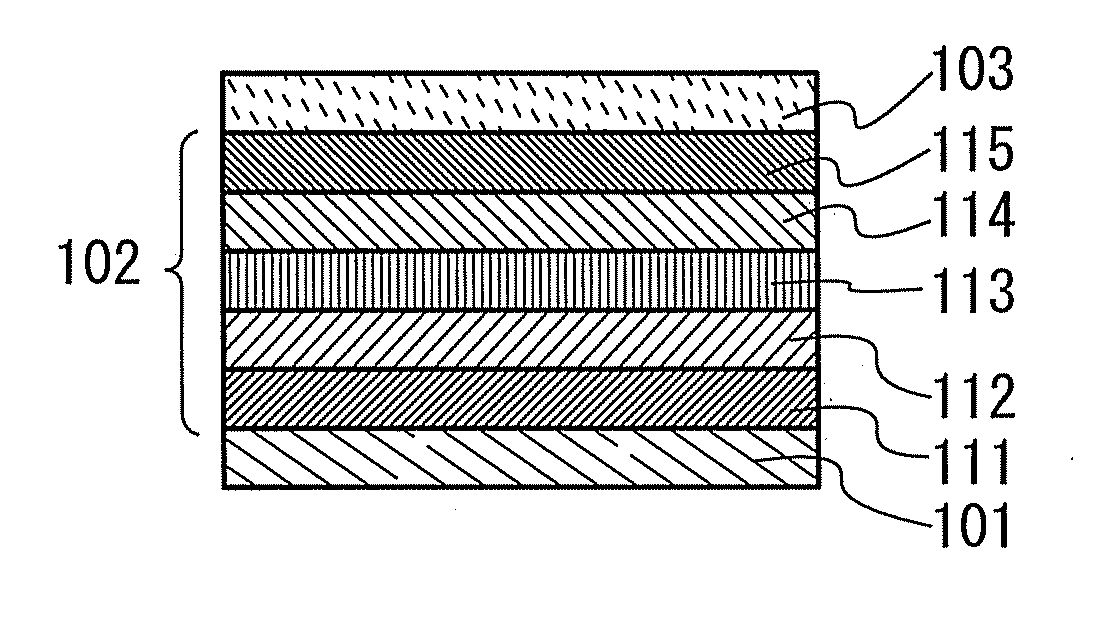
Provided is a light-emitting element with high emission efficiency. The light-emitting element includes a first organic compound, a second organic compound, and a guest material. The LUMO level of the first organic compound is lower than that of the second organic compound, and the HOMO level of the first organic compound is lower than that of the second organic compound. The LUMO level of a guest material is higher than that of the first organic compound, and the HOMO level of the guest material is lower than that of the second organic compound. The guest material has a function of converting triplet excitation energy into light emission. The first organic compound and the second organic compound form an exciplex.
Owner:SEMICON ENERGY LAB CO LTD
Organometallic Complex, Light-Emitting Element, Light-Emitting Device, Electronic Device and Lighting Device
ActiveUS20120098417A1Improve emission efficiencyReduce power consumptionGroup 5/15 element organic compoundsSolid-state devicesIridiumHydrogen
Provided is a novel substance that can emit phosphorescence. Alternatively, provided is a novel substance with high emission efficiency. An organometallic complex in which a 4-arylpyrimidine derivative is a ligand and iridium is a central metal is provided. Specifically, an organometallic complex having a structure represented by a general formula (G1) is provided. In the general formula (G1), R1 represents a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms or a substituted or unsubstituted aryl group having 6 to 10 carbon atoms, R2 represents any of hydrogen, a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms, and a substituted or unsubstituted phenyl group, R3 represents hydrogen or a substituted or unsubstituted alkyl group having 1 to 4 carbon atoms, and Ar1 represents a substituted or unsubstituted arylene group having 6 to 10 carbon atoms.
Owner:SEMICON ENERGY LAB CO LTD
Radiopaque markers for medical devices
InactiveUS20050060025A1High level of radiopacitySufficient radiopacityStentsBlood vesselsIridiumRhenium
An implantable medical device includes a structural body made from a superelastic material and includes one or more marker holders integrally formed on the structural body. Each marker holder is designed to hold a radiopaque marker which has a level of radiopacity greater than the superelastic material. The radiopaque marker can be made from a nickel-titanium alloy which includes a ternary element. The ternary element can be selected from the group of elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, and hafnium. In one form, the marker holder includes a pair of projecting fingers connected together at a notched region to cooperatively create a particular-shaped opening. This opening, in turn, is adapted to receive a similarly shaped portion formed on the radiopaque marker. In one form, the radiopaque marker includes an inner core which is partially, or completely, encased by an outer layer. This inner core can be made from a highly radiopaque material while the outer layer is formed from a material that is easier to weld to the marker.
Owner:ABBOTT VASCULAR SOLUTIONS
High-molecular compounds and organic luminescent devices
InactiveUS20030186080A1Improve efficiencyImprove stabilityDischarge tube luminescnet screensElectroluminescent light sourcesIridiumPolymer science
A main chain-type or side chain-type polymeric compound having a structure wherein at least one metal complex segment having a plurality ligands is introduced into a main chain or a side chain is provided. In the case where the polymeric compound is the main chain-type polymeric compound, the metal complex segment has at least one ligand constituting a polymer main chain of the polymeric compound and having a carbon atom and oxygen atom bonded to a metal atom. On the other hand, in the case where the polymeric compound is the side chain-type polymeric compound, a polymer main chain thereof has a conjugated structure, preferably a conjugated double bond. A ligand for the polymeric compound includes a chain or cyclic ligand, of which a bidentate ligand having an organic cyclic structure is preferred, and the ligand has at least one carbon atom or oxygen atom and is bonded to a center metal atom, preferably iridium, via the carbon atom or oxygen atom. In a case of forming a luminescence layer by using the polymeric compound as a luminescent material, a resultant organic luminescence device is less liable to cause a concentration extinction and is a high-luminescence efficiency device excellent in stability.
Owner:CANON KK
Processes for making ethanol from acetic acid
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
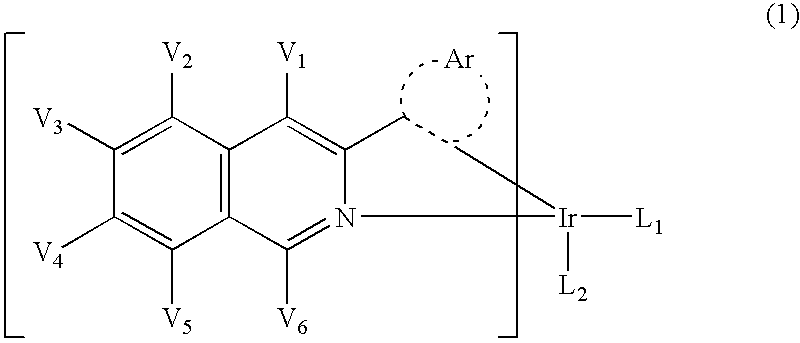
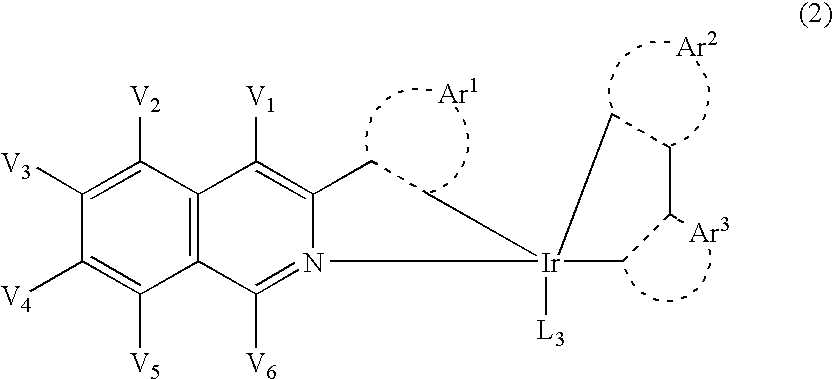
Light-emitting material comprising orthometalated iridium complex, light-emitting device, high efficiency red light-emitting device, and novel iridium complex
InactiveUS7238437B2Group 5/15 element organic compoundsSolid-state devicesIridiumLight emitting device
Owner:UDC IRELAND
Light-Emitting Element, Light-Emitting Device, Electronic Device, Lighting Device, and Novel Organic Compound
ActiveUS20130048964A1Easy transferHigh luminance regionOrganic chemistrySolid-state devicesIridiumOrganic compound
A light-emitting element includes an EL layer between a pair of electrodes. The EL layer contains a first compound and a second compound. The first compound is a phosphorescent iridium metal complex having a LUMO level of greater than or equal to −3.5 eV and less than or equal to −2.5 eV, and the second compound is an organic compound having a pyrimidine skeleton. The light-emitting element includes an EL layer between a pair of electrodes. The EL layer contains a first compound and a second compound. The first compound is a phosphorescent iridium metal complex having a diazine skeleton, and the second compound is an organic compound having a pyrimidine skeleton.
Owner:SEMICON ENERGY LAB CO LTD
Magnetic signal reproduction system and magnetic signal reproduction method
InactiveUS20090027812A1Stable reproduction propertyDeterioration of reproduction propertyManufacture unitary devices of plural headsRecord information storageIridiumAlloy
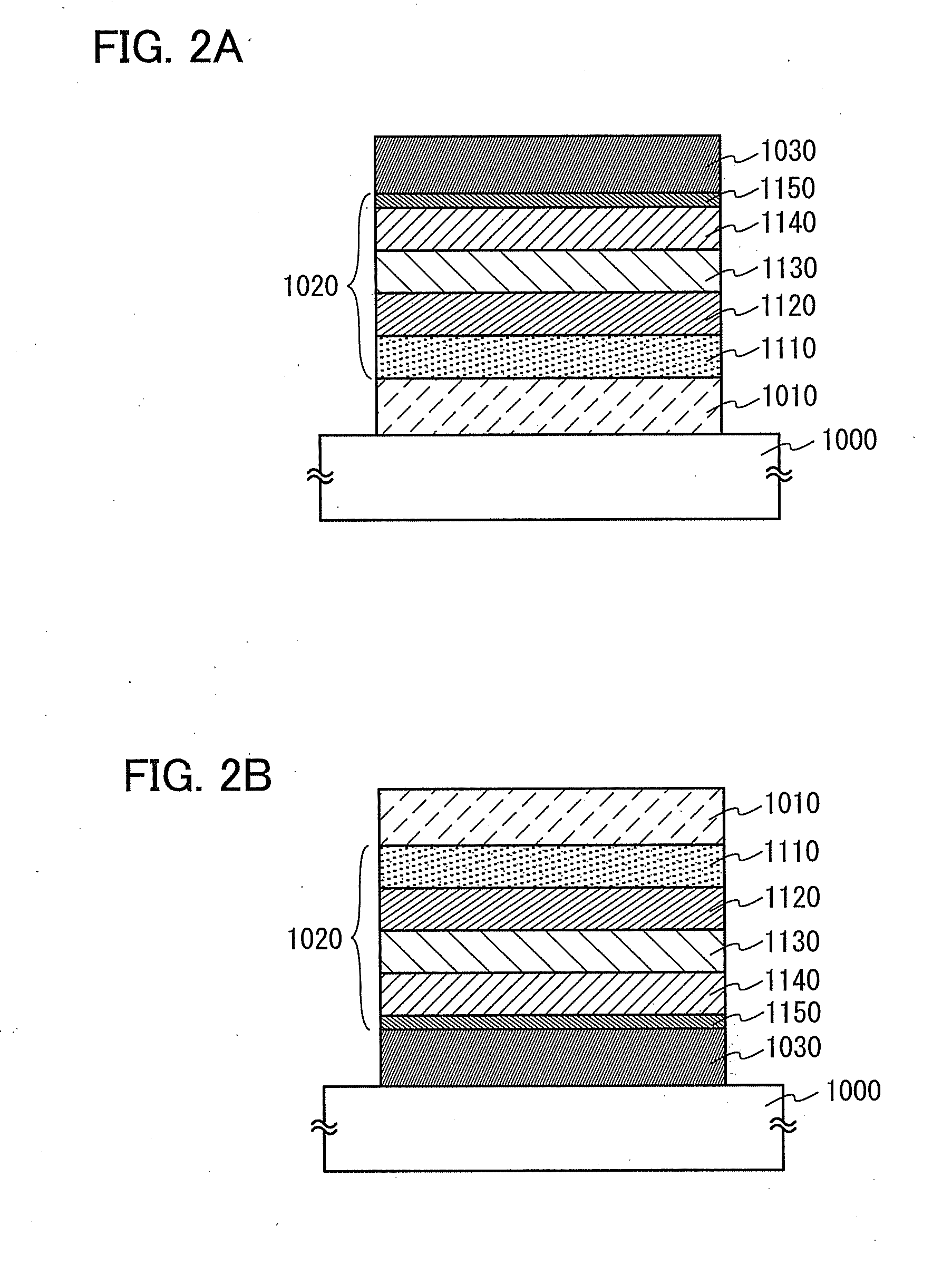
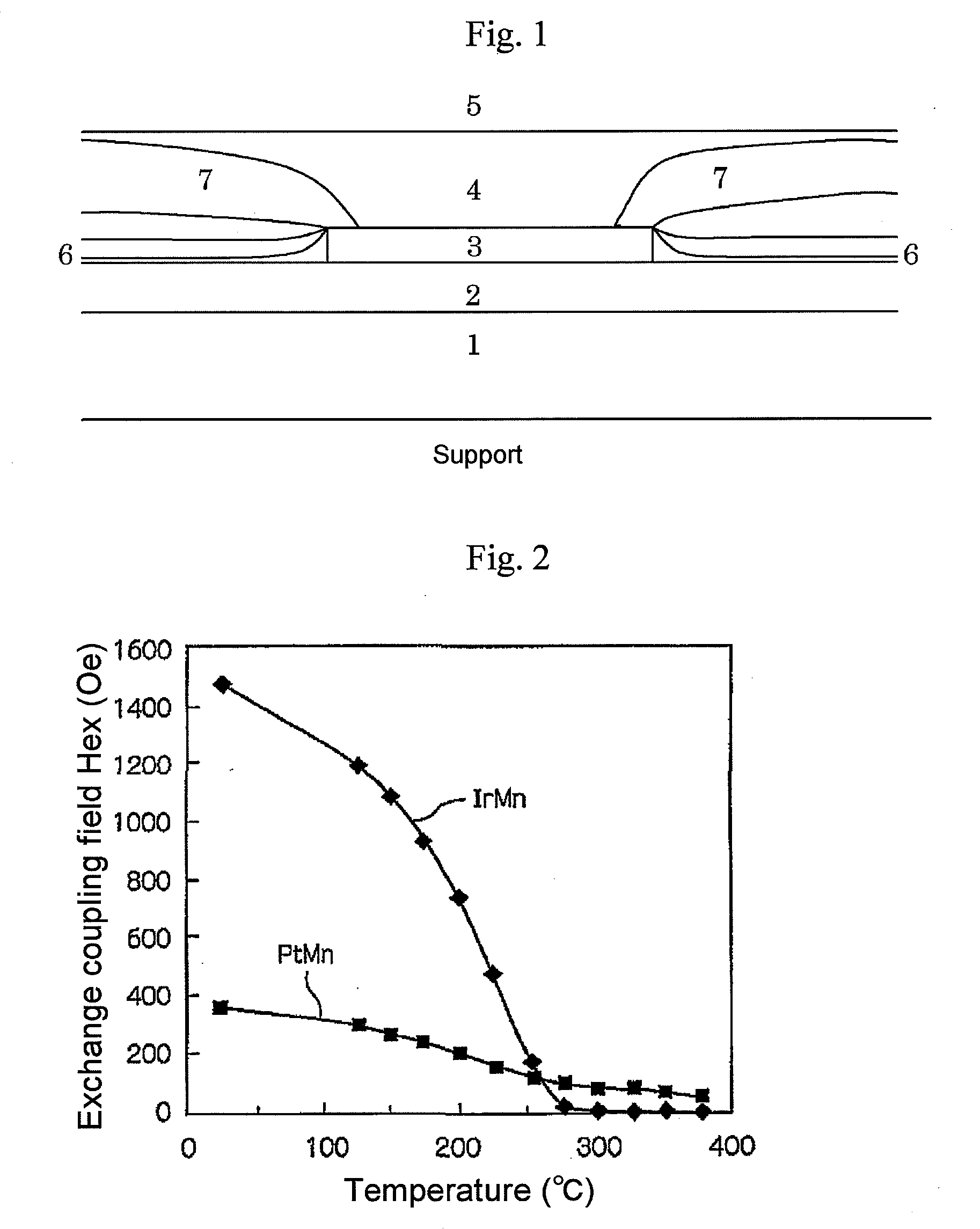
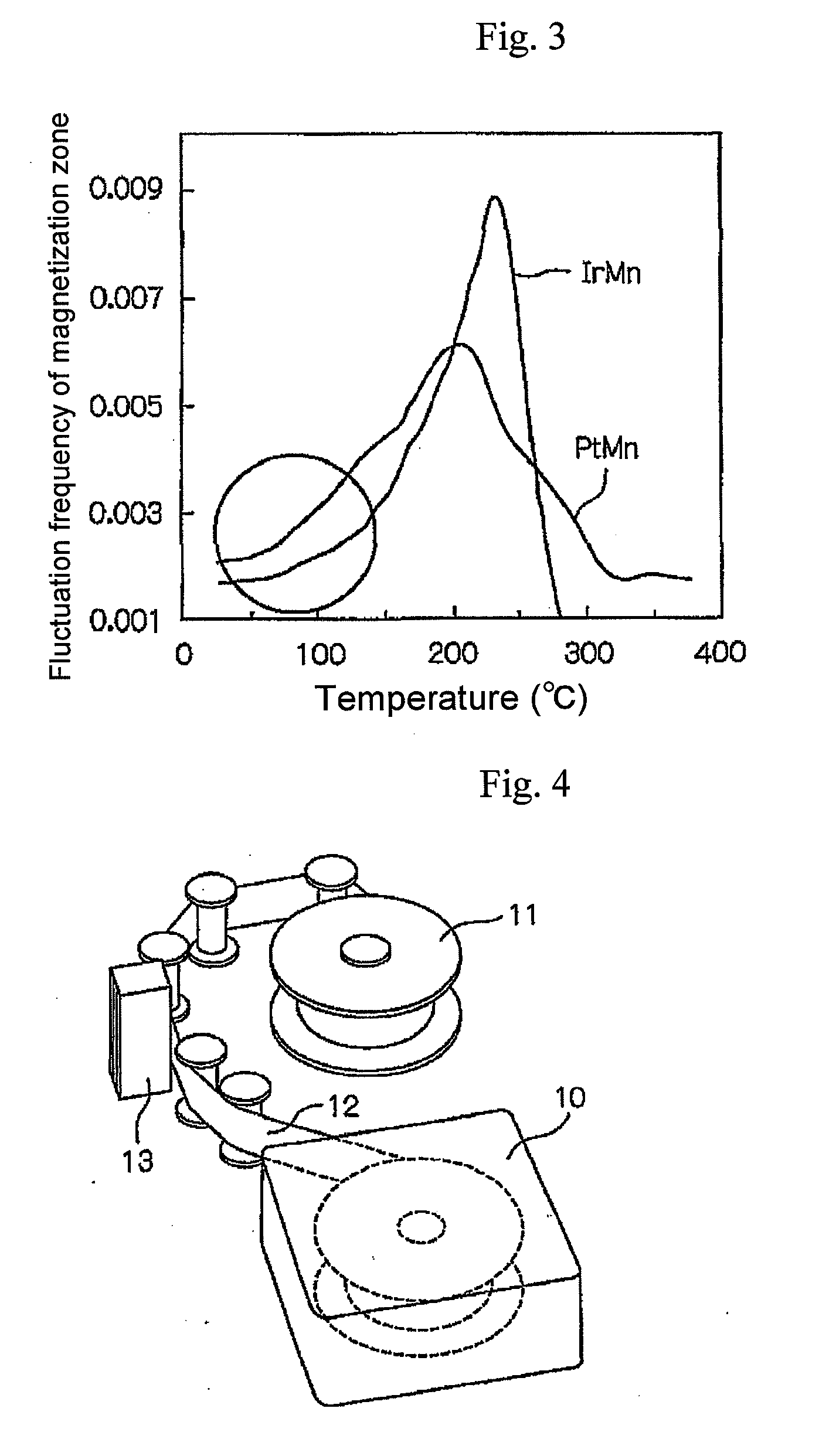
The magnetic signal reproduction system comprises a magnetic recording medium comprising a magnetic layer comprising a ferromagnetic powder and a binder on a nonmagnetic support and a reproduction head, wherein a number of protrusions equal to or greater than 10 nm in height on the magnetic layer surface, as measured by an atomic force microscope, ranges from 50 to 2500 / 10,000 μm2, a quantity of lubricant on the magnetic layer surface, denoted as a surface lubricant index, ranges from 0.5 to 5.0, a surface abrasive occupancy of the magnetic layer ranges from 2 to 20 percent, the reproduction head is a magnetoresistive magnetic head comprising a spin-valve layer, the spin-valve layer comprises a magnetization free layer, a magnetization pinned layer and an antiferromagnetic layer, and the antiferromagnetic layer is comprised of alloy comprising iridium and manganese, and the reproduction head comes in sliding contact with the magnetic recording medium during signal reproduction.
Owner:FUJIFILM CORP +1
Metal alloys for the reflective or the semi-reflective layer of an optical storage medium
InactiveUS6280811B1Moderate to high reflectivityImprove corrosion resistancePhotosensitive materialsRadiation applicationsIridiumMagnesium
A copper-based or silver-based alloy thin film is provided for the highly reflective or semi-reflective layer of optical discs. Alloy additions to silver include gold, palladium, copper, rhodium, ruthenium, osmium, iridium, beryllium and platinum. Alloy additions to copper include silver, cadmium, gold, magnesium, aluminum, beryllium, zirconium, and nickel. These alloys have moderate to high reflectivity and reasonable corrosion resistance in the ambient environment.
Owner:TARGET TECH
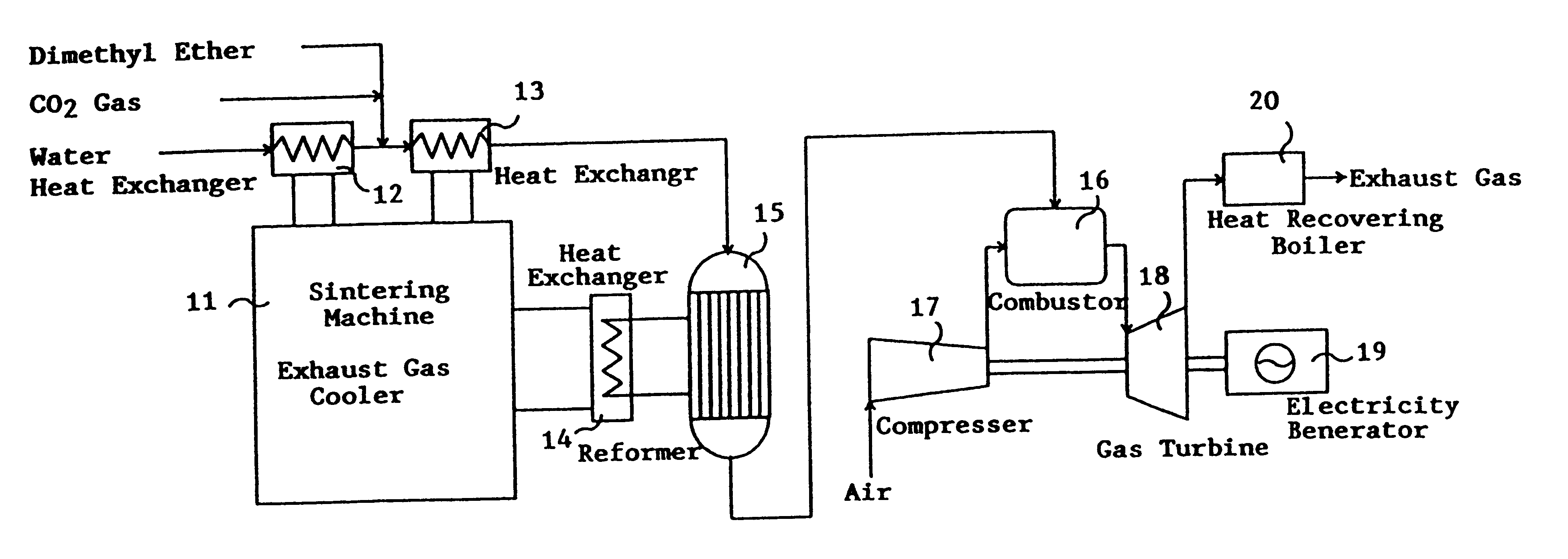
Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas
InactiveUS6361757B1Produce hydrogenEfficient productionIron compoundsCobalt compoundsIridiumForming gas
This invention provides a catalyst for producing hydrogen gas from a mixed gas comprising dimethyl ether and water vapor or carbon dioxide gas, which comprises copper, iron, cobalt, palladium, iridium, platinum, rhodium, or nickel as an active component, and a method of producing synthesis gas or hydrogen gas in a high yield at a low temperature. By using the catalyst, a fuel cell, electricity generation, reduction of iron ore and the like can be carried out.
Owner:NIPPON KOKAN KK
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
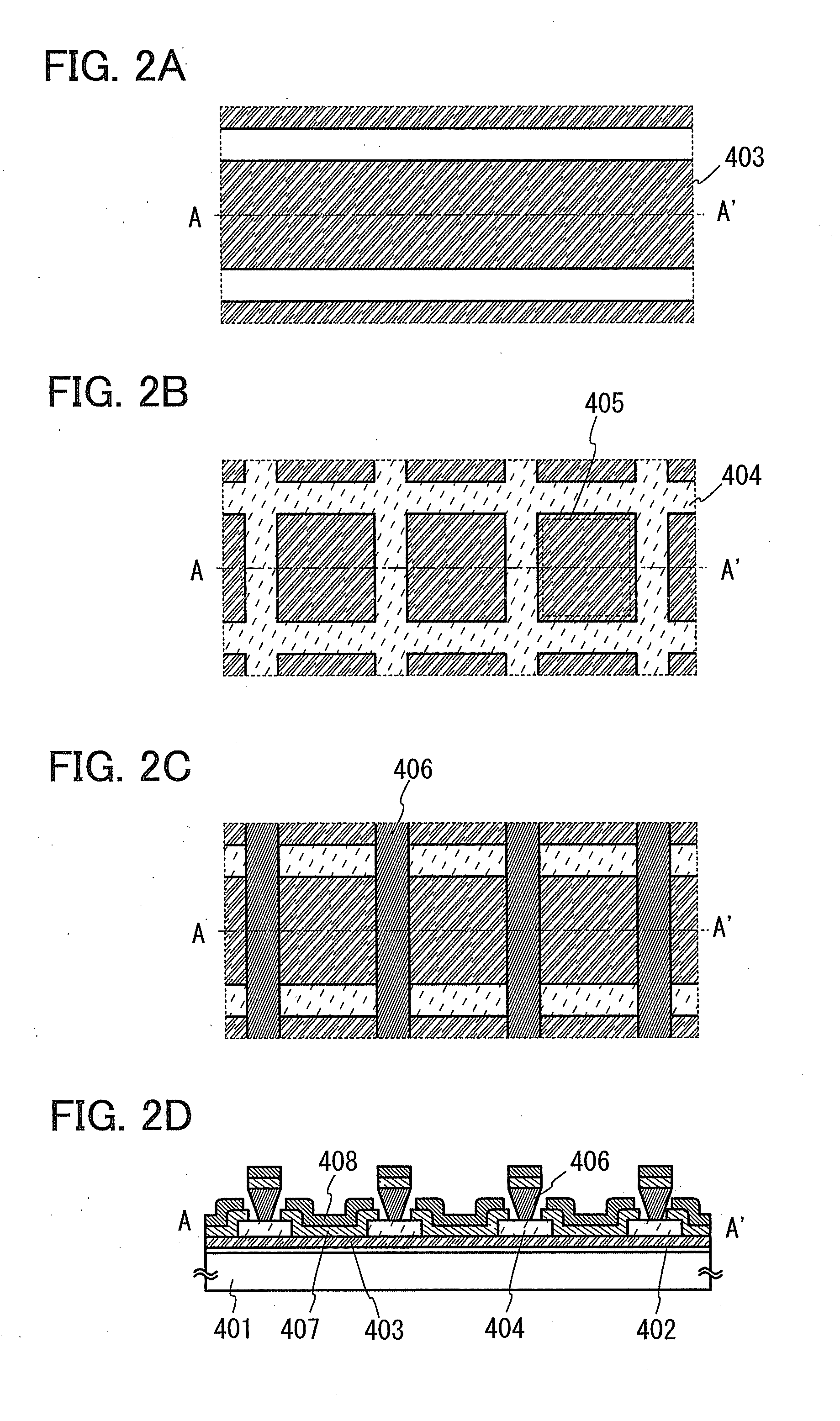
Light-emitting layer and light-emitting element
ActiveUS20120217486A1Increase energy levelInhibition transitionSolid-state devicesSemiconductor/solid-state device manufacturingIridiumExcited state
To provide a highly efficient organic light-emitting element. An extremely thin layer (a monomolecular film or the like) containing an organic light-emitting material such as an iridium complex is provided between a layer of an n-type organic material (an organic material having a high electron-transport property) and a layer of a p-type organic material (an organic material having a high hole-transport property). In a structure described above, in a layer of the organic light-emitting material, electrons are injected from the LUMO of the n-type organic material to the LUMO of the organic light-emitting material, and holes are injected from the HOMO of the p-type organic material to the HOMO of the organic light-emitting material, whereby the organic light-emitting material is brought into an excited state and emits light.
Owner:SEMICON ENERGY LAB CO LTD
Barriers for polymer-coated implantable medical devices and methods for making the same
InactiveUS6953560B1Reduce and prevent and inflammationReduce and prevent proliferationStentsSurgeryHafniumPt element
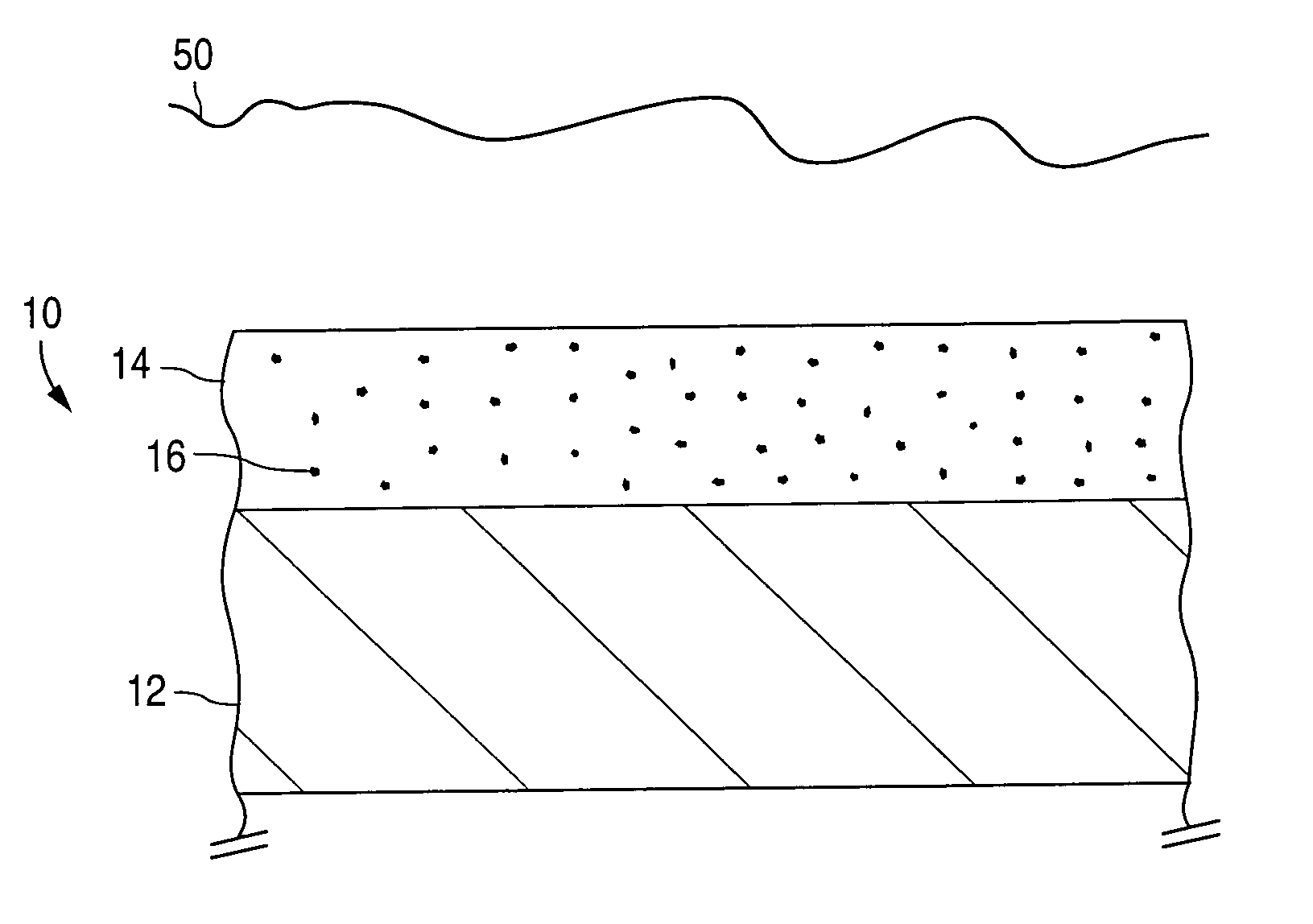
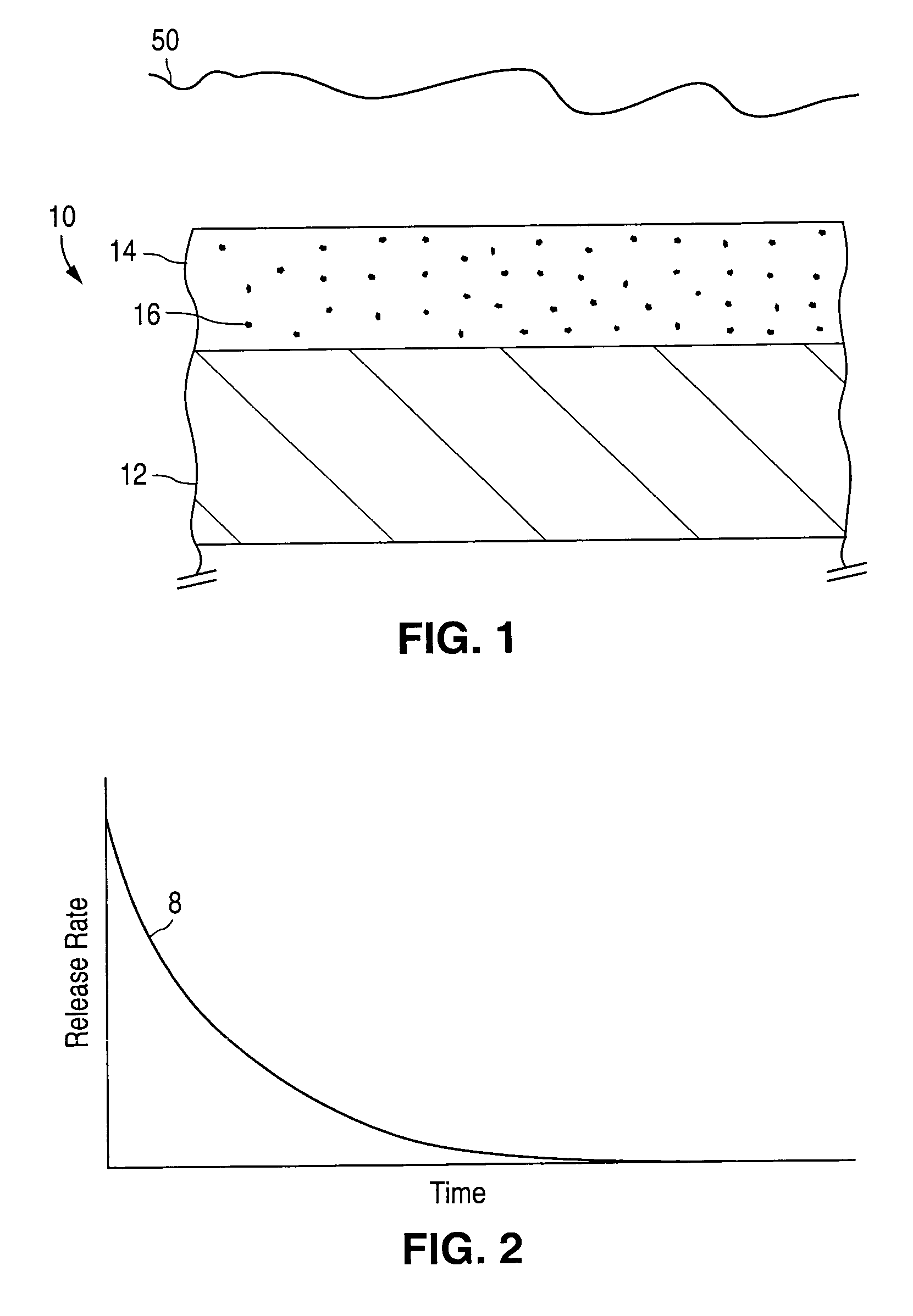
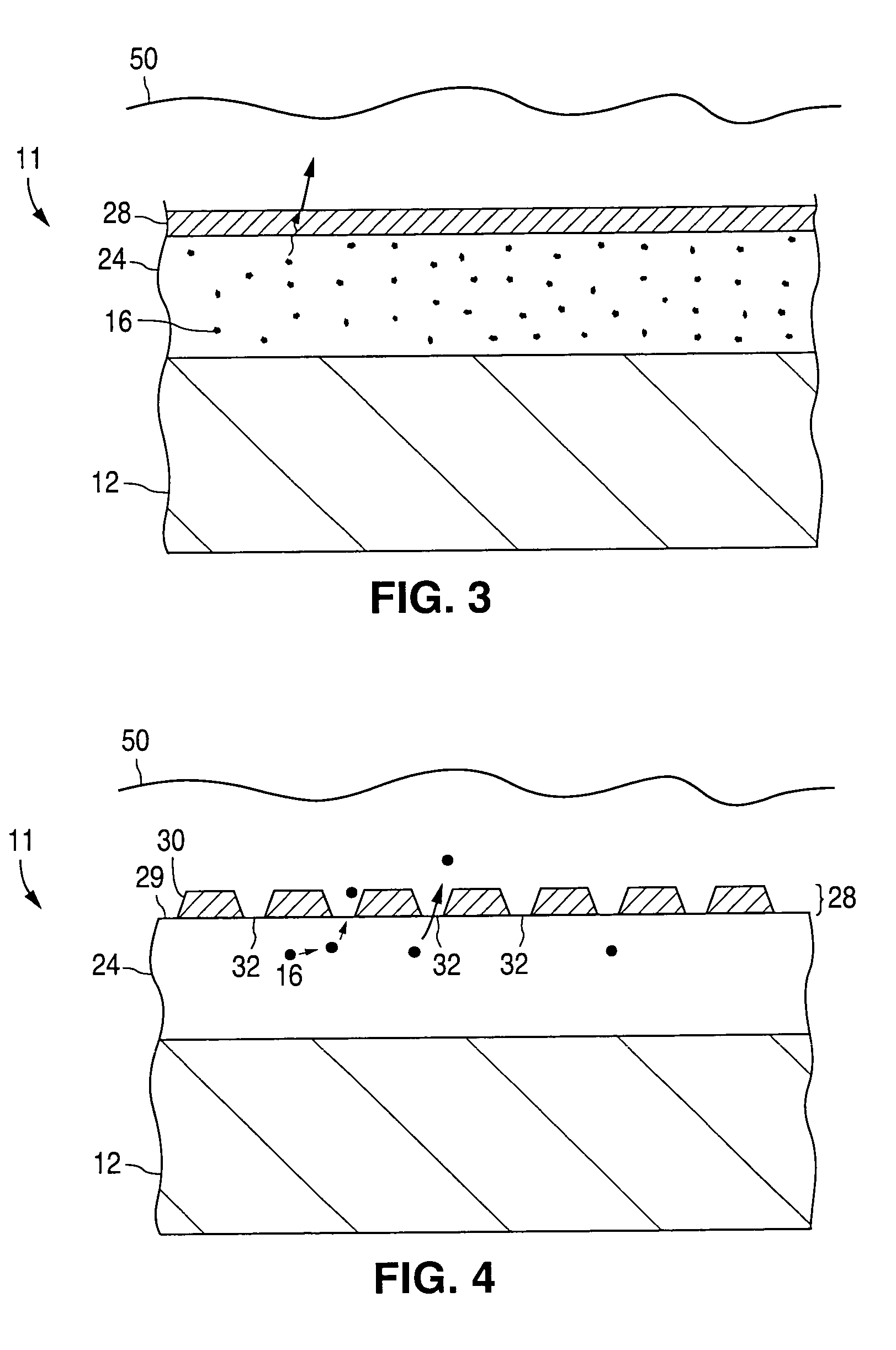
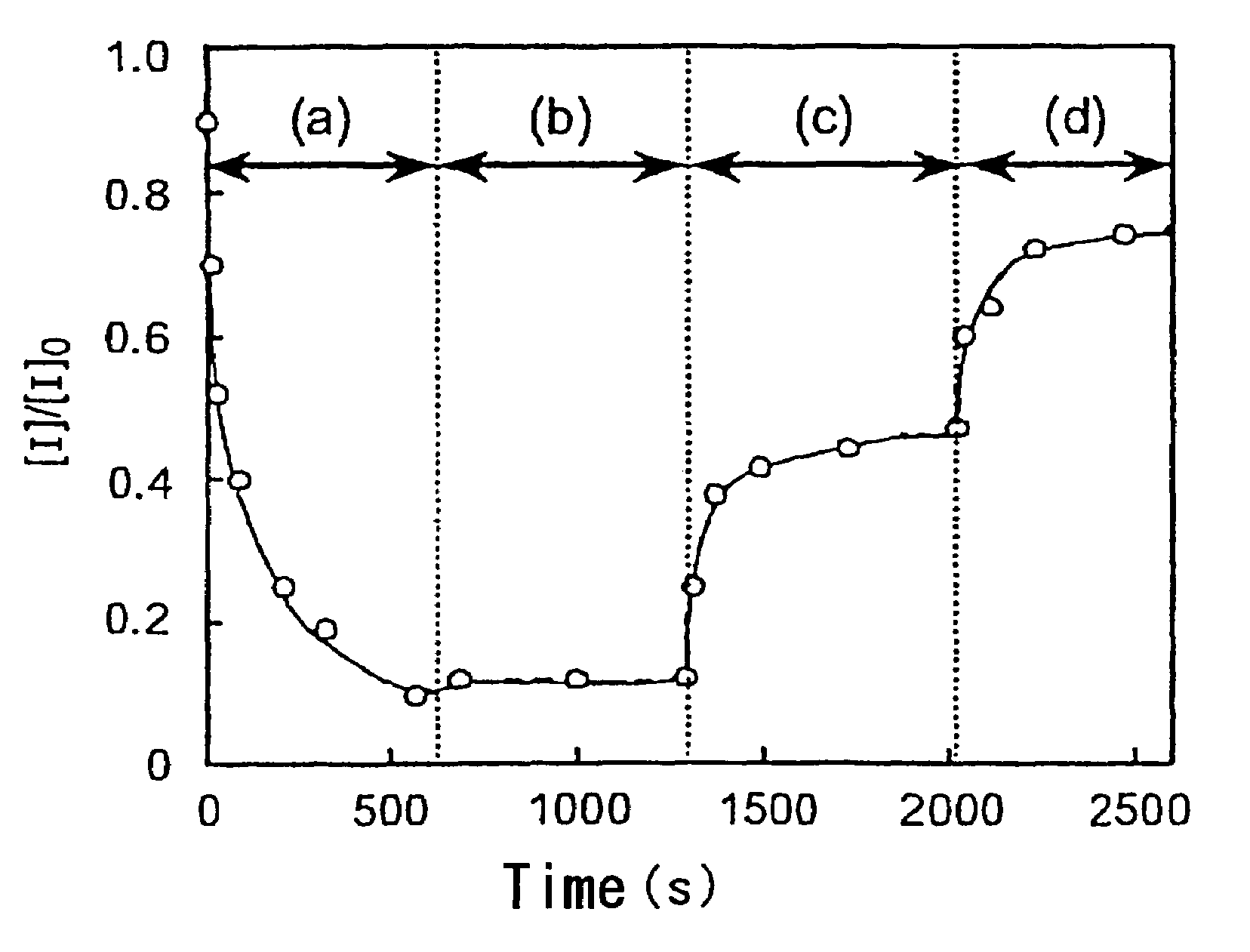
An implantable medical device and methods for making the implantable medical device are disclosed. The implantable medical device includes a substrate. At least a portion of the substrate is coated with a first layer including a polymer containing a drug. A barrier overlies the first layer. The barrier significantly reduces the rate of release of the drug from the polymer, thereby sustaining release of the drug from the medical device for a longer time.The barrier may be a homogeneous layer overlying the first layer, or a number of discrete deposits over the first layer. Alternatively, the barrier may be intermixed with an outer portion of the first layer. The barrier material is biocompatible, and typically has a thickness ranging from about 50 angstroms to about 20,000 microns. Suitable materials for the barrier include, but are not limited to, inorganic compounds, such as inorganic silicides, oxides, nitrides, carbides, as well as pure metals such as aluminum, chromium, gold, hafnium, iridium, niobium, palladium, platinum, tantalum, titanium, tungsten, zirconium, and alloys of these metals. The barriers disclosed may be applied to the first layer by several techniques, depending on the material being applied. Exemplary deposition techniques include physical vapor deposition, alkoxide hydrolysis, and electroless plating.The implantable device may be a stent or a graft, among other possibilities.
Owner:ABBOTT CARDIOVASCULAR
Photoacid generator
InactiveUS7550246B2Improve solubilitySensitive highPhotosensitive materialsGroup 8/9/10/18 element organic compoundsIridiumPhotoacid
Owner:JAPAN SCI & TECH CORP
Atraumatic stent with reduced deployment force, method for making the same and method and apparatus for deploying and positioning the stent
An implantable stent includes a plurality of elongate wires braided to form a hollow tubular structure having a tubular wall to define an interior surface and an exterior surface and having opposed open first and second ends, wherein the opposed open first and second ends are atraumatic ends The atraumatic ends of the stent are desirably free of any loose wire ends. The wires include composite wires to enhance visibility of the wires to provide improved external imaging of the wires in the body. The elongate composite wires of the stent may be metallic wires having an outer metallic portion including a first metal, such as nitinol, and an inner metallic core portion including a second metal, which is a radiopaque material, such as gold, barium sulfate, ferritic particles, platinum, platinum-tungsten, palladium, platinum-iridium, rhodium, tantalum or combinations thereof.
Owner:BOSTON SCI SCIMED INC
Organometallic complexes as phosphorescent emitters in organic LEDS
InactiveUS7291406B2Easy to combineDeterioration in emission qualityOrganic chemistryDischarge tube luminescnet screensIridiumPt element
Organic light emitting devices are described wherein the emissive layer comprises a host material containing an emissive molecule, which molecule is adapted to luminesce when a voltage is applied across the heterostructure, and the emissive molecule is selected from the group of phosphorescent organometallic complexes, including cyclometallated platinum, iridium and osmium complexes. The organic light emitting devices optionally contain an exciton blocking layer. Furthermore, improved electroluminescent efficiency in organic light emitting devices is obtained with an emitter layer comprising organometallic complexes of transition metals of formula L2MX, wherein L and X are distinct bidentate ligands. Compounds of this formula can be synthesized more facilely than in previous approaches and synthetic options allow insertion of fluorescent molecules into a phosphorescent complex, ligands to fine tune the color of emission, and ligands to trap carriers.
Owner:THE TRUSTEES OF PRINCETON UNIV +1
Cyclometallated iridium carbene complexes for use as hosts
An organic light emitting device is provided. The device has an anode, a cathode and an organic layer disposed between the anode and the cathode. The organic layer comprises a host and a dopant, and the host comprises a compound having at least one carbene atom coordinated to iridium, and the compound has the structure:
Owner:UNIVERSAL DISPLAY +1
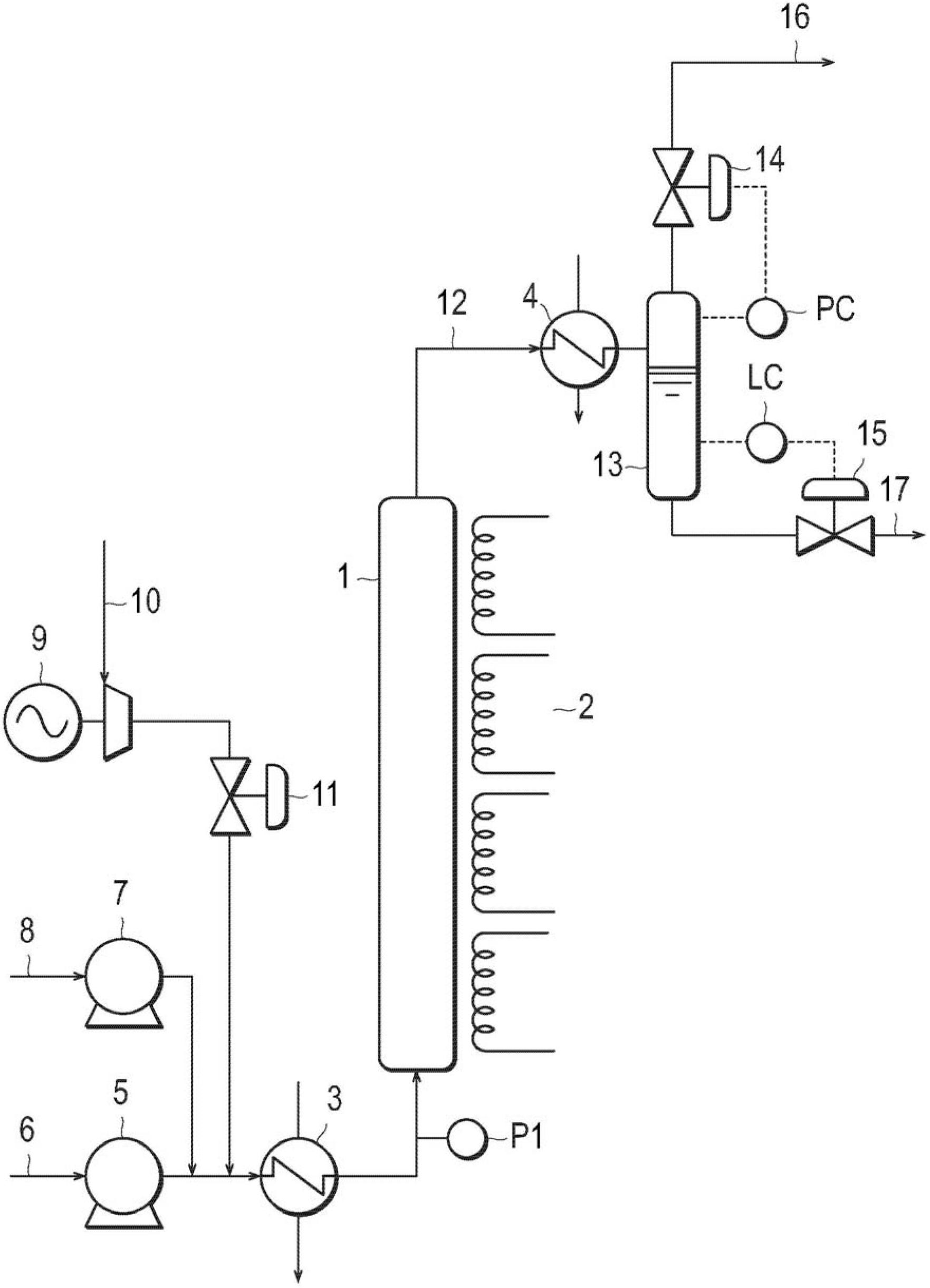
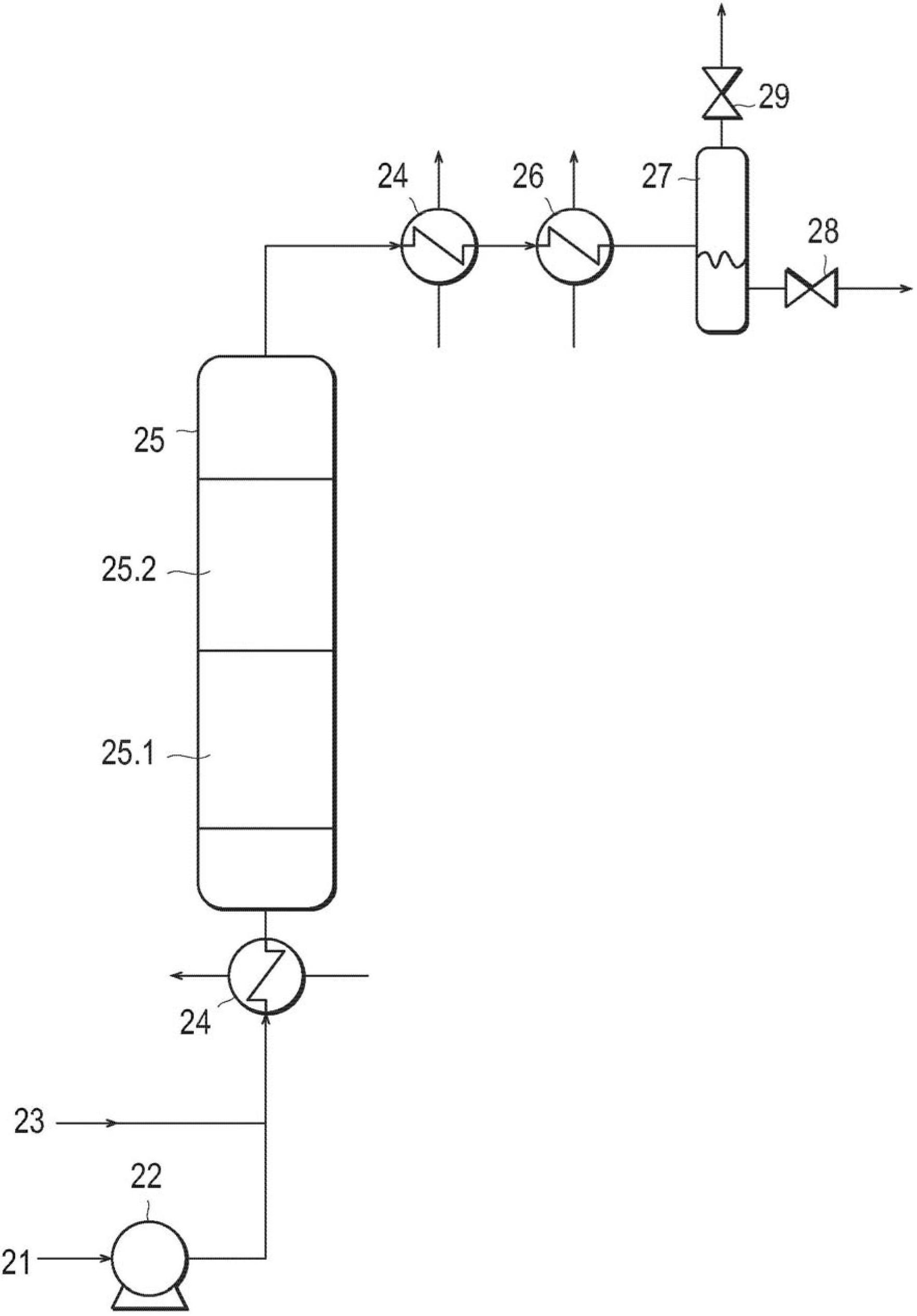
Catalyst for treatment of waste water, and method for treatment of waste water using the catalyst
InactiveCN102686521AStabilizationImprove purification effectWater contaminantsCatalyst activation/preparationIridiumWater use
Disclosed are: a catalyst which can exhibit an excellent catalytic activity and excellent durability for a long period in the wet oxidation treatment of waste water; a wet oxidation treatment method for waste water using the catalyst; and a novel method for treating waste water containing a nitrogenated compound, in which a catalyst to be used has a lower catalytic cost, the waste water containing the nitrogenated compound can be treated at high purification performance, and the high purification performance can be maintained. The catalyst for use in the treatment of waste water comprises an oxide of at least one element selected from the group consisting of iron, titanium, silicon, aluminum, zirconium and cerium as a component (A) and at least one element selected from the group consisting of silver, gold, platinum, palladium, rhodium, ruthenium and iridium as a component (B), wherein at least 70 mass% of the component (B) is present in a region positioned within 1000 [mu]m from the outer surface of the component (A) (i.e., the oxide), the component (B) has an average particle diameter of 0.5 to 20 nm, and the solid acid content in the component (A) (i.e., the oxide) is 0.20 mmol / g or more. The waste water treatment method uses a catalyst (a pre-catalyst) which is placed on an upstream side of the direction of the flow of the waste water and can convert the nitrogenated compound contained in the waste water into ammoniacal nitrogen in the presence of an oxidizing agent at a temperature of not lower than 100 DEG C and lower than 370 DEG C under a pressure at which the waste water can remain in a liquid state and a downstream-side catalyst (a post-catalyst) which is placed downstream of the direction of the flow of the waste water and can treat the waste water containing ammoniacal nitrogen.
Owner:NIPPON SHOKUBAI CO LTD
Isotropic dry cleaning process for noble metal integrated circuit structures
A method for removing from a microelectronic device structure a noble metal residue including at least one metal selected from the group consisting of platinum, palladium, iridium and rhodium, by contacting the microelectronic device structure with a cleaning gas including a reactive halide composition, e.g., XeF2, SF6, SiF4, Si2F6 or SiF3 and SiF2 radicals. The method may be carried out in a batch-cleaning mode, in which fresh charges of cleaning gas are successively introduced to a chamber containing the residue-bearing microelectronic device structure. Each charge is purged from the chamber after reaction with the residue, and the charging / purging is continued until the residue has been at least partially removed to a desired extent. Alternatively, the cleaning gas may be continuously flowed through the chamber containing the microelectronic device structure, until the noble metal residue has been sufficiently removed.
Owner:FELLOWS RES B V +1
Preparation method of catalyst with core-shell structure for low-temperature fuel cell
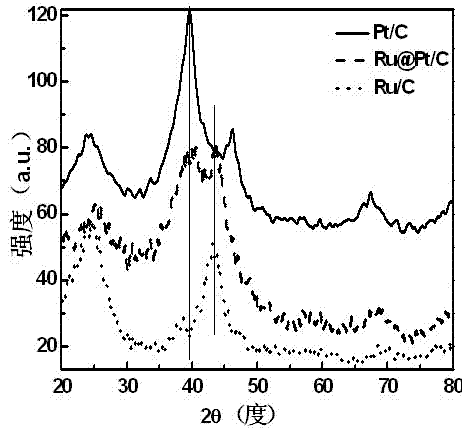
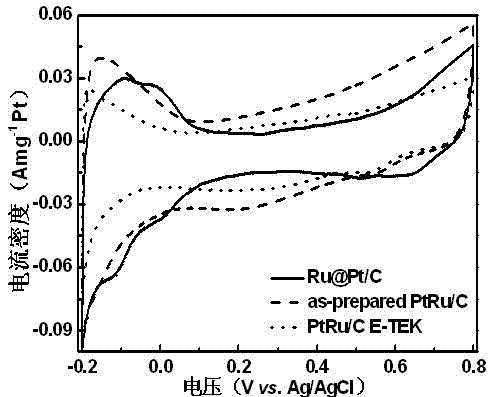
InactiveCN102500365AHigh catalytic activitySolve the costCell electrodesCatalyst activation/preparationIridiumPtru catalyst
The invention discloses a preparation method of a catalyst with a core-shell structure for a low-temperature fuel cell, belonging to the technical field of fuel cells. In the catalyst with the core-shell structure prepared with the preparation method, platinum is taken as a shell, a metal alloy consisting of more than one of metals including ruthenium, platinum, iron, cobalt, nickel, copper, tin, iridium, gold and silver is taken as an inner core, and the shell and the inner core are loaded on a carbon carrier. The preparation method comprises the following preparation steps of: reducing a metal chloride or a metal nitrate with a reducing agent, and forming a core on the carbon carrier with a large specific surface area; stabilizing the core; and precipitating Pt on a core layer with a impregnation reduction method, a high-pressure organic sol method, a microwave method or an electrodeposition process to form the catalyst with the core-shell structure. Due to the adoption of the preparation method, the utilization ratio of noble metal platinum is increased, the cost of an electro-catalyst is reduced effectively, and the methanol oxidizing capability and oxygen reducing activity of the obtained catalyst are increased by 10.8 times and 8.7 times in maximum respectively in comparison to the mass ratio and activity of a commercial JM4100Pt / C catalyst.
Owner:SOUTH CHINA UNIV OF TECH
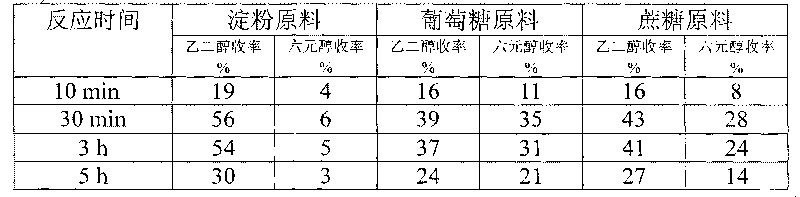
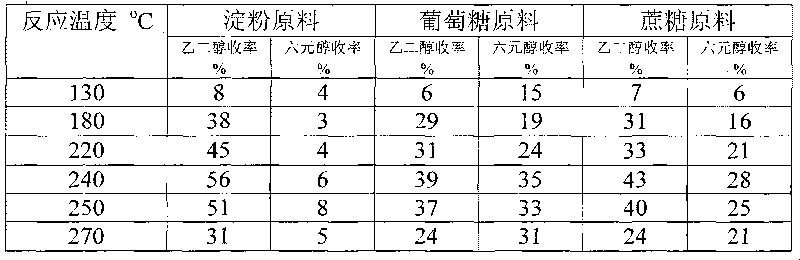
Method for preparing ethanediol from polyhydroxy compounds
ActiveCN101735014ARaw material resources are renewableMeet the requirements of sustainable developmentOrganic compound preparationCatalyst activation/preparationHydrogen pressureCobalt
The invention provides a method for preparing ethanediol from polyhydroxy compounds comprising starch, hemicellulose, cane sugar, glucose, fructose and fructosan. The method comprises the following steps of: taking the polyhydroxy compounds as reaction raw materials, and taking metals, carbides, nitrides, and phosphides of transition metals in families VIII, IX and X, such as ferrum, cobalt, nickel, ruthenium, rhodium, palladium, iridium, platinum, molybdenum and tungsten as catalytic active components to form a polymetallic catalyst; and performing further catalytic conversion under a hydrothermal condition that the temperature is 120 to 300 DEG C and the hydrogen pressure is 1 to 13 MPa to prepare the ethanediol from the polyhydroxy compounds with high efficiency, high selectivity and high yield. The method for preparing the ethanediol from the polyhydroxy compounds has the outstanding advantages of renewable raw materials, environment-friendly reaction process, and atom economical efficiency. Simultaneously, compared with other techniques taking biomasses as the raw materials, the method has the advantages of simple process and high yield.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
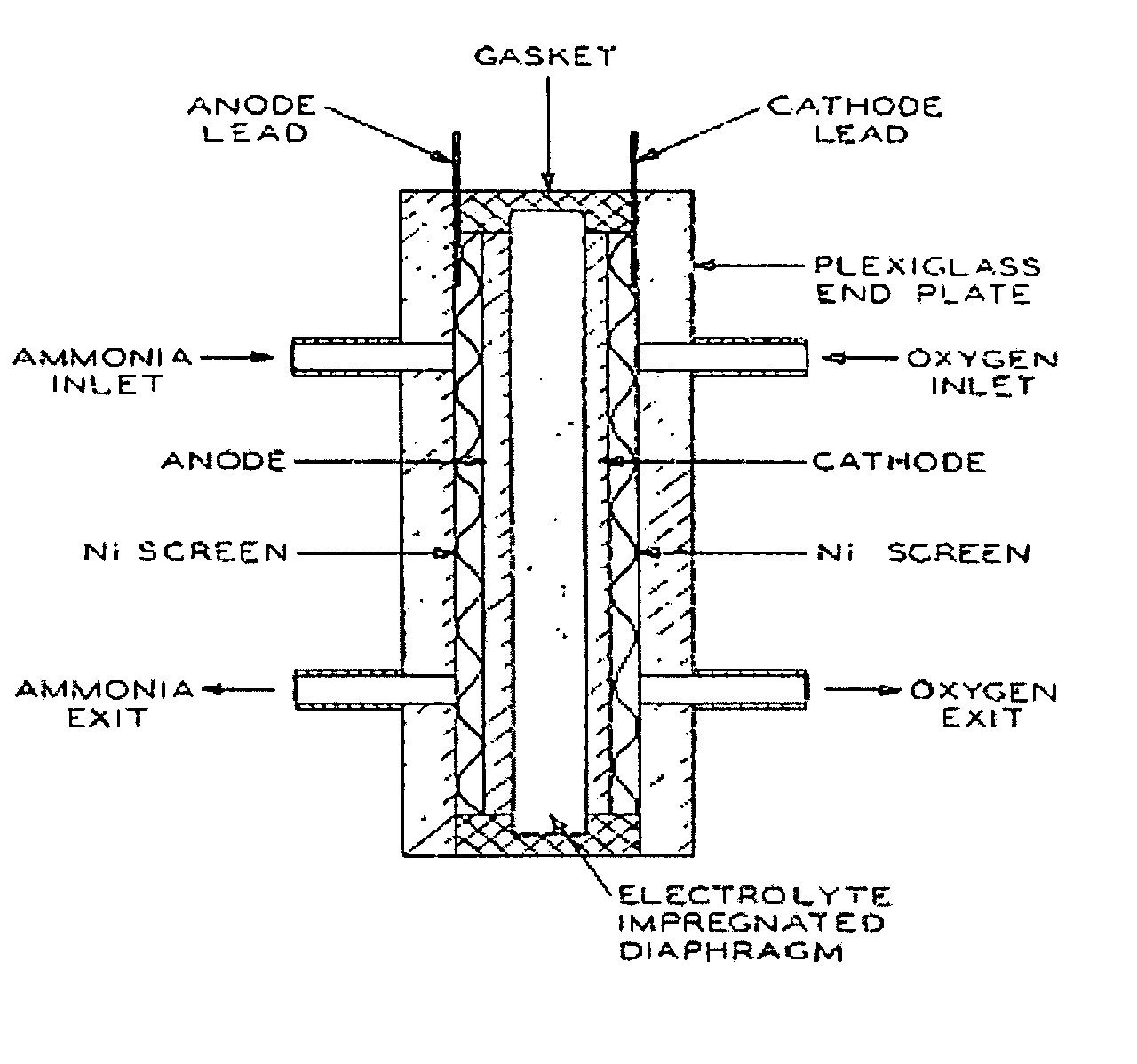
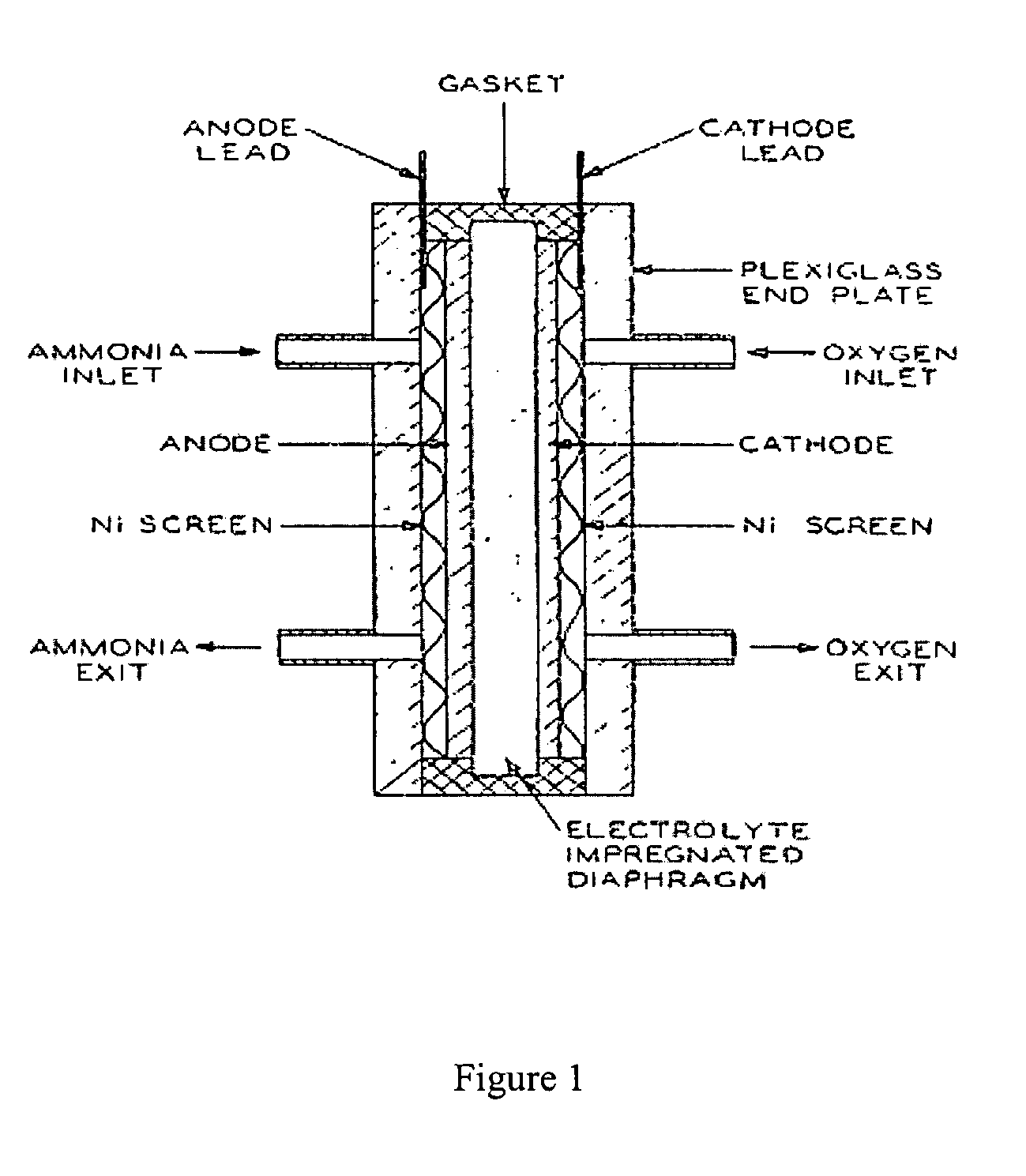
Electro-catalysts for the oxidation of ammonia in alkaline media
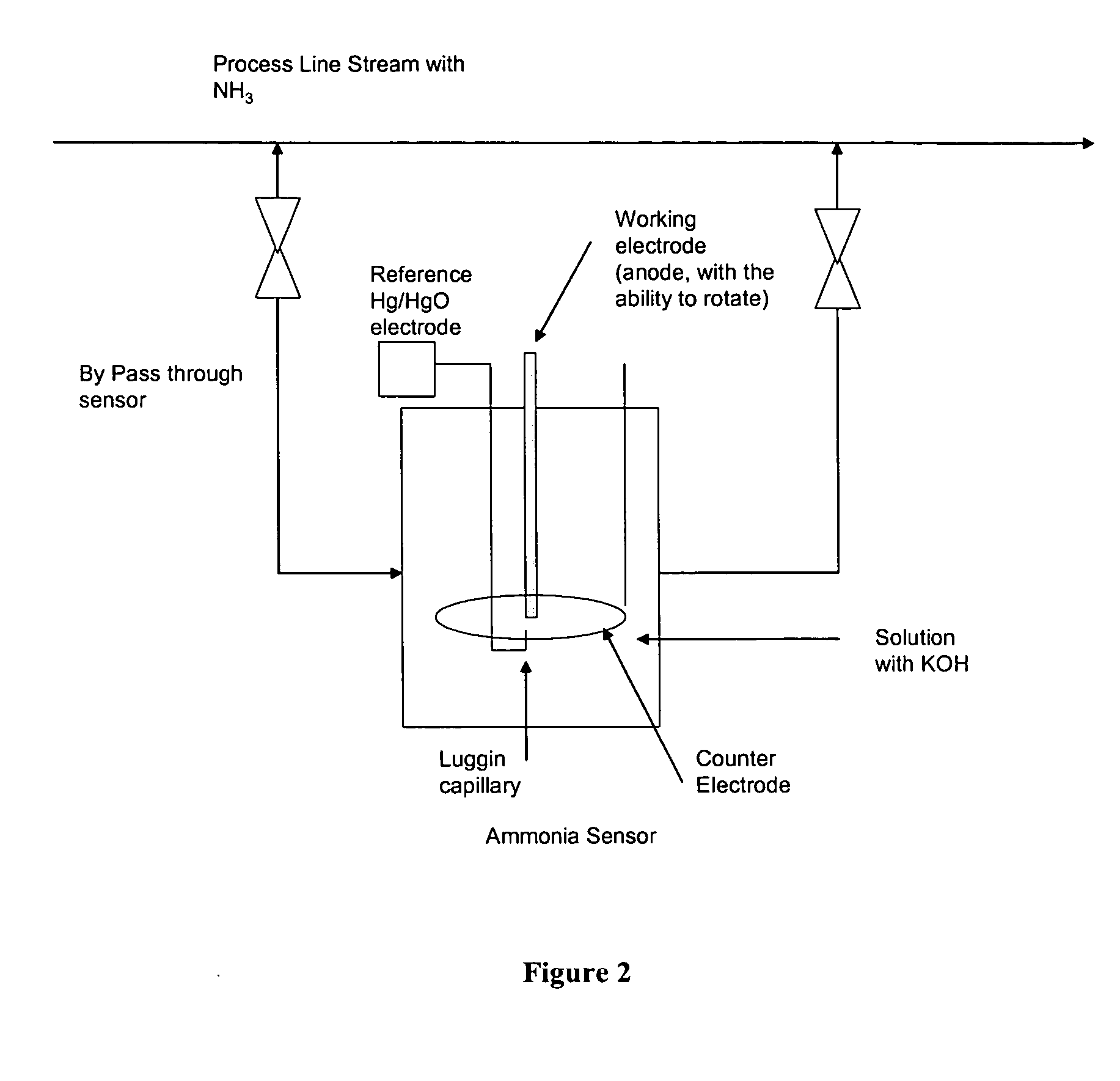
InactiveUS20050211569A1From normal temperature solutionsLiquid separation by electricityChemistryIridium
An electro-catalyst for the oxidation of ammonia in alkaline media; the electrocatalyst being a noble metal co-deposited on a support with one or more other metals that are active to ammonia oxidation. In some embodiments, the support is platinum, gold, tantalum, or iridium. In some embodiments, the support has a layer of Raney metal deposited thereon prior to the deposition of the catalyst. Also provided are electrodes having the electro-catalyst deposited thereon, ammonia electrolytic cells, ammonia fuel cells, ammonia sensors, and a method for removing ammonia contaminants from a contaminated effluent.
Owner:OHIO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com