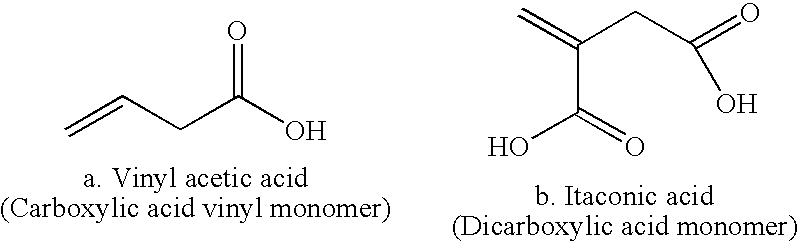
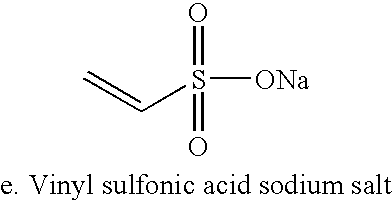
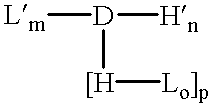Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33960 results about "Hydrolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrolysis (/haɪˈdrɒlɪsɪs/; from Ancient Greek hydro-, meaning 'water', and lysis, meaning 'to unbind') is any chemical reaction in which a molecule of water ruptures one or more chemical bonds. The term is used broadly for substitution, elimination, and fragmentation reactions in which water is the nucleophile.
Water-soluble copolymer film packet
Owner:MONOSOL LLC +1
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
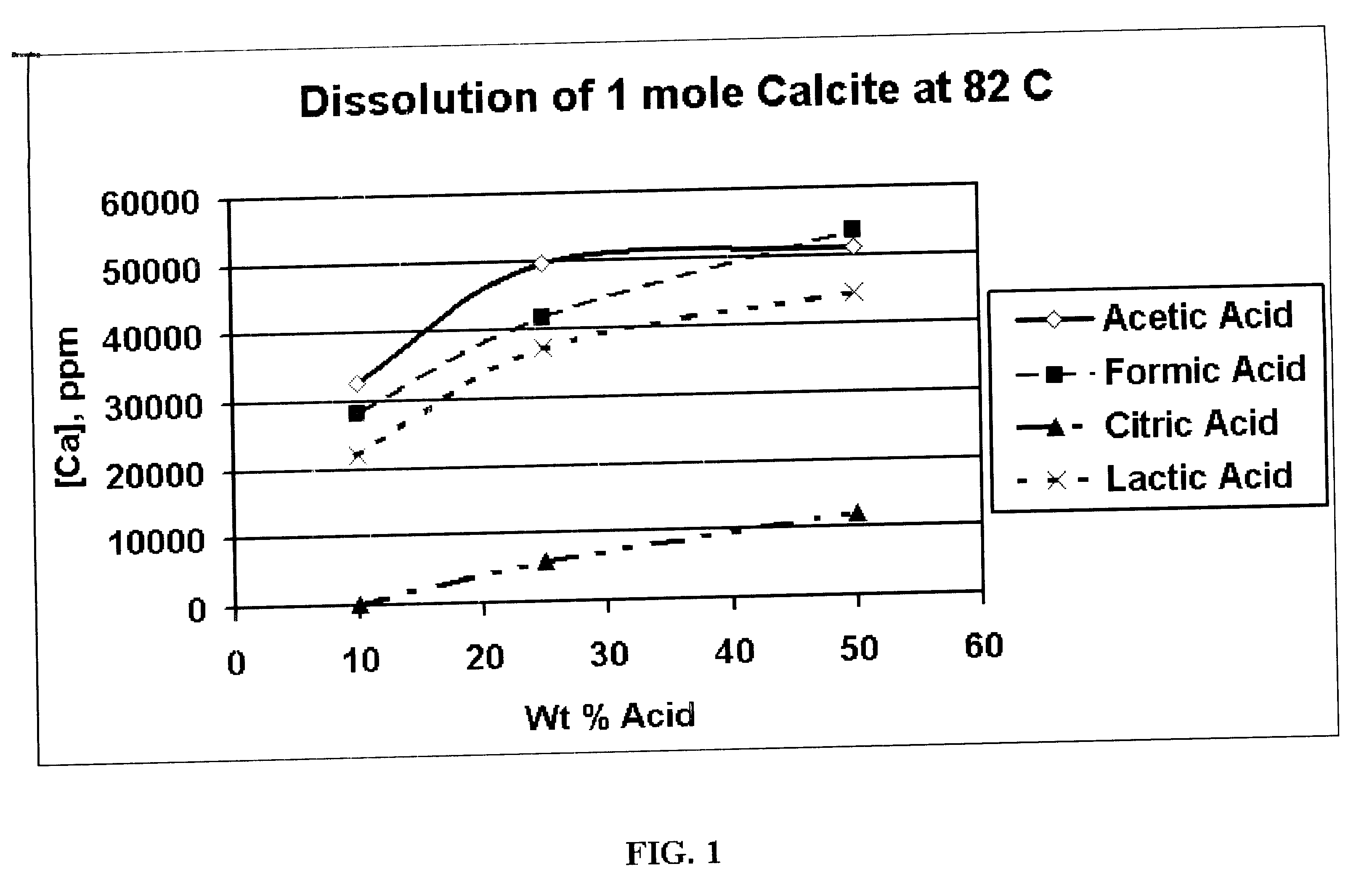
Generating Acid Downhole in Acid Fracturing
An acid fracturing method is provided in which the acid is generated in the fracture by hydrolysis of a solid acid-precursor selected from one or more than one of lactide, glycolide, polylactic acid, polyglycolic acid, a copolymer of polylactic acid and polyglycolic acid, a copolymer of glycolic acid with other hydroxy-, carboxylic acid-, or hydroxycarboxylic acid-containing moieties, and a copolymer of lactic acid with other hydroxy-, carboxylic acid or hydroxycarboxylic acid-containing moieties. The solid acid-precursor may be mixed with a solid acid-reactive material to accelerate the hydrolysis and / or coated to slow the hydrolysis. Water-soluble liquid compounds are also given that accelerate the hydrolysis. The method ensures that the acid contacts fracture faces far from the wellbore.
Owner:SCHLUMBERGER TECH CORP
Methods and compositions for the prevention and treatment of atherosclerosis, restenosis and related disorders
Methods and compositions for the prevention and treatment of all forms of atherosclerosis are described. Administration of compounds such as thalidomide, its analogs, hydrolysis products, metabolites, derivatives and precursors as well as additional compounds capable of inhibiting tumor necrosis factor alpha (TNF-alpha) are used in the invention. Also disclosed is the coating of prosthetic devices, such as stents, with the compounds of the invention for the prevention and / or treatment of restenosis.
Owner:CELGENE CORP
Nanoporous silicone resins having low dielectric constants
InactiveUS6541107B1Group 4/14 element organic compoundsDead plant preservationOrganic solventCarbon–carbon bond
Nanoporous silicone resins and silicone resin films having low dielectric constants and a method for preparing such nanoporous silicone resins. The silicone resin comprises the reaction product of a mixture comprising(A) 15-70 mol % of a tetraalkoxysilane described by formula where each R1 is an independently selected alkyl group comprising 1 to about 6 carbon atoms,(B) 12 to 60 mol % of a hydrosilane described by formula where each X is an independently selected hydrolyzable substituent,(C) 15 to 70 mole percent of an organotrialkoxysilane described by formula where R2 is a hydrocarbon group comprising about 8 to 24 carbon atoms or a substituted hydrocarbon group comprising a hydrocarbon chain having about 8 to 24 carbon atoms and each R3 is an independently selected alkyl group comprising 1 to about 6 carbon atoms; in the presence of(D) water,(E) hydrolysis catalyst, and(F) organic solvent for the reaction product.The silicone resin is cured and heated in an inert atmosphere at a temperature sufficient to effect thermolysis of carbon-carbon bonds of the R2 groups thereby forming a nanoporous silicone resin.
Owner:DOW CORNING CORP
Dissolving Filter Cake
InactiveUS20040094300A1Needed propertyWider fractureCleaning apparatusFluid removalSolid baseCarrier fluid
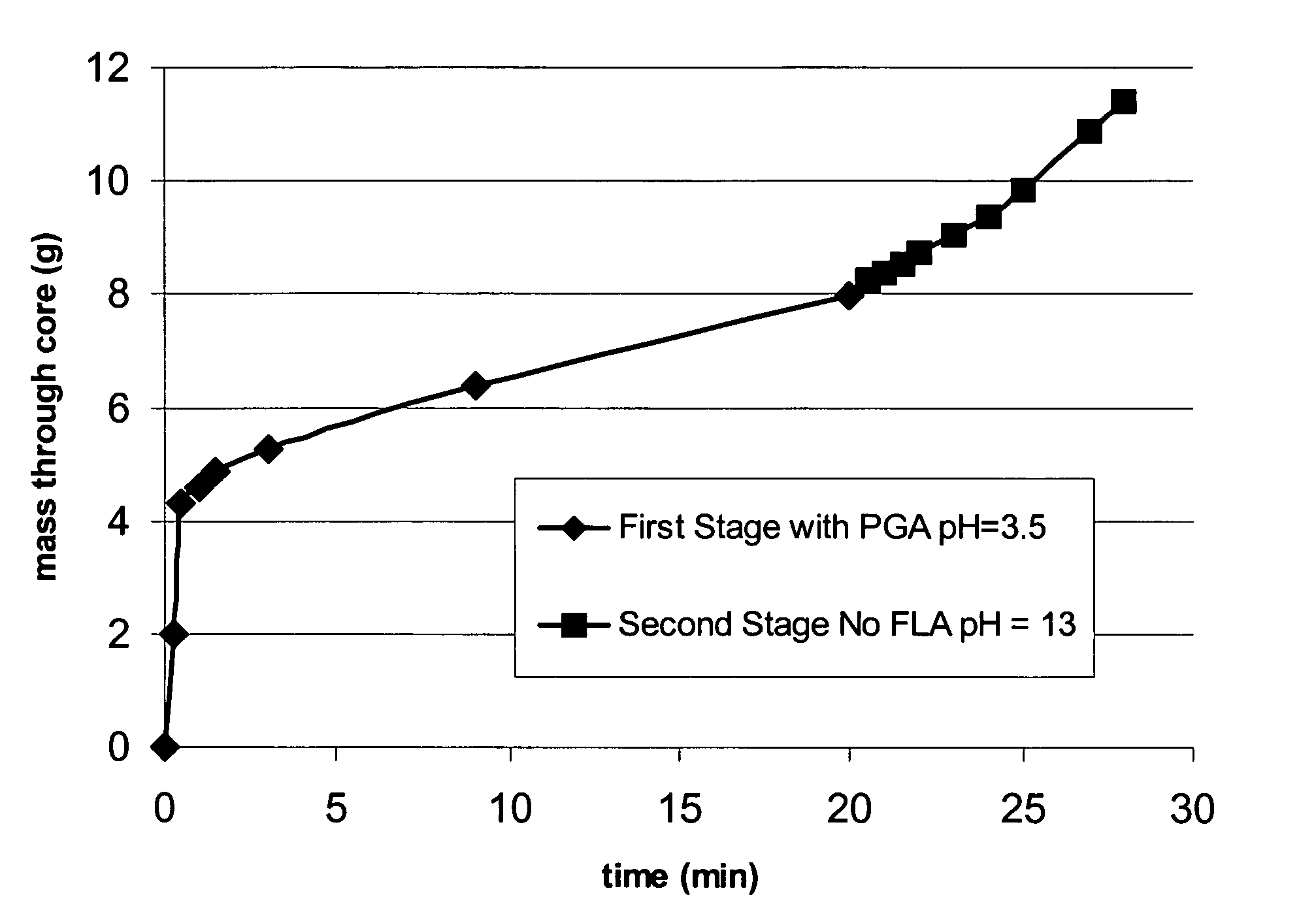
Methods are presented to induce a screenout during a subterranean formation fracturing or combined fracturing and gravel packing treatment having a viscoelastic surfactant-based carrier fluid by laying down a filter cake at least a portion of which is a base-soluble material, injecting proppant slurry, and causing hydrolysis and dissolution of the solid base-soluble material by adding base so that leak-off increases, the concentration of proppant in the fracture increases, and the proppant screens out. A method of gravel packing with a viscoelastic surfactant-based carrier fluid by laying down a filter cake at least a portion of which is a base-soluble material, injecting gravel, and causing hydrolysis and dissolution of the solid base-soluble material by adding base. Methods of slowing or accelerating the hydrolysis and dissolution are given, and addition of bridging-promoting materials is included.
Owner:SCHLUMBERGER TECH CORP
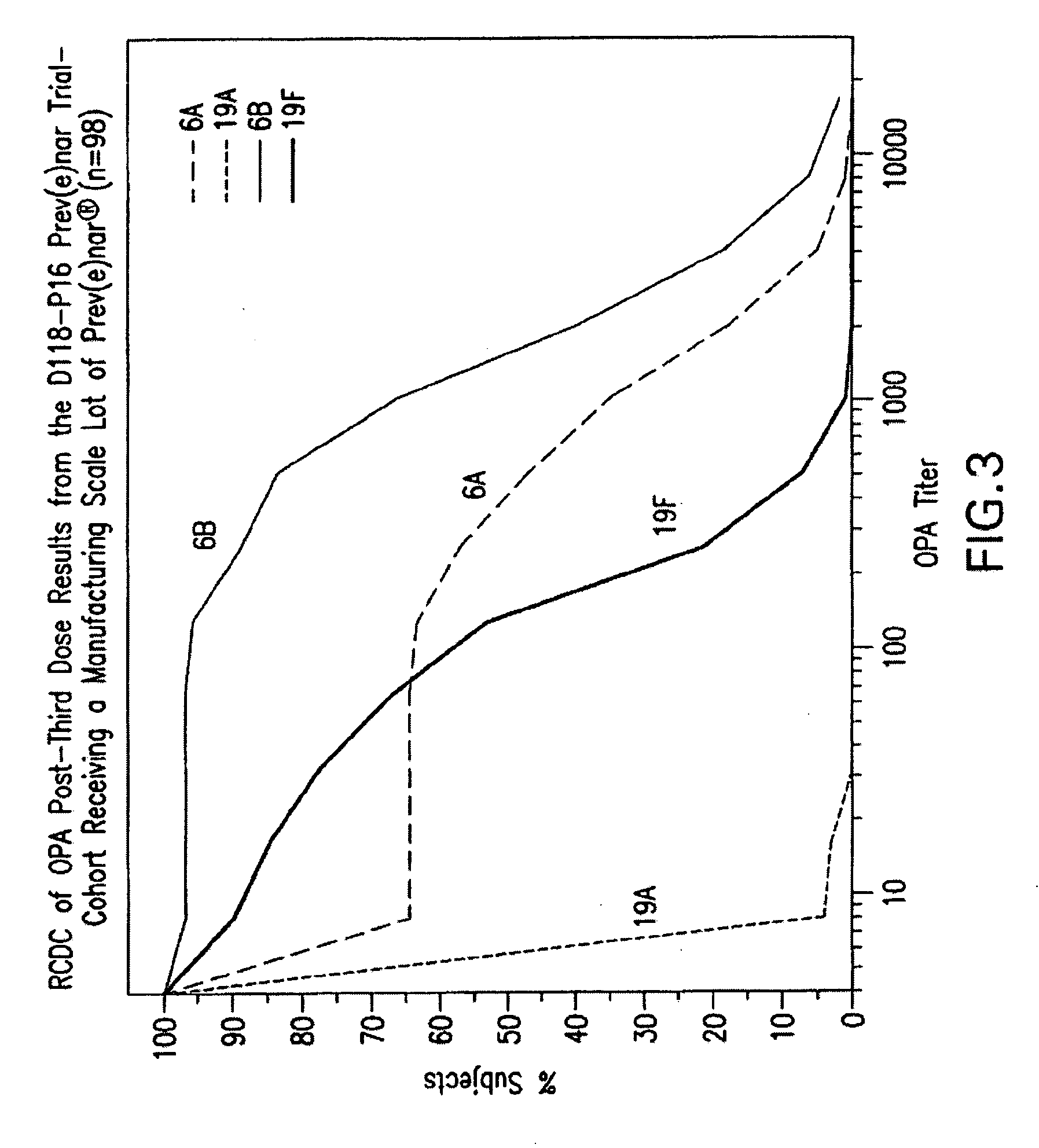
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
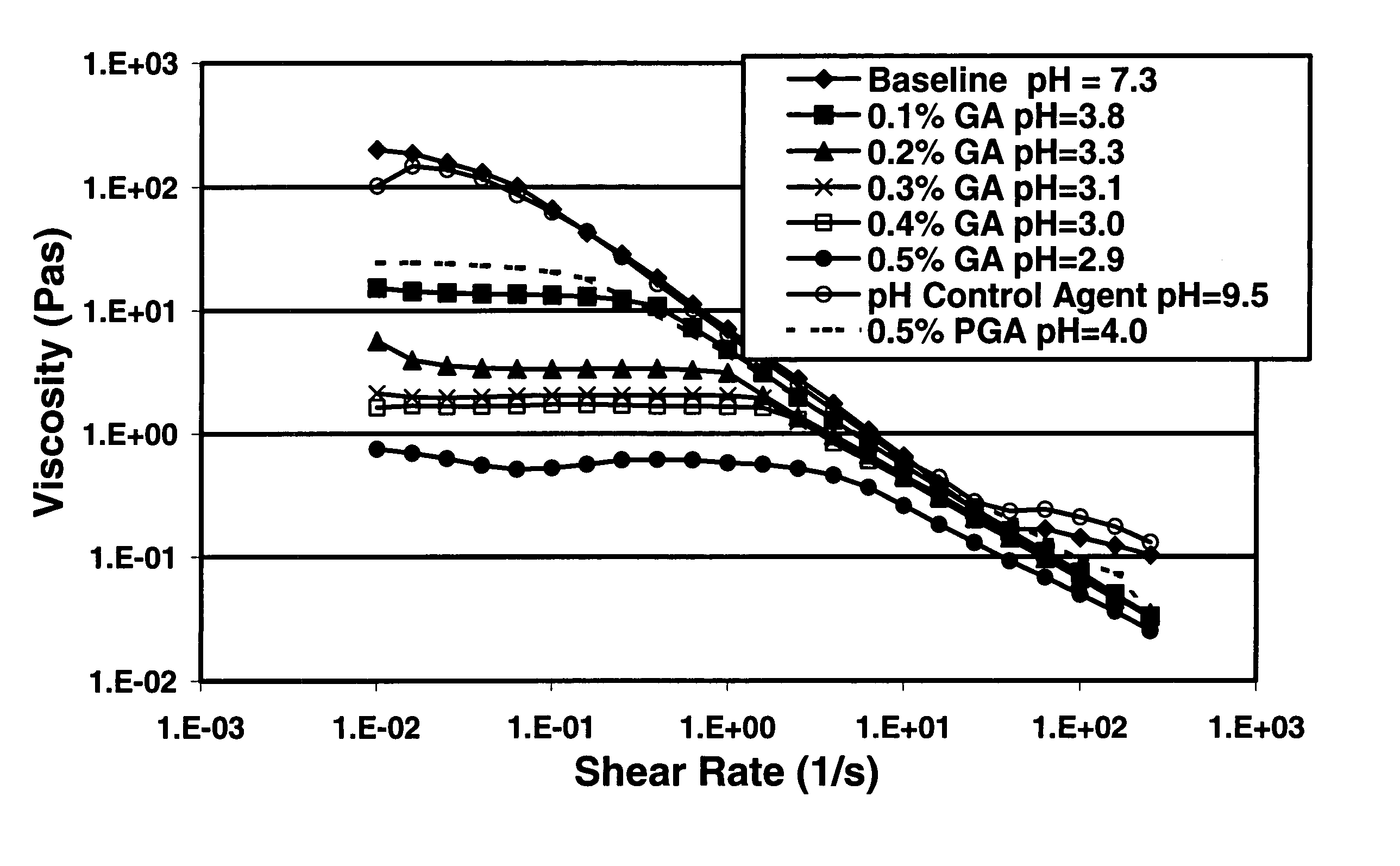
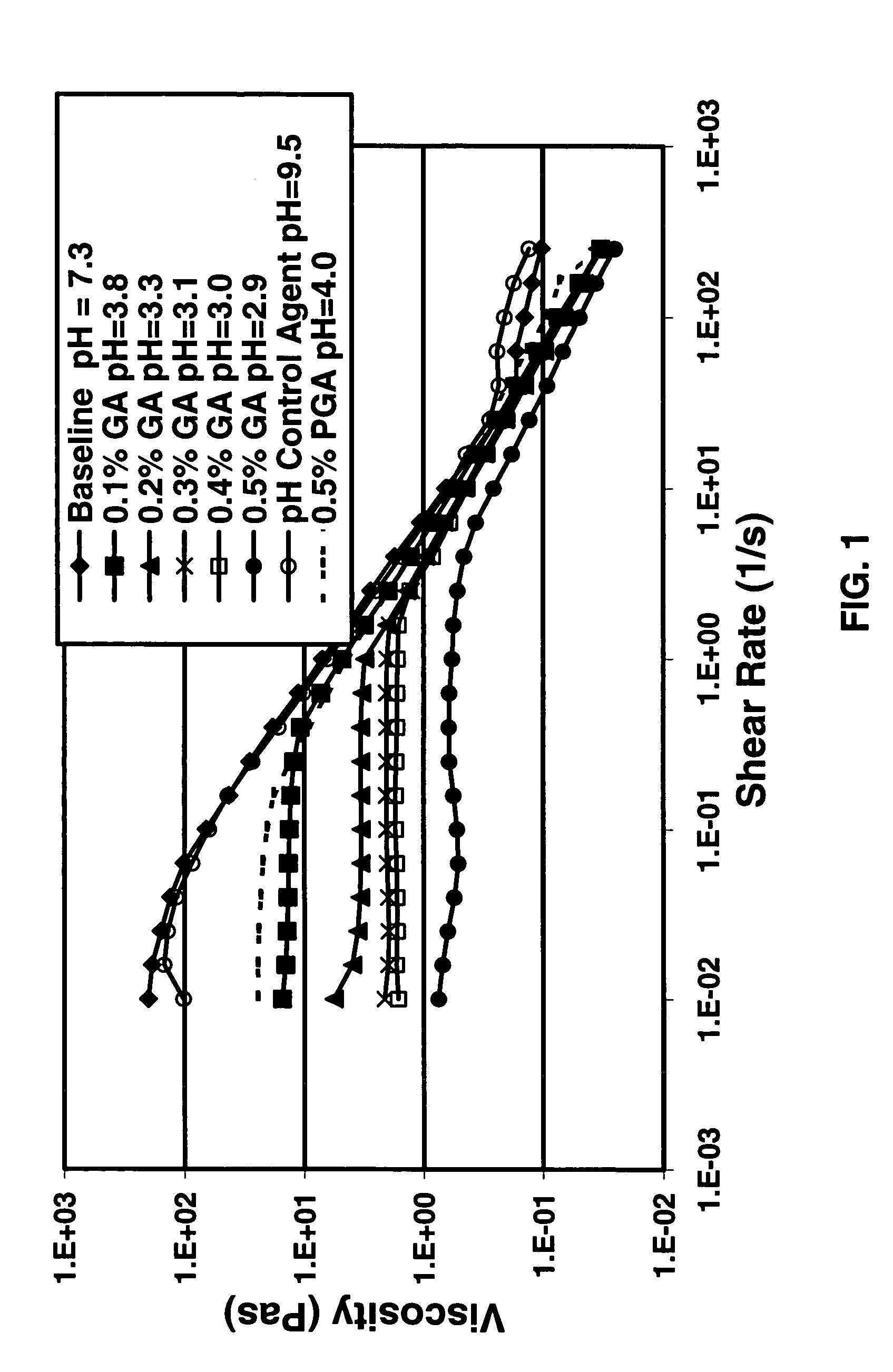
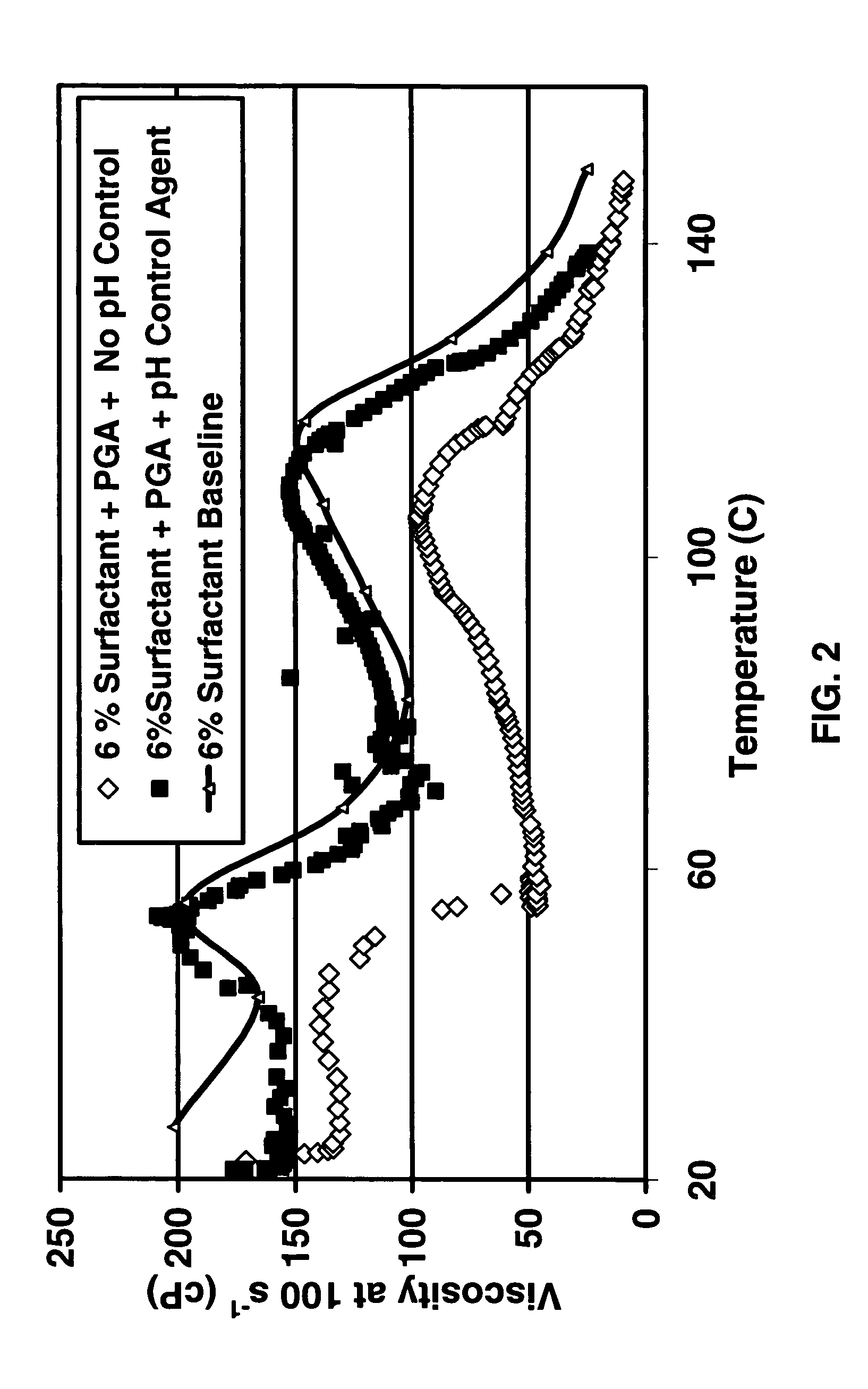
Degradable additive for viscoelastic surfactant based fluid systems
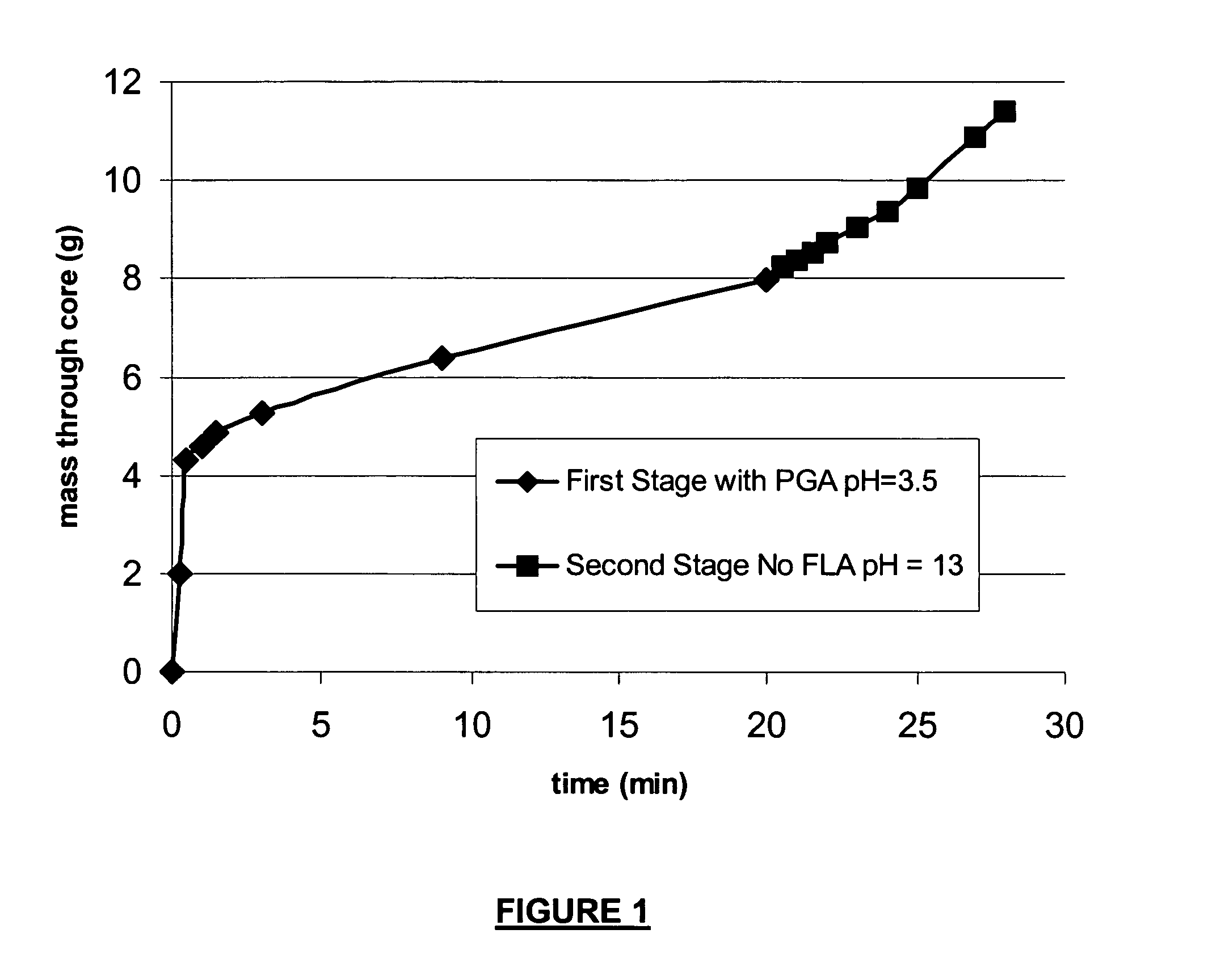
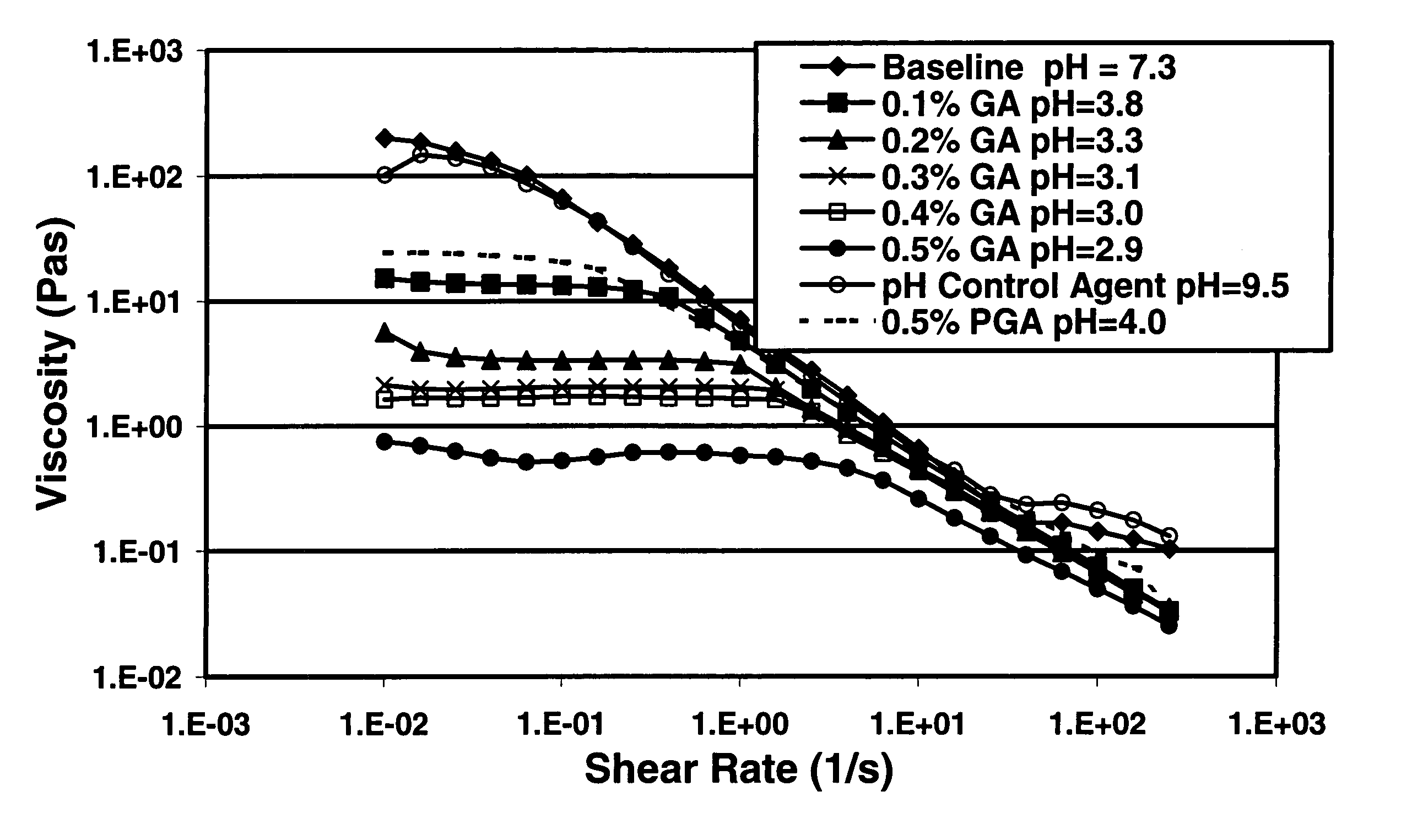
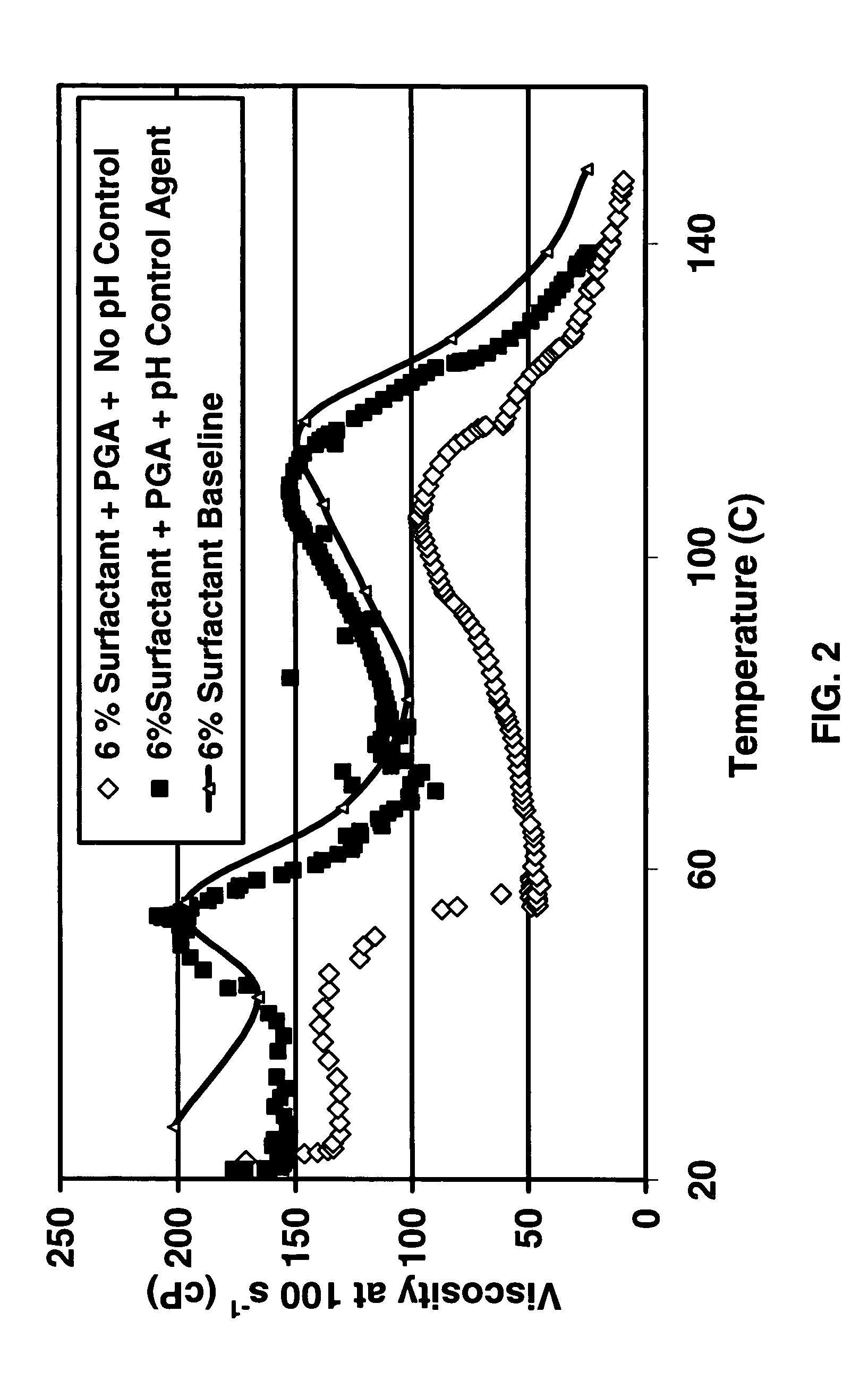
A method is given for treating a subterranean formation penetrated by a wellbore with a viscosified fluid. The fluid contains a solid hydrolysable polyacid that upon dissolution and hydrolysis releases an acid that is a breaker for the viscosifying system. Suitable solid hydrolysable polyacids include polylactic acid and polyglycolic acid. The fluid also contains a pH control agent, present in an amount sufficient to neutralize any acid present in the solid hydrolysable polyacid before the injection and to neutralize any acid generated by the solid hydrolysable polyacid during the injection, so that the acid breaker is not available to break the fluid during the injection. In one embodiment the viscosifier is a viscoelastic surfactant fluid system and the solid hydrolysable polyacid is of a size selected to be a fluid loss additive, for example in fracturing or gravel packing. In another embodiment, the solid hydrolysable polyacid is used in particles sufficiently small that they enter the pores of the formation. In either case, the viscosifier is broken after the solid releases more acid than can be neutralized by the pH control agent.
Owner:SCHLUMBERGER TECH CORP
Method and apparatus for sustainable energy and materials
A process for the production of hydrogen from anaerobically decomposed organic materials by applying an electric potential to the anaerobically decomposed organic materials, including landfill materials and sewage, to form hydrogen, and for decreasing the time required to treat these anaerobically decomposed organic materials. The organic materials decompose to volatile acids such as acetic acid, which may be hydrolyzed by electric current to form hydrogen. The process may be continuously run in sewage digestion tanks with the continuous feed of sewage, at landfill sites, or at any site having a supply of anaerobically decomposed or decomposable organic materials.
Owner:MCALISTER TECH LLC +1
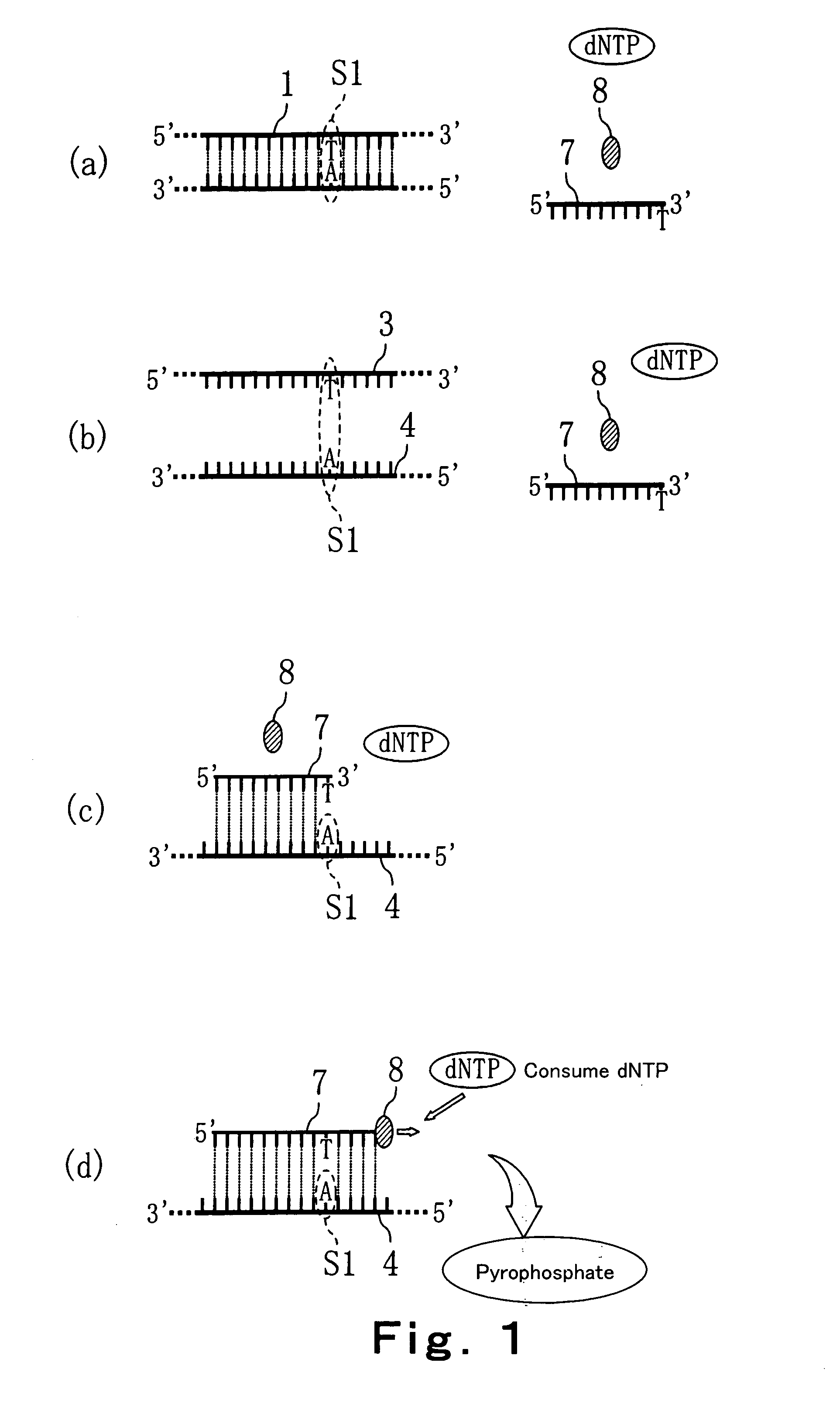
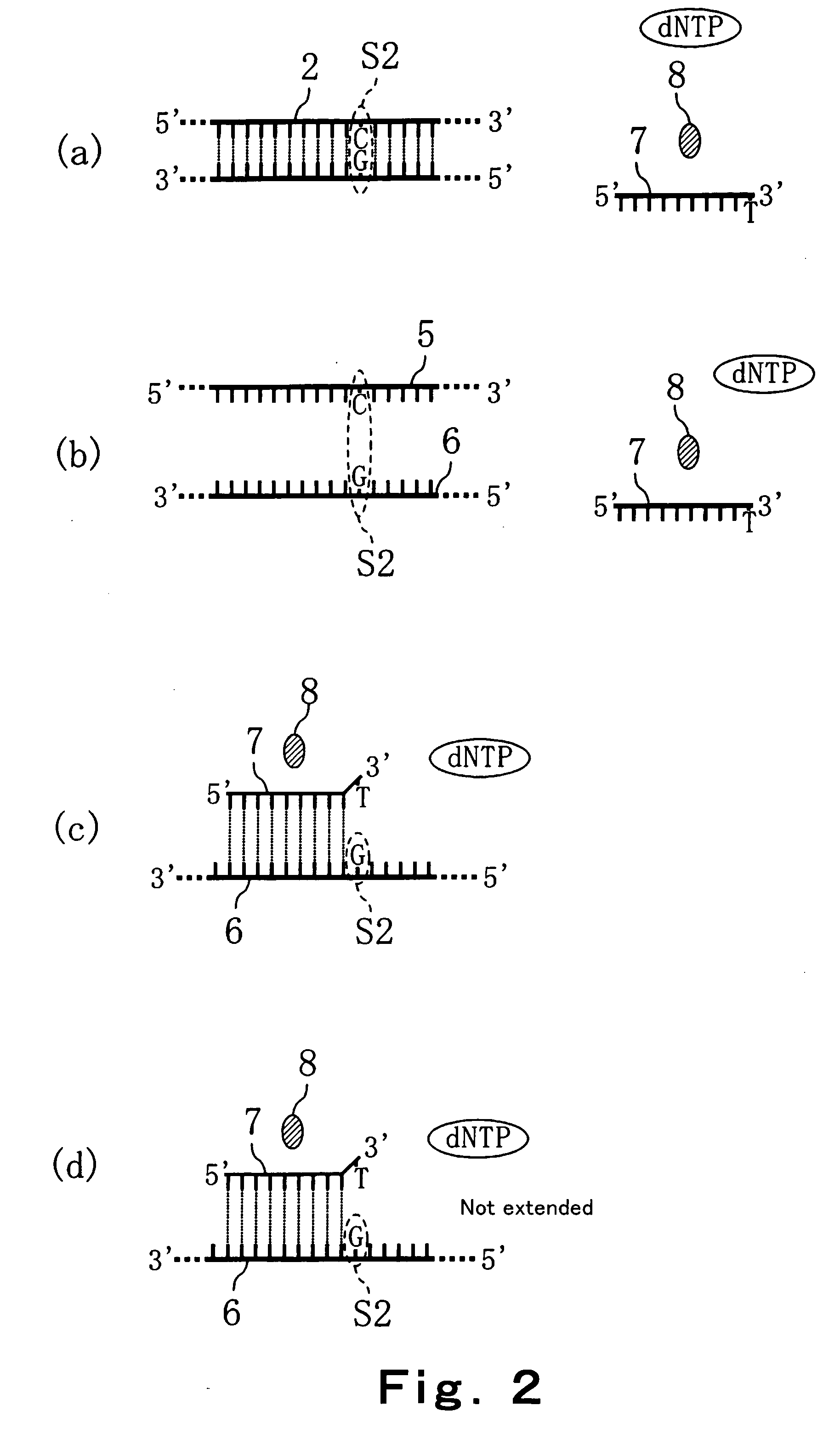
Method of detecting primer extension reaction, method of discriminating base type, device for discriminating base type, device for detecting pyrophosphate, method of detecting nucleic acid and tip for introducing sample solution
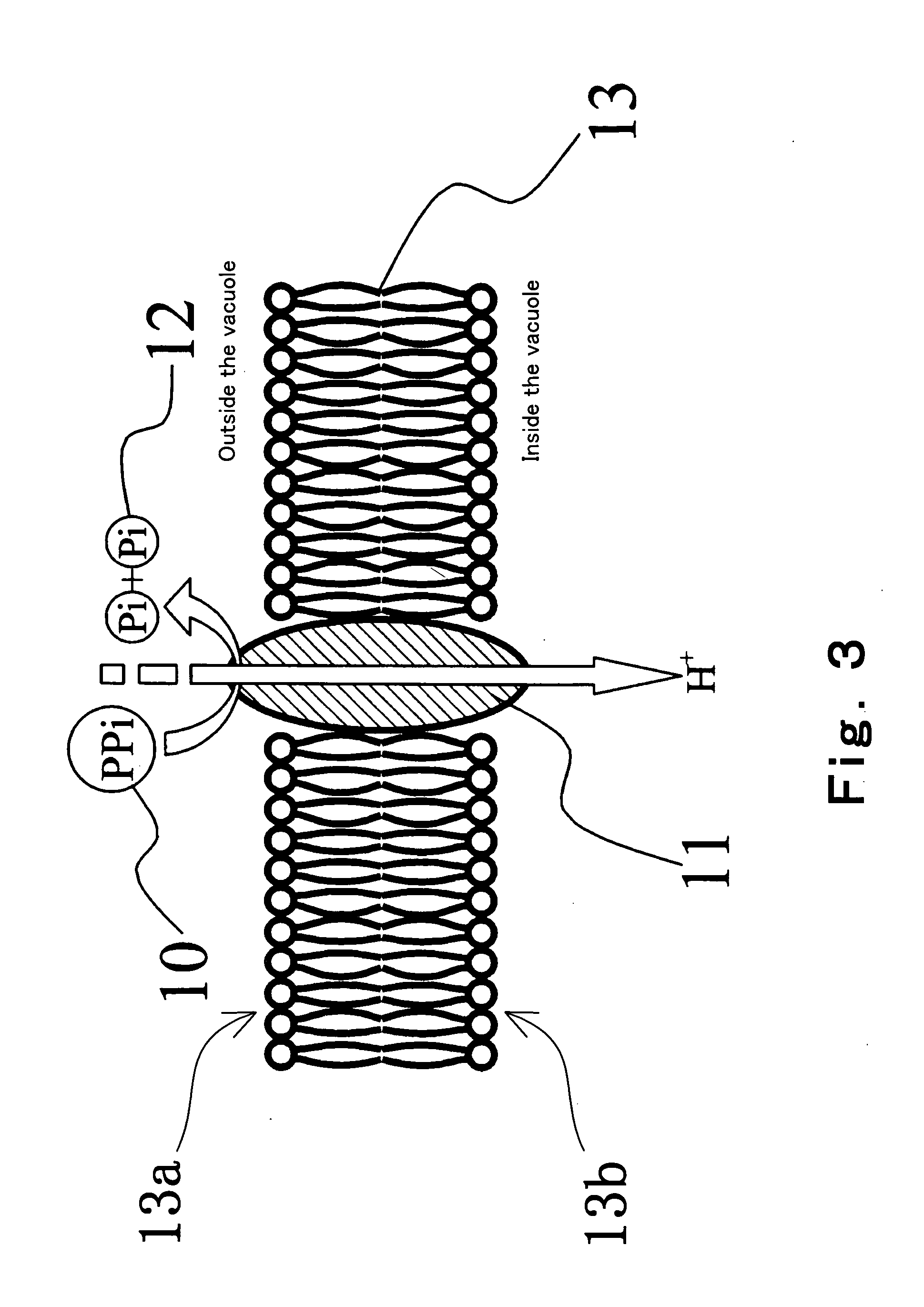
InactiveUS20050032075A1Decrease of H+ concentrationImprove concentrationMicrobiological testing/measurementMaterial analysisBase JNucleotide
Convenient techniques for discriminating the base type in a base sequence of a nucleic acid are provided. The technique includes the step (a) of preparing a sample solution containing a nucleic acid, a primer having a base sequence that includes a complementary binding region which complementarily binds to the nucleic acid, and a nucleotide; the step (b) of allowing the sample solution to stand under a condition to cause an extension reaction of the primer, and producing pyrophosphate when the extension reaction is caused; the step (c) of bringing the sample solution into contact with the front face of a H+ hardly permeable membrane having H+-pyrophosphatase, which penetrates from front to back of the membrane, of which active site that hydrolyzes pyrophosphate being exposed to the front face; the step (d) of measuring the H+ concentration of at least either one of the solution at the front face side of the H+ hardly permeable membrane or the solution at the back face side of the H+ hardly permeable membrane, in a state where the H+-pyrophosphatase is immersed in the solution; the step (e) of detecting the extension reaction on the basis of the result of measurement in the step (d) ; and the step (f) of discriminating the base type in the base sequence of the nucleic acid on the basis of the result of detection in the step (e).
Owner:PANASONIC CORP
Production of Peracids Using An Enzyme Having Perhydrolysis Activity
ActiveUS20080176783A1Efficient implementationReduce concentrationBiocideHydrolasesMedicinal chemistryPeroxide
A process is provided for producing peroxycarboxylic acids from carboxylic acid esters. More specifically, carboxylic acid esters are reacted with an inorganic peroxide, such as hydrogen peroxide, in the presence of an enzyme catalyst having perhydrolysis activity. The present perhydrolase catalysts are classified as members of the carbohydrate esterase family 7 (CE-7) based on the conserved structural features. Further, disinfectant formulations comprising the peracids produced by the processes described herein are provided.
Owner:DUPONT US HLDG LLC
Method of enhancing salty taste, salty taste enhancer, salty taste seasoning agent and salty taste-enhanced foods
The present invention relates to a method of enhancing the salty taste of a food or beverage containing salt which comprises adding an acidic peptide or a peptide obtained by subjecting a protein to hydrolysis and deamidation to the food or beverage, a salty taste enhancer comprising the peptide as an active ingredient, a salty taste seasoning agent comprising the peptide and salt, and a food or beverage comprising the salty taste enhancer or the salty taste seasoning agent.
Owner:KYOWA HAKKO FOOD SPECIALTIES +1
Elastomeric material compositions obtained from castor oil and epoxidized soybean oil
Elastomers are formed from castor oil and / or ricinoleic acid estolides and a polyester formed from an epoxidized vegetable oil such as ESO and a polycarboxylic acid such as sebacic acid, optionally in the presence of a peroxide initiator, or include crosslinked reaction products derived from ricinoleic acid or castor oil estolides, epoxy group-containing compounds such as epoxy resins and / or epoxidized vegetable oil, epoxy hardeners such as polyamine and polycarboxylic acid hardeners, thermally activated free radical initiators such as peroxides, and optionally but preferably include fillers such as limestone or wood flour. The elastomers can be prepared using a two-step, solvent-less procedure at elevated or ambient temperatures. These predominantly “all-natural” elastomers have physical properties comparable to conventional petroleum-based elastomers and composites and exhibit good flexibility, resiliency, abrasion resistance and inertness to hydrolysis. The resulting elastomers display good mechanical strength and resiliency, are resistant to abrasion and hydrolysis, and can be processed into sheet materials, which makes them attractive as floor covering components.
Owner:AFI LICENSING +1
Methods for the production of ammonia from urea and/or biuret, and uses for NOx and/or particulate matter removal
InactiveUS6077491ARisk minimizationReduce amountNitrogen compoundsDispersed particle separationHydrolysisAmmonia
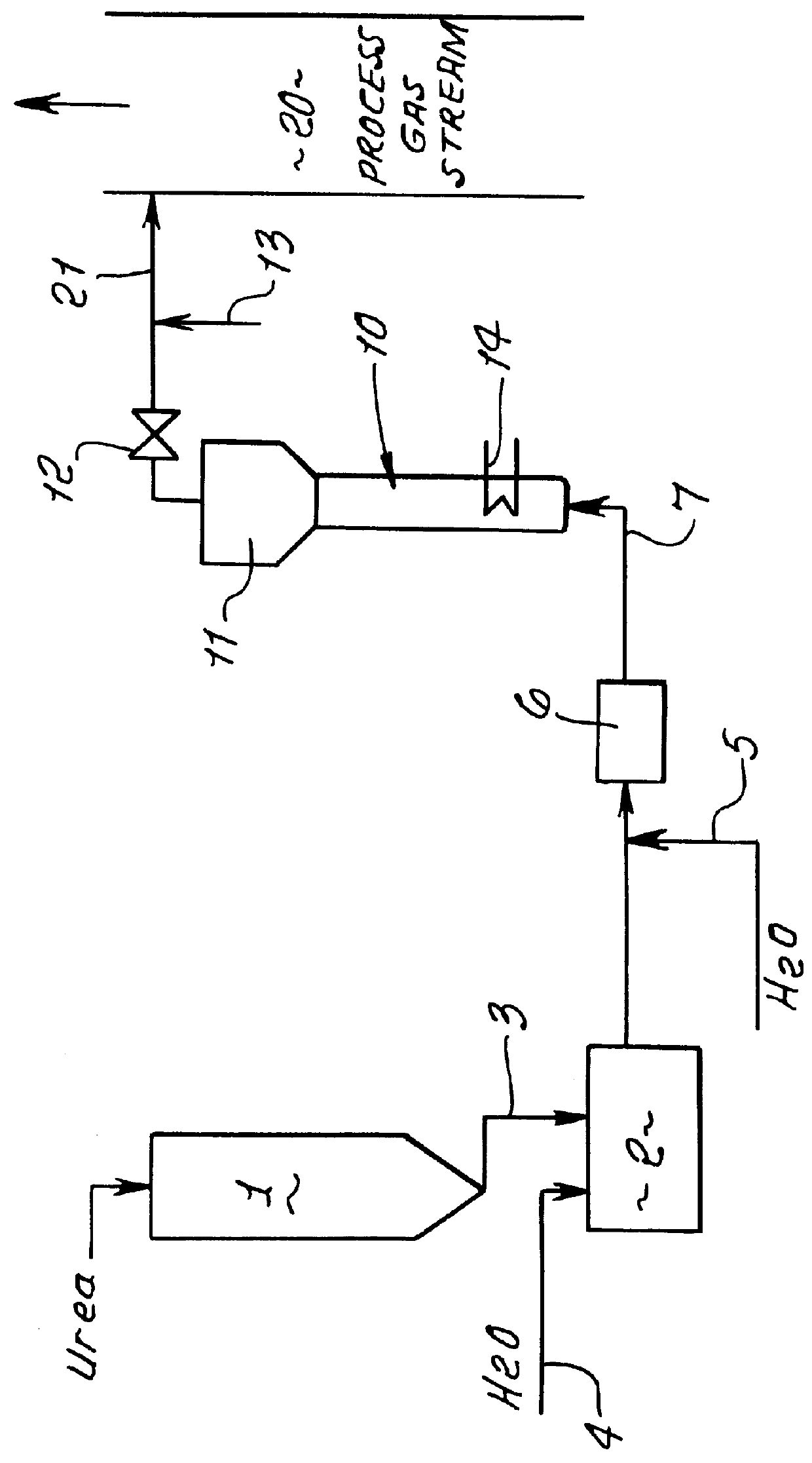
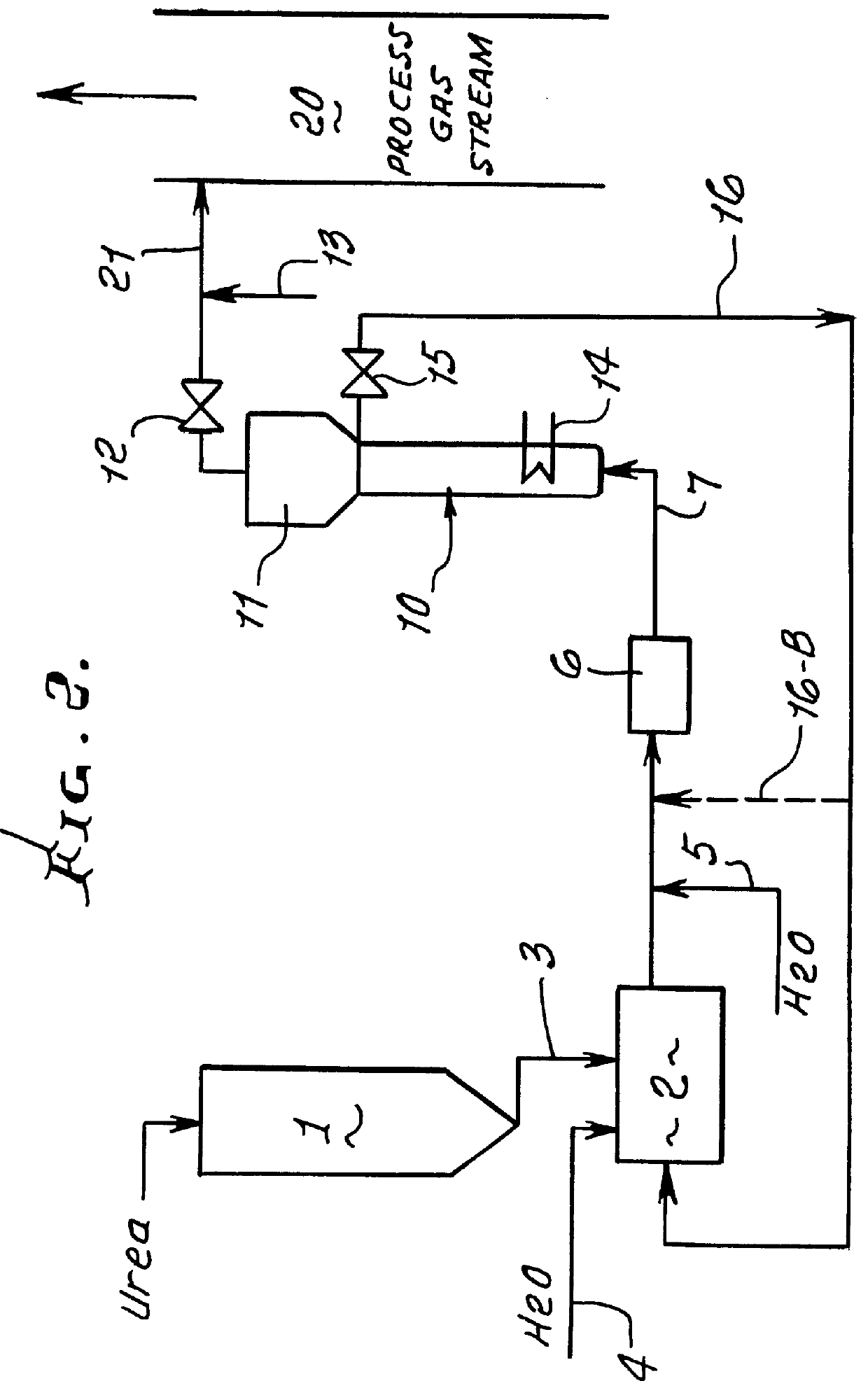
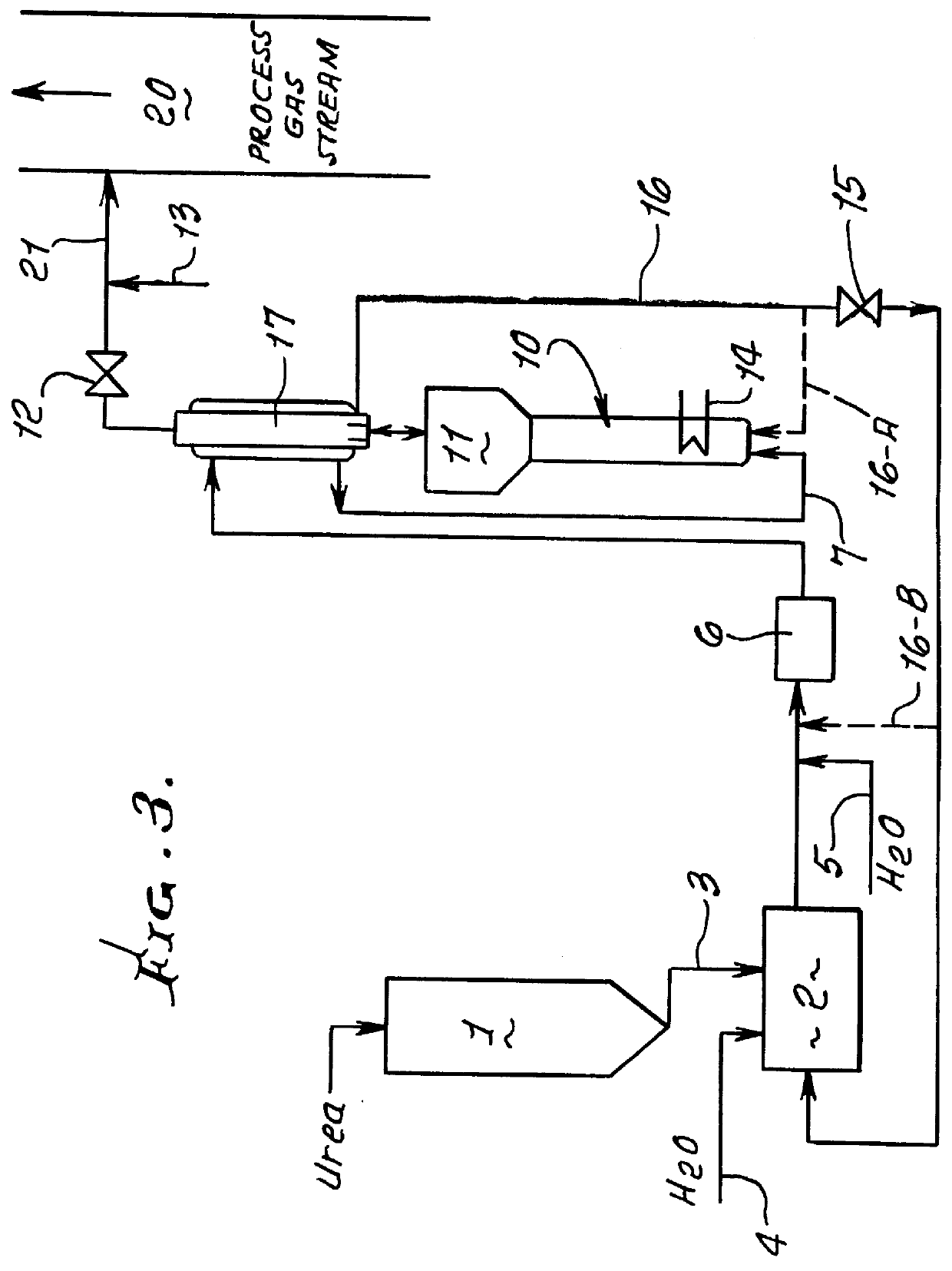
This patent describes technology for generating ammonia from urea. The method is based on the hydrolysis of an aqueous solution of urea and / or biuret by heating under pressure to form a mixture of ammonia, carbon dioxide and water. The gas mixtures produced are useful for supplying ammonia at controlled pressure and rate of flow for many industrial applications without the risks and hazards associated with the transportation and on-site storage of ammonia, thereby providing a significant safety advantage over present industrial practice.
Owner:EC&C TECH INC A
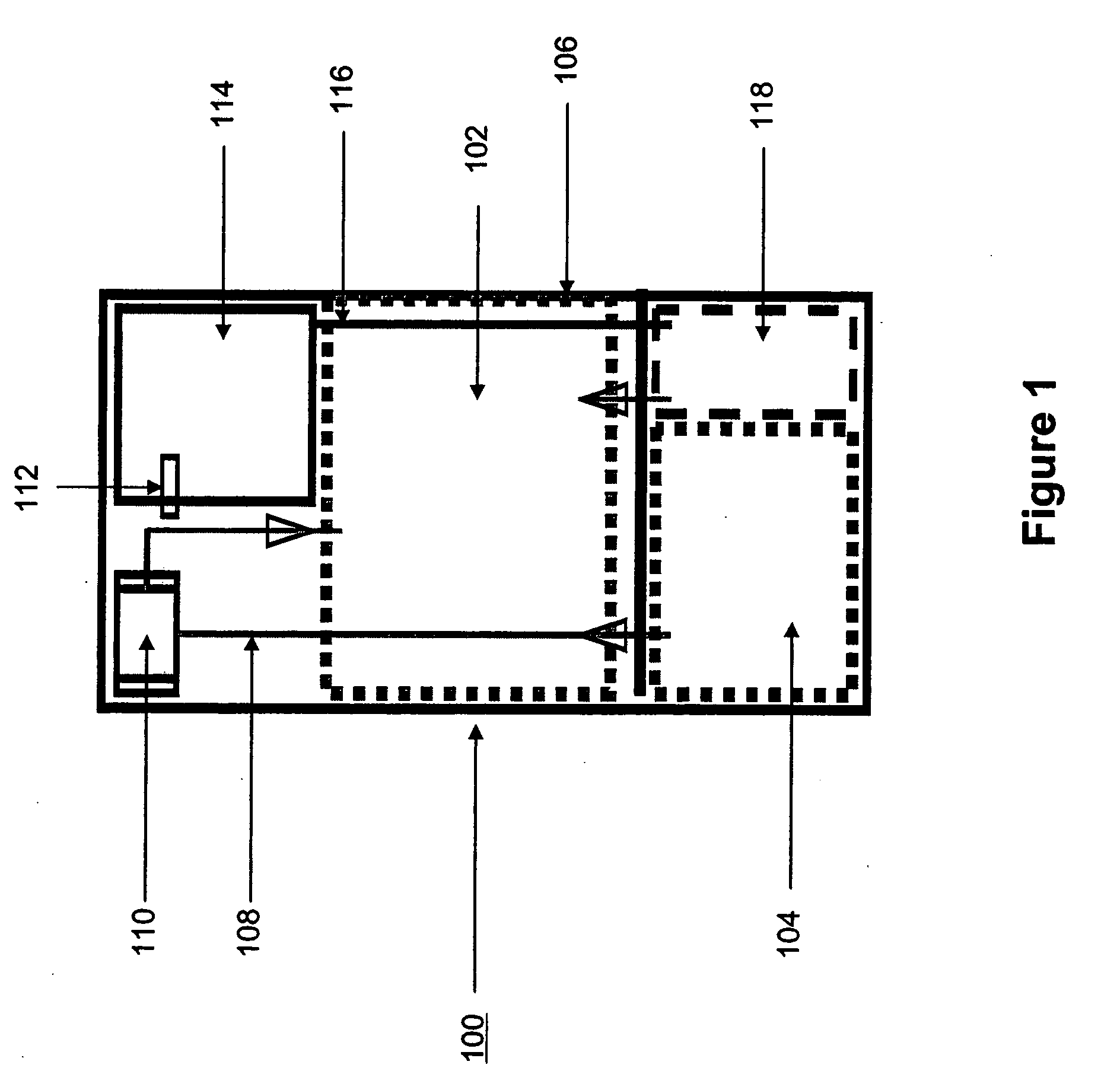
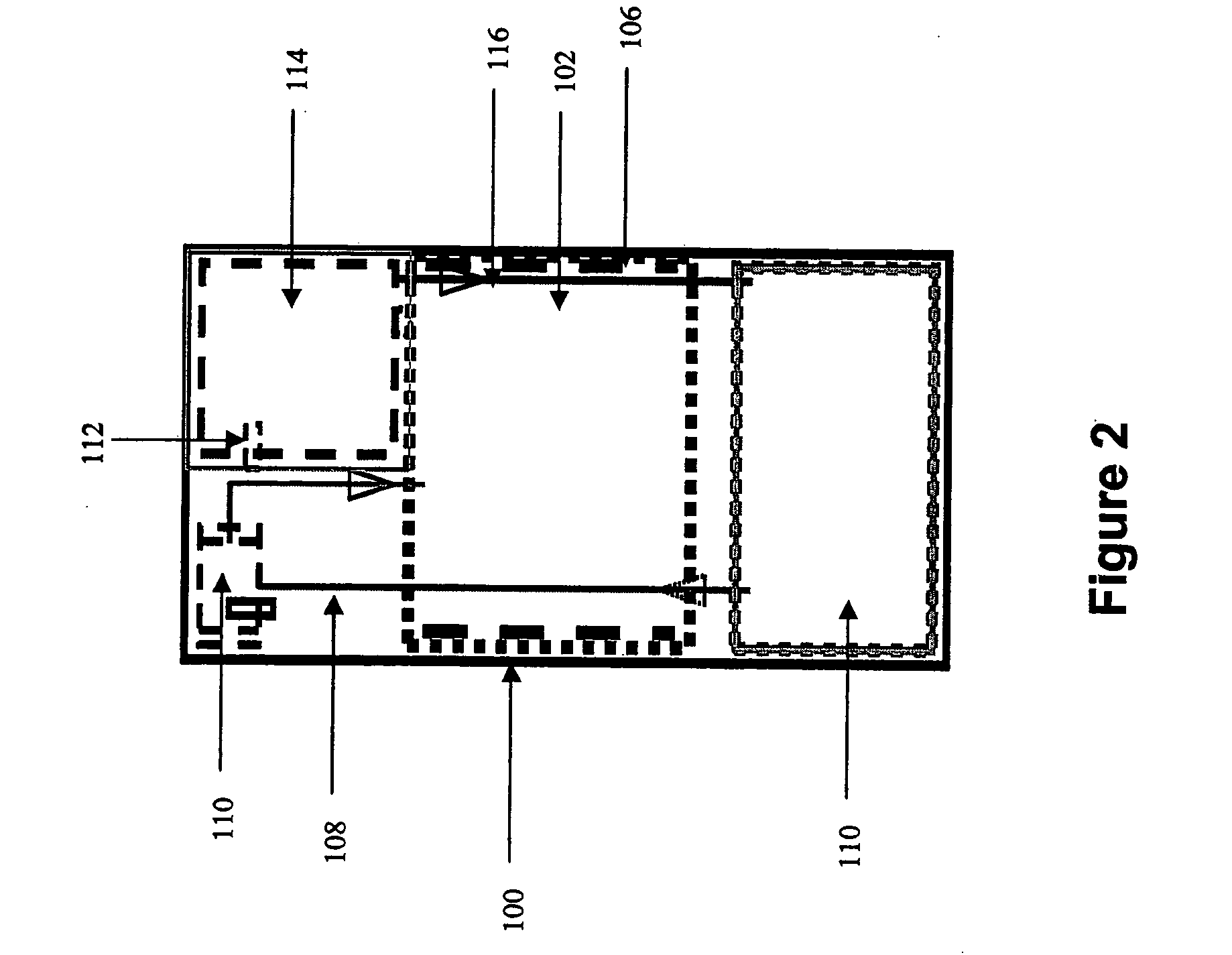
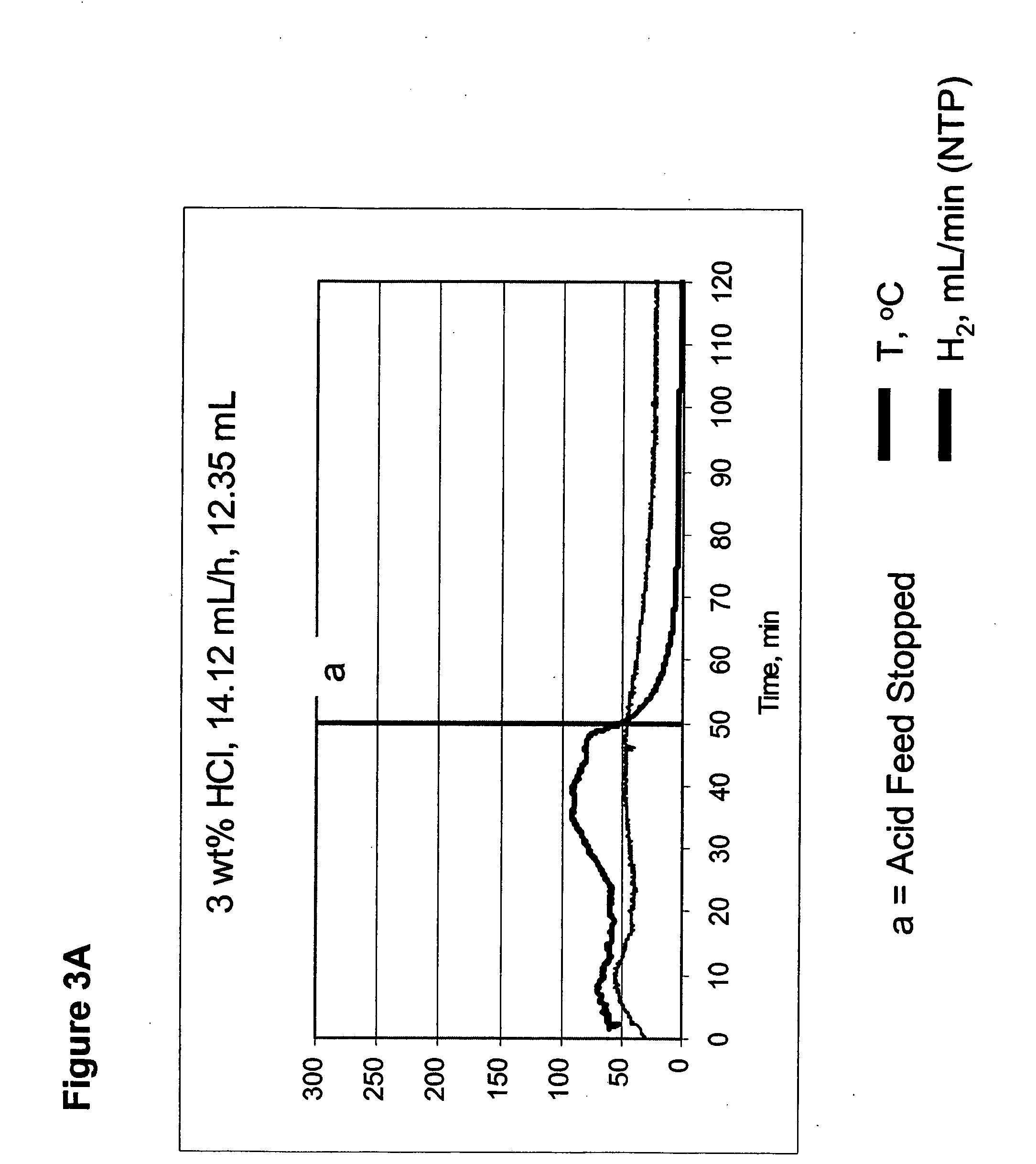
Systems and methods for hydrogen generation from solid hydrides
InactiveUS20050238573A1Regulate rateReactant parameters controlHydrogen productionO-Phosphoric AcidAlkaline earth metal
A system is disclosed for hydrogen generation based on hydrolysis of solid chemical hydrides with the capability of controlled startup and stop characteristics wherein regulation of acid concentration, acid feed rate, or a combination of both control the rate of hydrogen generation. The system comprises a first chamber for storing a solid chemical hydride and a second chamber for storing an acidic reagent. The solid chemical hydride is a solid metal borohydride having the general formula MBH4, where M is selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation. The acidic reagent may comprise inorganic acids such as the mineral acids hydrochloric acid, sulfuric acid, and phosphoric acid, and organic acids such as acetic acid, formic acid, maleic acid, citric acid, and tartaric acid, or mixtures thereof.
Owner:MILLENNIUM CELL
Fuel Composition
ActiveUS20080229654A1Maximize product yieldYield maximizationFatty acid isomerisationFatty acid oxidationIsomerizationVegetable oil
Compositions and methods for forming hydrocarbon products from triglycerides are disclosed. In one aspect, the methods involve the thermal decomposition of fatty acids, which can be derived from the hydrolysis of triglycerides. The thermal decomposition products can be combined with low molecular weight olefins, such as Fischer-Tropsch synthesis products, and subjected to molecular averaging reactions. Alternatively, the products can be subjected to hydrocracking reactions, isomerization reactions, and the like. The products can be isolated in the gasoline, jet and / or diesel fuel ranges. Thus, vegetable oils and / or animal fats can be converted using water, catalysts, and heat, into conventional products in the gasoline, jet and / or diesel fuel ranges. These products are virtually indistinguishable from those derived from their petroleum-based analogs, except that they can have virtually no aromatic, sulfur or nitrogen content, they are derived, in whole or in part, from renewable resources, and can also be derived from domestically available coal and / or natural gas.
Owner:BRADIN DAVID
Isolatable, water soluble, and hydrolytically stable active sulfones of poly(ethylene glycol) and related polymers for modification of surfaces and molecules
A poly(ethylene glycol) derivative is disclosed that is activated with a sulfone moiety for selective attachment to thiol moieties on molecules and surfaces. The activated PEG is water soluble, hydrolytically stable for extended periods, and forms hydrolytically stable linkages with thiol moieties. The linkages generally are not reversible in reducing environments. The PEG derivative is useful for modifying the characteristics of substances including modifying biologically active molecules and surfaces for biocompatibility. Methods for synthesizing the active PEG and for preparing conjugates of the active PEG and other substances, including biologically active substances, are also disclosed.
Owner:NEKTAR THERAPEUTICS INC
Dissolving filter cake
Owner:SCHLUMBERGER TECH CORP
Soluble silicone resin compositions having good solution stability
InactiveUS6232424B1Improve solution stabilityLow dielectric constantGroup 4/14 element organic compoundsCoatingsOrganic solventHydrolysis
Soluble silicone resin compositions having good solution stability and a method for their preparation. The silicone resin comprises the reaction product of a mixture comprising(A) 15-70 mol % of a tetraalkoxysilane described by formula where each R1, is an independently selected alkyl group comprising 1 to about 6 carbon atoms,(B) 12 to 60 mol % of an organosilane described by formula where R4, is selected from the group consisting of alkyl groups comprising 1 to about 6 carbon atoms and phenyl and each X is an independently selected hydrolyzable substituent,(C) 15 to 70 mole percent of an organotrialkoxysilane described by formula where R2, is a hydrocarbon group comprising about 8 to 24 carbon atoms or a substituted hydrocarbon group comprising a hydrocarbon chain having about 8 to 24 carbon atoms and each R3, is an independently selected alkyl group comprising 1 to about 6 carbon atoms; in the presence of(D) water,(E) hydrolysis catalyst, and(F) organic solvent for the reaction product.
Owner:DOW CORNING CORP
Degradable additive for viscoelastic surfactant based fluid systems
A method is given for treating a subterranean formation penetrated by a wellbore with a viscosified fluid. The fluid contains a solid hydrolysable polyacid that upon dissolution and hydrolysis releases an acid that is a breaker for the viscosifying system. Suitable solid hydrolysable polyacids include polylactic acid and polyglycolic acid. The fluid also contains a pH control agent, present in an amount sufficient to neutralize any acid present in the solid hydrolysable polyacid before the injection and to neutralize any acid generated by the solid hydrolysable polyacid during the injection, so that the acid breaker is not available to break the fluid during the injection. In one embodiment the viscosifier is a viscoelastic surfactant fluid system and the solid hydrolysable polyacid is of a size selected to be a fluid loss additive, for example in fracturing or gravel packing. In another embodiment, the solid hydrolysable polyacid is used in particles sufficiently small that they enter the pores of the formation. In either case, the viscosifier is broken after the solid releases more acid than can be neutralized by the pH control agent.
Owner:SCHLUMBERGER TECH CORP
Barriers for polymer-coated implantable medical devices and methods for making the same
InactiveUS6953560B1Reduce and prevent and inflammationReduce and prevent proliferationStentsSurgeryHafniumPt element
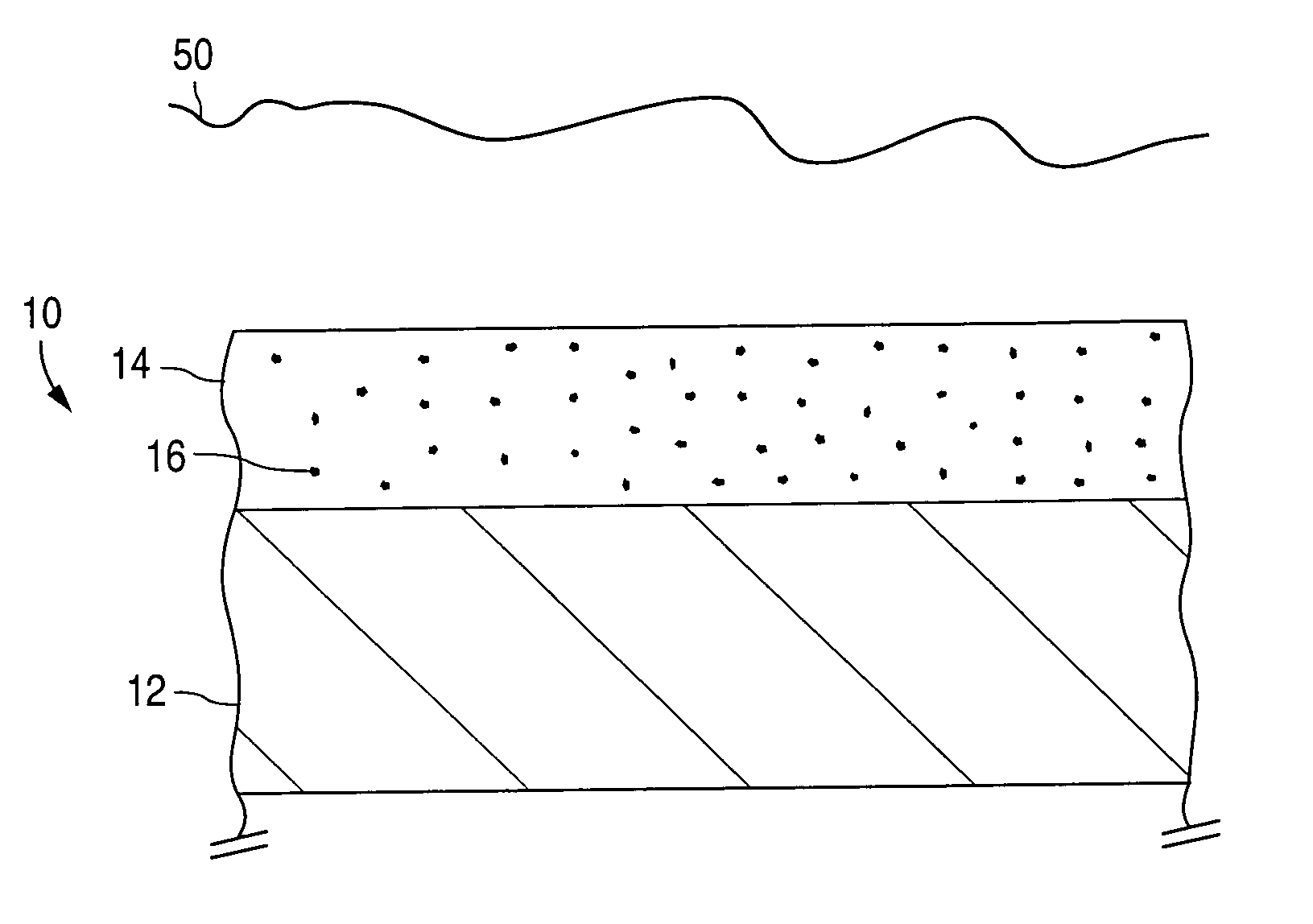
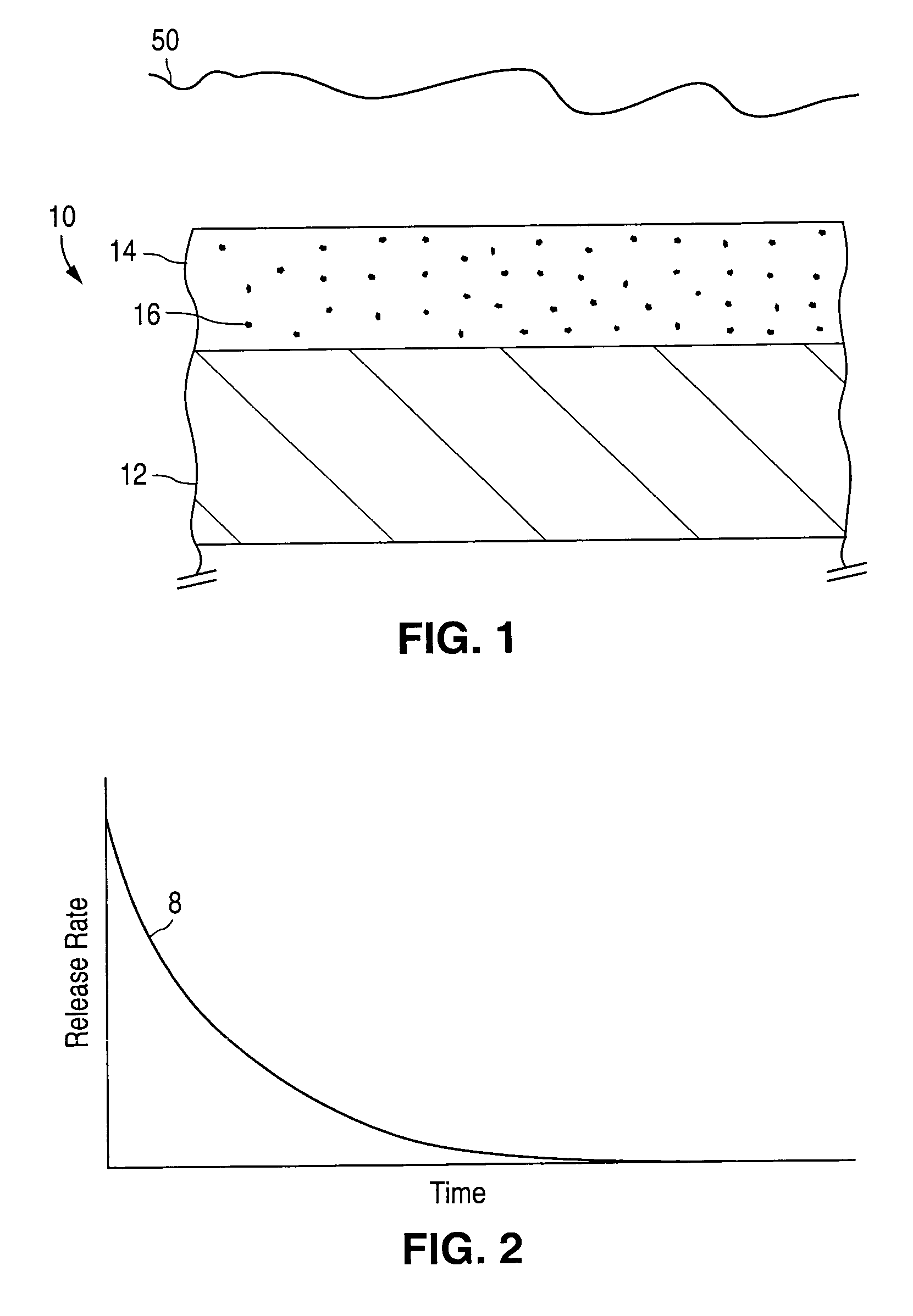
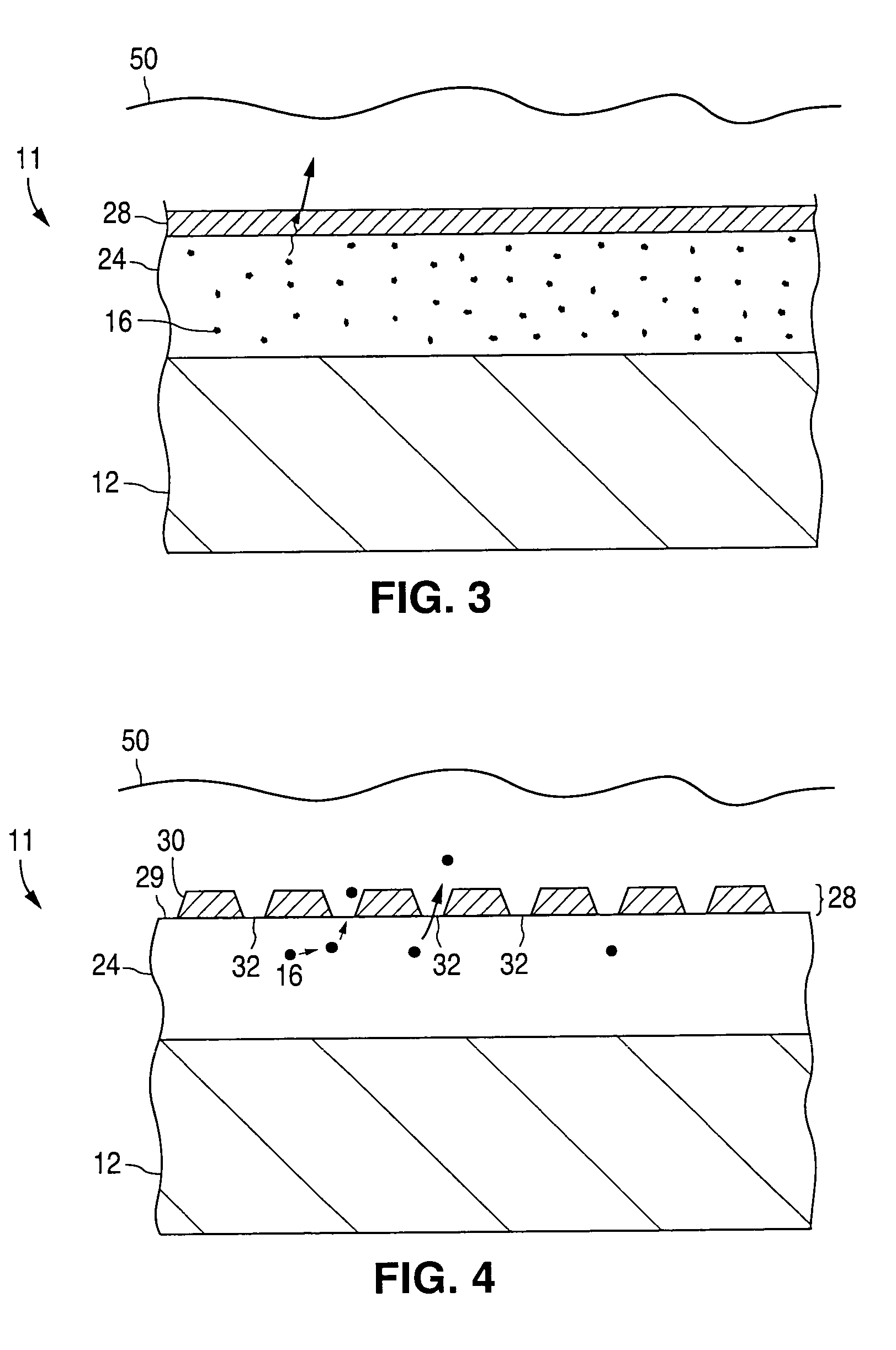
An implantable medical device and methods for making the implantable medical device are disclosed. The implantable medical device includes a substrate. At least a portion of the substrate is coated with a first layer including a polymer containing a drug. A barrier overlies the first layer. The barrier significantly reduces the rate of release of the drug from the polymer, thereby sustaining release of the drug from the medical device for a longer time.The barrier may be a homogeneous layer overlying the first layer, or a number of discrete deposits over the first layer. Alternatively, the barrier may be intermixed with an outer portion of the first layer. The barrier material is biocompatible, and typically has a thickness ranging from about 50 angstroms to about 20,000 microns. Suitable materials for the barrier include, but are not limited to, inorganic compounds, such as inorganic silicides, oxides, nitrides, carbides, as well as pure metals such as aluminum, chromium, gold, hafnium, iridium, niobium, palladium, platinum, tantalum, titanium, tungsten, zirconium, and alloys of these metals. The barriers disclosed may be applied to the first layer by several techniques, depending on the material being applied. Exemplary deposition techniques include physical vapor deposition, alkoxide hydrolysis, and electroless plating.The implantable device may be a stent or a graft, among other possibilities.
Owner:ABBOTT CARDIOVASCULAR
Compositions and methods using nicotinic acid for treatment of hypercholesterolemia, hyperlipidemia nd cardiovascular disease
InactiveUS20070105793A1BiocideCarbohydrate active ingredientsCardiovascular problemsImmediate release
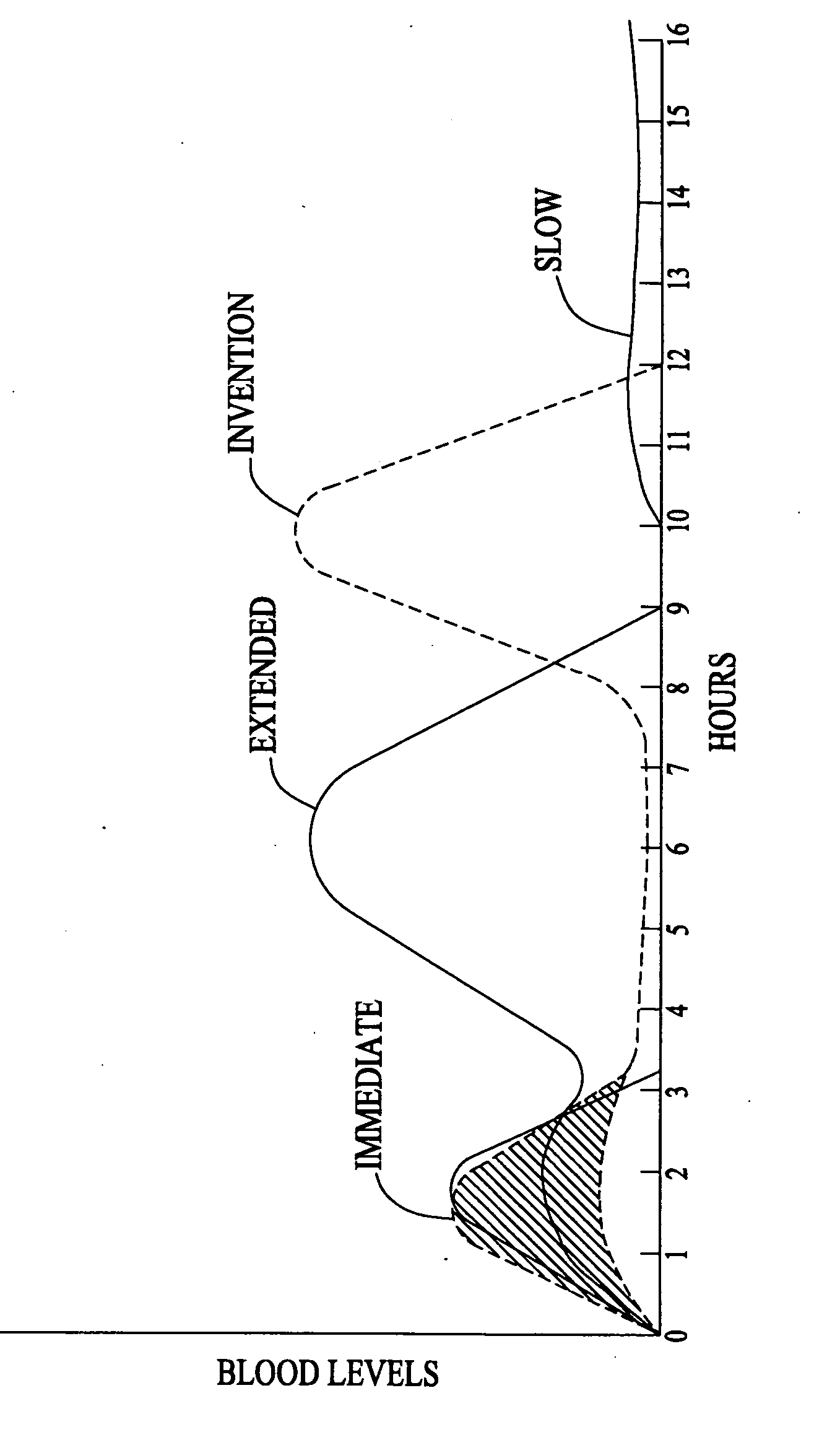
An improved method and composition for the treatment of hyperlipidemia, hypercholesterolemia, and hypertriglyceridemia as well as other disease states, particularly cardiovascular problems, uses a multiple release system that provides an immediate release of nicotinic acid followed by a subsequent release of nicotinic acid from the hydrolysis of a compound that generates nicotinic acid. In general, a composition according to the present invention comprises: (1) a quantity of nicotinic acid intended to saturate liver enzymes during a period from about 0.5 hours to about 2.5 hours after administration of the pharmaceutical composition but insufficient to trigger significant generation of nicotinuric acid; (2) a quantity of a derivative or analogue of nicotinic acid that is subject to hydrolysis after ingestion such that preferrably no more than about 10% of the derivative or analogue is hydrolyzed by about 8 hours after ingestion and such that substantially all of the derivative or analogue is hydrolyzed by about 12 hours after ingestion; and (3) at least one pharmaceutically acceptable carrier.
Owner:CONCOURSE HEALTH SCI
Hybrid organic-inorganic planar optical waveguide device
InactiveUS6511615B1Reduce stressStress induced polarization effects can be minimizedOptical articlesGlass shaping apparatusOptical radiationSilanes
A planar optical device is formed on a substrate. The device comprises an array of waveguide cores which guide optical radiation. A cladding layer is formed contiguously with the array of waveguide cores to confine the optical radiation to the array of waveguide cores. At least one of the array of waveguide cores and cladding layer is an inorganic-organic hybrid material that comprises an extended matrix containing silicon and oxygen atoms with at least a fraction of the silicon atoms being directly bonded to substituted or unsubstituted hydrocarbon moieties. This material can be designed with an index of refraction between 1.4 and 1.55 and can be deposited rapidly to thicknesses of up to 40 microns. In accordance with another embodiment of the invention, a method for forming a planar optical device obviates the need for a lithographic process. Illustratively, a method for forming an array of cores comprises the steps of: (1) preparing a waveguide core composition precursor material comprising at least one silane and a source of hydrocarbon moiety, (2) partially hydrolyzing and polymerizing the waveguide core precursor material to form a waveguide core composition, (3) using a mold, forming an array of waveguide cores comprising the waveguide core composition, and (4) completing hydrolysis and polymerization of the waveguide core composition under conditions effective to form an inorganic-organic hybrid material that comprises an extended matrix containing silicon and oxygen atoms with at least a fraction of the silicon atoms being directly bonded to substituted or unsubstituted hydrocarbon moieties. A cladding layer is then deposited over the array of waveguide cores. The use of the mold to pattern the array of waveguide cores obviates the need for a lithographic process.
Owner:CORNING INC
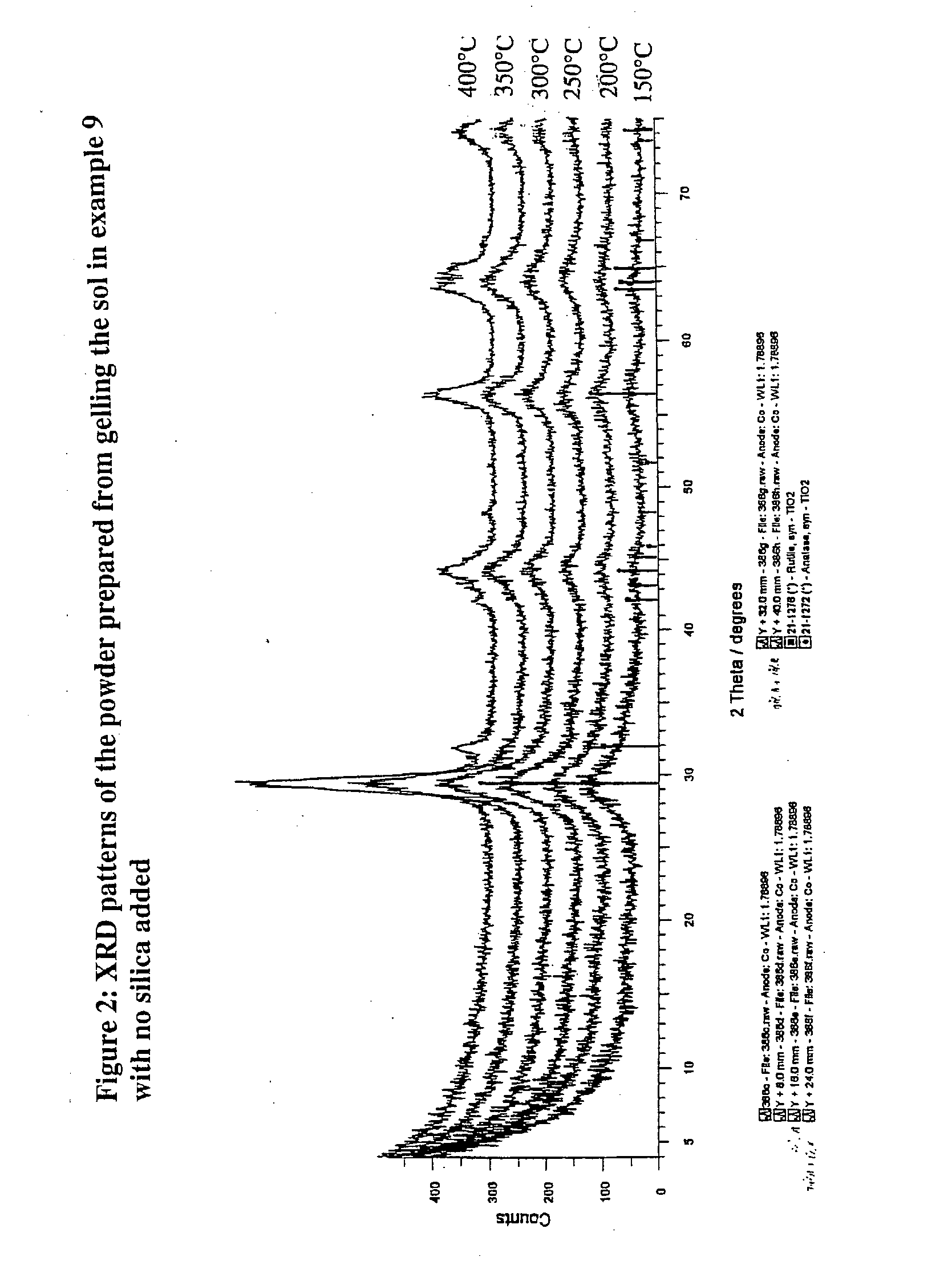
Titanium-containing materials
The invention relates to a method of preparing a solution containing colloidal particles which contain crystalline titanium dioxide wherein one or more hydrolysable titanium-containing compound(s) is stabilised by oxalic acid in a reaction medium. The reaction further relates to the preparation of titania materials (including particulate materials, coating solutions and films) which comprise or include anatase phase titania, and so are suitable in photocatalytic applications. The invention also deals with a method of preparing B-phase titania.
Owner:IND RES LTD
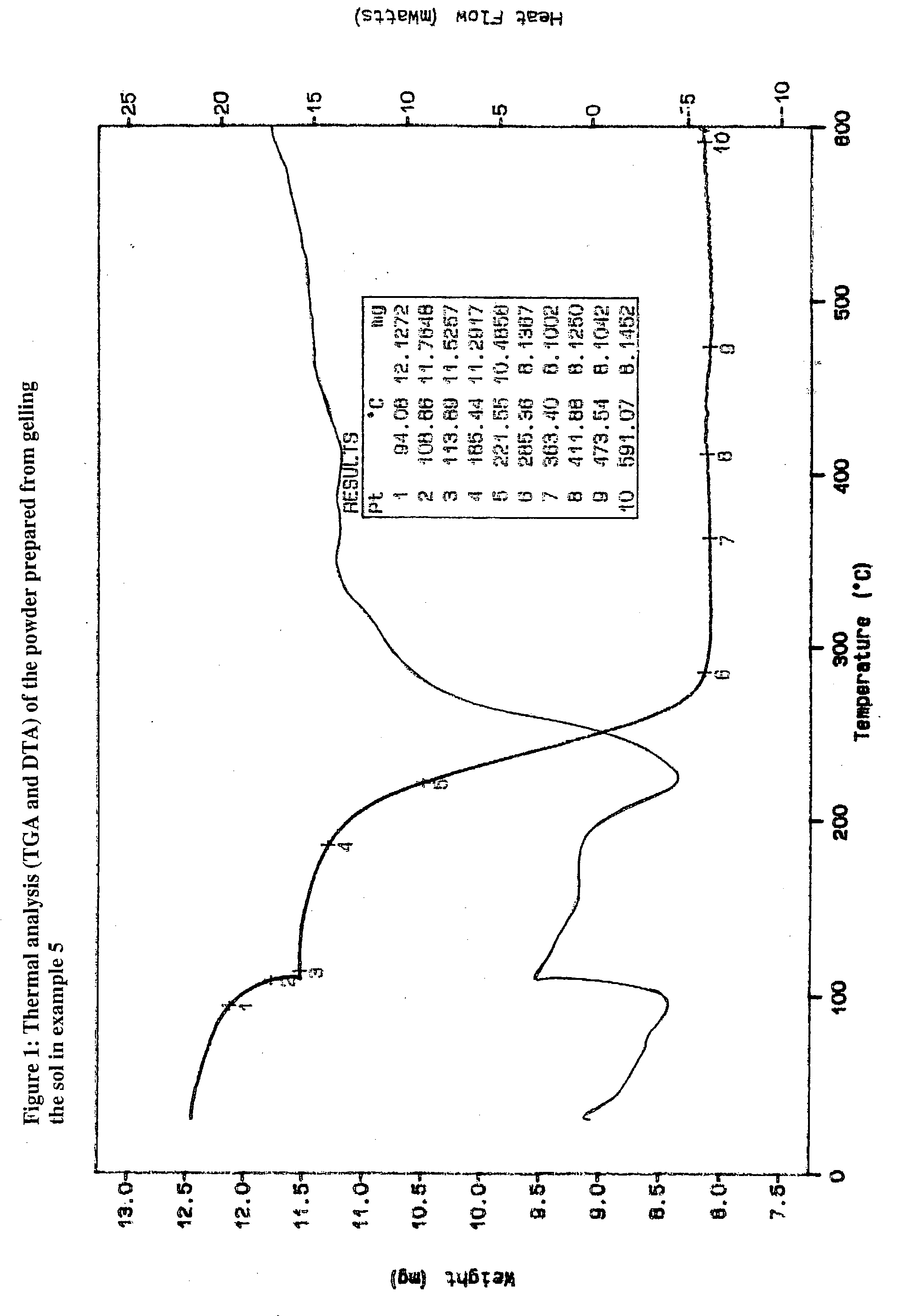
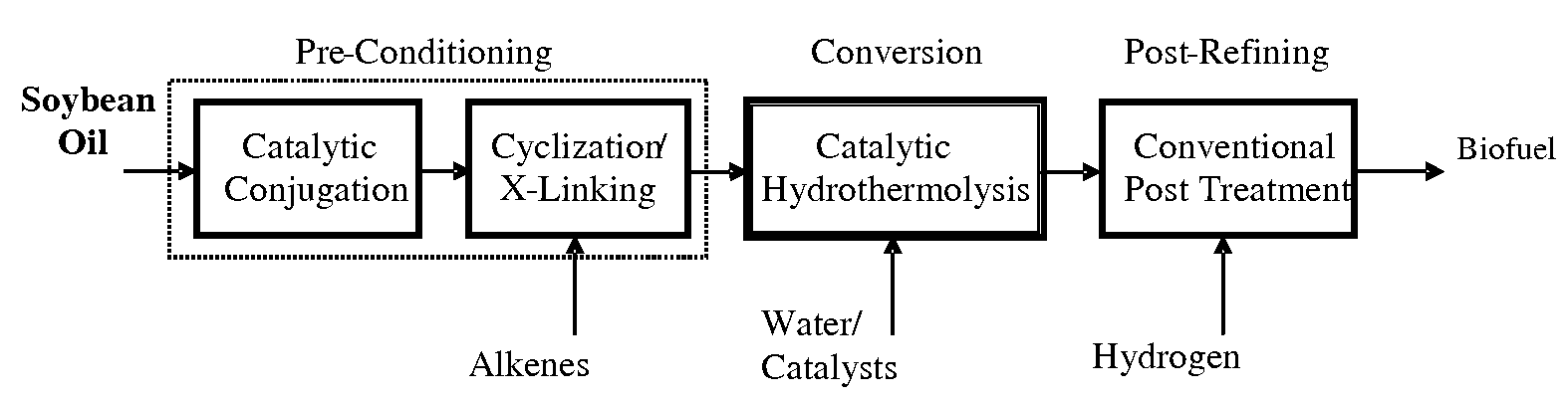
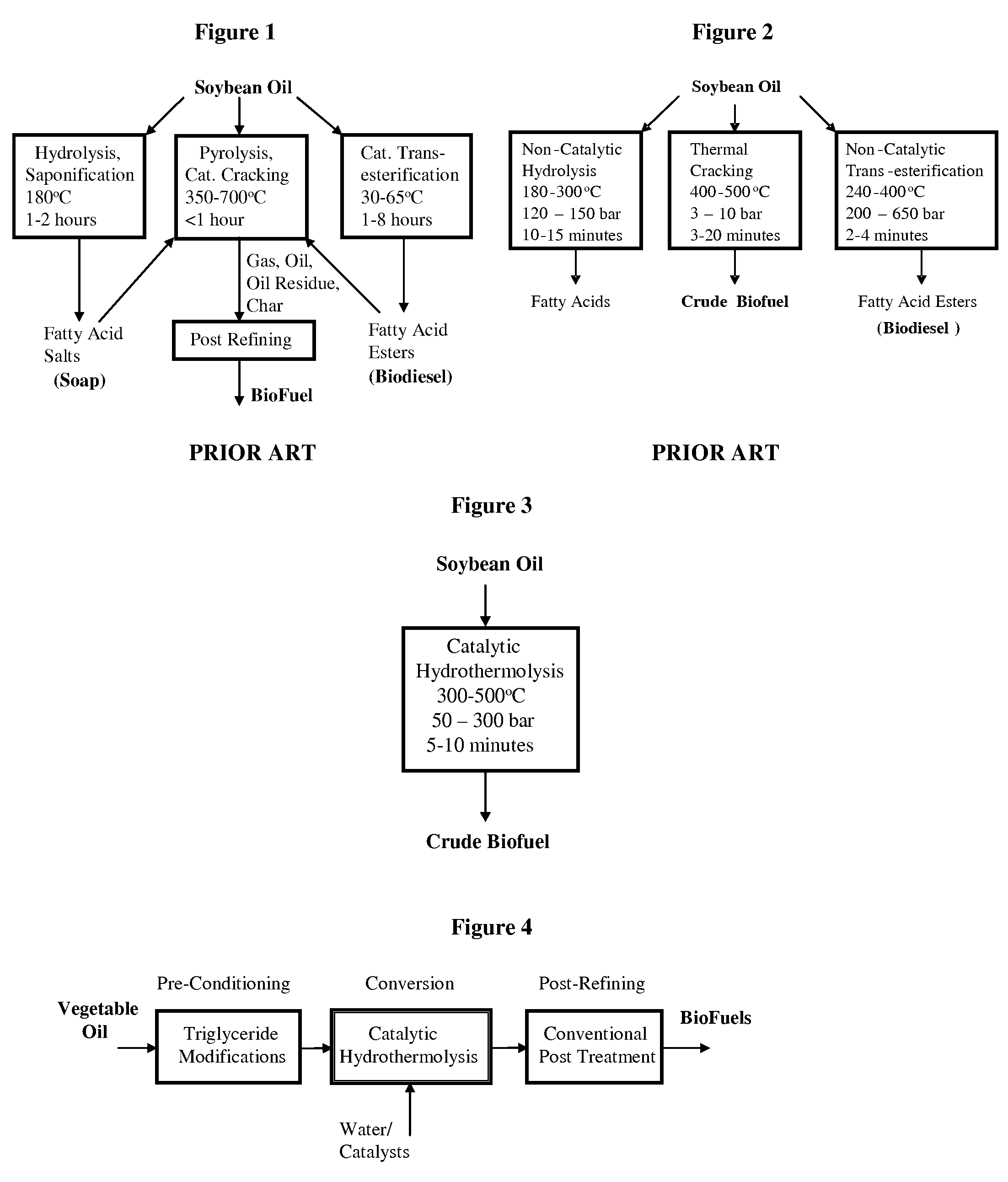
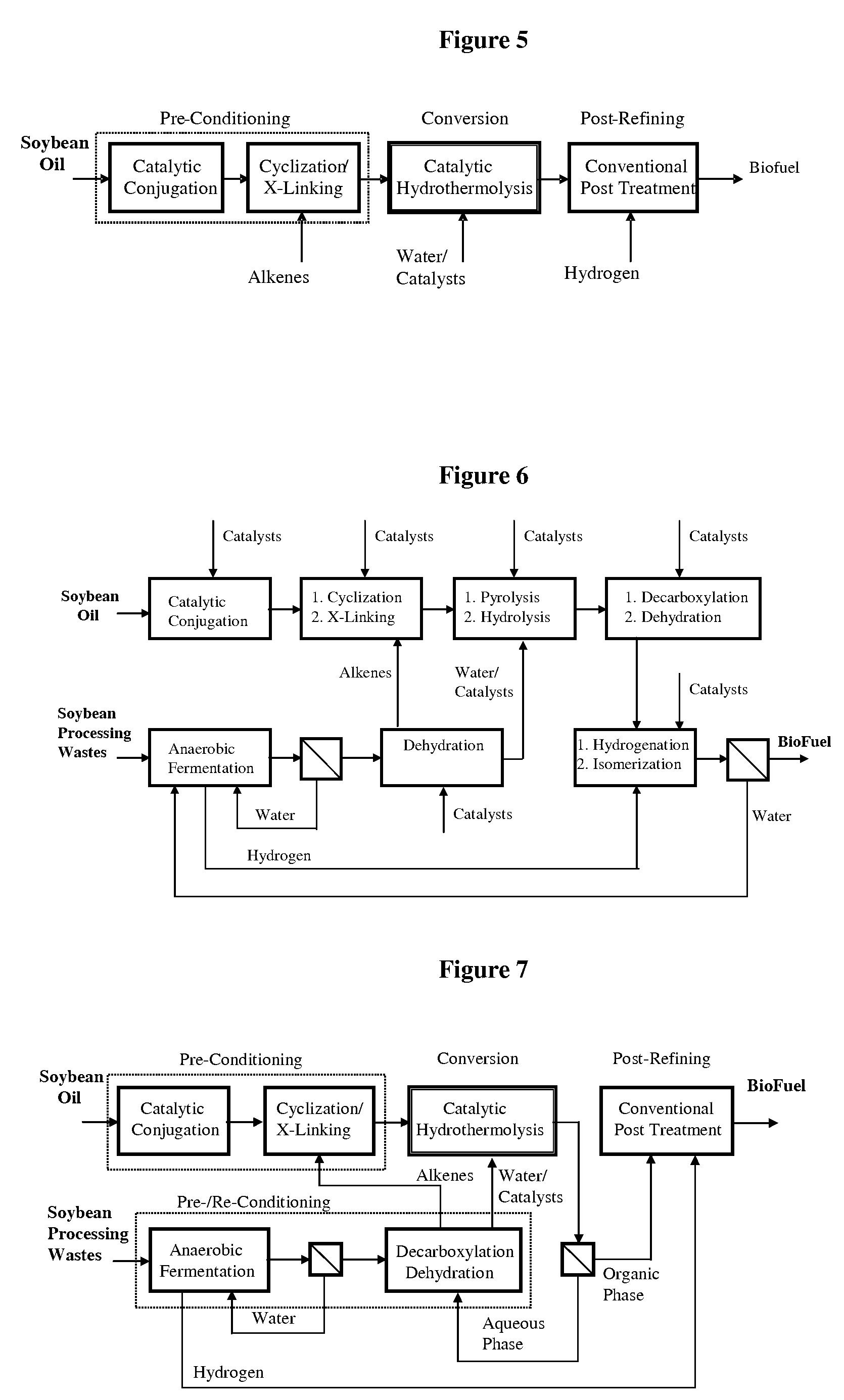
Method of converting triglycerides to biofuels
ActiveUS7691159B2Improve chemical and physical and combustion qualityImprove thermal stabilityFatty acid chemical modificationOrganic compound preparationCross-linkIsomerization
A triglyceride-to-fuel conversion process including the steps of (a) preconditioning unsaturated triglycerides by catalytic conjugation, cyclization, and cross-link steps; (b) contacting the modified triglycerides with hot-compressed water containing a catalyst, wherein cracking, hydrolysis, decarboxylation, dehydration, aromatization, or isomerization, or any combination thereof, of the modified triglycerides produce a crude hydrocarbon oil and an aqueous phase containing glycerol and lower molecular weight molecules, and (c) refining the crude hydrocarbon oil to produce various grades of biofuels. A triglyceride-to-fuel conversion process further including the steps of (a) carrying out anaerobic fermentation and decarboxylation / dehydration, wherein the anaerobic fermentation produces hydrogen, volatile acids, and alcohols from fermentable feedstocks, and the decarboxylation / dehydration produces alkenes from the volatile acids and alcohols, respectively; (b) feeding the alkenes to the cyclization process; (c) feeding the hydrogen to the post refining process; and (d) recycling the aqueous phase containing glycerol to the decarboxylation / dehydration process. A biofuel composition including straight-chain, branched and cyclo paraffins, and aromatics. The paraffins are derived from conversion of triglycerides. The aromatics are derived from conversion of either triglycerides, petroleum, or coal.
Owner:APPLIED RES ASSOCS INC
Doped, pyrogenically prepared oxides
InactiveUS6328944B1Germanium dioxidePipe protection by thermal insulationDoped oxideAqueous solution
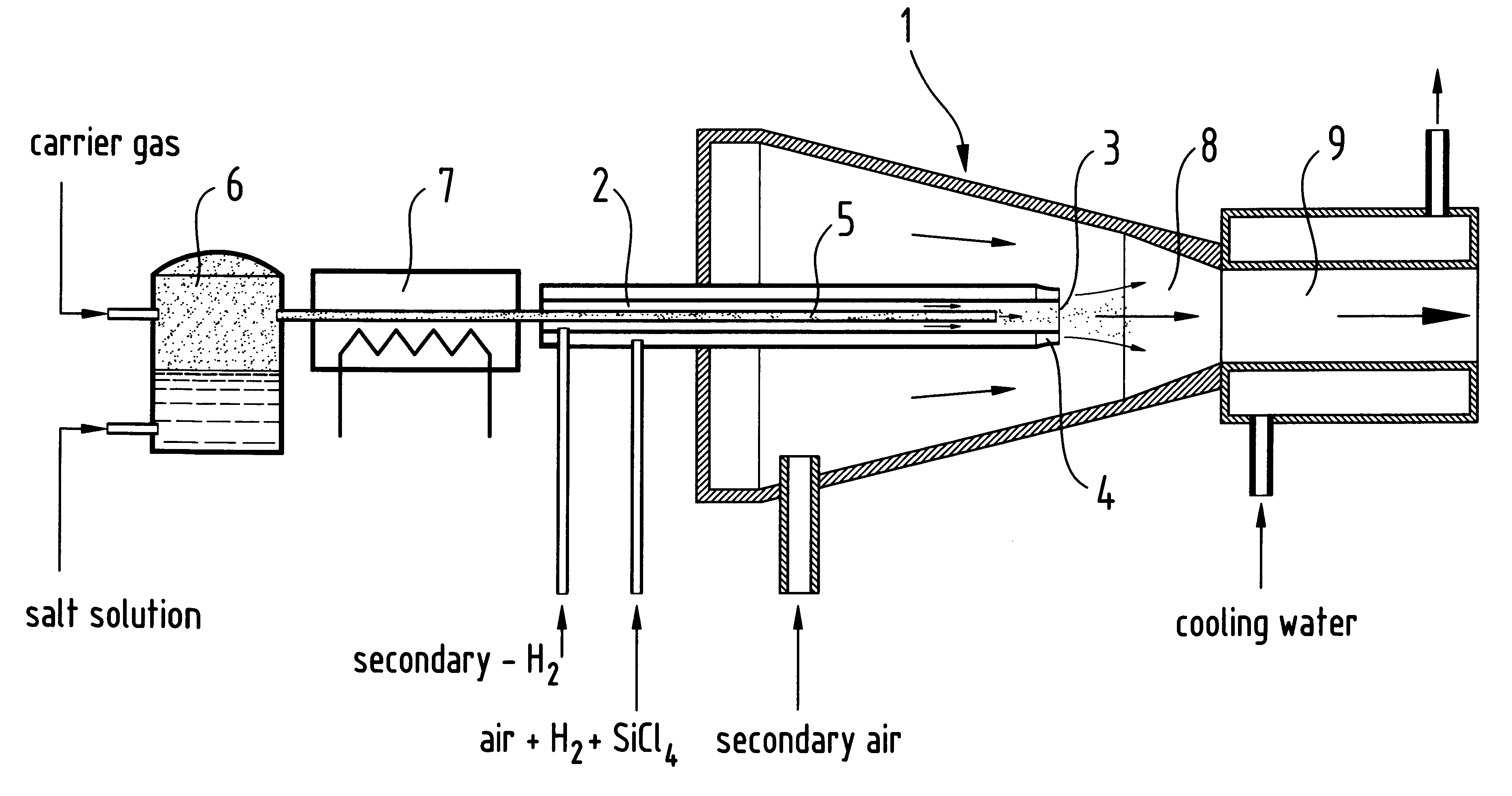
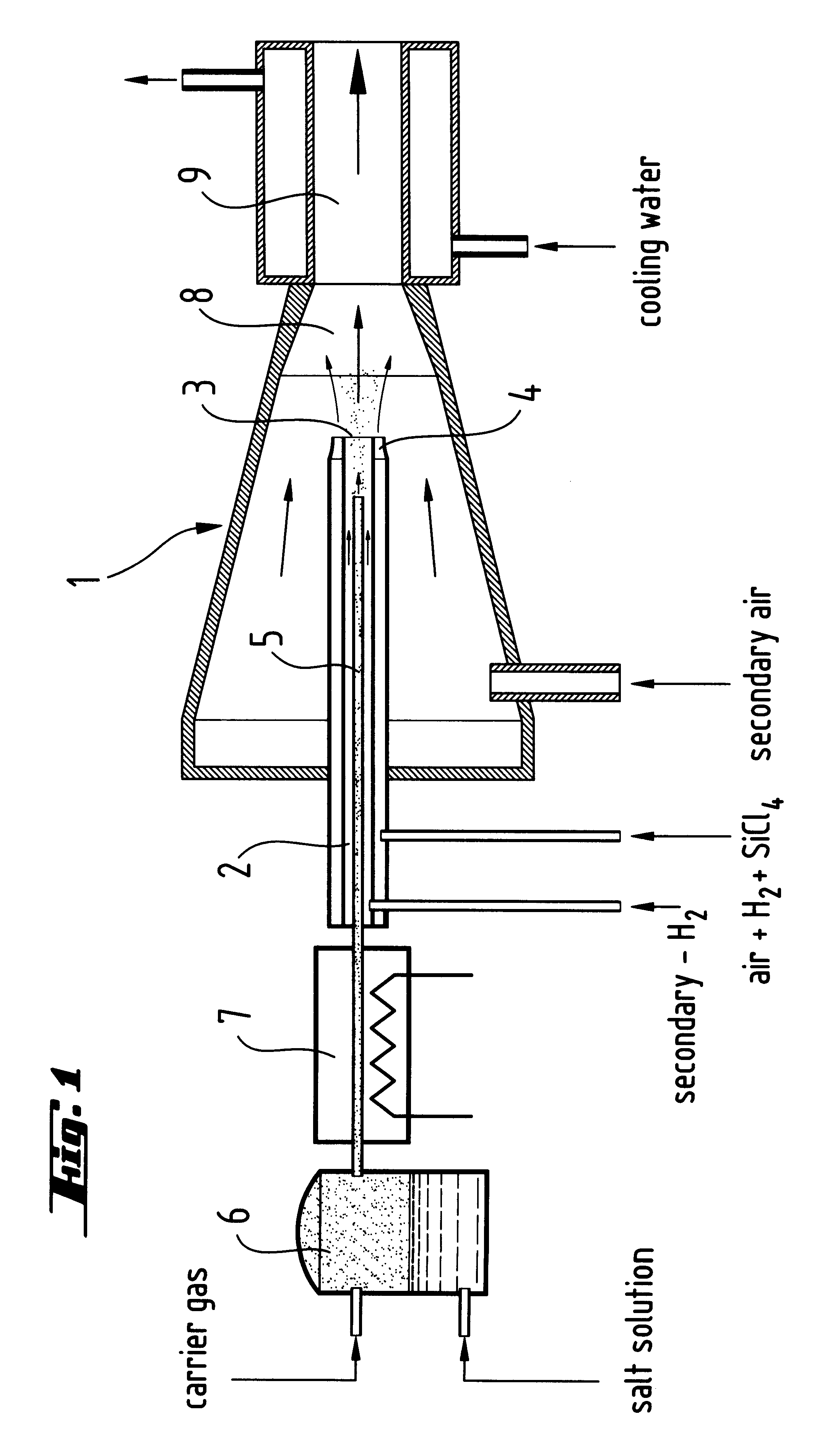
Doped, pyrogenically prepared oxides of metals and / or non-metals which are doped with one or more doping components in an amount of 0.00001 to 20 wt. %. The doping component may be a metal and / or non-metal or an oxide and / or a salt of a metal and / or a non-metal. The BET surface area of the doped oxide may be between 5 and 600 m2 / g. The doped pyrogenically prepared oxides of metals and / or non-metals are prepared by adding an aerosol which contains an aqueous solution of a metal and / or non-metal to the gas mixture during the flame hydrolysis of vaporizable compounds of metals and / or non-metals.
Owner:EVONIK DEGUSSA GMBH
Process for the preparation and purification of thiol-containing maytansinoids
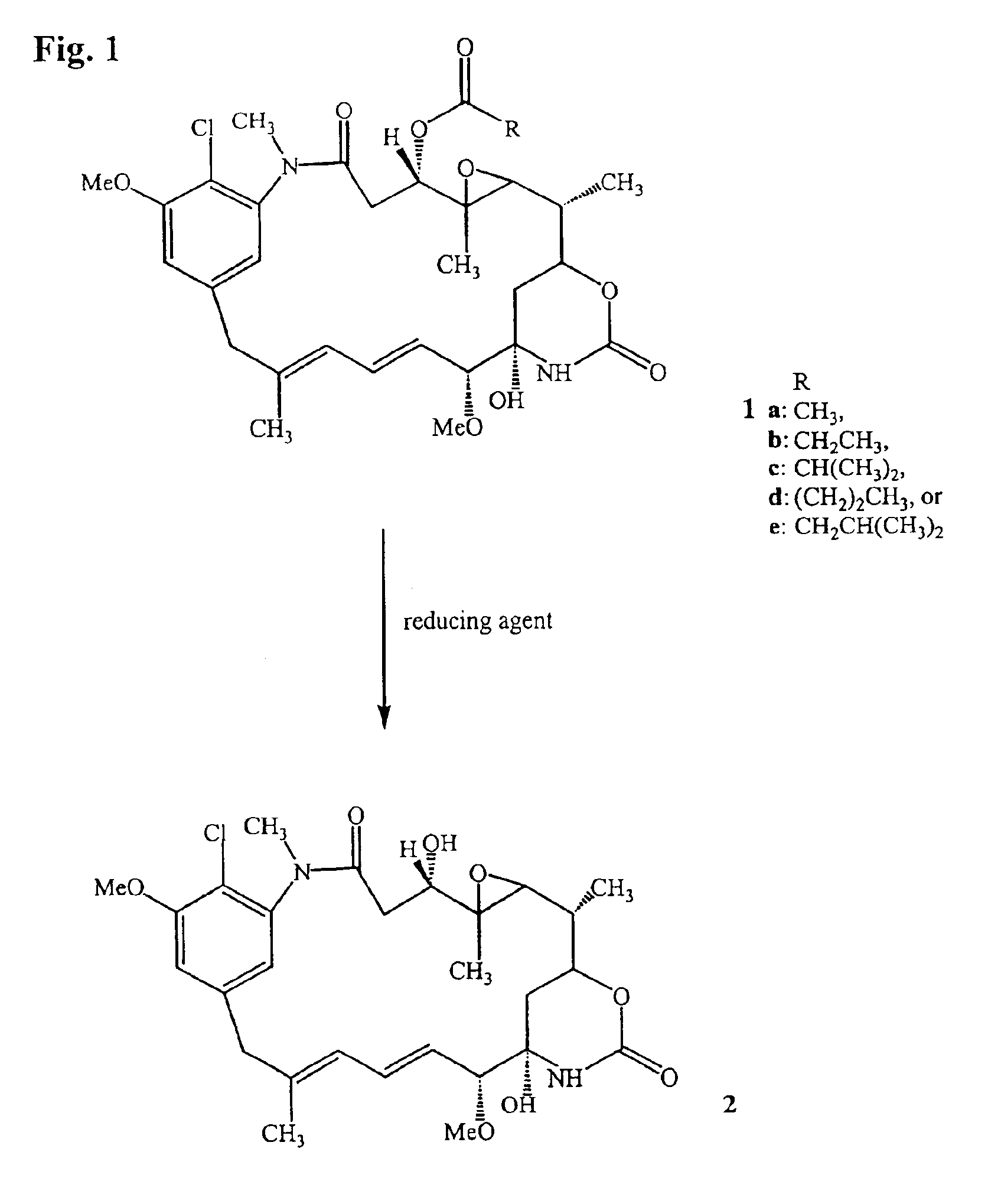
InactiveUSRE39151E1Reduce process complexityAllows scalabilityOrganic active ingredientsOrganic chemistryLithiumThiol
The present invention provides a process for the preparation and purification of thiol-containing maytansinoids comprising the steps of: (1) reductive hydrolysis of a maytansinoid C-3 ester with a reducing agent selected from the group consisting lithium trimethoxyaluminum hydride (LiAl (OMe)3H), lithium triethoxyaluminum hydride (LiAl(OEt)3H), lithium tripropoxyaluminum hydride (LiAl (OPr)3H), sodium trimethoxyaluminum hydride (NaAl (OMe)3H), sodium triethoxyaluminum hydride (NaAl(OEt)3H) and sodium tripropoxyaluminum hydride (NaAl(OPr)3H) to yield a maytansinol; (2) purifying the maytansinol to remove side products when present; (3) esterifying the purified maytansinol with a carboxylic acid to yield a mixture of an L- and a D-aminoacyl ester of maytansinol; (4) separating the L-aminoacyl ester of maytansinol from the reaction mixture in (3); (5) reducing the L-aminoacyl ester of maytansinol to yield a thiol-containing maytansinoid; and (5) purifying the thiol-containing maytansinoid.
Owner:IMMUNOGEN INC
Method and apparatus for producing ammonia (NH3)
InactiveUS6928807B2High activityNitrogen compoundsInternal combustion piston enginesHydrolysisRapid thermal processing
A method is provided for producing ammonia (NH3) and introducing the produced ammonia (NH3) into an exhaust gas stream as a reduction means for selectively catalytically reducing nitrogen oxides contained in the exhaust gas stream, which is an exhaust stream generated by the combustion process of a motor, a gas turbine, or a burner. The method comprises feeding dry urea from a supply container in a controlled amount to reactor and subjecting the dry urea in the reactor to a sufficiently rapid thermal treatment such that a gas mixture comprising the reaction products of ammonia (NH3) and isocyanic acid (HCNO) is created. Also, the method comprises immediately catalytically treating the thus produced gas mixture in the presence of water such that the isocyanic acid (HCNO) resulting from the rapid thermal treatment is converted, via quantitative hydrolysis treatment, into ammonia (NH3) and carbon dioxide (CO2).
Owner:MAN NUTZFAHRZEUGE AG
Vaccines comprising aluminium adjuvants and histidine
To improve the stability of vaccines comprising aluminum salt(s), the invention uses the amino acid histidine. This can improve pH stability and adjuvant adsorption and can be reduce antigen hydrolysis. Histidine is preferably presen during adsorption to the aluminium salt(s). The antigen in the vaccine may be a protein or a saccharide and is preferably from N. meningitidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Resin composition, method of its composition, and cured formulation
InactiveUS20060029811A1Improve flame retardant performanceMaintain good propertiesSemiconductor/solid-state device detailsSynthetic resin layered productsCarboxylic acidMicroparticle
It is an object of the present invention to provide a resin composition which can form cured formulations having various excellent properties such as an insulating property, thermal shock resistance, moldability / formability and strength, and exhibit an excellent appearance in which transparency is enhanced, a resin composition whose cured thin film has excellent flame retardancy, good mechanical property and heat resistance, a dispersing element containing an inorganic microfine particle which can give a flame retardancy to a resin, to which the inorganic microfine particle is added, and can reduce a hygroscopic property to the extent possible, a method for producing the same and a cured formulation obtained by using the resin composition. The present invention relates to a resin composition comprising a compound having at least one of a glycidyl group and / or an epoxy group and an inorganic microfine particle, a resin composition comprising three components of a phenolic compound, a compound having at least one of a glycidyl group and / or an epoxy group and an inorganic microfine particle, a flame retardant resin composition comprising a polyhydric phenol and an inorganic microfine particle, and a dispersing element containing an inorganic microfine particle obtained by a hydrolysis condensation reaction of alkoxide and / or metal carboxylate in a dispersion medium.
Owner:NIPPON SHOKUBAI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com