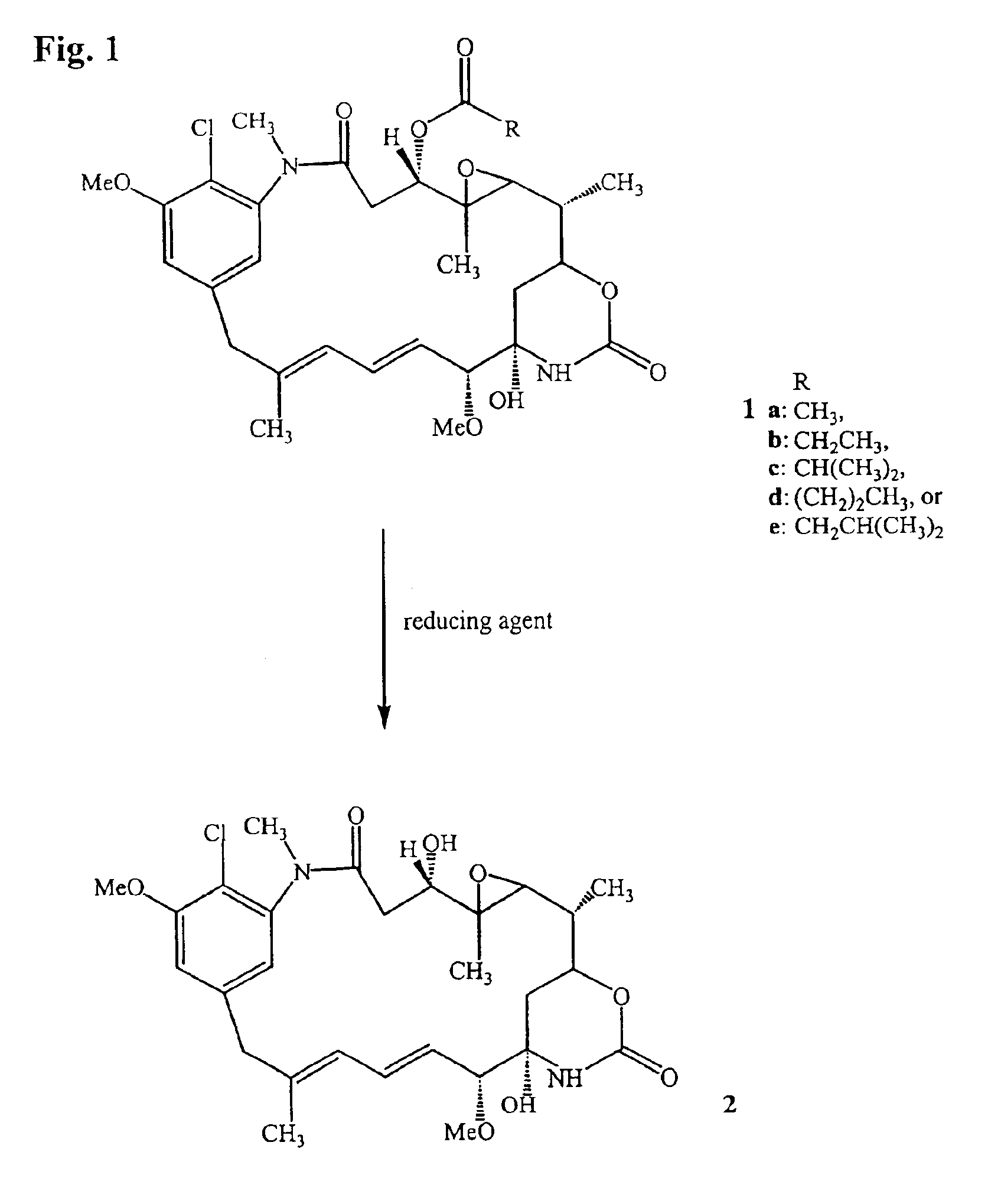
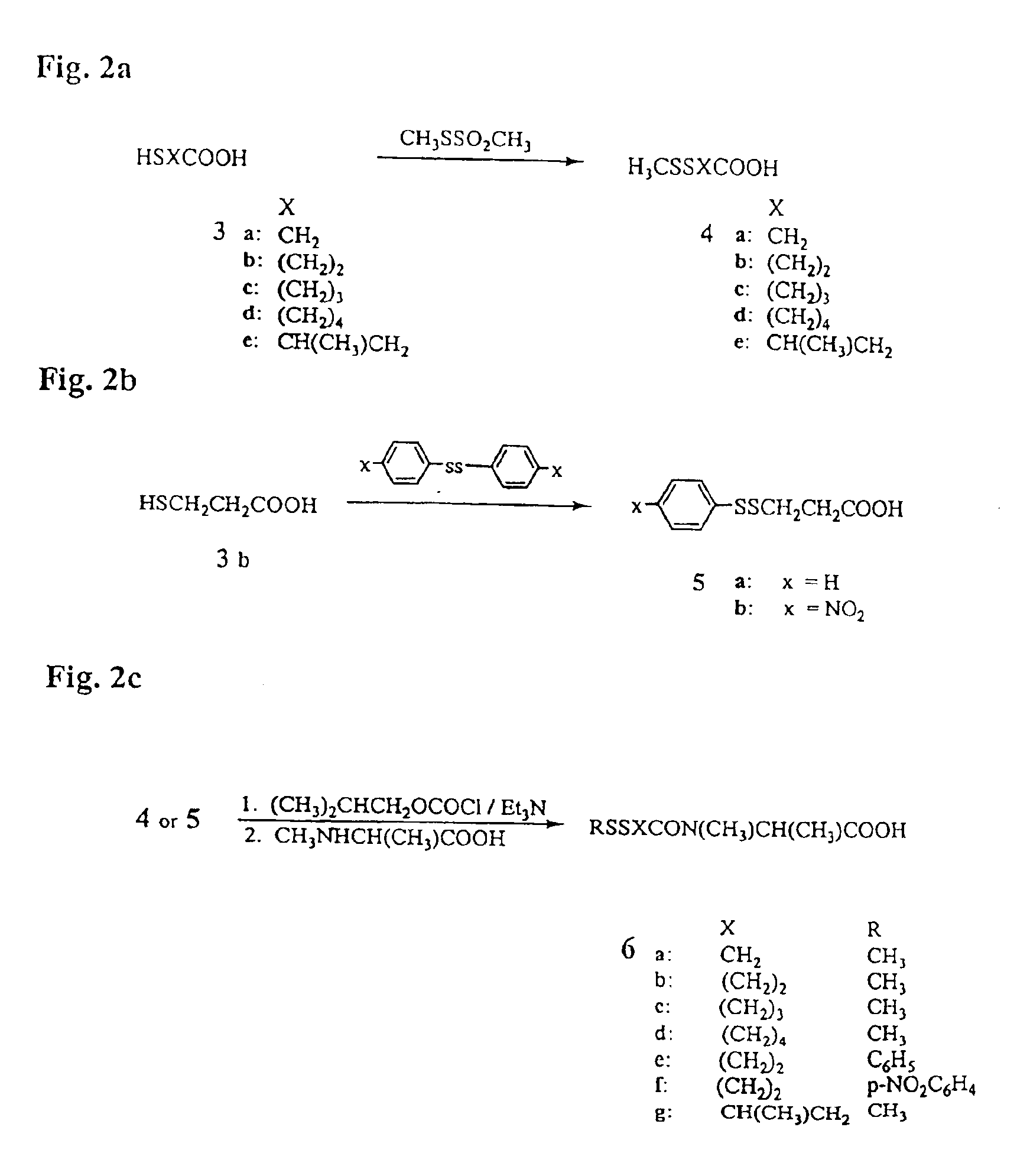
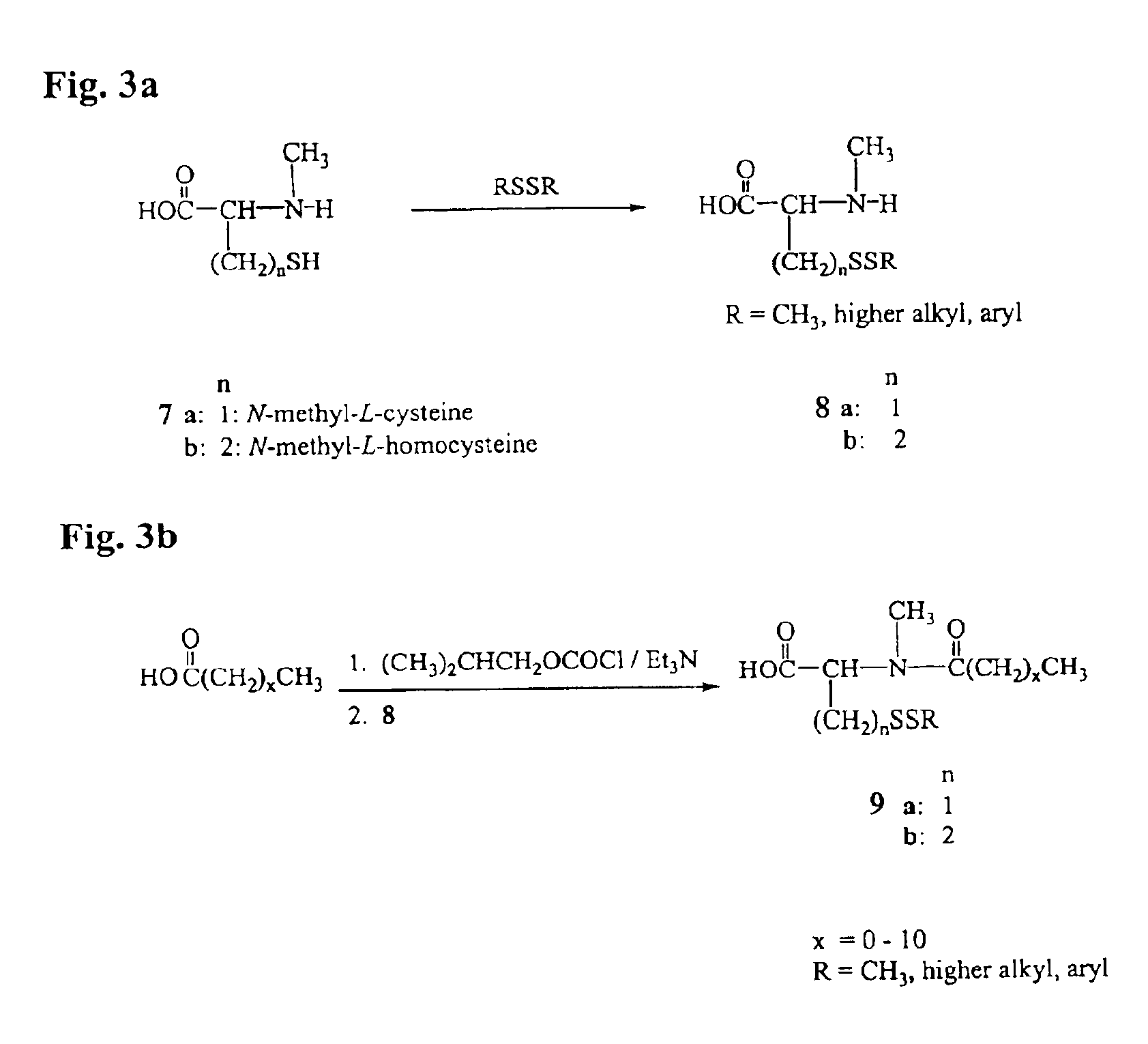
Process for the preparation and purification of thiol-containing maytansinoids
a technology of thiol-containing maytansinoids and purification process, which is applied in the field of purification process of cytotoxic agents comprising thiol-containing maytansinoids, can solve the problems of difficult chemical modification of existing drugs without reducing their cytotoxic potential, and the mechanism by which drug molecules are released from antibodies is very inefficient, so as to reduce the complexity of the process and improve the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Thiol-Containing Maytansinoid Derivatives
[0131]Melting points were determined on a electrothermal melting point apparatus. Proton magnetic resonance (1H NMR) spectra were obtained on a Varian™ EM360 spectrometer at 60 MHz or on a Bruker™ AM300 machine at 300 MHz. Chemical shifts are reported in δ values relative to an internal tetramethylsilane (TMS) standard. UV spectra were recorded on a Perkin Elmer™λ4A spectrophotometer. Optical rotations were determined using a Perkin Elmer™ model 241 polarimeter. A Rainin™ HP, Hewlett Packard™, or Hitachi™ instrument equipped with wavelength or diode array UV detector and a Waters™ Radialpak C-18 column or Diazem™ cyano or Chromasil™ cyano column was used for HPLC analyses and purification. Elemental analyses were performed by Atlantic Microlabs, Atlanta, Ga.
[0132]2-Mercaptoacetic acid (3a), 3-mercaptopropanoic acid (3b) and 4-mercaptobutanoic acid (3c) are commercially available.
[0133]5-Mercaptopentanoic acid (3d). 5-Mercaptopent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com