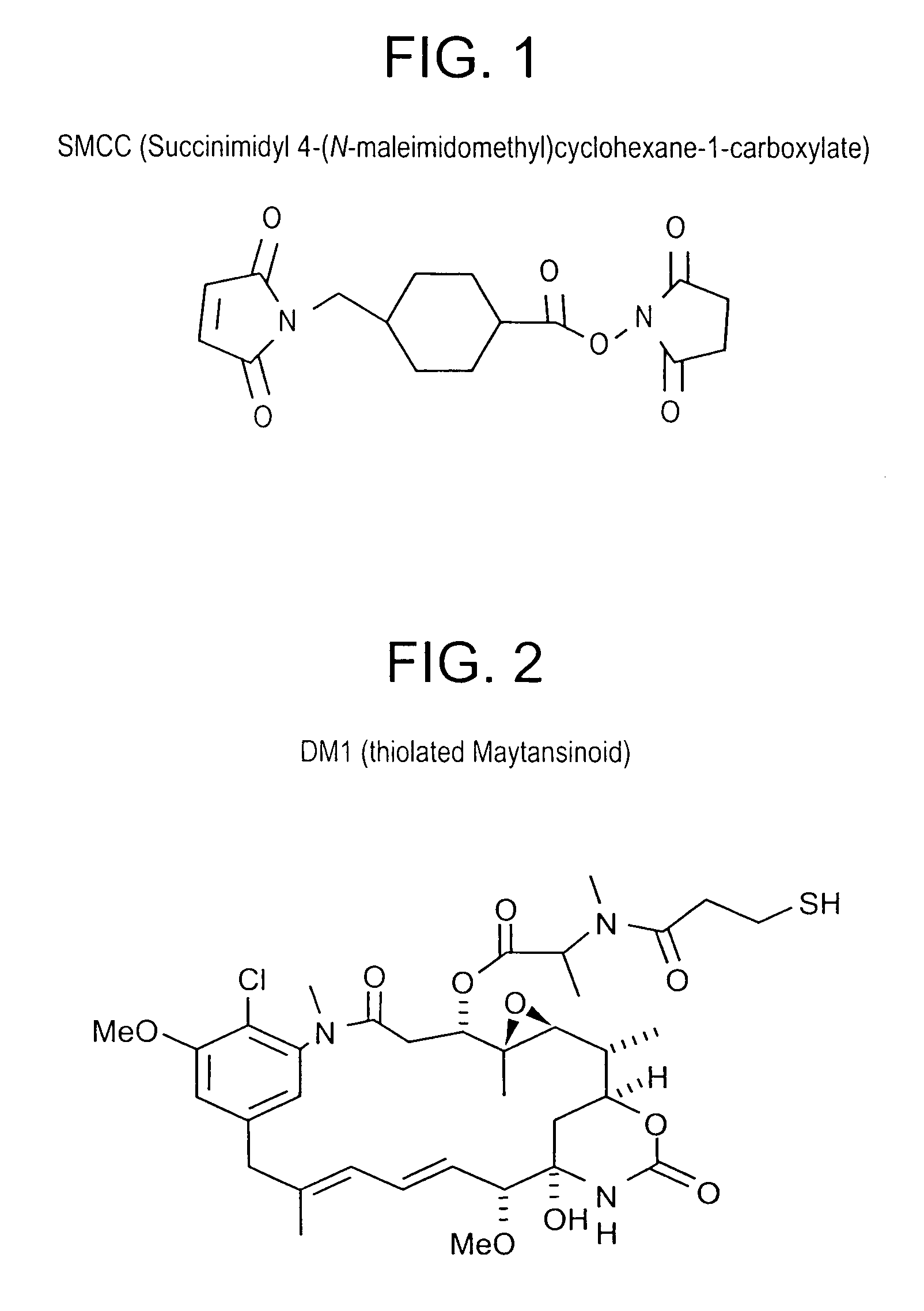
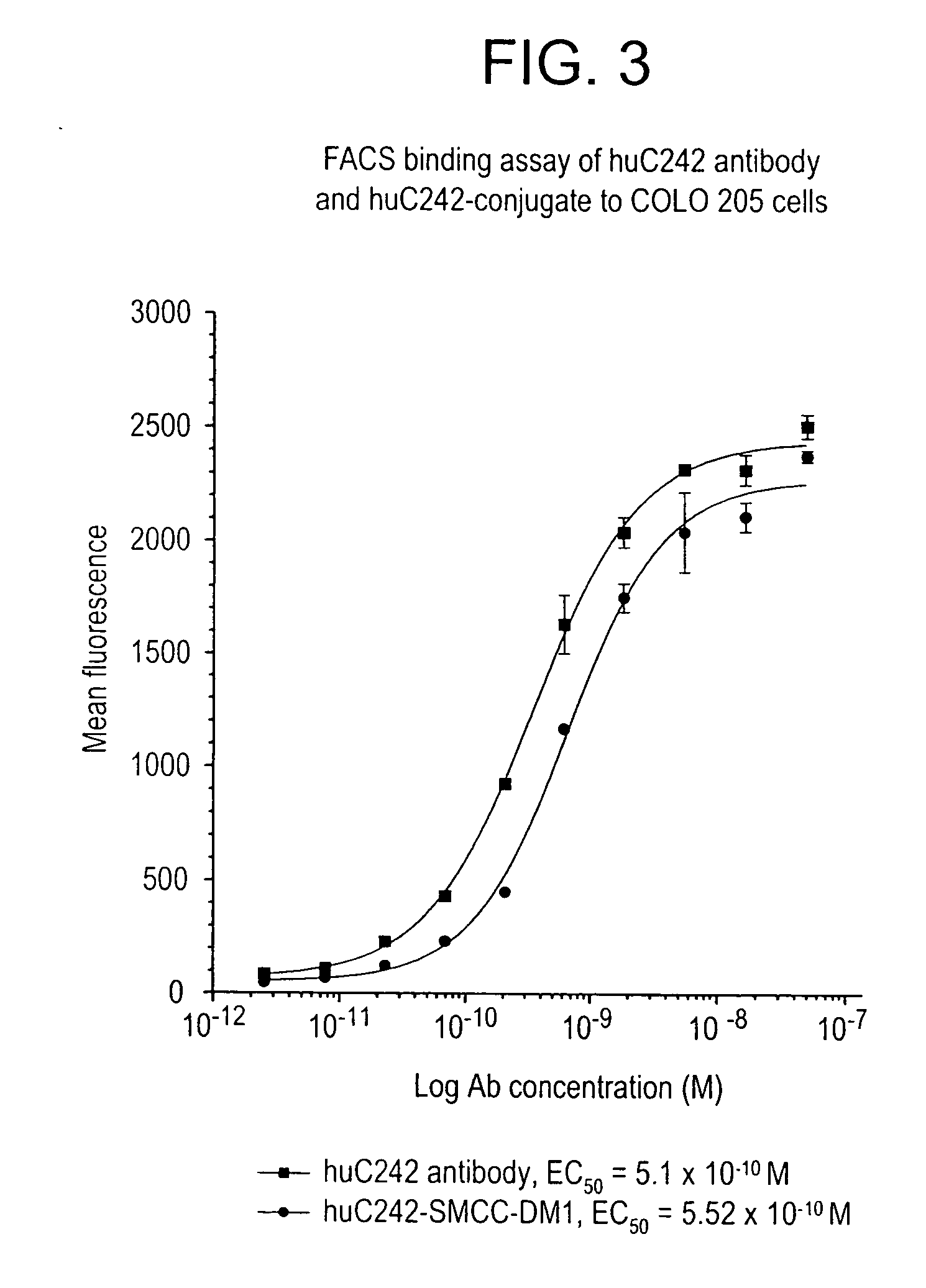
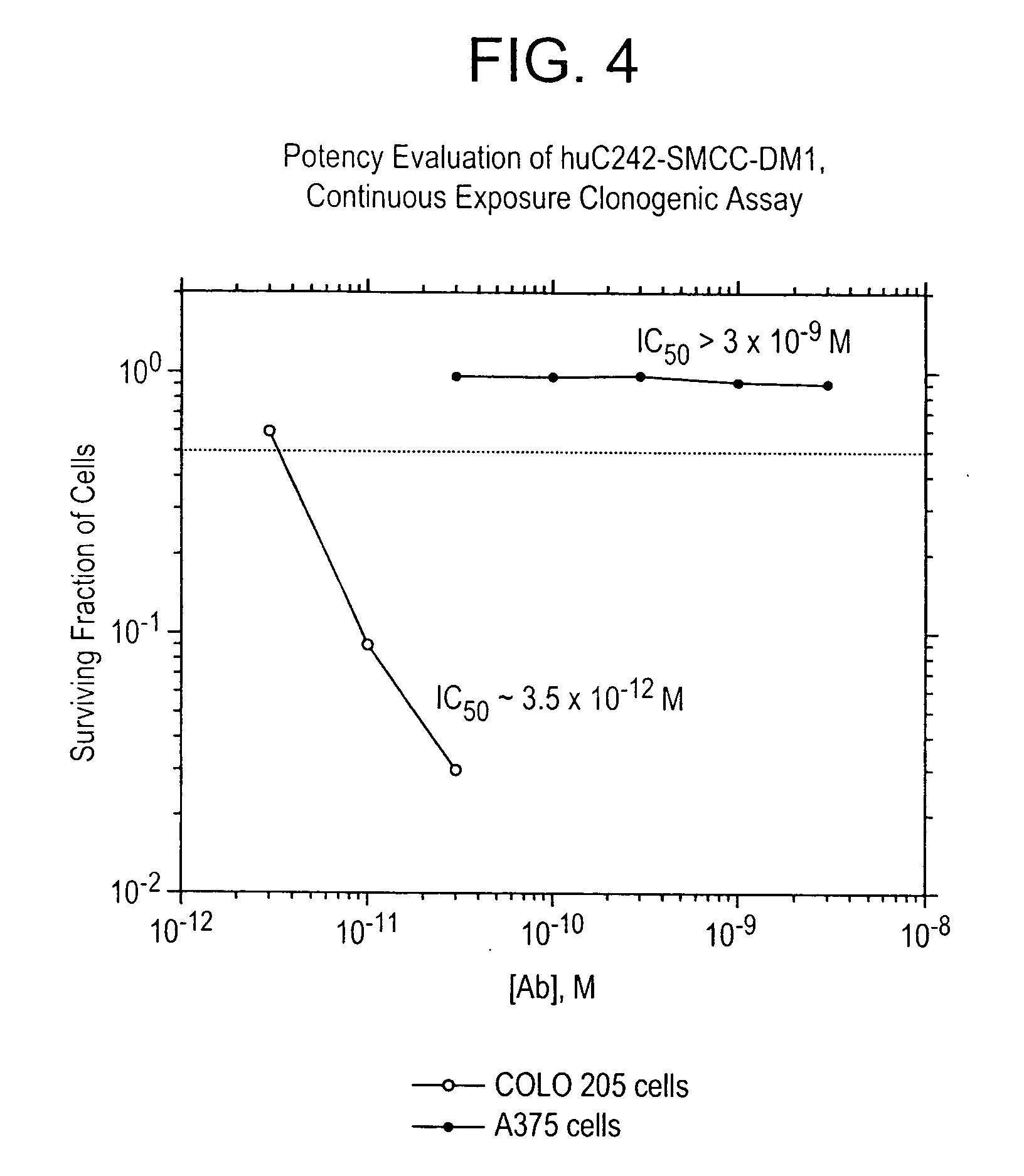
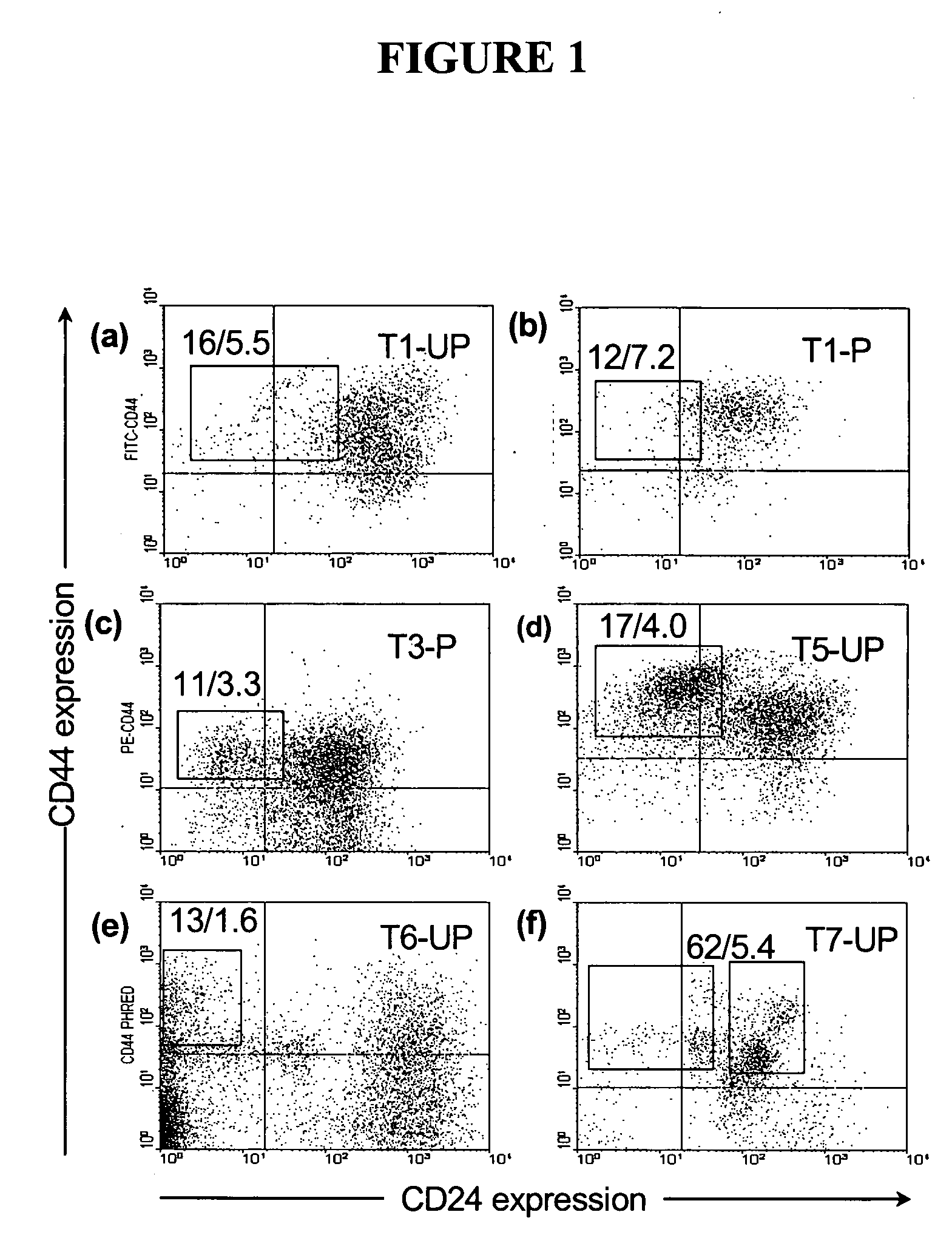
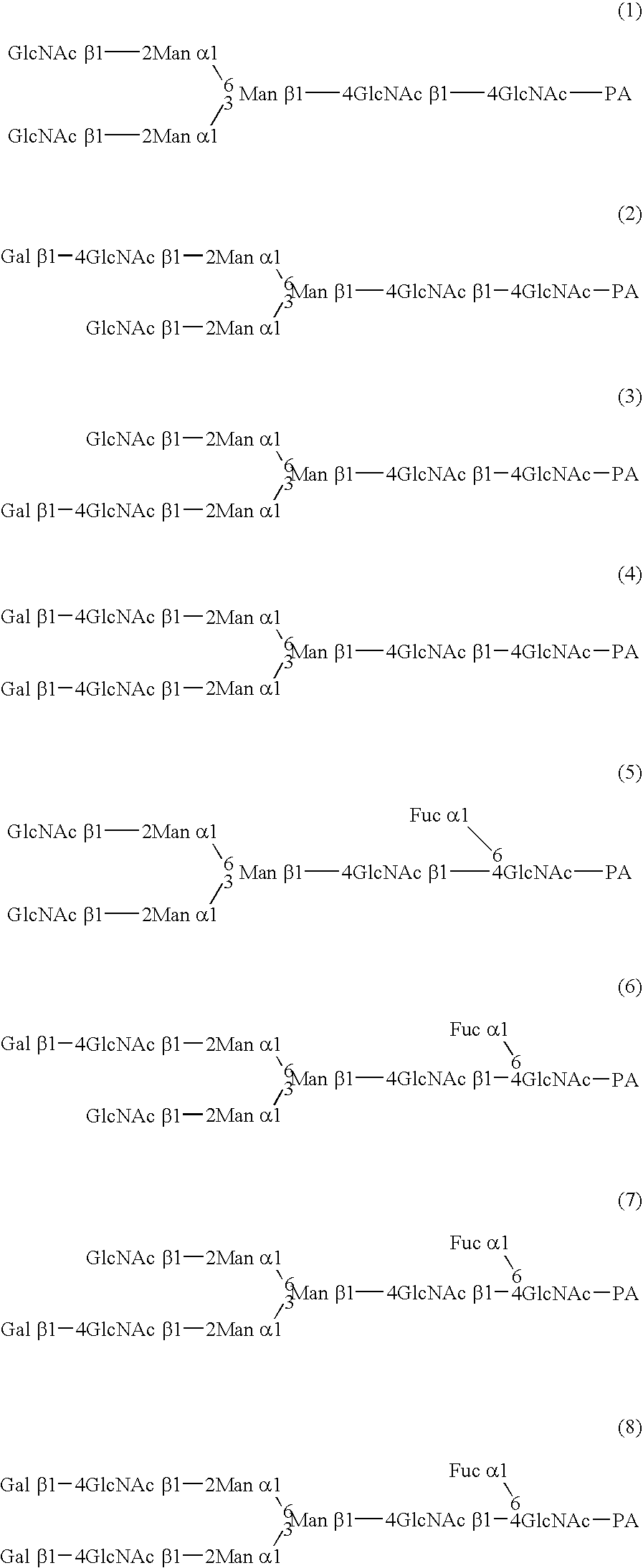Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146393results about "Antineoplastic agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
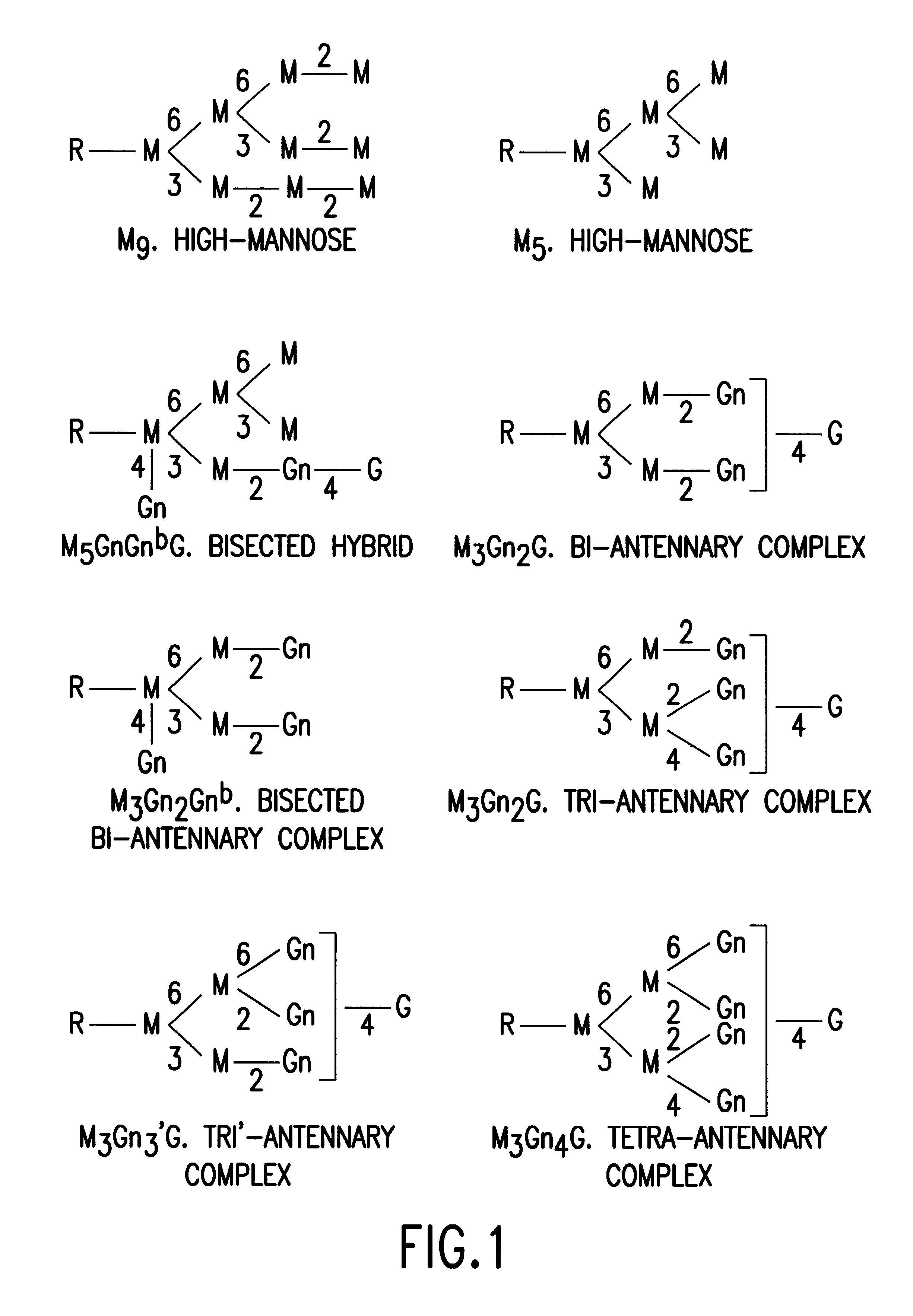
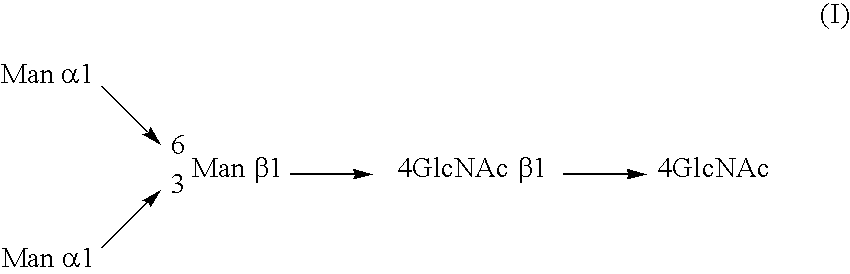
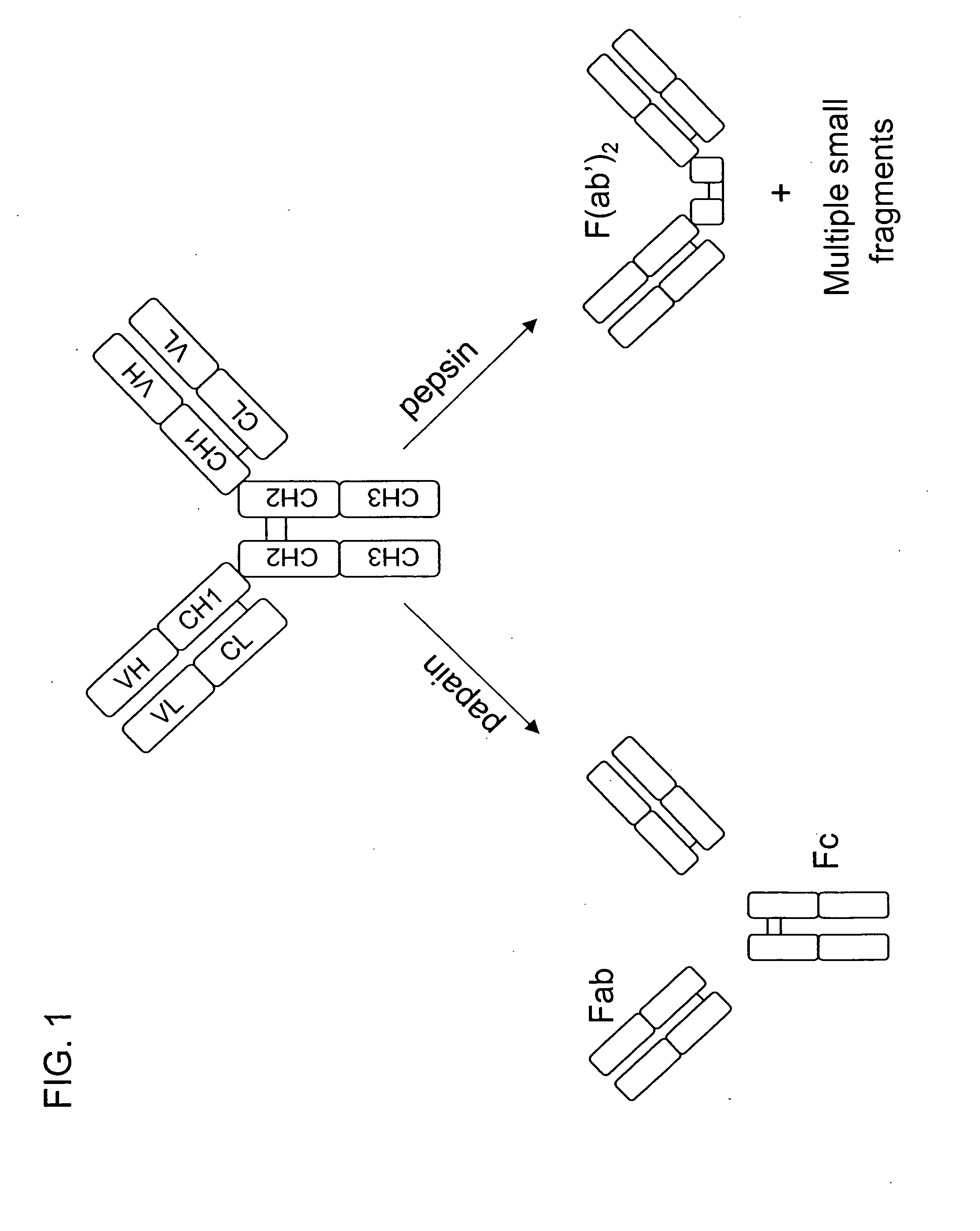
Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity
InactiveUS6602684B1Increase healing valueEnhanced Fc-mediated cellular cytotoxicityNanotechFungiAntibody fragmentsADAMTS Proteins
The present invention relates to the field glycosylation engineering of proteins. More particular, the present invention is directed to the glycosylation engineering of proteins to provide proteins with improved therapeutic properties, e.g., antibodies, antibody fragments, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin, with enhanced Fc-mediated cellular cytotoxicity.
Owner:ROCHE GLYCART AG
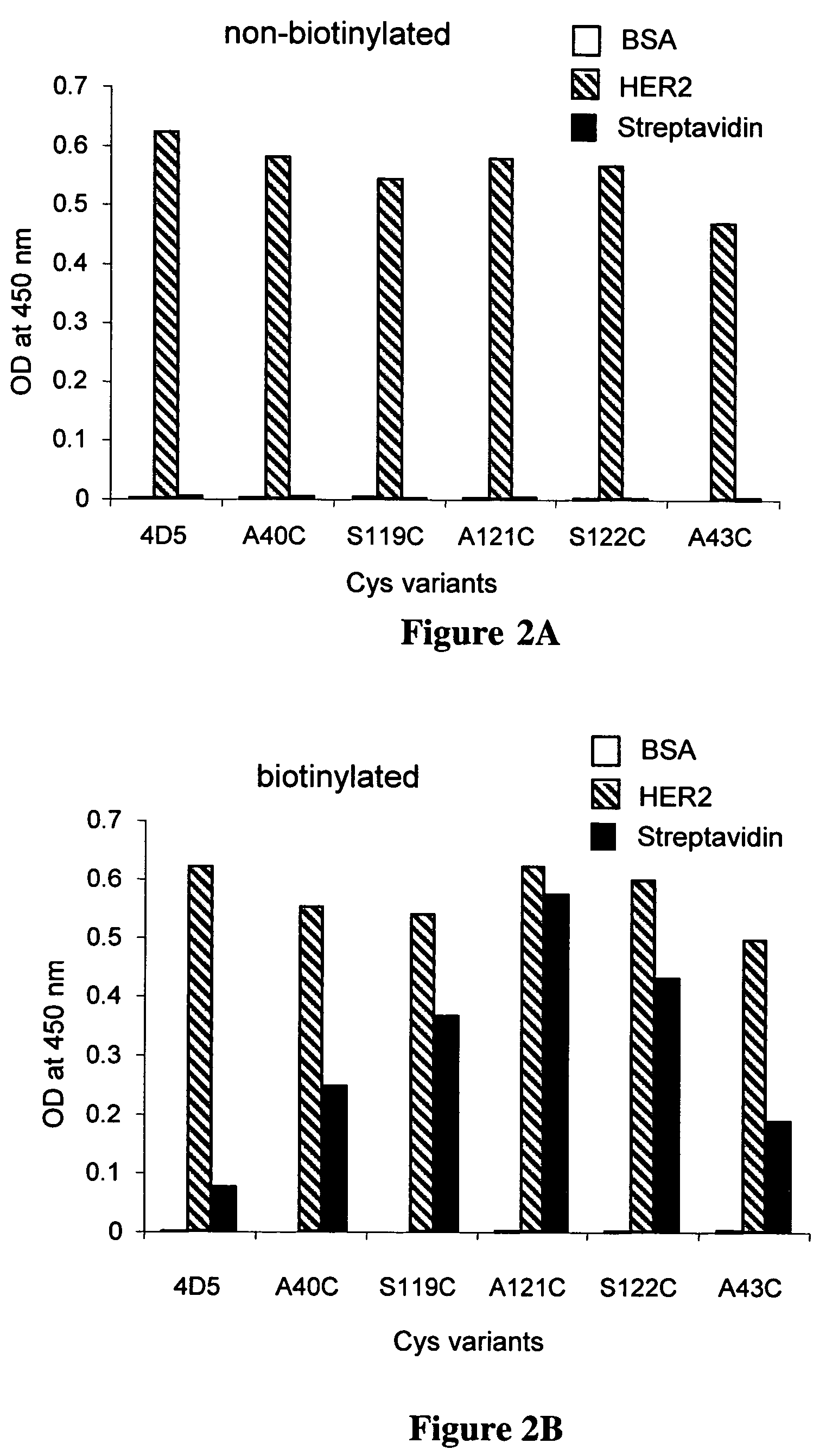
Cysteine engineered antibodies and conjugates
Owner:GENENTECH INC
Cells of which genome is modified
InactiveUS20040110704A1Raise the ratioDecreased and deleted activityAntibacterial agentsAntipyreticGlycosideN-Acetylglucosamine
A cell in which genome is modified so as to have a more decreased or deleted activity of an enzyme relating to modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain than its parent cell, and a process for producing an antibody composition using the cell.
Owner:KYOWA HAKKO KOGYO CO LTD
Antigen binding molecules with increased Fc receptor binding affinity and effector function
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies specific for human CD20. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
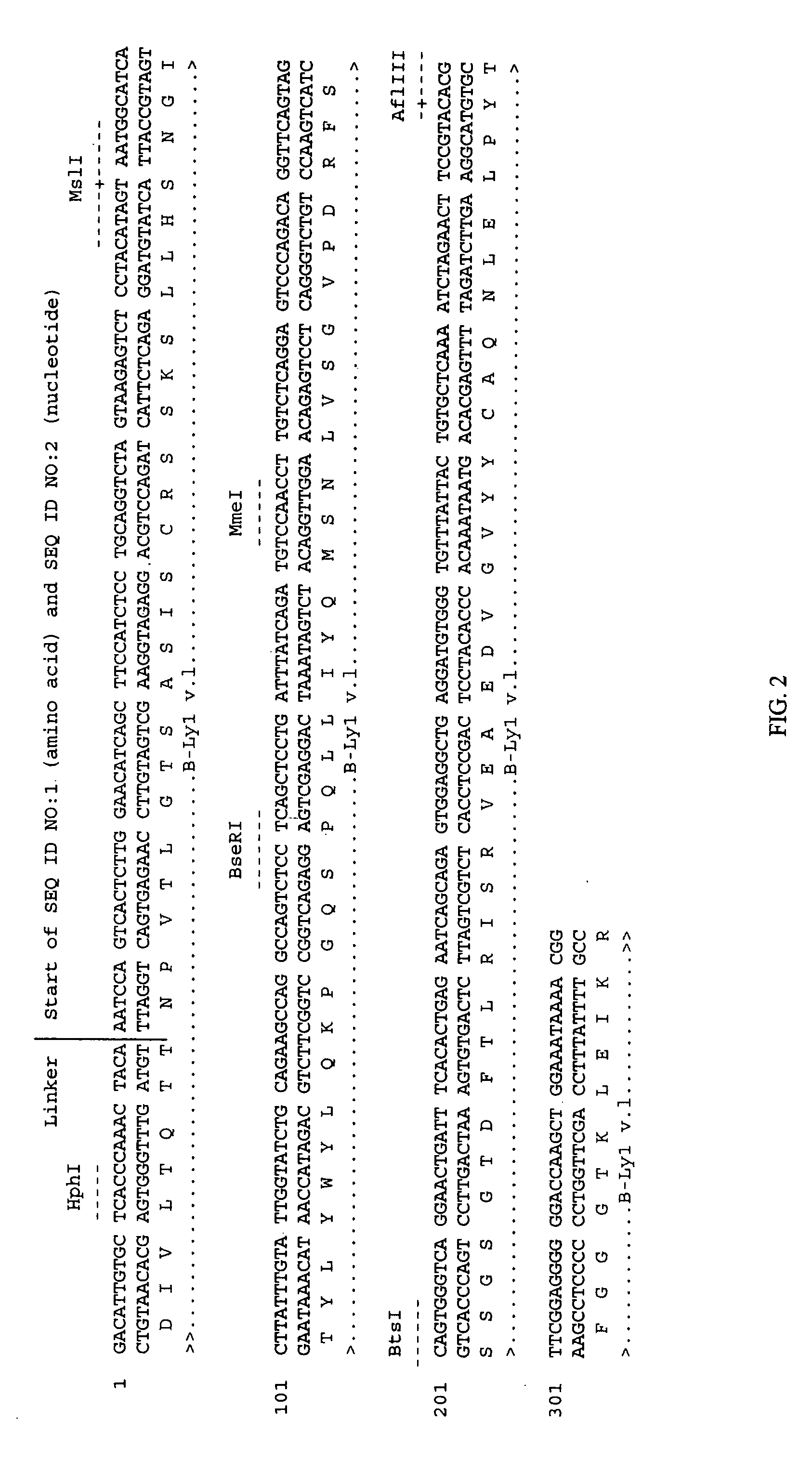
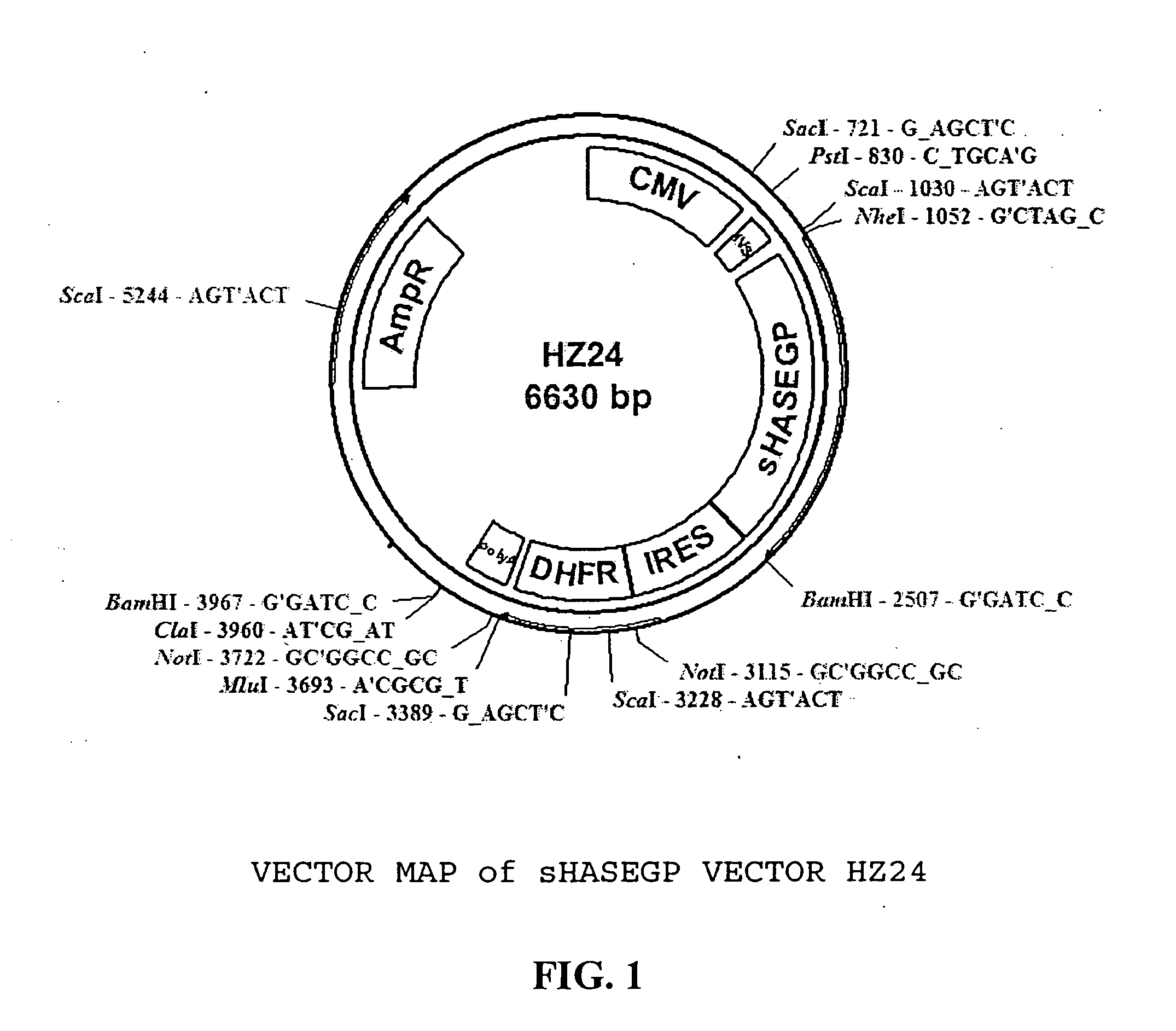
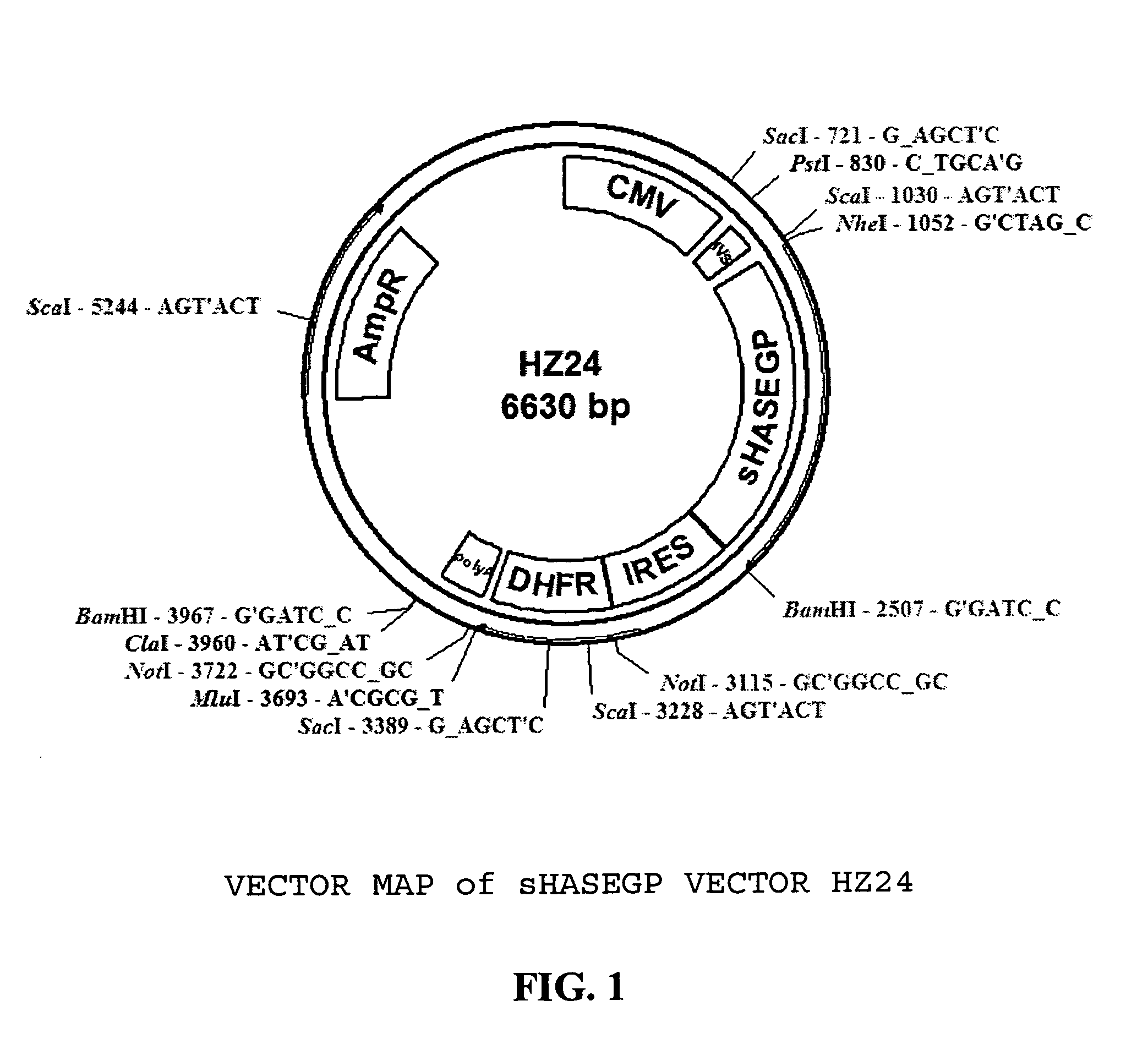
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
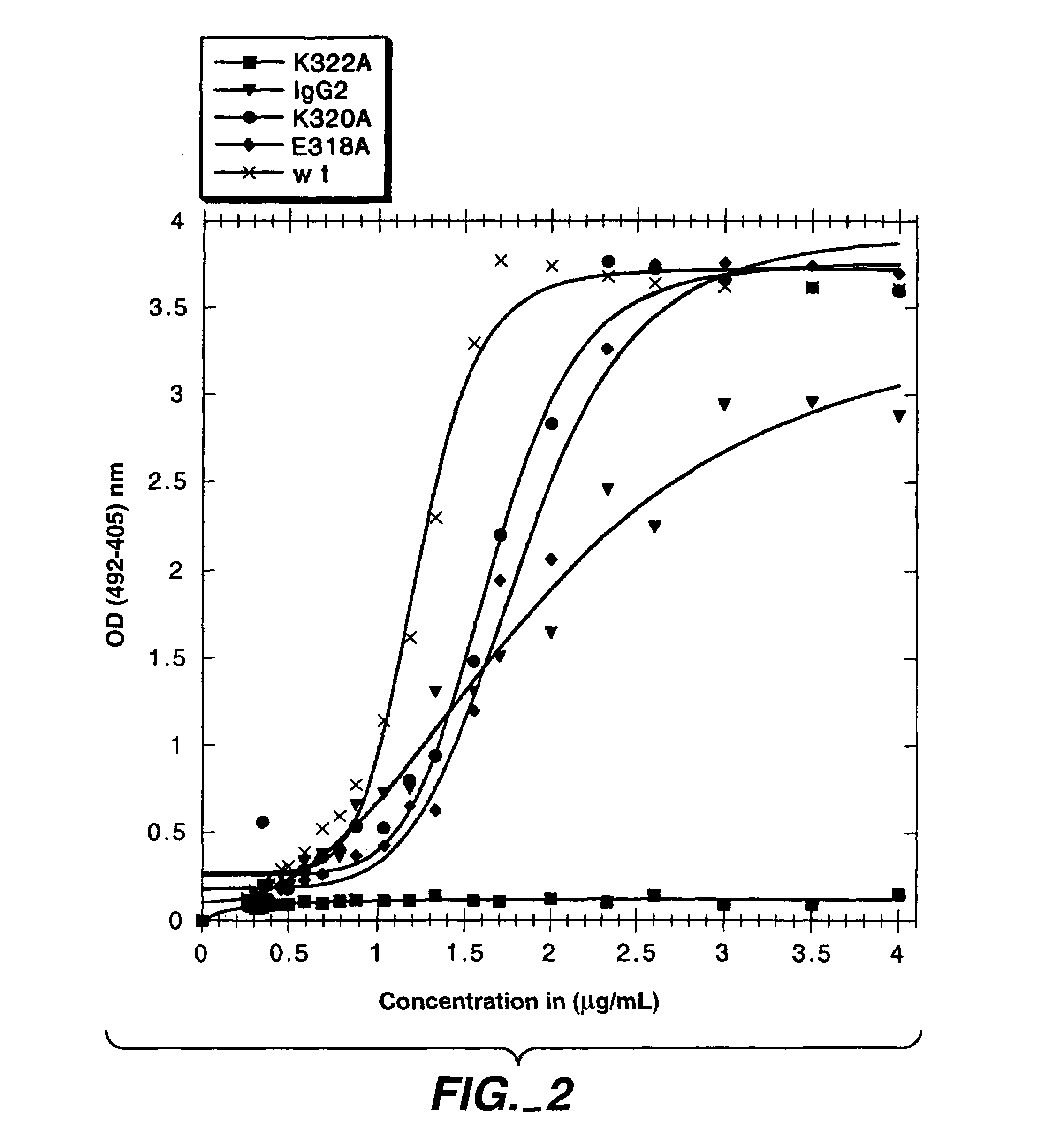
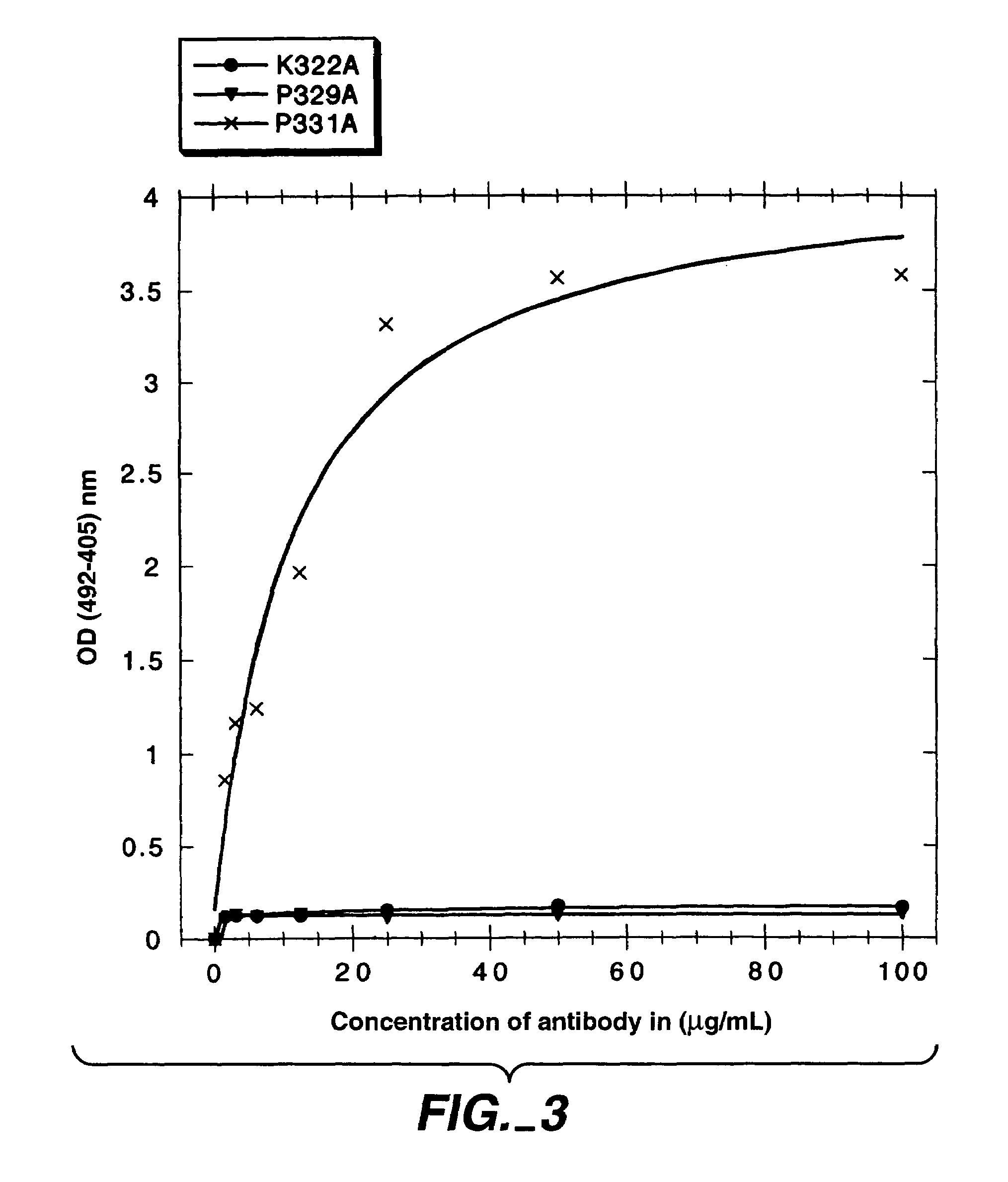
Polypeptide variants with altered effector function
The present invention concerns polypeptides comprising a variant Fc region. More particularly, the present invention concerns Fc region-containing polypeptides that have altered effector function as a consequence of one or more amino acid modifications in the Fc region thereof.
Owner:GENENTECH INC
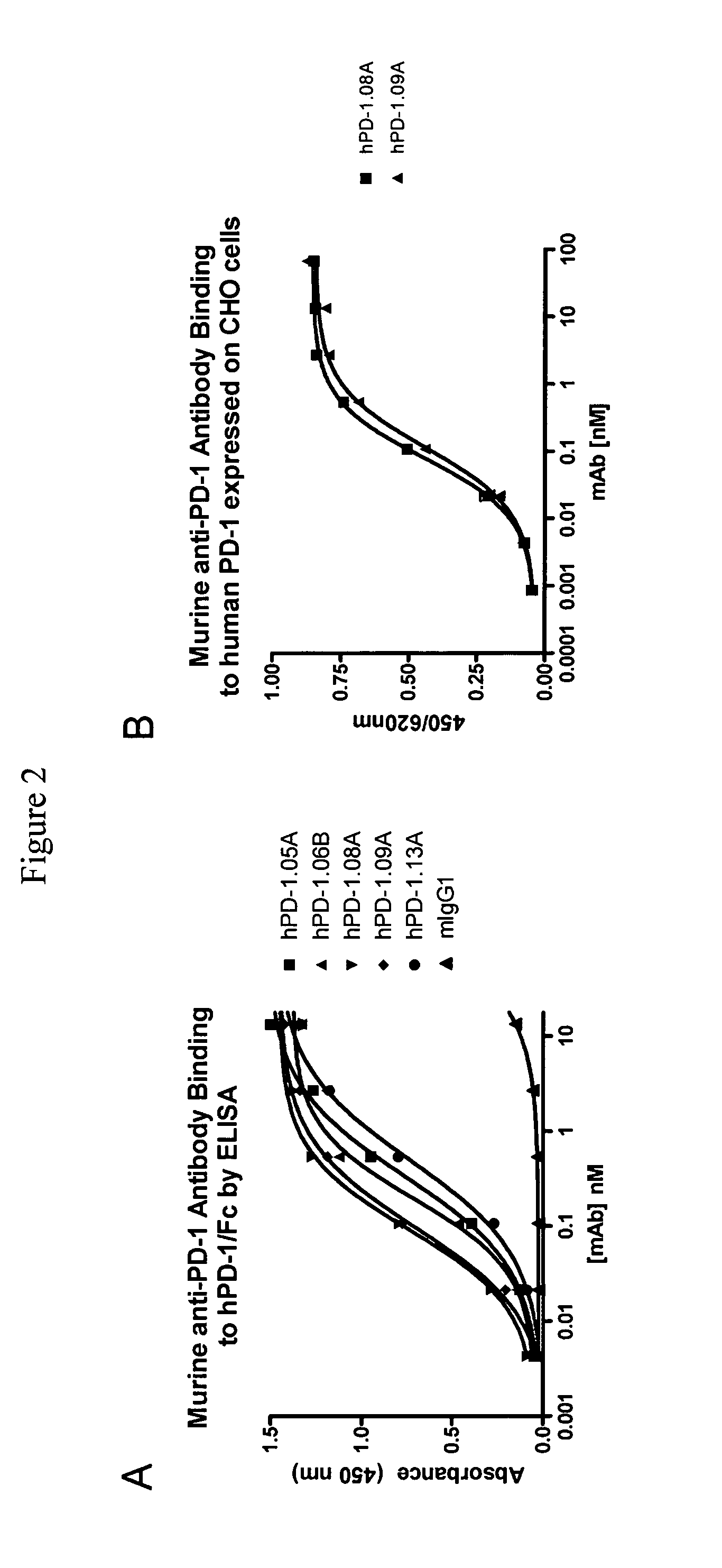
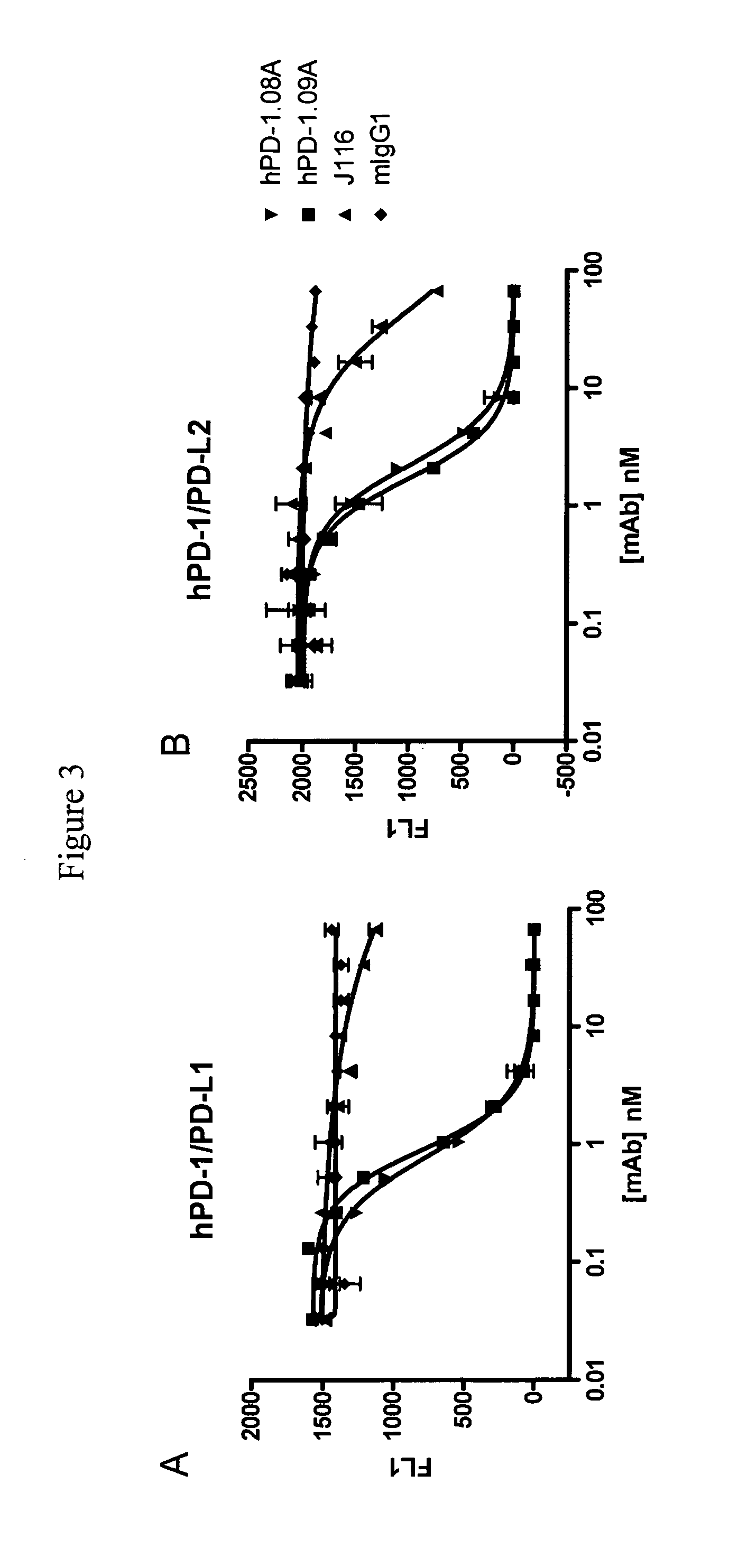
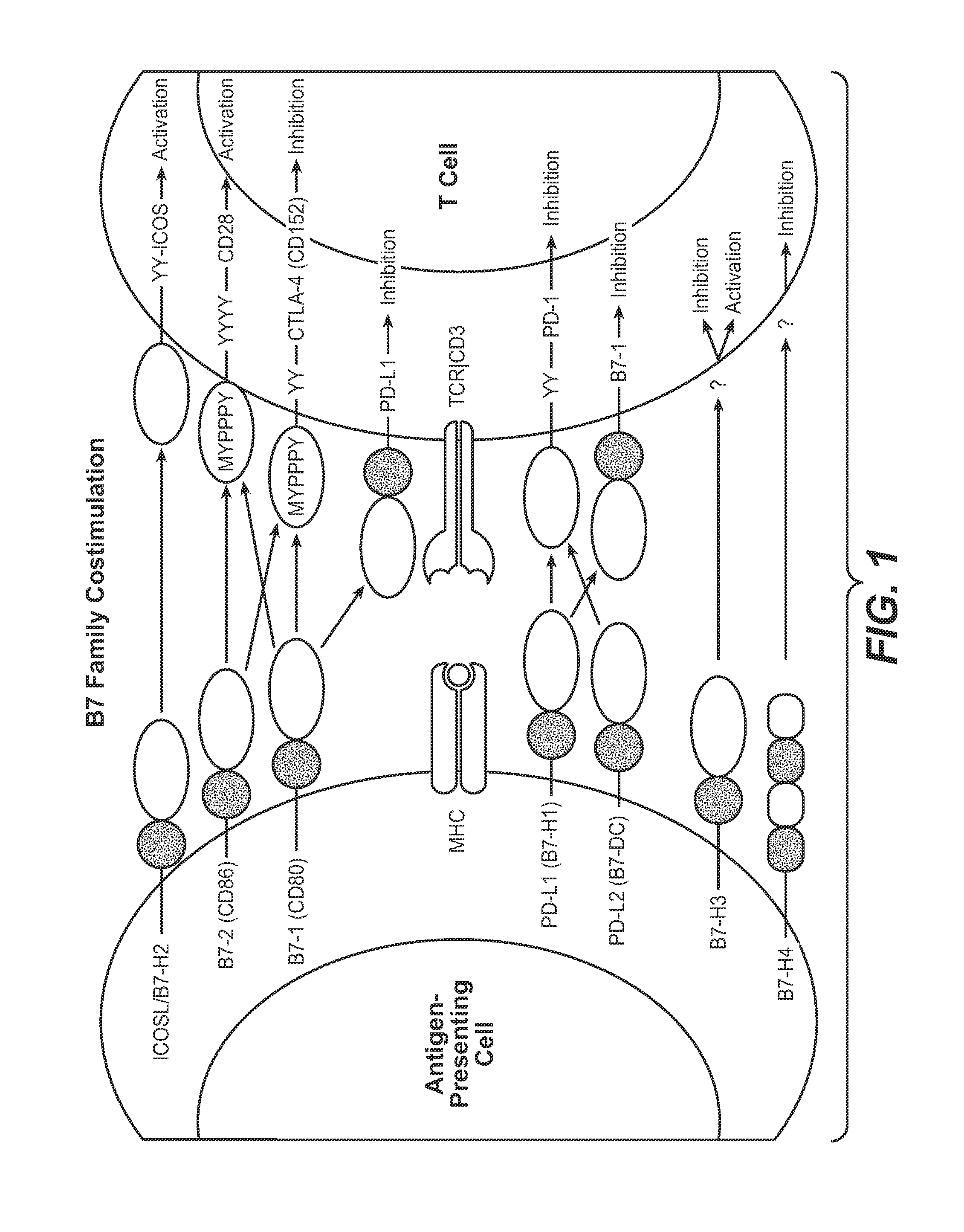
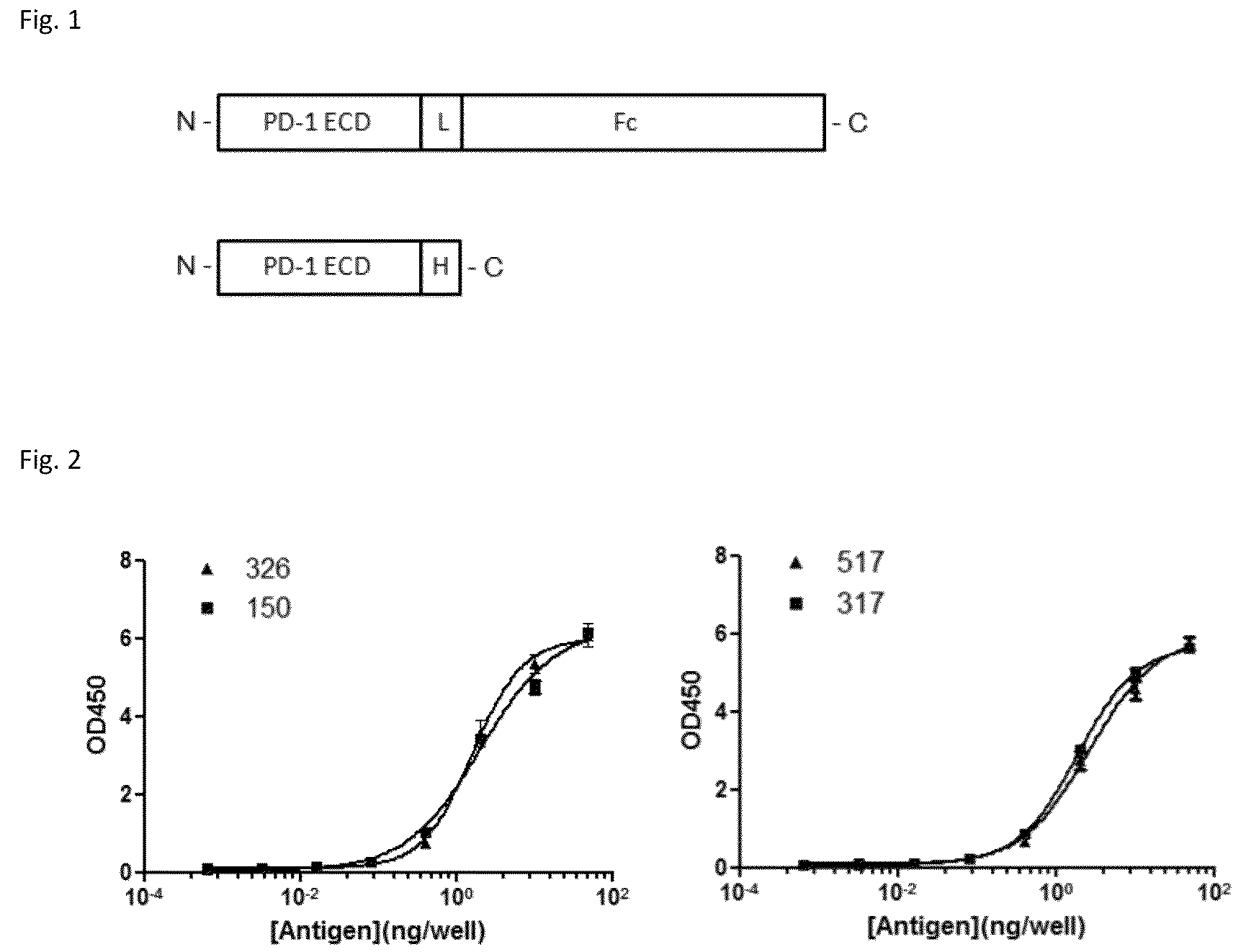
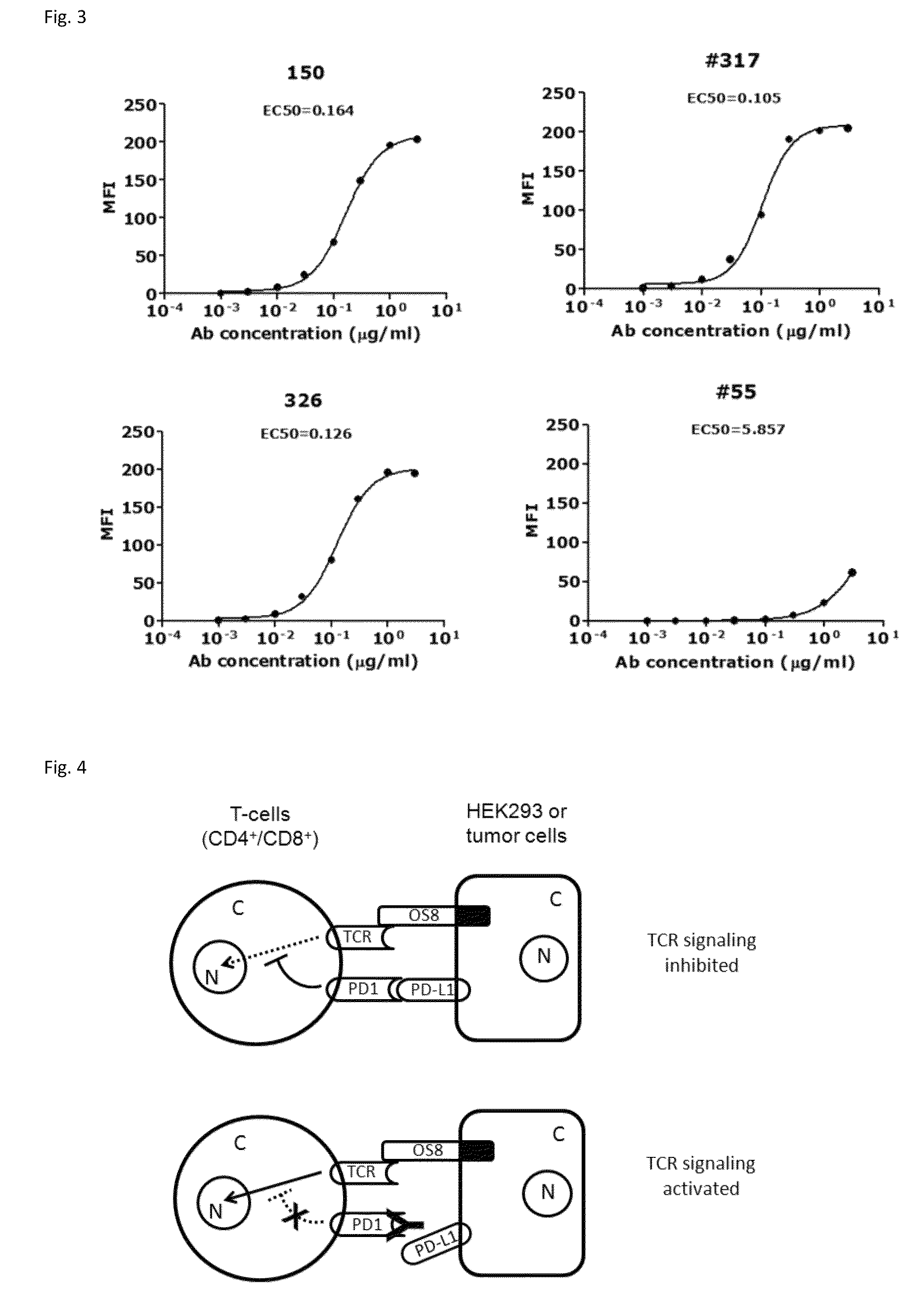
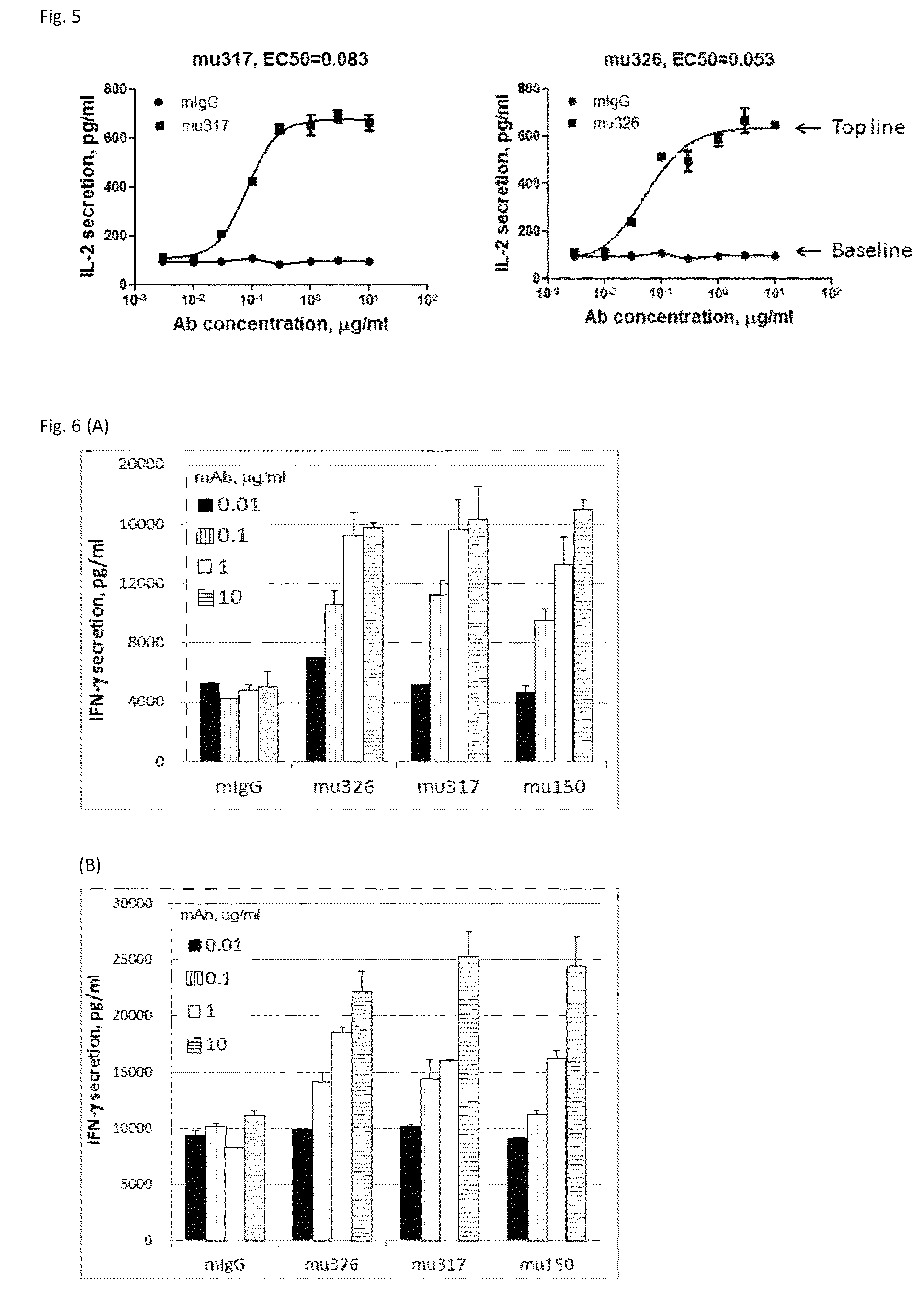
Antibodies to human programmed death receptor PD-1
ActiveUS8354509B2Increased activationIncreased proliferationSugar derivativesAntibody ingredientsProgrammed deathReceptor for activated C kinase 1
Antibodies which block the binding of human Programmed Death Receptor 1 (hPD-1) to its ligands (hPD-L1 or hPD-L2) and their variable region sequences are disclosed. A method of increasing the activity (or reducing downmodulation) of an immune response through the PD-I pathway is also disclosed.
Owner:MERCK SHARP & DOHME BV
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
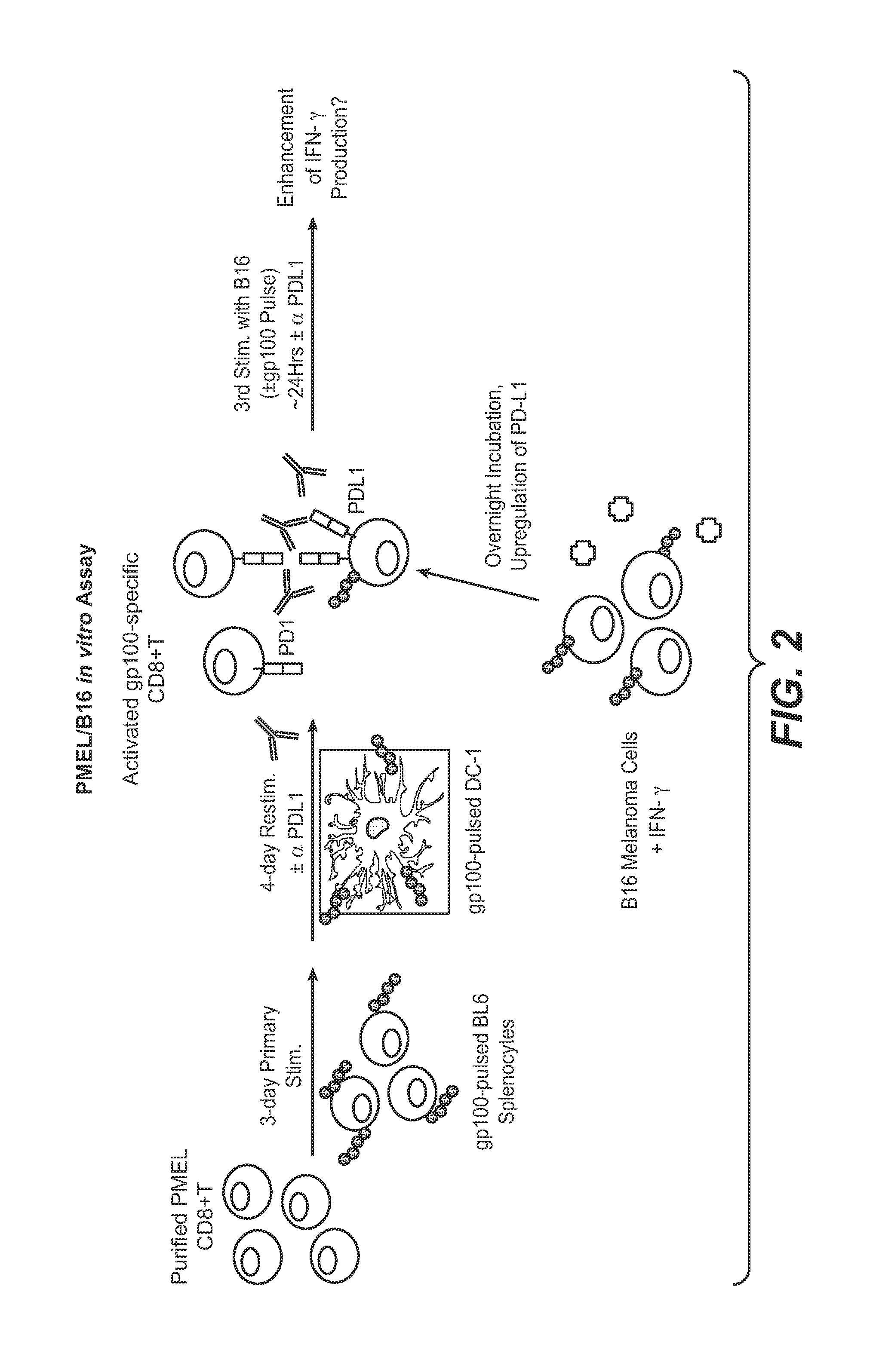
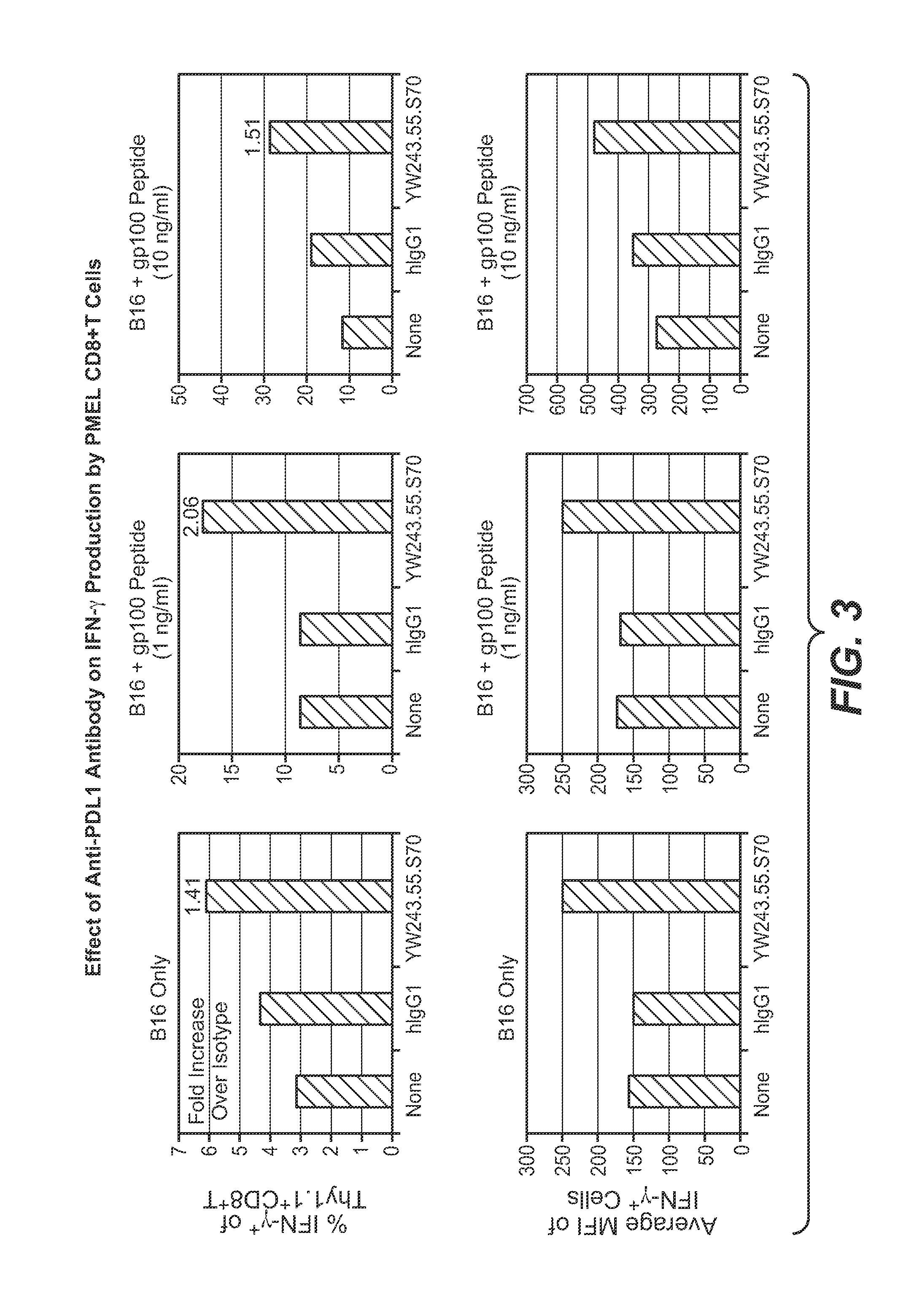
Anti-PD-L1 antibodies, compositions and articles of manufacture
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
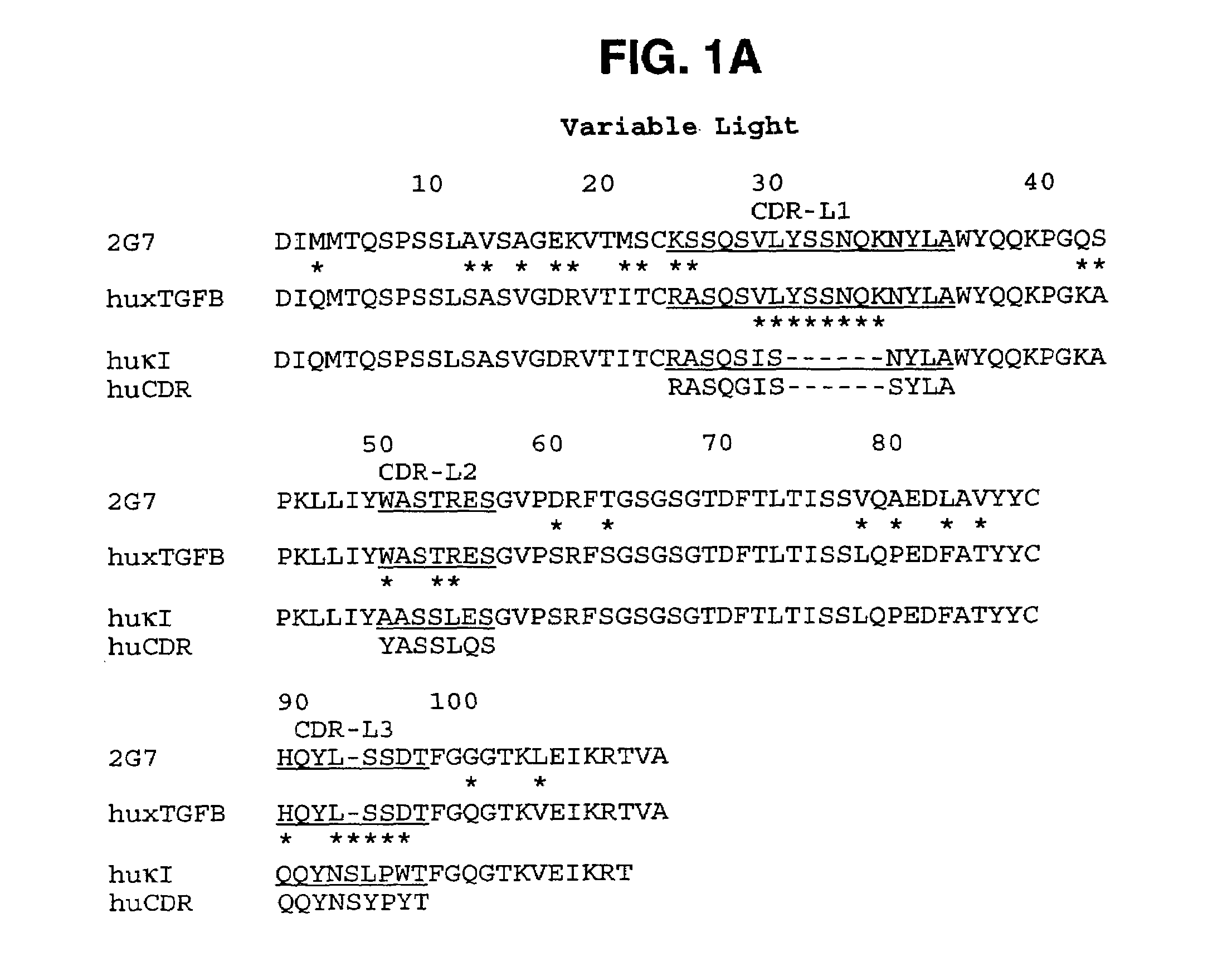
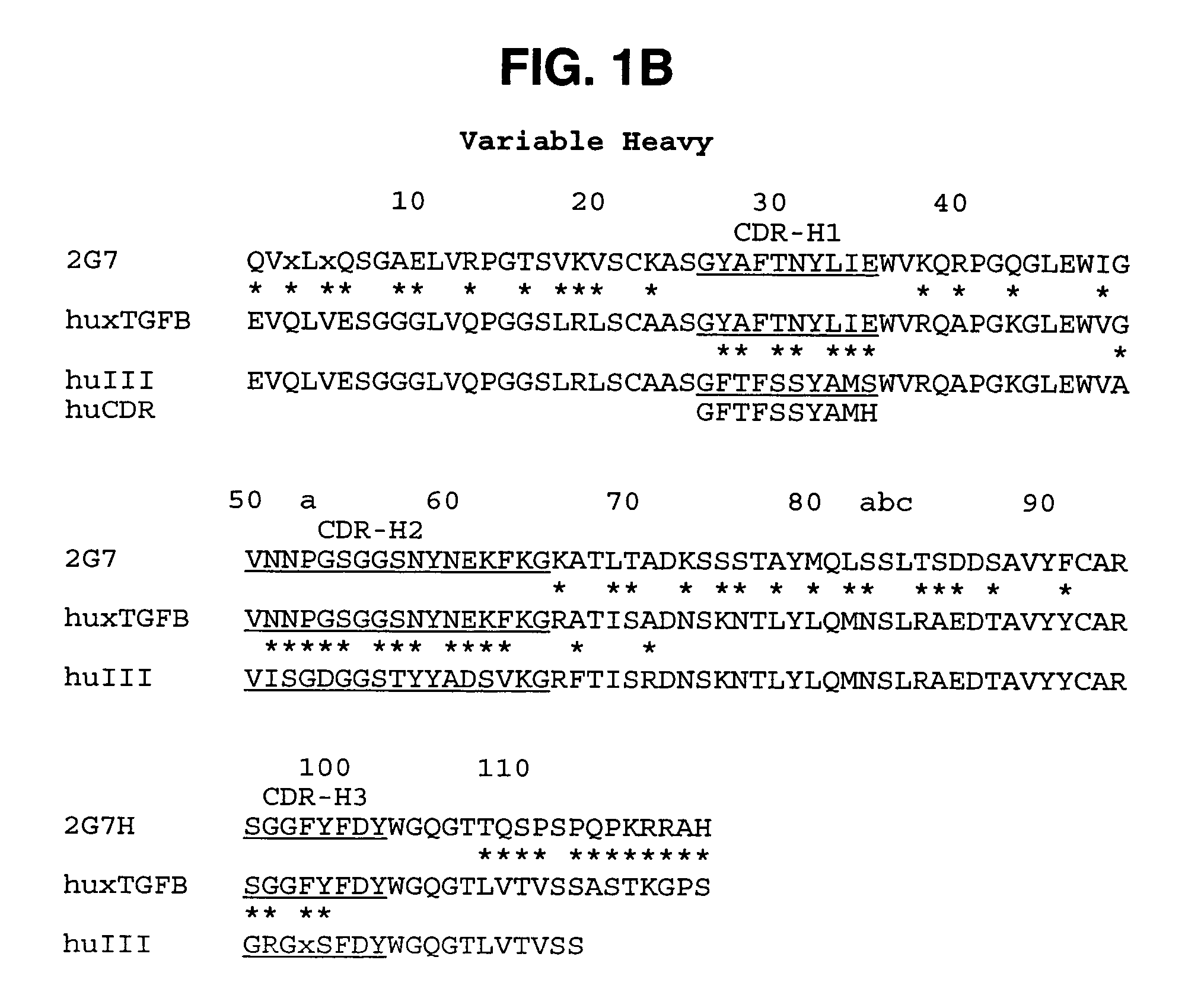
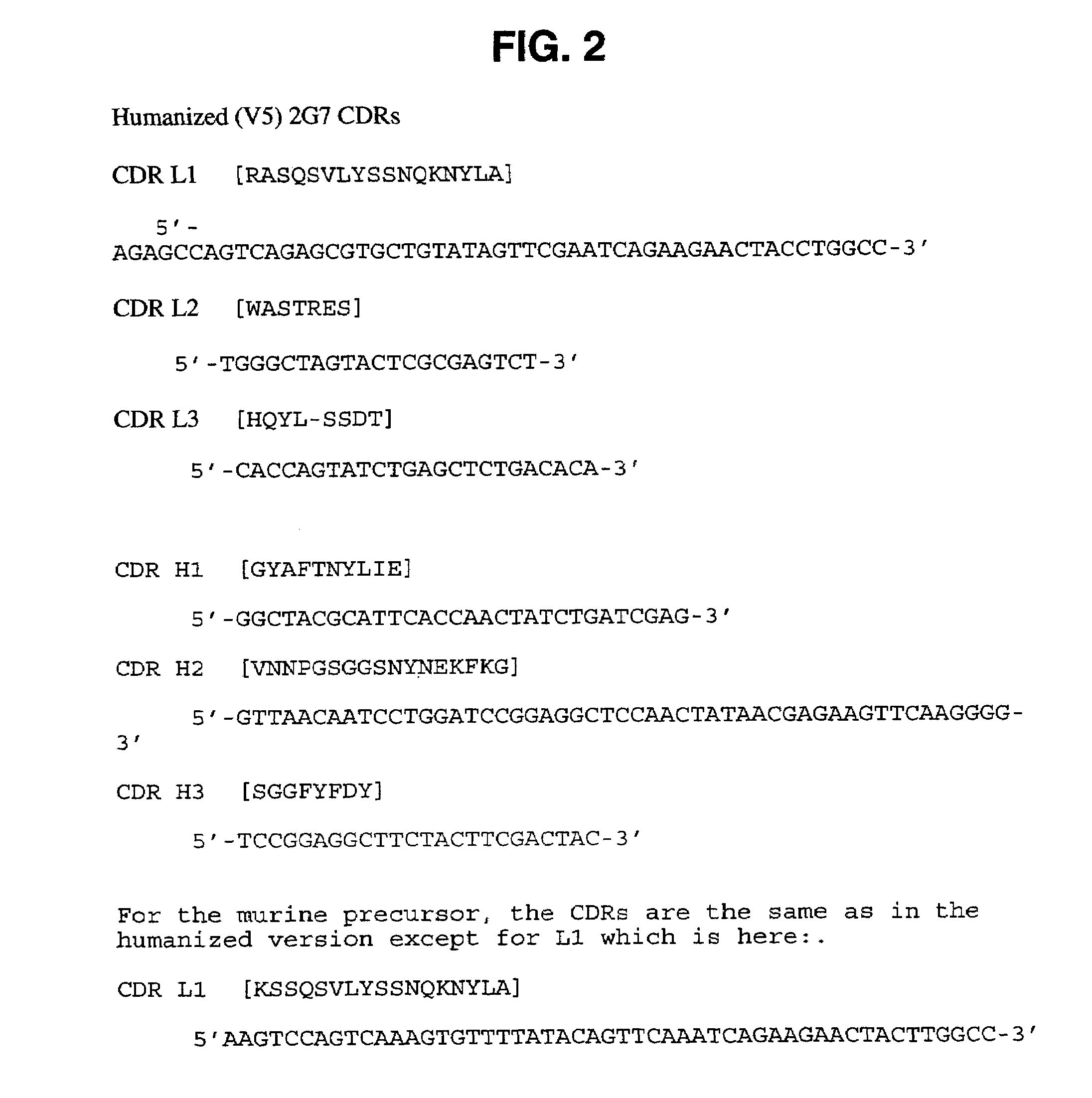
Humanized anti-TGF-beta antibodies
Humanized anti-TGF-beta antibodies are provided, as well as methods for their preparation and use, including methods for treating TGF-beta disorders, for example, cancer. Also provided are articles of manufacture designed for various uses that contain the humanized antibodies.
Owner:GENENTECH INC
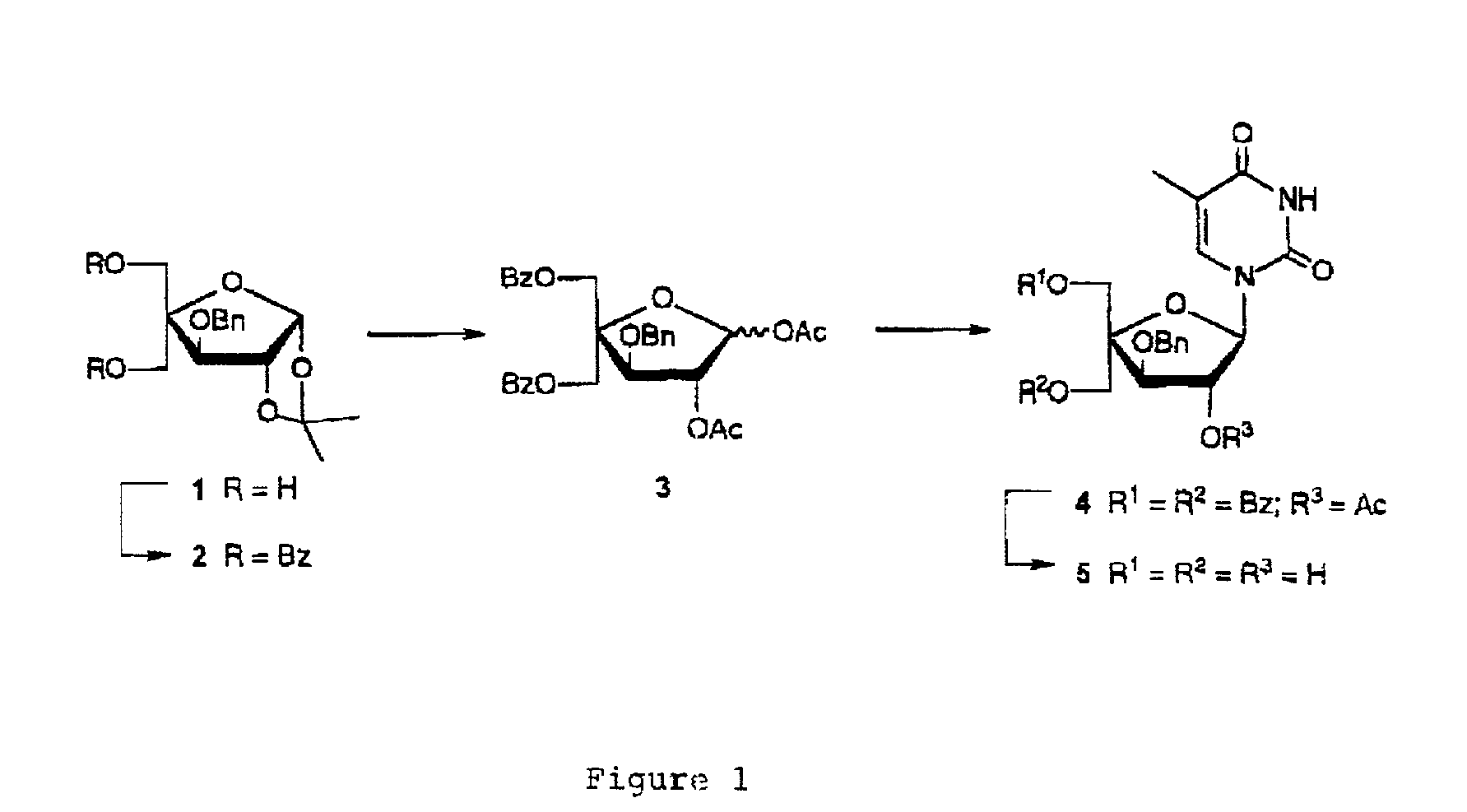
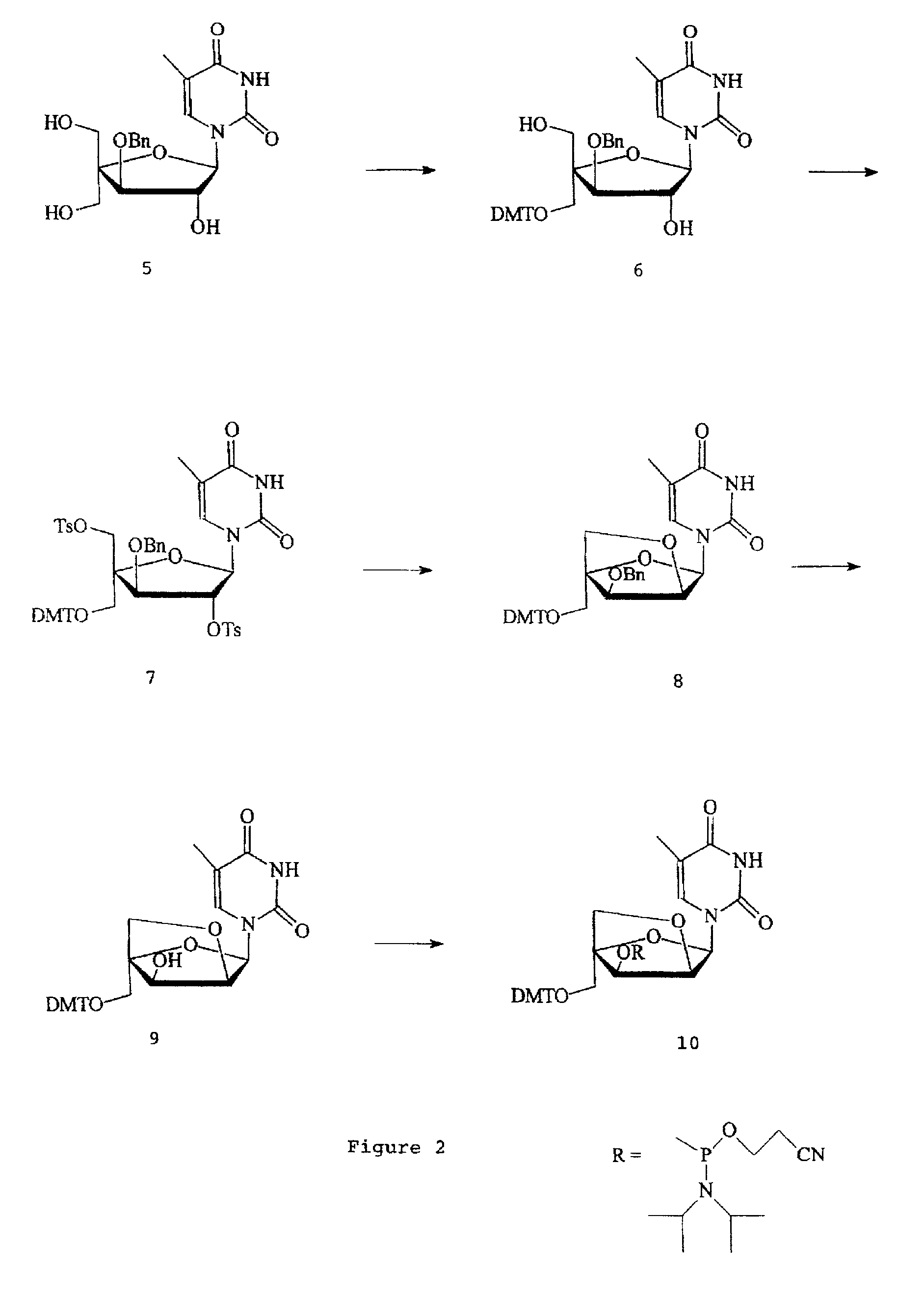
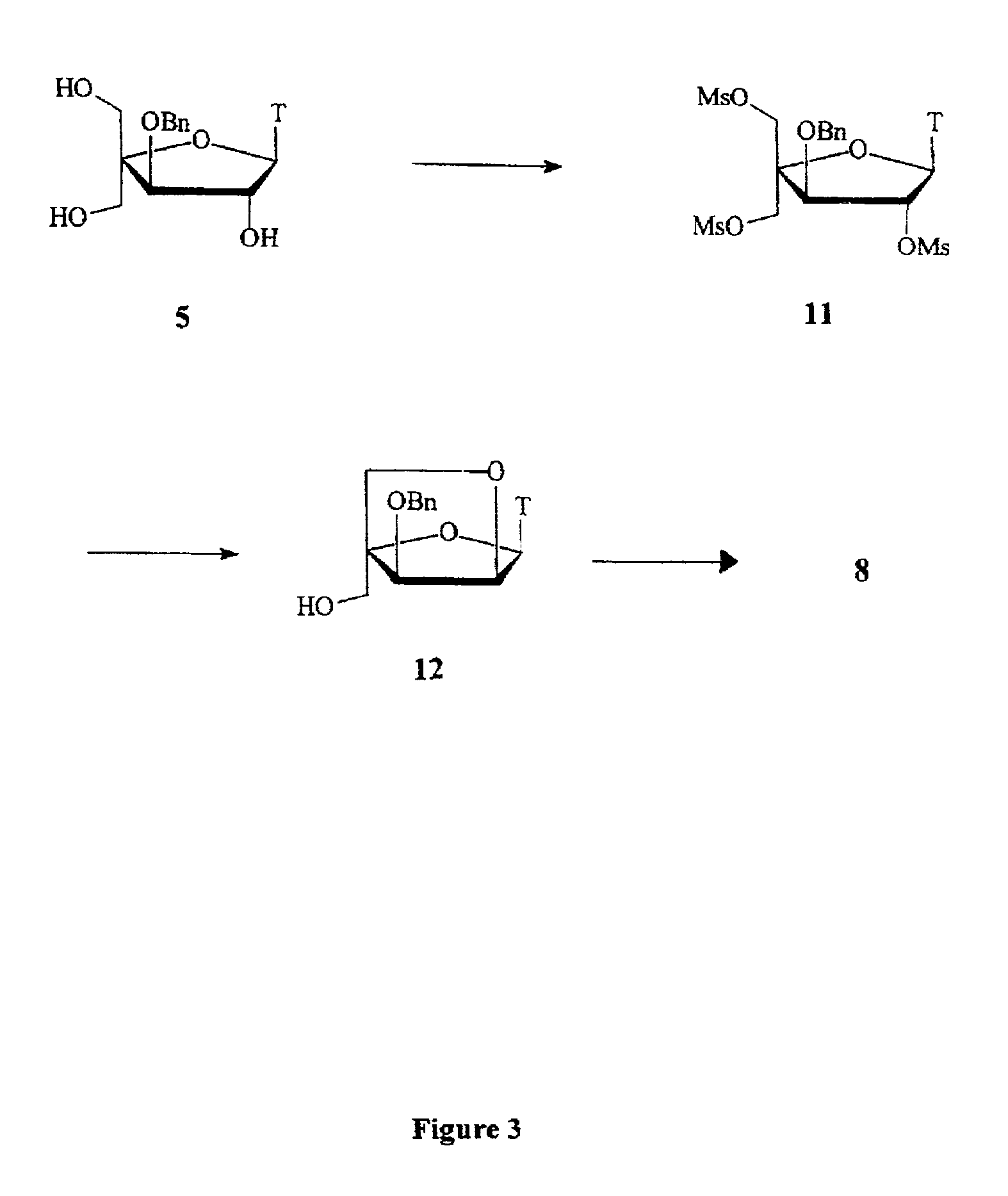
L-ribo-LNA analogues
Provided are L-ribo bicyclic nucleotide compounds as well as syntheses of such compounds. The nucleoside compounds of the invention are useful in forming oligonucleotides that can produce nucleobase specific duplexes with complementary single stranded and double stranded nucleic acids.
Owner:SANTARIS PHARMA AS
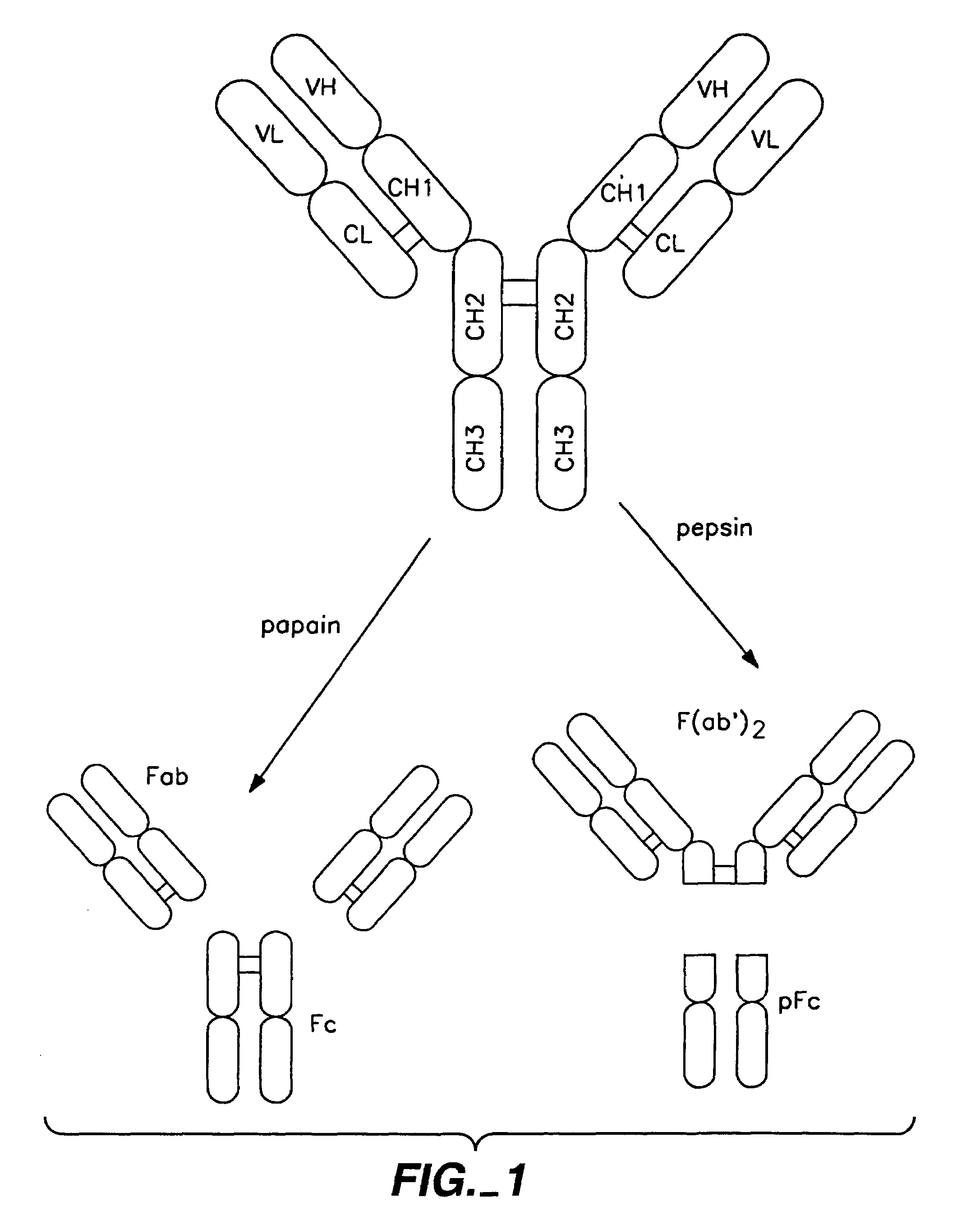
Multivalent antibodies and uses therefor
The present application describes engineered antibodies, with three or more functional antigen binding sites, and uses, such as therapeutic applications, for such engineered antibodies.
Owner:GENENTECH INC
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
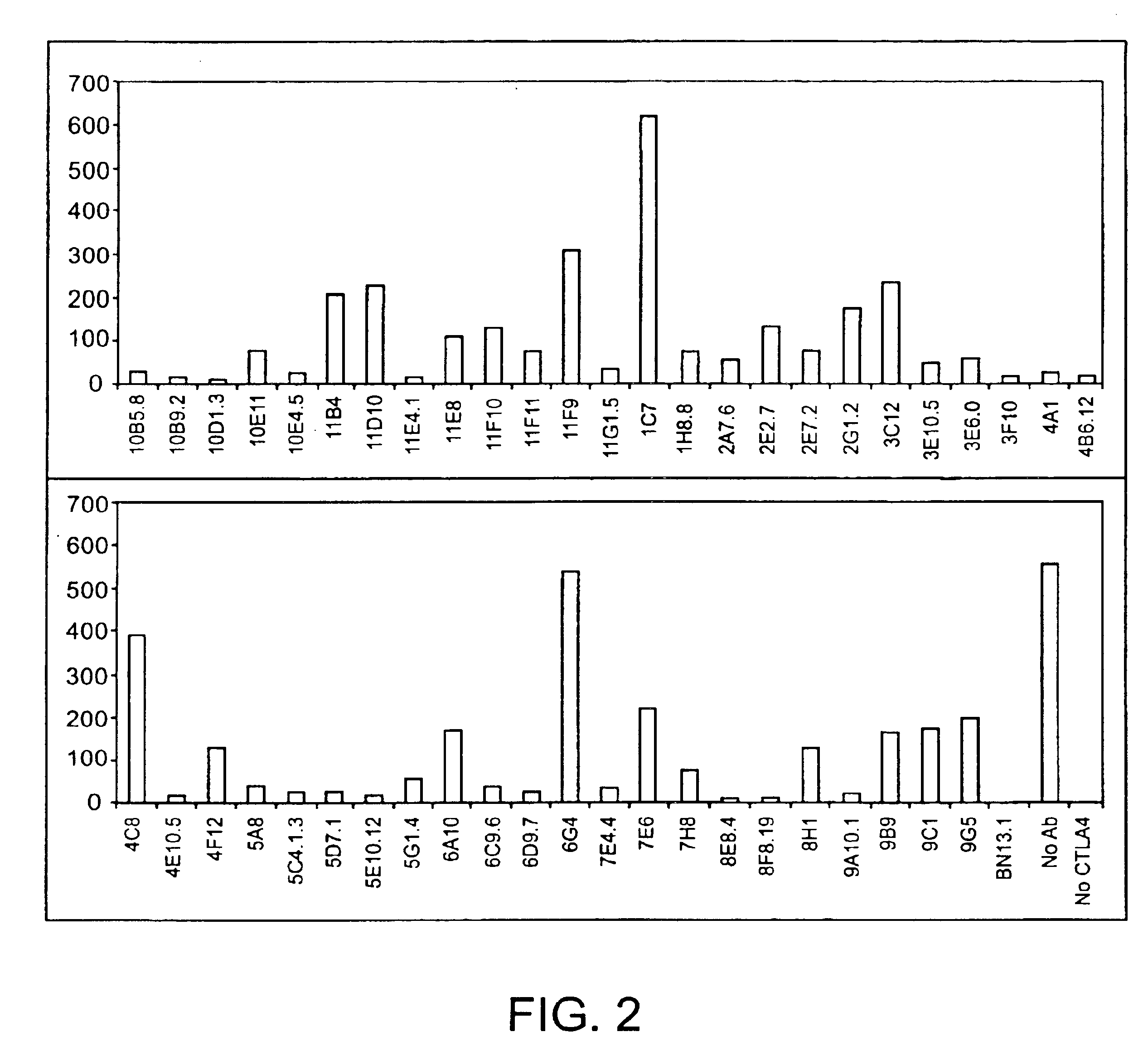
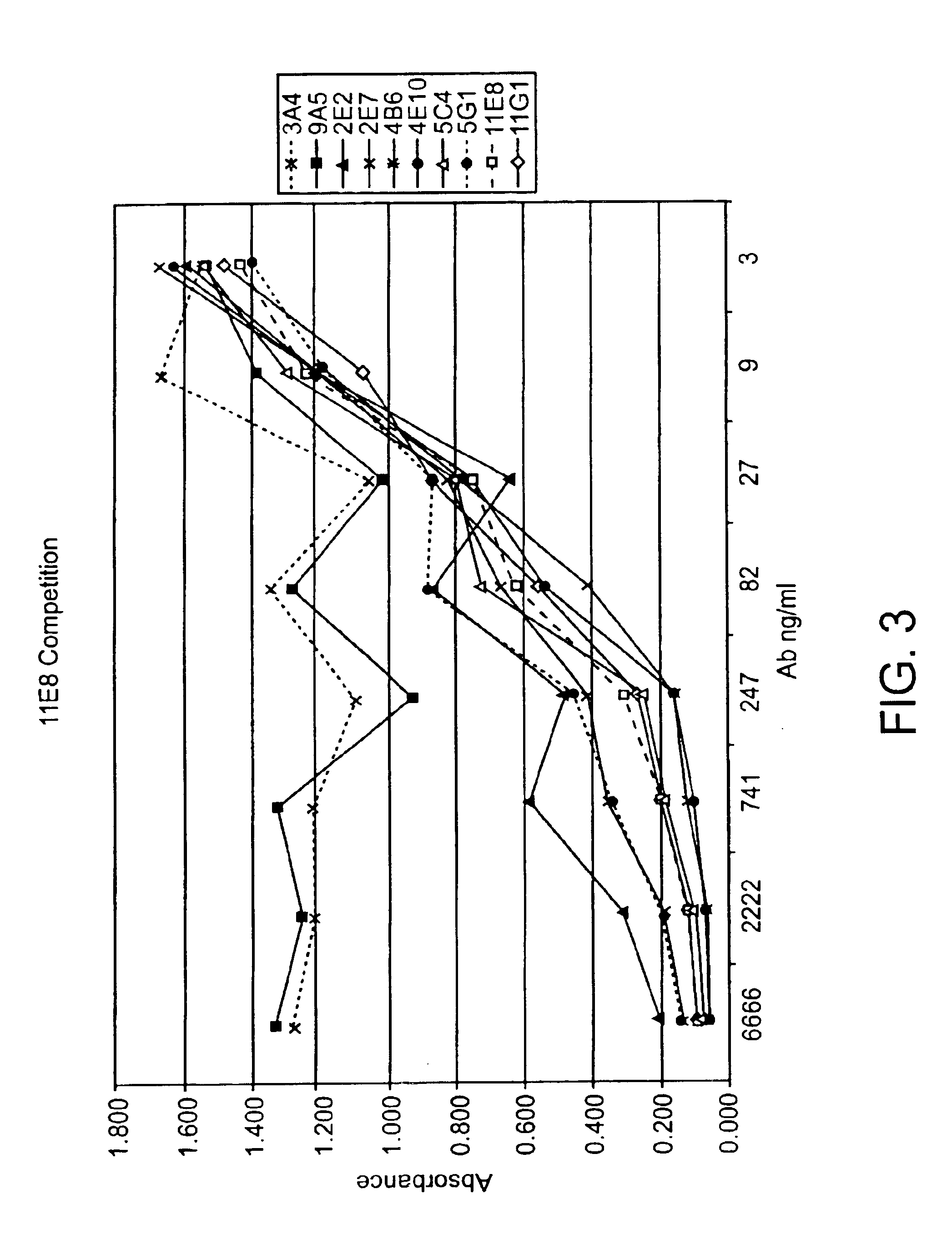
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
Simultaneous stimulation and concentration of cells
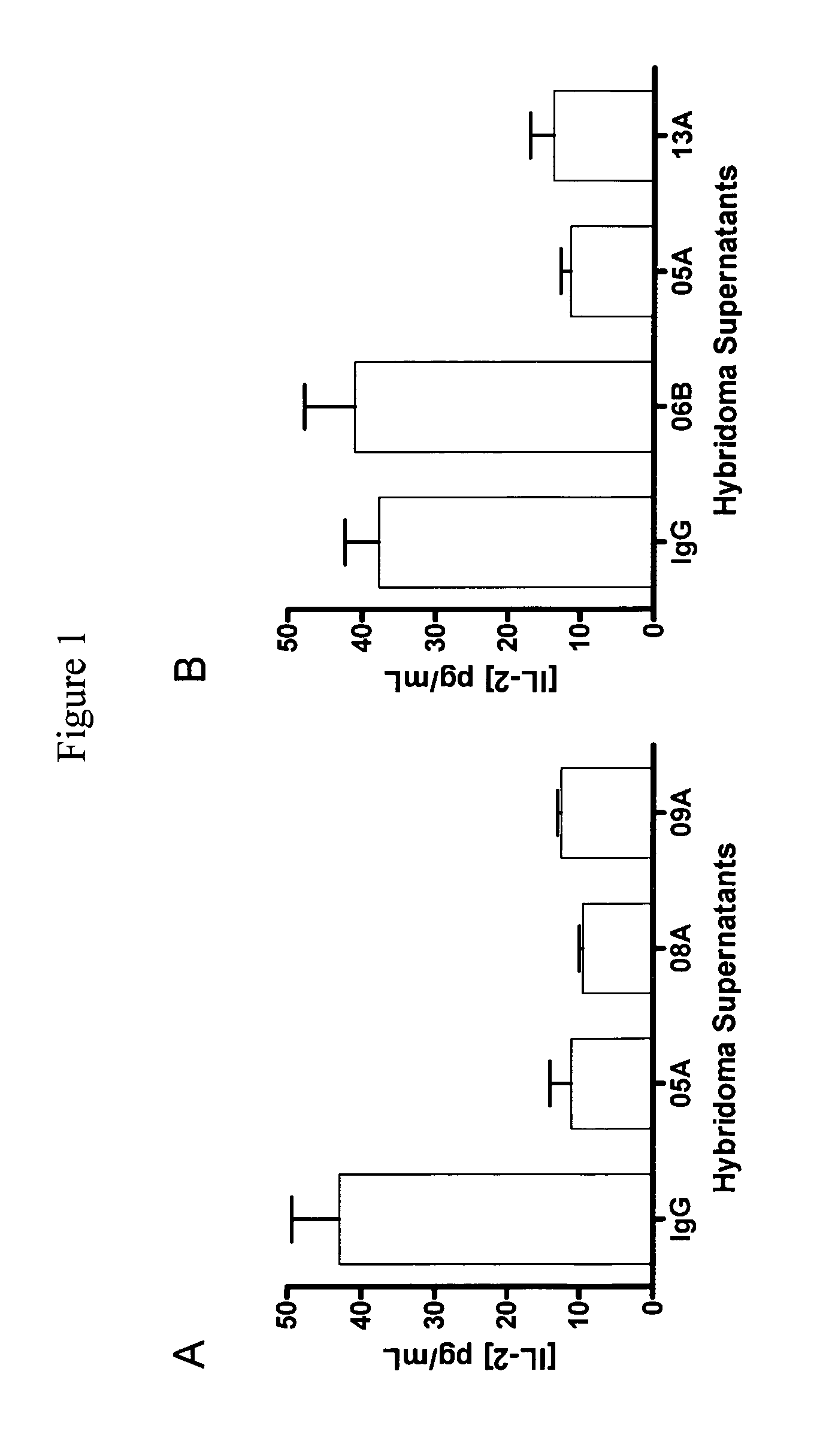
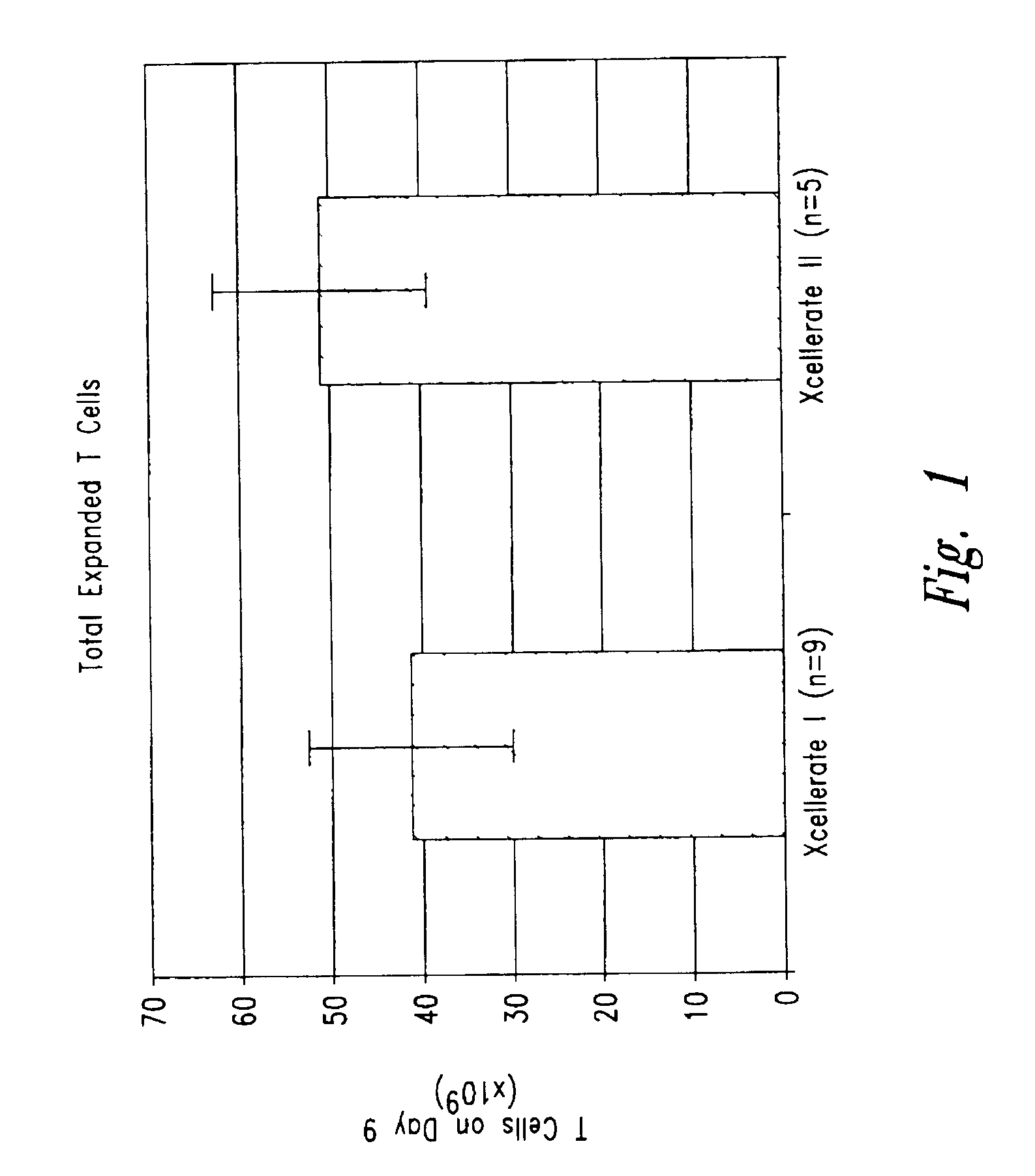
InactiveUS6905874B2Maximizes stimulationBiocideImmunoglobulins against cell receptors/antigens/surface-determinantsT cellCells signal
The present invention relates generally to methods for stimulating cells, and more particularly, to a novel method to concentrate and stimulate cells that maximizes stimulation and / or proliferation of such cells. In the various embodiments, cells are stimulated and concentrated with a surface yielding enhanced proliferation, cell signal transduction, and / or cell surface moiety aggregation. In certain aspects methods for stimulating a population of cells such as T-cells, by simultaneous concentration and cell surface moiety ligation are provided by contacting the population of cells with a surface, that has attached thereto one or more agents that ligate a cell surface moiety and applying a force that predominantly drives cell concentration and cell surface moiety ligation, thereby inducing cell stimulation, cell surface moiety aggregation, and / or receptor signaling enhancement. Also provided are methods for producing phenotypically tailored cells, including T-cells for the use in diagnostics, drug discovery, and the treatment of a variety of indications, including cancer, viral infection, and immune related disorders. Compositions of cells having specific phenotypic properties produced by these processes are further provided.
Owner:LIFE TECH CORP
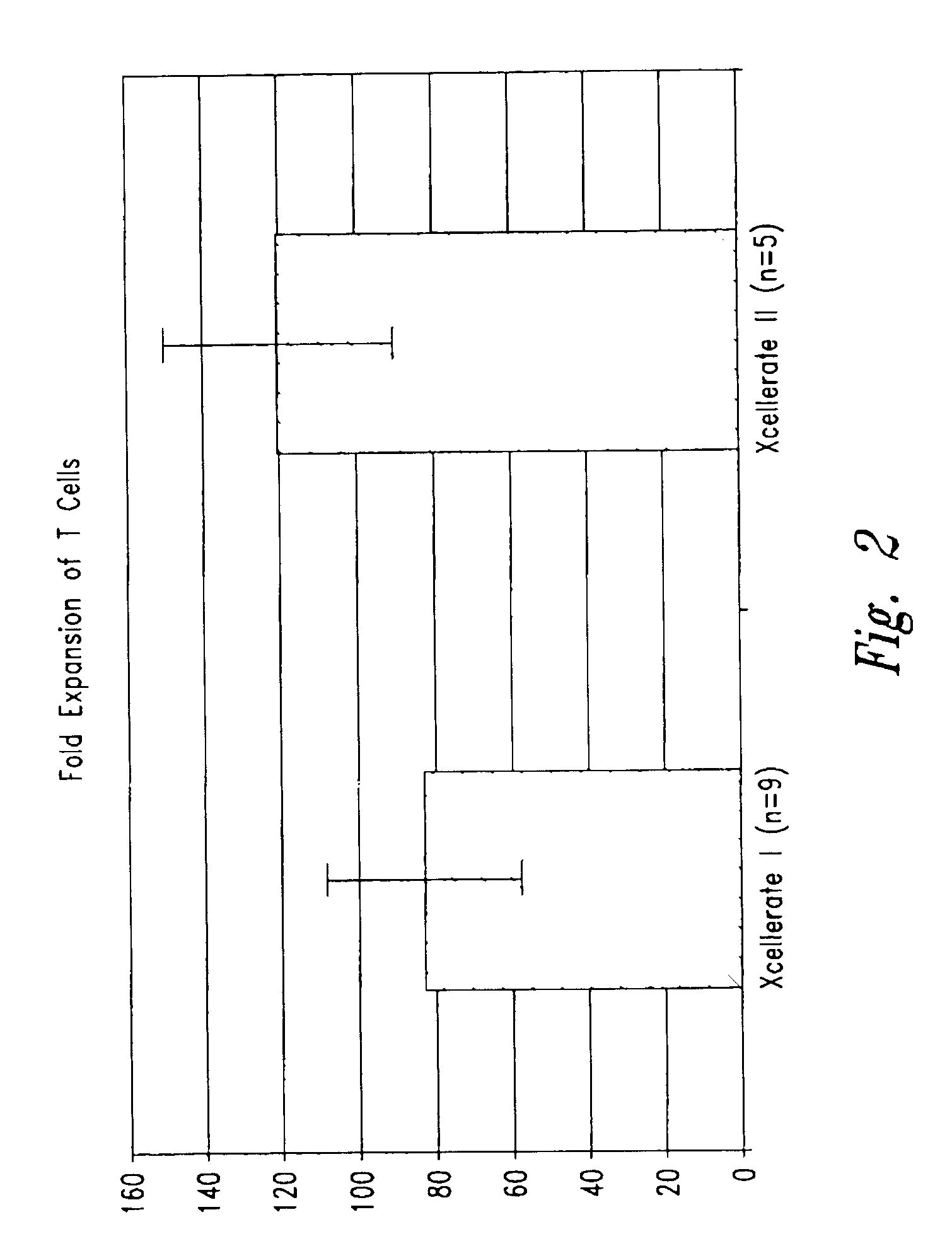
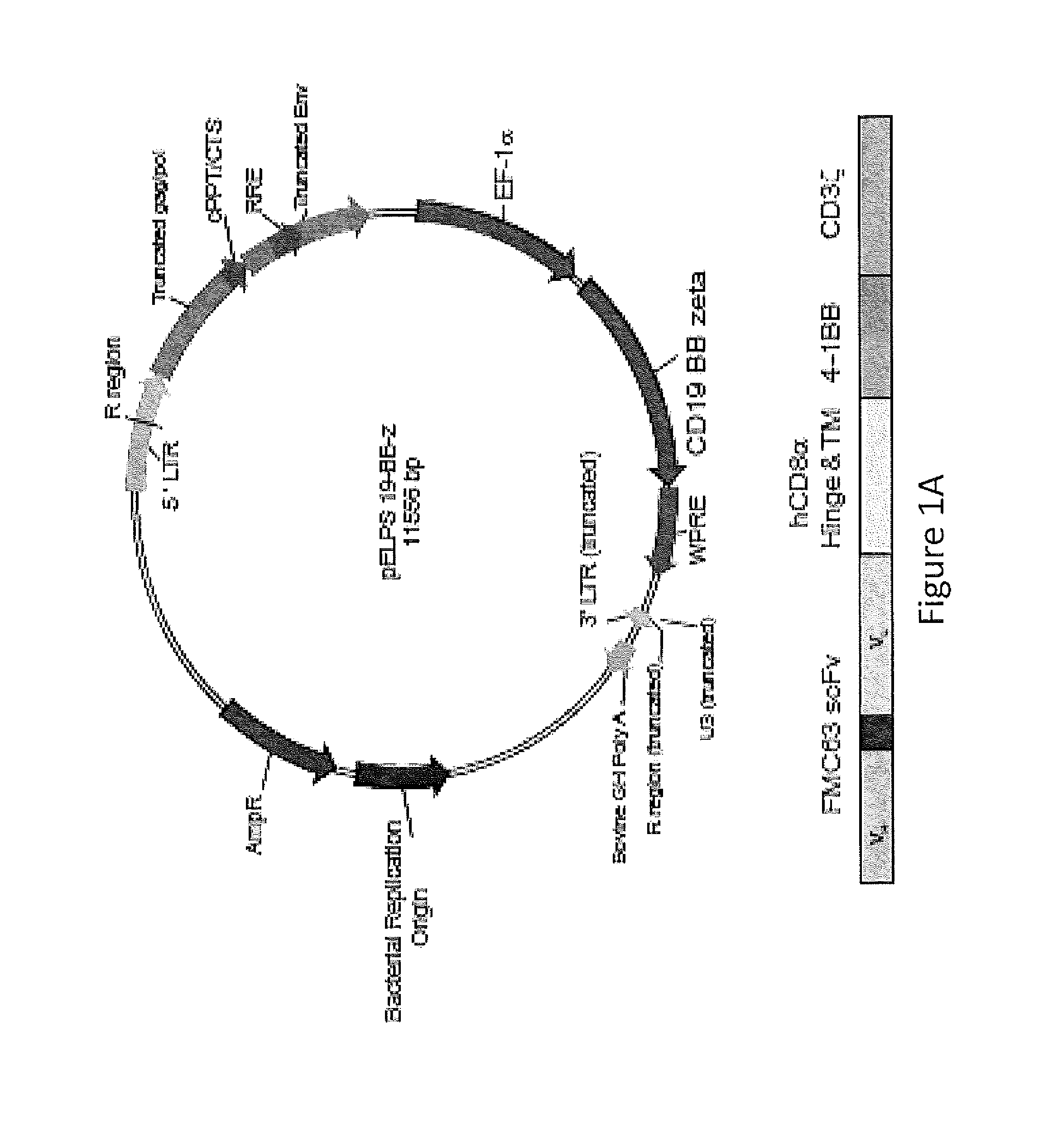
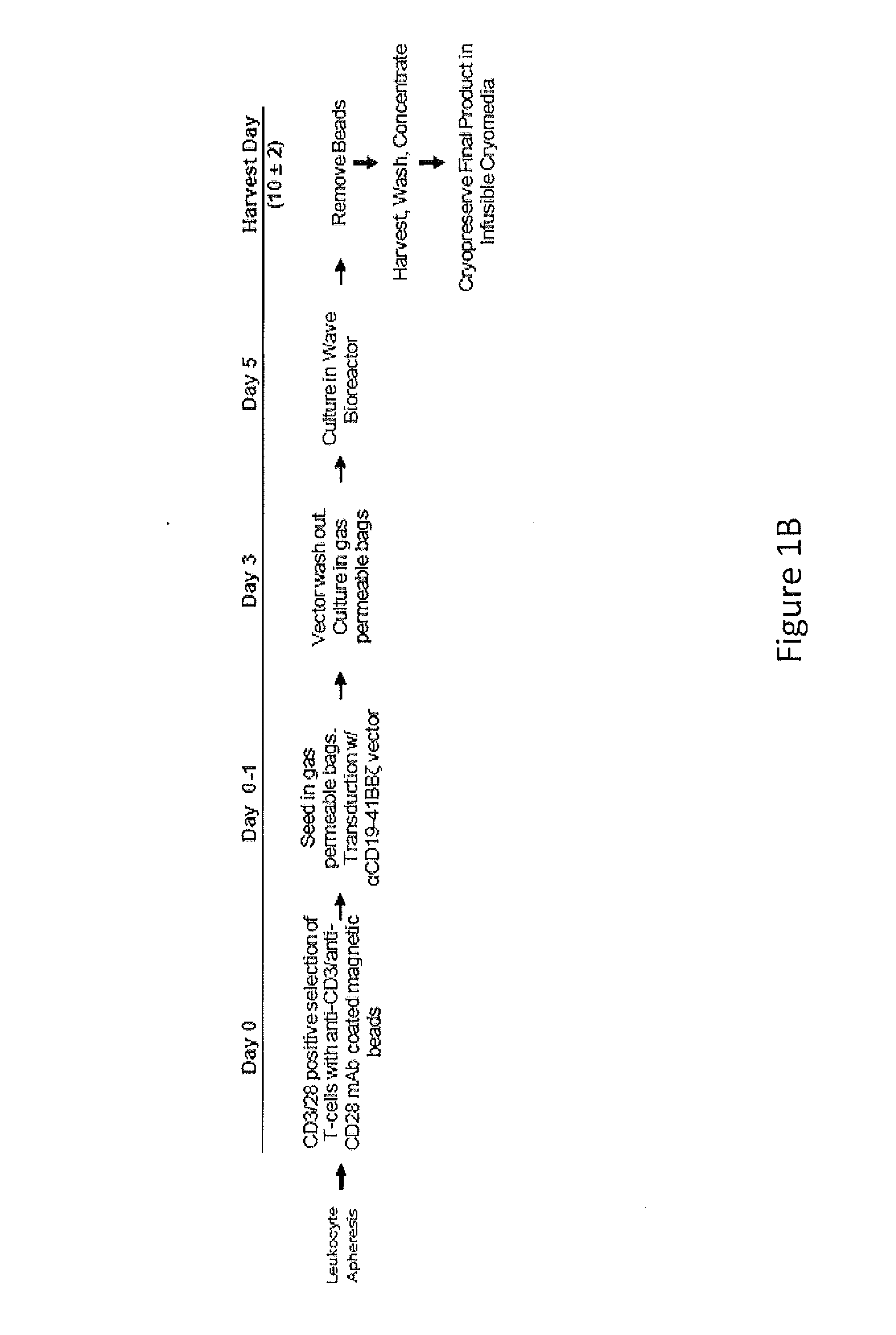
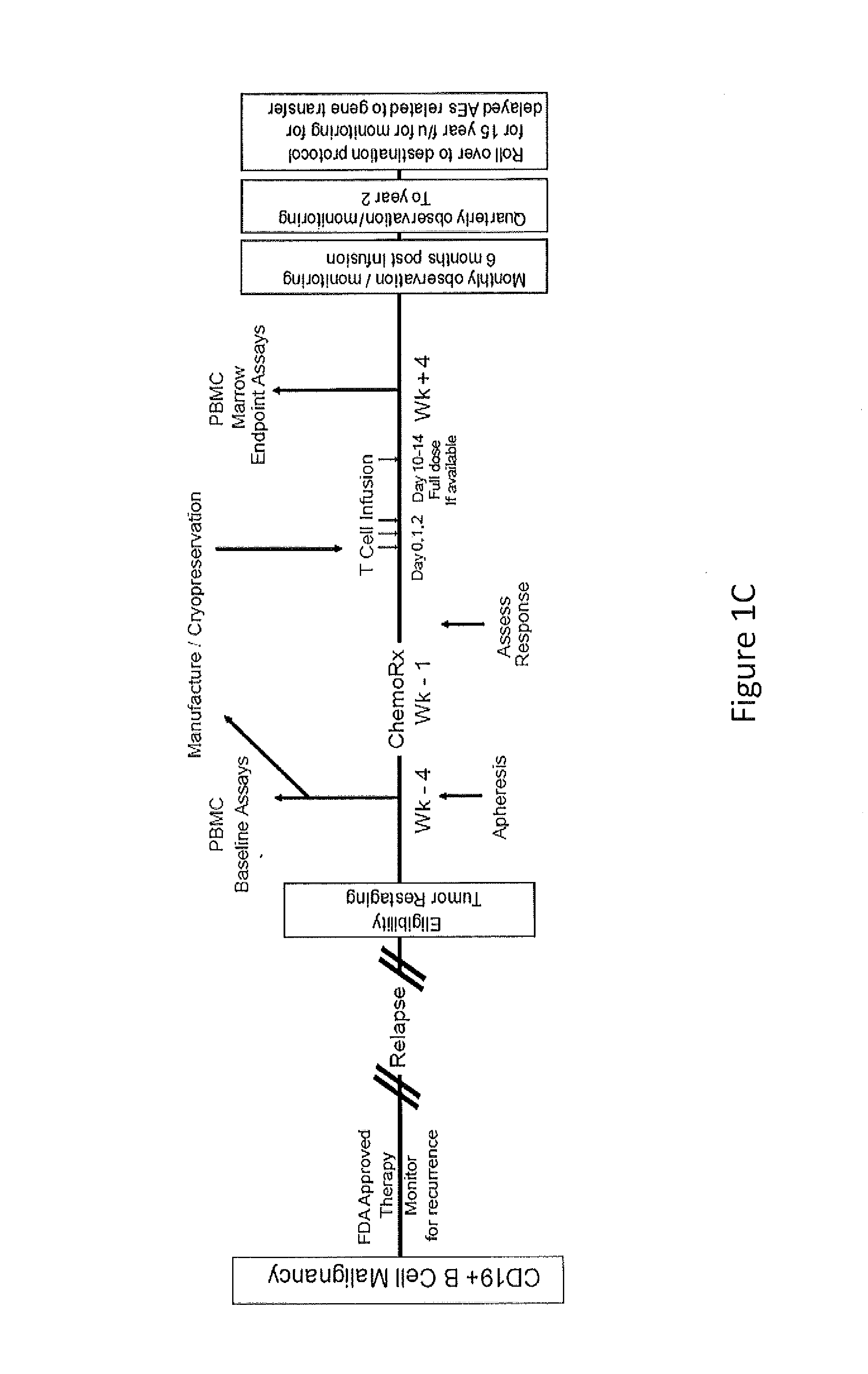
Use of Chimeric Antigen Receptor-Modified T-Cells to Treat Cancer
ActiveUS20130287748A1Stimulate immune responseVirusesPeptide/protein ingredientsBinding domainAntigen binding
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell to express a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
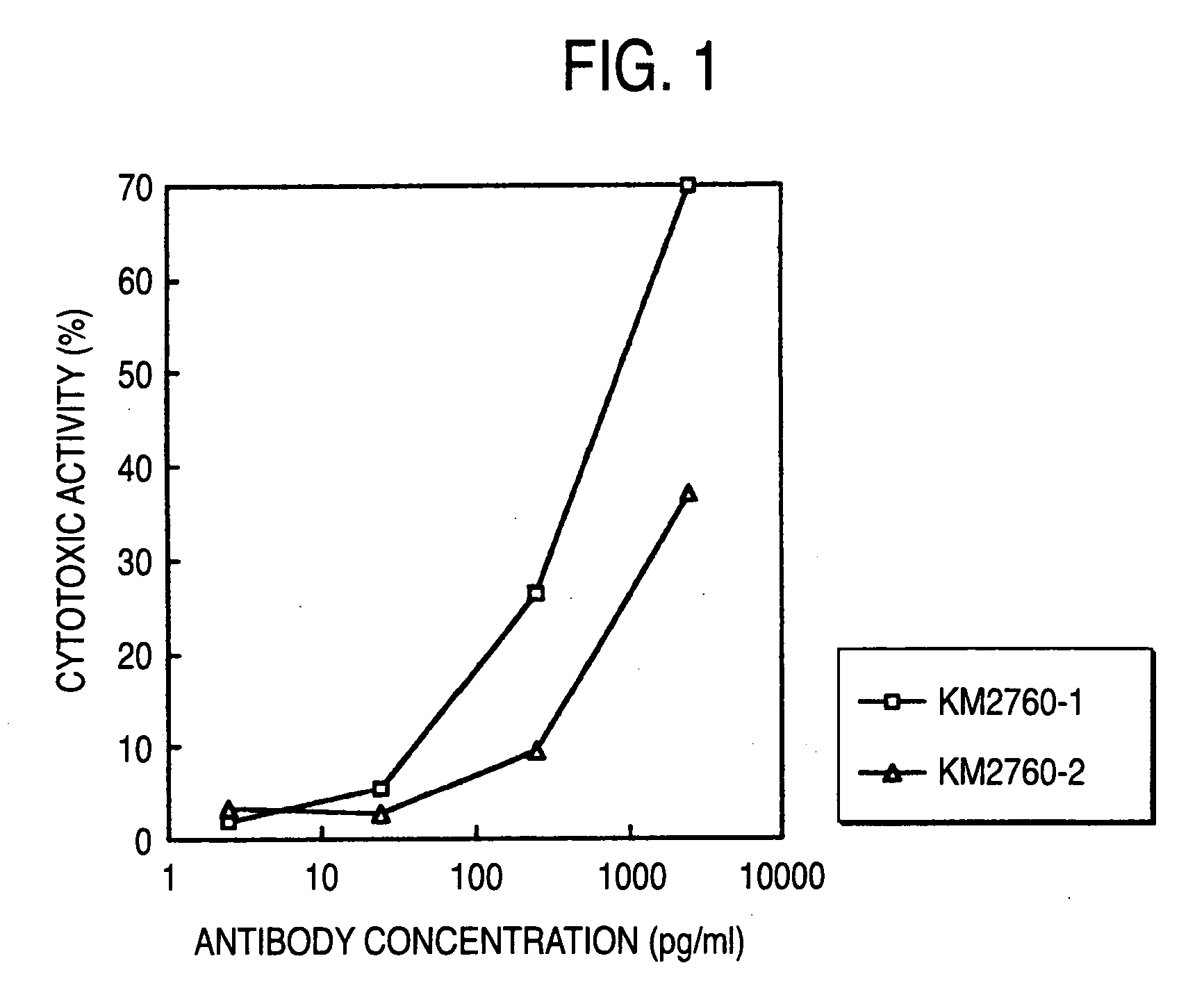
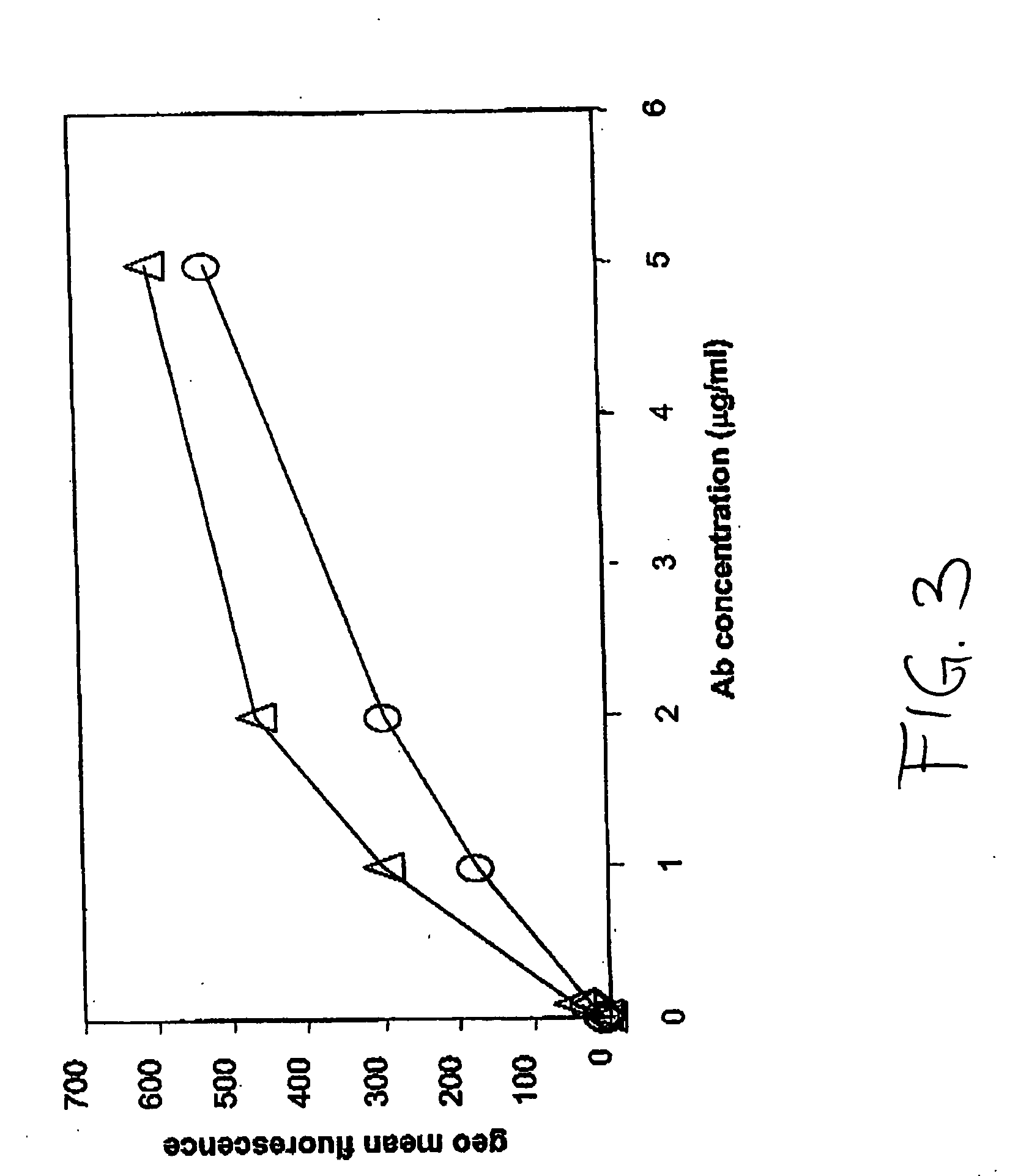
Cells producing antibody compositions with increased antibody dependent cytotoxic activity
The present invention relates to a cell for the production of an antibody molecule such as an antibody useful for various diseases having high antibody-dependent cell-mediated cytotoxic activity, a fragment of the antibody and a fusion protein having the Fc region of the antibody or the like, a method for producing an antibody composition using the cell, the antibody composition and use thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Method of treating androgen independent prostate cancer
The present invention is directed to a method treating prostate cancer. The method comprises administering to a patient in need thereof at least one compound selected from N-methyl-Δ3,3′-dihydroindole-2,2′ diketone; N-1-(β-D-O-triacetyl-xylopranosyl)-Δ3,3′-dihydroindole-2,2′ diketone; and N-1-(β-D-O-triacetyl-xylopranosyl)-N′-methyl-Δ3,3′-dihydroindole-2,2′ diketone. Preferably the compound is in an amount sufficient to inhibit growth, invasion, and / or metastasis of prostate cancer cells.
Owner:NATROGEN THERAPEUTICS INT
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
Pentapeptide compounds and uses related thereto
Pentapeptide compounds are disclosed. The compounds have biological activity, e.g., cytotoxicity. Prodrugs having targeting groups and pentapeptide moieities, as well as precursors thereof are also disclosed. For example, precursors having a reactive linker that can serve as a reaction site for joining to a targeting agent, e.g., an antibody, as disclosed.
Owner:SEAGEN INC
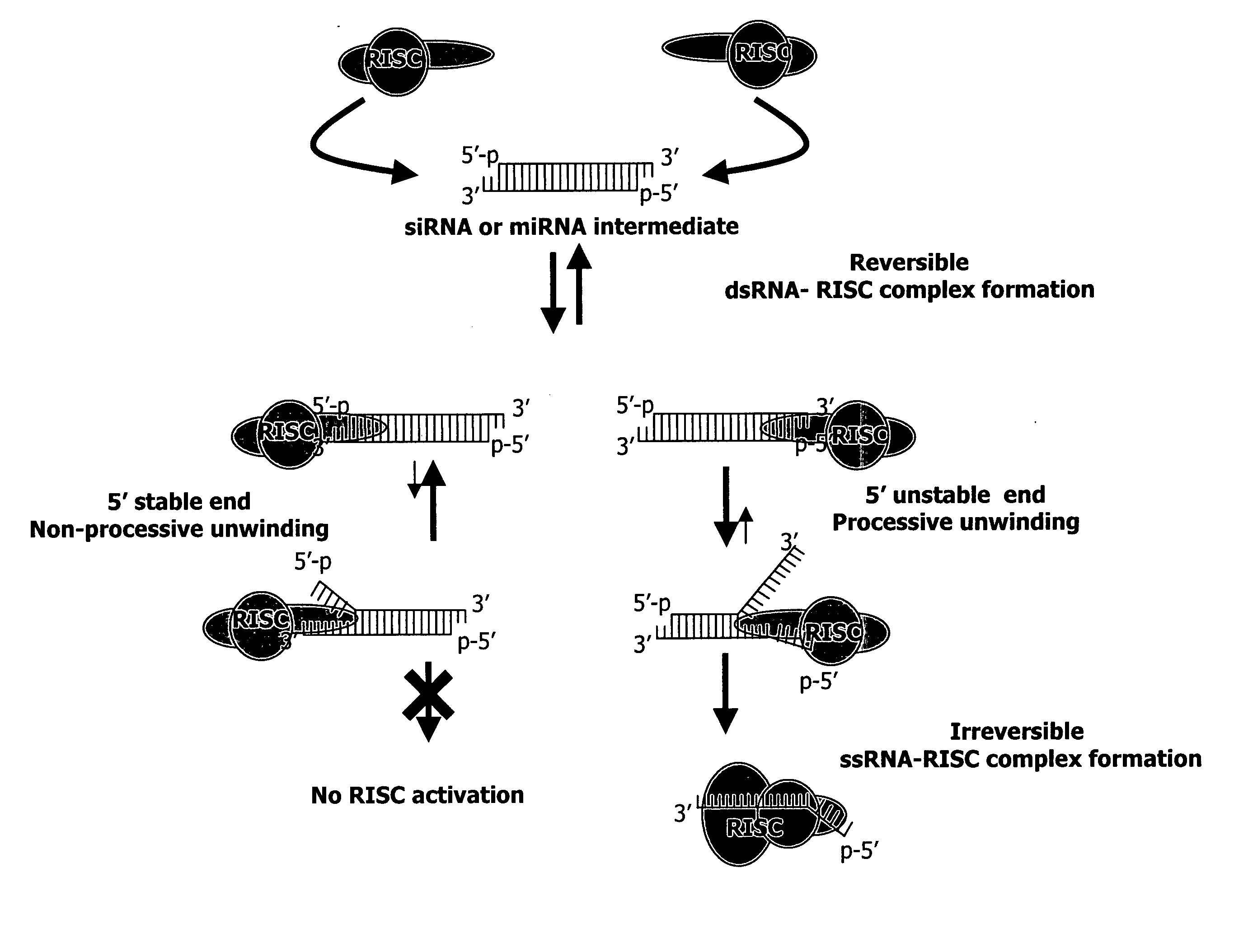
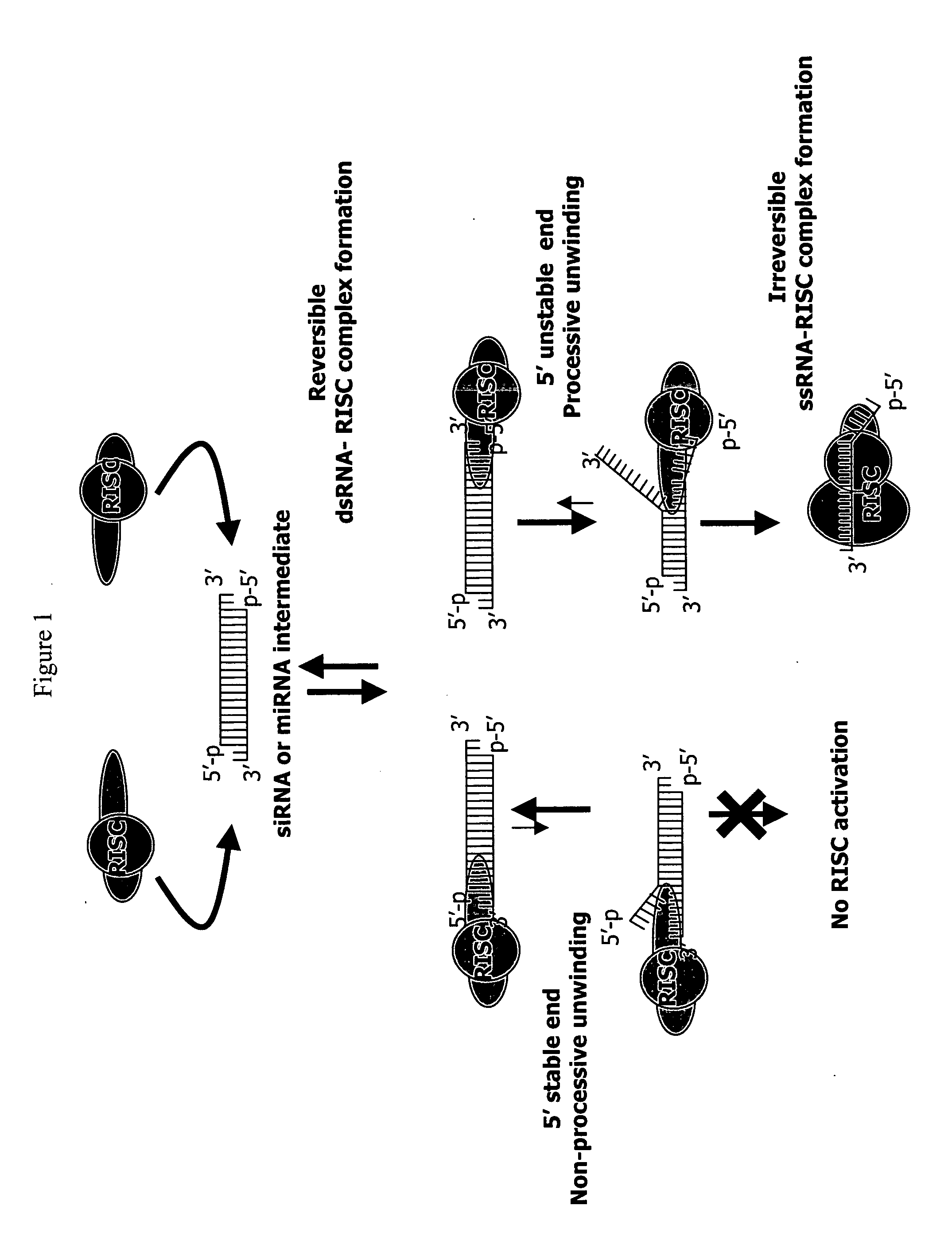
Methods and compositions for selecting siRNA of improved functionality
InactiveUS20050255487A1Improve efficiencyGood curative effectOrganic active ingredientsGenetic material ingredientsGene silencingSilencing gene
Efficient sequence specific gene silencing is possible through the use of siRNA technology. By selecting particular siRNAs by rational design, one can maximize the generation of an effective gene silencing reagent, as well as methods for silencing genes. Methods, compositions, and kits generated through rational design of siRNAs are disclosed.
Owner:THERMO FISHER SCIENTIFIC INC
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114649A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsT cellInfective disorder
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
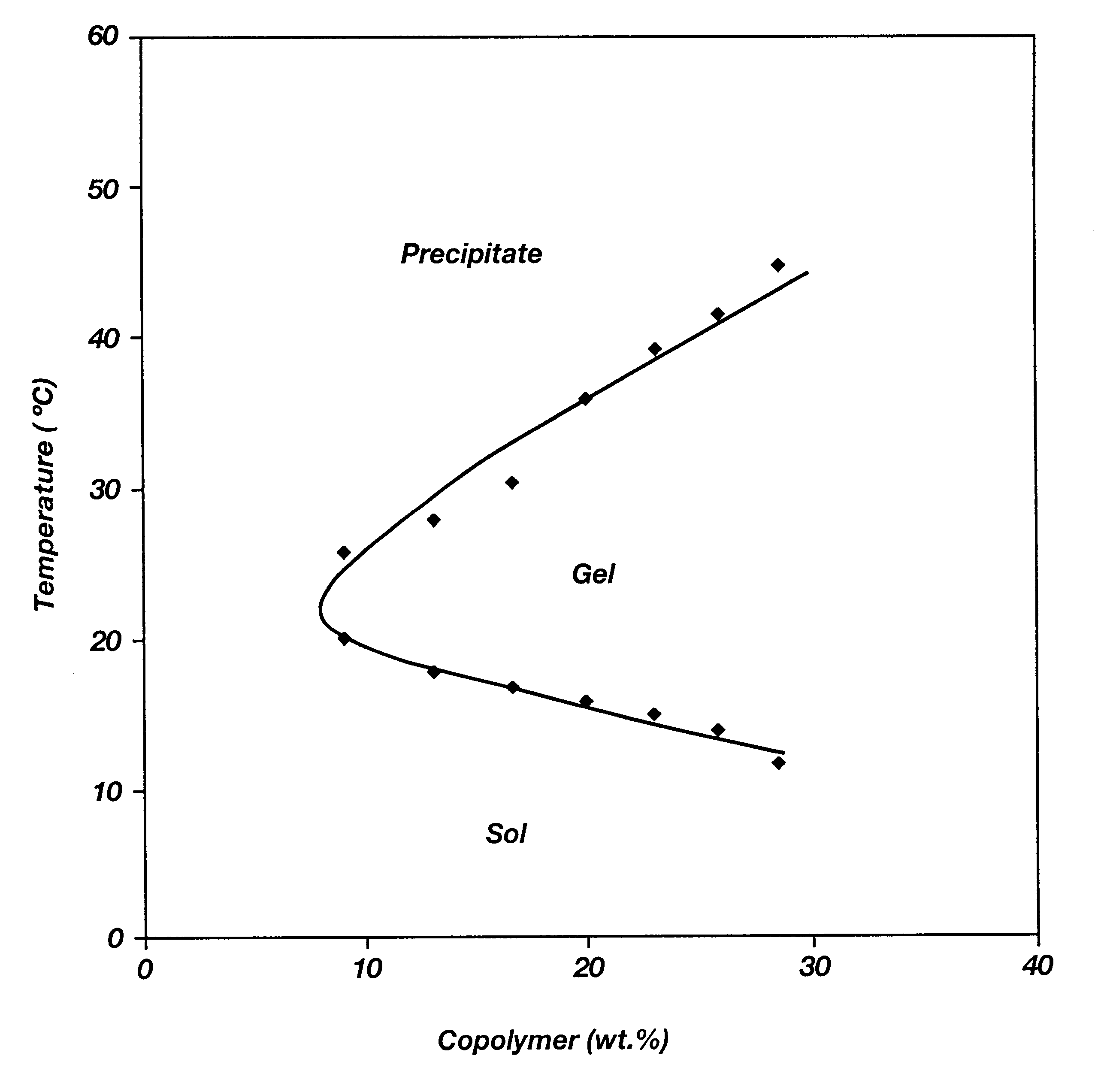
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
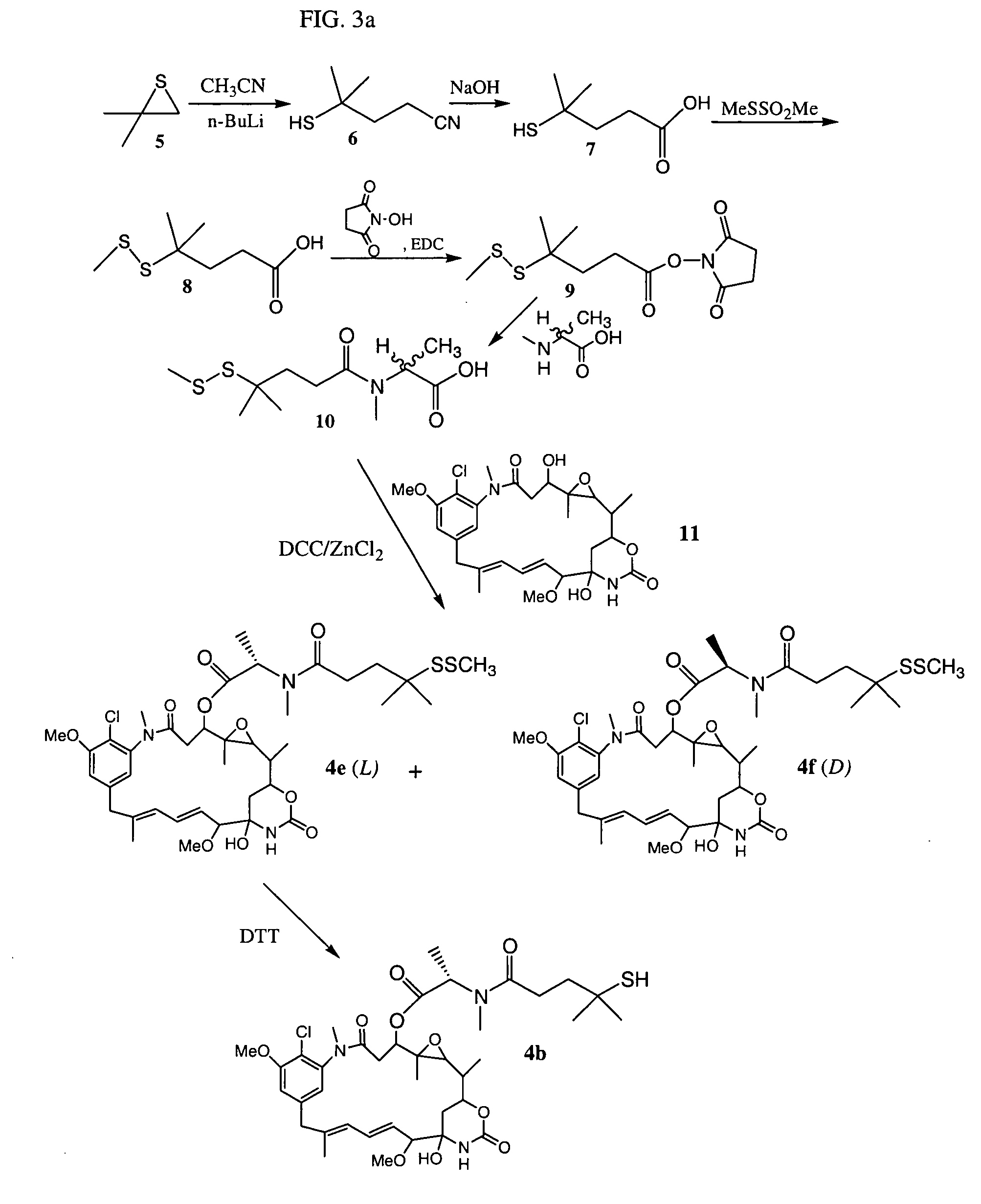
Cytotoxic agents comprising new maytansinoids
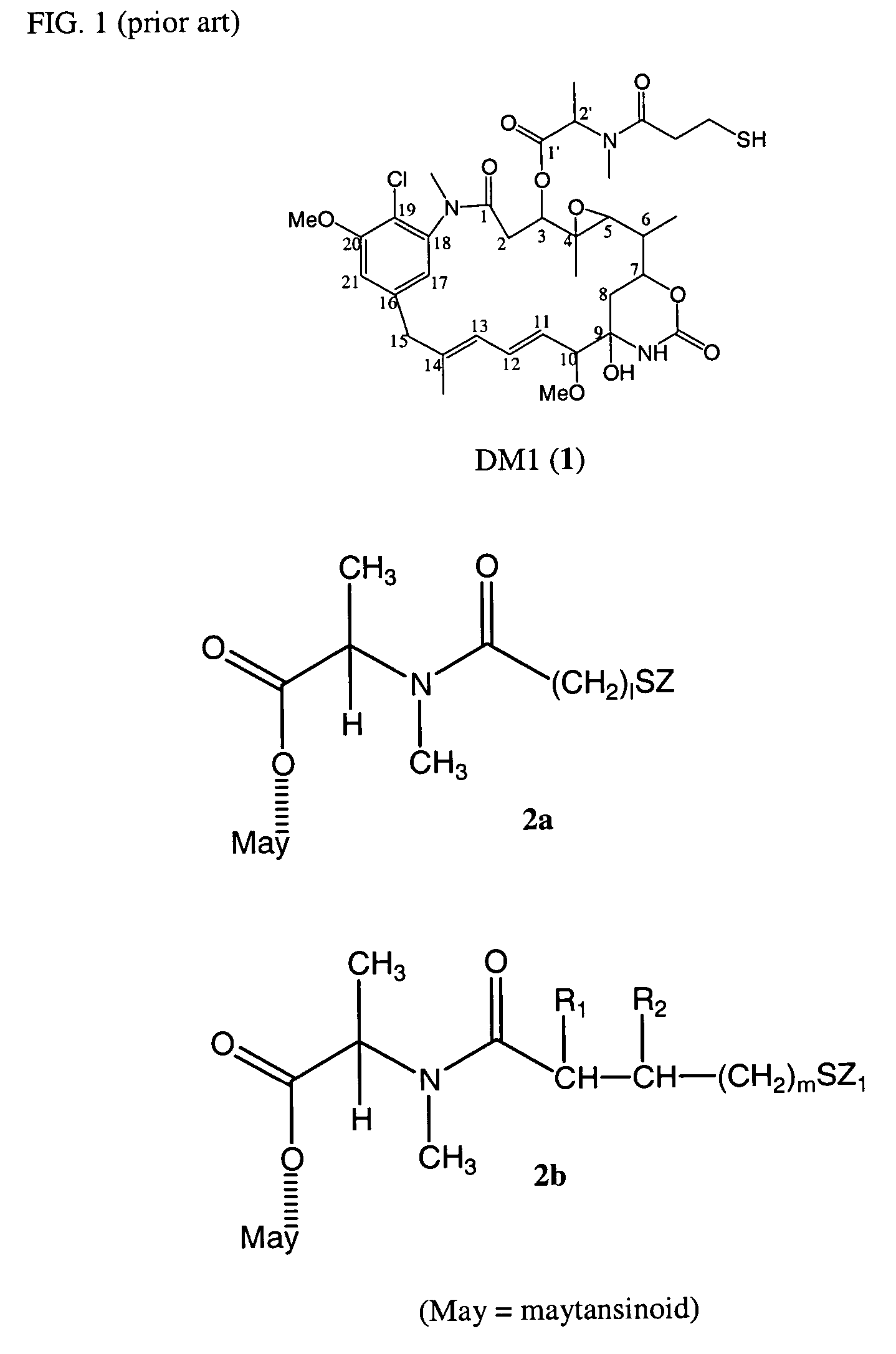
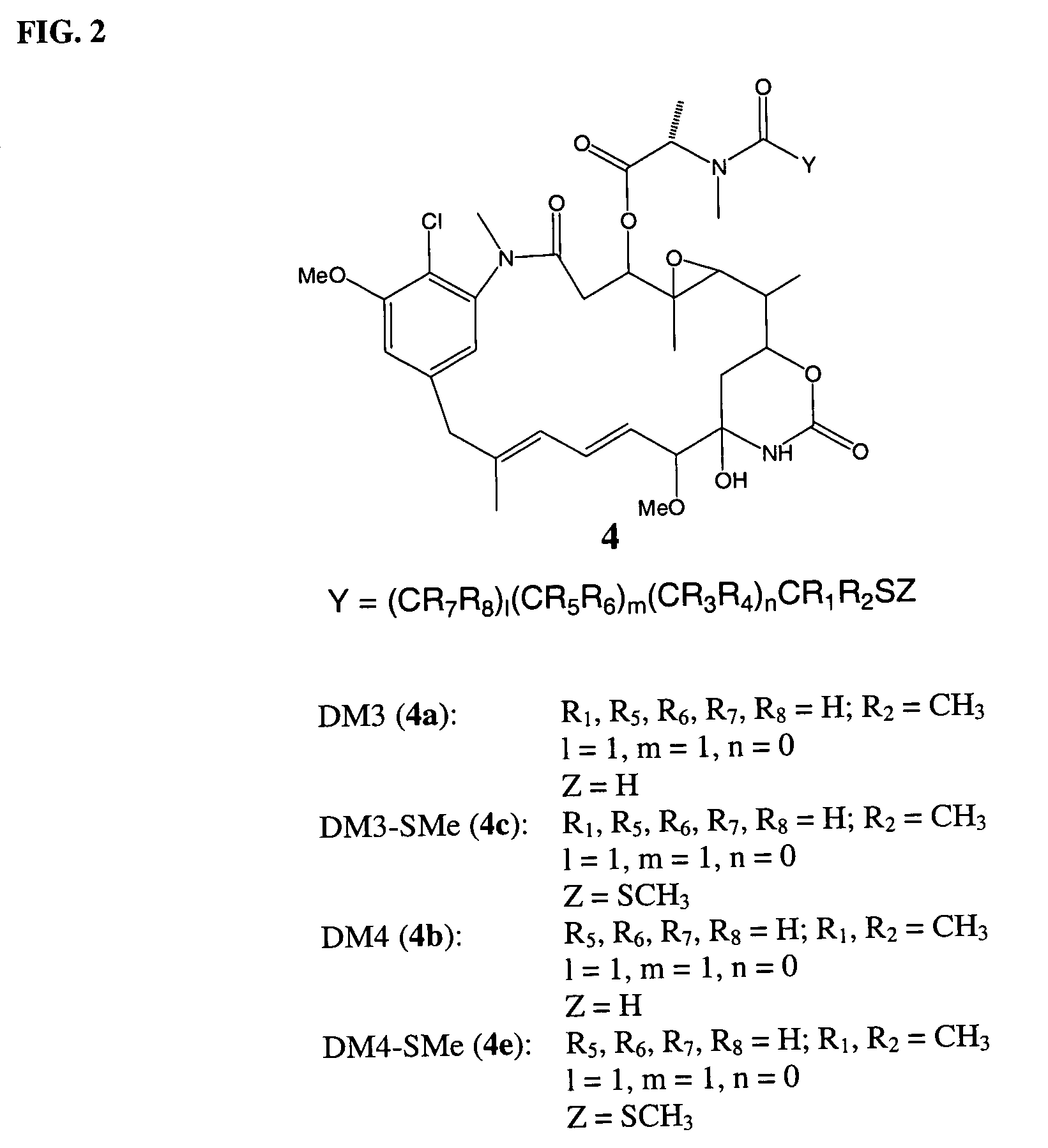
ActiveUS7276497B2Improve anti-tumor activityImprove biological activityOrganic active ingredientsOrganic chemistryAnimal tumorEfficacy
New thiol and disulfide-containing maytansinoids bearing a mono or di-alkyl substitution on the α-carbon atom bearing the sulfur atom are disclosed. Also disclosed are methods for the synthesis of these new maytansinoids and methods for the linkage of these new maytansinoids to cell-binding agents. The maytansinoid-cell-binding agent conjugates are useful as therapeutic agents, which are delivered specifically to target cells and are cytotoxic. These conjugates display vastly improved therapeutic efficacy in animal tumor models compared to the previously described agents.
Owner:IMMUNOGEN INC
Anti-PD1 antibodies and their use as therapeutics and diagnostics
ActiveUS8735553B1Inhibition of secretionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathAnti pd1
Owner:BEIGENE SWITZERLAND GMBH
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
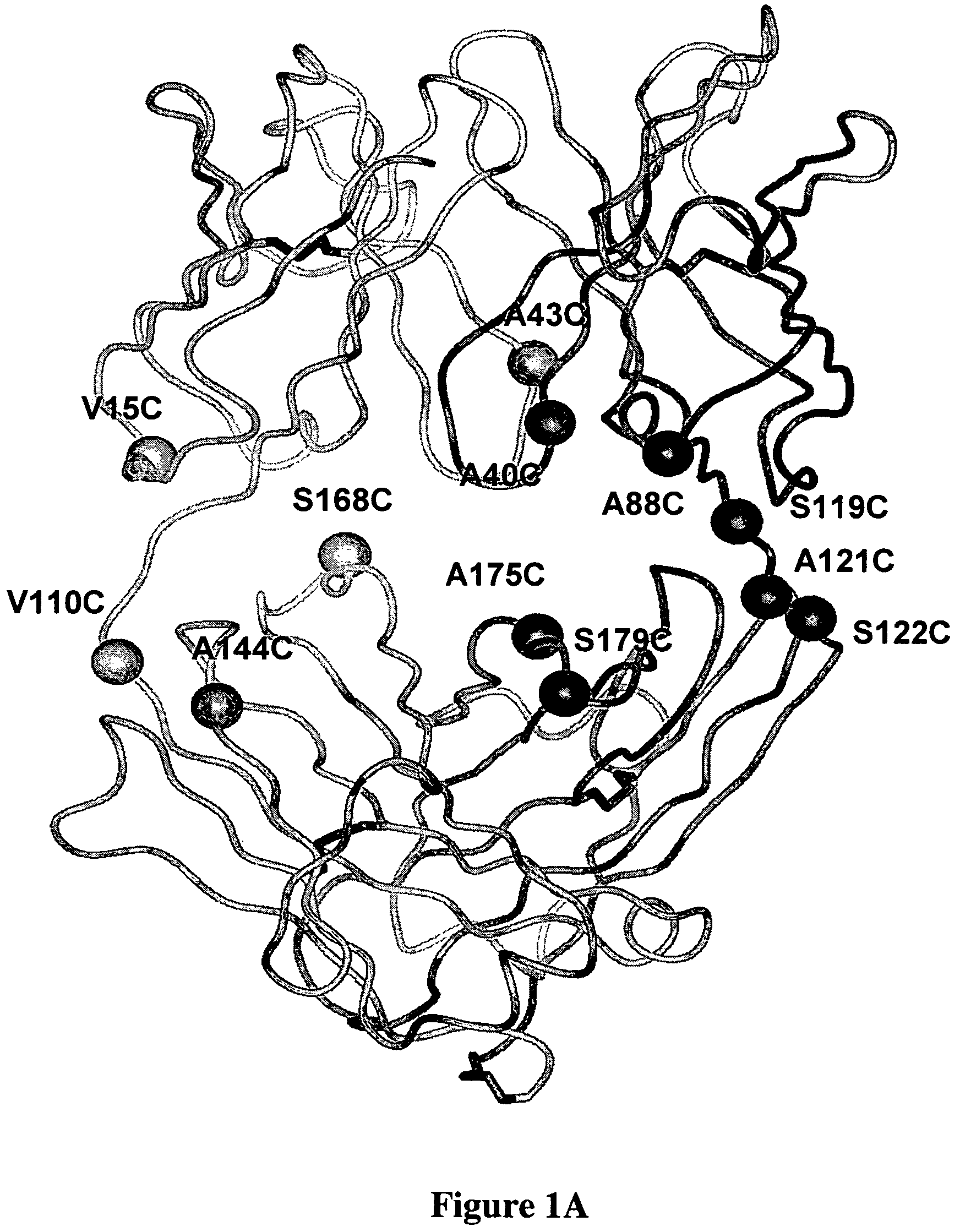
Stable Heterodimeric Antibody Design with Mutations in the Fc Domain
ActiveUS20120149876A1Promote formationImprove stabilityImmunoglobulinsImmunological disordersFc(alpha) receptorFc receptor
The provided scaffolds have heavy chains that are asymmetric in the various domains (e.g. CH2 and CH3) to accomplish selectivity between the various Fc receptors involved in modulating effector function, beyond those achievable with a natural homodimeric (symmetric) Fc molecule, and increased stability and purity of the resulting variant Fc heterodimers. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
Compositions and methods for treating and diagnosing cancer
InactiveUS20060019256A1Reduce the amount requiredMicrobiological testing/measurementAntiinfectivesSolid tumorCancer research
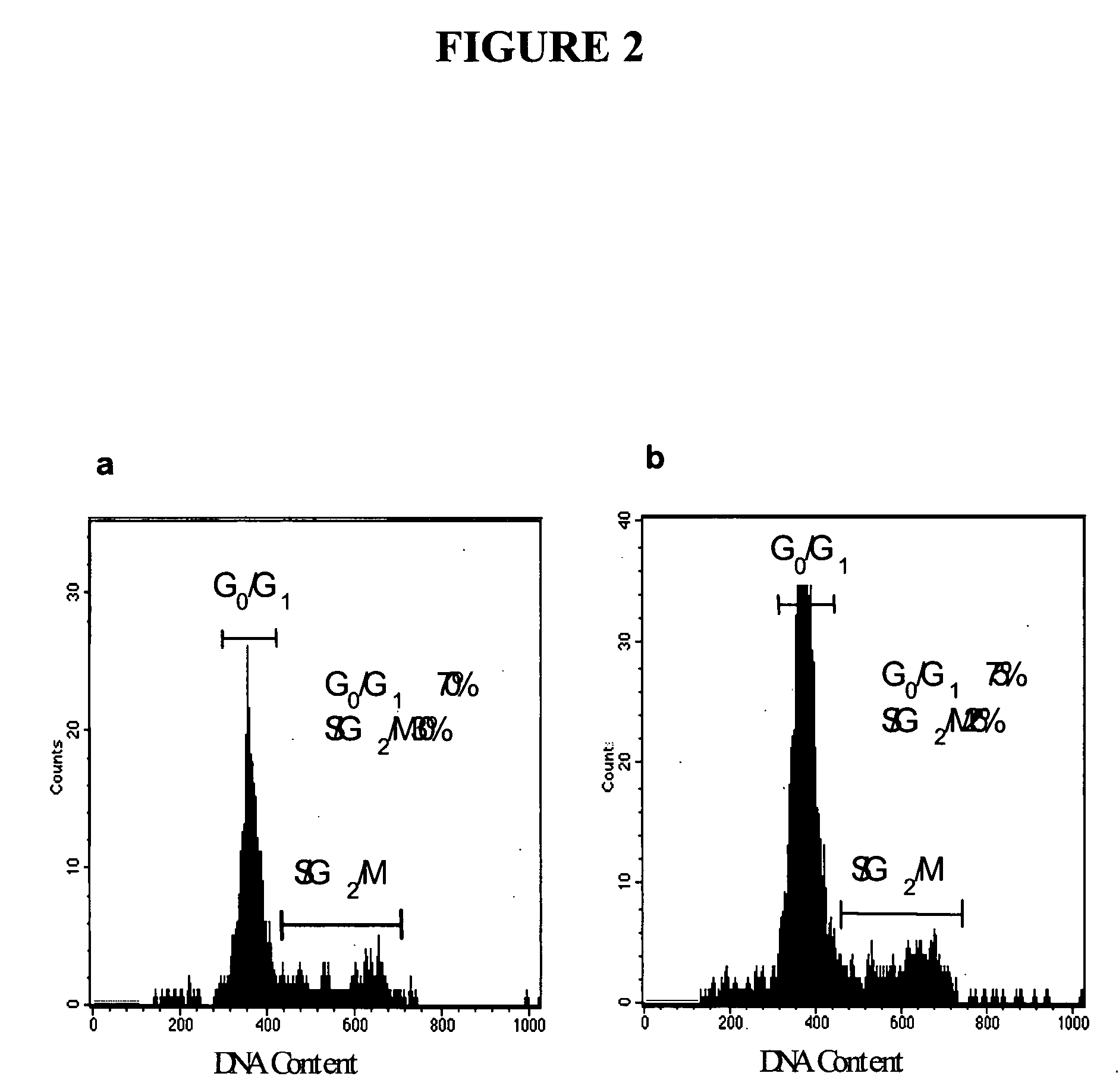
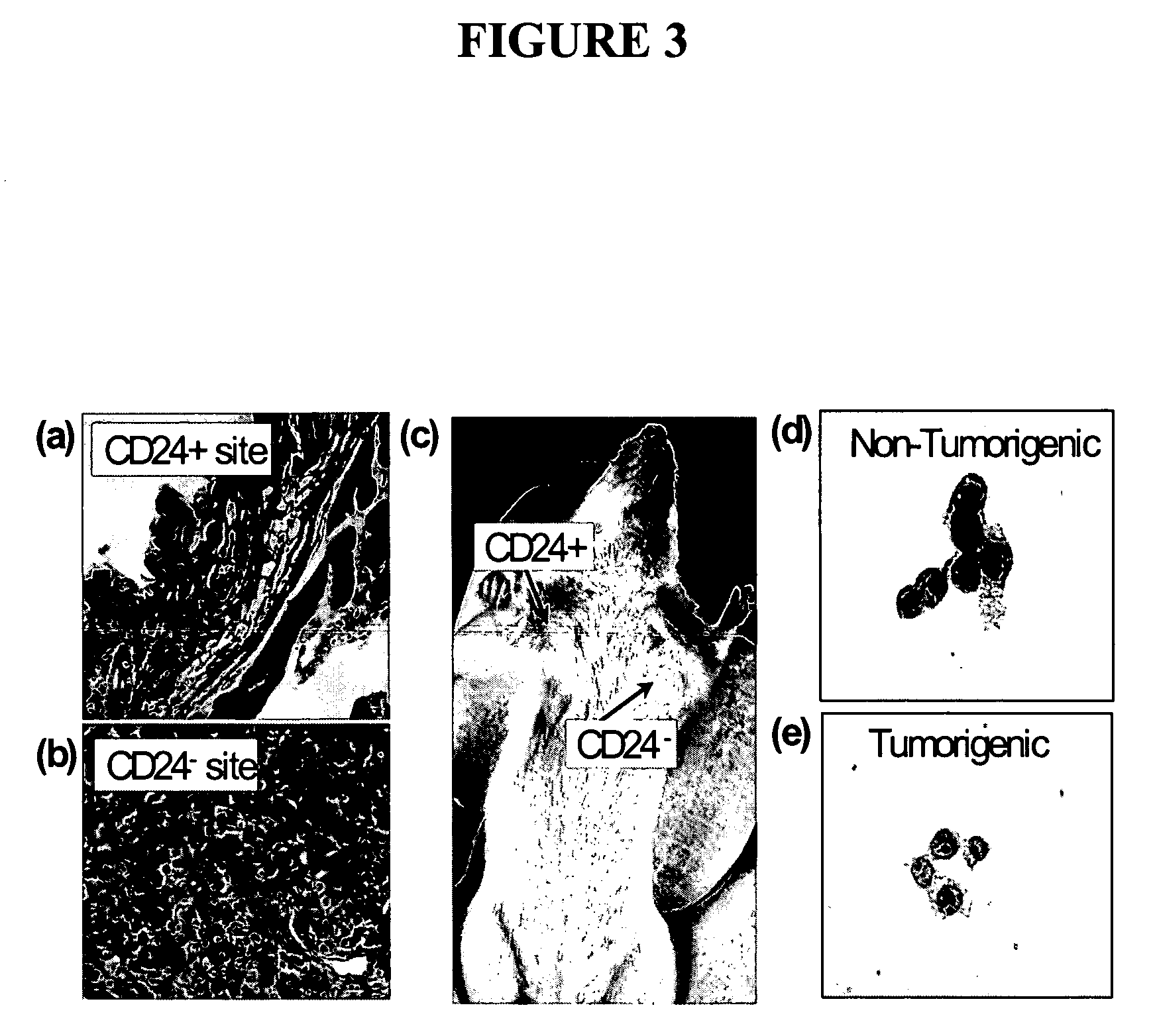
The present invention relates to compositions and methods for treating, characterizing, and diagnosing cancer. In particular, the present invention provides gene expression profiles associated with solid tumor stem cells, as well as novel stem cell cancer markers useful for the diagnosis, characterization, and treatment of solid tumor stem cells.
Owner:RGT UNIV OF MICHIGAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com