Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2099 results about "Treatment regimen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of Treatment Regimen. Treatment Regimen means administration of a Licensed Product as a single agent, or in combination with one or more approved pharmacological, anti-tumor agents, in each case, regardless of dosing, formulation and route of administration.
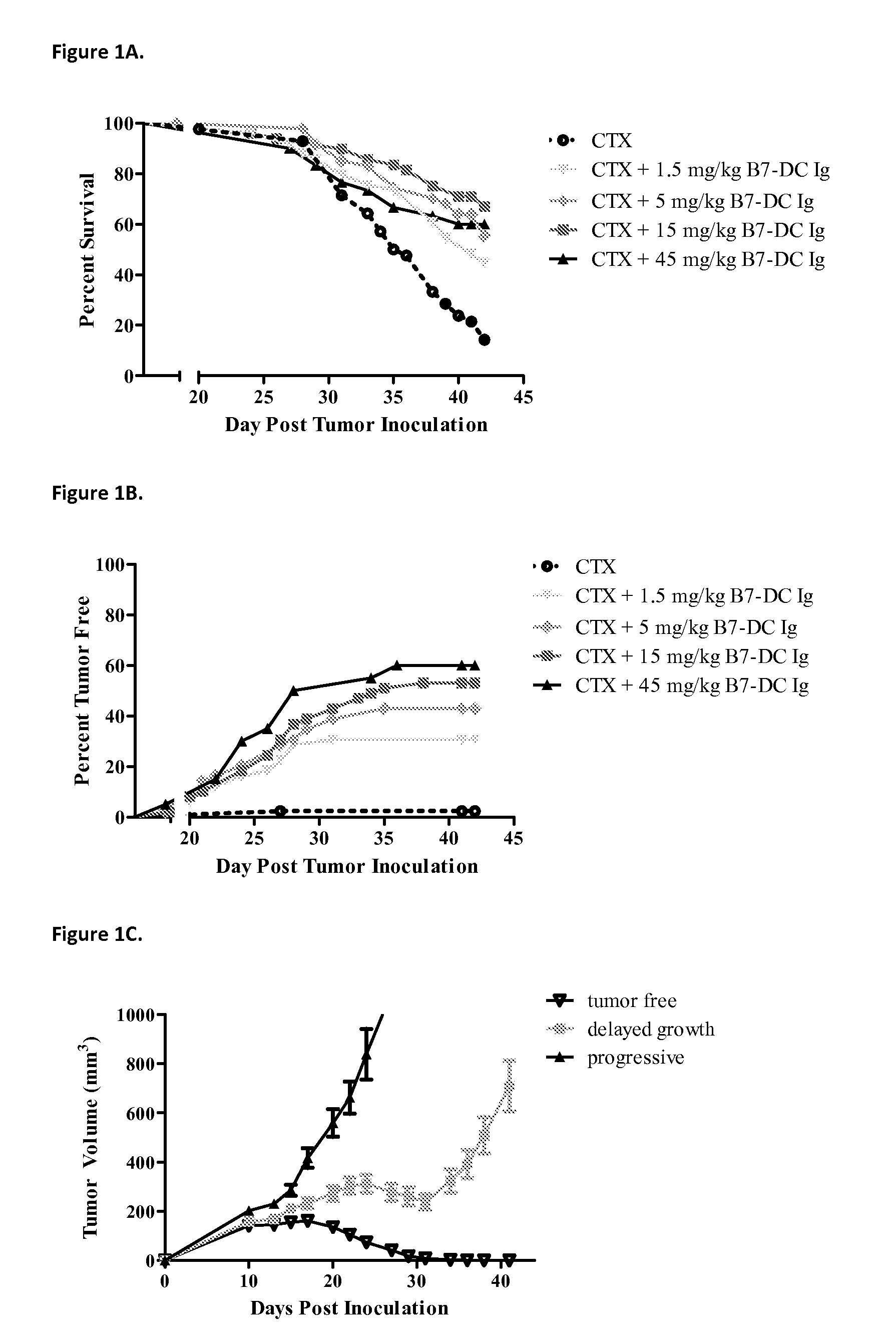
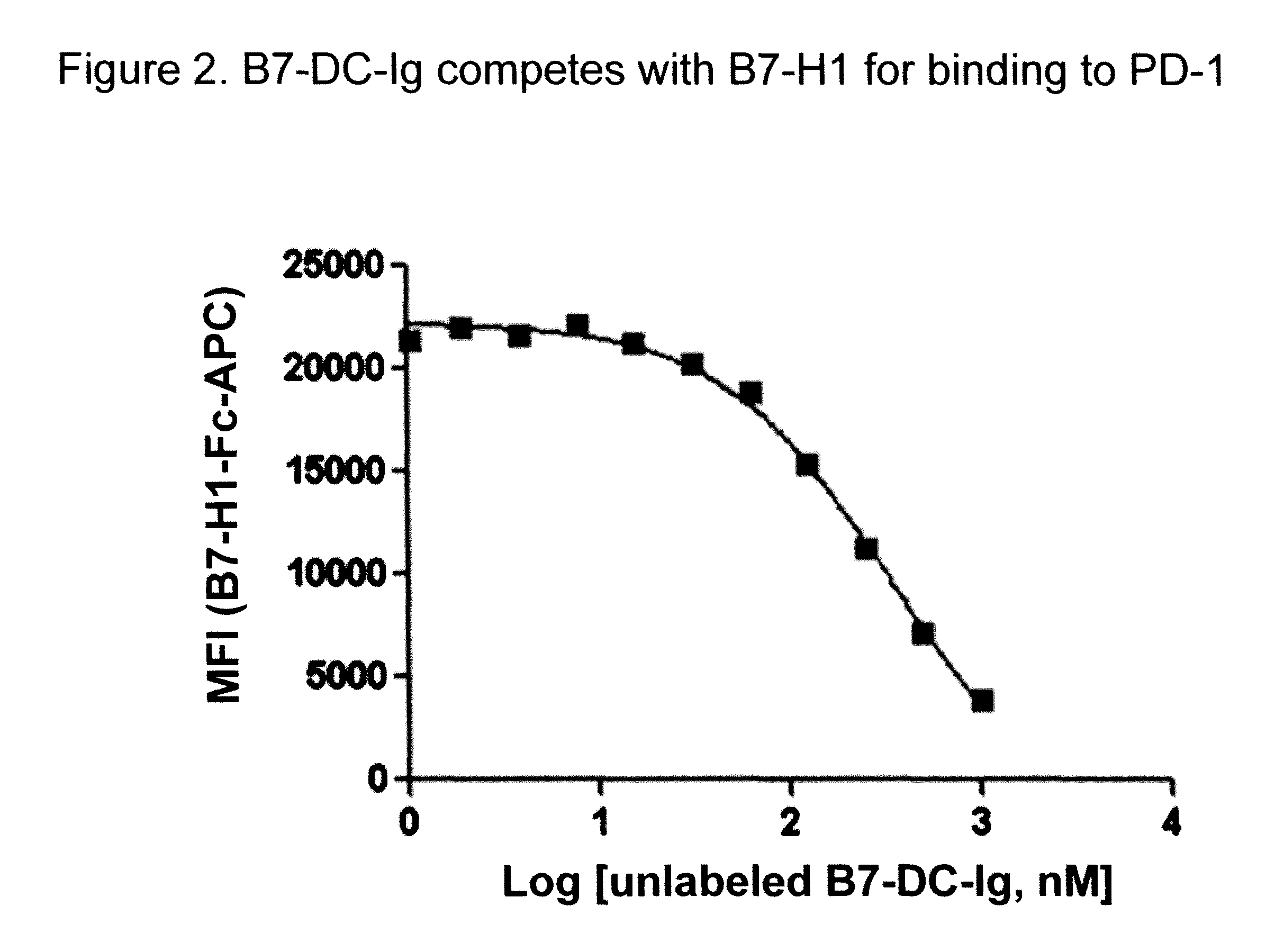
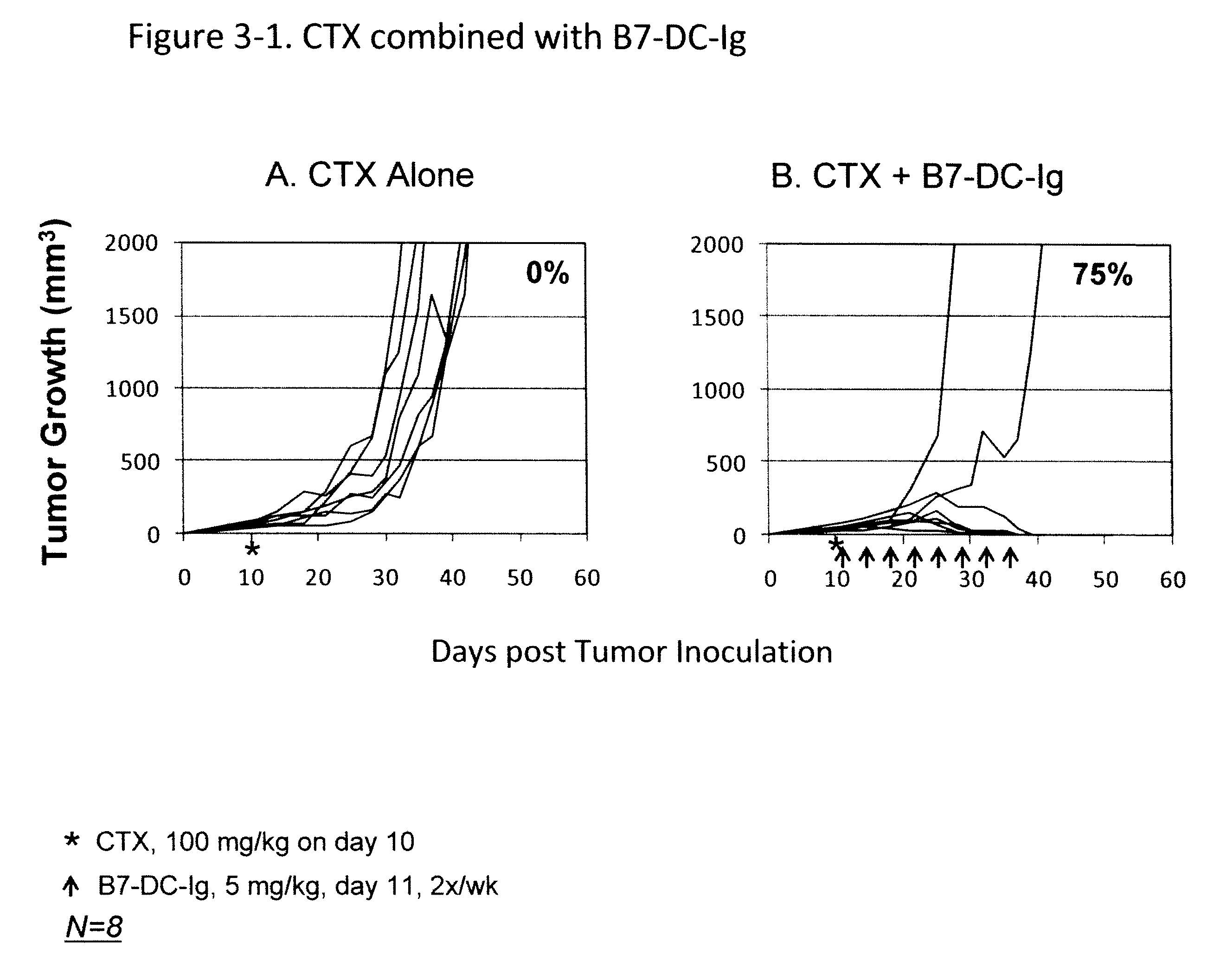
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114649A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsT cellInfective disorder
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
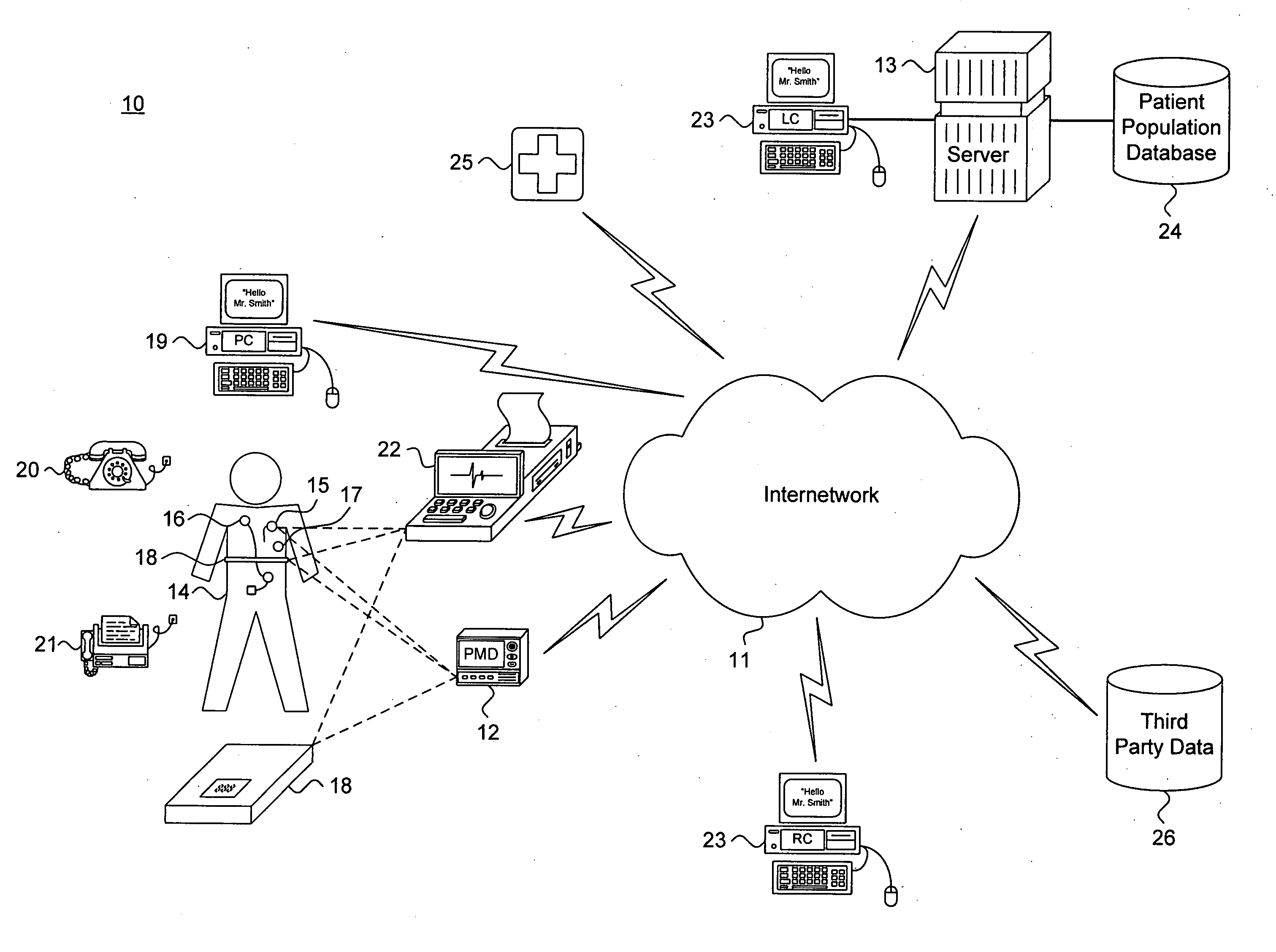
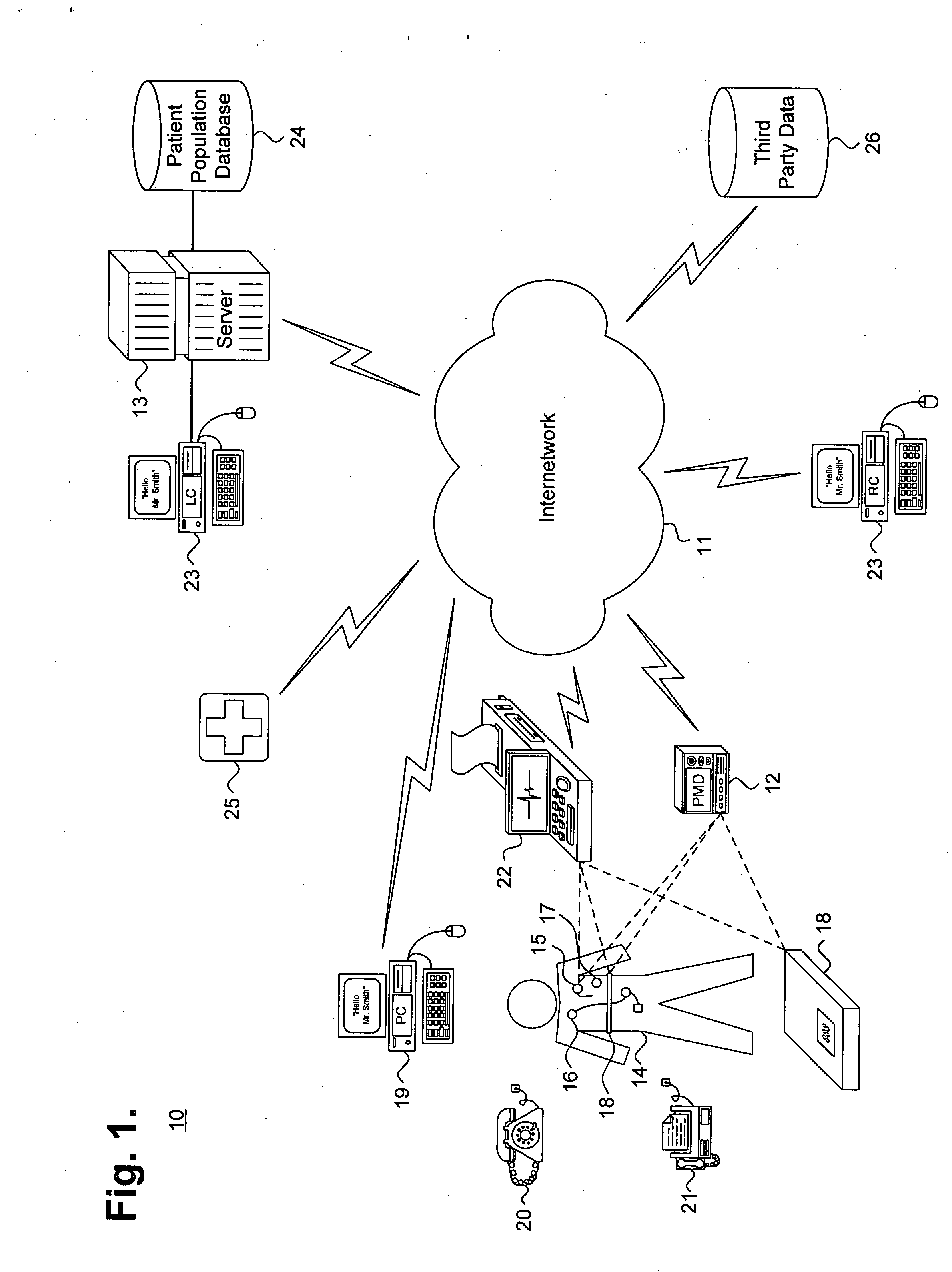
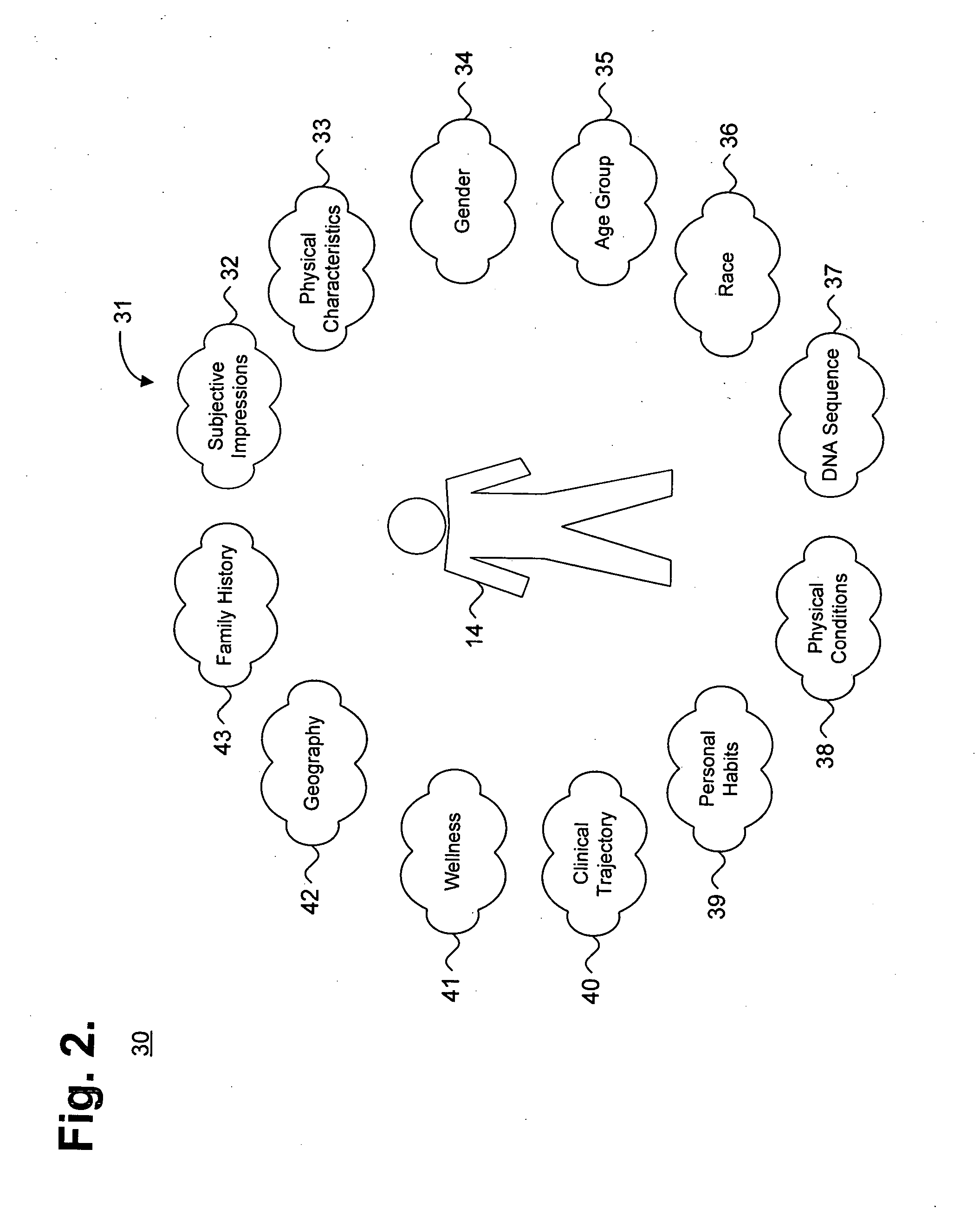
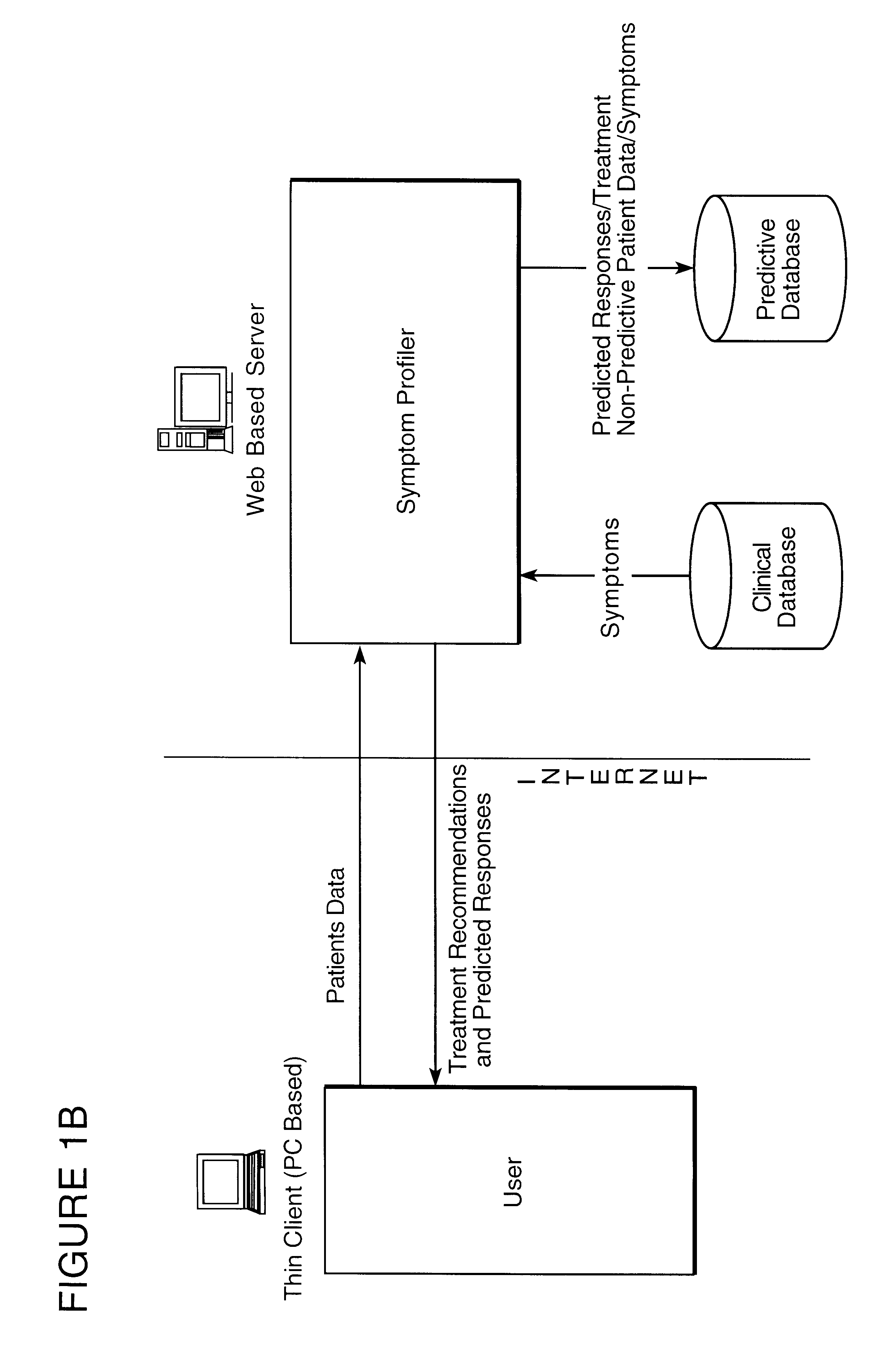
System and method for providing goal-oriented patient management based upon comparative population data analysis
A system and method for providing goal-oriented patient management based upon comparative population data analysis is presented. At least one therapy goal is defined to manage a disease state. A patient population is selected sharing at least one characteristic with an individual patient presenting with indications of the disease state. One or more treatment regimens associated with the patient population are identified as implementing actions under the at least one therapy goal. The implementing actions are followed through one or more quantifiable physiological indications monitored via data sources associated with the patient.
Owner:CARDIAC PACEMAKERS INC
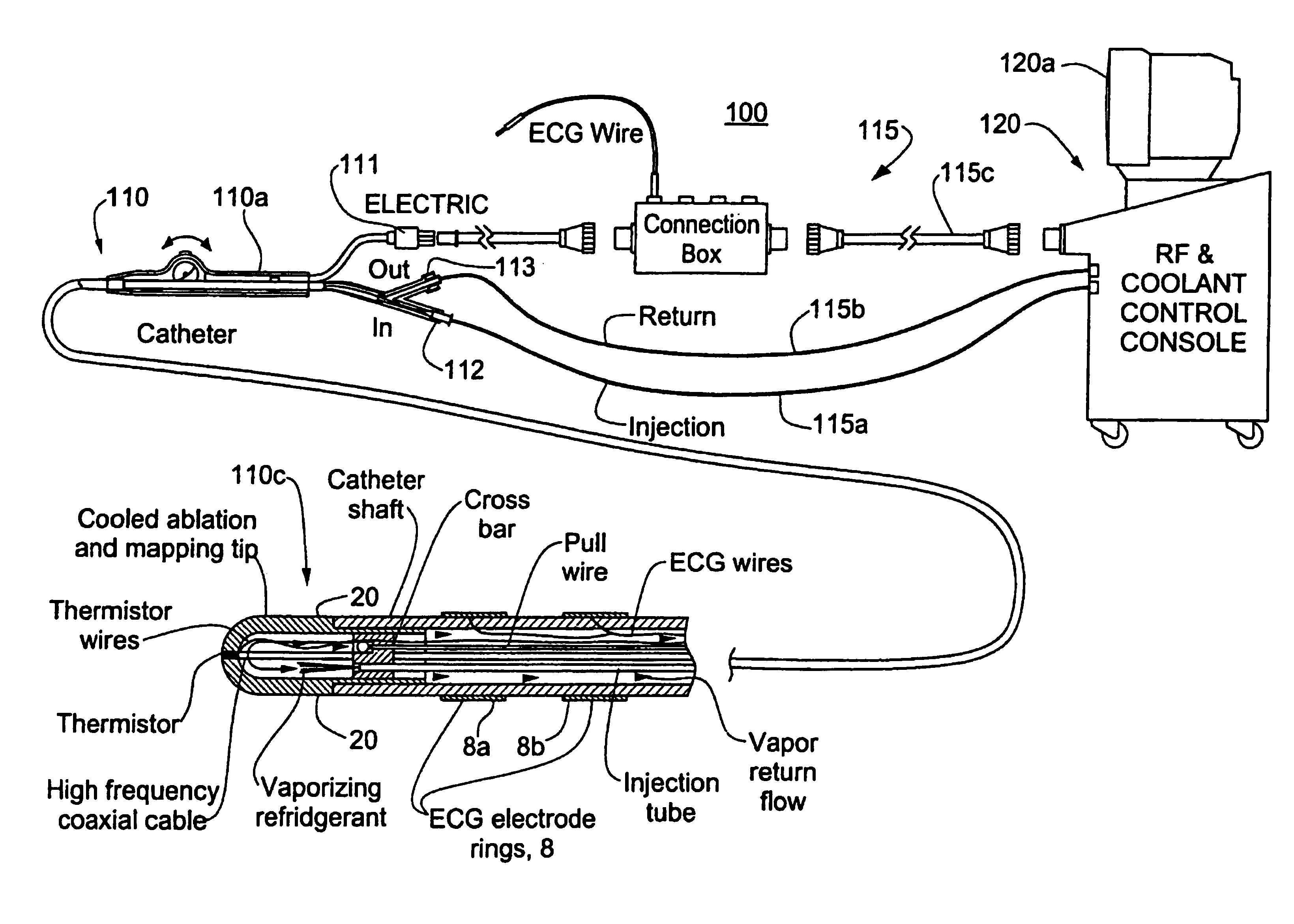
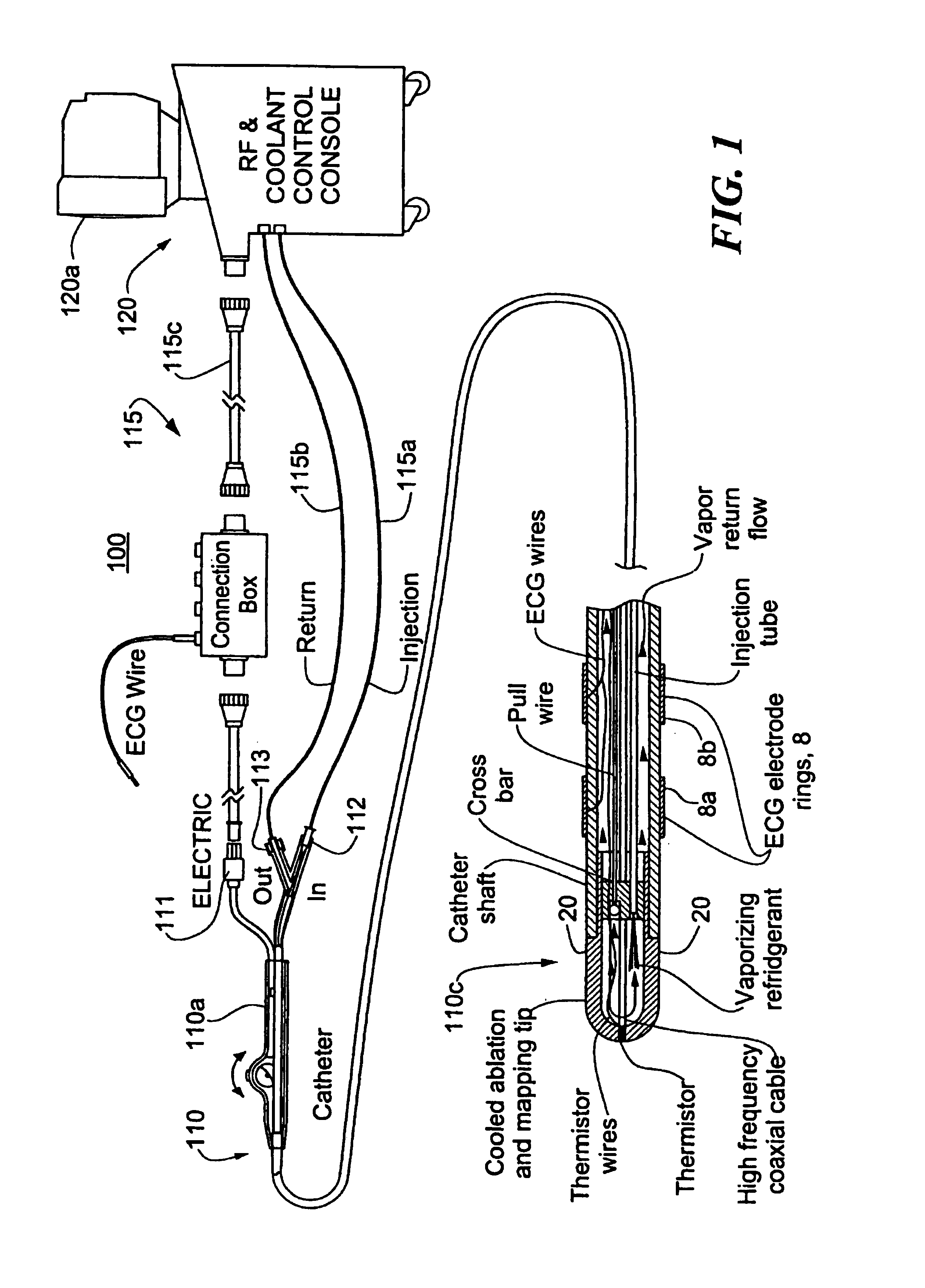
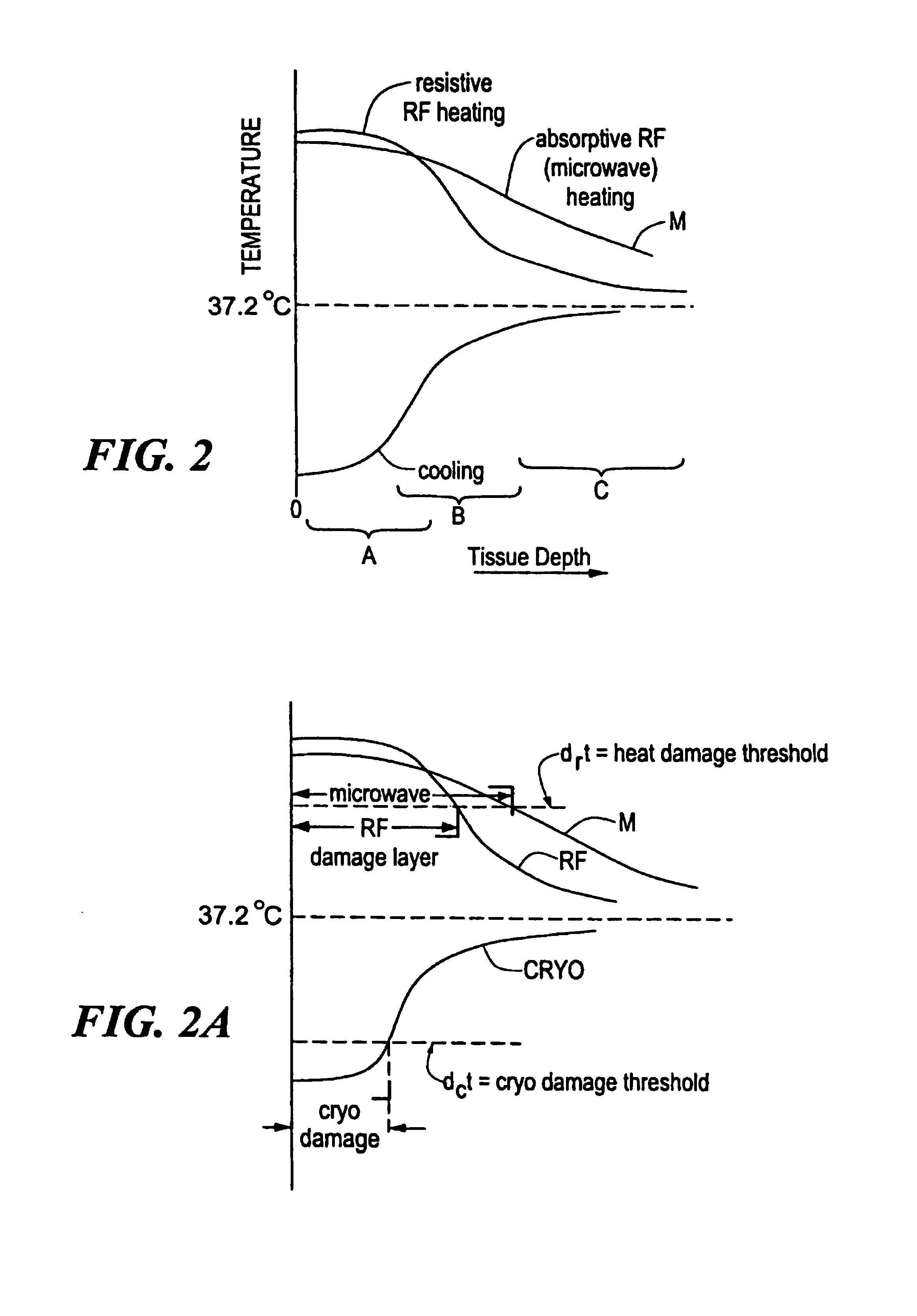
Catheter with cryogenic and heating ablation
InactiveUS7097641B1Improve versatilityEnhancing speed and placement lesionCatheterSurgical instruments for heatingTissue remodelingCelsius Degree
A catheter includes a cryoablation tip with an electrically-driven ablation assembly for heating tissue. The cryoablation tip may be implemented with a cooling chamber through which a controllably injected coolant circulates to lower the tip temperature, and having an RF electrode at its distal end. The RF electrode may be operated to warm cryogenically-cooled tissue, or the coolant may be controlled to conductively cool the tissue in coordination with an RF treatment regimen, allowing greater versatility of operation and enhancing the lesion size, speed or placement of multi-lesion treatment or single lesion re-treatment cycles. In one embodiment a microwave energy source operates at a frequency to extend beyond the thermal conduction depth, or to penetrate the cryogenic ice ball and be absorbed in tissue beyond an ice boundary, thus extending the depth and / or width of a single treatment locus. In another embodiment, the cooling and the application of RF energy are both controlled to position the ablation region away from the surface contacted by the electrode, for example to leave surface tissue unharmed while ablating at depth or to provide an ablation band of greater uniformity with increasing depth. The driver or RF energy source may supply microwave energy at a frequency effective to penetrate the ice ball which develops on a cryocatheter, and different frequencies may be selected for preferential absorption in a layer of defined thickness at depth in the nearby tissue. The catheter may operate between 70 and minus 70 degrees Celsius for different tissue applications, such as angioplasty, cardiac ablation and tissue remodeling, and may preset the temperature of the tip or adjacent tissue, and otherwise overlay or delay the two different profiles to tailor the shape or position where ablation occurs or to speed up a treatment cycle.
Owner:MEDTRONIC CRYOCATH LP
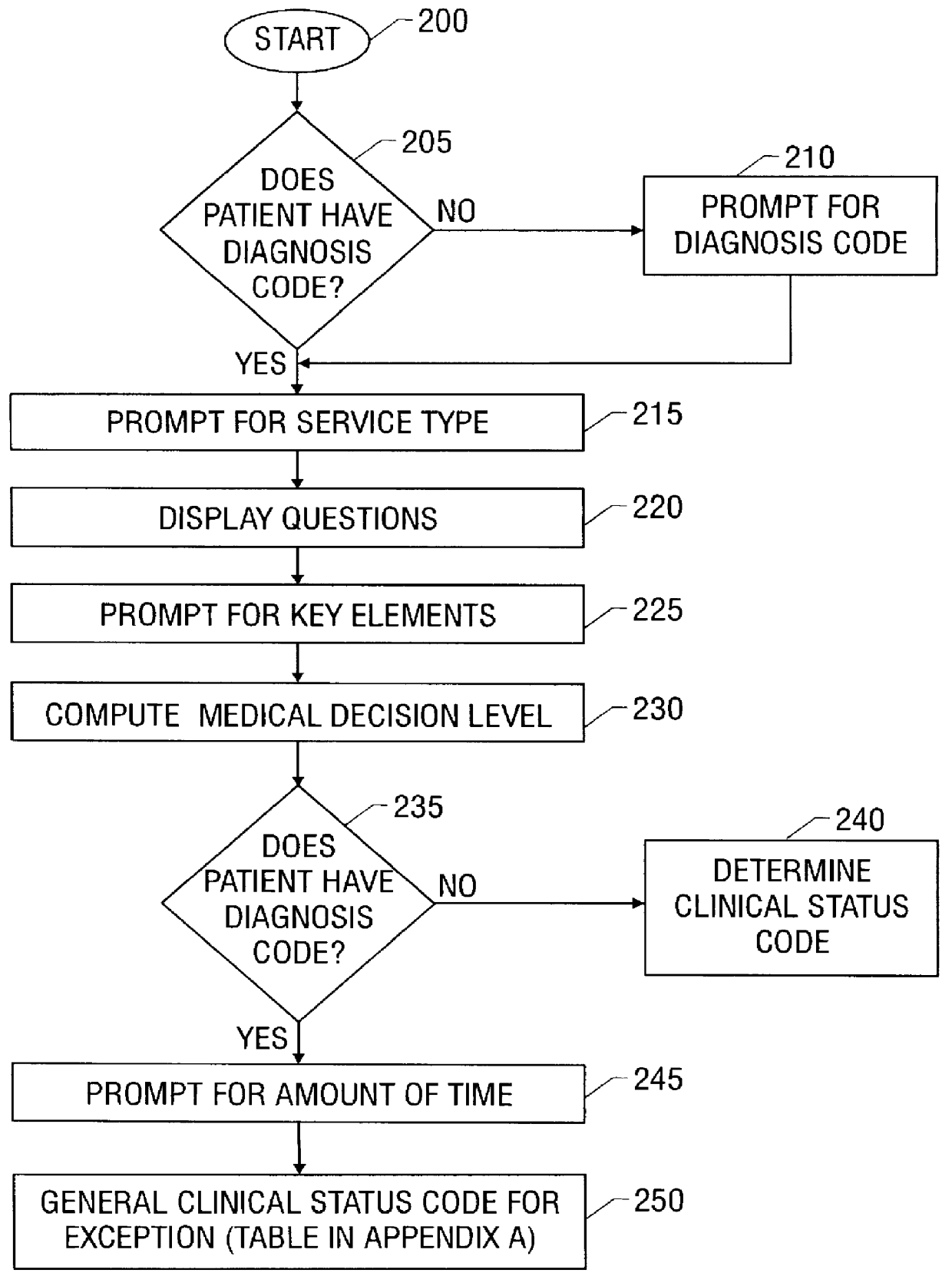
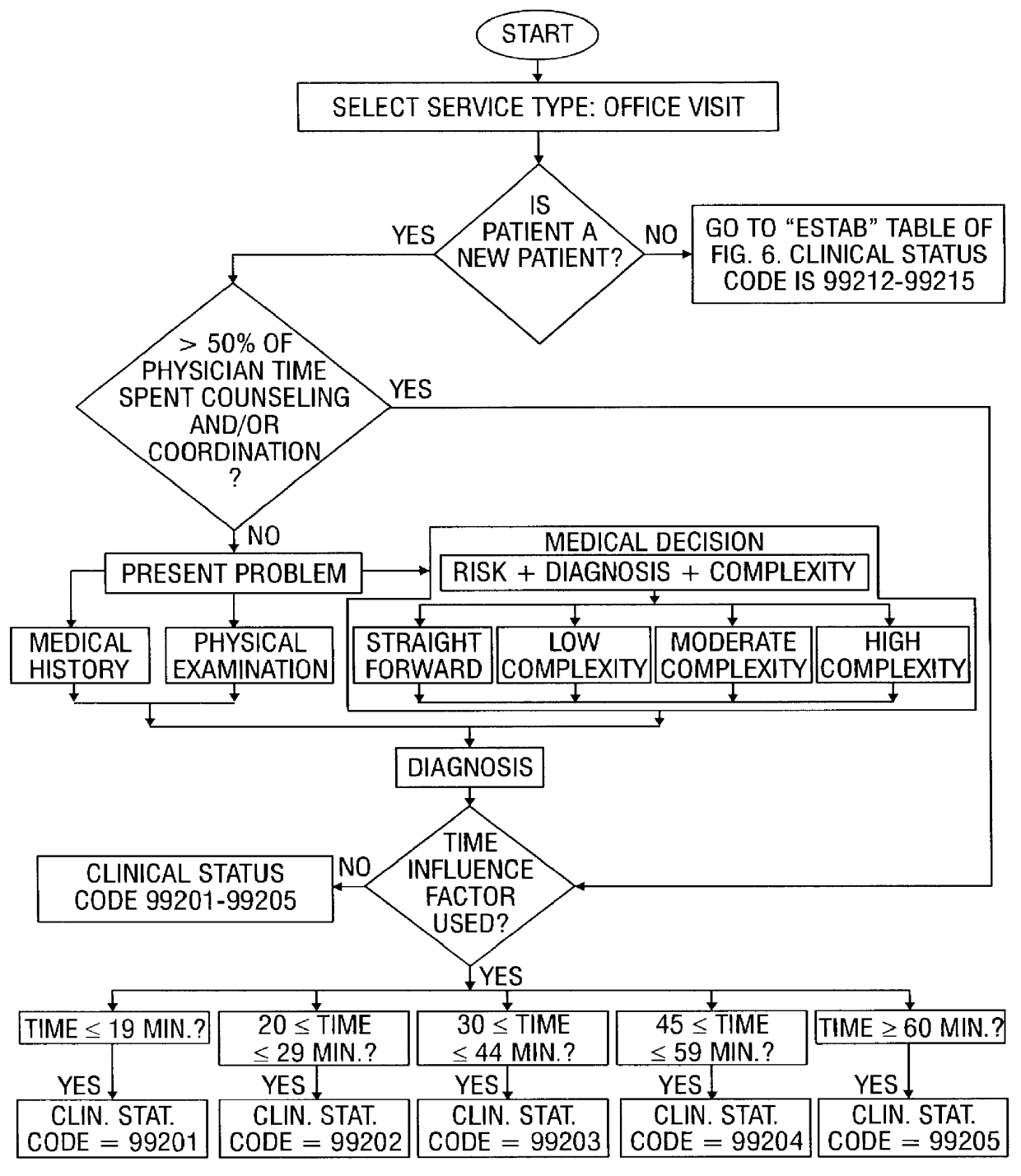
System and method for recording patient history data about on-going physician care procedures
InactiveUS6154726AConvenient recordingOptimize schedulingData processing applicationsComputer-assisted medical data acquisitionStaff timeEfficacy
A system and method for processing patient data permits physicians and other medical staff personnel to record, accurately and precisely, historical patient care information. An objective measure of a physician's rendered level of care, as described by a clinical status code, is automatically generated. Data elements used in the determination of the generated clinical status code include a level of history of the patient, a level of examination of the patient, a decision-making process of the physician treating the patient, and a "time influence factor." The quantity and quality of care information for a particular patient is enhanced allowing future care decisions for that patient to be based on a more complete medical history. Enhanced care information can be used in outcome studies to track the efficacy of specific treatment protocols. Archiving of patient information is done in a manner which allows reconstruction of the qualitative aspects of provided medical services. The medical care data can be recorded, saved, and transferred from a portable system to a larger stationary information or database system. Considerable physician and staff time are saved and precision and accuracy are significantly enhanced, by generating these clinical status codes automatically (at the point of service by the care-provider without any intermediary steps) from information recorded simultaneously with the provision of services.
Owner:RENSIMER ENTERPRISES
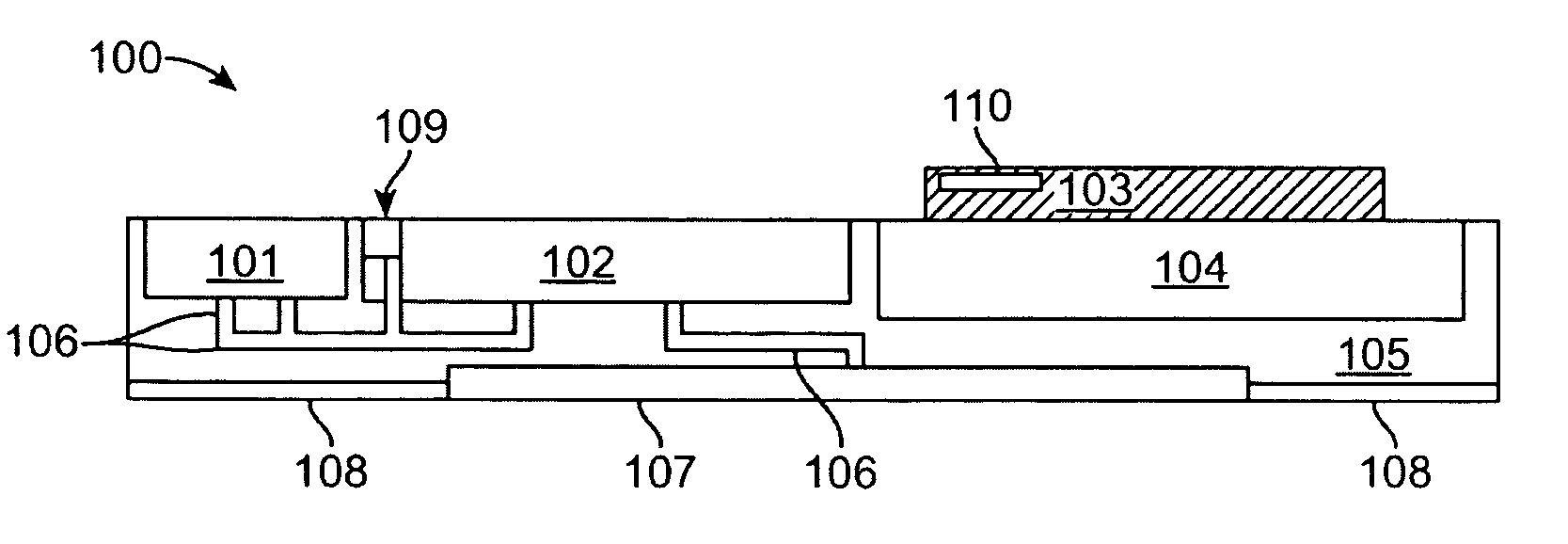
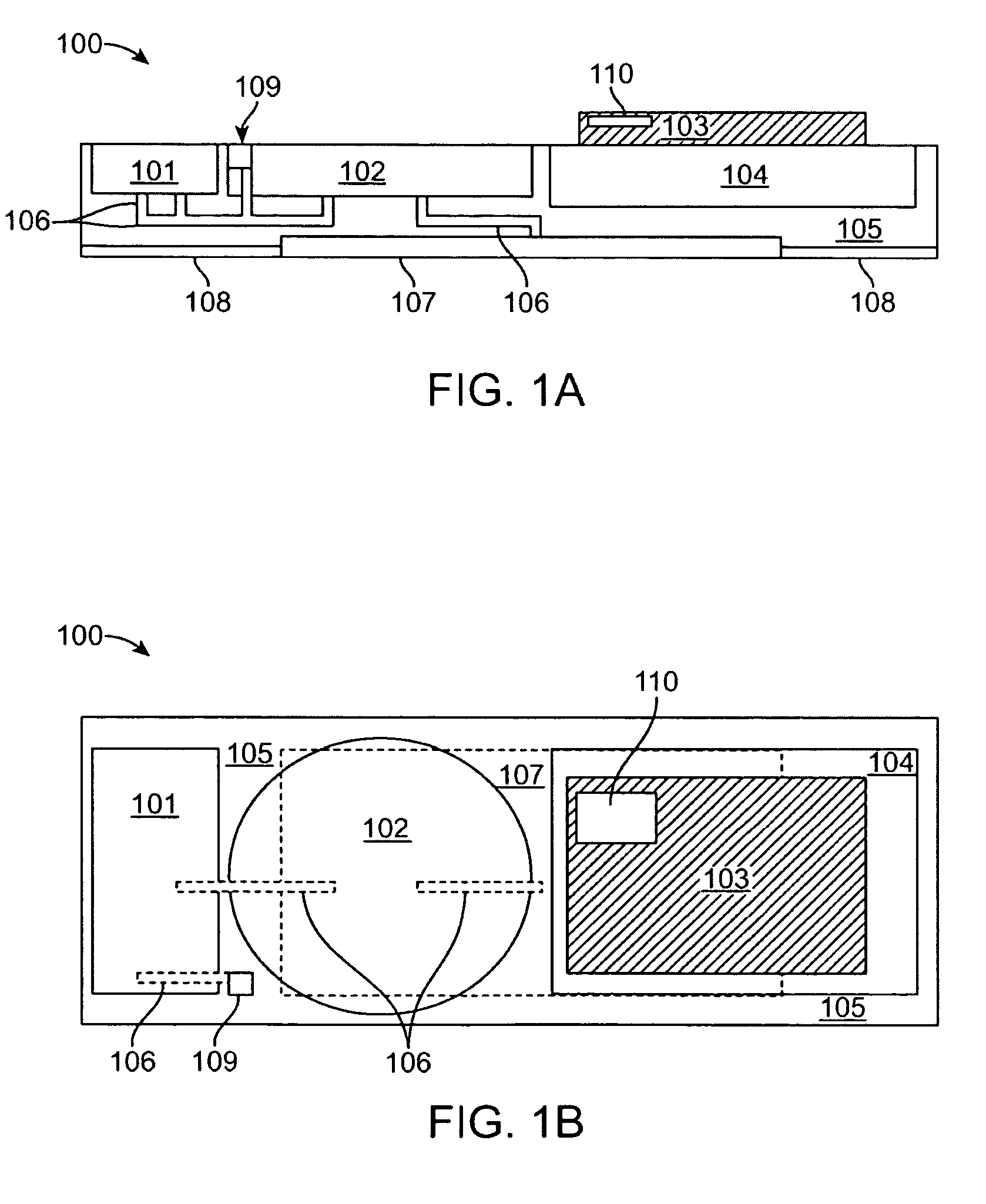
Transdermal patch system
InactiveUS20090259176A1Maintaining awarenessRemain alertElectrotherapyMicroneedlesTransdermal patchMedicine
A transdermal patch system configured as a patch or pump assembly may be placed into contact upon a skin surface to transport drugs or agents transdermally via any number of different mechanisms such as microporous membranes, microneedles, in-dwelling catheters, etc. The assembly may enclose or accommodate a reservoir configured as an elongate microchannel to contain the drug or agent suspended in a fluid vehicle. The reservoir may also be fluidly coupled via microchannels to transport the drugs into or against an underlying skin surface as driven or urged via a pump and controlled by an electronic control circuitry which may be programmed to affect any number of treatment regimens.
Owner:LOS GATOS RES
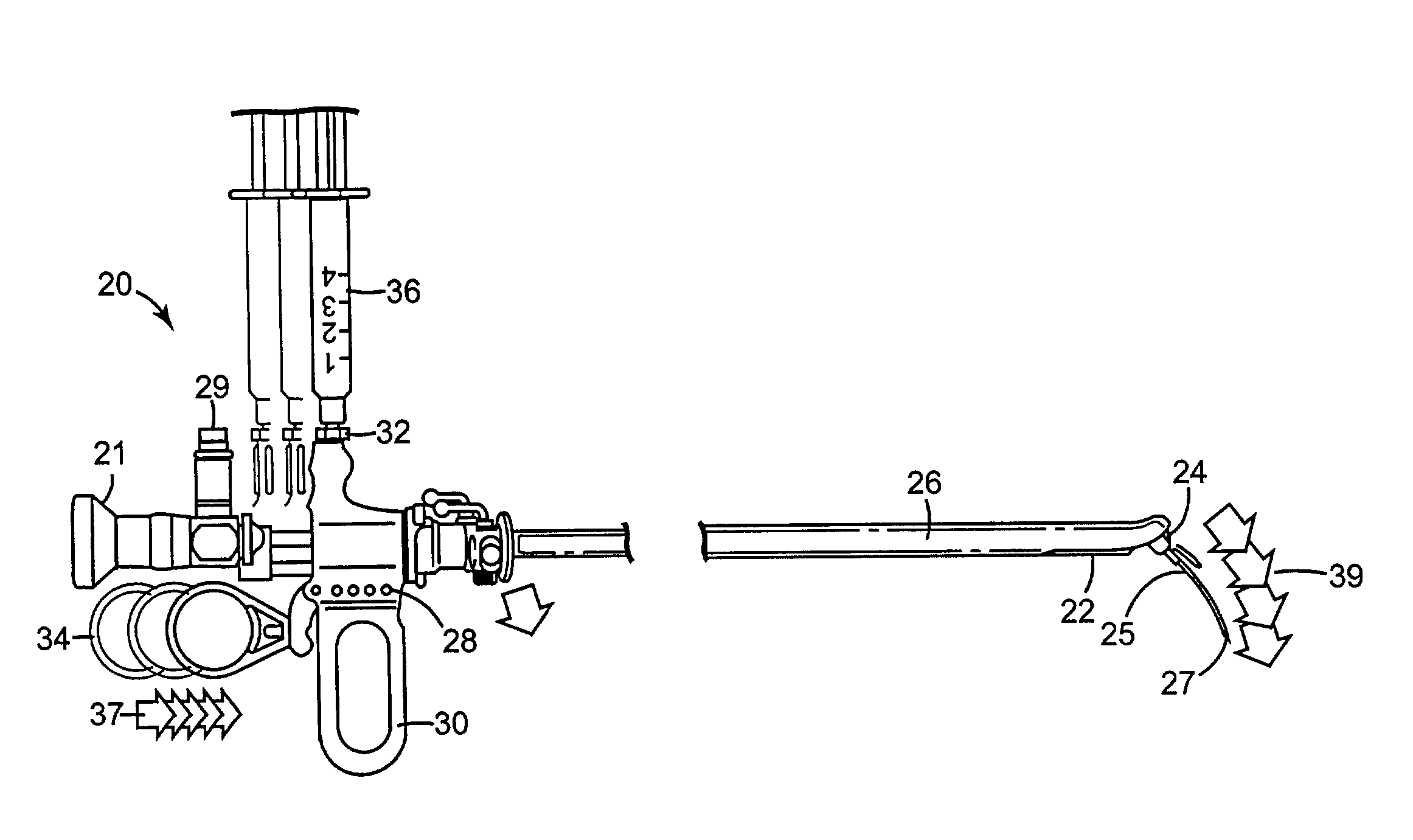
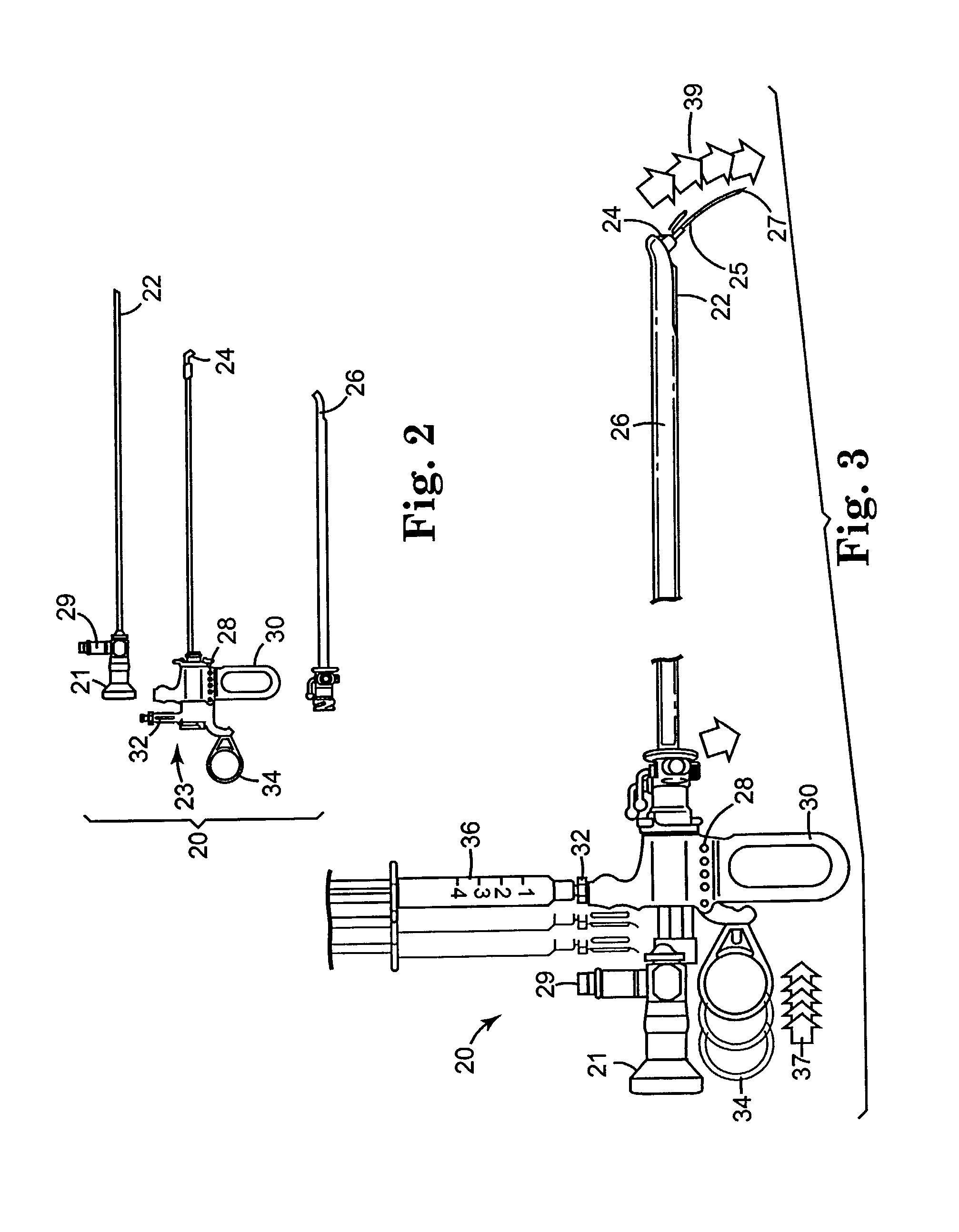
Regimen for treating prostate tissue and surgical kit for use in the regimen
InactiveUS7015253B2Decreasing prostate sizeSmall sizeBiocideHydroxy compound active ingredientsSteroidal antiandrogenRegimen
The present invention provides treatment regimens for treating diseased prostate tissue, including the steps of chemically ablating prostate tissue and coadministering an antiandrogen. In some embodiments, prostate tissue is chemically ablated by injection of ethanol, or an injectable gel comprising ethanol, into prostate tissue. Steroidal and non-steroidal antiandrogens are suitable antiandrogens. One suitable non-steroidal antiandrogen is bicalutamide. The treatment regimen is suitable for treatment of prostate tissue diseases including benign prostatic hyperplasia and prostatic carcinoma. The invention further provides a treatment regimen for treating benign prostatic hyperplasia, including the steps of damaging prostate tissue and coadministering an antiandrogen. Also provided by the present invention is a kit for treating a human male, including a means for necrosing prostate tissue, an antiandrogen drug, and a means for administering the antiandrogen drug. A kit including a first surgical device for delivering a chemoablation fluid to prostate tissue transurethrally, an antiandrogen drug such as bicalutamide, and a second surgical device for administering the antiandrogen drug, is further provided.
Owner:BOSTON SCI SCIMED INC
Compositions of PD-1 antagonists and methods of use
InactiveUS8114845B2Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsCompound (substance)T cell
Owner:MEDIMMUNE LLC
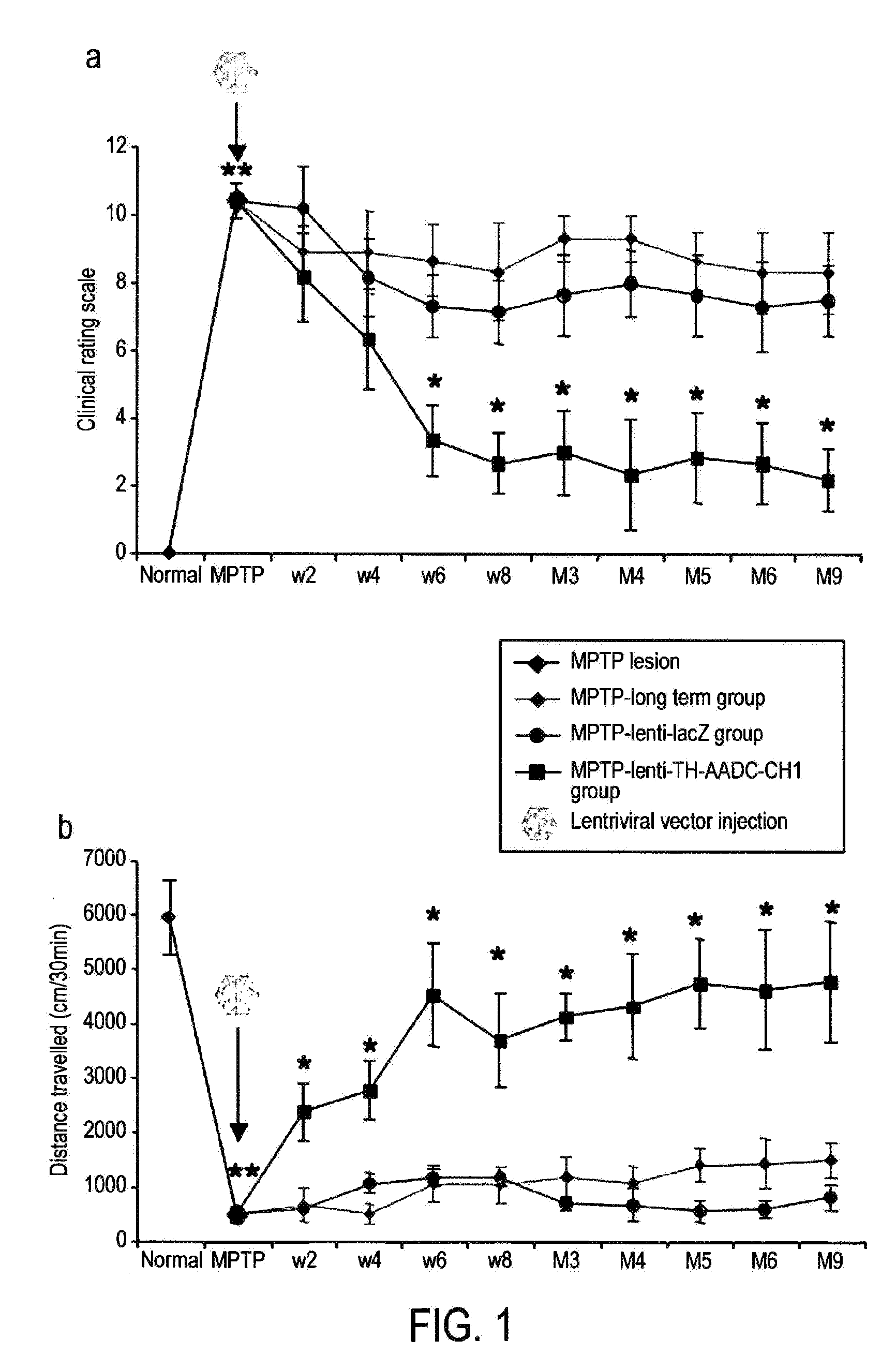
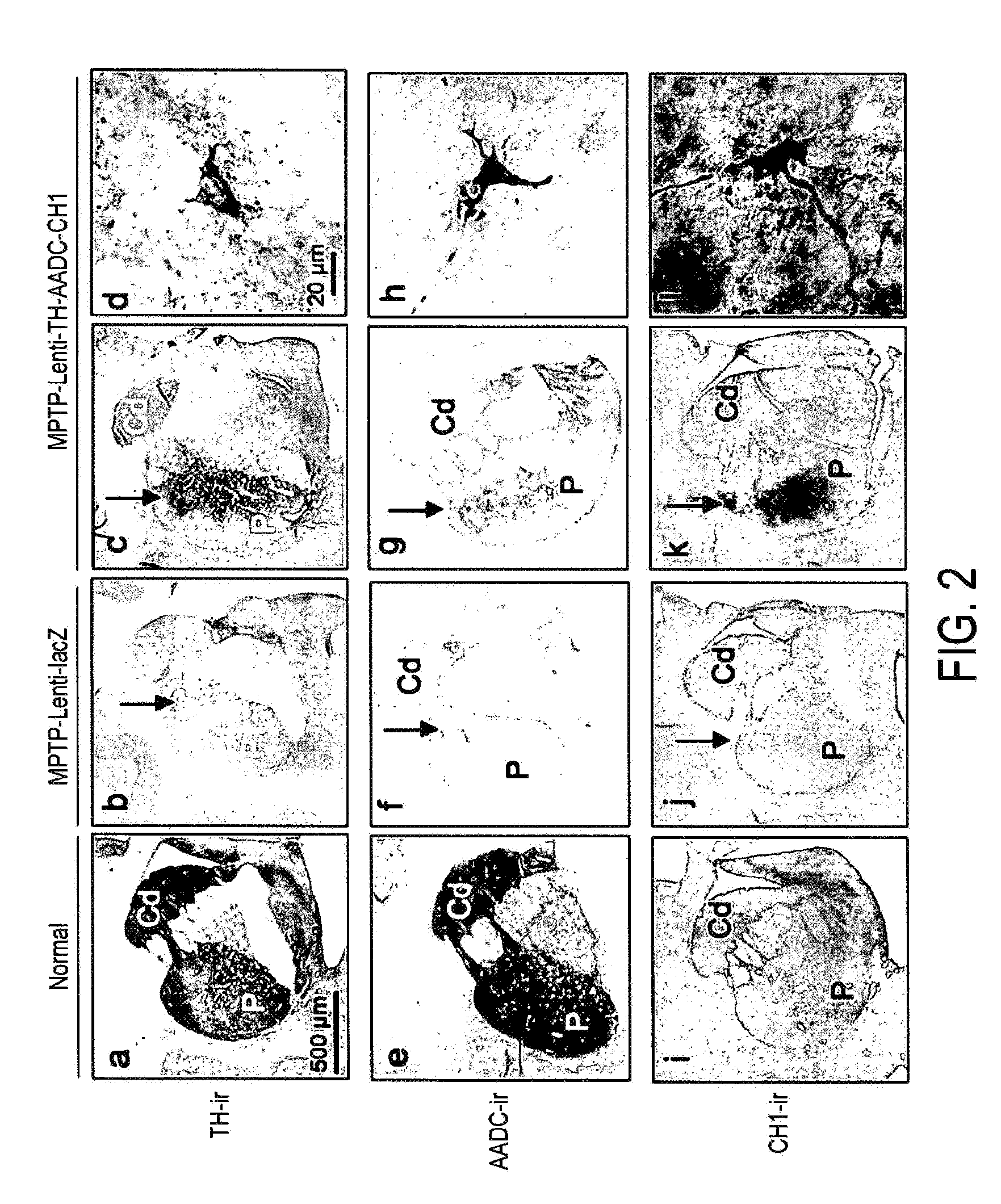
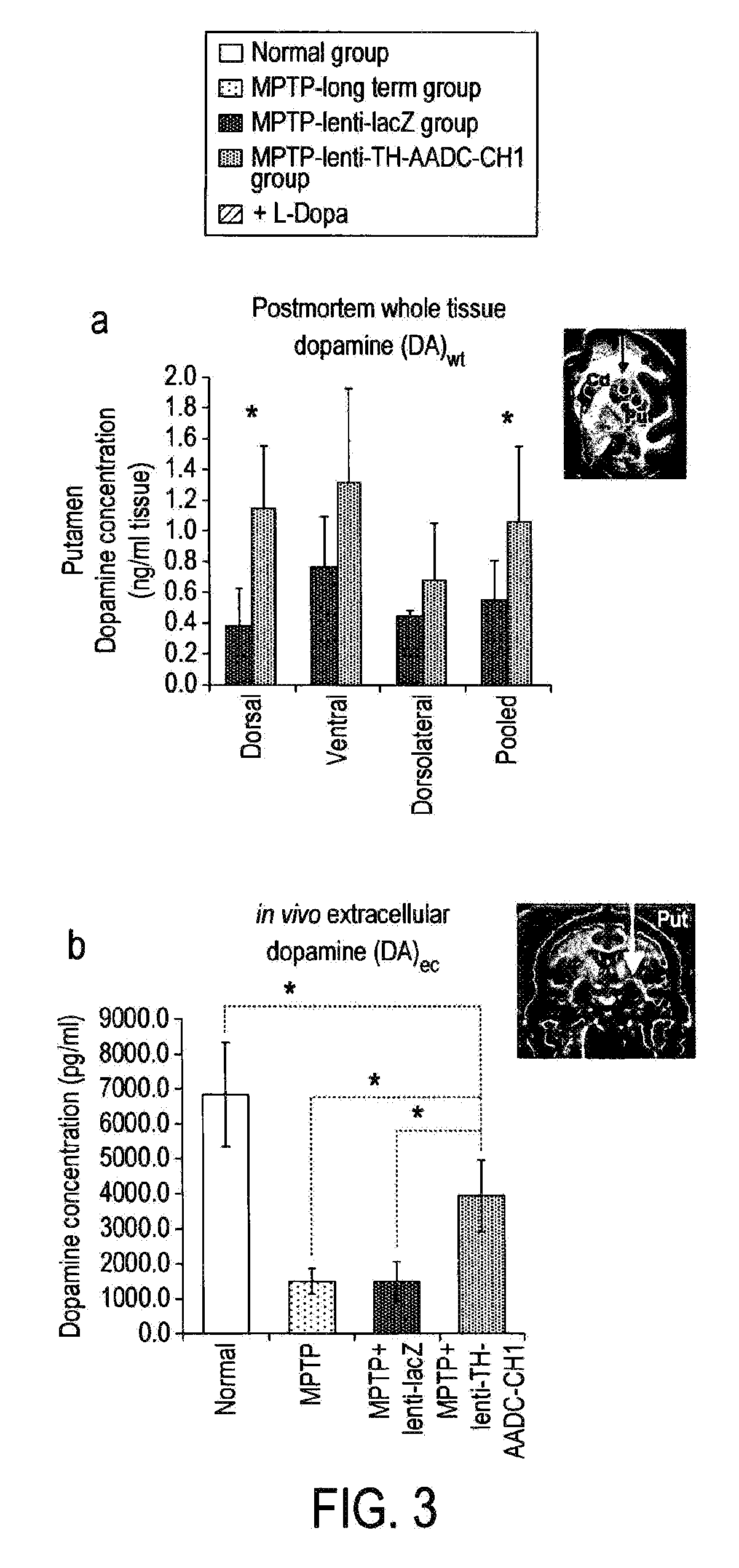
Treatment regimen for parkinson's disease
InactiveUS20120295960A1Reduce potential side effectsReduce maintenanceNervous disorderNucleic acid vectorSide effectCombination therapy
Provided is an improved treatment for Parkinson's Disease where the efficacy of L-Dopa treatment is increased by including gene therapy in the treatment regimen. The combination therapy results in long-term improvements in response to L-Dopa and diminished side effects caused by L-Dopa.
Owner:OXFORD BIOMEDICA (UK) LTD
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
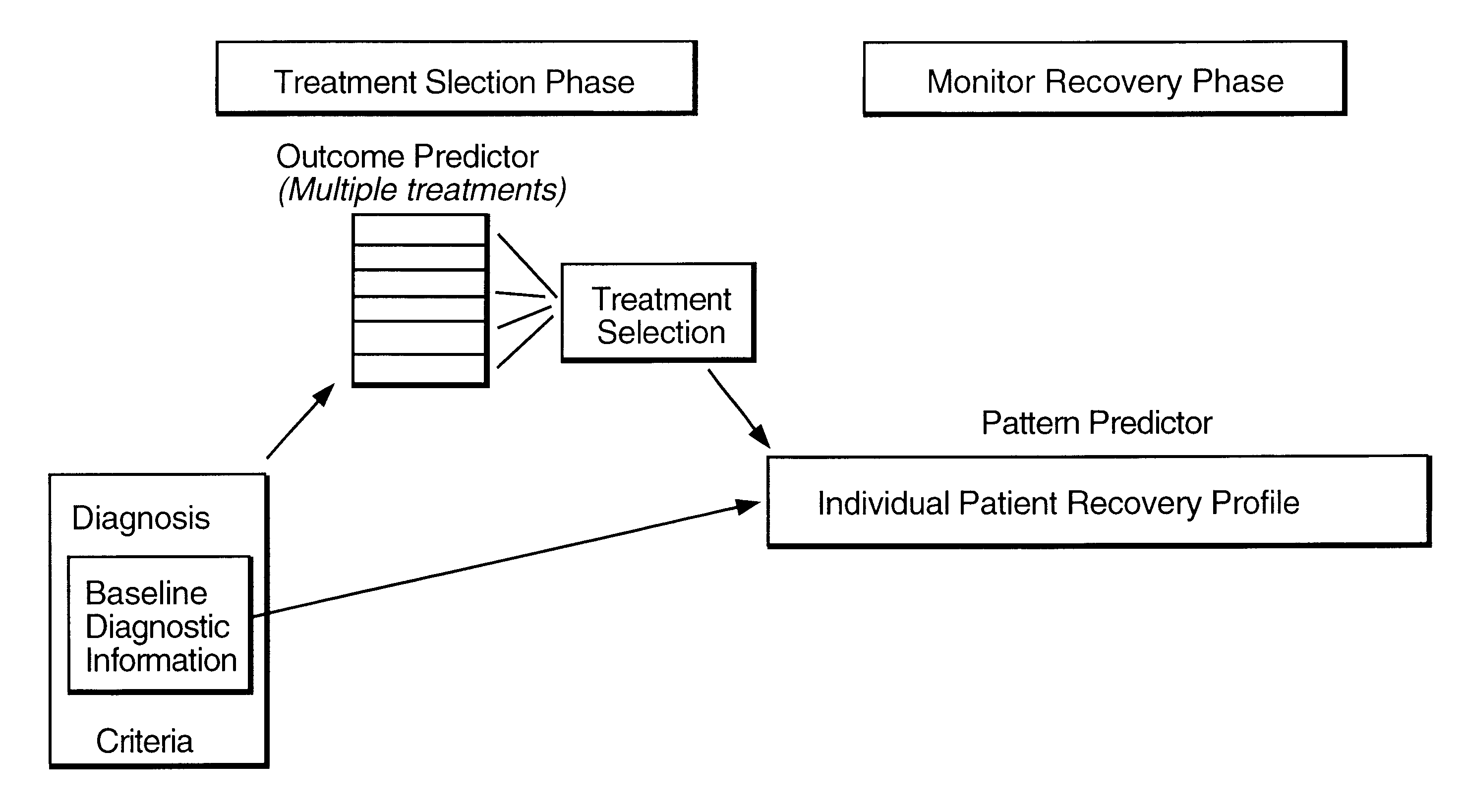
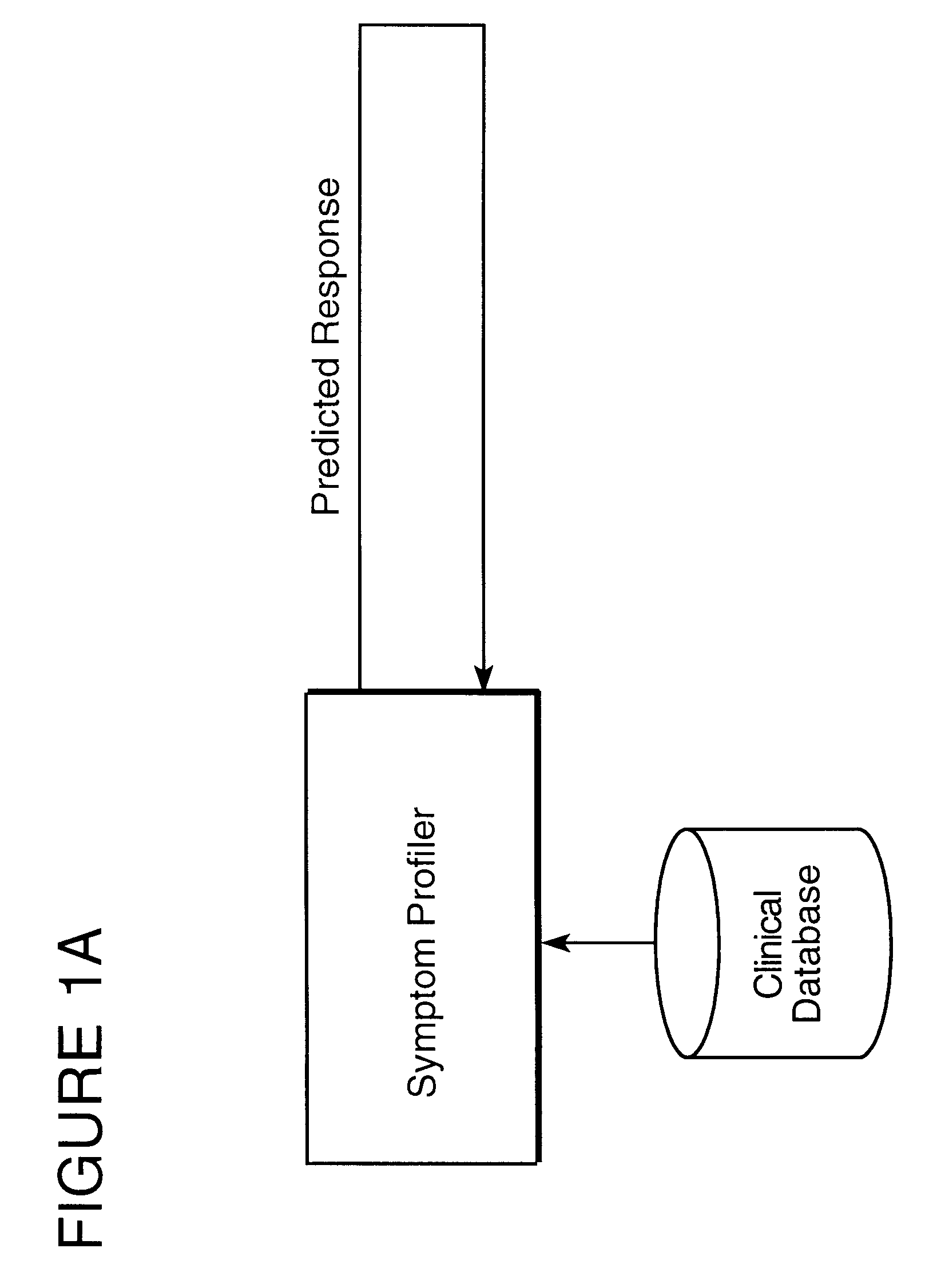
Method for predicting the therapeutic outcome of a treatment
InactiveUS6317731B1Improve accuracy and precisionHigh precisionBiological neural network modelsComputer-assisted medical data acquisitionDiseaseNerve network
A method useful for facilitating choosing a treatment or treatment regime and for predicting the outcome of a treatment for a disorder which is diagnosed and monitored by a physician or other appropriately trained and licensed professional, such as for example, a psychologist, based upon the symptoms experienced by a patient. Unipolar depression is an example of such a disorder, however the model may find use with other disorders and conditions wherein the patient response to treatment is variable. In the preferred embodiment, the method for predicting patient response includes the steps of performing at least one measurement of a symptom on a patient and measuring that symptom so as to derive a baseline patient profile, such as for example, determining the symptom profile with time; defining a set of a plurality of predictor variables which define the data of the baseline patient profile, wherein the set of predictor variables includes predictive symptoms and a set of treatment options; deriving a model that represents the relationship between patient response and the set of predictor variables; and utilizing the model to predict the response of said patient to a treatment. A neural net architecture is utilized to define a non-linear, second order model which is utilized to analyze the patient data and generate the predictive database from entered patient data.
Owner:ADVANCED BIOLOGICAL LAB
Method and system for combined energy therapy profile
ActiveUS20080221491A1Increase temperatureLess energy useUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyEffective treatmentEnergy Therapy
A method and system for treating tissue with a combined therapy profile is disclosed. In one exemplary embodiment, ultrasound energy is used to treat numerous depths of tissue within a region of interest and the spatial and temporal properties of the ultrasound energy are varied for more effective treatment. The method and system of the present invention are configured to treat all of the tissue from the surface on down and not spare intervening tissue.
Owner:GUIDED THERAPY SYSTEMS LLC
Chronic treatment regimen using glucagon-like insulinotropic peptides
InactiveUS7259233B2Avoids and minimizes side effectPeptide/protein ingredientsImmunoglobulinsDiseasePeptide
The present invention encompasses a method of treating a disease by maintaining chronic steady state serum levels of a GLP-1 compound within a specified range.
Owner:ELI LILLY & CO
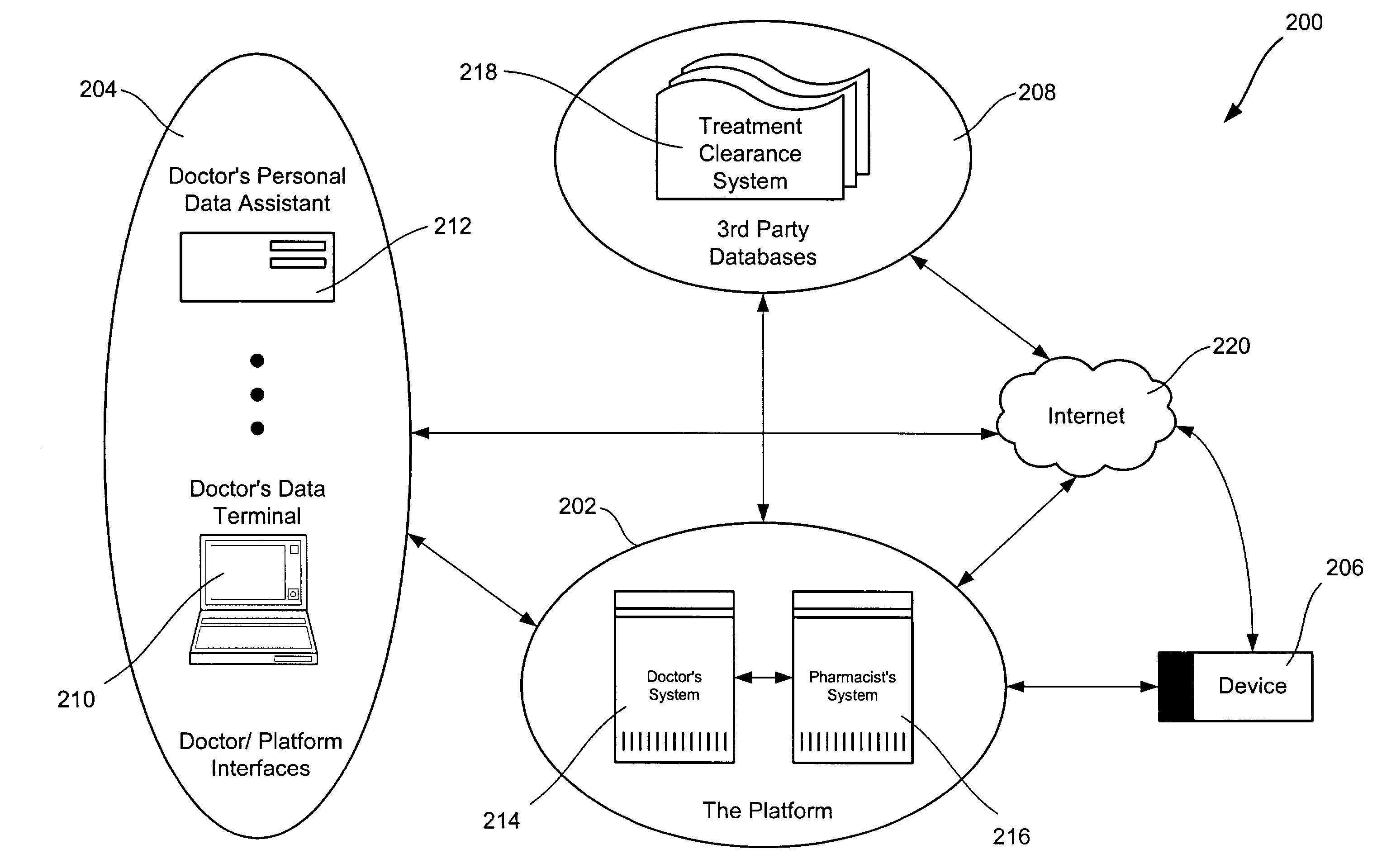
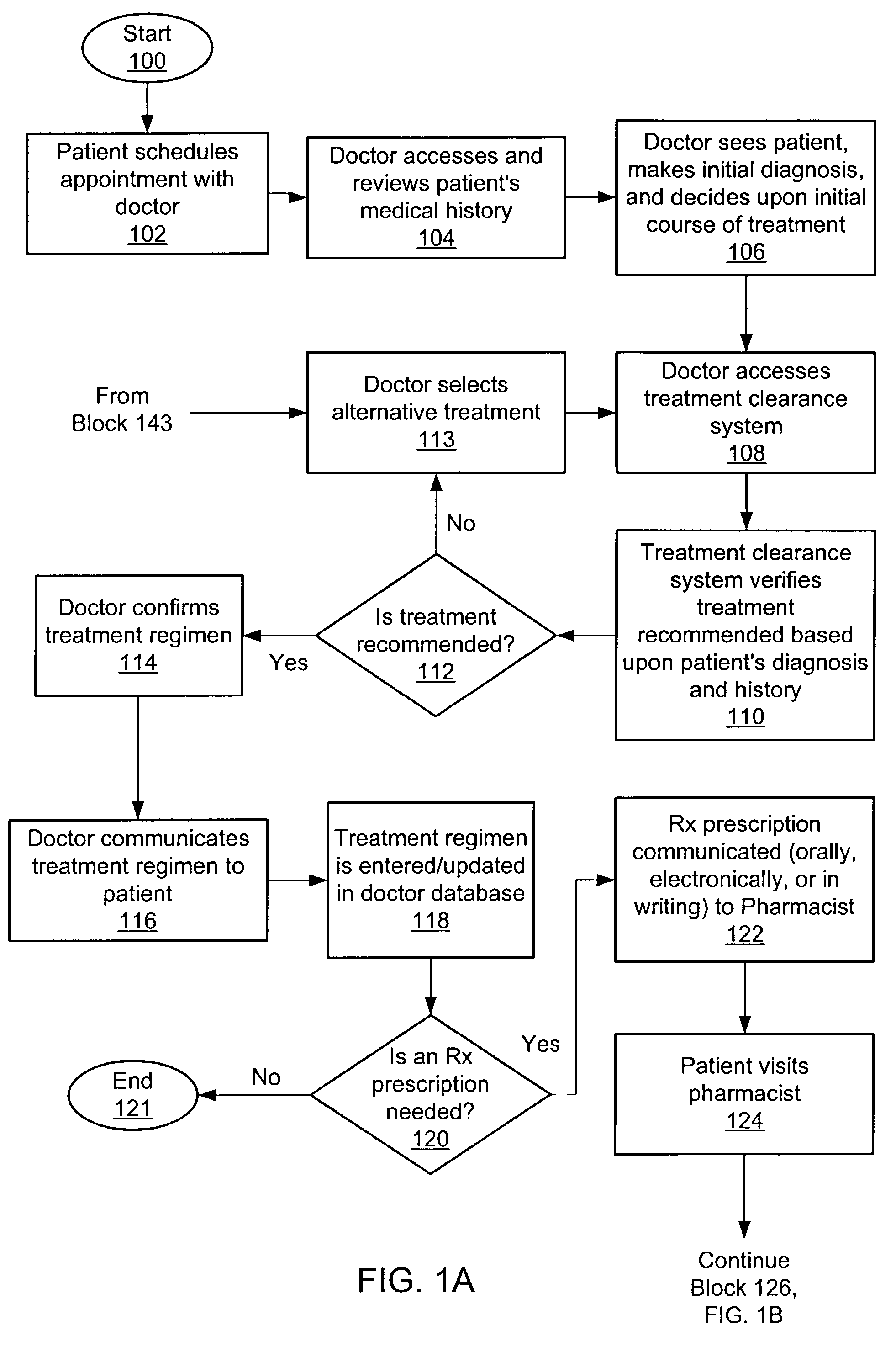
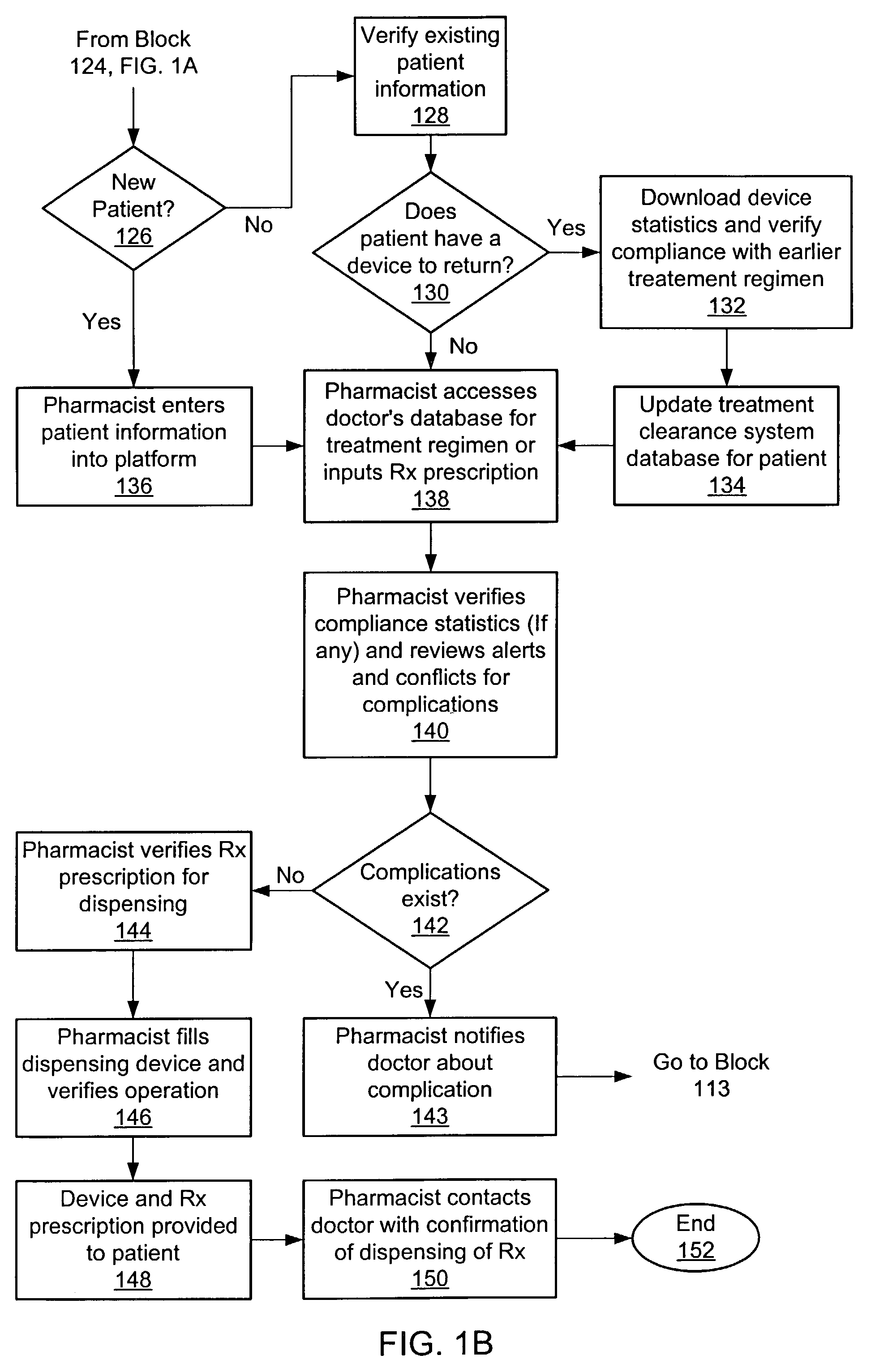
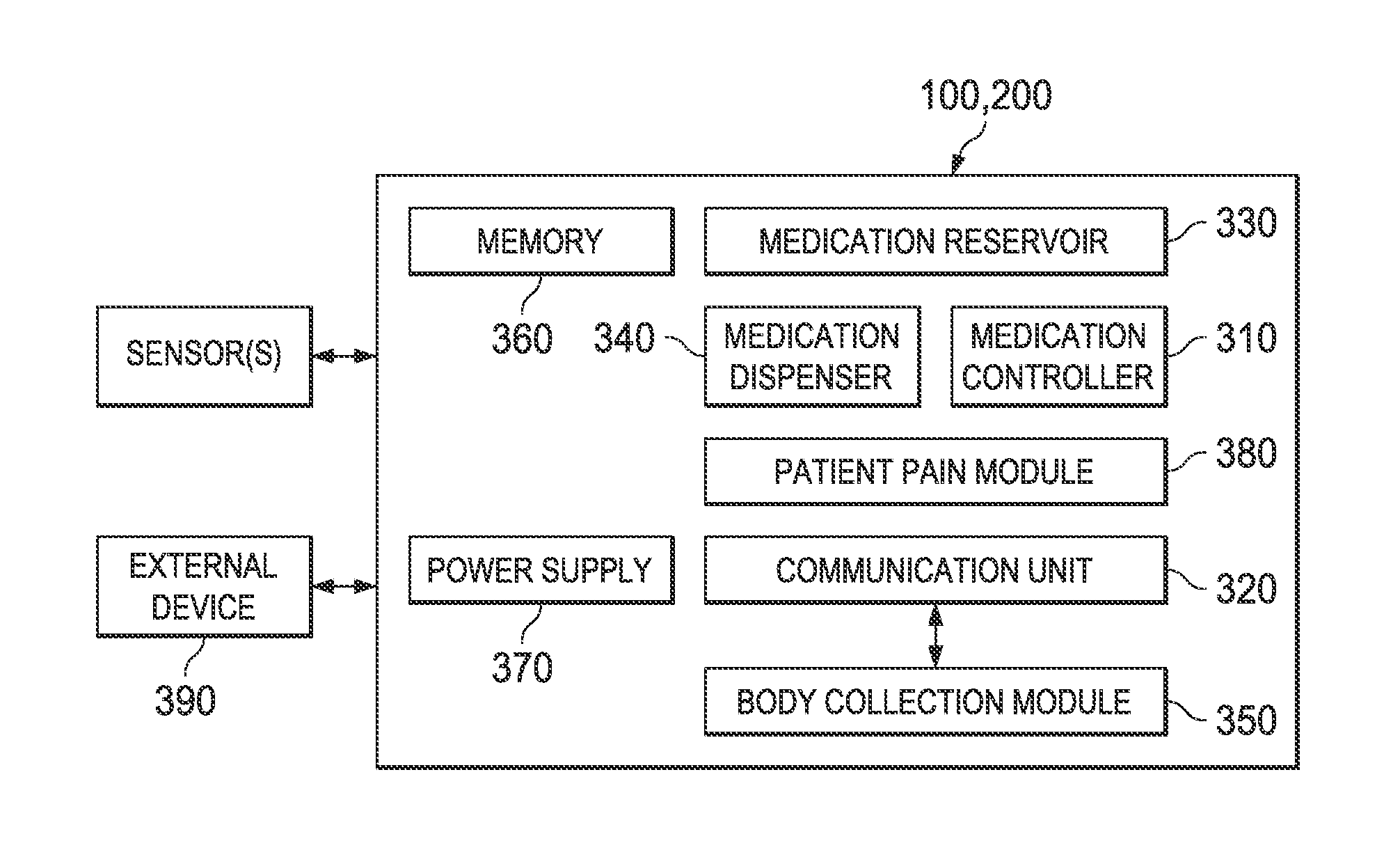
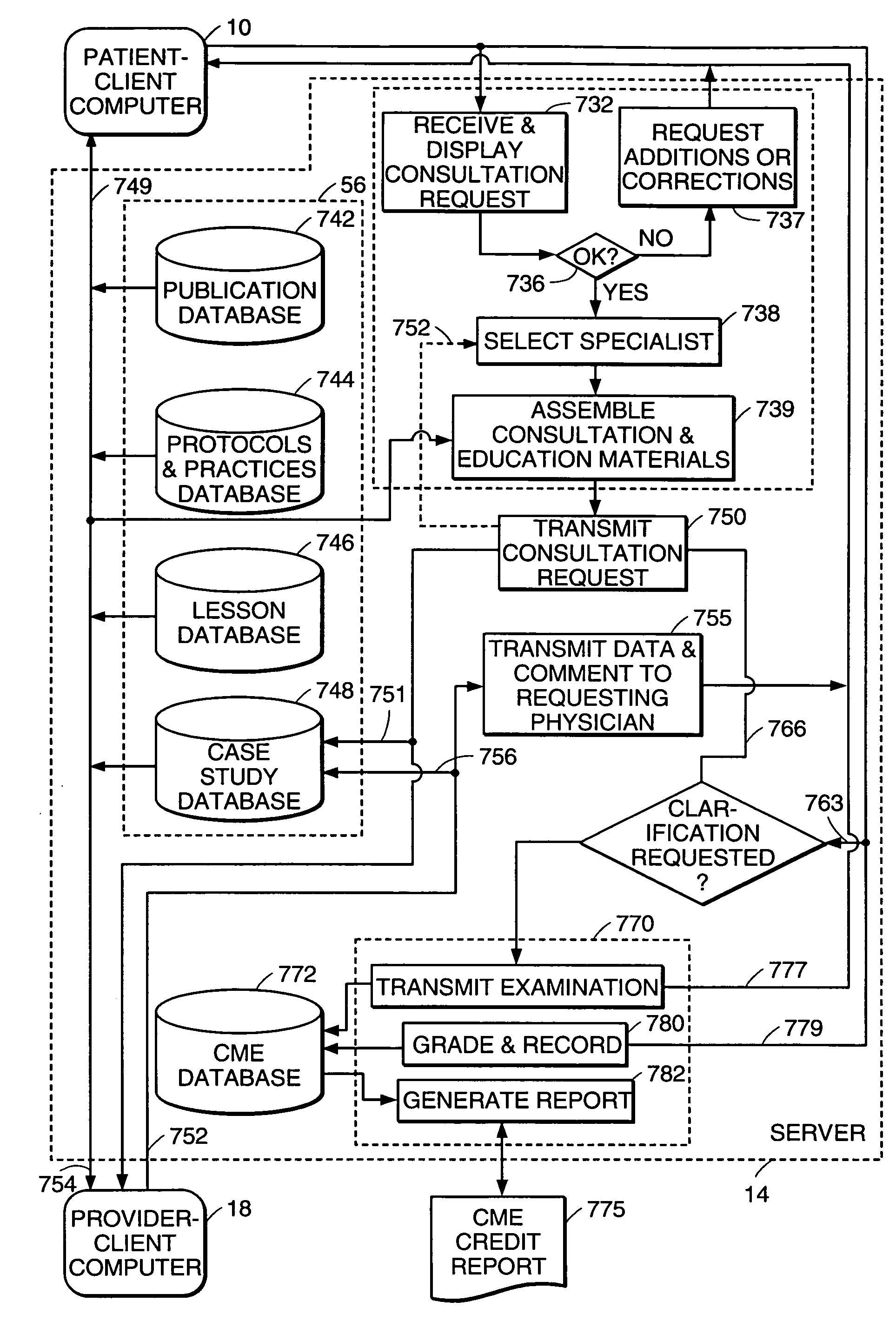
Apparatus, device and method for prescribing, administering and monitoring a treatment regimen for a patient
InactiveUS7395214B2To offer comfortEasy to useData processing applicationsDrug and medicationsTreatment SchedulePatient compliance
A treatment Device is provided which enables a Doctor / Pharmacist to provide patient specific instructions in a textual format to a patient. The instructions are converted by a speech synthesizer provided in the Device into an audibly perceptible format. When configured as a hand-held unit, the Device may store a plurality of medications. Upon activation or automatically, instructions saved in the device are communicated to a patient / user via various audible, visual and / or tactile indicators. The instructions are provided to the device via a Platform which enables Doctors and Pharmacists input instructions into the Platform, which, via a Platform interface, are saved into a storage device provided in the Device. In an additional embodiment, a removable medication dispensing cartridge is provided which facilitates the controlled and automatic dispensing of a medication to a patient based upon a treatment schedule. Patient compliance information with a treatment schedule may be provided by the Device.
Owner:SHILLINGBURG CRAIG P
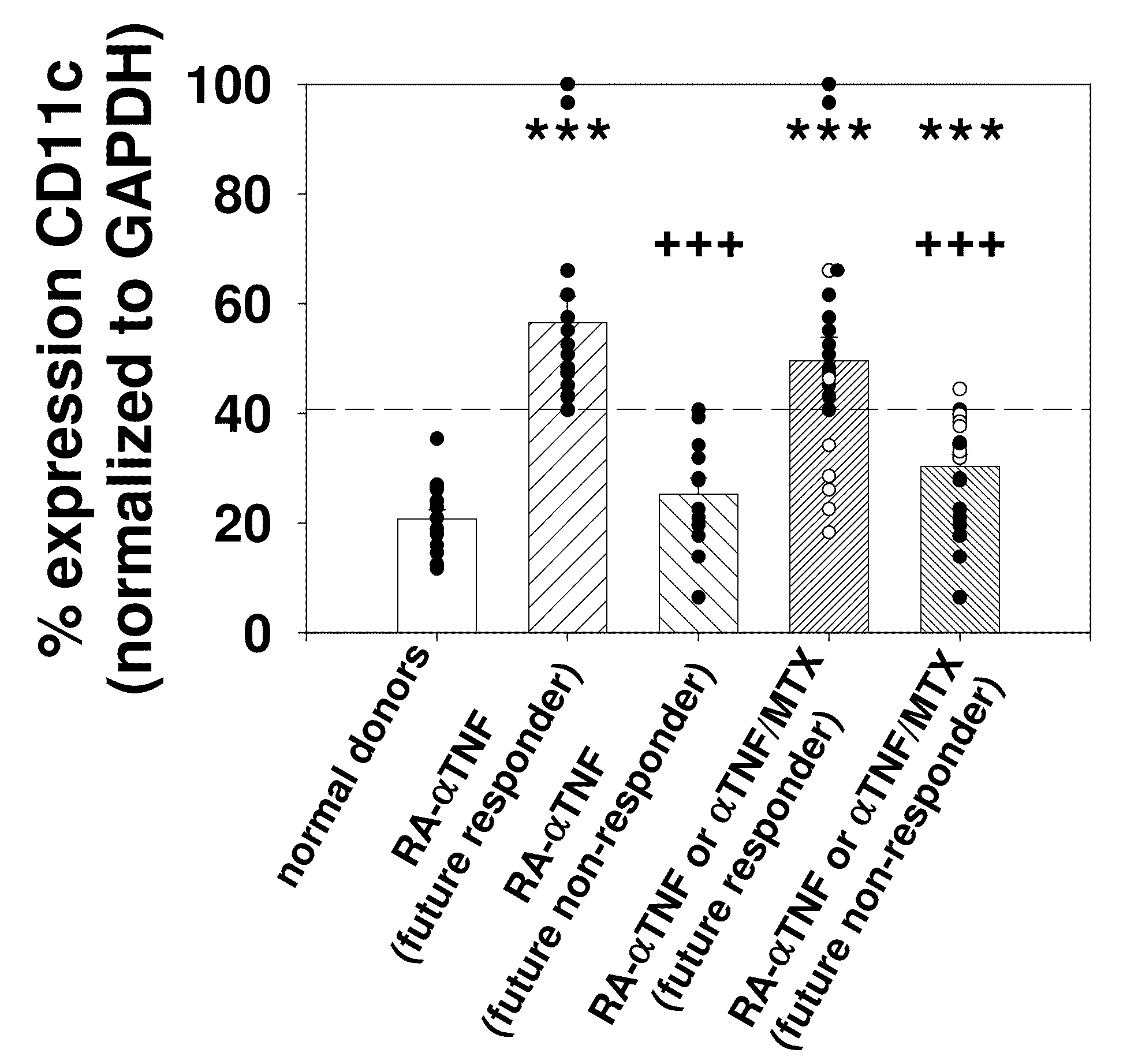
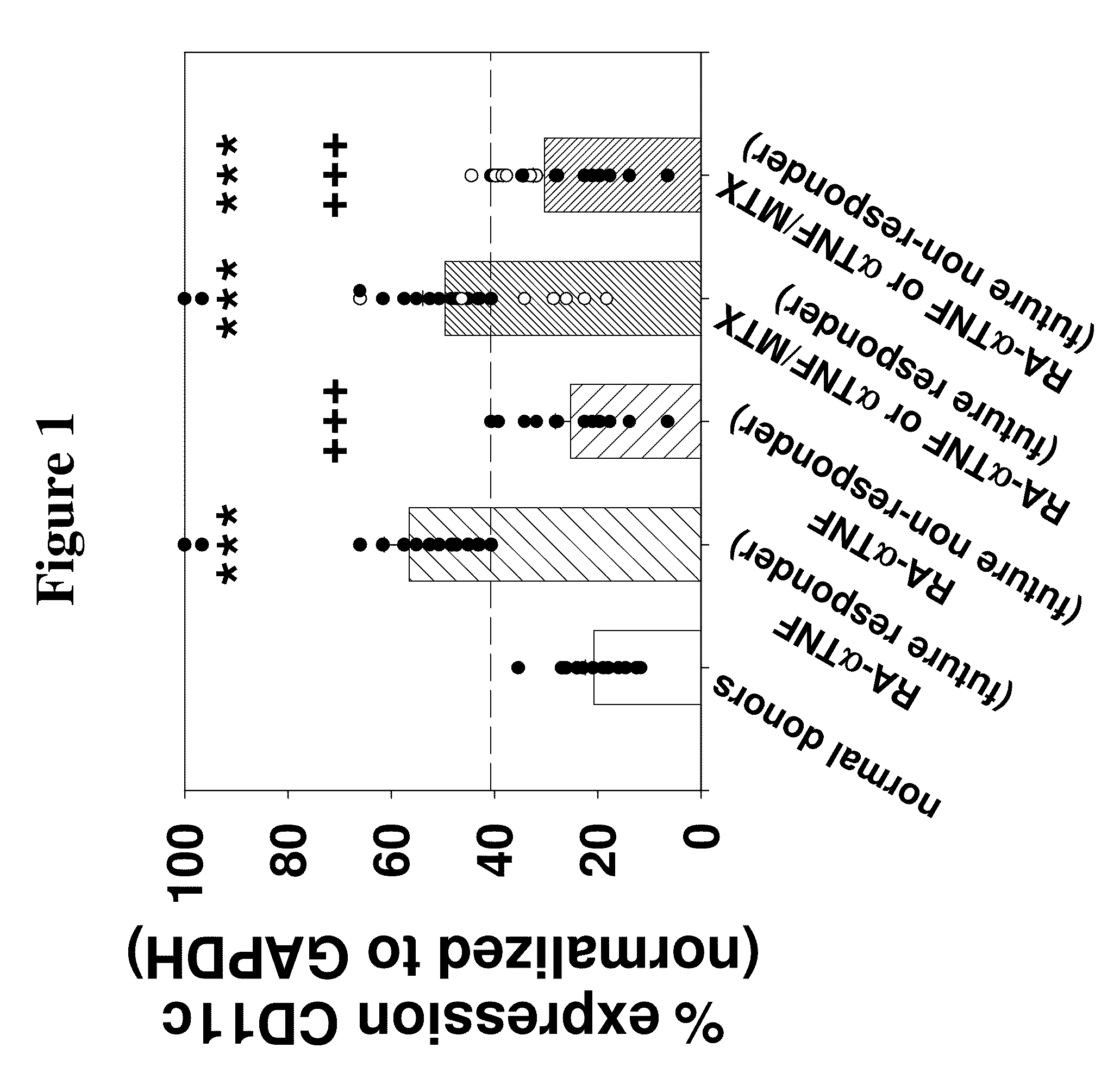
BIOMARKERS PREDICTIVE OF THE RESPONSIVENESS TO TNFalpha INHIBITORS IN AUTOIMMUNE DISORDERS
ActiveUS20090017472A1Prediction of responsivenessAntipyreticMicrobiological testing/measurementDiseaseAutoimmune responses
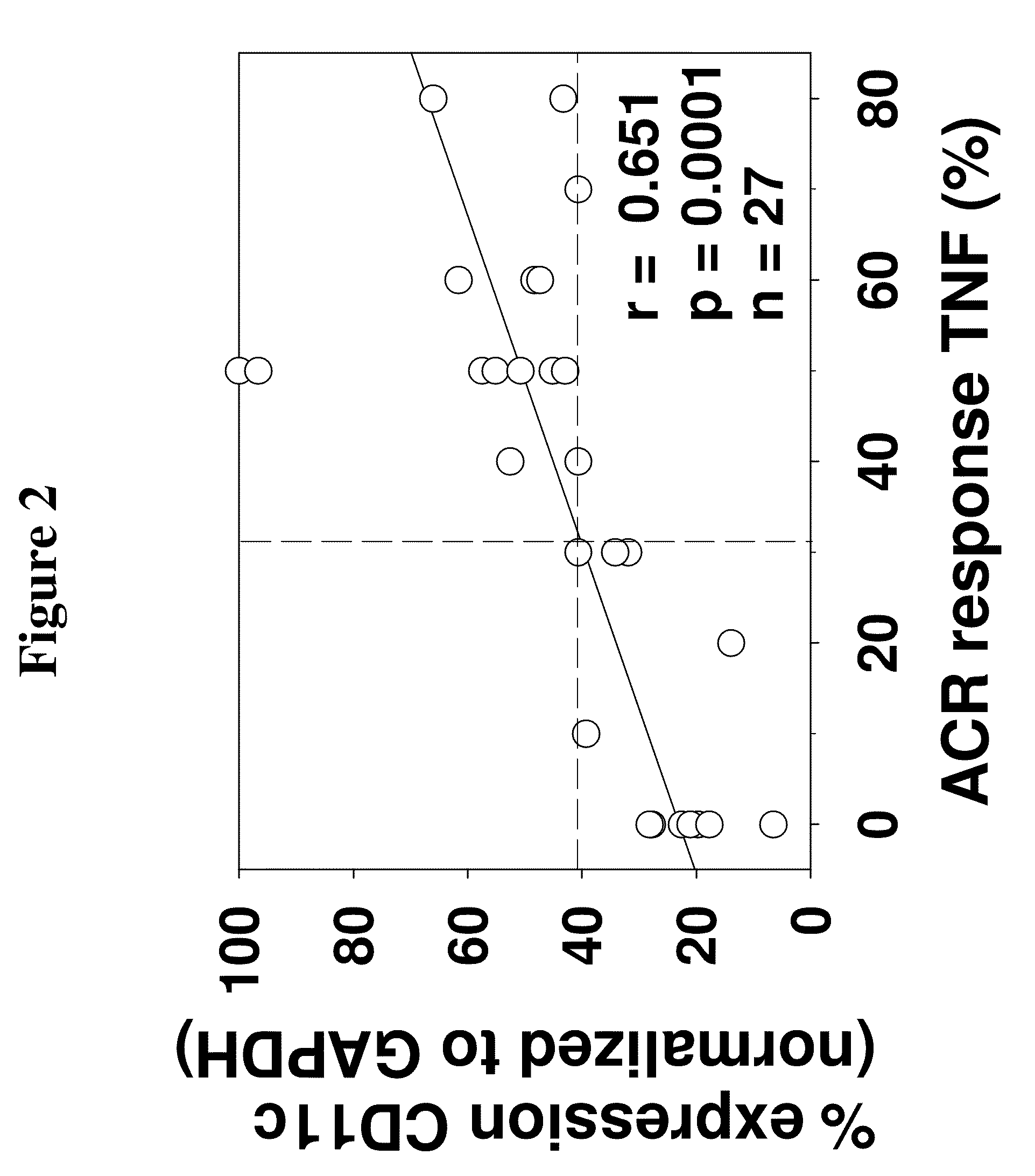
The invention provides methods for predicting responsiveness to TNFα inhibitors in a subject suffering from an autoimmune disorder, such as rheumatoid arthritis. The methods involve assaying for expression of one or more biomarkers in the subject that are predictive of responsiveness to TNFα inhibitors. A preferred biomarker of the invention is CD11c. The methods can further comprise selecting a treatment regimen with a TNFα inhibitor in an autoimmune disorder subject based upon expression of the biomarker(s) in the subject. The methods can further comprise administering a TNFα inhibitor to the subject according to the selected treatment regimen. Kits that include means for measuring expression of one or more biomarkers that are predictive of responsiveness to TNFα inhibitors for an autoimmune disorder are also provided. Methods of preparing and using databases, and computer program products therefore, for selecting an autoimmune disorder subject for treatment with a TNFα inhibitor are also provided.
Owner:ABBVIE INC
Method, apparatus and system for automatic treatment of pain
We disclose methods and medical device systems for automated delivery of therapies for pain and determination of need for and safety of treatment. In one embodiment, such a medical device system may comprise a sensor configured to sense at least one body signal from a patient; and a medical device configured to receive a first sensed body signal from the sensor; determine a patient pain index based at least in part on said first sensed body signal; determine whether said patient pain index is above at least a first pain index threshold; determine a safety index based at least in part on a second sensed body signal; select a pain treatment regimen based on at least one of said safety index and or a determination that said pain index is above said first pain index threshold; and deliver said pain treatment regimen.
Owner:FLINT HILLS SCI L L C
Methods for the identification, assessment, and treatment of patients with cancer therapy
ActiveUS20060281122A1Reduced growth rateEliminate ineffective or inappropriate therapeutic agentsMechanical/radiation/invasive therapiesMicrobiological testing/measurementAbnormal tissue growthRegimen
The present invention is directed to the identification of predictive markers that can be used to determine whether patients with cancer are clinically responsive or non-responsive to a therapeutic regimen prior to treatment. In particular, the present invention is directed to the use of certain individual and / or combinations of predictive markers, wherein the expression of the predictive markers correlates with responsiveness or non-responsiveness to a therapeutic regimen. Thus, by examining the expression levels of individual predictive markers and / or predictive markers comprising a marker set, it is possible to determine whether a therapeutic agent, or combination of agents, will be most likely to reduce the growth rate of tumors in a clinical setting.
Owner:MILLENNIUM PHARMA INC
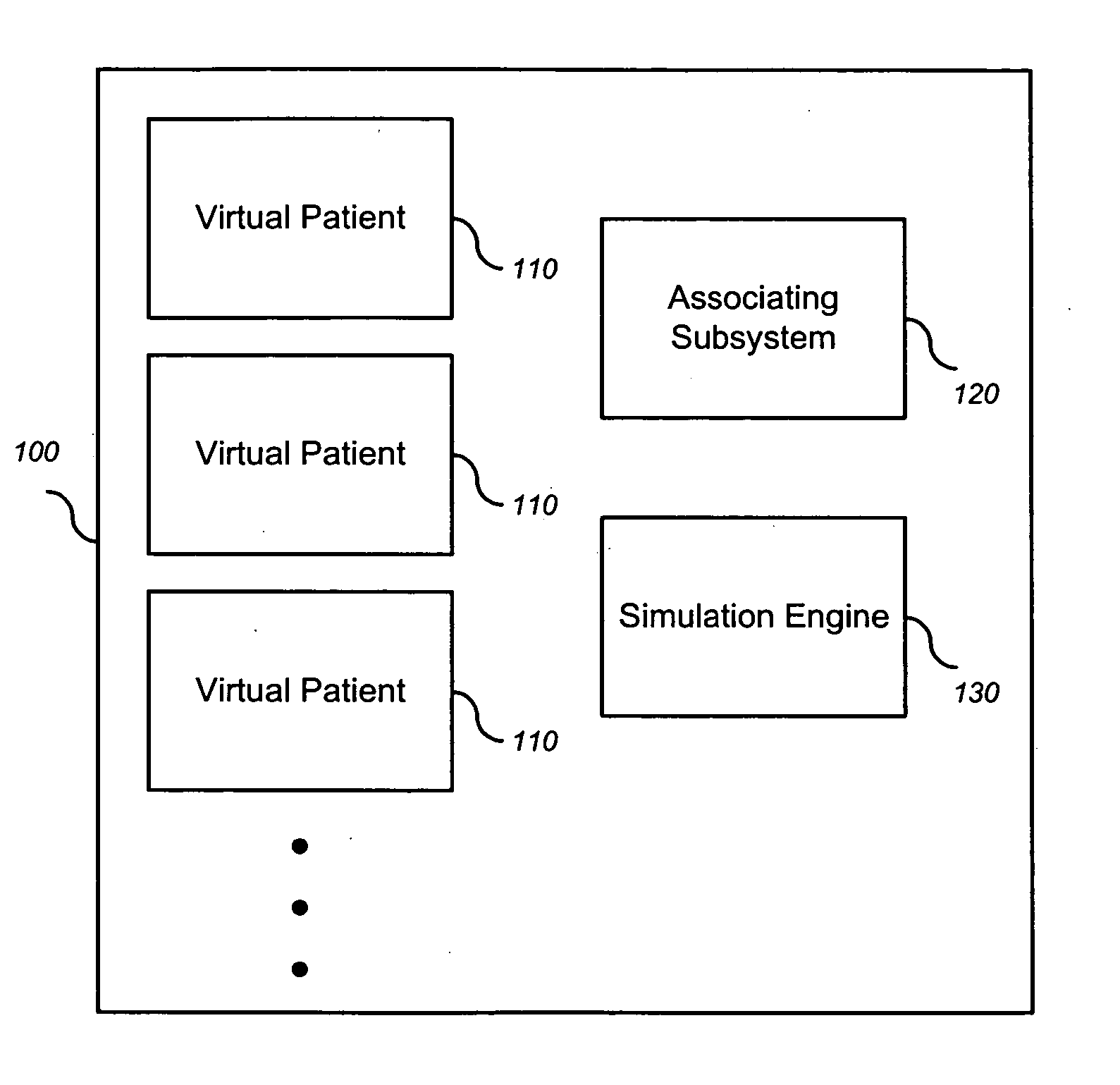
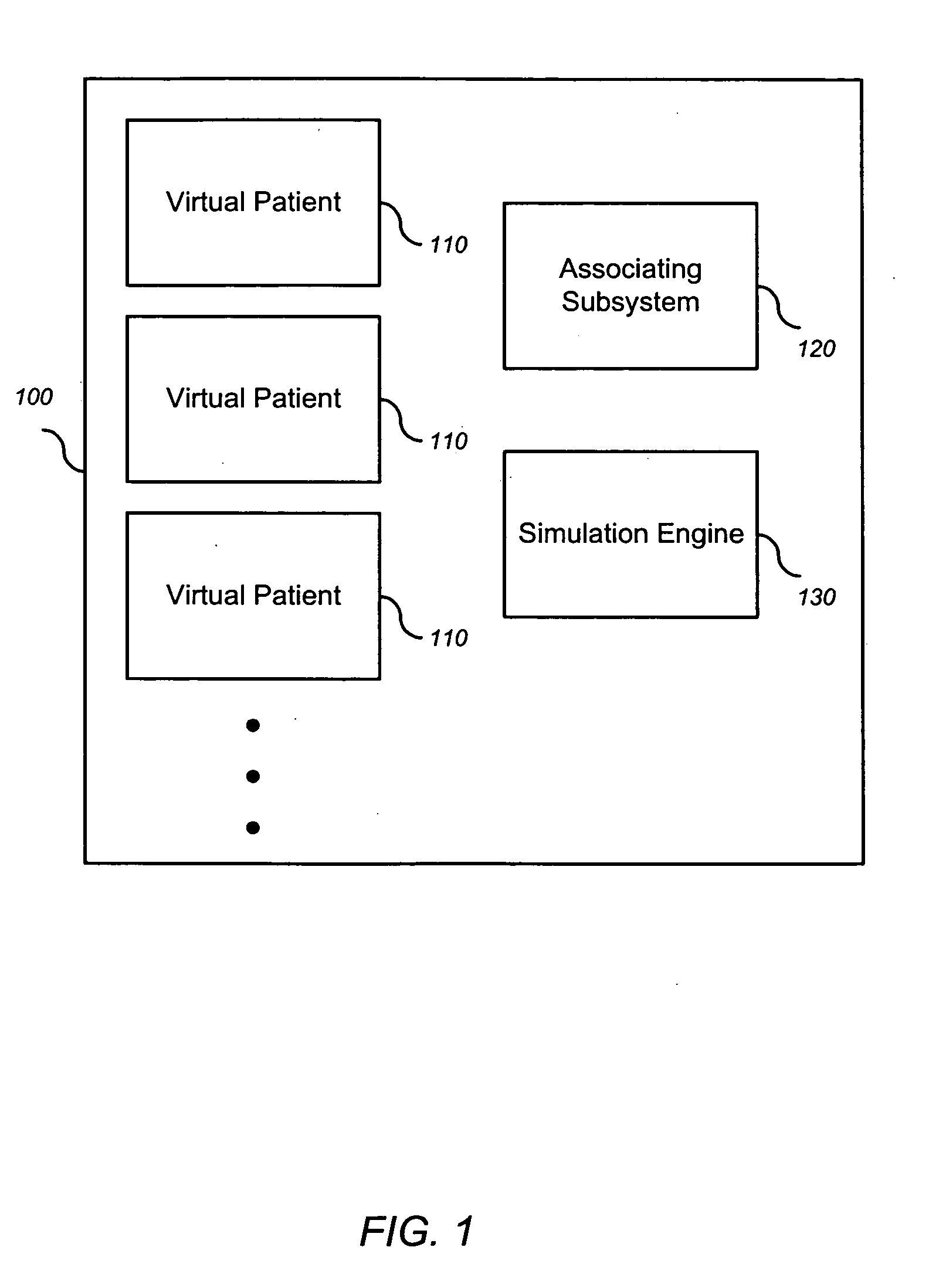
Simulating patient-specific outcomes
InactiveUS20050131663A1Timing is simpleMedical simulationAnalogue computers for chemical processesEfficacyBiomarker (petroleum)
The invention encompasses systems, methods, and apparatus for predicting and monitoring an individual's response to a therapeutic regimen. The invention includes multiple virtual patients, an associating subsystem operable to associate the subject with one or more of the virtual patients, and a simulation engine operable to apply one or more experimental protocols to the one or more virtual patients identified with the subject to generate a set of outputs. The set of outputs can represent therapeutic efficacy, identify biomarkers for monitoring therapeutic efficacy, or merely report the status of the biological system as it represents a particular individual
Owner:ENTELOS HLDG
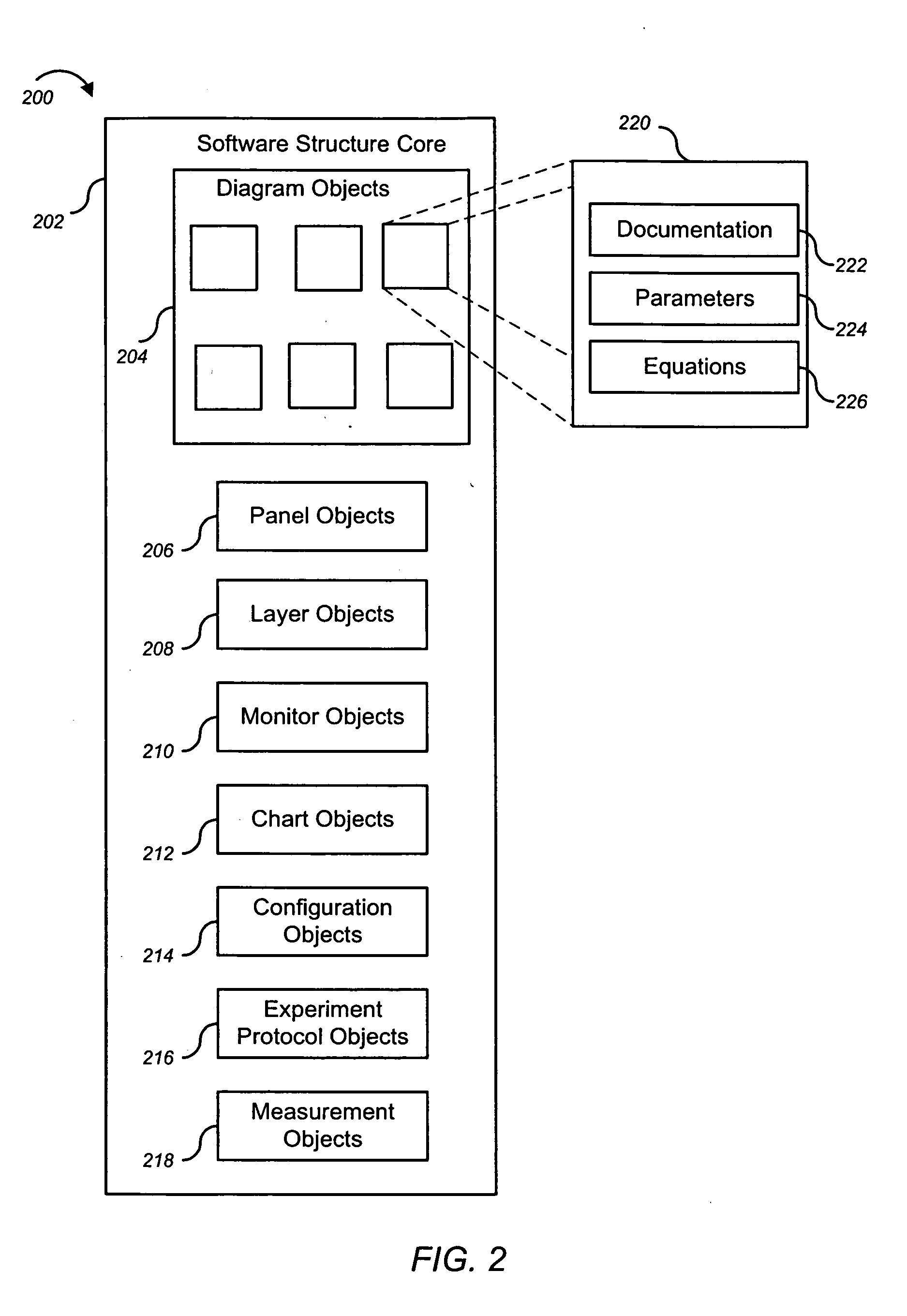
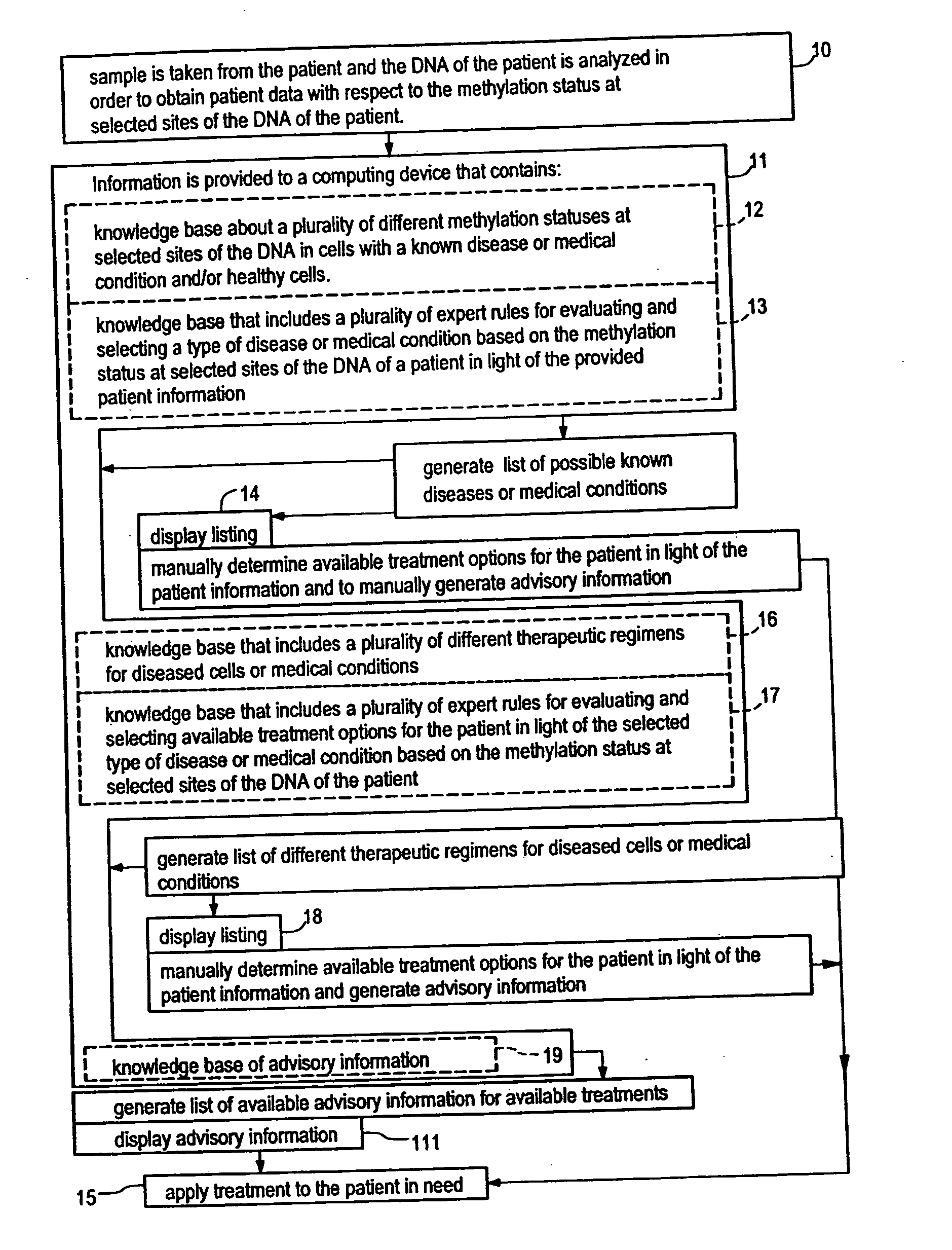
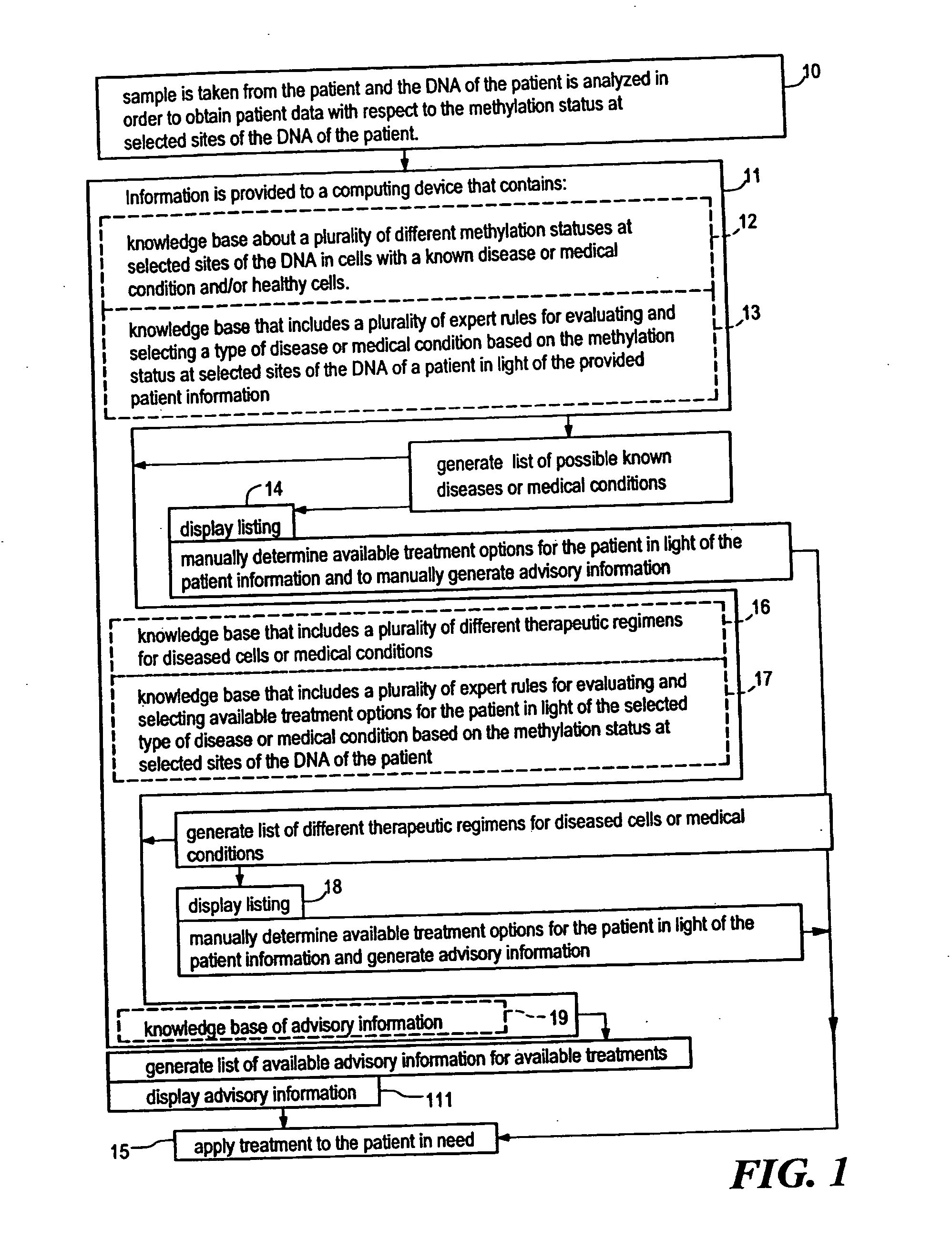
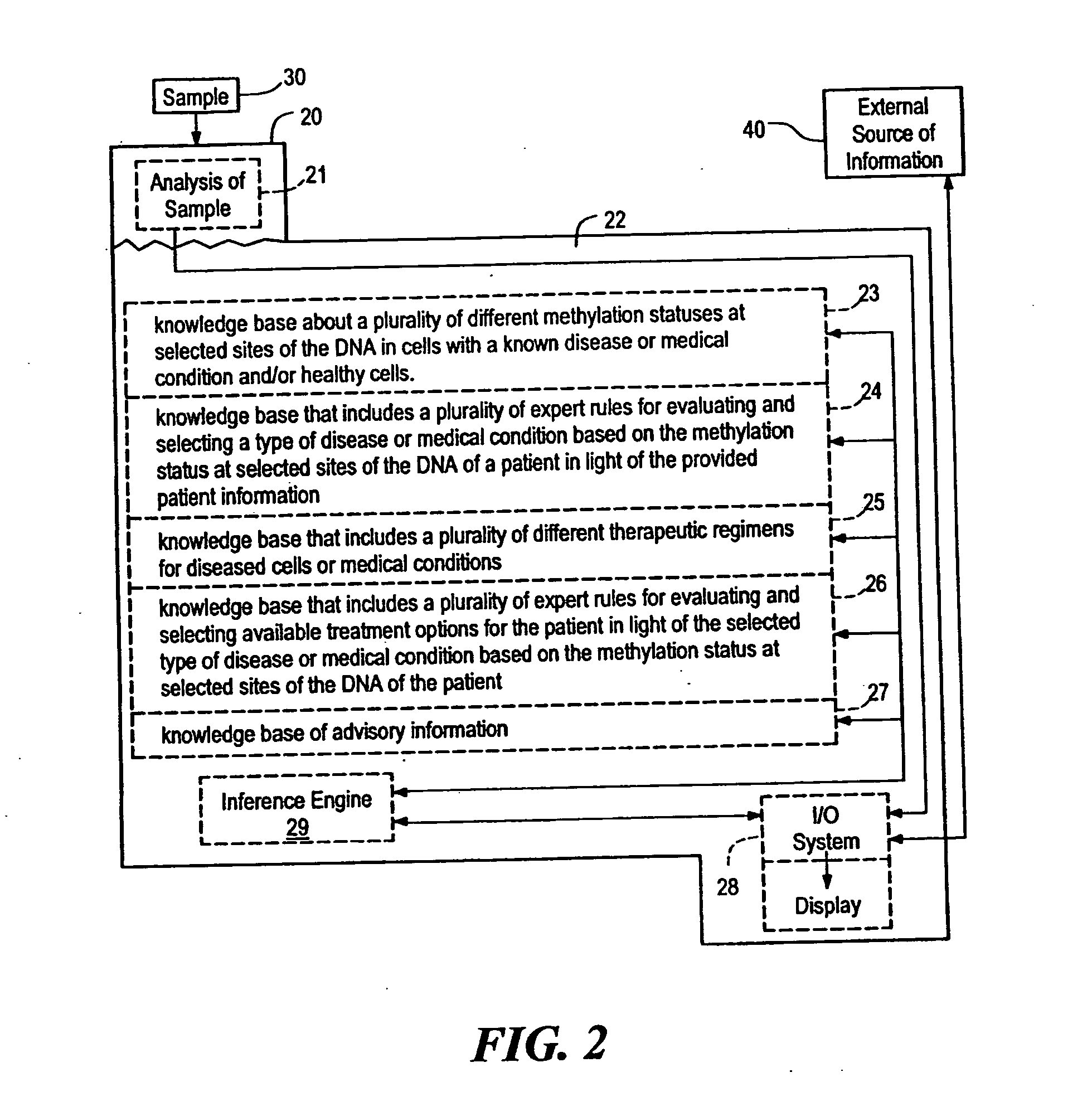
Systems, methods and computer program products for guiding selection of a therapeutic treatment regimen based on the methylation status of the DNA
InactiveUS20050021240A1Data processing applicationsMicrobiological testing/measurementRegimenProphylactic treatment
Systems, methods and computer program products for guiding selection of a therapeutic treatment regimen or a preventive therapeutic treatment regimen are disclosed. The method comprises (A) providing to a computing device comprising a first knowledge base comprising information about a plurality of different methylation statuses at selected sites of the DNA in cells with a known disease or medical condition and / or healthy cells, a second knowledge base comprising a plurality of expert rules for evaluating and selecting a type of disease or medical condition based on the methylation status at selected sites of the DNA of a patient, (B) generating in said computing device a ranked listing of diseases or medical conditions based on the information about the methylation status at selected sites of the DNA of the patient, the first knowledge base and the second knowledge base.
Owner:EPIGENOMICS AG
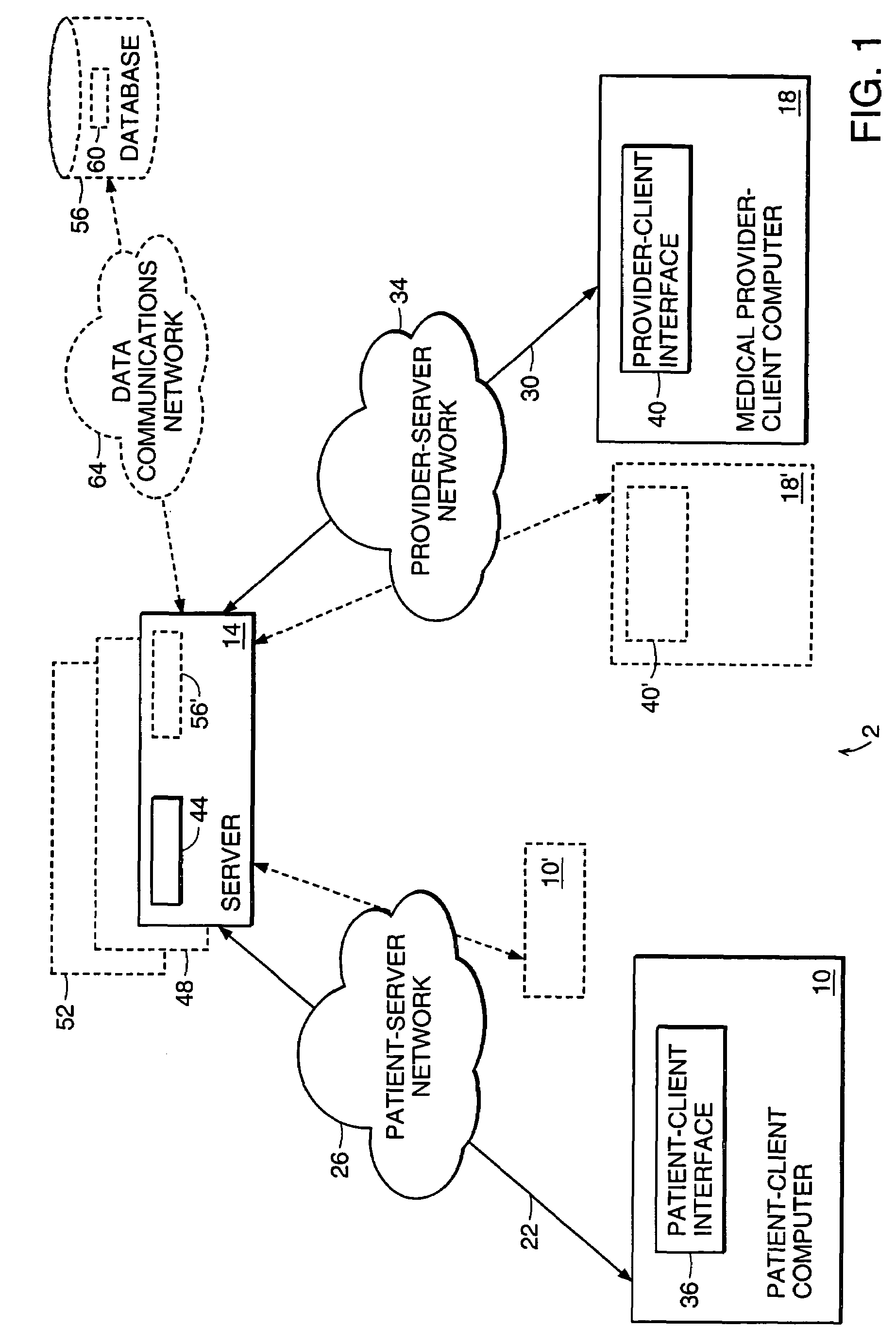
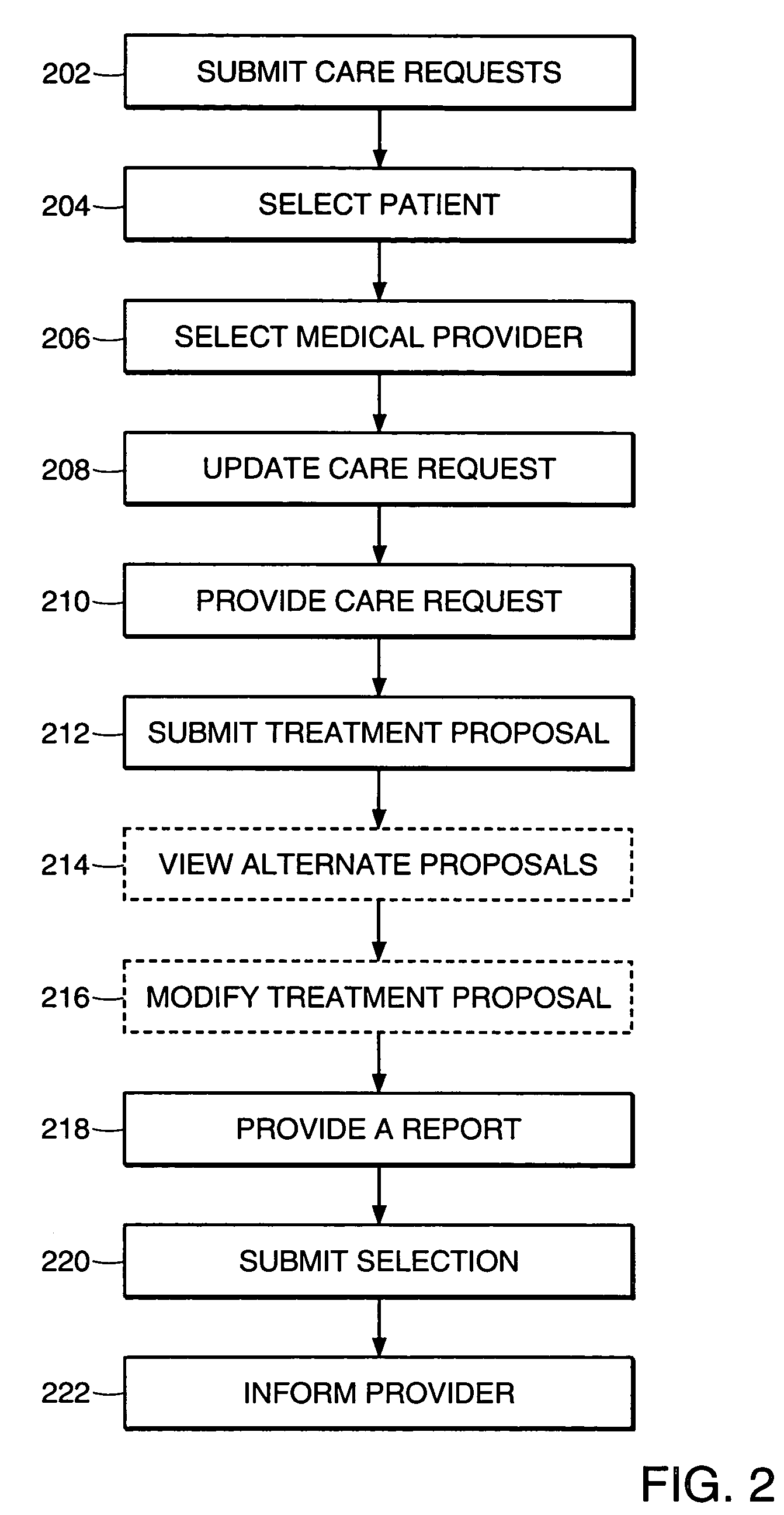
Health care management system
InactiveUS7756721B1Facilitating and managing health careFacilitate communicationFinanceTelemedicineManagement systemMedical treatment
The present invention relates to a system and method for facilitating and managing health care between a medical provider and a patient. In one aspect, the system and method includes providing a patient having a first criteria, which includes a medical symptom. The system and method also include selecting a subset of medical providers having expertise in treating the medical symptom, generating a care request to obtain a treatment proposal for the medical symptom of the patient, and updating the care request with medical information associated with the medical symptom. The system and method further include receiving at least one treatment proposal of the medical symptom from the medical providers and selecting a treatment proposal of the medical symptom from the medical providers.
Owner:BEST DOCTORS
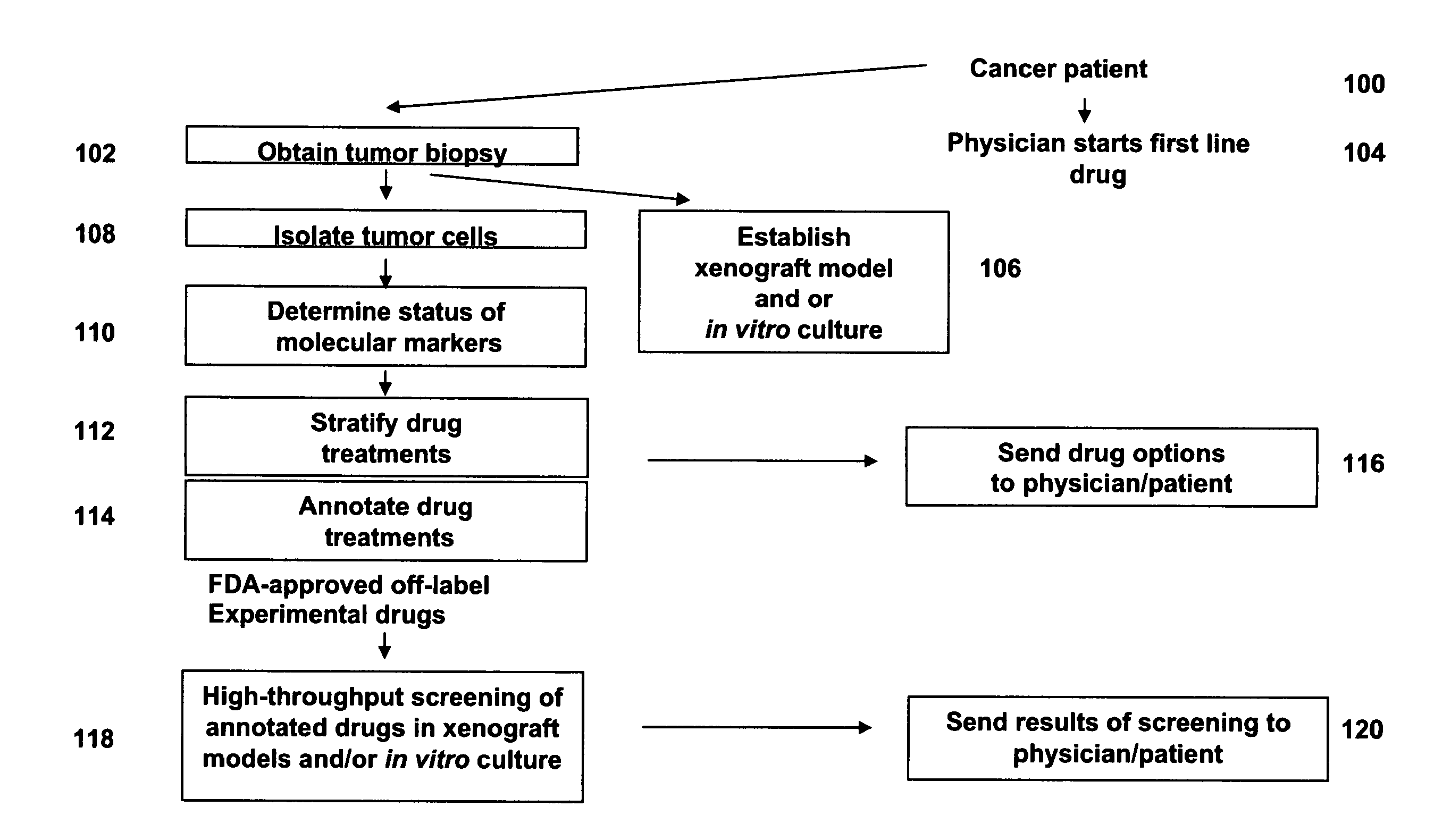
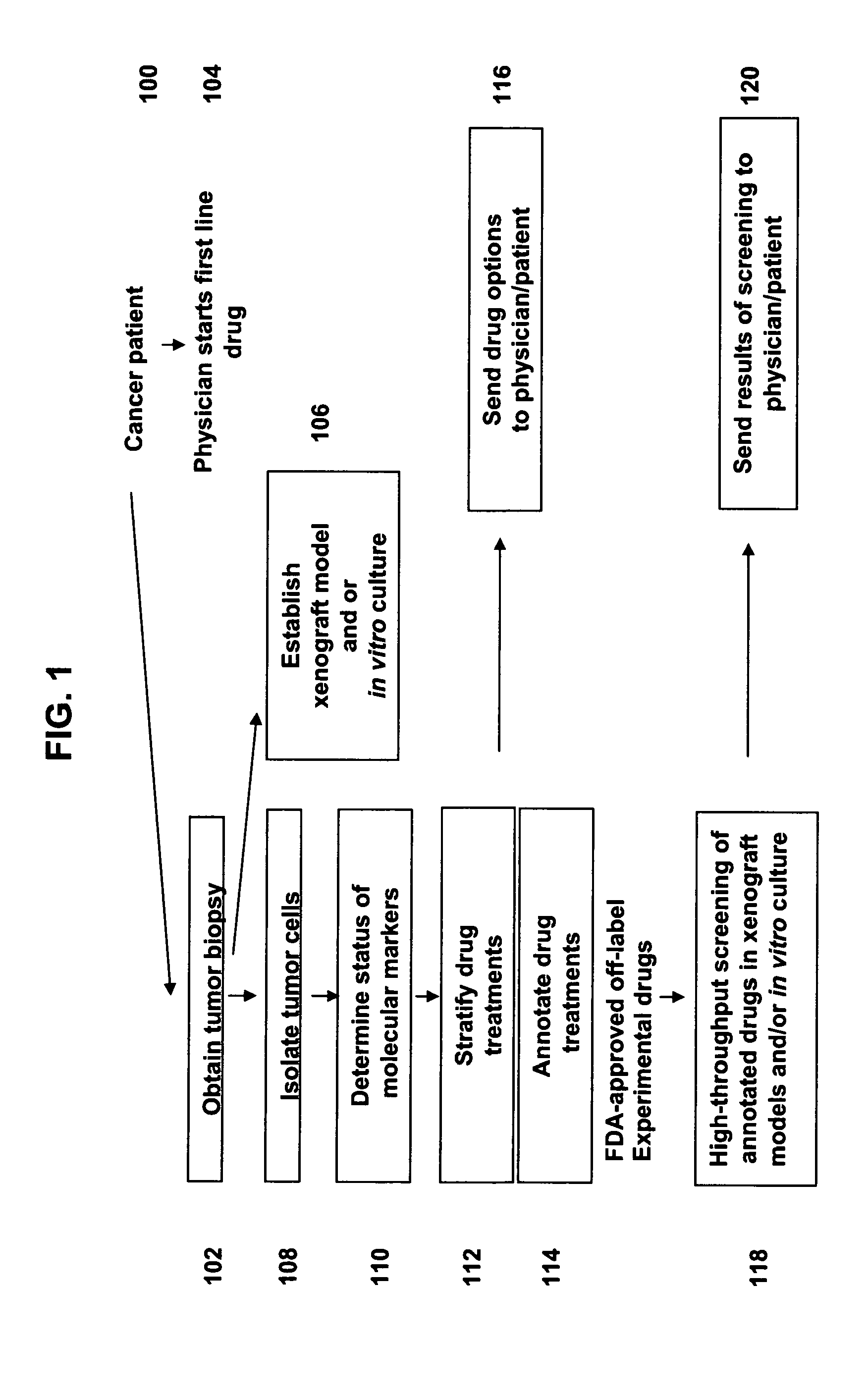
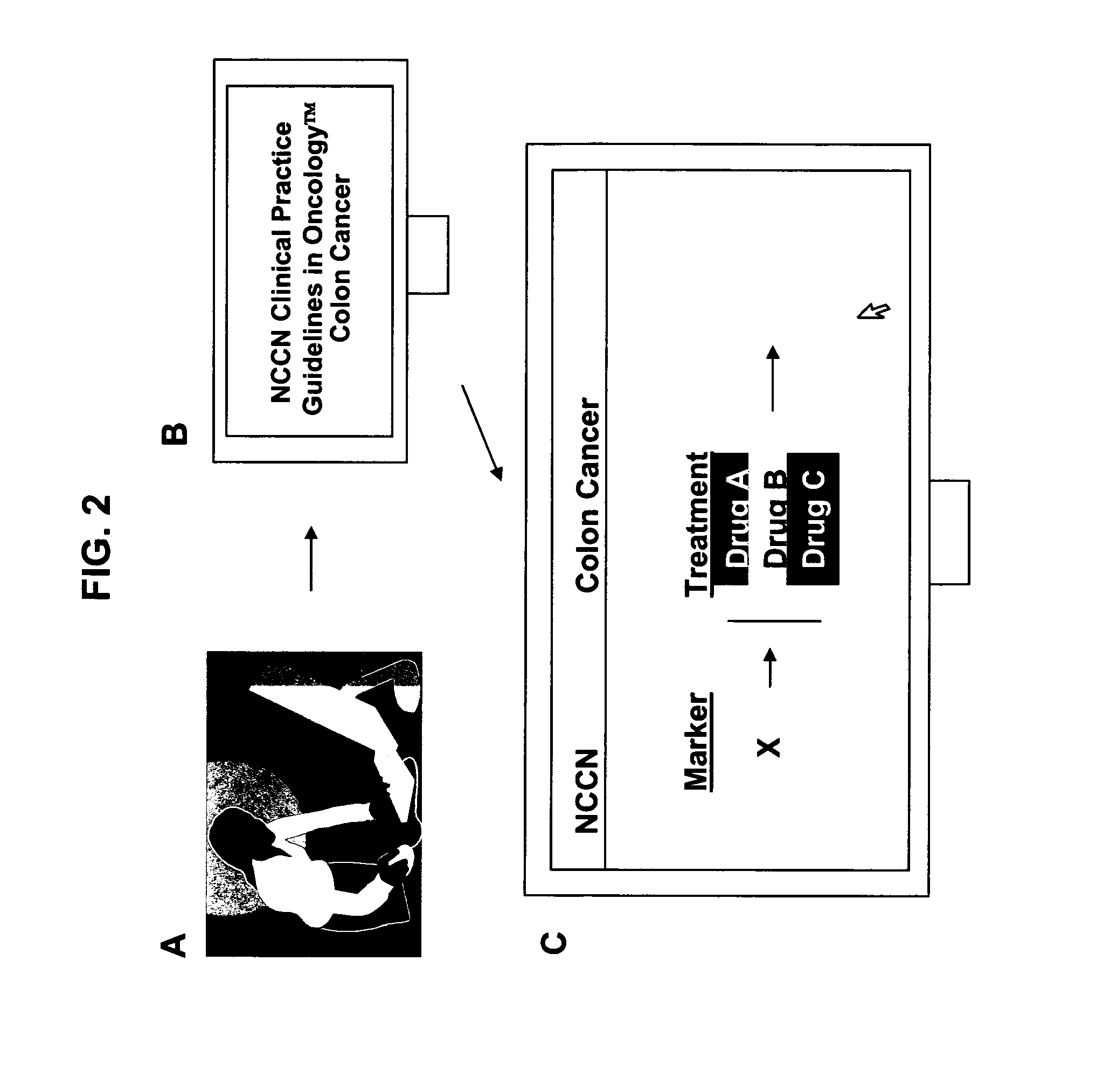
Methods for stratifying and annotating cancer drug treament options
ActiveUS20110230360A1Microbiological testing/measurementPreparing sample for investigationCancer pharmacotherapyCancer drugs
Personalized medicine involves the use of a patient's molecular markers to guide treatment regimens for the patient. The scientific literature provides multiple examples of correlations between drug treatment efficacy and the presence or absence of molecular markers in a patient sample. Methods are provided herein that permit efficient dissemination of scientific findings regarding treatment efficacy and molecular markers found in patient tumors to health care providers.
Owner:BLOODQ INC
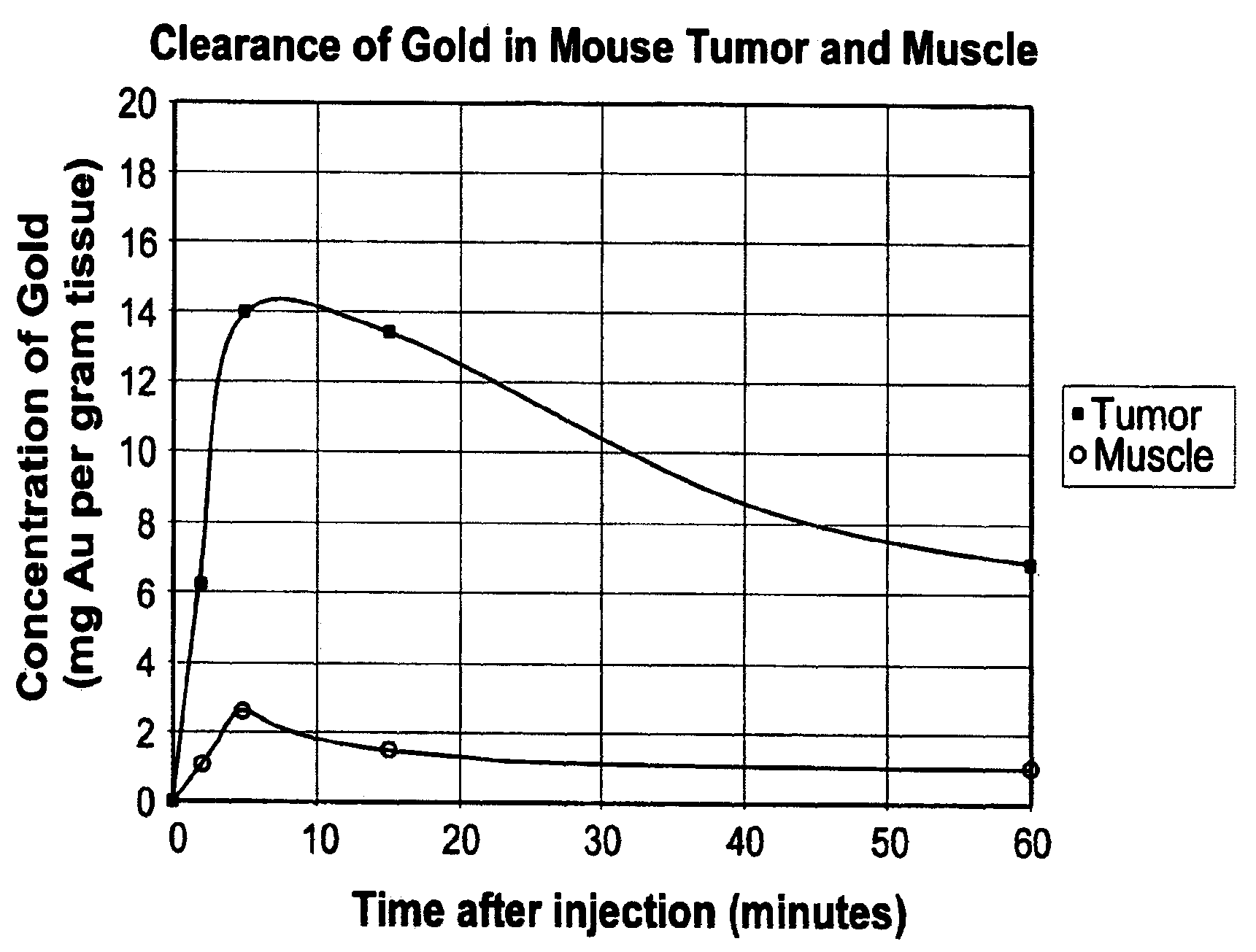
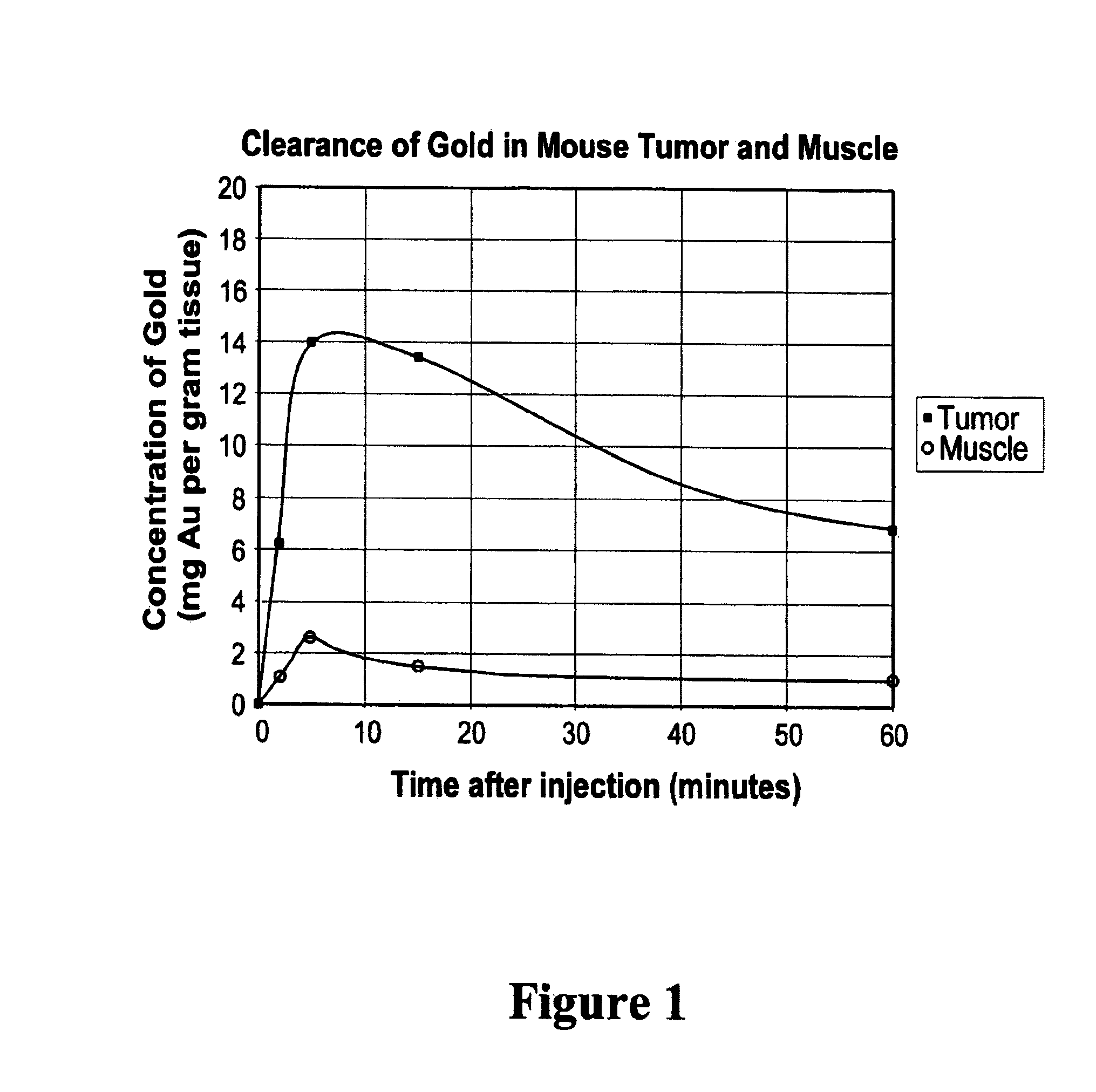
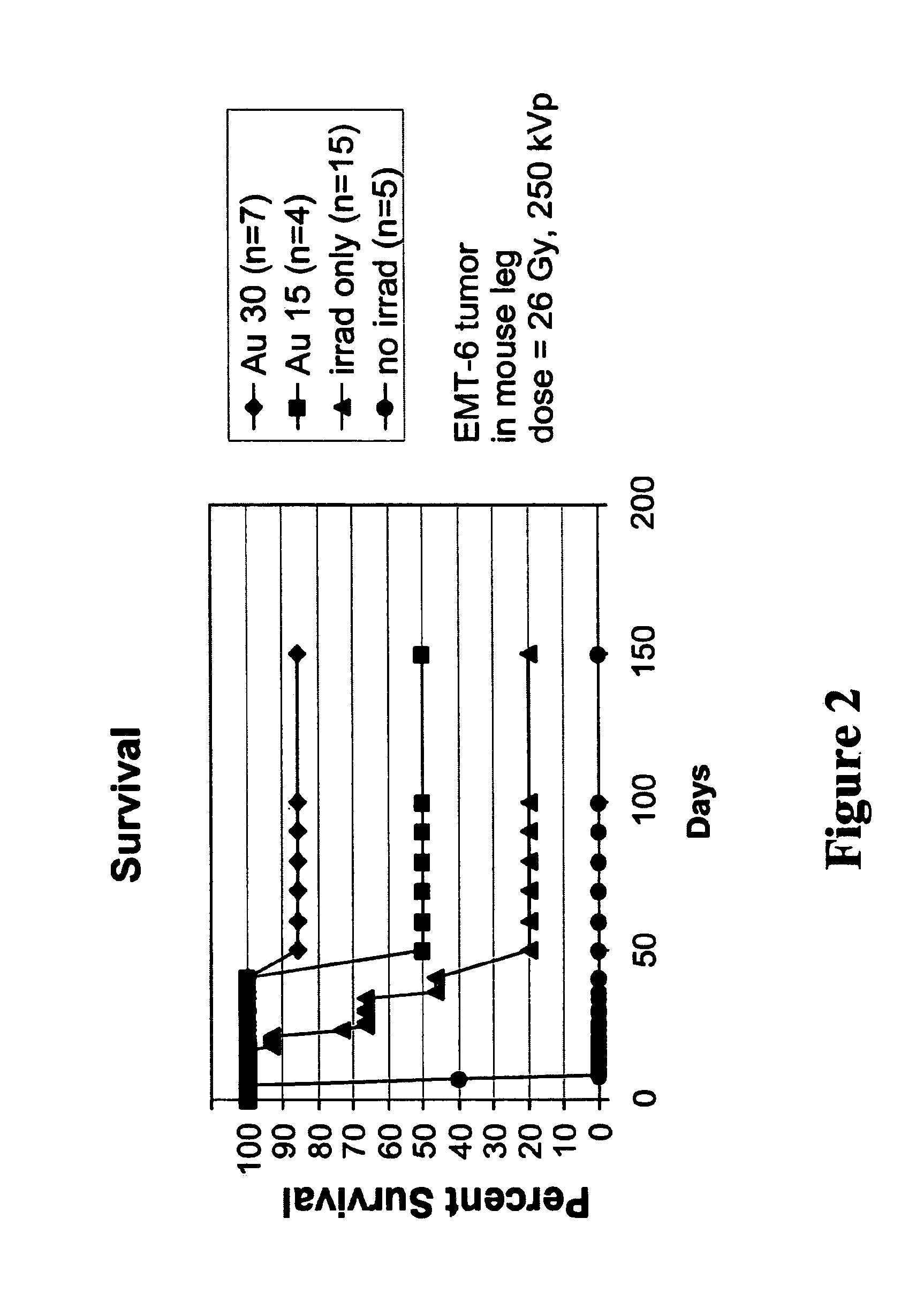
Methods of enhancing radiation effects with metal nanoparticles
Owner:NANOPROBES
Systems, methods, and devices for automatic closure of medical devices
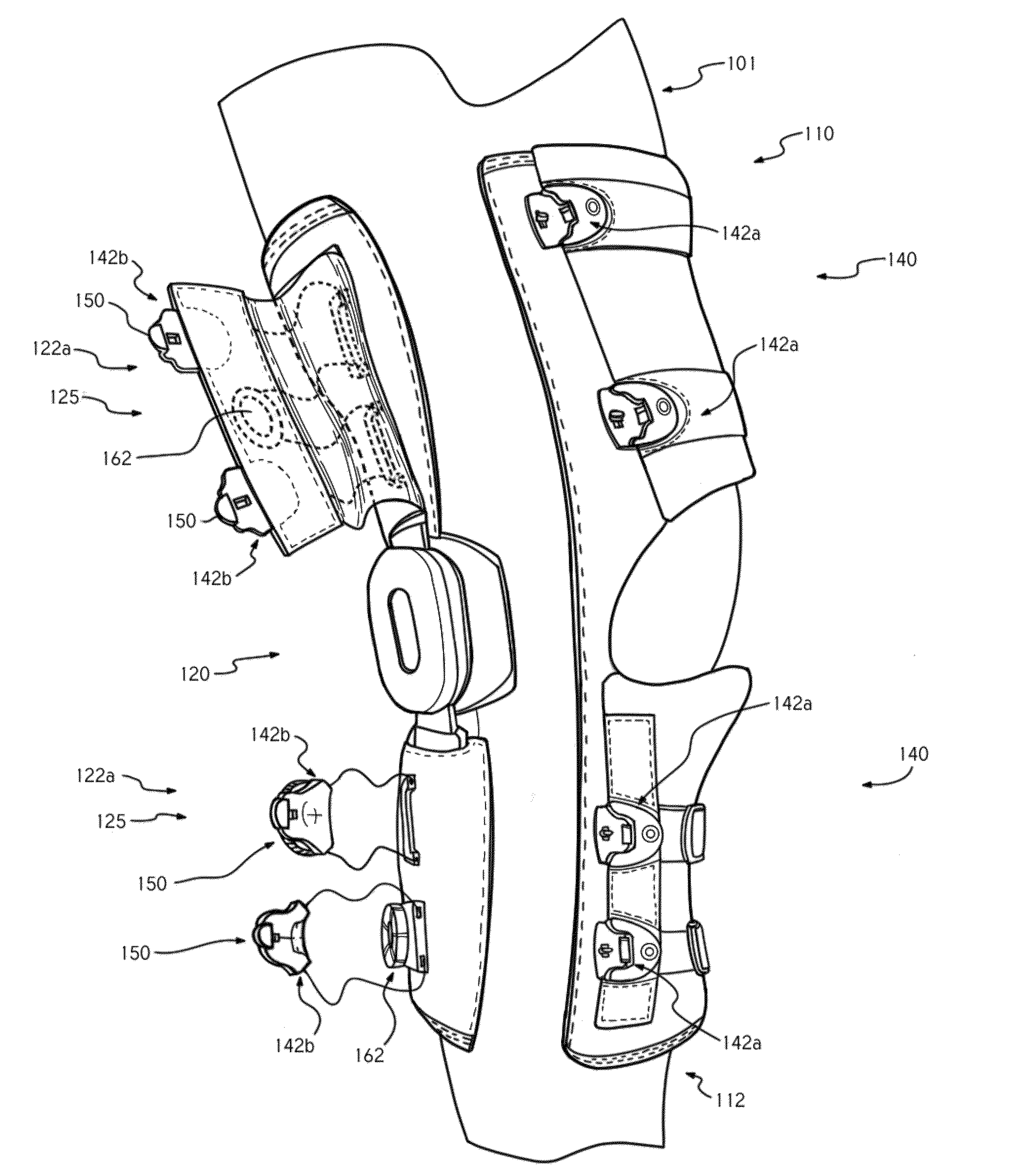
ActiveUS20140257156A1Reduce tensionPromote repetitive and cyclical movement and therapeutic healingNon-surgical orthopedic devicesFasteningsRepetitive movementsEngineering
According to an embodiment, a brace may include a motorized tensioning device, a tensioning member operationally coupled with the motorized tensioning device to tighten the brace about the limb, and a control unit communicatively coupled with the motorized tensioning device to control adjustment of a tension of the tensioning member. A method for providing therapy with the brace fitted about a limb may include communicating a first instruction from the control unit to the motorized tensioning device to adjust the tension of the tensioning member according to a therapeutic regimen that is designed to aid in recovery of the limb via repetitive movement of the limb.
Owner:BOA TECHNOLOGY
Methods and compositions for determining treatment regimens in systemic inflammatory response syndromes
InactiveUS20050148029A1Improve discriminationMicrobiological testing/measurementDisease diagnosisMedicineWhole body
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to rule in or out SIRS, or for differentiating sepsis, severe sepsis, septic shock and / or MODS from each other and / or from non-infectious SIRS.
Owner:BIOSITE INC
Dosage forms of bisphosphonates
ActiveUS20050260262A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyUpper gastrointestinal
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Treatment of B-cell associated diseases
InactiveUS6846476B2Enhanced killing and depletionReduce the possibilityRadioactive preparation carriersAntibody ingredientsDiseaseAutoimmune responses
Treatment of B-cell associated diseases including autoimmune and B-cell malignancies such as leukemias, lymphomas, using the combination of an anti-CD20 antibody, preferably RITUXAN® and a radiolabeled anti-CD22 antibody, preferably an 90Y labeled humanized anti-CD22 antibody, is described. These therapeutic regimens provide for enhanced depletion of B cells, and therefore reduce the risk in B cell malignancy treatment of relapse associated with RITUXAN® and, moreover, provide for prolonged immunosuppression of B-cell immune responses, especially in the context of autoimmune diseases and transplant.
Owner:BIOGEN INC
Biomarkers for predicting prostate cancer progression
InactiveUS20060234259A1Useful predictionCompound screeningApoptosis detectionProstate cancerOncology
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Selective neurostimulation for treating epilepsy
A method and device for treating epilepsy are disclosed which provide for electrical, chemical or magnetic stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of epilepsy. Deep brain stimulation is combined with vagus nerve stimulation to enhance symptomatic relief of the disorder. Some embodiments also employ a sensing capability to optimize the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
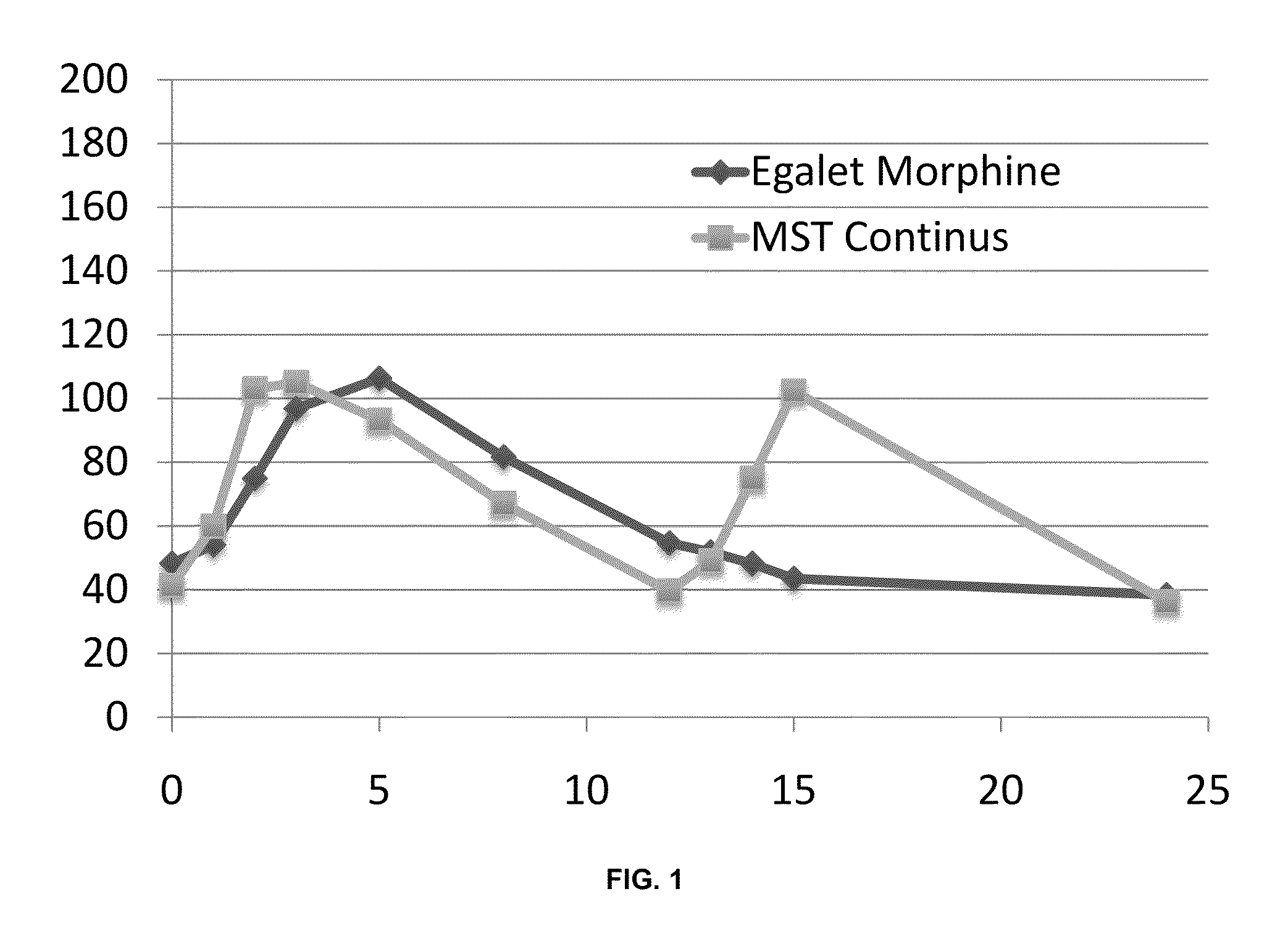
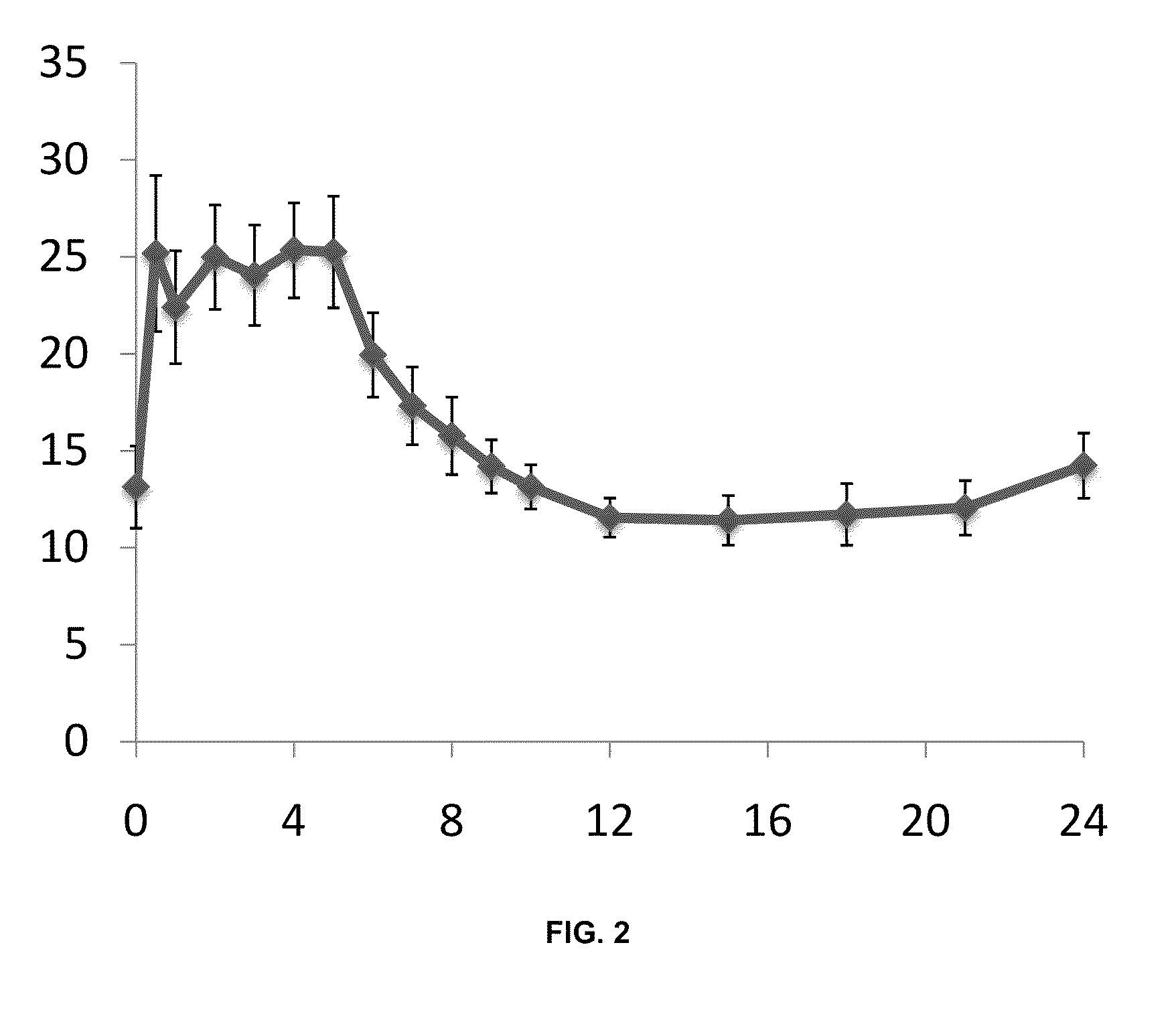
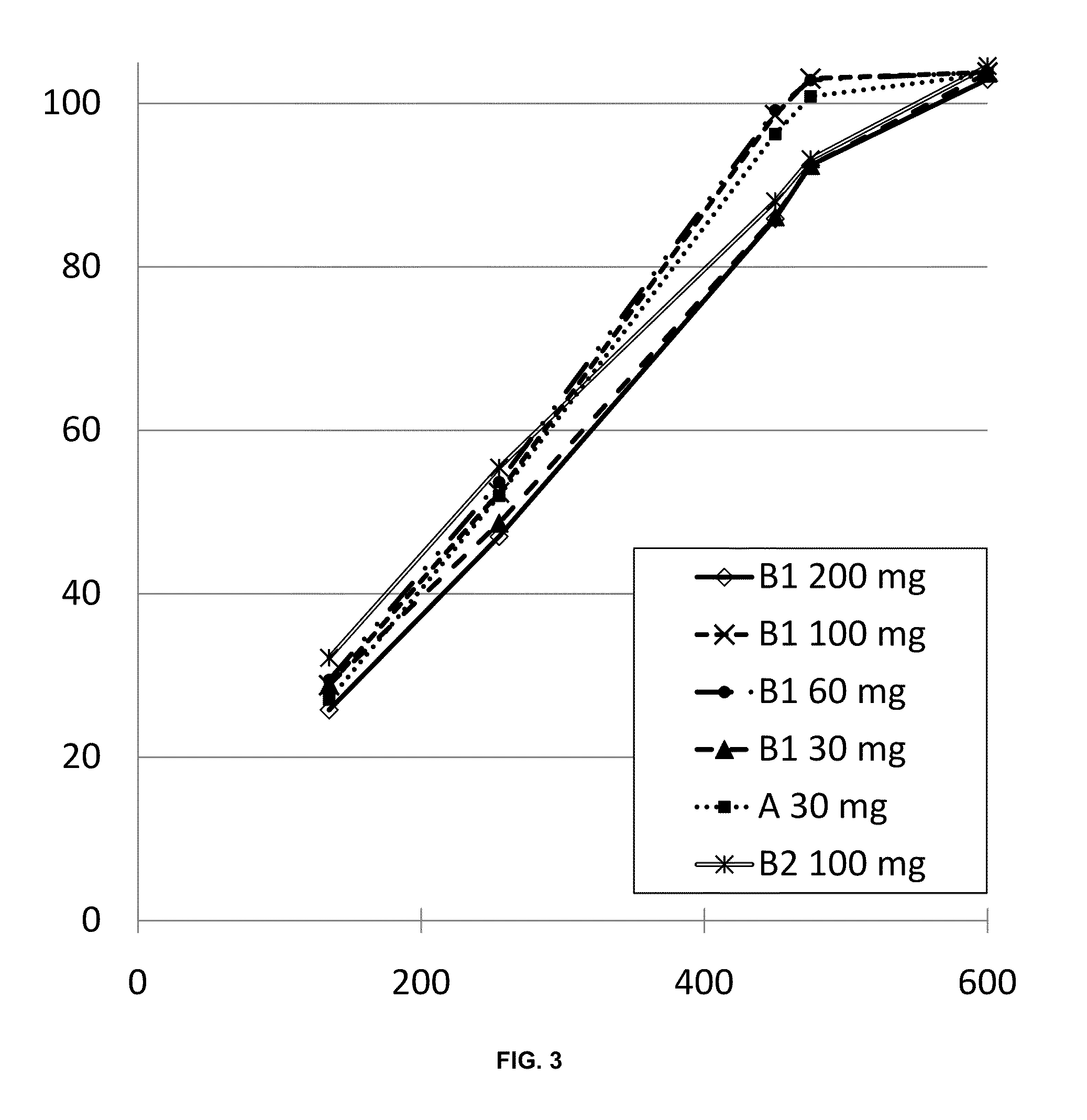
Controlled release formulations with continuous efficacy
InactiveUS20100203129A1Effective treatment regimenFacilitated releaseBiocidePowder deliveryControlled-Release FormulationsTreatment regimen
The present invention relates to pharmaceutical compositions, which provide controlled release of a drug. The compositions are suitable for continuous administration as they remain effective throughout the treatment regimen. The present invention also relates to the use of the compositions for preparation of a medicament for continuous treatment of an individual.
Owner:EGALET LTD
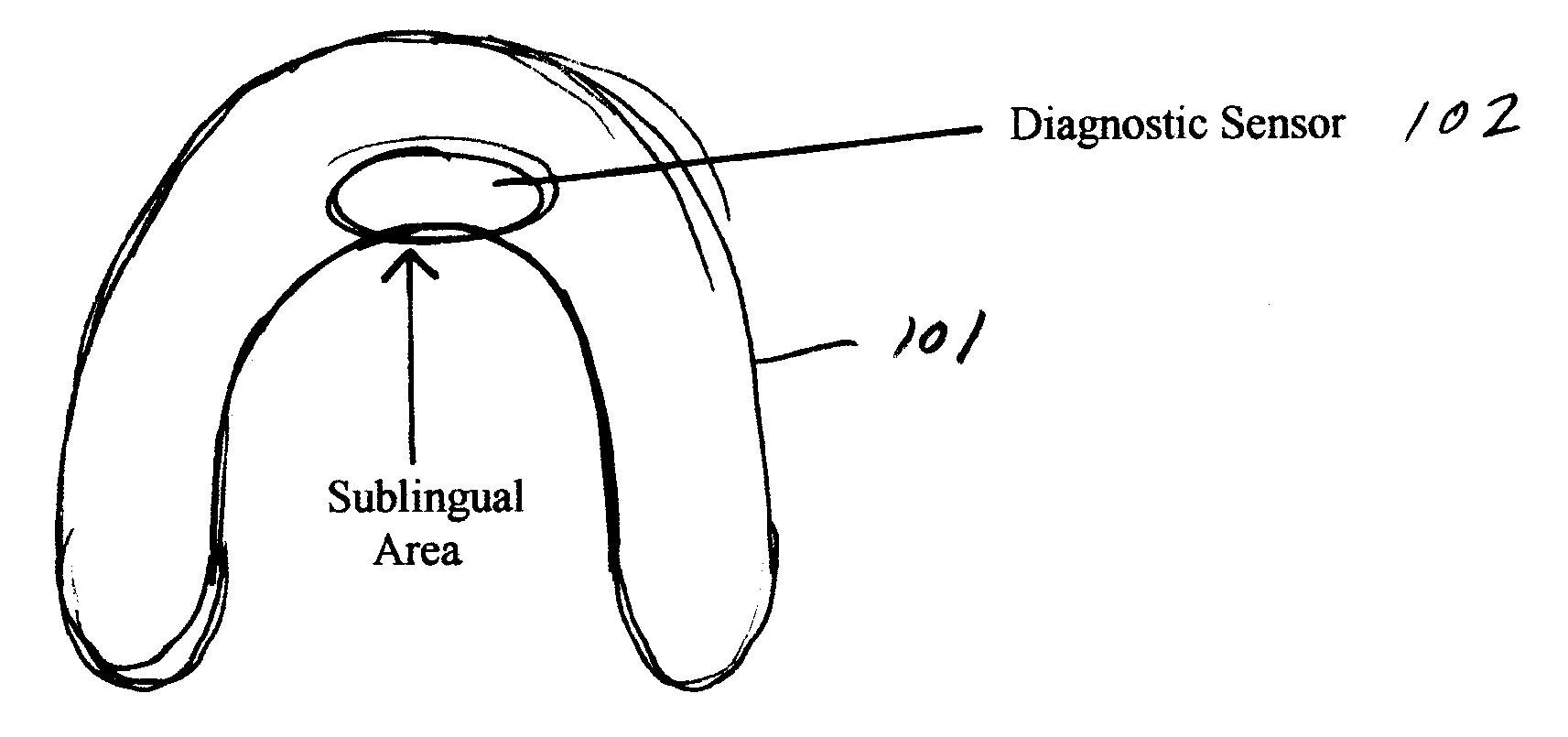
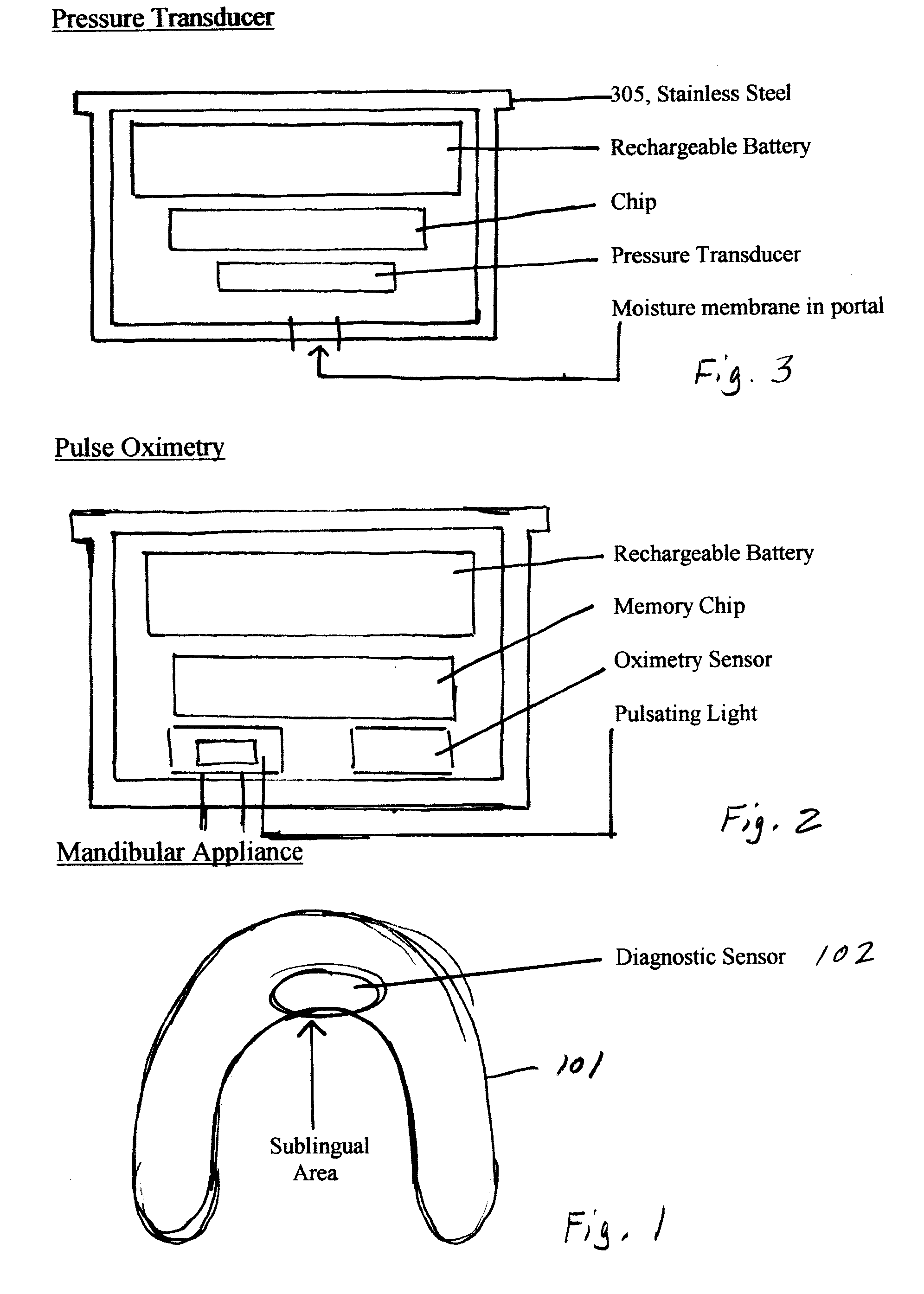
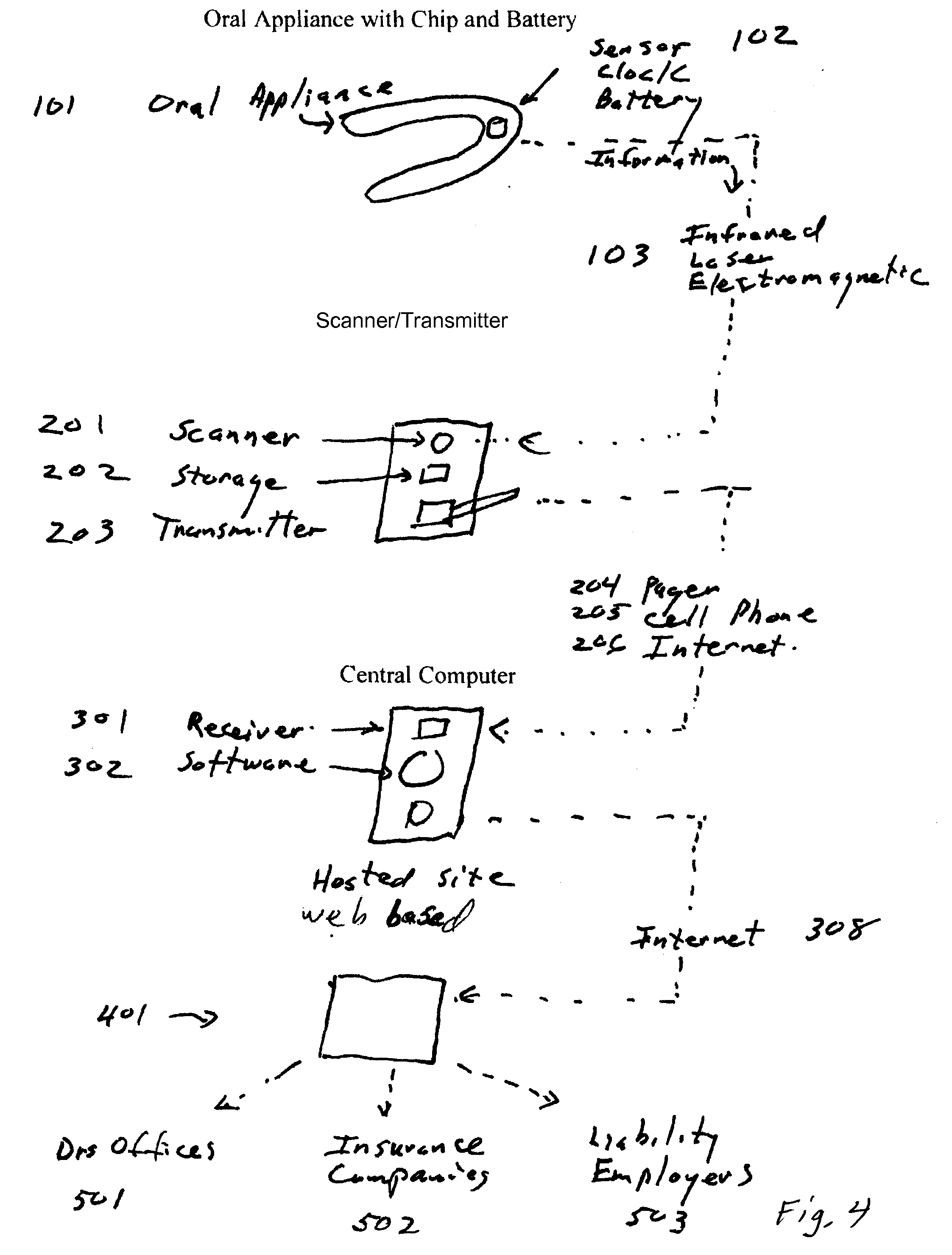
Oral appliance compliance monitoring system
An oral appliance compliance monitoring system and method comprises an oral appliance suitable for wearing in a patient's oral cavity during sleeping periods, the oral appliance having one or more sensors measuring a variety of conditions such as oxygen saturation levels in the oral cavity mucosa. The data generated by the sensor is continuously transmitted to a local scanner which is in communication with a central computer. The computer interprets the data to determine if the patent is wearing the oral appliance in compliance with a prescribed treatment regimen for breathing-related sleep disorders. Remotely located computers are authorized to receive the streamed data to enable remote monitoring of compliance in real time by a plurality of patients with treatment regimens.
Owner:DUHAMEL JAMES BRIAN +2
Mobile station and methods for diagnosing and modeling site specific full-scale effluent treatment facility requirements
InactiveUS20110257788A1Reduce financial riskHigh continuity of operationWater/sewage treatment by neutralisationSustainable biological treatmentInitial treatmentIon exchange
A mobile station and methods are disclosed for diagnosing and modeling site specific effluent treatment facility requirements to arrive at a treatment regimen and / or proposed commercial plant model idealized for the particular water / site requirements. The station includes a mobile platform having power intake, effluent intake and fluid outflow facilities and first and second suites of selectably actuatable effluent pre-treatment apparatus. An effluent polishing treatment array is housed at the station and includes at least one of nanofiltration, reverse osmosis and ion-exchange stages. A suite of selectively actuatable post-treatment apparatus is housed at the station. Controls are connected at the station for process control, monitoring and data accumulation. A plurality of improved water treatment technologies is also disclosed. The modeling methods include steps for analyzing raw effluent to be treated, providing a field of raw effluent condition entry values and a field of treated effluent condition goals entry values, and utilizing said fields to determine an initial treatment model including a selection of, and use parameters for, treatment technologies from the plurality of down-scaled treatment technologies at the facility, the model dynamically and continuously modifiable during treatment modeling.
Owner:ROCKWATER RESOURCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com