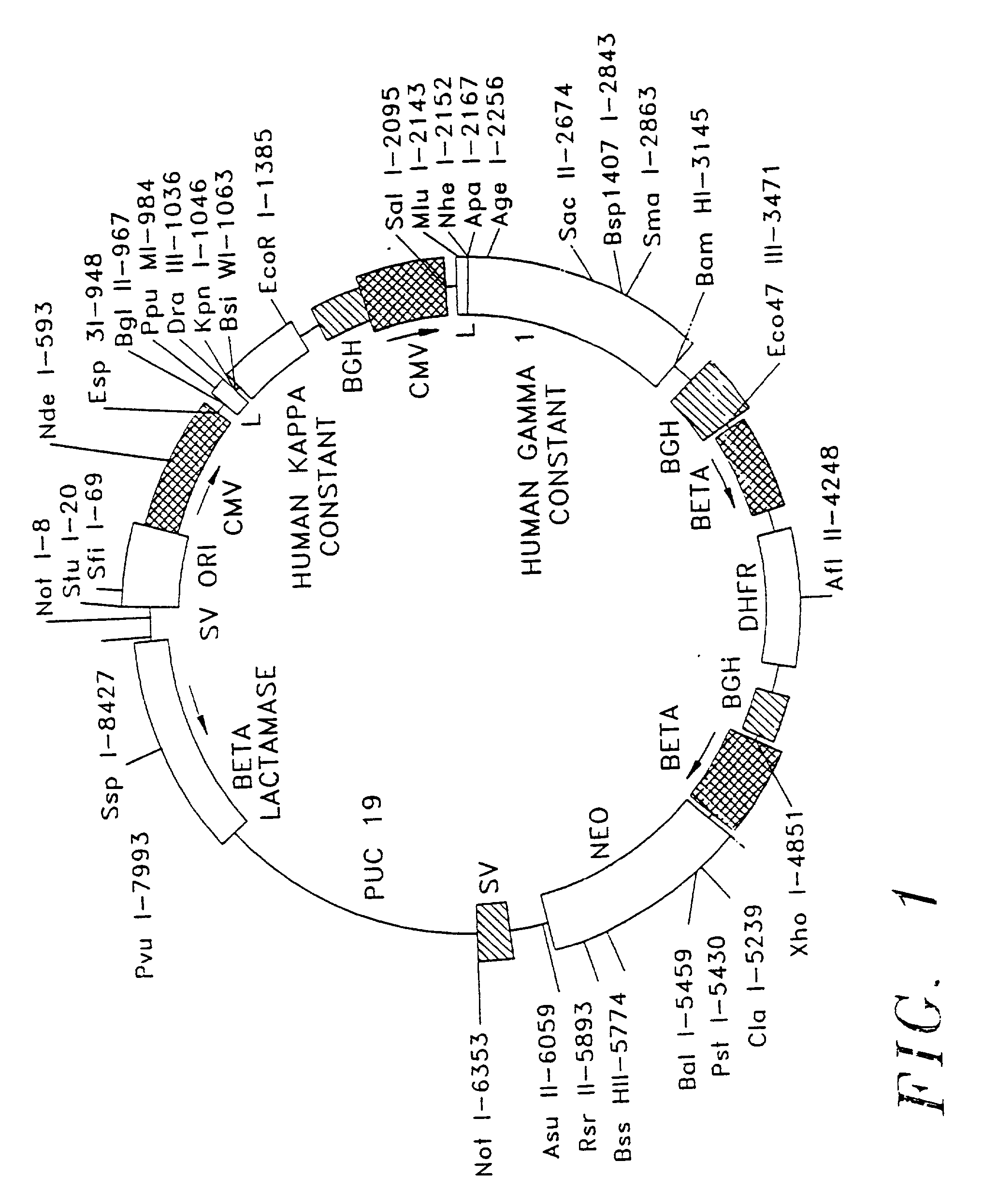
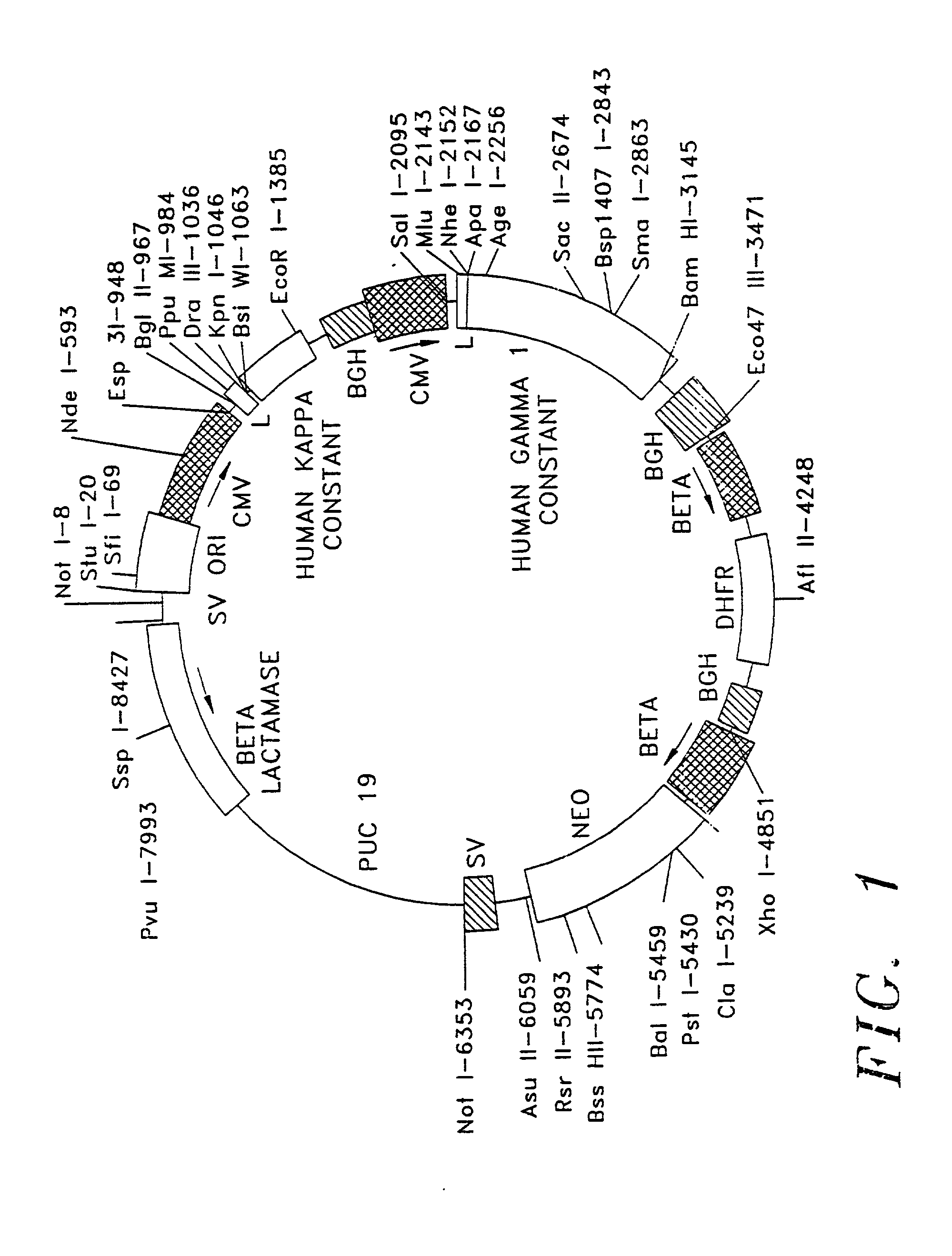
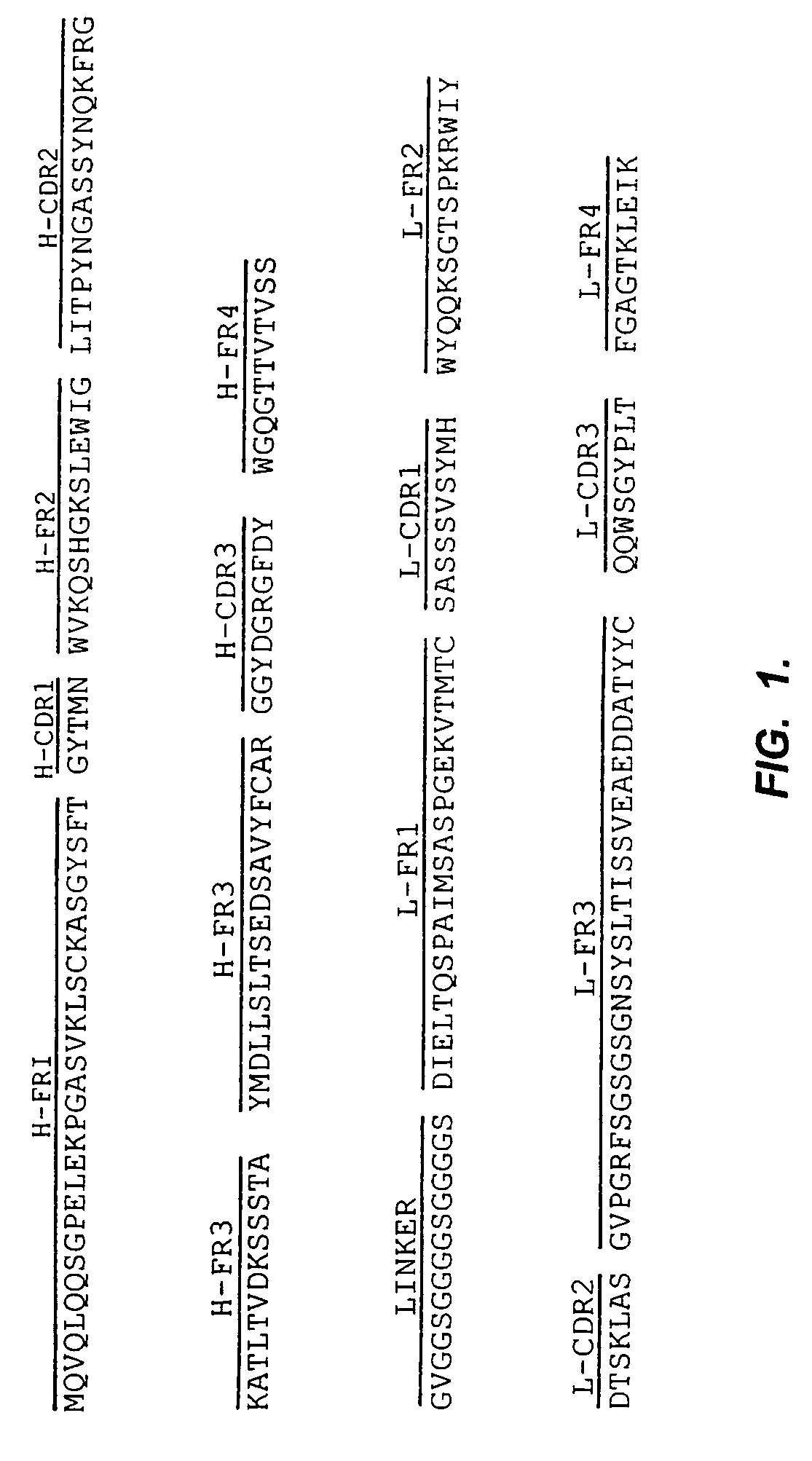
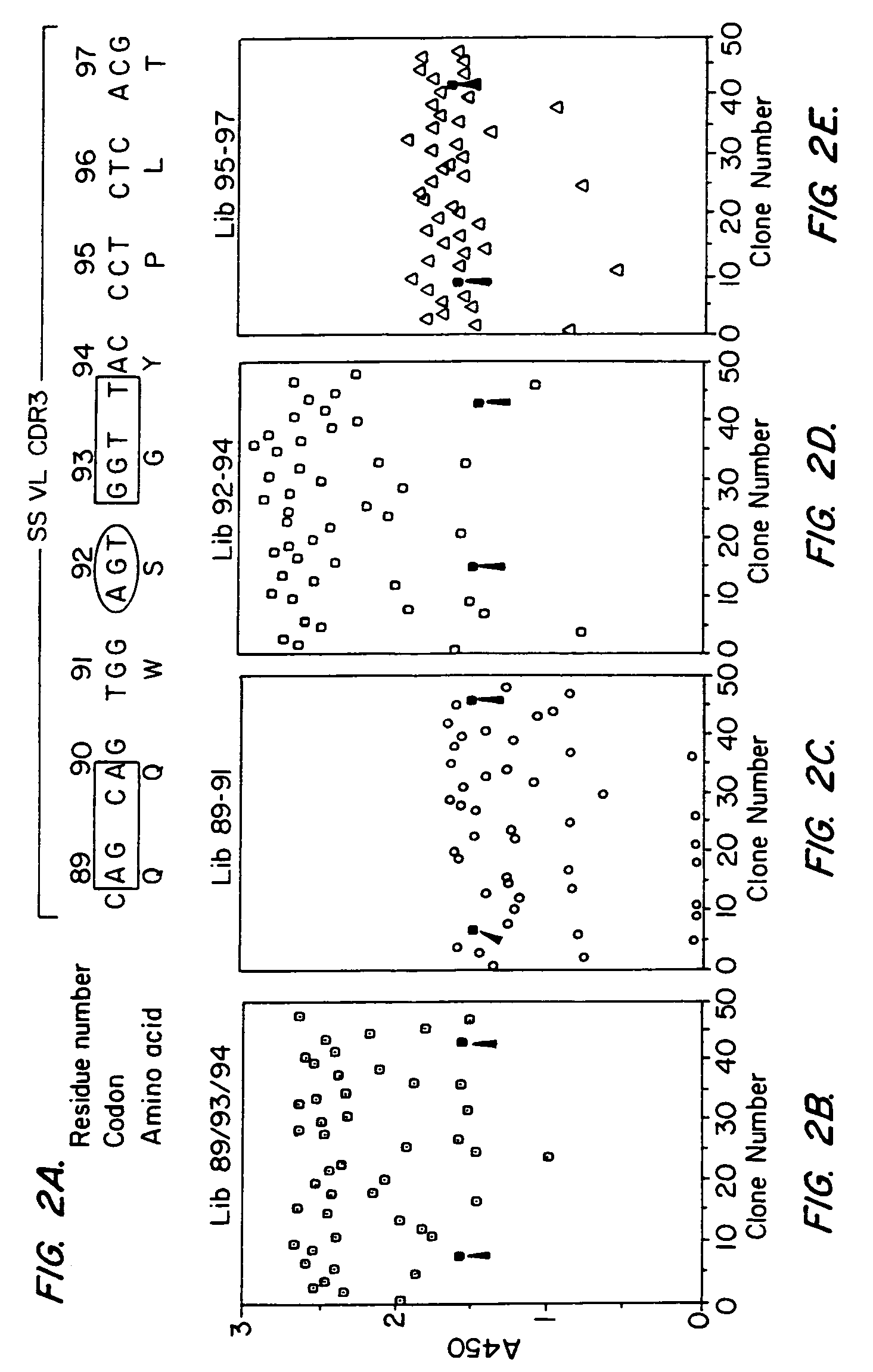
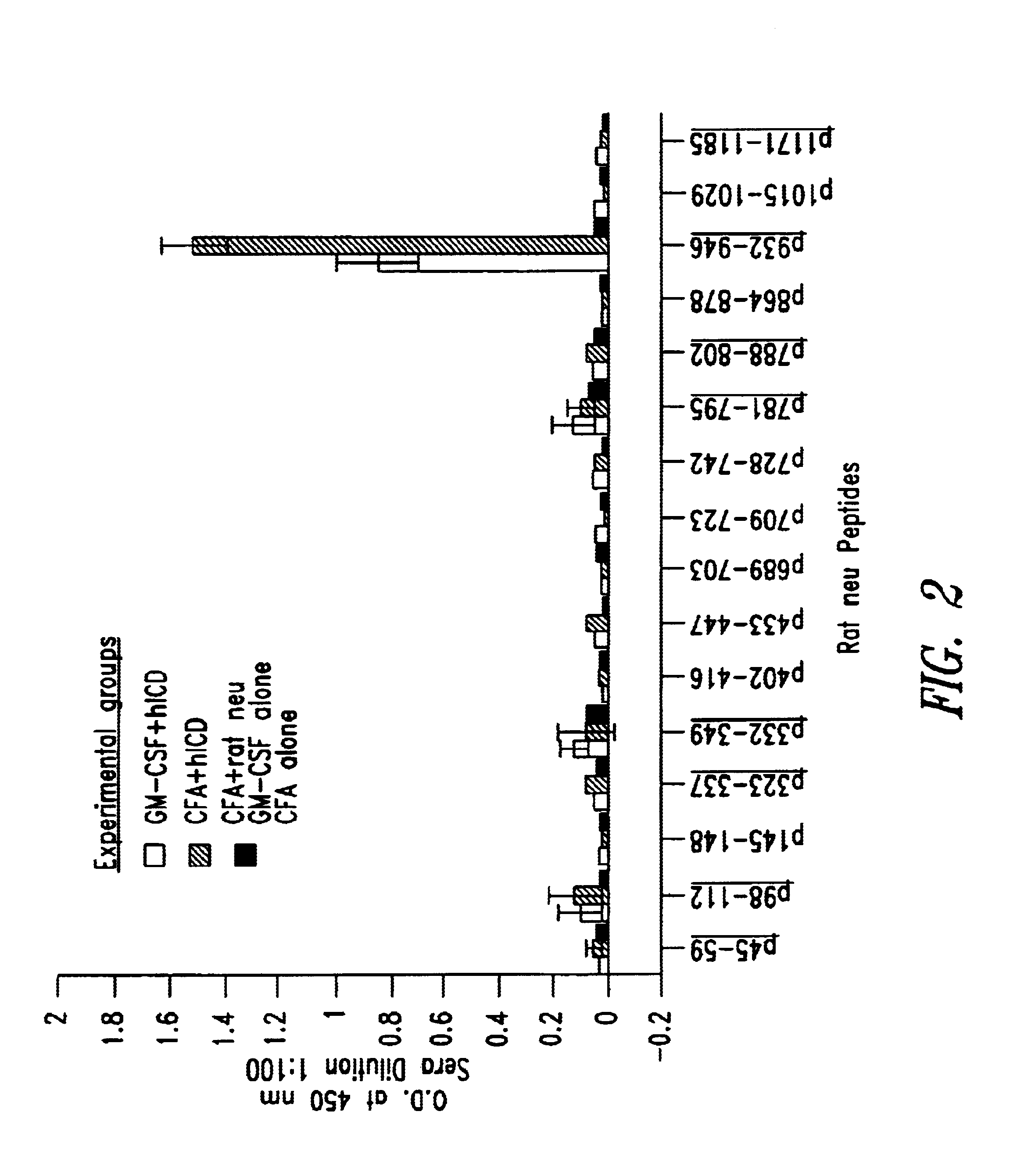
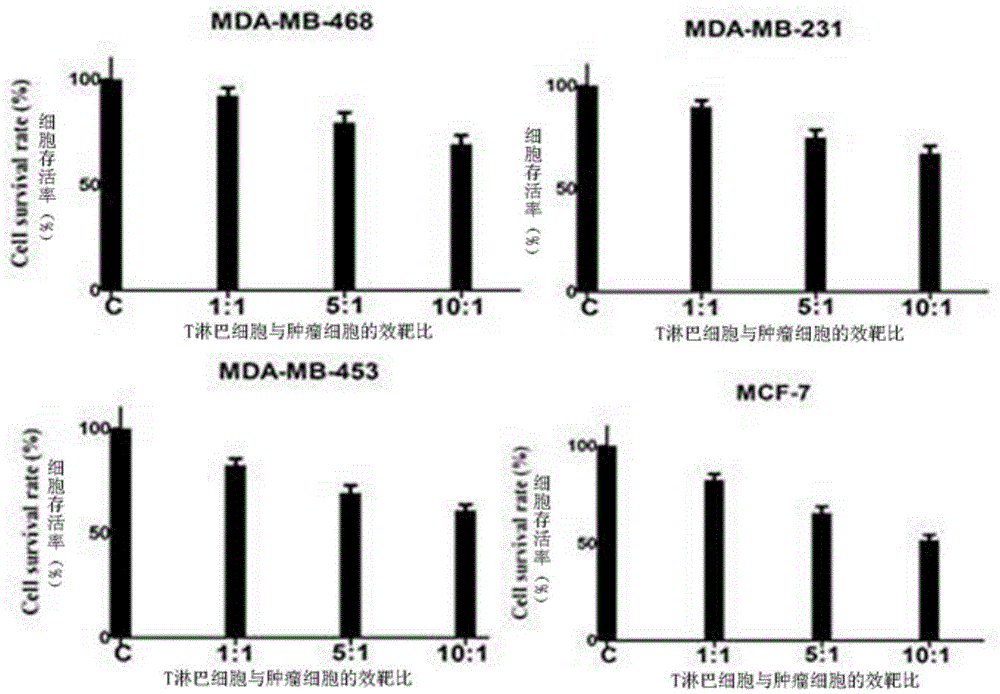
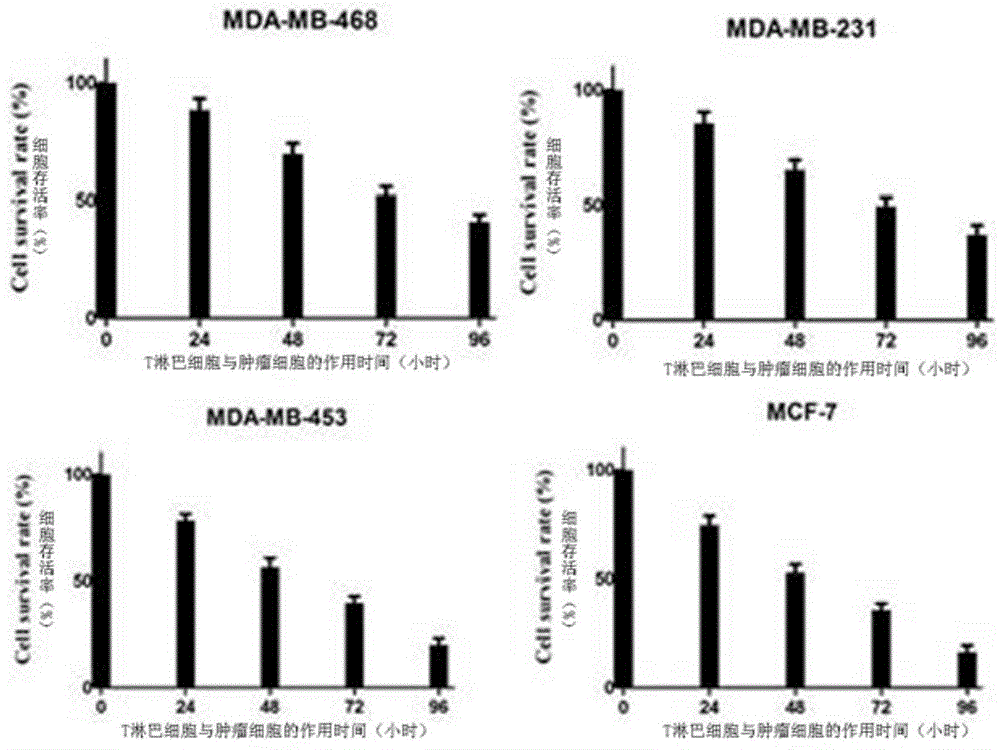
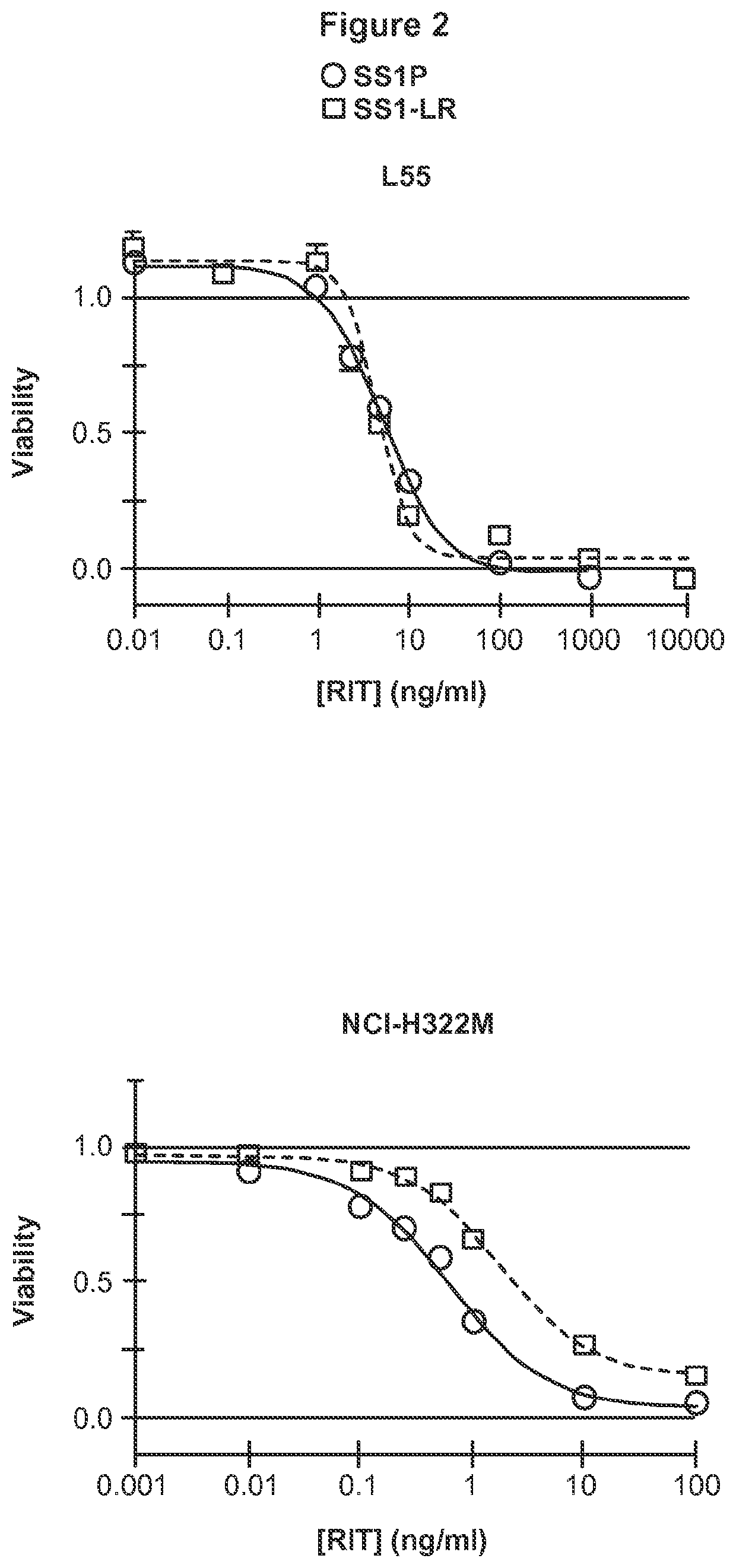
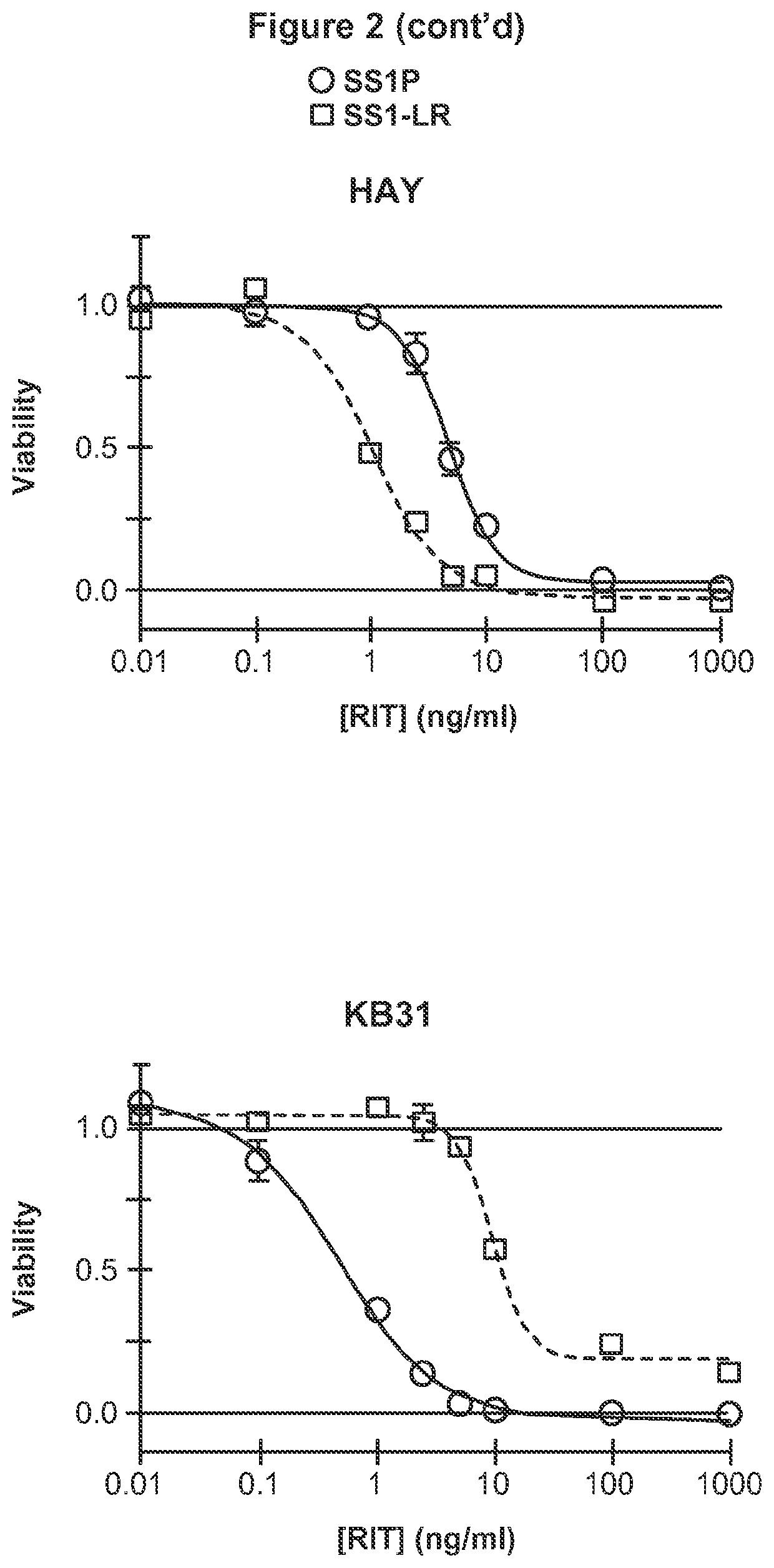
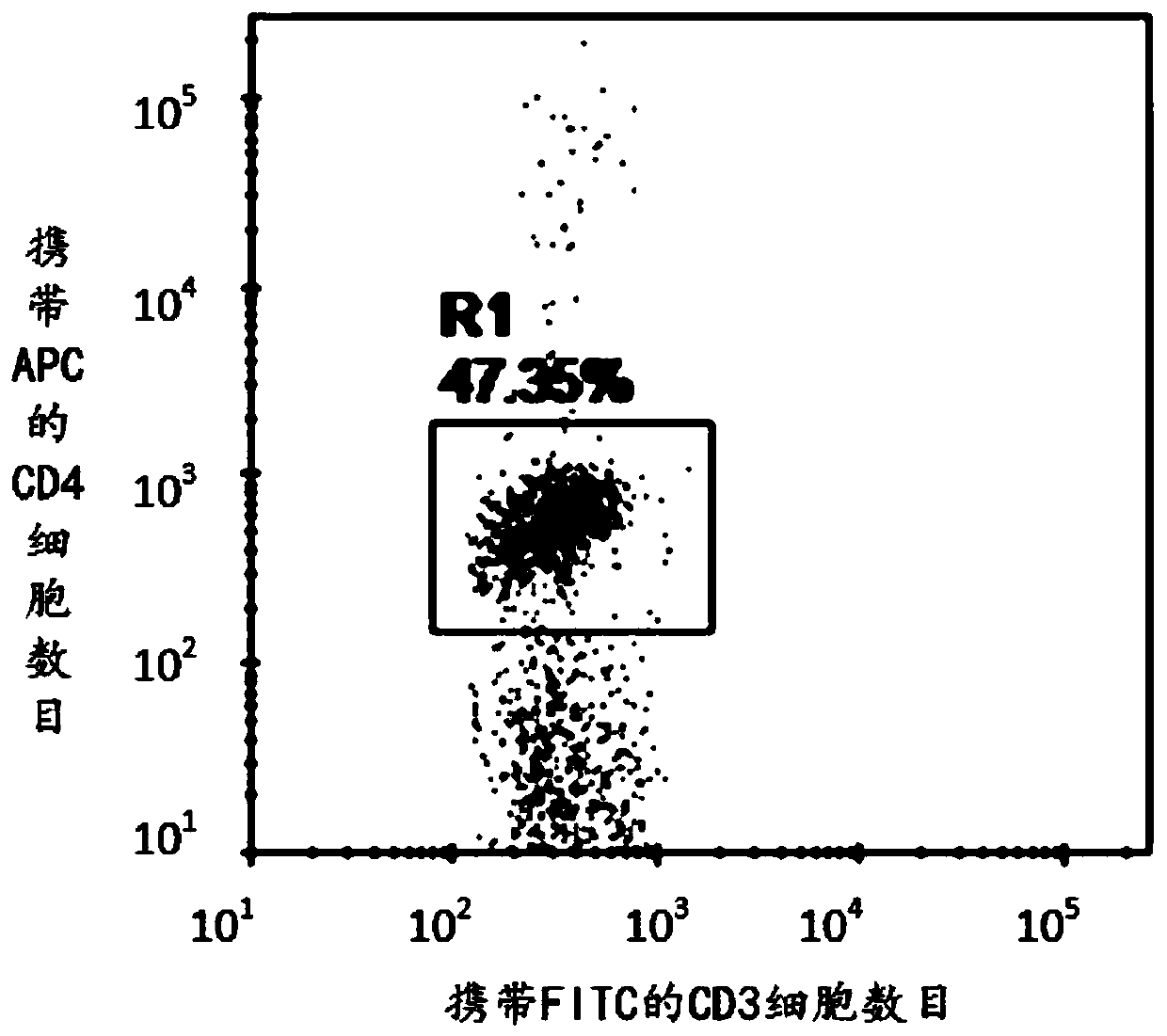
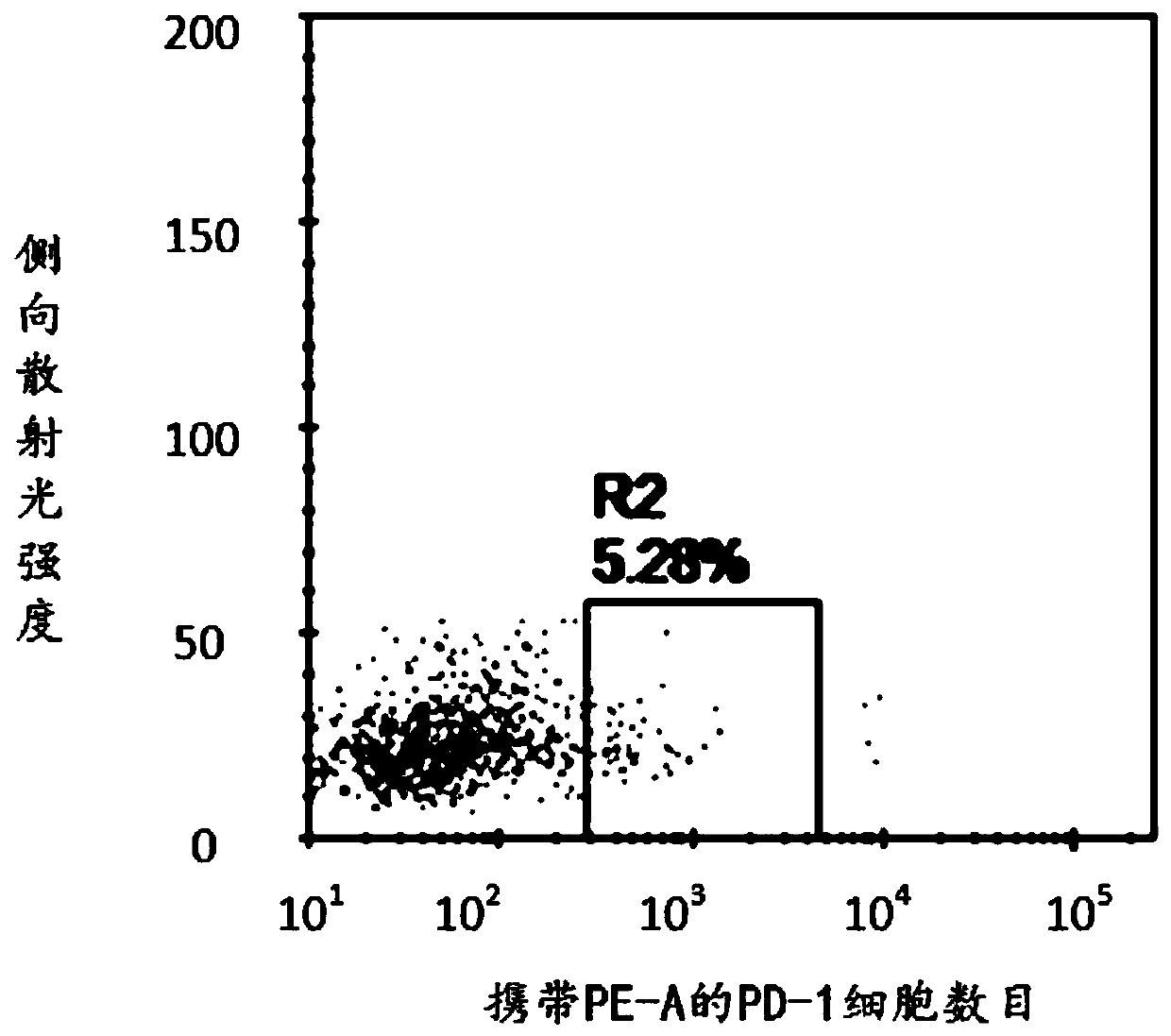
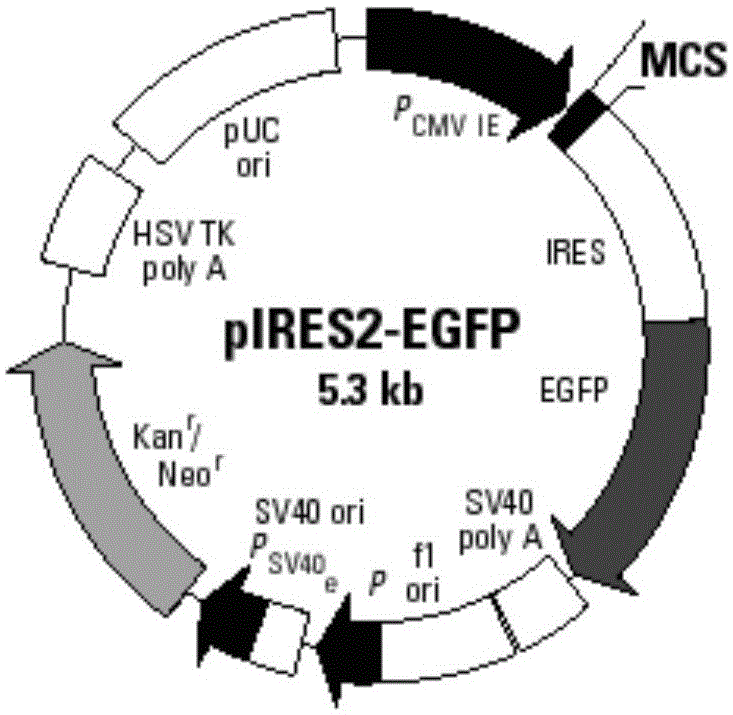
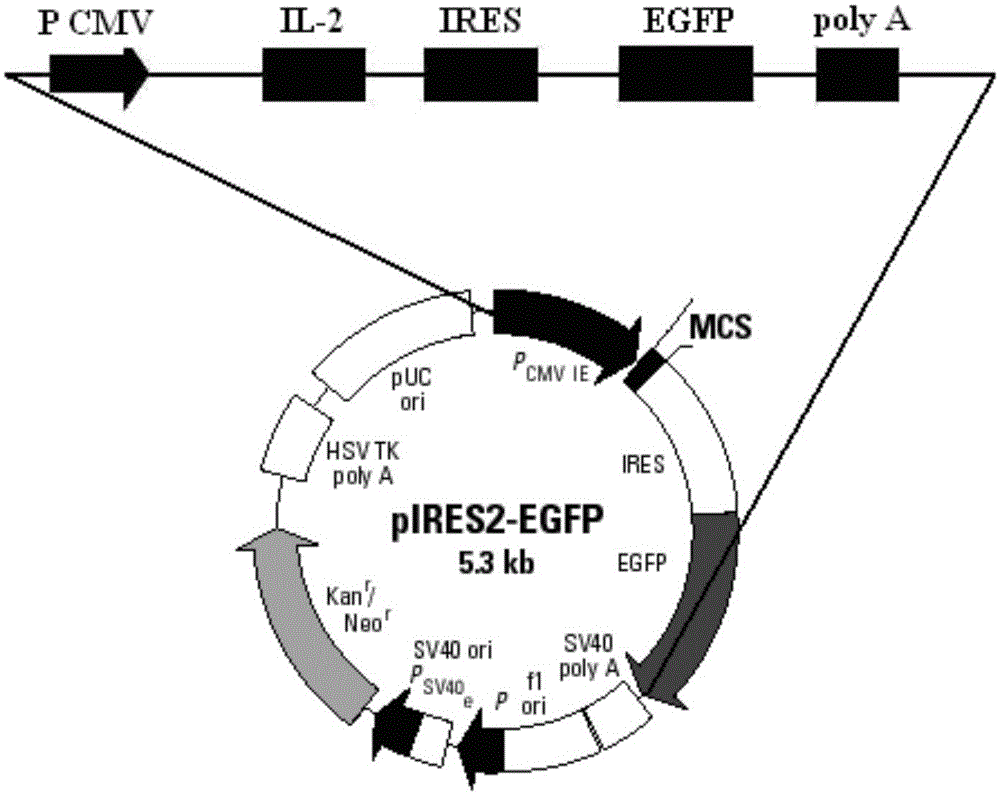
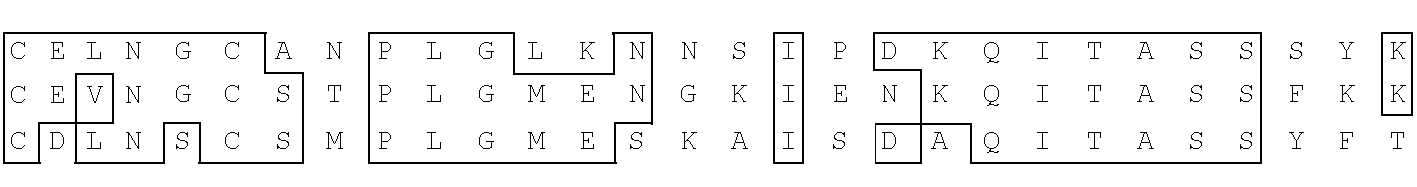Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Differentiation Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antigenic determinant located on a surface of certain lineage of cells during a particular developmental stage. It serves as an immunologic marker. DIFFERENTIATION ANTIGEN: "Monoclonal antibodies can be identified using distinct differentiation antigens.".
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:IDEC PHARM CORP
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
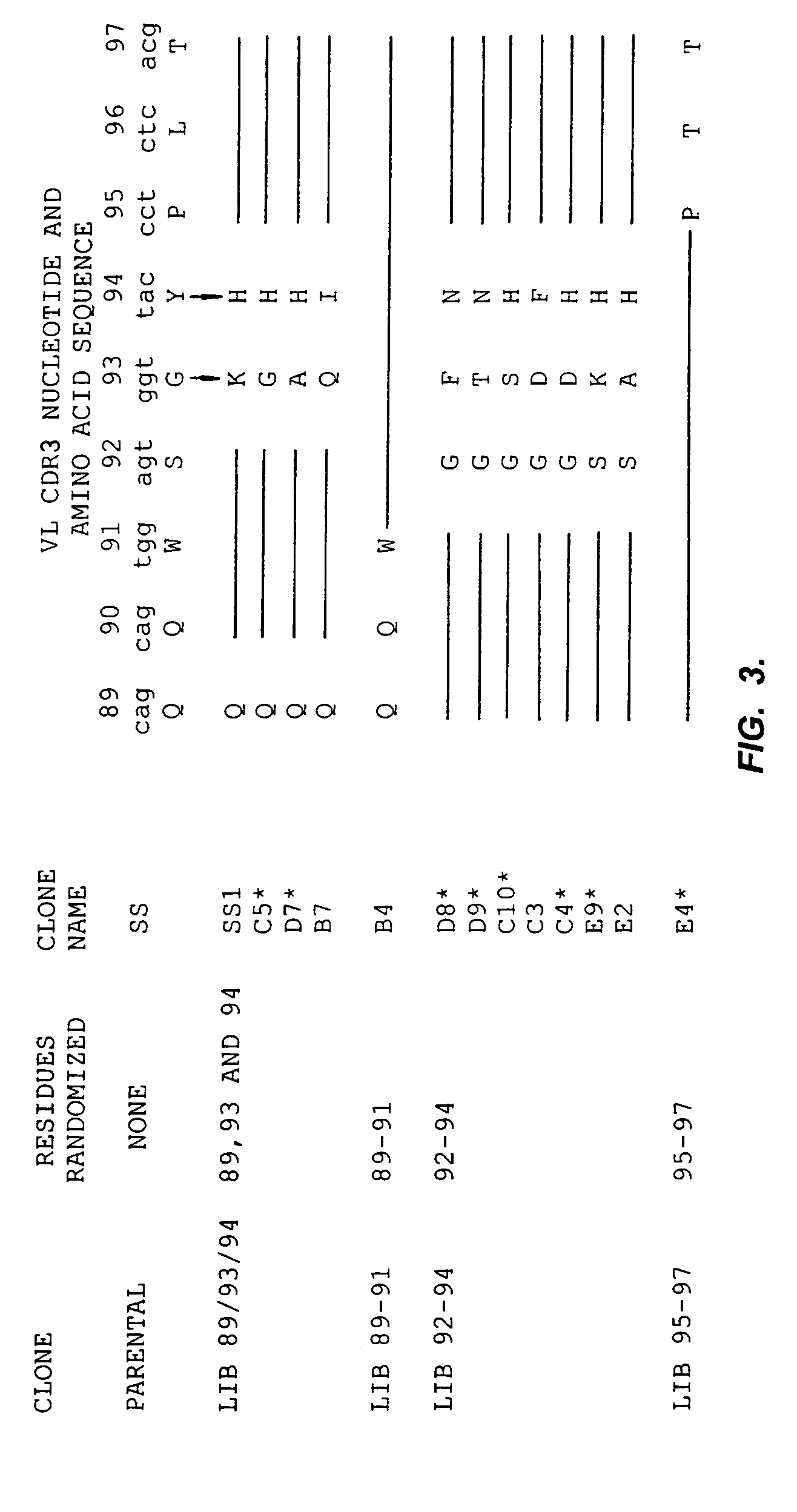
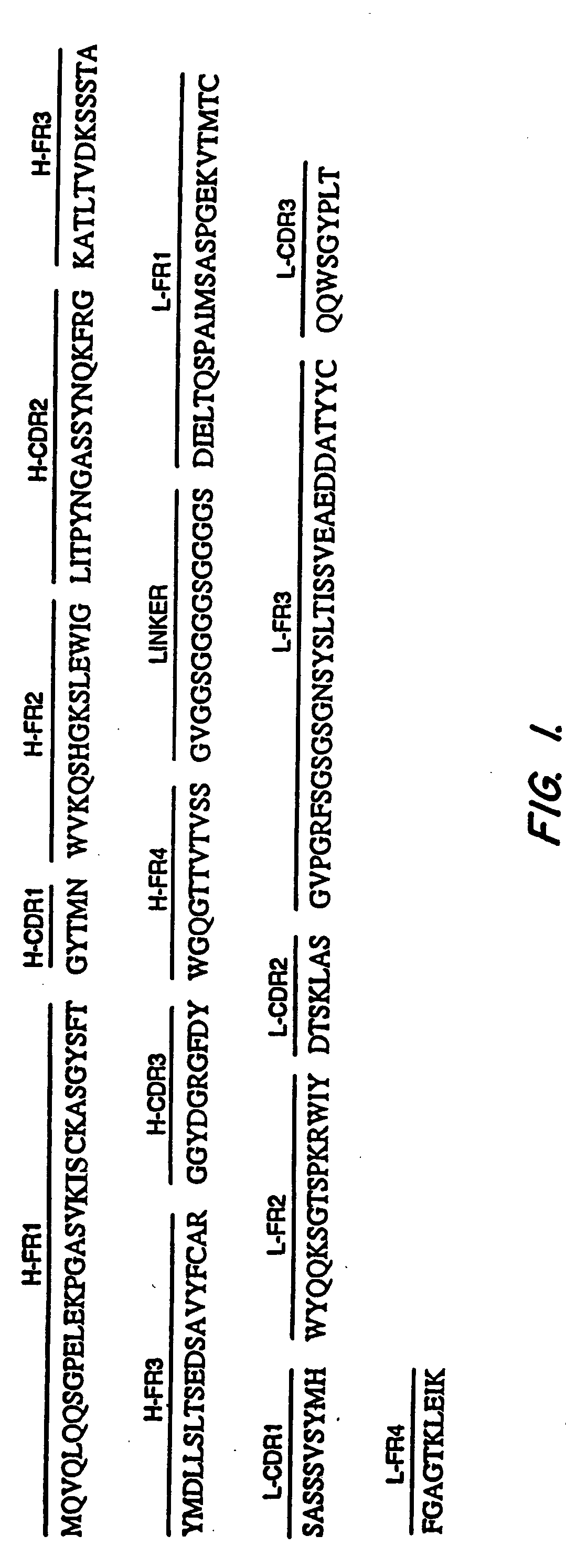
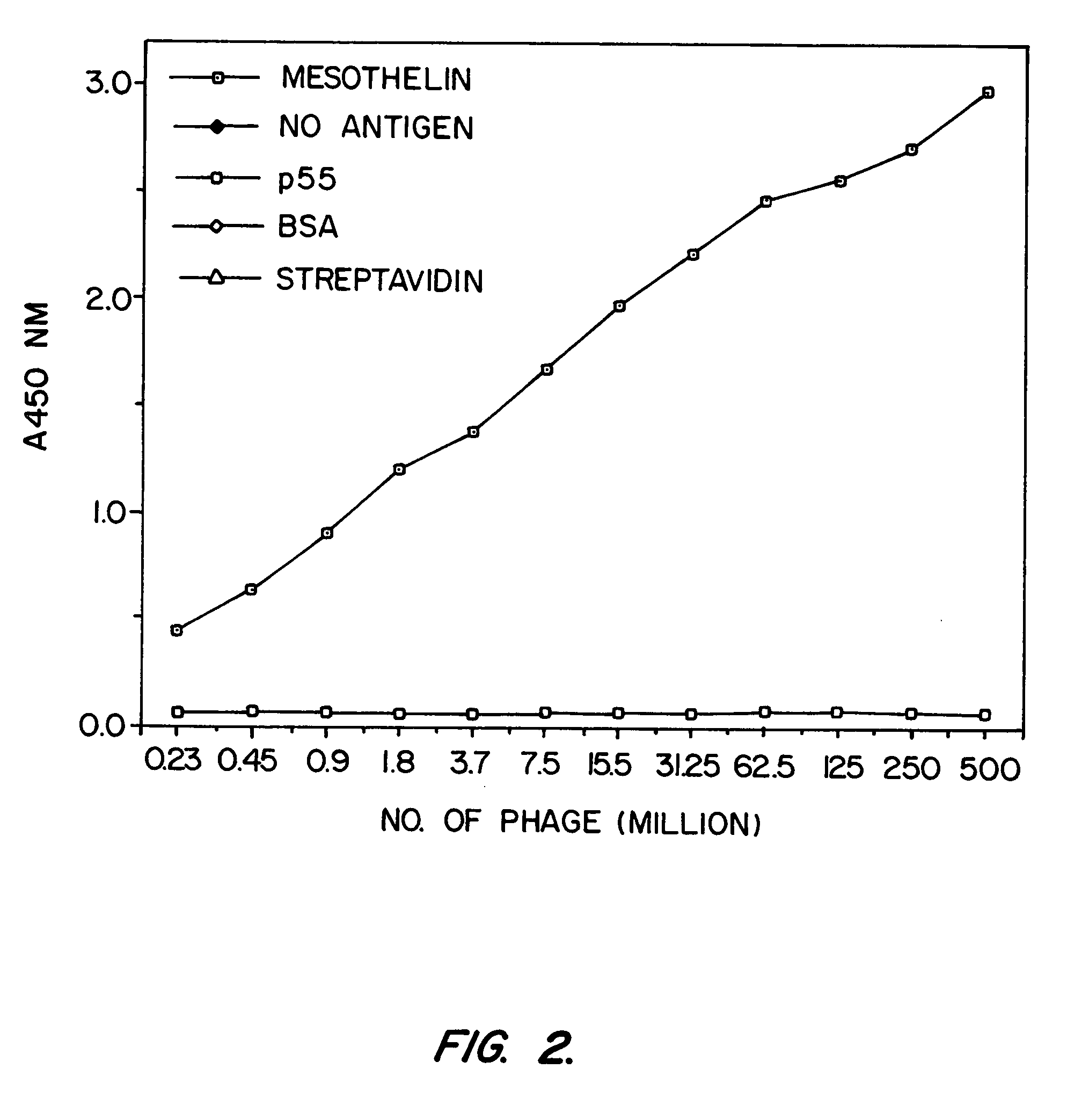
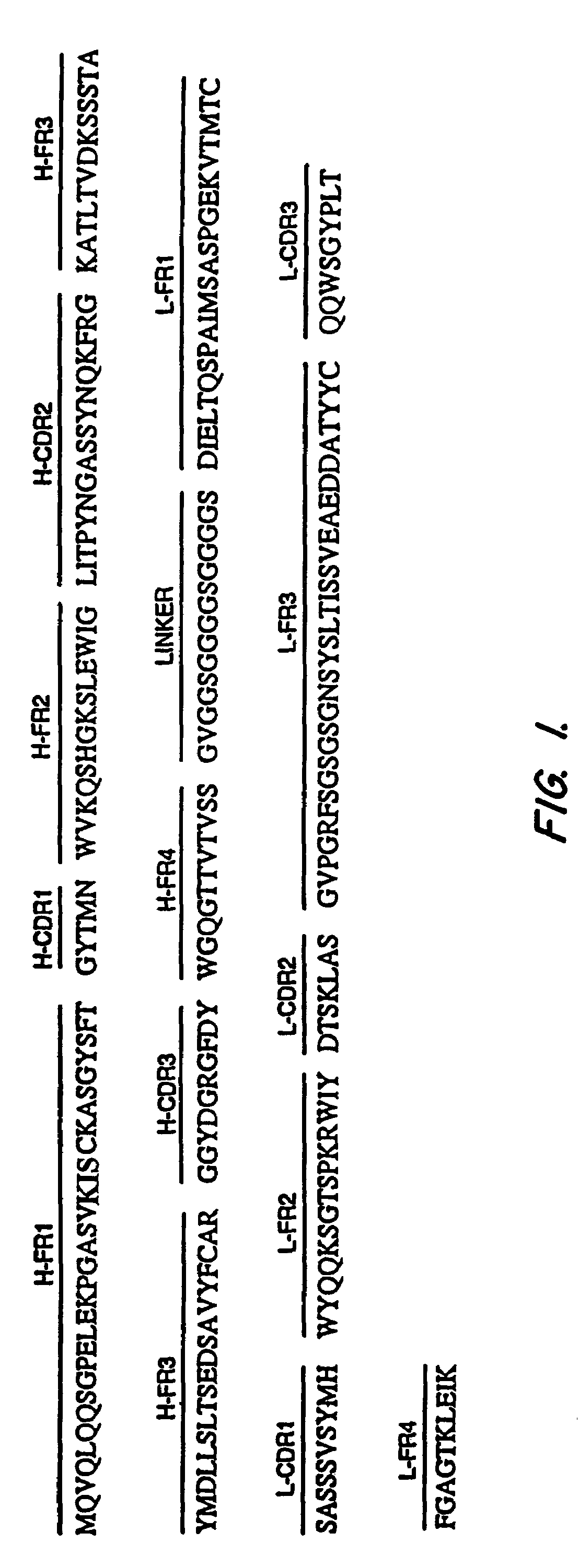
Anti-mesothelin antibodies having high binding affinity
InactiveUS7081518B1Peptide/protein ingredientsHybrid cell preparationAnti-Mesothelin AntibodyAntiendomysial antibodies
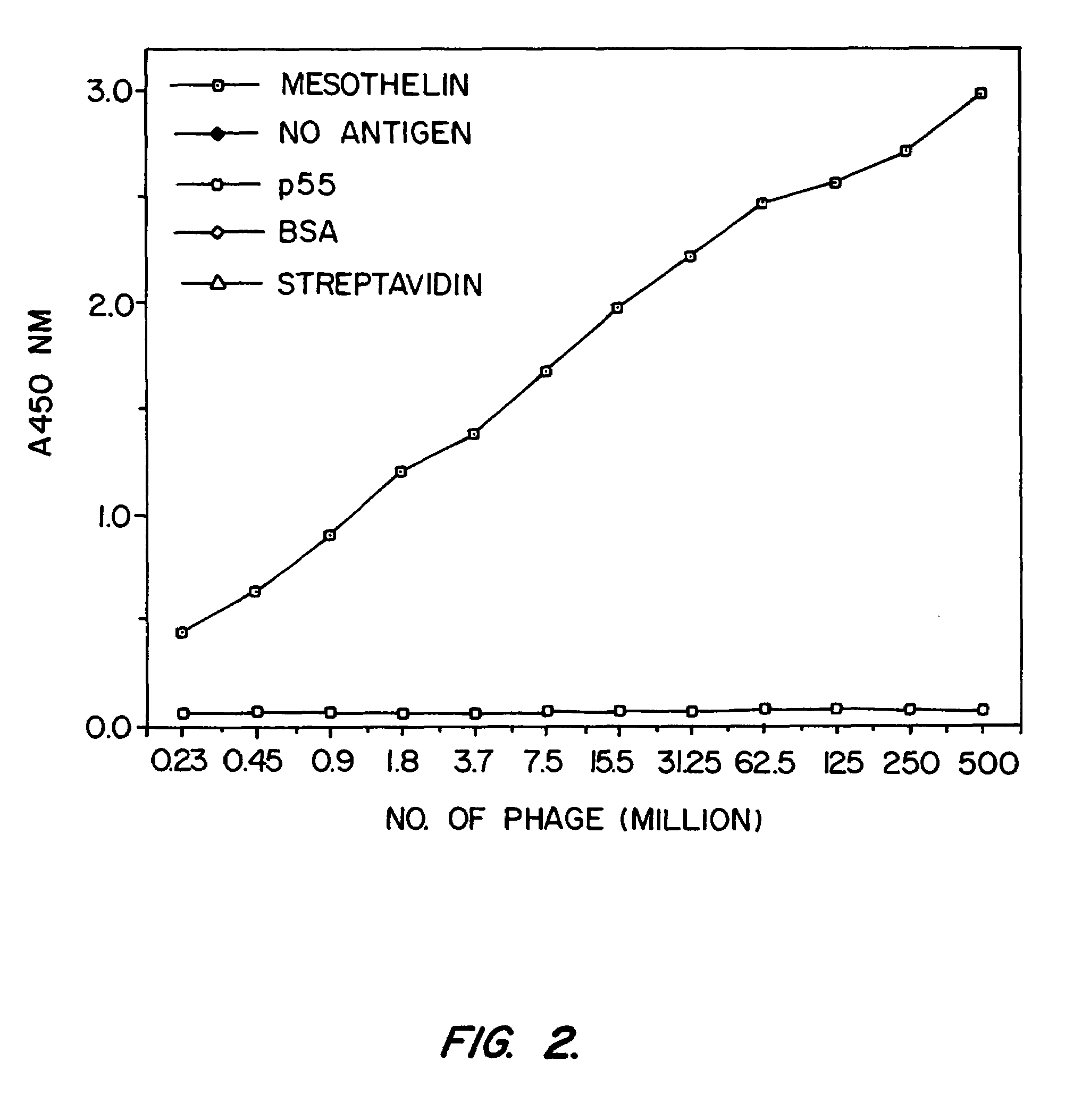
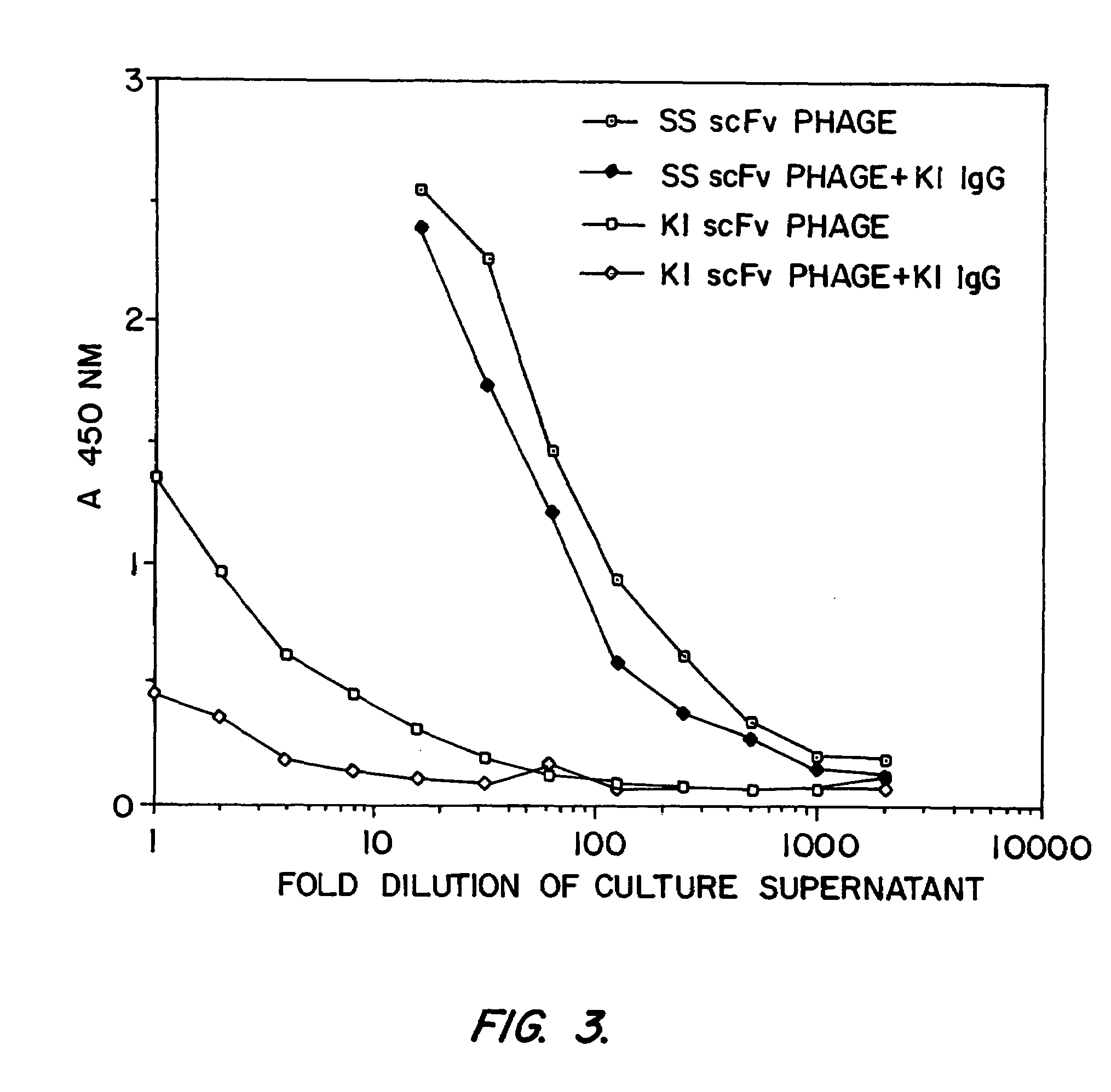
Mesothelin is a differentiation antigen present on the surface of ovarian cancers, mesotheliomas and several other types of human cancers. Because among normal tissues, mesothelin is only present on mesothelial cells, it represents a good target for antibody mediated delivery of cytotoxic agents. The present invention is directed to anti-mesothelin antibodies, including Fv molecules with particularly high affinity for mesothelin, and immunoconjugates employing them. Also described are diagnostic and therapeutic methods using the antibodies. The anti-mesothelin antibodies are well-suited for the diagnosis and treatment of cancers of the ovary, stomach, squamous cells, mesotheliomas and other malignant cells expressing mesothelin.
Owner:UNITED STATES OF AMERICA
Antibodies, including FV molecules, and immunoconjugates having high binding affinity for mesothelin and methods for their use
Mesothelin ins a differentiation antigen present on the surface of ovarian cancers, mesotheliomas and several other types of human cancers. Because among normal tissues, mesothelin is only present on mesothelial cells, it represents a good target for antibody mediated delivery of cytotoxic agents. The present invention is directed to anti-mesothelin antibodies, including Fv molecules with particularly high affinity for mesothelin, and immunoconjugates employing them. Also described are diagnostic and therapeutic methods using the antibodies. The anti-mesothelin antibodies are well-suited for the diagnosis and treatment of cancers of the ovary, stomach, squamous cells, mesotheliomas and other malignant cells expressing mesothelin.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Antibodies, including Fv molecules, and immunoconjugates having high binding affinity for mesothelin and methods for their use
Mesothelin ins a differentiation antigen present on the surface of ovarian cancers, mesotheliomas and several other types of human cancers. Because among normal tissues, mesothelin is only present on mesothelial cells, it represents a good target for antibody mediated delivery of cytotoxic agents. The present invention is directed to anti-mesothelin antibodies, including Fv molecules with particularly high affinity for mesothelin, and immunoconjugates employing them. Also described are diagnostic and therapeutic methods using the antibodies. The anti-mesothelin antibodies are well-suited for the diagnosis and treatment of cancers of the ovary, stomach, squamous cells, mesotheliomas and other malignant cells expressing mesothelin.
Owner:UNITED STATES OF AMERICA
Mesothelin, immunogenic peptides derived therefrom, and compositions comprising mesothelin, or immunogenic peptides thereof
InactiveUS7375183B1Tumor rejection antigen precursorsTumor specific antigensDifferentiation AntigensImmunogenic peptide
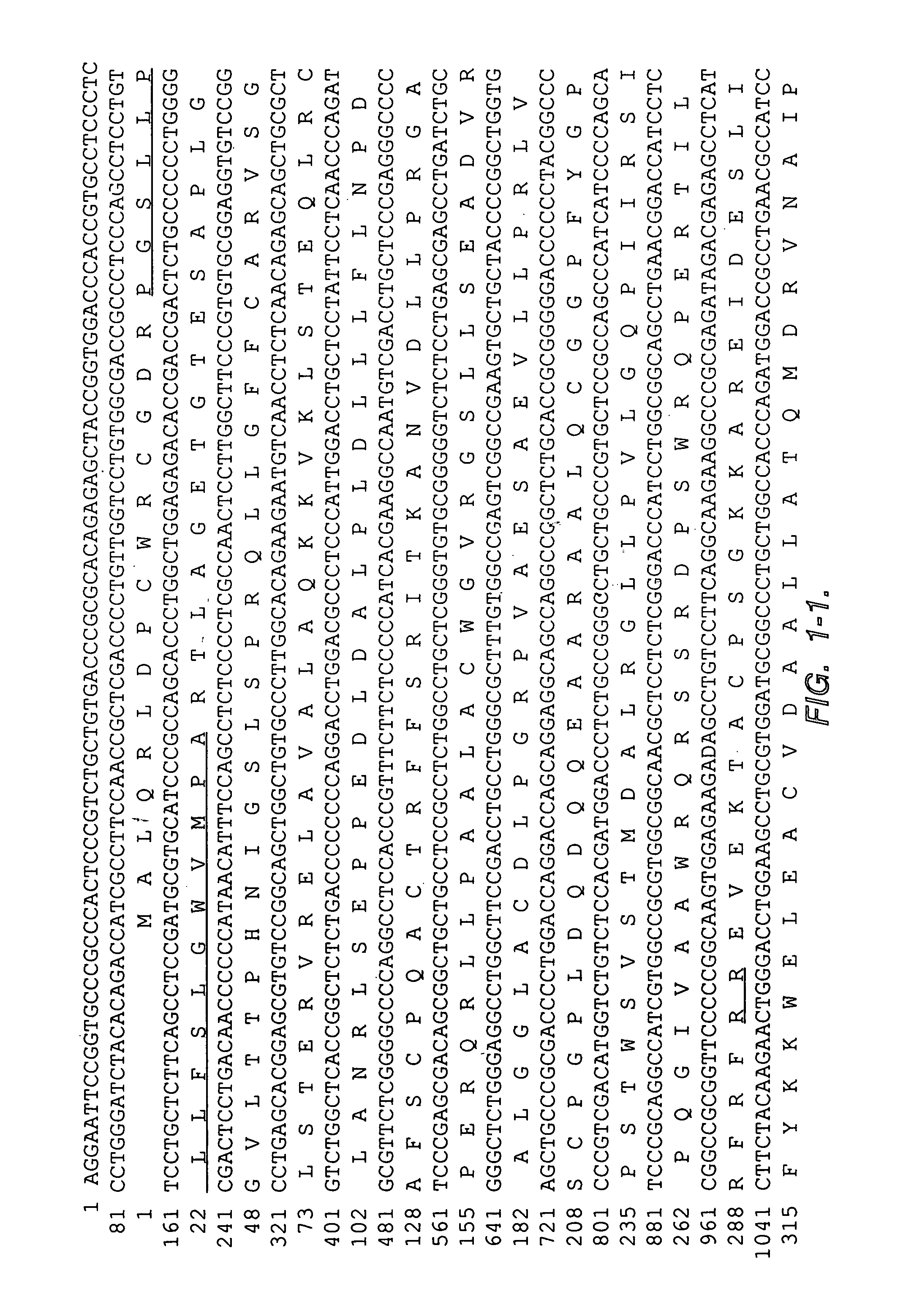
This invention relates to the discovery of a differentiation antigen termed mesothelin which is associated with mesotheliomas and ovarian cancers. Mesothelin is about 69 kD in its full-length form. The invention includes uses for the amino acid and nucleic acid sequences for mesothelin, recombinant cells expressing it, methods for targeting and / or inhibiting the growth of cells bearing mesothelin, methods for detecting the antigen and its expression level as an indication of the presence of tumor cells, and kits for such detection.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Methods and compositions to generate immunity in humans against self tumor antigens by immunization with homologous foreign proteins
InactiveUS6942862B2Enhancing and eliciting immune responsePeptide/protein ingredientsBiological material analysisForeign proteinDifferentiation Antigens
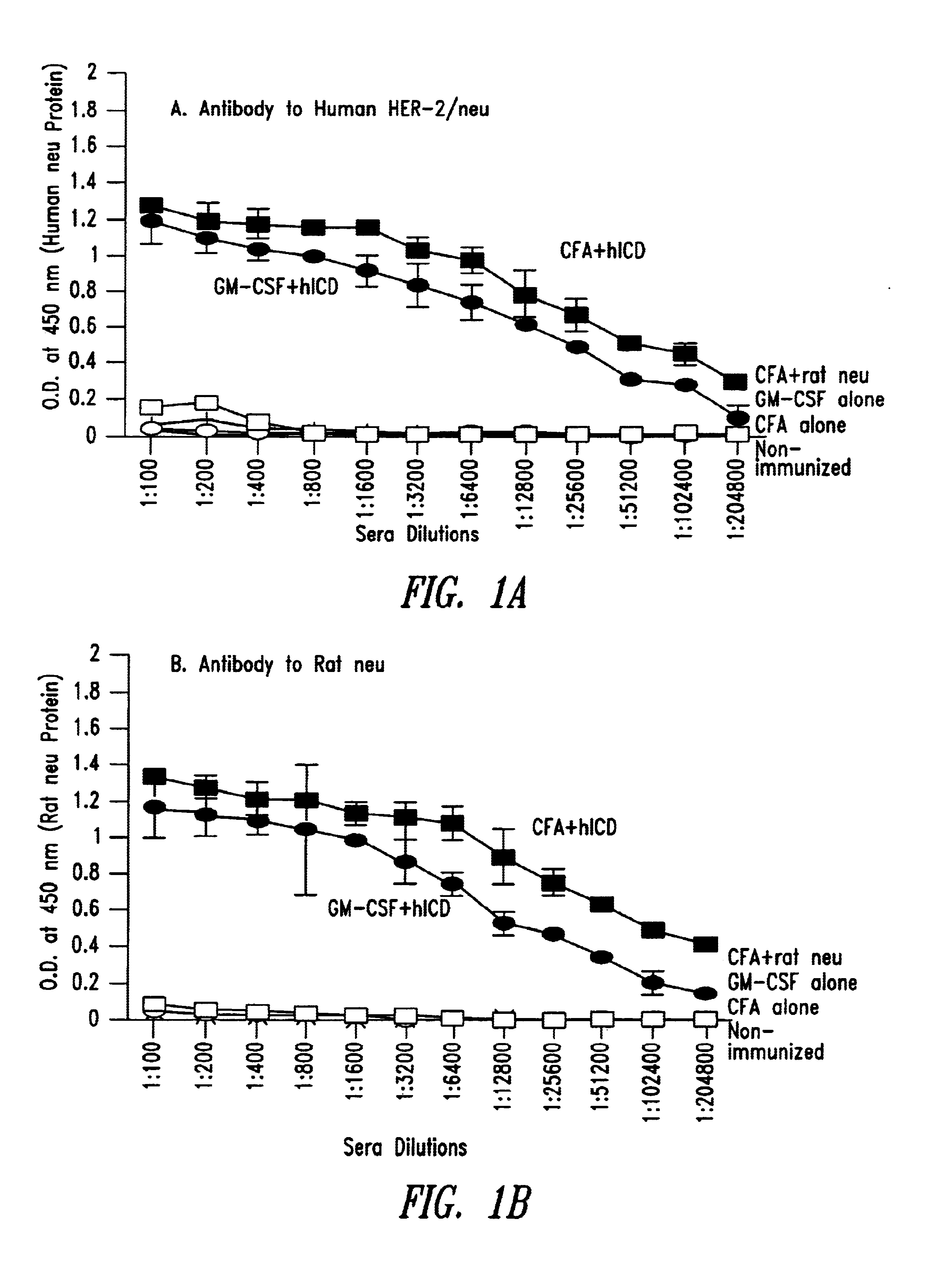
Methods and compositions to elicit or enhance immunity in humans against self tumor antigens are disclosed. Such immunity is generated by immunization with homologous foreign proteins. Self tumor antigens include protein expression products of overexpressed human oncogenes, such as human HER-2 / neu protein, and organ-specific or tissue-specific differentiation antigens, such as PAP or PSA, associated with tumor cells.
Owner:WASHINGTON OF UNIV
Monoclonal antibody against CK8 protein and cell strain, preparation method and application thereof
ActiveCN112940118AStrong specificityIncreased sensitivityImmunoglobulins against animals/humansBiological material analysisAntigenWhite blood cell
The invention relates to a monoclonal antibody capable of recognizing a human leukocyte differentiation antigen CK8, a secretory cell strain, a preparation method thereof and application in immunodetection. According to the technical scheme, 340-365 amino acid sequences at the C tail end of CK8 protein are selected as antigen peptides, and the antigen peptides are coupled with KLH carrier protein to obtain the immunogen; the amino acid sequence of the antigen peptide is shown as SEQID1, a mouse is immunized, and the mouse hybridoma cell strain 14C2 capable of efficiently secreting the anti-CK8 protein monoclonal antibody and the anti-CK8 protein monoclonal antibody secreted by the cell strain are obtained through cell fusion, screening and subcloning. The antibody obtained by the scheme has high specificity and sensitivity, can specifically recognize cells expressing the CK8 protein, and is suitable for immunological detection, especially immunohistochemical detection.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Pharmaceutical composition comprising an Anti-cd6 monoclonal antibody used in the diagnosis and treatment of rheumatoid arthritis
The present invention is related to the branch of immunology and particularly with the generation of pharmaceutical compositions containing a humanized monoclonal antibody recognizing the leukocyte differentiation antigen CD6. Accordingly with that statement, the purpose of this invention is to provide pharmaceutical compositions which contain a humanized anti-CD6 monoclonal antibody for the diagnosis and treatment of Autoimmune Diseases, particularly the Rheumatoid Arthritis.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Detection kit for minute residues of B-cell acute lymphocyte leukemia
InactiveCN109884313AImprove the detection rateReduce missed detection rateBiological testingCD20Leukocyte Differentiation
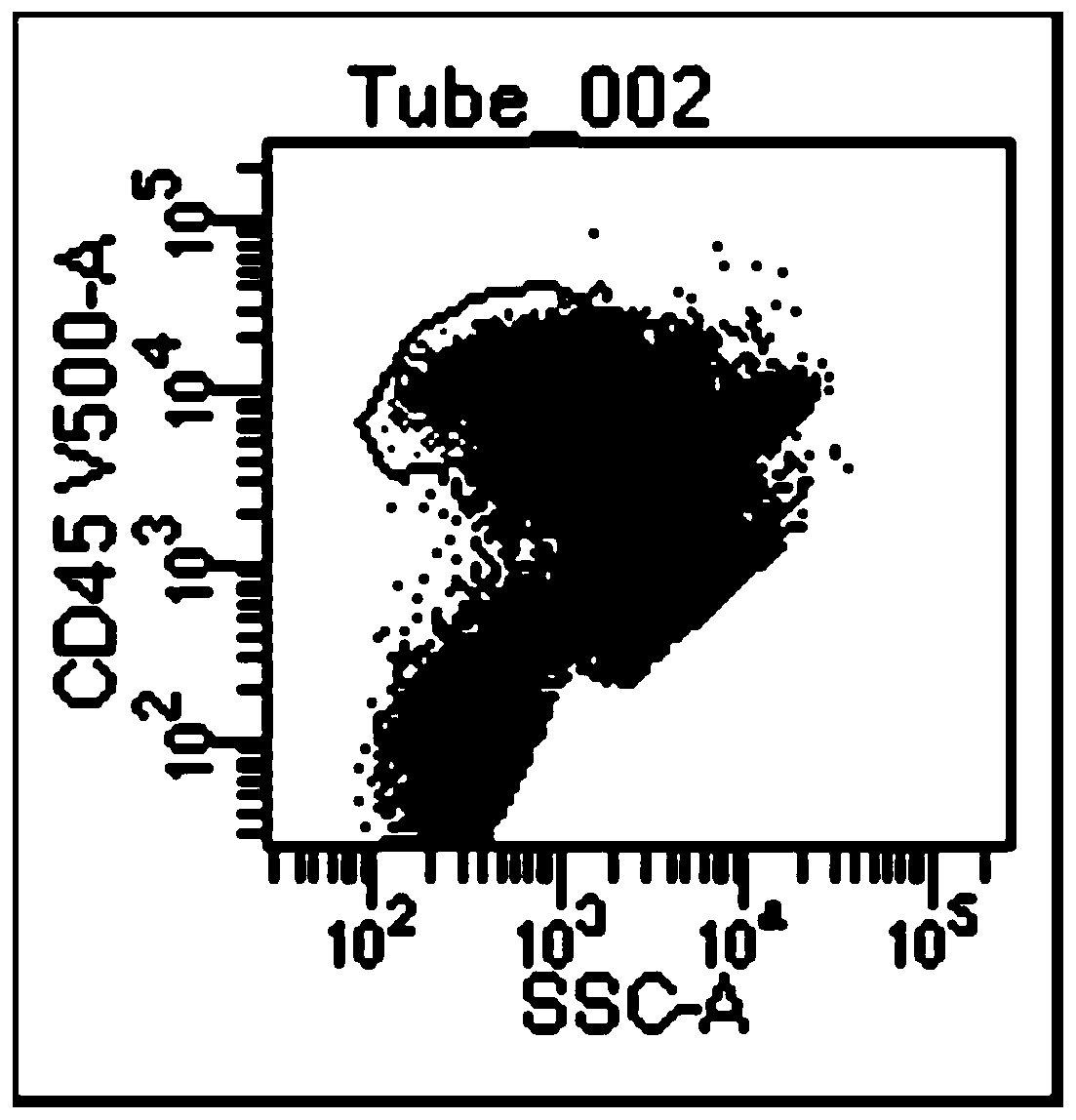
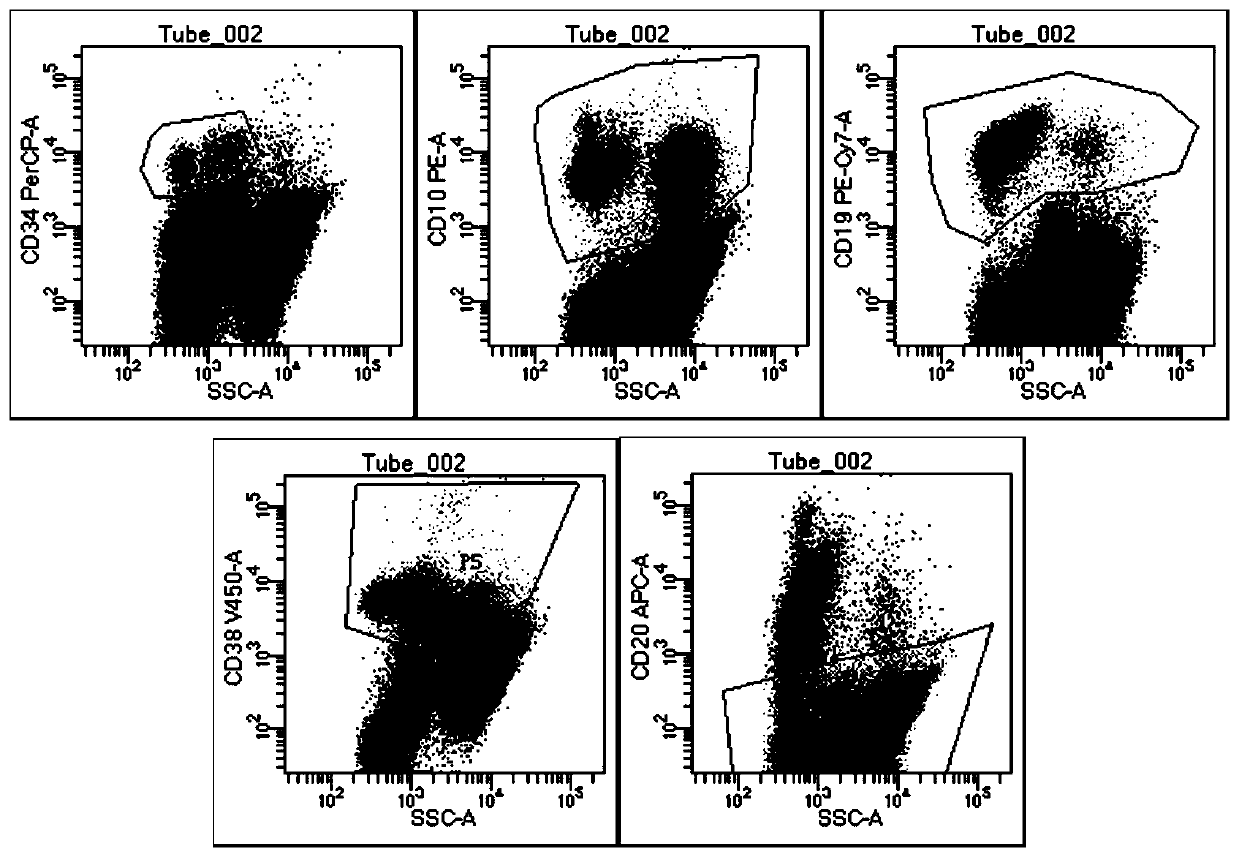
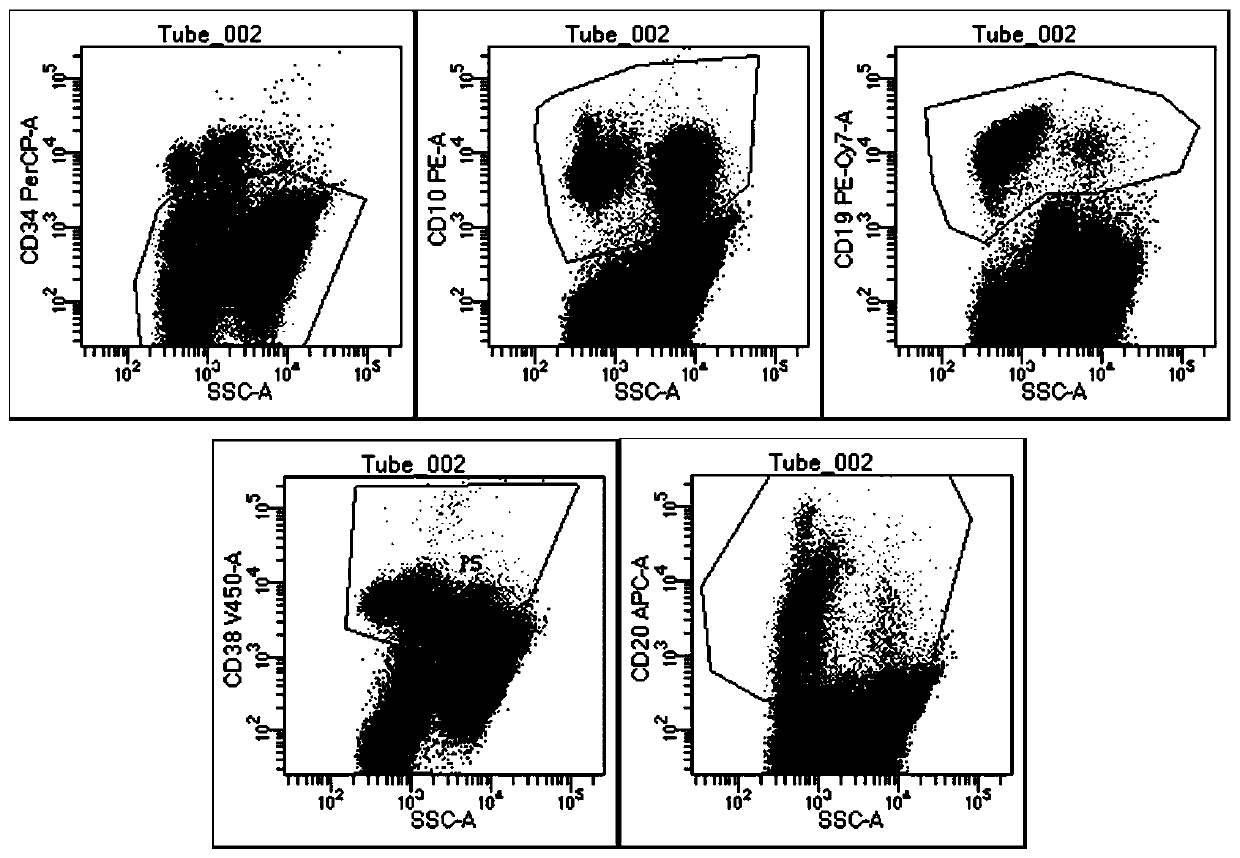
The invention relates to the technical field of medical detection, in particular to a detection kit for minute residues of B-cell acute lymphocyte leukemia. The detection kit comprises anti CD10, CD19, CD20, CD34, CD38 and CD45 antibodies labeled by different fluoresceins. The detection kit comprises six monoclonal antibody combinations of leukocyte differentiation antigen, and a fluorescence labeling and flow cytometric detection technology is combined to realize that one-time flow cytometric detection can quickly and accurately identify the level and typing of abnormal cells of the minute residues of the B-cell acute lymphocyte leukemia, the obtained detection index can be used as an intermediate result to intuitively judge the prognosis conditions of B-cell acute lymphocyte leukemia patients, and thus important guiding significance is provided for the formulation of clinical treatment schemes for the leukemia patients.
Owner:武汉康圣达医学检验所有限公司
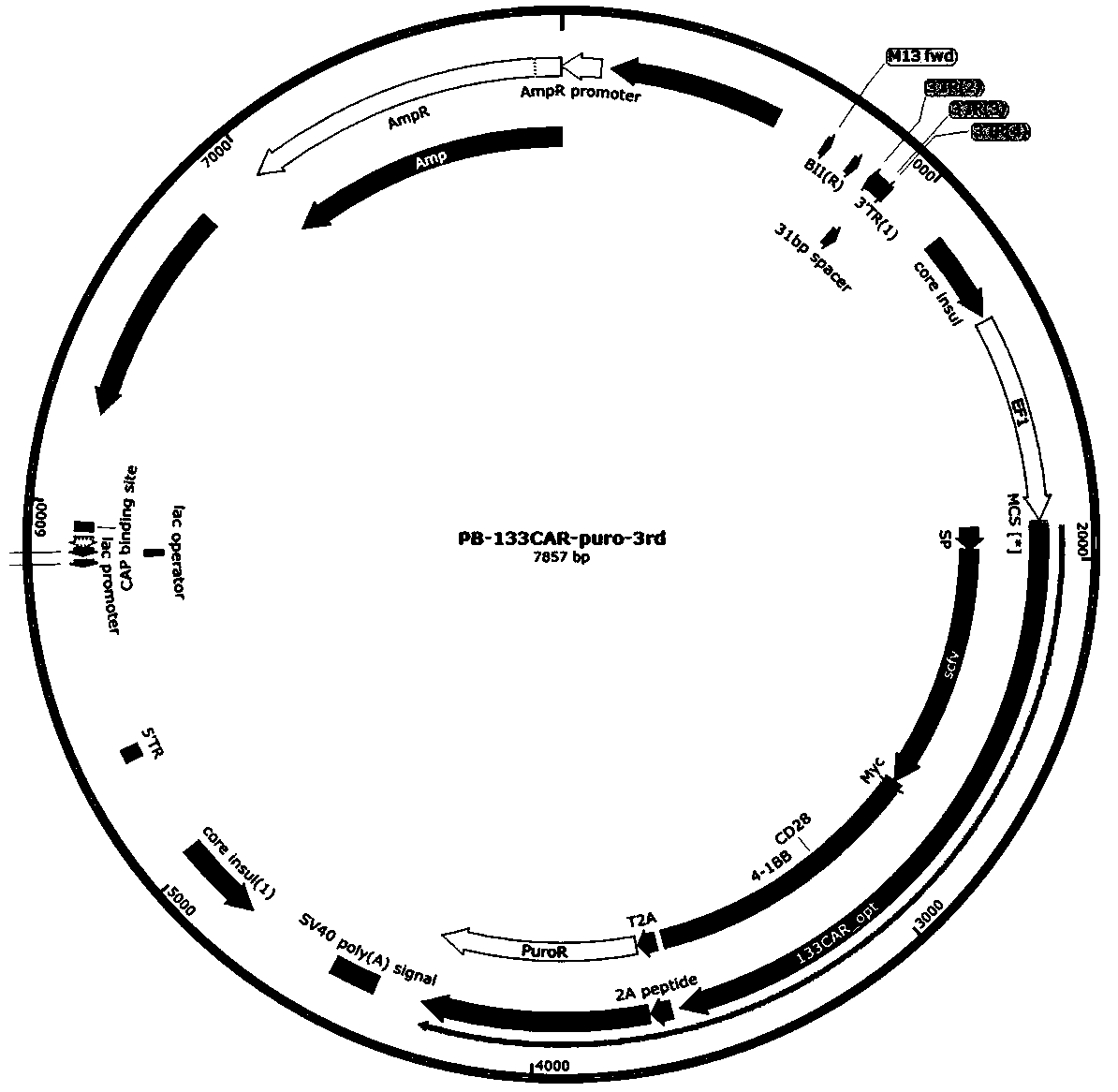
Preparation method and application of CAR-T cell based on base editing target CD133
InactiveCN109652381ASimple preparation processHigh activityImmunoglobulin superfamilyGenetically modified cellsAntigenLeukocyte Differentiation
The invention discloses a preparation method and application of a CAR-T cell based on PD-1 gene knock-out and base editing target CD133. The method comprises the steps that gene knocking out is conducted through a BE-Plus system while a plasmid vector expressing a leukocyte differentiation antigen CD133-CAR is guided into a T cell, and the obtained CAR-T cell can be applied to the preparation of anti-tumor drugs. The method has the advantages that the preparation technology is simple, and the method has higher application value in treating tumor cells.
Owner:SUZHOU MAXIMUM BIO TECH CO LTD
Compositions for treatment of melanoma and method of using same
InactiveUS7556805B2Overcome toleranceEffective immunityBiocideBacteriaDifferentiation AntigensMelanoma
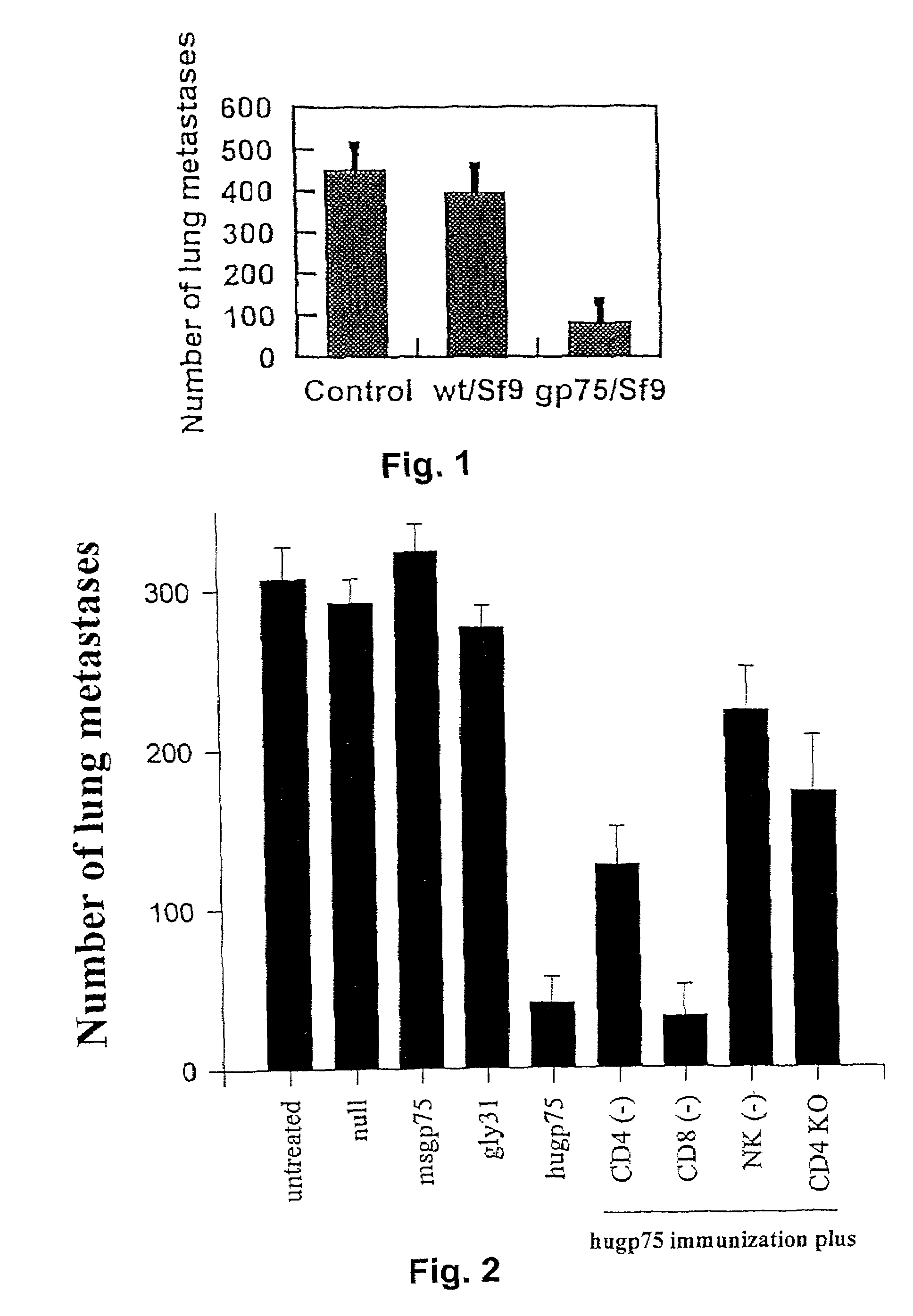
Melanoma can be treated in a mammalian subject by administering to the subject an immunologically-effective amount of a xenogeneic melanoma-associated differentiation antigen. For example, genetic immunization with a plasmid containing a sequence encoding human gp75 has been shown to be effective in treatment of dogs with melanoma.
Owner:ANIMAL MEDICAL CENT THE
Double-antibody combined fluorescence detection kit for liver cancer resection postoperative prognosis
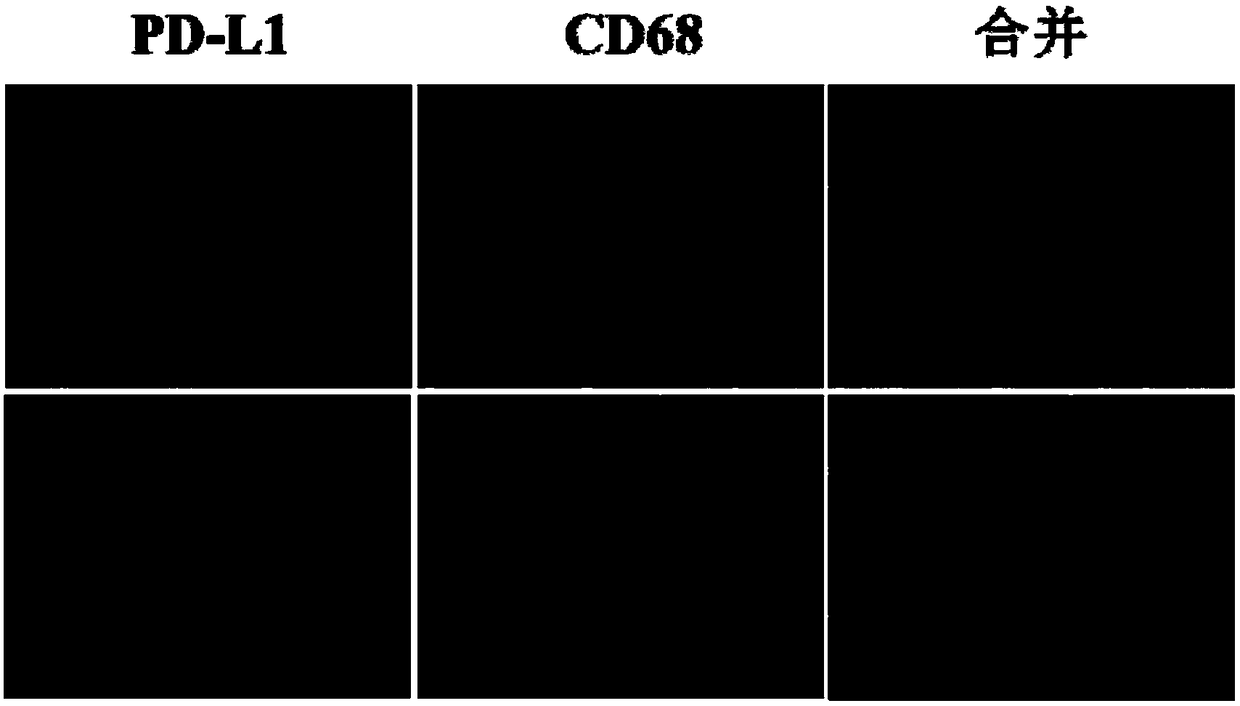
The invention relates to a double-antibody combined fluorescence detection kit for liver cancer resection postoperative prognosis. The double-antibody combined fluorescence detection kit for liver cancer resection postoperative prognosis comprises a first antibody and a second antibody, wherein the first antibody is selected from the antibody combination of the following protein molecular markers:CD68 (cell differentiation antigen 68) and PD-L1 (procedural death ligand 1). The kit uses double-antibody combination indexes; the expression mode of the PD-L1 in tissues can be precisely reflected,so that the prognosis of a patient is better evaluated; the kit can help doctors to select the proper treatment user groups and modes, so that the powerful support is provided for effective diagnosisand treatment.
Owner:SUN YAT SEN UNIV CANCER CENT
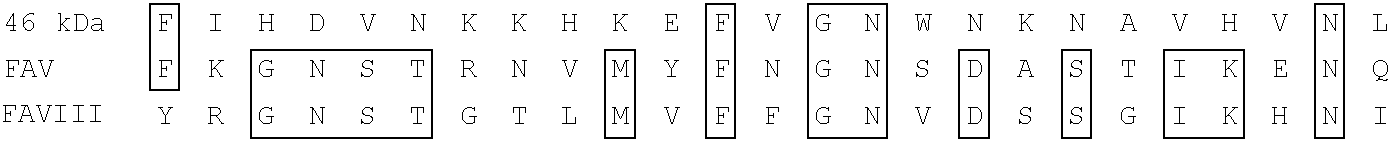
Antibody with 46 kdalton HMFG antigen binding specificity, immunoassay kit and diagnostic method
InactiveUS6939677B1Peptide/protein ingredientsAntibody mimetics/scaffoldsImmune profilingVaccination
A polypeptide has the antibody binding activity of the 46 Kdalton HMFG differentiation antigen and / or homology to at least one of the light chains of clotting factors V and VIII and is also provided as a fusion protein with a second antigenic polypeptide. An antibody has affinity for the polypeptide of the invention or a functional fragment thereof in vivo and in vitro methods for therapy vaccination and detecting the presence of the polypeptide, the antibody, the DNA and RNA of the invention are provided. DNA and RNA sequences encode the polypeptide of the invention or fragments thereof and immunoassay kits comprise the antibodies and / or polypeptides of the invention.
Owner:CANCER RES FUND OF CONTRA COSTA
Viral vector complexes having adapters of predefined valence
The invention concerns an improvement in the art of inserting and expressing foreign gene into eukaryotic cells. The invention particularly concerns methods and compositions whereby viral vectors can be used to insert and express foreign genes into specifically cells having particular differentiation antigens. A method of determining which differentiation antigens can be used is taught. The invention encompasses complexes of viral particles and adapters that cause the binding and internalization of the vector particles such that a gene of interest in the particle is expressed.
Owner:NEW YORK UNIV
Mesothelin, A Differentiation Antigen Present On Mesothelium, Mesotheliomas, and Ovarian Cancers and Methods and Kits for Targeting the Antigen
This invention relates to the discovery of a differentiation antigen termed mesothelin which is associated with mesotheliomas and ovarian cancers. Mesothelin is about 69 kD in its full-length form. The invention includes uses for the amino acid and nucleic acid sequences for mesothelin, recombinant cells expressing it, methods for targeting and / or inhibiting the growth of cells bearing mesothelin, methods for detecting the antigen and its expression level as an indication of the presence of tumor cells, and kits for such detection.
Owner:THE GOVT OF THE U S A AD REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
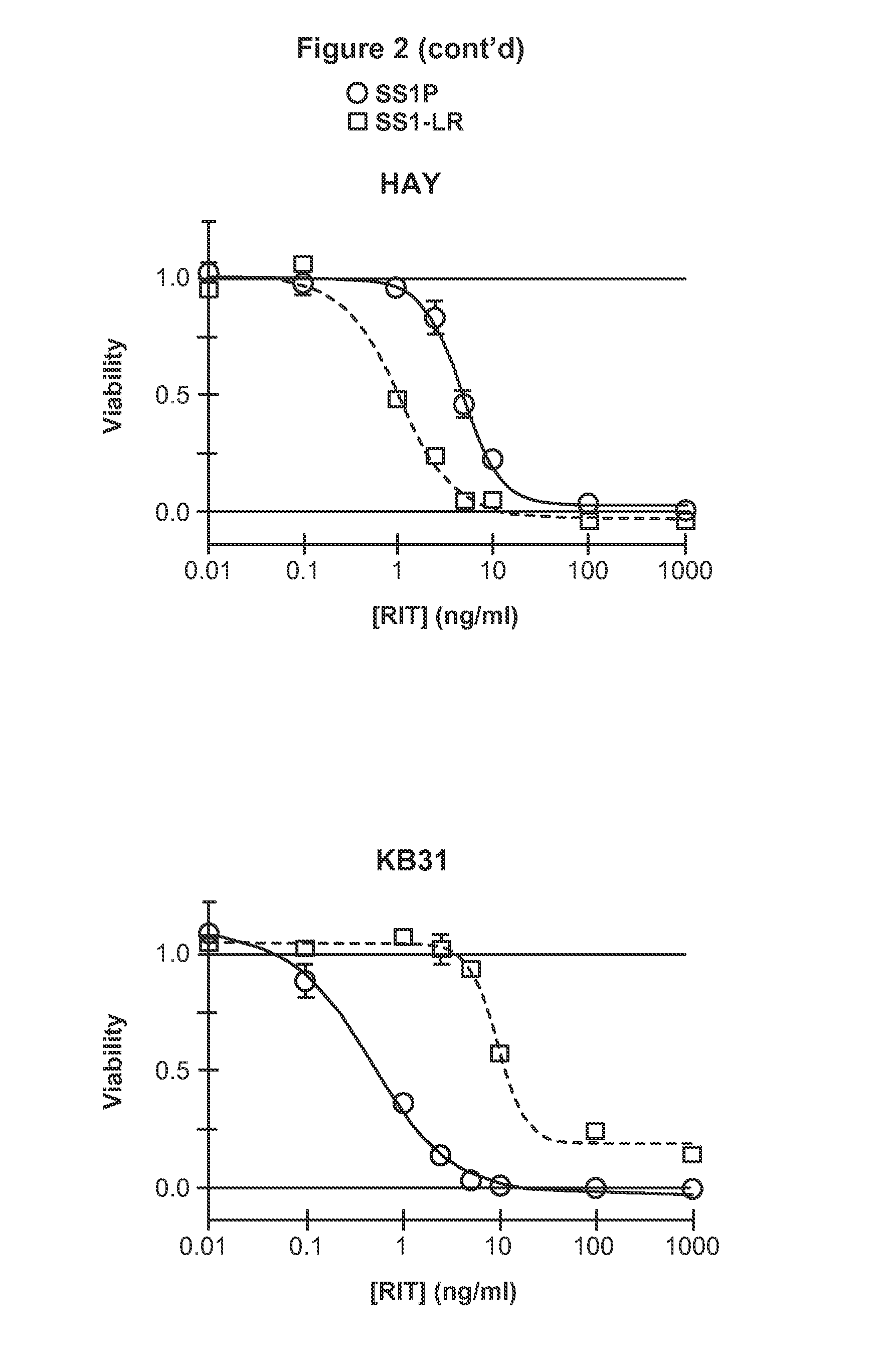
Recombinant immunotoxin targeting mesothelin
ActiveUS20140154248A1Low immunogenicityHigh cytotoxic activityBacteriaAntibody mimetics/scaffoldsAntiendomysial antibodiesAnti-Mesothelin Antibody
Mesothelin is a differentiation antigen present on the surface of ovarian cancers, mesotheliomas and several other types of human cancers. Because among normal tissues, mesothelin is only present on mesothelial cells, it represents a good target for antibody mediated delivery of cytotoxic agents. The present invention is directed to improved recombinant immunotoxins comprising anti-mesothelin antibodies, including Fv molecules with particularly high affinity for mesothelin, and a Pseudomonas Exotoxin moiety which has been modified to reduce its immunogenicity and protease sensitivity and providing a better cytotoxicity for cells which express mesothelin. The RITs are well-suited for the treatment of cancers of the ovary, stomach, squamous cells, mesotheliomas and other malignant cells expressing mesothelin.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
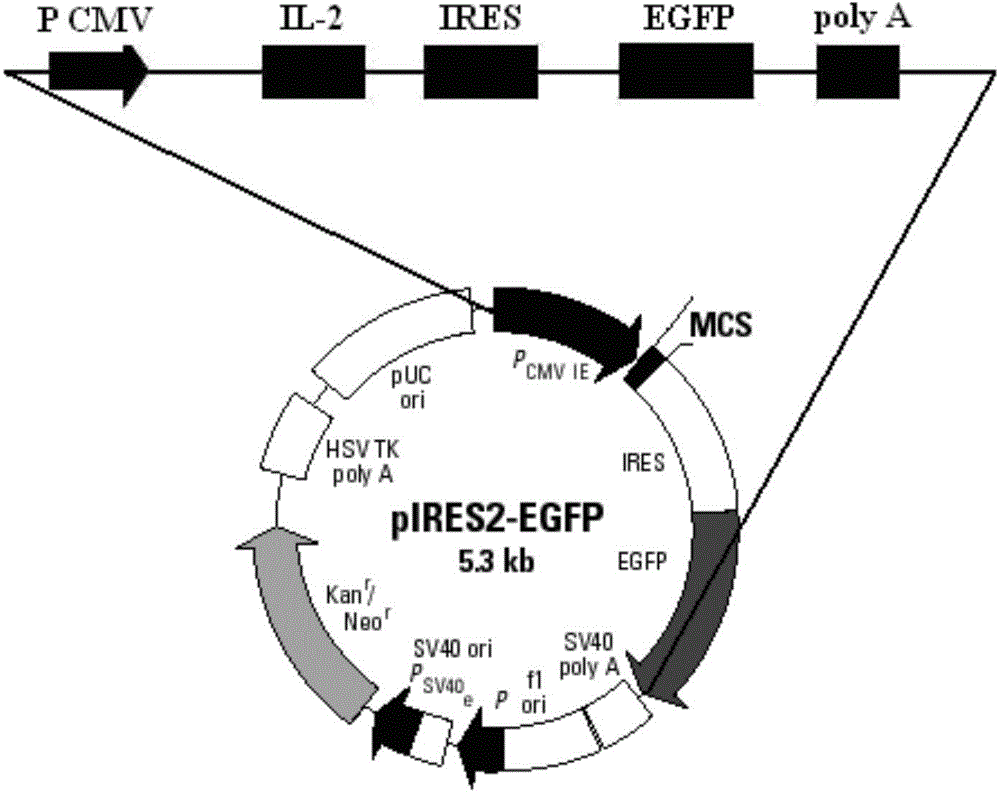
IL-2 and MART-1 dual-gene co-expression recombinant vector as well as preparation method and application of recombinant vector
ActiveCN103555763AGood immunotherapy effectReduce the introductionGenetic material ingredientsAntineoplastic agentsAntigenDifferentiation Antigens
The invention discloses an IL-2 and MART-1 dual-gene co-expression recombinant vector, which is sequentially linked with an IL-2 gene, an IRES sequence and an MART-1 gene in a vector transcription direction, or is sequentially linked with an MART-1 gene, an IRES sequence and an IL-2 gene in a vector transcription direction, wherein nucleotide sequence of the IL-2 gene is represented in SEQ ID NO: 1 in a sequence list, nucleotide sequence of the MART-1 gene is represented in SEQ ID NO: 2 in the sequence list, and nucleotide sequence of the IRES sequence is represented in SEQ ID NO: 3 in the sequence list. The dual-gene co-expression recombinant vector, which links the IL-2 gene and the MART-1 gene through the IRES sequence, can simultaneously express a human melanin differentiation antigen and interleukin-2; the recombinant vector, in immunogene therapy of melanin, not only can develop an immune regulating function of a cell factor but also can generate a specific anti-tumor effect aiming at the melanin.
Owner:XINXIANG MEDICAL UNIV
Pharmaceutical composition for inducing an immune response in a human or animal
InactiveUS20090029457A1Mammal material medical ingredientsArtificial cell constructsDendritic cellDifferentiation Antigens
The present invention relates to a pharmaceutical composition for inducing an immune response in a human or animal, comprising dendritic cells loaded with at least five cancer / testis antigen and no lineage specific differentiation antigens or substantially no lineage specific differentiation antigens provided from at least one cancer cell line, as well as to isolated cell lines expressing a multiplicity of cancer testis antigens and no differentiation antigens, and to a method of inducing an immune response in a human or animal using the composition of the invention.
Owner:DANDRIT BIOTECH AS
Separate culture method for human amniotic mesenchymal stem cells
PendingCN106676063APromote proliferationHigh cell puritySkeletal/connective tissue cellsEmbryonic cellsAntigenWhite blood cell
The invention discloses a separate culture method for human amniotic mesenchymal stem cells, and belongs to the technical field of biology. The separate culture method comprises the following steps: (I) cell separation; (II) cell culture: inoculating a single separated human amniotic karyocyte into a plastic culture bottle of 75cm<2>, culturing in a 5 percent CO2 incubator at the temperature 37 DEG C, wherein a culture solution is a culture medium special for the human amniotic mesenchymal stem cell; replacing the culture solution 24 hours later, discarding non-adherent cells, and replacing the culture solution every 2 to 3 days; fusing the cells after the human amniotic mesenchymal stem cells grow to 70 to 80 percent, digesting with pancreatin, and performing passage; performing passage once in the culture medium special for the human amniotic mesenchymal stem cells every 2 to 3 days at the temperature of 37 DEG C and in 5 percent CO2. The obtained human amniotic mesenchymal stem cells express by the following three kinds of cell membrane molecules, namely, a human leukocyte differentiation antigen CD73, a human leukocyte differentiation antigen CD90 and a human leukocyte differentiation antigen CD105, and do not express a human leukocyte differentiation antigen CD45 and a human leucocyte antigen HLA-DR.
Owner:北京天晟宇生物科技有限公司
Group of antitumor T lymphocytes and preparation method thereof
InactiveCN103981146AGrowth inhibitionReduce recurrenceMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthLeukocyte Differentiation
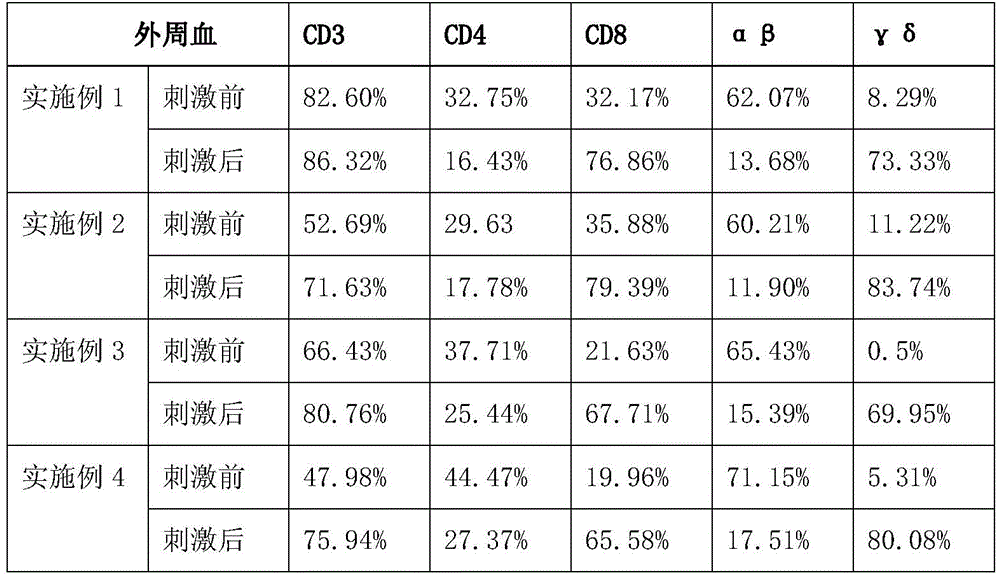
The invention provides a group of antitumor T lymphocytes. The group of antitumor T lymphocytes are used for representing the following five types of lymphocyte membrane molecules to a certain extent: human leukocyte differentiation antigens CD3, human leukocyte differentiation antigens CD4, human leukocyte differentiation antigens CD8, human Alpha Beta T cell receptors and human Gamma Delta T cell receptors, thereby inhibiting the proliferation of breast cancers and hepatoma cell lines, inducing the apoptosis of cancer cells as well as inhibiting the growth of the in-vivo tumors of tumor-bearing mice suffering from the hepatoma and the breast cancers. If the antitumor T lymphocytes provided by the invention are matched with the traditional surgery, chemotherapy and radiation therapy, a small quantity of residues and diffused tumor cells are removed and killed in a biological therapy mode after a large quantity of tumor cells are removed by the conventional therapy method, thereby improving and consolidating the tumor therapy and reducing the tumor relapse.
Owner:SHENZHEN KIVITA INNOVATIVE DRUG INST +1
Recombinant immunotoxin targeting mesothelin
ActiveUS10683362B2High cytotoxic activityStrong cytotoxicityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAnti-Mesothelin Antibody
Mesothelin is a differentiation antigen present on the surface of ovarian cancers, mesotheliomas and several other types of human cancers. Because among normal tissues, mesothelin is only present on mesothelial cells, it represents a good target for antibody mediated delivery of cytotoxic agents. The present invention is directed to improved recombinant immunotoxins comprising anti-mesothelin antibodies, including Fv molecules with particularly high affinity for mesothelin, and a Pseudomonas Exotoxin moiety which has been modified to reduce its immunogenicity and protease sensitivity and providing a better cytotoxicity for cells which express mesothelin. The RITs are well-suited for the treatment of cancers of the ovary, stomach, squamous cells, mesotheliomas and other malignant cells expressing mesothelin.
Owner:UNITED STATES OF AMERICA
Application of tunicamycin in preparing tumour cell reversing drug
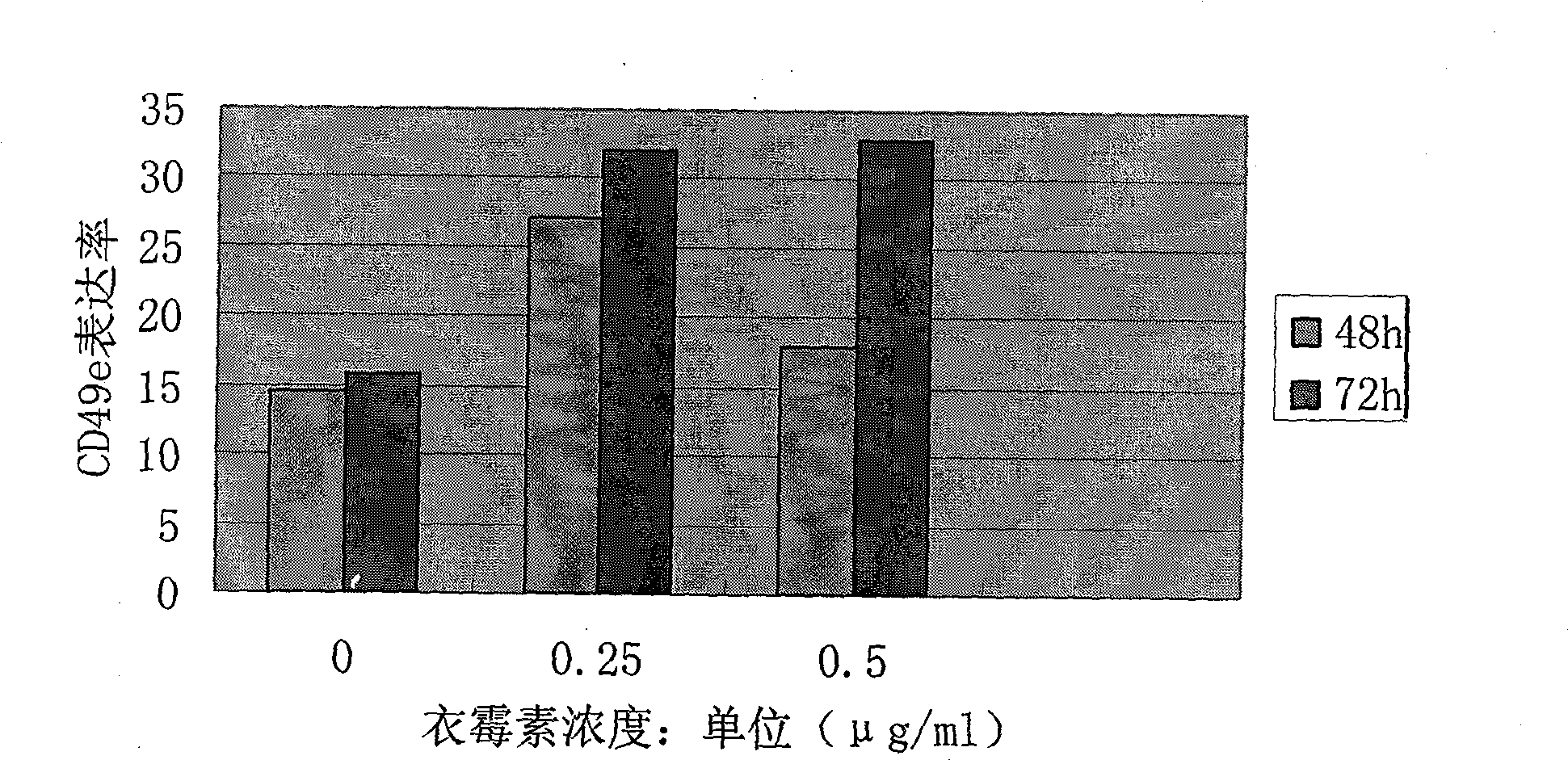
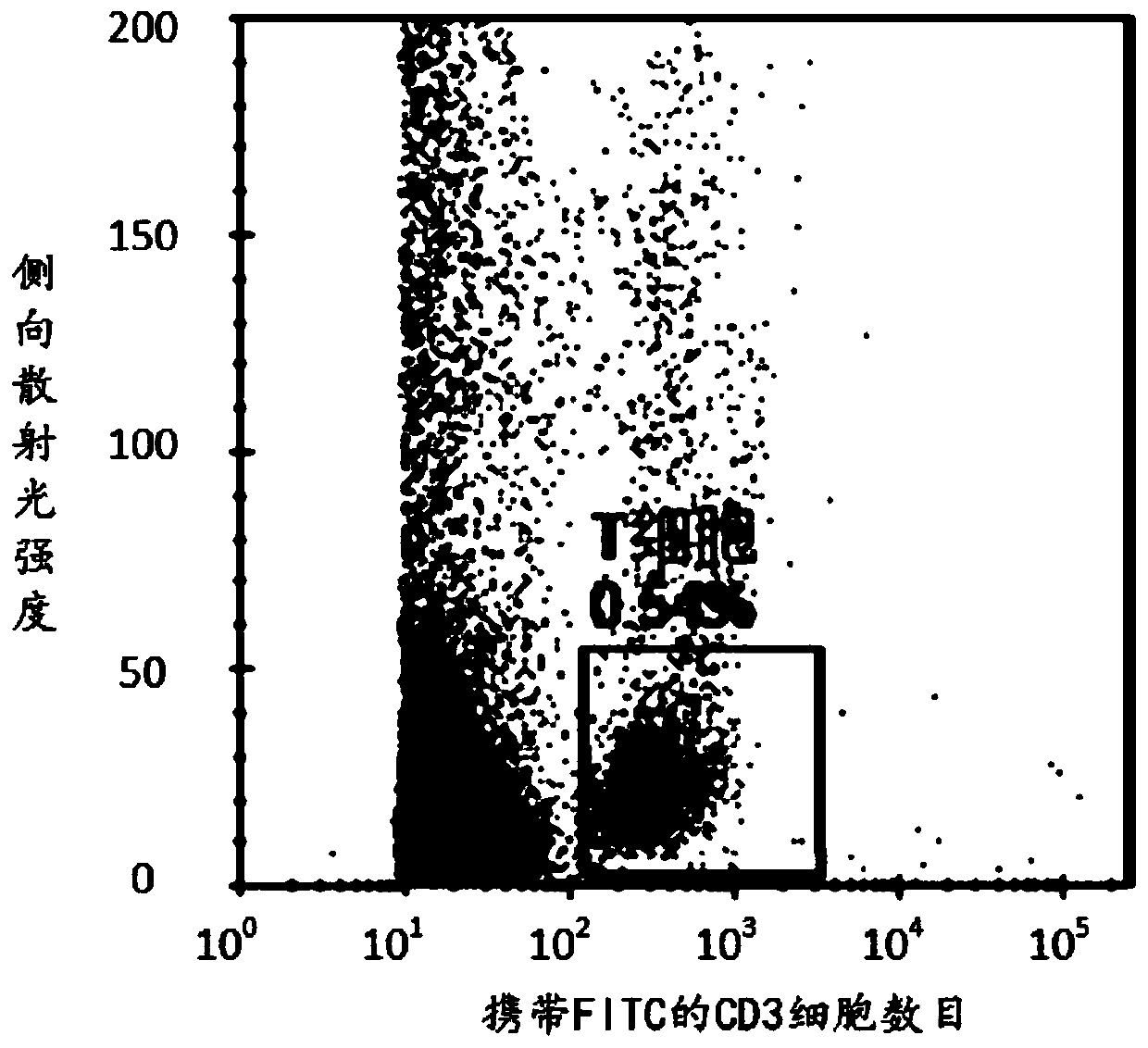
InactiveCN101530420AReduce drug useThe effect is accurateOrganic active ingredientsAntineoplastic agentsAntigenTumor therapy
The invention relates to the technical field of medication. The tumour is always cured by operation, irradiation and chemical medication, and the induction differentiation therapy of malignant tumour becomes the research hotpot and frontier field of cancer biology and tumor therapeutics at present. The invention aims at providing a now application of tunicamycin which is the application in preparing a tumour cell reversing drug. The concentration of low-dosage tunicamycin adopted by the invention is 0.1-0.5mug / ml. After a myeloma cell line is treated, myeloma cell strain is differentiated in state, which is shown as cells are transited into mature plasma cell state; cell differentiation antigen CD49e is raised; and excreted immune globulin light chains are also increased. The invention has the advantages of low drug dosage of the tunicamycin, exact effect, less influence on normal cell and benefit for improving the living state of tumour patients.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Antibody detection kit, and application thereof in immunoassay
PendingCN111537735AA quick look at expressionsAccurate and quick inspectionBiological material analysisBiological testingImmune profilingSurface marker
The invention provides an antibody detection kit. The antibody detection kit comprises an antibody of a programmed death receptor 1 and antibodies of at least three cluster differentiation antigens. The antibody of the programmed death receptor 1 and the antibody of each cluster differentiation antigen carry different fluoresceins, the at least three cluster differentiation antigens comprise a mature T cell surface marker, an auxiliary T cell surface marker and a cytotoxic T cell surface marker, and can be used for rapidly investigating the expression condition of PD-1 protein, and the staining index of fluorescein carried by the antibody of the programmed death receptor 1 is higher than that of fluorescein carried by the antibody of each cluster differentiation antigen, so that the expression condition of the PD-1 protein can be accurately investigated. The invention also provides application of the antibody detection kit in immunoassay.
Owner:江西赛基生物技术有限公司
il-2 and mart-1 double gene co-expression recombinant vector and preparation method and application thereof
ActiveCN103555763BOvercoming reciprocal inhibitionGuarantee structureGenetic material ingredientsAntineoplastic agentsAntigenDifferentiation Antigens
The invention discloses an IL-2 and MART-1 dual-gene co-expression recombinant vector, which is sequentially linked with an IL-2 gene, an IRES sequence and an MART-1 gene in a vector transcription direction, or is sequentially linked with an MART-1 gene, an IRES sequence and an IL-2 gene in a vector transcription direction, wherein nucleotide sequence of the IL-2 gene is represented in SEQ ID NO: 1 in a sequence list, nucleotide sequence of the MART-1 gene is represented in SEQ ID NO: 2 in the sequence list, and nucleotide sequence of the IRES sequence is represented in SEQ ID NO: 3 in the sequence list. The dual-gene co-expression recombinant vector, which links the IL-2 gene and the MART-1 gene through the IRES sequence, can simultaneously express a human melanin differentiation antigen and interleukin-2; the recombinant vector, in immunogene therapy of melanin, not only can develop an immune regulating function of a cell factor but also can generate a specific anti-tumor effect aiming at the melanin.
Owner:XINXIANG MEDICAL UNIV
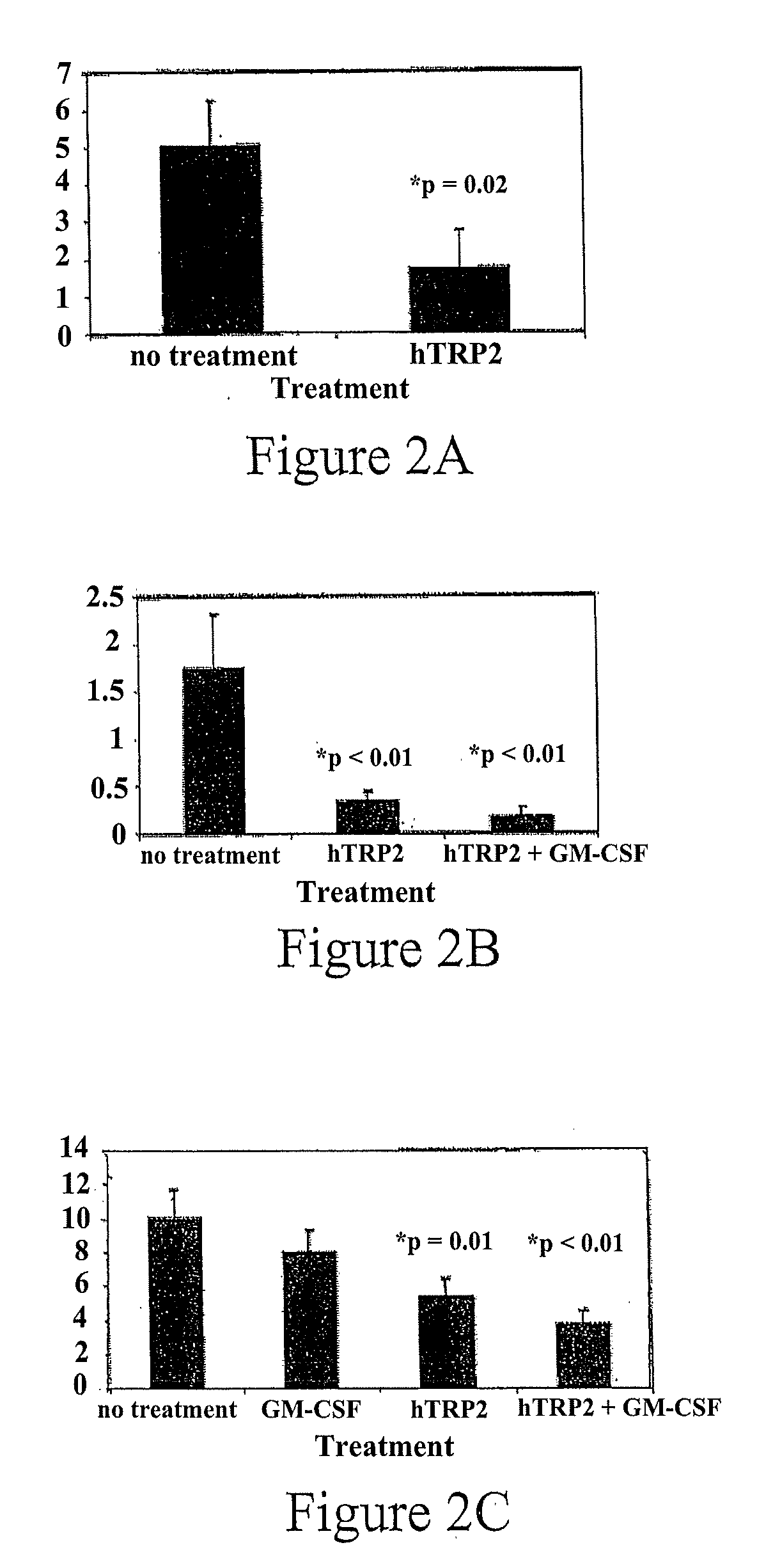
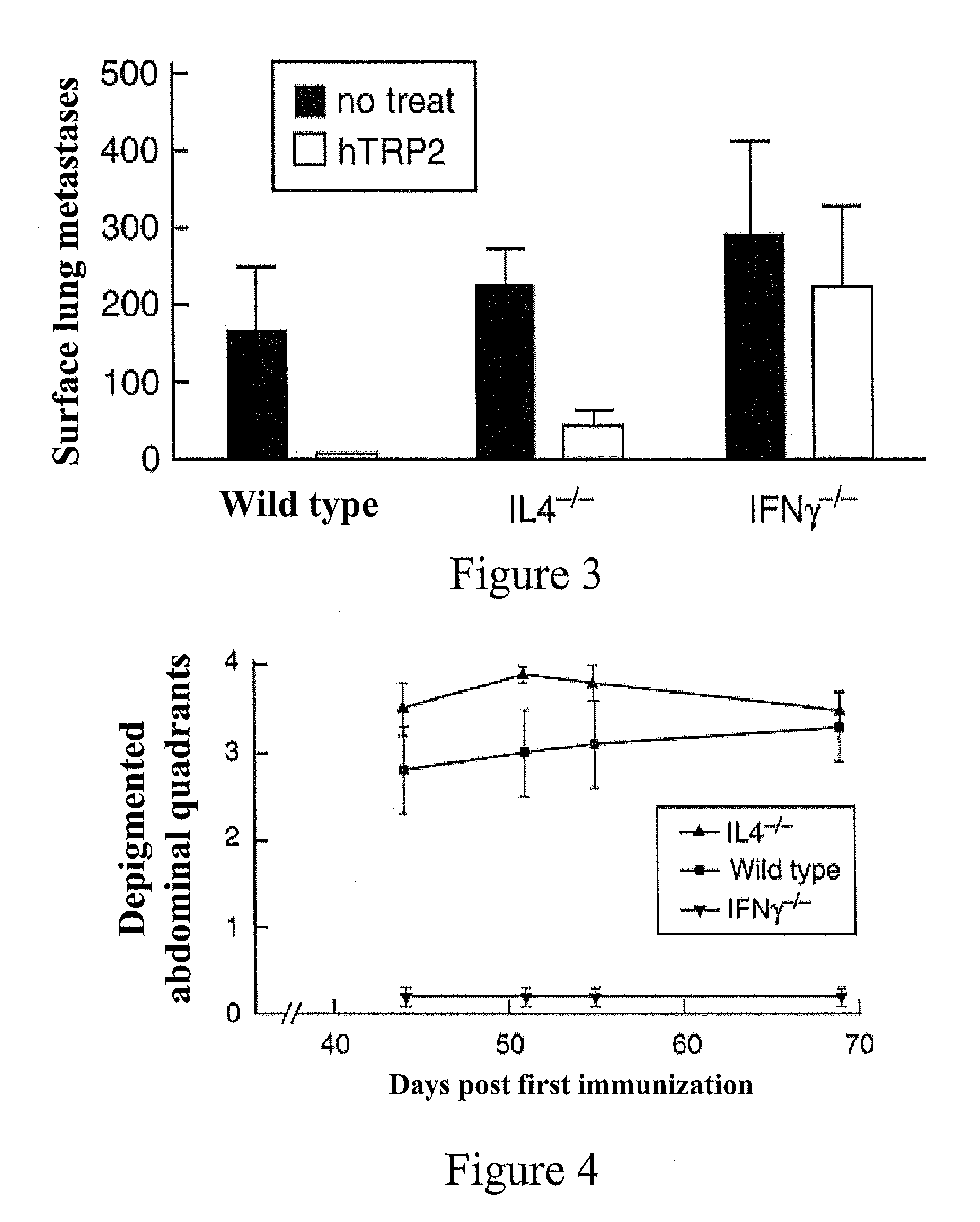
Method and Compositions for Stimulation of an Immune Response to TRP2 using a Xenogeneic TRP2 Antigen
InactiveUS20100068263A1Effective immunityOvercome toleranceOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsMelanomaDifferentiation Antigens
Tolerance of the immune system for endogenous TRP2 can be overcome and an immune response stimulated by administration of xenogeneic or xenoexpressed TRP2 antigen. For example, mouse TRP2, or antigenically-effective portions thereof, can be used to stimulate an immune response to the corresponding differentiation antigen in a human subject. Administration of xenogeneic antigens in accordance with the invention results in an effective immunity against TRP2 expressed by the cancer in the treated individual, thus providing a therapeutic approach to the treatment of cancers expressing TRP2, such as melanoma.
Owner:SLOAN KETTERING INST FOR CANCER RES
CD29<+> human umbilical cord-derived mesenchymal stem cell and application thereof in preparation of bone tissue engineering seed cell used for treating bone injury
InactiveCN107502587ALow immunogenicityGood treatment effectSkeletal disorderUnknown materialsAntigenTreatment effect
The invention discloses a CD29<+> human umbilical cord-derived mesenchymal stem cell and an application thereof in preparation of a bone tissue engineering seed cell used for treating bone injury. The CD29<+> human umbilical cord-derived mesenchymal stem cell expresses the following four mesenchymal stem cell membrane molecules: a human leukocyte differentiation antigen CD73, a human leukocyte differentiation antigen CD90, a human leukocyte differentiation antigen CD105 and a human leukocyte differentiation antigen CD29. The CD29<+> human umbilical cord-derived mesenchymal stem cell disclosed by the invention can be converted into a bone cell in a mouse to participate in fracture healing, has low immunogenicity and can be taken as a cell source of tissue-engineered bone, then the traditional surgical therapy scheme is combined, and a good therapeutic effect can be achieved, so that the CD29<+> human umbilical cord-derived mesenchymal stem cell disclosed by the invention has a broad application prospect.
Owner:JILIN TUO HUA BIOTECH
GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) and MART-1 (Melanoma Antigen Recognized By T-Cells 1) dual-gene co-expression recombinant vector and preparation method and application thereof
ActiveCN103602695AGood immunotherapy effectReduce dosageGenetic material ingredientsAntineoplastic agentsAntigenAgricultural science
The invention discloses a GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) and MART-1 (Melanoma Antigen Recognized By T-Cells 1) dual-gene co-expression recombinant vector. The GM-CSF and MART-1 dual-gene co-expression recombinant vector is characterized that a GM-CSF gene, an IRES (Internal Ribosome Entry Sequence) and an MART-1 gene are connected in sequence along the vector transcription direction, or the MART-1 gene, IRES and GM-CSF gene are connected in sequence along the vector transcription direction; a nucleotide sequence of the GM-CSF gene is as shown in SEQ ID NO:1 in a sequence table; the nucleotide sequence of the MART-1 gene is as shown in SEQ ID NO:2 in the sequence table; the nucleotide sequence of the IRES is as shown in SEQ ID NO:3 in the sequence table. According to the dual-gene co-expression recombinant vector, the IRES sequence is utilized for connecting the GM-CSF gene with the MART-1 gene, and thus human melanoma differentiation antigen and the granulocyte-macrophage colony-stimulating factor can be expressed in the same vector at the same time; the recombinant vector can be applied to immunogene therapy of melanoma, enables the immune regulation effect of cytokines to be played, and can also produce specific anti-tumor effect for malignant melanoma in a targeting way.
Owner:HENAN HUALONG BIOLOGICAL TECH
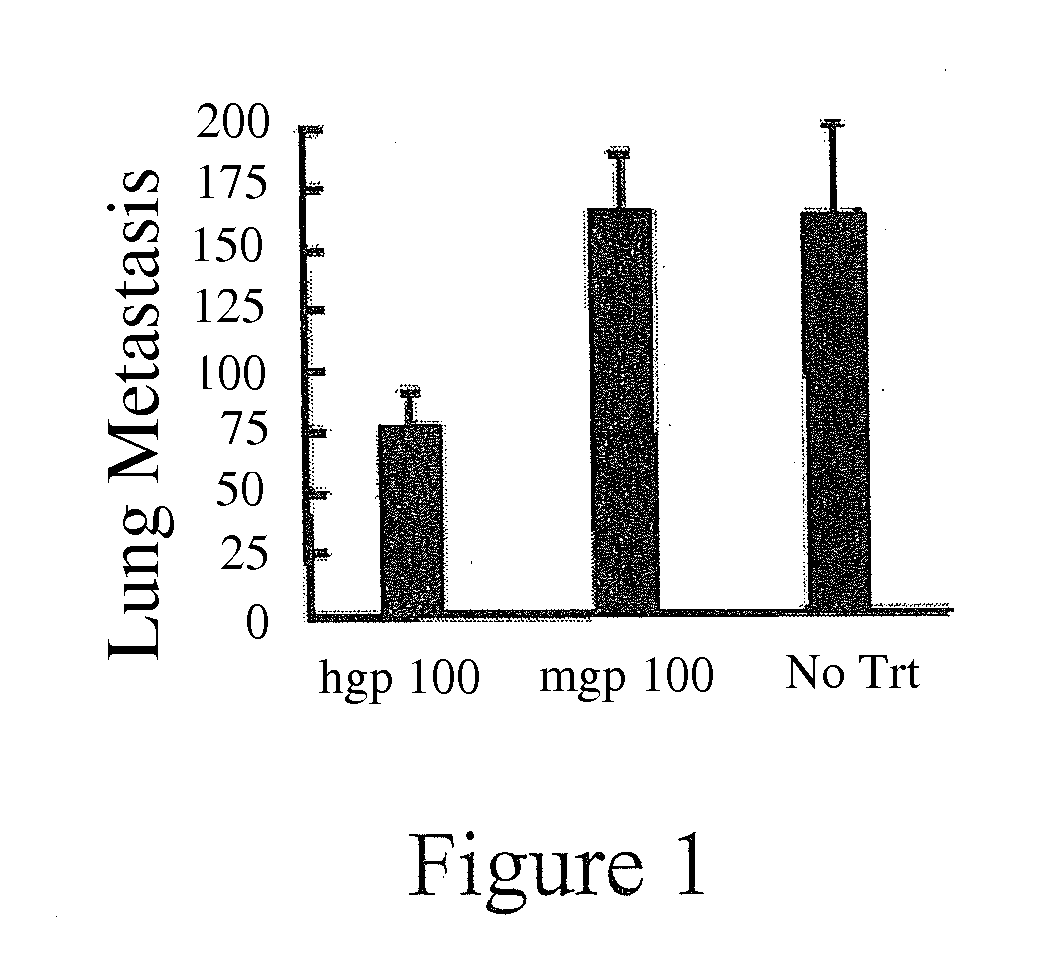
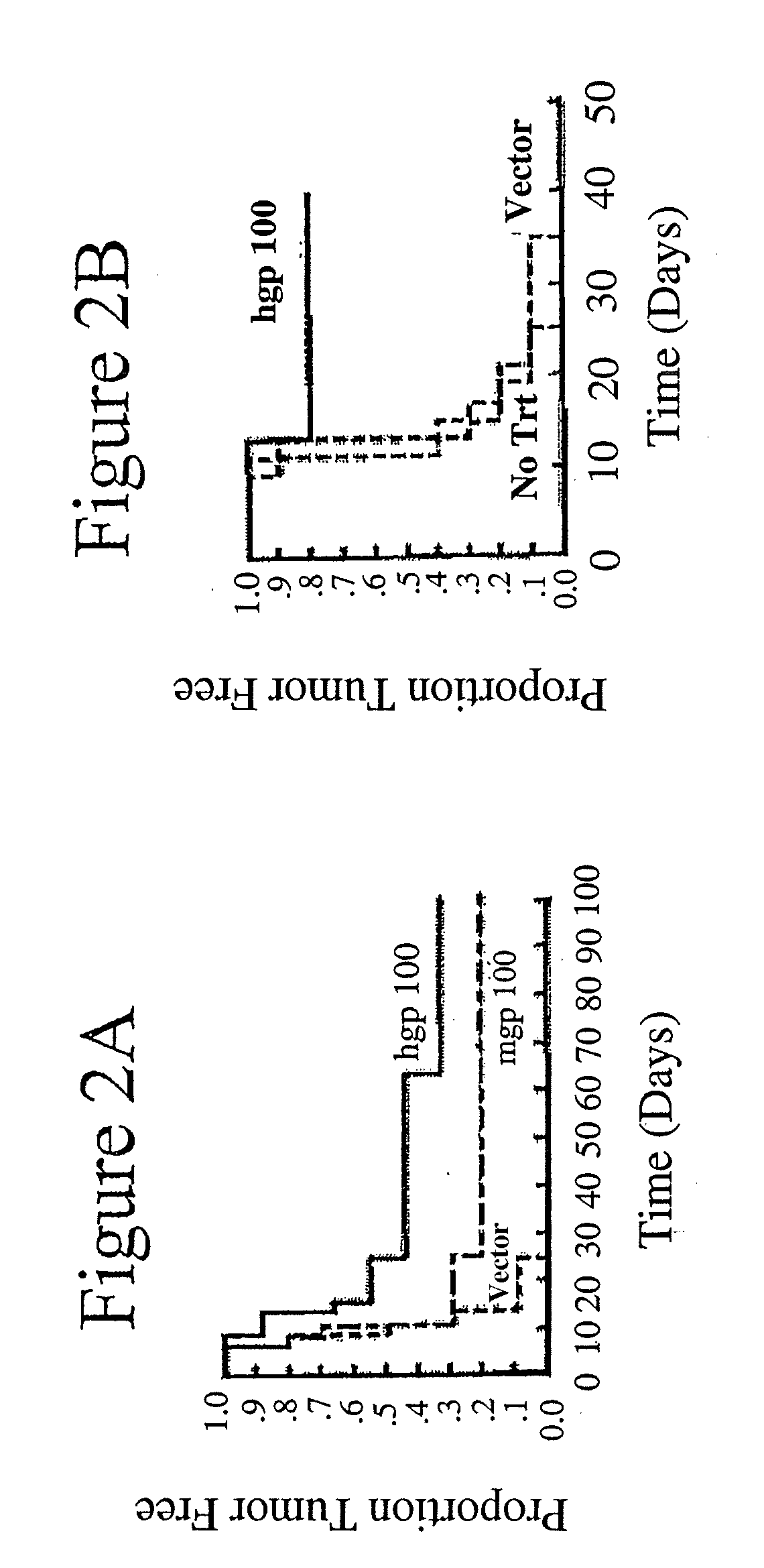
Method and Compositions for Stimulation of an Immune Response to gp100 using a Xenogeneic gp100 Antigen
InactiveUS20100068216A1Effective immunityTolerance of the immune system for endogenous gp100Organic active ingredientsTumor rejection antigen precursorsDifferentiation AntigensMelanoma
Tolerance of the immune system for endogenous gp100 can be overcome and an immune response stimulated by administration of xenogeneic or xenoexpressed gp100 antigen. For example, mouse gp100, or antigenically-effective portions thereof, can be used to stimulate an immune response to the corresponding differentiation antigen in a human subject. Administration of xenogeneic antigens in accordance with the invention results in an effective immunity against gp100 expressed by the cancer in the treated individual, thus providing a therapeutic approach to the treatment of cancers expressing gp100, such as melanoma.
Owner:SLOAN KETTERING INST FOR CANCER RES
cd29 + Human umbilical cord-derived mesenchymal stem cells and their use in the preparation of drugs for the treatment of skeletal muscle atrophy in high-sugar and high-fat environments
ActiveCN107557332BImprove hyperlipidemiaImprove symptoms of skeletal muscle atrophyMetabolism disorderMuscular disorderWhite blood cellHUMAN LEUKOCYTE DIFFERENTIATION ANTIGEN
The invention discloses a CD29<+> human umbilical cord source mesenchymal stem cell and use thereof in the preparation of a drug for treating skeletal muscle atrophy in high-sugar and high-fat environments. The CD29<+> human umbilical cord source mesenchymal stem cell represents the following mesenchymal stem cell cytomembrane molecules: a human leukocyte differentiation antigen CD73, a human leukocyte differentiation antigen CD90, a human leukocyte differentiation antigen CD105 and a human leukocyte differentiation antigen CD29. According to the CD29<+> human umbilical cord source mesenchymalstem cell, the hyperlipidemia and hyperglycemia of a db<- / -> mouse can be obviously improved, muscle fiber cross sections of soleus and gastrocnemius muscle of the db<- / -> mouse can be increased, andthe contents and cell number of each myotube are increased, so that the symptoms of the skeletal muscle atrophy of the db<- / -> mouse are improved. According to the CD29<+> human umbilical cord sourcemesenchymal stem cell, the theoretical foundation and experiment basis are laid for the subsequent research and development of the drug for treating skeletal muscle atrophy in the high-sugar and high-fat environments, and the CD29<+> human umbilical cord source mesenchymal stem cell has wide application prospects.
Owner:JILIN TUO HUA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com