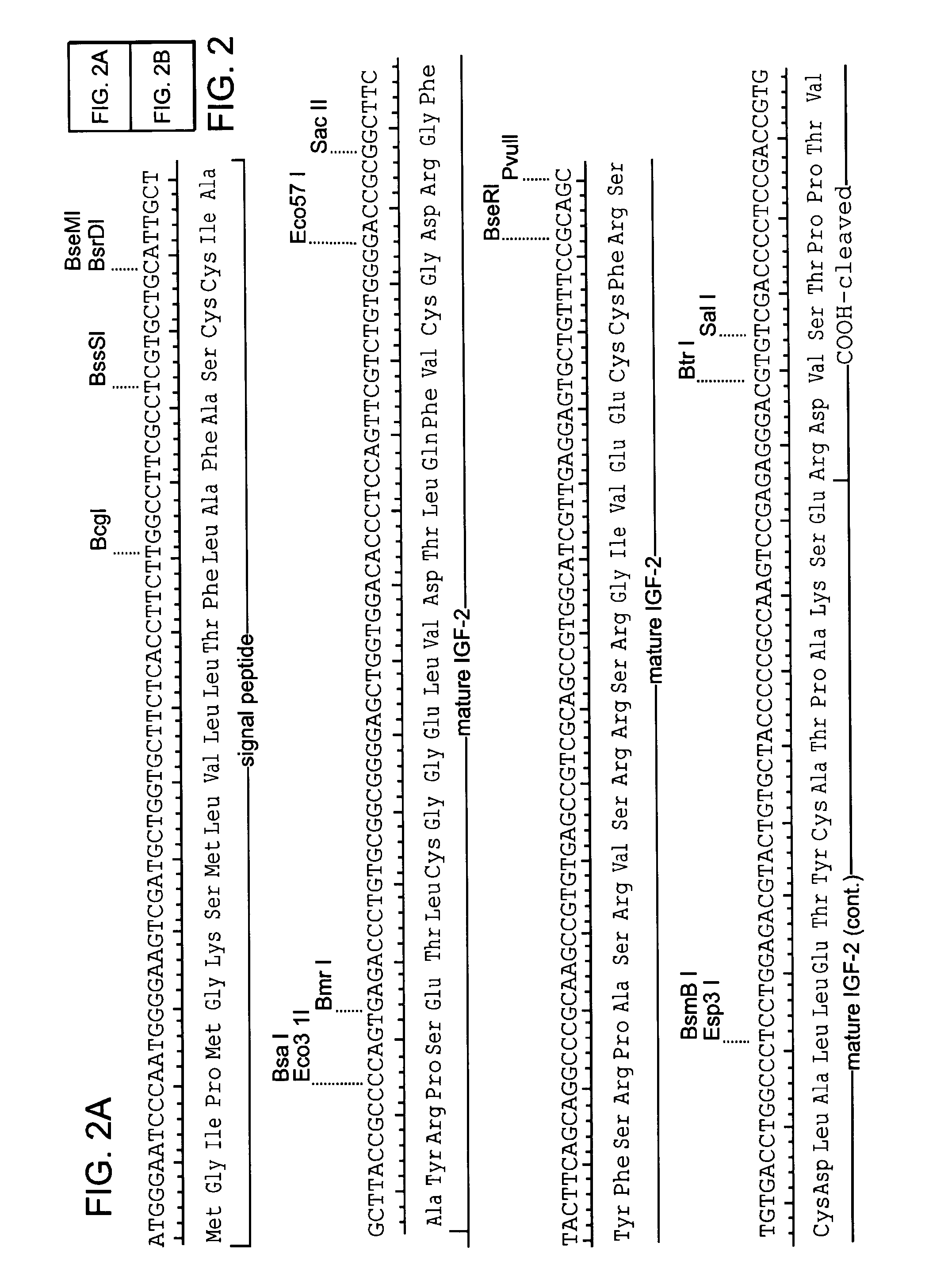
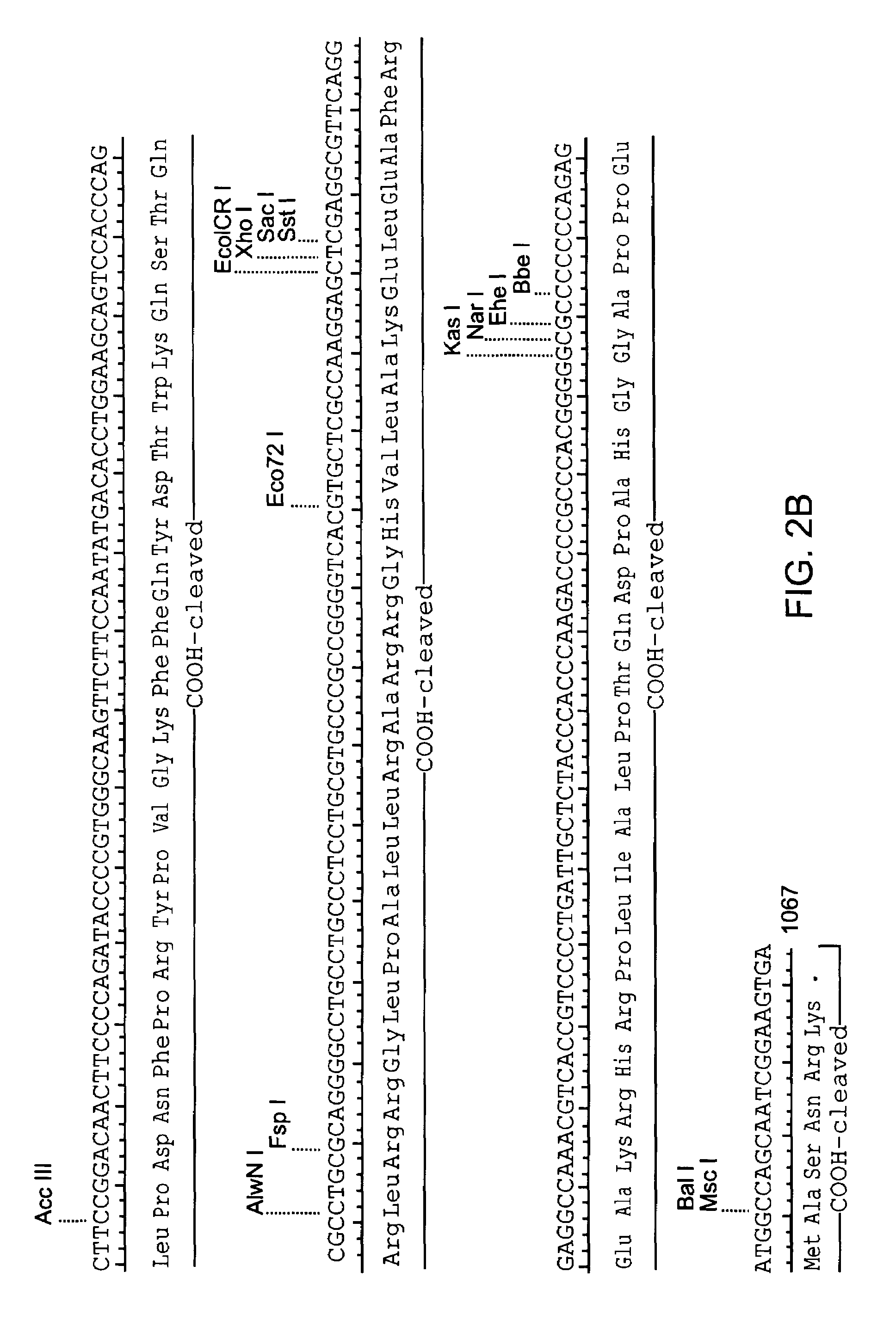
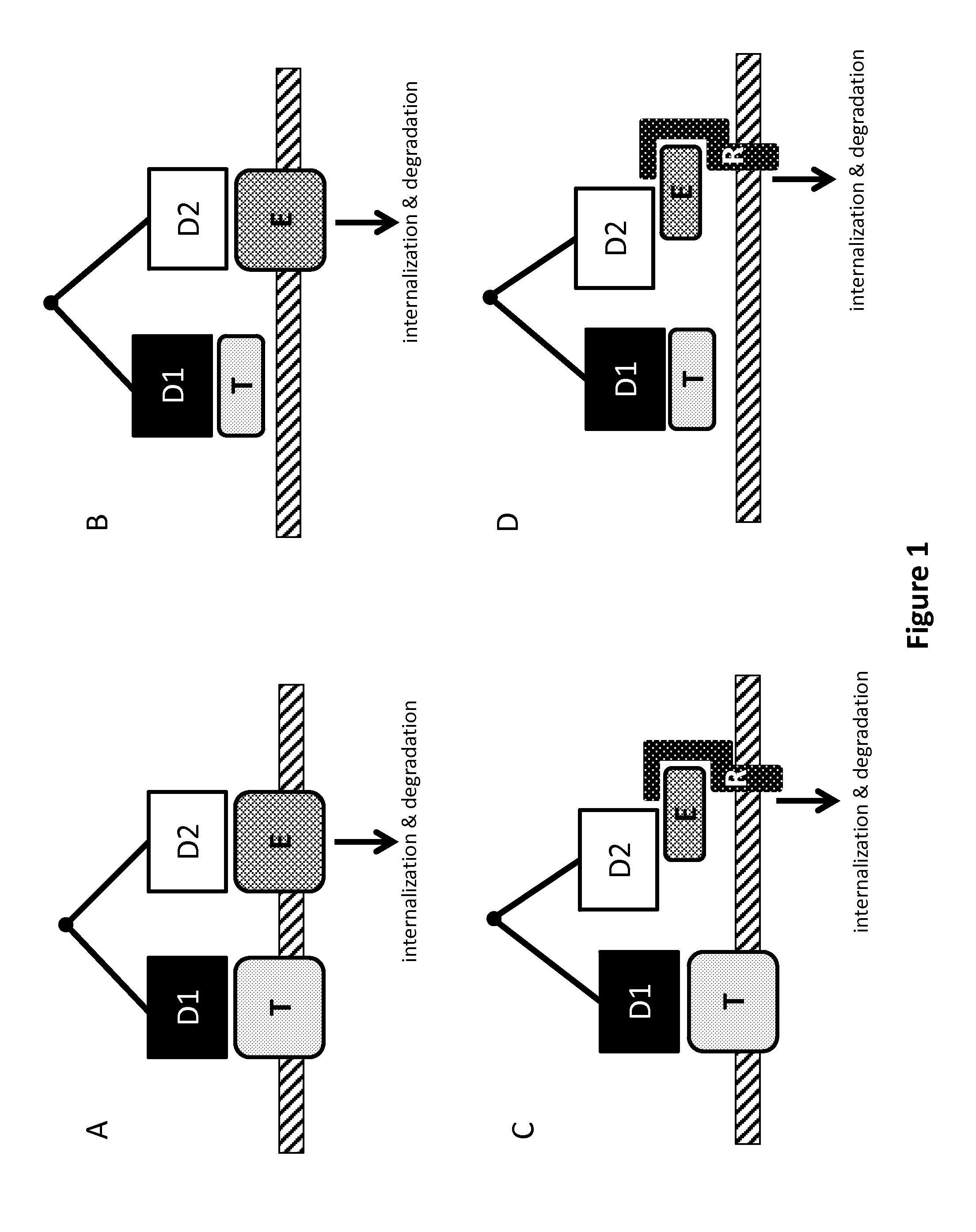
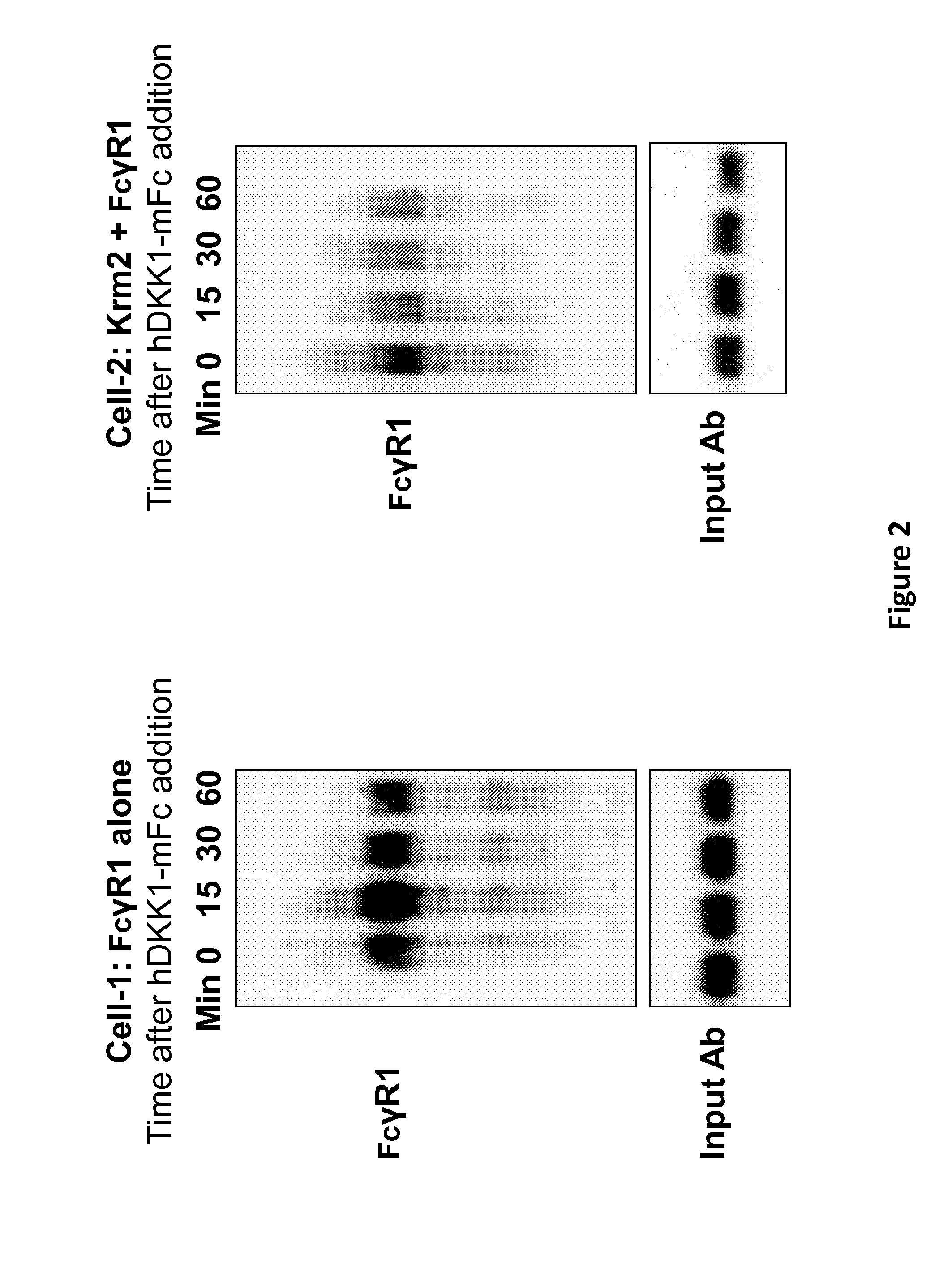
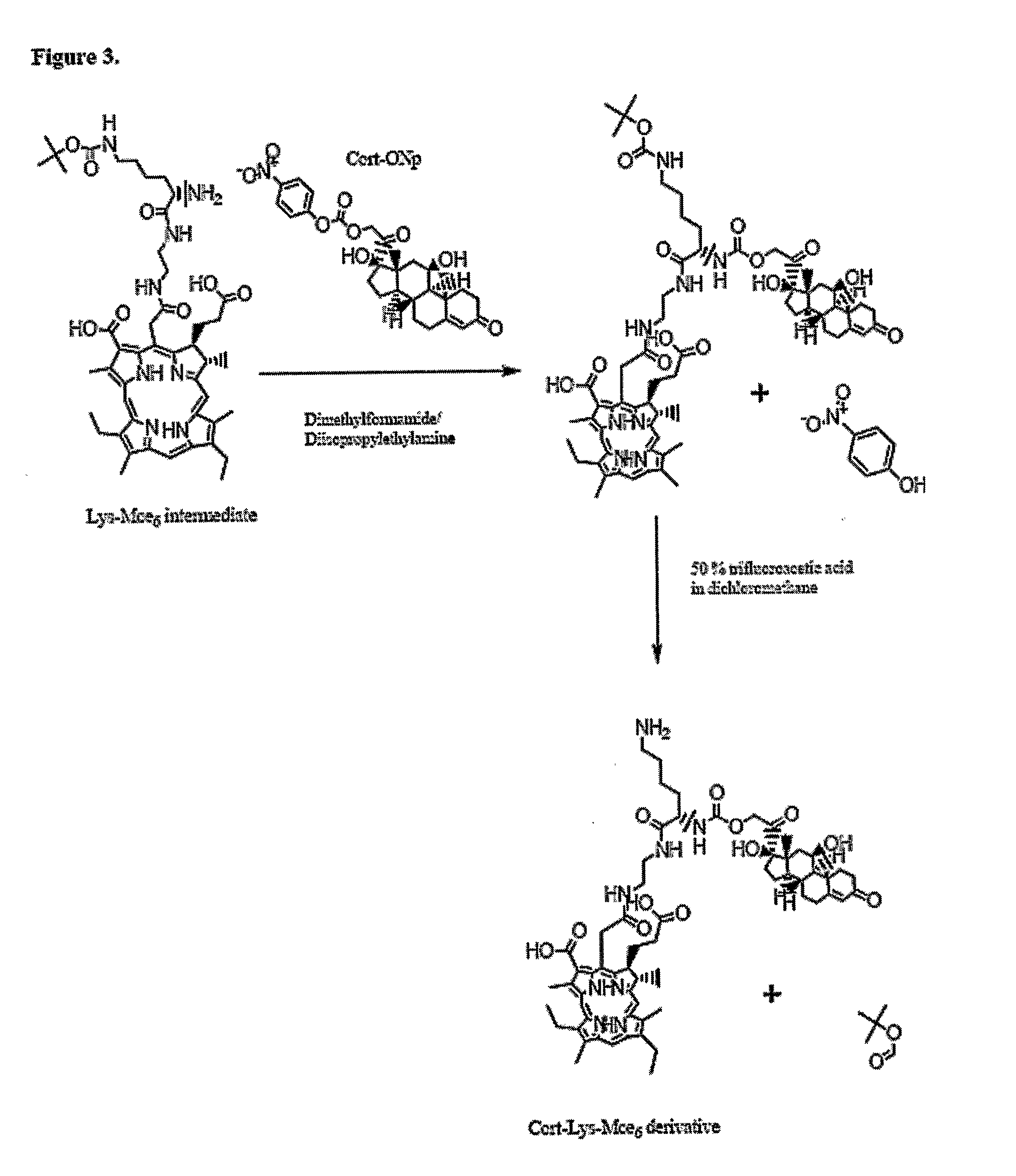
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
469 results about "Internalization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Internalization (or internalisation) is the process of making something internal, with more specific meanings in various fields. It is the opposite of externalization.
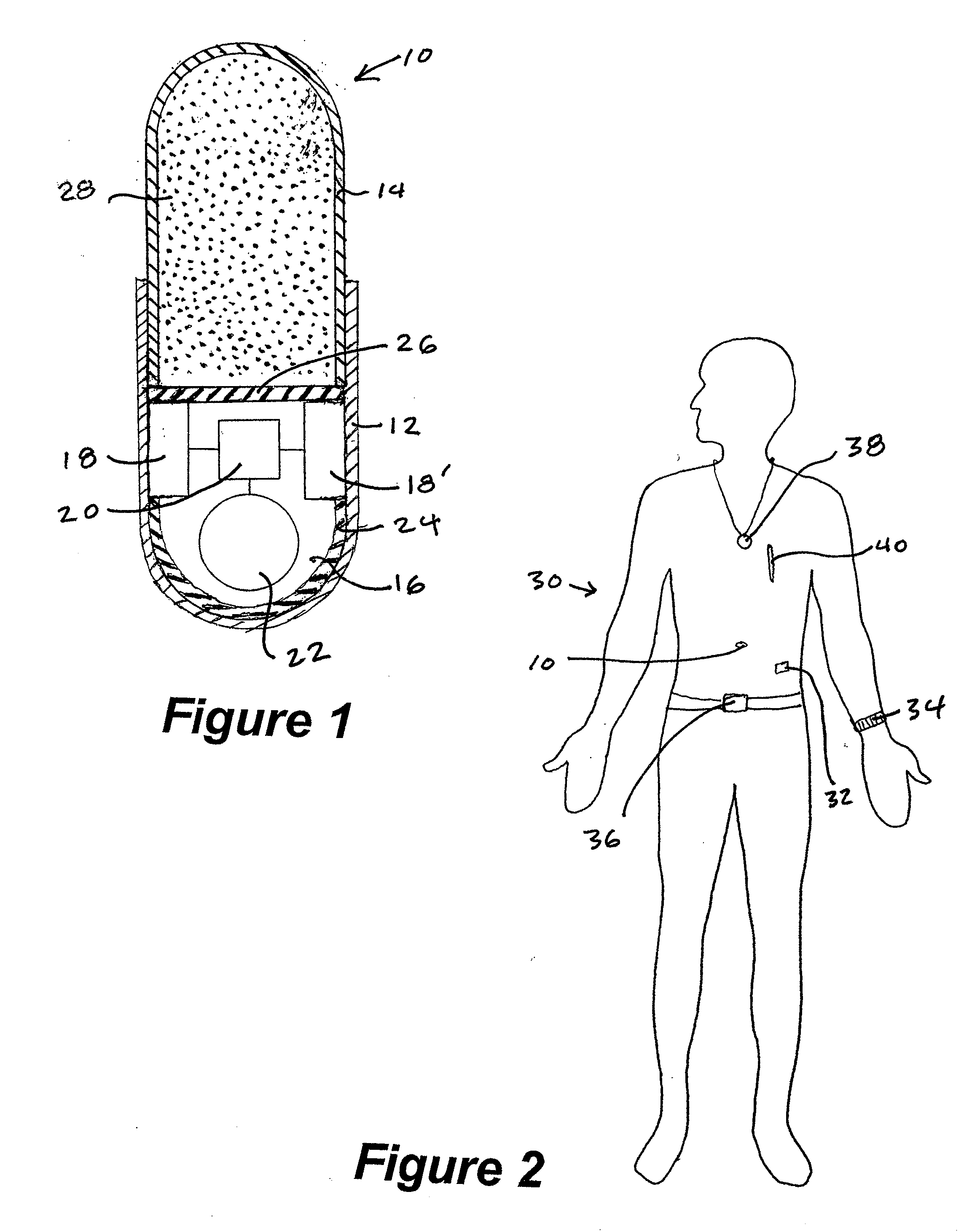
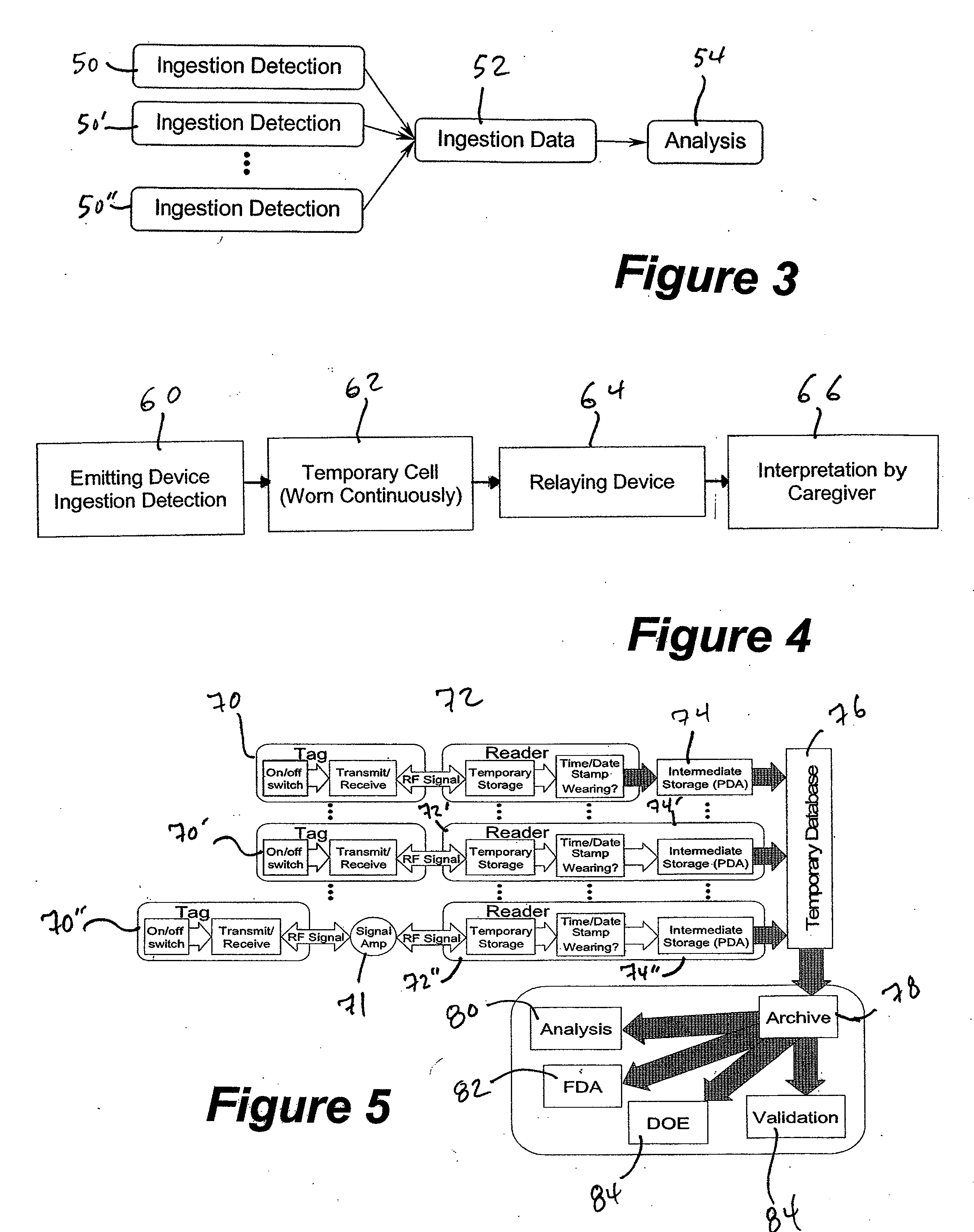
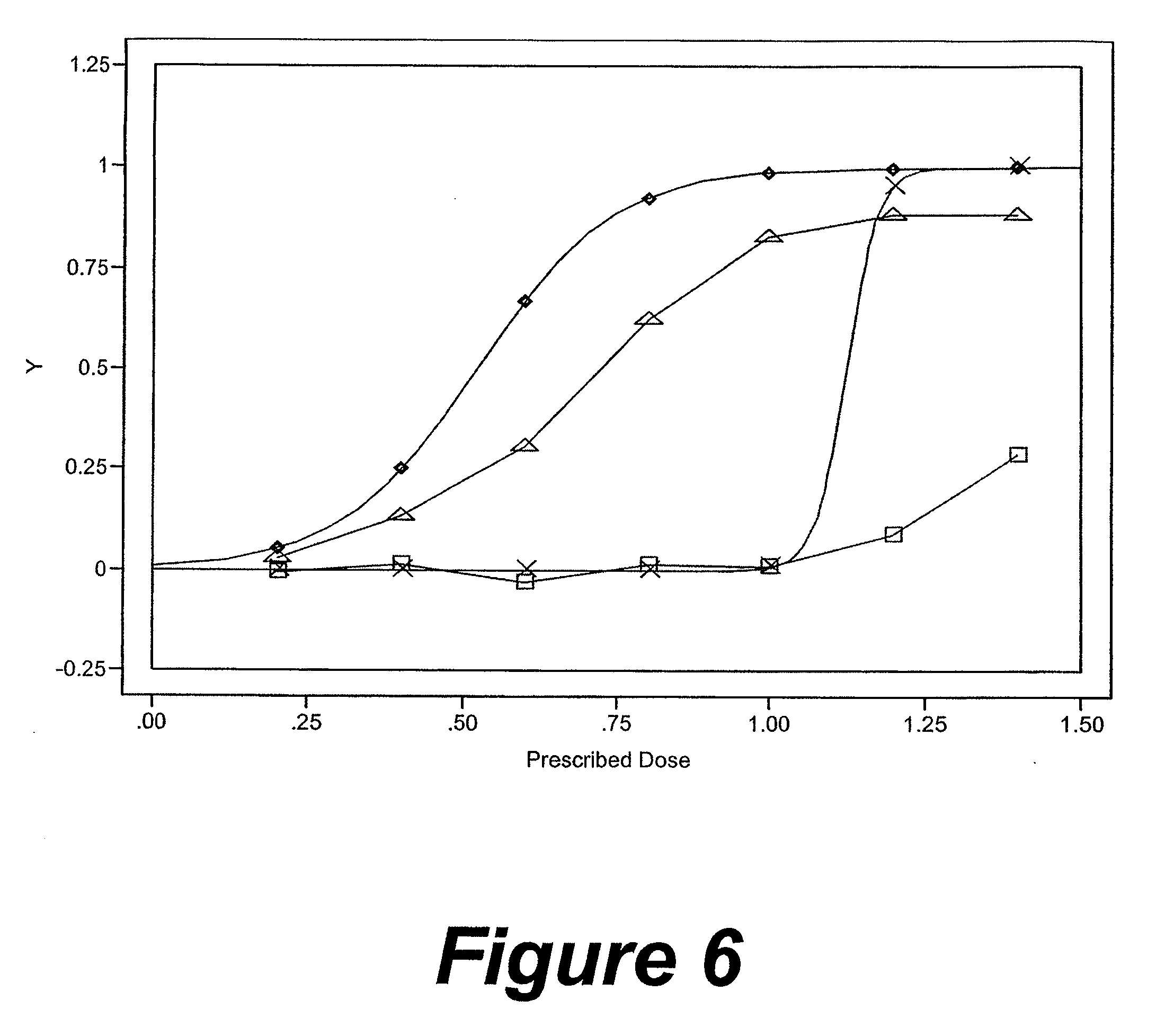
Method and system for monitoring and analyzing compliance with internal dosing regimen
InactiveUS20070237719A1Ultrasonic/sonic/infrasonic diagnosticsCompounds screening/testingDosing regimenMedicine
A method and system for monitoring and analyzing compliance with an internal dosing regimen prescribed to be taken in multiple dose forms includes the steps of detecting internalization of a first dose form to generate a first data point, detecting internalization of a second dose form to generate a second data point, and analyzing the first data point and the second data point. The step of analyzing the first and second data points generates a metric of a variety of possible metric types. The first and second dose forms may be two of any plural number of sequentially-internalized dose forms which generate a like number of sequential data points. Subsequent internalizations of dose forms result in at least a like number of data points being generated. To effect the disclosed method a system is provided which includes at least two dose forms, a time stamp identifier operatively associated with each dose form, a receiving device for receiving the time stamp identifier data, and an analyzer for analyzing the received data,
Owner:DOW GLOBAL TECH LLC
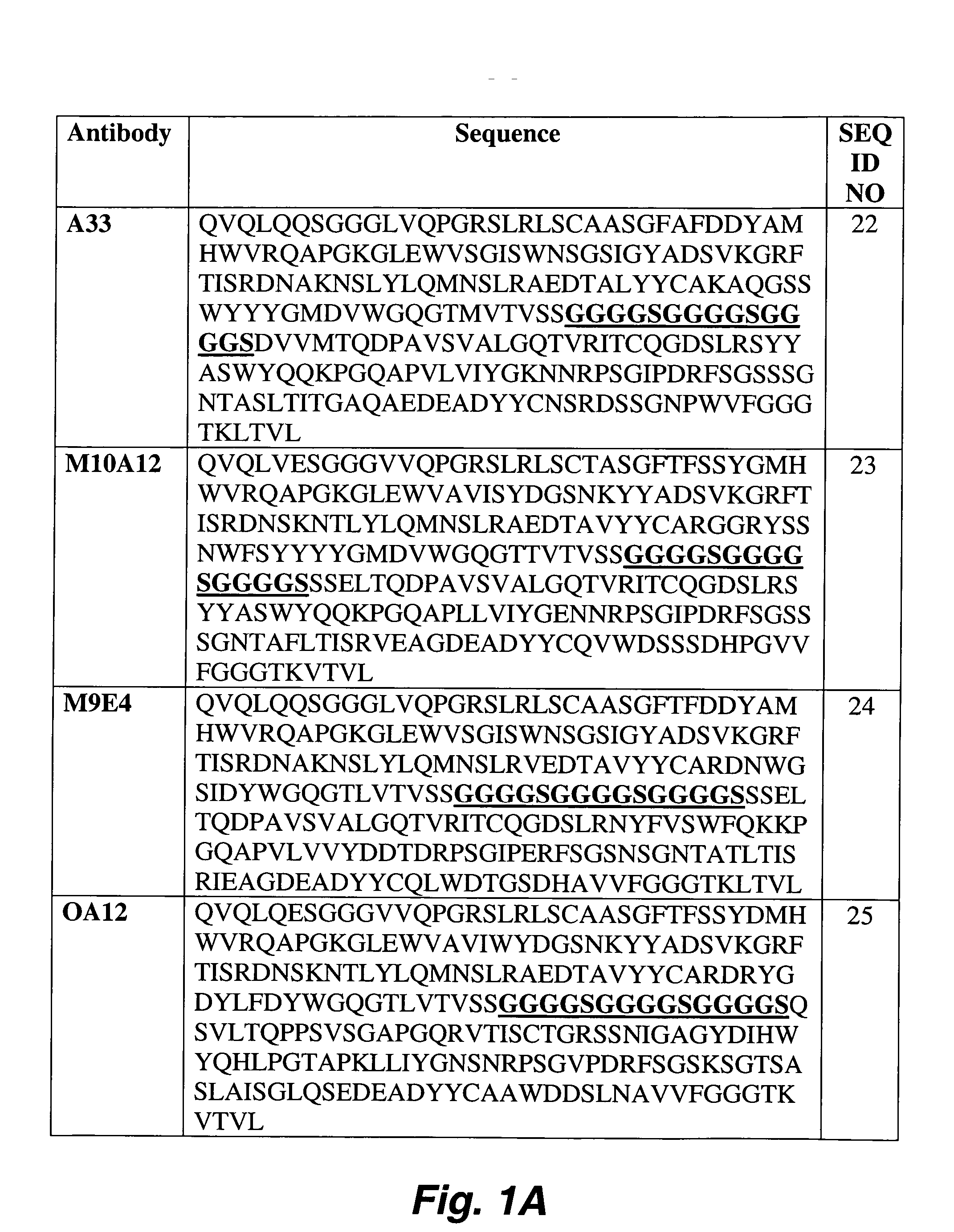
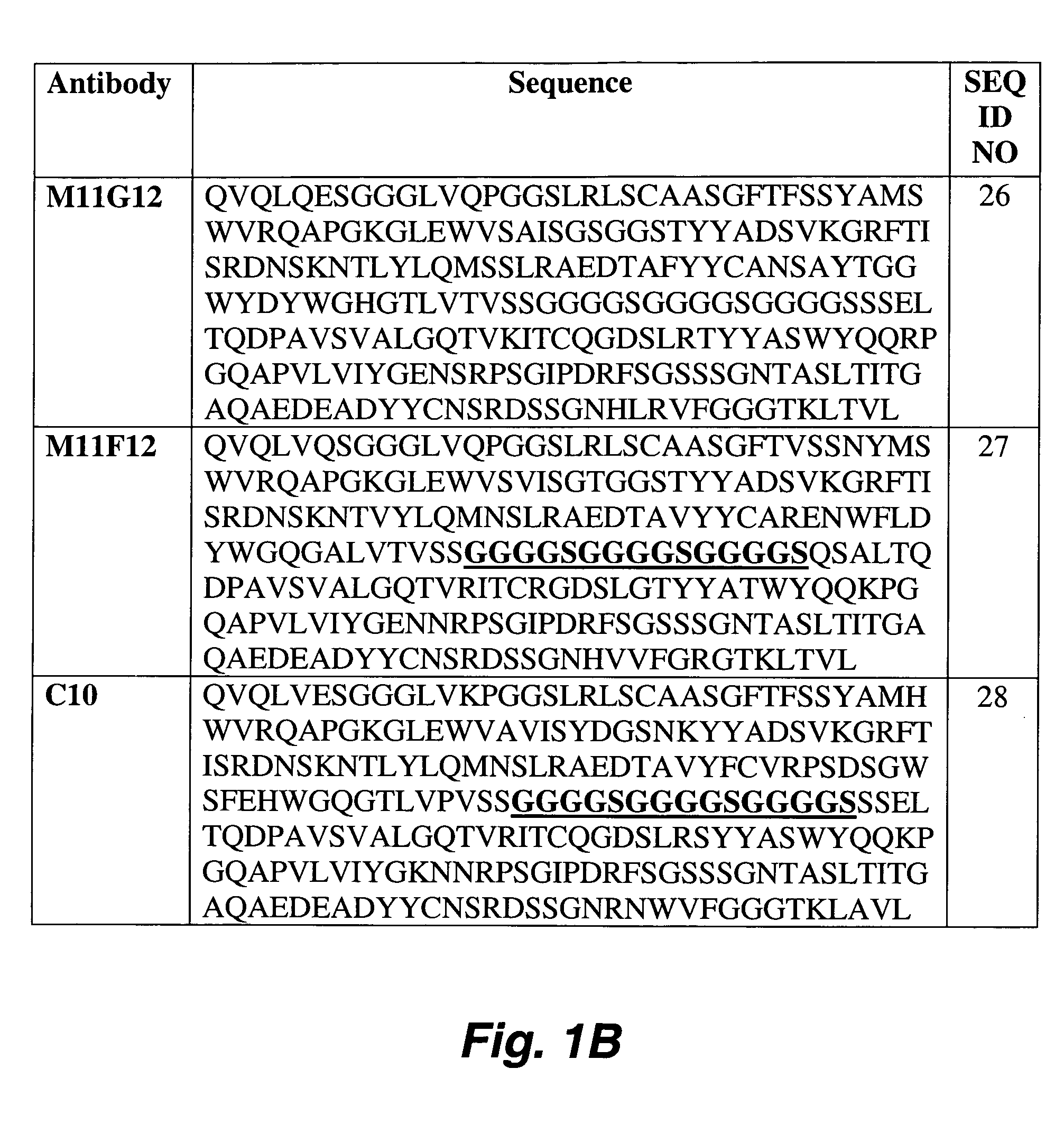
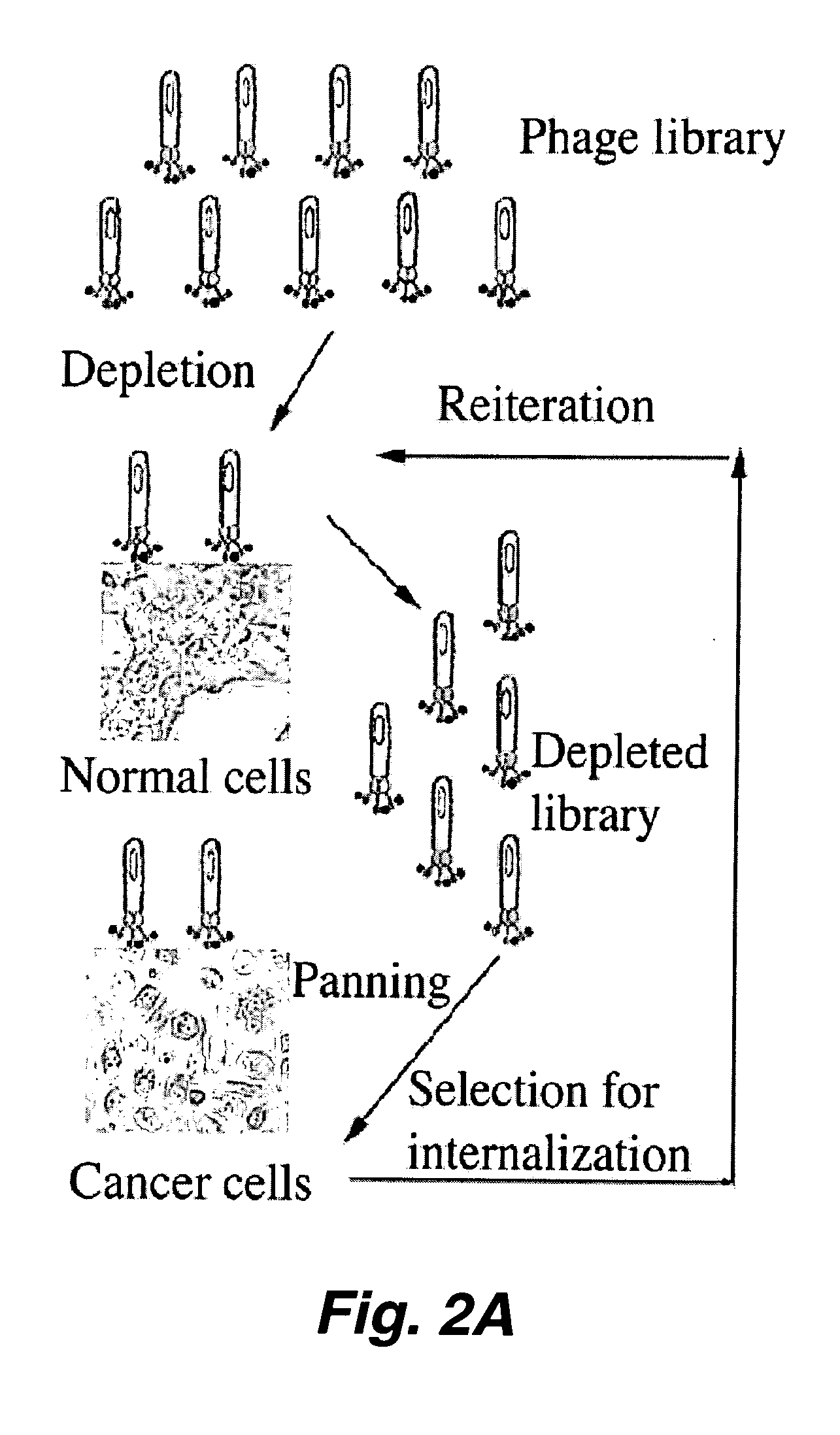
Identification of peptides that facilitate uptake and cytoplasmic and/or nuclear transport of proteins, DNA and viruses
The present invention relates to internalizing peptides which facilitate the uptake and transport of cargo into the cytoplasm and nuclei of cells as well as methods for the identification of such peptides. The internalizing peptides of the present invention are selected for their ability to efficiently internalize cargo into a wide variety of cell types both in vivo and in vitro. The method for identification of the internalizing peptides of the present invention comprises incubating a target cell with a peptide display library, isolating peptides with internalization characteristics and determining the ability of said peptide to internalize cargo into a cell.
Owner:UNIVERSITY OF PITTSBURGH
Targeted therapeutic proteins
InactiveUS20050281805A1Extended half-lifeReduce the binding forcePeptide/protein ingredientsAntibody mimetics/scaffoldsTherapeutic proteinLysosome
Targeted therapeutics that localize to a specific subcellular compartment such as the lysosome are provided. The targeted therapeutics include a therapeutic agent and a targeting moiety that binds a receptor on an exterior surface of the cell, permitting proper subcellular localization of the targeted therapeutic upon internalization of the receptor. Nucleic acids, cells, and methods relating to the practice of the invention are also provided.
Owner:BIOMARIN PHARMA INC
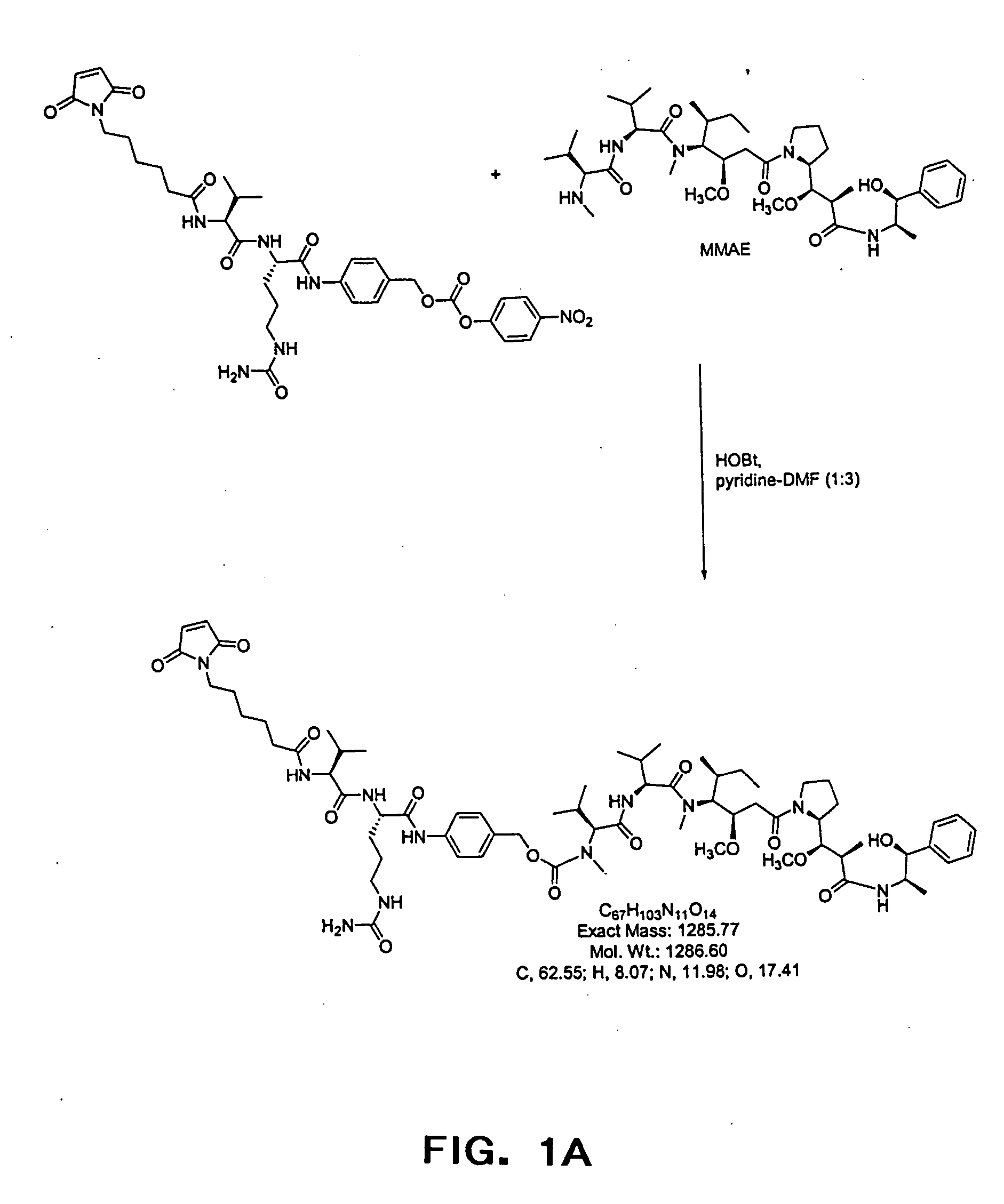
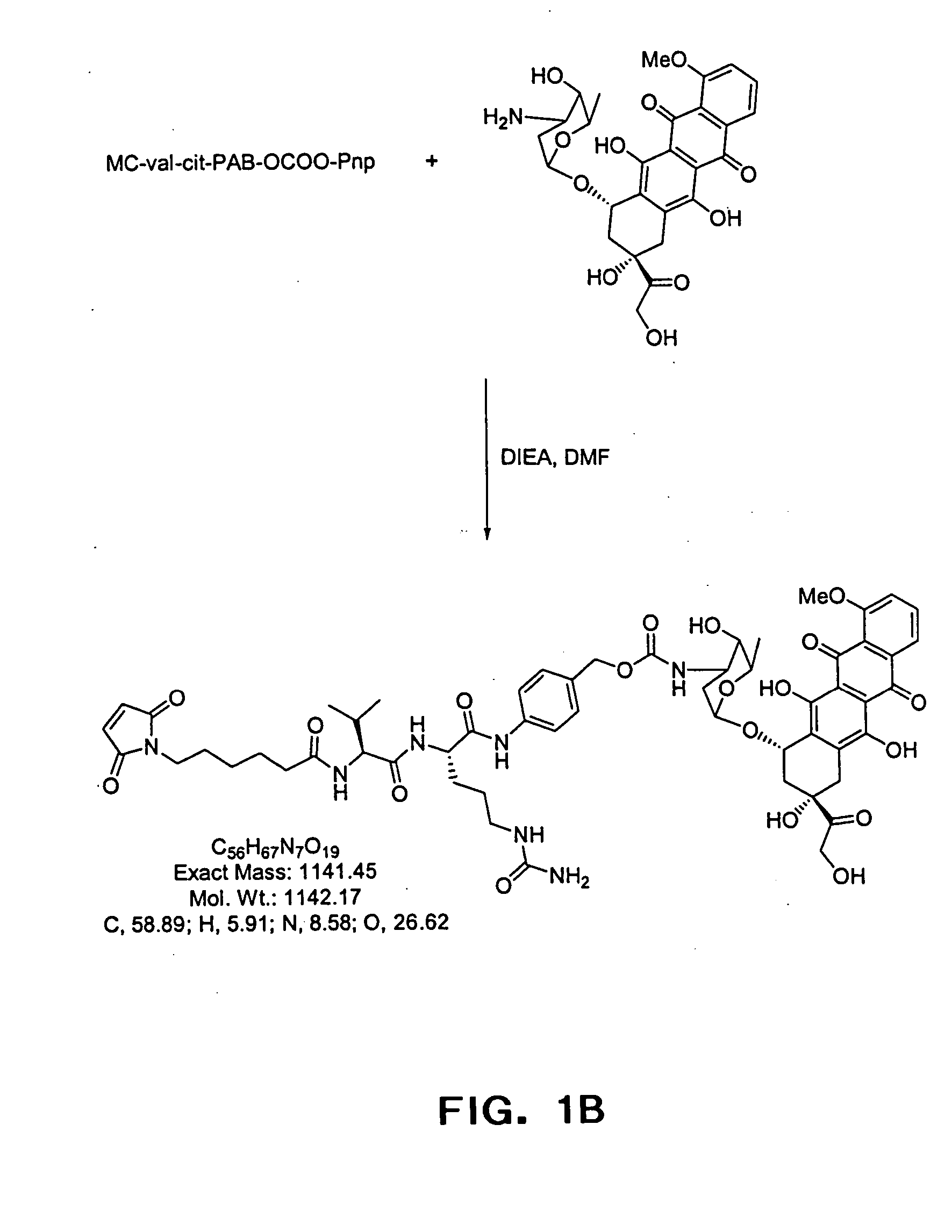
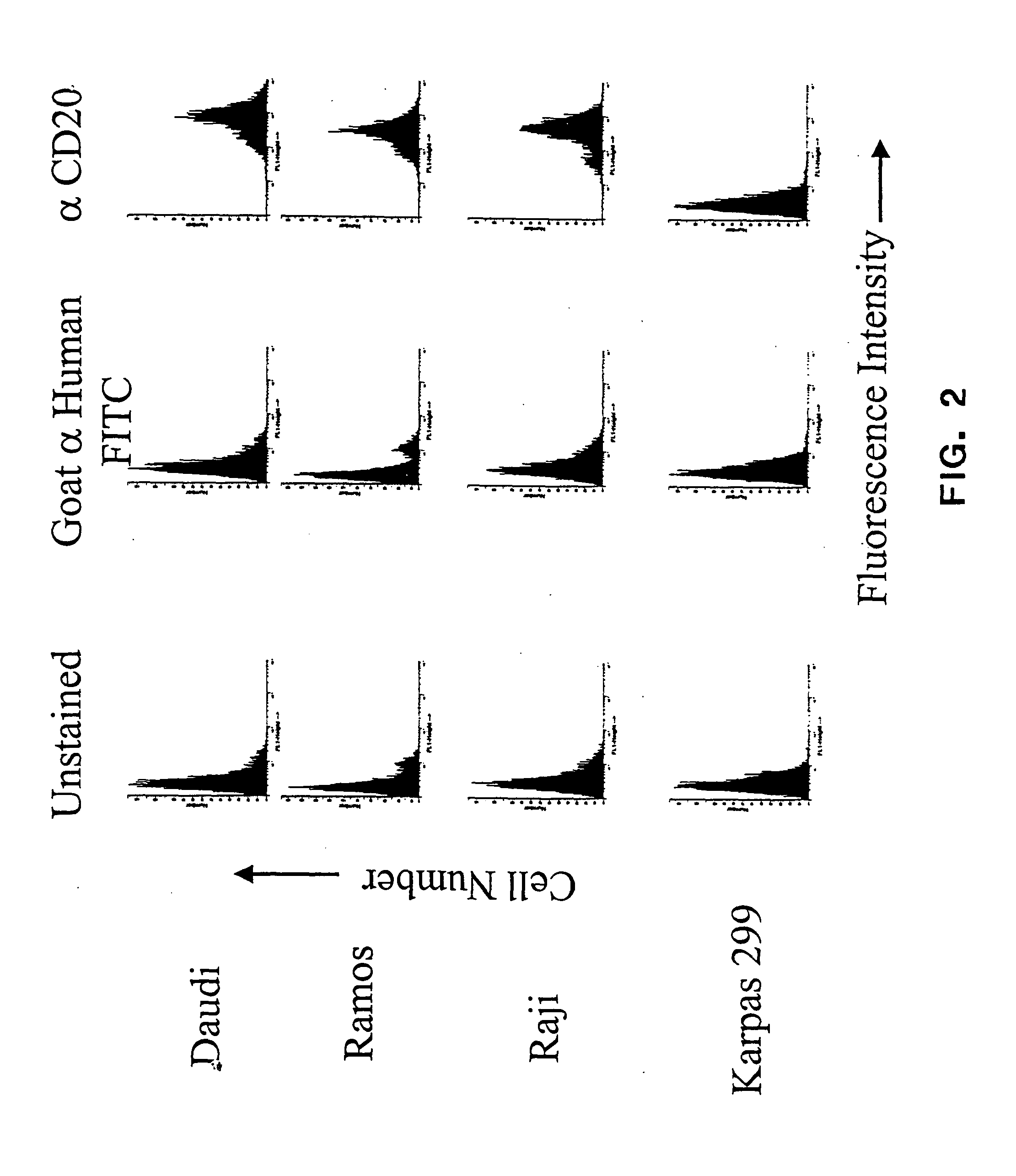
Anti-CD20 antibody-drug conjugates for the treatment of cancer and immune disorders
The present invention relates to methods and compositions for the treatment of CD20-expressing cancers and immune disorders involving CD20-expressing cells. The present methods comprise administering to a subject an anti CD20 antibody-drug conjugate that has a high potency and / or is capable of internalizing into CD20-expressing cells. The present invention further provides pharmaceutical compositions and kits comprising such conjugates. The present invention yet further provides methods of and compositions relating to combination therapy of cancer and immune disorders involving CD20-expressing cells using the anti-CD20 antibody-drug conjugates of the invention.
Owner:SEATTLE GENETICS INC
Antibodies with immune effector activity and that internalize in folate receptor alpha-positive cells
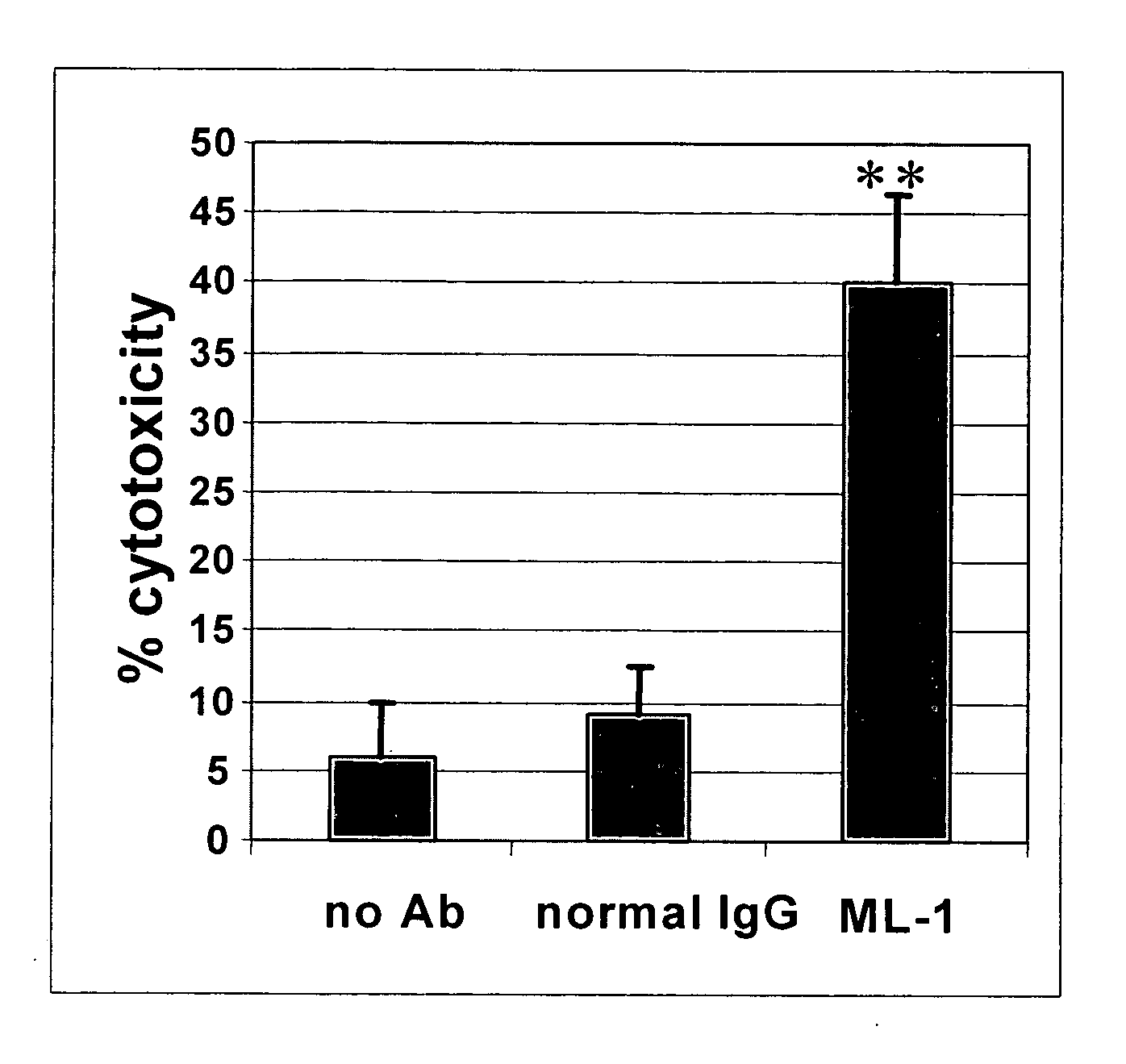
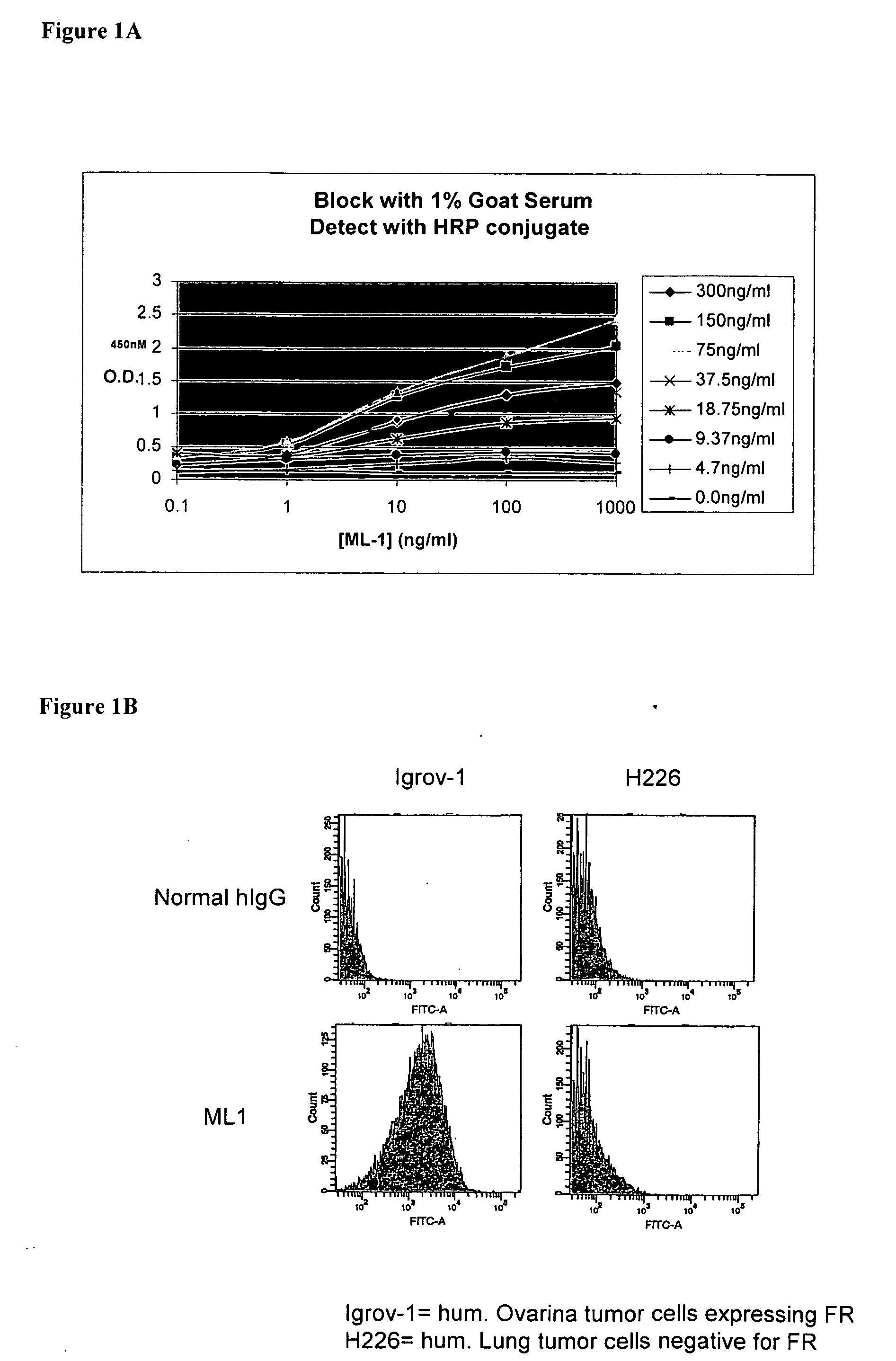
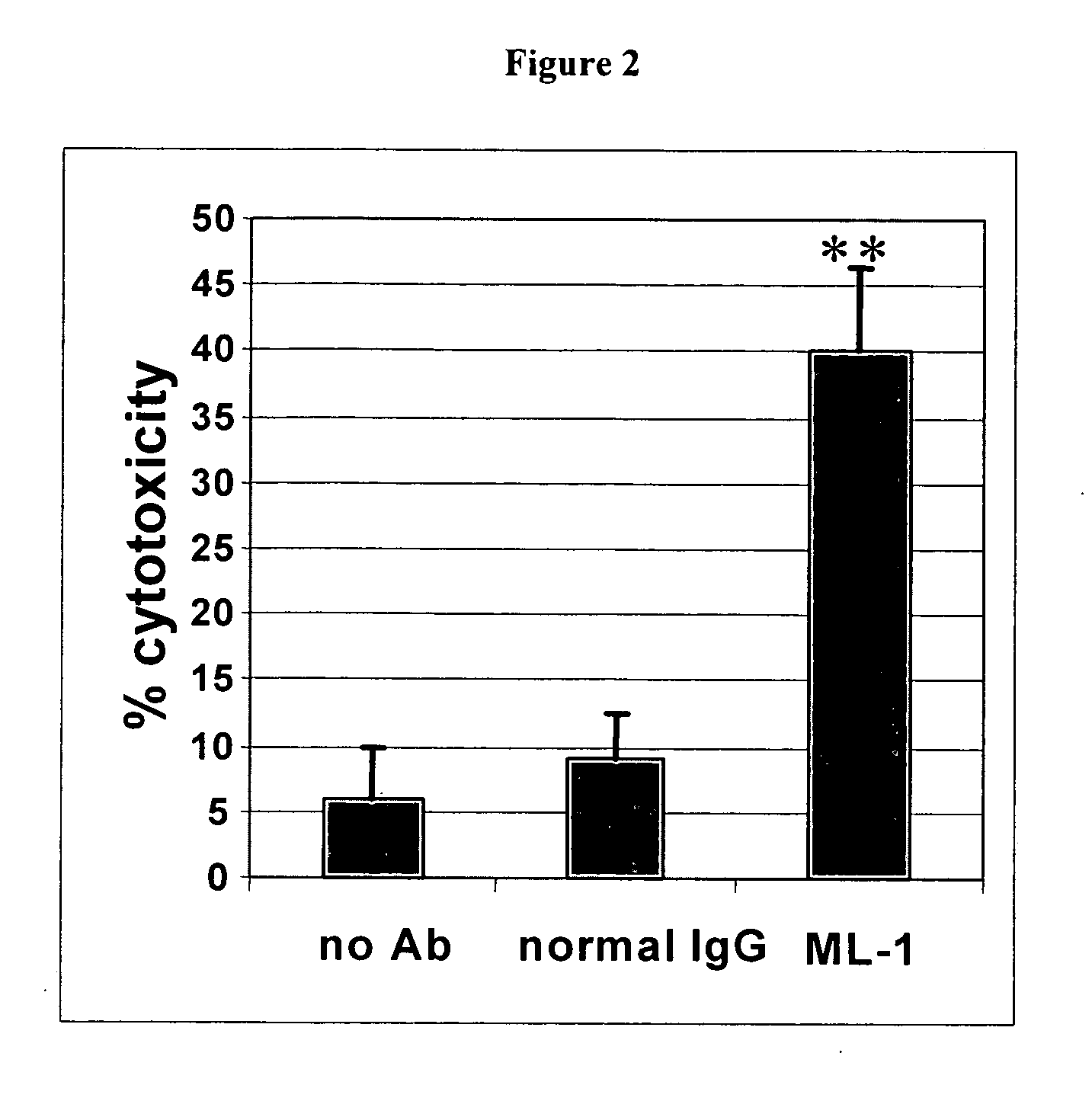
InactiveUS20060239910A1Improve anti-tumor activityEnhanced antibody-dependent cellular cytotoxicityOrganic active ingredientsNanomedicineDrug specific IgEFolate Receptor Alpha
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing folate receptor alpha (FRA) and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to FRA-expressing cells as well as in eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer cells; and methods of treating cancer using the antibodies.
Owner:EISAI INC
Detection of ion channel or receptor activity
InactiveUS20060148104A1Easy accessImprove throughputMicrobiological testing/measurementNanoinformaticsHeterologousBiological activation
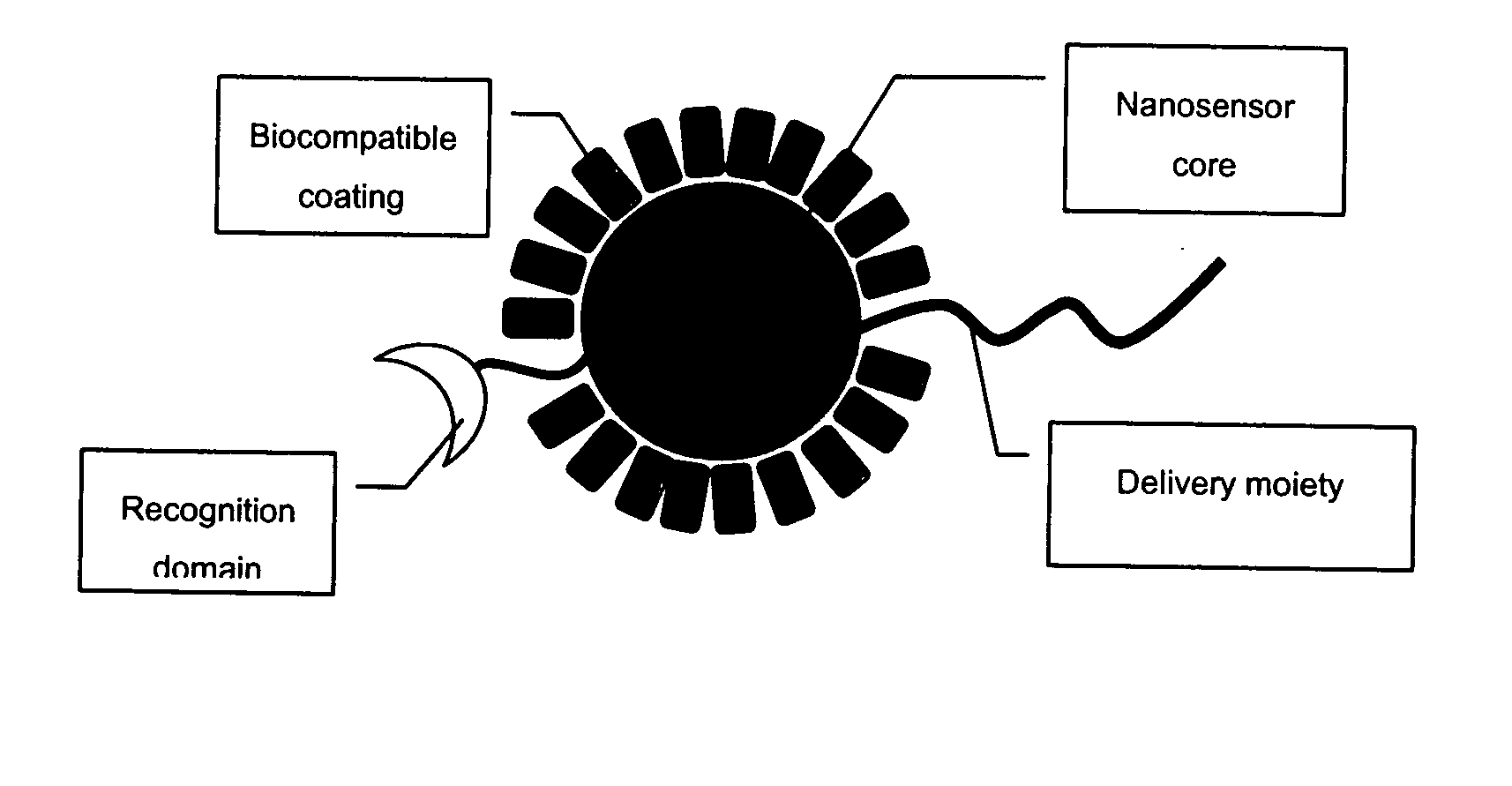
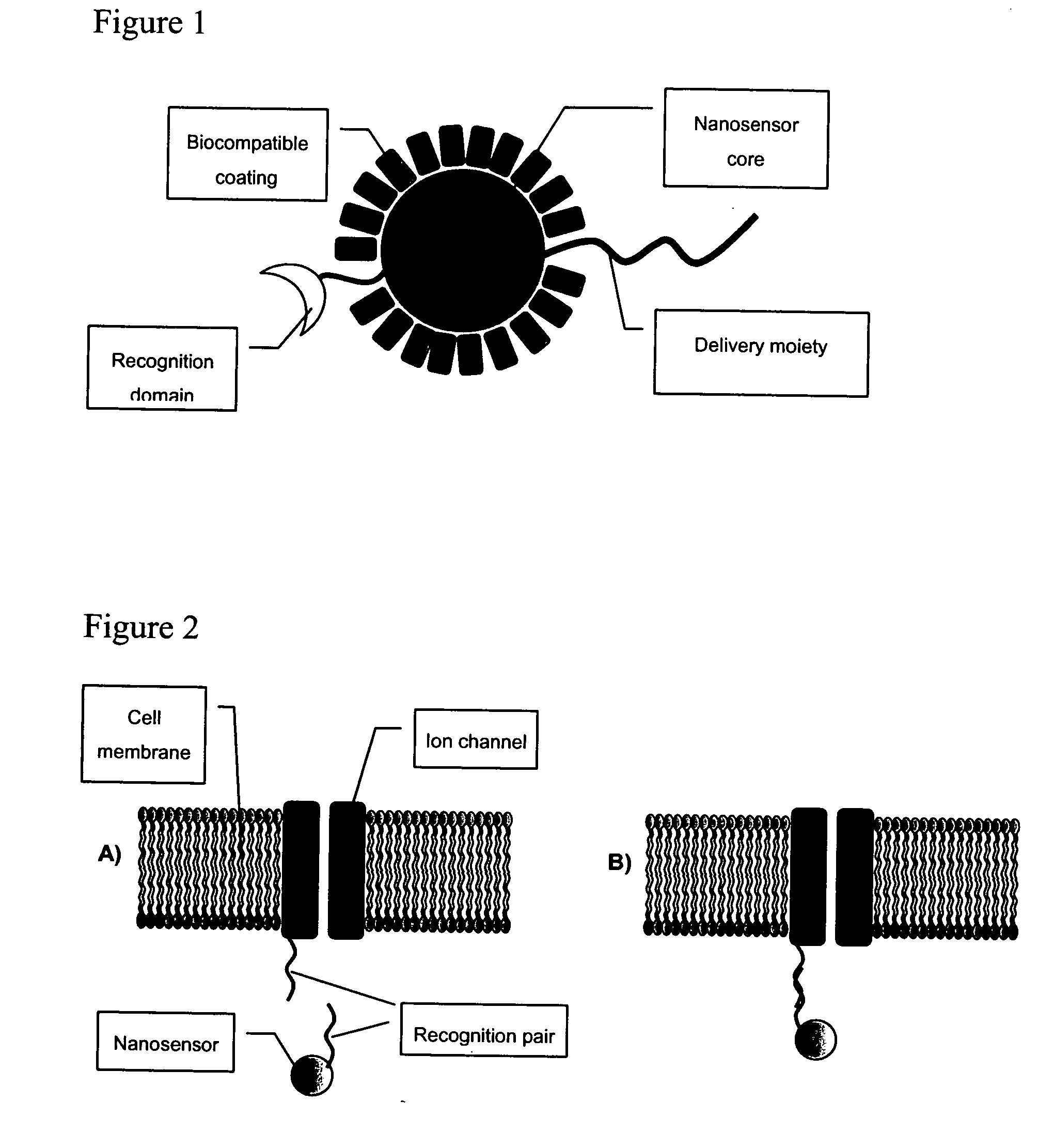
The invention provides nanosensors and nanosensor components for the detection of ion channel activity, receptor activity, or protein protein interactions. Certain of the nanosensor components comprise a nanoparticle and recognition domain. Following contact with cells and, optionally, internalization of the nanosensor component by a cell, the recognition domain binds to a target domain, e.g., a heterologous target domain, of a polypeptide of interest such as an ion channel subunit, G protein coupled receptor (GPCR), or G protein subunit. Ion channel activity, GPCR activity, or altered protein interaction results in a detectable signal. The nanoparticles may be functionalized so that they respond to the presence of an ion by altering their proximity. Certain of the nanosensors utilize the phenomenon of plasmon resonance to produce a signal while others utilize magnetic properties, RET, and / or ion-sensitive moieties. Also provided are polypeptides, e.g., ion channel subunits, comprising a heterologous target domain, and cell lines that express the polypeptides. Further provided are a variety of methods for detecting ion channel activity, receptor activity, or protein interaction and for identifying compounds that modulate one or more of these. In certain embodiments the invention allows the user to detect the activity of specific ion channels even in the presence of other channels that permit passage of the same ion(s) or result in activation of the same downstream targets, thereby achieving improved specificity in high throughput screens while at the same time providing a high signal to noise ratio.
Owner:CHILDRENS MEDICAL CENT CORP +1
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Subcellular targeting of therapeutic proteins
InactiveUS7396811B2Convenient treatmentSimple preparation processNervous disorderPeptide/protein ingredientsLysosomeTherapeutic protein
Targeted therapeutics that localize to a specific subcellular compartment such as the lysosome are provided. The targeted therapeutics include a therapeutic agent and a targeting moiety that binds a receptor on an exterior surface of the cell, permitting proper subcellular localization of the targeted therapeutic upon internalization of the receptor. Nucleic acids, cells, and methods relating to the practice of the invention are also provided.
Owner:BIOMARIN PHARMA INC
Ultrasonic concentration of carrier particles
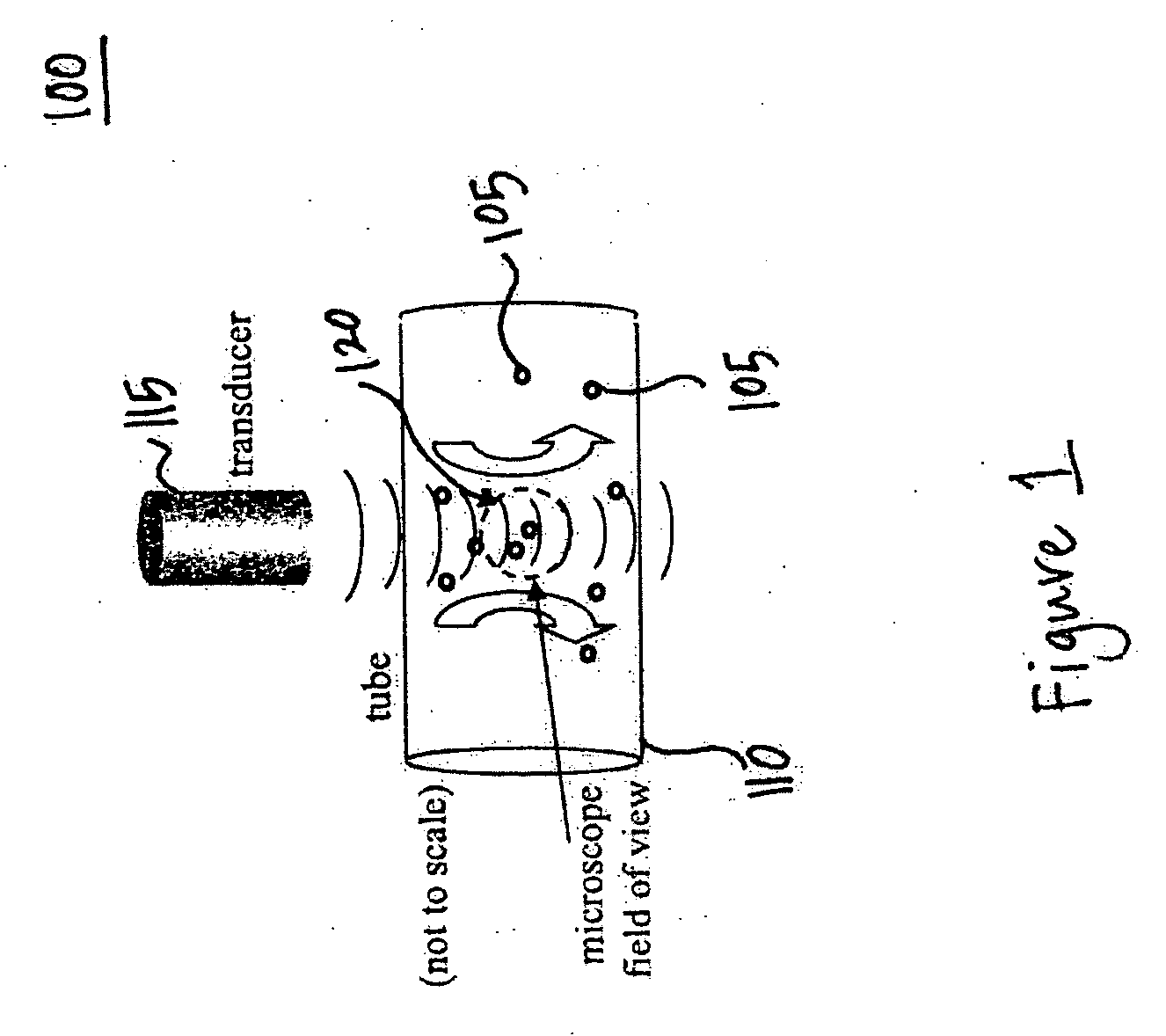
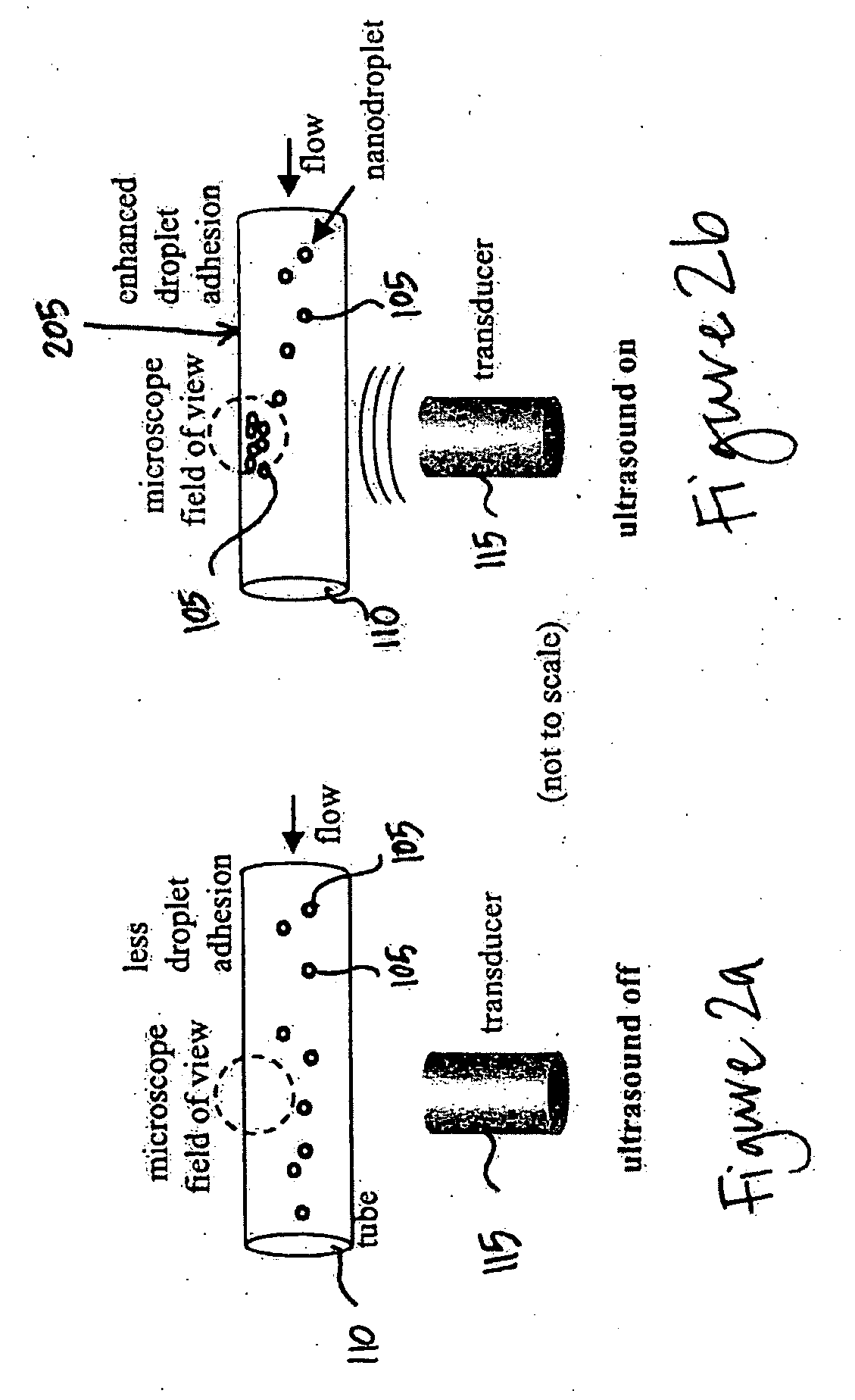
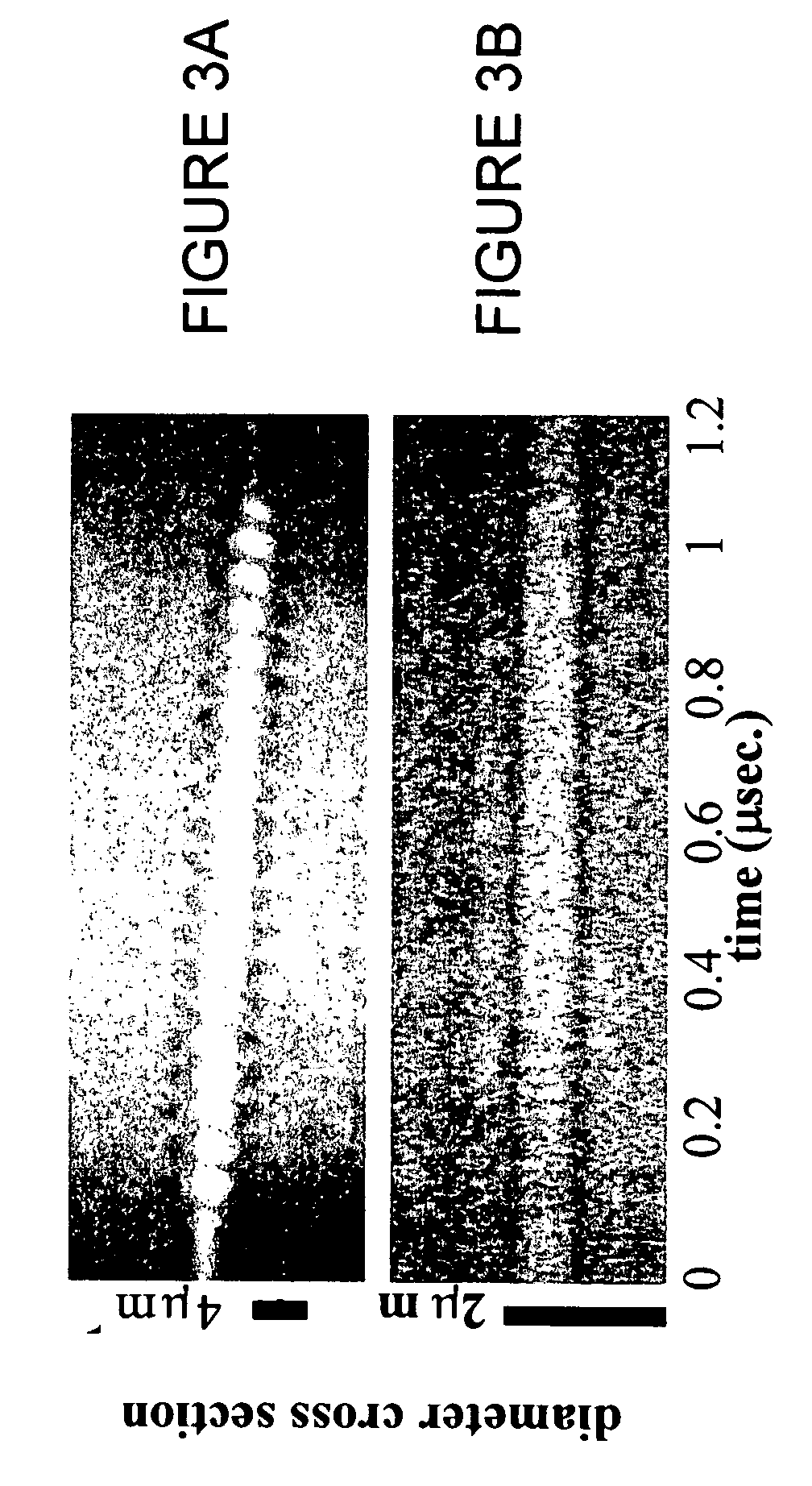
InactiveUS20070071683A1Ultrasonic/sonic/infrasonic diagnosticsBiocideMembrane permeabilityMembrane permeabilization
Methods, compositions, and apparatus for localized delivery of compounds are provided. In certain embodiments, acoustic streaming force is used to direct carrier particles to a target site, mediate particle internalization, and release associate compounds. Ultrasound radiation is preferred as the source for the acoustic streaming force. Also encompassed are embodiments in which targeting and membrane permeability enhancement are combined with imaging of the treatment site.
Owner:RGT UNIV OF CALIFORNIA +1
Targeted therapeutic proteins
InactiveUS7560424B2Convenient treatmentSimple preparation processPeptide/protein ingredientsHydrolasesLysosomeTherapeutic protein
Targeted therapeutics that localize to a specific subcellular compartment such as the lysosome are provided. The targeted therapeutics include a therapeutic agent and a targeting moiety that binds a receptor on an exterior surface of the cell, permitting proper subcellular localization of the targeted therapeutic upon internalization of the receptor. Nucleic acids, cells, and methods relating to the practice of the invention are also provided.
Owner:BIOMARIN PHARMA INC
Multispecific antigen-binding molecules and uses thereof
InactiveUS20130243775A1Cell receptors/surface-antigens/surface-determinantsSkeletal disorderBispecific antibodyAntigen binding
The present invention provides multispecific antigen-binding molecules and uses thereof. The multispecific antigen-binding molecules comprise a first antigen-binding domain that specifically binds a target molecule, and a second antigen-binding domain that specifically binds an internalizing effector protein. The multispecific antigen-binding molecules of the present invention can, in some embodiments, be bispecific antibodies that are capable of binding both a target molecule and an internalizing effector protein. In certain embodiments of the invention, the simultaneous binding of the target molecule and the internalizing effector protein by the multispecific antigen-binding molecule of the present invention results in the attenuation of the activity of the target molecule to a greater extent than the binding of the target molecule alone. In other embodiments of the invention, the target molecule is a tumor associated antigen, and the simultaneous binding of the tumor associated antigen and the internalizing effector protein by the multispecific antigen-binding molecule of the present invention causes or facilitates the targeted killing of tumor cells.
Owner:REGENERON PHARM INC
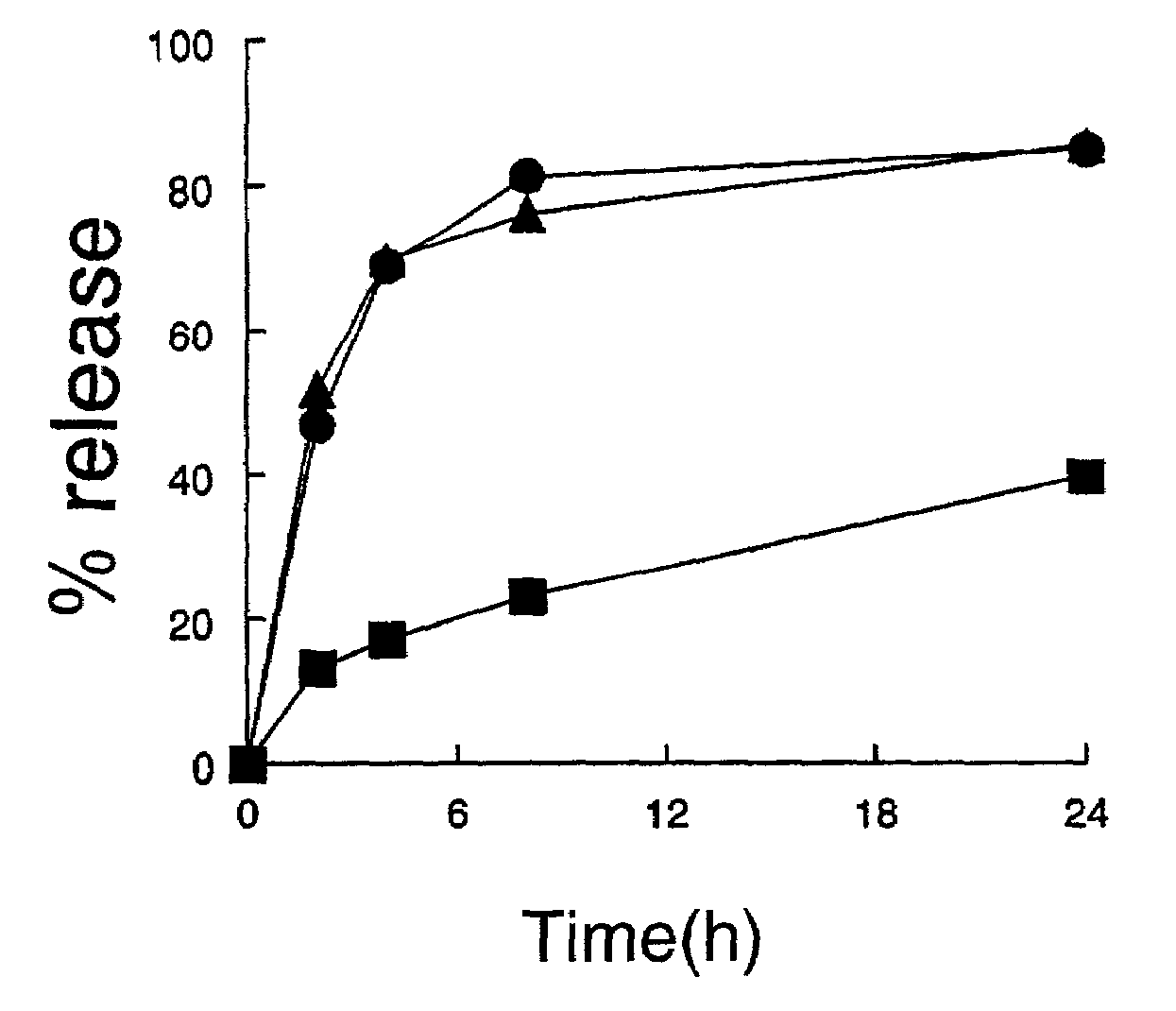
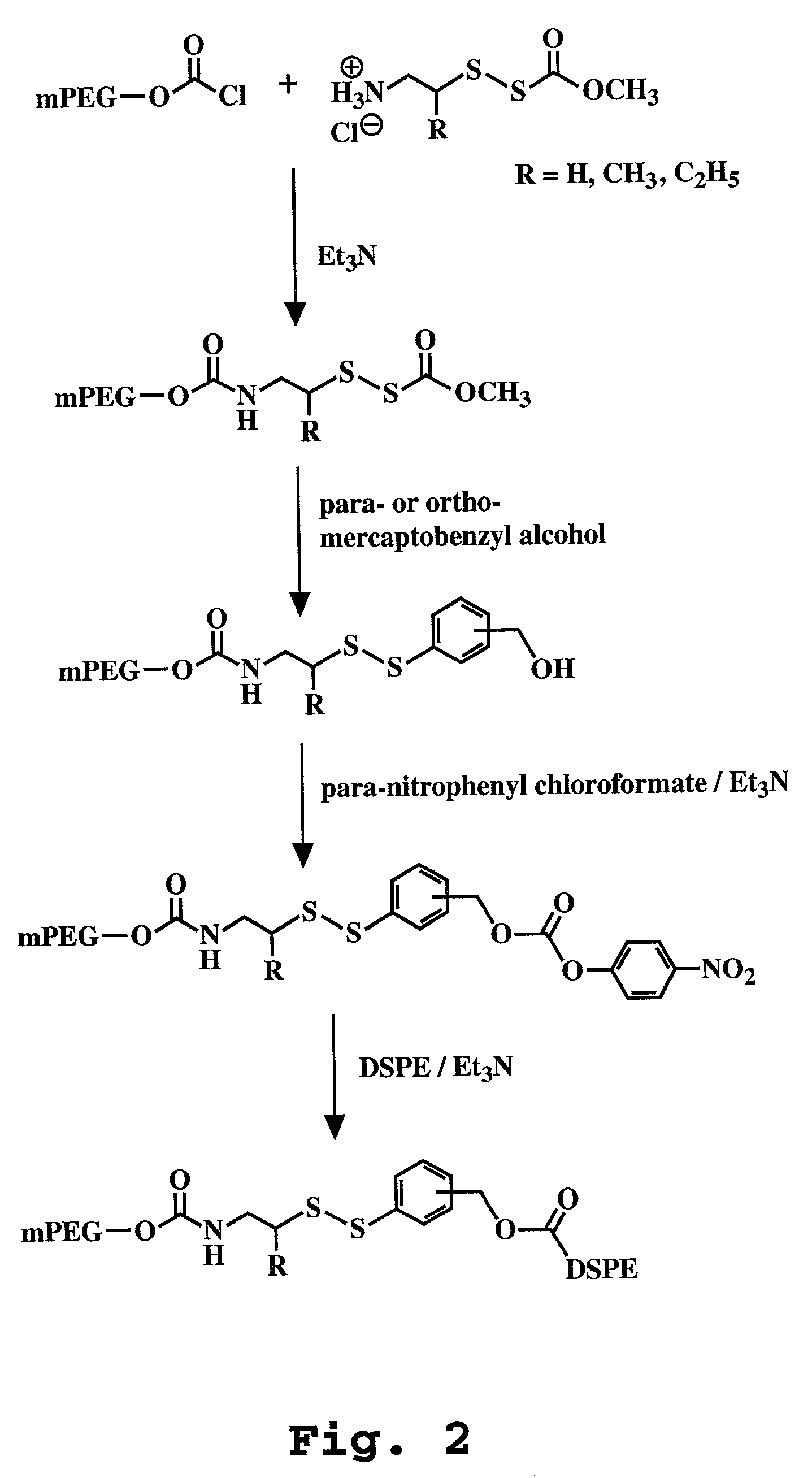
Liposome composition for improved intracellular delivery of a therapeutic agent
InactiveUS7108863B2Accelerate the accumulation processStrong cytotoxicityUltrasonic/sonic/infrasonic diagnosticsBiocideLipid formationLiposome
A liposomal composition and a method of using the same for achieving intracellular delivery of a liposome-entrapped agent is described. The liposomes are composed of a pH sensitive lipid and include a targeting ligand to direct the liposomes to a target cell. The liposomes also include a stabilizing component, such a polymer-derivatized lipid, where the polymer is attached to the lipid by a releasable linkage. Administration of the liposomes results in cellular internalization and destabilization of the liposome for intracellular delivery of the entrapped agent.
Owner:ALZA CORP
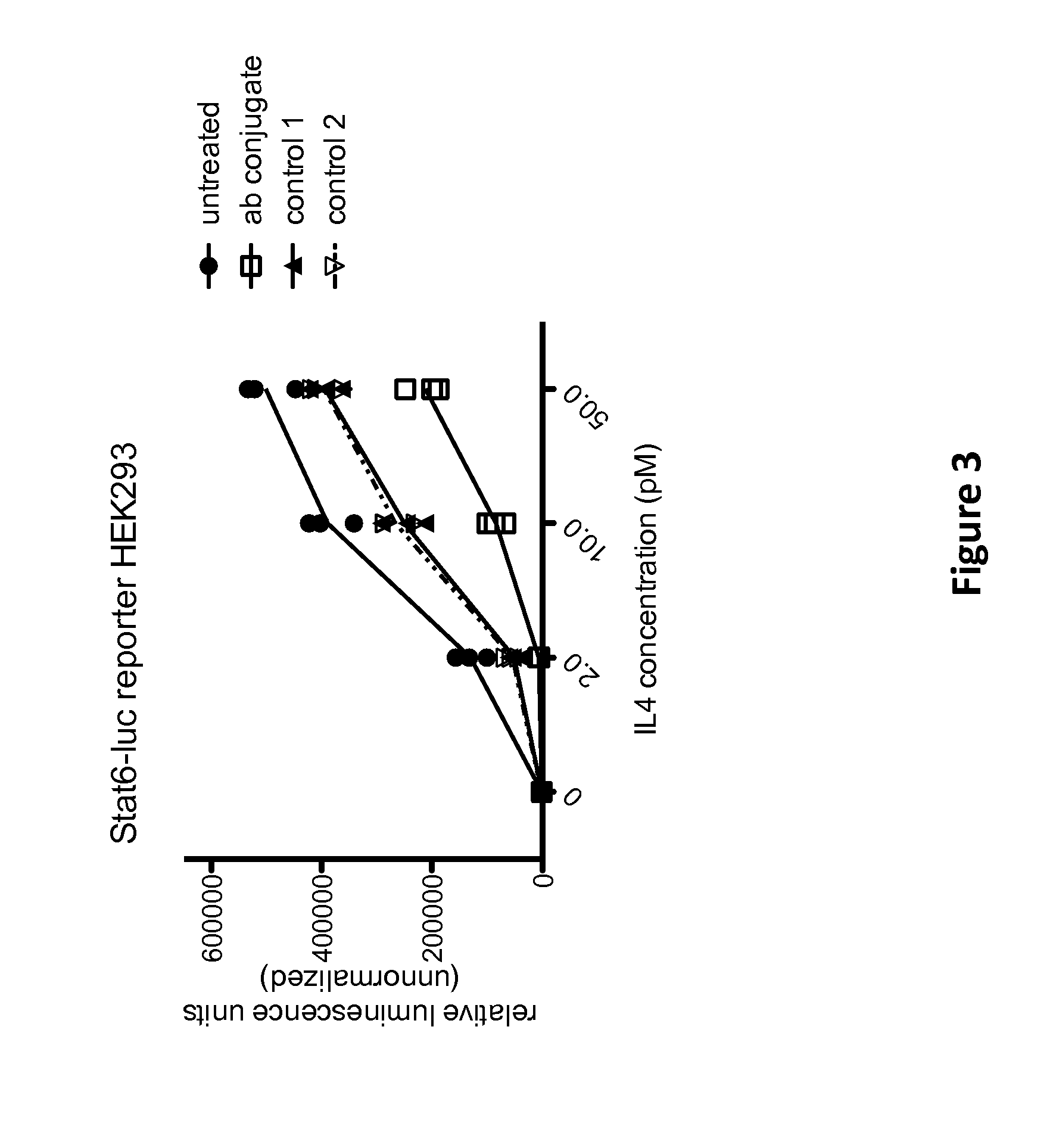
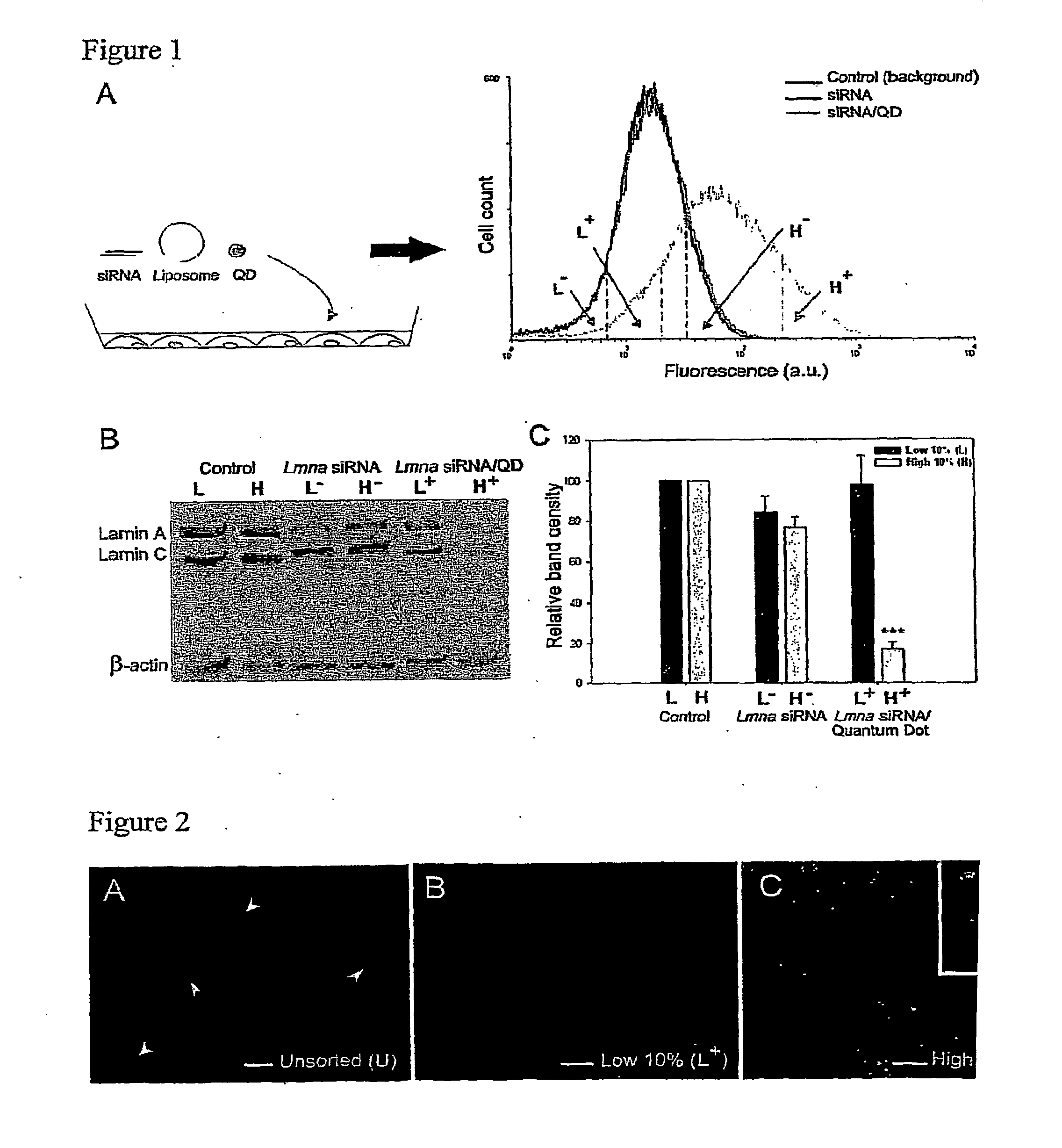
Compositions and Methods to Monitor RNA Delivery to Cells
InactiveUS20090317802A1Microbiological testing/measurementGeneral/multifunctional contrast agentsNanoparticleQuantum dot
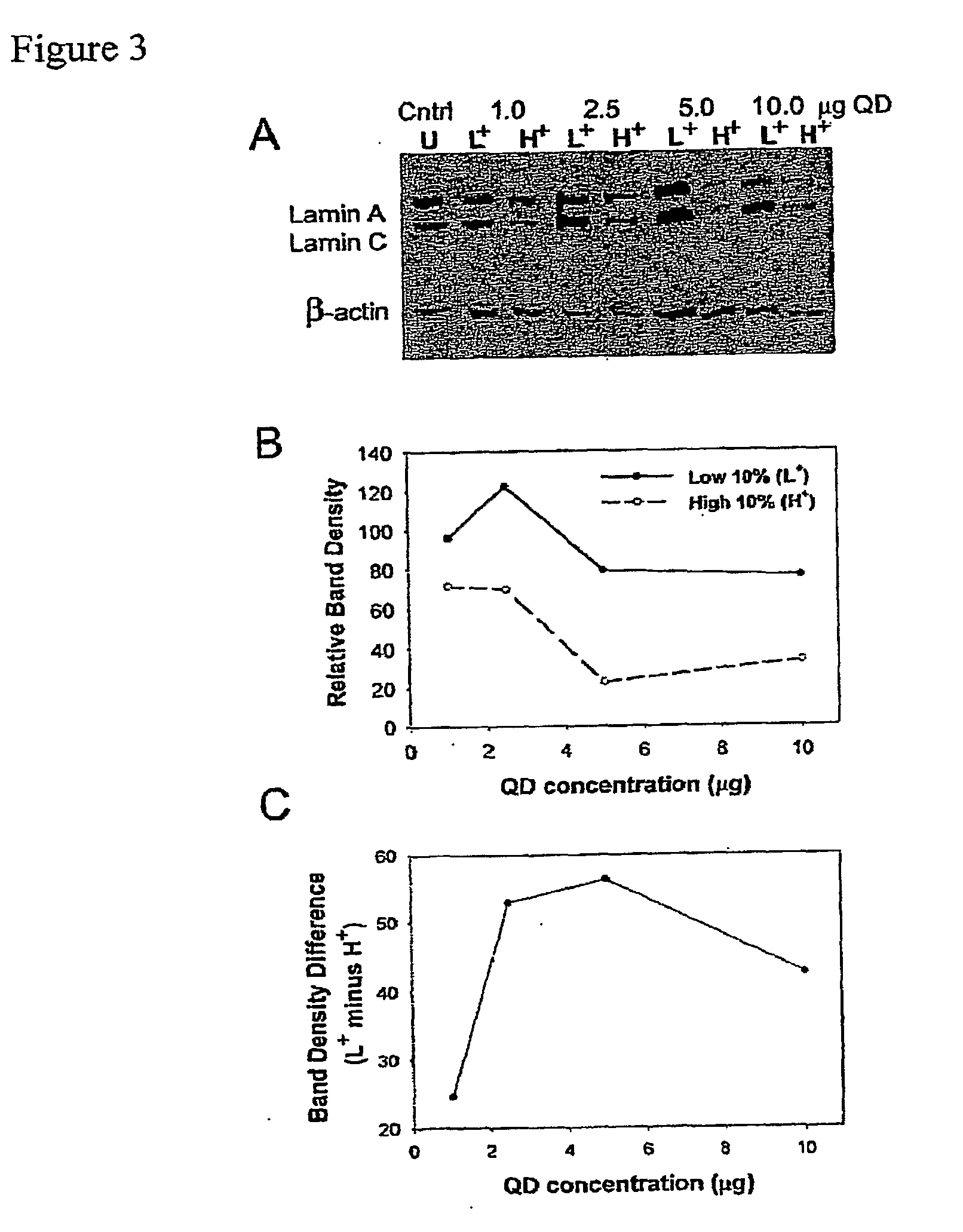
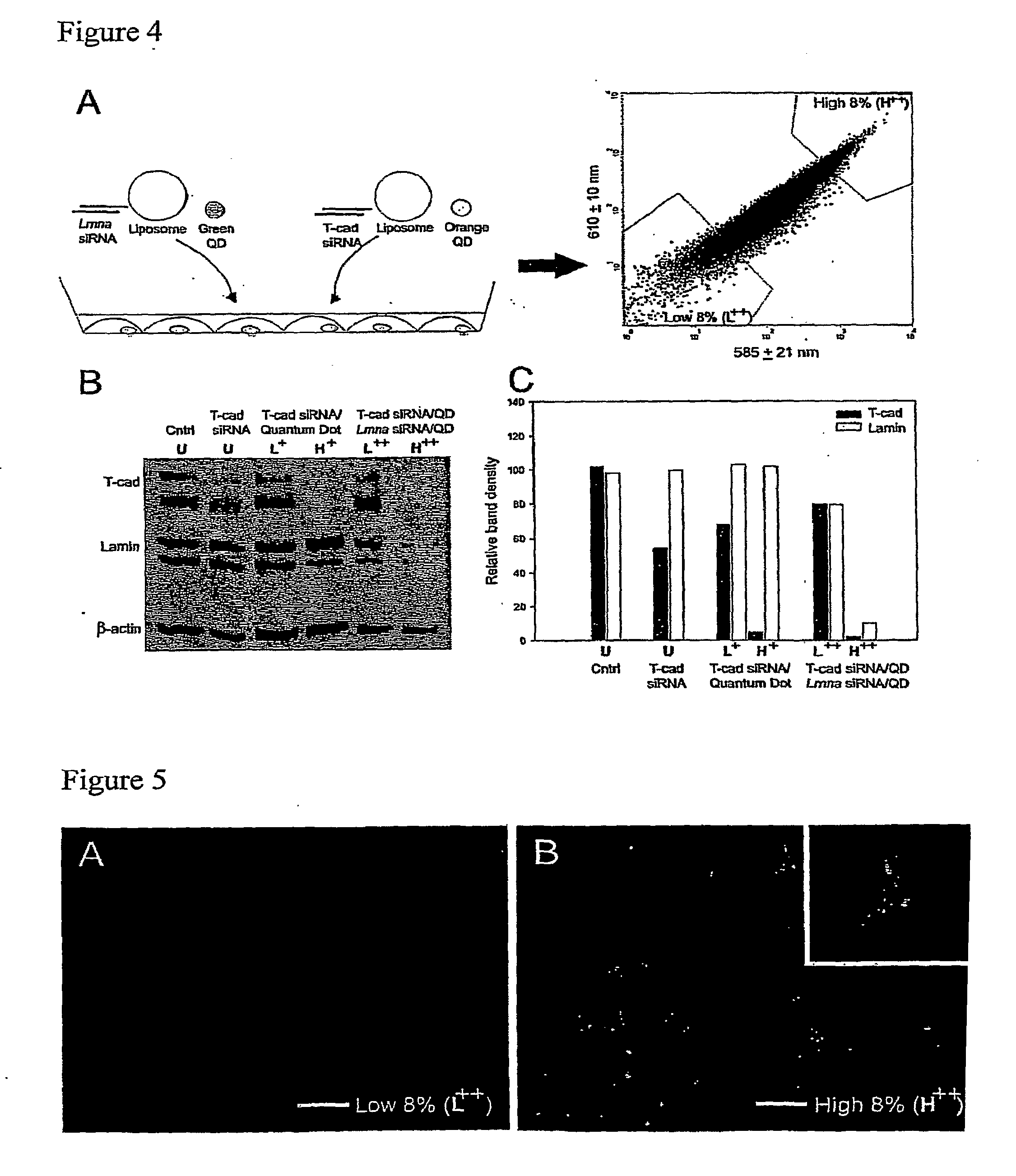
Methods and compositions for tracking or monitoring uptake of siRNA by mammalian cells are provided. The methods and compositions may be used to monitoring the silencing activity of the internalized siRNA. The compositions contain an siRNA, an optically or magnetically detectable nanoparticle such as a quantum dot and, optionally, a transfection reagent. Cells are contacted with an siRNA and an optically or magnetically detectable nanoparticle, optionally in the presence of a transfection reagent. Detection of internalized nanoparticles is indicative of siRNA uptake. The invention allows analysis, identification, processing, etc., of cells that have efficiently taken up siRNA. In one embodiment, cells are sorted into at least two populations based on the amount of siRNA taken up.
Owner:RGT UNIV OF CALIFORNIA
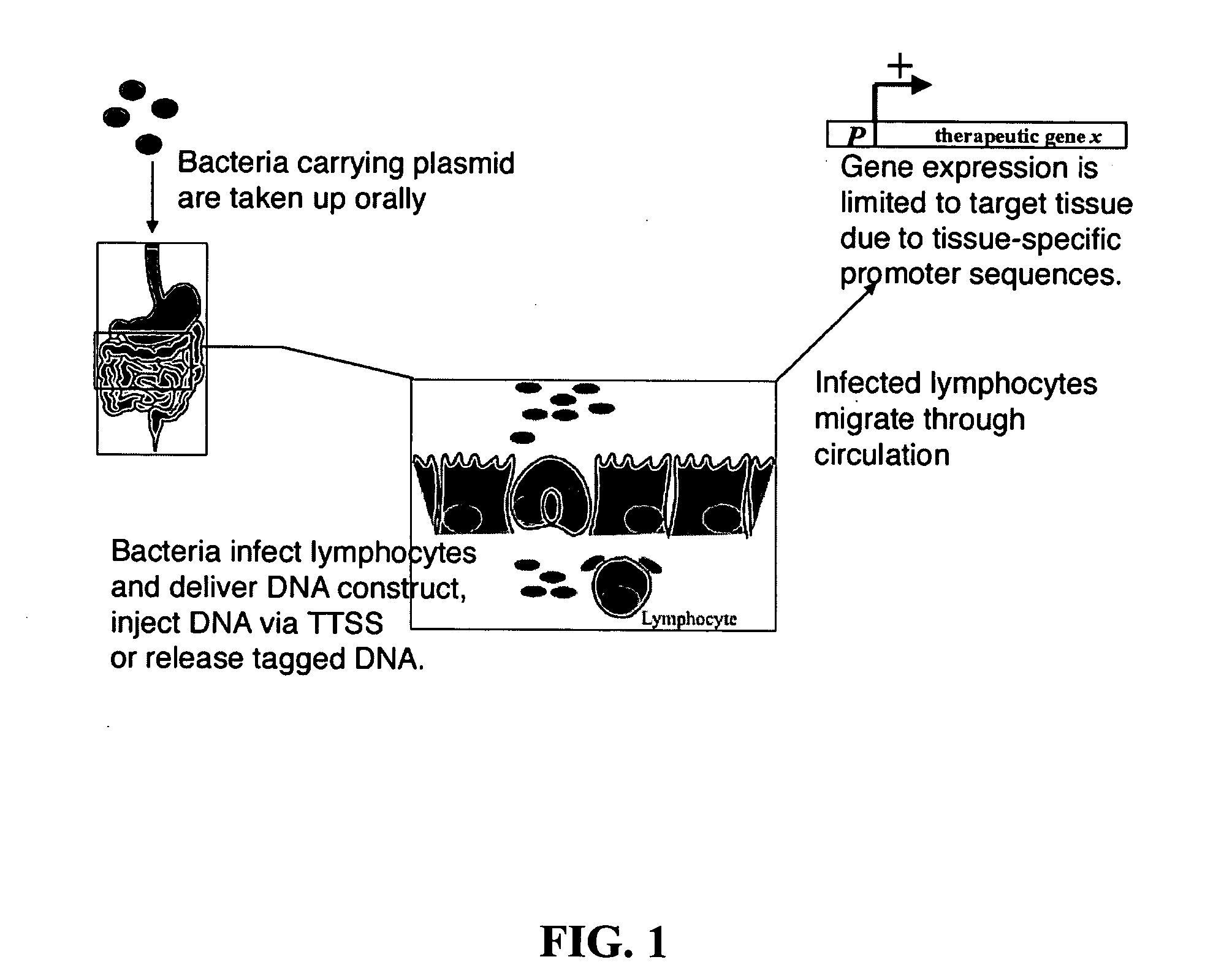
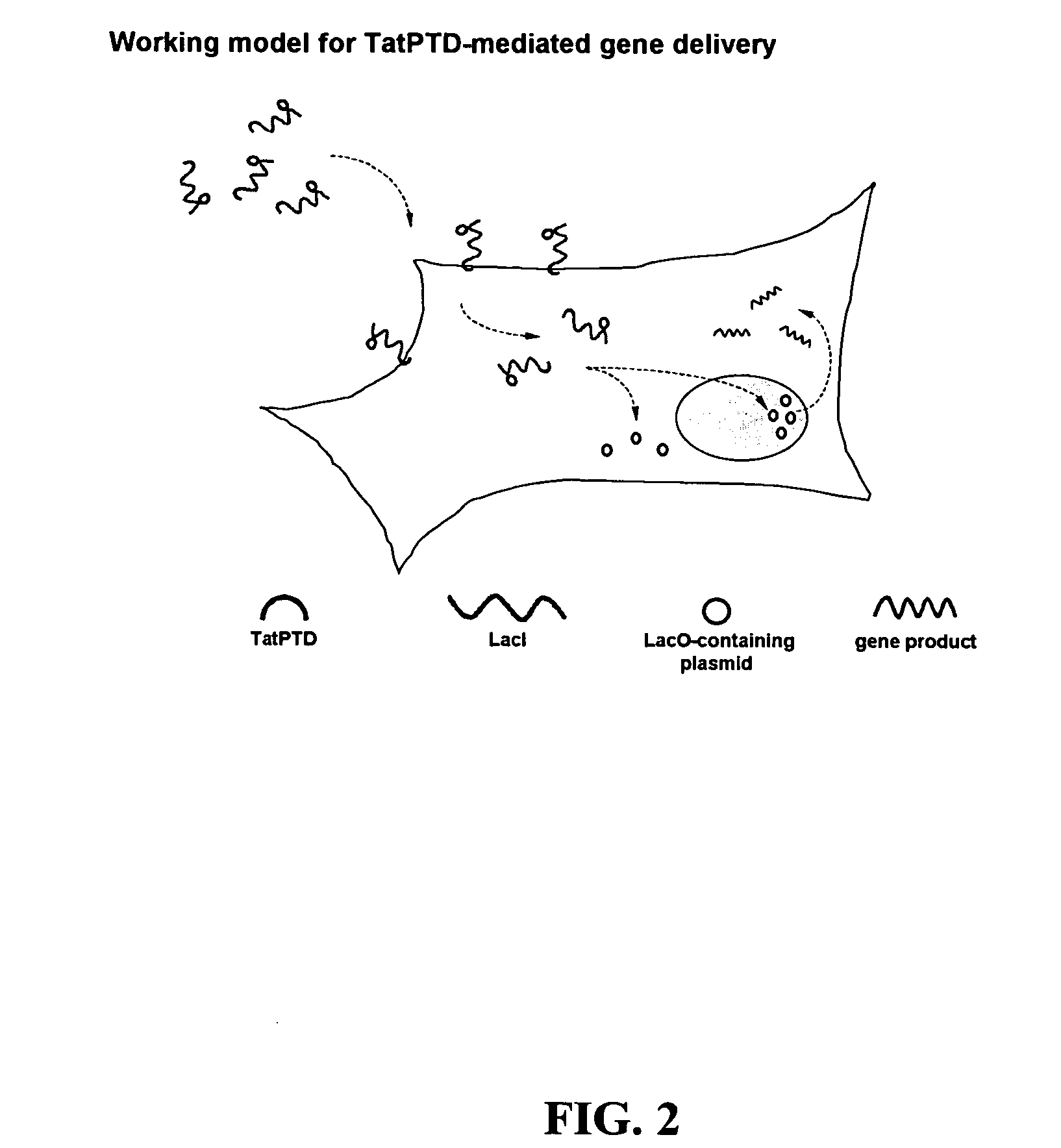
Bacterial vector systems
InactiveUS20060068469A1Efficient rapid deliveryFusion with DNA-binding domainBacteriaGene deliveryGene targets
The present invention provides bacterial vectors and fusion proteins containing a TTSS polypeptide, compositions of such fusion proteins including polynucleotides, and methods of delivering one or more genes into a target cell that involve contacting the cell with a composition that includes such a fusion protein. Compositions and methods of gene delivery that involve a bacterium and a TAT, Antp, or HSV VP22 polypeptide are also disclosed. The invention also concerns methods of delivering one or more genes into a target cell utilizing a bacterium capable of becoming internalized within the cell, wherein the bacterium includes one or more genes targeted for delivery to the cell, a gene encoding an RNA polymerase, and a gene that causes lysis of the bacterium.
Owner:RES DEVMENT FOUND
Antibodies against csf-1r
ActiveUS20110243947A1Prevent dimerizationInhibit tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDocetaxel-PNPDocetaxel
The invention provides a human antibody that binds human CSF-1R with high affinity. Antibodies of the present invention have significant advantages over the antibodies known in the art by being multifunctional: inhibiting signaling of CSF-1R, internalizing and inducing CSF-1R degradation and stimulating ADCC in cell including tumors, macrophages and monocytes. They are also shown to be effective in treating leukemia, breast, endometrial and prostate cancer alone or in combination with docetaxel, paclitaxel, Herceptin® or doxorubicin.
Owner:IMCLONE SYSTEMS
Antibodies That Bind OV064 and Methods of Use Therefor
InactiveUS20090208489A1Promote growthReduce induced immune suppressionAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeAntigen Binding Fragment
Antibodies and antigen-binding fragments of antibodies that bind OV064 are disclosed. The antibodies bind an extracellular domain of OV064. Some of the antibodies and antigen-binding fragments bind an epitope on OV064 sufficient to induce internalization. In some embodiments, the antibodies are humanized, chimeric or human. Nucleic acids and vectors encoding the antibodies or portions thereof, recombinant cells that contain the nucleic acids, and compositions comprising the antibodies or antigen-binding fragments are also disclosed. The invention also provides therapeutic and diagnostic methods utilizing the antibodies and antigen-binding fragments provided herein.
Owner:MILLENNIUM PHARMA INC
Targeted therapeutic proteins
InactiveUS7629309B2Convenient treatmentSimple preparation processPeptide/protein ingredientsAntibody mimetics/scaffoldsTherapeutic proteinLysosome
Targeted therapeutics that localize to a specific subcellular compartment such as the lysosome are provided. The targeted therapeutics include a therapeutic agent and a targeting moiety that binds a receptor on an exterior surface of the cell, permitting proper subcellular localization of the targeted therapeutic upon internalization of the receptor. Nucleic acids, cells, and methods relating to the practice of the invention are also provided.
Owner:BIOMARIN PHARMA INC
Methods and compositions using peptides and proteins with c-terminal elements
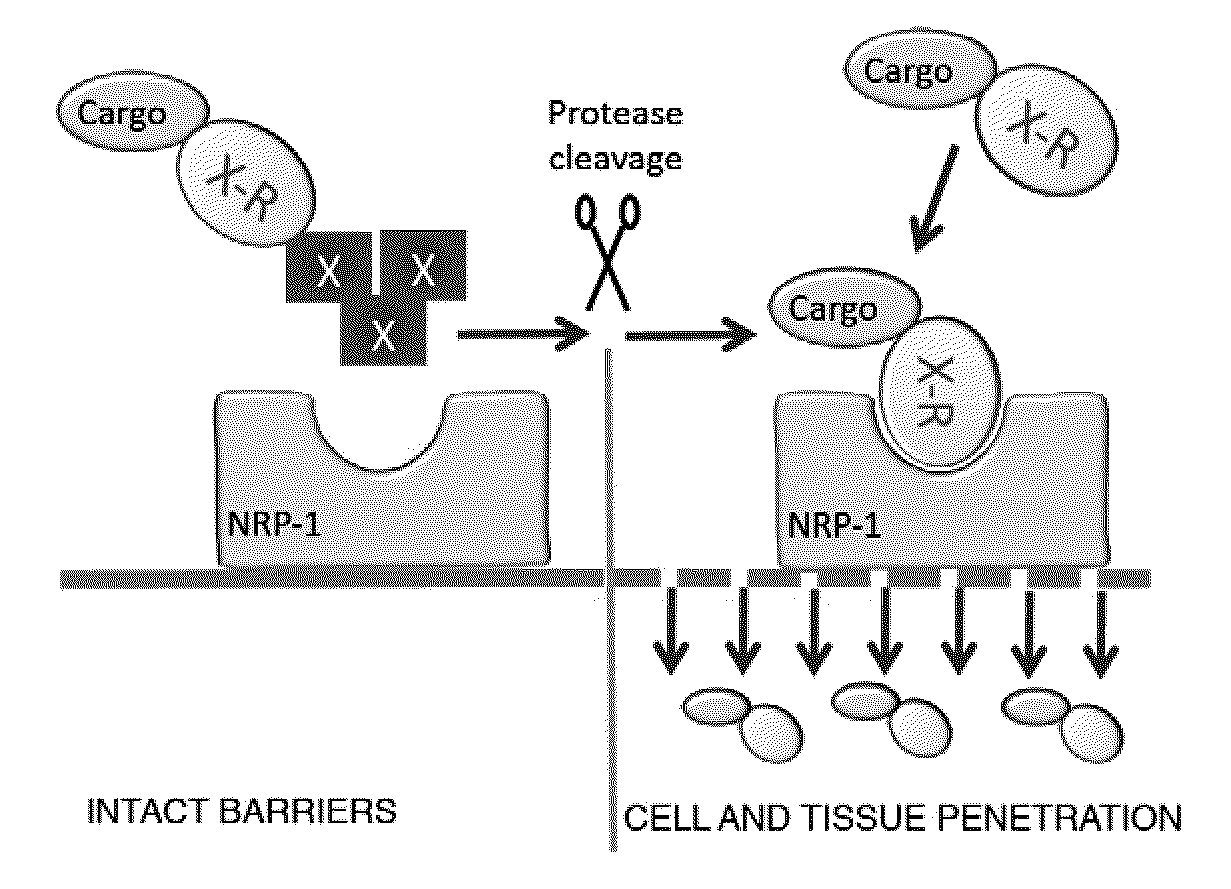
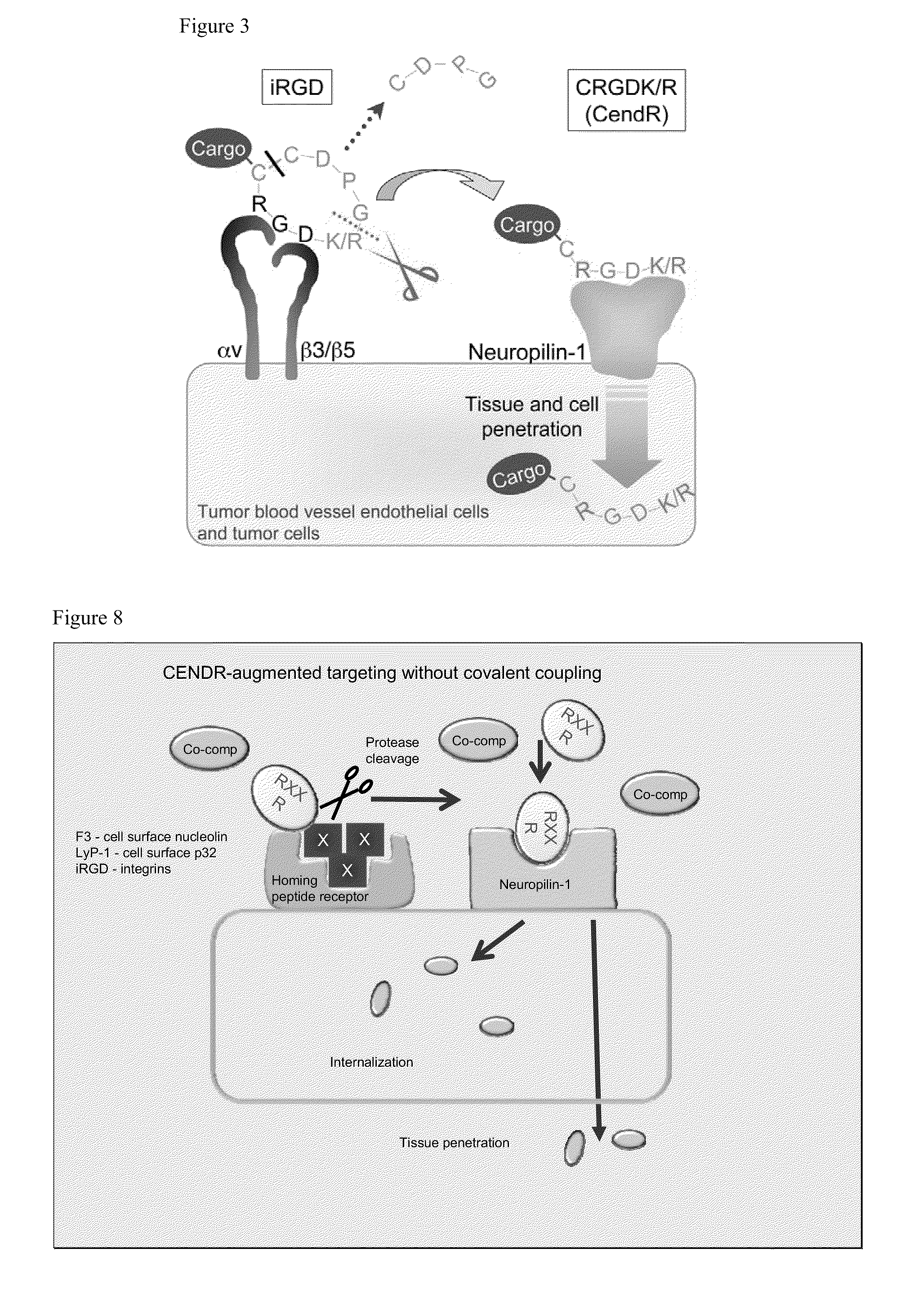
ActiveUS20100322862A1Improve permeabilityInternalization of enhancedOrganic active ingredientsPeptide/protein ingredientsCell selectivityPeptide sequence
Disclosed are compositions and methods useful for targeting and internalizing molecules into cells of interest and for penetration by molecules of tissues of interest. The compositions and methods are based on peptide sequences that are selectively internalized by a cell, penetrate tissue, or both. The disclosed internalization and tissue penetration is useful for delivering therapeutic and detectable agents to cells and tissues of interest.
Owner:RICOH KK +1
Compositions and methods for enhancing receptor-mediated cellular internalization
InactiveUS6908623B2Promote absorptionMaximizing expressionPowder deliveryOrganic active ingredientsDiagnostic agentWhole body
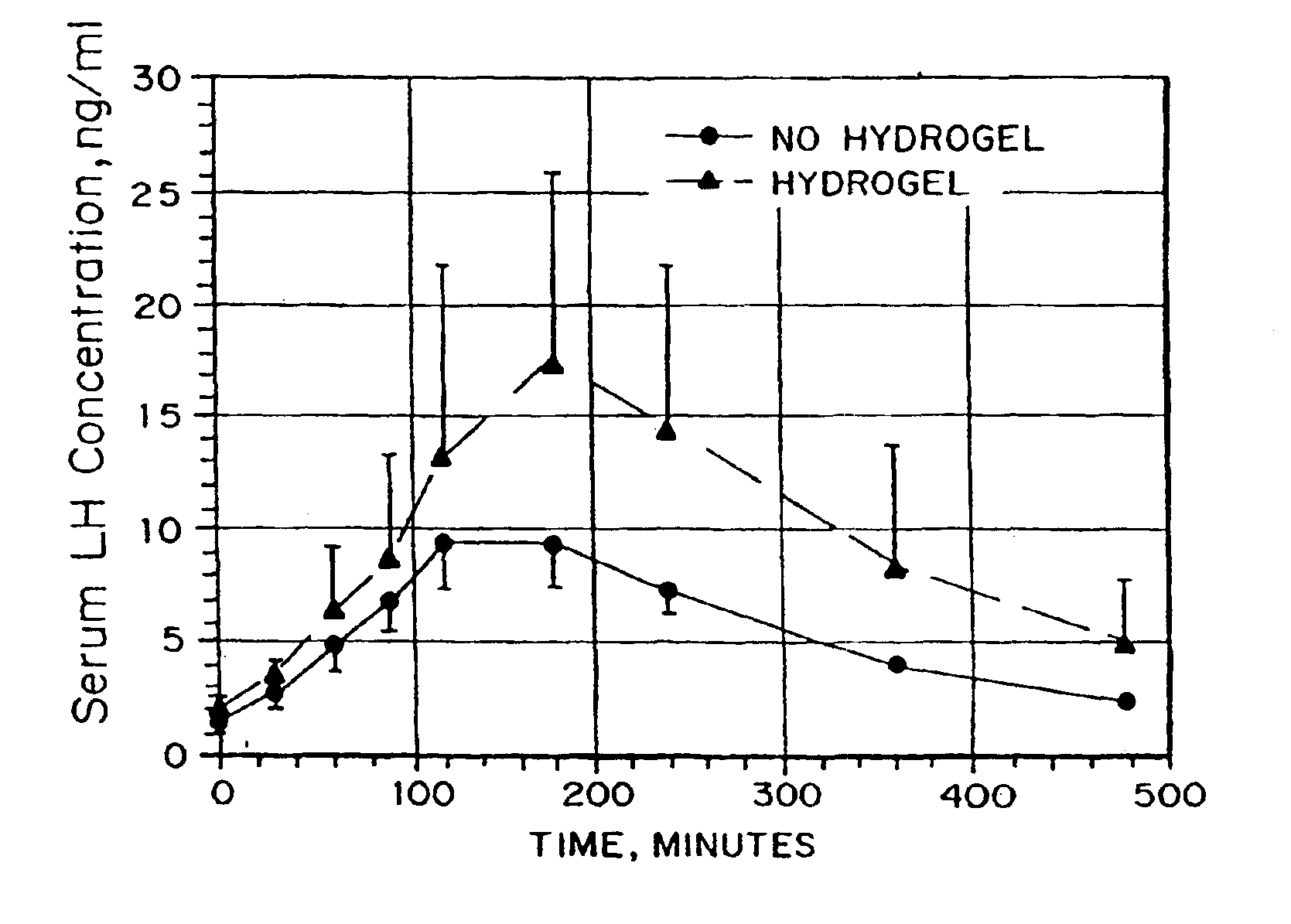
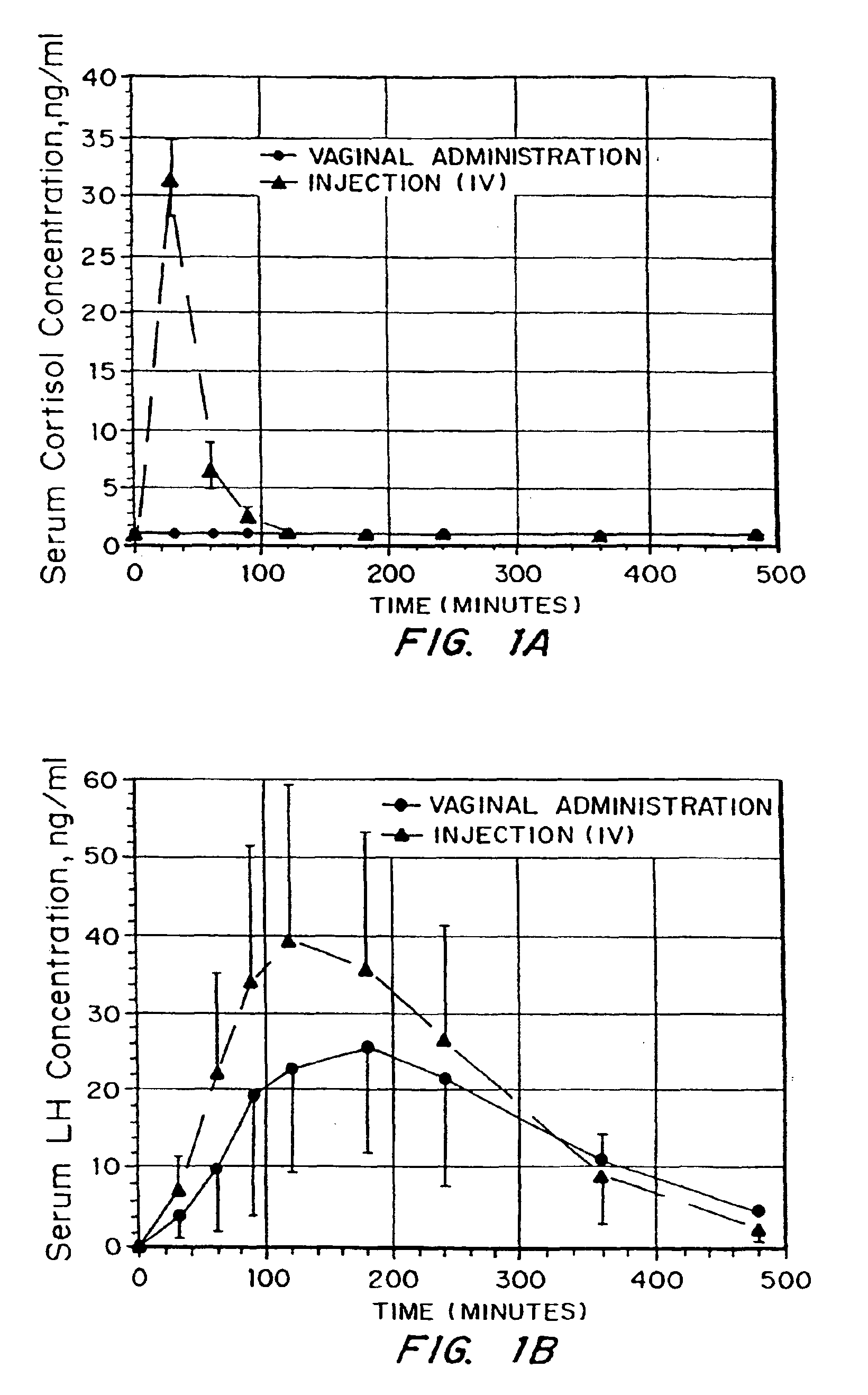
Compositions and methods for improving cellular internalization of one or more compounds are disclosed. The compositions include a compound to be delivered and a biocompatible viscous material, such as a hydrogel, lipogel, or highly viscous sol. The composition also include, or are administered in conjunction with, an enhancer in an amount effective to maximize expression of or binding to receptors and enhance RME of the compound into the cells. This leads to high transport rates of compounds to be delivered across cell membranes, facilitating more efficient delivery of drugs and diagnostic agents. Compositions are applied topically orally, nasally, vaginally, rectally, and ocularly. The enhancer is administered with the composition or separately, either systemically or preferably locally. The compound to be delivered can also be the enhancer.
Owner:PENN STATE RES FOUND
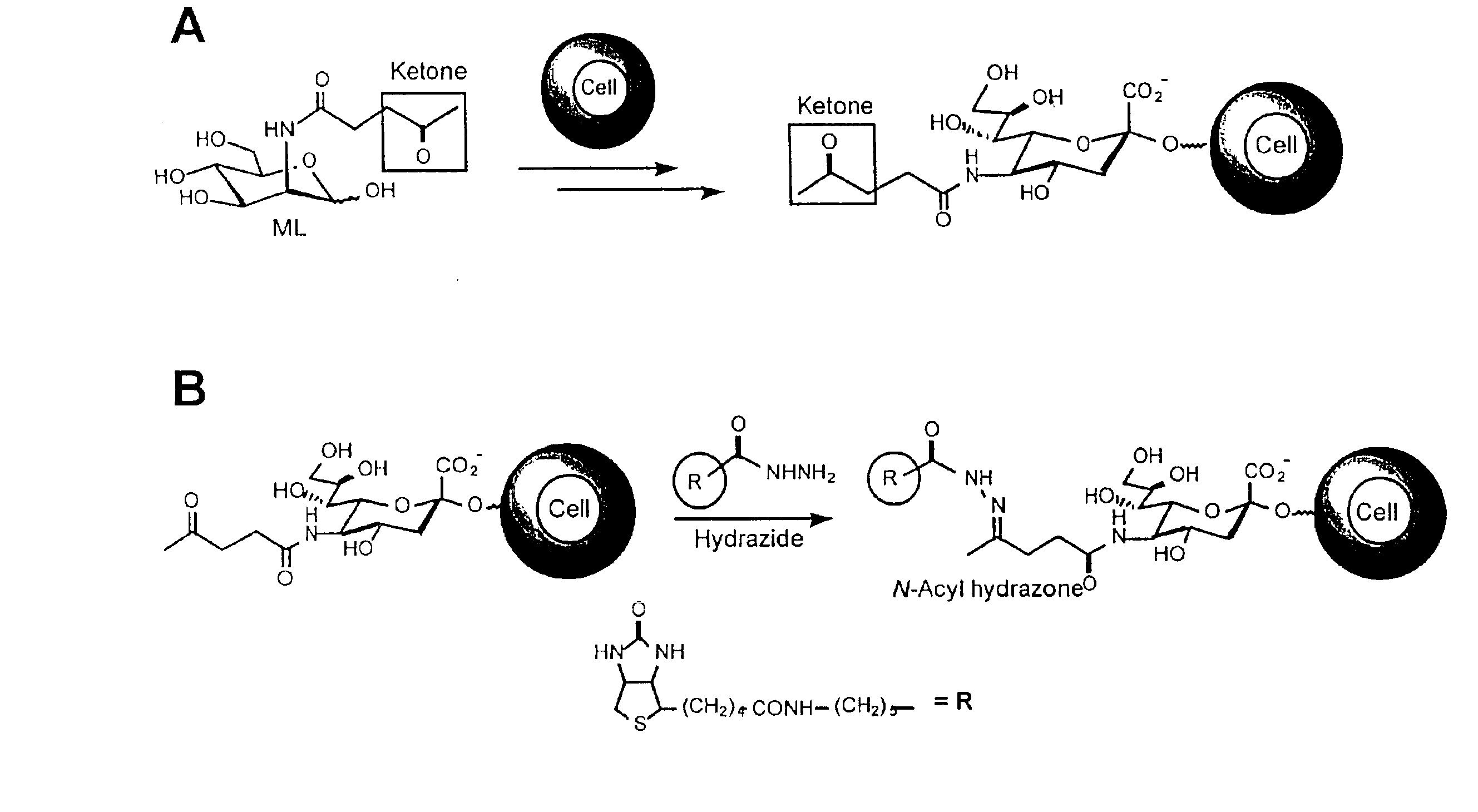
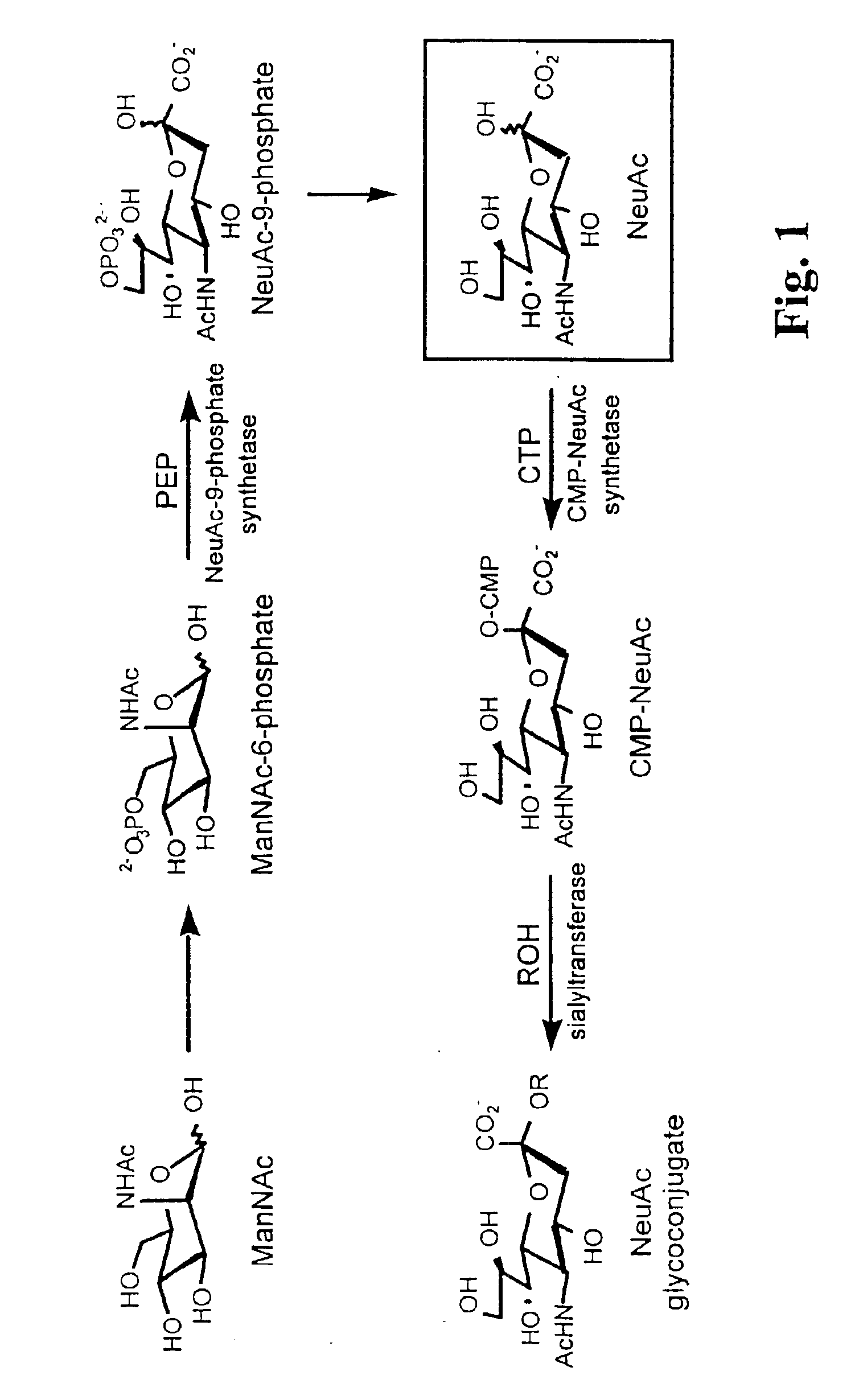
Glycoconjugates and methods
Methods for making the functionalized glycoconjugates include (a) contacting a cell with a first monosaccharide, and (b) incubating the cell under conditions whereby the cell (i) internalizes the first monosaccharide, (ii) biochemically processes the first monosaccharide into a second saccharide, (iii) conjugates the saccharide to a carrier to form a glycoconjugate, and (iv) extracellularly expresses the glycoconjugate to form an extracellular glycoconjugate comprising a selectively reactive functional group. Methods for forming products at a cell further comprise contacting the functional group of the extracellularly expressed glycoconjugate with an agent which selectively reacts with the functional group to form a product. Subject compositions include cyto-compatible monosaccharides comprising a nitrogen or ether linked functional group selectively reactive at a cell surface and compositions and cells comprising such saccharides.
Owner:RGT UNIV OF CALIFORNIA
Cell surface coupling of nanoparticles
ActiveUS20170080104A1Efficiently and stably couplingMaintenance of such surfaceAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsBiologySurface modified
The present disclosure is directed, in some embodiments, to methods and compositions of comprising a cell having a non-internalizing receptor, and a nanoparticle surface-modified with a ligand that binds to the non-internalizing receptor.
Owner:MASSACHUSETTS INST OF TECH
Combined Active and Passive Targeting of Biologically Active Agents
InactiveUS20070287680A1Good curative effectLow in DOPolypeptide with localisation/targeting motifOrganic active ingredientsActive agentEfficacy
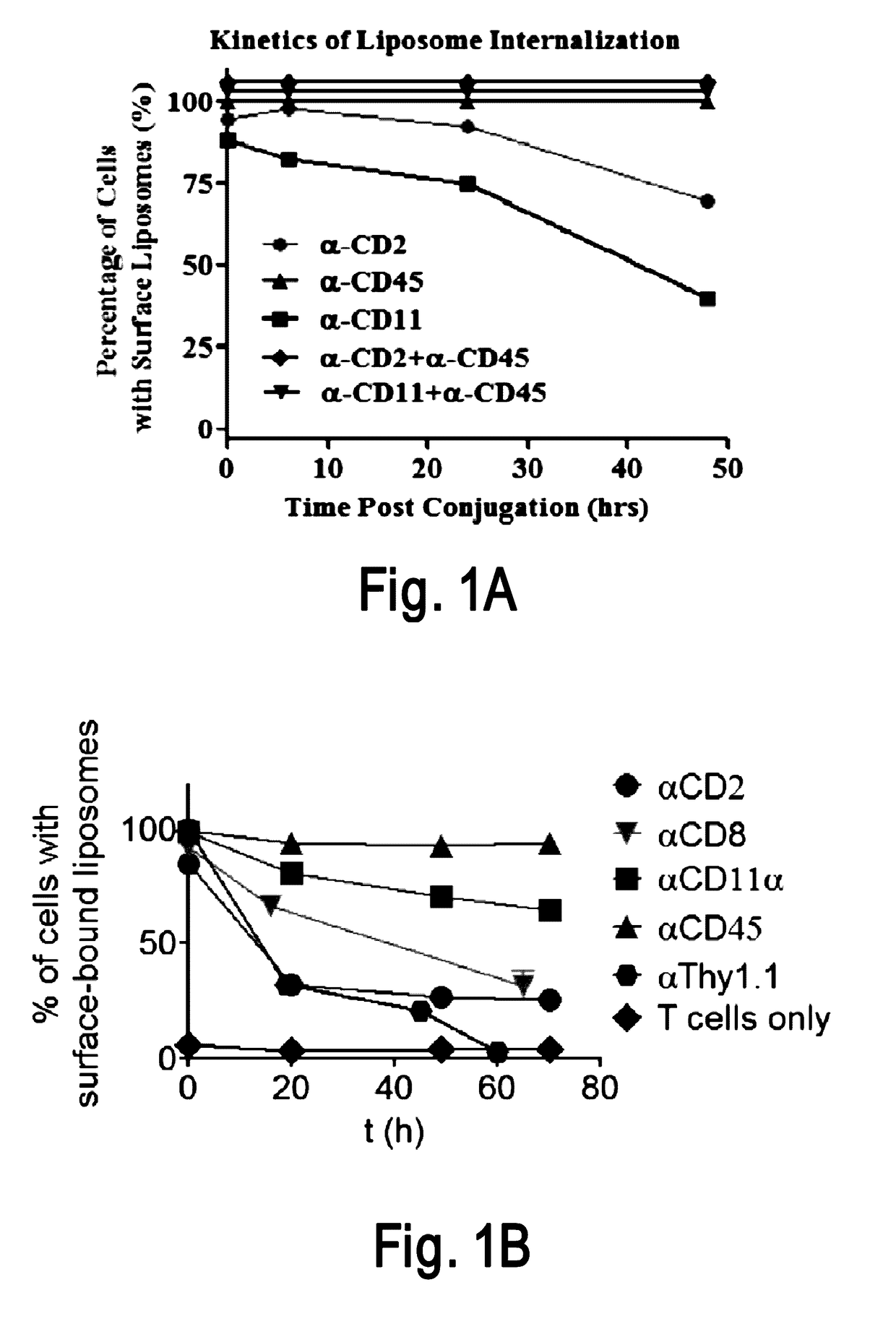
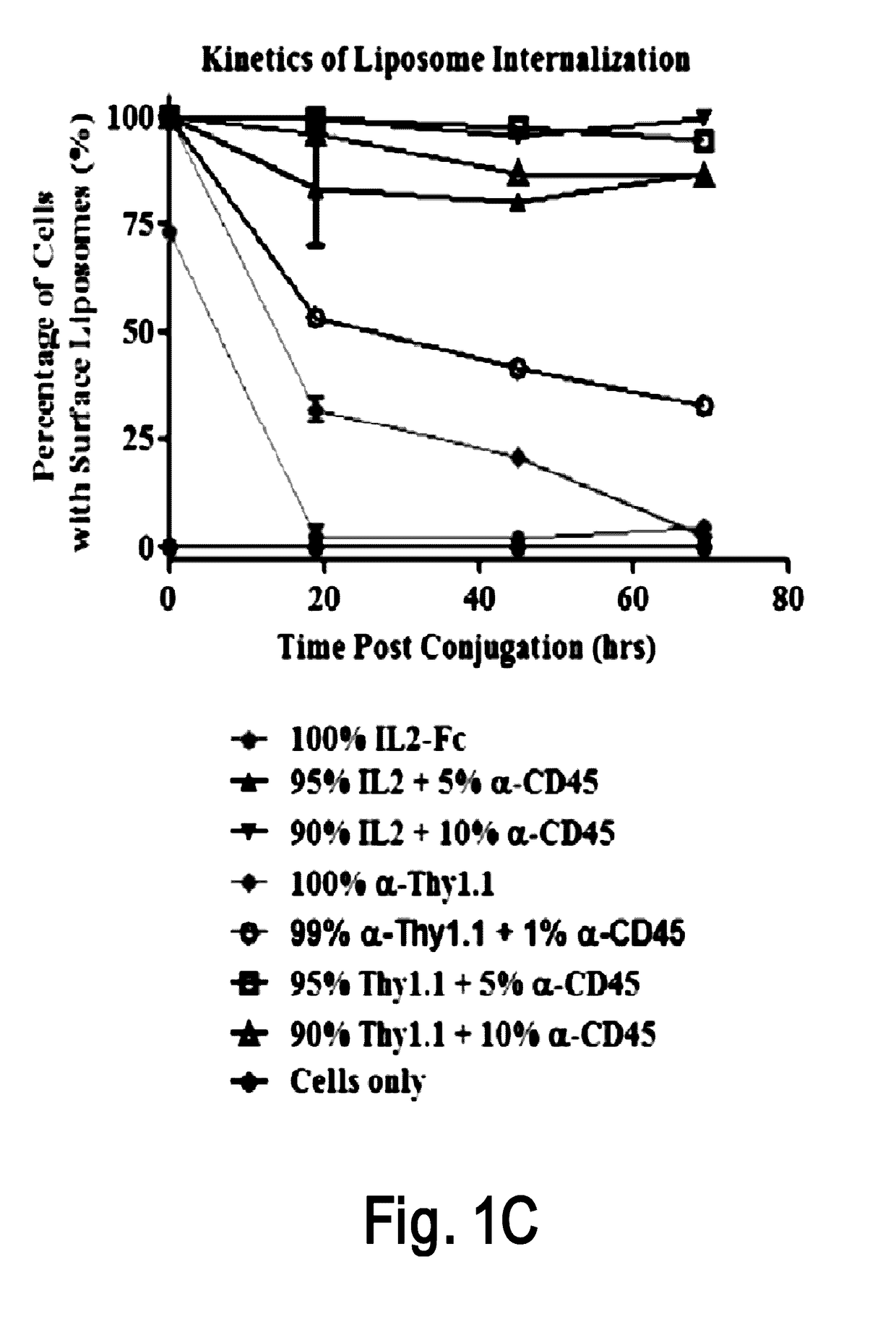
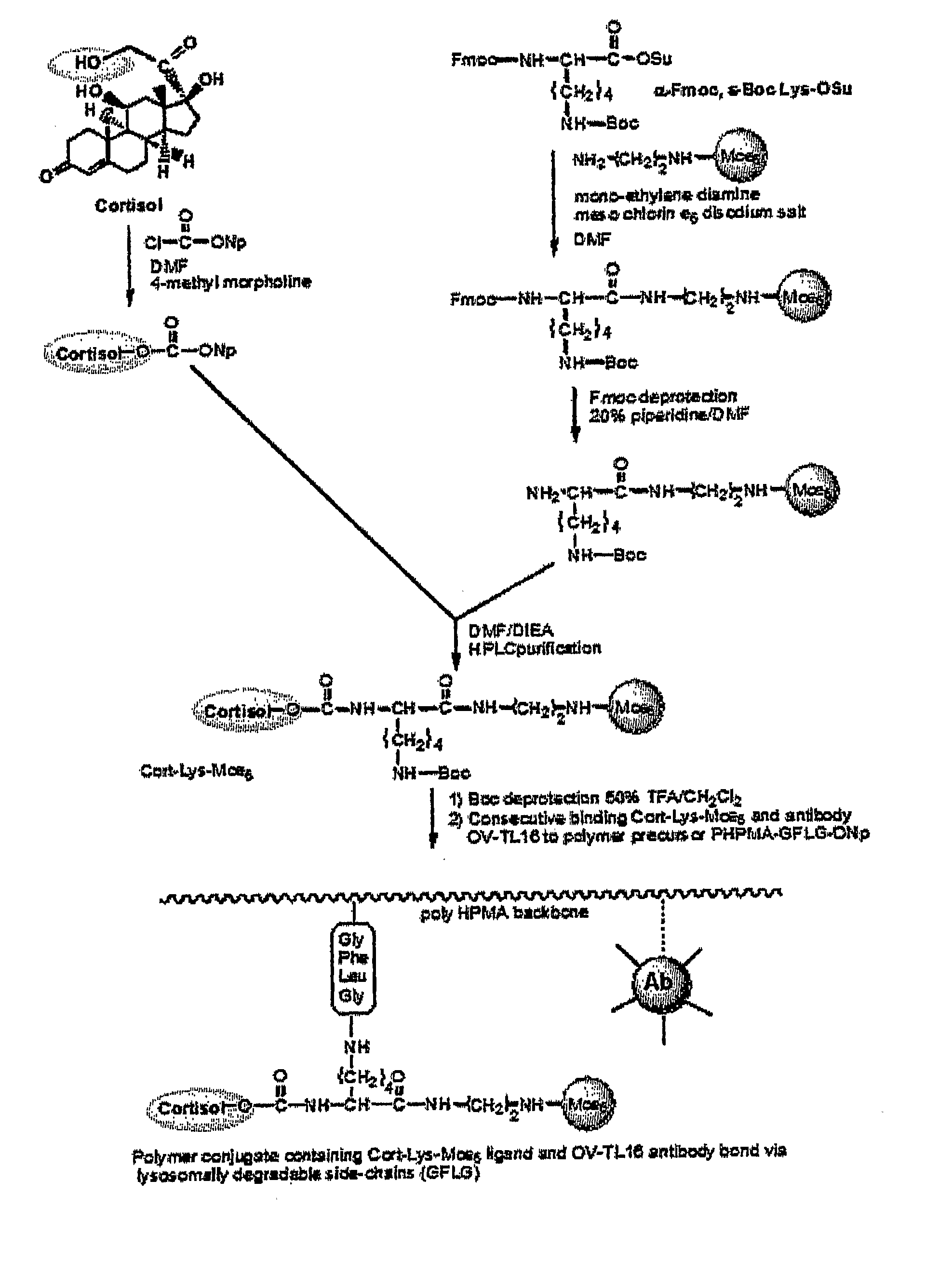
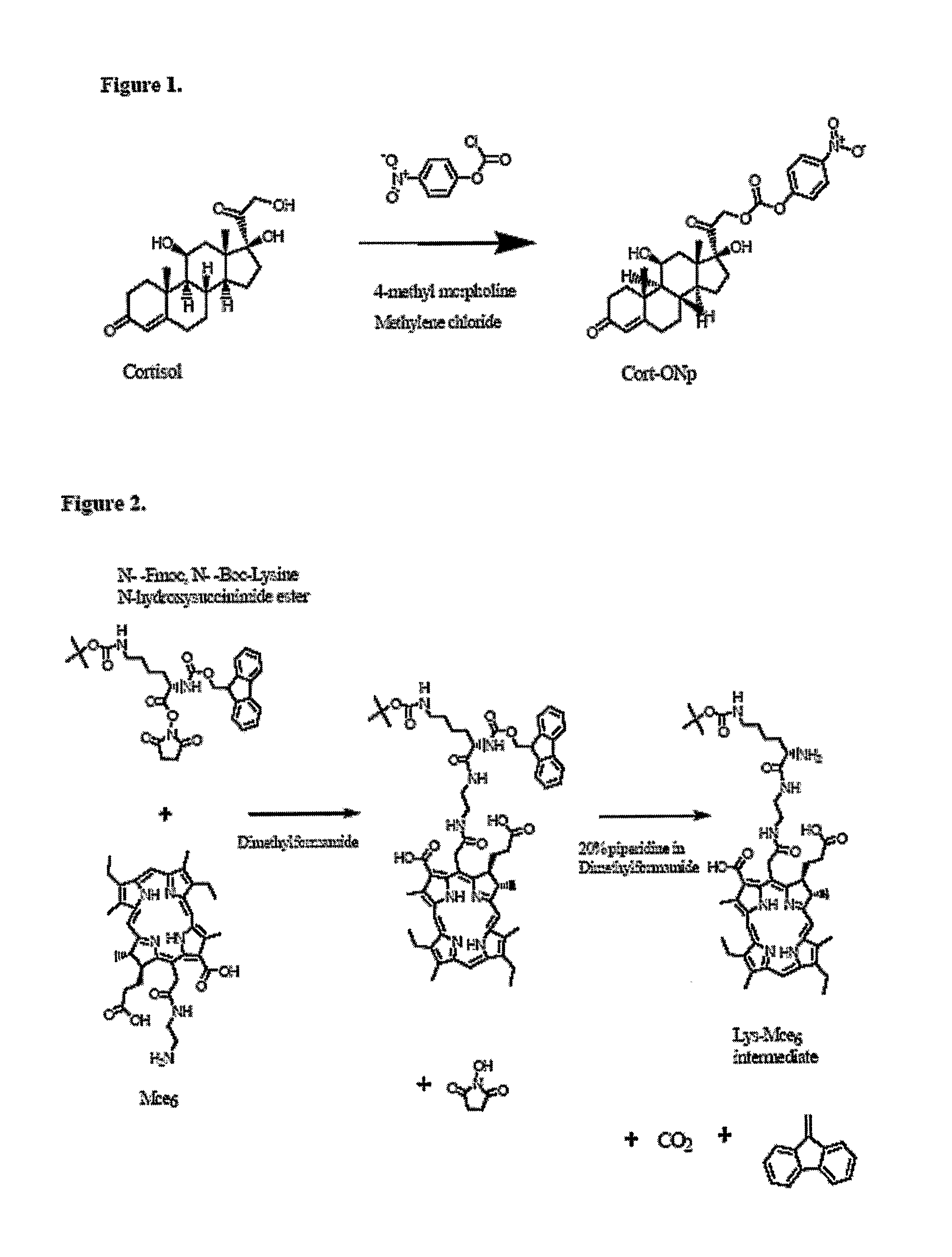
Disclosed is a conjugate comprising a biologically active agent (drug) linked to a subcellular targeting moiety that targets a drug specifically to the nucleus. Targeting is achieved by attaching a steroid hormone (or an analog) to the drug. The steroid hormone attached to the drug binds its corresponding receptor, the formation of the receptor-ligand complex results in the internalization of the complex into the nucleus, thus resulting in nuclear translocation of the drug. Also disclosed is a conjugate (comprising the complex of the drug and the steroid hormone) bound to a polymer by spacers allowing for concurrent passive targeting to the tumor cell (afforded by attachment to the polymer by the EPR effect) and nuclear targeting of the conjugate (due to the presence of the steroid). Using a suitable degradable spacer allows for the release of free drug in the tumor and enhances nuclear targeting efficacy. The polymer can be further linked to a cellular targeting molecule, where the targeting molecule directs the polymer to specific cells. One may thus be able to effectively target drugs to the nucleus of tumor cells. With little or modifications, several therapeutic agents can be targeted using the invention.
Owner:UNIV OF UTAH RES FOUND
Porous nanoparticle supported lipid bilayer nanostructures
ActiveUS20110268791A1Low release rateFacilitated releaseOrganic active ingredientsHeavy metal active ingredientsNanoparticleLipid bilayer
Various exemplary embodiments provide protocell nanostructures and methods for constructing and using the protocell nanostructures. In one embodiment, the protocell nanostructures can include a core-shell structure including a porous particle core surrounded by a shell of lipid bilayer(s). The protocell can be internalized in a bioactive cell. Various cargo components, for example, drugs, can be loaded in and released from the porous particle core of the protocell(s) and then delivered within the bioactive cell.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC +1
Targeted therapeutic proteins
InactiveUS20090203575A1Convenient treatmentSimple preparation processPeptide/protein ingredientsAntibody mimetics/scaffoldsLysosomeTherapeutic protein
Targeted therapeutics that localize to a specific subcellular compartment such as the lysosome are provided. The targeted therapeutics include a therapeutic agent and a targeting moiety that binds a receptor on an exterior surface of the cell, permitting proper subcellular localization of the targeted therapeutic upon internalization of the receptor. Nucleic acids, cells, and methods relating to the practice of the invention are also provided.
Owner:BIOMARIN PHARMA INC
Co-Administration of an Agent Linked to an Internalization Peptide with an Anti-Inflammatory
ActiveUS20090176713A1Reduce capacityInhibit the inflammatory responseOrganic active ingredientsNervous disorderCo administrationBiotin
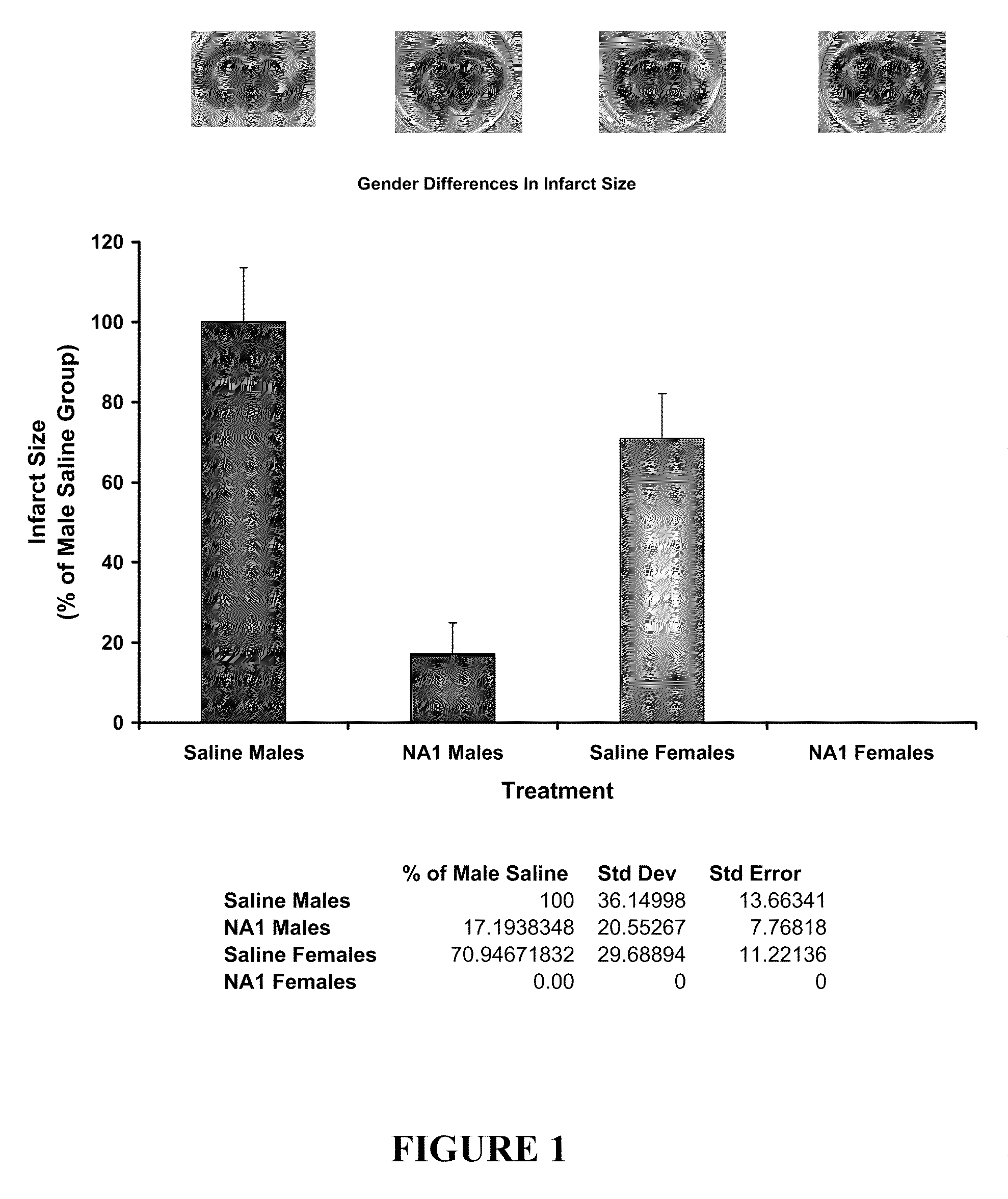
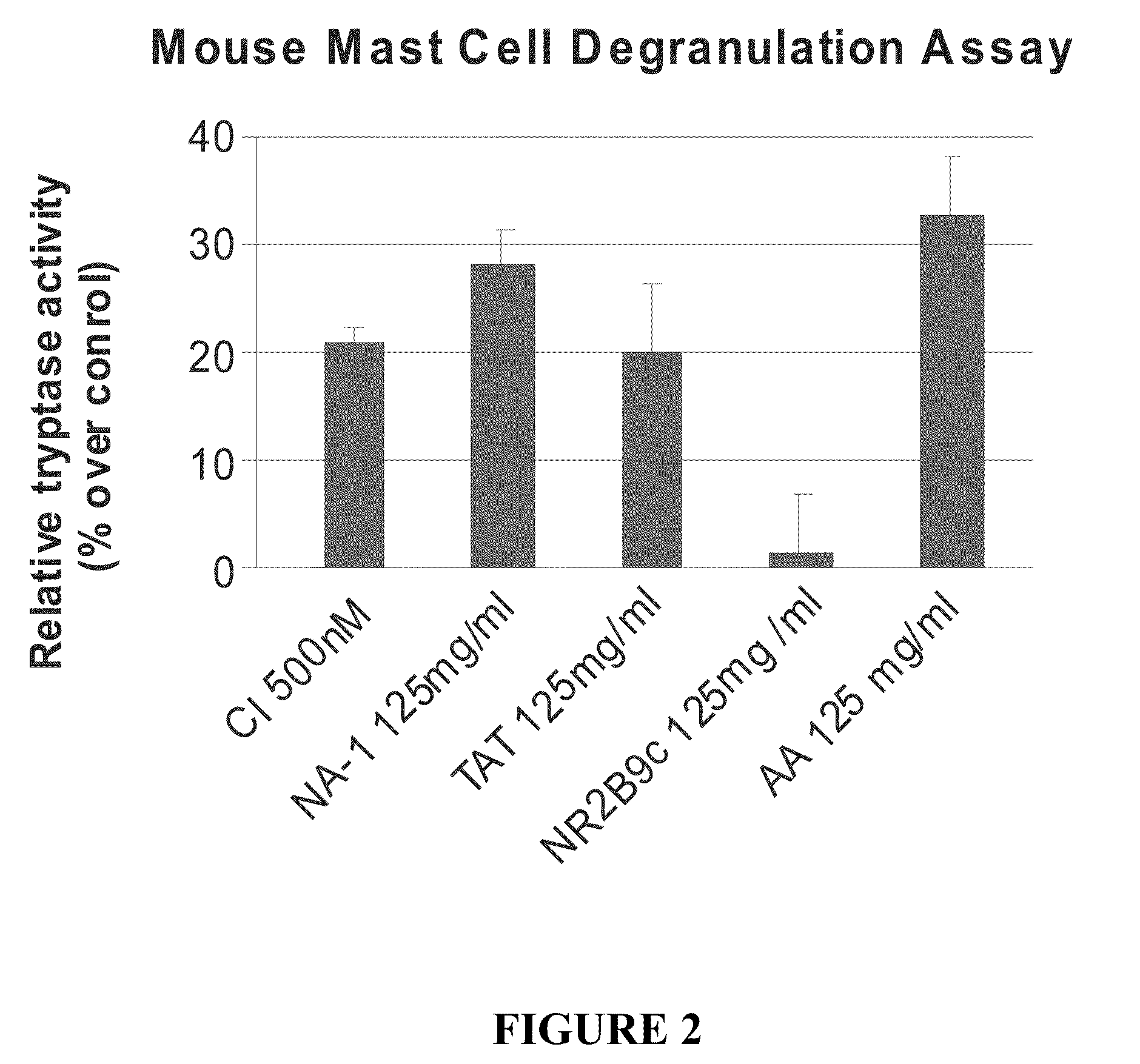
The invention provides methods of delivering pharmacologic agents linked to an internalization peptide, in which an inflammatory response inducible by the internalization peptide is inhibited by co-administration of an anti-inflammatory or by linking the internalization peptide to biotin or similar molecule. Such methods are premised in part on the results described in the examples whereby administration of a pharmacological agent linked to tat high dosages is closely followed by an inflammatory response, which includes mast cell degranulation, histamine release and the typical sequelae of histamine release, such as redness, heat, swelling, and hypotension.
Owner:NONO INC
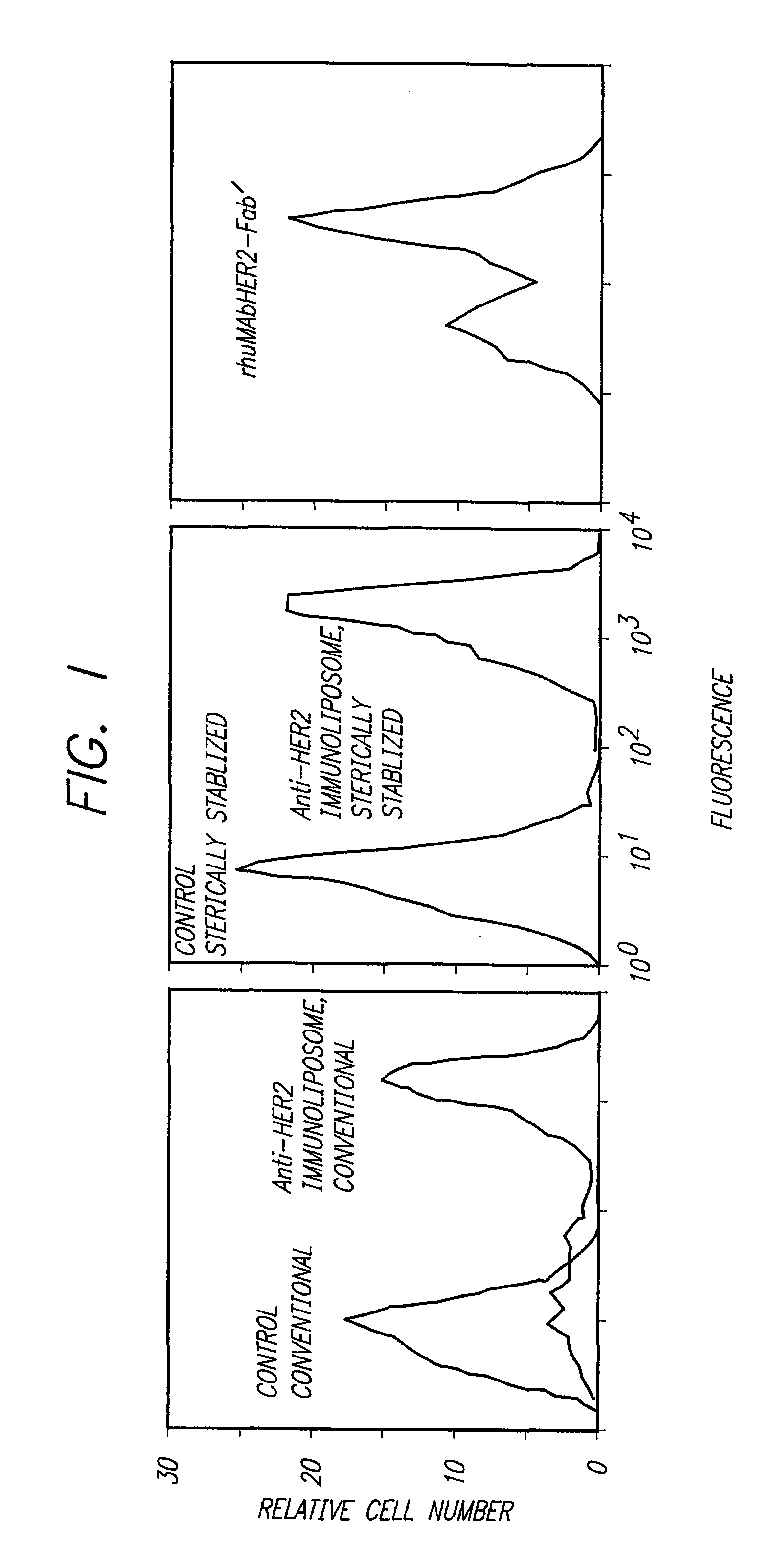
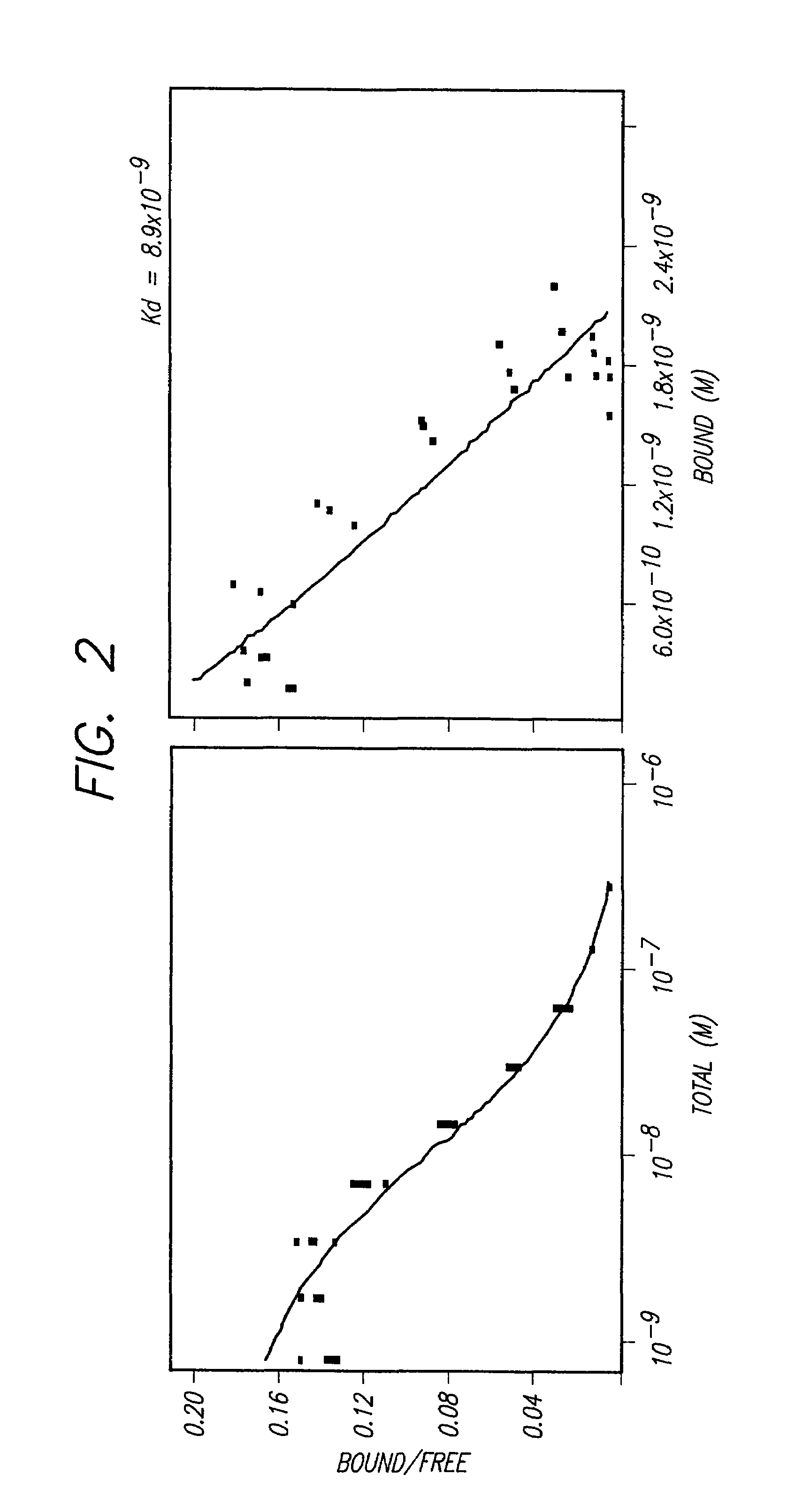
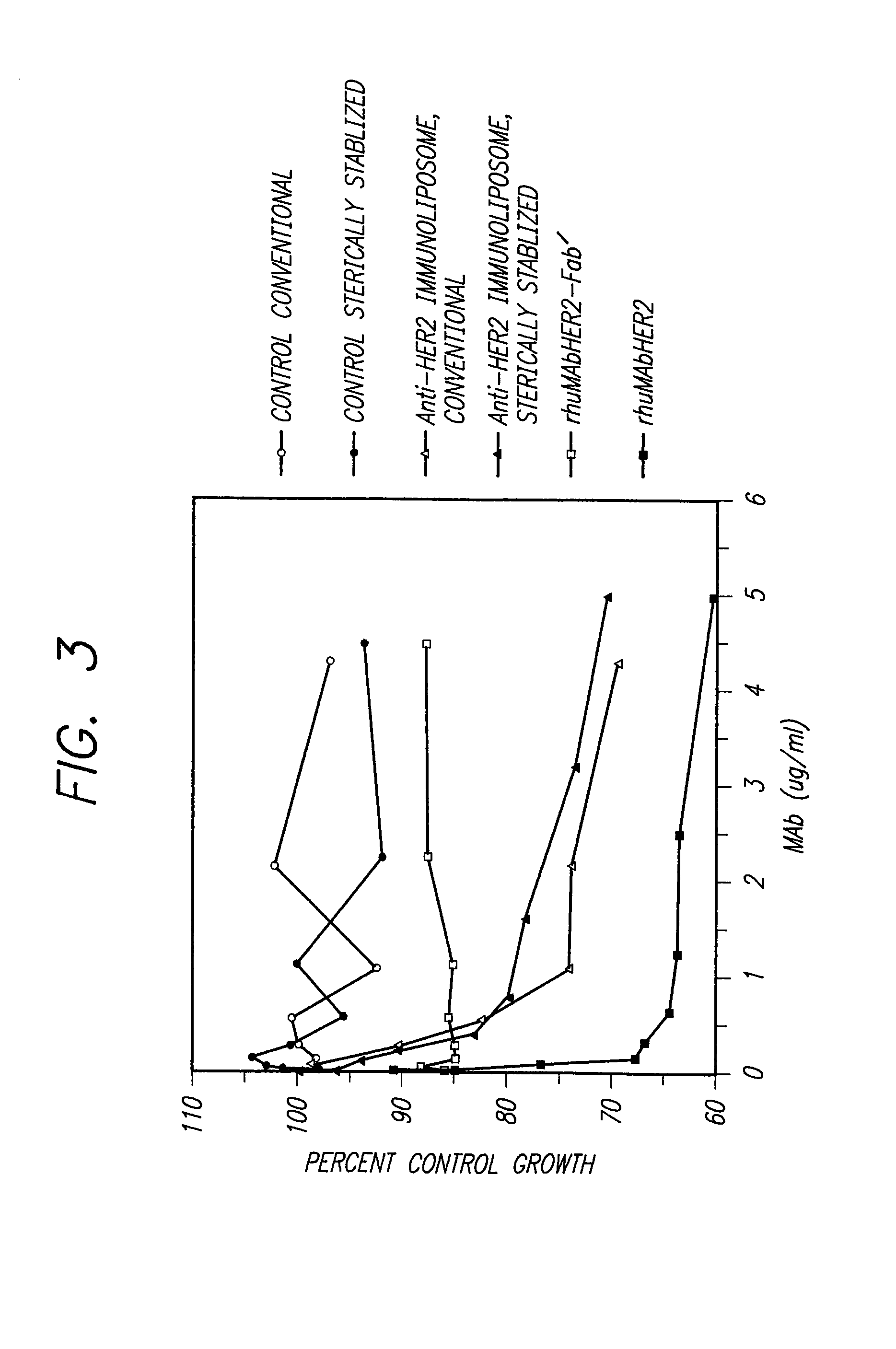
Immunoliposomes that optimize internalization into target cells
InactiveUS7135177B2Extended half-lifeAvoid lostBiocideHeavy metal active ingredientsSurface markerLipid formation
The present invention provides for immunoliposomes that optimizes internalization of a drug into target cells bearing a characteristic cell surface marker. The immunoliposomes comprise an Fab' domain of an antibody that specifically binds the characteristic marker, an amphipathic vesicle-forming lipid, and a polyethylene glycol derivatized lipid. The invention also provides for growth-inhibiting immunoliposomes that lack growth-inhibiting therapeutic agents and yet are capable of inhibiting the growth and proliferation of target cells.
Owner:RGT UNIV OF CALIFORNIA
Ligand/lytic peptide compositions and methods of use
InactiveUS20040018967A1Inhibition of maturationLysis of tumor cells is rapidAntibacterial agentsOrganic active ingredientsLytic peptideAutoimmune condition
Amphipathic lytic peptides are ideally suited to use in a ligand / cytotoxin combination to specifically inhibit cells that are driven by or are dependent upon a specific ligand interaction; for example, to induce sterility or long-term contraception, or to attack tumor cells, or to selectively lyse virally-infected cells, or to attack lymphocytes responsible for autoimmune diseases. The peptides act directly on cell membranes, and need not be internalized. Administering a combination of gonadotropin-releasing hormone (GnRH) (or a GnRH agonist) and a membrane-active lytic peptide produces long-term contraception or sterilization in animals in vivo. Administering in vivo a combination of a ligand and a membrane-active lytic peptide kills cells with a receptor for the ligand. The compounds are relatively small, and are not antigenic. Lysis of gonadotropes has been observed to be very rapid (on the order of ten minutes.) Lysis of tumor cells is rapid. The two components-the ligand and the lytic peptide-may optionally be administered as a fusion peptide, or they may be administered separately, with the ligand administered slightly before the lytic peptide, to activate cells with receptors for the ligand, and thereby make those cells susceptible to lysis by the lytic peptide. The compounds may be used in gene therapy to treat malignant or non-malignant tumors, and other diseases caused by clones or populations of "normal" host cells bearing specific receptors (such as lymphocytes), because genes encoding a lytic peptide or encoding a lytic peptide / peptide hormone fusion may readily be inserted into hematopoietic stem cells or myeloid precursor cells.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Prostate cancer specific internalizing human antibodies
ActiveUS20050186214A1Easy to addImprove stabilityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProstate cancer cellToxin
This invention provides novel prostate cancer specific internalizing human antibodies. The antibodies are useful by themselves to prevent growth and / or proliferation of prostate cancer cells. The antibodies can also be formulated as chimeric molecules to direct an effector (e.g. a cytotoxin, an imaging reagent, a drug, etc.) to a prostate tumor site.
Owner:RGT UNIV OF CALIFORNIA
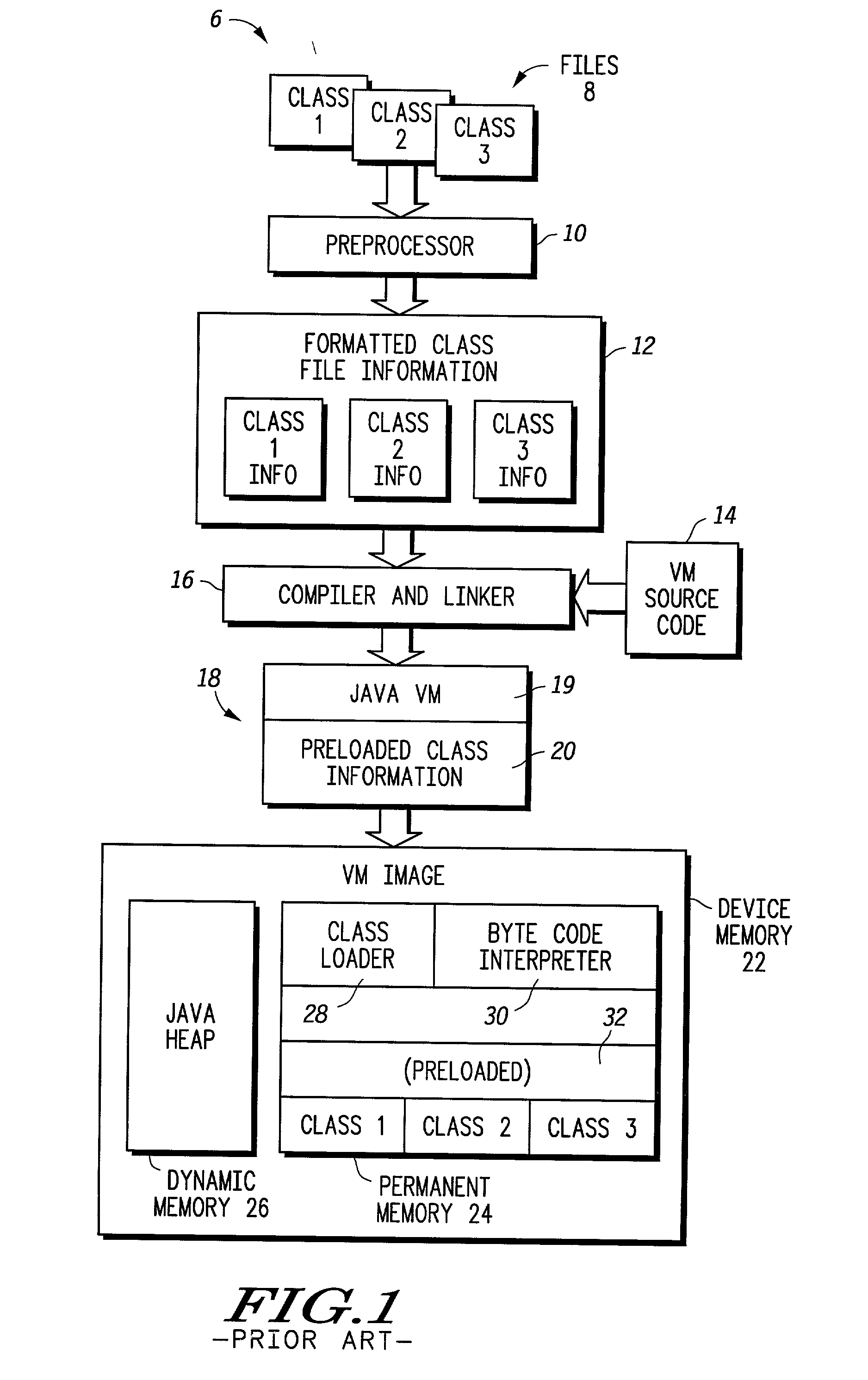
Method and device for creating and using pre-internalized program files
InactiveUS20020129078A1Software engineeringInterprogram communicationInternalizationComputer science
A device (45) receives new program files (46) and uses pre-internalized images to avoid having to internalize a program file every time that program execution occurs. In one embodiment, a software Virtual Machine (50) in the device functions to implement the pre-internalization. Once the program files are pre-internalized to create images that are stored in a permanent memory (56) of the device, the images may subsequently be executed without having to perform a pre-internalization operation. Additionally, use of dynamic memory (52) is reduced in connection with subsequent program execution and execution time of new program files is reduced.
Owner:NORTH STAR INNOVATIONS
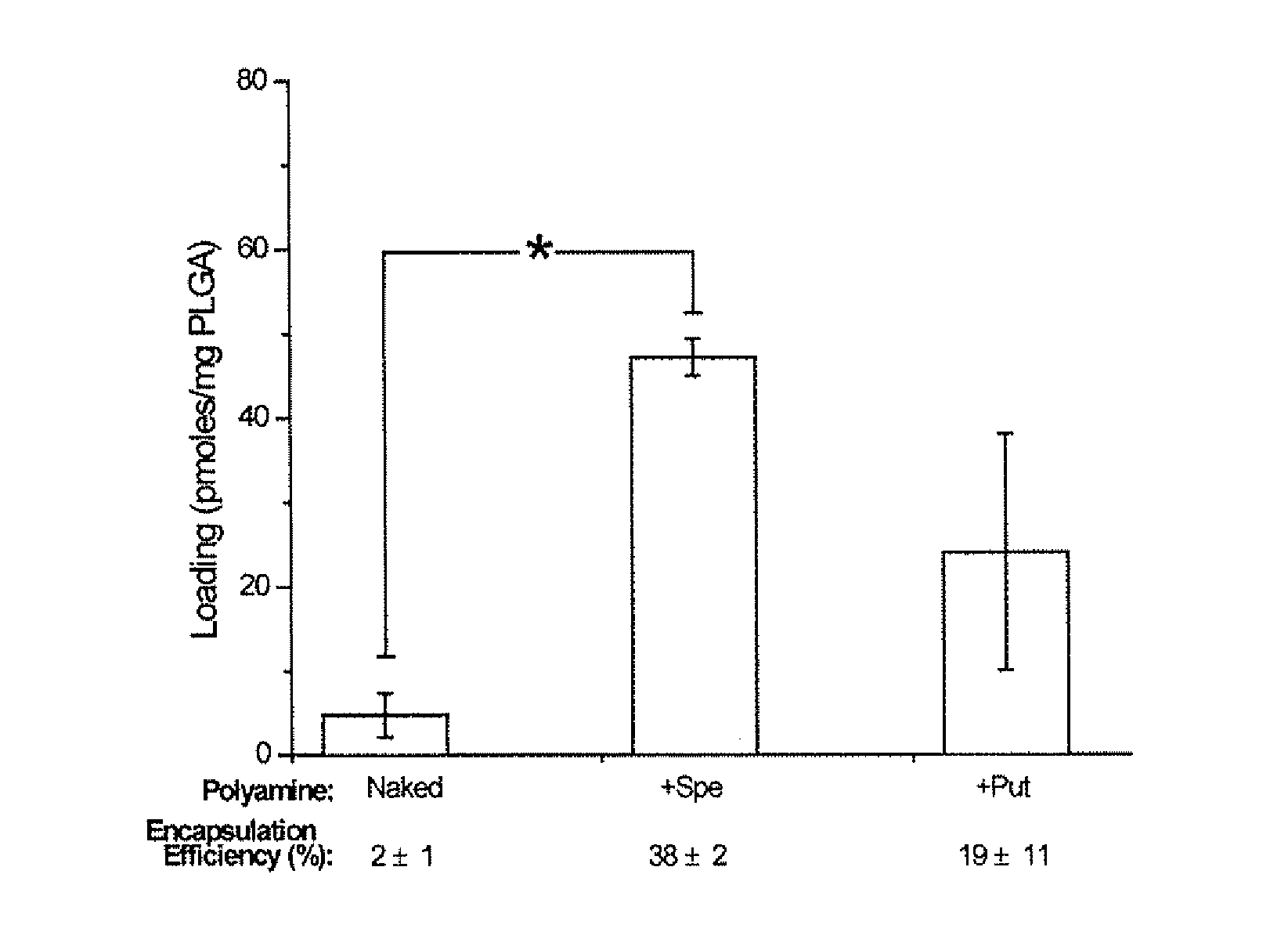
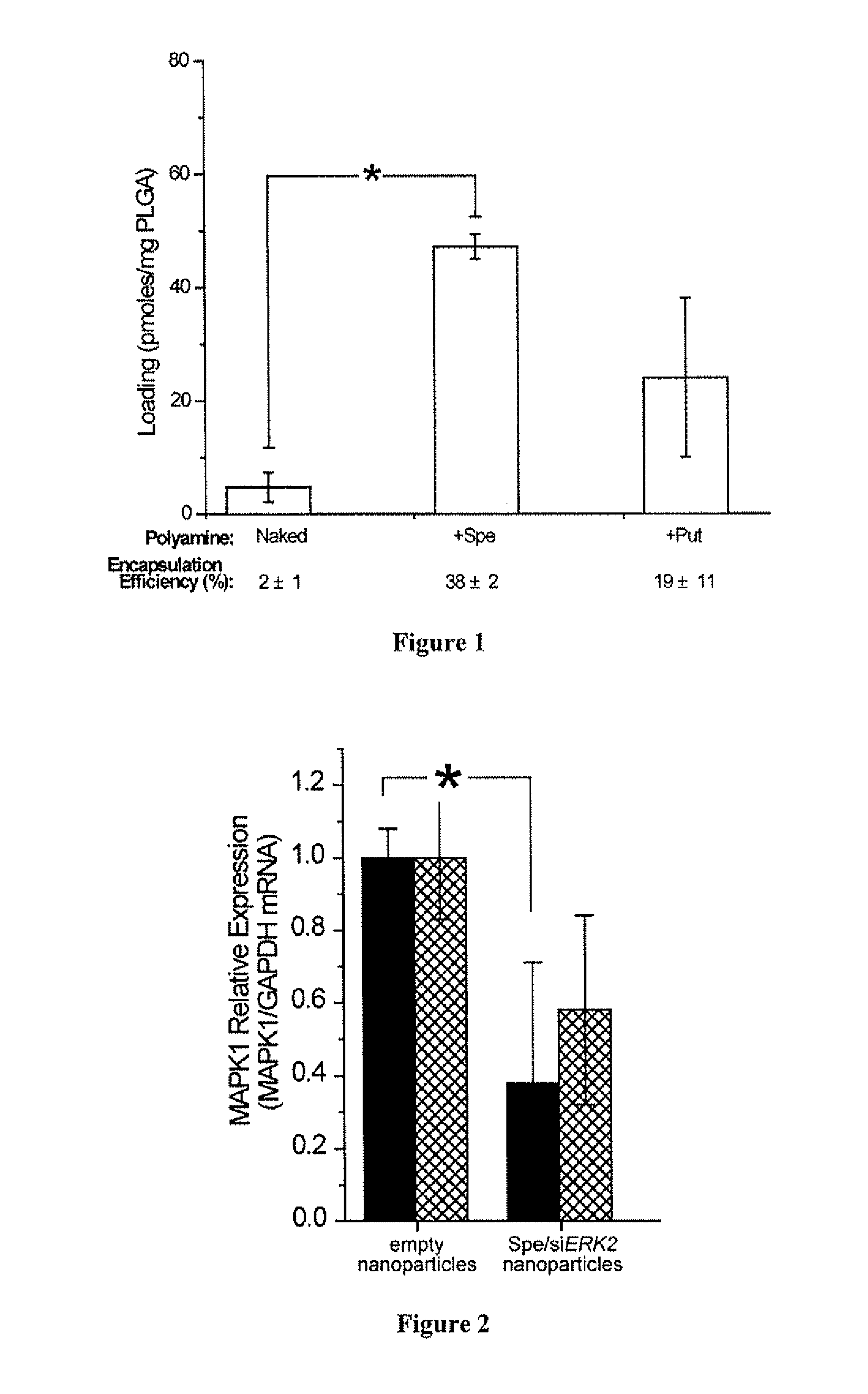
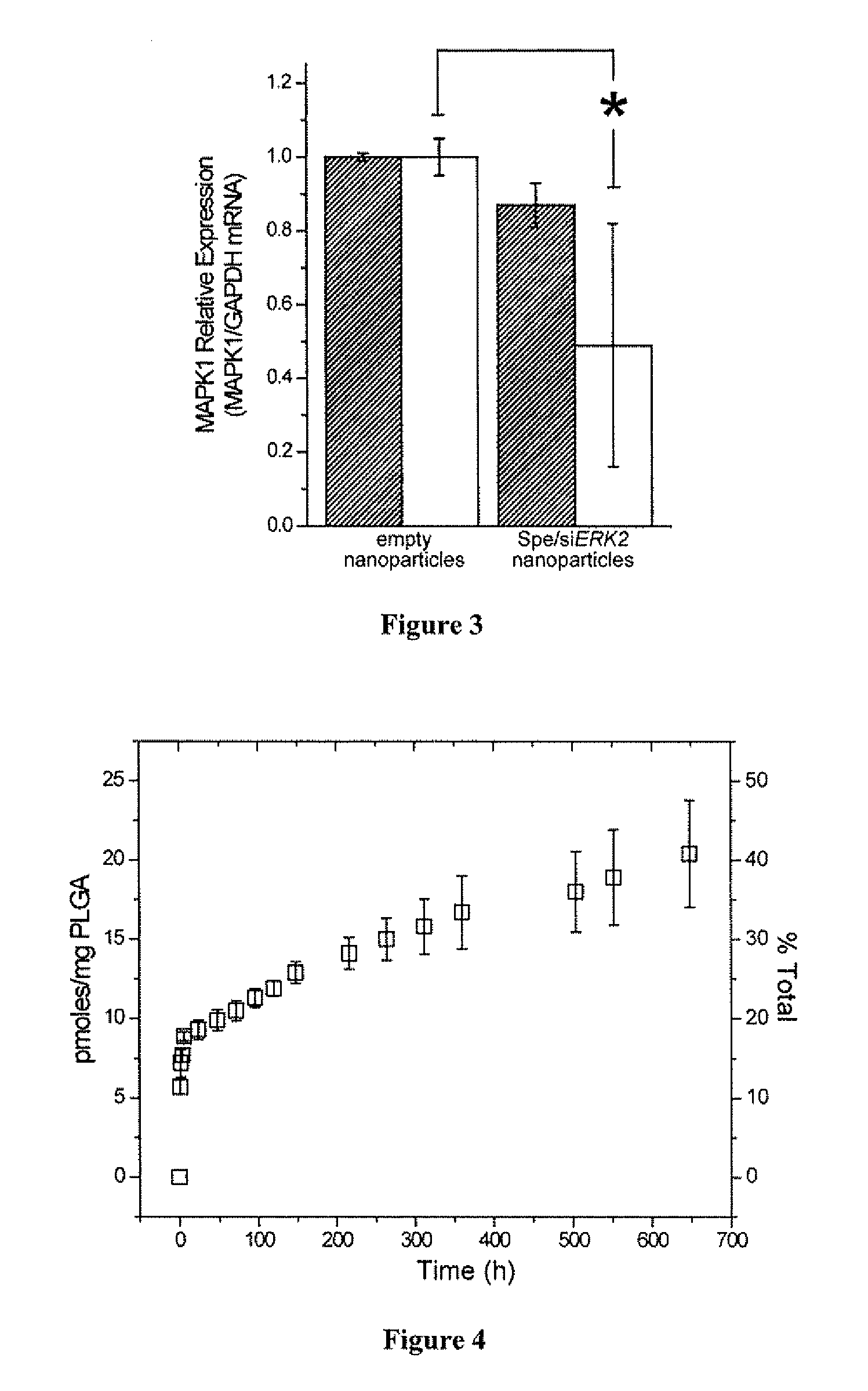
Compositions and methods for controlled delivery of inhibitory ribonucleic acids
ActiveUS20110008451A1Easy modular assemblyAvoid encapsulationPowder deliveryOrganic active ingredientsDiseaseInternalization
Polymeric nanoparticles encapsulating inhibitory ribonucleic acids (RNAs) and methods of their manufacture and use are provided. Advantageous properties of the nanoparticles include: 1) high encapsulation efficiency of inhibitory RNAs into the nanoparticles, 2) small size of the nanoparticles that increases cell internalization, and 3) sustained release of encapsulated inhibitory RNAs by the nanoparticles that allows for administration of an effective amount of inhibitory RNAs to cells or tissues over extended periods of time. Encapsulation efficiency of inhibitory RNAs into the nanoparticles is greatly increased by complexing the inhibitory RNAs to polycations prior to encapsulation. Methods of using the polymeric nanoparticles for treating or inhibiting diseases or disorders are provided.
Owner:YALE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com