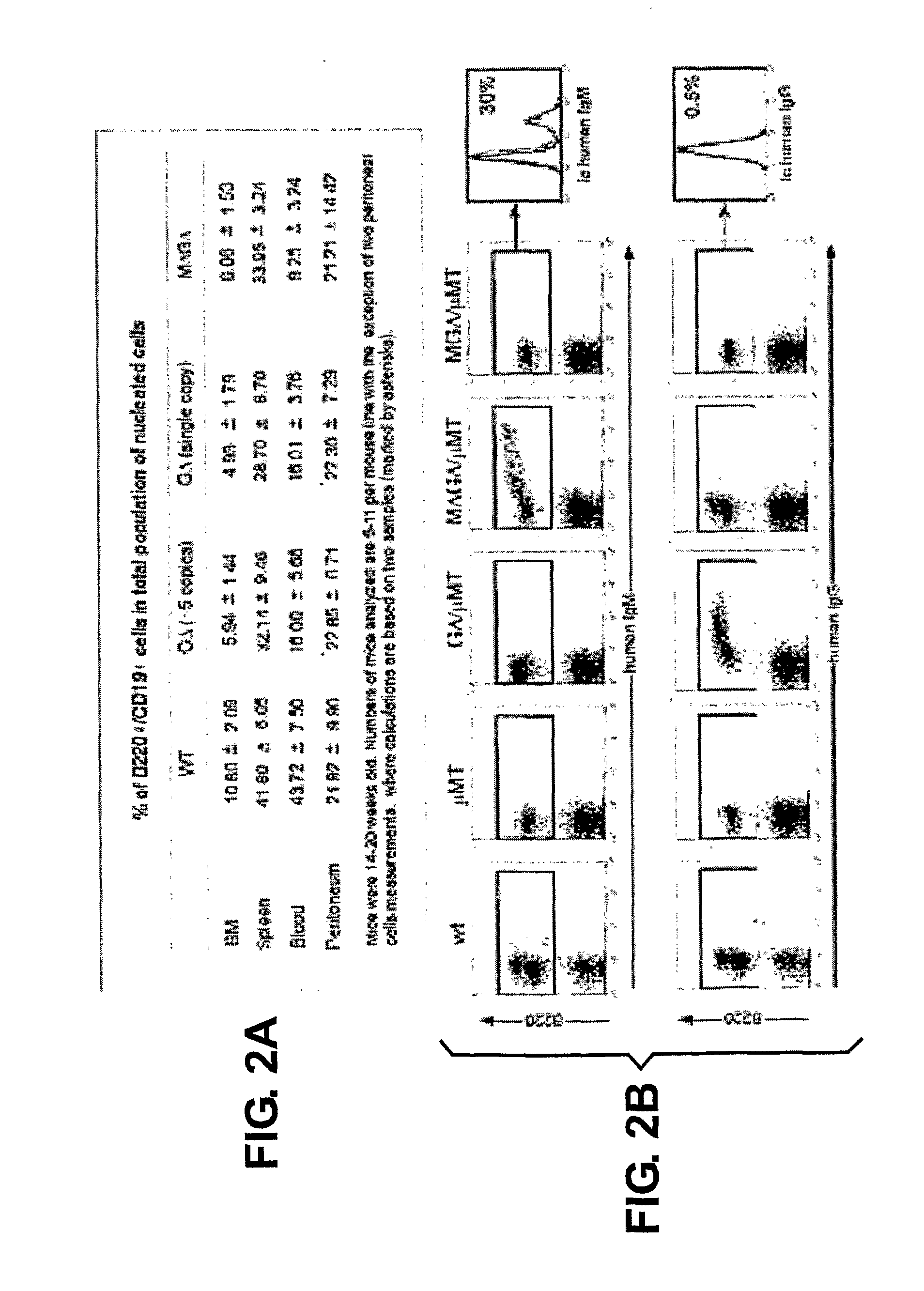
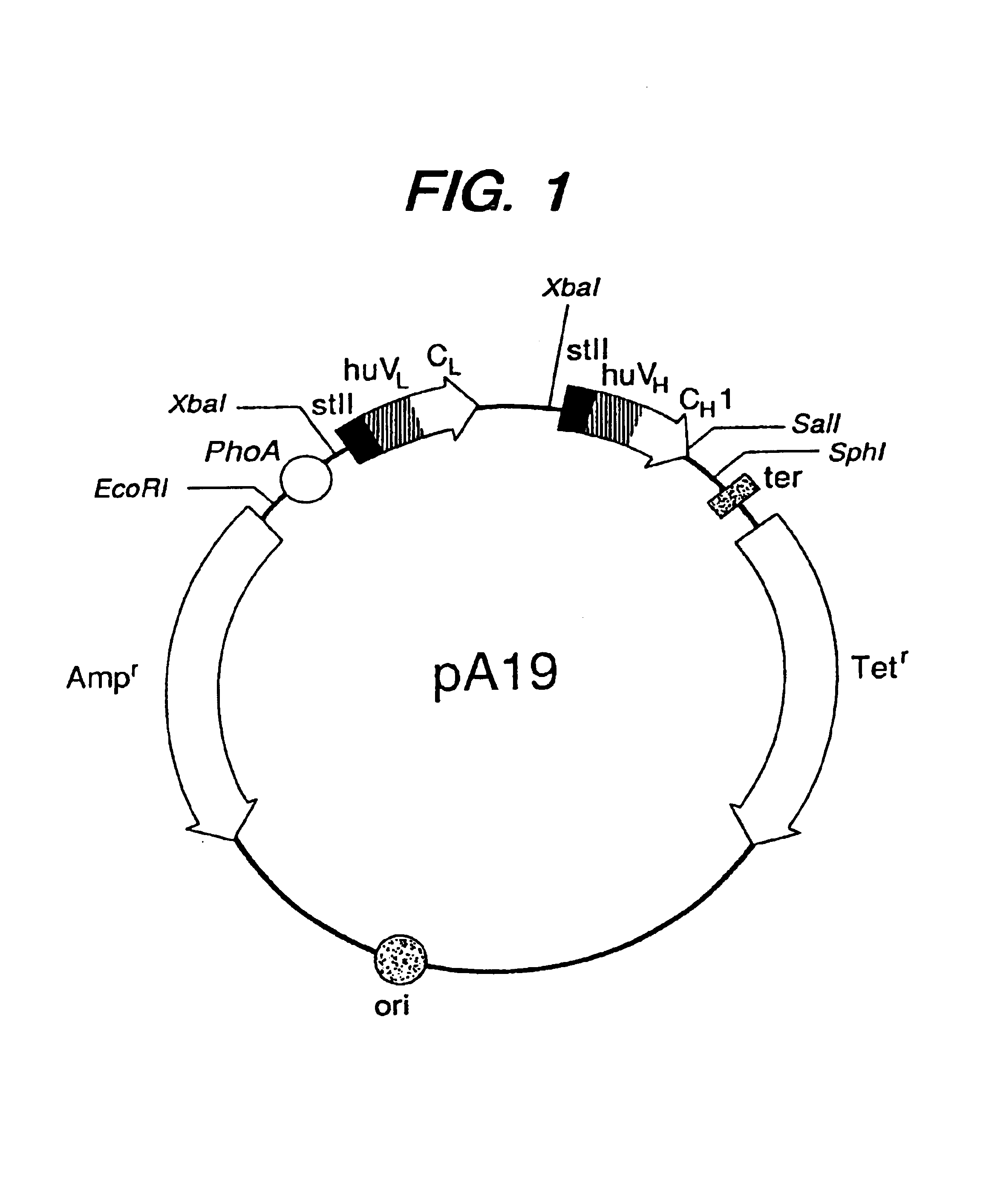
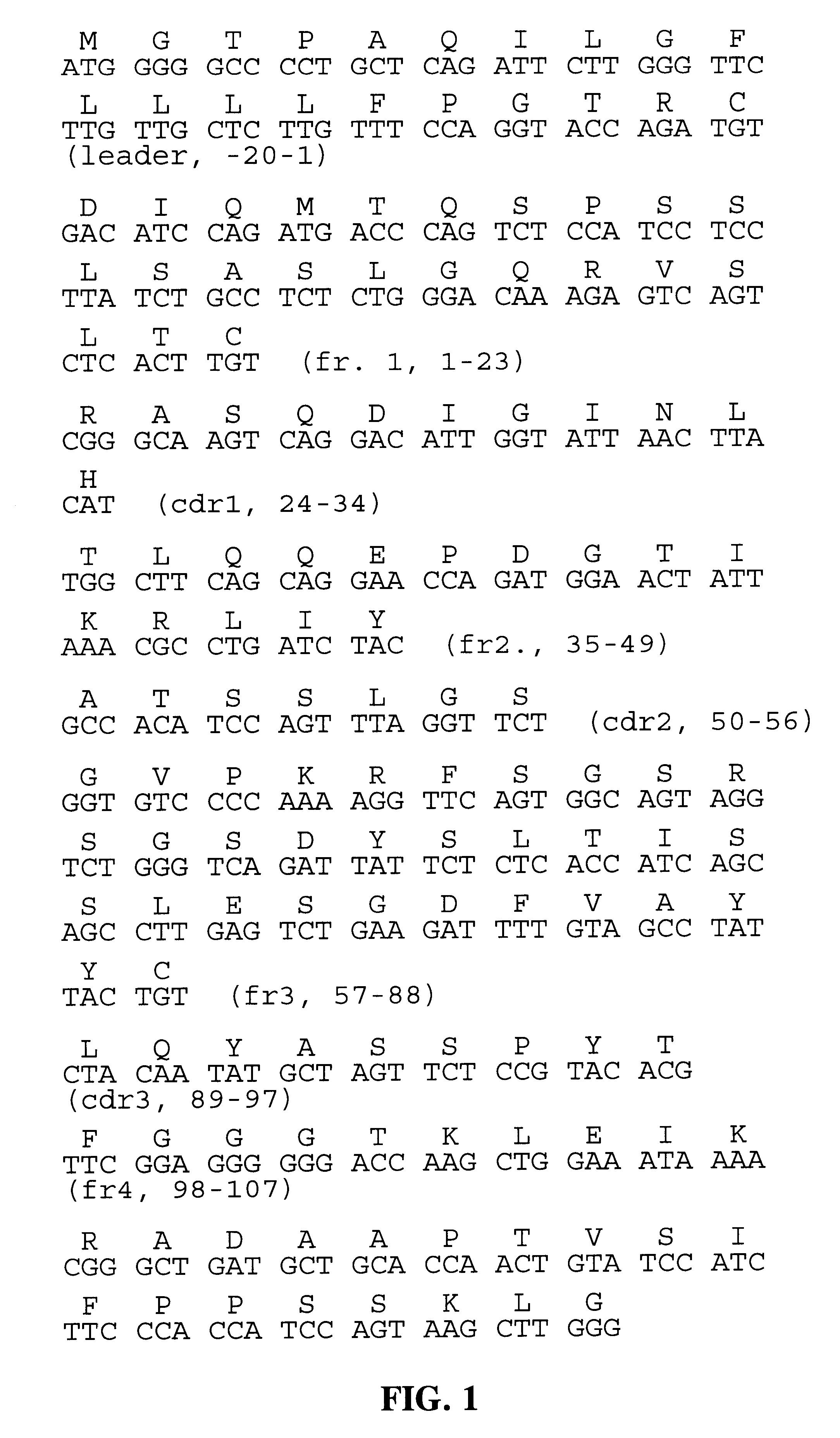Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3302 results about "Heavy chain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
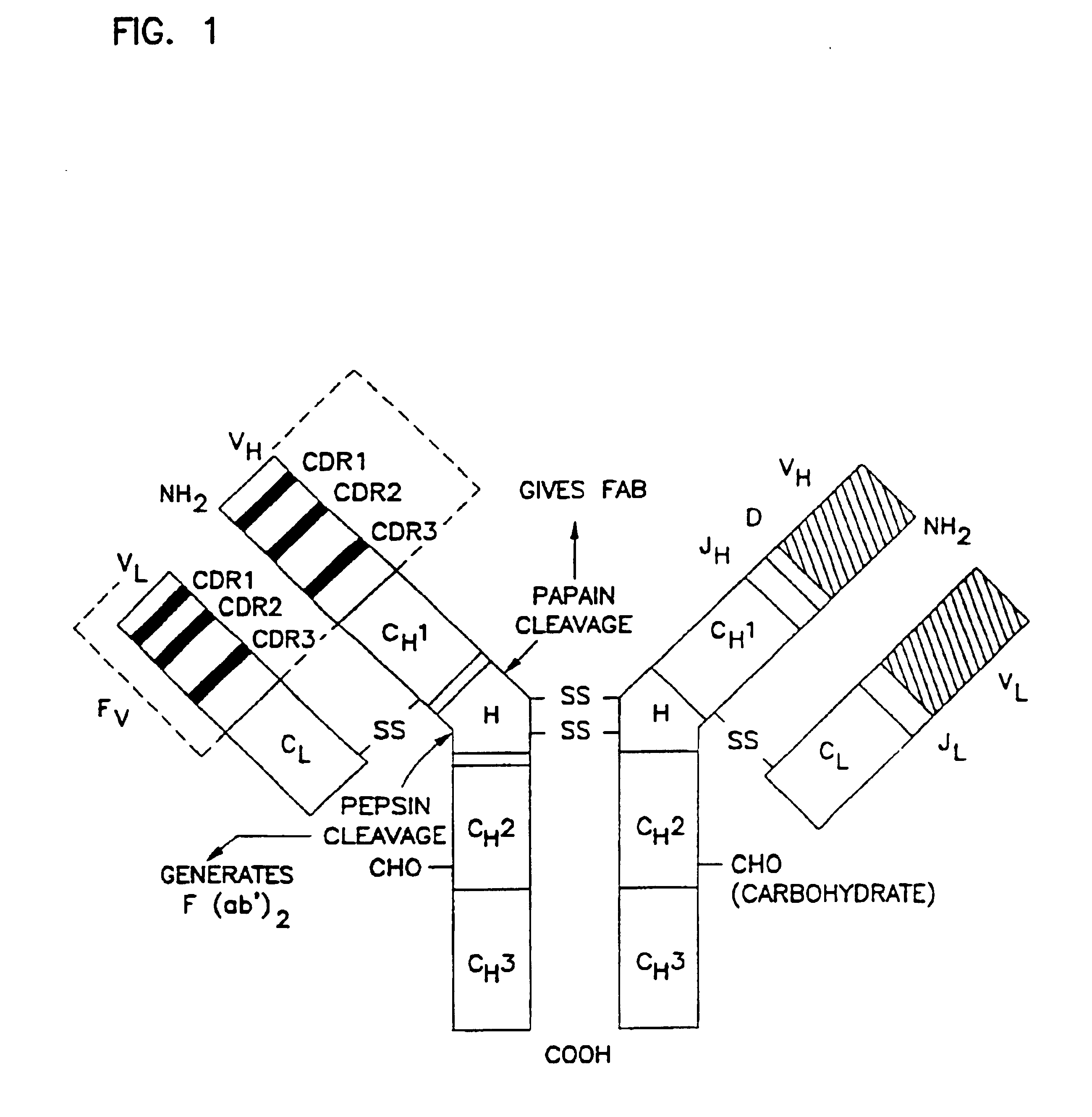
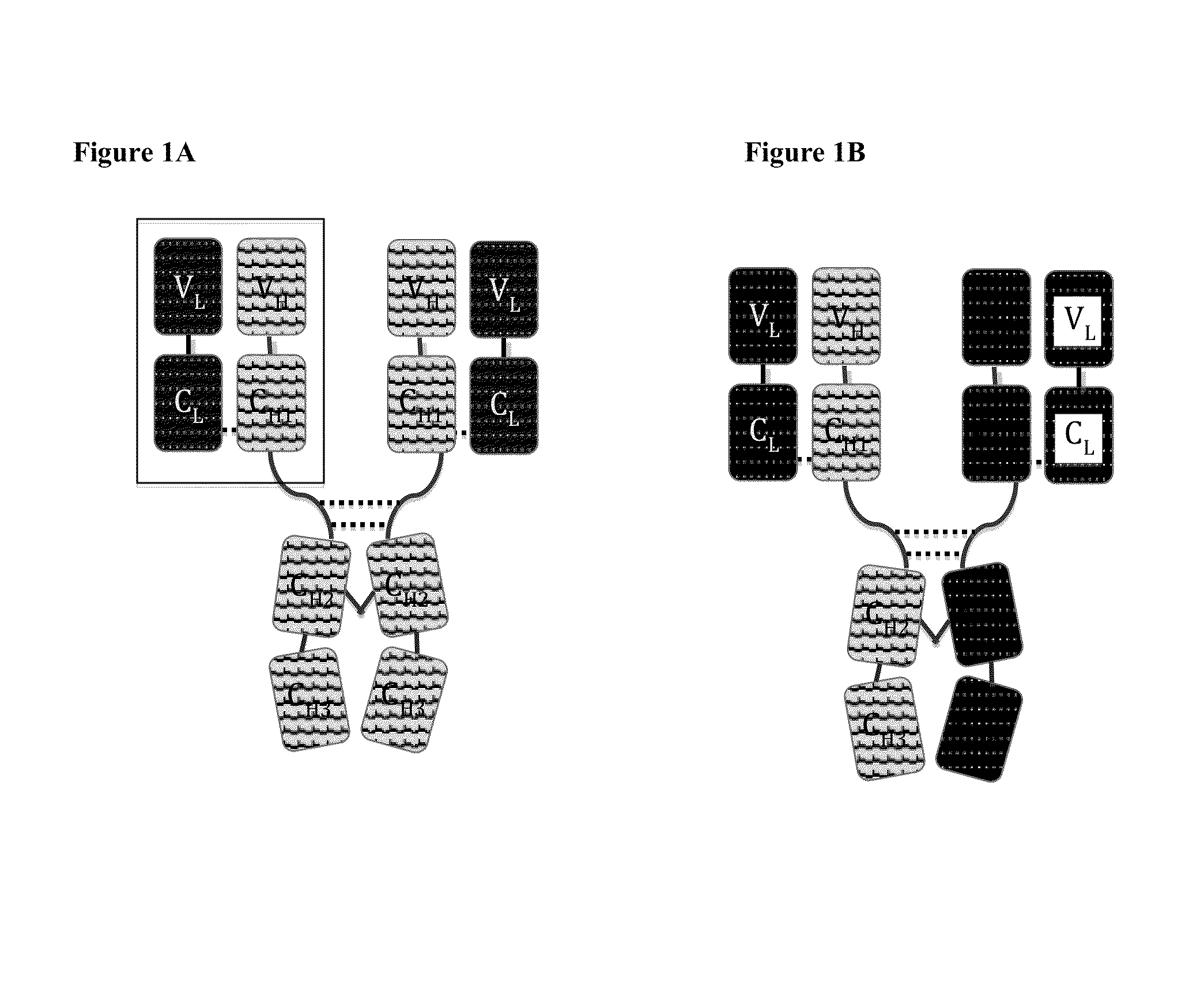
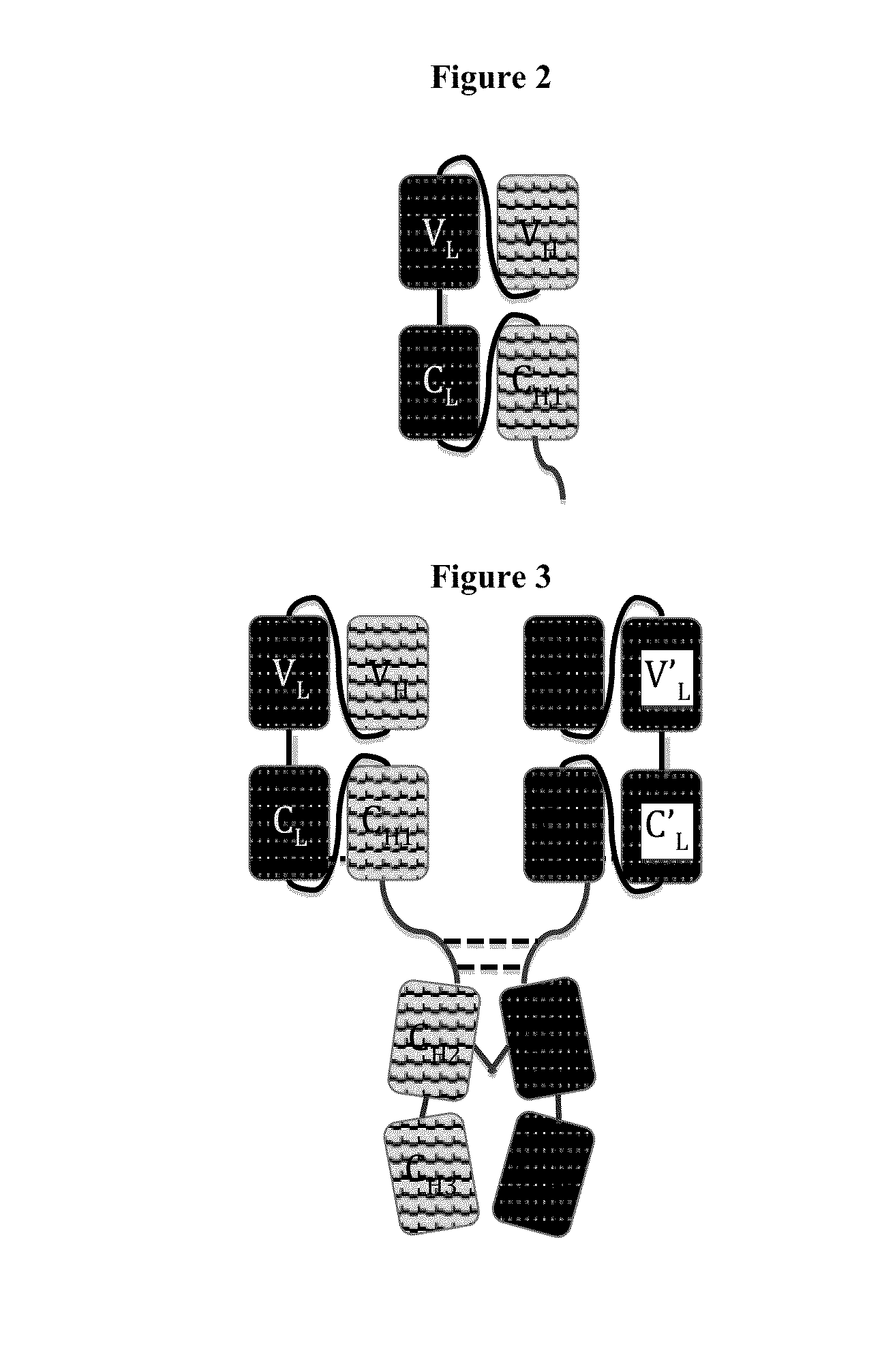
A heavy chain is the large polypeptide subunit of a protein complex, such as a motor protein or antibody. It commonly refers to the immunoglobulin heavy chain. The heavy chain is the larger of the two types of chains that comprise a normal immunoglobulin or antibody molecule. The heavy chain portion of an antibody contains 2 regions; the Fab and Fc. Amino acid sequence determines the type of heavy chains, and heavy chains define the isotype of Ig. Immunoglobulin G has γ gamma heavy chains, IgA has α alpha heavy chains, IgM has μ mu heavy chains, IgD has δ delta heavy chains, and IgE has ε epsilon heavy chains. In contrast, all light chains are either κ kappa or λ lambda light chains, either of which may be found on any Ig molecule, regardless of isotype. Each Ig unit is made up of 2 heavy chains, 2 light chains, and has 2 antigen-binding sites. Heavy chain is joined with the light chain with the help of disulphide bond.
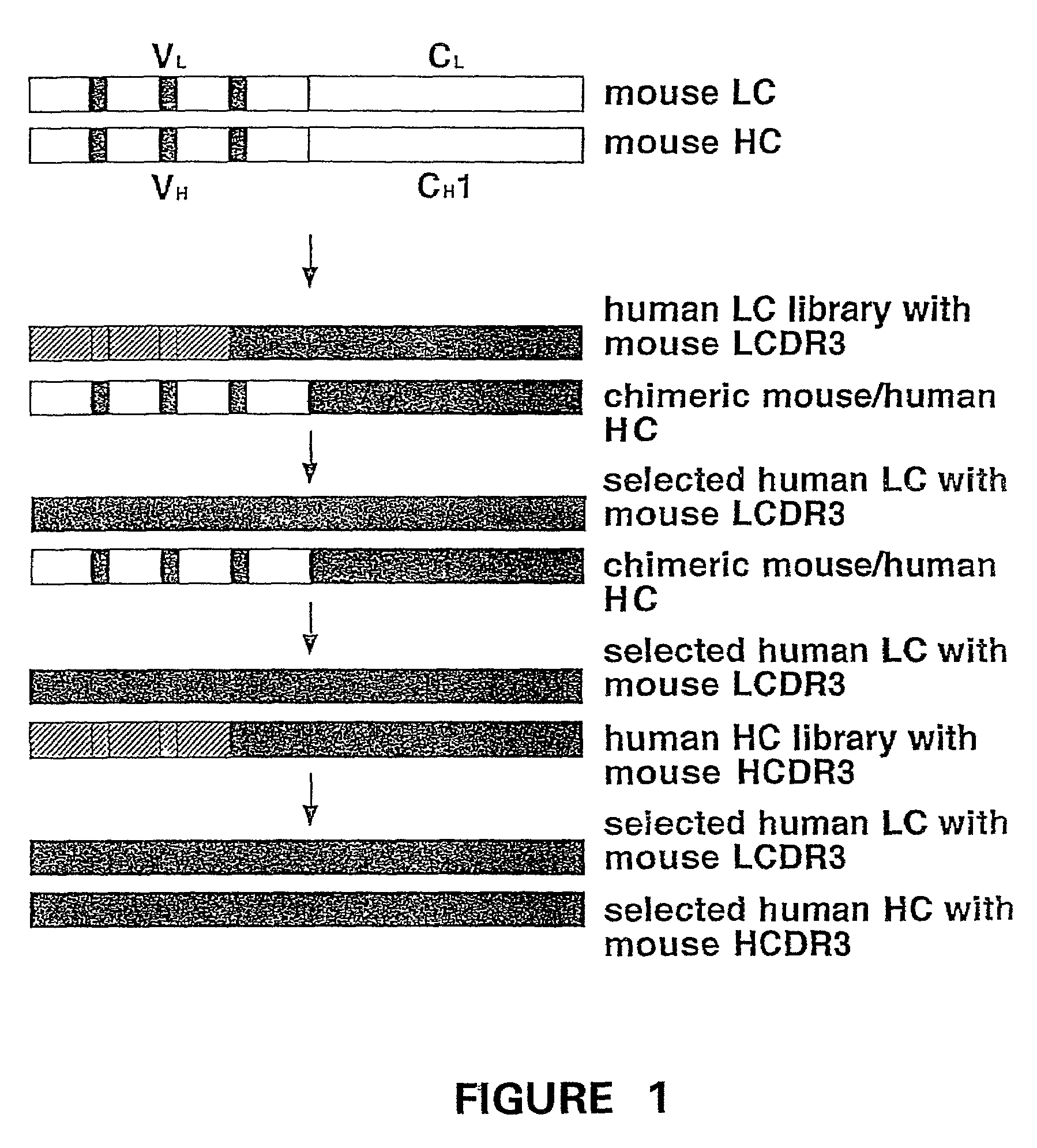
Humanization of murine antibody
InactiveUS7087409B2Hybrid immunoglobulinsSugar derivativesComplementarity determining regionHeavy chain
A humanized murine antibody is provided. The amino acid sequences of a light chain complementarity determining region from a mouse antibody are grafted onto a human light chain, and a heavy chain complementarity determining region from a mouse antibody are grafted onto a human antibody heavy chain to produce libraries from which a humanized murine antibody having the desired specificity is selected.
Owner:THE SCRIPPS RES INST
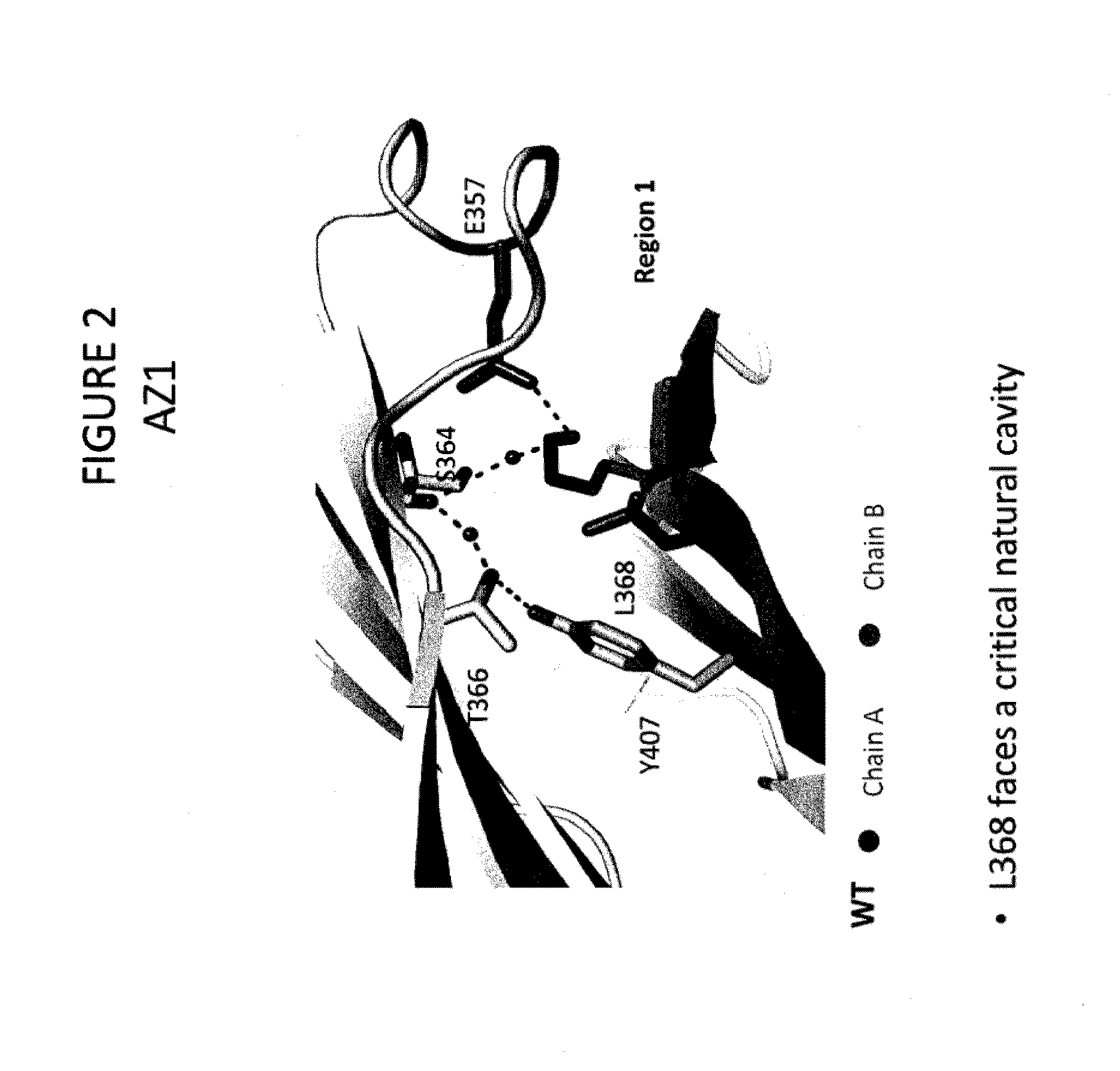
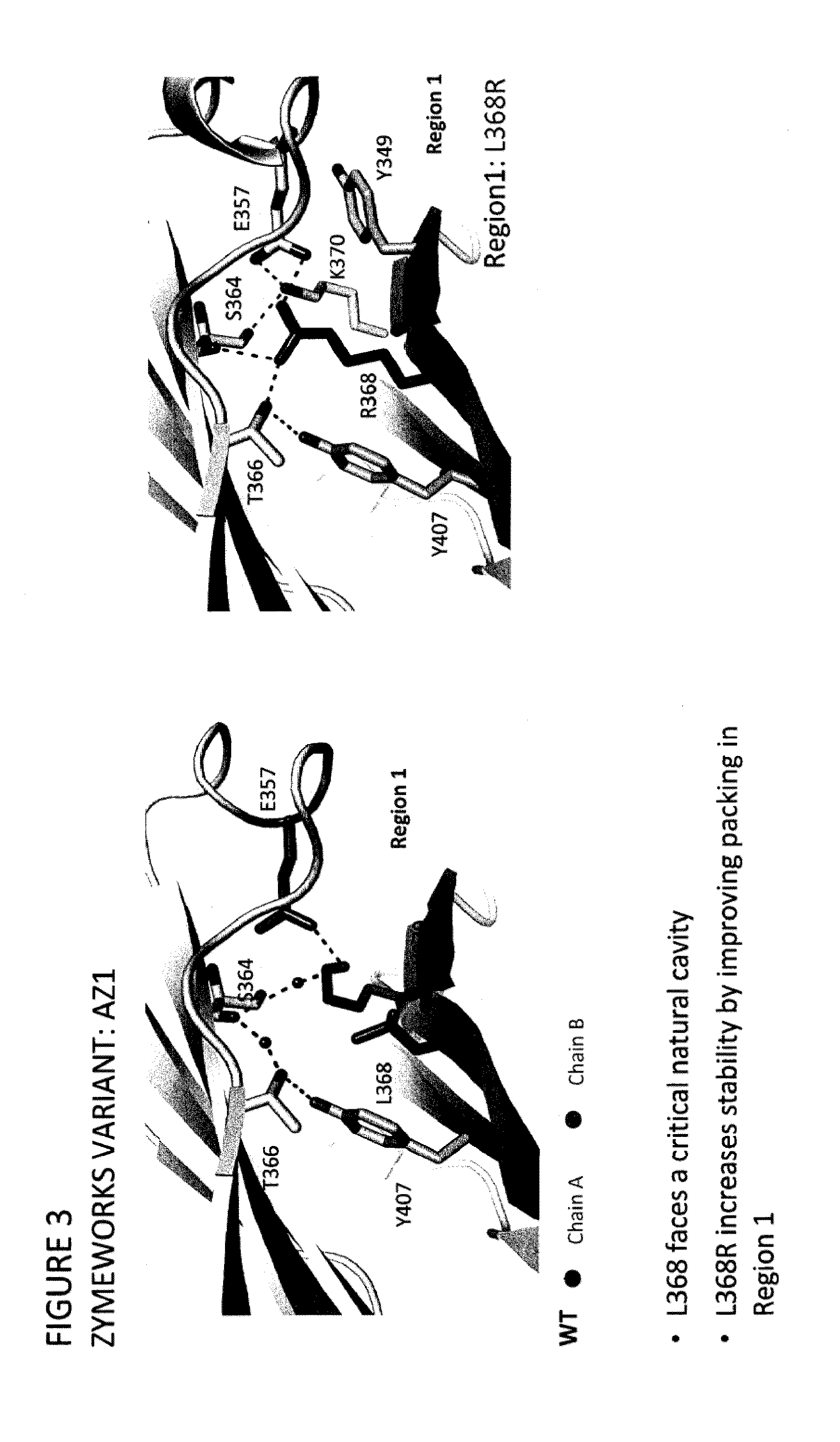
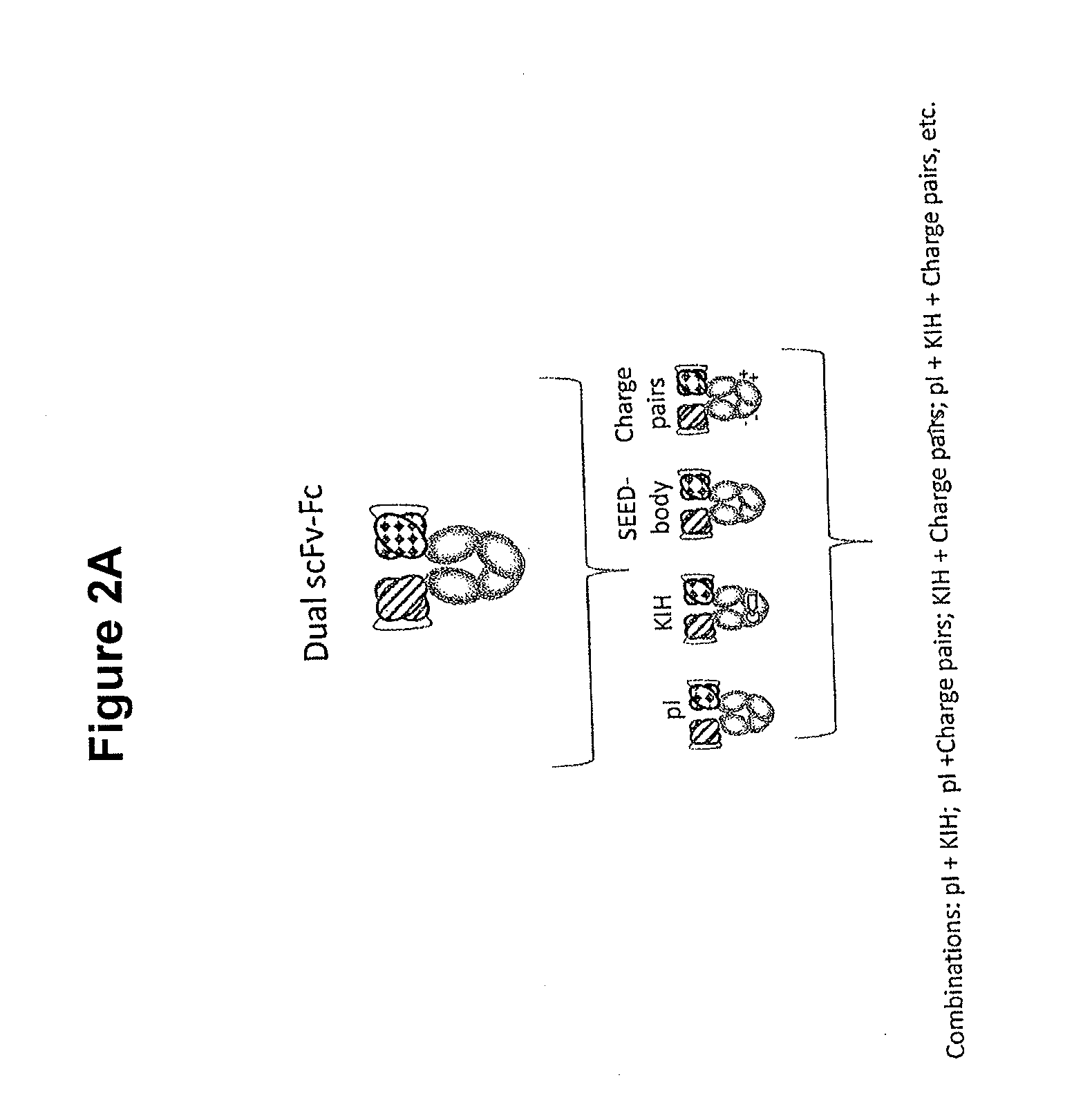
Stable Heterodimeric Antibody Design with Mutations in the Fc Domain
ActiveUS20120149876A1Promote formationImprove stabilityImmunoglobulinsImmunological disordersFc(alpha) receptorFc receptor
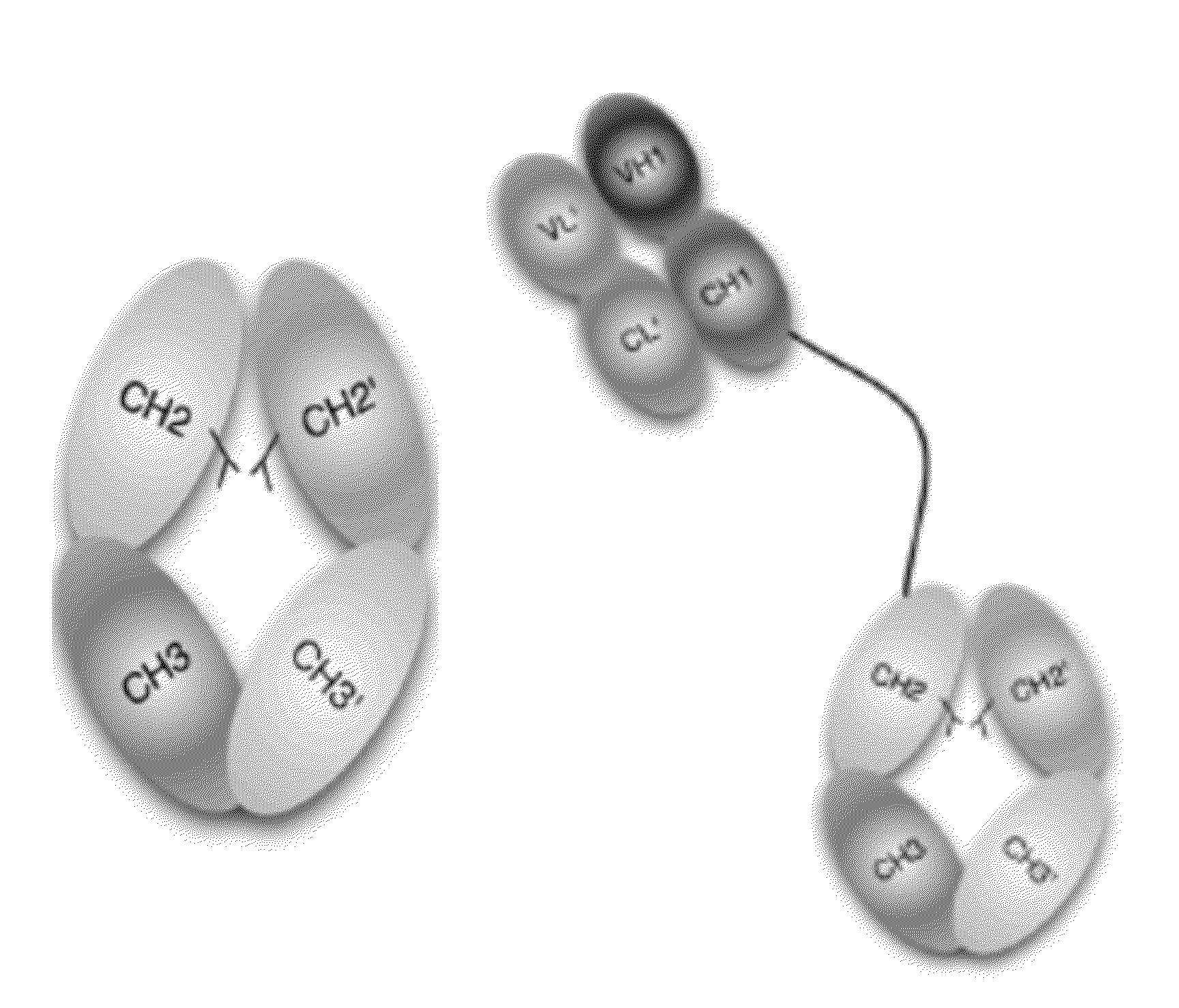
The provided scaffolds have heavy chains that are asymmetric in the various domains (e.g. CH2 and CH3) to accomplish selectivity between the various Fc receptors involved in modulating effector function, beyond those achievable with a natural homodimeric (symmetric) Fc molecule, and increased stability and purity of the resulting variant Fc heterodimers. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
Readily Isolated Bispecific Antibodies with Native Immunoglobulin Format
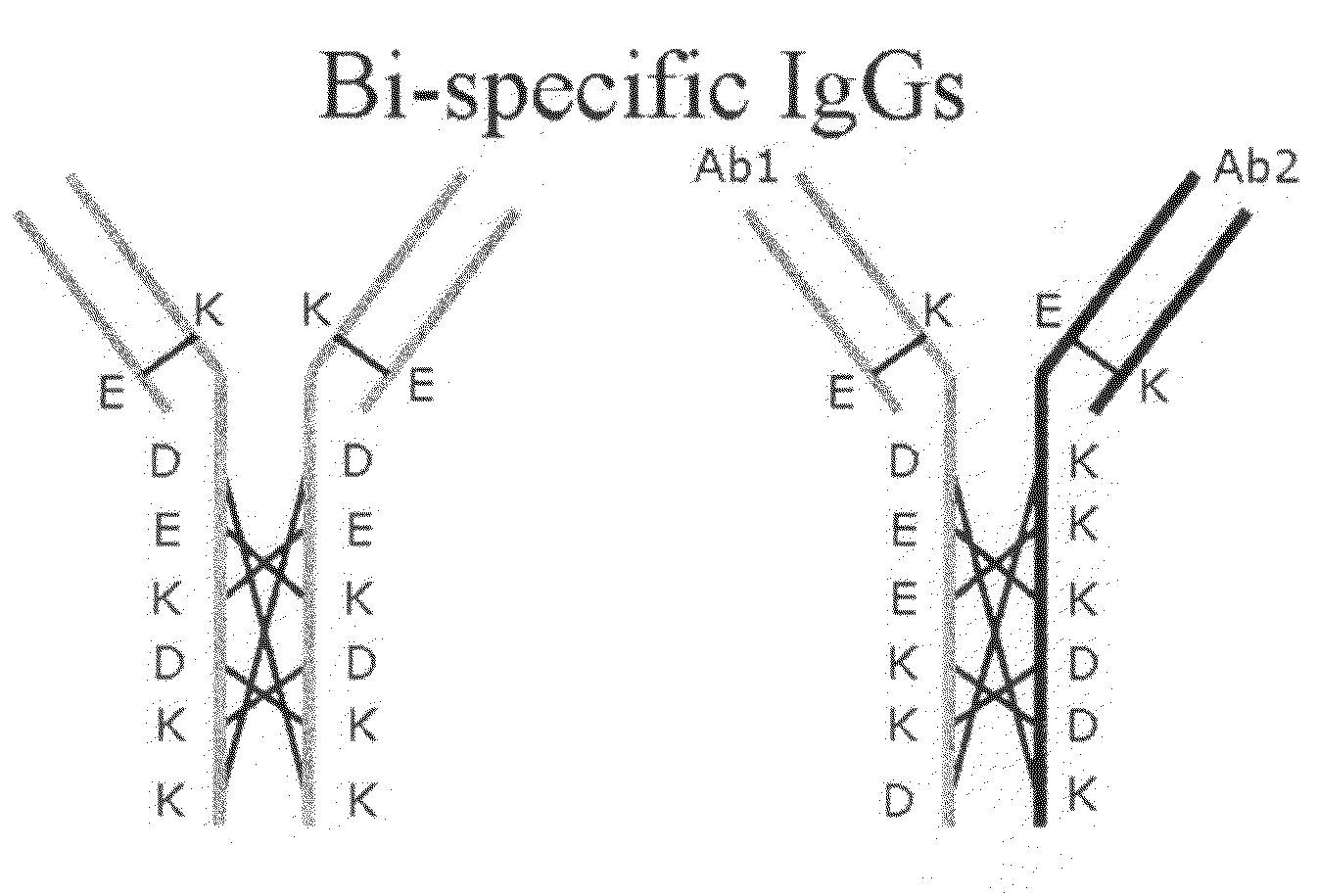
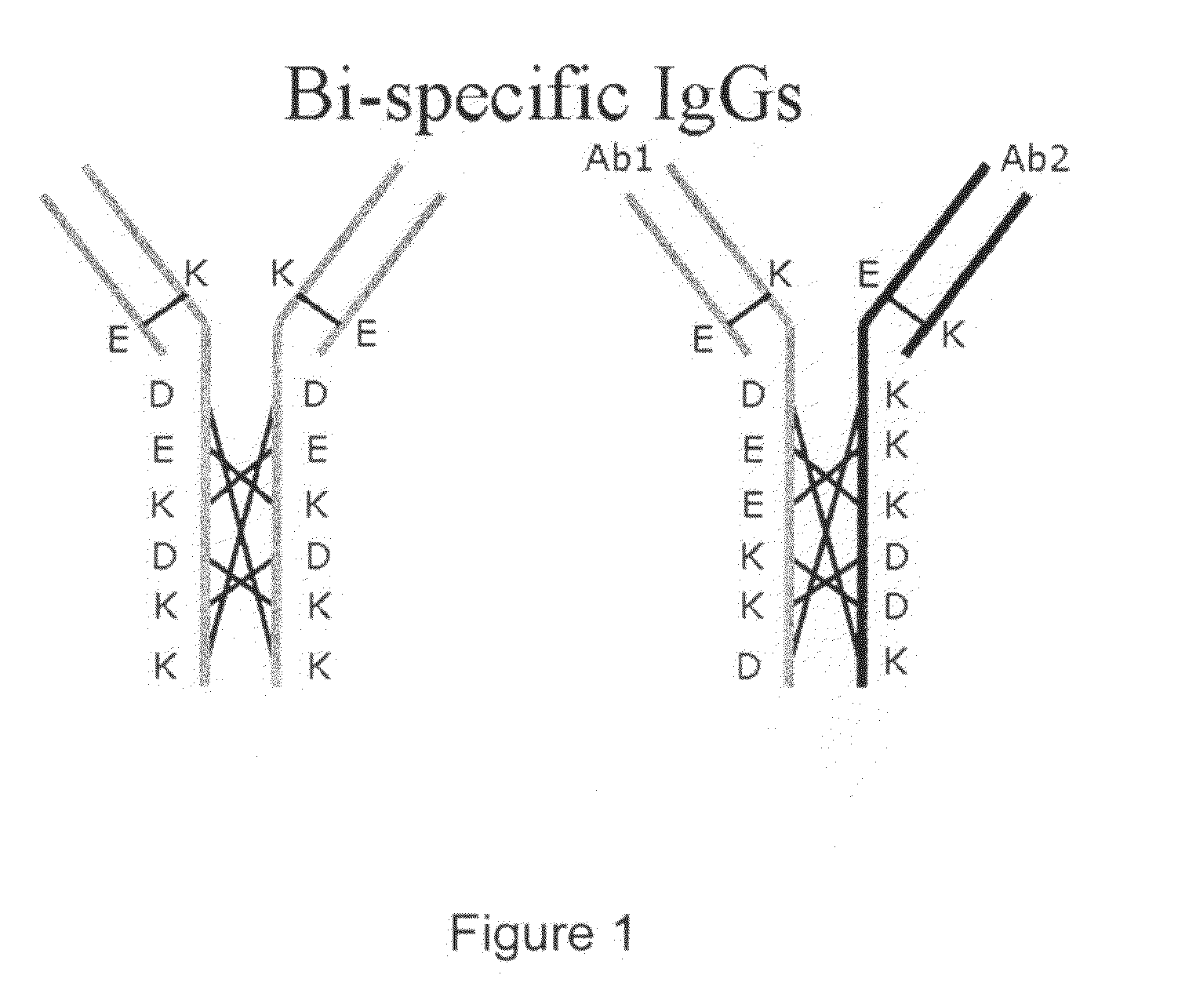
ActiveUS20100331527A1Improve abilitiesReduces and eliminates bindingHybrid immunoglobulinsSerum immunoglobulinsImmunoglobulin heavy chainHeavy chain
A bispecific antibody format providing ease of isolation is provided, comprising immunoglobulin heavy chain variable domains that are differentially modified in the CH3 domain, wherein the differential modifications are non-immunogenic or substantially non-immunogenic with respect to the CH3 modifications, and at least one of the modifications results in a differential affinity for the bispecific antibody for an affinity reagent such as Protein A, and the bispecific antibody is isolable from a disrupted cell, from medium, or from a mixture of antibodies based on its affinity for Protein A.
Owner:REGENERON PHARM INC
Production of Bispecific Antibodies
InactiveUS20090182127A1Reduce interactionImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsConstant domainHeavy chain
Bispecific antibodies comprising (a) a first light-heavy chain pair having specificity for a first target and a sufficient number of substitutions in its heavy chain constant domain with respect to a corresponding wild-type antibody of the same isotype to significantly reduce the formation of first heavy chain-first heavy chain dimers and (b) a second light-heavy chain pair comprising a heavy chain having a sequence that is complementary to the sequence of the first pair heavy chain sequence with respect to the formation of intramolecular ionic interactions, wherein the first pair or second pair comprises a substitution in the light chain and complementary substitution in the heavy chain that reduces the ability of the light chain to interact with the heavy chain of the other light chain-heavy chain pair are provided. Methods of producing such antibodies in one or more cells also are provided.
Owner:NOVO NORDISK AS
Stable Heterodimeric Antibody Design with Mutations in the Fc Domain
ActiveUS20130195849A1Improve stabilityHybrid immunoglobulinsImmunological disordersFc(alpha) receptorFc receptor
The provided scaffolds have heavy chains that are asymmetric in the various domains (e.g. CH2 and CH3) to accomplish selectivity between the various Fc receptors involved in modulating effector function, beyond those achievable with a natural homodimeric (symmetric) Fc molecule, and increased stability and purity of the resulting variant Fc heterodimers. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
Antibody specificity transfer using minimal essential binding determinants
The present invention provides methods of making antibodies having the binding specificity of a reference antibody. Antibodies generated by the methods of the inventions have at least one minimal essential binding specificity determinant from a heavy chain or light chain CDR3 from the reference antibody. The method can be used, e.g., in humanization procedures. The invention also provides libraries and antibodies made in accordance with the methods.
Owner:HUMANIGEN INC
Human B7.1-specific primatized antibodies and transfectomas expressing said antibodies
InactiveUS6113898AShrink tumorInhibit tumor growthPeptide/protein ingredientsAntipyreticDiseaseOrgan transplant rejection
The present invention relates to the identification of macaque antibodies to human B7.1 and B7.2 by screening of phage display libraries or monkey heterohybridomas obtained using B lymphocytes from B7.1 and / or B7.2 immunized monkeys. More specifically, the invention provides four monkey monoclonal antibodies 7B6, 16C10, 7C10 and 20C9 which inhibit the B7:CD28 pathway and thereby function as effective immunosuppressants. The invention further provides the complete DNA and amino acid sequences of the light and heavy chain of three primatized antibodies derived from those monkey monoclonal antibodies which bind B7.1 and possibly B7.2, primatized 7C10, primatized 7B6 and primatized 16C10. These primatized and monkey antibodies may be used as specific immunosuppressants, e.g., for the treatment of autoimmune diseases and to prevent organ transplant rejection.
Owner:BIOGEN INC
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
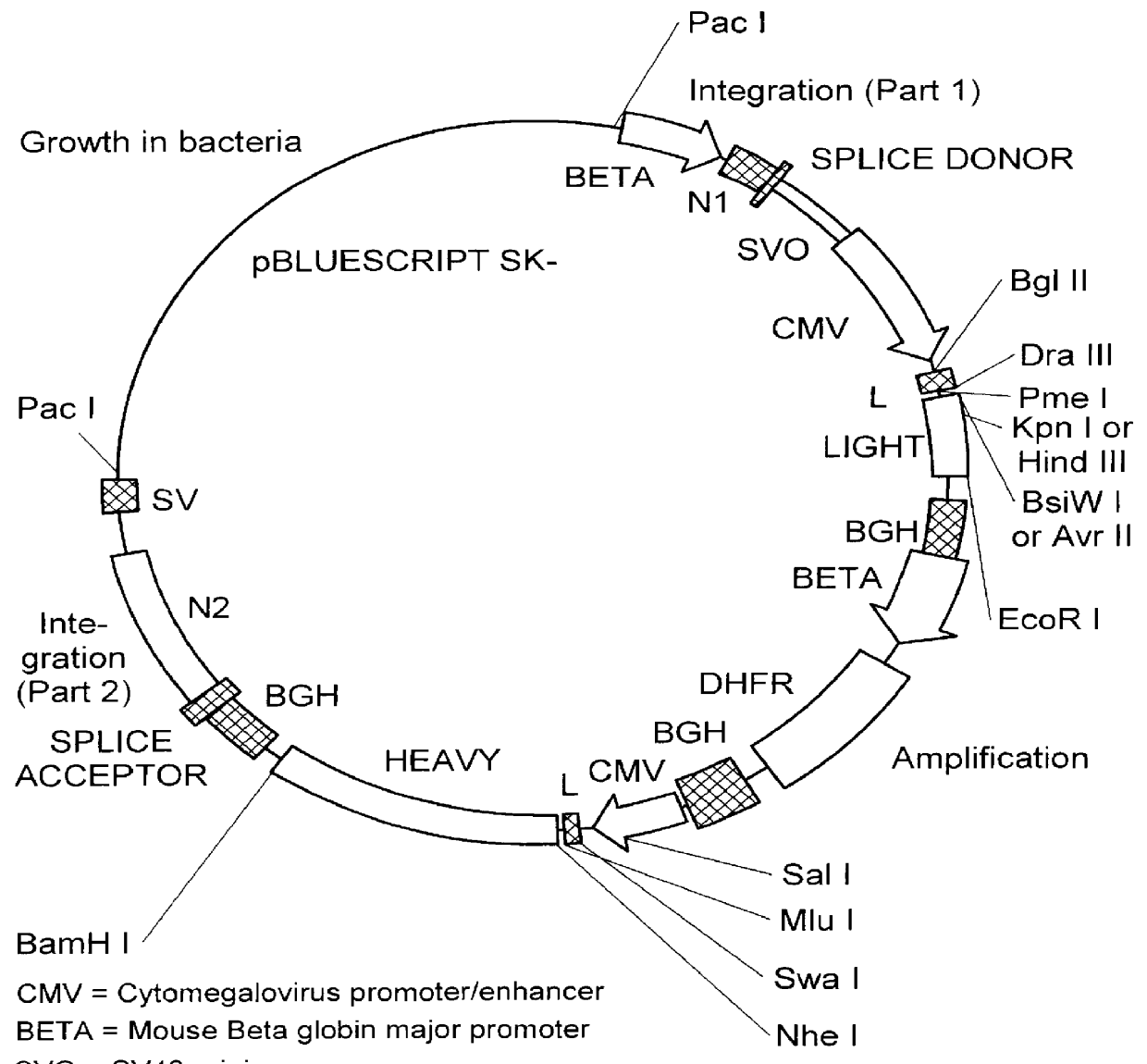
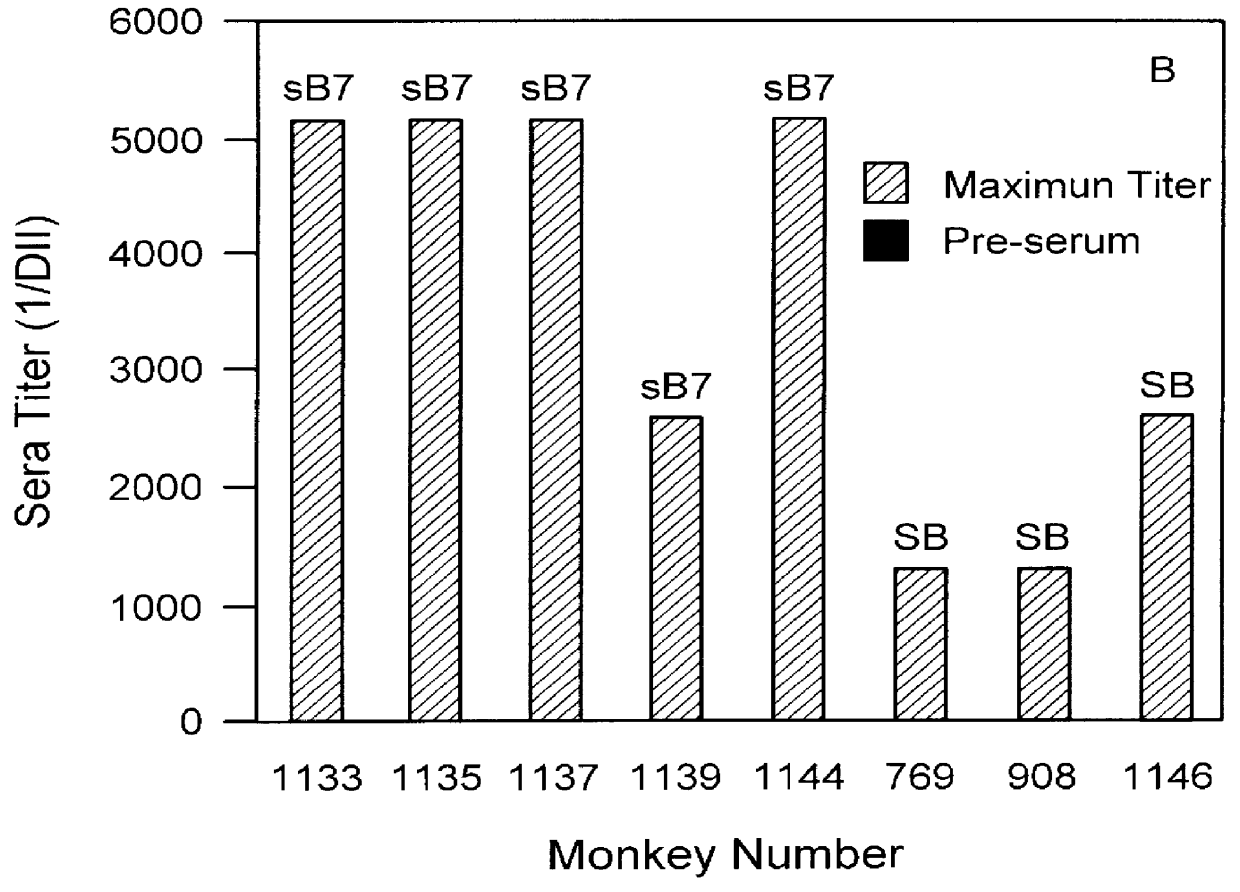
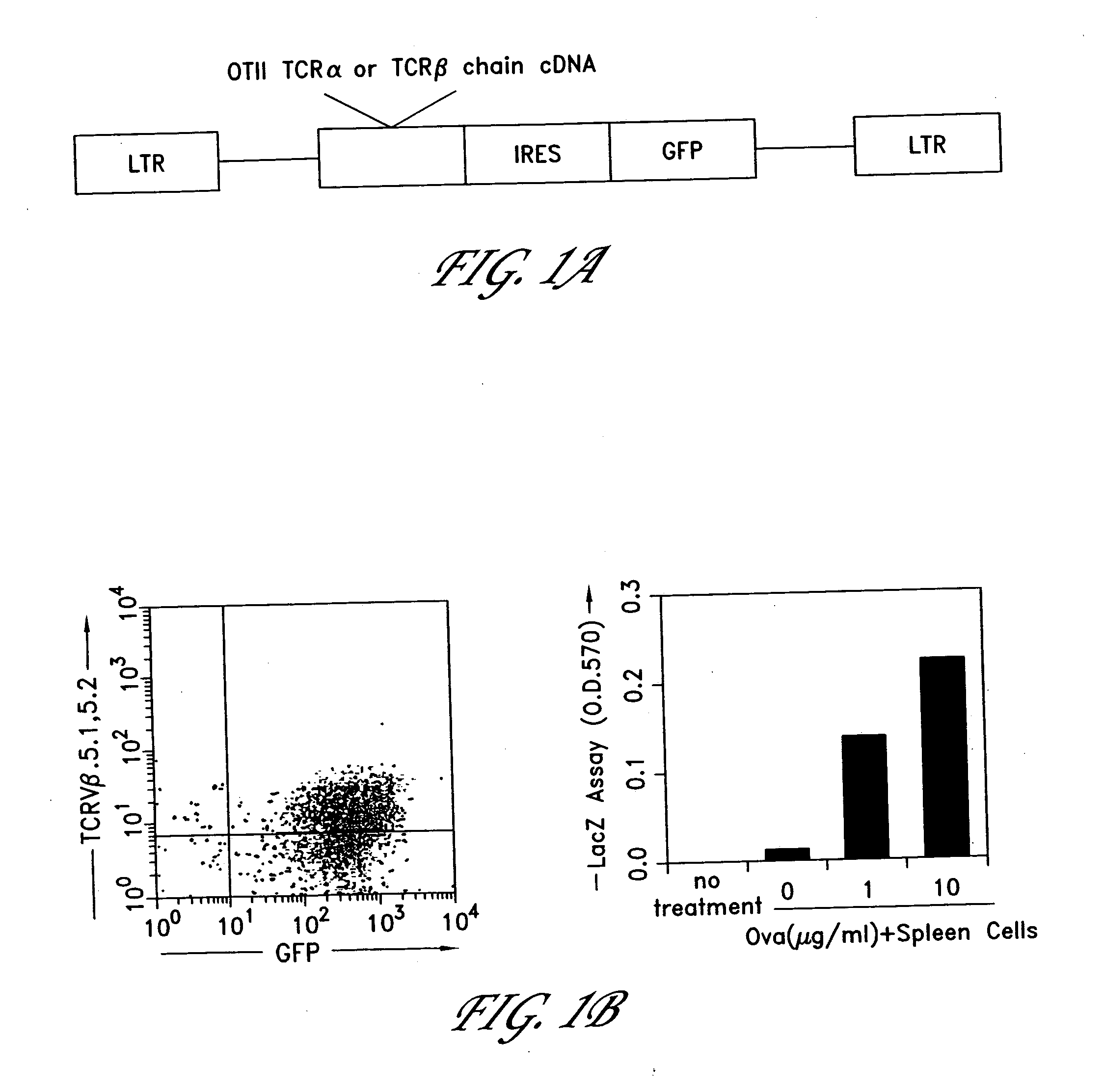
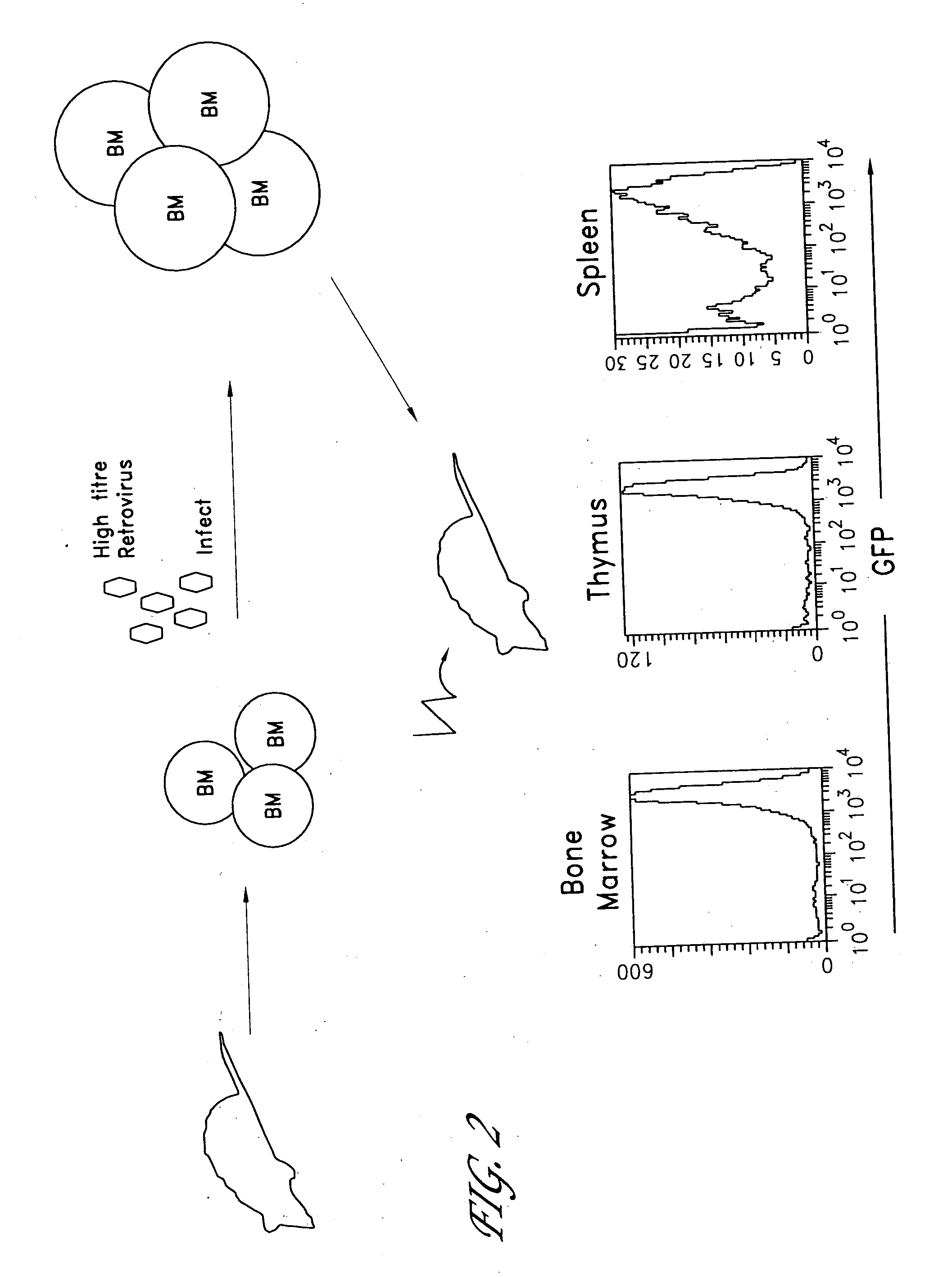
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
Antibodies to insulin-like growth factor I receptor
Owner:AMGEN FREMONT INC +1
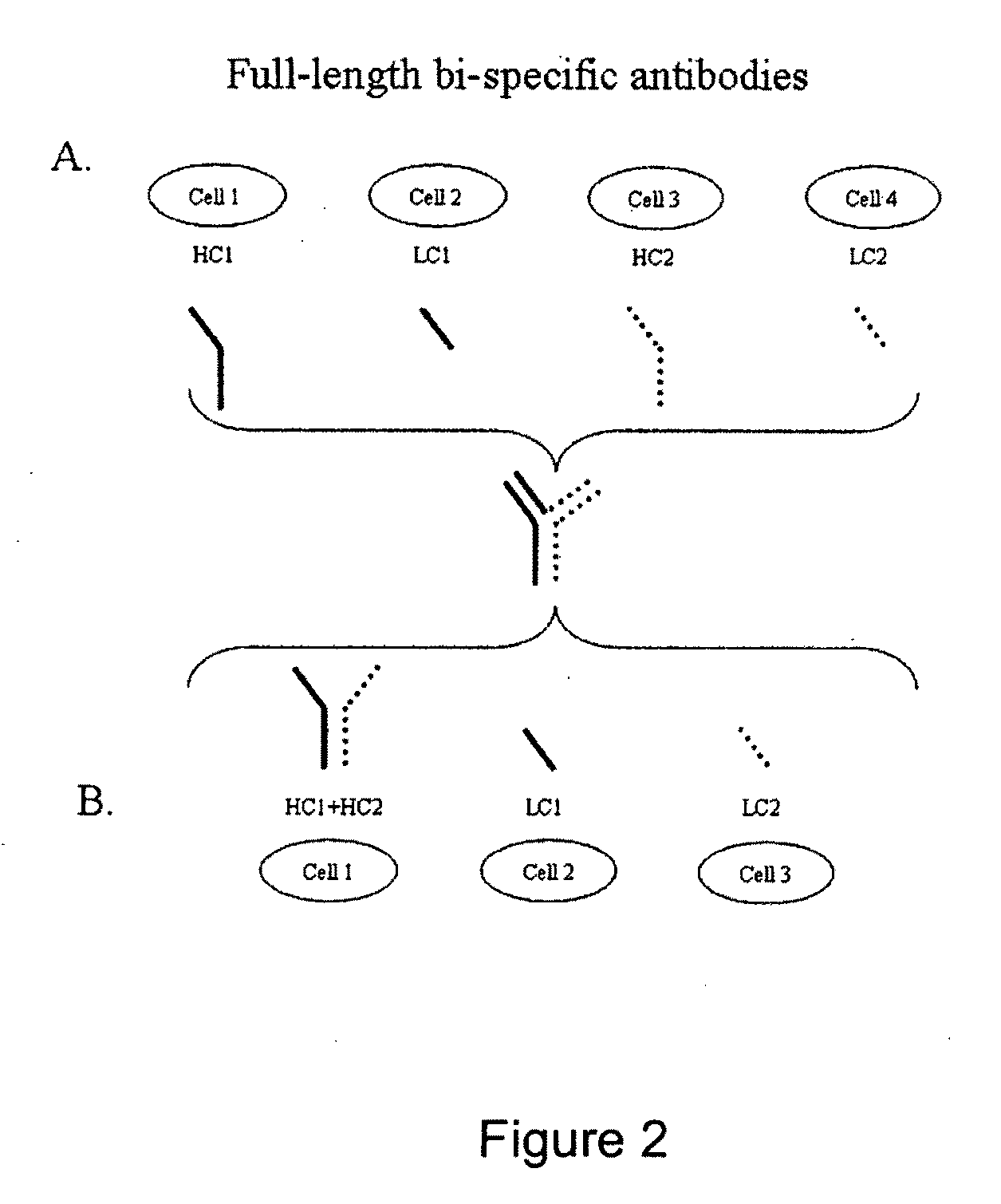
Methods for producing and identifying multispecific antibodies
The present invention relates to a high efficiency method of expressing multispecific antibodies in eukaryotic cells. The invention is further drawn to a method of producing immunoglobulin heavy and light chain libraries, particularly using the trimolecular recombination method, for expression in eukaryotic cells. The invention further provides methods of selecting and screening for multispecific antibodies, and antigen-binding fragments thereof. The invention also provides kits for producing, screening and selecting multispecific antibodies. Finally, the invention provides multispecific antibodies, and antigen-binding fragments thereof, produced by the methods provided herein.
Owner:VACCINEX
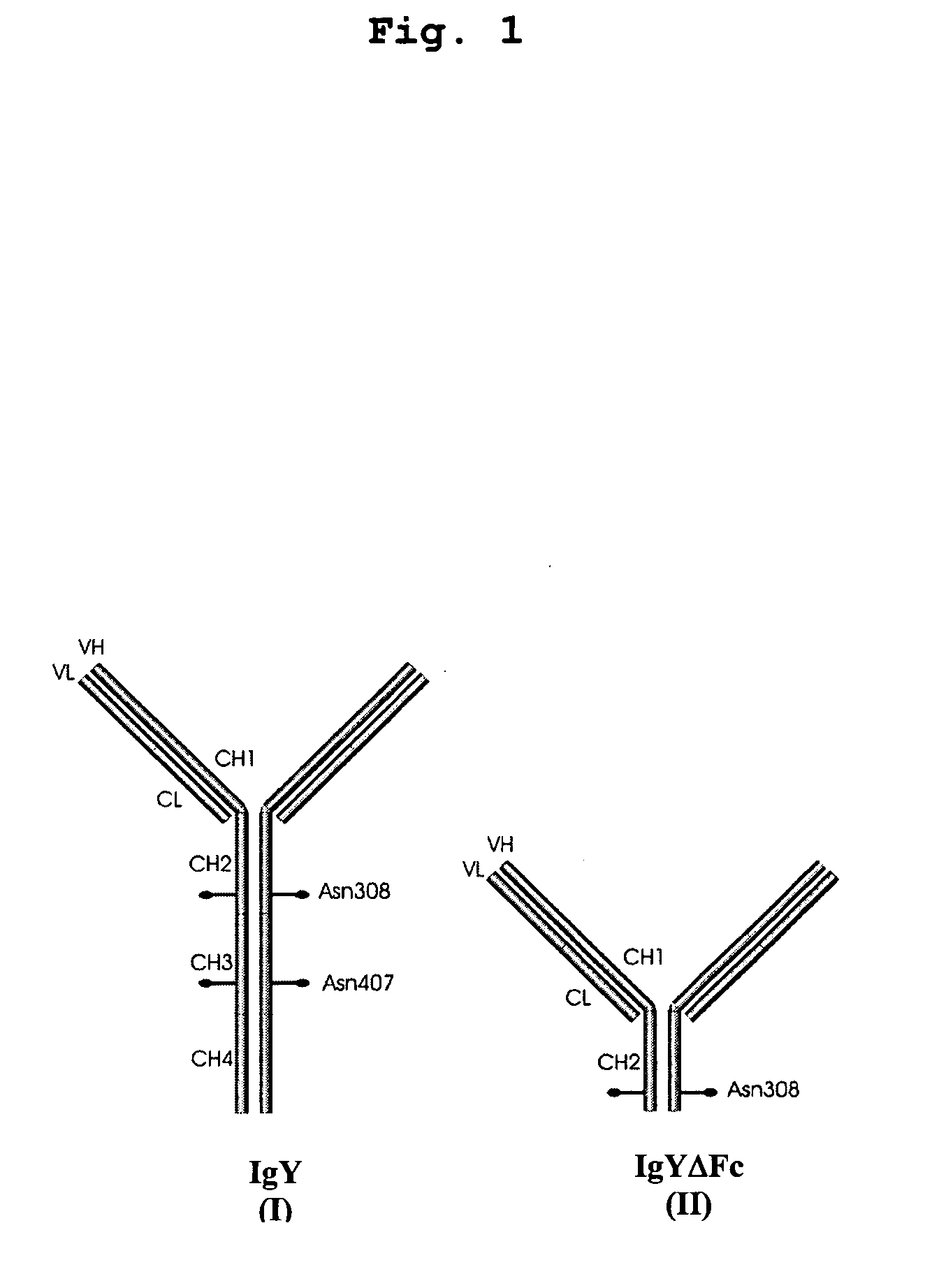
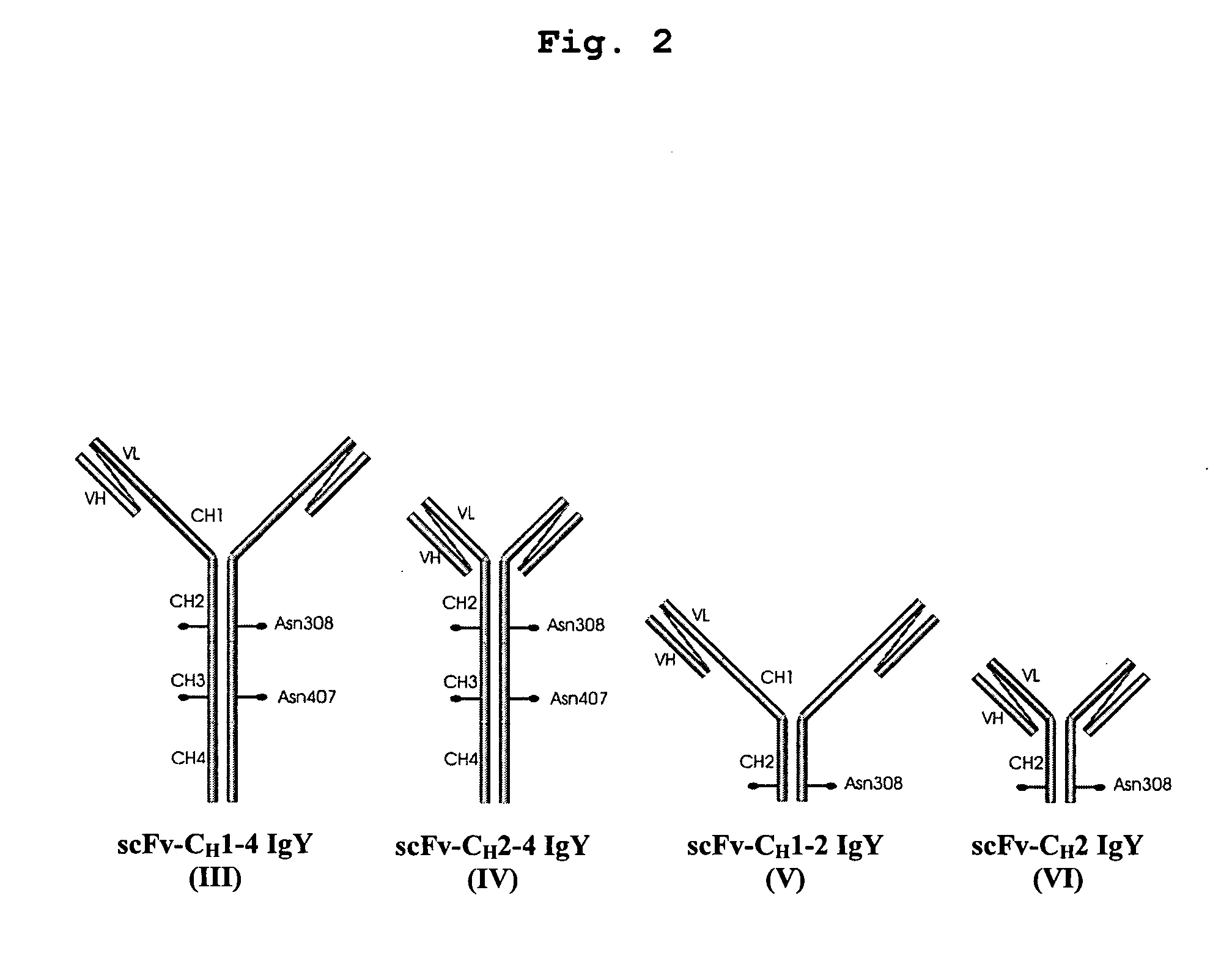
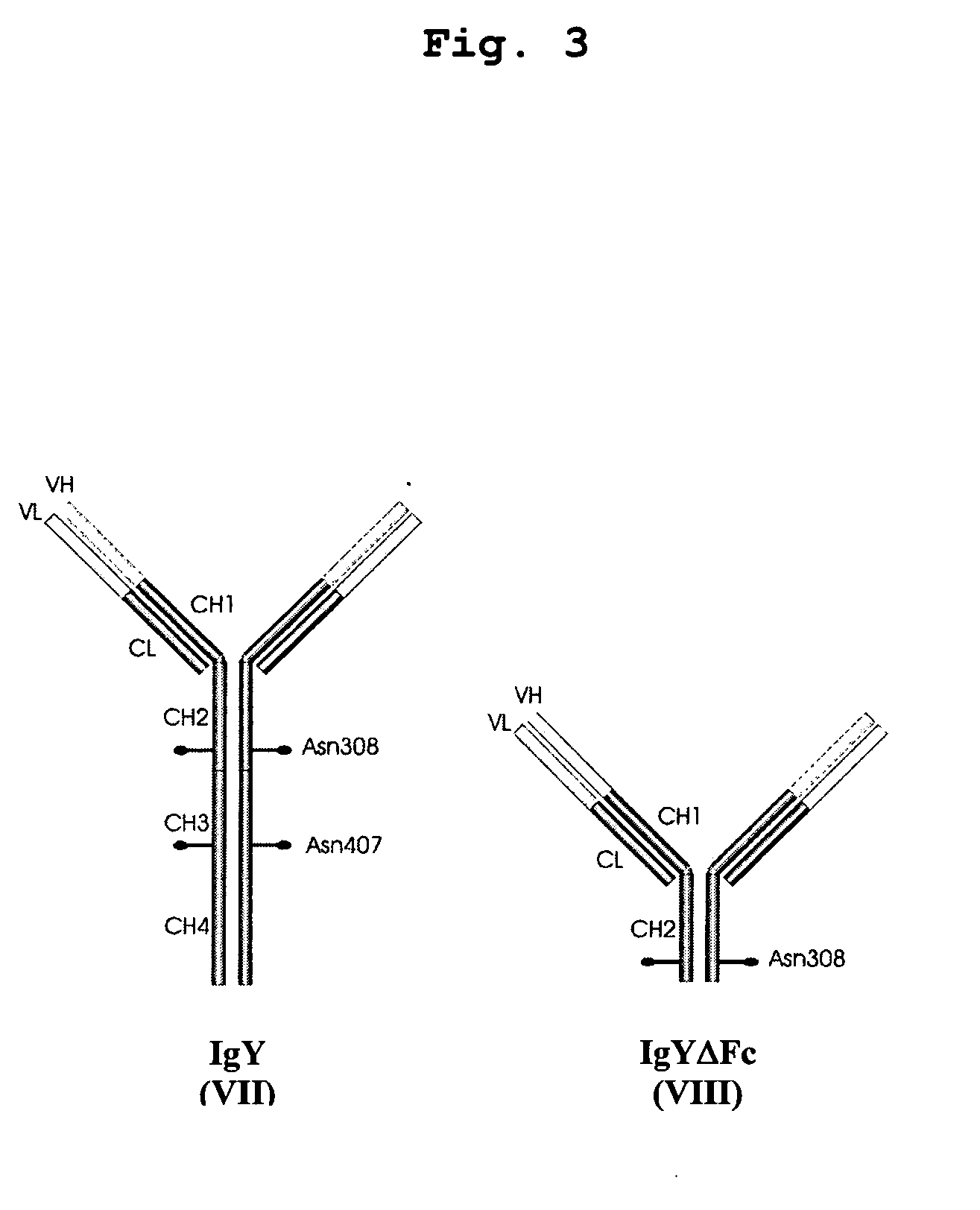
Bivalent IgY antibody constructs for diagnostic and therapeutic applications
InactiveUS20070141049A1Improve the immunityIncrease contentImmunoglobulins against virusesAntibody ingredientsHeavy chainMammal
This invention relates to the field of recombinant antibody technology. It provides novel recombinant IgY antibody constructs for diagnostic and therapeutical applications. The bivalent antibody constructs display a heterotetrameric or homodimeric format stabilized by disulfide bonds. The constant heavy chain domains CH2-CH4 are partly or completely of avian origin, whereas the VH, VL, CL, and CH1 domains as well as the hinge region may be of avian origin or derived from any other species. The invention allows to combine the advantages of IgY antibodies with those of established mammalian monoclonal antibodies. IgY antibody constructs comprising nonglycosylated IgY constant heavy chain domains allow to reduce unwanted interactions with C-type lectins, e.g., in human sera. Furthermore, chimeric IgY antibody containing mammalian VH, VL, CL, and CH1 domains as well as a mammalian hinge region provide a higher molecular stability than IgY antibodies in acidic conditions and, thereby, are especially suited for peroral therapeutic applications.
Owner:PLS DESIGN
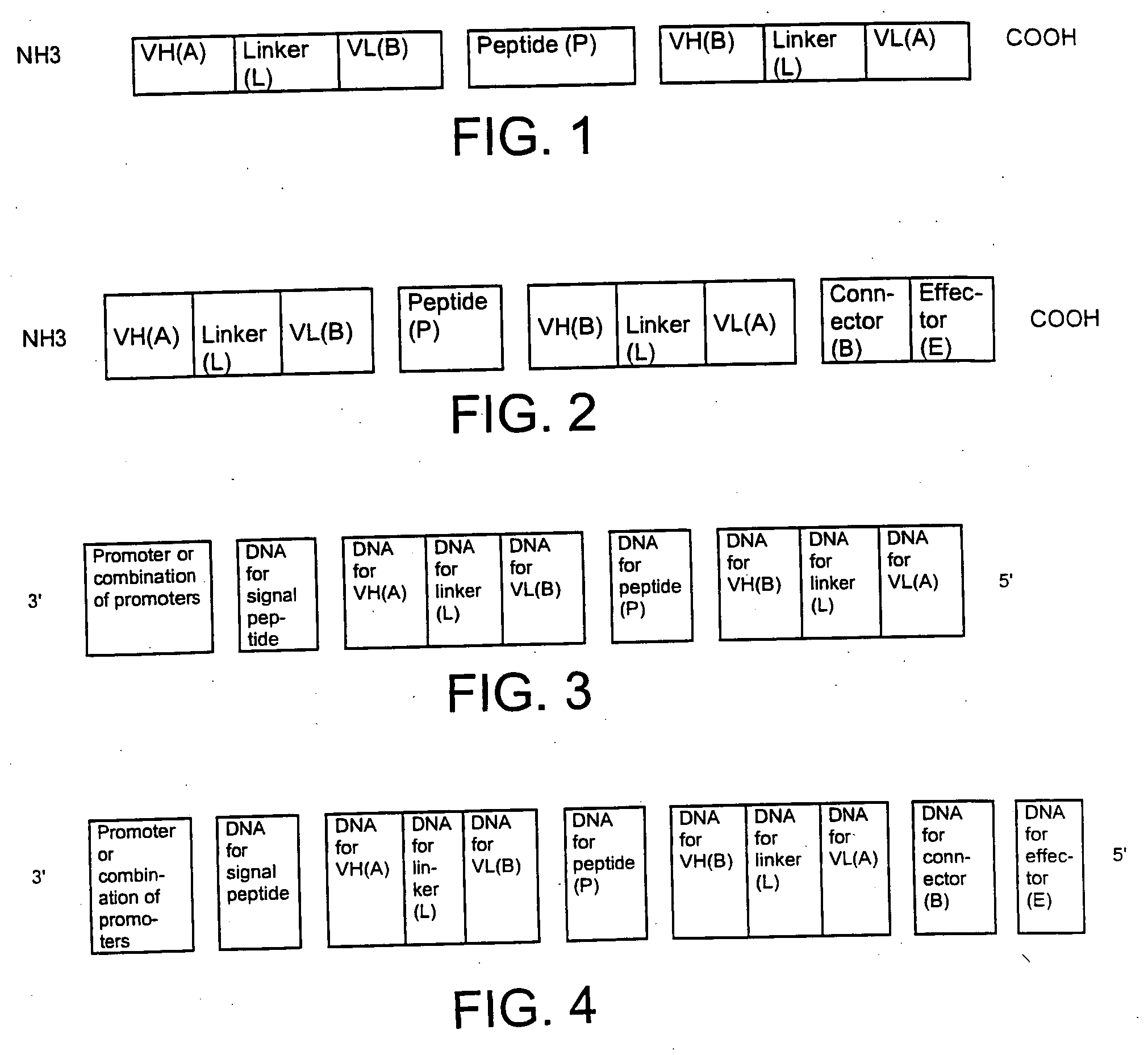
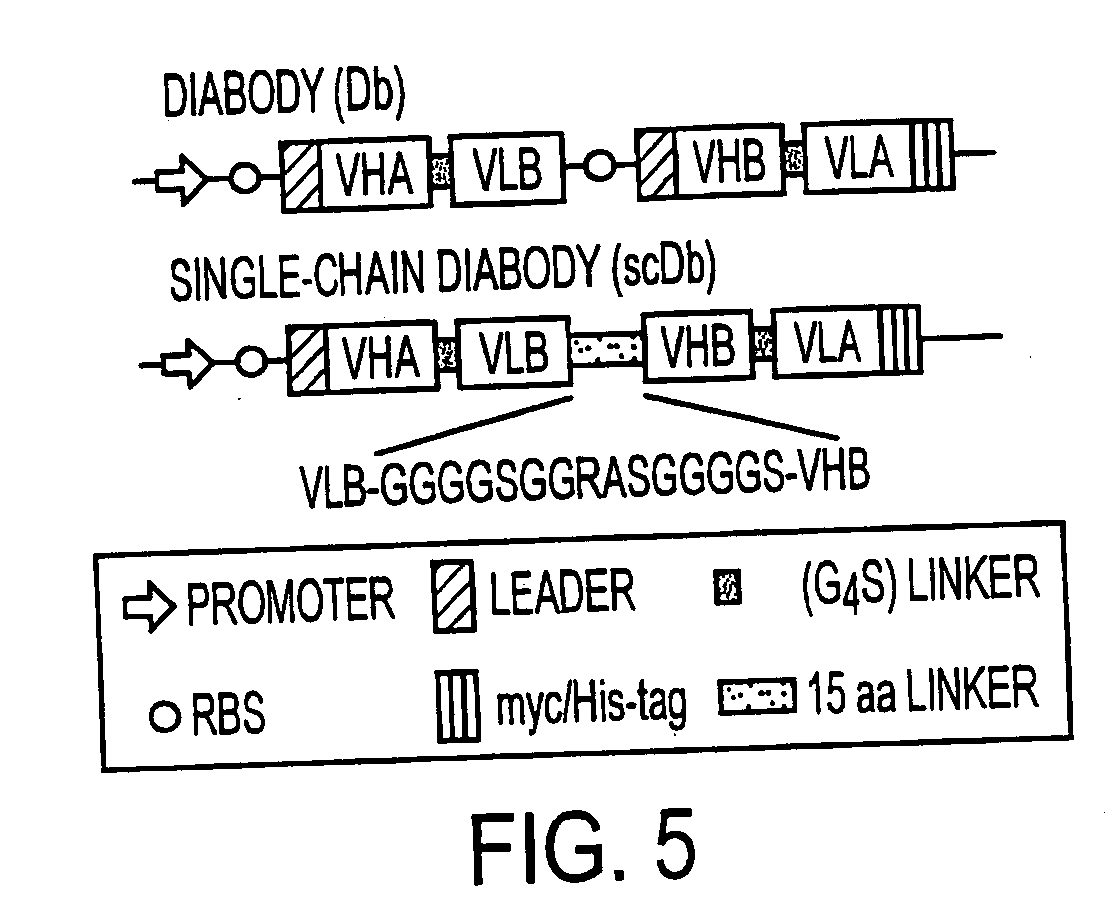
Single-chain multiple antigen-binding molecule, its preparation and use
InactiveUS20050004352A1Reduce dissociationLess complexOrganic active ingredientsFungiAntigen bindingVariable domain
The present invention relates to a single-chain, multiple antigen-binding molecule with diverse variable domains of a heavy and of a light chain of an immunoglobulin, which are connected in the form of a VH-VL construct, which are in turn connected together via a peptide, and to the preparation and use thereof as pharmaceutical or diagnostic aid.
Owner:AFFITECH RESEARCH AS
Fusion antibodies
ActiveUS20050069552A1Avoid stitchingHybrid immunoglobulinsAntibody mimetics/scaffoldsHeavy chainGene
The present invention provides novel antibodies. In particular, the present invention provides fusion antibodies comprising antibody heavy and light chain fusions. The present invention further provides multivalent antibodies comprising multiple fusion antibody chains. The present invention further provides methods of generating splice resistant antibody genes.
Owner:CATALENT USA WOODSTOCK INC +3
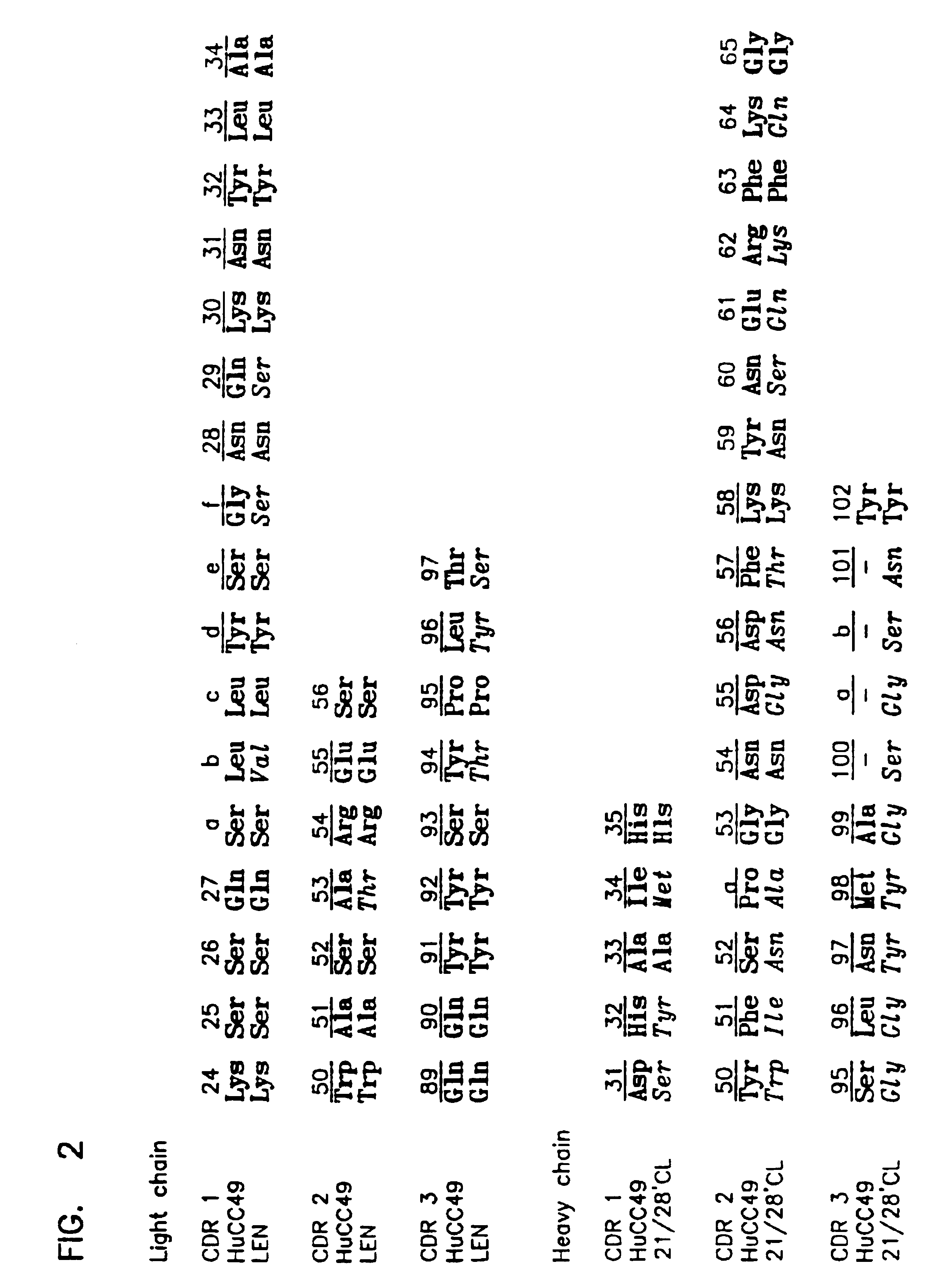
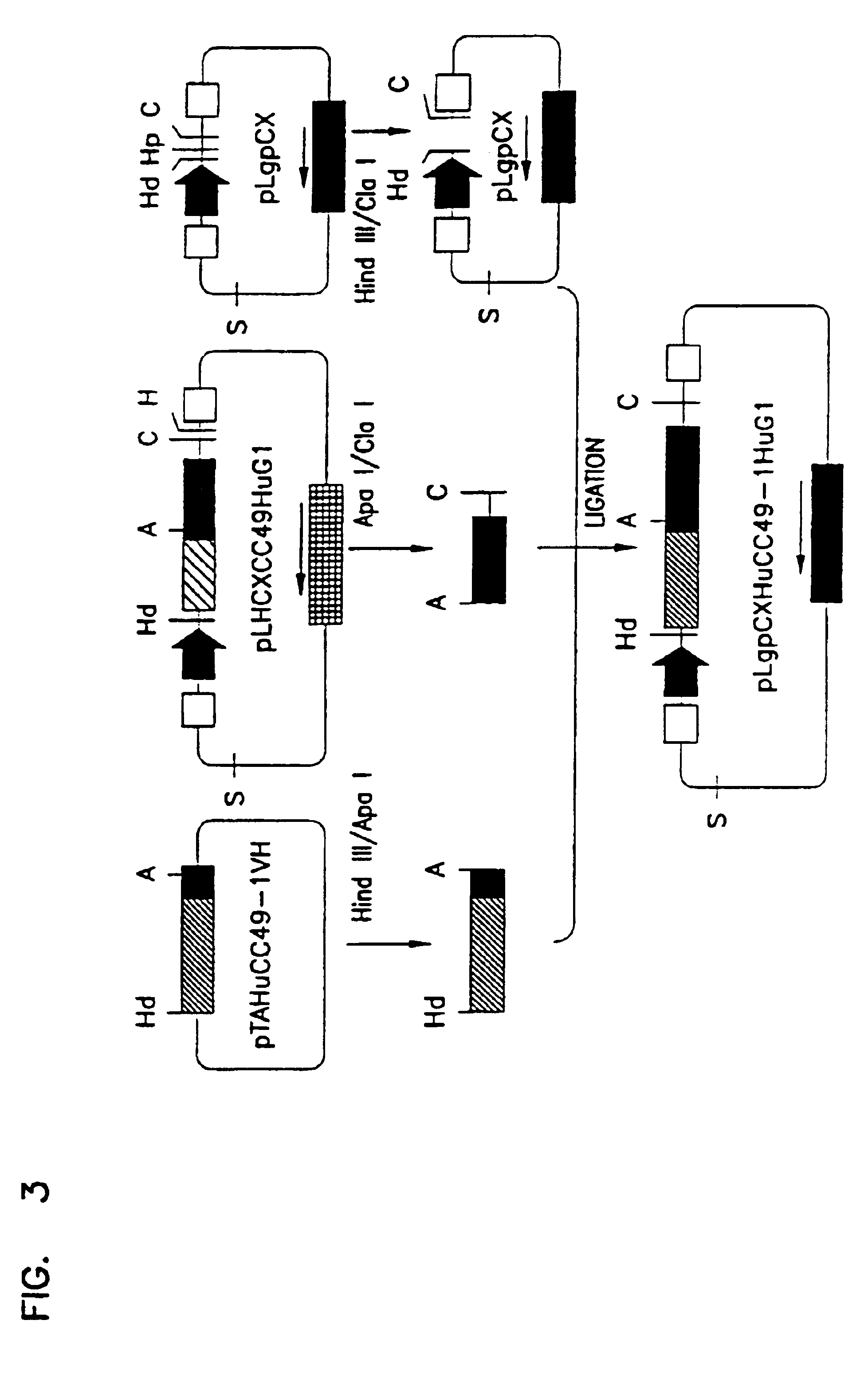
Variants of humanized anti carcinoma monoclonal antibody cc49
InactiveUS6818749B1Elicit adverse responseVirusesPeptide/protein ingredientsComplementarity determining regionHeavy chain
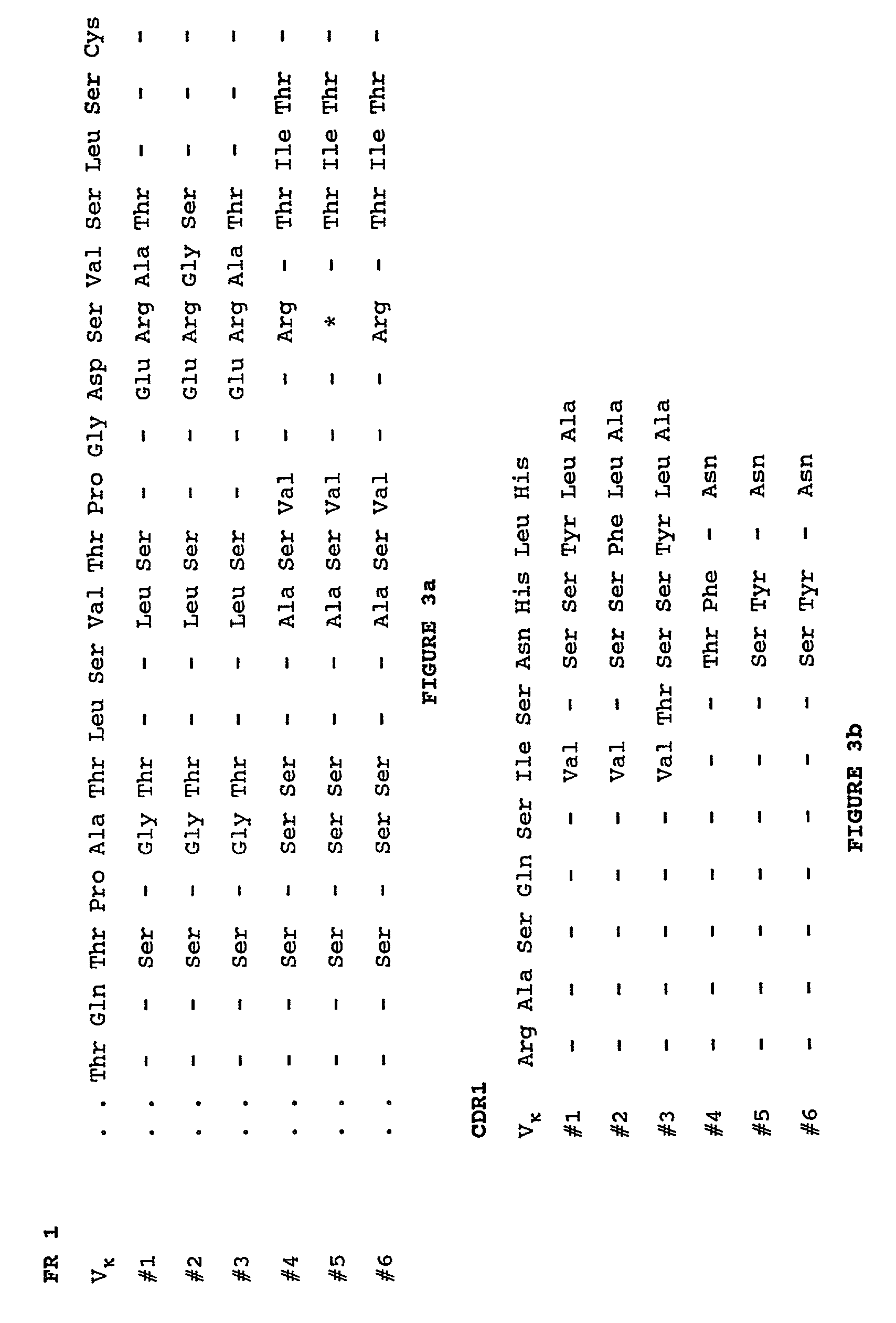
The invention is directed towards mouse-human chimeric variants of CC49 monoclonal antibodies with minimal murine content. A first aspect of the invention provides CDR variants of humanized monoclonal antibody (HuCC49) in which less than all six (three heavy chain and three light chain) Complementarity Determining Regions (CDRs) of CC49 are present. A second aspect of the invention provides SDR variants of humanized monoclonal antibody (HuCC49) in which only Specificity Determining Regions (SDRs) of at least one CDR from CC49 are present. The invention is also directed towards biotechnological methods of making the variants and therapeutic methods of using the variants.
Owner:UNITED STATES OF AMERICA
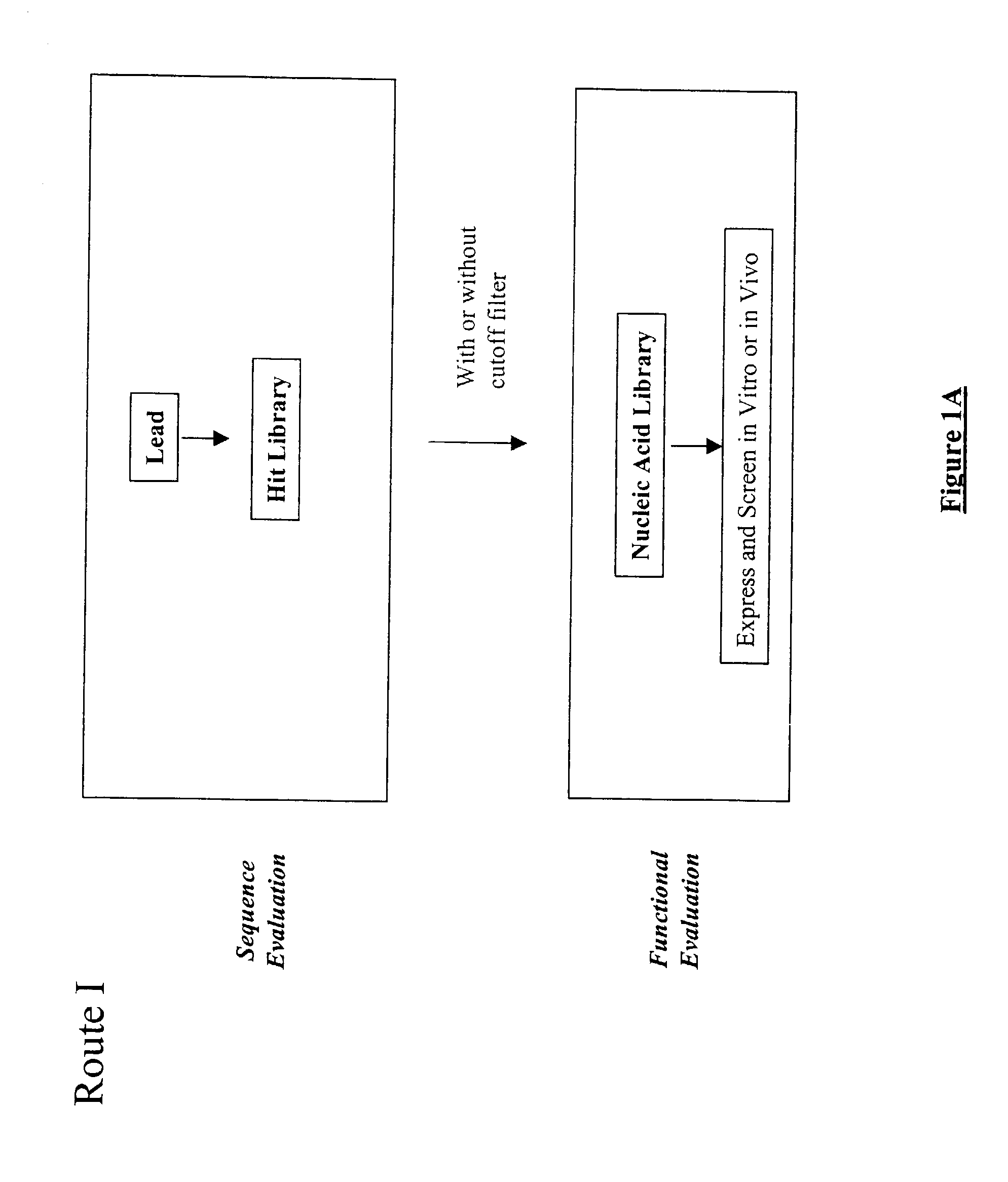
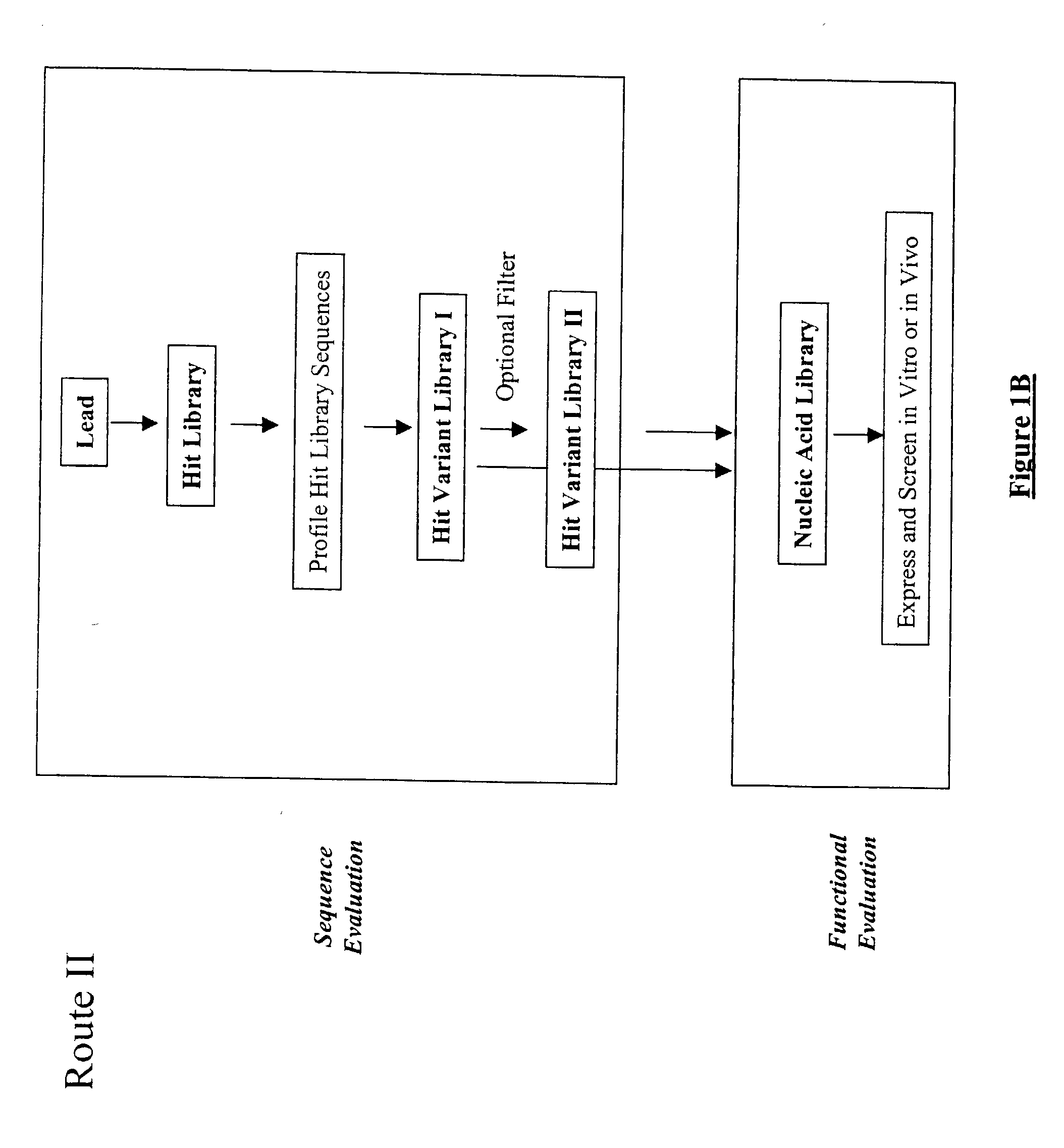
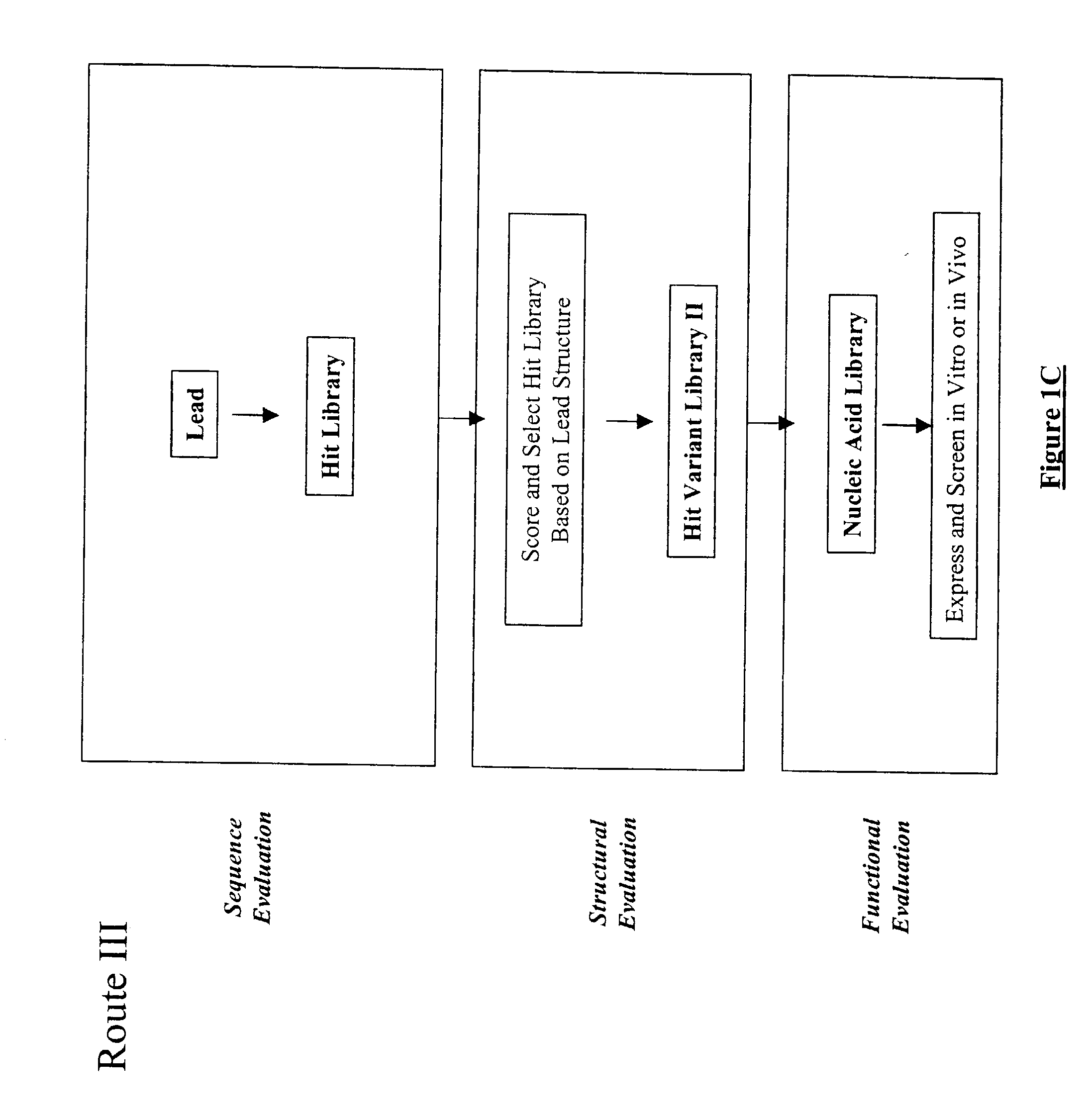
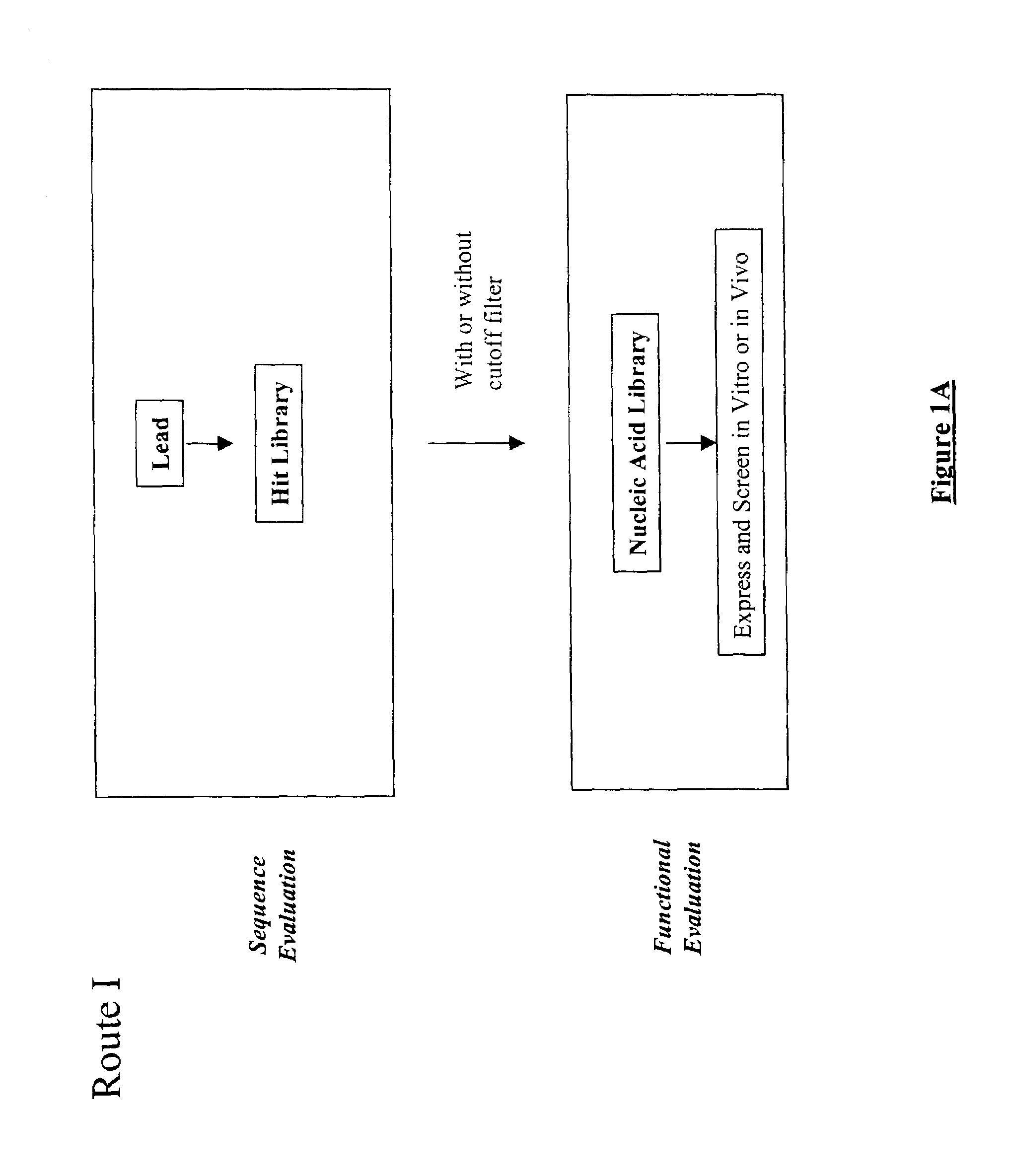
Structure-based selection and affinity maturation of antibody library
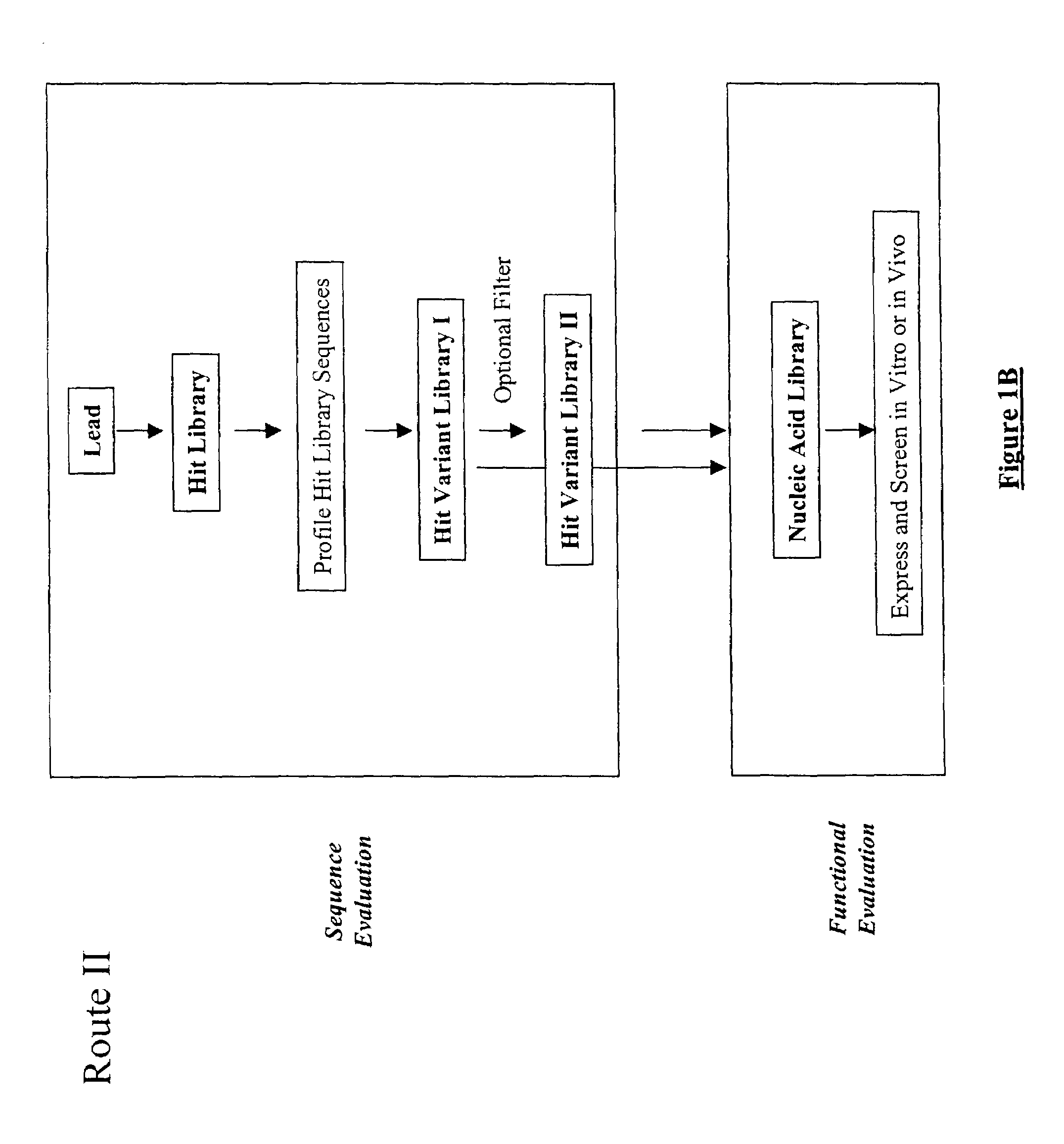
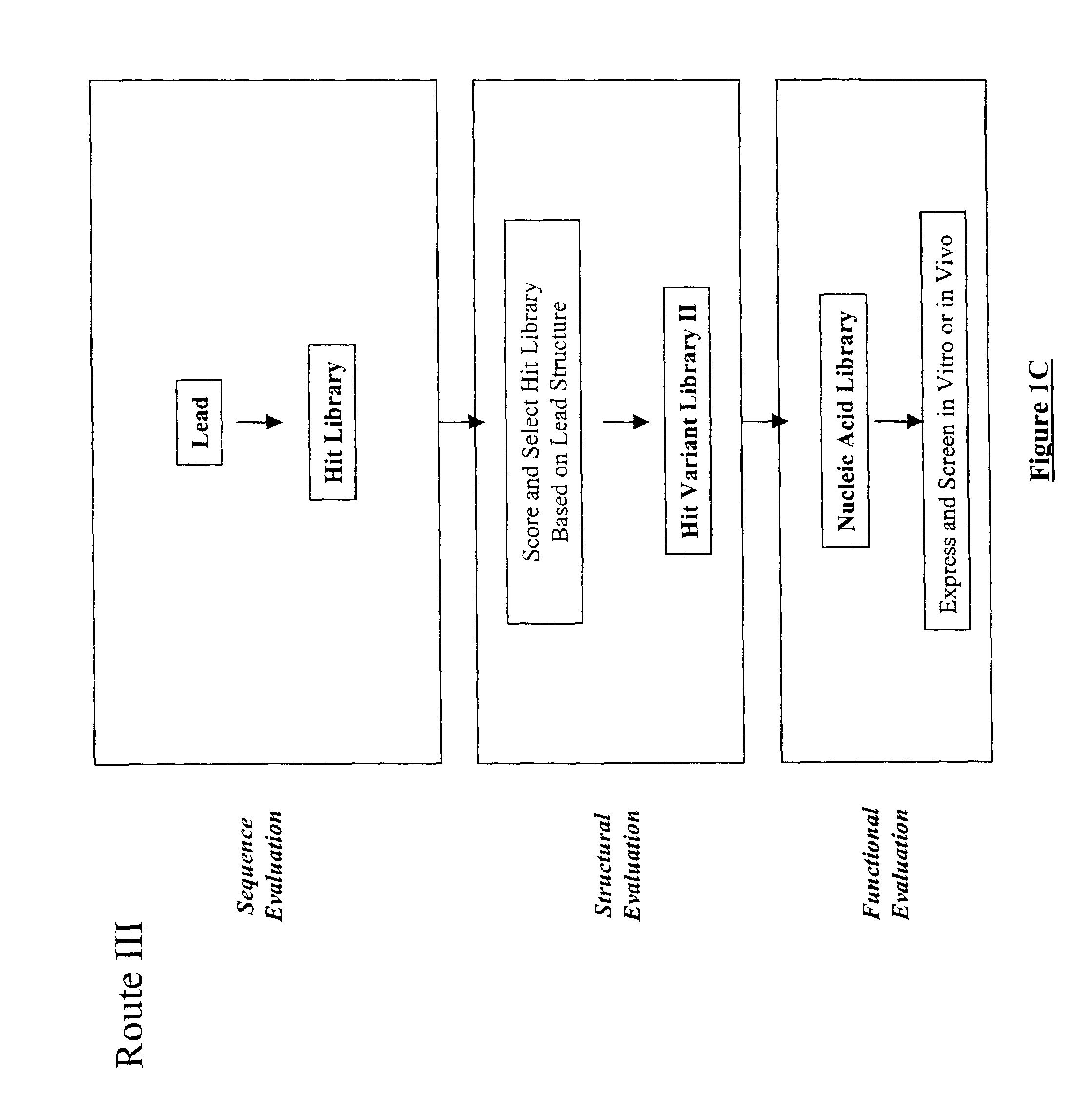
The present invention provides a structure-based methodology for efficiently generating and screening protein libraries for optimized proteins with desirable biological functions, such as antibodies with high binding affinity and low immunogenicity in humans. In one embodiment, a method is provided for constructing a library of antibody sequences based on a three dimensional structure of a lead antibody. The method comprises: providing an amino acid sequence of the variable region of the heavy chain (VH) or light chain (VL) of a lead antibody, the lead antibody having a known three dimensional structure which is defined as a lead structural template; identifying the amino acid sequences in the CDRs of the lead antibody; selecting one of the CDRs in the VH or VL region of the lead antibody; providing an amino acid sequence that comprises at least 3 consecutive amino acid residues in the selected CDR, the selected amino acid sequence being a lead sequence; comparing the lead sequence profile with a plurality of tester protein sequences; selecting from the plurality of tester protein sequences at least two peptide segments that have at least 10% sequence identity with lead sequence, the selected peptide segments forming a hit library; determining if a member of the hit library is structurally compatible with the lead structural template using a scoring function; and selecting the members of the hit library that score equal to or better than or equal to the lead sequence. The selected members of the hit library can be expressed in vitro or in vivo to produce a library of recombinant antibodies that can be screened for novel or improved function(s) over the lead antibody.
Owner:ABMAXIS
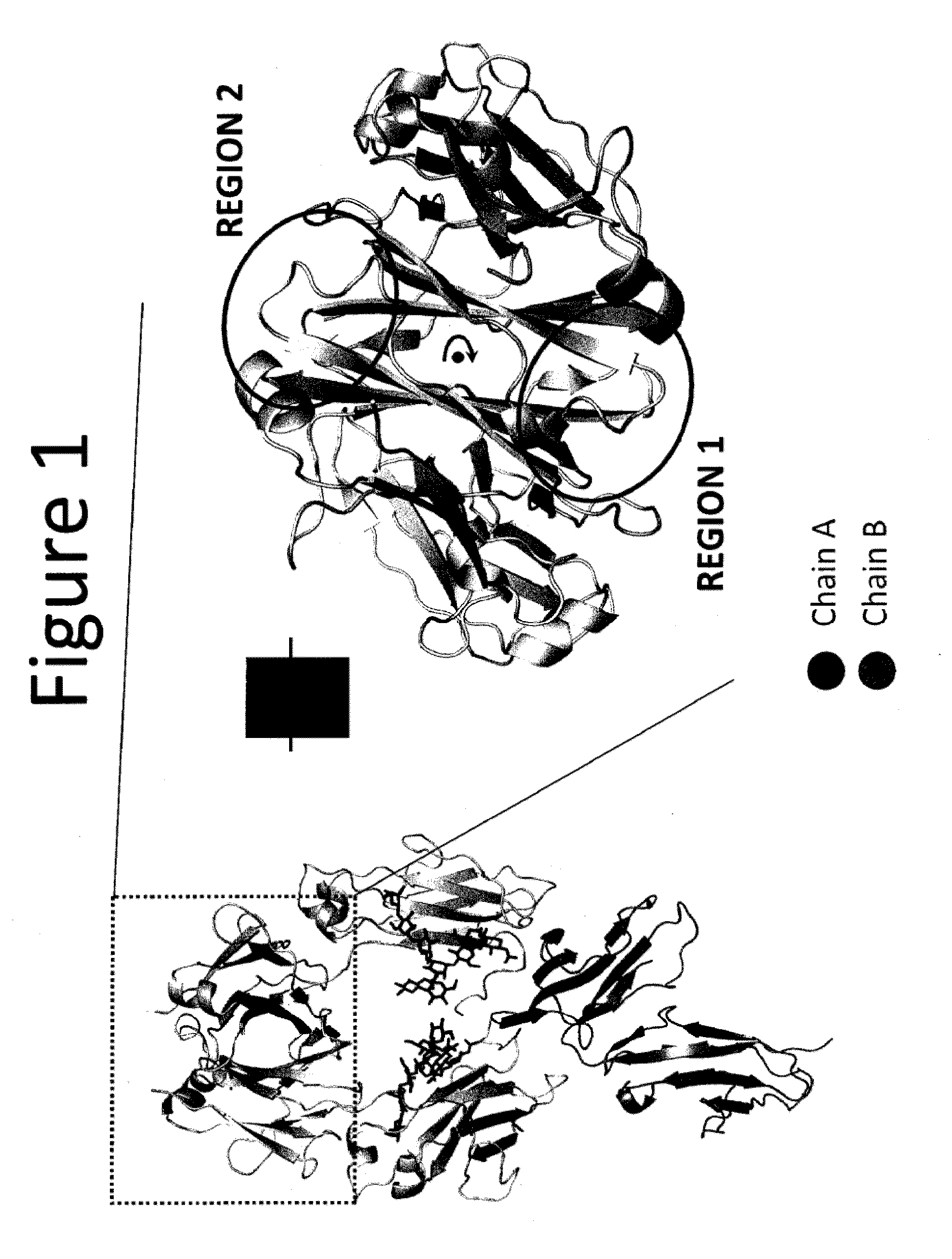
Heterodimeric proteins
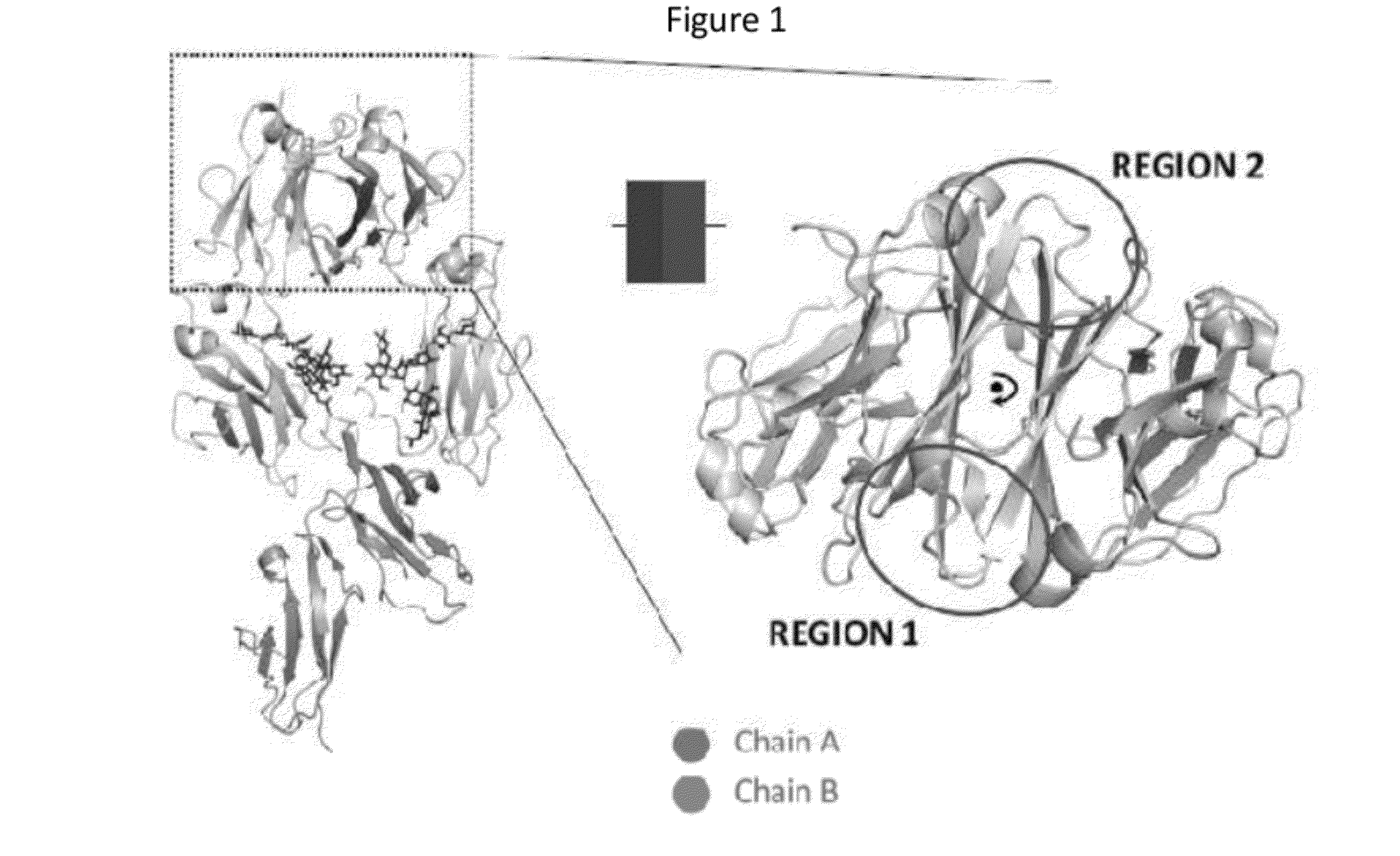
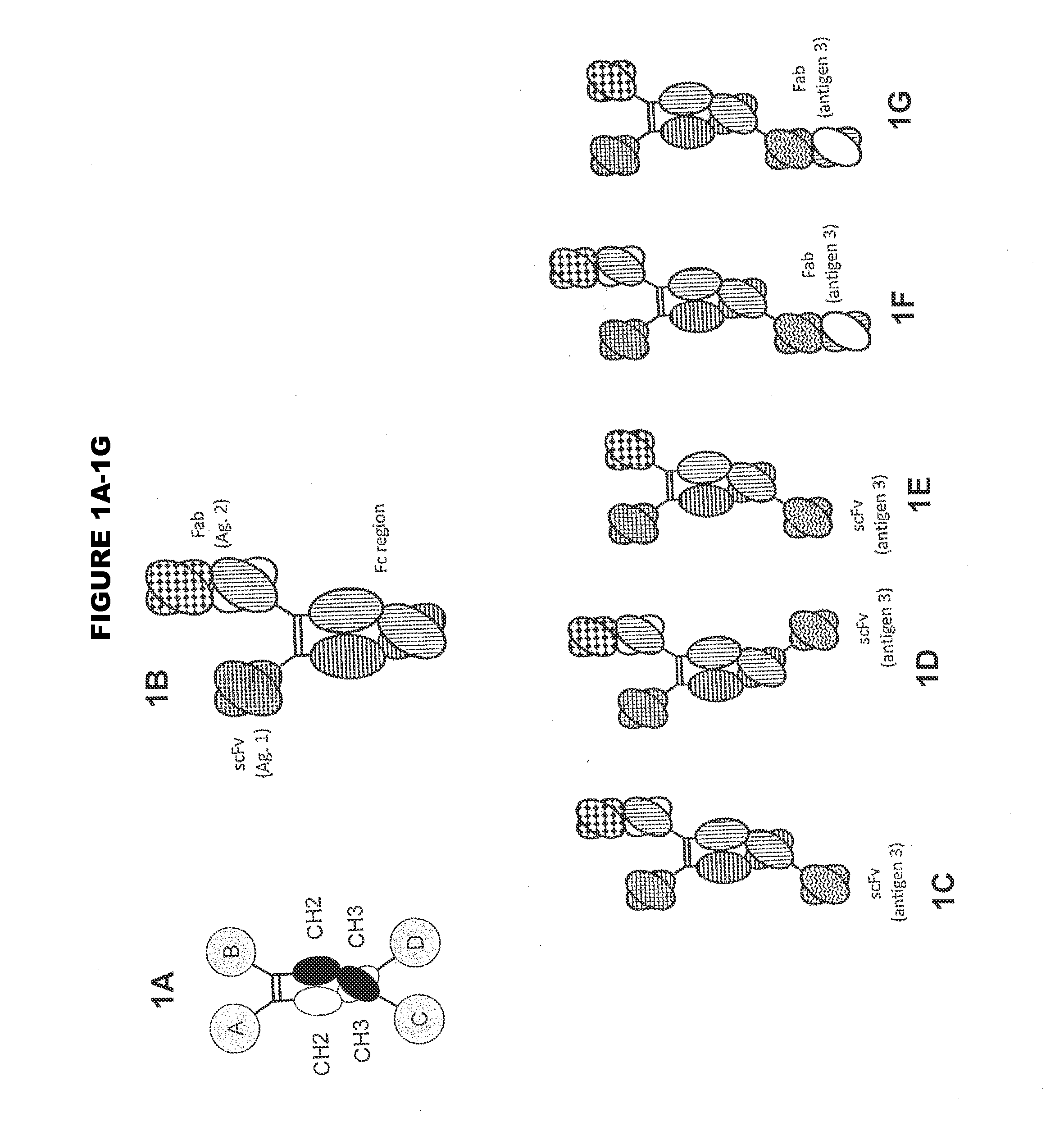
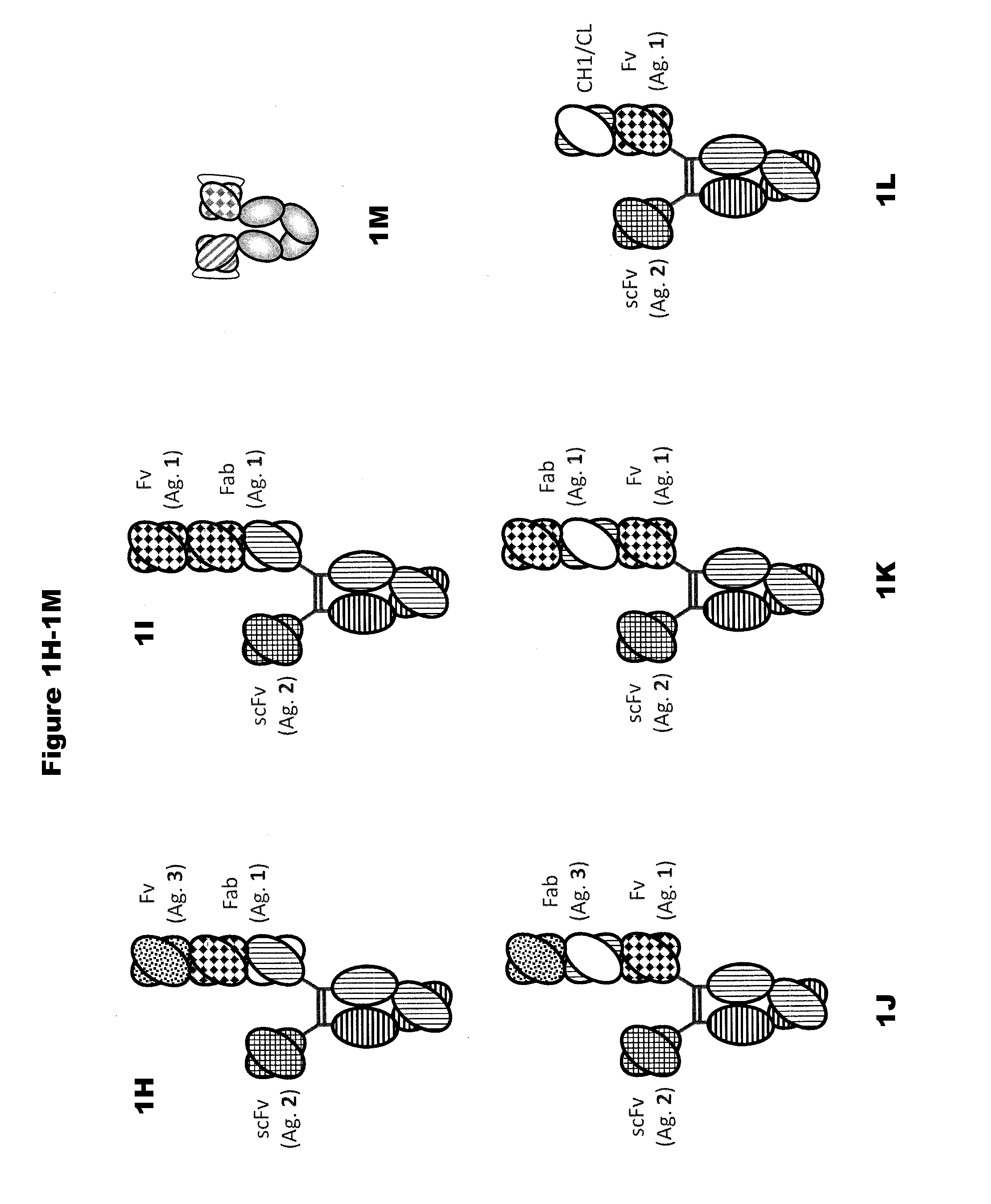
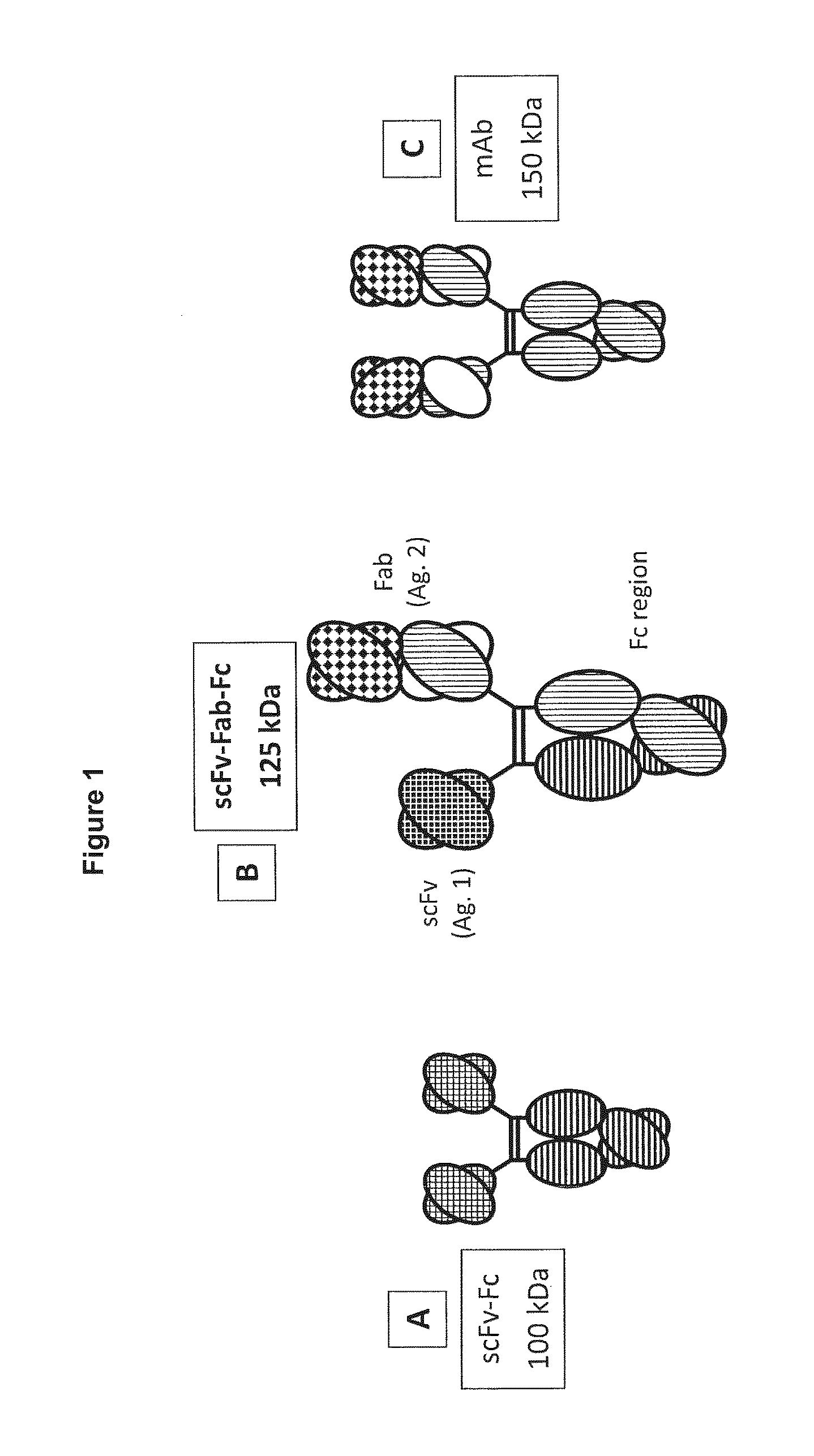
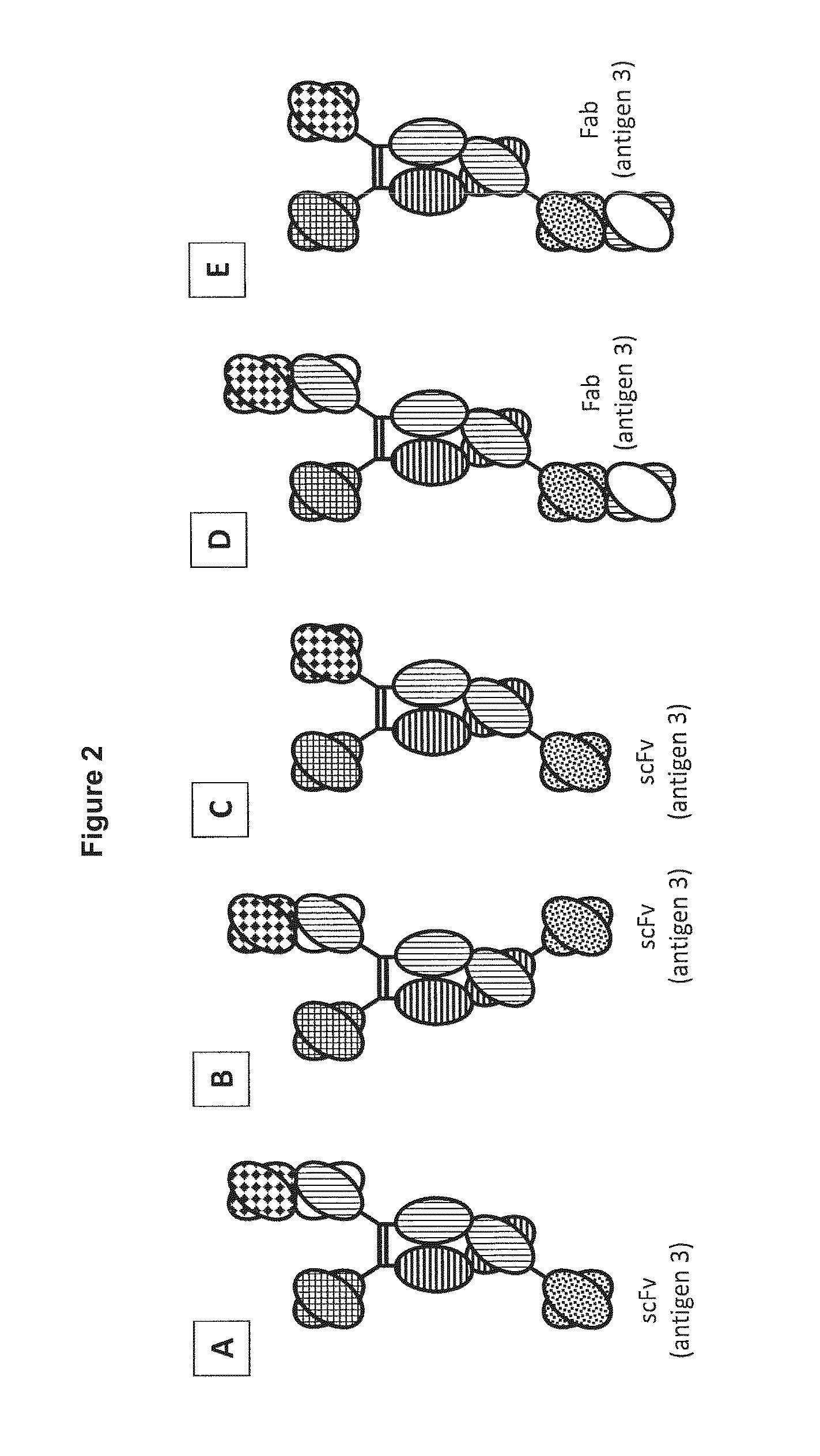
In one aspect, the present invention provides heterodimeric antibodies comprising a first monomer comprising a first heavy chain constant domain comprising a first variant Fc domain and a first antigen binding domain and a second monomer comprising a second heavy chain constant domain comprising a second variant Fc domain and a second antigen binding domain. In an additional aspect the heterodimeric antibody comprises a first monomer comprising a heavy chain comprising a first Fc domain and a single chain Fv region (scFv) that binds a first antigen, wherein the scFv comprises a charged scFv linker. The heterodimeric antibody further comprises a second monomer comprising a first heavy chain comprising a second Fc domain and a first variable heavy chain and a first light chain.
Owner:XENCOR INC
Polyclonal antibody composition for treating allergy
InactiveUS6849259B2Efficient removalPotential clinical advantageImmunoglobulins against animals/humansImmunoglobulins against plantsMicrosphereBULK ACTIVE INGREDIENT
A pharmaceutical composition for treating allergy is described. The composition comprises as an active ingredient a recombinant polyclonal antibody or a mixture of different monoclonal antibodies capable of reacting with or binding to an allergen together with one or more pharmaceutically acceptable excipients. The composition may be used topically as a solution, dispersion, powder, or in the form of microspheres. The polyclonal antibody is preferably a recombinant polyclonal antibody produced by phage display technology. The pairing of specific immunoglobulin variable region light chain and heavy chain maintained from the original polyclonal immune response or selected by panning using the allergen in question is preferably maintained by bulk transfer of the pairs into an expression vector.
Owner:SYMPHOGEN AS
Structure-based selection and affinity maturation of antibody library
InactiveUS7117096B2High affinityImprove throughputPeptide librariesPeptide/protein ingredientsProtein insertionIn vivo
The present invention provides a structure-based methodology for efficiently generating and screening protein libraries for optimized proteins with desirable biological functions, such as antibodies with high binding affinity and low immunogenicity in humans. In one embodiment, a method is provided for constructing a library of antibody sequences based on a three dimensional structure of a lead antibody. The method comprises: providing an amino acid sequence of the variable region of the heavy chain (VH) or light chain (VL) of a lead antibody, the lead antibody having a known three dimensional structure which is defined as a lead structural template; identifying the amino acid sequences in the CDRs of the lead antibody; selecting one of the CDRs in the VH or VL region of the lead antibody; providing an amino acid sequence that comprises at least 3 consecutive amino acid residues in the selected CDR, the selected amino acid sequence being a lead sequence; comparing the lead sequence profile with a plurality of tester protein sequences; selecting from the plurality of tester protein sequences at least two peptide segments that have at least 10% sequence identity with lead sequence, the selected peptide segments forming a hit library; determining if a member of the hit library is structurally compatible with the lead structural template using a scoring function; and selecting the members of the hit library that score equal to or better than or equal to the lead sequence. The selected members of the hit library can be expressed in vitro or in vivo to produce a library of recombinant antibodies that can be screened for novel or improved function(s) over the lead antibody.
Owner:ABMAXIS
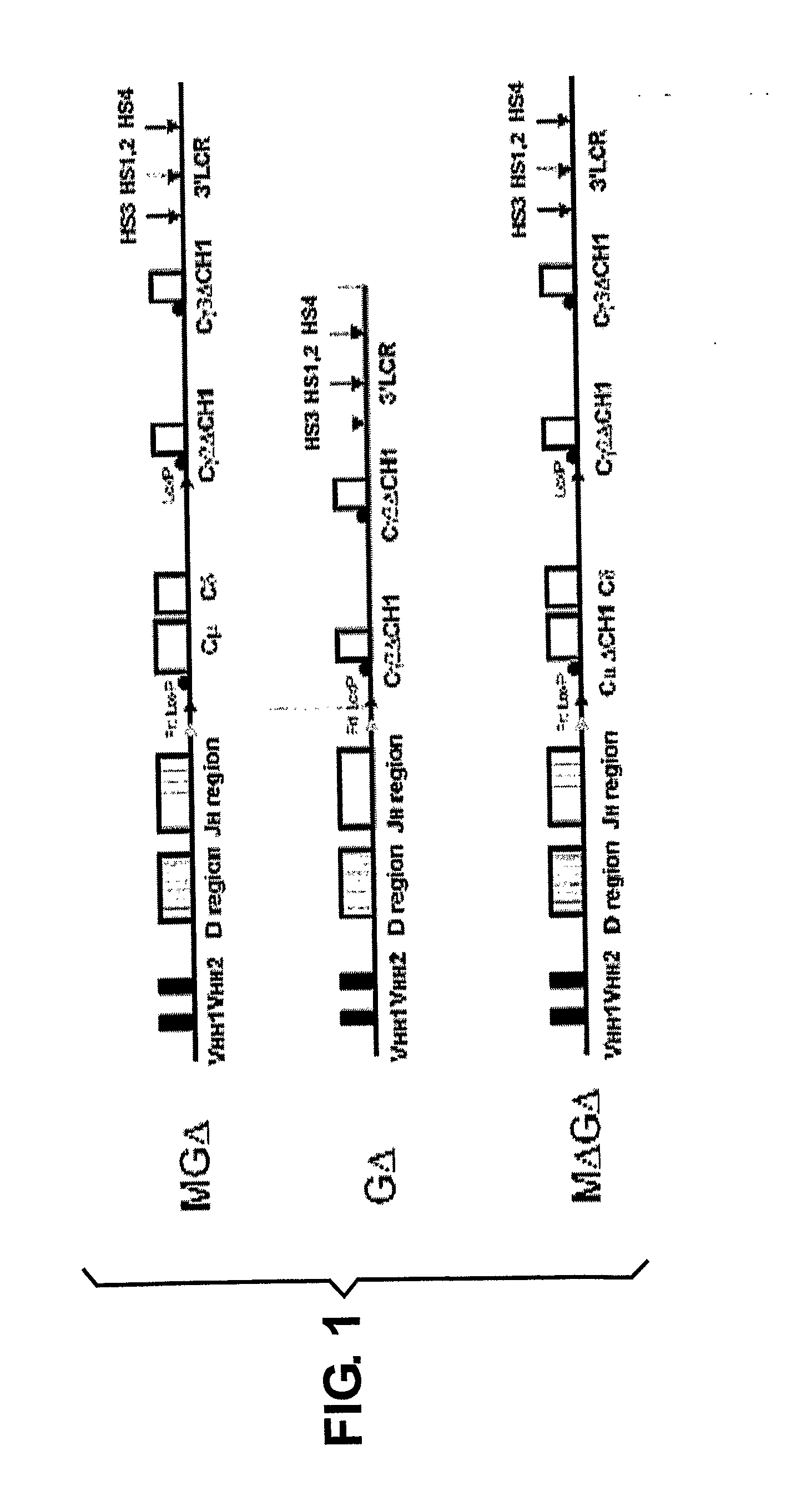
Generation of heavy-chain only antibodies in transgenic animals
InactiveUS20090307787A1Maximizing numberIncrease diversityHybrid cell preparationImmunoglobulinsHeavy chainMammal
The present invention relates to a method for the generation of VH heavy chain-only antibodies in a transgenic non-human mammal. In particular, the present invention relates to a method for the production of a VH heavy chain-only antibody in a transgenic non-human mammal comprising the step of expressing more than one heterologous VH heavy chain locus in that mammal.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
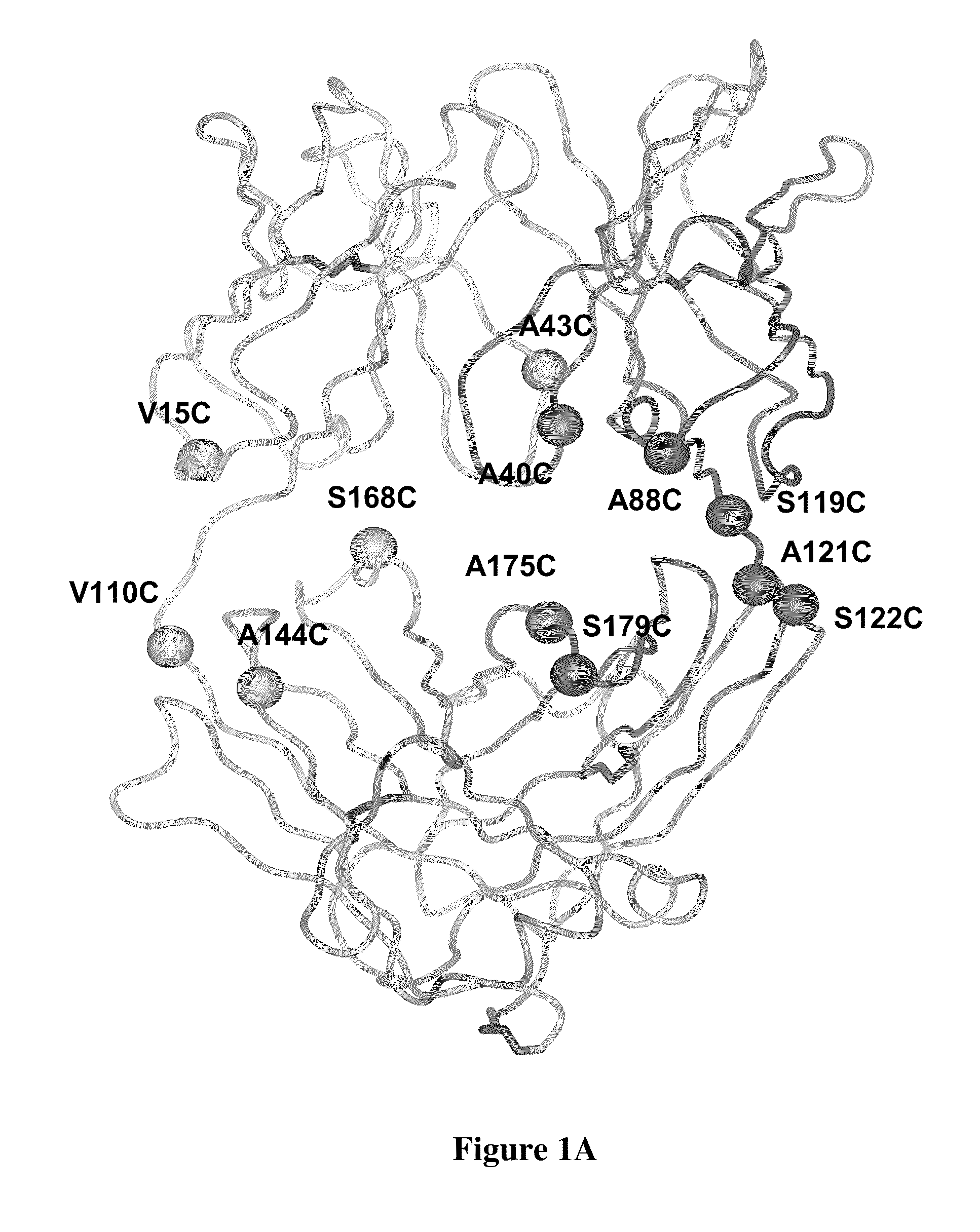
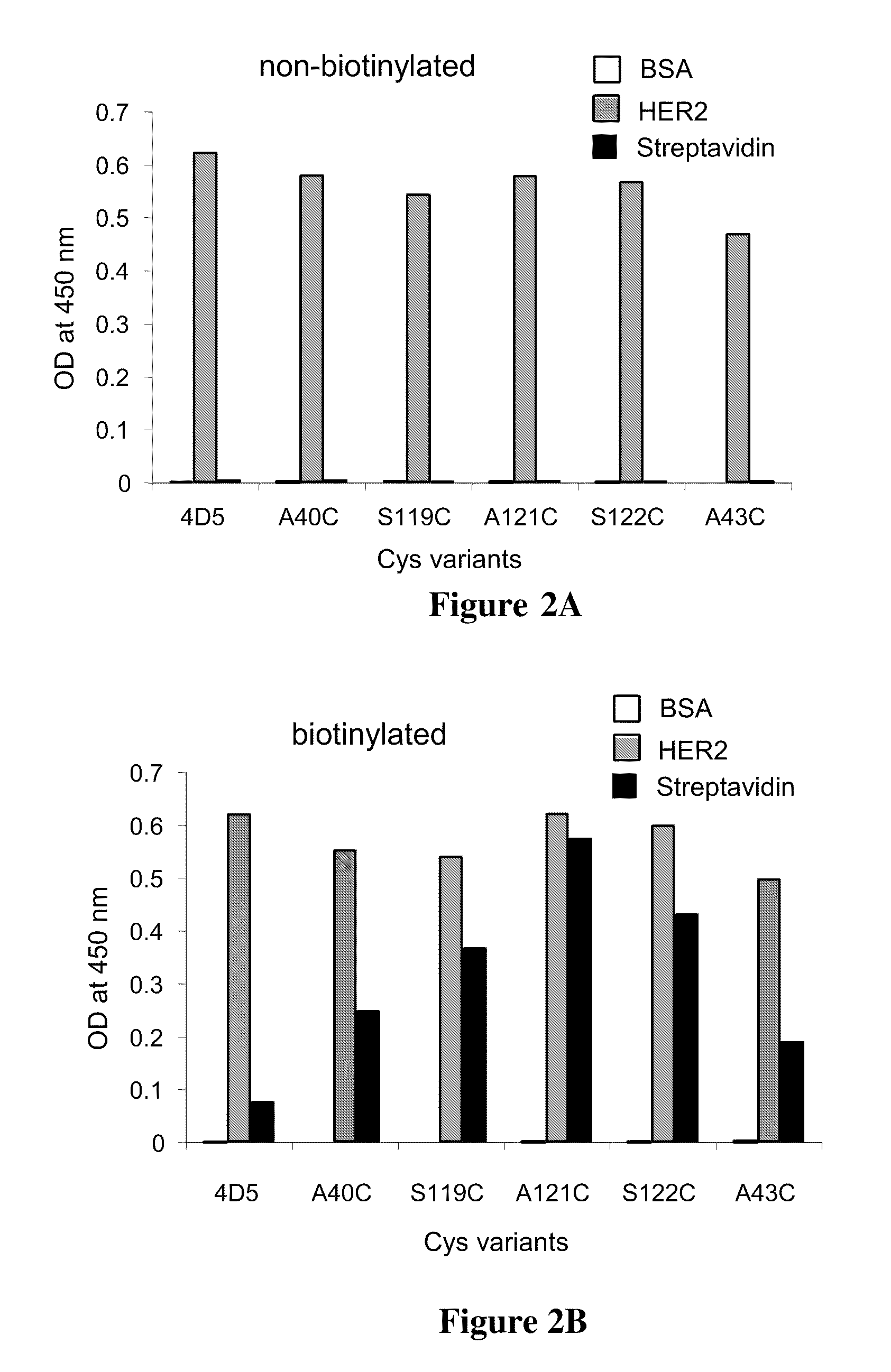
Cysteine engineered antibodies and conjugates
Cysteine engineered antibodies comprising a free cysteine amino acid in the heavy chain or light chain are prepared by mutagenizing a nucleic acid sequence of a parent antibody and replacing one or more amino acid residues by cysteine to encode the cysteine engineered antibody; expressing the cysteine engineered antibody; and isolating the cysteine engineered antibody. Certain highly reactive cysteine engineered antibodies were identified by the PHESELECTOR assay. Isolated cysteine engineered antibodies may be covalently attached to a capture label, a detection label, a drug moiety, or a solid support.
Owner:GENENTECH INC
Expression of functional antibody fragments
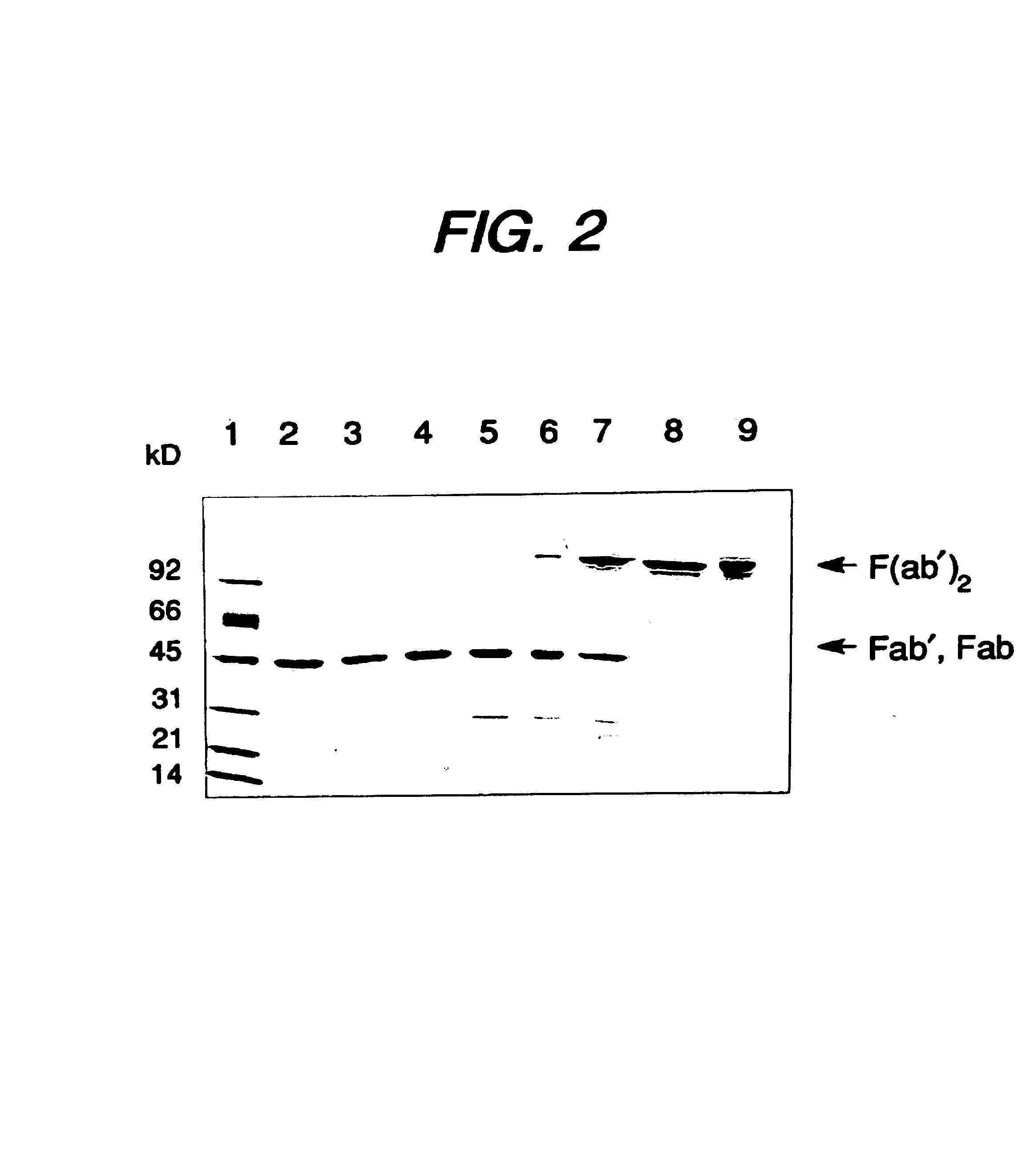
InactiveUS7018809B1Easy to prepareFacilitated releaseHybrid immunoglobulinsSerum immunoglobulinsMicroorganismHeavy chain
Methods for the high yield production of antibody Fv-containing polypeptides, especially Fab′ and F(ab′)2 antibody fragments are provided. Expression of heavy and light chain Fv in a microbial secretory system is followed by recovery of Fv from the periplasm under conditions that maintain a cysteine residue as a free thiol. The free thiol is reacted with free thiol of an antibody fragment of the same or differing specificity, or with agents such as diagnostic labels or therapeutic moieties. The products offer advantages of homogeneity and purity not available through the use of known methods for preparing such derivatives.
Owner:GENENTECH INC
Expression of heterologous multi-domain proteins in yeast
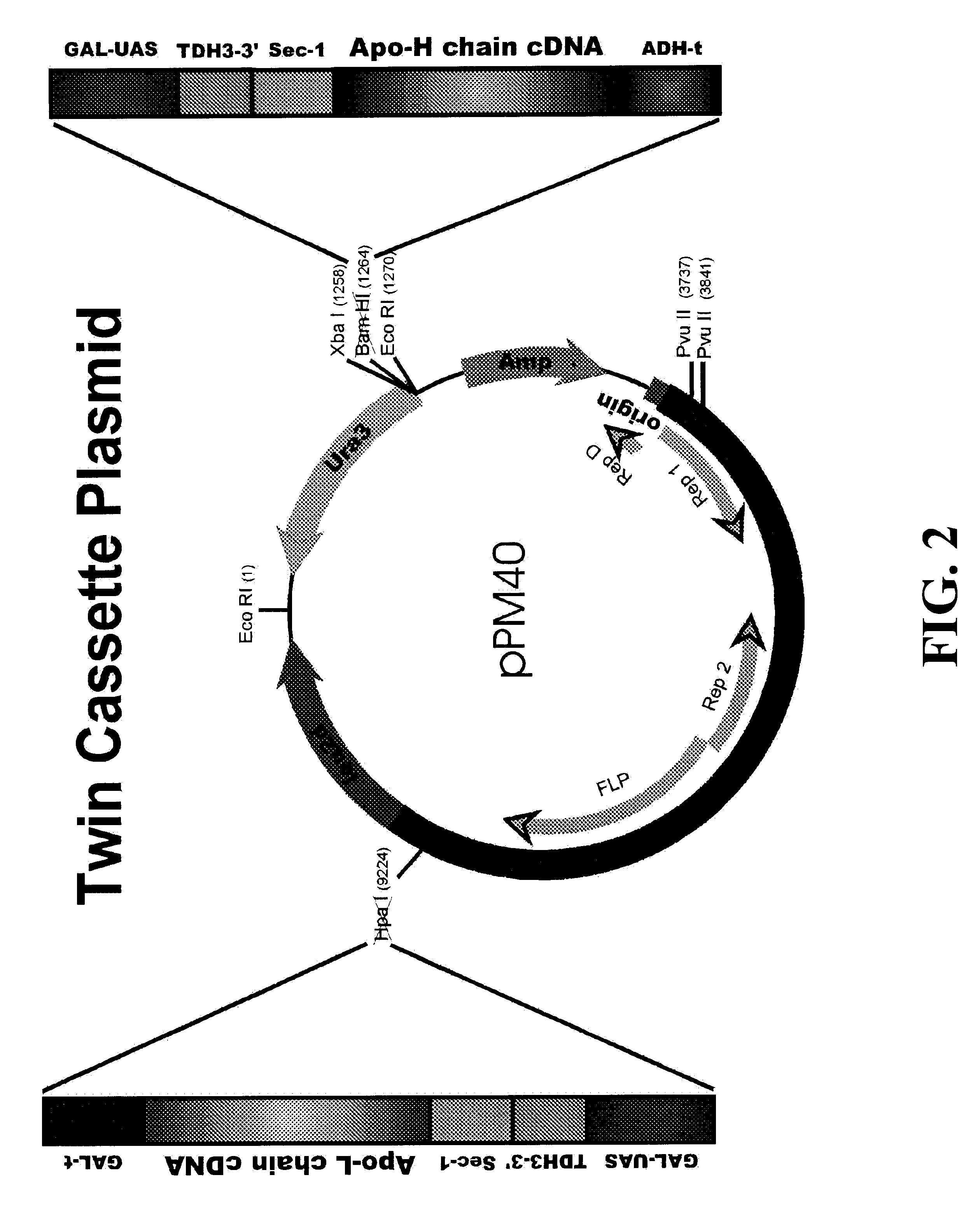
InactiveUS6358733B1Increase productionCost effective productionSugar derivativesAntibody mimetics/scaffoldsYeastSingle-Chain Antibodies
This invention demonstrates the utility of a yeast expression system for the expression of functional heterologous multi-domain proteins in yeast. The yeast expression system allows for the inclusion of a plurality of (up to three) modular expression cassettes which may encode multiple polypeptide chains of a heterologous multi-domain protein on a single plasmid (Twin Cassette). Because multiple polypeptide chains may be encoded for by the expression cassettes of the present invention in a single vector, the system can produce equivalent amounts of the multiple polypeptide chains, thereby enhancing the yield of a functional heterologous multi-domain protein. For example, functional monoclonal antibodies (MAbs) comprising a heavy chain and a light chain of an immunoglobulin (IgG), and functional immunotoxins comprising an antibody domain and an oxidase toxin may be produced using the Yeast expression system of the present invention. In addition, functional single chain antibodies, antibody fragments and chimeric antibodies may also be produced.
Owner:APOLIFE
Murine monoclonal anti-idiotype antibody 11D10 and methods of use thereof
Owner:UNIVERSITY OF KENTUCKY
Anti-FcRn antibodies for treatment of auto/allo immune conditions
Antibodies to heavy chain of human FcRn are provided which function as non-competitive inhibitors of IgG binding to FcRn. The antibodies may be polyclonal, monoclonal, chimeric or humanized, or antigen binding fragments thereof. These antibodies are useful for reducing the concentration of pathogenic IgGs in individuals and therefore used as a therapeutic tool in autoimmune and alloimmune conditions.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
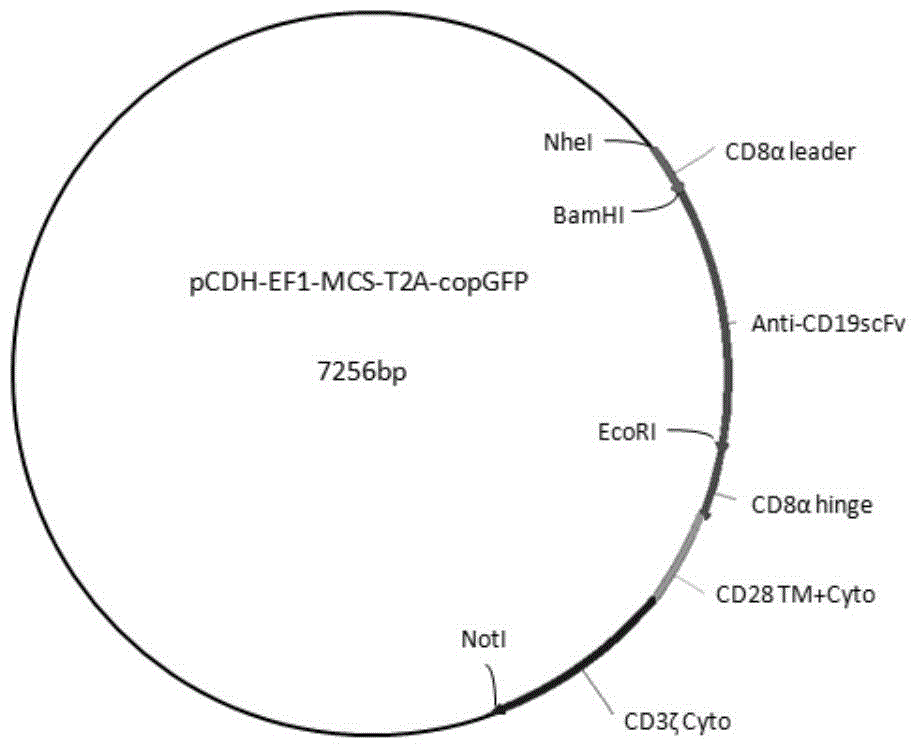
Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
ActiveCN104788573AConfirmed specific killing effectMammal material medical ingredientsHybrid peptidesAntigen receptorHeavy chain
The invention discloses a chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof. The chimeric antigen receptor is serially connected by an anti-human CD19 monoclonal antibody H119a light chain and heavy chain variable region (hCD19scFv), human CD8a hinge region, a human CD28 transmembrane region, an intracellular region and a human CD3zata intracellular region. The chimeric antigen receptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cell) can be used for treating the tumor with positive surface CD19.
Owner:JUVENTAS CELL THERAPY LTD
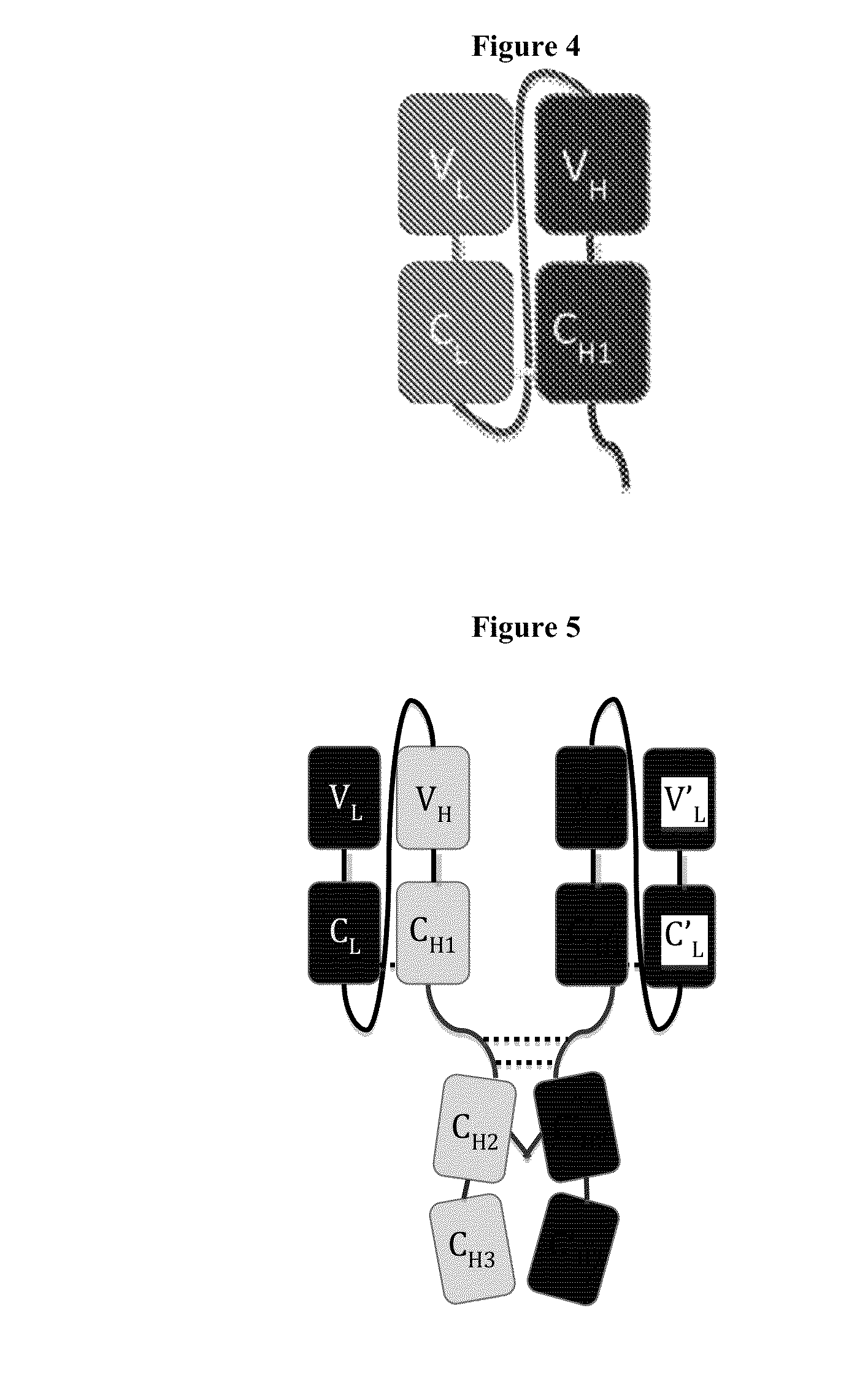
Immunoglobulin Constructs Comprising Selective Pairing of the Light and Heavy Chains
InactiveUS20140072581A1Promote formationImmunoglobulins against blood coagulation factorsSenses disorderHeavy chainSingle-Chain Fv
Disclosed herein is an isolated immunoglobulin construct comprising a first monomeric polypeptide comprising a first single chain Fv polypeptide connected to a first constant domain polypeptide; and a second monomeric polypeptide comprising a second single chain Fv polypeptide, connected to a second constant domain polypeptide; each said constant domain polypeptide comprising at least one each of a CL domain, a CH1 domain, a CH2 domain and a CH3 domain or fragments, variants or derivatives thereof; and wherein said first and second constant domain polypeptide form a Fc region.
Owner:ZYMEWORKS INC
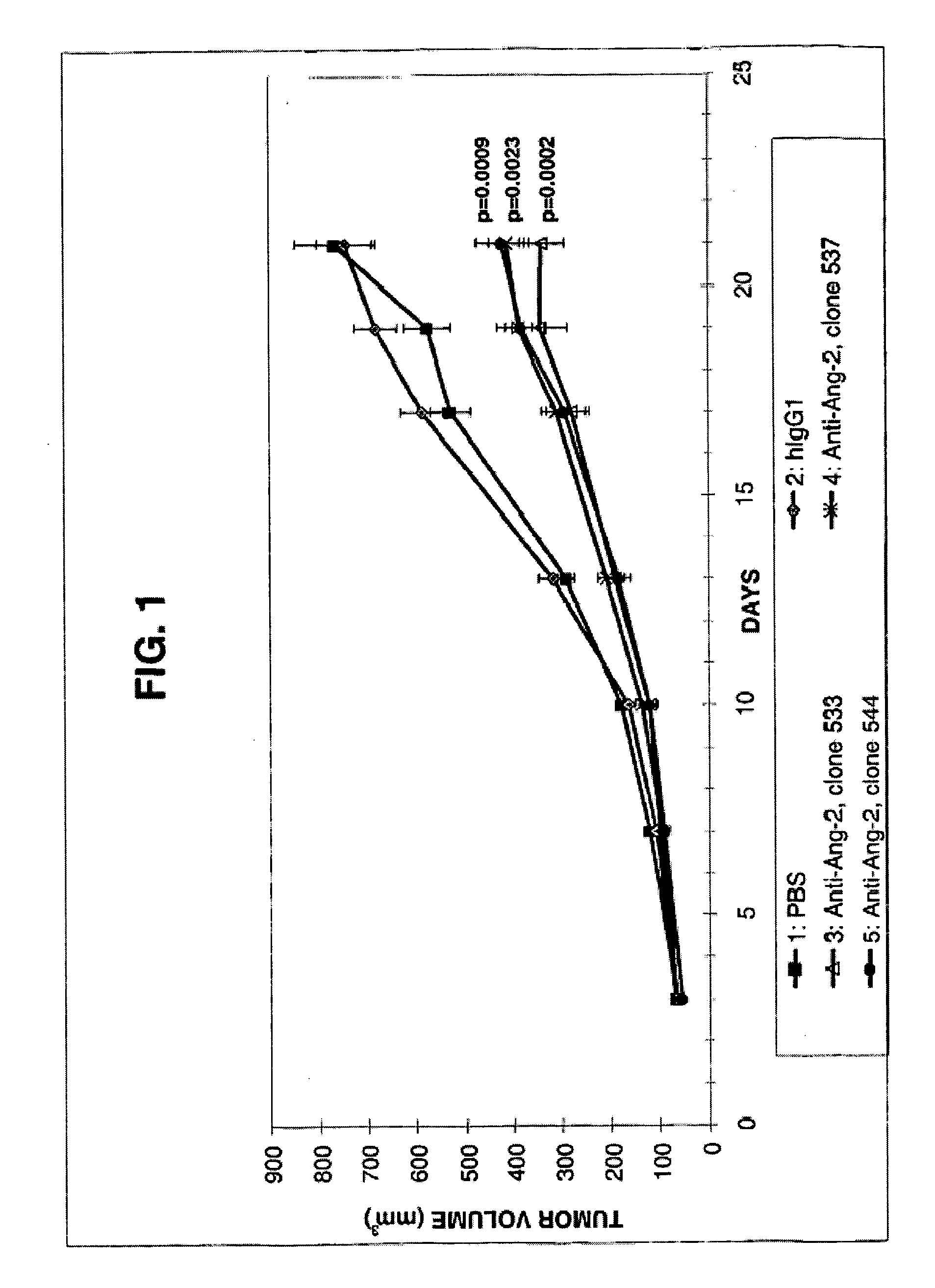
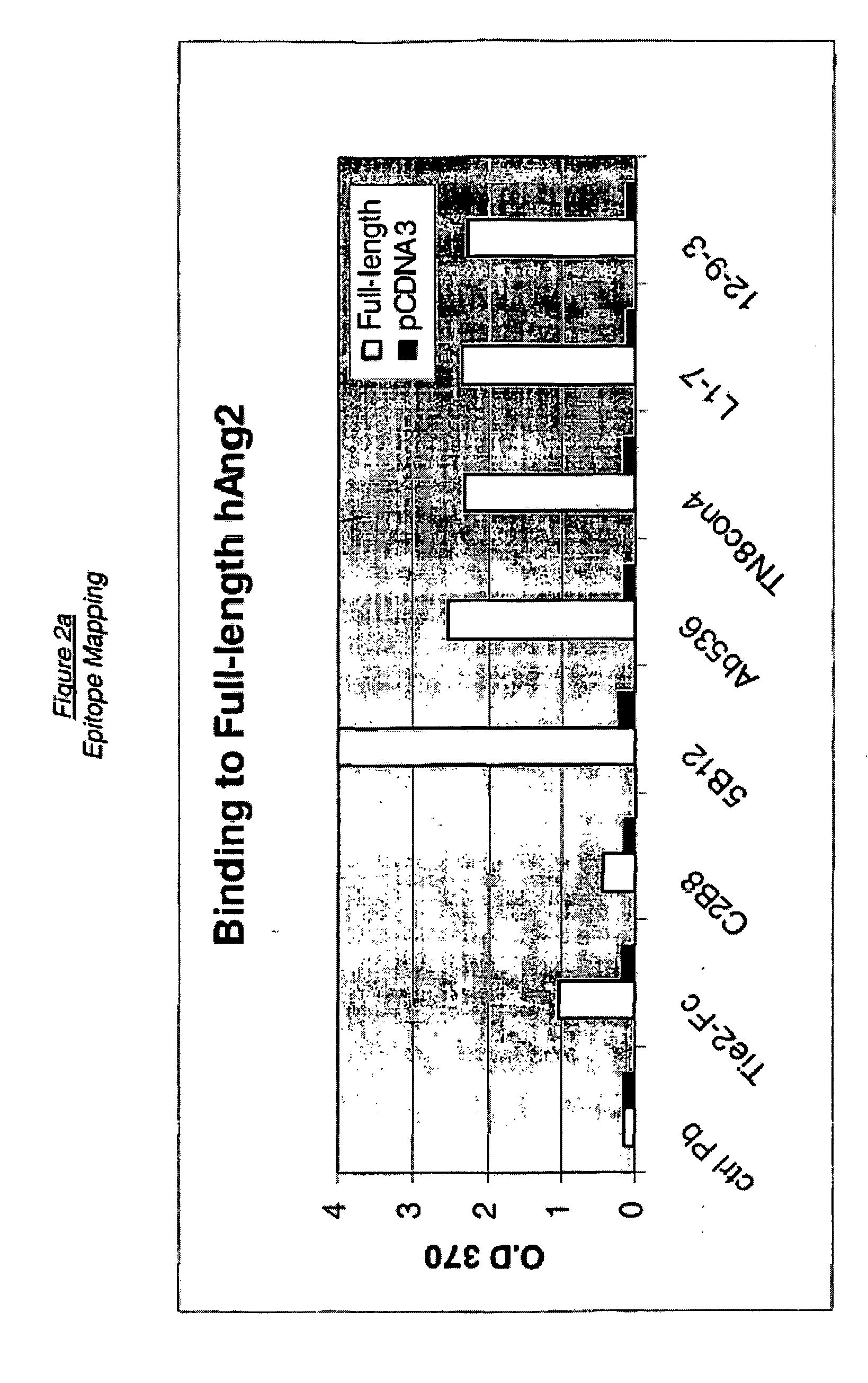
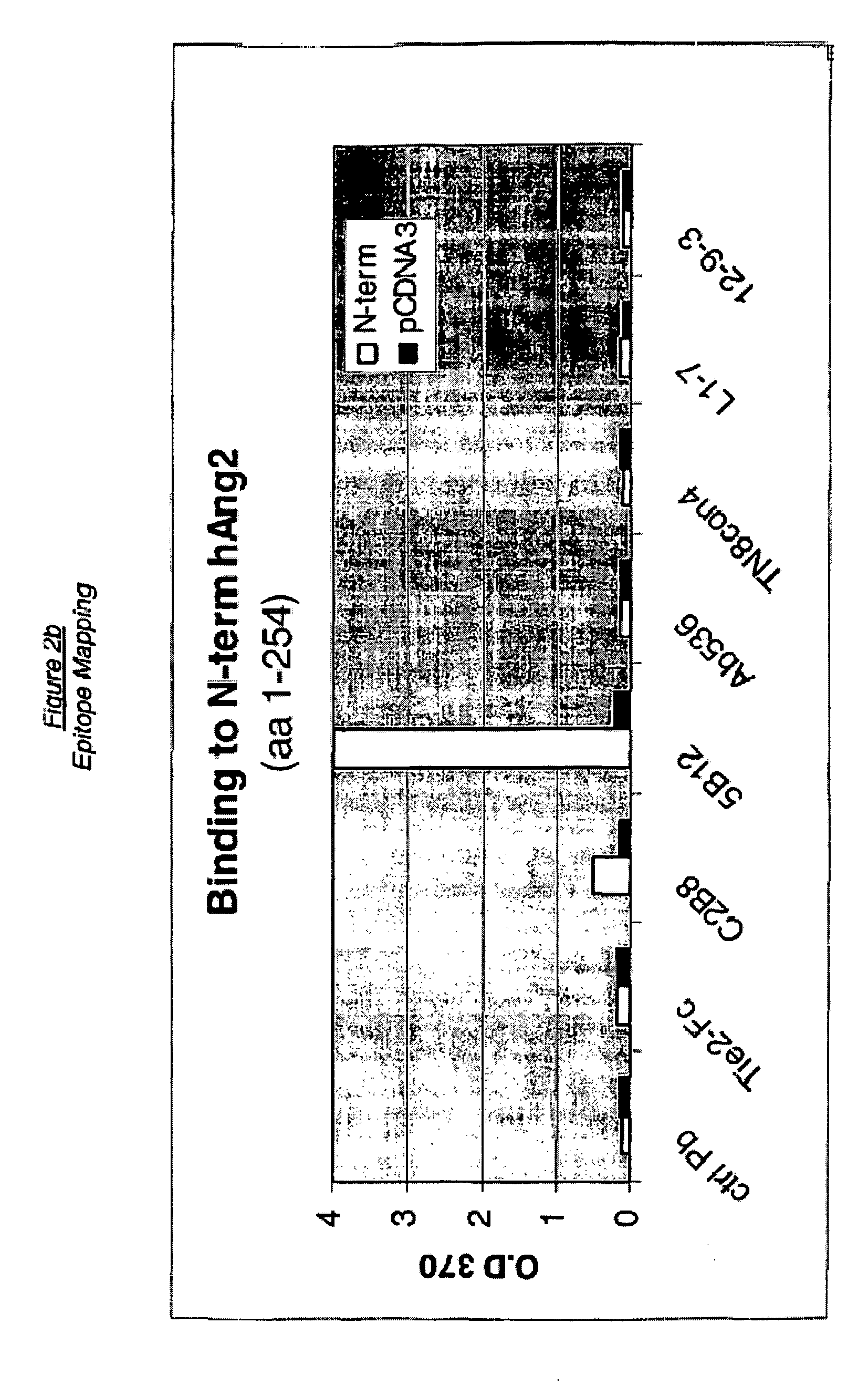
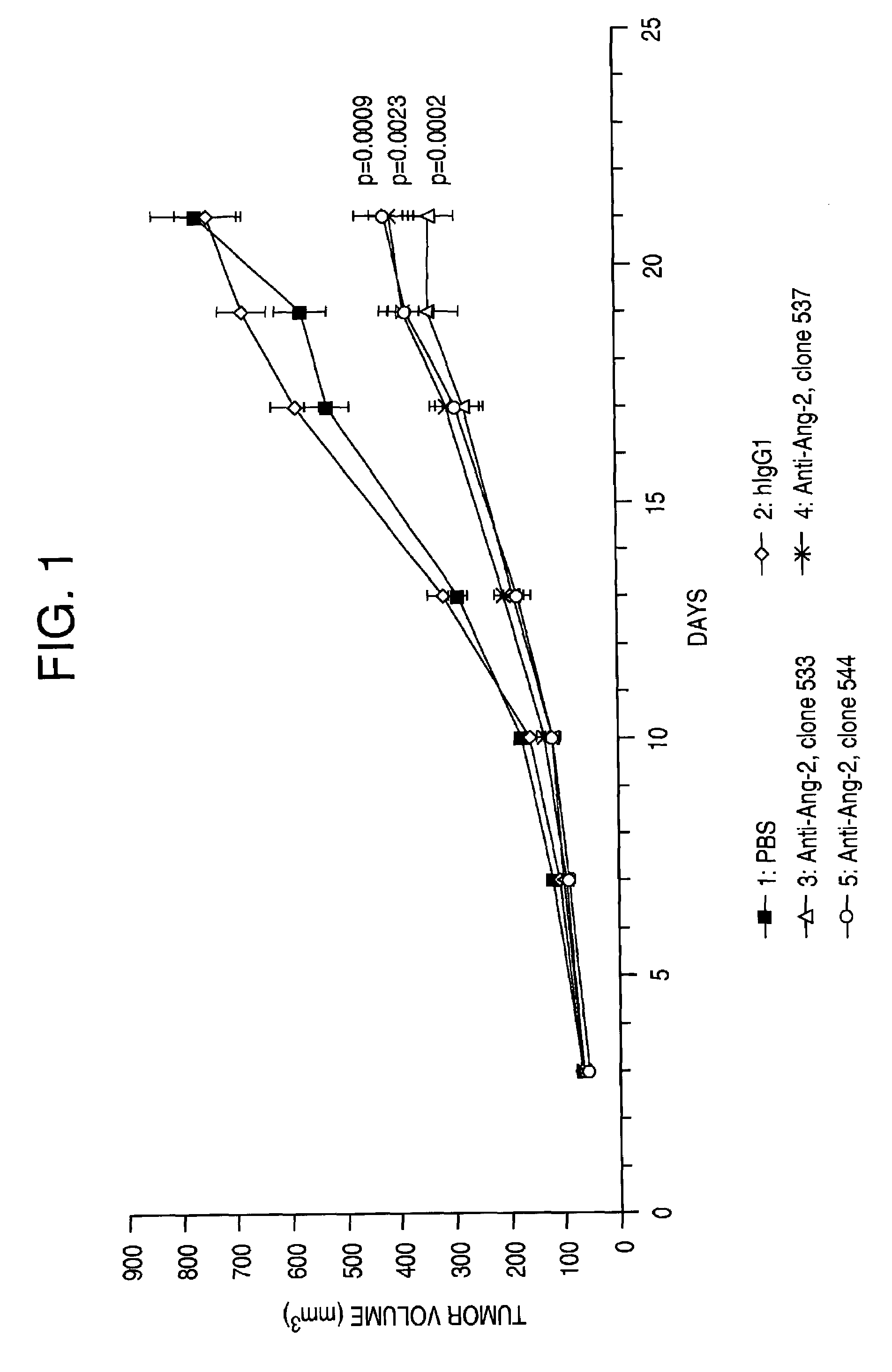
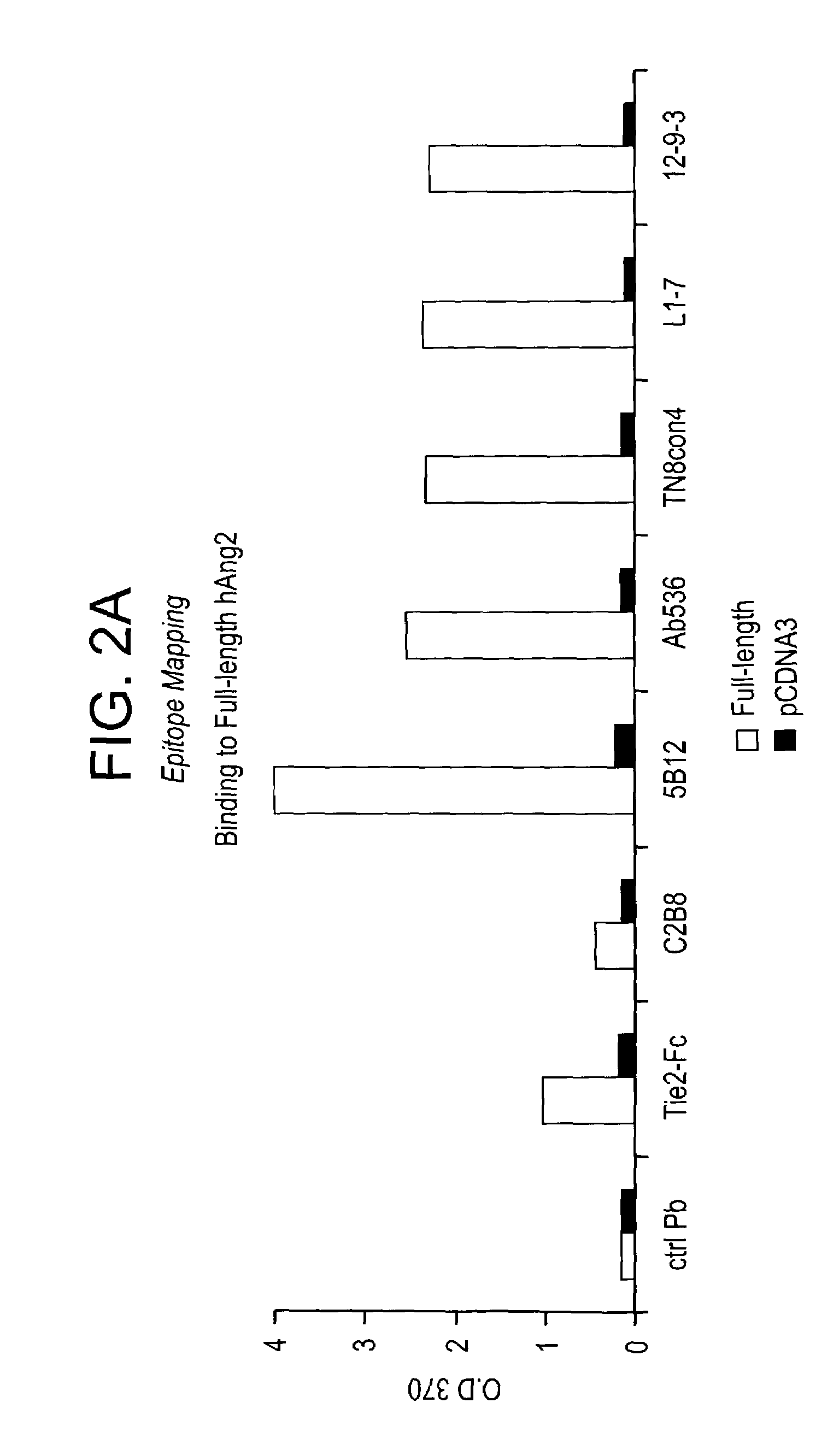
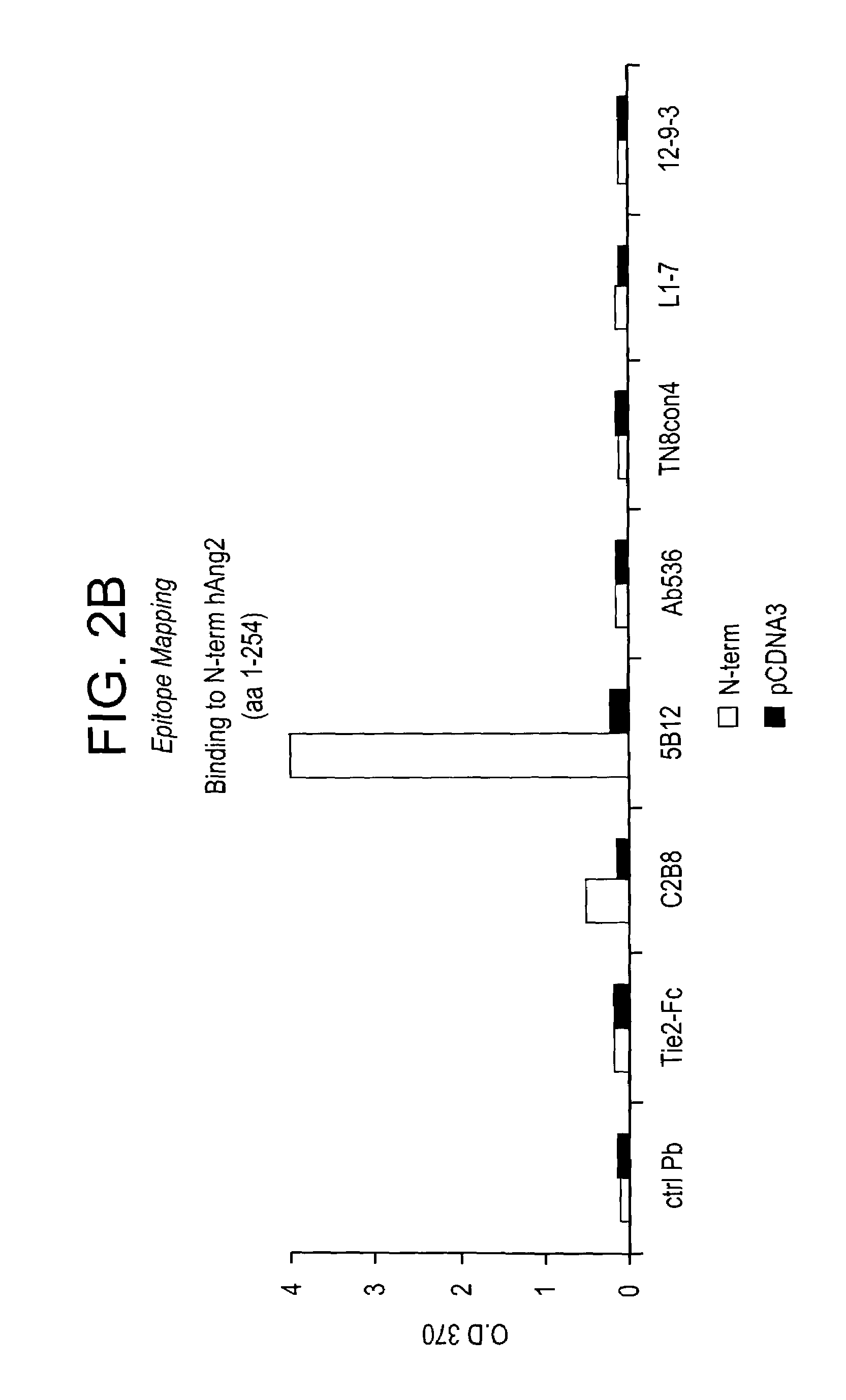
Angiopoietin-2 specific binding agents
Disclosed are specific binding agents, such as fully human antibodies, that bind to angiopoietin-2. Also disclosed are heavy chain fragments, light chain fragments, and CDRs of the antibodies, as well as methods of making and using the antibodies.
Owner:AMGEN INC
Angiopoietin-2 specific binding agents
Disclosed are specific binding agents, such as fully human antibodies, that bind to angiopoietin-2. Also disclosed are heavy chain fragments, light chain fragments, and CDRs of the antibodies, as well as methods of making and using the antibodies.
Owner:AMGEN INC
Chimeric antibodies
InactiveUS6020153ALow percentage and absence of bindingAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainIn vivo
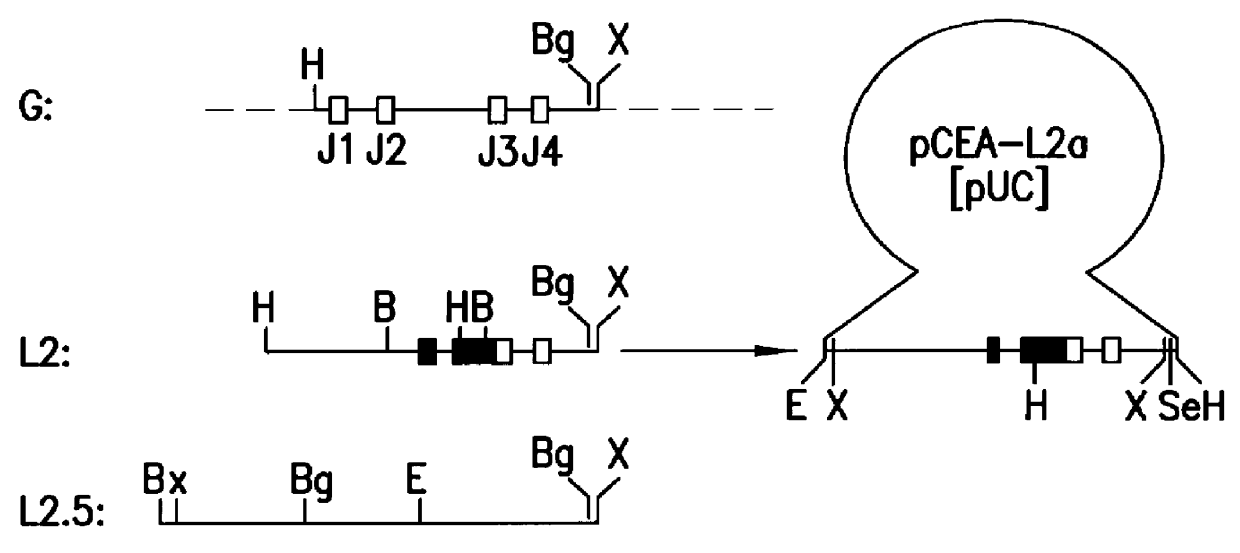
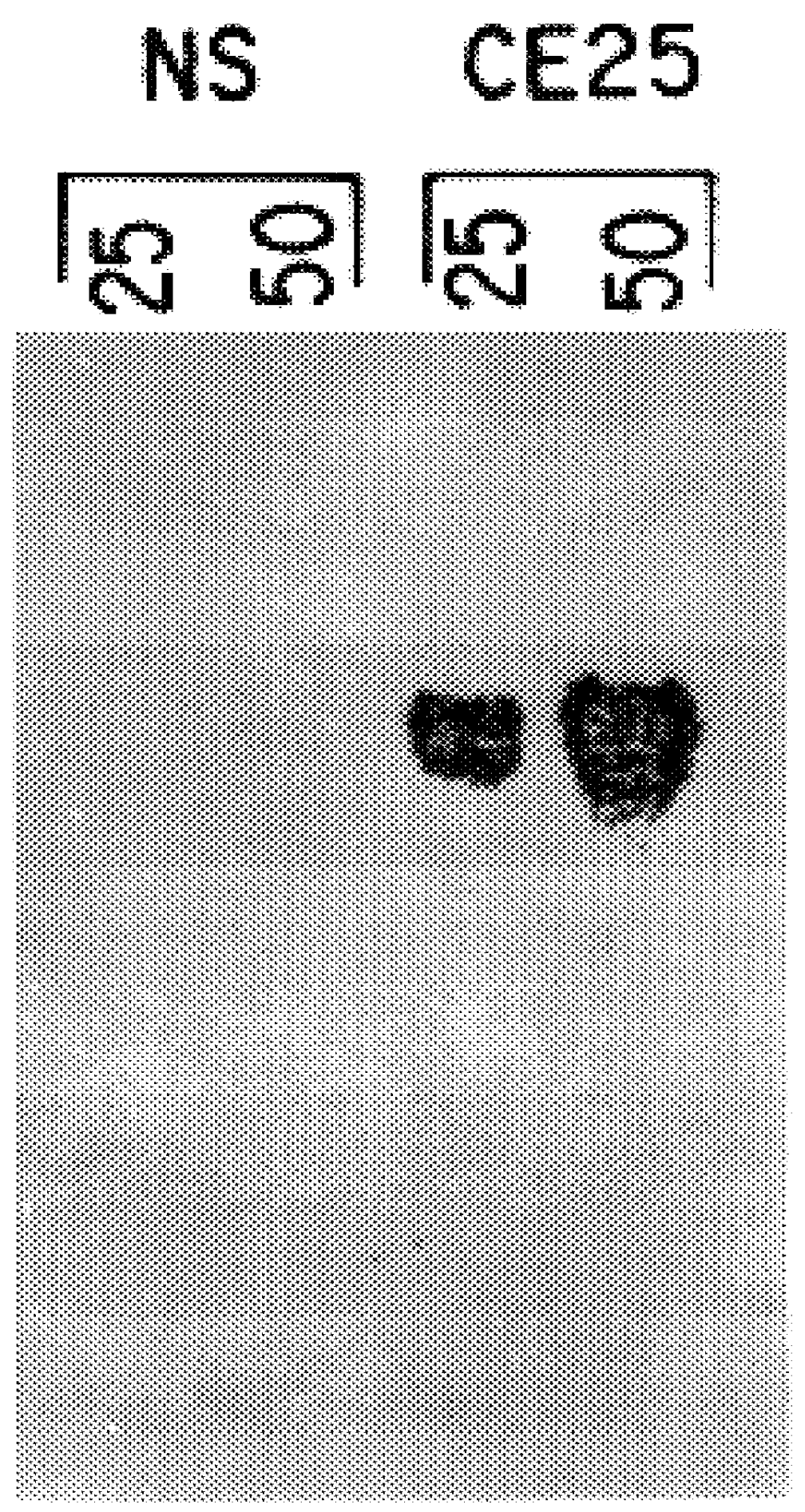
The invention relates to murine / human chimeric monoclonal antibodies with high specificity to and affinity for human carcinoembryonic antigen (CEA), derivatives thereof, processes for the preparation of these antibodies and their derivatives, DNAs coding for heavy and light chains of these antibodies, processes for the preparation of said DNAs, mammalian cell lines that produce and secrete the antibodies and processes for the preparation of said cell lines. The chimeric antibodies and their derivatives are used for clinical purposes in vitro and in vivo, especially for the diagnosis of cancer, for localization and in vivo imaging of tumors, for therapy, e.g. site-directed delivery of cytotoxins, and similar purposes. The invention also concerns test kits and pharmaceutical compositions containing said chimeric monoclonal antibodies and / or derivatives thereof.
Owner:CIBA GEIGY CORP
Novel heterodimeric proteins
ActiveUS20140322217A1Immunoglobulins against cell receptors/antigens/surface-determinantsFermentationAntigenHeavy chain
The invention provides heterodimeric antibodies comprising a first heavy chain comprising a first Fc domain and a single chain Fv region that binds a first antigen. The heterodimeric antibodies also comprise a second heavy chain comprising a second Fc domain, a first variable heavy chain and a first variable light chain, wherein the first and second Fc domains are different.
Owner:XENCOR INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com