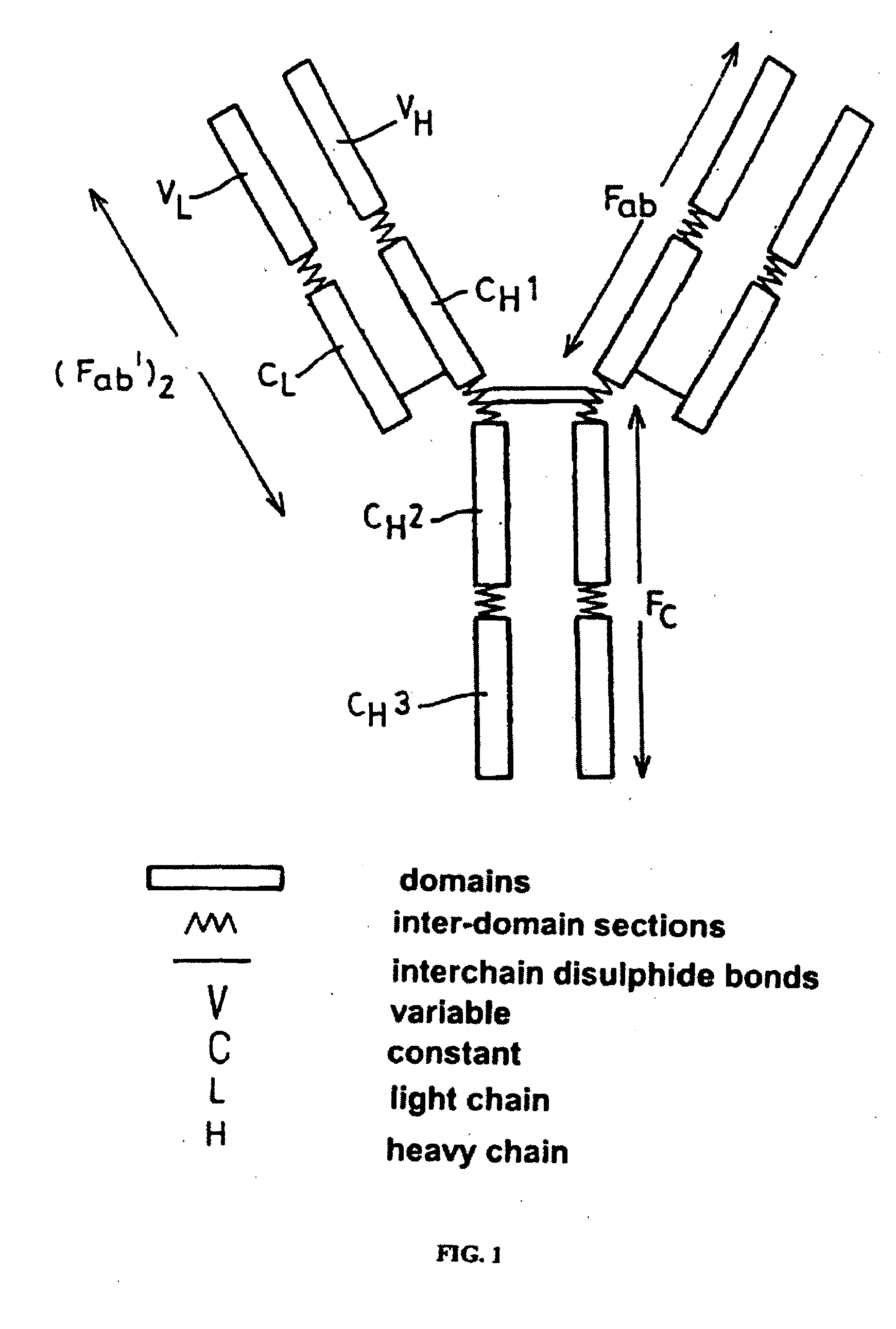
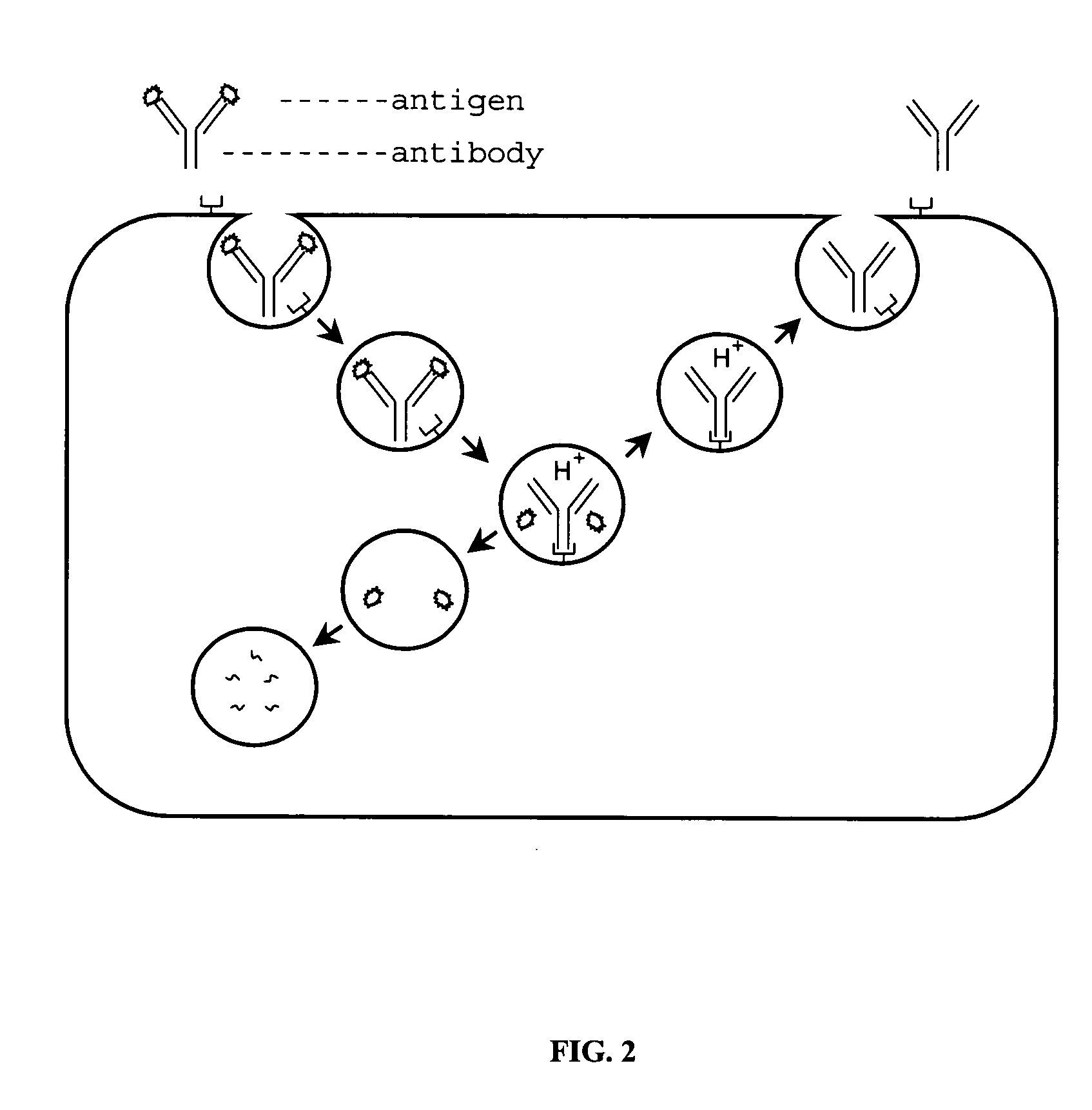
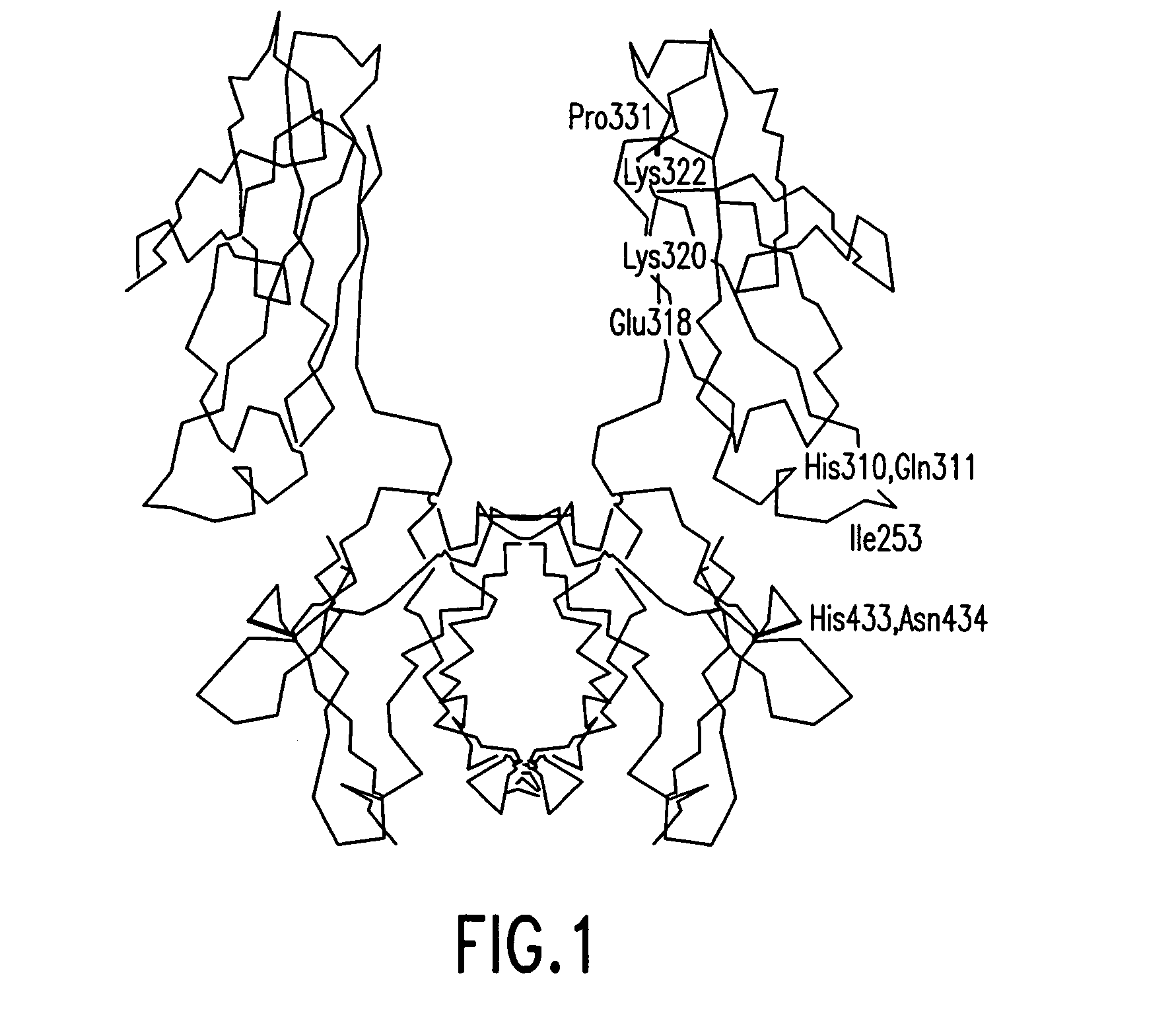
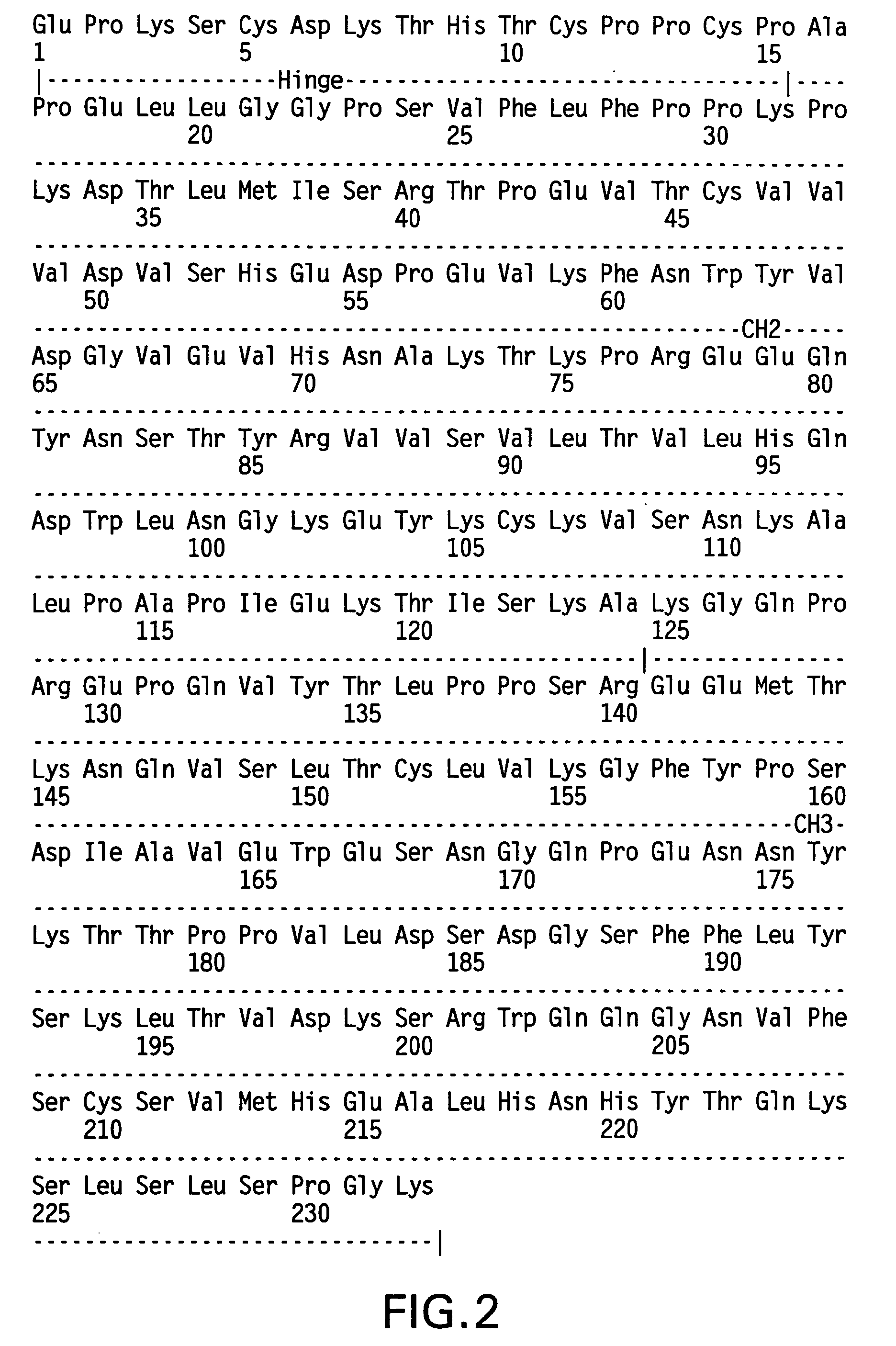
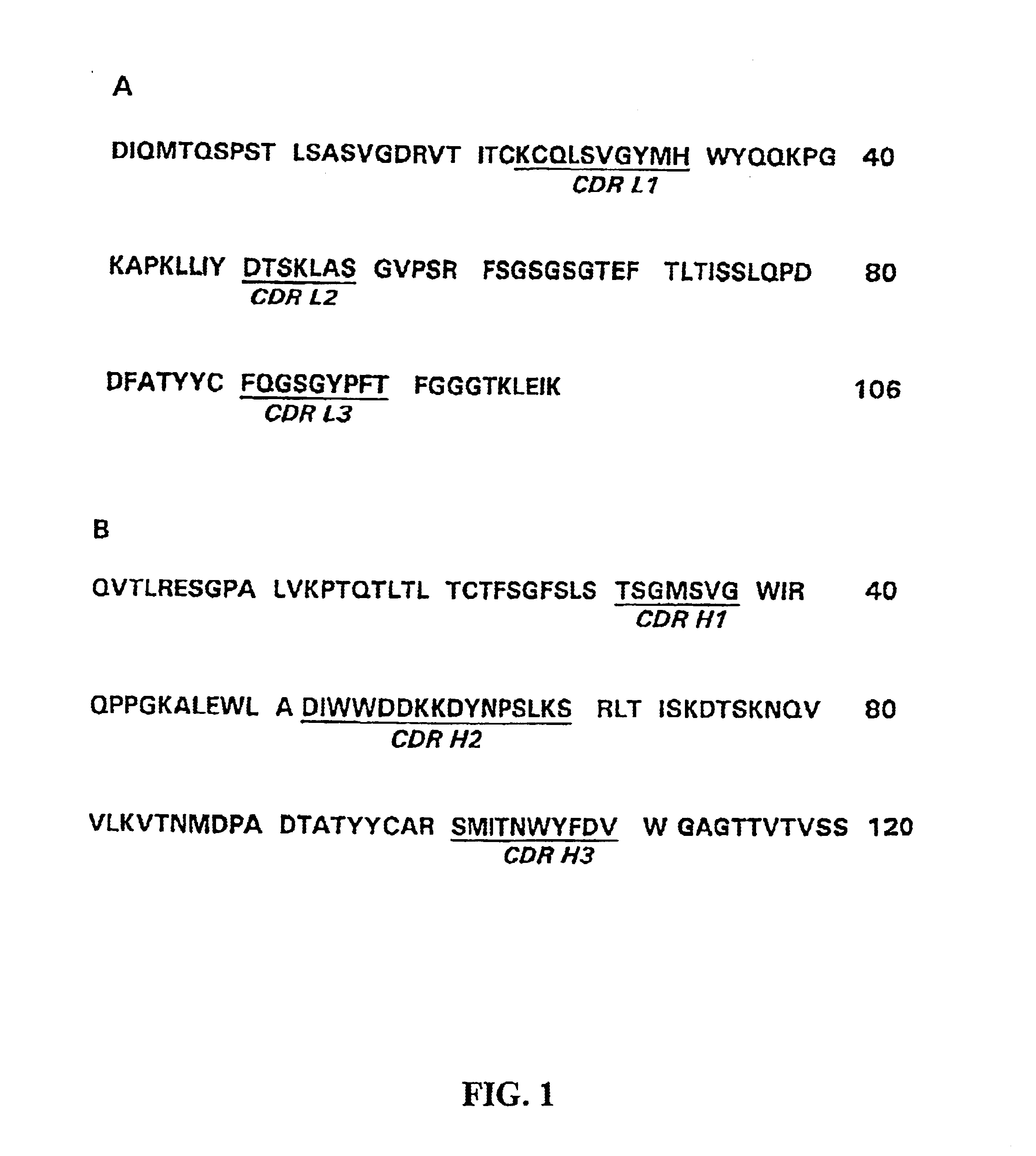
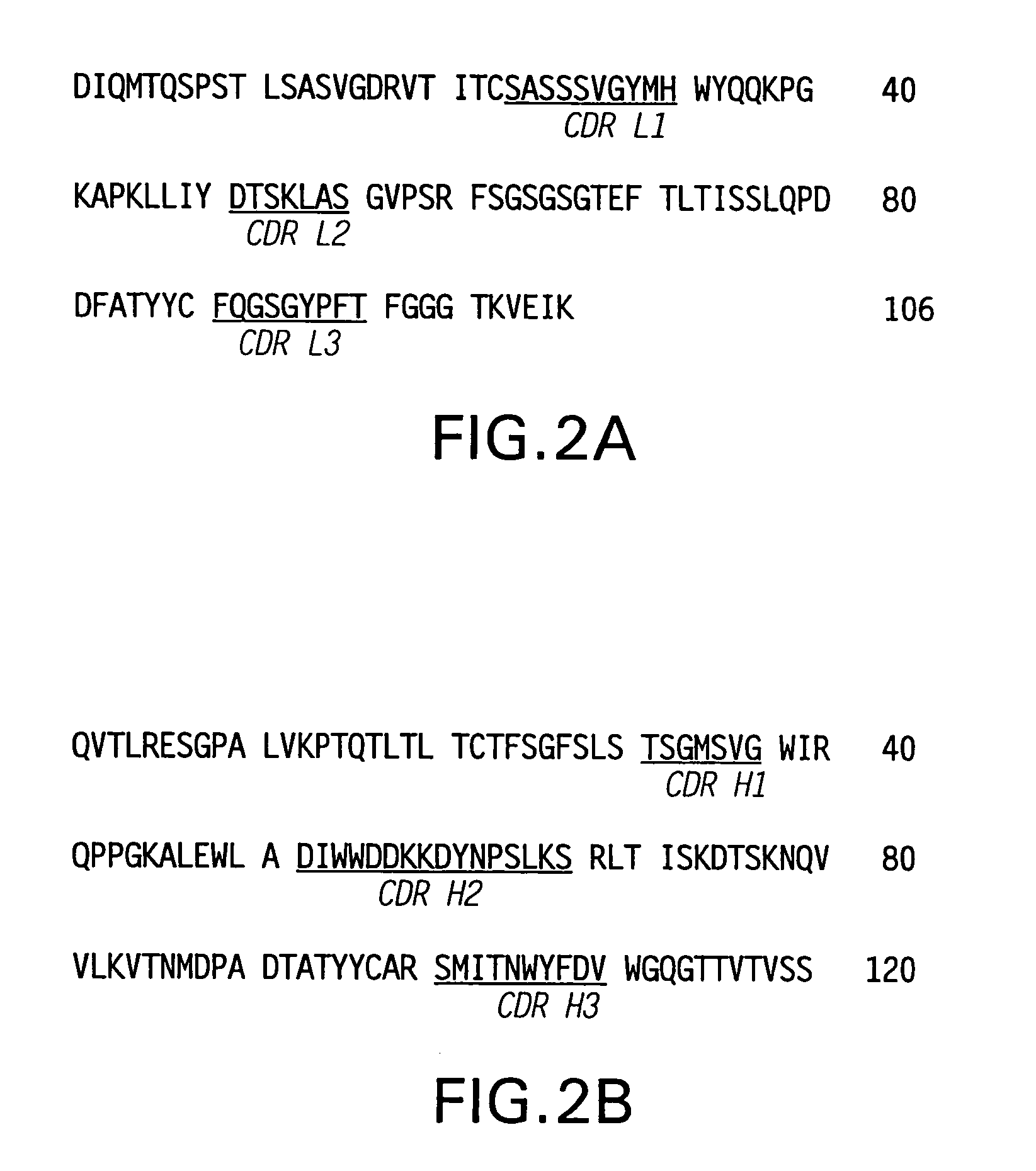
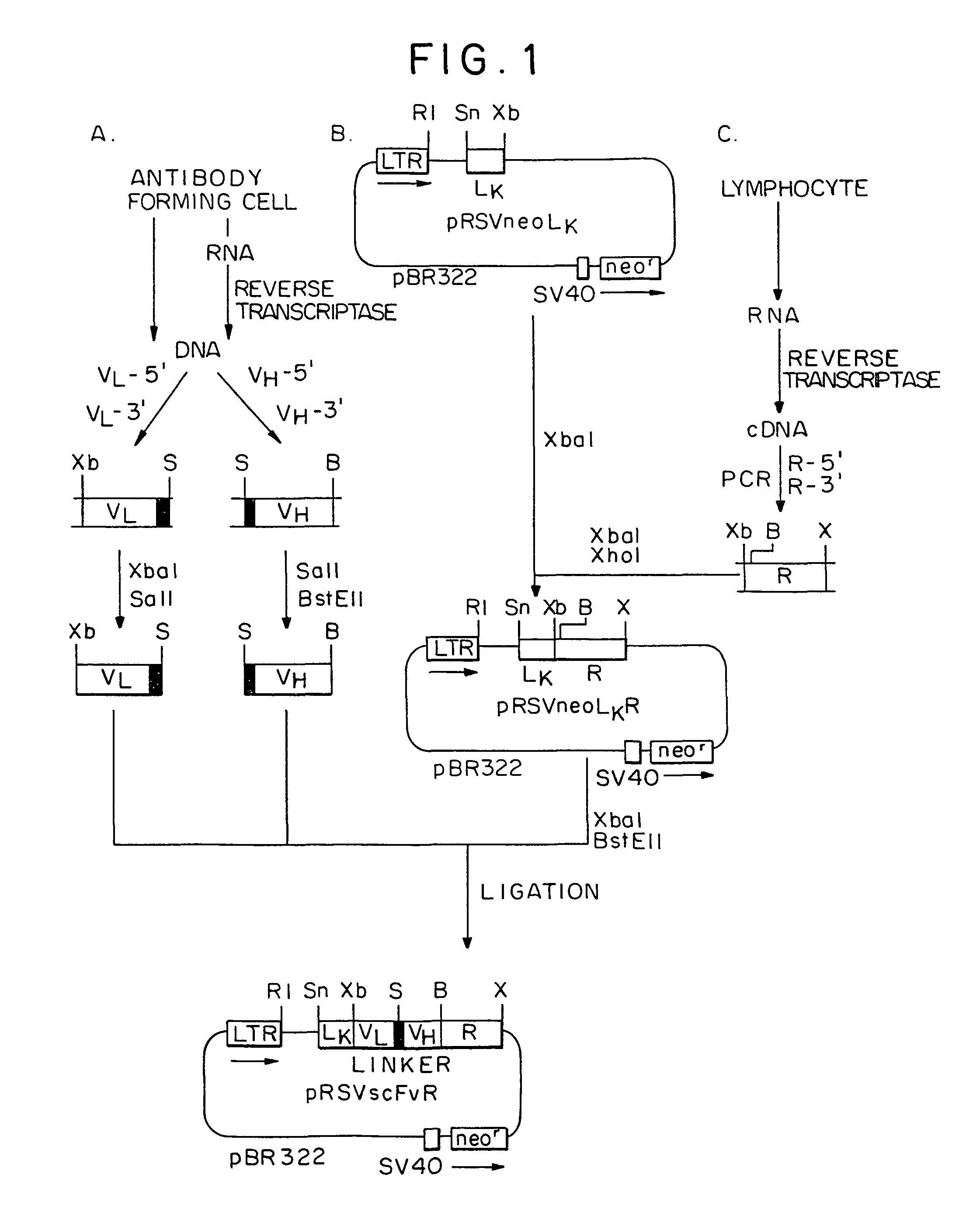
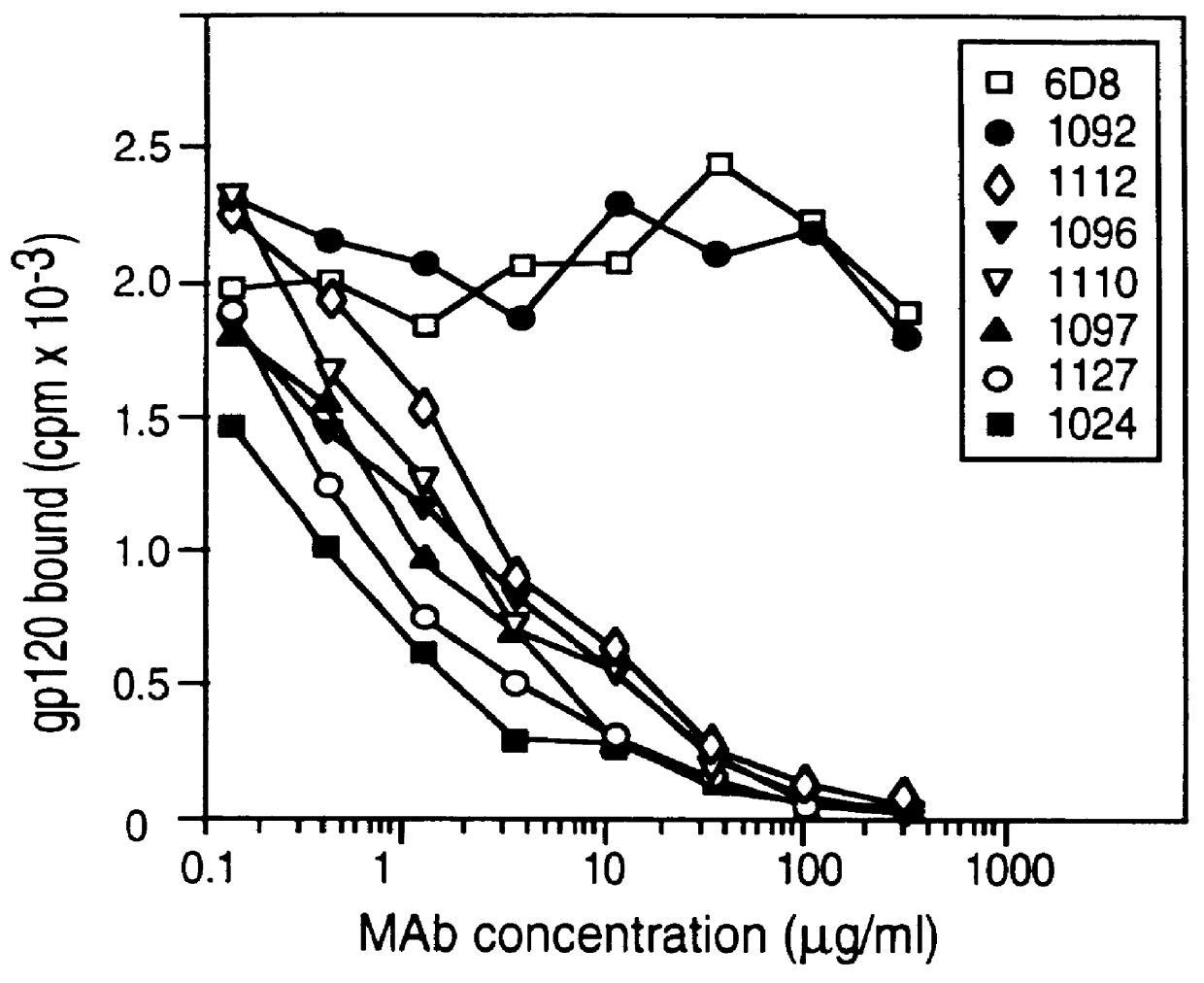
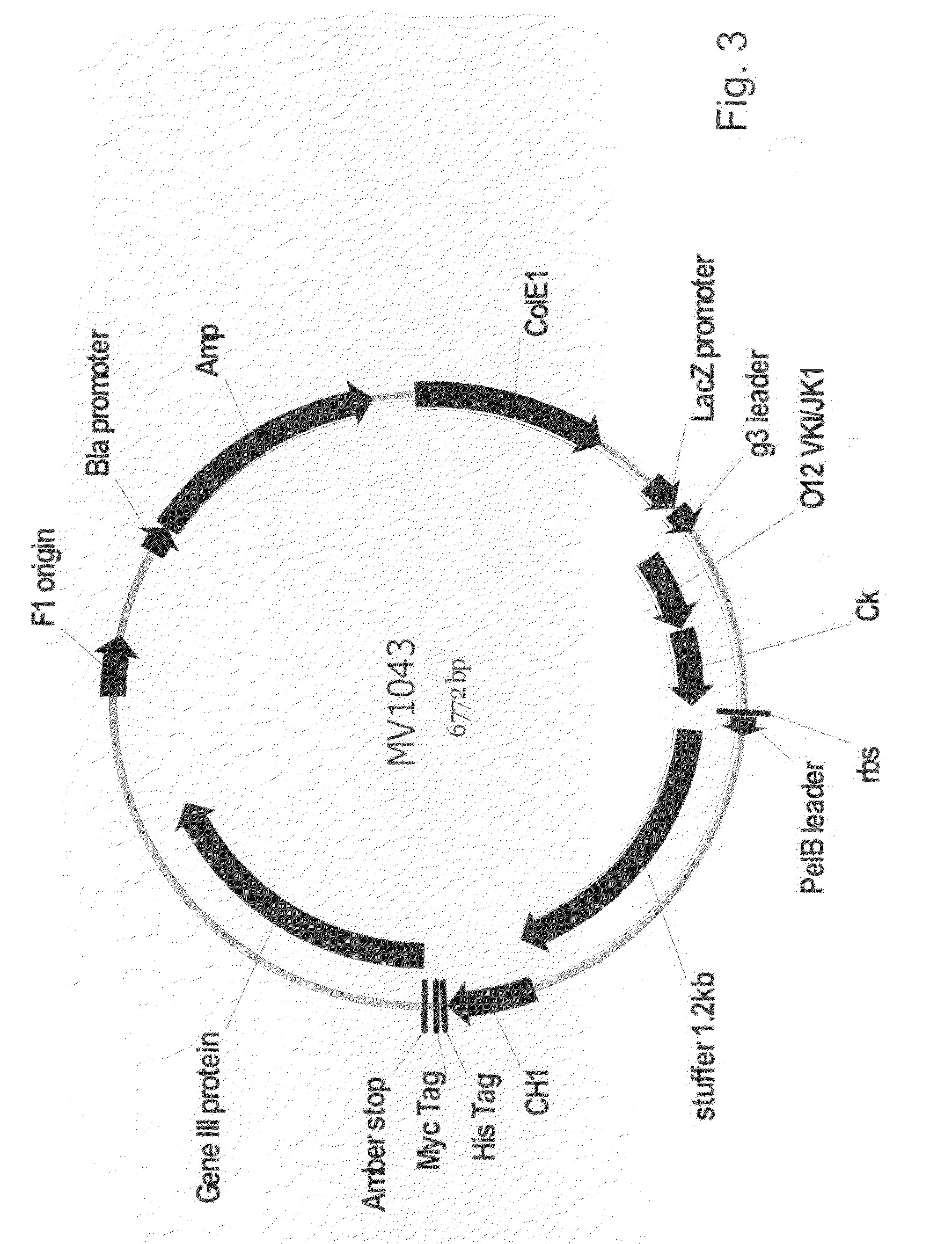
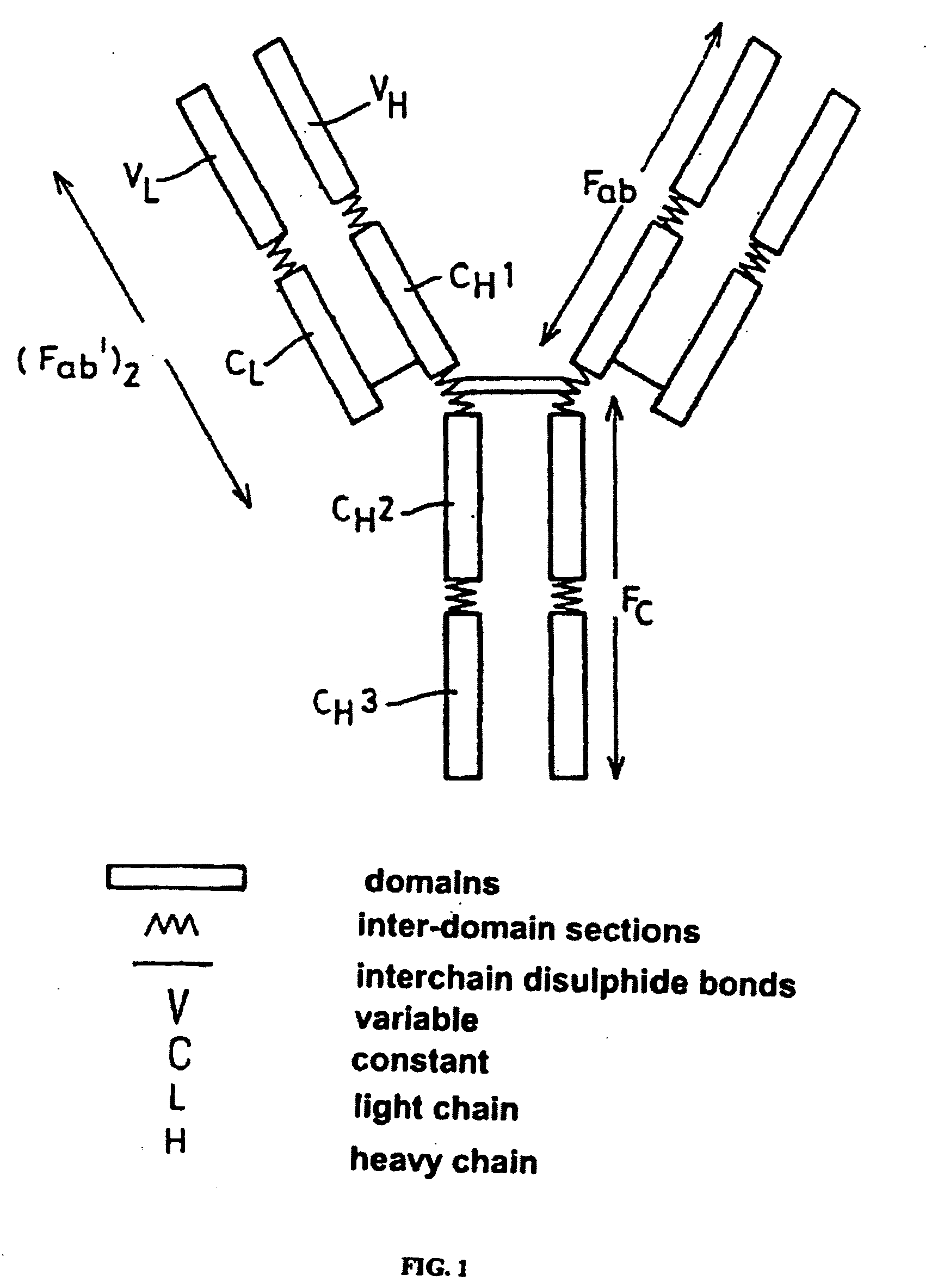
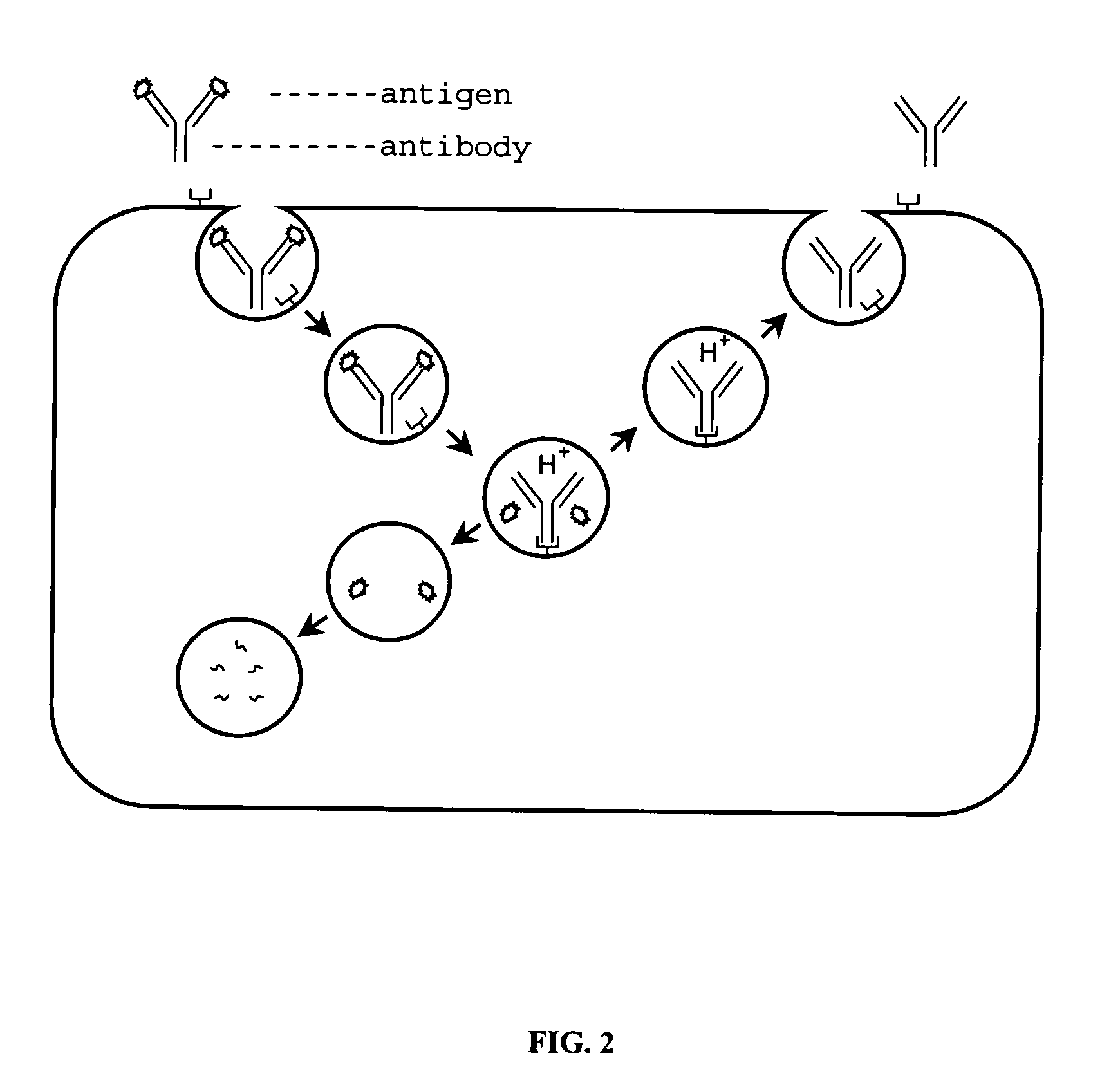
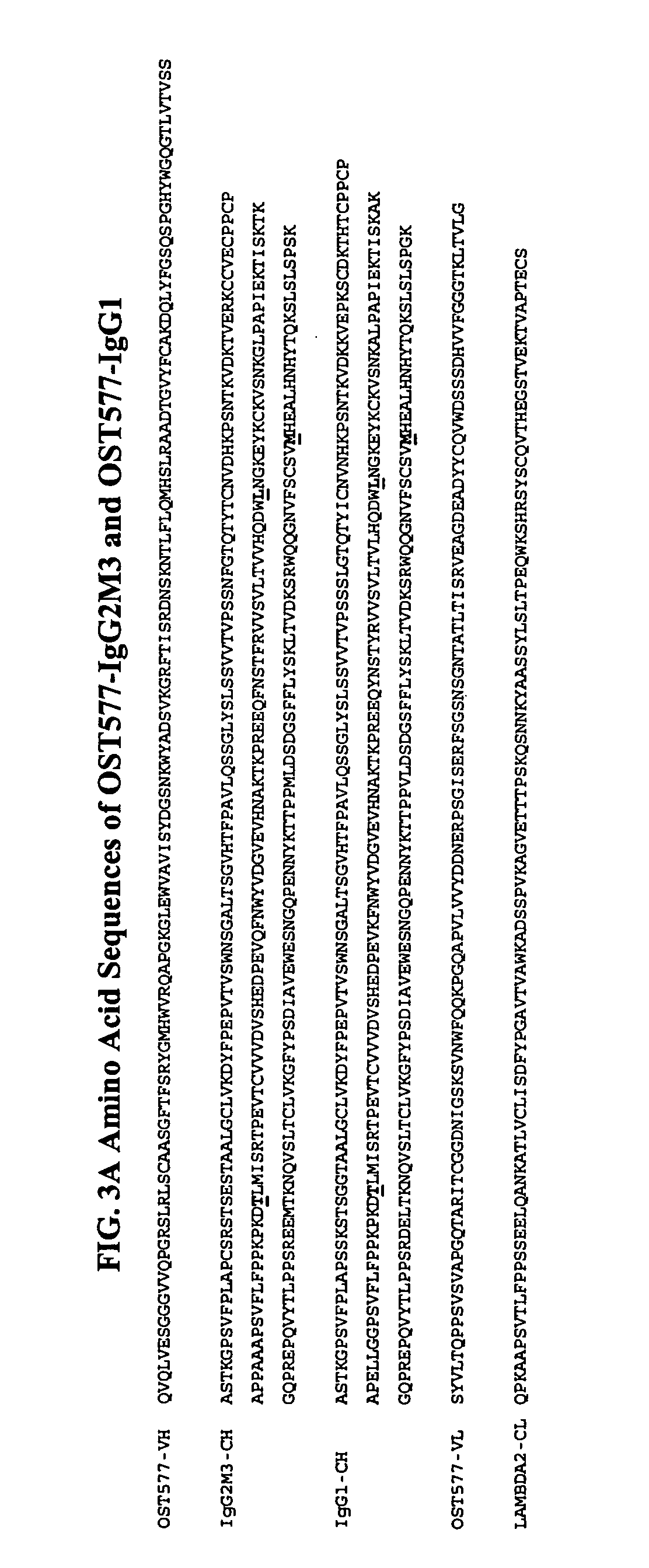
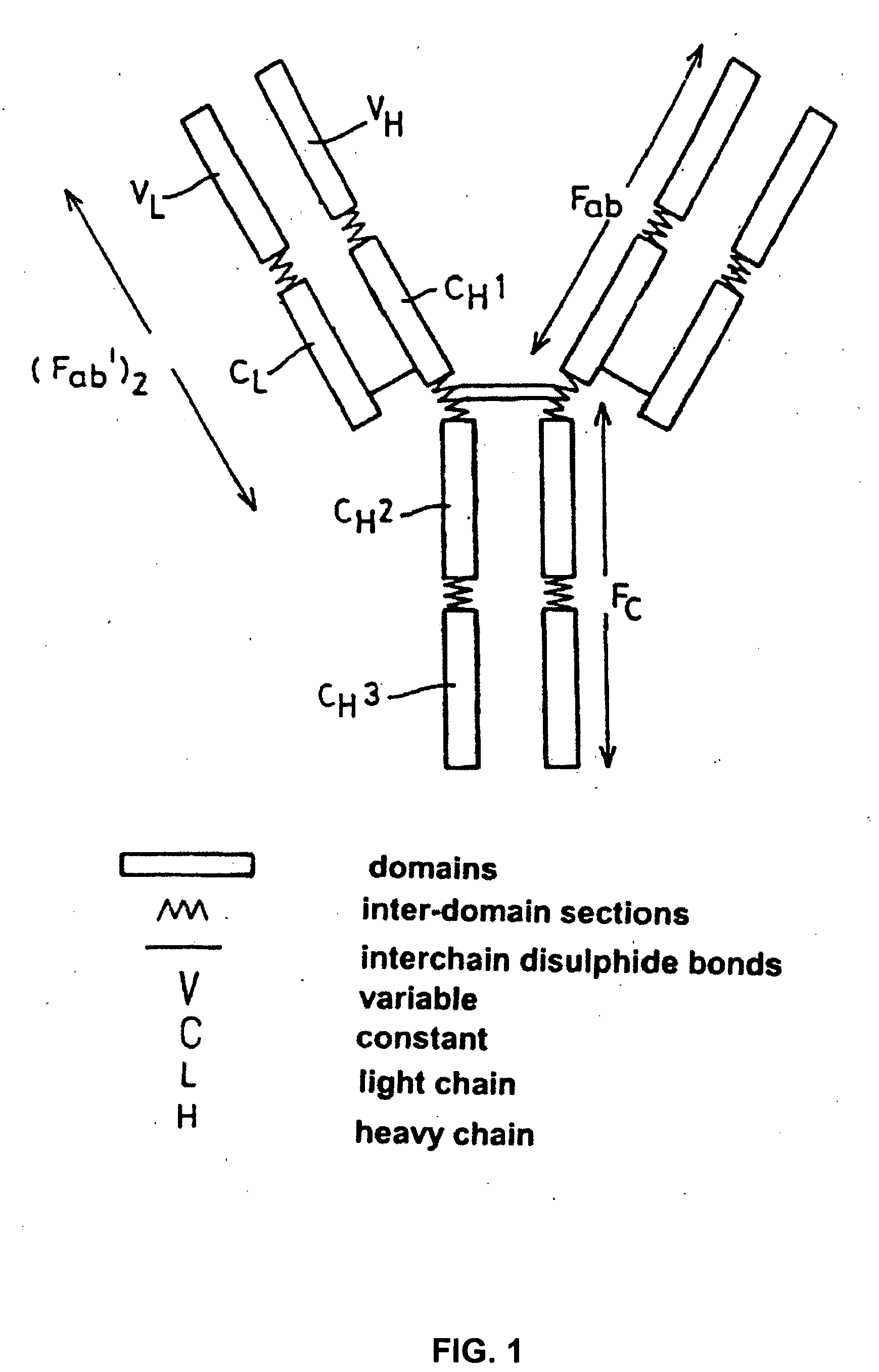
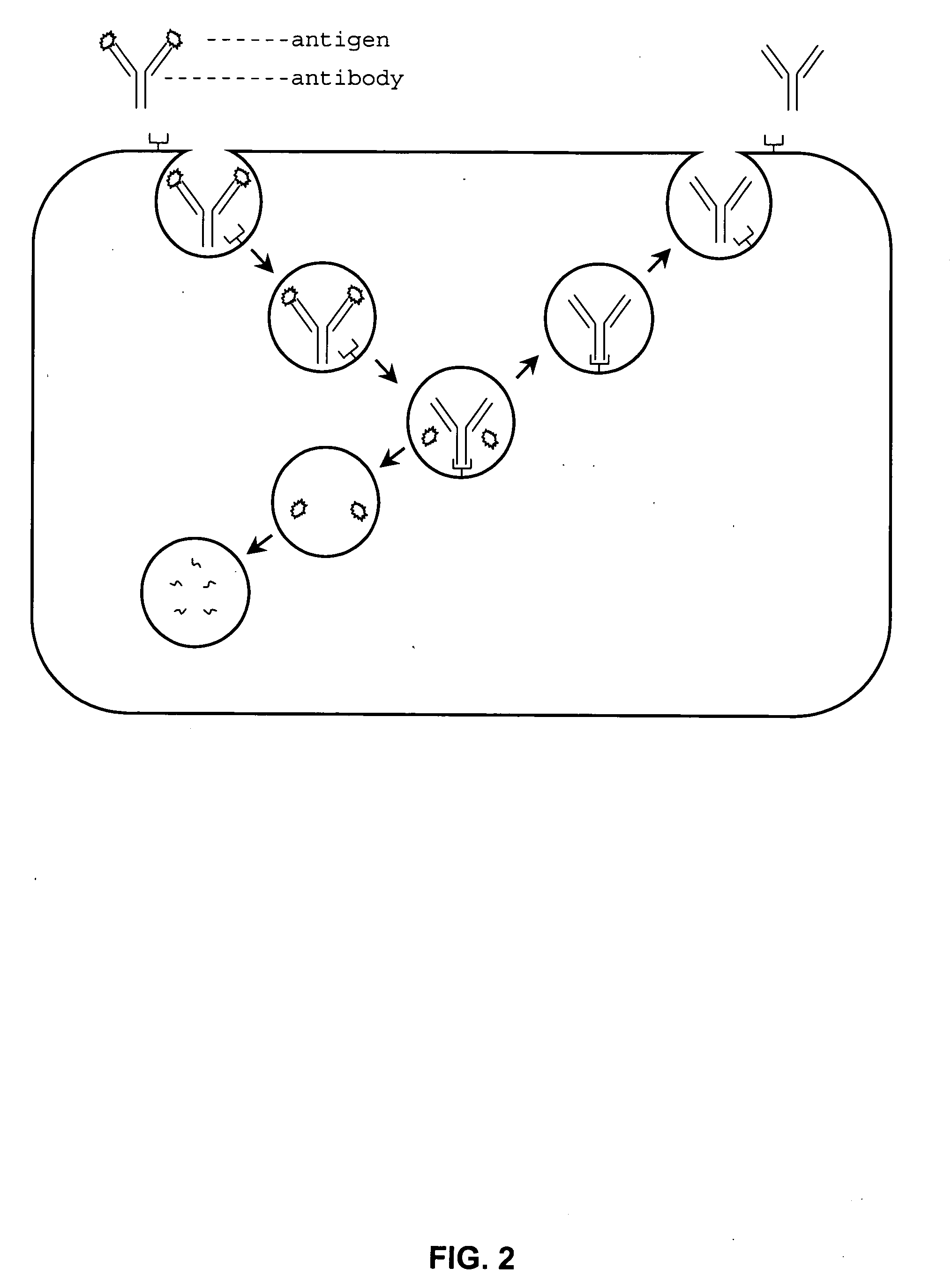
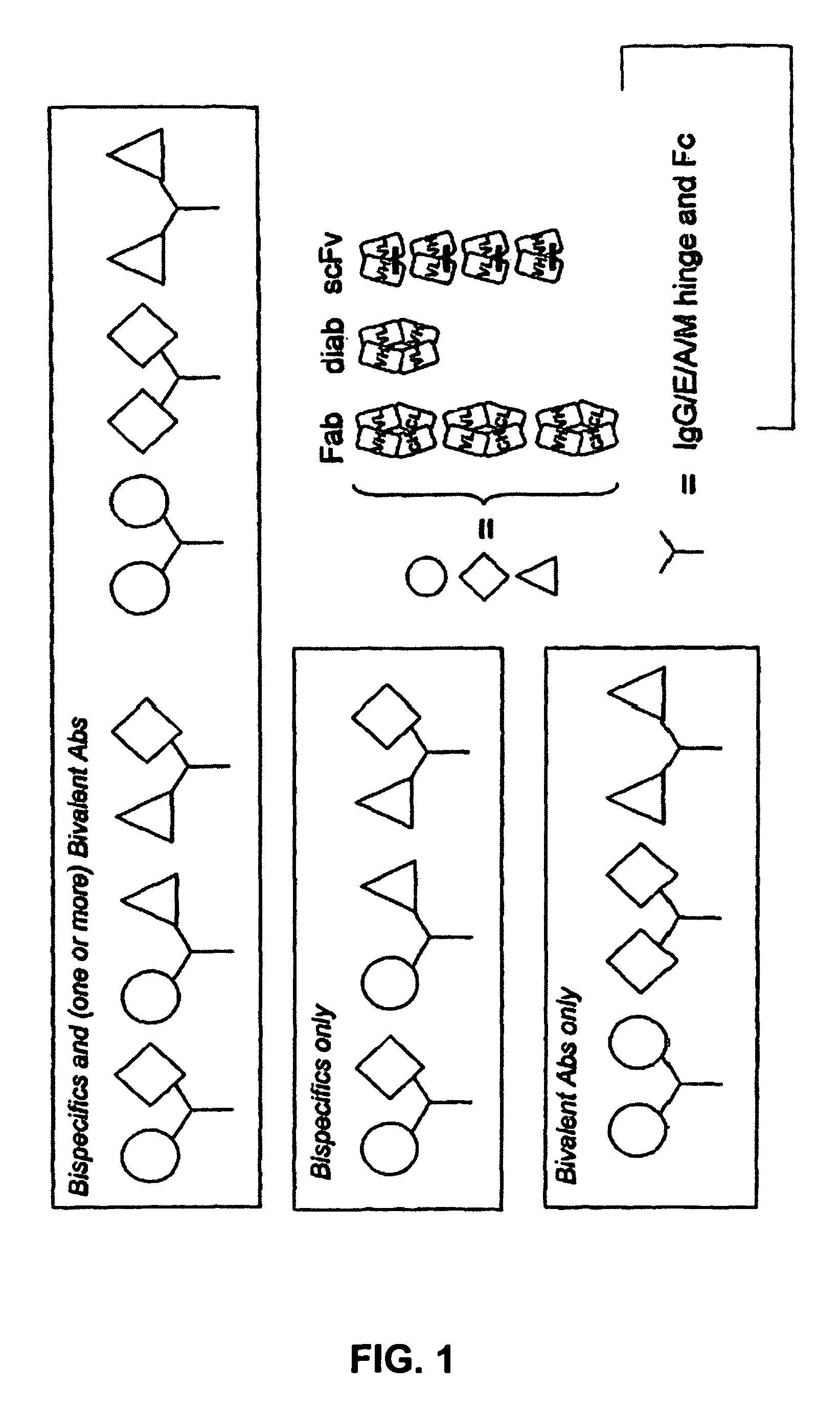
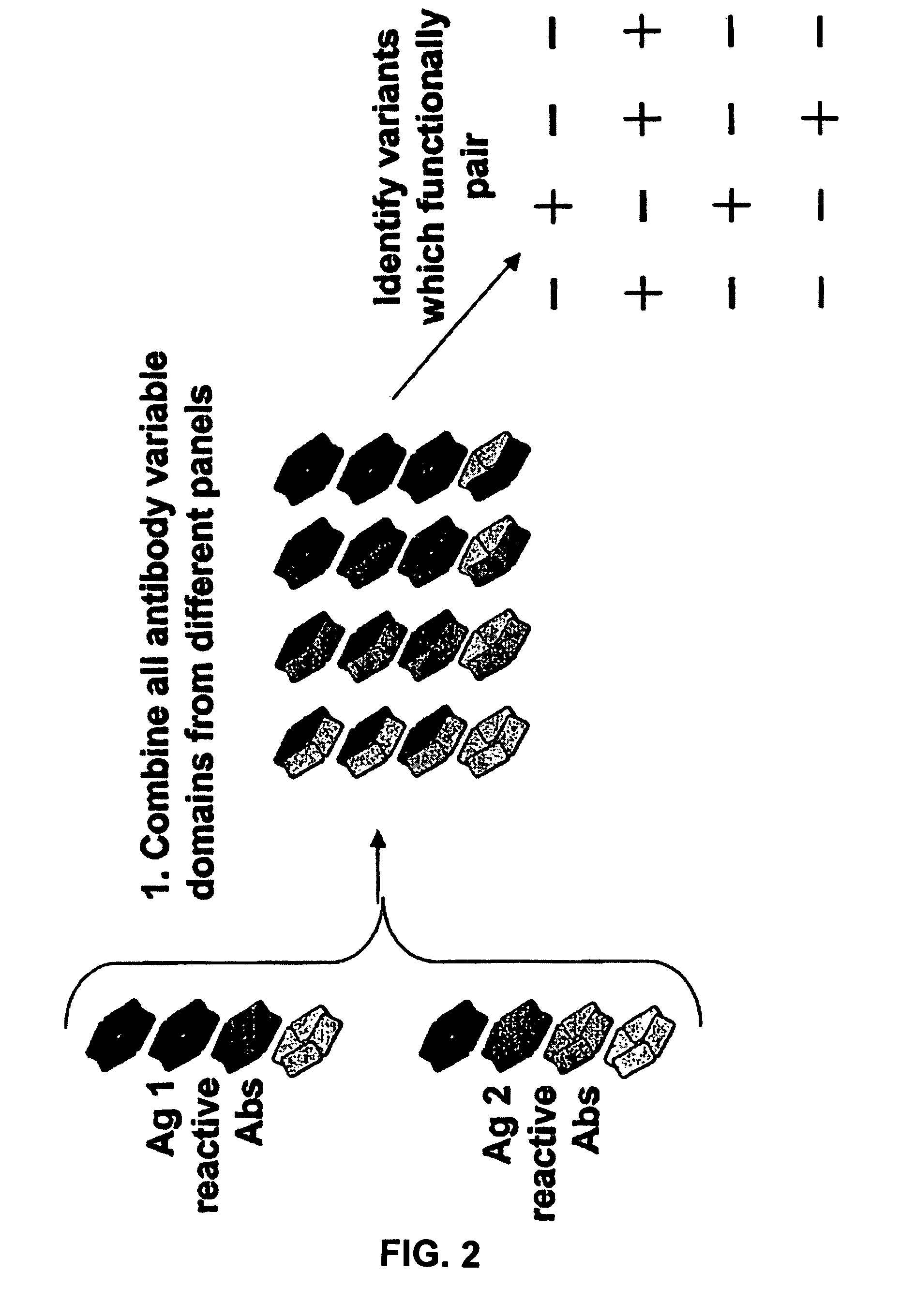
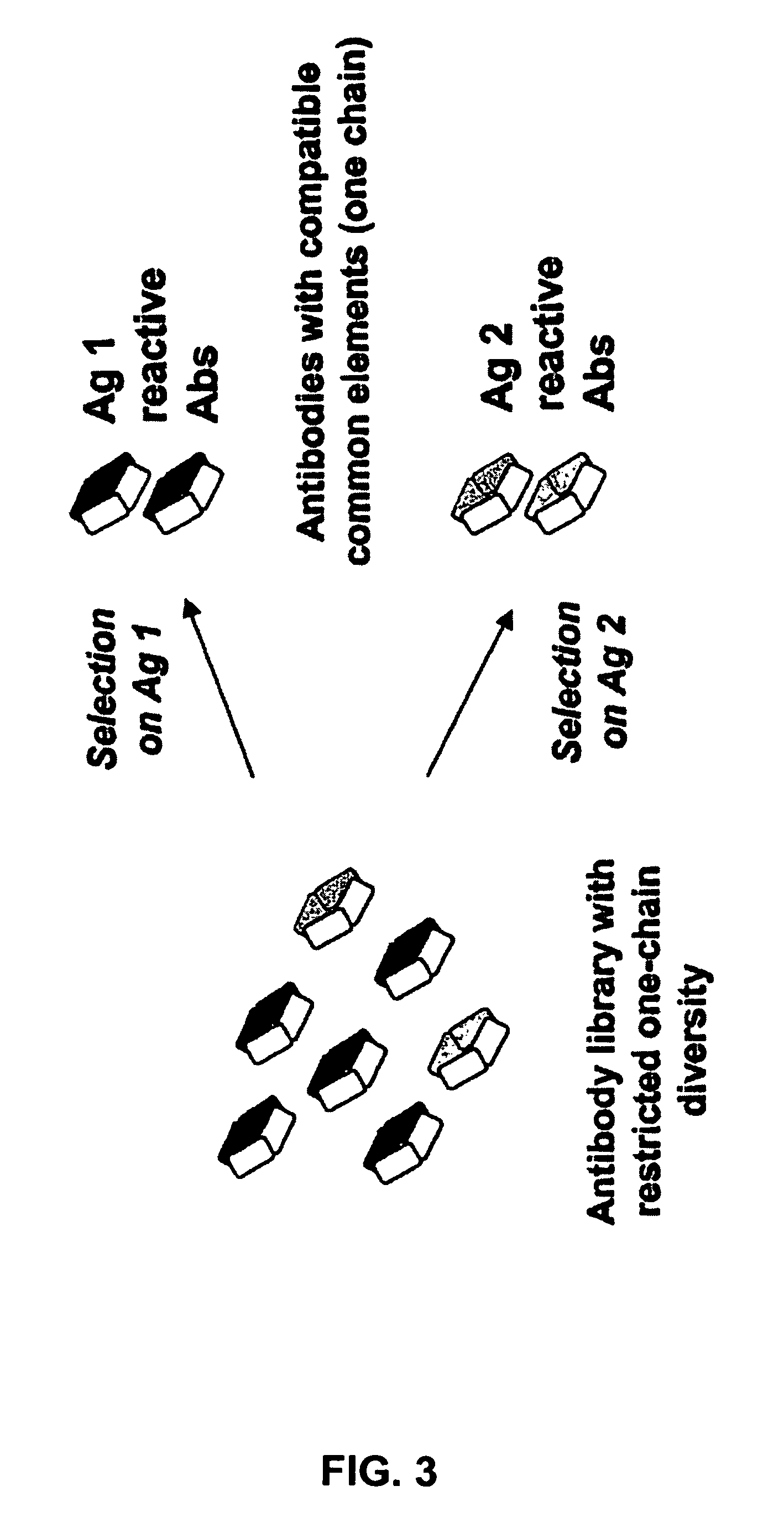
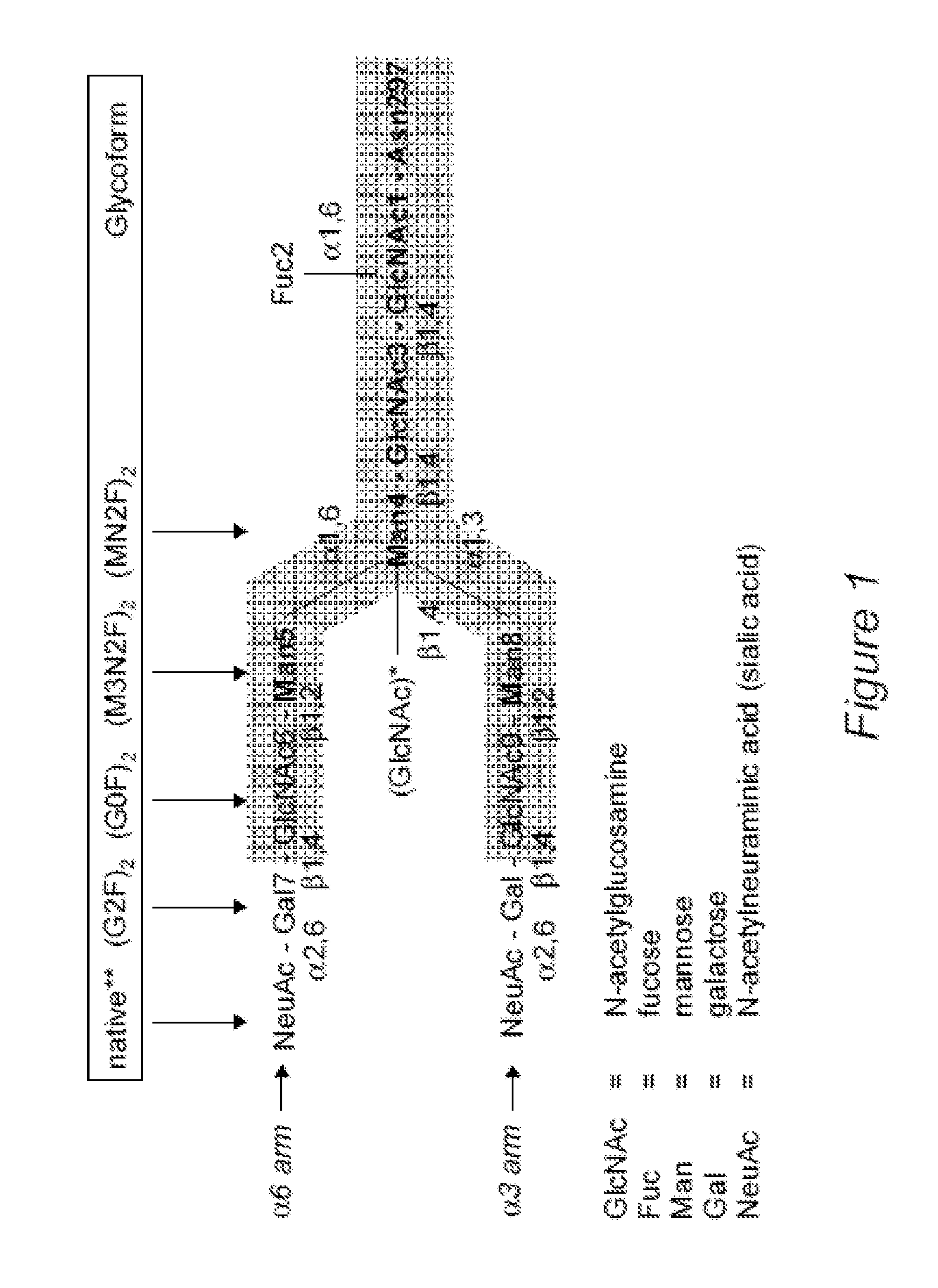
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4829results about "Immunoglobulins against viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050014934A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS7217797B2Function increaseExtended half-lifeAnimal cellsSugar derivativesAntiendomysial antibodiesBiochemistry
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Molecules with extended half-lives, compositions and uses thereof
InactiveUS20030190311A1High affinityExtended half-lifeCompounds screening/testingFungiIntravenous gammaglobulinIn vivo
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Anti-RSV antibodies
InactiveUS6818216B2Reduce dosing frequencyReduce dosageAnimal cellsSugar derivativesSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection.
Owner:MEDIMMUNE LLC
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS7229619B1Reduce dosing frequencyReduce dosageImmunoglobulins against virusesAntibody ingredientsSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection.
Owner:MEDIMMUNE LLC
Chimeric receptor genes and cells transformed therewith
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:UNITED STATES OF AMERICA +1
Antibody producing non-human mammals
ActiveUS20100146647A1Low variabilityLow immunogenicityAnimal cellsAntibody mimetics/scaffoldsHuman animalDNA rearrangement
Owner:MERUS NV
Bivalent IgY antibody constructs for diagnostic and therapeutic applications
InactiveUS20070141049A1Improve the immunityIncrease contentImmunoglobulins against virusesAntibody ingredientsHeavy chainMammal
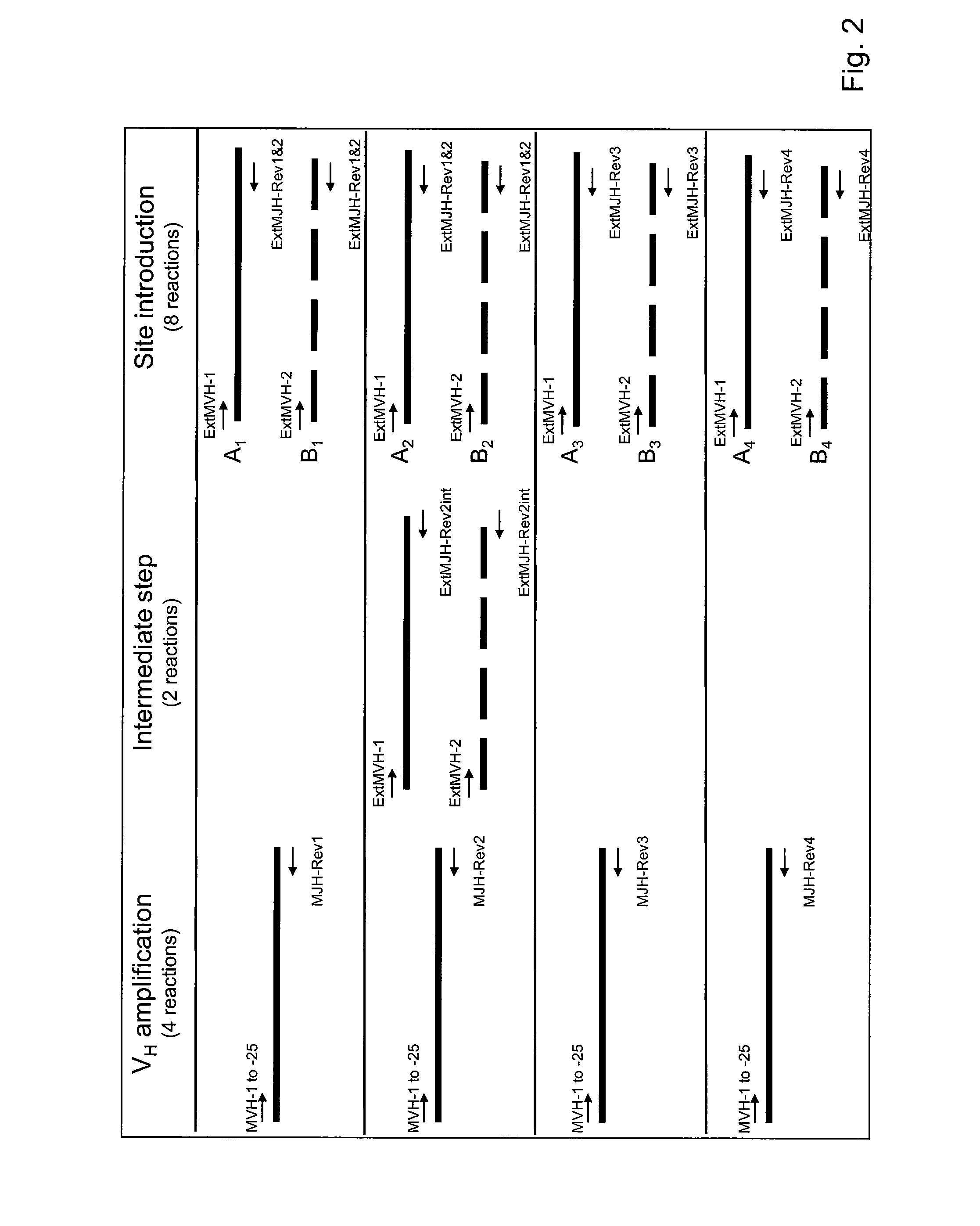
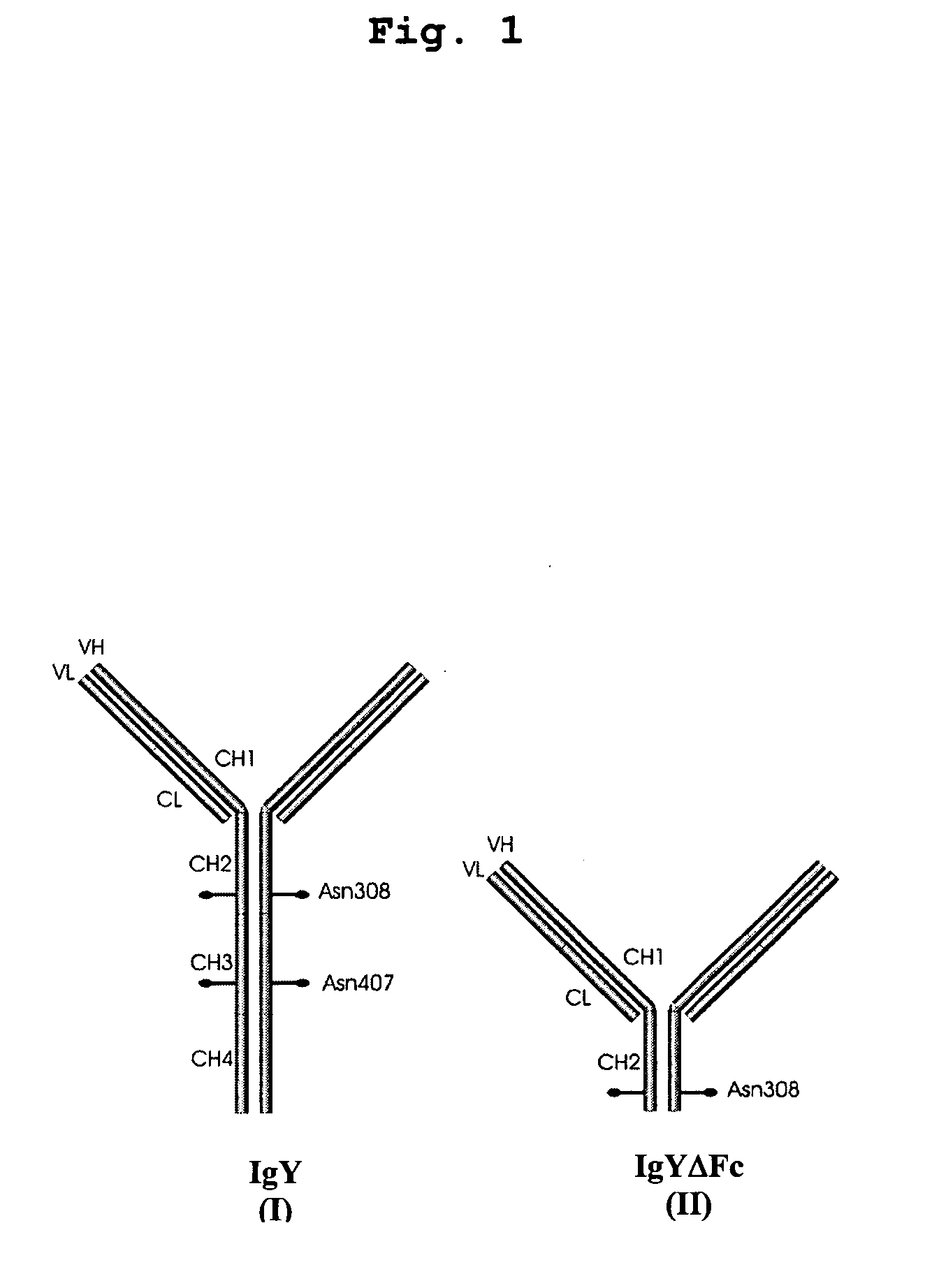
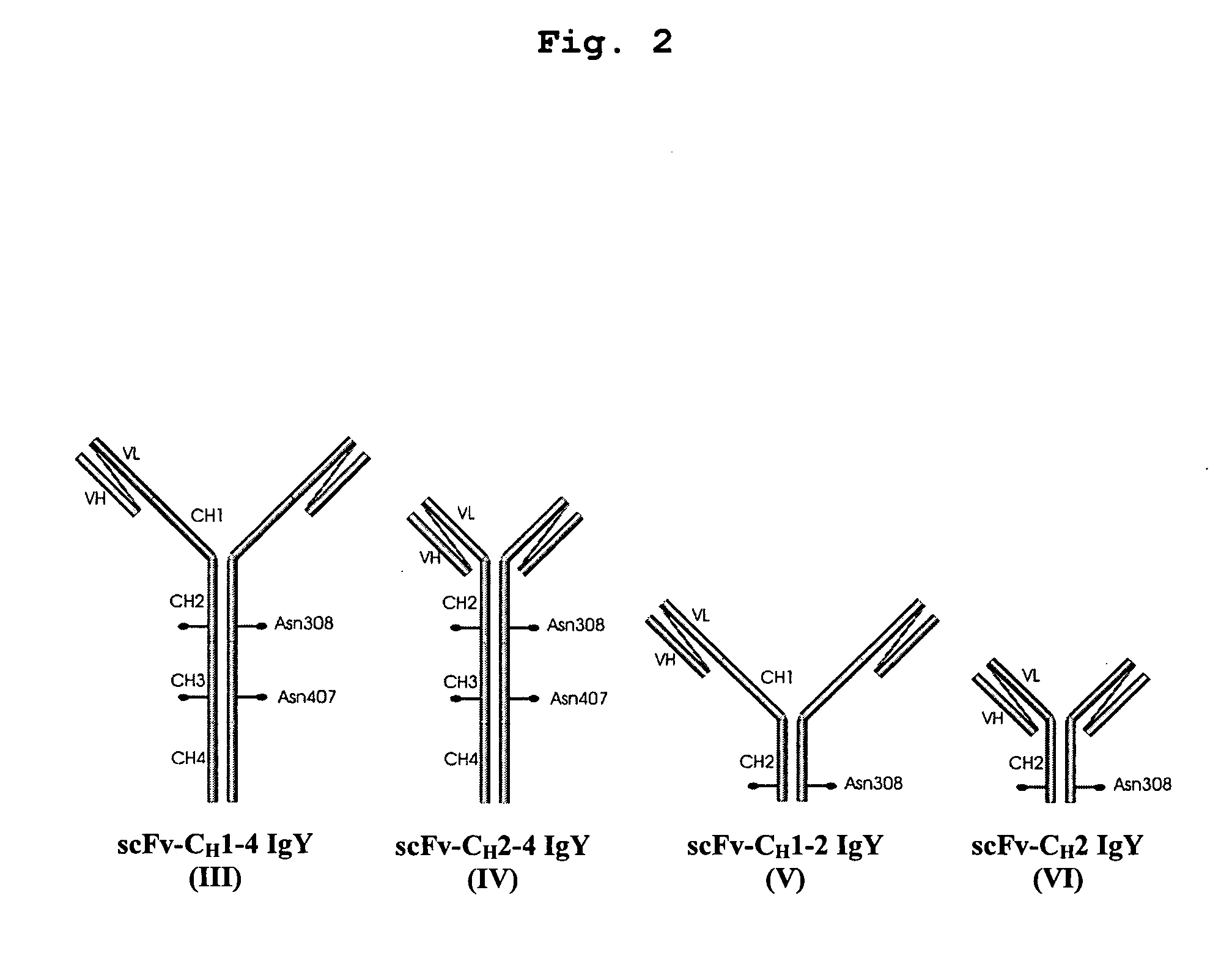
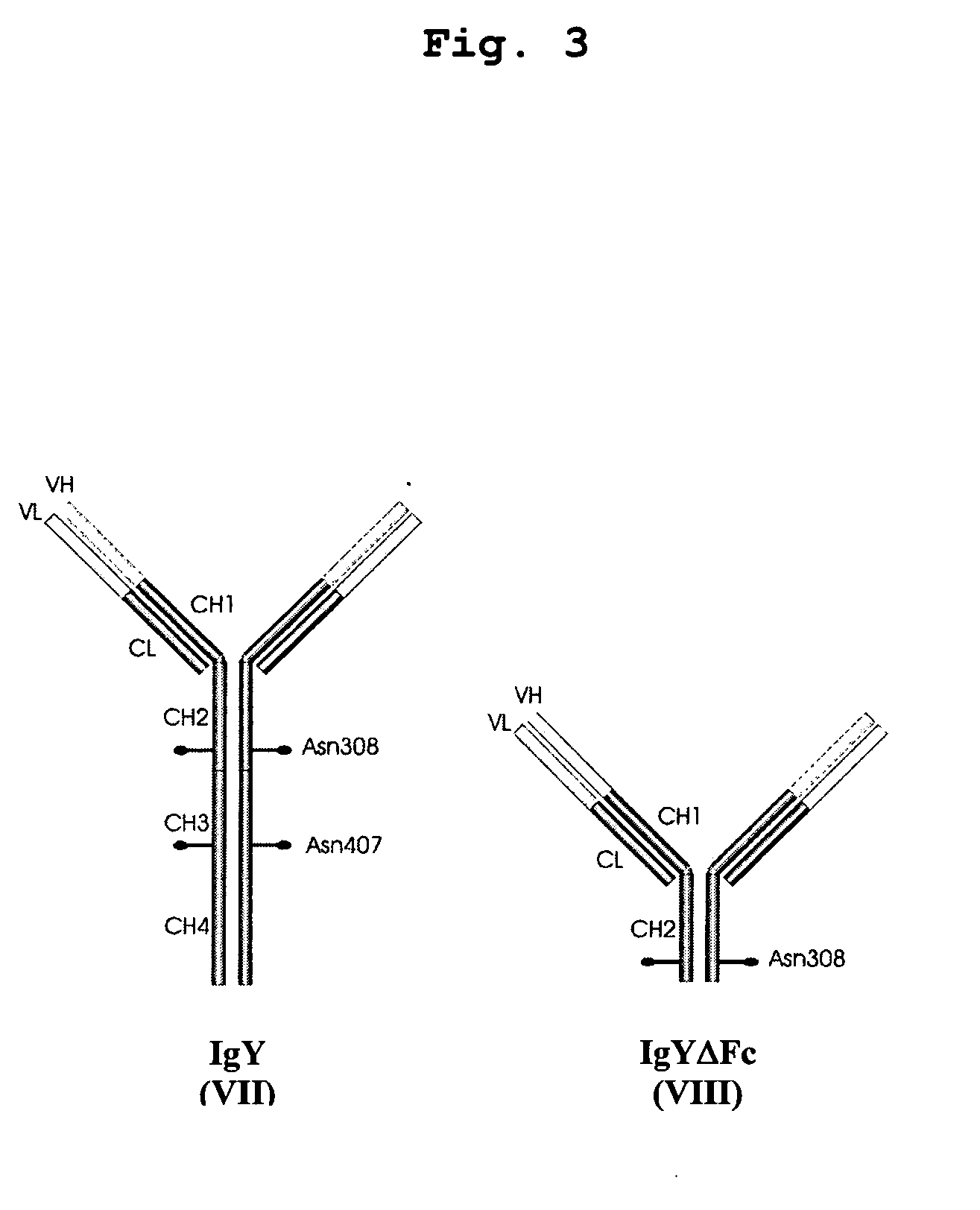
This invention relates to the field of recombinant antibody technology. It provides novel recombinant IgY antibody constructs for diagnostic and therapeutical applications. The bivalent antibody constructs display a heterotetrameric or homodimeric format stabilized by disulfide bonds. The constant heavy chain domains CH2-CH4 are partly or completely of avian origin, whereas the VH, VL, CL, and CH1 domains as well as the hinge region may be of avian origin or derived from any other species. The invention allows to combine the advantages of IgY antibodies with those of established mammalian monoclonal antibodies. IgY antibody constructs comprising nonglycosylated IgY constant heavy chain domains allow to reduce unwanted interactions with C-type lectins, e.g., in human sera. Furthermore, chimeric IgY antibody containing mammalian VH, VL, CL, and CH1 domains as well as a mammalian hinge region provide a higher molecular stability than IgY antibodies in acidic conditions and, thereby, are especially suited for peroral therapeutic applications.
Owner:PLS DESIGN
HIV envelope polypeptides
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
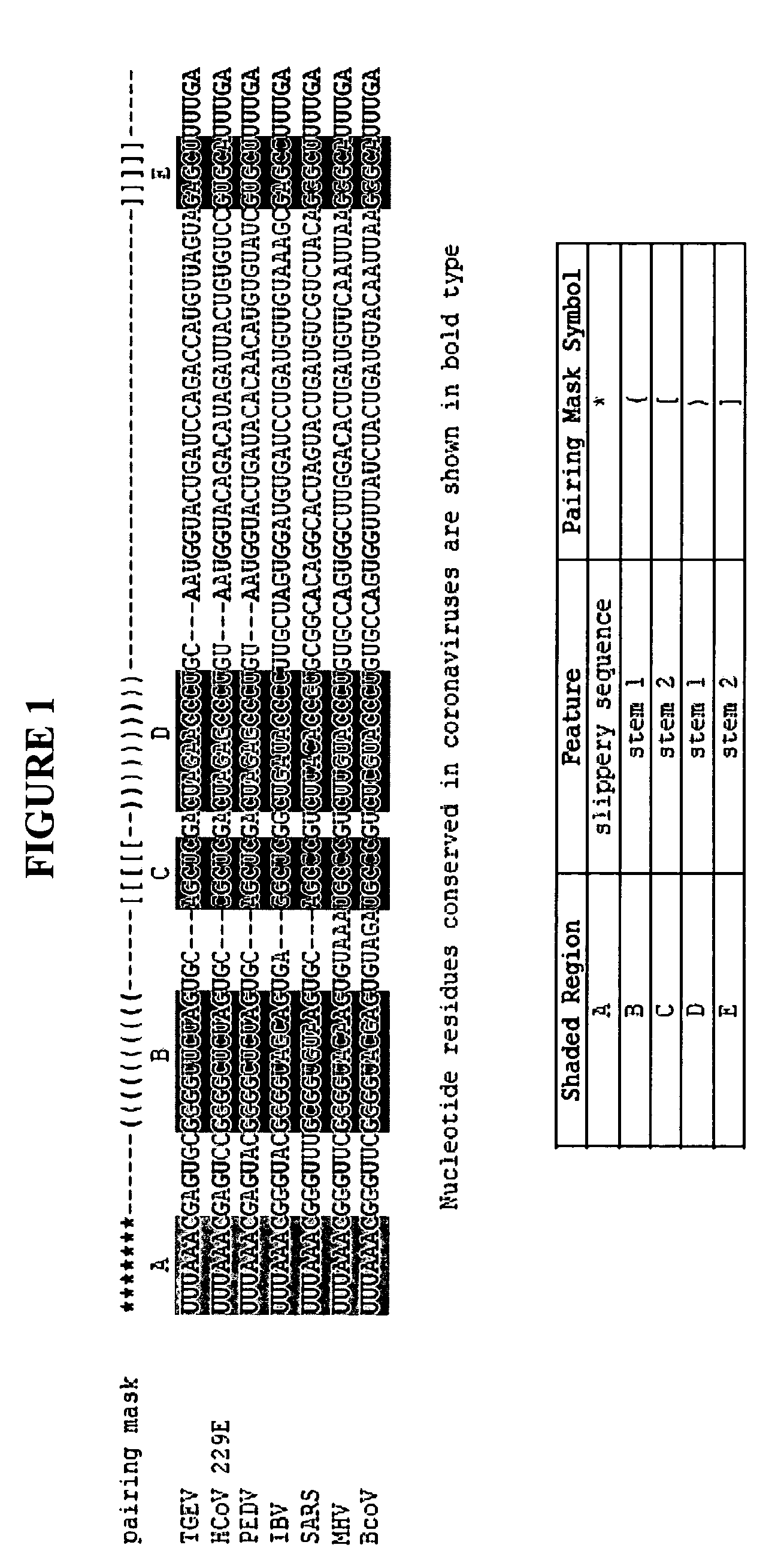
Compositions and methods for the treatment of severe acute respiratory syndrome (SARS)
The present invention provides design and synthesis of oligomeric compounds and compositions that can be administered to reduce the activity of SARS virus in vivo or in vitro, to prevent or treat SARS virus-associated disease, to detect AARS virus, and to diagnose SARS virus-associated diseases.
Owner:IONIS PHARMA INC
Antibody producing non-human mammals
InactiveUS20100069614A1Easy to openEasy maintenanceAntibody mimetics/scaffoldsImmunoglobulins against virusesHuman animalDNA rearrangement
Described are transgenic, non-human animals comprising a nucleic acid encoding an immunoglobulin light chain, whereby the immunoglobulin light chain is human, human-like, or humanized. The nucleic acid is provided with a means that renders it resistant to DNA rearrangements and / or somatic hypermutations. In one embodiment, the nucleic acid comprises an expression cassette for the expression of a desired molecule in cells during a certain stage of development in cells developing into mature B cells. Further provided is methods for producing an immunoglobulin from the transgenic, non-human animal.
Owner:MERUS NV
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050032114A1Reducing FcRn binding affinityReduced half-lifeAnimal cellsSugar derivativesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050276799A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesSerum igeHalf-life
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Production of humanized antibodies in transgenic animals
InactiveUS7129084B2Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Therapeutic using a bispecific antibody
Multivalent, multispecific molecules having at least one specificity for a pathogen and at least one specificity for the HLA class II invariant chain (Ii) are administered to induce clearance of the pathogen. In addition to pathogens, clearance of therapeutic or diagnostic agents, autoantibodies, anti-graft antibodies, and other undesirable compounds may be induced using the multivalent, multispecific molecules.
Owner:IMMUNOMEDICS INC
Prevention and treatment of HCV infection employing antibodies that inhibit the interaction of HCV virions with their receptor
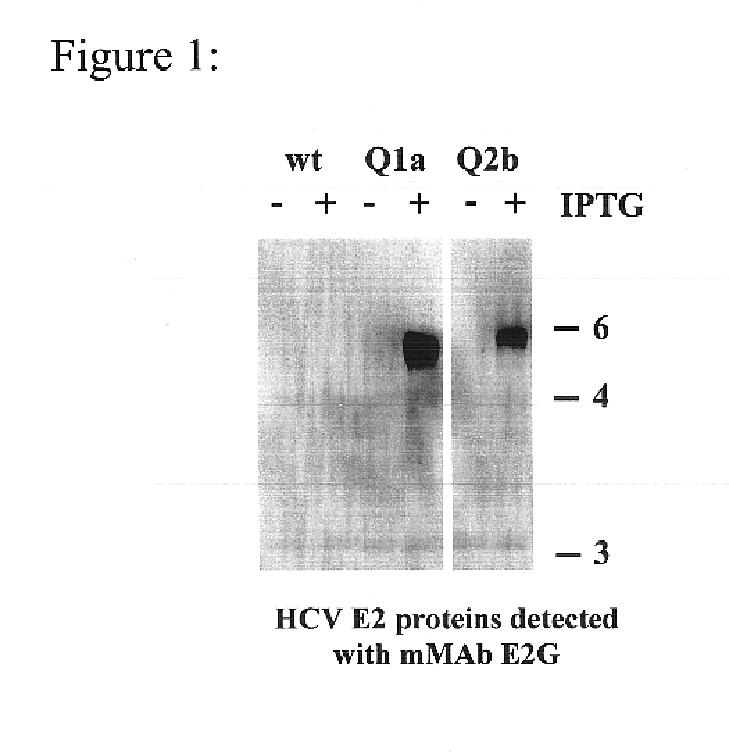
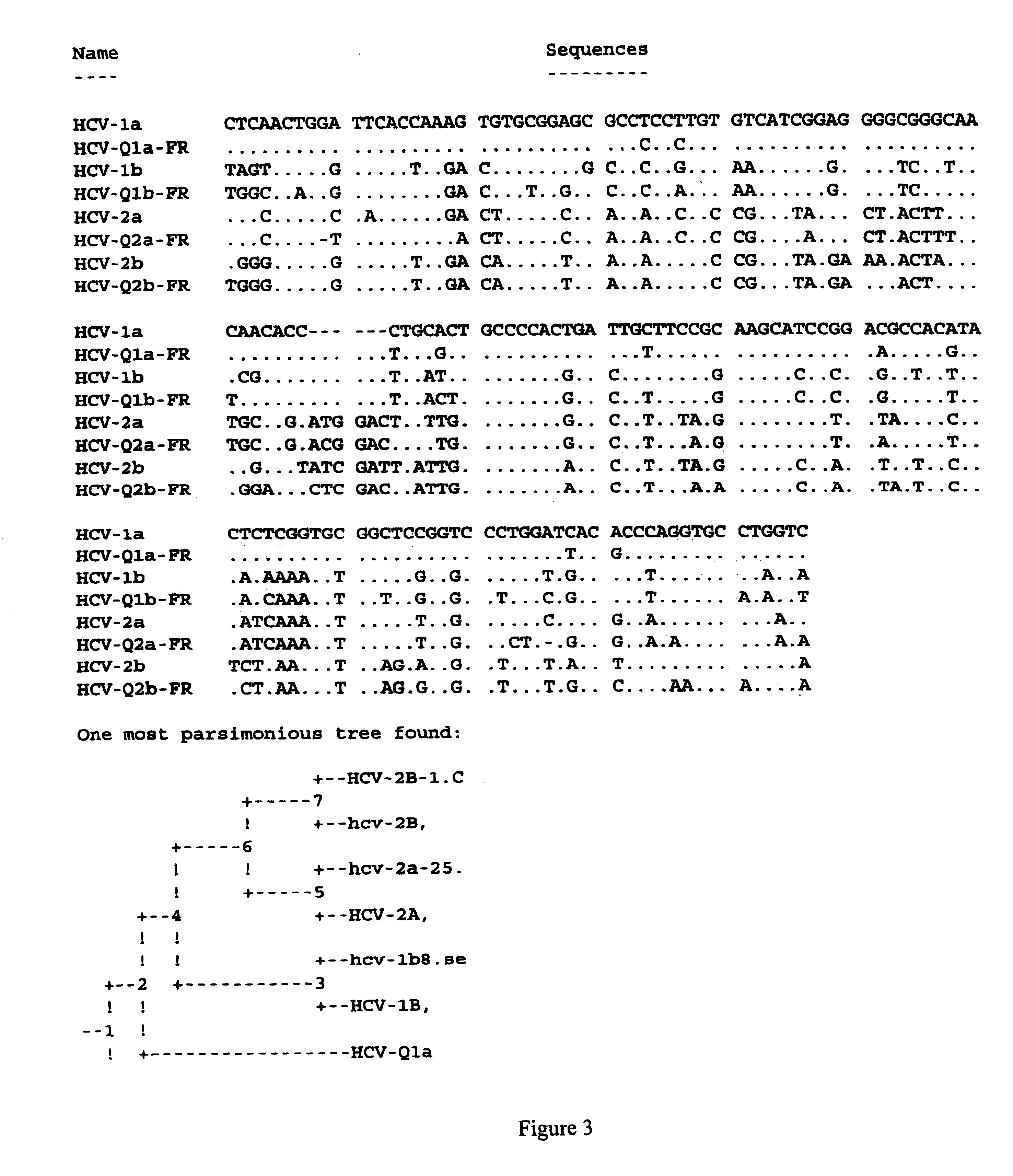
Human monoclonal antibodies binding to epitopes common to type 1 and 2 HCV are provided, as well as conformationally conserved HCV E2 2a and 2b proteins. Compositions comprising the antibodies find use in diagnosis and therapy. The antibodies recognize conformational epitopes that are conserved across multiple genotypes of HCV. Thus the antibodies have the potential to be useful in the prevention and treatment of the majority of HCV infections. A subset of the antibodies (CBH-2, CBH-5, CBH-7, CBH-8C, CBH-8E, and CBH-11) have the ability to prevent the binding of HCV E2 proteins of multiple genotypes to human CD81, a possible co-receptor for HCV infection. A subset of the antibodies (CBH-2 and CBH-5) have been shown to inhibit the binding of HCV virions (as opposed to purified E2 protein) to human CD81. A further subset of the antibodies (CBH-4D, CBH4B, CBH-8C, and CBH-9) have been shown to prevent HCV envelope mediated fusion using an HCV psuedotype system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Ligands to enhance cellular uptake of biomolecules
InactiveUS20060183886A1Effectively crossGain access to the cytoplasmOrganic active ingredientsSugar derivativesOligomerReceptor for activated C kinase 1
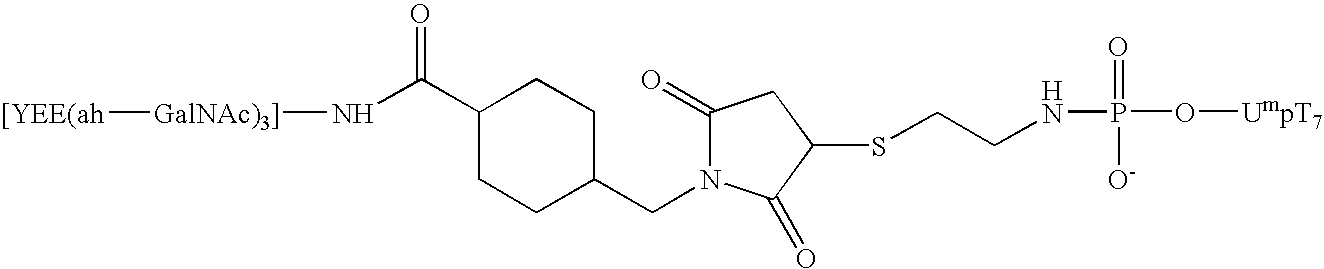
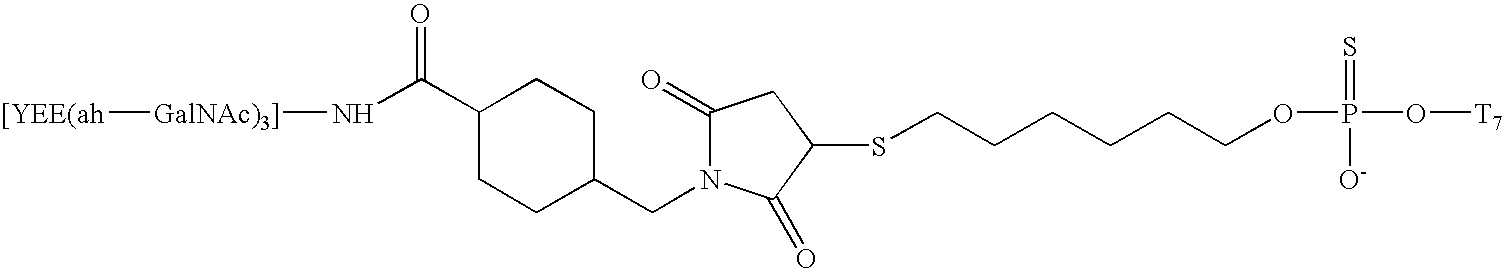
The present invention relates to the design and synthesis of homogeneous A-L-P constructs, which contain a hepatic ligand to direct an oligomer or “payload” to a hepatocyte intracellularly via a receptor-mediated, ligand-directed pathway.
Owner:CELLECTIVE DX CORP
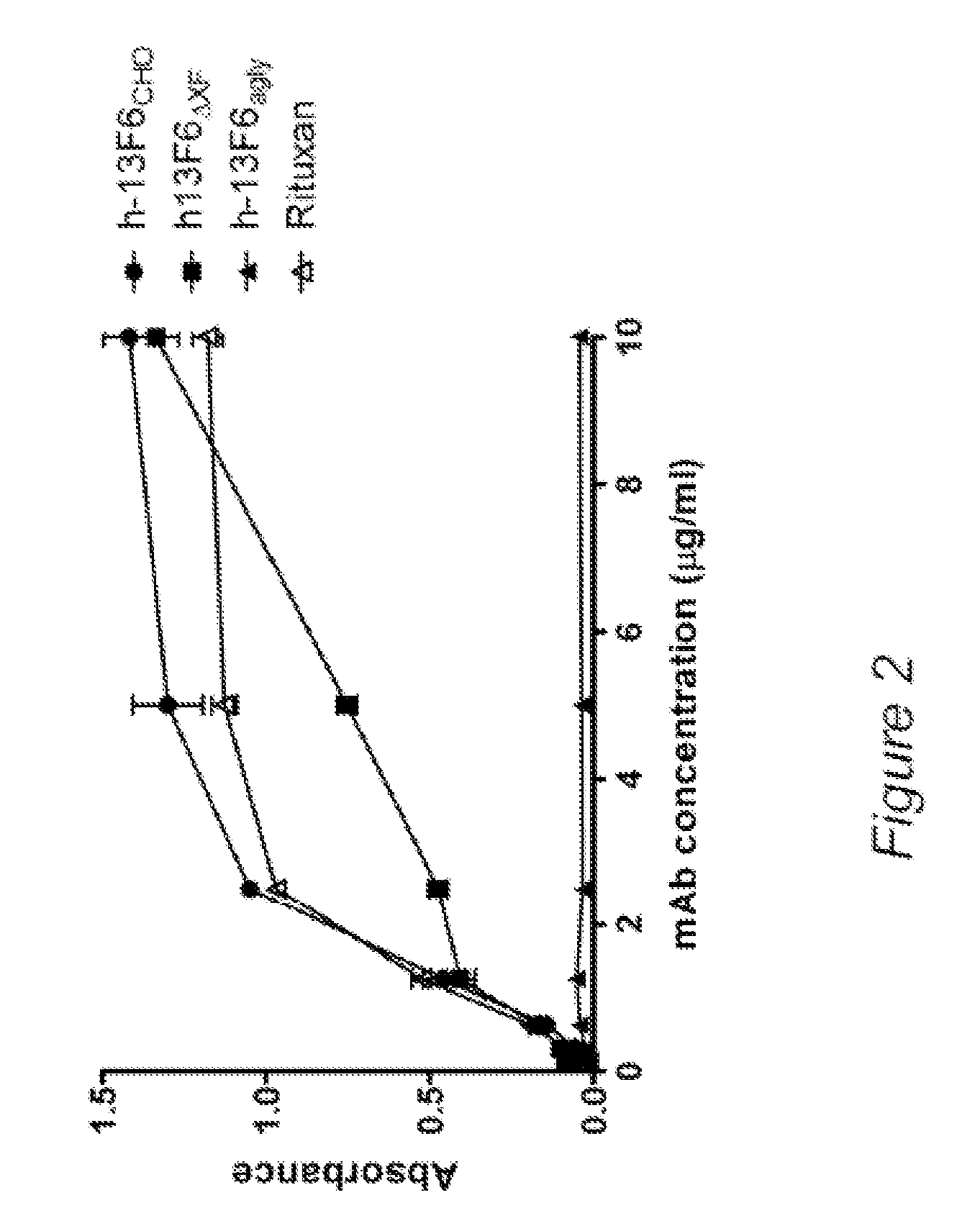
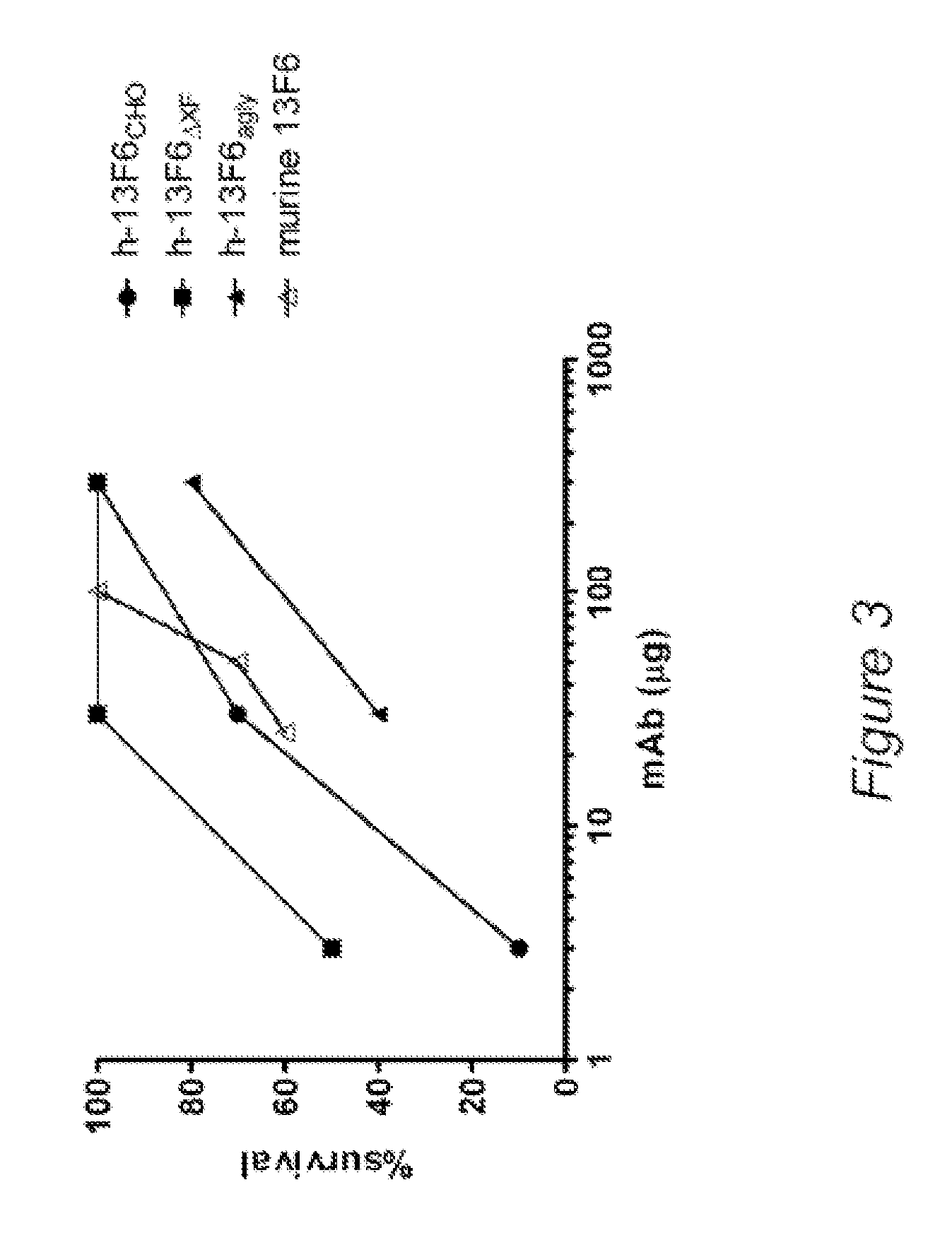
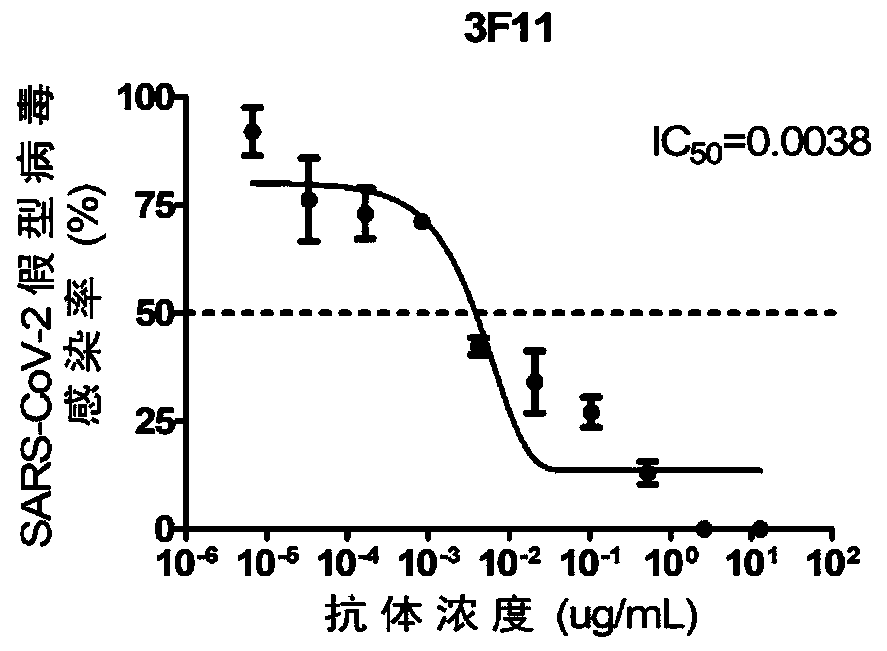
Anti-SARS-CoV-2-spike glycoprotein antibodies and antigen-binding fragments
ActiveUS10787501B1Growth inhibitionControl spreadViral antigen ingredientsImmunoglobulins against virusesAntigenAntiendomysial antibodies
The present disclosure provides antibodies and antigen-binding fragments thereof that bind specifically to a coronavirus spike protein and methods of using such antibodies and fragments for treating or preventing viral infections (e.g., coronavirus infections).
Owner:REGENERON PHARM INC
Fab library for the preparation of anti VEGF and anti rabies virus fabs
InactiveUS20060160184A1Maintain good propertiesOptimization mechanismAnimal cellsAntibody mimetics/scaffoldsProtein moleculesImmunoglobulin IgE
The present invention provides combinations of specific binding proteins, such as immunoglobulins, that are designed to be true combinations, essentially all components of the combination being functional and compatible with each other. The invention further provides a method for producing a composition comprising at least two different proteinaceous molecules comprising paired variable regions, the at least two proteinaceous molecules having different binding specificities, comprising paired variable regions, at least two proteinaceous molecules having different binding specificities, comprising contacting at least three different variable regions under conditions allowing for pairing of variable regions and harvesting essentially all proteinaceous molecules having binding specificities resulting from the pairing.
Owner:MERUS NV
Cross-linkers and their uses
ActiveUS8236319B2Good water solubilityHigh sensitivityOrganic active ingredientsTripeptide ingredientsCross-linkCross linker
Charged or pro-charged cross-linking moieties and conjugates of cell binding agents and drugs comprising the charged or pro-charged cross-linking moieties and method of making the same.
Owner:IMMUNOGEN INC
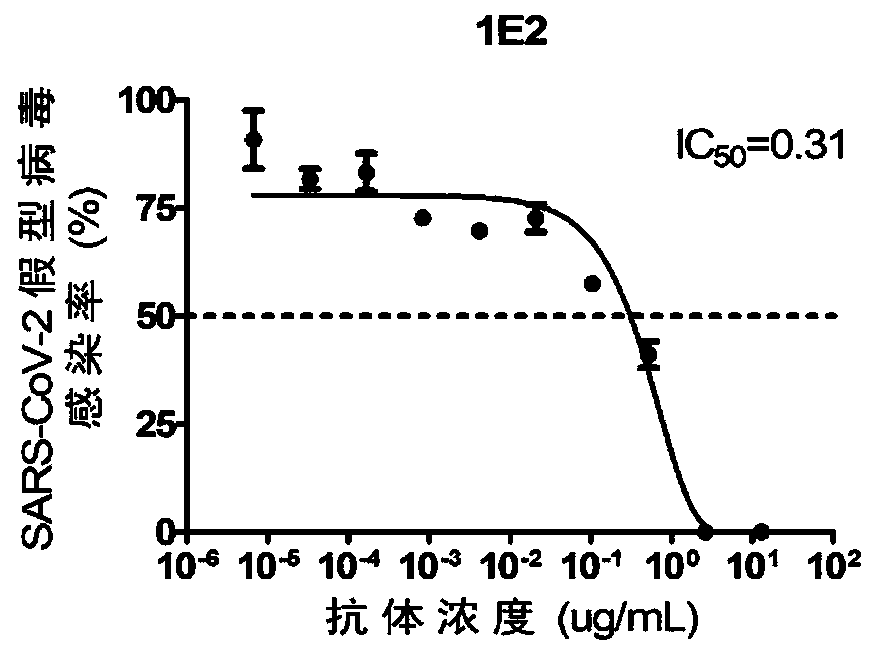
Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
PendingCN111153991AHigh affinityStrong specificitySsRNA viruses positive-senseVirus peptidesBALB/cMonoclonal antibody agent
The invention discloses a human SARS-CoV-2 monoclonal antibody. The preparation method of the human SARS-CoV-2 monoclonal antibody comprises the steps: adopting SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB / c mice, performing fusion and subcloning on spleen cells and myeloma cells of mice, then performing a large amount of repeated screening and domestication of cell lines through commercialized products SARS-CoV-2 Nucleocapsid and MERS Nucleocapsid so as to obtain a hybridoma cell line capable of secreting the SARS-CoV-2-resistant N monoclonal antibody with high affinity and high specificity finally and successfully, and finally performing ascites preparation and purification so as to obtain the monoclonal antibody, wherein the amino acid sequence of the SARS-CoVNucleocapsid recombinant protein is shown in SEQ ID No. 1. The invention also discloses application of the monoclonal antibody in preparation of SARS-CoV-2 virus detection products and preparation ofdrugs for inhibiting the SARS-CoV-2 viruses. The monoclonal antibody can be used for detecting the SARS-CoV-2 in human throat swabs / pulmonary secretions and other samples by using a double-antibody sandwich method, and can be applied to diagnosis and prevention and control of SARS-CoV-2 virus infection and scientific researches of viruses and other study.
Owner:BEIJING BIOSYNTHESIS BIOTECH
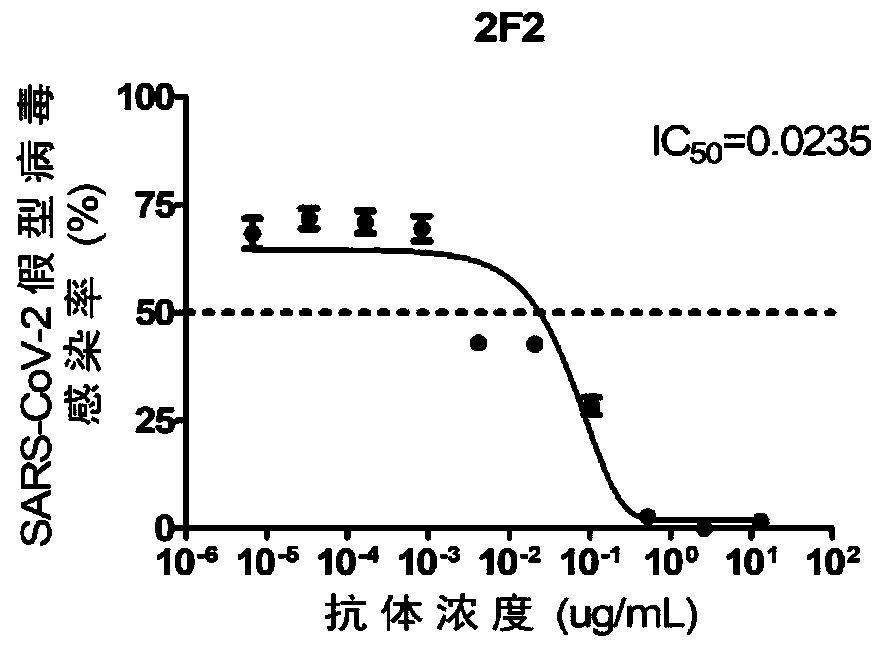
High-neutralizing-activity anti-SARS-CoV-2 fully-humanized monoclonal antibody and application thereof
ActiveCN111303280AGood neutralizing effectReduced responseImmunoglobulins against virusesAntiviralsDiseaseAntiendomysial antibodies
The invention discloses an anti-SARS-CoV-2 fully-humanized monoclonal antibody against SARS-CoV-2. The antibody is obtained by screening through a flow sorting-single cell PCR technology, and has a unique CDR partition. The invention further discloses an application of the antibody in preparation of medicines for treating the 2019-coronavirus disease. The monoclonal antibody disclosed by the invention has efficient and specific anti-SARS-COV-2 activity, also has the characteristics of high expression, complete humanization and good stability, and is suitable for industrial production.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method for selecting a single cell expressing a heterogeneous combination of antibodies
InactiveUS7919257B2Maintain good propertiesOptimization mechanismBiocideAntibody mimetics/scaffoldsProtein moleculesPairing
The present invention provides combinations of specific binding proteins, such as immunoglobulins, that are designed to be true combinations, essentially all components of the combination being functional and compatible with each other. The invention further provides a method for producing a composition comprising at least two different proteinaceous molecules comprising paired variable regions, the at least two proteinaceous molecules having different binding specificities, comprising paired variable regions, at least two proteinaceous molecules having different binding specificities, comprising contacting at least three different variable regions under conditions allowing for pairing of variable regions and harvesting essentially all proteinaceous molecules having binding specificities resulting from the pairing.
Owner:MERUS NV
MONOCLONAL ANTIBODIES WITH ALTERED AFFINITIES FOR HUMAN FCyRI, FCyRIIIa, AND C1q PROTEINS
InactiveUS20130149300A1Good curative effectLow toxicitySugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsDiseaseFc-Gamma Receptor
Disclosed herein are GNGN and G1 / G2 antibodies that recognize and bind various FcRs and C1q. Also disclosed herein are glycan-optiminzed antibodies, predominantly of the GNGN or G1 / G2 glycoform, with enhanced Fcγ receptor binding achieved through CHO, Nicotiana benthamiana and yeast manufacturing systems. Nucleic acids encoding these antibodies, as well as expression vectors and host cells including these nucleic acids are also disclosed herein. Methods and pharmaceutical compositions including the monoclonal antibodies are provided herein for the prevention and / or therapeutic treatment of viral infections, cancers and inflammatory diseases.
Owner:MAPP BIOPHARM +1
Single-domain antibodies for novel coronavirus and application of single-domain antibodies
ActiveCN111303279AHigh affinityAnd high activityImmunoglobulins against virusesAntiviralsAntigenReceptor
The invention discloses humanized single-domain antibodies for a novel coronavirus SARS-CoV-2 and an application of the humanized single-domain antibodies. The invention protects the single-domain antibodies described in any one of SEQ ID No.1 to SEQ ID No.5. Experimental results show that the single-domain antibodies provided by the invention have good affinity with receptor binding domain (RBD)antigens and have high neutralization activity on SARS-CoV-2 pseudovirus. The humanized single-domain antibodies have important scientific significance and application prospect for prevention and clinical treatment of the new coronavirus SARS-CoV-2 and for development of diagnostic reagents of the new coronavirus SARS-CoV-2.
Owner:BEIJING KAWIN TECH SHARE HLDG
Recombinant polyclonal antibody for treatment of respiratory syncytial virus infections
InactiveUS20100040606A1Reduce the possibilityMinimizing developmentSsRNA viruses negative-senseSugar derivativesEpitopeProtein G
Disclosed are novel polyclonal antibodies, which target respiratory syncyticilal virus (RSV), and novel high affinity antibody molecules reactive with RSV. The polyclonal antibodies may comprise antibody molecules which are reactive with both RSV protein F and RSV protein G, and preferably the polyclonal antibodies target a variety of epitopes on these proteins. The single antibody molecules of the invention are shown to exhibit affinities which provide for dissociation constants as low as in the picomolar range. Also disclosed are methods of producing the antibodies of the invention as well as methods of their use in treatment for RSV infection.
Owner:SYMPHOGEN AS
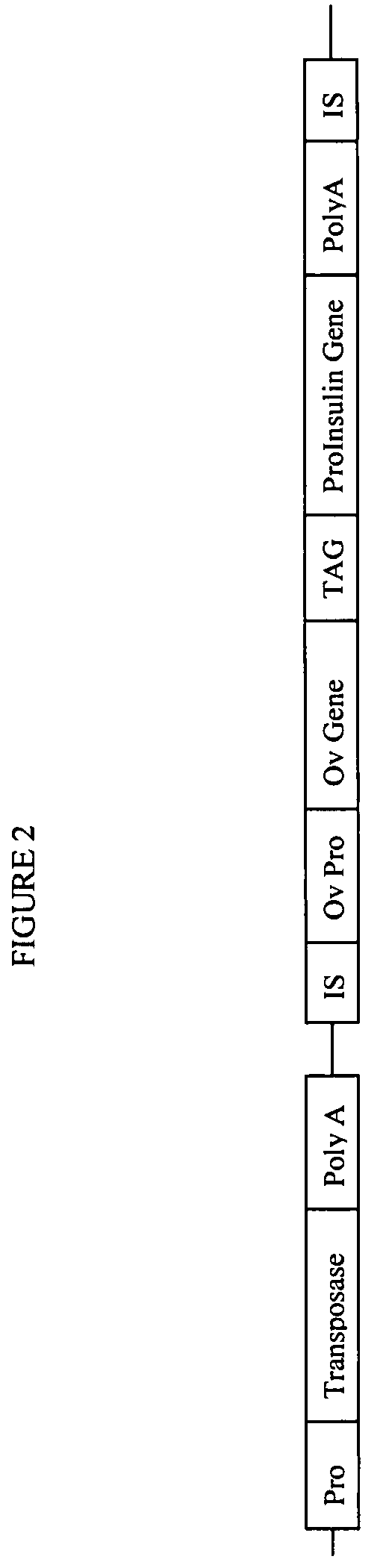
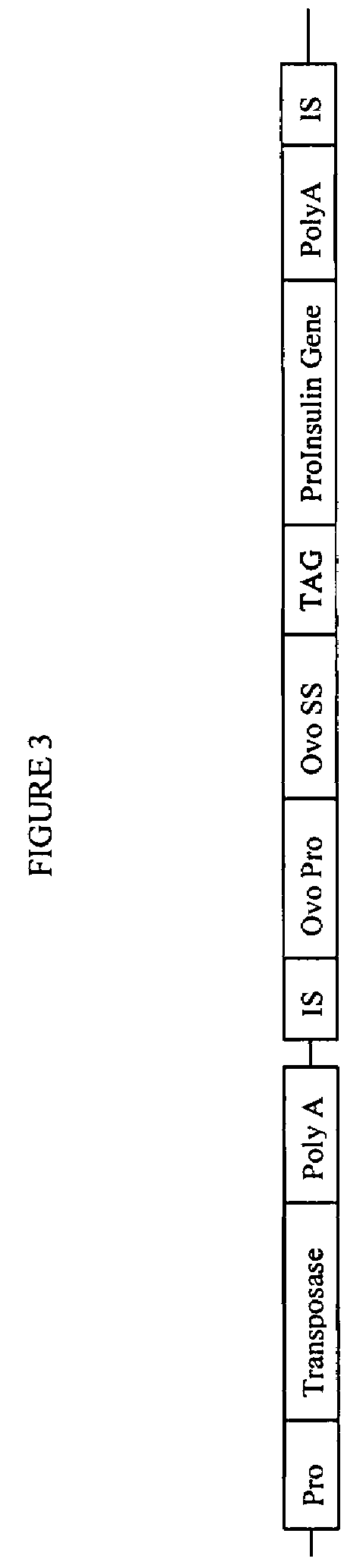
Gene regulation in transgenic animals using a transposon-based vector
InactiveUS7608451B2Improve efficiencyHigh copy numberEgg immunoglobulinsFood genetic modificationAnimal useTransgene
Administration of modified transposon-based vectors has been used to achieve stable incorporation of exogenous genes into animals. These transgenic animals produce transgenic progeny. Further, these transgenic animals produce large quantities of desired molecules encoded by the transgene. Transgenic egg-laying animals produce large quantities of desired molecules encoded by the transgene and deposit these molecules in the egg.
Owner:PROTEOVEC HLDG L L C
Anti-novel coronavirus monoclonal antibody and application thereof
ActiveCN111592594AStrong neutralizing activityImmunoglobulins against virusesAntiviralsDiseaseInfection induced
The invention relates to the fields of immunology and molecular virology, in particular to the fields of diagnosis, prevention and treatment of novel coronaviruses. Specifically, the present inventionrelates to an anti-novel coronavirus monoclonal antibody, and a composition (e.g., a diagnostic agent and a therapeutic agent) comprising the antibody. Furthermore, the invention also relates to an application of the antibody. The antibody provided by the invention can be applied to diagnosis, prevention and / or treatment of infection of the novel coronavirus and / or diseases (e.g., novel coronavirus pneumonia) caused by the infection.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com