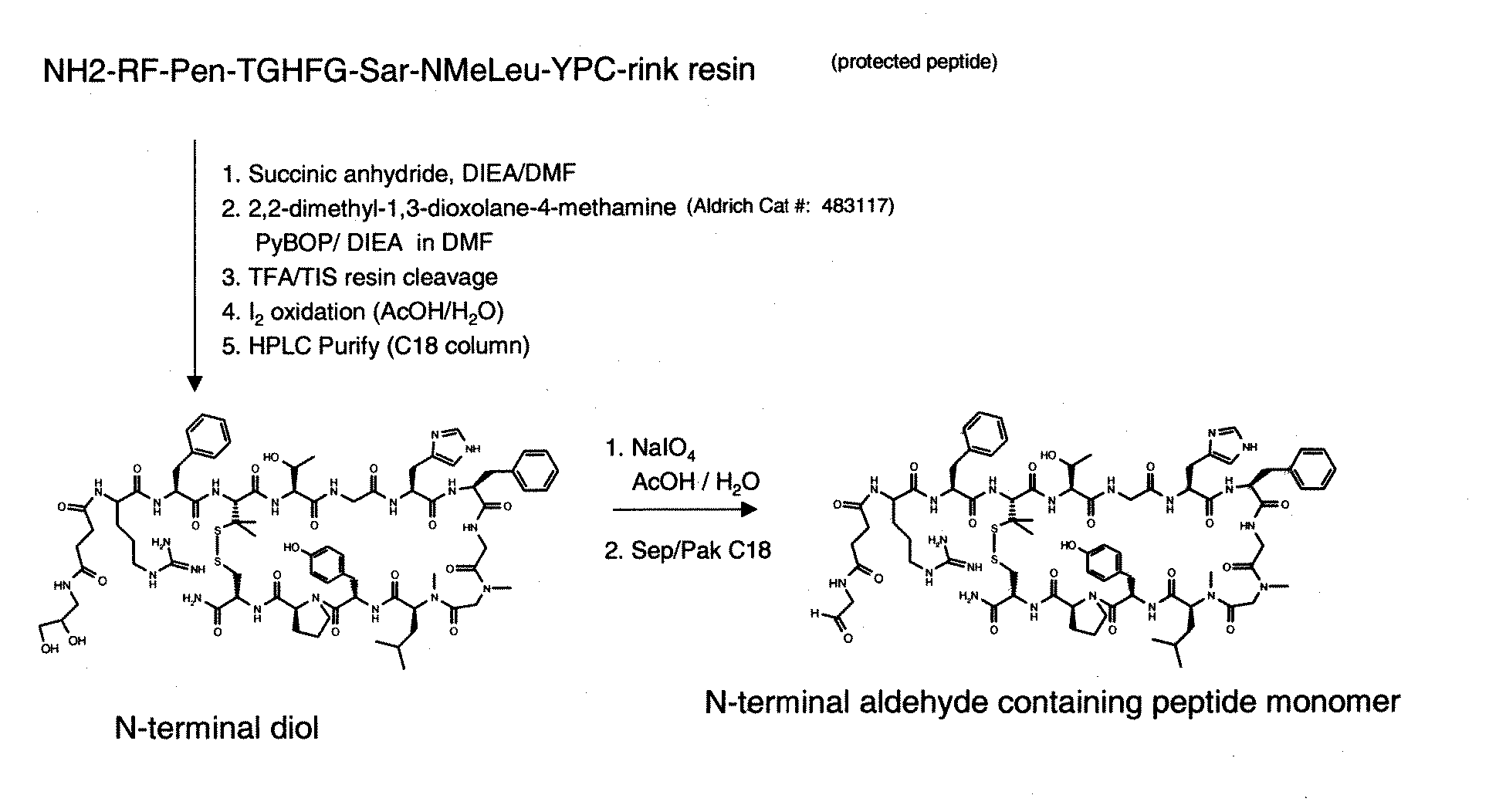
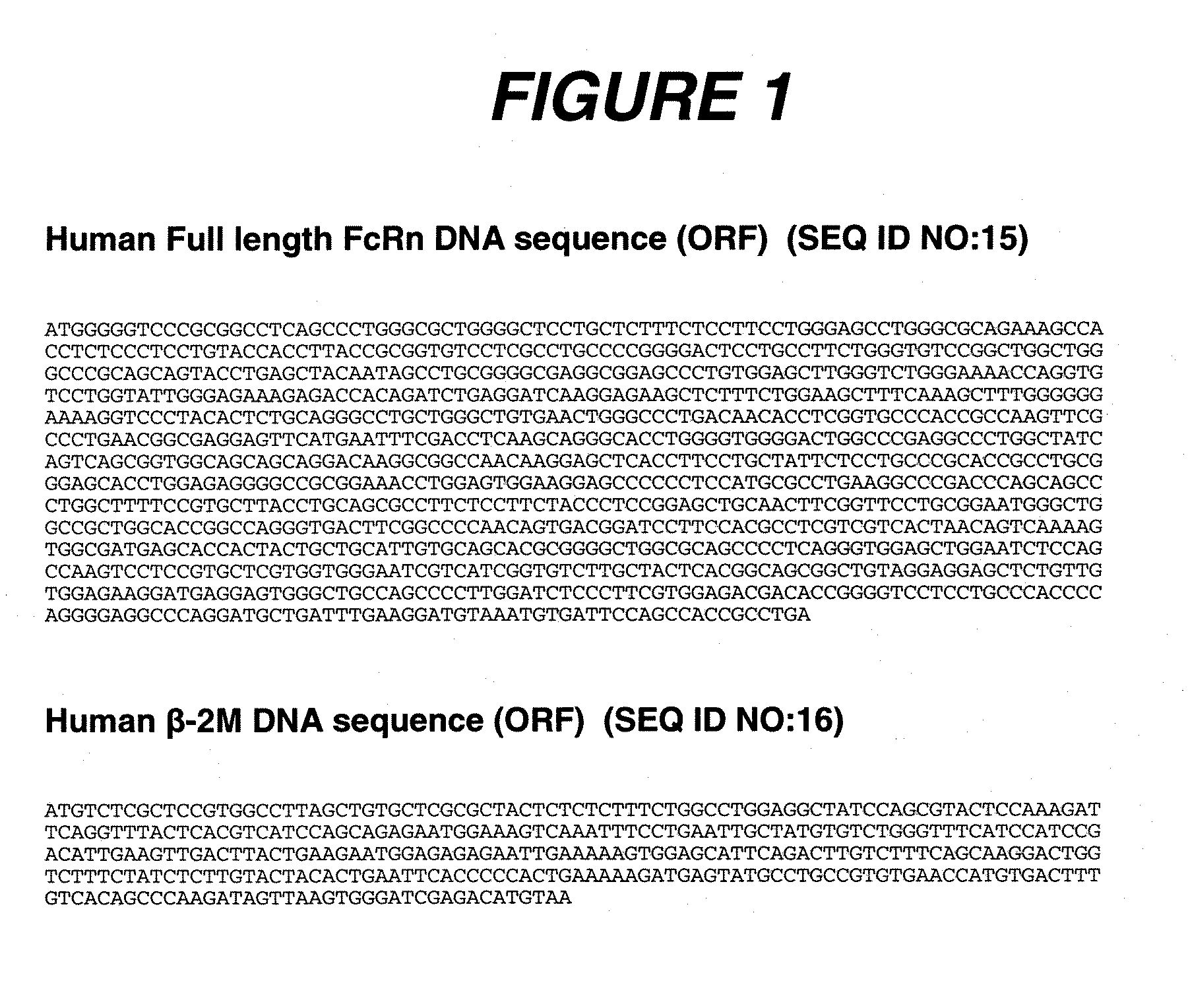
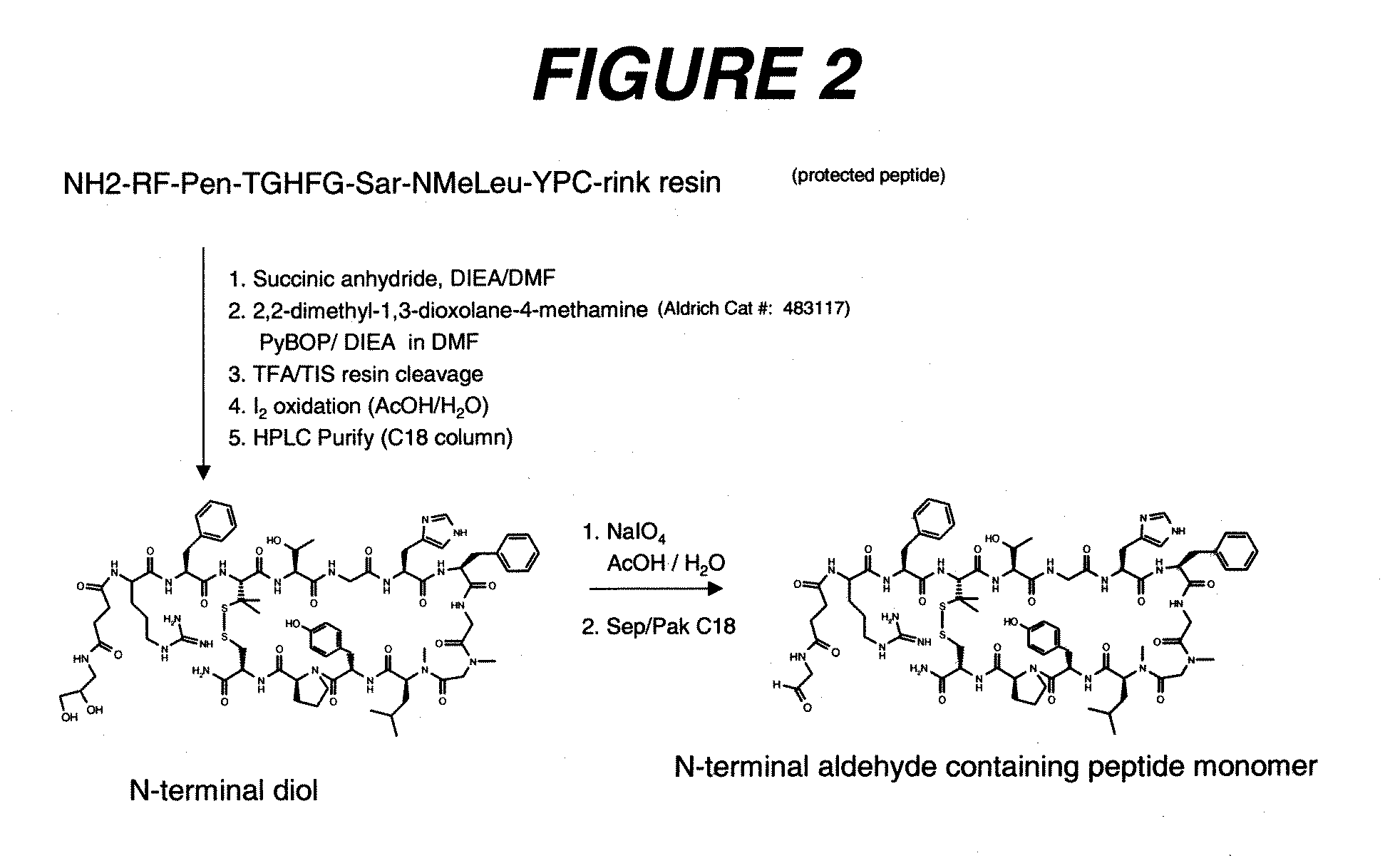
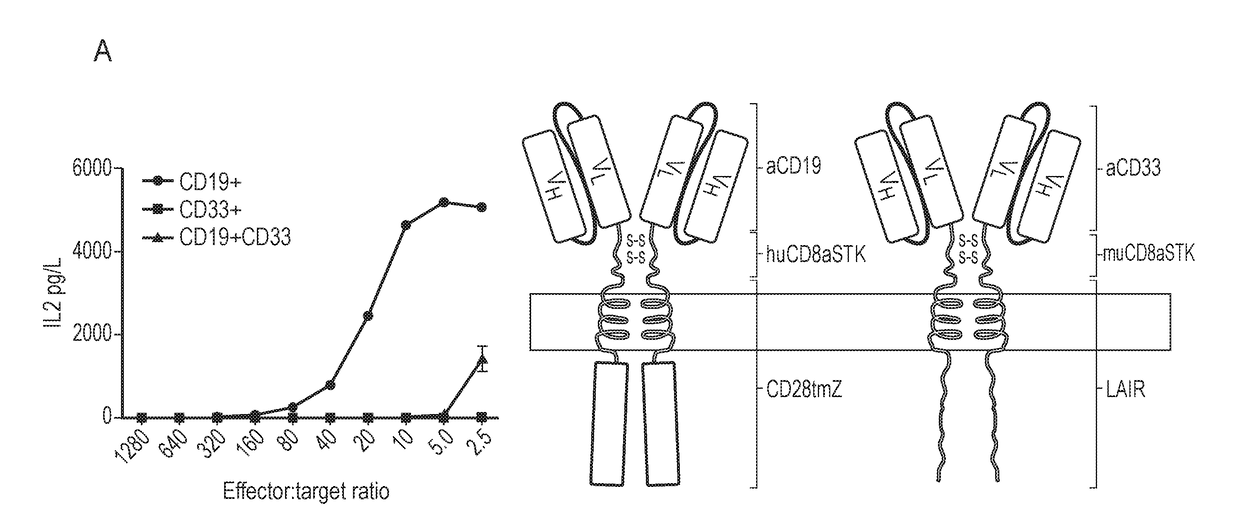
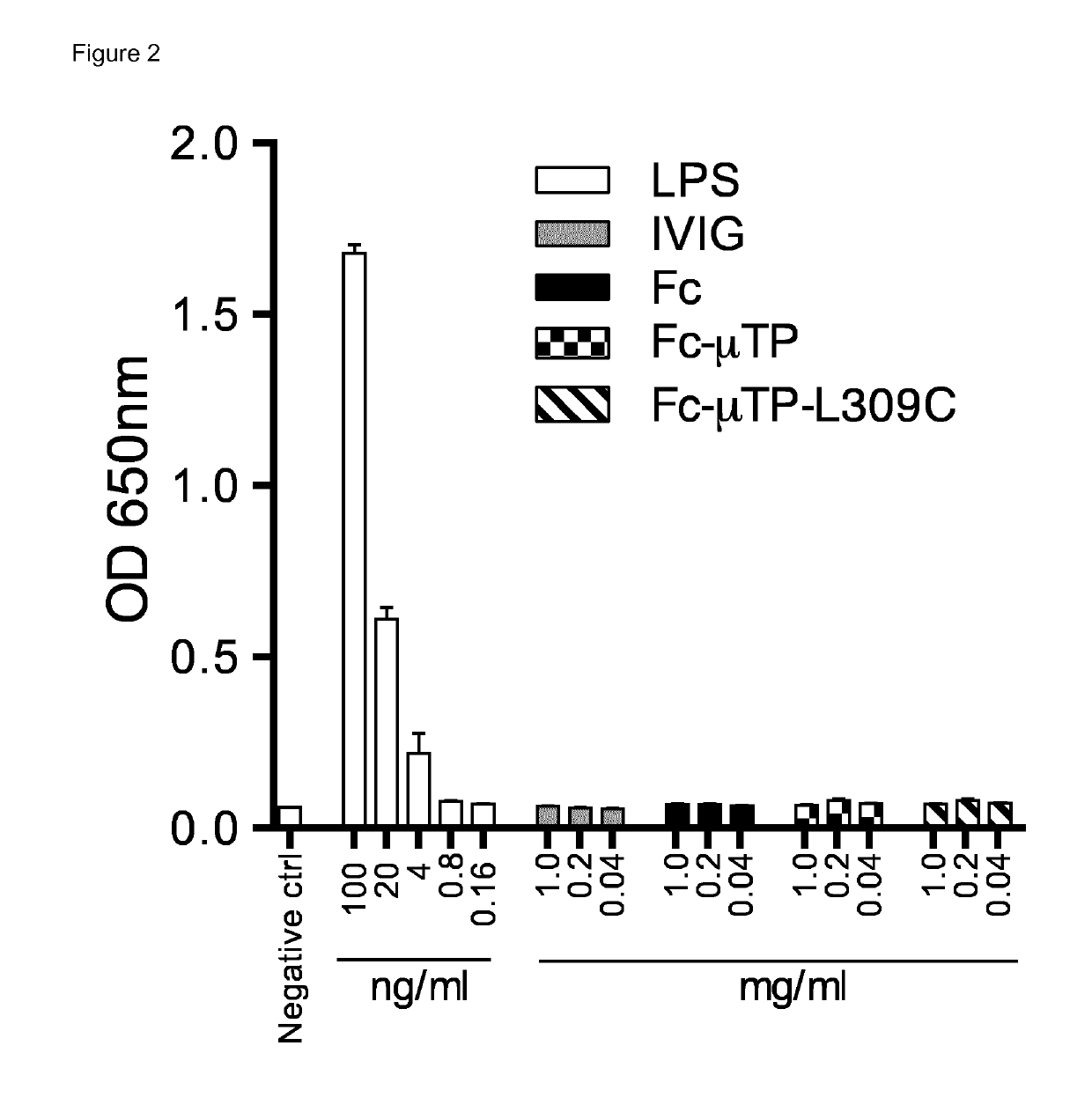
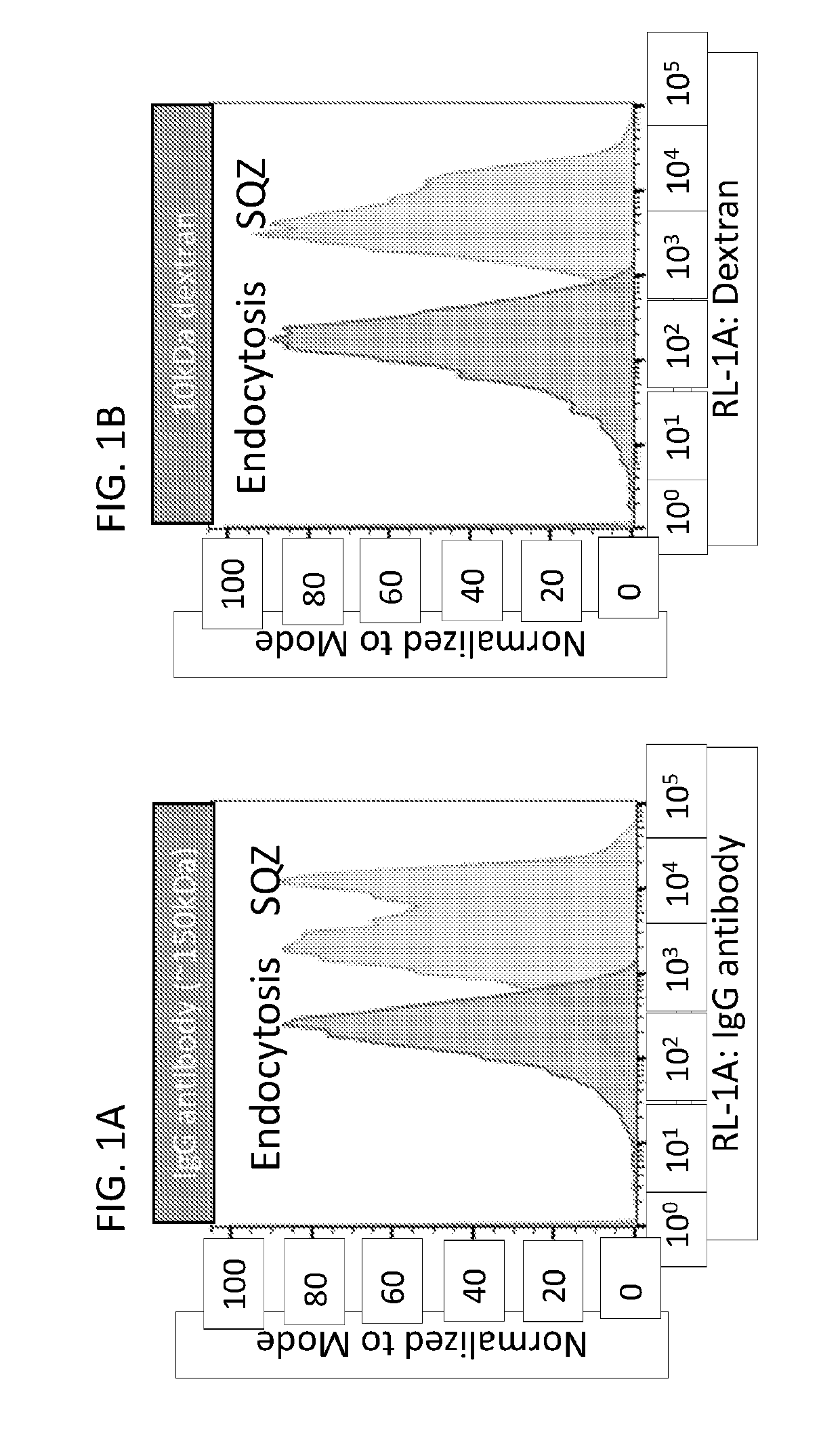
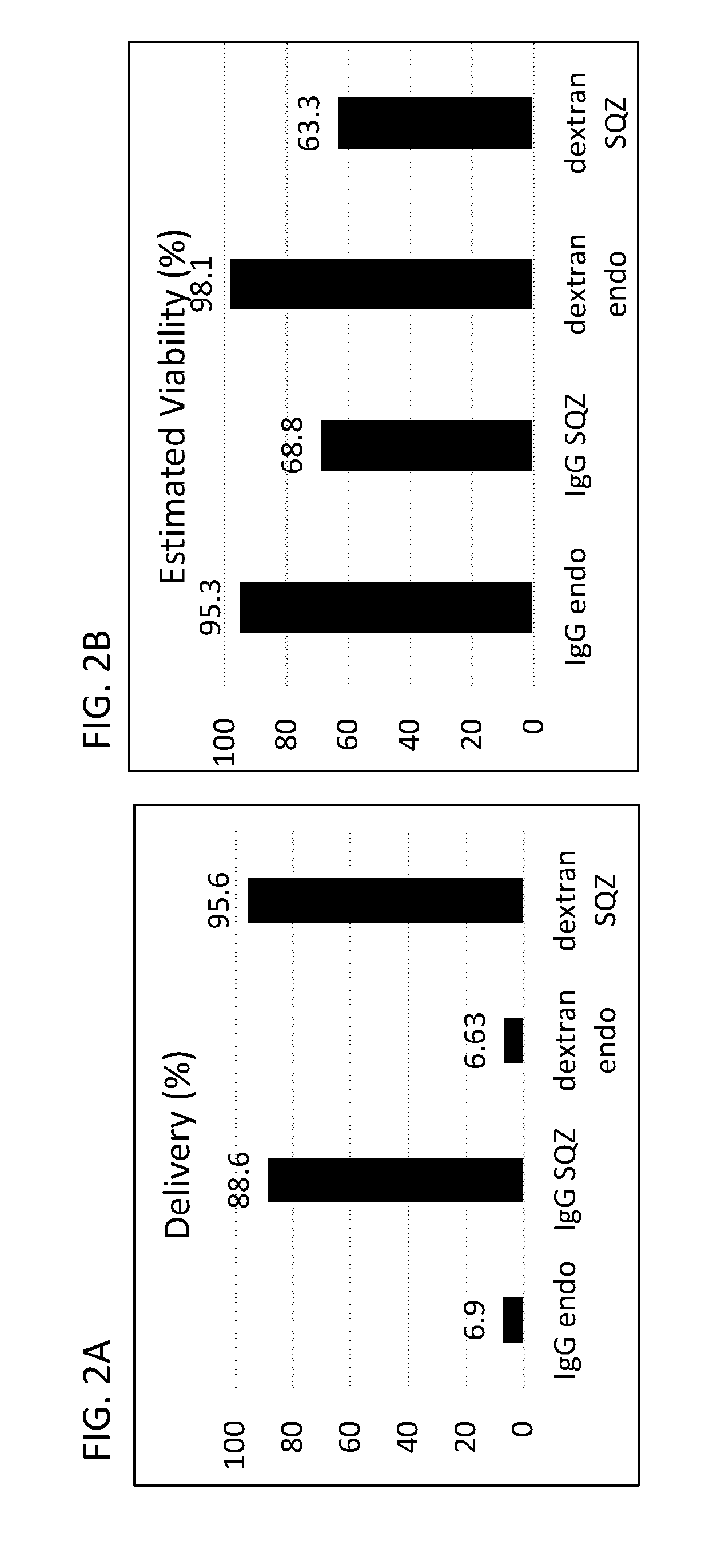
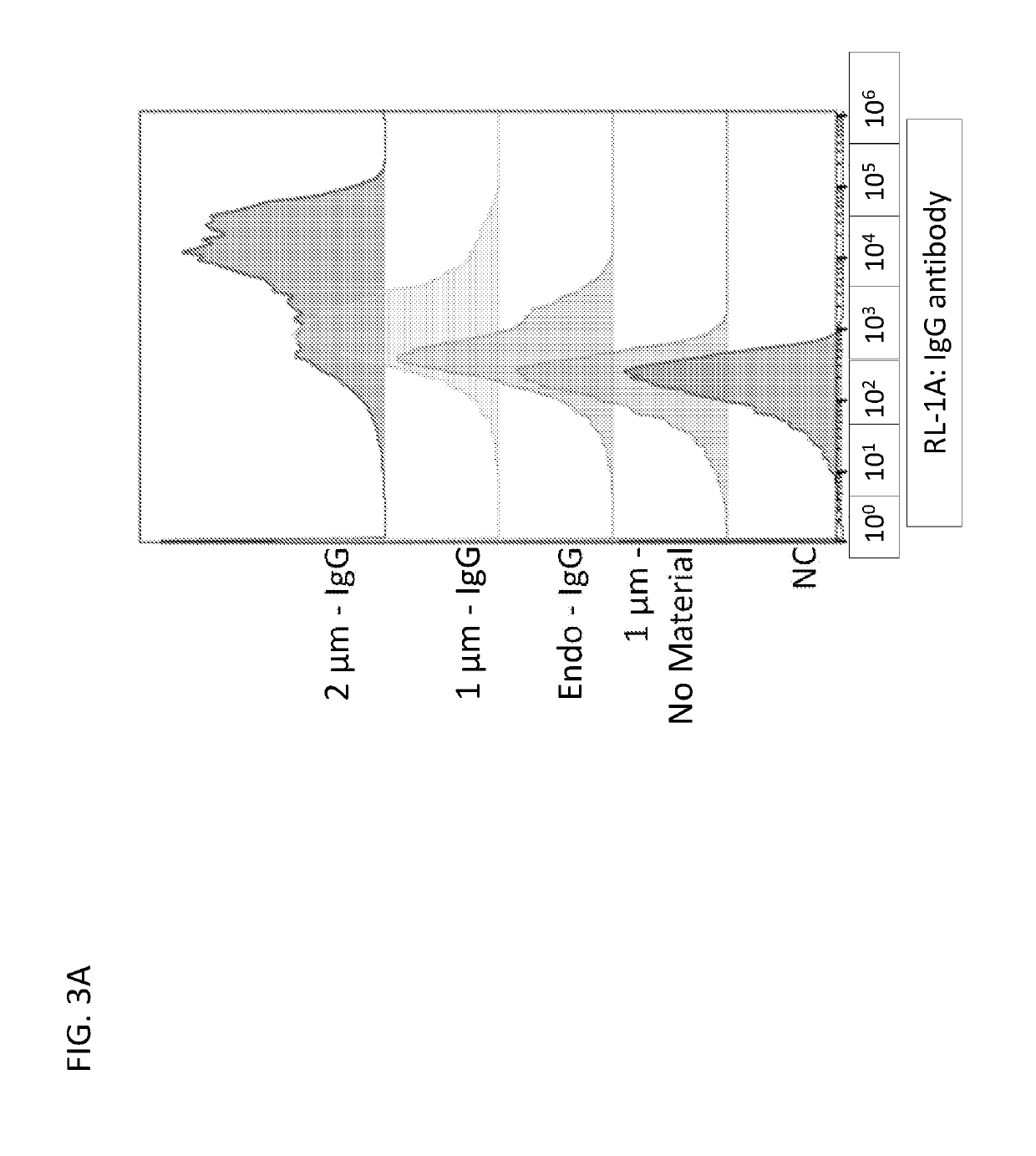
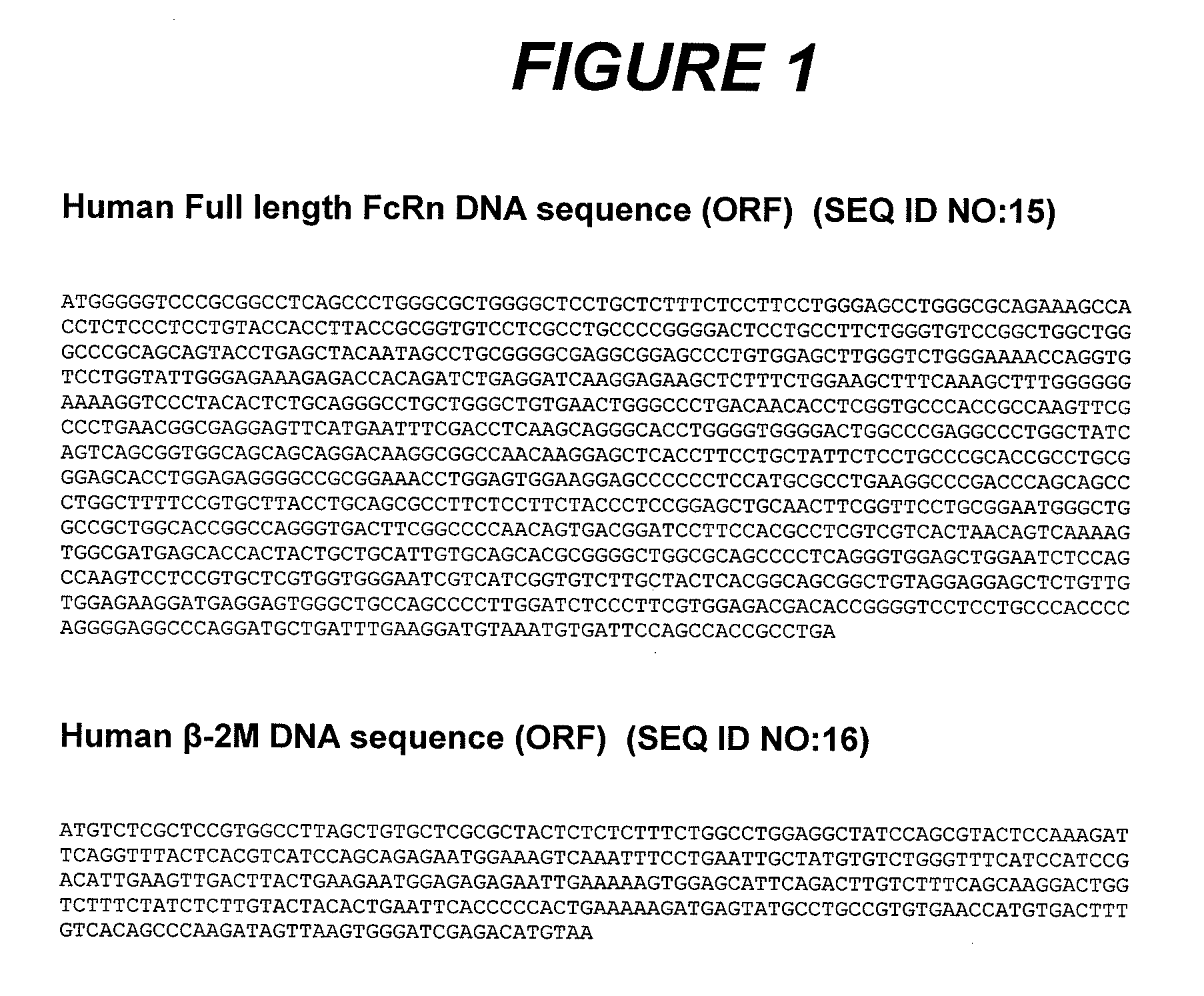
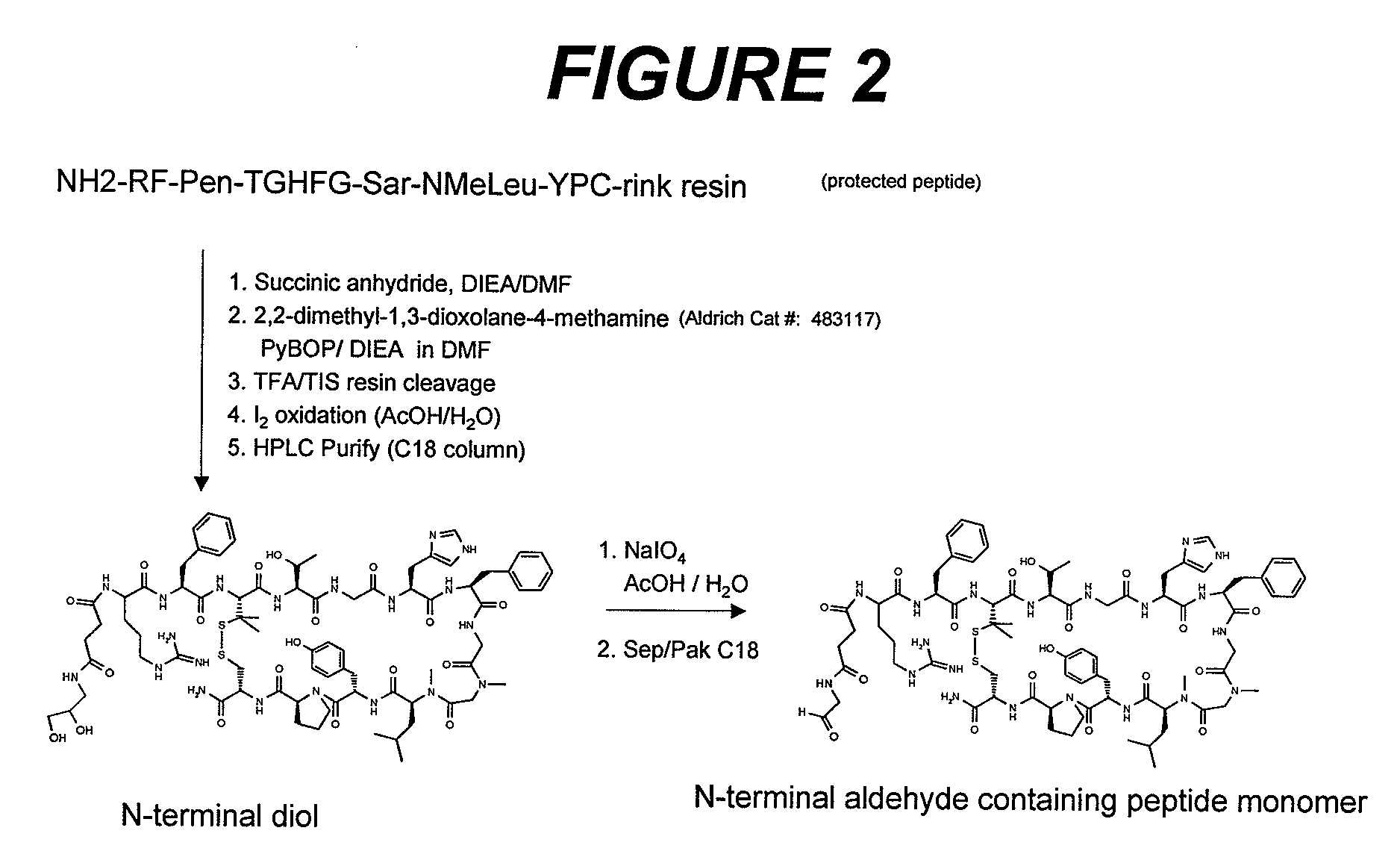
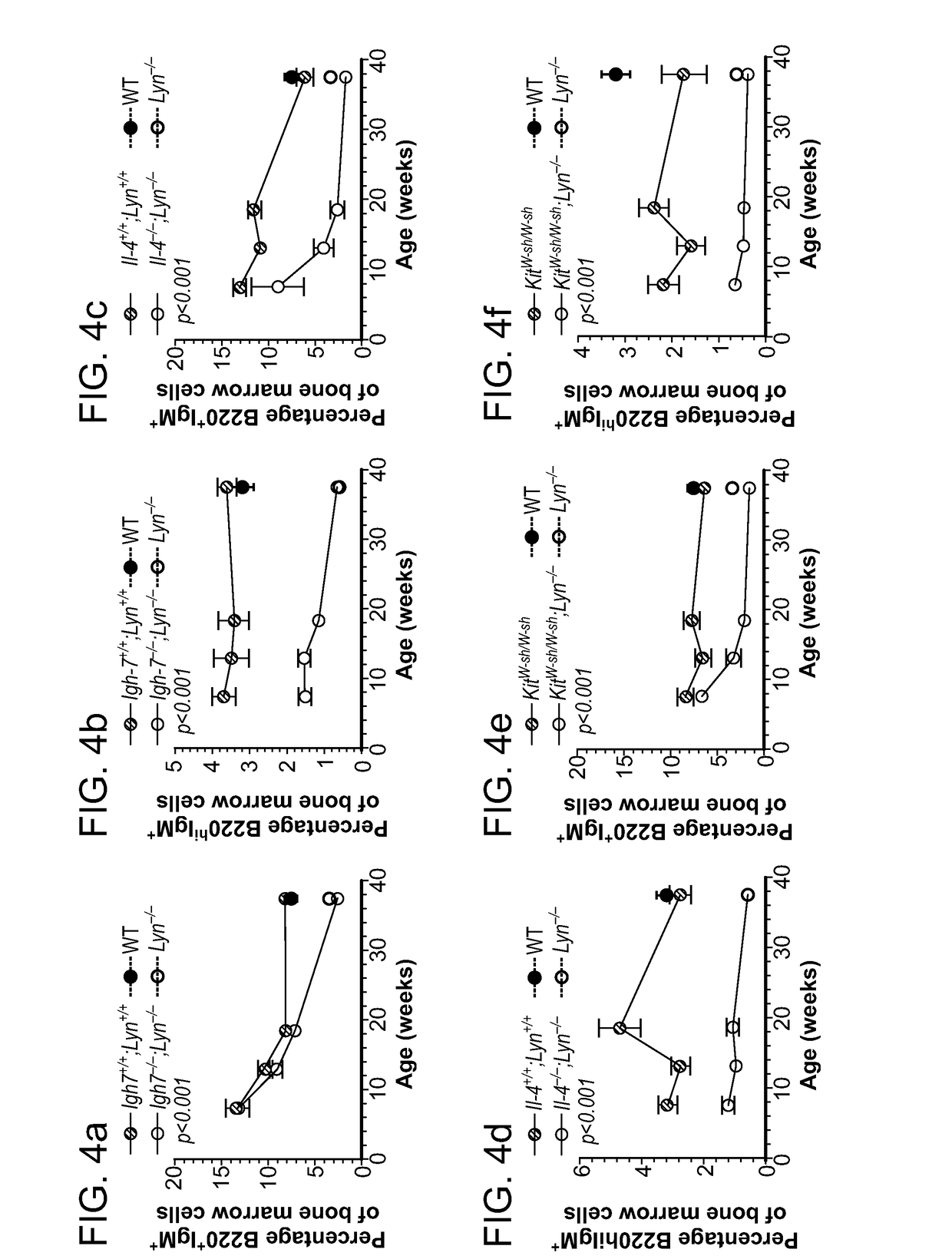
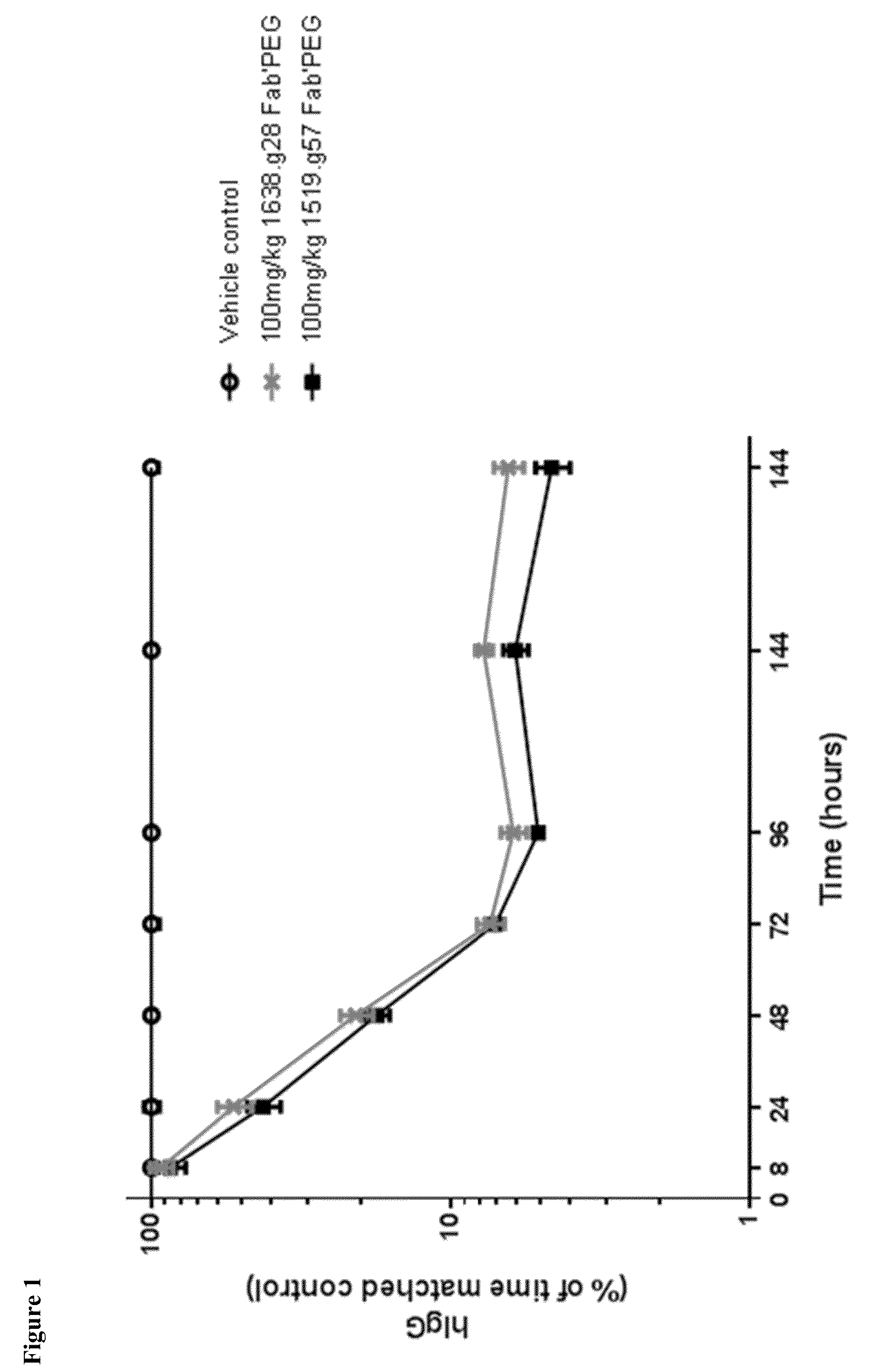
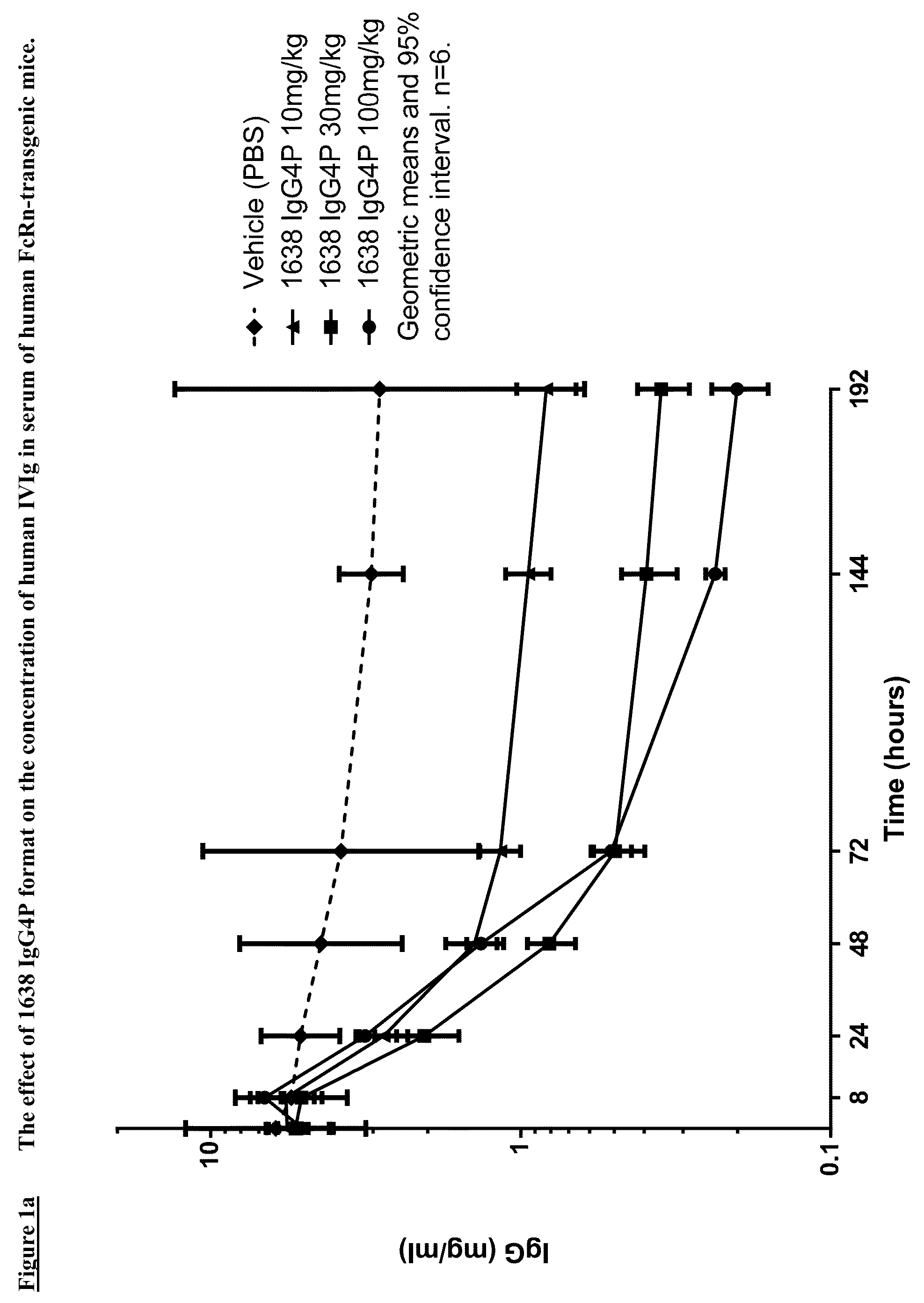
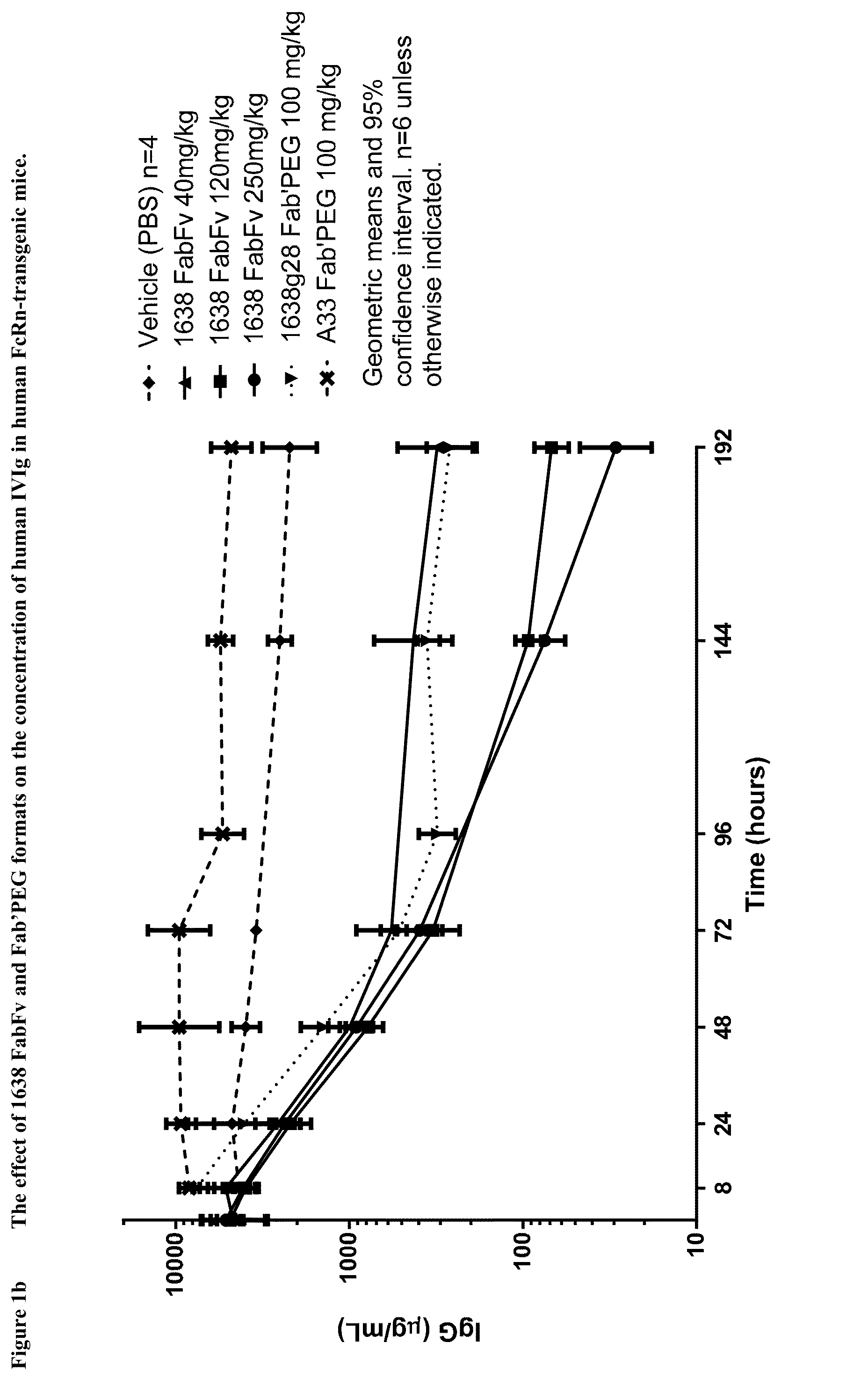
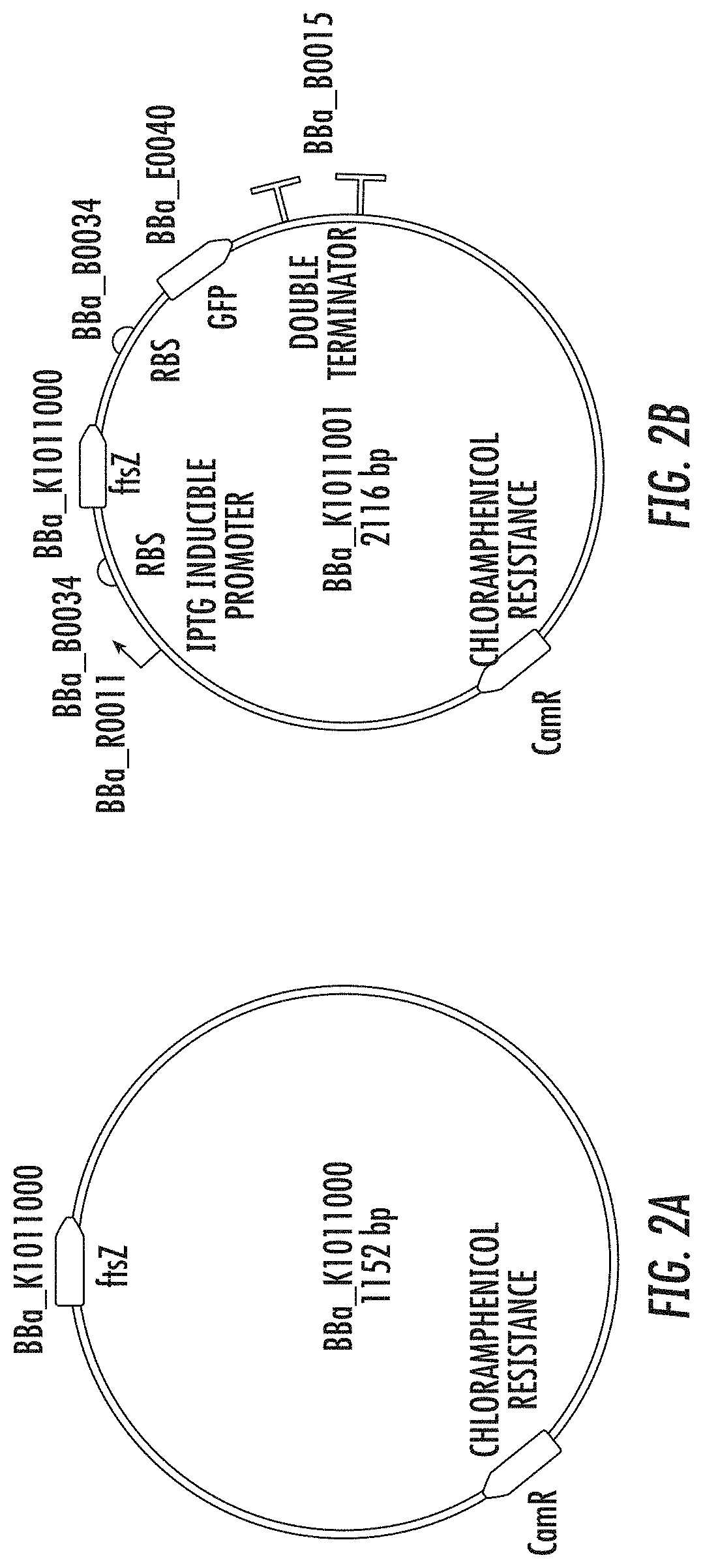
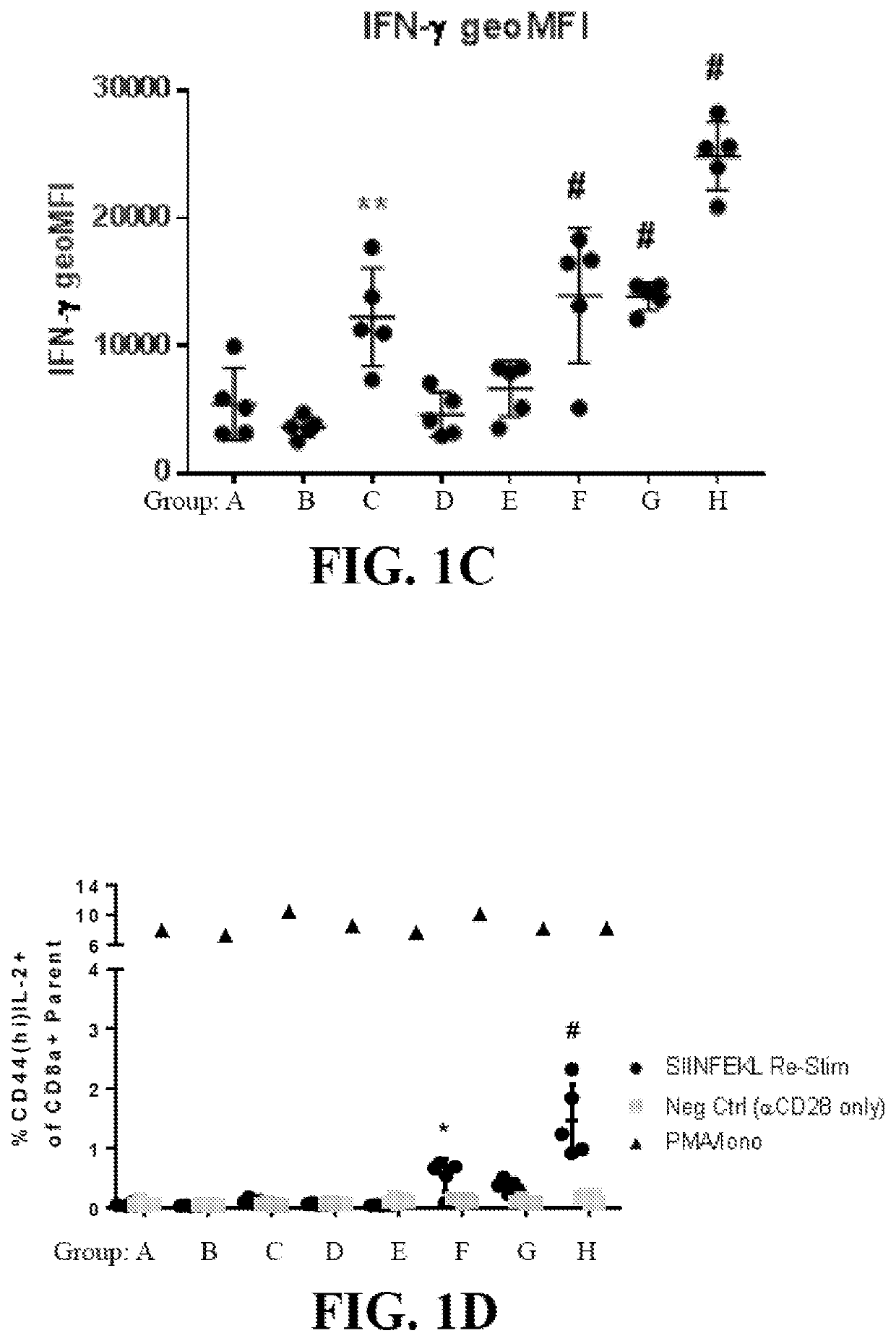
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57results about How to "Reduced half-life" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
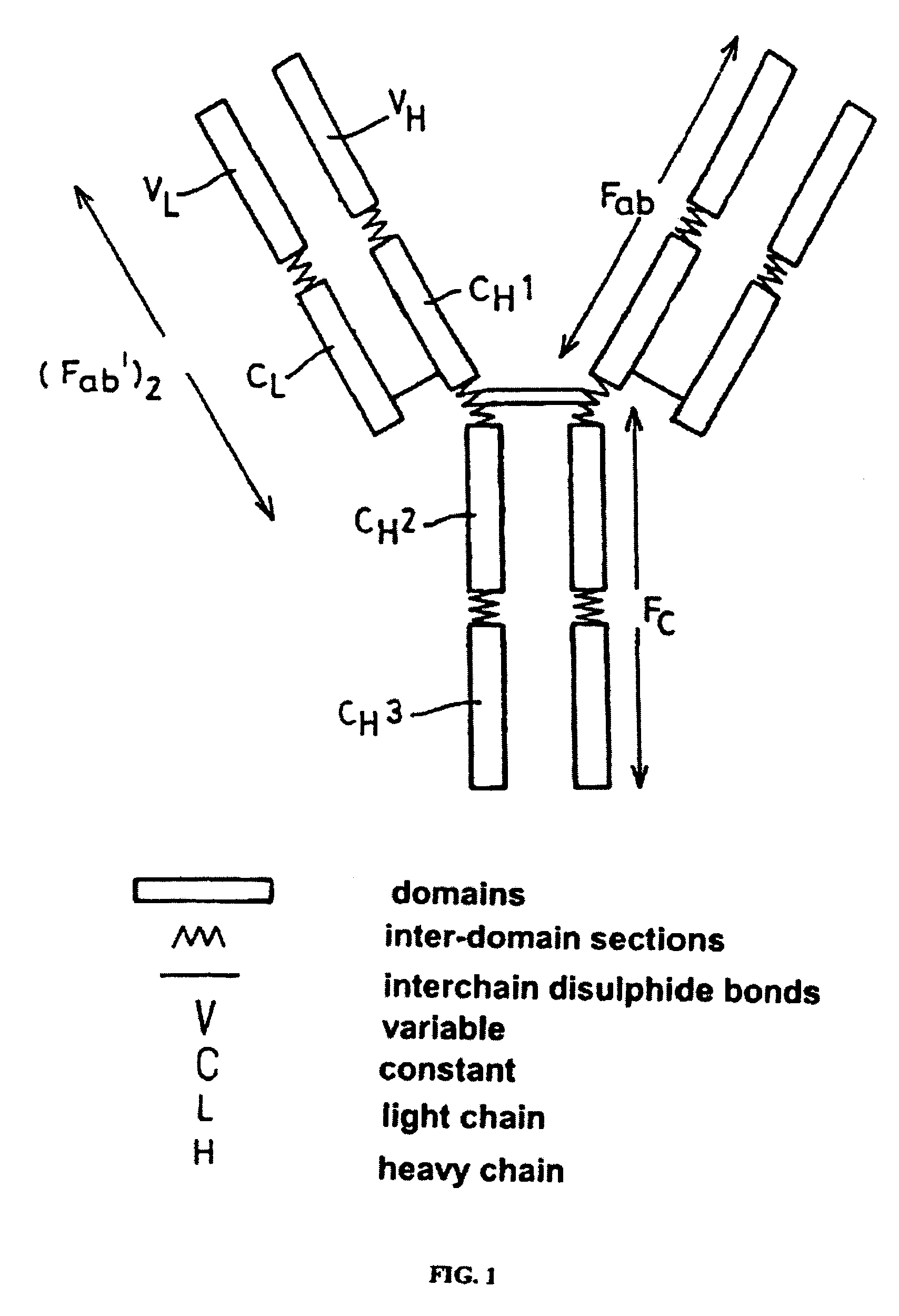
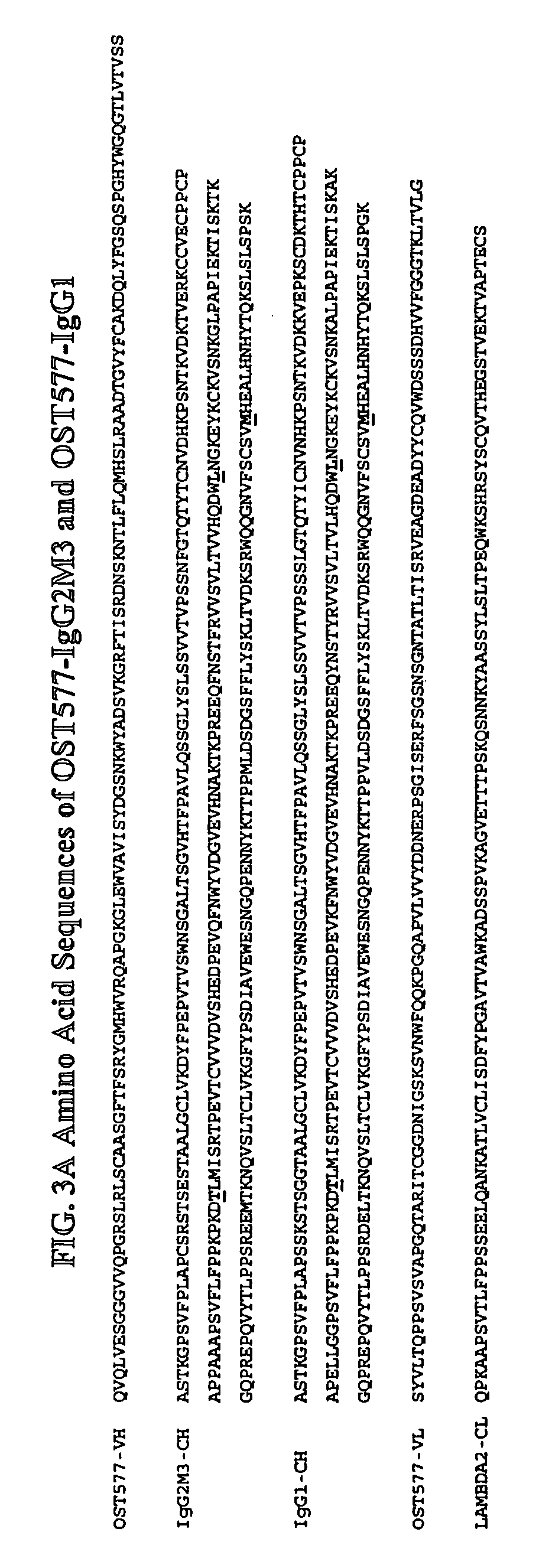
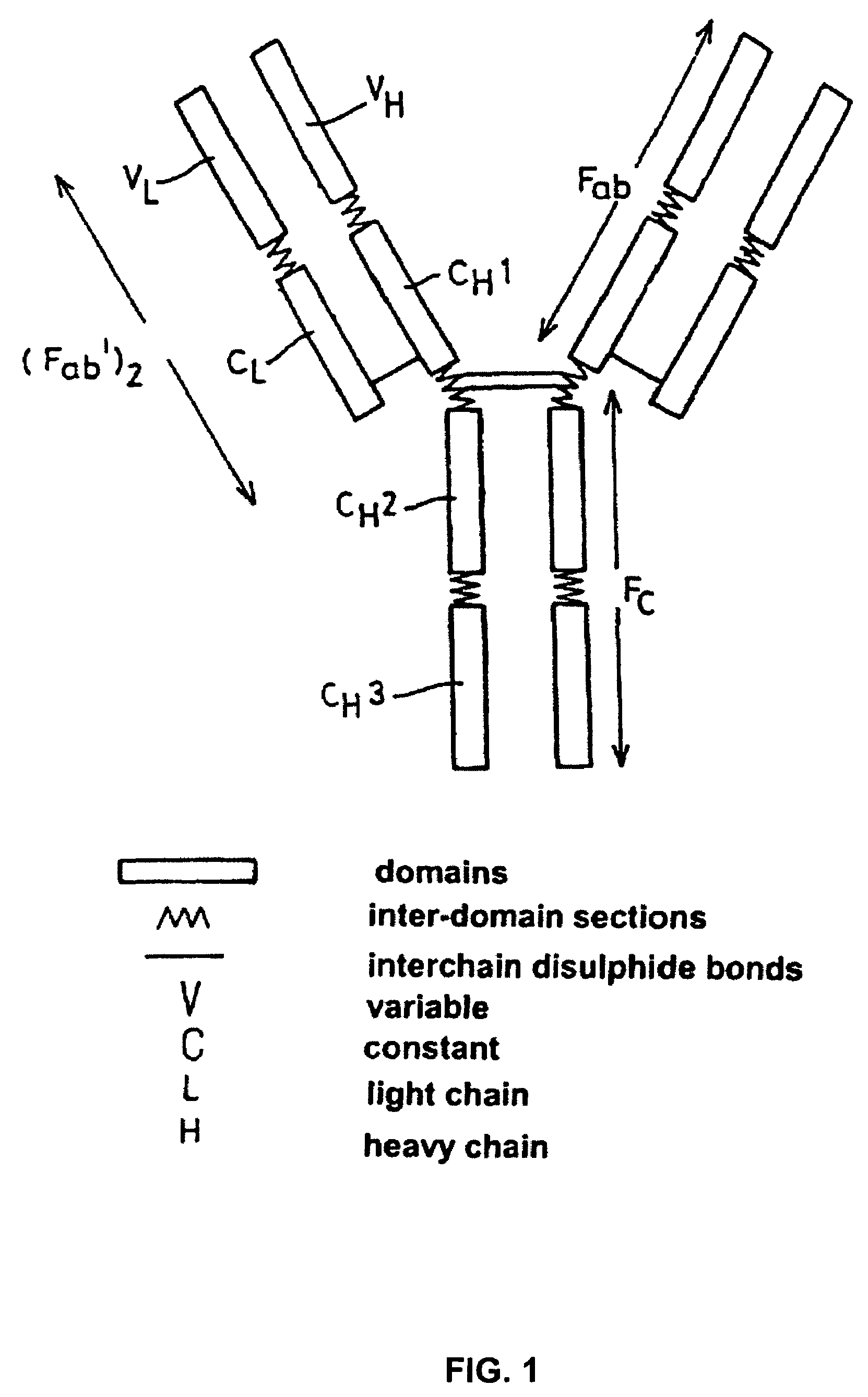
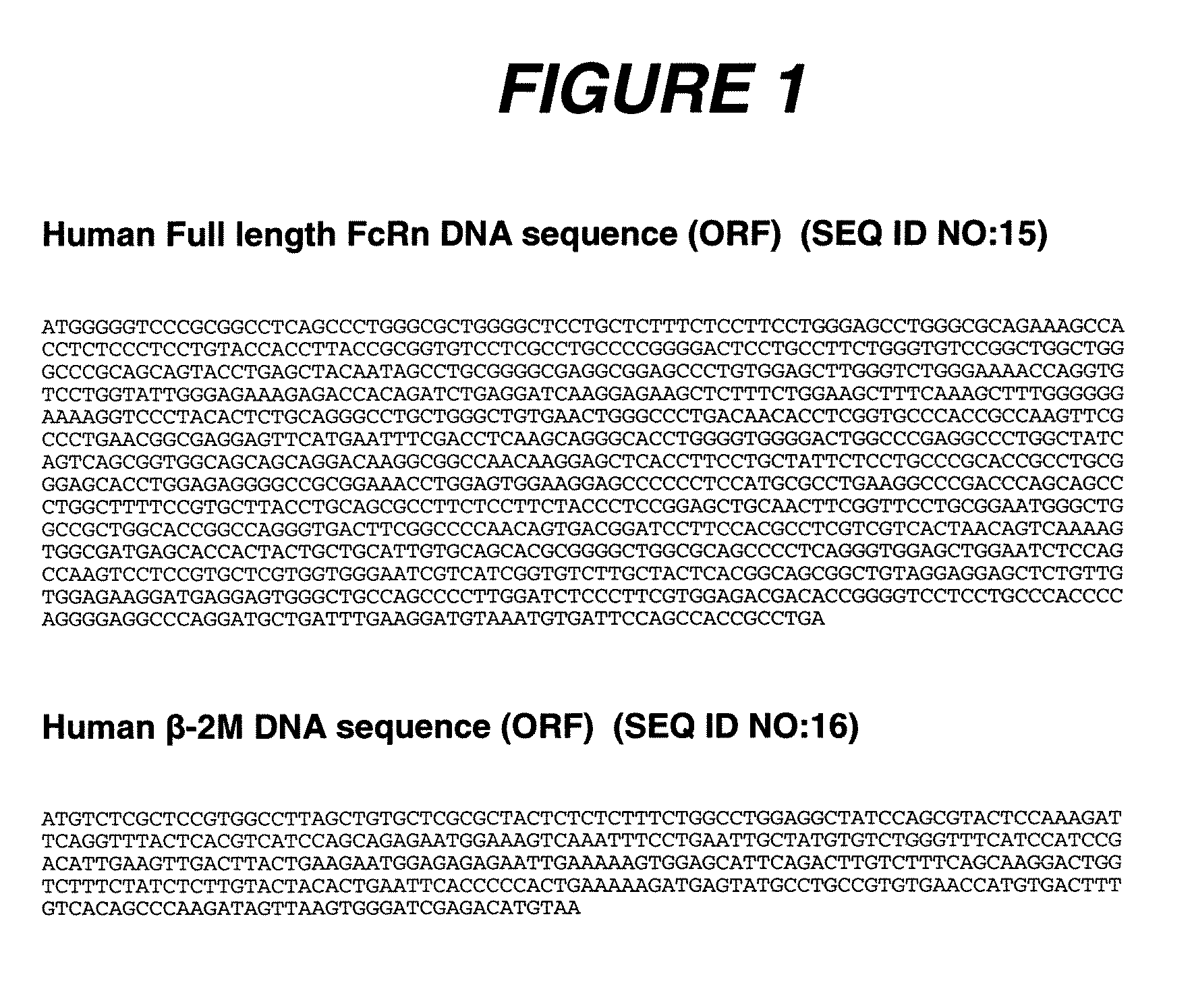
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
InactiveUS7361740B2Function increaseExtended half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesSerum igeHalf-life
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
InactiveUS7365168B2Function increaseExtended half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesSerum igeHalf-life
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
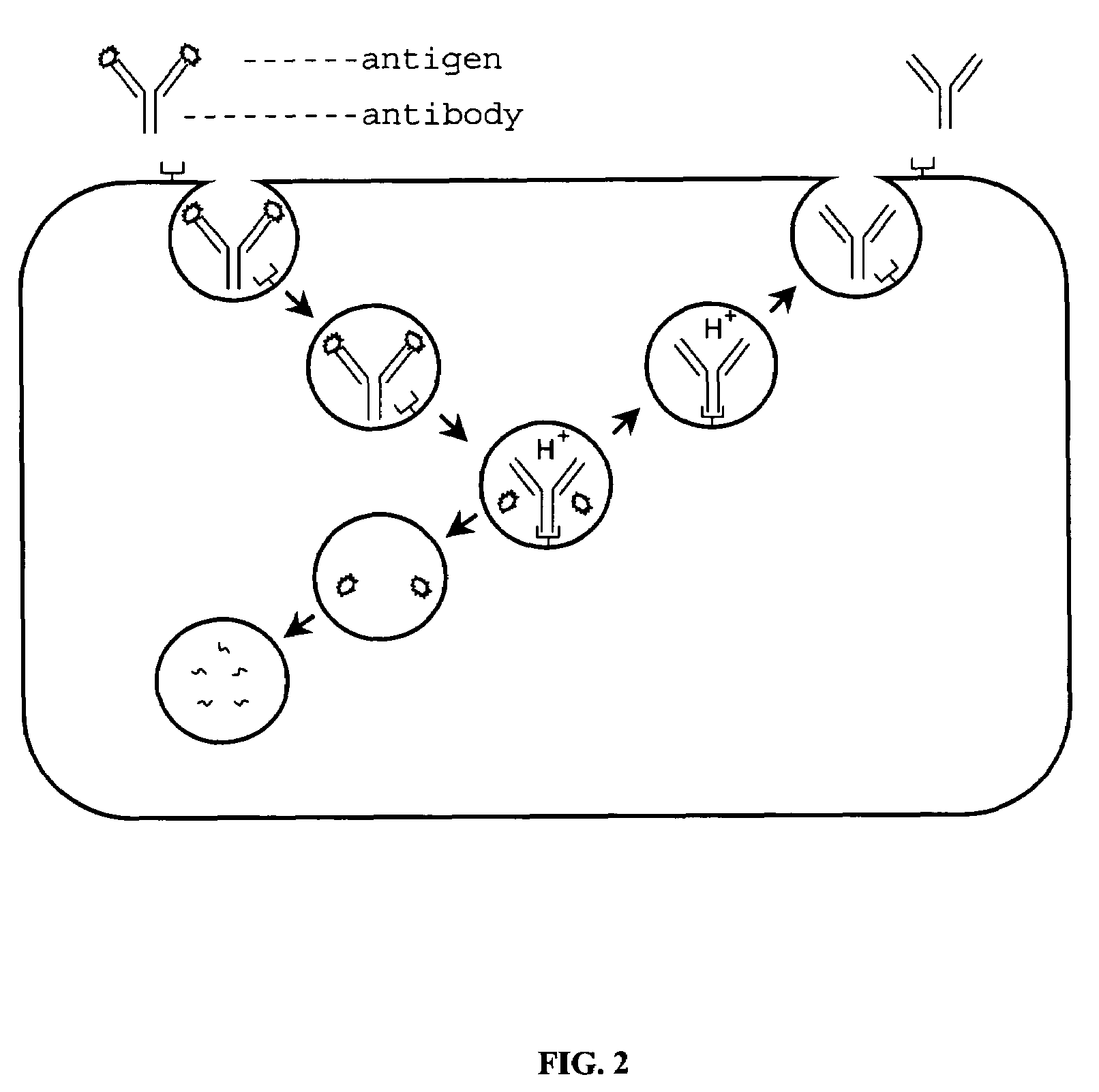
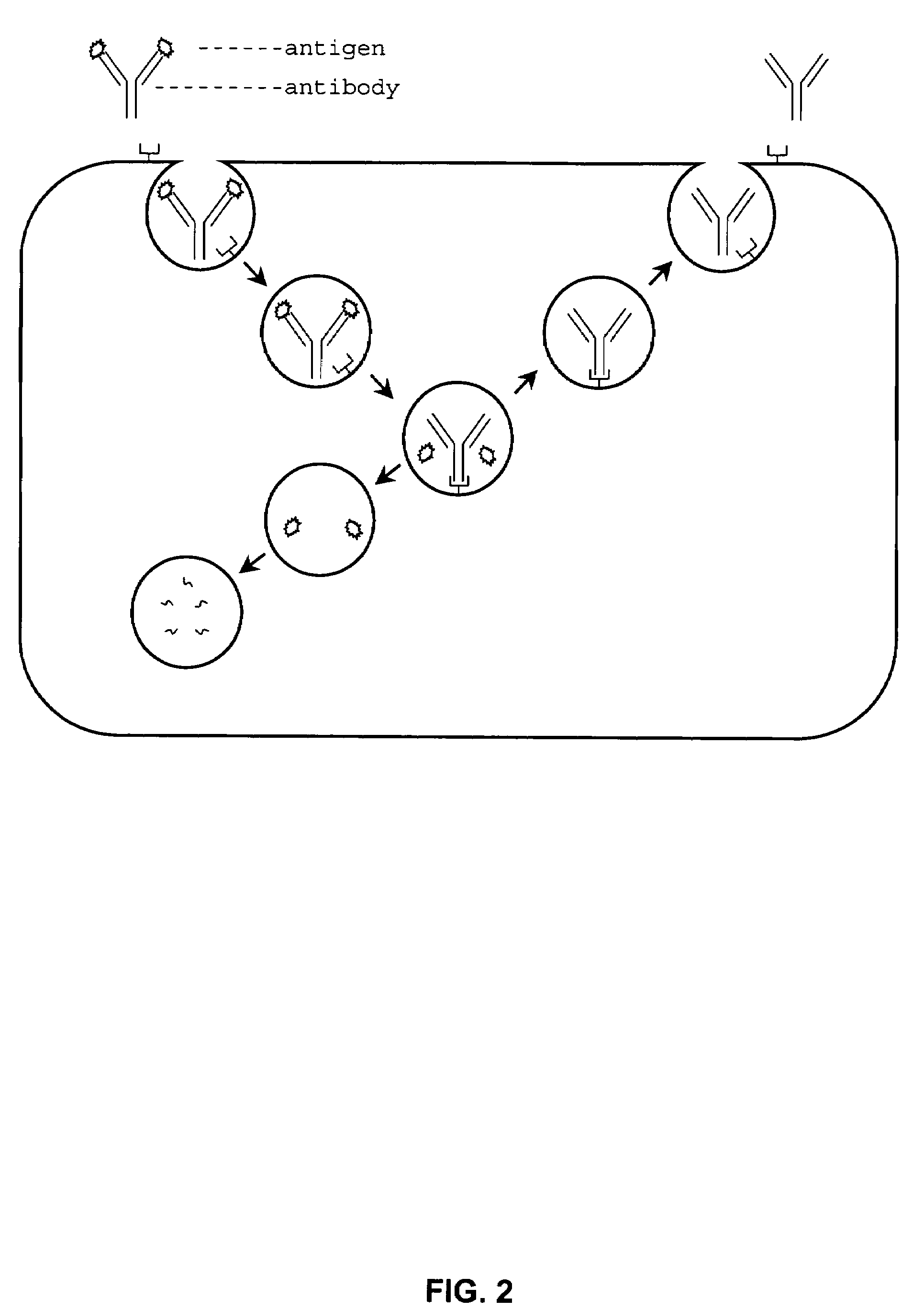
Peptides that block the binding of IgG to FcRn
InactiveUS8101186B2Reduced half-lifePeptide/protein ingredientsAntipyreticMedicineAutoimmune disease
Owner:BIOGEN HEMOPHILIA
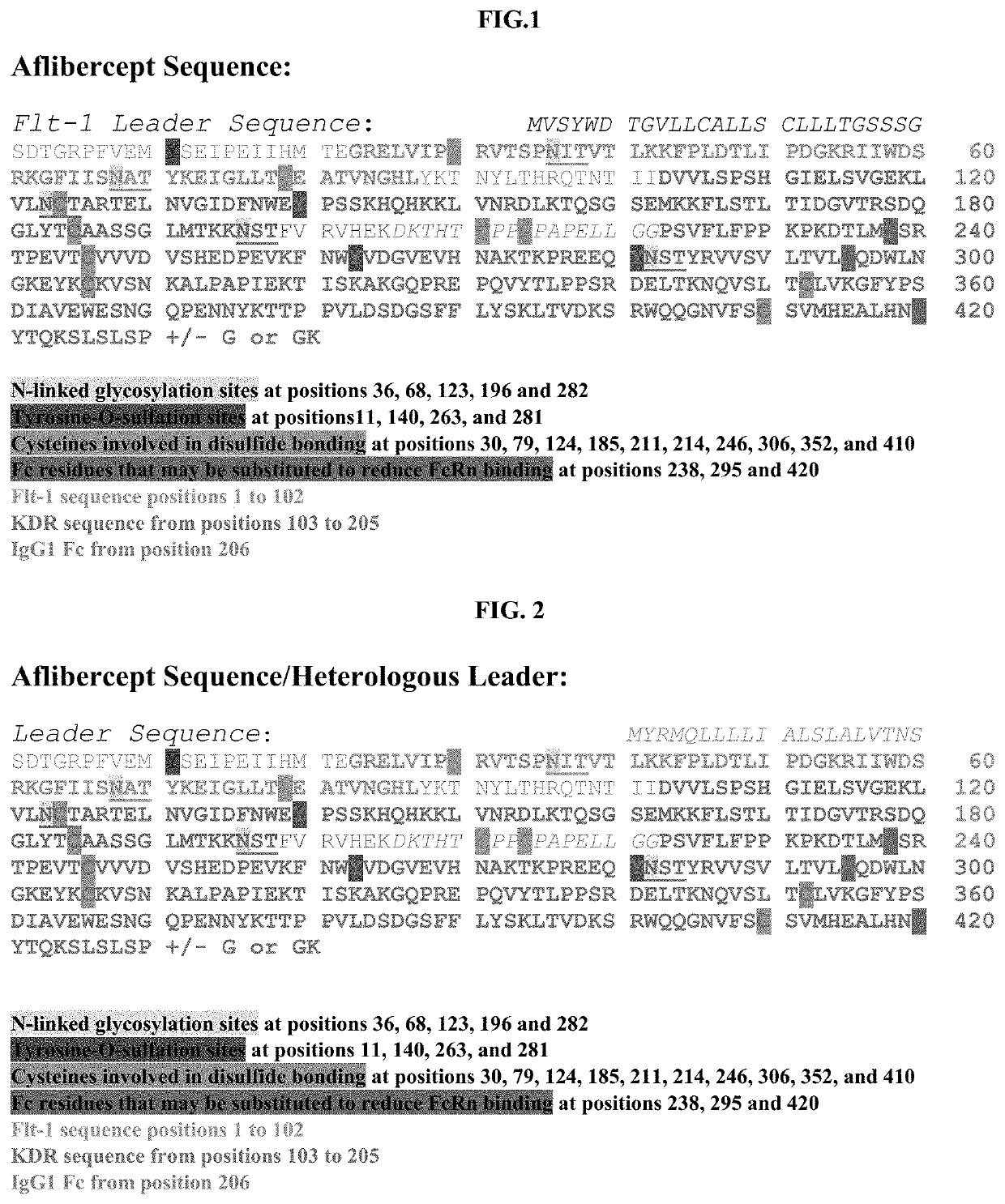
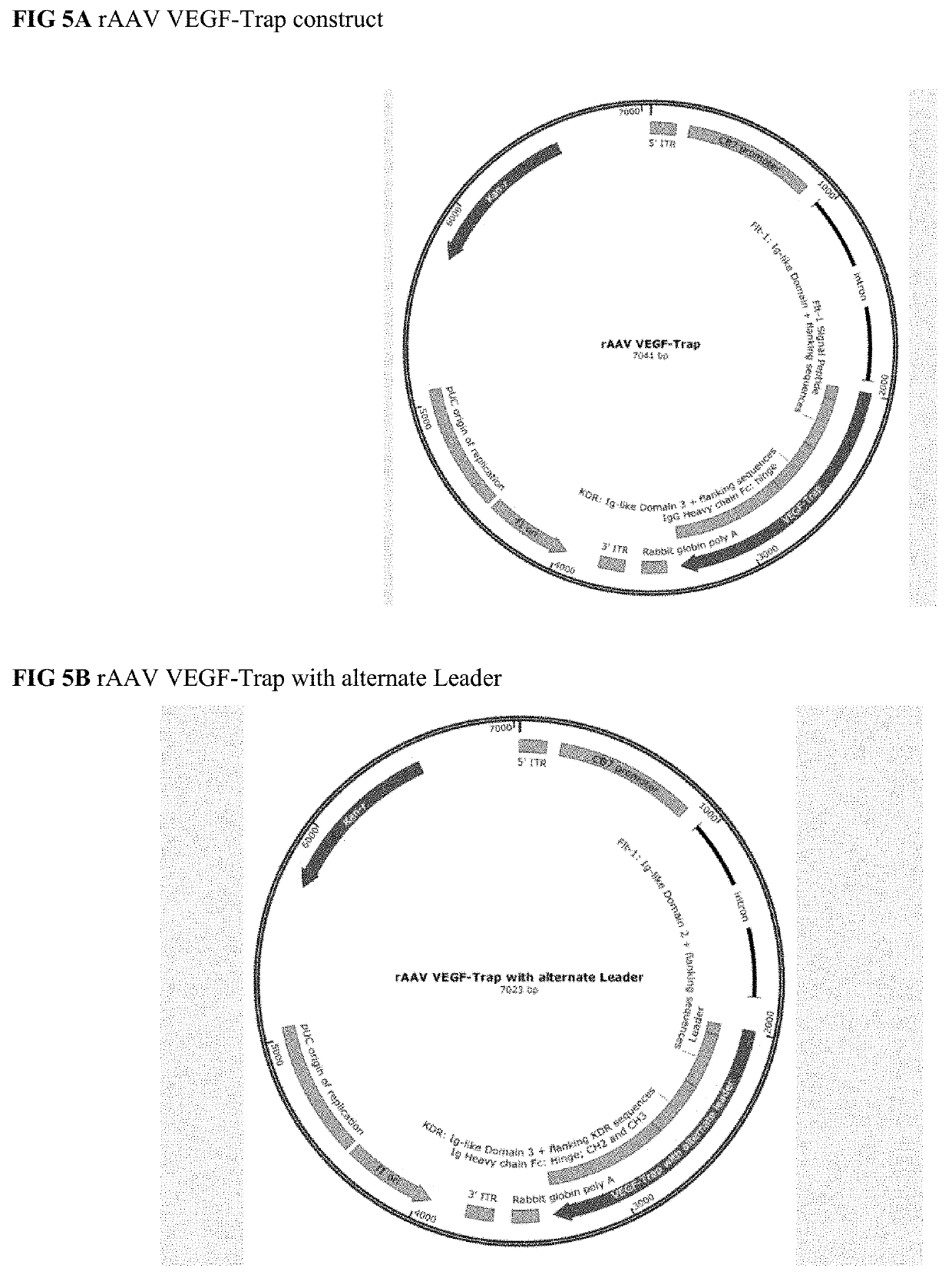
Treatment of ocular diseases with human post-translationally modified vegf-trap
PendingUS20210010025A1Maintain stabilityReduced half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseOncology
Compositions and methods are described for the delivery of a fully human post-translationally modified (HuPTM) therapeutic VEGF-Trap (VEGF-TrapHuPTM)—to a human subject diagnosed with an ocular disease or condition or cancer associated with neovascularization and indicated for treatment with the therapeutic mAb. Delivery may be advantageously accomplished via gene therapy—e.g., by administering a viral vector or other DNA expression construct encoding the VEGF-TrapHuPTM to a patient (human subject) diagnosed with an ocular condition or cancer indicated for treatment with the VEGF-Trap—to create a permanent depot in a tissue or organ of the patient that continuously supplies the VEGF-TrapHuPTM, i.e., a human-glycosylated transgene product. Alternatively, the VEGF-TrapHuPTM, for example, produced in cultured human cell culture, can be administered to the patient for treatment of the ocular disease or cancer.
Owner:REGENXBIO
Therapy for kidney disease and/or heart failure by intradermal infusion
InactiveUS20150080844A1Improve bioavailabilityReduced half-lifePeptide/protein ingredientsMicroneedlesNephrosisNephropathy
Intradermal delivery devices, systems and methods thereof for the administration of a natriuretic or chimeric peptide are described. The described delivery devices, systems and methods provide for the treatment of pathological conditions such as kidney disease alone, heart failure alone, concomitant kidney disease and heart failure, or cardiorenal syndrome by delivery of a natriuretic or chimeric peptide through a microneedle array using a delivery pump. The described delivery devices, systems and methods can provide for greater availability of a natriuretic or chimeric peptide and improved pharmacokinetics.
Owner:CAPRICOR THERAPEUTICS
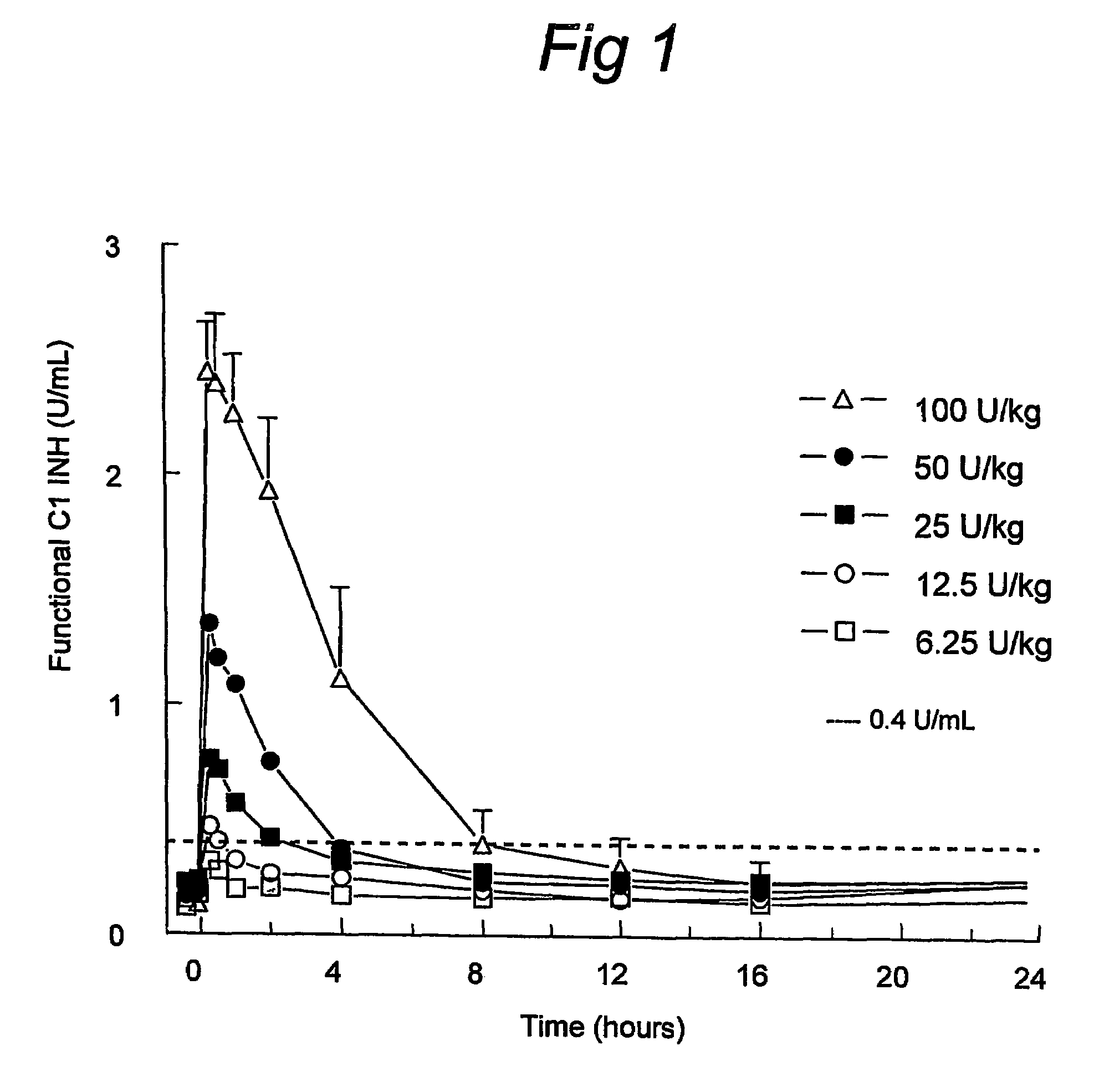
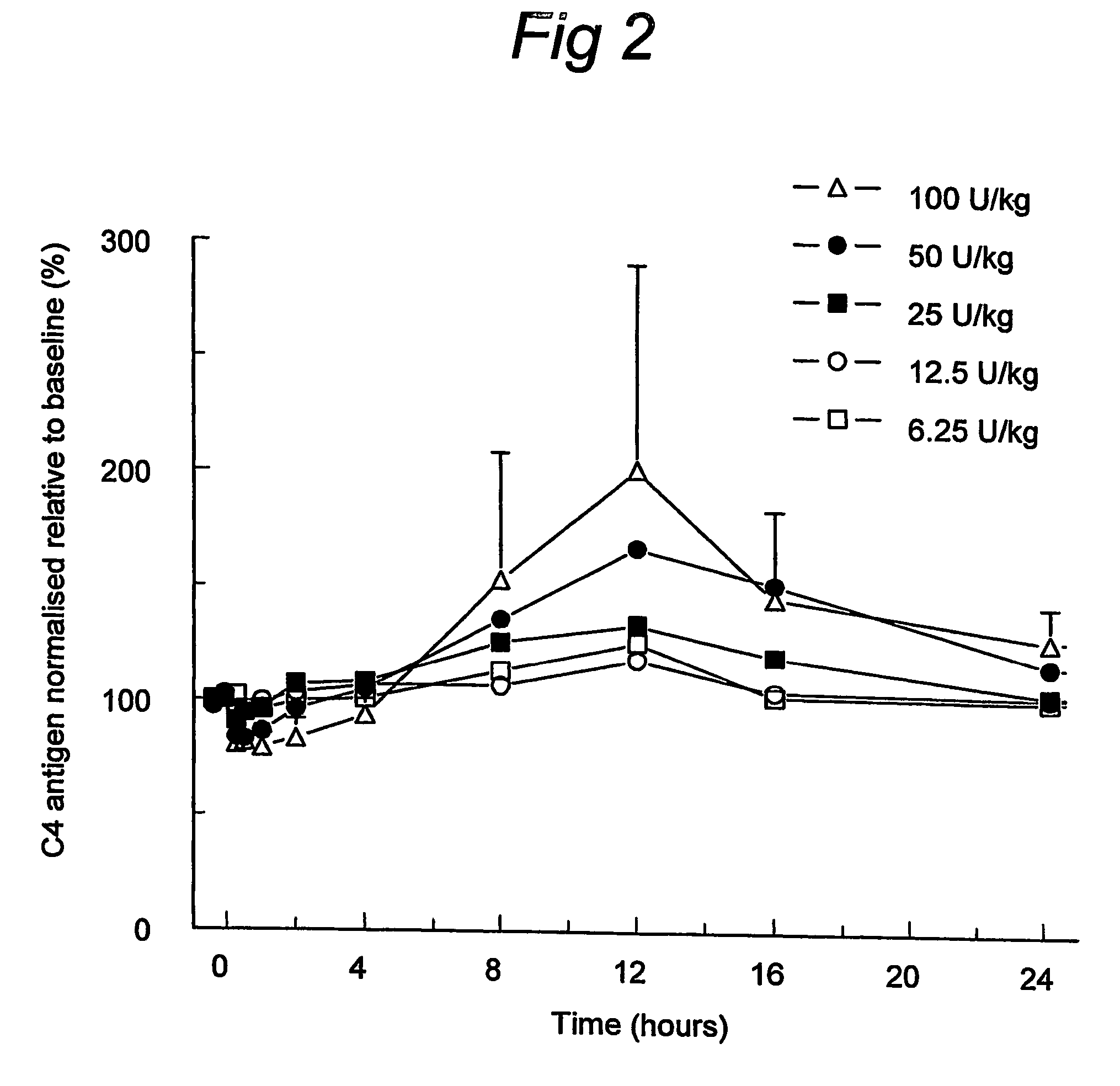
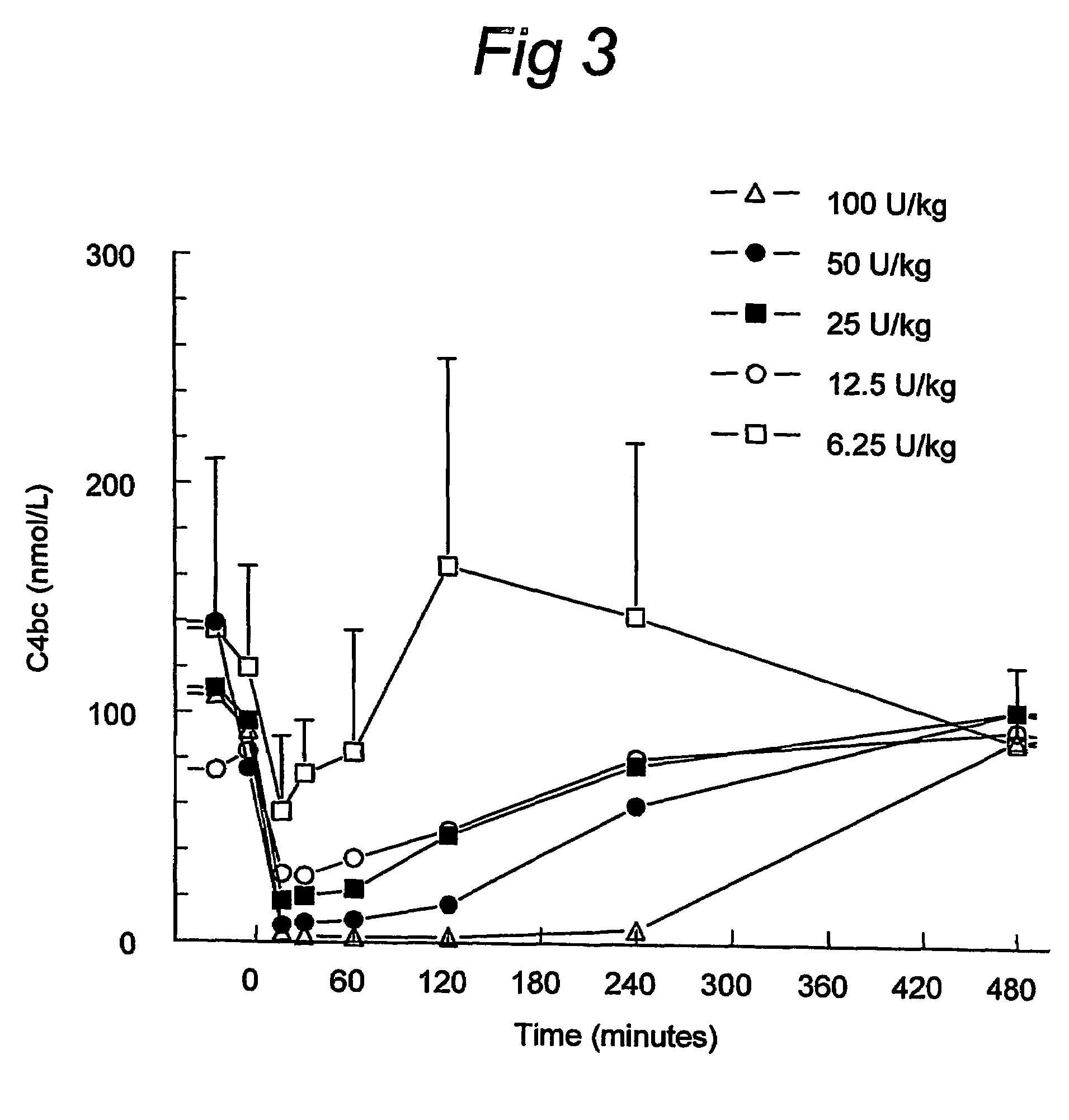
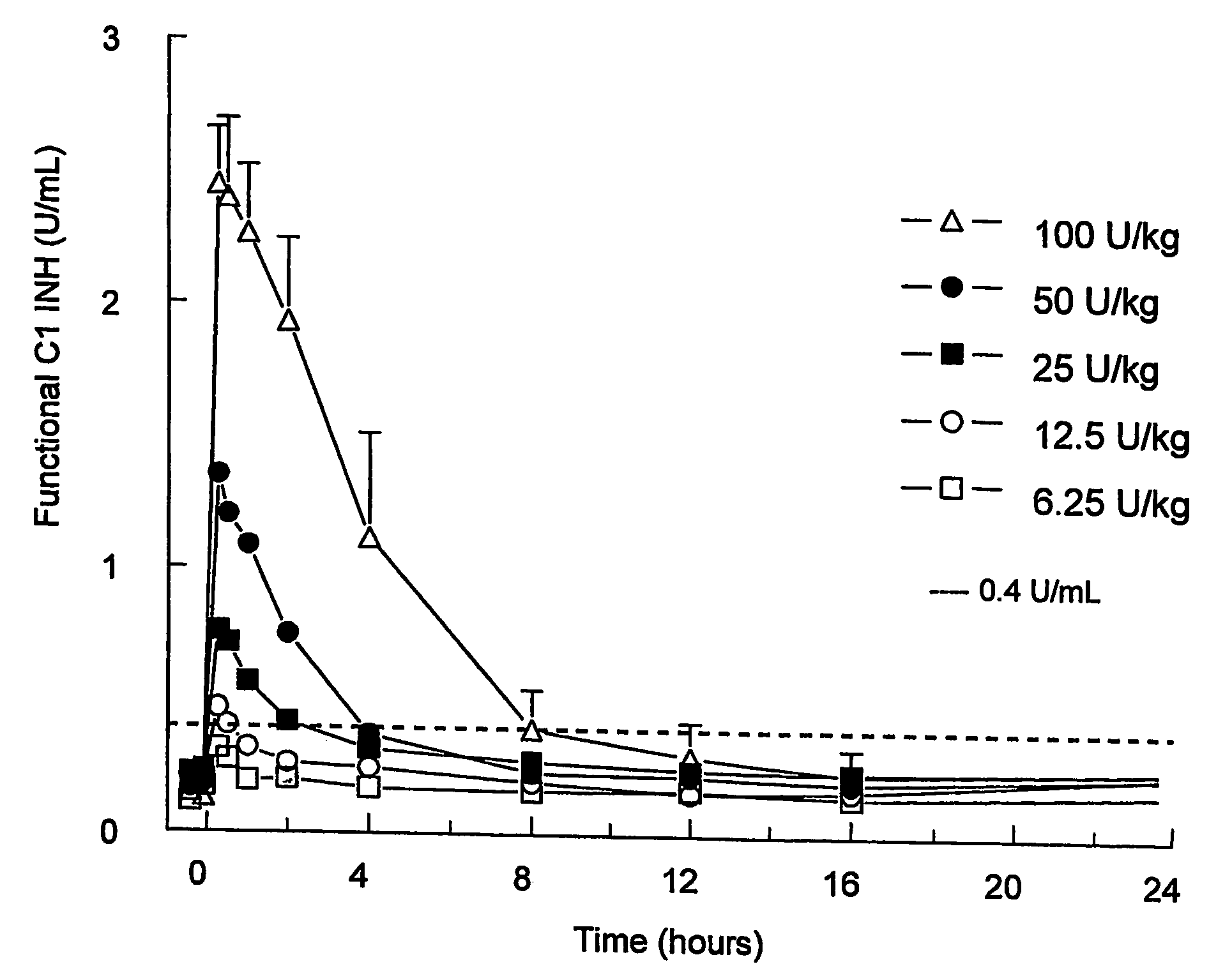
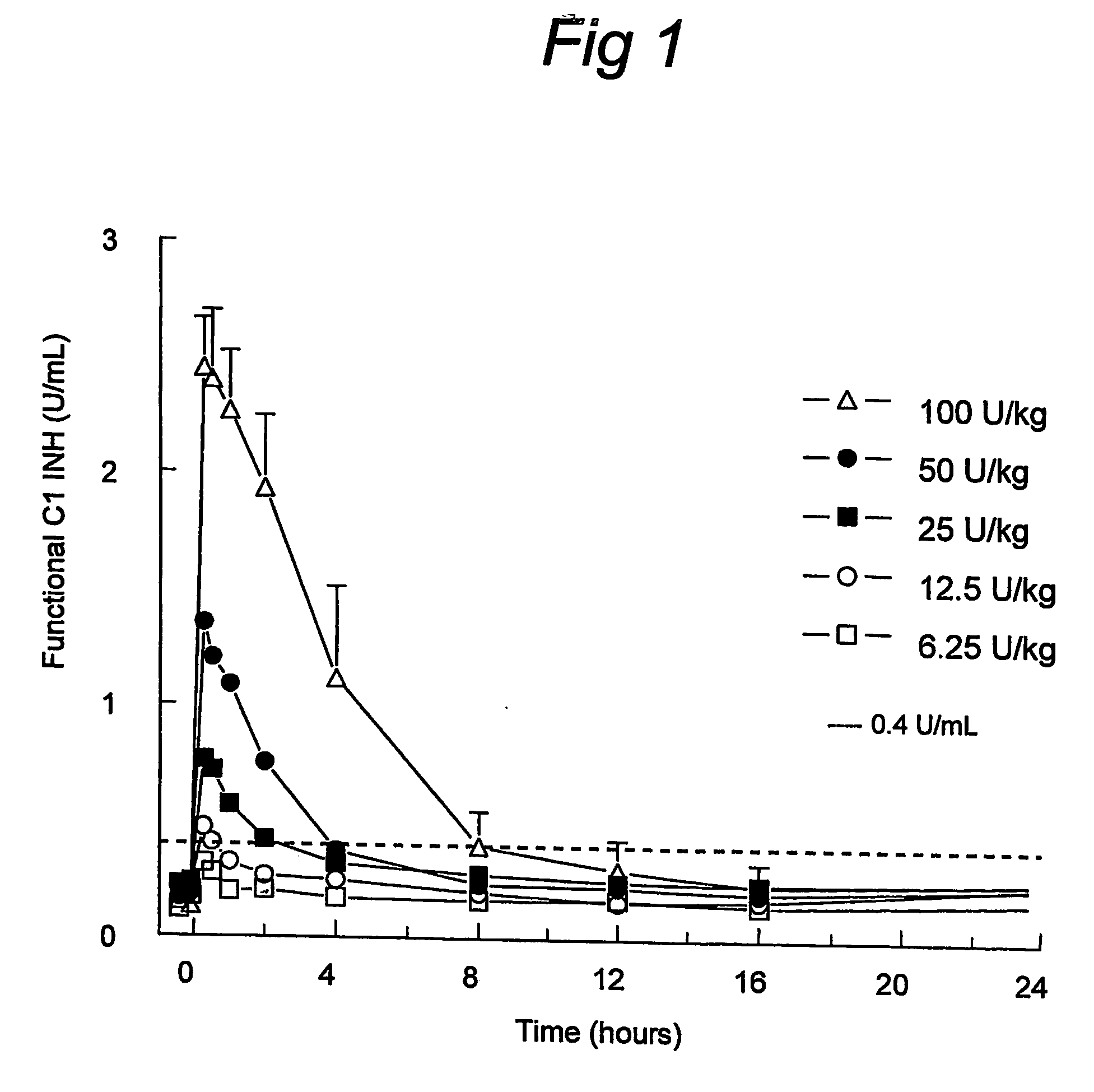
C1 inhibitor with short half-life transient treatment
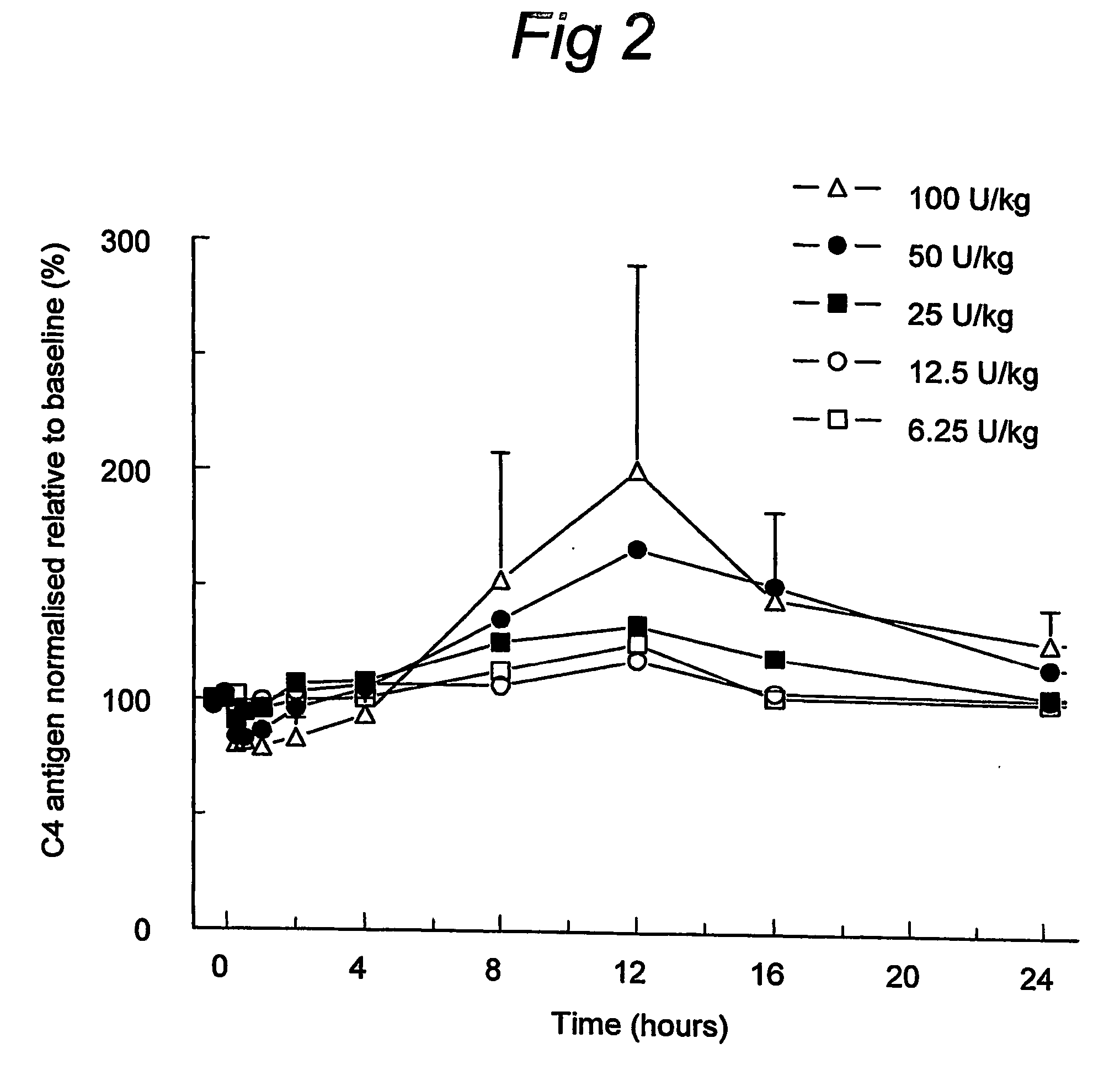
ActiveUS7544853B2Long exposureAvoid detrimental side-effectsPeptide/protein ingredientsAntipyreticPlasma derivedHalf-life
Owner:PHARMING INTPROP BV
PEPTIDES THAT BLOCK THE BINDING OF IgG to FcRn
InactiveUS20070254831A1Reduced half-lifeReduce serum concentrationPeptide/protein ingredientsAntipyreticCrystallographyAutoimmune condition
Owner:BIOGEN HEMOPHILIA
Biocompatible compounds for sustained release pharmaceutical drug delivery systems
InactiveUS7687054B2Improving physical and degradation characteristicReduce molecular weightPowder deliveryDispersion deliverySulfurMedicine
Methods, compounds, and medicinal formulations utilizing biocompatible polymers for delivery of a drug, particularly for solubilizing, stabilizing and / or providing sustained release of drug from topical, implantable, and inhalation systems. Many of the methods, compounds, and medicinal formulations are particularly suitable for oral and / or nasal inhalation and use polymers of the formula —[X—R1—C(O)]— wherein each R1 is an independently selected organic group that links the —X— group to the carbonyl group, and each X is independently oxygen, sulfur, or catenary nitrogen.
Owner:3M INNOVATIVE PROPERTIES CO
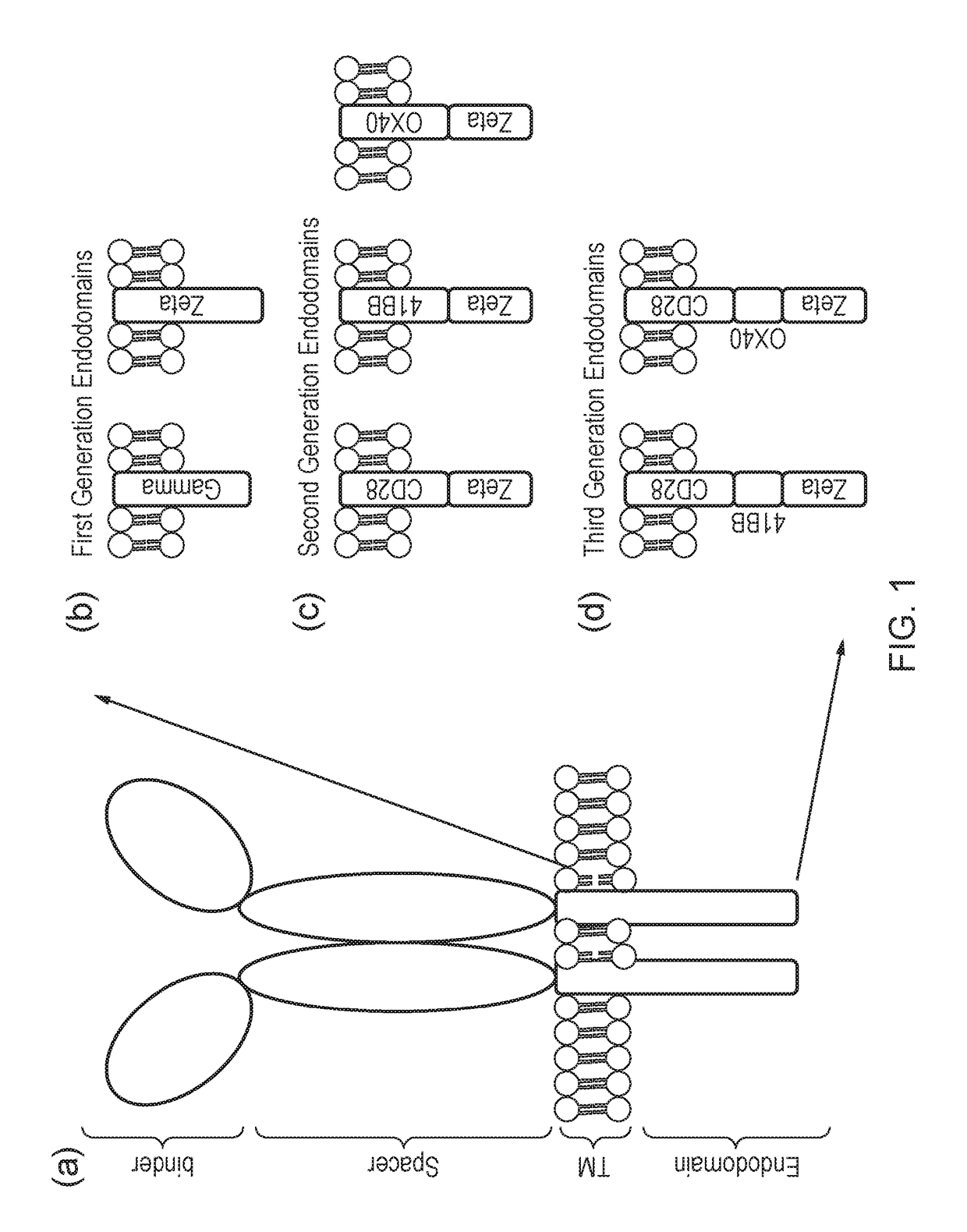
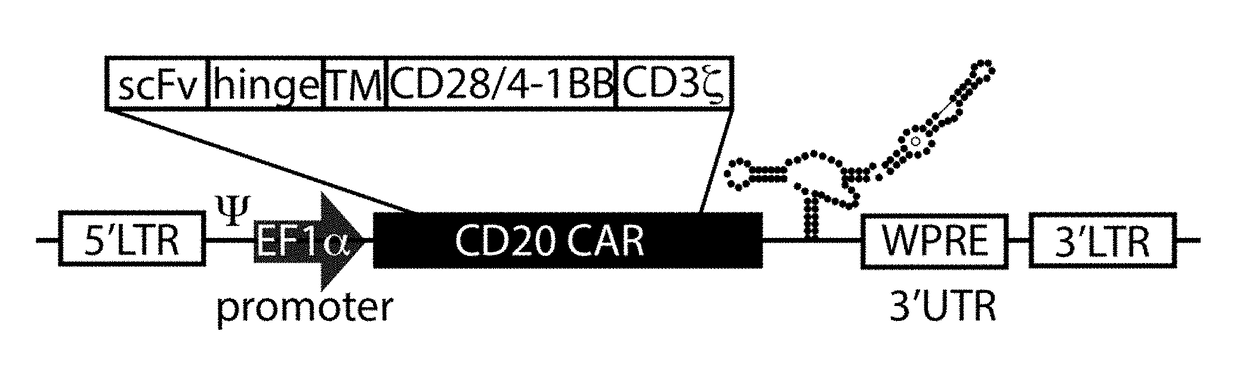
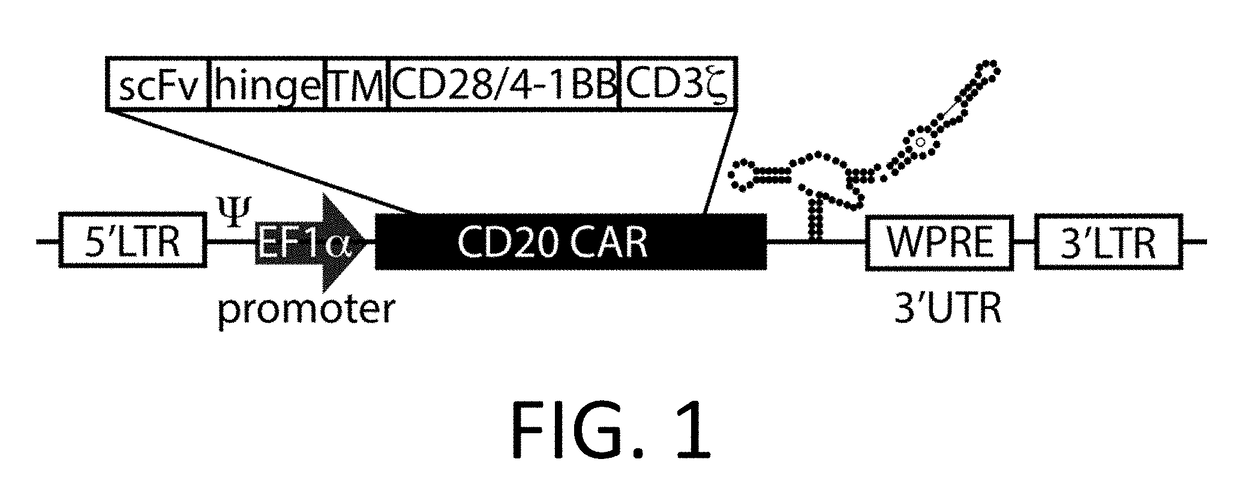
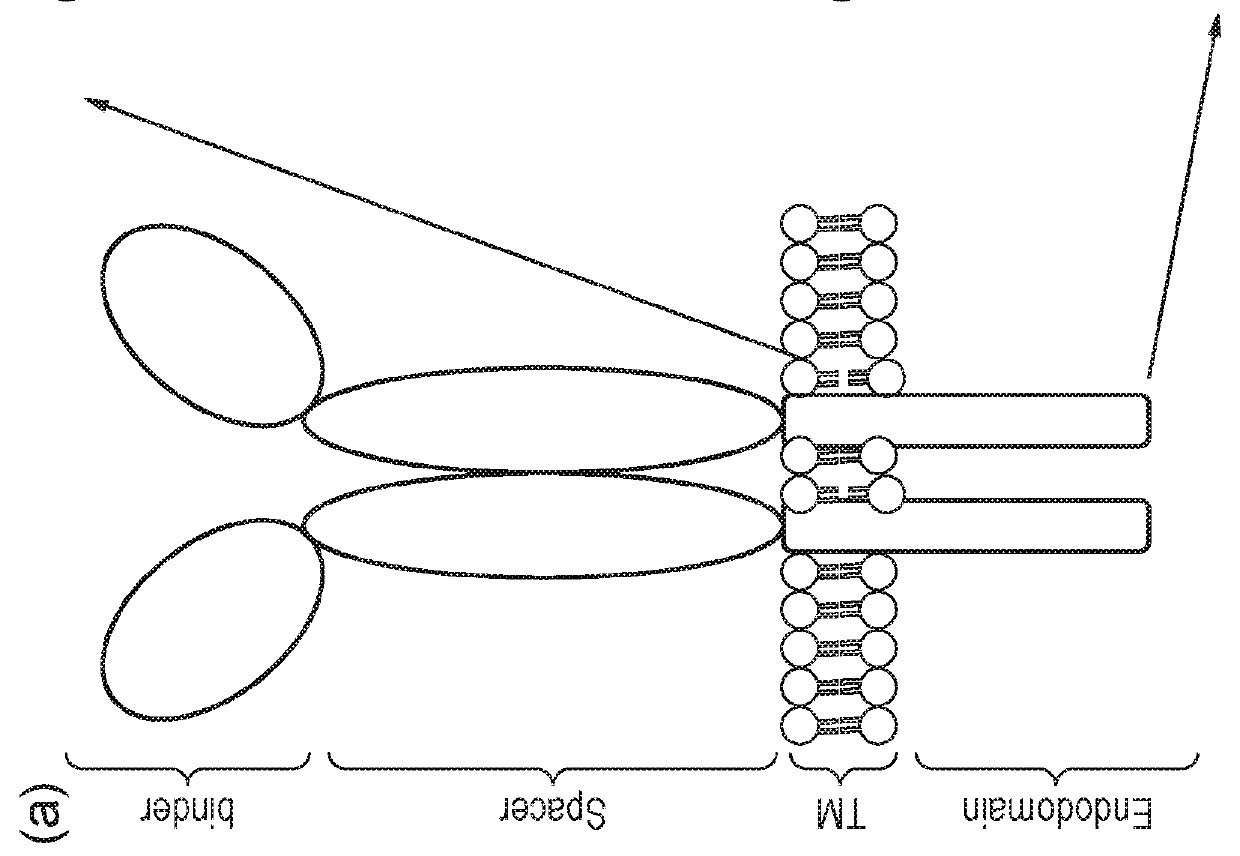
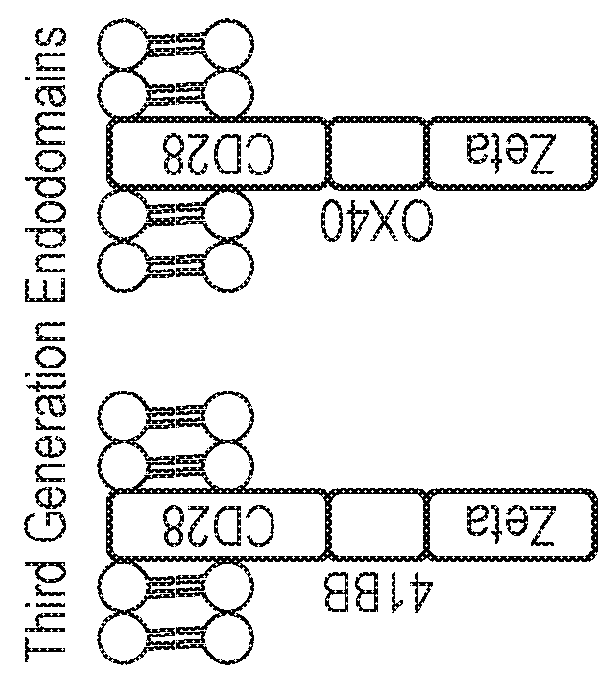
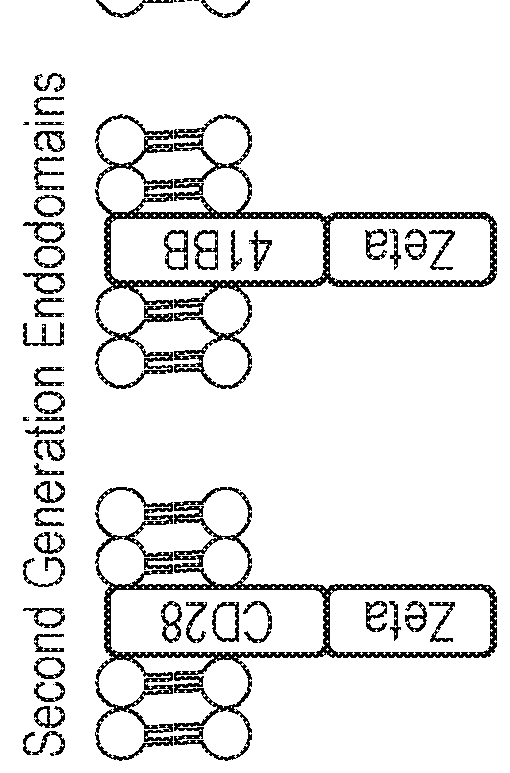
Nucleic acid construct for expressing more than one chimeric antigen receptor
InactiveUS20180111993A1Modulate relative cell surface expressionReduce traffic problemsPolypeptide with localisation/targeting motifImmunoglobulin superfamilySequence signalAntigen binding
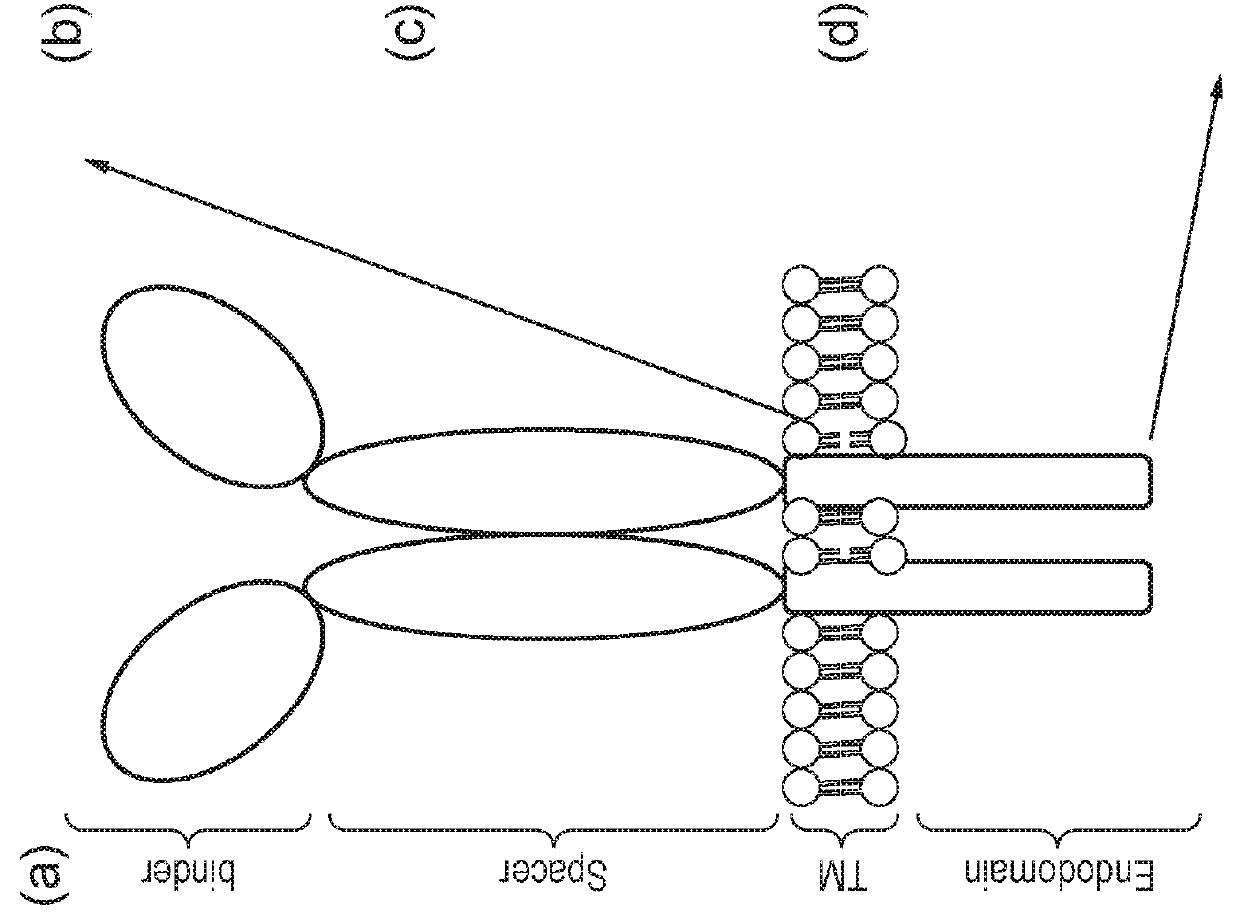
The present invention provides a nucleic acid construct comprising the following structure: A-X-B in which X is a nucleic acid sequence which encodes a cleavage site; and A and B are nucleic acid sequences encoding a first and a second chimeric antigen receptor (CAR), each CAR comprising: (i) an antigen-binding domain; (ii) a spacer (iii) a trans-membrane domain; and (iv) an endodomain wherein the antigen binding domains of the first and second CARs bind to different antigens; wherein one of the first or second CARs is an activating CAR comprising an activating endodomain and the other CAR is an inhibitory CAR comprising a ligation-off inhibitory endodomain; and wherein: (a) the first and / or second CAR comprises an intracellular retention signal; and / or (b) the signal peptide of the first or second CAR comprises one or more mutation(s) such that it has fewer hydrophobic amino acids.
Owner:AUTOLUS LIMIED
C1 inhibitor with short half-life transient treatment
ActiveUS20070185011A1Long exposureAvoid detrimental side-effectsPeptide/protein ingredientsAntipyreticPlasma derivedMedicine
The present invention relates to the use of a C1 inhibitor (C1INH) with shorter half-life than plasma-derived C1INH for the preparation of a medicament for the transient treatment of an individual. It relates to both therapeutic and prophylactic treatment. The method of the invention allows for the administration of C1INH at certain therapeutic levels for a concise pre-determined time span. Pharmaceutical compositions based on C1INH with shorter half-lives may be used both in situations where transient treatment is merely and advantage. The advantage of the use according to the invention is that an individual is not exposed to C1INH for longer than required, since the levels of the C1INH more rapidly subsides after administration has stopped. In contrast, levels of plasma-derived C1INH would remain elevated for a prolonged period of time.
Owner:PHARMING INTPROP BV
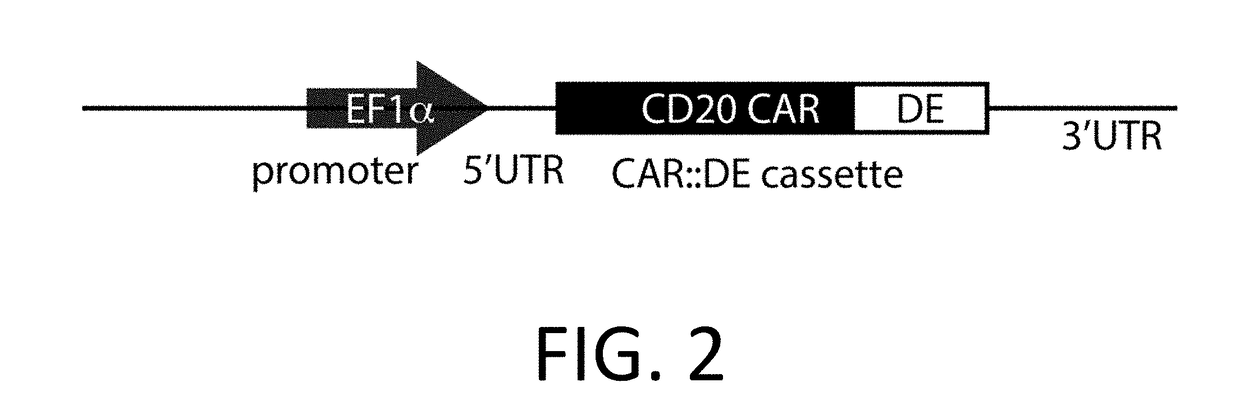
Gold optimized CAR T-cells
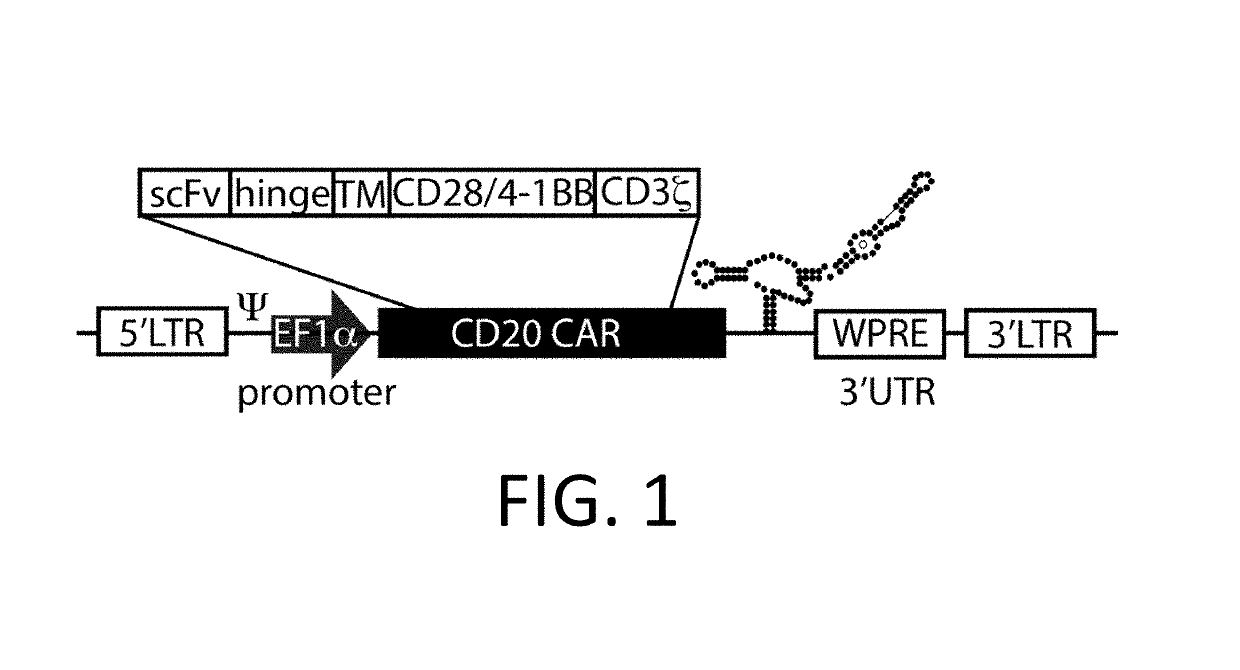
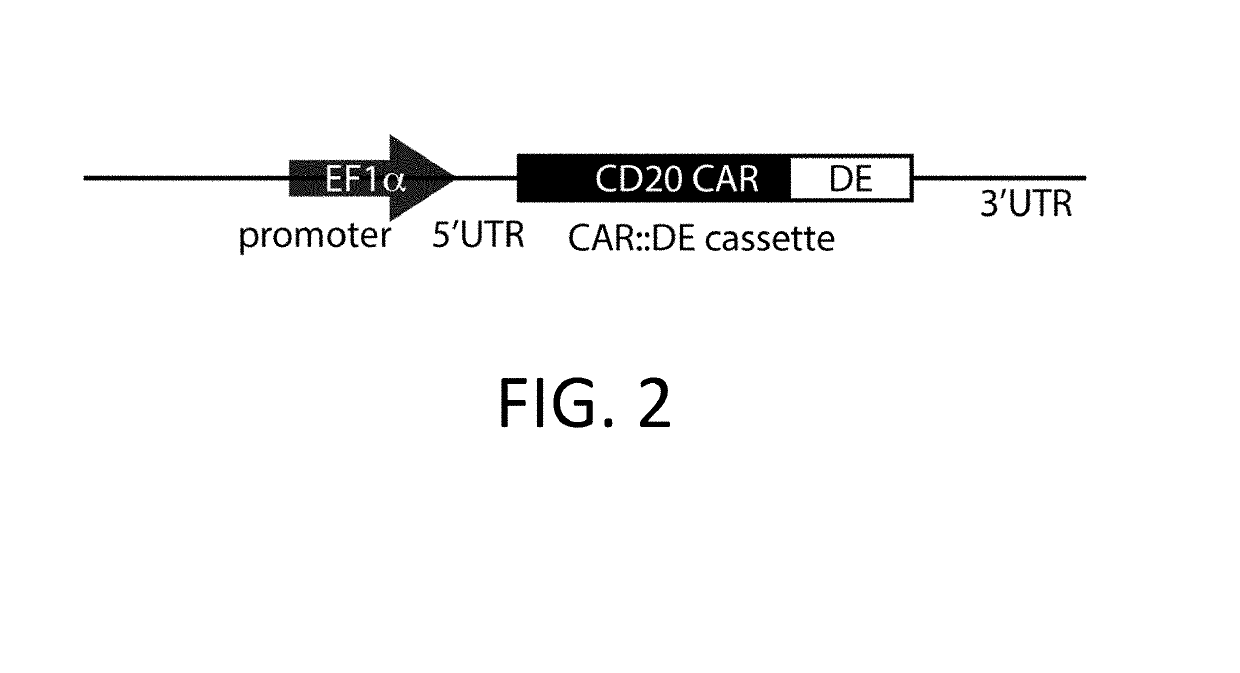
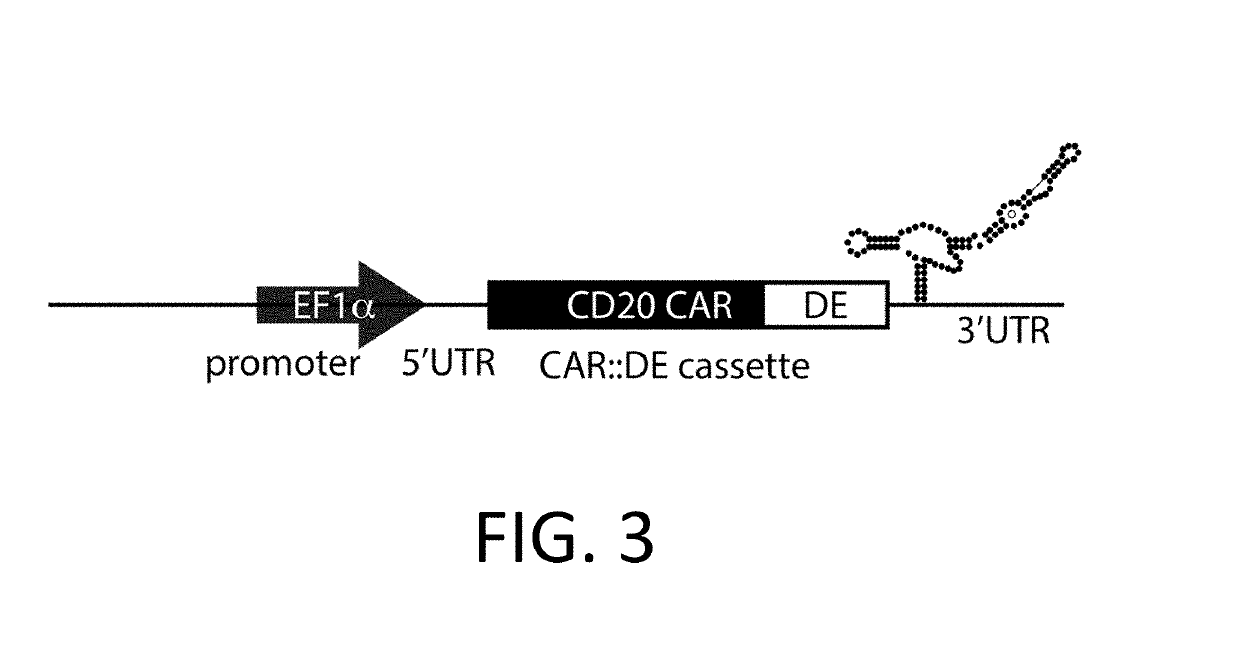
ActiveUS10323249B2Reduced half-lifeReduces GAPDH bindingImmunoglobulin superfamilyVectorsAntigen receptorsTransgene
Control Devices are disclosed including RNA destabilizing elements (RDE), RNA control devices, and destabilizing elements (DE) combined with Chimeric Antigen Receptors (CARs) or other transgenes in eukaryotic cells. Multicistronic vectors are also disclosed for use in engineering host eukaryotic cells with the CARs and transgenes under the control of the control devices. These control devices can be used to optimize expression of CARs in the eukaryotic cells so that, for example, effector function is optimized. CARs and transgene payloads can also be engineered into eukaryotic cells so that the transgene payload is expressed and delivered after stimulation of the CAR on the eukaryotic cell.
Owner:CHIMERA BIOENG INC
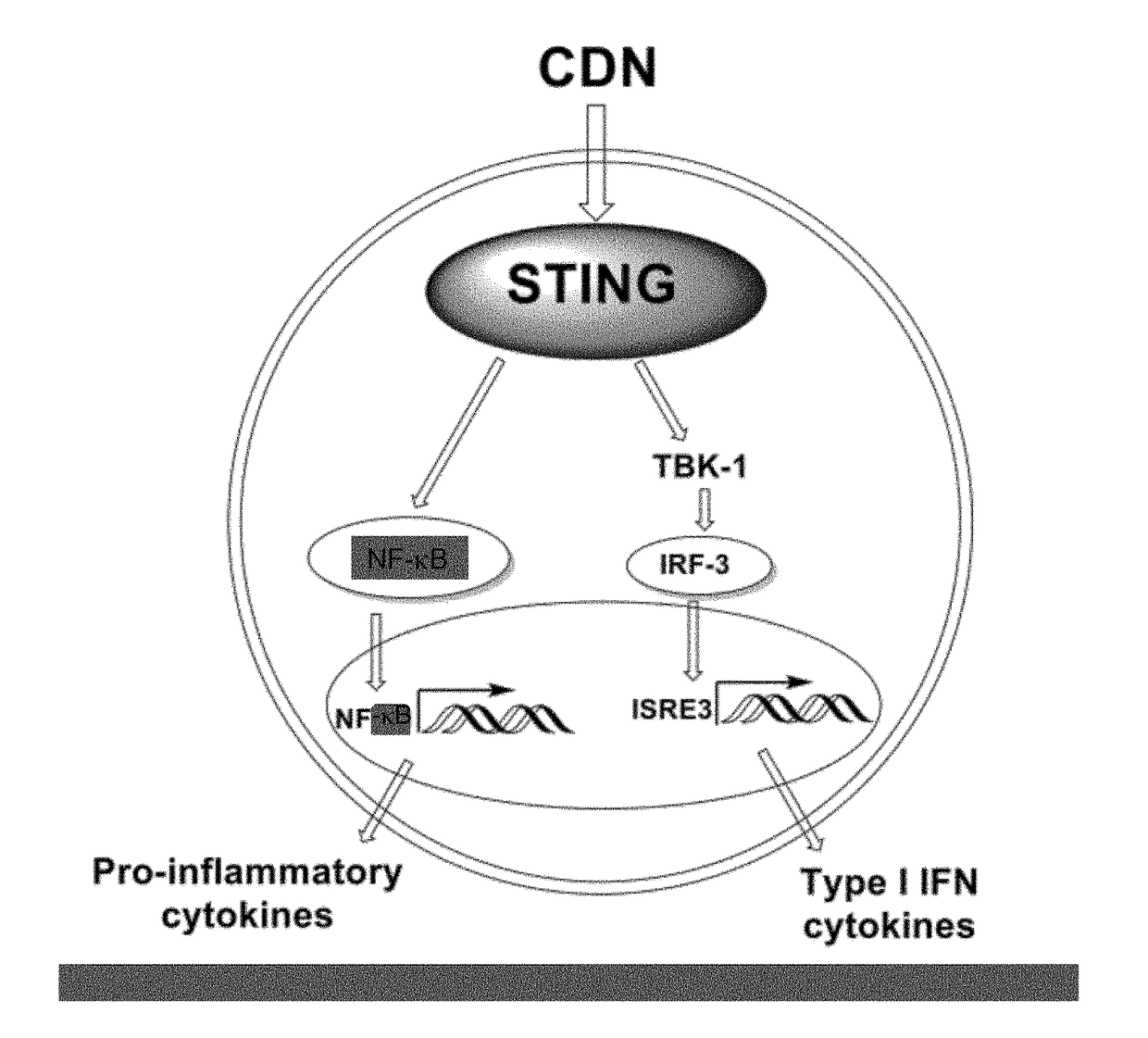
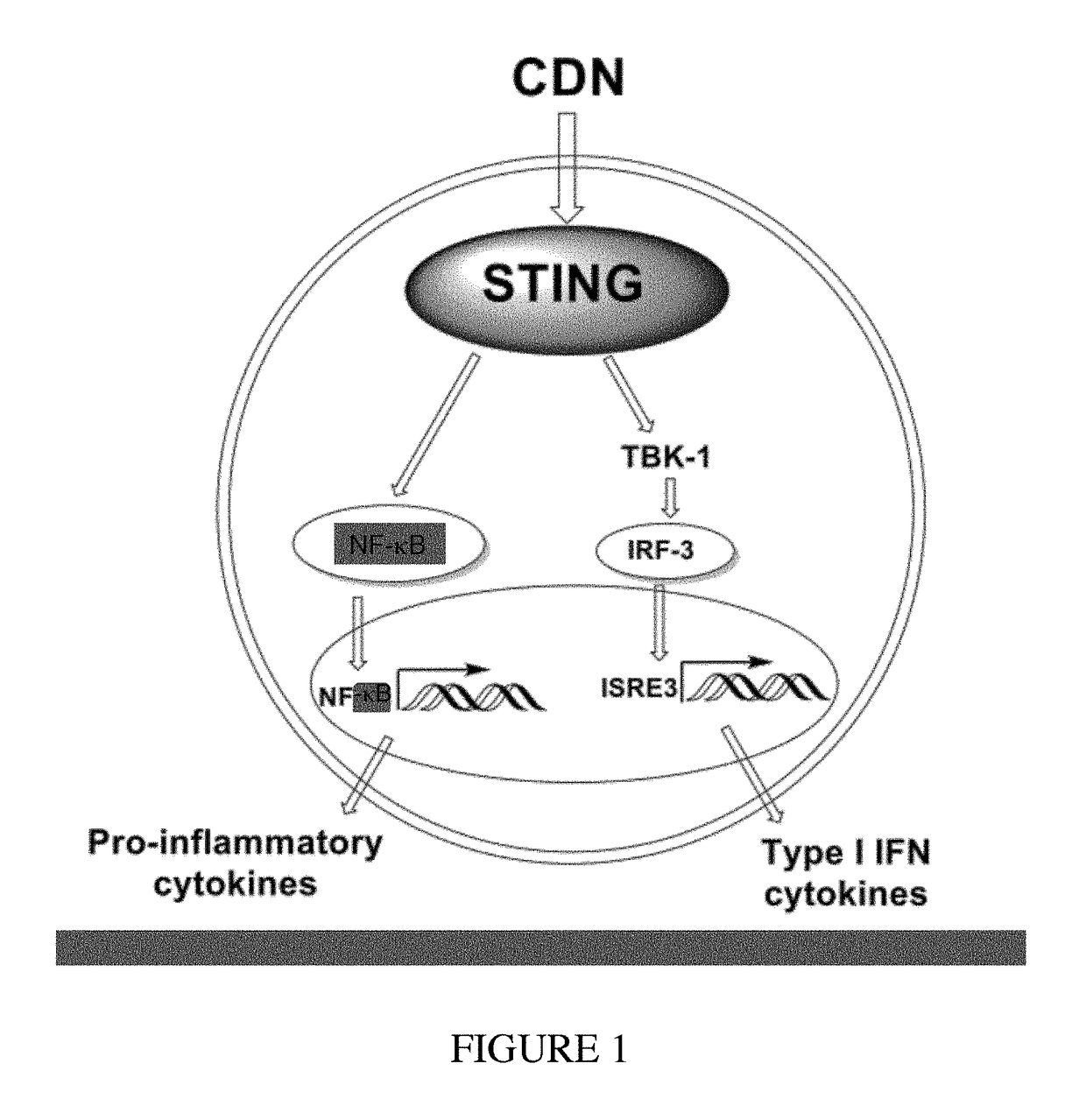
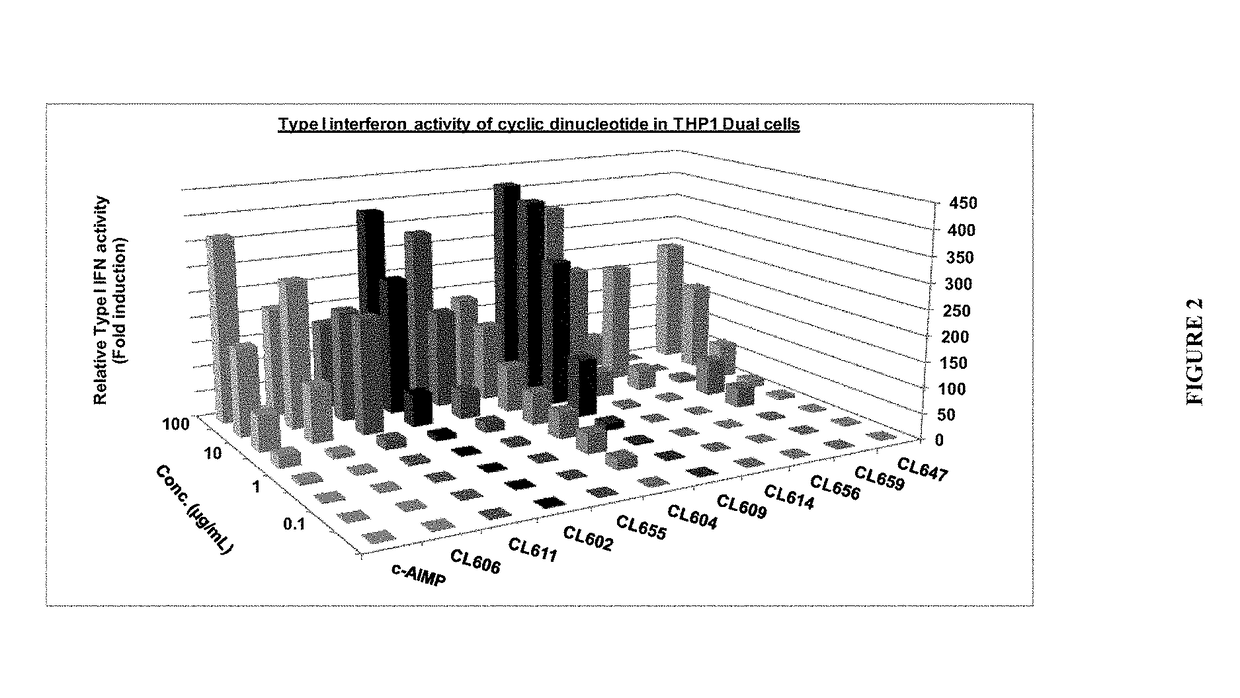
Combined use of a chemotherapeutic agent and a cyclic dinucleotide for cancer treatment
InactiveUS20170340658A1Good treatment effectEffective treatmentOrganic active ingredientsAntineoplastic agentsCombined useAgonist
A kit of parts includes a) gemcitabine or a pharmaceutically acceptable salt thereof and b) a cyclic dinucleotide or pharmaceutically acceptable salt thereof, wherein the cyclic dinucleotide or pharmaceutically acceptable salt thereof is an agonist of the receptor known as “stimulator of interferon genes” (STING), for use in the treatment of solid pancreatic cancer.
Owner:INVIVOGEN
Rapid Generation of Anti-Idiotypic Antibodies
ActiveUS20130330323A1Reduces and eliminates side effectReduce adverse effectsMuscular disorderAntibody ingredientsIn vivoAnti-idiotypic antibodies
Owner:BIOGEN MA INC
Recombinant igg fc multimers
InactiveUS20190119377A1Optimization parametersPrevents upregulationPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsInflammatory bowel diseaseDisease injury
Owner:CSL BEHRING LENGNAU AG
Intracellular delivery of biomolecules to induce tolerance
PendingUS20190111082A1Reduced half-lifeExtended half-lifeApparatus sterilizationMammal material medical ingredientsAntigen deliveryAnucleated cell
The present invention provides methods for inducing tolerance and / or suppressing an immune response to an antigen by passing a cell suspension containing an anucleate cell through a constriction, wherein the constriction deforms the cell thereby causing a perturbation of the cell such that an antigen and / or tolerogenic factor enters the cell. In some embodiments, the anucleate cell is delivered to an individual and the antigen is delivered to and processed in a tolerogenic environment to induce tolerance and / or suppress an immune response to the antigen.
Owner:SQZ BIOTECH CO
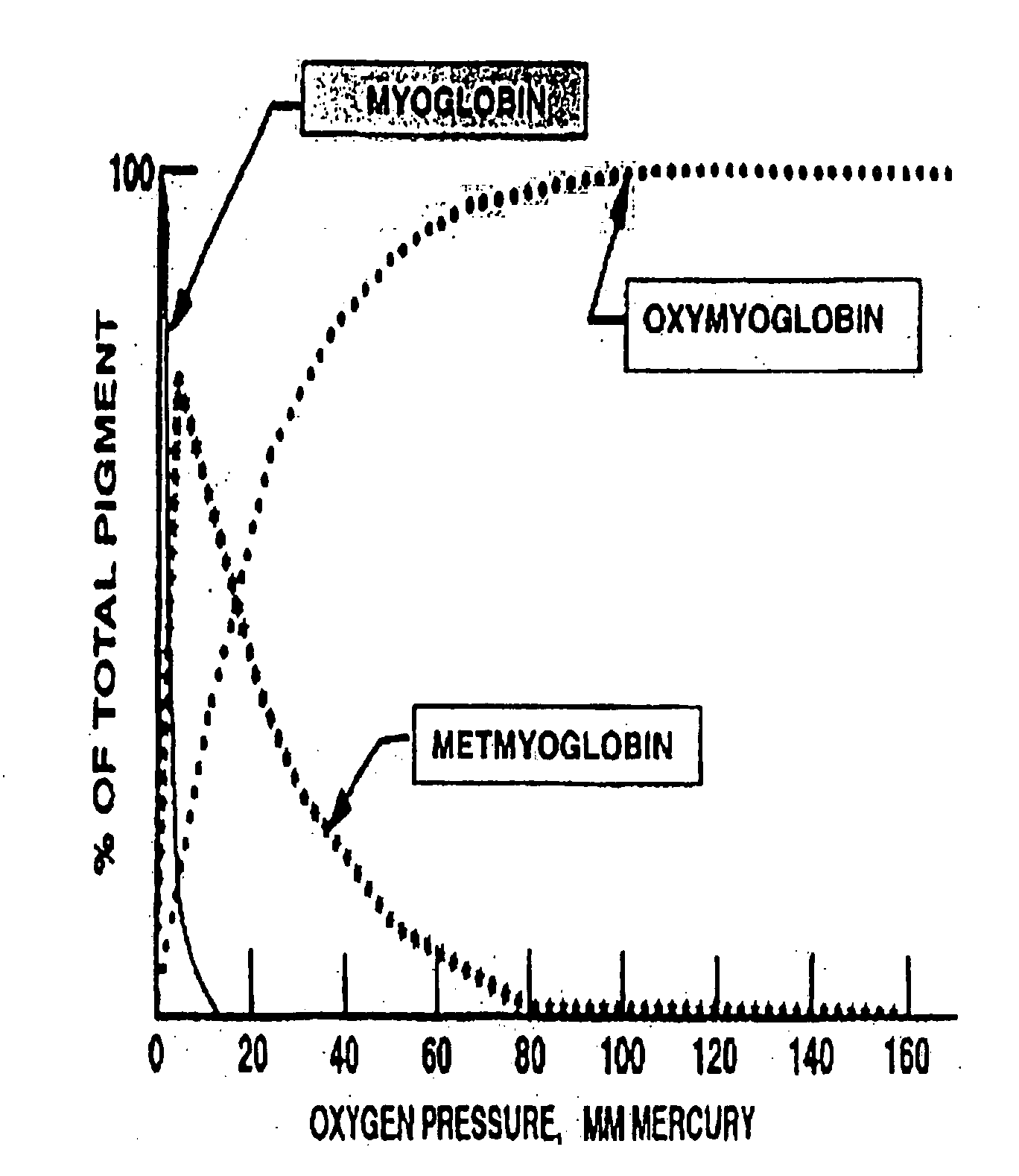
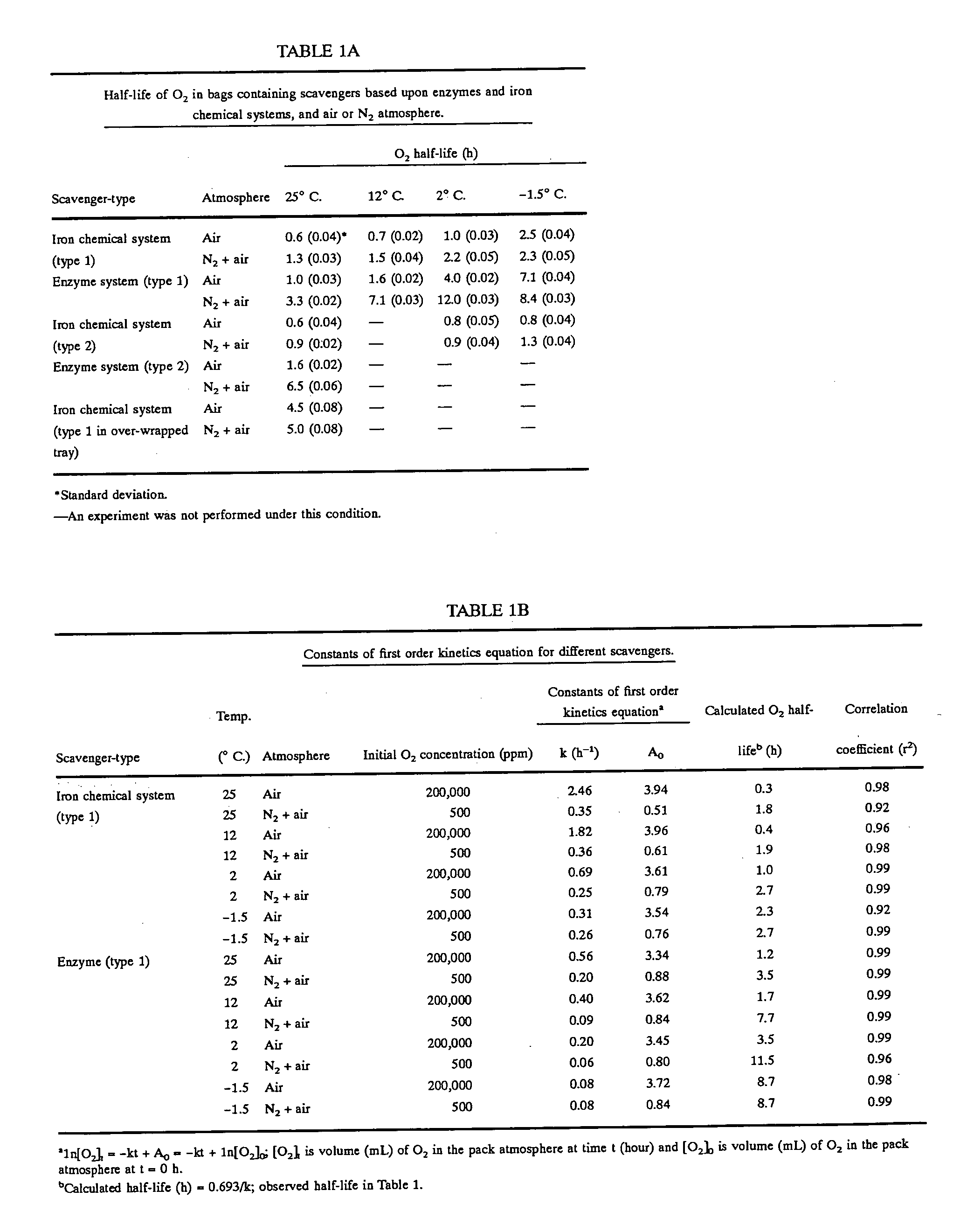
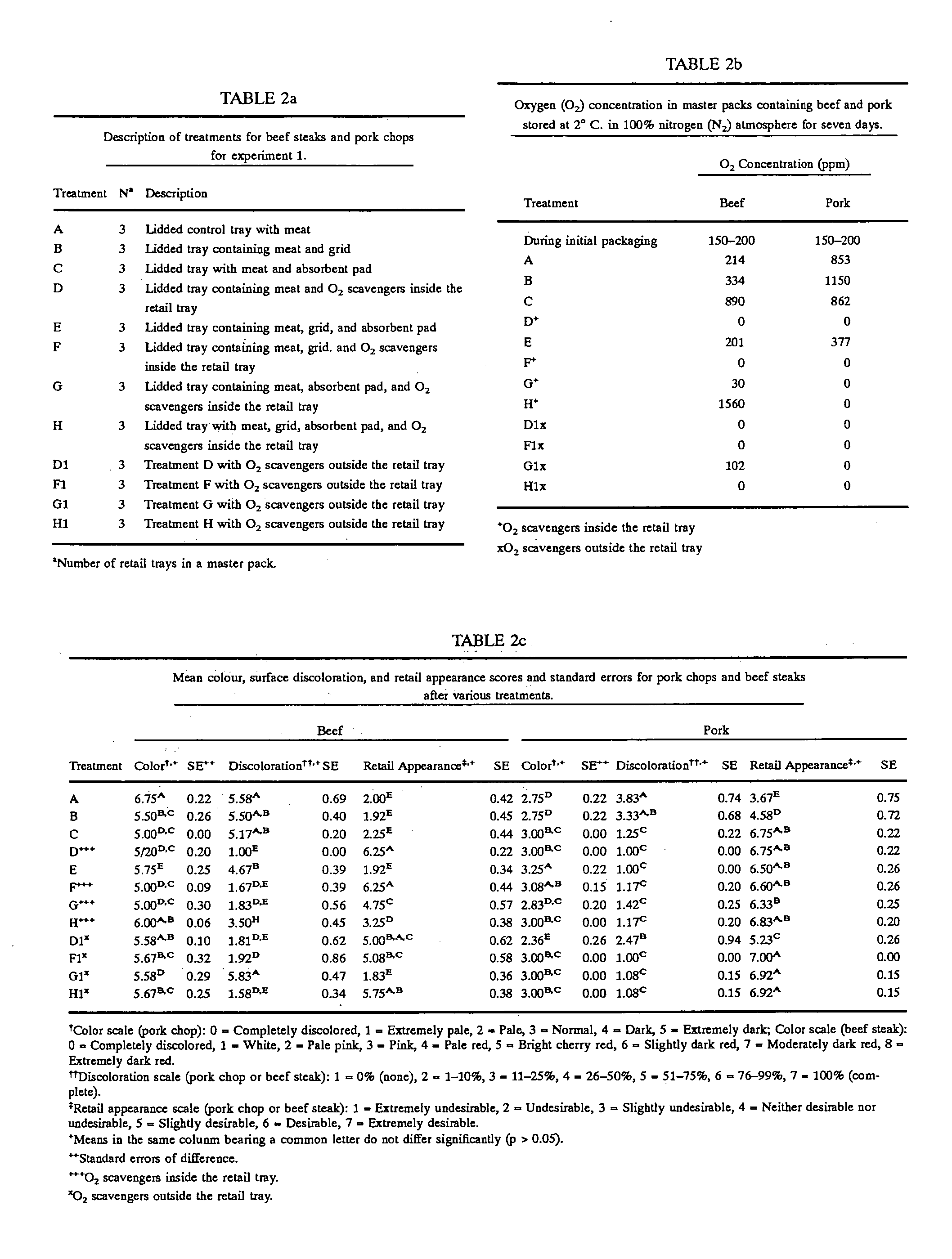
Method for extending shelf-life and prevention of discoloration of meat
InactiveUS20060147586A1Reduces temperature fluctuationRapid spoilage be restrictReady-for-oven doughsPackaging meatChemistryWrapping procedure
The current invention covers an improved meat-packaging procedure and machine for packaging meat cuts for long-term storage at temperatures of between 28° and 32° F. The process includes sealing meat cuts within a master bag containing oxygen scavenger materials capable of reducing the residual oxygen content of the atmosphere within the bag to 0 ppm within 24 hours of sealing. Gas is injected into the master bag to form a nitrogen-rich storage environment of at least 50% nitrogen. A small amount of carbon monoxide gas (0.1% to 5%) is preferred for the storage environment, as this helps to preserve the red coloration of meat under long-term storage conditions. The over-wrap of the meat trays can be perforated so that gas exchange occurs within the master bag between the interior and exterior of the meat tray to absorb the residual oxygen inside the meat trays. For meat trays containing meat with poor color stability, oxygen scavengers are preferably placed within the meat trays. For cuts with good color stability, the oxygen scavengers may be placed outside the meat trays. Meat can be stored by this system for up to 15 weeks and up to nine days of retail display life.
Owner:TEXAS MEAT PACKAGING SYST
PEPTIDES THAT BLOCK THE BINDING OF IgG TO FcRn
InactiveUS20110059889A1Reduced half-lifePeptide/protein ingredientsAntipyreticMedicineAutoimmune disease
Owner:BIOGEN HEMOPHILIA
Gold Optimized CAR T-cells
ActiveUS20180057822A1Reduced half-lifeReduces GAPDH bindingImmunoglobulin superfamilyVectorsAntigen receptorsTransgene
Control Devices are disclosed including RNA destabilizing elements (RDE), RNA control devices, and destabilizing elements (DE) combined with Chimeric Antigen Receptors (CARs) or other transgenes in eukaryotic cells. Multicistronic vectors are also disclosed for use in engineering host eukaryotic cells with the CARs and transgenes under the control of the control devices. These control devices can be used to optimize expression of CARs in the eukaryotic cells so that, for example, effector function is optimized. CARs and transgene payloads can also be engineered into eukaryotic cells so that the transgene payload is expressed and delivered after stimulation of the CAR on the eukaryotic cell.
Owner:CHIMERA BIOENG INC
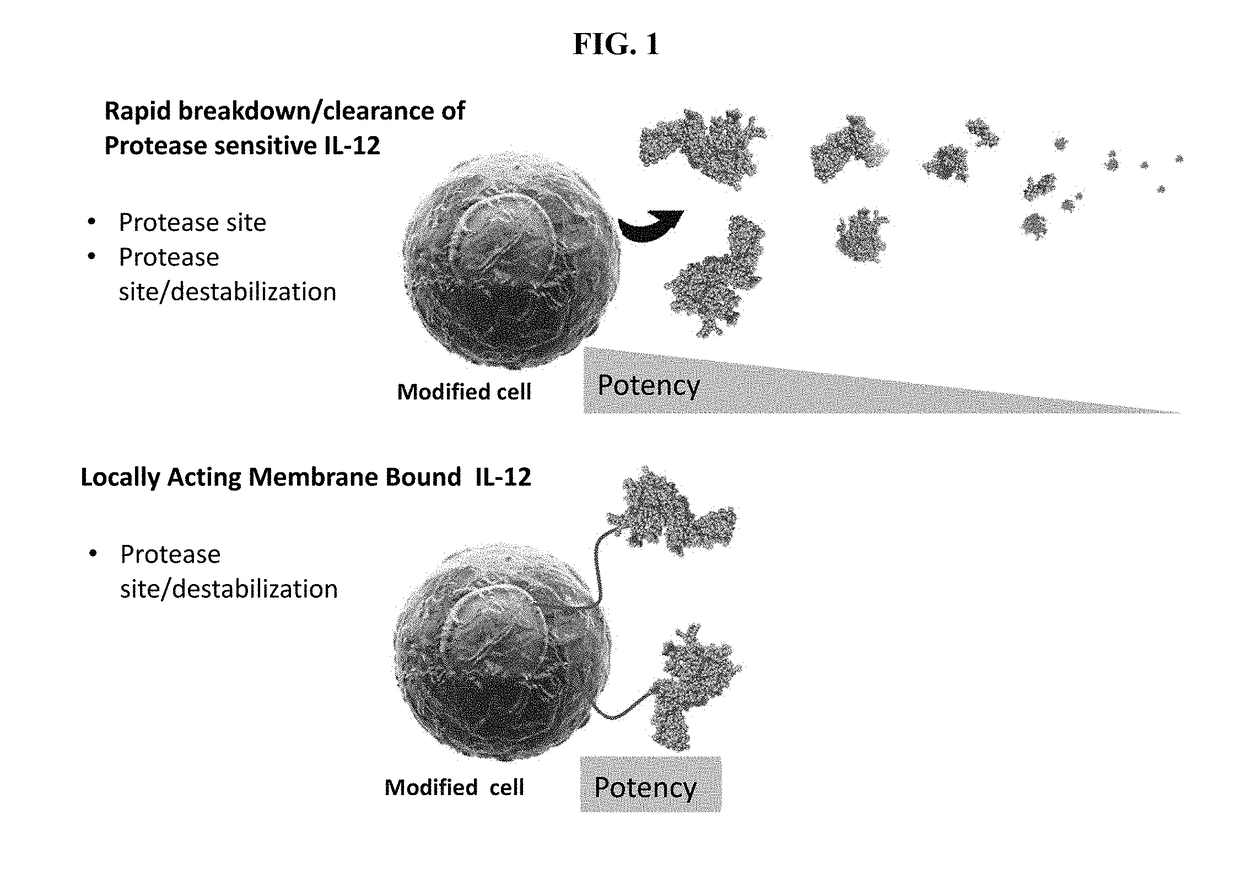
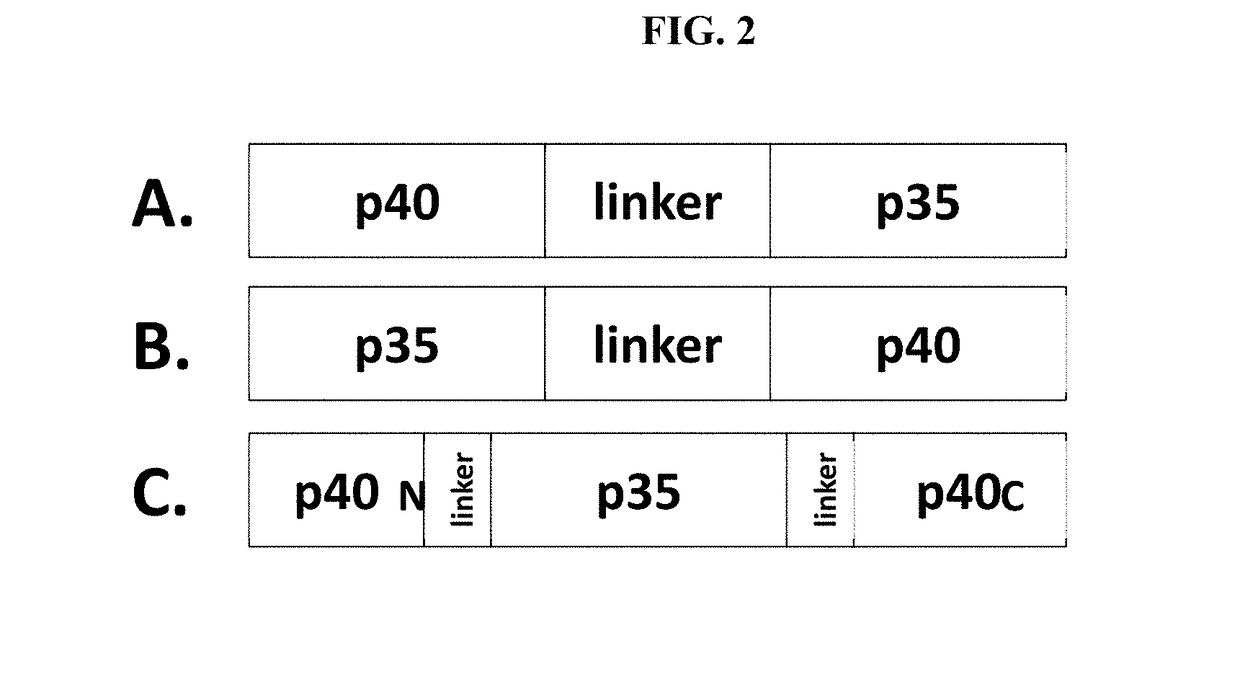
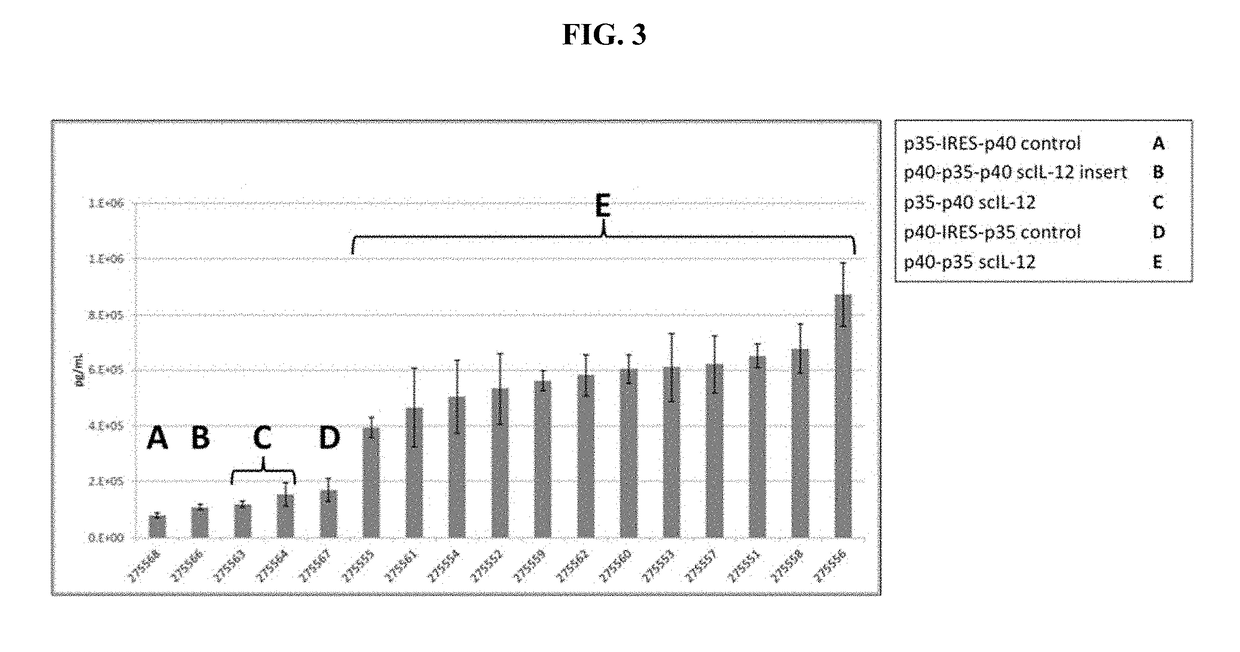
Improved Therapeutic Control of Proteolytically Sensitive, Destabilized Forms of Interleukin-12
InactiveUS20190062394A1Reduced half-lifeReduced activityPolypeptide with localisation/targeting motifFusion with protease siteWhite blood cellHalf-life
The present invention relates to modified forms of IL-12. These modified forms of IL-12 may be engineered to have a shortened in vivo half-life compared and / or enhanced localization of biological effects compared to that of corresponding non-modified form of IL-12. Short half-life and membrane bound forms of IL-12 may provide greater therapeutic control for in vivo therapeutic delivery, in particular when used in combination with ligand inducible delivery of IL-12. Modified forms of IL-12 engineered to have shortened in vivo half-life and / or enhanced localization of biological effects include heterodimeric p35 / p40, single chain and membrane bound forms of IL-12 wherein a naturally occurring IL-12 amino acid sequence is genetically modified to destabilize IL-12 tertiary structure / polypeptide folding and enhance susceptibility of the IL-12 molecule to in vivo proteolytic degradation.
Owner:PRECIGEN INC
Nucleic acid construct for expressing more than one chimeric antigen receptor
InactiveUS20180104321A1Modulate relative cell surface expressionReduce traffic problemsPolypeptide with localisation/targeting motifImmunoglobulin superfamilySequence signalAntigen receptor
The present invention provides a nucleic acid construct comprising the following structure: A-X-B in which X is a nucleic acid sequence which encodes a cleavage site; and A and B are nucleic acid sequences encoding a first and a second chimeric antigen receptor (CAR), each CAR comprising: (i) an antigen-binding domain; (ii) a spacer (iii) a trans-membrane domain; and (iv) an endodomain wherein the antigen binding domains of the first and second CARs bind to different antigens, wherein the spacer of the first CAR is different to the spacer of the second CAR and wherein one of the first or second CARs is an activating CAR comprising an activating endodomain and the other CAR is an inhibitory CAR comprising a ligation-off inhibitory endodomain; and wherein: (a) the first and / or second CAR comprises an intracellular retention signal; and / or (b) the signal peptide of the first or second CAR comprises one or more mutation(s) such that it has fewer hydrophobic amino acids.
Owner:AUTOLUS LIMIED
Nanocarriers for cancer treatment
InactiveUS20170165382A1Enhanced EPR effectPromote accumulationOrganic active ingredientsRadioactive preparation carriersNanocarriersOrganic chemistry
The present invention provides conjugates containing metal binding ligands, as well as nanocarriers prepared from the conjugates.
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for treating or preventing lupus
ActiveUS9657292B2Lower Level RequirementsReduce expressionOrganic active ingredientsAntipyreticSystemic lupus erythematosusRelated disorder
The invention features compositions comprising agents that inhibit or reduce self-reactive IgE and / or basophils, and related methods of using the compositions for treating or preventing lupus, lupus nephritis, and lupus-related disorders.
Owner:UNITED STATES OF AMERICA
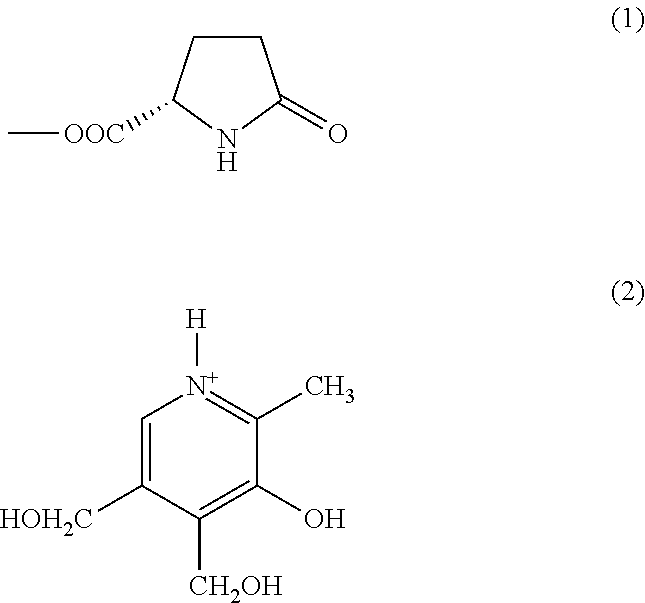
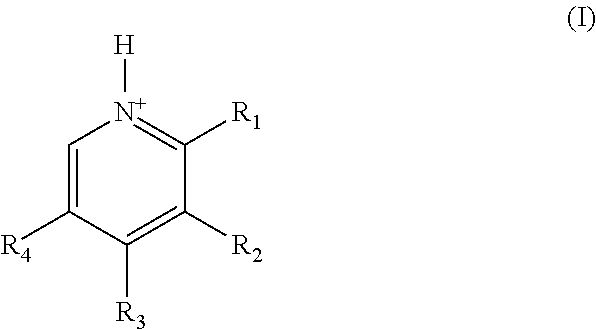
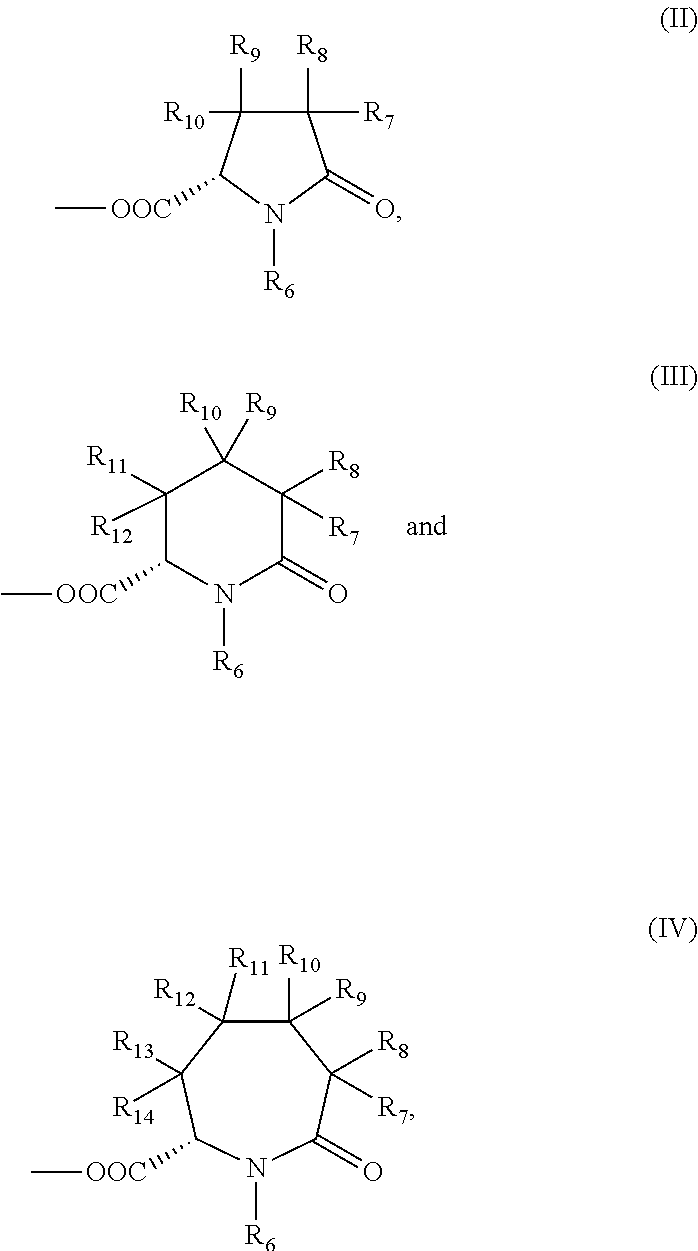
Substituted pyridoxine-lactam carboxylate salts
Owner:ALCOBRA LTD
Antibodies specific to fcrn
InactiveUS20160264668A1Many functionsQuick clearSenses disorderNervous disorderDna encodingSpecific antibody
Owner:UCB PHARMA SRL
Compositions and Methods for Pesticide Degradation
InactiveUS20200399618A1Reduced half-lifeHydrolasesAntibody mimetics/scaffoldsMicrobiologyPesticide degradation
Compositions and methods related to anucelate cells (e.g., bacterial minicells) for pesticide degradation applications including related cells, polypeptides, and vectors.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Anucleate cell-derived vaccines
PendingUS20220105166A1Decreased hemoglobin levelImprove the level ofCell dissociation methodsPeptide/protein ingredientsDiseaseAntigen delivery
The present invention provides methods for stimulating an immune response to an antigen comprising administering to an individual, an anucleate cell-derived vesicle comprising an antigen and / or an adjuvant. In some embodiments, the anucleate cell-derived vesicle comprising the antigen and / or adjuvant is generated by passing a cell suspension containing an input anucleate cell through a constriction, wherein the constriction deforms the input anucleate cell thereby causing a perturbation of the cell to form an anucleate cell-derived vesicle such that an antigen and / or an adjuvant enters the anucleate cell-derived vesicle. In some embodiments, the anucleate cell-derived vesicle comprising the antigen and / or adjuvant is delivered to an individual and the antigen is delivered to and processed in an immunogenic environment to treat a disease, prevent a disease, and / or vaccinate an individual against an antigen.
Owner:SQZ BIOTECH CO
Short-acting factor vii polypeptides
ActiveUS20140363419A1Increase the gapOpportunities decreasePeptide/protein ingredientsBlood disorderDiseaseSialic acid aldolase
Short-acting Factor VII peptides are disclosed. A shortened half-life is desirable for treatment of acute bleeding and similar disorders. Modification of the sialylation and / or glycosylation of Factor VII and variants thereof produced peptides useful in treating conditions of acute bleeding.
Owner:COAGULANT THERAPEUTICS CORP
Nucleic acid construct for expressing more than one chimeric antigen receptor
InactiveUS20180105573A1Good treatment effectAvoid problemsPolypeptide with localisation/targeting motifImmunoglobulin superfamilySequence signalAntigen
The present invention provides a nucleic acid construct comprising the following structure: A-X—B in which A and B are nucleic acid sequences encoding a first and a second chimeric antigen receptor (CAR); and X is a nucleic acid sequence which encodes a cleavage site, wherein the first and second CAR recognise different antigens; the first and second CAR comprise or associate with activating endodomains; and (a) the first and / or second CAR comprises an intracellular retention signal; and / or (b) the signal peptide of the first or second CAR comprises one or more mutation(s) such that it has fewer hydrophobic amino acids.
Owner:AUTOLUS LIMIED
Stabilized aqueous dispersion of fluoropolymer
InactiveUS20040171726A1Reduced half-lifeLong half-lifeFibre treatmentSpecial tyresPolymer scienceFluoropolymer
The present invention relates to a stabilized fluoropolymer aqueous dispersion, wherein the stabilizer is polysiloxane polyoxyethylene copolymer preferably having a cloud point of no greater than 25° C.
Owner:EI DU PONT DE NEMOURS & CO
Orodispersible film composition comprising enalapril for the treatment of hypertension in a pediatric population
ActiveUS20200360461A1Reduce the amount requiredProcess stabilityOrganic active ingredientsDipeptide ingredientsPharmaceutical medicinePediatric population
The present invention relates to an oral applicable therapeutic dosage form, in particular an orodispersible film comprising Enalapril or pharmaceutically acceptable salts thereof for use in the treatment of hypertension in a pediatric population. The pediatric population is defined from 1 to 18 years of age. The present invention also provides a method of manufacturing of such a dosage form.
Owner:PHARMATHEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com