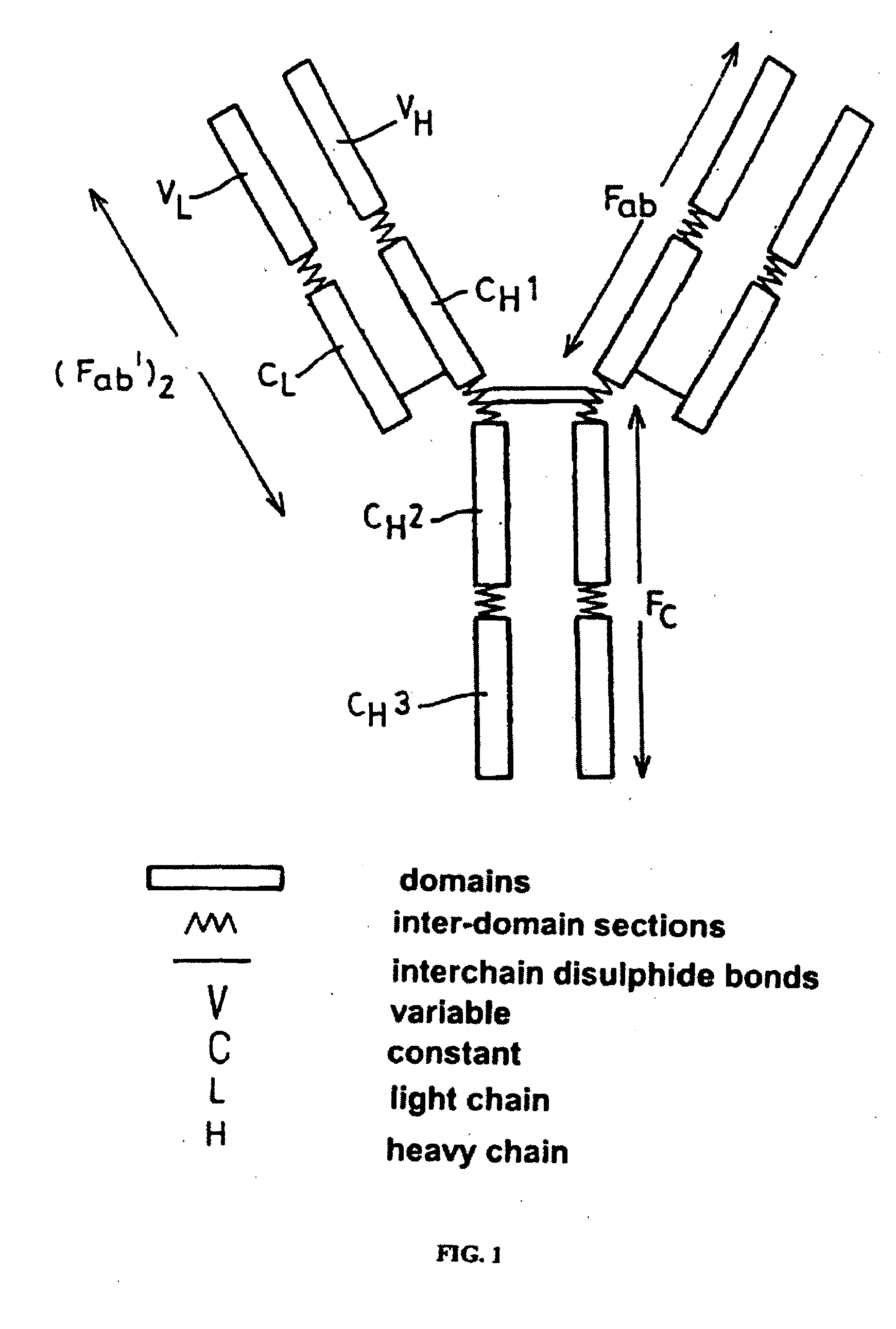
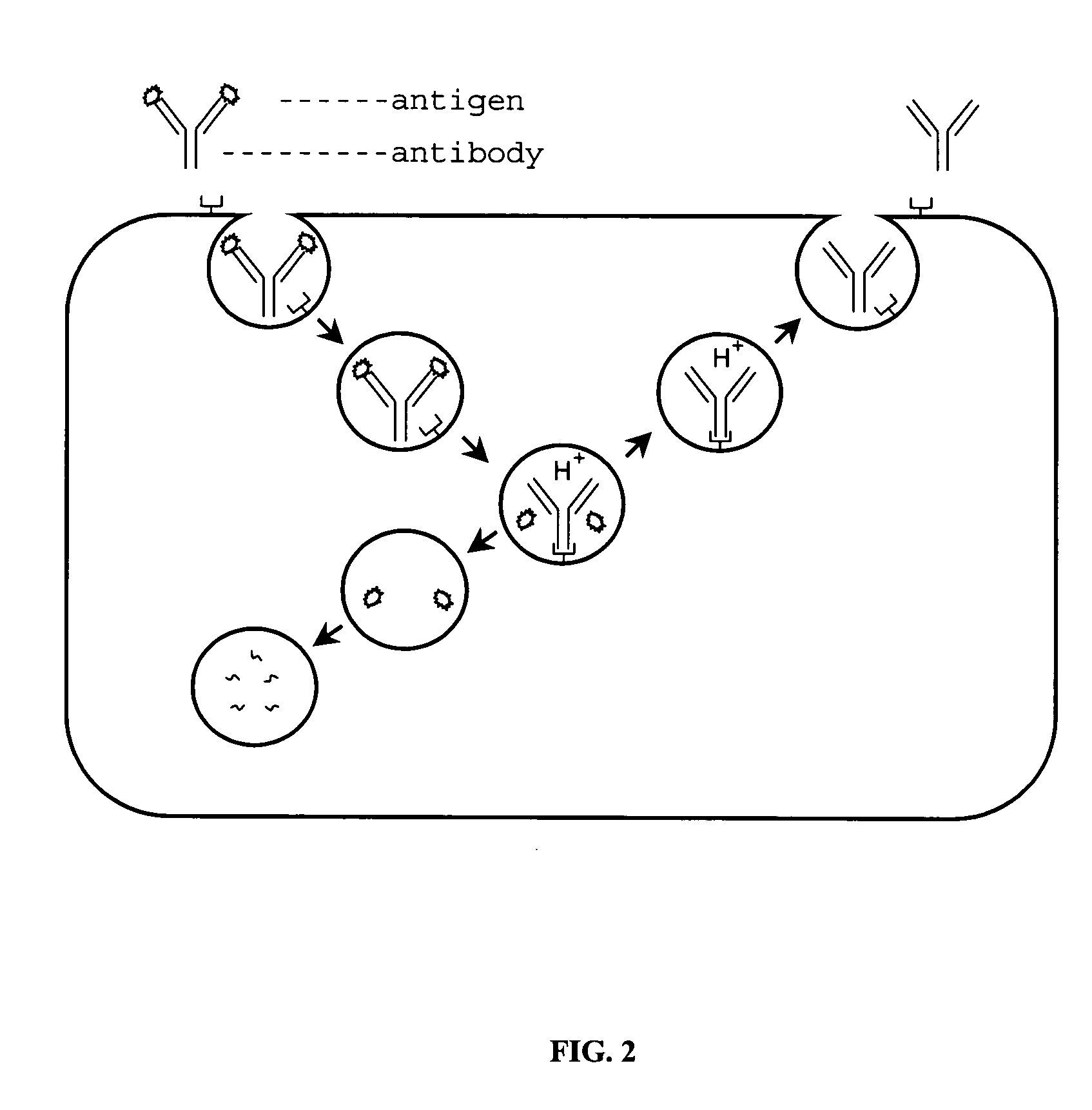
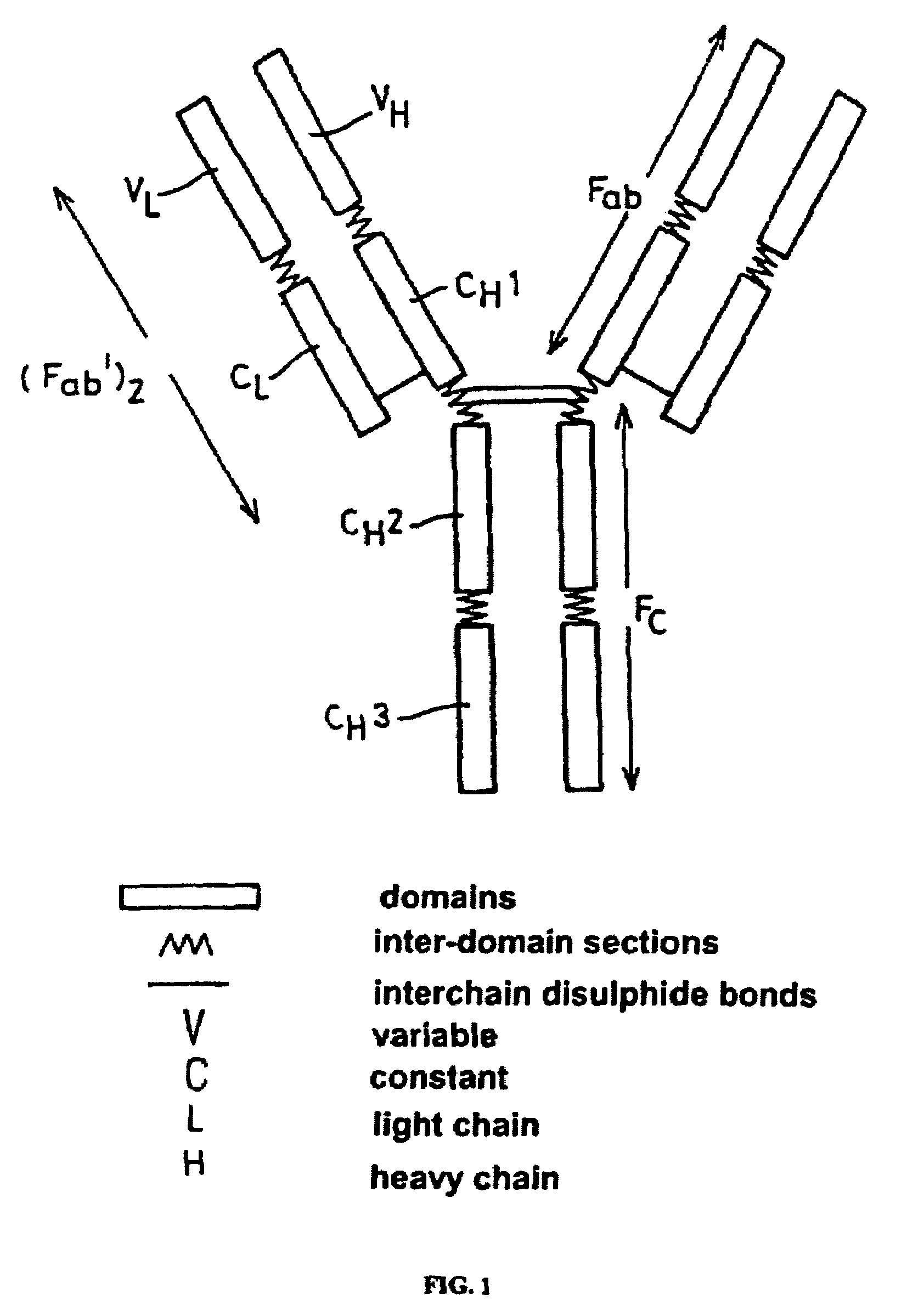
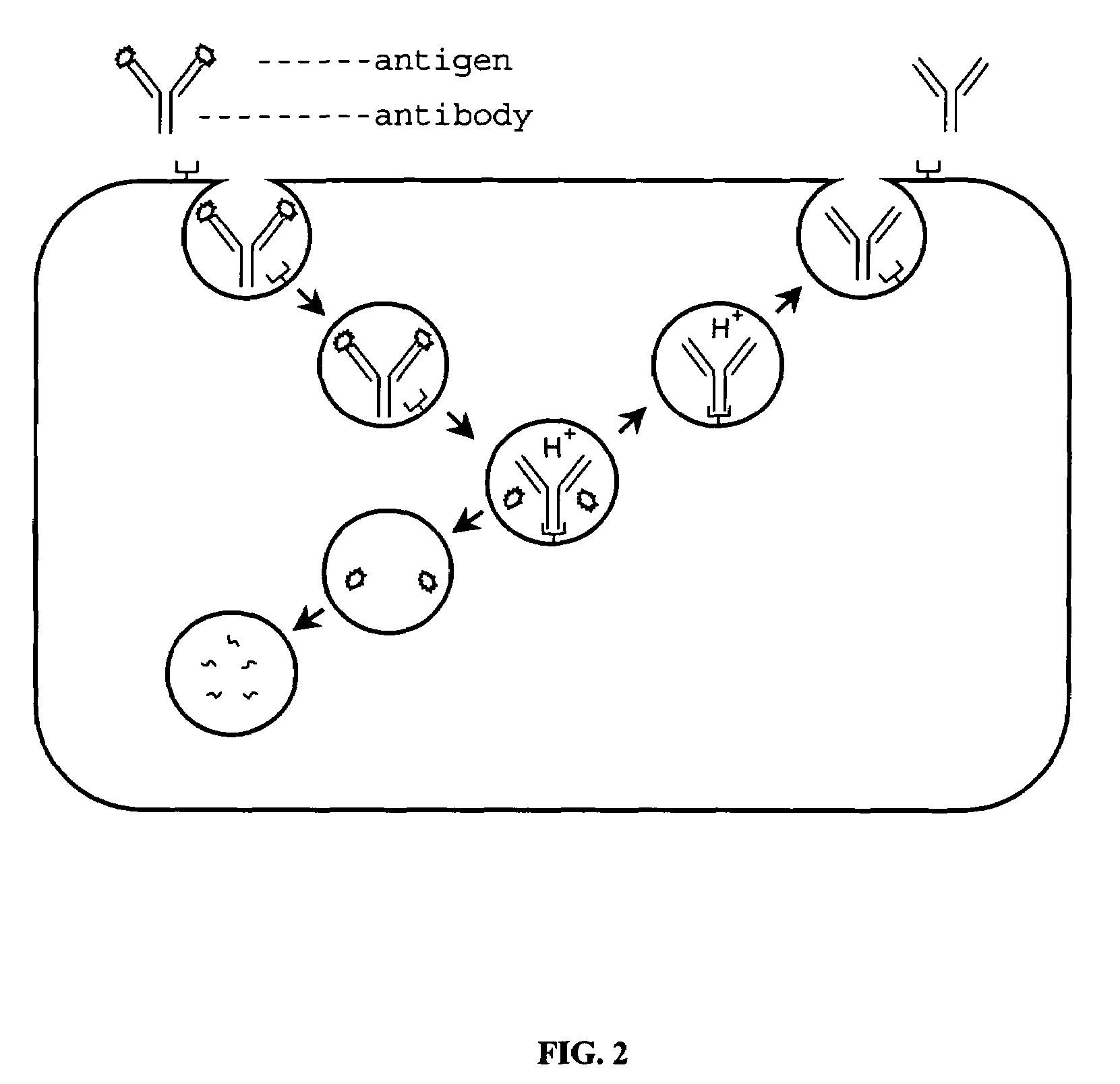
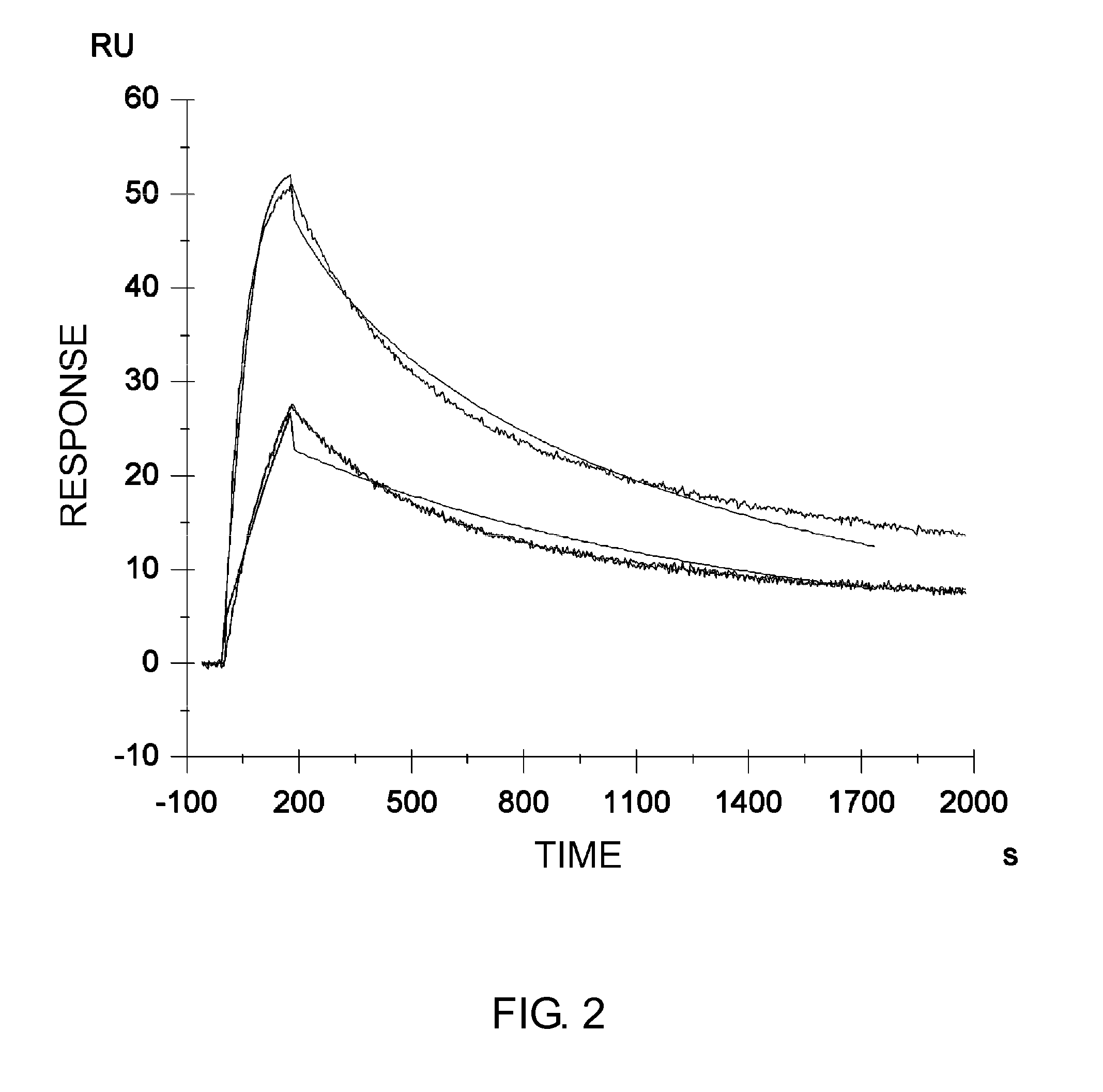
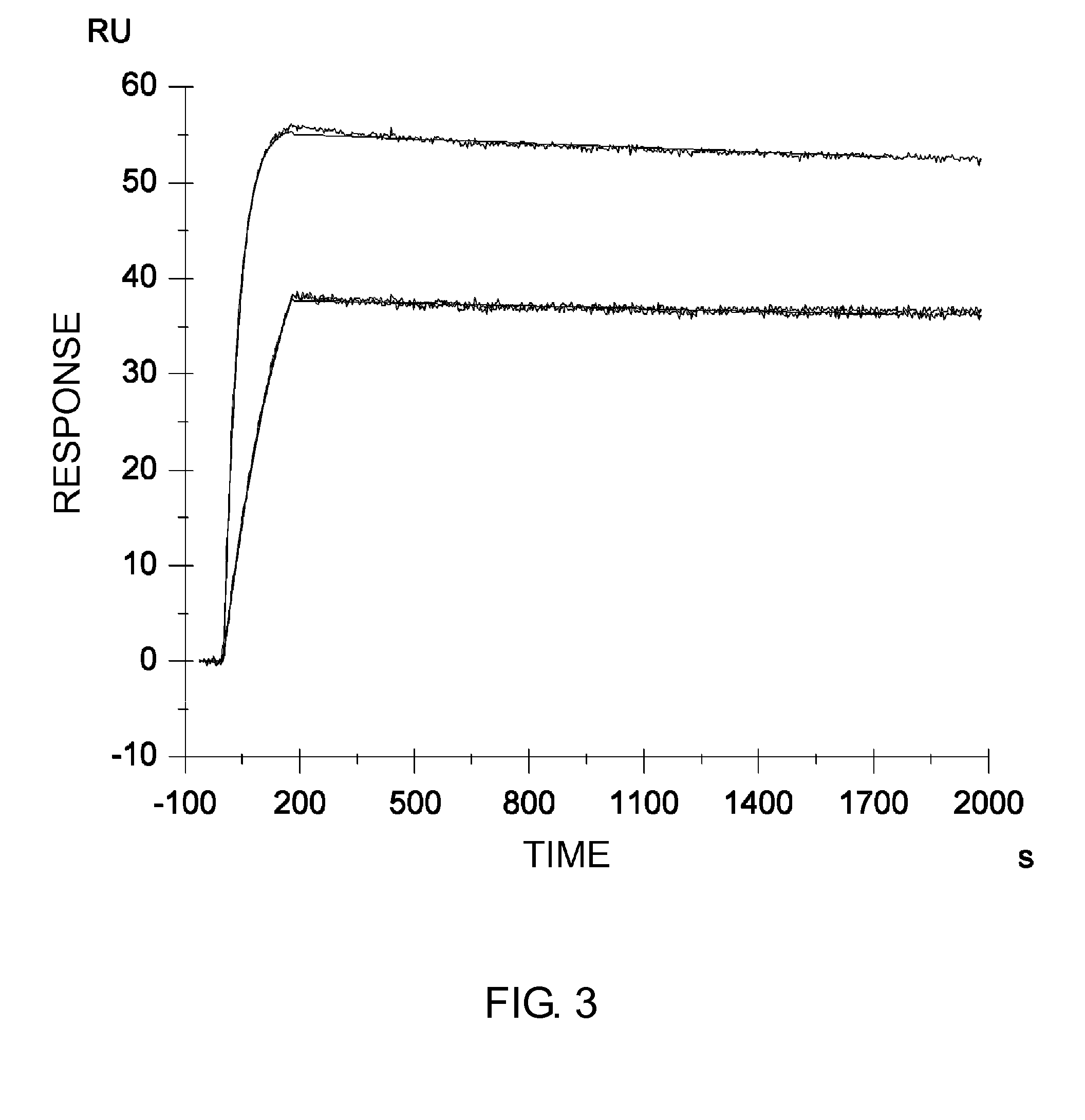
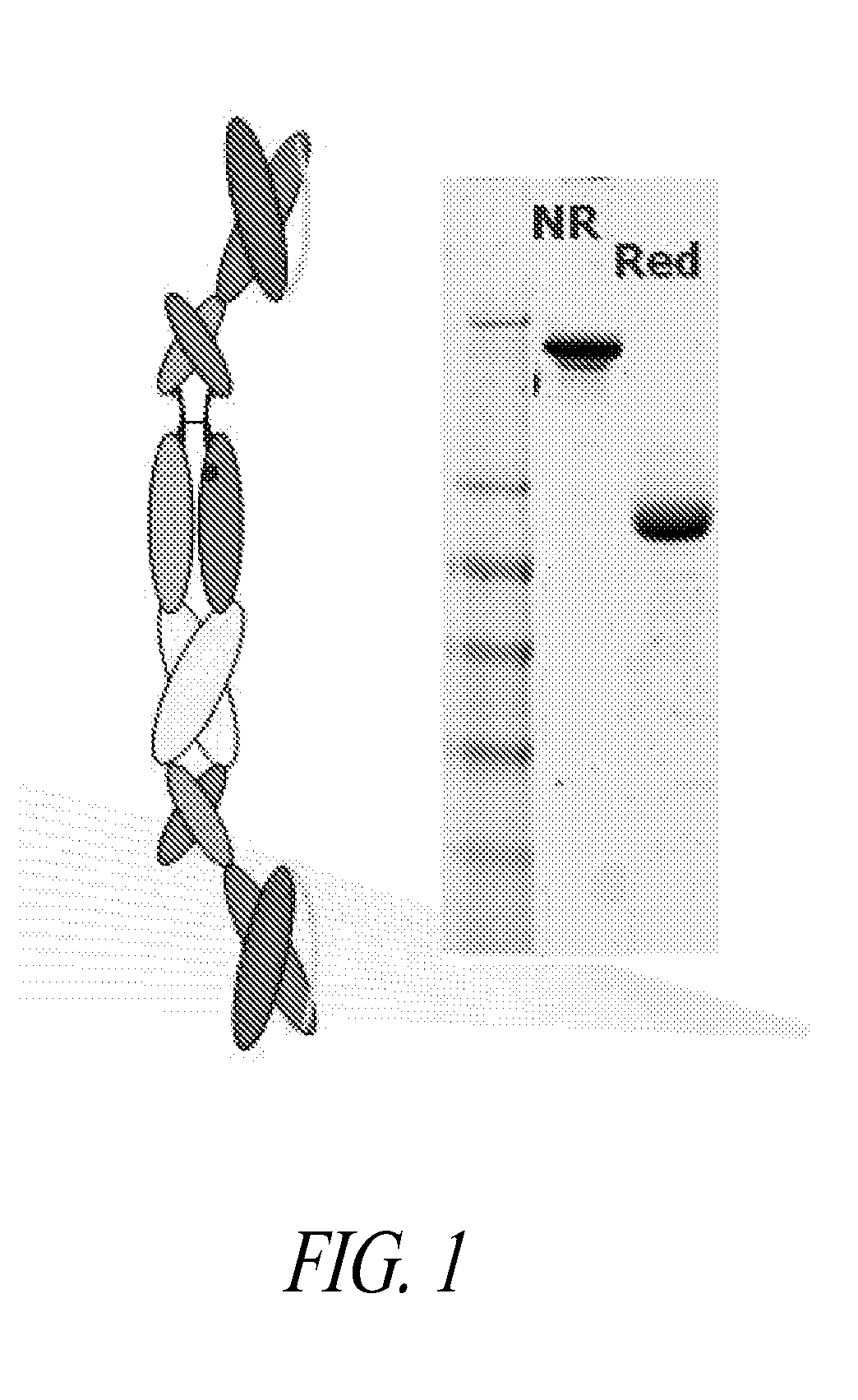
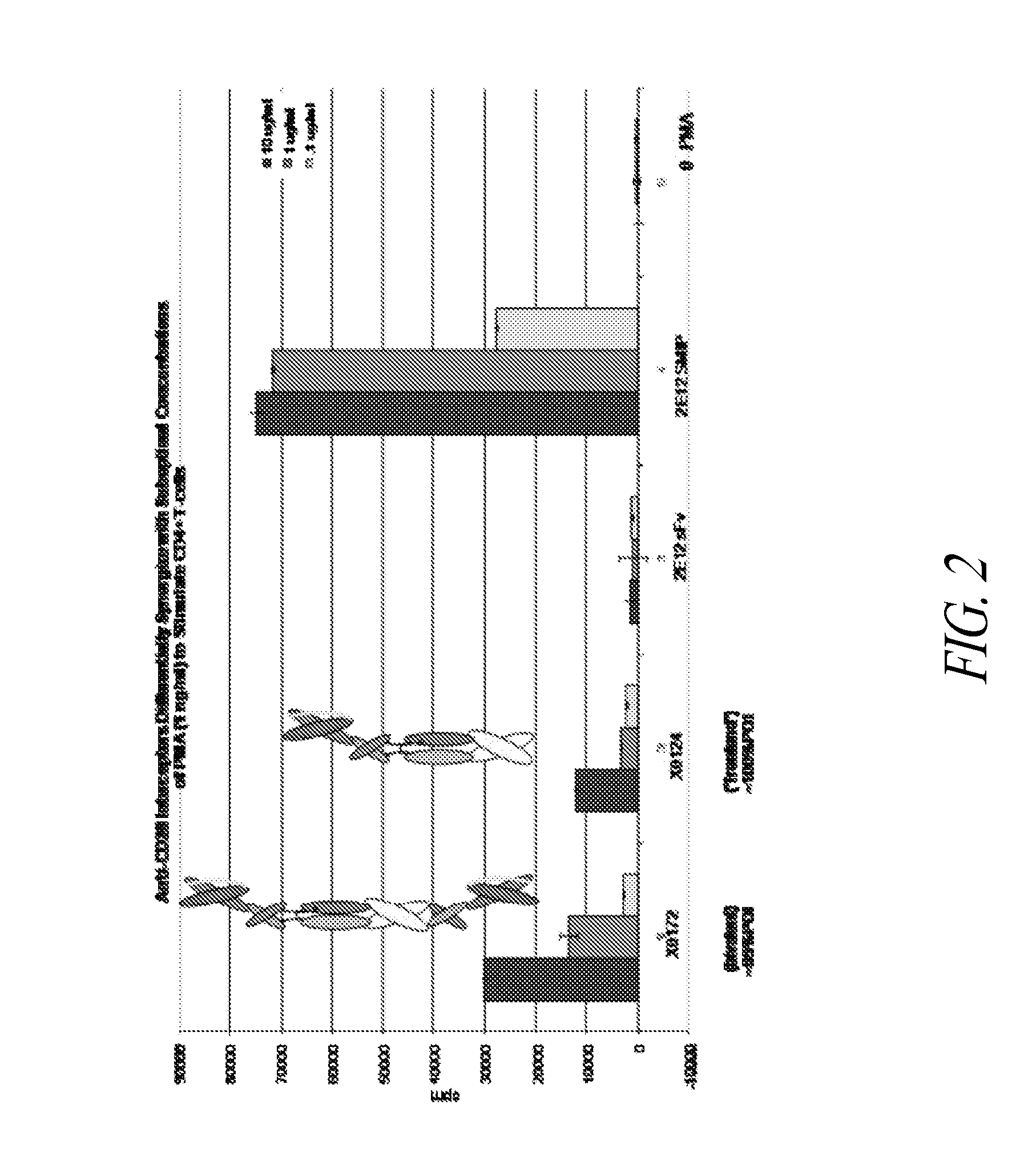
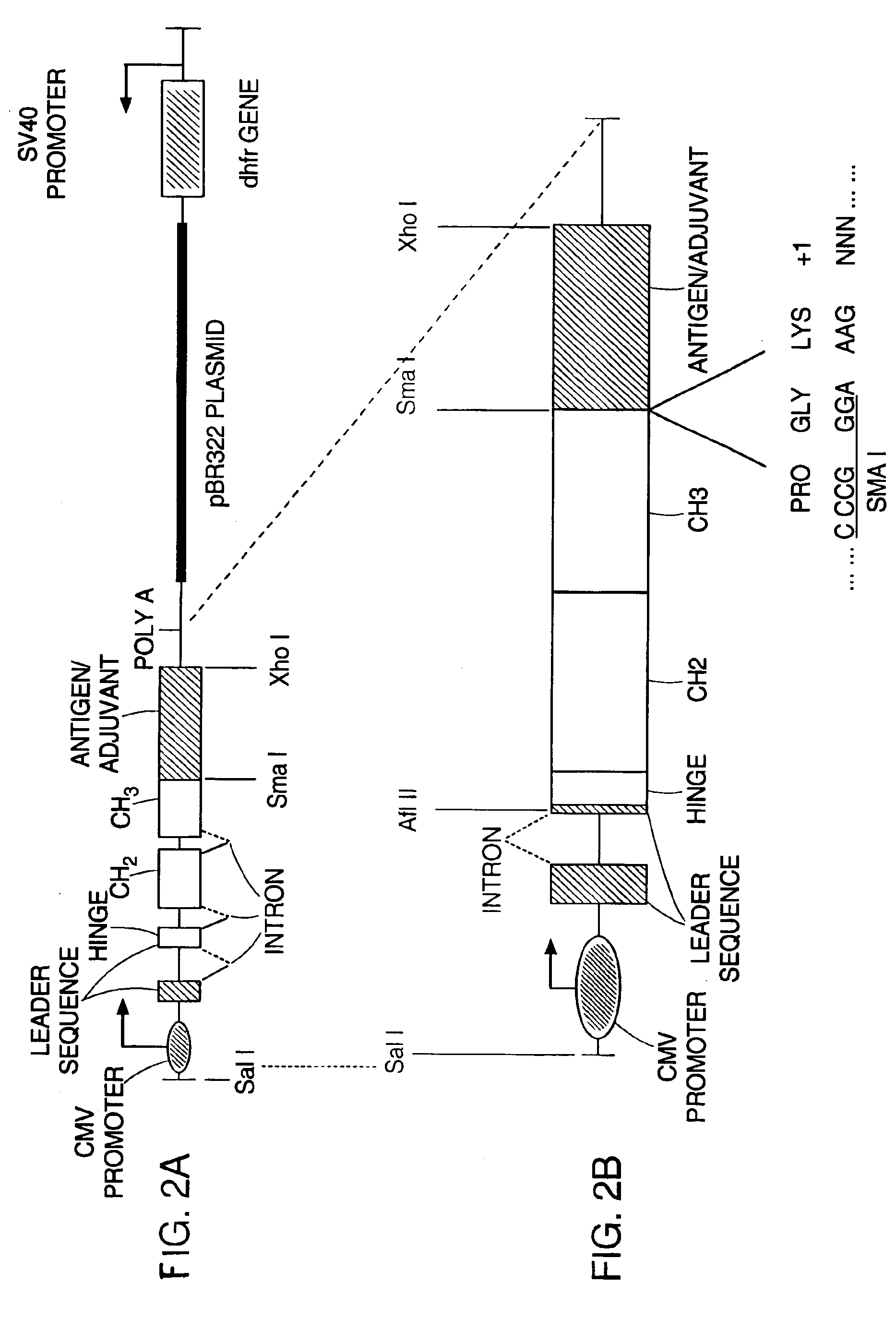
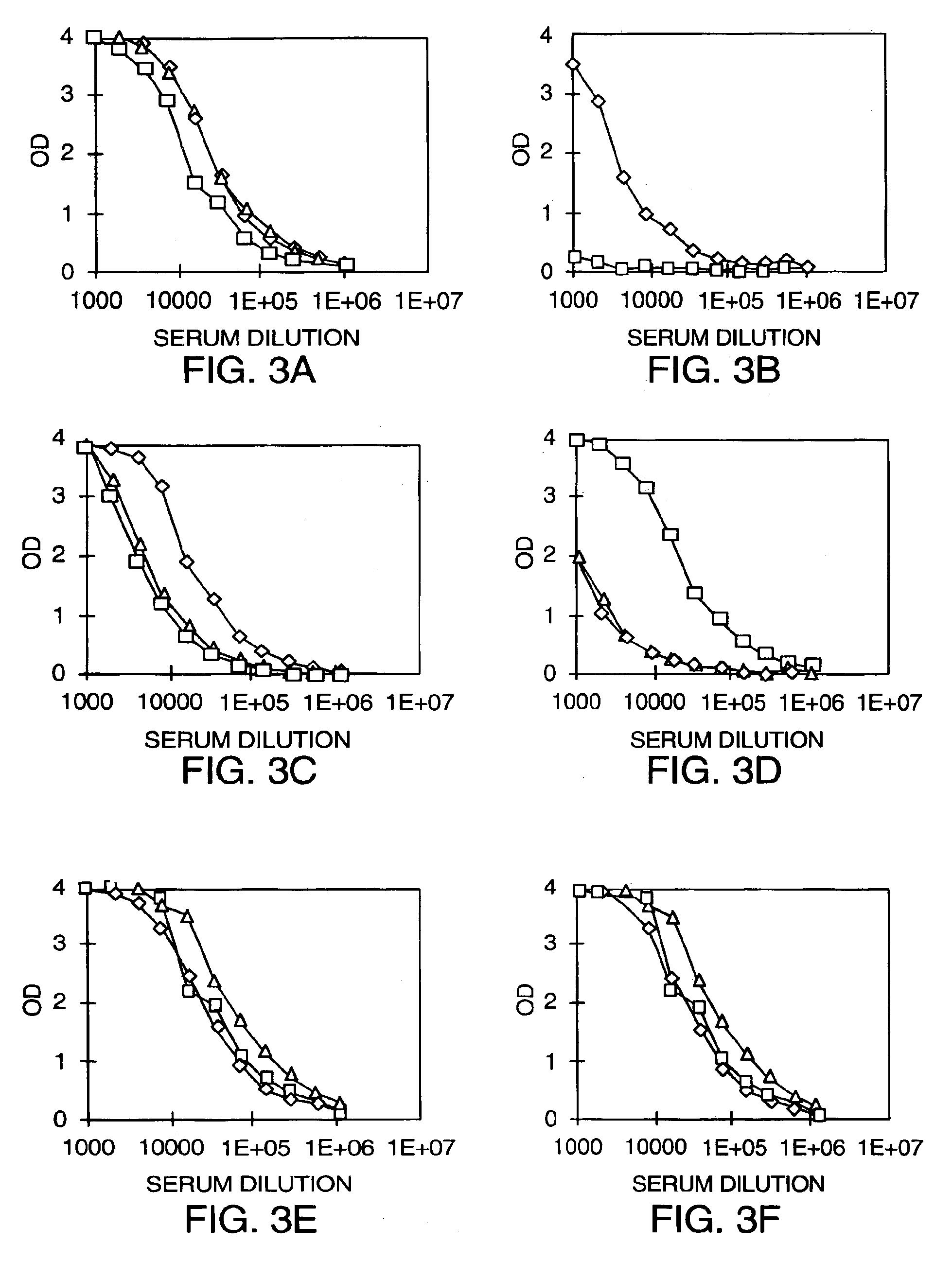
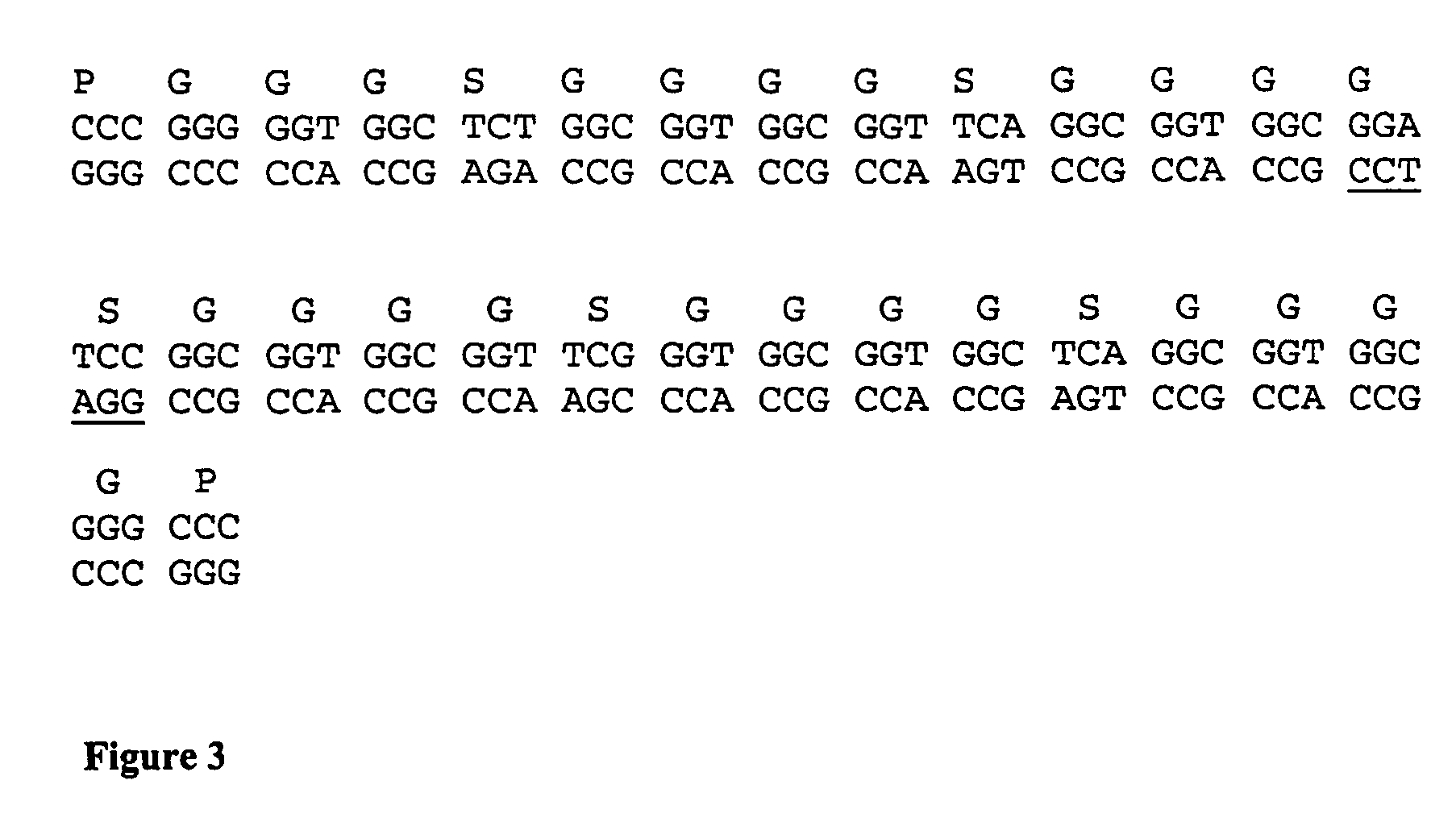
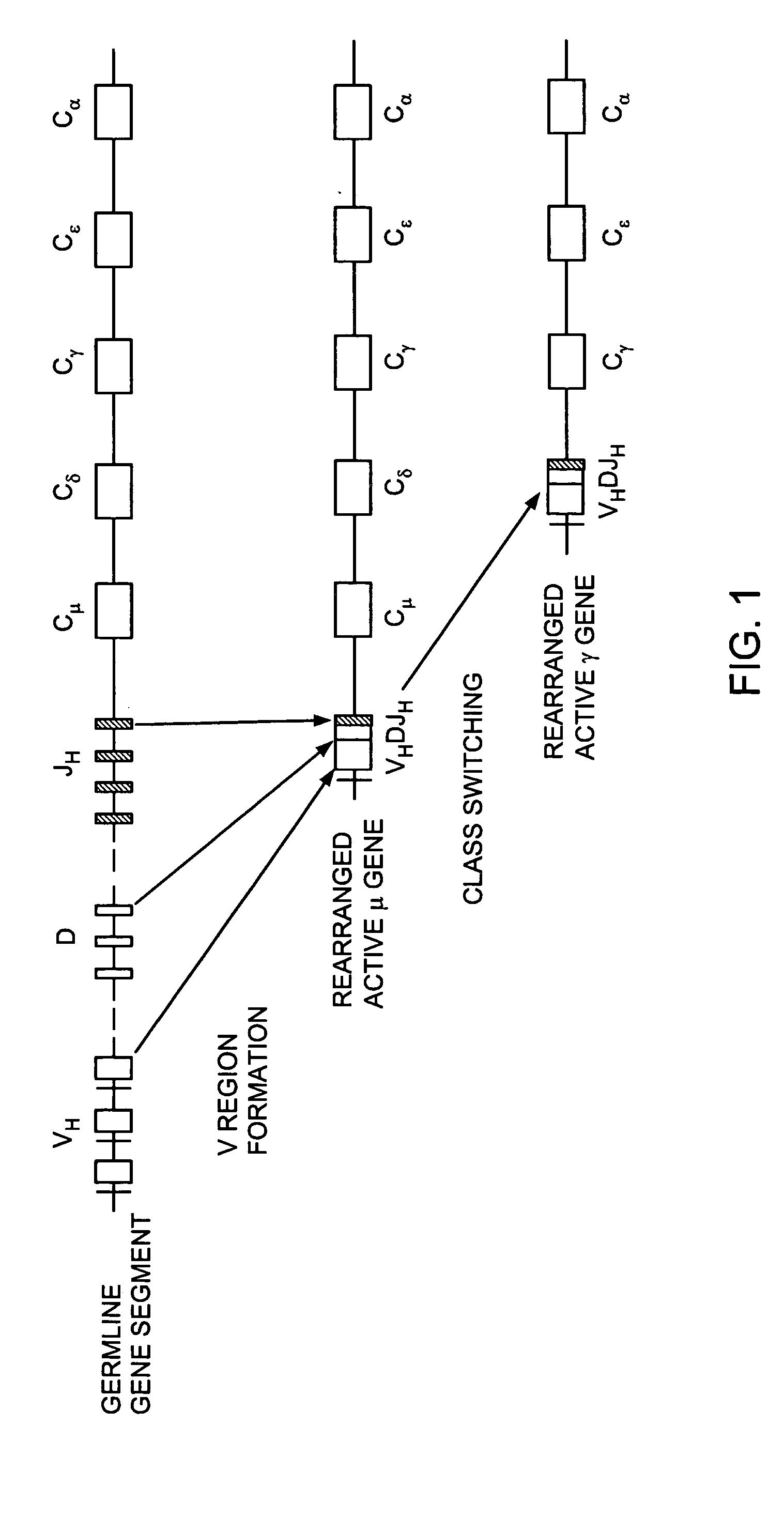
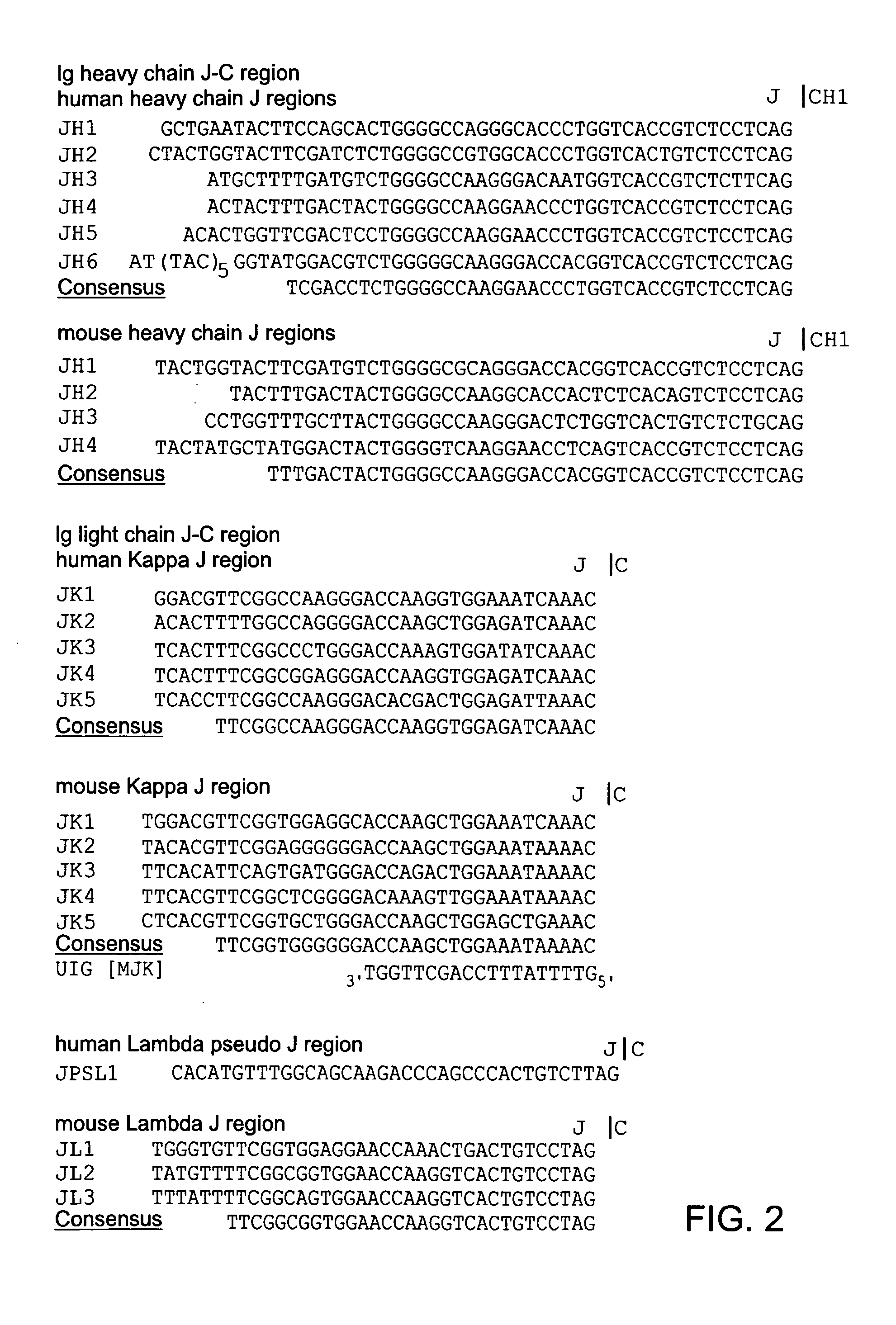
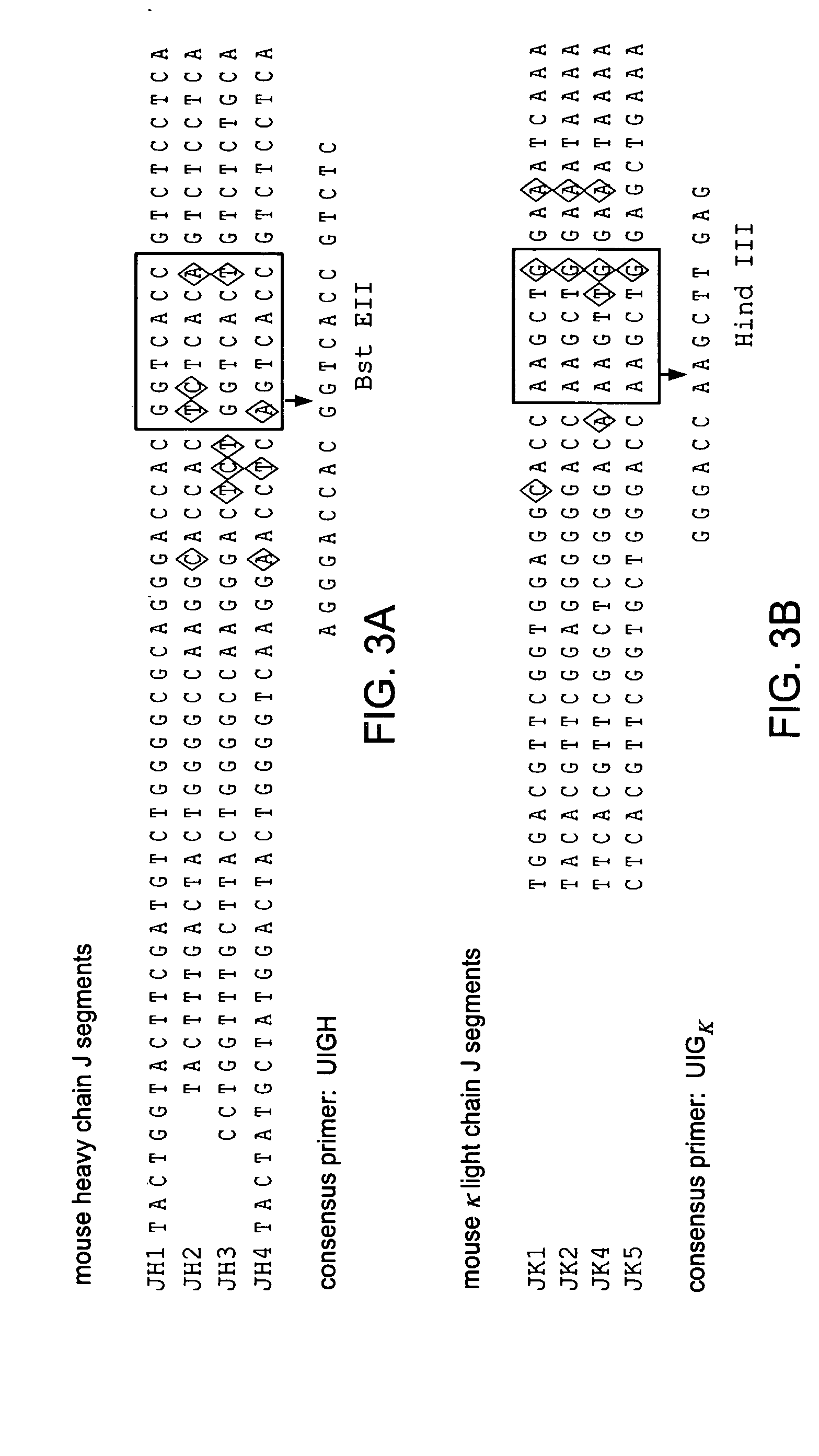
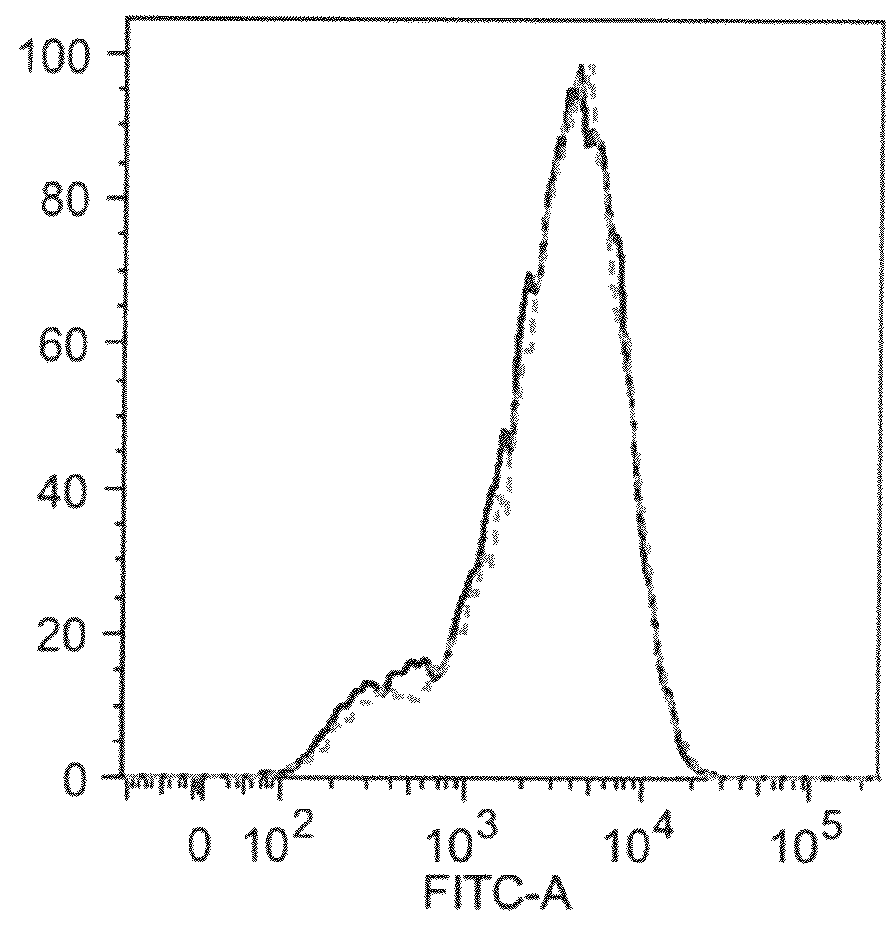
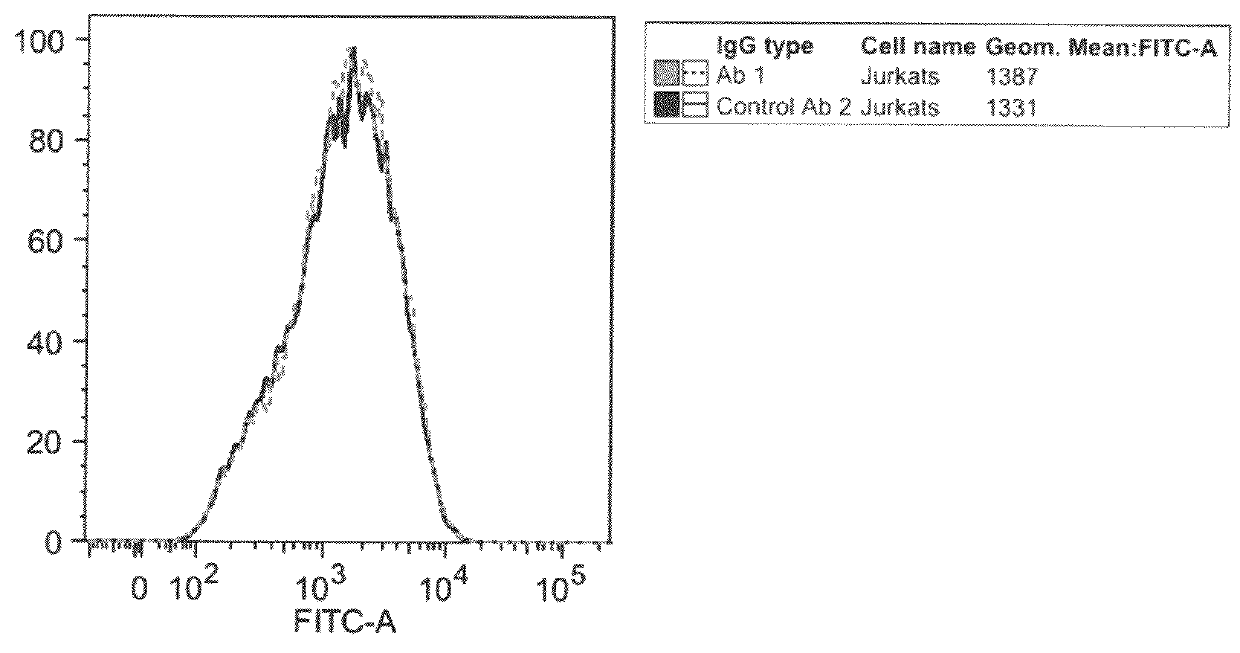
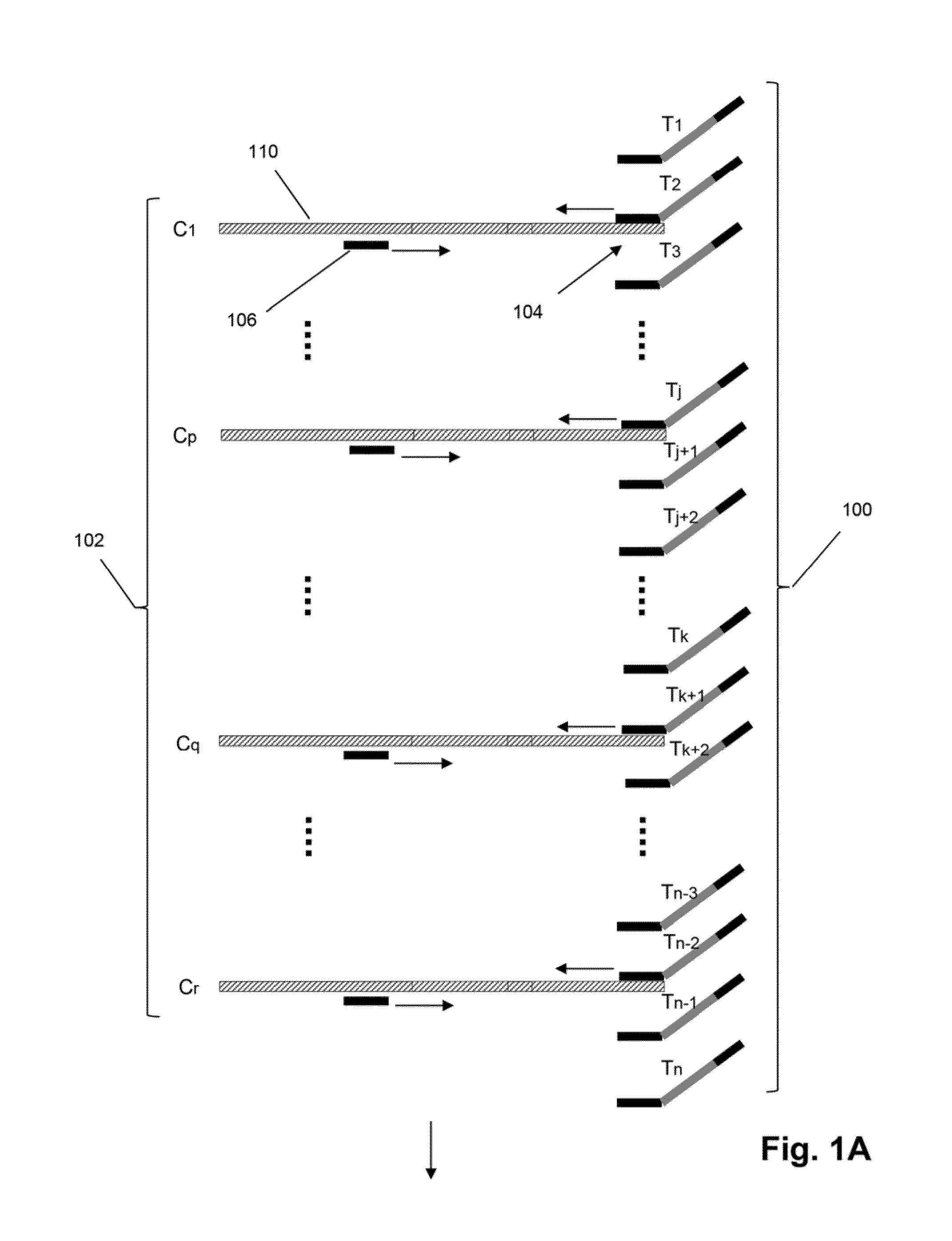
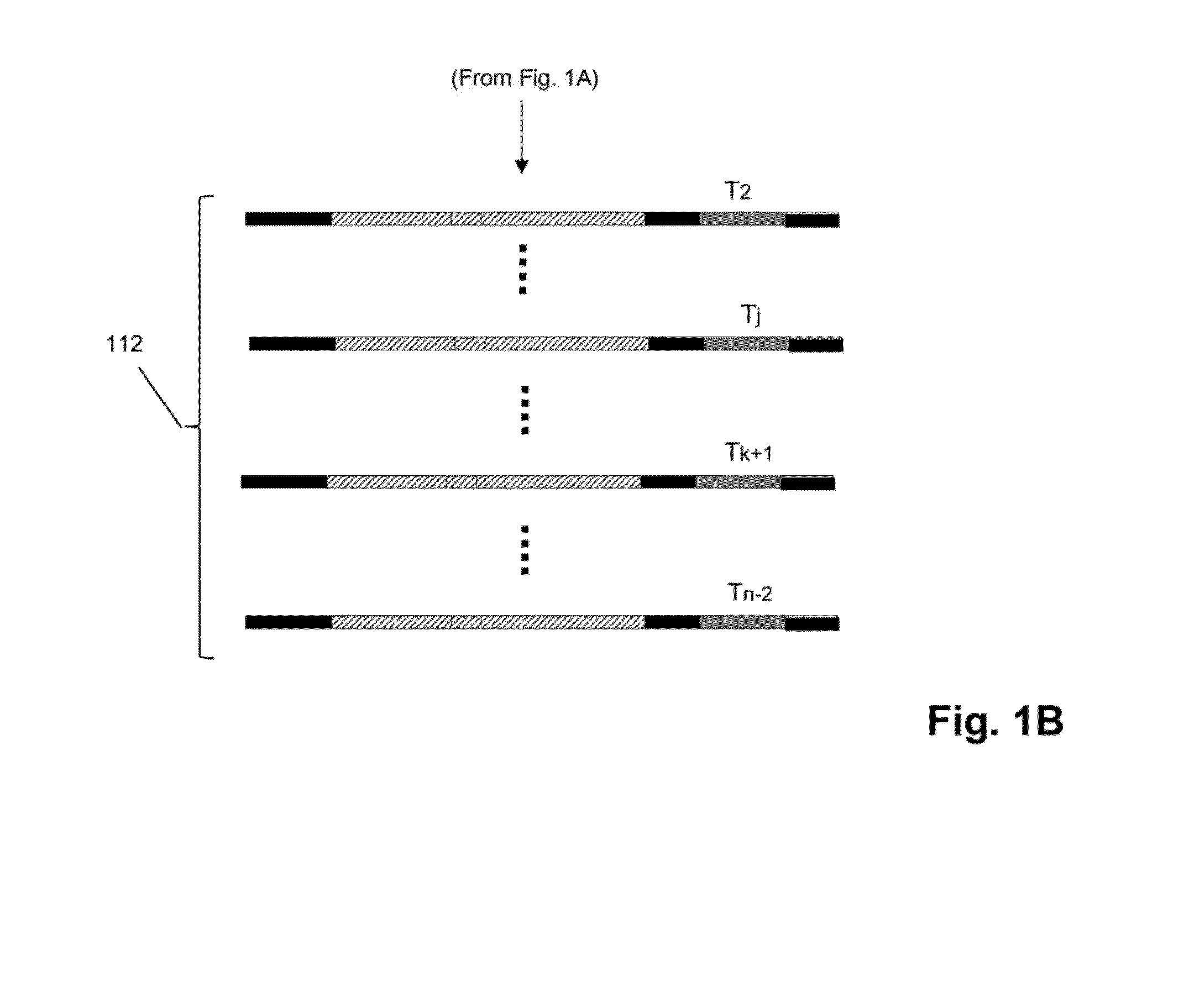
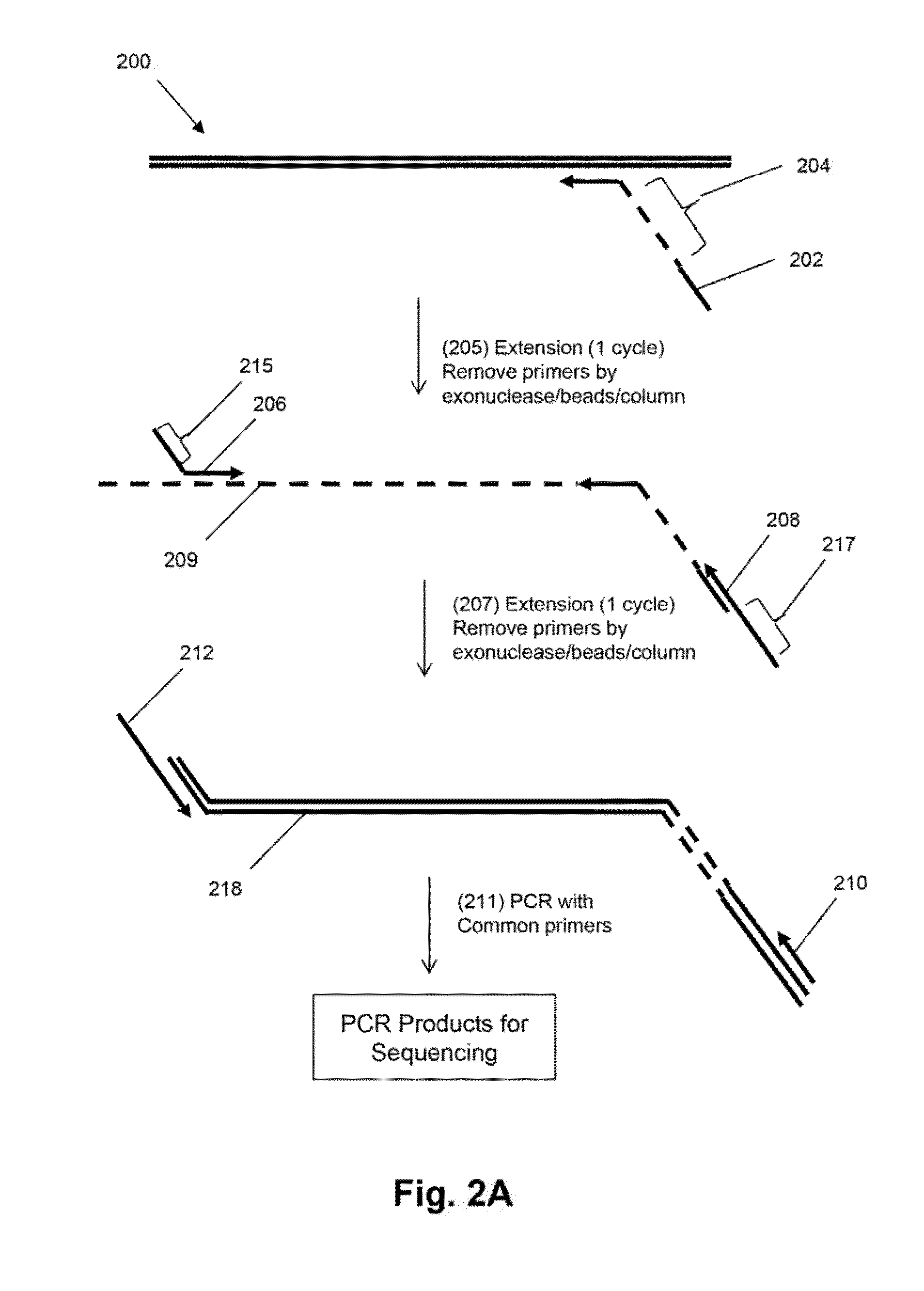
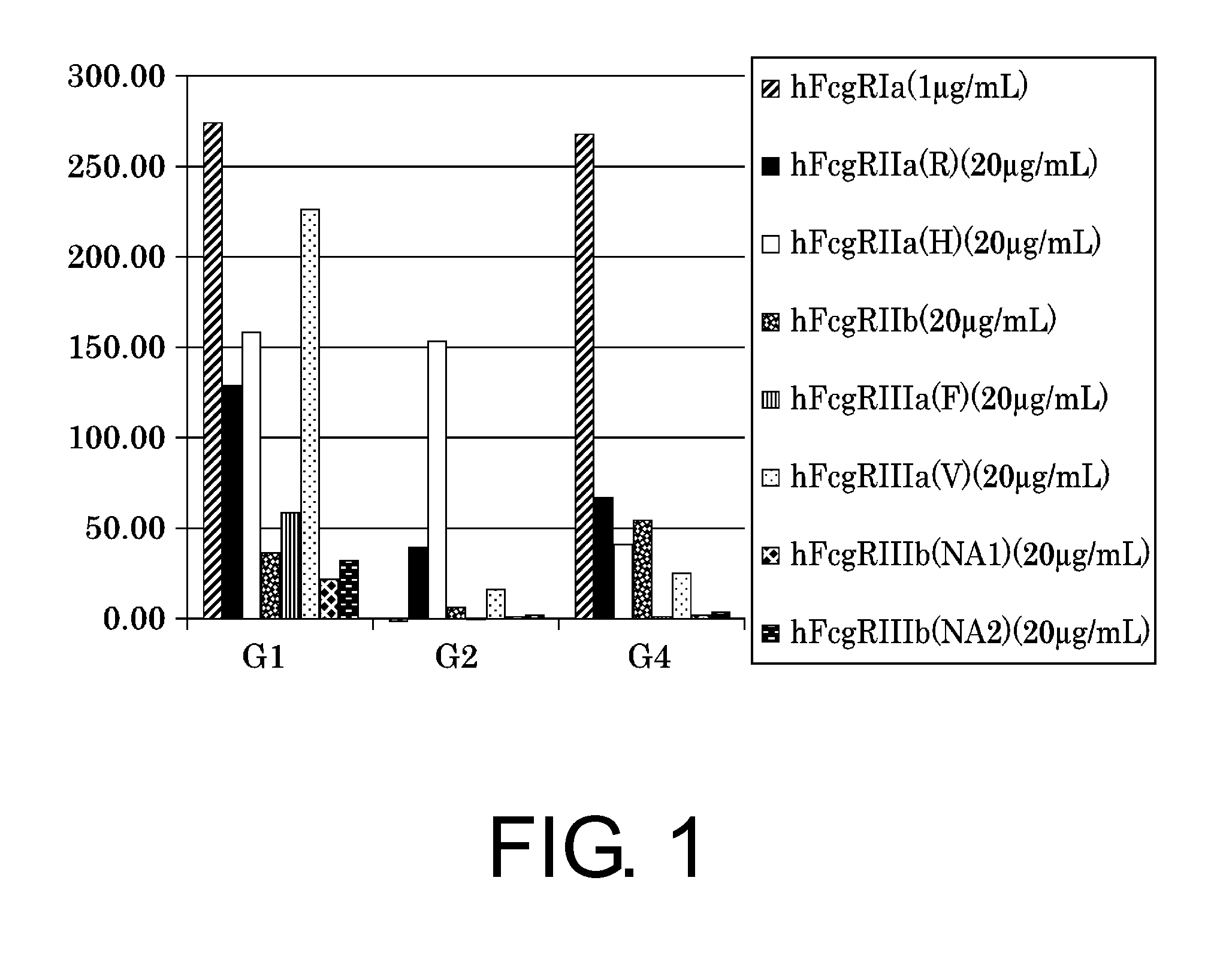
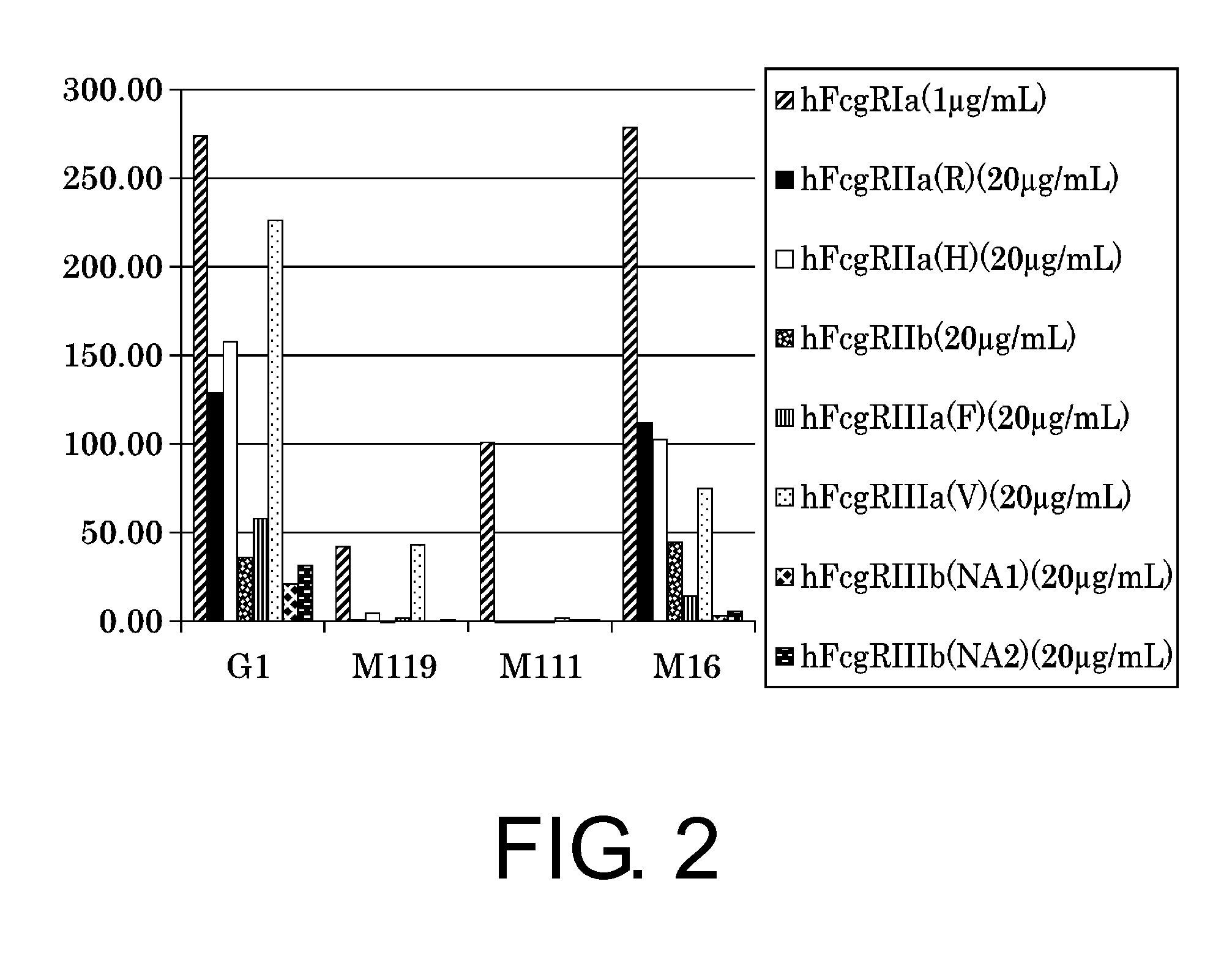
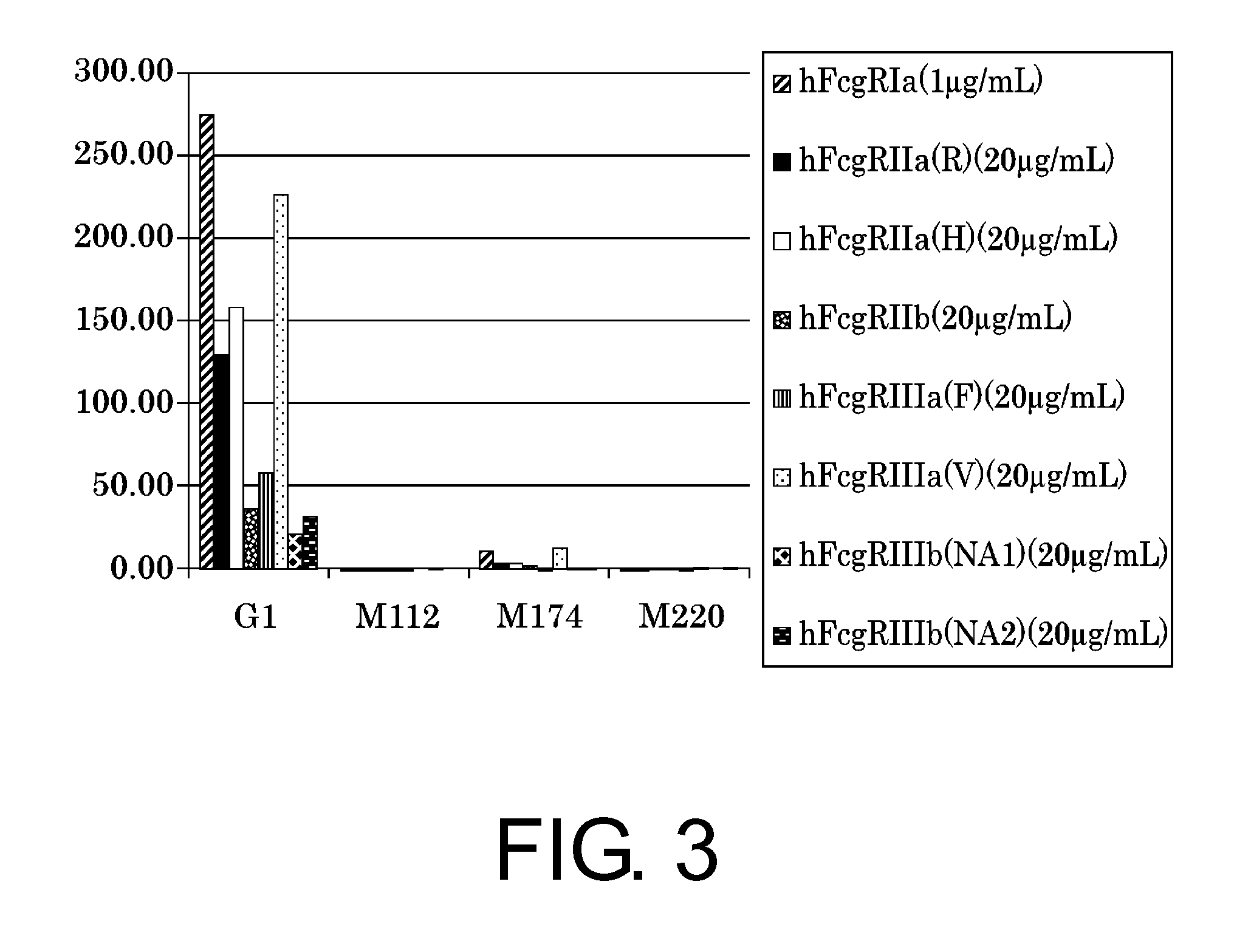
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
535 results about "Constant region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of constant region. : the part of the polypeptide chain of a light or heavy chain of an antibody that ends in a free carboxyl group −COOH and that is relatively constant in its sequence of amino acid residues from one antibody to another.
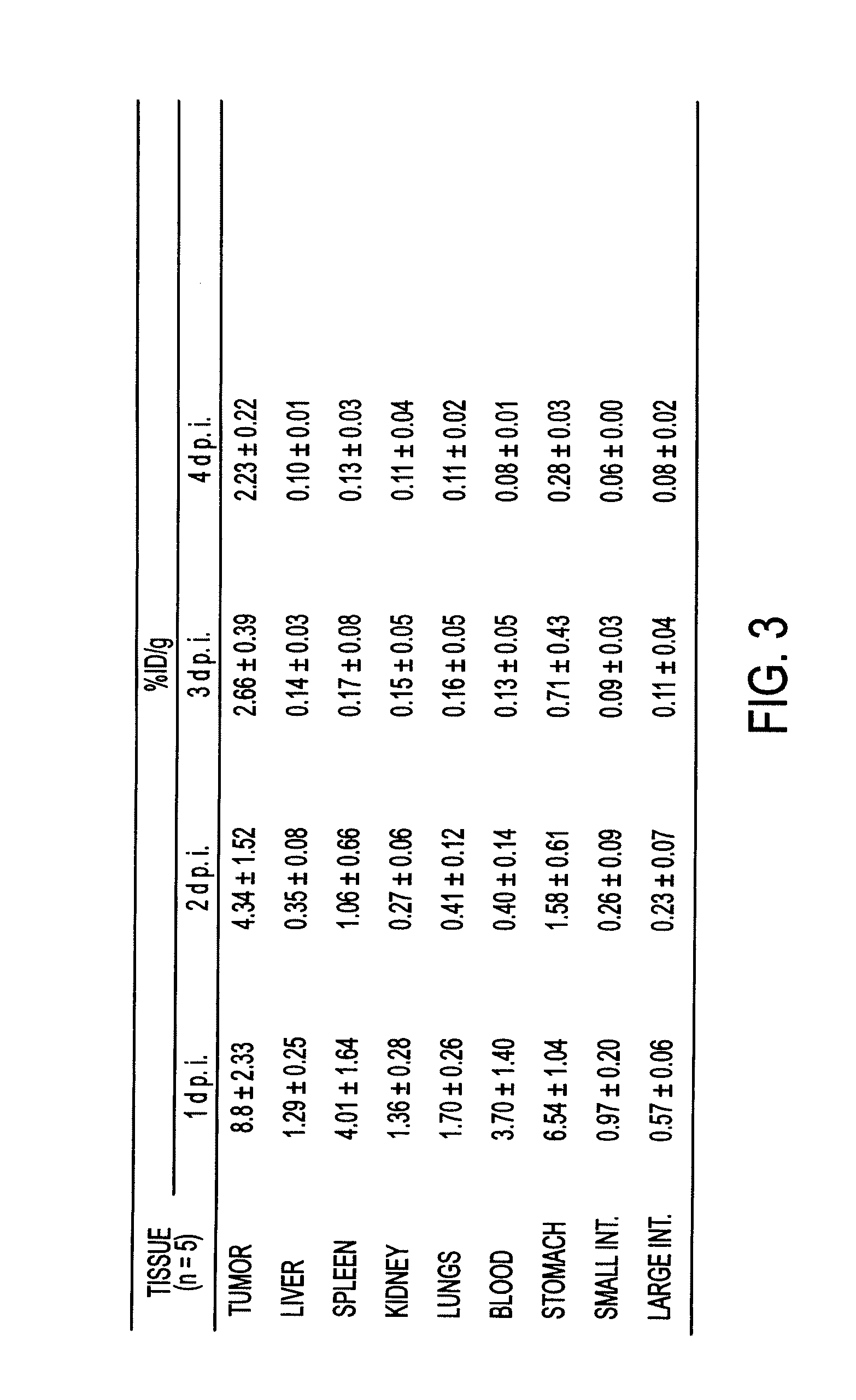
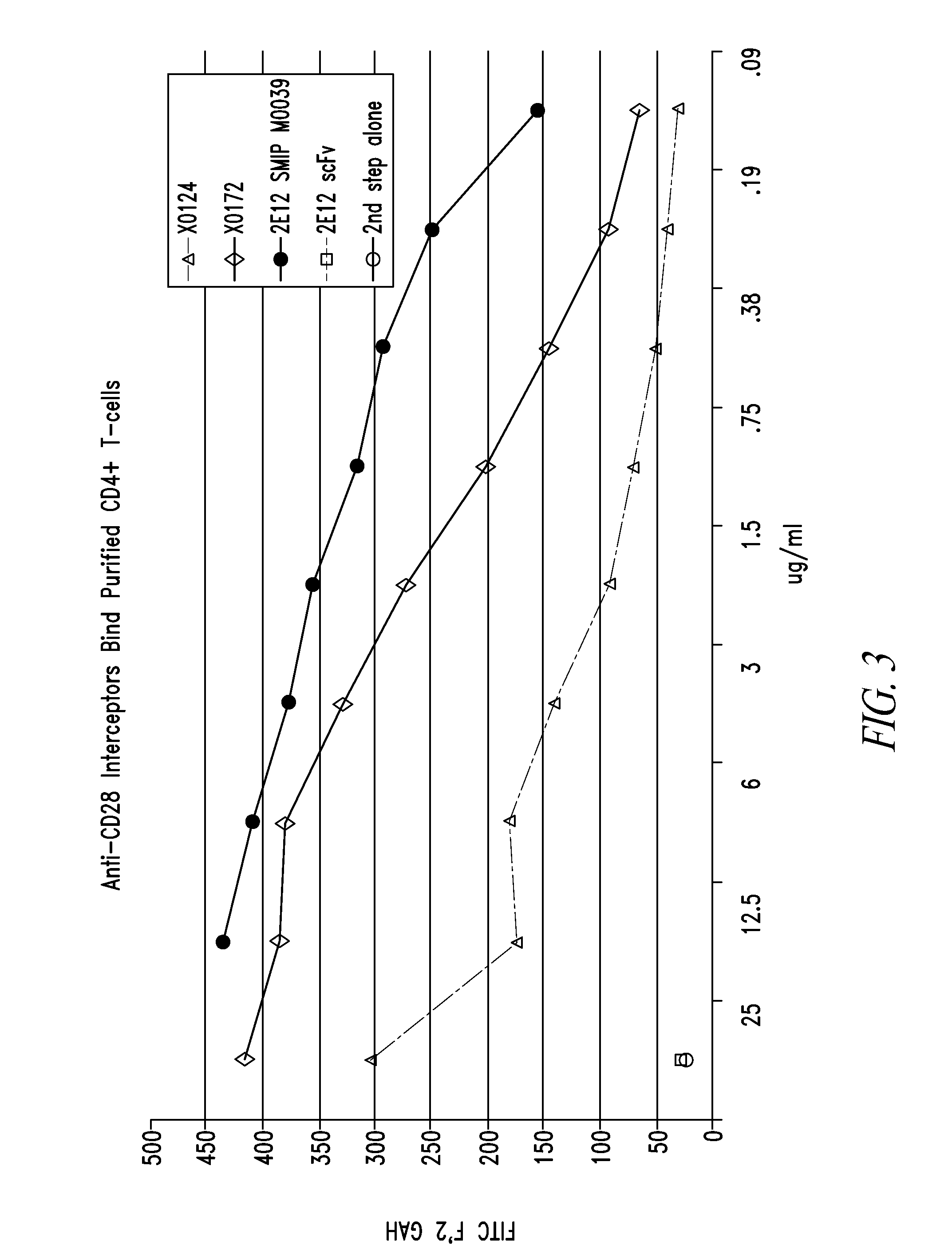
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050014934A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesHalf-lifeAntibody
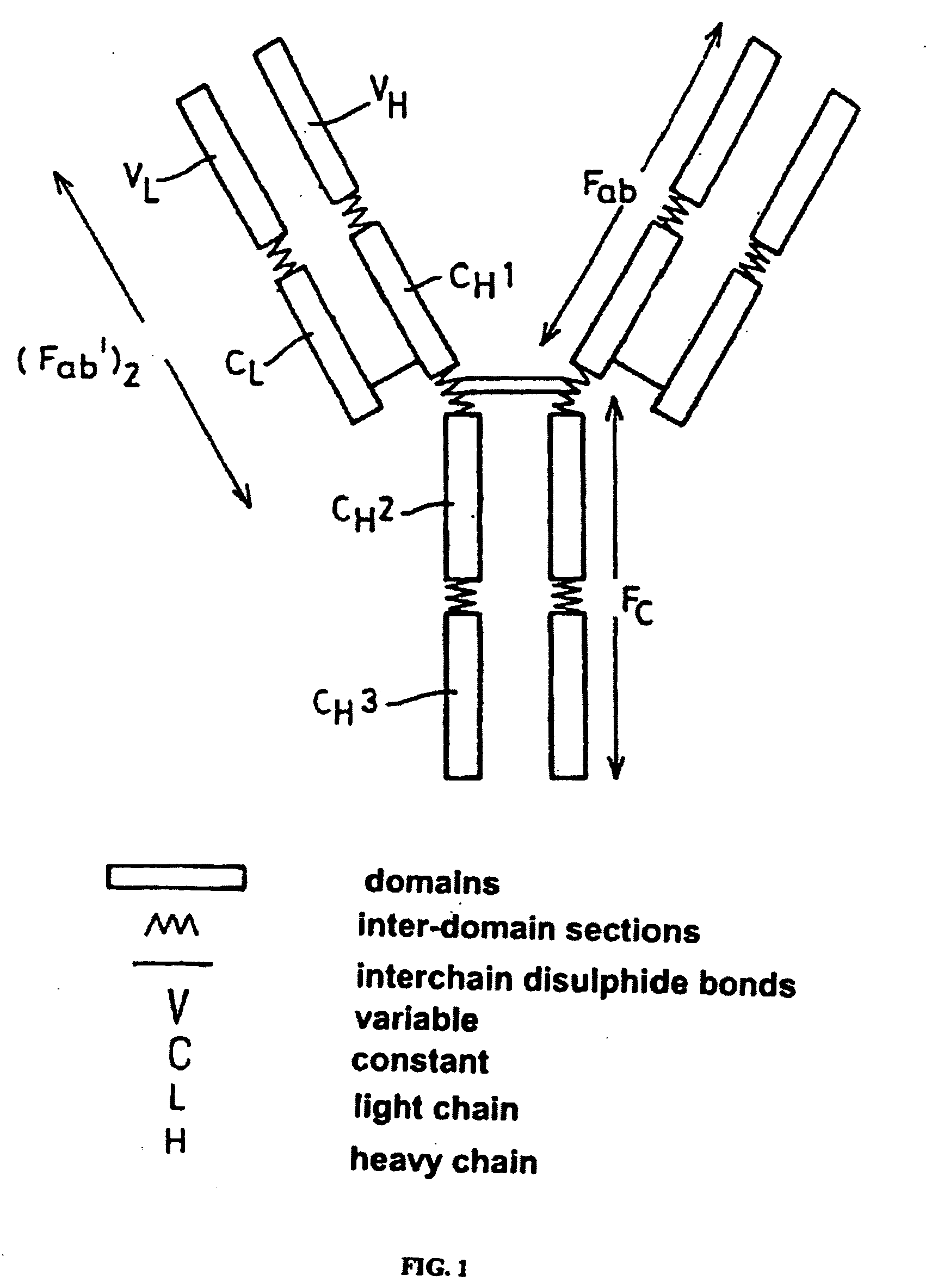
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS7217797B2Function increaseExtended half-lifeAnimal cellsSugar derivativesAntiendomysial antibodiesBiochemistry
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
Chimeric antibody with specificity to human B cell surface antigen
A chimeric antibody with human constant region and murine variable region, having specificity to a 35 kDA polypeptide (Bp35(CD20)) expressed on the surface of human B cells, methods of production, and uses.
Owner:ROYALTY PHARMA FINANCE TRUST
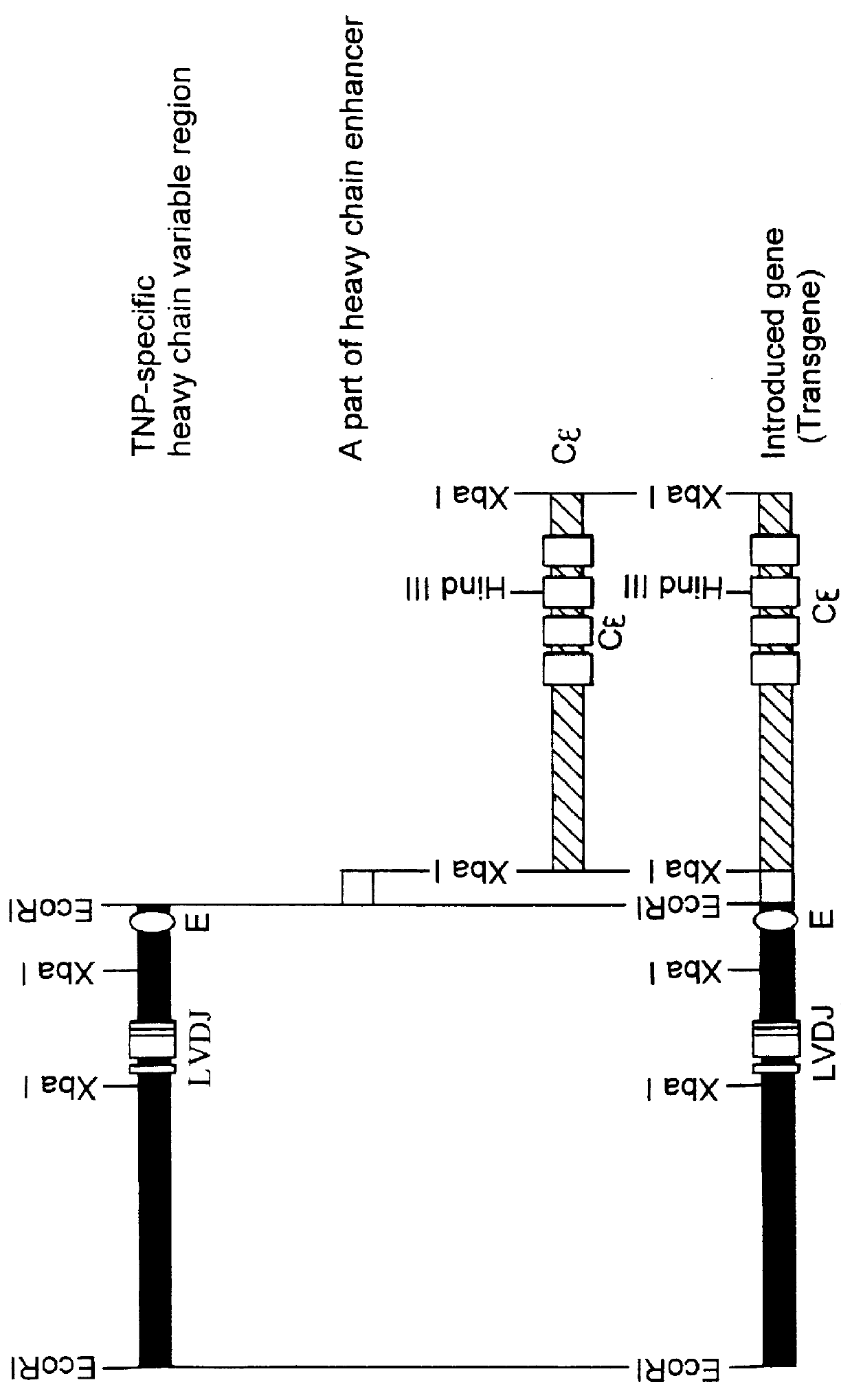
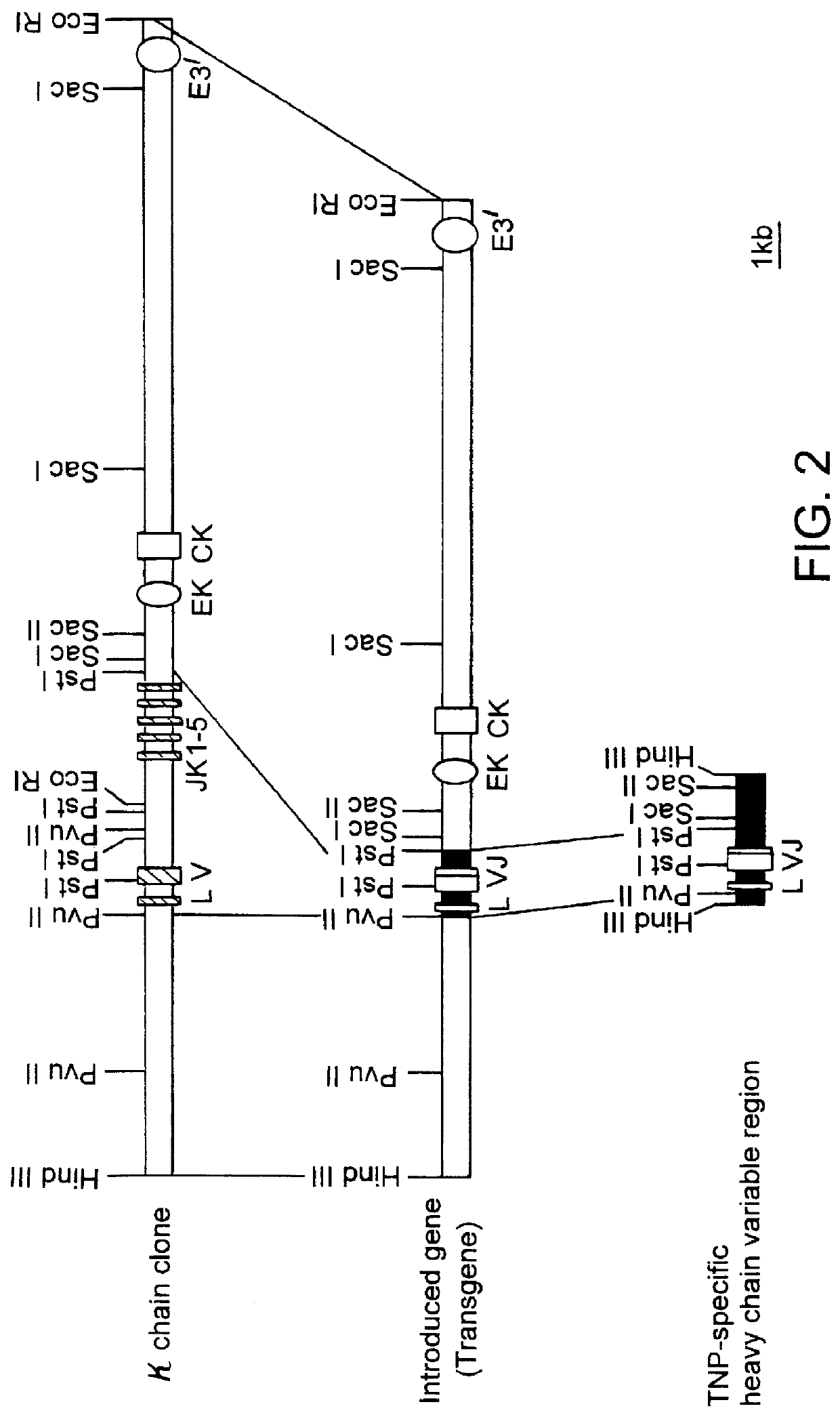
Transgenic mouse allergy models and methods for their use
InactiveUS6118044AImprove import efficiencySufficient amountMicrobiological testing/measurementTissue cultureAntibody typesAllergy
Transgenic mice which constitutively express an antibody-type molecule encoded by the transgene and which has an IgE heavy chain constant region and is specific for a pre-defined antigen, provide an allergic reaction to that antigen without prior sensitization and are useful as allergy models.
Owner:SANKYO CO LTD +1
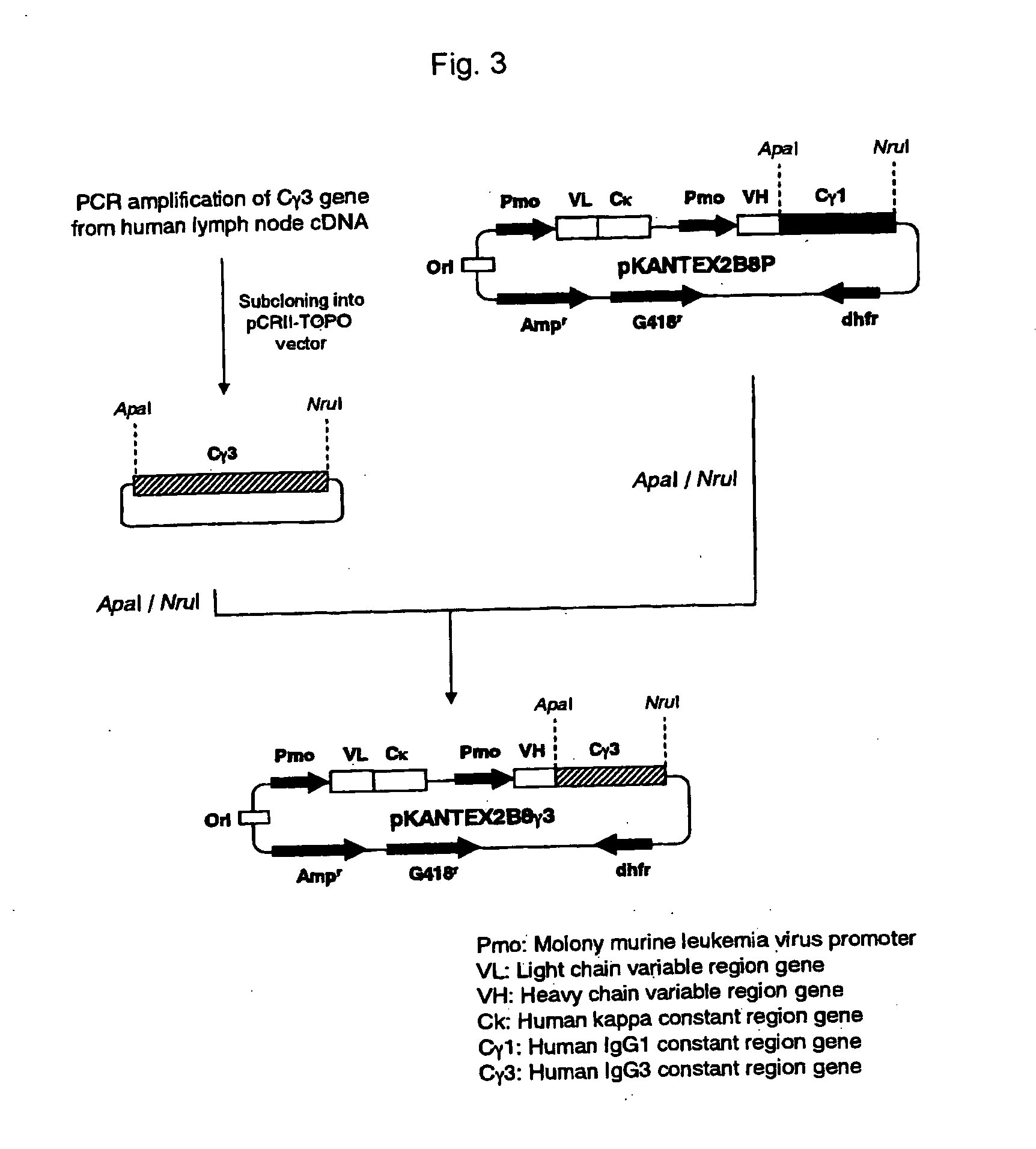
Recombinant antibody composition
ActiveUS20070148165A1Enhanced effector functionGood treatment effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsA-DNABULK ACTIVE INGREDIENT
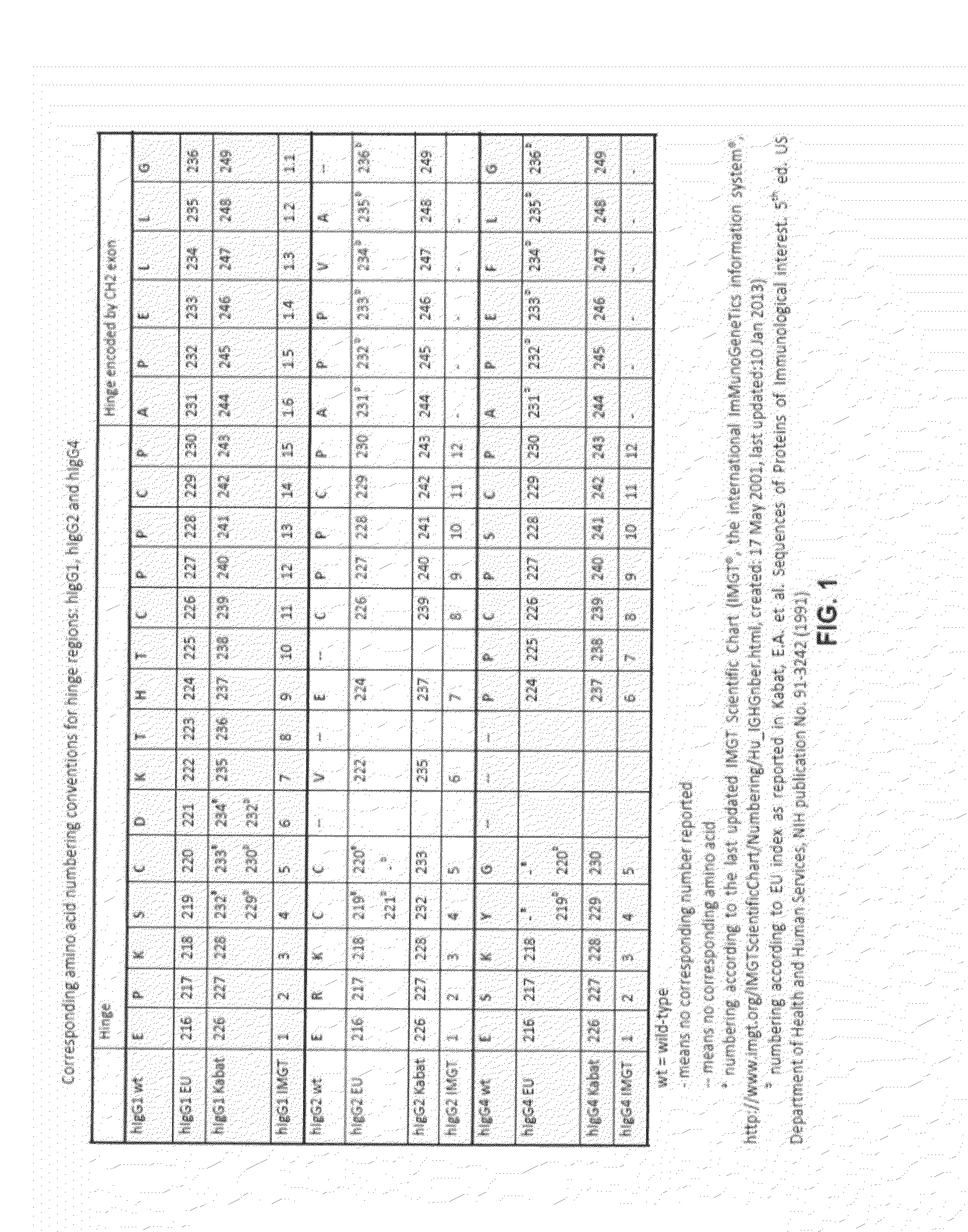
The present invention relates to a recombinant antibody composition having higher complement-dependent cytotoxic activity than a human IgG1 antibody and a human IgG3 antibody, wherein a polypeptide comprising a CH2 domain in the Fc region of a human IgG1 antibody is replaced by a polypeptide comprising an amino acid sequence which corresponds to the same position of a human IgG3 antibody indicated by the EU index as in Kabat, et al.; a DNA encoding the antibody molecule or a heavy chain constant region of the antibody molecule contained in the recombinant antibody composition; a transformant obtainable by introducing the recombinant vector into a host cell; a process for producing the recombinant antibody composition using the transformant; and a medicament comprising the recombinant antibody composition as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
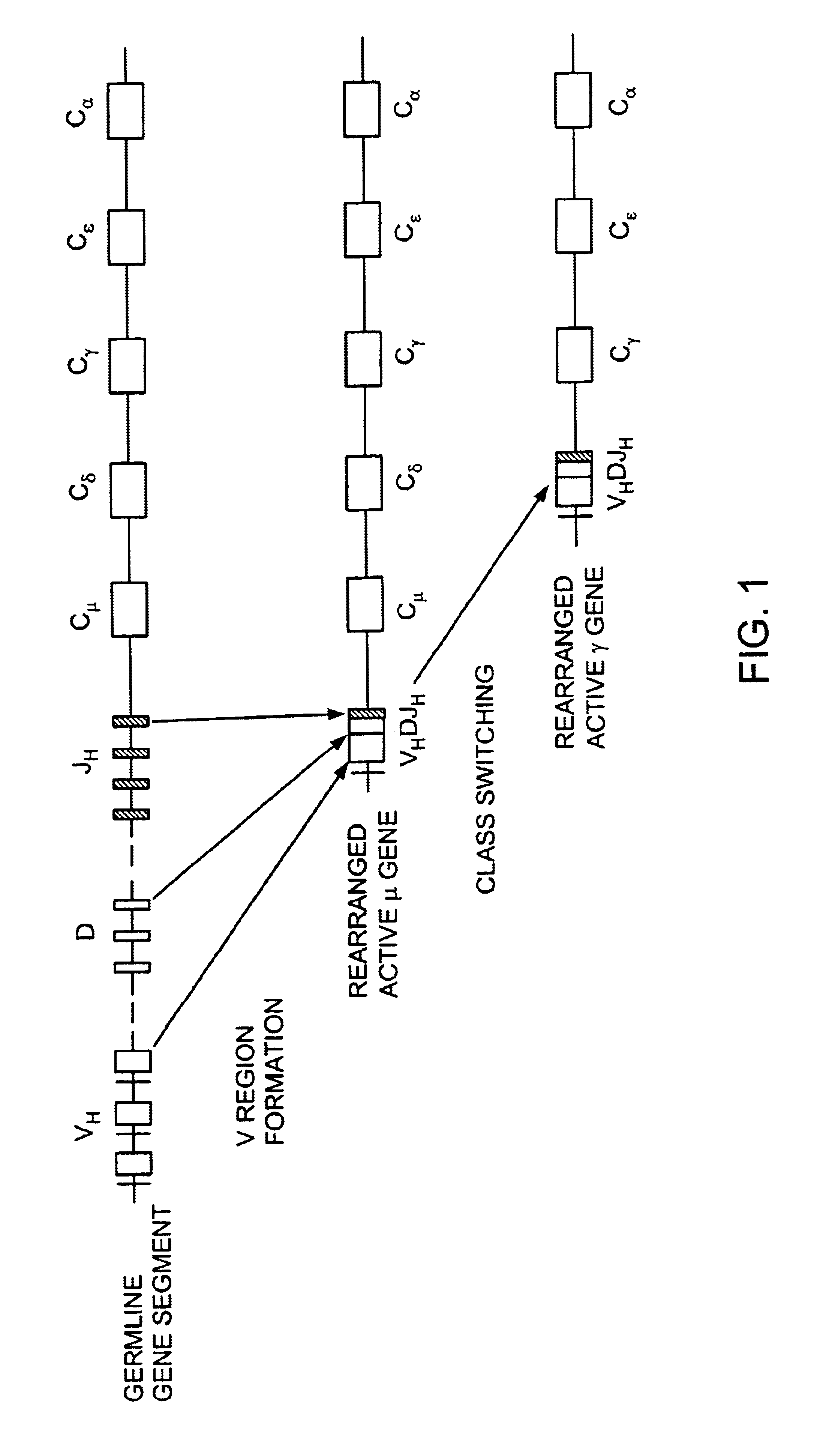
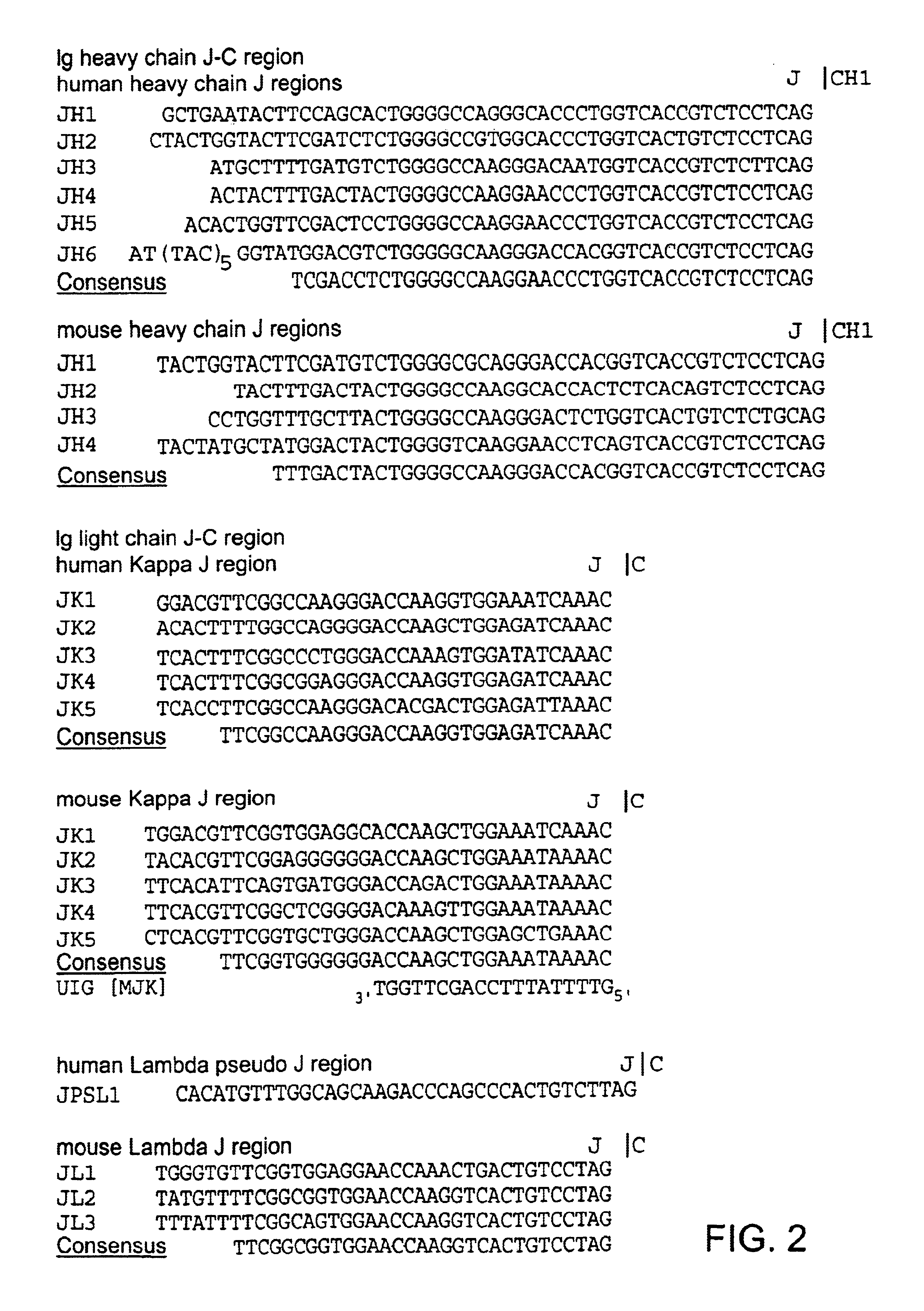
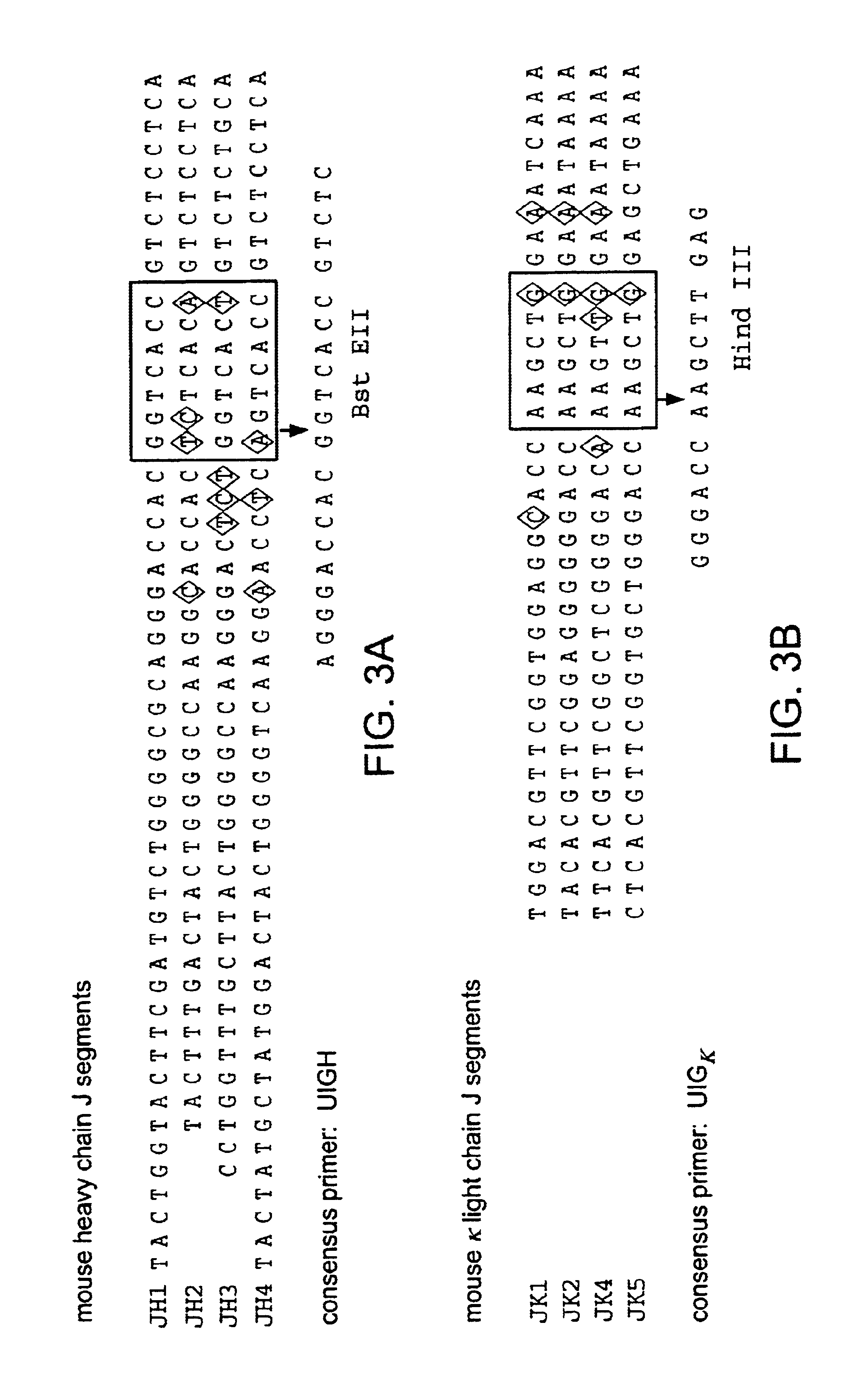
Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions
InactiveUS7049426B2Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenExon
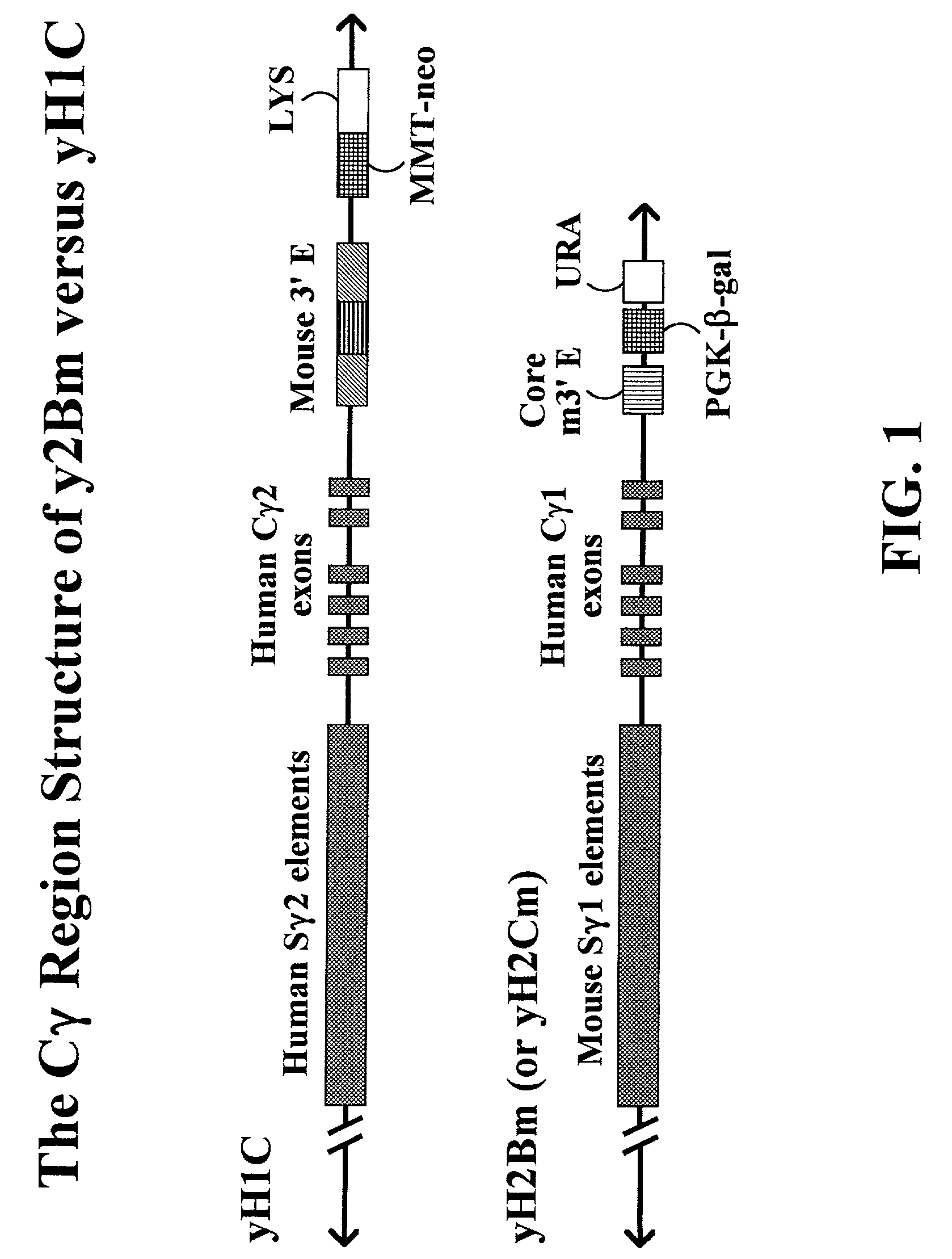
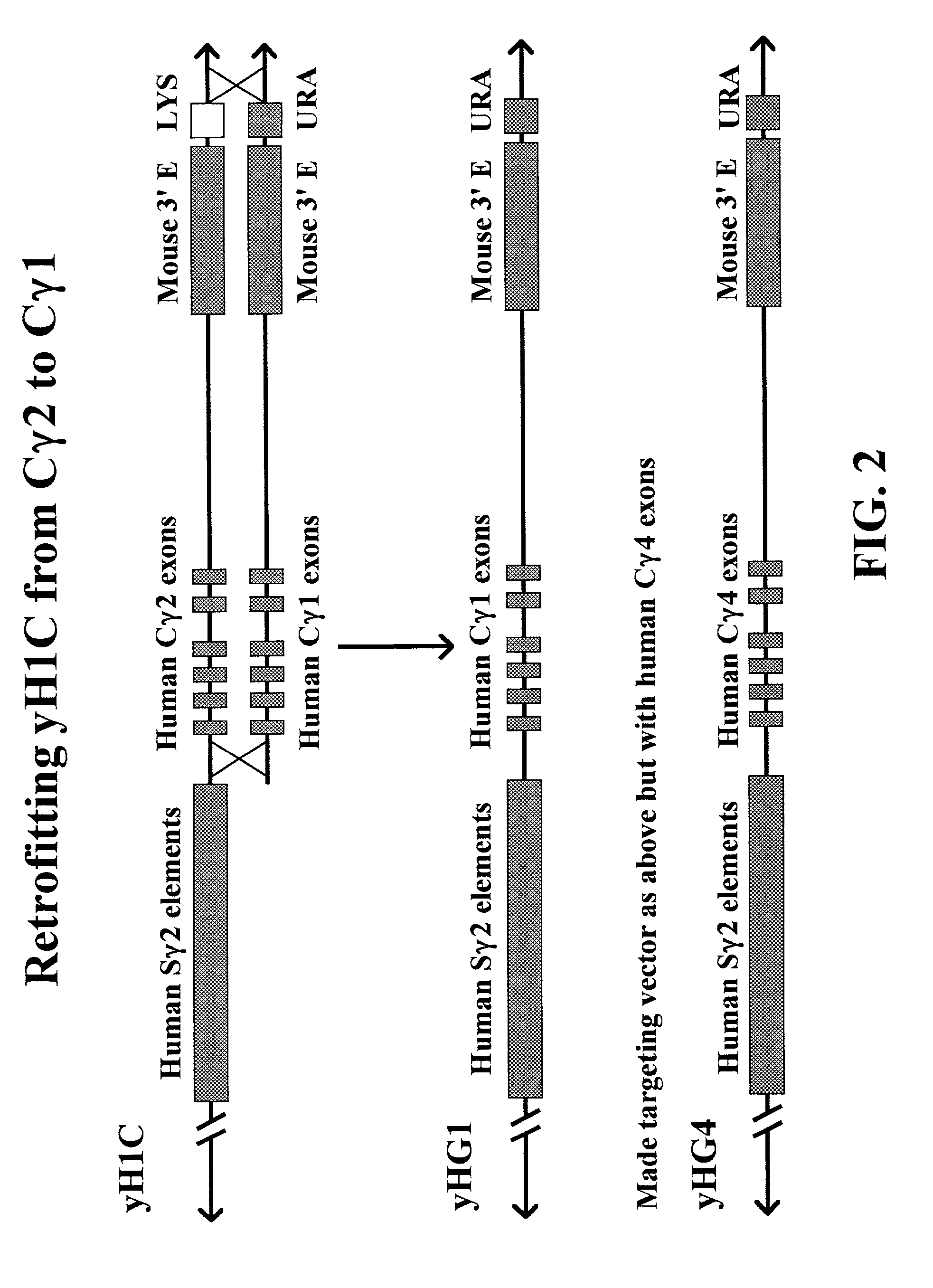
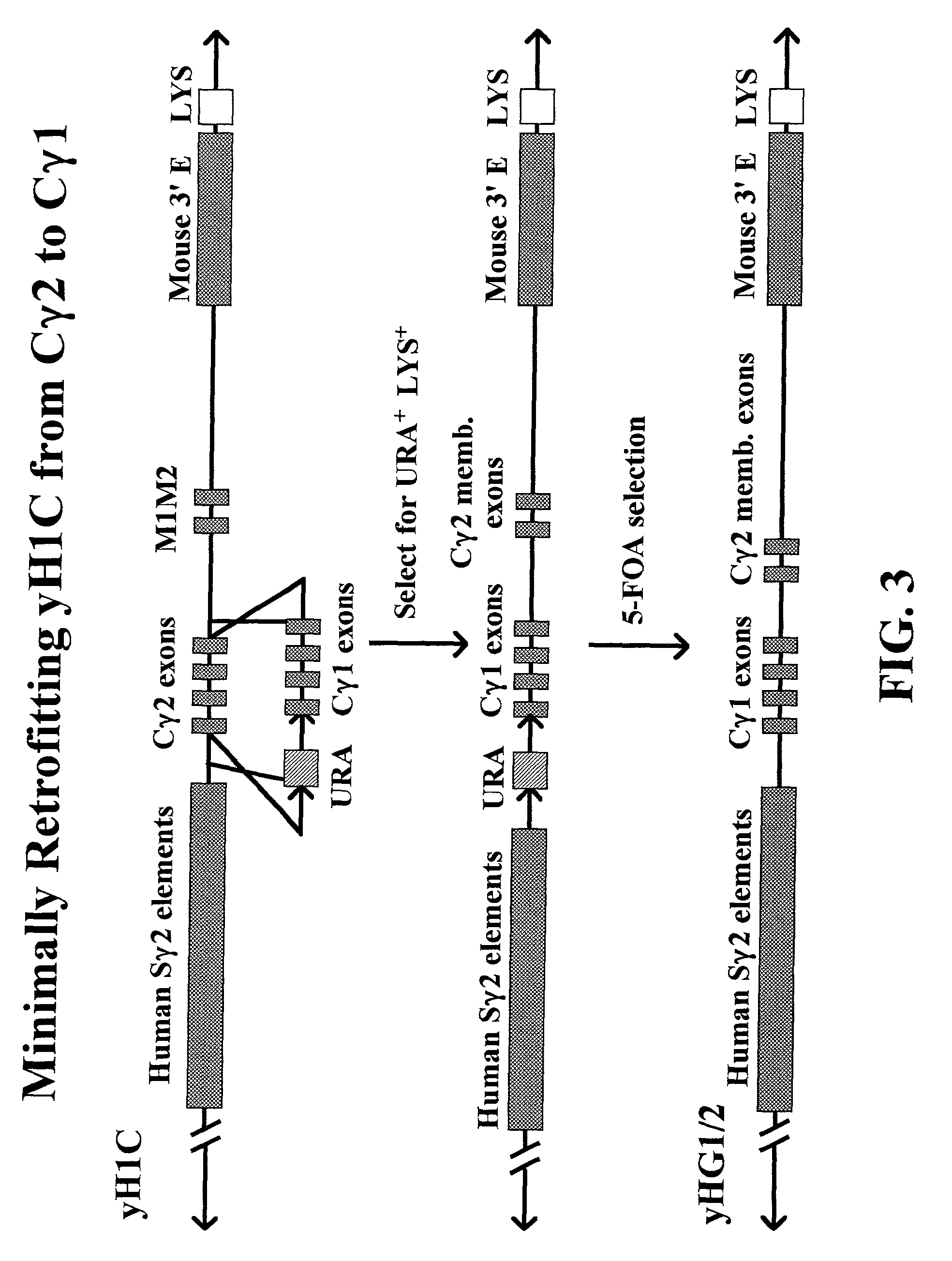
The present invention provides fully human antibodies in a transgenic animal of a desired isotype in response to immunization with any virtually any desired antigen. The human immunoglobulin heavy chain transgene in the foregoing animals comprises a human constant region gene segment comprising exons encoding the desired heavy chain isotype, operably linked to switch segments from a constant region of a different heavy chain isotype, i.e., a non-cognate switch region. Said additional constant region segment comprises a switch region and human constant region coding segment, wherein the constant region coding segment is operably linked to a switch region that it is not normally associated with, i.e., a non-cognate switch region. In the transgenes of the invention, the non-cognate switch region may be a switch region from a different species than the constant region coding segment. The switch region and membrane exons of the invention may comprise a human gamma-2 constant region and the secreted constant region exons are from a human gamma-1 or a human gamma-4 constant region.
Owner:ABQENIX INC
Bispecific Antibody Point Mutations for Enhancing Rate of Clearance
A mutant bispecific antibody that includes (a) a human hinge constant region from IgG having one or more amino acid mutations in the CH2 domain, (b) two scFvs; and (c) two Fvs has been constructed. This type of antibody displays enhanced clearance, which has been found to be particularly useful in the context of pre-targeting methods.
Owner:IMMUNOMEDICS INC
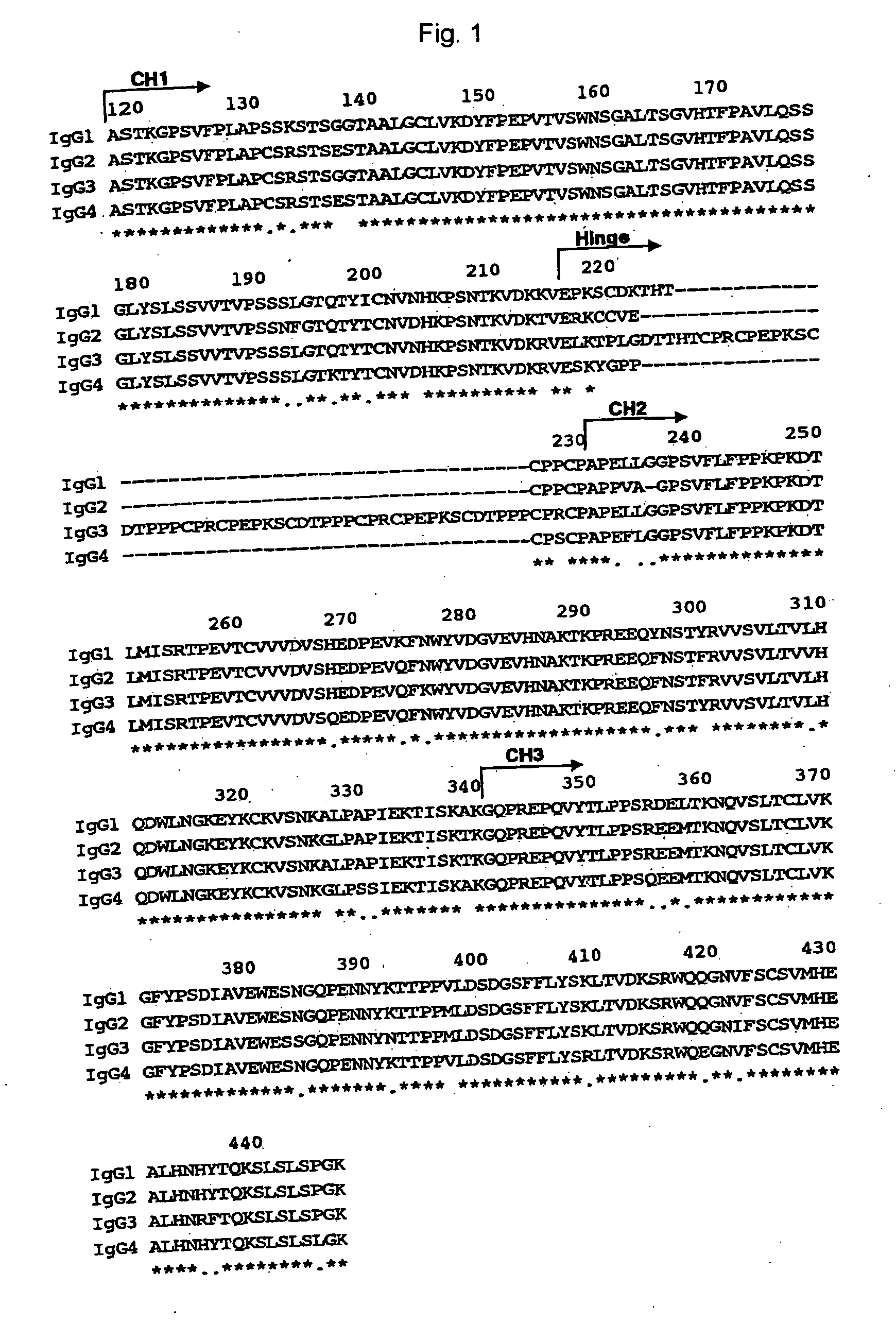
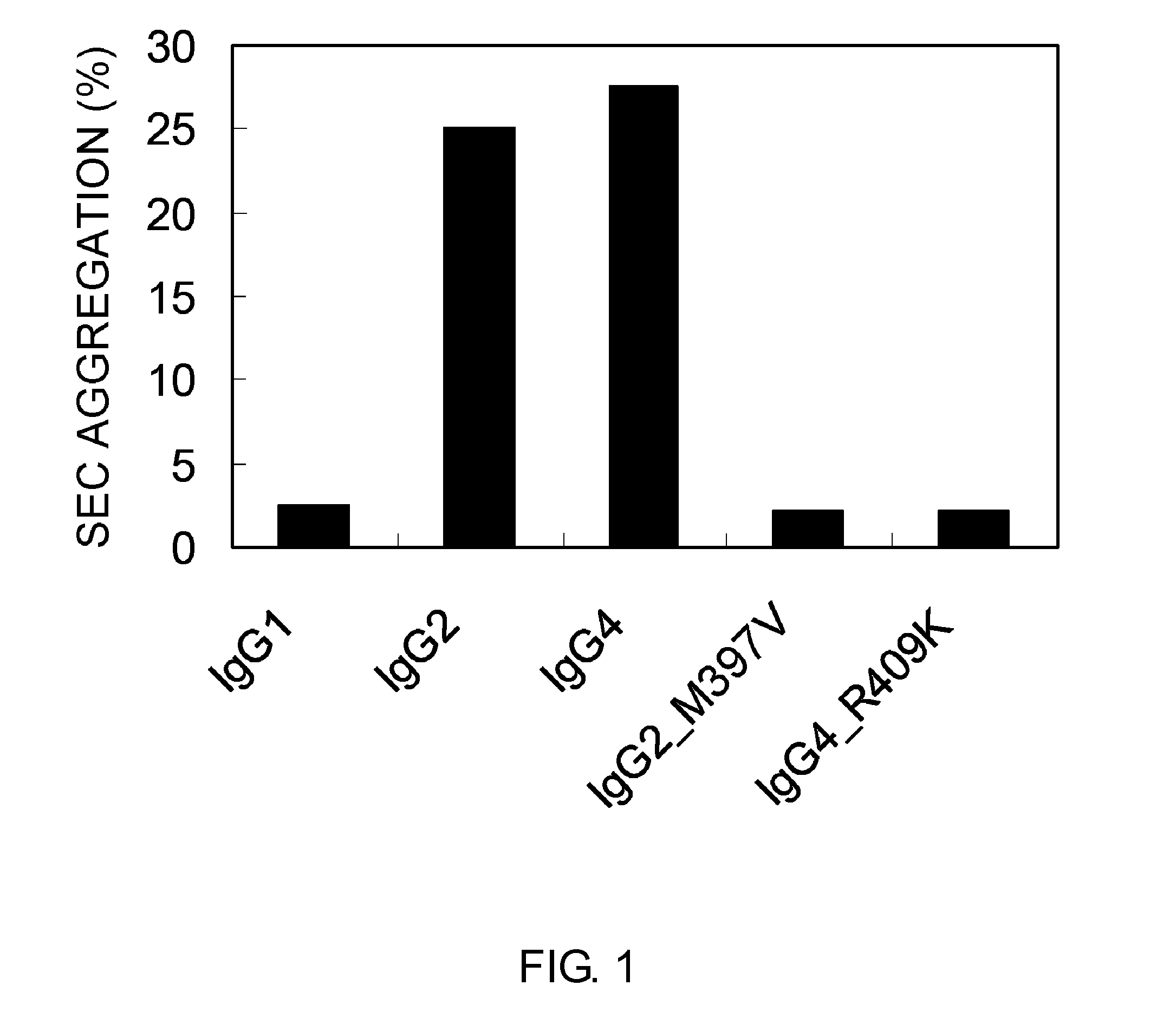
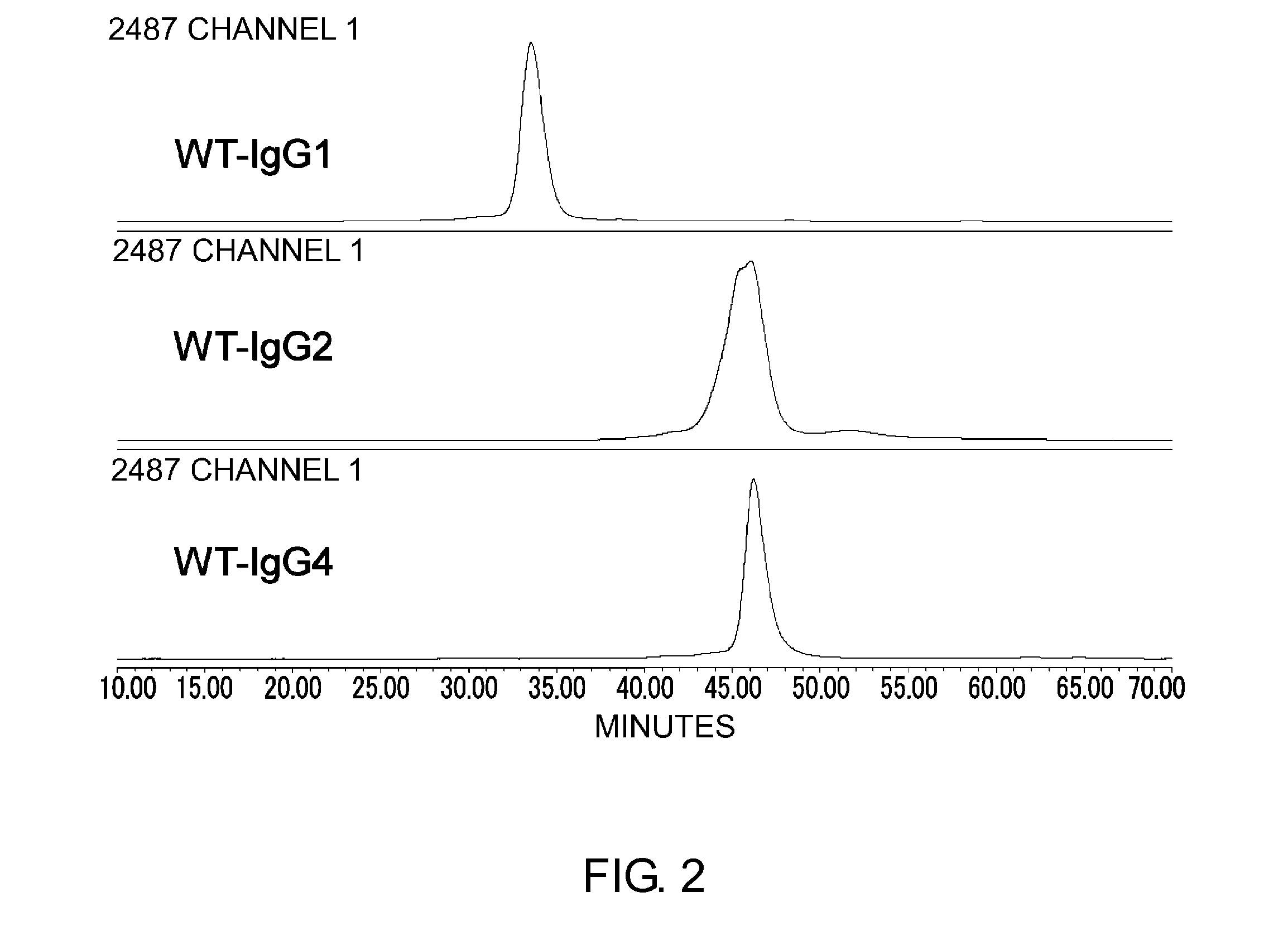
Modified Antibody Constant Region
ActiveUS20100298542A1Improving immunogenicityImprove propertiesAntipyreticAnalgesicsHigh concentrationHinge region
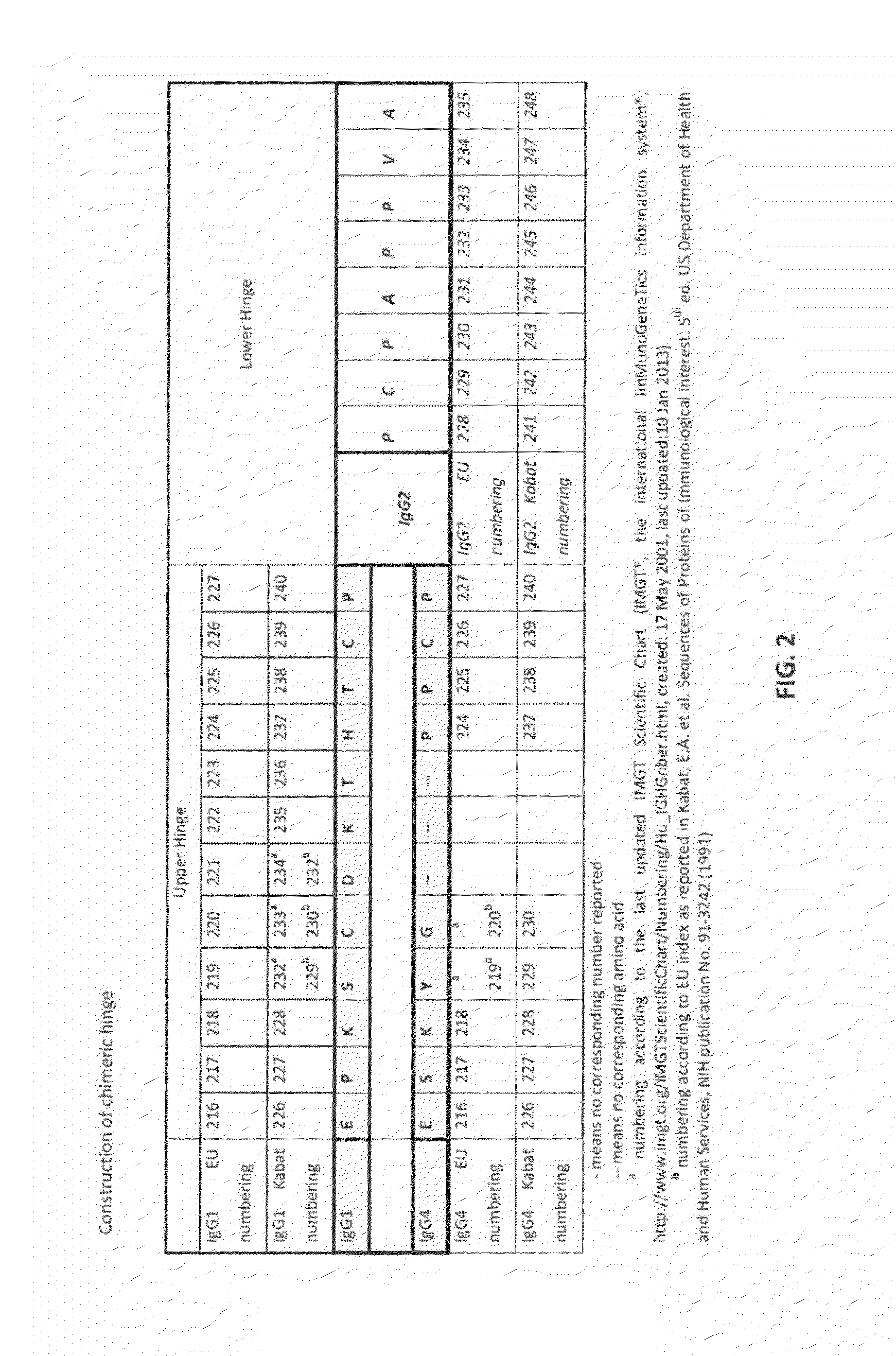
The present inventors succeeded in improving the antibody constant region to have increased stability under acid conditions, reduced heterogeneity originated from disulfide bonds in the hinge region, reduced heterogeneity originated from the H chain C terminus, and increased stability at high concentrations as well as in discovering novel constant region sequences having reduced Fcγ receptor-binding, while minimizing the generation of novel T-cell epitope peptides. As a result, the present inventors successfully discovered antibody constant regions with improved physicochemical properties (stability and homogeneity), immunogenicity, safety, and pharmacokinetics.
Owner:CHUGAI PHARMA CO LTD
Antibodies comprising chimeric constant domains
ActiveUS20140243504A1Reduced effector functionAnimal cellsHybrid immunoglobulinsFc(alpha) receptorFc receptor
Antibodies, antigen-binding proteins and Fc-fusion proteins that comprise recombinant polypeptides containing a chimeric heavy chain constant region sequence are provided that bind to certain Fc receptors however have reduced effector functions. Methods of making constructs for expression of such chimeric Fc-containing antibodies, antigen-binding proteins and Fc-fusion proteins in cell systems, and methods of producing and isolating the chimeric Fc-containing proteins are provided.
Owner:REGENERON PHARM INC
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050032114A1Reducing FcRn binding affinityReduced half-lifeAnimal cellsSugar derivativesHalf-lifeAntibody
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
ActiveUS20050276799A1Reducing FcRn binding affinityReduced half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesSerum igeHalf-life
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Heterodimer Binding Proteins and Uses Thereof
InactiveUS20130129723A1Animal cellsFused cellsImmunoglobulin Joining RegionImmunoglobulin light chain
The present disclosure provides polypeptide heterodimers formed between two different single chain fusion polypeptides via natural heterodimerization of an immunoglobulin CH1 region and an immunoglobulin light chain constant region (CL). The polypeptide heterodimer comprises two or more binding domains that specifically bind one or more targets (e.g., a receptor). In addition, both chains of the heterodimer further comprise an Fc region portion. The present disclosure also provides nucleic acids, vectors, host cells and methods for making polypeptide heterodimers as well as methods for using such polypeptide heterodimers, such as in directing T cell activation, inhibiting solid malignancy growth, and treating autoimmune or inflammatory conditions.
Owner:EMERGENT PRODUCTS DEVELOPMENT SEATTLE LLC
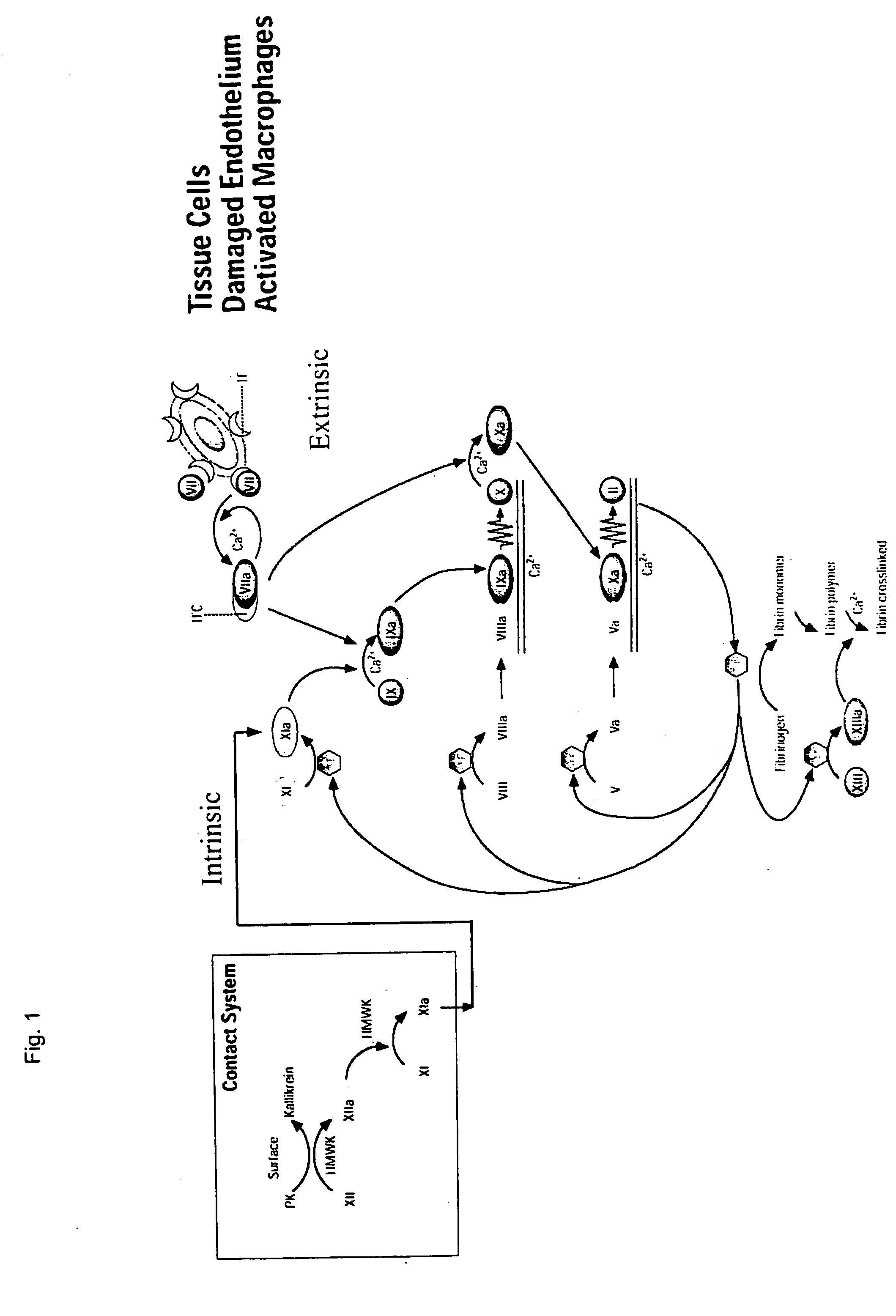
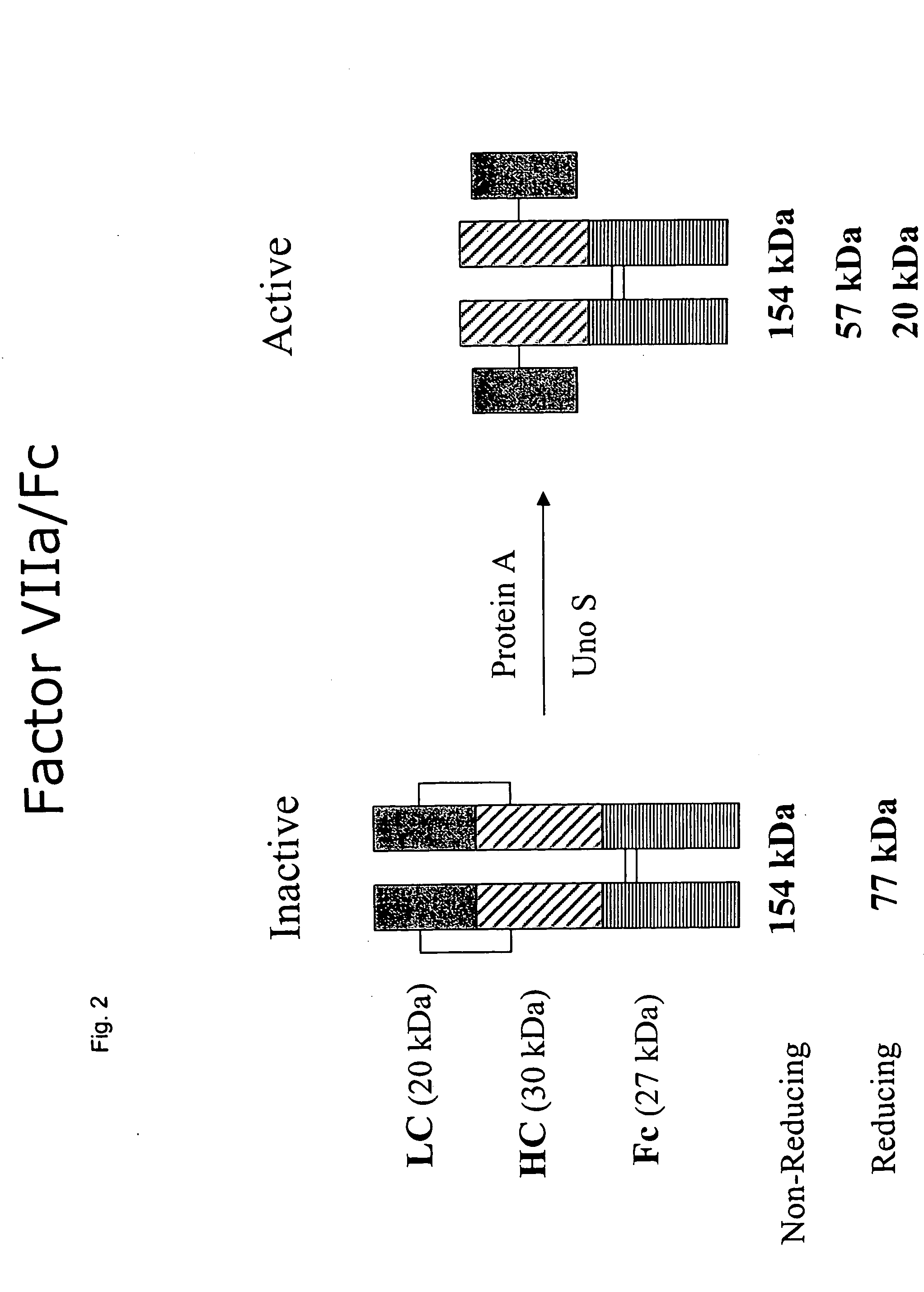
Clotting factor-Fc chimeric proteins to treat hemophilia
InactiveUS20050147618A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHemostatic DisordersChimera Protein
The invention relates to a chimeric protein comprising at least one clotting factor and at least a portion of an immunoglobulin constant region. The invention relates to a method of treating a hemostatic disorder comprising administering a therapeutically effective amount of a chimeric protein wherein the chimeric protein comprises at least one clotting factor and at least a portion of an immunoglobulin constant region.
Owner:BIOVERATIV THERAPEUTICS INC
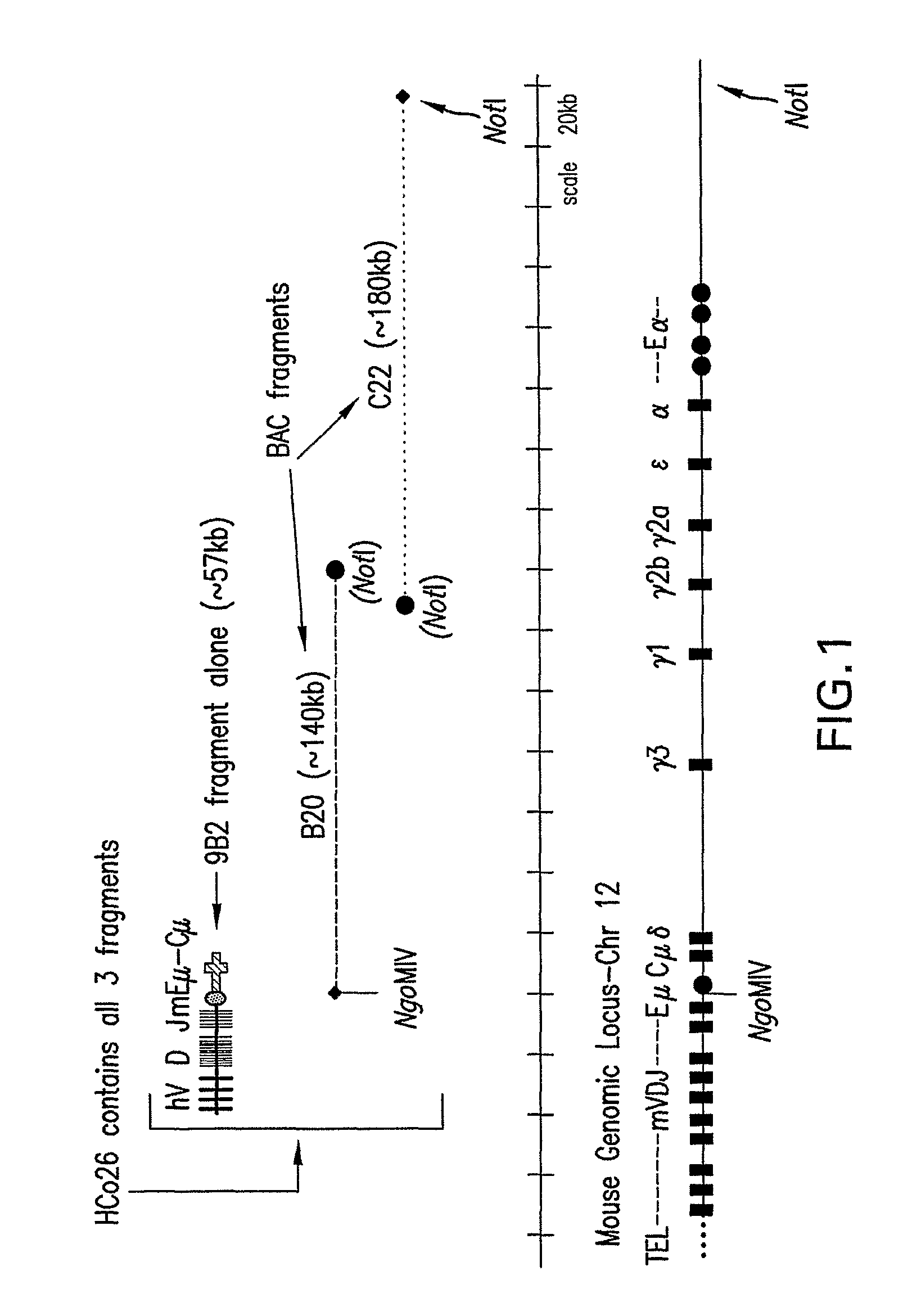
Transgenic animals expressing chimeric antibodies for use in preparing human antibodies
ActiveUS7910798B2Improved trafficking developmentStrengthen associationSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsTransgenesisIn vivo
The invention provides transgene constructs for expressing chimeric antibodies, and transgenic non-human host animals carrying such constructs, wherein the chimeric antibodies comprise human variable regions and constant regions of the non-human transgenic host animal. The presence of immunoglobulin constant regions of the host animal allows for generation of improved antibodies in such transgenic host animals. Subsequently, the chimeric antibodies can be readily converted to fully human antibodies using recombinant DNA techniques. Thus, the invention provides compositions and methods for generating human antibodies in which chimeric antibodies raised in vivo in transgenic mice are used as intermediates and then converted to fully human antibodies in vitro.
Owner:ER SQUIBB & SONS INC
Natural human antibody
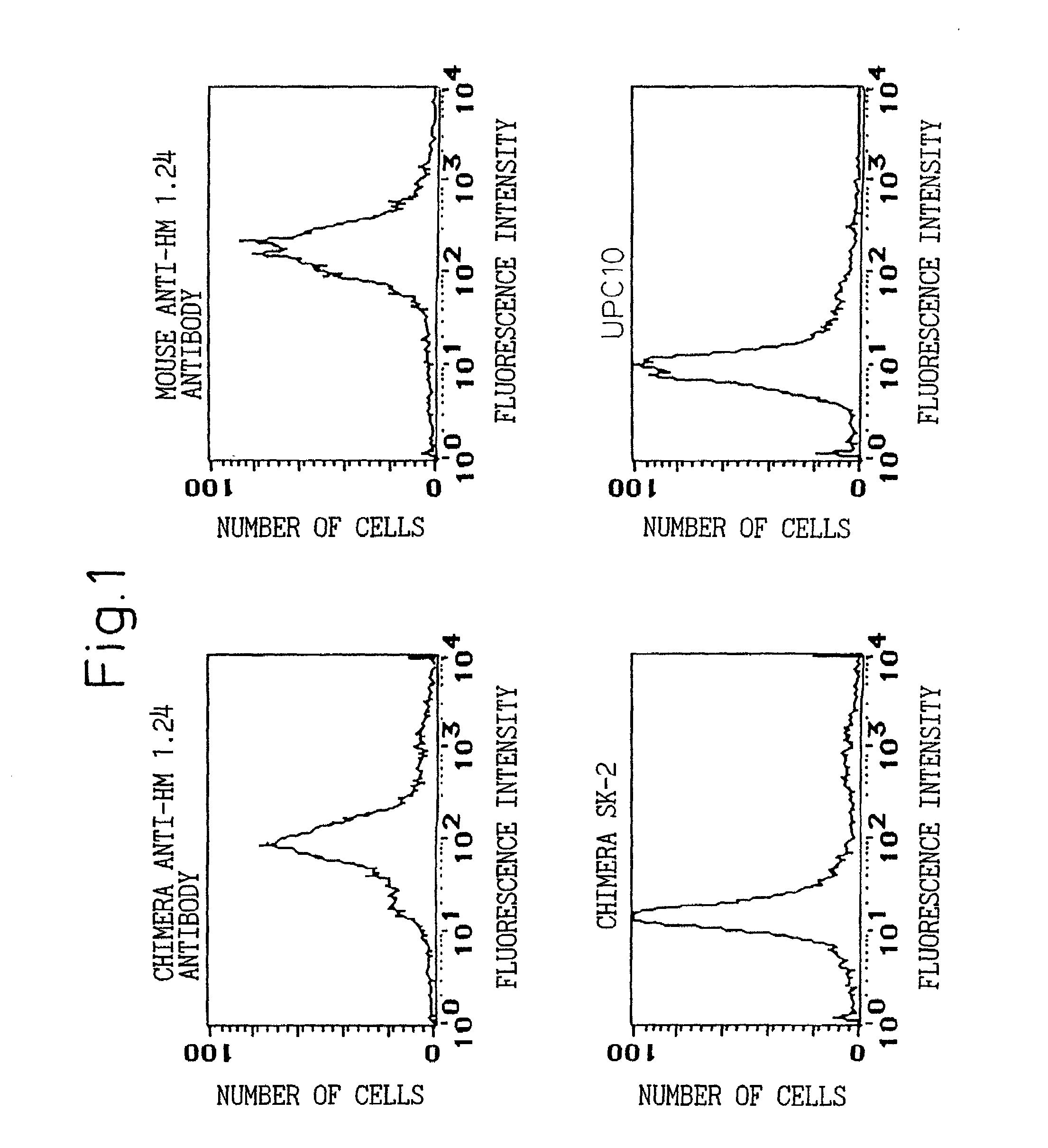
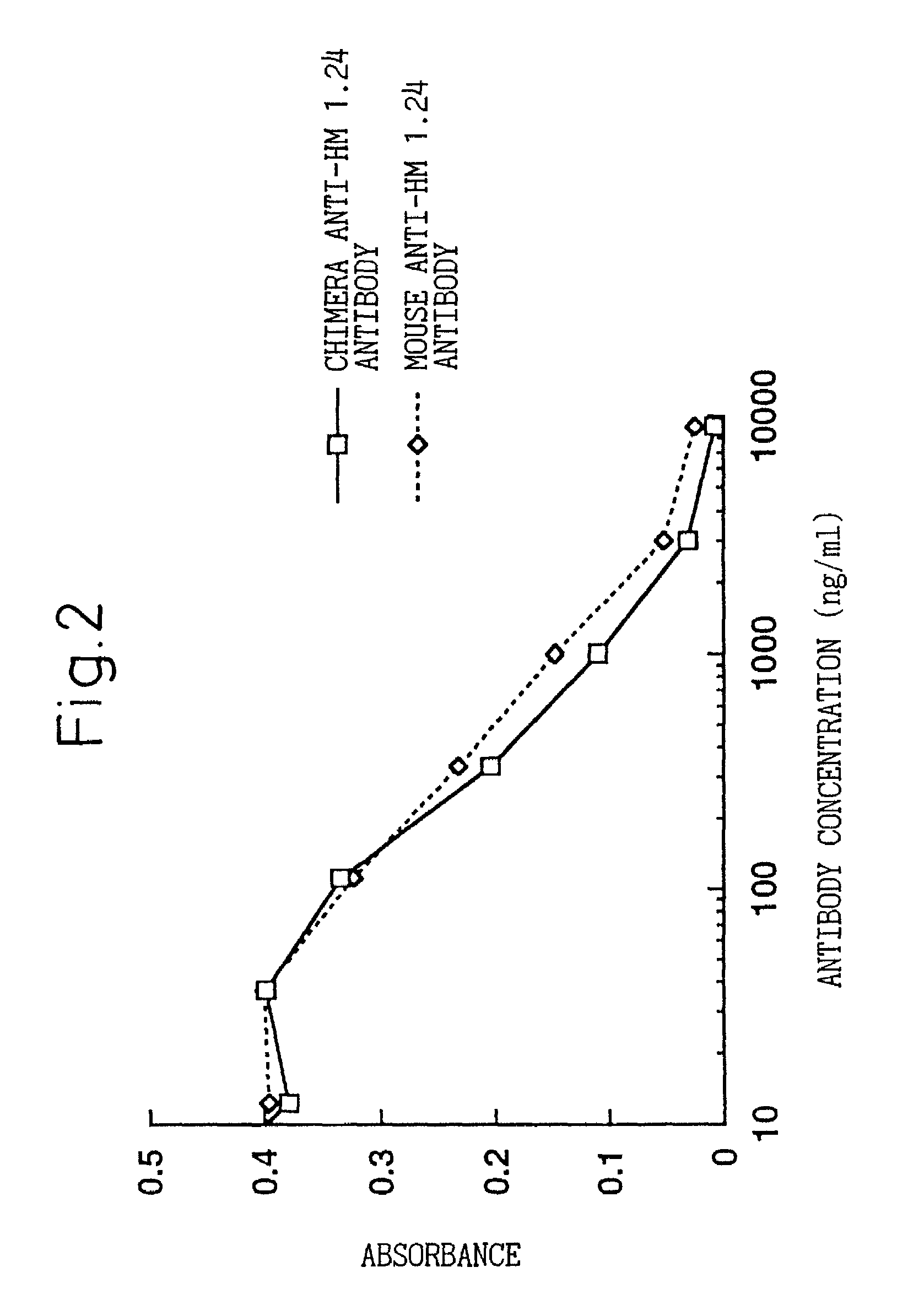
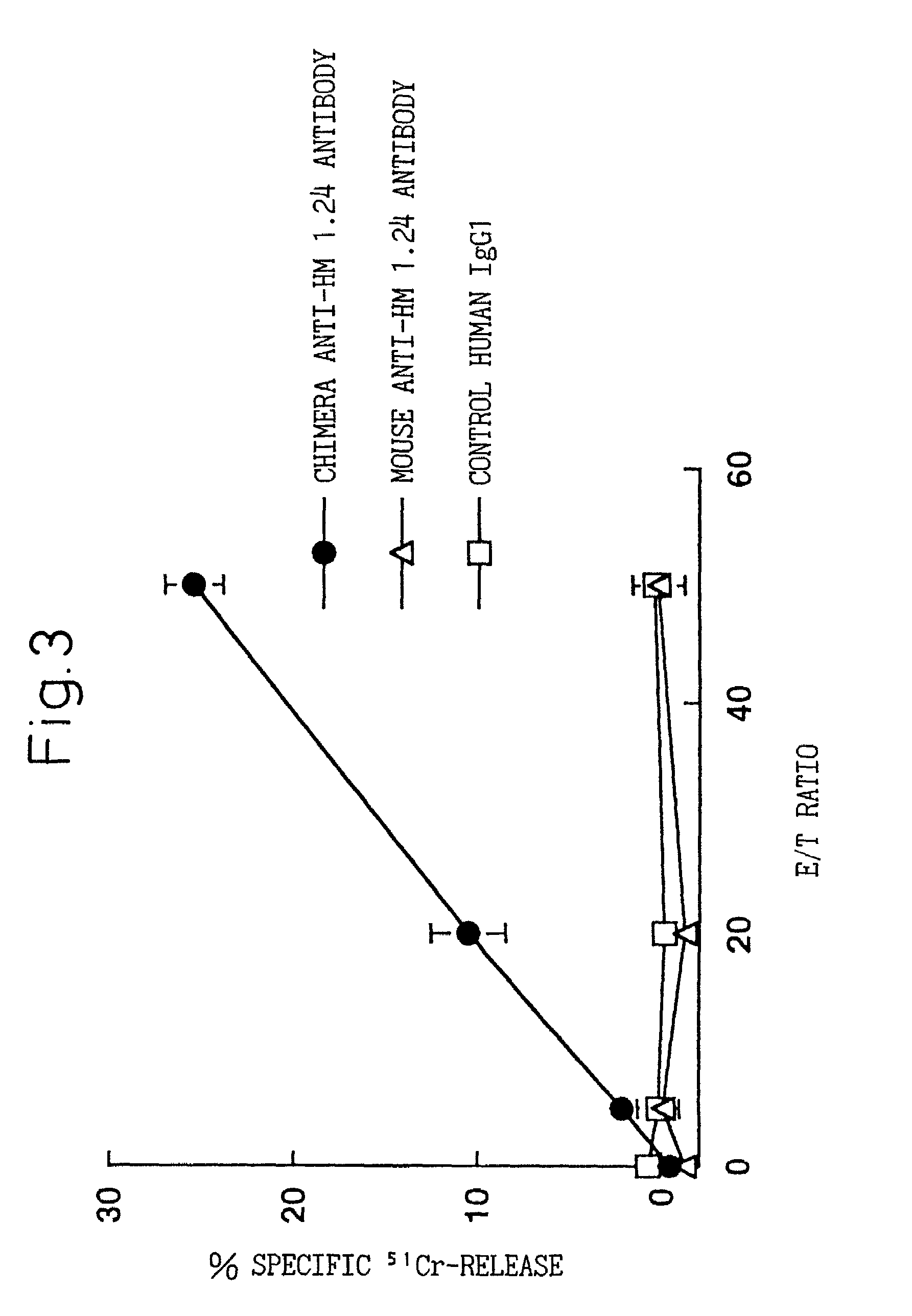
A reshaped human anti-HM1.24 antibody comprising:(A) L chains each comprising (1) a constant region of a human L chain, and (2) FRs of a human L chain, and CDRs of L chain of mouse anti-HM1.24 monoclonal antibody; and(B) H chains each comprising (2) a constant region of a human H chain, and (2) FRs of a human H chain, and CDRs of H chain of mouse anti-HM1.24 monoclonal antibody. Since the majority of the reshaped human antibody is derived from human antibody and the CDR has a low antigenicity, the reshaped human antibody of the present invention has low antigenicity and therefore is very promising in medical and therapeutic applications.
Owner:CHUGAI PHARMA CO LTD
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS7067110B1Improving immunogenicityStrengthIn-vivo radioactive preparationsAntibody mimetics/scaffoldsPeptide antigenFc(alpha) receptor
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
Single chain recombinant T cell receptors
InactiveUS7569664B2Improve stabilityUseful purposeCompound screeningApoptosis detectionExtracellularC-terminus
A single chain T cell receptor (scTCR) comprising an a segment constituted by a TCR α chain variable region sequence fused to the N terminus of a TCR α chain constant region extracellular sequence, a β segment constituted by a TCR β chain variable region fused to the N terminus of a TCR β chain constant region extracellular sequence, and a linker sequence linking the C terminus of the α segment to the N terminus of the β segment, or vice versa, the constant region extracellular sequences of the α and β segments being linked by a disulfide bond, the length of the linker sequence and the position of the disulfide bond being such that the variable region sequences of the α and β segments are mutually orientated substantially as in native αβ T cell receptors. Complexes of two or more such scTCRs, and use of the scTCRs in therapy and in various screening applications are also disclosed.
Owner:IMMUNOCORE LTD +1
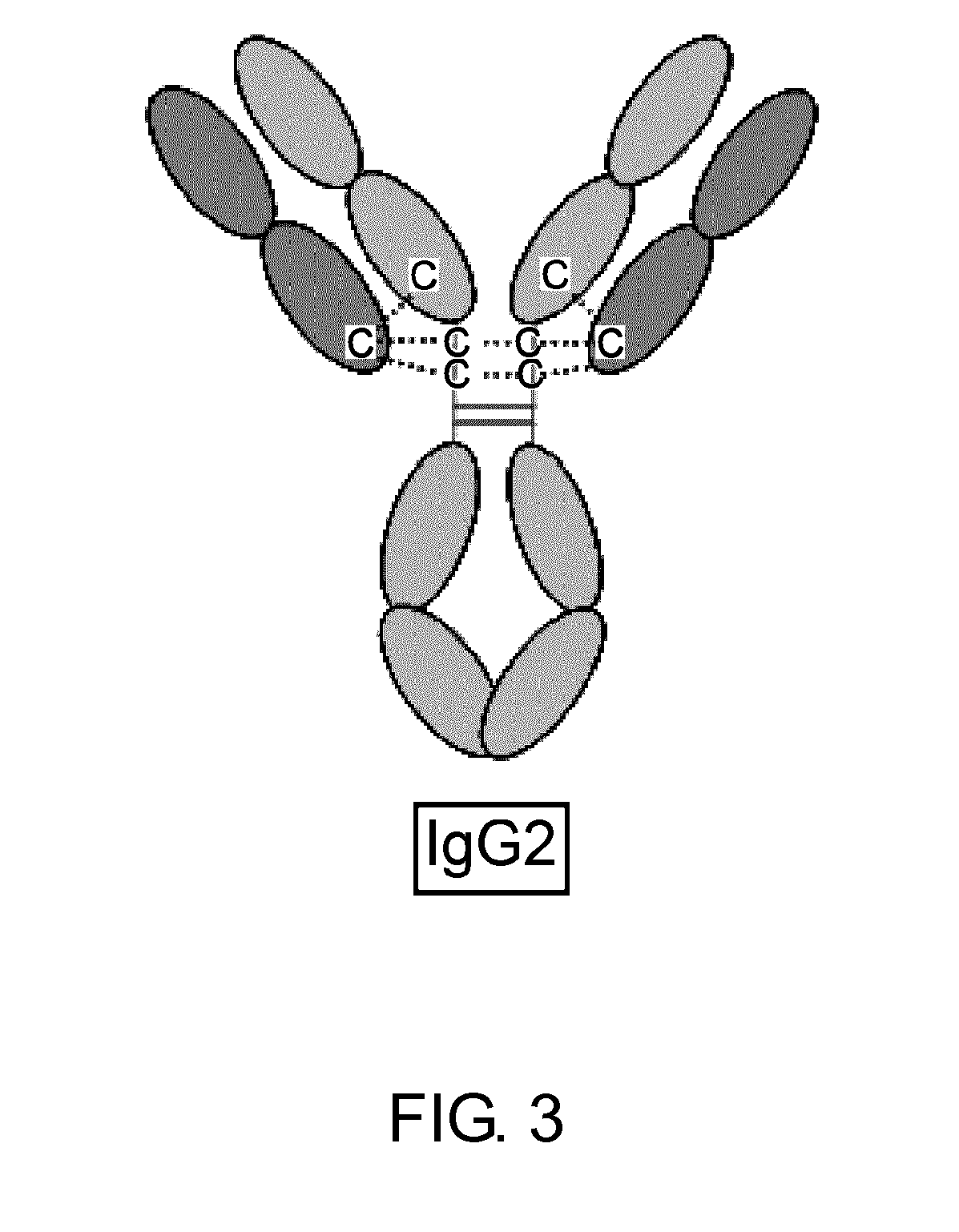
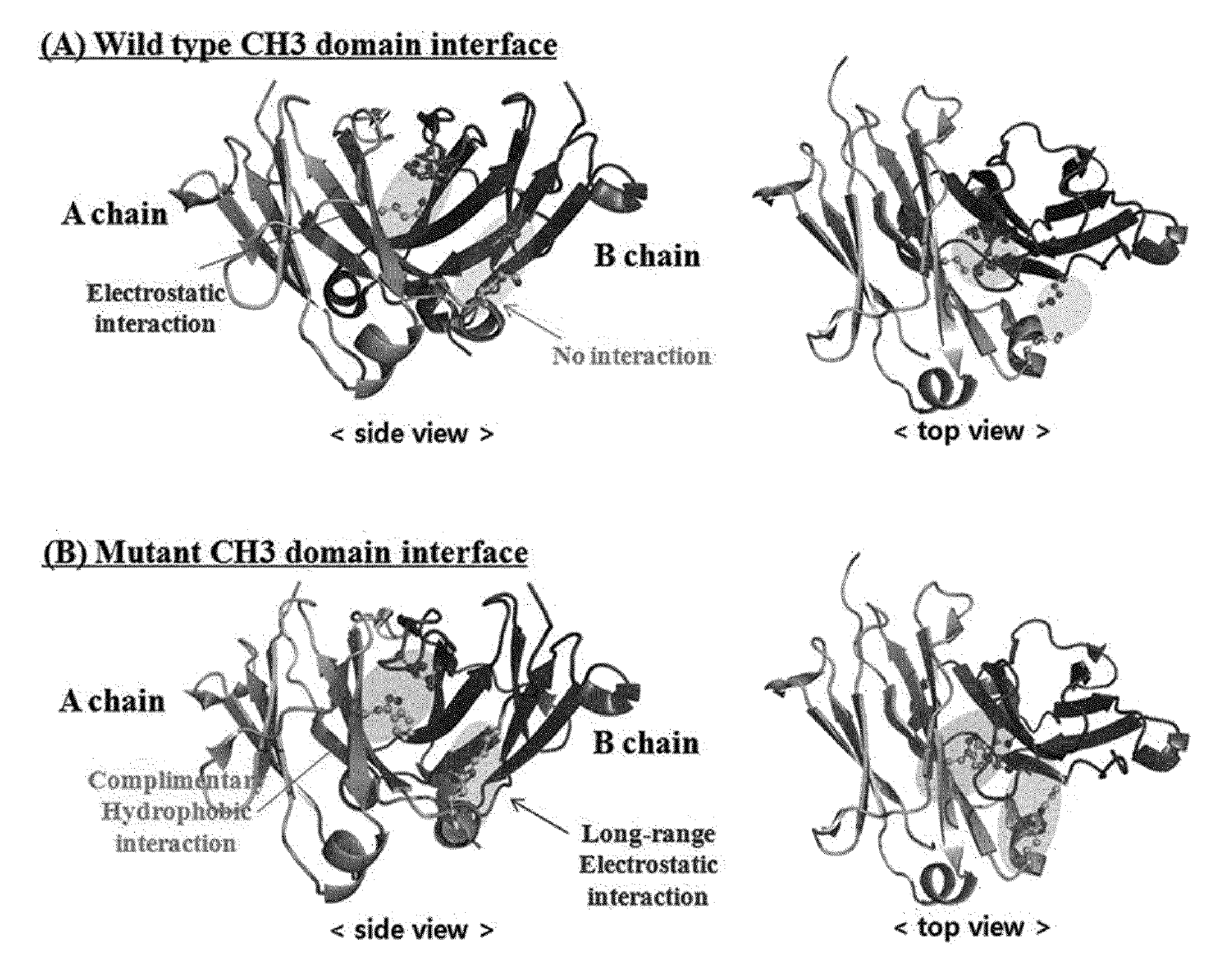
Ch3 domain variant pair inducing formation of heterodimer of heavy chain constant region of antibody at high efficiency, method for preparing same, and use thereof
ActiveUS20150307628A1High yieldMinimize formationHybrid immunoglobulinsAntibody mimetics/scaffoldsDiseaseProtein target
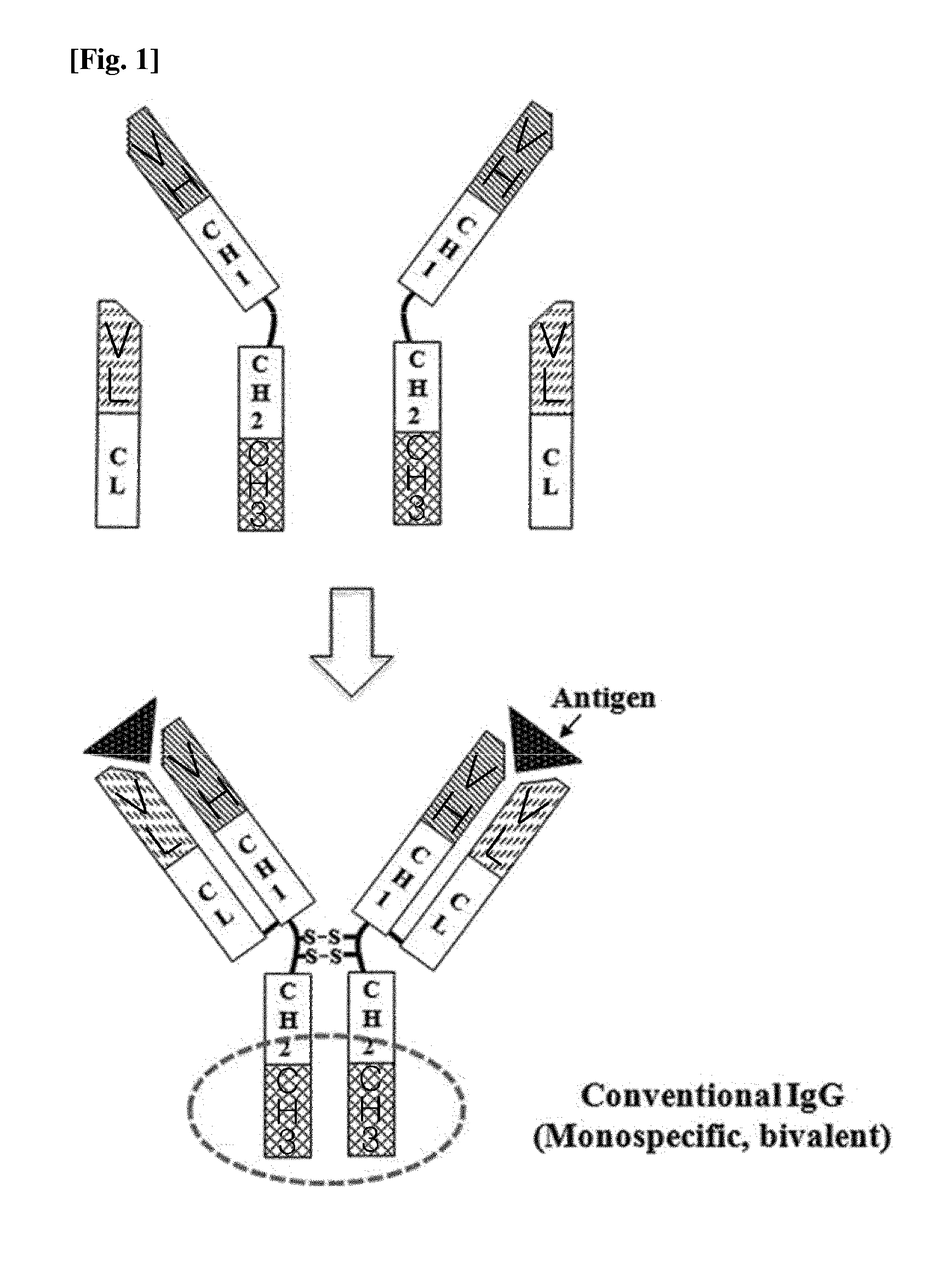
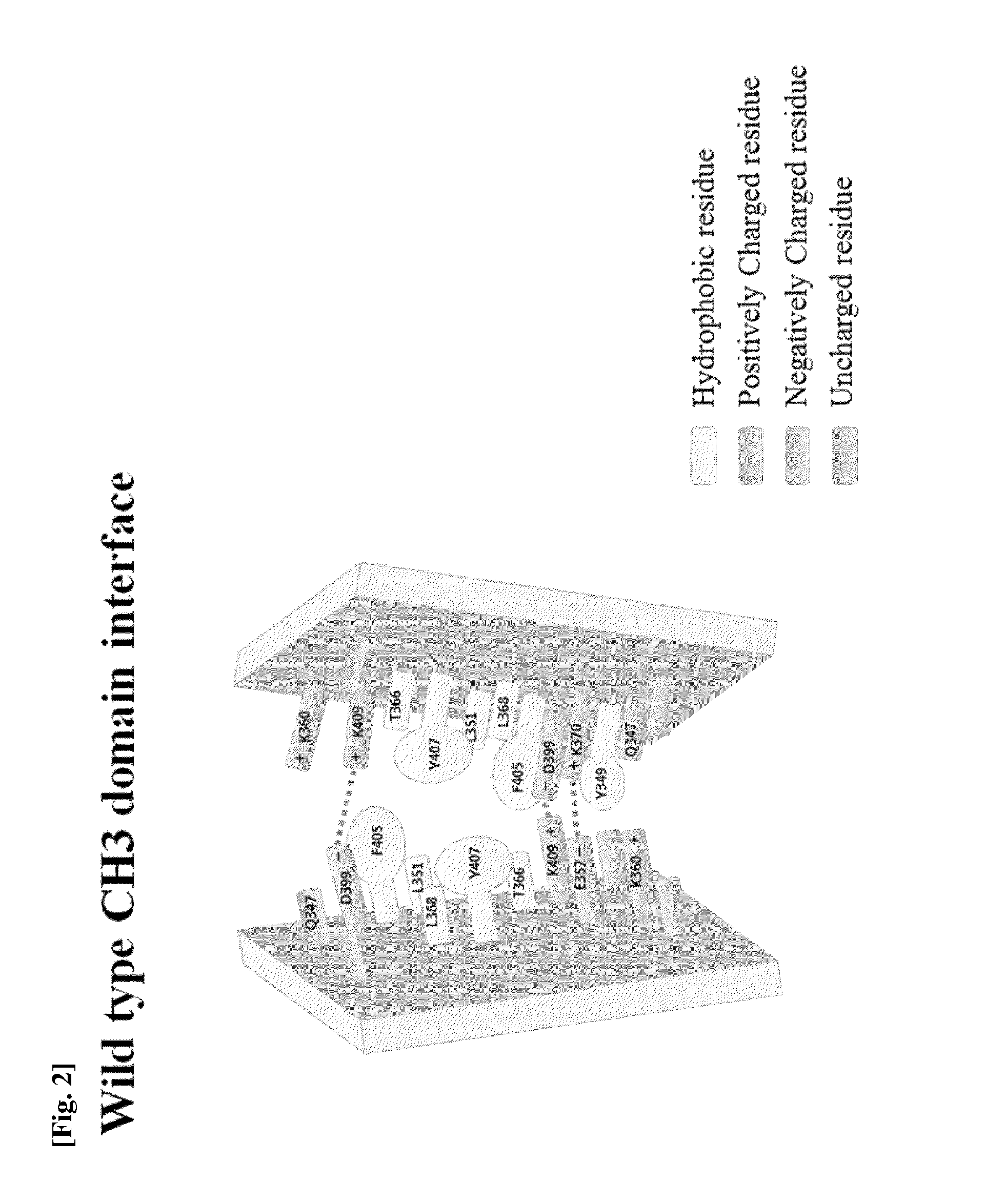
Disclosed are a CH3 domain variant pair of an antibody, a method for preparing same, and a use thereof. A mutation is induced in the CH3 domain so as to improve a yield of forming a heterodimer heavy chain constant region of an antibody. The CH3 domain heterodimer forms a heterodimer heavy chain constant region with a high efficiency of 90 to 95% or more and also has outstanding heat stability. A heterodimer heavy chain constant region including the CH3 domain heterodimer can construct a bispecific monoclonal antibody which simultaneously recognizes two kinds of antigens. The CH3 domain heterodimer and the bispecific antibody or fusion protein of an antibody constant region comprising same can be usefully applied to the treatment or prevention of a disease associated with a target antigen or a target protein.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Recombinant antibody composition
ActiveUS7923538B2Enhanced effector functionGood treatment effectAntibody ingredientsImmunoglobulinsA-DNAActive ingredient
The present invention relates to a recombinant antibody composition having higher complement-dependent cytotoxic activity than a human IgG1 antibody and a human IgG3 antibody, wherein a polypeptide comprising a CH2 domain in the Fc region of a human IgG1 antibody is replaced by a polypeptide comprising an amino acid sequence which corresponds to the same position of a human IgG3 antibody indicated by the EU index as in Kabat, et al.; a DNA encoding the antibody molecule or a heavy chain constant region of the antibody molecule contained in the recombinant antibody composition; a transformant obtainable by introducing the recombinant vector into a host cell; a process for producing the recombinant antibody composition using the transformant; and a medicament comprising the recombinant antibody composition as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
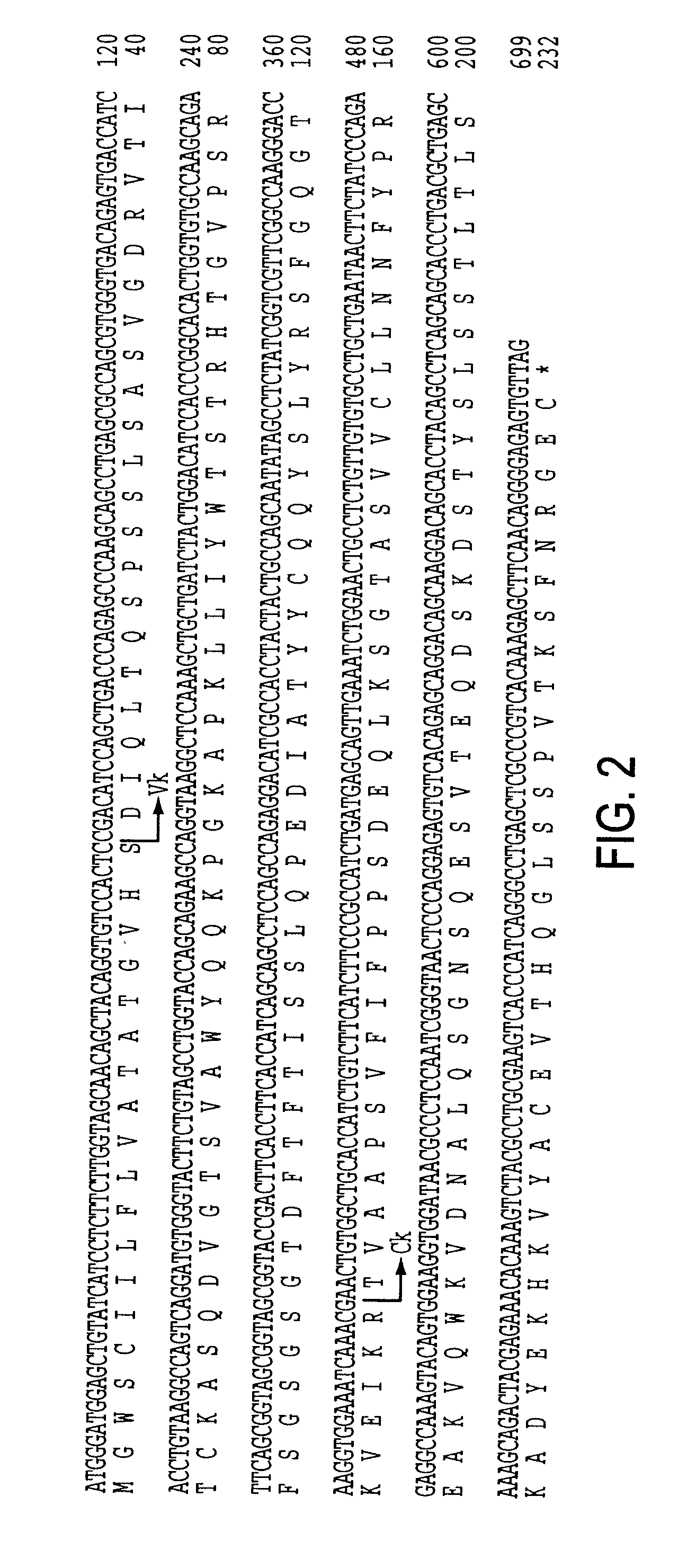
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Humanized immunoglobulin reactive with α4β7 integrin
InactiveUS7147851B1Reduce inflammationLess immunogenicFungiBacteriaComplementarity determining regionAntigen binding
The present invention relates to humanized immunoglobulins having binding specificity for α4β7 integrin, comprising an antigen binding region of nonhuman origin (e.g., rodent) and at least a portion of an immunoglobulin of human origin (e.g., a human framework region, a human constant region). In one embodiment, the humanized immunoglobulin can compete with murine Act-1 for binding to human α4β7 integrin. In a preferred embodiment, the antigen binding region of the humanized immunoglobulin comprises each of the complementarity determining regions of the light and heavy chains of the murine Act-1 antibody.
Owner:MILLENNIUM PHARMA INC
Mutants of anti-cd40 antibody
ActiveUS20070148163A1High therapeutic effectImmunoglobulins against bacteriaImmunoglobulins against virusesHinge regionMutant
A mutant of a potentially therapeutic anti-CD40 antibody is provided which mutant has reduced ADCC and CDC activities designed to be optimized as a pharmaceutical agent. A mutant of an agonistic anti-CD40 antibody, comprising mutation and / or substitution of at least one amino acid in the constant region to reduce the ADCC and / or CDC activities therein, and a mutant of an antagonistic anti-CD40 antibody, comprising at least one mutation or substitution in the constant region to reduce the ADCC and / or CDC activities therein, both mutants having at least a hinge region derived from a human IgG2.
Owner:KYOWA HAKKO KIRIN CO LTD
Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis
InactiveUS7361740B2Function increaseExtended half-lifeImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against virusesSerum igeHalf-life
The present invention provides for a modified antibody of class IgG, in which at least one amino acid from the heavy chain constant region selected from the group consisting of amino acid residues 250, 314, and 428 is substituted with another amino acid which is different from that present in the unmodified antibody, thereby altering the binding affinity for FcRn and / or the serum half-life in comparison to the unmodified antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Binding proteins comprising immunoglobulin hinge and fc regions having altered fc effector functions
Provided herein are binding proteins comprising one or more immunoglobulin Fc region hinge, CH2, and / or CH3 domain wherein one or more hinge and / or constant region CH2 and / or CH3 domain is modified to alter the binding protein's binding affinity and / or specificity for a cognate receptor (e.g., an Fc receptor) and / or to impart one or more new binding specificity(ies) to the hinge and / or constant region that the corresponding unmodified immunoglobulin does not possess (e.g., affinity for distinct class of cognate receptor distinct from the class of cognate receptor to which the unmodified binding protein specifically binds). Binding proteins according to the present invention include, for example, modified antibodies, antibody fragments, recombinant binding proteins, and molecularly engineered binding domain-immunoglobulin fusion proteins, including small modular immunopharmaceutical products (SMIP™ products).
Owner:TRUBION PHARM INC
Chimeric antibody with specificity to human B cell surface antigen
A chimeric antibody with human constant region and murine variable region, having specificity to a 35 kDA polypeptide (Bp35(CD20)) expressed on the surface of human B cells, methods of production, and uses.
Owner:ROBINSON RANDY +2
Antibodies comprising chimeric constant domains
ActiveUS9359437B2Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsFc(alpha) receptorFc receptor
Owner:REGENERON PHARM INC
Mosaic tags for labeling templates in large-scale amplifications
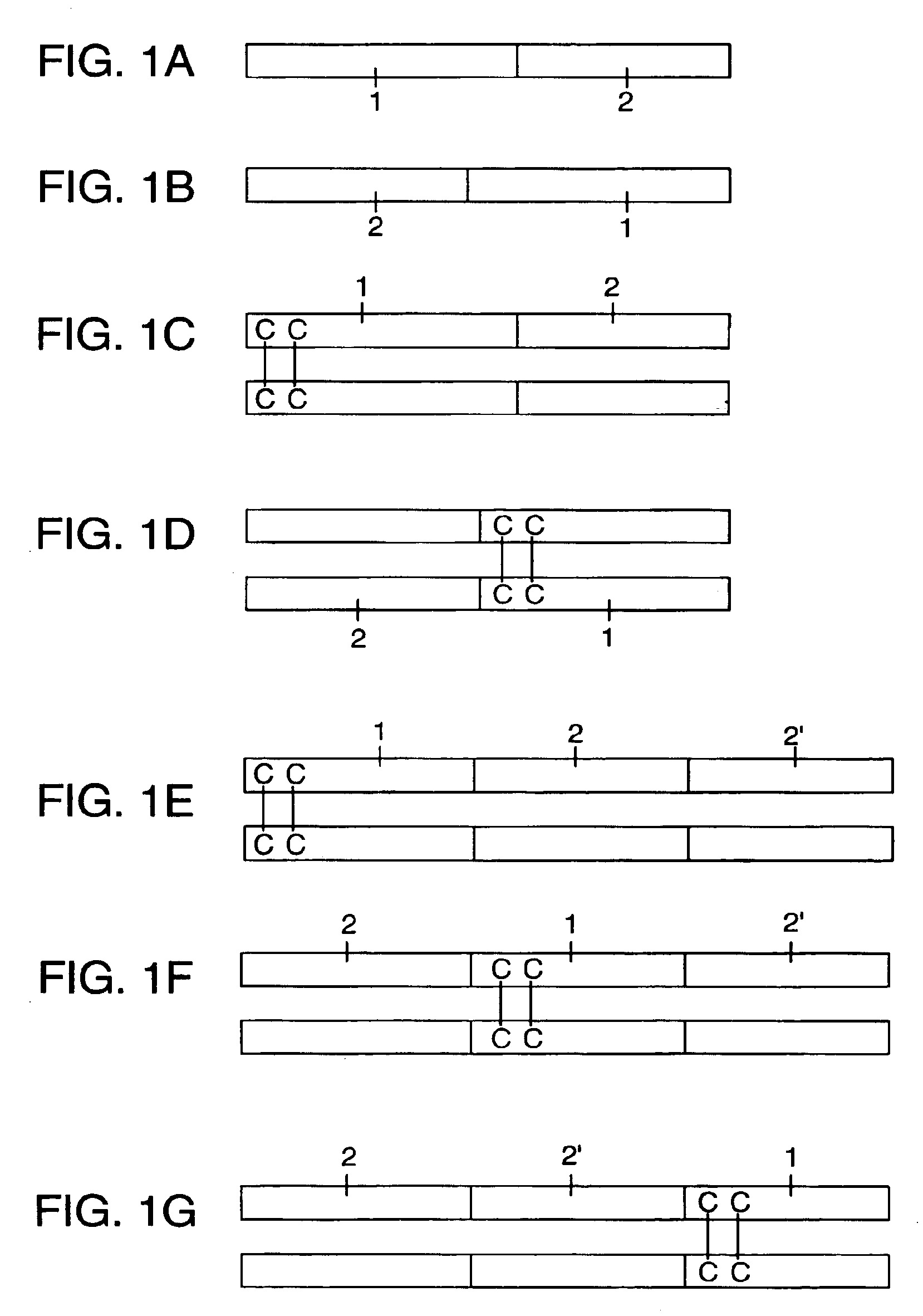
The invention relates to methods of labeling nucleic acids, such as fragments of genomic DNA, with unique sequence it referred to herein as “mosaic tag,” prior to amplification and / or sequencing. Such sequence tags are useful for identifying amplification and sequencing errors. Mosaic tags minimize sequencing and amplification artifacts due to inappropriate annealing priming, hairpin formation, or the like, that may occur with completely random sequence tags of the prior art. In one aspect, mosaic tags are sequence tags that comprise alternating constant regions and variable regions, wherein each constant region has it position in the mosaic tag and comprises a predetermined sequence of nucleotides and each variable region has a position in the mosaic tag and comprises a predetermined number of randomly selected nucleotides.
Owner:ADAPTIVE BIOTECH
Antibody constant region variant
ActiveUS20130101581A1Improve propertiesIncreased riskImmunoglobulinsImmunological disordersAntibodyReceptor for activated C kinase 1
The present inventors carried out dedicated research to generate antibody constant regions with reduced Fcγ receptor-binding activity by altering amino acid sequences in the antibody constant region. As a result, the present inventors successfully identified novel constant region sequences with reduced Fcγ receptor-binding activity compared to conventional antibody constant regions.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com