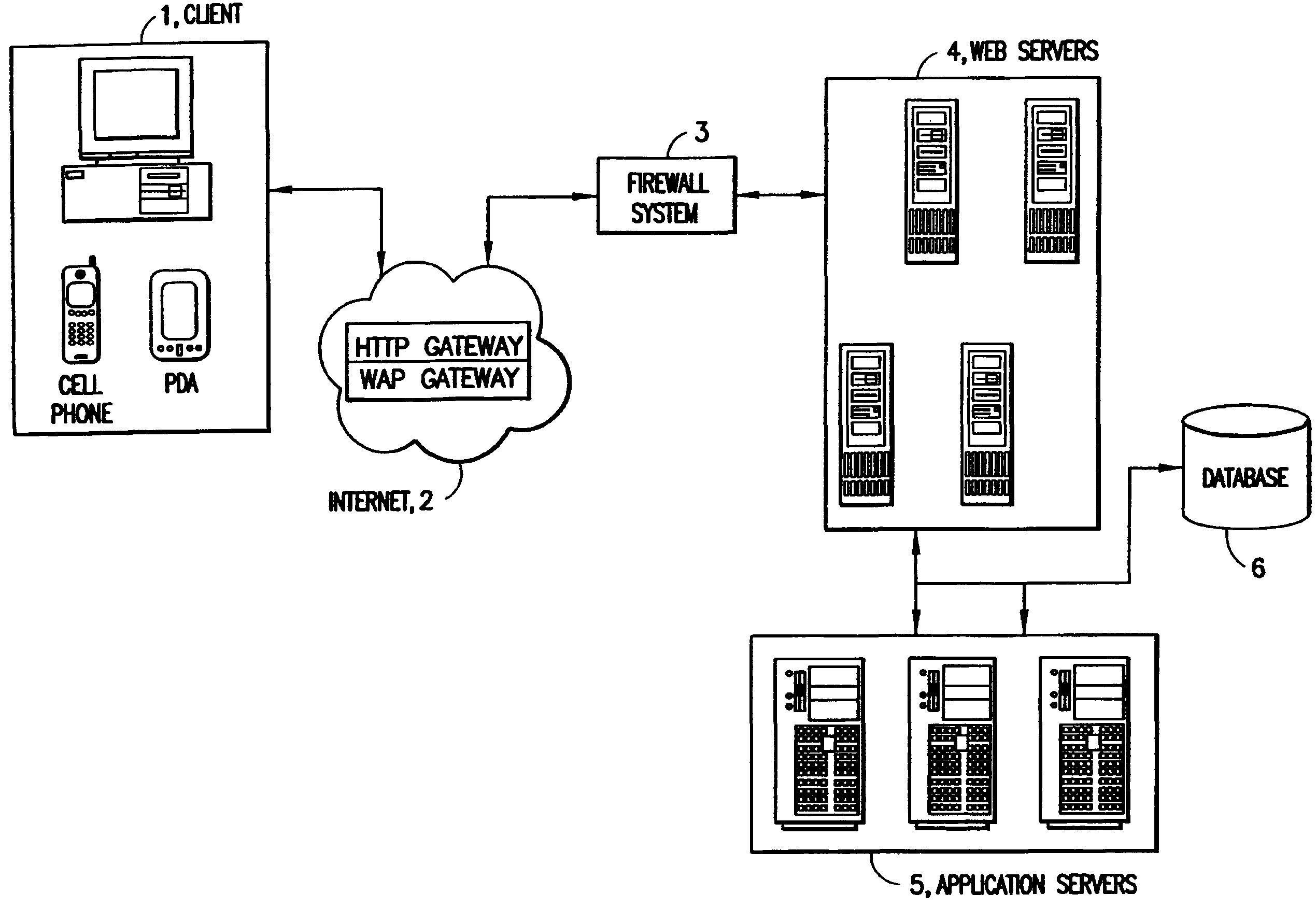
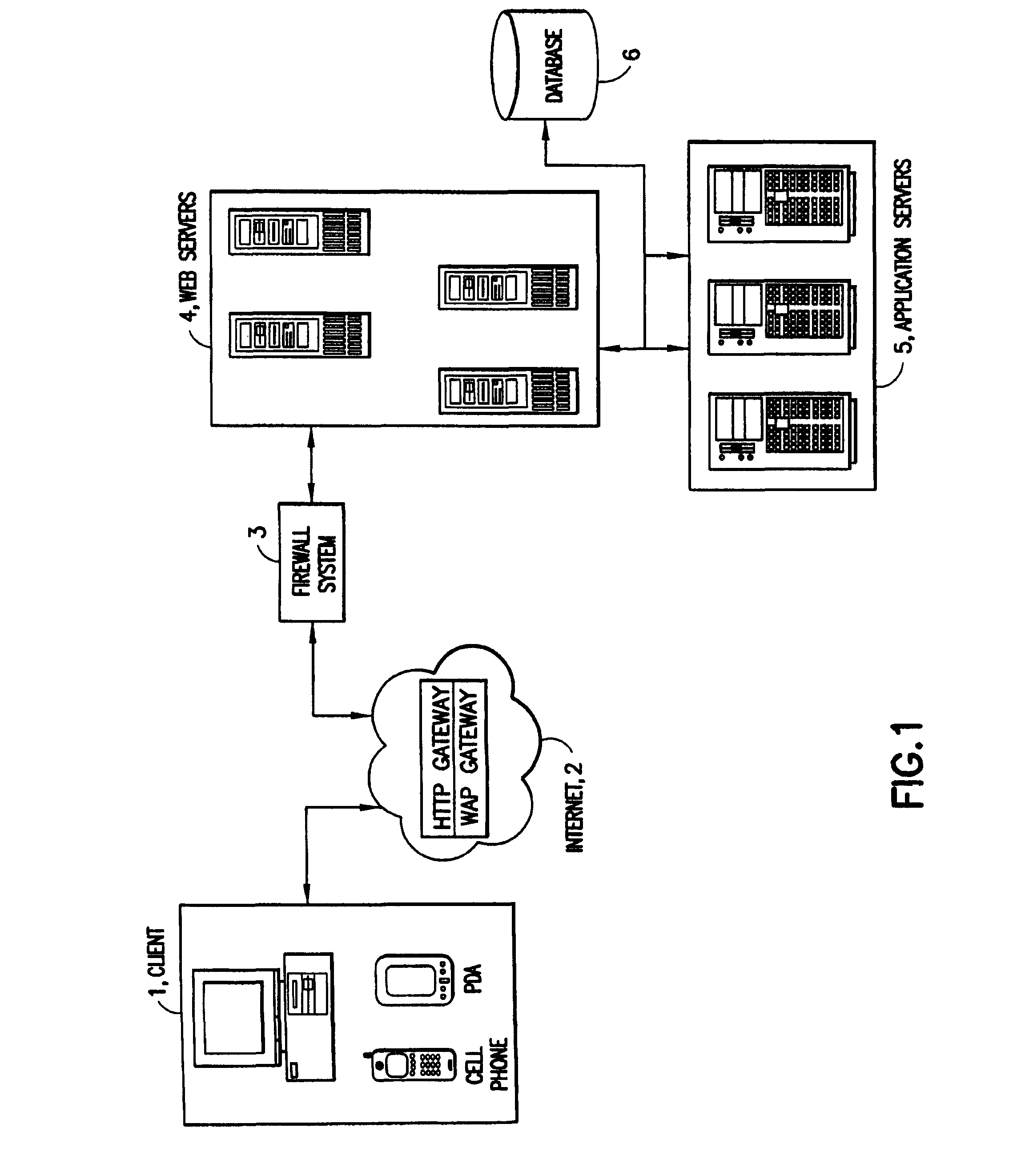
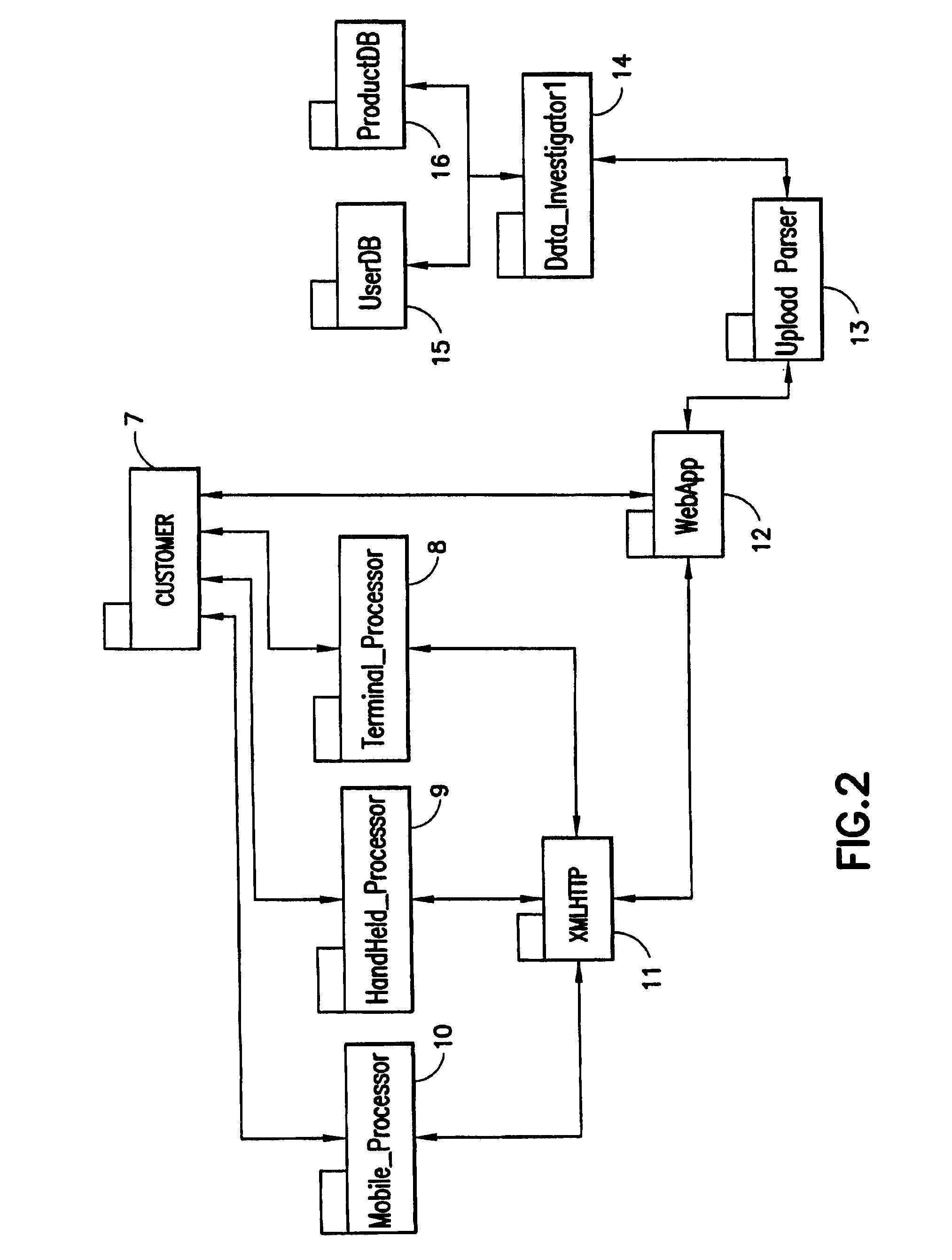
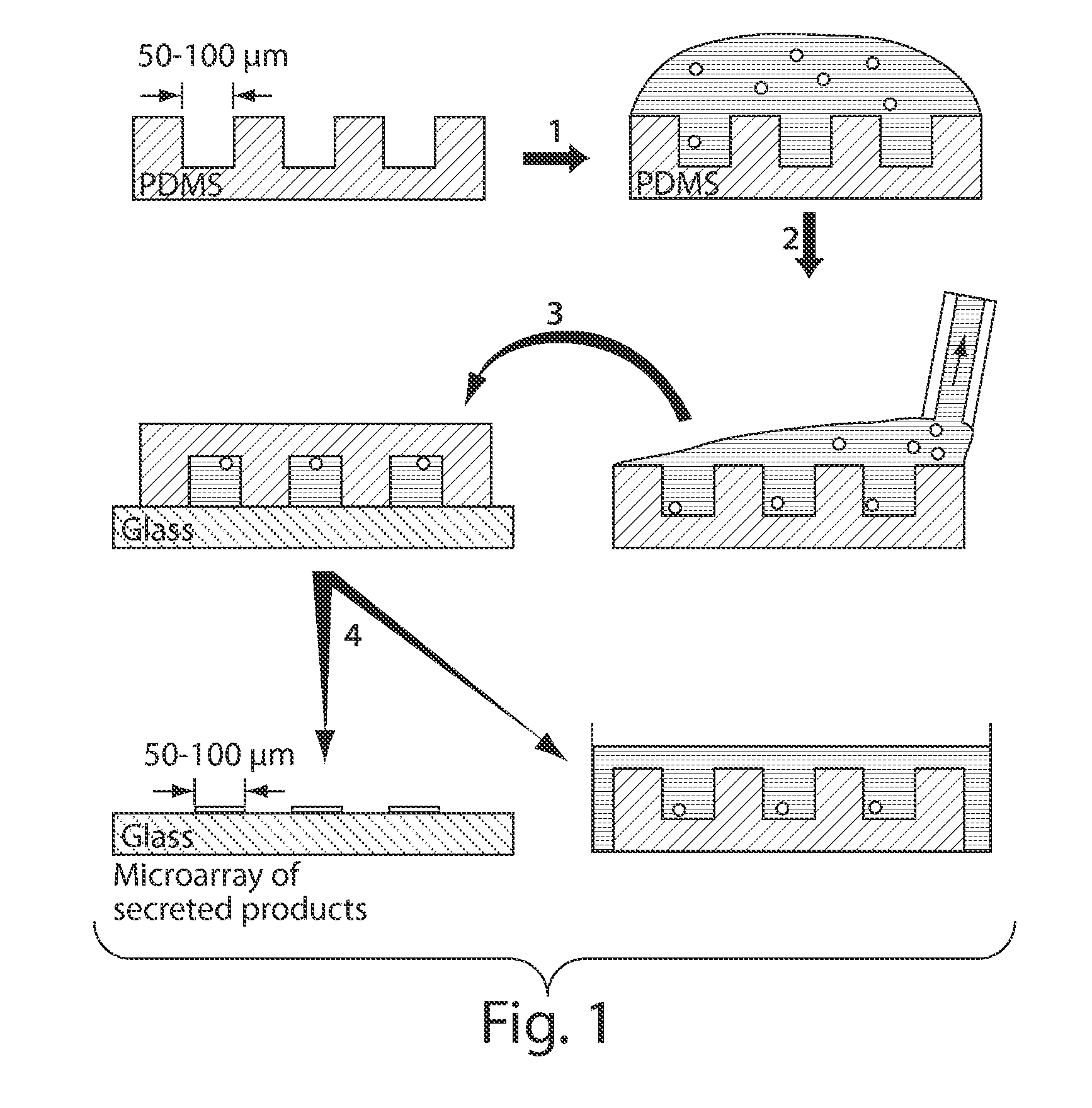
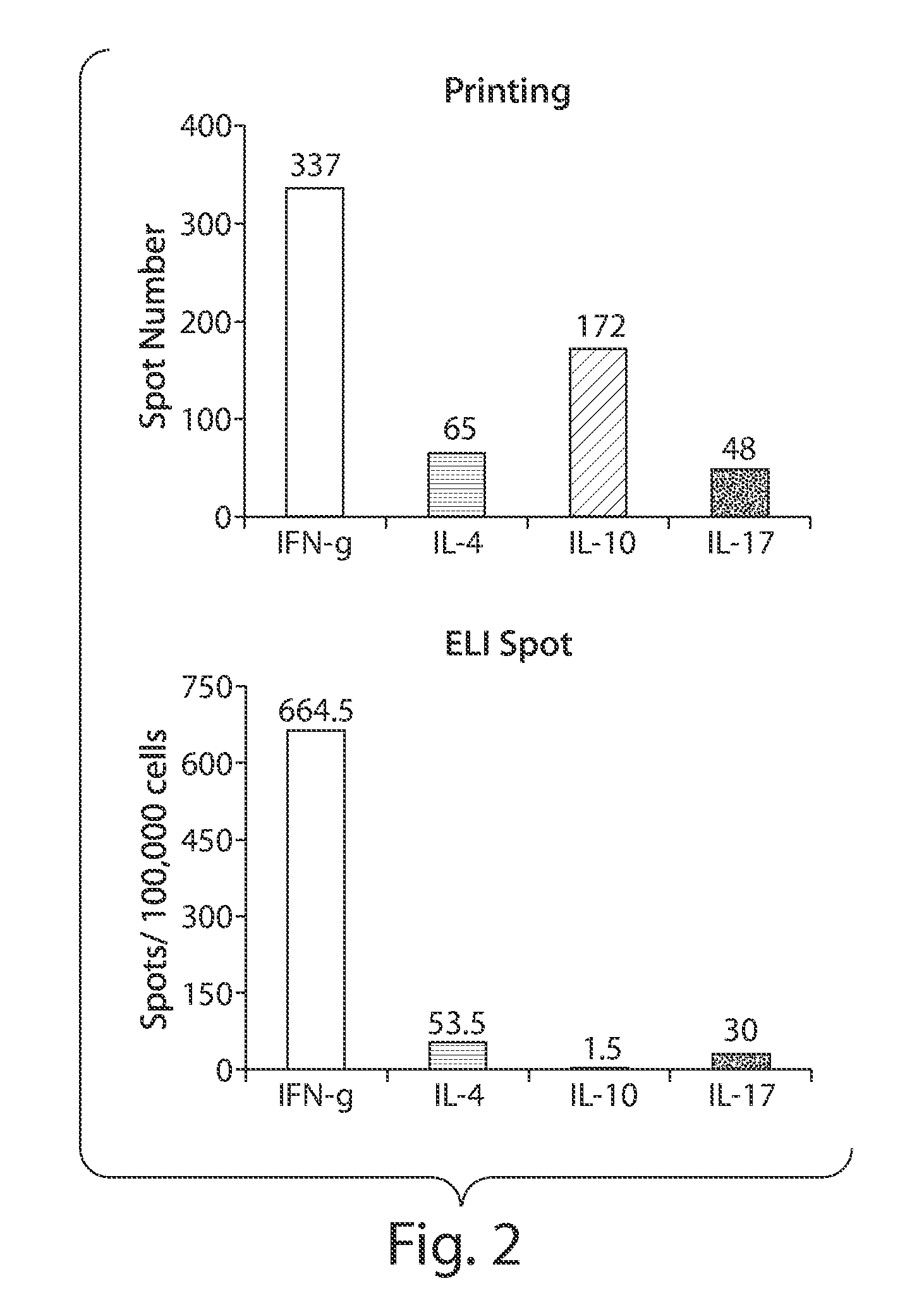
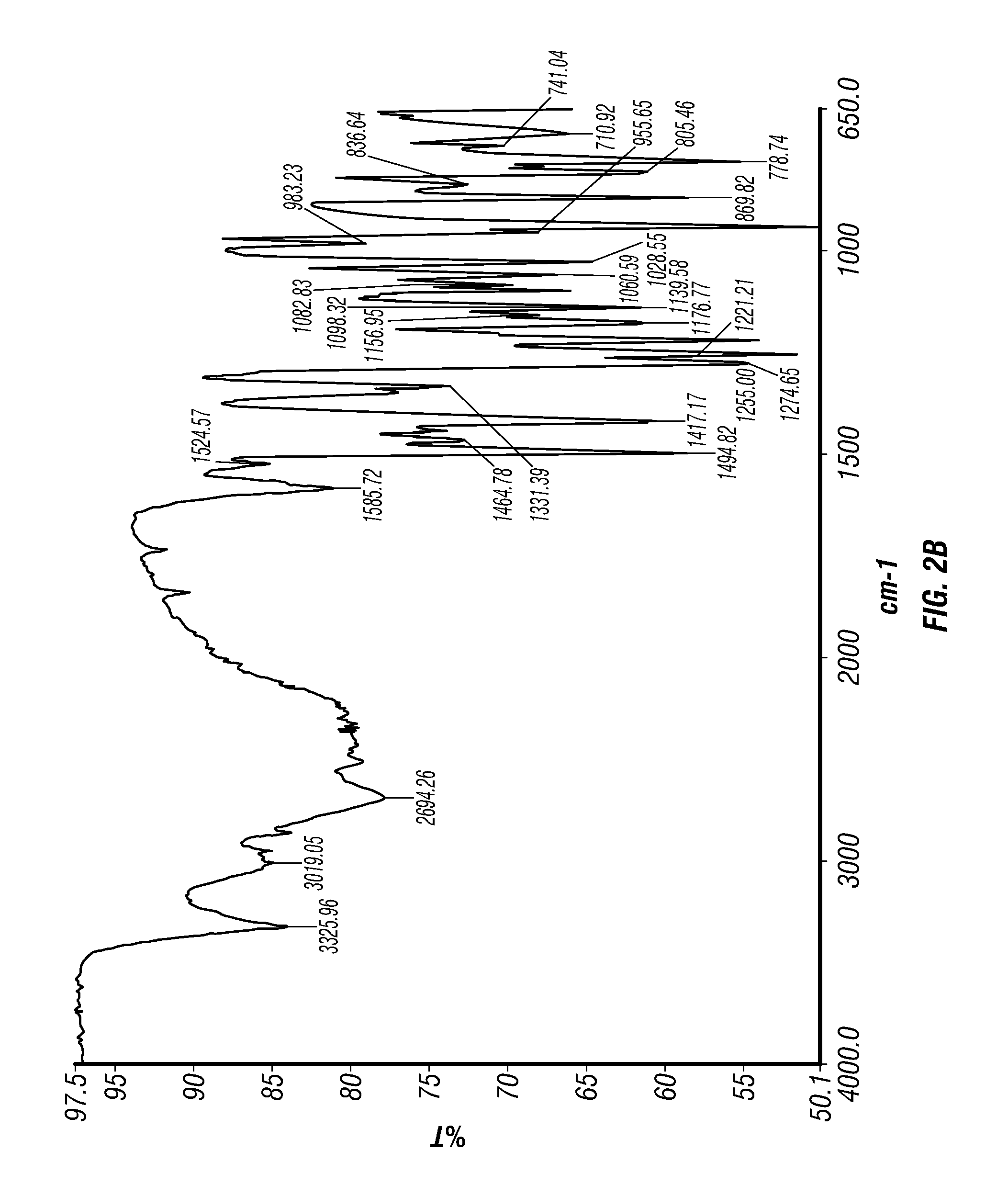
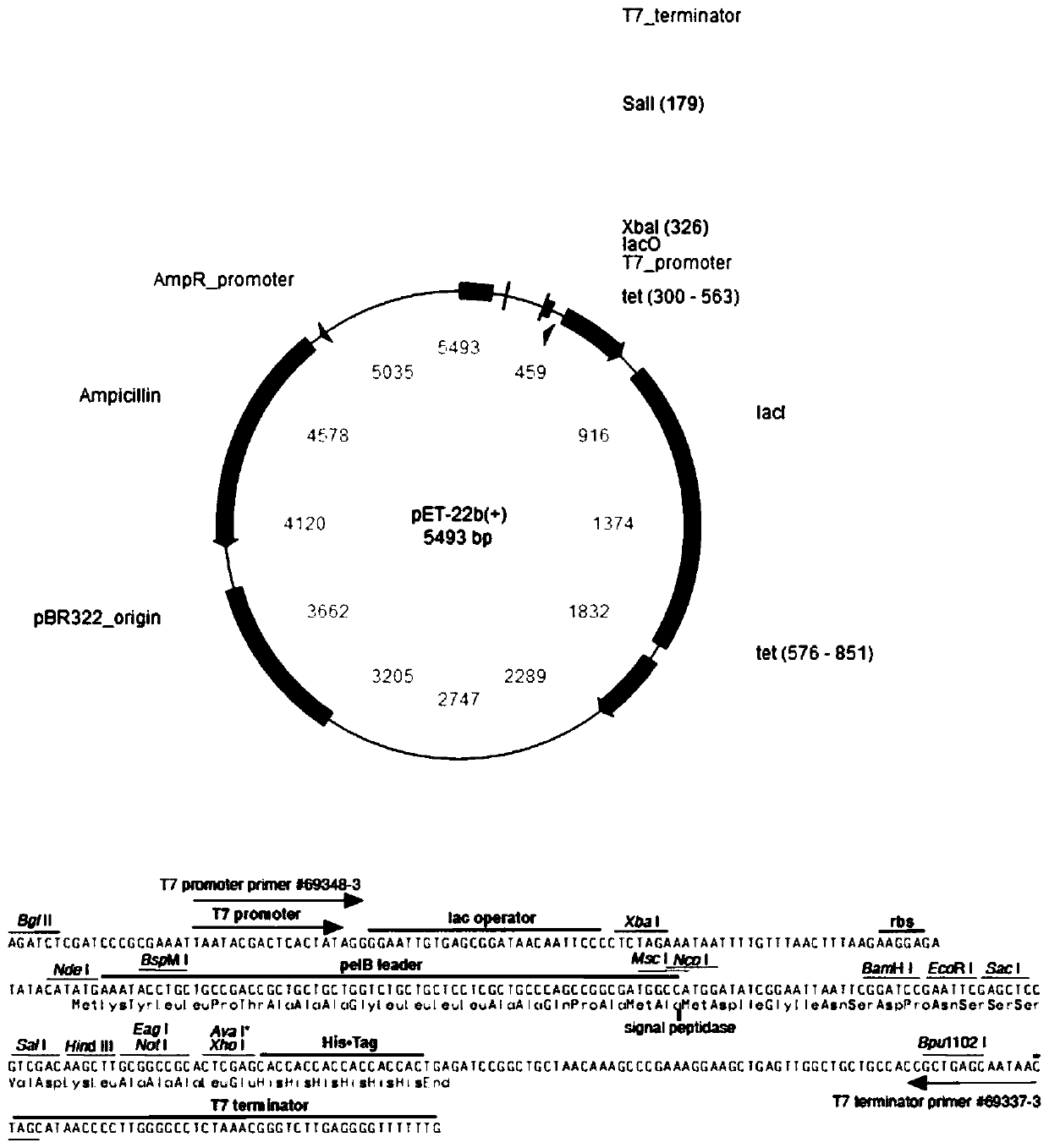
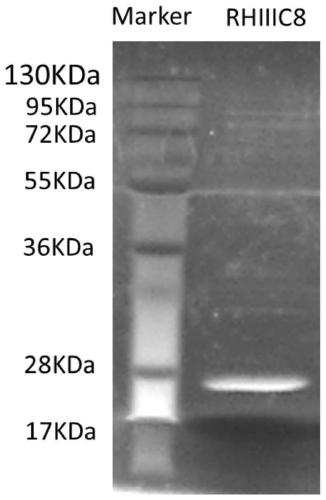
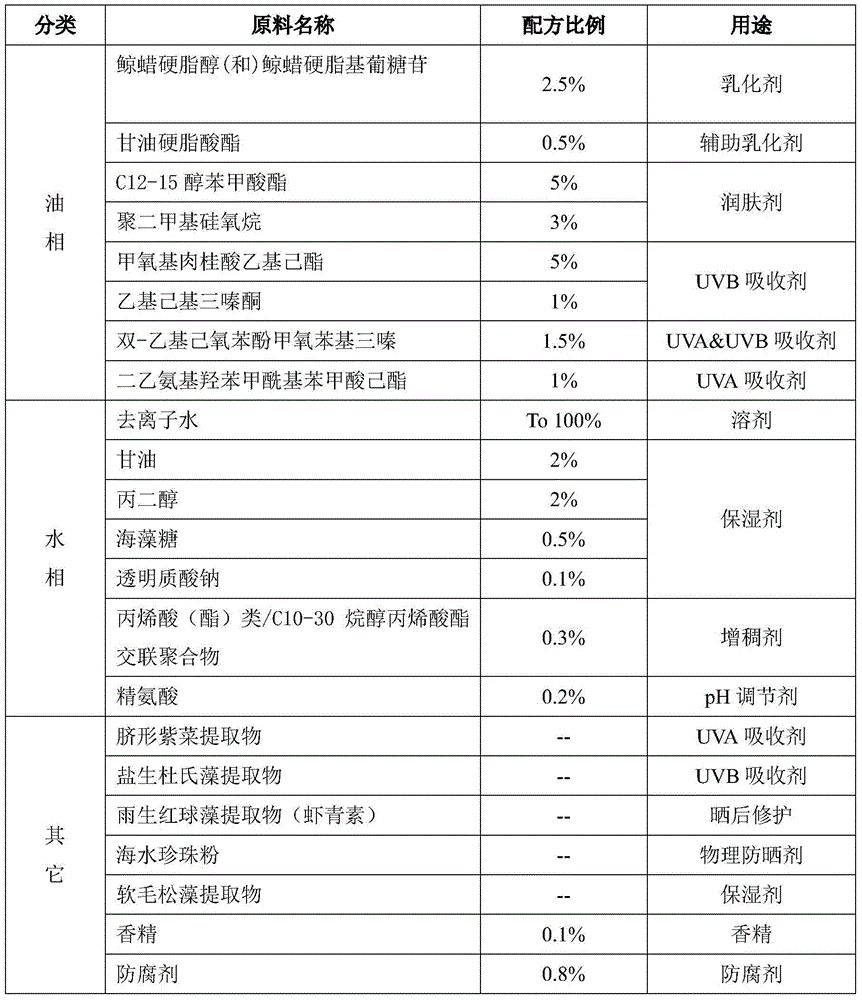
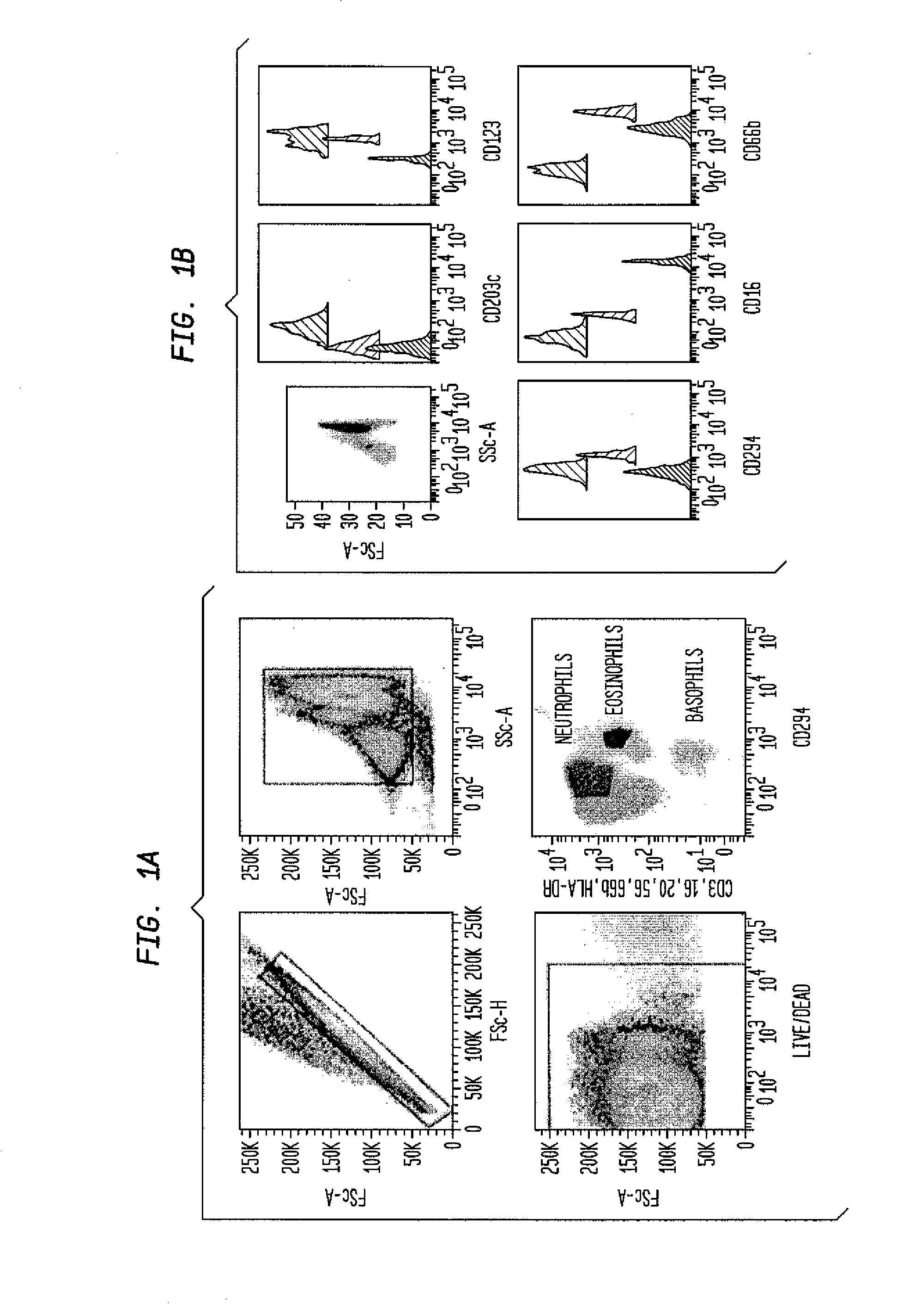
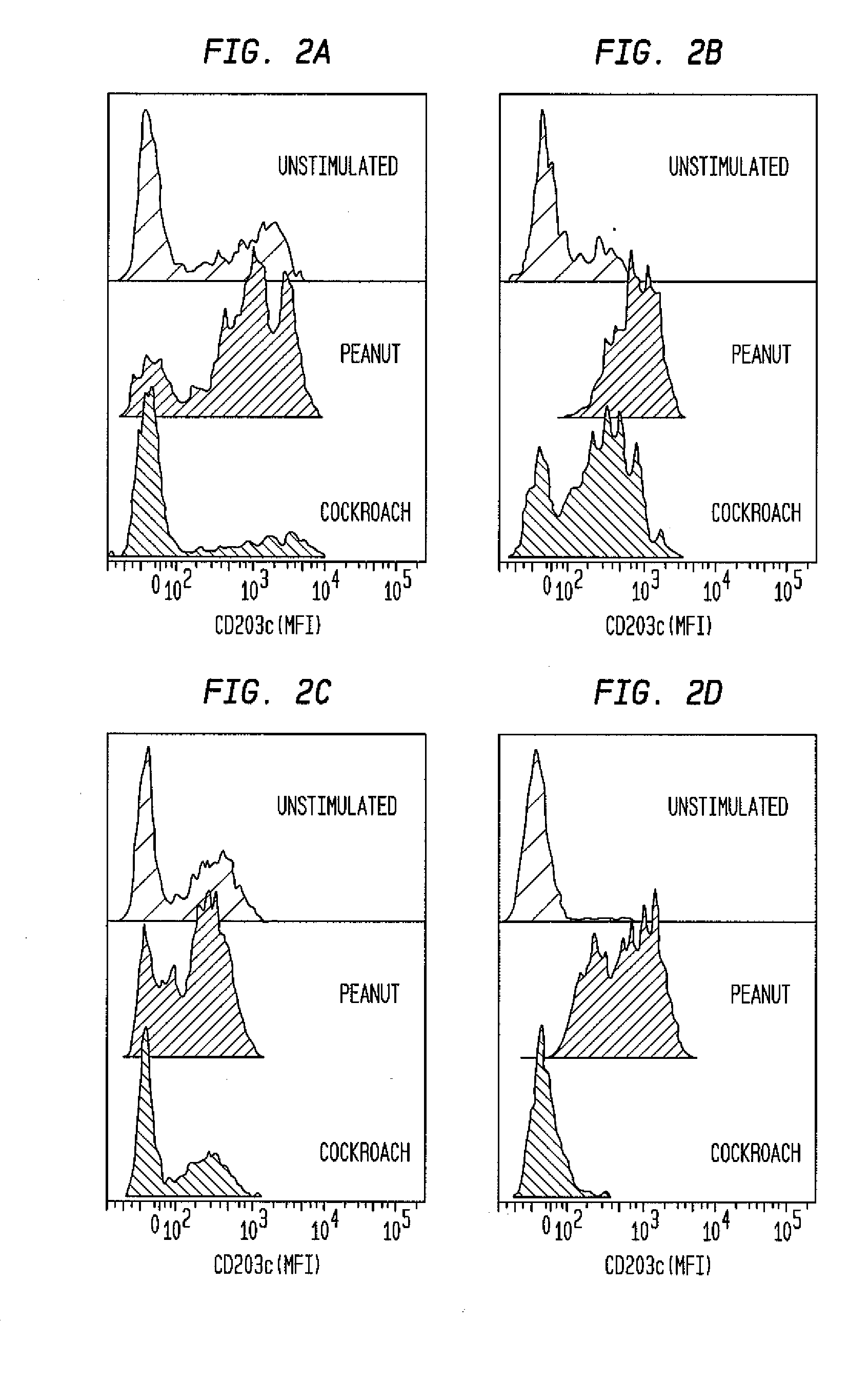
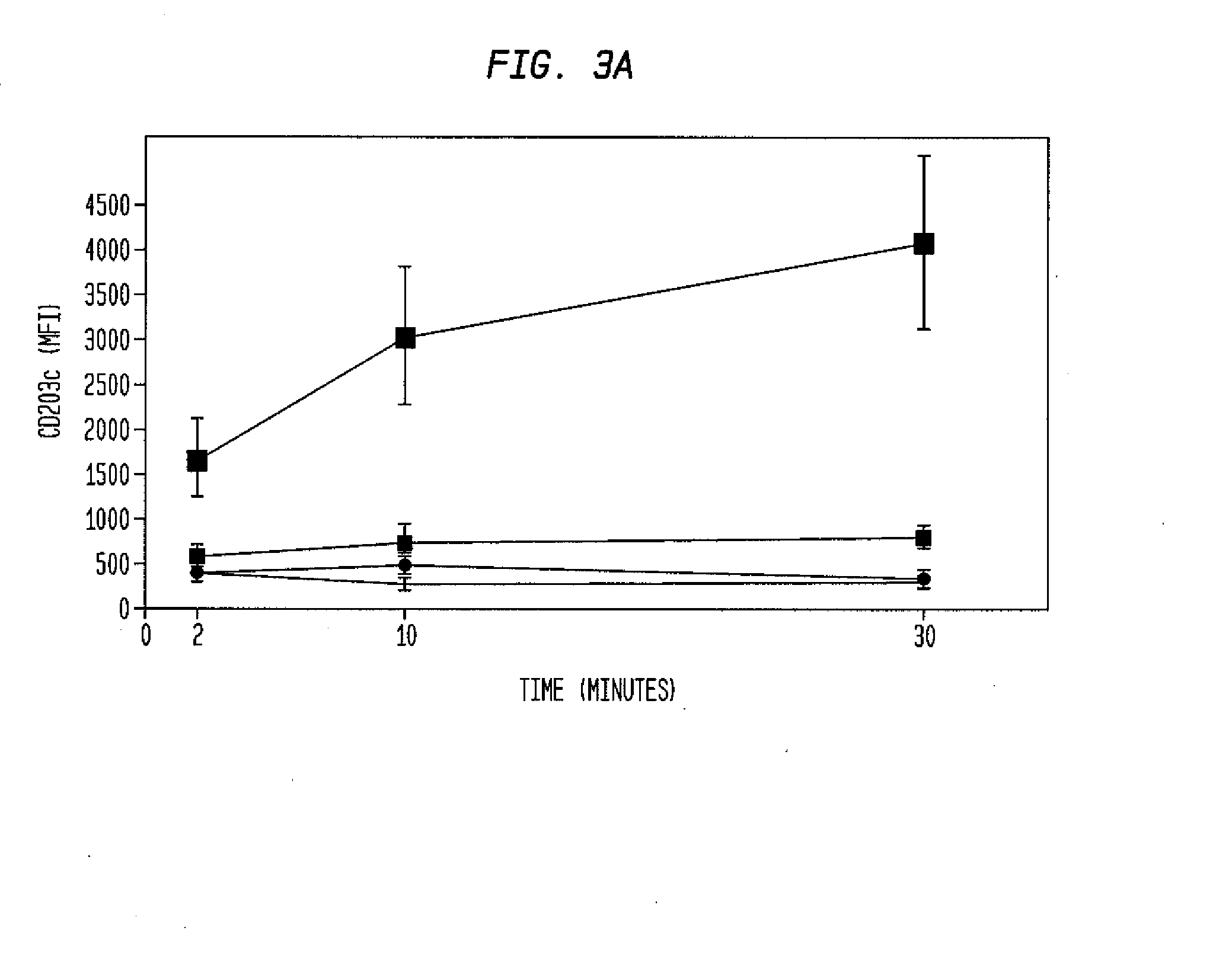
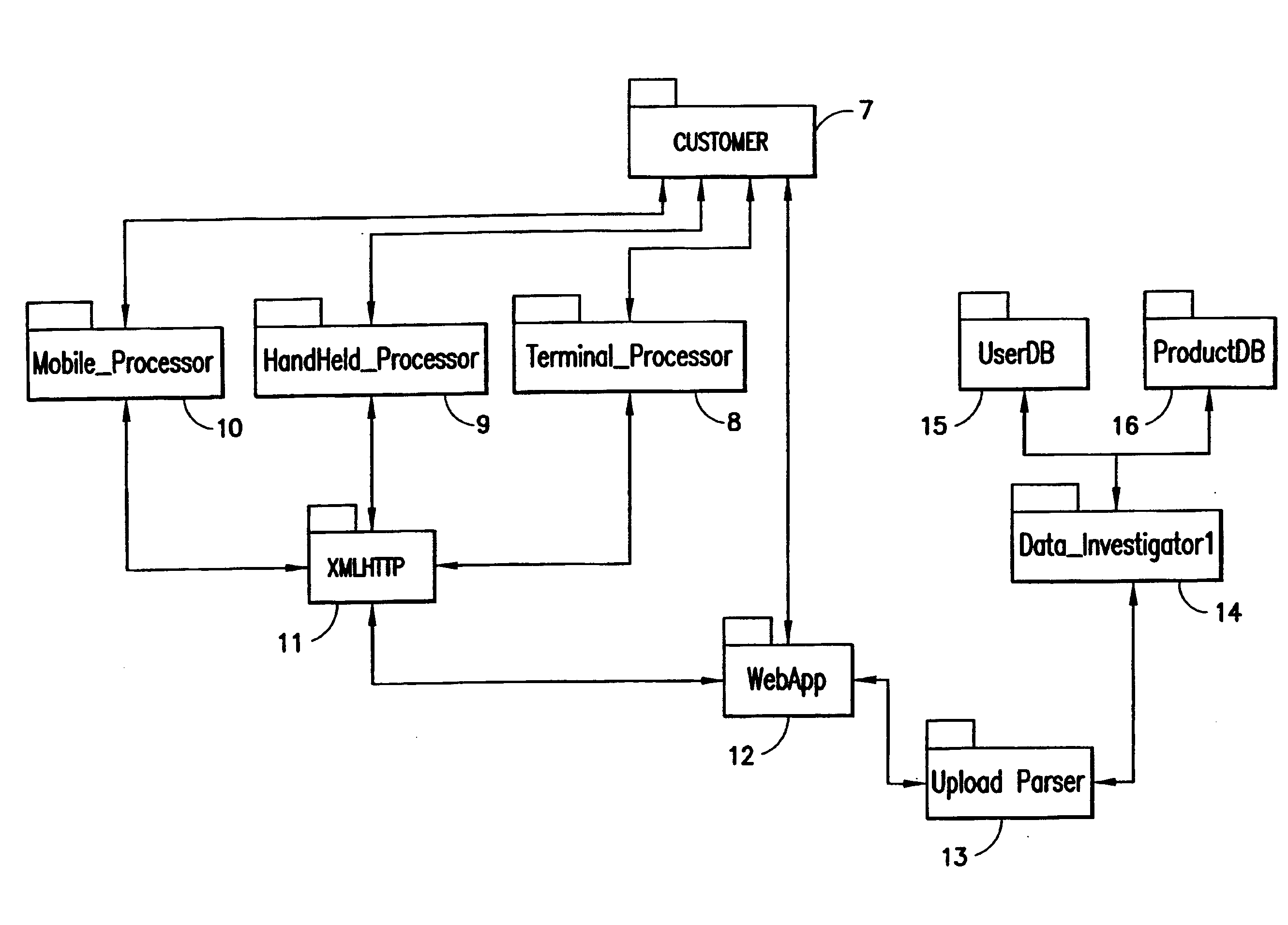
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1133 results about "Allergic reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fabric or garment with integrated flexible information infrastructure
InactiveUS6381482B1Raise the ratioImprove high temperature stabilityWeft knittingOrnamental textile articlesInformation processingEngineering
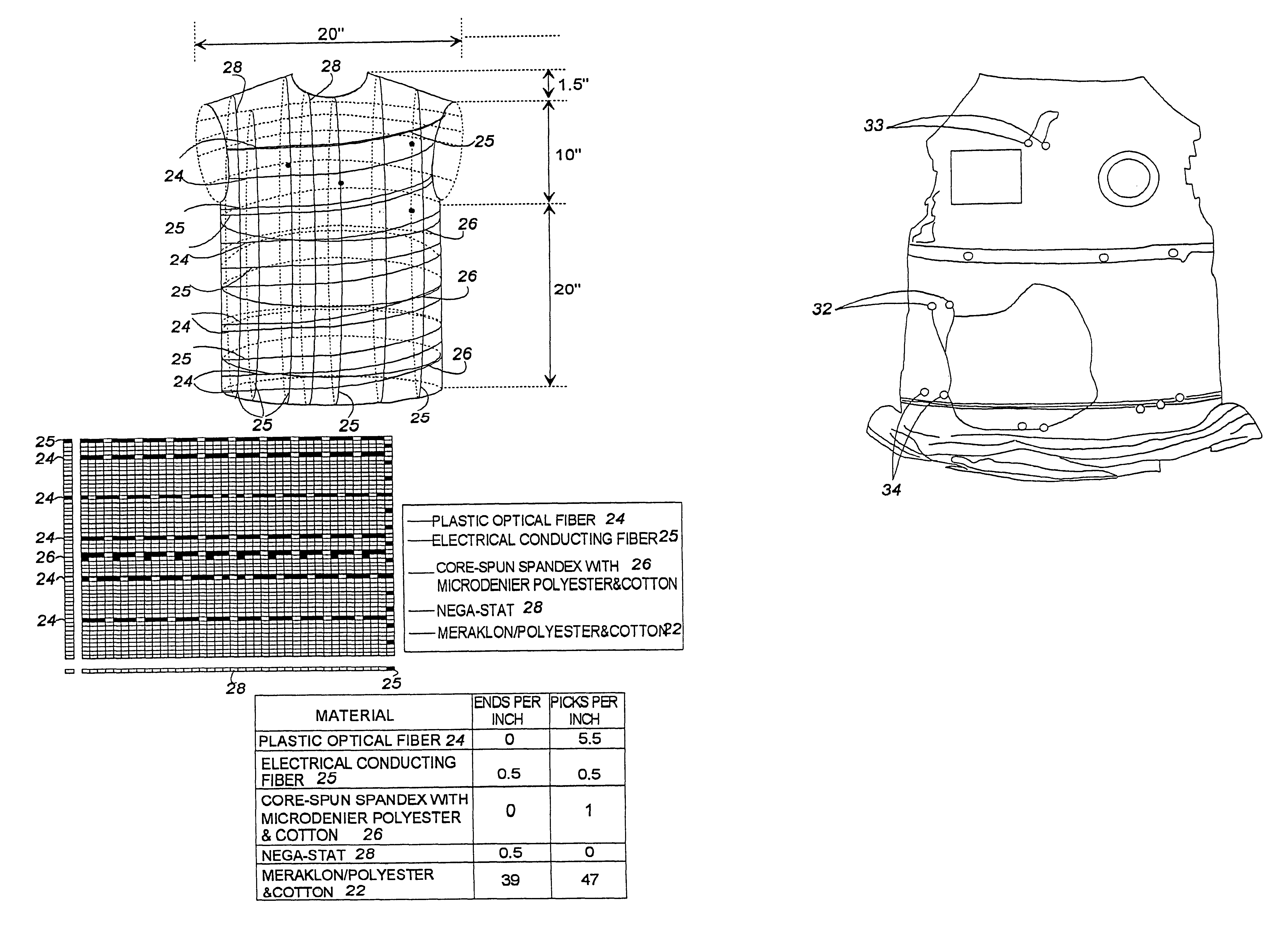
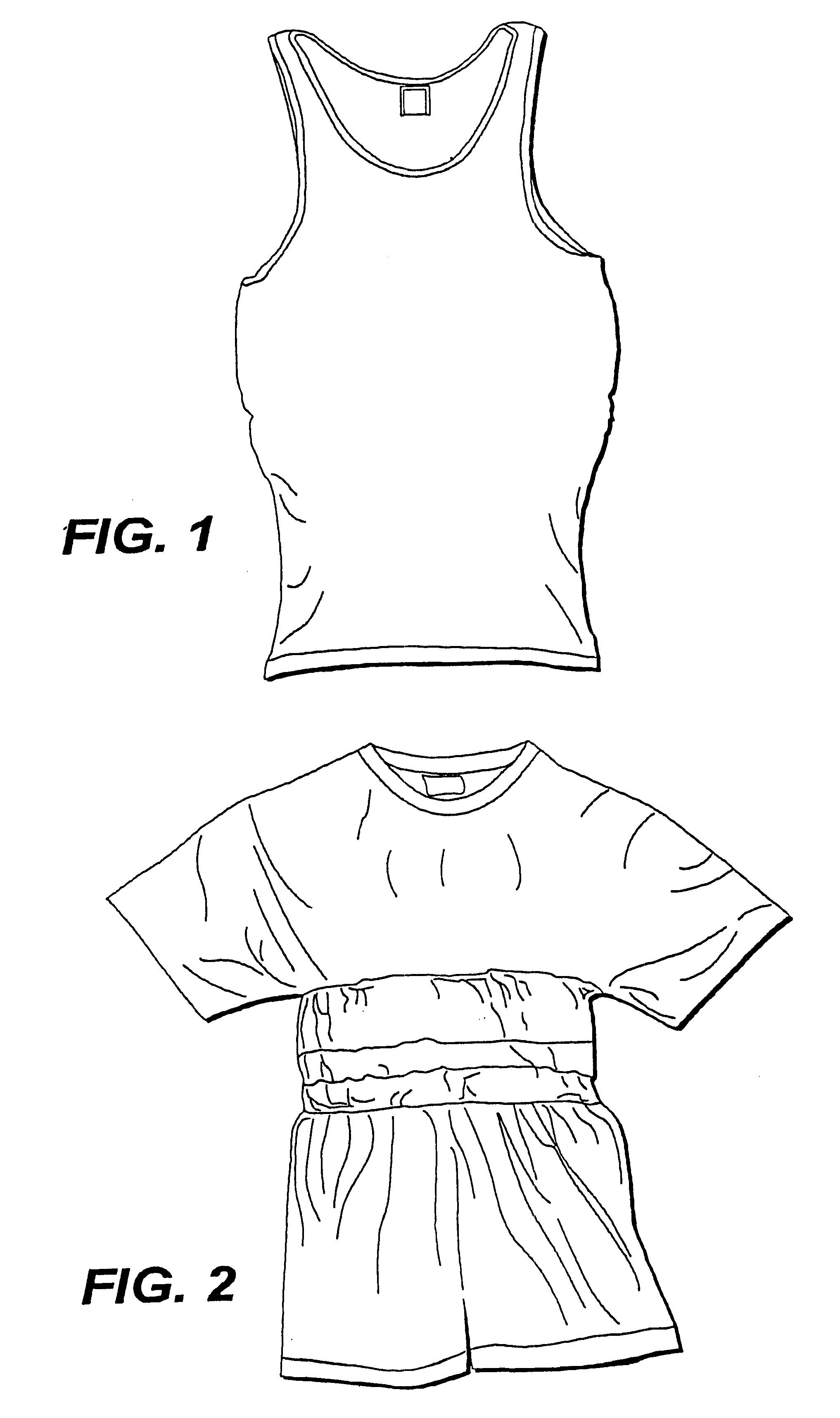
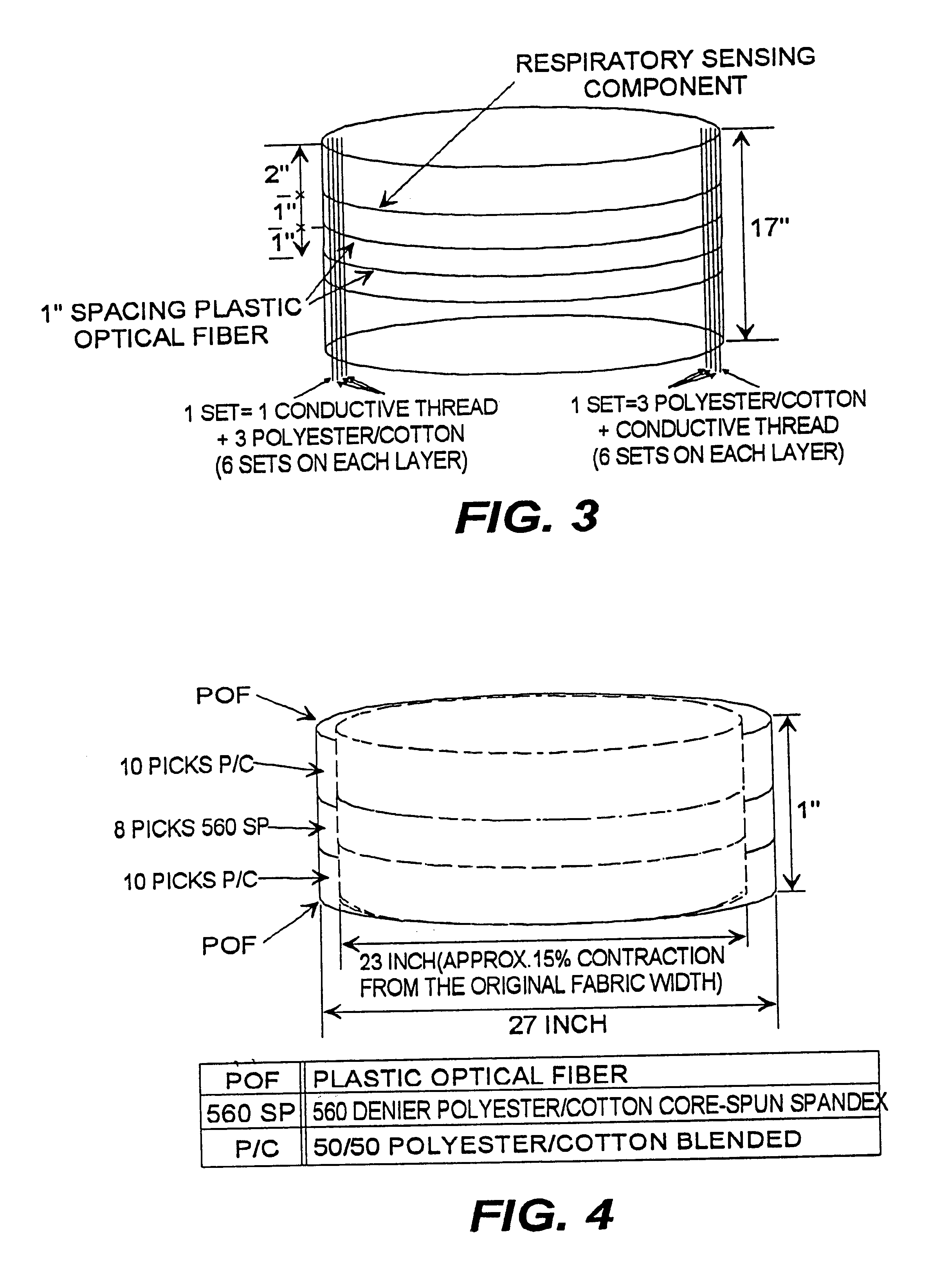
A fabric, in the form of a woven or knitted fabric or garment, including a flexible information infrastructure integrated within the fabric for collecting, processing, transmitting and receiving information concerning-but not limited to-a wearer of the fabric. The fabric allows a new way to customize information processing devices to "fit" the wearer by selecting and plugging in (or removing) chips / sensors from the fabric thus creating a wearable, mobile information infrastructure that can operate in a stand-alone or networked mode. The fabric can be provided with sensors for monitoring physical aspects of the wearer, for example body vital signs, such as heart rate, EKG, pulse, respiration rate, temperature, voice, and allergic reaction, as well as penetration of the fabric. The fabric consists of a base fabric ("comfort component"), and an information infrastructure component which can consist of a penetration detection component, or an electrical conductive component, or both. The preferred penetration detection component is a sheathed optical fiber. The information infrastructure component can include, in addition to an electrically conductive textile yarn, a sensor or a connector for a sensor. A process is provided for making an electrical interconnection between intersecting electrically conductive yarns. Furthermore, a process is established for sheathing the plastic optical fiber and protecting it.
Owner:GEORGIA TECH RES CORP
Cosmetic or dermopharmaceutical composition comprising an enzyme which is insoluble in an aqueous medium, as well as its uses
InactiveUS20040120917A1Lowering melanic indexEffective formulationCosmetic preparationsHair cosmeticsIrritationAqueous medium
The invention relates to a cosmetic or dermopharmaceutical composition, as well as these uses. The invention relates mainly to a cosmetic or dermopharmaceutical composition, notably an anti-wrinkle cosmetic or dermopharmaceutical composition, comprising an enzyme which is insoluble in an aqueous medium, in admixture with at least one cosmetically or dermopharmaceutically acceptable excipient. This composition is mainly used for limiting reactions of irritation and / or of allergy during topical use of this composition.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
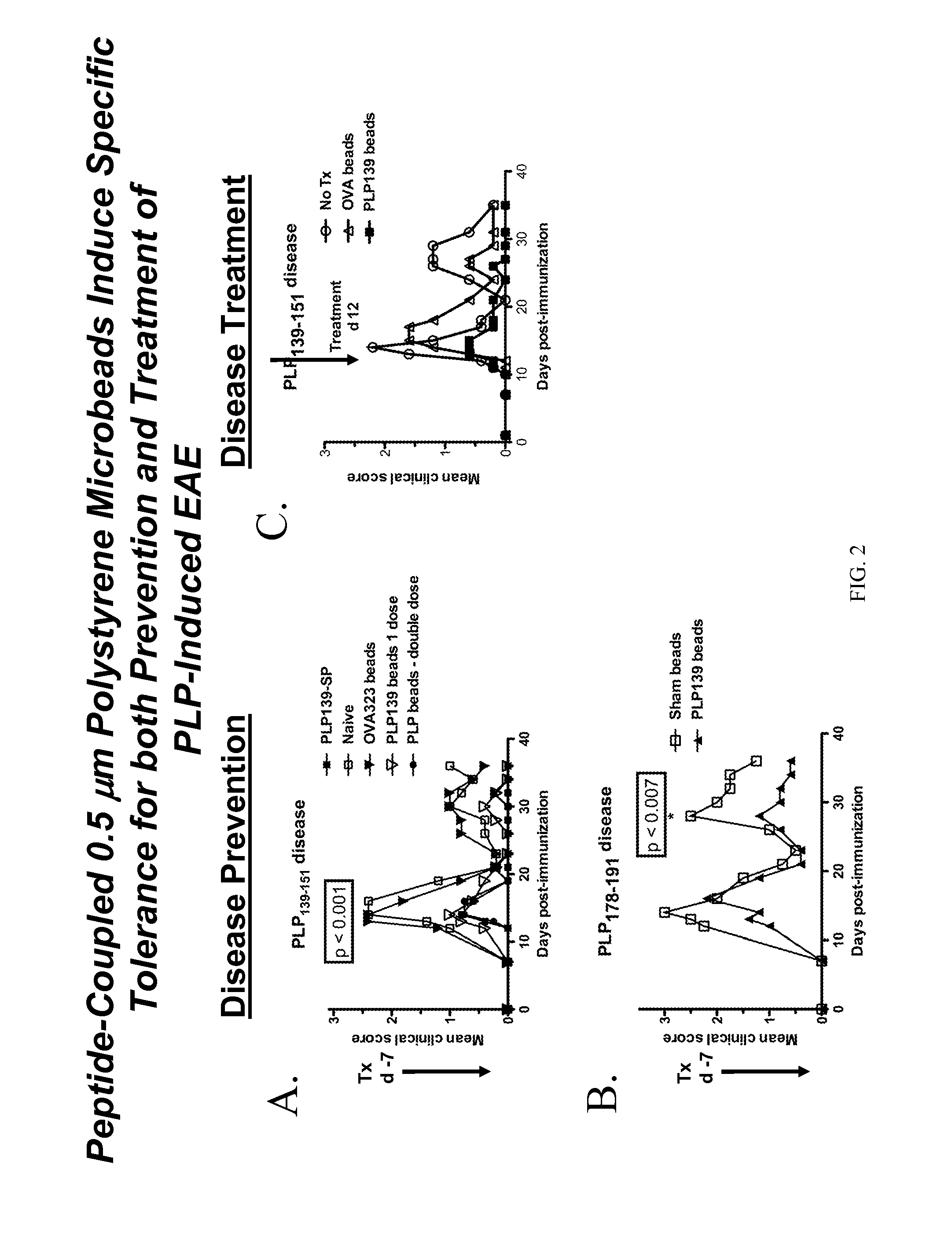
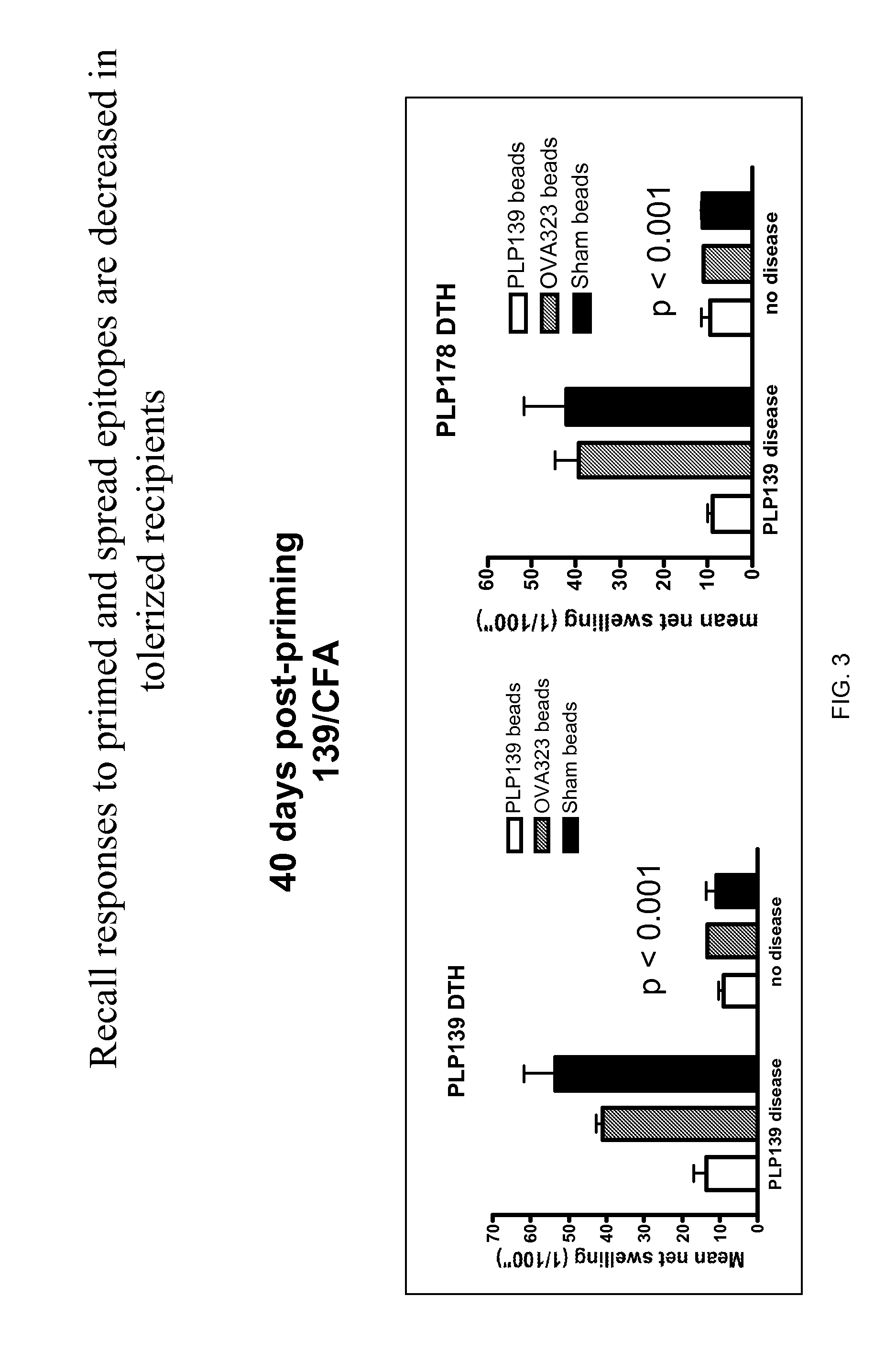
Compositions and methods for induction of antigen-specific tolerance
InactiveUS20120076831A1Efficiently inducing long-term immune tolerancePowder deliveryNervous disorderDiseaseTransplant rejection
The present invention utilizes carrier particles to present antigen peptides and proteins to the immune system in such a way as to induce antigen specific tolerance. The carrier particle is designed in order to trigger an immune tolerance effect. The invention is useful for treatment of immune related disorders such as autoimmune disease, transplant rejection and allergic reactions.
Owner:MILLER STEPHEN +4
Biocompatible cemented carbide articles and methods of making the same
InactiveUS20070082229A1Eliminate and minimize toxic effectReduce concentrationSurgeryJewelleryBiocompatibility TestingAlloy
This invention relates to cemented carbide articles that are characterized by substantially improved biocompatibility with human skin, tissue, organs, etc., compared with articles made from conventional cemented carbides. The essential feature of these improved biocompatible cemented carbide articles is a binder-depleted zone at and near the exposed surfaces of the articles. By depleting the binder (which mainly consists of Co and / or Ni, as well as their alloys) at and near the surface, the toxic effects of Co and Ni (known carcinogens), as well as the allergic reactions that these metals can cause when in contact with human skin, are eliminated. By depleting the binder only at and near the surface, the bulk properties of the cemented carbide article are not compromised or altered in any manner. Applications of these binder-depleted cemented carbides could include articles that human skin may experience prolonged exposure to, for example, jewelry articles such as rings, bracelets, bangles, chains, necklaces, pendants, watches, watch cases, watch straps, etc. In addition, the cemented carbides of this invention may be used in applications where the article comes directly in contact with human skin, tissue, organs, etc. such as surgical and other medical instruments, razor blades, etc. Also, other applications could include knives, tools, dies, and other wear components that are used to process and handle, and hence, come into direct contact with materials meant for human consumption and / or ingestion. Examples of such materials include foodstuffs and pharmaceuticals.
Owner:MIRCHANDANI RAJINI P +1
Carboxylic acid-modified nitrile copolymer latex and latex composition for dip forming comprising the same
ActiveUS20100152365A1Good oil resistanceHigh mechanical strengthGroup 4/14 element organic compoundsSpecial tyresVulcanizationSulfur
A carboxylic acid-modified nitrile copolymer latex is provided. The copolymer latex comprises a carboxylic acid-modified nitrile copolymer of an unsaturated monomer having at least one crosslinkable functional group selected from vinyl and epoxy groups. The use of the copolymer latex enables the production of a molded article that does not undergo long stirring and maturation, causes no allergic reactions due to the absence of sulfur and a vulcanization accelerator, has good oil resistance and high mechanical strength, and produces a soft feeling. Further provided is a latex composition for dip forming comprising the copolymer latex, and an article produced from the latex composition by dip forming.
Owner:LG CHEM LTD
Adjuvants Of Immune Response
InactiveUS20070298051A1Improving immunogenicityImprove efficacyBiocideOrganic active ingredientsAdjuvantImmunogen
The present invention features methods to substantially increase the immunogenicity of a vaccine, preferably a DNA vaccine, and involves providing a mammal with a vaccine regimen, which includes an immunogen and Flt3L in combination with MIP-1α or MIP-3α. The methods of the present invention can be used for the prevention and treatment of various pathological states, including for example, cancer, microbial infections, autoimmune diseases, tissue rejection, and allergic reactions.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Systems and methods for a consumer to determine food/medicine interactions
InactiveUS7805319B2Reduce errorsEasily check compatibilityDrug and medicationsNutrition controlBiochemical engineeringPharmaceutical drug
One embodiment of the present invention relates to systems and methods for detecting harmful and / or hazardous ingredients that may cause an allergic reaction, interfere with the effectiveness of a prescription drug, exacerbate symptoms associated with a chronic illness, and / or cause another undesired reaction.
Owner:BADINELLI ELLEN
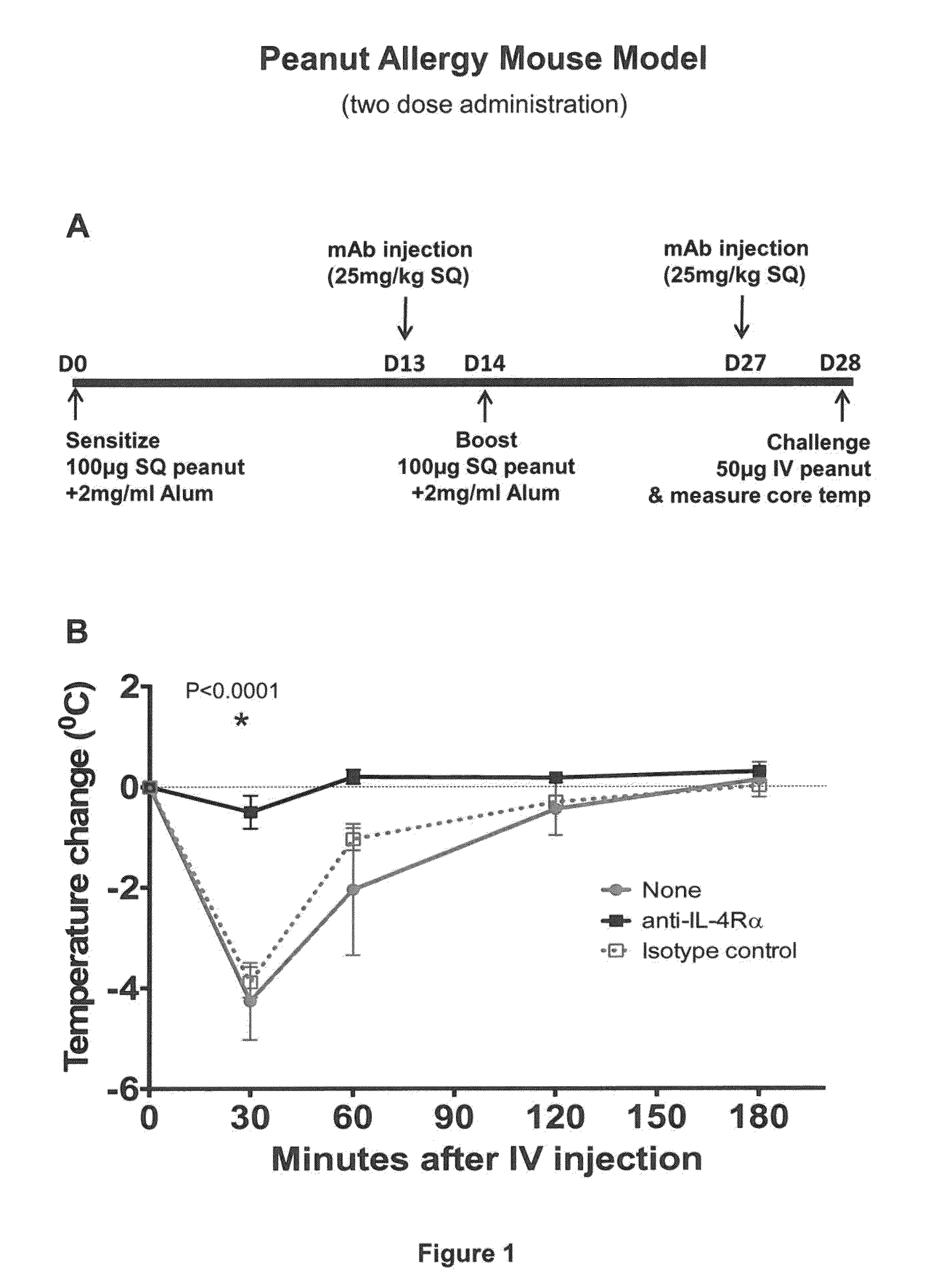
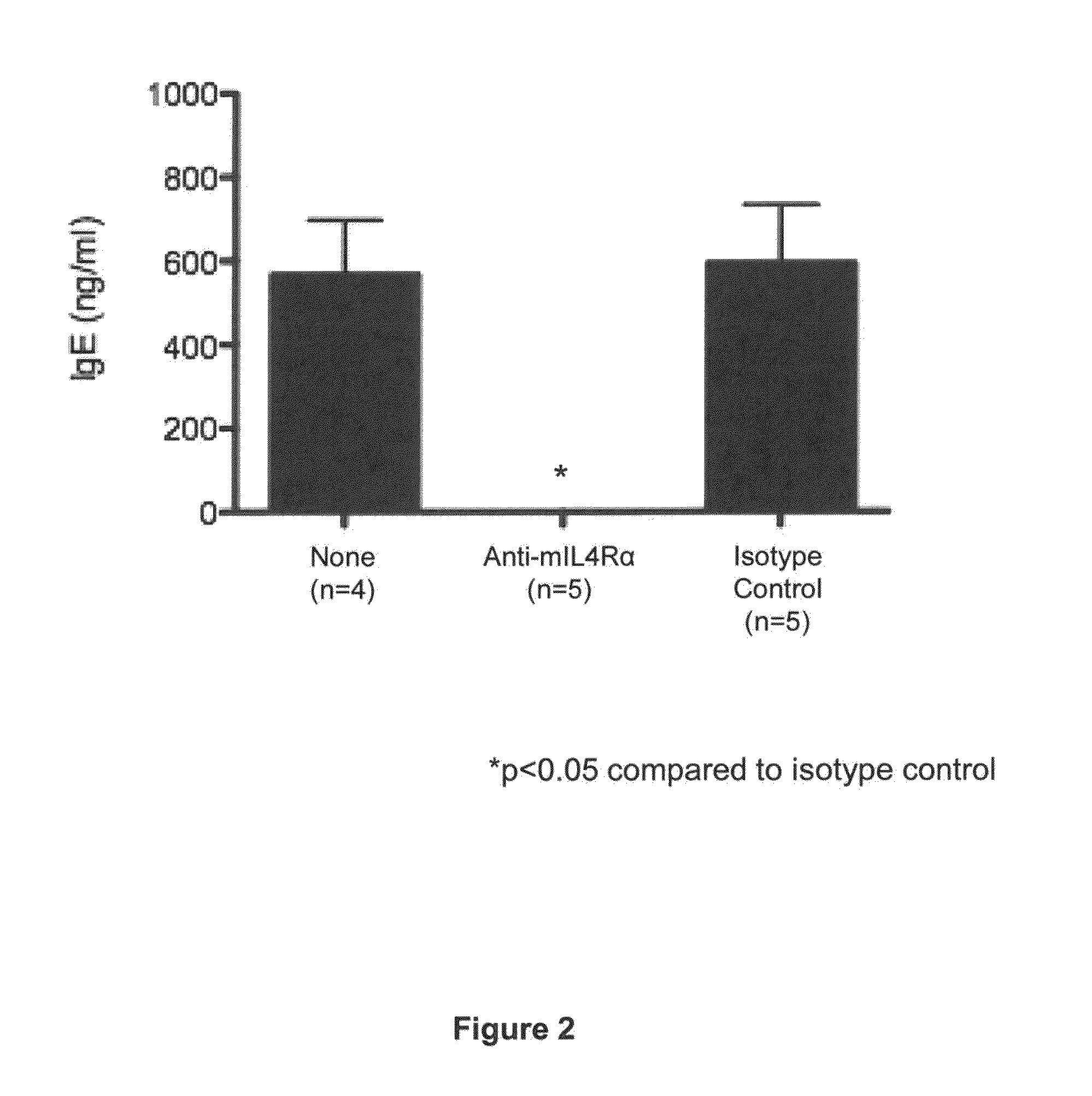
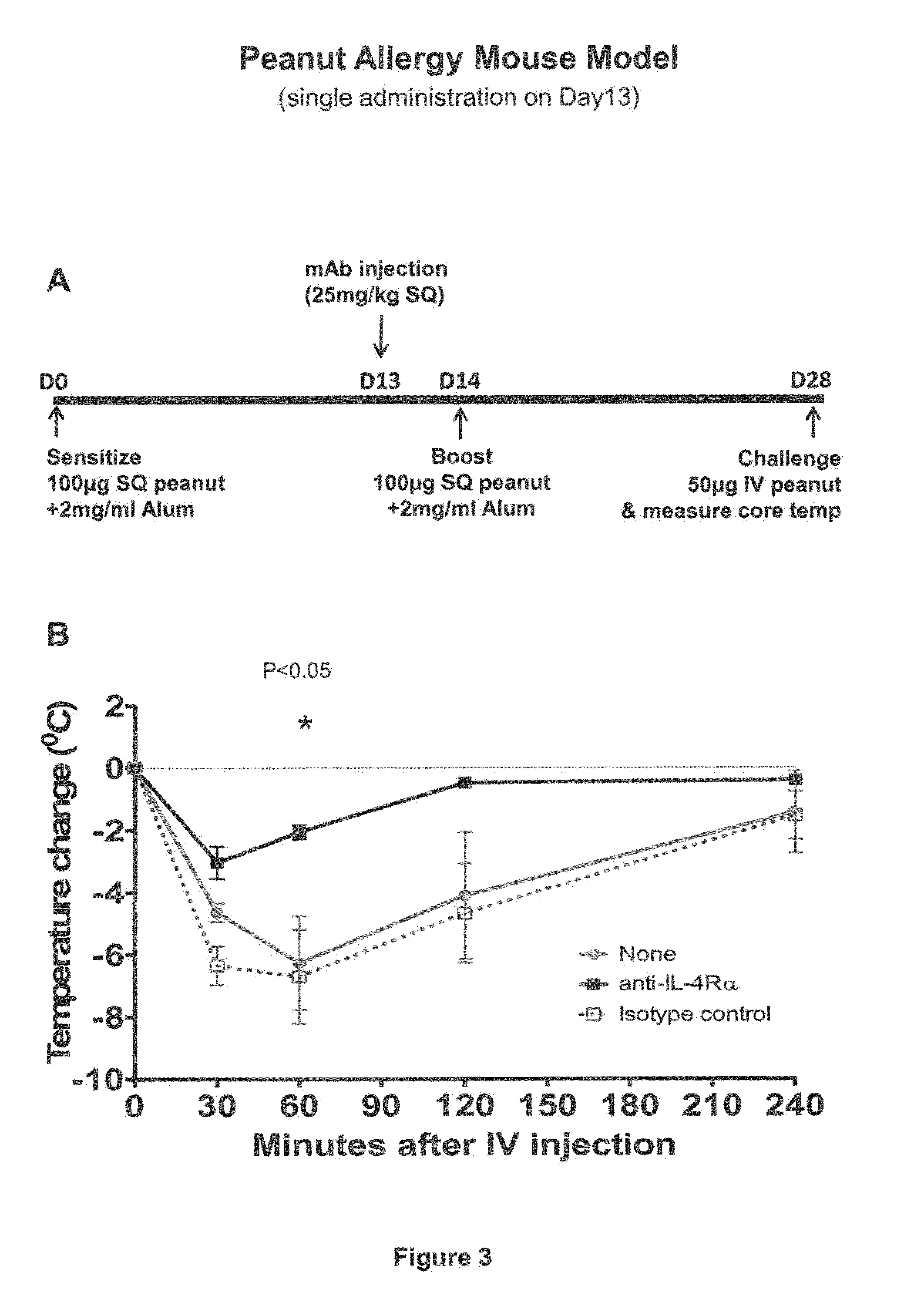
Methods for treating allergy and enhancing allergen-specific immunotherapy by administering an IL-4R inhibitor
ActiveUS20140356372A1Prevention and reduction of severityImprove efficacyPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsRegimenSeverity/Intensity
The present invention provides methods for treating, preventing or reducing the severity of allergic reactions. The present invention also provides methods for enhancing the efficacy and / or safety of an allergen-specific immunotherapy (SIT) regimen. The methods of the present invention comprise administering to a subject in need thereof a therapeutic composition comprising an interleukin-4 receptor (IL-4Rα) antagonist such as an anti-IL-4Rα antibody.
Owner:REGENERON PHARM INC
Multipotent Adult Stem Cells And Uses of Multipotent Adult Stem Cells To Treat Inflammation
InactiveUS20100172885A1Effective treatmentIncrease the number ofBiocideBone marrow stroma cellsDiseaseAutoimmune responses
Disclosed are cell preparations comprising multipotent adult stem cells and methods for using multipotent adult stem cells to treat autoimmune diseases, treat allergic responses, treat cancer, treat inflammatory diseases, treat fibrotic disorders, reduce inflammation and / or fibrosis, promote would healing, repair epithelial damage, and / or promote angiogenesis.
Owner:MESOBLAST INT
High molecule bonding adriamycin medicine, nano capsule and preparation thereof
InactiveCN101234204AReduce manufacturing costPlay a role in isolationOrganic active ingredientsPharmaceutical non-active ingredientsPolymer scienceEnd-group
The invention provides a macromolecule bonding adriamycin medicine, a nanometer capsule and a preparation method thereof. The bonding medicine is bonded by a block copolymer of poly ethylene glycol-polylactic acid and adriamycin. Firstly, the block copolymer of poly ethylene glycol-polylactic acid is acquired by ring-opening polymerization of aliphatic cyclic esters with poly ethylene glycol, solvent and catalyst being in existence; then a carboxyl end group is transformed by a hydroxyl-terminated; and then with a condensing agent being in existence, the carboxyl end group carries out amidation reaction with the adriamycin; thus acquiring the macromolecule adriamycin bonding medicine. The bonding medicine has amphiphilic property, thus being able to self assembly to prepare the nanometer capsule with a core-shell structure in the water solution. The adriamycin is wrapped inside the capsule to play a role of isolation and protection, which overcomes the deficiencies of short circulation time in vivo, large dosage and severe allergic reaction existing in the current adriamycin preparation. In addition, the nanometer particles are expected to congregate in the blood circulation through the so-called 'enhanced infiltration and retention effect', so as to improve the targeting action of the adriamycin on the tumor location.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method For Diagnosing Allergic Reactions
ActiveUS20120015824A1Library screeningImmunoglobulins against cytokines/lymphokines/interferonsDiseaseCytokine
The invention provides a method for multiple cytokine detection from single cells for the purpose of generating immunological profiles of diseases.
Owner:MASSACHUSETTS INST OF TECH
Large scale parallel immuno-based allergy test and device for evanescent field excitation of fluorescence
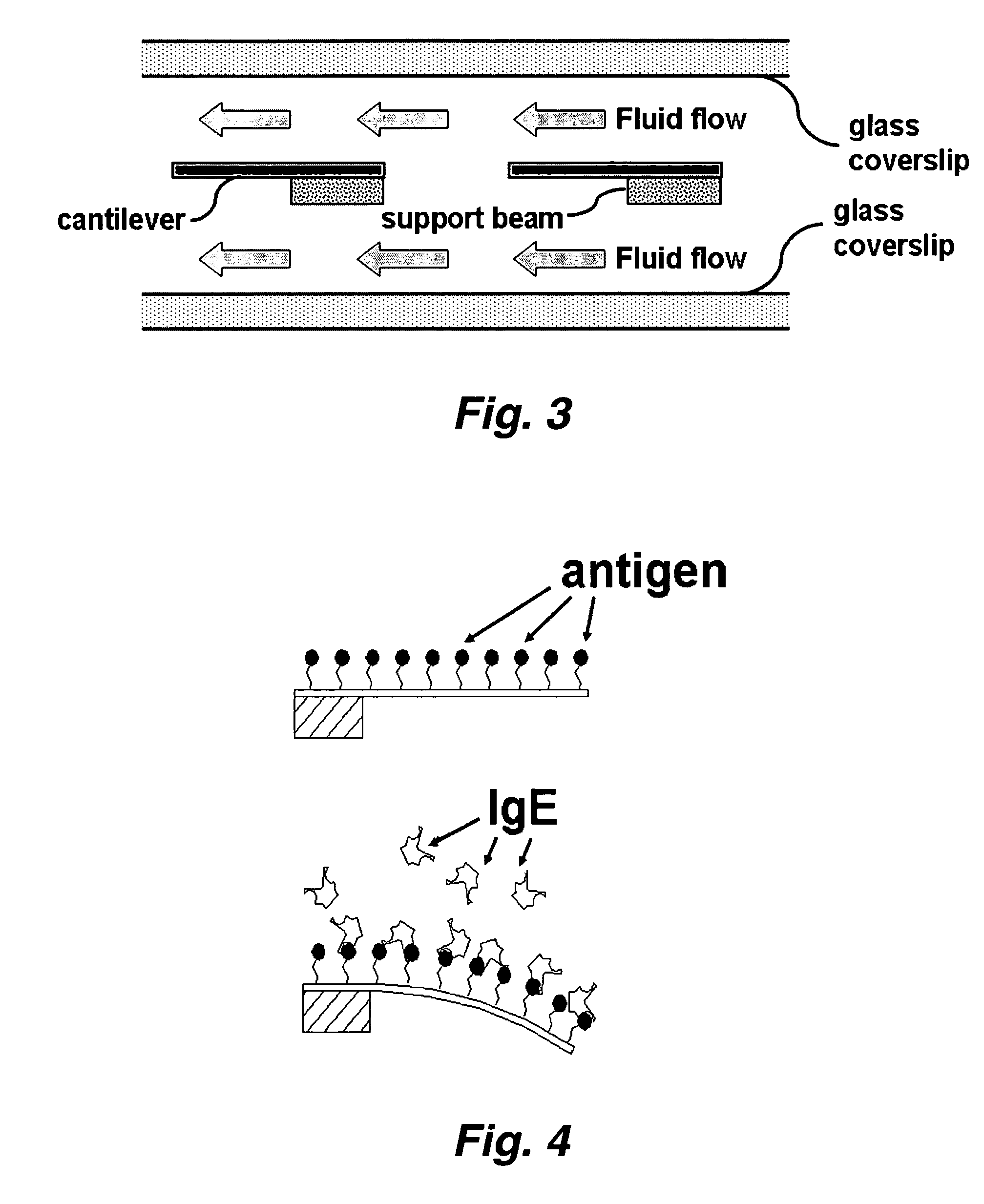
InactiveUS20070117217A1Quick checkRapid diagnosisMaterial analysis by optical meansDisease diagnosisAntigenMassively parallel
This invention provides a device and methods for the rapid detection and / or diagnosis and / or characterization of one or more allergies (e.g., causes IgE mediated allergic reaction (immediate hypersensitvity) in a mammal (e.g., a human or a non-human mammal). In certain embodiments, the device comprises a microcantilever array where different cantilevers comprising the array bear different antigens. Binding of IgE to the antigen on a cantilever causes bending of the cantilever which can be readily detected.
Owner:RGT UNIV OF CALIFORNIA
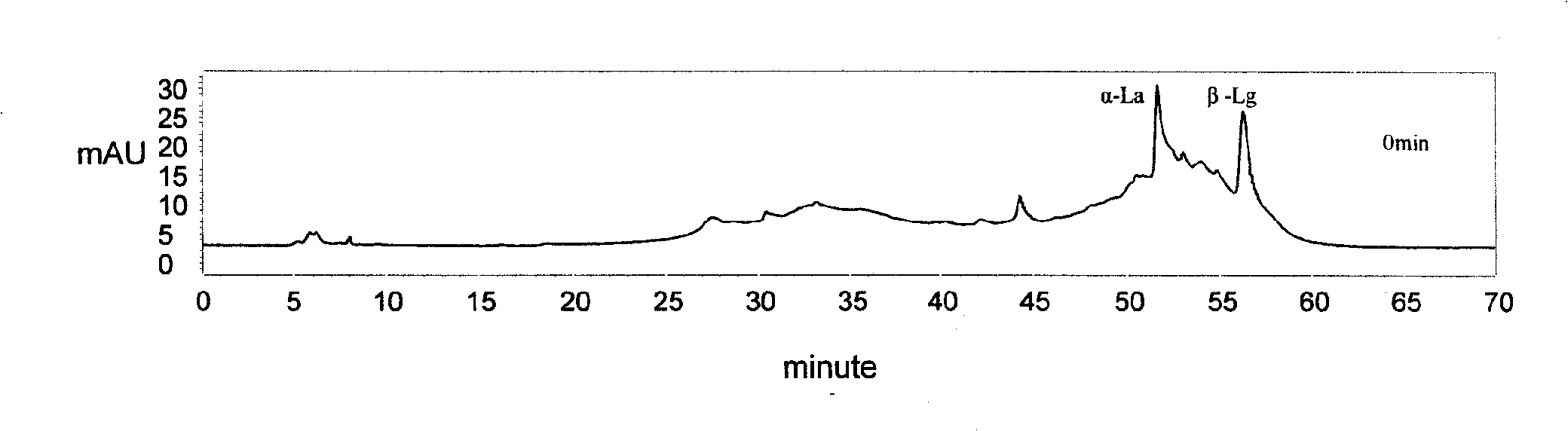
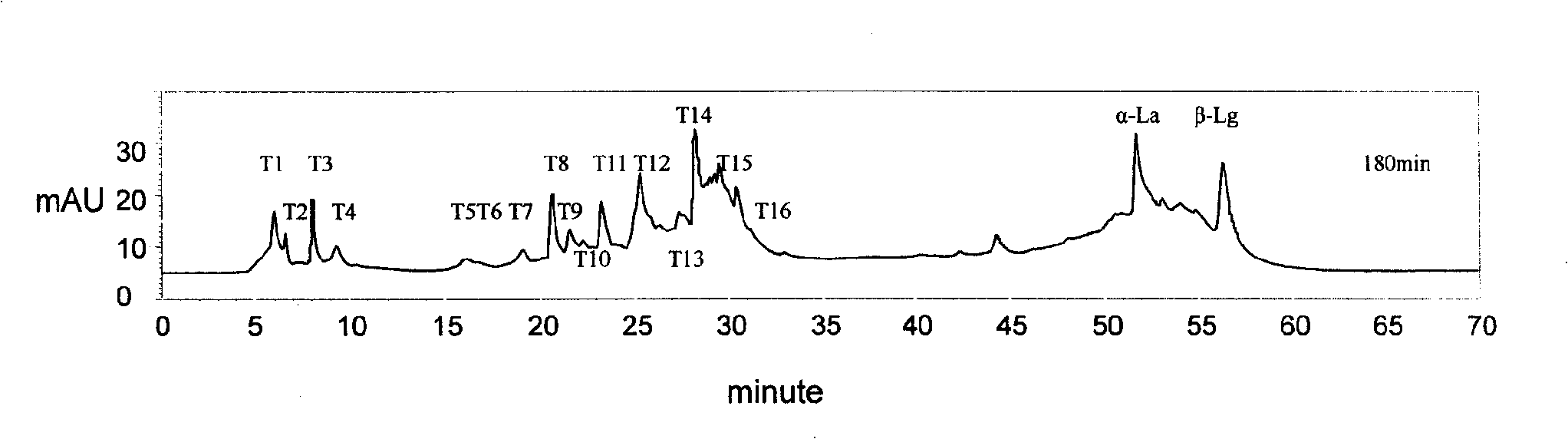
Subsensitive lactalbumin hydrolysate and preparation method thereof
The invention discloses a muting sensitive lactalbumin hydrolysate and a preparation method thereof. The method mainly comprises the following steps: dissolving condensed whey protein in the water, then carrying out denaturation under high temperature, carrying out hydrolysis by using the protamex composed of alkali protease, papain and flavourzyme, treating the lactalbumin hydrating solution with desalinization and drying to obtain the lactalbumin hydrolysate of the invention. The lactalbumin hydrolysate of the invention has high suppression ratio of Beta-lactoglobulin and Alpha- lactalbumin, can reduce or eliminate cow milk allergic reaction; furthermore, the lactalbumin hydrolysate can also produce other active materials, thus also having the functions of promoting immunity, being easy to digest and absorb and reducing blood pressure; and the lactalbumin hydrolysate has high security and no side effect, bitter taste or other bad flavors.
Owner:CHINA AGRI UNIV
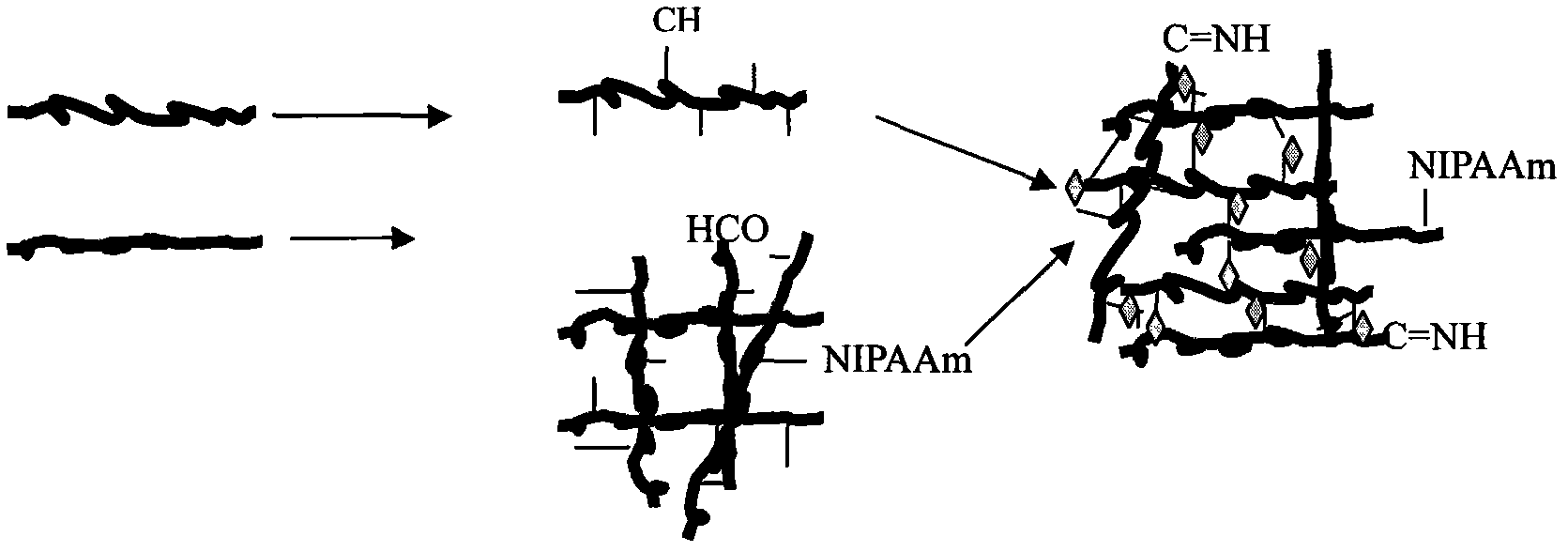
Injectable temperature sensitive gel used for filling and repairing damaged tissues
The invention relates to an injectable temperature sensitive gel used for filling and repairing damaged tissues. The gel is characterized in that: medical-grade hyaluronic acid and chitosan are used as gel raw materials; the gel swelling ratio is 2-500; and a temperature sensitive temperature is 32-37 DEG C. The gel provided by the invention has the advantages that the injection temperature sensitive gel has physical and chemical properties of a general gel, also has injectable and temperature sensitive properties and a good fluidity at a room temperature and is easy for injection; the formability in organisms is good and the degradation is slow so that the general gel can not be compared; a cross-linking agent is not used for the gel so that harms of the cross-linking agent to a human body are avoided; a gel system can prolong a persistence time of the gel in a body, and also can continuously stimulate the body to produce reactions so that the tissue in-situ regeneration and repair is promoted and the filling quality is increased; and the gel simultaneously has the advantages of reducing bacterial invansion, anticoagulation and low allergic reaction and the like.
Owner:冯淑芹
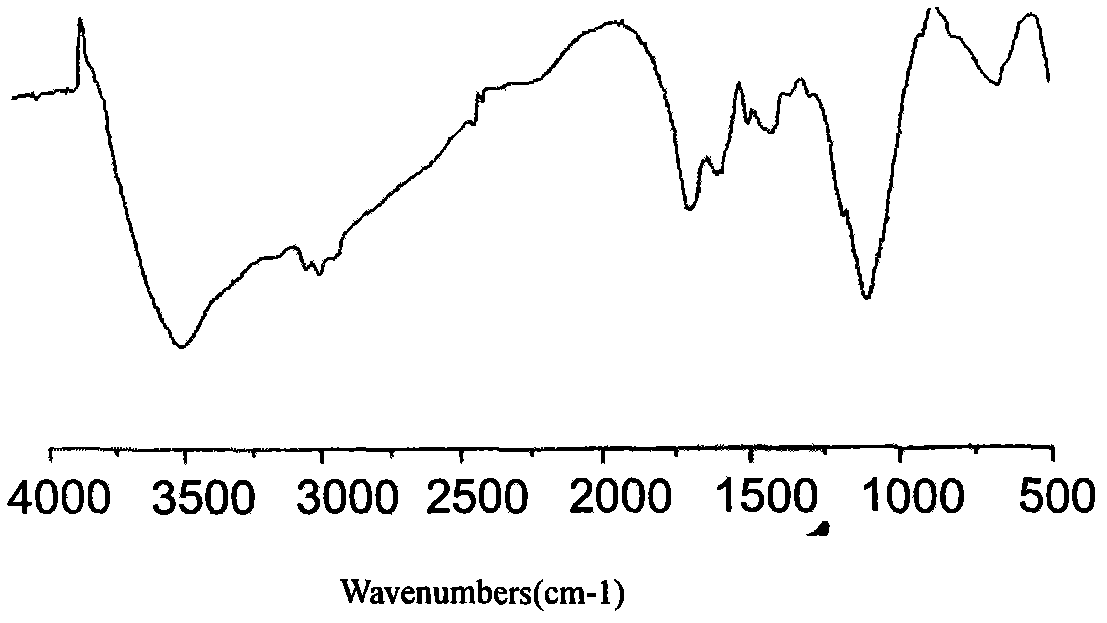
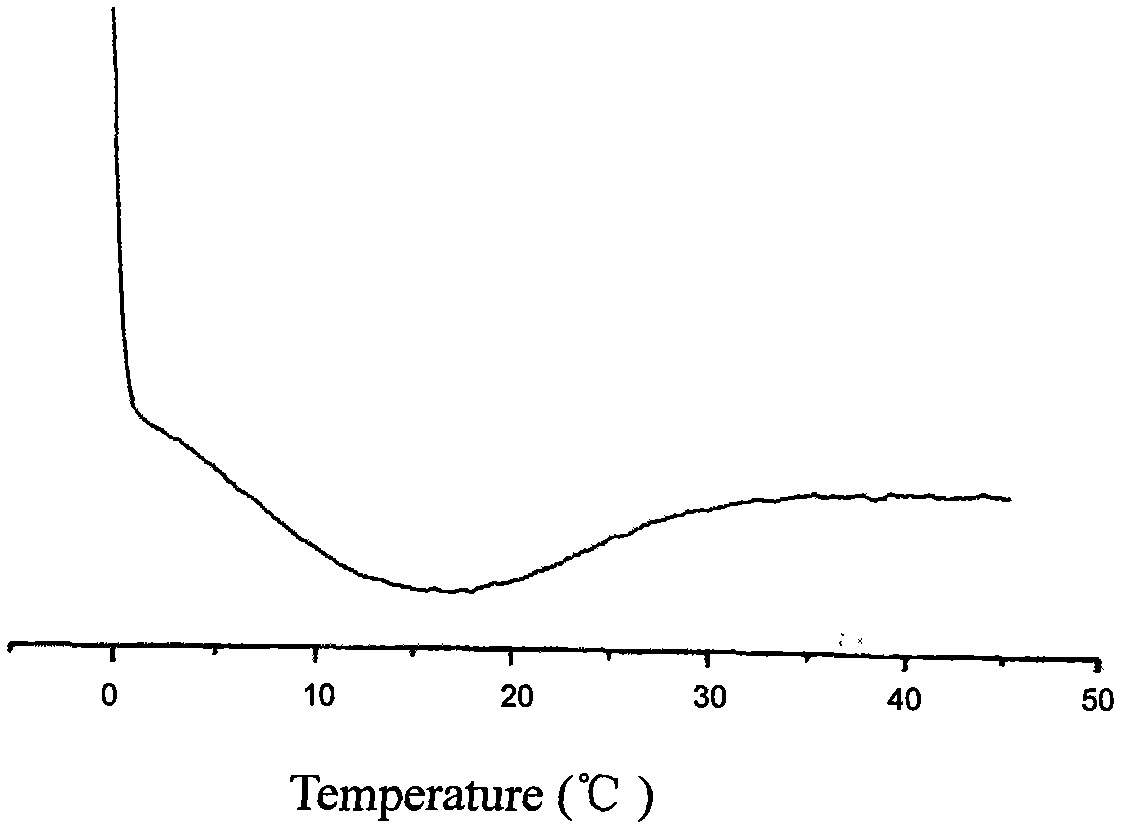
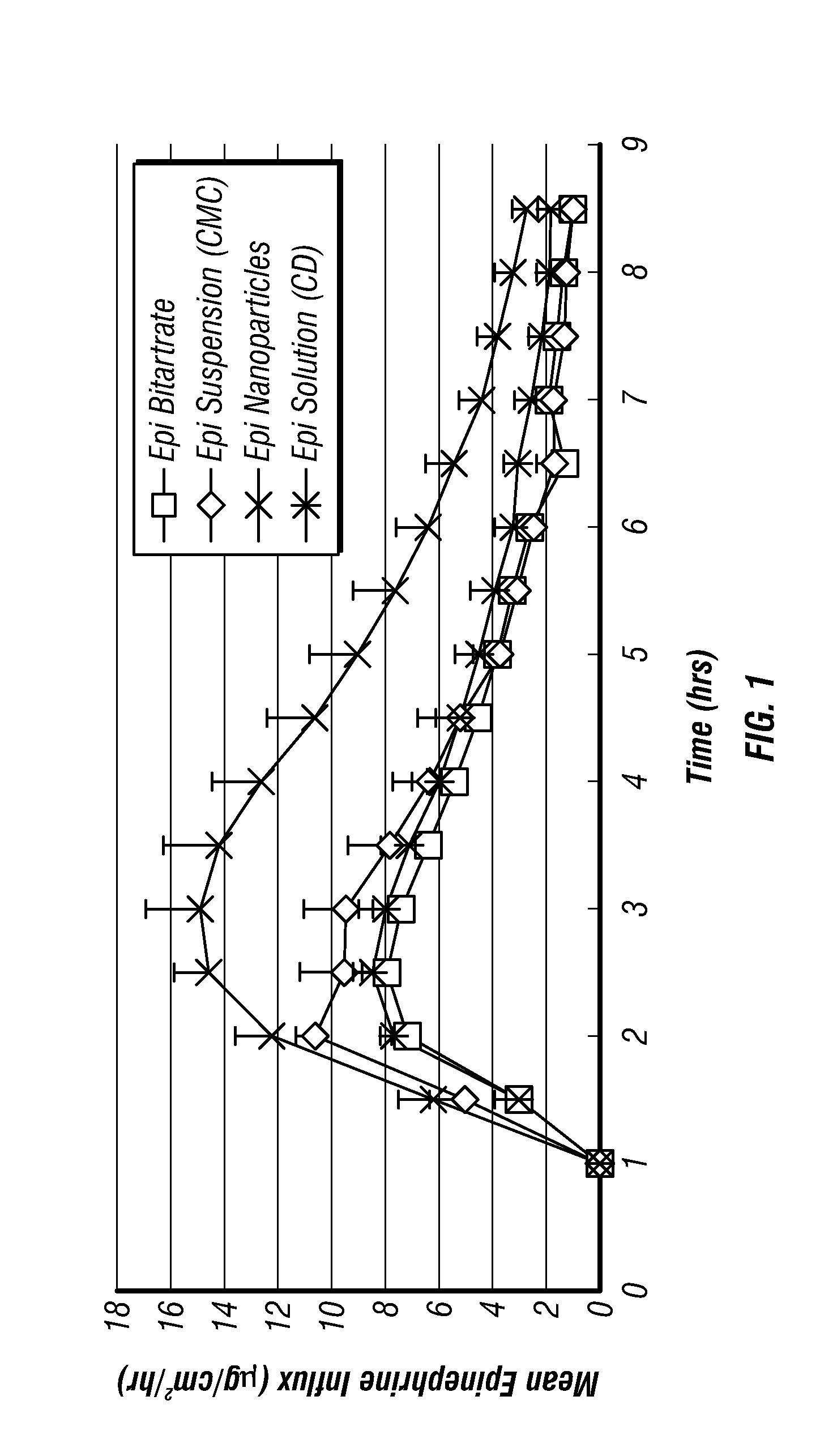
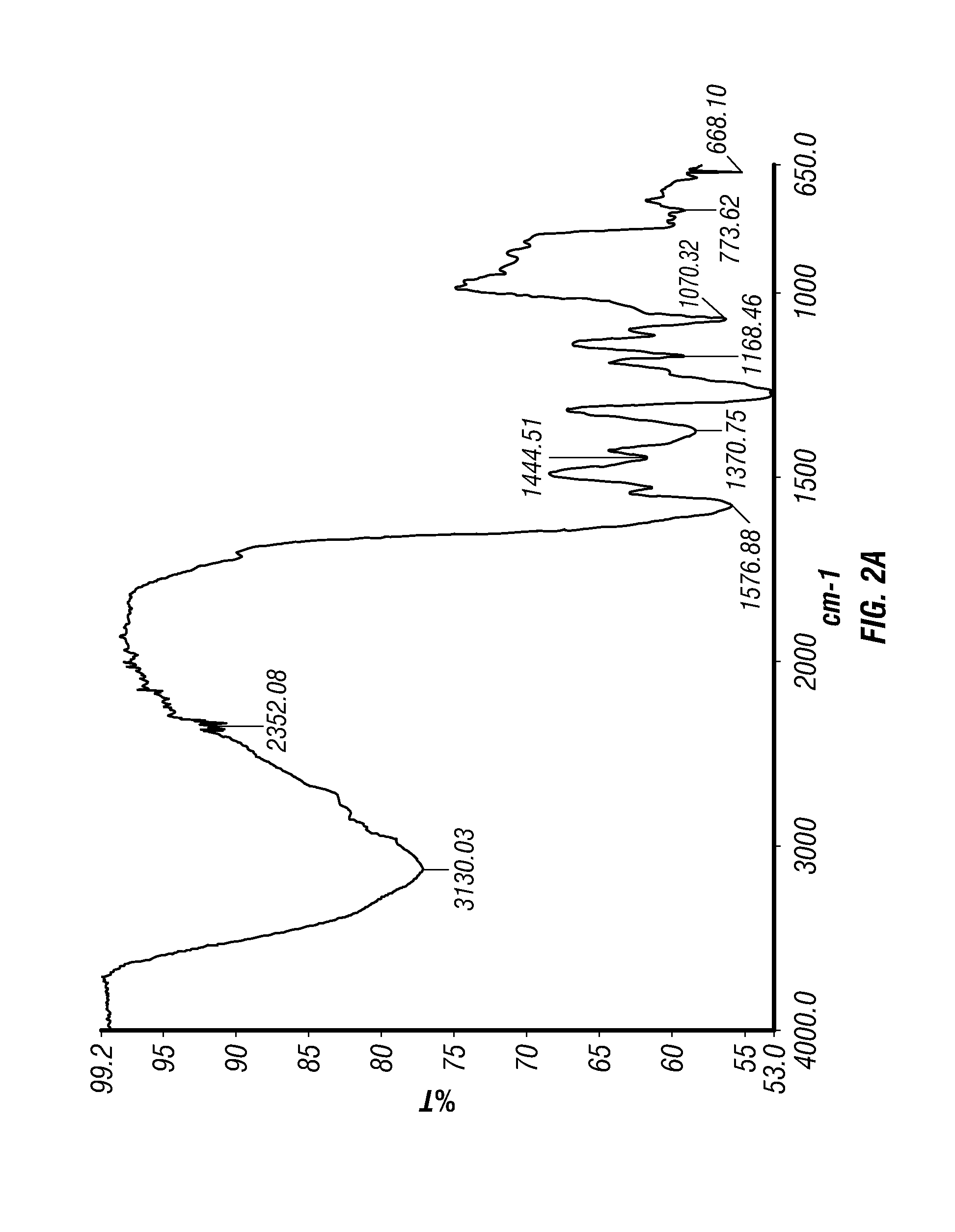
Epinephrine nanoparticles, methods of fabrication thereof, and methods for use thereof for treatment of conditions responsive to epinephrine
InactiveUS20120322884A1Enhancing sublingual bioavailabilityImprove bioavailabilityBiocideOrganic active ingredientsNanoparticleBioavailability
The invention provides a composition including epinephrine nanoparticles and methods for therapeutic use of the composition in the treatment of conditions responsive to epinephrine such as a cardiac event or an allergic reaction, particularly anaphylaxis. The epinephrine nanoparticles can be incorporated into orally-disintegrating and fast-disintegrating tablet pharmaceutical formulations and can significantly increase the sublingual bioavailability of epinephrine, and thereby reduce the epinephrine dose required. Additionally, the invention provides methods for fabrication of stabilized epinephrine nanoparticles for use in the described compositions.
Owner:NOVA SOUTHEASTERN UNIVERSITY
Natural pigment lipstick
InactiveCN105310907AIncrease contentImprove antioxidant capacityCosmetic preparationsMake-upHuman bodyWax
The invention discloses a natural pigment lipstick. The natural pigment lipstick is mainly prepared from, by weight, 3-7 parts of natural pigment, 15-20 parts of natural wax, 40-70 parts of natural oil, 2-5 parts of natural flavors and 1-3 parts of other natural functional additives. The lipstick uses natural plant extract juice as a pigment dye, other components do not contain any artificially synthesized compound, and the lipstick is non-toxic to a human body, can lubricate lip mucosa, does not produce allergic reaction, promotes blood circulation and body health and is wide in raw material source, simple and convenient to produce and low in cost.
Owner:GUANGZHOU JUZHU GENERAL TECH INST CO LTD
Recombinant human collagen and application thereof
InactiveCN110194795AHigh purityIncrease productionConnective tissue peptidesBacteriaBiotechnologyProtein target
The invention discloses a recombinant human collagen and an application thereof. An amino acid sequence of the protein is shown as SEQ ID NO. 3, the nucleotide sequence of the protein-coding gene is shown as SEQ ID NO. 1. The recombinant human collagen of the invention has very good hydrophilicity and stability, and the amino acid composition thereof is 100% identical to the corresponding amino acid sequence of the natural collagen, and the amount of expressed protein can account for about 25% of the total protein of the thalline, per milliliter of the bacteria liquid precipitate contains 0.25mg of the target protein, and the production cost is very low and the cycle is short. The prepared collagen is applied to the human body without immunological rejection and allergic reaction, and canbe widely applied to the biomedicine and cosmetics industries.
Owner:郭伟
Sunscreen cosmetic composition and method for preparing seaweed sunscreen components of composition
ActiveCN104644511AEnsure safetyEnsure effectivenessCosmetic preparationsToilet preparationsHaematococcus pluvialis extractIrritation
The invention discloses a sunscreen cosmetic composition and a method for preparing seaweed sunscreen components of the composition. The sunscreen cosmetic composition comprises the following components in percentage by weight based on the total weight of the composition: 0.01-10 percent of a porphyra umbilicalis extract, preferably 5 percent, 0.01-10 percent of a dunaliella salina extract, preferably 5 percent, 0.0001-1 percent of a haematococcus pluvialis extract, preferably 0.001 percent, 0.01-10 percent of seawater pearl powder, preferably 1 percent, and 0.01-10 percent of codium tomentosum extract, preferably 10 percent. According to the composition disclosed by the invention, the irritation and allergic reactions of organic sun-screening agents brought to the human body can be effectively reduced, the injured skin can be repaired during sun protection, and water is replenished to the skin. According to the preparation method disclosed by the invention, a wetting agent propylene glycol for cosmetics serves as an extraction solvent, the porphyra umbilicalis extract, dunaliella salina extract and haematococcus pluvialis extract are obtained under specific preparation conditions, and hazards of organic solvents for conventional extraction are avoided.
Owner:GUANGZHOU KENENG COSMETICS RES CO LTD +1
Prinsepia utilis Royle oil-containing functional skin care product and preparation method thereof
ActiveCN102406576AMild textureImprove securityCosmetic preparationsToilet preparationsAllergic dermatitisDisease
The invention discloses a Prinsepia utilis Royle oil-containing functional skin care product and a preparation method thereof. The Prinsepia utilis Royle oil-containing functional skin care product is prepared from the following raw materials in part by weight: 1 to 2 parts of Prinsepia utilis Royle oil, 10 to 25 parts of purslane extracting solution, 5 parts of glycerol, 2 to 3 parts of 1,2-pentanediol, 5 to 7 parts of caprylic / caprictriglycerides, 2 parts of synthetic squalane, 1 to 5 parts of tocopheryl acetate, 1 to 3 parts of cetearyl alcohol, 0.1 to 0.2 part of allantoin, 0.6 to 0.9 part of coconut oil-base glucoside, 0.5 to 1 part of coconut oil alcohol, 0.5 to 2 parts of glyceryl stearate, 0.1 to 1 part of sodium hyaluronate and the balance of water. The functional skin care product has mild property and high safety without toxic or side reaction and anaphylactic reaction, can repair the destructed skin barrier, resists inflammation and skin ageing, and has a good effect and high safety of assisting in treating skin diseases related with skin barrier destruction, such as facial dermatitis (hormone dependence dermatitis, allergic dermatitis and acne), atopic dermatitis and the like.
Owner:YUNNAN BOTANEE BIO TECH GRP CO LTD
Methods and assays for detecting and quantifying pure subpopulations of white blood cells in immune system disorders
ActiveUS20100112628A1Determining susceptibility to an allergic reactionBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseWhite blood cell
Methods for detecting nonactivated basophils in a whole blood sample obtained from a normal healthy subject, methods for determining a subject's susceptibility to an allergic reaction to an allergen, where the subject has no known allergy to the allergen, methods for measuring a response to challenge with a potential allergen in a whole blood sample obtained from a subject with known allergic reactivity to allergens other than the potential allergen; and an in vitro system for reliable detection or quantification of a specific white blood cell population in a whole blood sample are described.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Systems and methods for a consumer to determine food/medicine interactions
InactiveUS20080263011A1Reduce errorsEasily check compatibilityDigital data processing detailsDrug and medicationsDiseaseAdditive ingredient
One embodiment of the present invention relates to systems and methods for detecting harmful and / or hazardous ingredients that may cause an allergic reaction, interfere with the effectiveness of a prescription drug, exacerbate symptoms associated with a chronic illness, and / or cause another undesired reaction.
Owner:BADINELLI ELLEN
Nano microcapsule type dry garment piece, and preparation method and application thereof
The invention relates to a fragrant microcapsule type finishing agent which comprises the following constituents by mass percentage: 35-45 percent of fragrant nano microcapsule, 35-45 percent of thickening agent, 15-25 percent of adhesive and 1-5 percent of color paste, wherein the sum of the percentages of all the constituents is 100 percent. The invention also discloses a nano microcapsule type dry garment piece which has lasting fragrance more than 1 year, is sterile, has high safety, has no stimulation to the skin and no allergic response, and is suitable for fragrance finishing and storing of fabrics and ready-to-wear clothing made of cotton, wool, silk, linen and chemical fiber; and the fragrant nano microcapsule has good particle dispersivity on base cloth and is extremely easy to embed into the base cloth tissue structure.
Owner:无锡翔隆高分子面料有限公司
Method of preparing Chinese medicine for treating children's bronchopneumonia
InactiveCN101244161ASmall side effectsNot easy to reboundRespiratory disorderPlant ingredientsPenicillinSide effect
The invention relates to a herbal medicine preparation method for the children bronchopneumonia treatment, belonging to the technical field of Chinese herbal medicine preparation methods; which adopts the technical proposal that: herbs like ginkgo seed, ephedra, apricot kernel, perilla, pinellia ternate, Swallowwort Rhizome (SRh), aster, Tussilago farfara, cortex mori, Chinese wolfberry root-bark, scutelarria, blackberry lily, dyers woad leaf, isatis root, bupleurum, dandelion, honeysuckle, houttuynia cordata, sargentgloryvine stem, sophora tonkinensis, APN (Andrographis Paniculta), Franchet Groundcherry Fruit, balloonflower and liquorice are immersed in 1000ml water for half an hour, and then decocted on mild fire; after decoctation, the herbal liquor is filtered to get 300ml herbal medicine for the treatment of children bronchopneumonia. In prior art, penicillin, streptomycin, and gentamicin are used for the treatment of children bronchopneumonia; the application of penicillin can cause allergic reaction, as a result, the life of the patient may be in danger if rescue is not timely. The preparation method has the advantages of simple manufacturing technology, small side effect and durable and stable therapeutic effect.
Owner:郭新美
Methods of treating diseases which are mediated by cutaneous lymphocyte antigen positive cells
ActiveUS8388964B2Improves and prevents and inhibits and reduces skinImproves and prevents and inhibits and reduces pruritisCompounds screening/testingLuminescence/biological staining preparationDiseaseContact dermatitis
The present invention relates to methods of treating patients suffering from itching and puritis mediated by cutaneous lymphocyte antigen positive T cell. In particular, diseases or disorders including contact dermatitis, drug induced delayed type cutaneous allergic reactions, toxic epidermal necrolysis, cutaneous T cell lymphoma, bullous pemphigoid, alopecia aereata, vitiligo, acne rosacea, prurigo nodularis, and herpes simplex virus, or combination thereof will benefit from the administration of an IL-31 antagonist. The invention also includes methods of predicting a therapeutically responsive patient population.
Owner:ZYMOGENETICS INC +1
Casein and methods of use thereof
InactiveUS20100223682A1Reduce allergiesSugar derivativesPeptide/protein ingredientsHypersensitive responseWild type
This invention provides an altered kosher kappa casein polypeptide having at least one altered amino acid in a defined amino acid stretch. The defined amino acid stretch contributes to an allergic reaction induced by a kosher wild-type casein polypeptide in a human subject. Moreover, the defined amino acid stretch is expressed solely in kosher animals. The invention further provides an expression system for expressing the altered kosher casein polypeptide.
Owner:KATZ YITZHAK +1
Immunomodulatory compounds and methods of use thereof
The present invention is directed to methods of treating diseases and disorders related to immune responses by administering one or more immunomodulatory compounds. In particular, the invention is directed to methods of stimulating and reducing immune responses, treating autoimmune conditions, treating allergic reactions and asthma, and preventing ischemic damage and asthma by administering one or more immunomodulatory compounds.
Owner:EISIA R&D MANAGEMENT CO LTD
Directed Cell-Based Therapy Using Microbubble Tagged Cells
ActiveUS20110208113A1Promote tissue growthImprove satisfactionBiocideUltrasound therapyAuditory radiationProgenitor
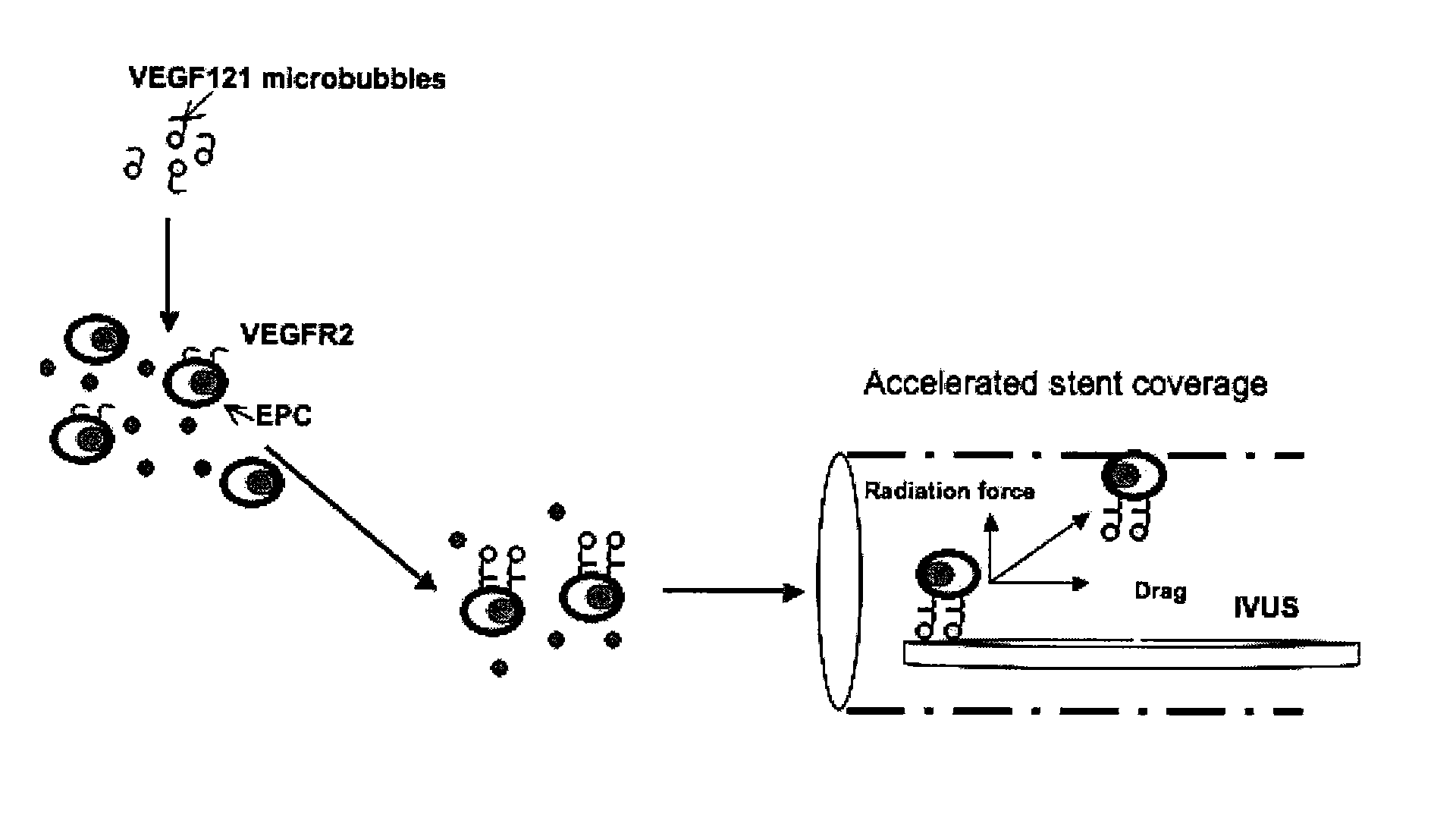
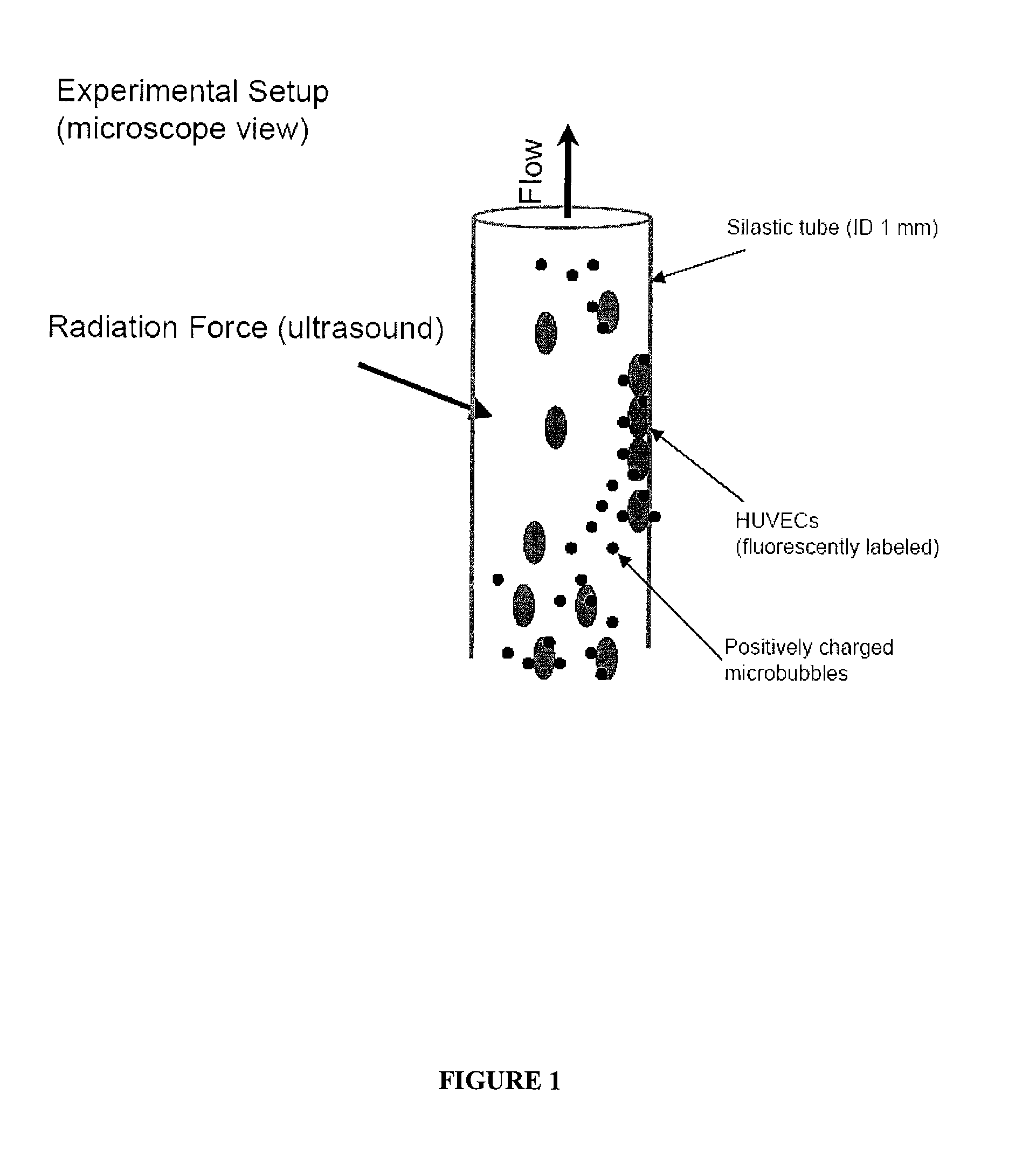
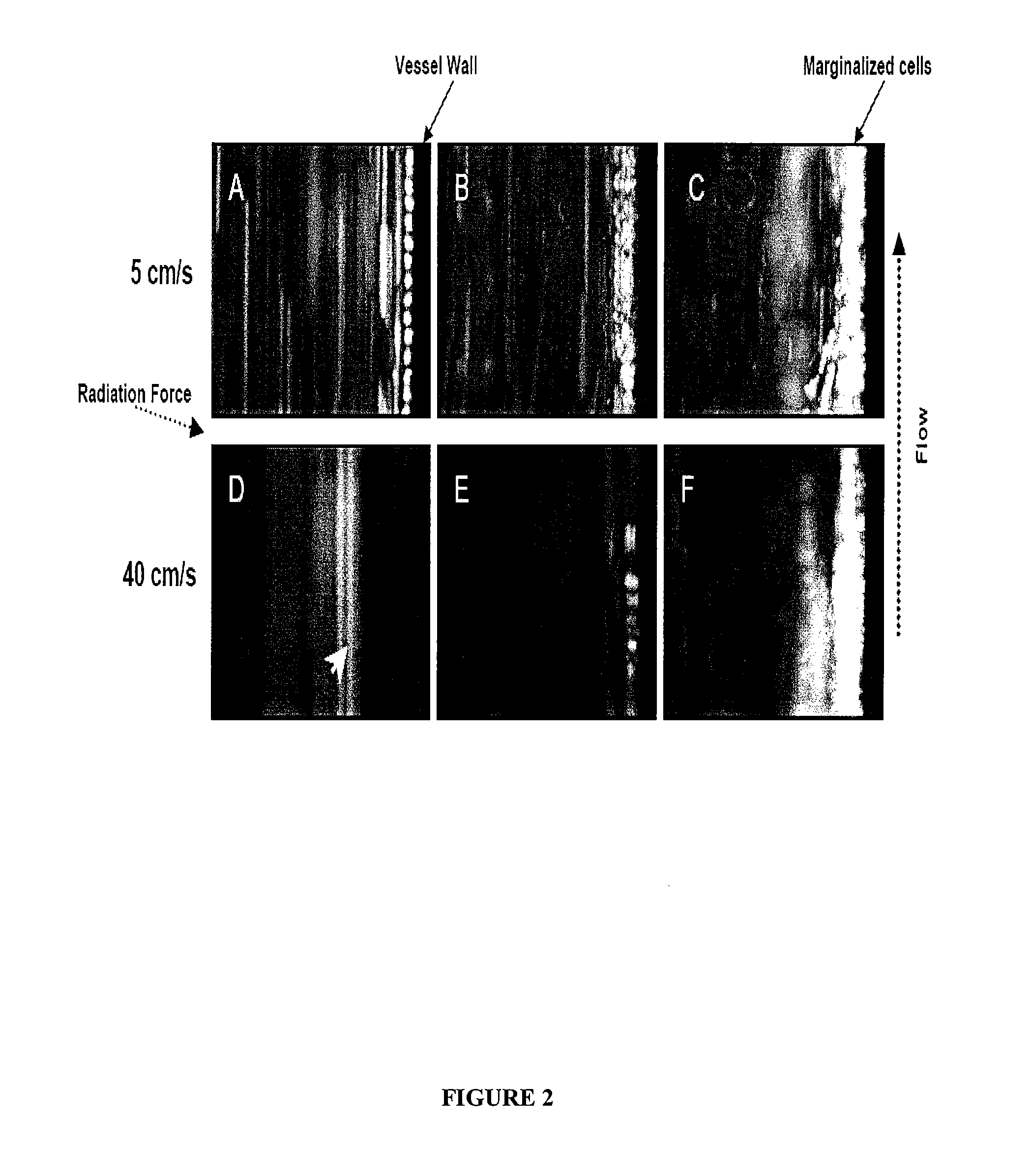
The disclosed technology describes compositions and methods useful for providing cell based therapy. For example, one embodiment of cell based therapy involves the regeneration of injured tissue and / or promoting wound healing. Certain embodiments provide improved therapeutic compositions using microbubbles by delivering biological progenitor cells to the injured tissues. The administration of the microbubbles is directed by acoustic radiation forces that interact with embodiments of microbubbles comprising an acoustically active gas. As such, a high efficiency of progenitor cell delivery to injured tissue is realized. One advantage of this technique over targeted delivery of pharmaceutical compounds, is that the delivered progenitors cells may be derived from the patient (i.e., personalized therapy), thereby avoiding side effects, allergic reactions, and overall problems associated with refractive drug responses.
Owner:UNIVERSITY OF PITTSBURGH
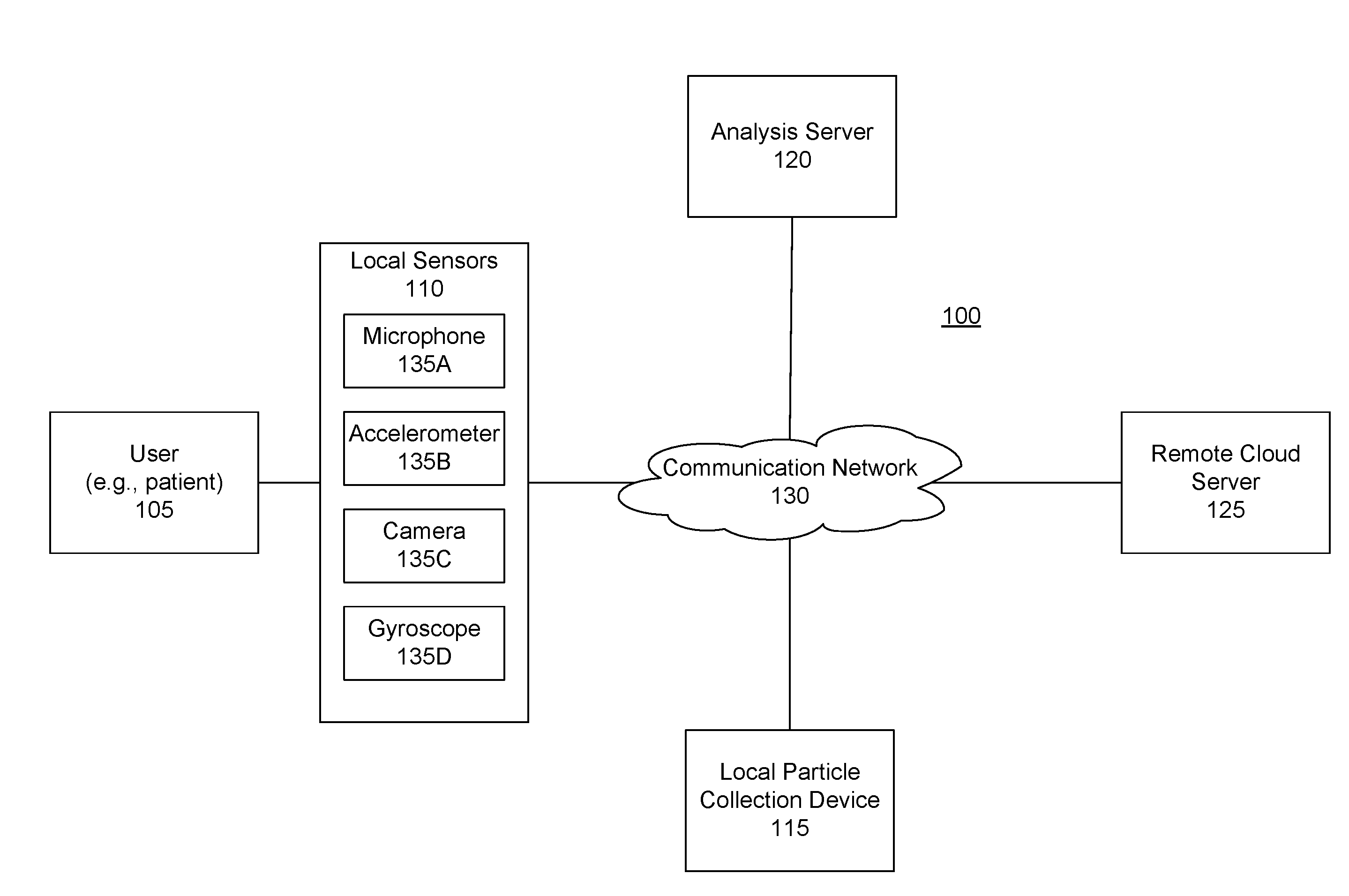
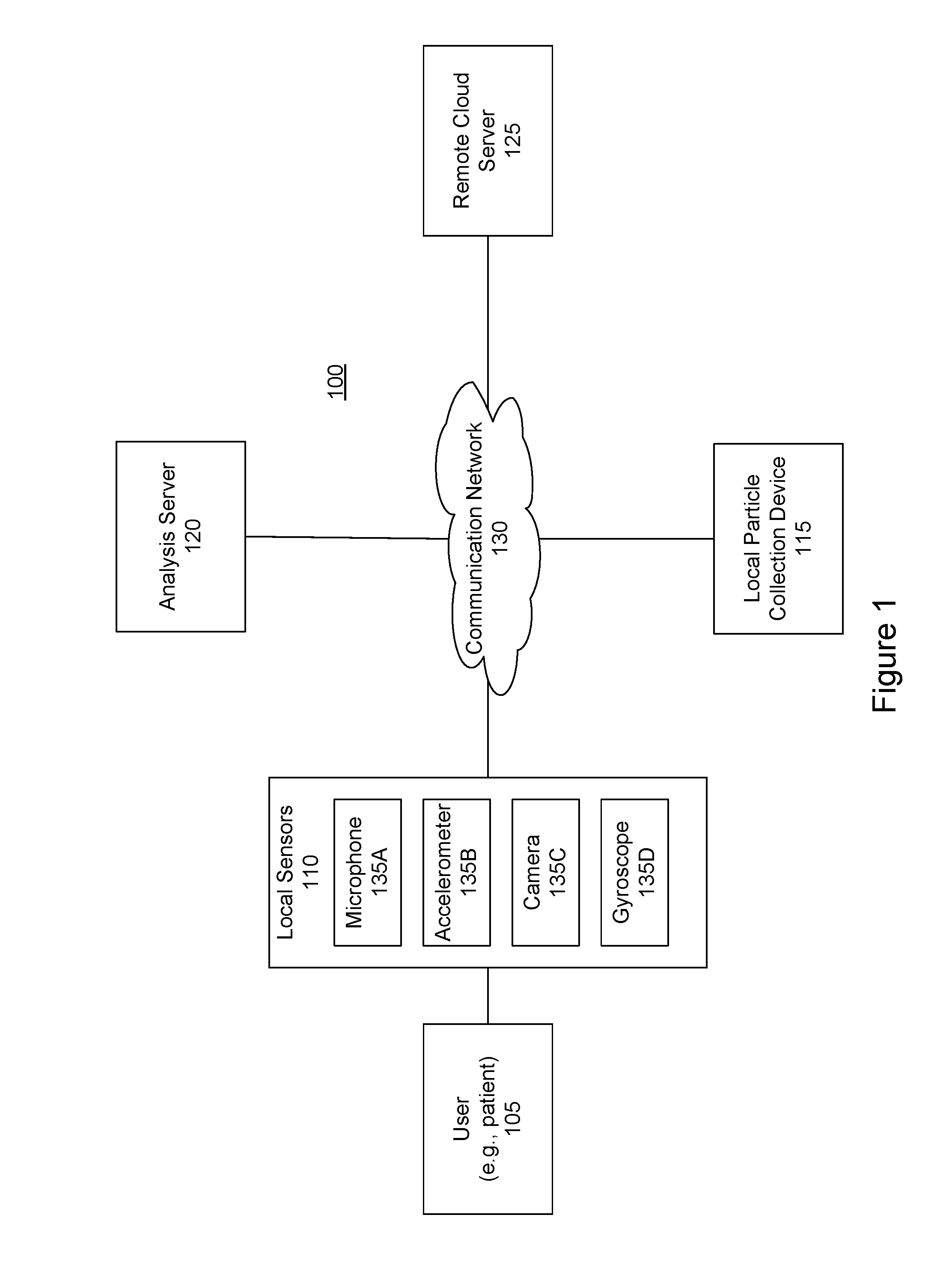
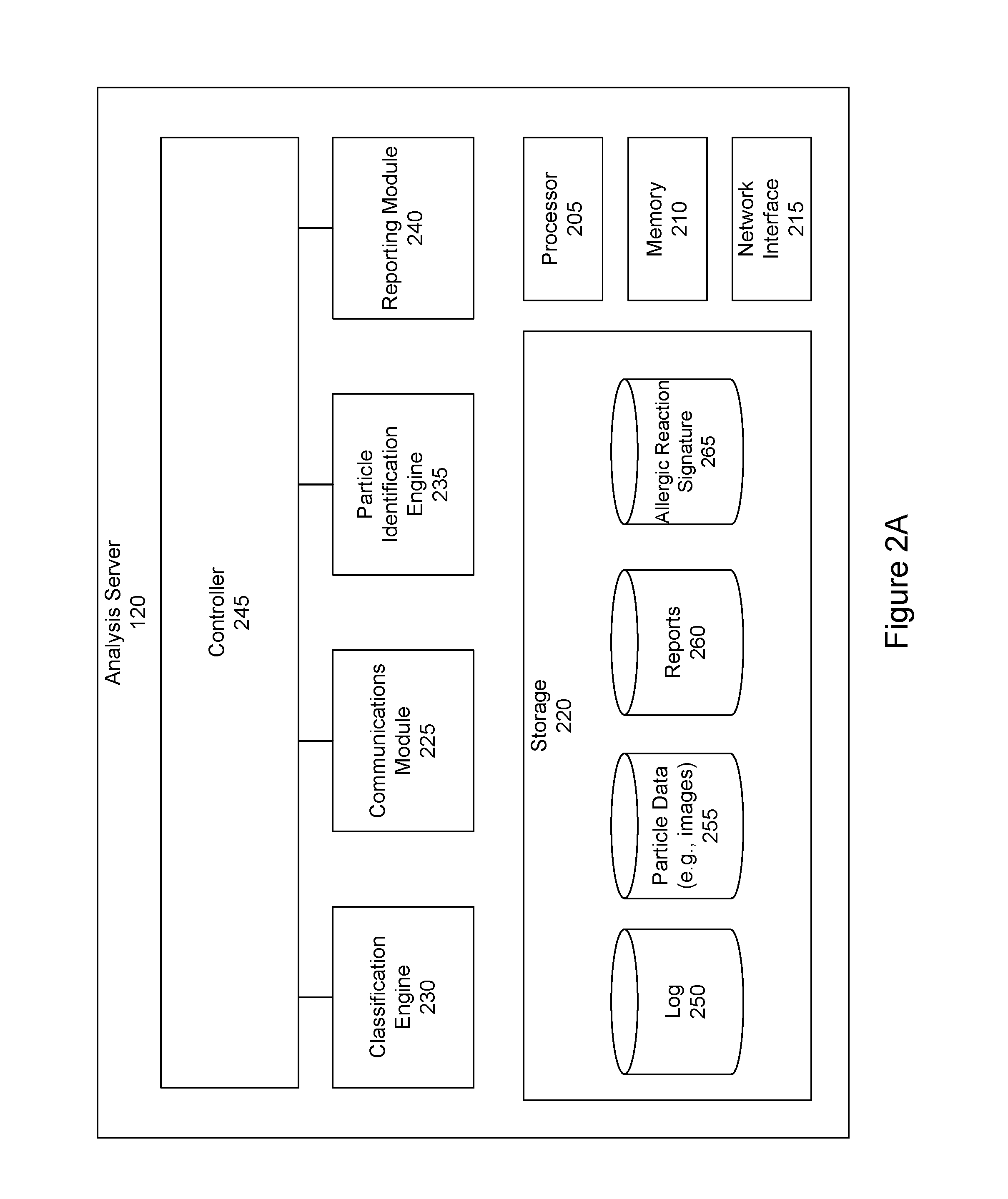
Pollen sampling and retrieval triggered by a user's allergic reactions
Information from a sensor that is local to a user is received. The information may indicate that the user has experienced a physiological event. The information is analyzed to determine whether the physiological event should be classified as an allergic reaction. If the physiological event should be classified as an allergic reaction, an action is taken such as particles currently present in the environment local to the user are collected.
Owner:SCANIT TECH INC
Supression of allergic reactions by transdermal administration of allergens conjugated to cholera toxin or fragments thereof
InactiveUS20050074462A1Suppress allergic reactionsBacterial antigen ingredientsPeptide/protein ingredientsAdjuvantCo administration
The present invention discloses the use of the non-toxic cell-binding B subunit of CT (CTB), and holotoxin CT that is devoid of ADP-ribosylating activity, as adjuvants for enhancing transcutaneous immune response to a co-administered protein allergen. It was found that topical administration of CTB to mice induced serum antibody response against itself comparable to those evoked by CT, but was inefficient at promoting systemic antibody responses against an admixed prototype protein allergen. To the contrary co-administration of either CT or CTB with allergen led to vigorous antigen-specific T cell proliferative responses in lymph nodes draining the cutaneous site of administration and at distant systemic sites. Consistent with these observations, it was found that CTB selectively potentiated Th1-driven responses without affecting Th2-dependent responses. Cutaneously applied CT enhanced serum IgE responses to a co-administered allergen, while CTB partially suppressed epicutaneously induced IgE responses to the same allergen.
Owner:DUOTOL
Acne removing and antiallergic cream and preparation method thereof
InactiveCN103565714ANo drug resistanceAnti-acidificationCosmetic preparationsToilet preparationsPolyethylene glycolGlycerol
The invention discloses an acne removing and antiallergic cream which is prepared by mixing stearyl alcohol polyether-2, stearyl alcohol polyether-20, glycerin stearate, PEG (Polyethylene Glycol)-100 stearate, stearic acid, cetearyl alcohol, aloe oil, polydimethyl siloxane, isopropyl myristate, propyl p-hydroxybenzoate, methyl p-hydroxybenzene, tocopherol, azone, bisabolol, glycerol, xanthan gum, EDTA (Ethylene Diamine Tetraacetic Acid)-2Na, propylene glycol, ethanol, menthol, salicylic acid, a golden cypress extractive, a scutellaria baicalensis extractive, an India chinaberry leaf extractive, a spiraea ulmaria extractive, a bee milk extractive, an aloe leaf extractive, tea tree oil and sodium hydroxide. The acne removing and antiallergic cream disclosed by the invention can be used for removing acnes, promoting the facial blood circulation and skin cell regeneration, improving the skin tolerance and reducing or eliminating the allergic reaction of the skin; the allergic tolerance of the skin is improved, so that the cream is safe and effective; no any spices are added, so that allergens are greatly reduced.
Owner:SHENZHEN GUAFEN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com