Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Allergic response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An allergic response is a hypersensitive harmless or would not cause an immune response in everyone. An allergic response may cause harmful symptoms such as itching or inflammation or tissue injury.
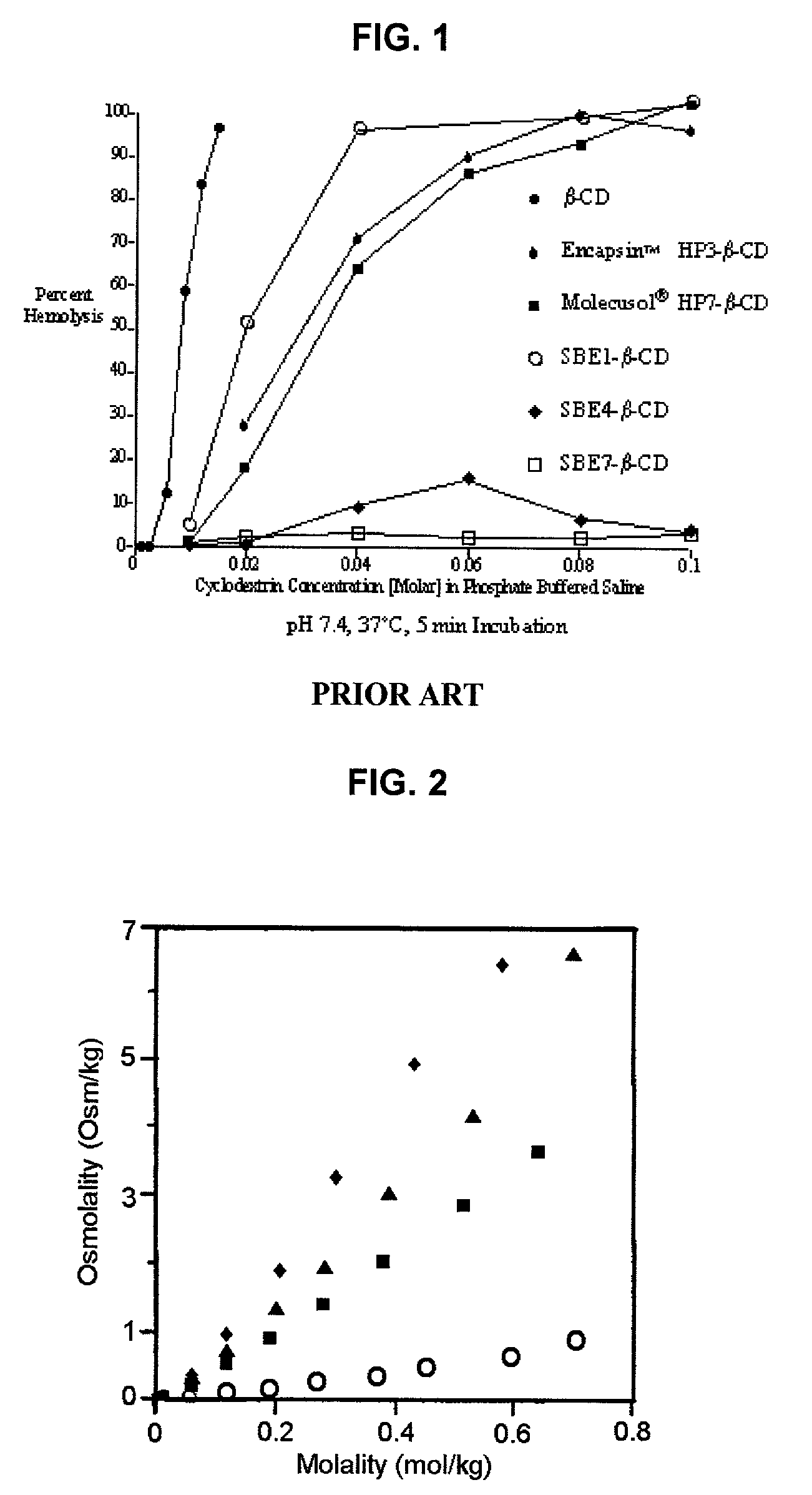
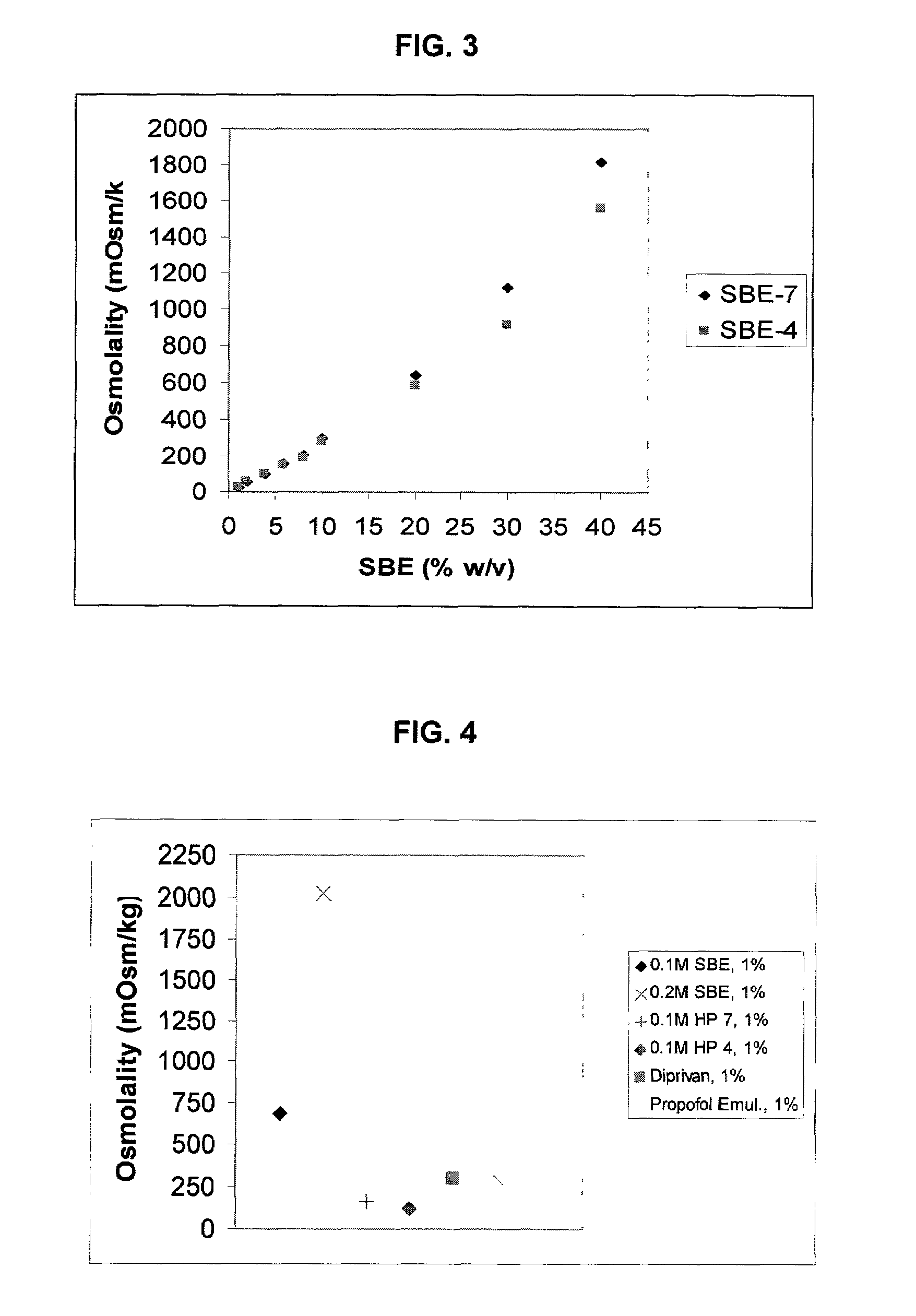
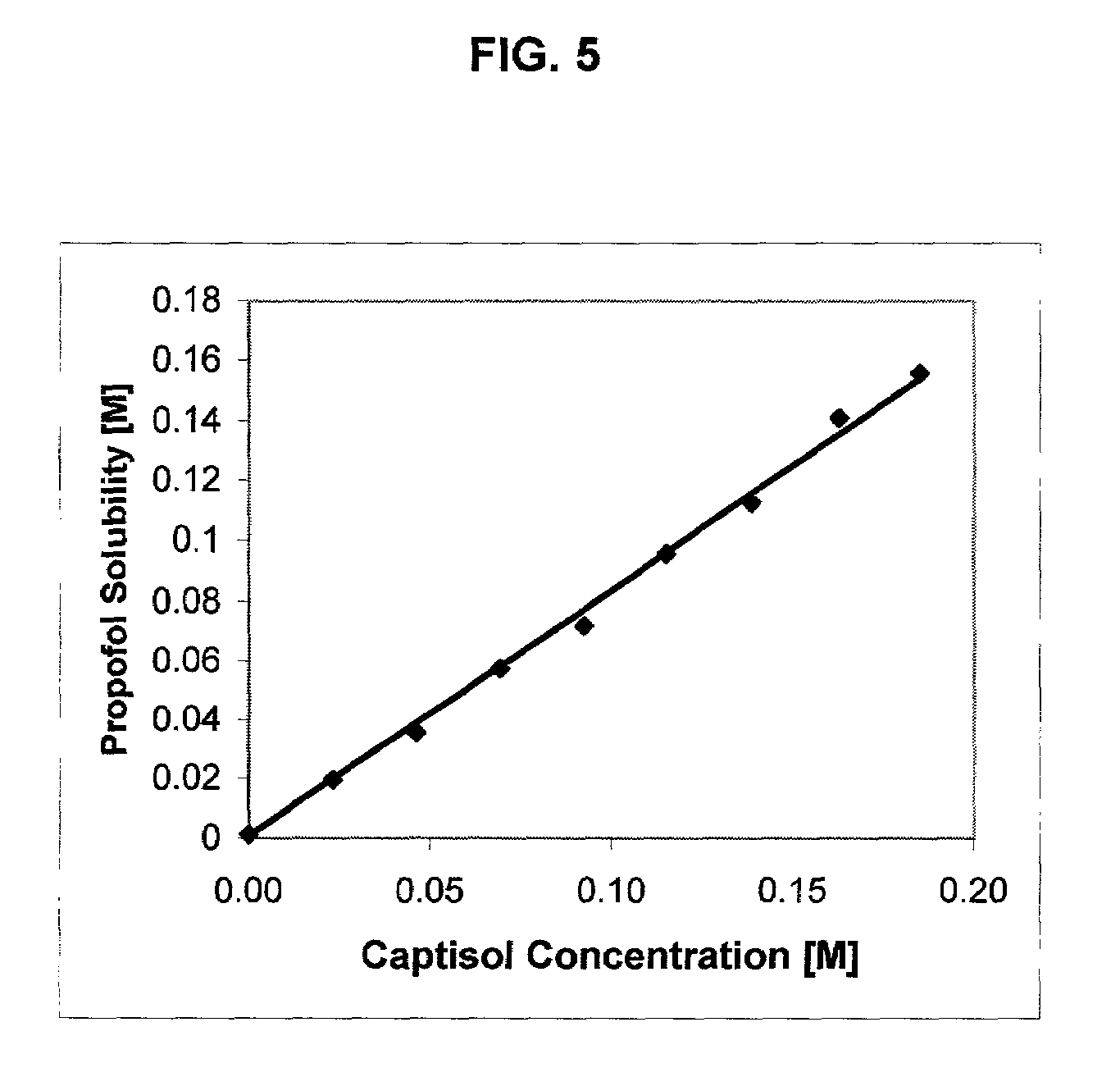
Formulations containing propofol and a sulfoalkyl ether cyclodextrin
InactiveUS7034013B2Reduce bacterial contaminationHigh photochemical stabilityBiocideHydroxy compound active ingredientsEmulsionAllergic response
An injectable formulation of a sedative hypnotic drug, such as the anesthetic drug propofol, that is pharmaceutically stable and demonstrates a reduced incidence of pain upon injection. The formulation of the present invention employs a sulfoalkyl ether cyclodextrin solubilizing and complexing excipient, such as CAPTISOL® cyclodextrin (sulfobutyl ether β-cyclodextrin) to form a true aqueous solution and not a suspension. This formulation minimizes the allergic response and microbial contamination issues typically associated with propofol parenteral formulations. The present formulation may also reduce pain on injection as compared to the known emulsion type propofol formulations. The liquid formulation can be sterile filtered unlike emulsion-type formulations of sedative hypnotics. The liquid formulation can be lyophilized or otherwise dried to yield a solid formulation.
Owner:CUDA PHARMA LLC
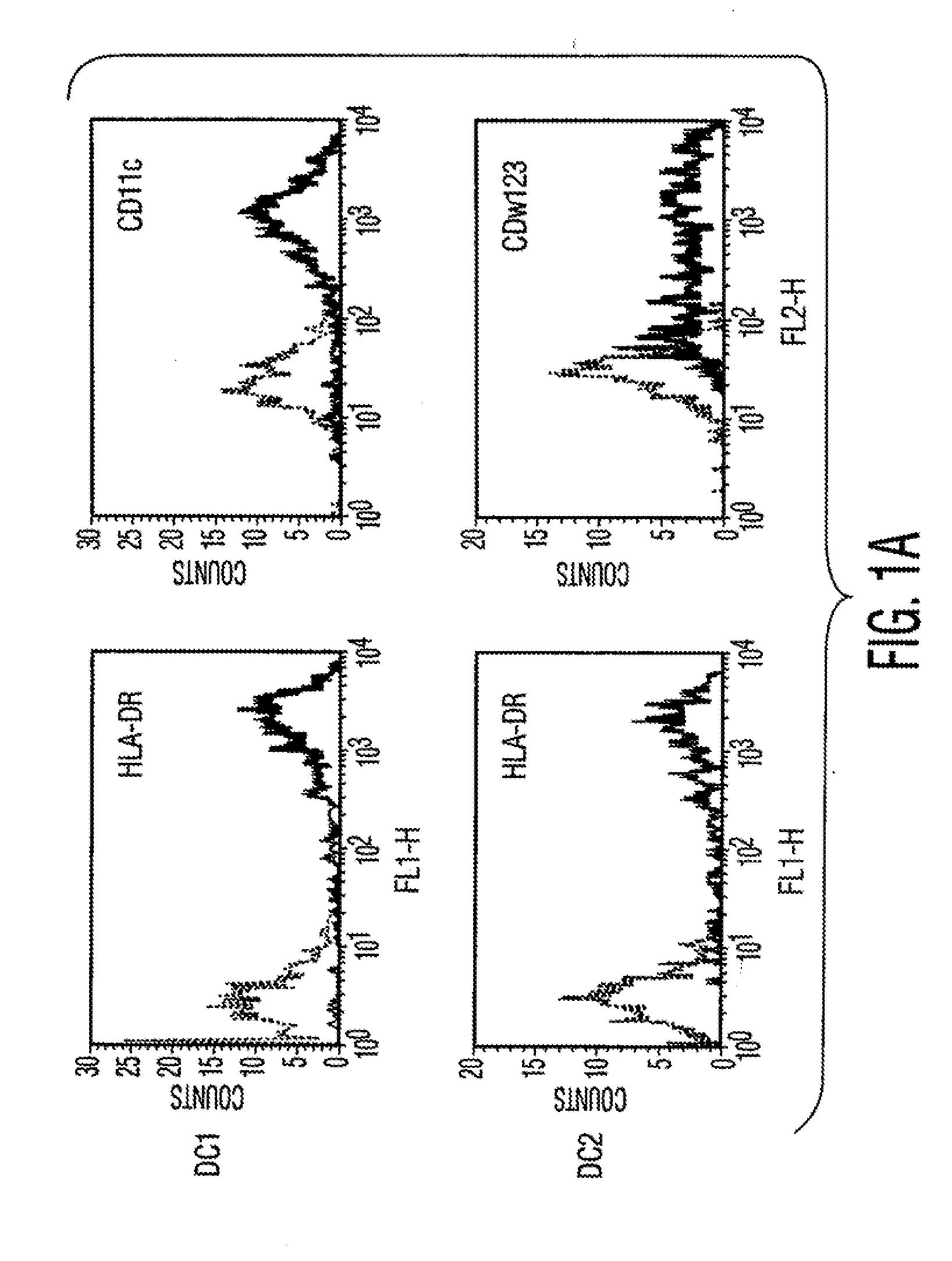
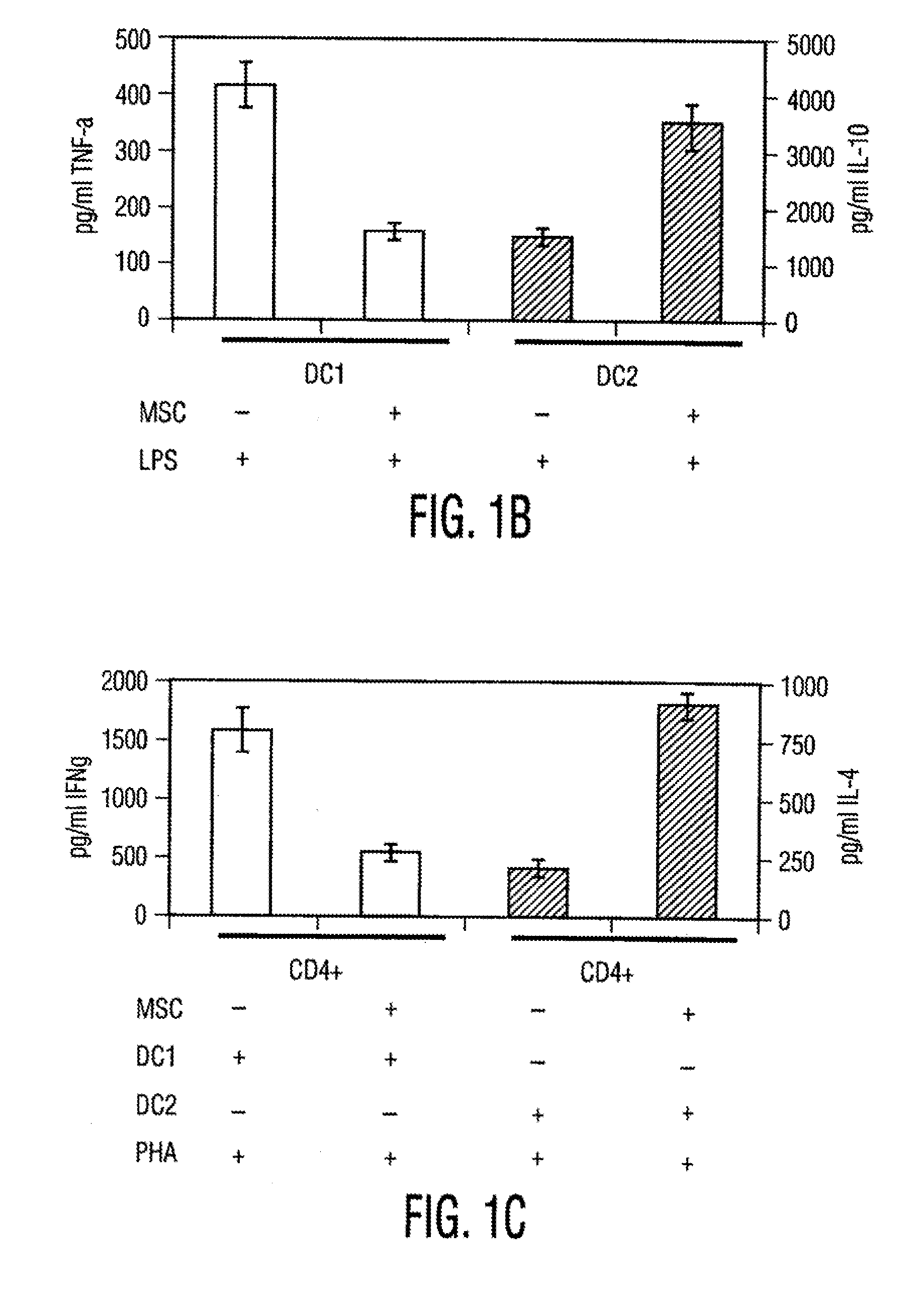
Multipotent Adult Stem Cells And Uses of Multipotent Adult Stem Cells To Treat Inflammation
InactiveUS20100172885A1Effective treatmentIncrease the number ofBiocideBone marrow stroma cellsDiseaseAutoimmune responses
Disclosed are cell preparations comprising multipotent adult stem cells and methods for using multipotent adult stem cells to treat autoimmune diseases, treat allergic responses, treat cancer, treat inflammatory diseases, treat fibrotic disorders, reduce inflammation and / or fibrosis, promote would healing, repair epithelial damage, and / or promote angiogenesis.
Owner:MESOBLAST INT
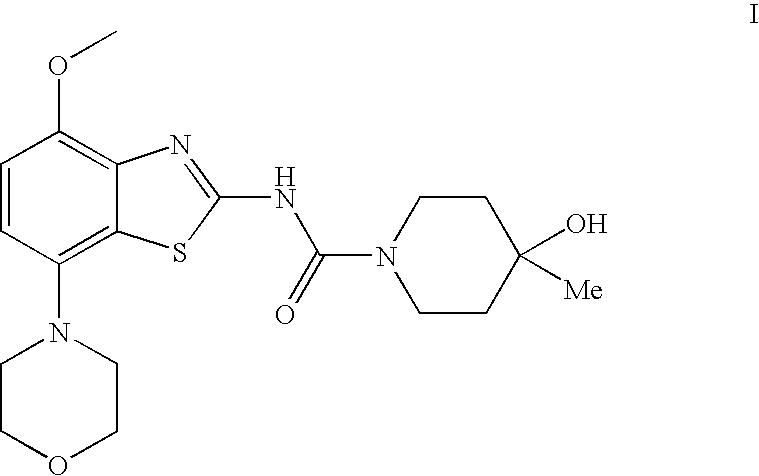
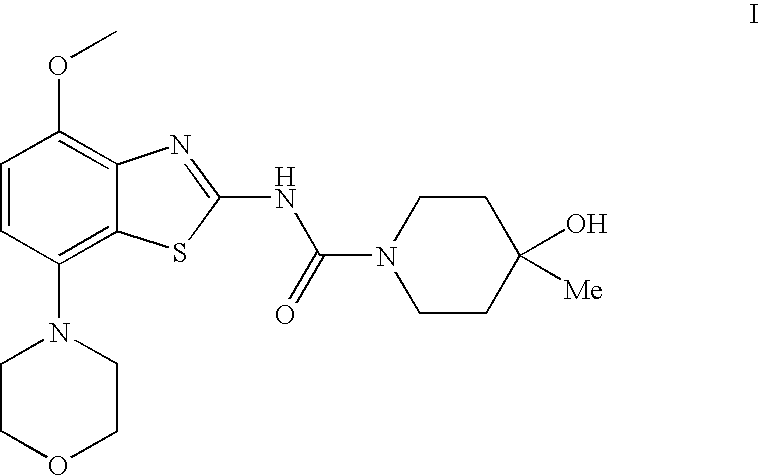
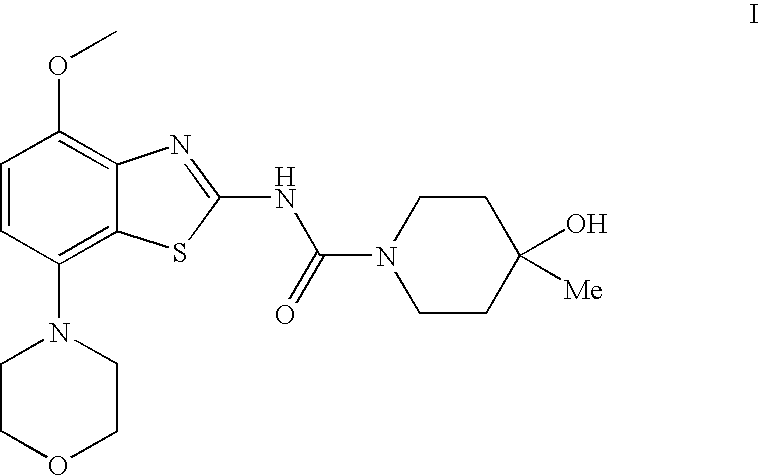
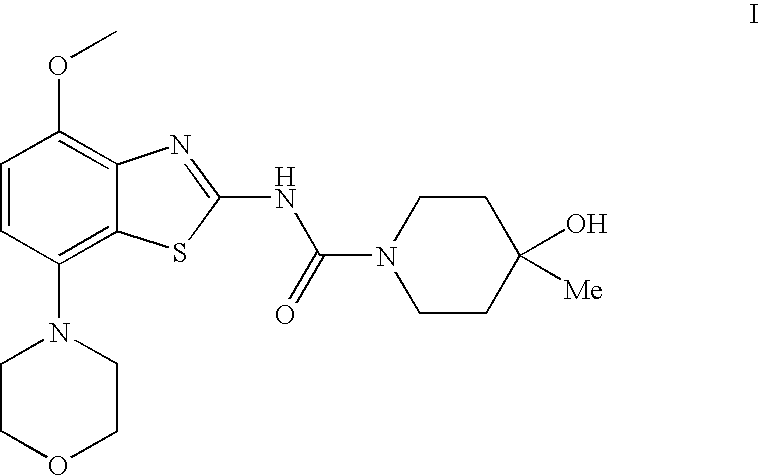
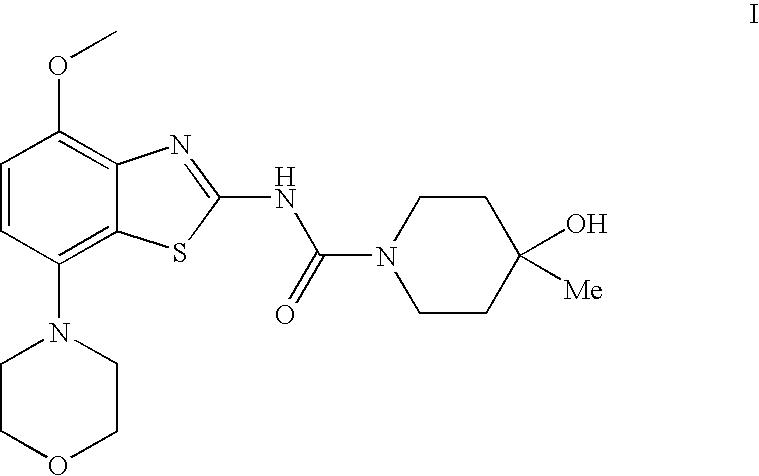
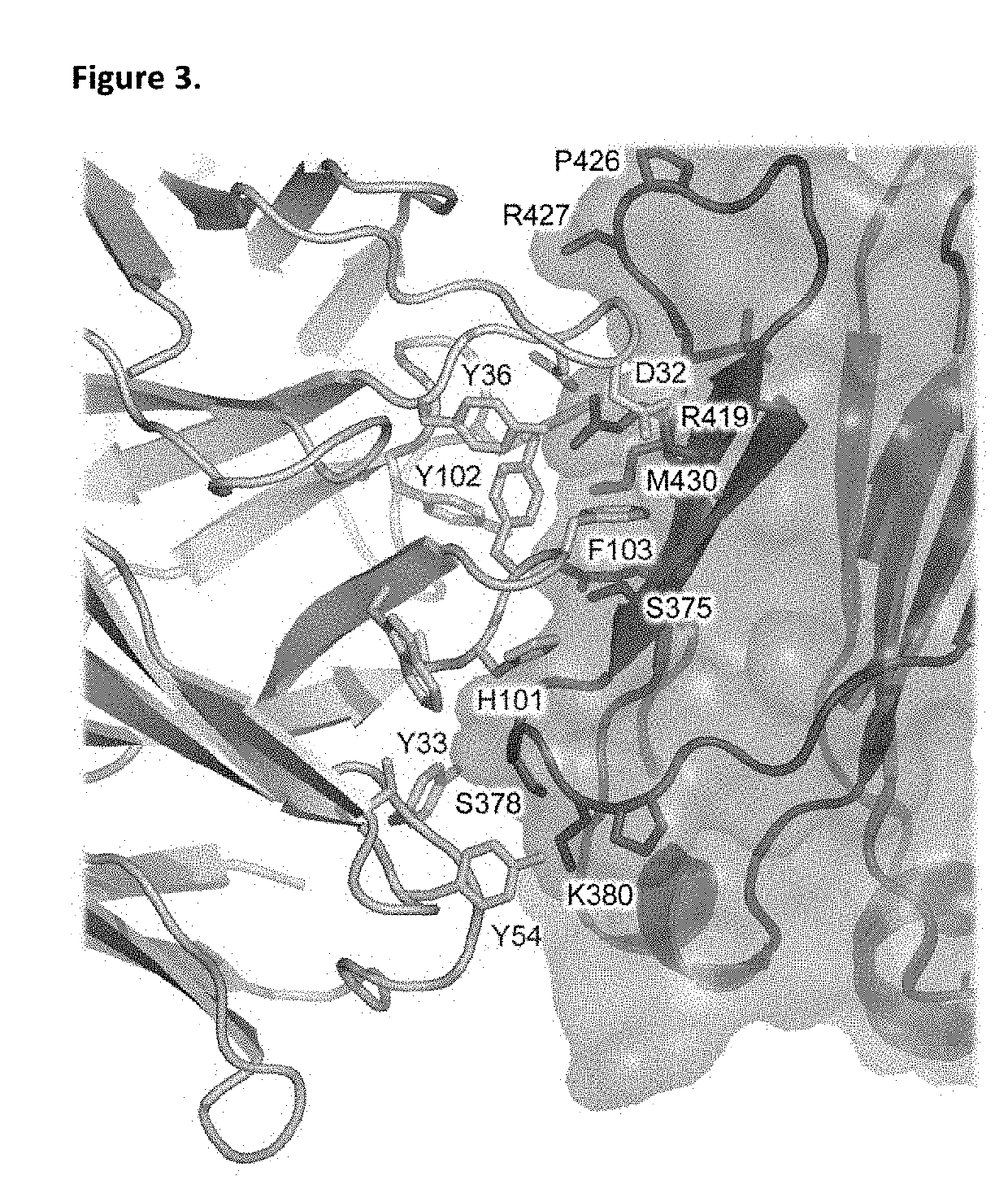
4-Hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide
ActiveUS20050261289A1High affinityOrganic active ingredientsNervous disorderSubstance abuserDisease cause
The present invention relates to the compound of formula which is 4-hydroxy-4-methyl-piperidine-1-carboxylic acid(4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide, and to pharmaceutically acceptable acid addition salts thereof. It has been found that the compound is useful for the treatment or prevention of Alzheimer's disease, Parkinson's disease, Huntington's disease, neuroprotection, schizophrenia, anxiety, pain, respiration deficits, depression, ADHD (attention deficit hyper-activity disorder), drug addiction to amphetamines, cocaine, opioids, ethanol, nicotine, or cannabinoids, or for the treatment of asthma, allergic responses, hypoxia, ischemia, seizure, substance abuse, or for use as muscle relaxants, antipsychotics, antiepileptics, anticonvulsants and cardioprotective agents.
Owner:F HOFFMANN LA ROCHE INC
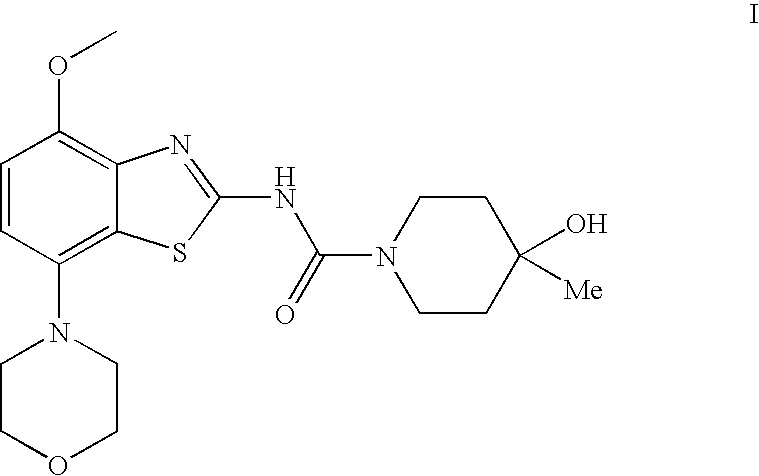
4-hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide
The present invention relates to the compound of formulawhich is 4-hydroxy-4-methyl-piperidine-1-carboxylic acid(4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide, and to pharmaceutically acceptable acid addition salts thereof. It has been found that the compound is useful for the treatment or prevention of Alzheimer's disease, Parkinson's disease, Huntington's disease, neuroprotection, schizophrenia, anxiety, pain, respiration deficits, depression, ADHD (attention deficit hyper-activity disorder), drug addiction to amphetamines, cocaine, opioids, ethanol, nicotine, or cannabinoids, or for the treatment of asthma, allergic responses, hypoxia, ischemia, seizure, substance abuse, or for use as muscle relaxants, antipsychotics, antiepileptics, anticonvulsants and cardioprotective agents.
Owner:F HOFFMANN LA ROCHE INC
Composition and methods for treating allergic response
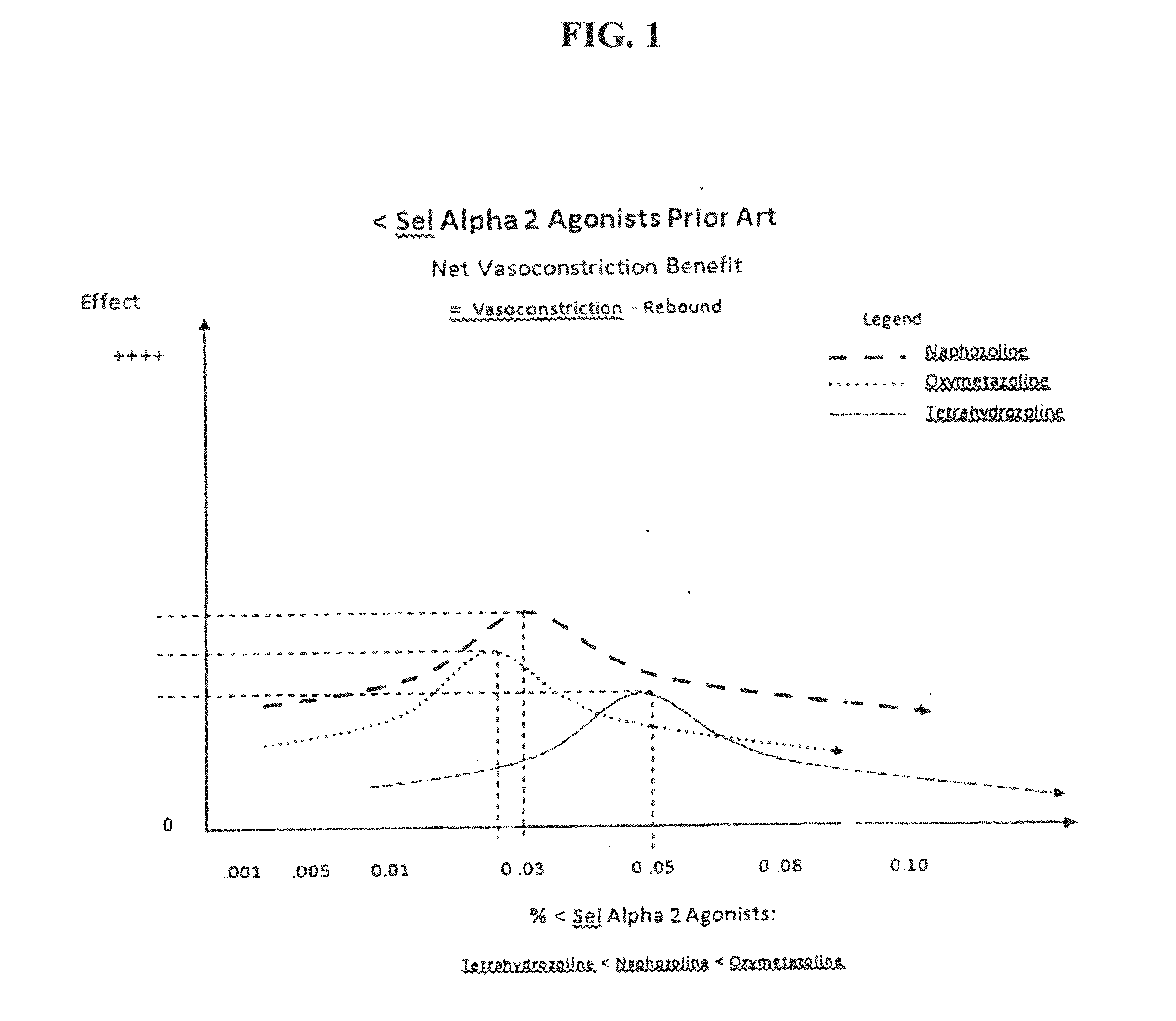
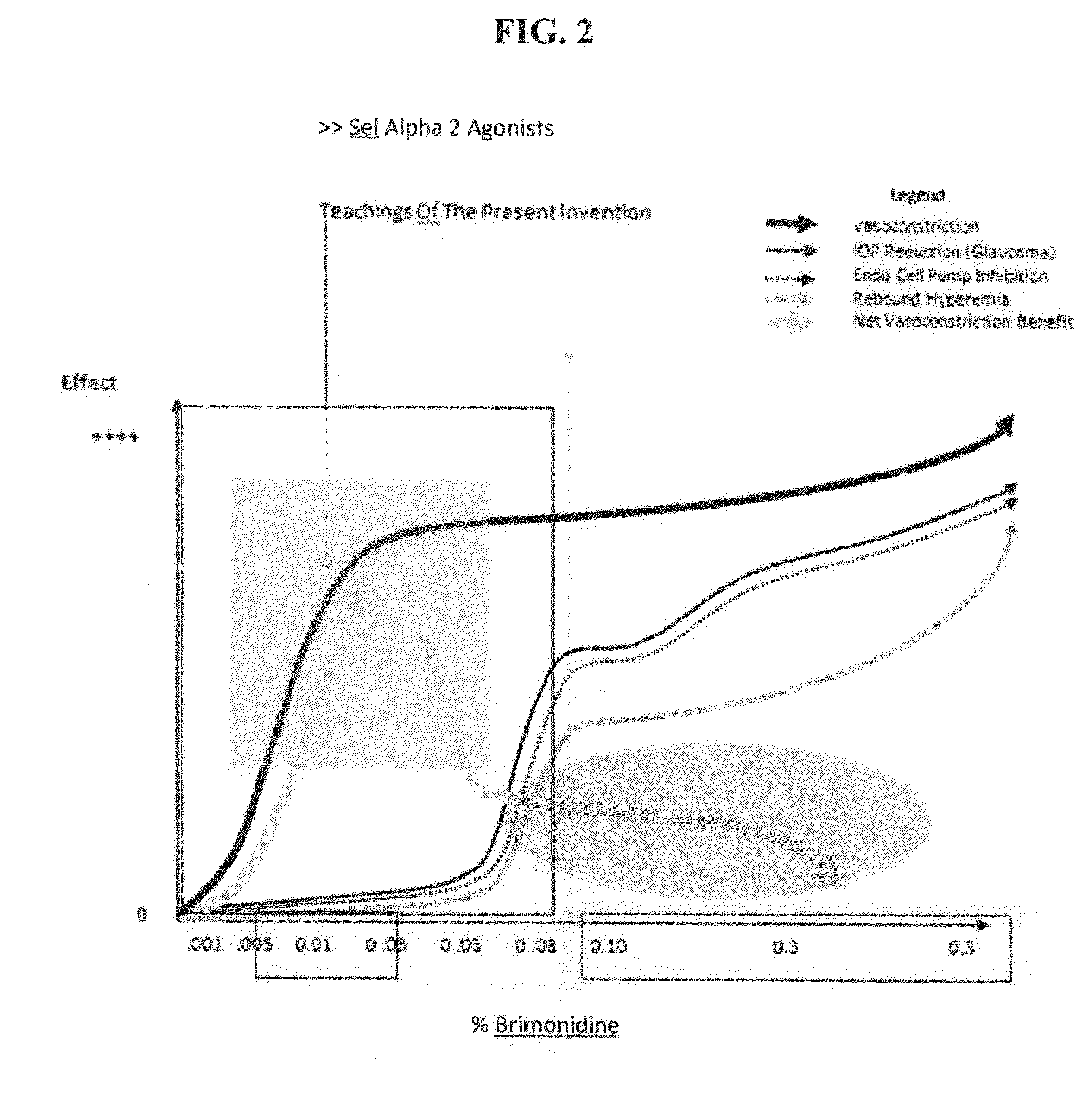
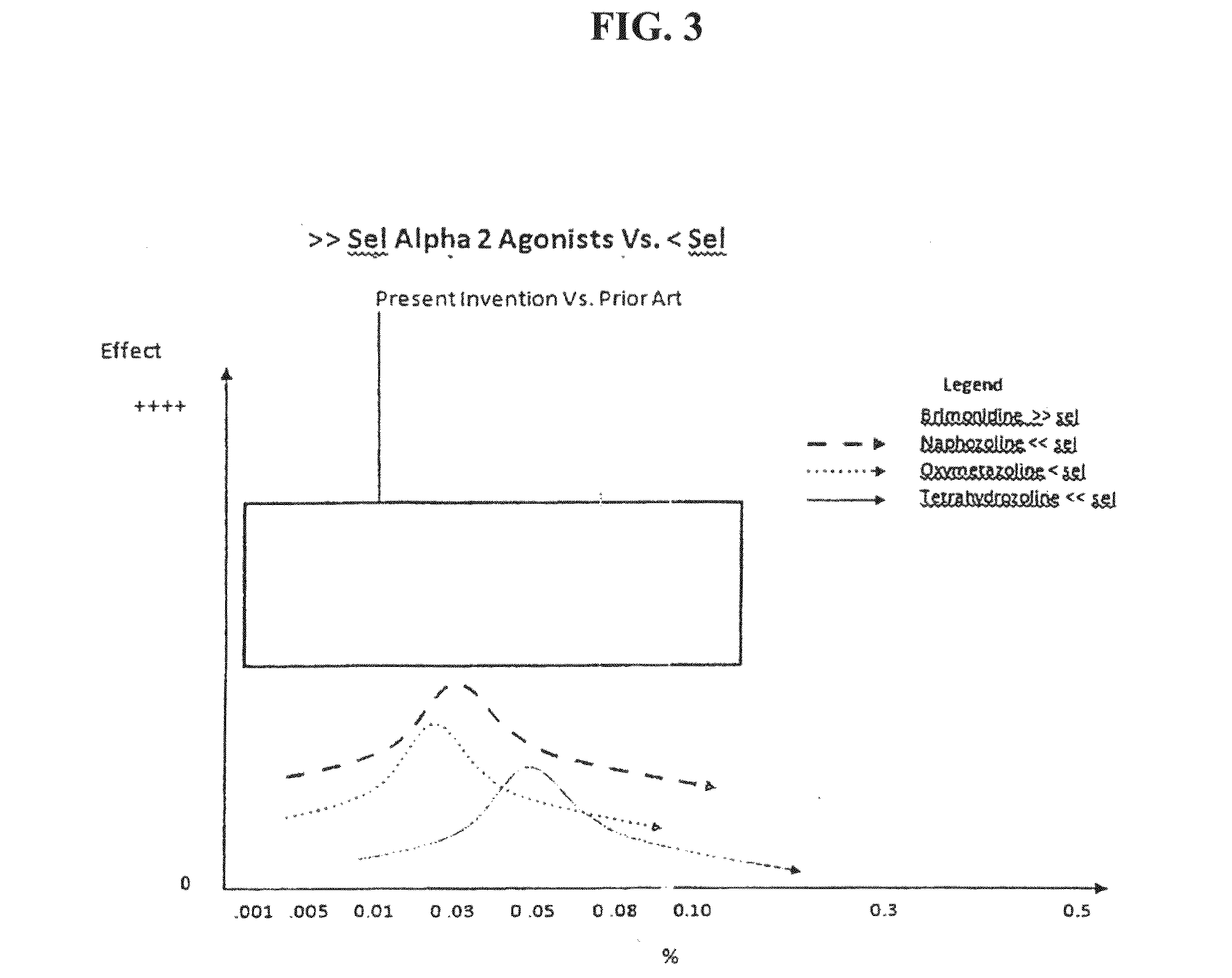
The invention generally relates to compositions and methods for treating and / or preventing an allergic response with reduced rebound hyperemia, comprising highly selective alpha-2 adrenergic receptor agonists, at low concentrations, such as below 0.05% weight by volume, and histamine antagonists. The compositions preferably comprise brimonidine. The compositions preferably have pH between about 5.5 and about 6.5.
Owner:EYE THERAPIES
Enteric coated preparation for preventing and treating hyperuricemia and gout
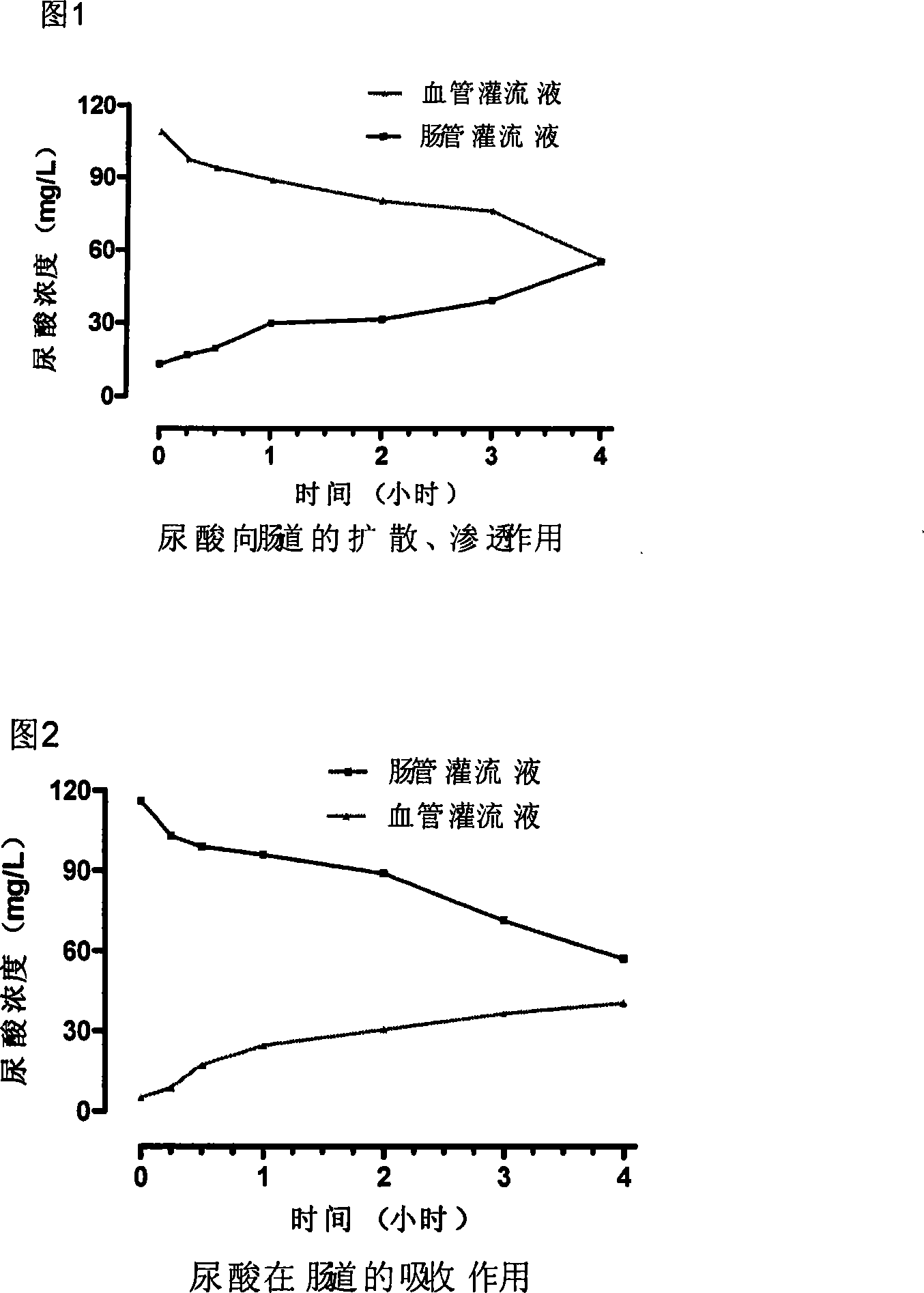
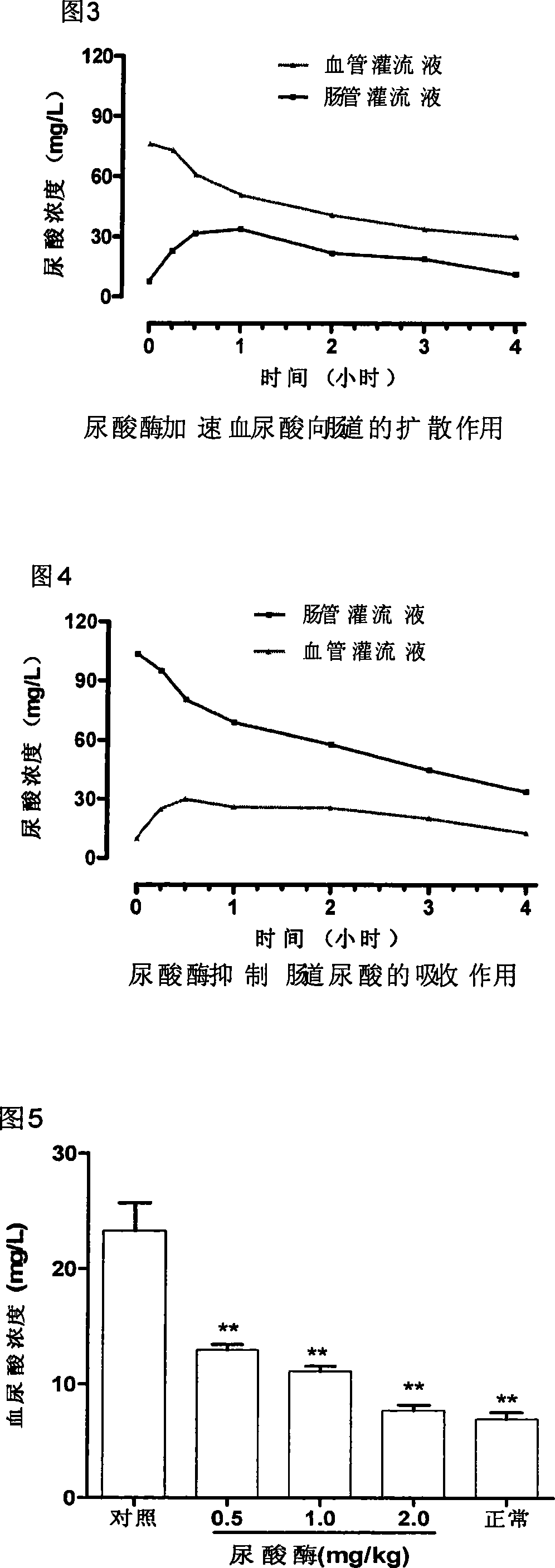
InactiveCN101168052APromote decompositionHigh molecular weightPeptide/protein ingredientsSkeletal disorderSerum uric acidAllergic response
Provided is an enteric preparation for preventing and curing hyperuricemia and gout. The employing essential component of the preparation is enteric capsules of uricase. The causes of the hyperuricemia and the gout are that uric acid is over generated and / or the uric acid is diminished in discharging, and metabolic diseases increasing the density of serum uric acid. About one third of the uric acid of the human body is defecated from intestinal canals, and the uric acid entering the canals still can be reabsorbed. When the hyperuricemia and the gout happen, the proportion of the uric acid defecated from the intestinal canals is increased. The uricase is capable of rapidly effectively dissociating the uric acid. The uricase of the enteric preparation after being orally taken is disintegrated in the intestinal canals and is diffused from blood into the uric acid of the intestinal canals, preventing the uric acid from reabsorbing, thereby reducing the density of the serum uric acid, achieving the purposes of preventing and curing the hyperuricemia and the gout. The oral uricase has the characteristics of non-absorbent property by the intestinal canals, keeping out of blood circulation, no producing of antibodies of organisms and allergic response, providing a new method for preventing and curing the hyperuricemia and the gout.
Owner:XI AN JIAOTONG UNIV
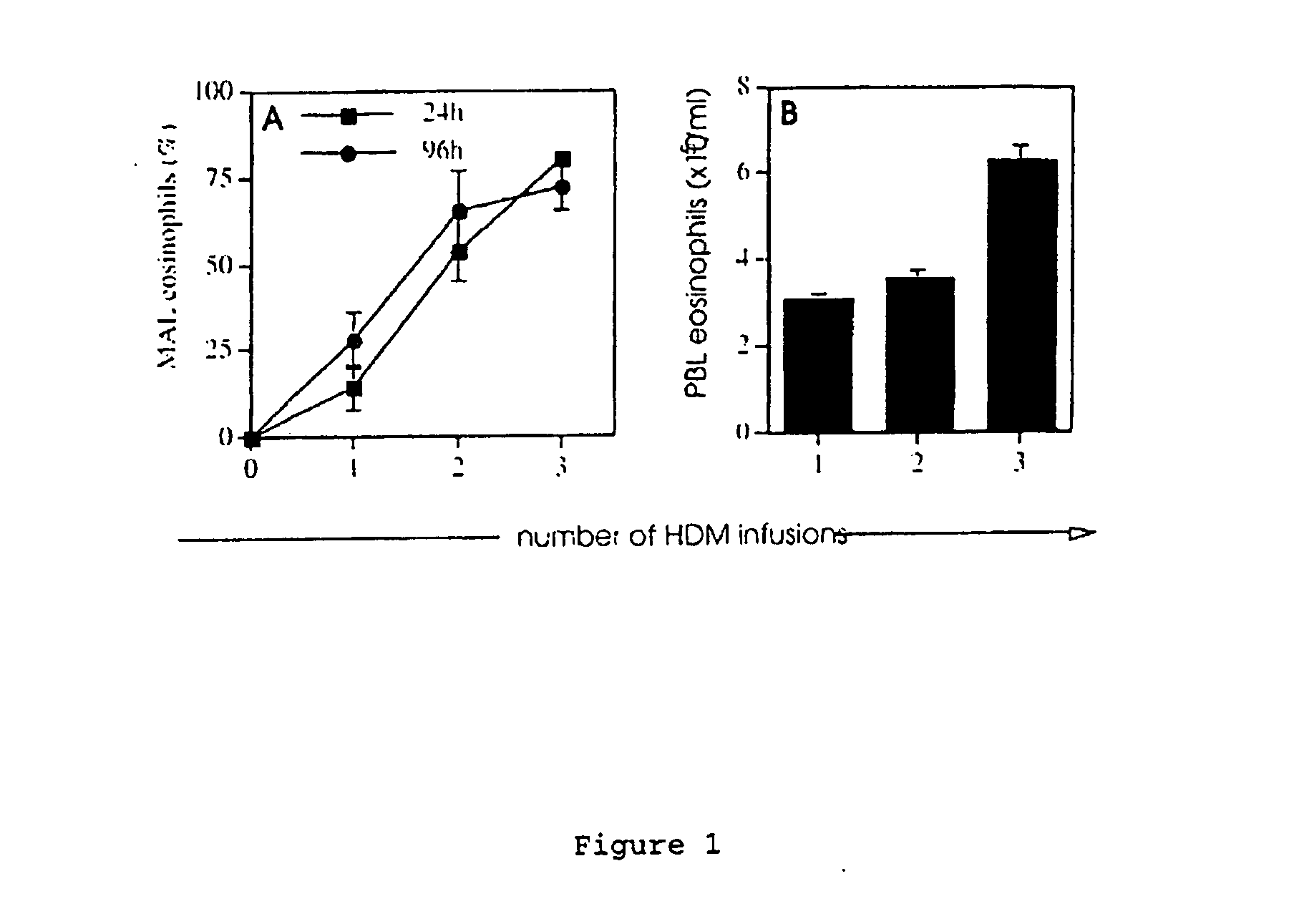
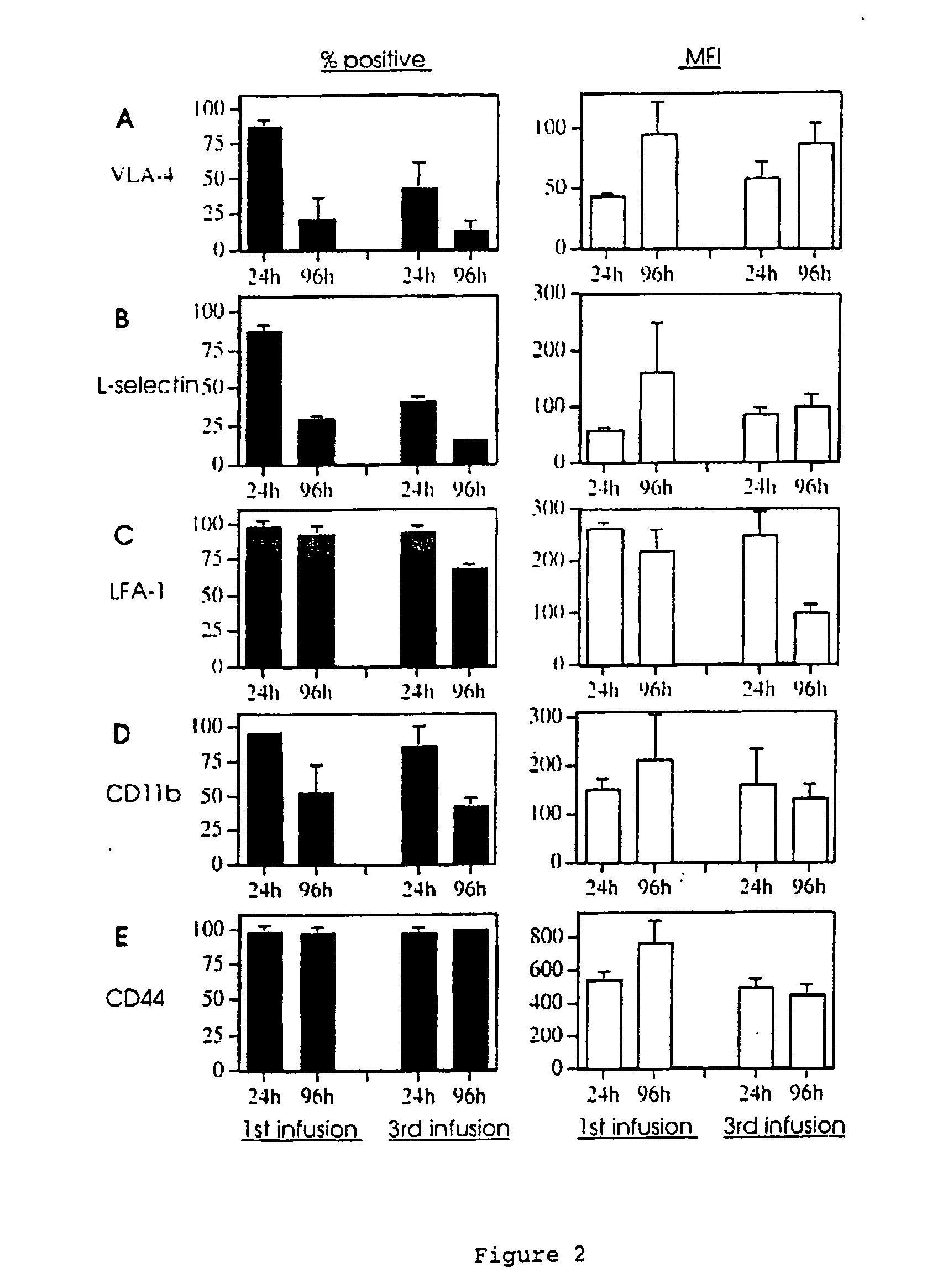
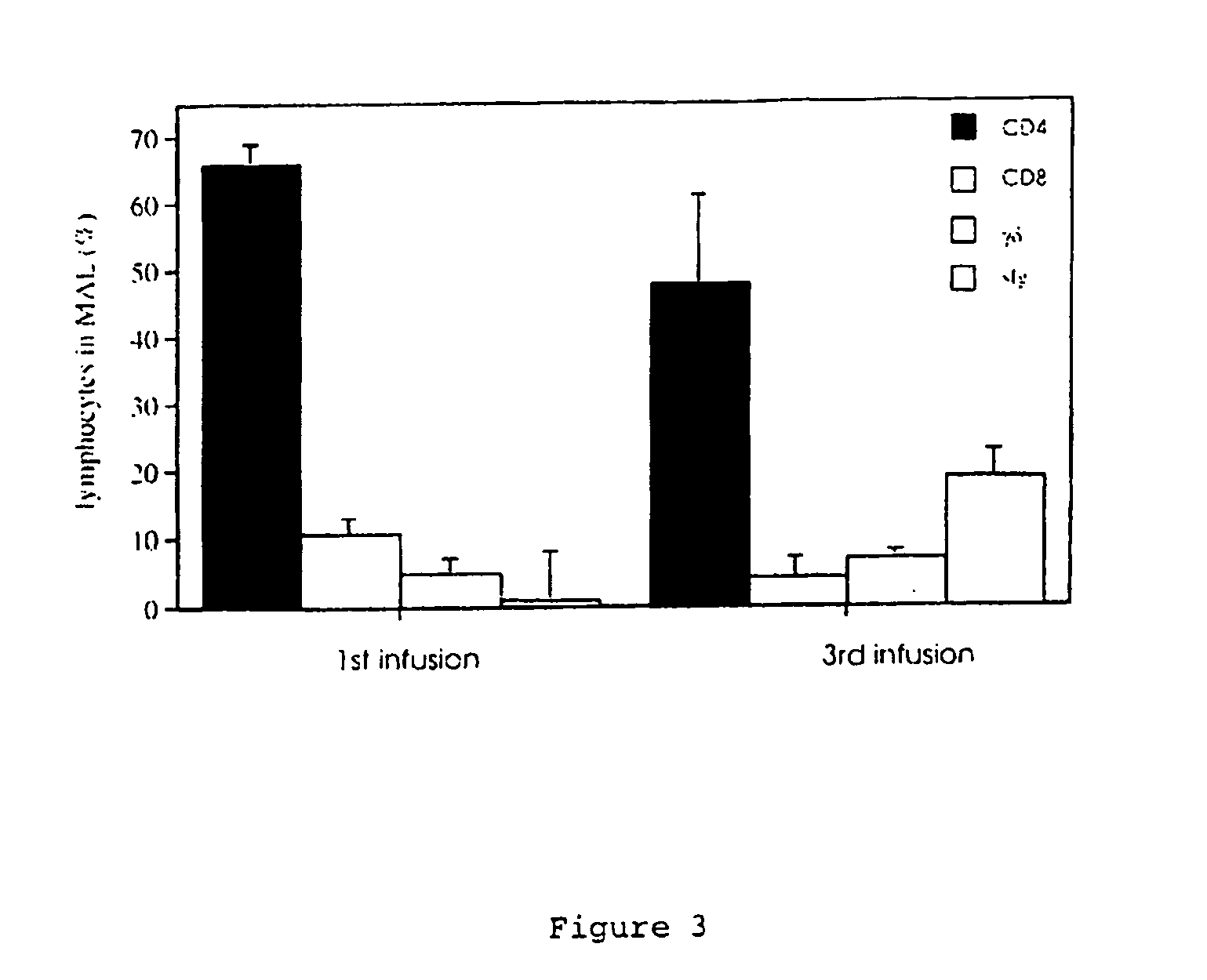
Animal model for allergy
The invention relates to model systems for allergic conditions, and in particular to in vivo model systems in a large animal. The model systems of the invention are especially useful for providing large numbers of activated or non-activated eosinophils, for the discovery and evaluation of novel anti-inflammatory drug targets and for providing a model for the in vivo study of asthma and the effects of allergy treatments. In a preferred embodiment the animal is a sheep. In one embodiment, repeated infusion of house dust mite allergen (HDM) into the mammary gland is used to induce a specific allergic response, which is characterised by the recruitment of inflammatory cells, particularly eosinophils, into the mammary lumen; these cells can be harvested from peripheral blood and mammary lavage (MAL). In a second embodiment, the mammal is immunised with soluble antigen, for example by repeated subcutaneous immunisation, and then subjected to a single challenge with the same antigen administered directly to the lung.
Owner:ALLERGENIX
Emulsified wax compositions and uses thereof
The present invention discloses emulsified wax compositions having a wide range of uses, including as cosmetic, pharmaceutical and agricultural compositions, particularly for improving skin moisture, appearance, and wound healing, for the treatment of inflammation and allergic responses of the skin, and for preservation of agricultural fresh produce. The composition of the invention comprises an aqueous phase and at least one wax at a concentration of at least 5%, at least one fatty acid and at least one basic amino acid or a salt thereof.
Owner:AIGIS LABS LTD
Emulsified wax compositions and uses thereof
ActiveUS20100297273A1Improving skin moistureGood lookingBiocideCosmetic preparationsWound healingAllergic response
The present invention discloses emulsified wax compositions having a wide range of uses, including as cosmetic, pharmaceutical and agricultural compositions, particularly for improving skin moisture, appearance, and wound healing, for the treatment of inflammation and allergic responses of the skin, and for preservation of agricultural fresh produce. The composition of the invention comprises an aqueous phase and at least one wax at a concentration of at least 5%, at least one fatty acid and at least one basic amino acid or a salt thereof.
Owner:AIGIS LABS LTD
Phospholipid membrane preparation
InactiveUS20050266066A1Inhibit productionLess prone to allergic reactionsCarrier-bound antigen/hapten ingredientsLiposomal deliveryAntigenAllergic response
The present invention aims at providing a phospholipid membrane preparation wherein an antigen or an allergen is bound onto the surface of a phospholipid membrane comprising a phospholipid containing an acyl group or a hydrocarbon group having 10 to 12 carbon atoms, and a stabilizer of a phospholipid membrane. The present invention provides a phospholipid membrane preparation having an immune response controlling function that suppresses production of IgE antibody to increases production of practically sufficient IgG antibody and usable as a vaccine that does not easily cause an allergic response.
Owner:NOF CORP +1
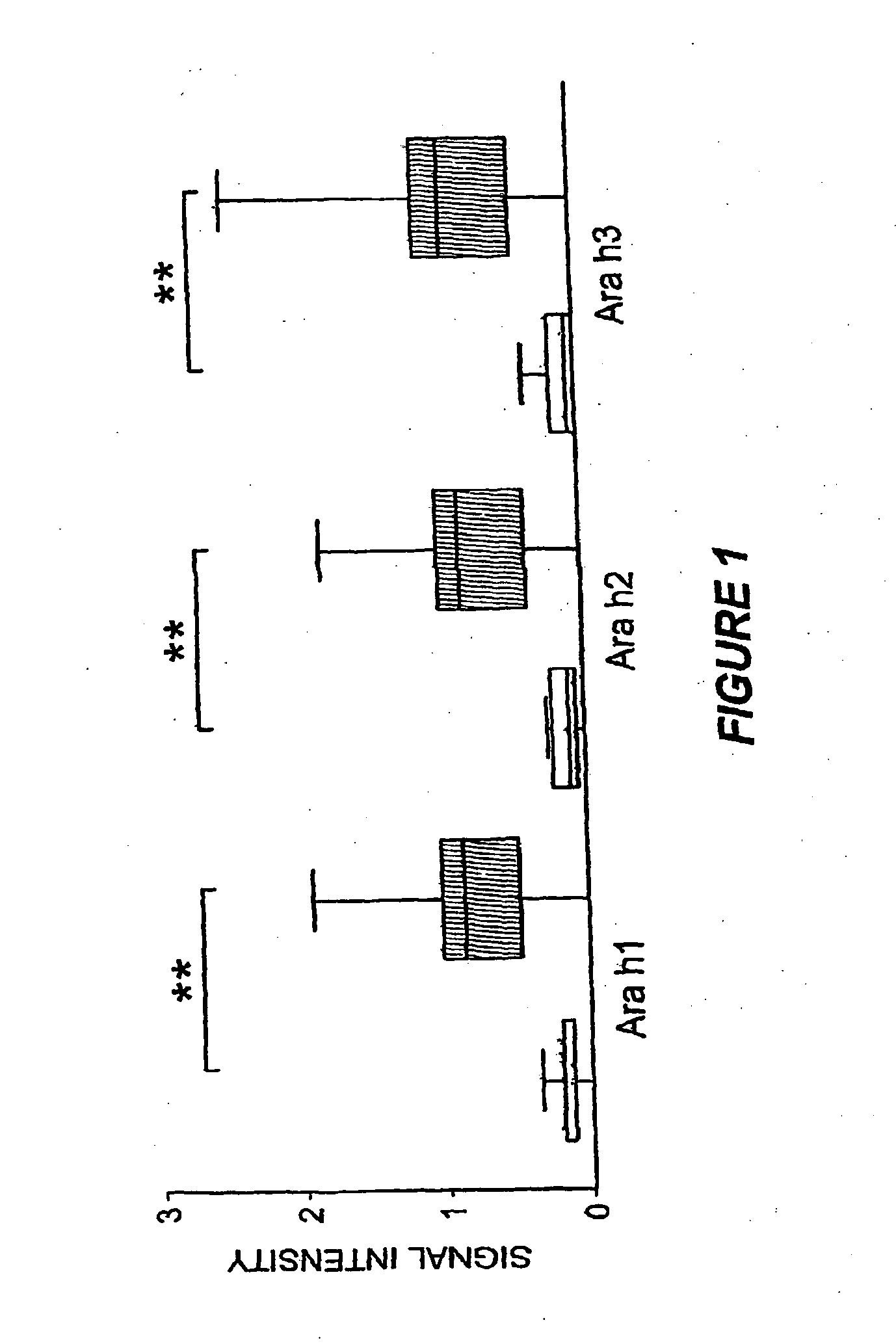
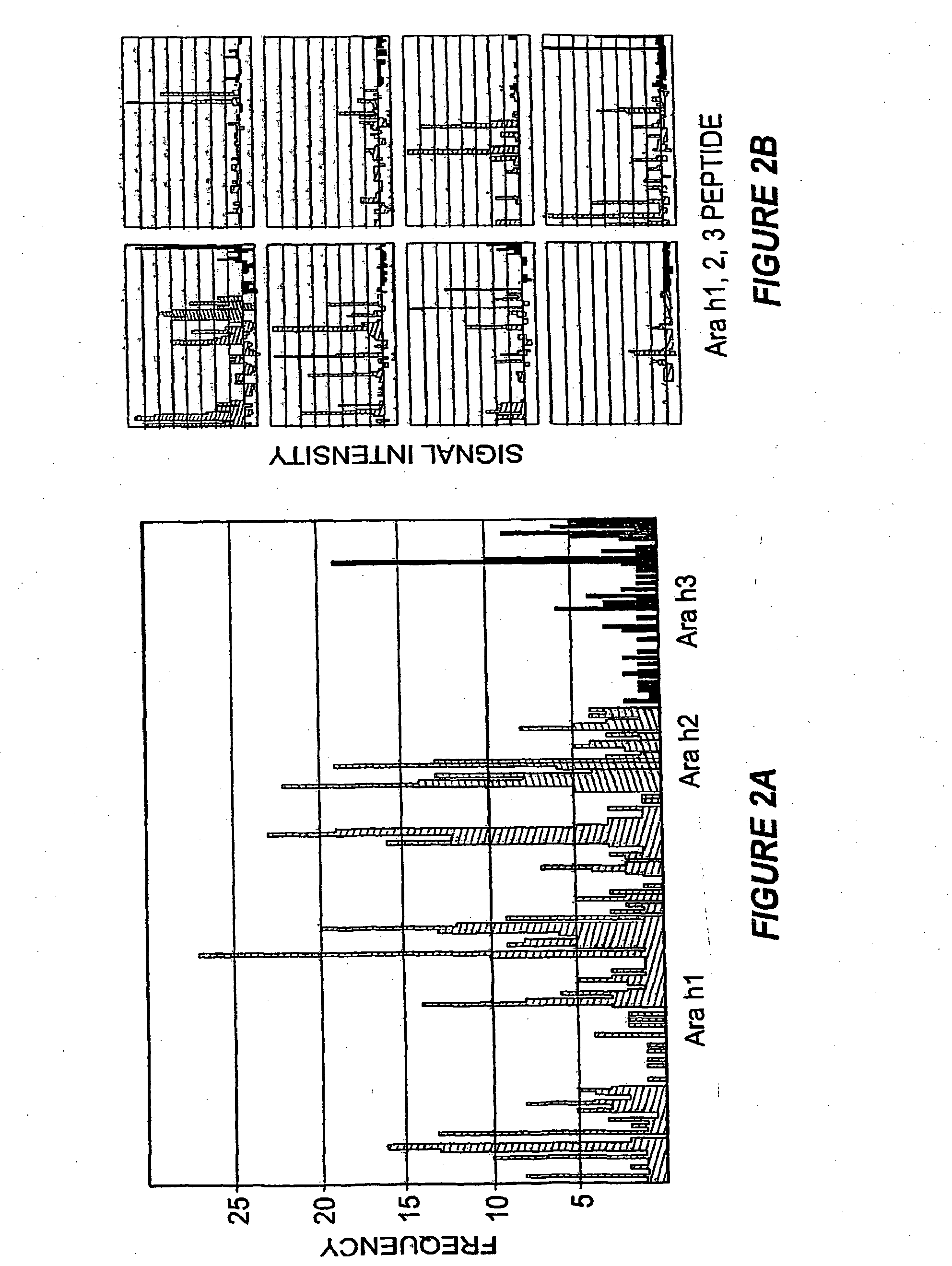
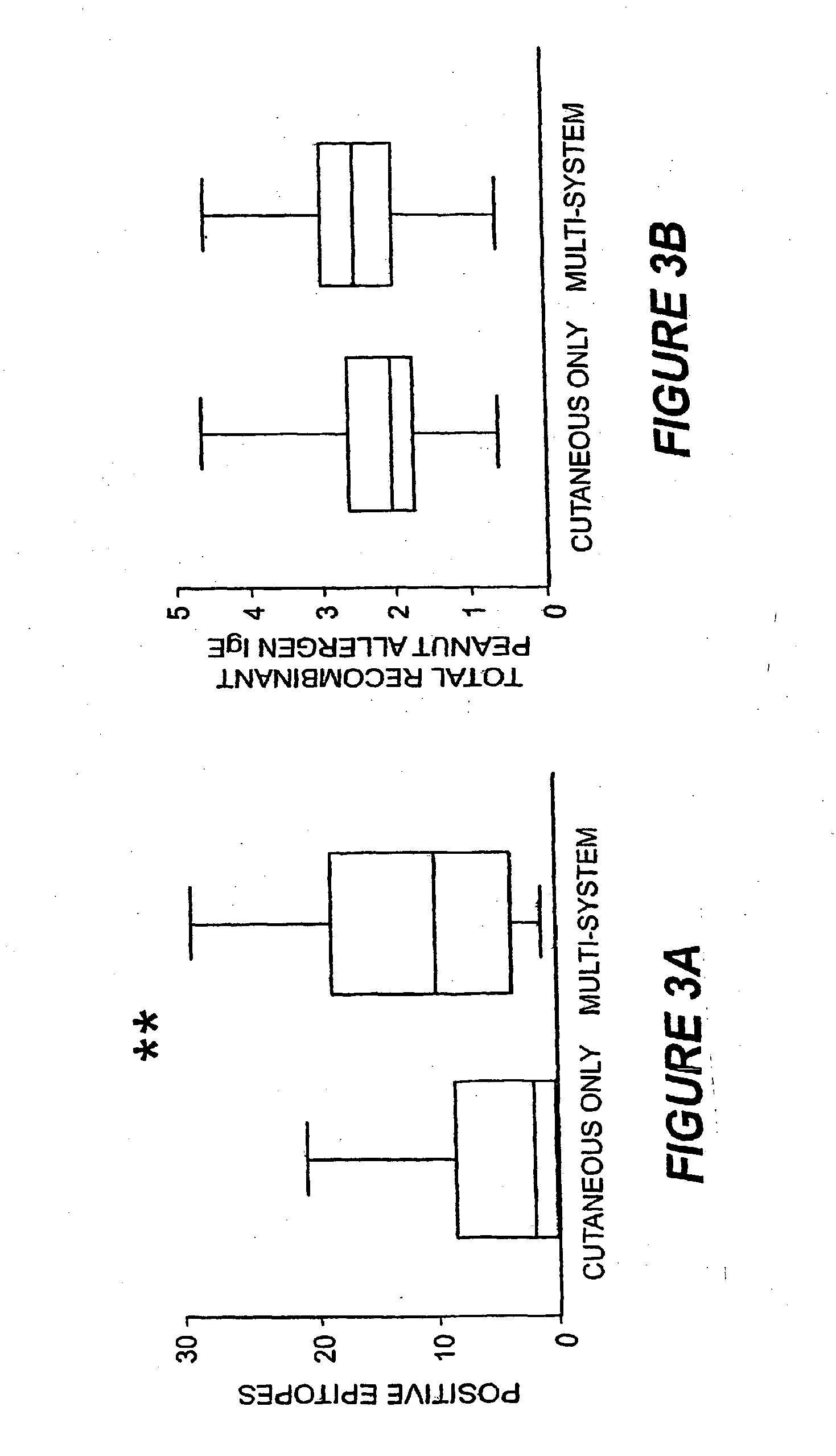
Methods of determining allergen response using microarray immunoassay techniques
The present invention is directed to materials and methods that may be used in diagnosing and / or characterizing allergies. More specifically, the specification describes methods and compositions for making and using a plurality of peptides having allergen epitopes that may be used in immunoassays e.g., microarray-based immunoassays to predict the severity of an allergic response.
Owner:NIH DEITR
Anti-allergy composition and related method
ActiveUS20050175718A1Prevent delay sensitizationLower potentialBiocideSenses disorderAllergic responseAdditive ingredient
An composition and related method that prevents, inhibits and / or mitigates an allergic response by down regulating the production of IgE, down regulating the binding of IgE antibodies to receptors on cells, and / or inhibiting allergy mediators, for example, histamine, prostoglandin D2, or luekotriene C4 release. The composition comprises at least one of the following ingredients: luteolin from Perilla leaf or seed, Cinnamon, Kiwi, Picao preto, Hesperidin, Acerola cherry, Guaco, Holy Basil, Kakadu, Solamum, Rosmarinic acid, Tinospora and Aframomum. In one embodiment, the composition at least three different ingredients selected from Cinnamon, Acerola, Luteolin and Picao preto. Optionally, these ingredients can be combined with at least one of Aframomum, Rosmarinic acid, and Tinospora. The composition is administered with effective amounts to prevent, inhibit and / or mitigate allergic responses.
Owner:ACCESS BUSINESS GRP INT LLC
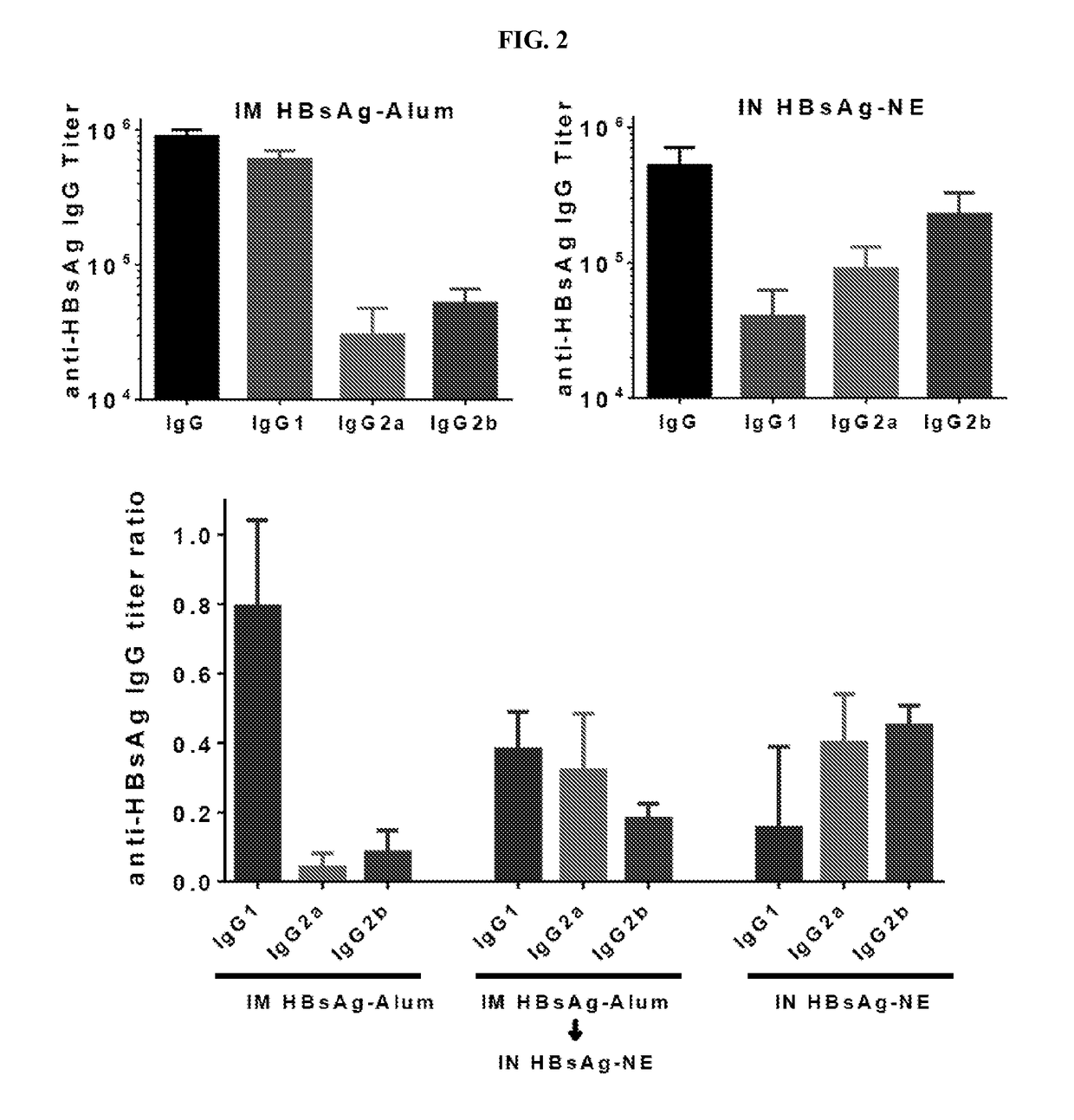
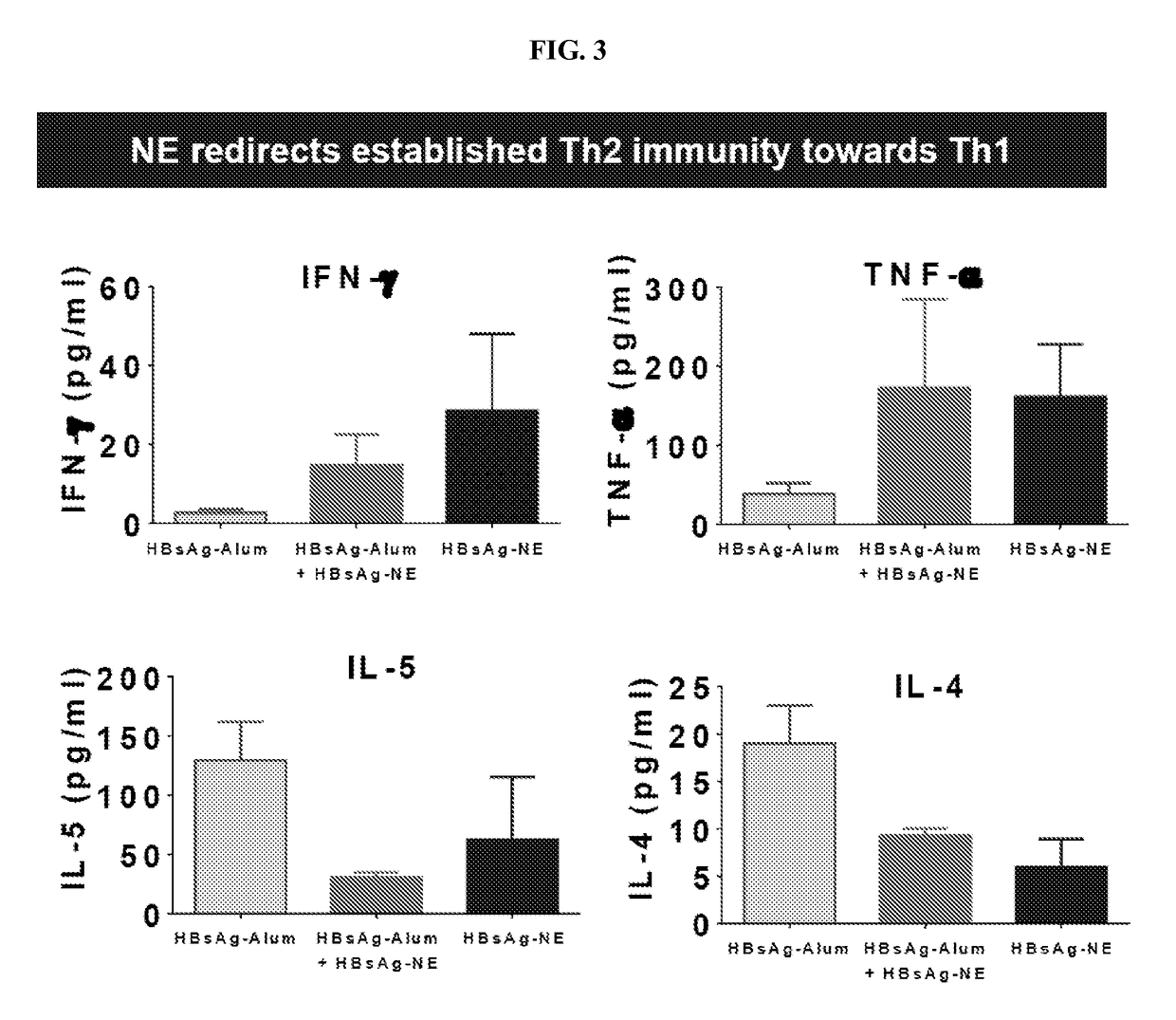
Nanoemulsion compositions for preventing, suppressing or eliminating allergic and inflammatory disease
The present invention provides methods and compositions for the stimulation of immune responses and for treating or preventing allergic disease and responses and inflammatory disease and responses. In particular, the present invention provides nanoemulsion compositions and methods of using the same for the induction of immune responses that prevent or treat allergic disease by reducing allergic response. Compositions and methods of the invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine (e.g., vaccination)) and research applications.
Owner:RGT UNIV OF MICHIGAN
Use of parasitic biological agents for prevention and control of allergic and other IgE-mediated disorders
InactiveUS20060024333A1Reduce excessive immune responseLimit magnitudeProtozoa antigen ingredientsLeech/worm material medical ingredientsDiseaseInterleukin 10
The present invention describes using, on a repetitive basis, a non-human colonizing helminth compound, in an amount sufficient to establish as needed a transitory helminth infection and or to simulate in a parasitic helminth infection with helminth excretory and secretory products (ESP), thereby having immunosuppressive effect against benign antigens and or stimulating a regulatory immune response characterized by the production of T helper cells 2 (Th2), T regulatory helper cells (TReg) and certain cytokines, including, but not limited to interleukin 10 (IL-10), as a therapy or prophylaxis of allergy and other IgE-mediated disorders, which are marked by an inappropriate immunoglobulin E (IgE) immune response including, but not limited to an abnormal IgE antibody production to benign antigens. The invention presents using helminth compound by administering it in a frequency and amount sufficient to eliminate or ameliorate the inappropriate immune response in an asthmatic and or allergic individual. This invention is generally directed to diseases IgE antibody-mediated disorders, including asthma, allergies, hypersensitivity and anaphylactic reactions. More specifically, the present invention is directed toward the treatment of certain common food allergies, such as an allergy to peanut, tree nut, milk, egg, wheat, and shellfish (CFA's).
Owner:OCONNOR DANIEL J
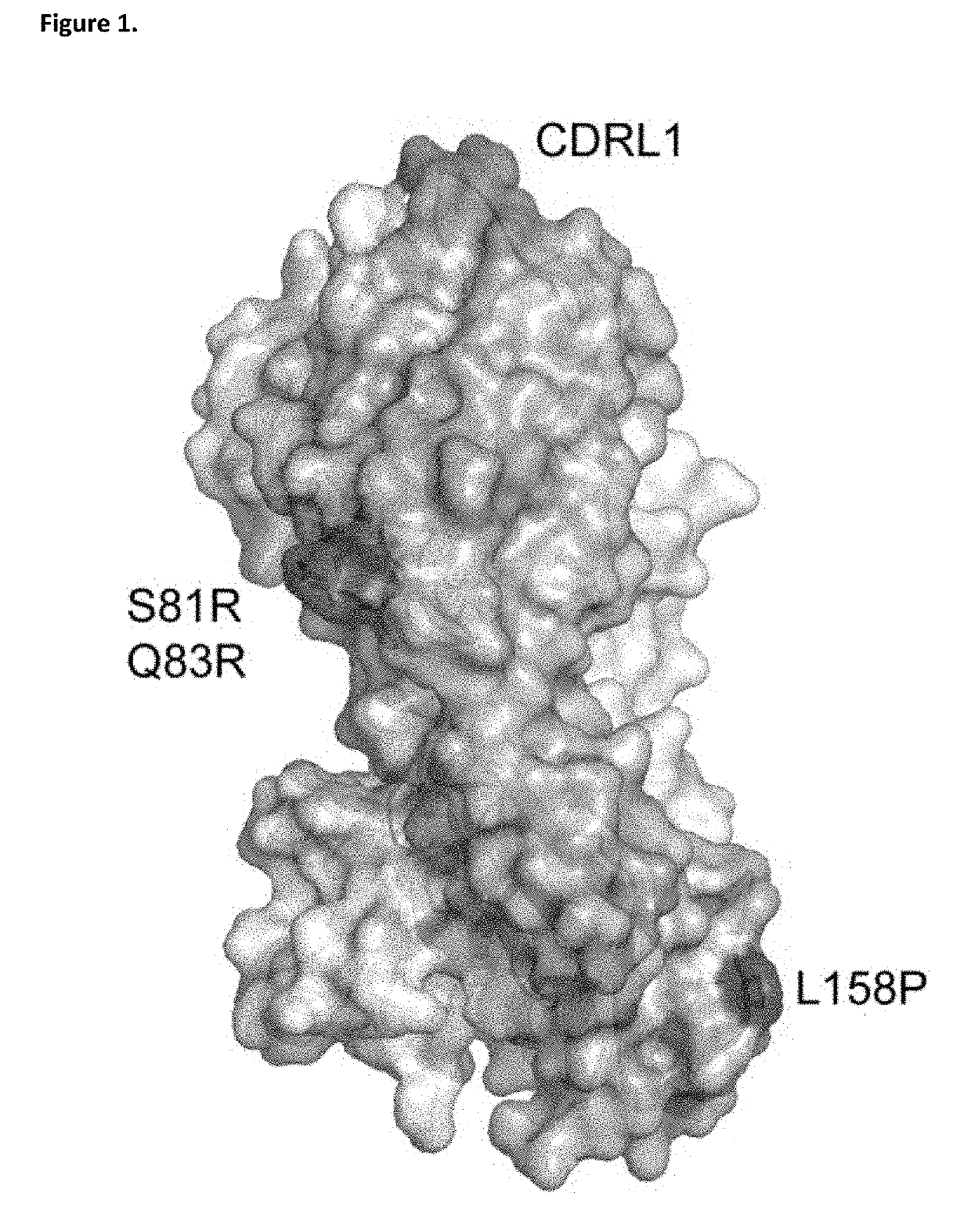
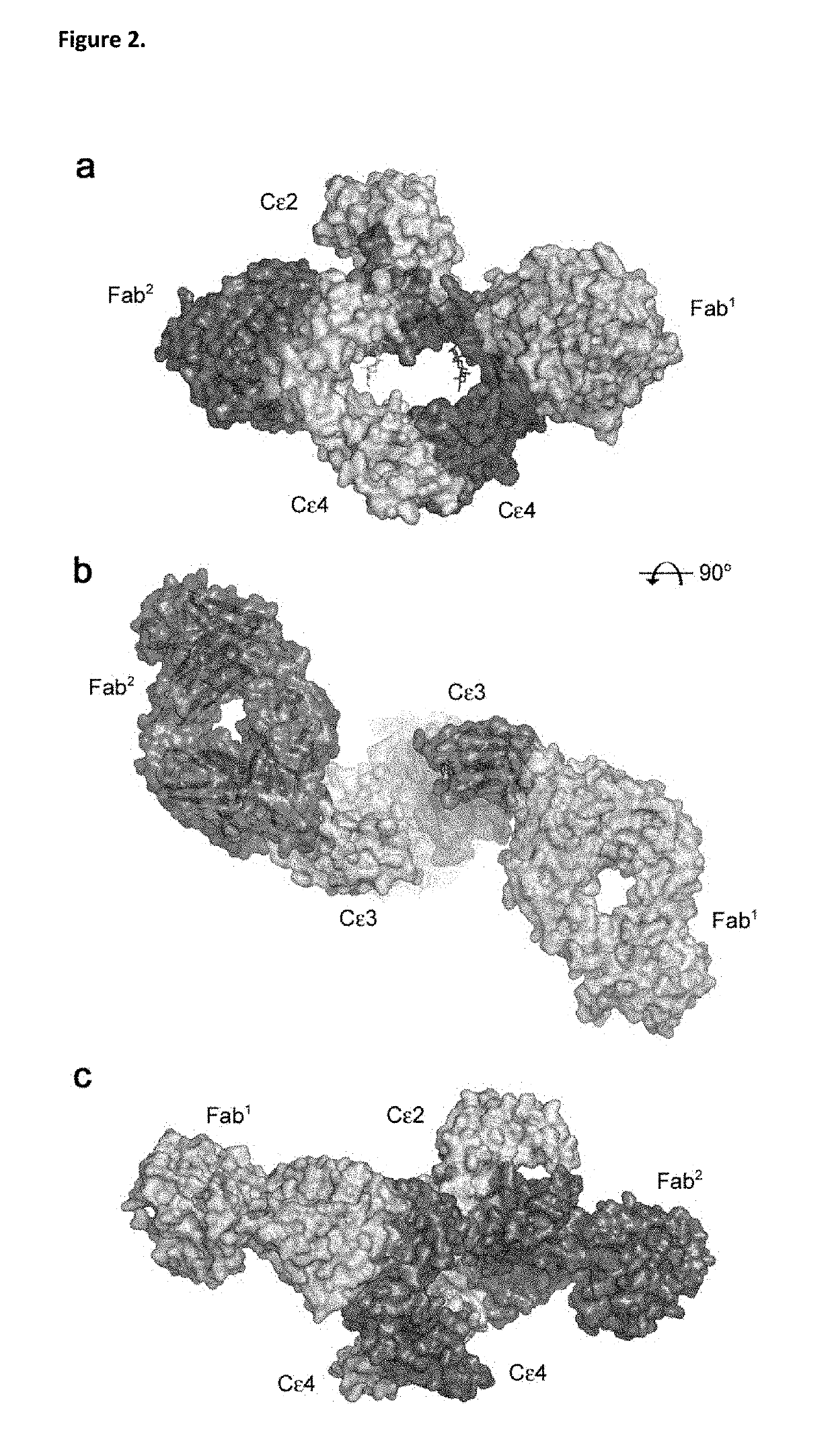
ANTI-IgE ANTIBODIES
ActiveUS20190144565A1Enhanced interactionHigh affinityPharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAutoimmune Reactions
The present invention relates to the area of improved anti-IgE antibodies and antigen binding agents, and compositions thereof, which target IgE, for instance: for use in treating disorders caused by IgE (such as allergic responses, or certain autoimmune responses); and, in particular, disorders caused by the interaction of IgE with the FcεRI receptor. In particular, this invention relates to improved anti-IgE antibodies and antigen binding agents related to novel mutants of omalizumab (Xolair®). The improved anti-IgE antibodies and antigen binding agents of the invention may have improved affinity for IgE and / or an improved interaction with the Cε2 domain of IgE and / or an improved modified epitope on IgE (for instance further involving the Cε2 domain of IgE) and / or the ability to disassociate IgE from the FcεRI receptor for instance at pharmaceutically-relevant concentrations. In one aspect, improved or novel treatments for IgE mediated disorders are disclosed in which IgE is targeted (for instance free IgE and / or IgE complexed with the FcεRI receptor).
Owner:UCB PHARMA SRL
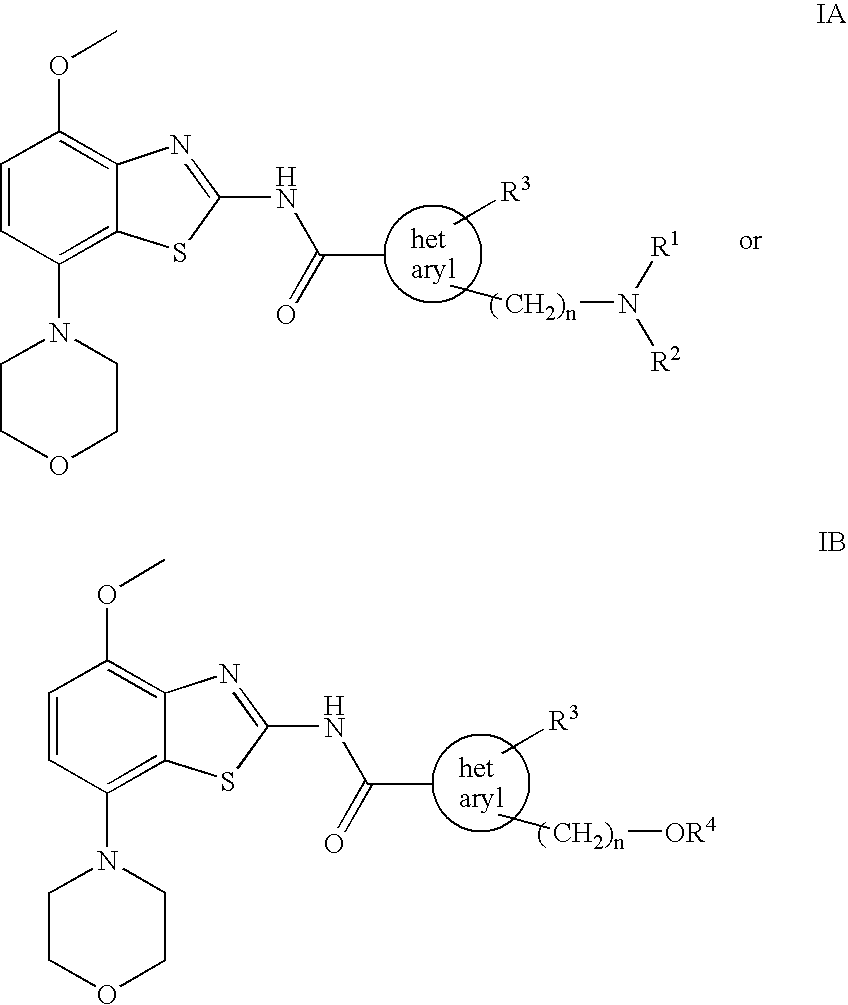
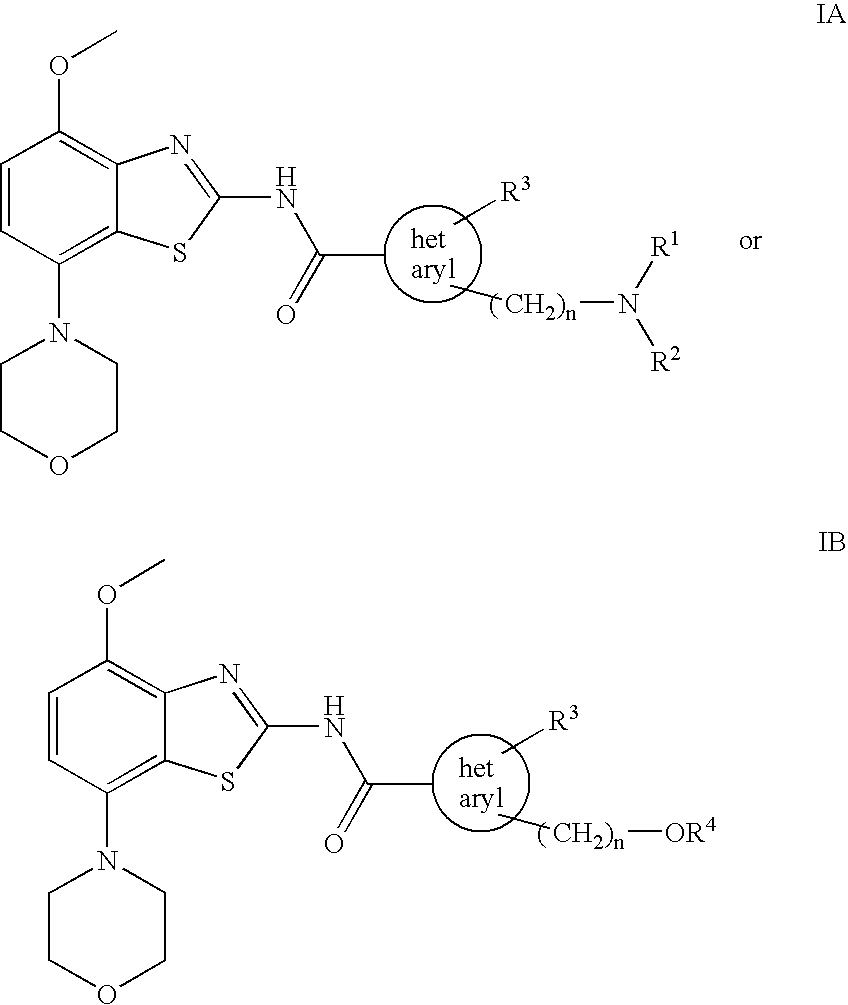
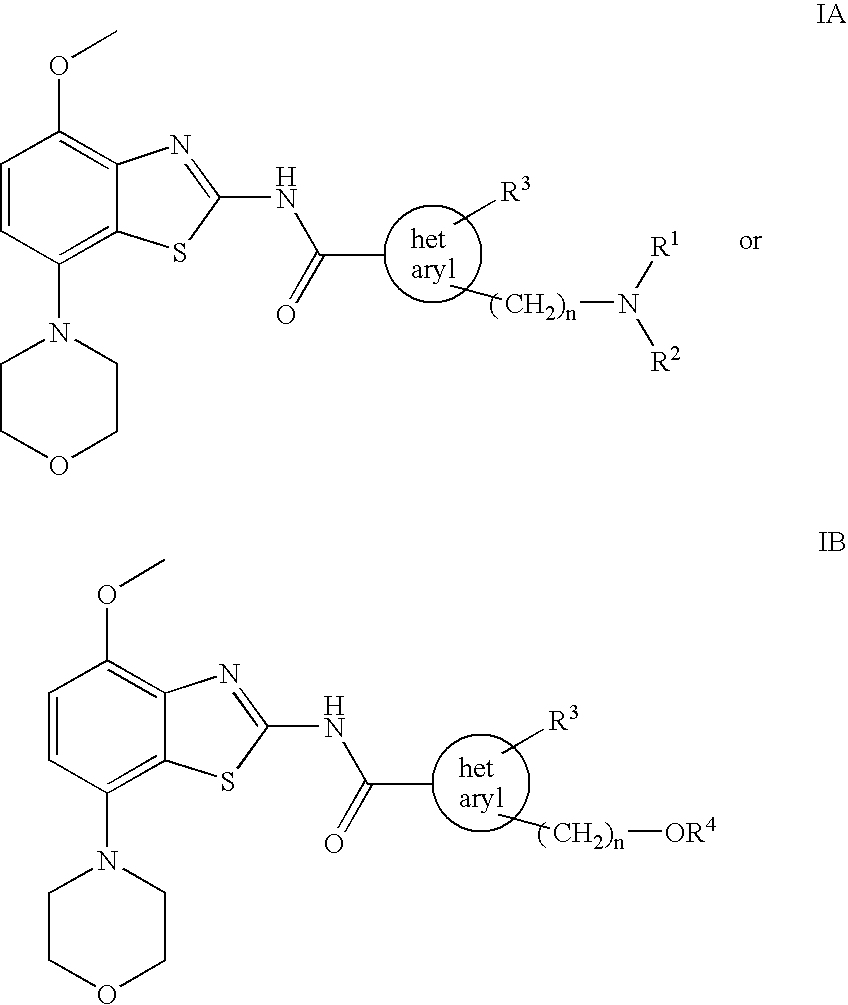
Benzothiazole derivatives
InactiveUS20060019949A1High affinityHigh selectivityOrganic active ingredientsNervous disorderTranquilizing AgentsDisease cause
The present invention relates to compounds of formulae IA and IB wherein R1 and R2 are each independently lower alkyl or —(CH2)m—O-lower alkyl, or together with the N atom to which they are attached form a heterocyclic ring; R3 is hydrogen or lower alkyl; R4 is lower alkyl; hetaryl is 3H-imidazole-2,4-diyl or 1H-pyrazole-1,4-diyl; n is 1 or 2; and m is 1 or 2; and to pharmaceutically acceptable acid addition salts thereof. These compounds may be used for the treatment of Alzheimer's disease, Parkinson's disease, Huntington's disease, neuroprotection, schizophrenia, anxiety, pain, respiration deficits, depression, ADHD, drug addiction, such as amphetamine, cocaine, opioids, ethanol, nicotine, cannabinoids, or for the treatment of asthma, allergic responses, hypoxia, ischaemia, seizure and substance abuse, or for use as sedatives, muscle relaxants, antipsychotics, antiepileptics, anticonvulsants and cardiaprotective agents for coronary artery disease and heart failure.
Owner:F HOFFMANN LA ROCHE INC
Composition and method for reducing allergic response
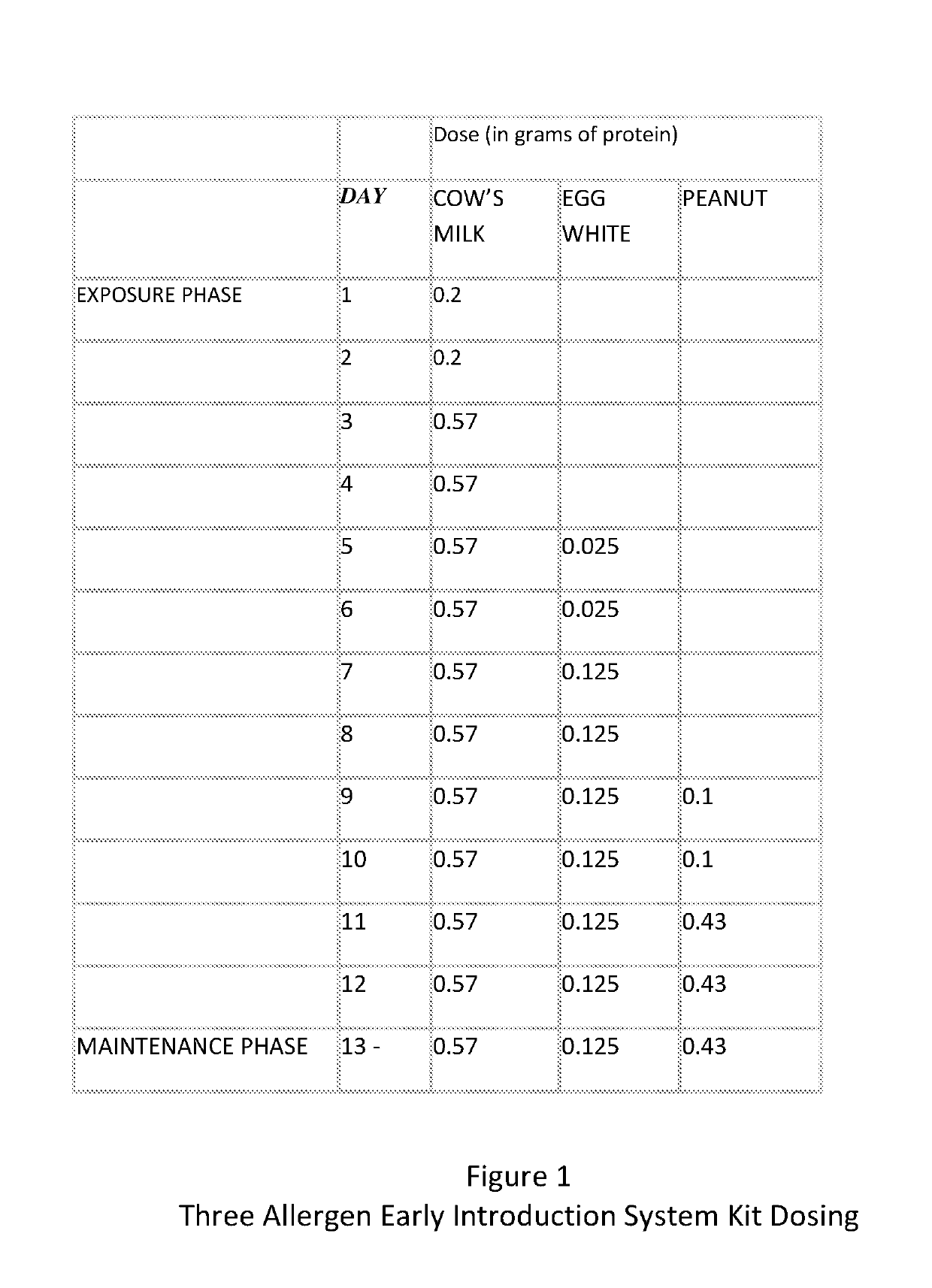
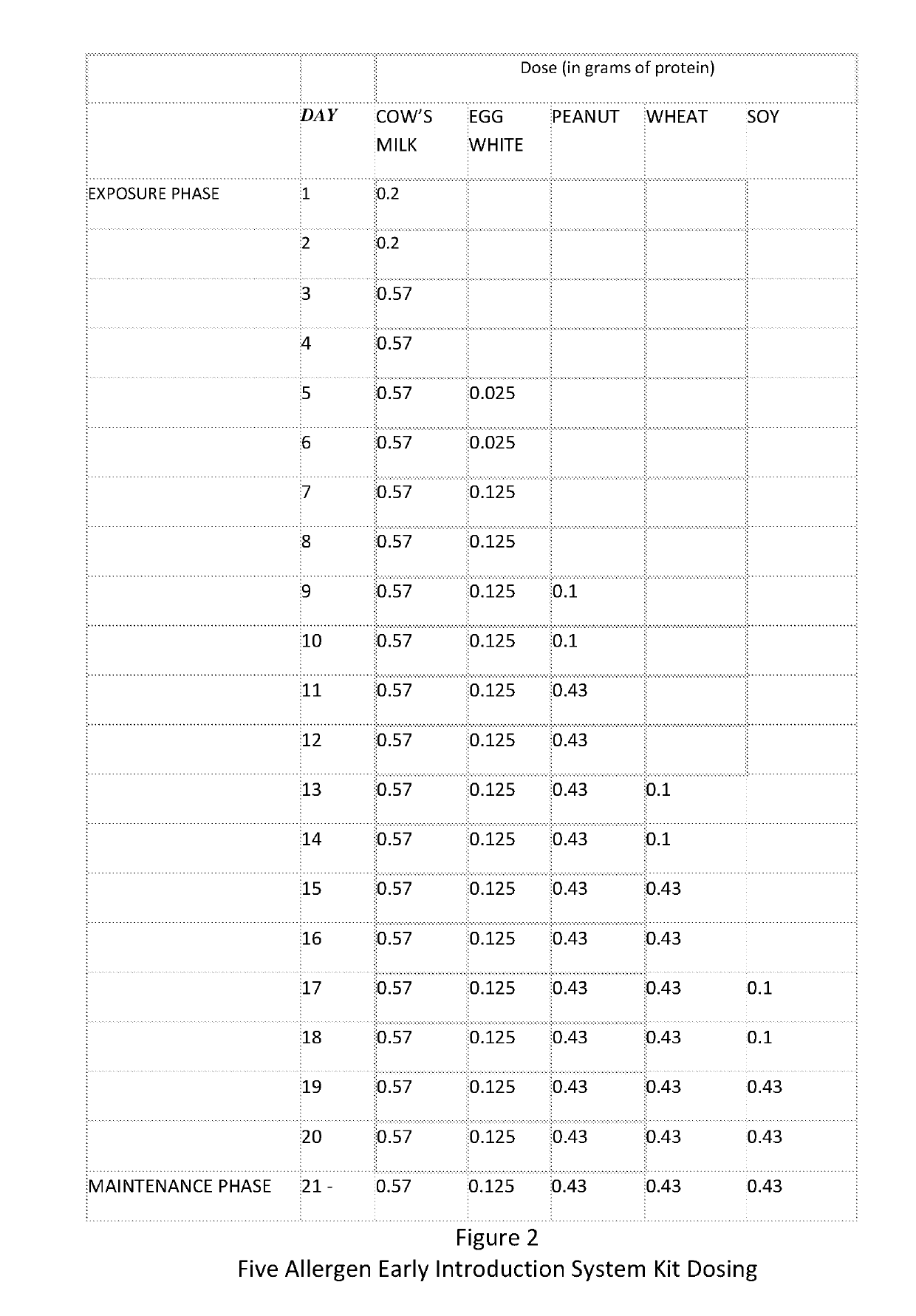
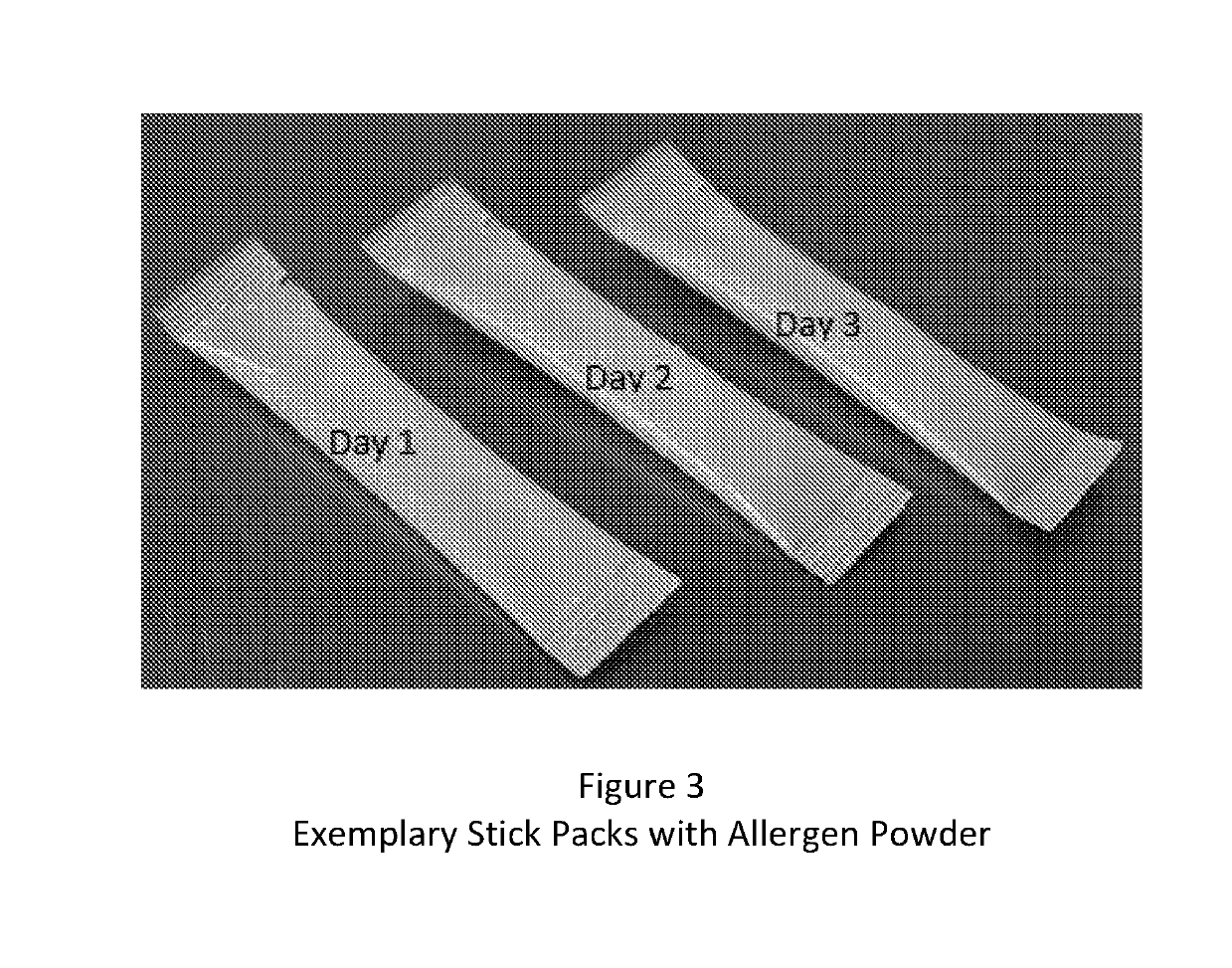
A method and kit for the sequential early introduction to an infant of at least two allergens to decrease the infant's risk for developing allergies, the method involving administering an initial lower, exposure dose of a first allergen for a day or two, followed by administering a higher maintenance dose of the first allergen for several days, followed by administering the maintenance dose of the first allergen and an initial lower, exposure dose of a second allergen for a day or two, followed by administering the higher maintenance dose of the first allergen and a higher maintenance does of the second allergen for several days. The allergens can be provided in powdered protein form in premeasured pouches for addition to baby formula or to mother's milk. Alternatively, the allergens can already be provided in baby formula, or in other foods such as snack bars, cookies, or gels.
Owner:PROLLERGY CORP
Injection for treating hyperthyroidism
InactiveCN101474397AReduce volumeHigh remission rateOrganic active ingredientsCyclic peptide ingredientsLarynxSevere hypothyroidism
The invention relates to an injection medicament for treating hyperthyroid, which comprises the following components by weight part: 2 to 5 milligrams of dexamethasone injection, 0.5 to 1 milligram of octreotide injection, and 10 to 20 milligrams of ciclosporin A. Immune preparations such as the dexamethasone injection, the octreotide injection, the ciclosporin A and the like are adopted for treating the hyperthyroid by locally injecting the thyroid, the volume of the tumid thyroid is remarkably shrunk, and the function of the thyroid basically recovers within two months, so the injection medicament has remarkable curative effect, and can remarkably improve the remission rate of hyperthyroid and reduce the rate of relapse. Serious abnormal change, hypothyroidism and parathyroid hypofunction are not caused, and laryngeal recurrent nerve injury is also not caused. Through more than 580 cases of clinical therapy in related hospitals, the volume of the tumid thyroid is remarkably shrunk after therapy, clinical symptoms disappear, allergic response and complications are not caused, the effective percentage is nearly 100 percent, and the cure rate is as high as 98 percent.
Owner:陈小国
Cell-specific signaling biomarker analysis by high parameter cytometry; sample processing, assay set-up, method, analysis
ActiveUS20180252708A1Facilitate data interpretationEasy to explainDisease diagnosisIndividual particle analysisMarker selectionProgenitor cell
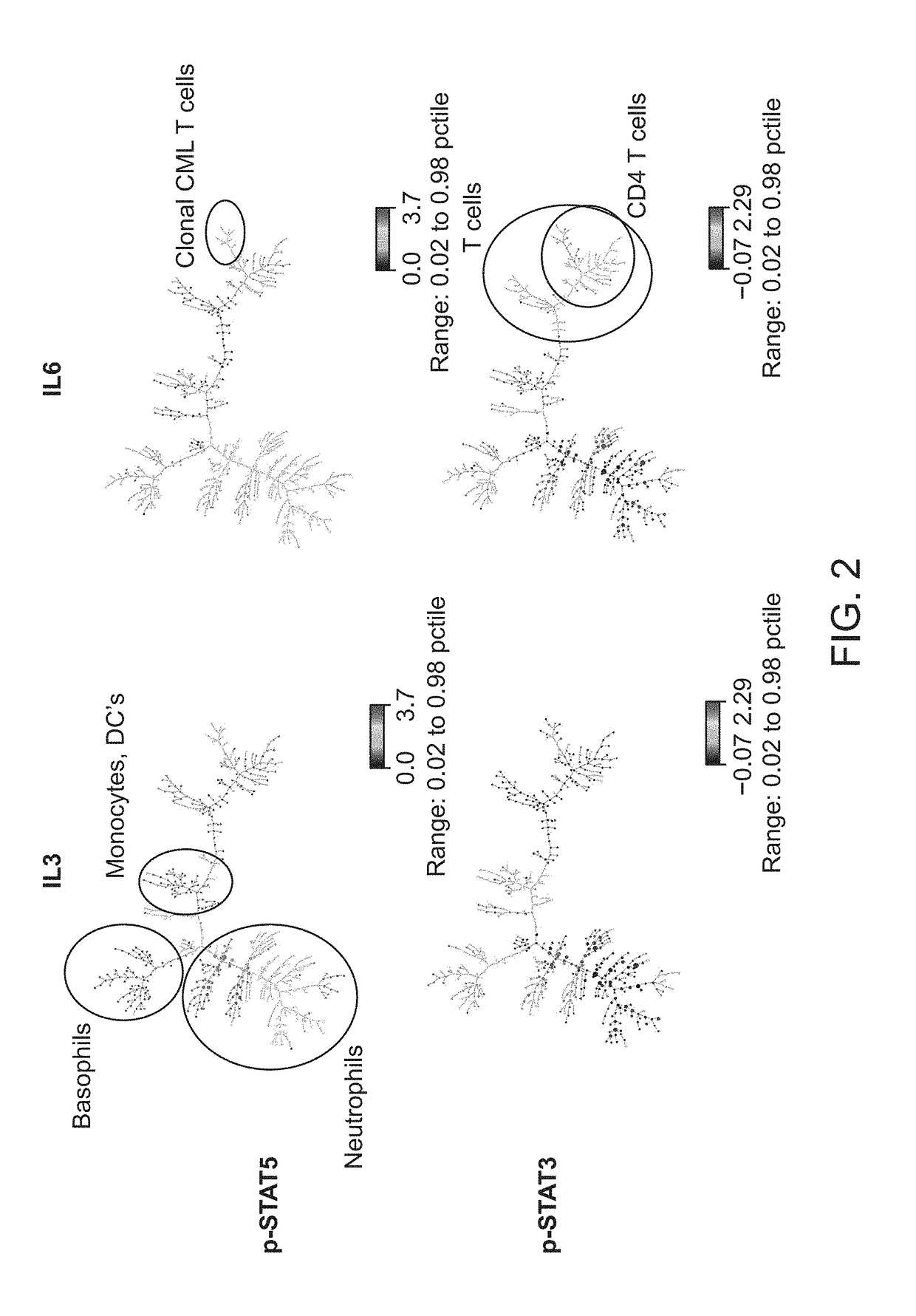
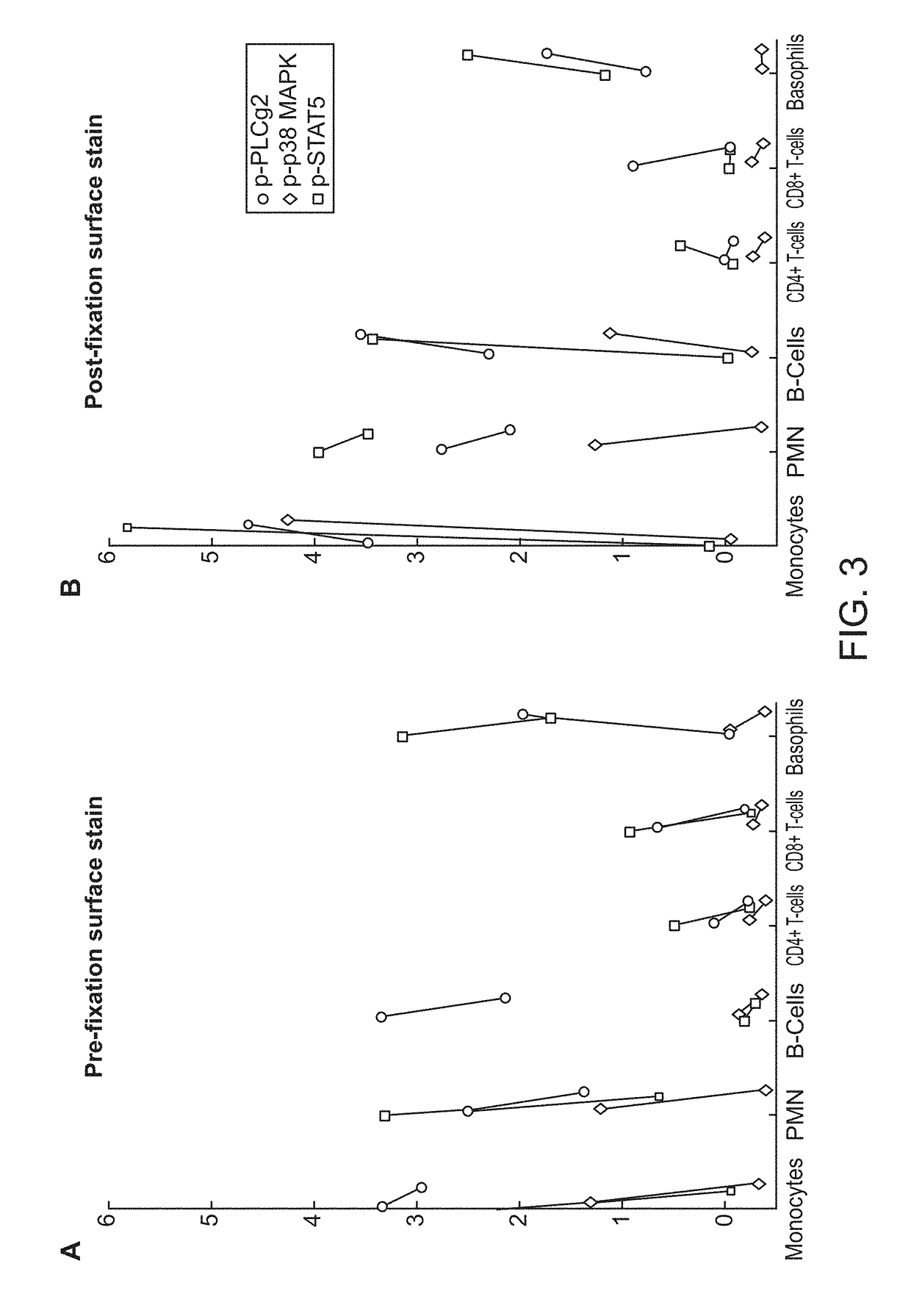
The present invention recognizes that current clinical laboratory testing methods for multiparametric single cell analysis are limited to analysis of intact live cells, and are insufficient for identification of signaling activation profile defining certain cell types, including but not limited to neoplastic and immunologically activated cell subsets. One aspect of the present invention generally relates to marker selection in panels to include proteins routinely assessed in standard FCM, while preferably also incorporating markers for surface receptor proteins within activated signaling cascades. A further aspect of the present invention generally relates to panel design for the following indications in neoplastic and non-neoplastic clinical applications as examples of the technology: (a) identification of CML progenitor cell subsets in the setting of disease recurrence after treatment discontinuation or relapse due to treatment resistance, and (b) characterization of activated basophils to predict the severity of an allergic response. Another aspect of the present invention generally relates to methods to measure levels of surface and IC biomarkers in separate or combined assays for robust characterization of each or select cell compartment, and data analysis based on results from each or all method(s) used for optimal detection of the markers. A further aspect of the present invention generally relates to the identification and profiling of cell subpopulations based on analysis of surface markers including those associated with lineage and maturation of cell types and receptor proteins, and the corresponding IC phosphoproteins including those in activated signaling cascades to predict certain disease states or response to treatment.
Owner:DEEPATH MEDICAL
Method for treating disorders of the skin
ActiveUS20130310309A1Symptoms improvedReduce severityCosmetic preparationsBiocideSerum rashSide effect
A topical pharmaceutical composition for treating a skin disorder selected from the group consisting of Herpes viral infection, Varicella viral infection, rash, insect bites, jellyfish stings, burns, psoriasis, itching, skin allergic response, skin lesions as a result of drug or medical treatment side effects or complications, and hypopigmantation. The composition comprises a peptide of the formula pGLU-X—Y—Z, where X, Y and Z are amino acids, with or without an alkyl group, and a pharmaceutically acceptable excipient.
Owner:S I S SHULOV INST FOR SCI LTD
Gab2 (p97) gene and methods of use thereof
InactiveUS20060265769A1Narrow downAvoid adjustmentPeptide/protein ingredientsNucleic acid vectorAntigenAllergic response
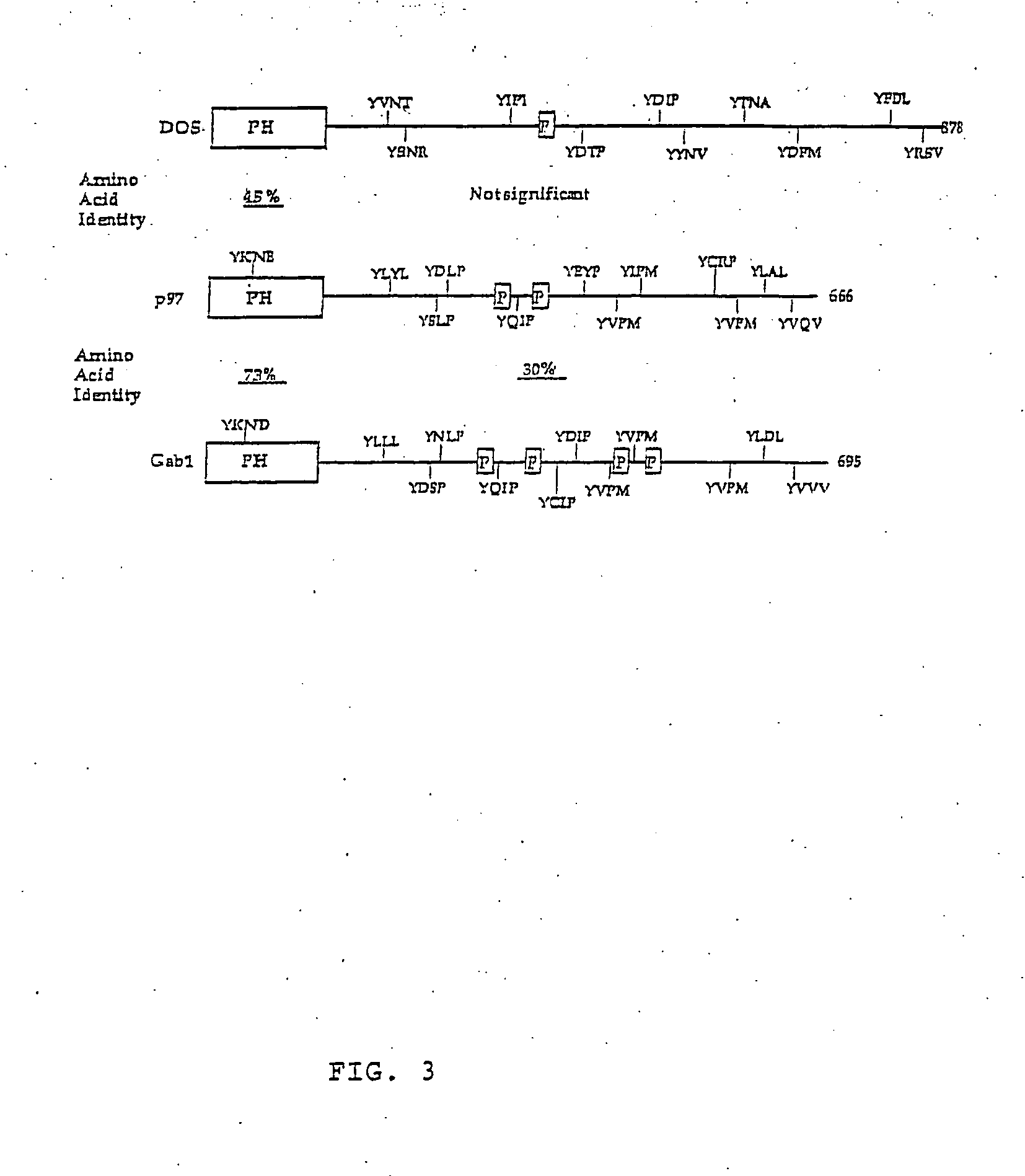
This invention relates to the purification, cloning and characterization of a novel gene, Gab2. In response to extracellular stimuli (e.g., cyokines, growth factors, hormones and antigens), Gab2 binds several signal relay molecules, including the protein-tyrosine phosphatase SHP-2 and phosphatidylinositol-3-OH kinase (PI-3K), which results in the initiation of multiple signaling cascades. Gab2 nucleic acid molecules, peptides, vectors, host cells, probes, antibodies, knockout and transgenic animals are provided. The invention also relates to methods of diagnosis, prevention and treatment of Gab2-mediated conditions such as allergic responses, neoplastic disorders and immune disorders. The invention further relates to diagnostic kits for disorders associated with altered Gab2 expression.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
FcE-PE chimeric protein for targeted treatment of allergy responses a method for its production and pharmaceutical compositions containing the same
InactiveUS20090082549A1Efficient killingUseful in therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsEscherichia coliDisease
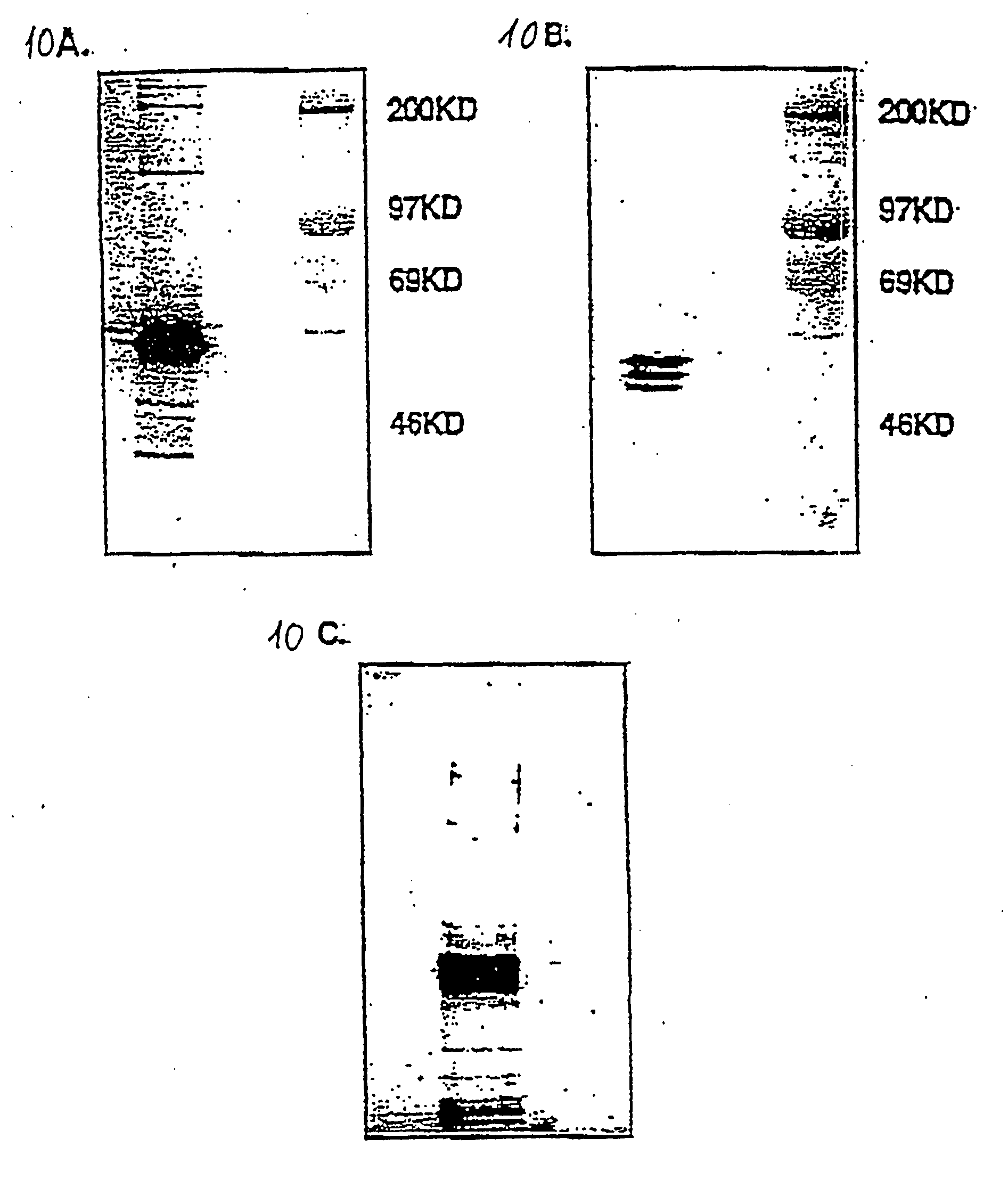
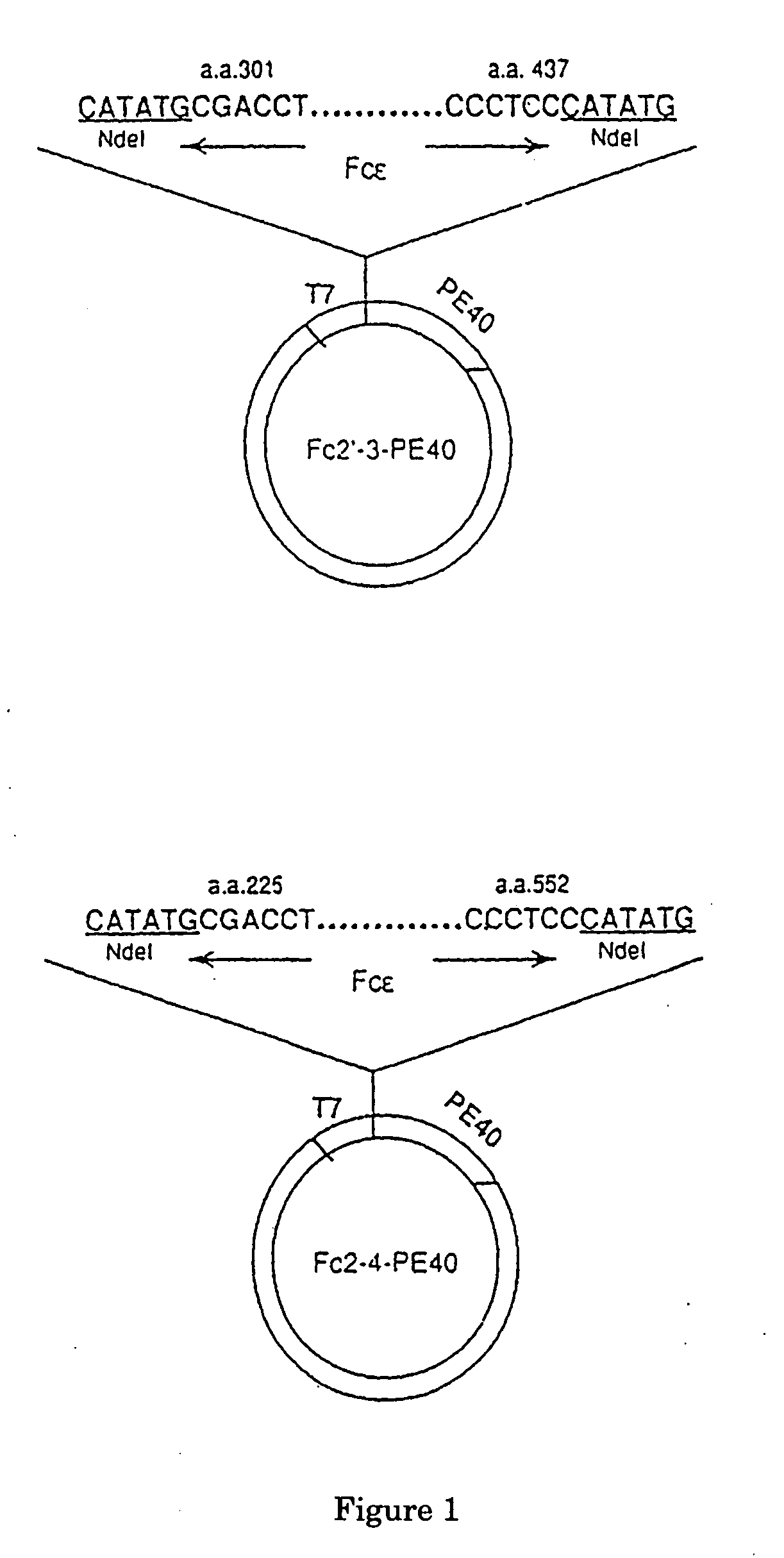
The present invention generally relates to a new approach for the therapy of allergic responses, based on targeted elimination of cells expressing the FcεRI receptor by a chimeric cytotoxin FC2′-3-PE40. A sequence encoding amino acids 301-437 of the Fc region of the mouse IgE molecule was genetically fused to PE40—a truncated form of PE lacking the cell binding domain. The chimeric protein, produced in E. coli, specifically and efficiently kills mouse mast cell lines expressing the FcεRI receptor, as well as primary mast cells derived from bone marrow. The present invention provides a chimeric protein for targeted elimination of FcεRI expressing cells especially useful for the therapy of allergic responses. The said chimeric protein is comprised of a cell targeting moiety for FcεRI expressing cells and a cell killing moiety. The preferred killing moiety is the bacterial toxin Pseudomonas exotoxin (PE). This Pseudomonas exotoxin is a product of Pseudomonas aeruginosa. The present invention also relates to a method for the preparation of said protein. This chimeric protein is prepared by genetically fusing the Fc region of the mouse IgE molecule to PE40, a truncated form of PE lacking the cell binding domain. The present invention also provides pharmaceutical compositions, for the treatment of allergic diseases and for the treatment of hyperplasias and malignancies, comprising as an active ingredient the above mentioned chimeric protein and a conventional adjuvant product.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
An antibacterial antiinflammation pain-relieving tissue-regenerating medicine, its preparation method and use
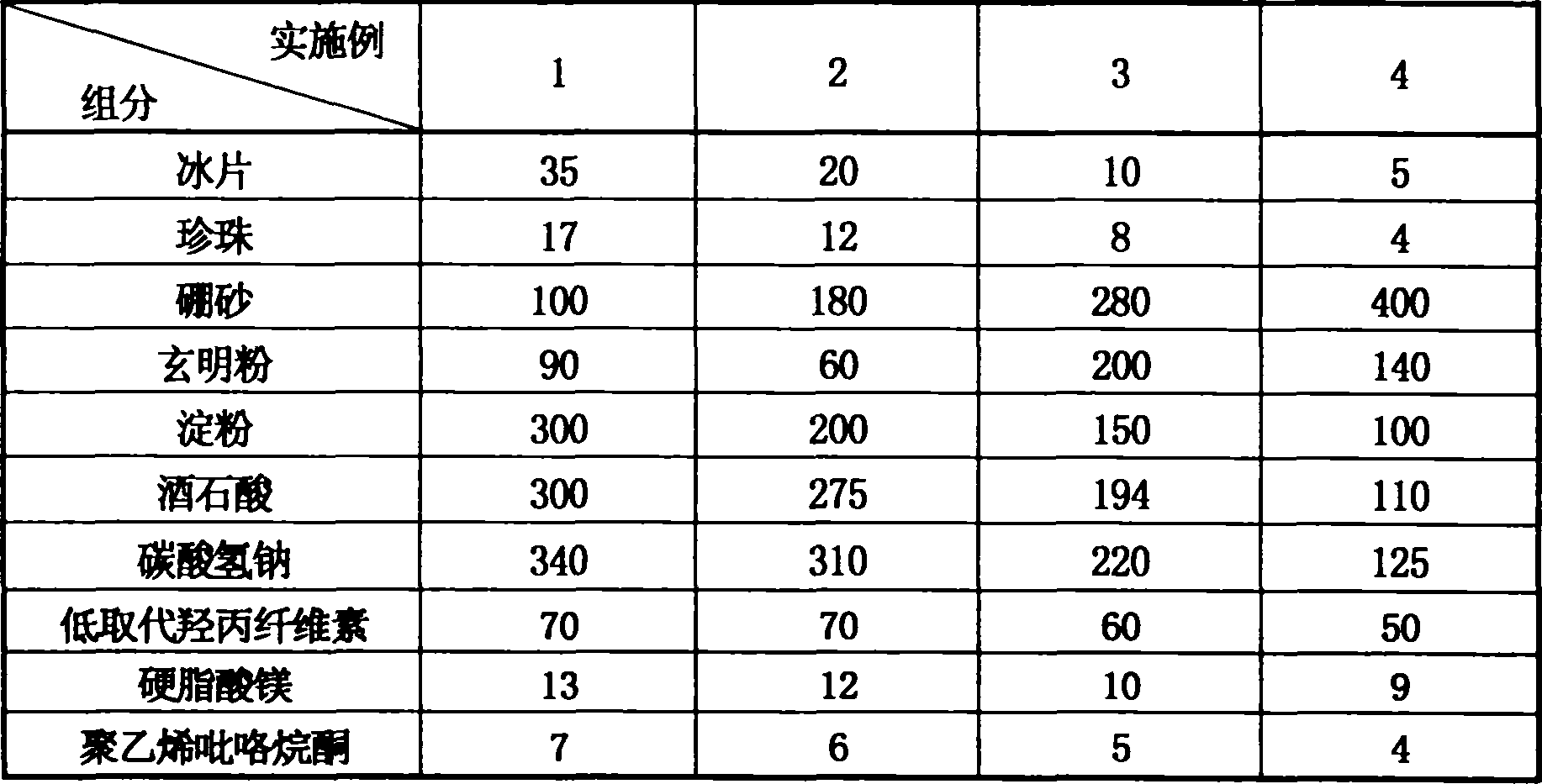
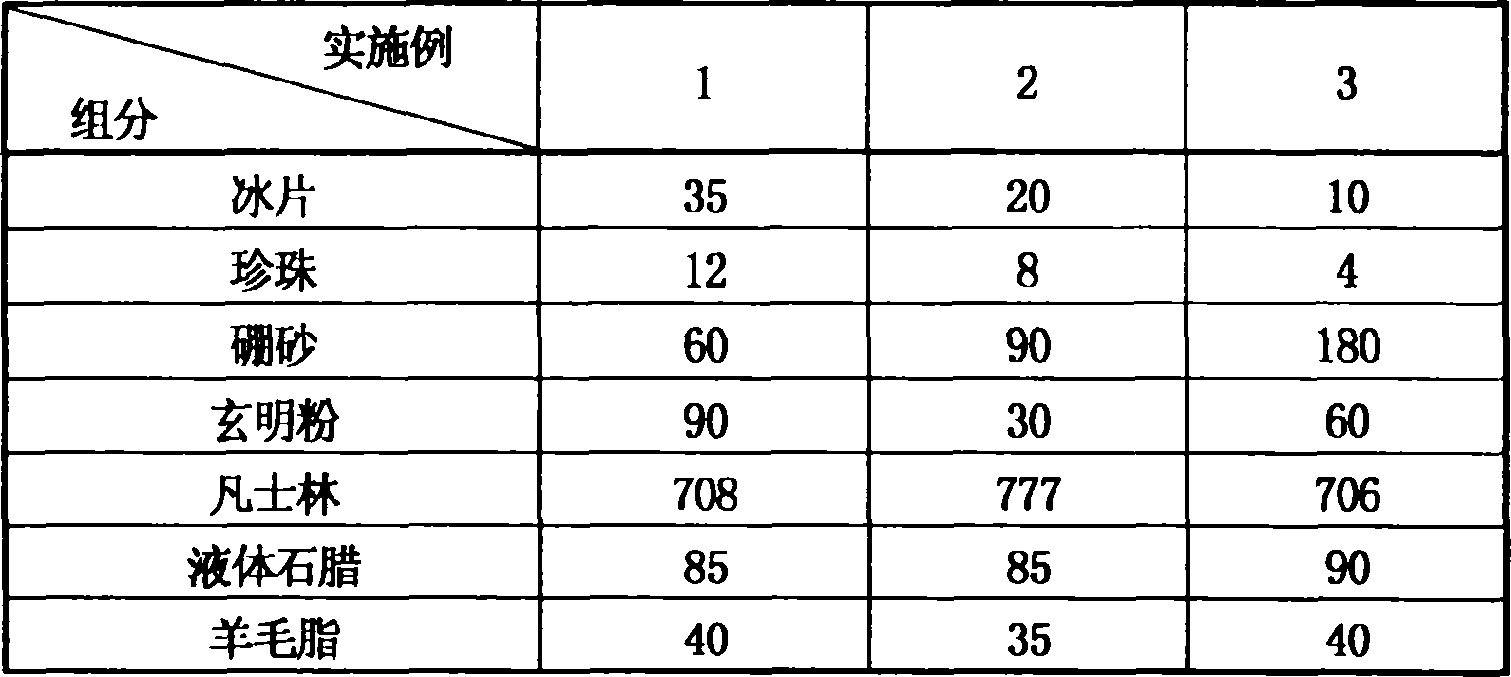
ActiveCN1879654AEvidence clearReasonable compositionInorganic boron active ingredientsHydroxy compound active ingredientsEffervescent tabletAllergic response
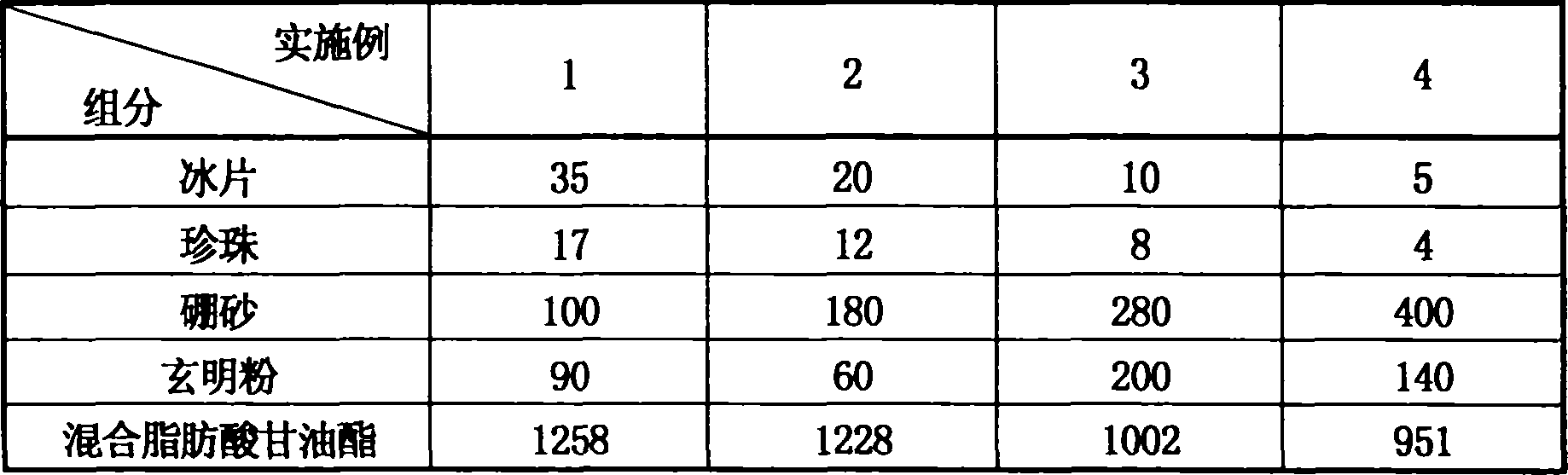
Disclosed is an antibiotic medicament for diminishing inflammation and reducing pain, its process and use, wherein the medicament is prepared from boneol, pearl powder, borax and compound of glauber-salt and liquorice through the steps of drying, disintegrating, grinding, sieving and mixing. The medicament can be prepared into suppository, effervescent tablet, soft electuary and coating film.
Owner:王伟东 +1
Mimotopes for use in immunotherapy for shellfish and/or arthropod allergy
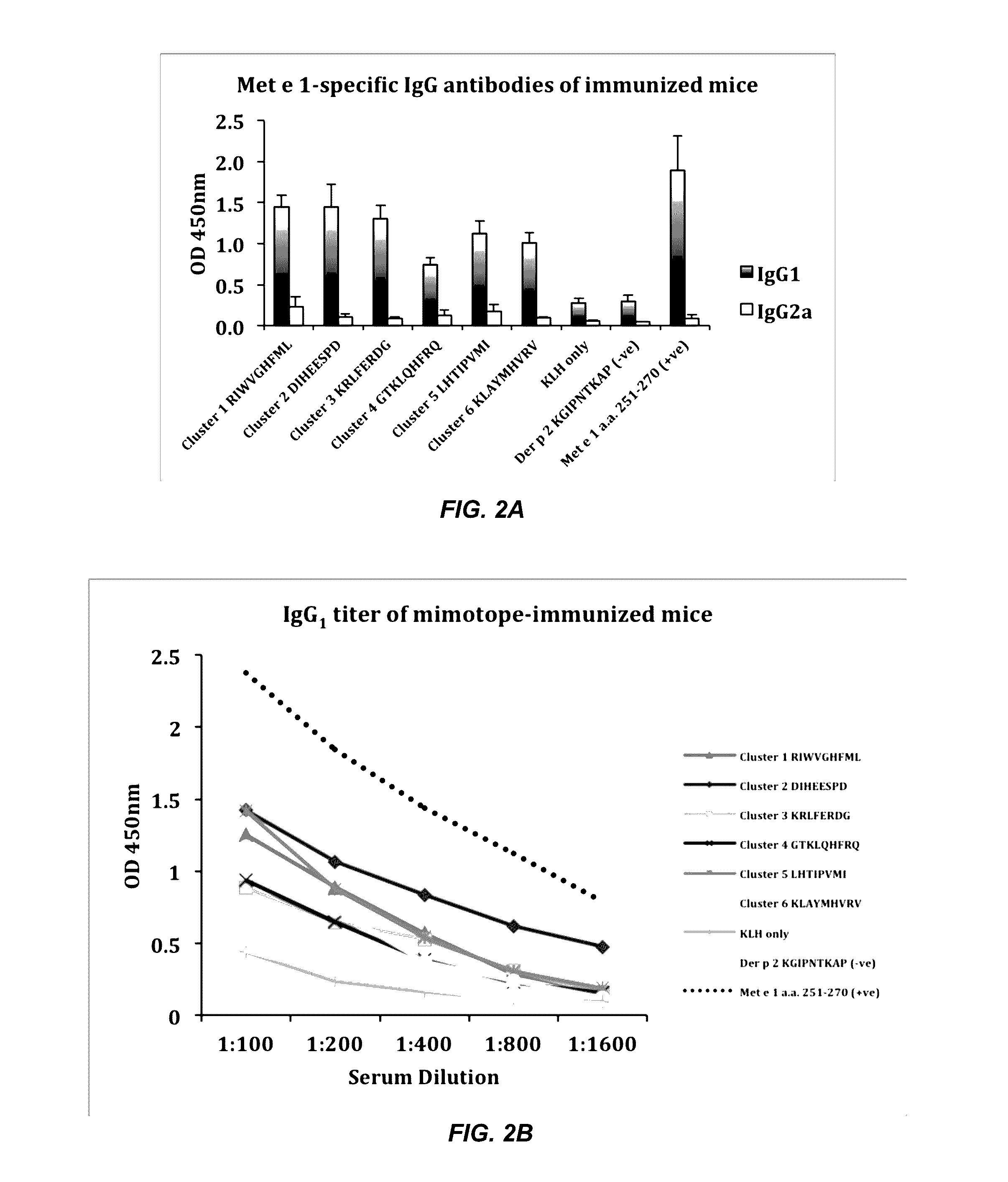
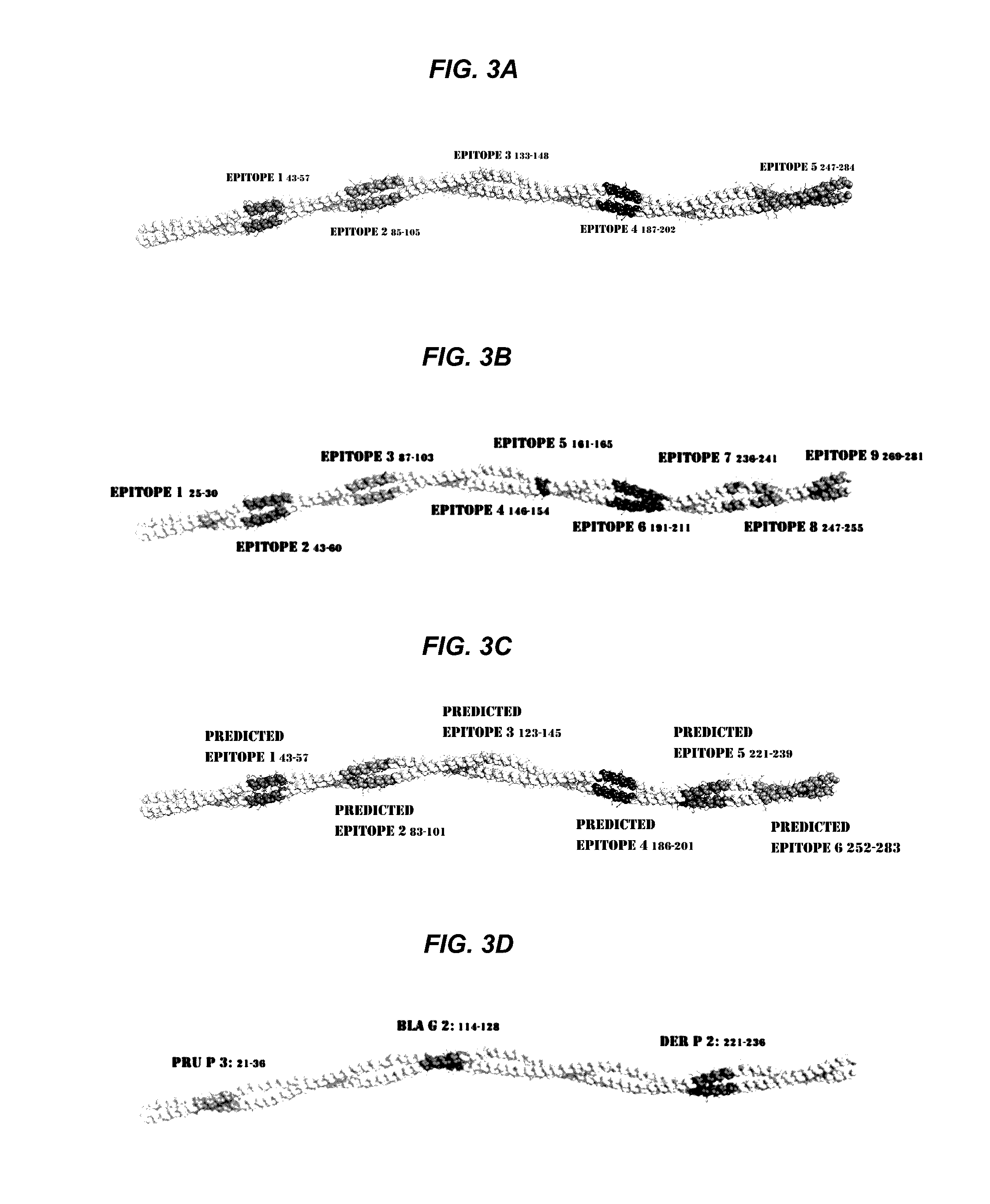
The present invention provides isolated peptides and nucleic acids encoding the isolated peptides that can modify a subject's immune response to tropomyosin. The isolated peptides correspond to peptide epitope mimics that are based on an invertebrate tropomyosin allergen, e.g., the shrimp tropomyosin Met e 1. Also provided are compositions and methods of use thereof to reduce, minimize or eliminate an allergic response to arthropods and / or shellfish.
Owner:RGT UNIV OF CALIFORNIA +1
Use of winged euony twigs in preparing anti-IV type allergy medicine
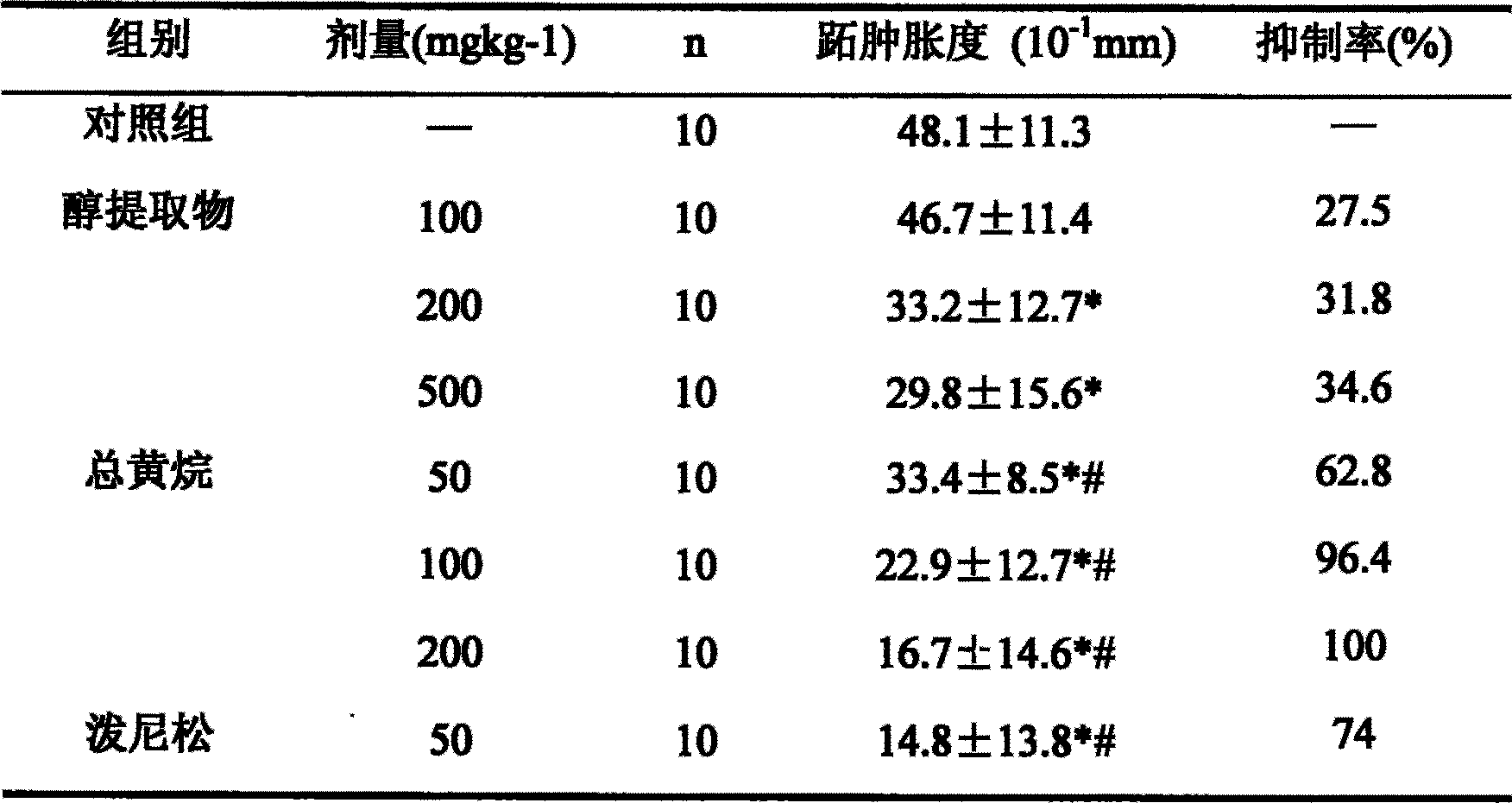
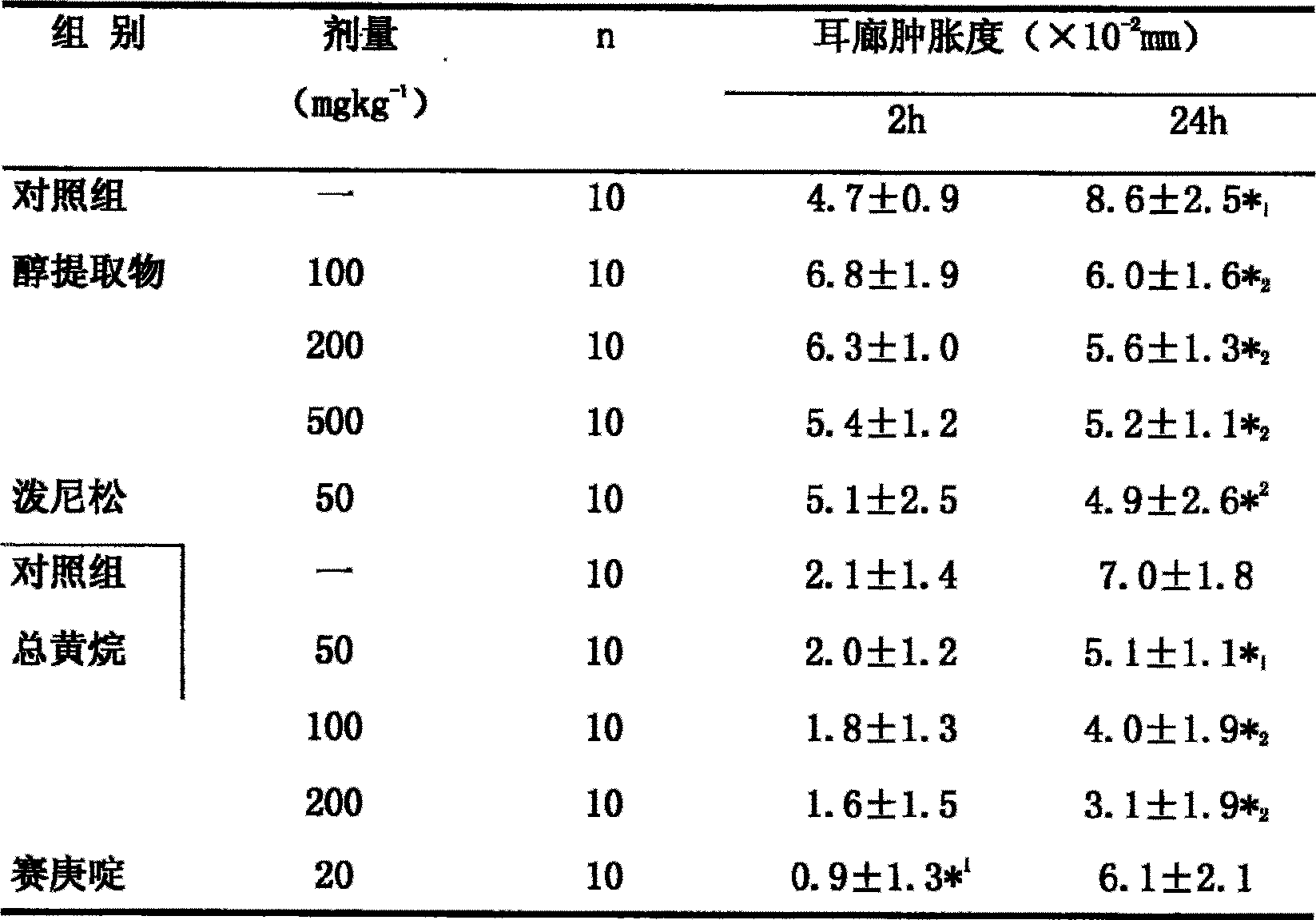
The invention relates to the medical technique field and discloses an application for preparing IV-type allergic response resistance medicament of an extract of ramulus euonymi which is a celastraceae plant. The animal models (sheep red blood cell causing mice delayed type pedal swelling, olive oil causing mice dermatitis contacta, 5-HT causing rat mastocyte in abdominal cavity releasing histamine, 5-HT inducing guinea pig ileum shrinking) which are broadly used for screening IV-type allergic response resistance in at home and abroad at present, verifies that the extract of the ramulus euonymi can obviously inhibit the mice delayed type pedal swelling caused by sheep red blood cells, the mice dermatitis contacta caused by olive oil, the rat mastocyte in abdominal cavity releasing histamine caused by 5-HT and the guinea pig ileum shrinking caused by 5-HT, thereby proving that the ramulus euonymi has exact effect in resisting IV-type allergic response, and therefore the ramulus euonymi can be used for preparing IV-type allergic response resistance medicament.
Owner:黄德斌 +2
T cell epitopes from cockroach and methods of making and using same
ActiveUS20150110819A1Reduce riskProvide protectionBacteriaPeptide/protein ingredientsDiseaseAllergic response
The invention provides Cockroach proteins, peptides, subsequences, portions, homologues, variants and derivatives thereof, and methods and uses and medicaments of such proteins, peptides, subsequences, portions, homologues, variants and derivatives thereof. Such methods, uses and medicaments include modulating an immune response, protecting a subject against or treating a subject for an allergic response, allergic disorder or allergic disease and inducing immunological tolerance to the allergen (e.g., Cockroach allergen) in a subject.
Owner:LA JOLLA INST FOR ALLERGY & IMMUNOLOGY
Anti-allergy composition and related method
ActiveUS20060134236A1Reduce compositionMethod reduceBiocideOrganic active ingredientsAdditive ingredientAllergic response
An composition and related method that prevents, inhibits and / or mitigates an allergic response by down regulating the production of IgE, down regulating the binding of IgE antibodies to receptors on cells, and / or inhibiting allergy mediators, for example, histamine, prostoglandin D2, or luekotriene C4 release. The composition comprises at least one of the following ingredients: luteolin from Perilla leaf or seed, Cinnamon, Kiwi, Picao preto, Hesperidin, Acerola cherry, Guaco, Holy Basil, Kakadu, Solamum, Rosmarinic acid, Tinospora and Aframomum. In one embodiment, the composition at least three different ingredients selected from Cinnamon, Acerola, Luteolin and Picao preto. Optionally, these ingredients can be combined with at least one of Aframomum, Rosmarinic acid, and Tinospora. The composition is administered with effective amounts to prevent, inhibit and / or mitigate allergic responses.
Owner:ACCESS BUSINESS GRP INT LLC
Method for treating disorders of the skin
A topical pharmaceutical composition for treating a skin disorder selected from the group consisting of Herpes viral infection, Varicella viral infection, rash, insect bites, jellyfish stings, burns, psoriasis, itching, skin allergic response, skin lesions as a result of drug or medical treatment side effects or complications, and hypopigmantation. The composition comprises a peptide of the formula pGLU—X—Y—Z, where X, Y and Z are amino acids, with or without an alkyl group, and a pharmaceutically acceptable excipient.
Owner:S I S SHULOV INST FOR SCI LTD
Method for Determining an Allergic Response
InactiveUS20080145871A1Prevent anaphylactic shockSuitable for useTesting eggsArtificial cell constructsSensitized cellAllergic response
The present invention concerns a method for determining an allergic response by determining the extent of degranulation of human IgE sensitized cells upon activation by allergens in food products.
Owner:NV NUTRICIA
Methods Of Determining Allergen Response Using Microarray Immunoassay Techniques
The present invention is directed to materials and methods that may be used in diagnosing and / or characterizing allergies. More specifically, the specification describes methods and compositions for making and using a plurality of peptides having allergen epitopes that may be used in immunoassays e.g., microarray based immunoassays to predict the severity of an allergic response.
Owner:MT SINAI SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com