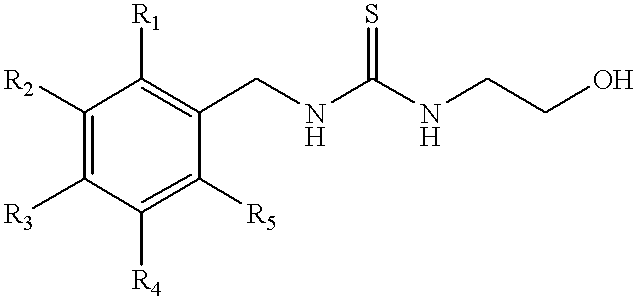
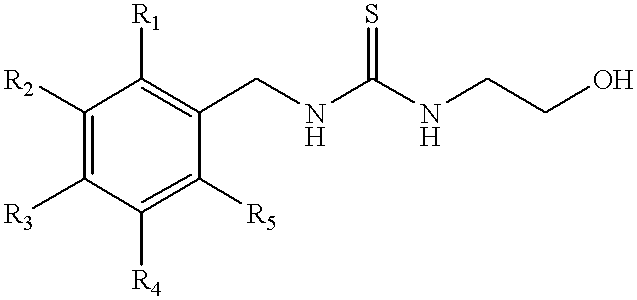
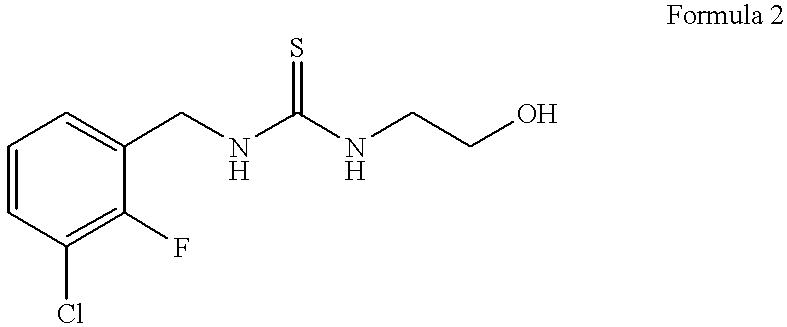
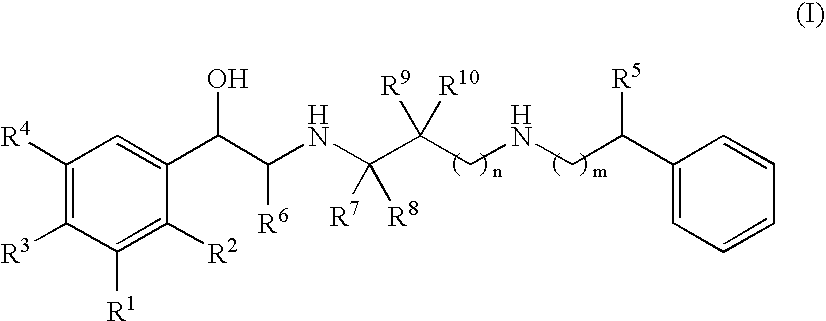
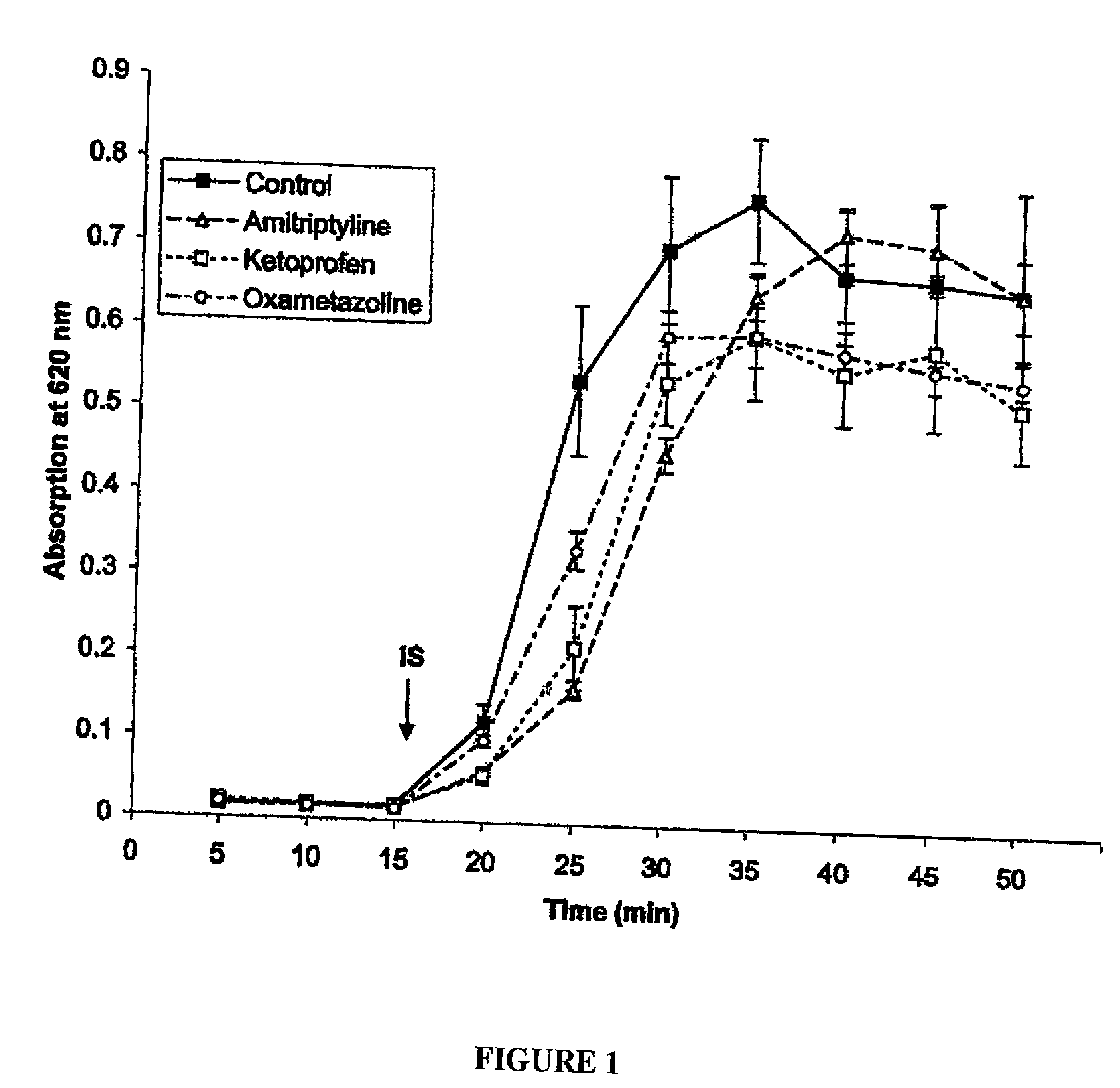
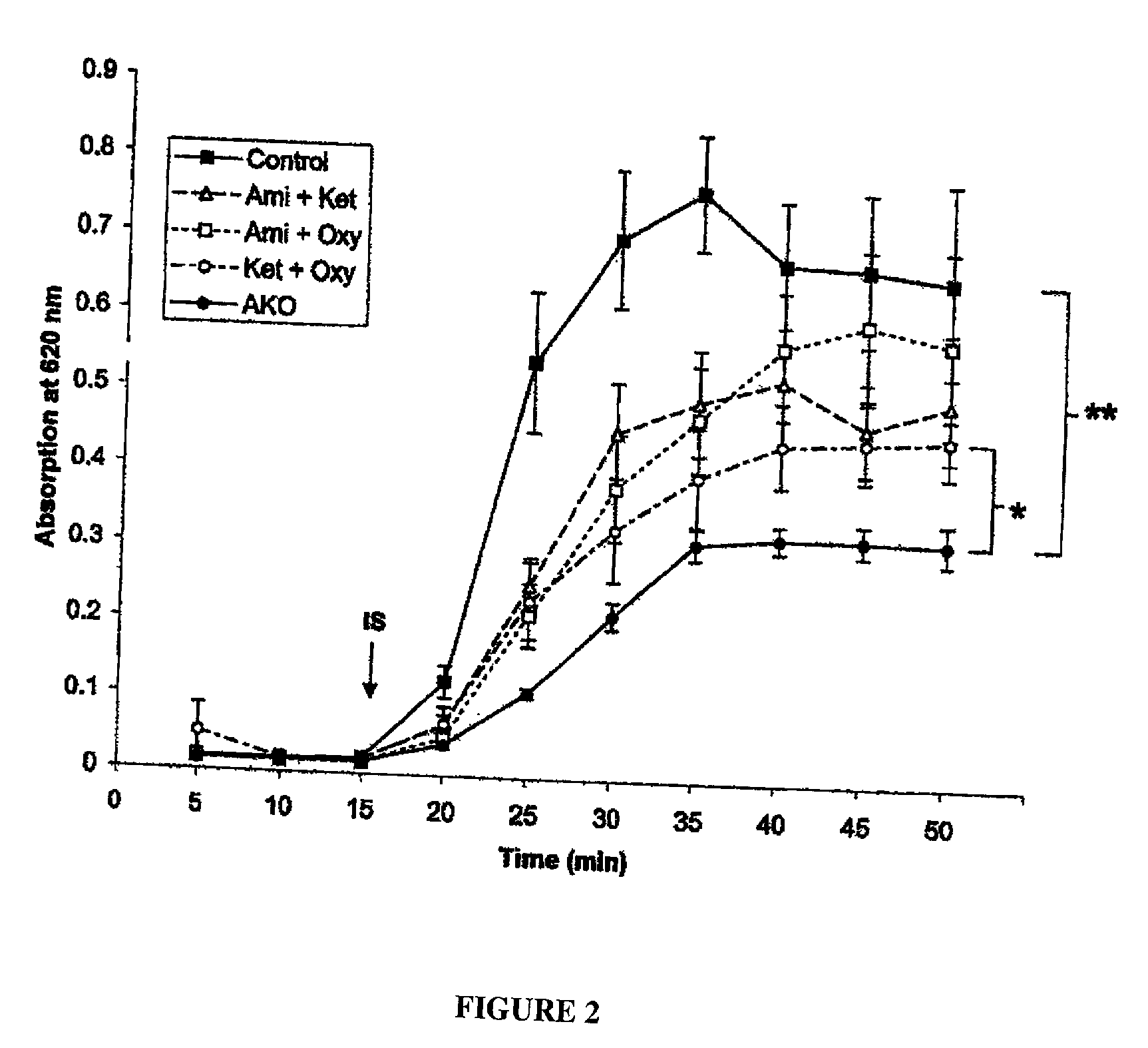
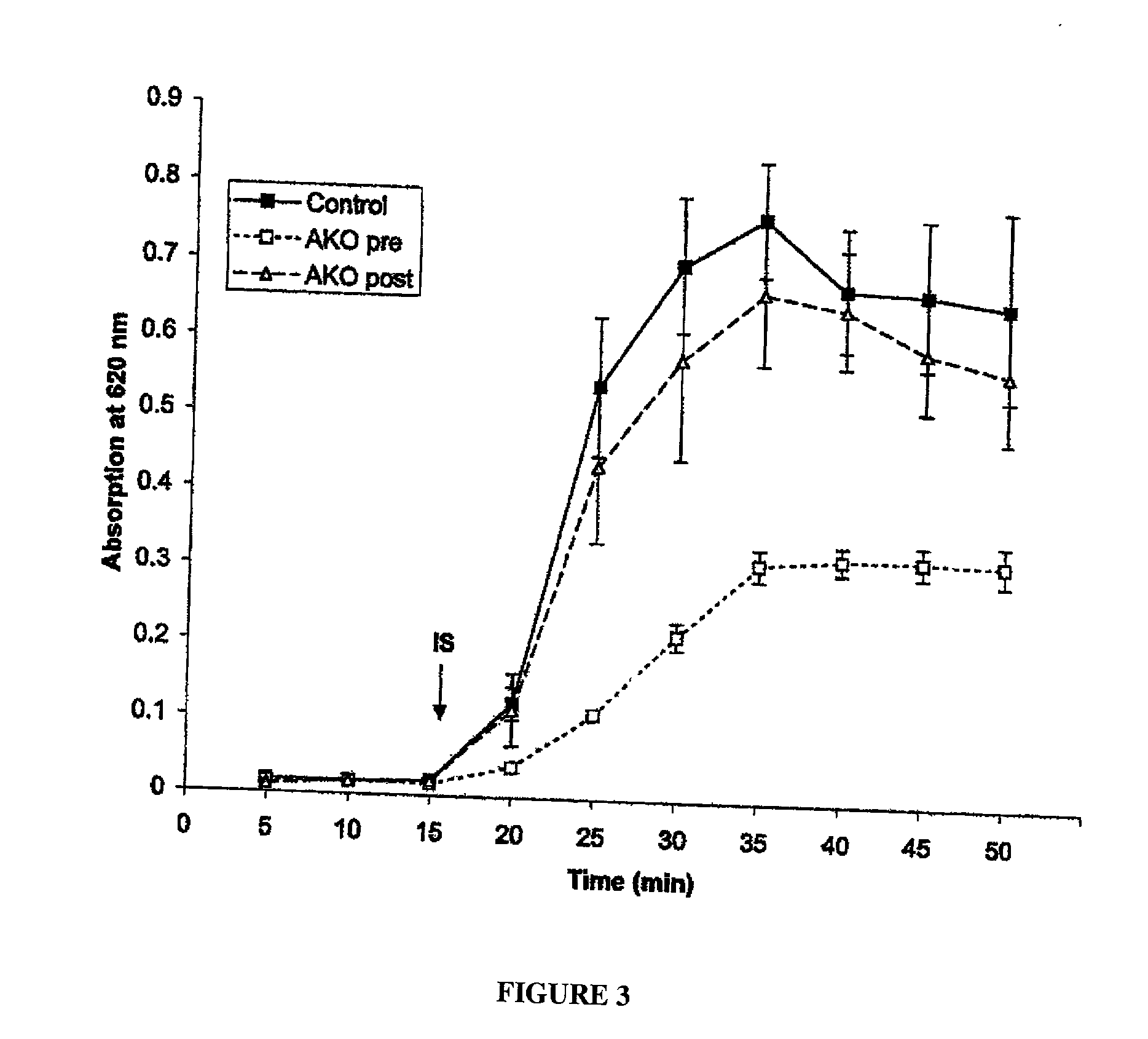
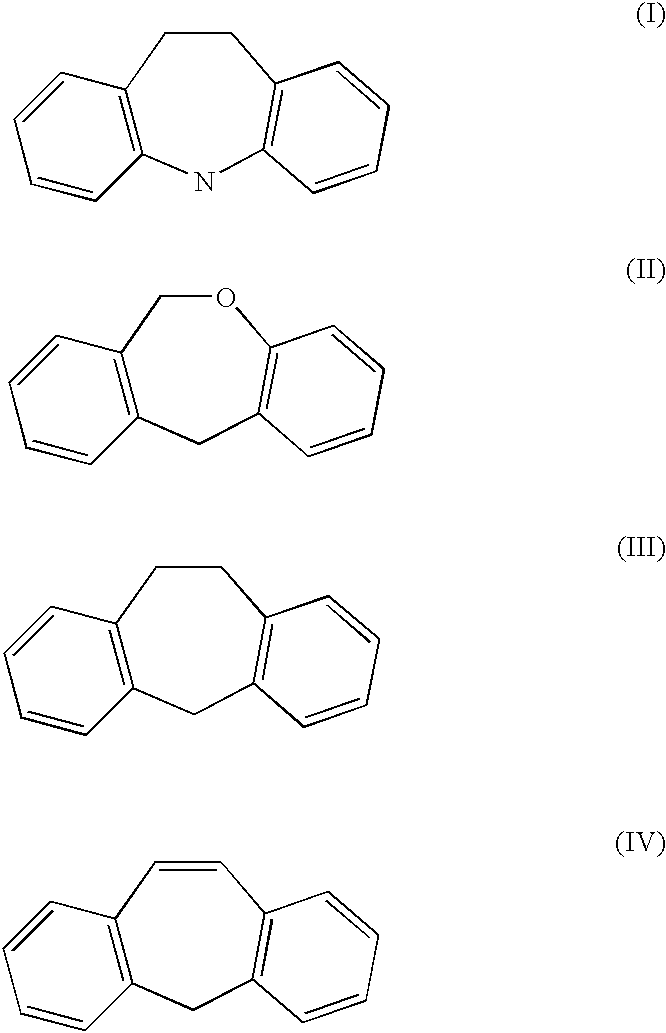
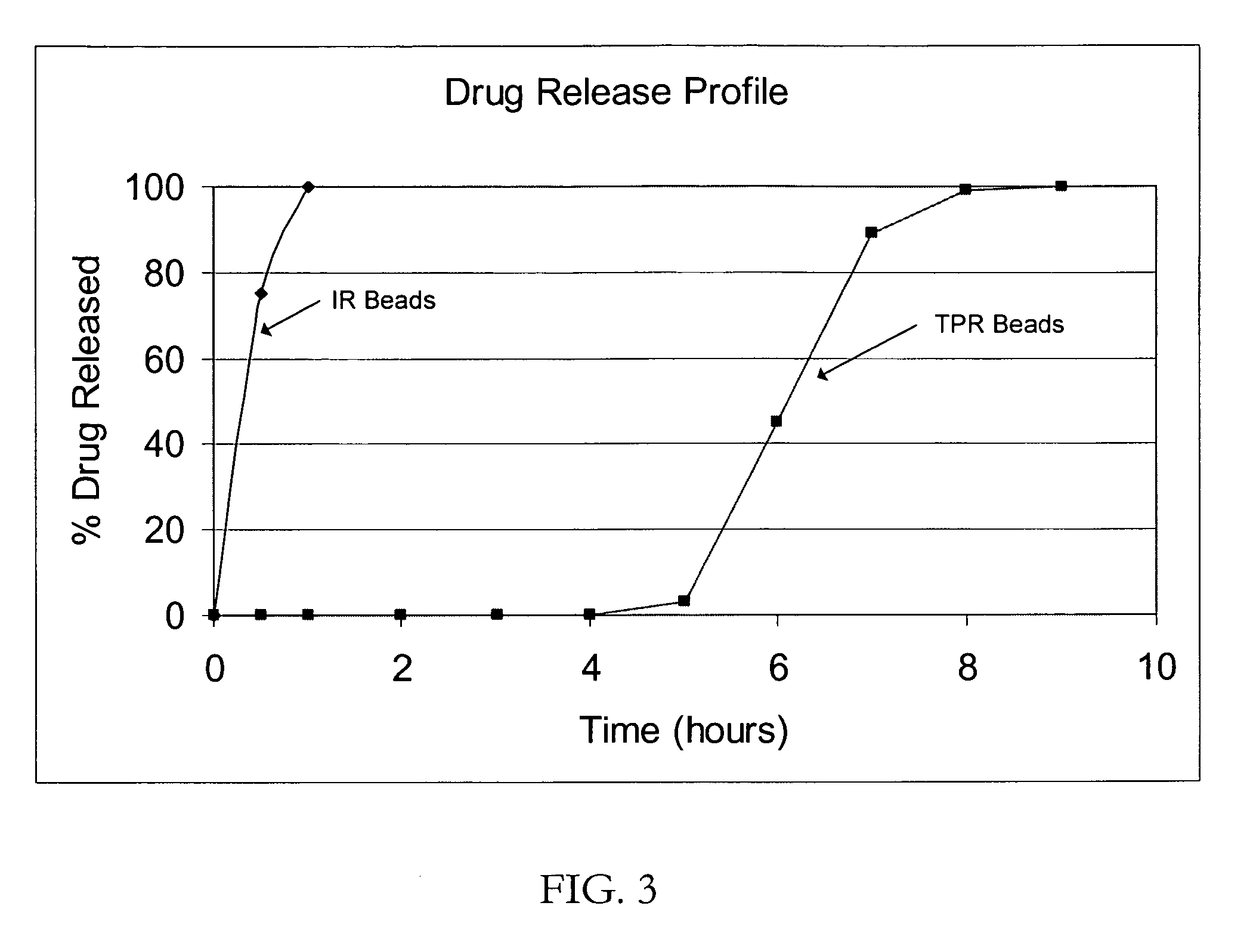
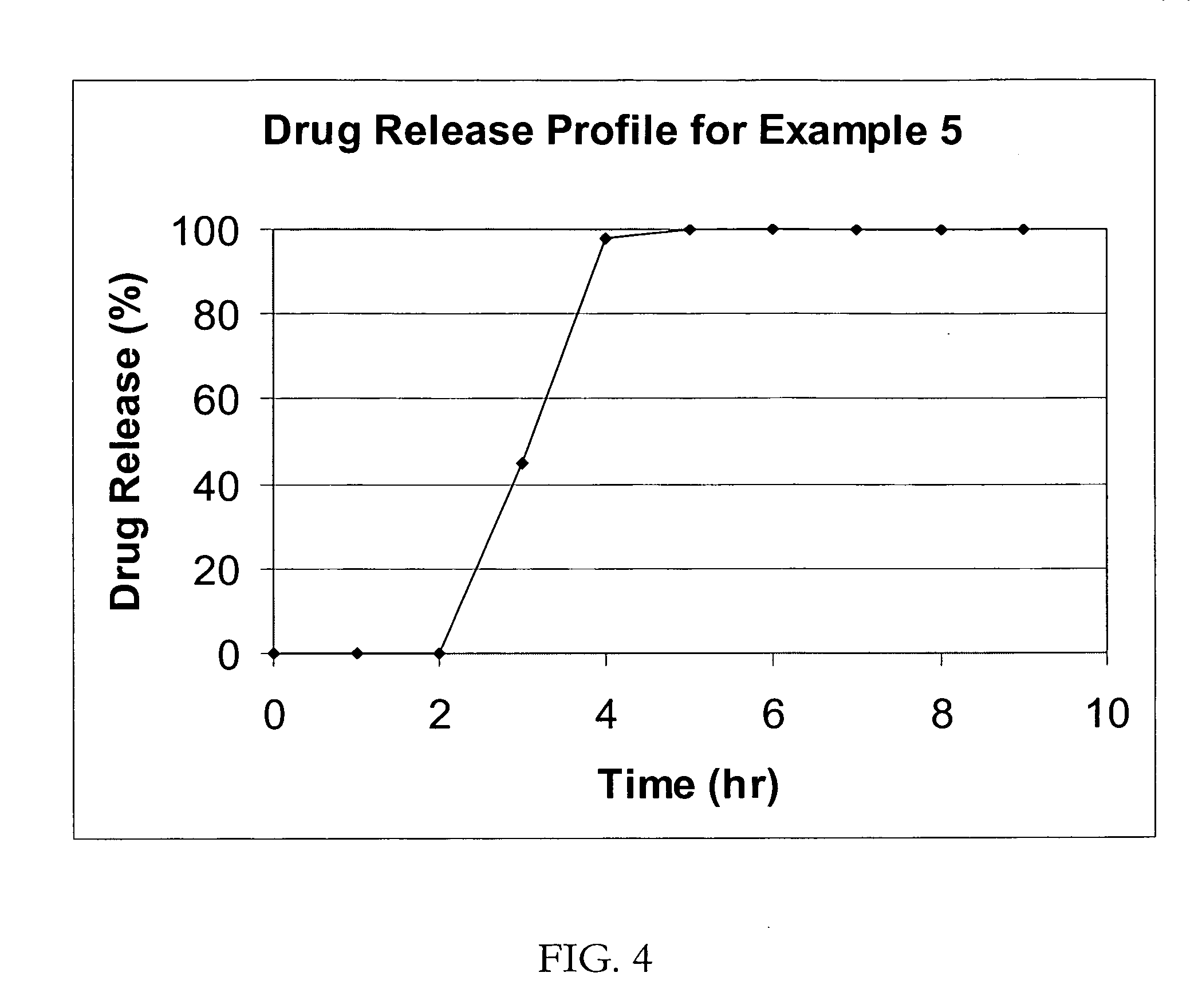
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1101 results about "Adrenergic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adrenergic means "working on adrenaline (epinephrine) or noradrenaline (norepinephrine)".
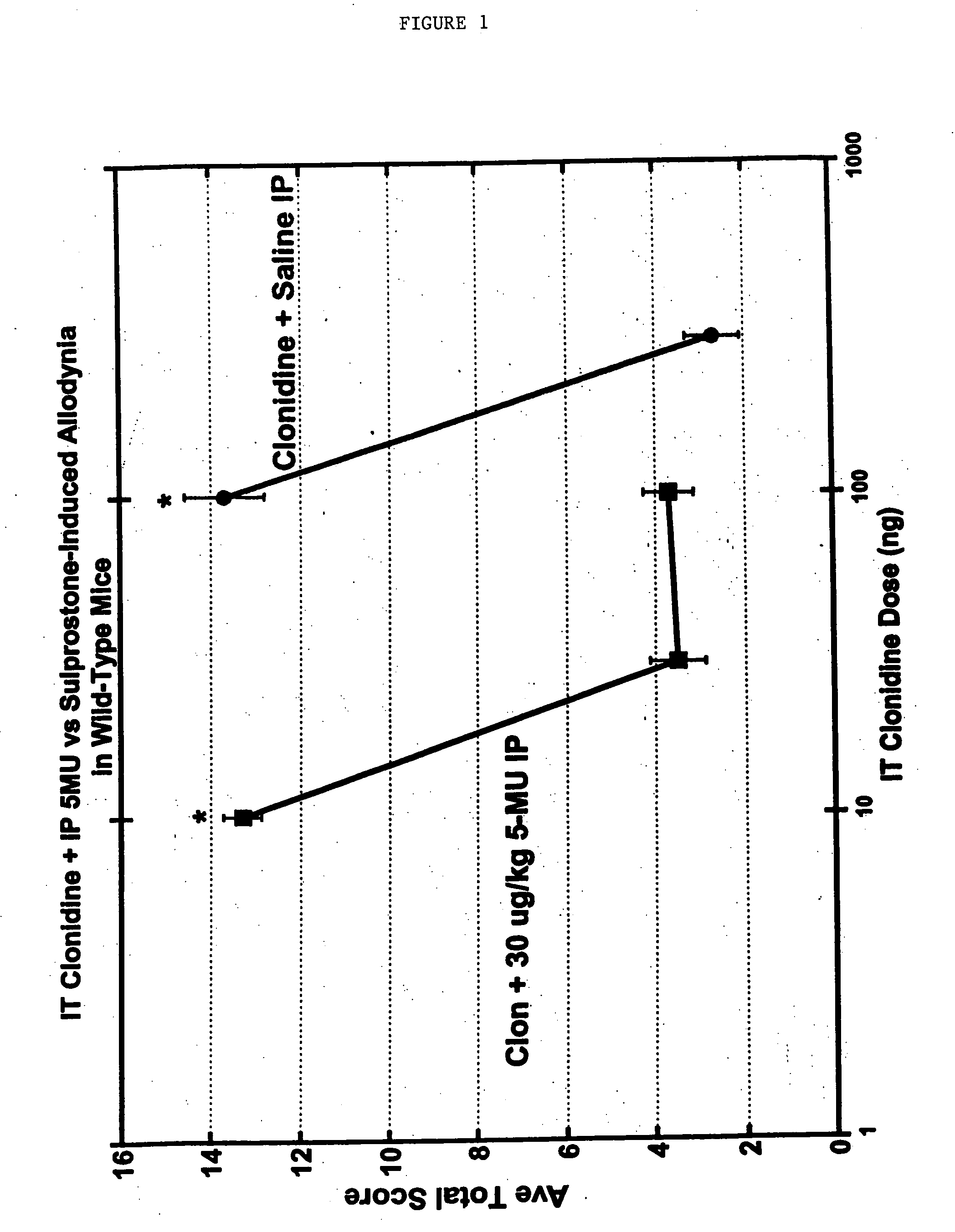
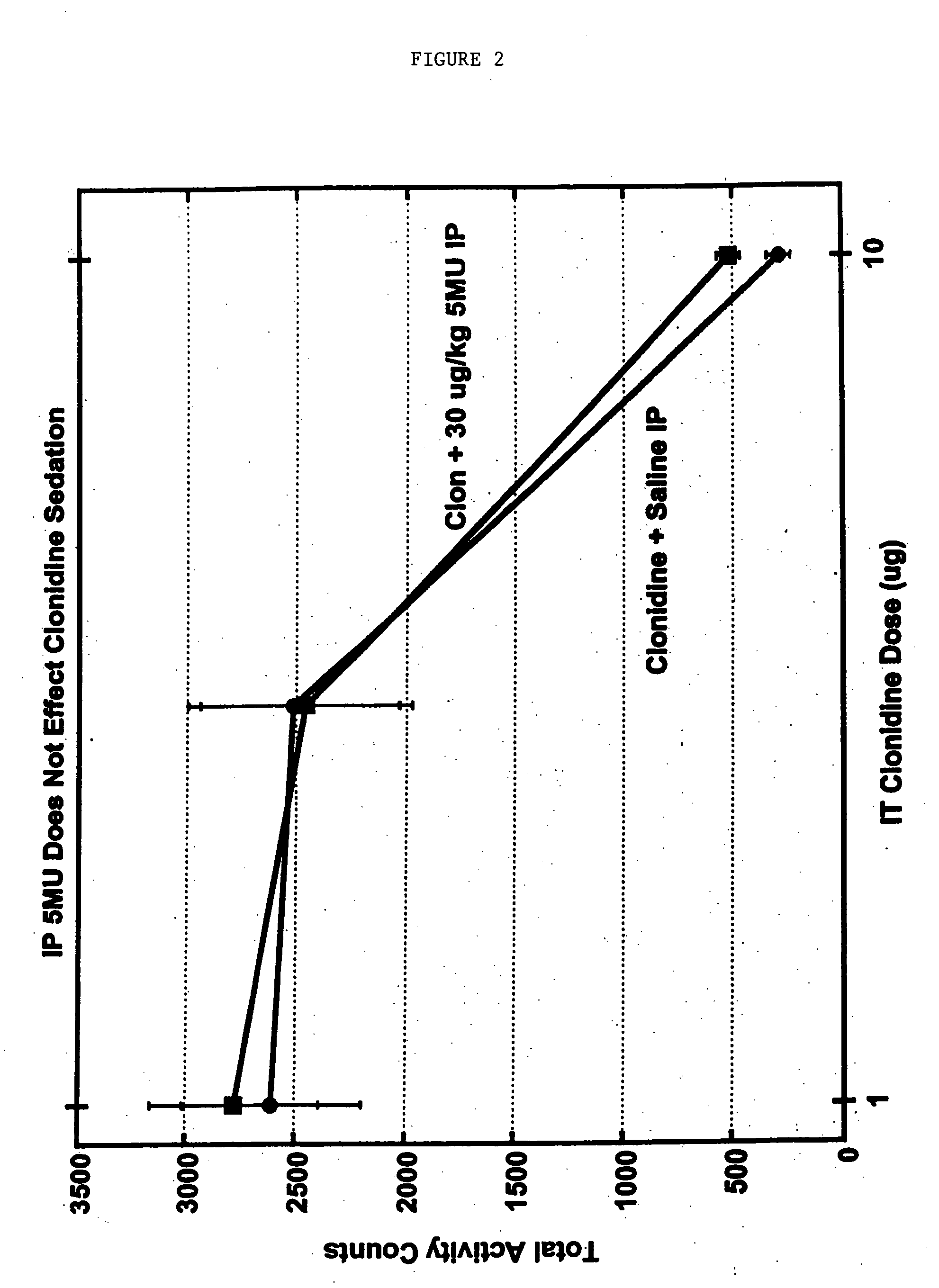
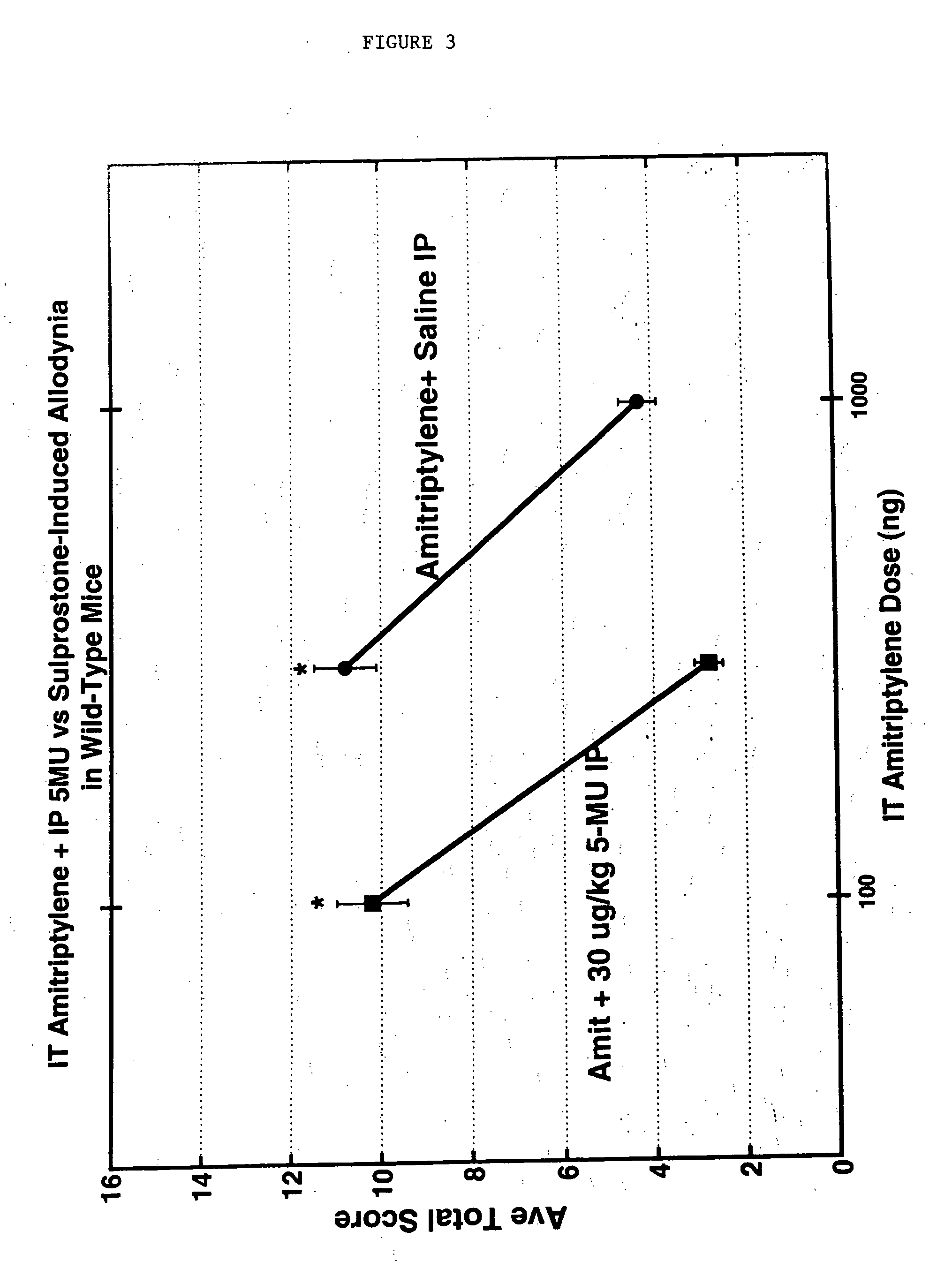
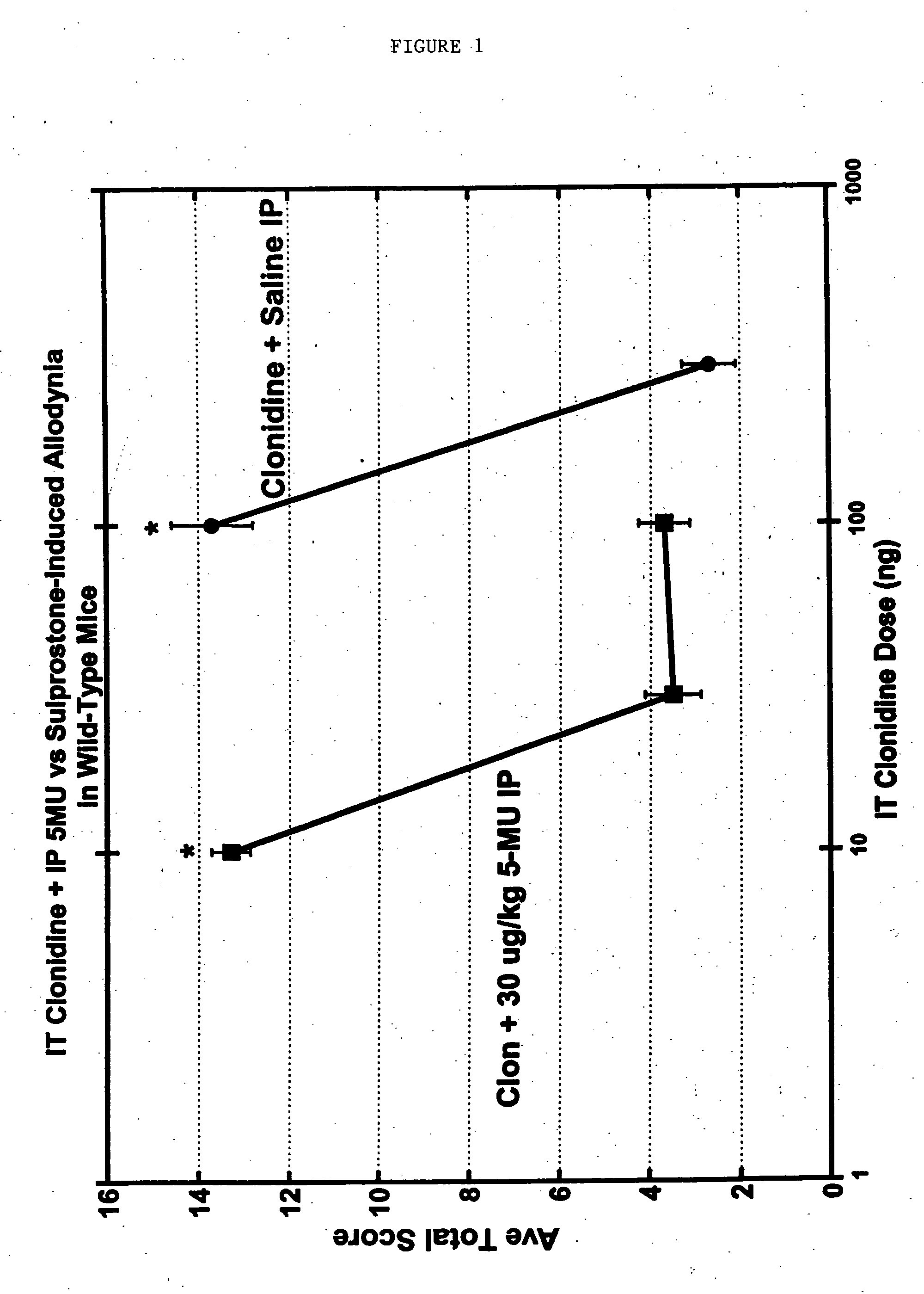
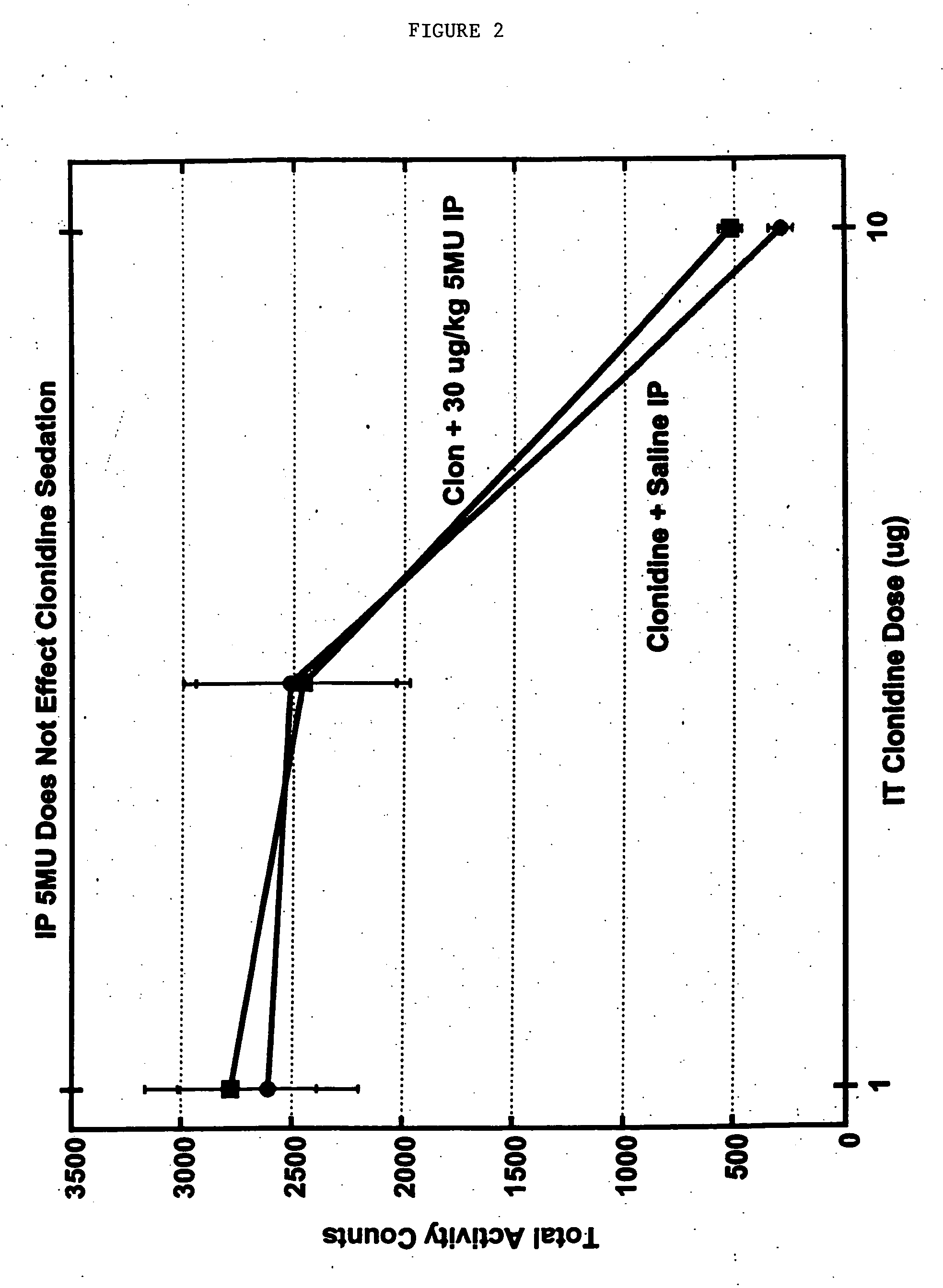
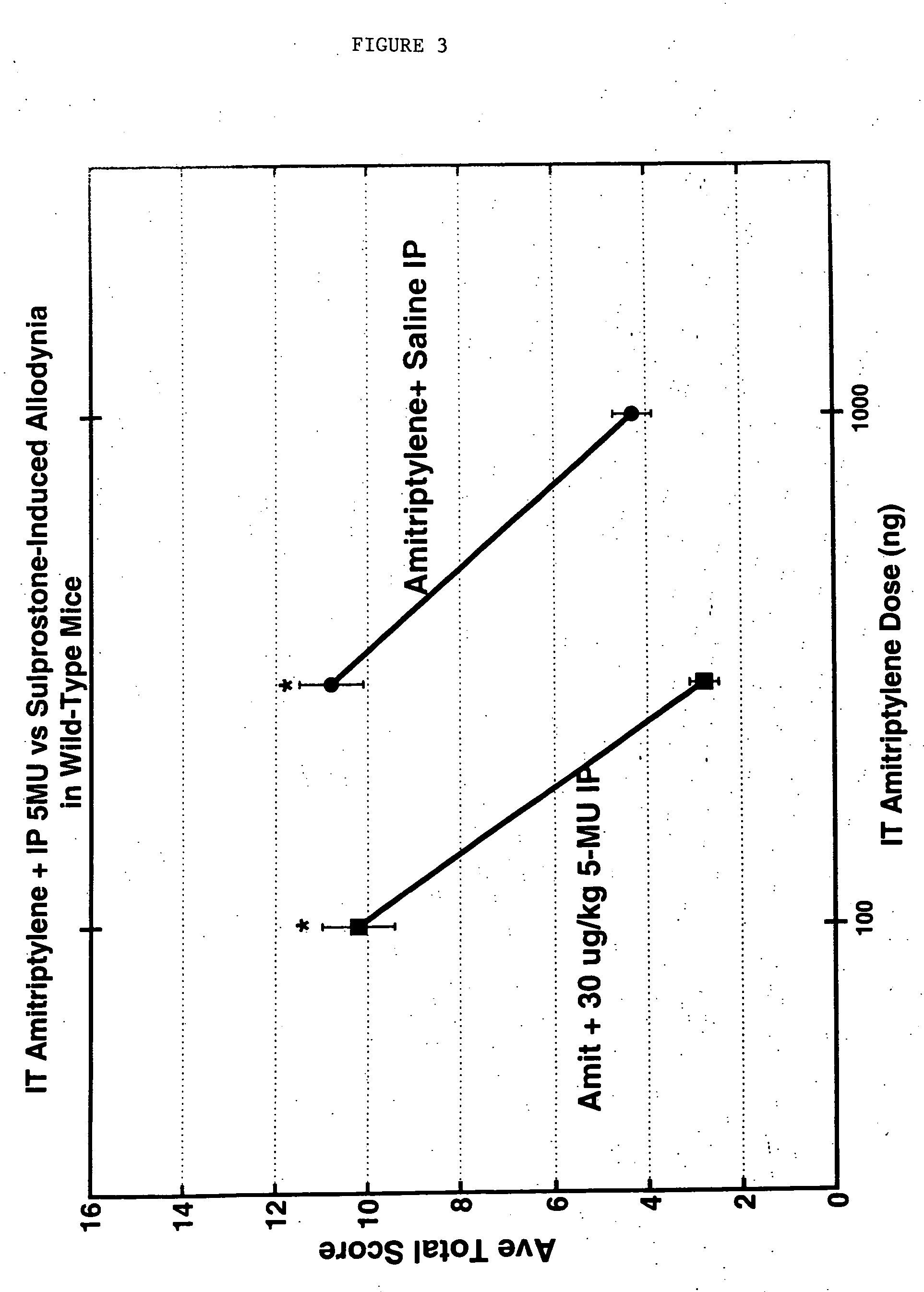
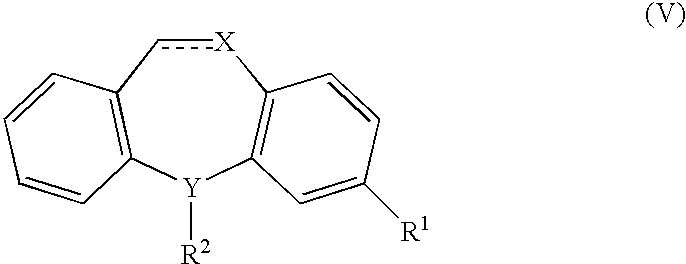
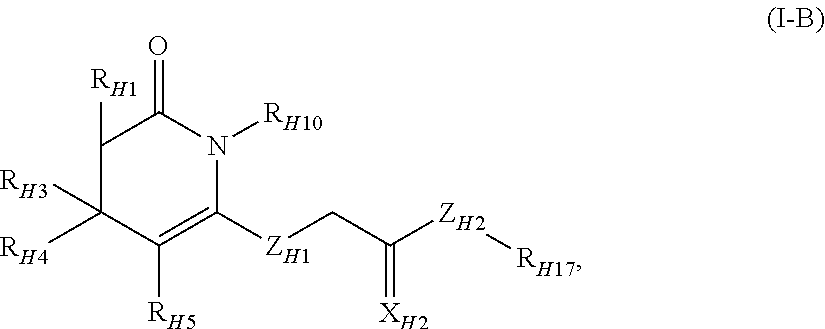
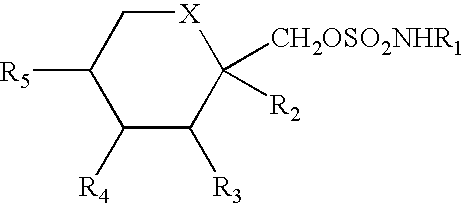
Methods and compositions for the treatment of pain and other alpha 2 adrenergic-mediated conditions
InactiveUS20050059744A1Improve effectivenessHigh activityBiocideOrganic active ingredientsSedative EffectsSide effect
Owner:ALLERGAN INC
Dimeric small molecule potentiators of apoptosis
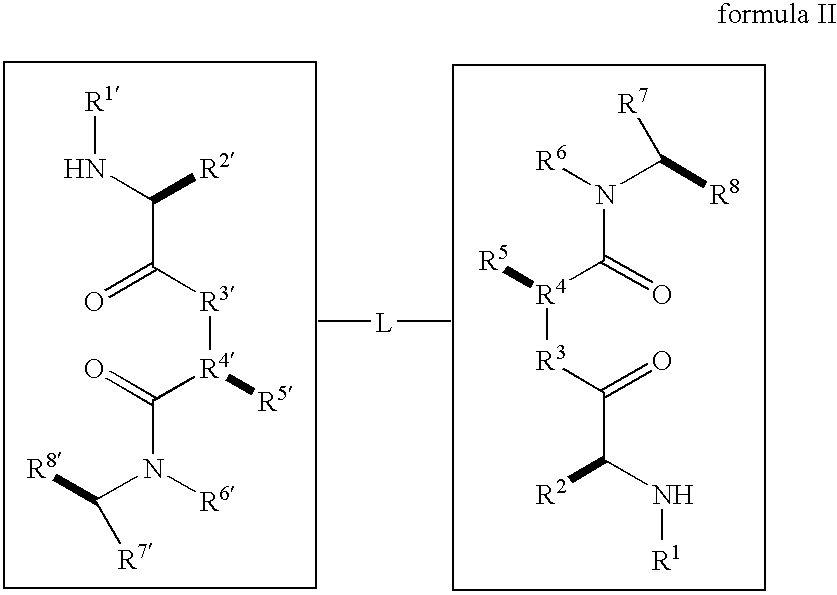
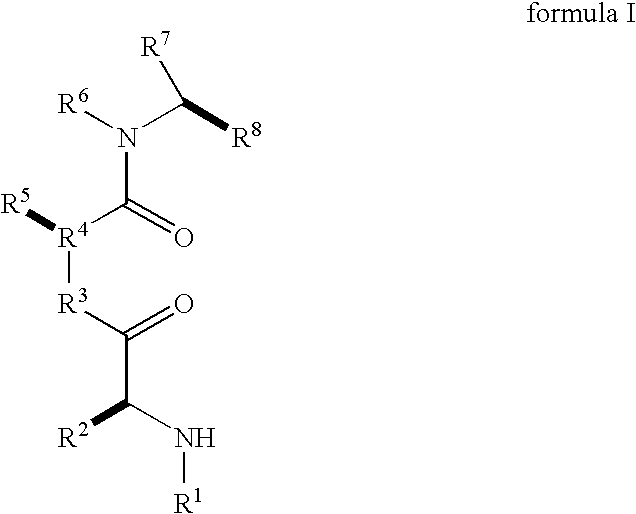
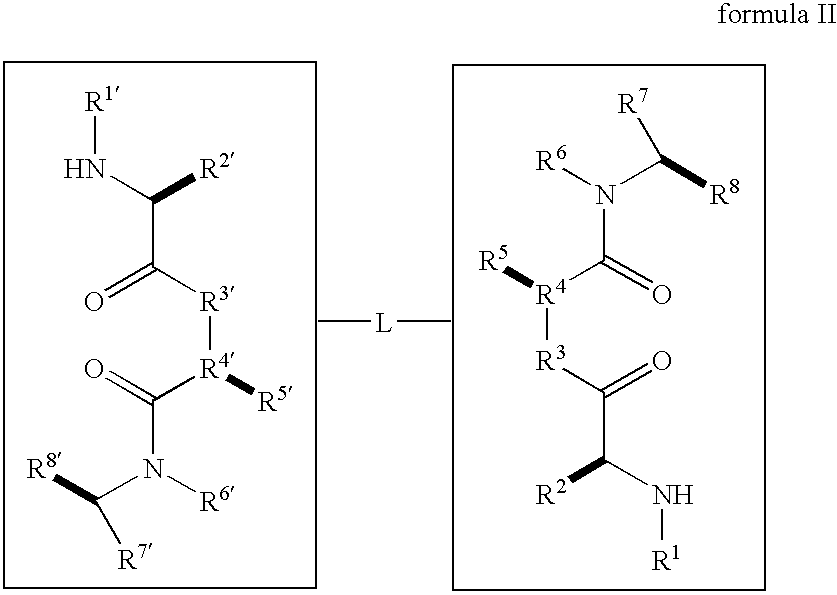
Caspase activity and apoptosis are promoted using active, dimeric Smac peptide mimetics of the general formula M1-M2, wherein moieties M1 and M2 are monomeric Smac mimetics and L is a covalent linker. Target cancerous or inflammatory cells are contacted with an effective amount of an active, dimeric Smac mimetic, and a resultant increase in apoptosis of the target cells is detected. The contacting step may be effected by administering to a pharmaceutical composition comprising a therapeutically effective amount of the dimeric mimetic, wherein the individual may be subject to concurrent or antecedent radiation or chemotherapy for treatment of a neoproliferative pathology.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods of treating cutaneous flushing using selective alpha-2-adrenergic receptor agonists
InactiveUS20050020600A1Effective treatmentTreatment safetyBiocidePharmaceutical delivery mechanismQuinoxalineVasomotor instability
The present invention relates to a method of treating, reducing, inhibiting, preventing and / or reversing cutaneous facial flushing caused by abnormal, endogenously-induced vasomotor instability associated with, but not limited to acne rosacea, menopause-associated hot flashes, hot flashes resulting from orchiectomy or ingestion of substances capable of inducing a cutaneous facial flushing reaction (e.g.: alcohol, chocolate, spices) by topical dermatological application of an effective dose of a composition comprising at least one α2 adrenergic receptor agonist (such as a (2-imidazolin-2-ylamino) quinoxaline derivative such as brimonidine tartrate)and a suitable carrier.
Owner:GALDERMA LAB LP
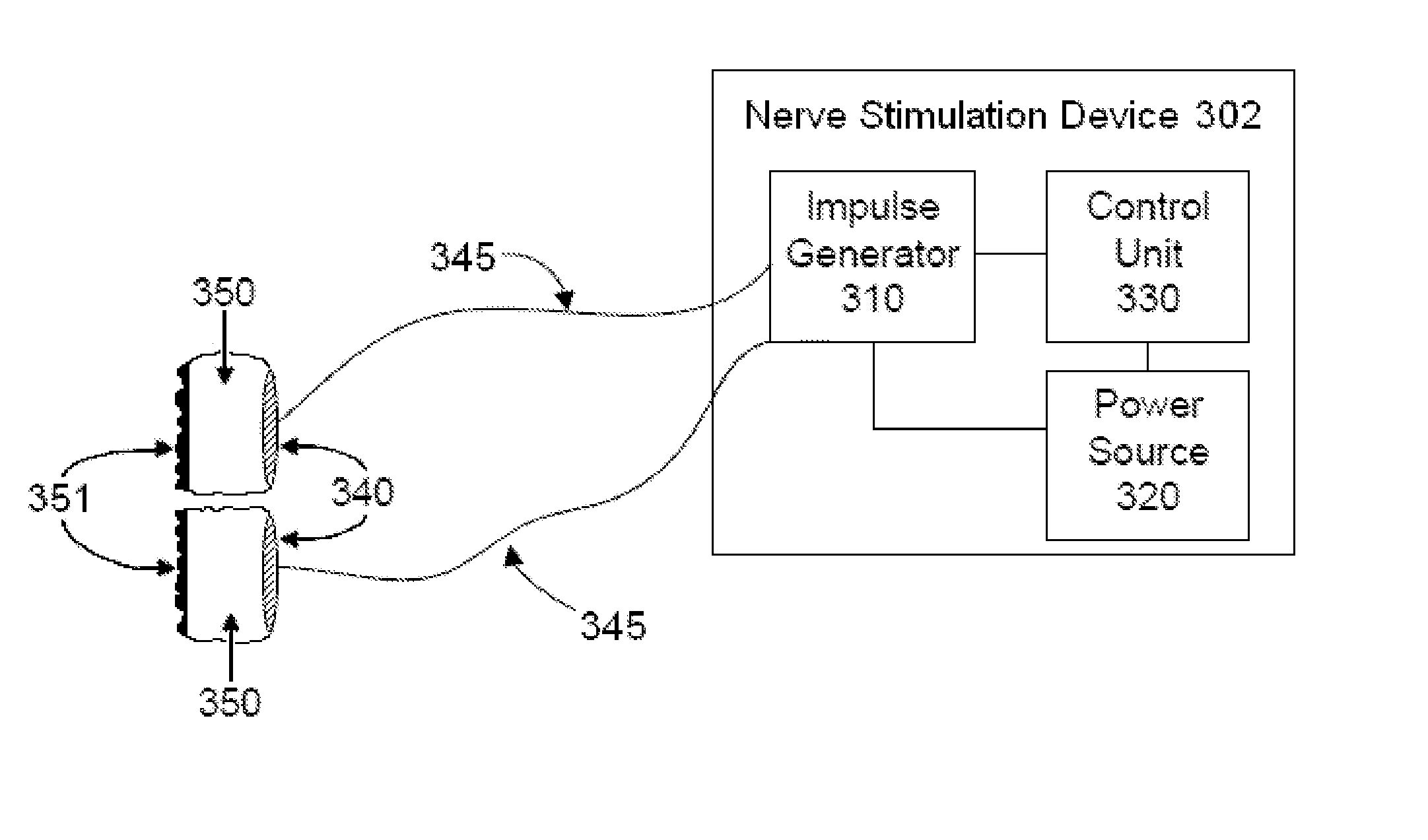
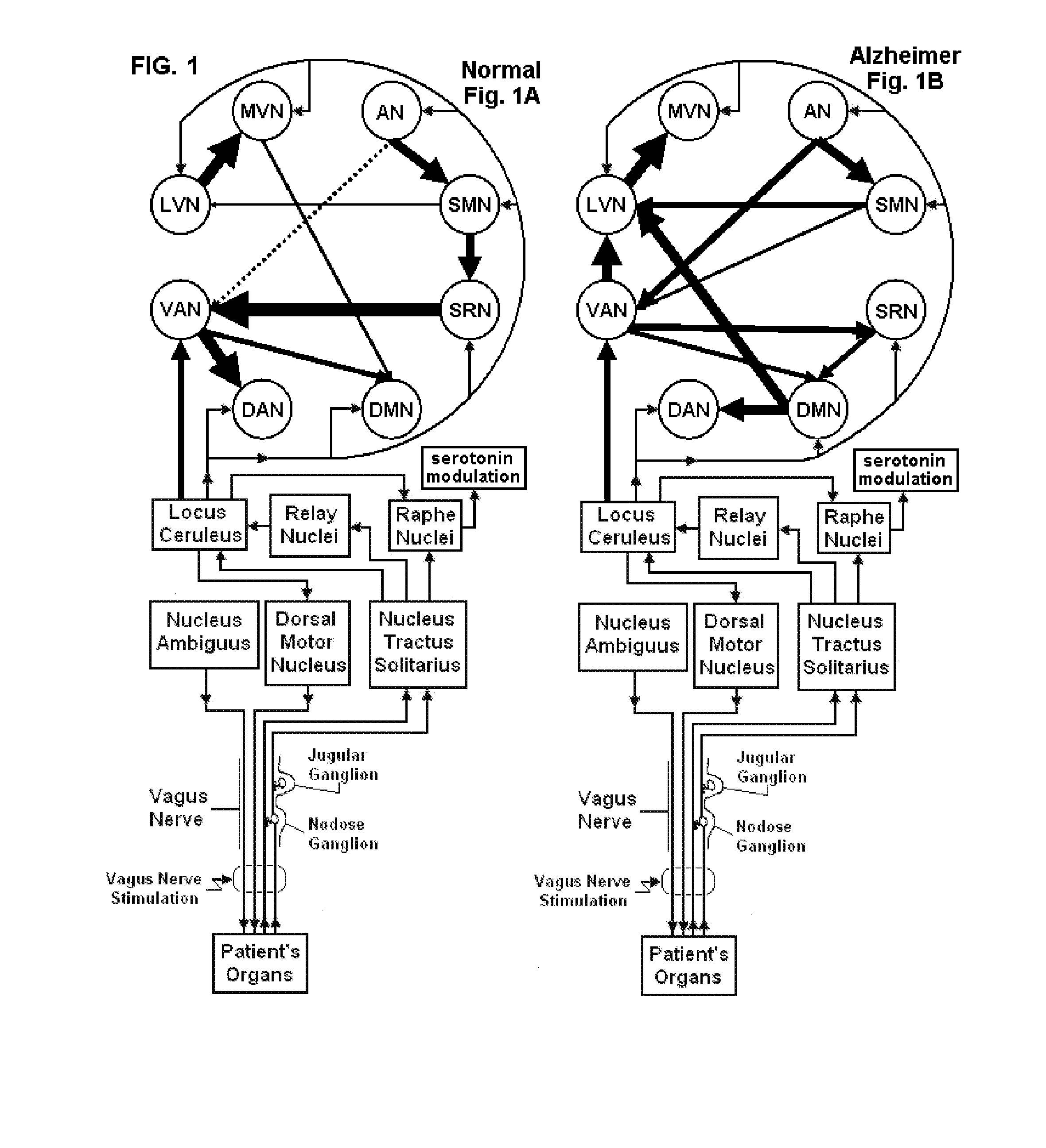
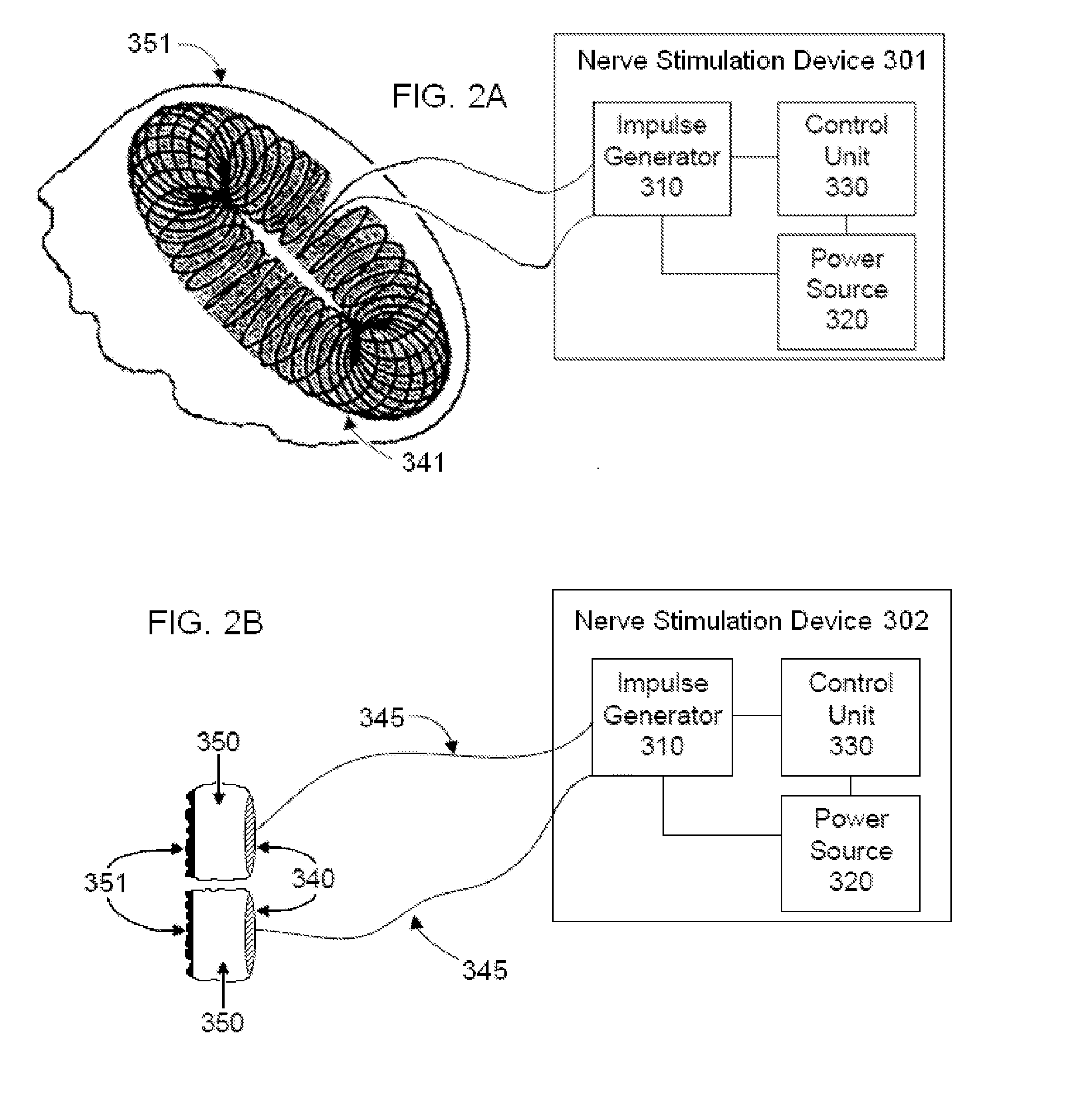
Non-invasive magnetic or electrical nerve stimulation to treat or prevent dementia
ActiveUS20130066392A1Inhibition of excitementAvoid stimulationElectroencephalographyElectrotherapyAdrenergicMedicine
Devices, systems and methods are disclosed for treating or preventing dementia, such as Alzheimer's disease. The methods comprise transmitting impulses of energy non-invasively to selected nerve fibers, particularly those in a vagus nerve, that modulate the activity of a patient's locus ceruleus. The transmitted energy impulses, comprising magnetic and / or electrical energy, stimulate the selected nerve fibers to cause the locus ceruleus to release norepinephrine into regions of the brain that contain beta-amyloids. The norepinephrine counteracts neuroinflammation that would damage neurons in those regions and the locus ceruleus, thereby arresting or slowing the progression of the disease in the patient.
Owner:ELECTROCORE
Methods and compositions for the treatment of pain and other alpha 2 adrenergic-mediated conditions
InactiveUS20050058696A1Improve effectivenessHigh activityOrganic active ingredientsSenses disorderAdrenergicSide effect
Owner:ALLERGAN INC
Method and therapeutic/cosmetic topical compositions for the treatment of rosacea and skin erythema using a1-adrenoceptor agonists
ActiveUS20050165079A1ConstrictMinimize rednessSalicyclic acid active ingredientsBiocideAdrenergicHyper reactivity
The present invention is directed to the treatment of skin erythema as exhibited in rosacea and other conditions characterized by increased erythema (redness) of the skin. These conditions exhibit dilation of blood vessels due to a cutaneous vascular hyper-reactivity. In particular, the present invention is directed to a novel composition and method for the treatment of skin erythema using α1-adrenergic receptor (α1-adrenoceptor) agonists incorporated into cosmetic, pharmacological or dermatological compositions for topical application to the skin.
Owner:ALLERGAN INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
Methods and compositions for modulating alpha adrenergic receptor activity
InactiveUS6545182B2High activityHighly effectiveBiocideSenses disorderAdrenergicAlpha-2 adrenergic receptor
Methods and compositions for the treatment of pain using thiourea derivatives. Particularly disclosed are new compositions for the treatment of chronic pain, and methods for their use.
Owner:ALLERGAN INC
Diamine beta2 adrenergic receptor agonists
The invention provides novel β2 adrenergic receptor agonist compounds. The invention also provides pharmaceutical compositions comprising such compounds, methods of using such compounds to treat diseases associated with β2 adrenergic receptor activity, and processes and intermediates useful for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
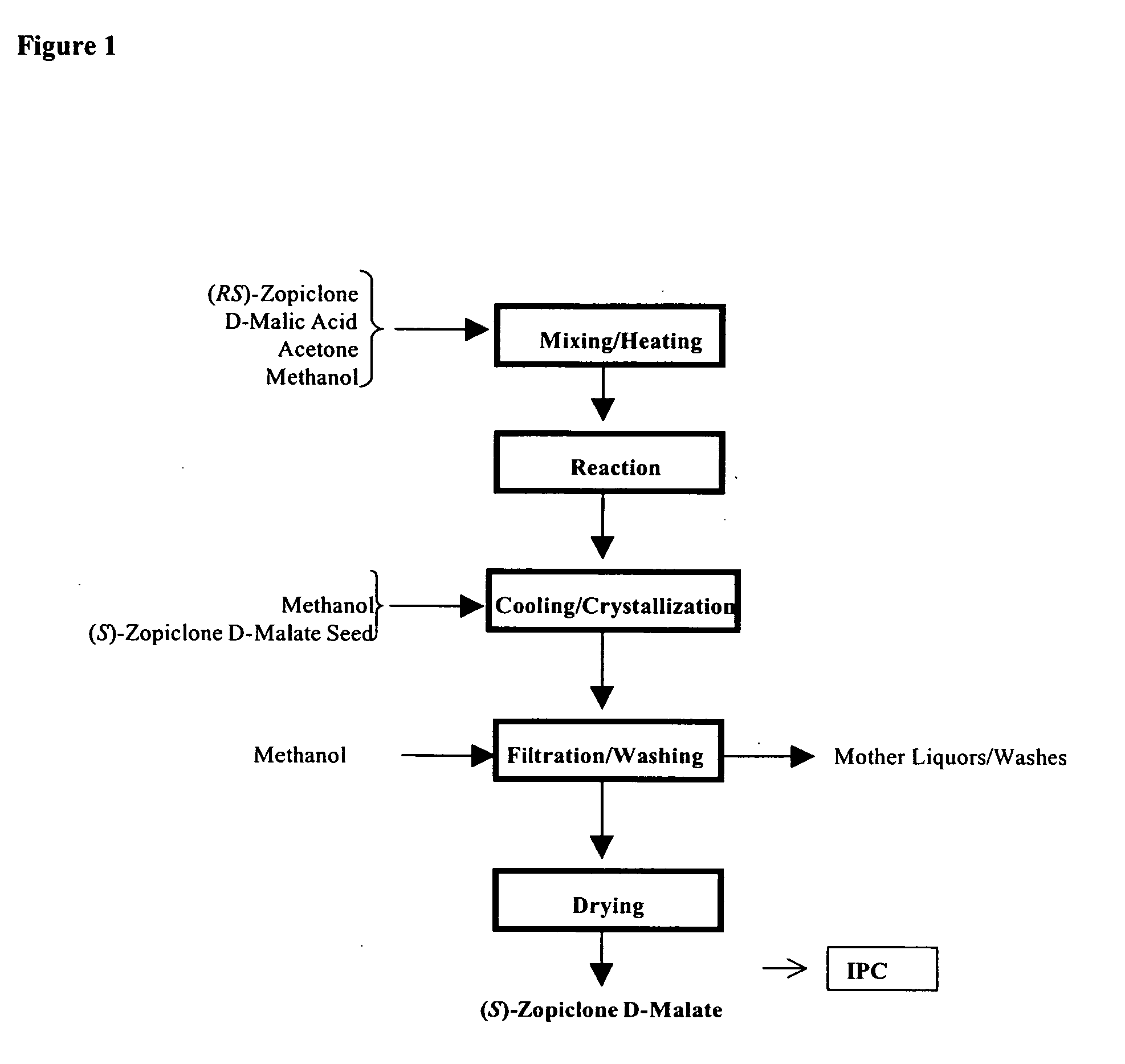
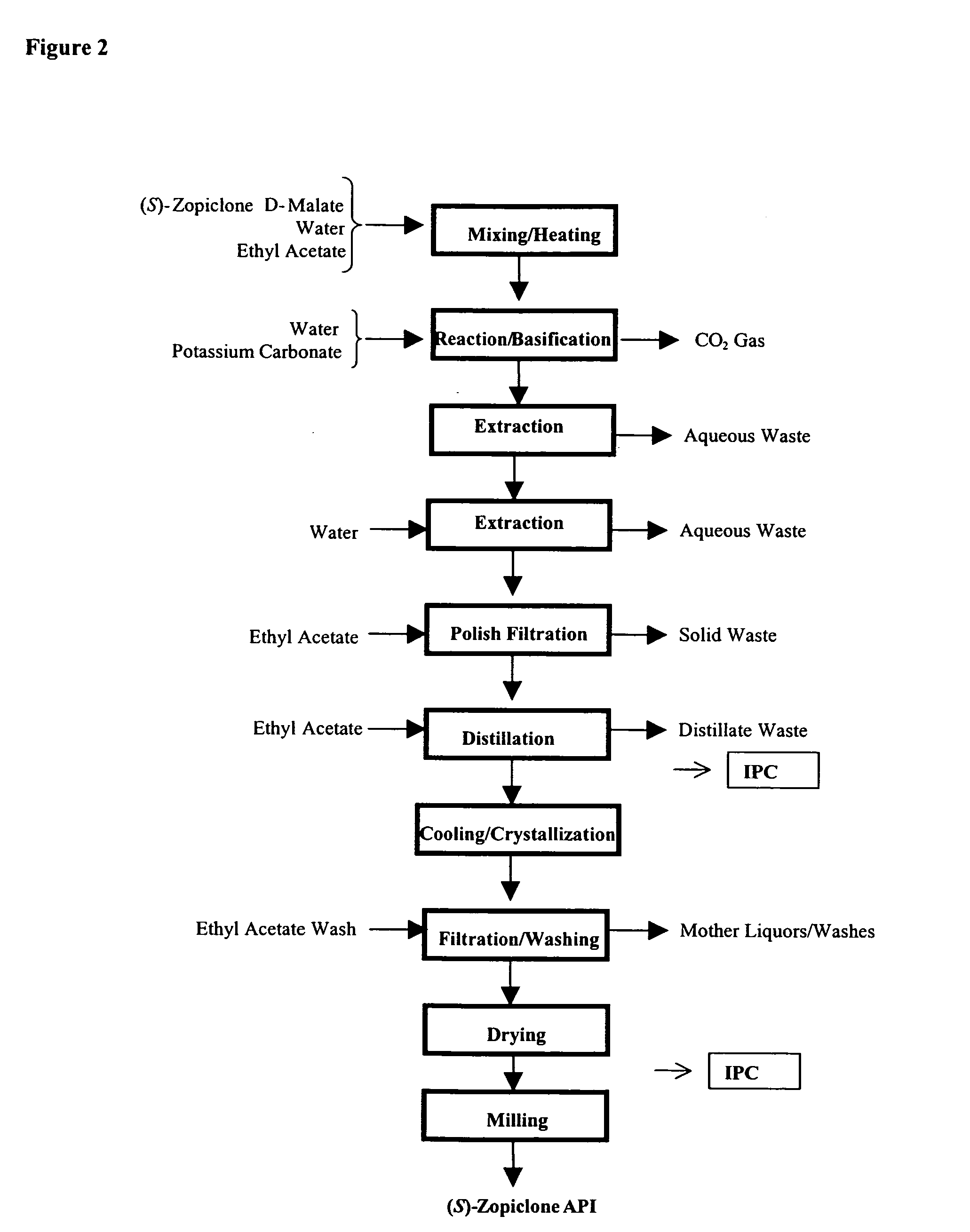
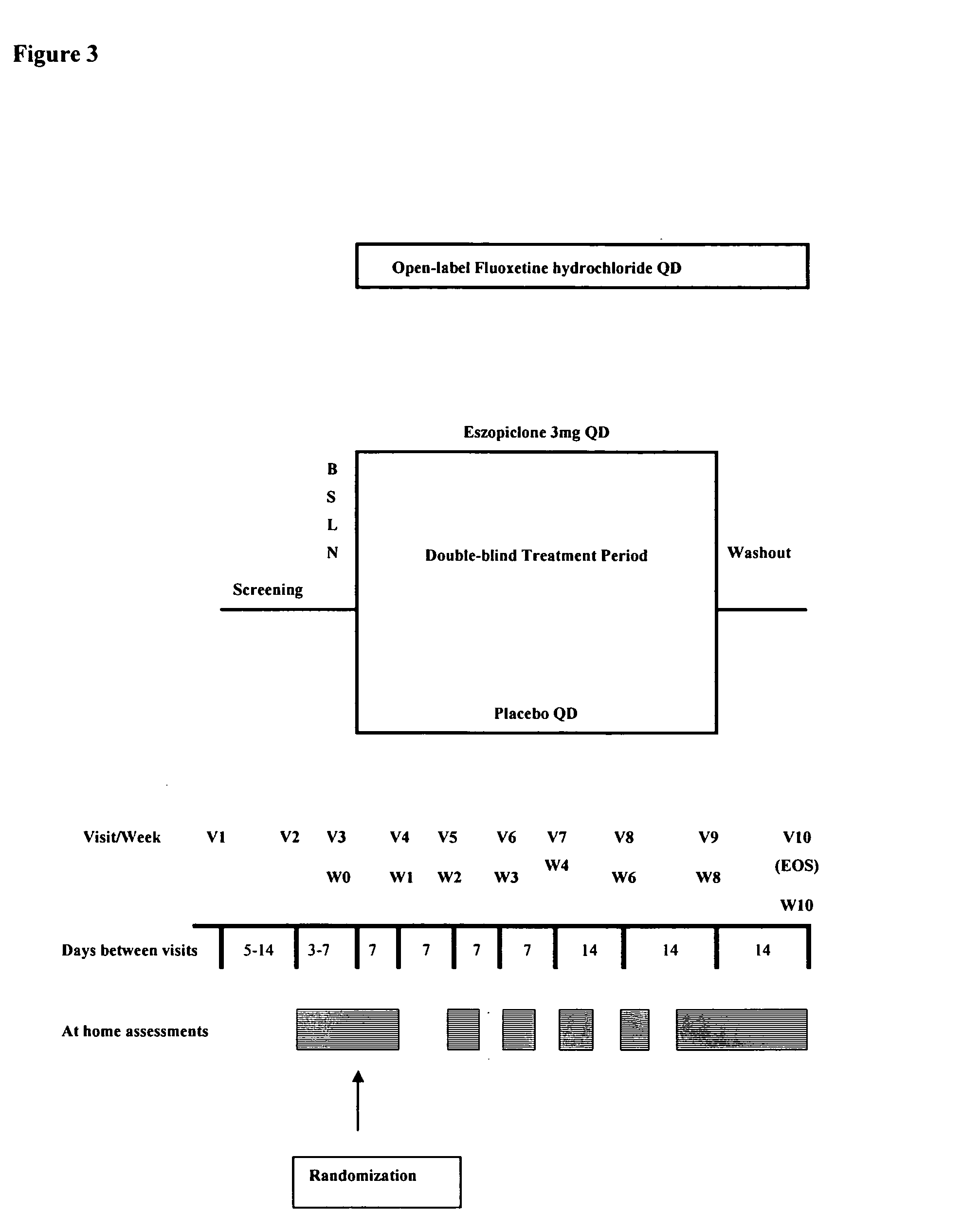
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:SEPACOR INC
Arthroscopic irrigation solution and method for peripheral vasoconstriction and inhibition of pain and inflammation
InactiveUS20030087962A1Control inflammationPain controlBiocideKallidin/bradykinin ingredientsArteriolar VasoconstrictionInflammation Process
A method and solution for perioperatively inhibiting a variety of pain and inflammation processes during arthroscopic procedures. The solution preferably includes a vasoconstrictor that demonstrates substantial agonist activity at alpha adrenergic receptors and that is selected for peripheral (local) vasoconstriction and one or more additional pain and inflammation inhibitory agents at dilute concentration in a physiologic carrier, such as saline or lactated Ringer's solution. The solution is applied by continuous irrigation of a wound during a surgical procedure for peripheral vasoconstriction and inhibition of pain and / or inflammation while avoiding undesirable side effects associated with systemic application of larger doses of the agents.
Owner:OMEROS CORP
Compositions having enhanced pharmacokinetic characteristics
InactiveUS7491383B2Reduced activityImprove bioavailabilityBiocideSenses disorderAdrenergicFatty acid
Compositions comprising a therapeutic component and an efficacy enhancing component, that enhances the pharmacokinetic disposition of the therapeutic component, are disclosed. The therapeutic component may include an alpha-2-adrenergic agonist and the efficacy enhancing component may include fatty acids. In one embodiment, the therapeutic component and the efficacy enhancing component form a complex.
Owner:ALLERGAN INC
Method of treating chronic fatigue syndrome
The present invention provides a method of treating fibromyalgia syndrome (FMS), chronic fatigue syndrome (CFS), and pain in an animal subject comprising administering a therapeutically effective amount of a dual serotonin norepinephrine reuptake inhibitor compound or a pharmaceutically acceptable salt thereof, wherein said dual serotonin norepinephrine reuptake inhibitor compound is characterized by a non-tricyclic structure and a greater inhibition of norepinephrine reuptake than serotonin reuptake, and wherein said compound is not administered adjunctively with phenylalanine or tyrosine. In particular, the use of milnacipran to treat FMS, CFS, and pain is disclosed.
Owner:FOREST LAB HLDG LTD
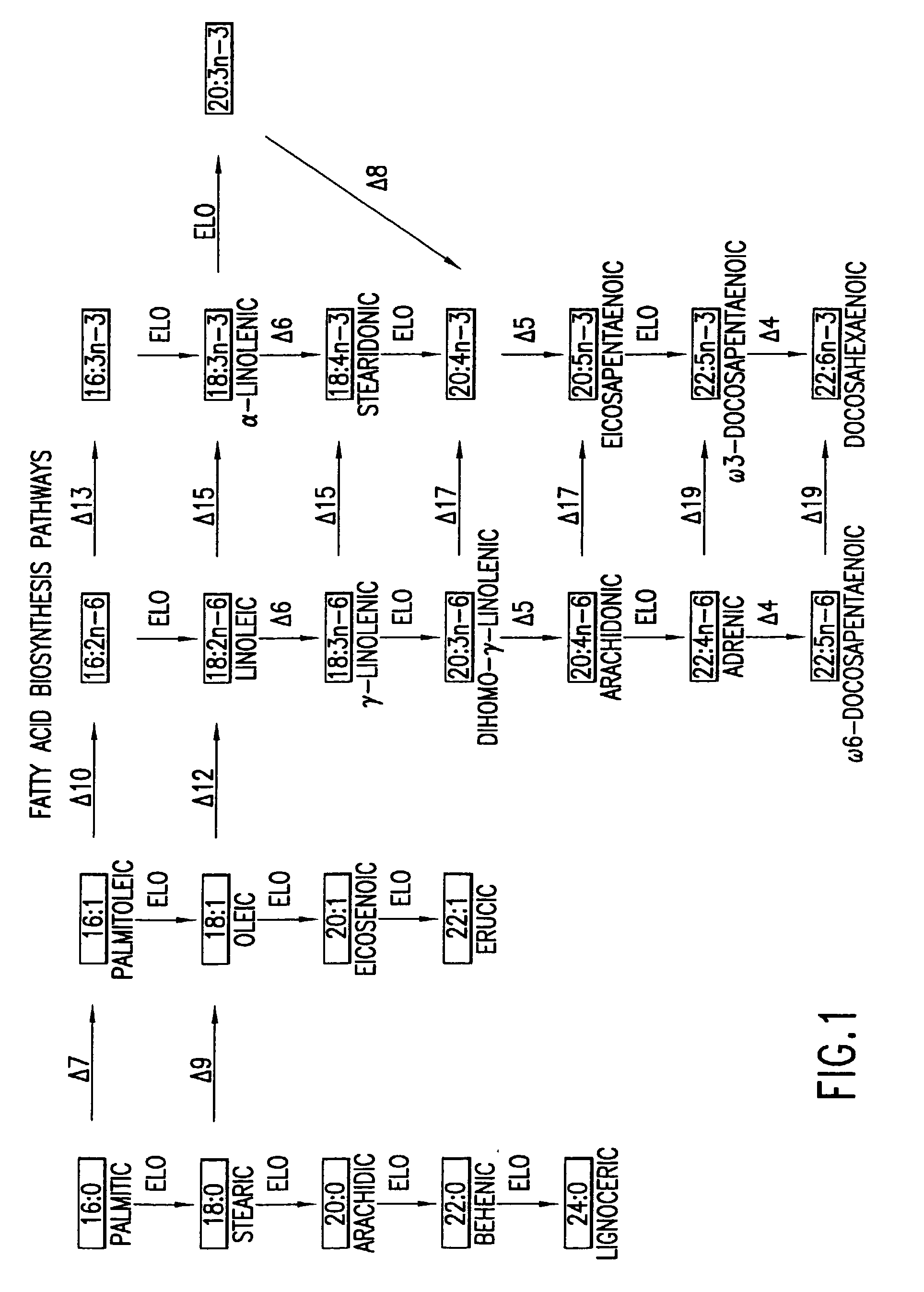
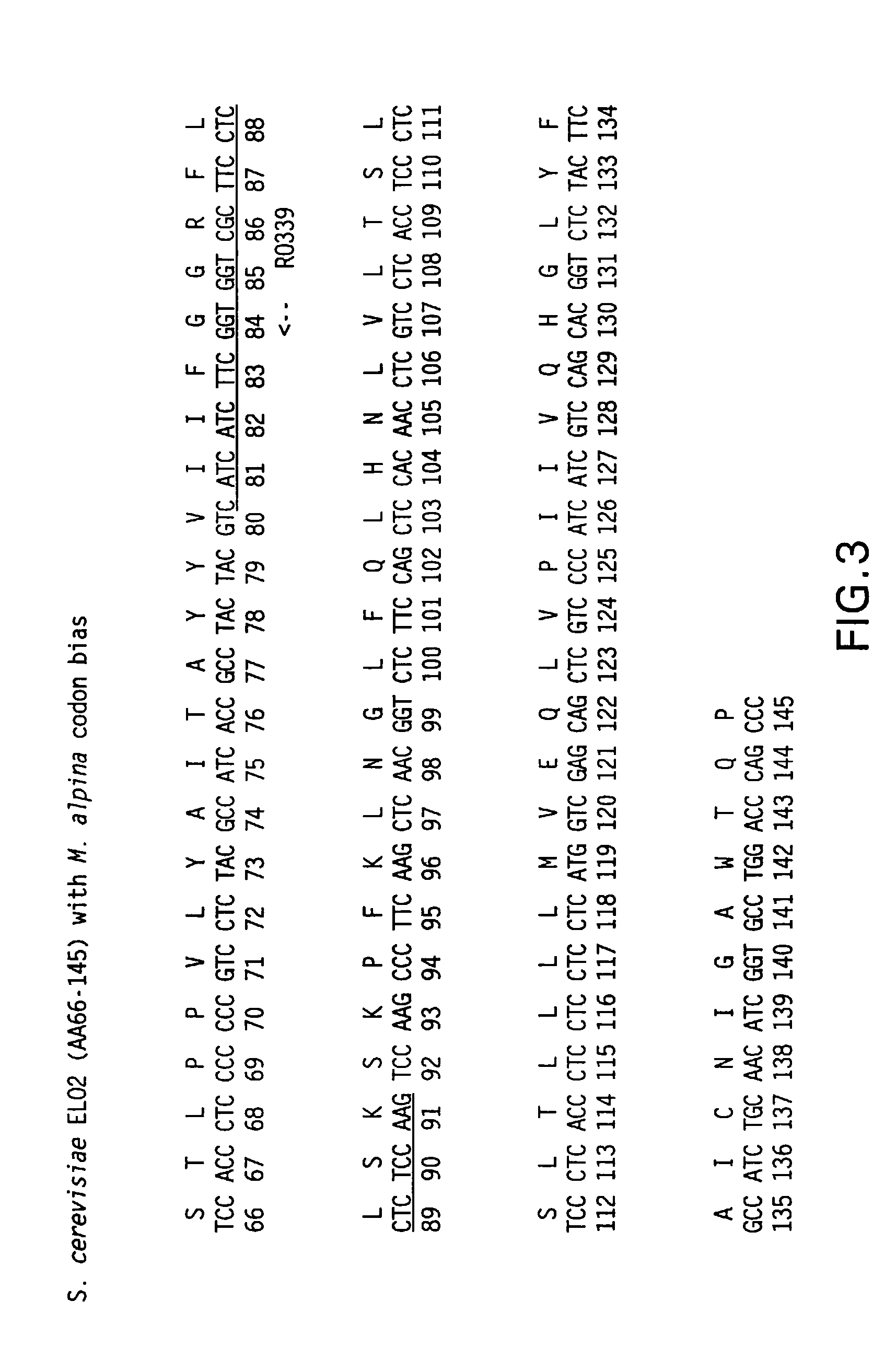
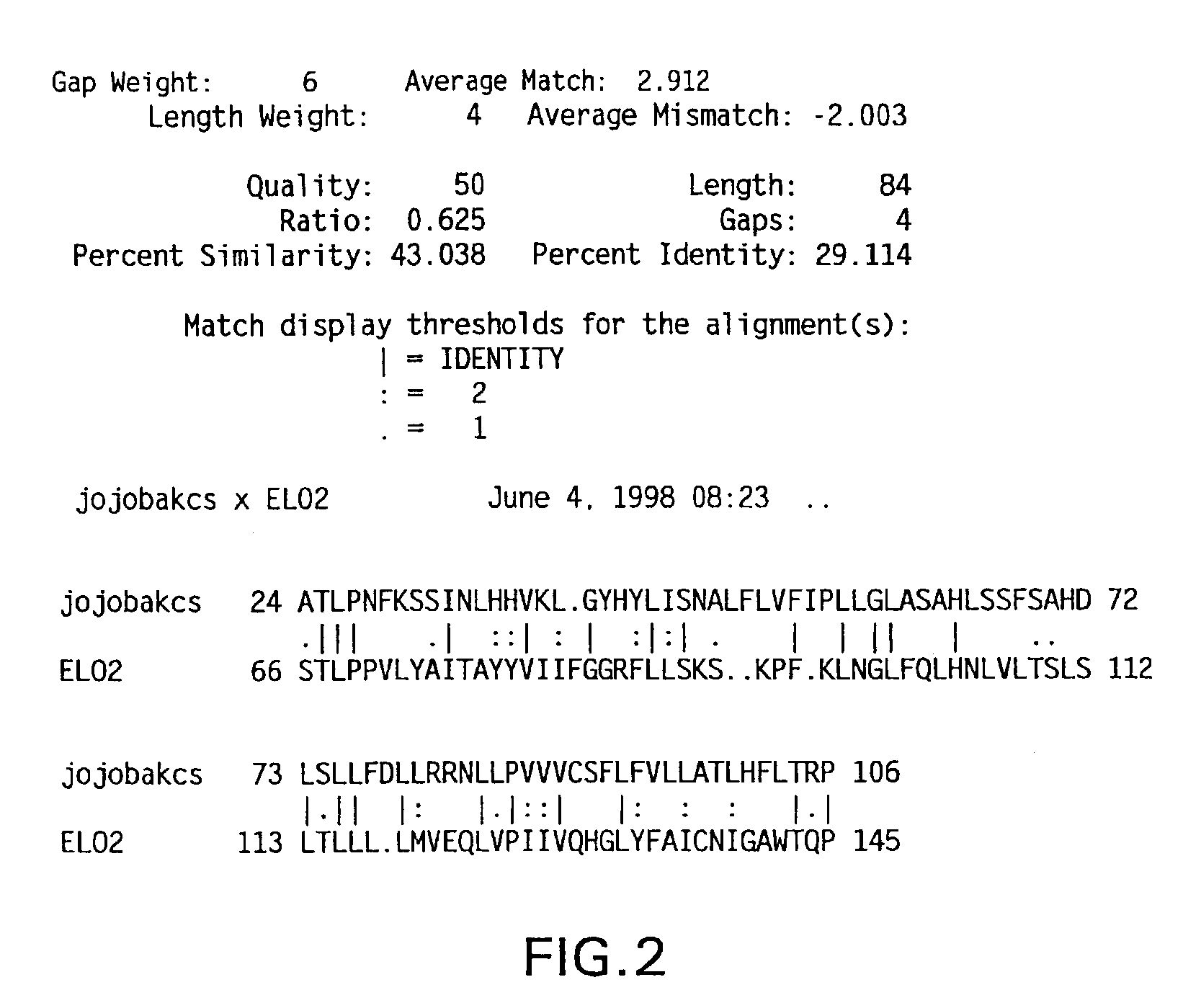
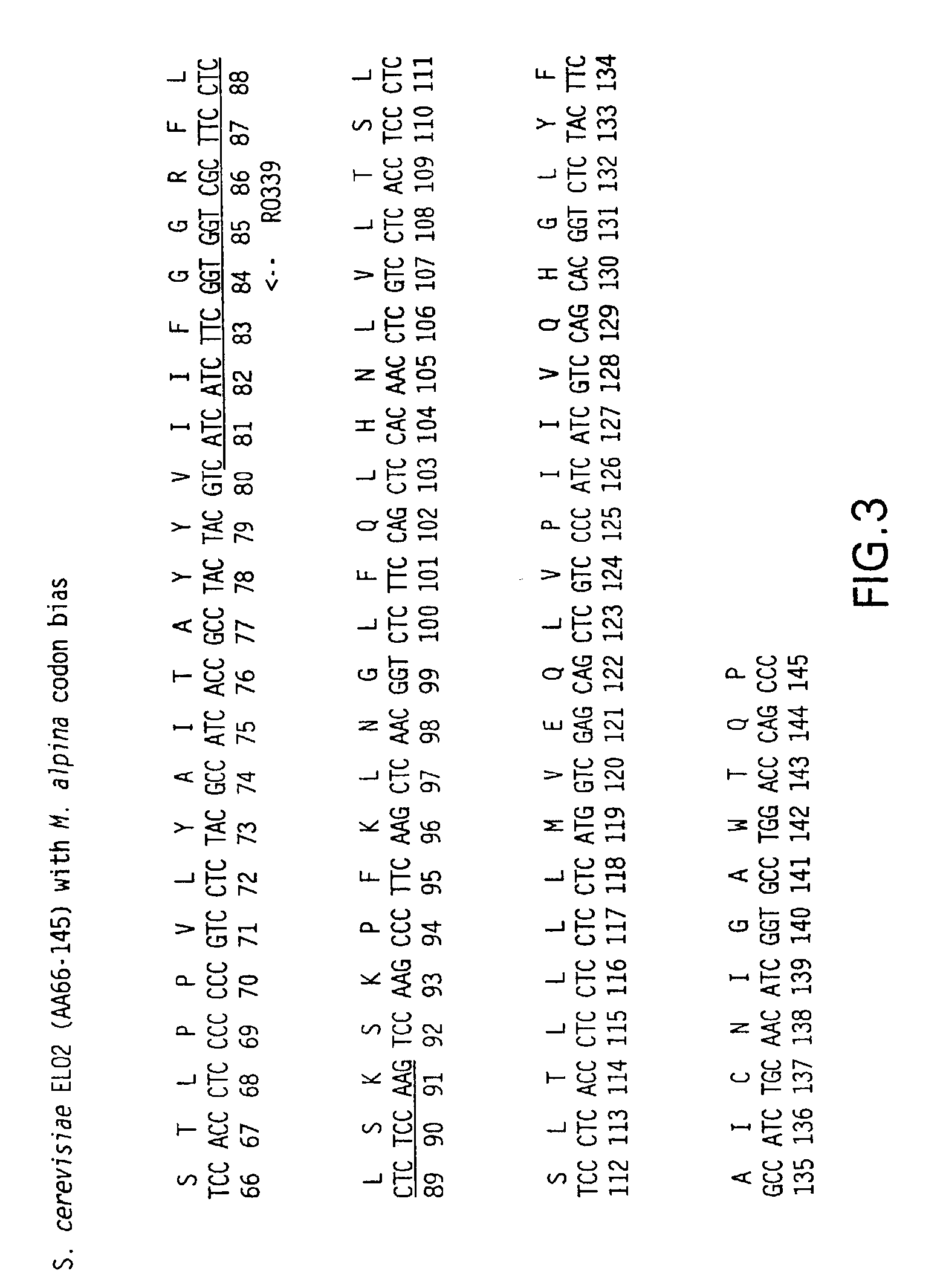
Elongase genes and uses thereof
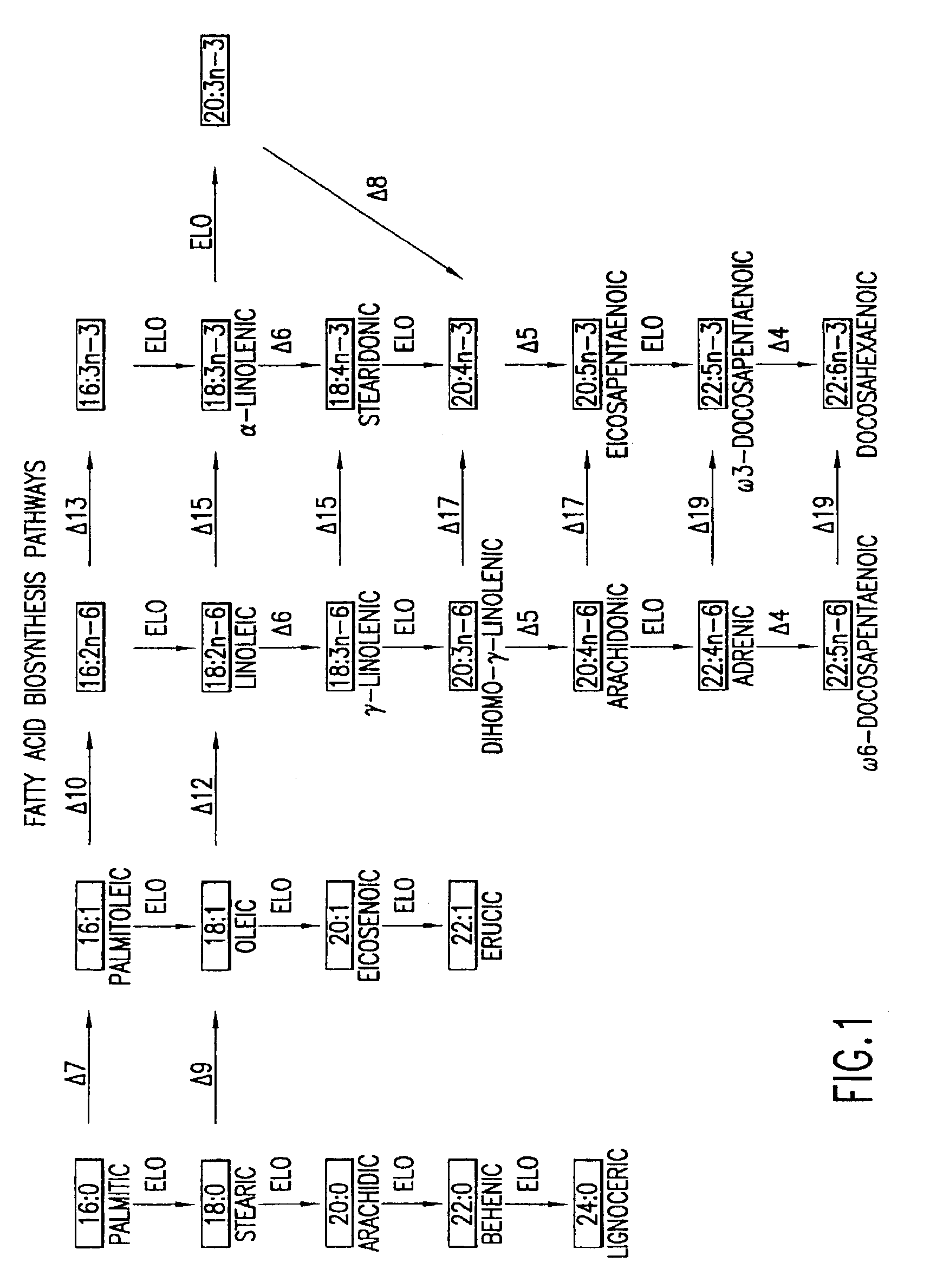
The subject invention relates to the identification of several genes involved in the elongation of polyunsaturated acids (i.e., "elongases") and to uses thereof. At least two of these genes are also involved in the elongation of monounsaturated fatty acids. In particular, elongase is utilized in the conversion of gamma linolenic acid (GLA) to dihomogamma linolenic acid (DGLA) and in the conversion of AA to adrenic acid (ADA), or eicosapentaenoic acid (EPA) to omega3-docosapentaenoic acid (DPA). DGLA may be utilized in the production of polyunsaturated fatty acids, such as arachidonic acid (AA), docosahexaenoic acid (DHA), EPA, adrenic acid, omega6-docosapentaenoic acid or omega3-docosapentaenoic acid which may be added to pharmaceutical compositions, nutritional compositions, animal feeds, as well as other products such as cosmetics.
Owner:ABBOTT LAB INC
Treating skin disorders
InactiveUS20070082070A1Reduce severitySolve skin problemsBiocideHydroxy compound active ingredientsAdrenergicAlpha-2 adrenergic receptor
This document relates to methods and materials involved in treating skin disorders. For example, methods and materials for using an alpha adrenergic receptor agonist (e.g., midodrine) to treat mammals having a skin disorder are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6469055B2Increased formationPromote activationBiocideNervous disorderGlial fibrillary acidic proteinDisease
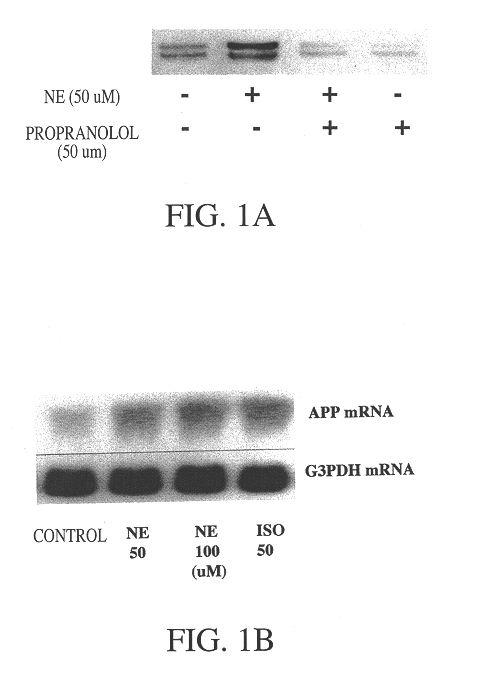
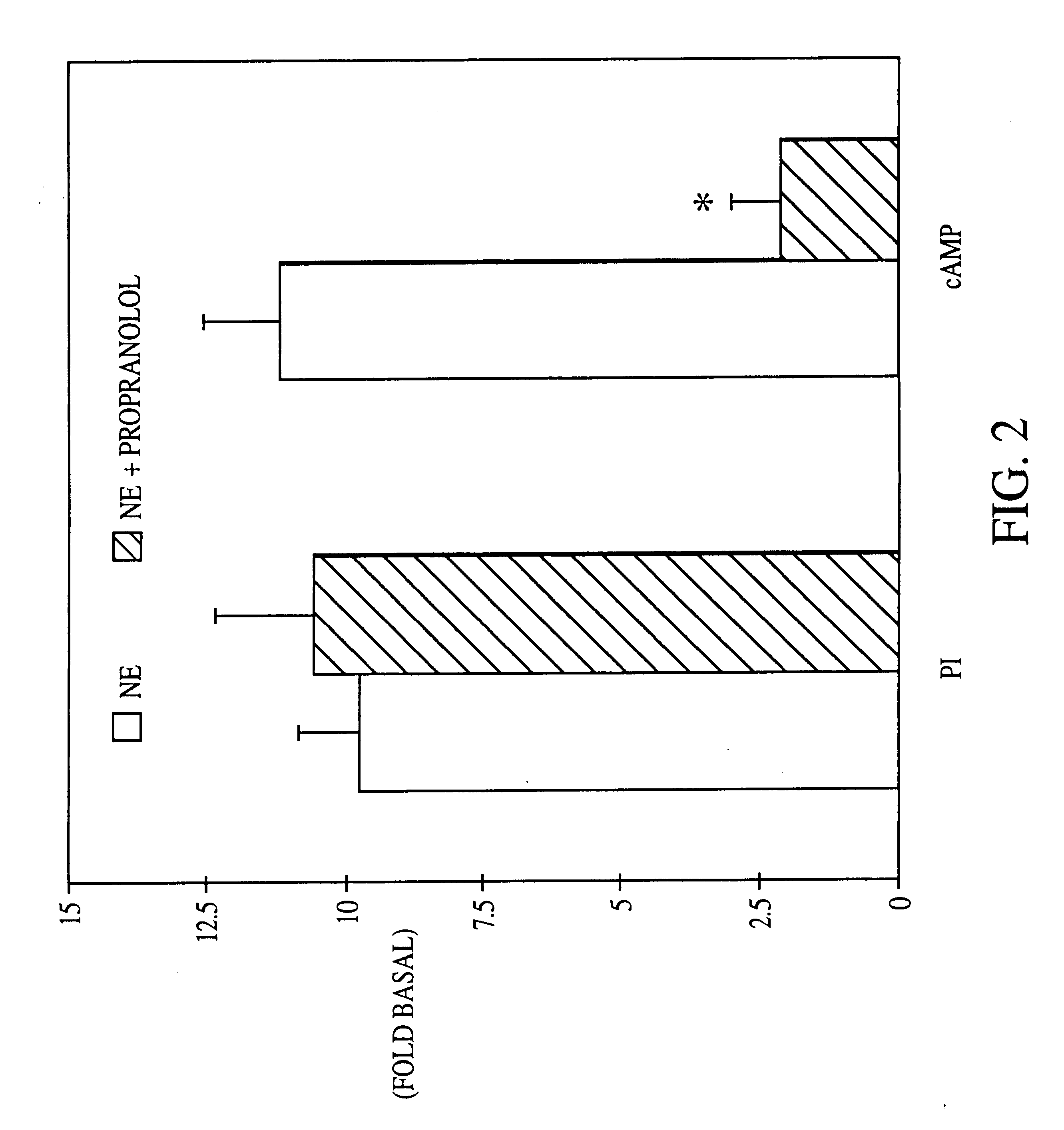
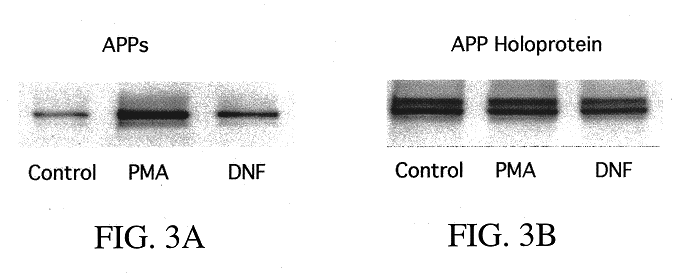
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, or forskolin is inhibited by immunosuppressants, immunophilin ligands, or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
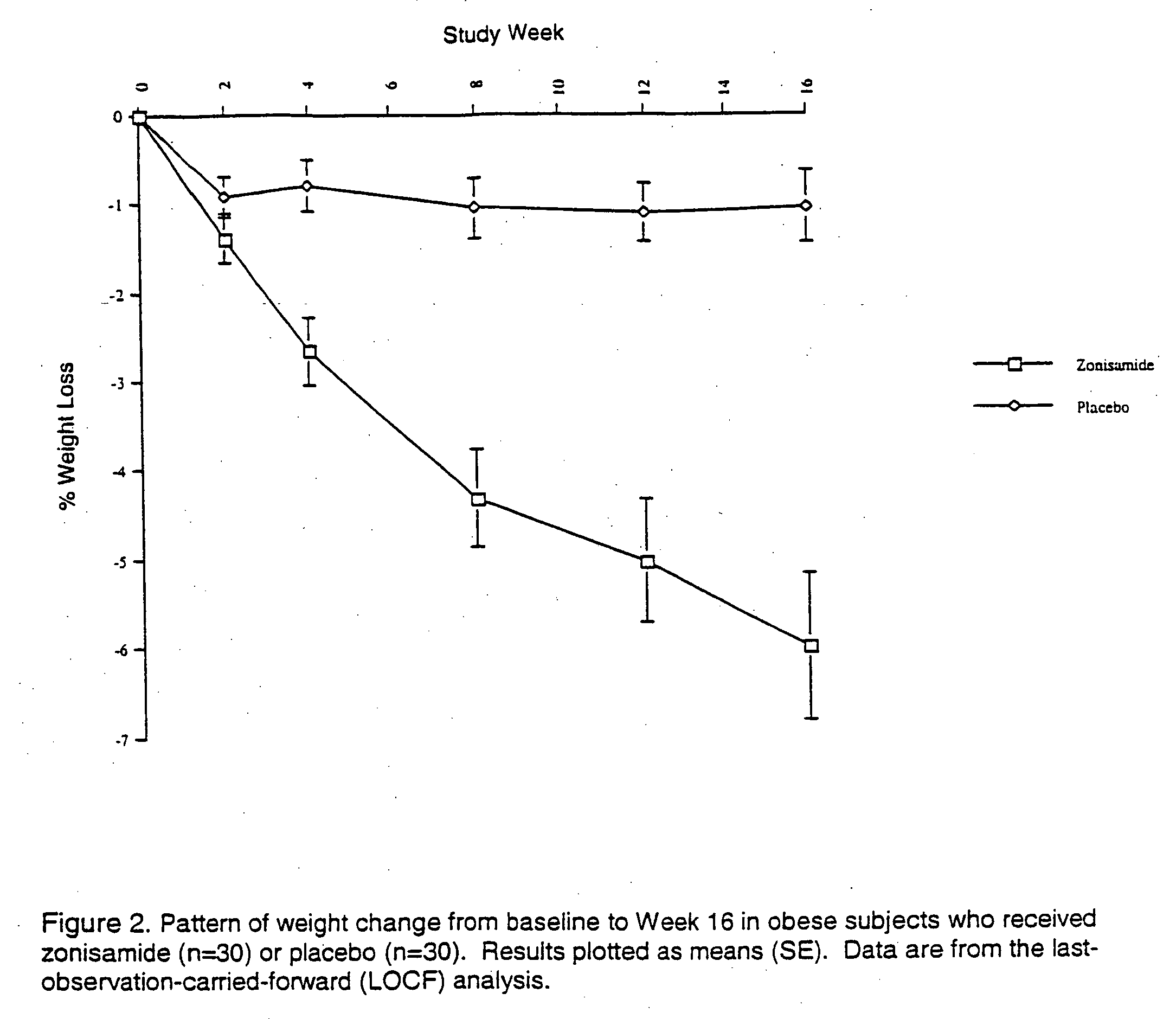
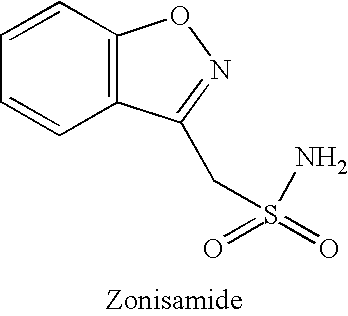
Method for treating obesity
InactiveUS20050143322A1Minimize risk factorHigh activityAntibacterial agentsBiocideAdrenergicCompound (substance)
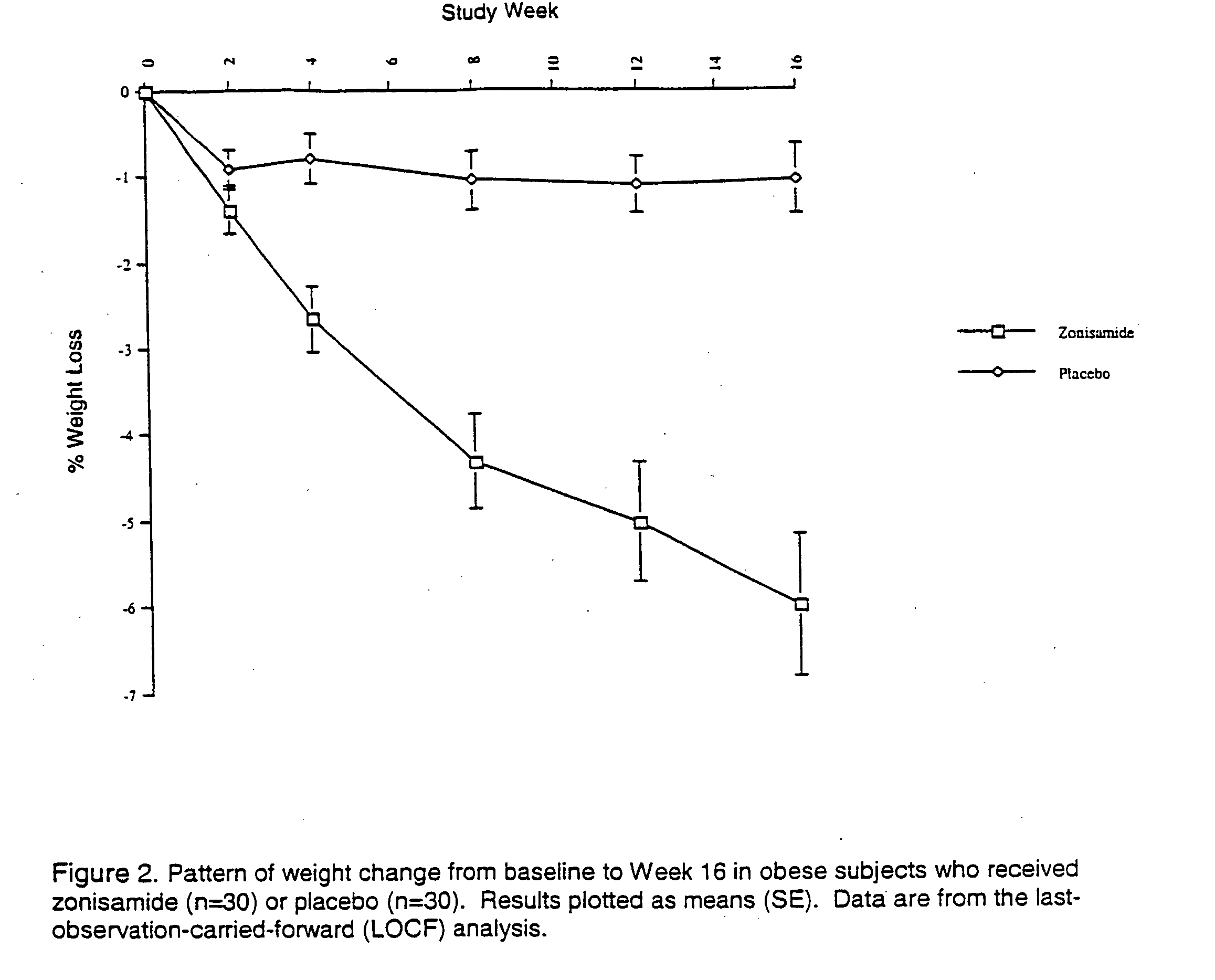
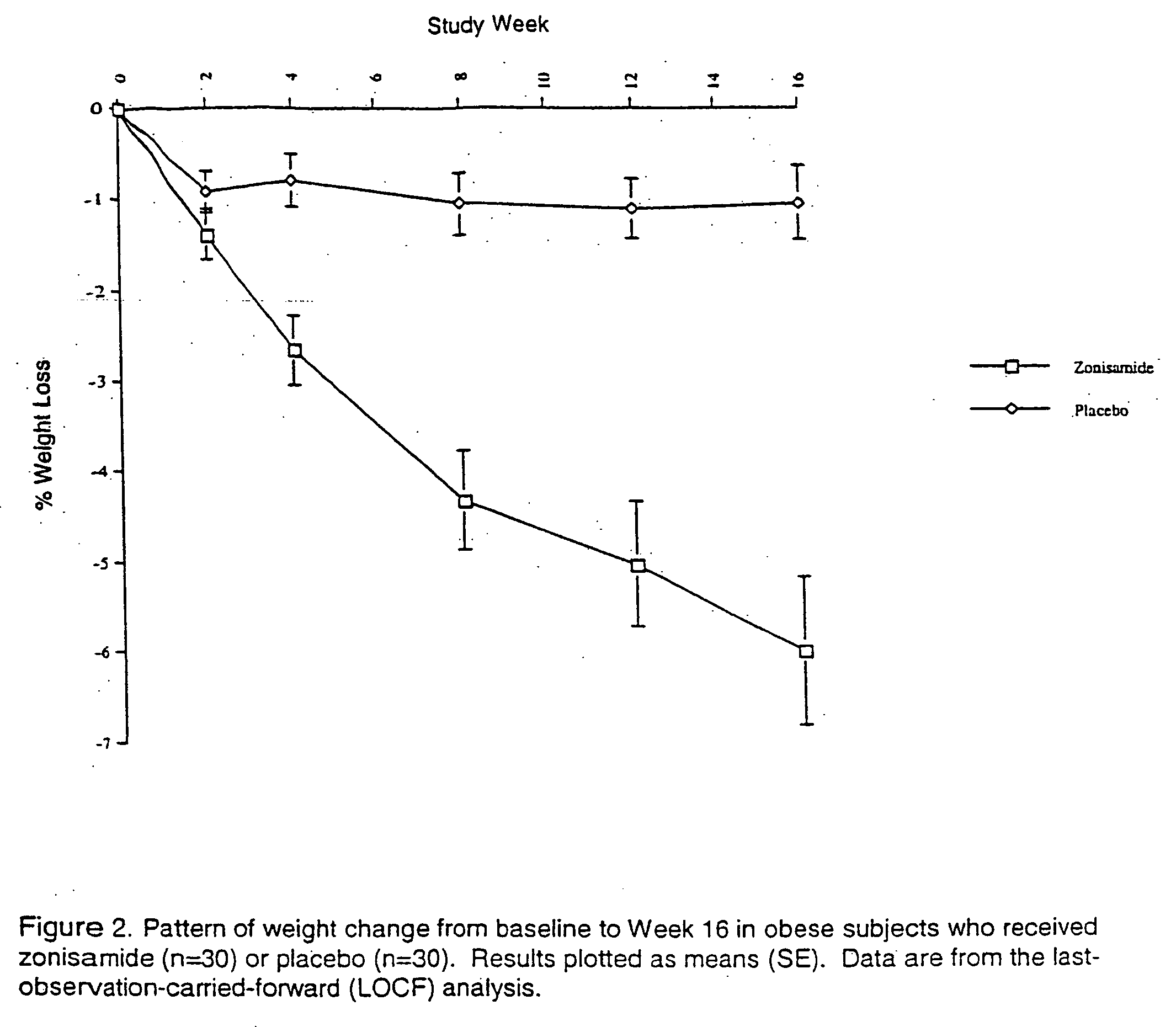
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:DUKE UNIV
Orally disintegrating tablets of atomoxetine
InactiveUS20060057199A1Low incidenceHighly irritateCosmetic preparationsToilet preparationsOral medicationPopulation
A coated multi-particulate pharmaceutical dosage form such as an orally disintegrating tablet (ODT) presentation for delivering atomoxetine or a pharmaceutically acceptable salt thereof, a selective norepinephrine reuptake inhibitor indicated for the treatment of ADHD, into the body to maintain a therapeutically effective amount of atomoxetine in the plasm. The dosage form may comprise one or more populations of coated atomoxetine-containing particles (beads, pellets, granules etc.) providing a pre-designed rapid release profile after a predesigned lag-time of about 0 to 6 hours following oral administration.
Owner:ADARE PHARM INC
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
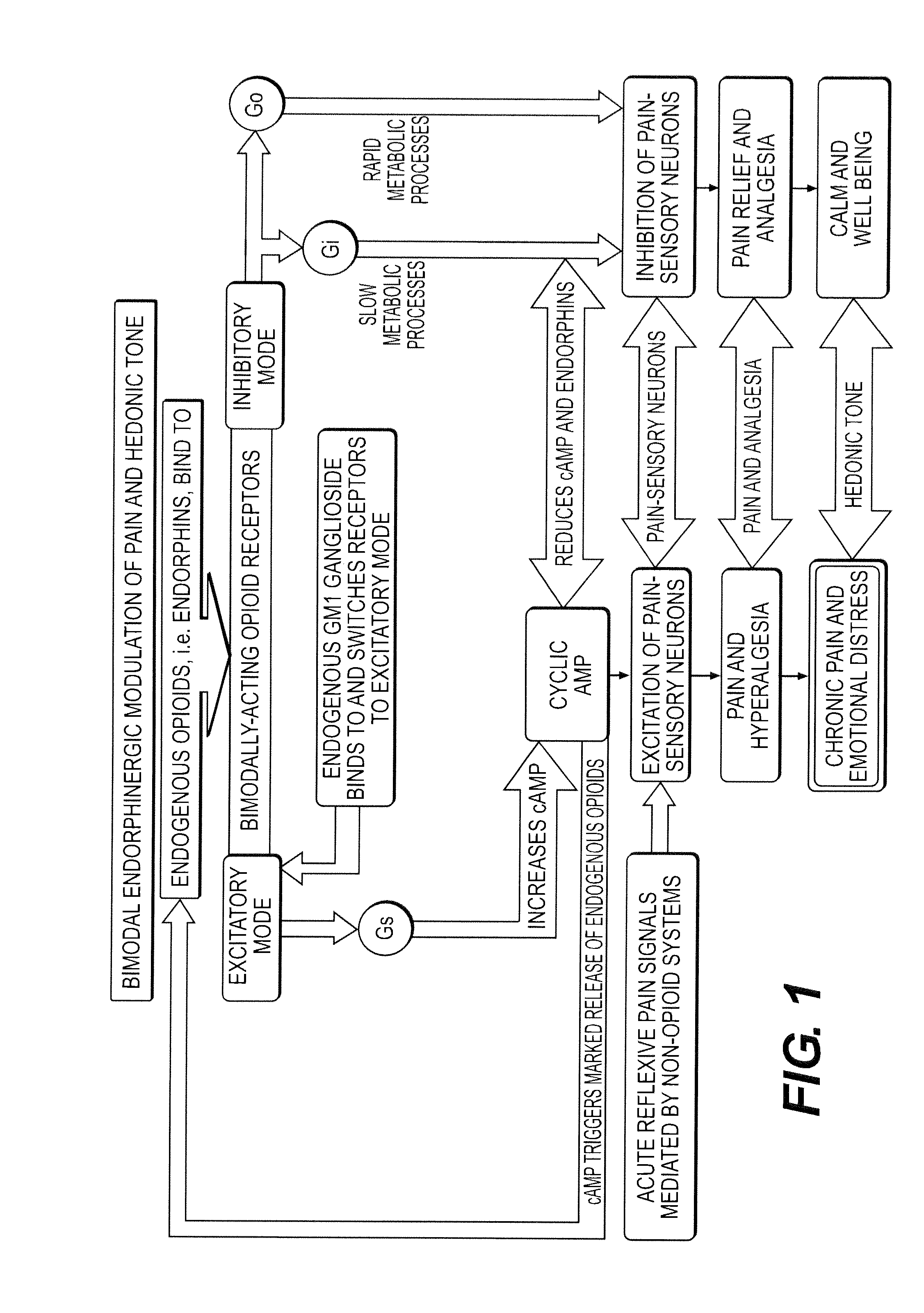
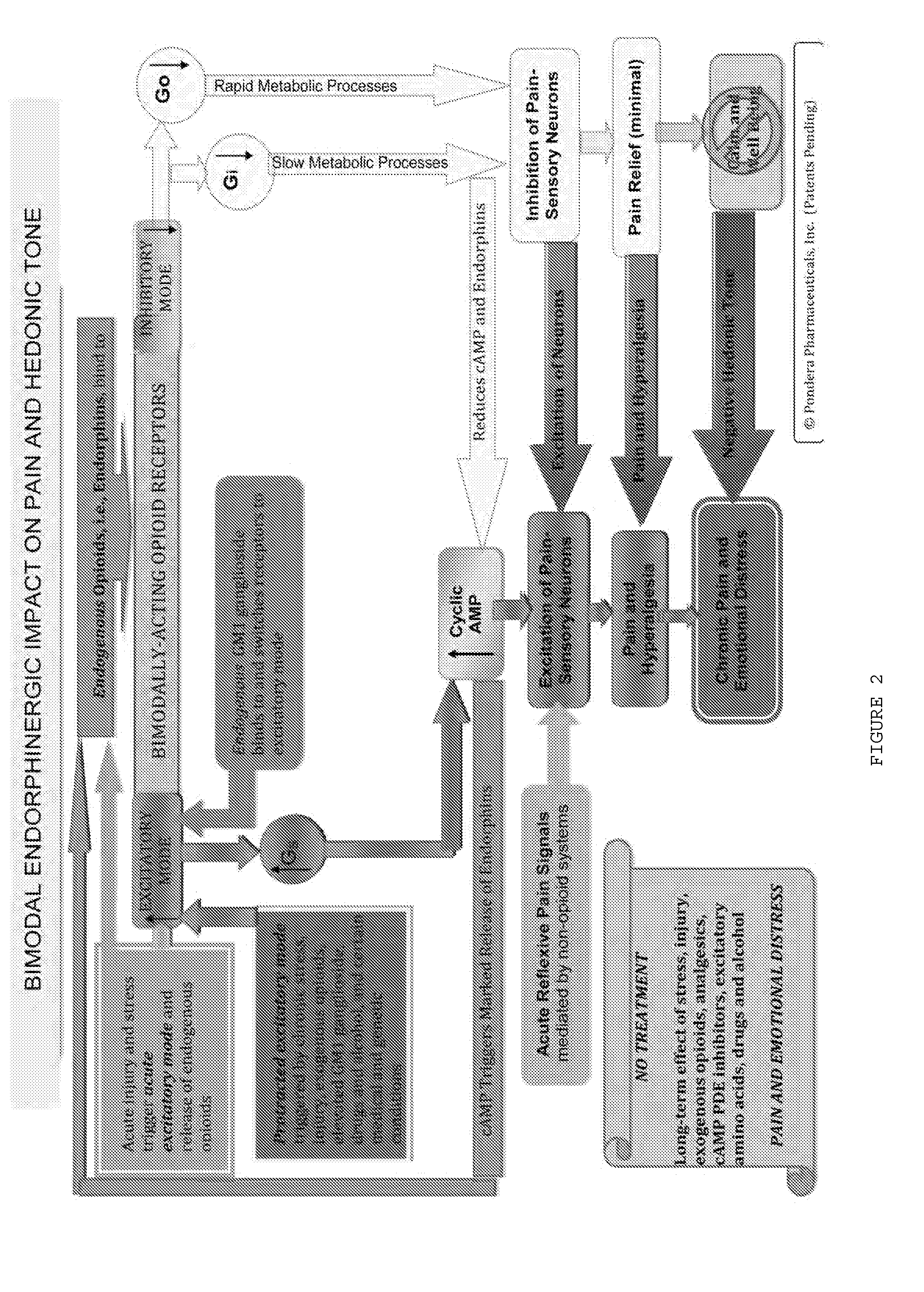
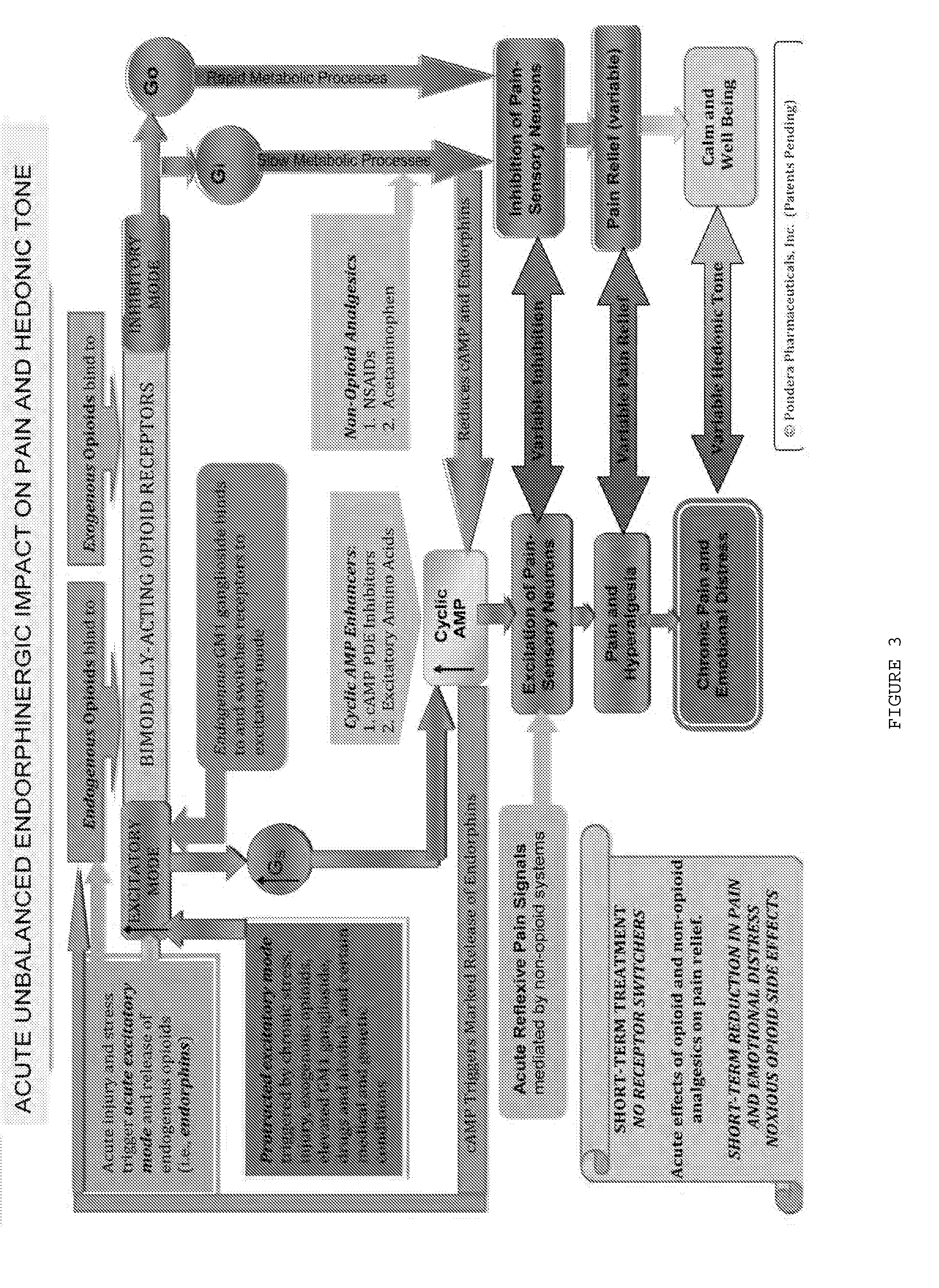
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
Method for treating obesity
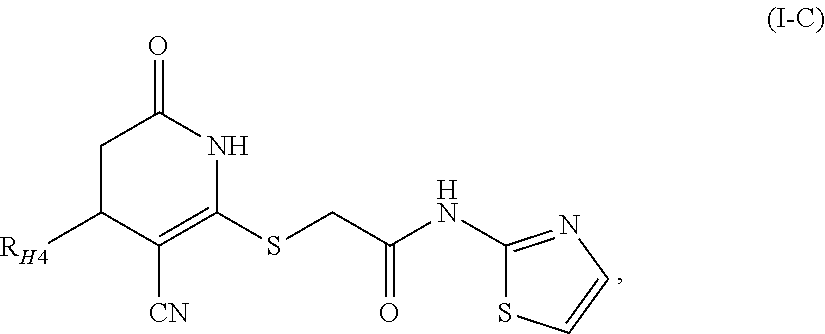
InactiveUS20050137144A1Minimizing metabolic risk factorHigh activityAntibacterial agentsBiocideZonisamideAdrenergic
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:GADDE KISHORE M +1
Elongase genes and uses thereof
The subject invention relates to the identification of four genes involved in the elongation of polyunsaturated acids (i.e., “elongases”) and to uses thereof. Two of these genes are also involved in the elongation of monounsaturated fatty acids. In particular, elongase is utilized in the conversion of gamma linolenic acid (GLA) to dihomogamma linolenic acid (DGLA) and in the conversion of DGLA or 20:4n-3 to eicosapentaenoic acid (EPA). DGLA may be utilized in the production of polyunsaturated fatty acids, such as arachidonic acid (AA), docosahexaenoic acid (DHA), EPA, adrenic acid, ω6-docosapentaenoic acid or ω3-docosapentaenoic acid which may be added to pharmaceutical compositions, nutritional compositions, animal feeds, as well as other products such as cosmetics.
Owner:ABBOTT LAB INC
1-(4-arylpiperazin-1-yl)- omega -[n-( alpha . omega -dicarboximido)]-alkanes useful as uro-selective alpha 1-adrenoceptor blockers
Novel piperzine derivatives substituted on one nitrogen by an aromatic system and on the other nitrogen by (2,5-dioxopyrrolidin)-1-yl) alkanes or (2,6-dioxopiperidin-1-yl) alkanes have been found to exhibit selective alpha 1A adrenergic activity. The compounds are useful for treatment of disease conditions, such as peripheral vascular disease, congestive heart failure, hypertension and especially benign prostatic hypertrophy.
Owner:RANBAXY LAB LTD
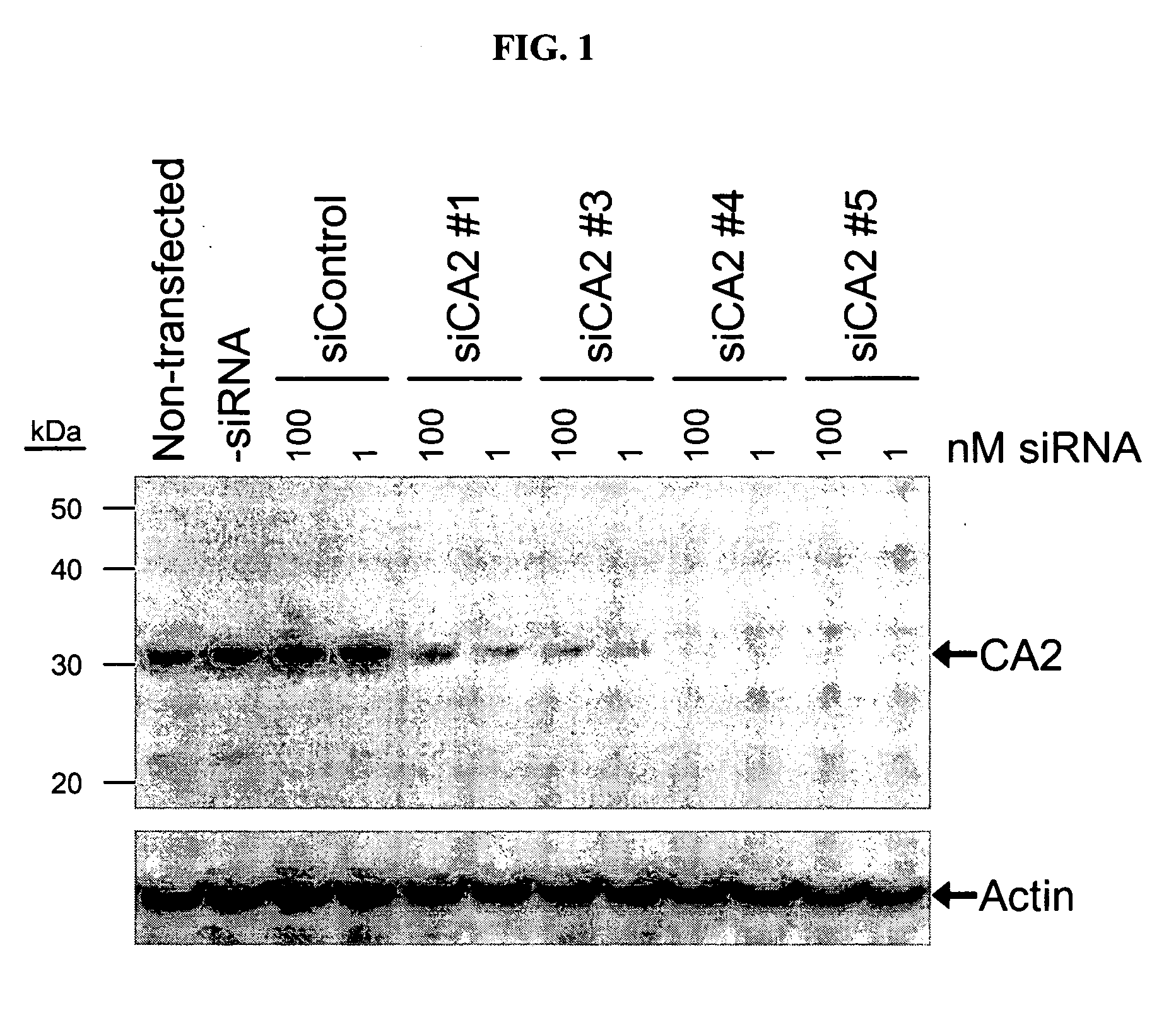
RNAi-mediated inhibition of ocular hypertension targets
InactiveUS20060172963A1Lower eye pressureOrganic active ingredientsSenses disorderIntra ocular pressureATPase
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:ARROWHEAD RES CORP +1
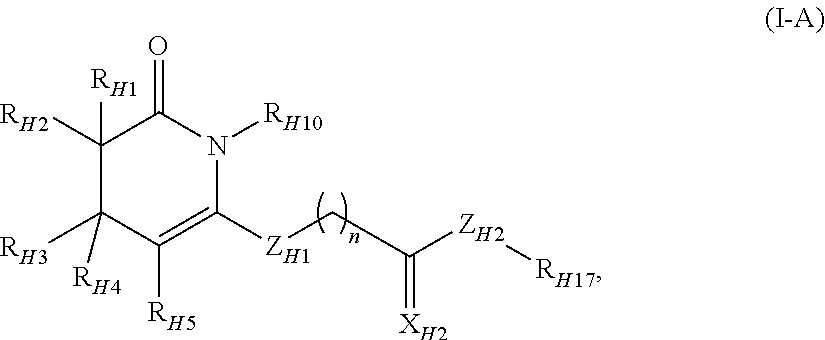
Small molecule inhibitors of necroptosis
The invention features a series of heterocyclic derivatives that inhibit tumor necrosis factor alpha (TNF-α) induced necroptosis. The heterocyclic compounds of the invention are described by Formulas (I)-(VIII) and by Compounds (I)-(I), (13)-(26), (27)-(33), (48)-(57), and (58)-(70). These necrostatins are shown to inhibit TNF-α induced necroptosis in FADD-deficient variant of human Jurkat T cells. The invention further features pharmaceutical compositions featuring necrostatins. The compounds and compositions of the invention may also be used to treat disorders where necroptosis is likely to play a substantial role.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for treating obesity
InactiveUS20060009514A1Minimize risk factorHigh activityAntibacterial agentsBiocideAdrenergicMetabolic risk
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:GADDE KISHORE M +1
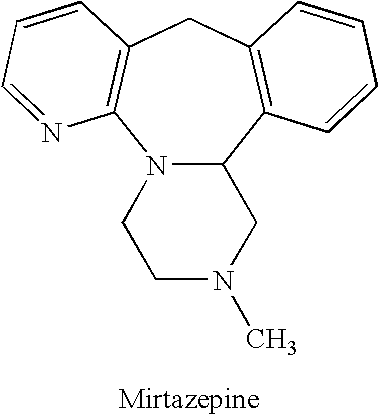
Methods for reducing the side effects associated with mirtzapine treatment
InactiveUS20060122127A1Eliminate side effectsBiocideCarbohydrate active ingredientsDiseaseNorepinephrine reuptake inhibitor
Compositions, and methods of use thereof, are provided for the prevention or treatment of side effects associated with the use of drugs that act as 5HT2 / 5HT3 serotonin receptor antagonists and alpha-2 adrenergic receptor antagonists (5HT2 / 5HT3 antagonist / alpha-2 antagonist). The method involves using dopamine-releasing compounds, such as amantadine, anticonvulsants, such as zonisamide, or dopamine / norepinephrine reuptake inhibitors, such as bupropion, in combination with 5HT2 / 5HT3 antagonist / alpha-2 antagonists, such as mirtazapine, to reduce the excessive daytime drowsiness and / or weight gain associated with 5HT2 / 5HT3 antagonist / alpha-2 antagonist use for the treatment of disorders, such as, depression, schizophrenia, anxiety disorders, sleep-related breathing disorders, insomnia, migraine headache, chronic tension-type headache, hot flashes, lower back pain, neuropathic pain and functional somatic syndromes. Formulations of dopamine-releasing compounds or anticonvulsants with 5HT2 / 5HT3 antagonist / alpha-2 antagonists are provided. In particular embodiments, combination therapy with mirtazapine and zonisamide provides relief from chronic low back pain, while reducing or avoiding side effects associated with monotherapy with mirtazapine or zonisamide.
Owner:CYPRESS BIOSCI
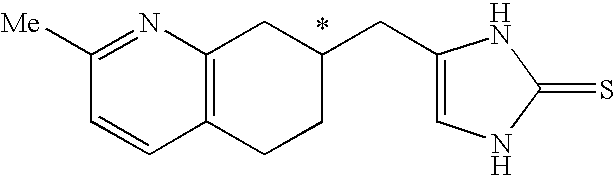
4-(2-Methyl-5,6,7,8-tetrahydro-quinolin-7-ylmethyl)-1,3-dihydro-imidazole-2-thione as specific alpha2B agonist and methods of using the same
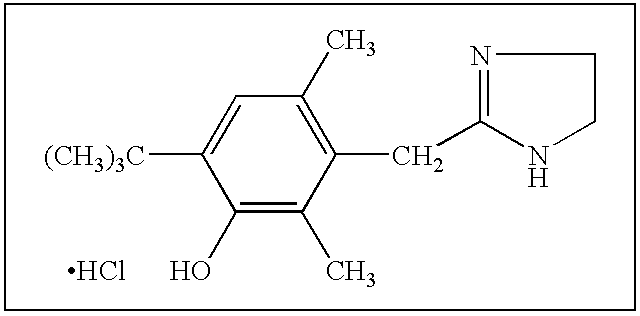
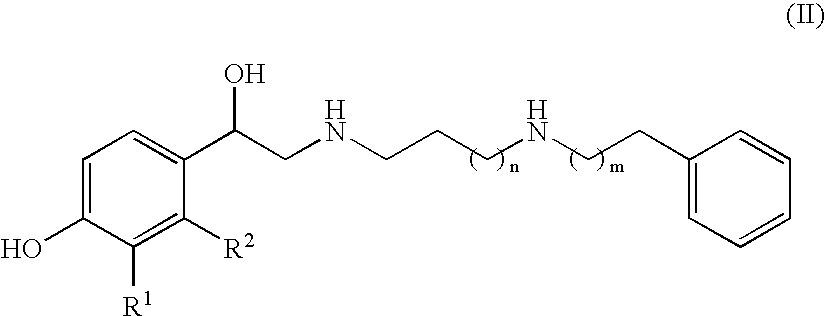
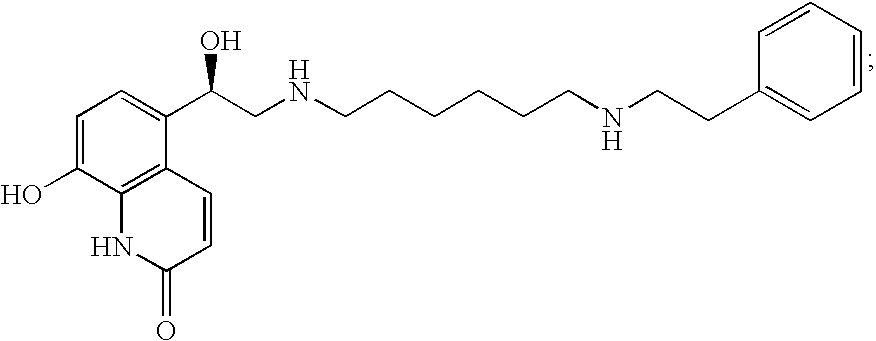
The compound of the formula wherein the * indicates an asymmetric carbon, is specific to alpha2B adrenergic receptors in preference over alpha2A and alpha2C adrenergic receptors, and as such has no or only minimal cardivascular and / or sedatory activity. The compound is useful as medicament in mammals, including humans, for treatment of diseases and or alleviations of conditions which are responsive to treatment by agonists of alpha2B adrenergic receptors.
Owner:ALLERGAN INC
Novel antipsychotic combination therapies and compositions useful therein
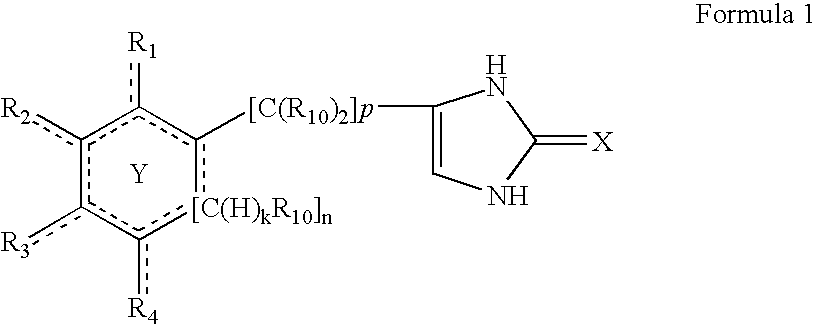
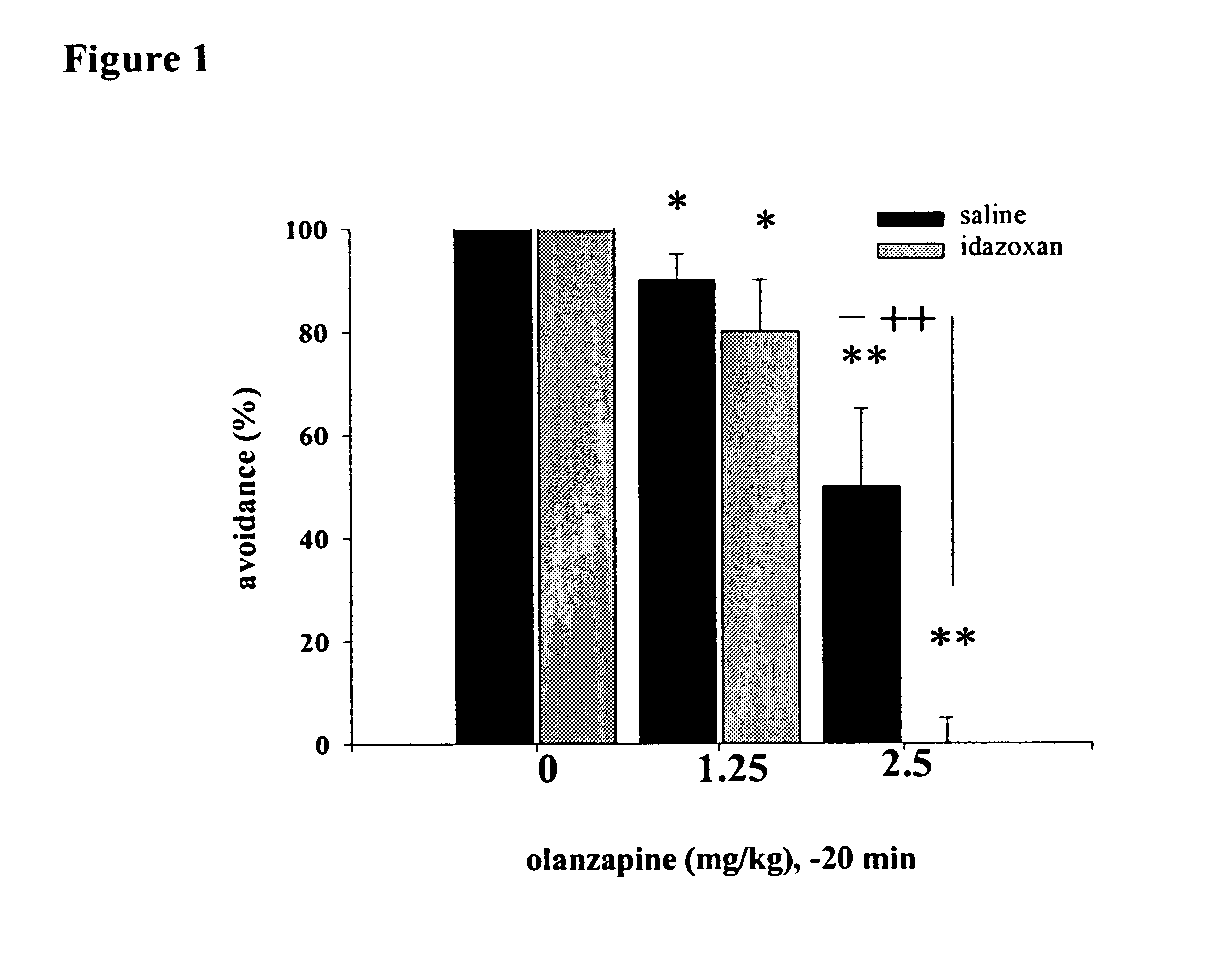
InactiveUS20040127489A1Minimize adverse eventReduce doseBiocideNervous disorderAdrenergicCombination therapy
The present invention provides novel antipsychotic therapies and compositions useful therein and provides methods for identifying new candidate molecules for the treatment of psychosis based on the proportional binding affinities for alpha2 adrenergic and D2 dopamine receptors.
Owner:THE STANLEY MEDICAL RES INST
6-(2-imidazolinylamino)quinoxaline compounds useful as alpha-2 adrenoceptor agonists
InactiveUS6117871ANo adverse side effectsSubstantial activityBiocideAnimal repellantsQuinoxalineThiol
The subject invention relates to methods of treating alpha-2 adenoreceptor modulated disorders, comprising administration, to a mammal in need of such treatment, of a safe and effective amount of a compound having the following structure: wherein: (a) R is unsubstituted C1-C3 alkanyl or alkenyl; and (b) R' is selected from hydrogen; unsubstituted C1-C3 alkanyl or alkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thiol; and halo. The subject invention also relates compounds and compositions for preventing or treating of disorders modulated by alpha-2 adrenoreceptors.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com