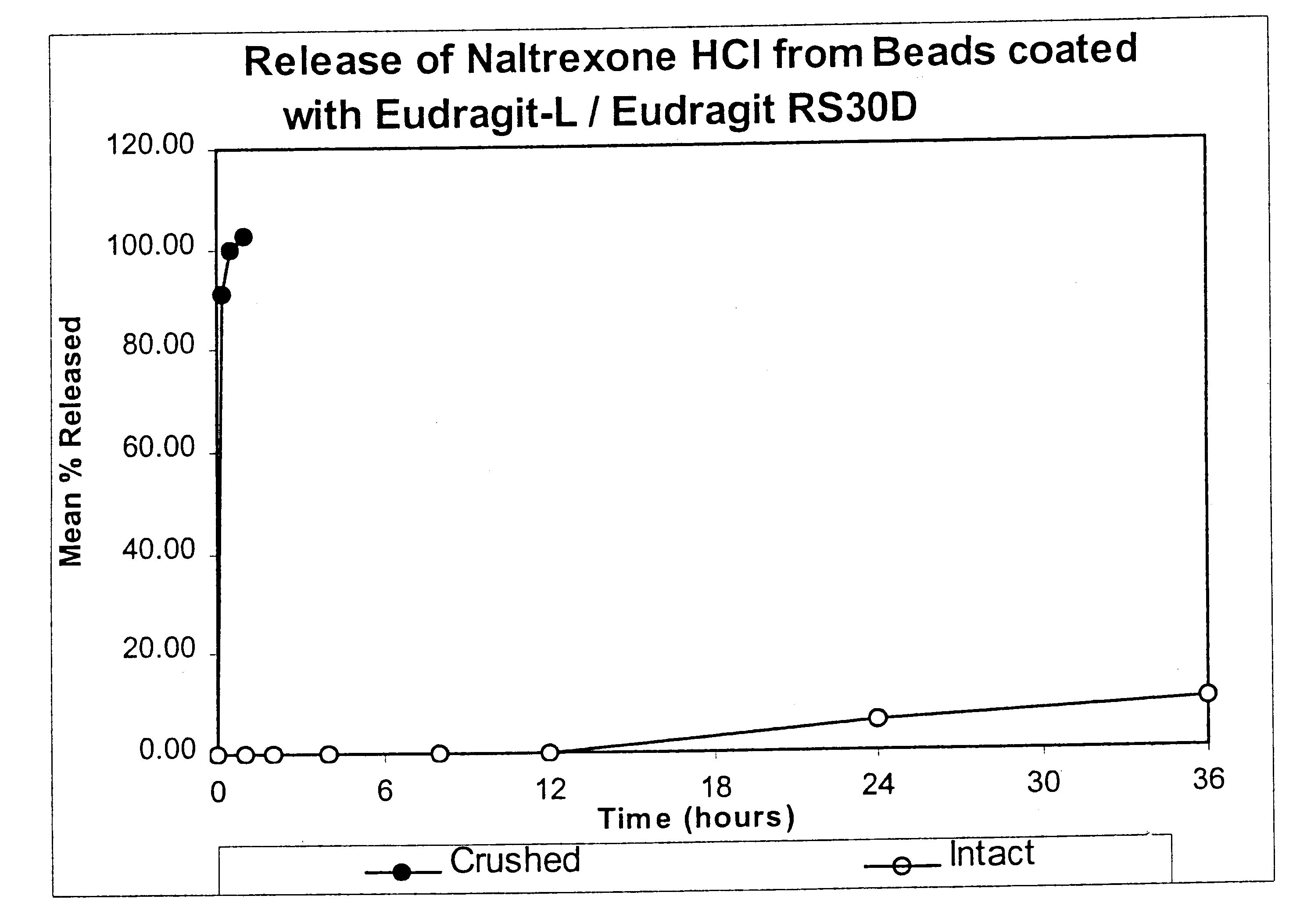
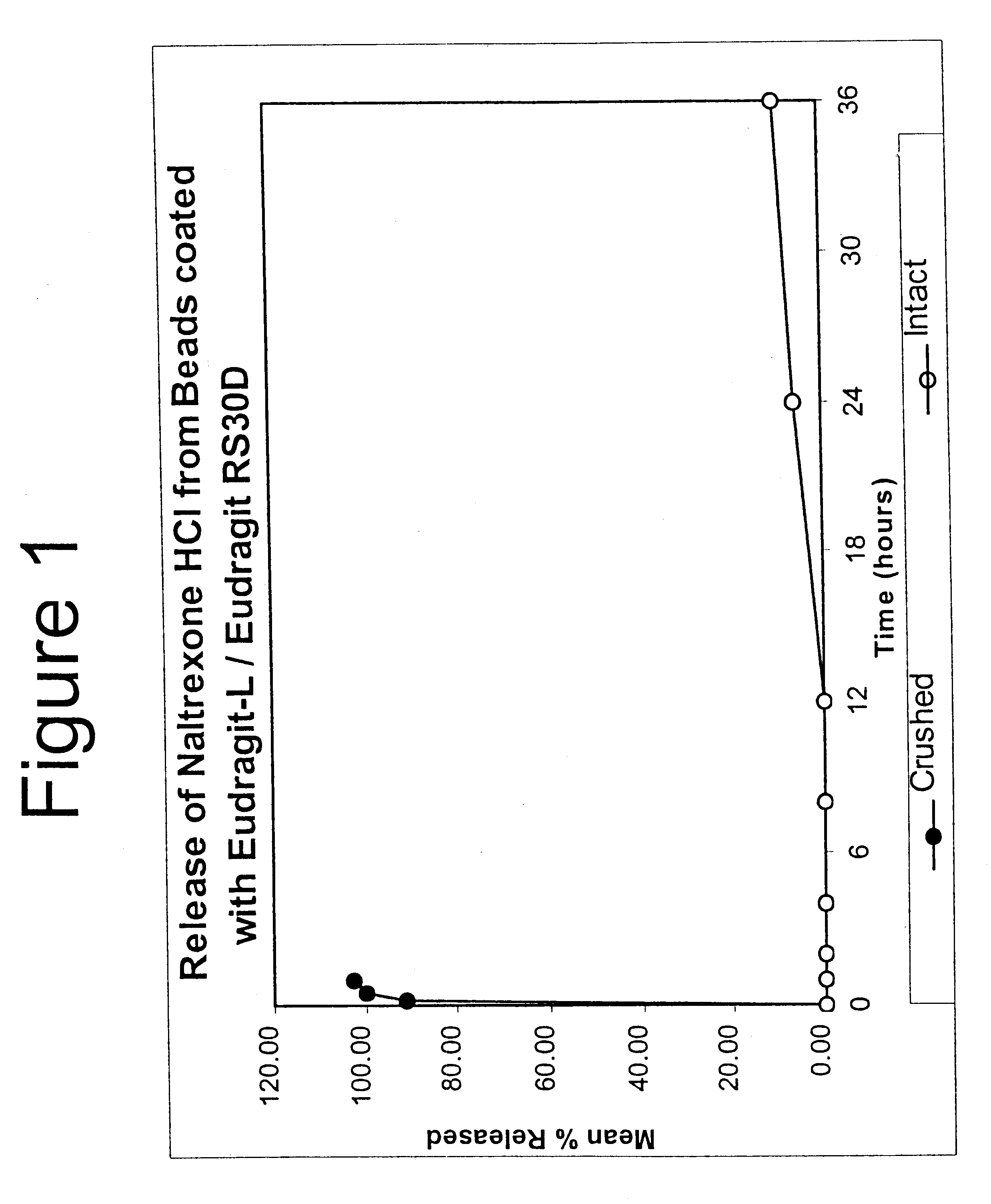
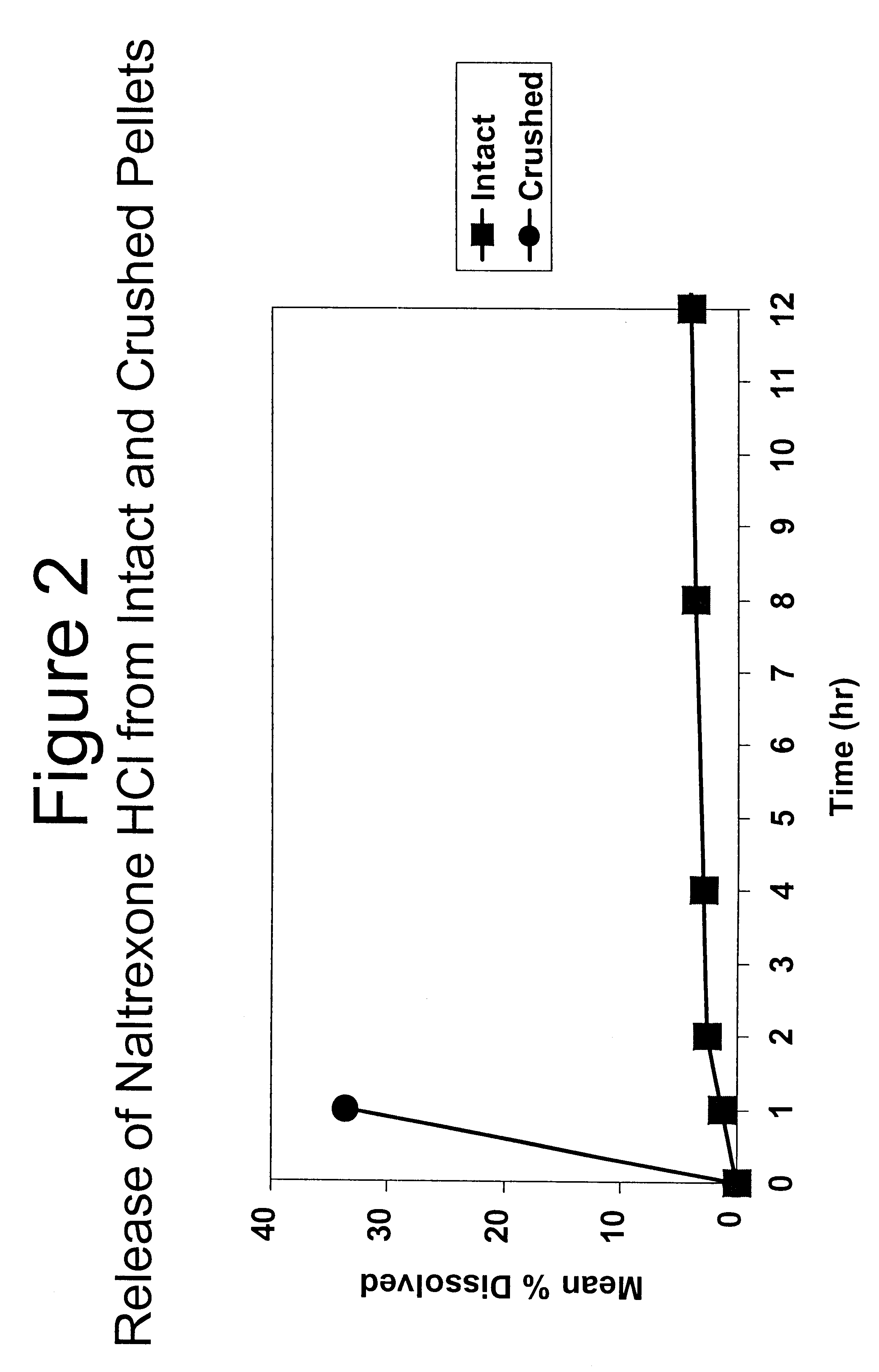
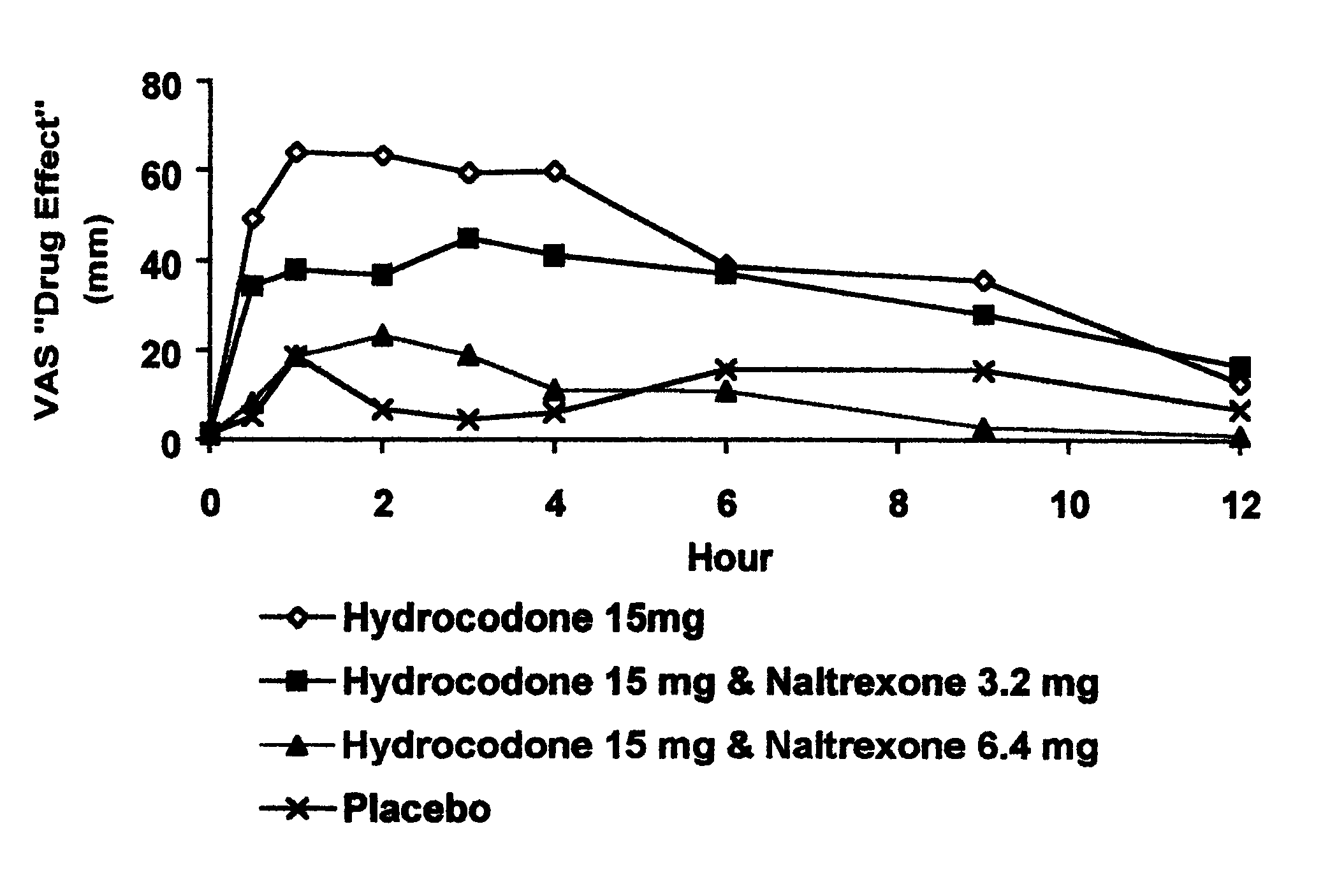
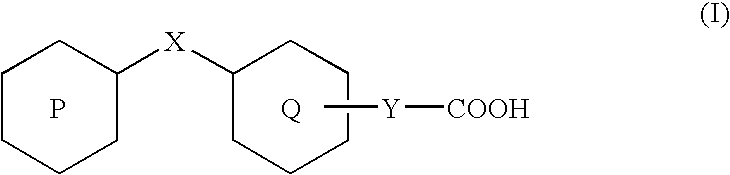
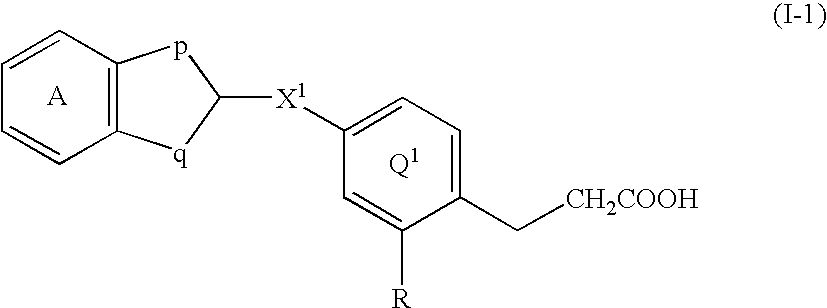
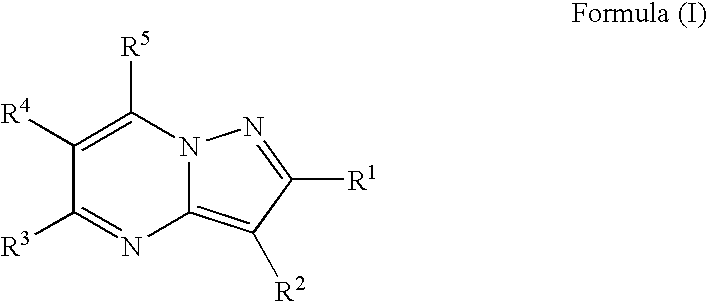
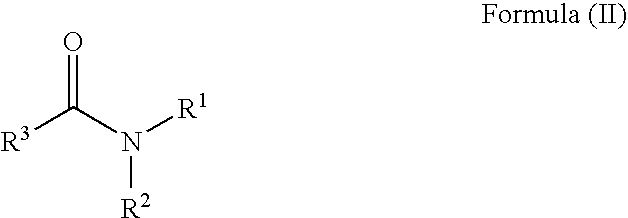Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6702 results about "Agonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. Whereas an agonist causes an action, an antagonist blocks the action of the agonist, and an inverse agonist causes an action opposite to that of the agonist.
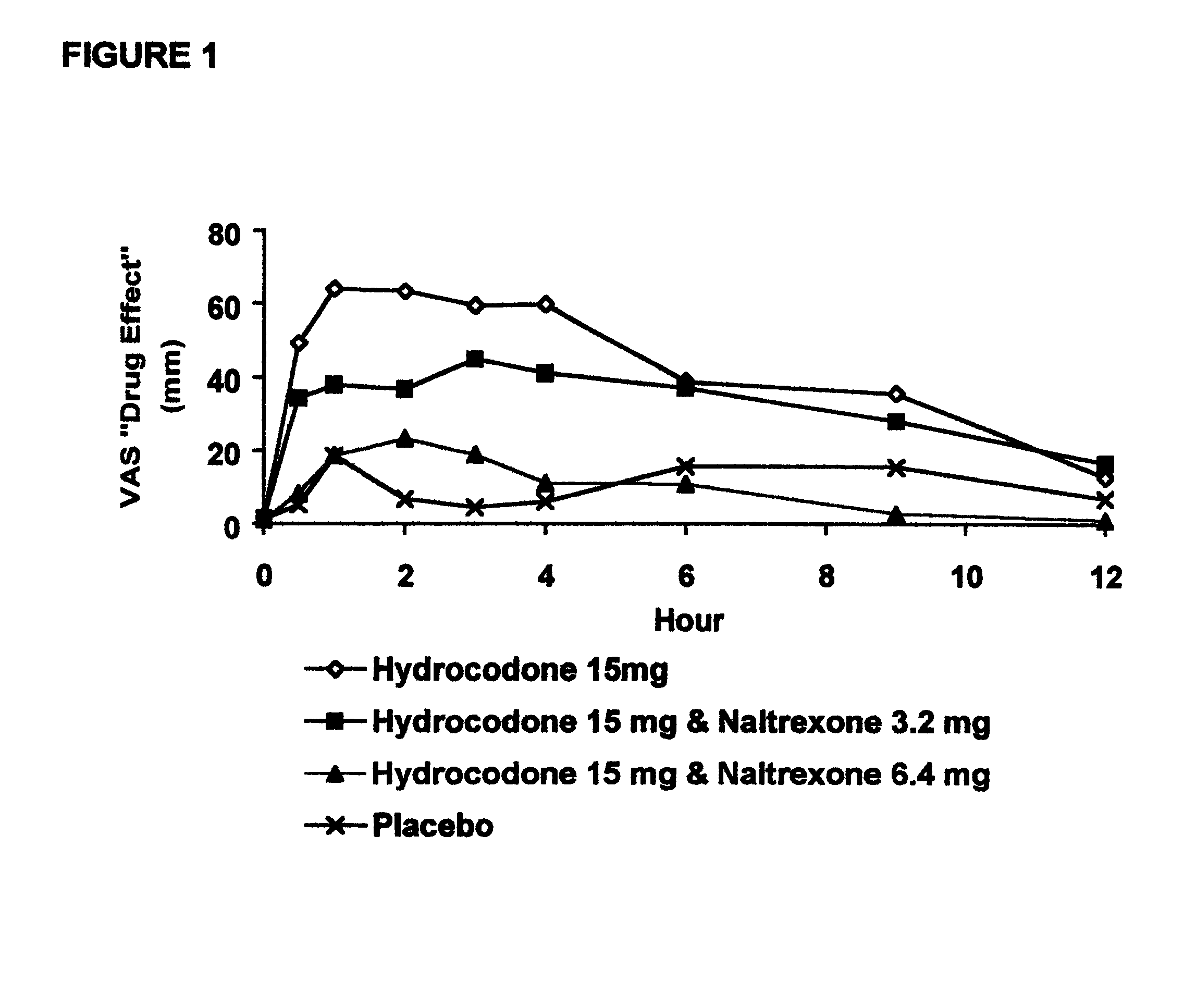
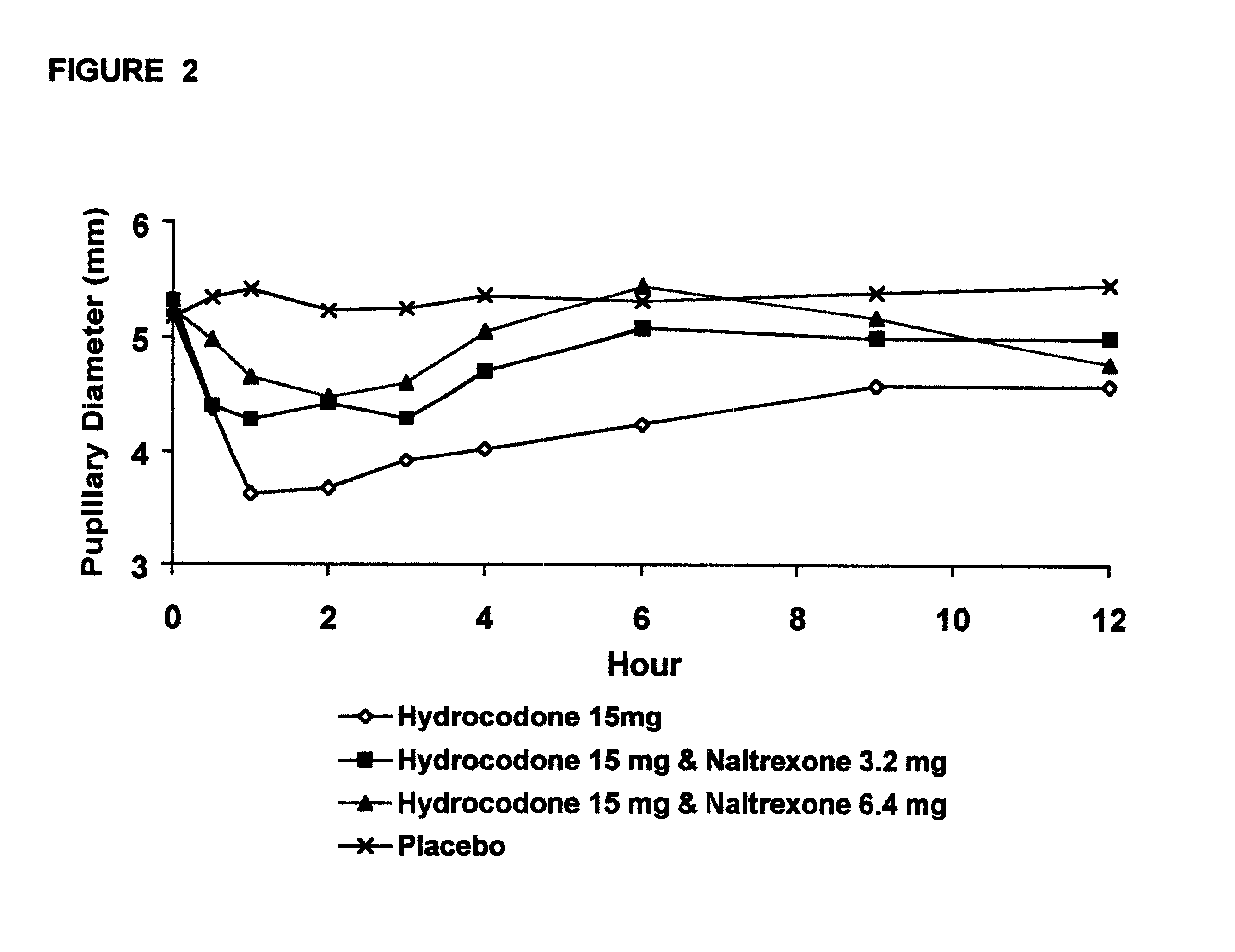
Method of preventing abuse of opioid dosage forms
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders
The present invention provides peptides and peptide analogues that mimic the structural and pharmacological properties of human ApoA-I. The peptides and peptide analogues are useful to treat a variety of disorders associated with dyslipidemia.
Owner:DASSEUX JEAN LOUIS +5
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Peptide agonists of GLP-1 activity
InactiveUS6528486B1Lower blood sugar levelsEffective and stablePeptide/protein ingredientsMetabolism disorderD-GlucoseGlycemic
The present invention relates to novel peptide conjugates which have increased stability and are useful in the treatment of excess levels of blood glucose.
Owner:ZP HLDG SPV
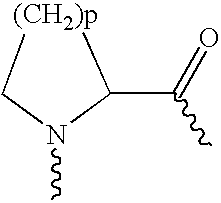
Modified exendins and exendin agonists
InactiveUS6924264B1Increase ratingsDecrease amount of potassiumMetabolism disorderSaccharide peptide ingredientsGastric emptyingPolyethylene glycol
Novel modified exendins and exendin agonist analogs having an exendin or exendin agonist analog linked to one or more polyethylene glycol polymers, for example, and related formulations and dosages and methods of administration thereof are provided. These modified exendins and exendin agonist analogs, compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
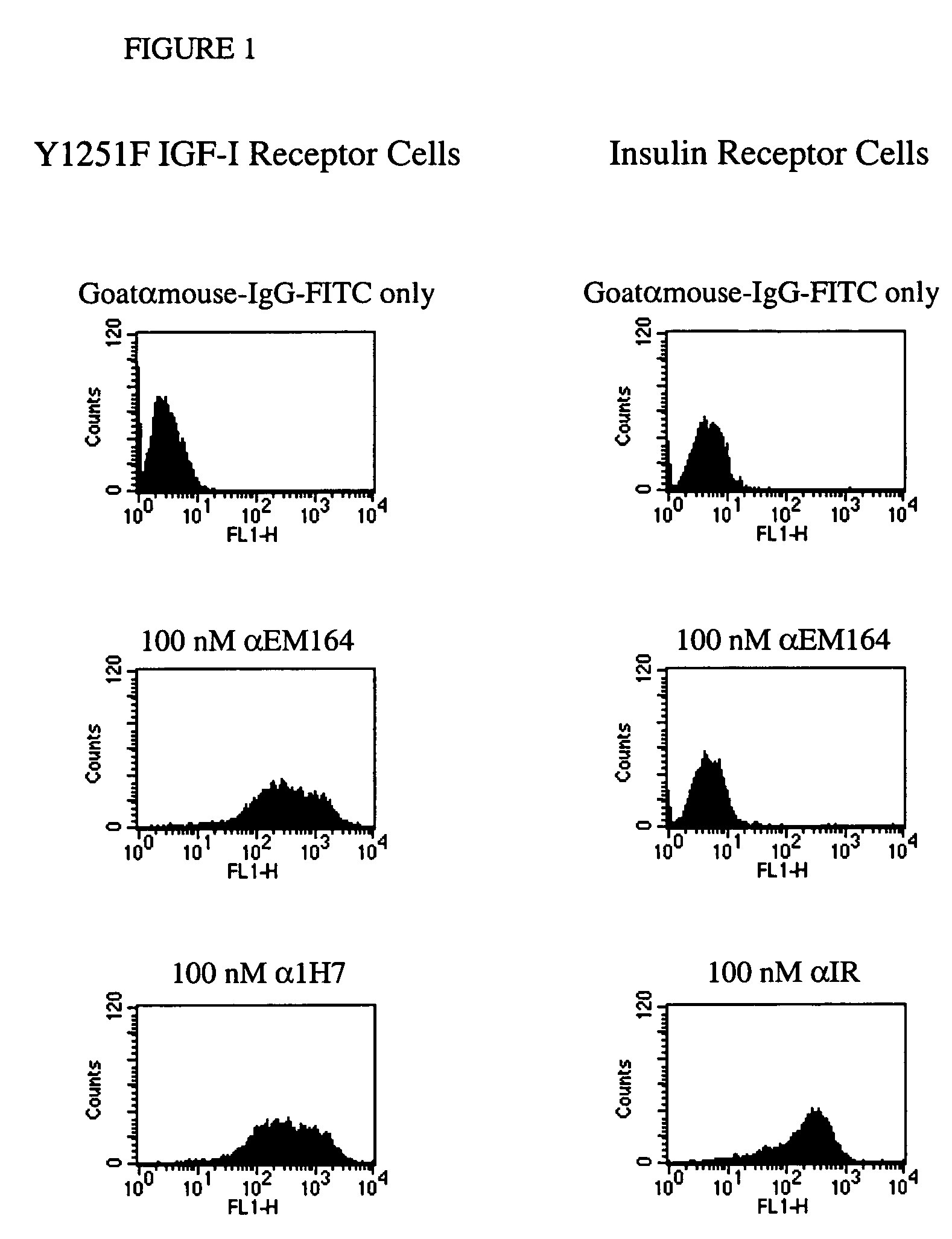
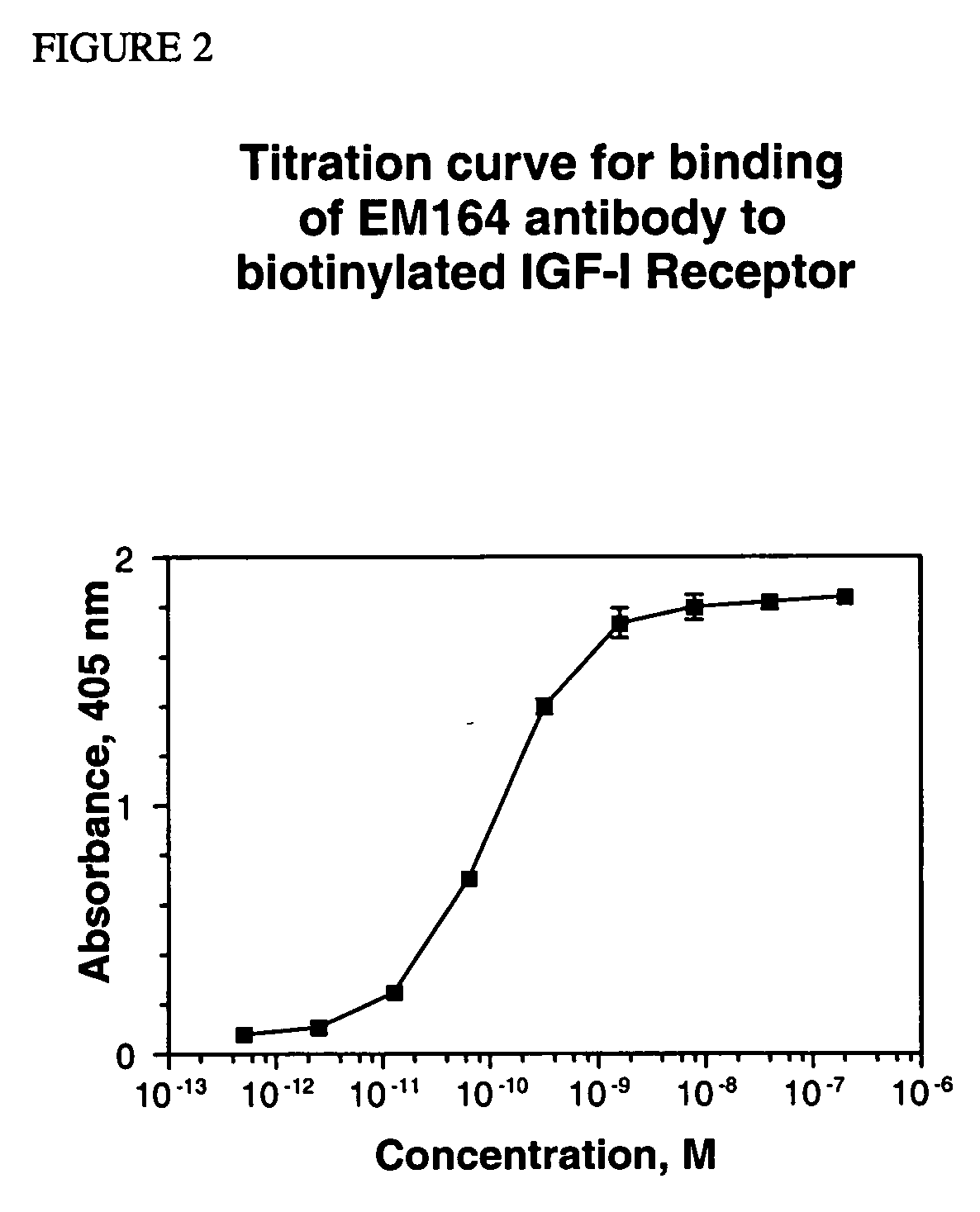
Anti-IGF-I receptor antibody
InactiveUS20050249728A1Increase profitOrganic active ingredientsFungiSynovial sarcomaAbnormal tissue growth
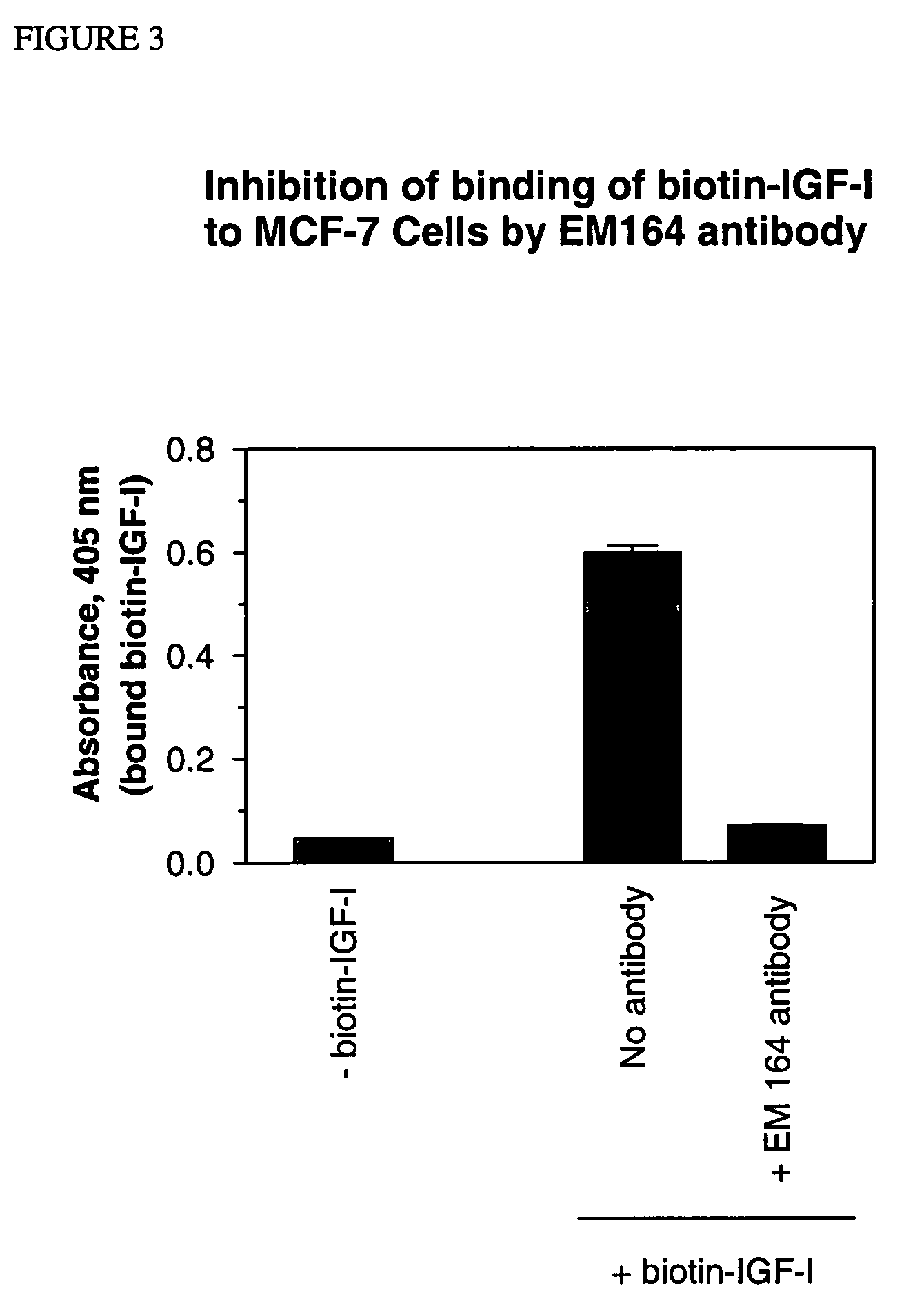
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to, and inhibit, insulin-like growth factor-I receptor, antagonize the effects of IGF-I, IGF-II and serum on the growth and survival of tumor cells, and which are substantially devoid of agonist activity. Said antibodies and fragments thereof may be used, optionally in conjunction with other therapeutic agents, in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma, prostate cancer and pancreatic cancer, and said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
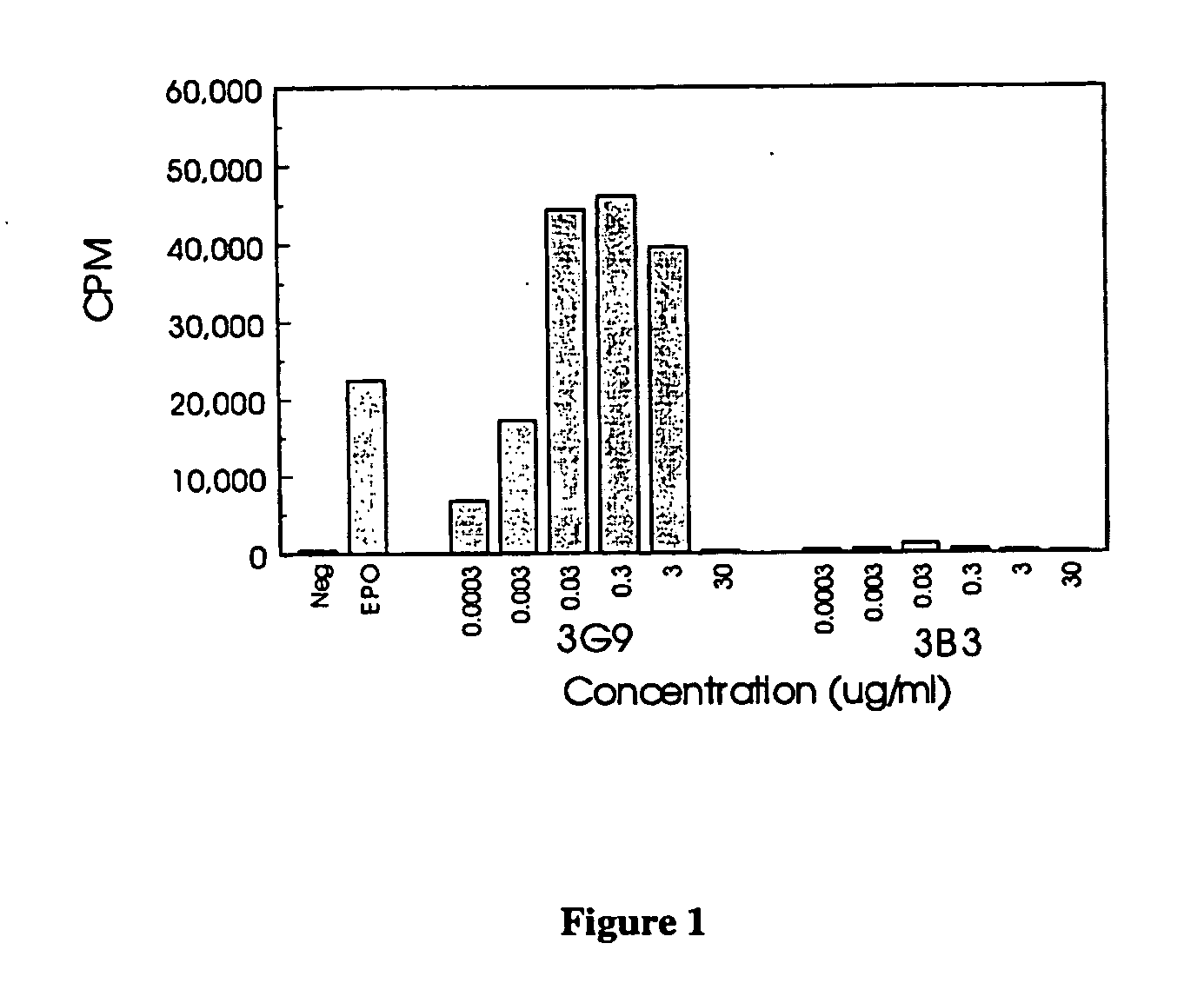
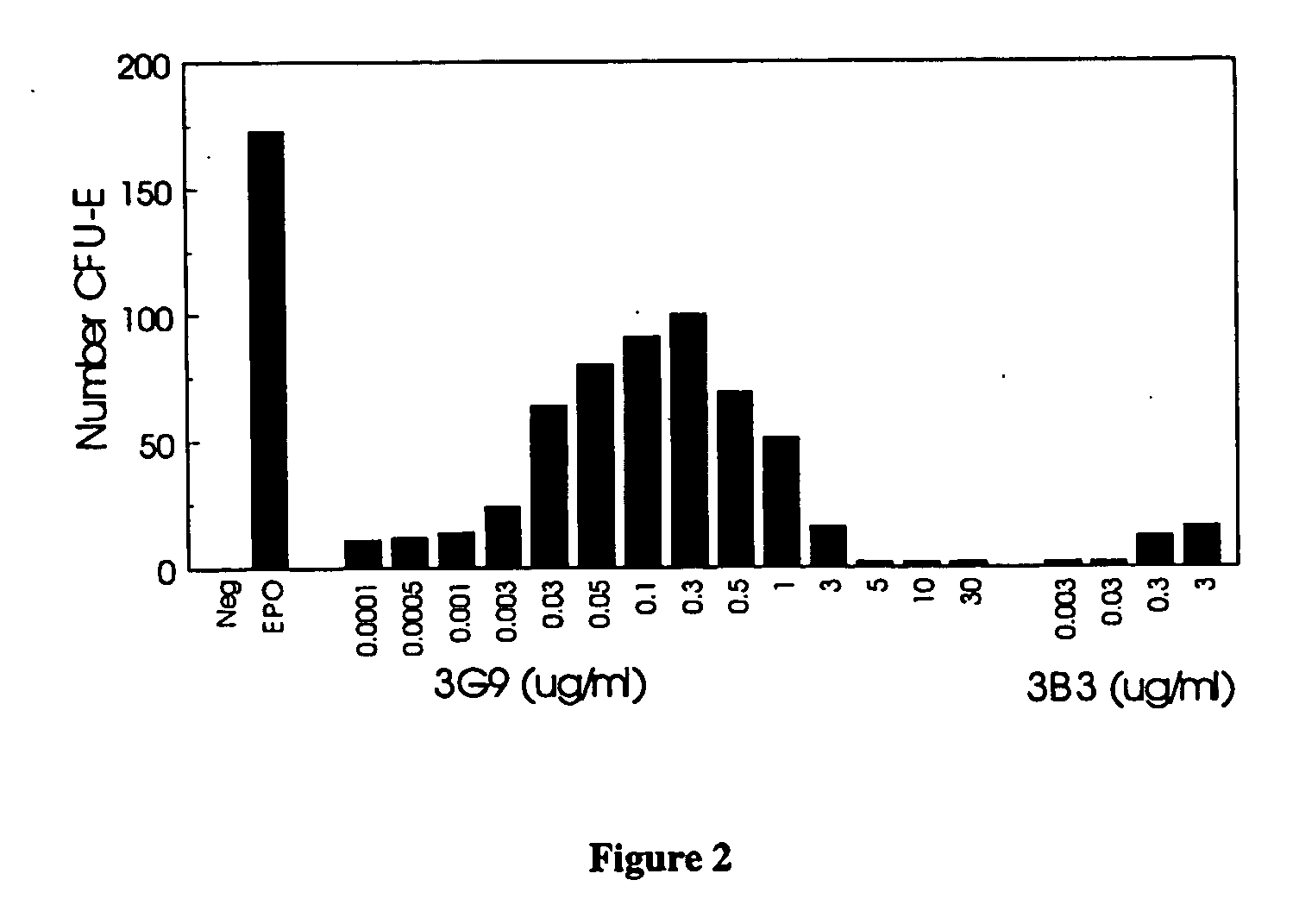
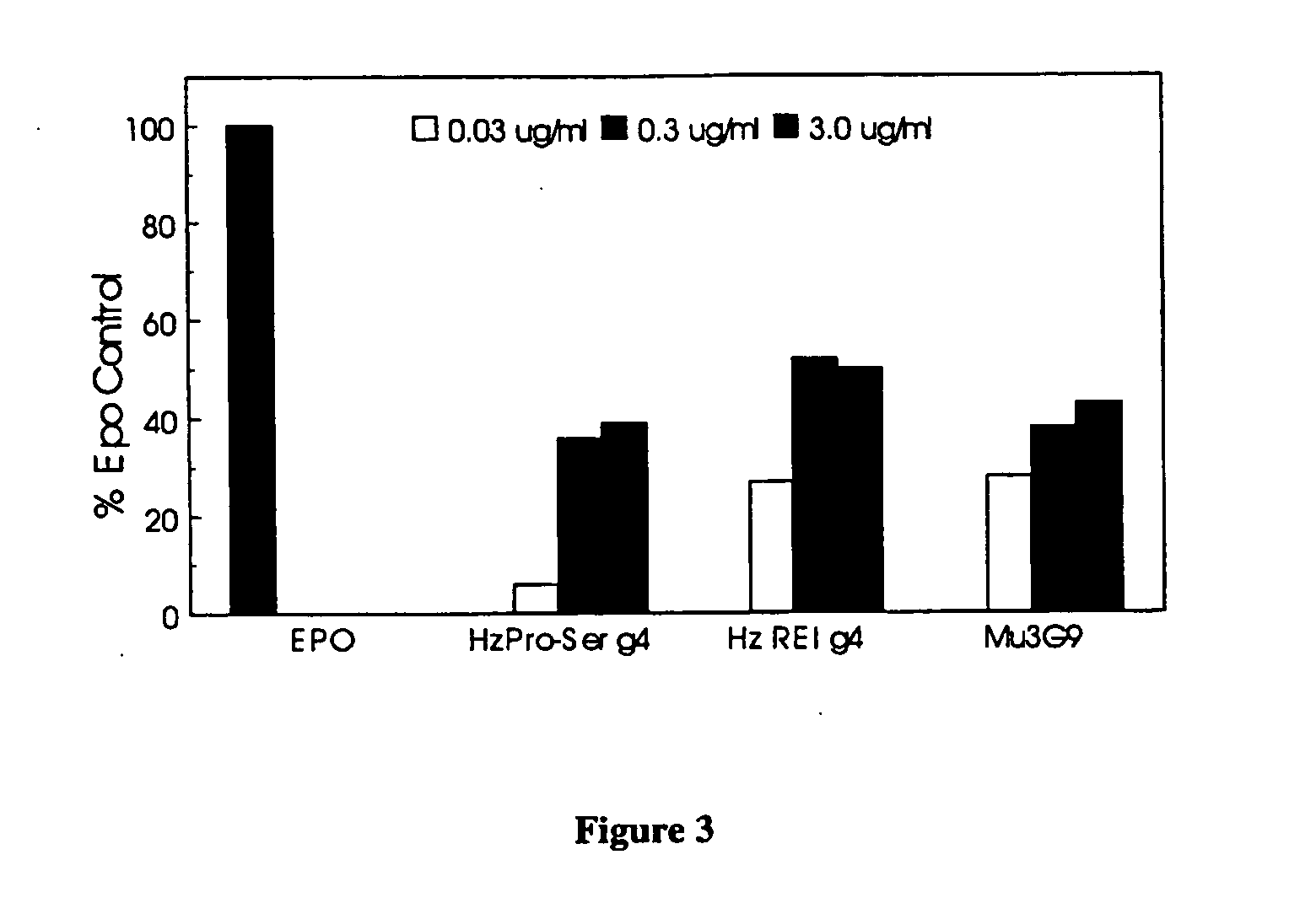
Erythropoietin receptor antibodies
InactiveUS20050244409A1Increased erythropoiesisAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsErythropoietin receptorMedicine
Erythropoietin receptor agonist and antagonist antibodies and their use in enhancing erythropoiesis are disclosed.
Owner:SMITHKLINE BECKMAN CORP
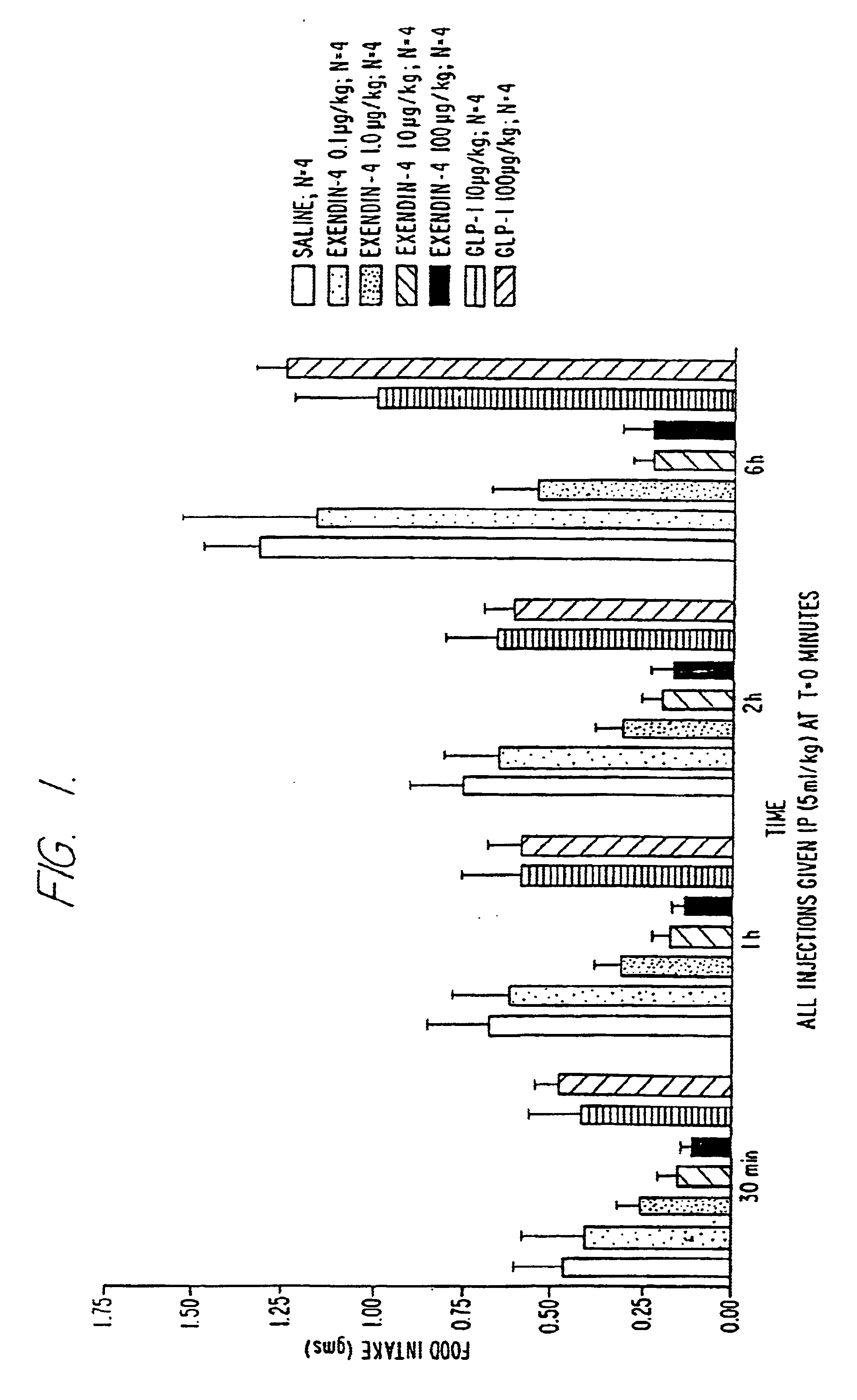
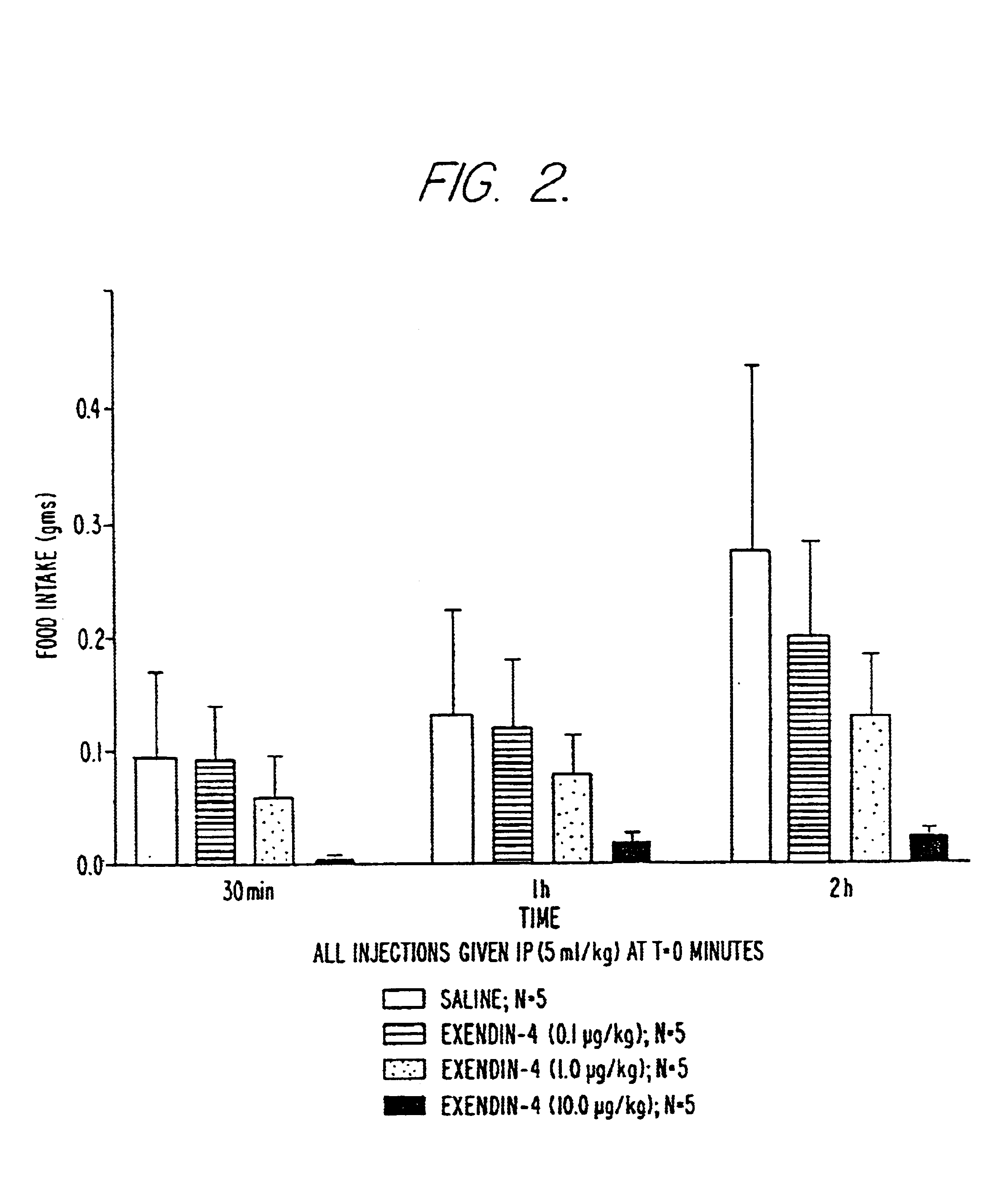
Use of exendins for the reduction of food intake
InactiveUS6956026B2Reduce appetiteReduce cardiac riskPeptide/protein ingredientsPharmaceutical delivery mechanismFeeding disabilityCvd risk
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
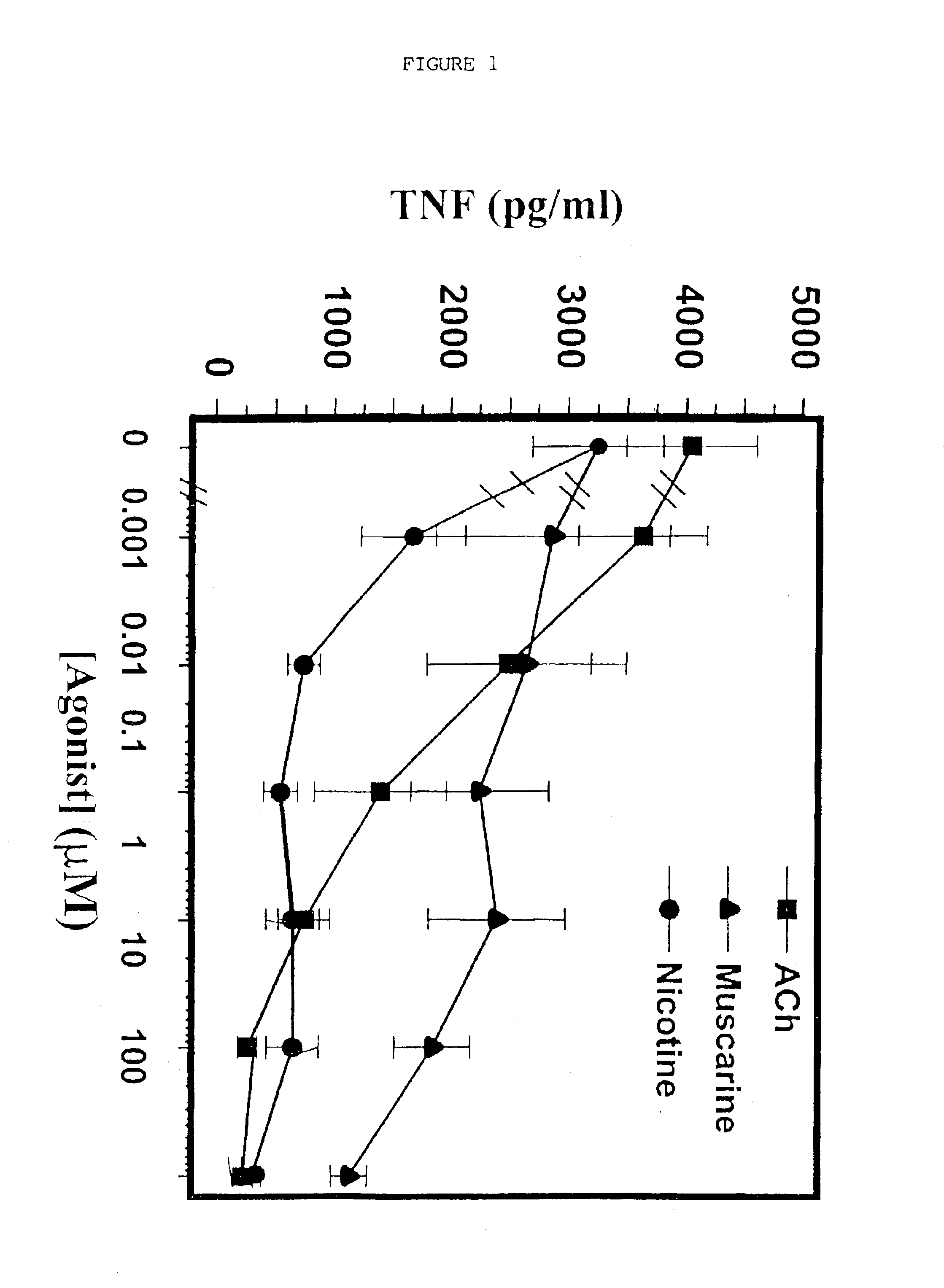
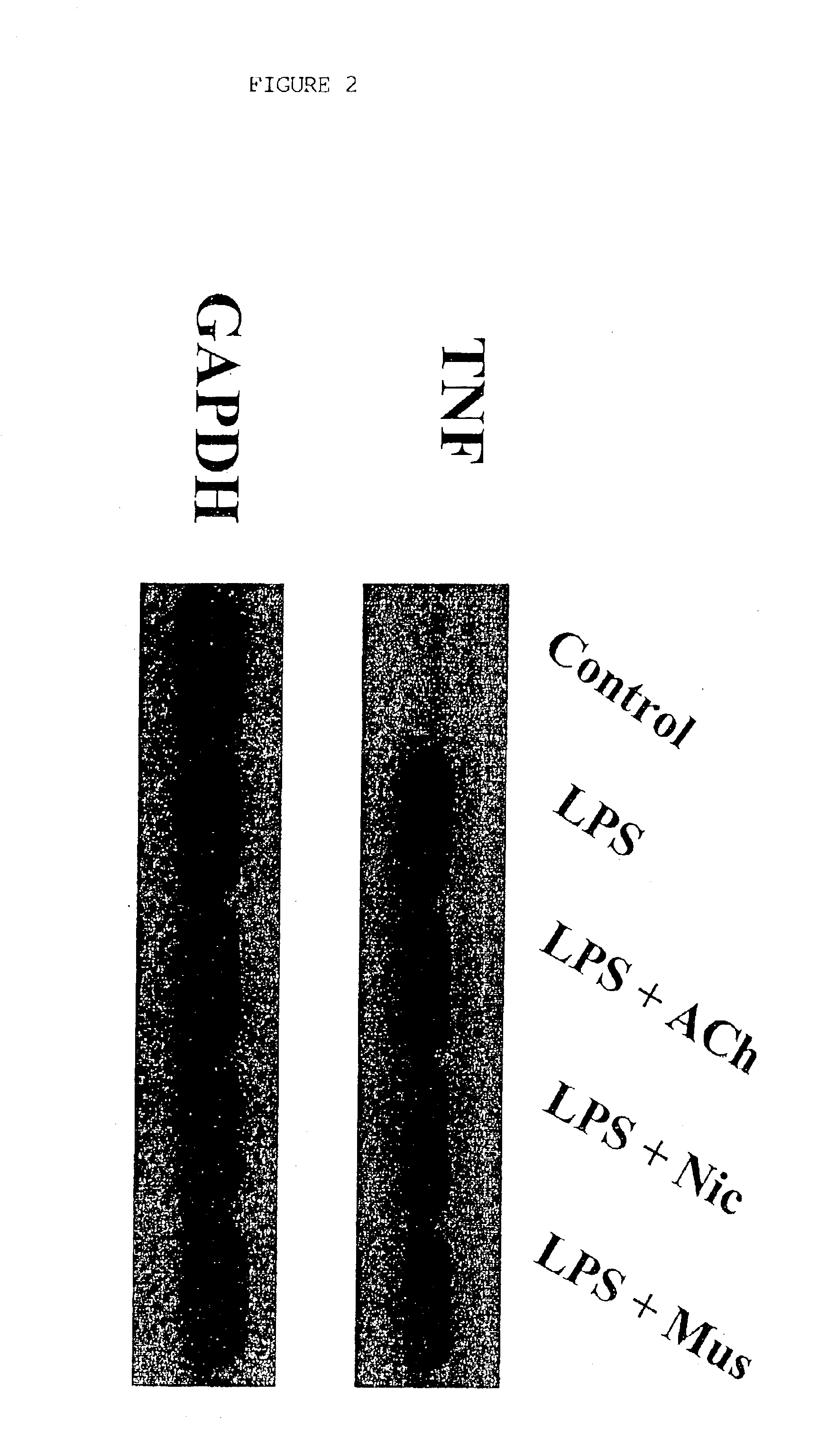
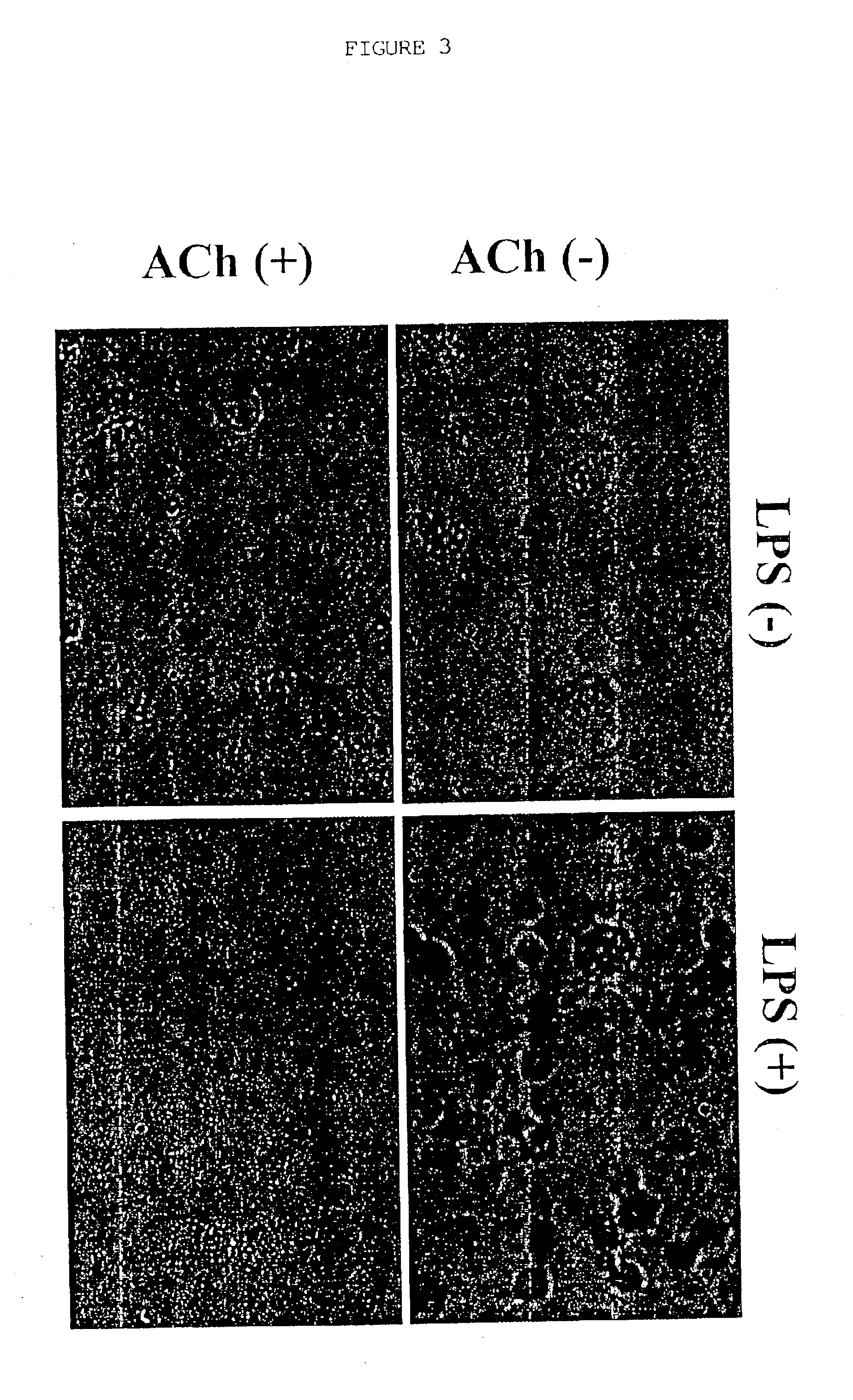
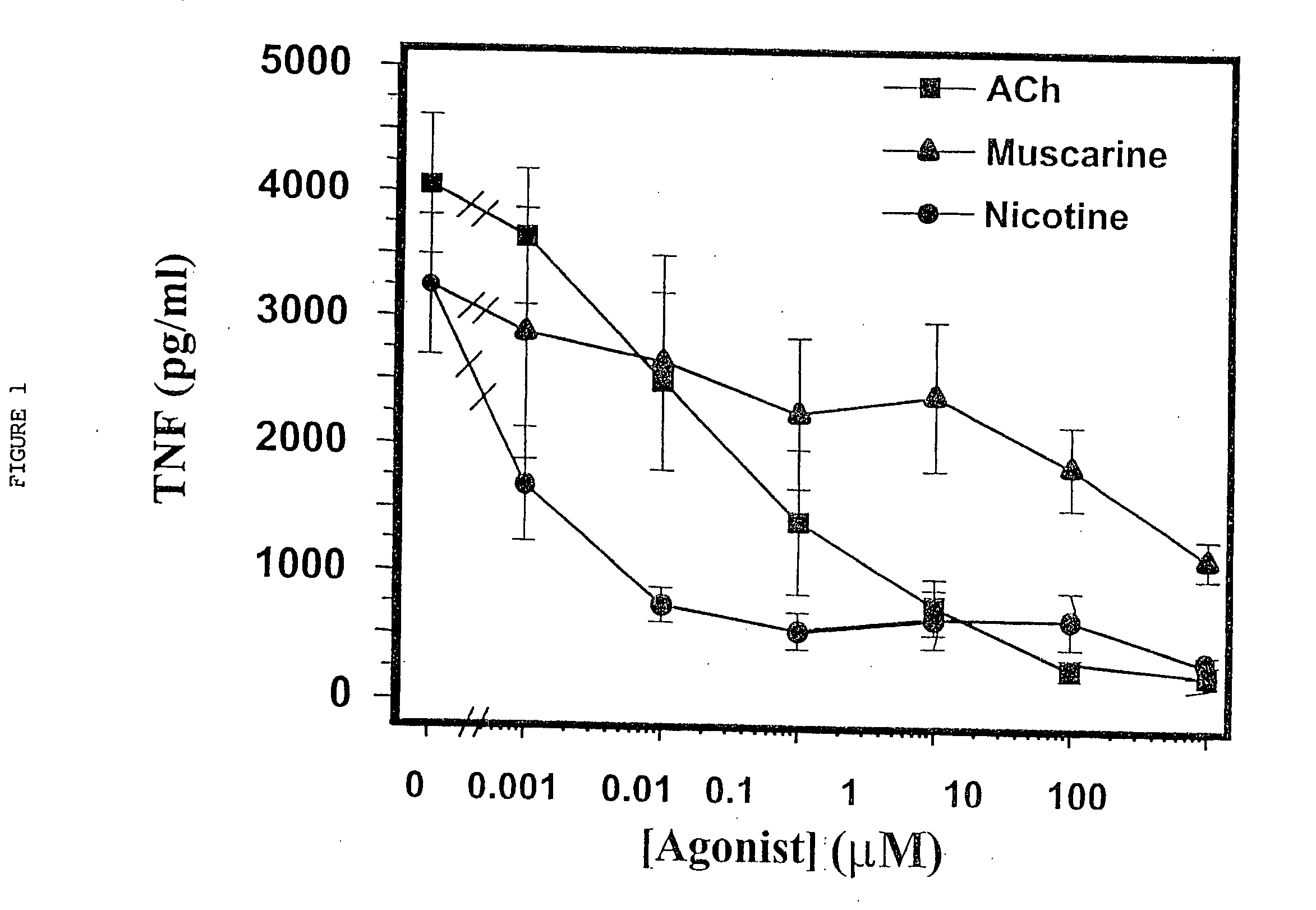
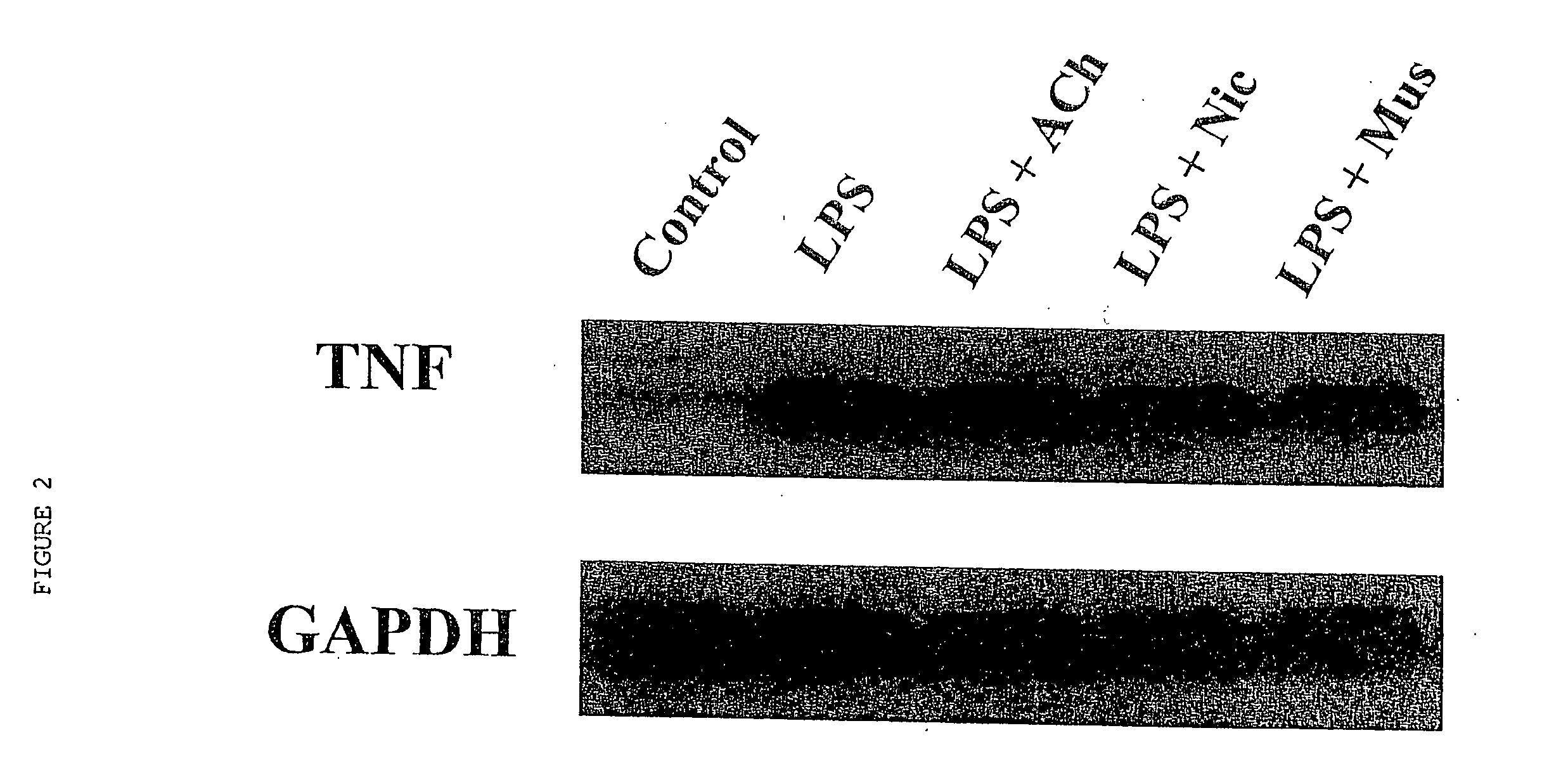
Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation
A method of inhibiting the release of a proinflammatory cytokine in a cell is disclosed. The method comprises treating the cell with a cholinergic agonist. The method is useful in patients at risk for, or suffering from, a condition mediated by an inflammatory cytokine cascade, for example endotoxic shock. The cholinergic agonist treatment can be effected by stimulation of an efferent vagus nerve fiber, or the entire vagus nerve.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Degraded agonist antibody
InactiveUS20040242847A1Excellent antigen-binding propertyExcellent agonist activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAntiendomysial antibodies
The invention relates to a modified antibody which contains two or more H chain V regions and two or more L chain V regions of monoclonal antibody and can transduce a signal into cells by crosslinking a cell surface molecule(s) to thereby serve as an agonist. The modified antibody can be used as a signal transduction agonist and, therefore, useful as a preventive and / or remedy for various diseases such as cancer, inflammation, hormone disorders and blood diseases.
Owner:CHUGAI PHARMA CO LTD
Anti-IGF-I receptor antibody
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to, and inhibit, insulin-like growth factor-I receptor, antagonize the effects of IGF-I, IGF-II and serum on the growth and survival of tumor cells, and which are substantially devoid of agonist activity. Said antibodies and fragments thereof may be used, optionally in conjunction with other therapeutic agents, in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma, prostate cancer and pancreatic cancer, and said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Therapeutic Use of a TLR Agonist and Combination Therapy
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
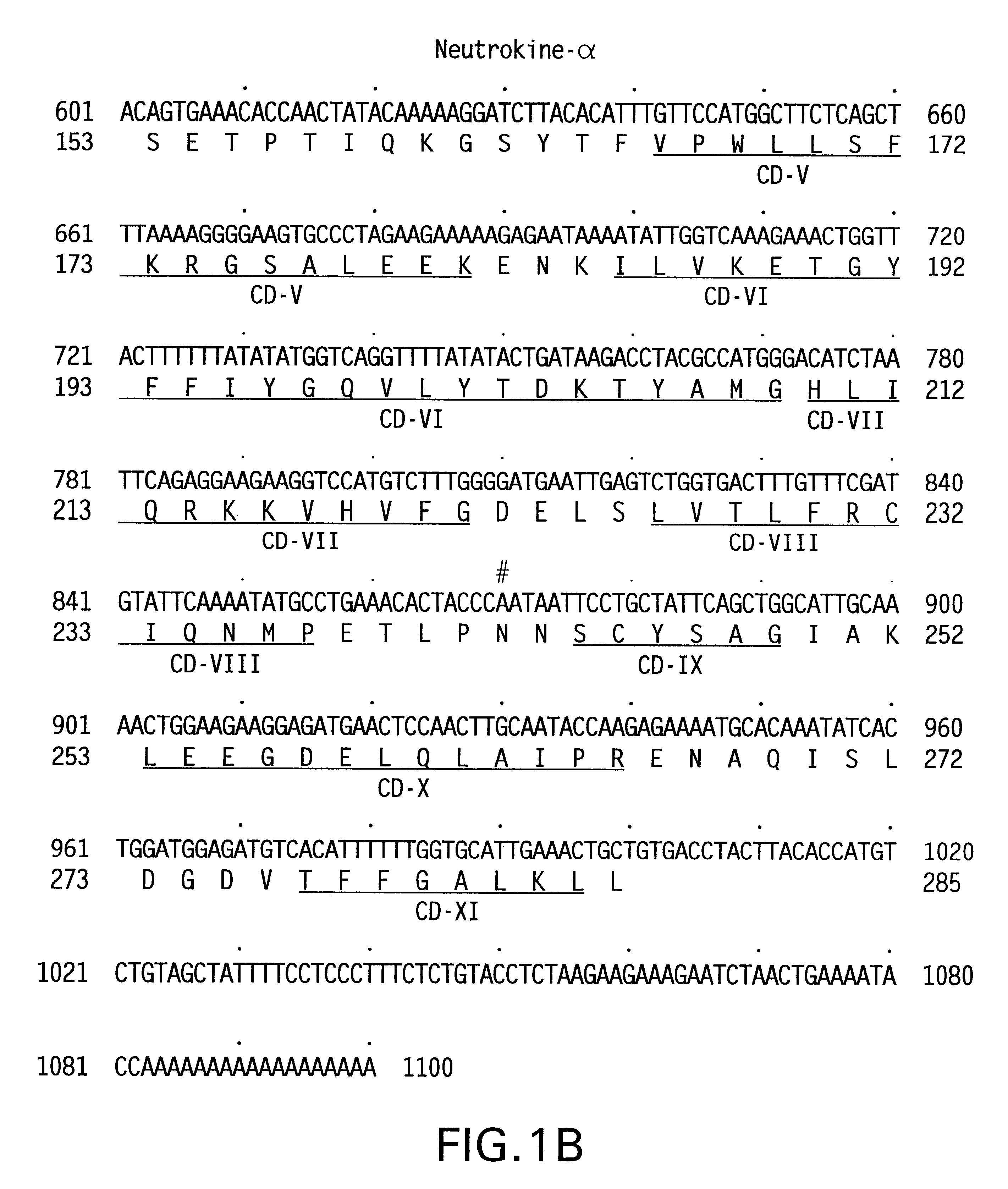
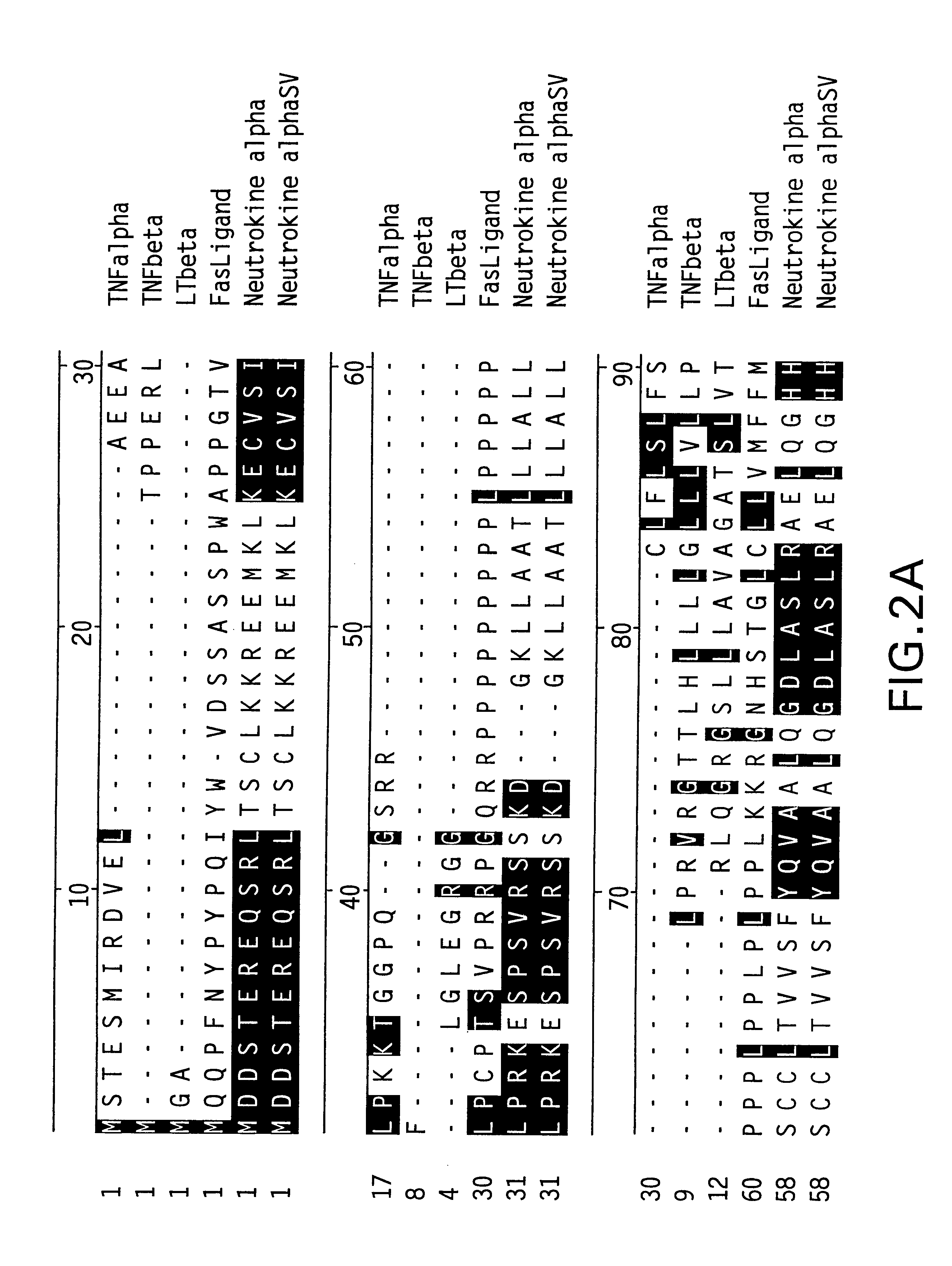
Antibodies to neutrokine-alpha
InactiveUS6403770B1Peptide/protein ingredientsGenetic material ingredientsScreening methodTnf family
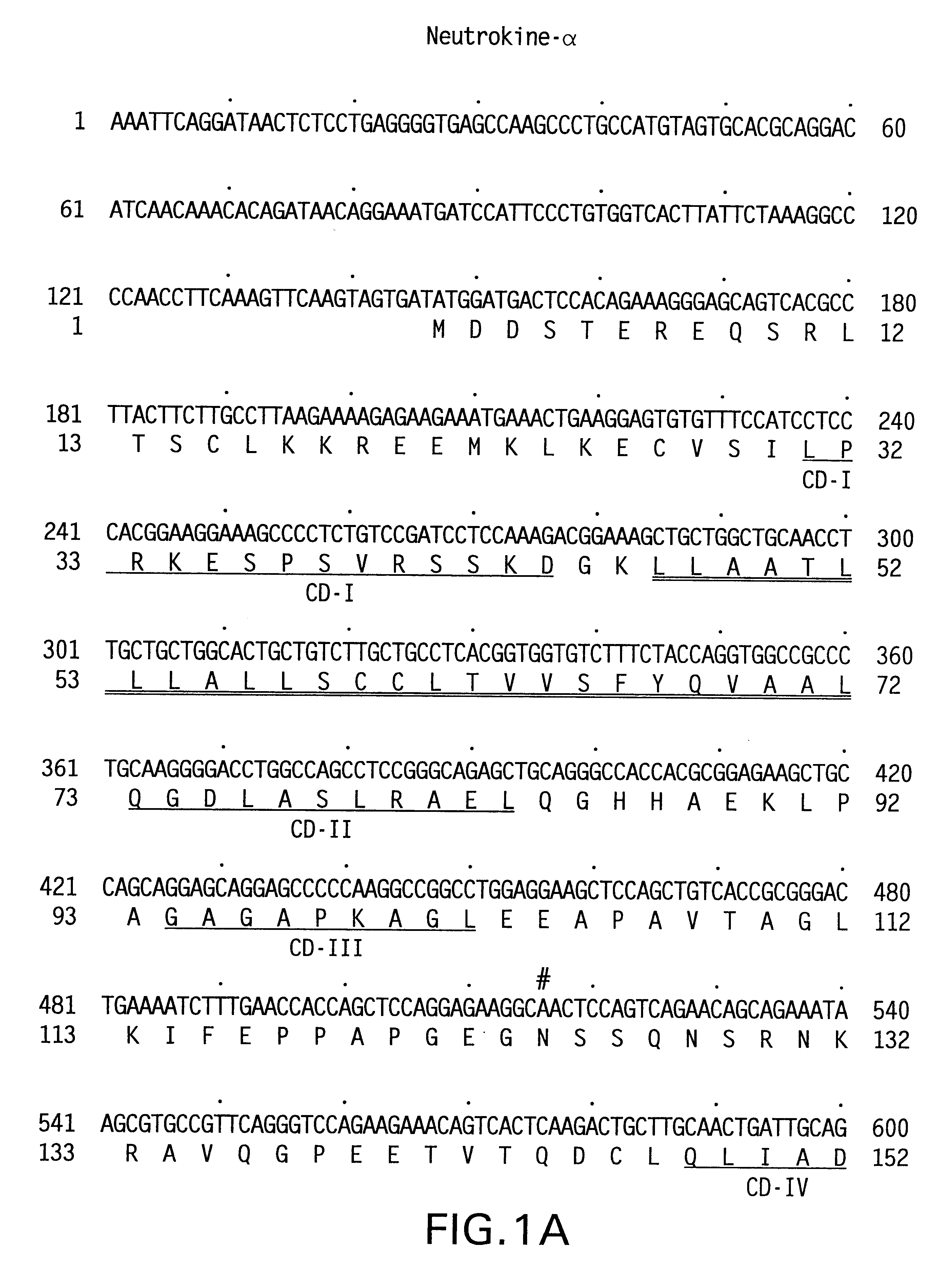
The present invention relates to a novel Neutrokine-alpha, and a splice variant thereof designated Neutrokine-alphaSV, polynucleotides and polypeptides which are members of the TNF family. In particular, isolated nucleic acid molecules are provided encoding the human Neutrokine-alpha and / or Neutrokine-alphaSV polypeptides, including soluble forms of the extracellular domain. Neutrokine-alpha and / or Neutrokine-alphaSV polypeptides are also provided as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists and antagonists of Neutrokine-alpha and / or Neutrokine-alphaSV activity. Also provided are diagnostic methods for detecting immune system-related disorders and therapeutic methods for treating immune system-related disorders.
Owner:HUMAN GENOME SCI INC
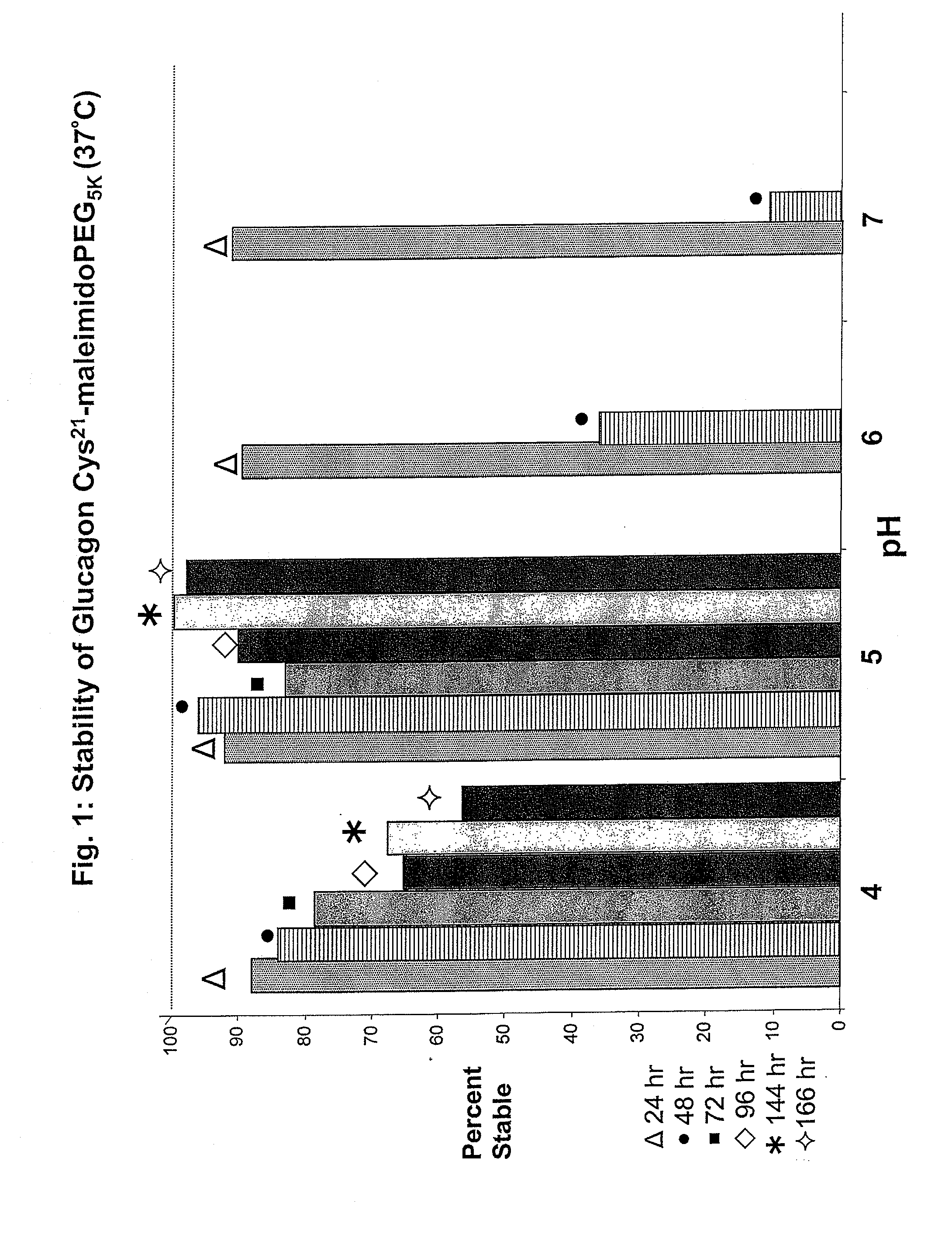
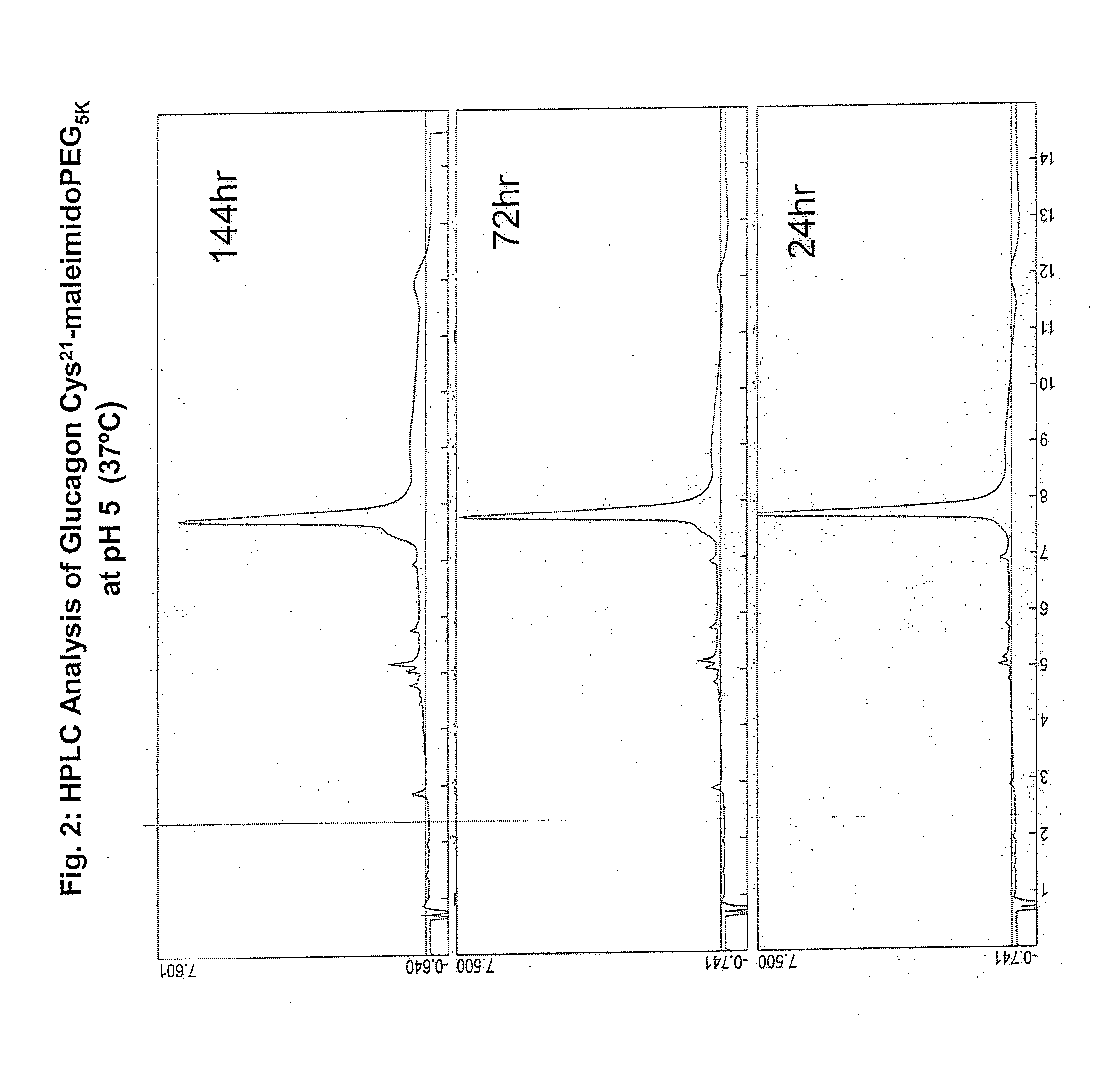
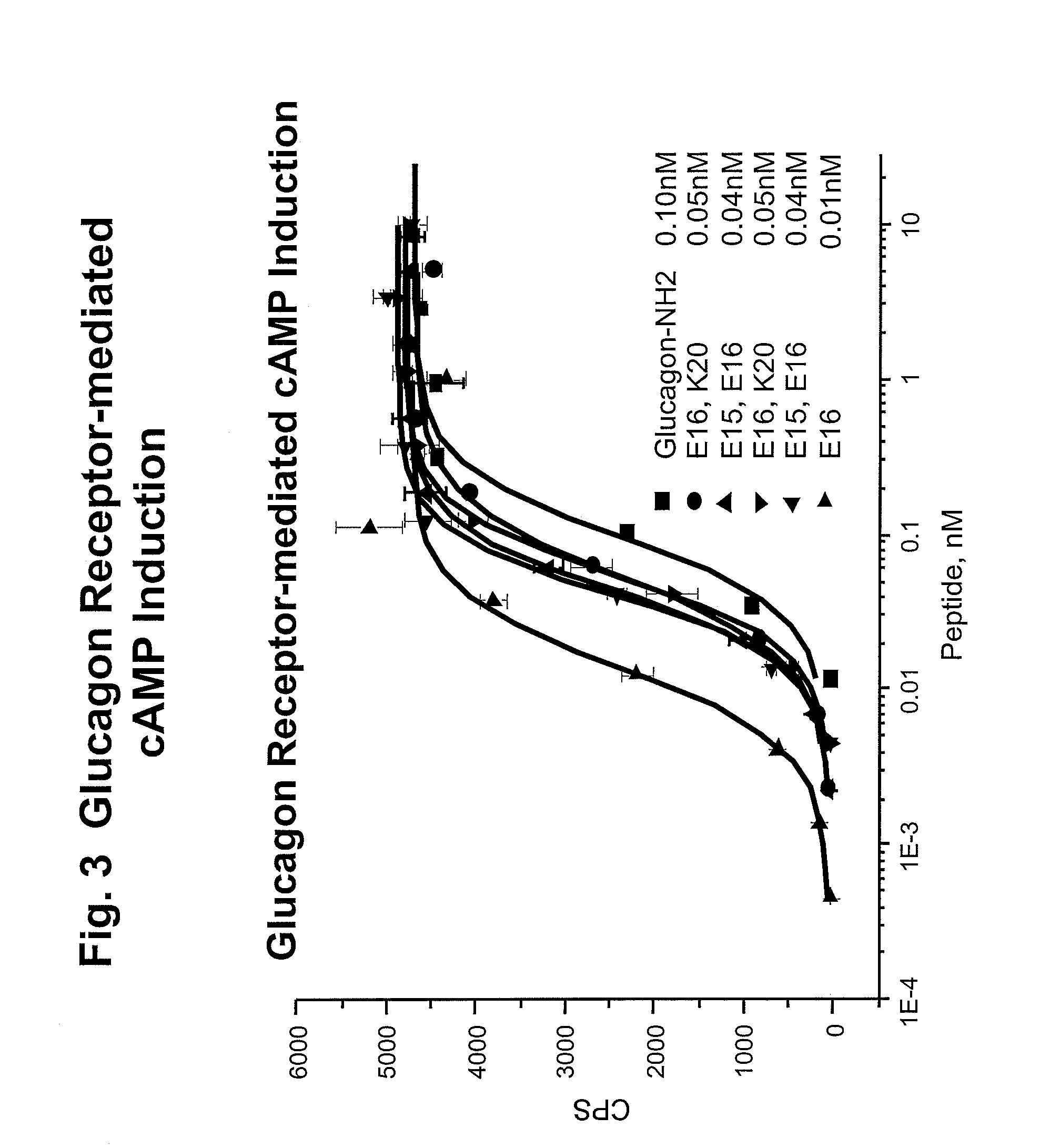
Glucagon/glp-1 receptor co-agonists
InactiveUS20100190701A1High activityEnhanced biophysical stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityCarboxylic acid
Modified glucagon peptides are disclosed having enhanced potency at the glucagon receptor relative to native glucagon. Further modification of the glucagon peptides by forming lactam bridges or the substitution of the terminal carboxylic acid with an amide group produces peptides exhibiting glucagon / GLP-1 receptor co-agonist activity. The solubility and stability of these high potency glucagon analogs can be further improved by modification of the polypeptides by pegylation, substitution of carboxy terminal amino acids, or the addition of a carboxy terminal peptide selected from the group consisting of SEQ ID NO: 26 (GPSSGAPPPS), SEQ ID NO: 27 (K-RNRNNIA) and SEQ ID NO: 28 (KRNR).
Owner:INDIANA UNIV RES & TECH CORP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
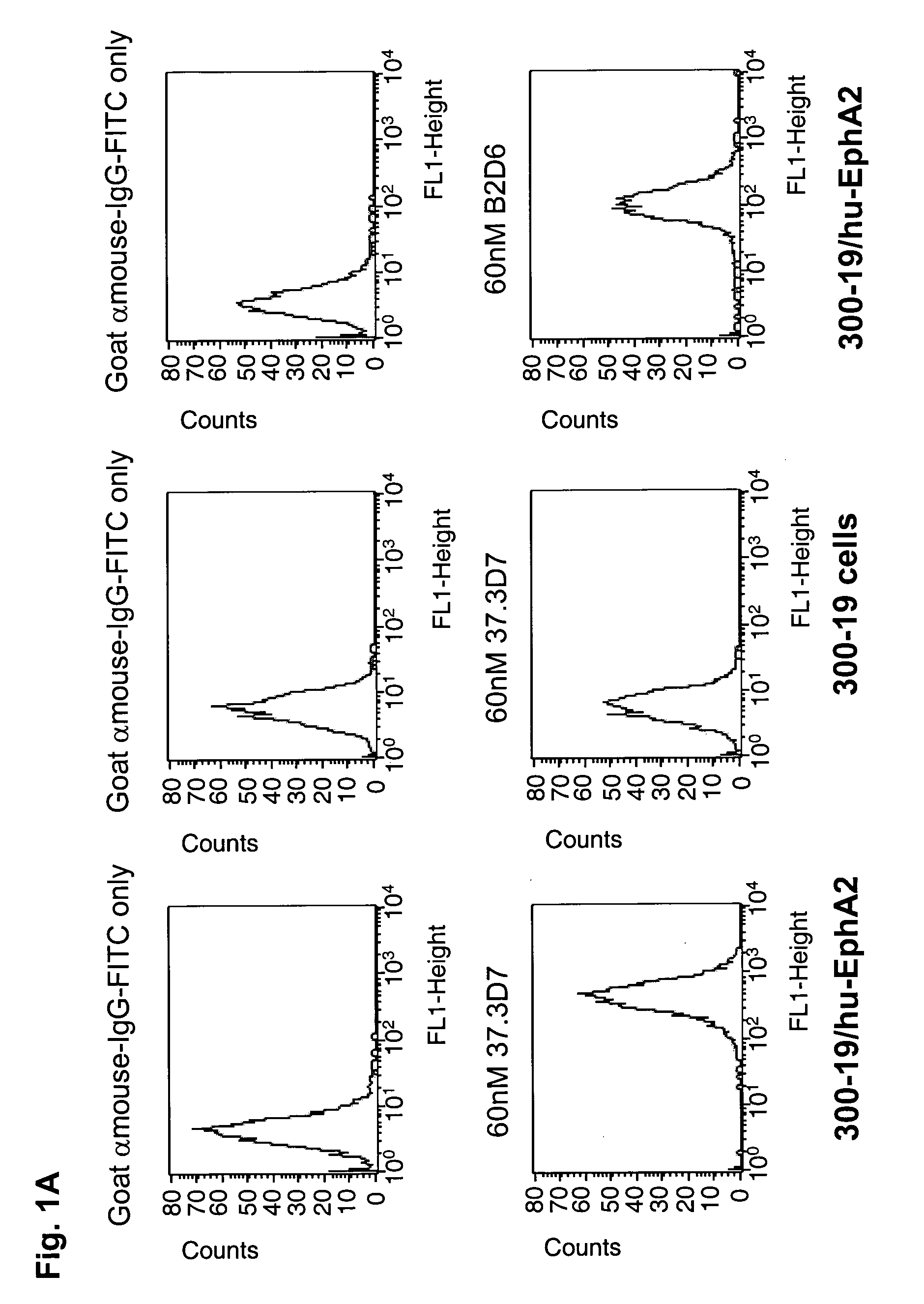
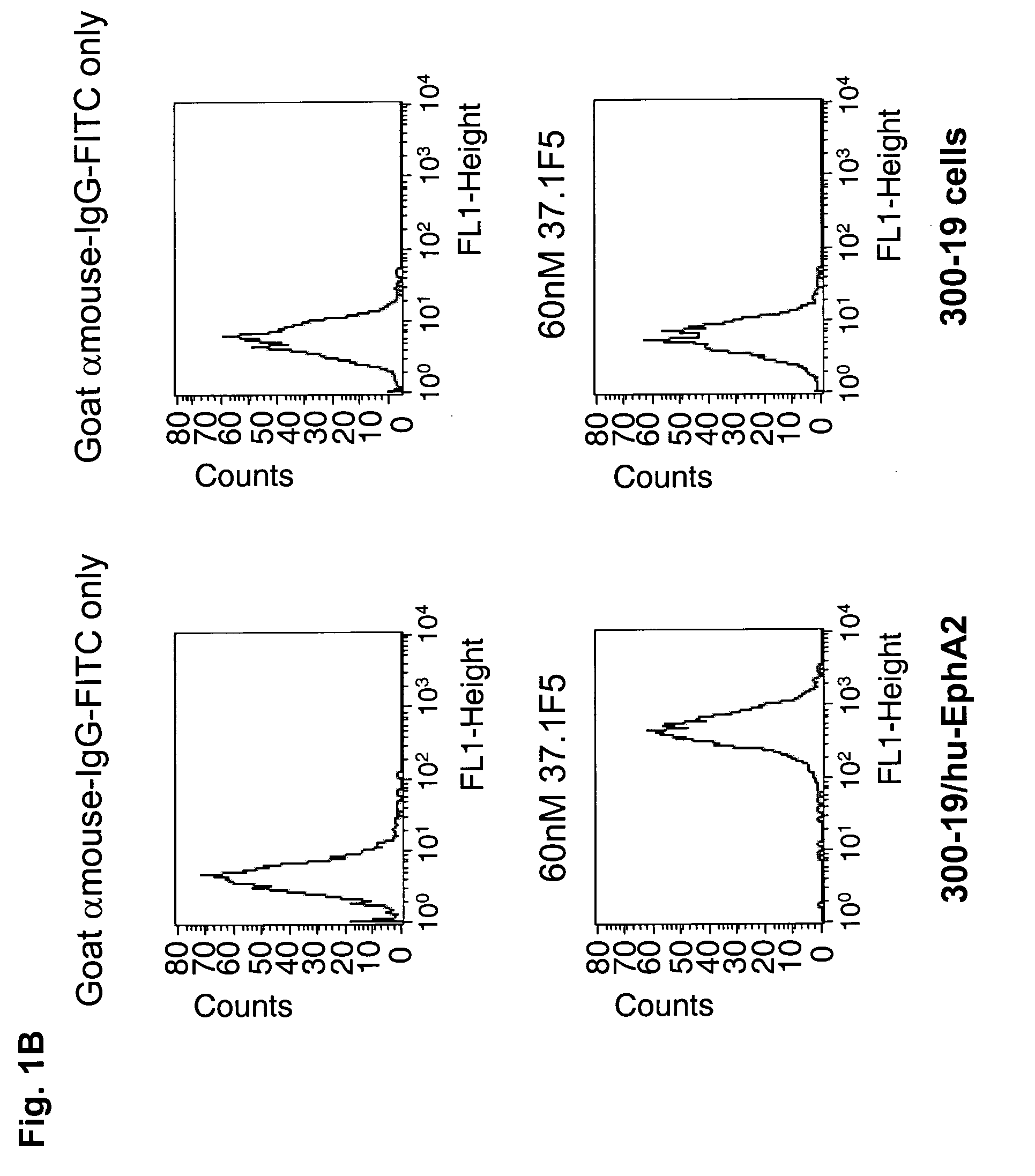
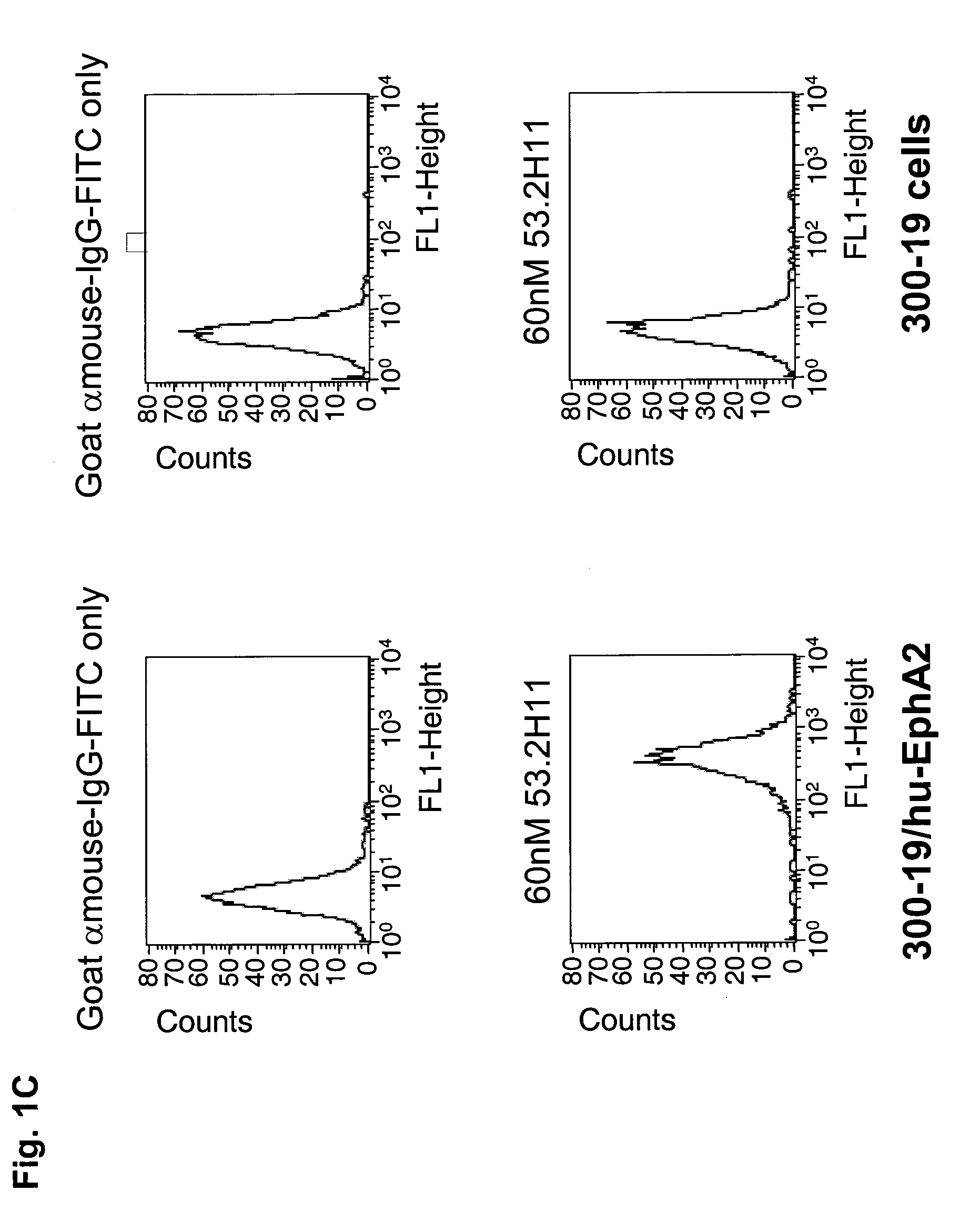
Antagonist antibody for the treatment of cancer
ActiveUS20100047257A1High degreeStrong cytotoxicitySugar derivativesBiological material analysisSynovial sarcomaAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to, and inhibit A class of Eph receptors, antagonize the effects of growth factors on the growth and survival of tumor cells, and which have minimal agonistic activity or are preferentially devoid of agonist activity. Said antibodies and fragments thereof may be used in the treatment of tumors that express elevated levels of A class of Eph receptors, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma and pancreatic cancer, and said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of A class of Eph receptors. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate are disclosed are all embodiments of the invention. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the A class of Eph receptors.
Owner:SANOFI SA
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
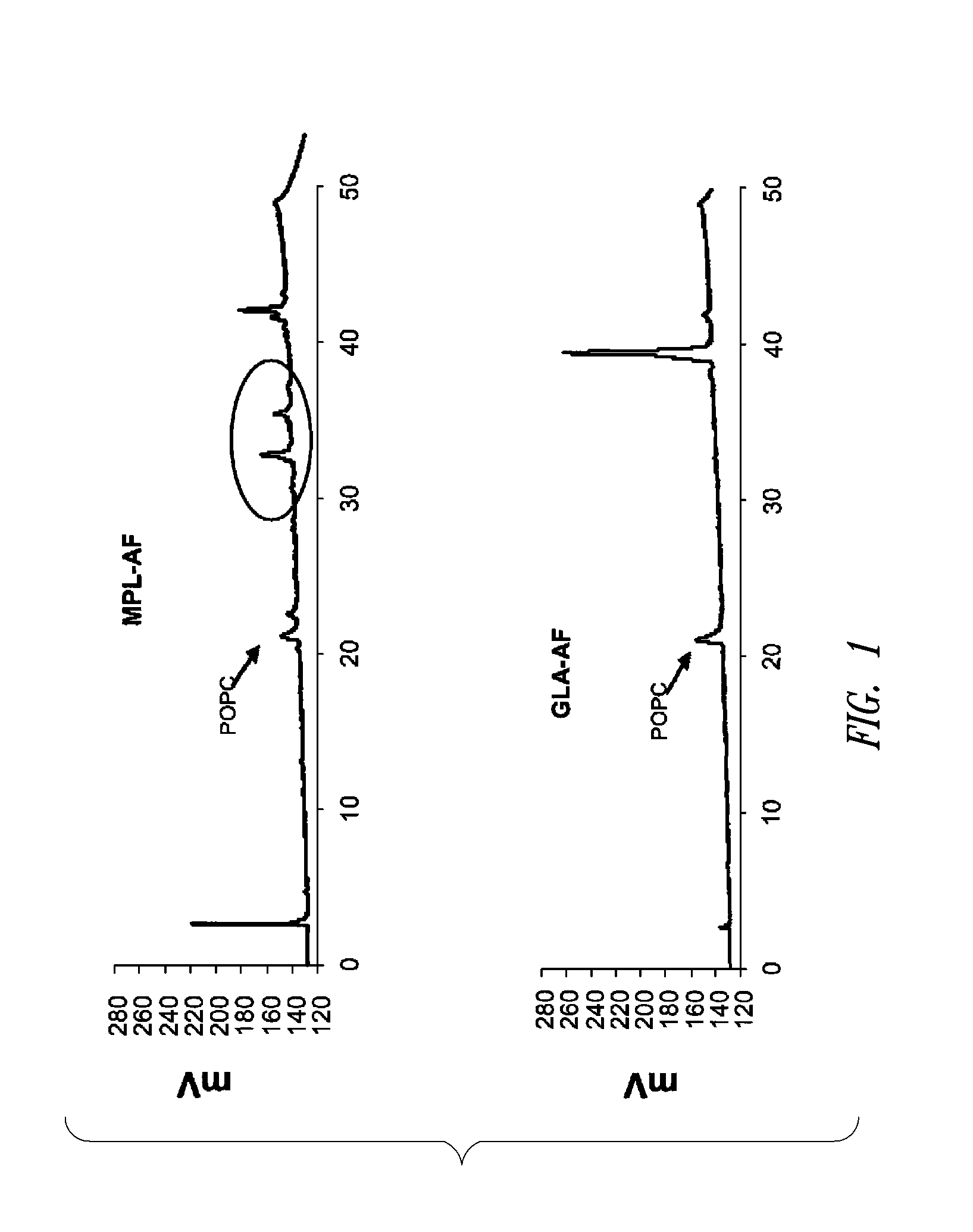
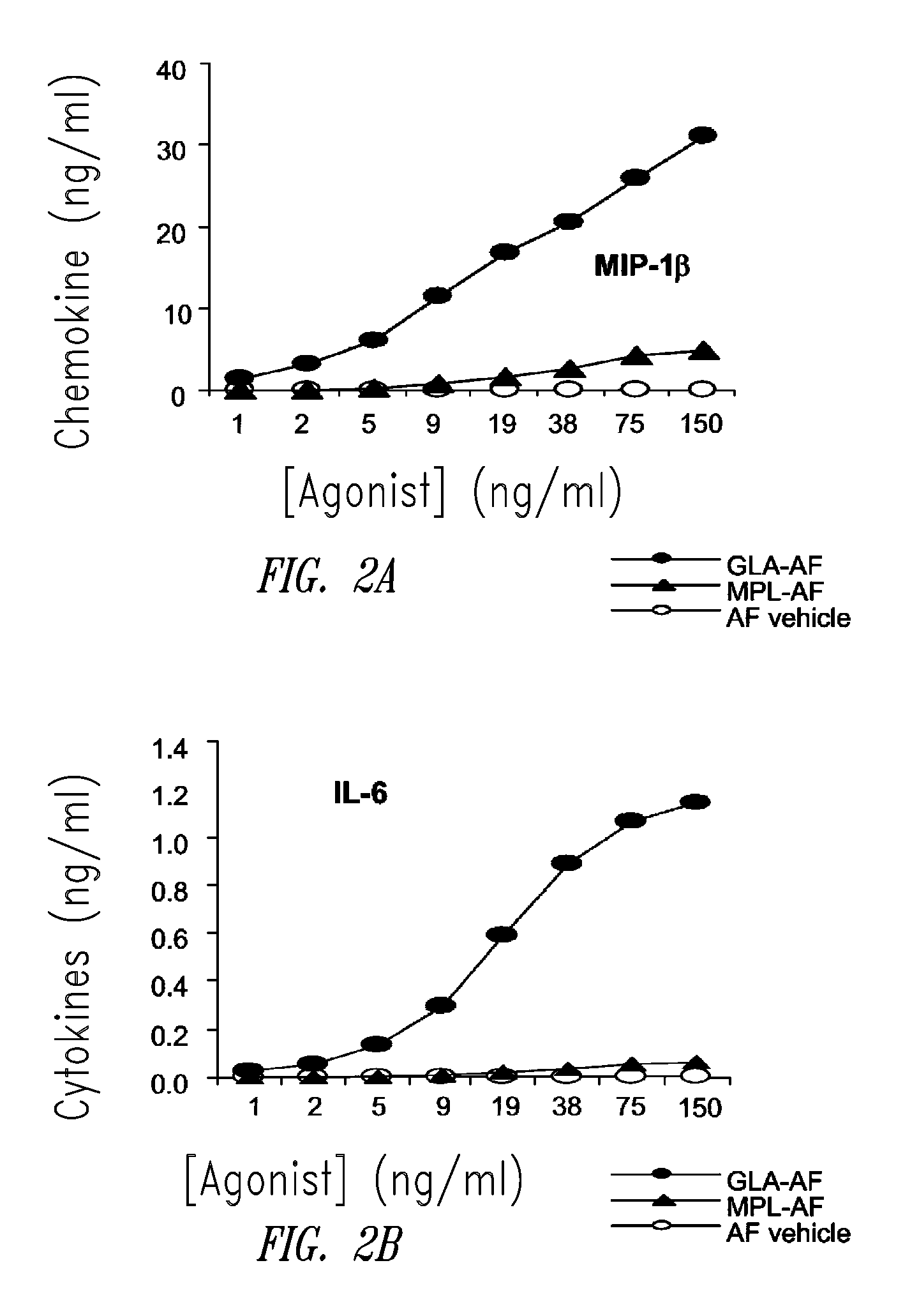
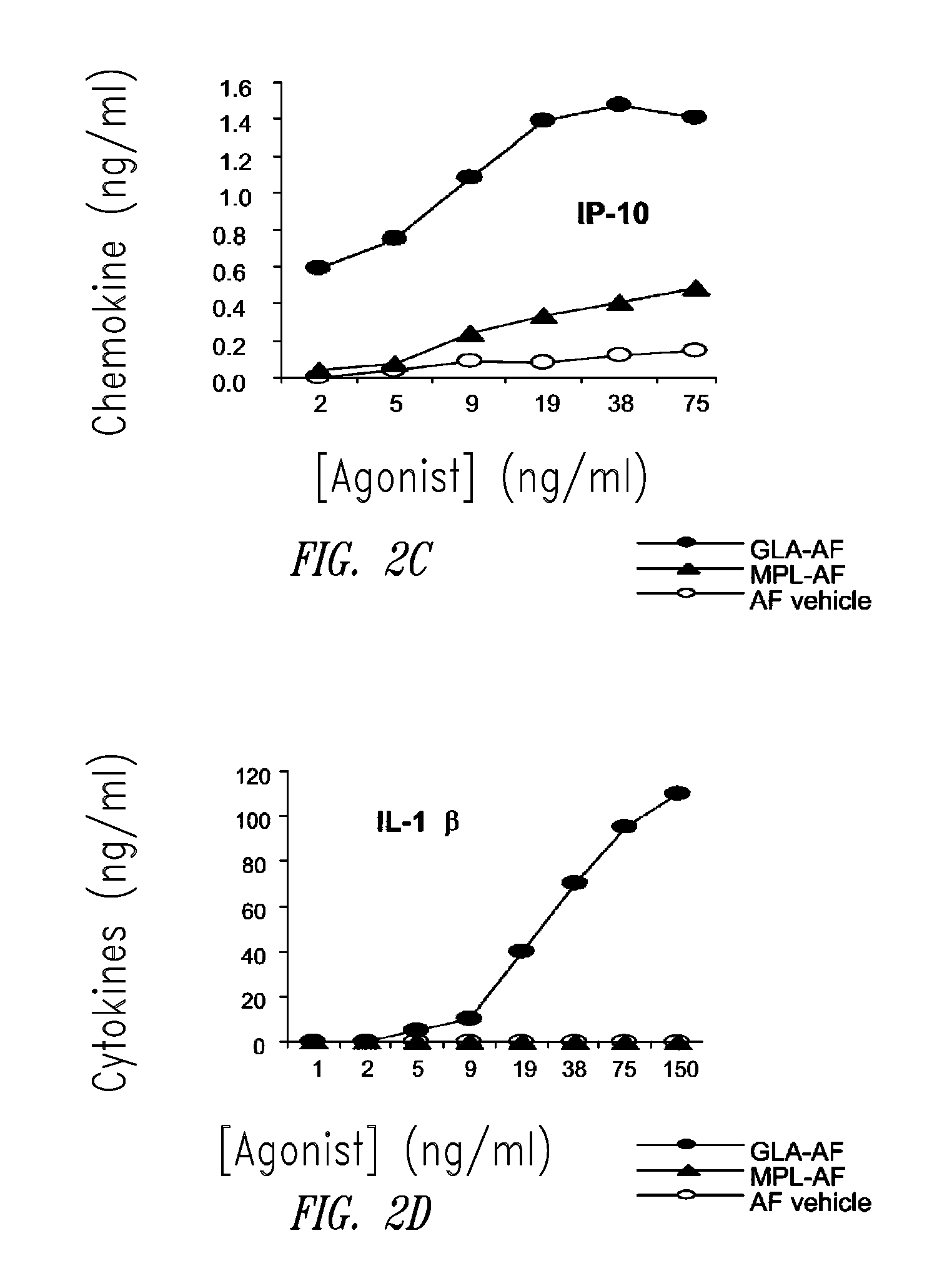
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation
InactiveUS20050125044A1Inhibition releaseImplantable neurostimulatorsHeterocyclic compound active ingredientsEfferentEndotoxic shock
A method of inhibiting the release of a proinflammatory cytokine in a cell is disclosed. The method comprises treating the cell with a cholinergic agonist. The method is useful in patients at risk for, or suffering from, a condition mediated by an inflammatory cytokine cascade, for example endotoxic shock. The cholinergic agonist treatment can be effected by stimulation of an efferent vagus nerve fiber, or the entire vagus nerve.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
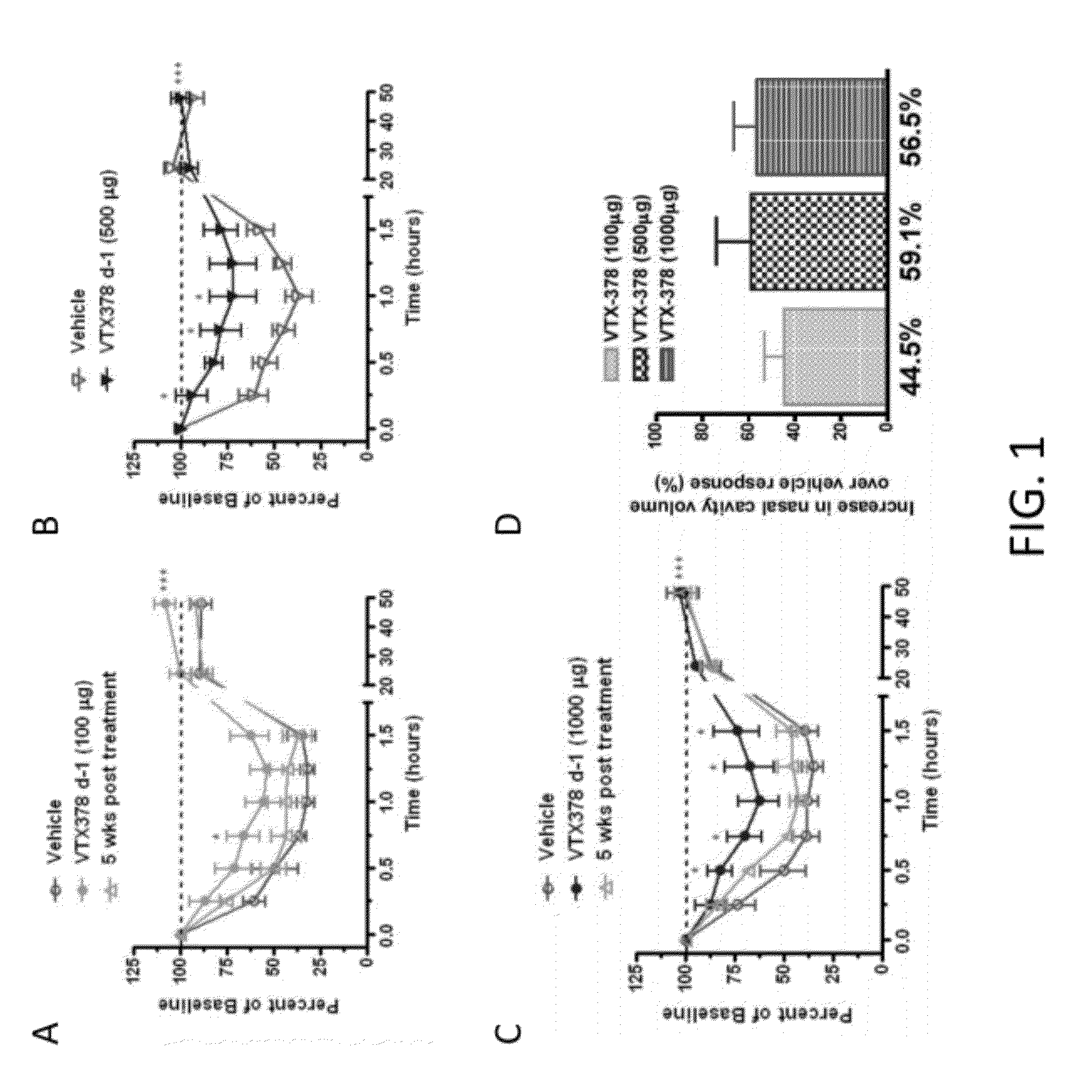
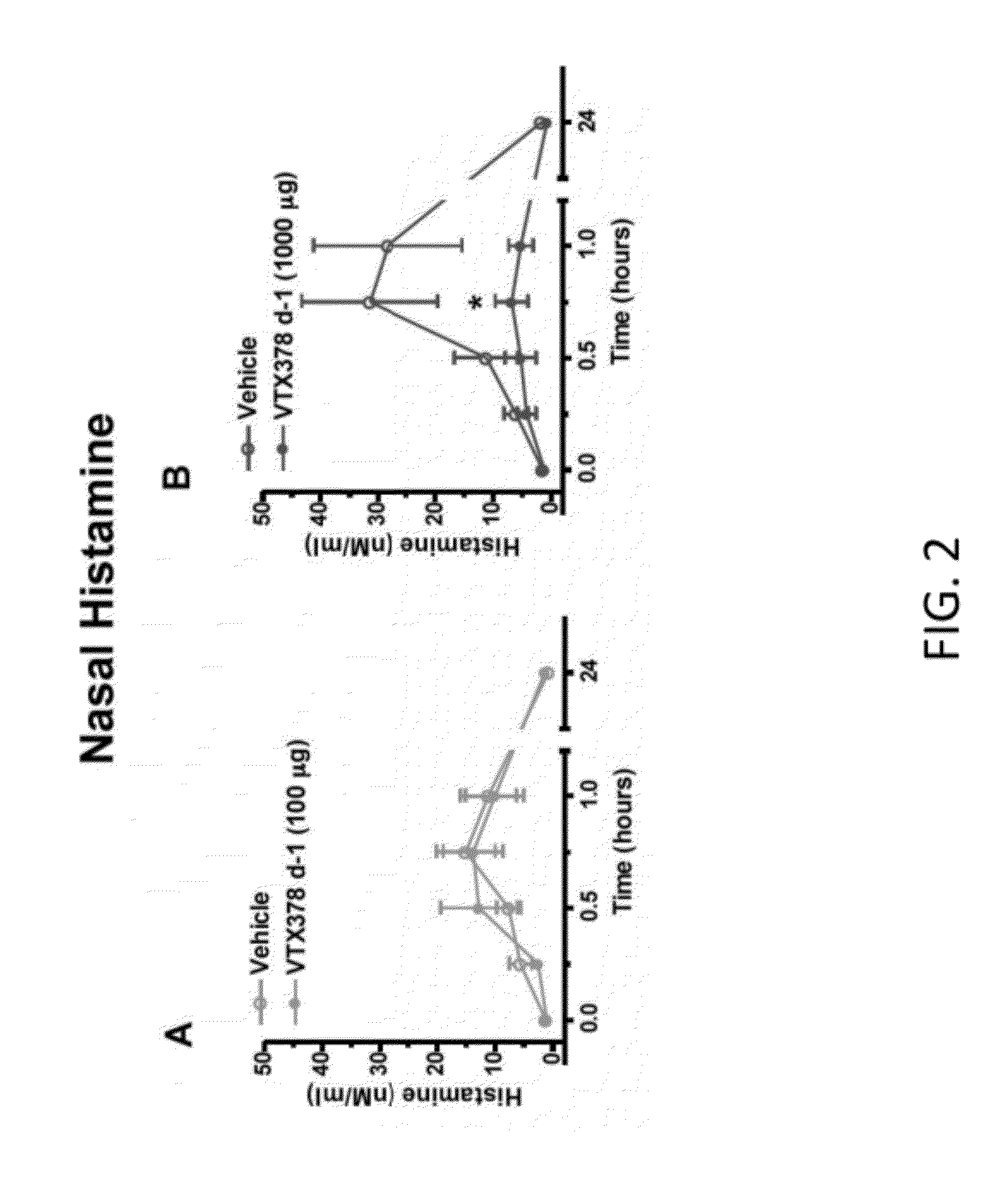
Methods for the Treatment of Allergic Diseases
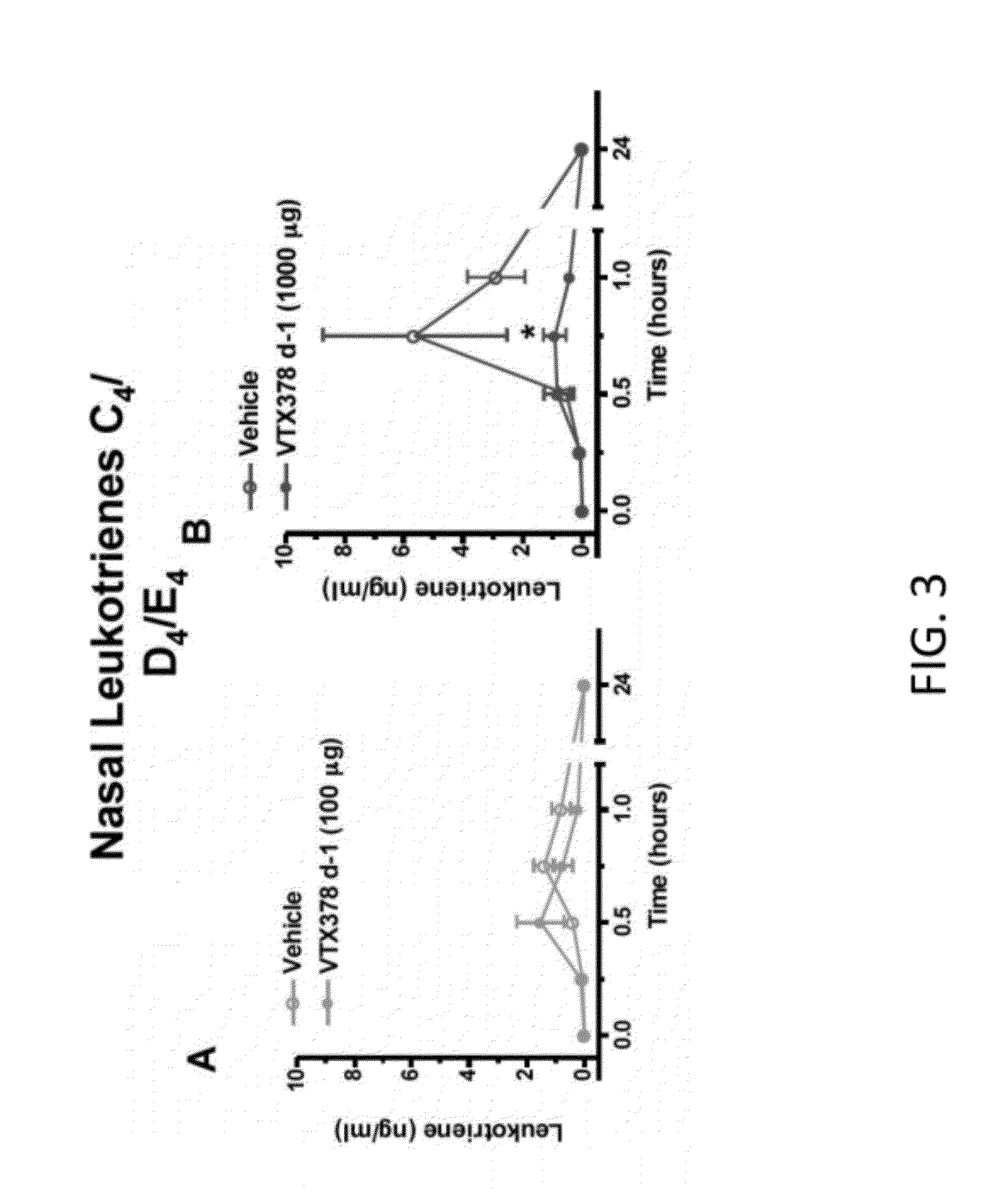
InactiveUS20120082658A1Rapid symptomsTreating, ameliorating, or preventing seasonal or perennial allergic diseasesPowder deliveryBiocideTLR8Agonist
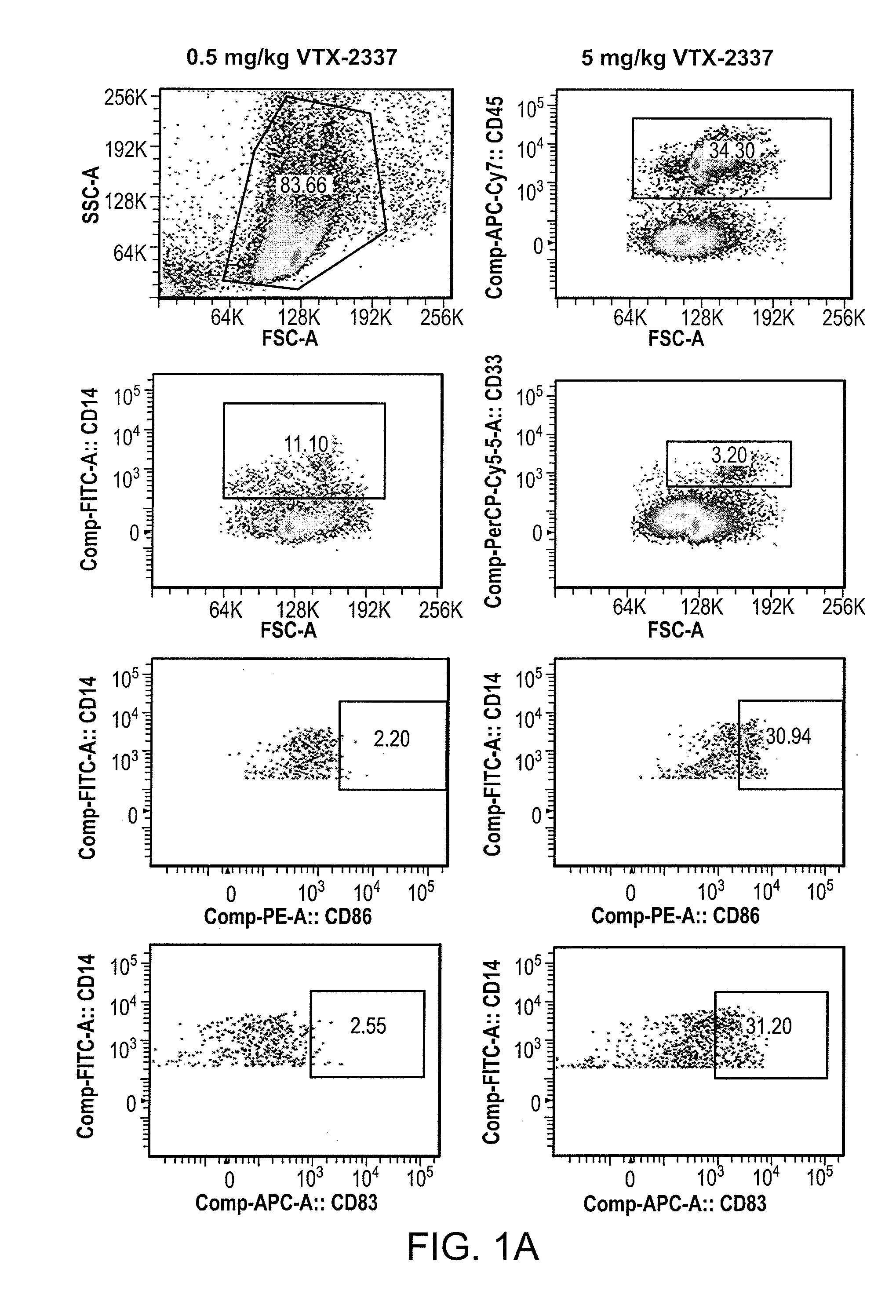
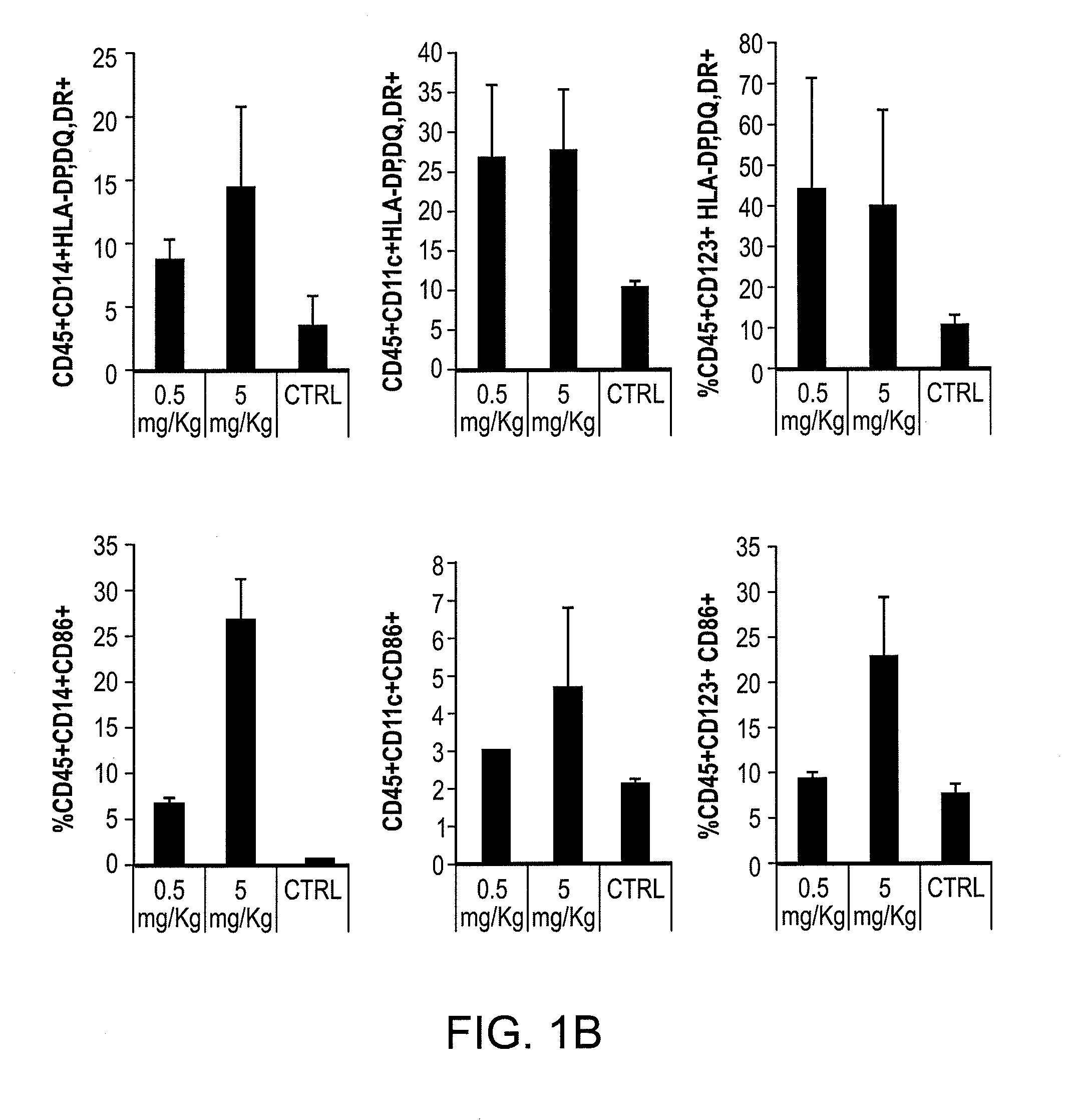
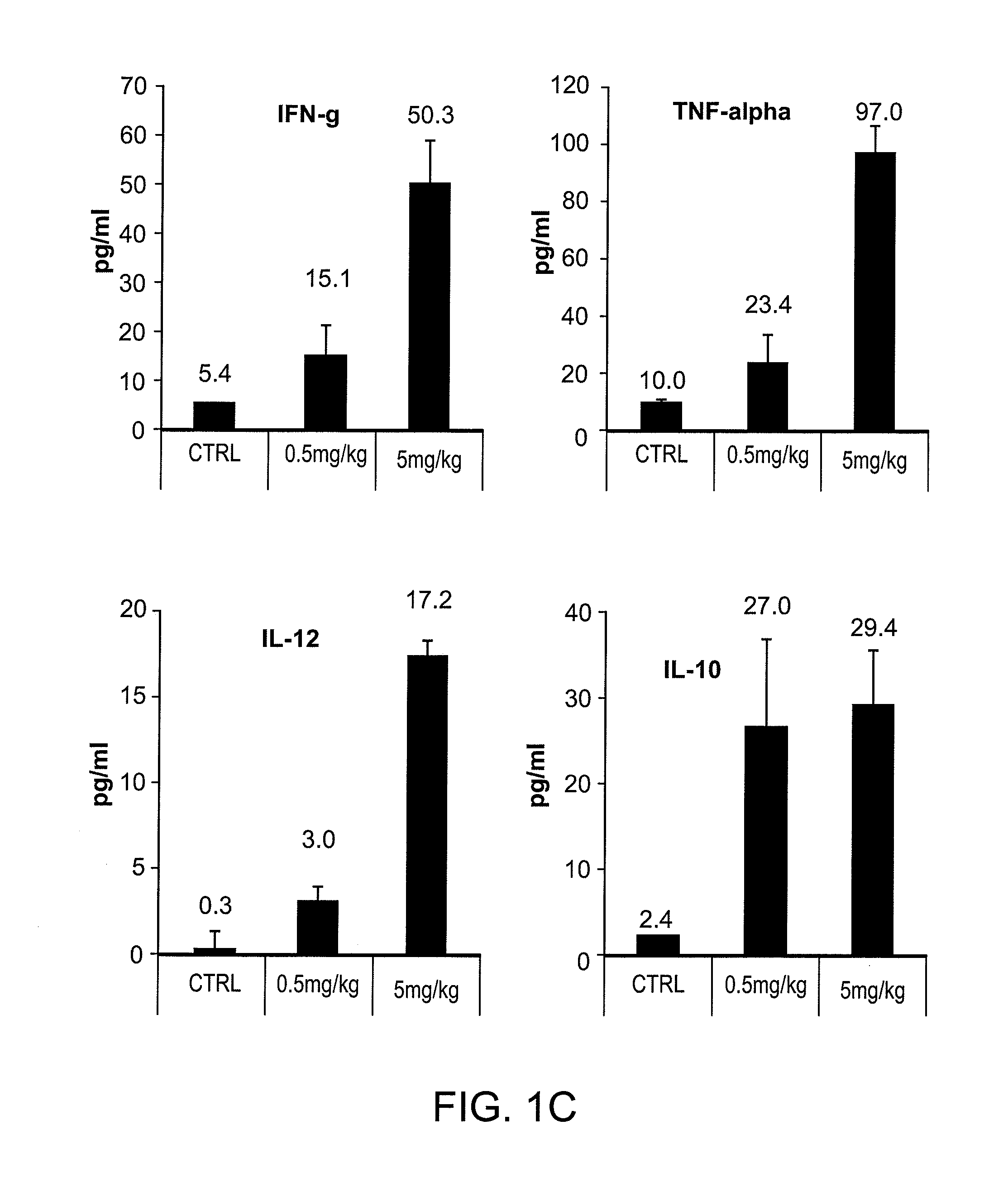
The present invention is directed generally to a TLR8 agonist VTX-378, for use in the treatment or prevention of allergic diseases, including allergic rhinitis.
Owner:VENTIRX PHARMA
Colon and colon cancer associated polynucleotides and polypeptides
InactiveUS20030109690A1Inhibiting and promoting productionInhibiting and promoting and functionSugar derivativesMicrobiological testing/measurementAntigenCancer antigen
The present invention relates to novel colon or colon cancer related polynucleotides and the polypeptides encoded by these polynucleotides herein collectively known as "colon or colon cancer antigens," and the use of such colon or colon cancer antigens for detecting disorders of the colon, particularly the presence of colon cancer and colon cancer metastases. More specifically, isolated colon or colon cancer associated nucleic acid molecules are provided encoding novel colon or colon cancer associated polypeptides. Novel colon or colon cancer polypeptides and antibodies that bind to these polypeptides are provided. Also provided are vectors, host cells, and recombinant and synthetic methods for producing human colon or colon cancer associated polynucleotides and / or polypeptides. The invention further relates to diagnostic and therapeutic methods useful for diagnosing, treating, preventing and / or prognosing disorders related to the colon, including colon cancer, and therapeutic methods for treating such disorders. The invention further relates to screening methods for identifying agonists and antagonists of polynucleotides and polypeptides of the invention. The present invention further relates to methods and / or compositions for inhibiting the production and function of the polypeptides of the present invention.
Owner:HUMAN GENOME SCI INC
Composition and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6187756B1Increased formationPromote activationBiocideElcosanoid active ingredientsDiseaseGlial fibrillary acidic protein
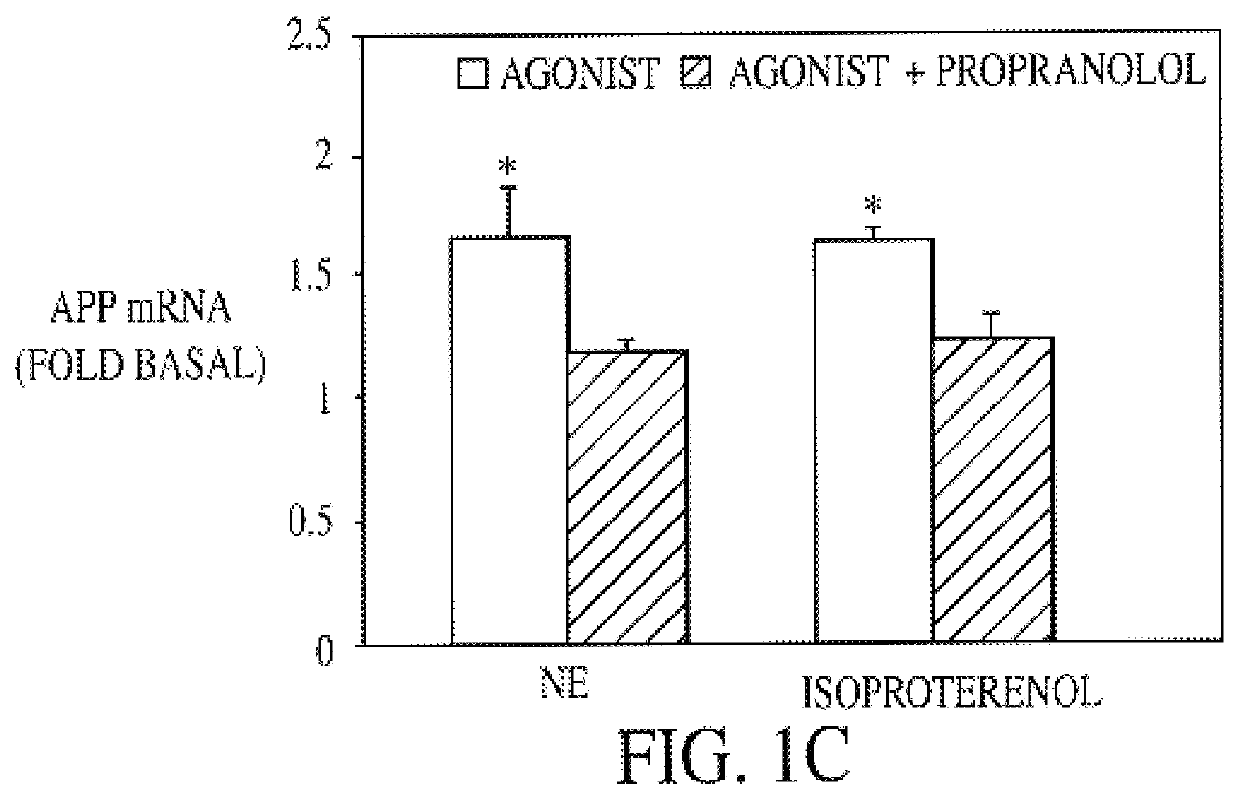
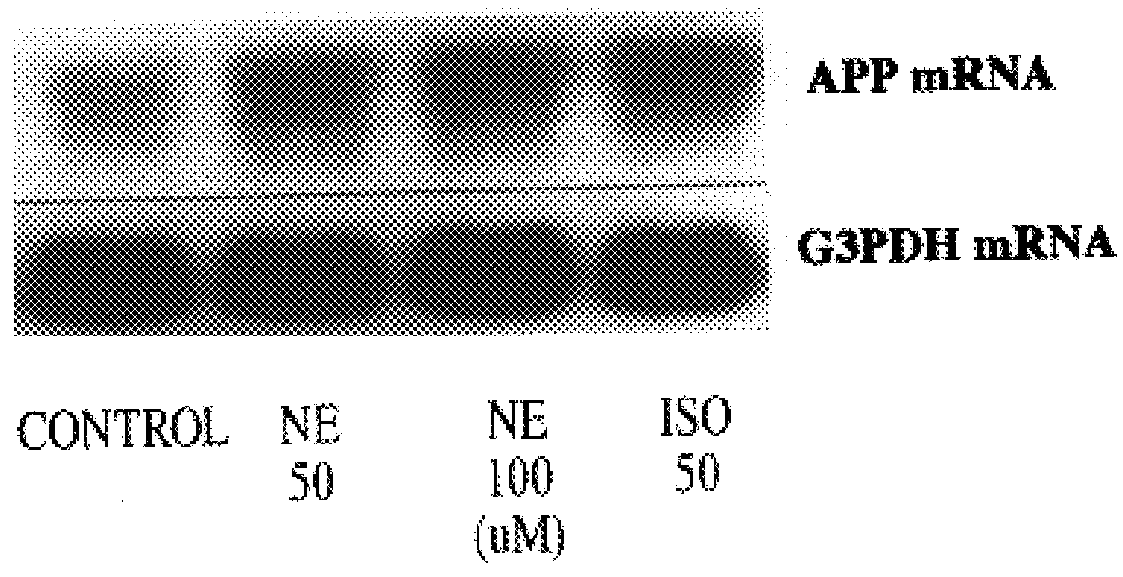
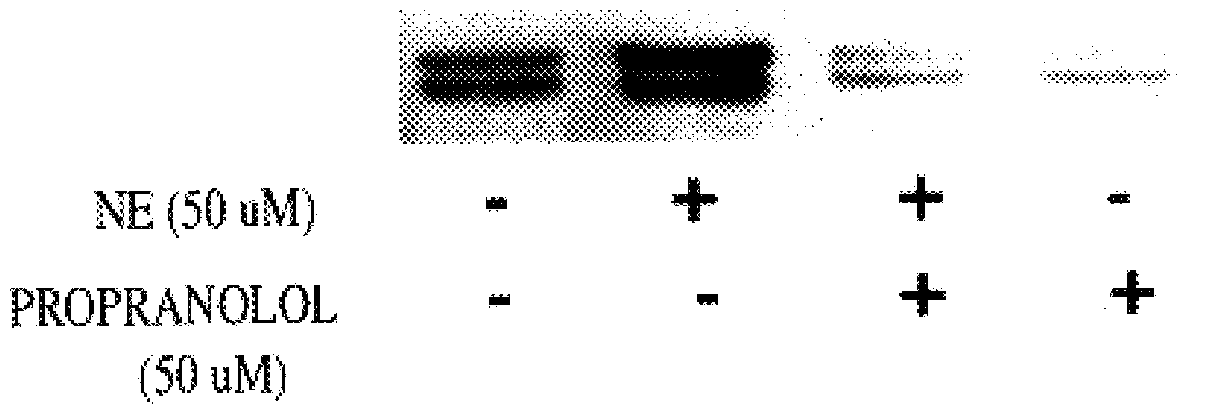
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Amine Compounds
InactiveUS20080200535A1Potent immunosuppressive actionBiocideSenses disorderUveitisAutoimmune disease
There is provided a compound exhibiting an activity of suppressing immune response with reduced adverse drug reactions, which compound is useful in the chemotherapy for preventing or treating, for example, a wide range of various autoimmune diseases including systemic erythematodes, chronic rheumatoid arthritis, Type I diabetes, inflammatory bowel disease, biliary cirrhosis, uveitis, multiple sclerosis or other disorders, or chronic inflammatory diseases, or cancers, lymphoma or leukemia, or resistance to organ or tissue transplantation or rejection against transplantation.Novel amine compounds having an S1P1 / Edg1 receptor agonist effect, possible stereoisomers or racemic bodies of the compounds, or pharmacologically acceptable salts, hydrates or solvates of the compound, the stereoisomers or the racemic bodies, or prodrugs of the compounds, the stereoisomers, the racemic bodies, the salts, the hydrates or the solvates, are provided.
Owner:ASAHI KASEI PHARMA
Remedy for Diabetes
ActiveUS20080319077A1Good secretion effectGood hypoglycemic effectBiocideMetabolism disorderAgonistDiabetic patient
The present invention relates to a therapeutic agent for diabetes with sulfonylurea secondary failure, which contains a GPR40 agonist. According to the present invention, a therapeutic agent for diabetes with sulfonylurea secondary failure that affords a superior insulin secretion effect and a superior hypoglycemic effect even in diabetic patients for whom a sulfonylurea compound or a fast-acting insulin secretagogue fails to provide an insulin secretion effect and therefore, fails to provide a sufficient hypoglycemic effect can be provided.
Owner:TAKEDA PHARMA CO LTD
Combination therapy using an 11beta-hydroxysteroid dehydrogenase type 1 inhibitor and a glucocorticoid receptor agonist to minimize the side effects associated with glucocorticoid receptor agonist therapy
InactiveUS20060094699A1Reduce adverse side effectsUndesirable side-effectBiocideAnimal repellants11beta hydroxysteroid dehydrogenaseSide effect
Combination therapy comprising the administration of an 11β-hydroxysteroid dehydrogenase type 1 inhibitor and a glucocorticoid receptor agonist for treating some forms of cancer, diseases and disorders having inflammation as a component, and to minimize the side effects associated with glucorticoid receptor agonist therapy.
Owner:HIGH POINT PHARMA
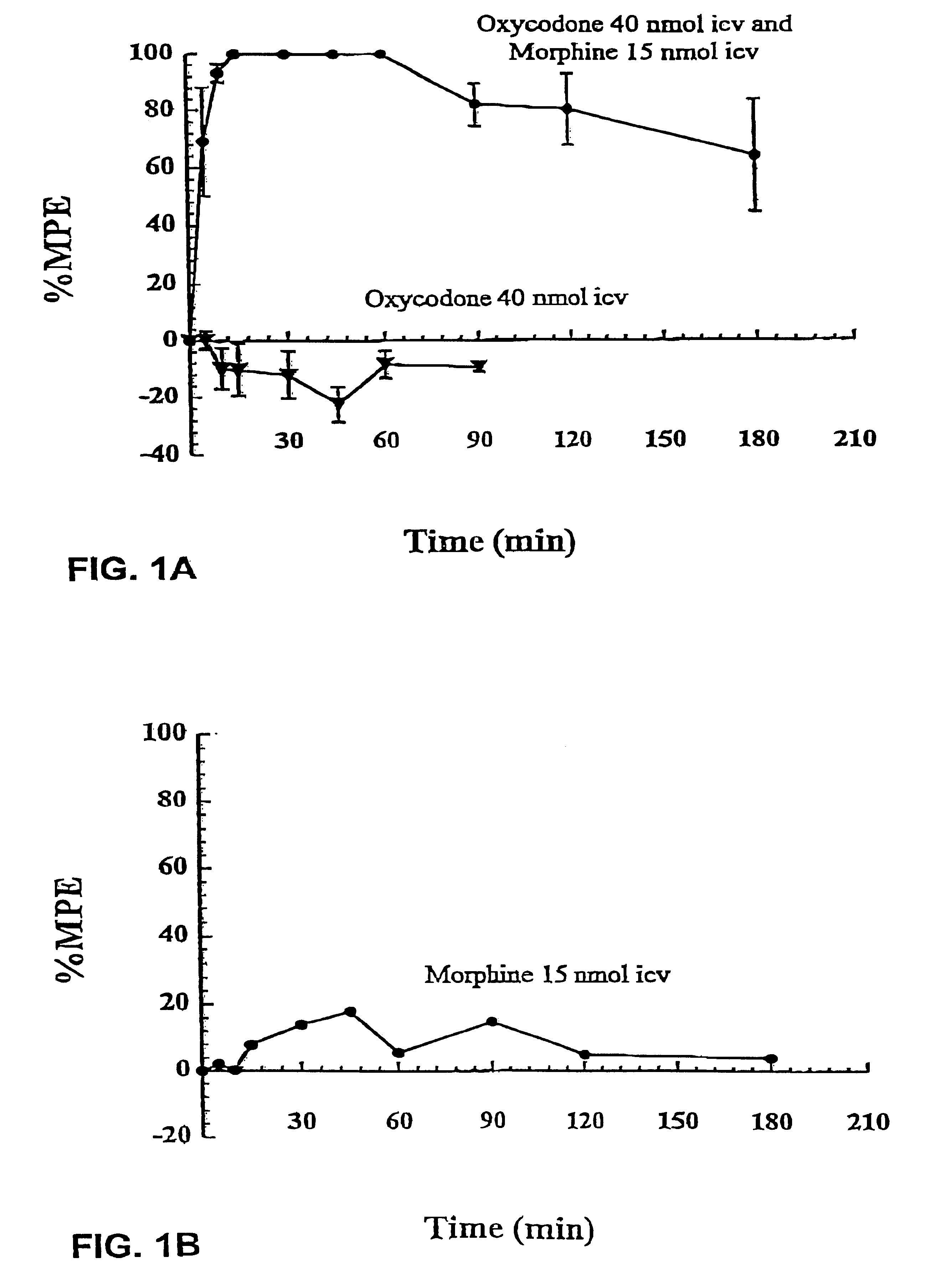
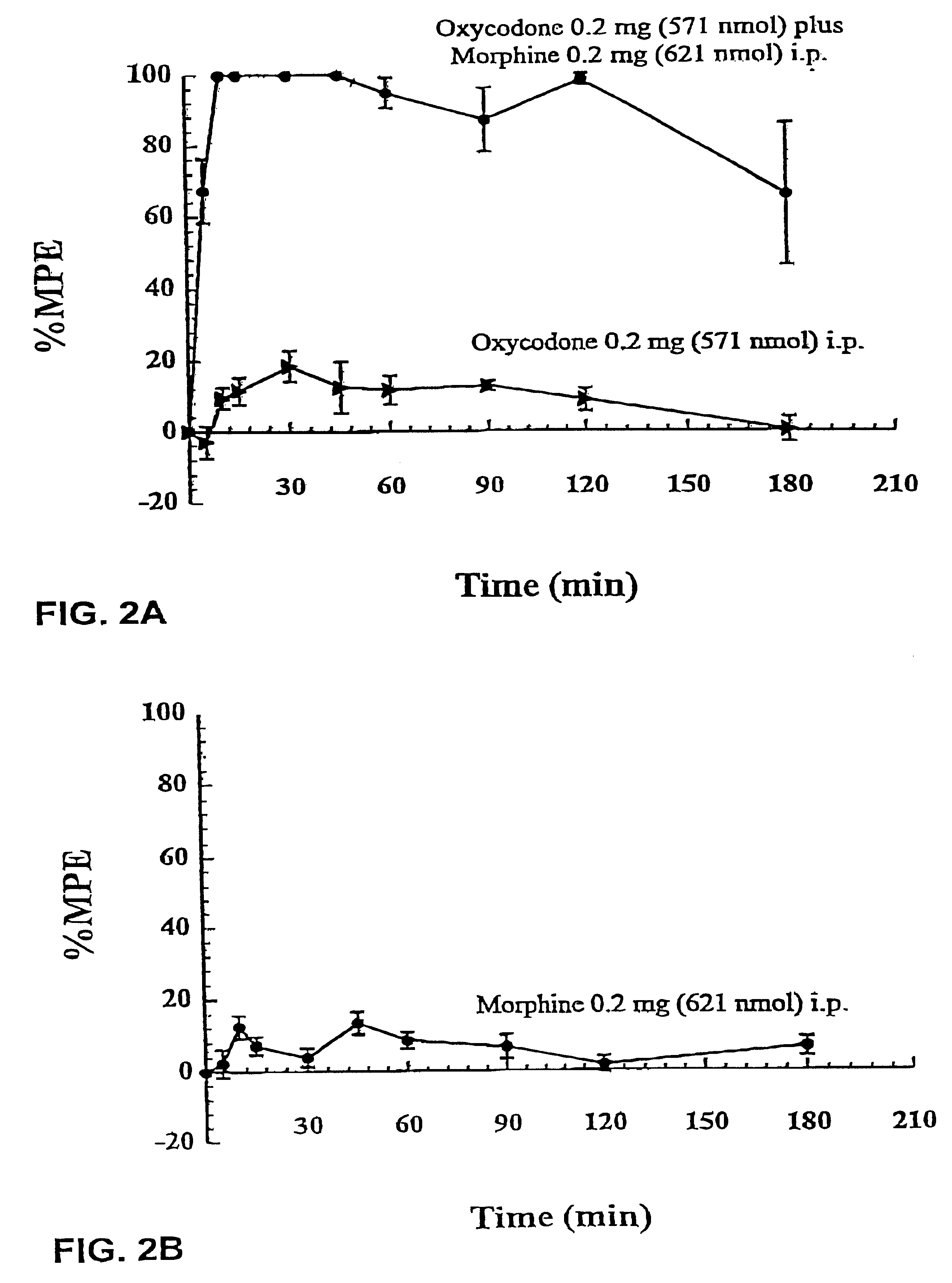
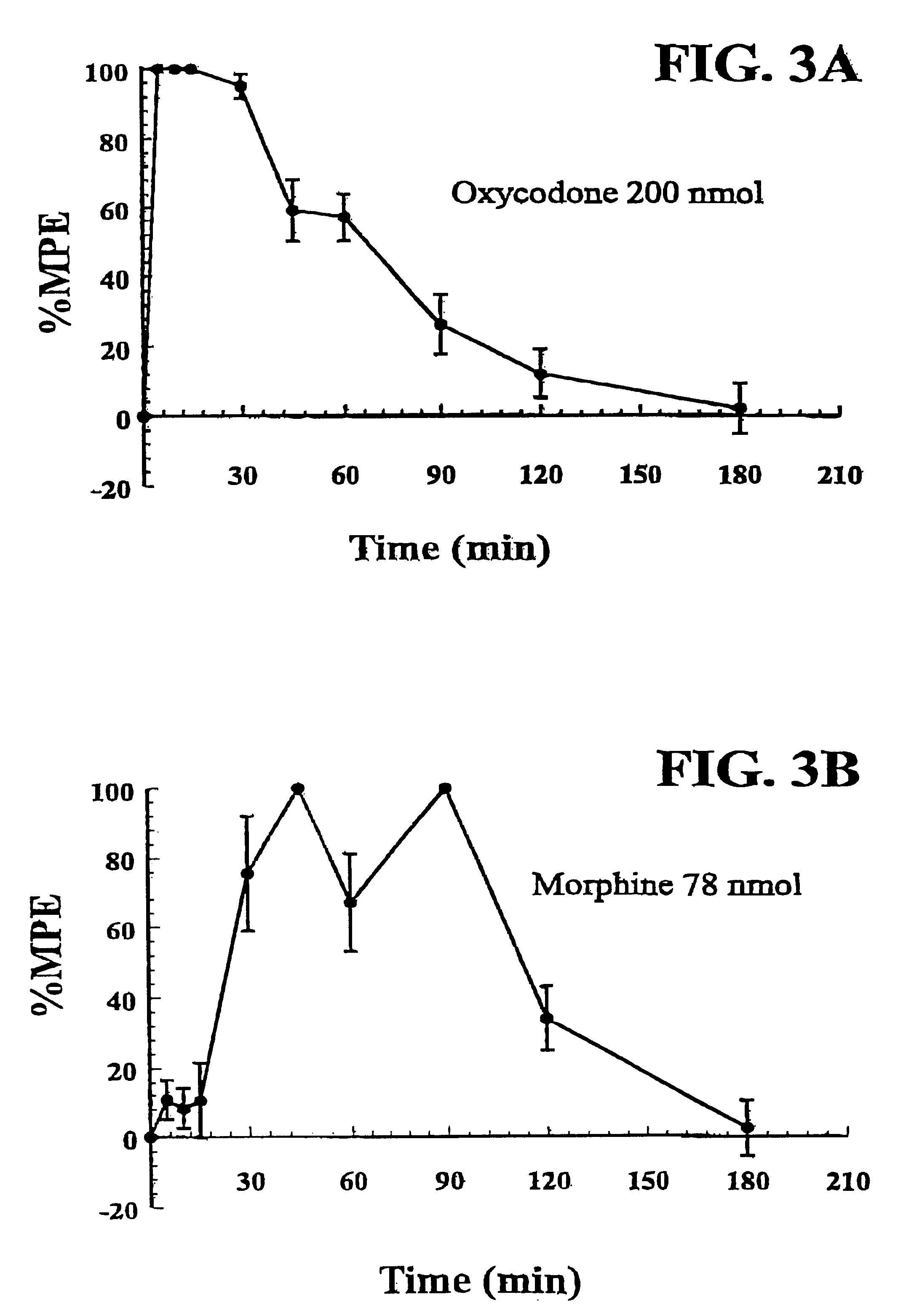
Production of analgesic synergy by co-administration of sub-analgesic doses of a MU opioid agonist and a kappa-2 opioid agonist
InactiveUS6310072B1High analgesic potencyReduction tendencyBiocideNervous disorderOpioid AgonistCo administration
An analgesic composition is disclosed comprising a sub-analgesic dosage of a mu-opioid agonist, optionally in the form of a pharmaceutically acceptable salt, and a sub-analgesic dosage of a kappa2-opioid agonist, optionally in the form of a pharmaceutically acceptable salt. There is also disclosed a method for producing analgesia in humans and lower animals which comprises administering concurrently to a human or lower animal in need of such treatment an analgesic composition of the invention.
Owner:THE LYNX PROJECT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com