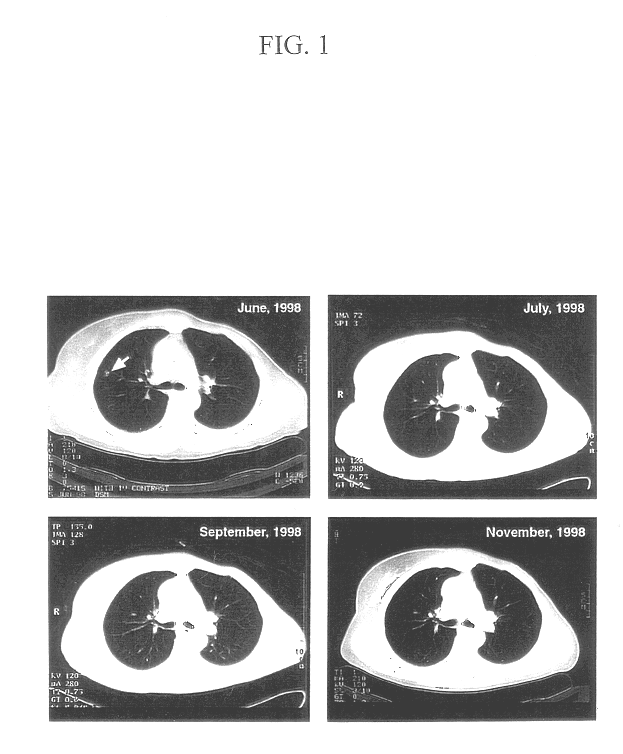
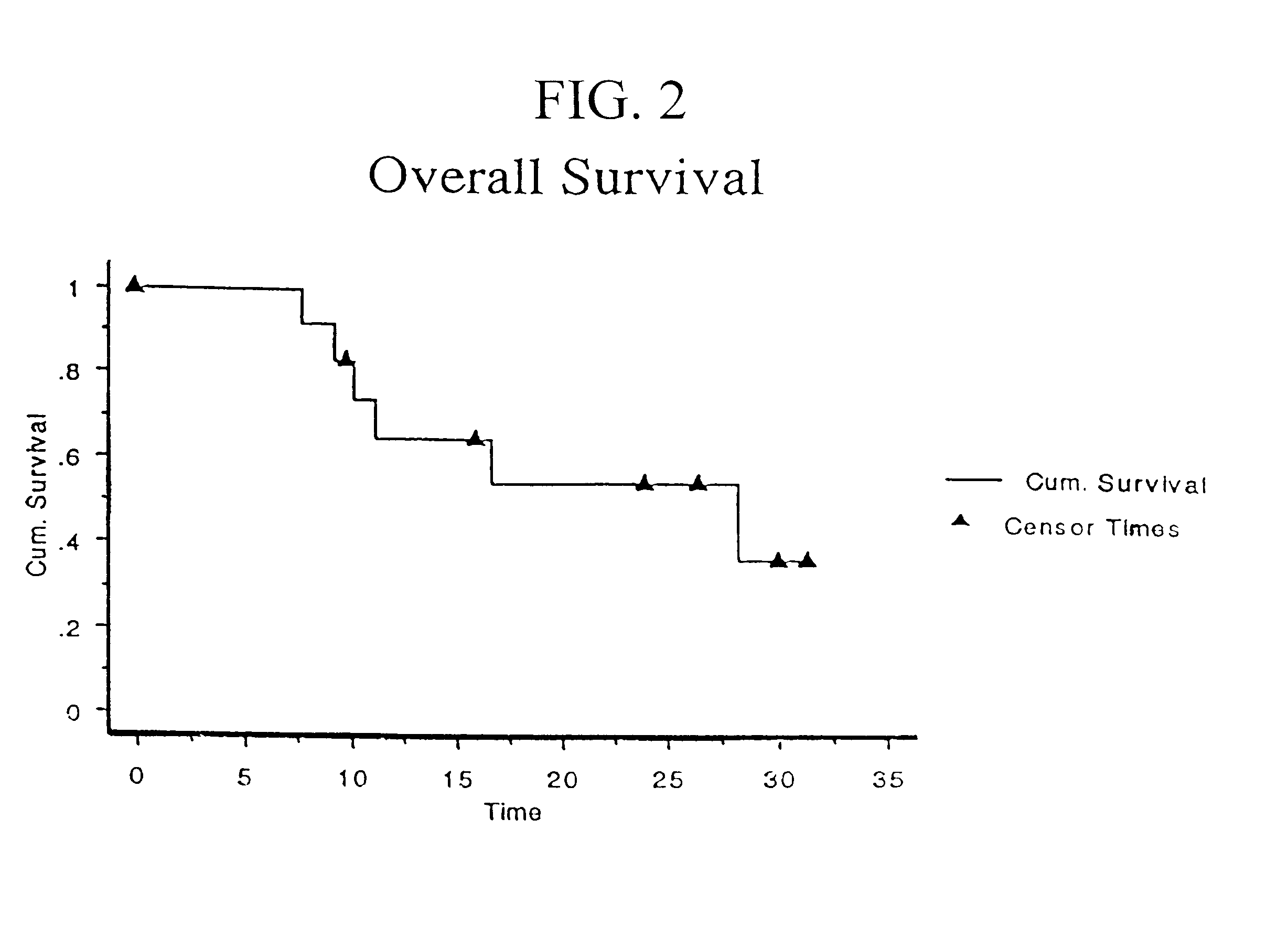
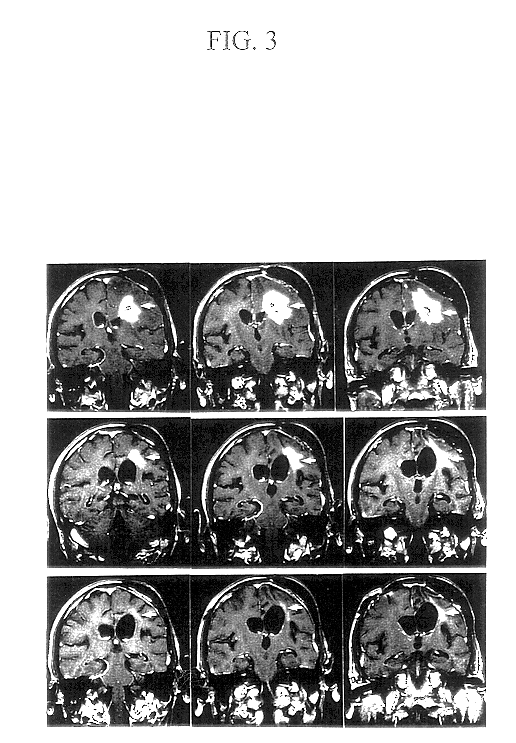
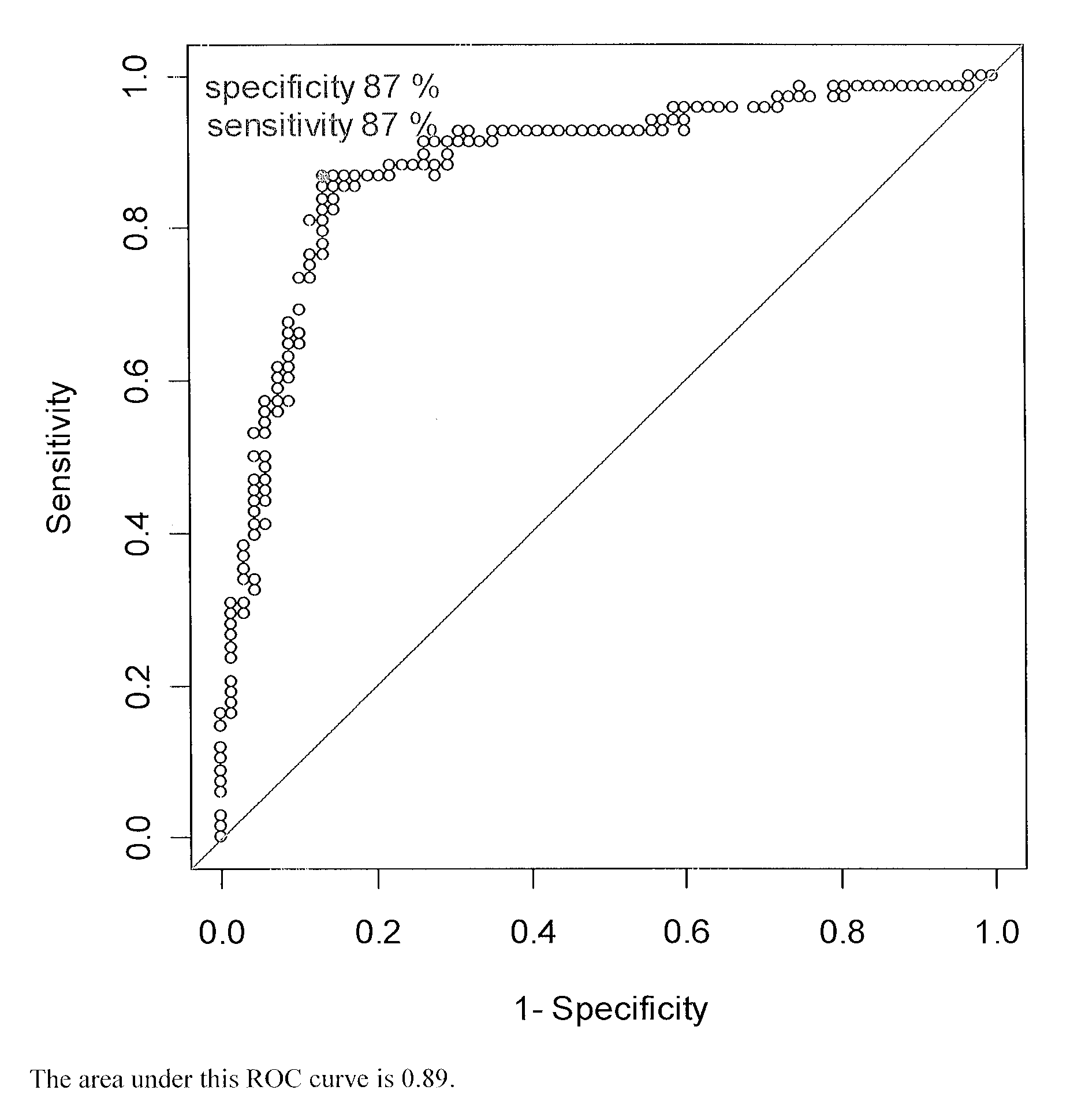
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
310 results about "Cancer antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
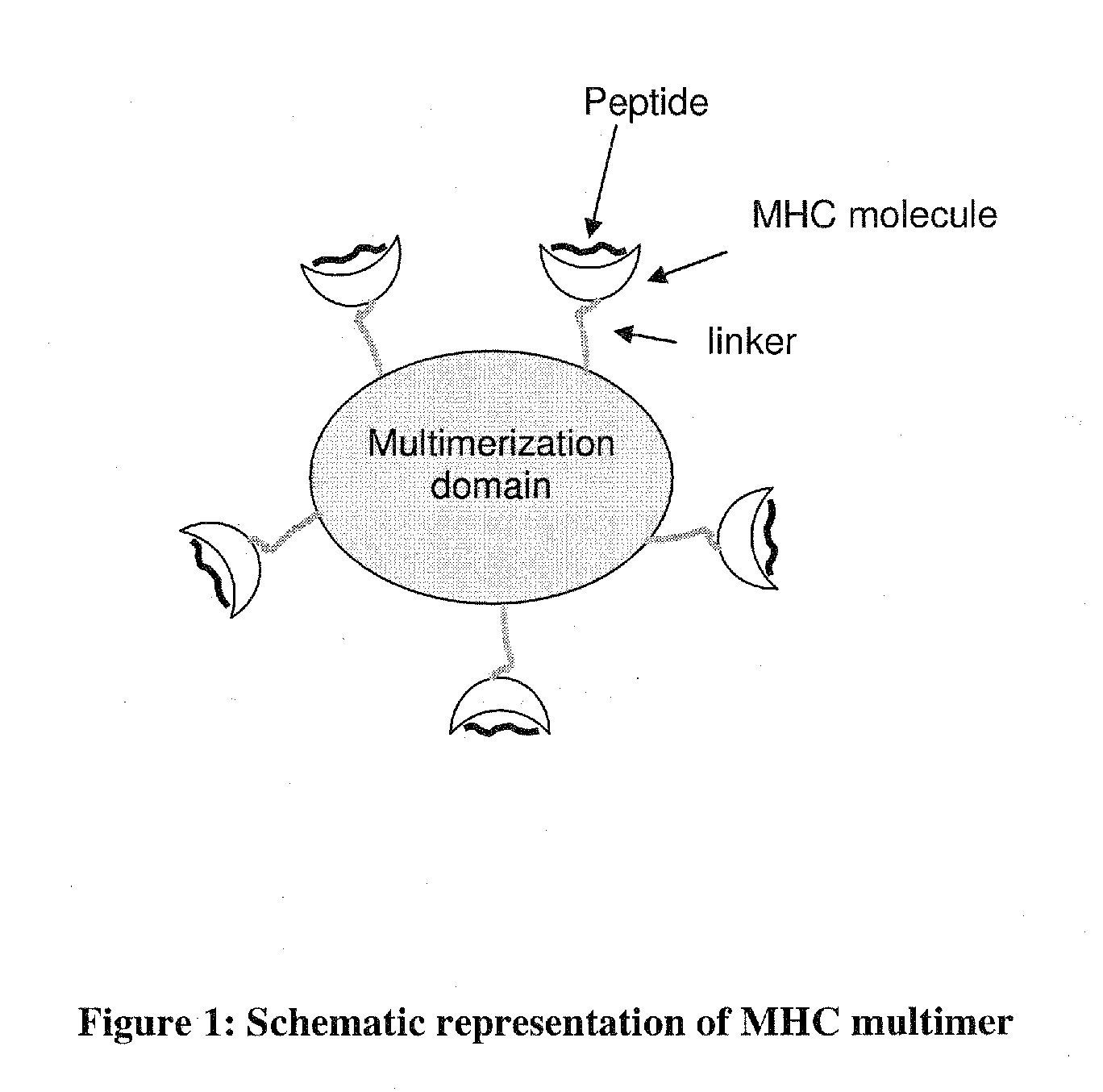
MHC Multimers in Cancer Vaccines and Immune Monitoring
InactiveUS20110318380A1Reduces infectious titerImprove efficacyPeptide/protein ingredientsImmunoglobulinsAntigenDisease
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising cancer antigenic peptides and uses there of.
Owner:AGILENT TECH INC
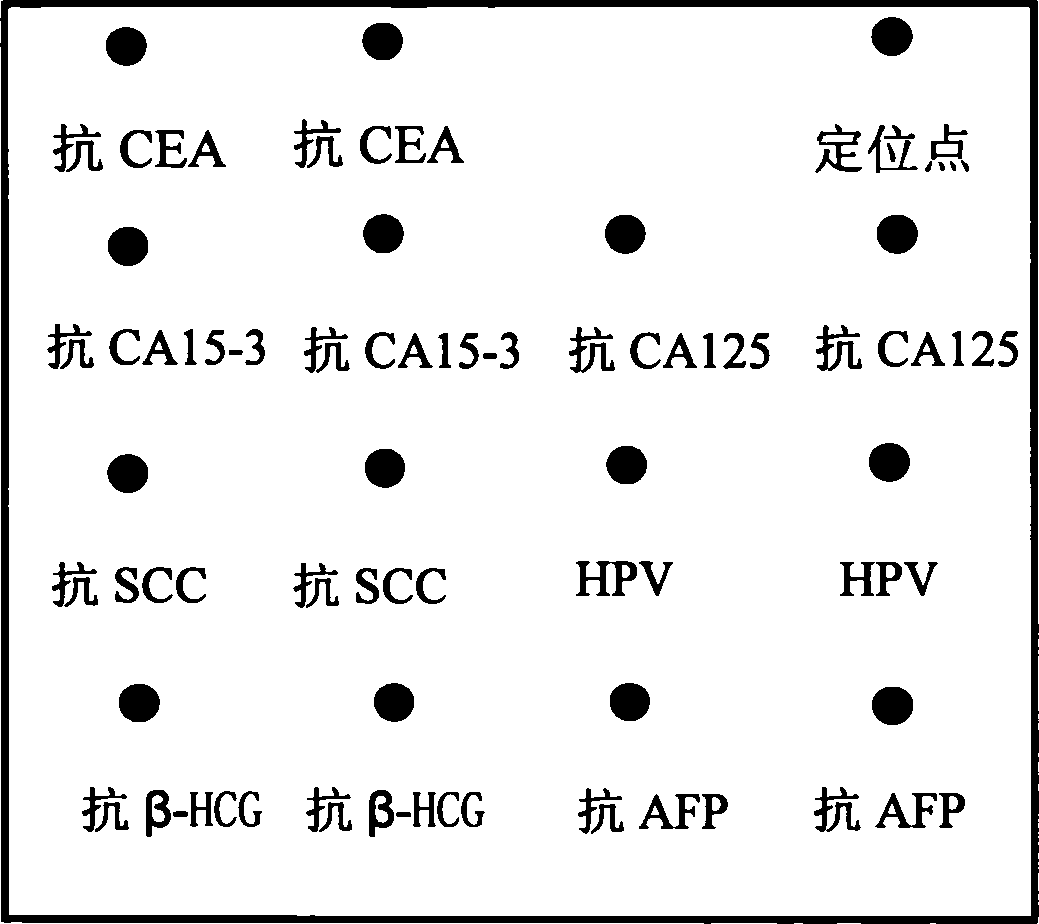
Reaction plate and protein chip kit for integrated detection of multiple gynecologic tumor markers
InactiveCN1880960AChemiluminescene/bioluminescenceColor/spectral properties measurementsCarbohydrate antigenGynecologic Tumor
The invention discloses an integral detecting reacting board and protein chip agent box of Carcinoembryonic antigen,CEA, 15-3 CA15-3(Carbohydrate Antigen 15-3,CA15-3), CA 125 (Cancer antigen 125,CA125), Squamous cell carcinoma antigen,SCC, Human papillomavirus antibody,HPVAb, beta-human chorionic gonadotropin, beta-HCG and alpha-fetoprotein,AFP, wherein the reacting hole board contains base and reacting hole on the base; the reacting hole contains 2-384 sample holes, 2-200 standard sample holes; the fixing phase carrier is set on the bottom of each reacting hole, which is covered by seven antigen or antibody molecule arrays with anti-CEA, anti-CA15-3,anti-CA125,anti-SCC,HPV,anti- beta-HCG,anti-AFP.
Owner:汪宁梅
Colon and colon cancer associated polynucleotides and polypeptides
InactiveUS20030109690A1Inhibiting and promoting productionInhibiting and promoting and functionSugar derivativesMicrobiological testing/measurementAntigenCancer antigen
The present invention relates to novel colon or colon cancer related polynucleotides and the polypeptides encoded by these polynucleotides herein collectively known as "colon or colon cancer antigens," and the use of such colon or colon cancer antigens for detecting disorders of the colon, particularly the presence of colon cancer and colon cancer metastases. More specifically, isolated colon or colon cancer associated nucleic acid molecules are provided encoding novel colon or colon cancer associated polypeptides. Novel colon or colon cancer polypeptides and antibodies that bind to these polypeptides are provided. Also provided are vectors, host cells, and recombinant and synthetic methods for producing human colon or colon cancer associated polynucleotides and / or polypeptides. The invention further relates to diagnostic and therapeutic methods useful for diagnosing, treating, preventing and / or prognosing disorders related to the colon, including colon cancer, and therapeutic methods for treating such disorders. The invention further relates to screening methods for identifying agonists and antagonists of polynucleotides and polypeptides of the invention. The present invention further relates to methods and / or compositions for inhibiting the production and function of the polypeptides of the present invention.
Owner:HUMAN GENOME SCI INC
Anti-MUC-1 single chain antibodies for tumor targeting
InactiveUS7183388B2Easy to addImprove stabilityTumor rejection antigen precursorsTumor specific antigensAntigenSingle-Chain Antibodies
Owner:RGT UNIV OF CALIFORNIA
SPAS-1 cancer antigen
InactiveUS20020150588A1Tumor rejection antigen precursorsPeptide/protein ingredientsCancer antigenProtective immunity
Compounds and methods for inducing protective immunity against cancer are disclosed. The compounds provided include polypeptides that contain at least one immunogenic portion of one or more SPAS-1 proteins and DNA molecules encoding such polypeptides. Such compounds may be formulated into vaccines and pharmaceutical compositions for immunization against cancer, or can be used for the diagnosis of cancer and the monitoring of cancer progression
Owner:RGT UNIV OF CALIFORNIA
Personalized cancer vaccines and adoptive immune cell therapies
InactiveCN104662171AMicrobiological testing/measurementBiological material analysisCancer cellWild type
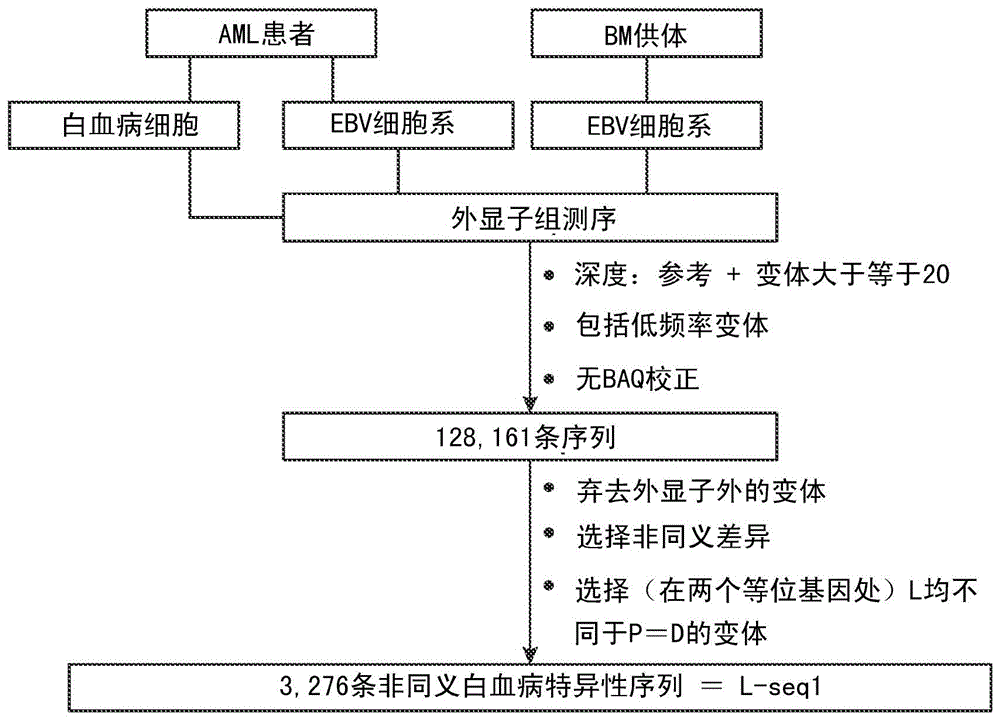
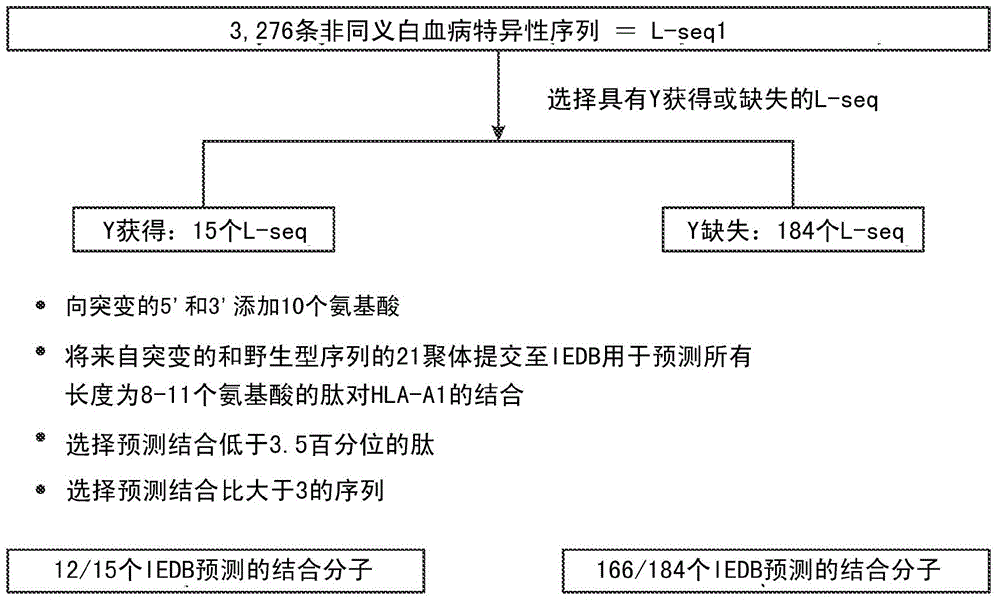
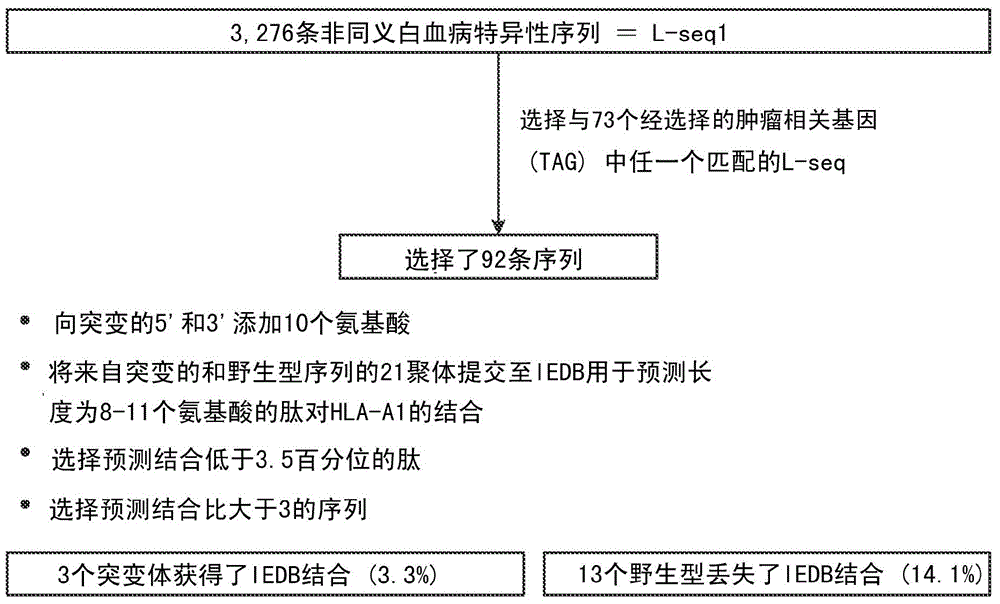
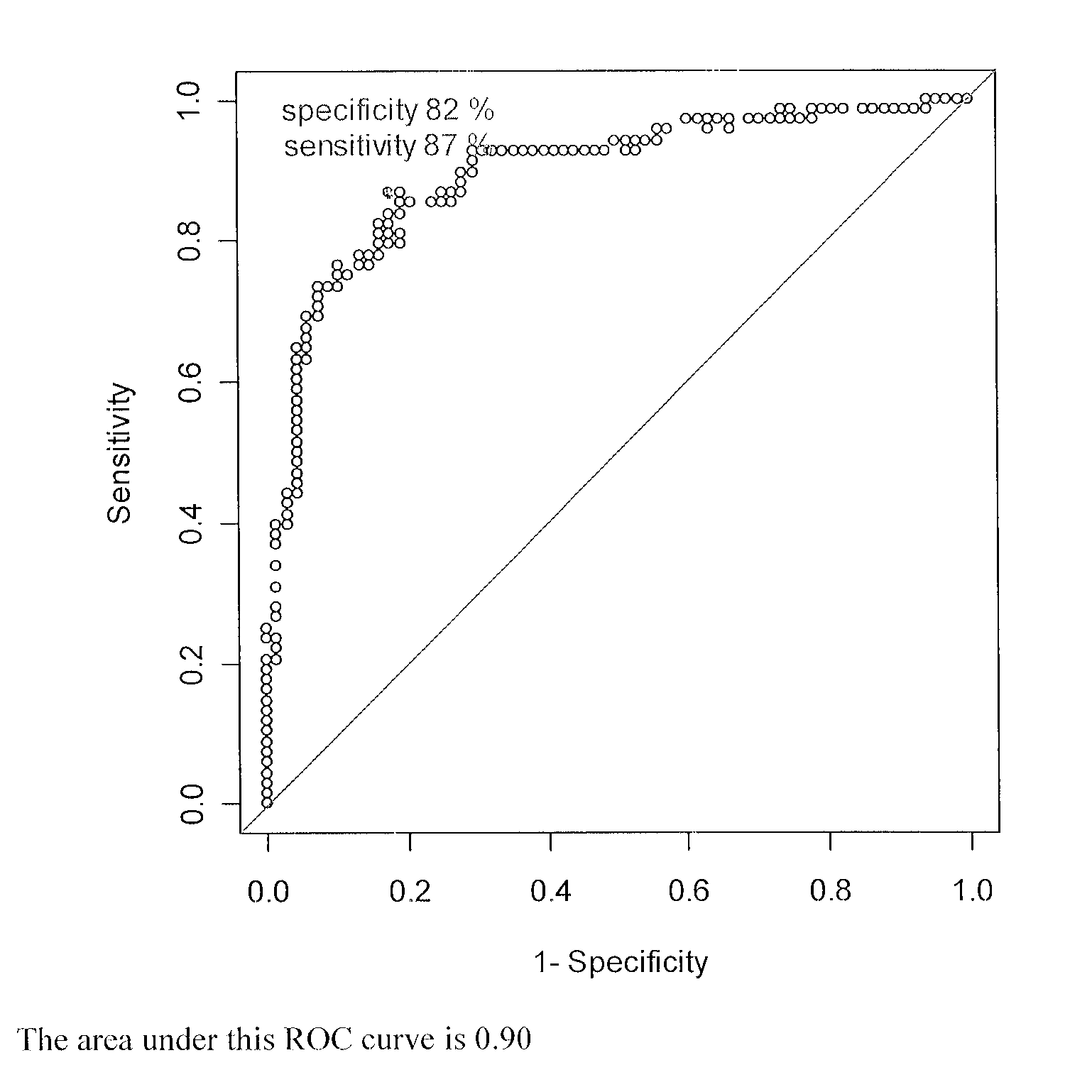
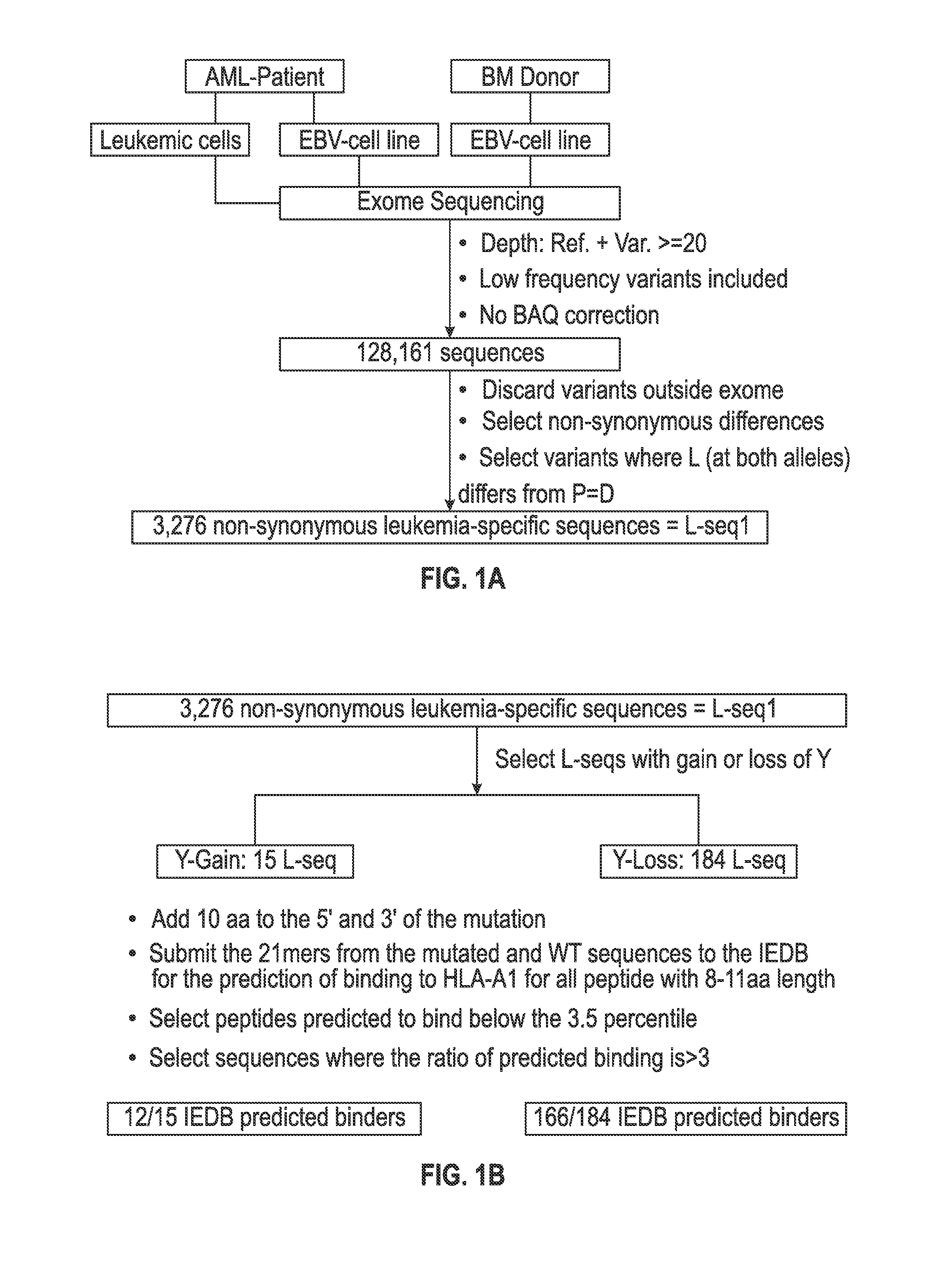
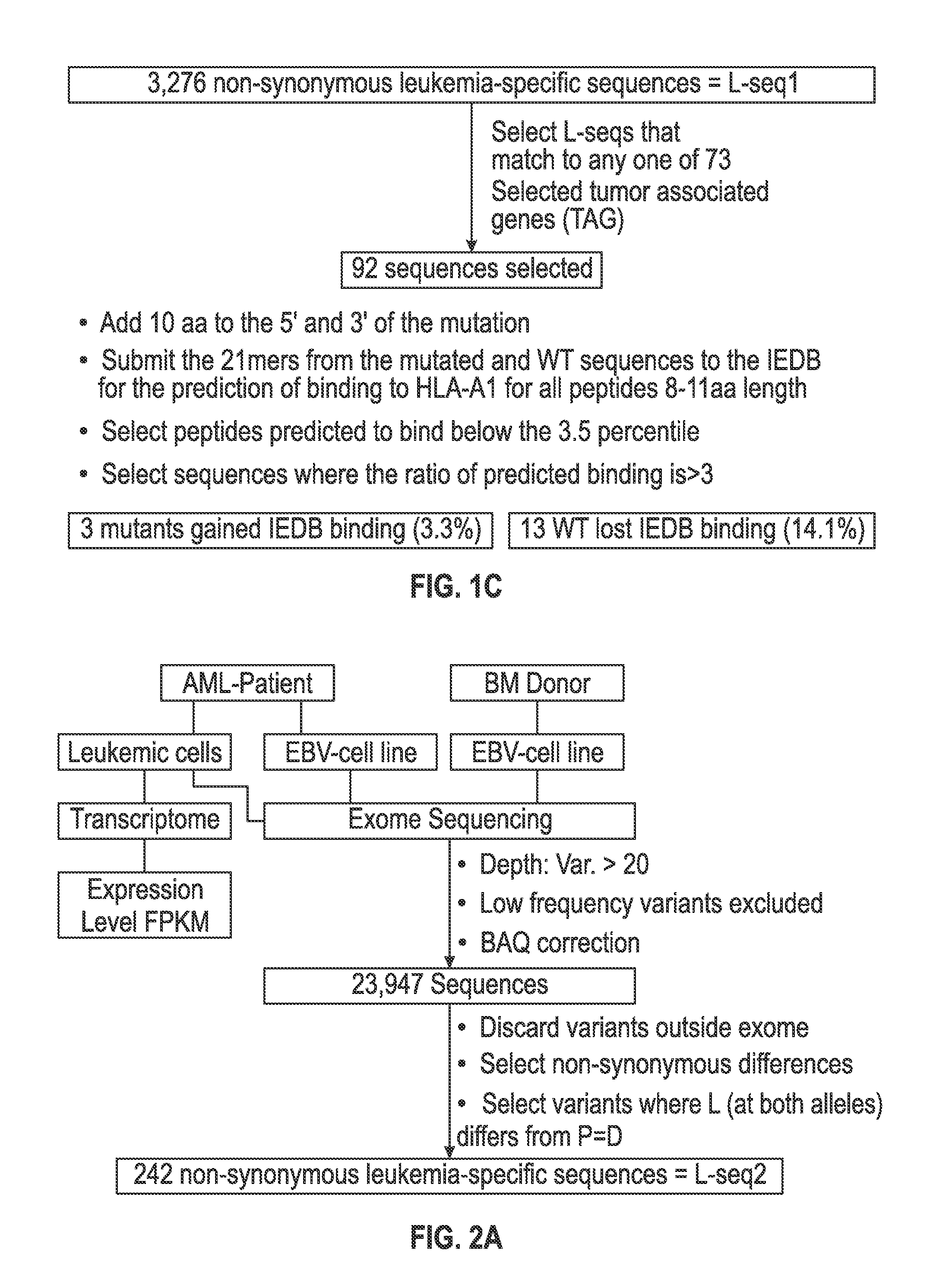
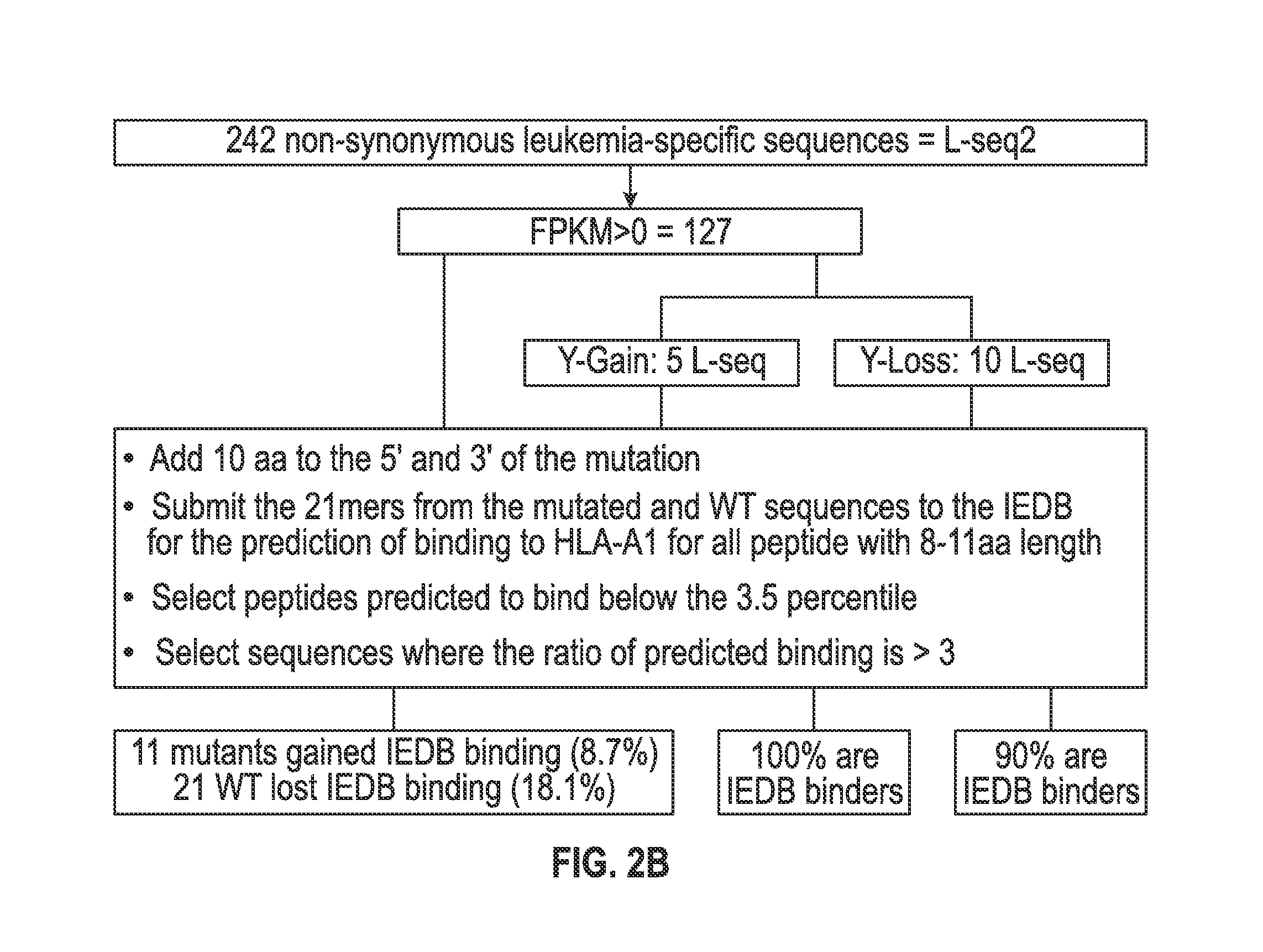
Cancer antigens containing mutations in an expressed gene of cancer cells from a cancer patient are identified. Sequences from cancer cells obtained using a parallel sequencing platform are selected by comparing to the patient's normal genes or to normal genes from an HLA-matched individual. Sequences are further selected by identifying an HLA supertype of the cancer patient and selecting for that HLA supertype, sequences that have a particular amino acid at the mutant position and / or corresponding wild-type position in the effected gene. Peptides containing cancer antigens (i.e., mutations—once a mutation is defined, what makes it an immunogen is its ability to induce an immune response) are optionally tested for binding to HLA antigens of the cancer patient. Peptides containing the cancer antigens are evaluated for activating T cells (e.g., helper T lymphocytes and cytotoxic T lymphocytes (CTL)) cell lines from the cancer patient or from an HLA-matched donor. The cancer antigen(s) identified for a cancer patient are used to prepare a cancer vaccine and to treat the cancer patient.
Owner:PERSIMMUNE
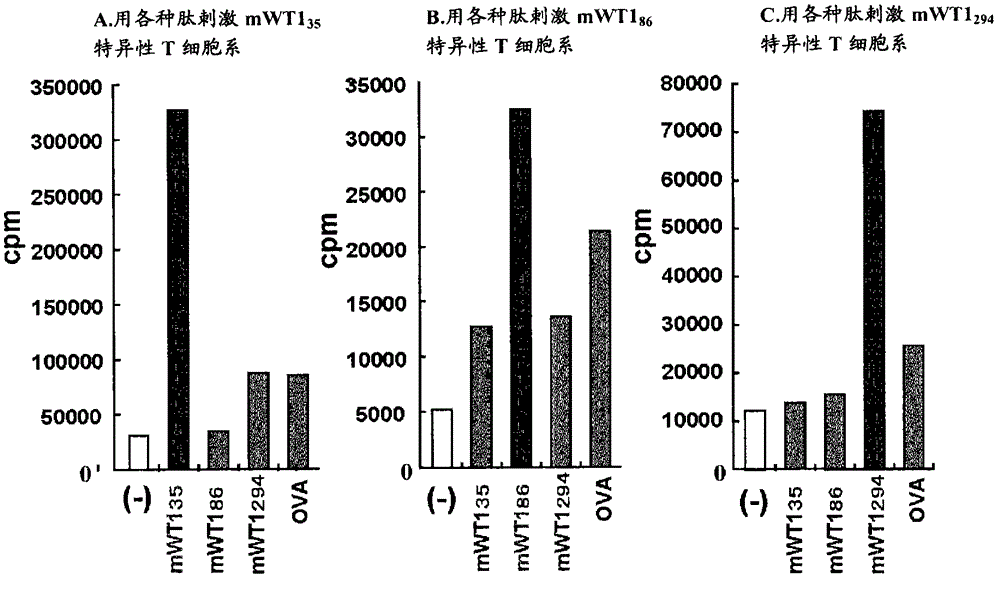
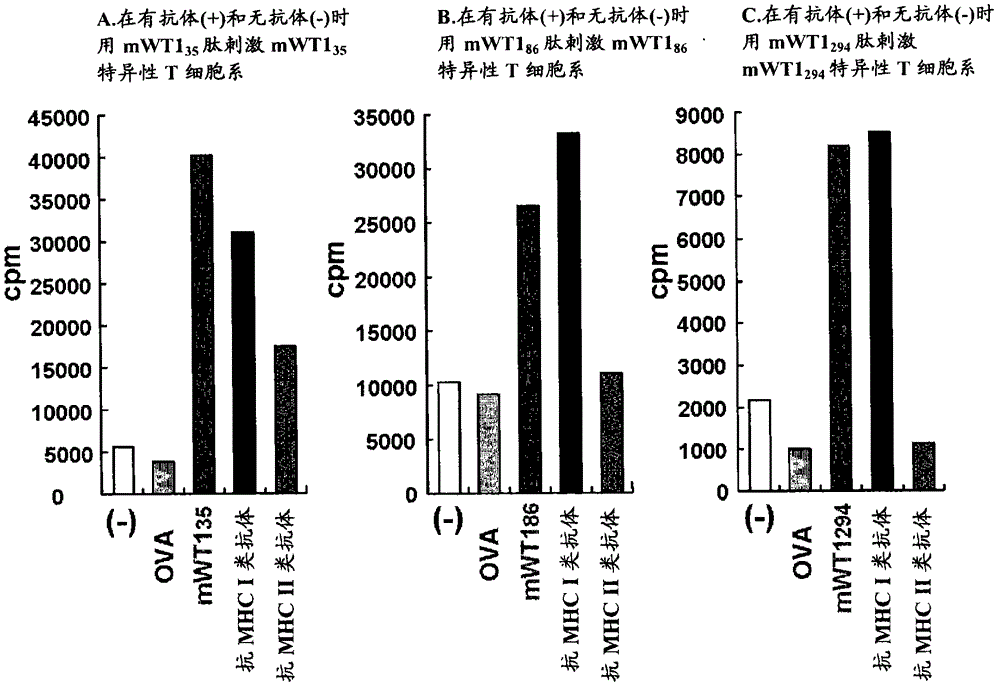
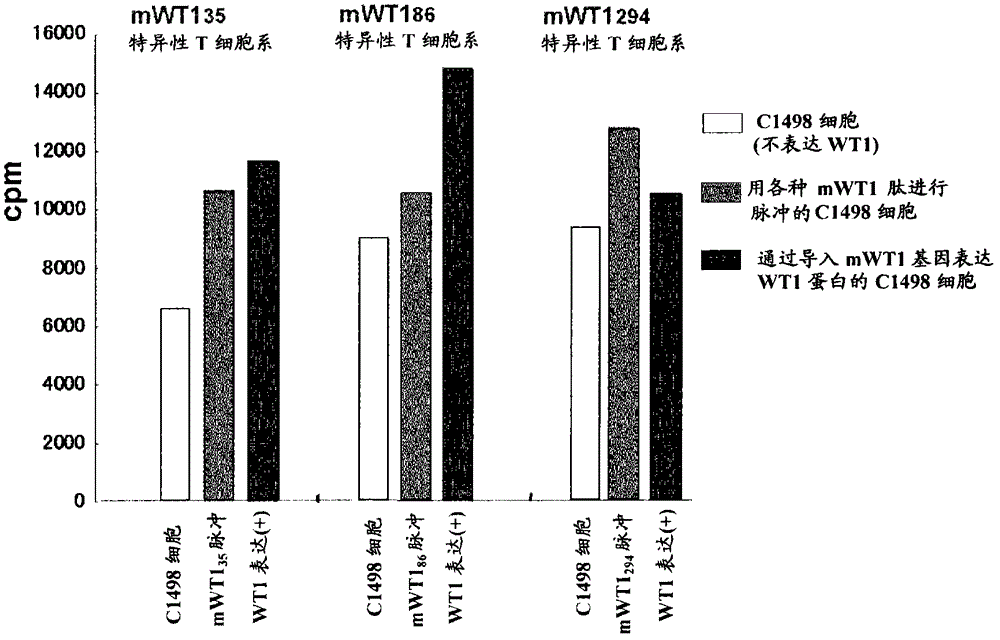
Hla-a24-restricted cancer antigen peptides
HLA-A24-restricted peptides derived from WT1 which have an activity to induce CTLs in vivo, polynucleotides encoding said peptides, cancer vaccines using those peptides or polynucleotides in vivo or in vitro, or the like are provided. The cancer vaccines of the present invention may be used to treat many cancer patients.
Owner:INT INST OF CANCER IMMUNOLOGY INC
T cell receptors and related materials and methods of use
InactiveUS7820174B2Peptide/protein ingredientsMammal material medical ingredientsMajor histocompatibilityCancer antigen
The invention provides an isolated or purified T cell receptor (TCR) having antigenic specificity for a cancer antigen, e.g., a renal cell carcinoma antigen, wherein the TCR recognizes the cancer antigen in a major histocompatibility complex (MHC)-independent manner. Also provided are related polypeptides, proteins, nucleic acids, recombinant expression vectors, isolated host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions. The invention further provides a method of detecting the presence of cancer in a host and a method of treating or preventing cancer in a host using the inventive TCRs or related materials.
Owner:UNITED STATES OF AMERICA
Method of image guided intraoperative simultaneous several ports microbeam radiation therapy with microfocus X-ray tubes
This invention pertains to a method of low-cost intraoperative all field simultaneous parallel microbeam single fraction few seconds duration 100 to 1,000 Gy and higher dose radiosurgery with micro-electro-mechanical systems (MEMS)-carbon nanotube based microaccelerators. It ablates cancer cells including the mesenchymal epithelial transformation associated cancer stem cells. Microbeam brachy-therapeutic radiosurgery is performed. Microaccelerators are configured for simultaneous parallel microbeam emission from varying angels to an isocentric tumor. Their additive dose rate at the isocentric tumor is in the range of 10,000 to 20,000 Gy / s. It eliminates most tumor recurrence and metastasis which enhances cancer cure rates. It also exposes cancer antigens which induces cancer immunity. Stereotactic breast core biopsy is combined with, positron emission tomography and computerized tomography and phase-contrast imaging. Parallel microbeam brachytherapy preserves normal breast appearance. Migration of normal stem cells from unirradiated valley regions heals the radiation damage to the normal tissue.
Owner:SAHADEVAN VELAYUDHAN
Cancer antigen peptide formulations
Cancer antigen peptides derived from WT1 which have an in vivo efficacy, particularly a clinical usefulness, and cancer vaccines as dosage forms suitable for said cancer antigen peptides, are provided.The invention relates to water-in-oil emulsions which comprise as an effective ingredient either one or both of peptides selected from the group consisting of a peptide having an amino acid sequence of Cys Met Thr Trp Asn Gln Met Asn Leu (SEQ ID NO: 2), and a peptide having an amino acid sequence of Cys Tyr Thr Trp Asn Gln Met Asn Leu (SEQ ID NO: 3), as well as processes for preparation of said emulsion.
Owner:INT INST OF CANCER IMMUNOLOGY INC
HLA-A24-restricted cancer antigen peptides
HLA-A24-restricted peptides derived from WT1 which have an activity to induce CTLs in vivo, polynucleotides encoding said peptides, cancer vaccines using those peptides or polynucleotides in vivo or in vitro, or the like are provided. The cancer vaccines of the present invention may be used to treat many cancer patients.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Tat-Based vaccine Compositions and Methods of Making and Using Same
A Tat-based vaccine composition comprising at least one antigen coupled to at least one immunostimulatory lentivirus trans-activator of transcription (Tat) molecule wherein the antigen is a cancer antigen an infectious disease antigen or a fragment thereof and methods to treat disease by administering the Tat-based vaccine composition. An additional Tat-based vaccine composition comprising immunostimulatory lentivirus Tat is provided.
Owner:CHERRY MED +1
Composition and method of cancer antigen immunotherapy
InactiveUS6406699B1Snake antigen ingredientsPharmaceutical delivery mechanismVaccinationCancer antigen
A cancer immunotherapy method and composition for treating cancer in a patient comprised of vaccinating a patient with a vaccine comprised of the patient's own malignancy and an immunologic adjuvant, removing primed peripheral blood T lymphocytes from the patient, stimulating the primed T lymphocytes to differentiate into effector lymphocytes in vitro, stimulating the effector T lymphocytes to proliferate in vitro, and infusing the effector T lymphocytes back into the patient.
Owner:TVAX BIOMEDICAL LLC
Methods for detecting major adverse cardiovascular and cerebrovascular events
InactiveUS20110045514A1Raise the possibilityMicrobiological testing/measurementDisease diagnosisTissue factorAnalyte
The present teachings relate to a method of assessing the probability of a major adverse cardiovascular or cerebrovascular event in a human. The method can include measuring a concentration, in a blood-based sample of a human, of a set of analytes, for example, alpha-fetoprotein, cancer antigen 125, glutathione S-transferase, and tissue factor. The method also can include determining a MACCE index for the set of analytes and identifying the human as having an increased likelihood of a major adverse cardiovascular or cerebrovascular event if the MACCE index is greater than zero, or a decreased likelihood of a major adverse cardiovascular or cerebrovascular event if the MACCE index is less than or equal to zero.
Owner:BG MEDICINE
Personalized cancer vaccines and adoptive immune cell therapies
Cancer antigens containing mutations in an expressed gene of cancer cells from a cancer patient are identified. Sequences from cancer cells obtained using a parallel sequencing platform are selected by comparing to the patient's normal genes or to normal genes from an HLA-matched individual. Sequences are further selected by identifying an HLA supertype of the cancer patient and selecting for that HLA supertype, sequences that have a particular amino acid at the mutant position and / or corresponding wild-type position in the effected gene. Peptides containing cancer antigens (i.e., mutations—once a mutation is defined, what makes it an immunogen is its ability to induce an immune response) are optionally tested for binding to HLA antigens of the cancer patient. Peptides containing the cancer antigens are evaluated for activating T cells (e.g., helper T lymphocytes and cytotoxic T lymphocytes (CTL)) cell lines from the cancer patient or from an HLA-matched donor. The cancer antigen(s) identified for a cancer patient are used to prepare a cancer vaccine and to treat the cancer patient.
Owner:PERSIMMUNE
T cell receptors and related materials and methods of use
The invention provides T cell receptors (TCRs) having antigenic specificity for a cancer antigen, e.g., tyrosinase. Also provided are related polypeptides, proteins, nucleic acids, recombinant expression vectors, isolated host cells, populations of cells, and pharmaceutical compositions. The invention further provides a method of detecting the presence of cancer in a host and a method of treating or preventing cancer in a host using the inventive TCRs or related materials.
Owner:UNITED STATES OF AMERICA
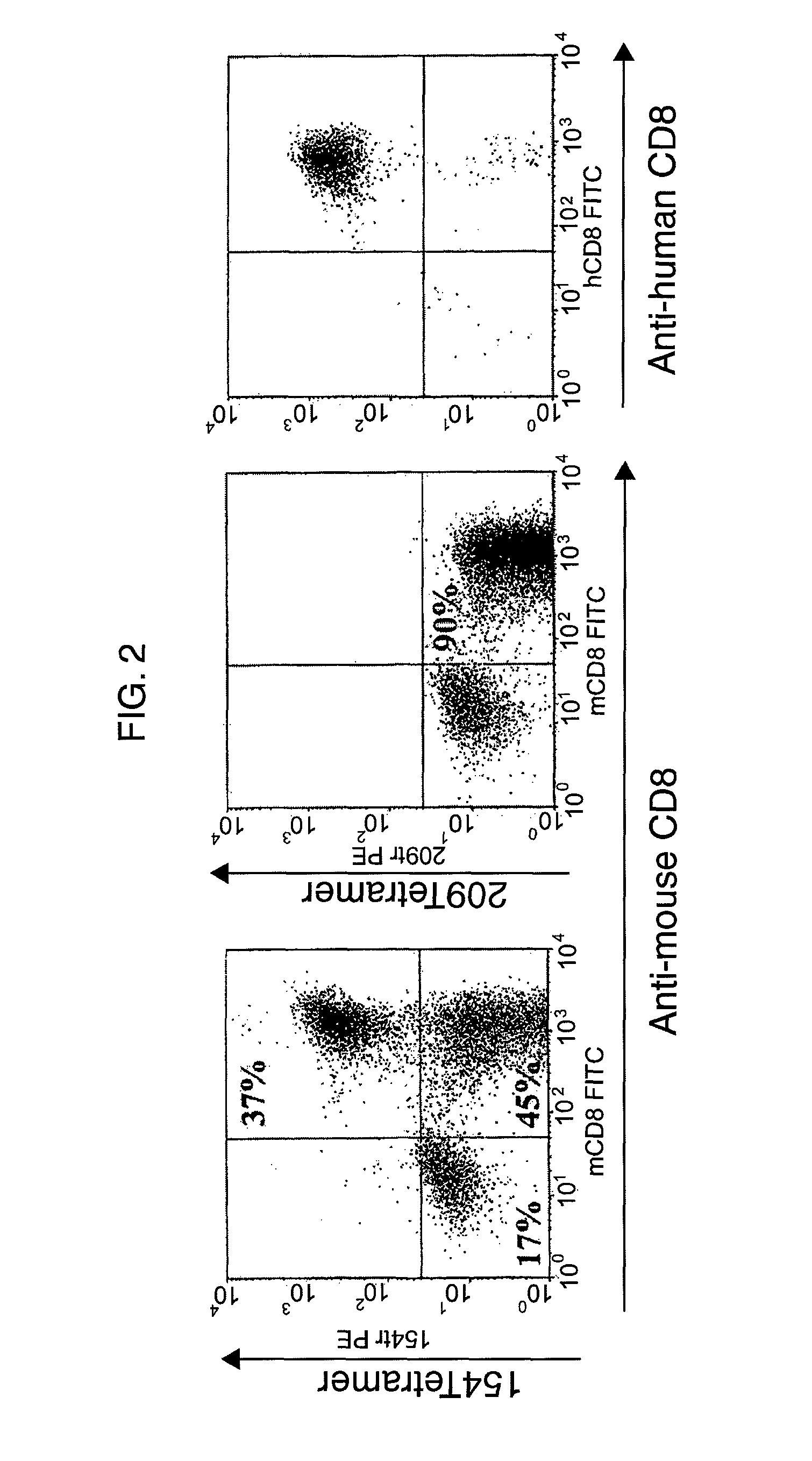
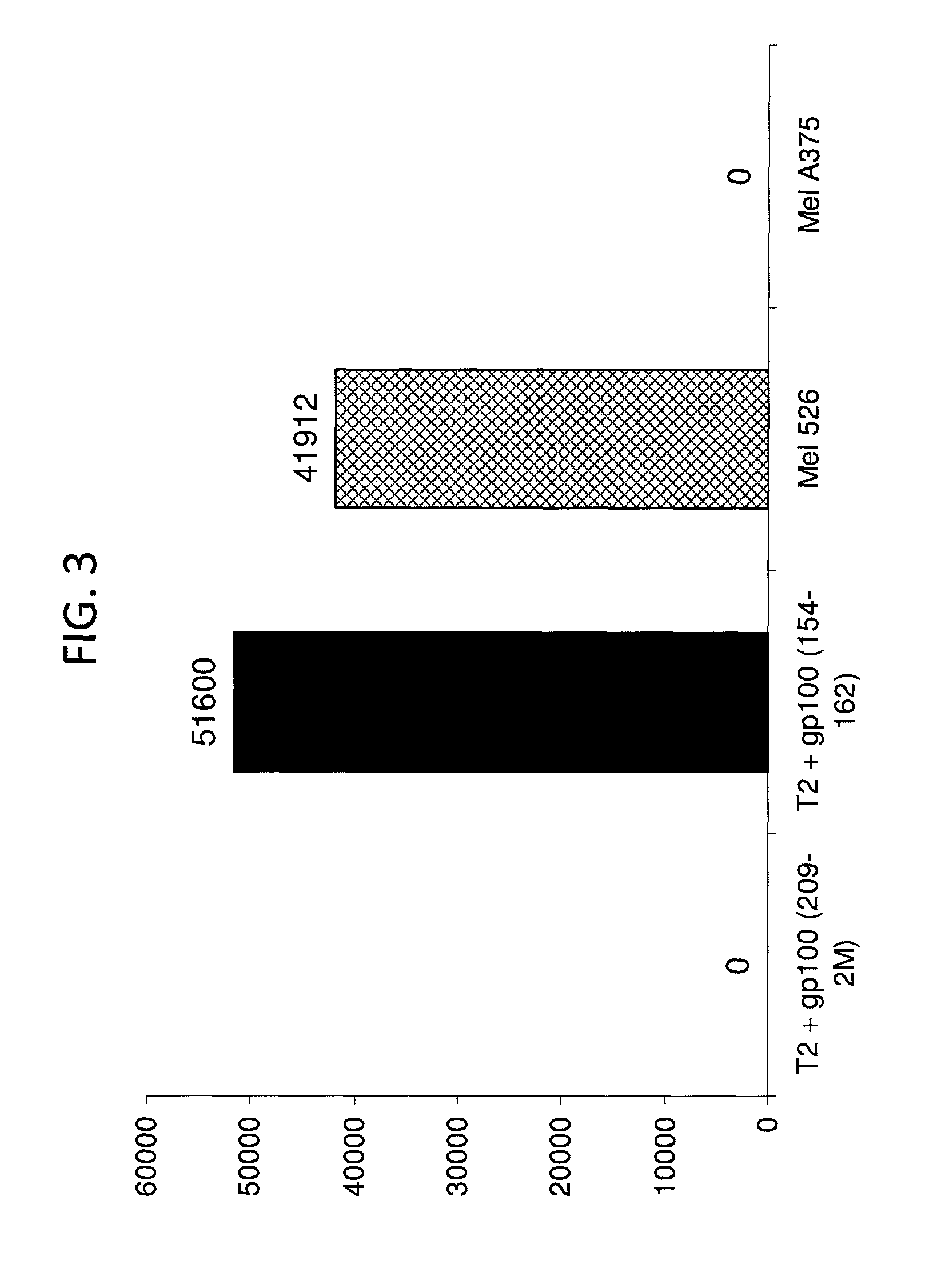
GP100-specific T cell receptors and related materials and methods of use
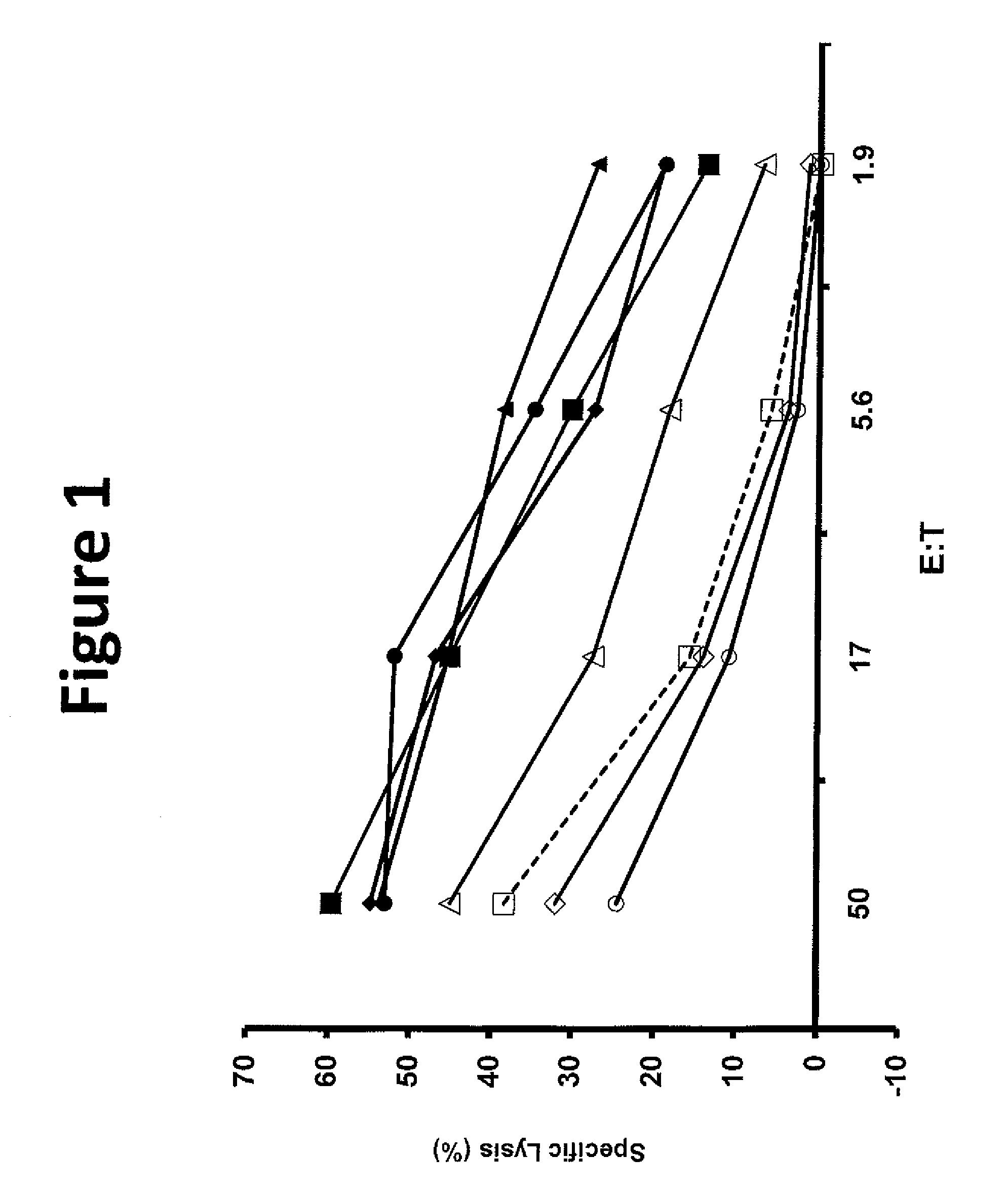
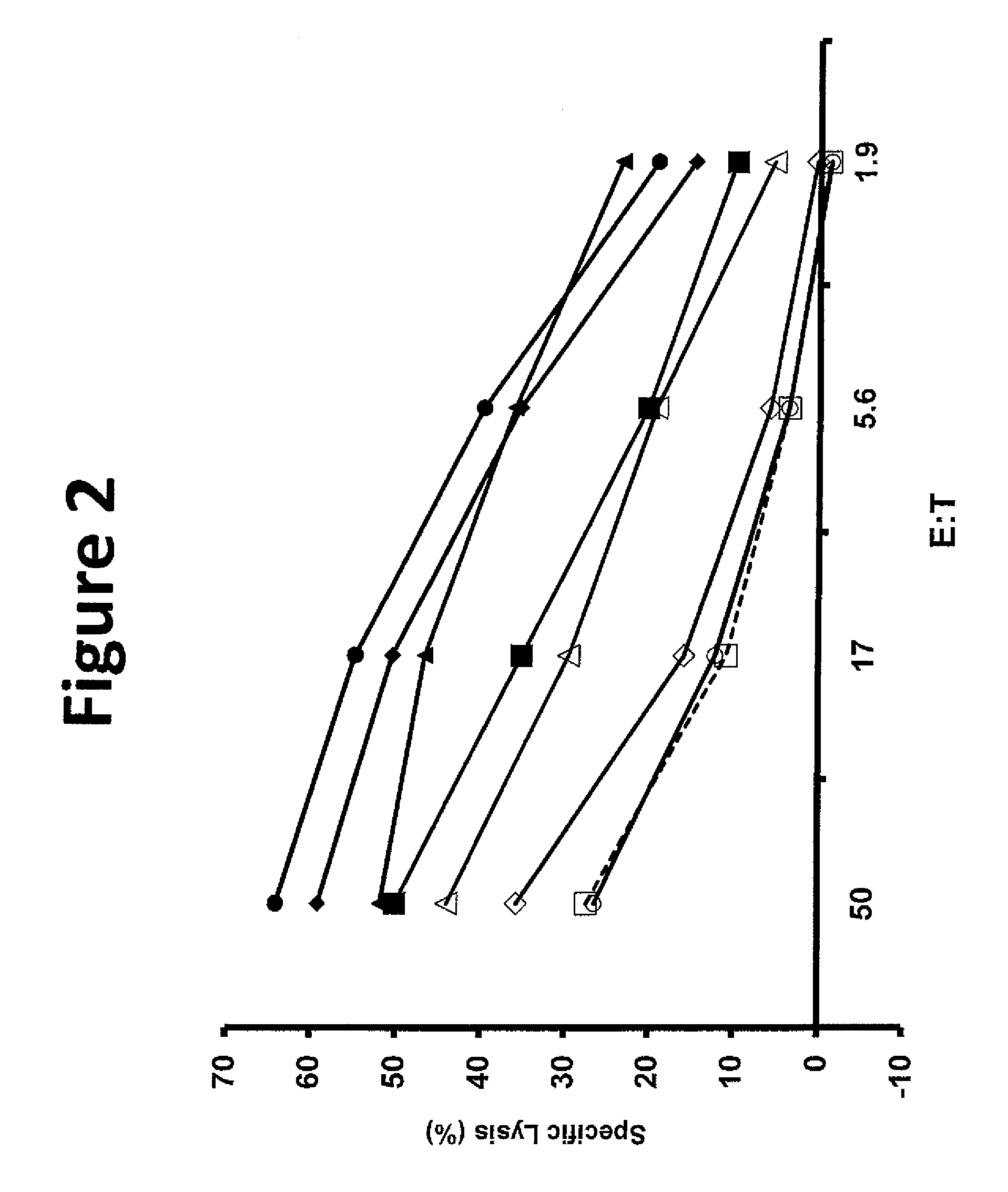
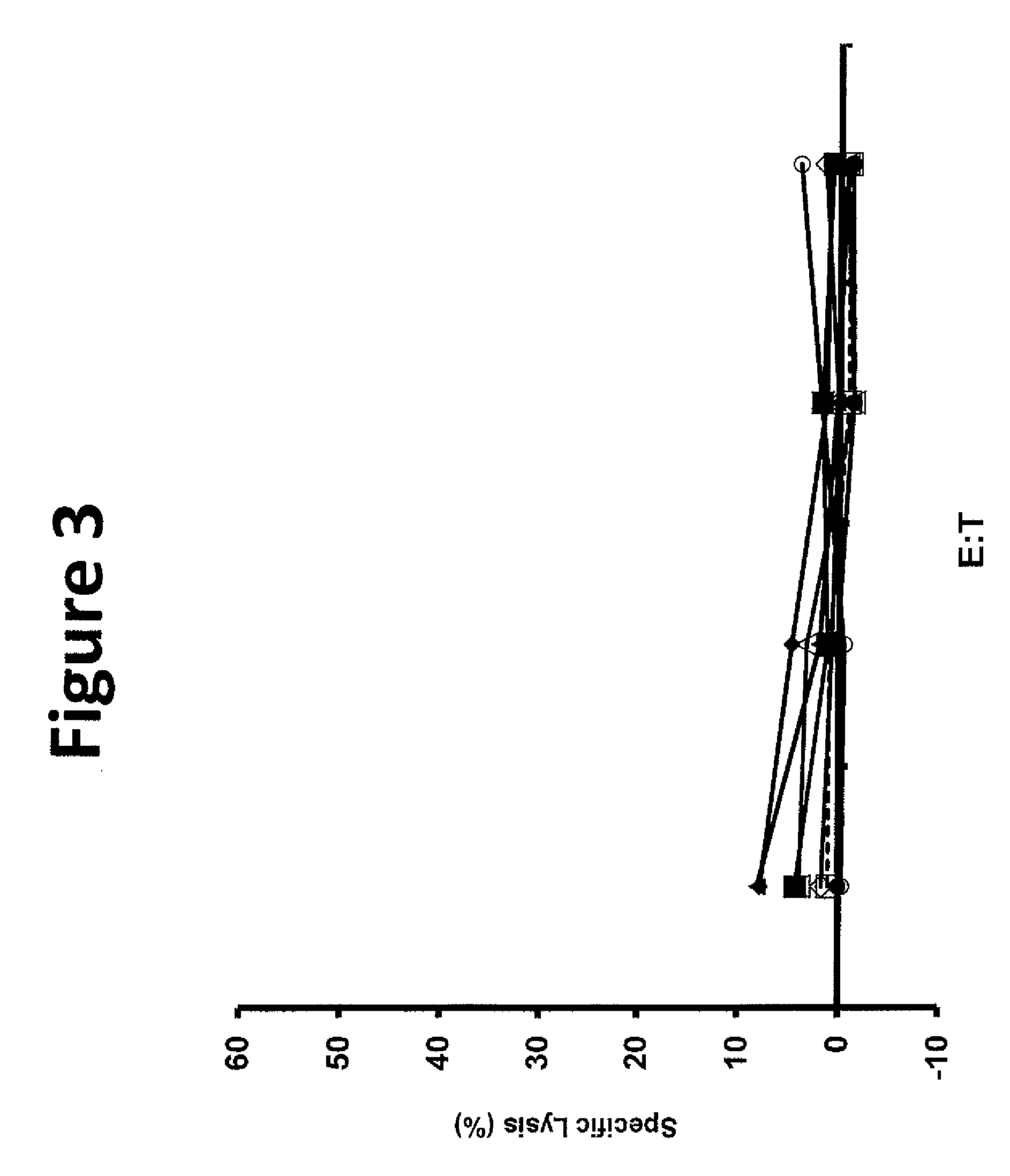
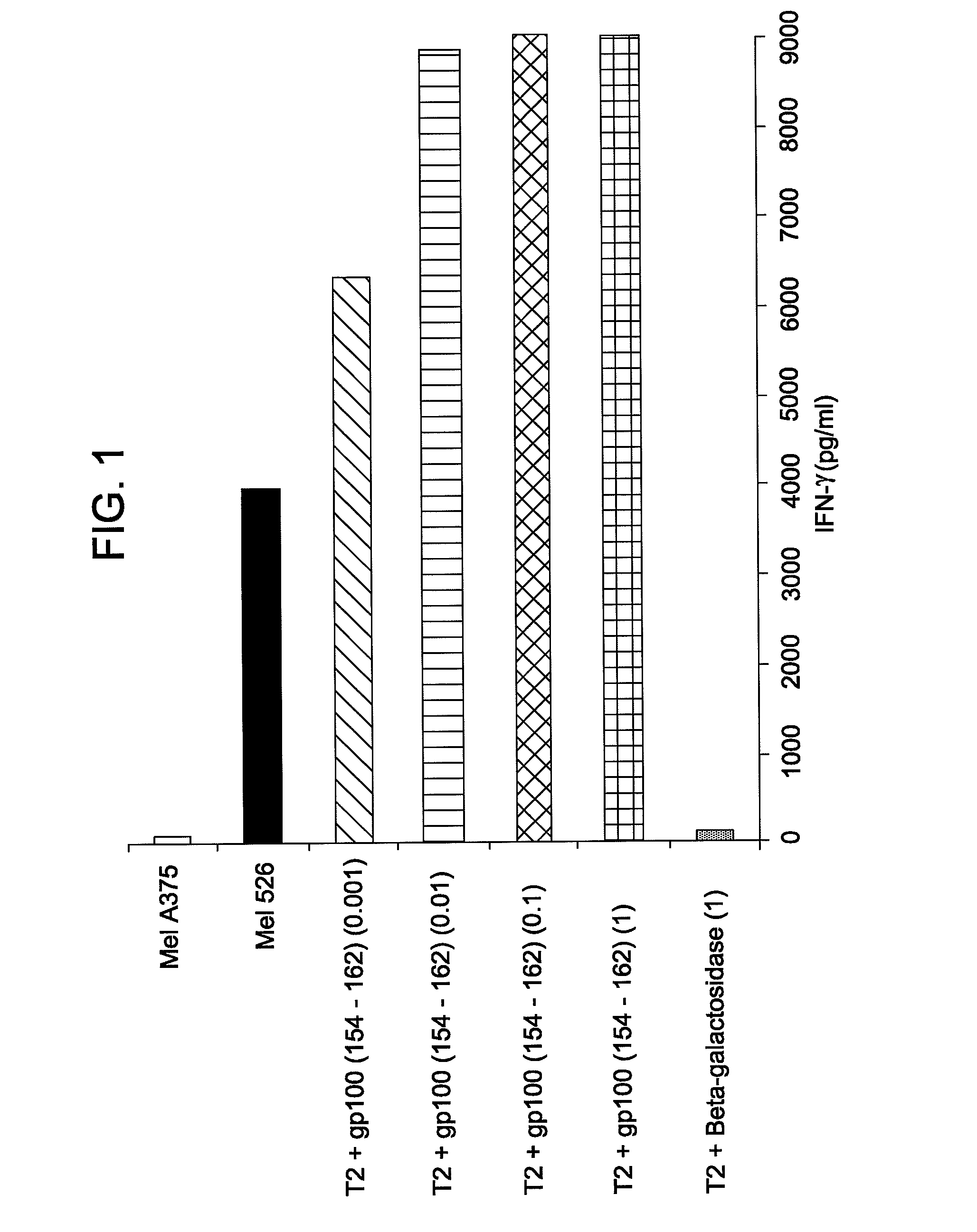
The invention provides human cells, particularly human T cells, comprising a murine T Cell Receptor (TCR) having antigen specificity for the cancer antigen gp100. Isolated or purified TCRs having antigenic specificity for amino acids 154-162 of gp100 (SEQ ID NO: 1), as well as related polypeptides, proteins, nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding fragments thereof, conjugates, and pharmaceutical compositions, are further provided. The invention further provides a method of detecting the presence of cancer in a host and a method of treating or preventing cancer in a host comprising the use of the inventive materials described herein.
Owner:UNITED STATES OF AMERICA
Edible vaccines expressed in soybeans
The present invention relates to vaccines that are made in transgenic soybeans for use in humans, animals of agricultural importance, pets, and wildlife. These vaccines are used as vaccines against viral, bacterial, fungal, parasitic or prion related diseases, cancer antigens, toxins, and autologous or self proteins. The transgenic soybeans of the instant invention also can be used for inducing tolerance to allergens or tolerance to autoimmune antigens, wherein an individual shows hypersensitivity to said allergen or has developed autoimmunity to autologous or self proteins, respectively. The invention also relates to prophylatically treating individuals and / or populations prior to showing hypersensitivity to allergens. Other aspects of the invention include using the transgenic soybeans as an oral contraceptive, and the expression of protein adjuvants in transgenic soybeans.
Owner:SOYMEDS
Cancer antigen-specific t-cell receptor gene, peptide encoded by the gene, and use of them
InactiveUS20100190163A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAntigenNucleotide
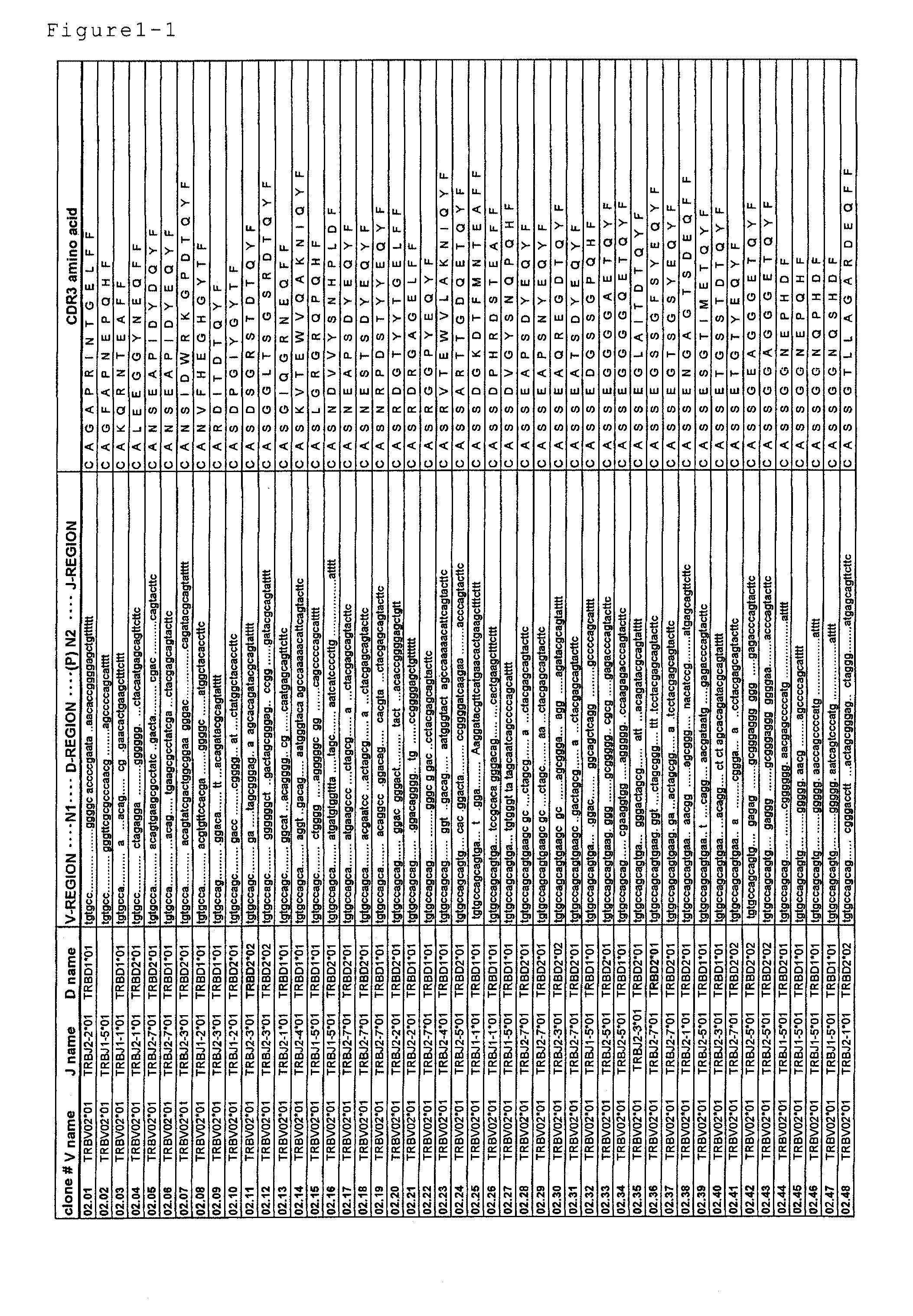
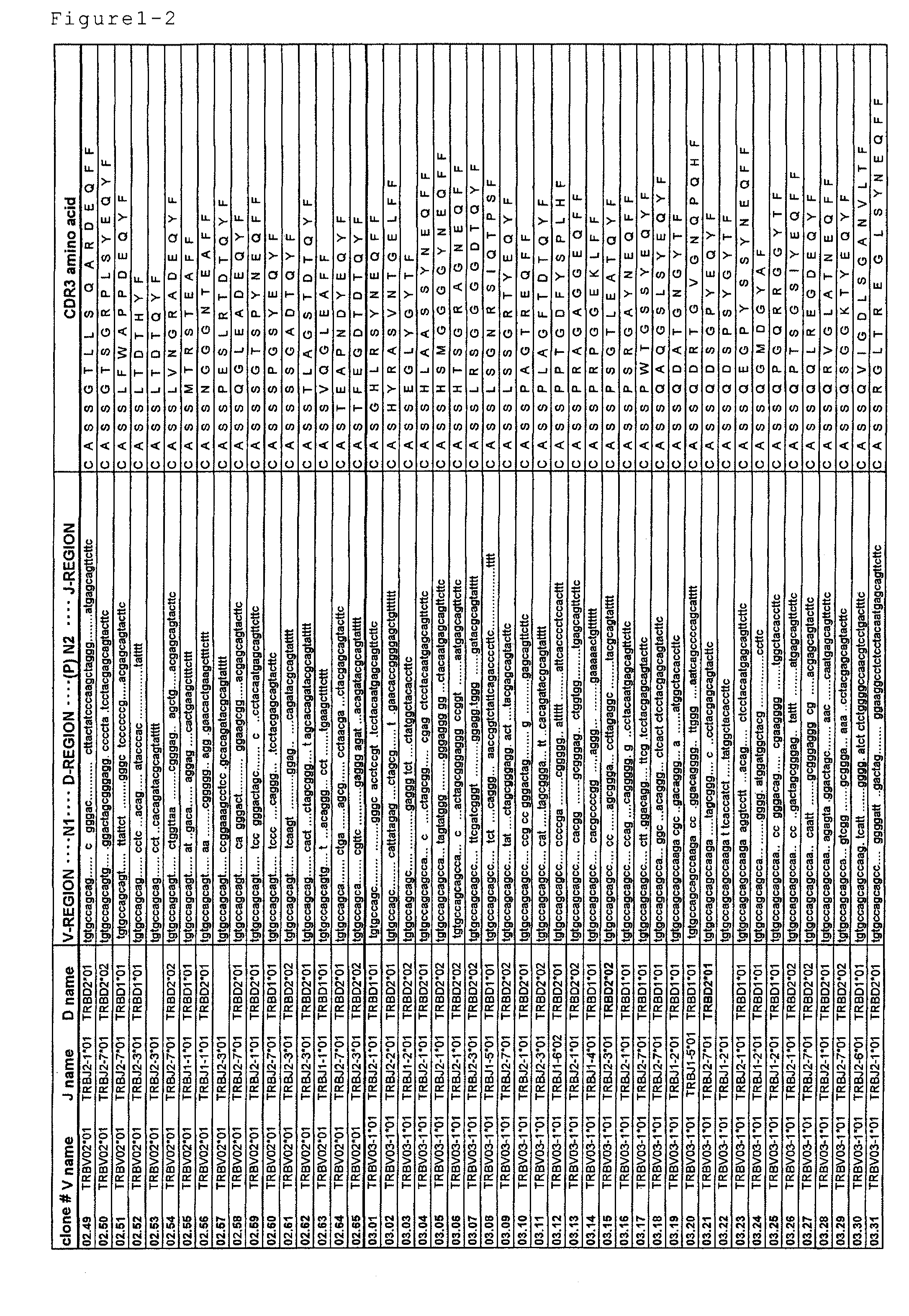
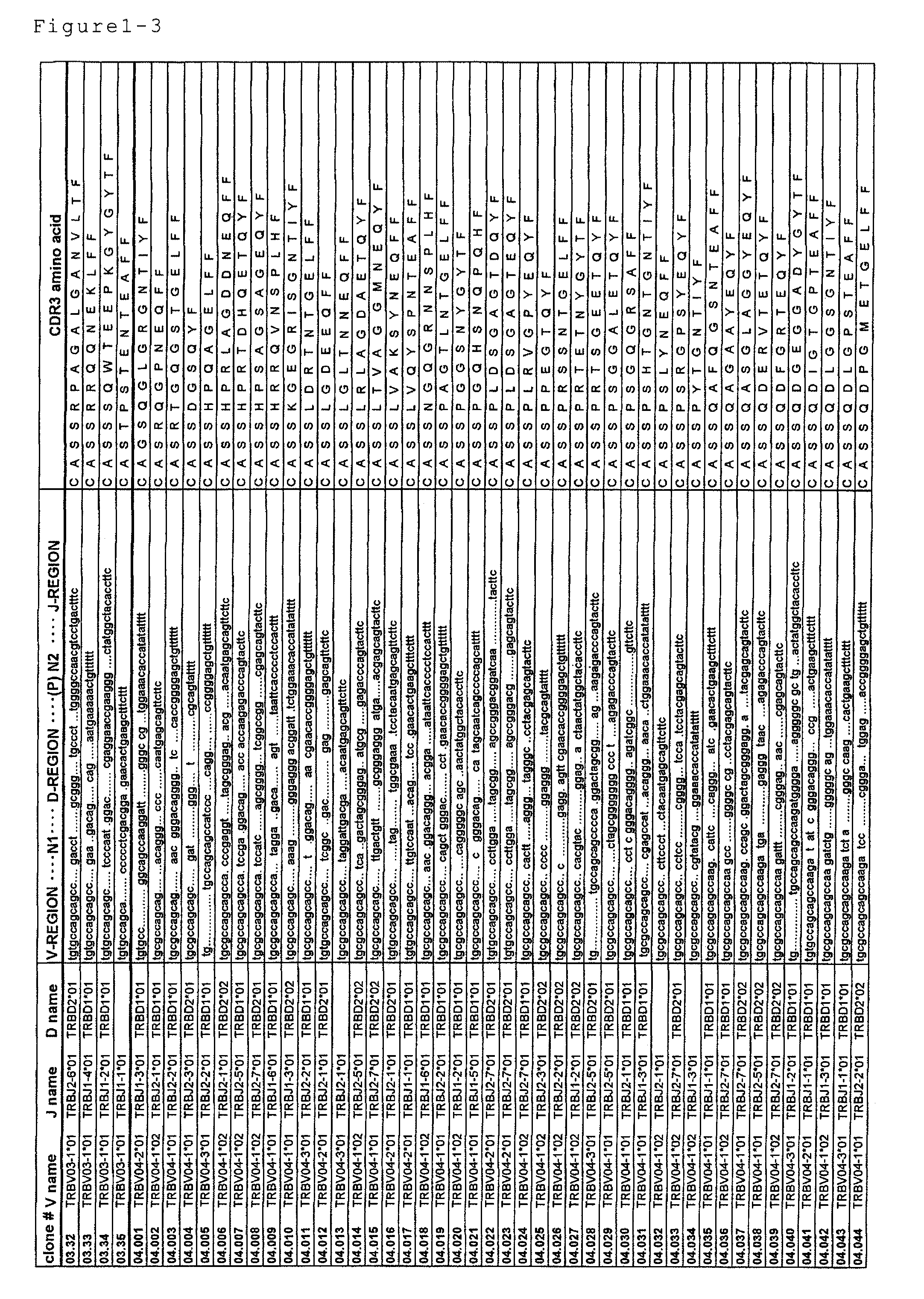
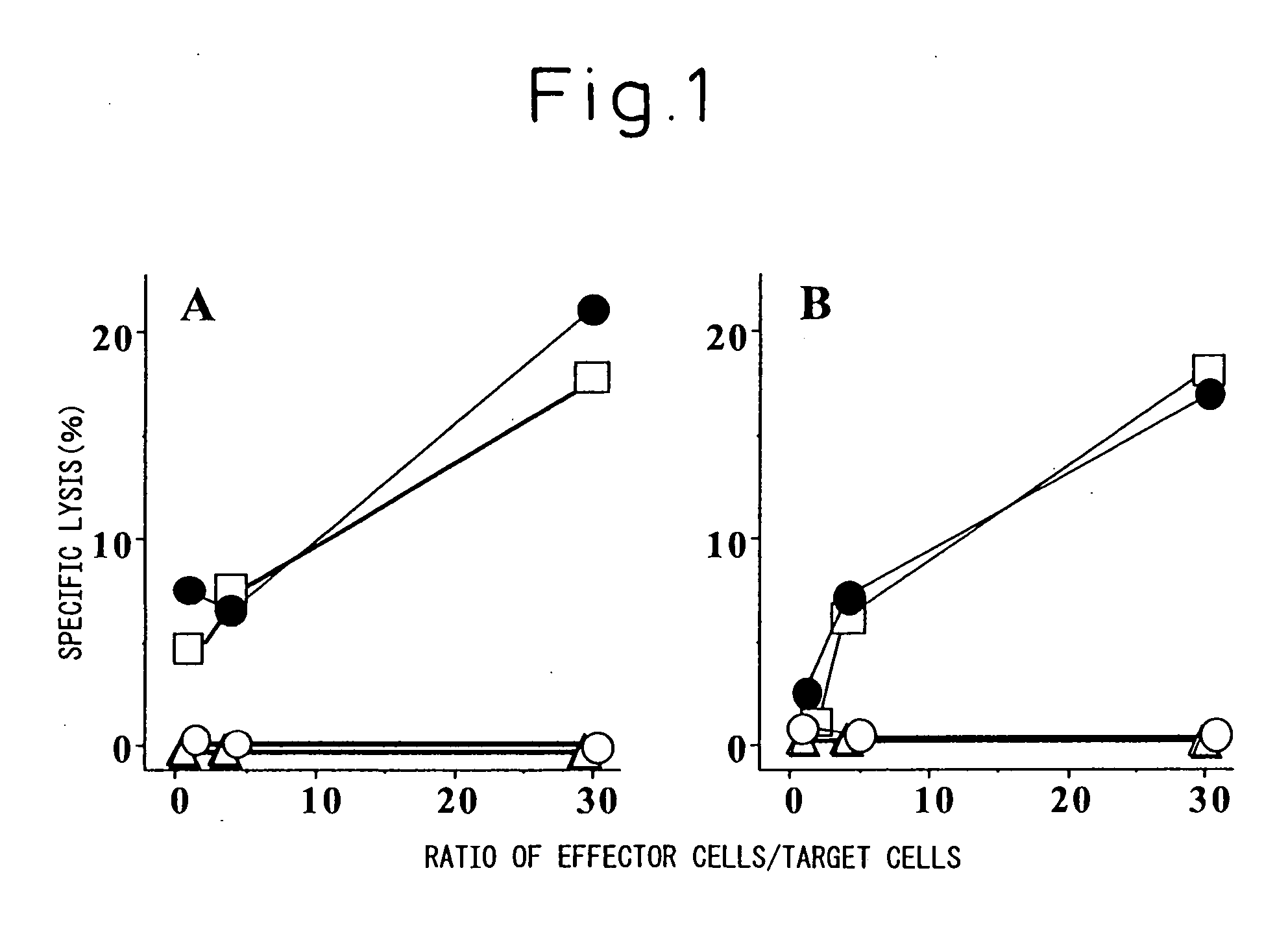
Disclosed are: a nucleotide sequence and an amino acid sequence for CDR3 region of T-cell receptor (TCR) gene of WT1-specific cytotoxic T-cell (CTL) for WT1 protein; a method for the detection or treatment of cancer using the nucleotide sequence or the amino acid sequence; and a chip, a primer set, a kit, an apparatus and the like for use in the detection of cancer, each of which comprises the nucleotide sequence or the amino acid sequence.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Cancer vaccine containing cancer antigen based on tumor suppressor gene wt1 product and cationic liposomes
InactiveUS20060165708A1Tumor rejection antigen precursorsTumor specific antigensAntigenCancer antigen
A cancer vaccine comprising a cancer antigen which comprises, as an active ingredient, the product of a tumor suppressor gene WT1, a partial peptide or a modified version thereof, and a cationic liposome.
Owner:MAYUMI TADANORI +2
Antibodies against cancer antigen TMEFF2 and uses thereof
ActiveUS7288248B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer antigenAntibody
Described herein are methods and compositions that can be used for diagnosis and treatment of cancer.
Owner:ABBVIE BIOTHERAPEUTICS
Cancer antigens and utilization thereof
An object of the present invention to provide: a human pancreatic cancer antigen and / or a human colon cancer antigen that can be applied to the diagnosis and / or treatment of various types of cancers or tumors including pancreatic cancer and colon cancer as representative examples; a gene encoding the same; an anti-cancer vaccine using the same; or the like. The present invention provides a cancer antigen comprising a protein having the amino acid sequence shown in SEQ ID NO: 1; a peptide comprising a portion of said protein and having immune-stimulating activity; an anti-cancer vaccine comprising said peptide; a DNA having the nucleotide sequence shown in SEQ ID NO: 2, or its complementary sequence or a part or full length of these sequence; an anti-cancer vaccine comprising said DNA; and use thereof.
Owner:MEDINET CO LTD
Immune cell compositions and methods of use
ActiveUS20180273601A1Restores effector functionEnhances tumor burden controlPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorT cell mediated immunity
Disclosed herein are cells that are immune cells or precursor cells thereof, which cells recombinantly express a chimeric antigen receptor (CAR), and a dominant negative form of an inhibitor of a cell-mediated immune response of the immune cell, wherein the CAR binds to a cancer antigen. Also disclosed herein are T cells that recognize and are sensitized to a cancer antigen, which T cells recombinantly express a dominant negative form of an inhibitor of a T cell-mediated immune response. Additionally provided are methods of using such cells to treat cancer in a subject in need thereof.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
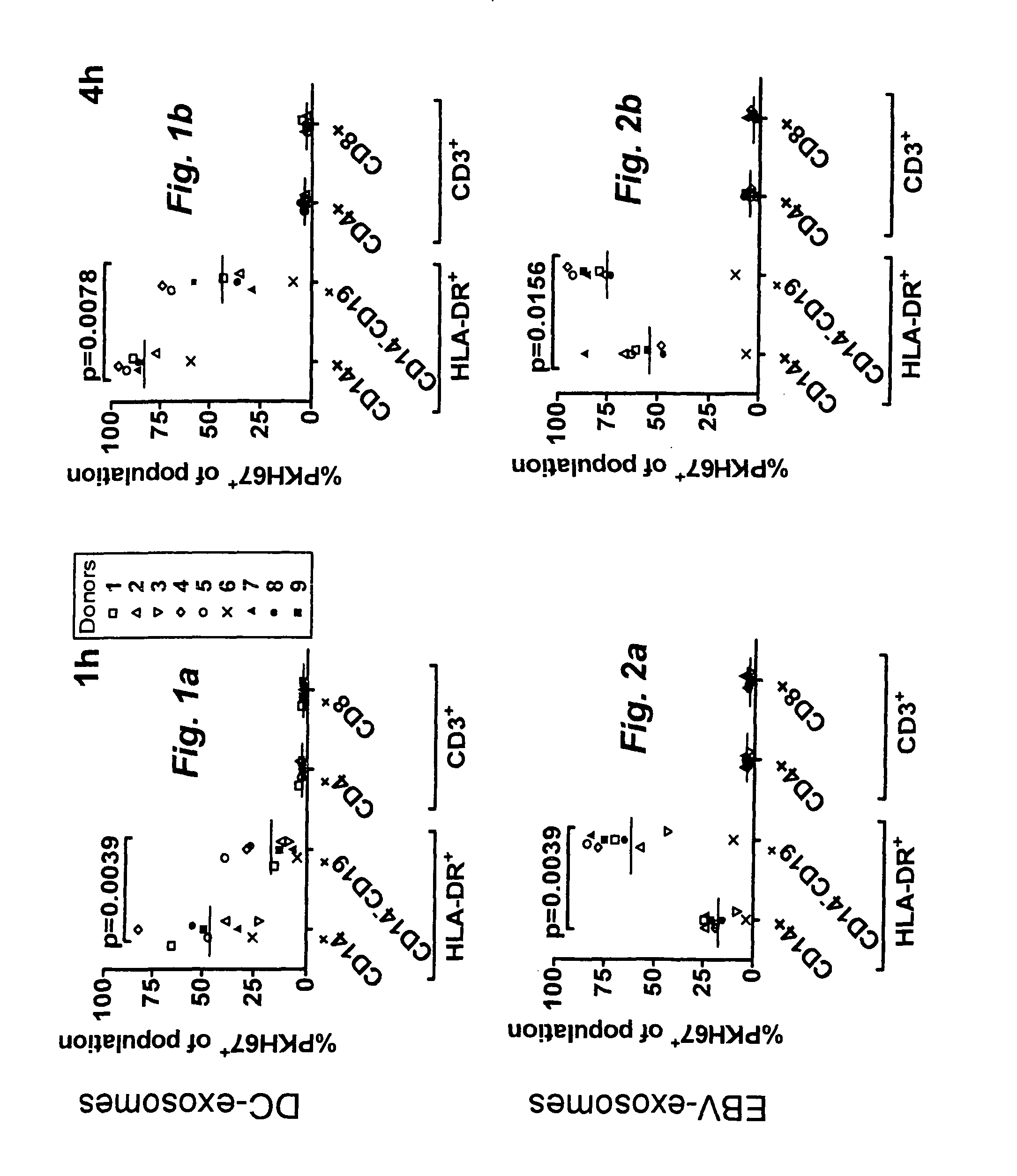
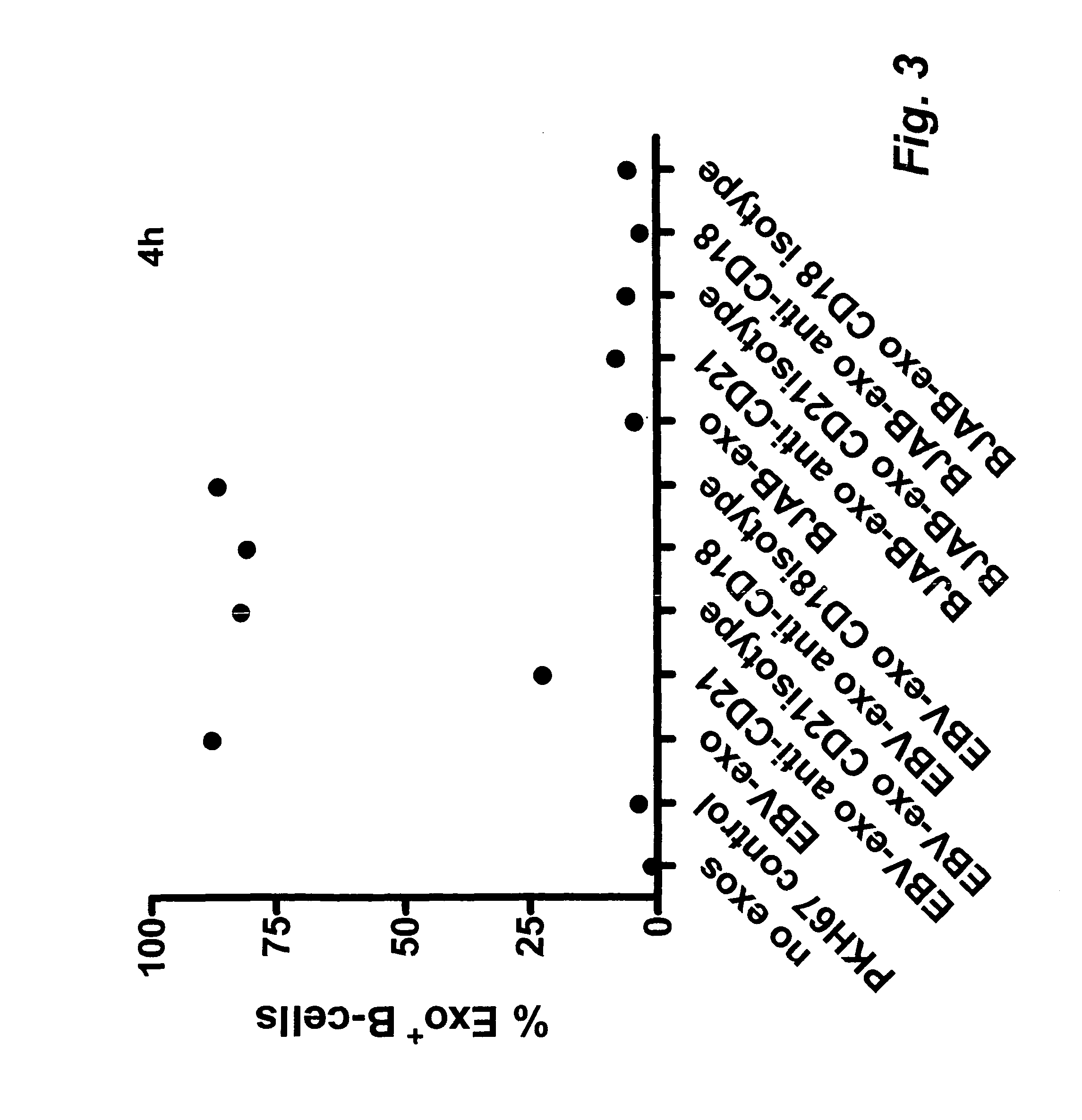
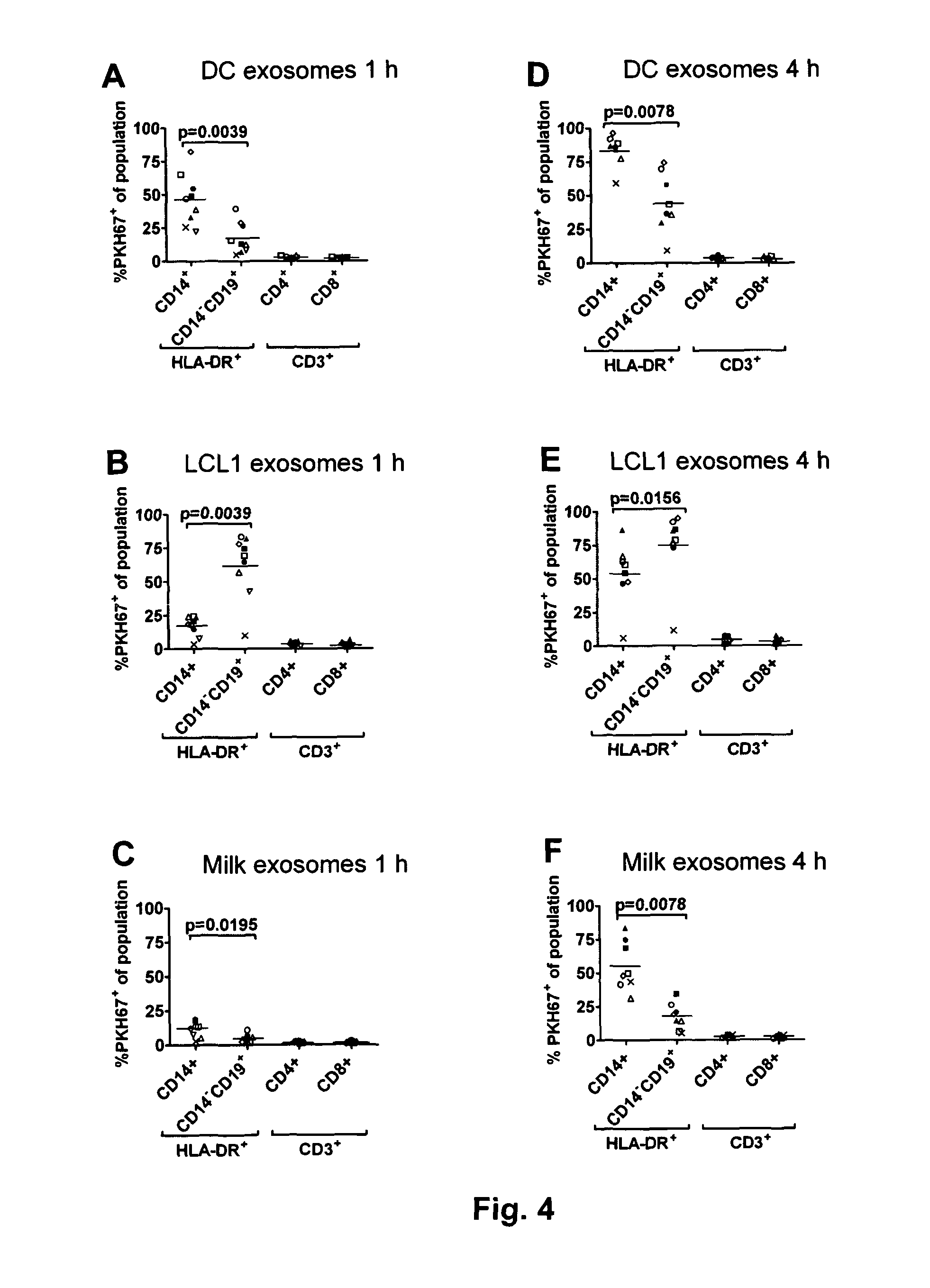
Exosome based treatment of cancer
ActiveUS20120183575A1Efficient and specific immune responseStrong T-cell responseAntibacterial agentsBacterial antigen ingredientsAntigenCancer antigen
A method of treating cancer in a patient comprises immortalizing B cells collected from the patient by infection with Epstein Barr virus, transforming the cells to a latent stage, culturing the cells in the presence of a cancer antigen, harvesting exosomes released from the cells, administering the exosomes to the patient. Alternatively the harvested exosomes are loaded with cancer antigen.
Owner:ITH IMMUNE THERAPY HLDG
Pharmaceutical composition for treatment and/or prevention of cancer
ActiveUS20150050283A1Reduce usageUseful in treatmentSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenic proteinActive ingredient
The invention provides an antibody targeting a cancer antigenic protein specifically expressed on the surface of cancer cells and use thereof in a therapeutic and / or preventive agent for cancer. Specifically, this invention provides an antibody or a fragment thereof which has immunological reactivity with a partial CAPRIN-1 polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 5 or an amino acid sequence having 80% or higher sequence identity to the amino acid sequence, and a pharmaceutical composition for treatment and / or prevention of cancer, comprising the antibody or fragment thereof as an active ingredient.
Owner:TORAY IND INC
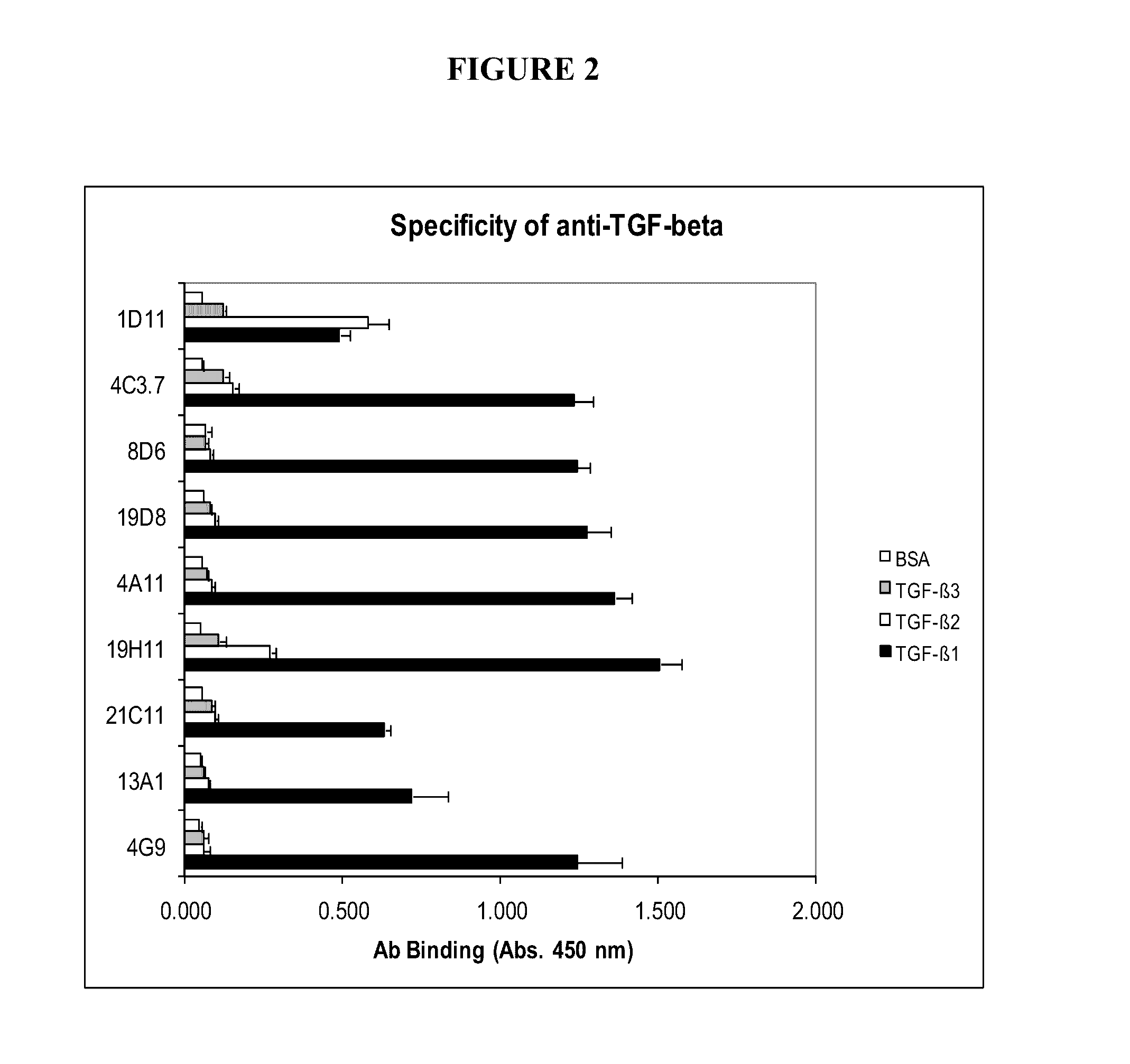
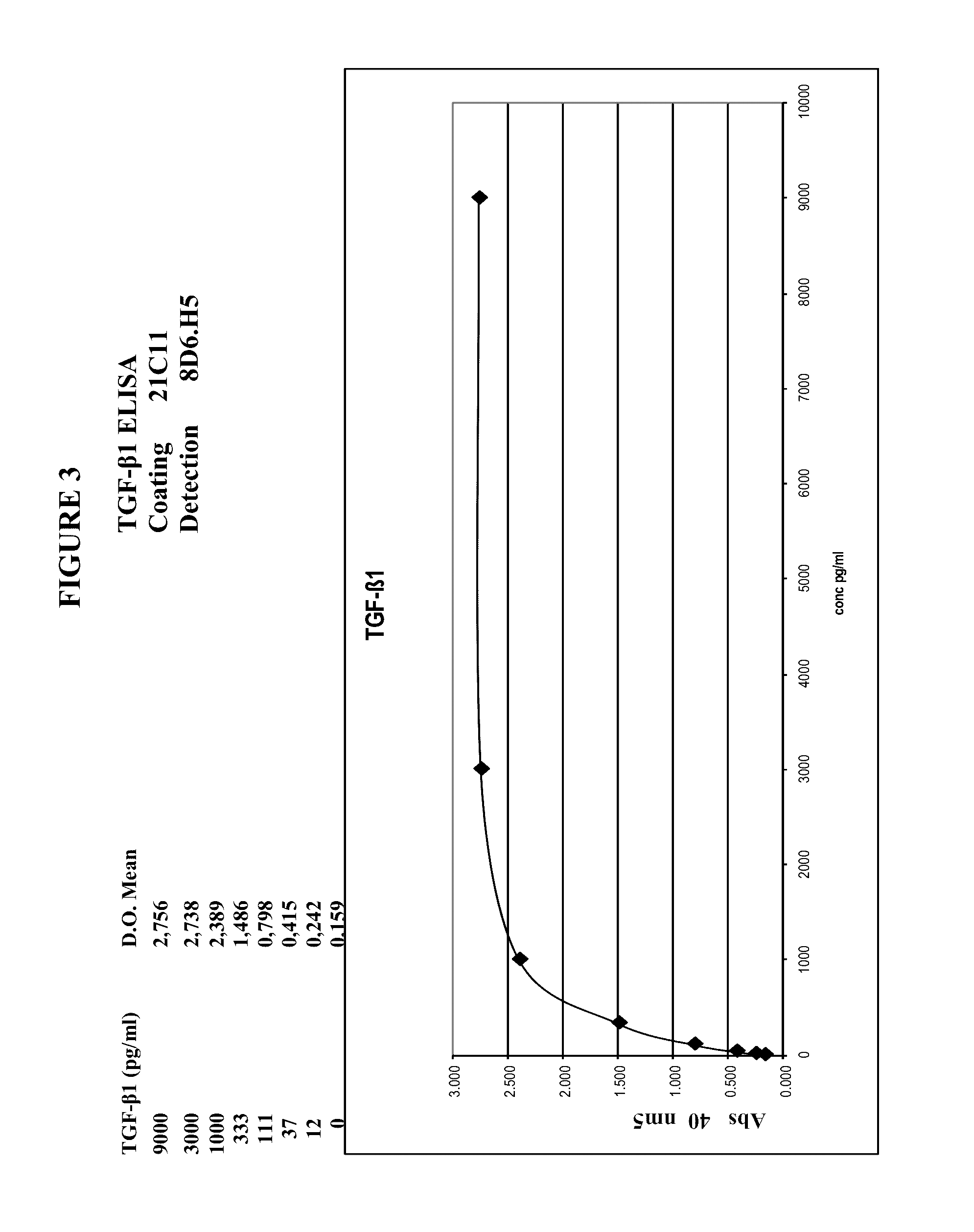
TGF-β1 specific antibodies and methods and uses thereof
ActiveUS9518112B2Less responseLess toxicityImmunoglobulins against growth factorsAntibody ingredientsDiseaseAnticarcinogen
Specific binding members, particularly antibodies and fragments thereof, which bind to transforming growth factor beta 1 (TGF-β1) are provided, particularly recognizing human and mouse TGF-β1 and not recognizing or binding TGF-β2 or TGF-β3. Particular antibodies are provided which specifically recognize and neutralize TGF-β1. These antibodies are useful in the diagnosis and treatment of conditions associated with activated or elevated TGF-β1, including cancer, and for modulating immune cells and immune response, including immune response to cancer or cancer antigens. The anti-TGF-β1 antibodies, variable regions or CDR domain sequences thereof, and fragments thereof may also be used in therapy in combination with chemotherapeutics, immune modulators, or anti-cancer agents and / or with other antibodies or fragments thereof. Antibodies of this type are exemplified by the novel antibodies hereof, including antibody 13A1, whose sequences are provided herein.
Owner:LUDWIG INST FOR CANCER RES LTD
Cancer antigen helper peptide
ActiveCN102803487AEfficient activationOrganic active ingredientsTumor rejection antigen precursorsAntigenCancer antigen
Disclosed are: a WT1 peptide which comprises an amino acid sequence composed of contiguous amino acid residues derived from a WT1 protein and can bind to an MHC class-II molecule to induce a WT1-specific helper T cell; a pharmaceutical composition containing the peptide; and others.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Cancer vaccination with antigen evolution
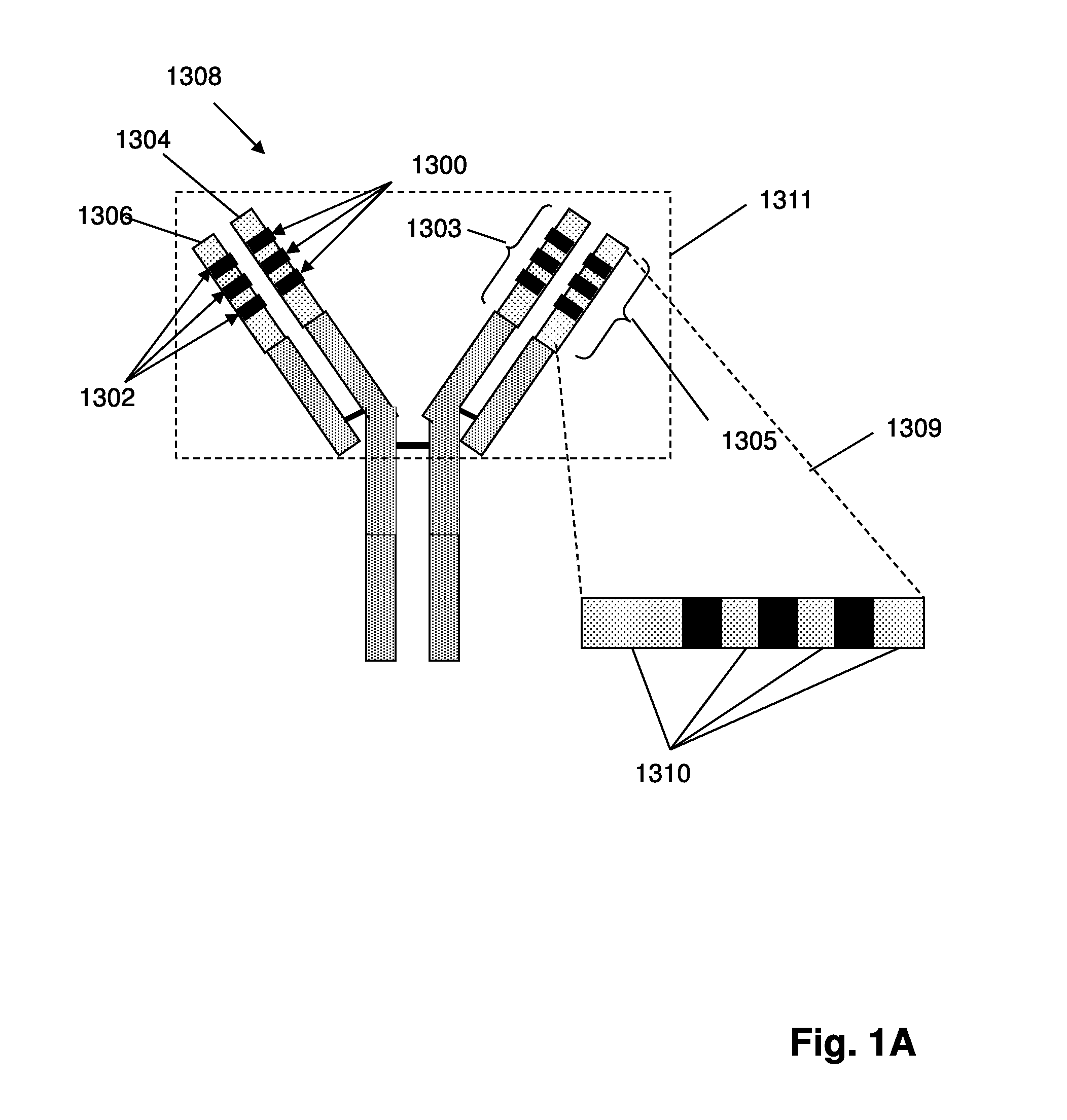
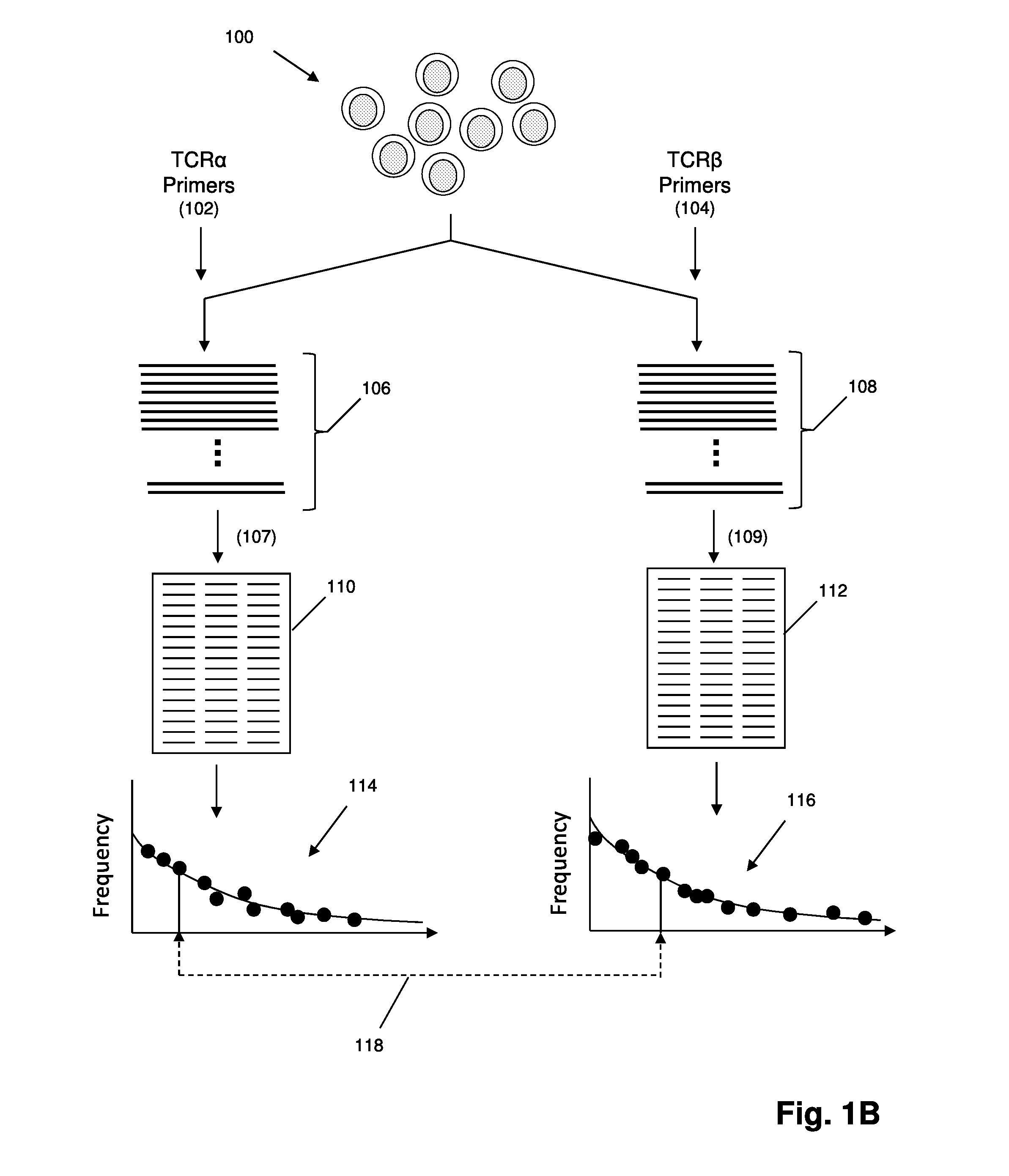
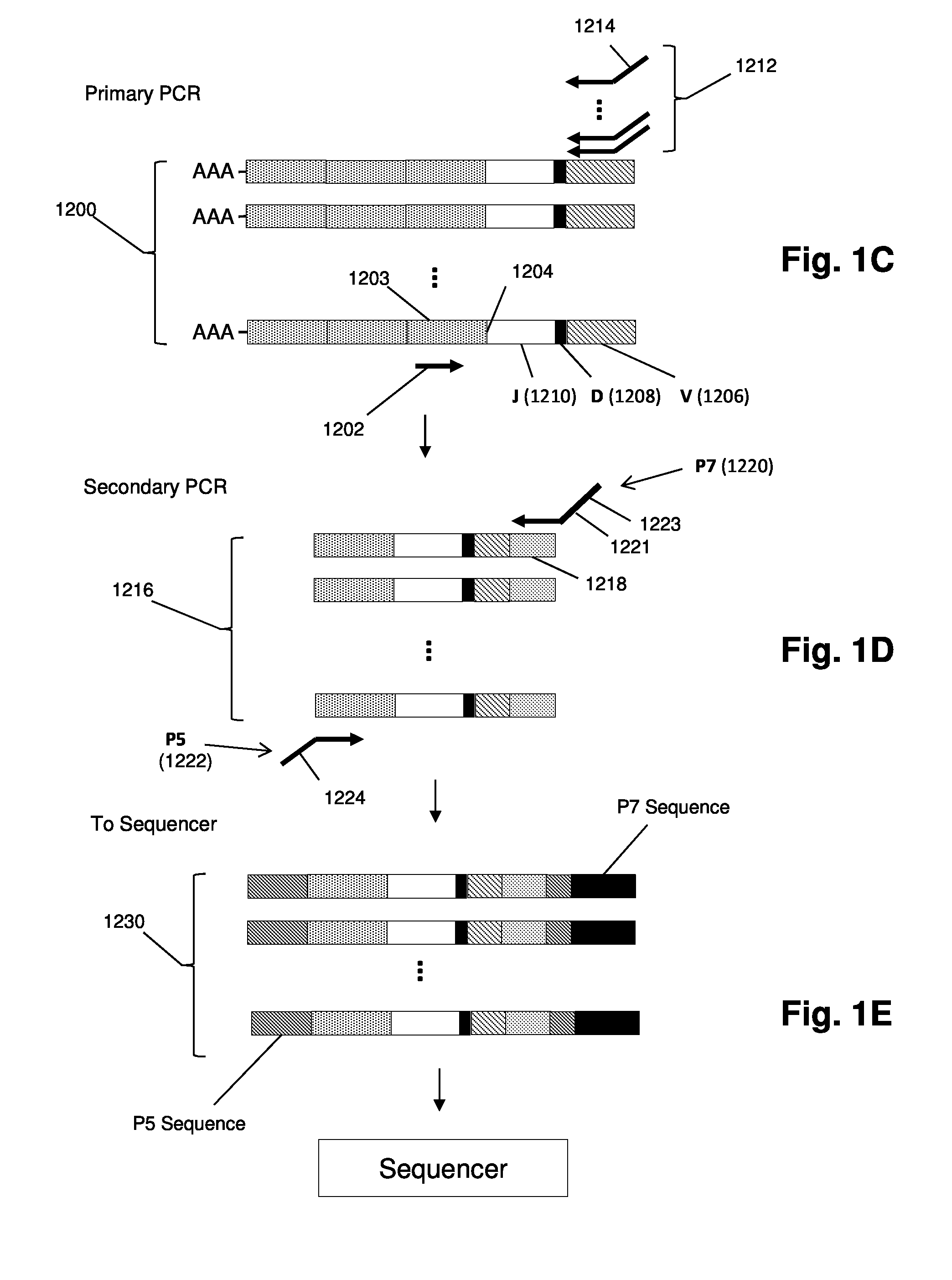
The invention provides methods for treating with cancer vaccines patients whose cancers undergo clonal evolution. The invention makes use of a series of cancer vaccines to stimulate a patient's immune system to mount both a humoral and cellular immune response against cancer cells as cancer-specific antigens on the cancer cells change by clonal evolution. Vaccines used in the invention are derived from antigens unique to the cancer. In one aspect of the invention, such unique antigens are determined by generating sequence-based profiles of cancer related nucleic acids. In some embodiments, cancer antigens may be identified in sequence-based profiles of exon sequences from a sample suspected of containing cancer cells; in other embodiments in which lymphoid or myeloid cancers are being treated, cancer antigens may be identified in sequence-based clonotype profiles.
Owner:ADAPTIVE BIOTECH
Preparation method and application of gastric cancer antigen electrogenerated chemiluminescence sensor based on PP<y>-NH2GO-Ag2Se@CdSe
InactiveCN104391117AMaterial analysis by optical meansBiological testingAntigenAntiendomysial antibodies
The invention relates to a preparation method and the application of a gastric cancer antigen electrogenerated chemiluminescence sensor based on PP<y>-NH2GO-Ag2Se@CdSe, belonging to the field of an electrochemical luminescence sensor. An Ag2Se@CdSe nano pin is taken as an electrogenerated chemiluminescence signal source; the Ag2Se@CdSe nano pin is fixedly loaded on the surface of PP<y>-NH2GO by utilizing the large specific surface area of the PP<y>-NH2GO, so that an antibody capture substrate can be prepared; the luminescent signals of the sensor can be effectively enhanced by the excellent electrical conductivity of the PP<y>-NH2GO. The CA72-4 can be detected according to different electrogenerated chemiluminescence signal intensities for the gastric cancer antigen CA72-4 with different concentrations.
Owner:UNIV OF JINAN
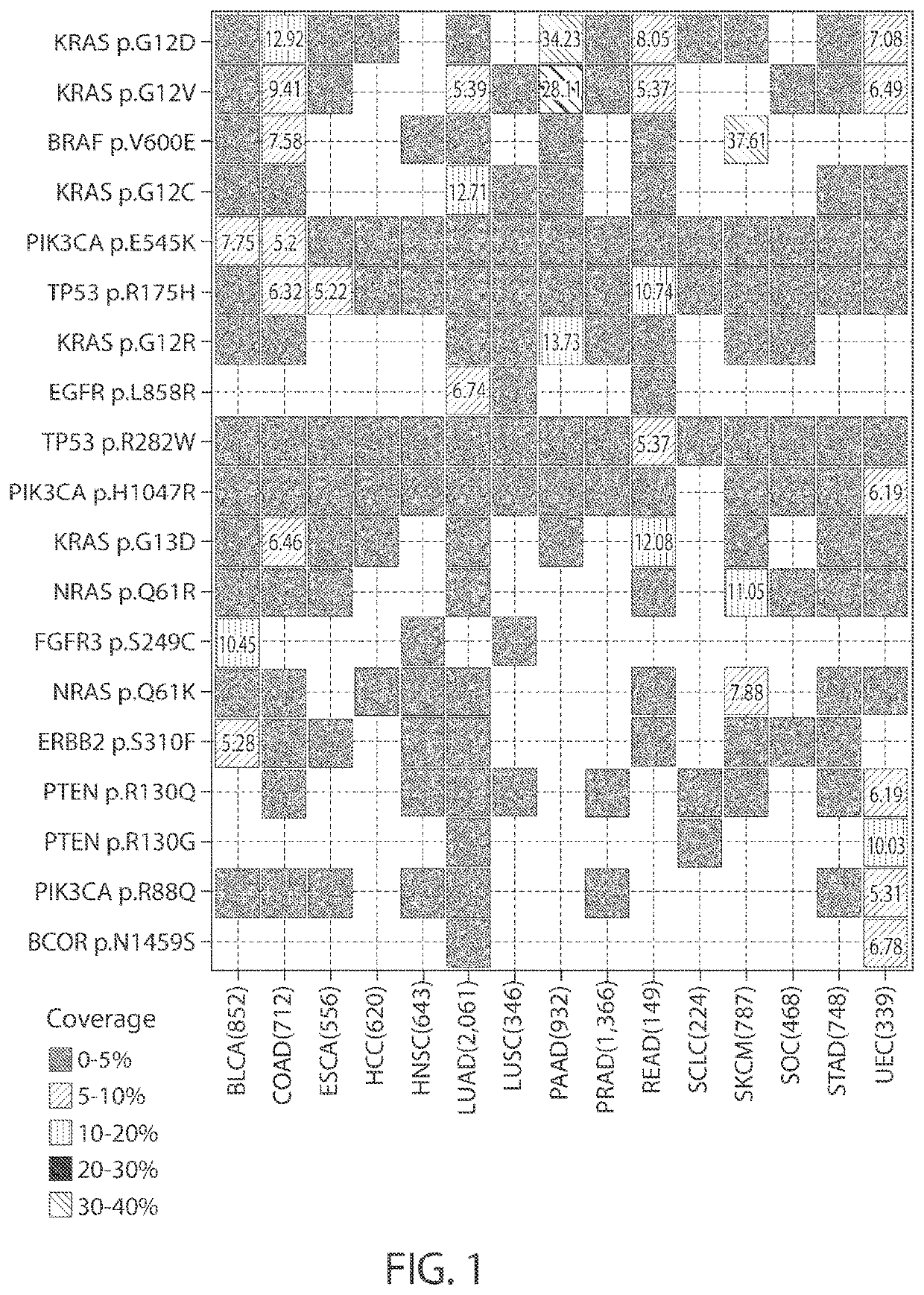
Personalized cancer vaccine epitope selection
The disclosure relates to optimized cancer vaccines, as well as methods of making the vaccines, using the vaccines, and compositions comprising the vaccines. The cancer vaccines comprise personalized cancer antigens or portions of cancer hotspot antigens. Additionally, the disclosure relates to a computerized system for selecting nucleic acids to include in an optimized cancer vaccine.
Owner:MODERNATX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com