Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
470 results about "Human papillomavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for detecting human papillomavirus mrna
InactiveUS20050118568A1High expressionIncrease probabilityMicrobiological testing/measurementEnzymologyHuman papillomavirusHuman Females
An in vitro method is provided for screening human female subjects to assess their risk of developing cervical carcinoma which comprises screening the subject for expression of mRNA transcripts from the E6 and optionally the L1 gene of human papillomavirus, wherein subjects positive for expression of L1 and / or E6 mRNA are scored as being at risk of developing cervical carcinoma. Kits for carrying out such methods are also provided.
Owner:NORCHIP AS
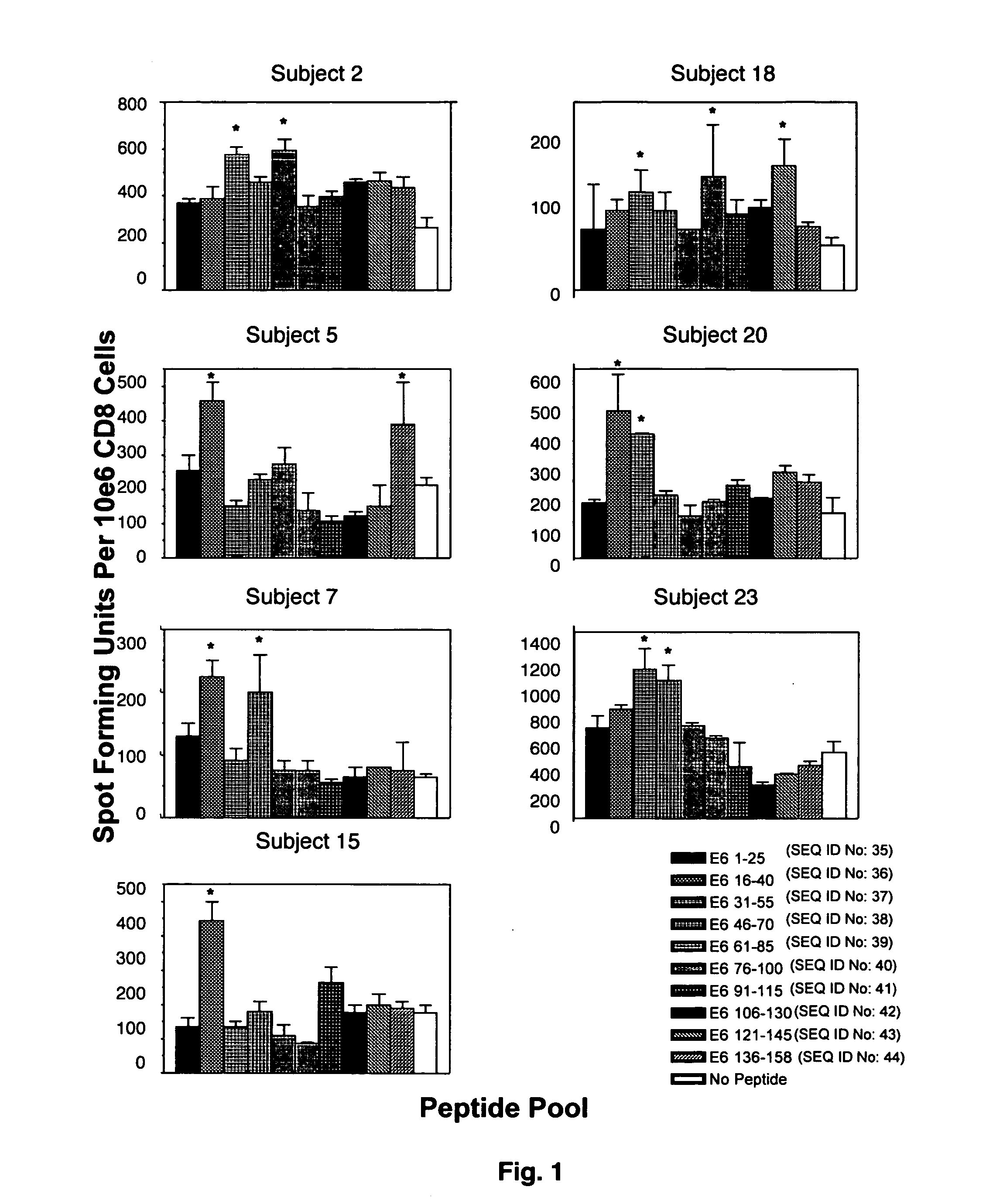
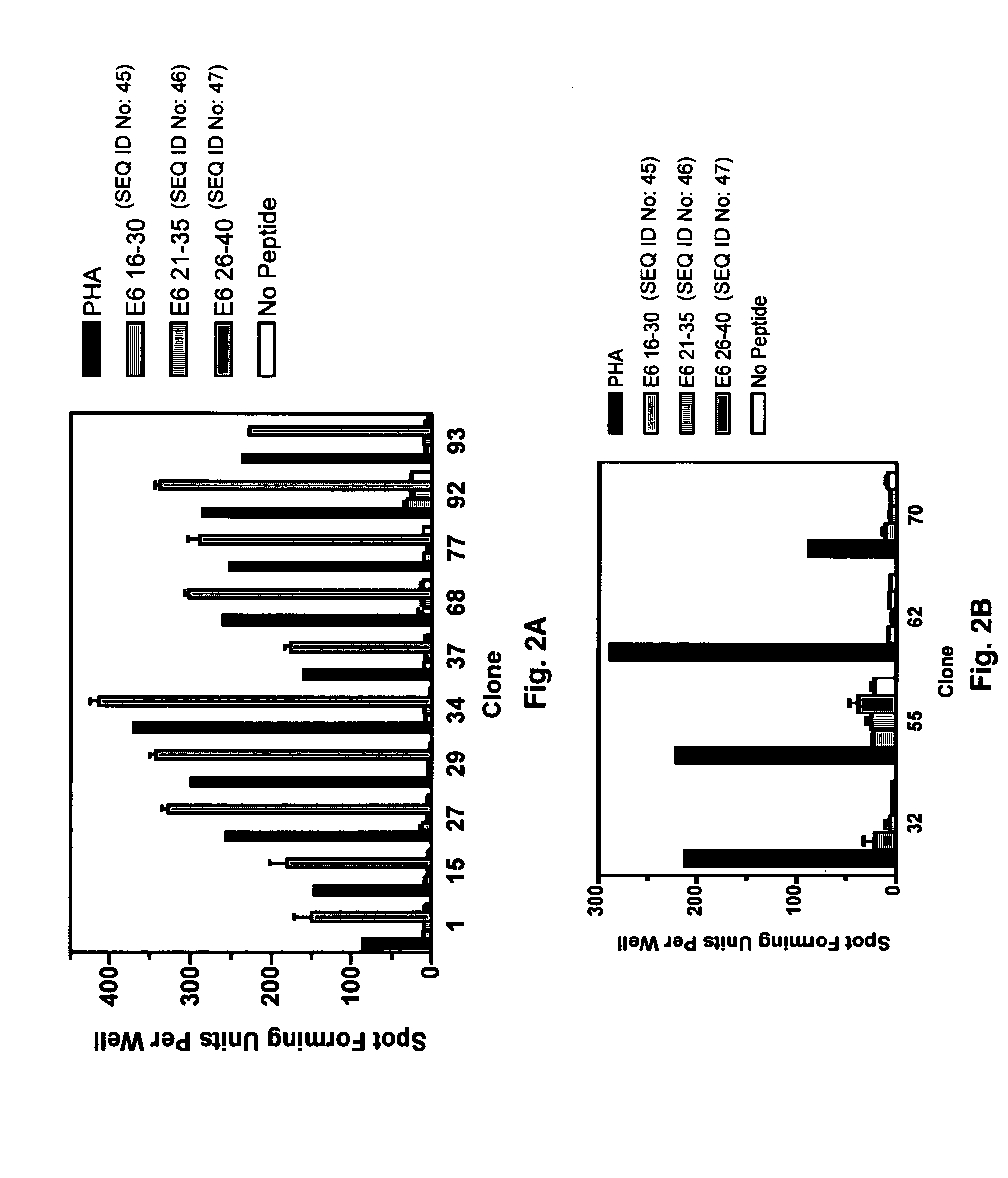
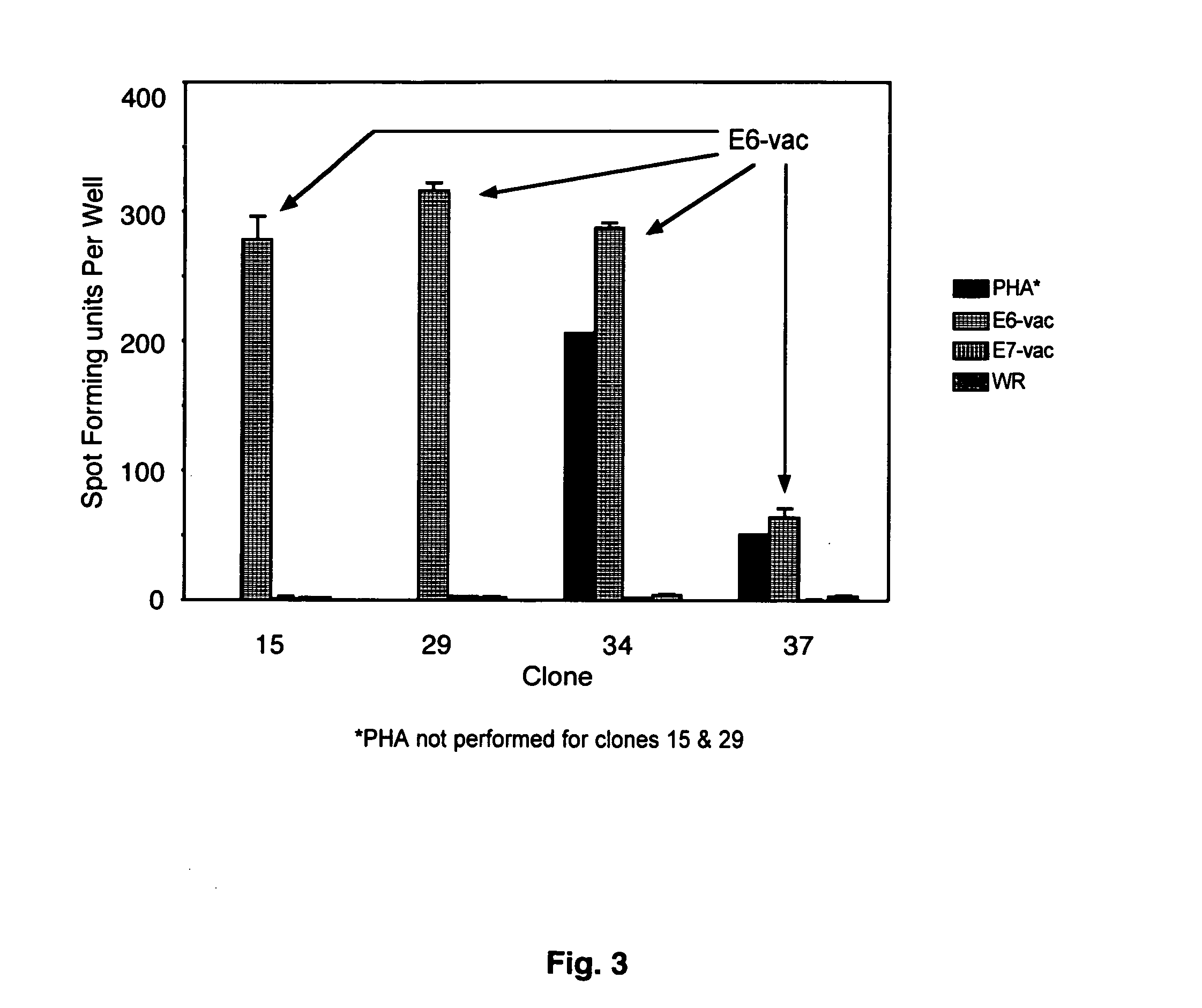
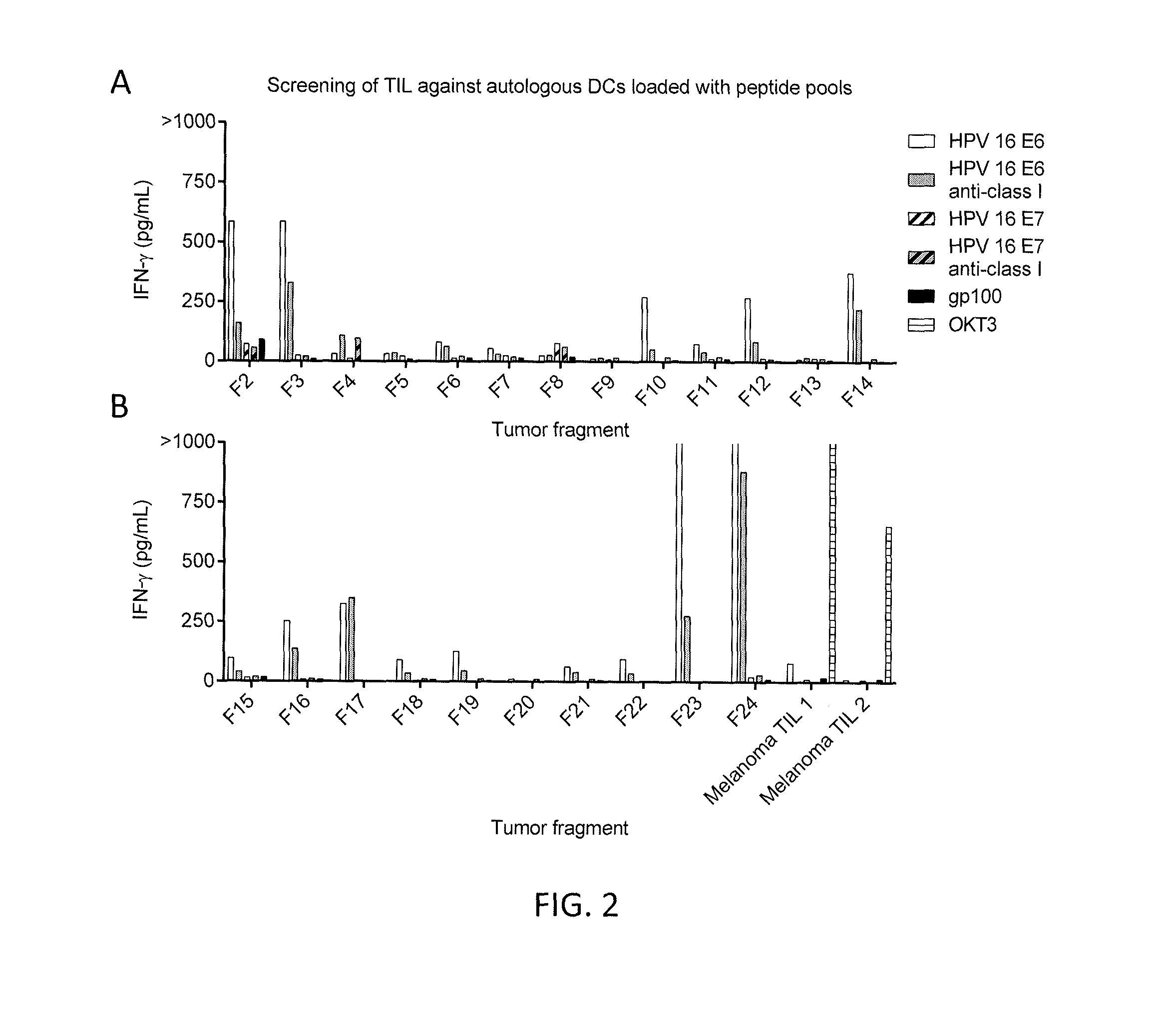
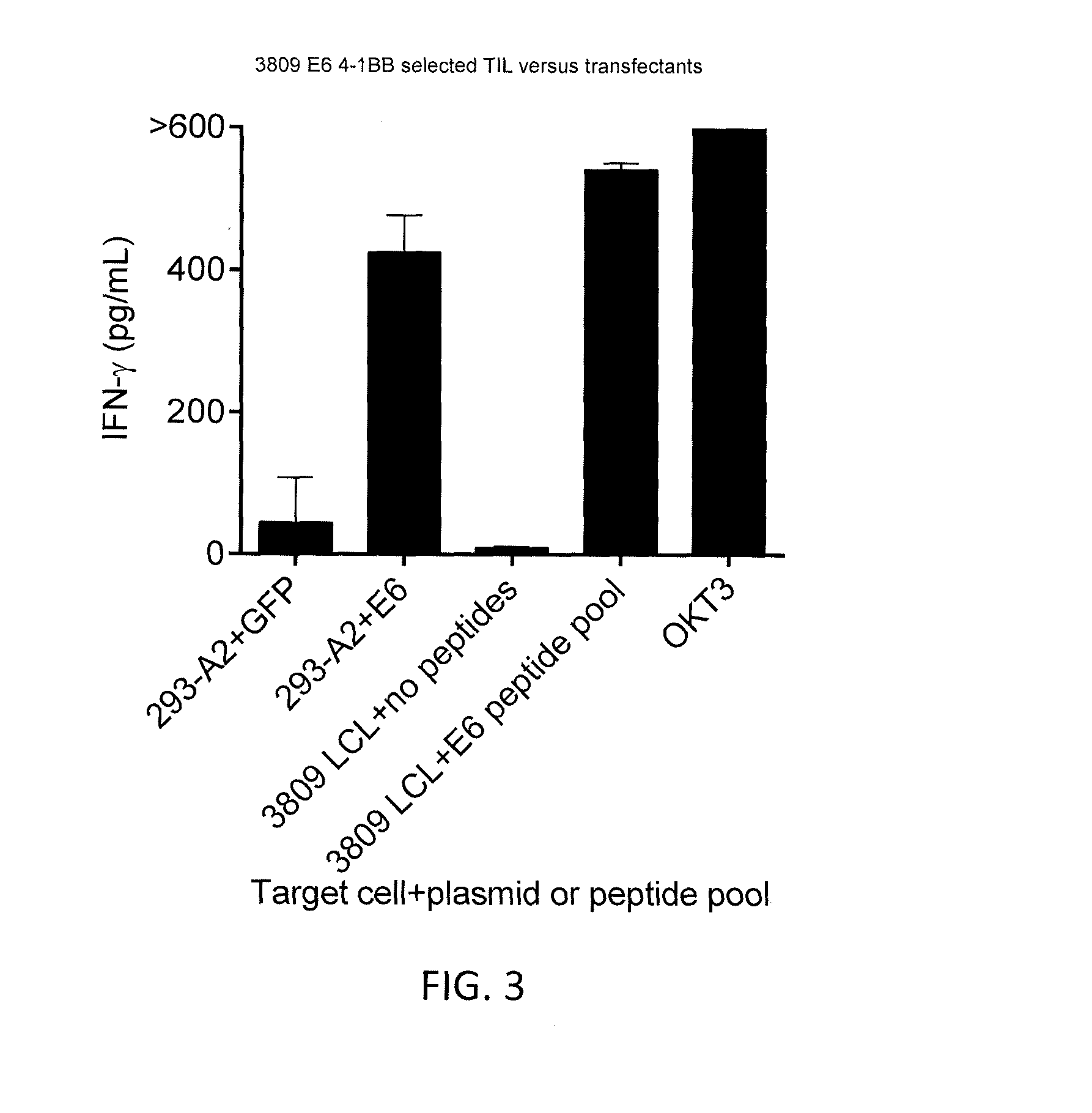
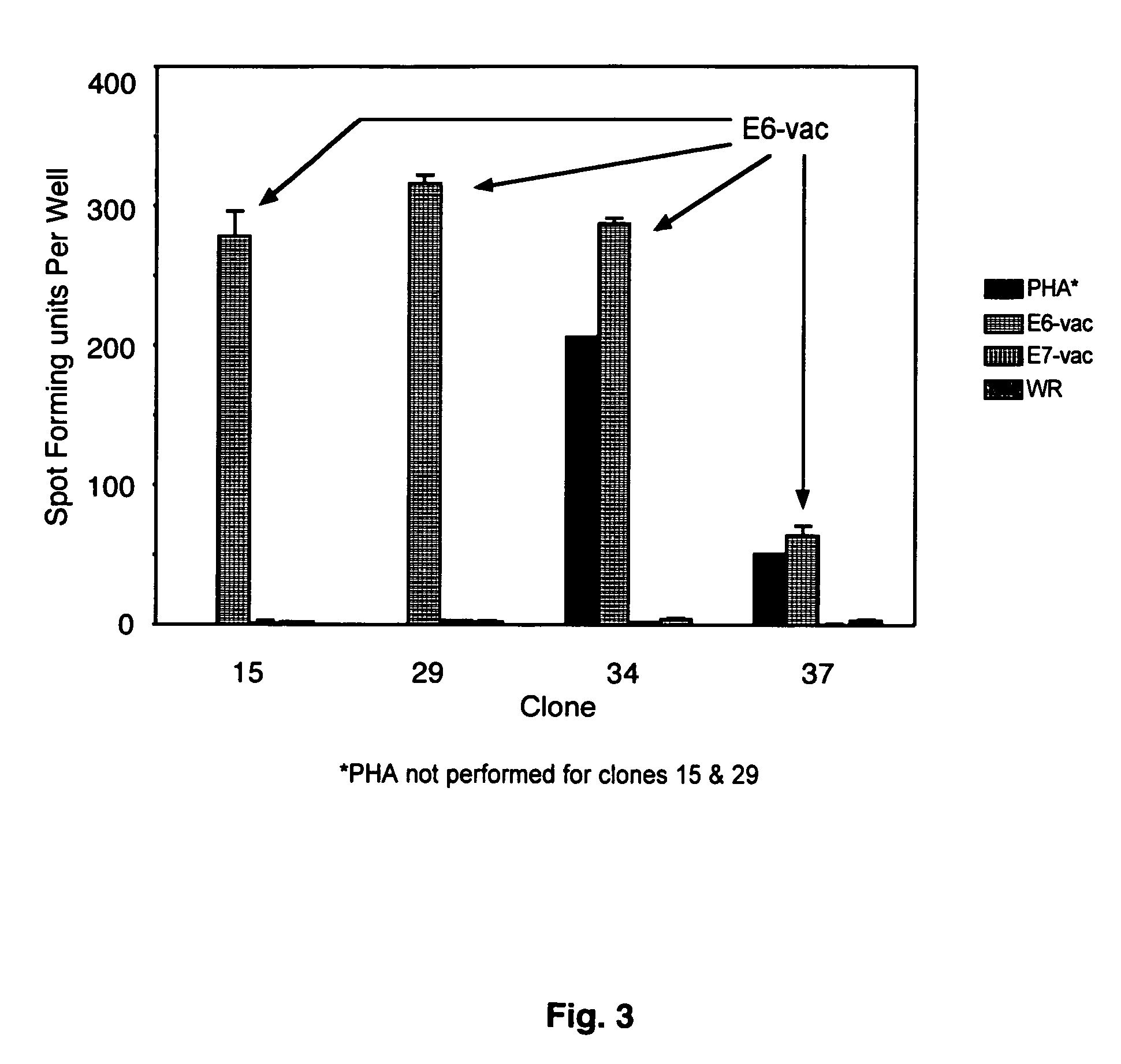
CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof
InactiveUS20060182763A1Peptide/protein ingredientsViral antigen ingredientsHuman papillomavirusDendritic cell
The present invention is directed to the examination of the pattern of immunodominant CD8 T cell epitopes in the E6 and E7 protein of Human Papillomavirus (HPV) and its further characterization in terms of its amino acid sequence and HLA restriction. These epitopes are identified based on their ability to induce strong CD8 T cell response and therefore, are important as sources of antigens for dendritic cell immunotherapy to treat cervical cancer. The present invention contemplates identifying a number of similar epitopes restricted by a wide variety of HLA types so that they can be used in concert to develop a preventative vaccine, which can be used for general population.
Owner:BIOVENTURES LLC
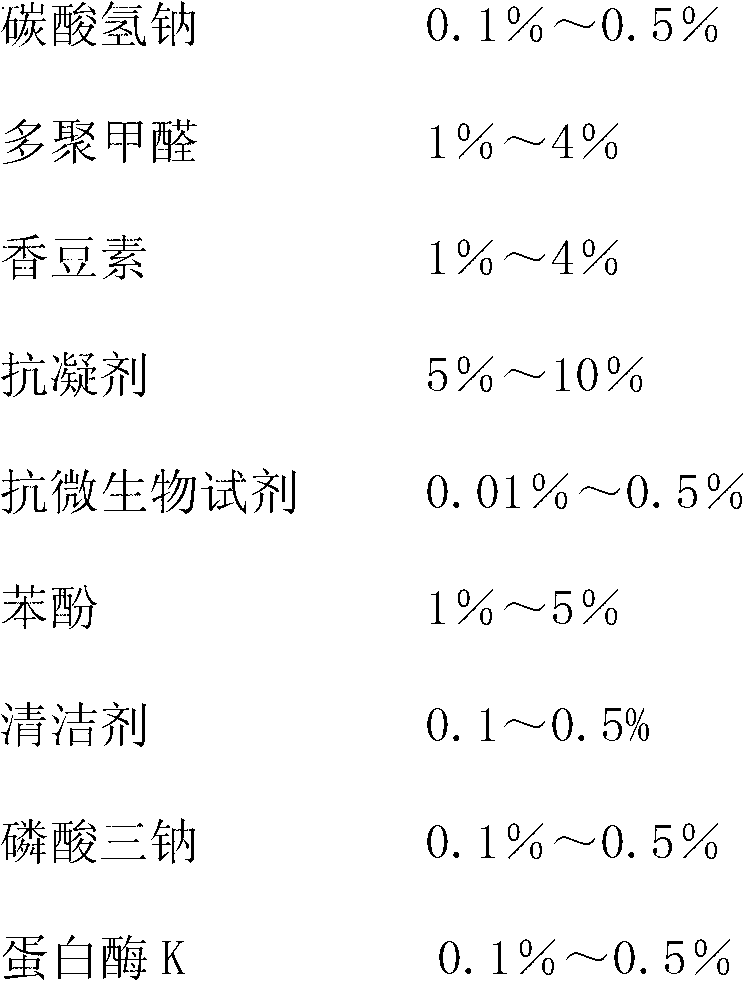
Exfoliative cells preserving fluid
InactiveCN103120153AHigh transparencyClear boundariesDead animal preservationHuman papillomavirusProduction effect
The invention discloses an exfoliative cells preserving fluid which is prepared from a pH buffering agent, an osmotic pressure maintenance agent, preservatives, a fixing agent for maintaining cellular morphology, an anticoagulant, a mucus softener, an antimicrobial reagent, a cleaning agent, a humectant and red blood cell destroying components. The components in the preserving fluid are reasonable in proportioning, and the exfoliative cells can be preserved at a long time under normal temperature, wherein the longest time can reach 2 years; mucus can be sufficiently dissolved and the red cells can be partially destroyed; a film production effect is good, cellular distribution is very even, cellular morphology is perfect, cytoplasm and cell nucleus demarcation is distinct, gradation is clear, and cytoplasm and cell nucleus transparency is very good, and the exfoliative cells preserving fluid can be simultaneously used for the HPV-DNA (human papillomavirus-deoxyribonucleic acid), Chlamydia and immunohistochemical test; the special liquid base preserving fluid used for the cytologic examination of other parts can be provided, an imported product can be completely replaced, and the cellular constituent with diagnostic significance can be sufficiently preserved; and the exfoliative cells preserving fluid is low in configuration cost and easy to popularize.
Owner:刘召宏
Human papillomavirus inhibitors
The present invention provides systems for identifying anti-viral agents. In particular, the invention encompasses reagents and strategies for identifying agents that inhibit or disrupt key protein-protein interactions that are important in the life cycle of papillomaviruses. The invention allows identification, production, and / or use of agents that reduce or inhibit the replication of HPV by inhibiting (e.g., precluding, reversing, or disrupting) the formation of the E1-E2 protein-protein complex. The invention also provides specific inhibitory agents, pharmaceutical compositions, and methods of using these inhibitors and pharmaceutical compositions for inhibiting viral replication in vitro. Methods are also described for the treatment and prevention of HPV infections and HPV-related diseases in patients.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Compositions, methods and products comprising human papillomavirus for detecting and treating a cancer
InactiveUS20060029943A1Microbiological testing/measurementBiological material analysisHuman papillomavirusAlphapapillomavirus
Owner:HERMONAT PAUL +2
Tissue specific promoters and transgenic mouse for the screening of pharmaceuticals
InactiveUS6313373B1Restores promoter activityVectorsSugar derivativesHuman papillomavirusPromoter activity
The present invention provides human involucrin (hINV) sequences having tissue specific and cell type specific promoter activity. The sequences provided herein direct expression to suprabasal cells of stratifying epithelia. The invention further provides methods for the production of transgenic animals which contain a hINV promoter sequence which directs the expression of human papillomavirus 16 oncogenes (or other oncogenes). These animals display cervical and epidermal hyperplasias as well as cancer of the trachea, esophagus, colon, epidermis, anus / rectum, lymph nodes, spleen and lung. The animals of the invention provide a useful model for screening potential anti-neoplastic compounds, carcinogens, and co-carcinogens for a number of cancers.
Owner:CASE WESTERN RESERVE UNIV
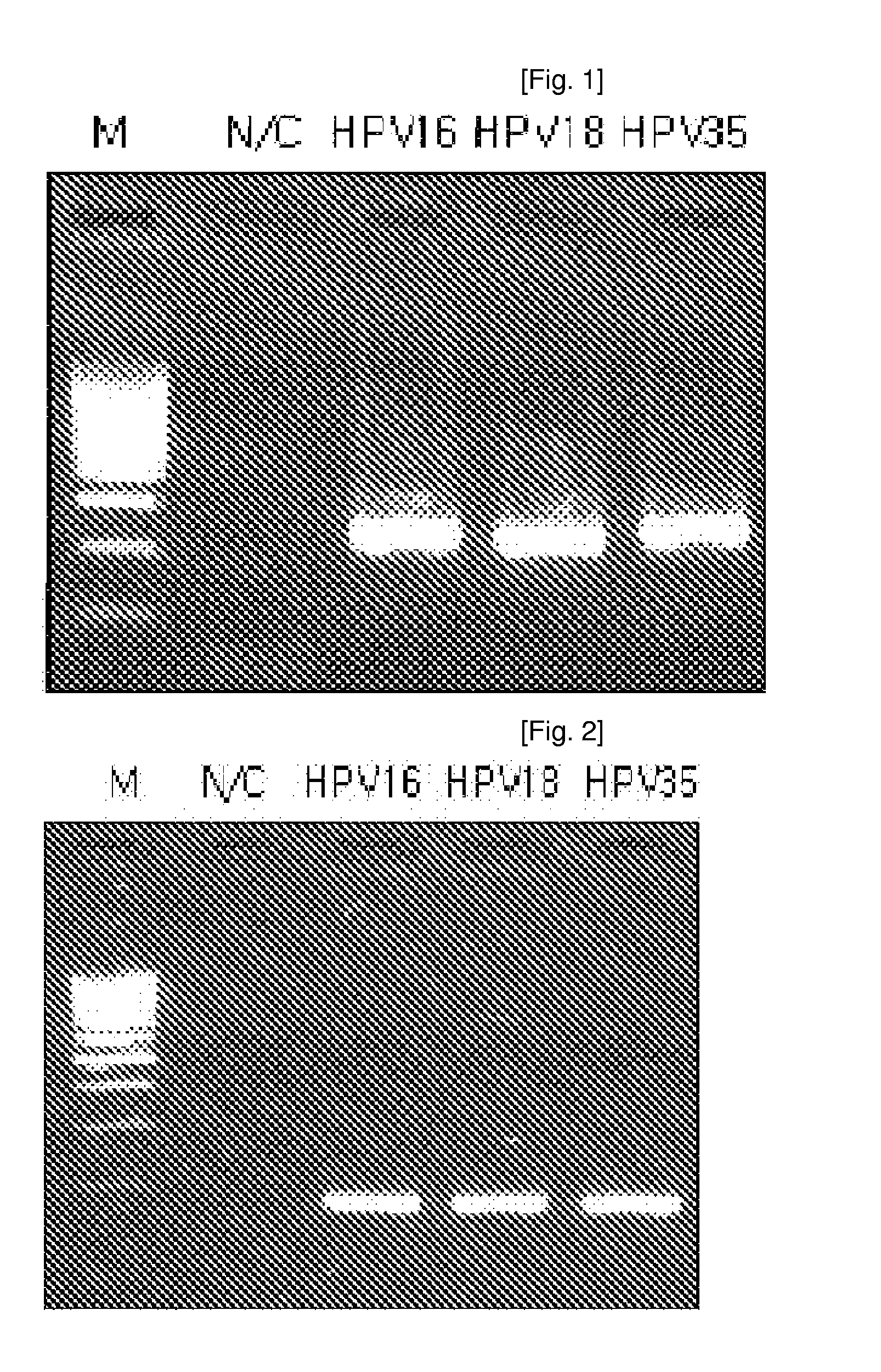
Polymerase chain reaction (PCR) method for diagnosing human papillomavirus (HPV) and reagent kit thereof
InactiveCN101017141AEfficient, systematic, economical and simpleShorten the timeMicrobiological testing/measurementChemiluminescene/bioluminescenceHuman bodyHuman papillomavirus
This invention relates to one polymer enzyme linkage reaction fluorescence test method to dialogue dangerous human body nipple shape virus and to isolate DNA sample HPV gene type to test one set of dangerous HPV infection from patient by the method, wherein, it belongs to life science and biological technique. This invention agent case comprises one fluorescence meter PCR technique as base of multi-layer polymer enzyme reaction composed of multiple HPV positive lead object, reaction lead object and fluorescence detector.
Owner:GENETEL PHARMA SHENZHEN
Synthetic human papillomavirus genes
InactiveUS7001995B1Easy to insertEasy to removeBiocideGenetic material ingredientsPolynucleotide VaccinesHuman papillomavirus
Synthetic DNA molecules encoding papillomavirus proteins are provided. The codons of the synthetic molecules are codons preferred by the projected host cell. The synthetic molecules may be used as a polynucleotide vaccine which provides effective immunoprophylaxis against papillomavirus infection through stimulation of neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME CORP
Methods of diagnosing cervical cancer
The invention provides reagents and methods for detecting pathogen infections in human samples. This detection utilizes specific proteins to detect the presence of pathogen proteins or abnormal expression of human proteins resulting from pathogen infections. Specific methods, compositions and kits are disclosed herein for the detection of oncogenic Human papillomavirus E6 proteins in clinical samples.
Owner:ARBOR VITA CORP
Protein delivery system using human papillomavirus virus-like particles
Human Papillomavirus virus like particles (VLPs) have been constructed so that they contain a modified L2 protein. The L2 protein has been minimized and is fused to a second protein or peptide. The fused protein is incorporated into the VLP and the VLP can deliver the protein to a cell. The modified VLPs can be used to increase the breadth of immune response in vaccine preparations or to deliver other proteins of interest.
Owner:MERCK SHARP & DOHME CORP
Detection and identification of human papillomavirus by PCR and type-specific reverse hybridization
InactiveUS20030165821A1Rapid and reliable for detectionMicrobiological testing/measurementFermentationHuman papillomavirusType specific
A method for detection and / or identification of HPV present in a biological sample comprising amplification of HPV polynucleic acids and of hybridization of said amplified polynucleic acids to a number of probes whereby a short fragment of the L1 gene of HPV is amplified after which, the amplimers are contacted with probes that specifically hybridize to the said short fragment of the L1 gene of at least one HPV type and a diagnostic kit to perform said method and primers and probes used in the said method.
Owner:INNOGENETICS NV
Method for detecting and typing 26 human papillomaviruses
InactiveCN1814796AImprove detection efficiencyShorten detection timeMicrobiological testing/measurementFluorescence/phosphorescenceHuman papillomavirusType specific
This invention relates to a suspension chip technology for the gene test to human papillomavirus used in testing and typing 26 kinds of papillomaviruses, in which, specific probes of which are crosslinked on 26 kinds of fluorescent microspheres to be reacted with the tested specimens then reacted with the report molecules labeled by the fluorescein to test the type specific nucleic acid of the virus by a fluorescent test device, which can test 26 kinds of ordinary HPV once and increases the test efficiency greatly and overcomes the shortcoming of missing testing the potential infections in the serology and immunity test method to realize early diagnosis to HPA diseases.
Owner:ZHEJIANG UNIV
Multiple-input Analytical System
ActiveUS20110159578A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHuman papillomavirusComputer science
The present disclosure provides an automated sample processing system that can receive samples in different first and second formats and process both sample formats. The disclosure also provides a human papillomavirus testing apparatus. The apparatus has a first input to receive first test specimens in the form of pre-processed cervical samples, and a second input to receive unprocessed cervical samples. A first subsystem prepares second test specimens from the unprocessed cervical samples, and a second subsystem selectively processes and tests first specimens, second test specimens, or first and second test specimens to determine the presence of one or more human papillomavirus indicators.
Owner:BECTON DICKINSON & CO
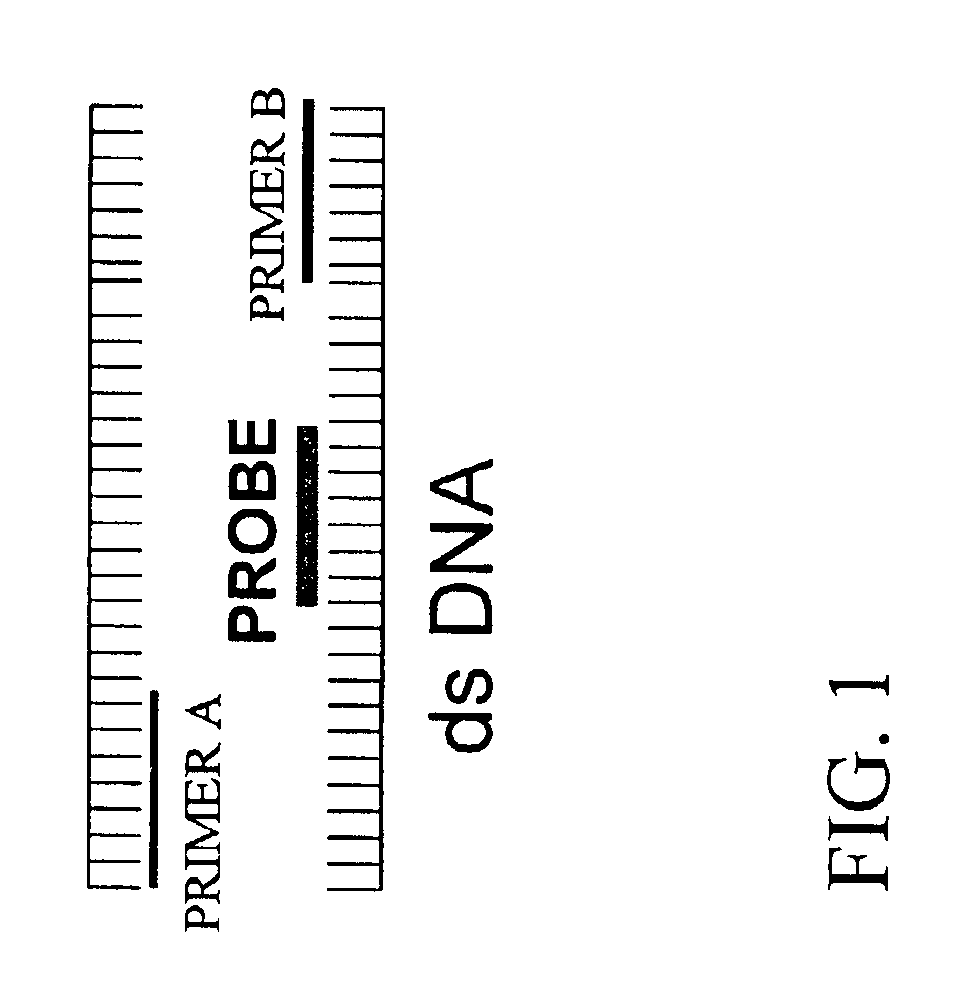
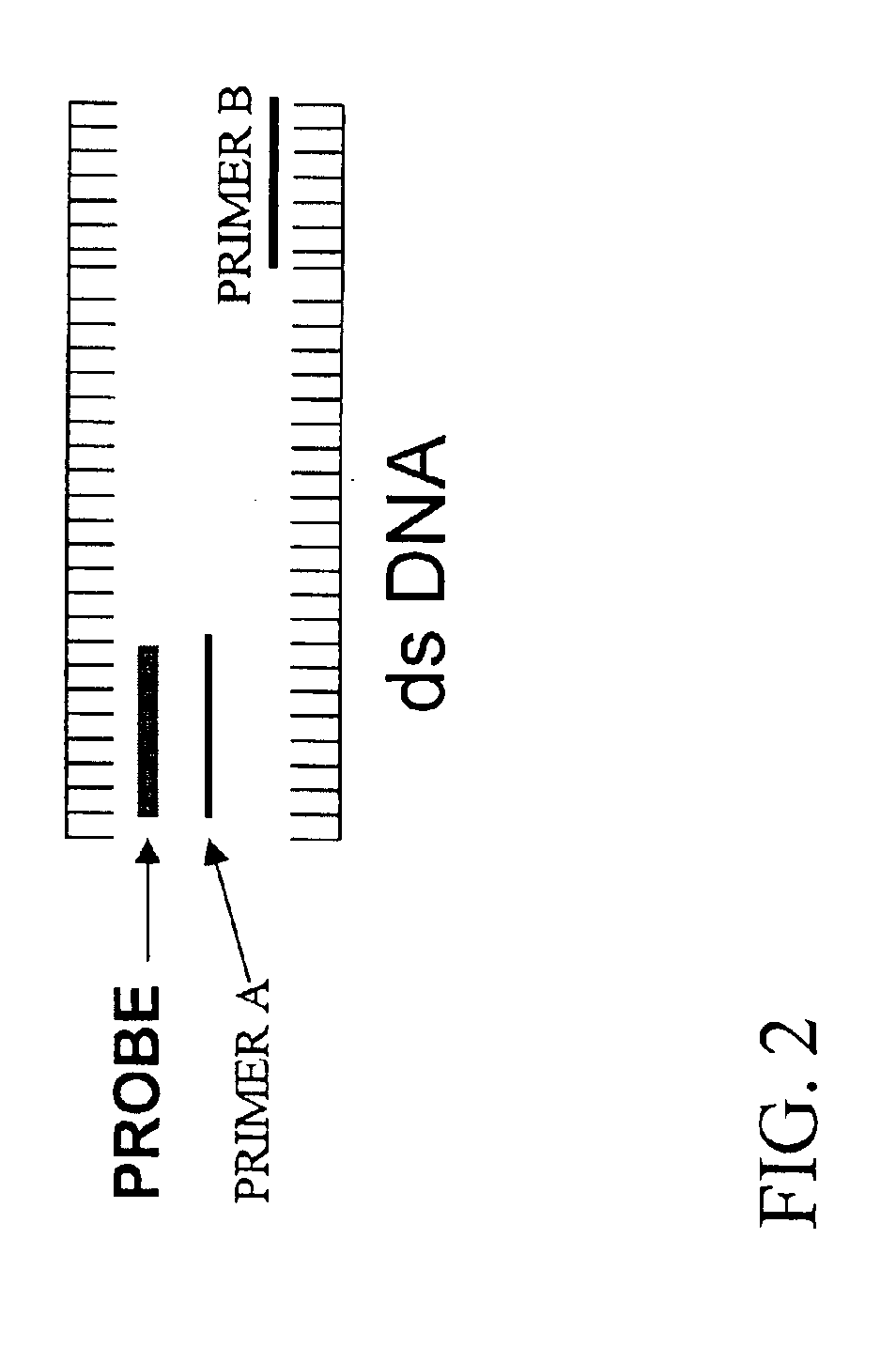
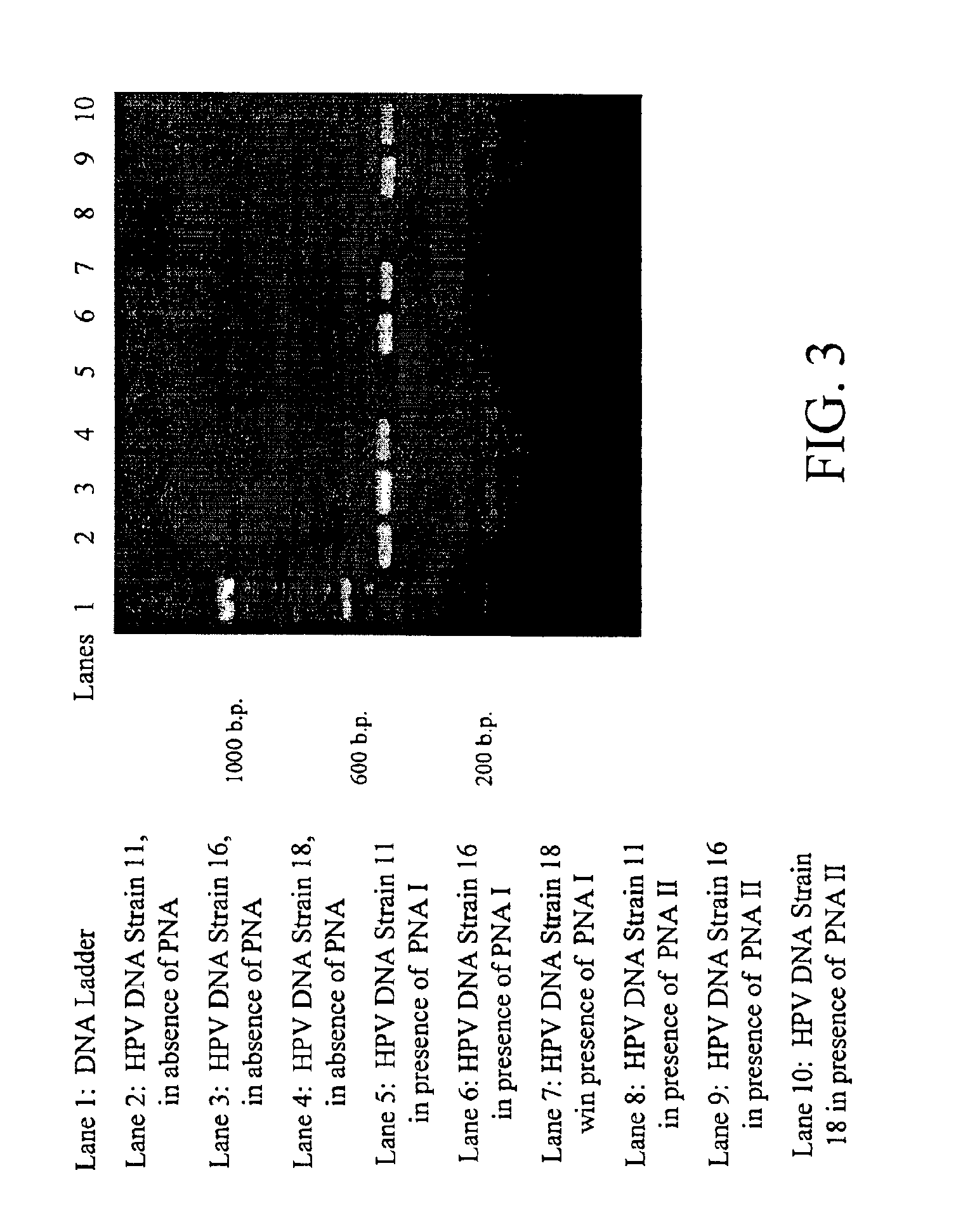
Detection and typing of human papillomavirus using PNA probes
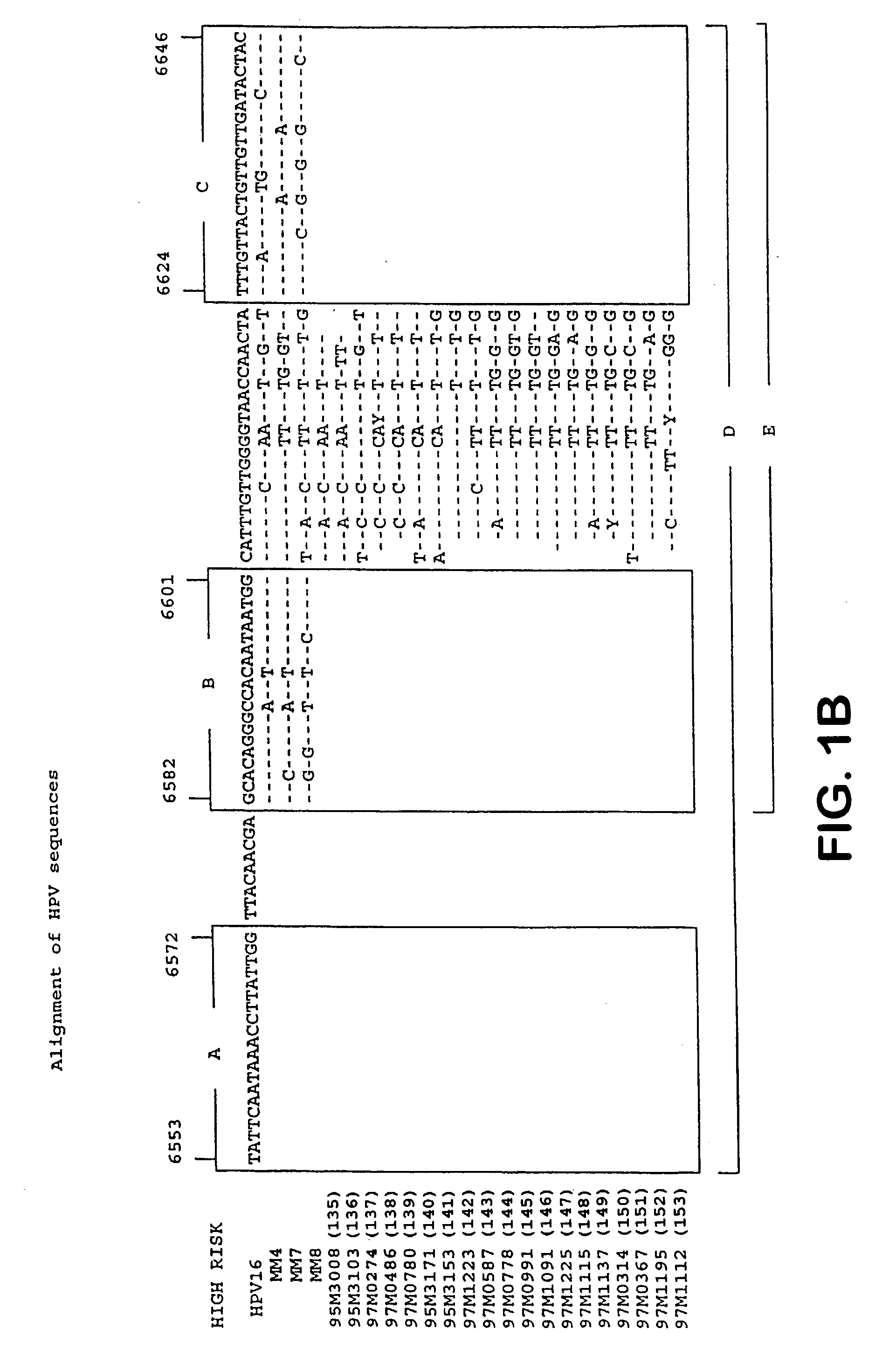
InactiveUS6936443B2Quantity minimizationSugar derivativesMicrobiological testing/measurementHuman papillomavirusTyping
The invention provides materials and methods for detection and typing of HPV infection using PNA probes. More specifically, methods are provided for detecting high-risk types of HPV infection with minimal numbers of PNA probes or using PNA probes to selectively amplify only high-risk types of HPV. Novel primer sequences are also provided.
Owner:CYTYC CORP
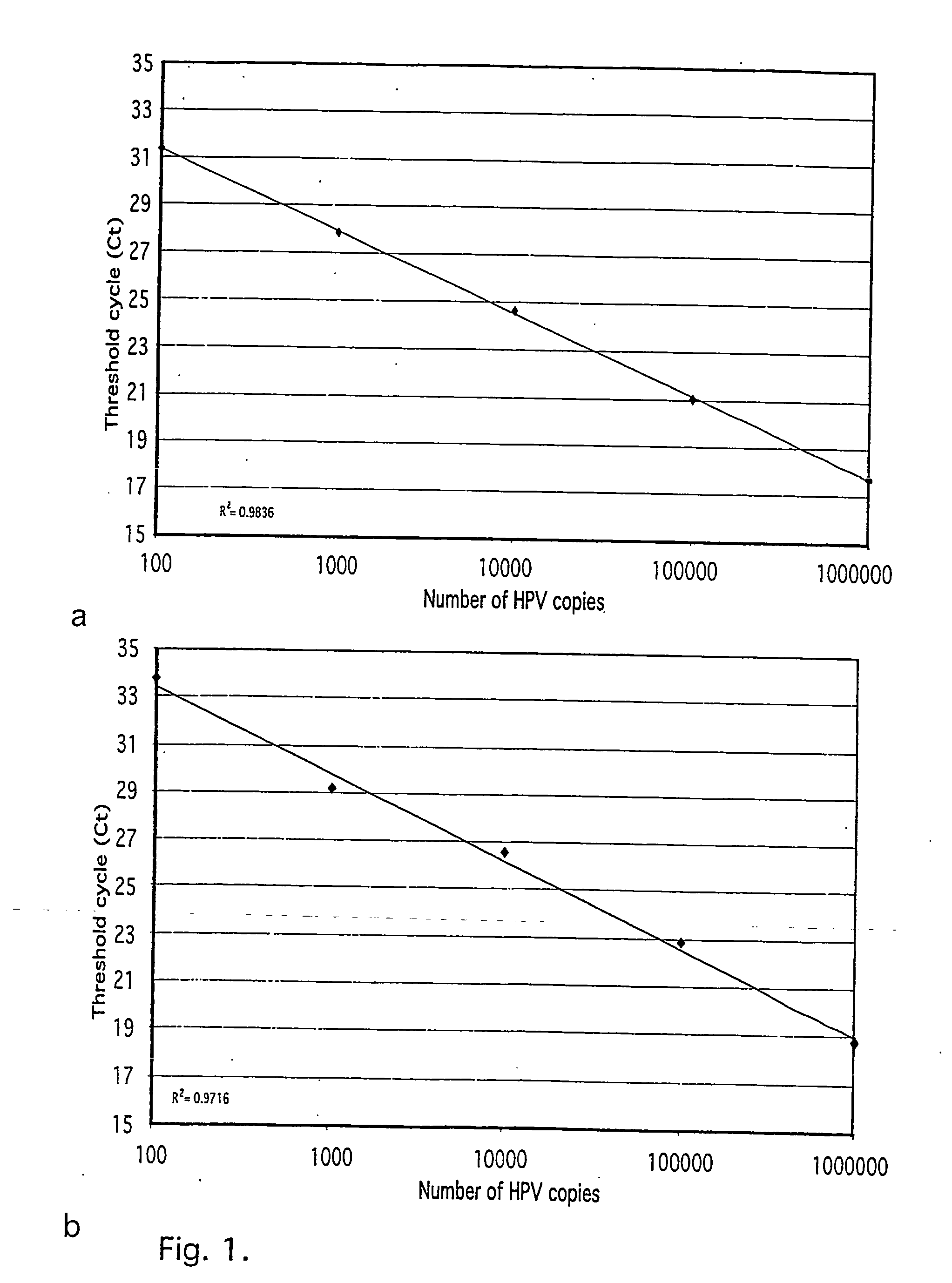
Method and kit for quantitative and qualitative determination of human papillomavirus
ActiveUS20070037137A1Minimize the numberRoutinely usedSugar derivativesMicrobiological testing/measurementHuman papillomavirusBiology
The present invention relates to a method and kit for quantitative and qualitative determination of human papillomavirus, HPV, in a sample. More precisely, for quantitative and qualitative determination of oncogenic HPV to predict the risk of HPV infection resulting in cervical carcinoma. The method and kit enable simultaneous measurement of several oncogenic HPV types.
Owner:CEPHEID INC
Polypeptides useful as immunotherapeutic agents and methods of polypeptide preparation
InactiveUS6123948AGood effectStimulate immune responseAntibody mimetics/scaffoldsVirus peptidesAdjuvantHuman papillomavirus
Fusion polypeptides and aggregates of polypeptides comprising papillomavirus-derived antigens, and compositions thereof and their use e.g. with adjuvants for immunogenic and vaccine purposes in eliciting e.g. HPV-specific immune responses. The polypeptides can be purified to result in aggregates which when in solution or dispersion can pass through a sterilisation filter, and in amorphous aggregates. An example of such a polypeptide is a fusion protein of human papillomavirus proteins L2 and E7.
Owner:CANTAB PHARMA RES
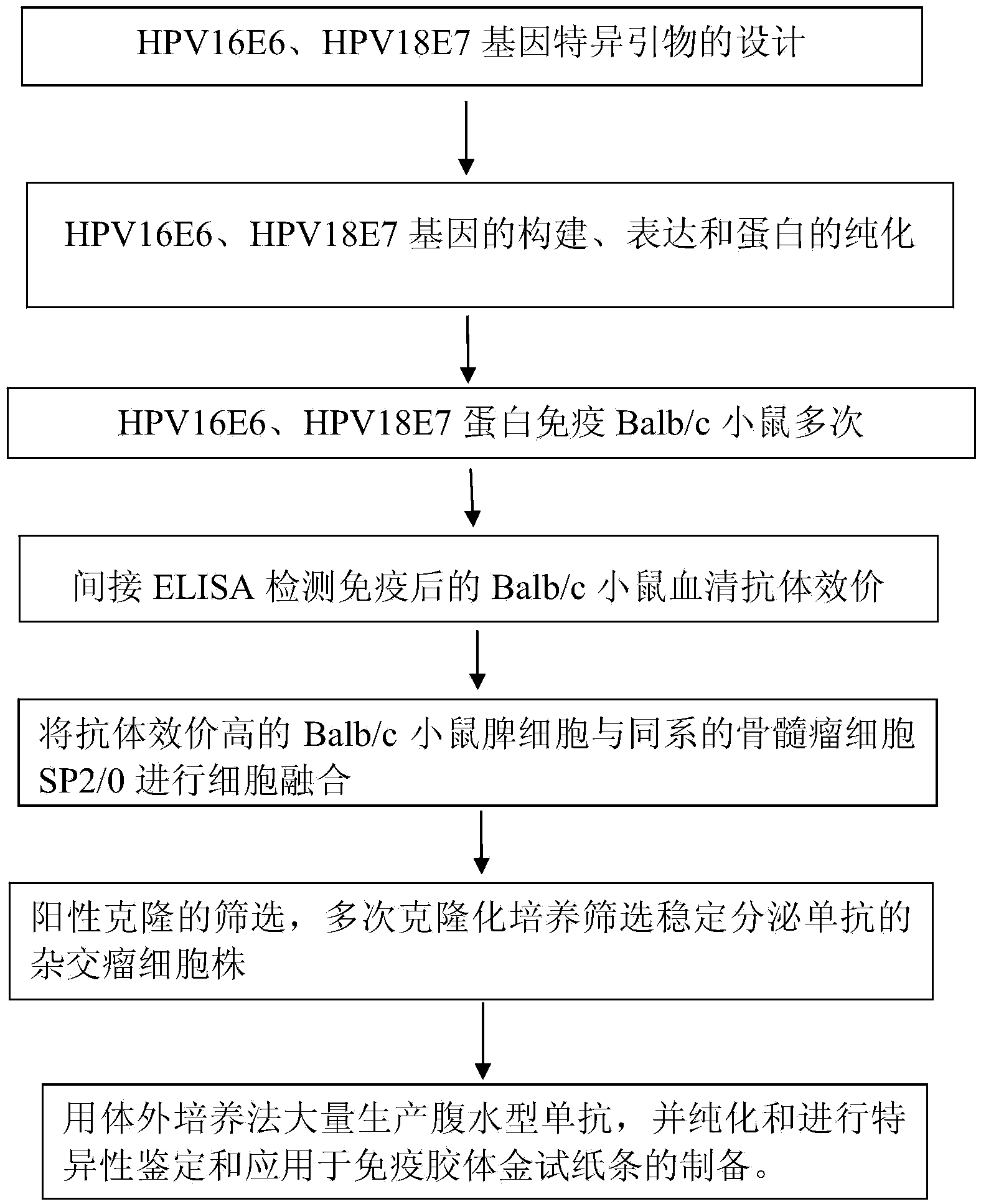
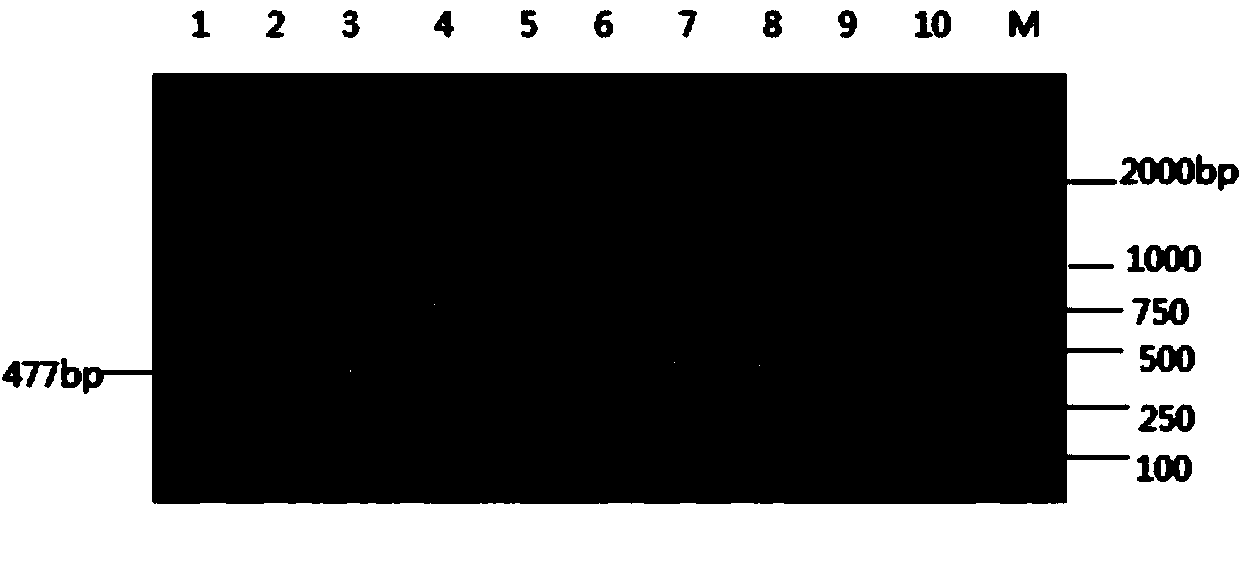
Monoclonal antibodies for resisting high-risk human papillomavirus proteins and application of monoclonal antibodies
InactiveCN103865883AStrong specificityImprove bindingImmunoglobulins against virusesMicroorganism based processesHuman papillomavirusLatex particle
The invention relates to monoclonal antibodies for resisting high-risk human papillomavirus proteins HPV16E6 and HPV18E7, a hybridoma cell strain secreting the monoclonal antibodies and the application of the monoclonal antibodies. The monoclonal antibodies can be used for specifically detecting the proteins HPV16E6 and HPV18E7. The two antibodies can be prepared into immunochromatographic test strips for rapidly detecting the proteins HPV16E6 and HPV18E7 by virtue of labeled colloidal gold or color latex particles. The monoclonal antibodies for detecting the proteins HPV16E6 and HPV18E7 have the characteristics of high speed (results can be obtained within 10 minutes), simplicity, specificity, sensitivity, low cost and easiness in popularization.
Owner:CHONGQING UNIV OF TECH
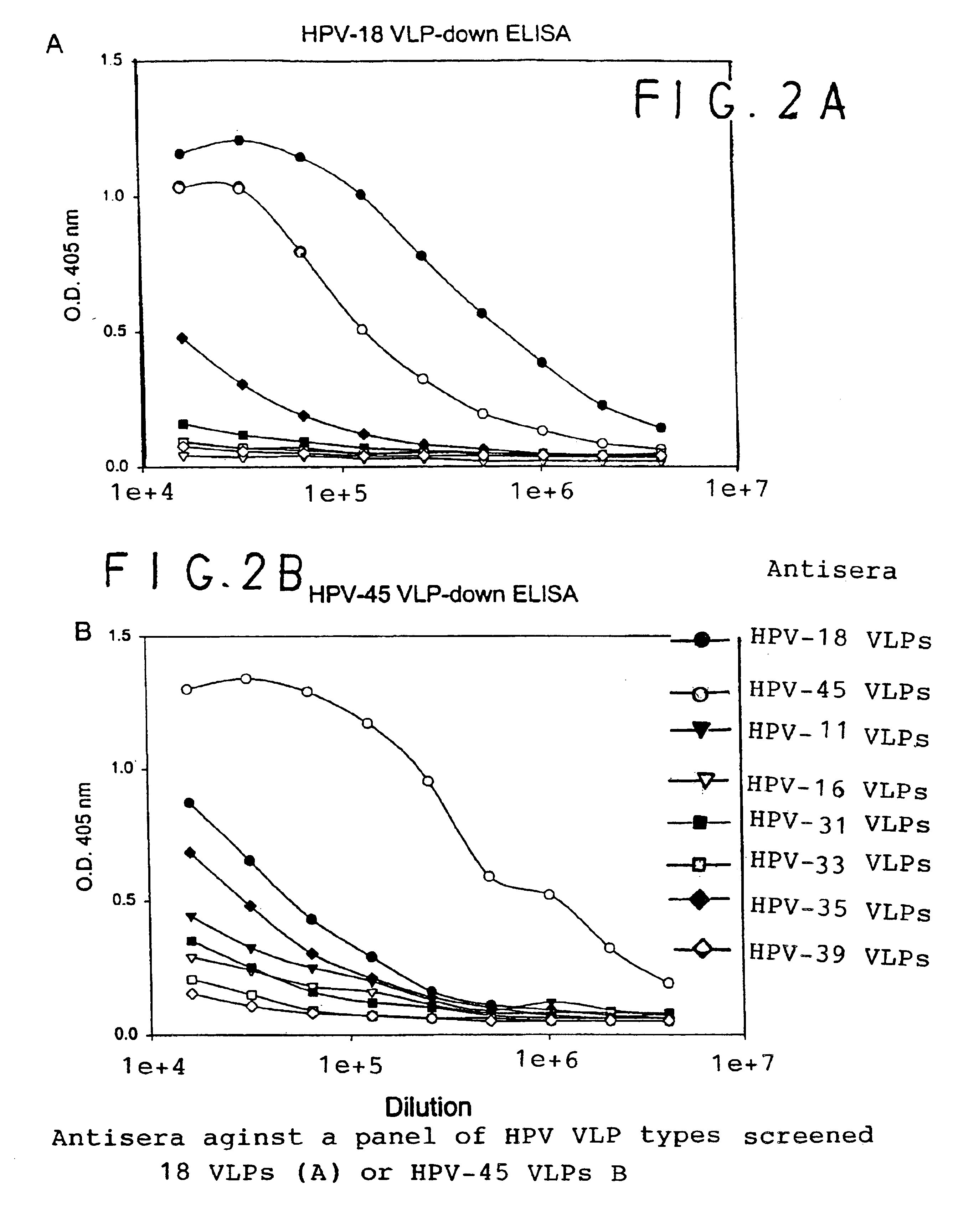
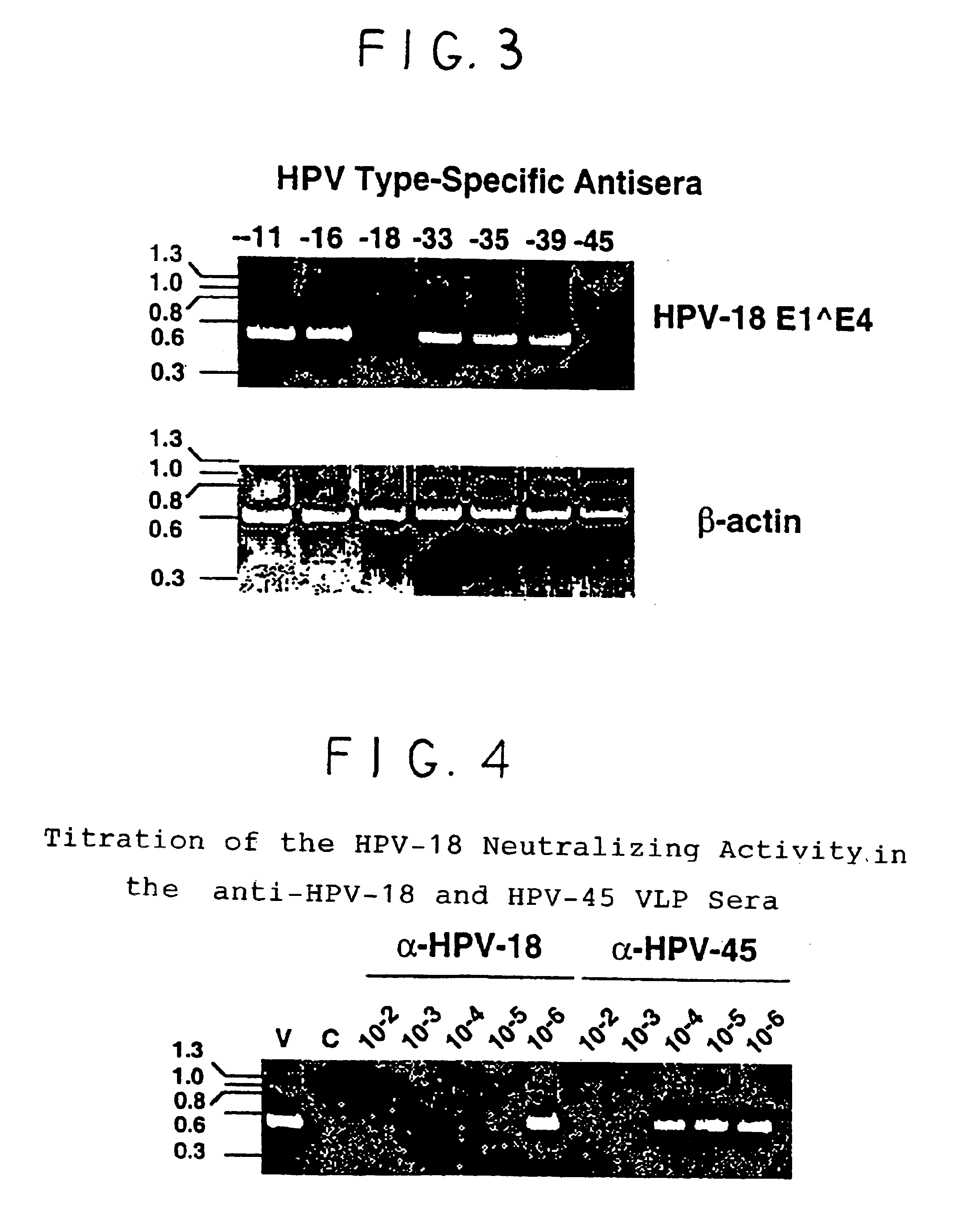
Chimeric human papillomavirus (HPV) L1 molecules and uses therefor
InactiveUS6908613B2Need can be quite largeLevel of protectionPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman papillomavirusVirus-like particle
Owner:MEDIMMUNE LLC
Method for detecting human papillomavirus mRNA
An in vitro method is provided for screening human female subjects to assess their risk of developing cervical carcinoma which comprises screening the subject for expression of mRNA transcripts from the E6 and optionally the L1 gene of human papillomavirus, wherein subjects positive for expression of L1 and / or E6 mRNA are scored as being at risk of developing cervical carcinoma. Kits for carrying out such methods are also provided.
Owner:NORCHIP AS
Self-assembling recombinant papillomavirus capsid proteins
Recombinant papillomavirus capsid proteins that are capable of self assembly into capsomer structures and viral capsids that comprise conformational antigenic epitopes are provided. The capsomer structures and viral capsids, consisting of the capsid proteins that are expression products of a bovine, monkey or human papillomavirus L1 conformational coding sequence proteins, can be prepared as vaccines to induce a high titer neutralizing antibody response in vertebrate animals. The self assembling capsid proteins can also be used as elements of diagnostic immunoassay procedures for papillomavirus infection.
Owner:DEUTES KREBSFORSCHUNGSZENT THE GERMAN CANCER RES CENT
Detection and parting method of human papillomavirus and reagent box
ActiveCN1948503AHigh sensitivityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationHuman papillomavirusInfection rate
A testing and typing method and its reagent boxes for Human Papillomavirus(HPV), relating to exciters and nucleic acid probes which are especially for testing and typing of HPV. The invention fixes three types of special probes including different subgroups of low-hazard HPV and high-hazard HPV, subgroups with extra-low infection rate of low-hazard or high-hazard HPV, onto the same position or mediator. The invention also fixes oligomeric nucleoside probes of special HPV onto microballoons based on Luminex xMAP technical platform, which forms microballoons probes for testing HPV subgroups and typing slugs of HPV. A testing and typing method with simplicity of operator and its reagent boxes are invented. The method has much strongpoint including high-sensitivity, high-flux, stable result and good repeatability.
Owner:SHANGHAI TELLGEN LIFE SCI CO LTD
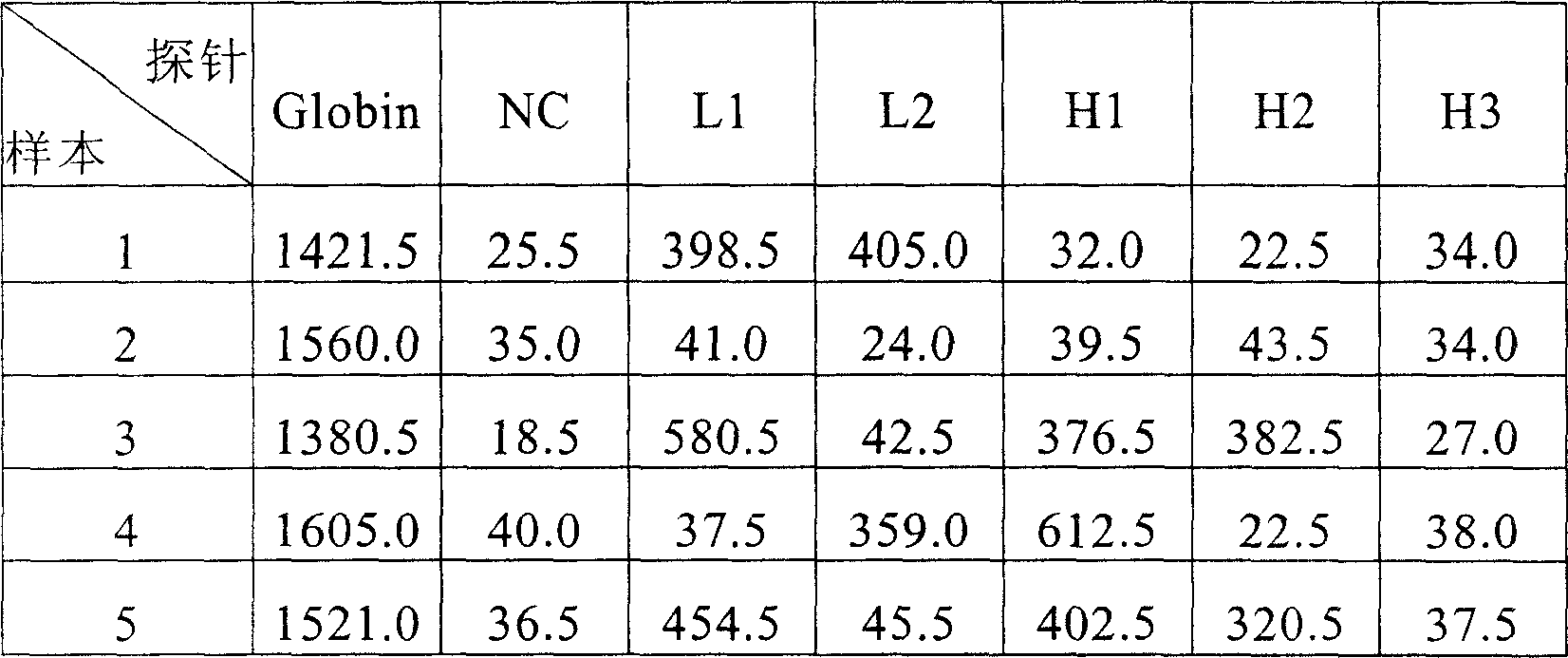
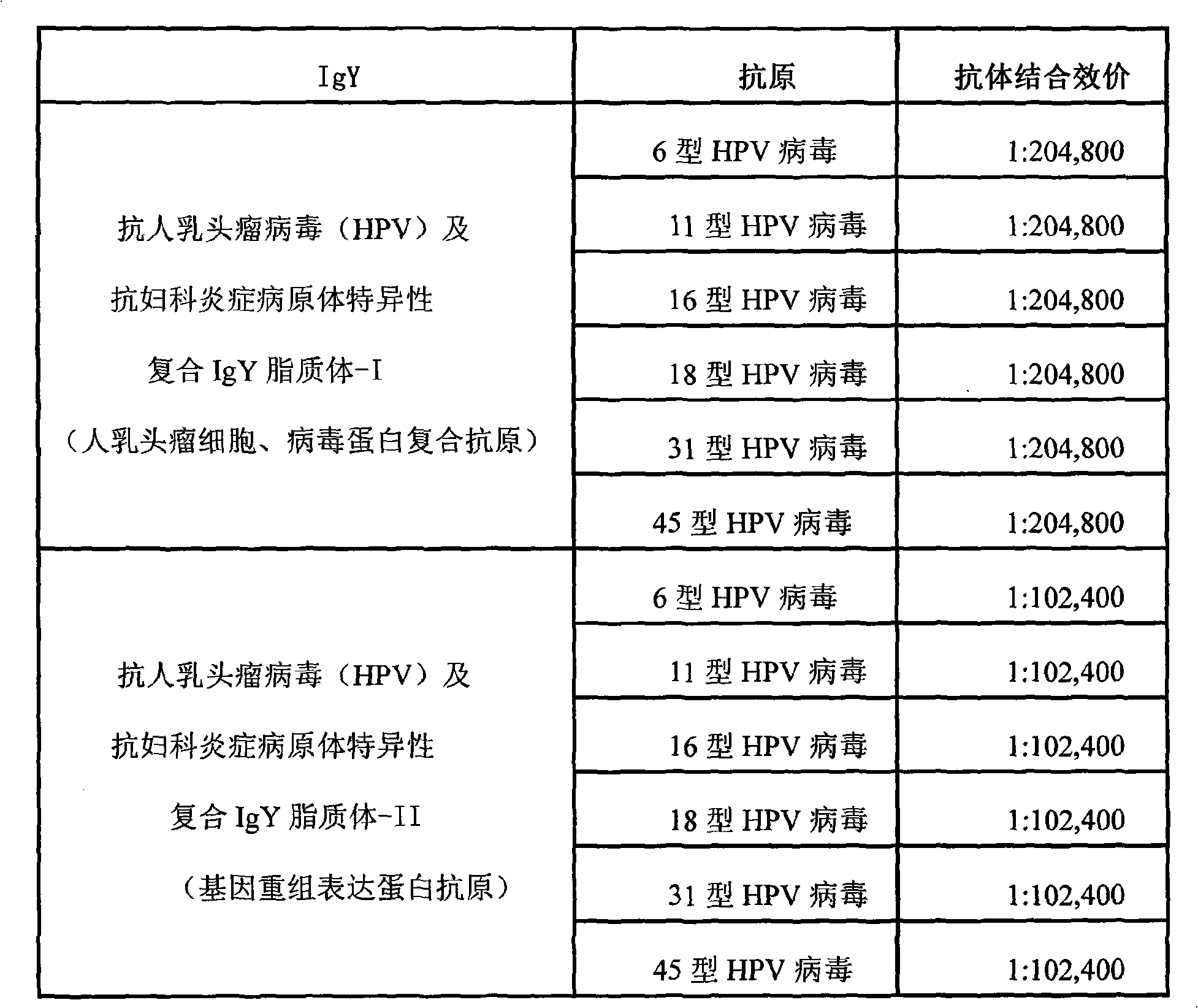
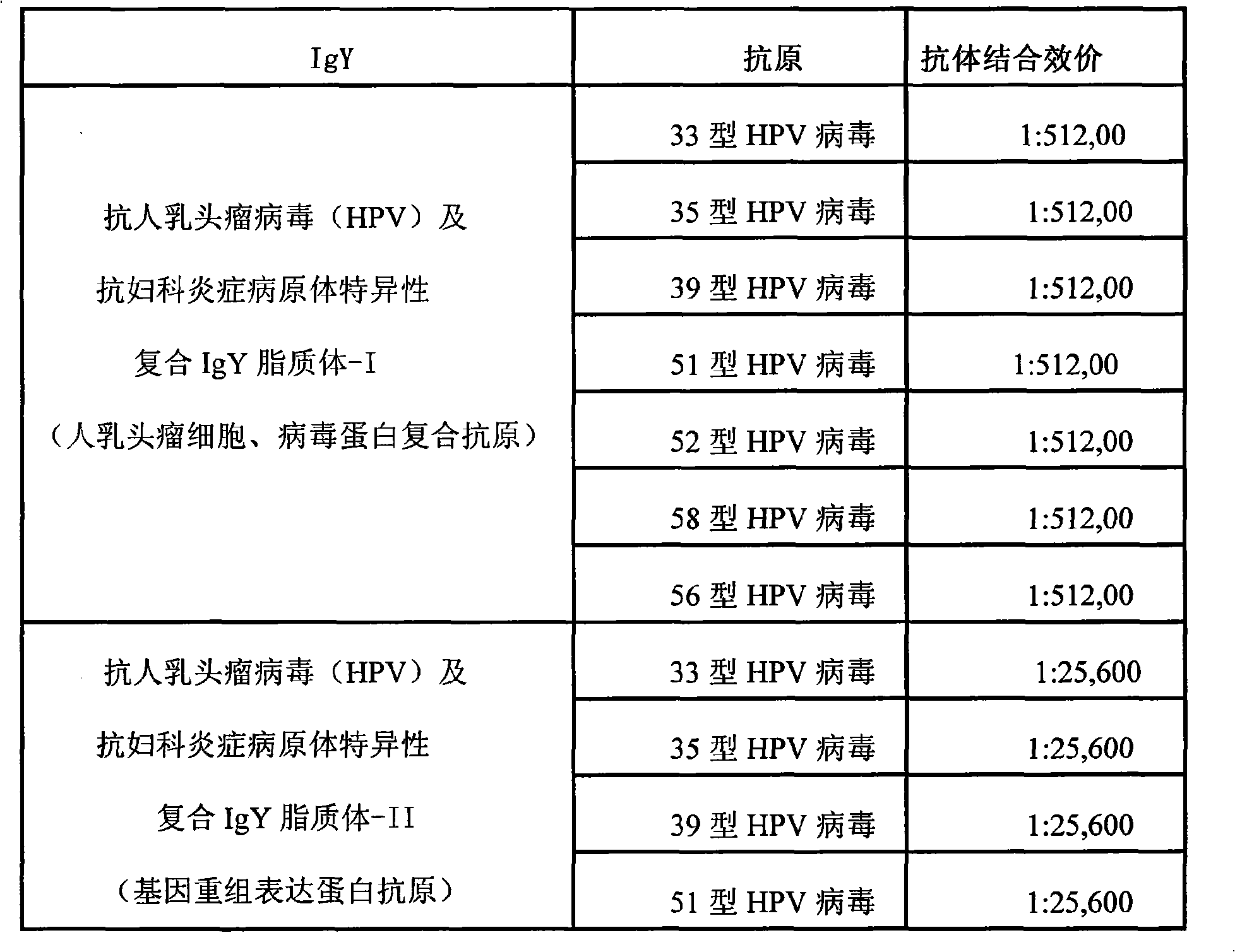
Nano liposome anti-HPV, gynecological inflammation pathogen specific compound IgY and combined preparation thereof
ActiveCN101328219AStrong targetingImprove permeabilityAntibacterial agentsEgg immunoglobulinsBacterial virusHuman papillomavirus
The invention relates to nanoliposome human papillomavirus (HPV) resistant and gynecological inflammation pathogen resistant specific complex IgY and a combined preparation for the same. Laying poultry is preferred to respectively prepare laying poultry for immunity of tumor somatic cells, viral protein complex antigens, gene recombination protein antigens and bacterial virus complex antigens; and human papillomavirus resistant special IgY and gynecological inflammation pathogen resistant specific IgY undergo affinity chromatography and purification and are converted into nanoliposomes, and then the nanoliposome special complex IgY is prepared. The nanoliposome human papillomavirus (HPV) resistant and gynecological inflammation pathogen resistant specific complex IgY and the combined preparation for the same have the advantages that: the nanoliposome technology is applied to increase the infiltration capacity and the prolongation effect and improve the curative effect. The nanoliposome human papillomavirus (HPV) resistant and gynecological inflammation pathogen resistant specific complex IgY and the combined preparation for the same can be prepared into various vaginal spraying agents, other spraying agents, lotion, gel, capsules, suppository, vaginal membranes, tablets, effervescent tablets, ointment, cream and so on. The nanoliposome complex IgY can infiltrate into a vagina mucosa to effectively remove HPV viruses and gynecological inflammation pathogens which are absorbed on the vaginal surface layer and in the vagina mucosa, is absorbed through the vagina mucosa and enters into the body to give play to the functions of prevention and treatment, and also can effectively prevent recrudescence.
Owner:SHENZHEN JASON INTELLIGENT BIOTECH CO LIMLTED PRC
Probe of human papillomavirus and DNA chip comprising the same
ActiveUS7670774B2High selectivityHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsHuman papillomavirusBiology
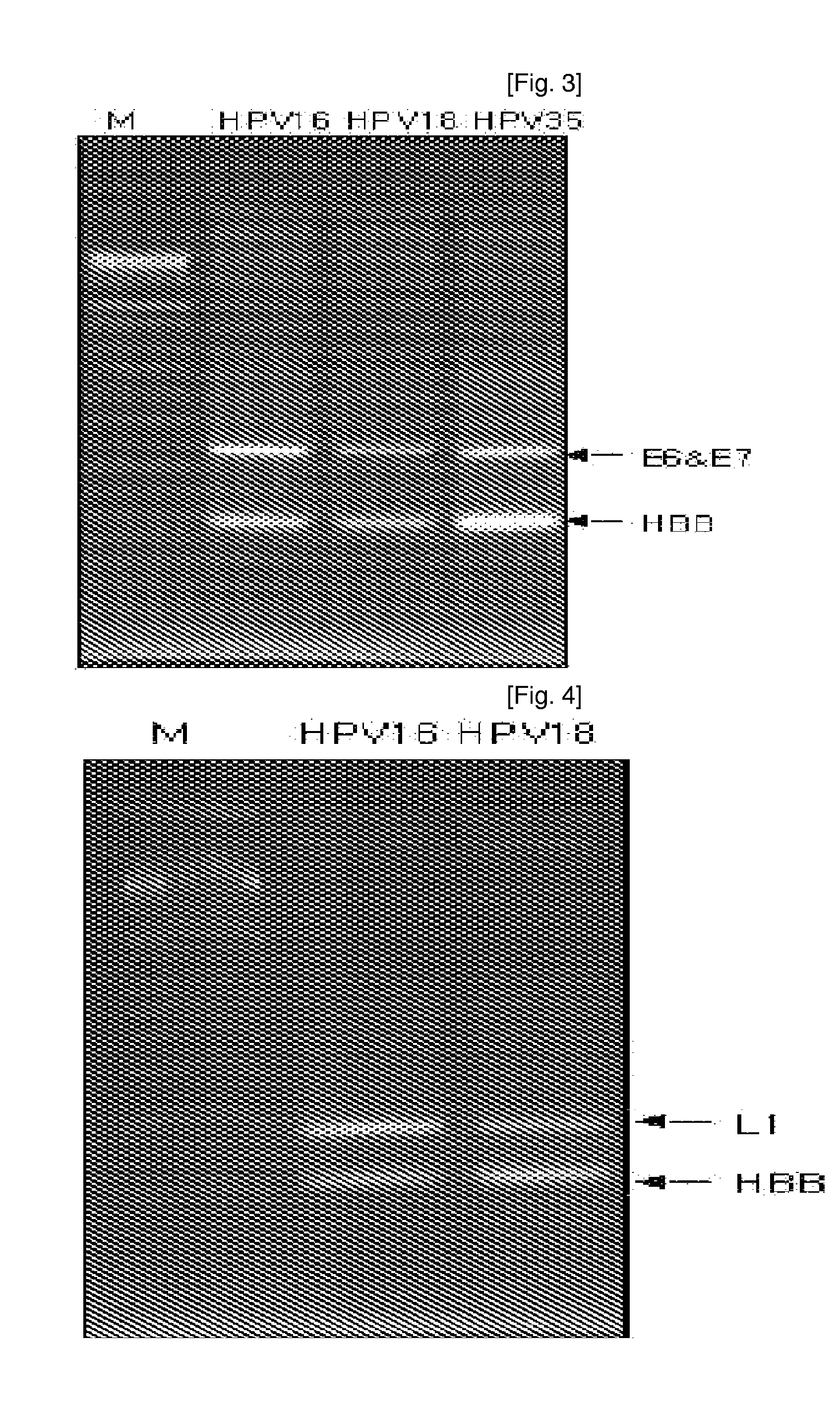
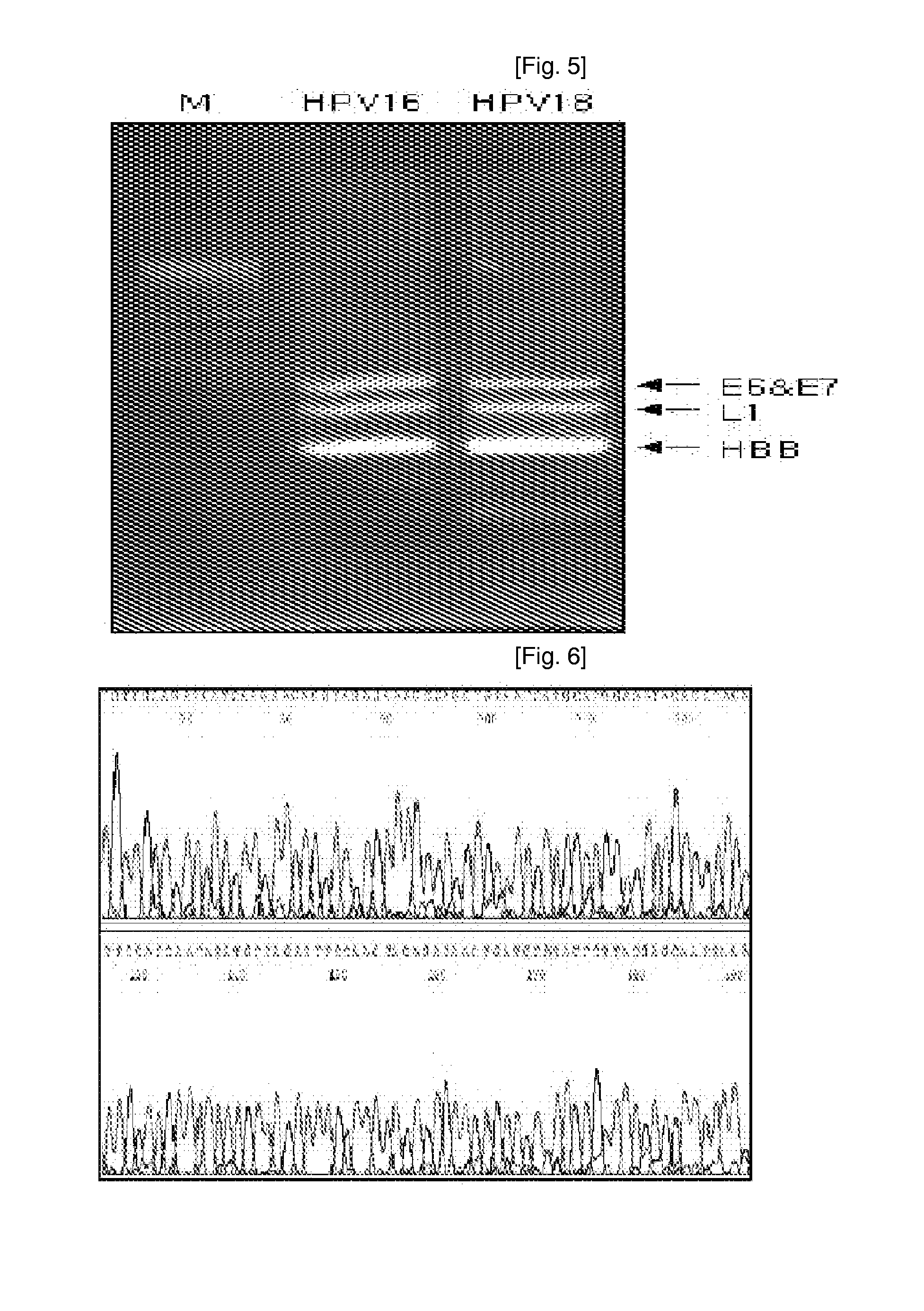
Oligonucleotide probes for analyzing 40 types of HPV were synthesized, and DNA chips were produced by using the oligonucleotide probes. The synthesis of the oligonucleotide probes is based on clones of L1 and E6 / E7 genes of 35 types of HPV obtained from cervical cell specimens from 4,898 Korean adult women and tissue specimens from 68 cervical cancer cases in addition to information based on American and European cases. The DNA chips can analyze the 40 types of HPV found in cervical, diagnose complex infection by at least one type of HPV, and have excellent diagnostic sensitivity and specificity on HPV genetic type up to 100% and reproducibility. Also, the DAN chips are superior to conventional analytic method, and very economical, since they can analyze numerous specimens in shortest time. Accordingly, the DNA chips are useful for predicting cervical cancer and precancerous lesion.
Owner:GOODGENE
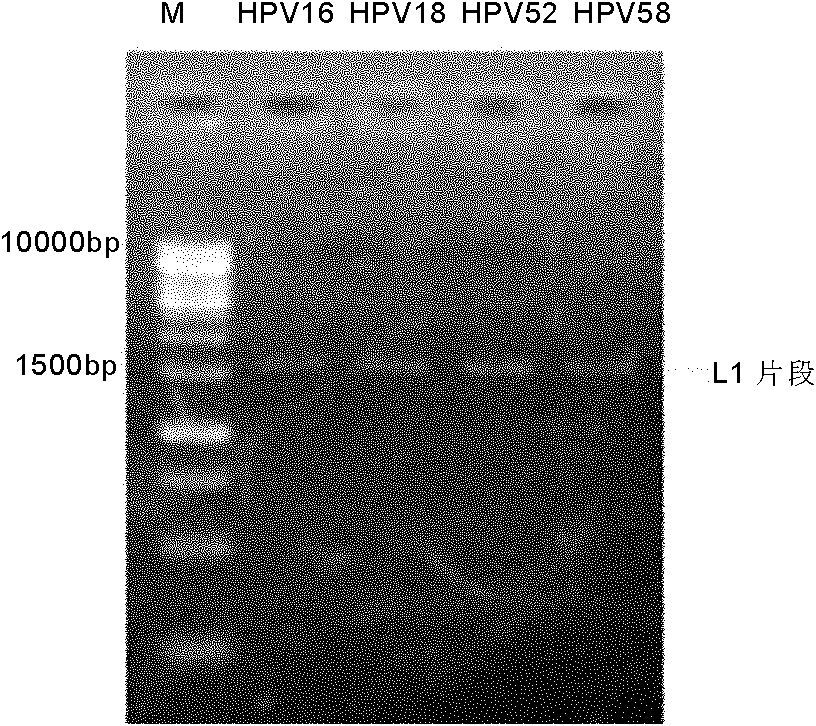
Human papillomavirus HPV DNA fragment, specific primer and application thereof
InactiveCN101851630ASimple methodMature technologyMicrobiological testing/measurementMicroorganism based processesDiseaseHuman papillomavirus DNA
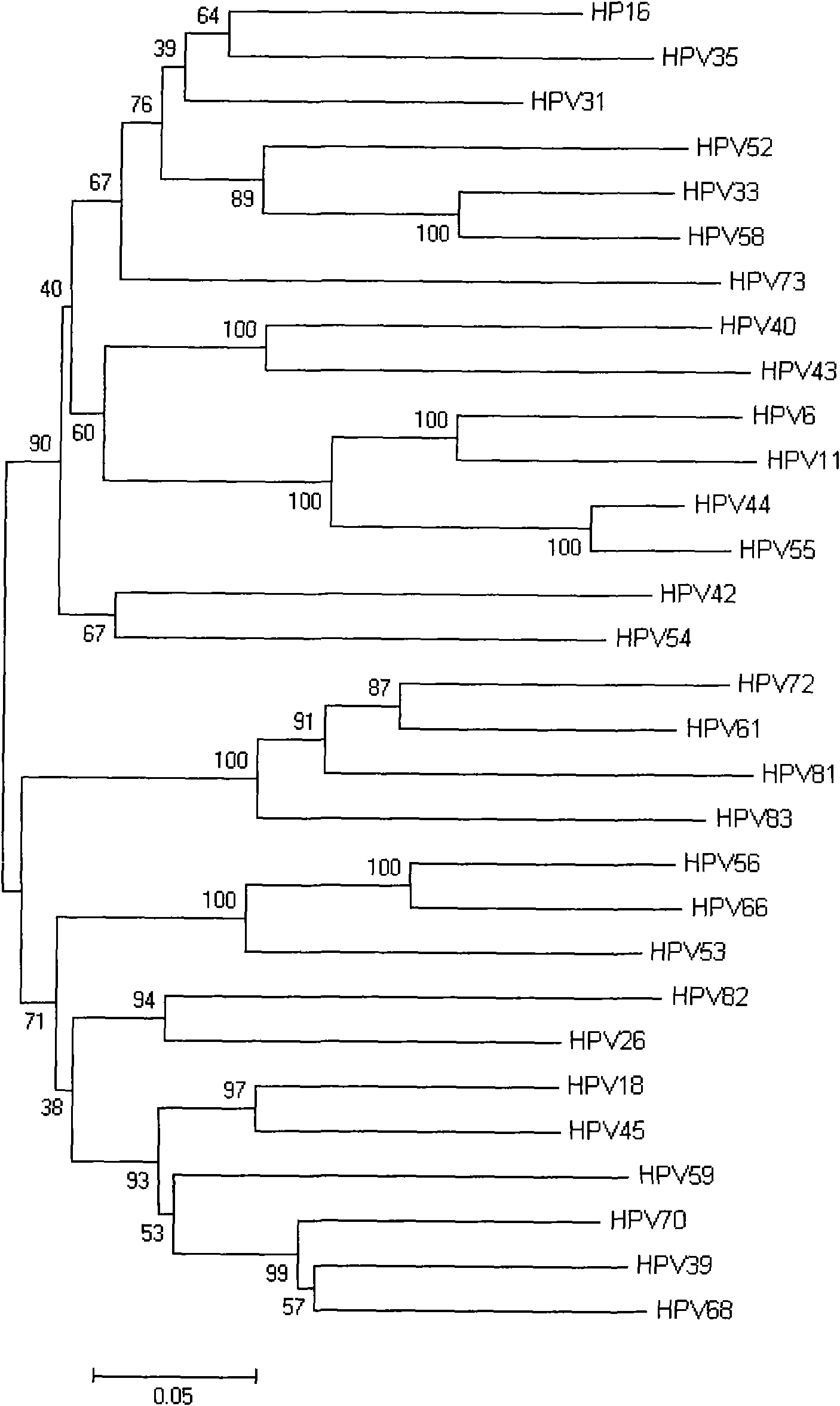
The invention provides a human papillomavirus HPV DNA fragment, a mix primer for human papillomavirus HPV DNA detection and / or parting and application thereof. The mixed primer can specifically amplify to obtain one or a plurality of DNA sequences from HPV6, HPV 11, HPV 16, HPV 18, HPV 26, HPV 31, HPV 33, HPV 35, HPV 39, HPV 40, HPV 42, HPV 43, HPV 44, HPV45, HPV 51, HPV 52, HPV 53, HPV 54, HPV 55, HPV 56, HPV 58, HPV 59, HPV 61, HPV 66, HPV 68, HPV 70, HPV 72, HPV 73, HPV 81, HPV 82 and HPV 83, can be used for the human papillomavirus HPV DNA detection and / or parting and prepared into a PCR kit. The invention can detect common types of HPV at a time, greatly improve the HPV detection efficiency, overcome the defect of missed detection of latent infection by a serology and IHC detection method, realize early diagnosis of HPV diseases, and is suitable for clinical HPV gene detection and parting and guides prevention and cure of cervical carcinoma.
Owner:白向阳 +1
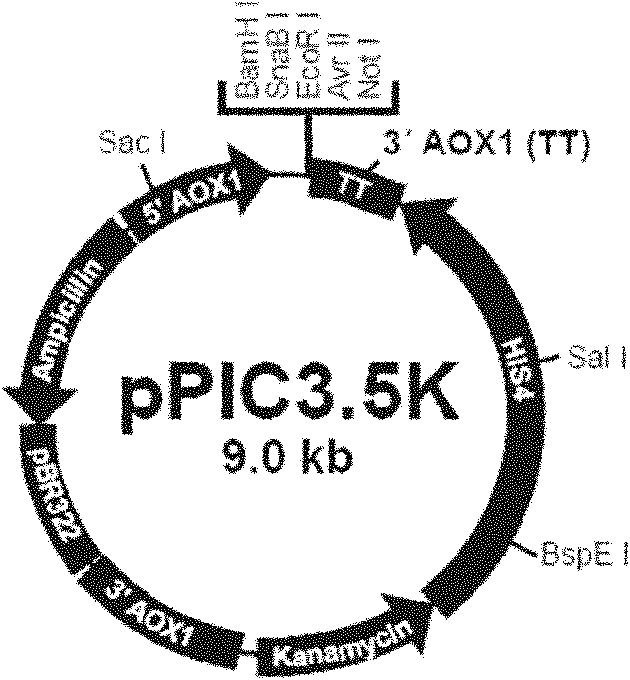
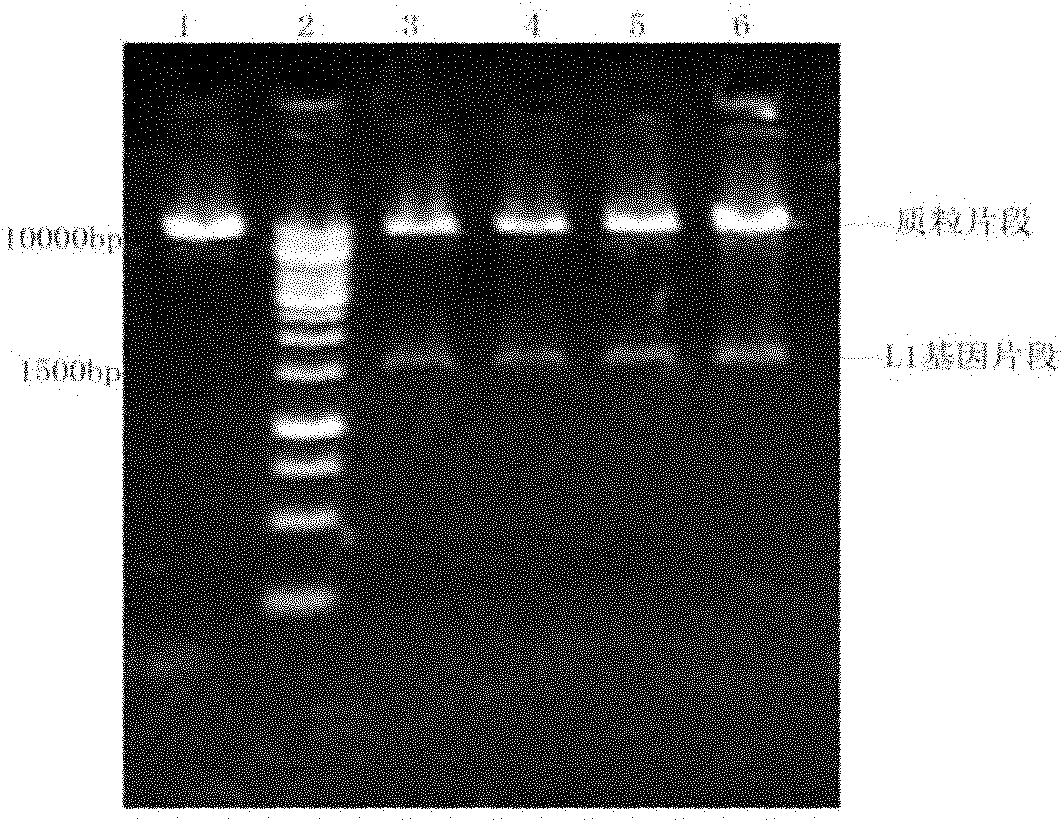
HPV16L1 polynucleotide sequence and expression vector, host cell and application thereof
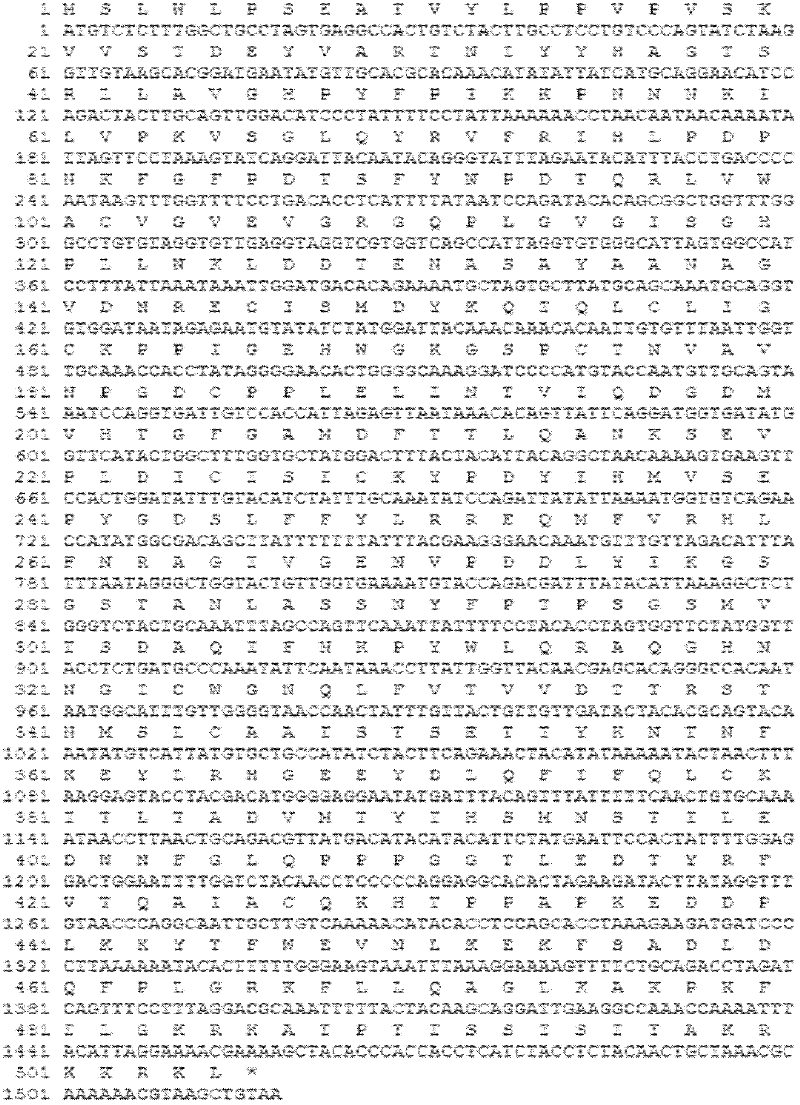
InactiveCN102586287AHigh yieldLow costFungiViral antigen ingredientsHuman papillomavirusSynthetic nucleotide
The invention relates to an HPV16L1 polynucleotide sequence, and an expression vector, a host cell and application of the HPV16L1 polynucleotide sequence. The HPV16L1 polynucleotide sequence comprises an amino acid sequence of recombinant human papillomavirus (HPV) L1 capsid protein, a synthetic nucleotide sequence coding the amino acid sequence, and a recombinant expression vector and a hansenula polymorpha expression host strain comprising the nucleotide sequence. The invention also relates to the application of HPV16L1 protein consisting of the amino acid sequence and derivatives of the HPV16L1 polynucleotide sequence in preparing vaccine. According to the invention, by transforming the nucleotide sequence of HPV16L1 wild type virogene, the recombinant HPV16L1 capsid protein in a hansenula polymorpha system is efficiently expressed, and the HPV16L1 capsid protein can be industrially produced by using the hansenula polymorpha expression system; and compared with the conventional other eukaryotic expression systems, the hansenula polymorpha expression system has the advantages of high yield, low cost and the like.
Owner:王昌华
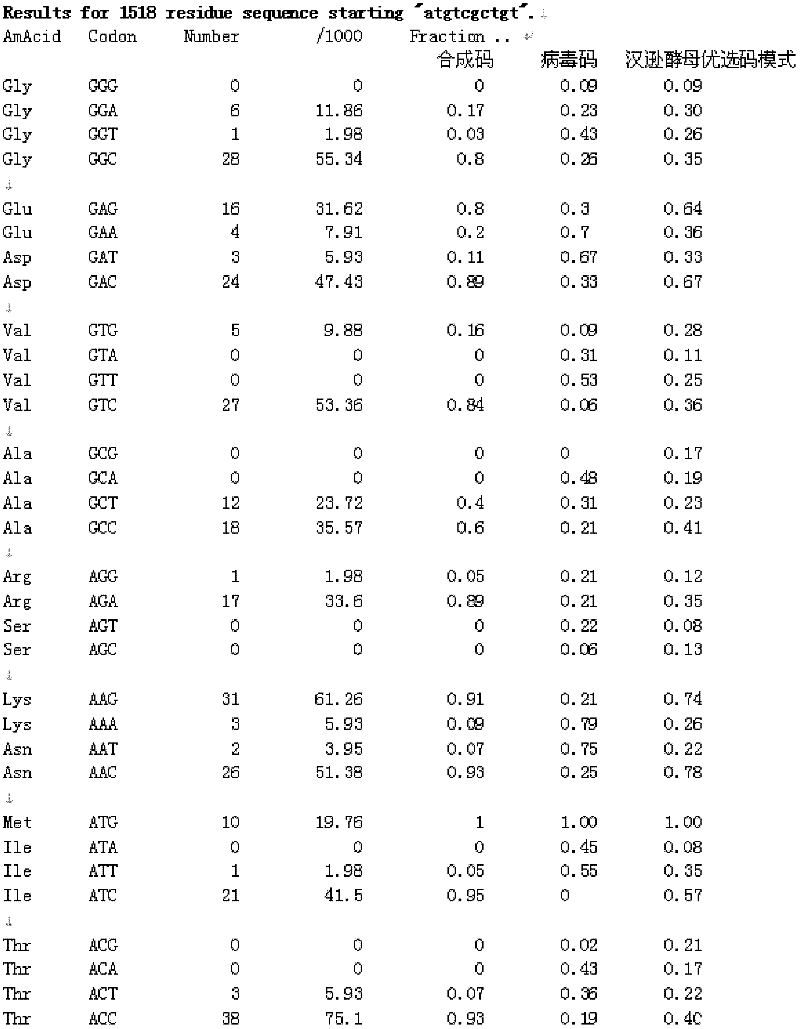
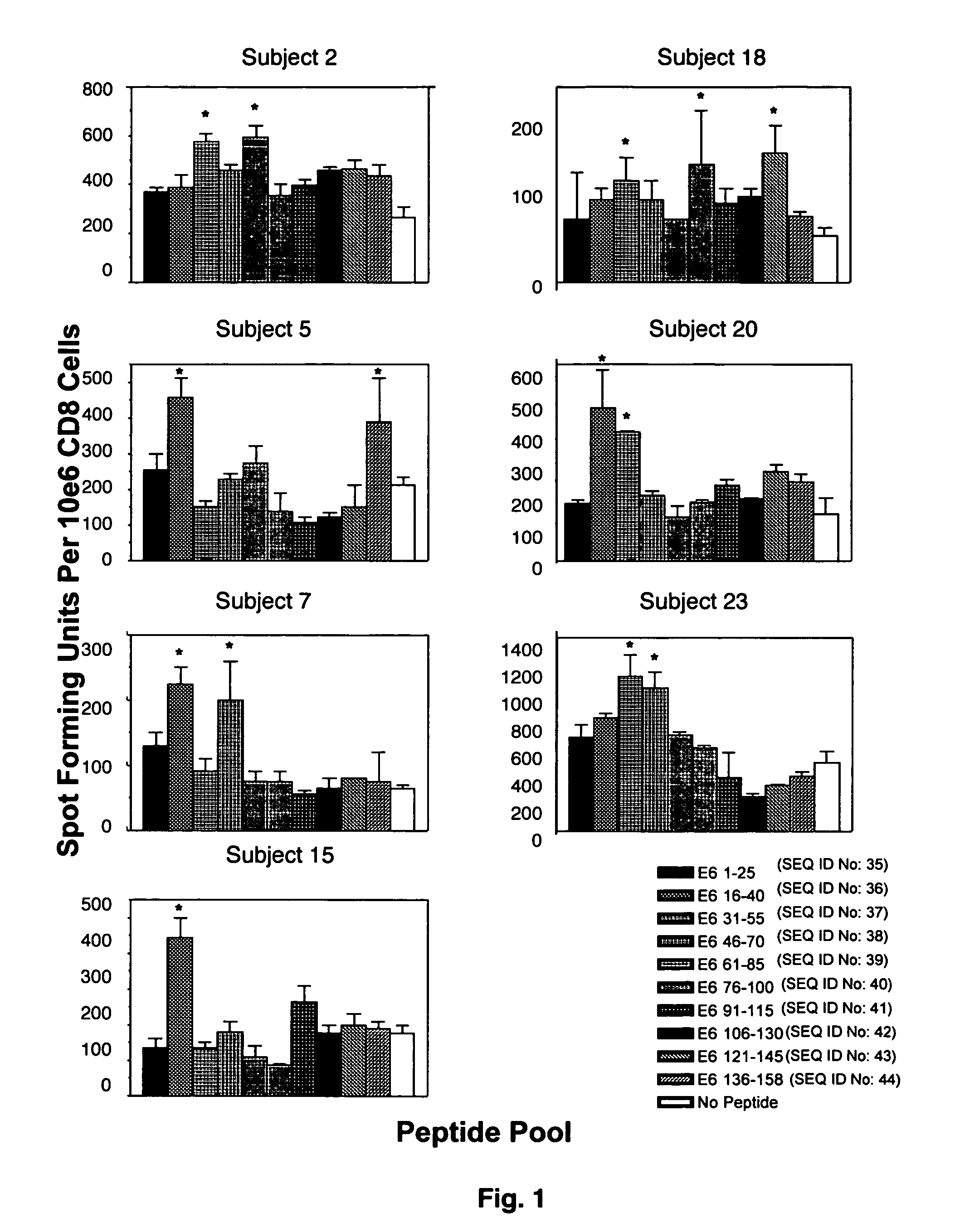
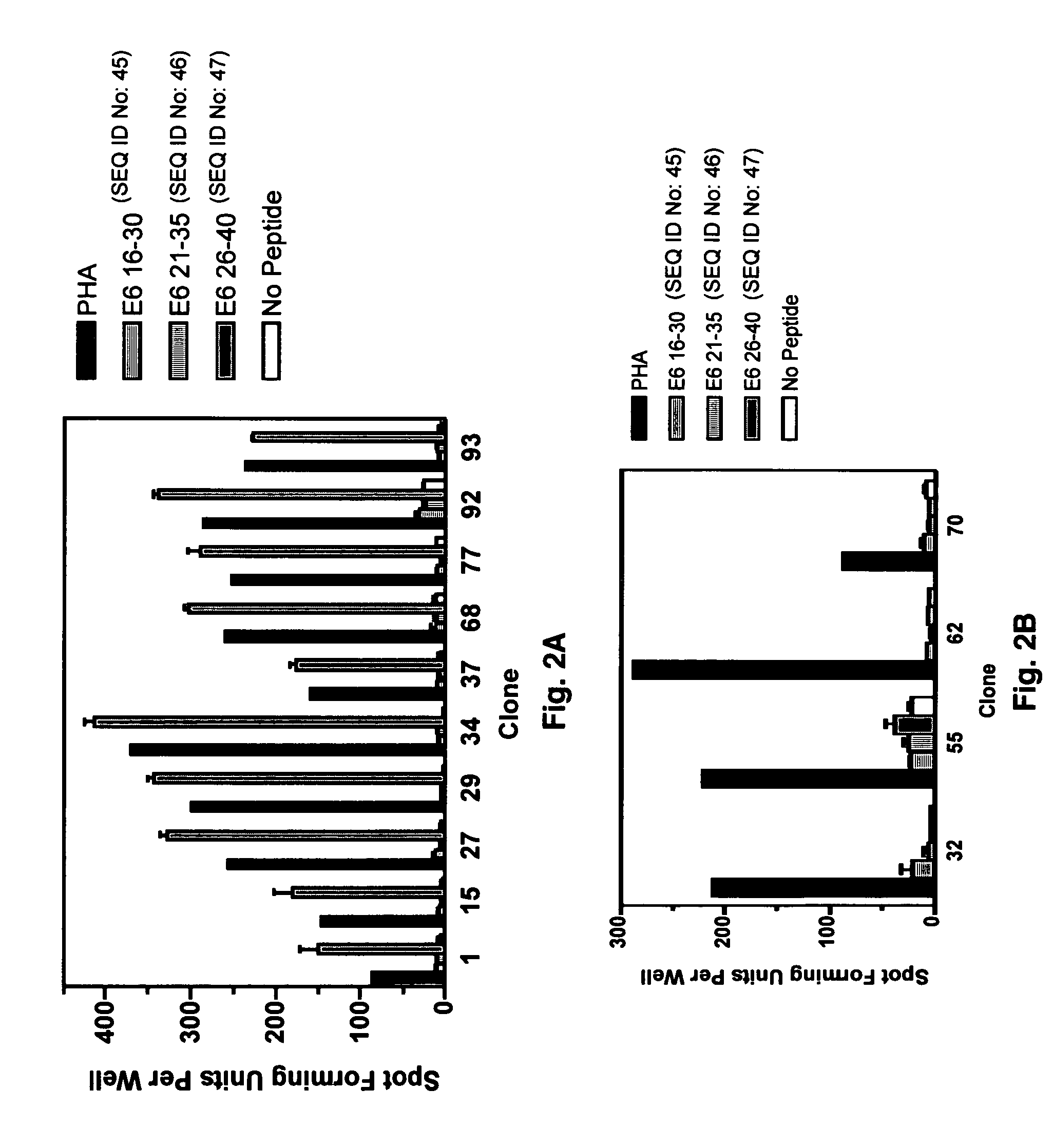
Anti-human papillomavirus 16 e7 t cell receptors
ActiveUS20170145070A1Minimize ToxicityImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
Disclosed is a synthetic T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E7, E711-19. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
Monoclonal antibody capable of specifically identifying HPV18 L1 protein and application of monoclonal antibody
ActiveCN108276491AEasy to detectStrong specificityImmunoglobulins against virusesMaterial analysisHeavy chainHuman papillomavirus
The invention relates to a monoclonal antibody capable of specifically identifying HPV18 L1 protein. The monoclonal antibody comprises a heavy-chain region and a light-chain region, wherein the sequence of the heavy-chain region is SEQ ID NO: 1 or SEQ ID NO: 3, and a sequence which has 80 percent consistency with the SEQ ID NO: 1 or the SEQ ID NO: 3; the sequence of the light-chain region is SEQ ID NO: 2 or SEQ ID NO: 4, and a sequence which has 80 percent consistency with the SEQ ID NO: 1 or the SEQ ID NO: 3; the invention further relates to application of the monoclonal antibody to preparation of an HPV18 detection reagent, and further relates to an HPV18 detection reagent kit comprising the monoclonal antibody. The monoclonal antibody and the reagent and the kit, which are prepared fromthe monoclonal antibody, can be used for detecting patients in an HPV (Human Papillomavirus) active infection period; the detection is convenient and the specificity is high.
Owner:Y CLONE MEDICAL SCI CO LTD
Anti-human papillomavirus 16 e6 t cell receptors
ActiveUS20160152681A1Highly avid recognitionMinimize destructionPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
Disclosed is a T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E6, E629-38. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof
The present invention is directed to the examination of the pattern of immunodominant CD8 T cell epitopes in the E6 and E7 protein of Human Papillomavirus (HPV) and its further characterization in terms of its amino acid sequence and HLA restriction. These epitopes are identified based on their ability to induce strong CD8 T cell response and therefore, are important as sources of antigens for dendritic cell immunotherapy to treat cervical cancer. The present invention contemplates identifying a number of similar epitopes restricted by a wide variety of HLA types so that they can be used in concert to develop a preventative vaccine, which can be used for general population.
Owner:BIOVENTURES LLC
Vaccine against human papillomavirus (HPV) as well as preparation method and application thereof
ActiveCN102154325AHigh expressionImprove stabilityFungiViral antigen ingredientsPichia pastorisHuman papillomavirus
The invention relates to a vaccine against human papillomavirus (HPV) as well as a preparation method and an application thereof. Specifically, the invention provides the encoding sequence of an optimized human papillomavirus capsid protein L1 (HPV L1) suitable for expression in Pichia pastoris, and a method for efficiently producing HPV L1. The optimized HPV L1 gene is very suitable for expression in Pichia pastoris, and has the characteristic of high expression level and high stability. According to the invention, HPV L1 proteins and self-assembled virus-like particles can be obtained in an efficient, simple and cheap way. The invention also provides a vaccine composition comprising HPV virus-like particles.
Owner:SHANGHAI WELLVAC BIOTECHNOLOGIES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com