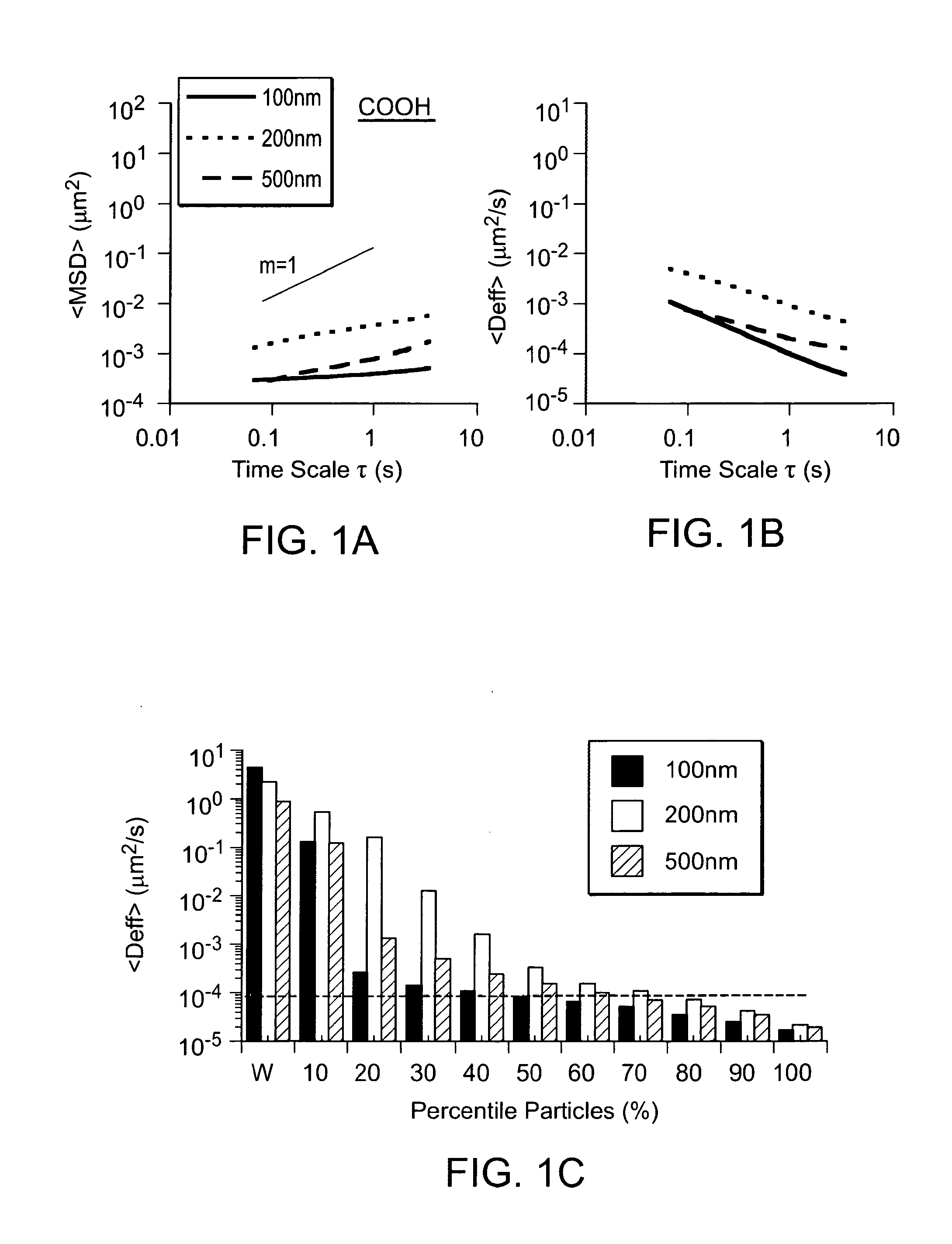
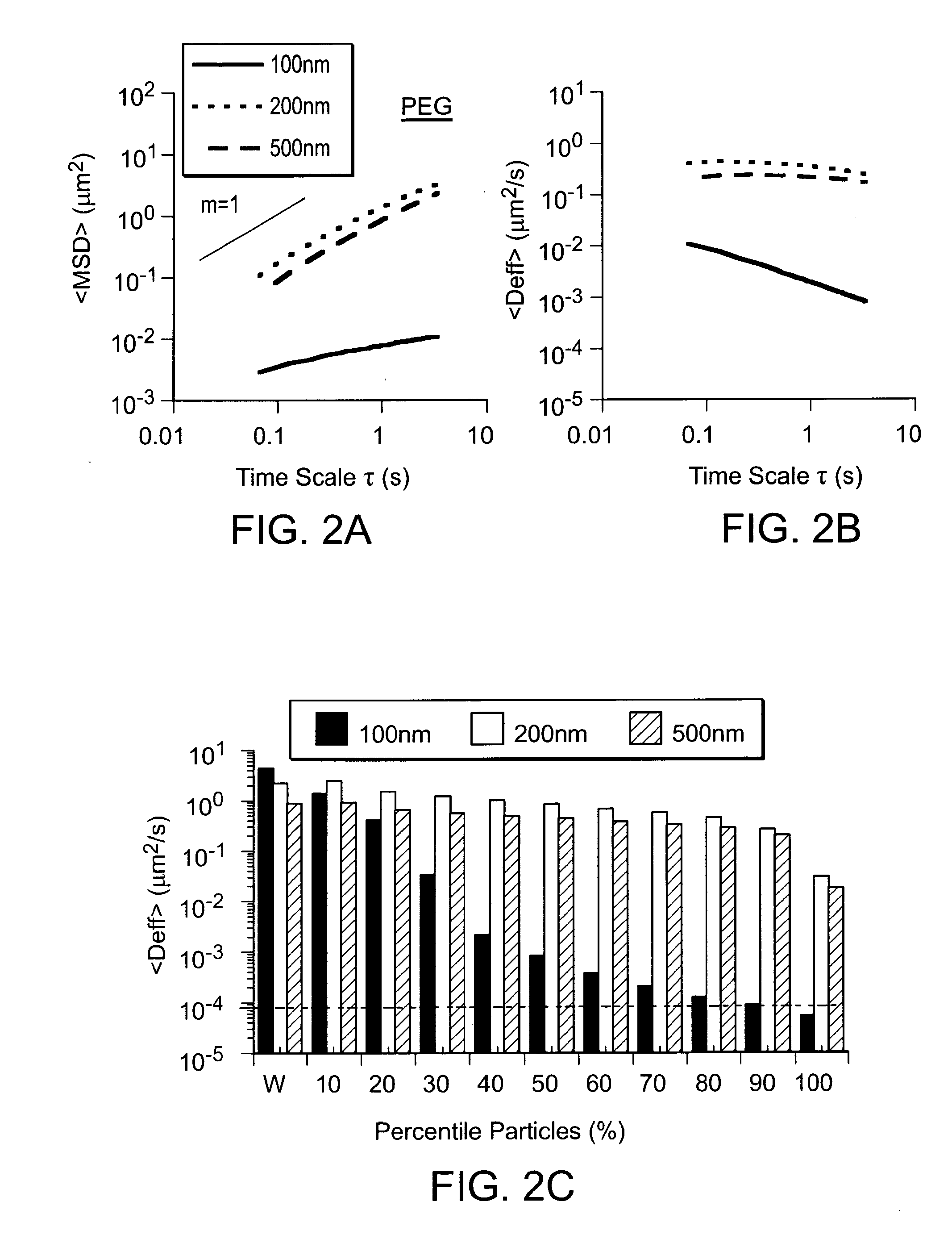
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1224 results about "Mucus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
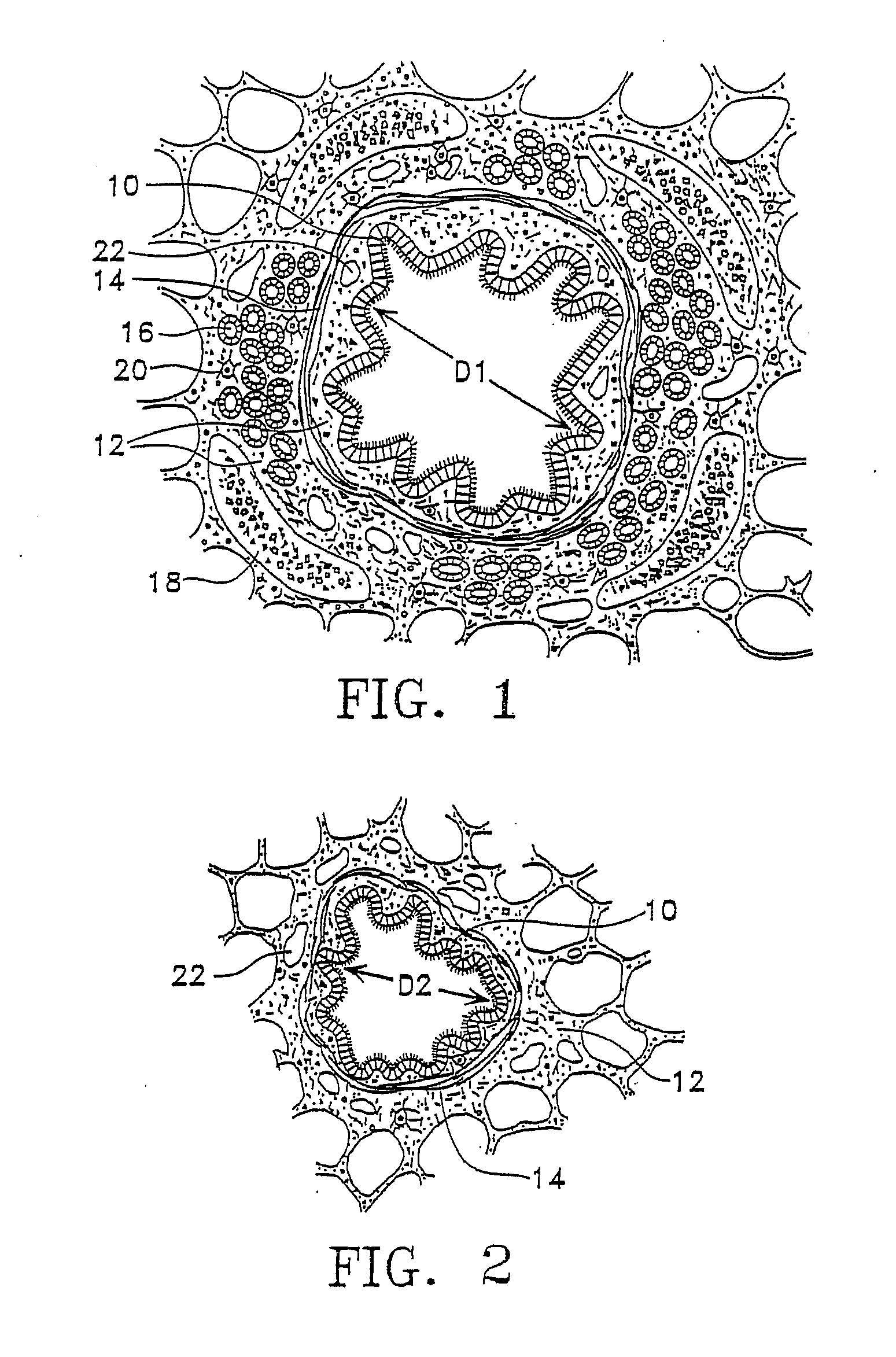
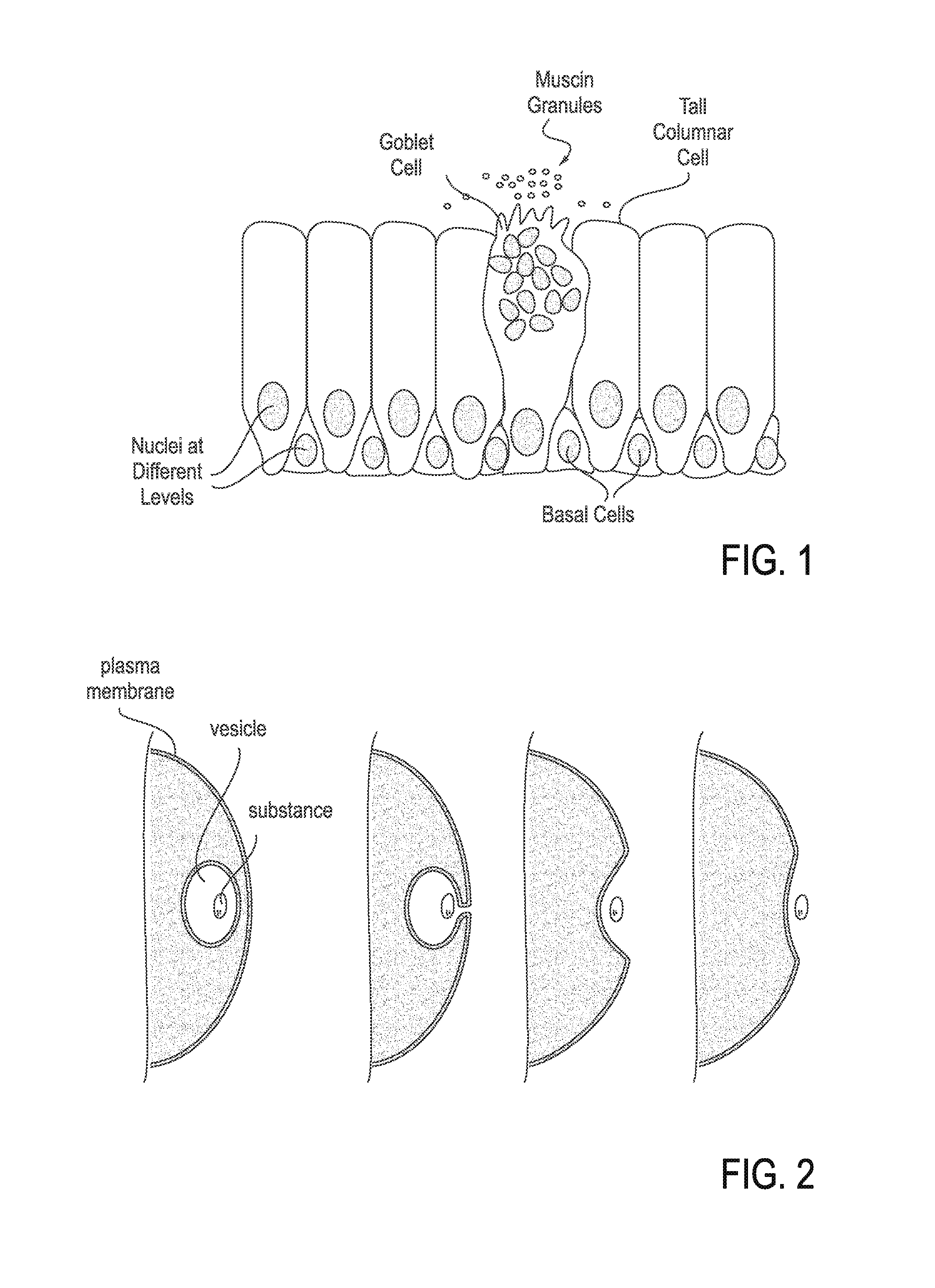
Mucus (/ˈmjuːkəs/ MEW-kəs) is a polymer. It is a slippery aqueous secretion produced by, and covering, mucous membranes. It is typically produced from cells found in mucous glands, although it may also originate from mixed glands, which contain both serous and mucous cells. It is a viscous colloid containing inorganic salts, antimicrobial enzymes (such as lysozymes), immunoglobulins, and glycoproteins such as lactoferrin and mucins, which are produced by goblet cells in the mucous membranes and submucosal glands. Mucus serves to protect epithelial cells in the linings of the respiratory, digestive, and urogenital systems, and structures in the visual, and auditory systems from pathogenic fungi, bacteria and viruses. Most of the mucus in the body is produced in the gastrointestinal tract.
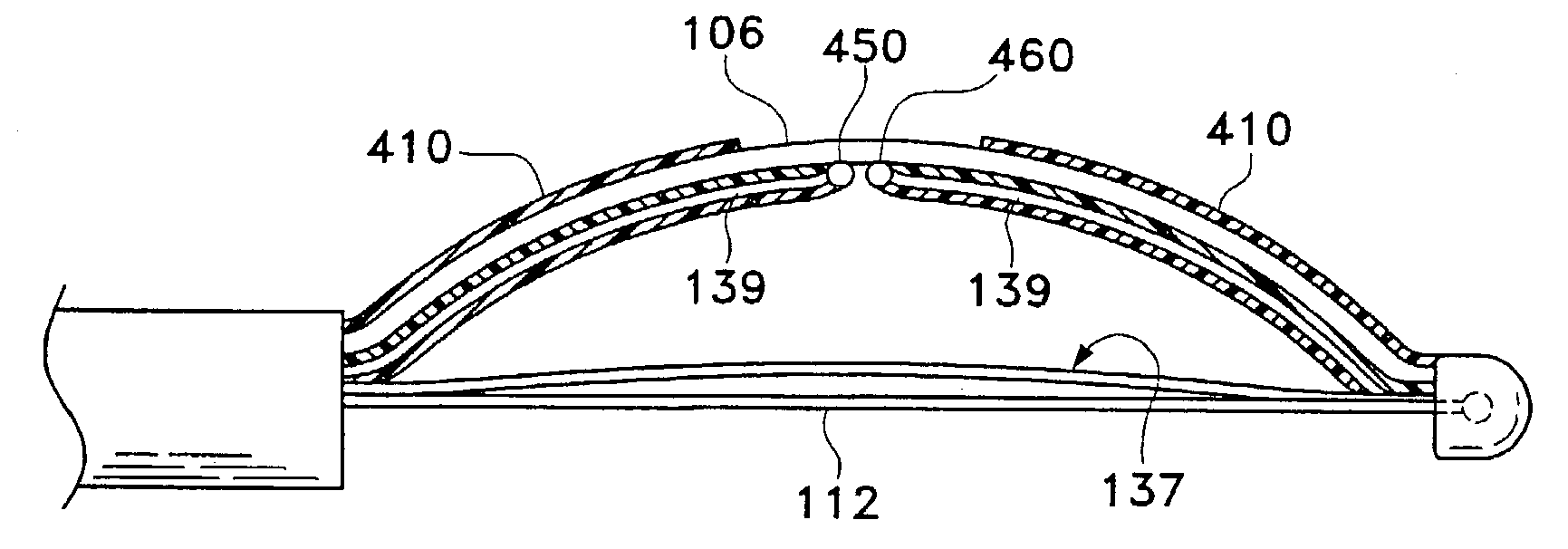
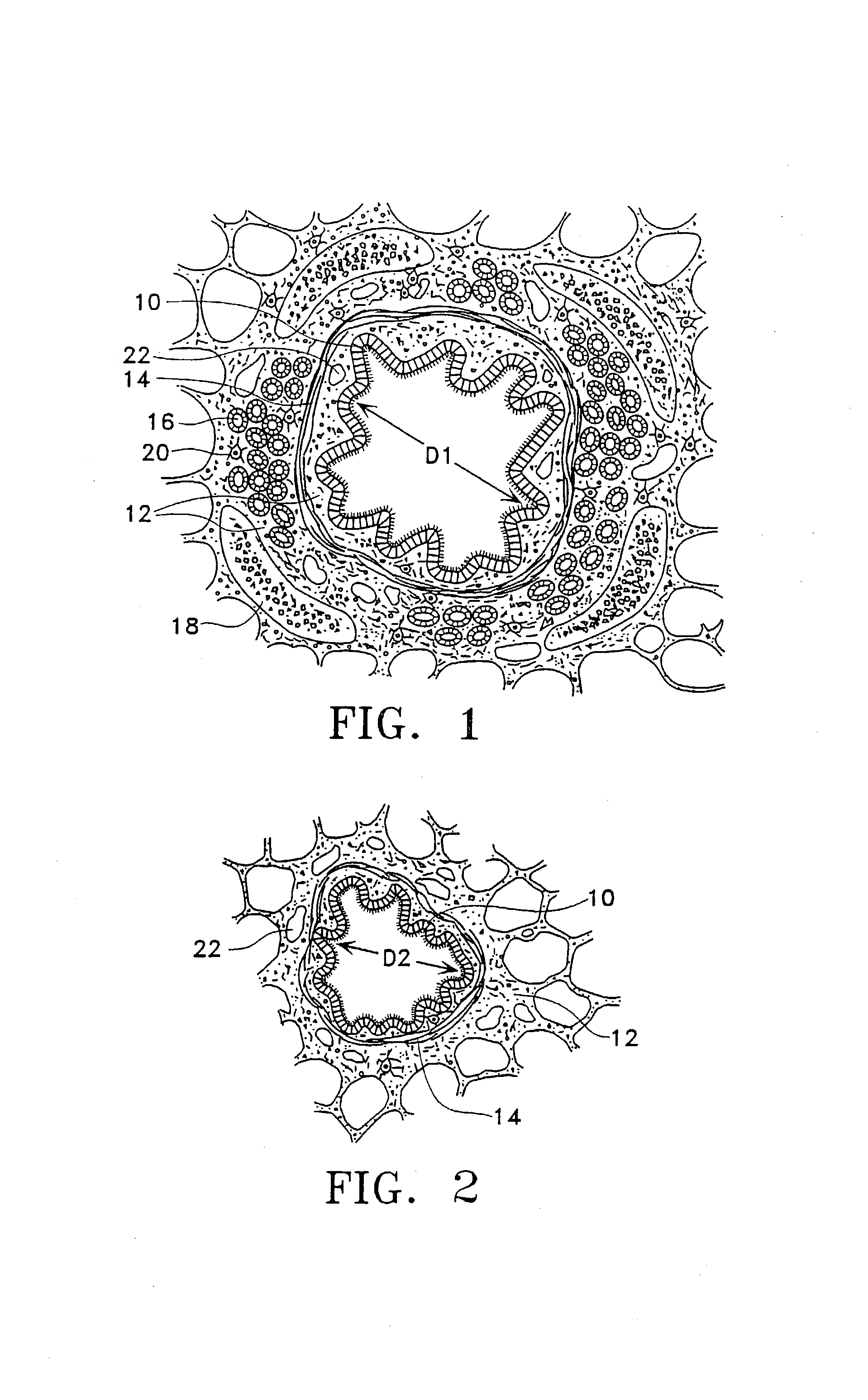
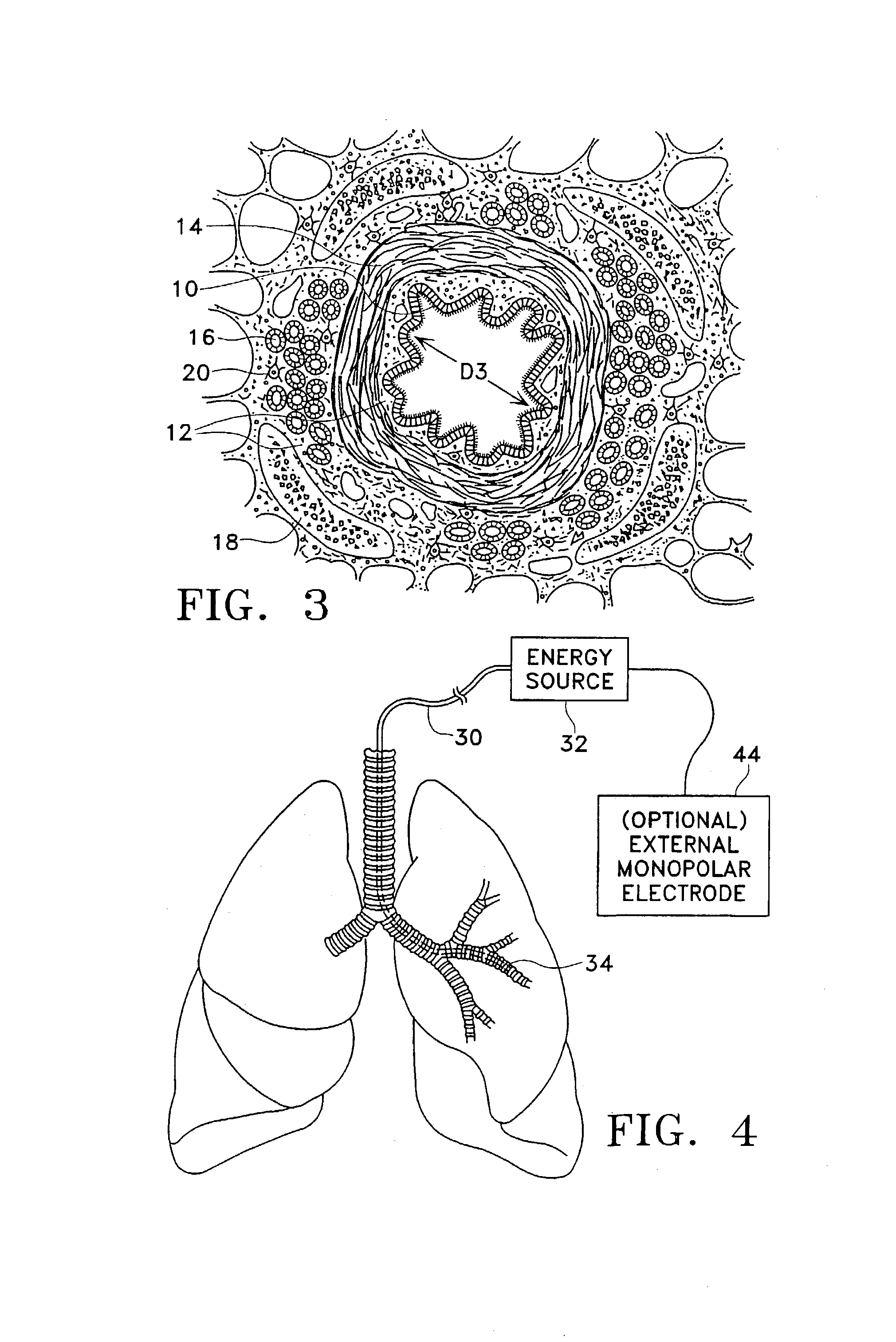
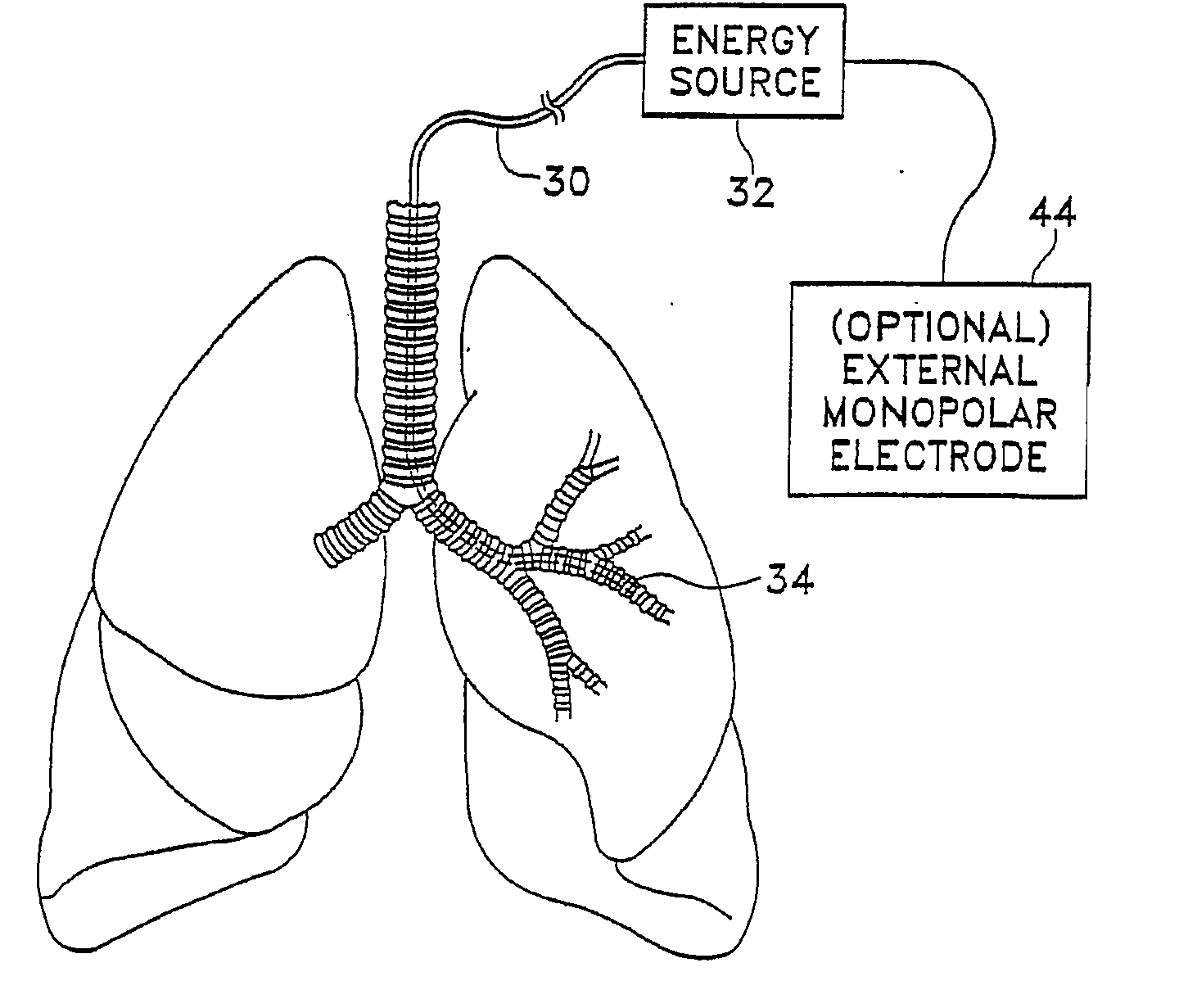
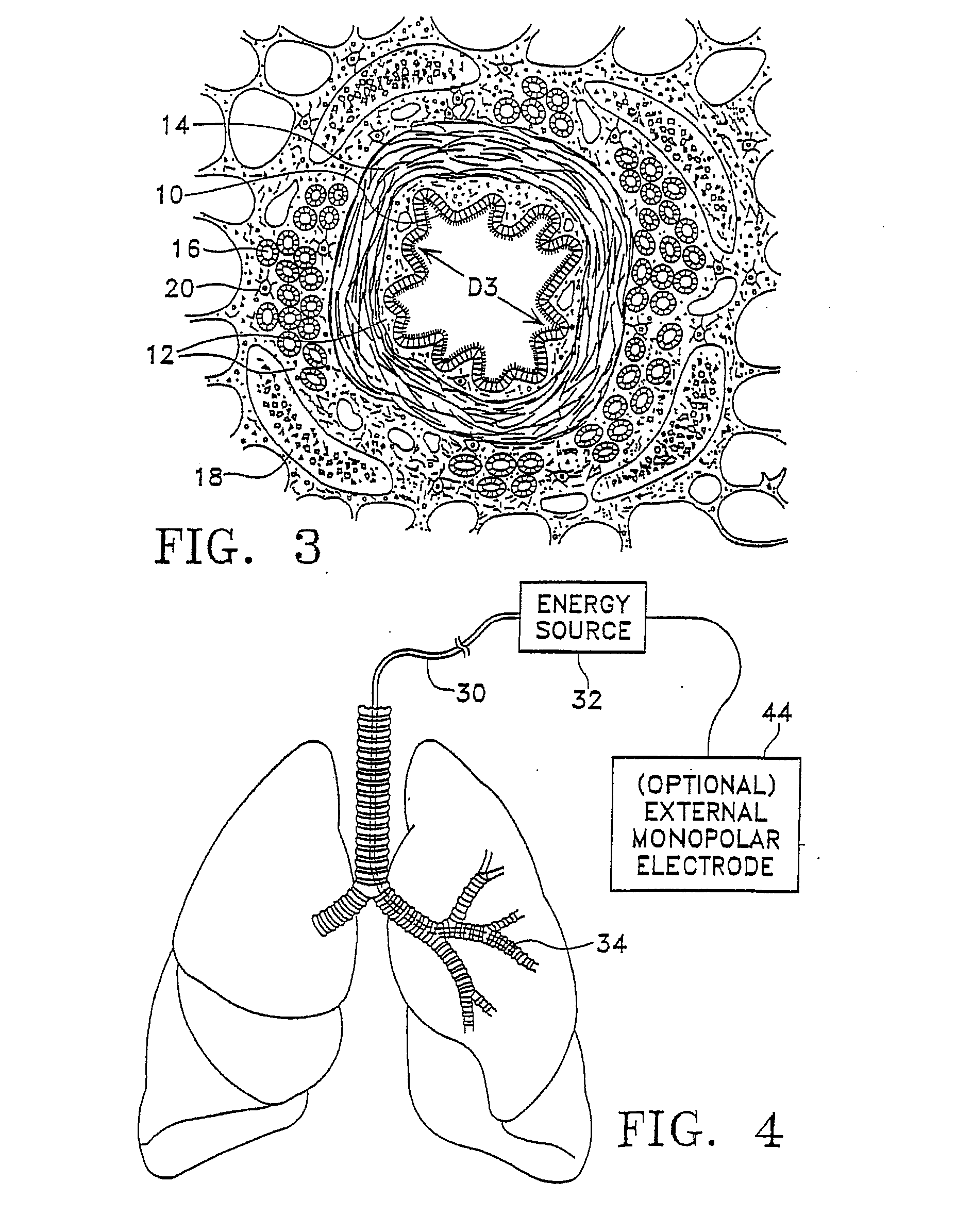
Modification of airways by application of energy
InactiveUS7198635B2Reduce plugging of the airwayPrevent the airway from being able to constrictElectrotherapySurgical needlesPatient complianceObstructive Pulmonary Diseases
This relates to methods and devices for treating reversible chronic obstructive pulmonary disease, and more particularly, relates to a device for exchanging energy with airway tissue such as that found in the airway of human lungs. The exchange of energy with this airway tissue in the airways reduces the ability of the air ways to constrict and / or reduces the resistance within the airway to the flow of air through the airway. This also relates to a method for decreasing responsiveness or decreasing resistance to airflow of airways involves the transfer of energy to or from the airway walls to prevent or reduce airway constriction and other symptoms of lung diseases. The treatment reduces the ability of the airway to contract during an acute narrowing of the airways, reduces mucus plugging of the airways, and / or increases the airway diameter. The methods according to the present invention provide a longer duration and / or more effective treatment for lung diseases than currently used drug treatments, and obviate patient compliance issues. This also includes additional steps that reduce the ability of the lung to produce at least one of the symptoms of reversible obstructive pulmonary disease and to reduce the resistance to the flow of air through a lung.
Owner:BOSTON SCI SCIMED INC
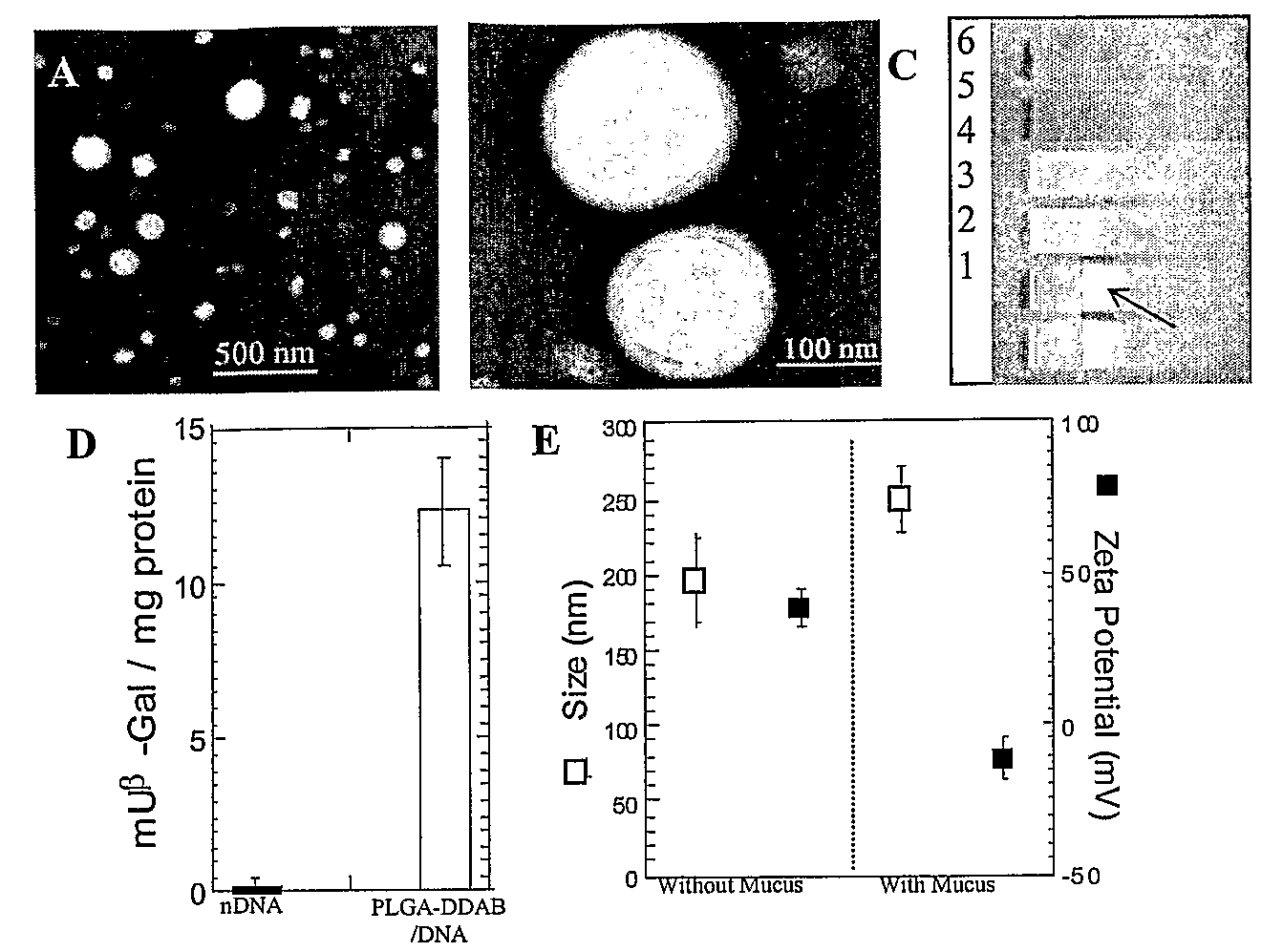
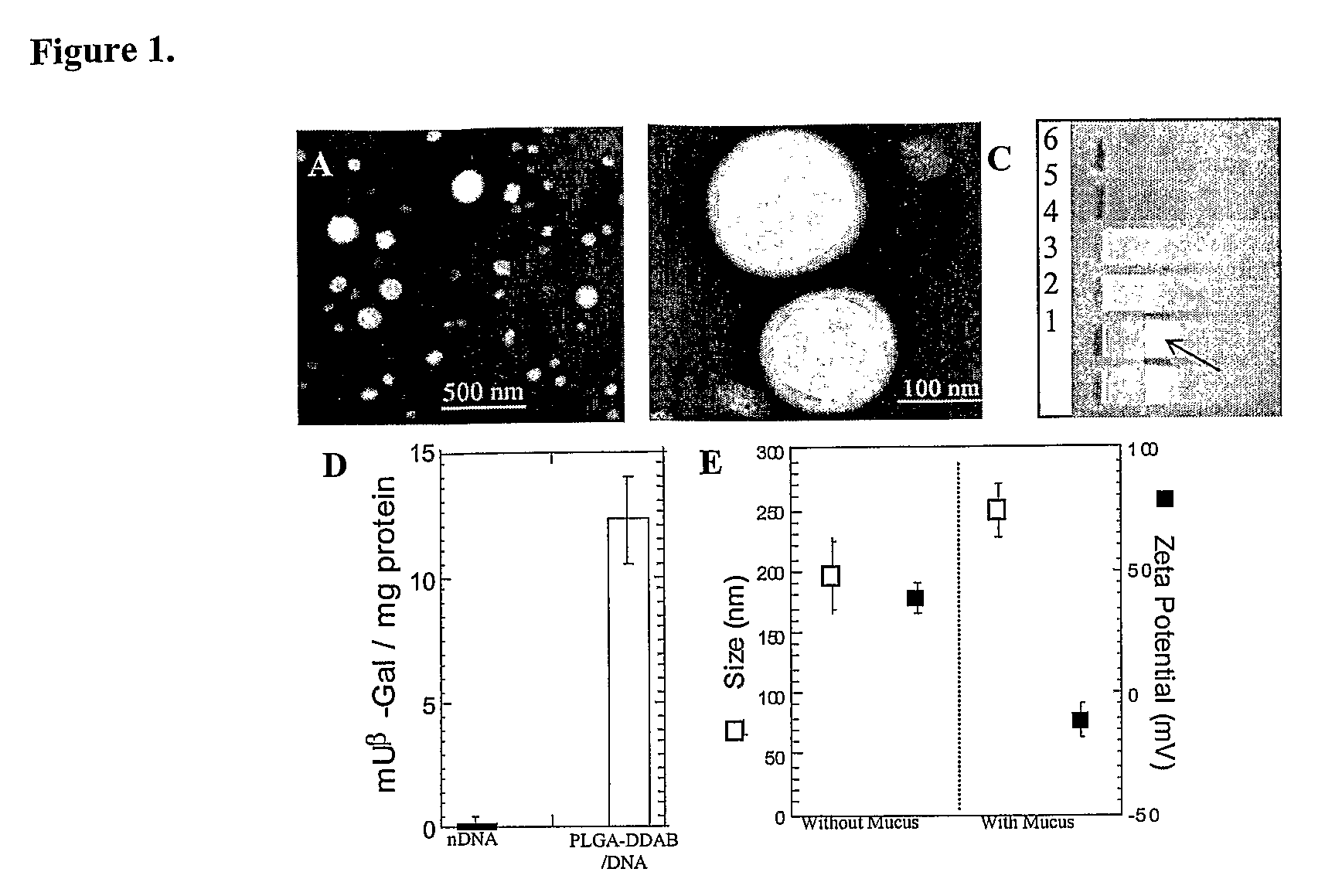
Compositions and methods for enhancing transport through mucus
InactiveUS20100215580A1Reduce mucoadhesivenessImprove hydrophilicityBiocidePowder deliveryChemistryRespiratory mucus
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
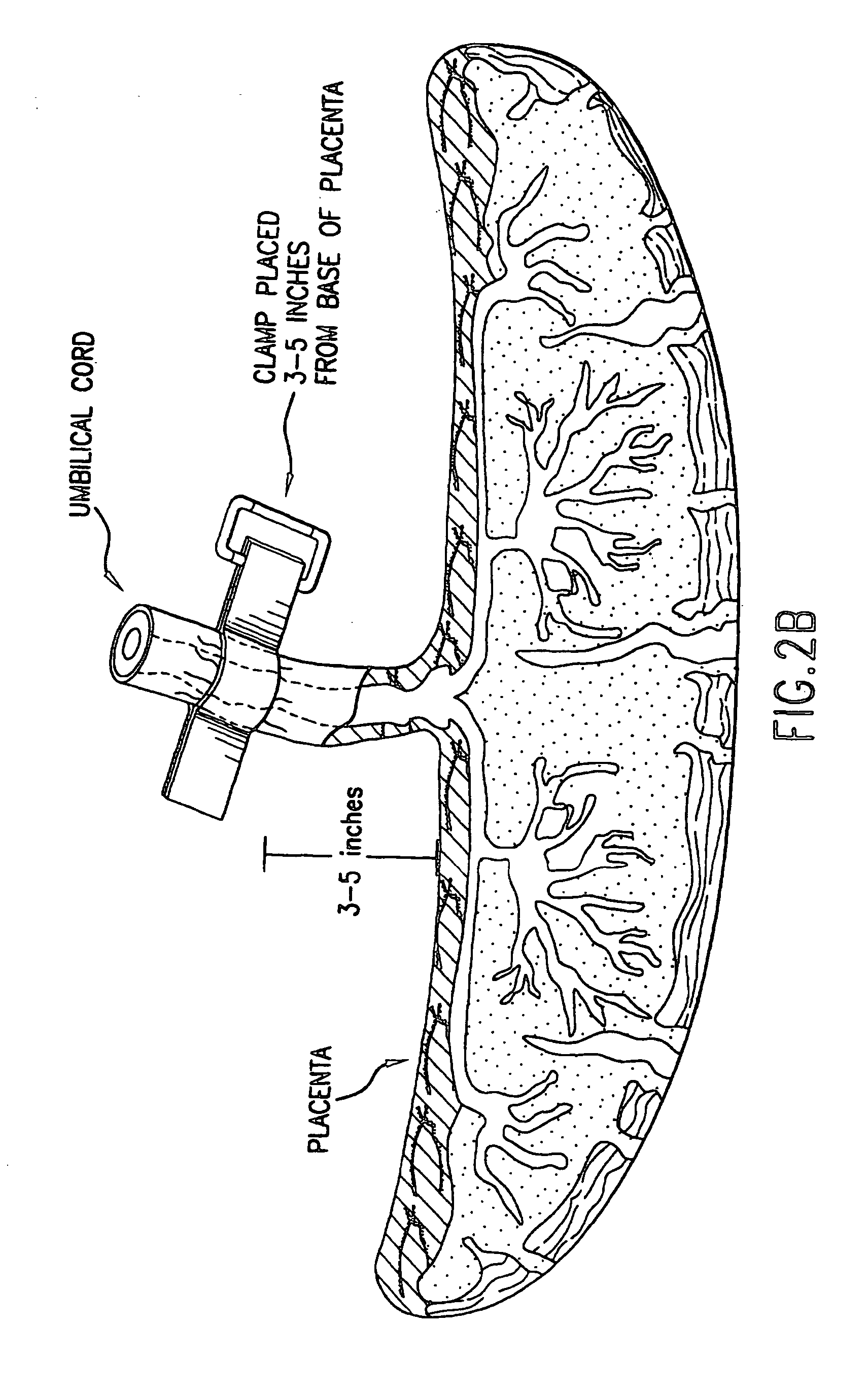
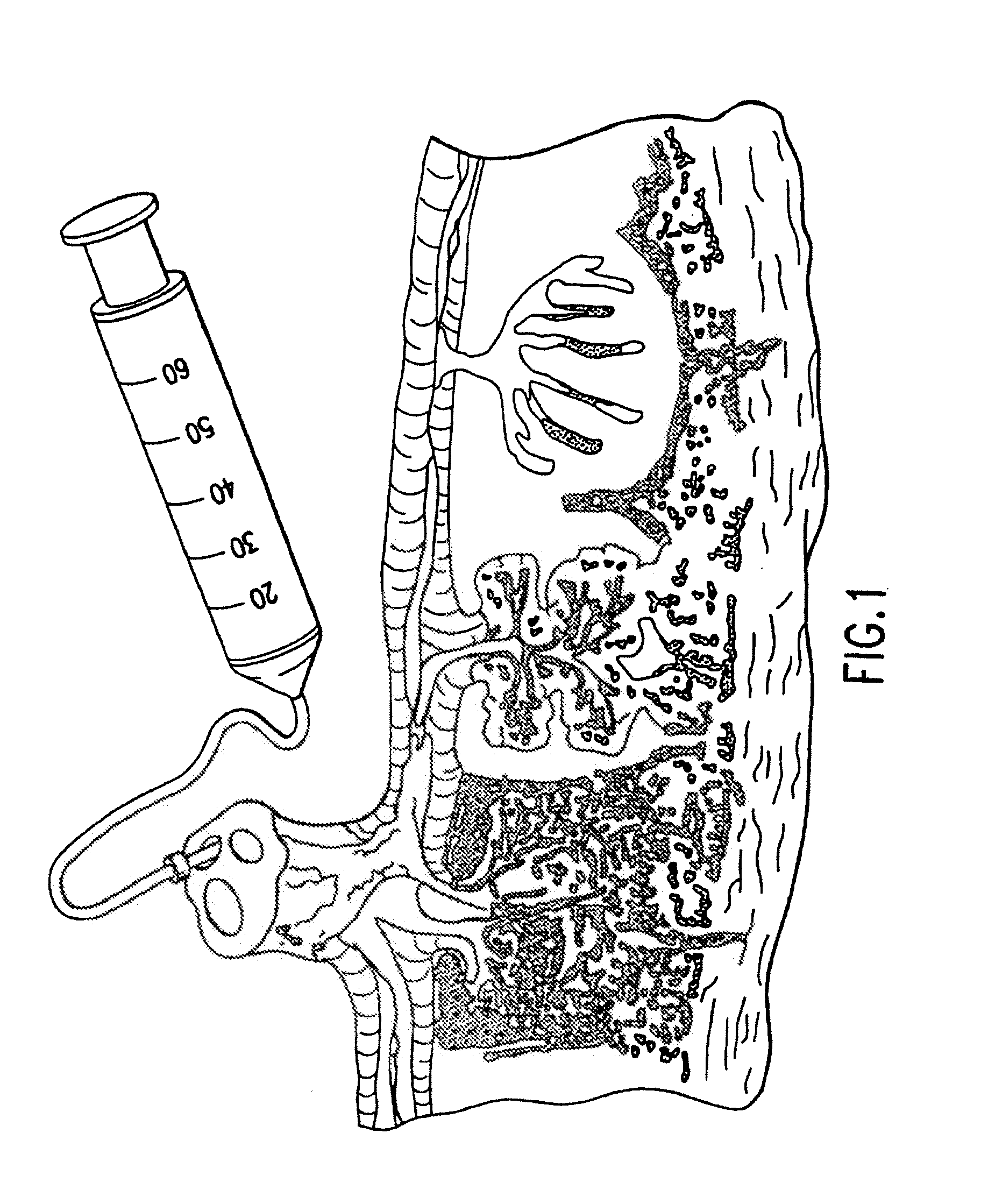
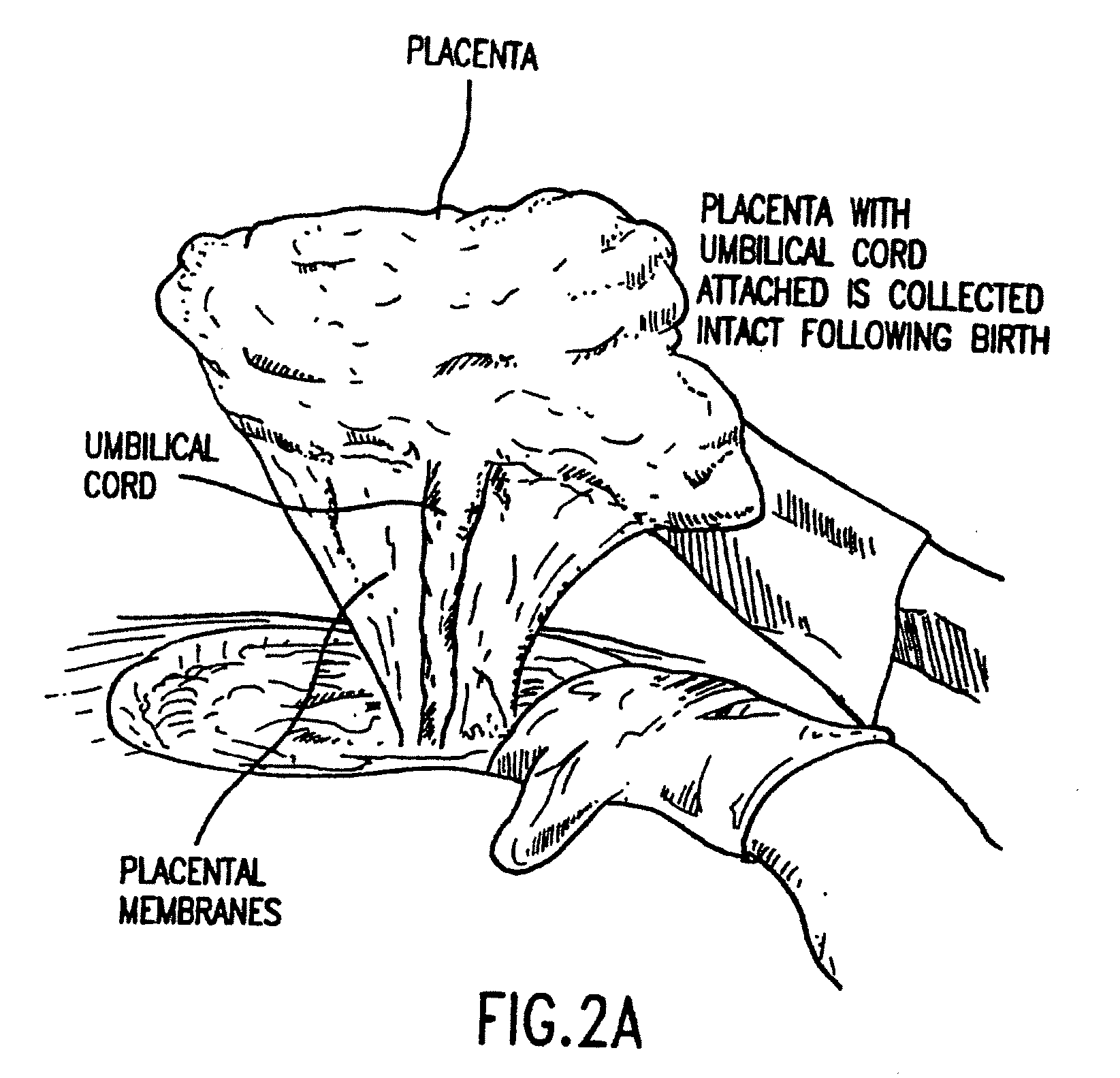
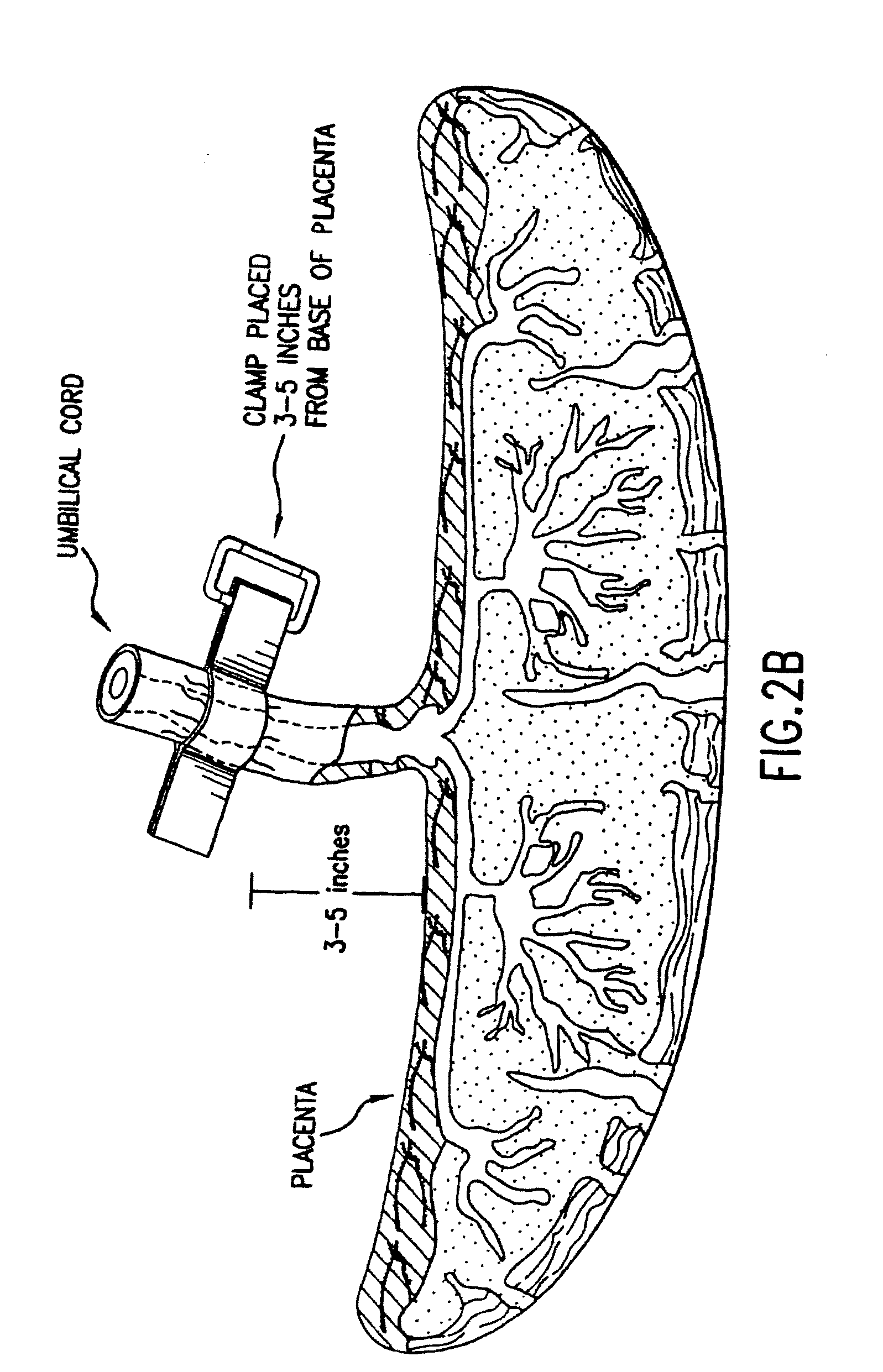
Composition for collecting and preserving placental stem cells and methods of using the composition
The present invention provides improved compositions and methods for the collection of stem cells from an organ, e.g., placenta. The invention provides a stem cell collection composition comprising an apoptosis inhibitor and, optionally, an enzyme such as a protease or mucolytic enzyme, vasodilator, necrosis inhibitor, oxygen-carrying perfluorocarbon, or an organ preserving compound. The invention provides methods of using the stem cell collection composition to collect stem cells and to preserve populations of stem cells.
Owner:CELULARITY INC
Drugs And Gene Carrier Particles That Rapidly Move Through Mucous Barriers
ActiveUS20080166414A1Easy adhesionPromote complexationPowder deliveryOrganic active ingredientsCrystallographyMucus
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
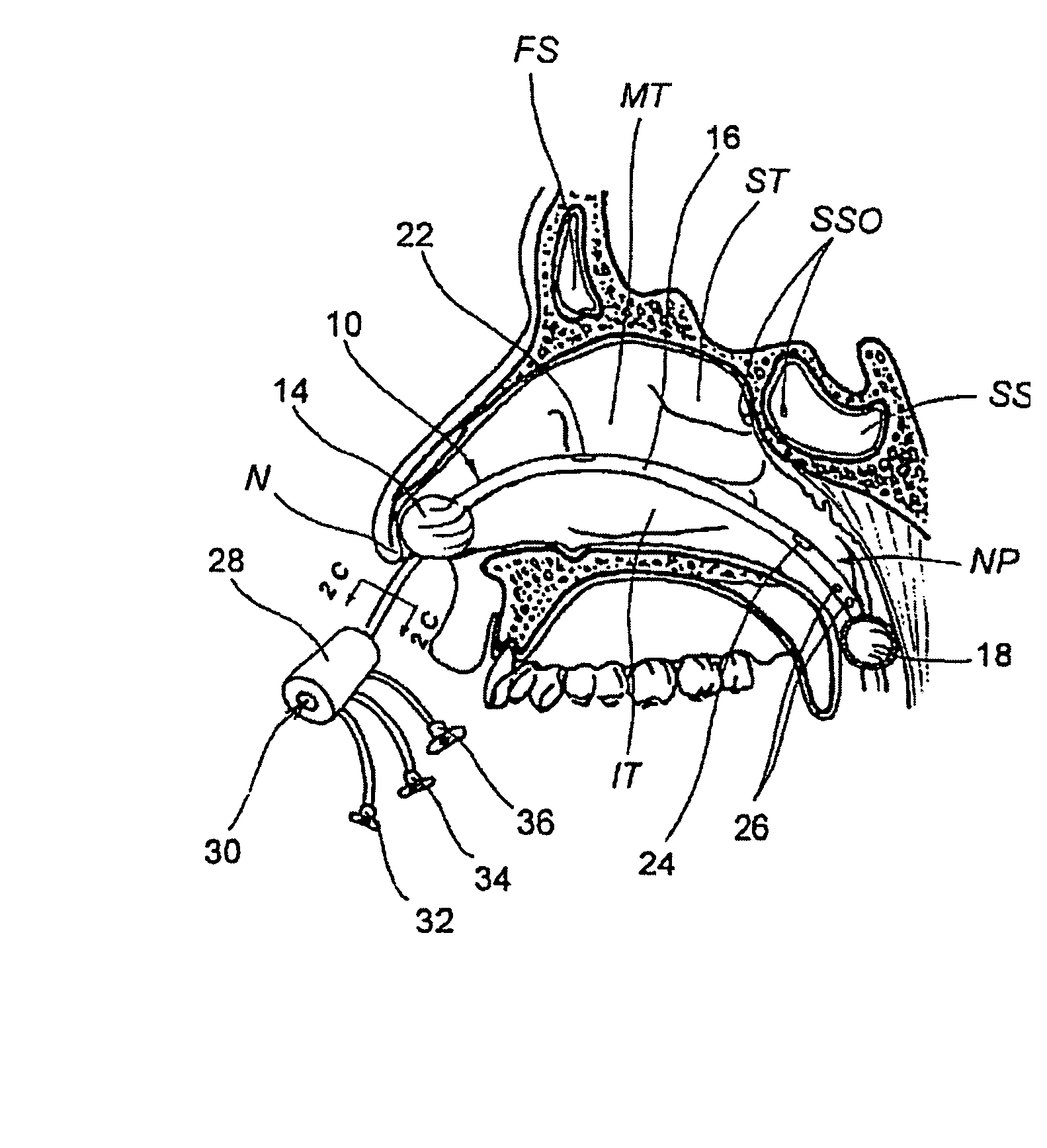
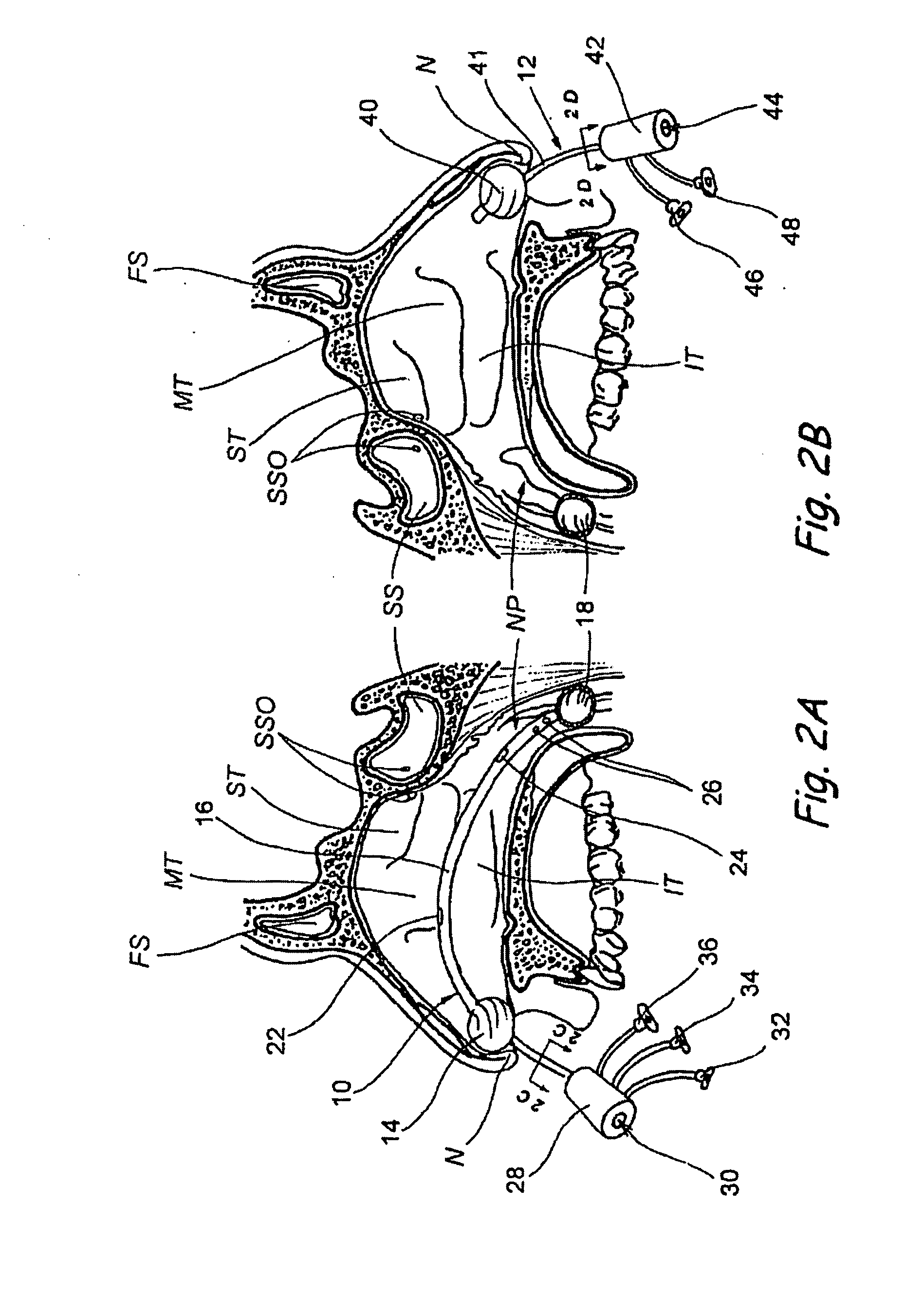
Devices, systems and methods for diagnosing and treating sinusitis and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
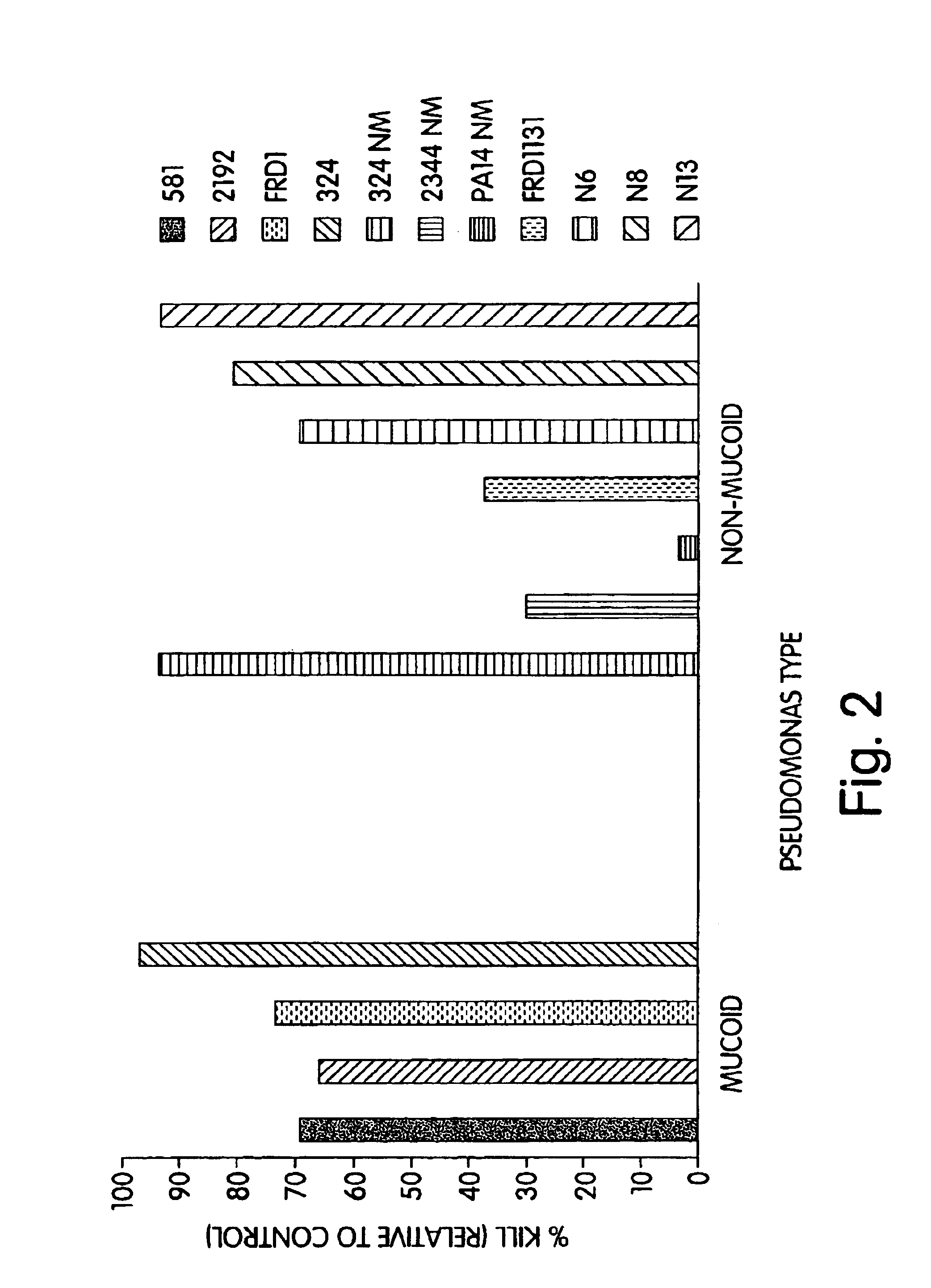
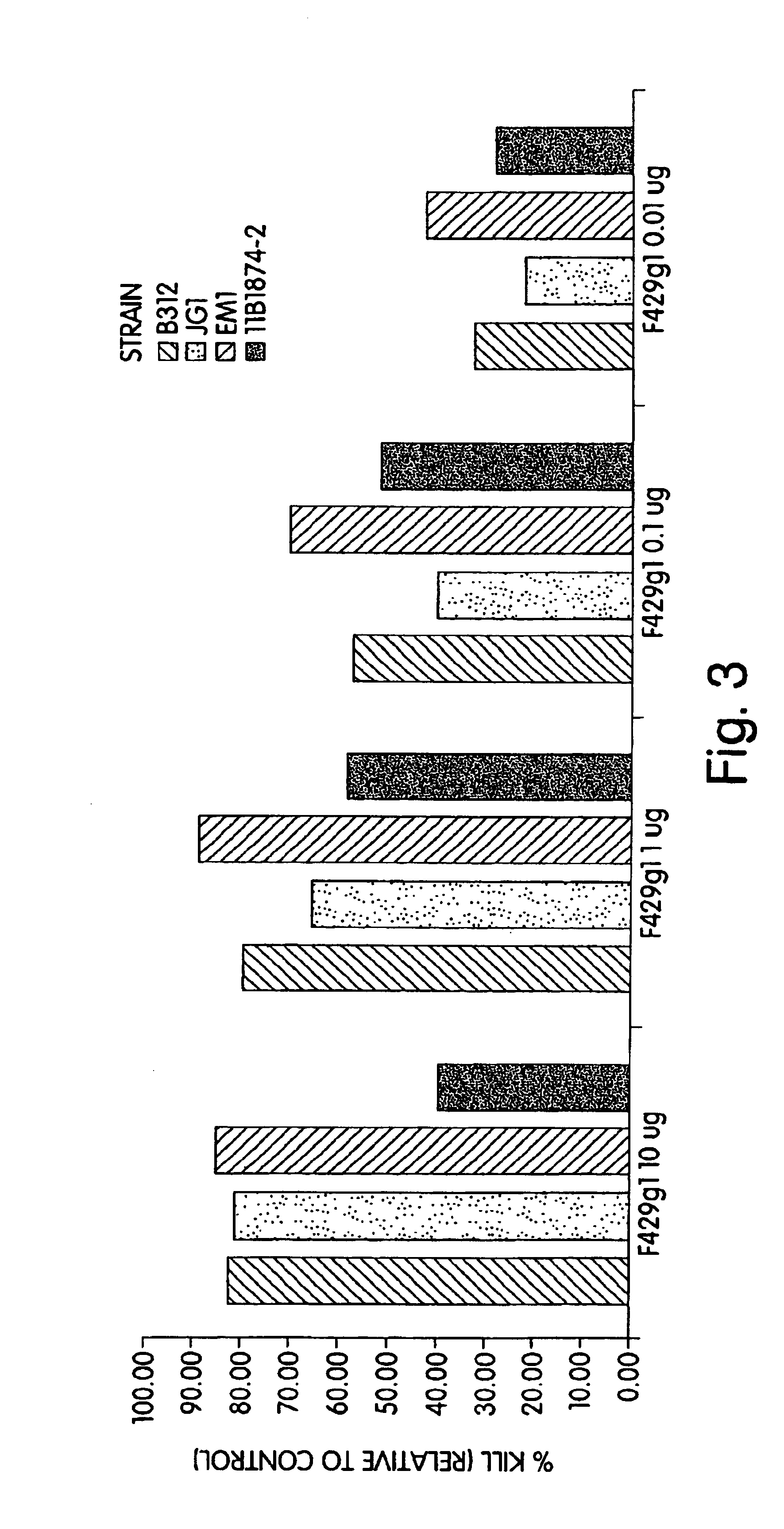
P. aeruginosa mucoid exopolysaccharide specific binding peptides
InactiveUS6962813B2Enhance opsonization and phagocytosisEnhance cytocidal effectAnimal cellsImmunoglobulins against bacteriaDiseaseMonoclonal antibody
The present invention relates to peptides, particularly human monoclonal antibodies, that bind specifically to P. aeruginosa mucoid exopolysaccharide. The invention further provides methods for using these peptides in the diagnosis, prophylaxis and therapy of P. aeruginosa infection and related disorders (e.g., cystic fibrosis). Some antibodies of the invention enhance opsonophagocytic killing of multiple mucoid strains of P. aeruginosa. Compositions of these peptides, including pharmaceutical compositions, are also provided, as are functionally equivalent variants of such peptides.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Modification of airways by application of energy
InactiveUS20070100390A1Reduce plugging of the airwayPrevent the airway from being able to constrictElectrotherapySurgical needlesPatient complianceObstructive Pulmonary Diseases
This relates to methods and devices for treating reversible chronic obstructive pulmonary disease, and more particularly, relates to a device for exchanging energy with airway tissue such as that found in the airway of human lungs. The exchange of energy with this airway tissue in the airways reduces the ability of the air ways to constrict and / or reduces the resistance within the airway to the flow of air through the airway. This also relates to a method for decreasing responsiveness or decreasing resistance to airflow of airways involves the transfer of energy to or from the airway walls to prevent or reduce airway constriction and other symptoms of lung diseases. The treatment reduces the ability of the airway Lo contract during an acute narrowing of the airways, reduces mucus plugging of the airways, and / or increases the airway diameter. The methods according to the present invention provide a longer duration and / or more effective treatment for lung diseases than currently used drug treatments, and obviate patient compliance issues. This also includes additional steps that reduce the ability of the lung to produce at least one of the symptoms of reversible obstructive pulmonary disease and to reduce the resistance to the flow of air through a lung.
Owner:BOSTON SCI SCIMED INC
Nanocrystals, compositions, and methods that aid particle transport in mucus
ActiveUS20130323179A1Lower aqueous solubilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryNanocrystalAqueous solubility
Nanocrystals, compositions, and methods that aid particle transport in mucus are provided. In some embodiments, the compositions and methods involve making mucus-penetrating particles (MPP) without any polymeric carriers, or with minimal use of polymeric carriers. The compositions and methods may include, in some embodiments, modifying the surface coatings of particles formed of pharmaceutical agents that have a low water solubility. Such methods and compositions can be used to achieve efficient transport of particles of pharmaceutical agents though mucus barriers in the body for a wide spectrum of applications, including drug delivery, imaging, and diagnostic applications. In certain embodiments, a pharmaceutical composition including such particles is well-suited for administration routes involving the particles passing through a mucosal barrier.
Owner:ALCON INC +1
Particles, compositions and methods for ophthalmic and/or other applications
Particles, compositions, and methods that aid particle transport in mucus are provided. The particles, compositions, and methods may be used, in some instances, for ophthalmic and / or other applications. In some embodiments, the compositions and methods may involve modifying the surface coatings of particles, such as particles of pharmaceutical agents that have a low aqueous solubility. Such compositions and methods can be used to achieve efficient transport of particles of pharmaceutical agents though mucus barriers in the body for a wide spectrum of applications, including drug delivery, imaging, and diagnostic applications. In certain embodiments, a pharmaceutical composition including such particles is well-suited for ophthalmic applications, and may be used for delivering pharmaceutical agents to the front of the eye and / or the back of the eye.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Method for promoting tissue regeneration on wound surfaces as device and treatment instrument or implant for carrying out method
InactiveUS20060122543A1Small lossDental implantsUltrasound therapyConnective tissue fiberMucous cell
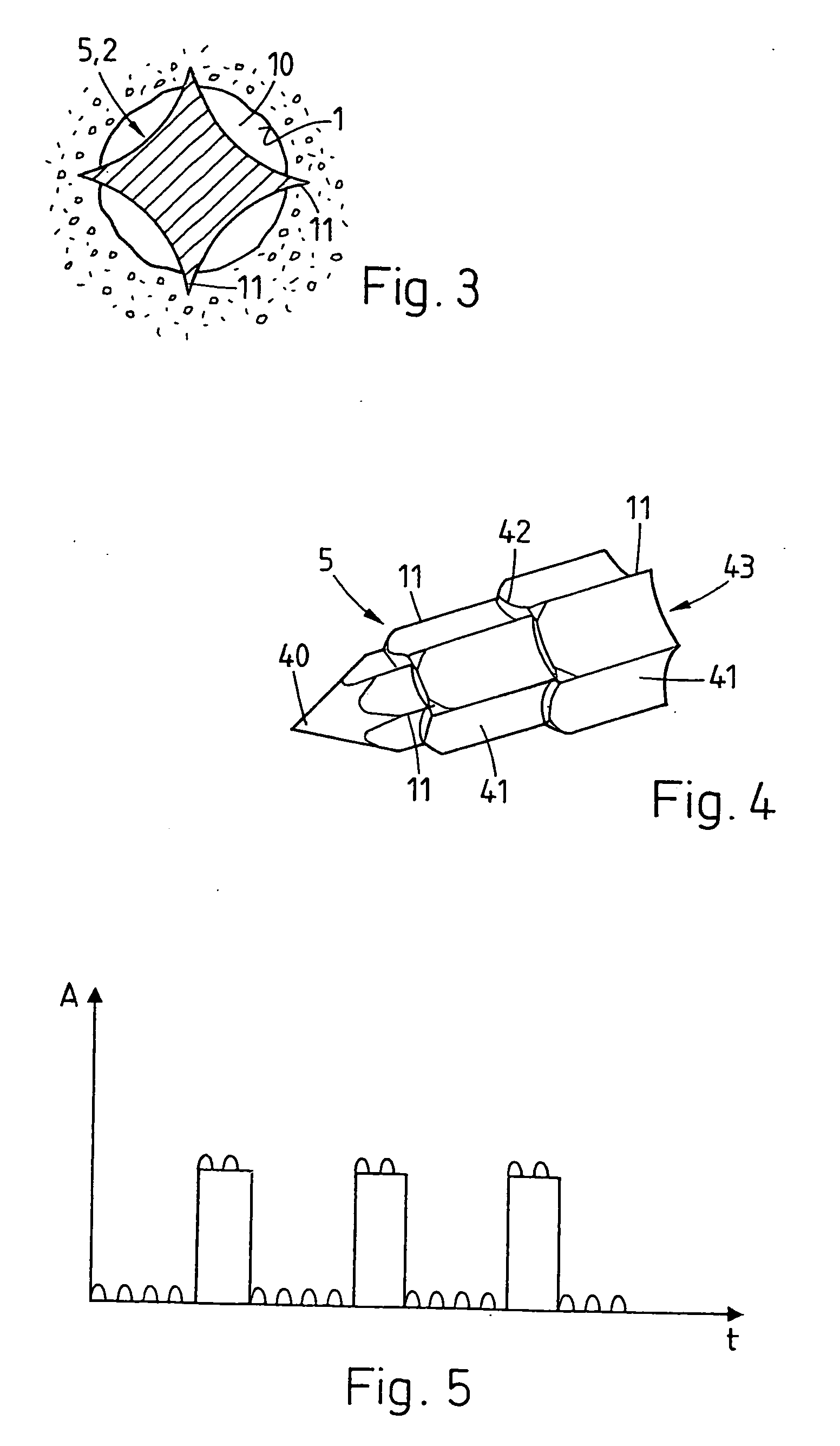
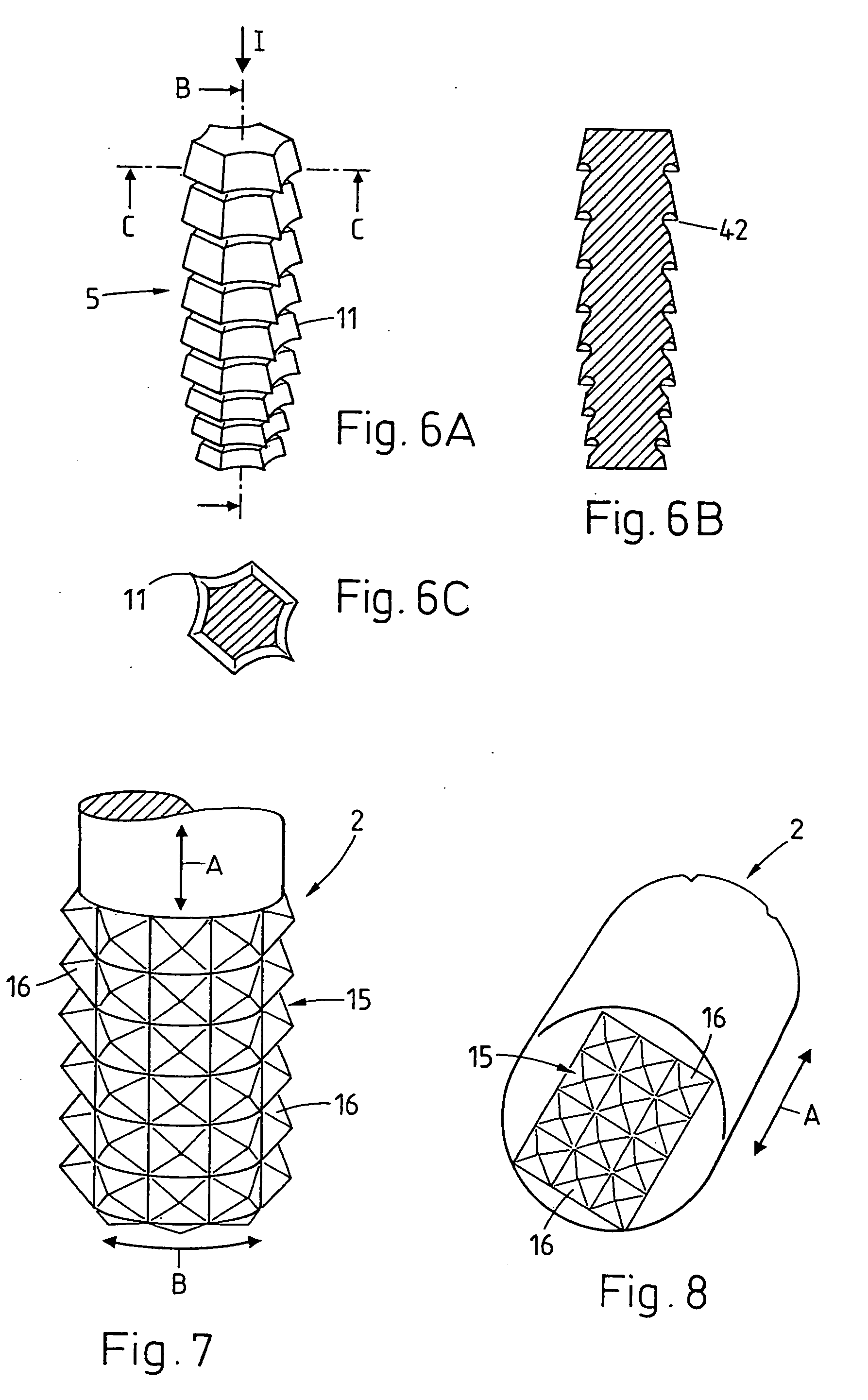
For promoting tissue regeneration on wound surfaces (1) mechanical oscillation is coupled into the wound surfaces. A treatment instrument (2) coupled to an oscillation drive is brought into contact with the wound surface (1), or an implant is impinged with oscillation during and / or after being positioned in the tissue. The oscillation acts mechanically and thermally on the tissue in the region of the treated wound surface (1), and according to the intensity acts in a stimulating, traumatic, necrotic or cell-destroying manner. Therefore, biological elements inhibiting tissue regeneration are destroyed or denatured and the metabolism in the region of the wound surface is stimulated. The effect may also be a mechanical one, slightly compacting or regionally dislocating the tissue. Since the treatment can be effected during or after positioning an implant, necrosis in particular effects undesired cells, such as connective tissue cells, mucous cells and diseased cells having been brought to the wound surface with the implant, which cells may inhibit the intergrowth between tissue and implant.
Owner:WOODWELDING
Post nasal drip treatment
ActiveUS9415194B2Absolute reductionAmeliorate PNDS and/or UACS symptomsUltrasound therapyBalloon catheterNasal cavityNostril
Systems and methods for treating a patient's mucus hypersecretion condition are disclosed herein. Certain implementations may involve a method for reducing mucus secretion in an upper airway of a patient to treat at least one of post nasal drip or chronic cough. The method may include advancing a treatment delivery portion of an energy-based treatment device into a nostril of the patient. The treatment delivery portion may contact mucosal tissue of the upper airway without piercing the mucosal tissue. The treatment delivery portion may deliver treatment to at least one tissue selected from the group of the mucosal tissue and another tissue underlying the mucosal tissue to modify a property of the at least one tissue and thus treat at least one of post nasal drip or chronic cough in the patient.
Owner:AERIN MEDICAL
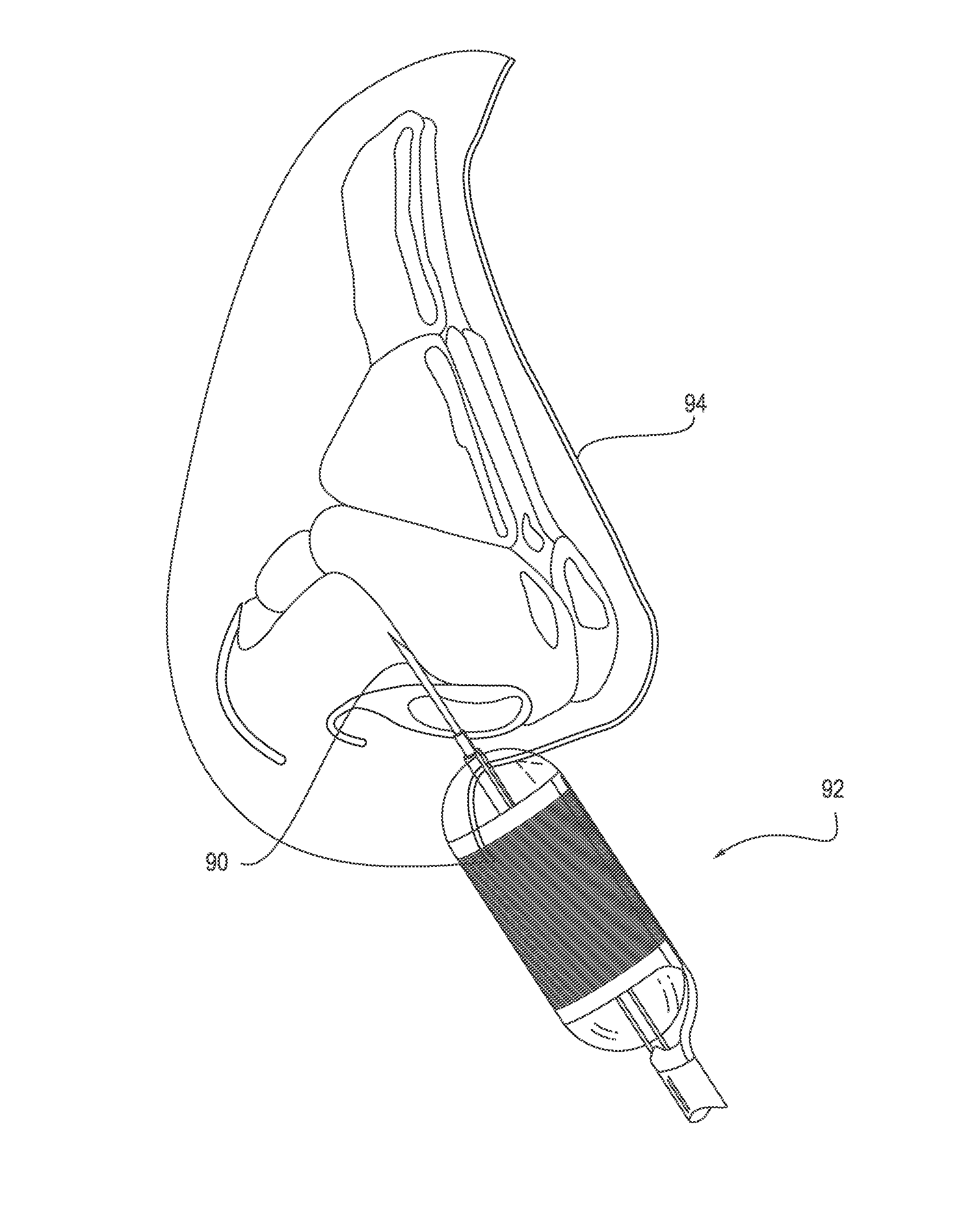
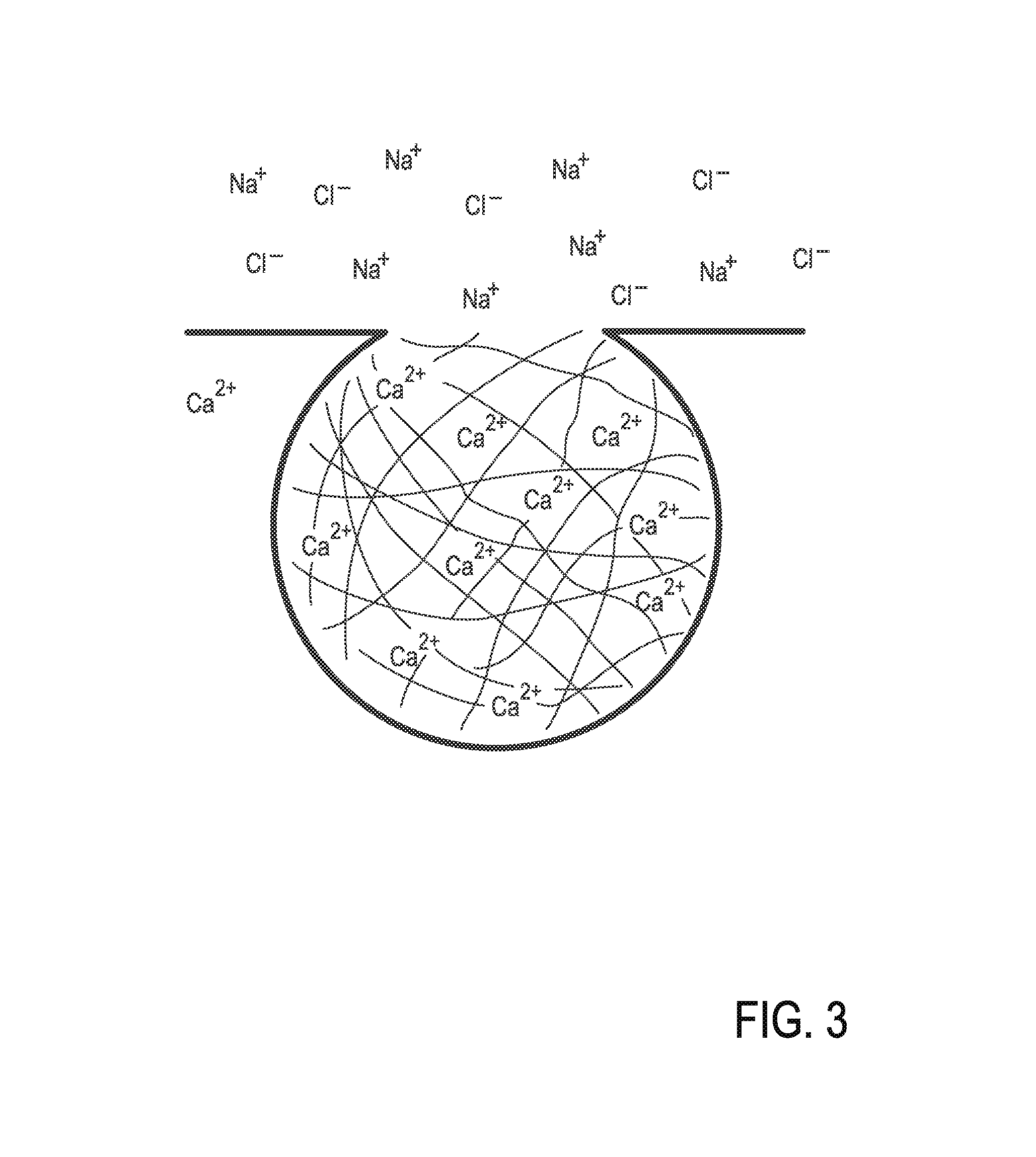
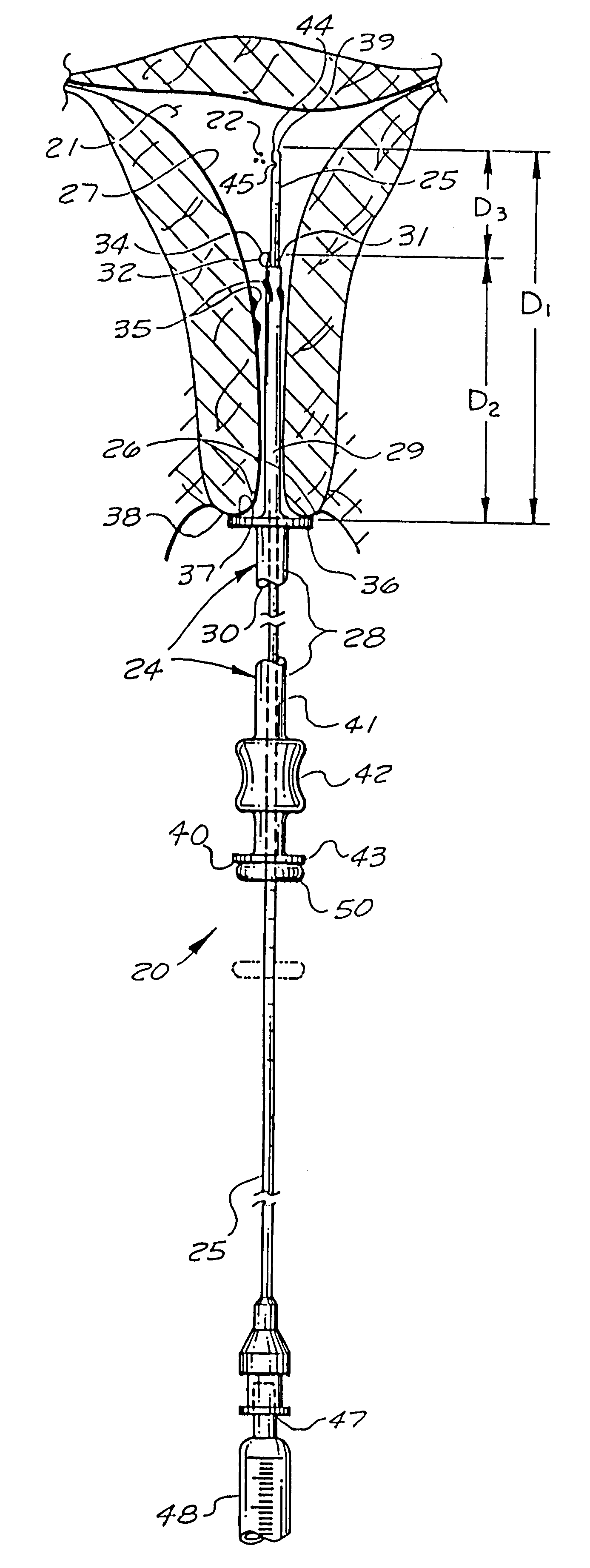
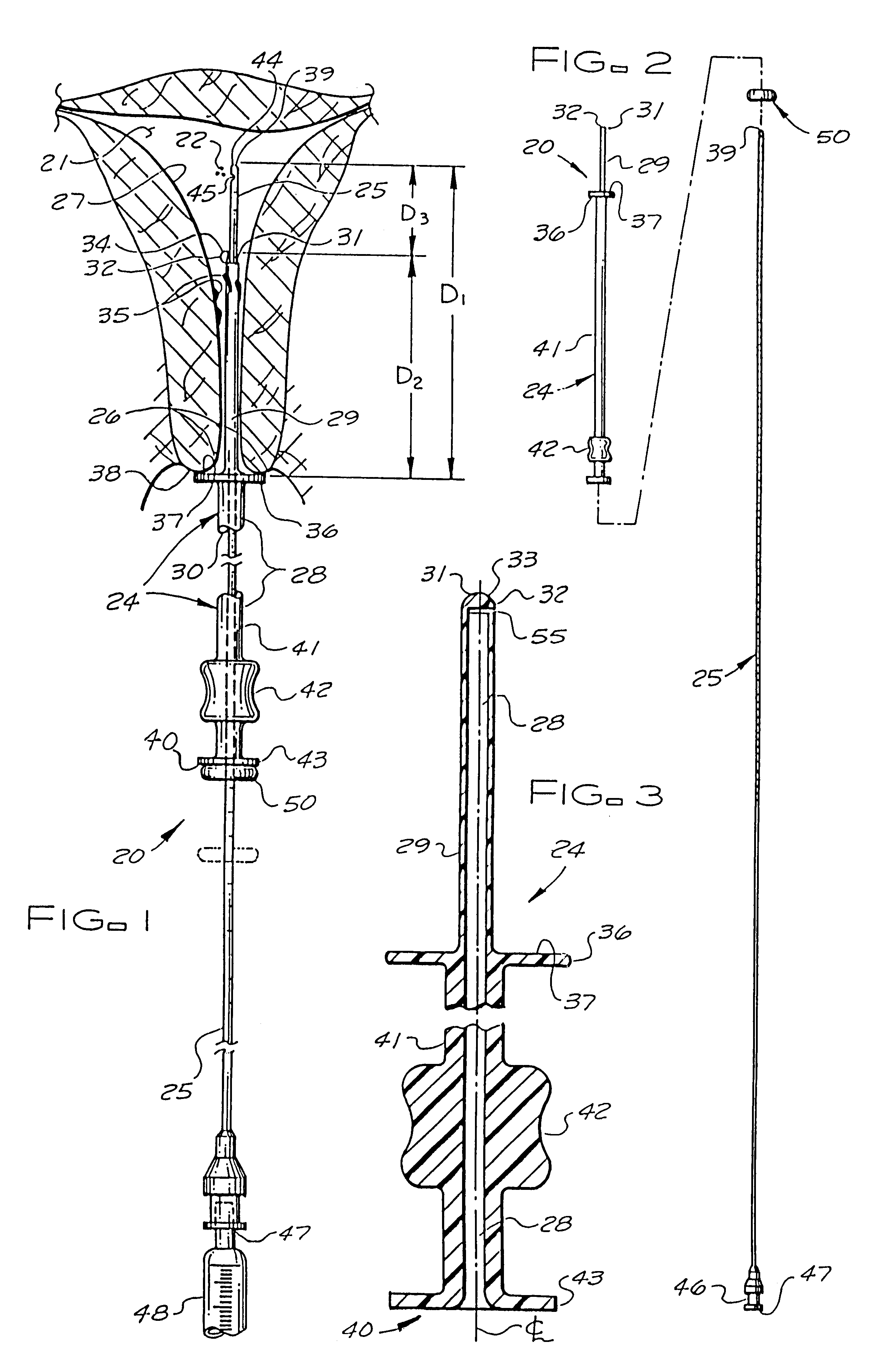
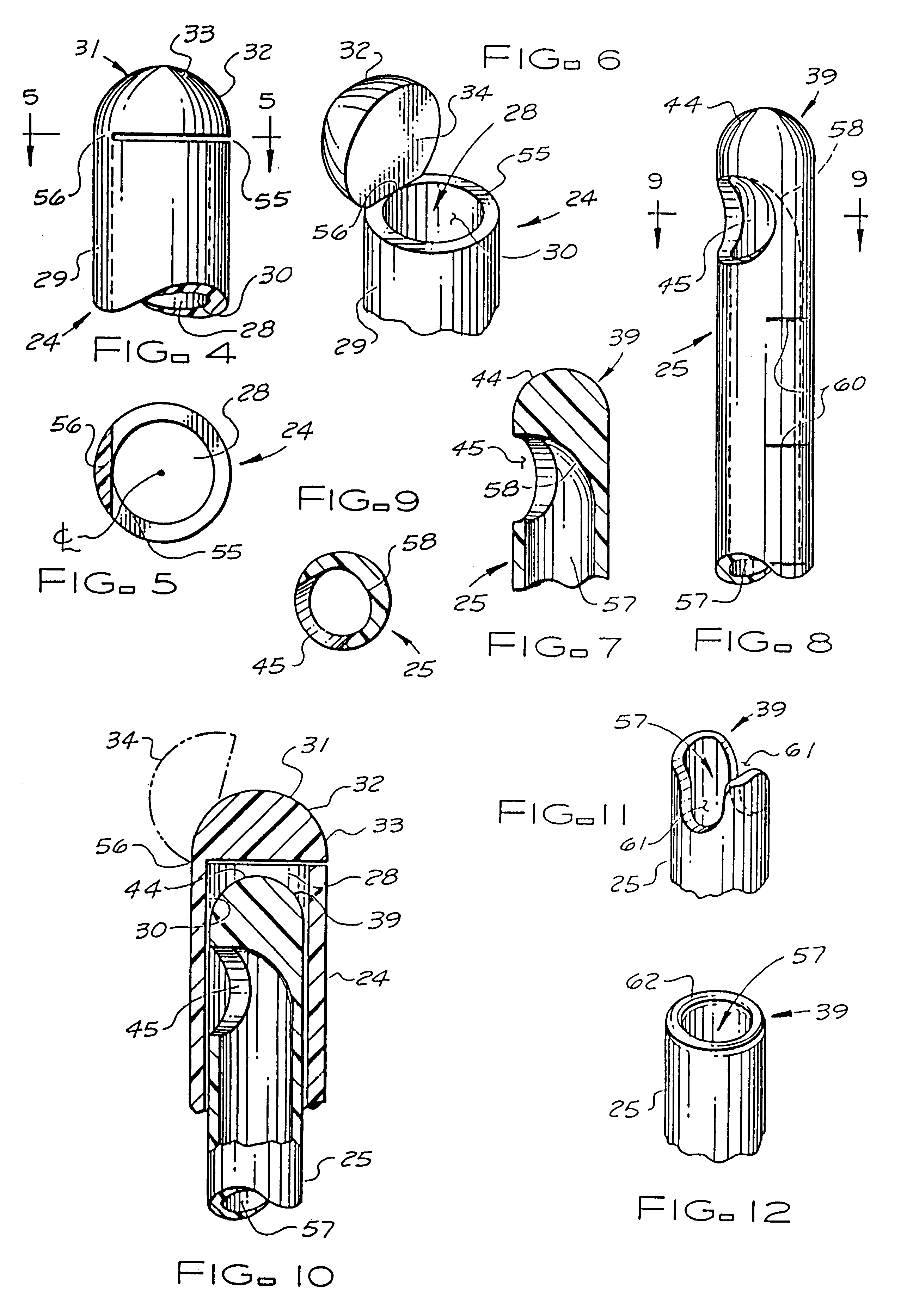
Catheter system for implanting embryos
Described is a catheter system for implanting embryos into a woman's uterus. The catheter system utilizes a protective catheter sleeve for introducing a catheter into the uterus without mucus contamination of an inner catheter. Once the sleeve containing the inner catheter is introduced into the uterus, the protected inner catheter, carrying the embryos, is pushed through a swivelable distal end cap on the sleeve to a desired implanting location. The distal end of the inner catheter has a protective cap and a side opening for embryo release. Also, stiffness and indicia features of the outer sleeve and inner catheter assist in the physician's handling of the catheter system and in ensuring a desired uterus location for implanting.
Owner:TAO JUN
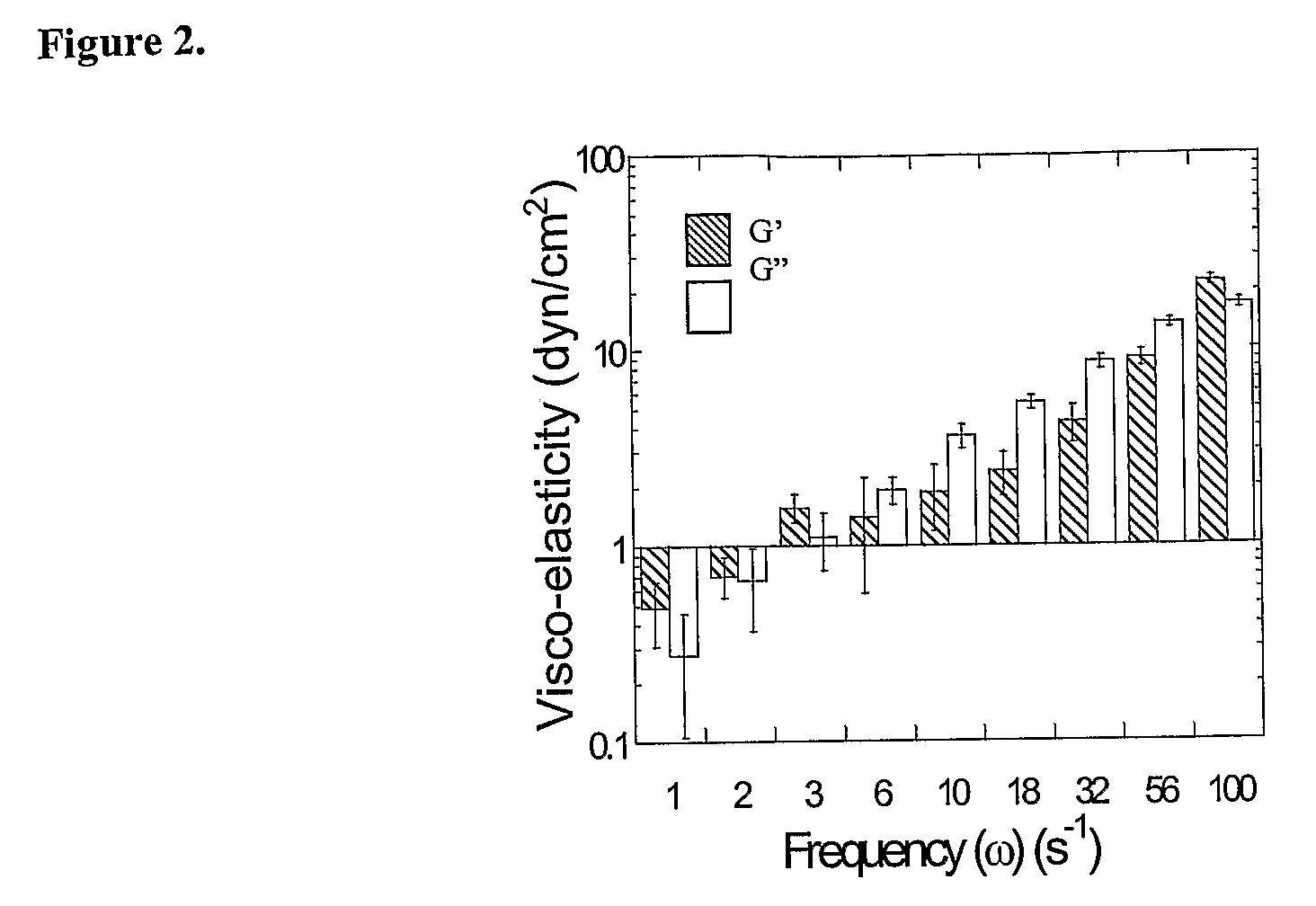
Use of glycosaminoglycans degrading enzymes for management of airway associated diseases
InactiveUS6153187AIncrease the gapReduce frequencyPowder deliveryPeptide/protein ingredientsDiseaseMedicine
A method of managing a patient having an accumulation of mucoid, mucopurulent or purulent material containing glycosaminoglycans, the method comprising the step of administering at least one glycosaminoglycans degrading enzyme to the patient in an amount therapeutically effective to reduce at least one of the following: the visco-elasticity of the material, pathogens infectivity and inflammation. An article of manufacture comprising an inhaler including, as an active ingredient, at least one glycosaminoglycans degrading enzyme for generating aerosols including the enzyme for management a patient having an accumulation of mucoid, mucopurulent or purulent material containing glycosaminoglycans.
Owner:INSIGHT BIOPHARMLS
Self-cleaning and sterilizing endotracheal and tracheostomy tube
ActiveUS20080257355A1Trend downMaintain airwayTracheal tubesPhysical/chemical process catalystsEndotracheal tubeGuide tube
The self-cleaning and sterilizing endotracheal and tracheostomy tube may include a combination of an endotracheal tube or a tracheostomy tube and a suction catheter that decreases the tendency of mucus and bacteria to adhere to the inner surfaces of the thereof. The endotracheal tube and the catheter may have a hydrophobic surface exhibiting the lotus effect, which may be formed either by femtosecond laser etching or by a coating of ploy (ethylene oxide). Alternatively, the endotracheal tube and the catheter may have a lumen coated with a photocatalyst. The endotracheal tube may also have a light source and a fiberoptic bundle mounted thereon, the optical fibers extending into the lumen to illuminate the photocatalyst.
Owner:RAO CHAMKURKISHTIAH P
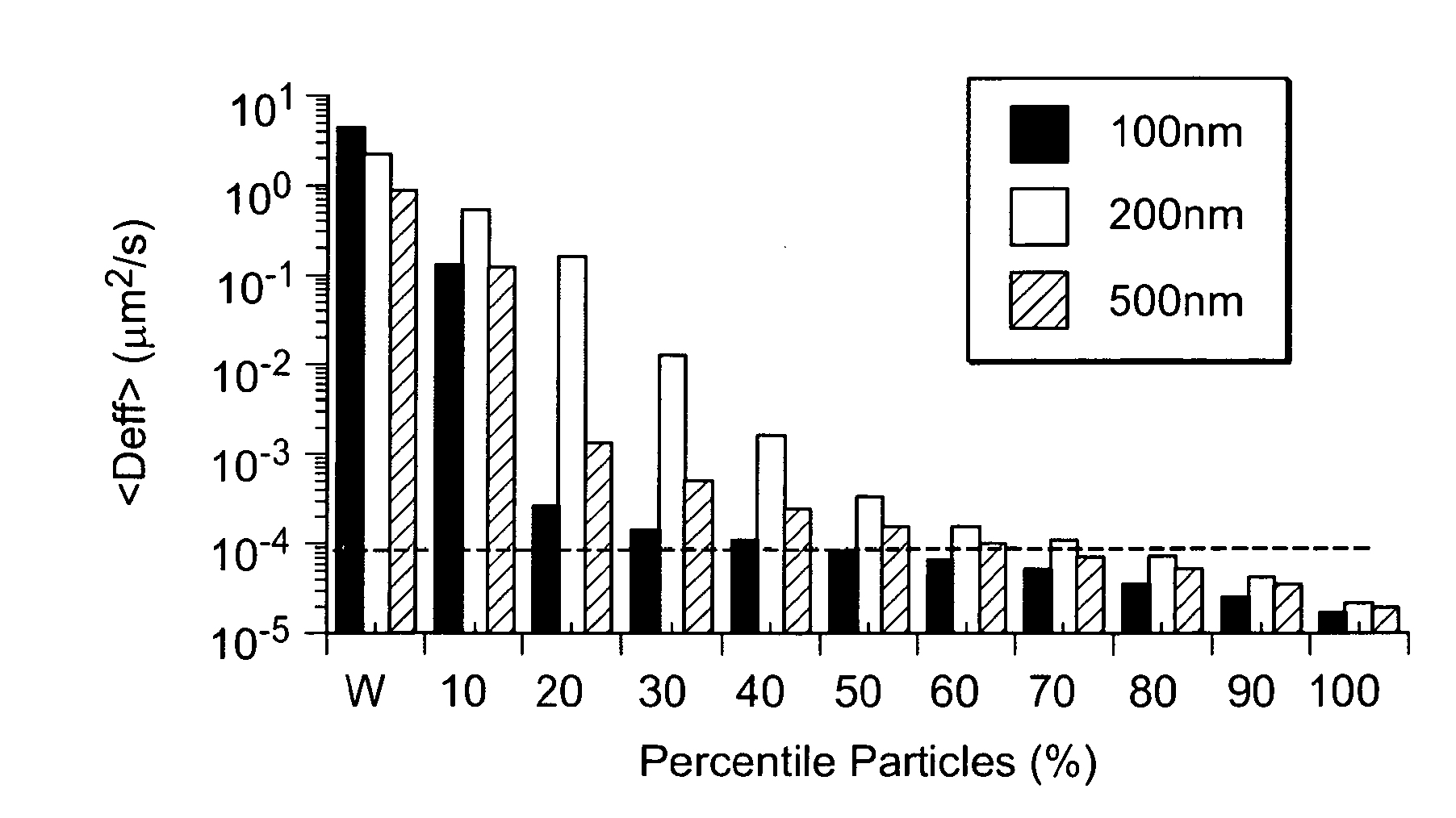
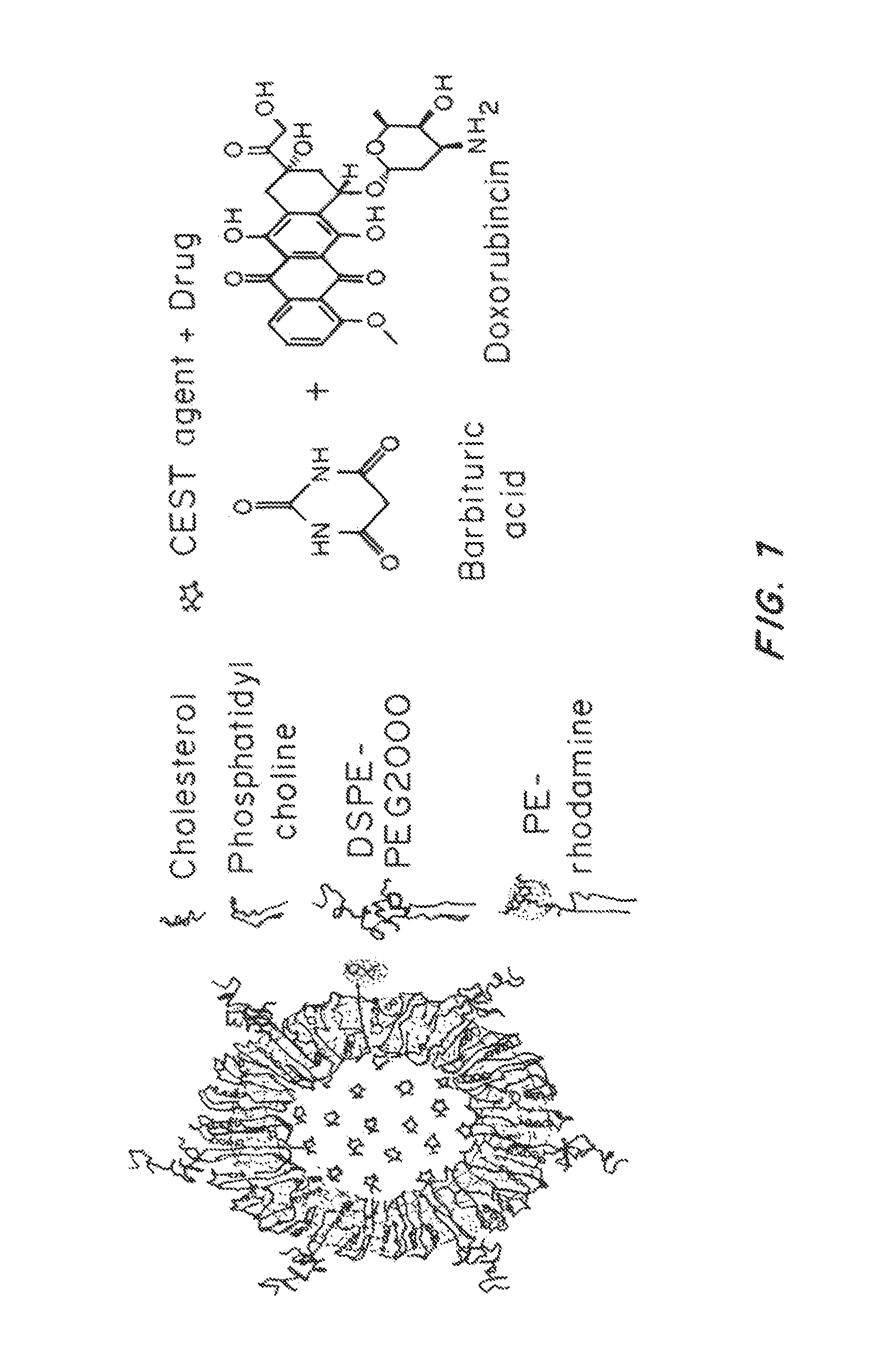
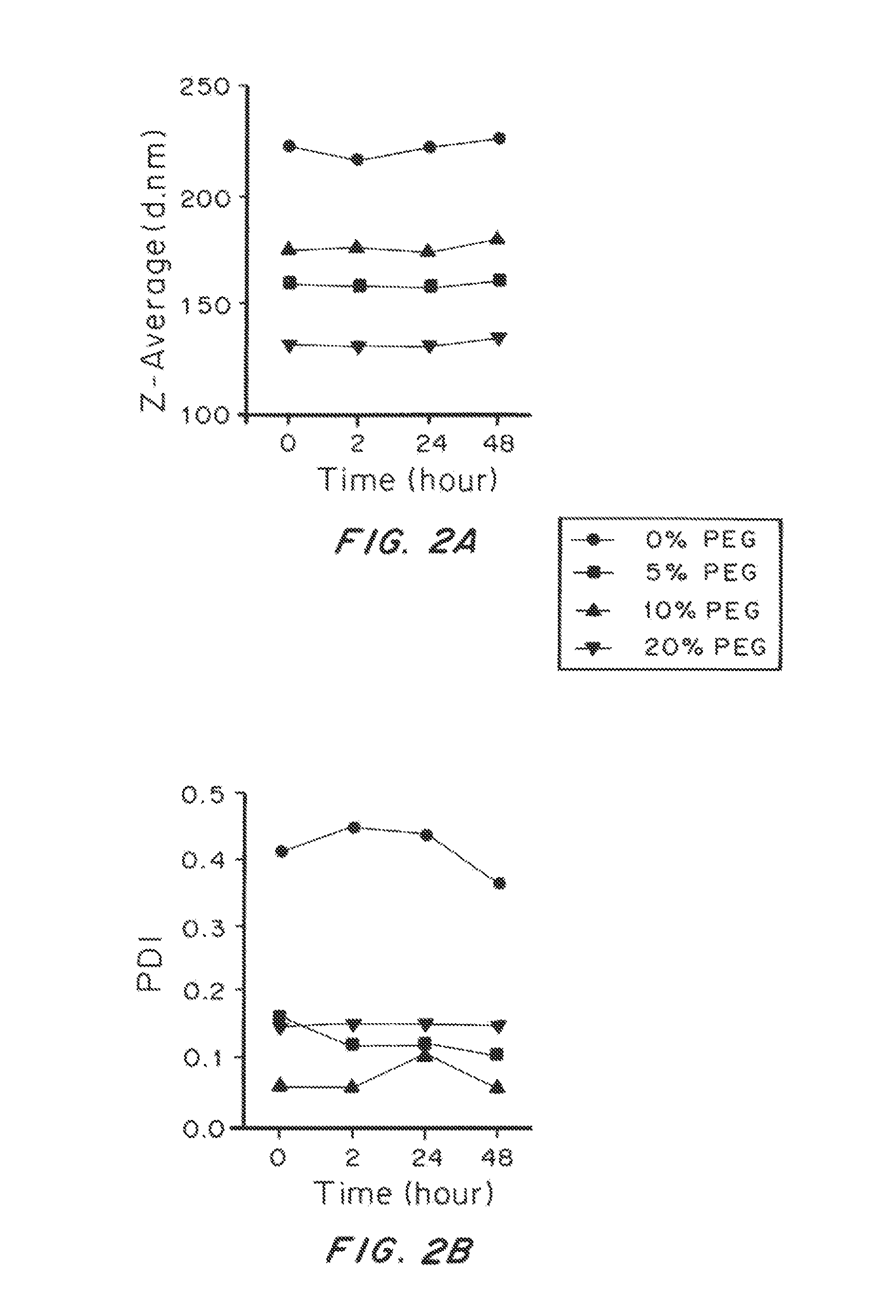
Lipid-Based Drug Carriers for Rapid Penetration Through Mucus Linings
Mucus-penetrating liposomal nanoparticles and methods of making and using thereof are described herein. The nanoparticles contain one or more lipids, one or more PEG-conjugated lipids, and optionally one or more additional materials that physically and / or chemically stabilize the particles. The nanoparticle have an average diameter of about 100 nm to about 300 nm, preferably from about 100 nm to about 250 nm, more preferably from about 100 nm to about 200 nm. The particles are mobile in mucus. The liposomes can further contain one or more therapeutic, prophylactic, and / or diagnostic agent to be delivered to a mucosal surface, such as the CV tract, the colon, the nose, the lungs, and / or the eyes. The liposomes can further contain one or more CEST agents to allow real time imaging of the particles in a live animal. The particles may also further contain an imaging agent, such as a fluorescent label.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
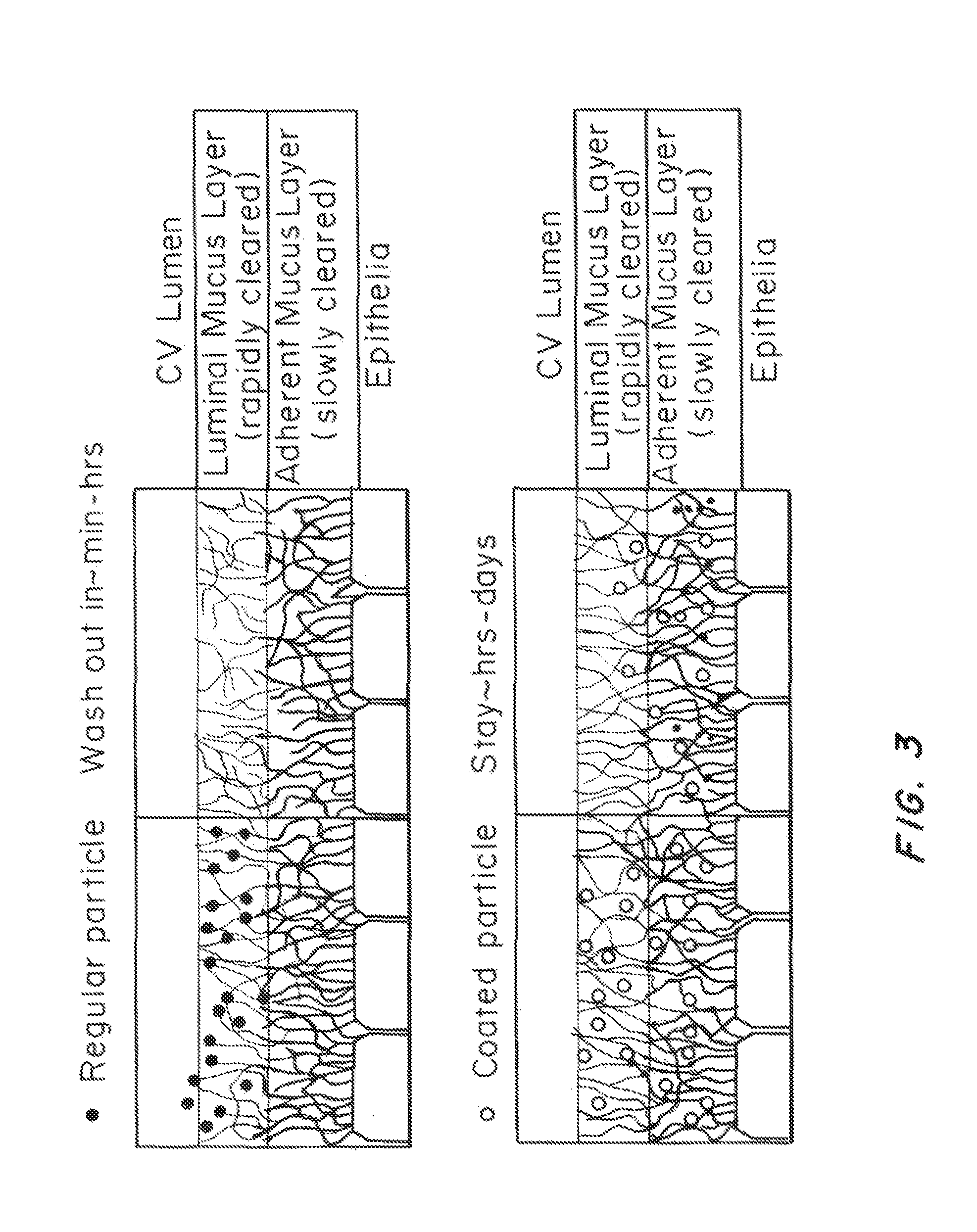
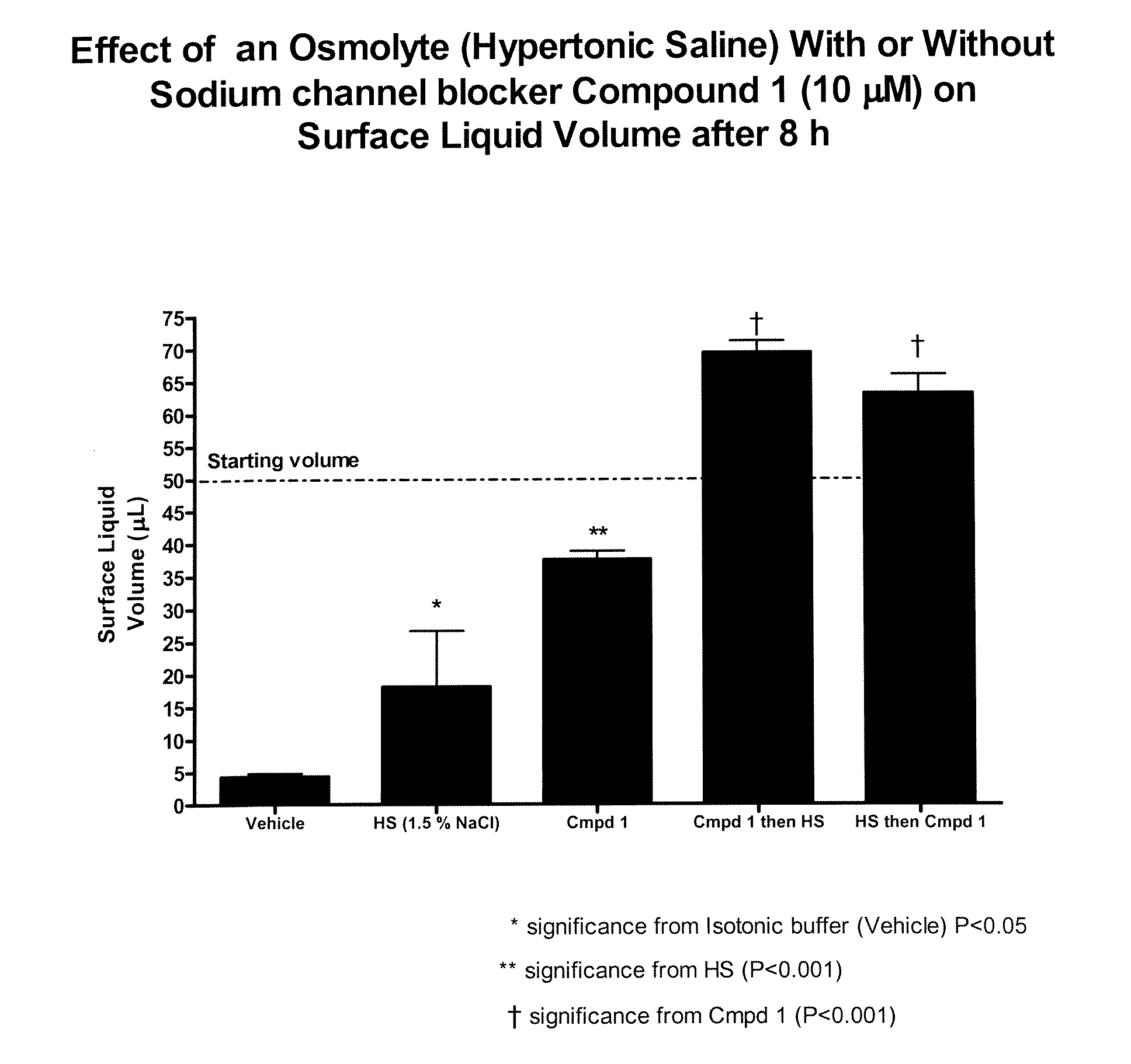
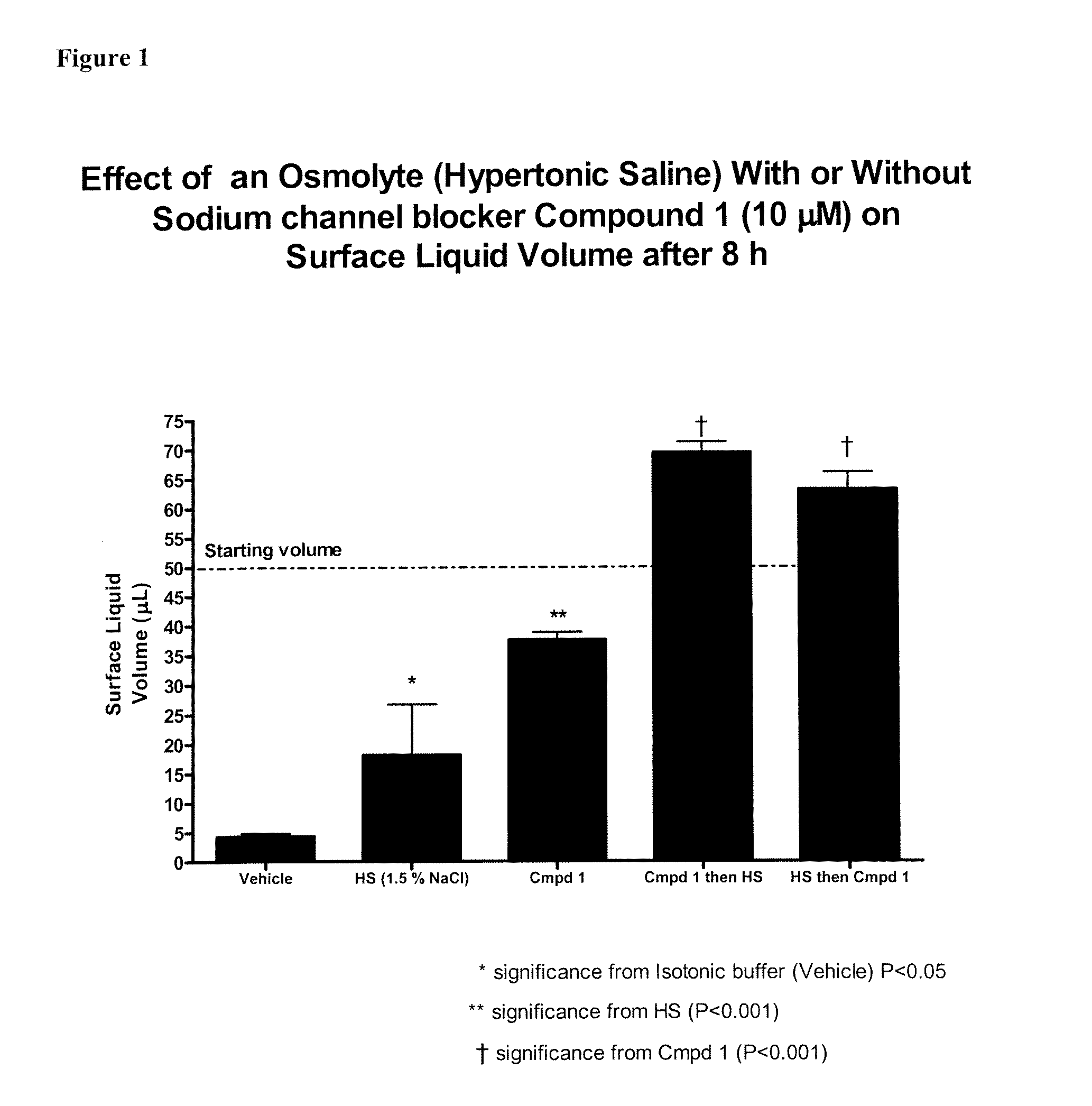
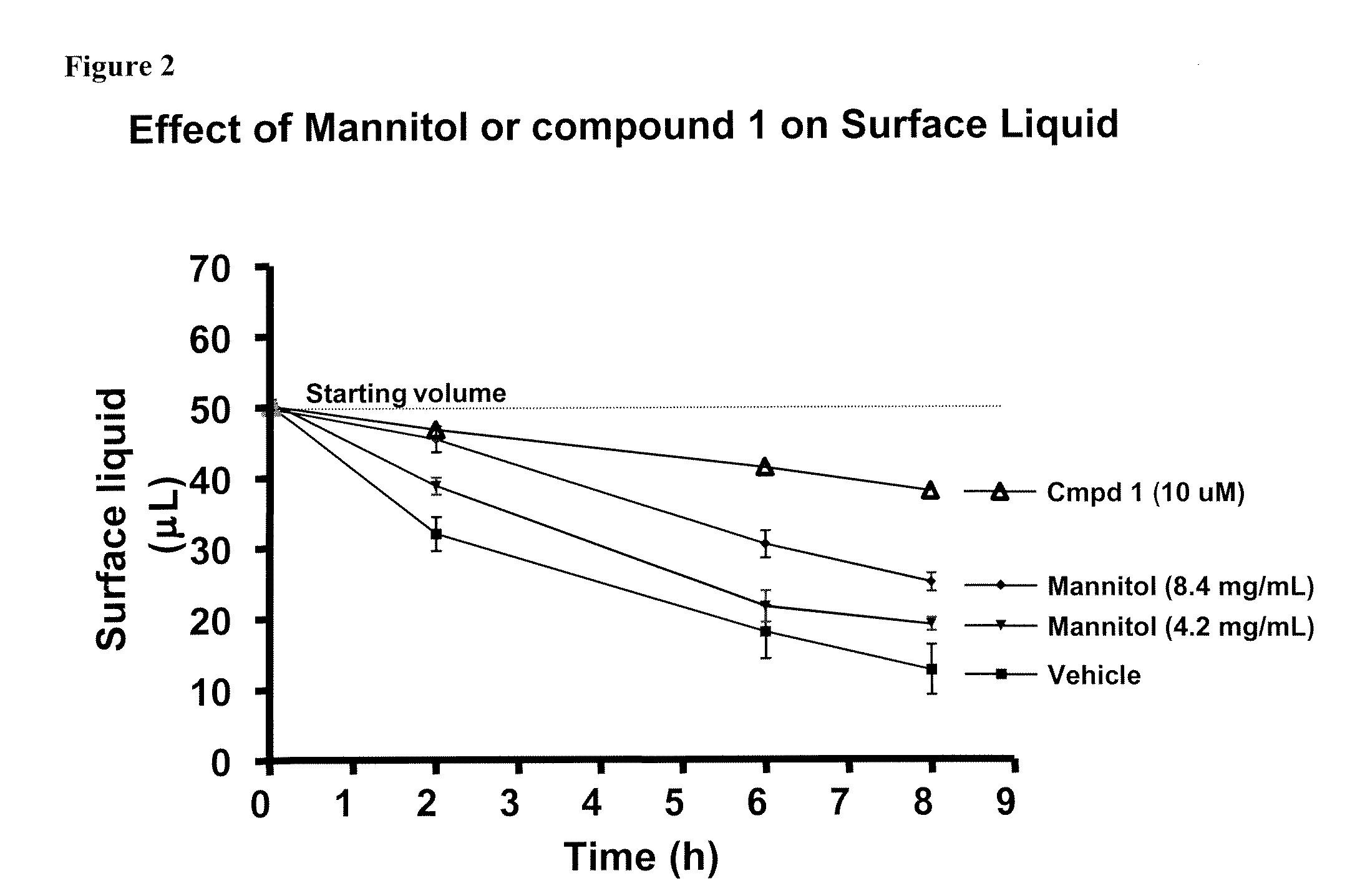
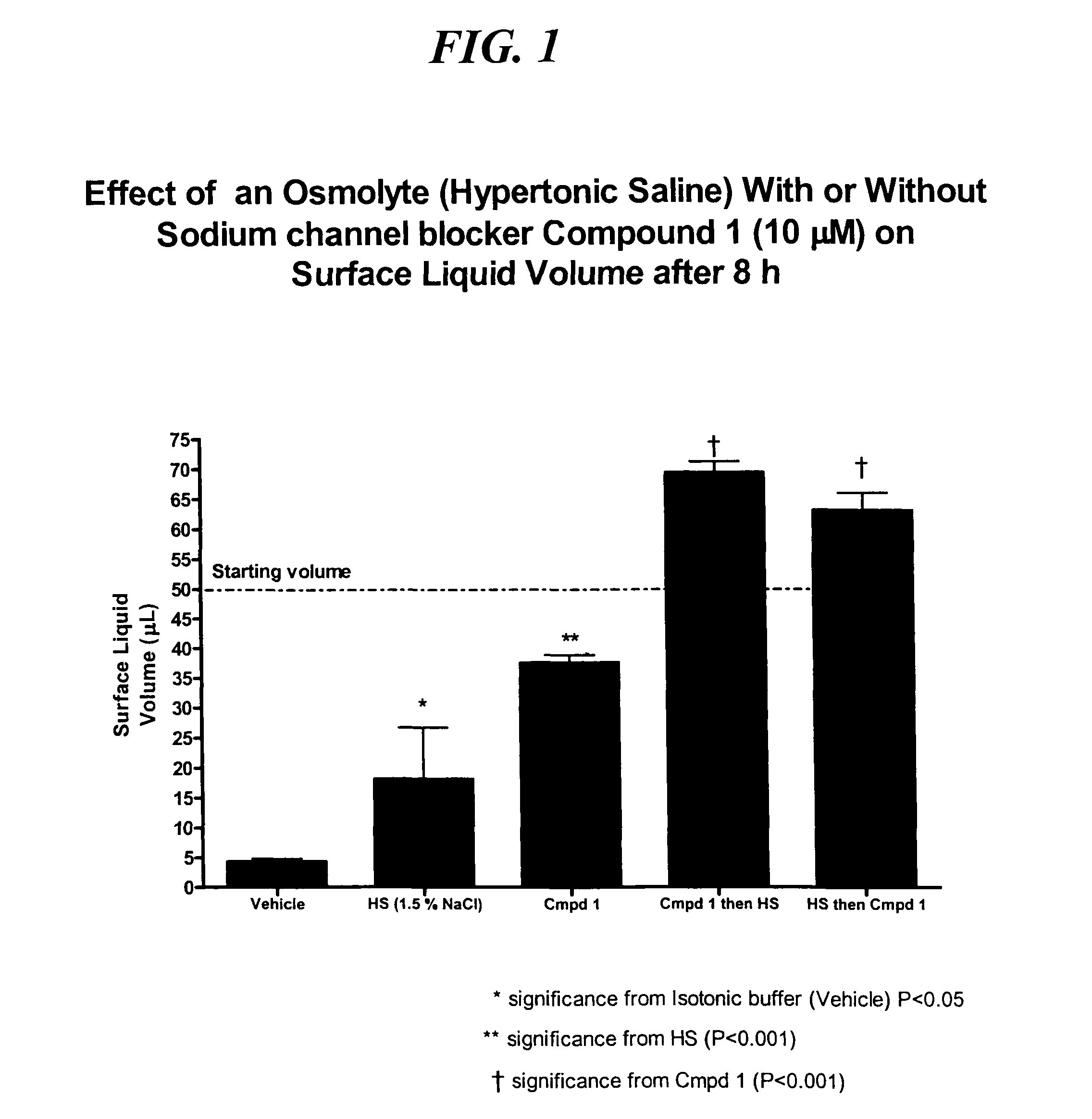
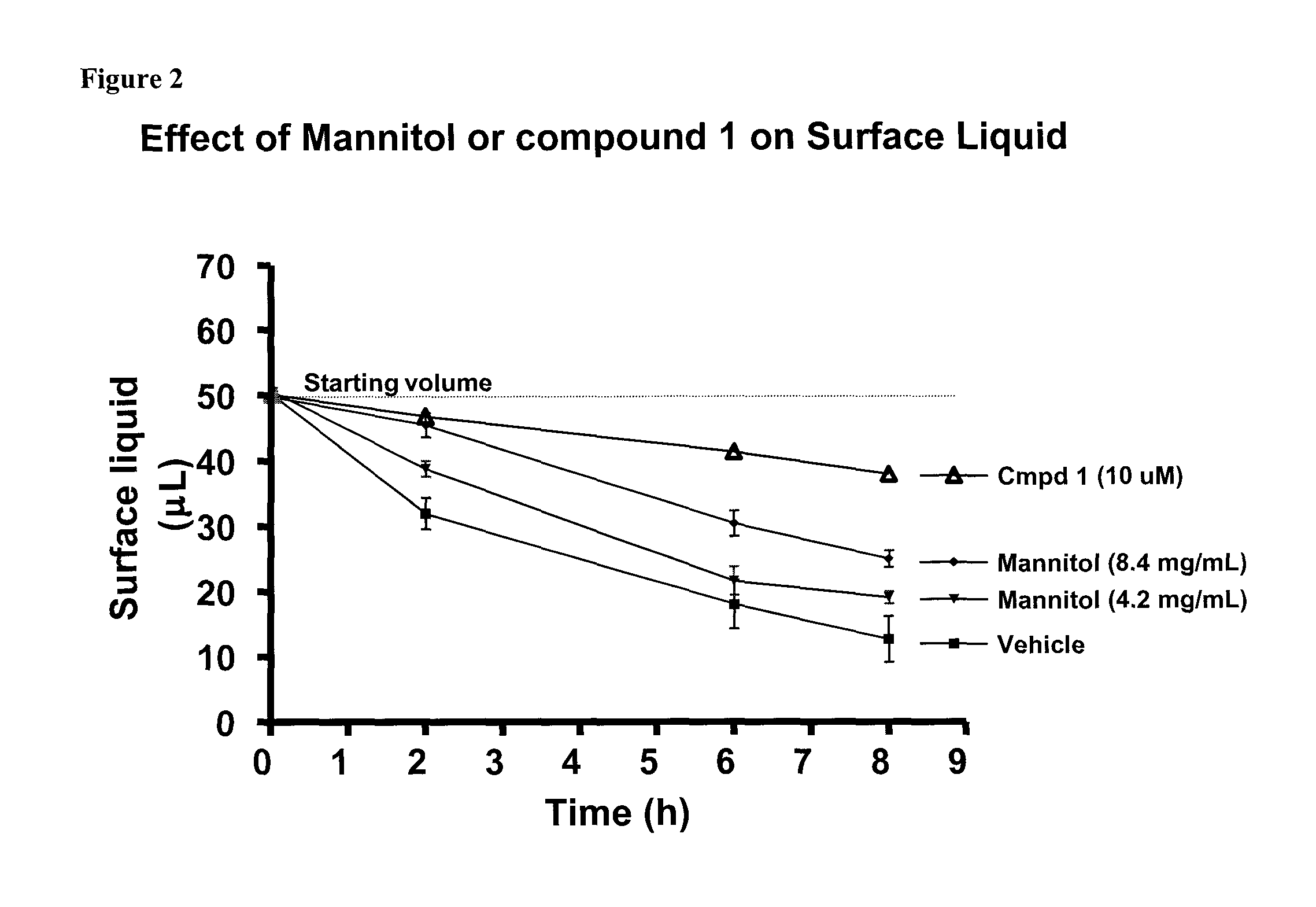
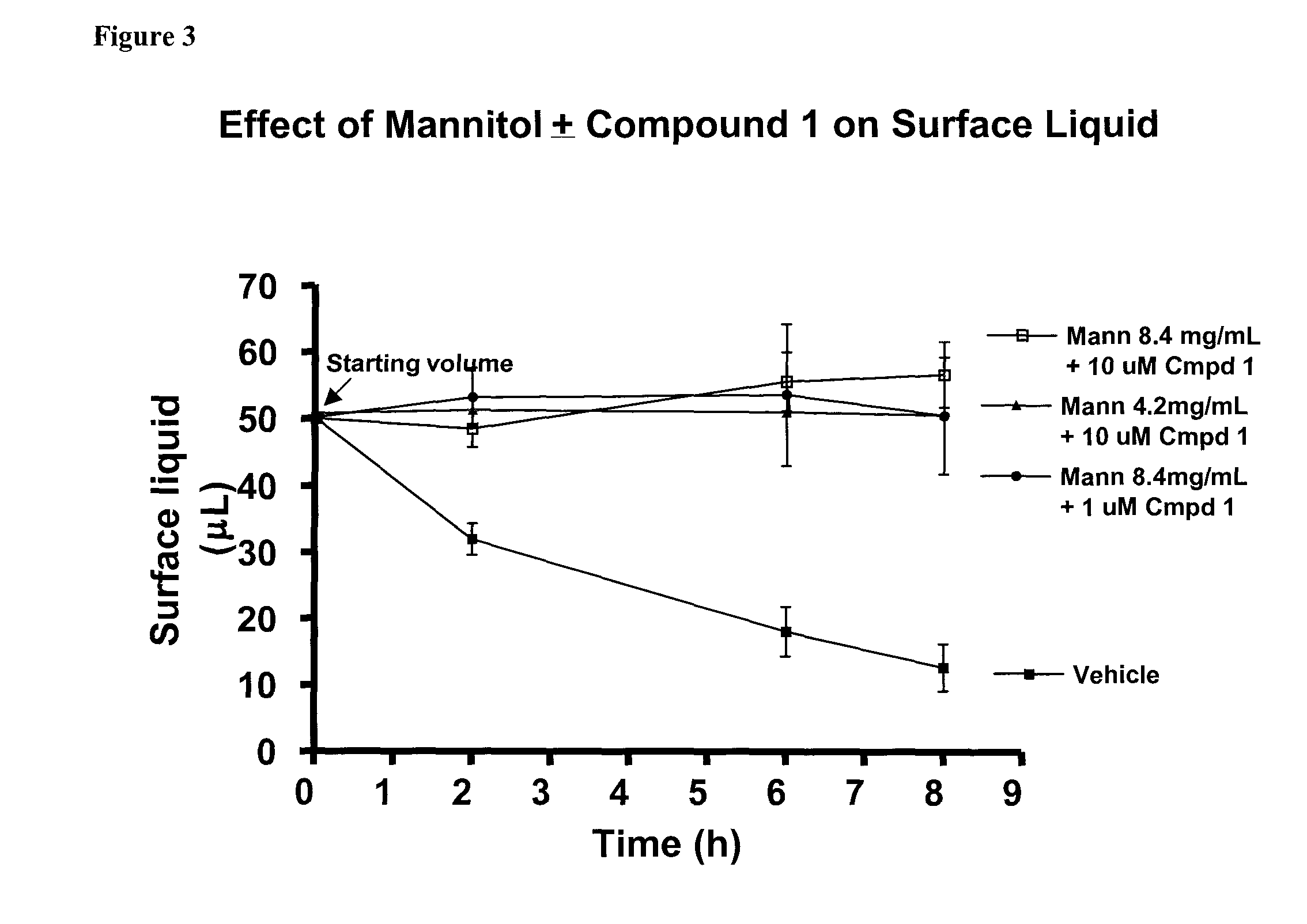
Methods of enhancing mucosal hydration and mucosal clearance by treatment with sodium channel blockers and osmolytes
ActiveUS20080090841A1More specificLess reversibleAntibacterial agentsOrganic active ingredientsOtic AgentsOsmolyte
The present invention relates to methods and compositions for treating diseases ameliorated by increased mucociliary clearance and mucosal hydration by administering an effective amount of a sodium channel blocker as defined herein and an osmolyte to a subject to a subject in need of increased mucociliary clearance and mucosal hydration.
Owner:PARION SCI DURHAM NC
Composition for collecting and preserving placental stem cells and methods of using the composition
ActiveUS20100291679A1Cell culture active agentsNon-embryonic pluripotent stem cellsVascular dilatationBiology
The present invention provides improved compositions and methods for the collection of stem cells from an organ, e.g., placenta. The invention provides a stem cell collection composition comprising an apoptosis inhibitor and, optionally, an enzyme such as a protease or mucolytic enzyme, vasodilator, necrosis inhibitor, oxygen-carrying perfluorocarbon, or an organ preserving compound. The invention provides methods of using the stem cell collection composition to collect stem cells and to preserve populations of stem cells.
Owner:CELULARITY INC
Catheter with snap on feature
An embryo transfer catheter with a protective sheath is disclosed. The use of a protective sheath shields a guide catheter from cervical mucus. The retracted open flaps of the outer protective sheath draw the mucus and blood away from the inner guide catheter. This allows an inner embryo transfer sheath to enter the uterus unobstructed. The embryo is then transferred to the uterus by means of fluid pressure with a culture medium. The protective sheath may be snap fit onto the guide catheter. The sheaths and catheters may be manufactured in a variety of lengths for individual convenience and then snap-fit onto each other for better control during an embryo implantation procedure. Other catheters and related instruments may also be manufactured with snap-on features.
Owner:COOK MEDICAL TECH LLC
High-fidelity shark-imitating anti-drag structure capable of slowly releasing drag reducer instantly and manufacturing method thereof
InactiveCN102381435AEfficient coupling drag reduction effectImprove high-speed maneuverabilityWatercraft hull designHydrodynamic/hydrostatic featuresControl systemCoupling
The invention relates to bionic technical field, and specifically relates to a shark-imitating anti-drag structure and a manufacturing method thereof. The high-fidelity shark-imitating anti-drag structure capable of slowly releasing drag reducer instantly comprises a shark skin-imitating layer, the upper surface of the shark skin-imitating layer is a scutate surface, the lower surface of the shark skin-imitating layer is dug with at least two grooves, the at least two grooves are interconnected with each other, and through holes which interconnects the upper surface with the lower surface areprovided in the grooves; and the shark-imitating anti-drag structure further comprises a slow release electric hydraulic control system. Because the technical solution is used, the high-fidelity shark-imitating anti-drag structure not only possesses a high-fidelity shark-imitating squama groove appearance, but also can imitate instantaneous fluid-secreting system of living shark under high speed maneuvering, for realizing high-efficient coupling drag reduction of squama groove sand self-lubricating mucus.
Owner:SHANDONG UNIV OF TECH
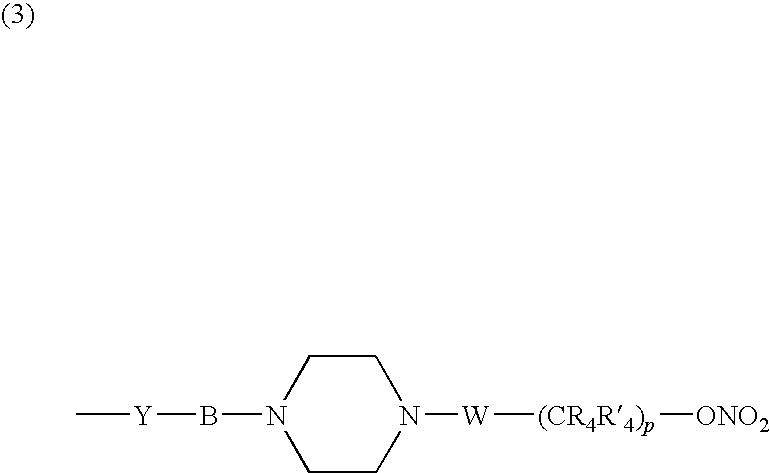
Nitrosated and/or nitrosylated compounds, compositions and methods of use
The invention describes novel nitrosated and / or nitrosylated compounds of the invention and pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitrosated and / or nitrosylated compound of the invention, and, optionally, at least one nitric oxide donor and / or at least one therapeutic agent. The invention also provides novel compositions comprising at least one compound of the invention, and at least one nitric oxide donor and / or at least one therapeutic agent. The invention also provides novel kits comprising at least one compound of the invention, that is optionally nitrosated and / or nitrosylated, and, optionally, at least one nitric oxide donor and / or at least one therapeutic agent. The invention also provides methods for (a) treating bacterial infections; (b) treating viral infections; (c) treating fungal infections; and (d) treating lesions. The nitrosated and / or nitrosylated compounds of the invention are preferably nitrosated and / or nitrosylated antimicrobial compounds, nitrosated and / or nitrosylated adenosine antagonists, nitrosated and / or nitrosylated LTB4 antagonists, nitrosated and / or nitrosylated mucoregulators and nitrosated and / or nitrosylated purine agonists. The methods of the invention are preferably for the treatment of bacterial infections associated with pulmonary diseases such as cystic fibrosis.
Owner:NICOX SA
Compositions and methods for reducing blood and fluid loss from open wounds
InactiveUS7303759B2Provide resistanceProvide protectionPowder deliveryAdhesive dressingsBurned skinPolyvinyl alcohol
The invention described herein relates to methods for reducing and / or stopping bleeding or fluid loss from open wound, denuded tissue, or burned skin, comprising the step of applying to the open wound, denuded tissue or burned skin a gel-forming composition comprising at least one of the following compositions: a polyacrylic acid having the structural formula [CH2═CHCO2H]n, where n is between 10,000 and 70,000; a polyacrylic acid and a desiccated water soluble organic or inorganic base; polyacrylic acid and a desiccated poorly soluble basic salt, and a polyvinyl alcohol having the structural formula of [CH2═CHOH]n, where n is between 15,000 and 150,000. When the gel-forming composition is applied to the open wound, denuded tissue, or burned skin, its ions react therein in the presence of water from blood or body fluid therein to form an aqueous gel or mucilage having sufficient viscosity and adhesiveness to cover and adhere to the open wound, denuded tissue, or burned skin so that bleeding or fluid loss is thereby reduced and / or stopped.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Nanocrystals, compositions, and methods that aid particle transport in mucus
Nanocrystals, compositions, and methods that aid particle transport in mucus are provided. In some embodiments, the compositions and methods involve making mucus-penetrating particles (MPP) without any polymeric carriers, or with minimal use of polymeric carriers. The compositions and methods may include, in some embodiments, modifying the surface coatings of particles formed of pharmaceutical agents that have a low water solubility. Such methods and compositions can be used to achieve efficient transport of particles of pharmaceutical agents though mucus barriers in the body for a wide spectrum of applications, including drug delivery, imaging, and diagnostic applications. In certain embodiments, a pharmaceutical composition including such particles is well-suited for administration routes involving the particles passing through a mucosal barrier.
Owner:ALCON INC +1
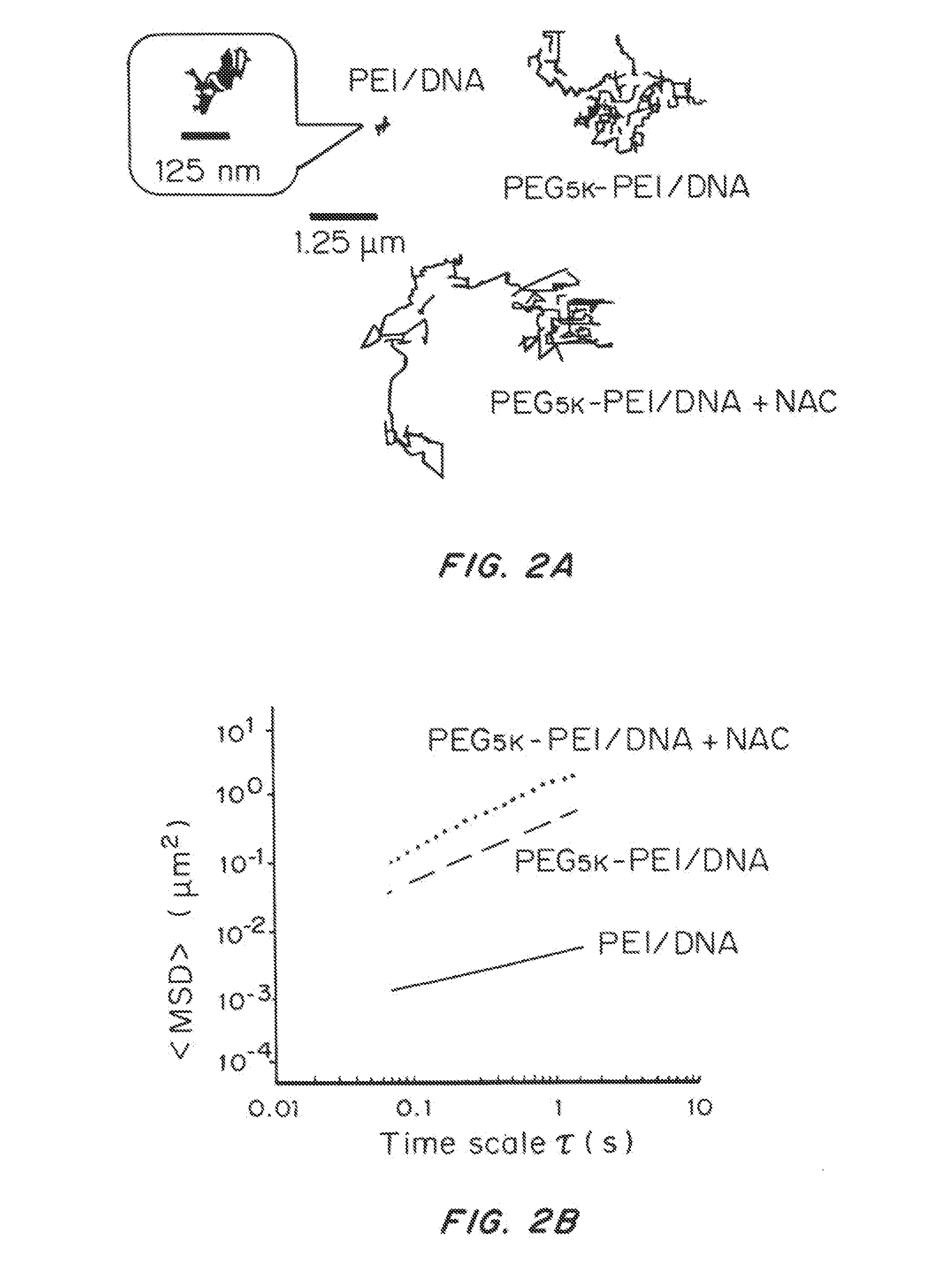
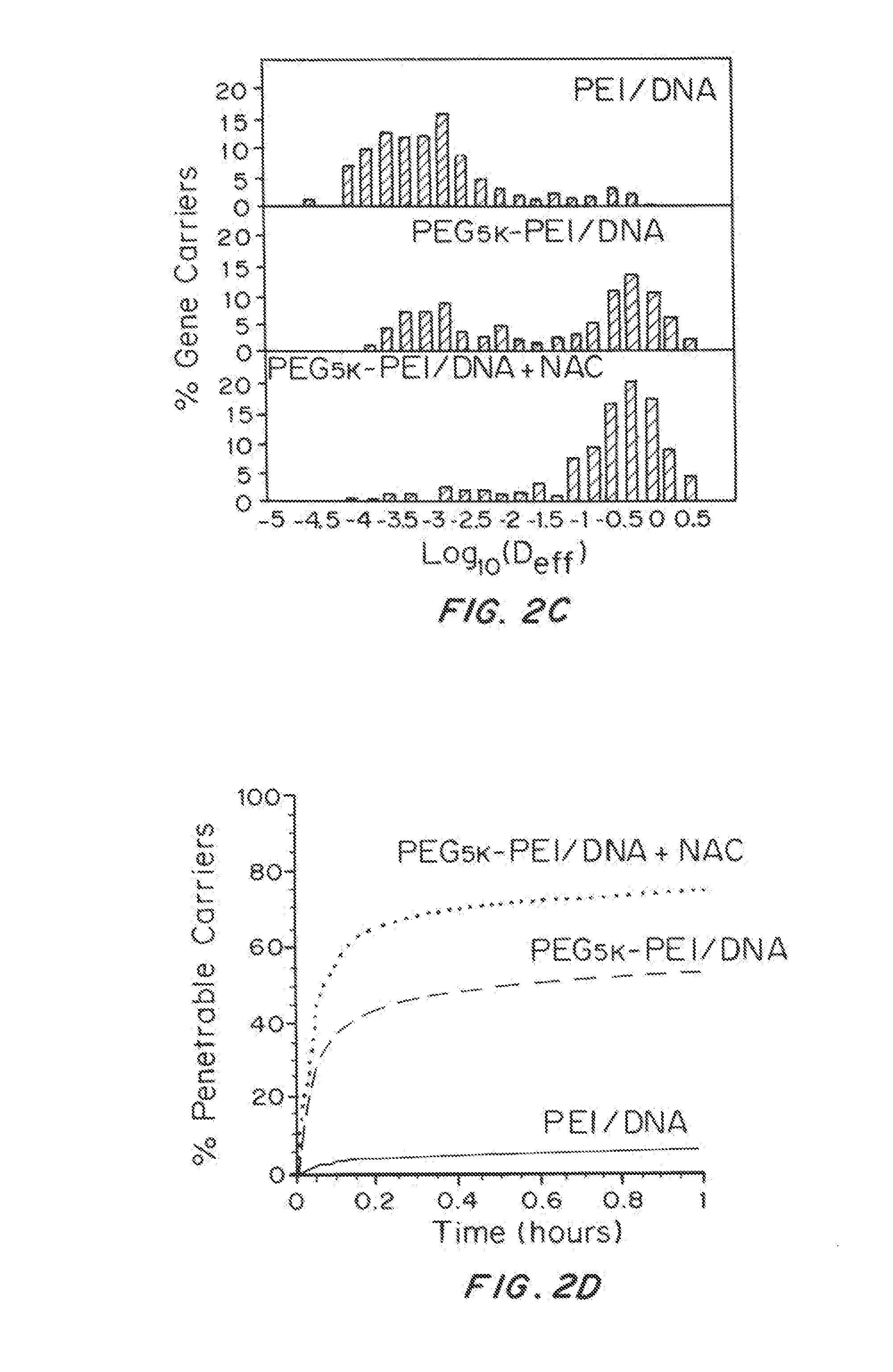
Mucus Penetrating Gene Carriers
ActiveUS20130323313A1Prevent complete digestionPowder deliveryPeptide/protein ingredientsNanoparticleSide chain
Nanoparticles gene carriers, particularly nanoparticle gene carriers which exhibit increased rates of diffusion through cystic fibrosis (CF) mucus, as well as methods of making and using thereof, are described herein. The nanoparticle gene carriers are formed from a nucleic acid complexed to one or more biocompatible, polycationic polymers. The nanoparticle gene carriers also contain one or more mucus resistant polymers. In a particularly preferred embodiment, the nanoparticle gene carrier is a nanoparticle formed from one or more nucleic acids, PEI, and a mucus-resistant / diffusive graft copolymer composed of a PEI backbone functionalized by one or more PEG side chains. The nanoparticle gene carriers can efficiently diffuse through CF mucus, and can effectively serve as a vehicle to administer one or more nucleic acids to a patient suffering from CF.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
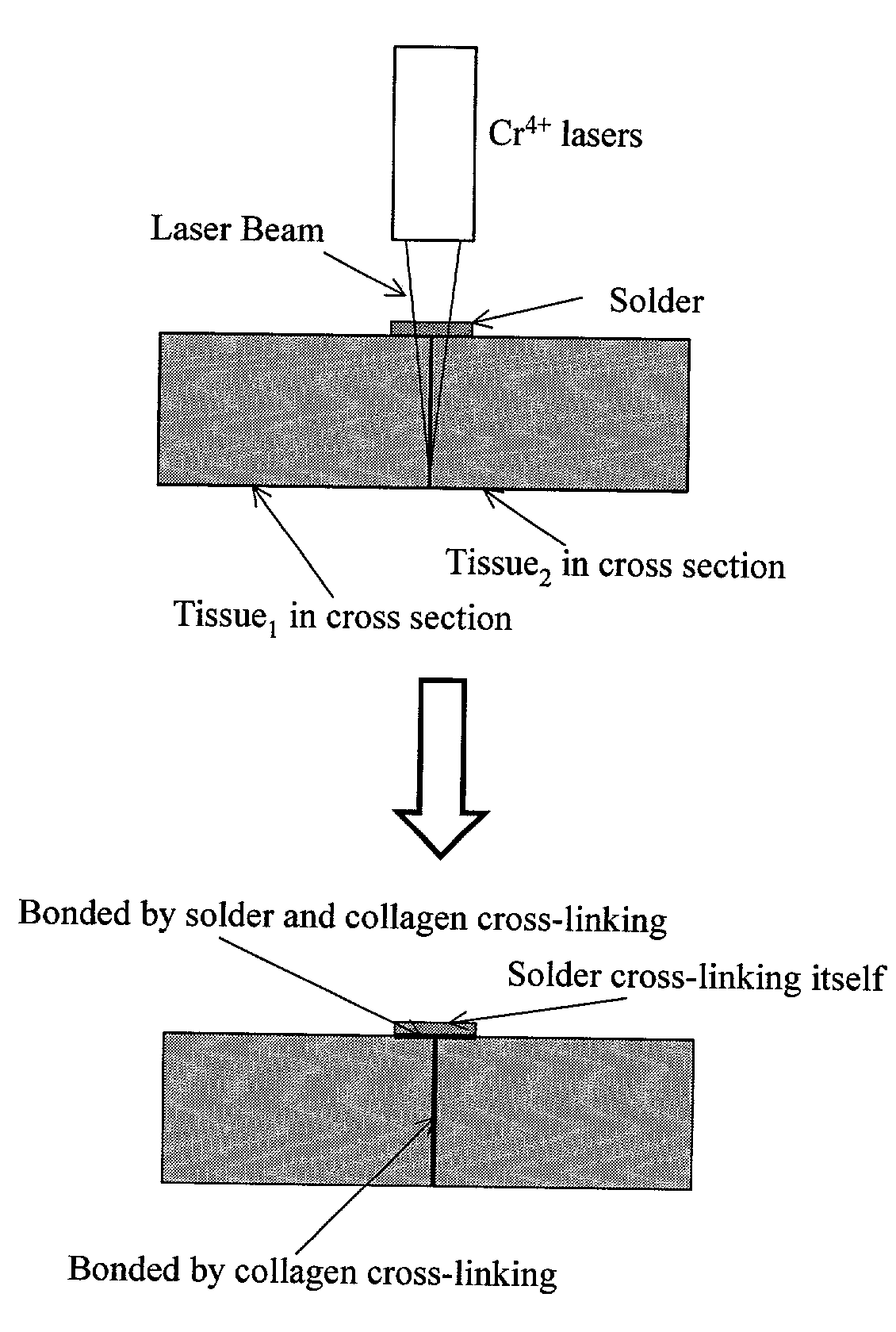
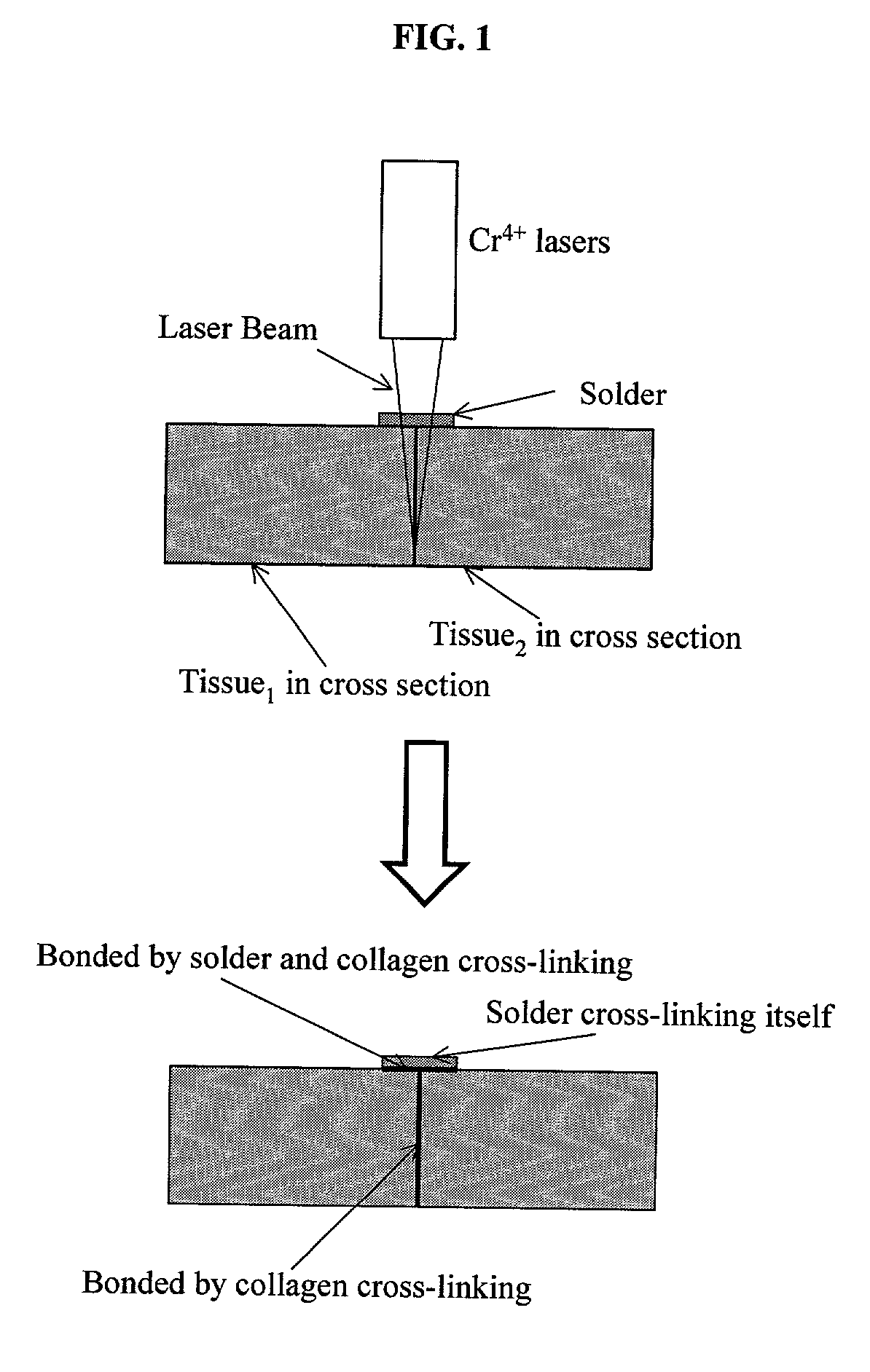
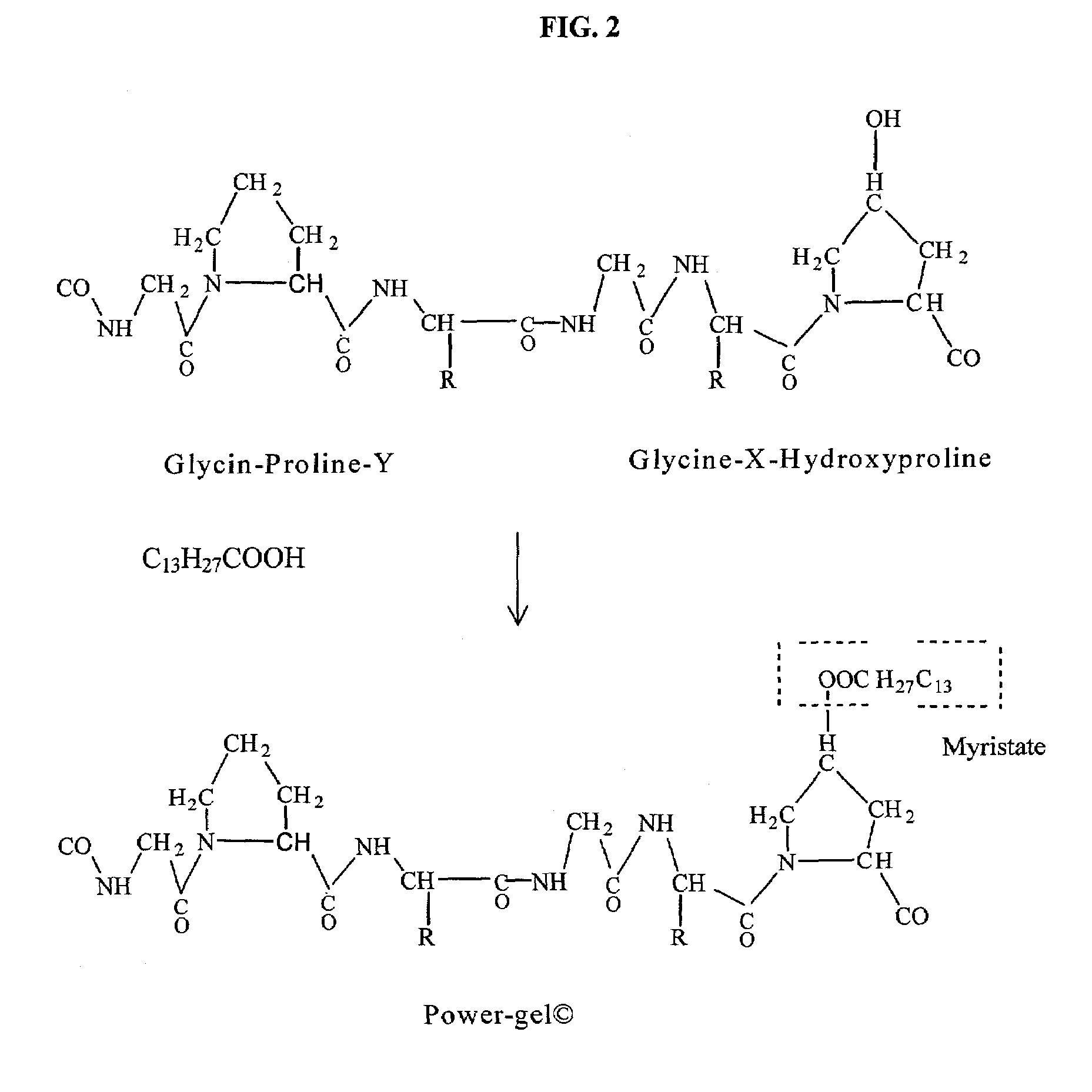
Gelatin based on Power-gel(TM) as solders for Cr4+laser tissue welding and sealing of lung air leak and fistulas in organs
InactiveUS7033348B2Enhancement in post-procedure effectivenessImprove welding strengthDiagnosticsSurgical instrument detailsEngineeringWeld strength
Laser tissue welding can be achieved using tunable Cr4+ lasers, semiconductor lasers and fiber lasers, where the weld strength follows the absorption spectrum of water. The use of gelatin and esterified gelatin as solders in conjunction with laser inducted tissue welding impart much stronger tensile and torque strengths than albumin solders. Selected NIR wavelength from the above lasers can improve welding and avoid thermal injury to tissue when used alone or with gelatin and esterified gelatin solders. These discoveries can be used to enhance laser tissue welding of tissues such as skin, mucous, bone, blood vessel, nerve, brain, liver, pancreas, spleen, kidney, lung, bronchus, respiratory track, urinary tract, gastrointestinal tract, or gynecologic tract and as a sealant for pulmonary air leaks and fistulas such as intestinal, rectal and urinary fistulas.
Owner:RES FOUND OF THE CITY UNIV OF NY THE
Methods of enhancing mucosal hydration and mucosal clearance by treatment with sodium channel blockers and osmolytes
The present invention relates to methods and compositions for treating diseases ameliorated by increased mucociliary clearance and mucosal hydration by administering an effective amount of a sodium channel blocker as defined herein and an osmolyte to a subject to a subject in need of increased mucociliary clearance and mucosal hydration.
Owner:PARION SCI DURHAM NC
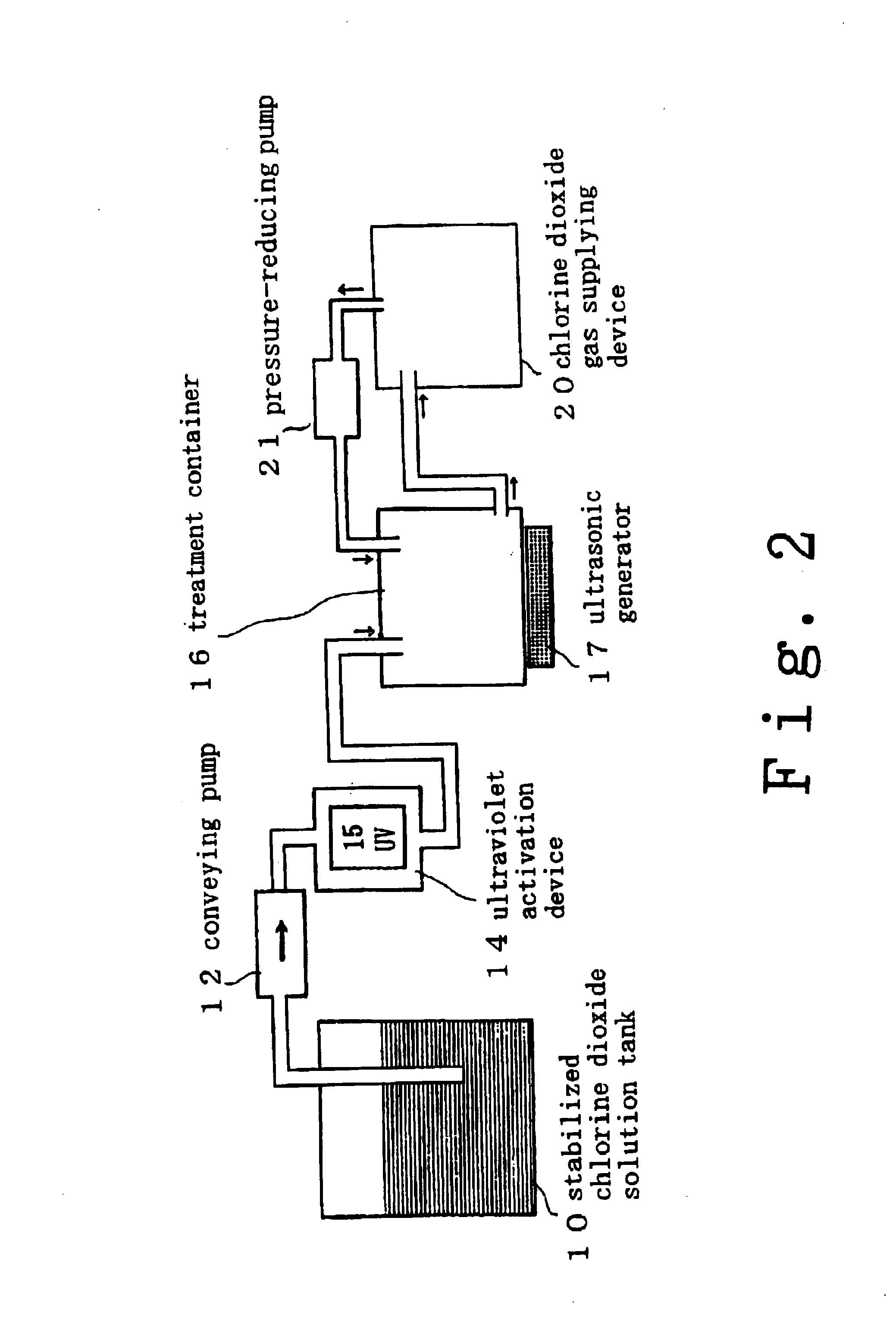
Method for cleaning and sterilizing medical equipment after use
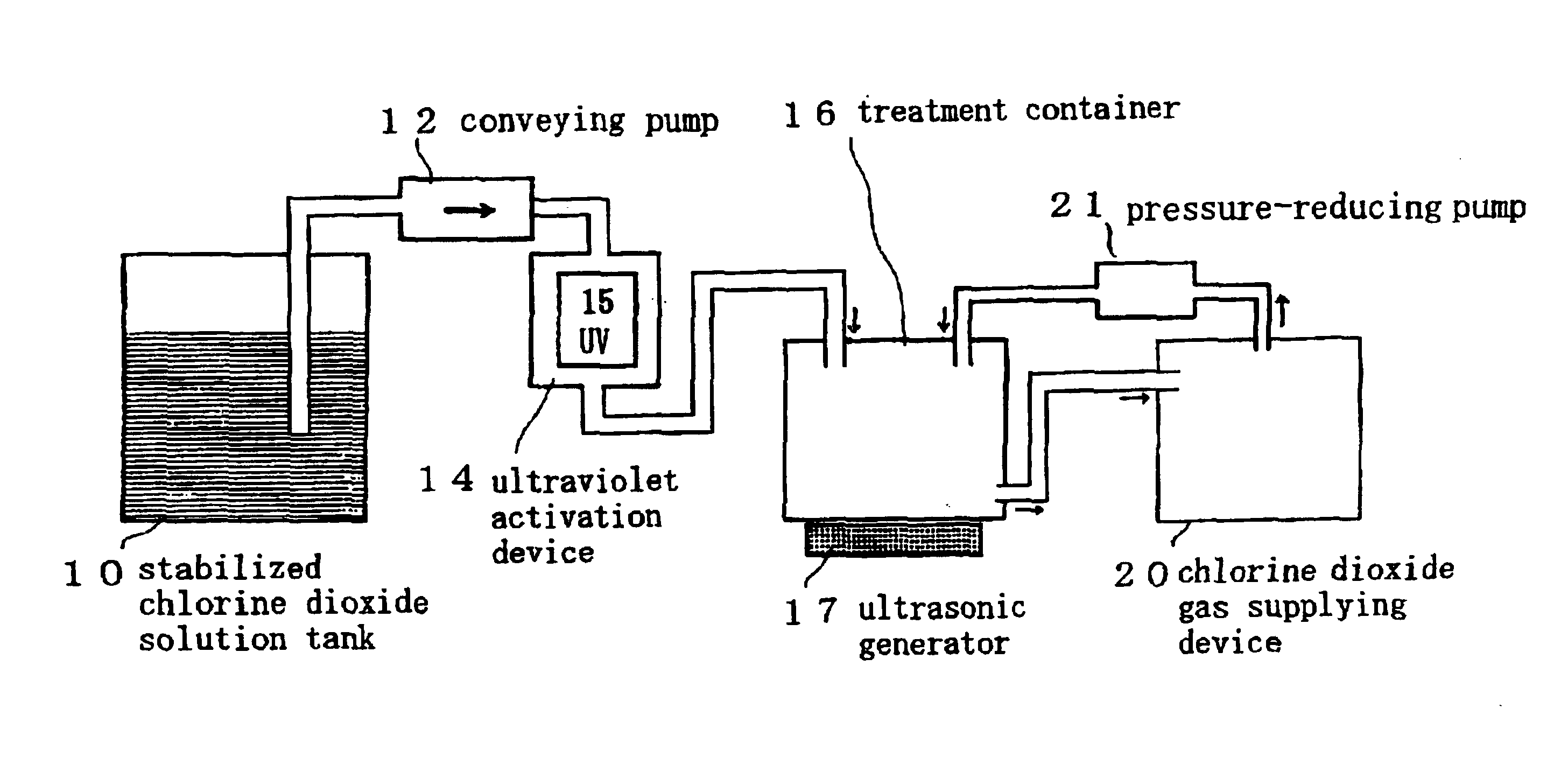
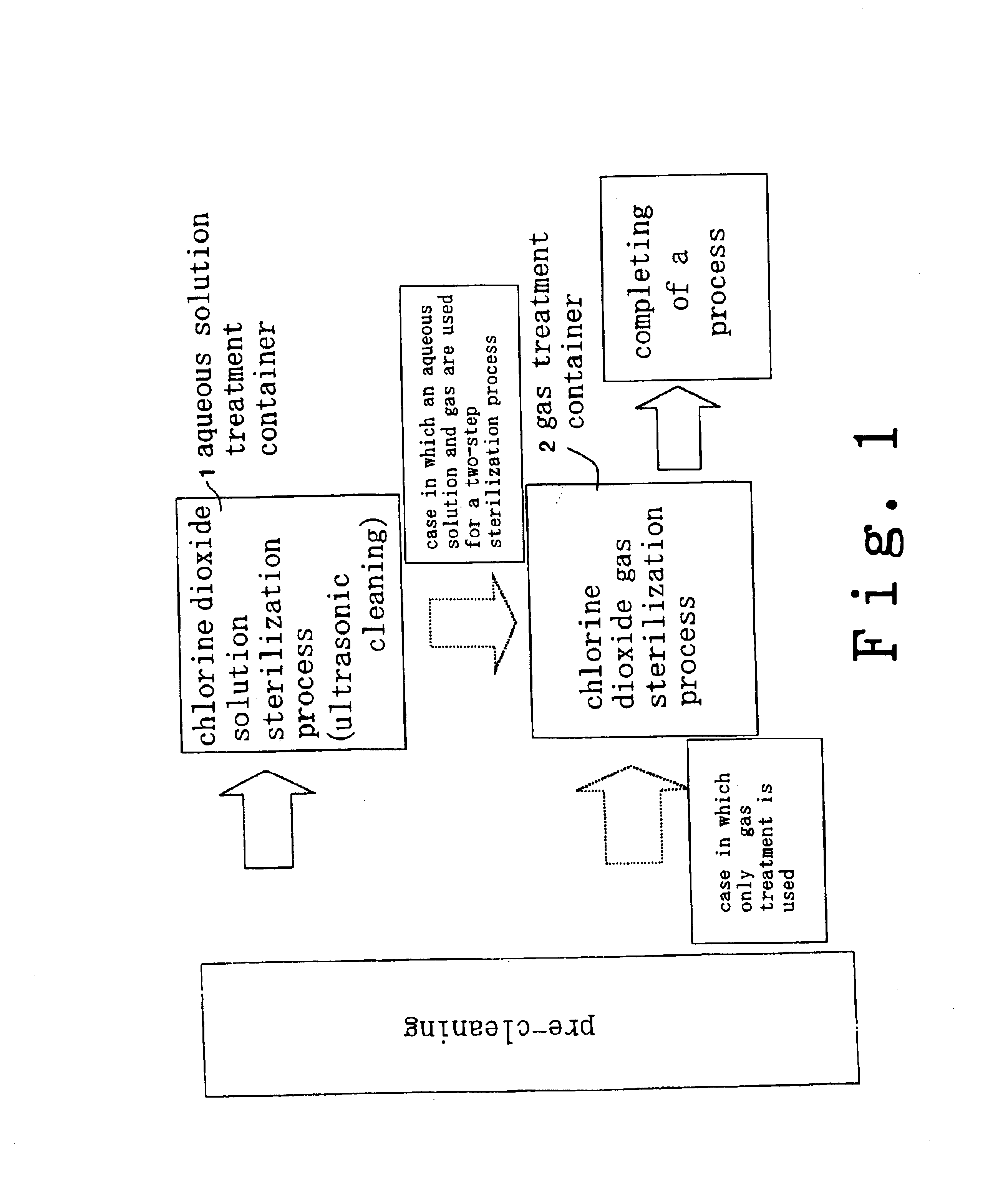
InactiveUS6858181B2Effective deodorizationEffective disinfectionEndoscopesMechanical vibrations separationChlorine dioxideMucus
According to the present invention, a pre-cleaning process is performed for removing a physical substance such as blood, mucus or tiny pieces of tissue that are attached to the exterior of the medical device and are inside a duct or a hollow portion. Then, the pre-cleaned medical device is immersed in a chlorine dioxide solution and / or a chlorine dioxide solution is passed through the duct or the hollow portion. Thereafter, the medical device is placed in a chlorine dioxide gas atmosphere and / or a chlorine dioxide gas is passed through the duct or the hollow portion. In this manner, the exterior of the medical device and the interiors of the duct and the hollow portion are deodorized, disinfected and sterilized.
Owner:KYORIN PHARMA CO LTD
Combination lung ventilation and mucus clearance apparatus and method
ActiveUS20100122699A1Improves patient comfortImproves device efficacyOperating means/releasing devices for valvesBreathing filtersPressure generationActivity daily
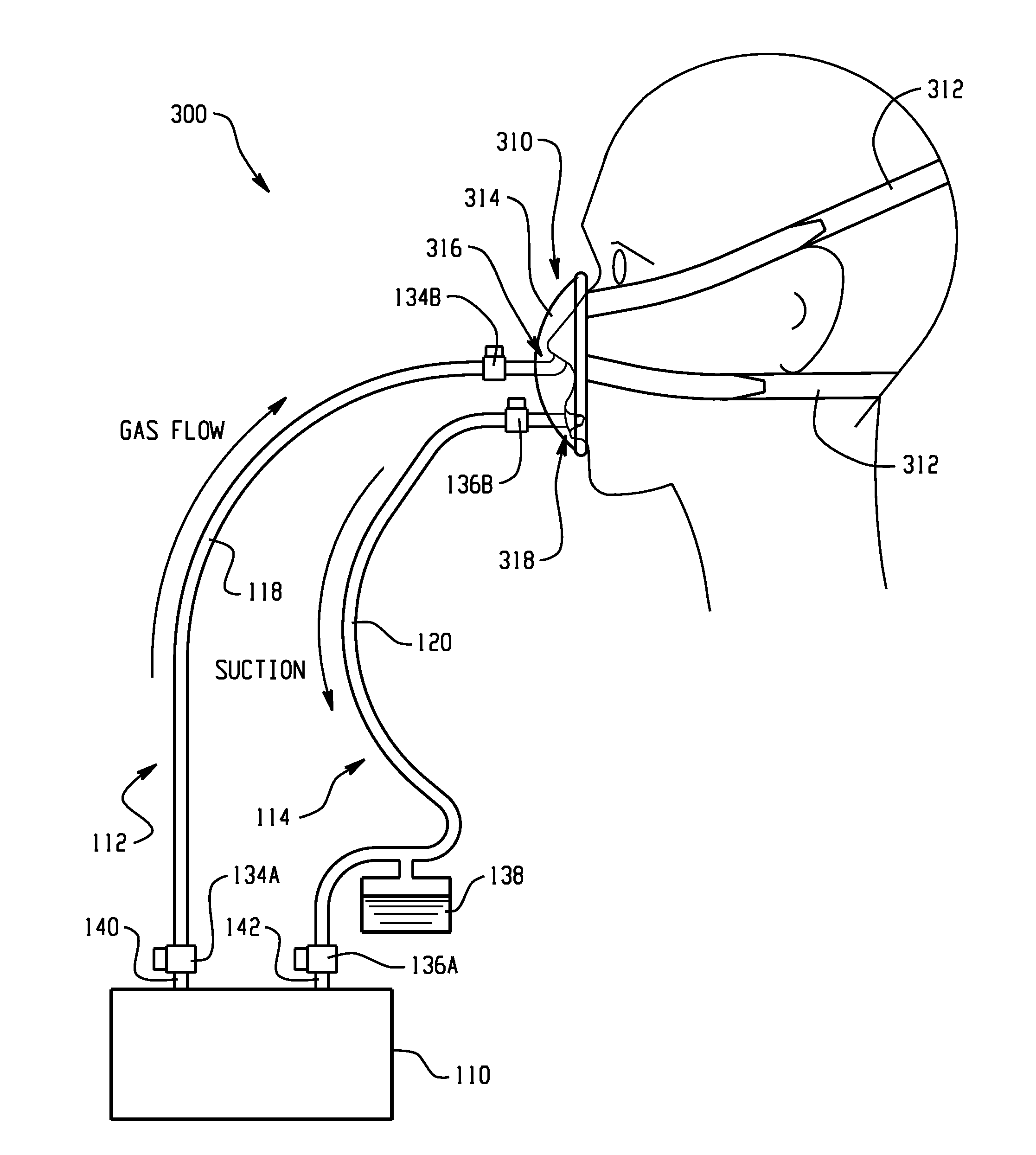
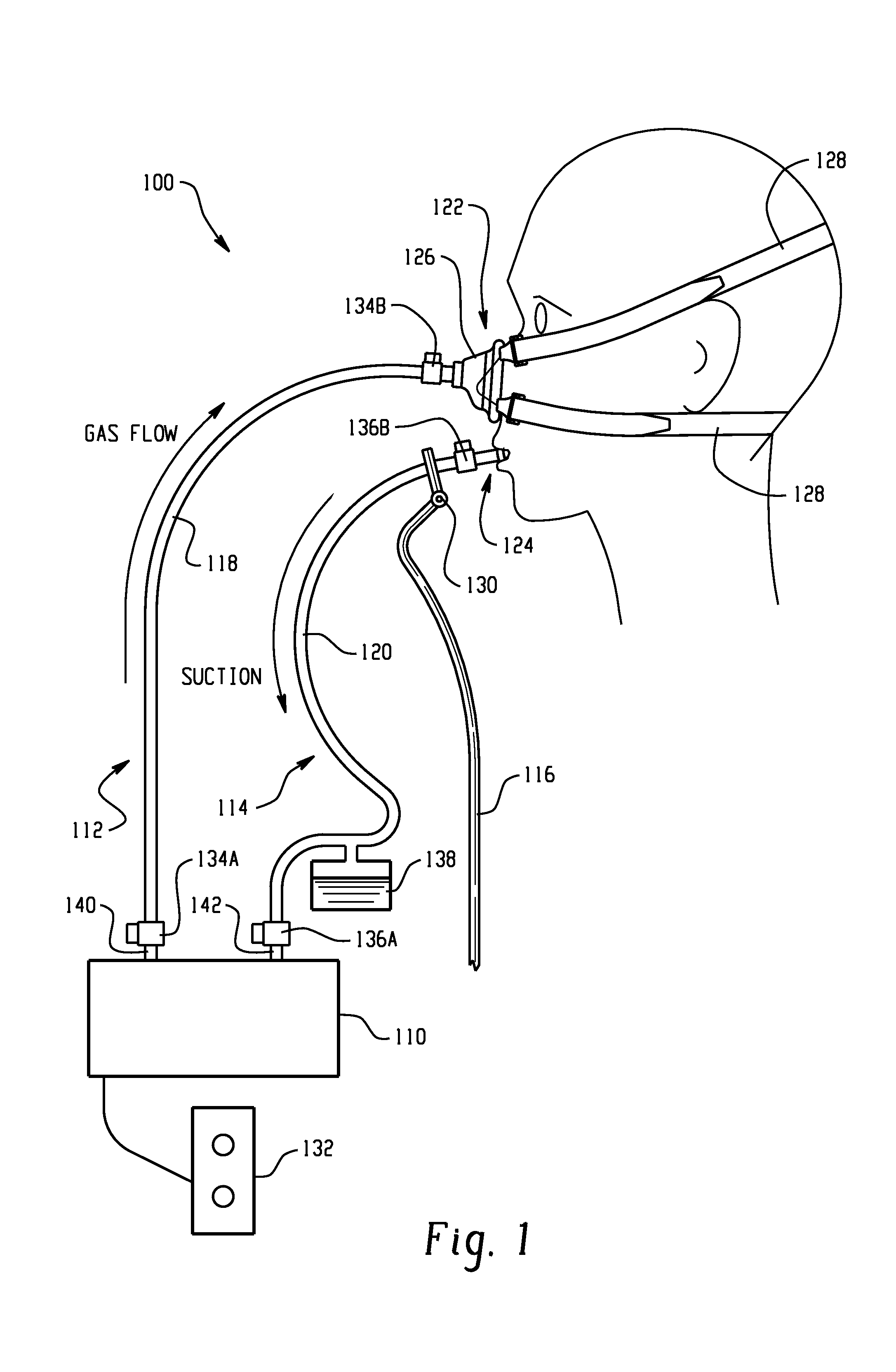
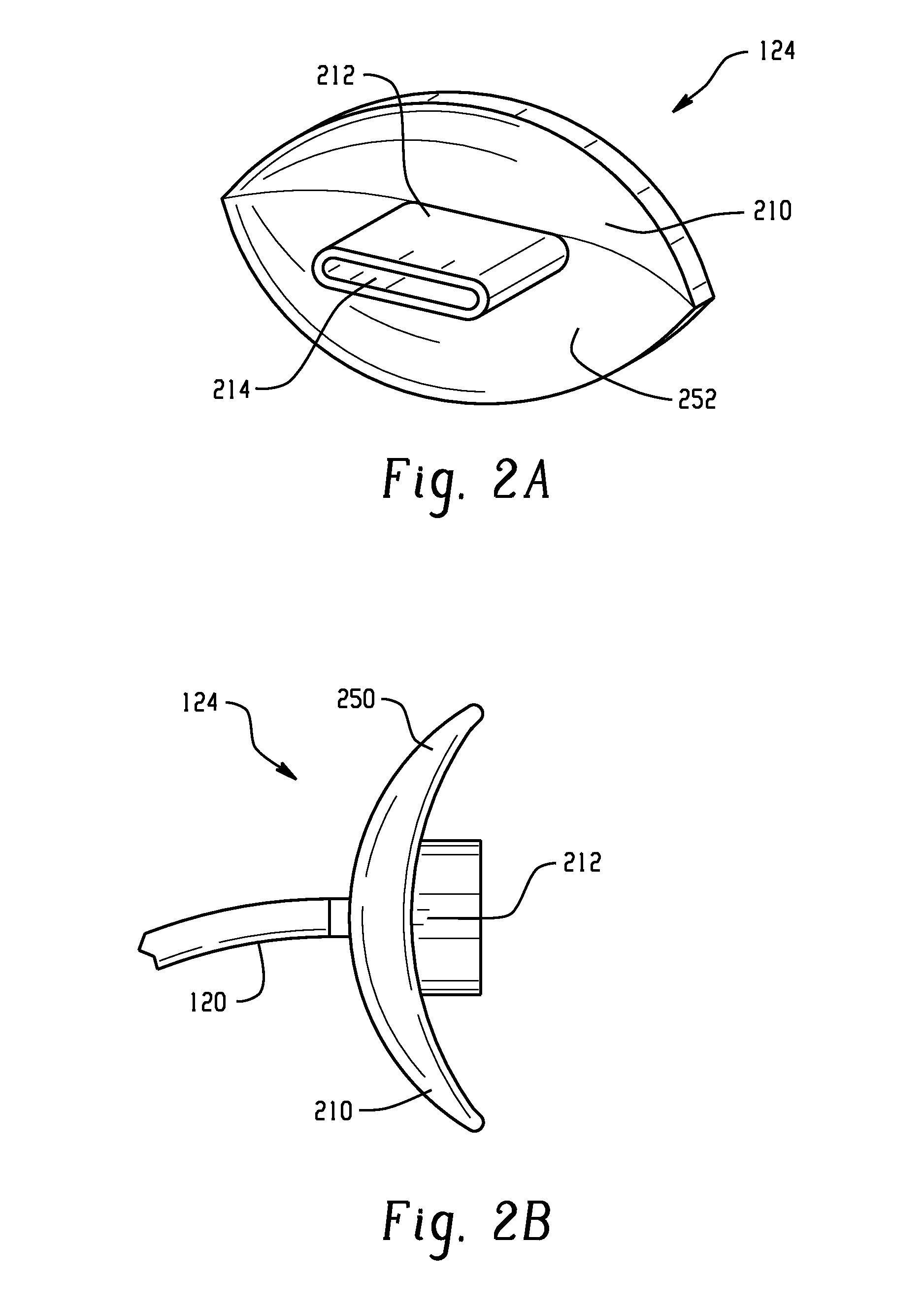
A medical device and method for combination lung ventilation and mucus clearance of a patient is disclosed. The medical device may be configured to provide lung ventilation with intermittent, on-demand mucus clearance, freeing the patient's mouth for activities of daily living such as when the patient is awake; and, in another configuration, the device can provide lung ventilation with automated mucus clearance, such as when the patient is sedated or asleep. The medical device may comprise a pressure generation source, a ventilation portion, and a mucus clearance portion. The mucus clearance portion may be separable from or separate from the ventilation portion such that the medical device is capable of maintaining lung ventilation with the mouth interface removed from the patient.
Owner:METROHEALTH VENTURES LLC
Combined nasal spray and aspirator device
ActiveUS20080312674A1Good for healthPresent invention is physically more favorable for usersEar treatmentCannulasNasal cavityNebulizer
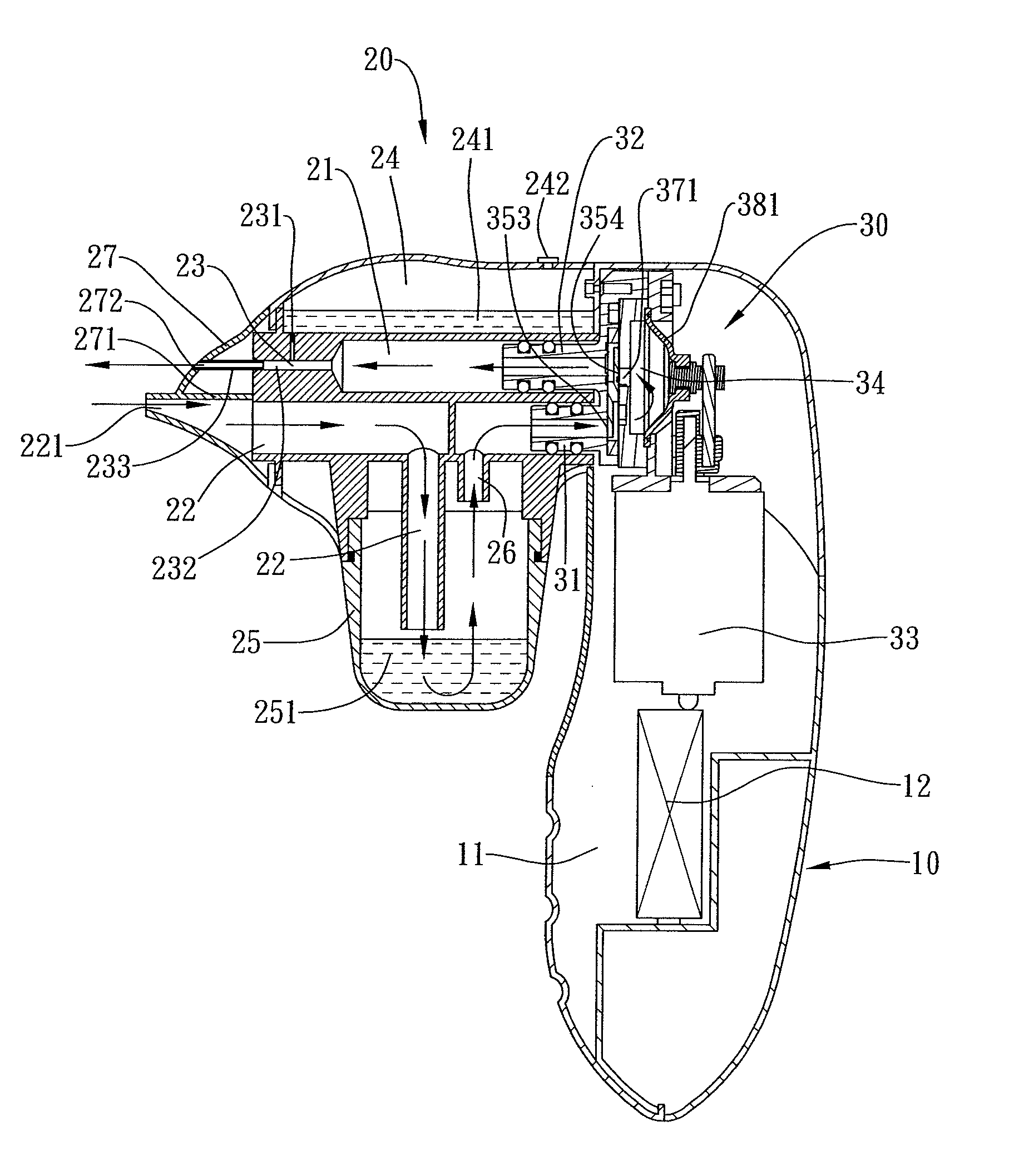
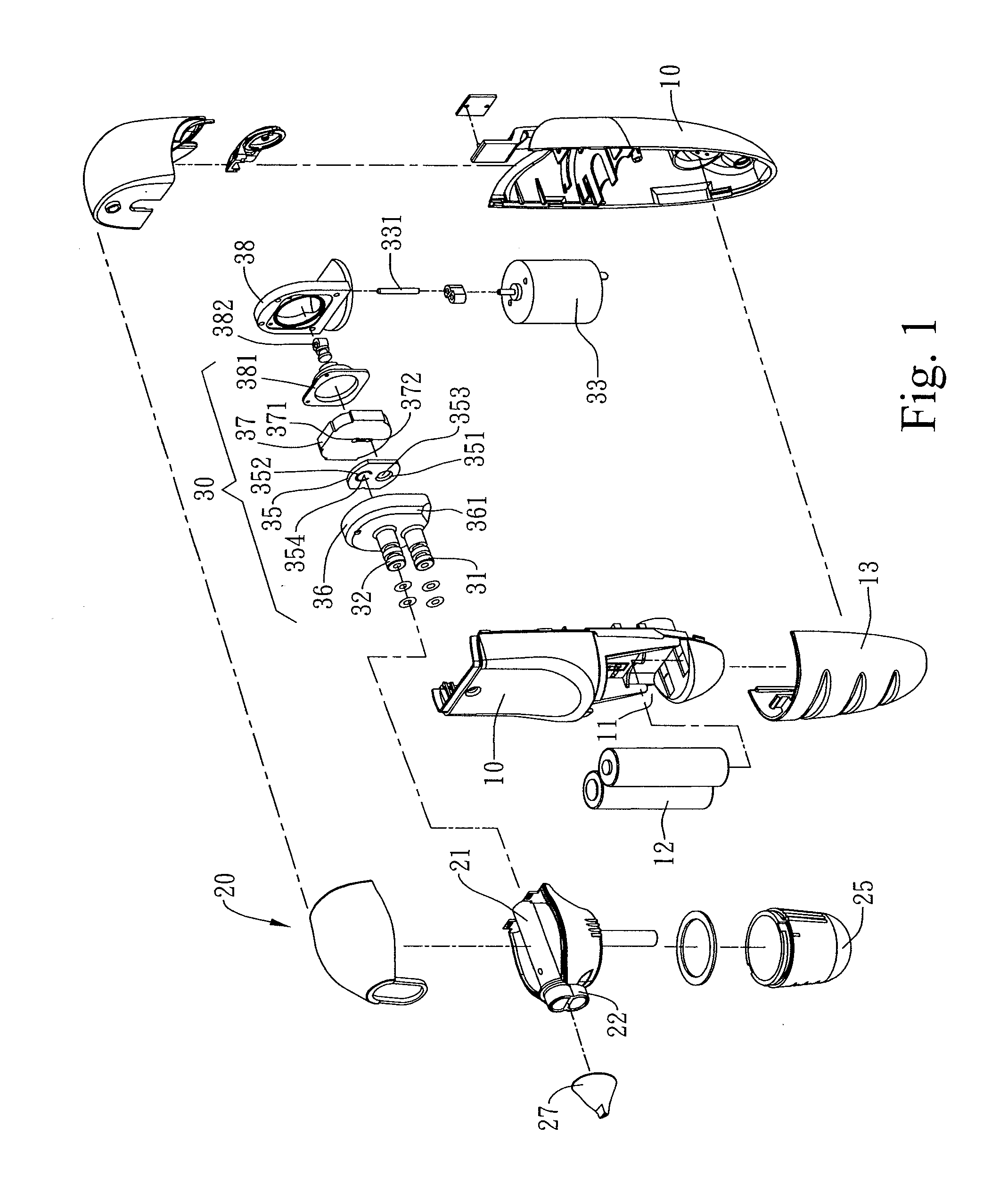
A combined nasal spray and aspirator device includes an air pump disposed within a housing and comprises a spray and aspirator assembly. The air pump, at which an air suction connector and an air discharge connector are disposed, is driven by a driving component to perform air suction and discharge actions. The spray and aspirator assembly, mounted to the air suction connector and the air discharge connector, comprises a spray conduit and a first aspiration conduit; the spray conduit connects to an atomizer which is provided with liquids from a liquid storage container, and the first aspiration conduit connects to a mucus collector so that when some air carrying mucus enters the mucus collector, the mucus is left behind and the air enters the air pump through a second aspiration conduit. The device of the present invention integrates the operations of a nasal spray and a nasal aspirator, sucking the mucus away right after it sprays and liquefies the mucus, with said actions occurring almost simultaneously. The present invention is physically more favorable for users.
Owner:AVITA
Use of inhaled gaseous nitric oxide as a mucolytic agent or expectorant
InactiveUS8518457B2Effective treatmentReducing severity and pathologyRespiratorsBiocideMedicineInhalation
Methods and devices for treating excess mucus accumulation in mammals by administering gaseous inhaled nitric oxide or nitric oxide releasing compounds as a mucolytic agent or expectorant are provided. Delivery of gaseous nitric oxide can be made nasally or orally and is preferably substantially coincident with inhalation of the mammal or based on a synchronous parameter of the mammal's respiratory cycle. Varying therapeutic profiles may be used for the delivery of gaseous nitric oxide depending on the severity of the excess mucus accumulation. Parameters for the therapeutic profiles may include flow rate of nitric oxide containing gas, duration of administration of nitric oxide containing gas, number of breaths for which nitric oxide containing gas is to be administered, and concentrations of therapeutic NO delivered to the airways.
Owner:BEYOND AIR LTD
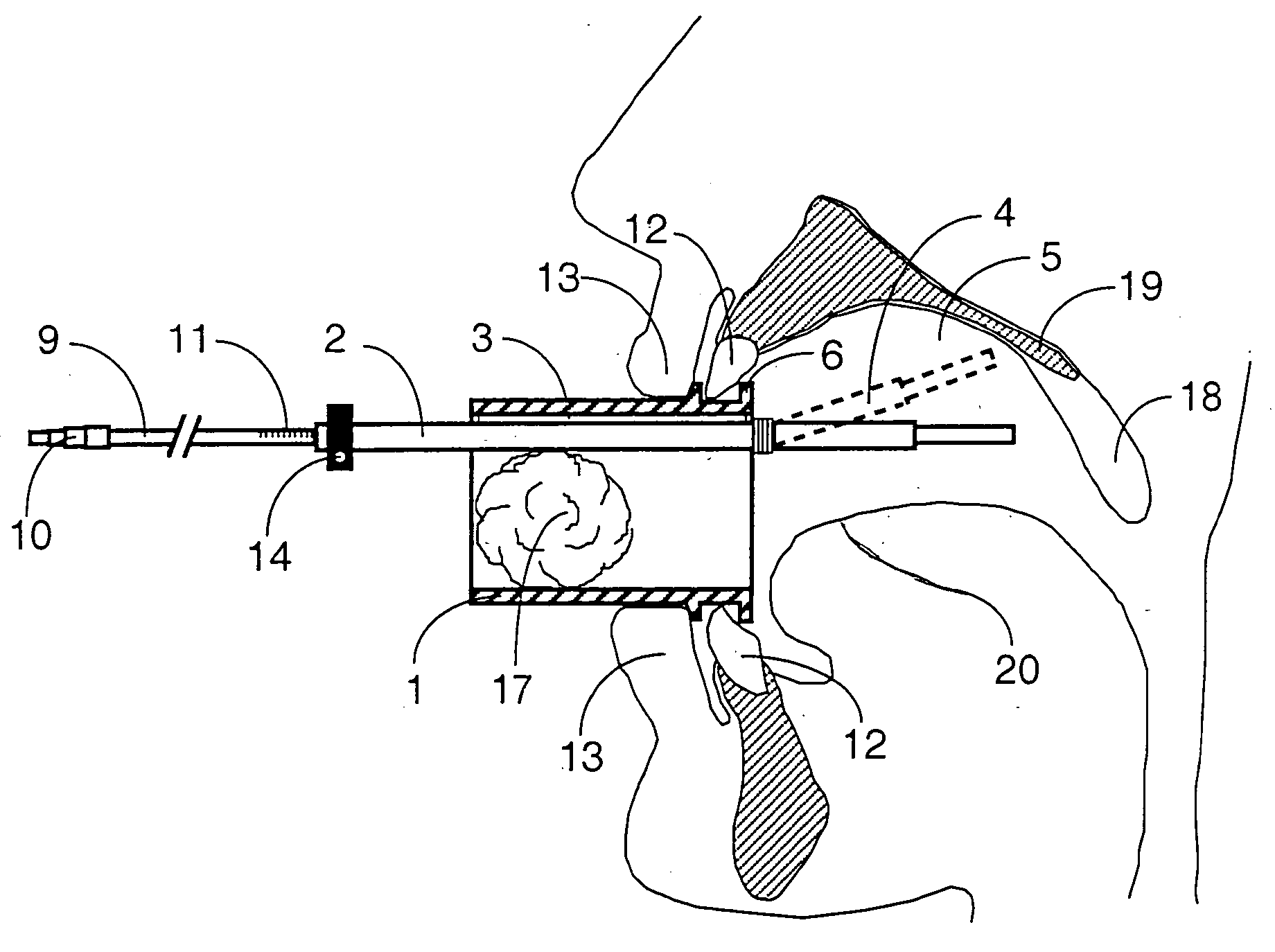
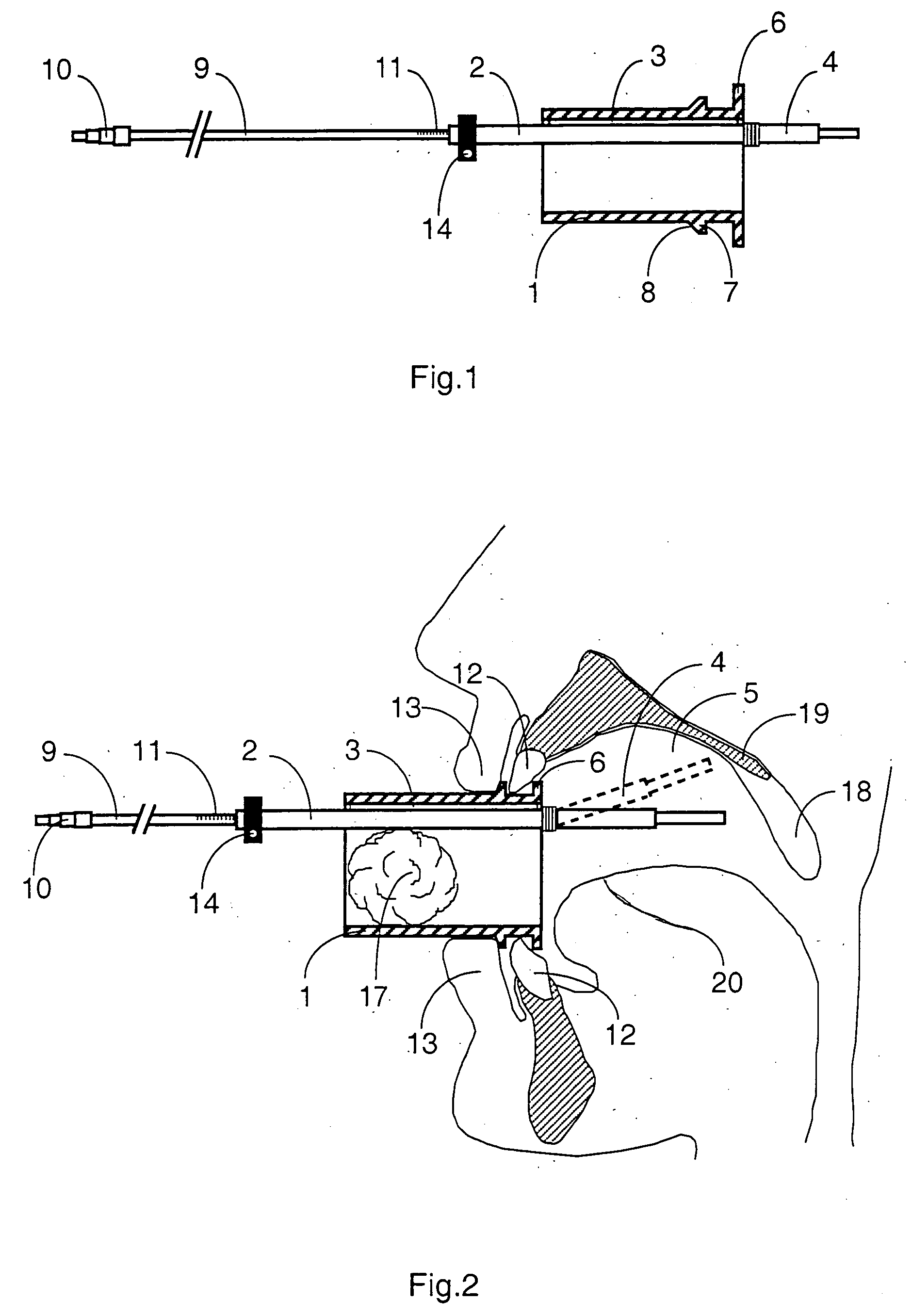
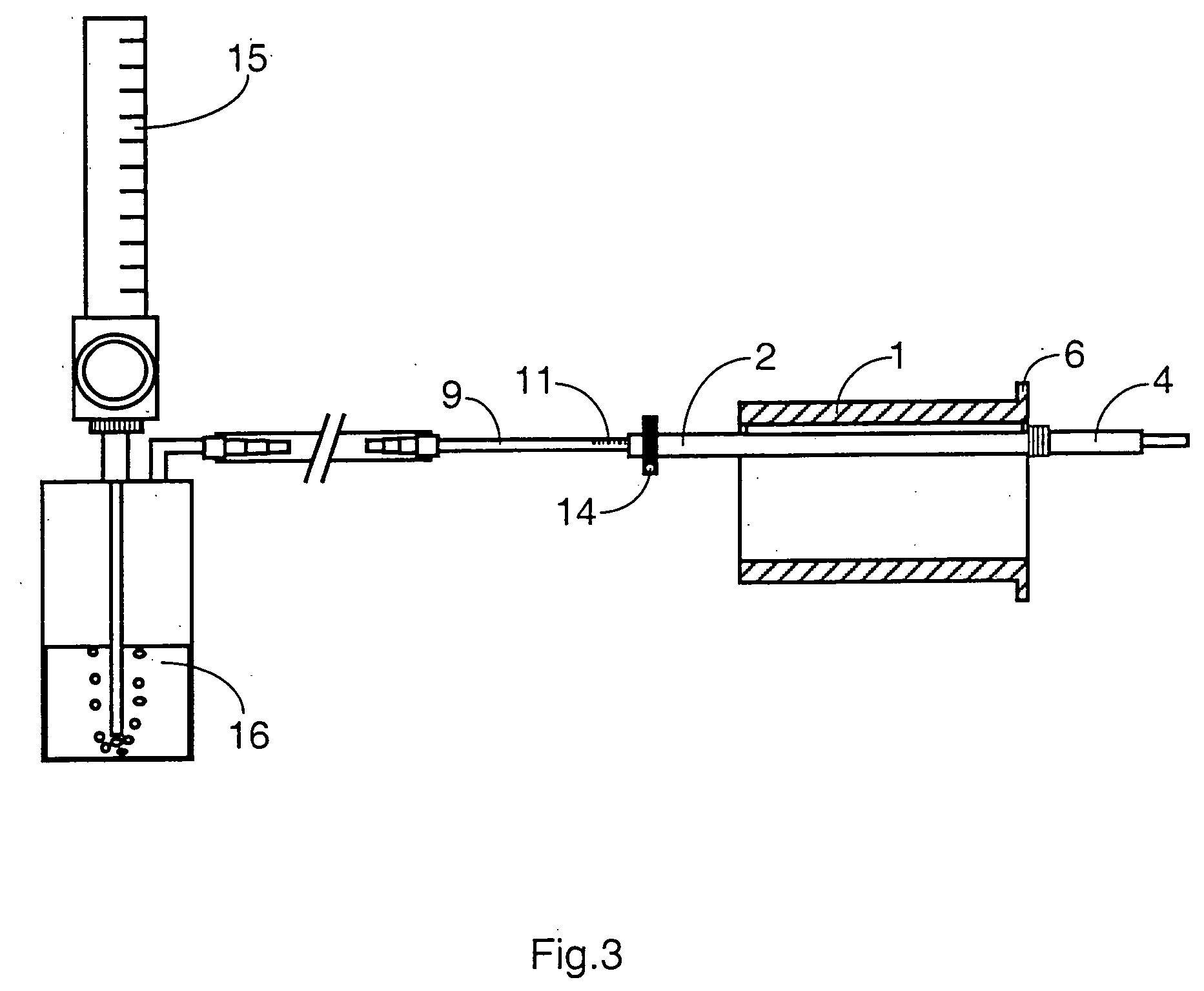
Mouthpeice intended for a device used to assess the sensitivity of the pharynx and a device comprising same
InactiveUS20040138585A1Limit undesirable effectLimiting undesirable effectBronchoscopesLaryngoscopesMeasurement scalesAirflow
To assess the sensitivity of a subject's pharynx, an open mouthpiece (1) fitted with a thin guide tube (2), having an articulated end (4), is inserted into the subject's mouth. A pipe (9) is inserted into the guide tube, under visual observation through the mouthpiece, until it touches the subject's palate. The position on a measurement scale (11) provided on the pipe facing a second end of the guide tube is noted, and the pipe is withdrawn over a preset distance, for example 1 cm, and fixed in this position onto the guide tube. A variable gas flow is injected into the pipe and reaches the pharyngeal mucous situated facing the pipe. The sensitivity of the subject's pharynx, measured by the threshold of sense perception, is determined by the lowest flow value perceived by the subject.
Owner:UNIV JOSEPH FOURIER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com