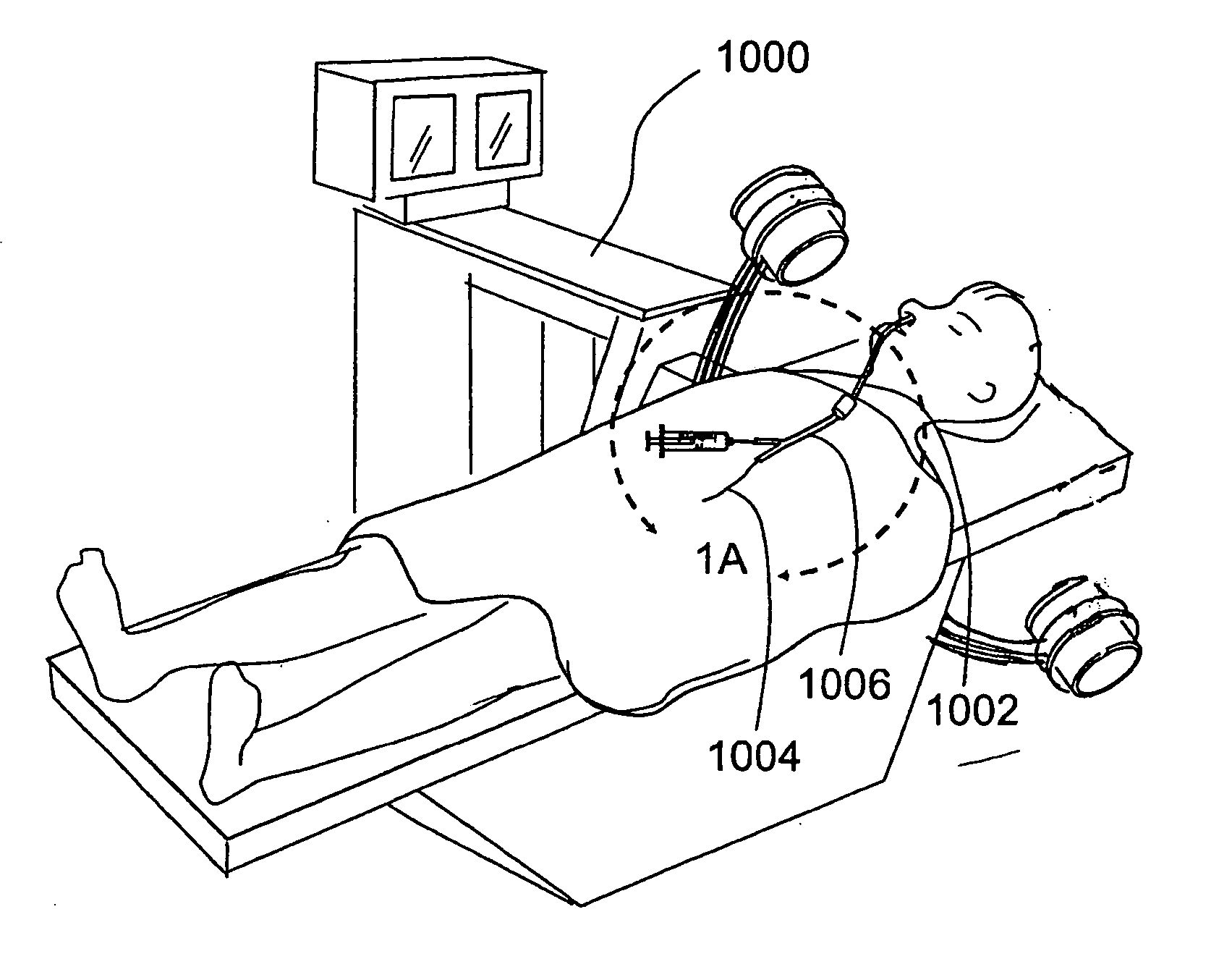
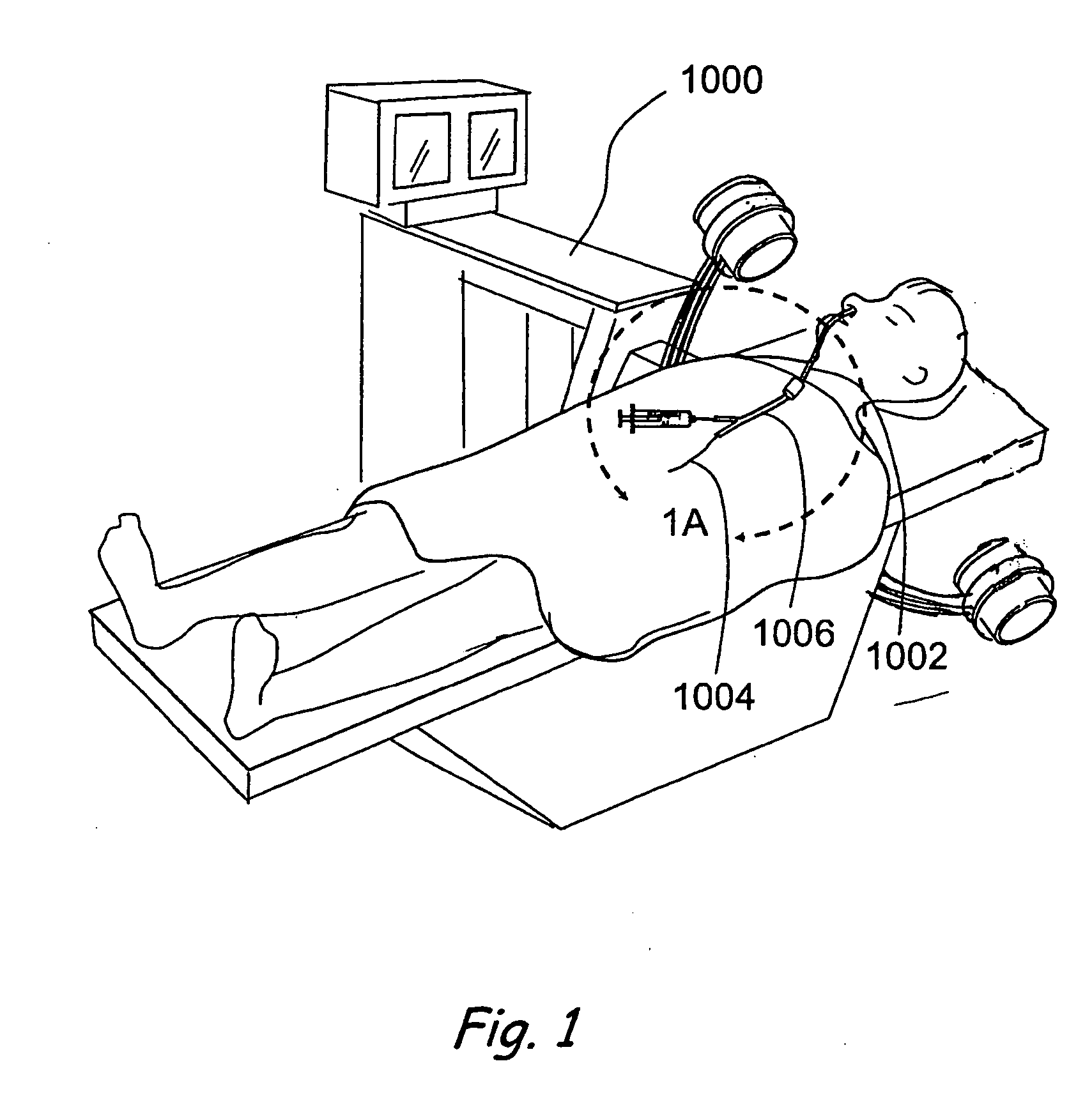
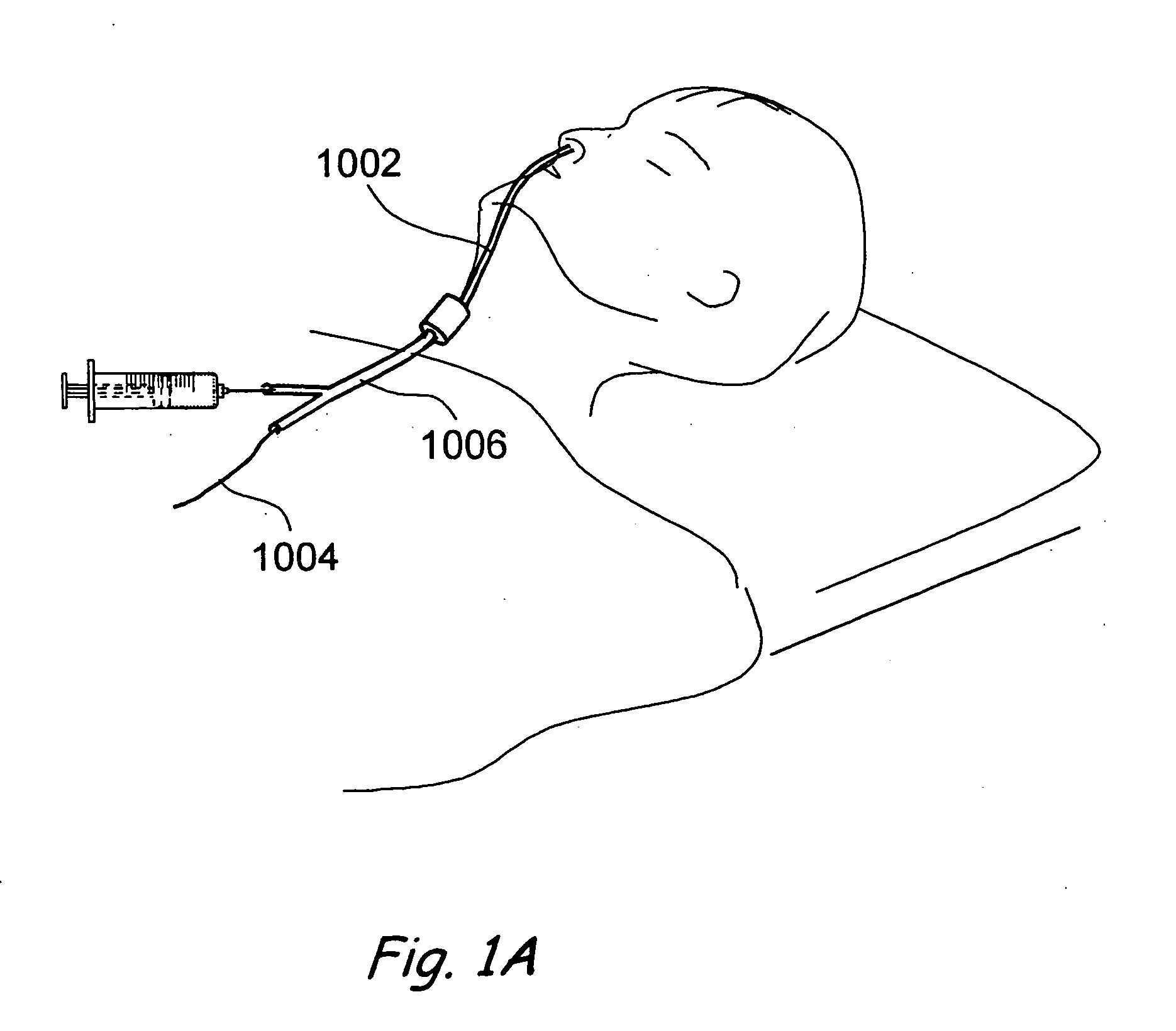
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6545 results about "Nose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A nose is a protuberance in vertebrates that houses the nostrils, or nares, which receive and expel air for respiration alongside the mouth. Behind the nose are the olfactory mucosa and the sinuses. Behind the nasal cavity, air next passes through the pharynx, shared with the digestive system, and then into the rest of the respiratory system. In humans, the nose is located centrally on the face and serves as an alternative respiratory passage especially during suckling for infants.
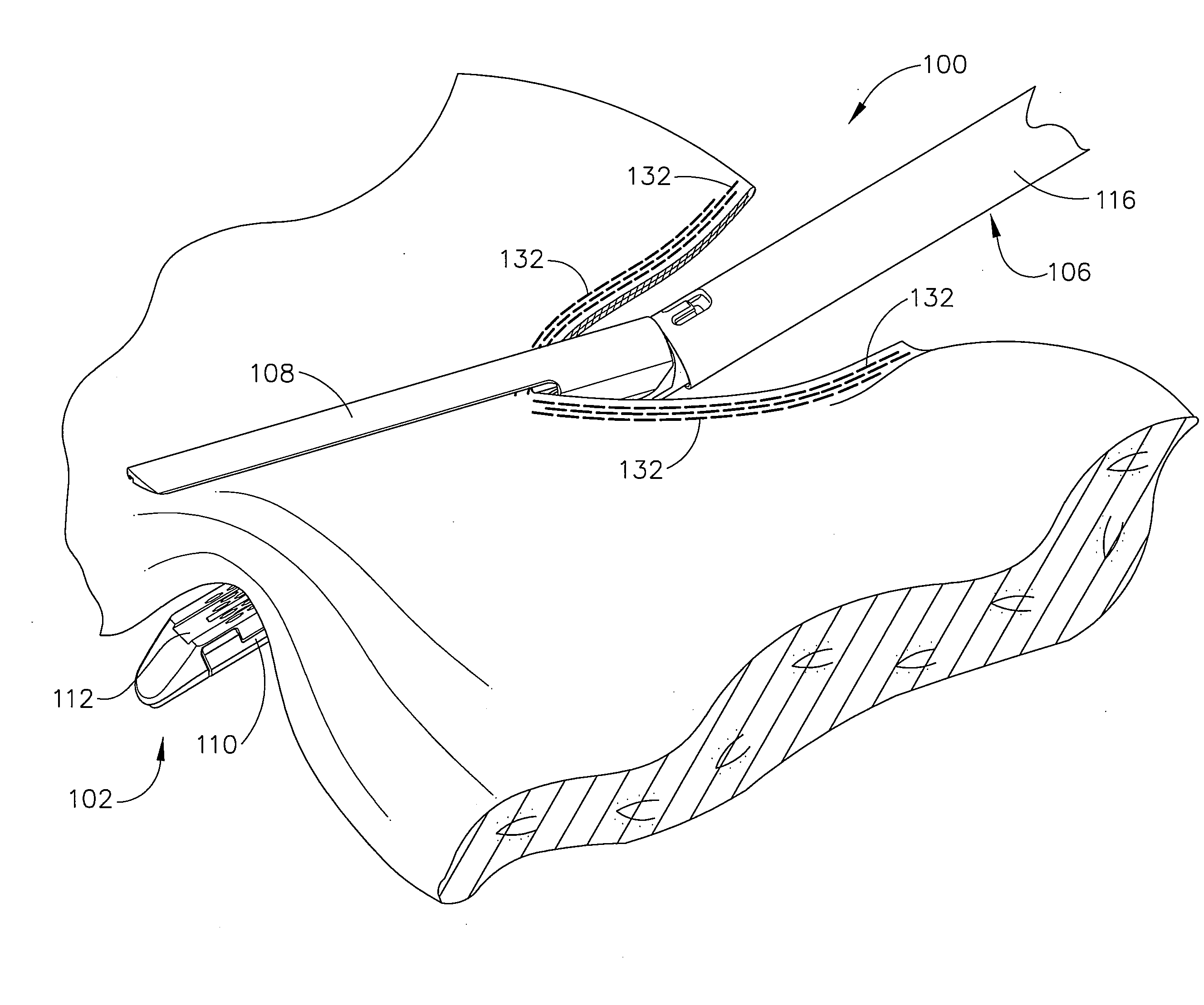
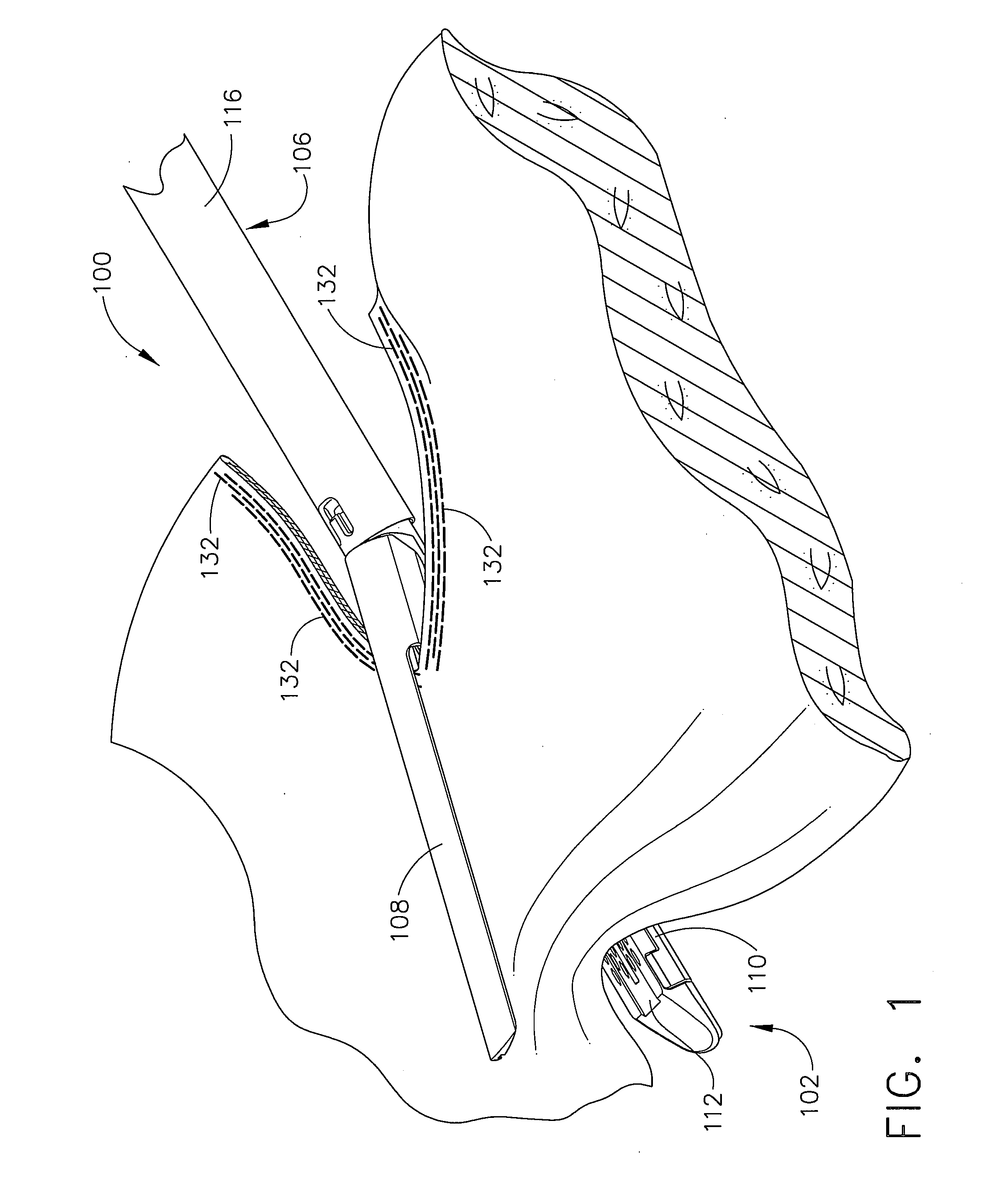
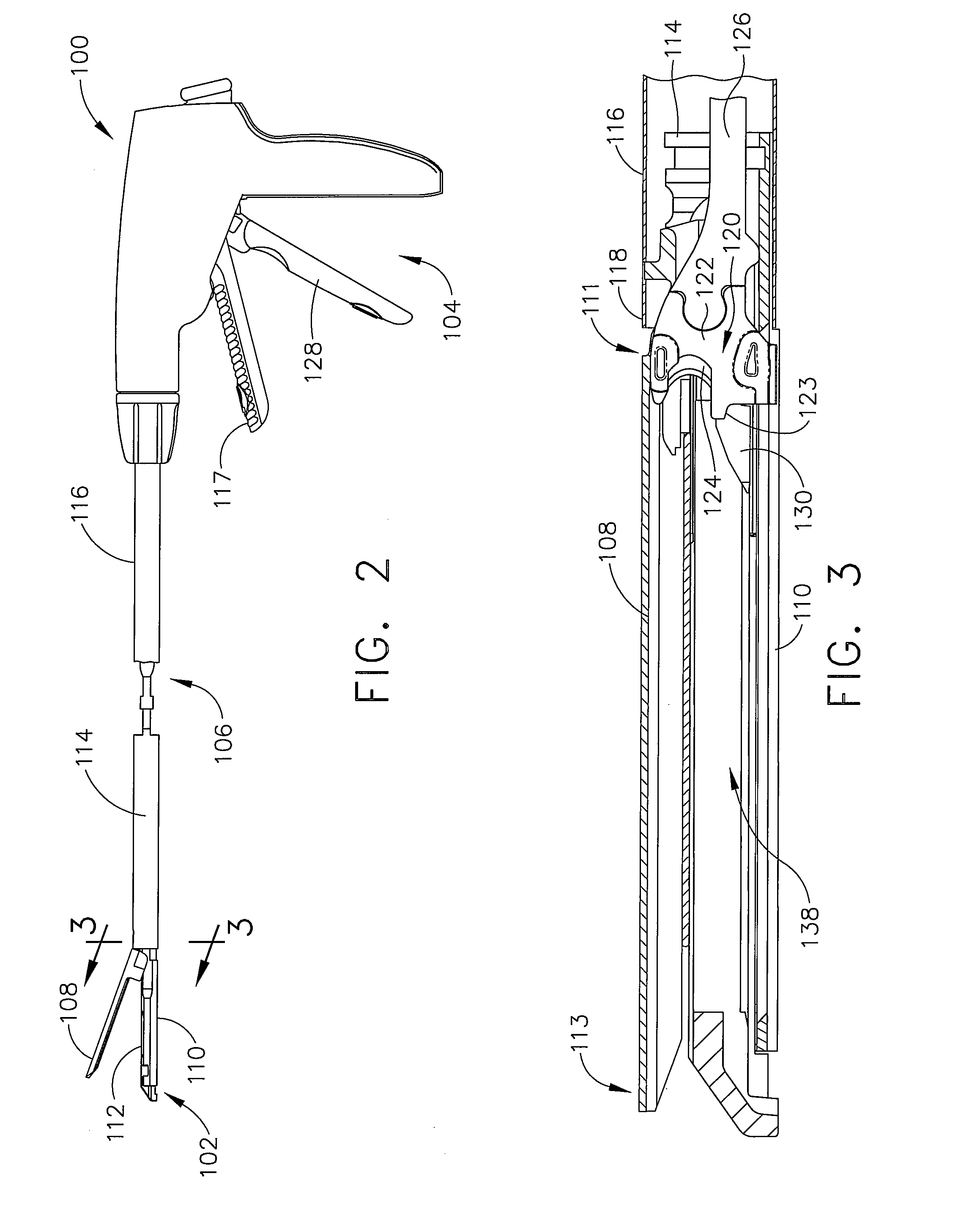
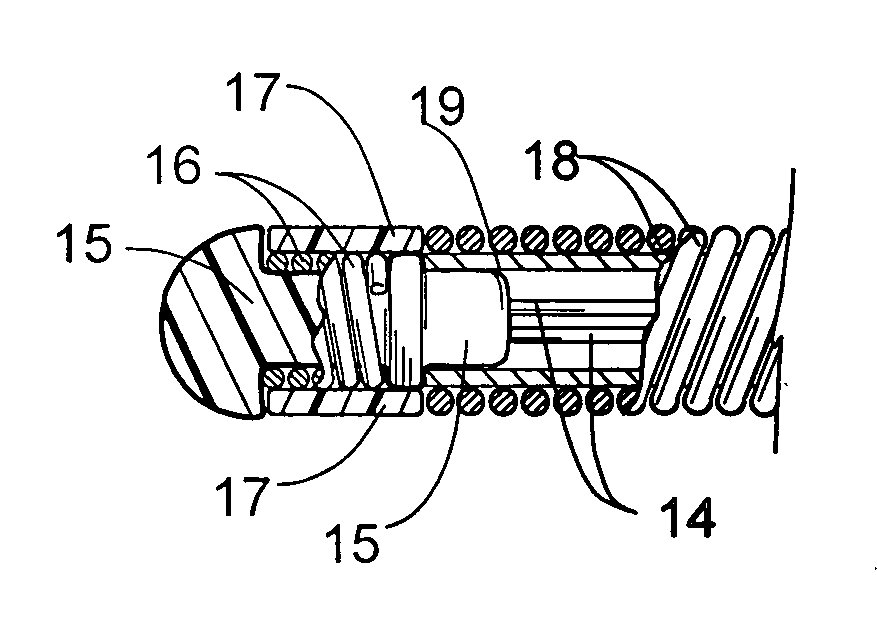
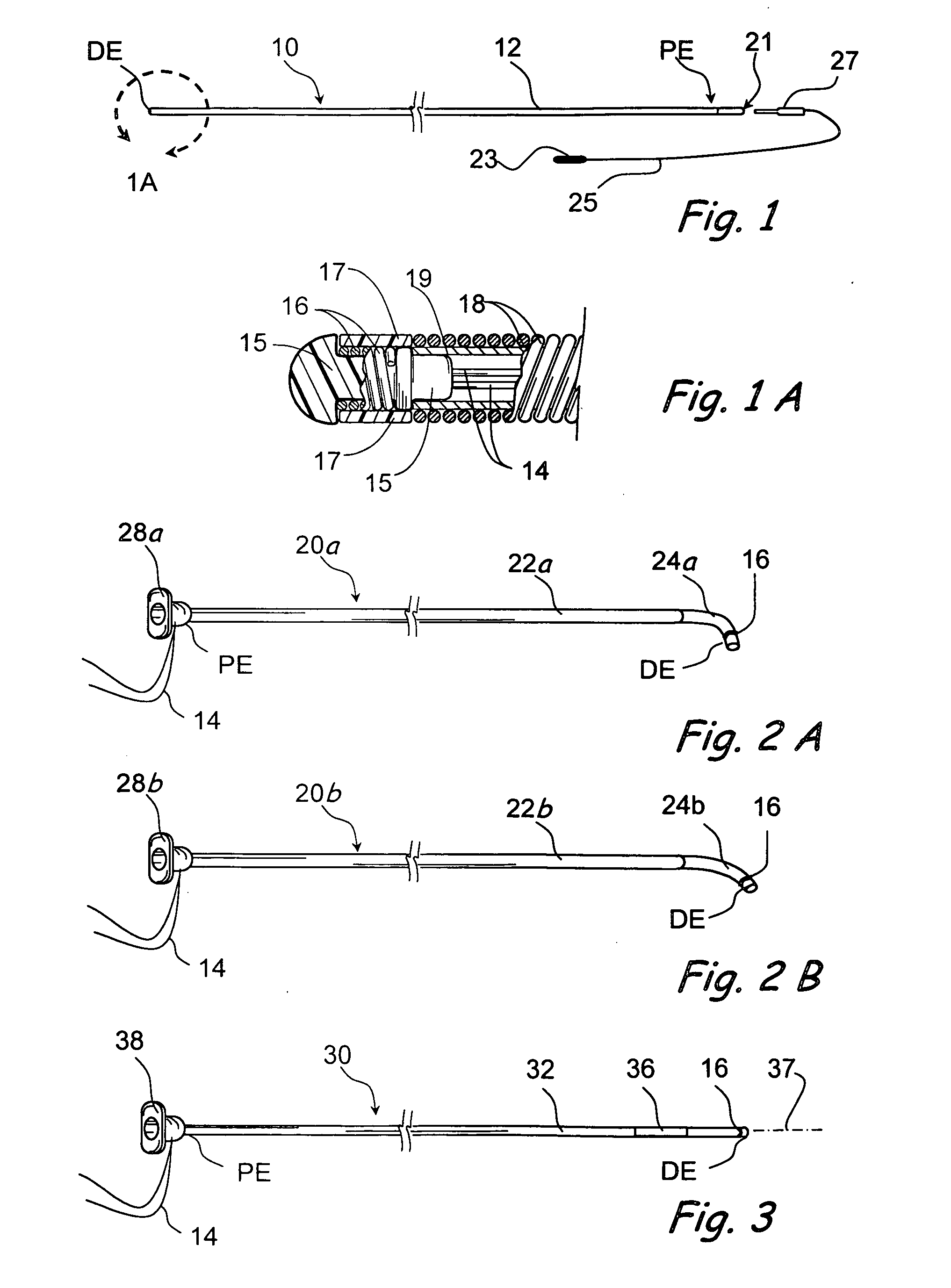
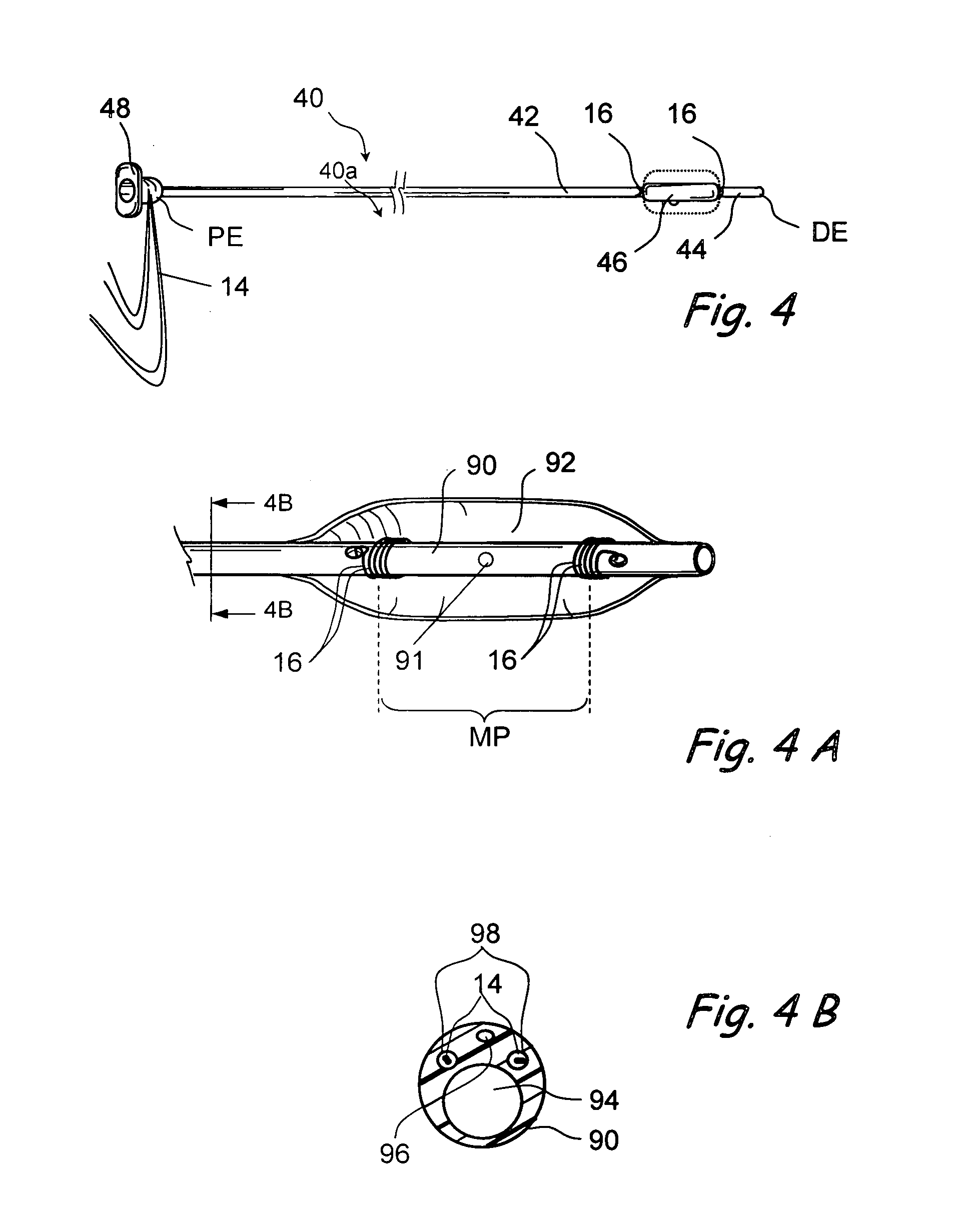
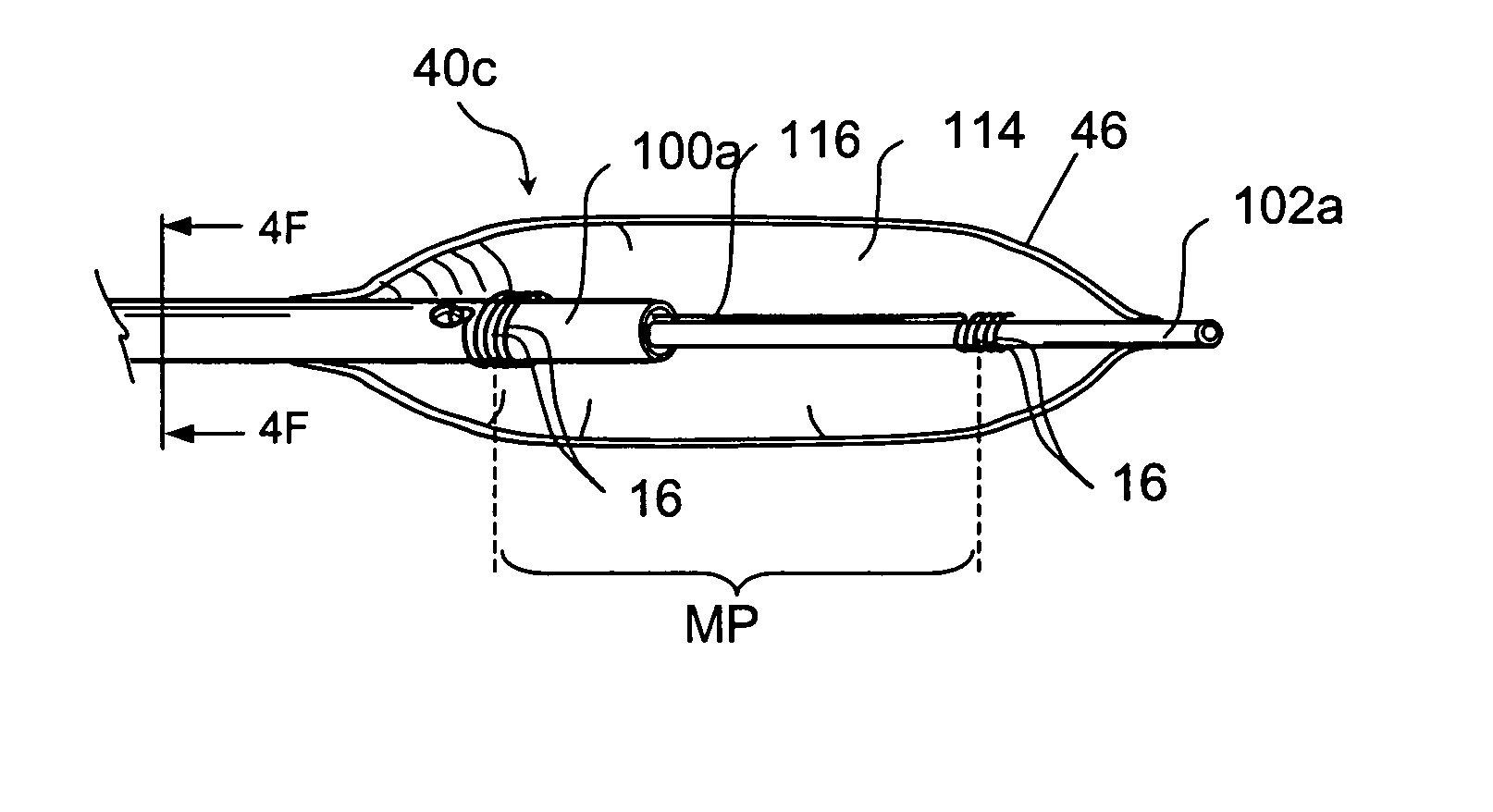
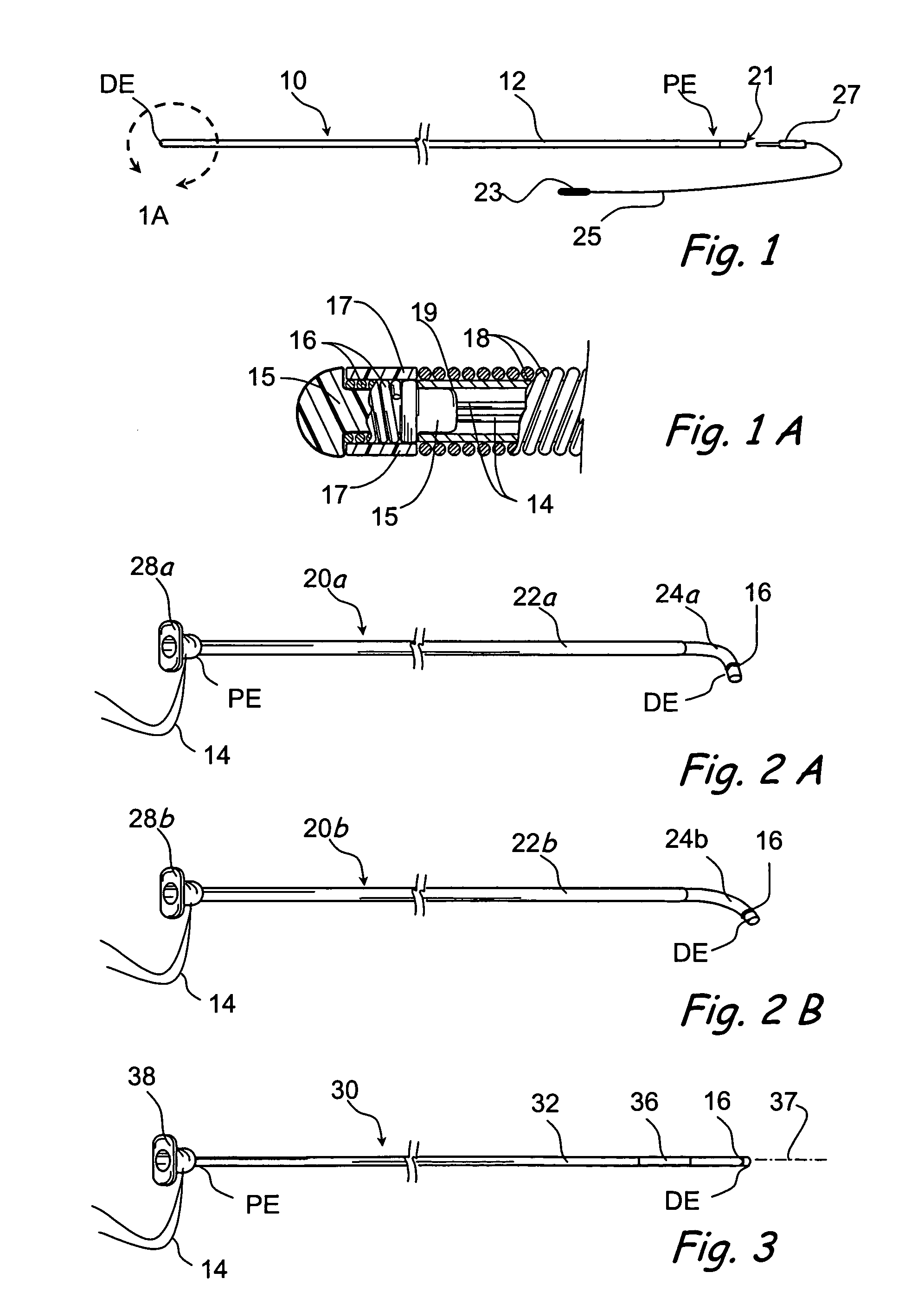
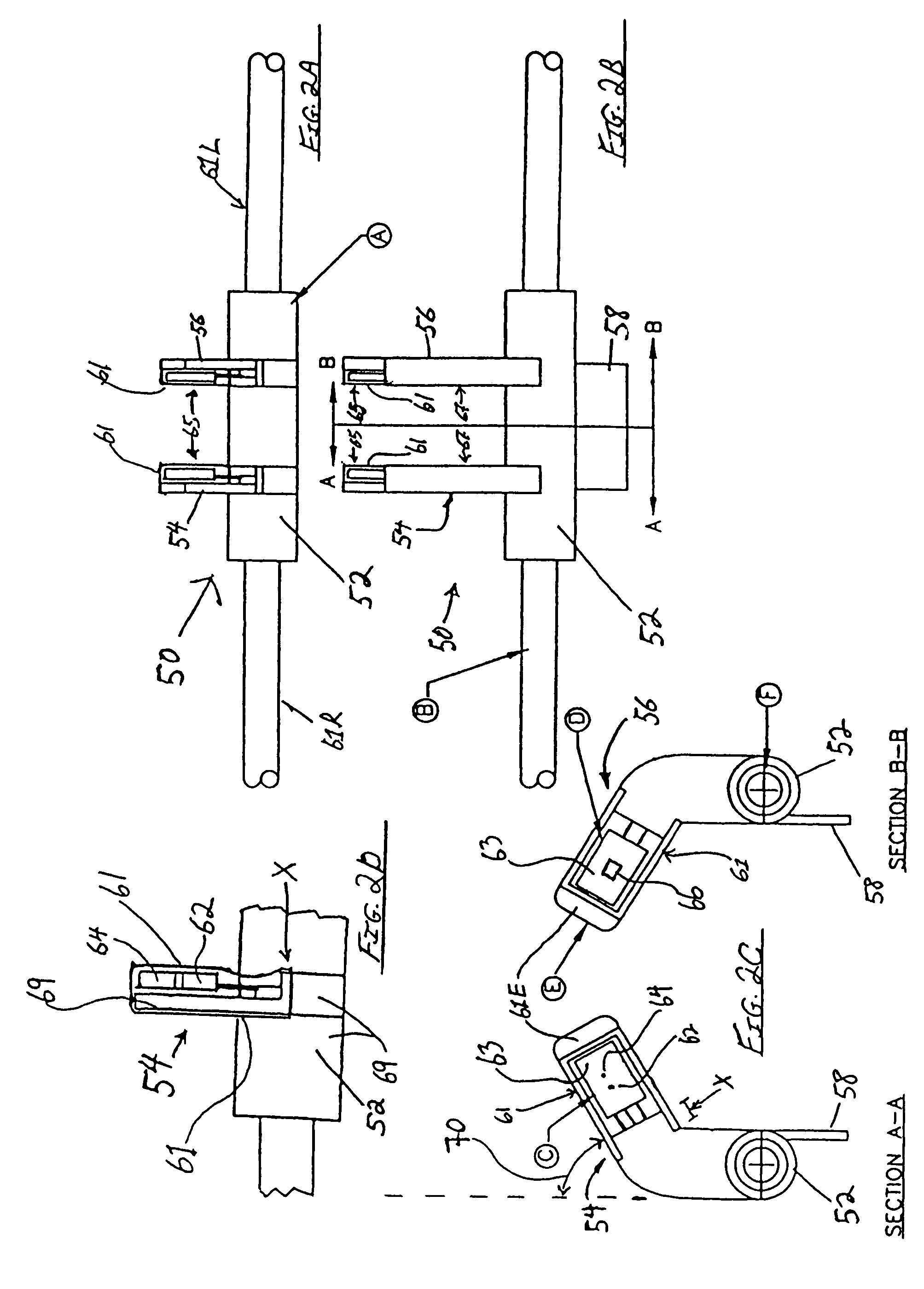
Surgical stapler end effector with tapered distal end
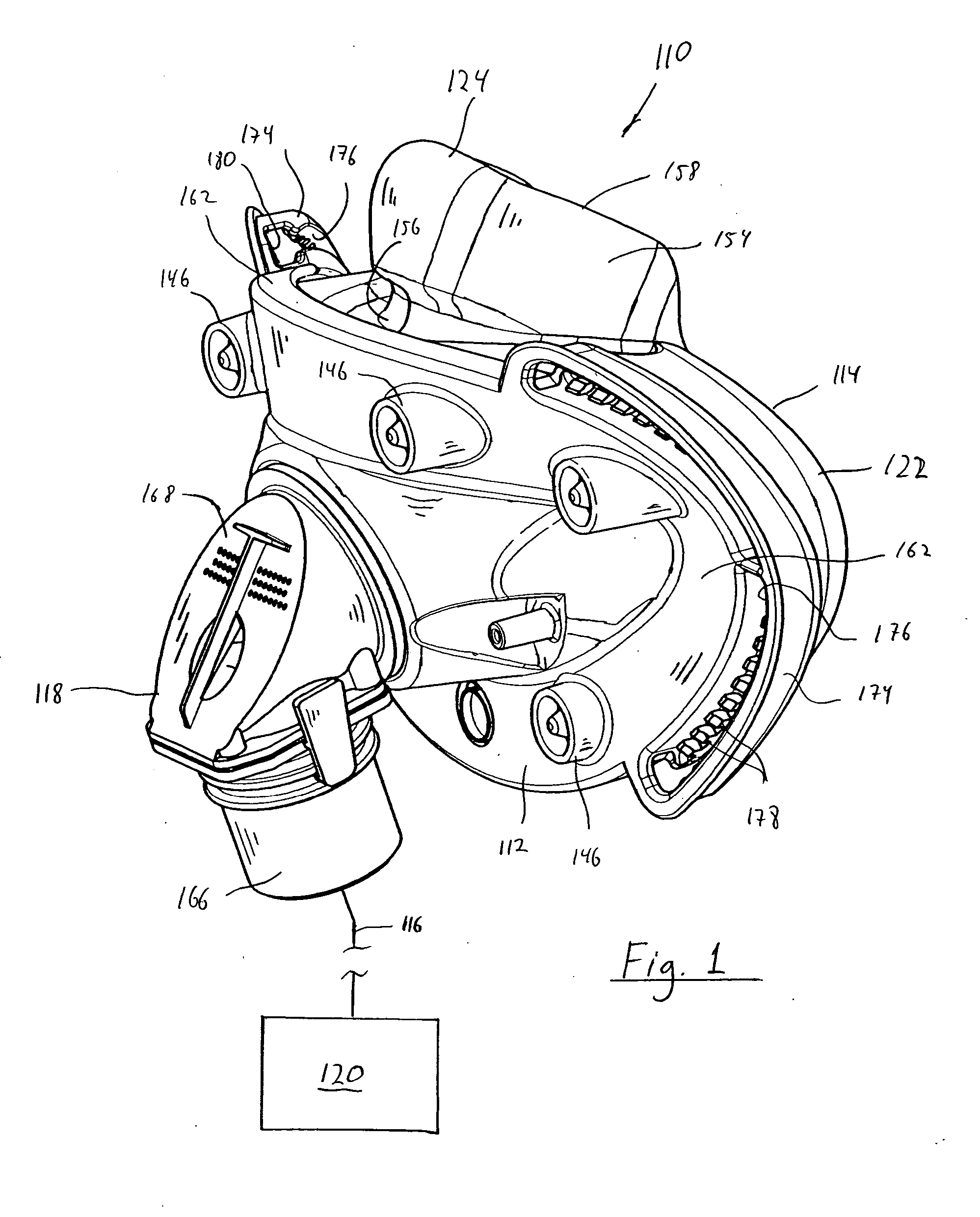
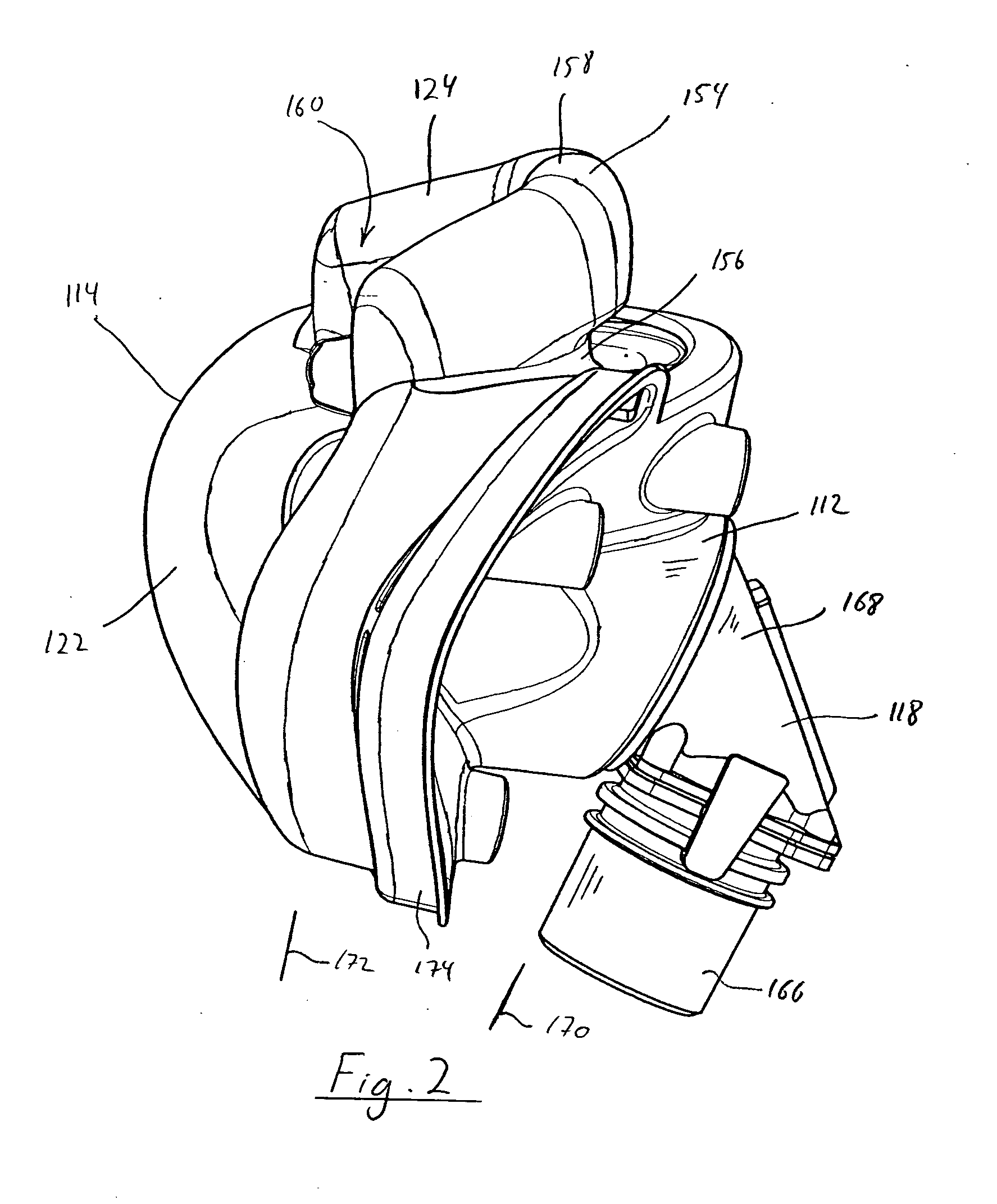
An end effector for a surgical stapler. Various embodiments include a channel configured to operably support a staple cartridge therein. A first tip portion is attached to or formed on a distal end of one of the channel and staple cartridge wherein the first tip portion may have a first upwardly extending curved outer surface. An anvil is movably coupled to the channel and is selectively movable between open and closed positions relative to the staple cartridge in response to opening and closing motions applied to the anvil. A second tip portion is attached to or formed on a distal end thereof. The second tip portion may have a second downwardly extending curved surface such that when the anvil is in the closed position, the second tip portion cooperates with the first tip portion to define a blunt end effector nose. In various embodiments, the end effector nose has a substantially paraboloid outer surface.
Owner:ETHICON ENDO SURGERY INC +1
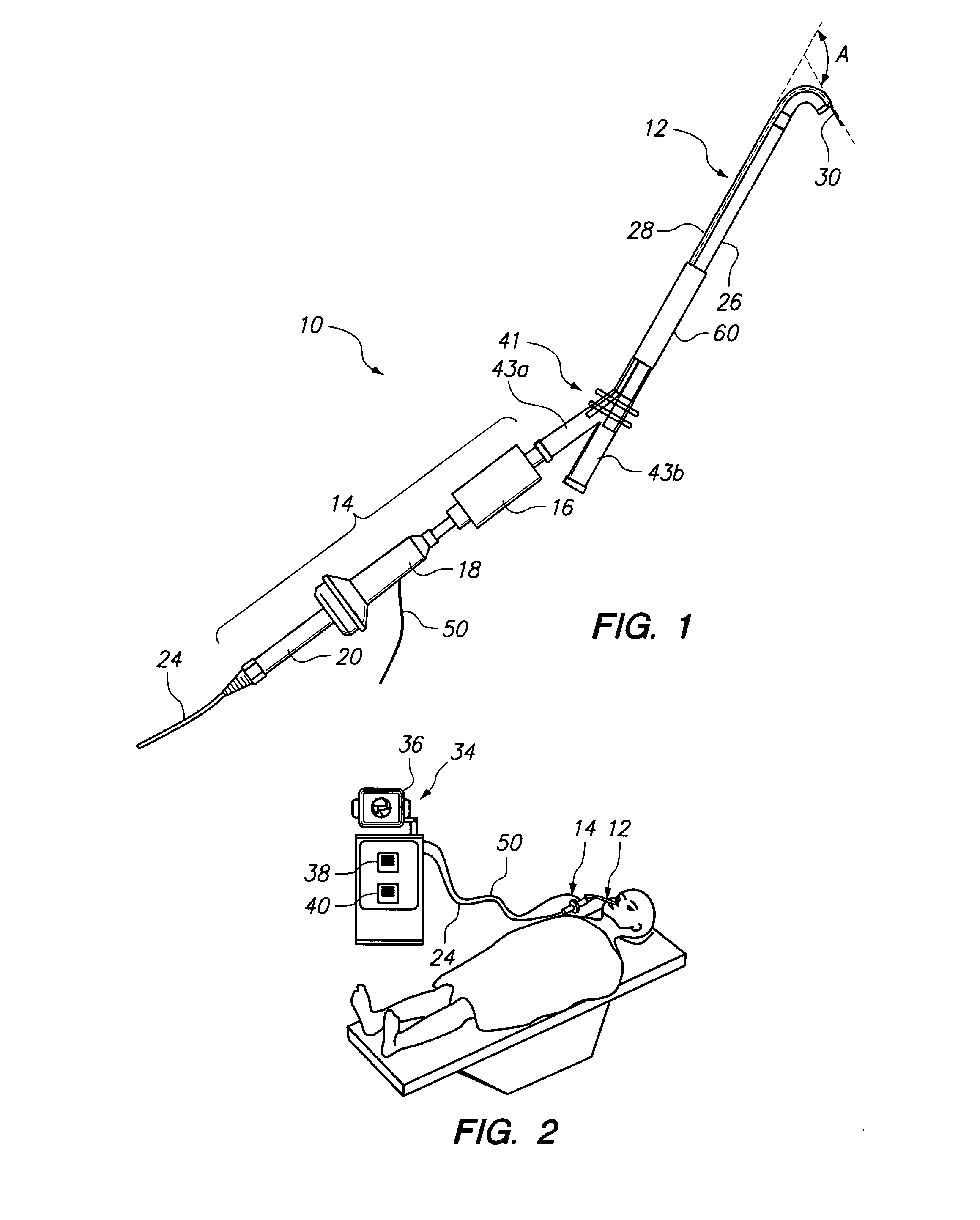
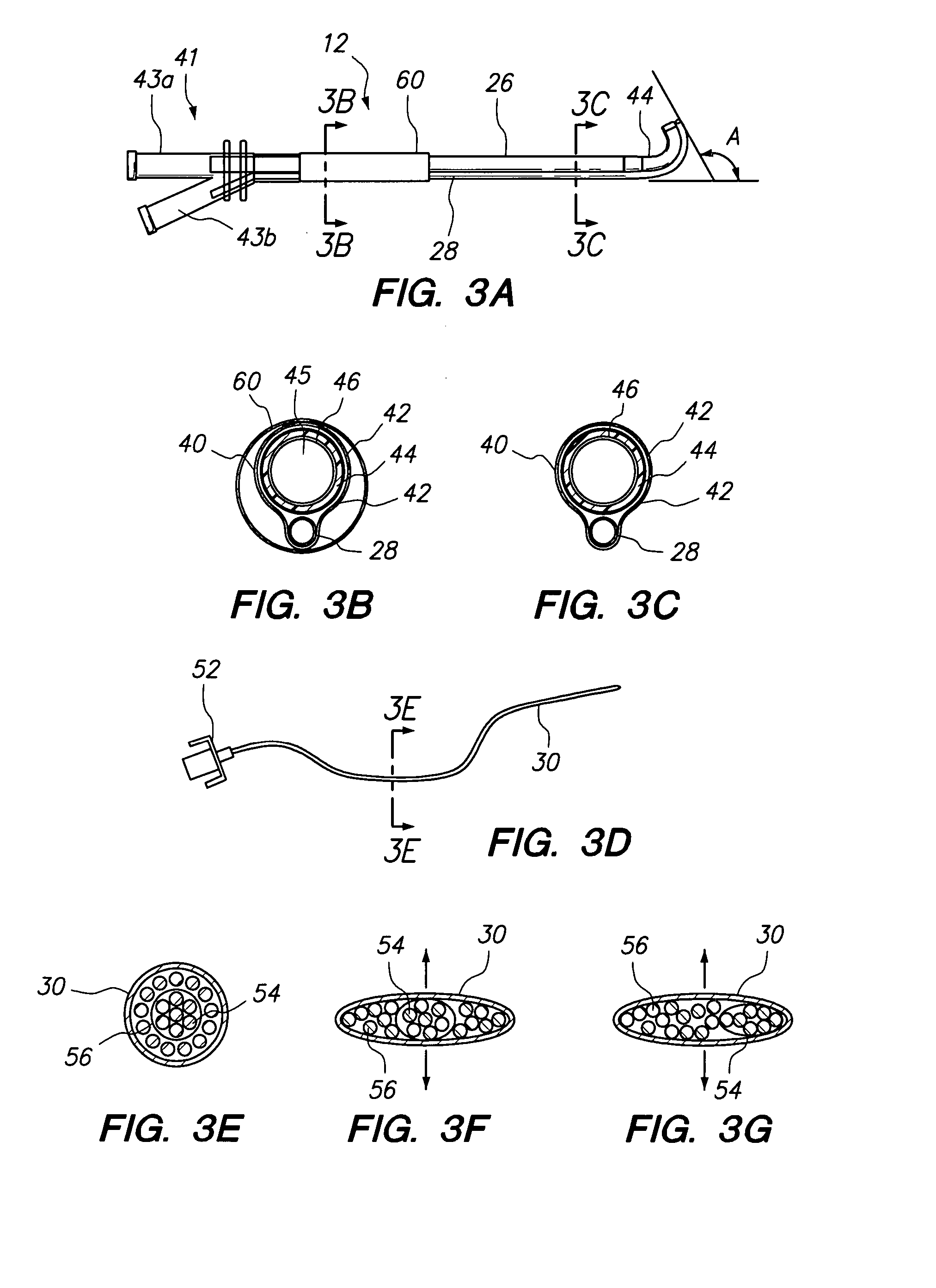
Electrosurgical system and method
InactiveUS7001380B2Lower impedanceMinimizing char formationCannulasDiagnosticsBenign conditionEnlarged tonsils
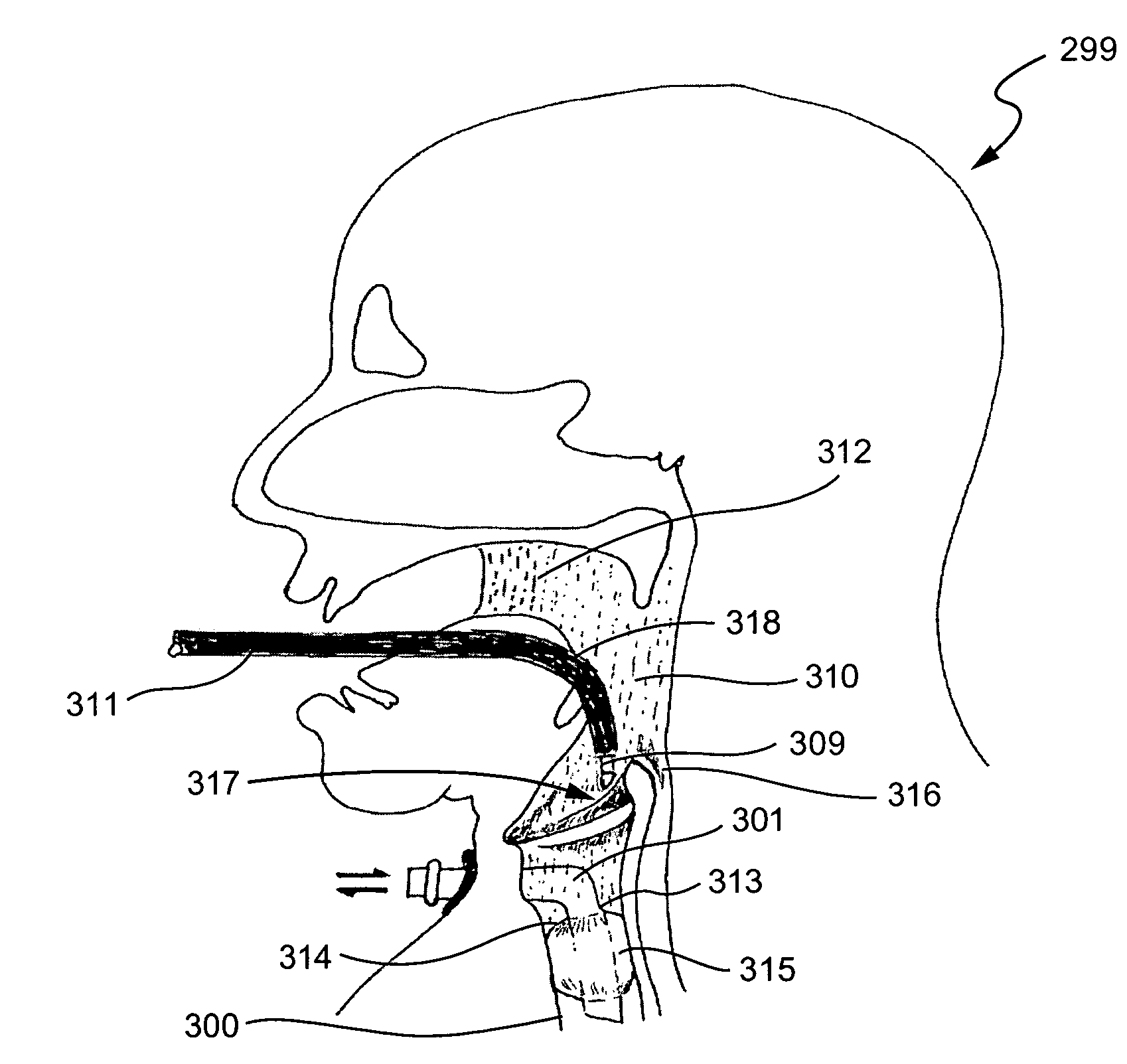
A method is disclosed for treating benign conditions, such as enlarged tonsils and / or adenoids located in a patient's throat or nasopharynx, or soft tissue lesions located in a patient's oropharynx or larynx. According to the method, a space containing the patient's nasopharynx, oropharynx or pharynx and larynx is isolated from the patient's trachea and lungs using an inflatable cuff tracheostomy tube or nasotracheal tube inserted in the patient's trachea. The cuff is inflated to occlude the trachea. The patient is placed in a supine position, whereupon at least a portion of the space containing the nasopharynx and / or oropharynx and larynx is filled with saline. An endoscope is then inserted into the space to view the operative site in which the tonsils or tissue lesion are to be treated. An electrosurgical instrument having an active tissue treatment electrode and a return electrode connected to an electrosurgical generator is then inserted into the space, either along side the endoscope or through the endoscope's working channel. The generator is then operated to apply a radio frequency voltage between the active and return electrodes of the electrosurgical instrument, whereby a conduction path is formed between the active and return electrodes, at least partially through the saline, whereupon the active electrode is manipulated to debulk or otherwise treat the soft tissue lesion or enlarged tonsils and / or adenoids.
Owner:GYRUS MEDICAL LTD
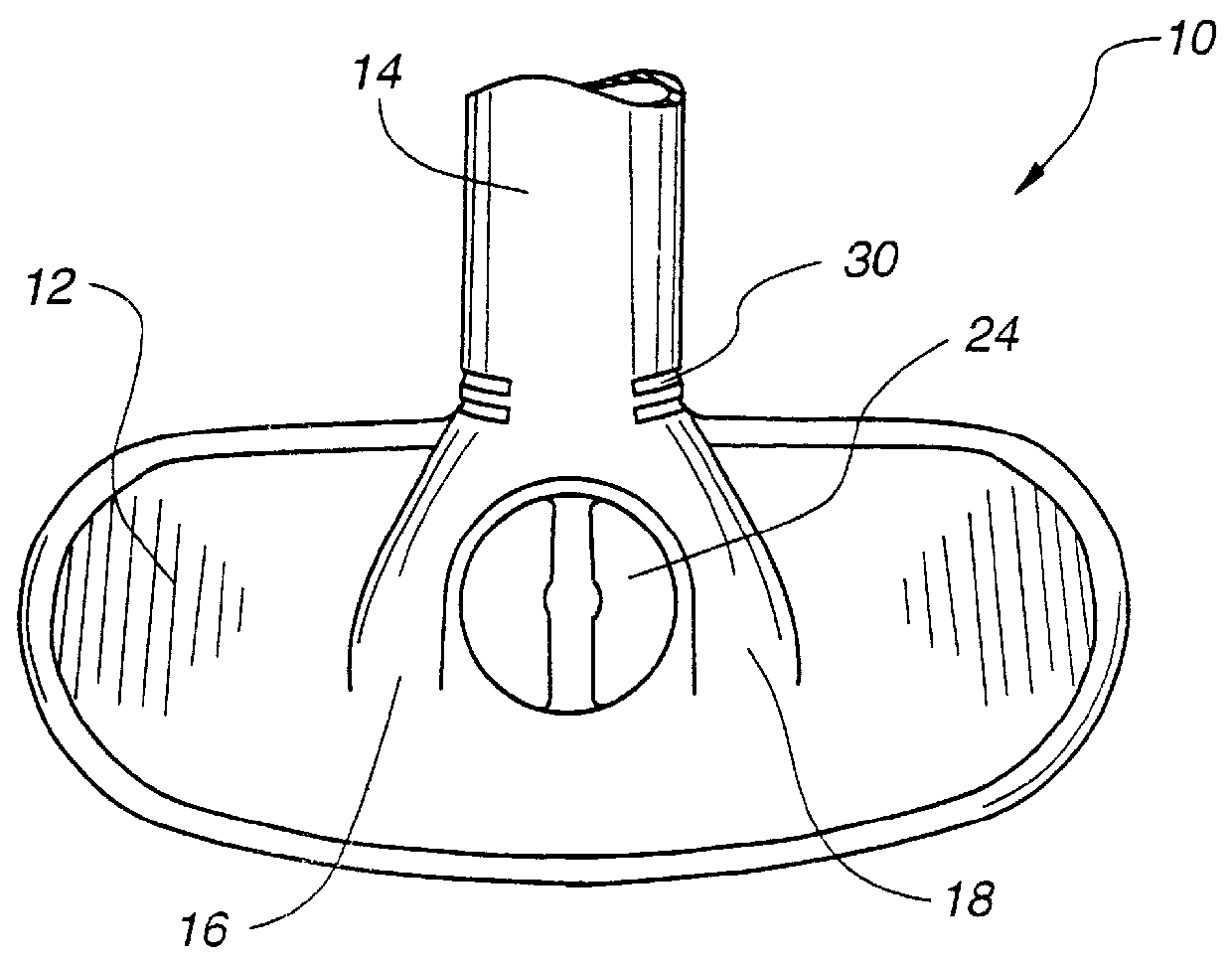
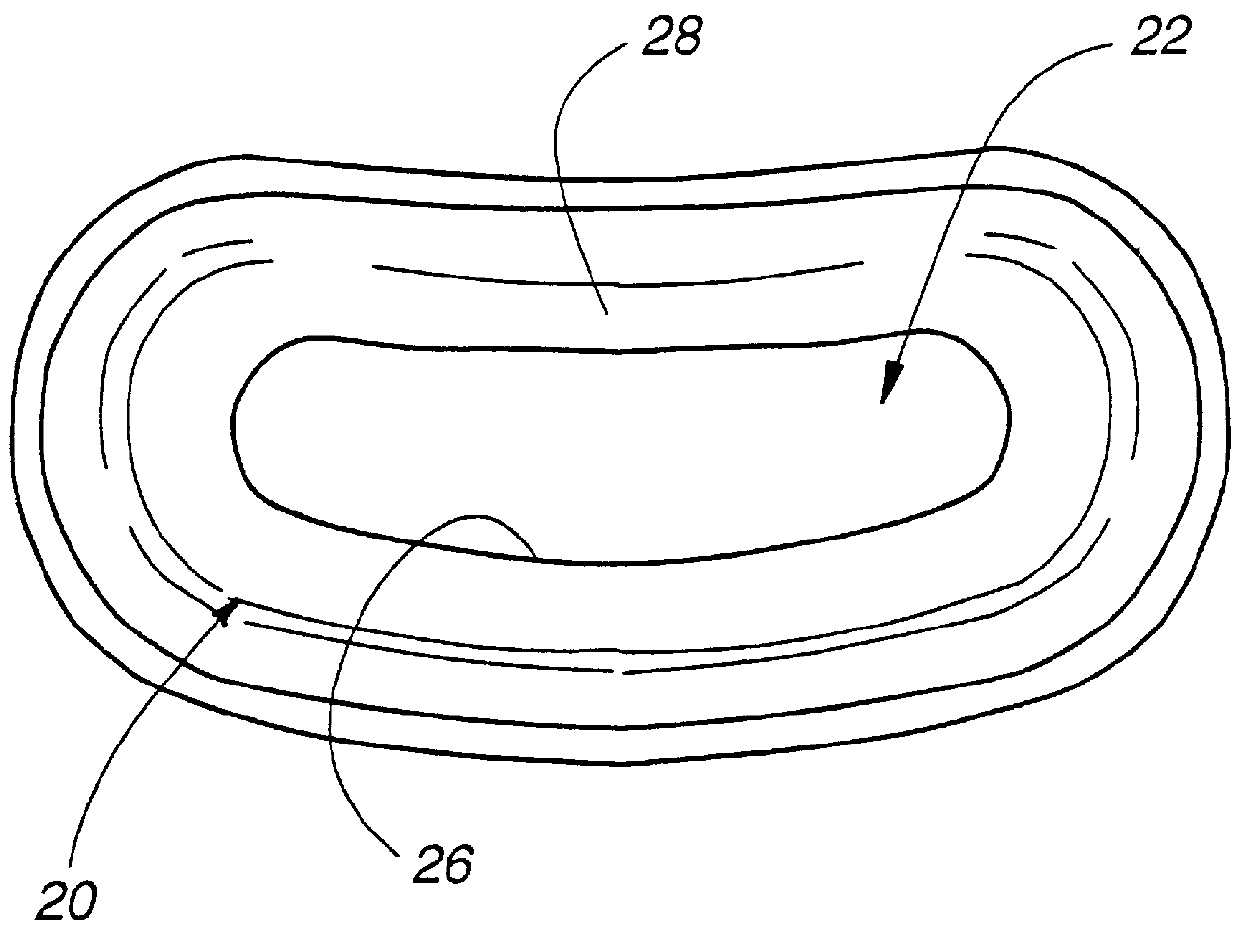
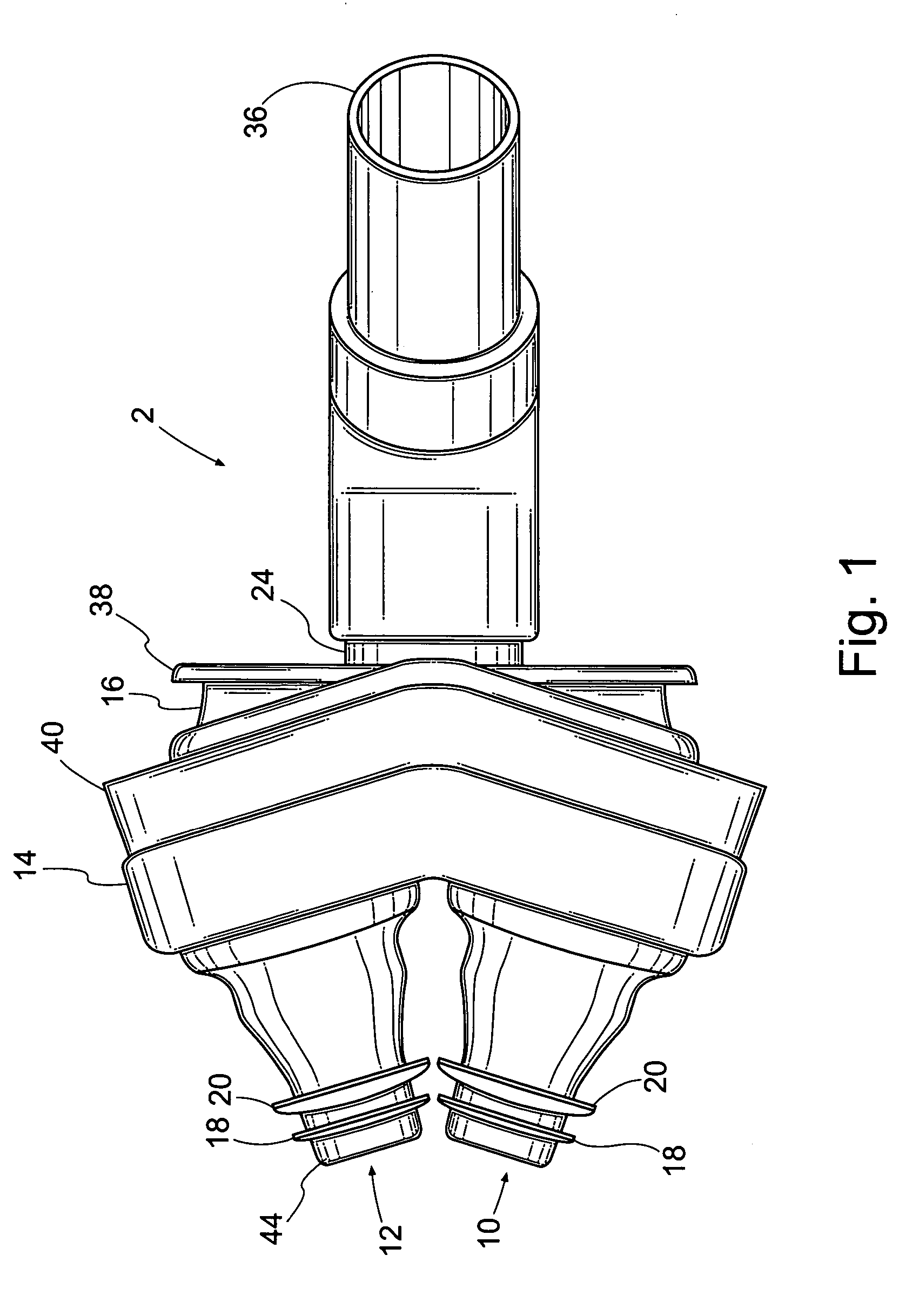
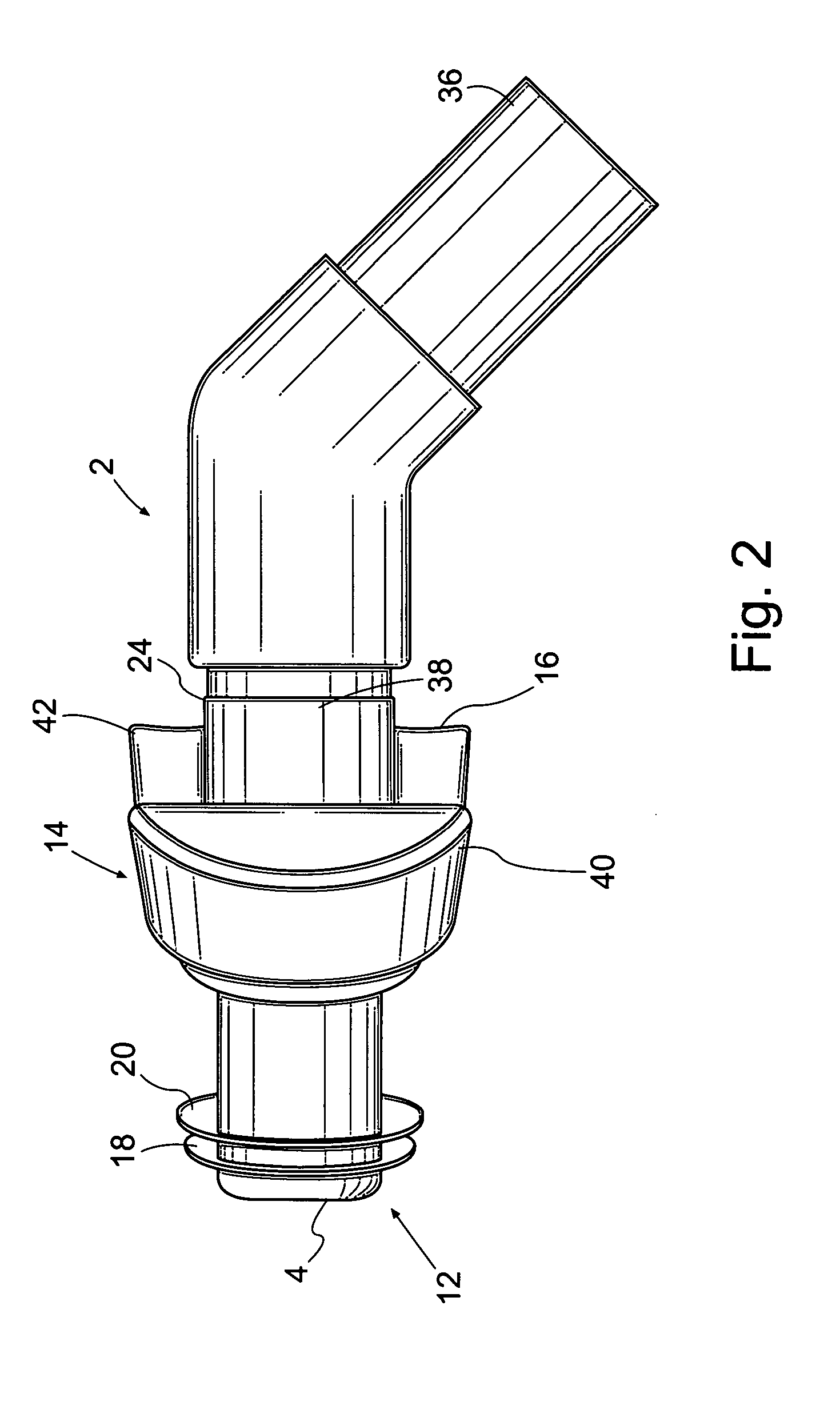
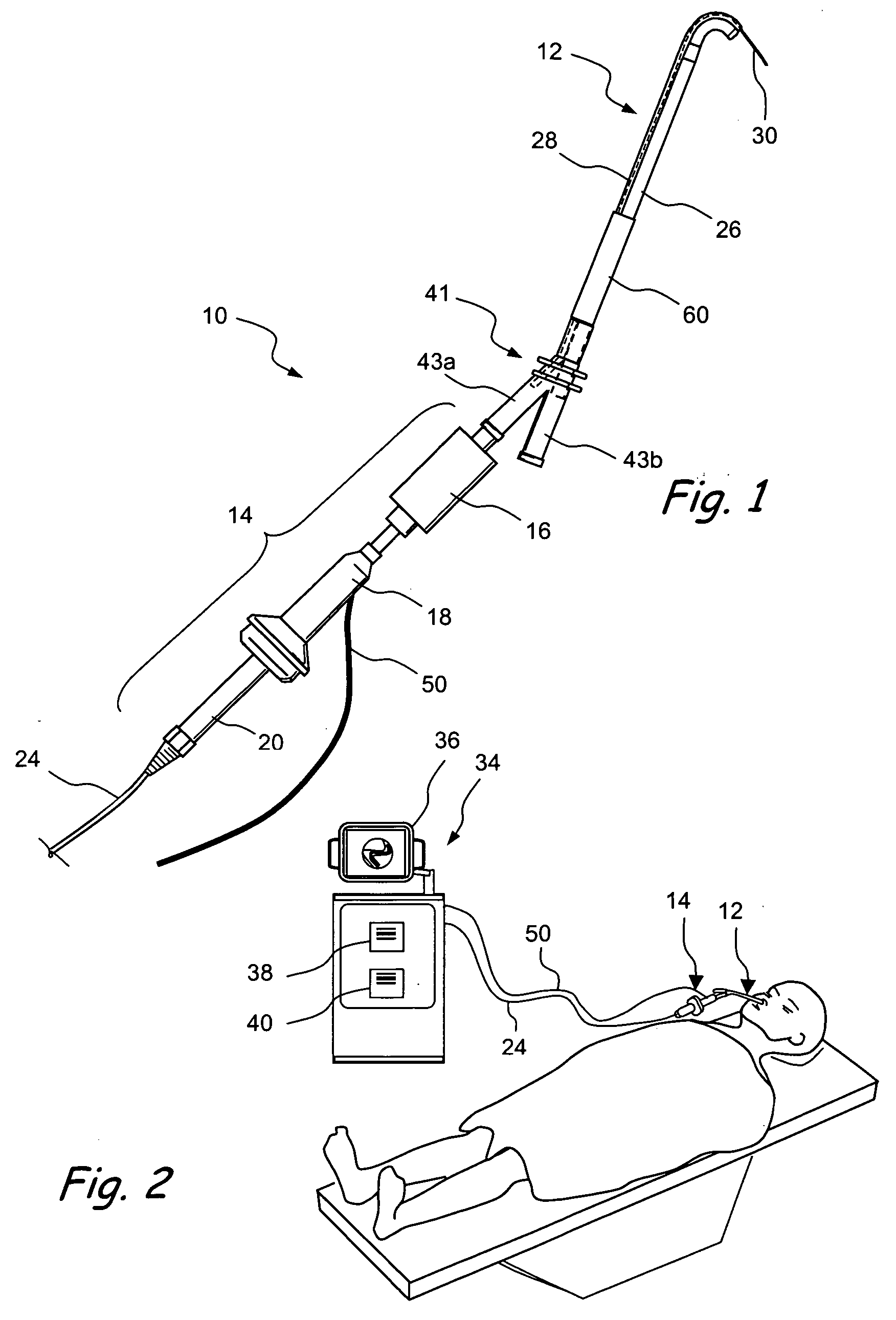
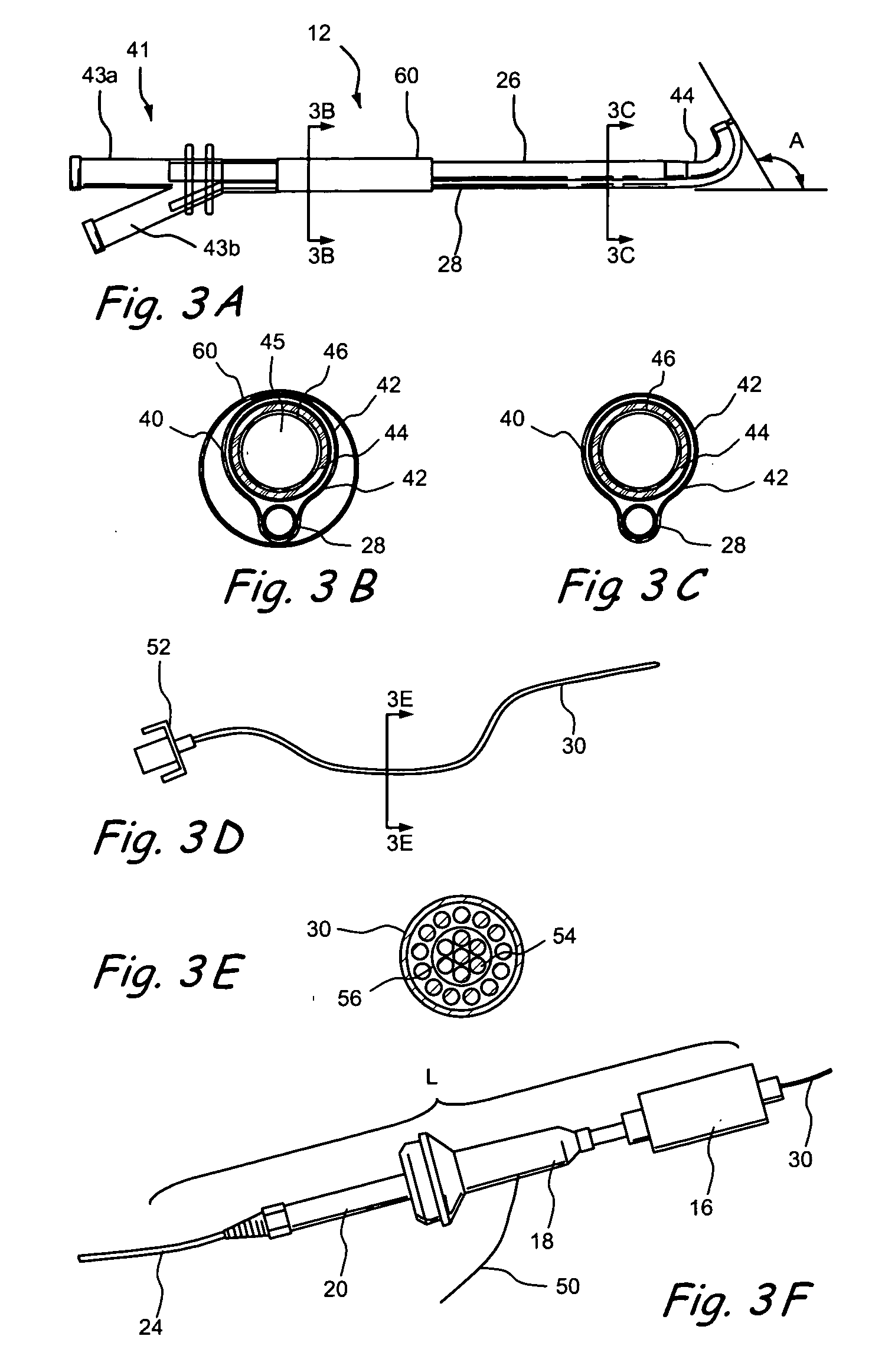
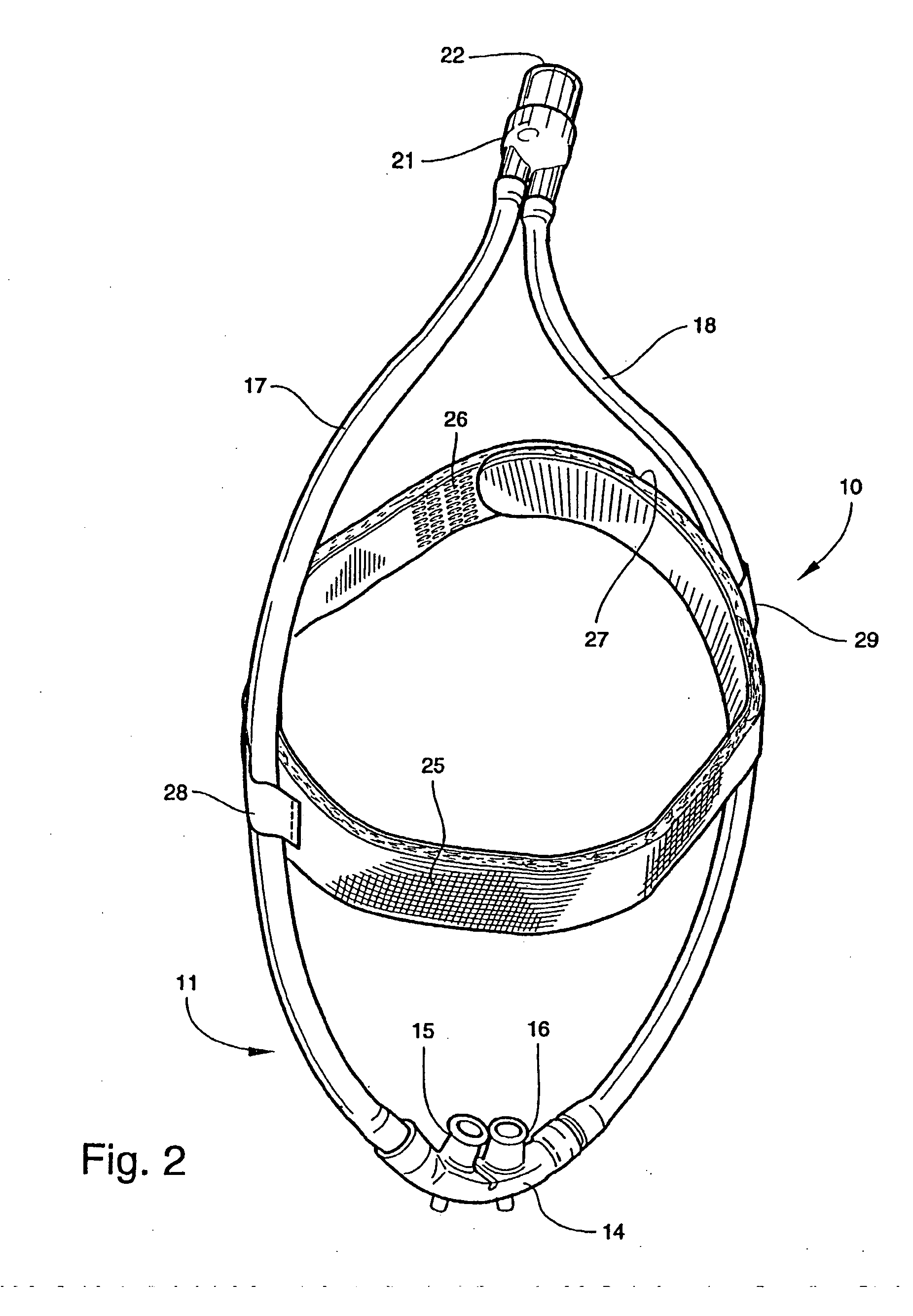
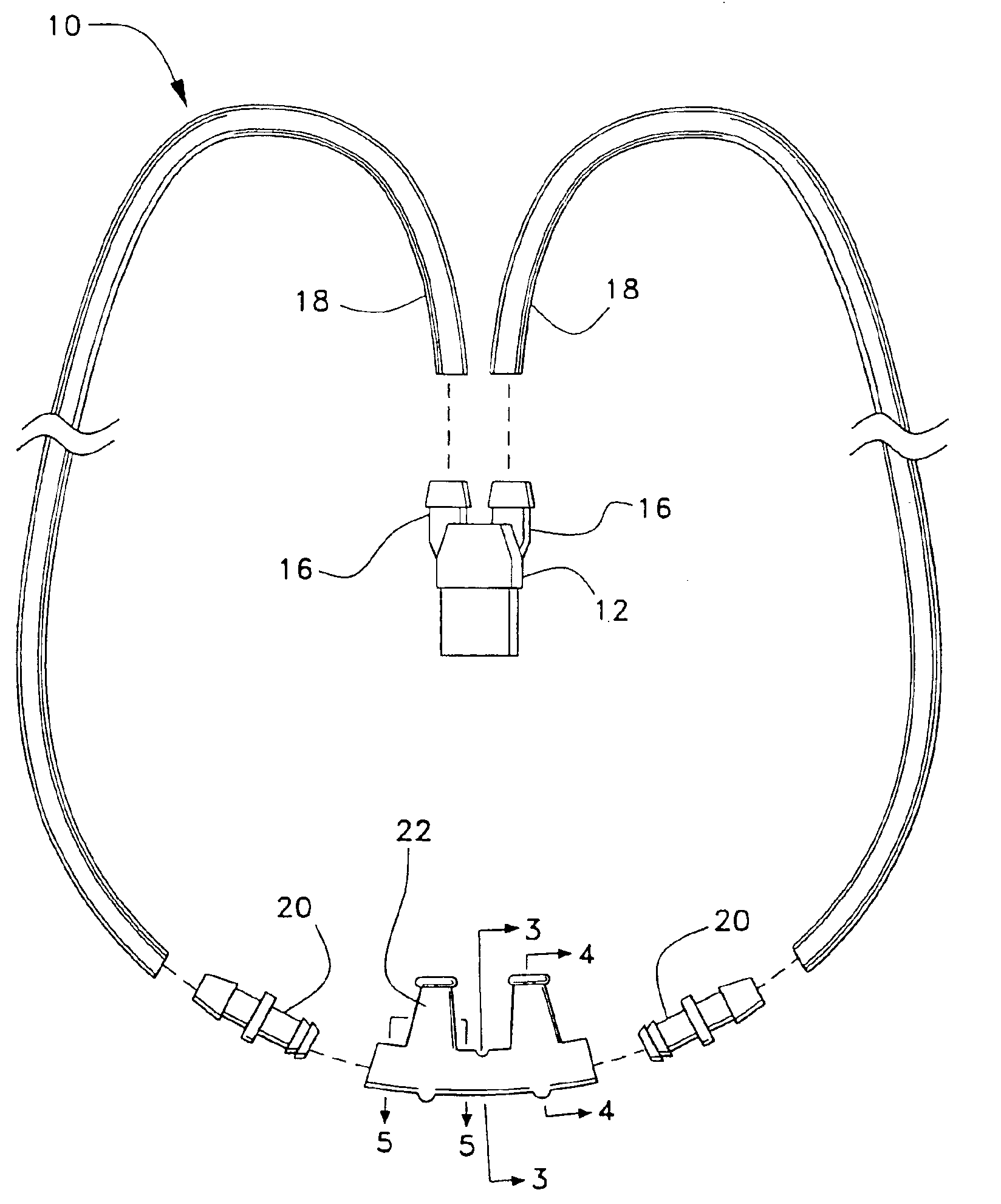
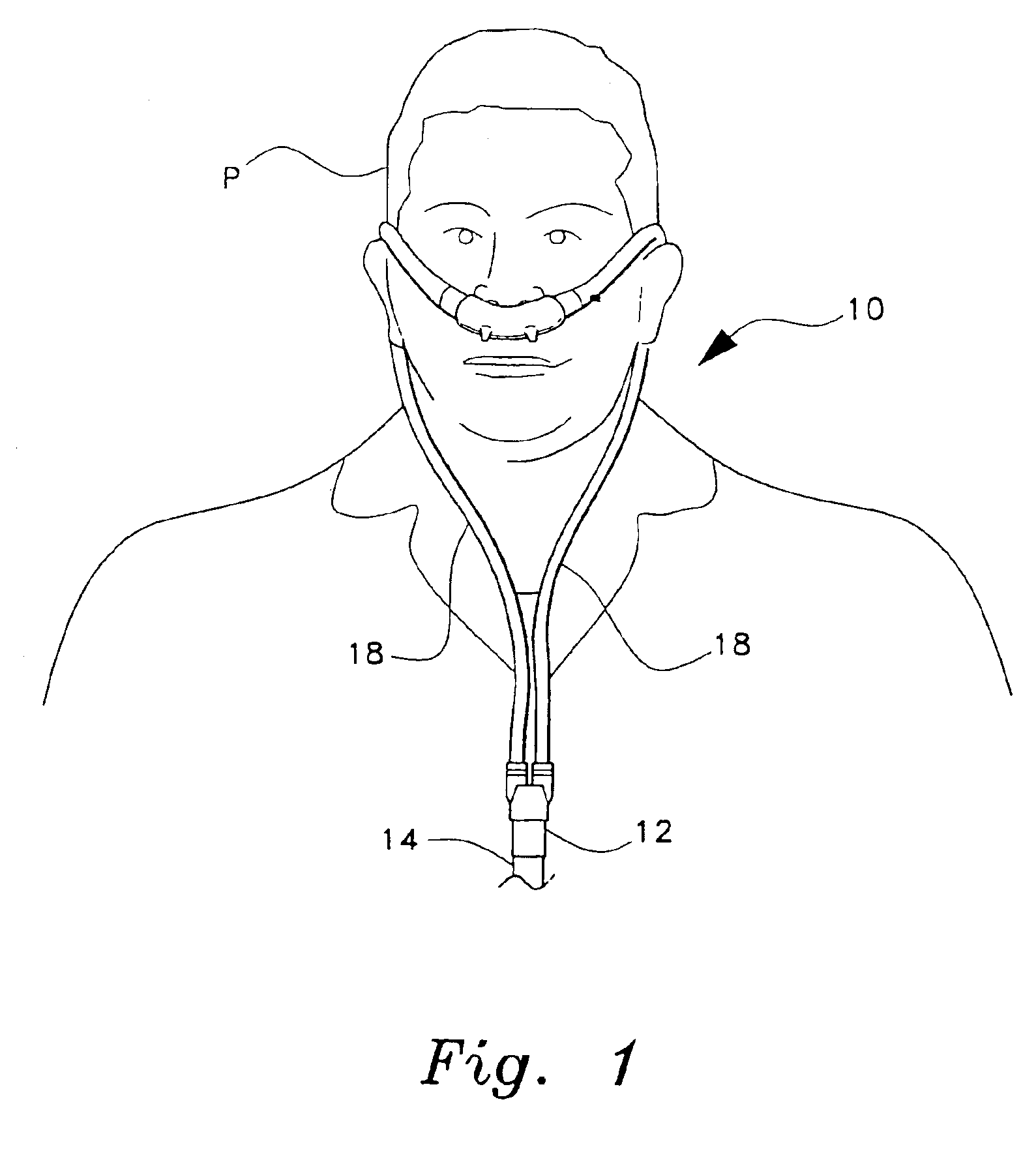
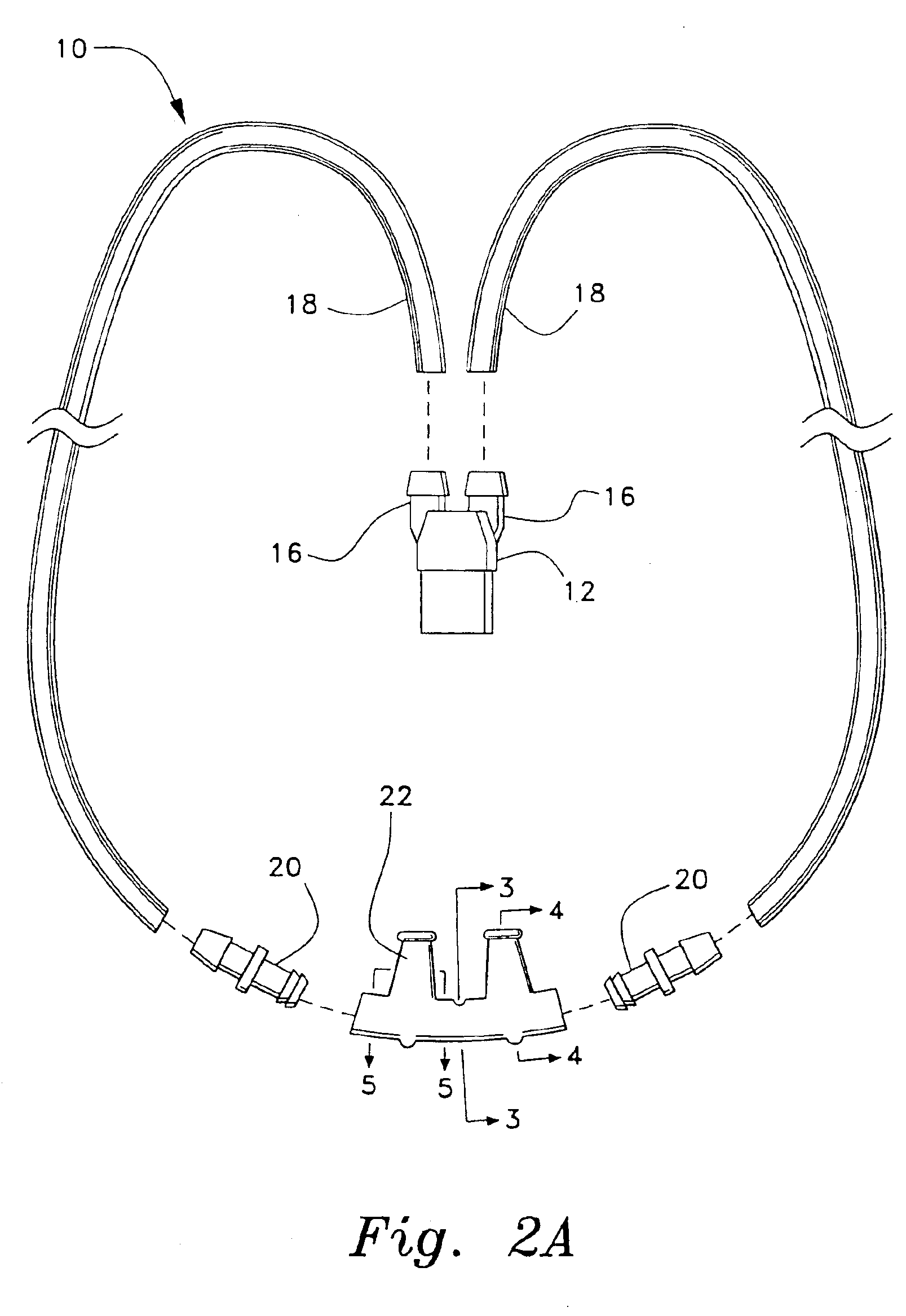
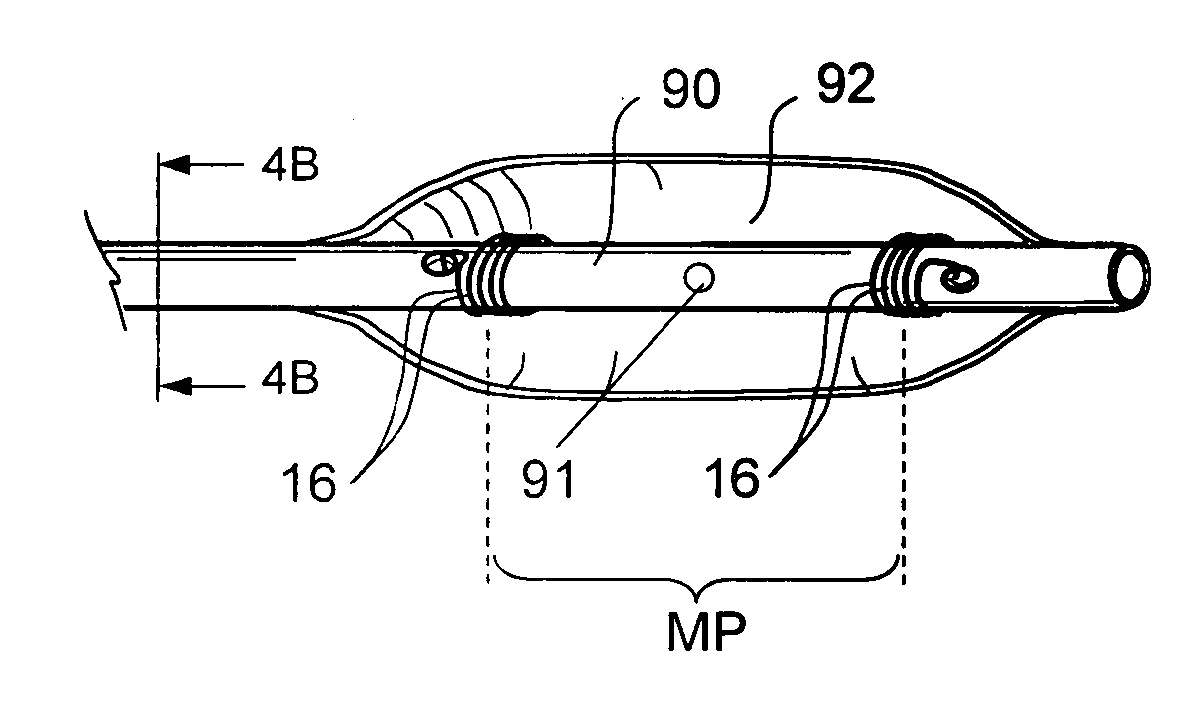
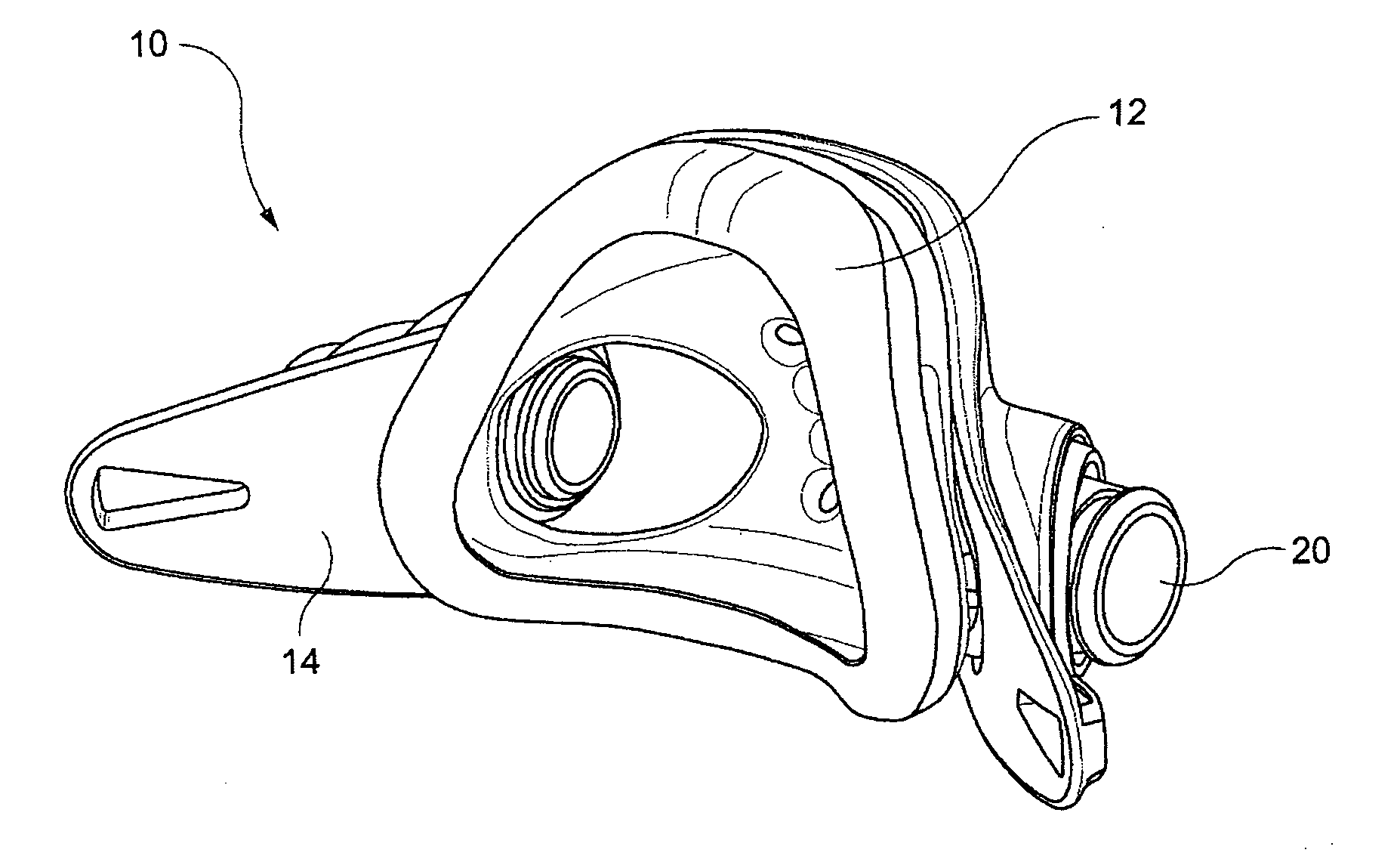
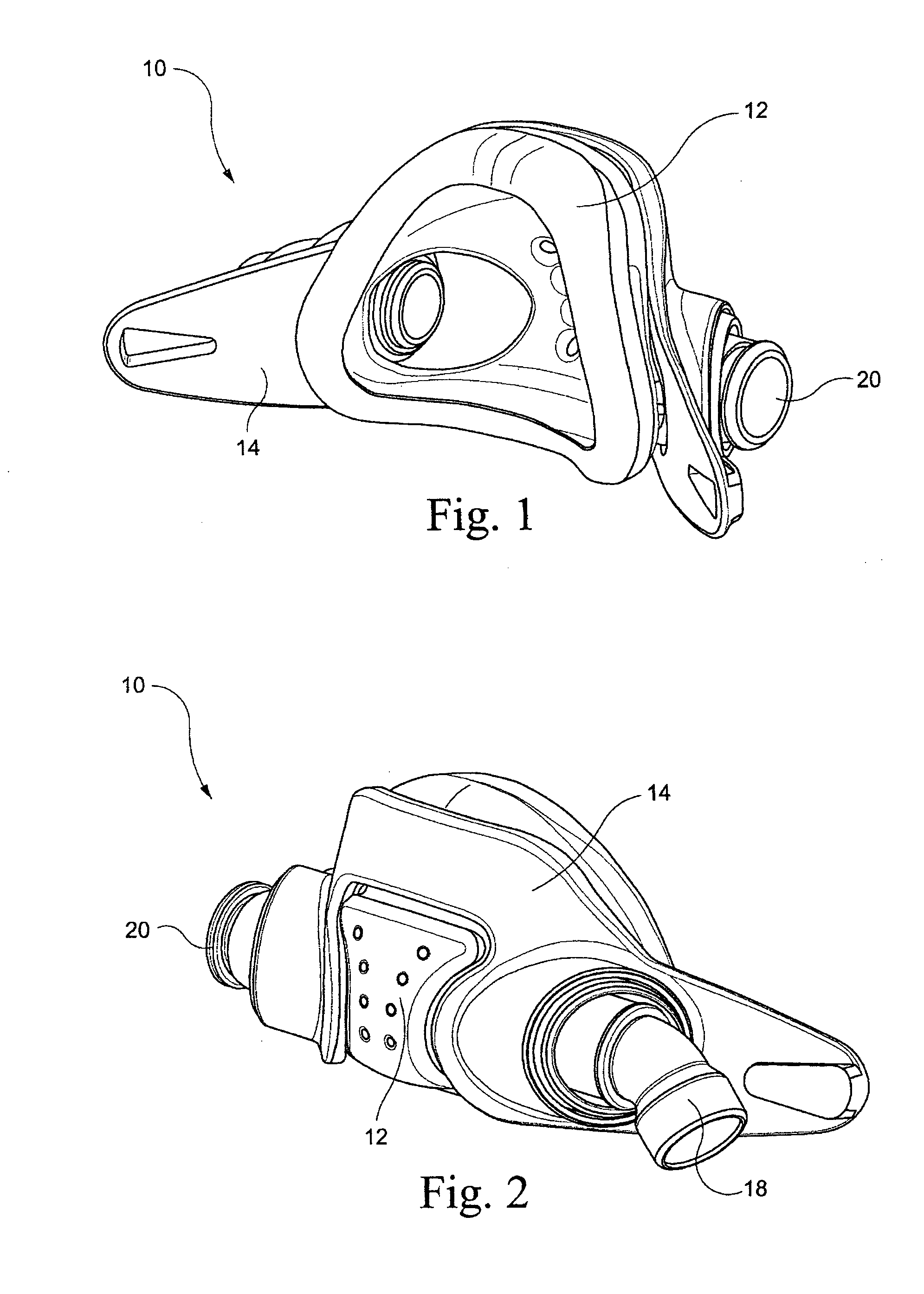
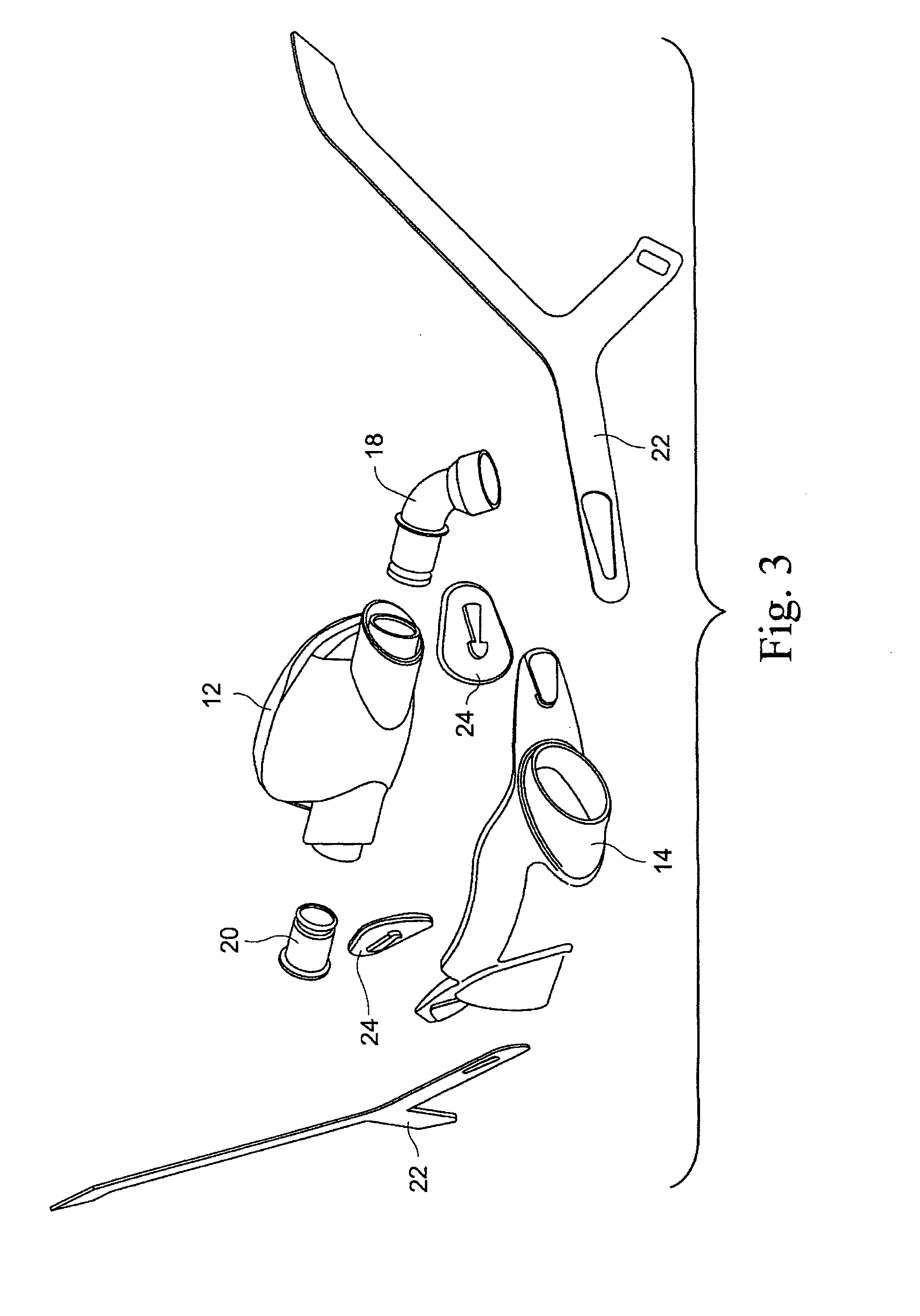
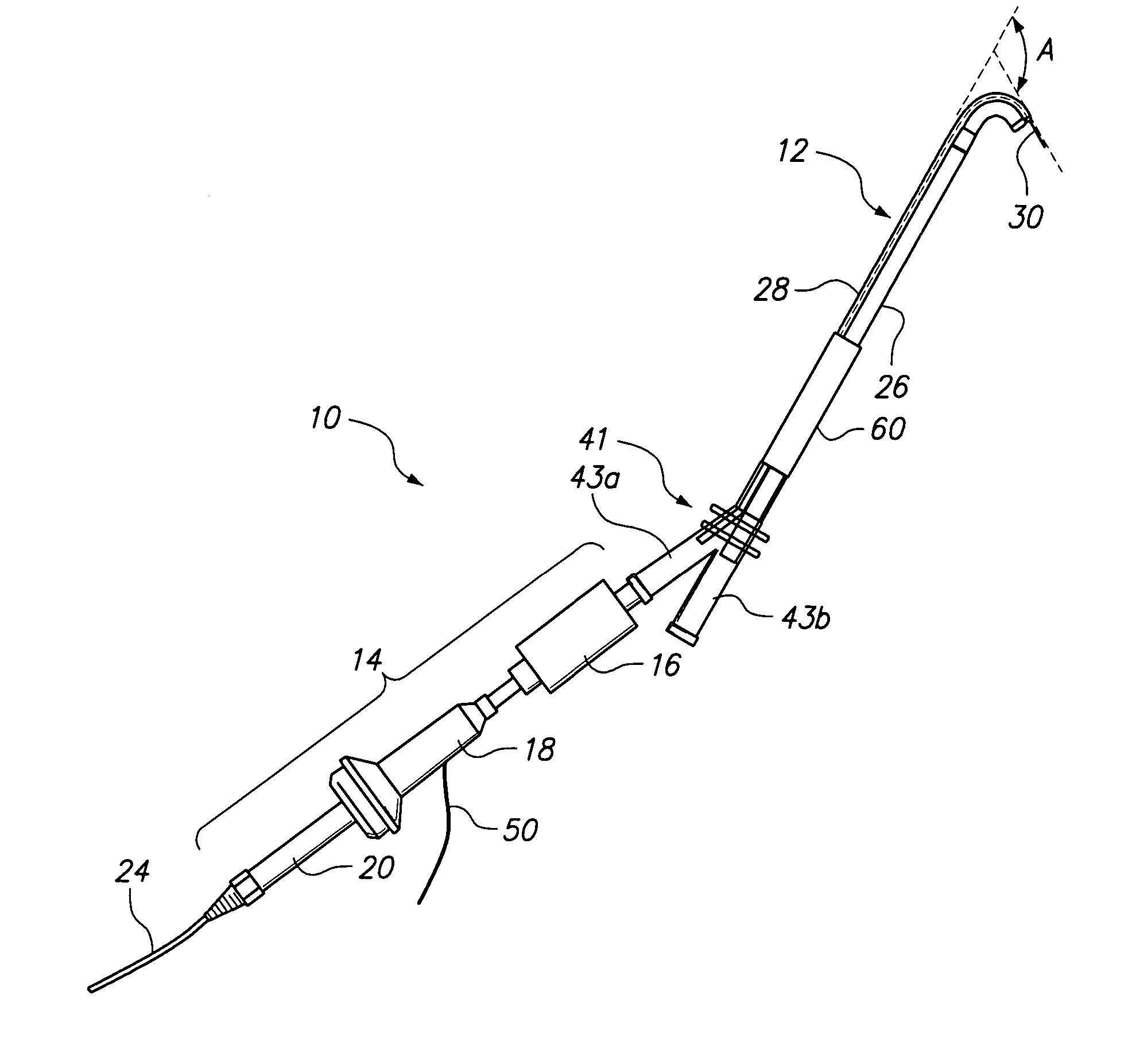
Patient interface systems
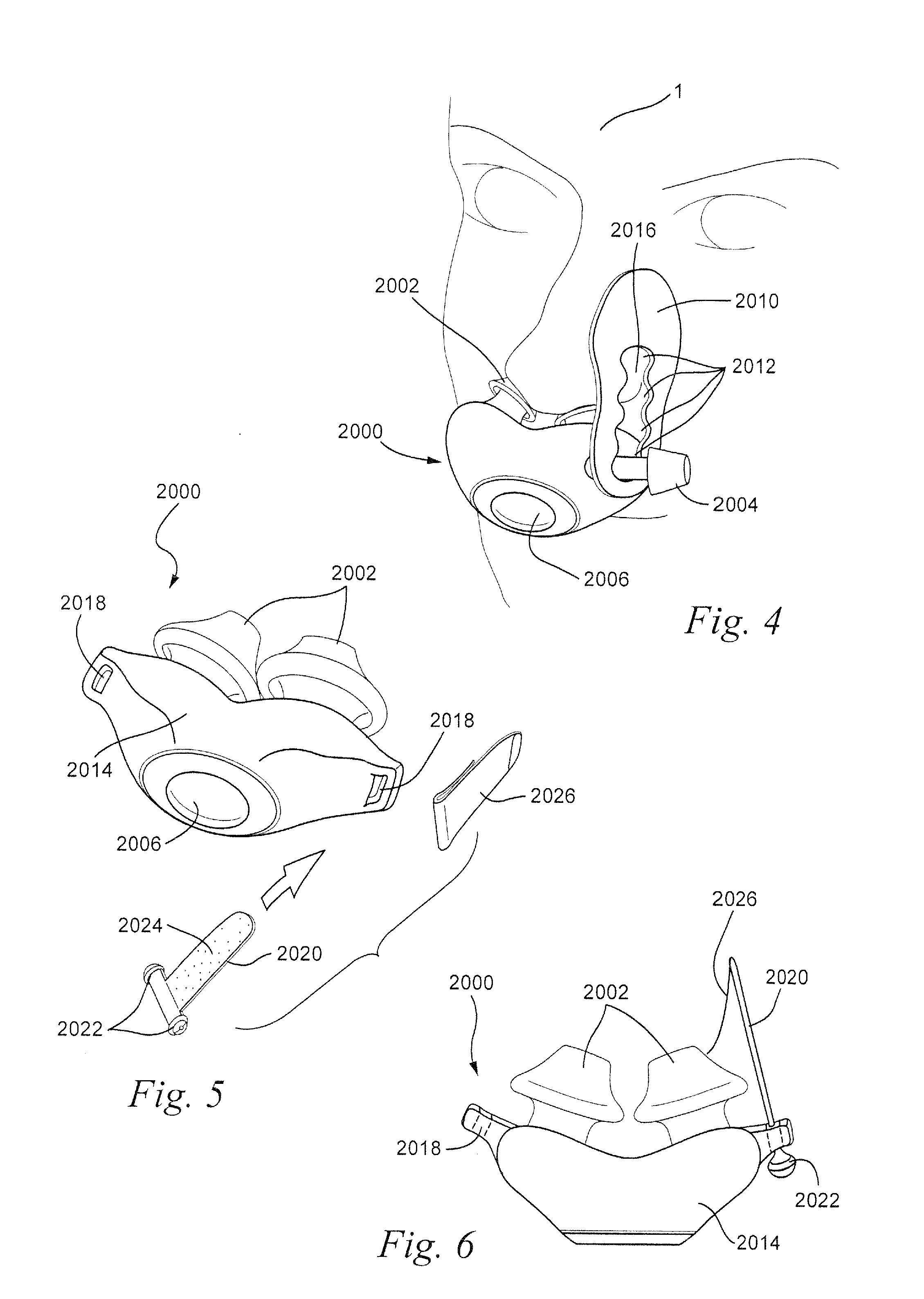
A patient interface system for delivering a flow of breathable gas to a patient includes a patient interface structure configured to sealingly engage the patient's nares; a pair of strips configured to be connected to opposite sides of the patient interface structure; and adhesive configured to secure the patient interface structure in sealing engagement with the patient's nares. The adhesive may be provided on the strips, and the strips are configured to be adhered to sides of the patient's nose by the adhesive. Another patient interface system includes a first component including adhesive on a first side and hook or loop fastener material on a second side, wherein the adhesive is configured to adhere the component to the face of the patient; and a patient interface structure configured to sealingly engage the patient's airways. The patient interface structure includes a second component including a corresponding loop or hook fastener material configured to engage the corresponding hook or loop fastener material of the first strip to secure the patient interface structure in sealing engagement with the patient's face. A patient interface system for delivering a flow of breathable gas to a patient includes a patient interface structure including a pair of nasal prongs or pillows configured to sealingly engage the patient's nares; and at least one spring configured to bias the nasal prongs or pillows outwards into engagement with the nares of the patient.
Owner:RESMED LTD
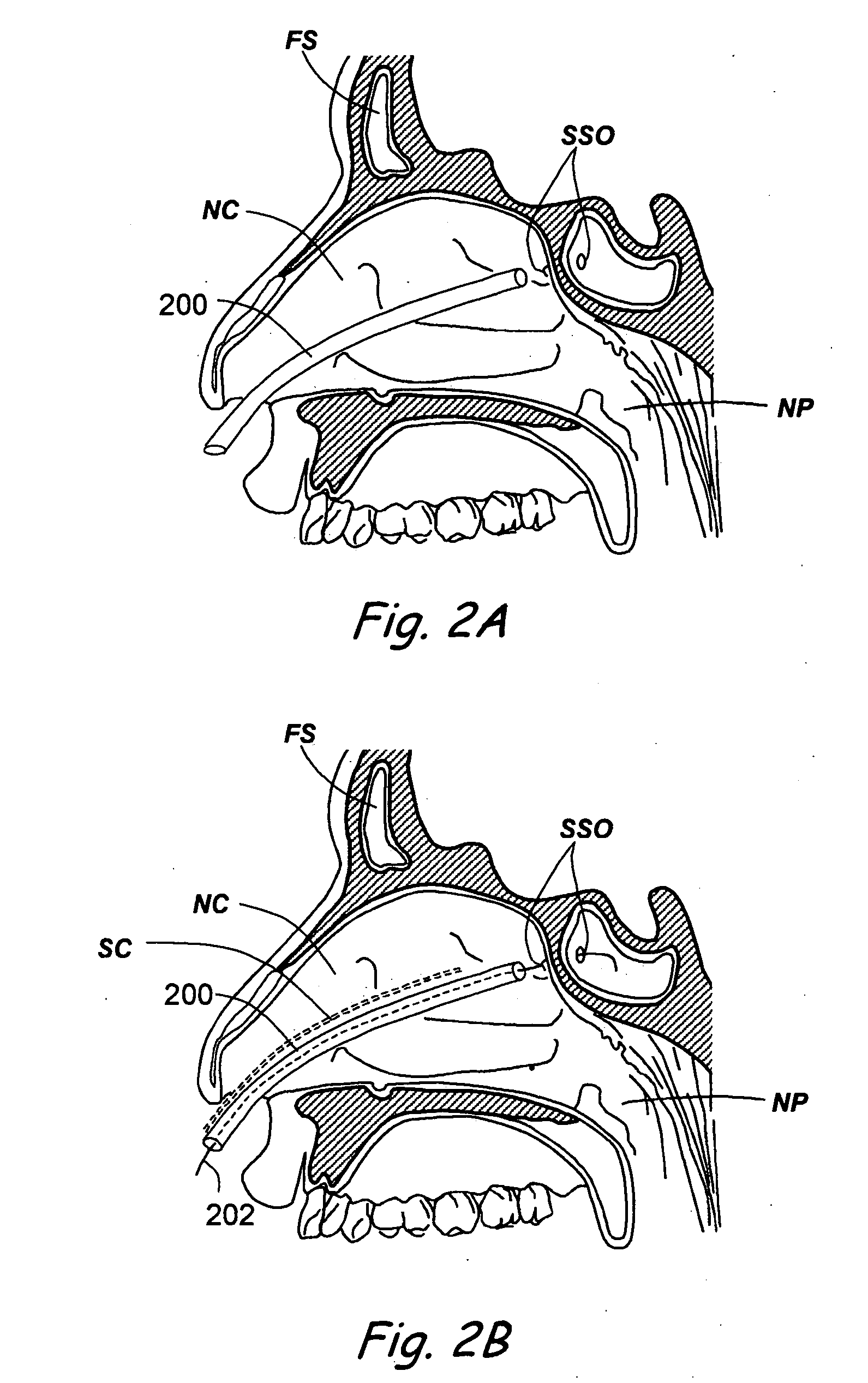
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
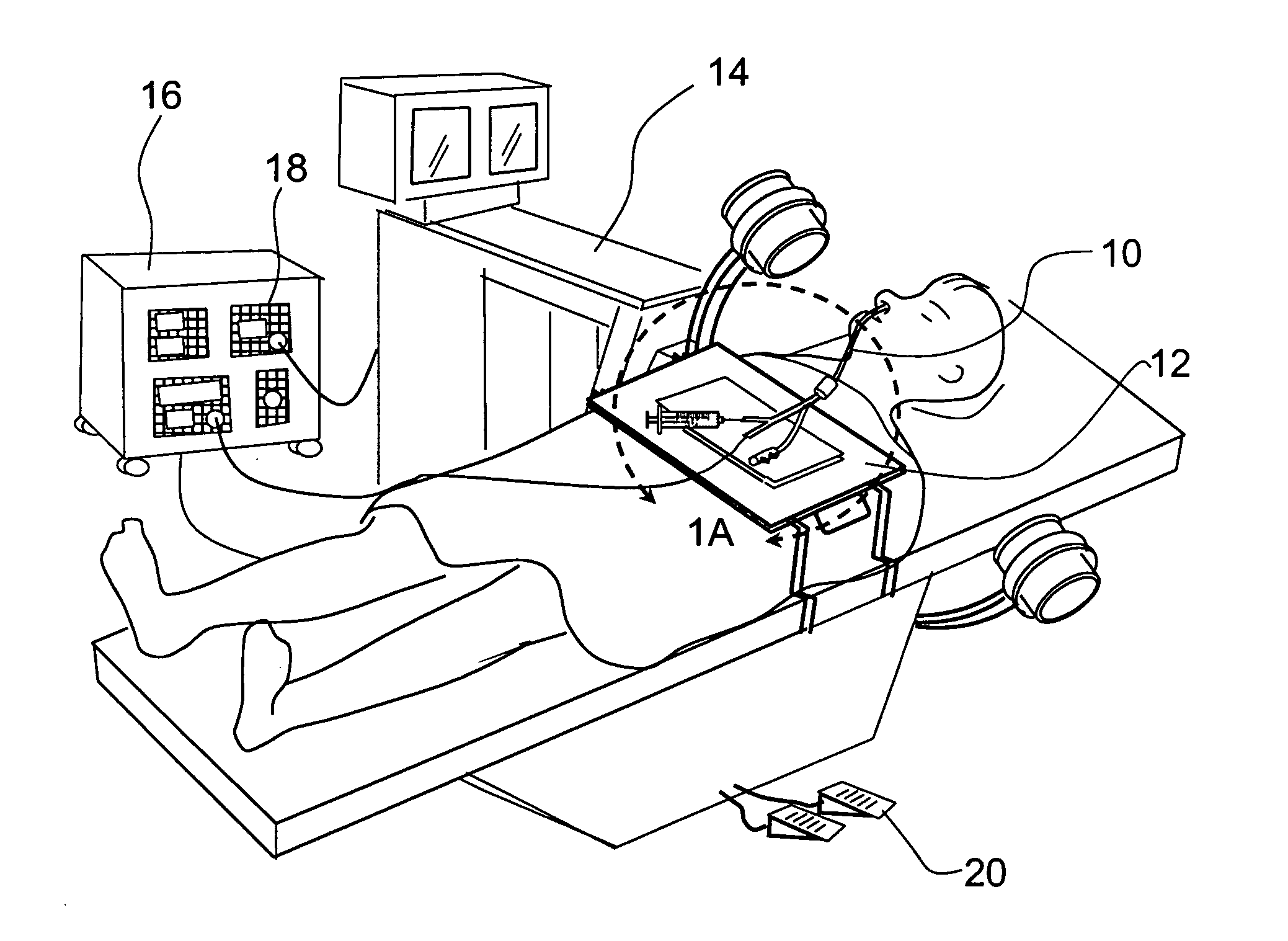
Systems and methods for performing image guided procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Devices, systems and methods for treating disorders of the ear, nose and throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
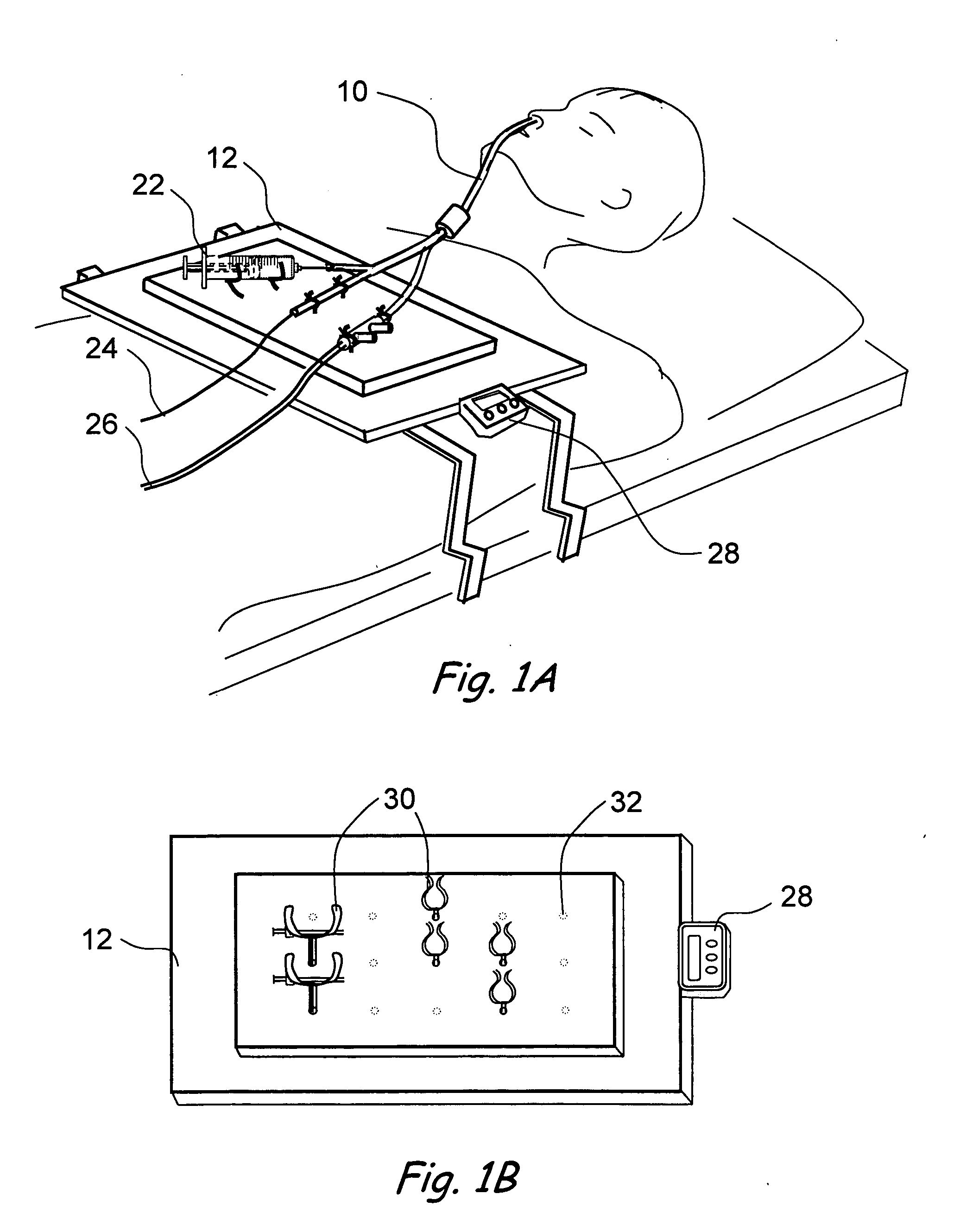
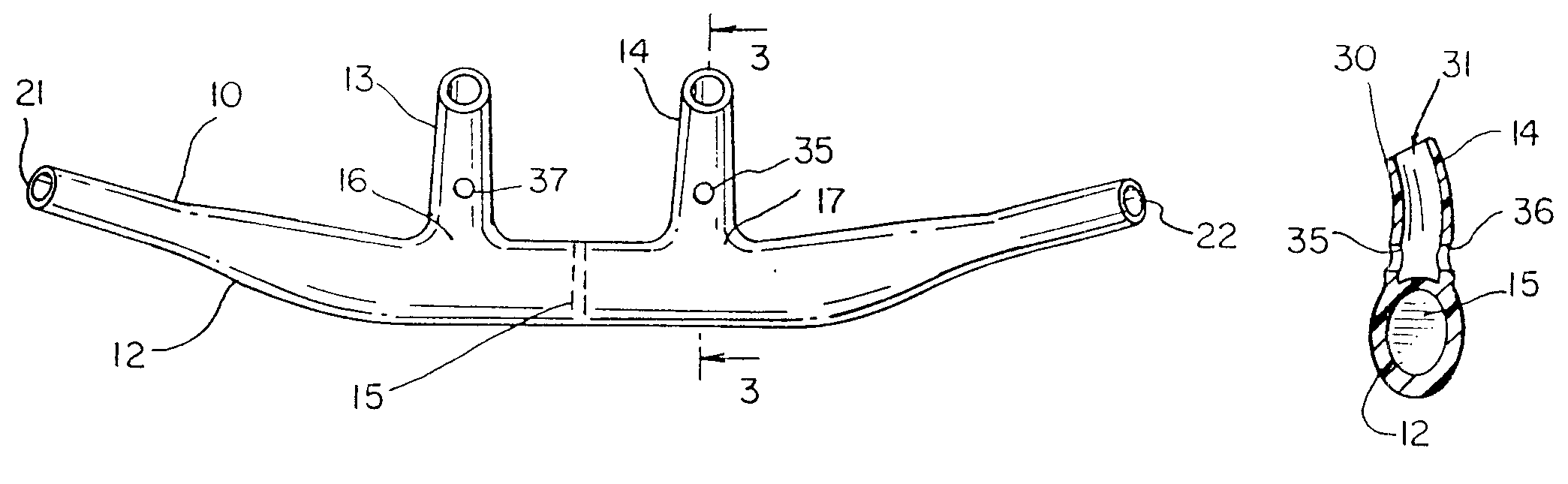
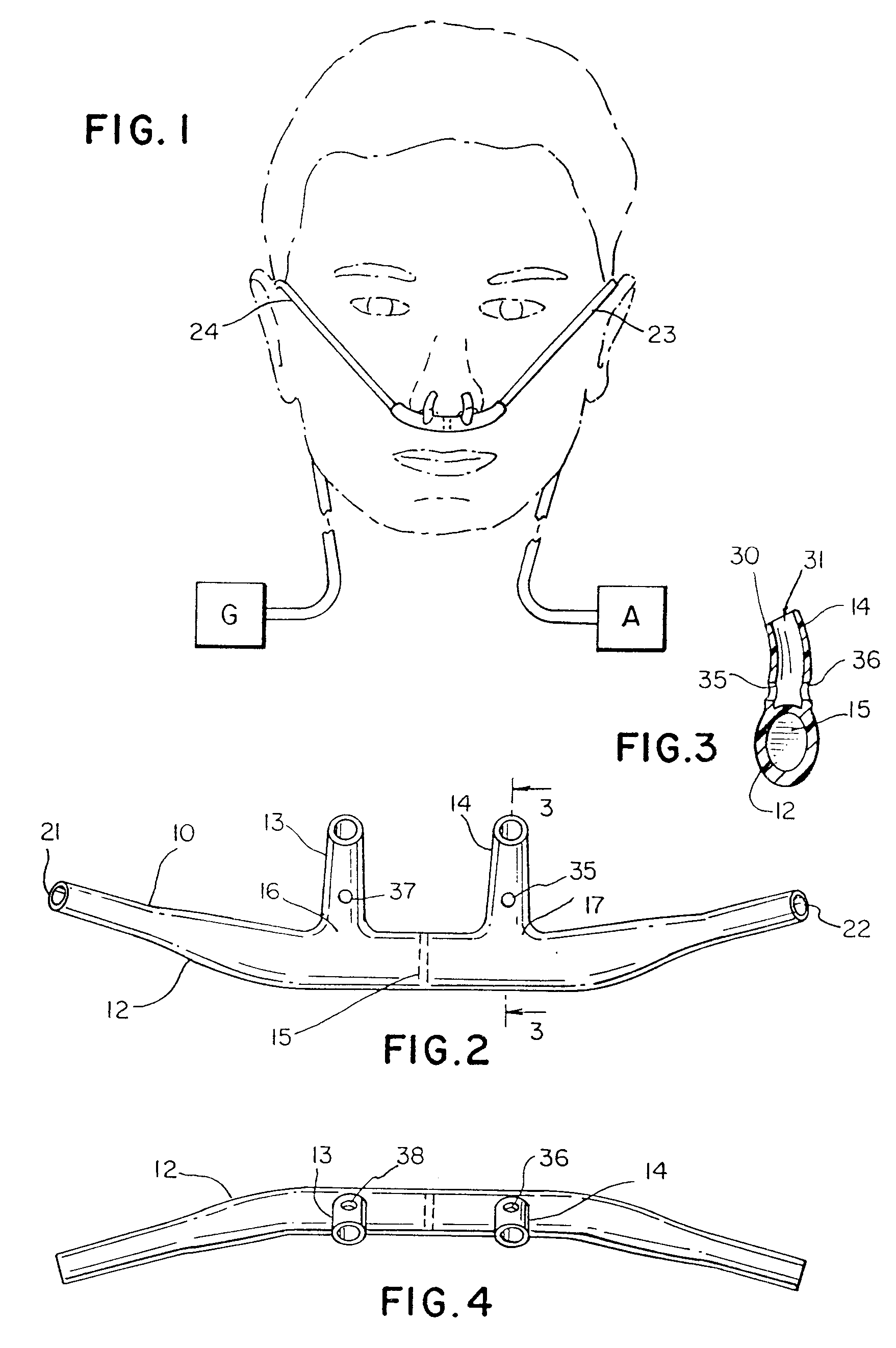
Hybrid ventilation mask with nasal interface and method for configuring such a mask
ActiveUS20060124131A1Improve comfortEliminate requirementsRespiratory masksBreathing masksNasal cavityNose
A nasal ventilation interface and method for providing nasal ventilation to a patient includes a hybrid face mask, covering only the mouth, coupled with removable nasal inserts extending from the upper surface of the mask. The nasal interface has modular, removable, and disposable nasal pillows connecting the upper surface of the mouth-portion of the mask to the user's nares. Each part of the hybrid mask is modular and can be formed in various shapes and sizes. The ventilation interface has differing gas line entry ports and can be shallow with a relatively deep soft facial interface or deep with a relatively shallow facial interface. In either case, the upper surface receives the nasal interface. The upper surface can have a removable ceiling in which is defined a nasal interface connector. The nasal interface can be integral with a removable ceiling. The entire hybrid mask can be made in one piece.
Owner:SALTER LABS LLC
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
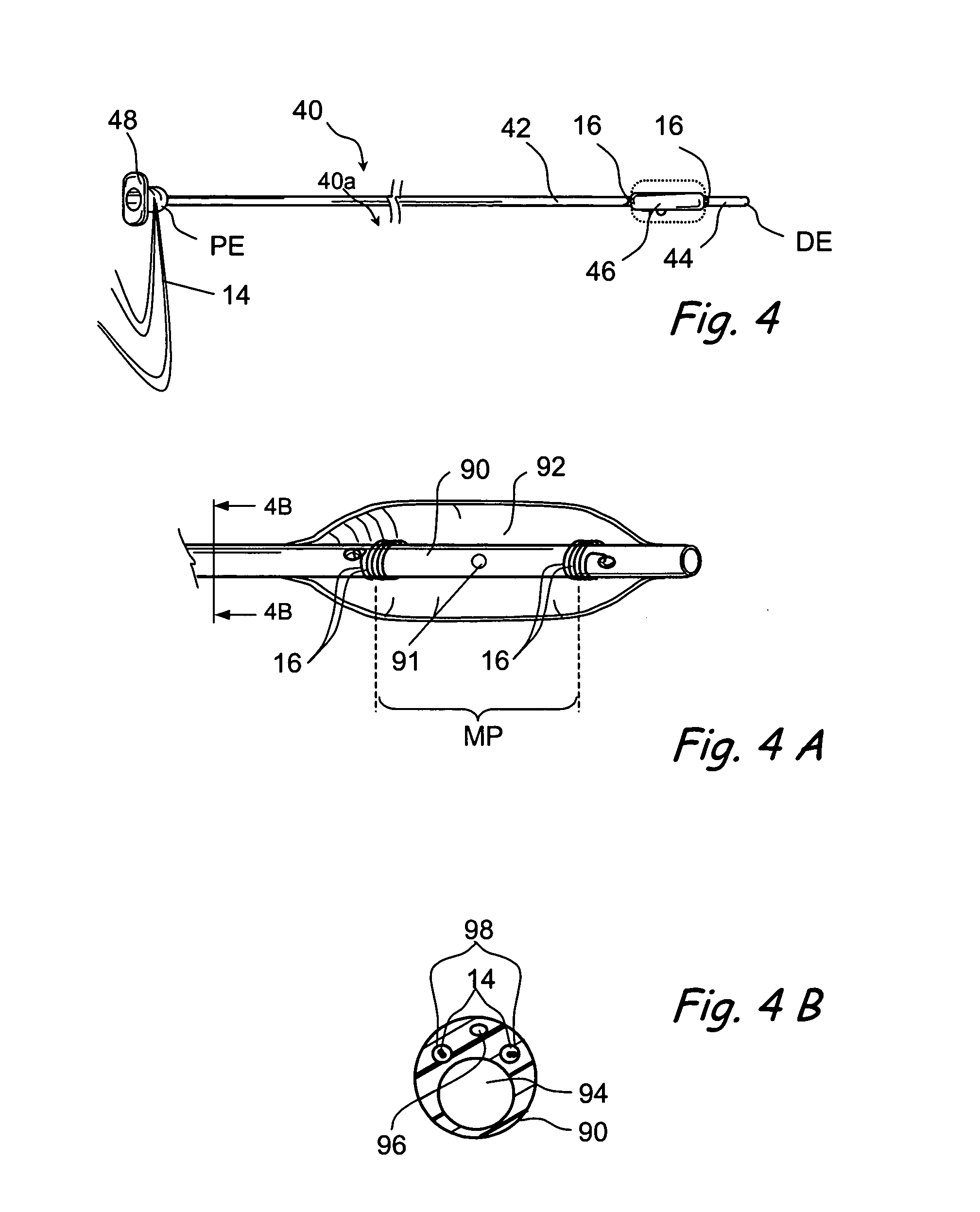
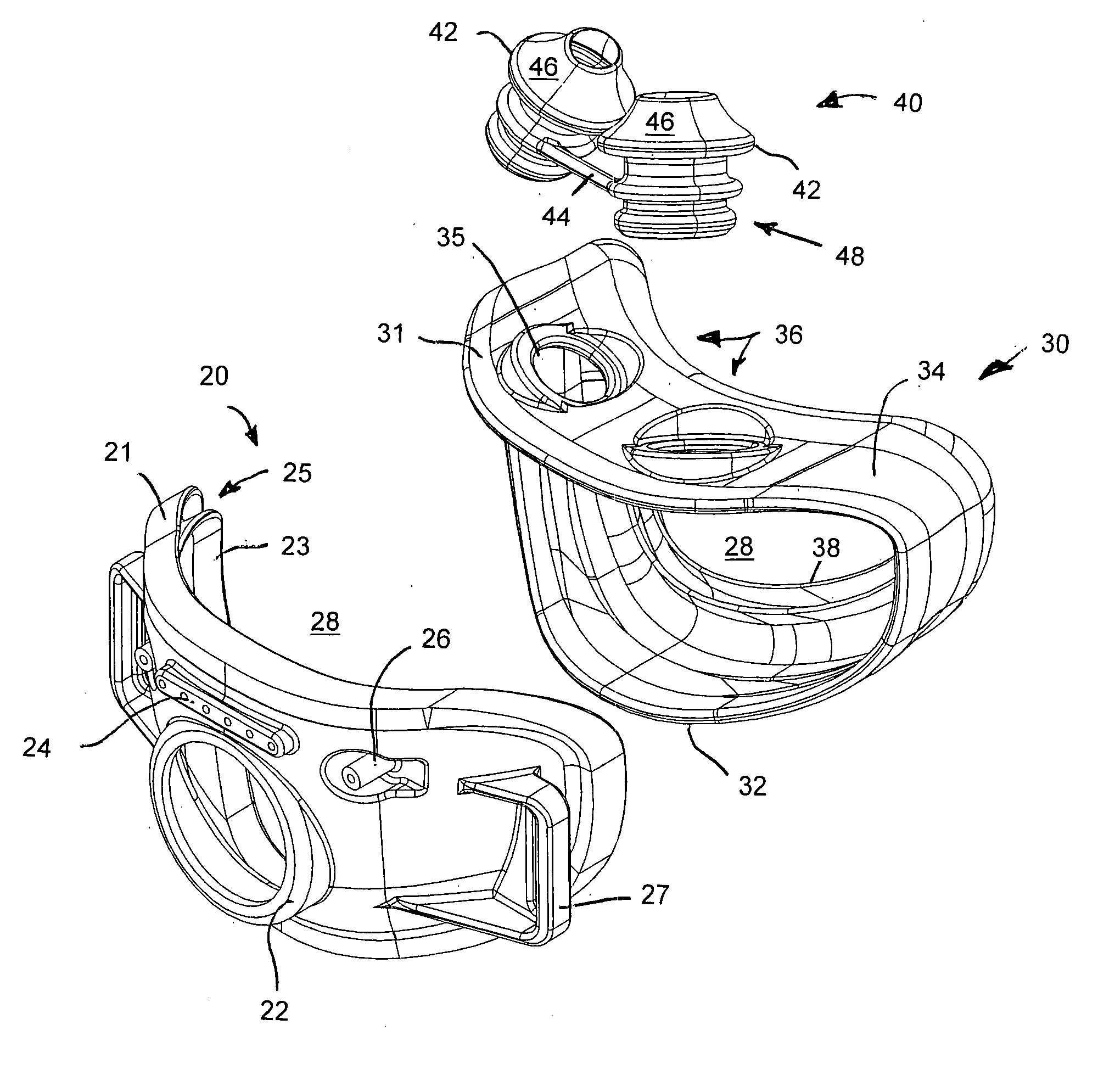
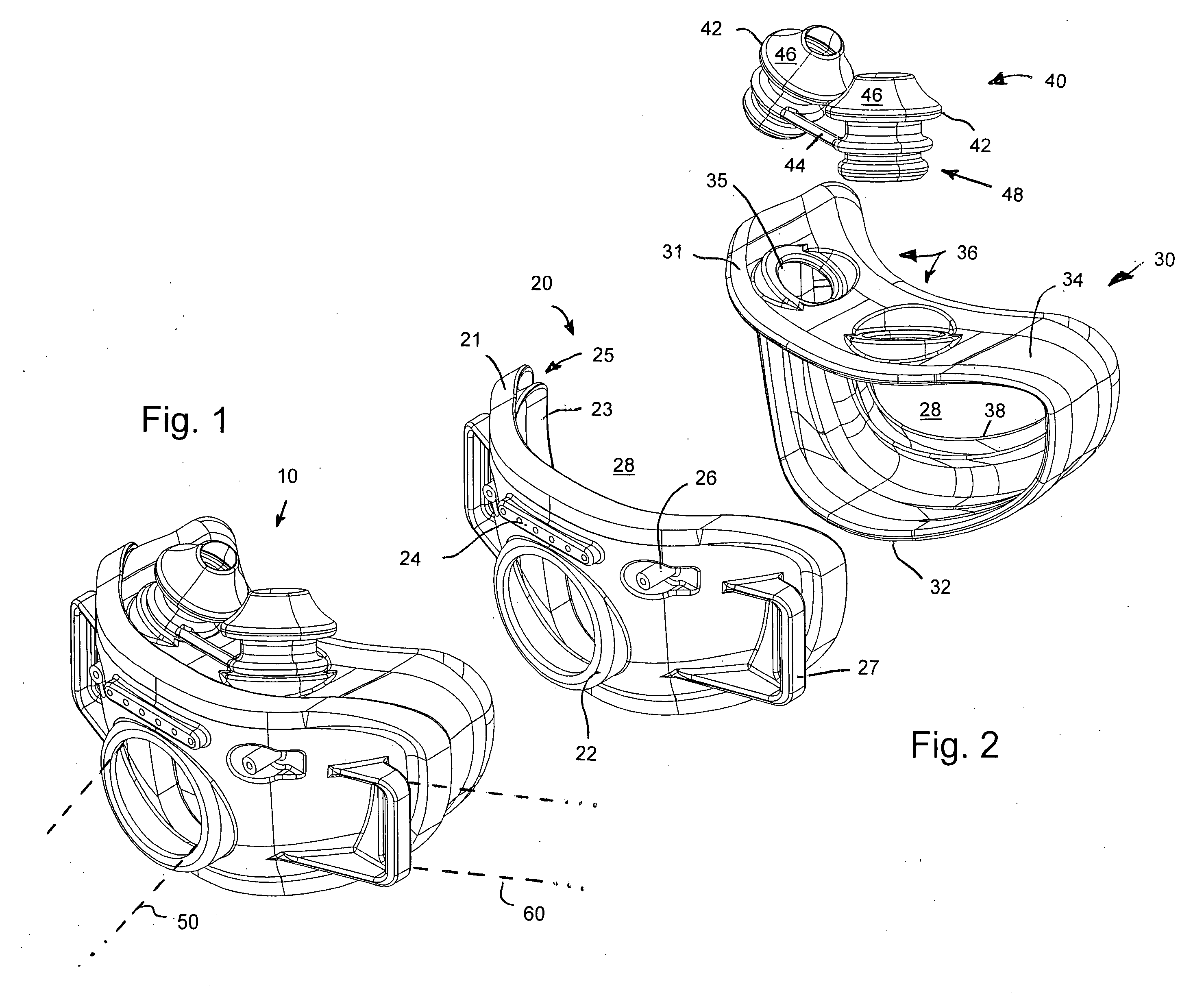
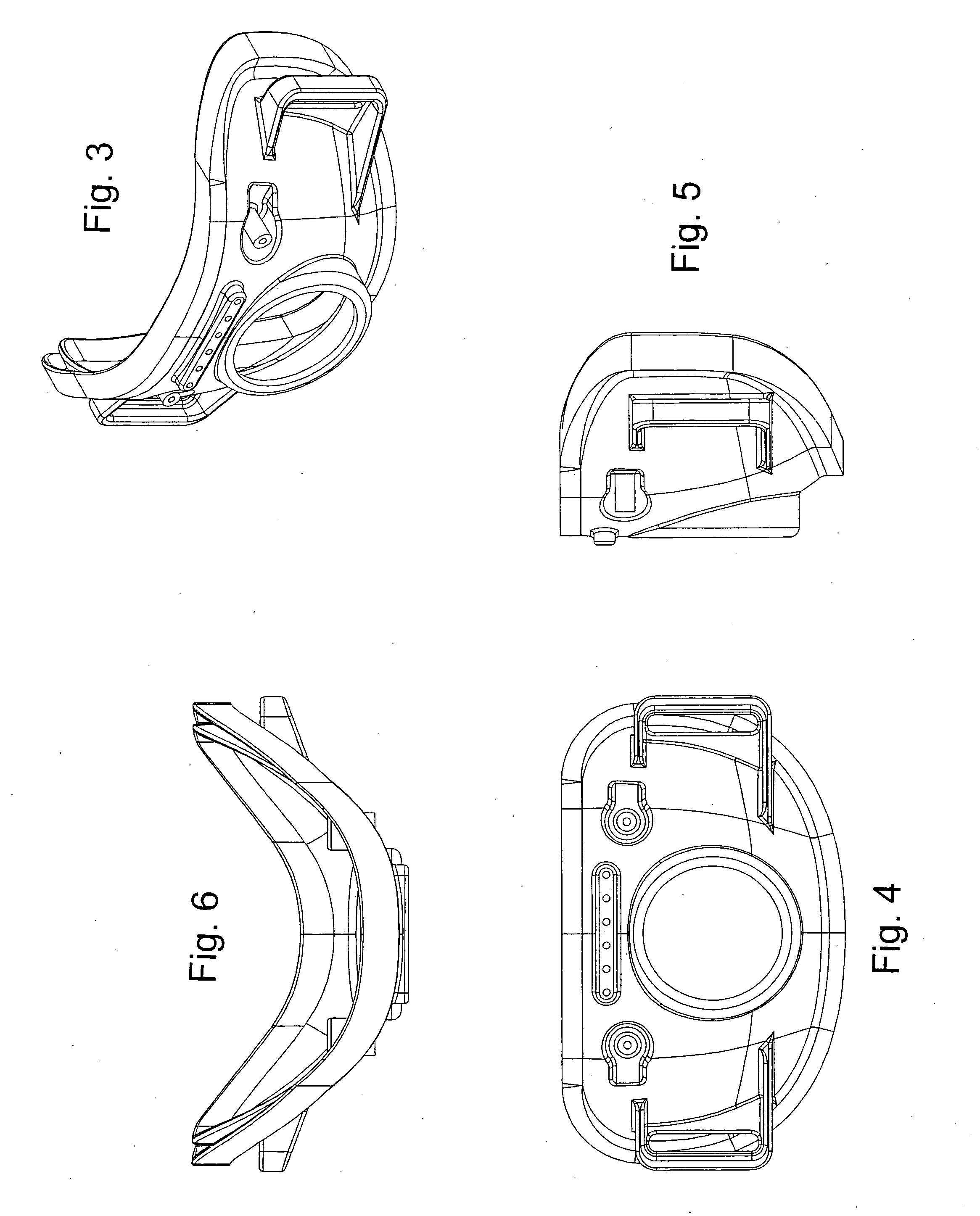
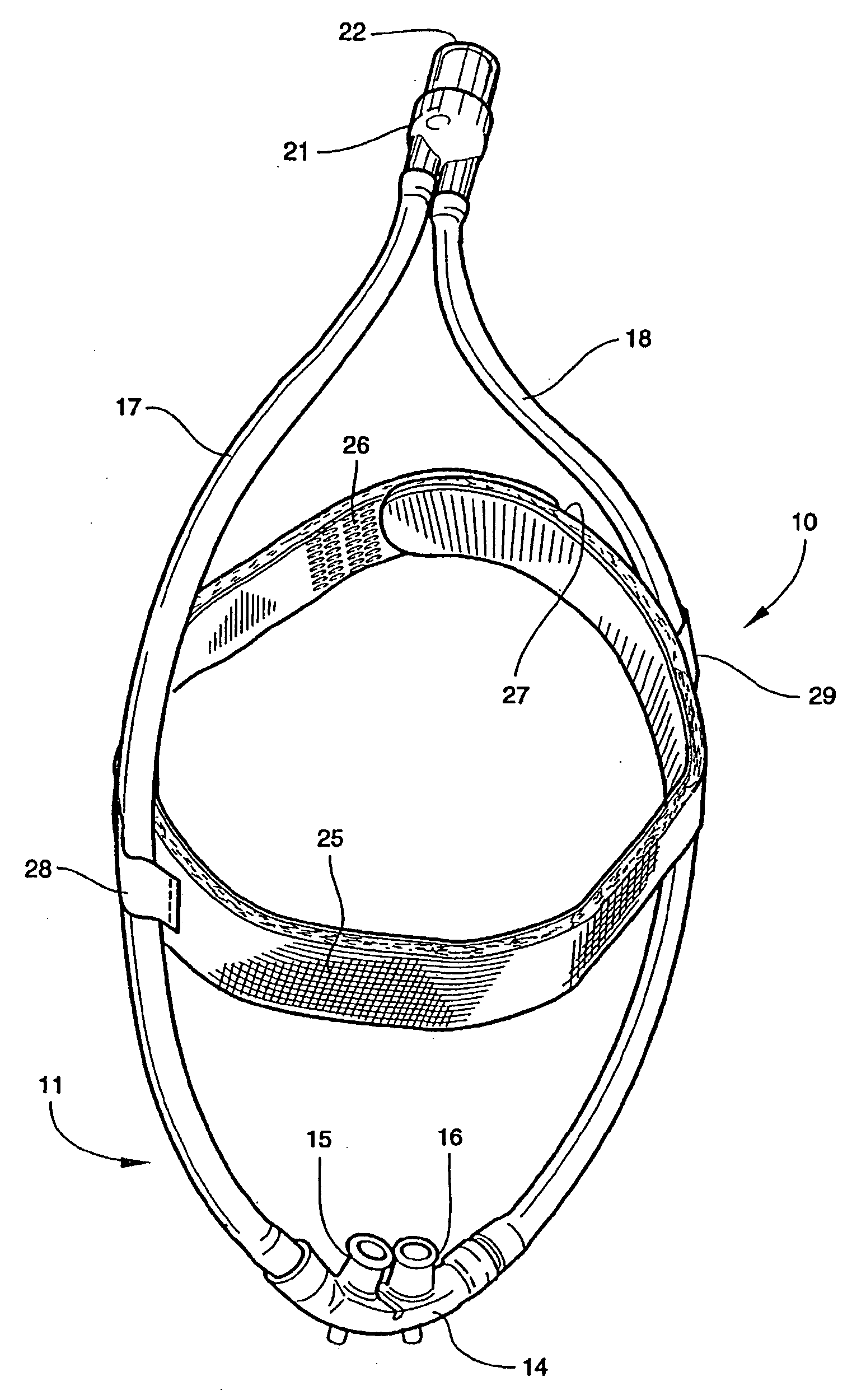
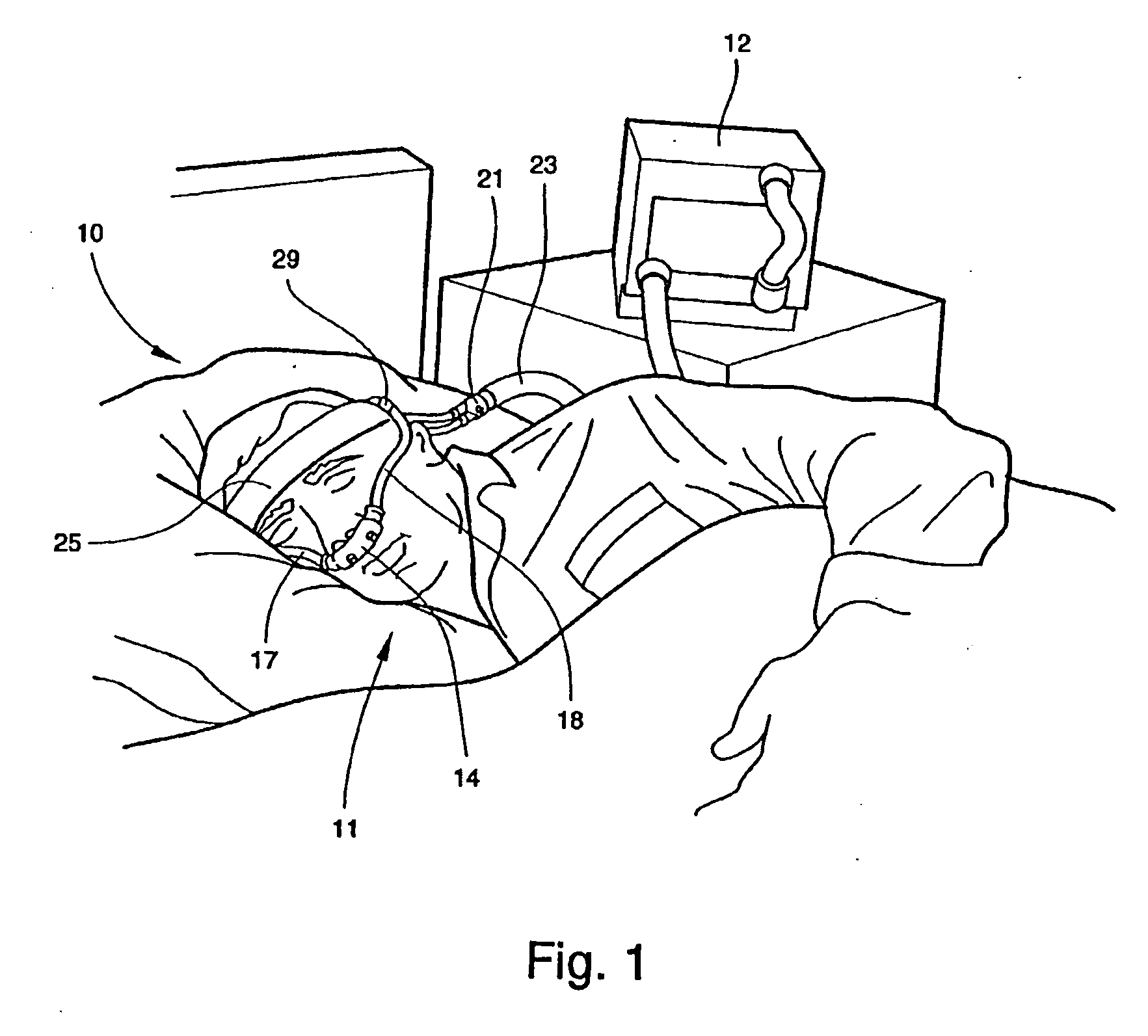
Facial masks for assisted respiration or CPAP
A combination mouth and nasal mask (40) for assisted respiration or CPAP is disclosed. The combination mask (40) has a frame (42) to the rear side of which is mounted a separate nasal cushion (44) and mouth cushion (46). Both the nasal cushion (44) and the mouth cushion (46) separately form a rolled edge seal around the patient's face in the vicinity of the nose and in the vicinity of the lips. Inlet air is communicated from within the housing (48) to the mouth chamber, circumscribed by the mouth cushion (46) by means of one or more interconnecting tubes (62). The proportion of air communicated to the mouth chamber (48) is controlled by the sizing and number of the tube(s), thus providing for free flow of air, restricted flow of air and pressure equalization, or zero flow of air. A mouth mask (10) for assisted respiration or CPAP also is disclosed.
Owner:RESMED LTD
Devices, systems and methods useable for treating sinusitis
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Compositions for nasal drug delivery, methods of making same, and methods of removing residual solvent from pharmaceutical preparations
InactiveUS6391452B1Taller in heightSlow down the mixing speedLiquid surface applicatorsPeptide/protein ingredientsNasal cavityNose
The present invention relates to pharmaceutical compositions for delivery of drugs intended to reside in the nose, compositions for nasal administration of drugs, e.g., antiviral agents, and particularly antiviral agents comprising the human major rhinovirus receptor, also known as intercellular adhesion molecule-1 (ICAM-1); to methods of making said nasal drug compositions, and to an improved process for the removal of residual solvent from pharmaceutical matrices.
Owner:BAYER PHARMA CORP
Systems and methods for electrosurgical treatment of obstructive sleep disorders
InactiveUS7131969B1Increase surface areaImprove sealingCannulasEnemata/irrigatorsThroatSleeping disorders
The present invention provides systems and methods for selectively applying electrical energy to a target location within the head and neck of a patient's body, particularly including tissue in the ear, nose and throat. The present invention applies high frequency (RF) electrical energy to one or more electrode terminals in the presence of electrically conductive fluid to remove and / or modify the structure of tissue structures. The present invention is particularly useful for treating sleep obstructive disorders, such as sleep apnea and snoring.
Owner:ARTHROCARE
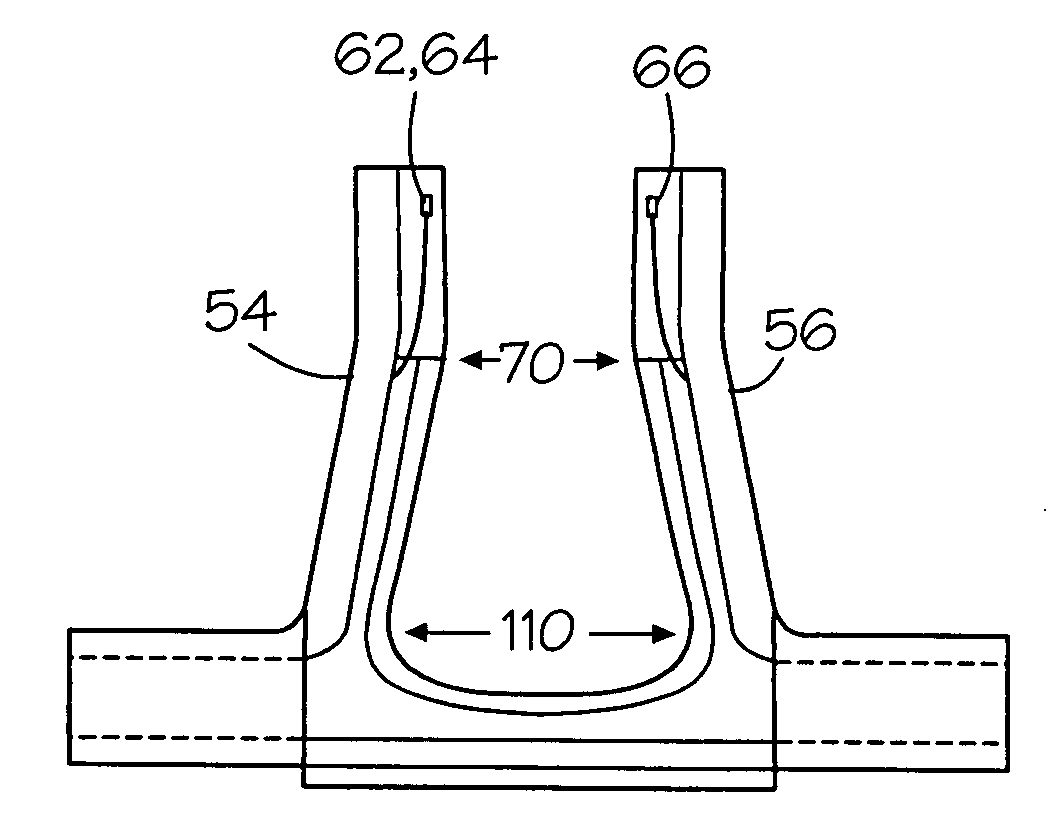
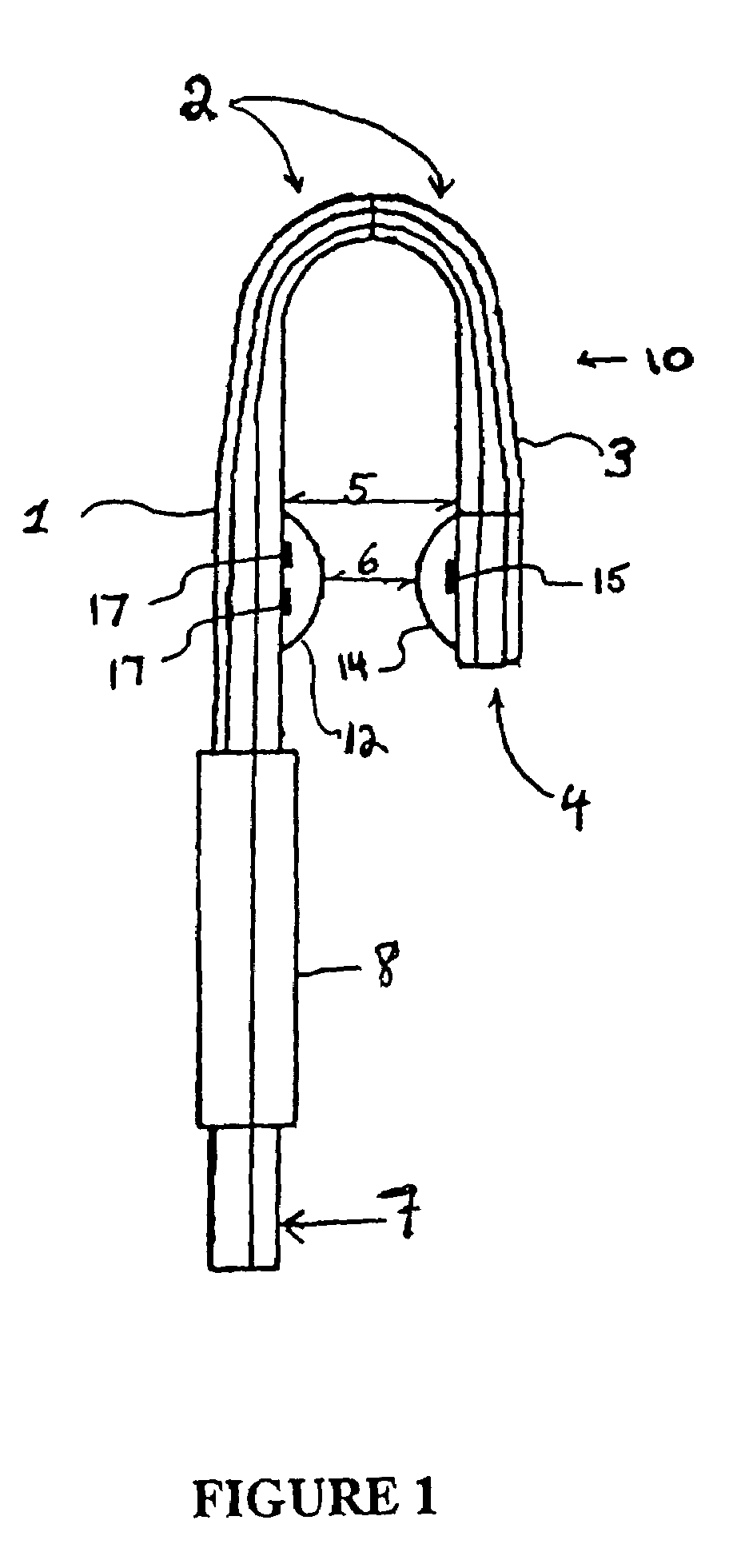
Nasal interface apparatus
A nasal interface device for use in the nares of a patient for positive airway pressure applications includes a pair of nasal prongs, each prong having a bore, a first end, a second end, and at least one deformable flap disposed proximate to the first end of each prong. The device further includes a body having a distal portion and a proximal portion forming a chamber, the proximal portion having apertures to receive the second ends of the nasal prongs, the chamber being in communication with the bores of the nasal prongs, and at least one exhalation port disposed within the body. The device includes at least one gas inlet on the distal portion of the body, the at least one gas inlet in communication with the chamber. In one preferred aspect of the invention, the at least one flap is deformable within the nares of a patient thereby creating a substantially airtight seal.
Owner:VIASYS HEALTHCARE
Nasal cannula
InactiveUS6439234B1Reduces and eliminates incidenceAccurate monitoringOperating means/releasing devices for valvesRespiratory masksGas analysisNose
Owner:SALTER LABS
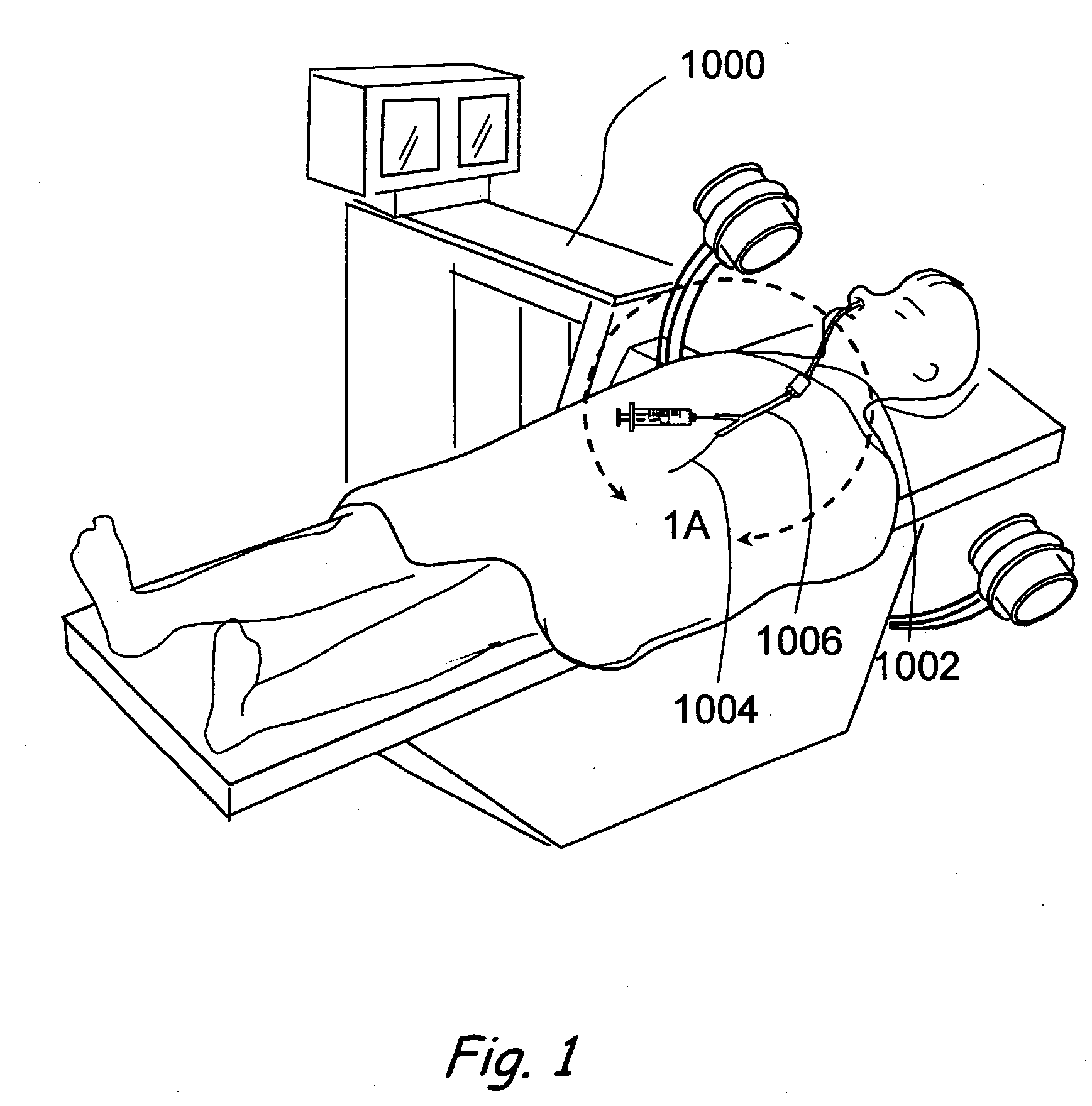
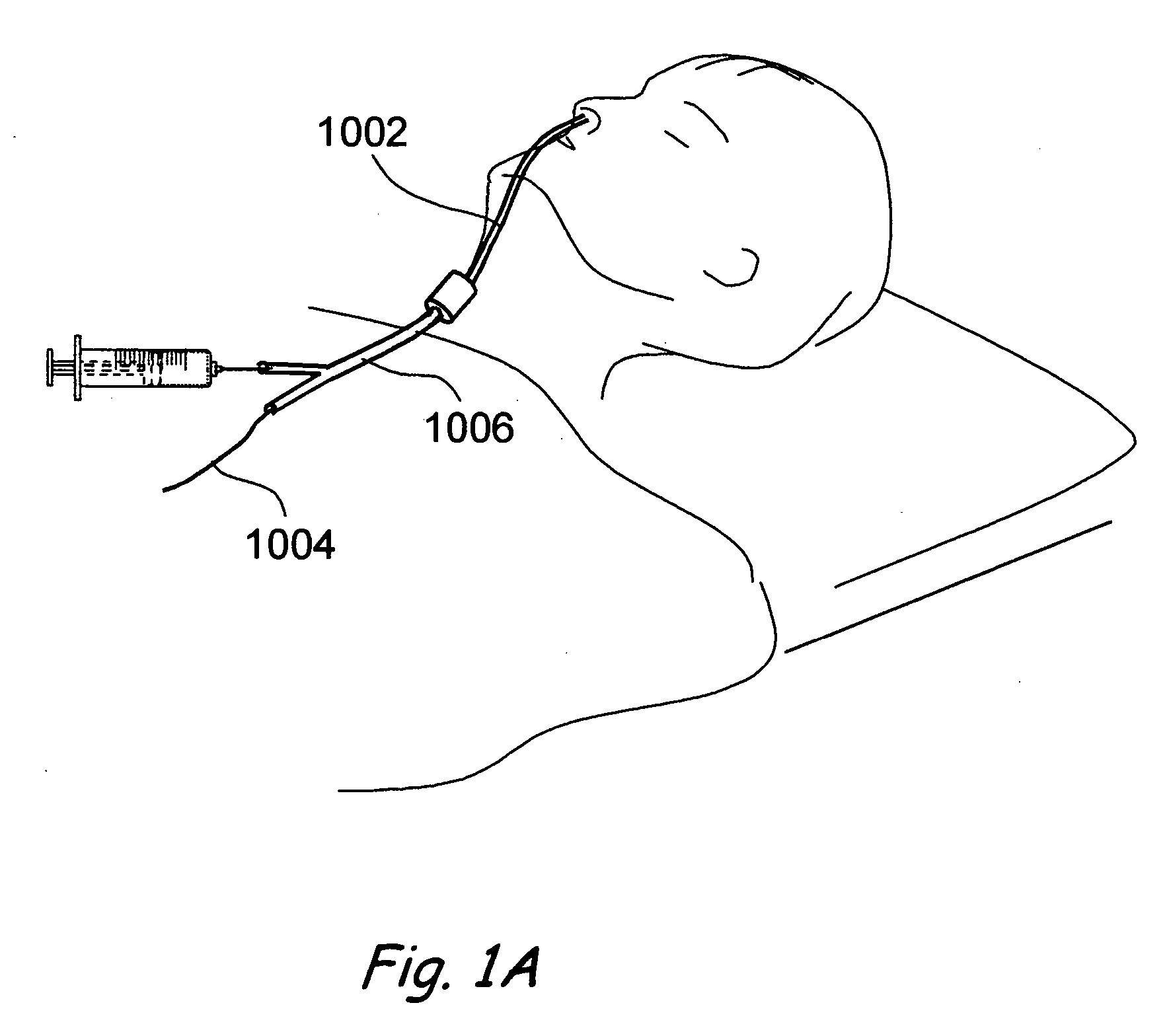
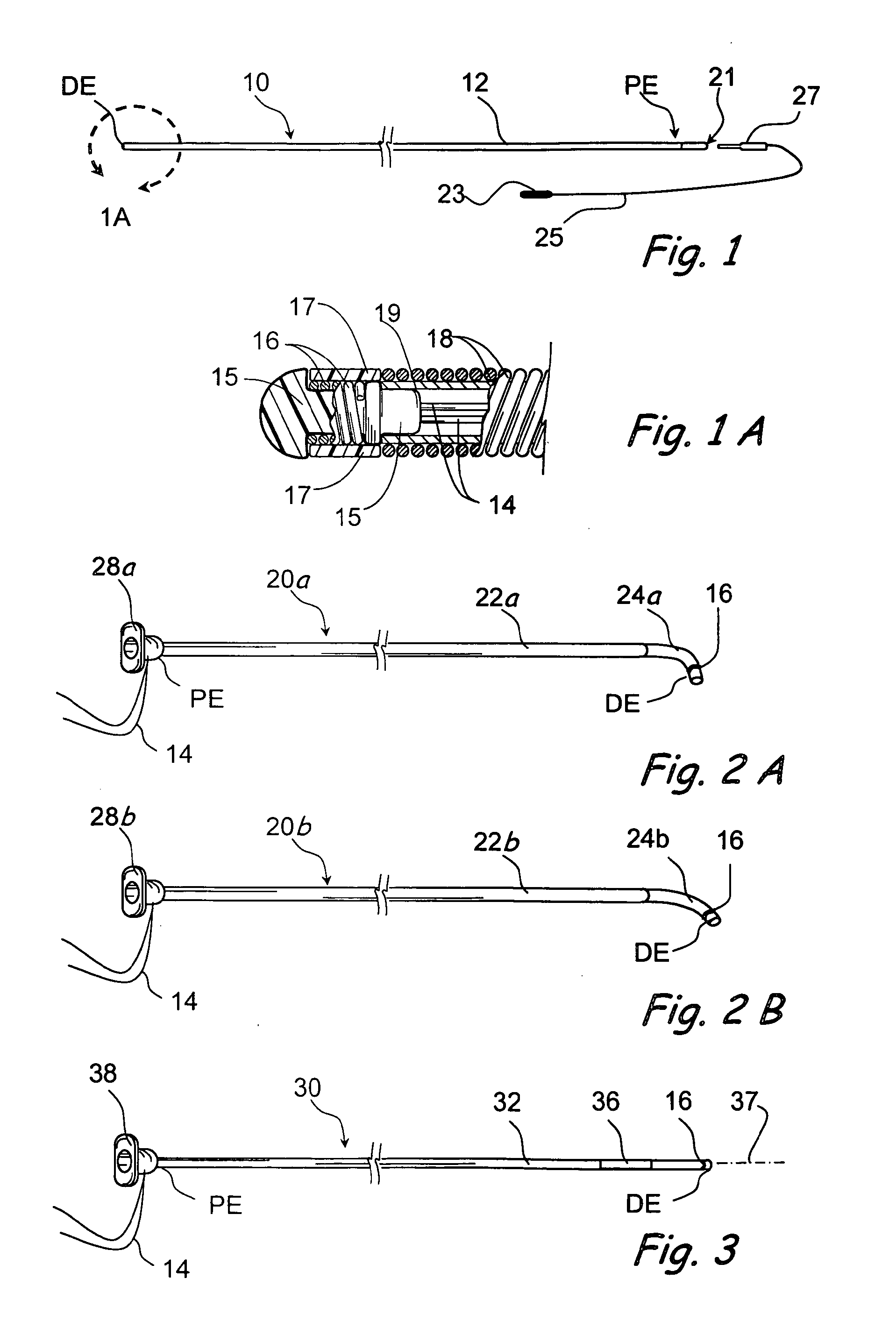
Endoscopic methods and devices for transnasal procedures
Medical devices, systems and methods that are useable to facilitate transnasal insertion and positioning of guidewires and various other devices and instruments at desired locations within the ear, nose, throat, paranasal sinuses or cranium.
Owner:ACCLARENT INC
Endoscopic methods and devices for transnasal procedures
Medical devices, systems and methods that are useable to facilitate transnasal insertion and positioning of guidewires and various other devices and instruments at desired locations within the ear, nose, throat, paranasal sinuses or cranium.
Owner:ACCLARENT INC
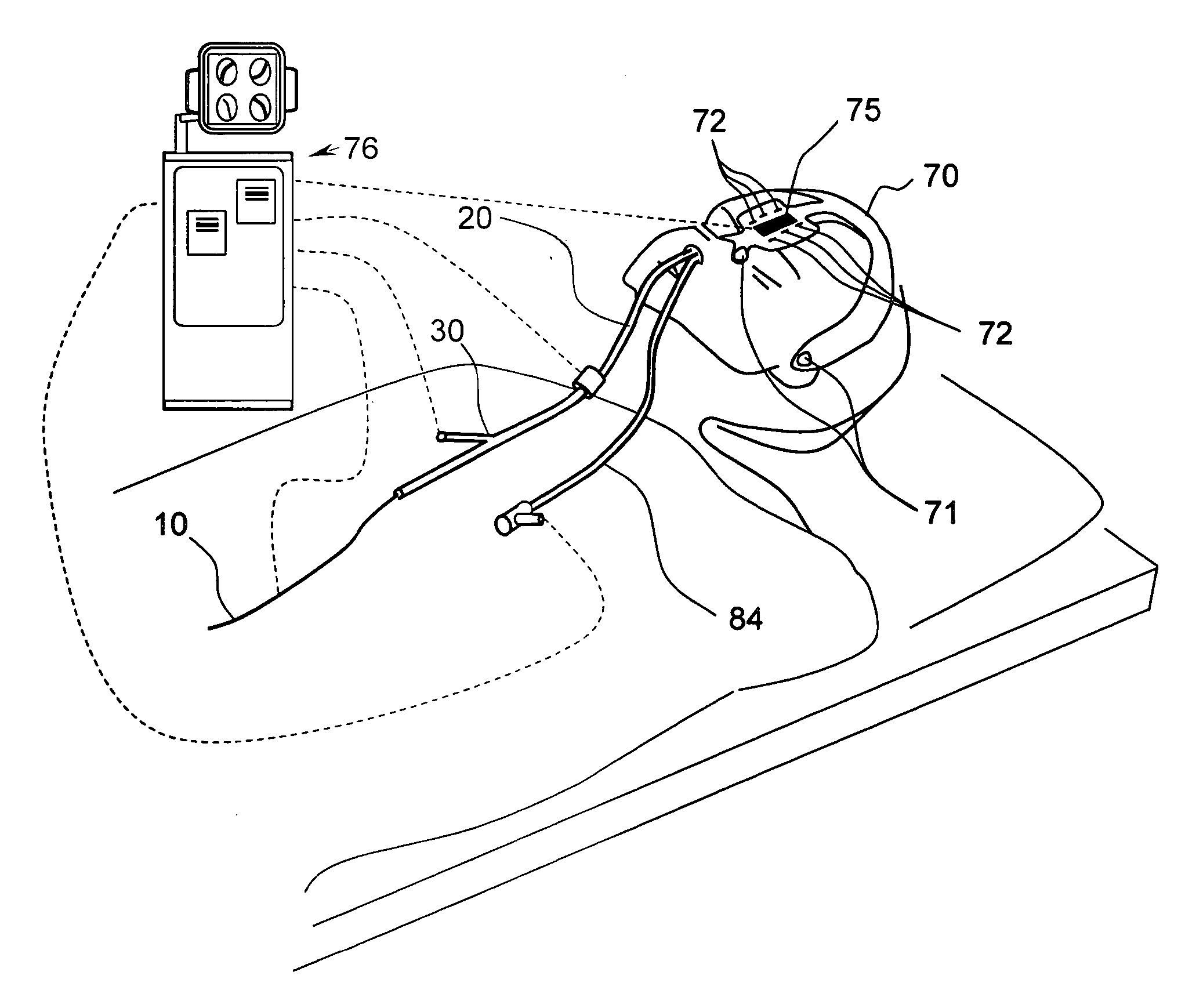
Specially configured nasal pulse oximeter/photoplethysmography probes, and combined nasal probe/cannula, selectively with sampler for capnography, and covering sleeves for same
InactiveUS7024235B2Easy to makeLow costRespiratorsOperating means/releasing devices for valvesPulse oximetersNose
The present invention relates to novel nasal pulse oximeter probes that are configured to be placed across the septum of the nose. These probes are fabricated to provide signals to obtain arterial oxygen saturation and other photoplethysmographic data. The present invention also relates to a combined nasal pulse oximeter probe / nasal cannula. The present invention also relates to other devices that combine a pulse oximeter probe with a device supplying oxygen or other oxygen-containing gas to a person in need thereof, and to sampling means for exhaled carbon dioxide in combination with the novel nasal probe. In certain embodiments, an additional limitation of a control means to adjust the flow rate of such gas is provided, where such control is directed by the blood oxygen saturation data obtained from the pulse oximeter probe.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
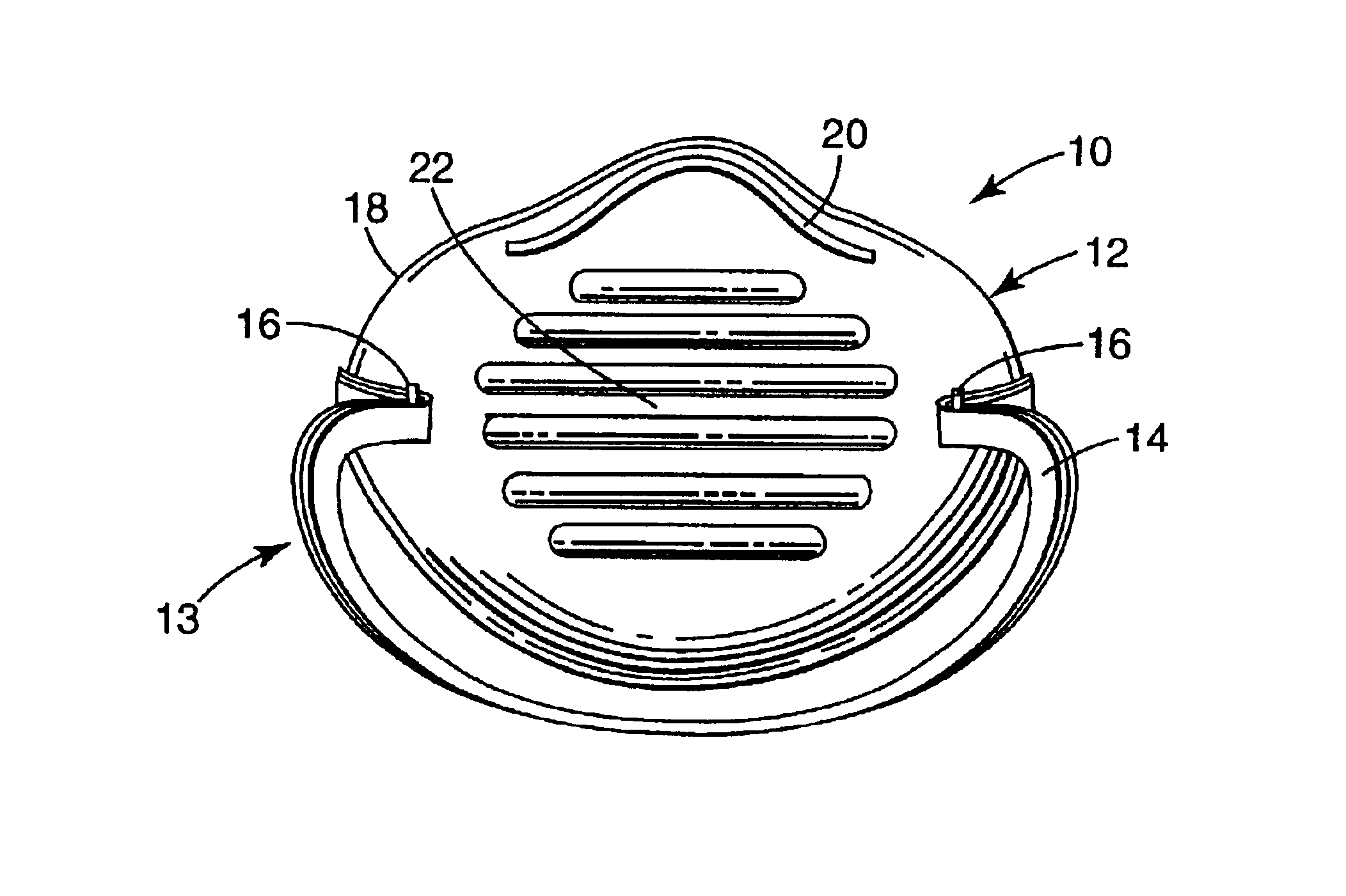
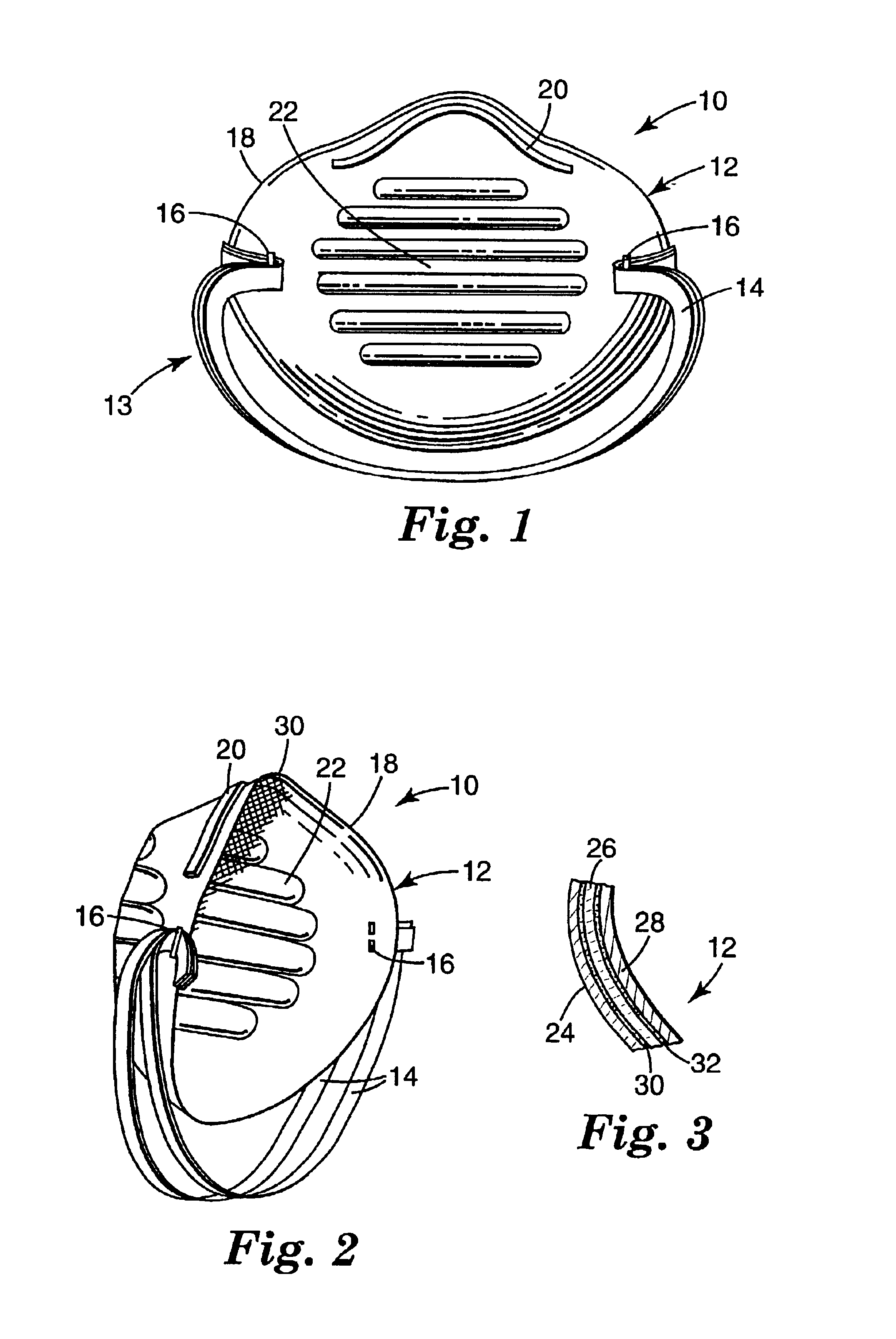
Crush resistant filtering face mask
InactiveUS6923182B2Reduce the possibilityImprove securityBreathing filtersBreathing masksFiltrationNose
A filtering face mask that includes a mask body that is adapted to fit over the nose and mouth of a person and a harness that is attached to the mask body. The mask body comprises i) a first shaping layer that has been molded; ii) a second shaping layer that has been molded; iii) a filtration layer that is disposed between the first and second shaping layers; iv) a first adhesive layer that adheres the first shaping layer to the filtration layer; and v) a second adhesive layer that adheres the second shaping layer to the filtration layer.
Owner:3M INNOVATIVE PROPERTIES CO
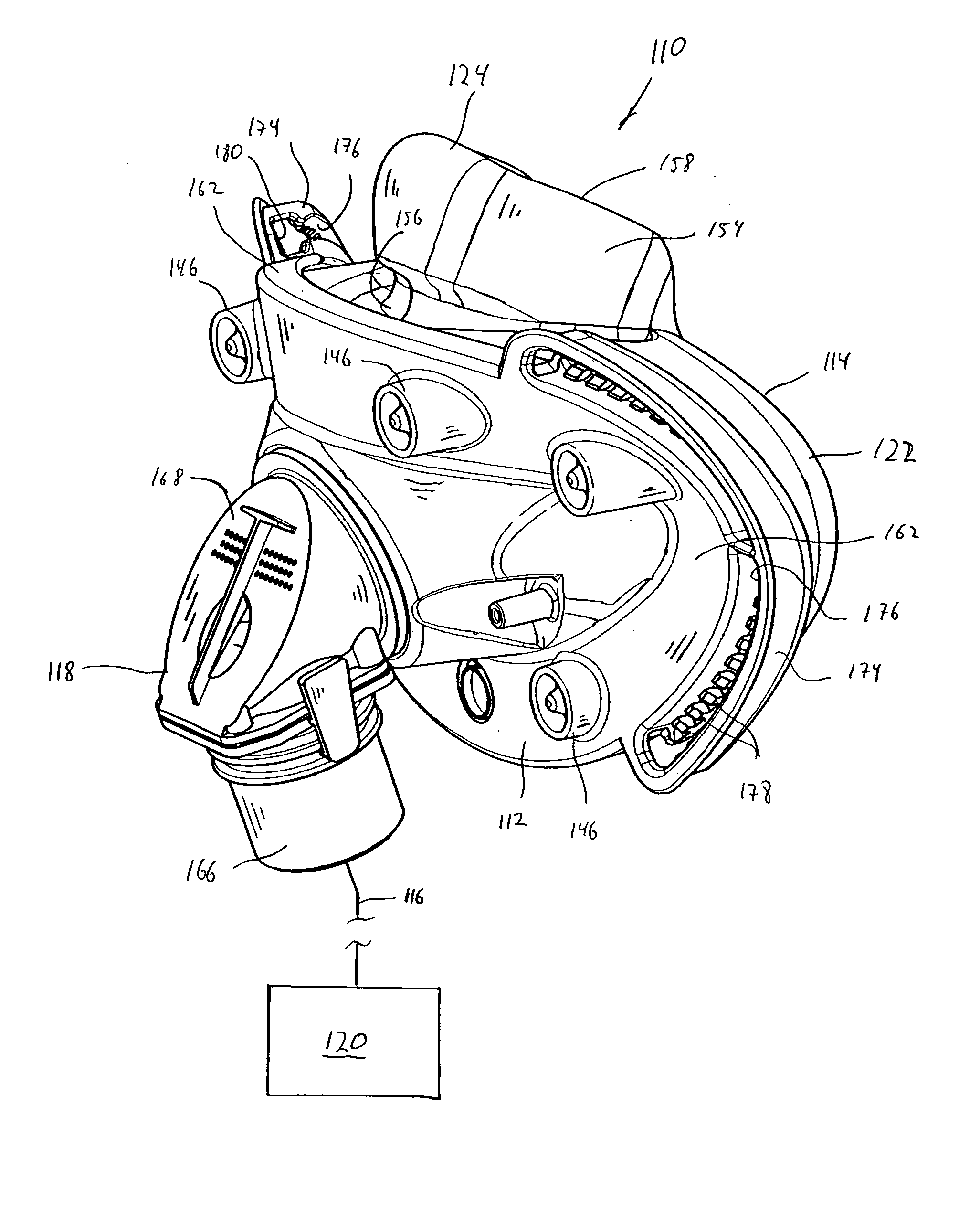
Full face respiratory mask with integrated nasal interface
ActiveUS20070125385A1Reduce complexityCut surfaceRespiratory masksBreathing masksNoseRespiratory mask
A patient interface that reliably and comfortably seals a user's face by providing an oral-nasal mask that includes an integrated nasal interface. The patient interface includes a seal member having an oral cushion portion configured to surround the user's mouth and a nasal interface portion that provides an interface with the user's nose. The seal member, including the oral cushion portion and the nasal interface portion, is a unitary member. Finally, the nasal interface portion remains below the bridge of the nose.
Owner:PHILIPS RS NORTH AMERICA LLC
Headwear for use by a sleep apnea patient
InactiveUS20050061326A1Reduce manufacturing costBreathing filtersBreathing masksNosePositive airway pressure device
Headwear is adapted for use by a patient to position airway tubes of a nasal interface operatively connected to a positive airway pressure device. The headwear includes an elongated head strap for being worn around a head of the patient. Cooperating sets of front and rear tube holders are attached to the head strap, and are adapted for engaging and holding respective airway tubes of the nasal interface to retain the tubes in a desired position during use. The tube holders include at least one elastic strip extending along a longitudinal dimension of the head strap.
Owner:HEADWEAR
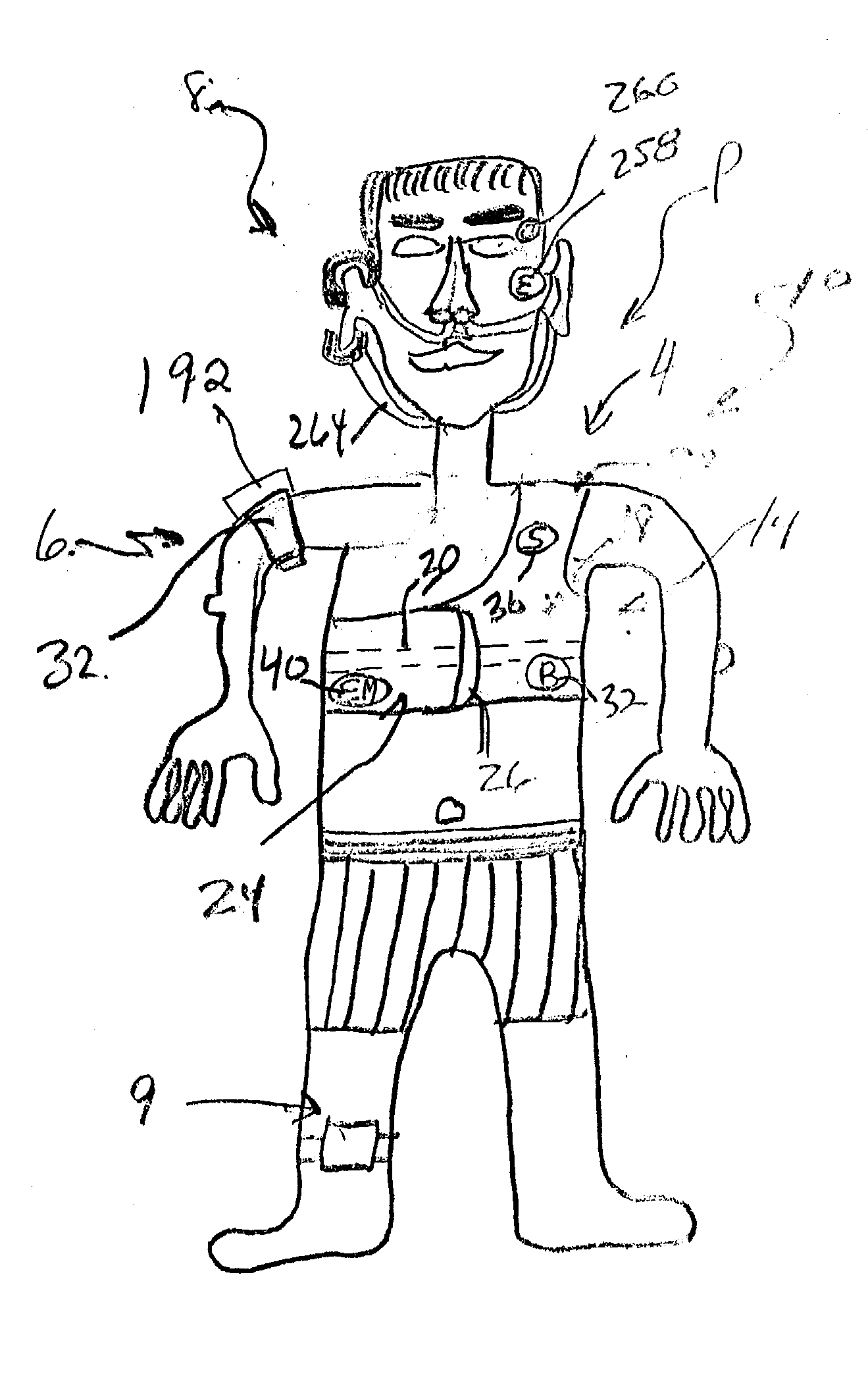
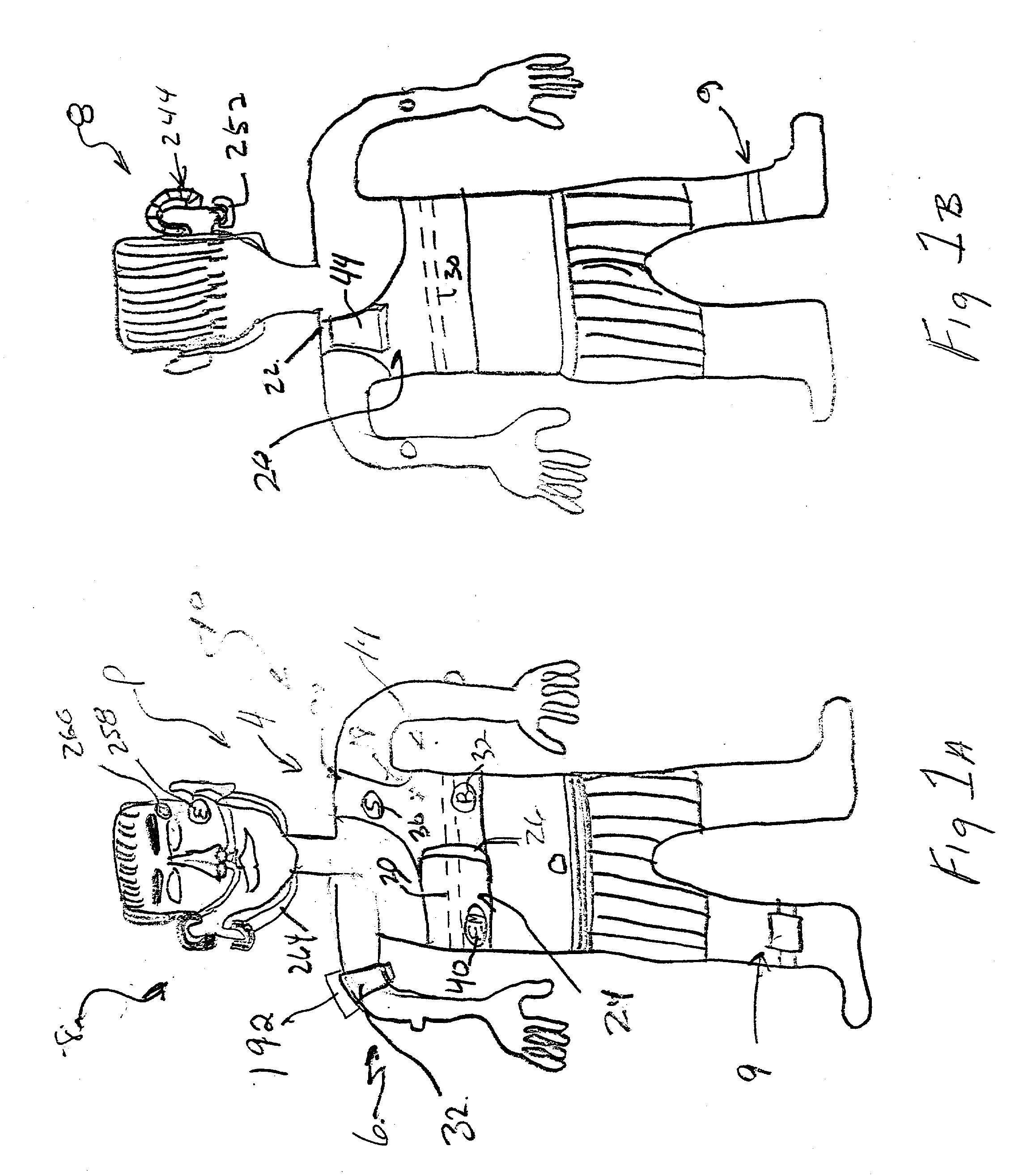
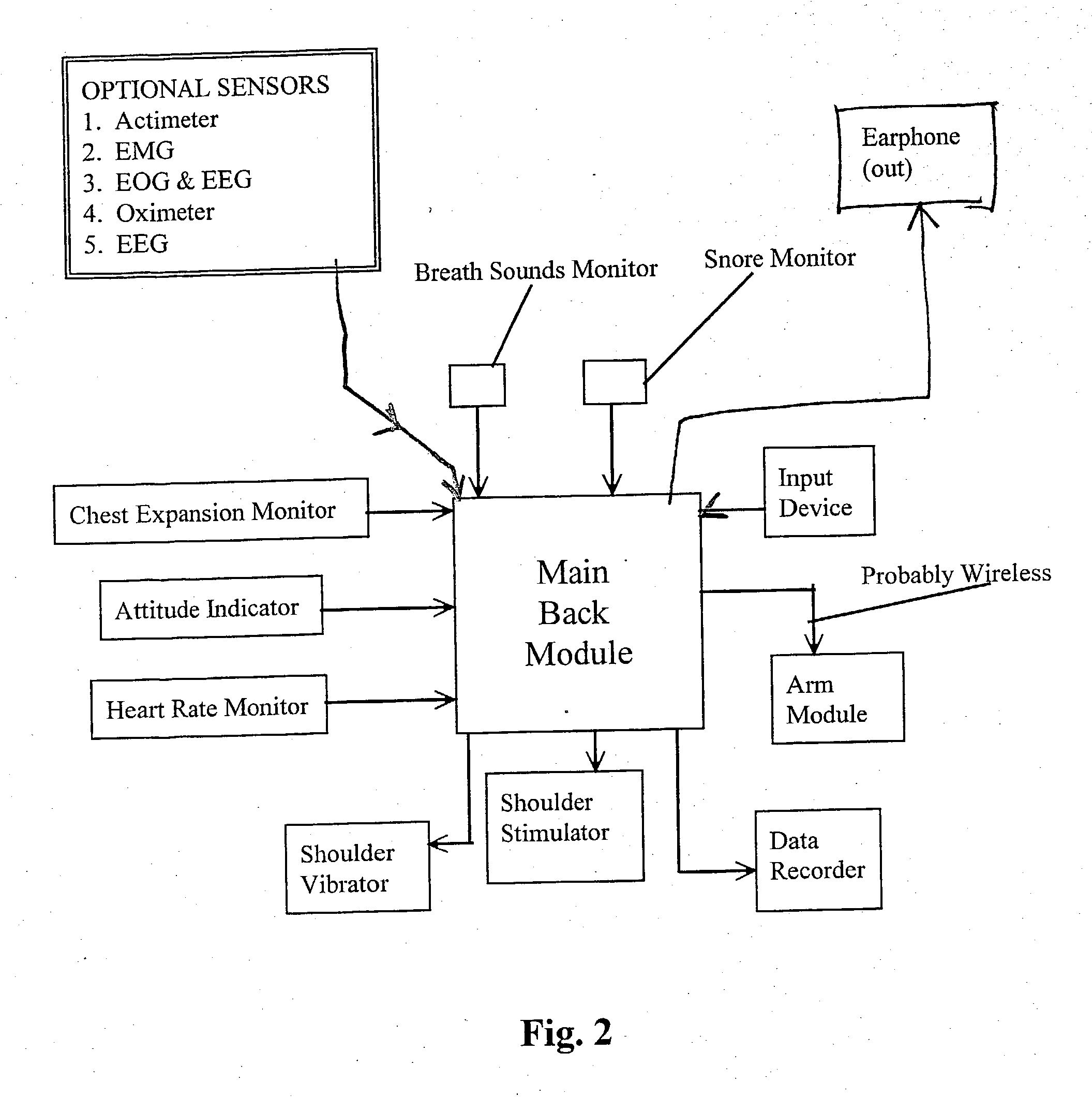
Device and method for treating disordered breathing
InactiveUS20030199945A1Promote properPrecise positioningElectrotherapySnoring preventionNoseSleep patterns
A device is disclosed for treating sleep and breathing disorders of a patient, along with the method of using the device. The device includes a processor for receiving sensor inputs, processing the received sensor inputs, and generating commands through output devices. A first sensor is positionable for receiving breathing sound information emitted from one of the mouth and nose of a patient. The second sensor is positionable on a patient for receiving breathing sounds information from a patient's chest cavity. A third sensor is positionable for receiving information relating to the amount of chest expansion of a patient. A first output device is provided that is capable of providing an auditory signal to a patient. A second output device is capable of providing an electrical signal to a muscle group of a patient that simulates a human touching event. The first, second and third sensors, and the first and second output devices are operatively coupled to the processor to permit the processor to receive information input from the sensors, process the input information to the detect the existence of a sleep-breathing disorder event, and to generate command to at least one of the first and second output devices. The command is capable of directing the at least one output device to provide a series of progressively intrusive stimuli designed to condition the patient to terminate the sleep breathing disorder event, and ultimately, return to a more normal sleep pattern.
Owner:CIULLA JAMES
Ventilation interface for sleep apnea therapy
InactiveUS6997177B2Large diameterAvoiding drying and burningRespiratory masksBreathing masksPositive airway pressureNasal cavity
The ventilation interface for sleep apnea therapy interfaces a ventilation device to the patient's airways. The ventilation interface includes a pair of nasal inserts made from flexible, resilient silicone which are oval shaped in cross-section and slightly tapered from a base proximal the ventilation supply to the distal tip end. A bead flange is disposed about the exterior of each insert at the distal end of the insert. In one embodiment, a valve is disposed between the nasal inserts and a source of positive airway pressure, the valve having a rim with a one-way diaphragm pivotally attached to the valve body with an inflatable bladder depending from the rim which seals against an exit port during inspiration and deflates to uncover the exit port on expiration. Another embodiment has nasal inserts without positive airway pressure but with a removable filter in the inserts for filtering inspired air.
Owner:SALTER LABS LLC
Systems and Methods for Performing Image Guided Procedures Within the Ear, Nose, Throat and Paranasal Sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Methods for treating ethmoid disease
ActiveUS7419497B2Low profileMinimize traumaGuide needlesBalloon catheterParanasal sinusitisHuman animal
Devices, systems and methods for stenting, spacing, draining, ventilating and / or delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects, including methods and systems for treating paranasal sinusitis and ethmoid disease.
Owner:ACCLARENT INC
Systems and methods for transnasal dilation of passageways in the ear, nose and throat
Devices, systems and methods useable for dilating the ostia of paranasal sinuses and / or other passageways within the ear, nose or throat. A dilation catheter device and system is constructed in a manner that facilitates ease of use by the operator and, in at least some cases, allows the dilation procedure to be performed by a single operator. Additionally, the dilation catheter device and system may be useable in conjunction with an endoscope and / or a fluoroscope to provide for easy manipulation and positioning of the devices and real time visualization of the entire procedure or selected portions thereof. In some embodiments, an optional handle may be used to facilitate grasping or supporting a device of the present invention as well as another device (e.g., an endoscope) with a single hand.
Owner:ACCLARENT INC
Respiratory mask
ActiveUS20100313891A1Solve the small frictionWeight bulkRespiratory masksBreathing masksNoseRespiratory mask
A respiratory mask for use with a patient, and that is suited for use with children, includes a flexible cushion arranged to interface with and deliver air to the patient's nose. The cushion has a tube connection portion at one or both sides adjacent the patient's nares, the tube connection portion being arranged to connect to an air delivery tube. This location, plus the very low profile of the mask, allows a patient (e.g. an infant) to sleep on their face more comfortably. A more rigid support structure adjacent the cushion is provided to stabilize the cushion and prevent it from collapsing. Headgear is also provided and arranged for releasable attachment to the support structure.
Owner:RESMED LTD
Endoscopic methods and devices for transnasal procedures
Medical devices, systems and methods that are useable to facilitate transnasal insertion and positioning of guidewires and various other devices and instruments at desired locations within the ear, nose, throat, paranasal sinuses or cranium. Direct viewing of such placements via an endoscope.
Owner:ACCLARENT INC
Systems and methods for performing image guided procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Use of mechanical dilator devices to enlarge ostia of paranasal sinuses and other passages in the ear, nose, throat and paranasal sinuses
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com