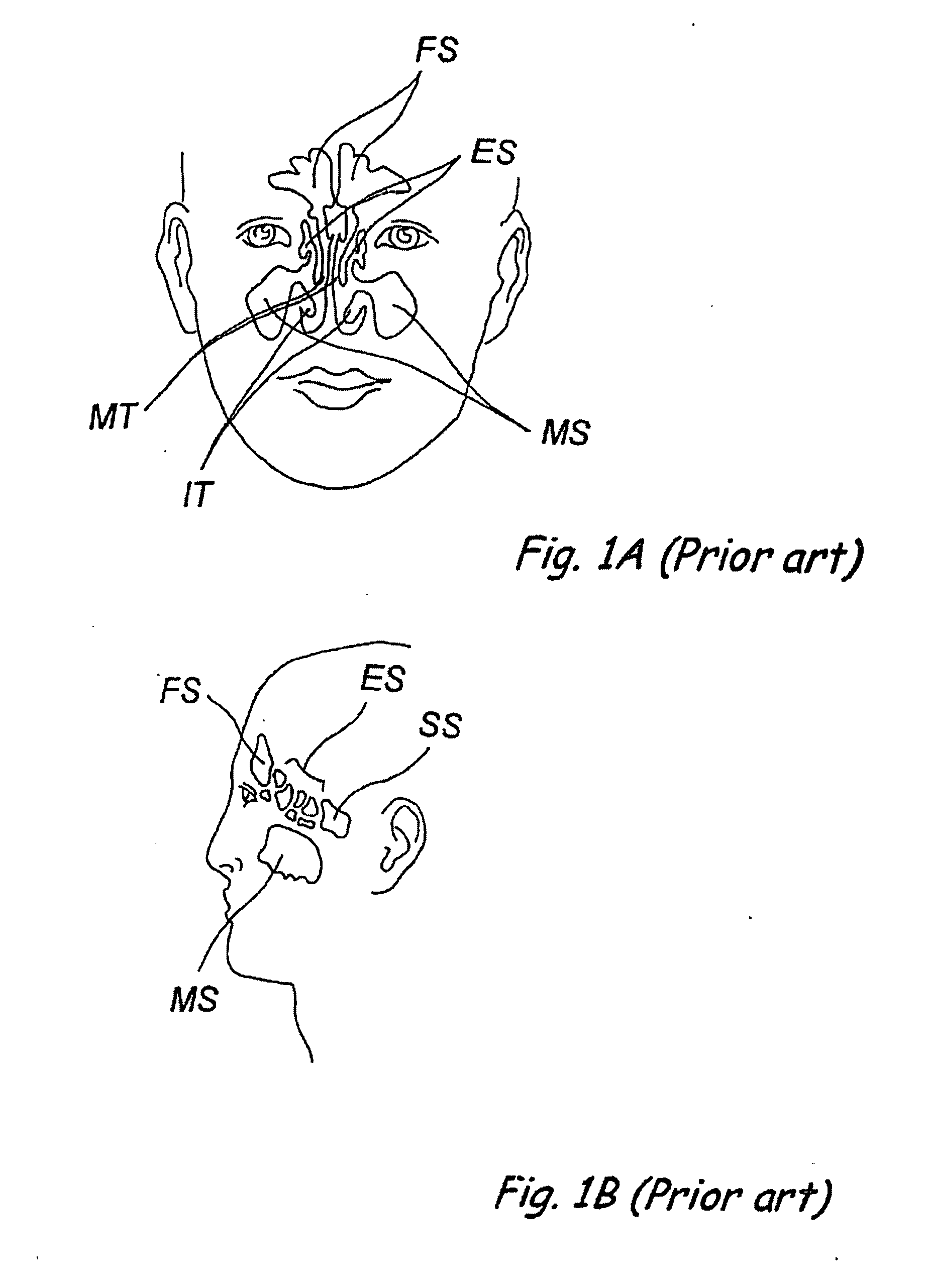
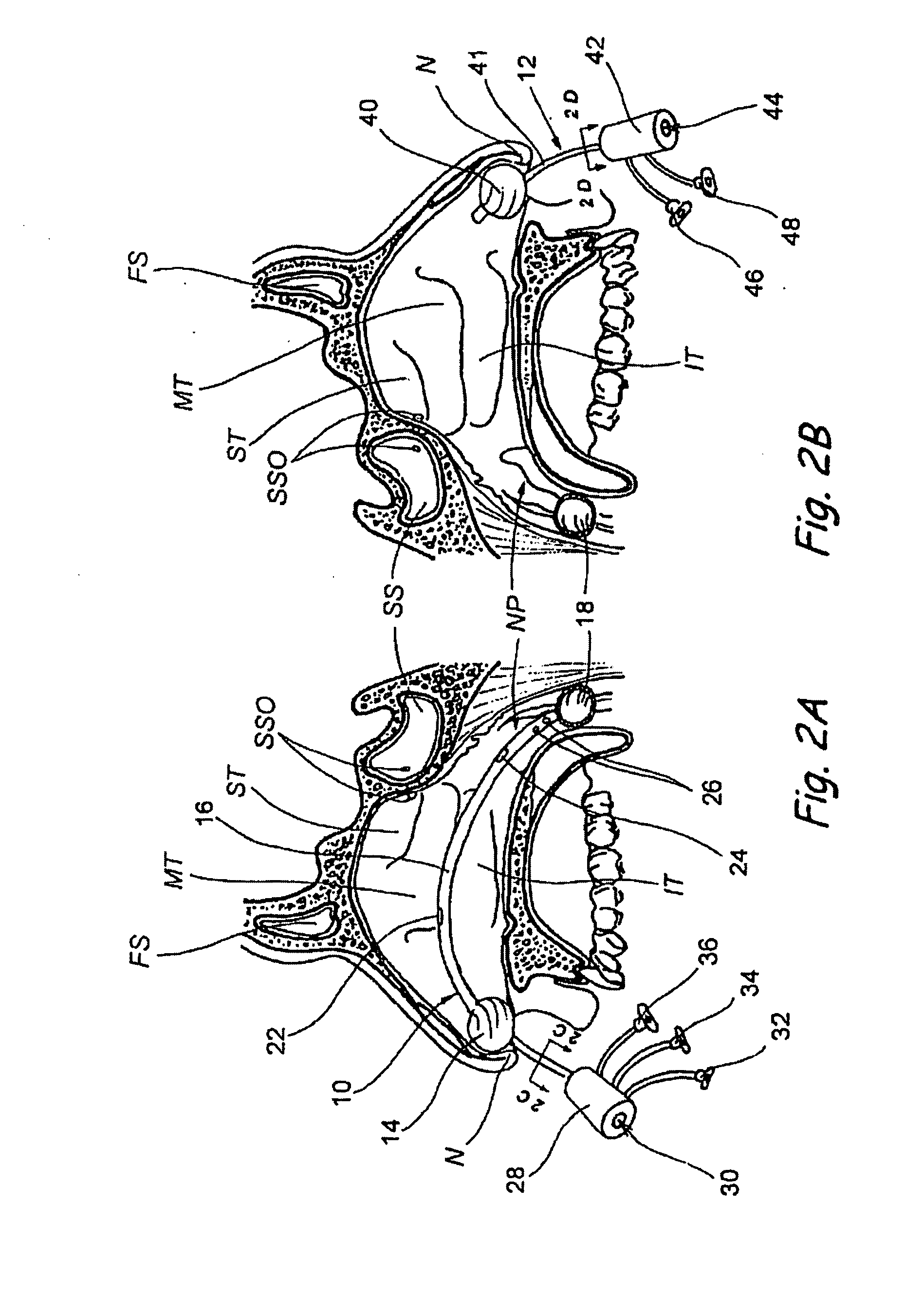
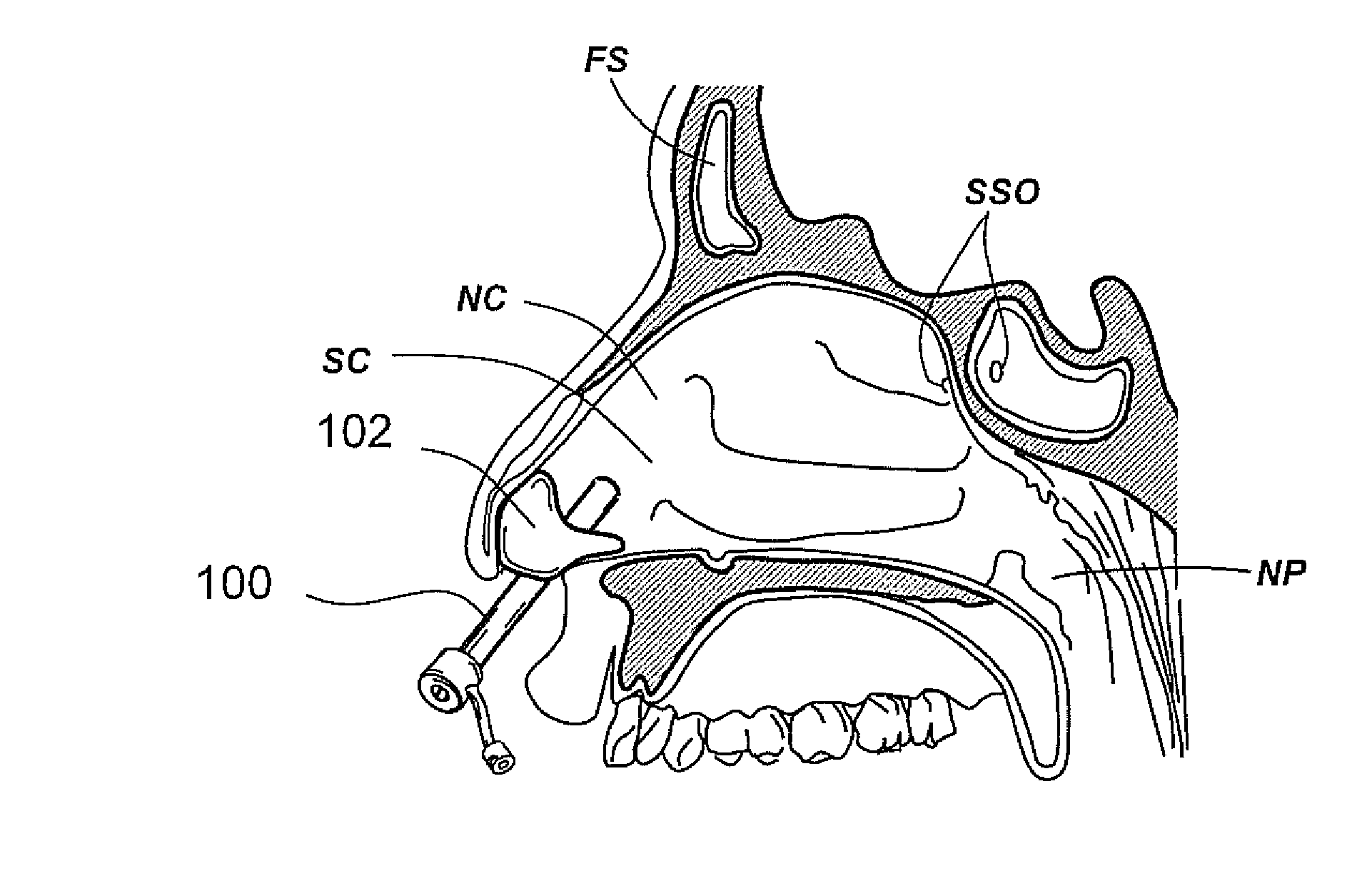
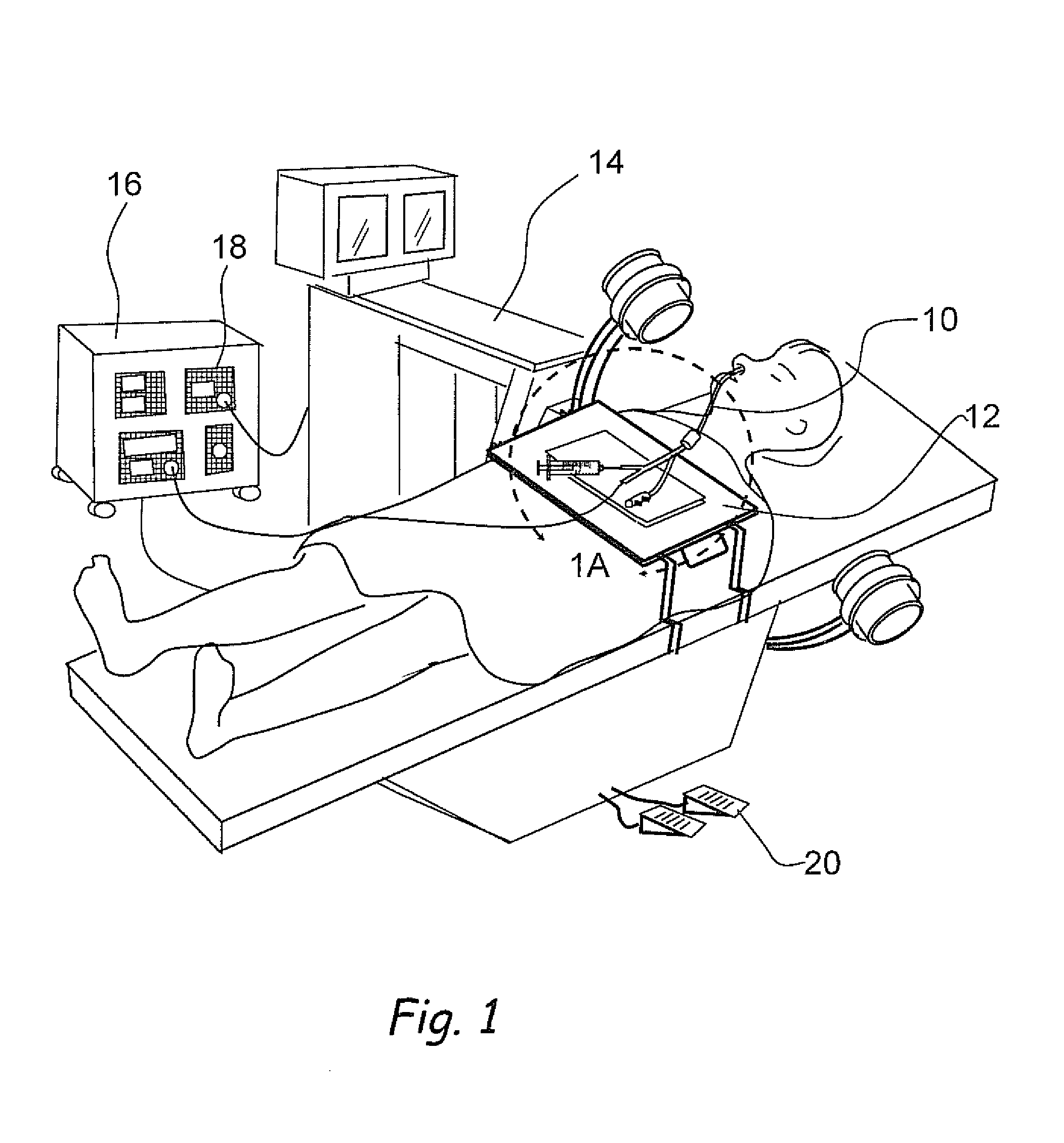
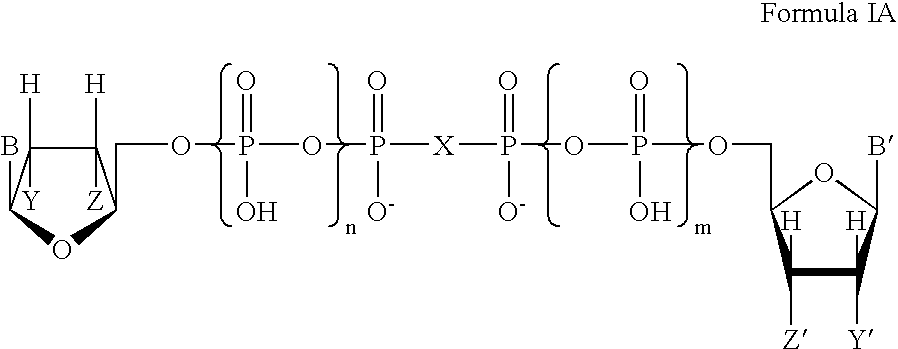Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
493 results about "Sinusitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Short-term inflammation of mucous membranes of the paranasal sinuses that surround the nasal cavity.
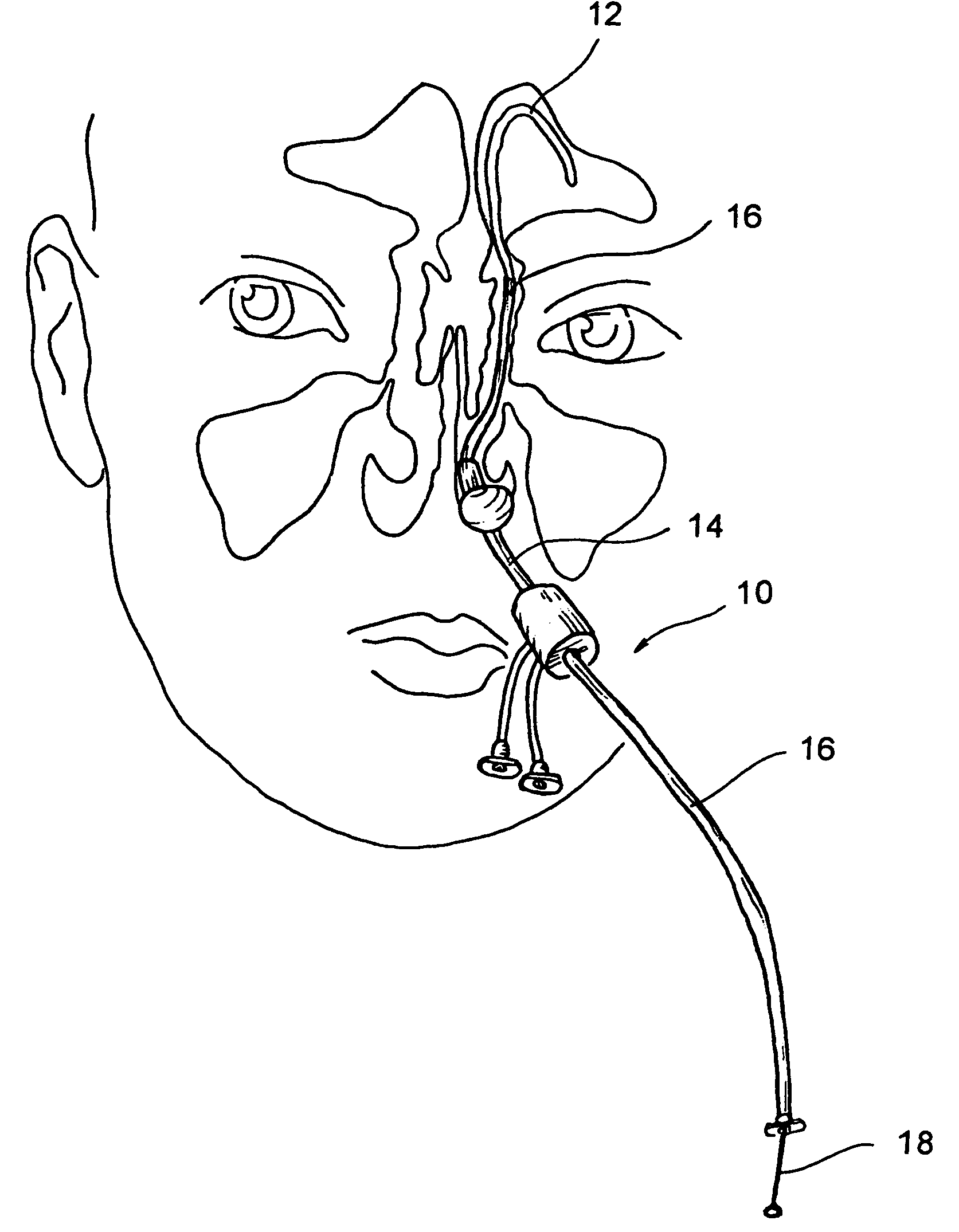
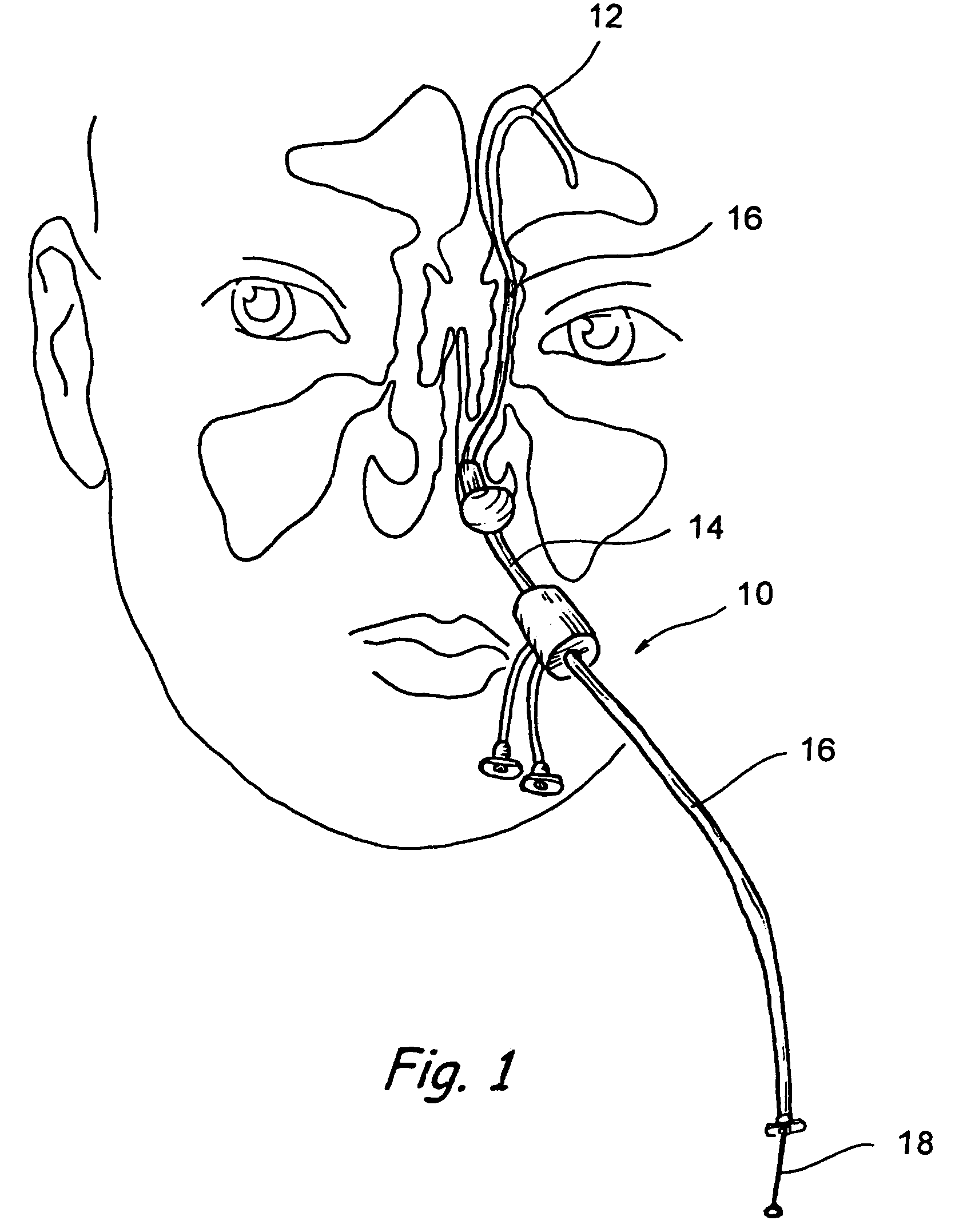
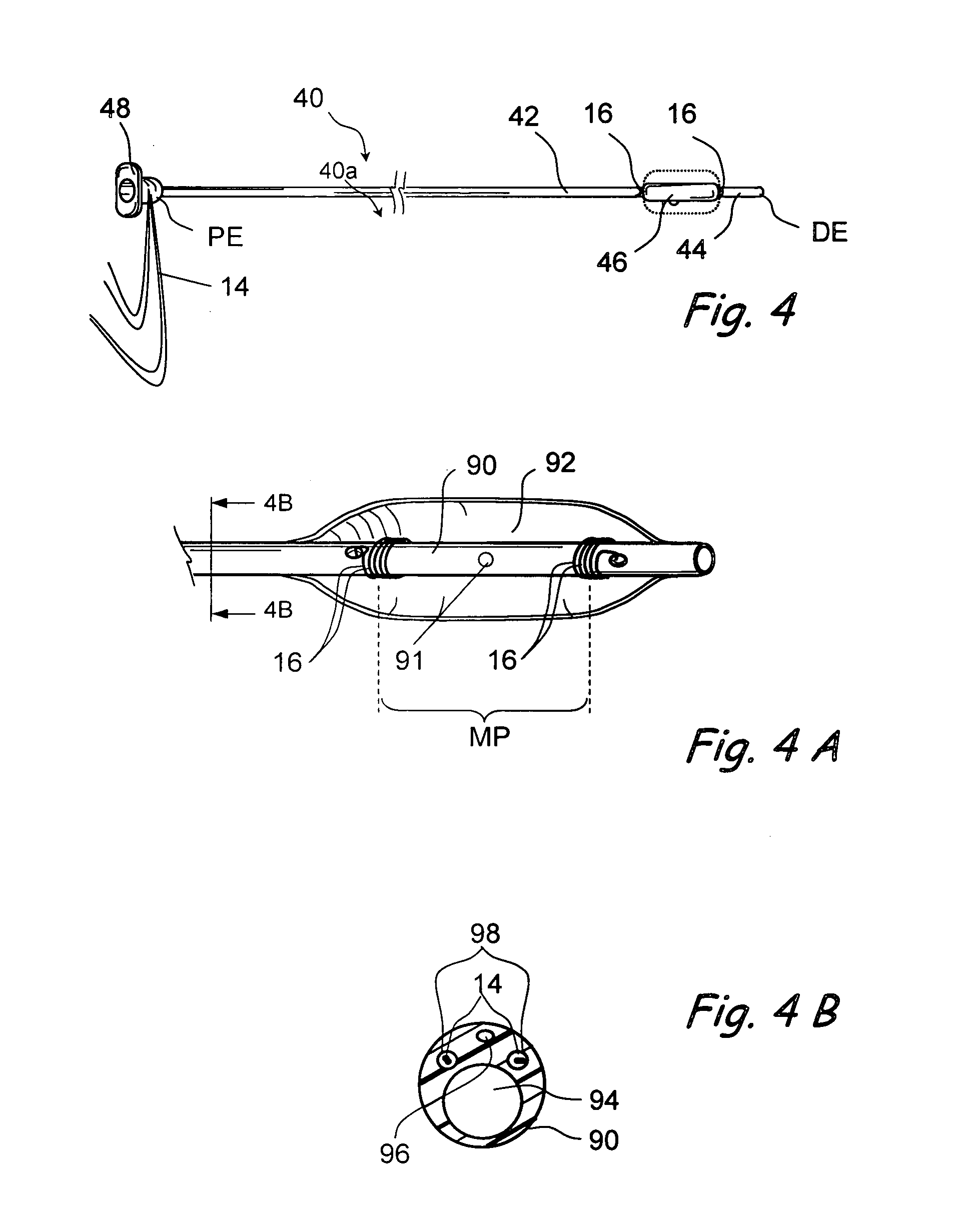
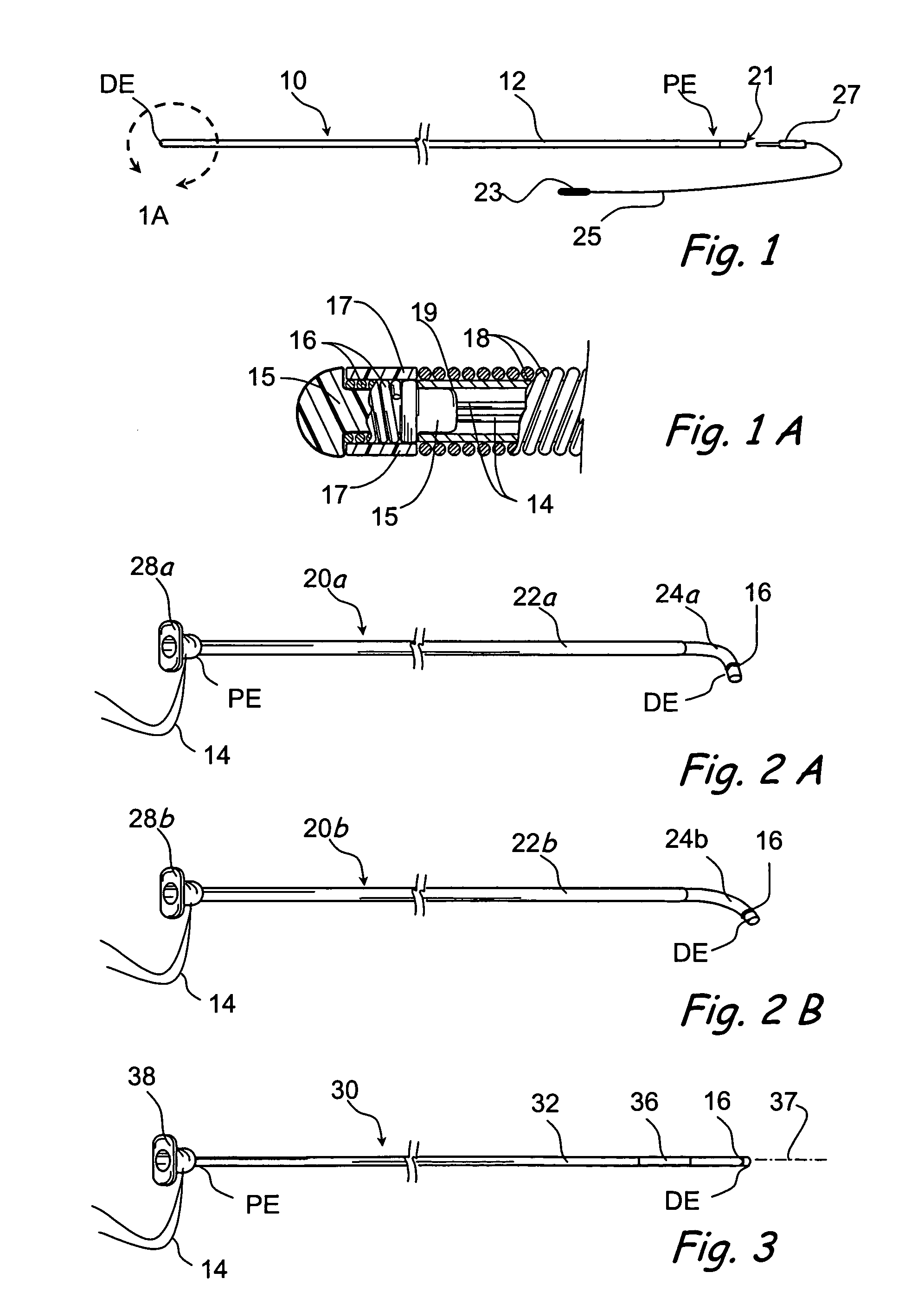
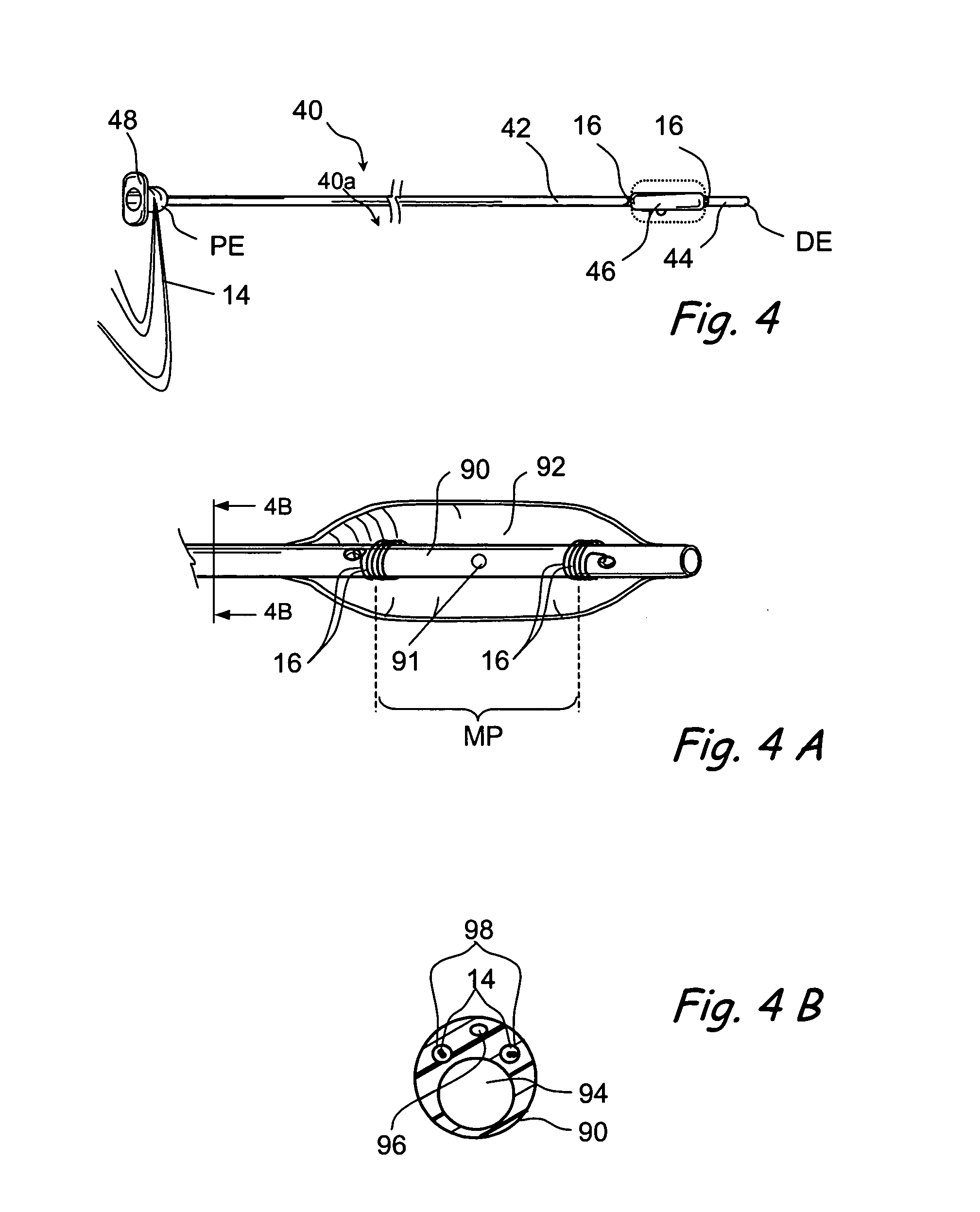
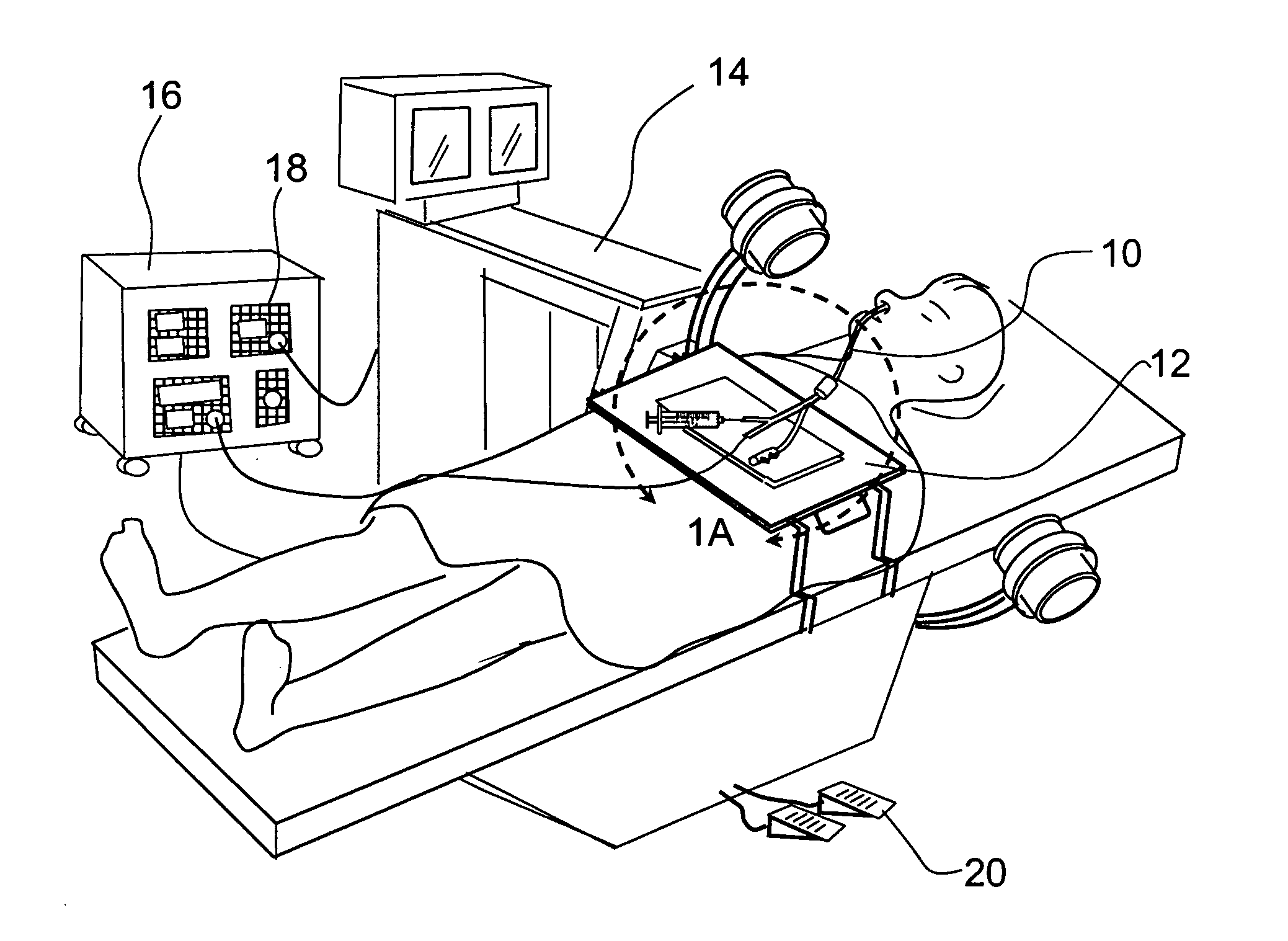
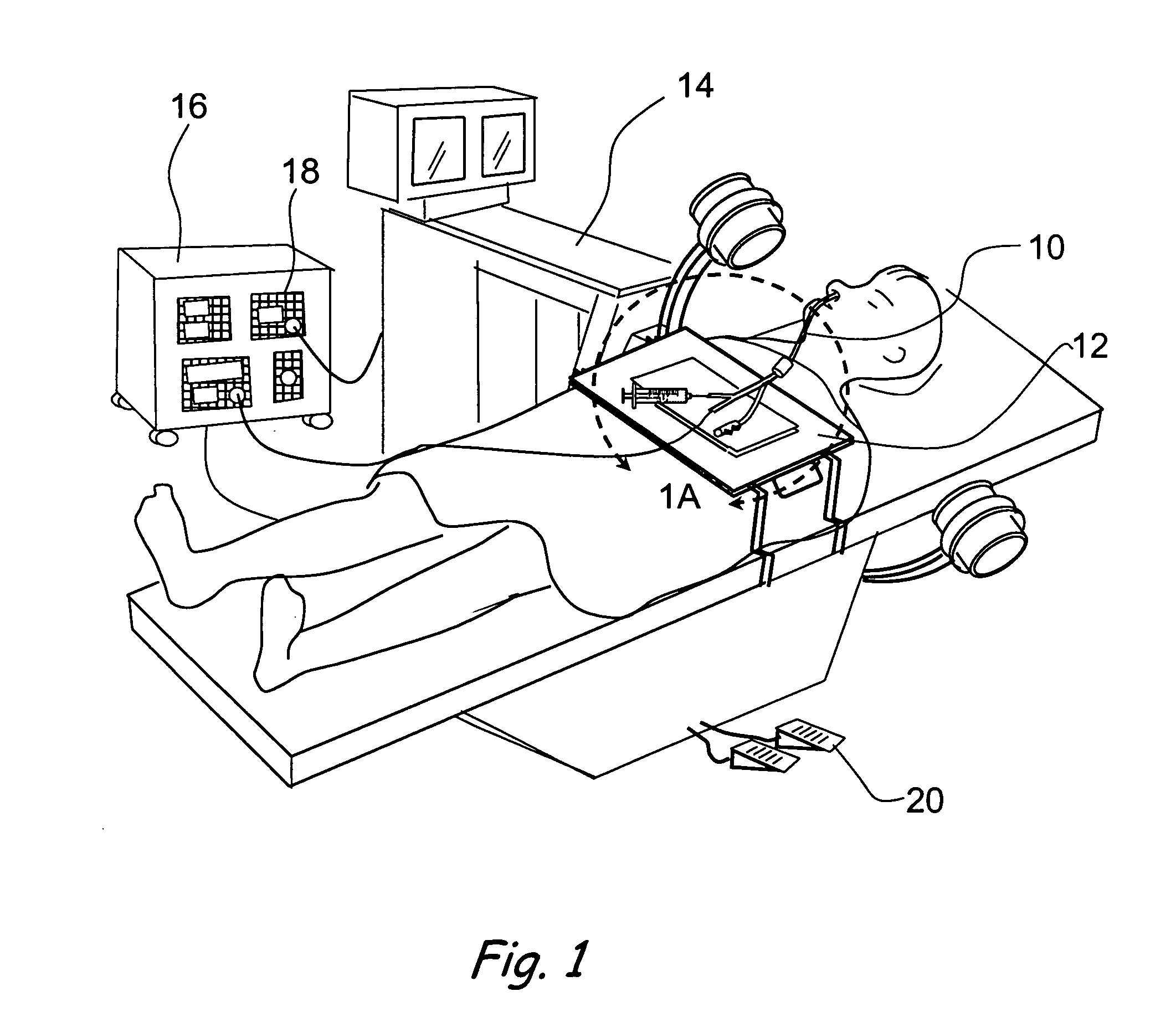
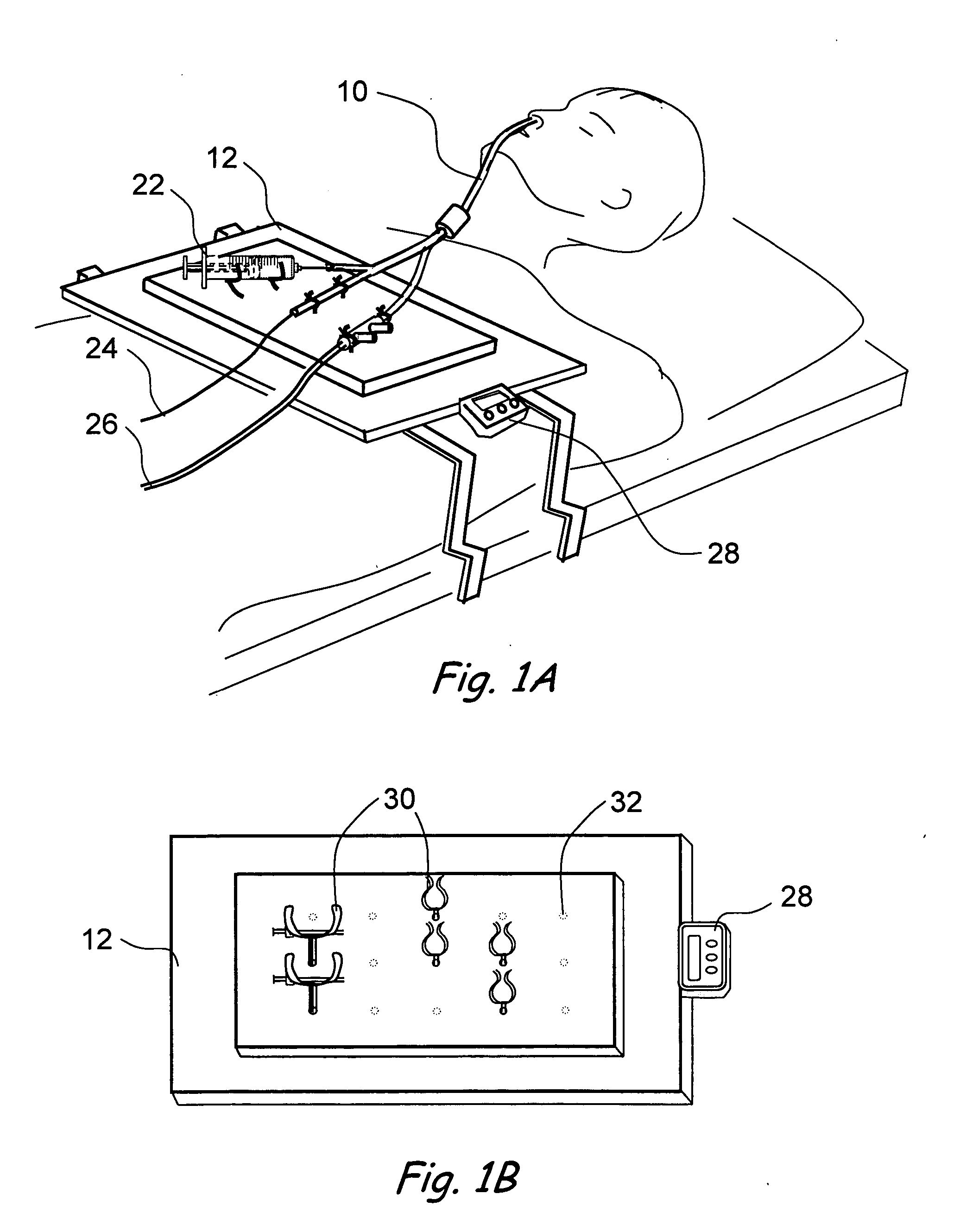
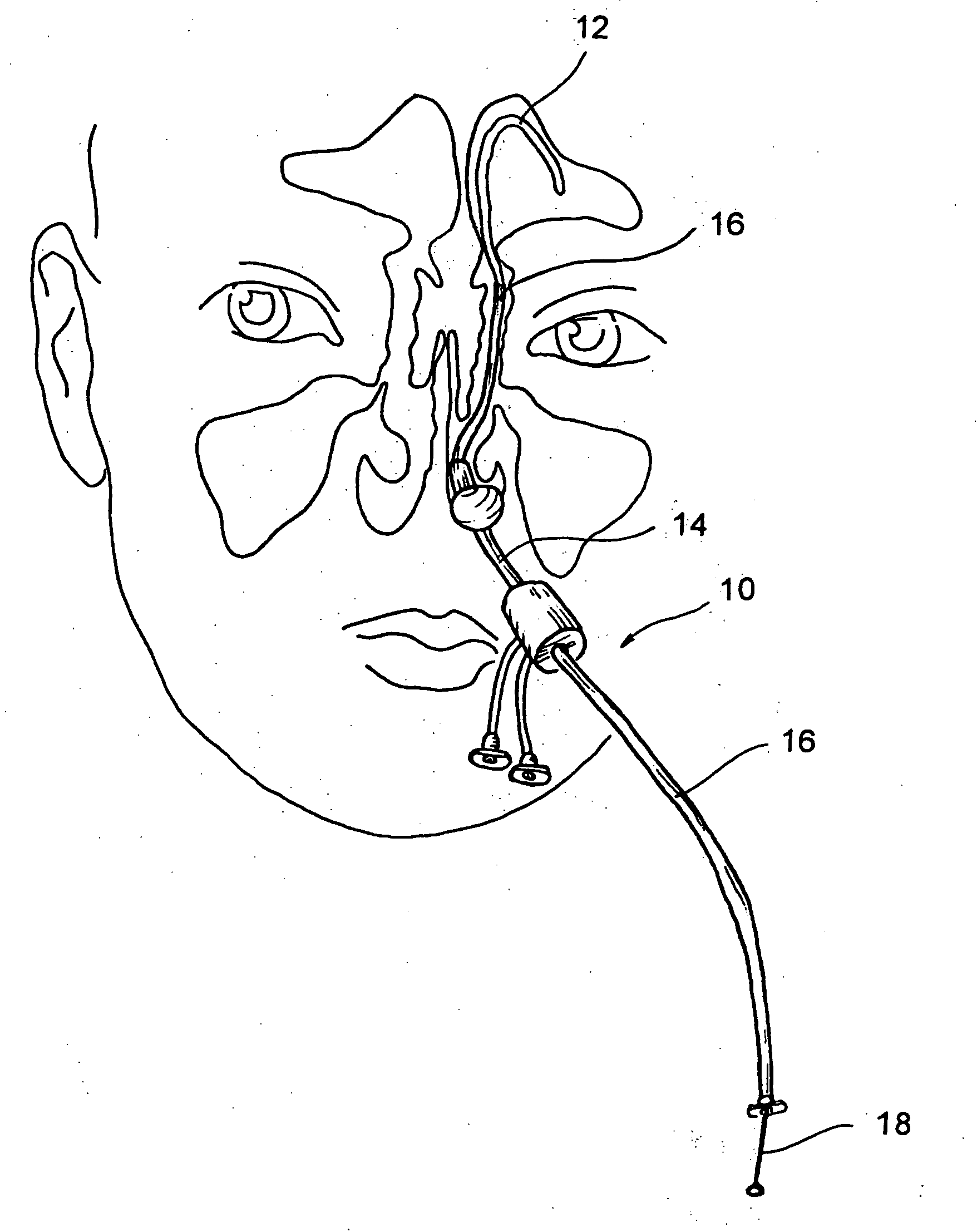
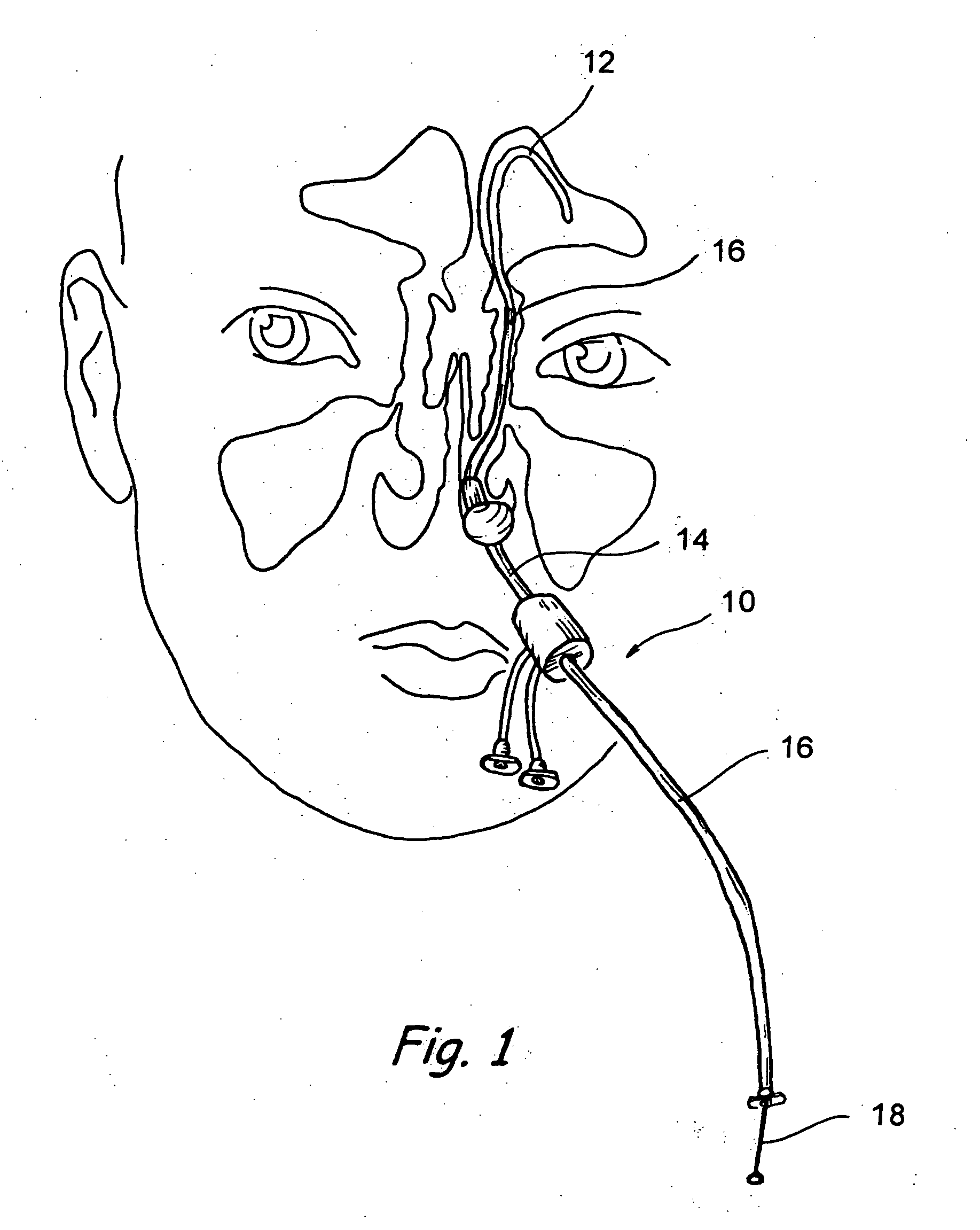
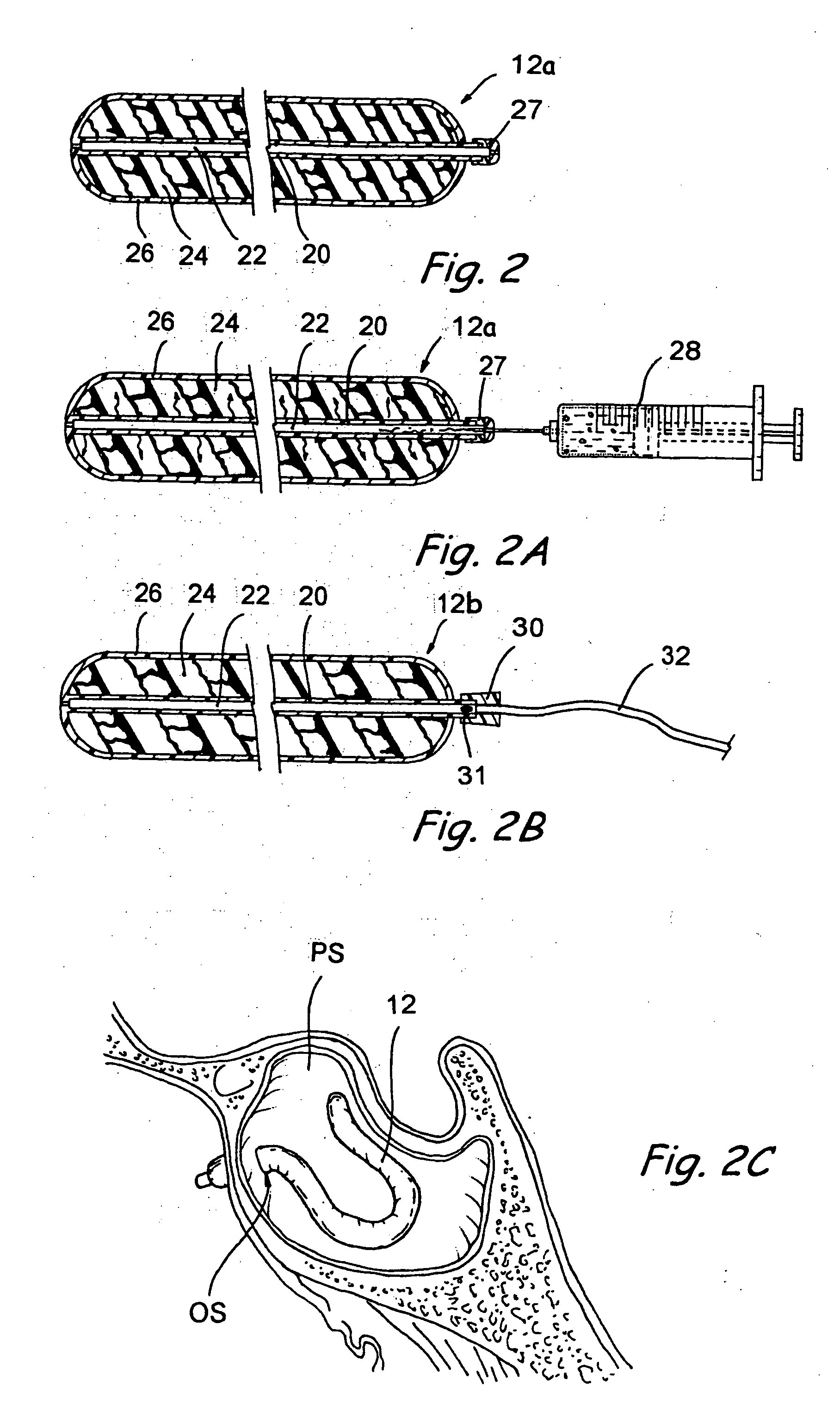
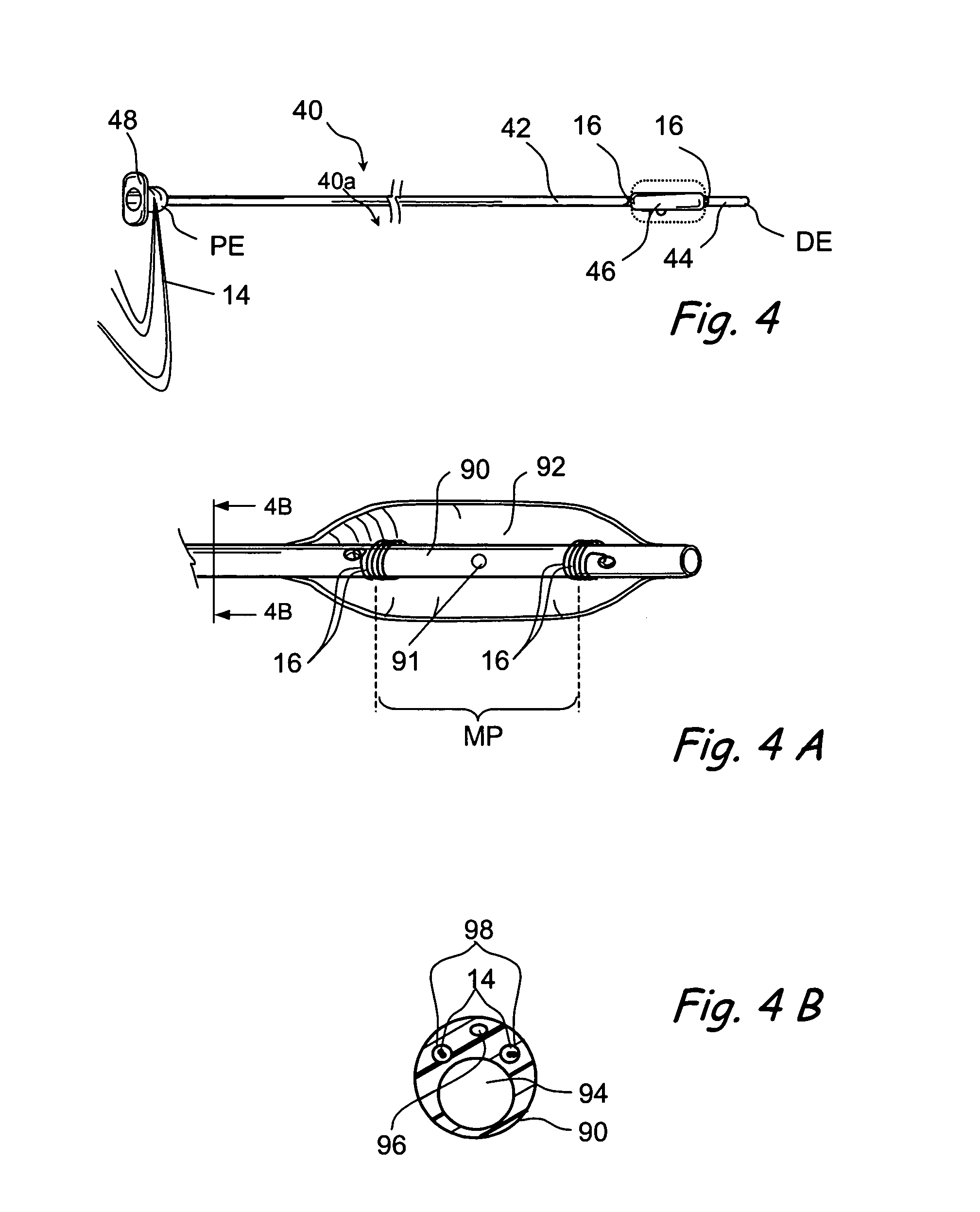
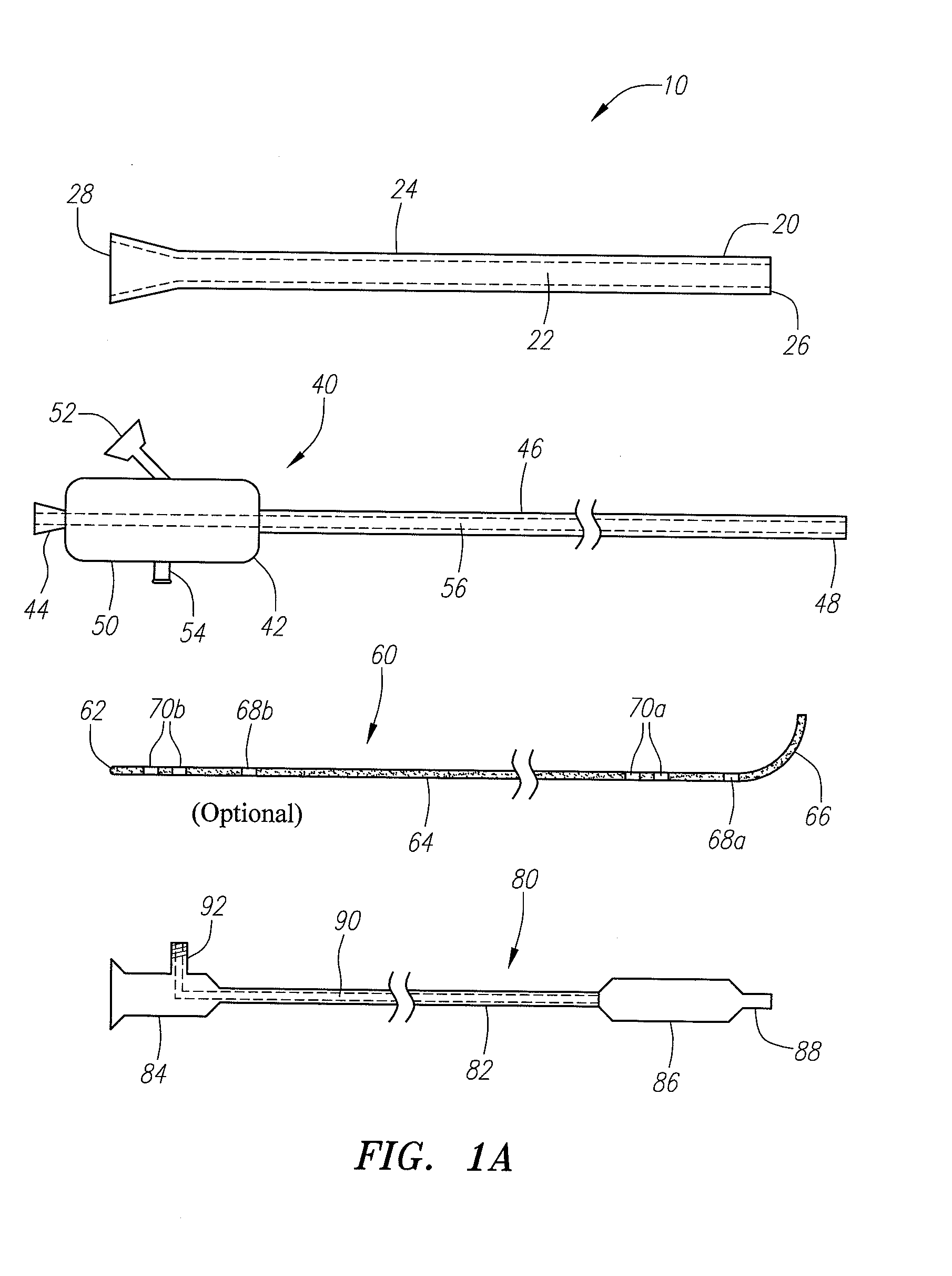
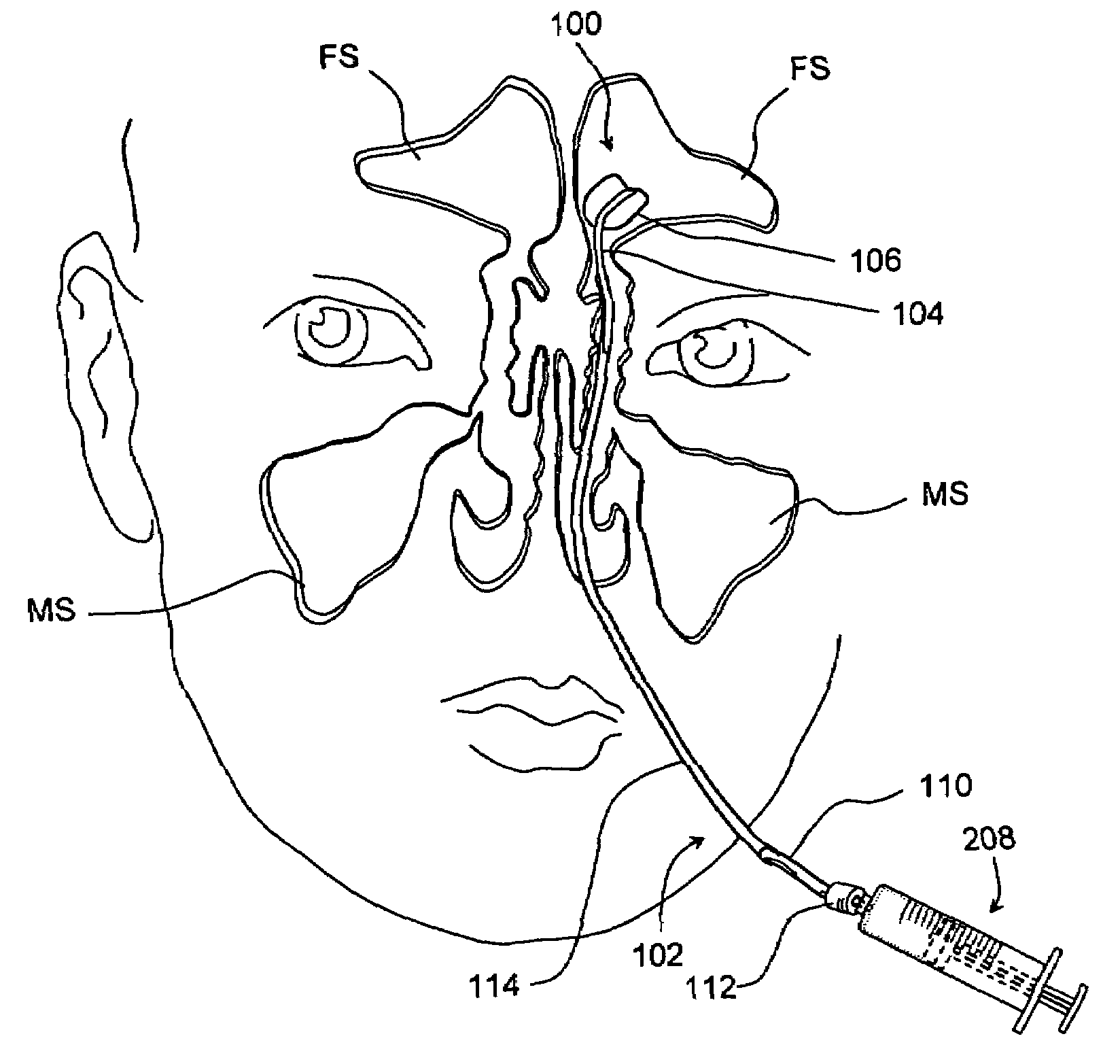
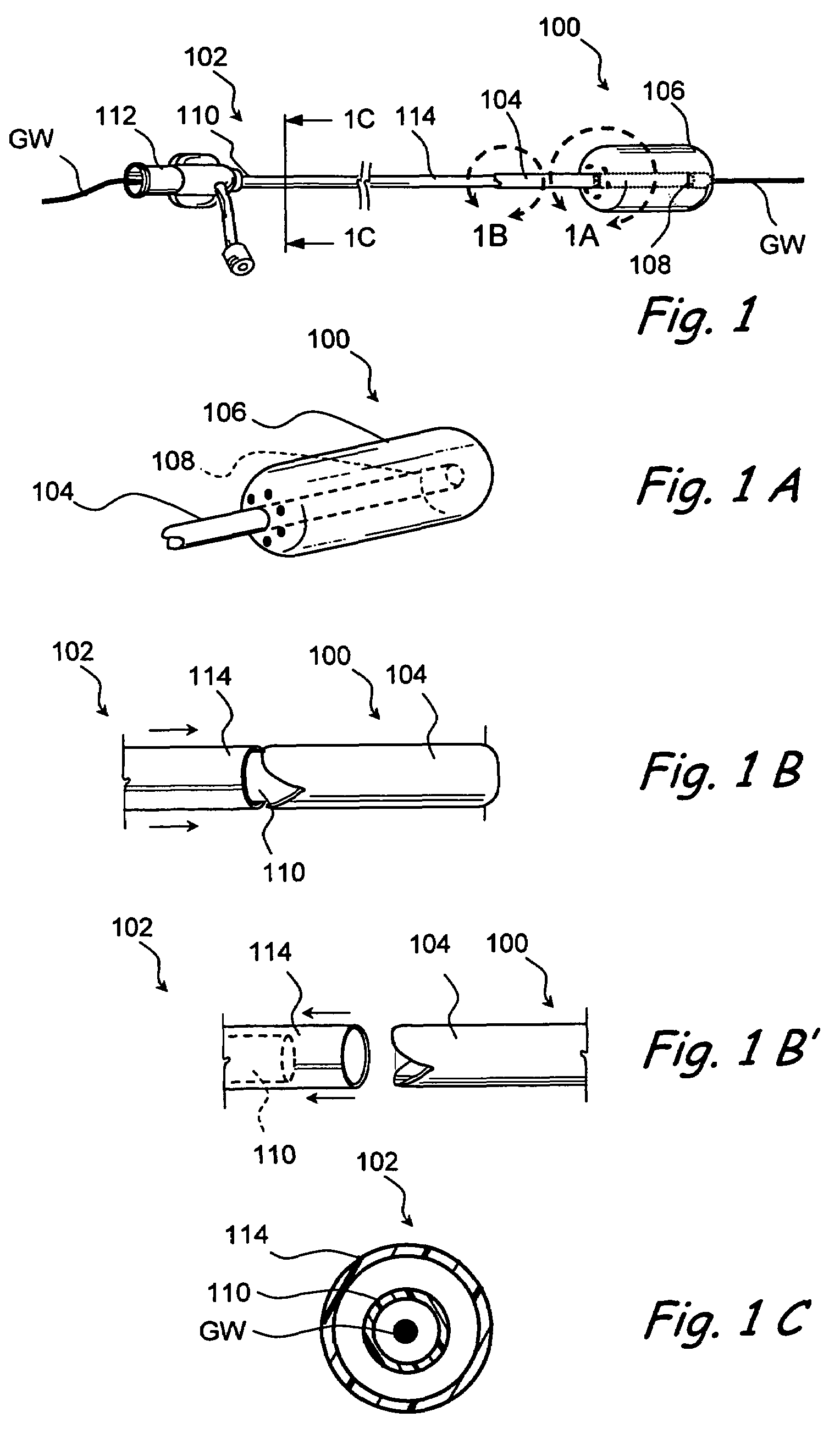
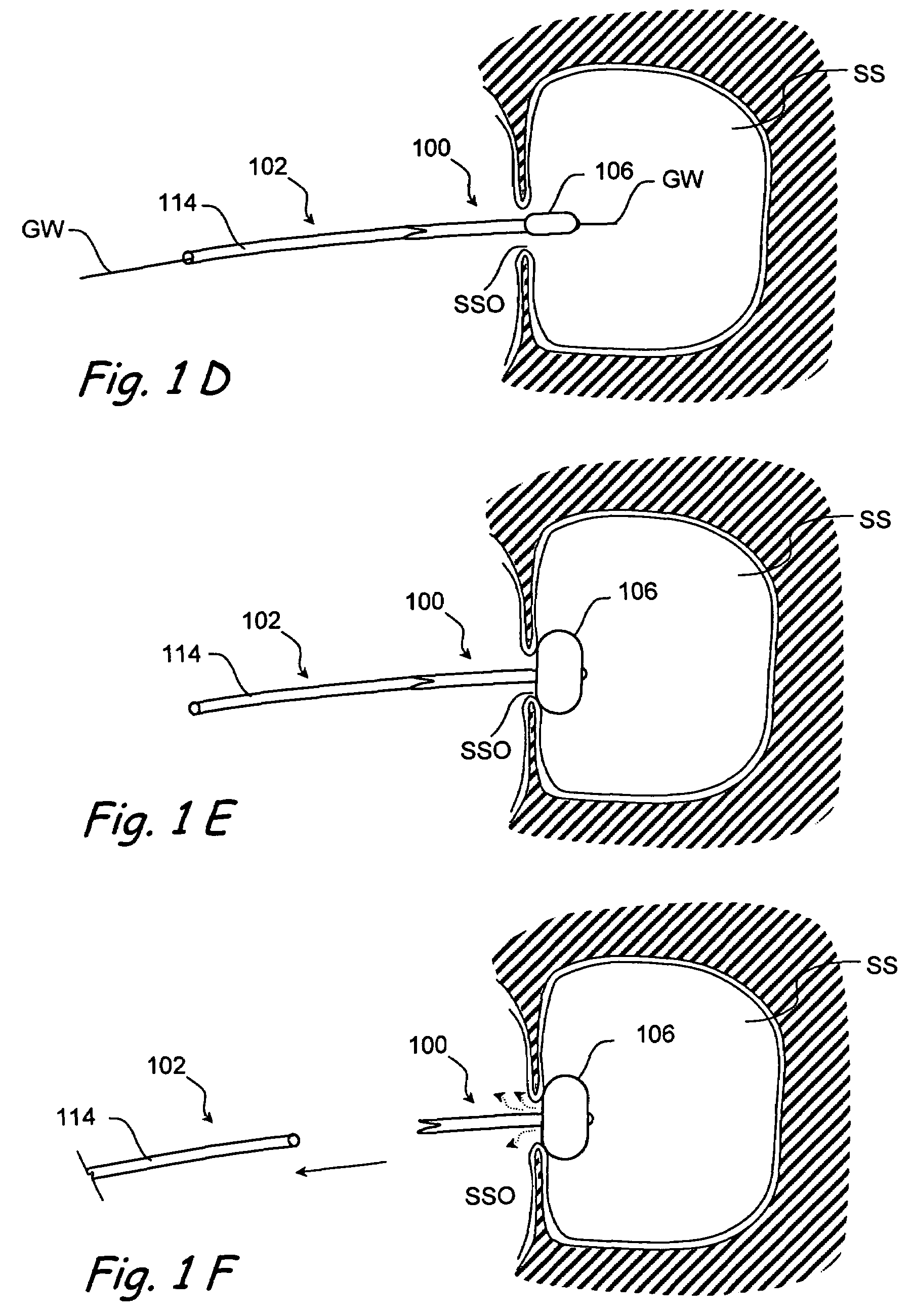
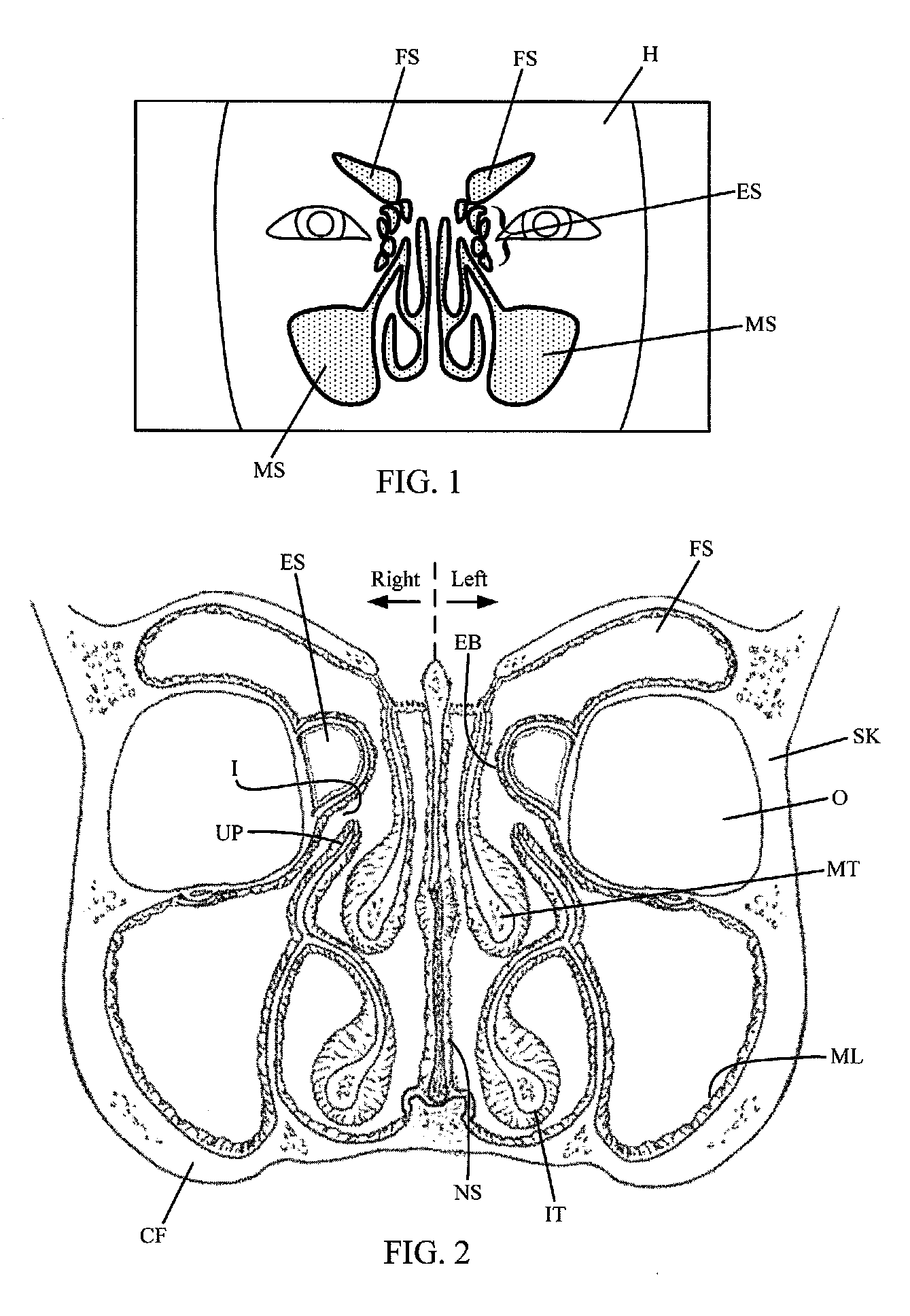
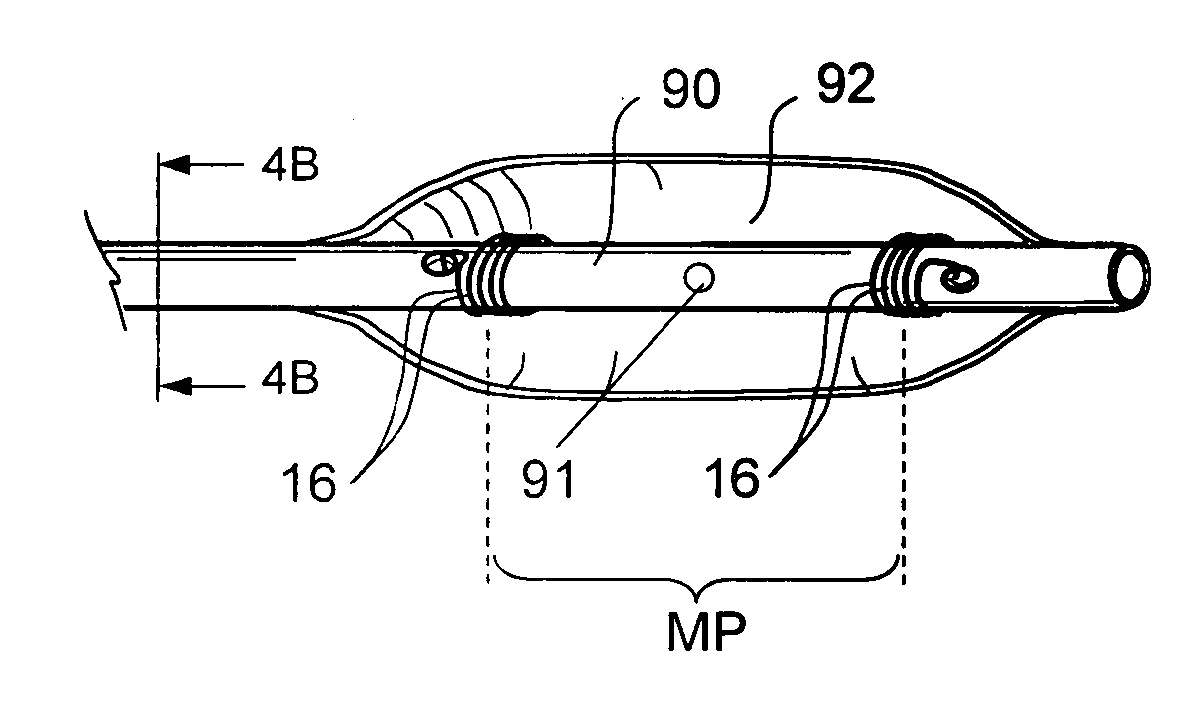
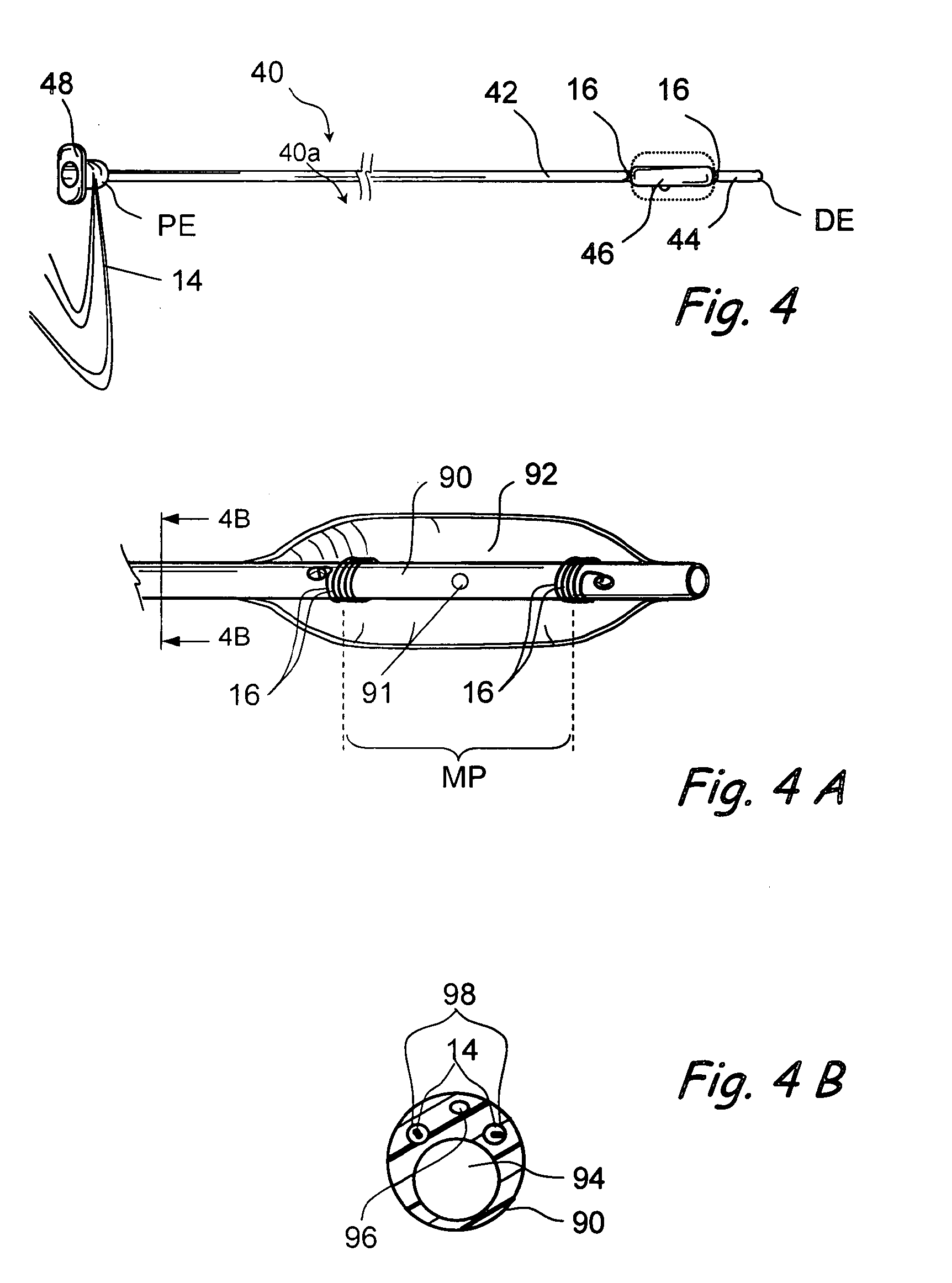
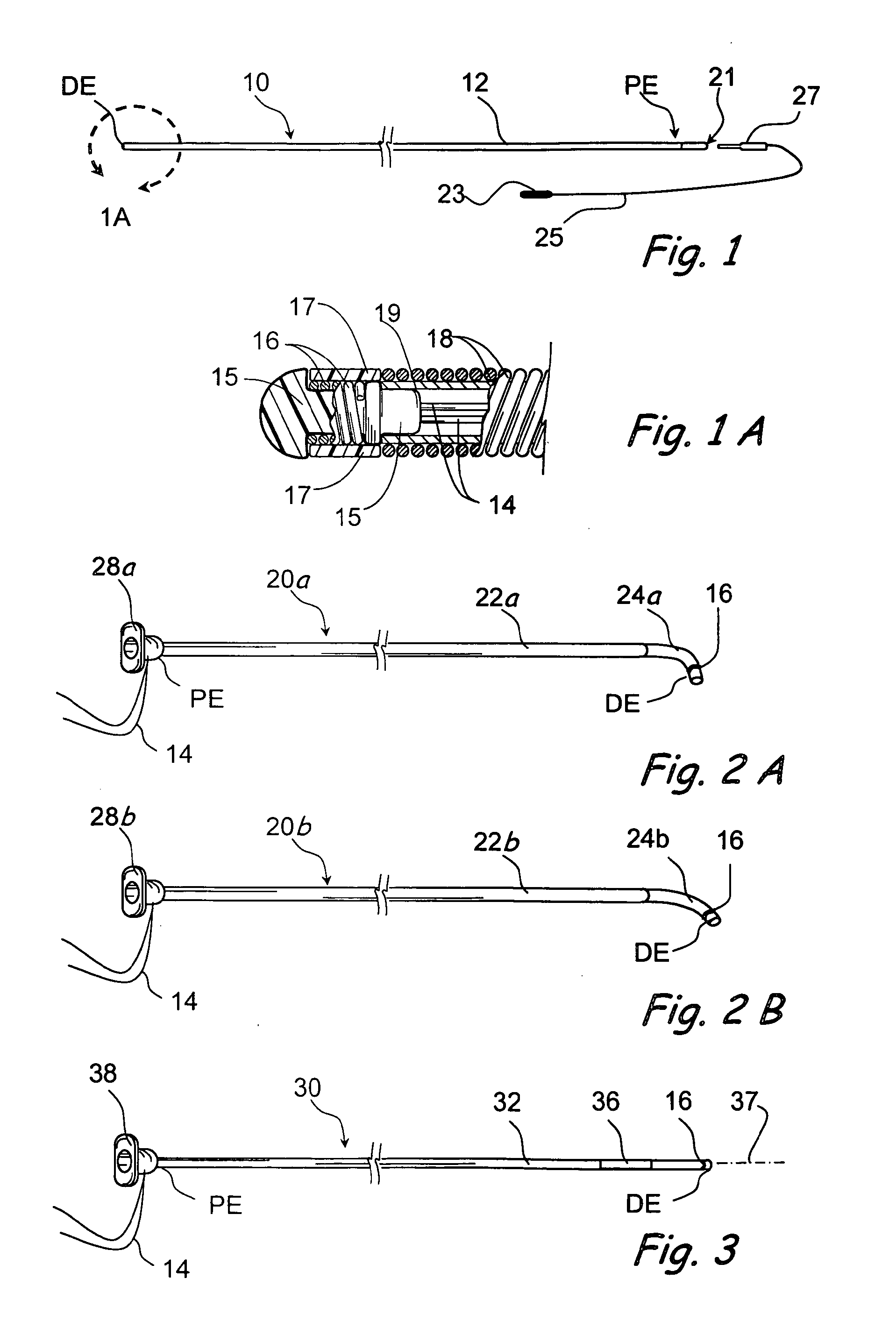
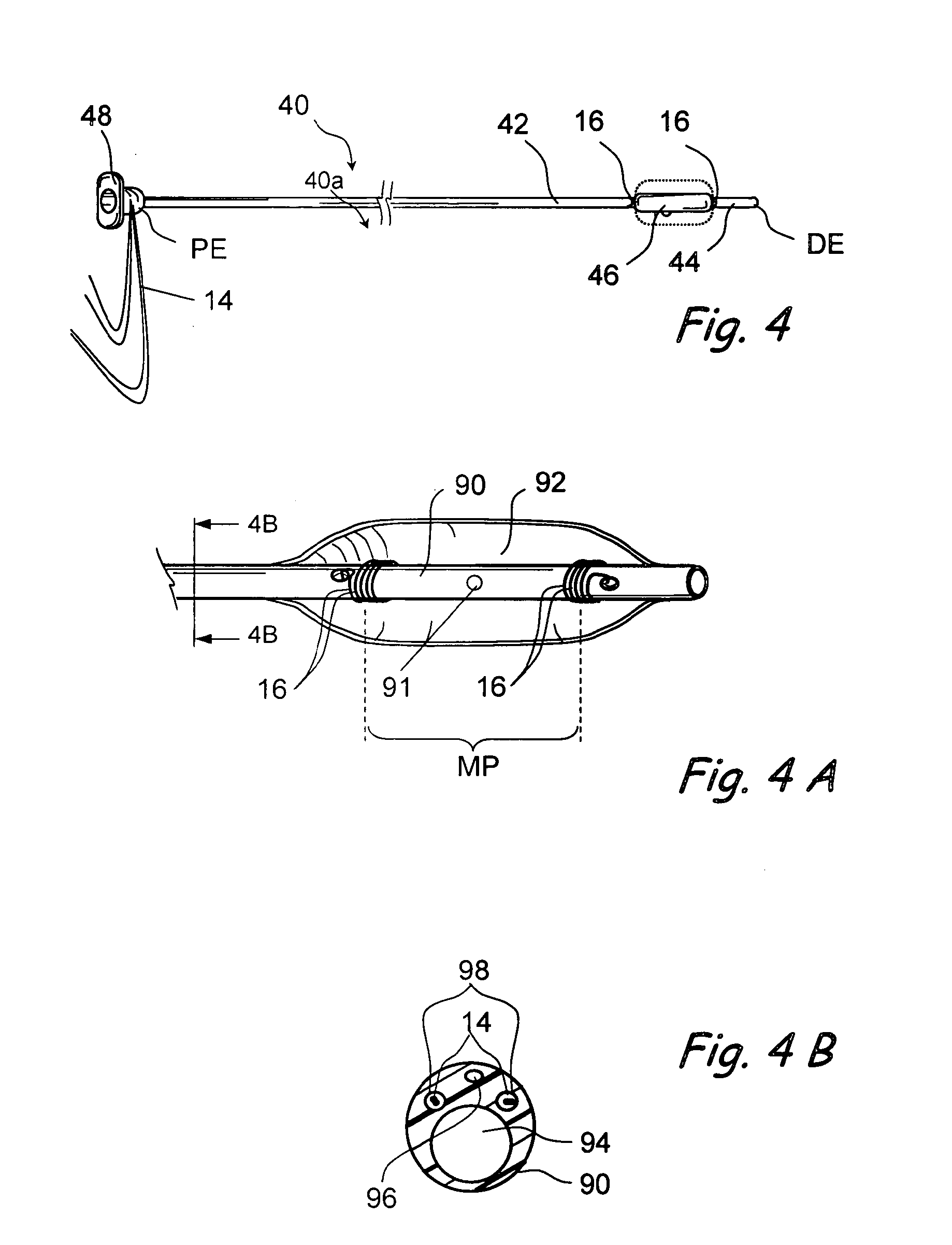
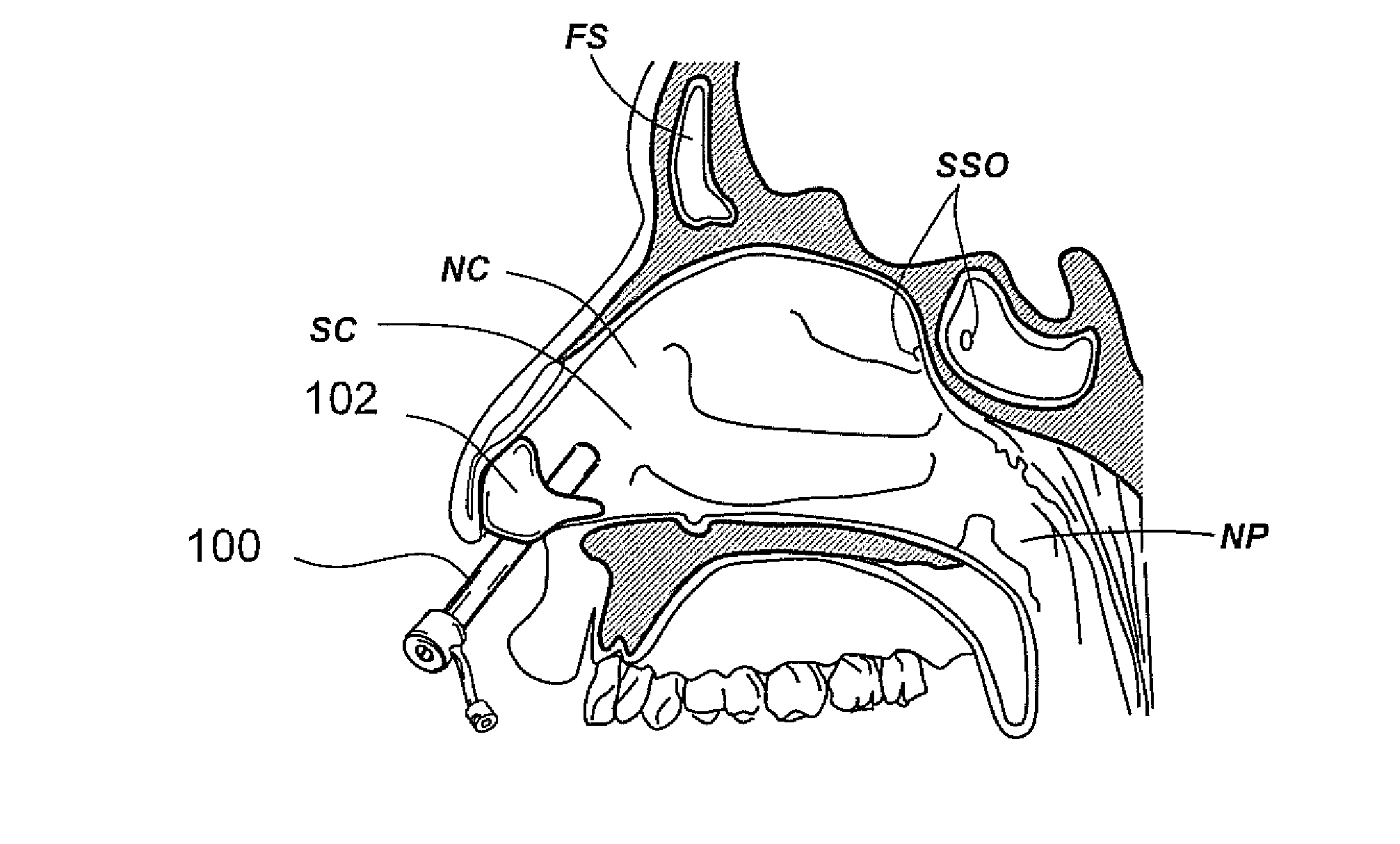
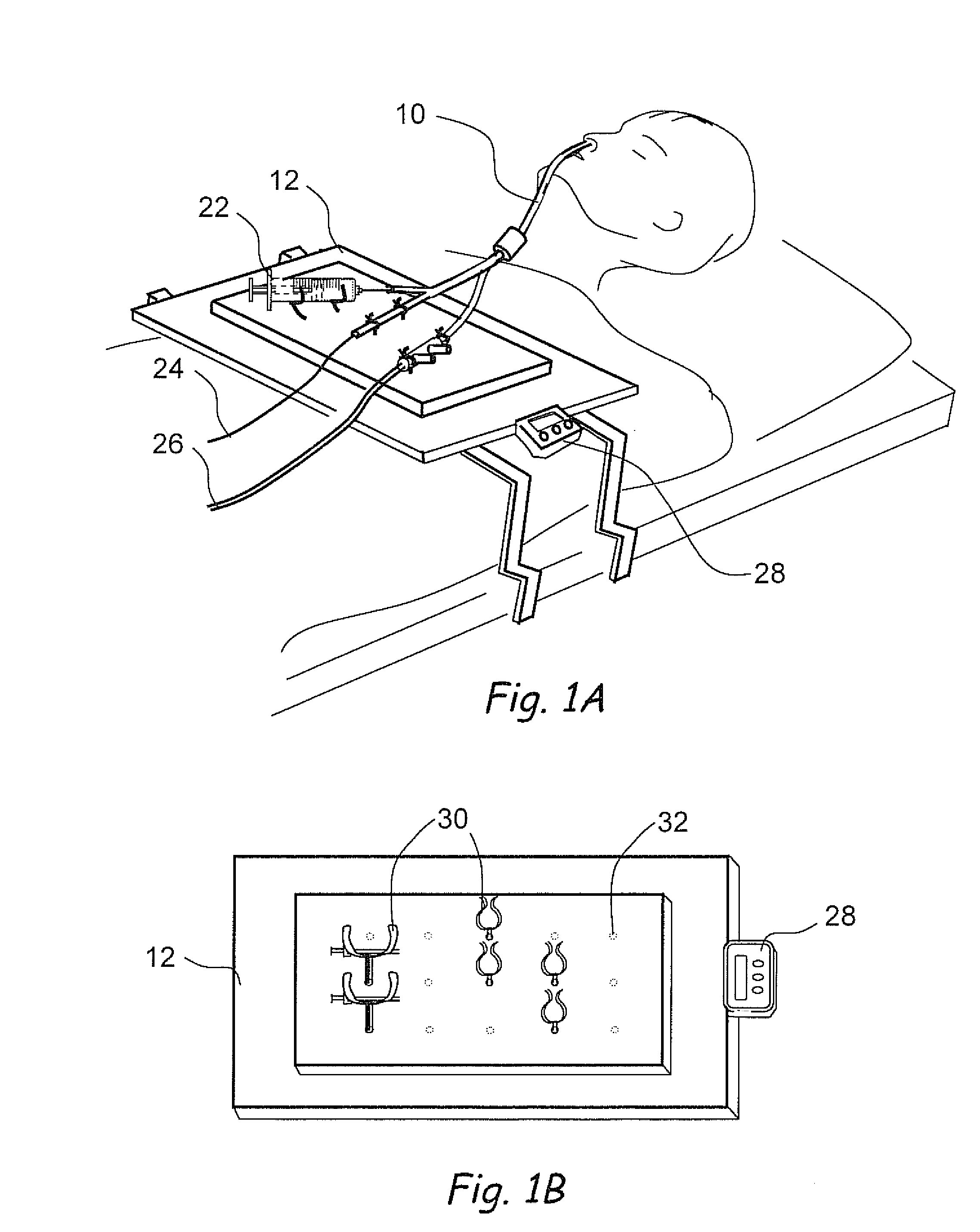
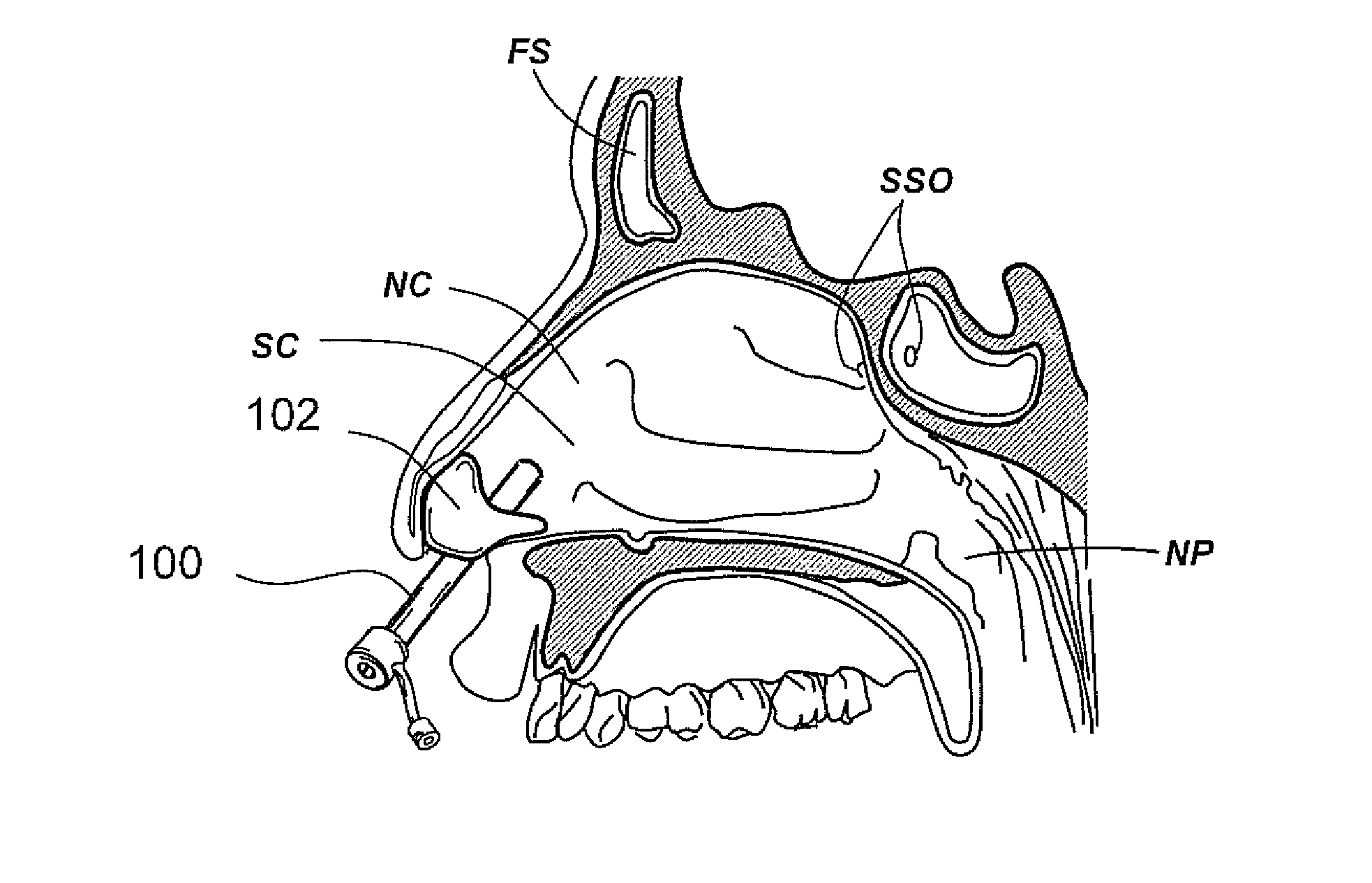
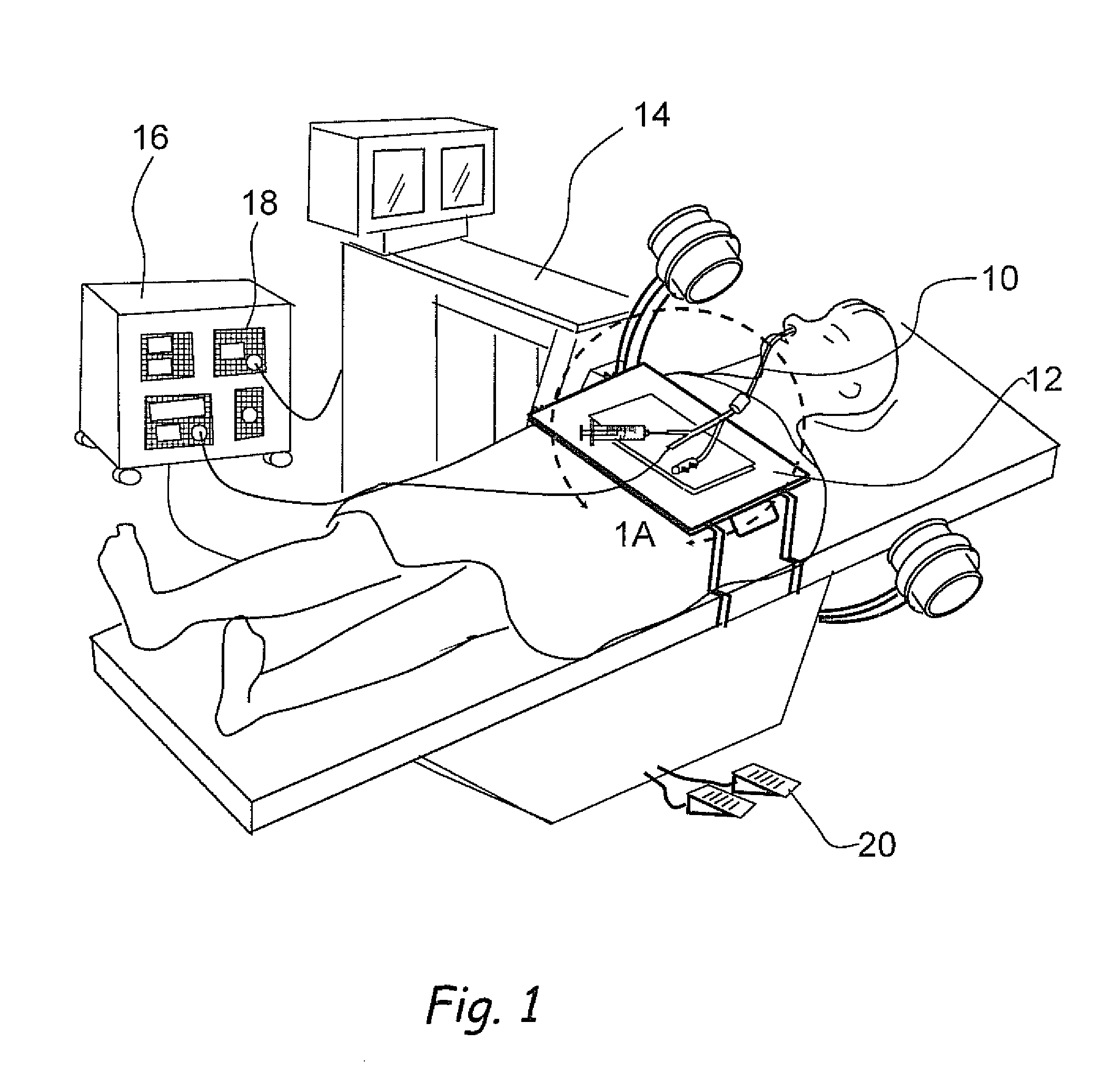
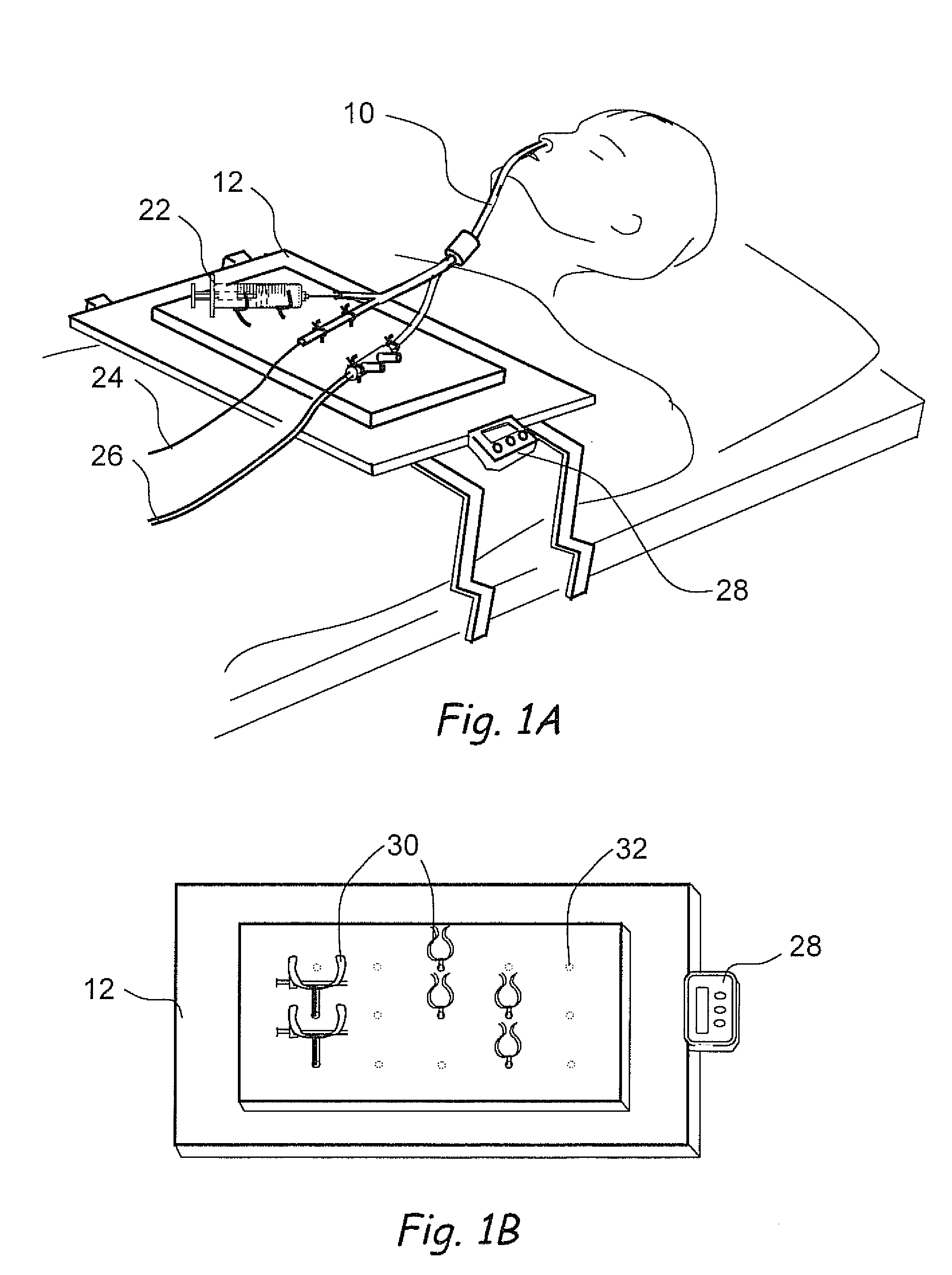
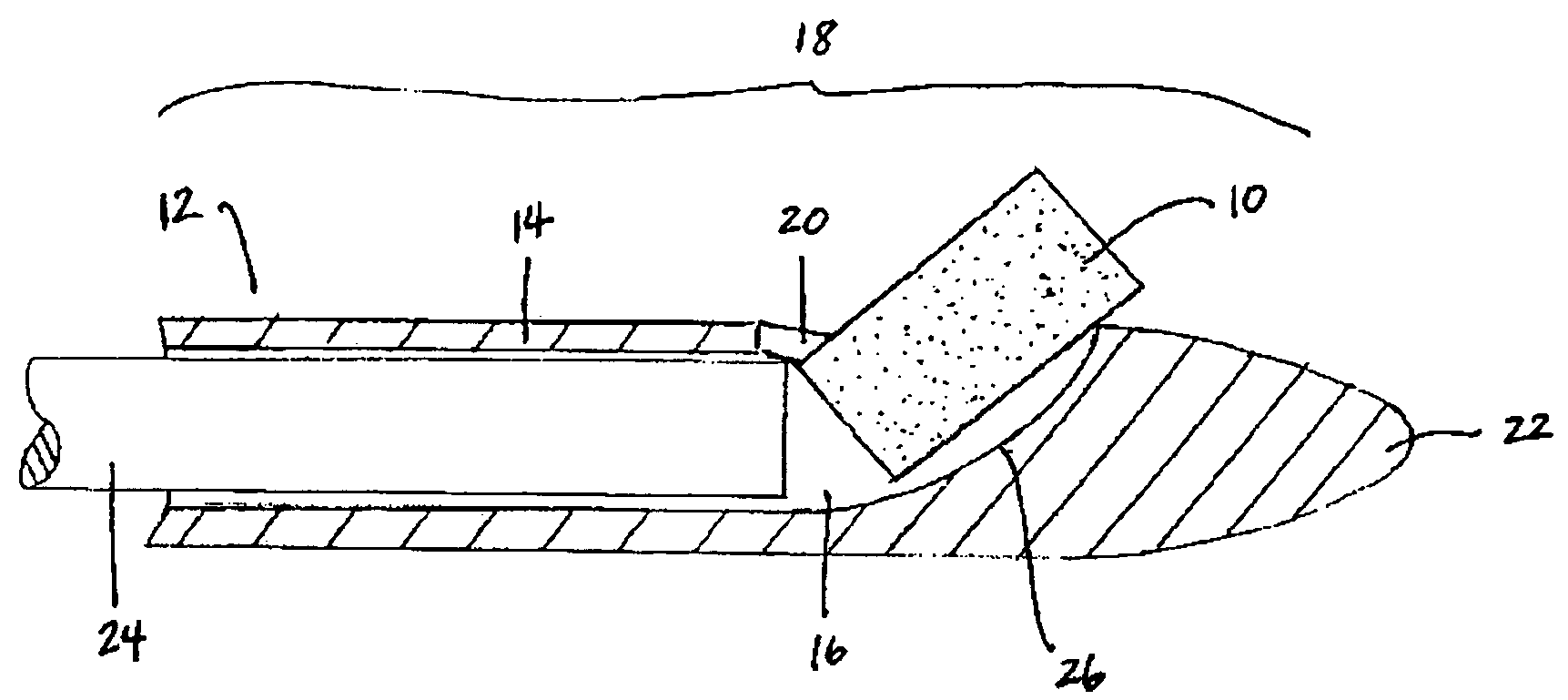
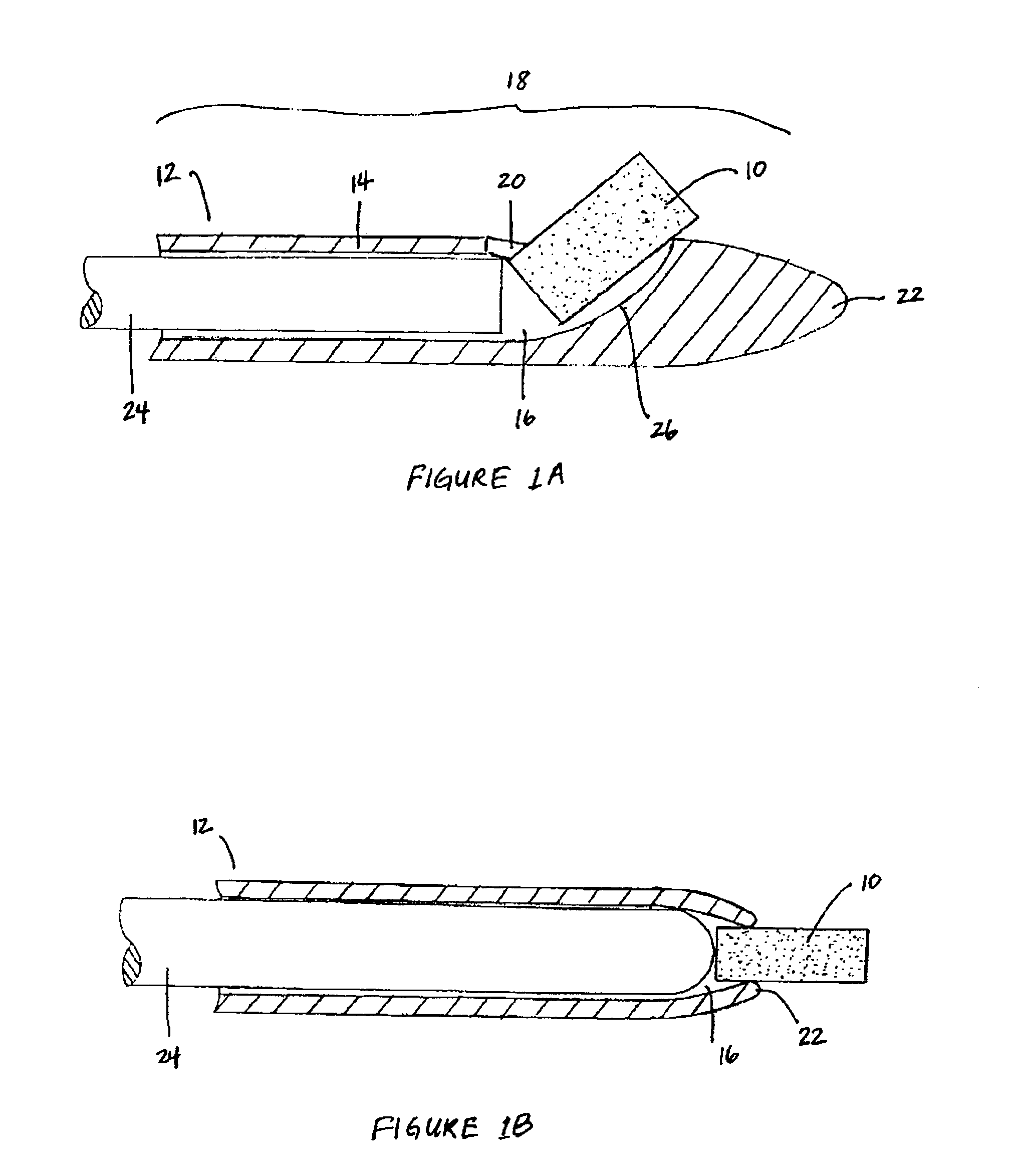
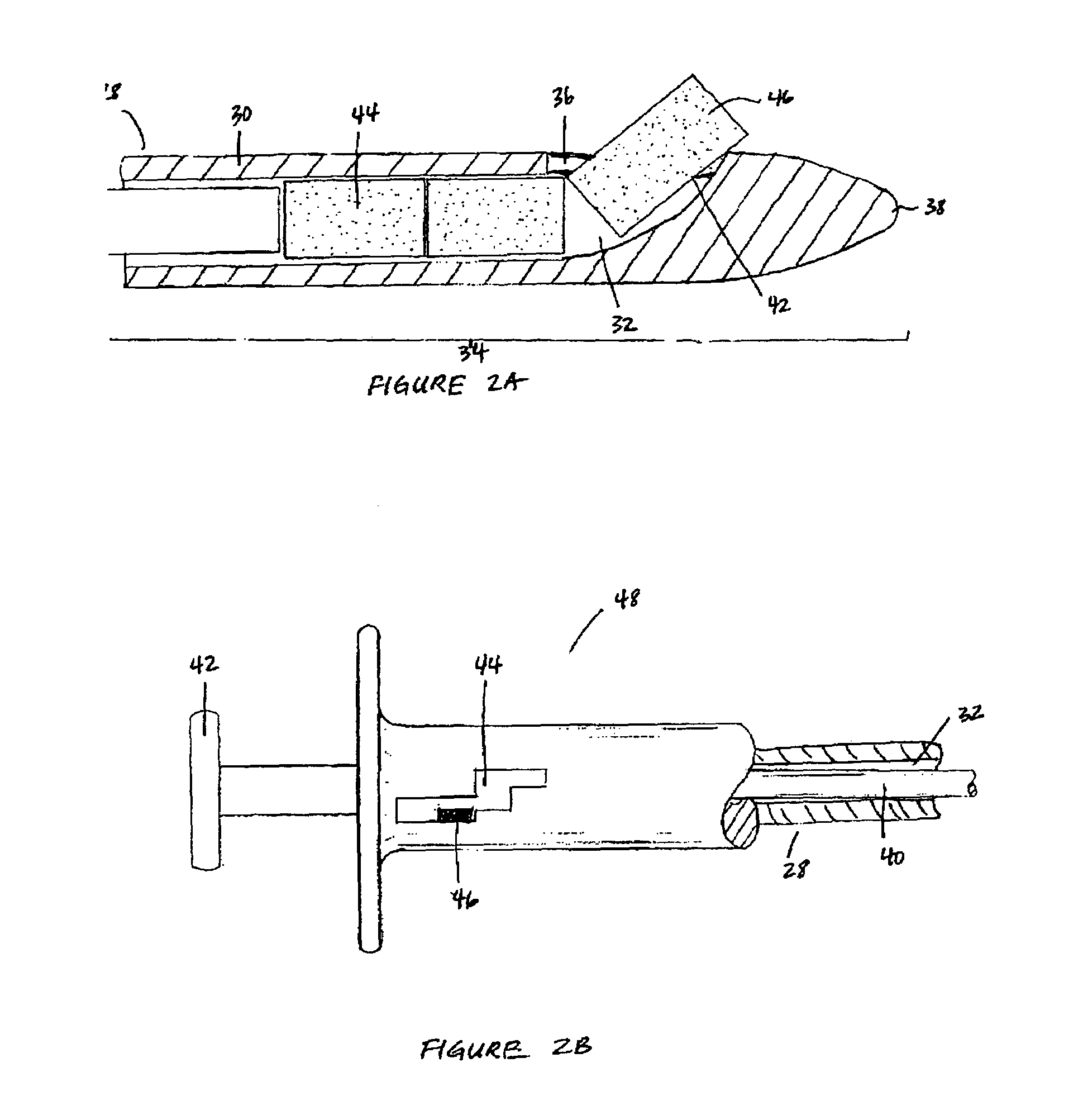
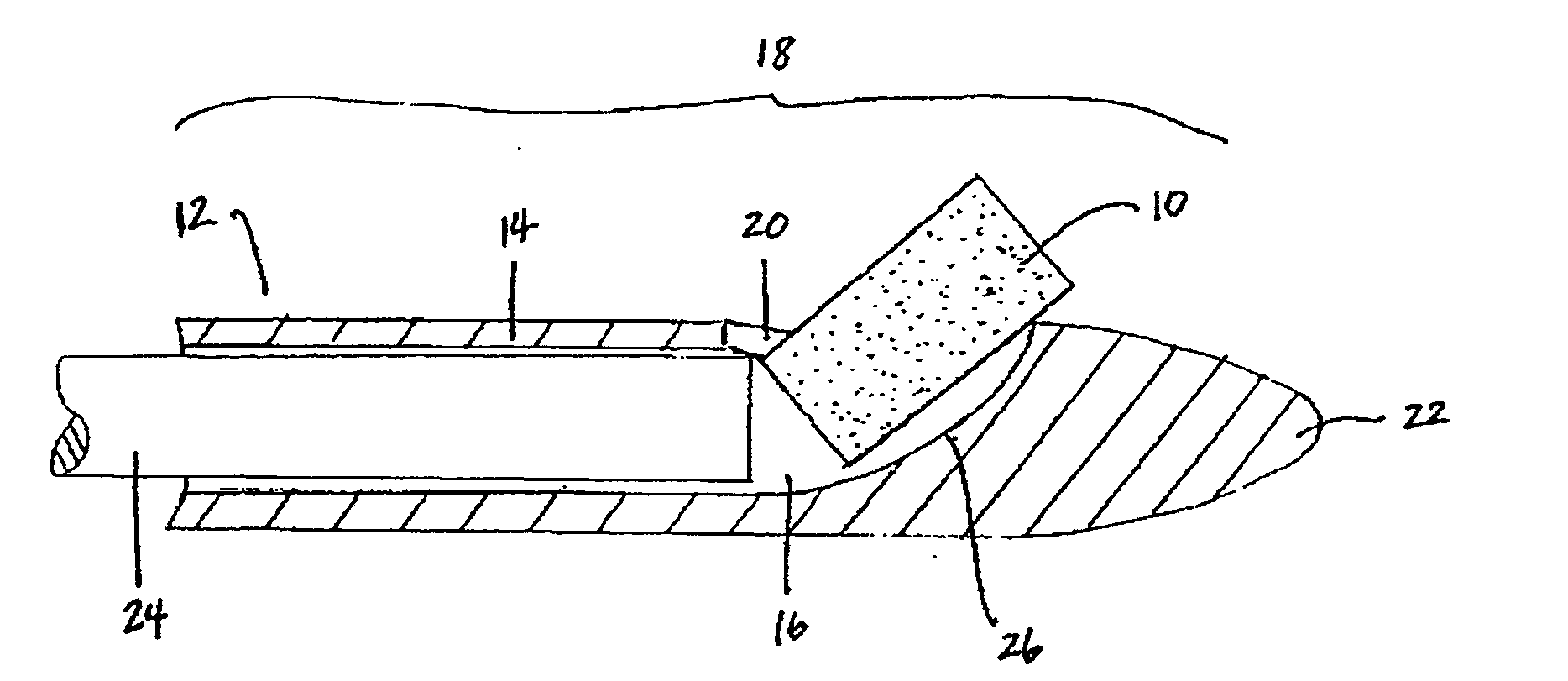
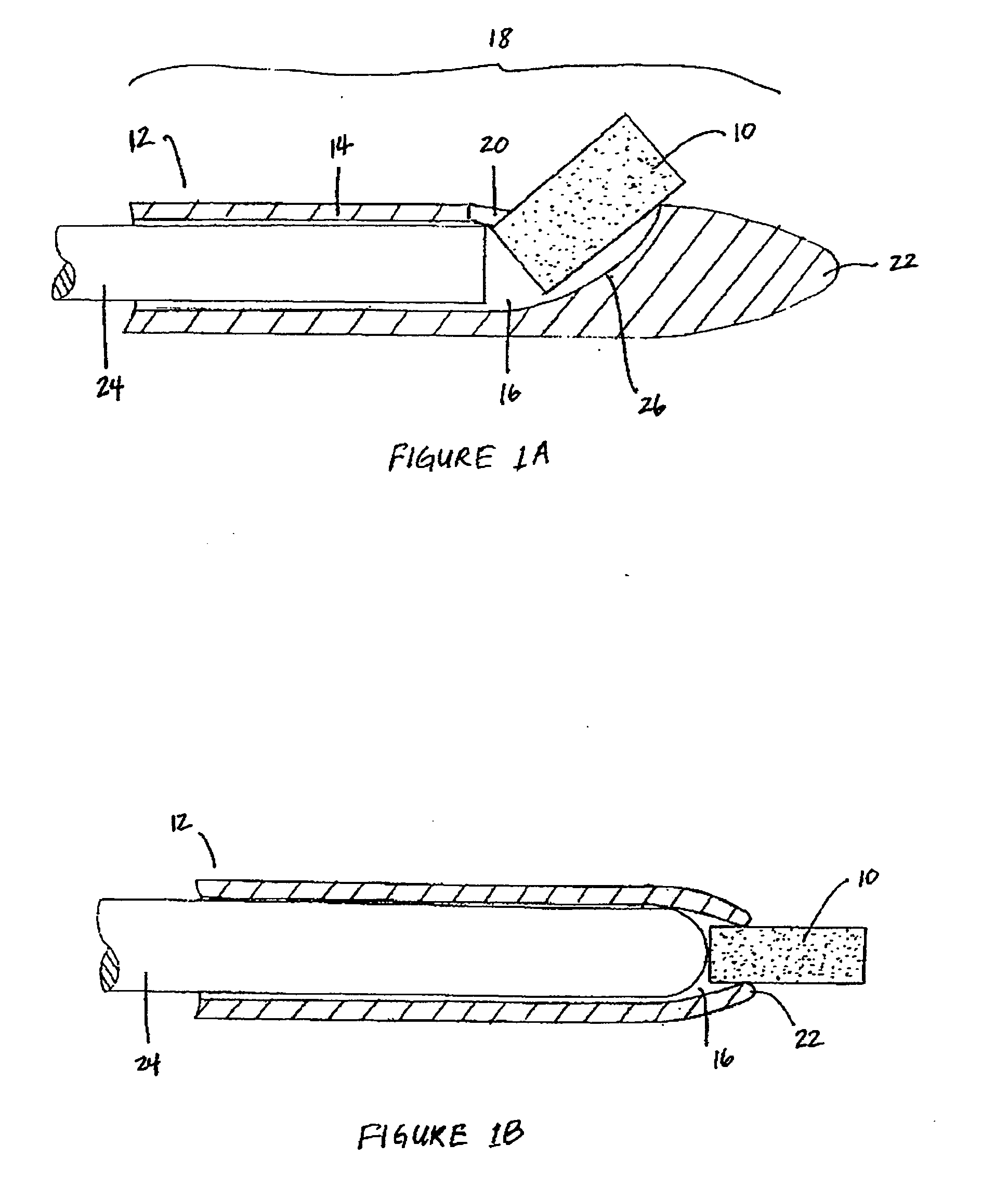
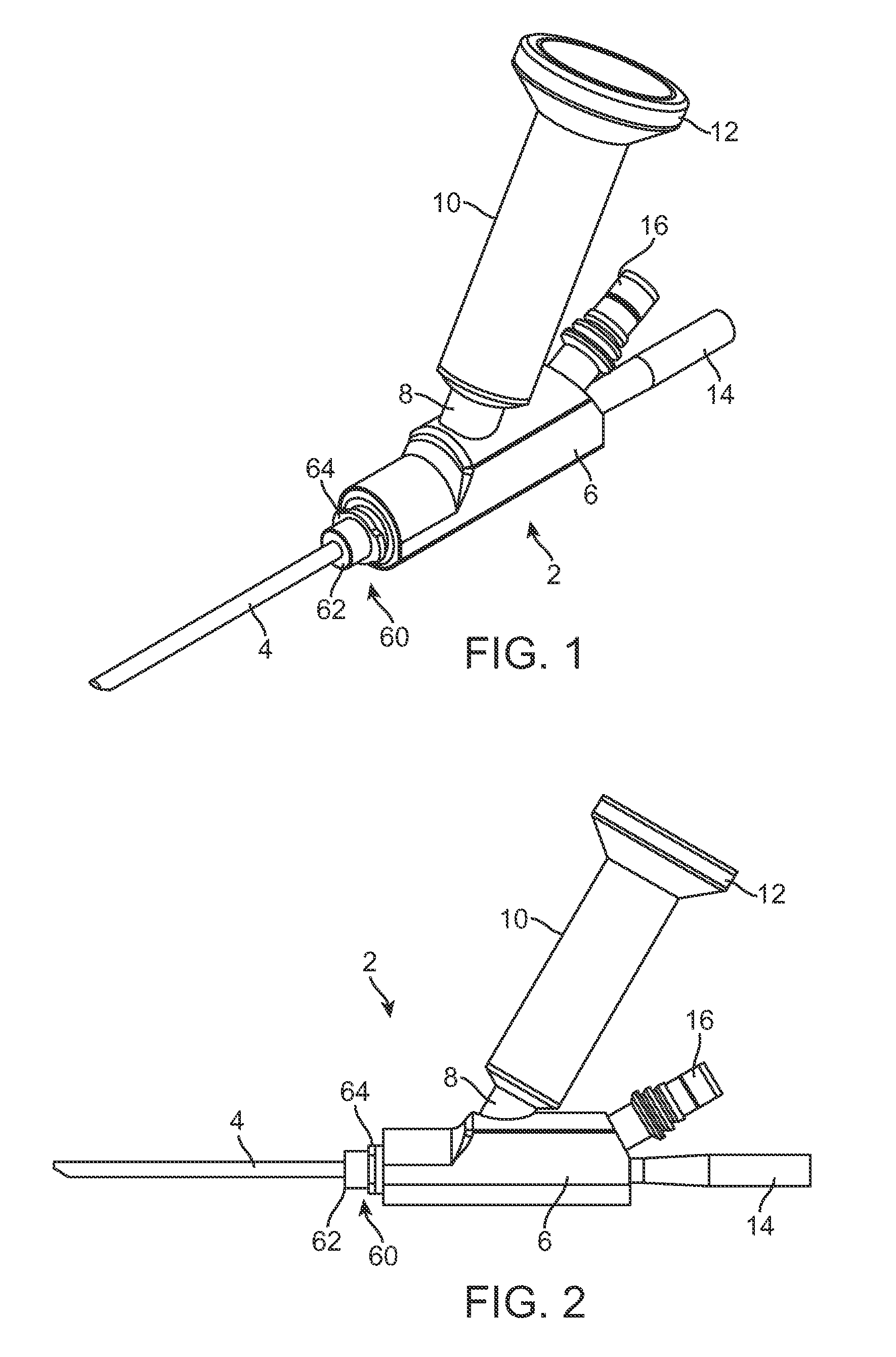
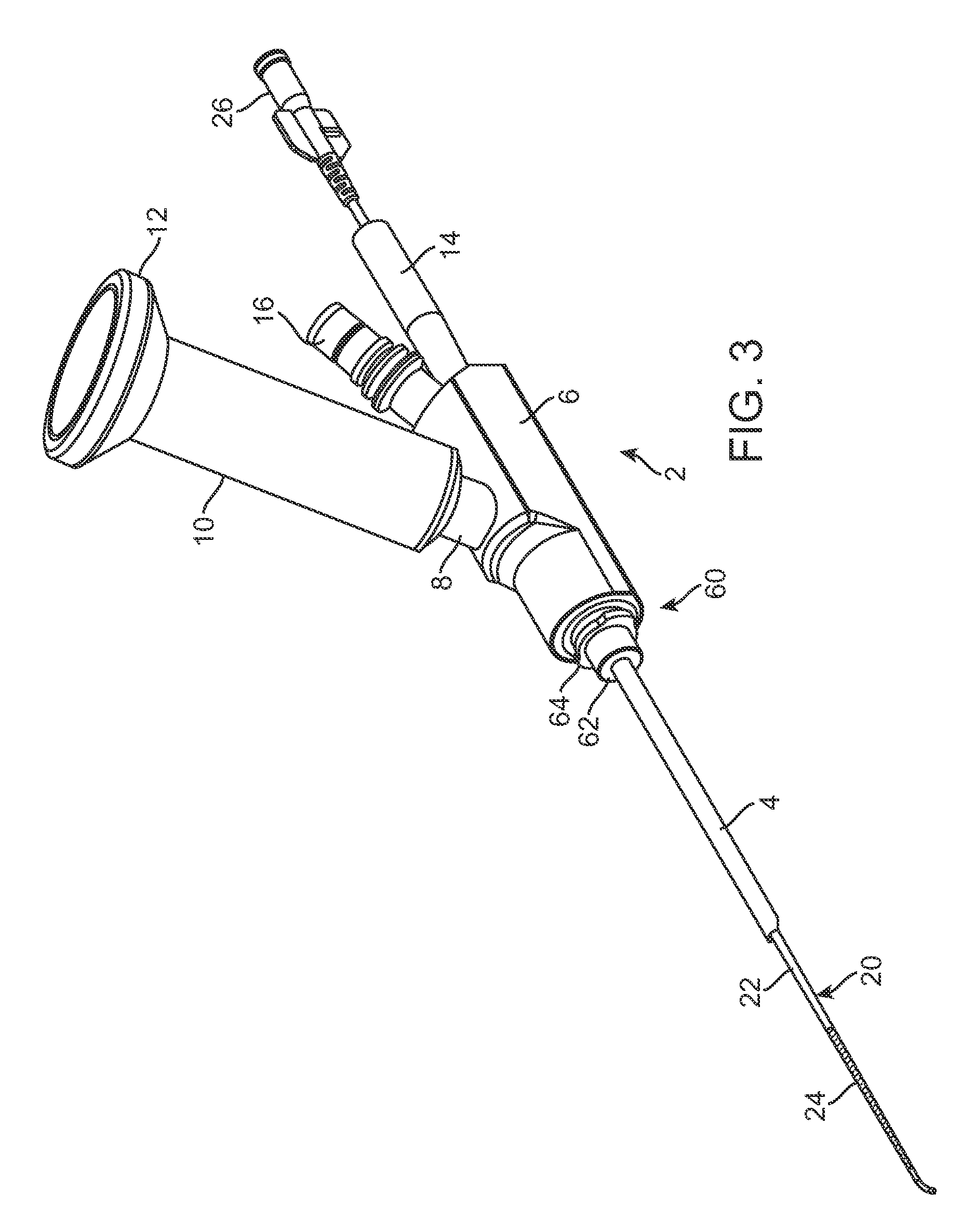
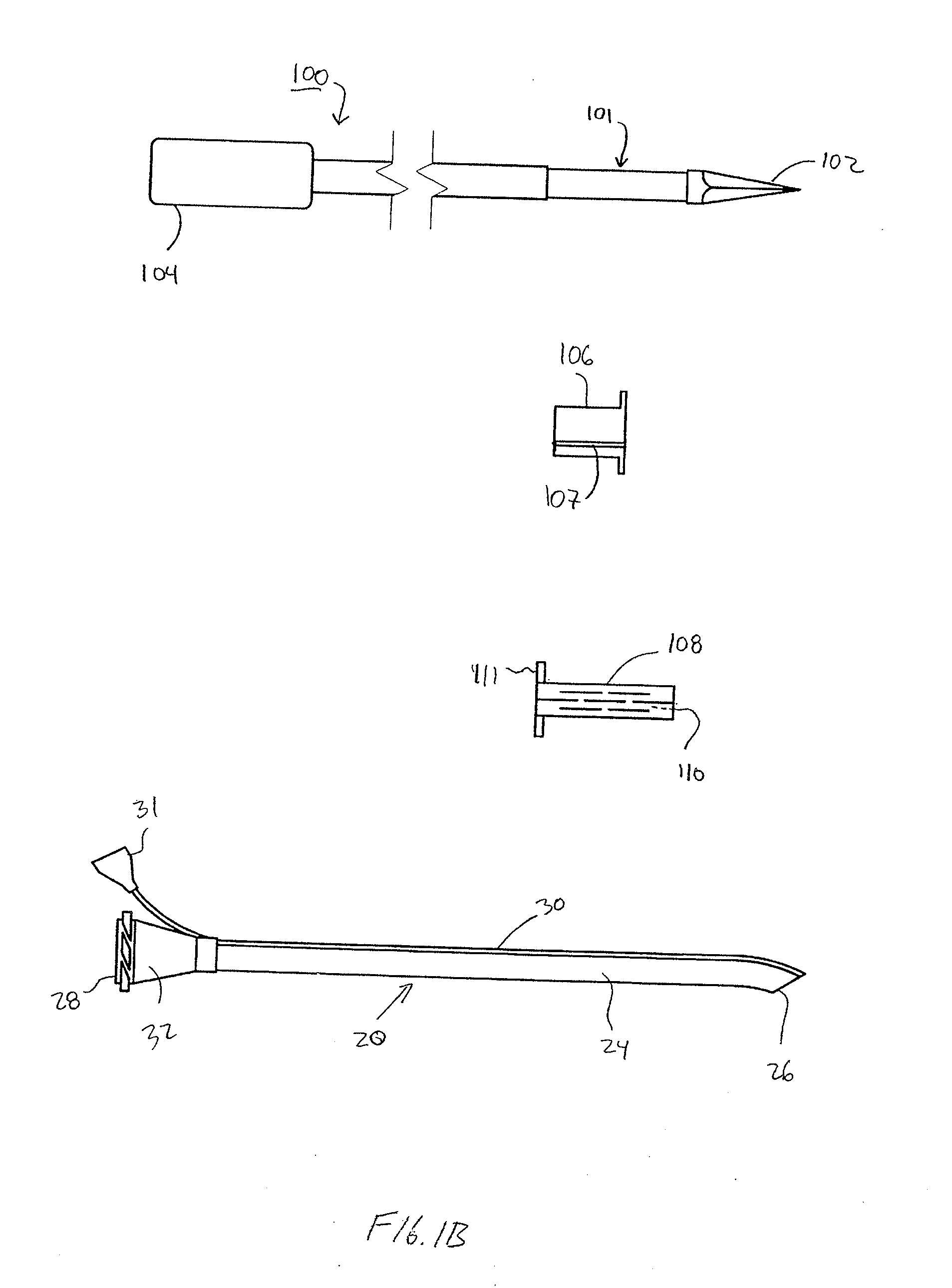
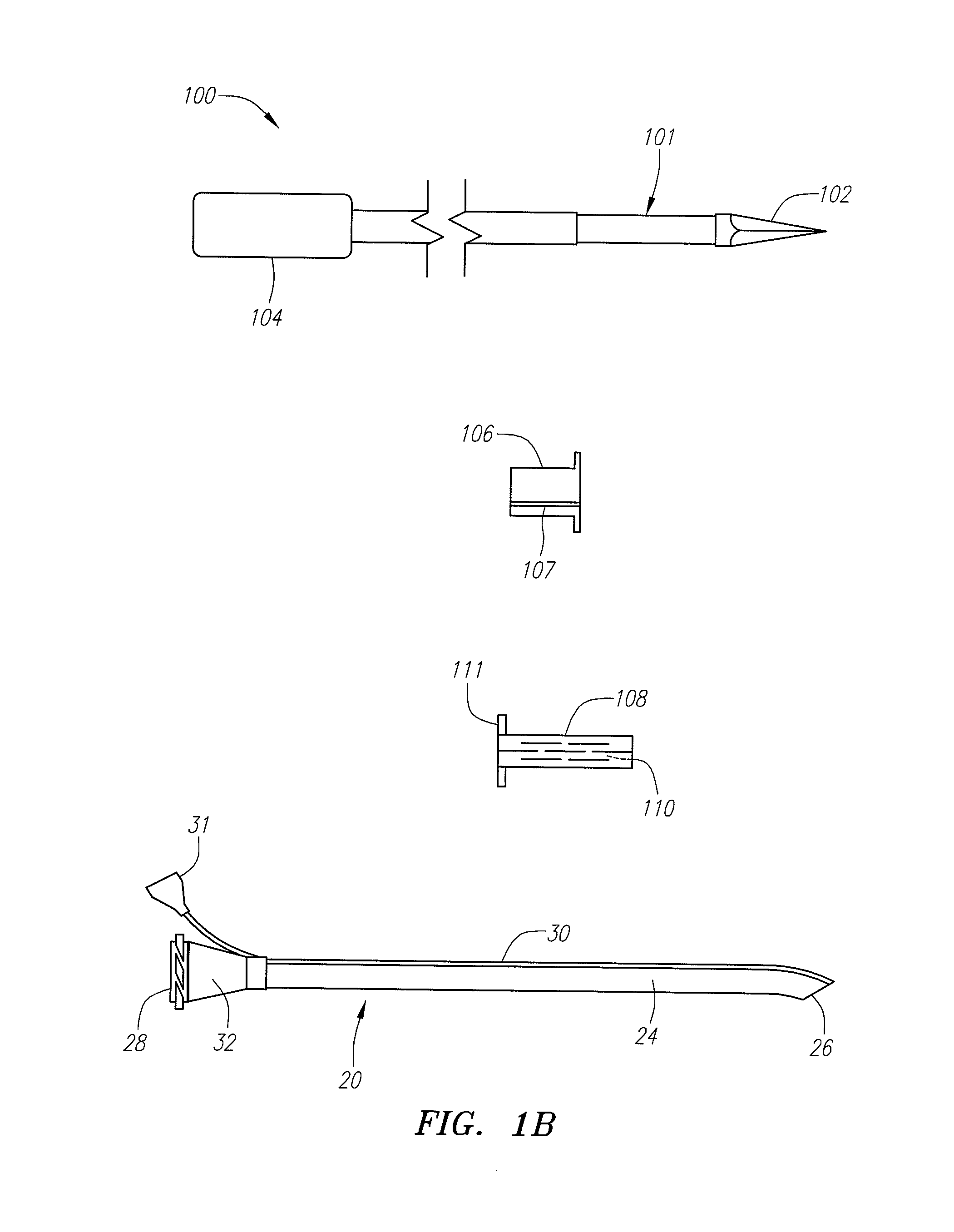
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
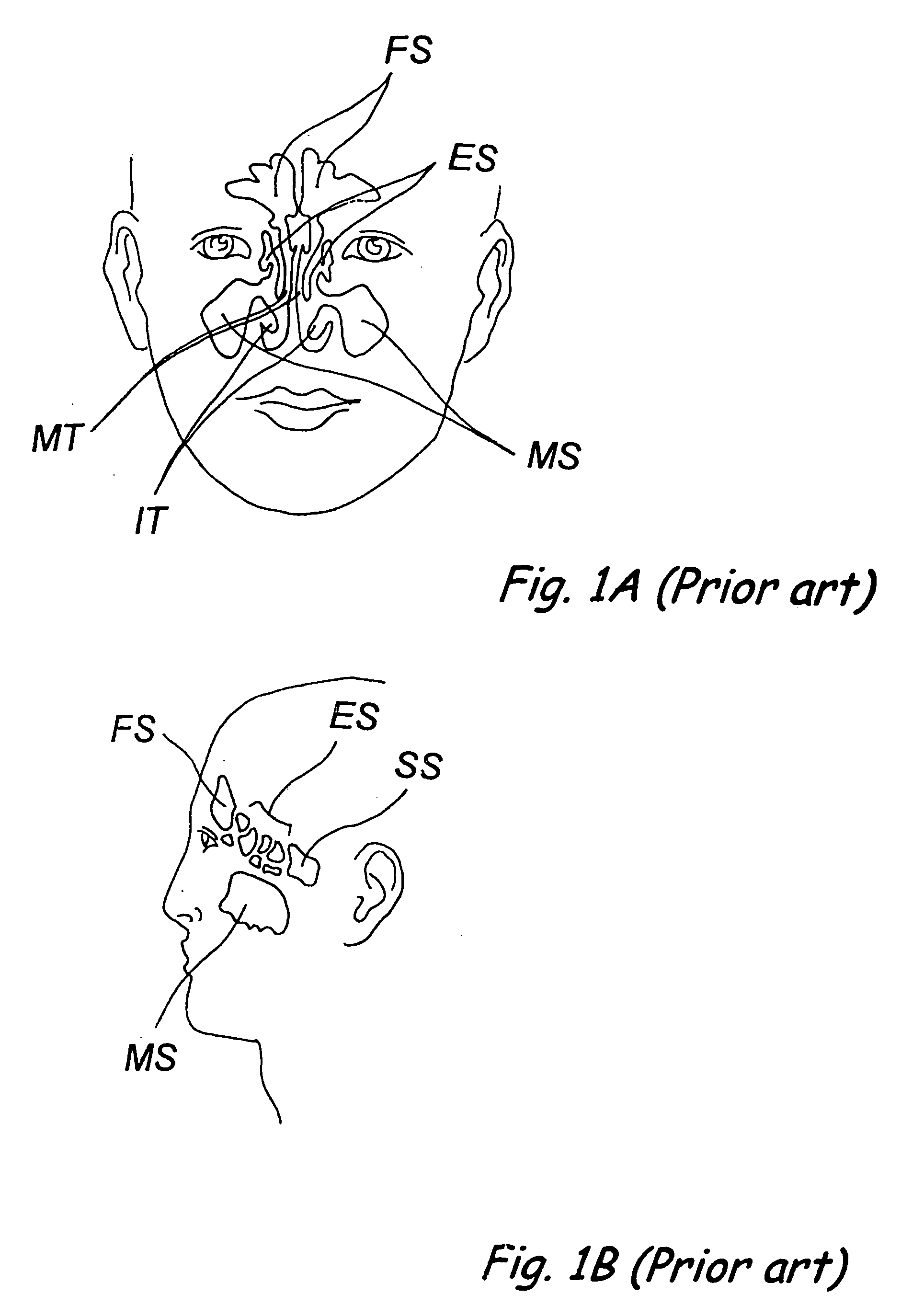
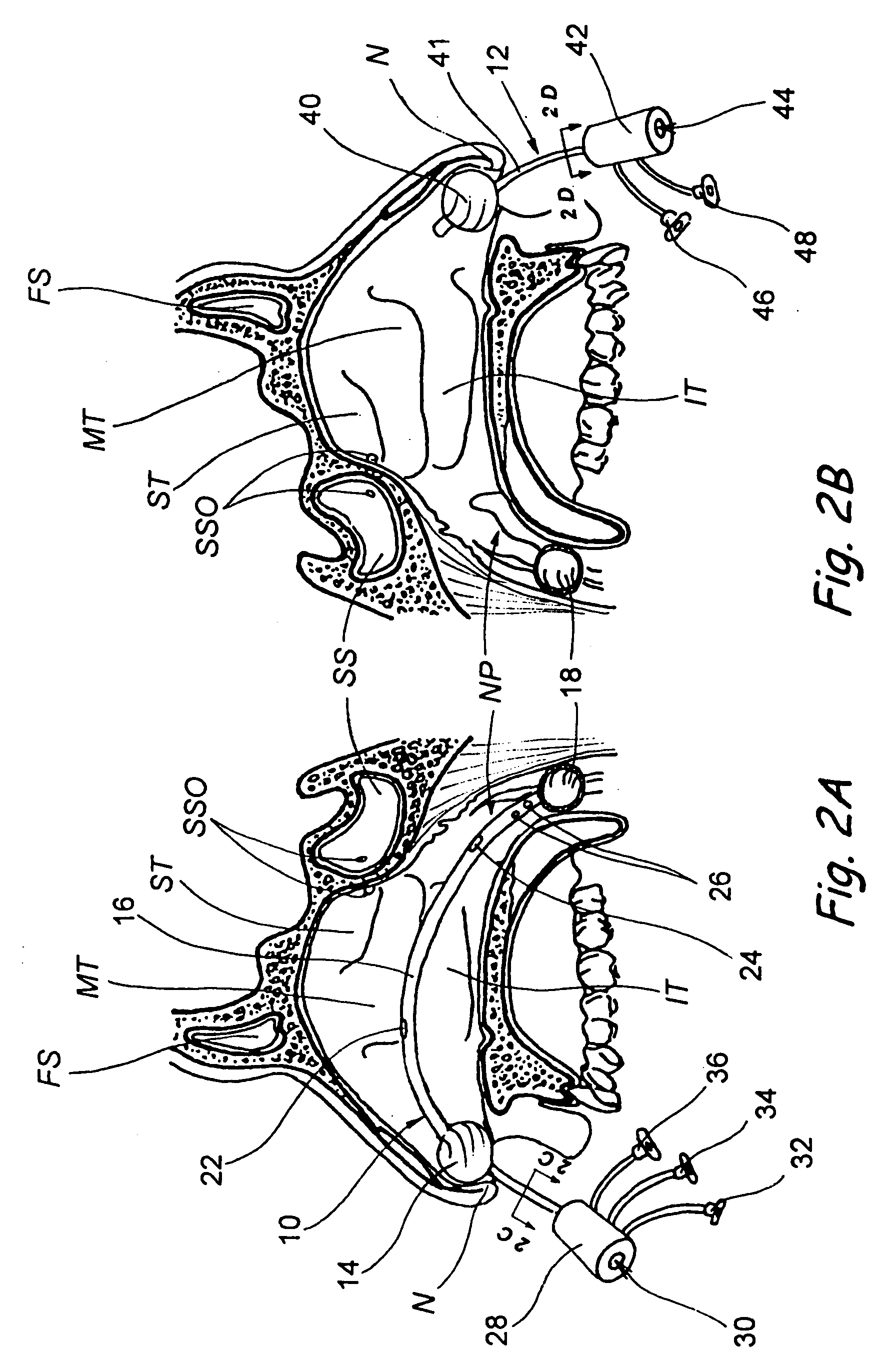
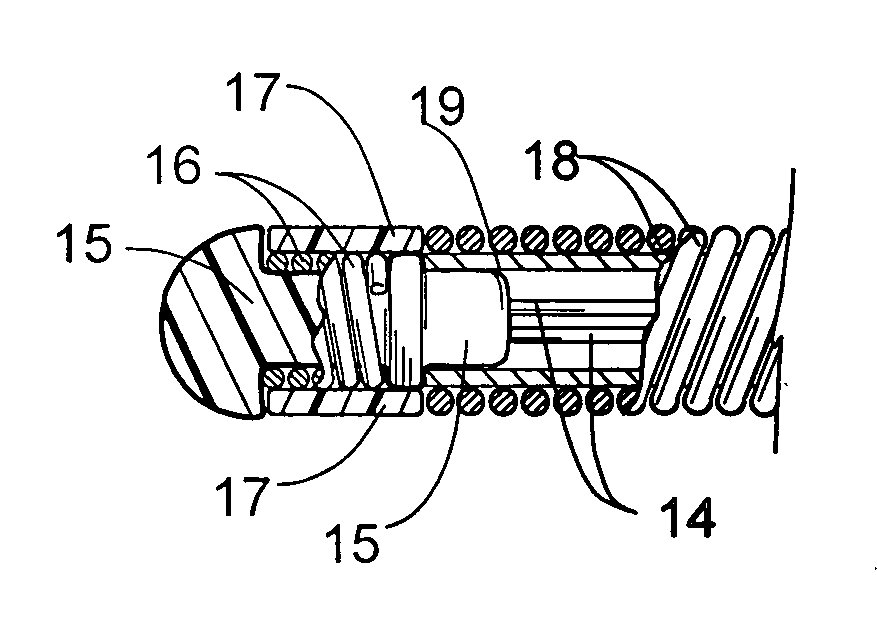
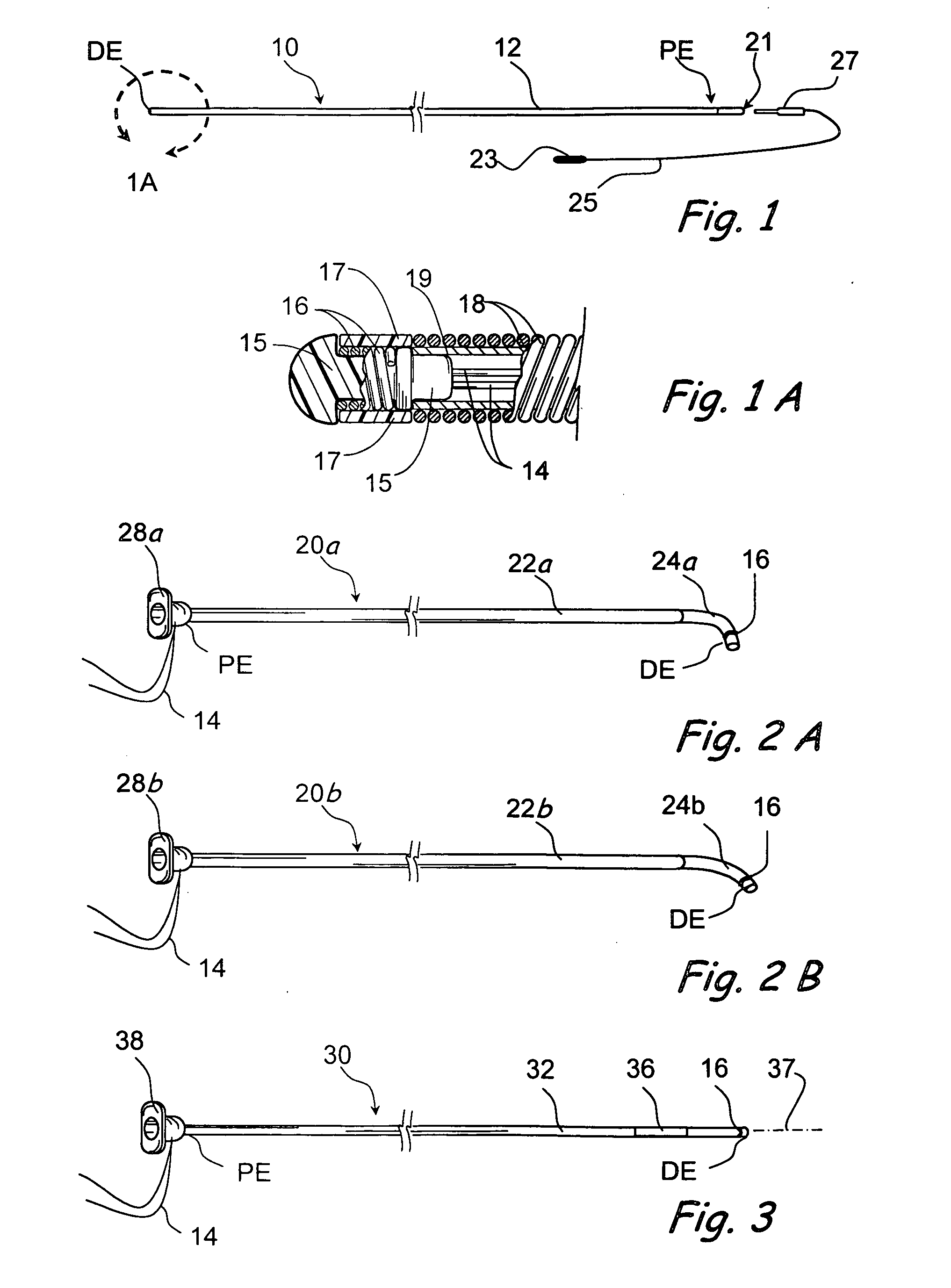
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
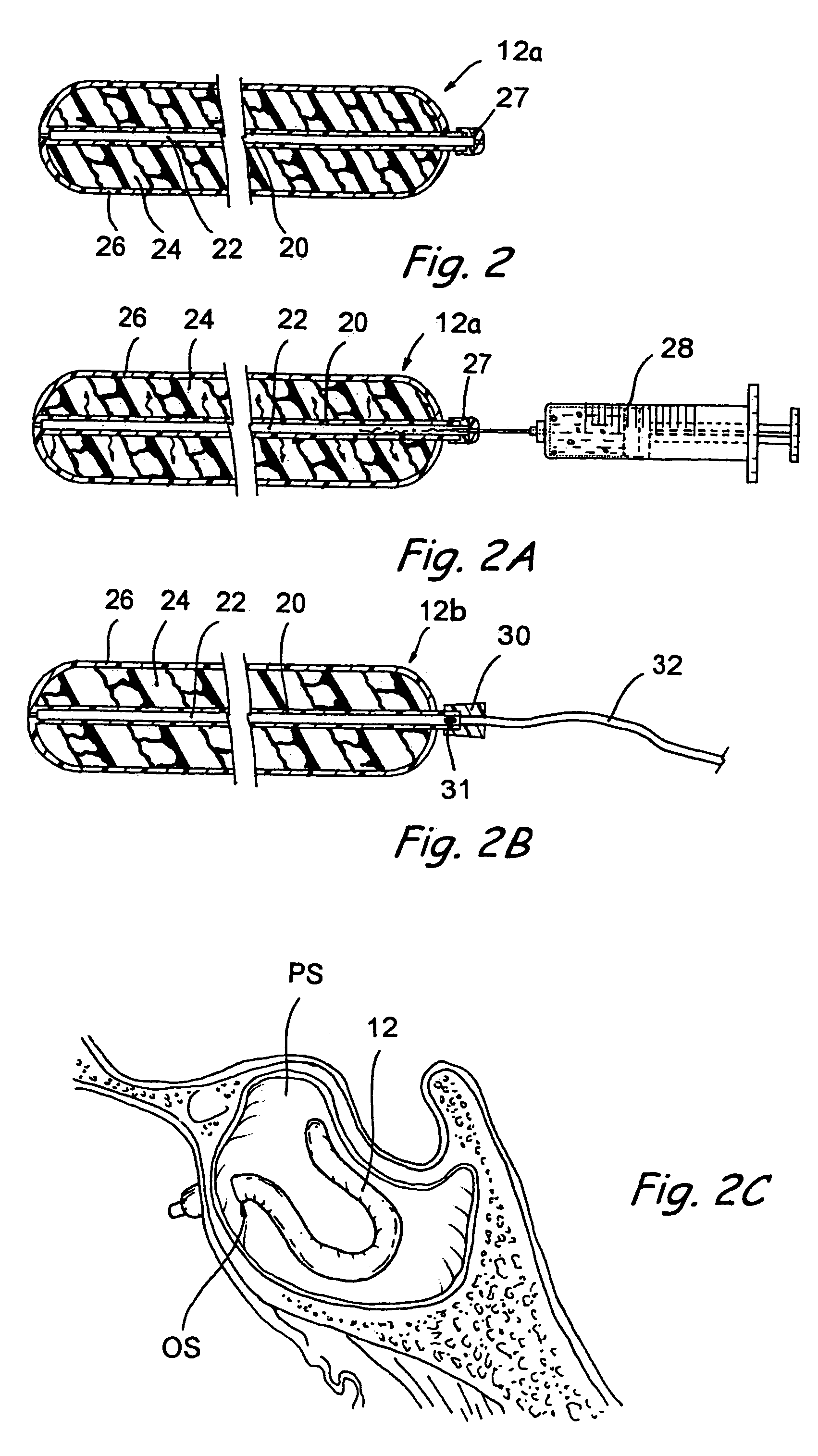
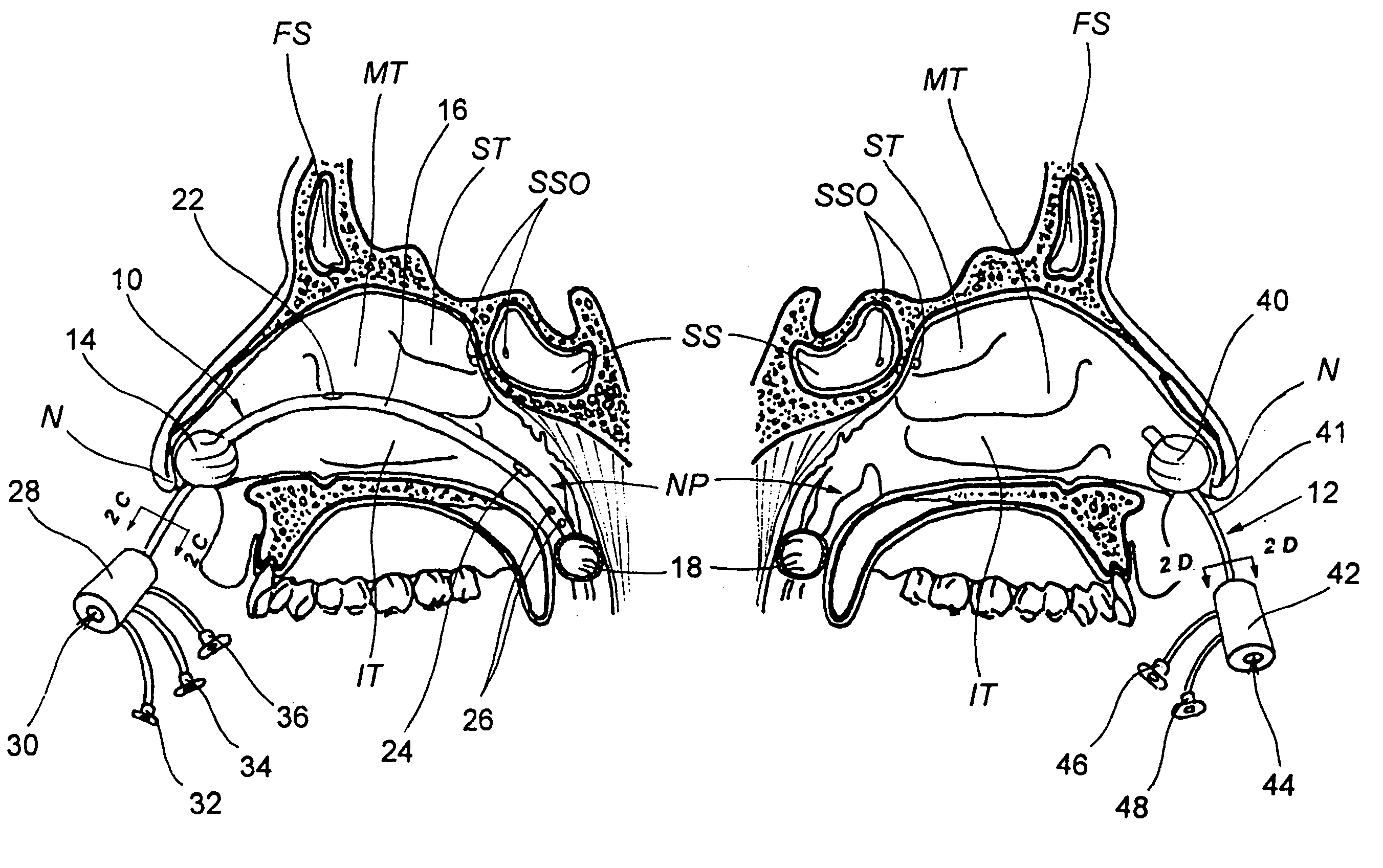
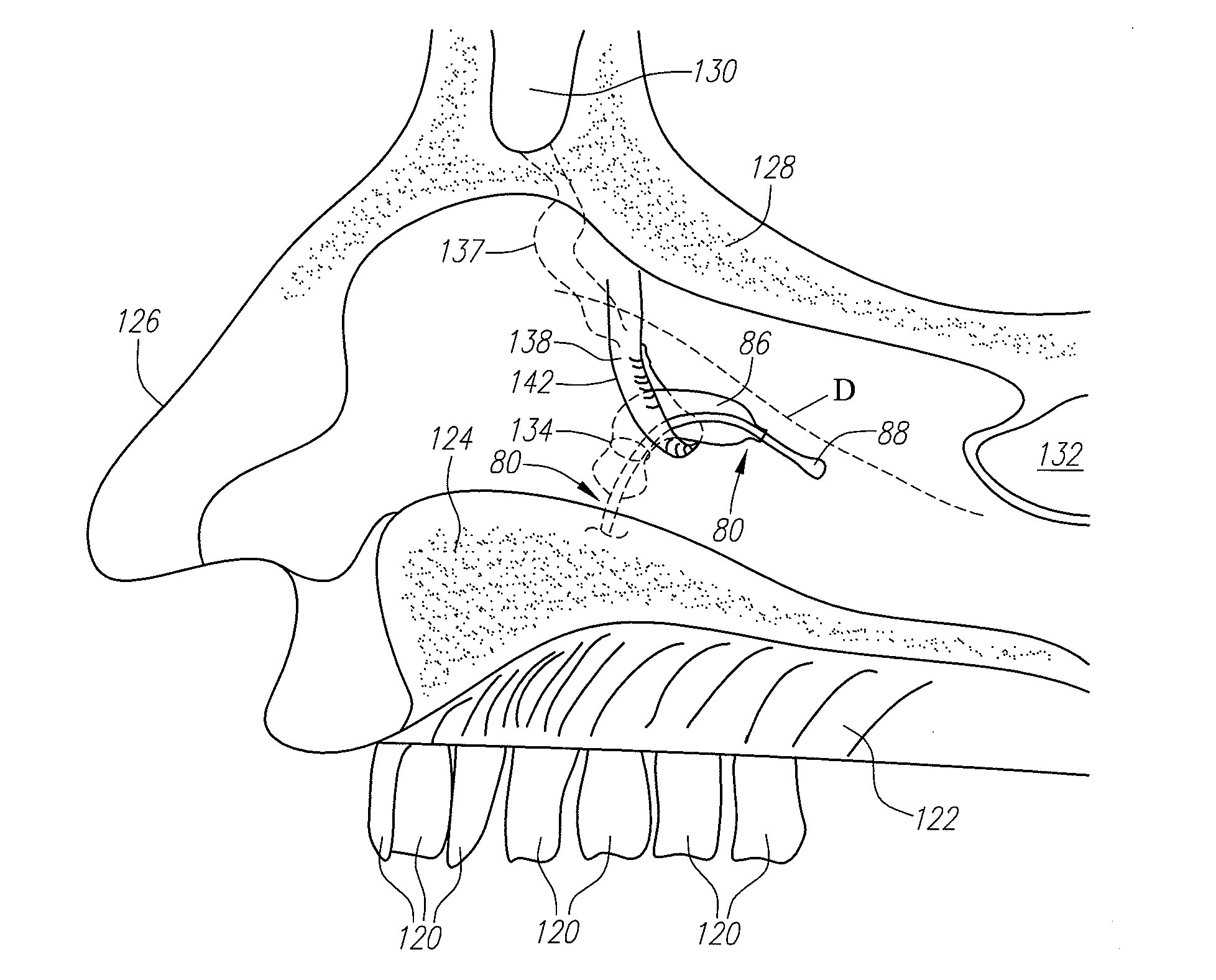
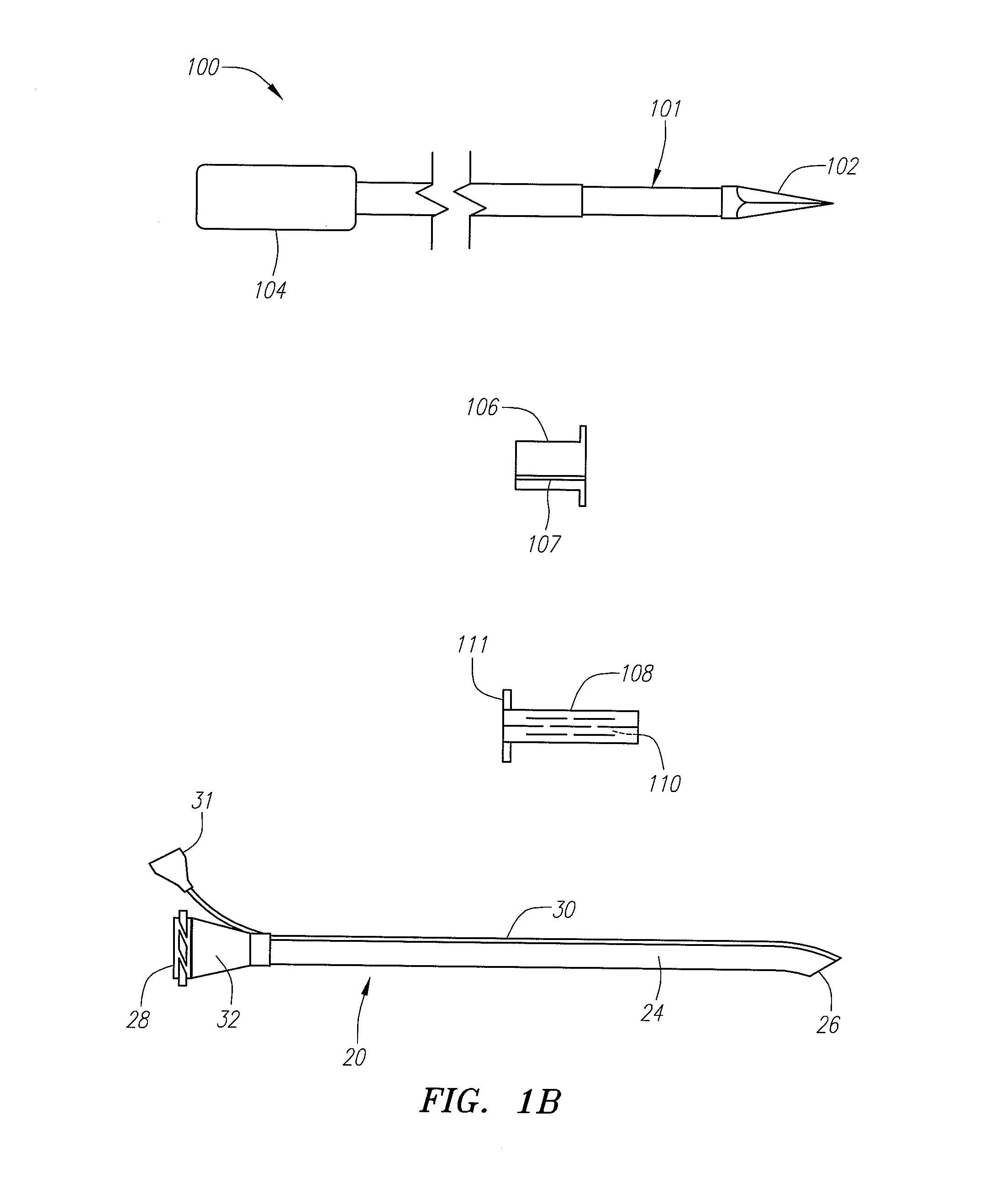
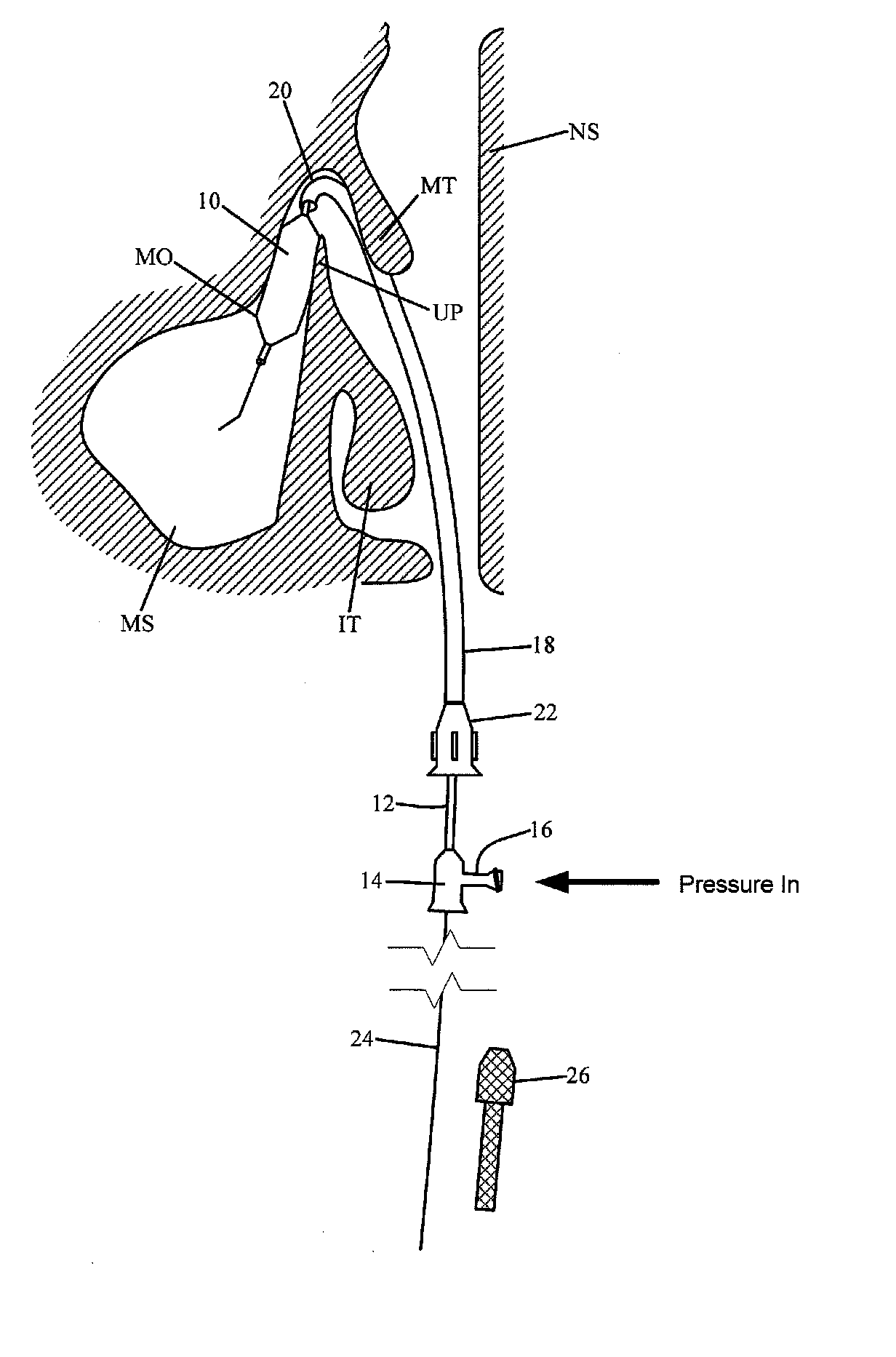
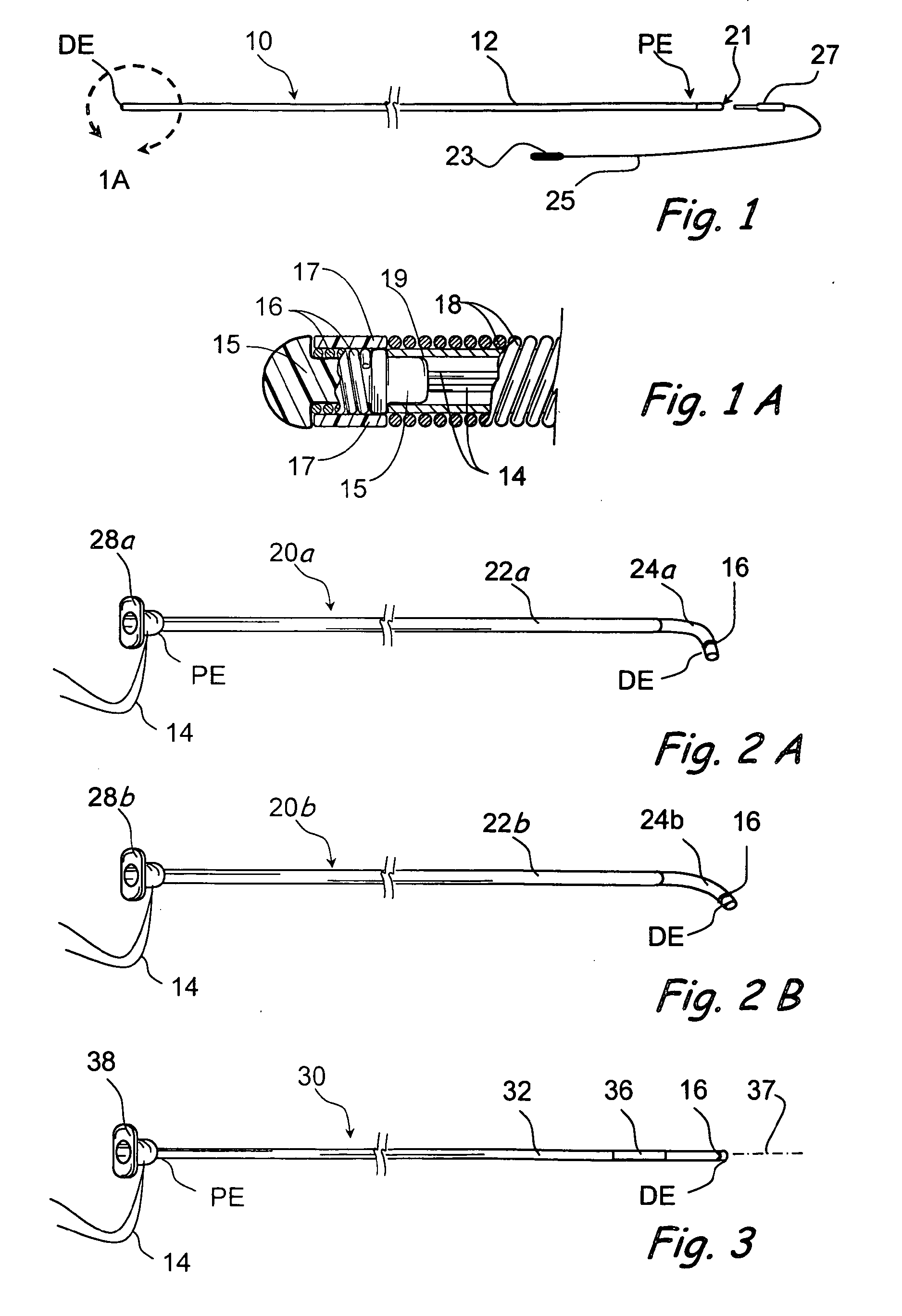
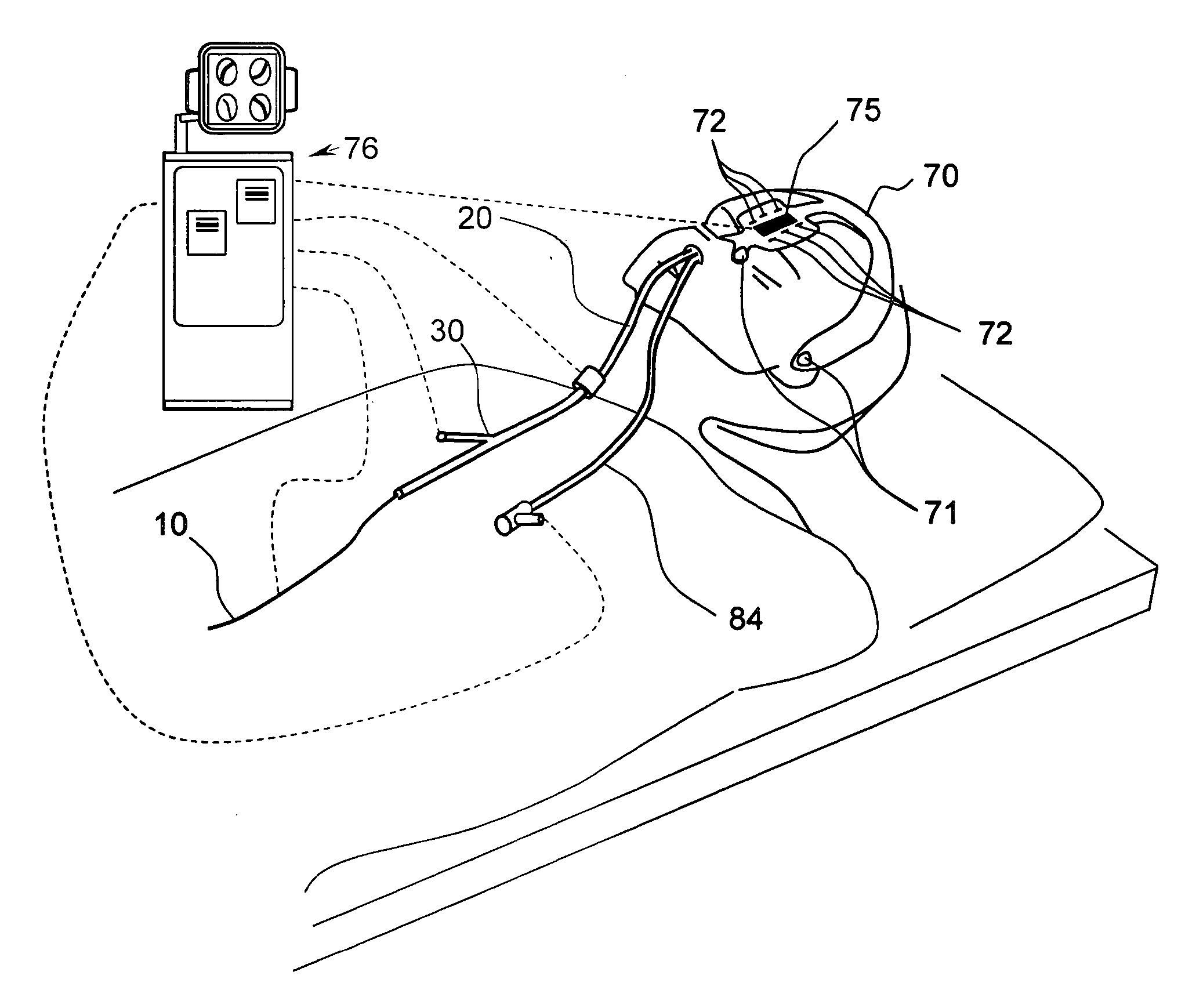
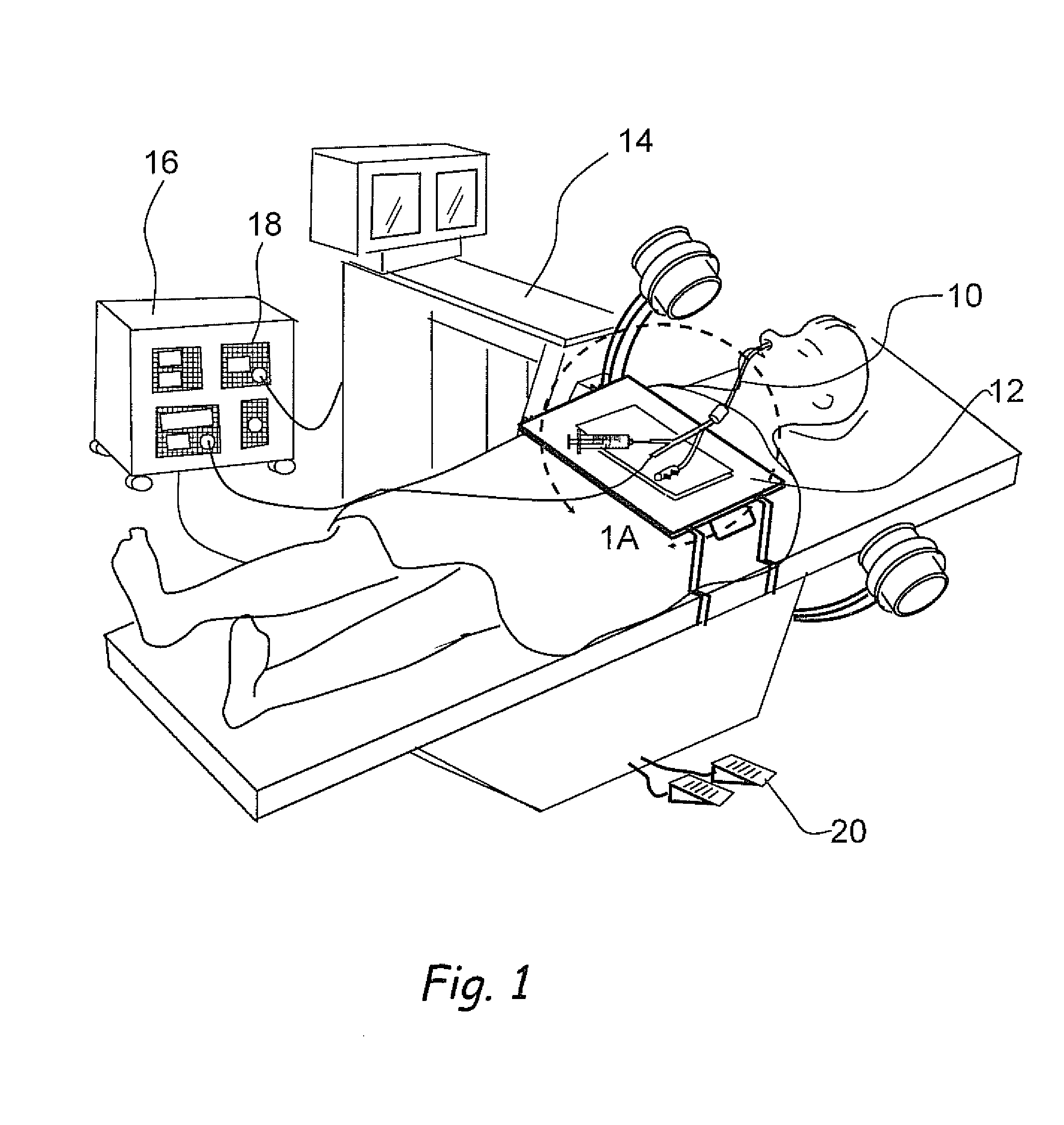
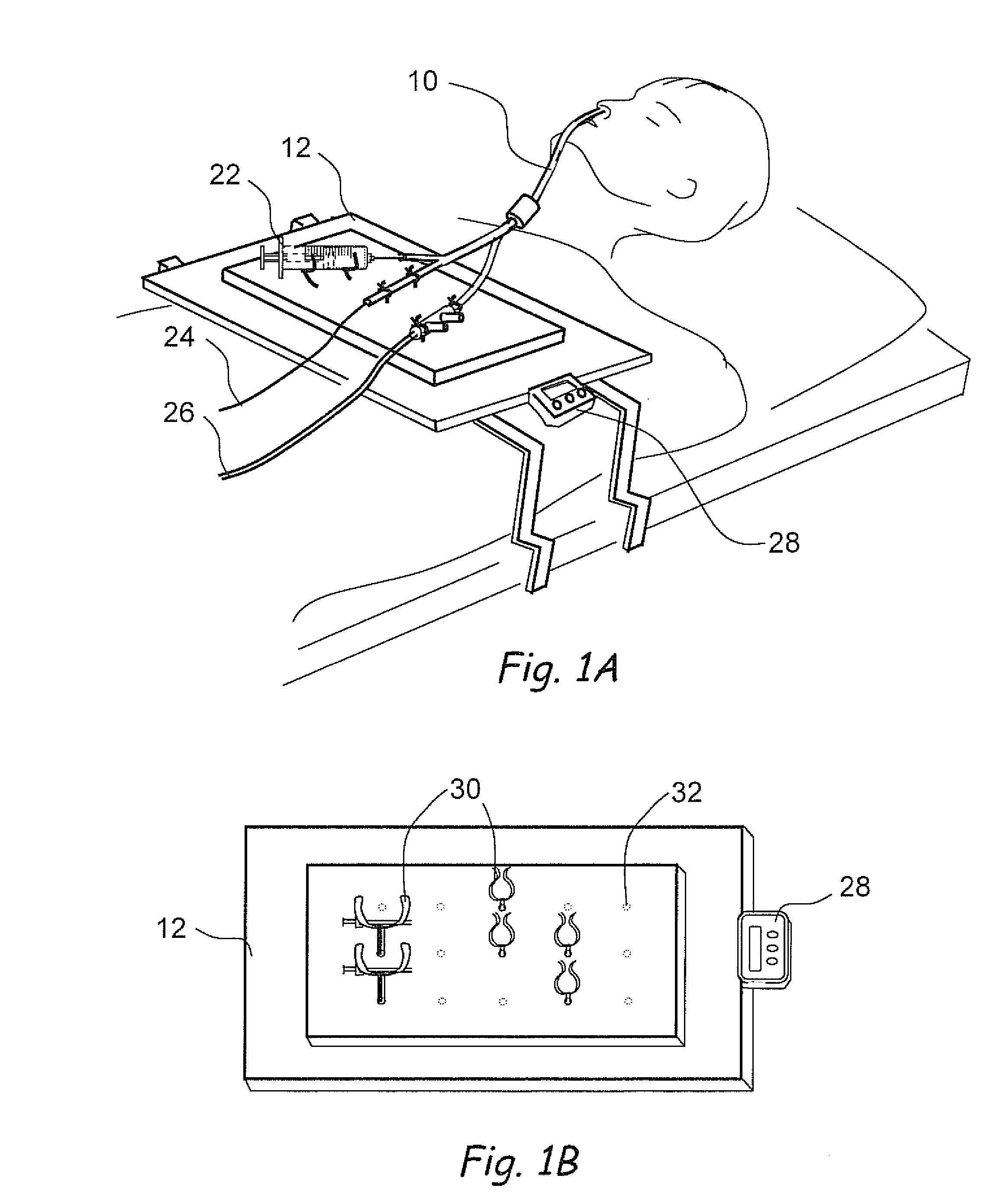
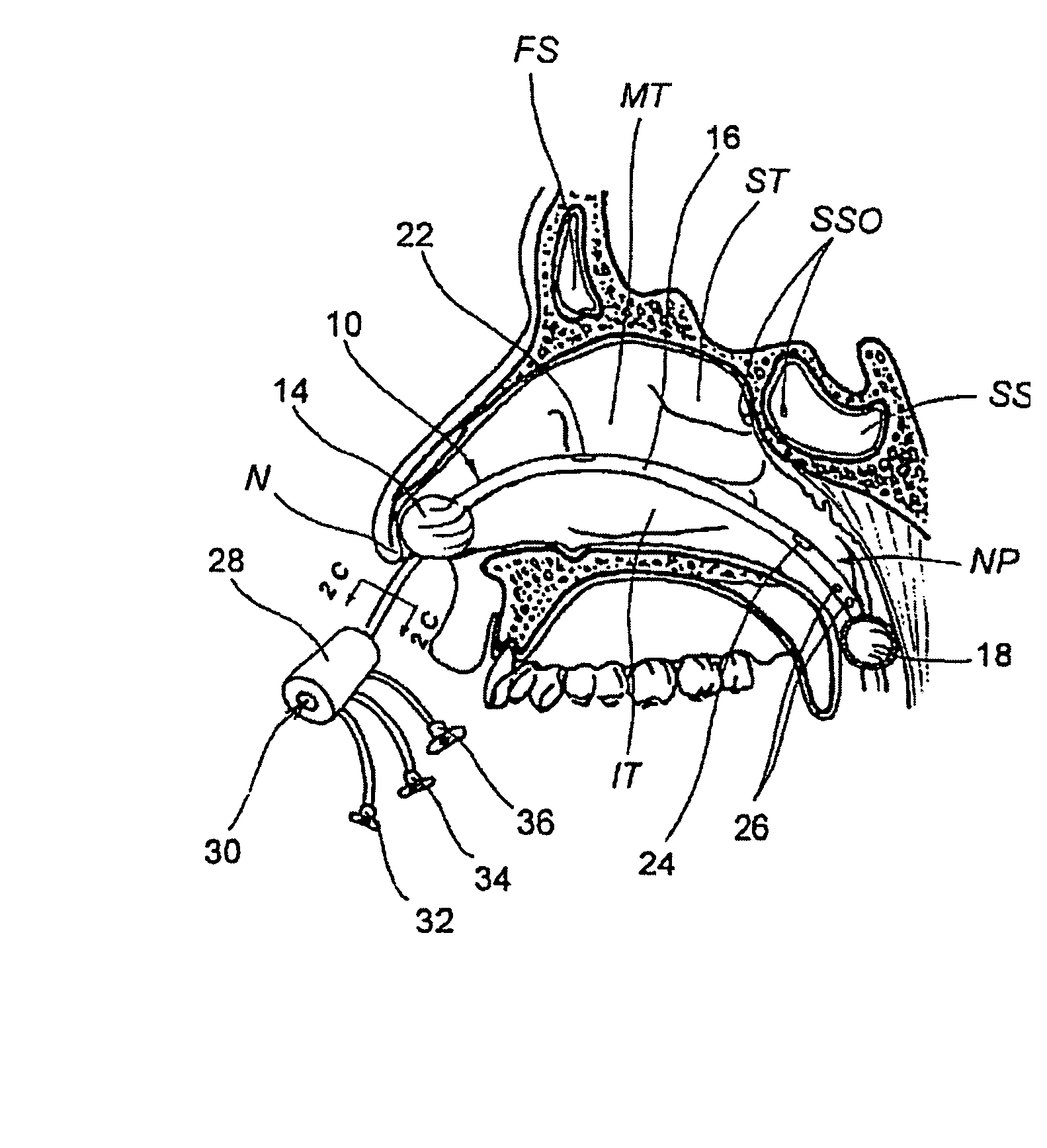
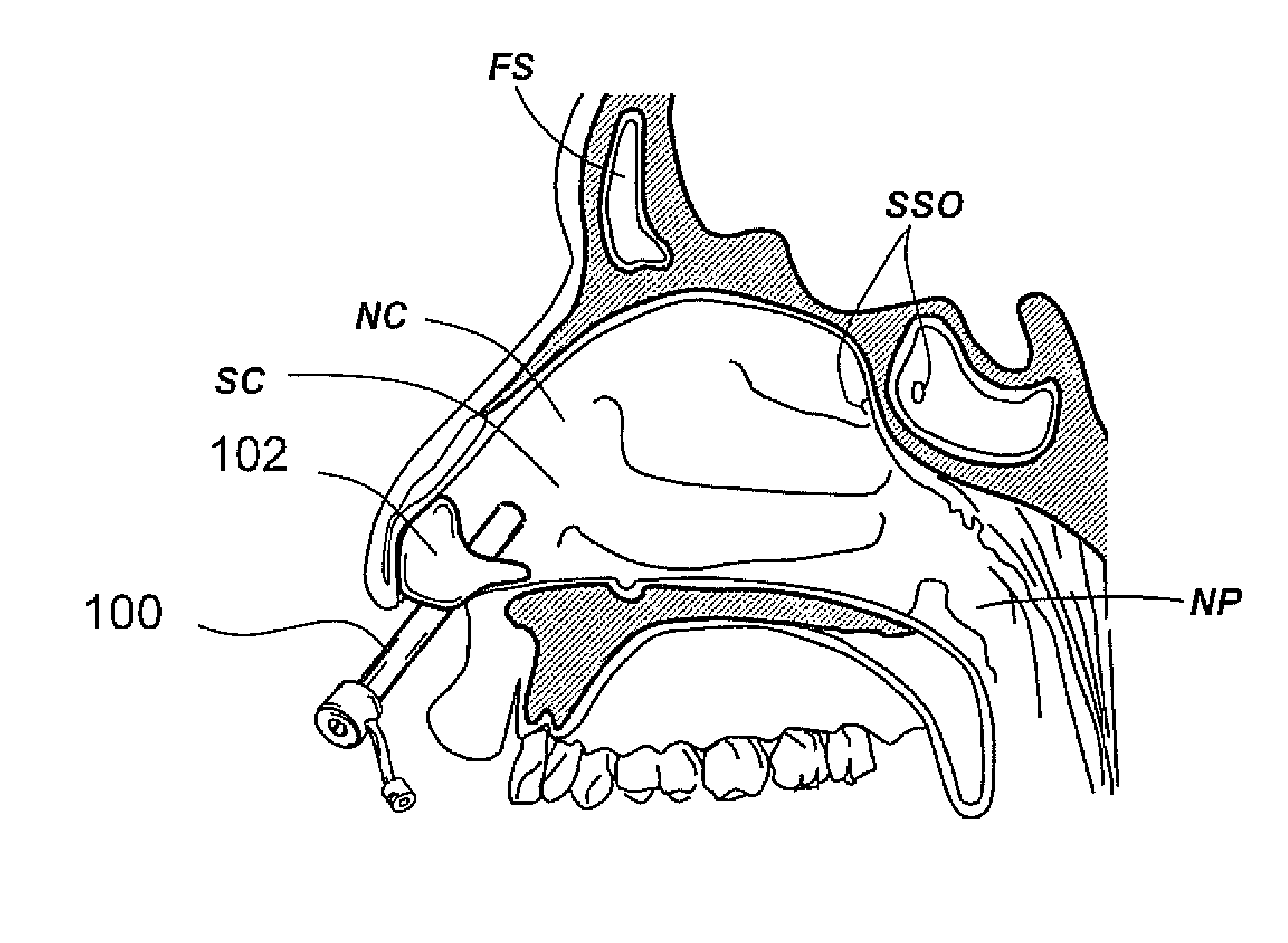
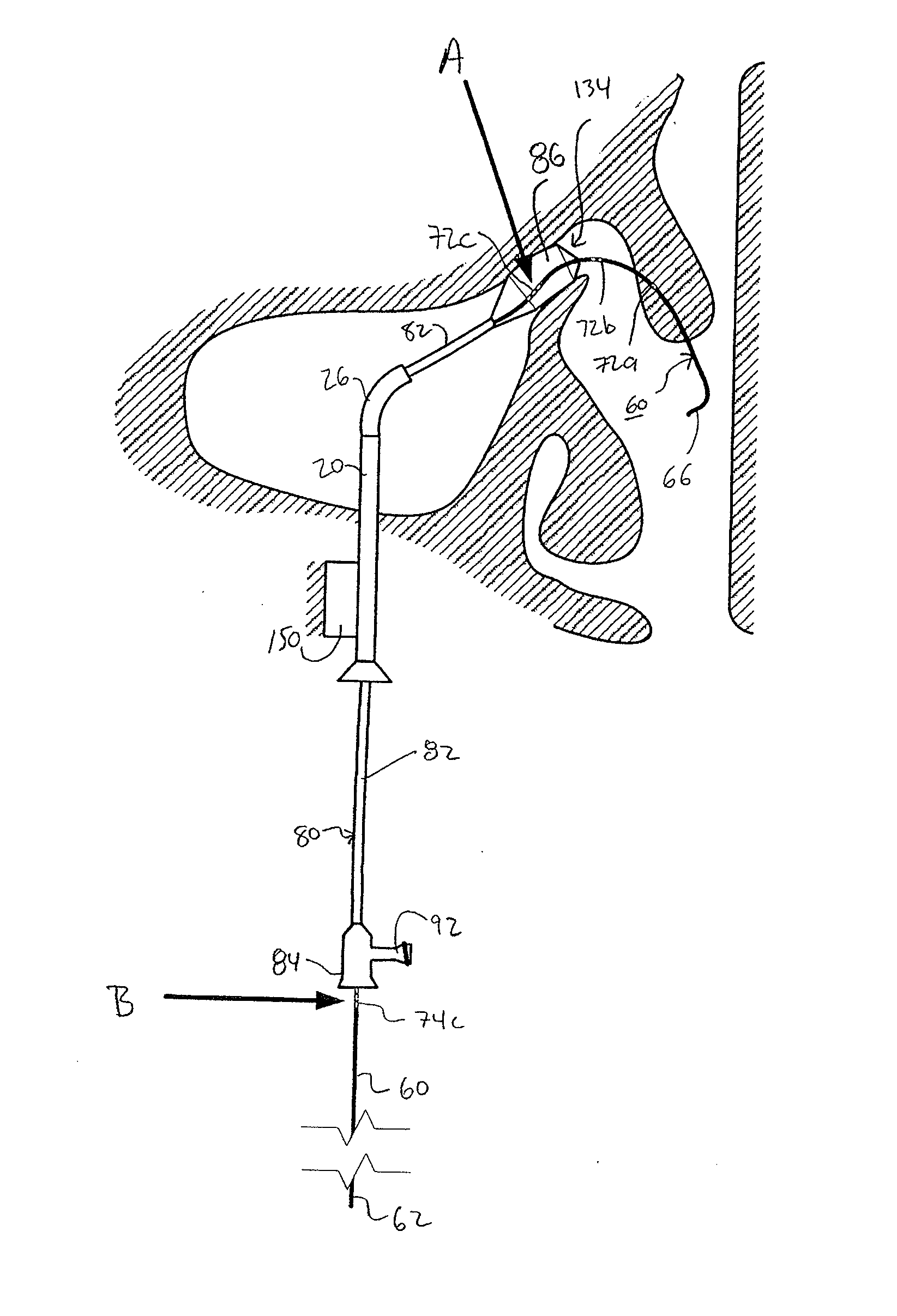
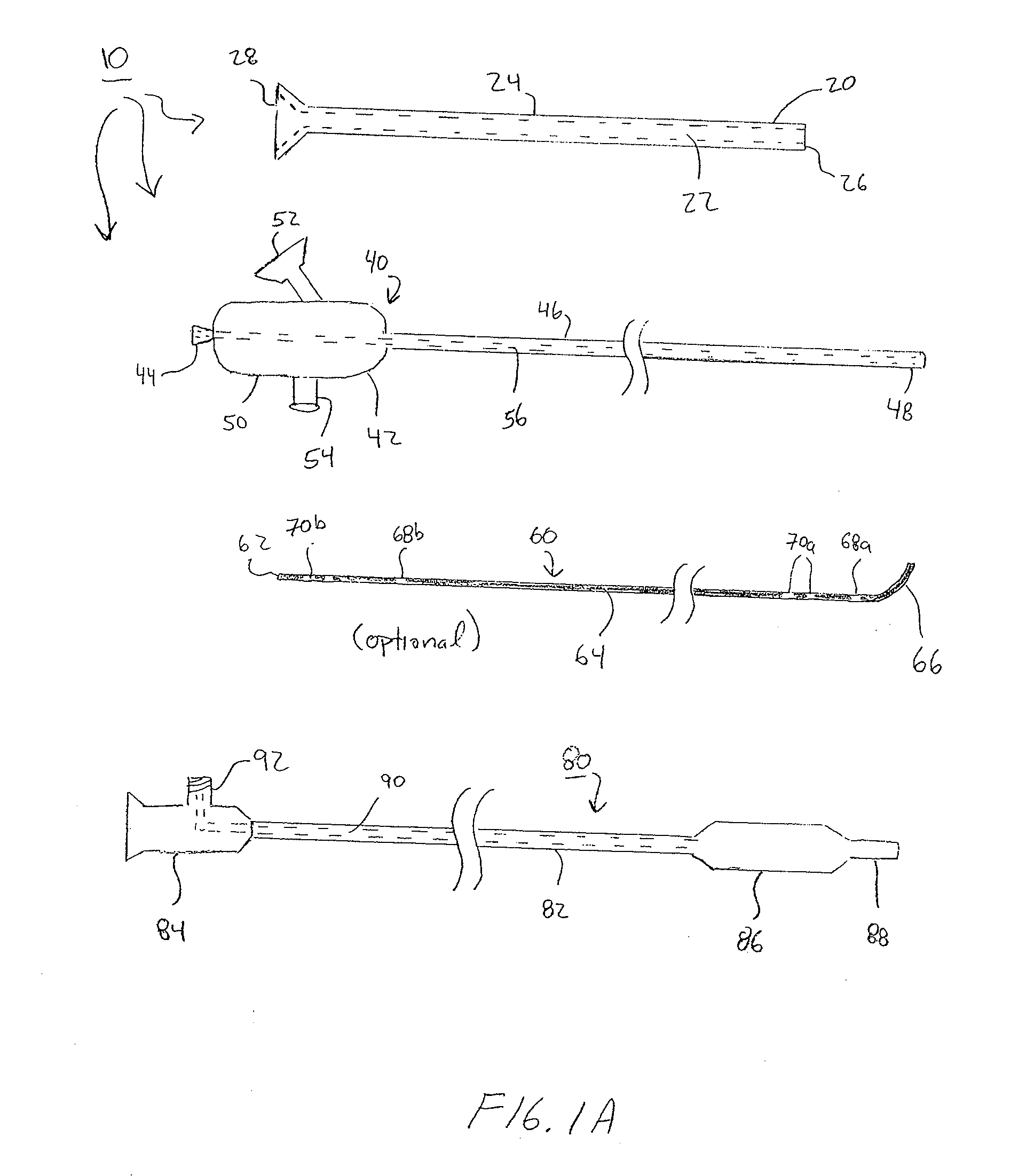
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
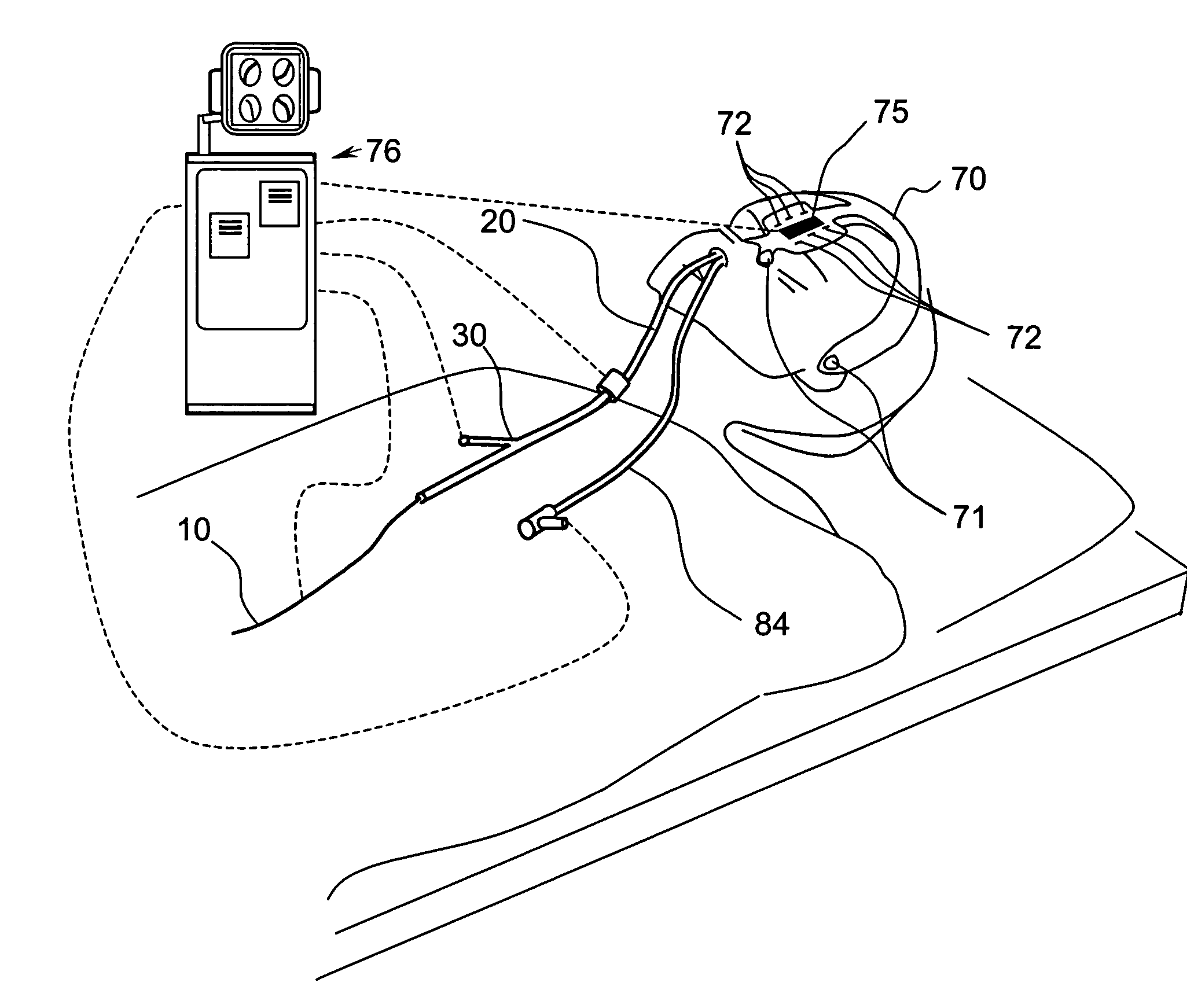
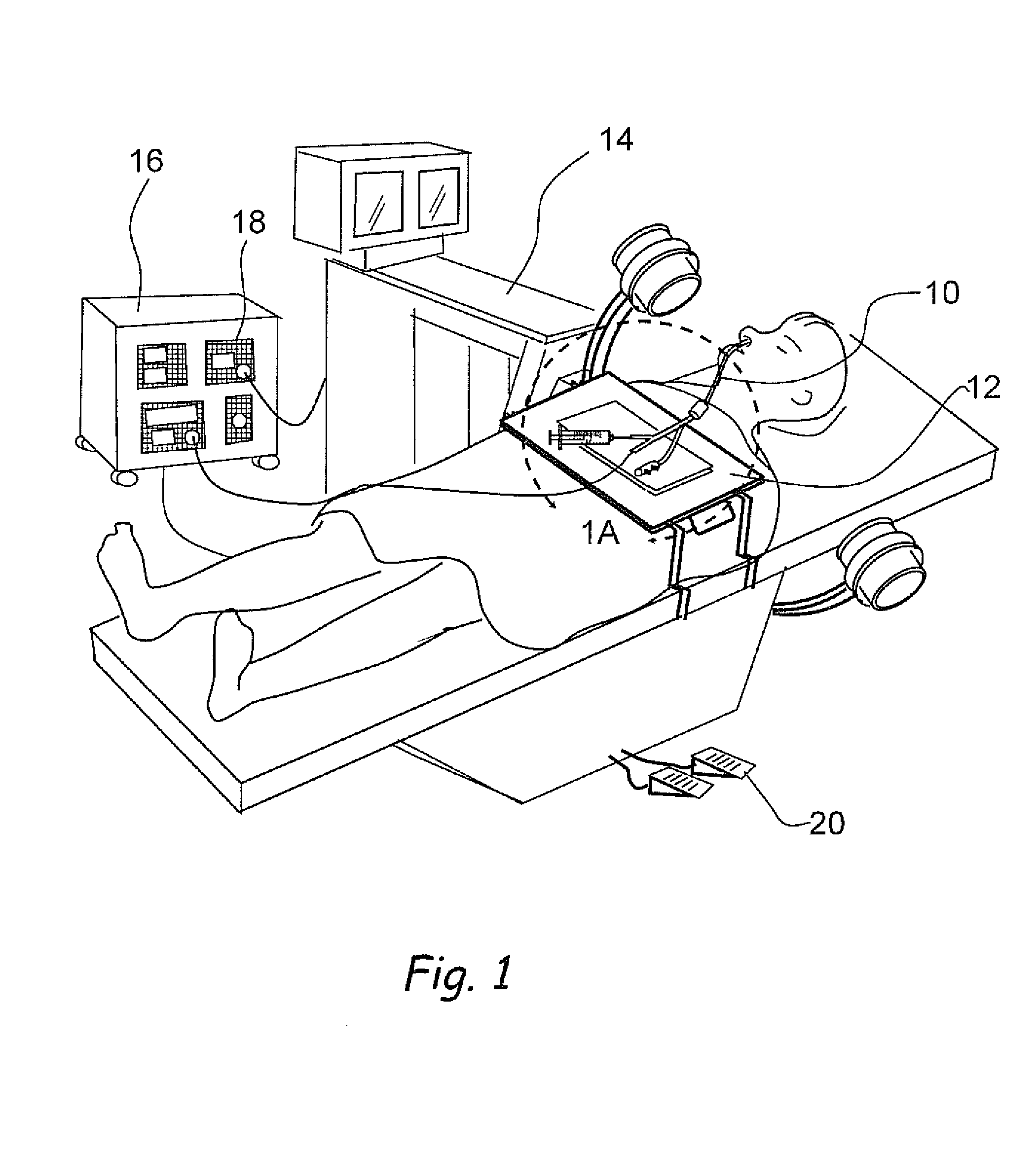
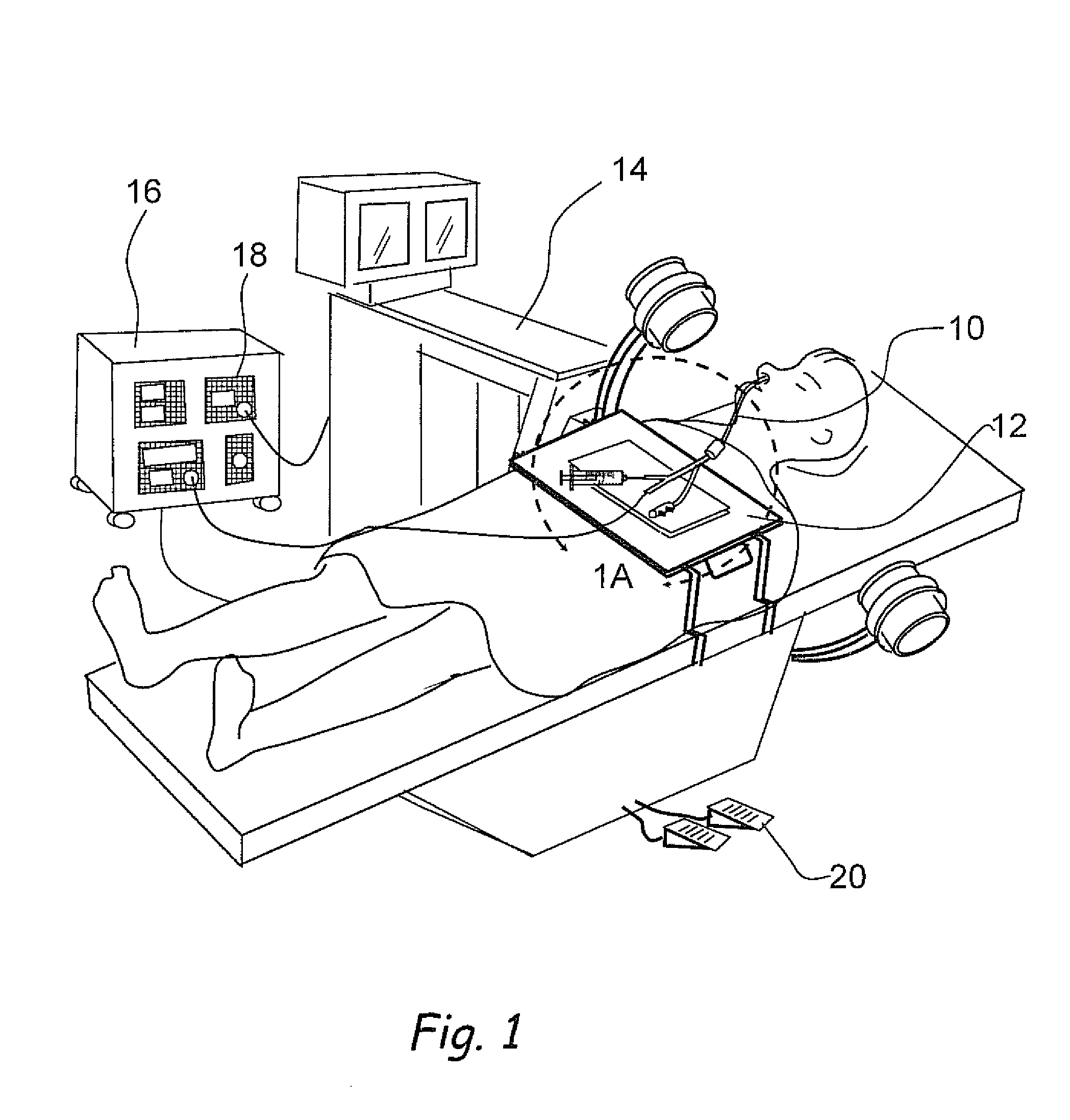
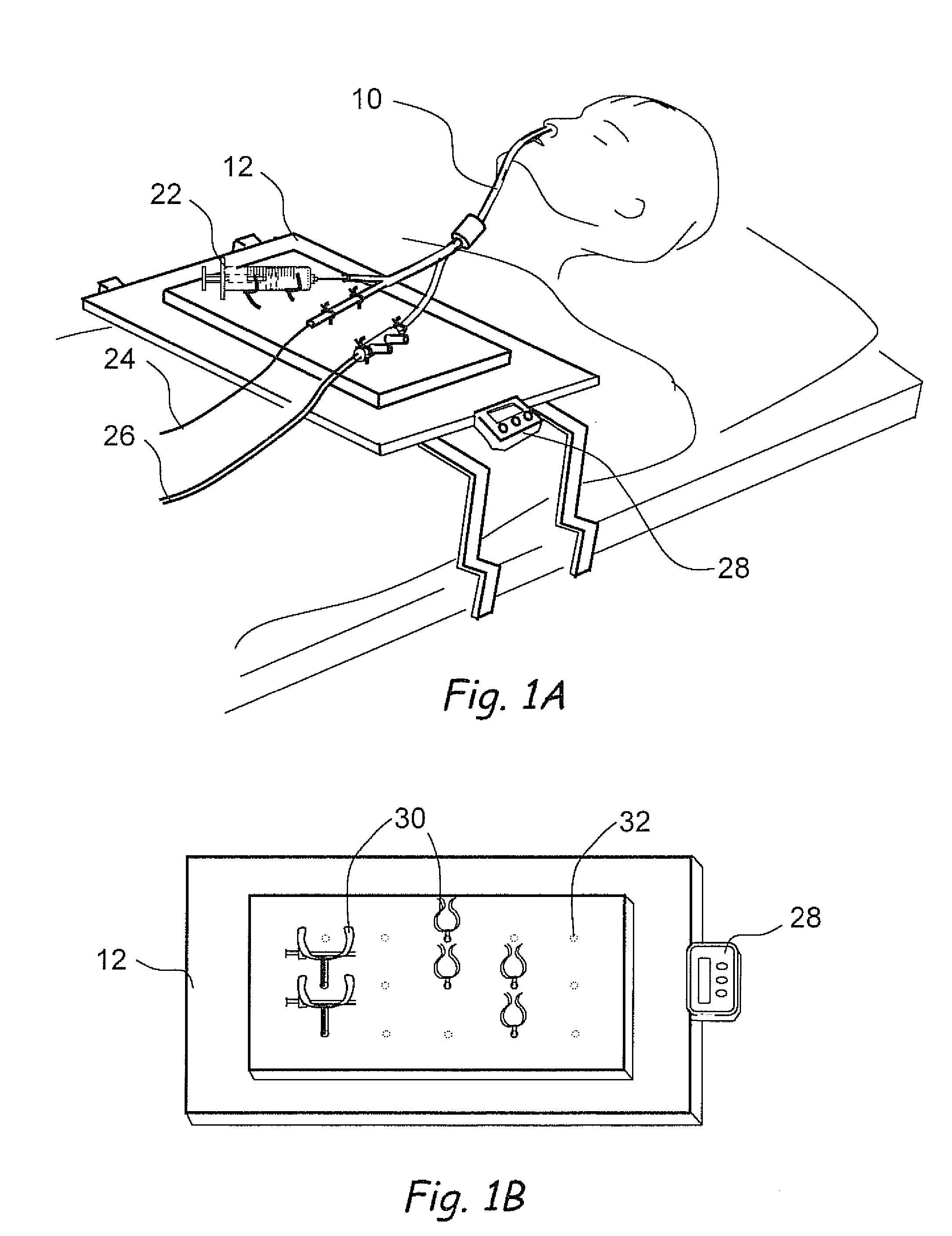
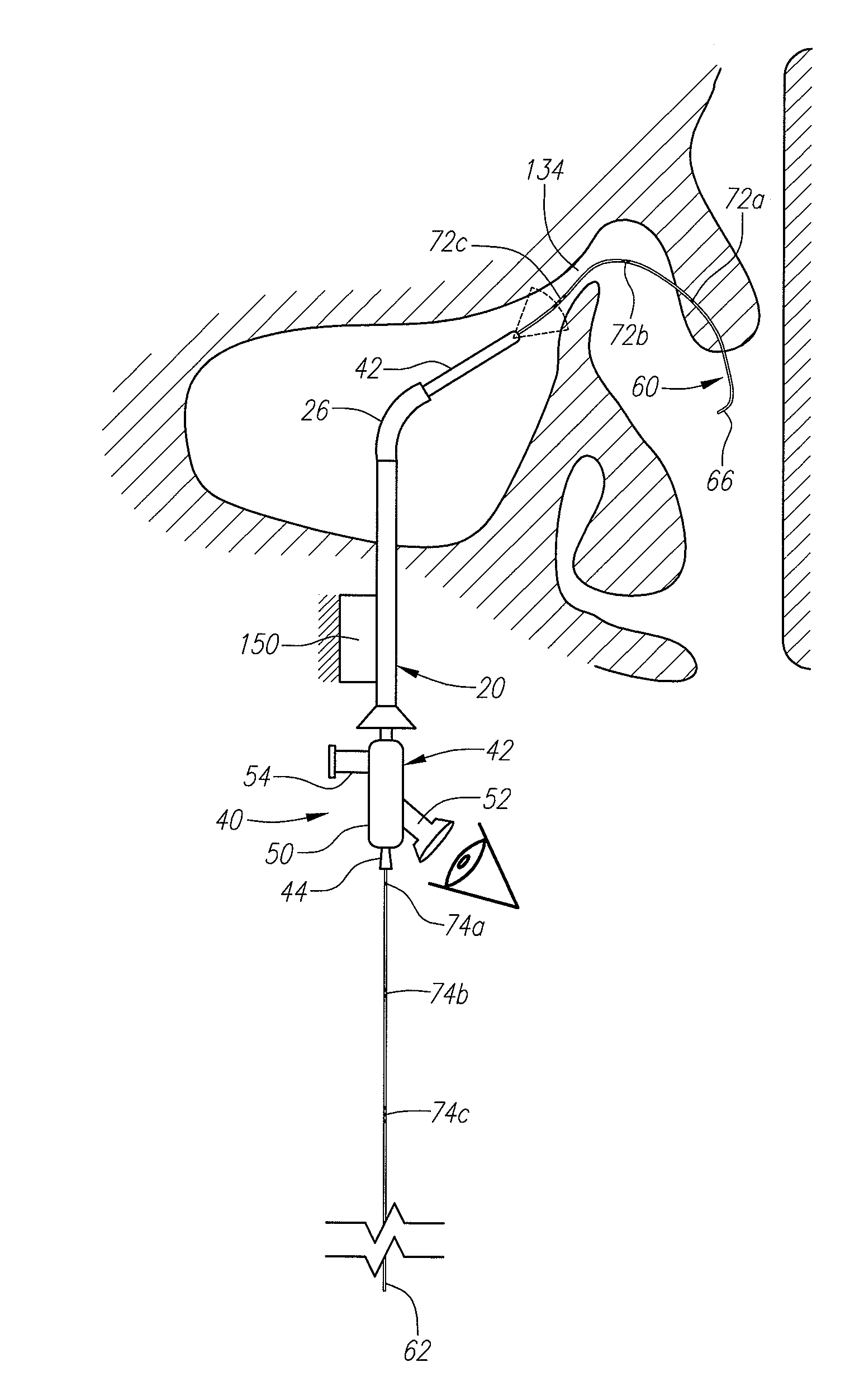
Systems and methods for performing image guided procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
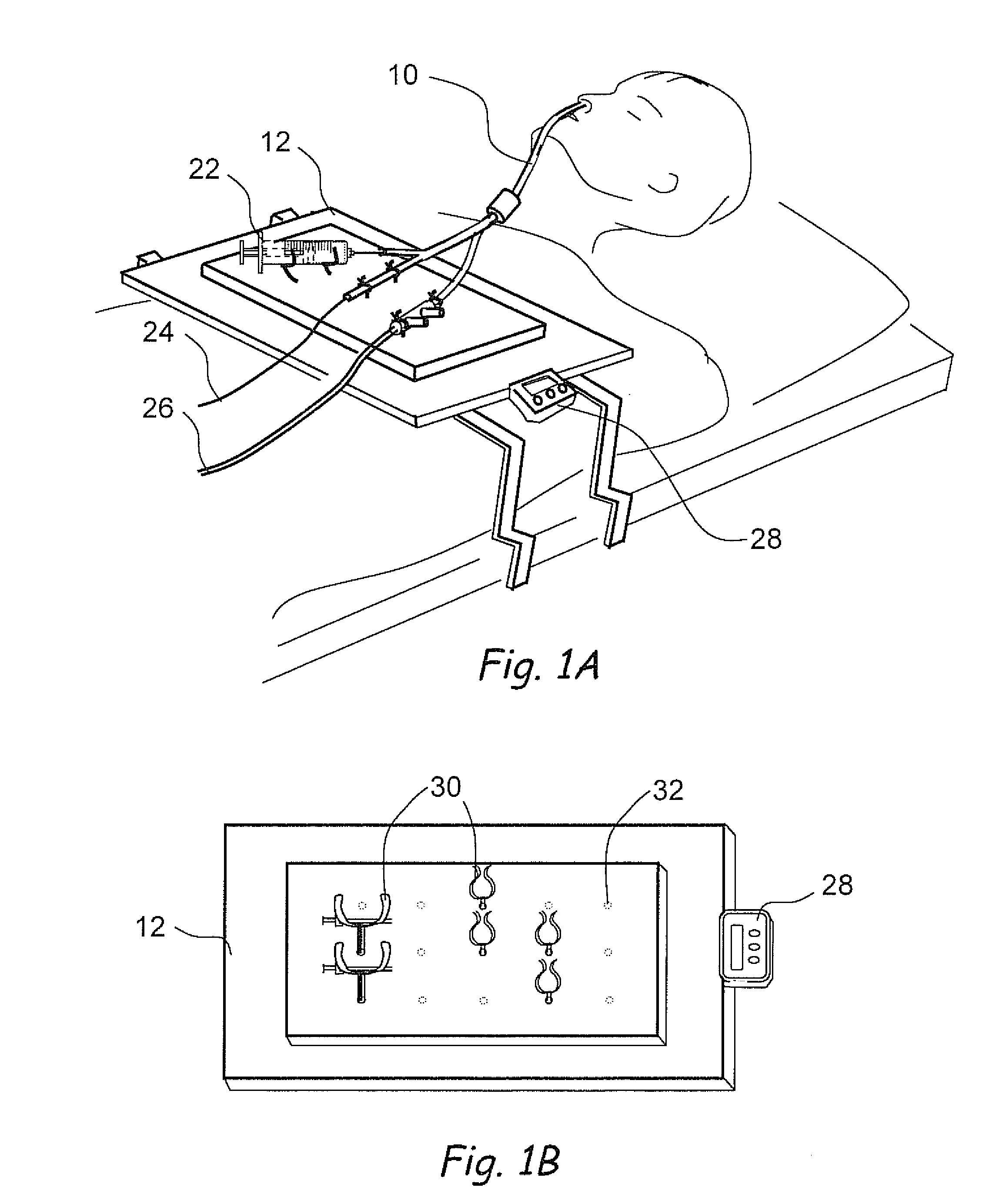
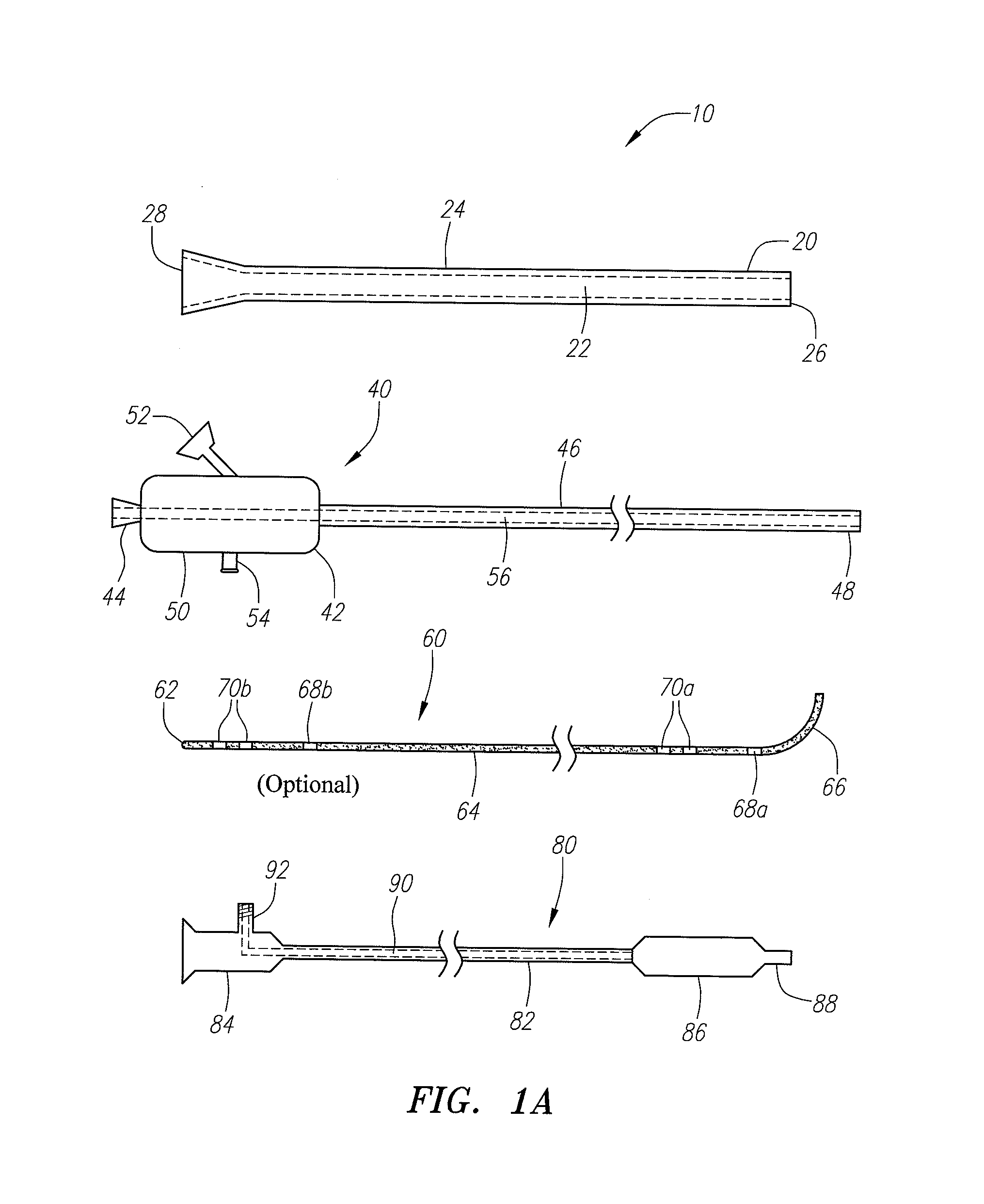
Devices, systems and methods for treating disorders of the ear, nose and throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
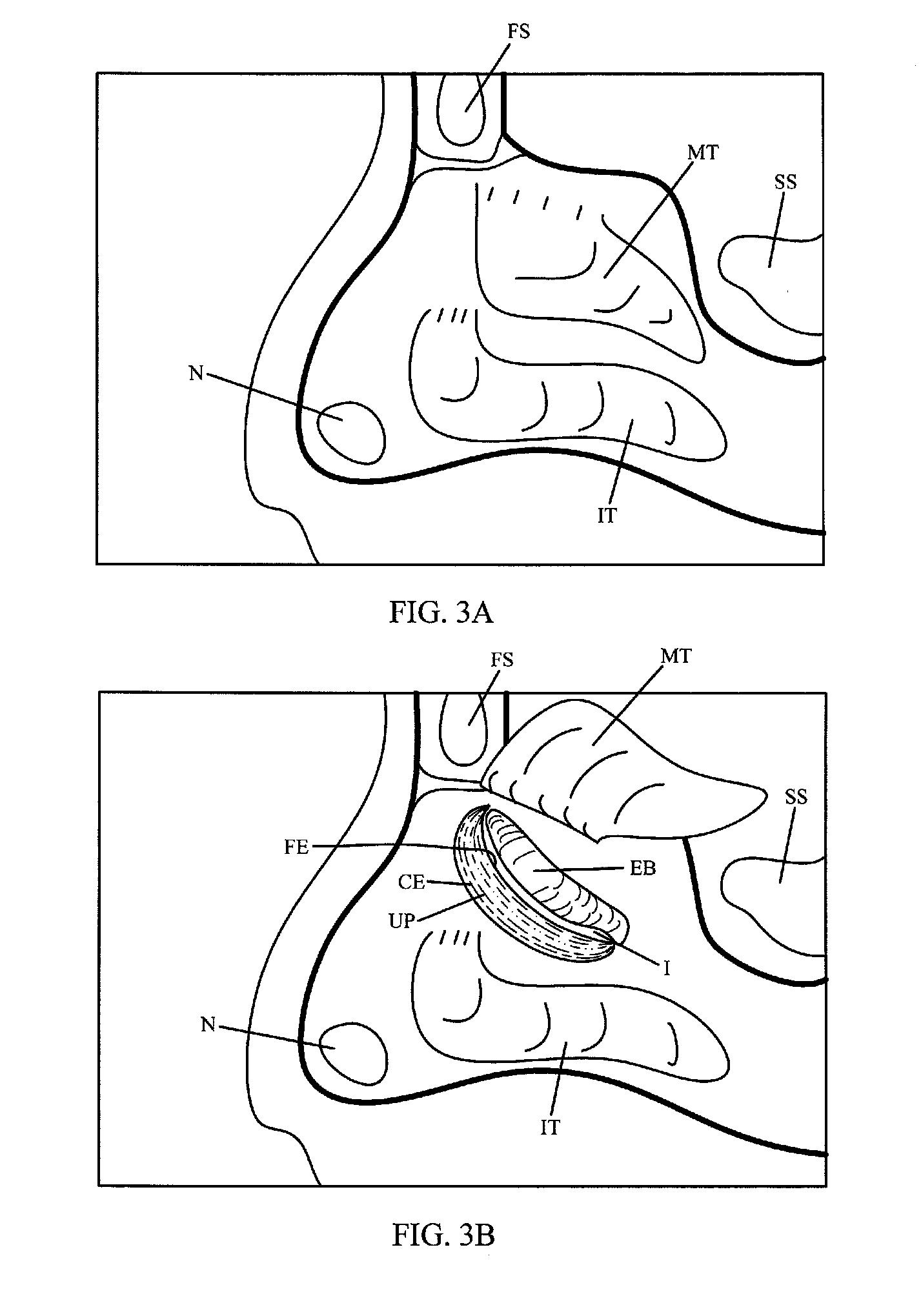
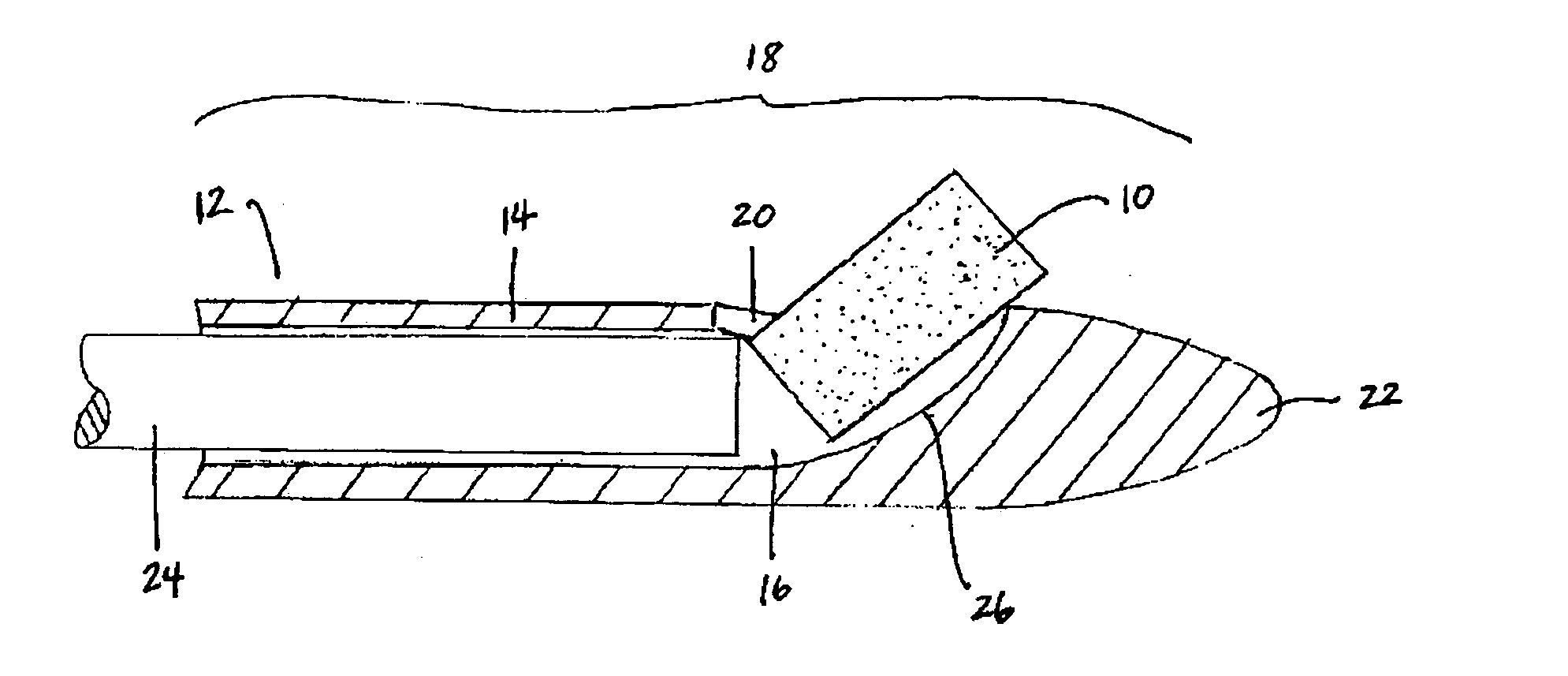
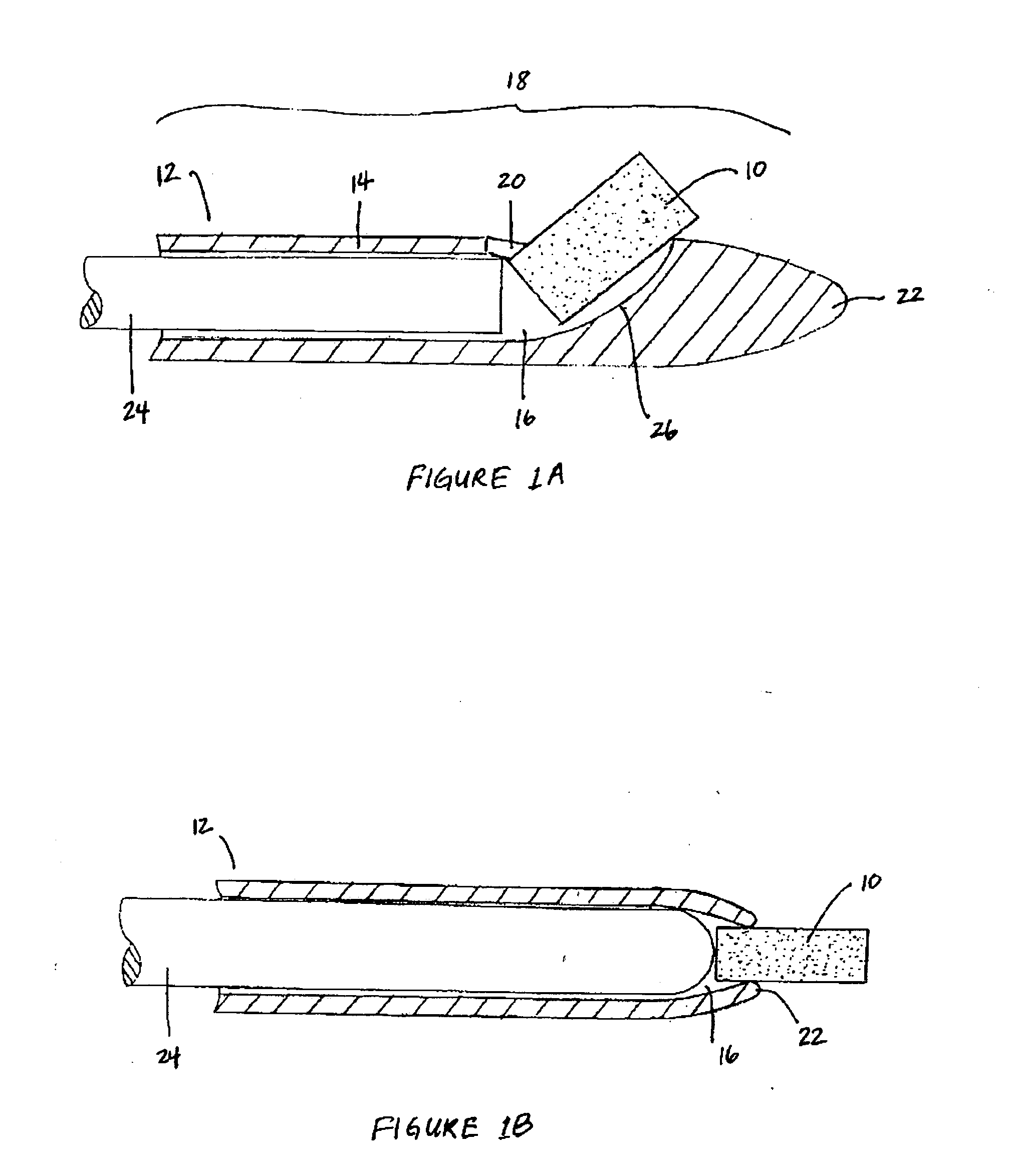
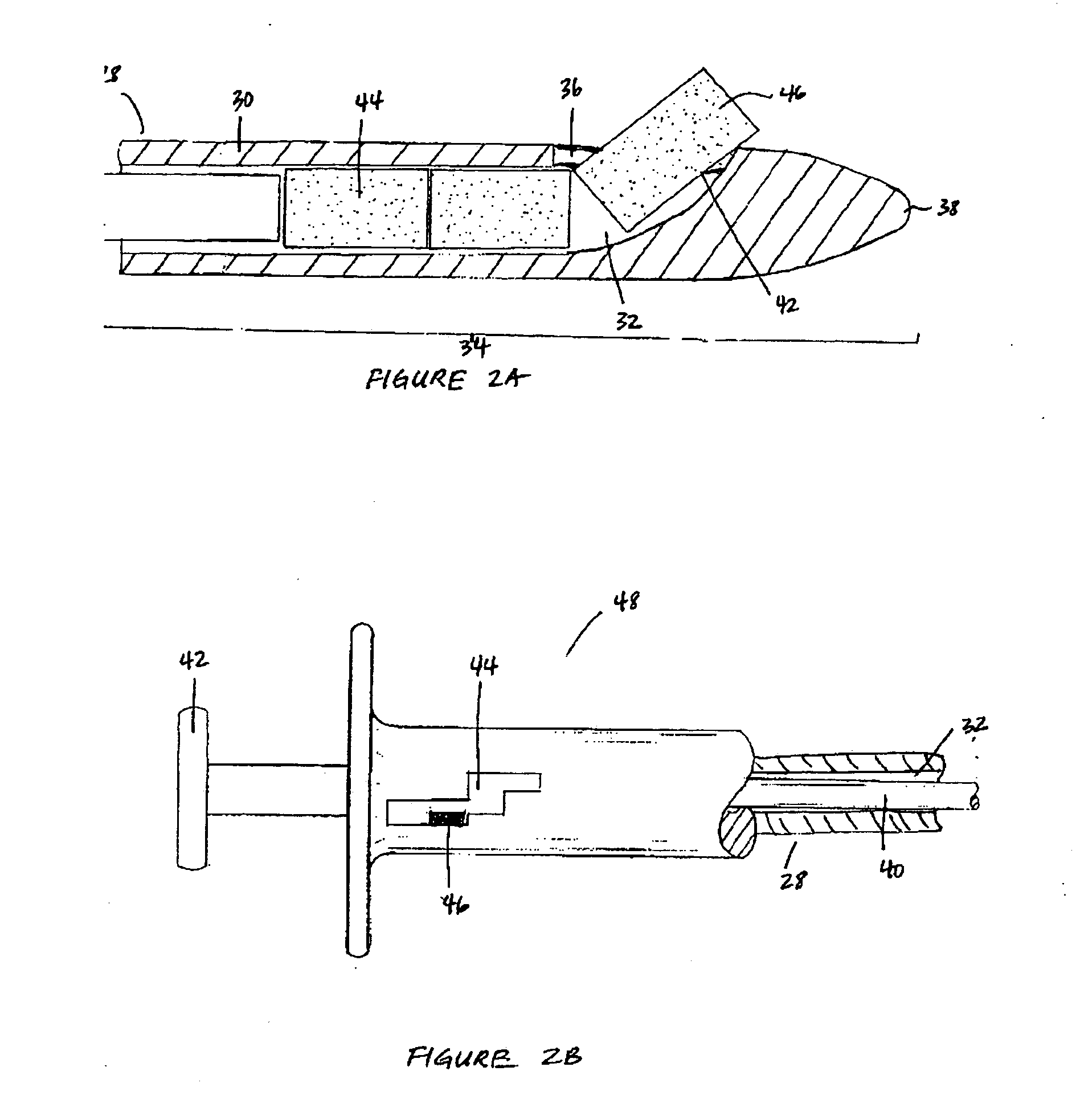
Apparatus and method for treatment of sinusitis
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. A cannula is positioned in the passageway. A visualization tool such as an endoscope is passed through a lumen or channel in the cannula to aid in visualization of the anatomical site of interest. A balloon dilation catheter is then deployed through or along the cannula so as to place the balloon within or across the constricted anatomical space (e.g., ostium). The balloon is then expanded so as to expand at least a portion of the constricted anatomical space. Alternative embodiments include the use of an optional guide wire and incorporating a endoscope lumen through the balloon dilation catheter.
Owner:ENTELLUS MEDICAL
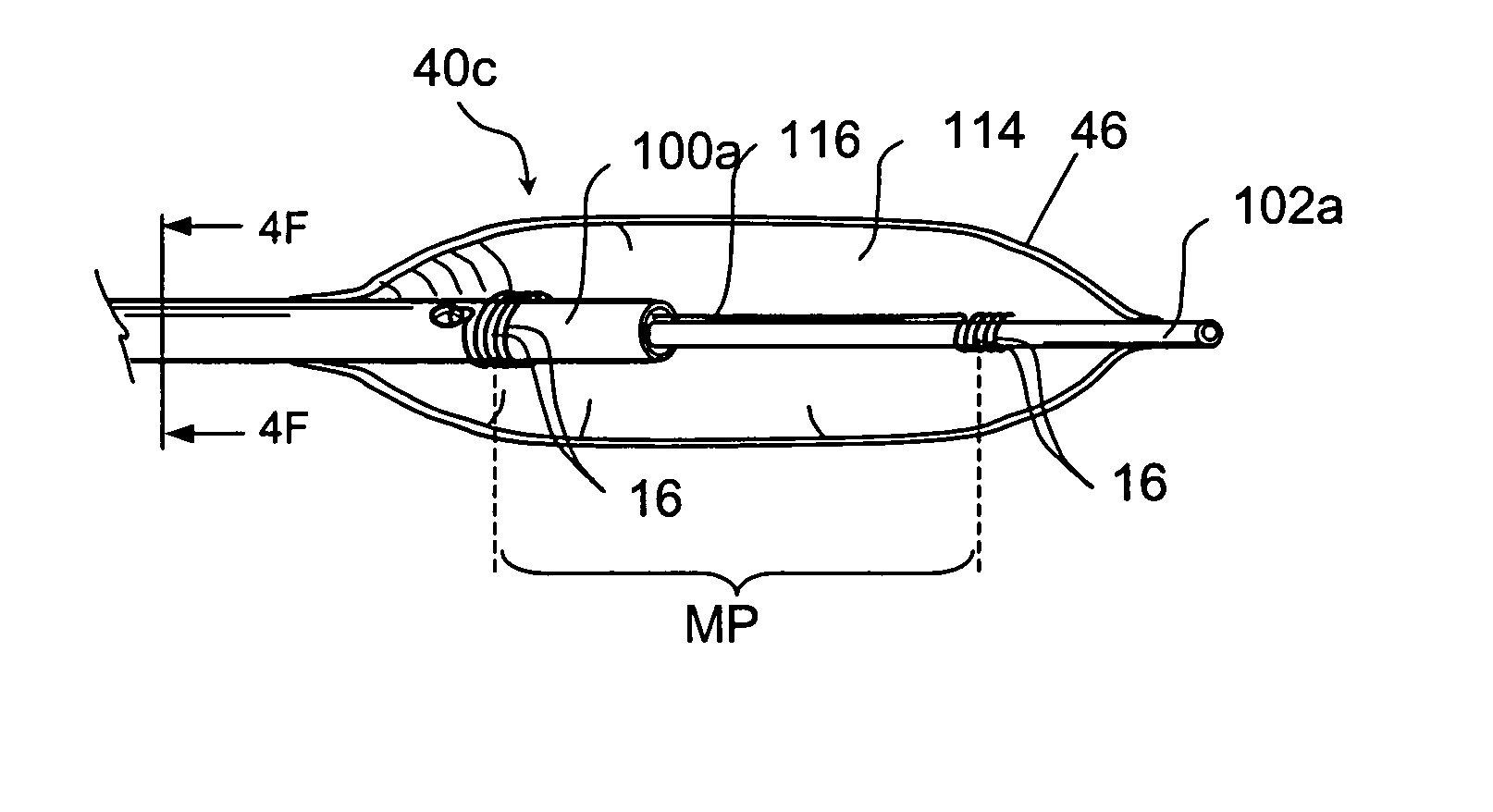
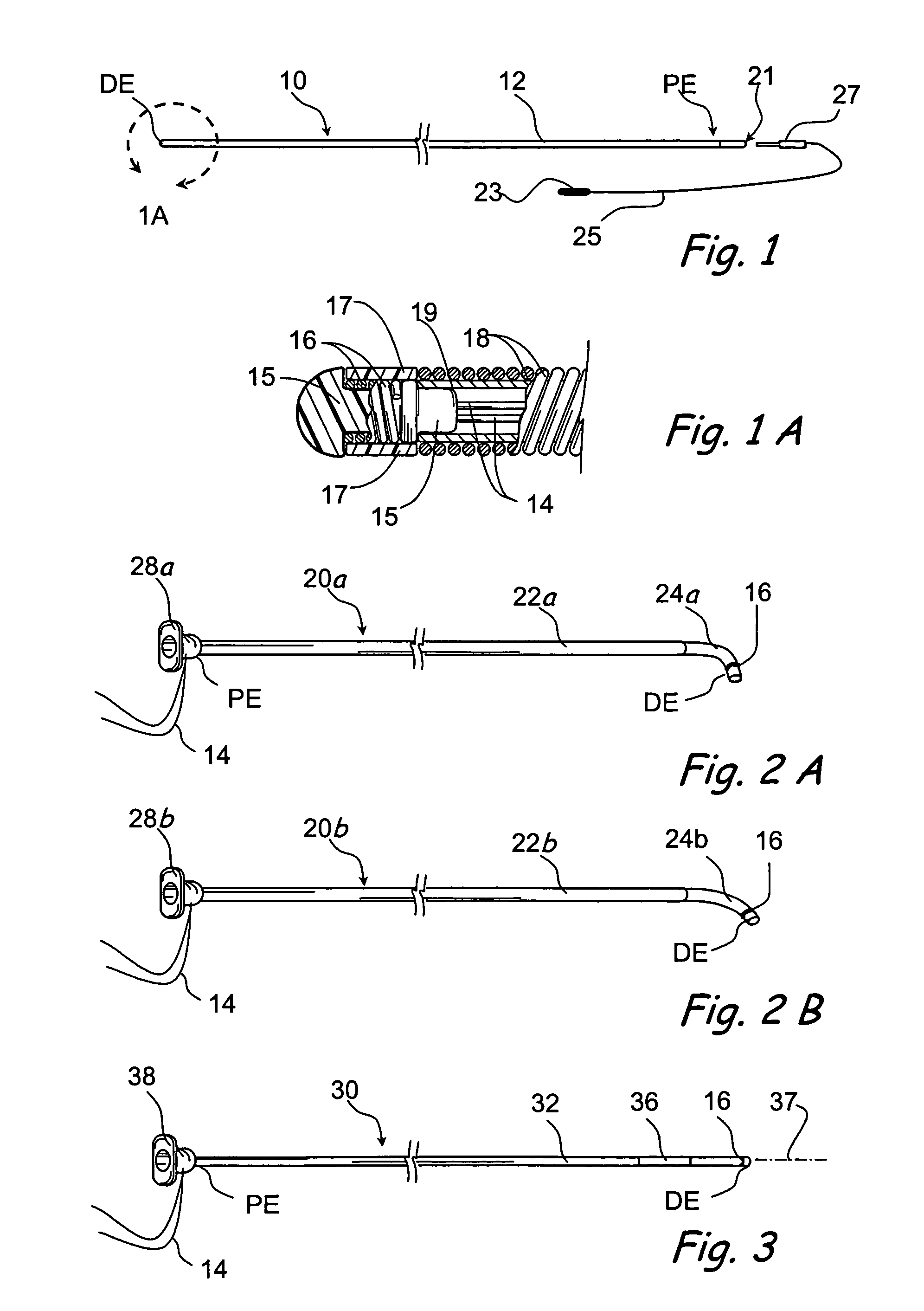
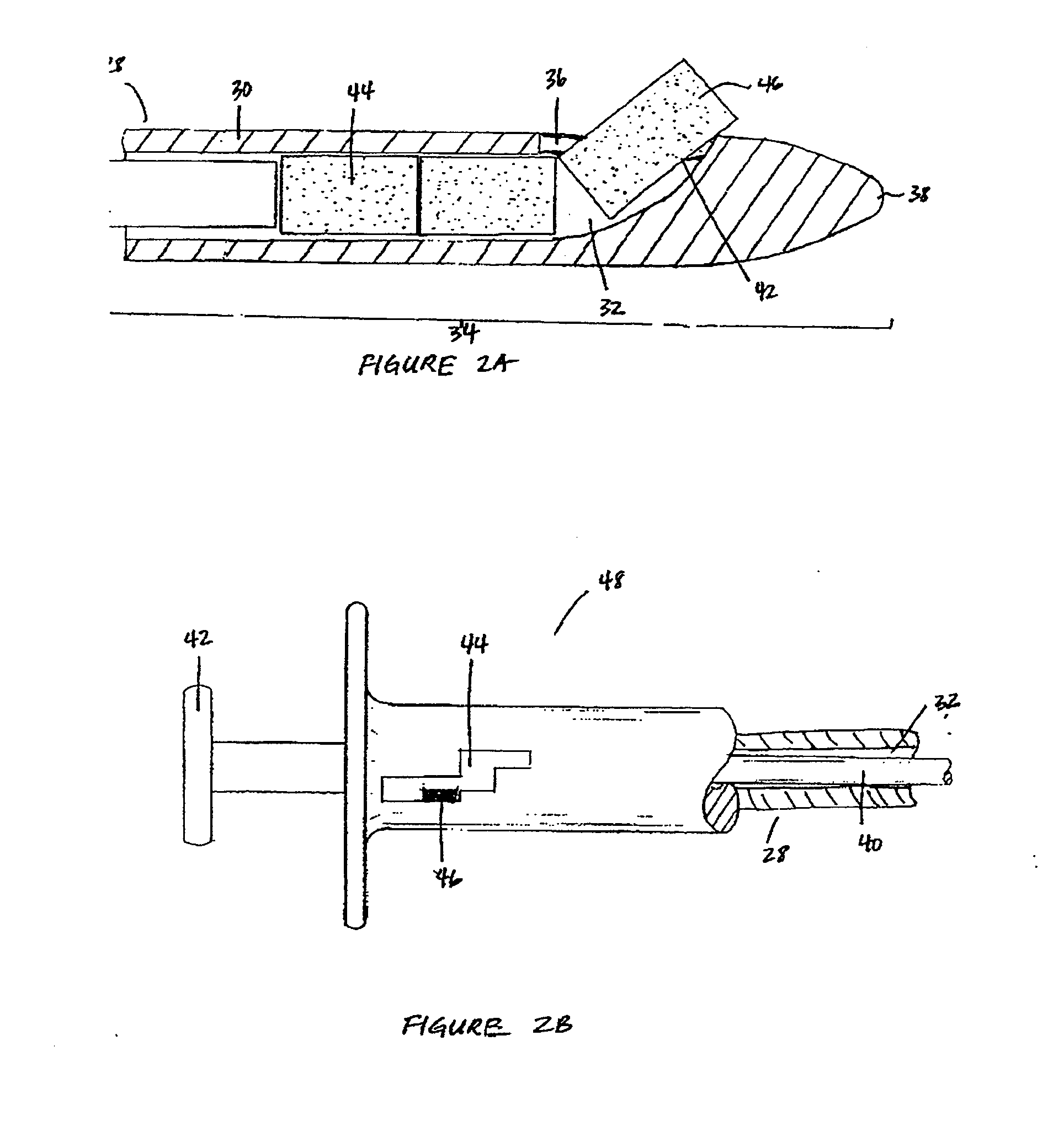
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
Device and method for treatment of sinusitus
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. An elongate member is inserted through the passageway, the elongate member having an inflation member such as a balloon disposed thereon. The inflation member is positioned within the constricted passageway. The inflation member is expanded so as to expand at least a portion of the constricted sinus passageway.
Owner:ENTELLUS MEDICAL
Systems and Methods for Performing Image Guided Procedures Within the Ear, Nose, Throat and Paranasal Sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Systems and methods for performing image guided procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Devices, Systems and Methods For Diagnosing and Treating Sinusitis and Other Disorders of the Ears, Nose and/or Throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Devices, systems and methods for diagnosing and treating sinusitis and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Sinus delivery of sustained release therapeutics
ActiveUS20050043706A1Easy accessReduce inflammationMedical devicesPharmaceutical delivery mechanismSinusitisMicroparticle
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
Devices, Systems and Methods for Treating Disorders of the Ear, Nose and Throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Sinus delivery of sustained release therapeutics
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
Devices, Systems and Methods for Treating Disorders of the Ear, Nose and Throat
Sinusitis, mucocysts, tumors, infections, hearing disorders, choanal atresia, fractures and other disorders of the paranasal sinuses, Eustachian tubes, Lachrymal ducts and other ear, nose, throat and mouth structures are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies and endoscopic studies. Access and occluding devices may be used to facilitate insertion of working devices such asendoscopes, wires, probes, needles, catheters, balloon catheters, dilation catheters, dilators, balloons, tissue cutting or remodeling devices, suction or irrigation devices, imaging devices, sizing devices, biopsy devices, image-guided devices containing sensors or transmitters, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting or delivering devices and implants, etc.
Owner:ACCLARENT INC
Sinus delivery of sustained release therapeutics
The invention provides biodegradable implants for treating sinusitis. The biodegradable implants have a size, shape, density, viscosity, and / or mucoadhesiveness that prevents them from being substantially cleared by the mucociliary lining of the sinuses during the intended treatment period. The biodegradable implants include a sustained release therapeutic, e.g., an antibiotic, a steroidal anti-inflammatory agent, or both. The biodegradable implants may take various forms, such as rods, pellets, beads, strips, or microparticles, and may be delivered into a sinus in various pharmaceutically acceptable carriers.
Owner:INTERSECT ENT INC
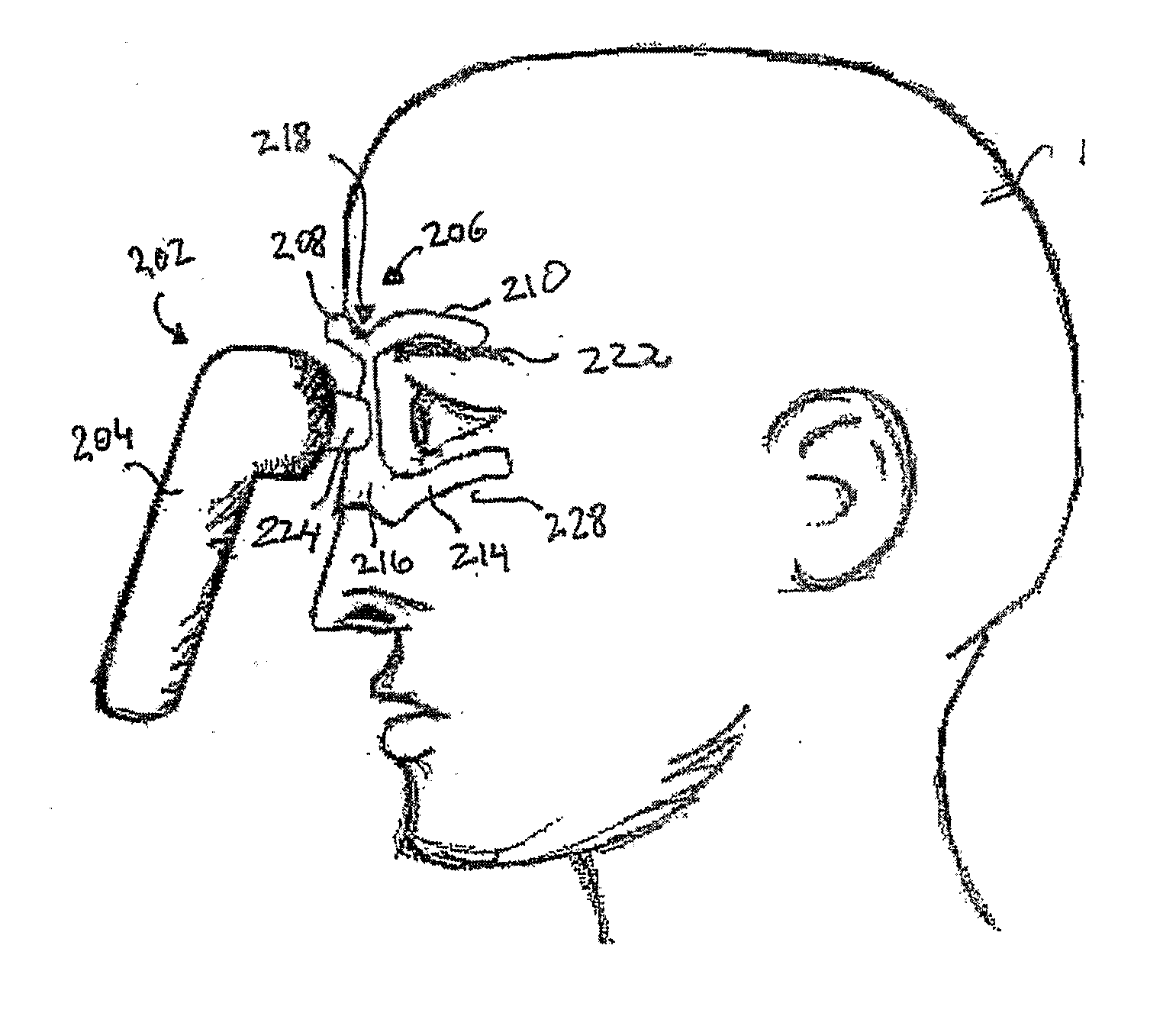
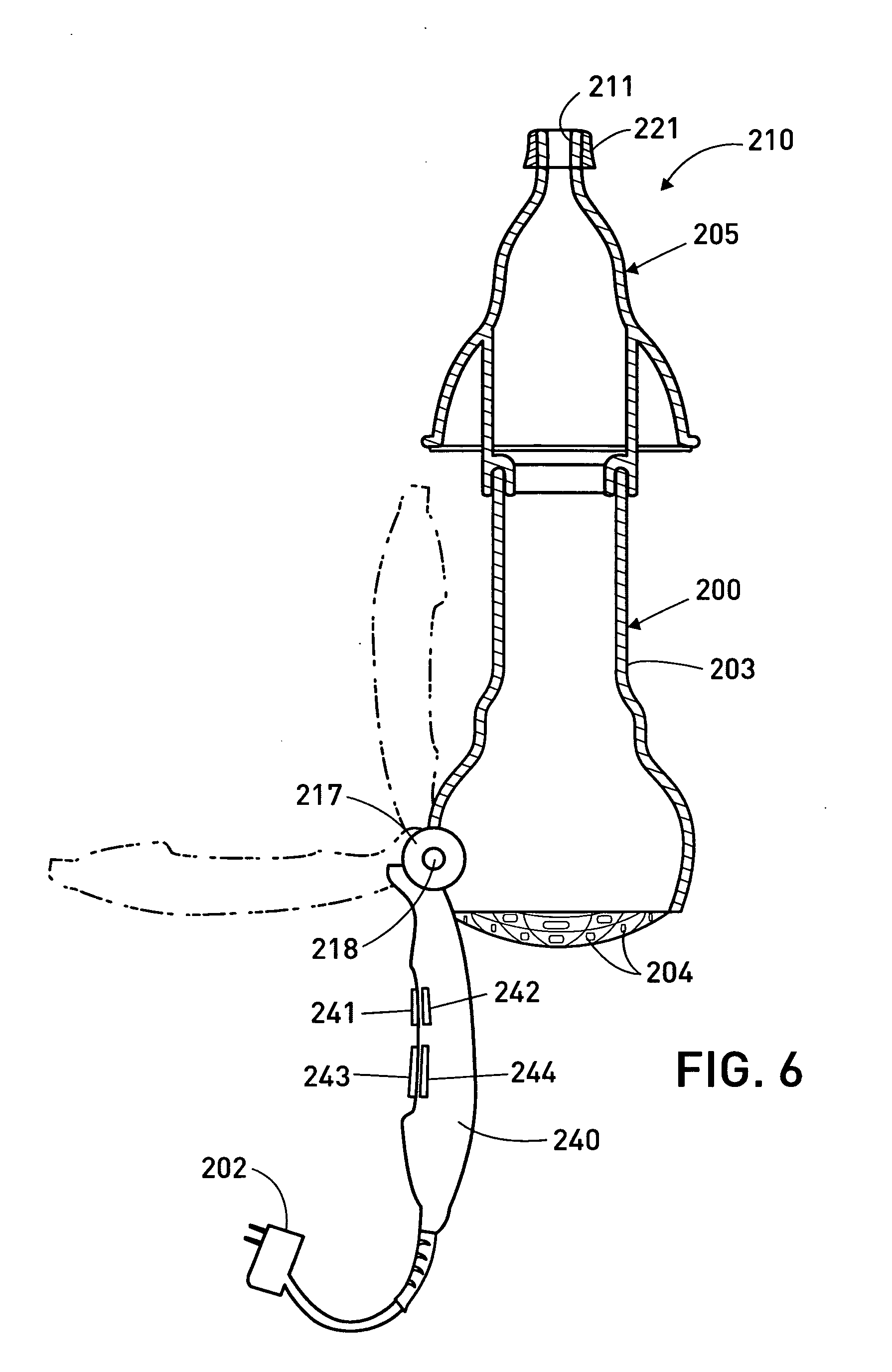
Vibrating Device For Treating Nasal Congestion and Sinusitis Symptoms and Method Thereof
The present invention discloses a method of treating nasal congestion and / or relieving sinusitis symptoms in a patient comprising attaching a vibration generating means to the patient head, at a location adjacent to sinuses to be treated, generating a vibration by said vibration generating means, and delivering the same to said patient. The present invention also discloses a device for treating nasal congestion and / or relieving sinusitis symptoms in a patient, comprising attaching means and a vibration generating means; said attaching means are in communication with the patient's head in a location adjacent to said nasal cavity, nasal passageway or sinuses to be treated; said generating means are adapted to vibrate said attaching means, to vibrate the location adjacent to said nasal cavity or sinuses and thus improved mucociliary clearance of secretions in nasal cavity or sinuses.
Owner:ADS & B INVESTMENT FUND
Treatment of disease states and adverse physiological conditions utilizing anti-fungal compositions
InactiveUS20060177424A1Effectively ameliorateEffective regulationBiocideBacteria material medical ingredientsSinusitisVaginal Yeast Infections
A method for treatment or prophylaxis of a disease state or other physiological condition, e.g., autism, delayed development, acid reflux disease, vaginal yeast infections, impaired hearing, chronic ear infections, seasonal allergies, Fibromyalgia syndrome, Crohn's disease, colitis, irritable bowel syndrome, interstitial cystitis, acne, sinusitis, rheumatoid arthritis, chronic fatigue syndrome, asthma, attention deficit disorder, attention deficit / hyperactivity disorder, rosacea, multiple sclerosis, hyperglycemia, or Ménière's disease, by administration of an anti-fungal composition that includes at least one of the bacilli (1) Bacillus subtilis, (2) Lactobacillus sporogenes, and (3) Streptococcus faecalis.
Owner:COBB AND CO
Device, method and system for treatment of sinusitis
InactiveUS20110307039A1Preventing facilitating treatmentEasy to install and removeTherapeutic coolingTherapeutic heatingNasal passageSinusitis
A device, method and system for facilitating treatment of a sinusitis condition, including a heat source configured to deliver an output of heated air to the nasal passages of an individual, and a delivery component having a space through which the heated air output of the heat source may pass, the delivery component being configured for placement in the individual's nostrils. The system provides a heated air output source and one or more delivery components configured and sized to fit within the nostril of an individual, which may be used to deliver one or more courses of heated air to a user's sinuses through a nostril.
Owner:CORNELL THOMAS H
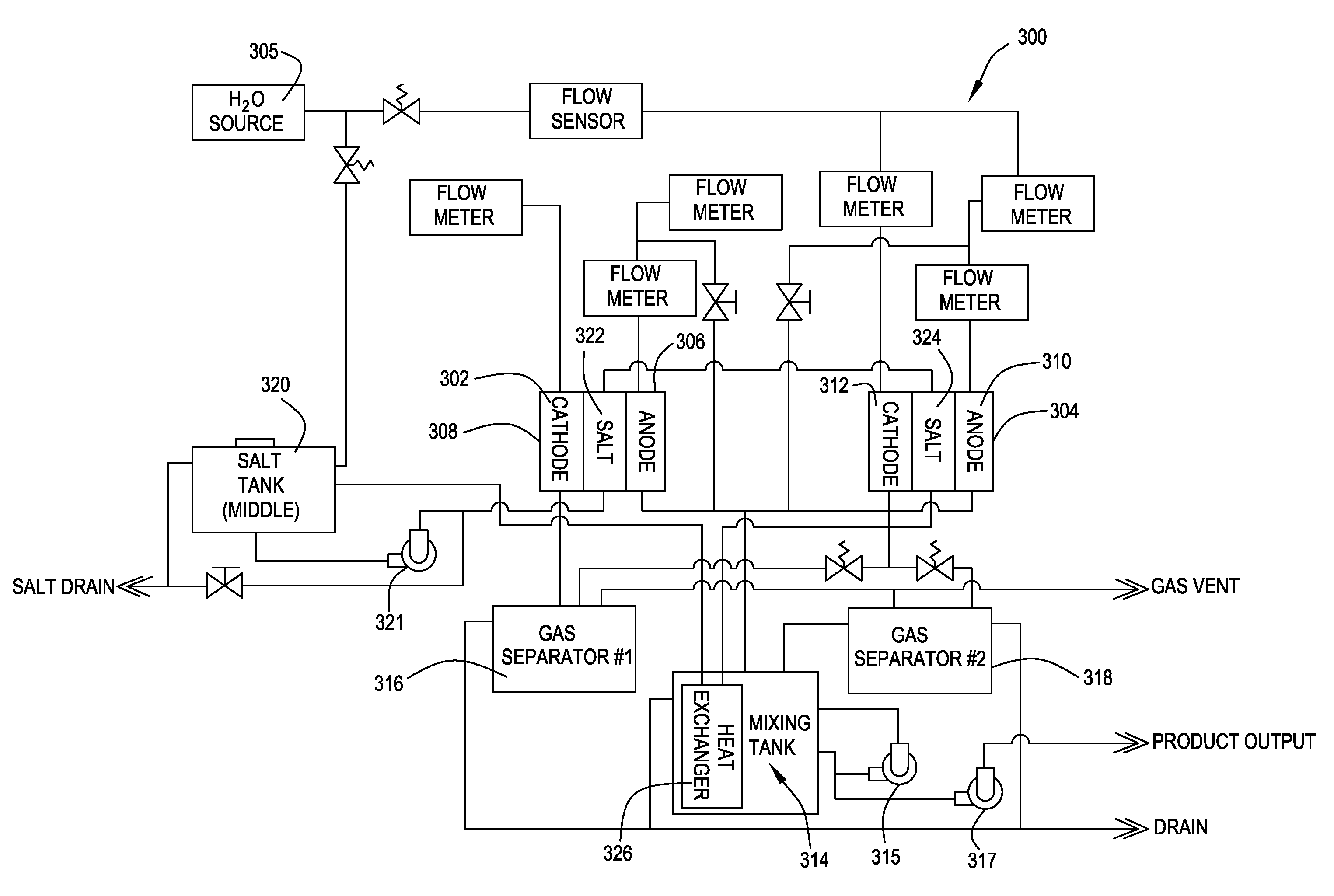
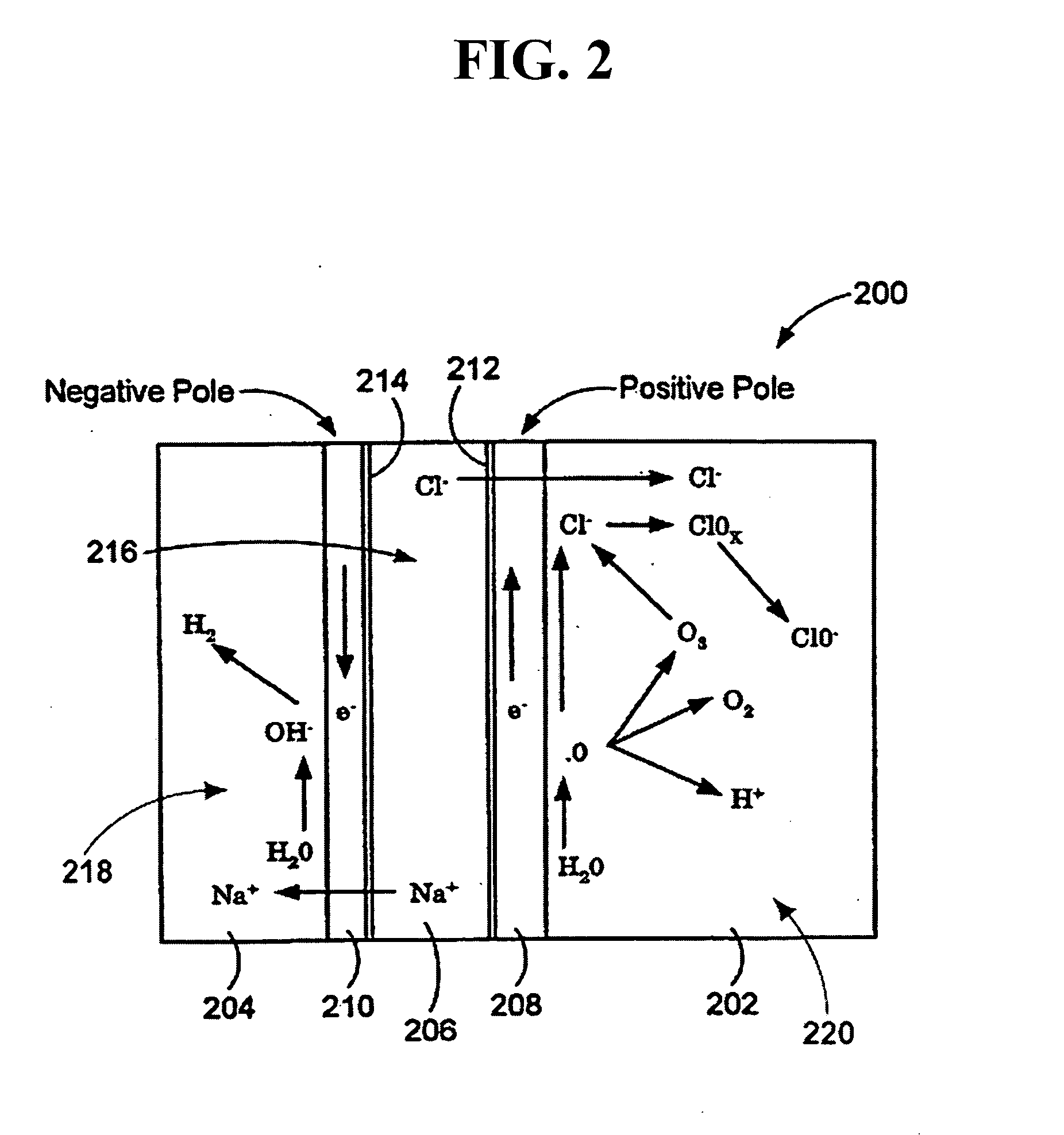
Methods of preventing or treating sinusitis with oxidative reductive potential water solution
InactiveUS20070196434A1Treating and preventing sinusitisAntibacterial agentsBiocideSinusitisMedicine
Provided is a method for preventing or treating sinusitis by administering a therapeutically effective amount of an oxidative reduction potential (ORP) water solution that is stable for at least about twenty-four hours. The ORP water solution administered in accordance with the invention can be combined with one or more suitable carriers. The ORP water solution can be administered alone or, e.g., in combination with one or more additional therapeutic agents.
Owner:SONOMA PHARMA INC
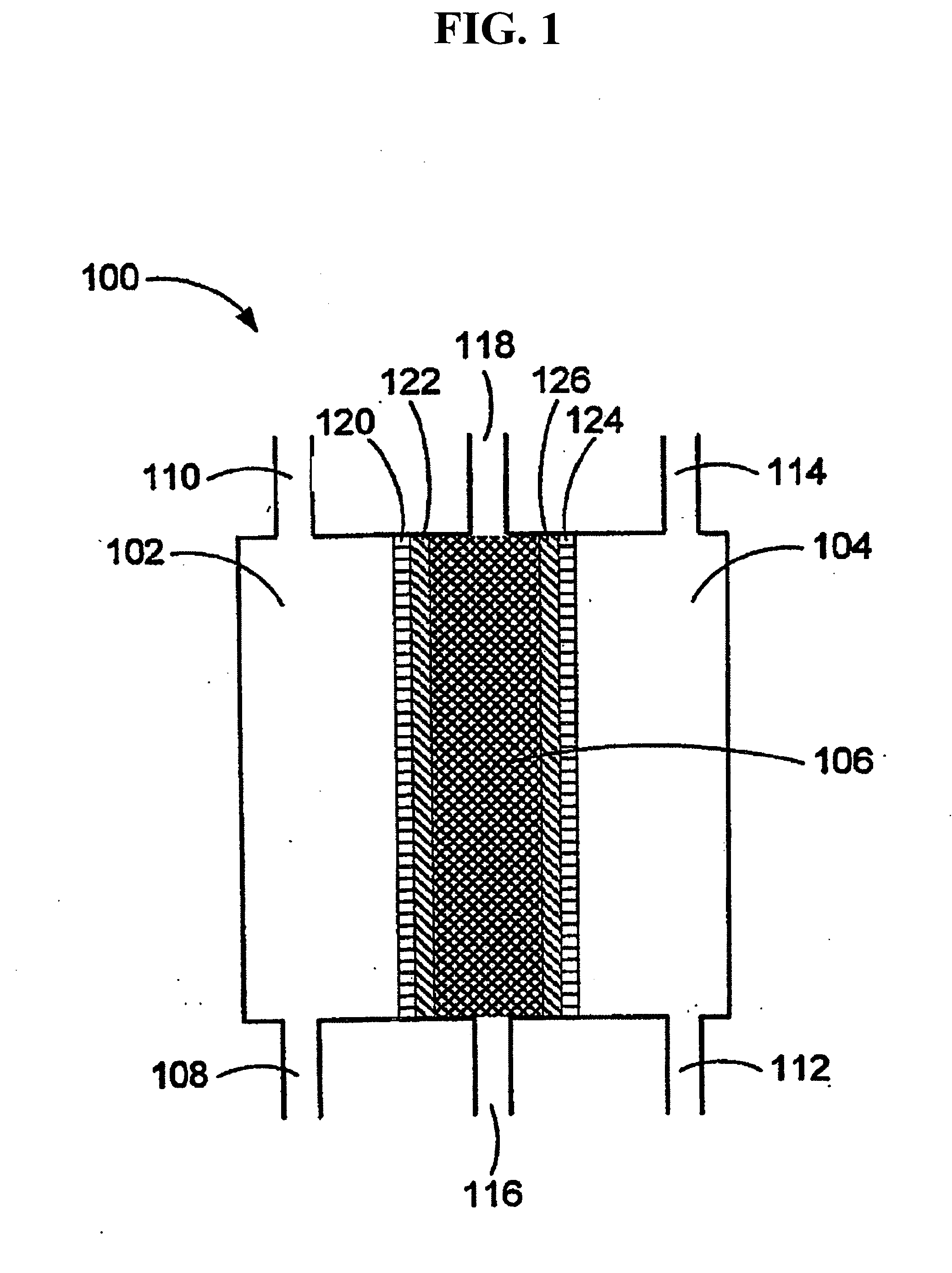
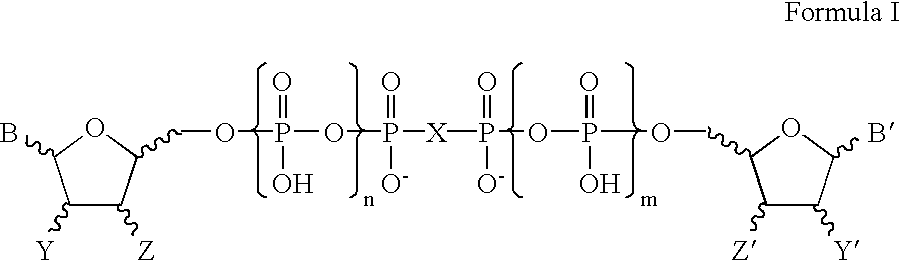
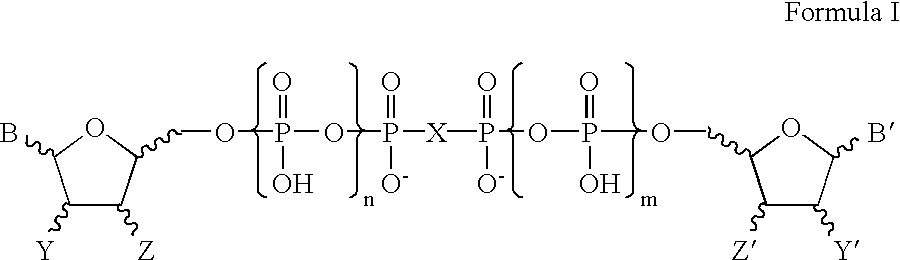
Certain dinucleotides and their use as modulators of mucociliary clearance and ciliary beat frequency
The present invention relates to certain novel dinucleotides and formulations thereof which are highly selective agonists of the P2Y2 and / or P2Y4 purinergic receptor. They are useful in the treatment of chronic obstructive pulmonary diseases such as chronic bronchitis, PCD, cystic fibrosis, as well as prevention of pneumonia due to immobility. Furthermore, because of their general ability to clear retained mucus secretions and stimulate ciliary beat frequency, the compounds of the present invention are also useful in the treatment of sinusitis, otitis media and nasolacrimal duct obstruction. They are also useful for treatment of dry eye disease and retinal detachment as well as facilitating sputum induction and expectoration.
Owner:MERCK SHARP & DOHME CORP
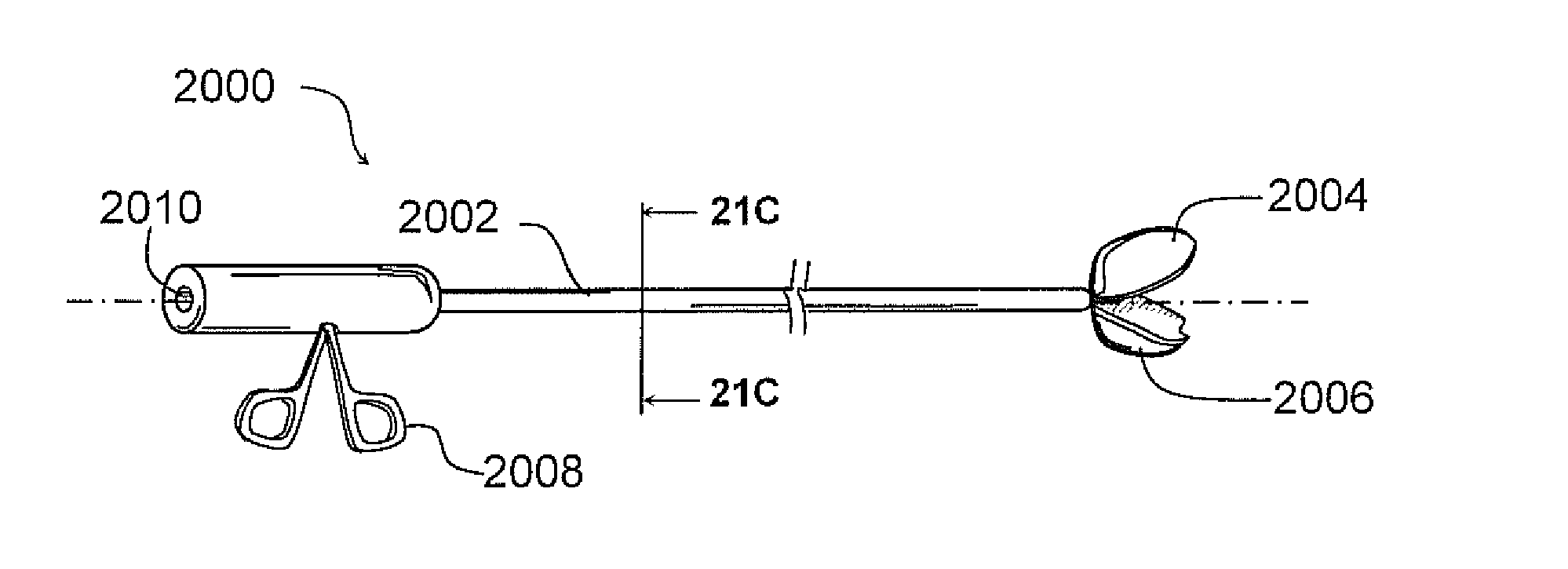
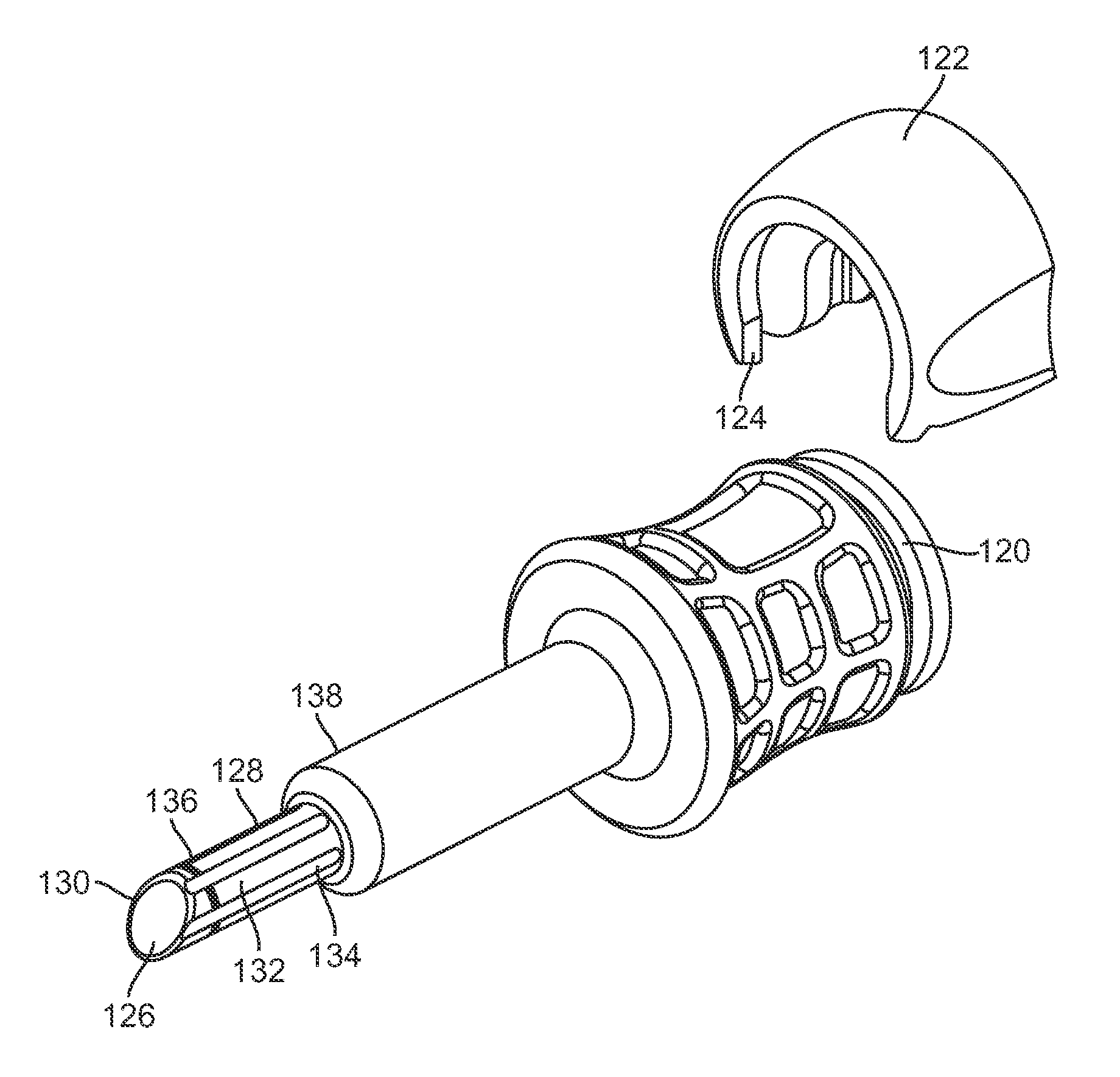
Endoscope system for treatment of sinusitis
An endoscope includes a substantially rigid shaft having a distal end and a proximal portion, the shaft having a first lumen and a second lumen separate from the first lumen, the second lumen containing one or more objective lenses disposed at the distal end thereof. A housing is mounted on the proximal portion of the shaft, the housing including an eyepiece mount and a light input port. An image fiber bundle is disposed in the second lumen, the image fiber bundle extending proximally from adjacent the one or more objective lenses to the eyepiece mount. An illumination fiber bundle is disposed in the second lumen, the illumination fiber bundle extending proximally from the distal end of the shaft to the light input port.
Owner:ENTELLUS MEDICAL
Apparatus and method for treatment of sinusitis
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. A cannula is positioned in the passageway. A visualization tool such as an endoscope is passed through a lumen or channel in the cannula to aid in visualization of the anatomical site of interest. A balloon dilation catheter is then deployed through or along the cannula so as to place the balloon within or across the constricted anatomical space (e.g., ostium). The balloon is then expanded so as to expand at least a portion of the constricted anatomical space. Alternative embodiments include the use of an optional guide wire and incorporating a endoscope lumen through the balloon dilation catheter.
Owner:ENTELLUS MEDICAL
Apparatus and method for treatment of sinusitis
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. A cannula is positioned in the passageway. A visualization tool such as an endoscope is passed through a lumen or channel in the cannula to aid in visualization of the anatomical site of interest. A balloon dilation catheter is then deployed through or along the cannula so as to place the balloon within or across the constricted anatomical space (e.g., ostium). The balloon is then expanded so as to expand at least a portion of the constricted anatomical space. Alternative embodiments include the use of an optional guide wire and incorporating a endoscope lumen through the balloon dilation catheter.
Owner:ENTELLUS MEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com