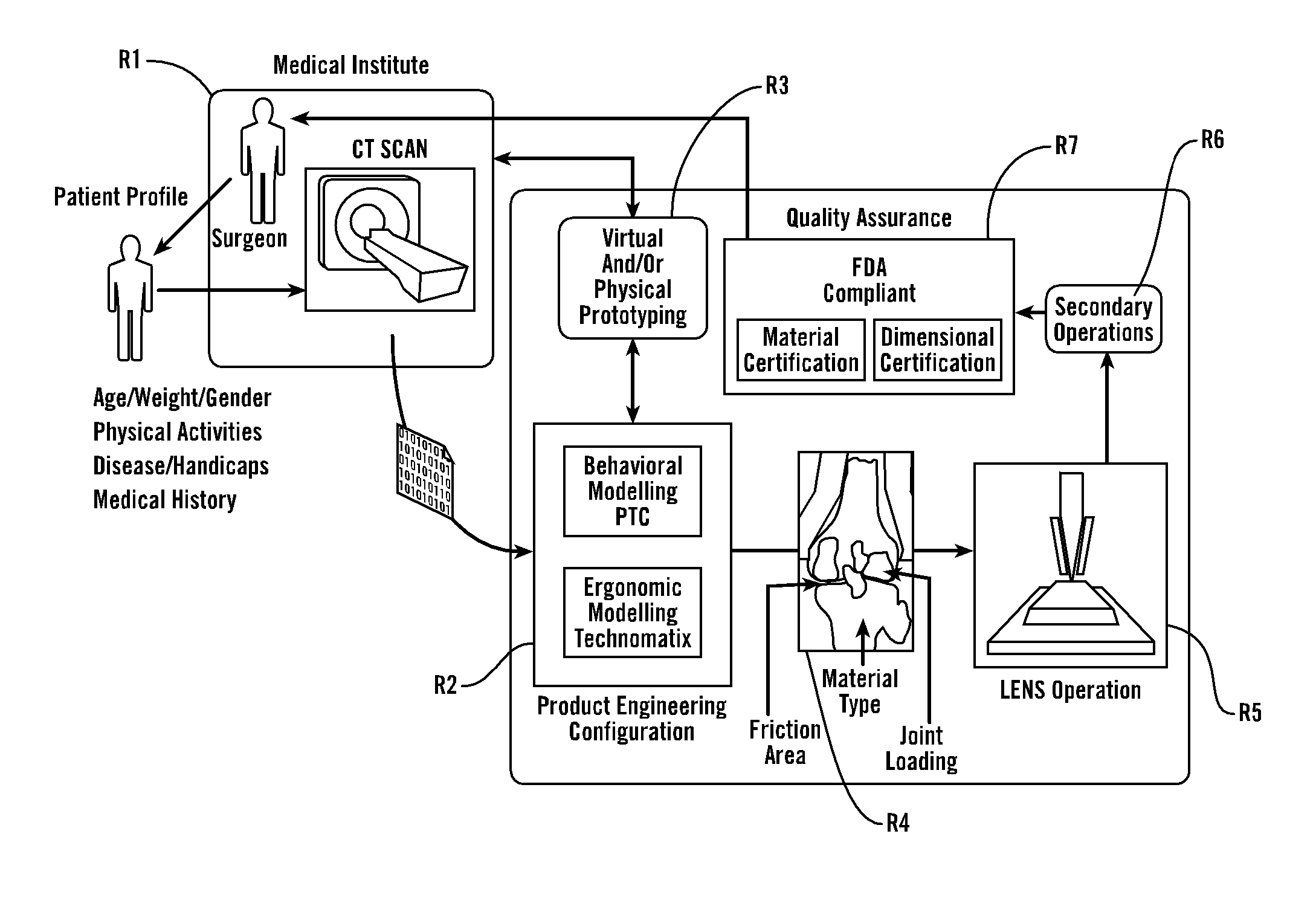
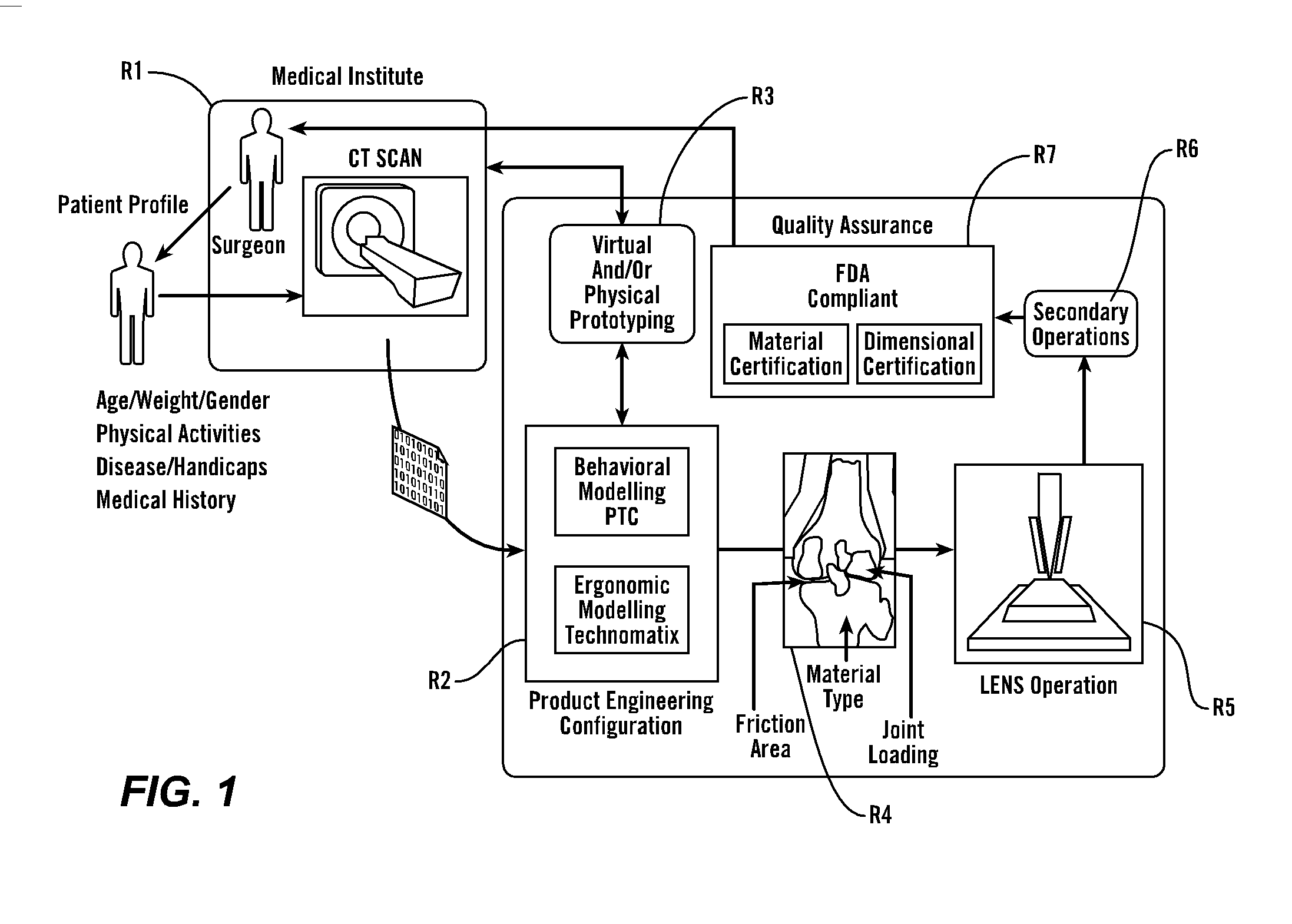
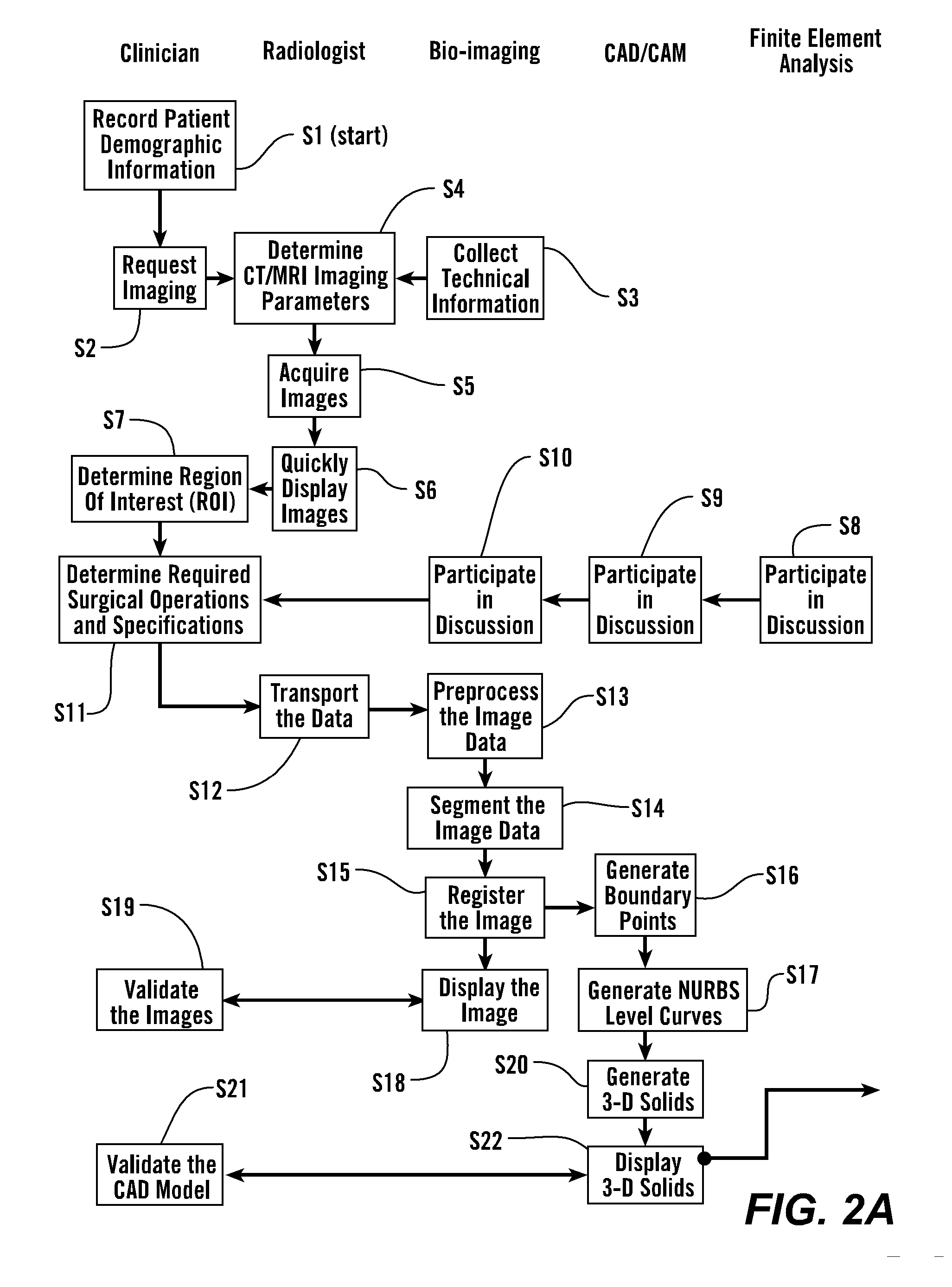
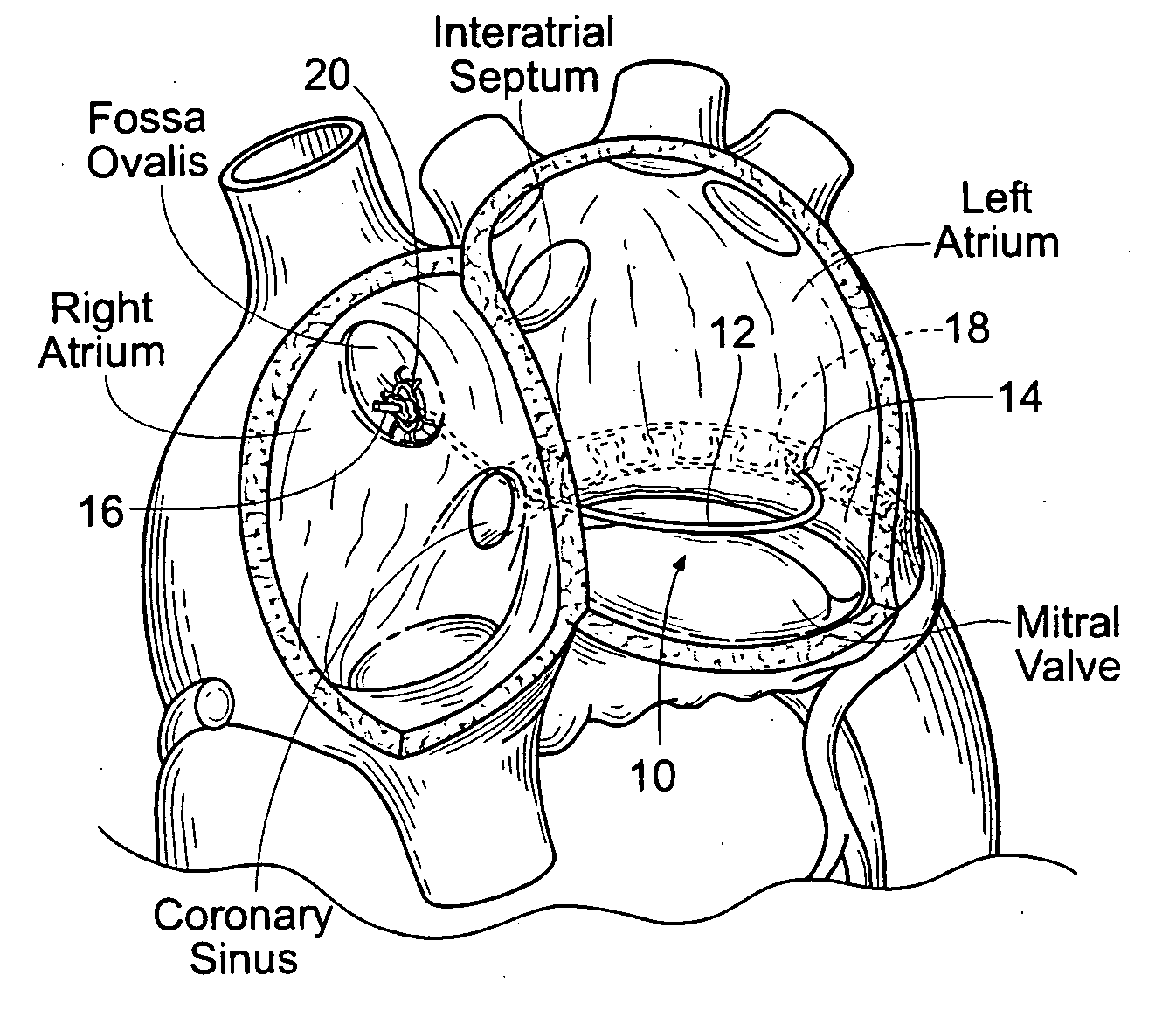
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3360 results about "Implant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
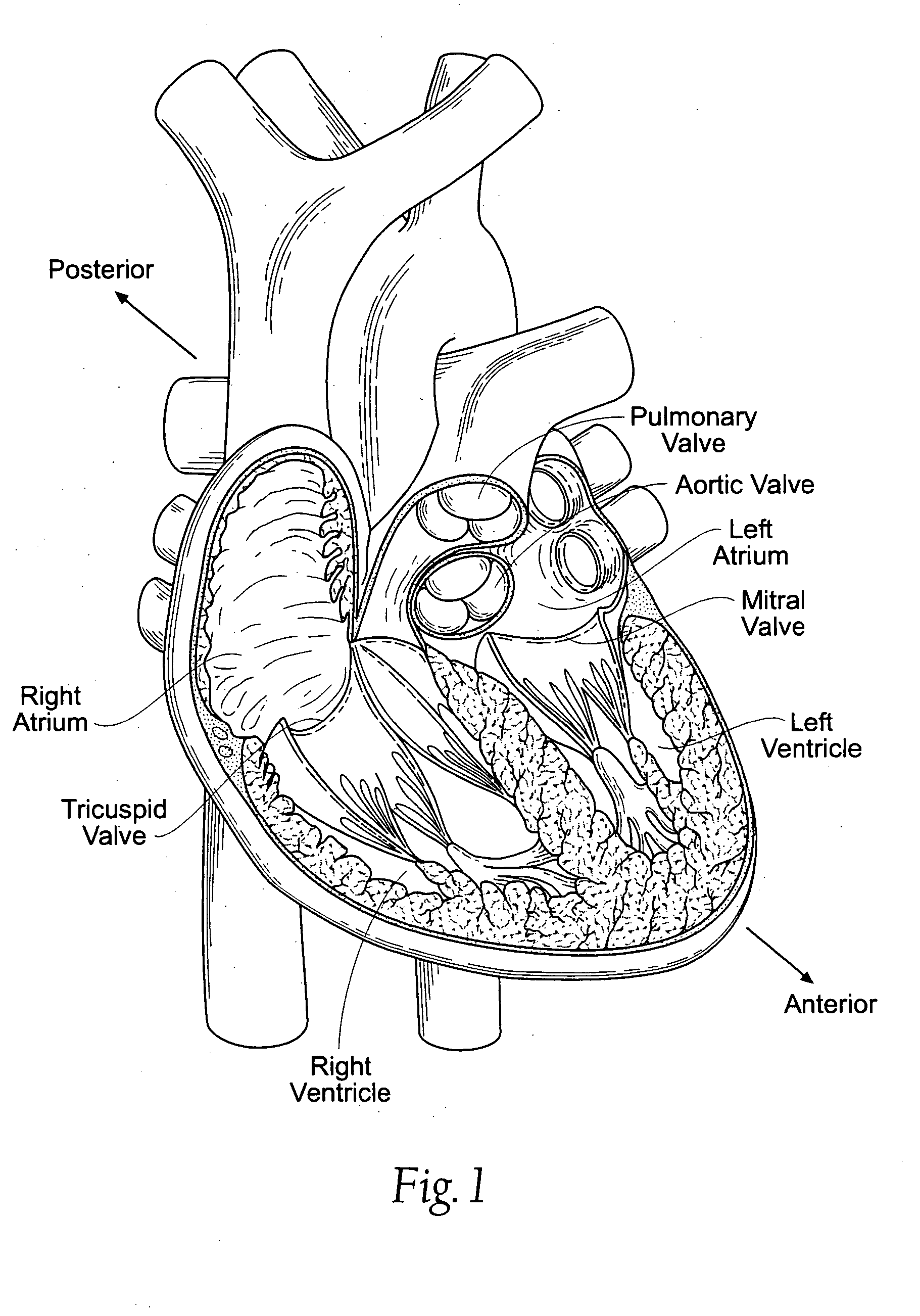
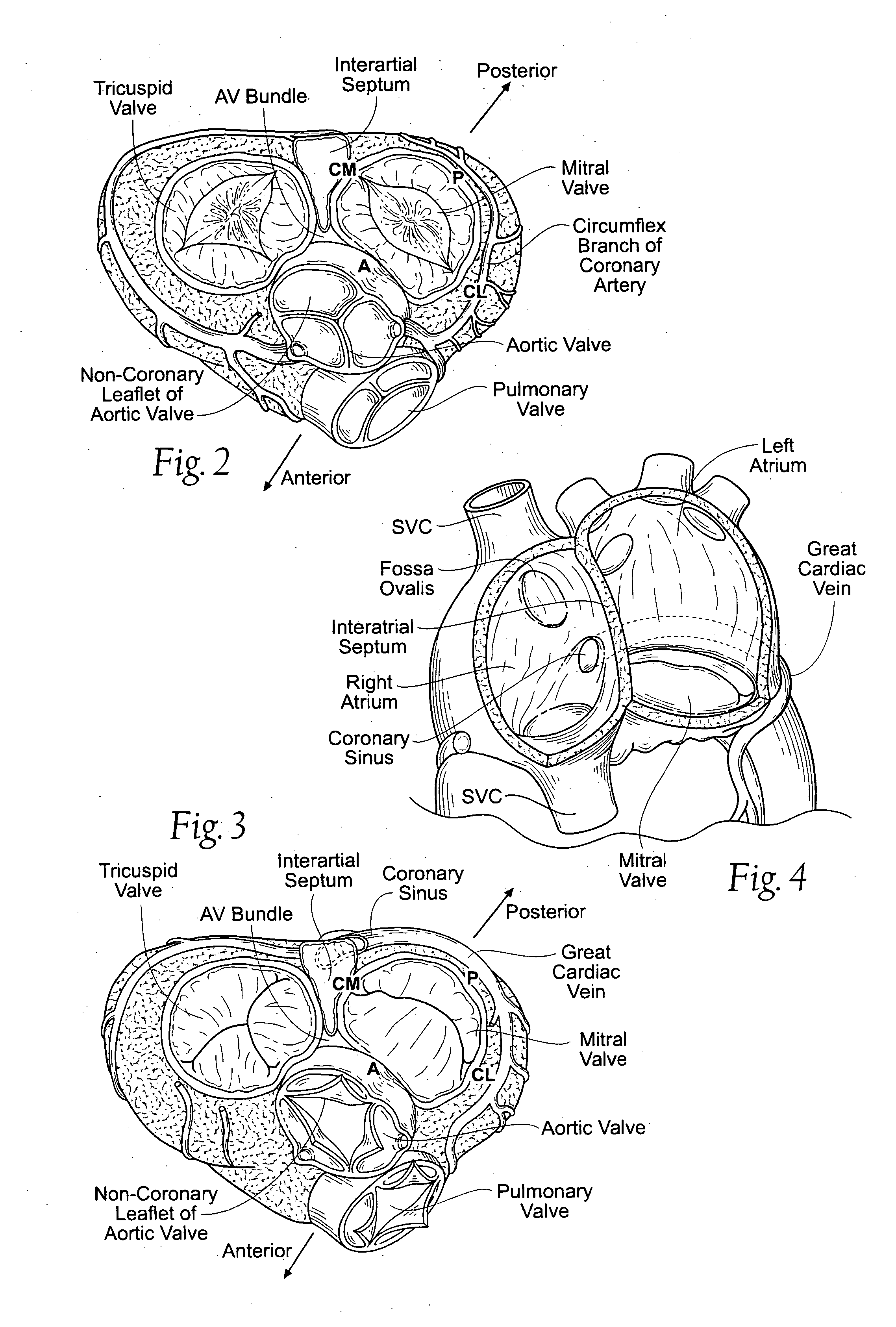
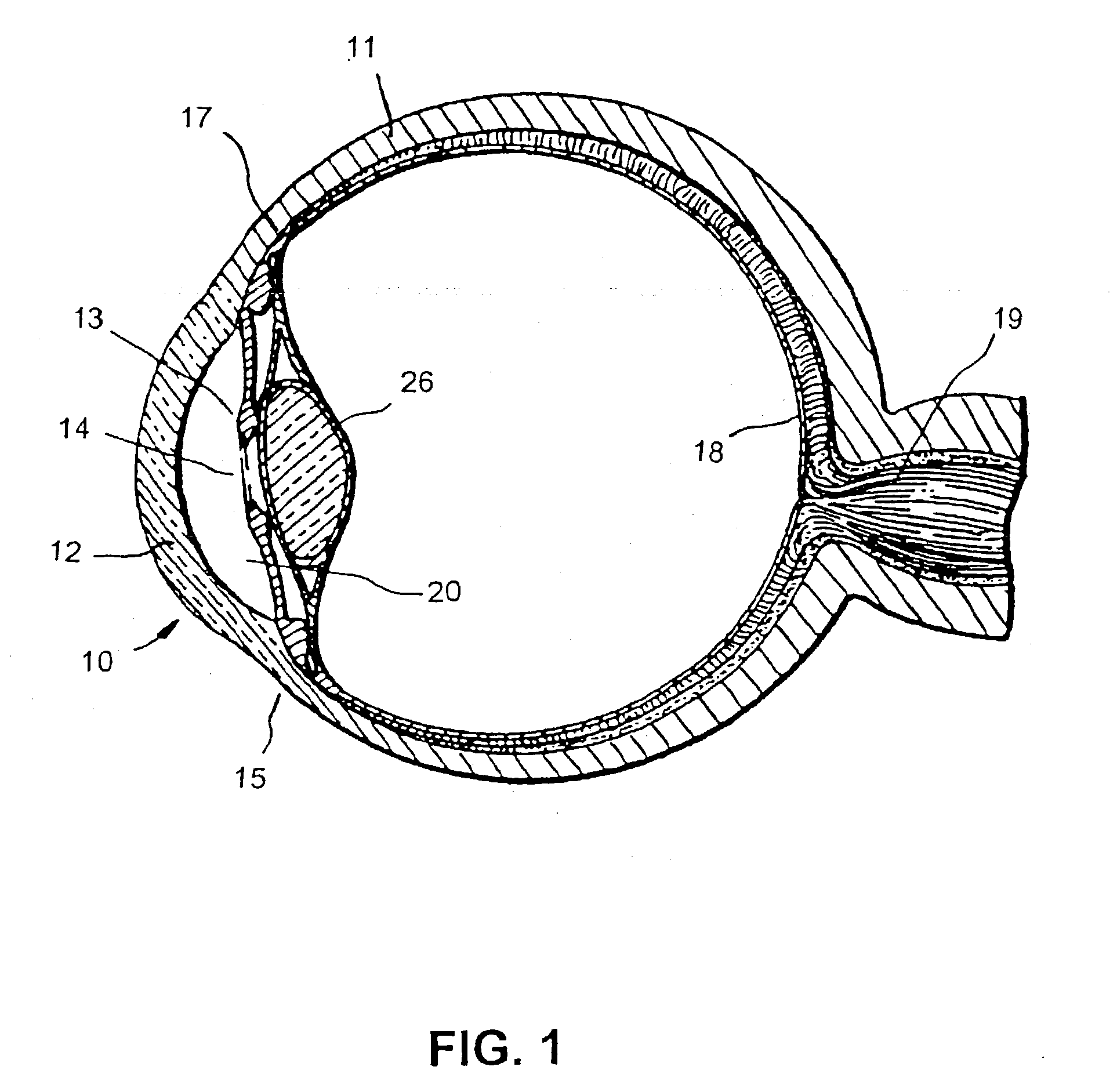
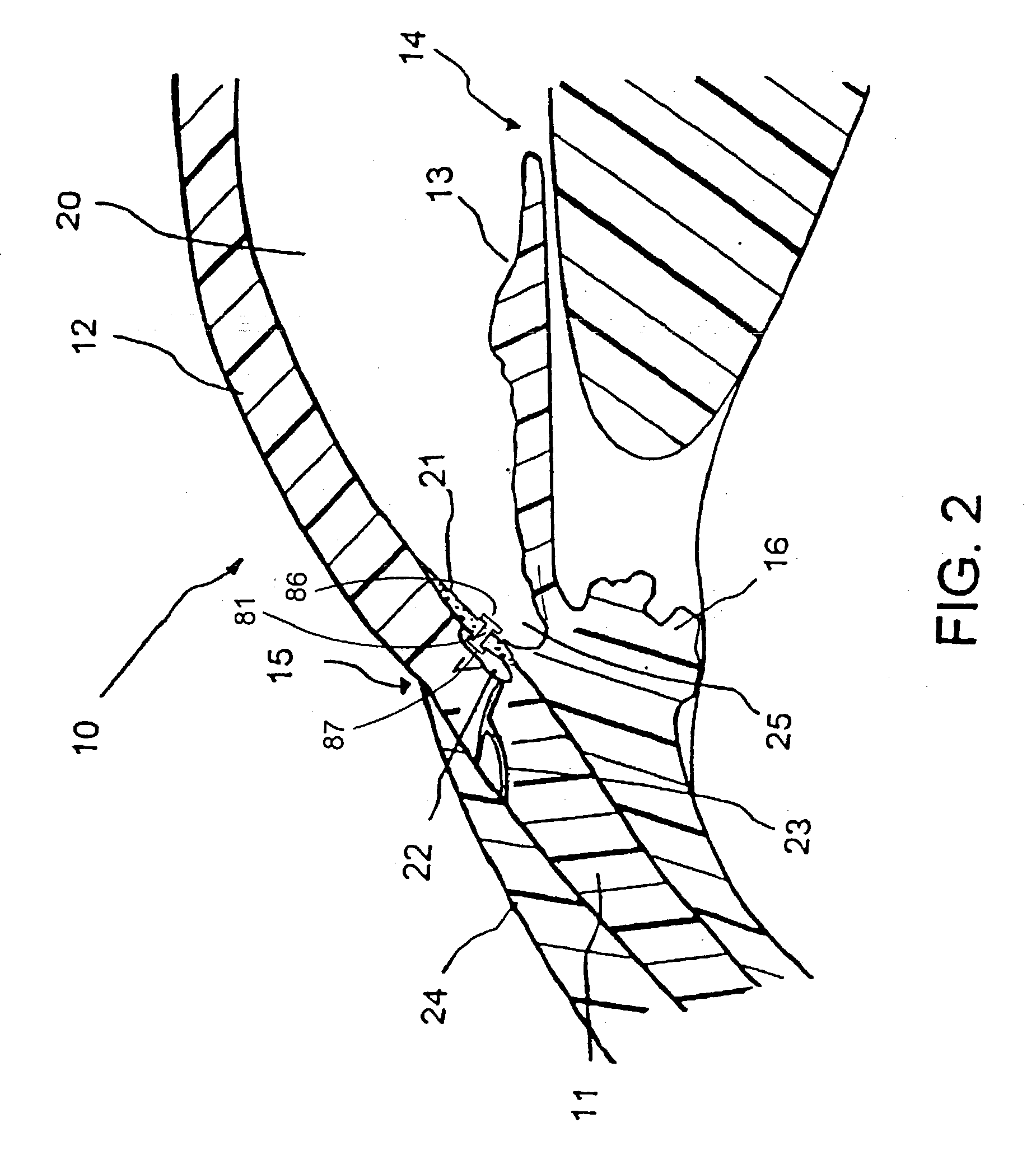
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In some cases implants contain electronics e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
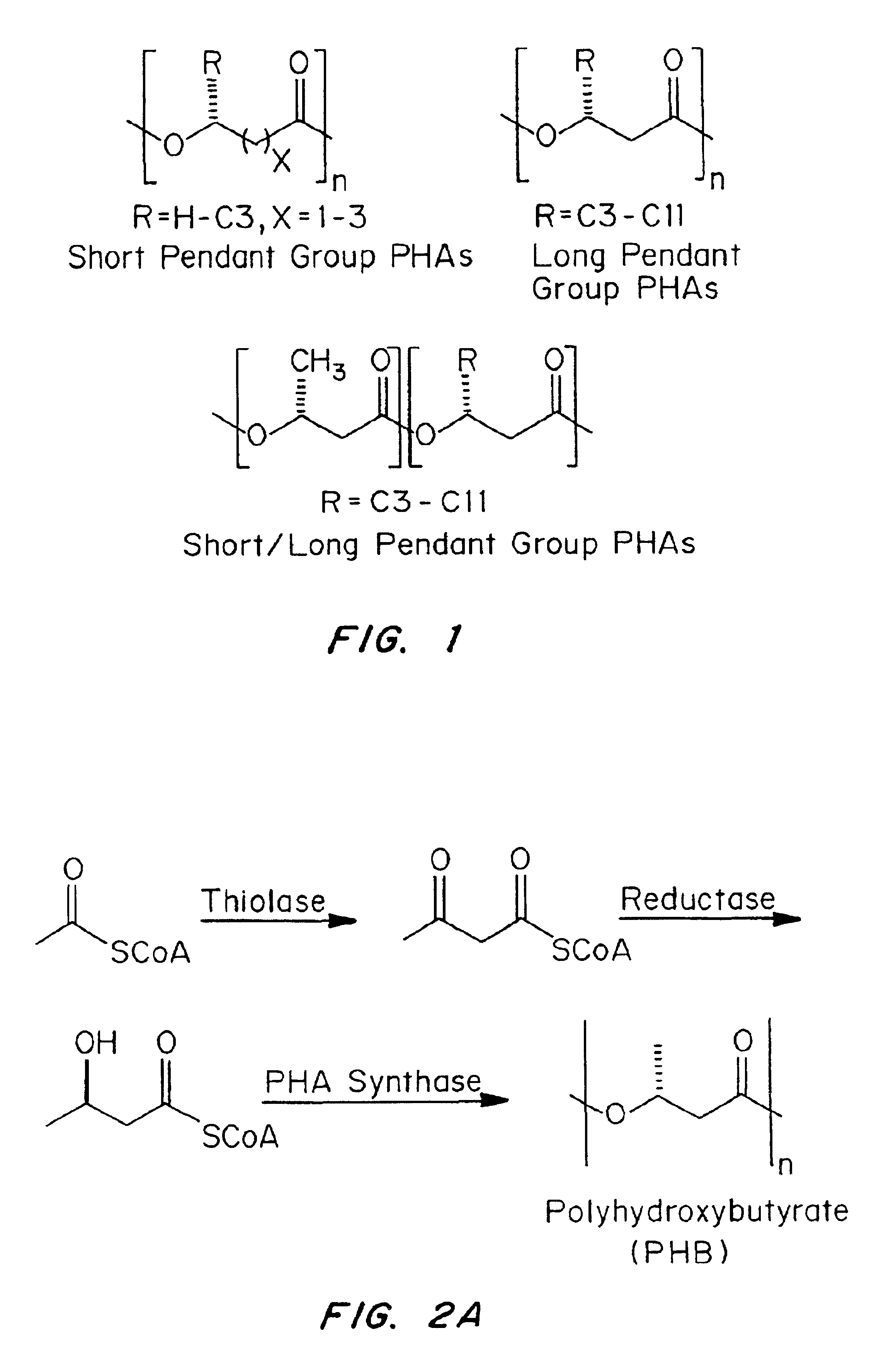
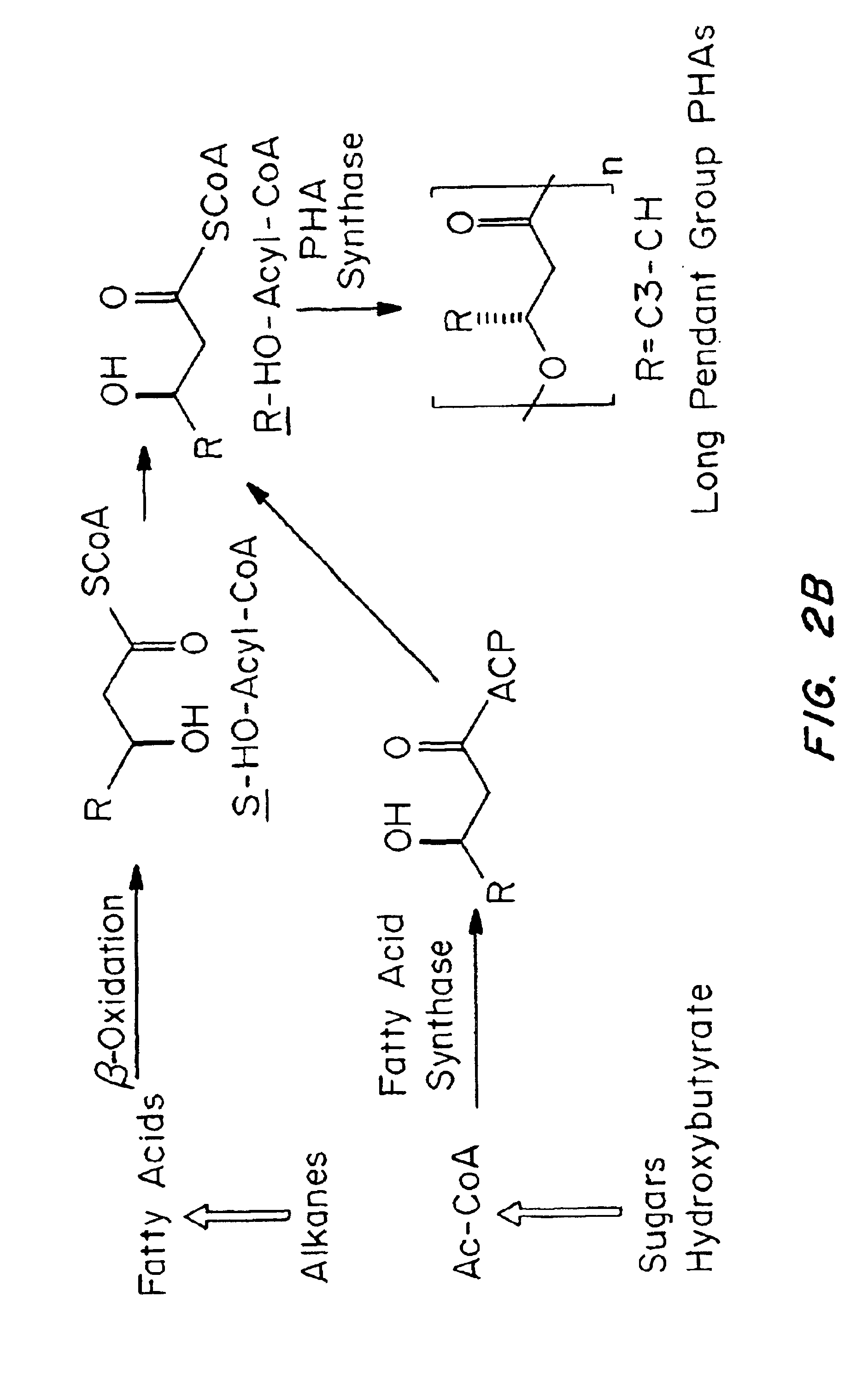
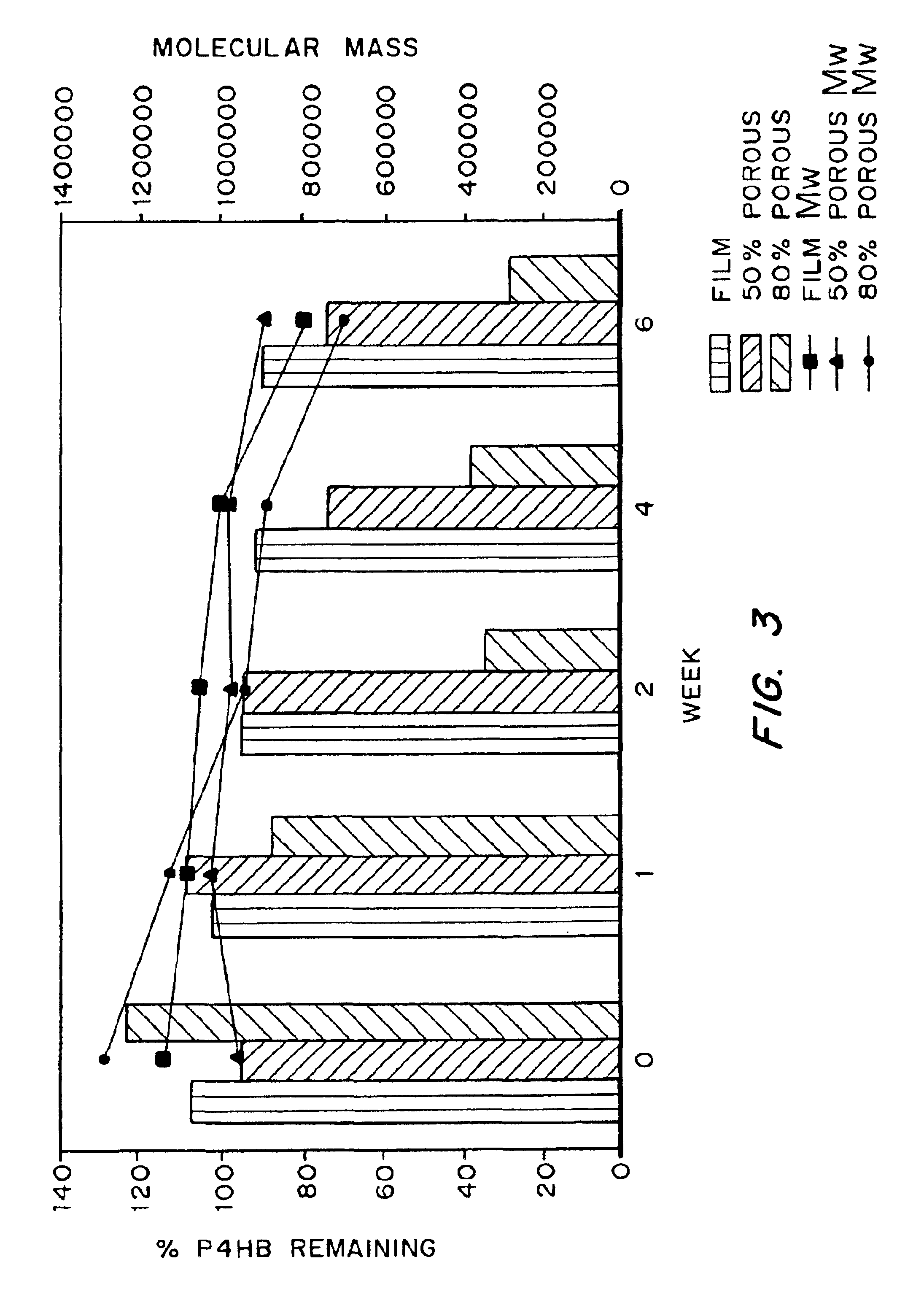
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
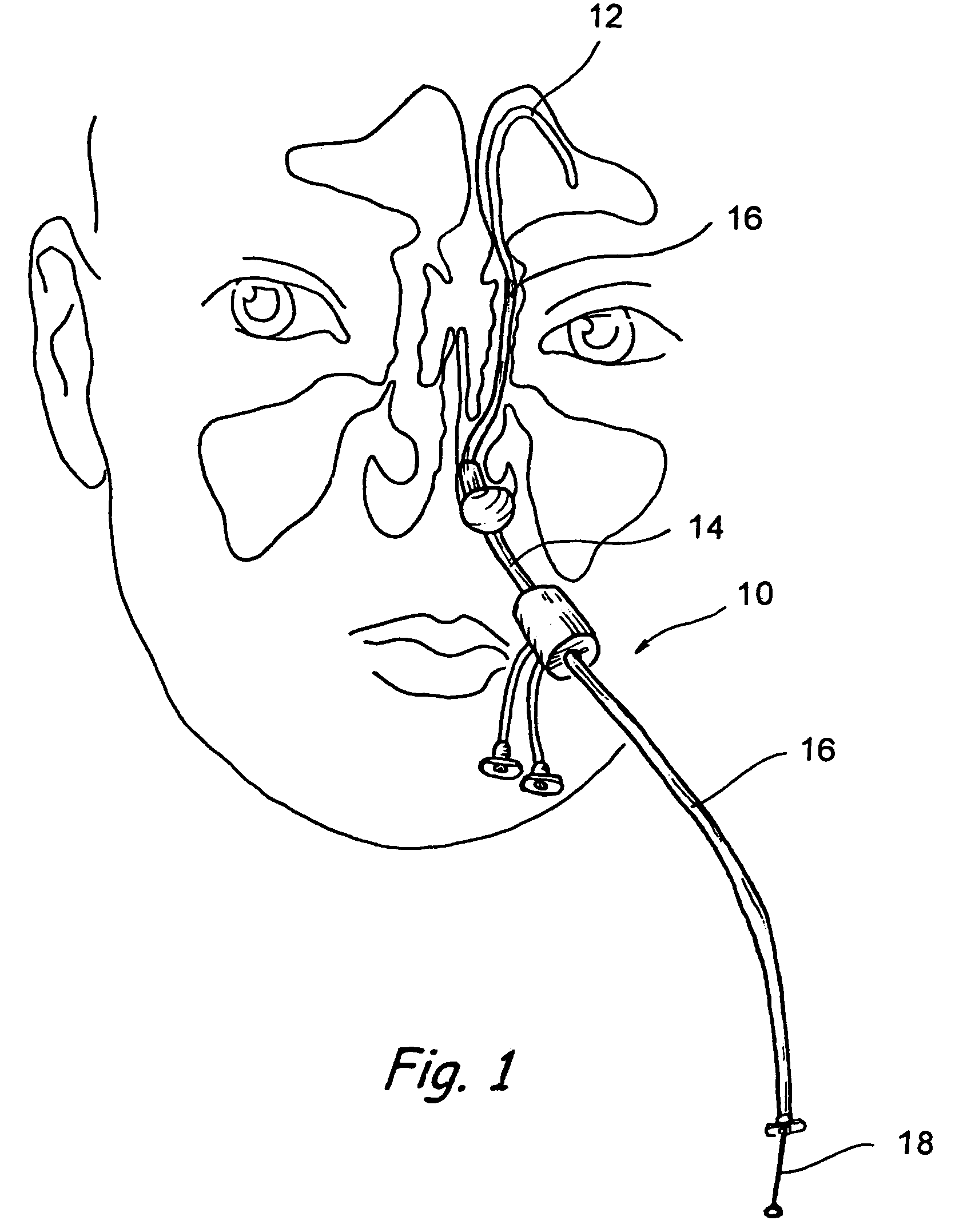
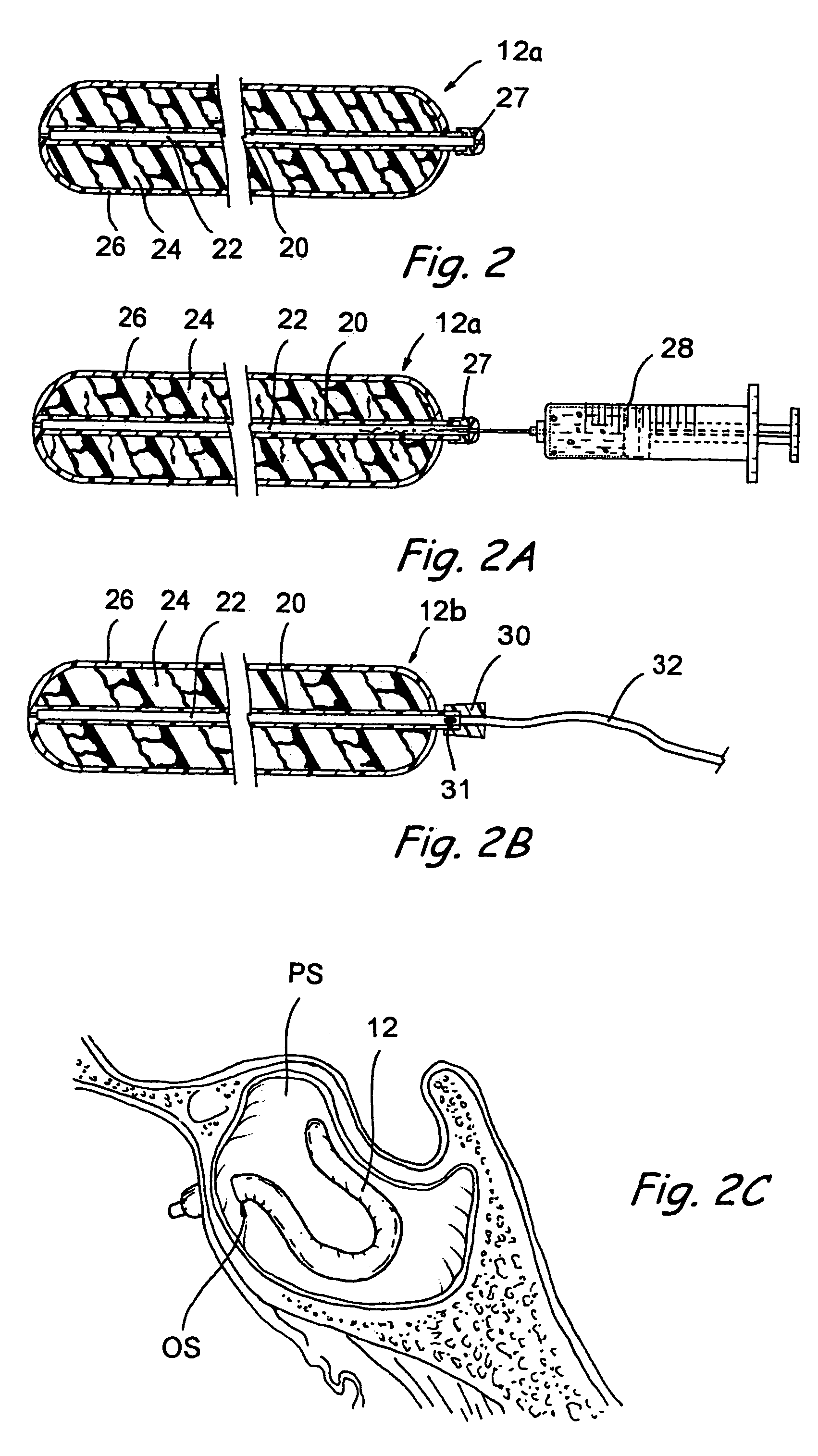
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
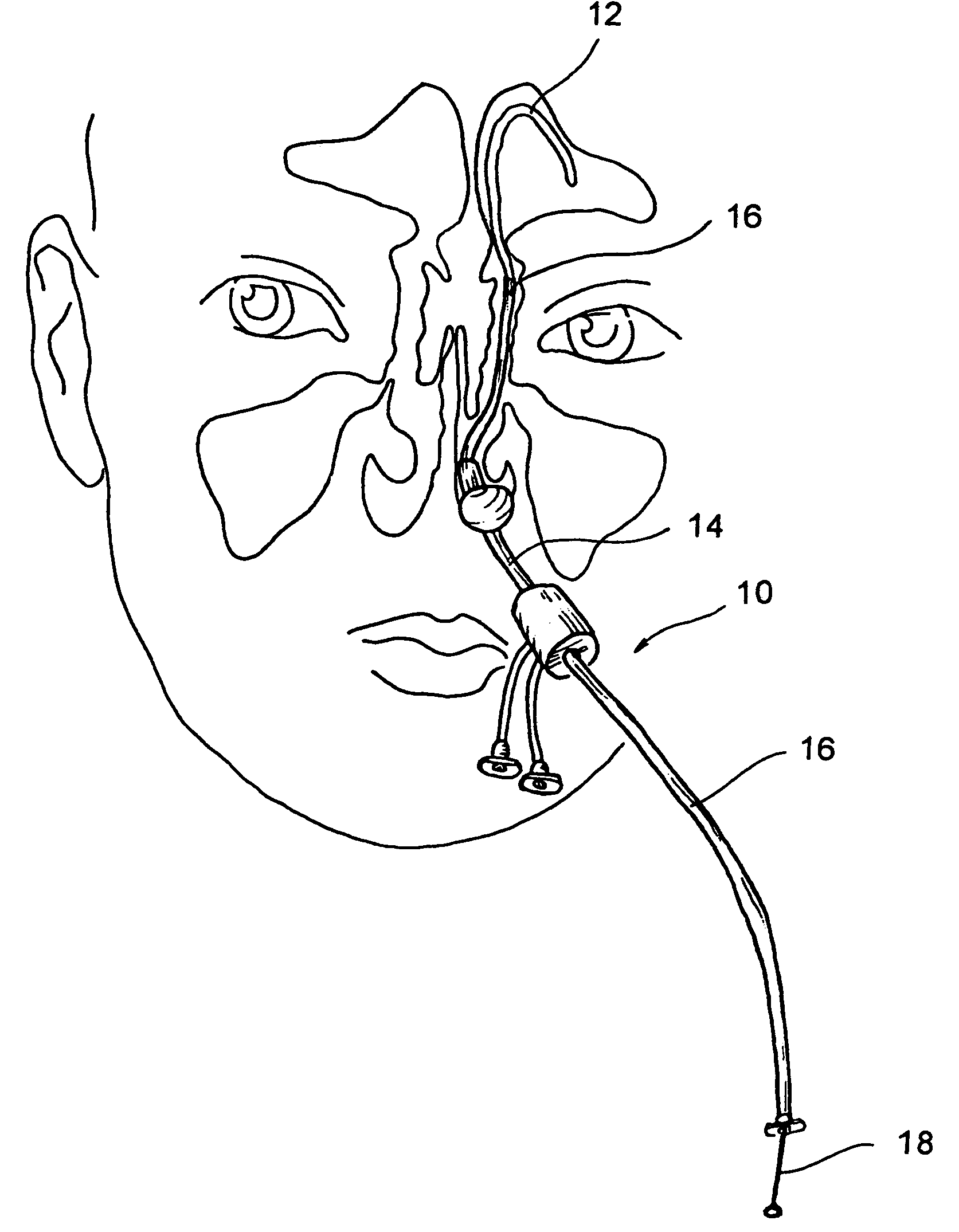
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
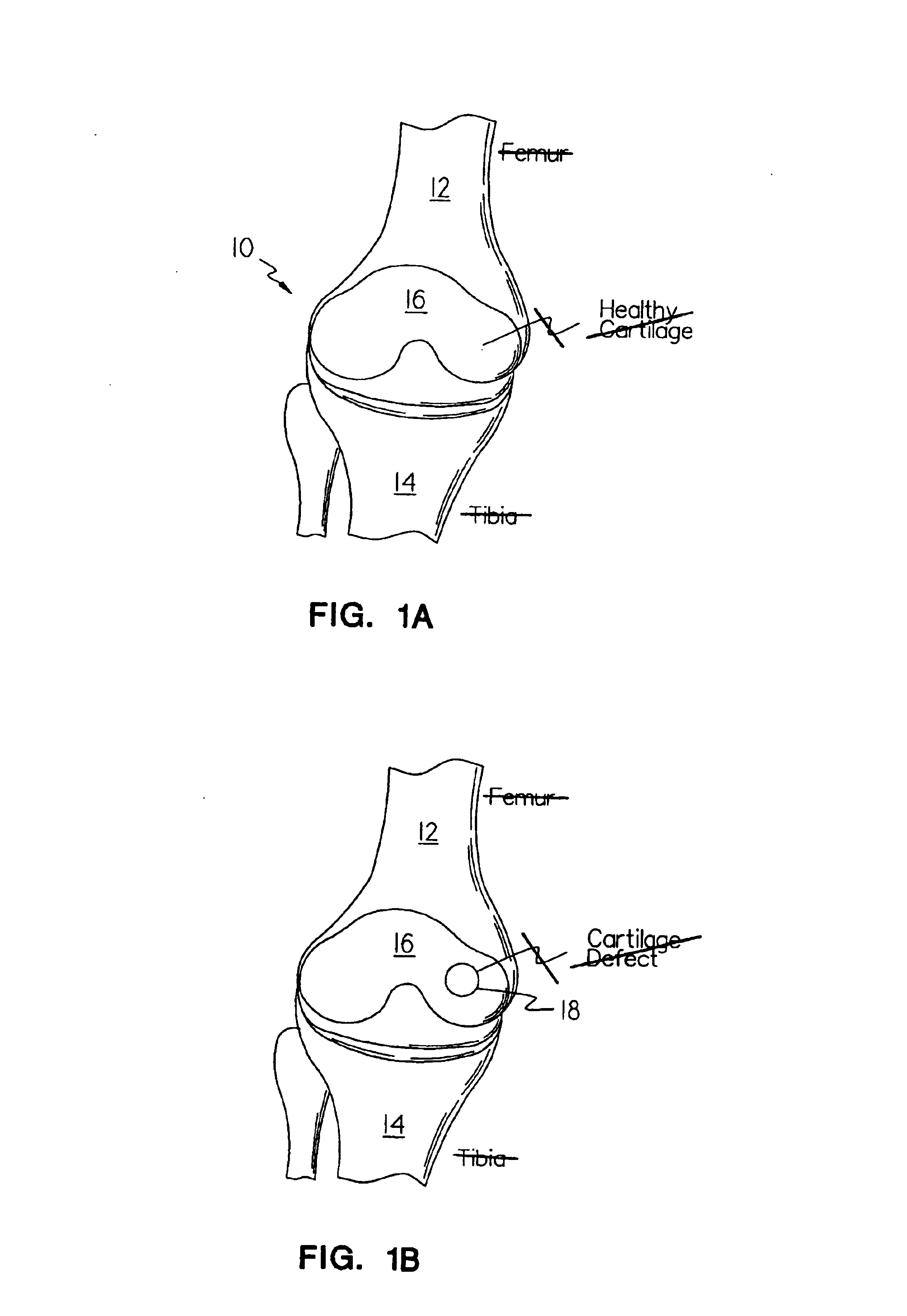
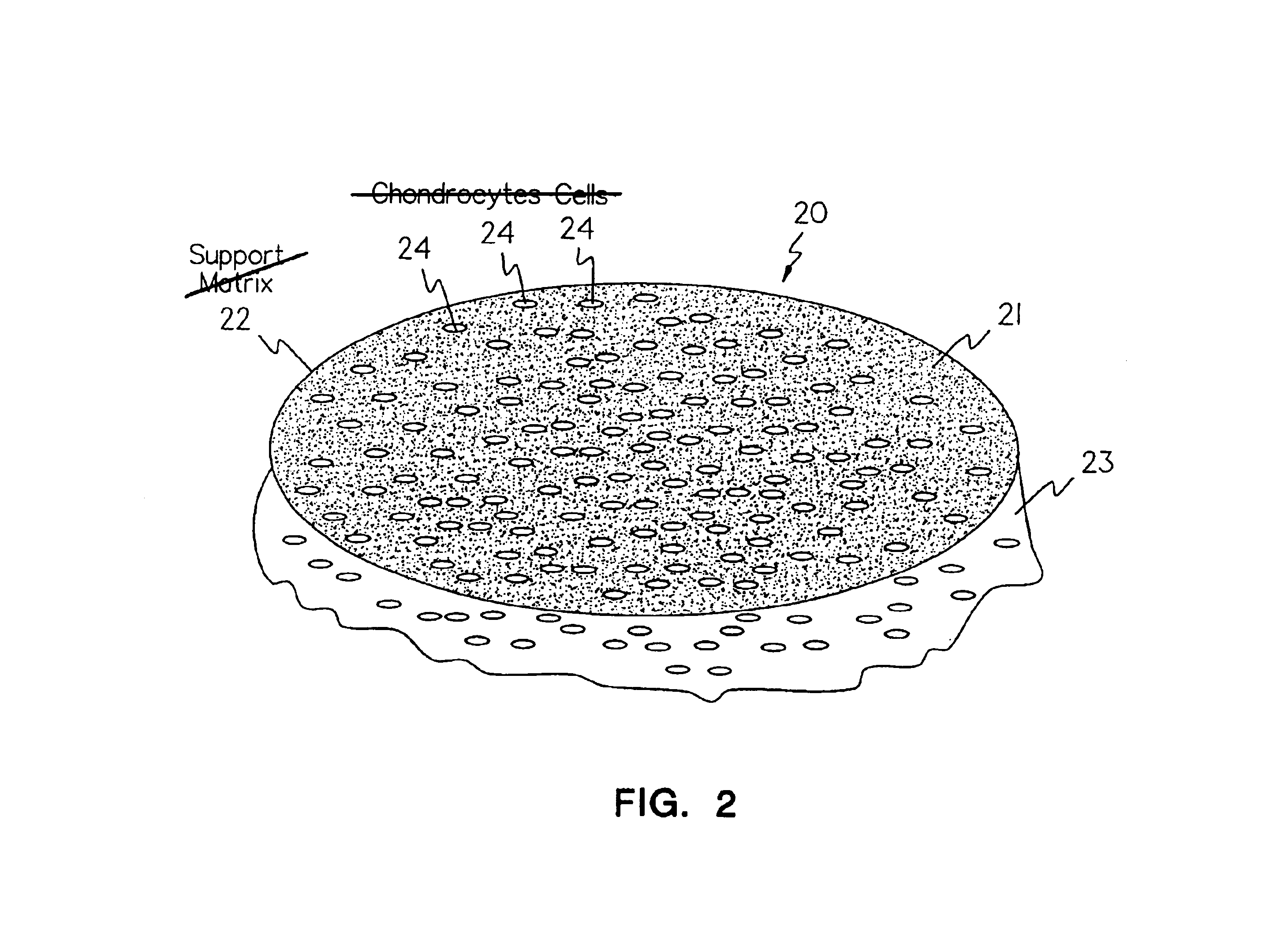
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
Method and apparatus for catheter-based annuloplasty
Owner:EDWARDS LIFESCIENCES CORP
Personal fit medical implants and orthopedic surgical instruments and methods for making
InactiveUS20070118243A1Minimizing Ni toxicityImprove visualizationElectrotherapyMechanical/radiation/invasive therapiesPersonalizationManufacturing technology
The present invention provides methods, techniques, materials and devices and uses thereof for custom-fitting biocompatible implants, prosthetics and interventional tools for use on medical and veterinary applications. The devices produced according to the invention are created using additive manufacturing techniques based on a computer generated model such that every prosthesis or interventional device is personalized for the user having the appropriate metallic alloy composition and virtual validation of functional design for each use.
Owner:VANTUS TECH CORP
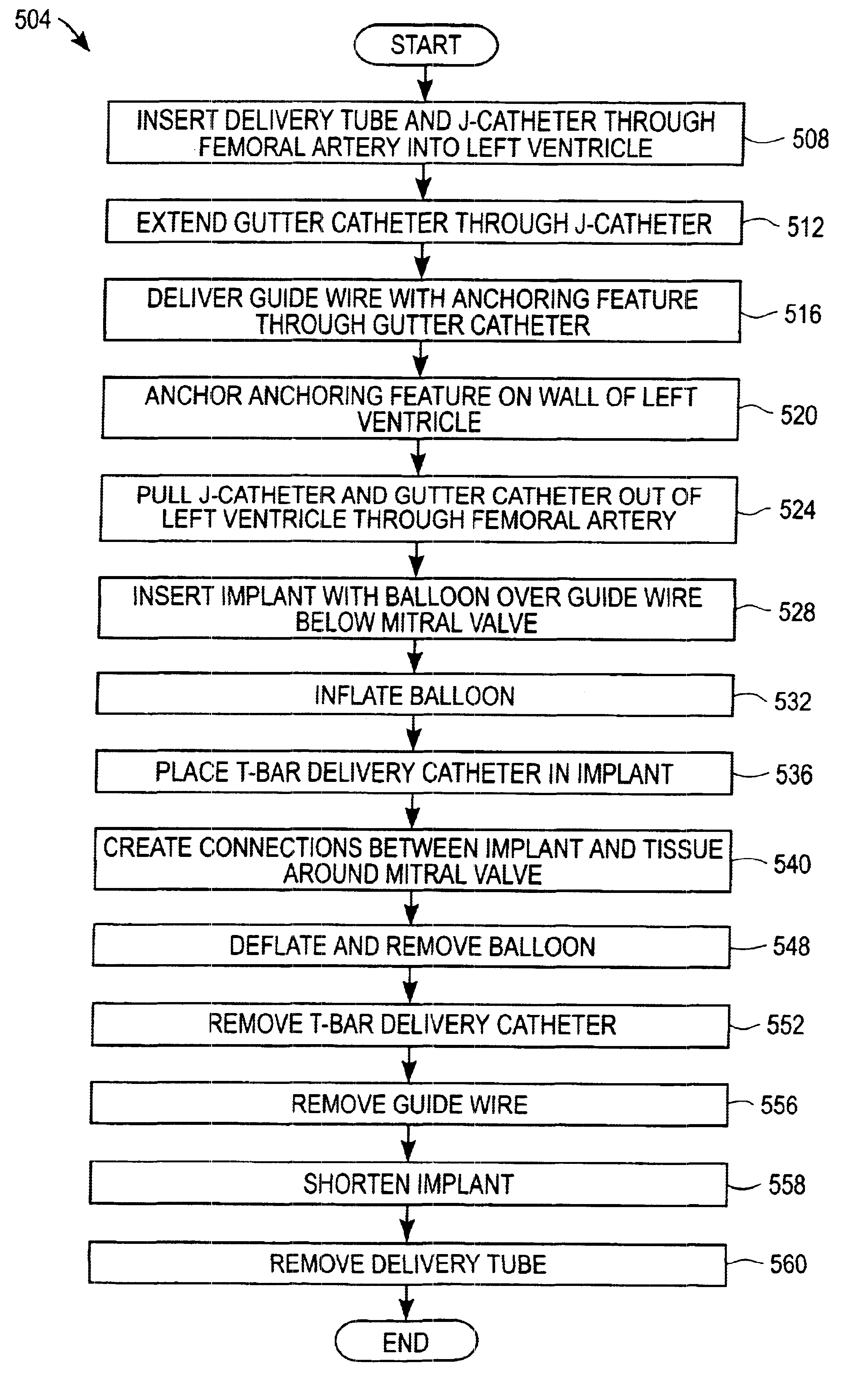
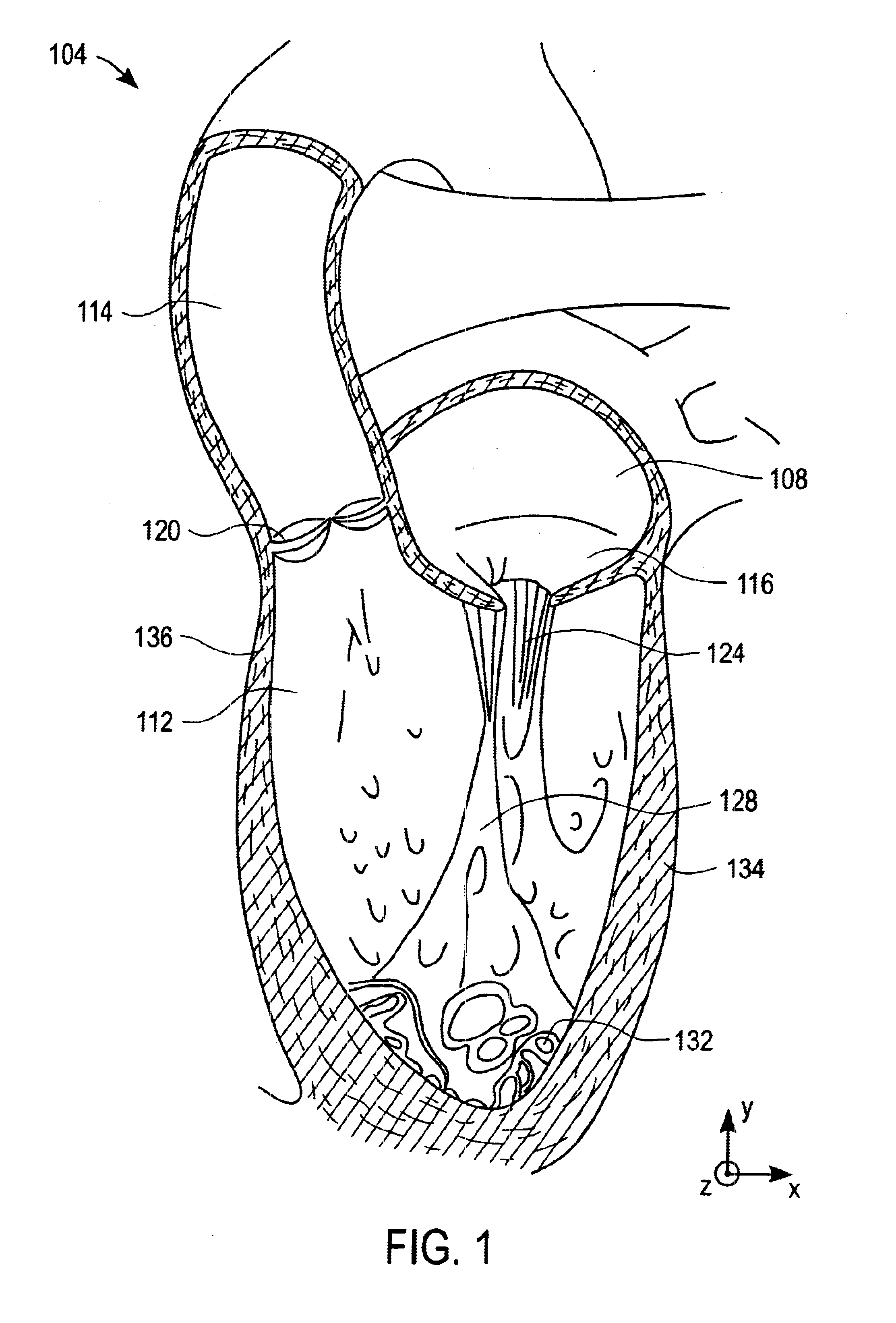
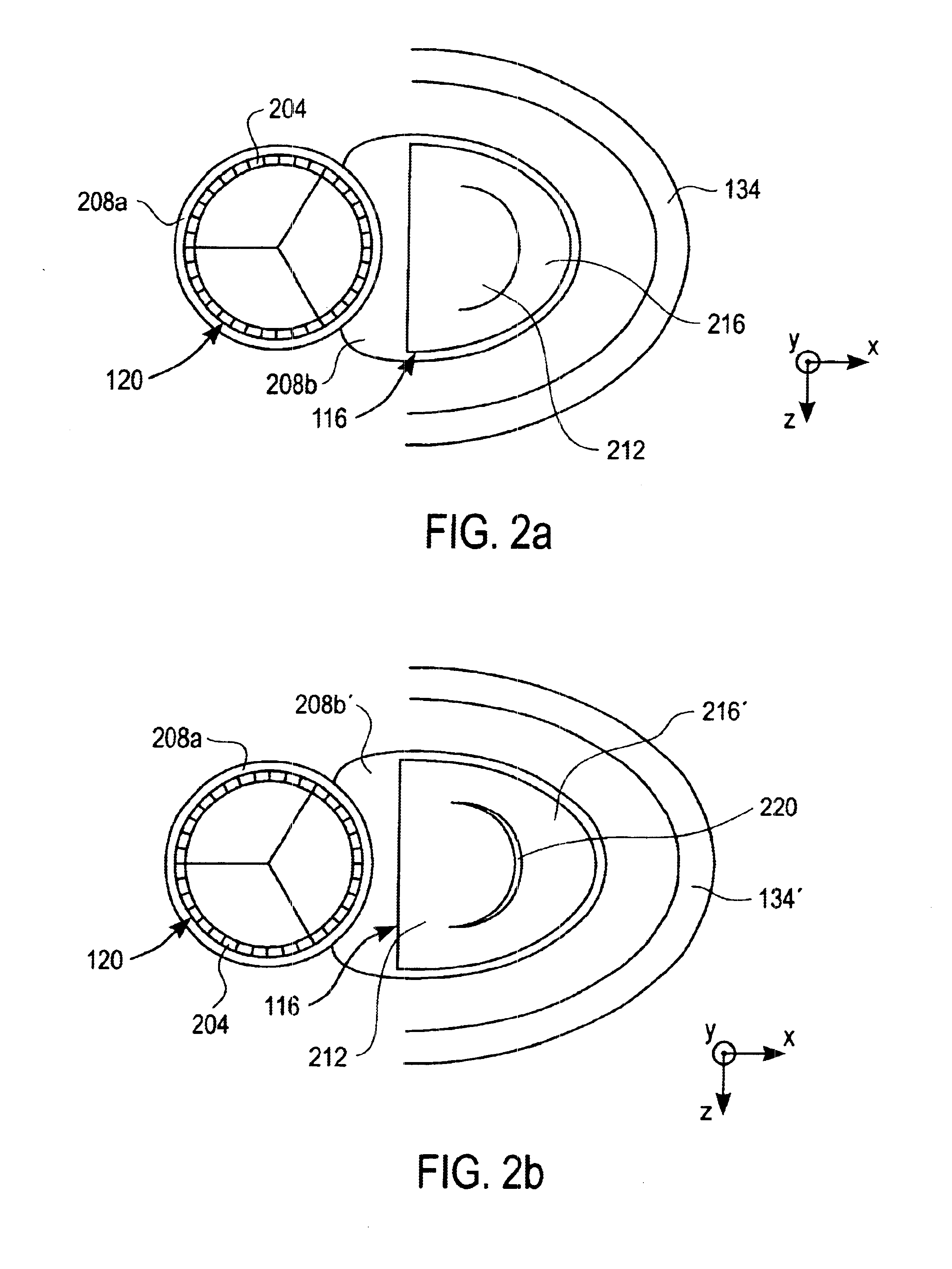
Devices, systems, and methods for reshaping a heart valve annulus
ActiveUS20050055089A1Improve septal-to-lateral dimensionImproved leaflet coaptionSuture equipmentsAnnuloplasty ringsMitral valve leafletLeft atrium
Implants or systems of implants apply a selected force vector or a selected combination of force vectors within or across the left atrium, which allow mitral valve leaflets to better coapt. The implants or systems of implants make possible rapid deployment, facile endovascular delivery, and full intra-atrial retrievability. The implants or systems of implants also make use of strong fluoroscopic landmarks.
Owner:VENTURE LENDING & LEASING IV
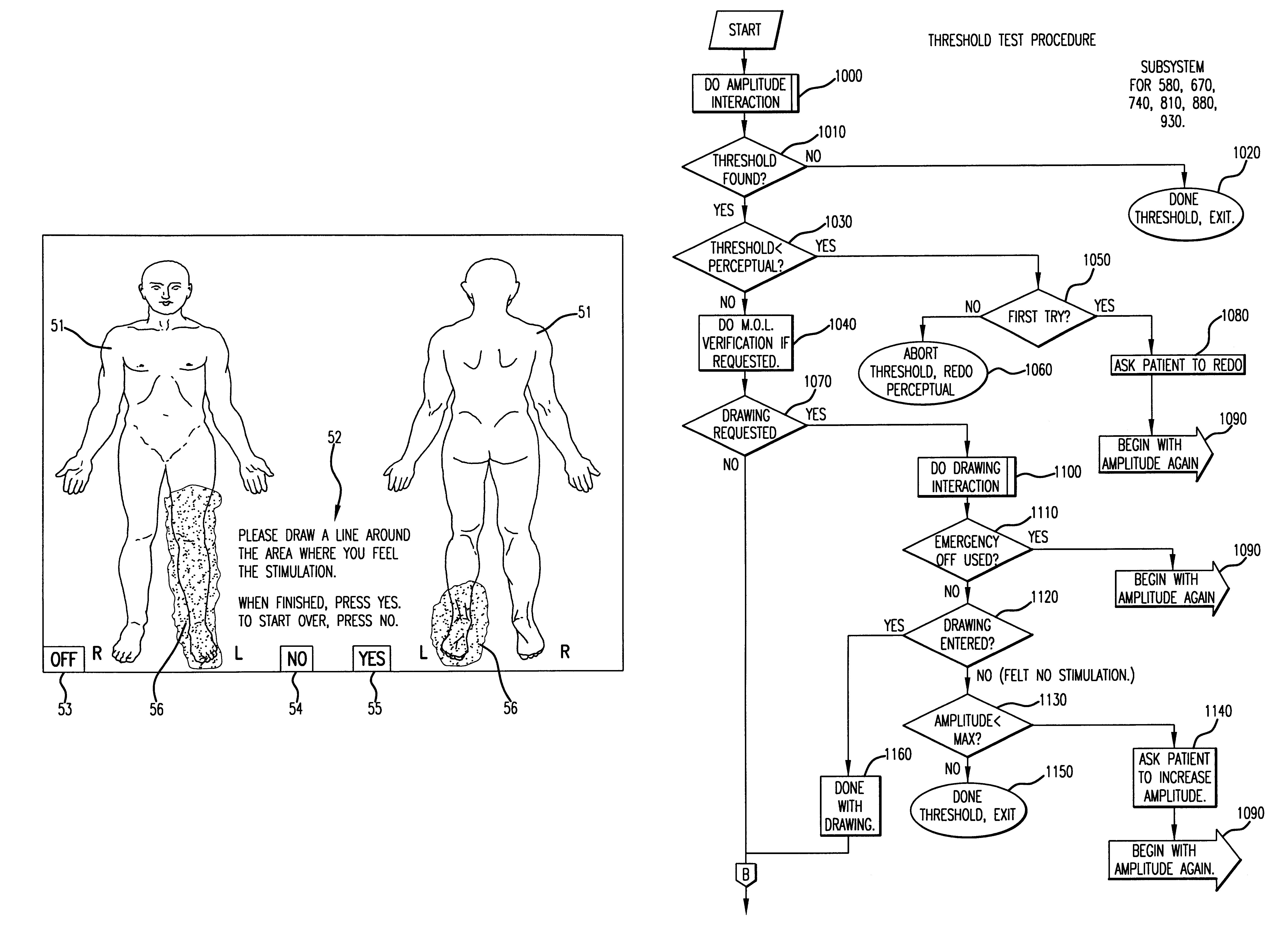
Patient interactive neurostimulation system and method
The present invention is a fully automated computer controlled system for adjustment of neurostimulation implants used in pain therapy and in treating neurological dysfunction which includes a patient interactive computer, and a universal transmitter interface integrally embedded in the patient interactive computer, or built into the antenna which is capable of stimulating any type of implanted neurostimulation devices by imitating any of the proprietary programming codes. The patient interacts with the system through the patient interactive computer (containing a unique software) which provides for consistency check of the data entered by the patient, deleting the entered data if the consistency check was not satisfactory, and requesting the patient to re-enter the data.
Owner:MEDTRONIC INC
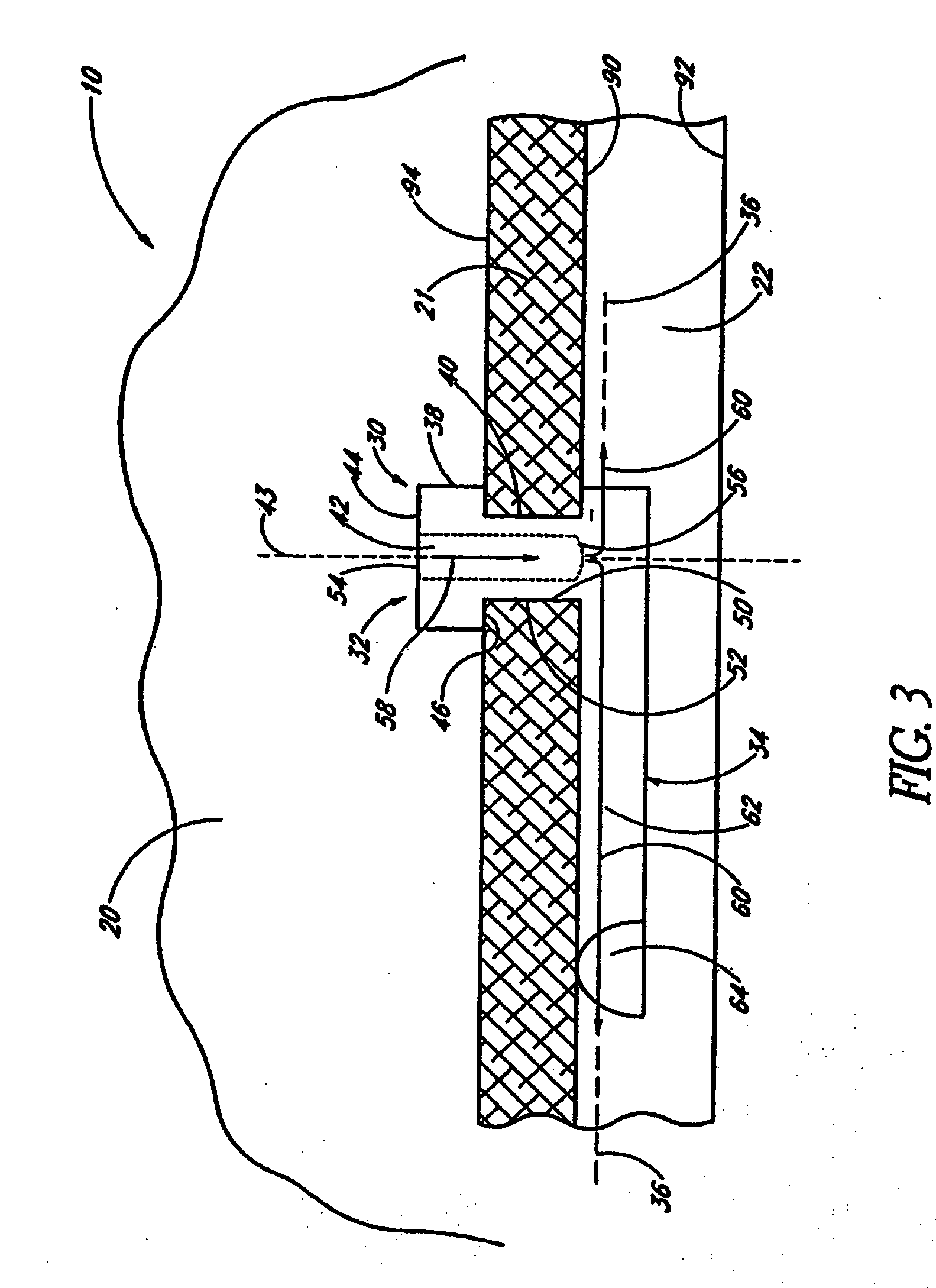
Devices, systems and methods useable for treating sinusitis
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Instruments and method for inserting an intervertebral implant
InactiveUS20050021042A1Implant stabilityDifferent sizeJoint implantsSpinal implantsBiomedical engineeringImplant
Instruments and methods for inserting an intervertebral implant. Insertion of the implant is accomplished by grasping the implant between the arms of an insertion instrument having arms which separate from each other and close against each other onto the implant, such that the ends of the arms of the insertion instrument engage recesses of the implant. A spacer may be provided between the arms of the insertion instrument to help position the implant while held by the arms.
Owner:CENTINEL SPINE LLC
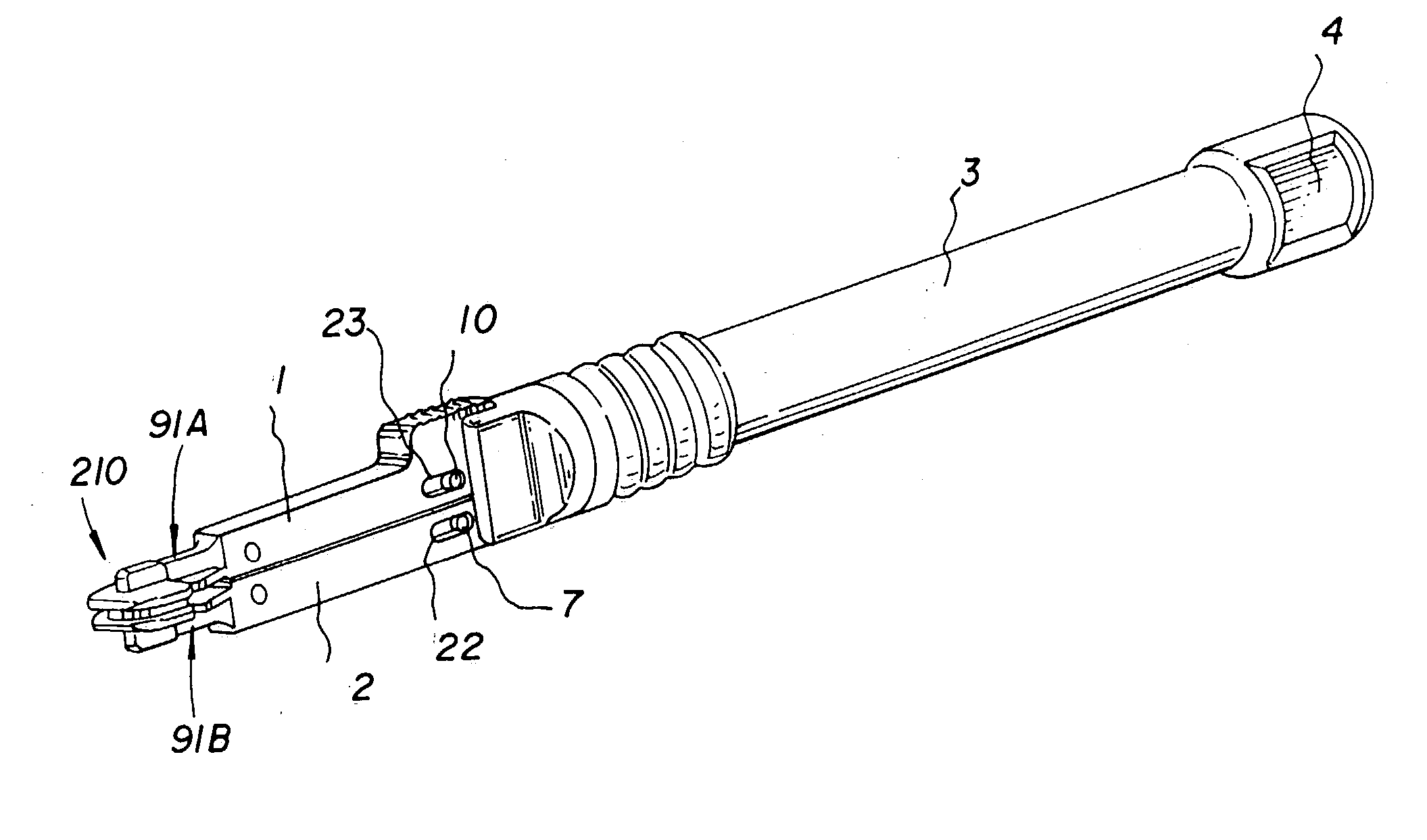
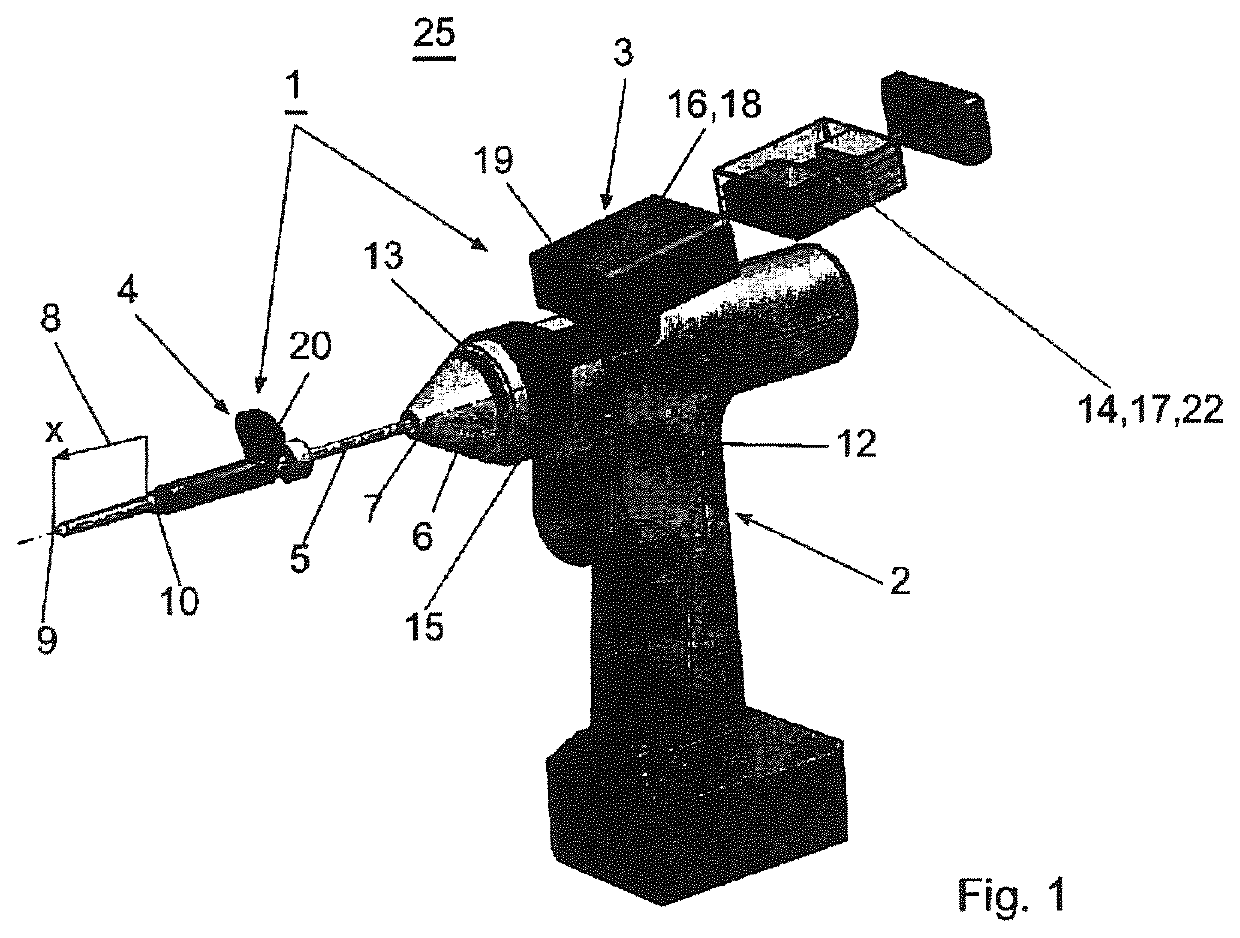
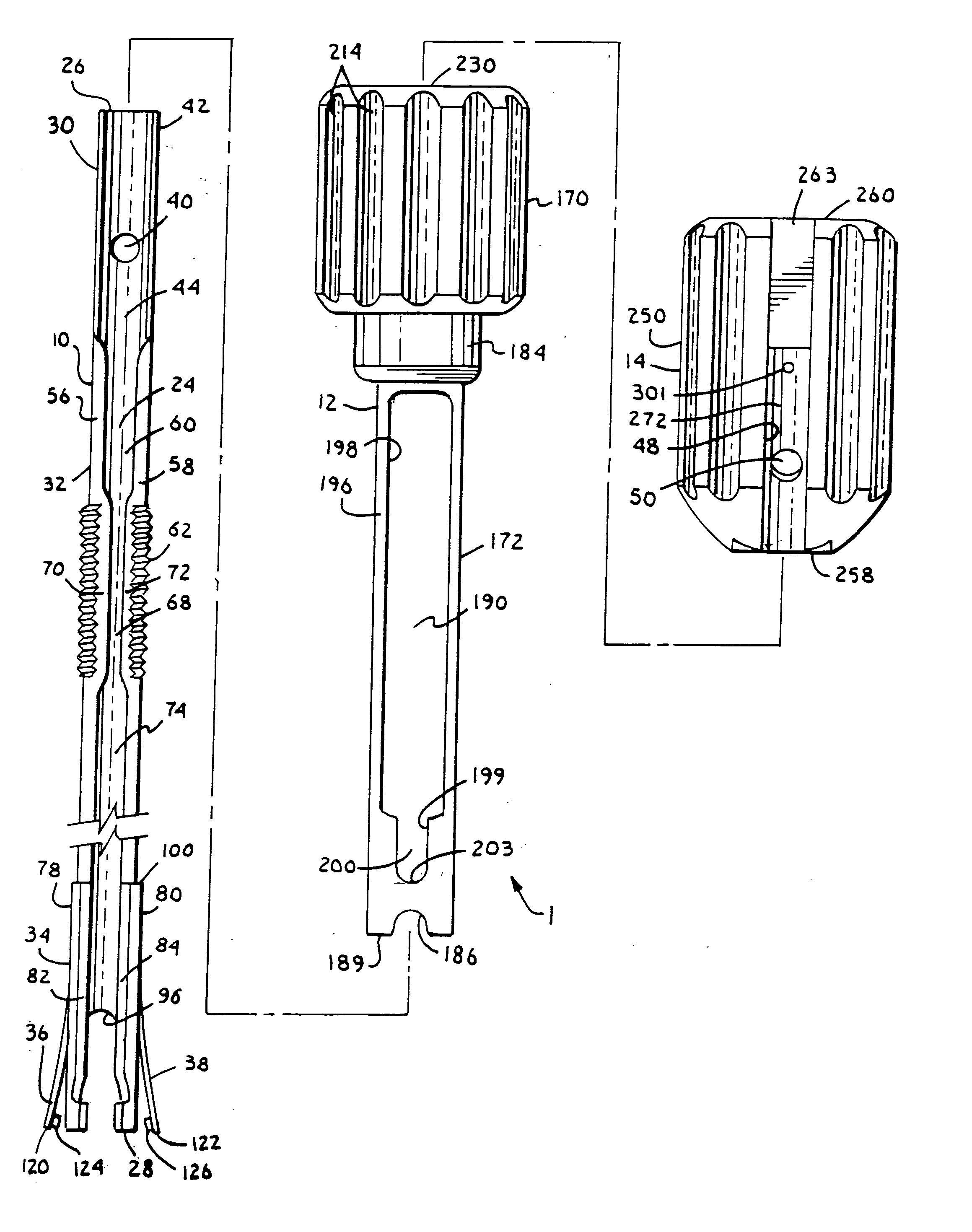
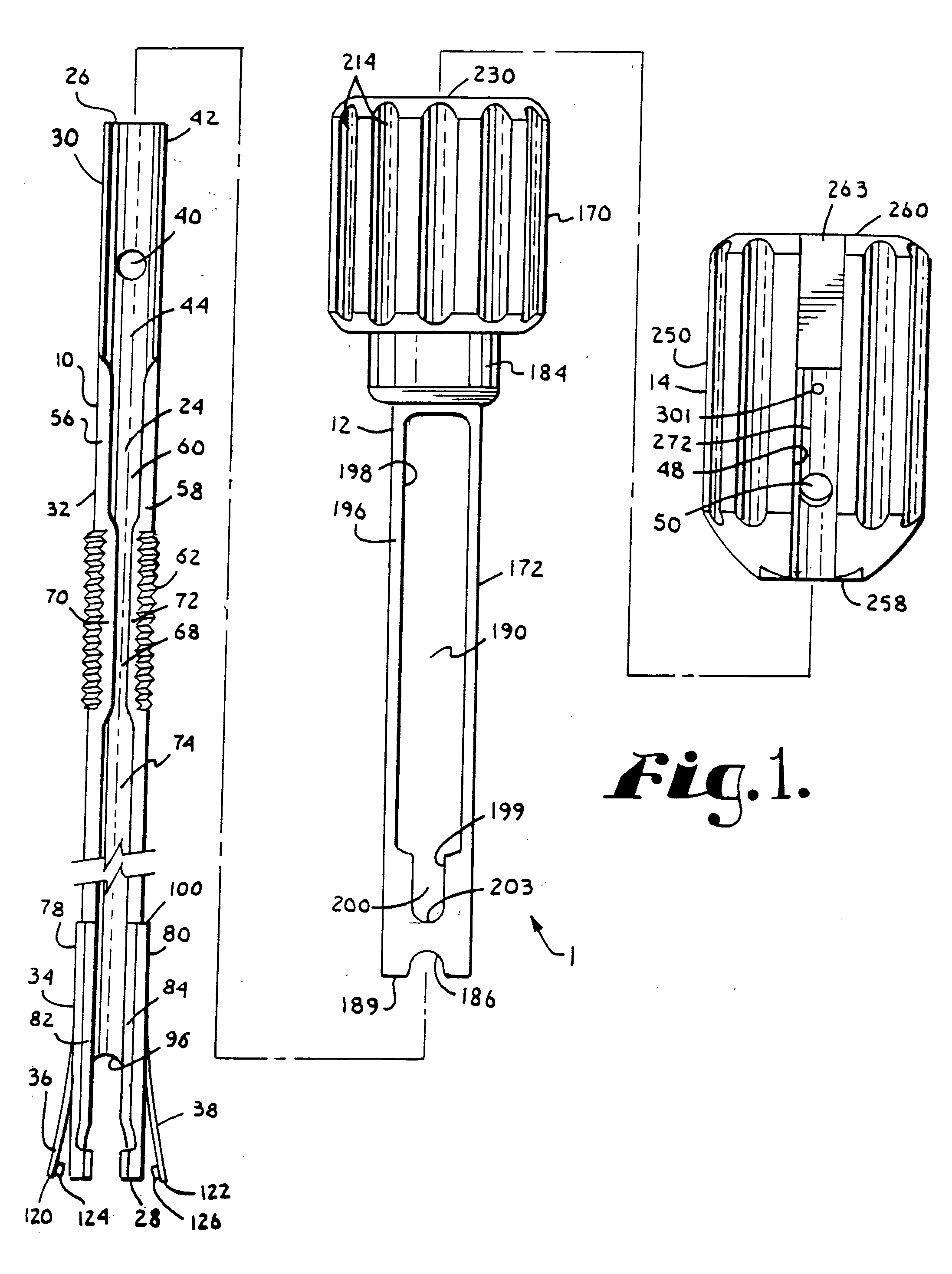
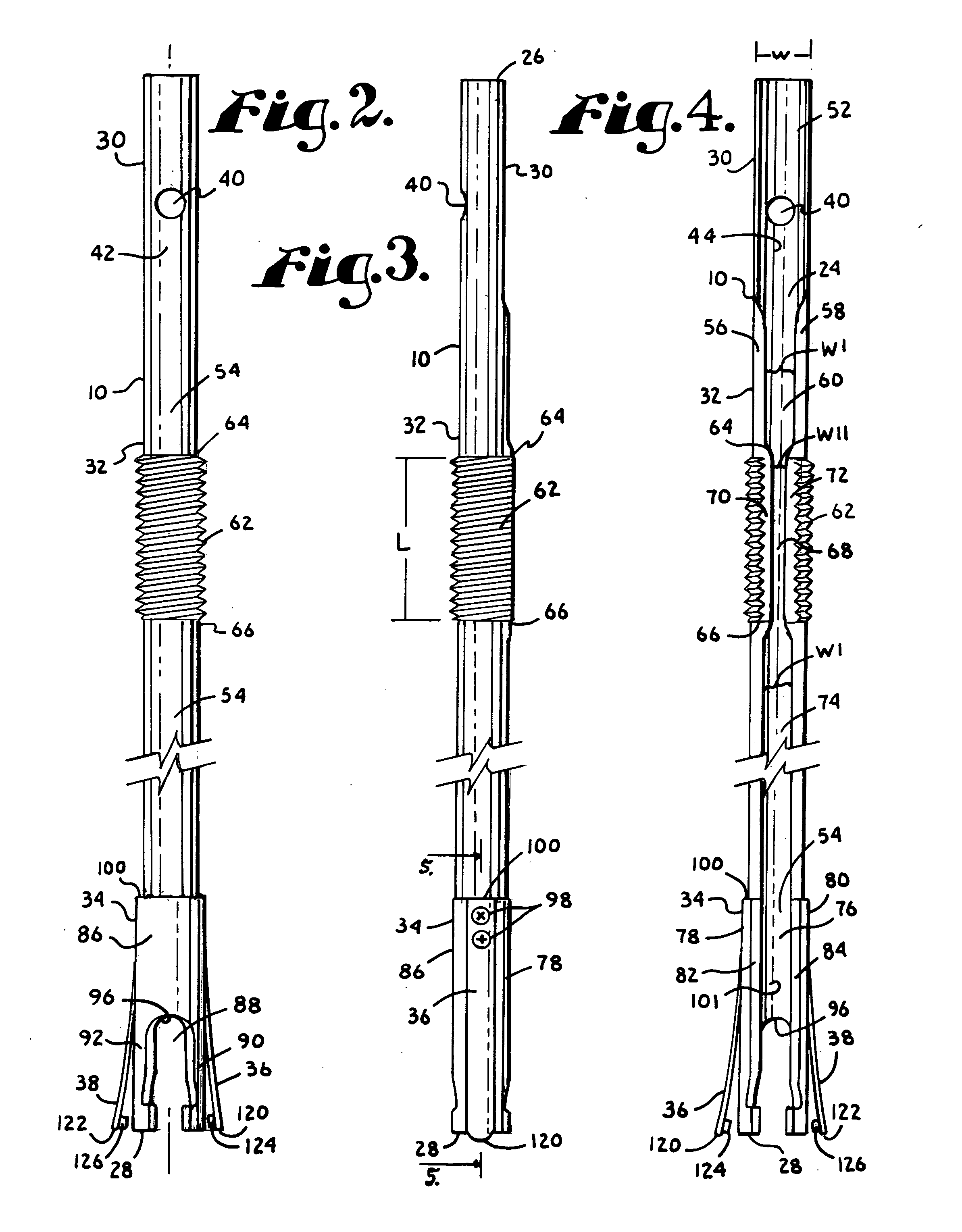
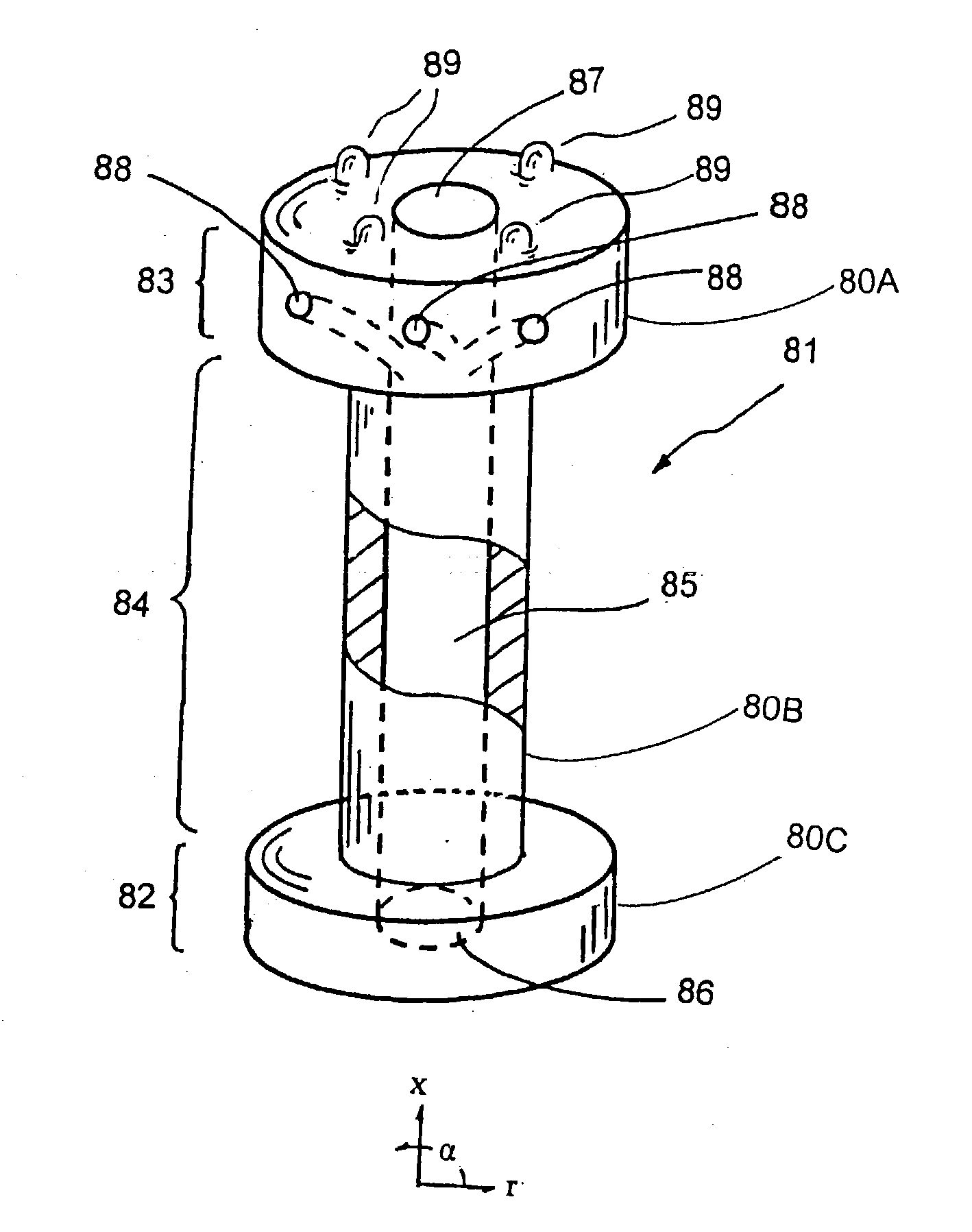
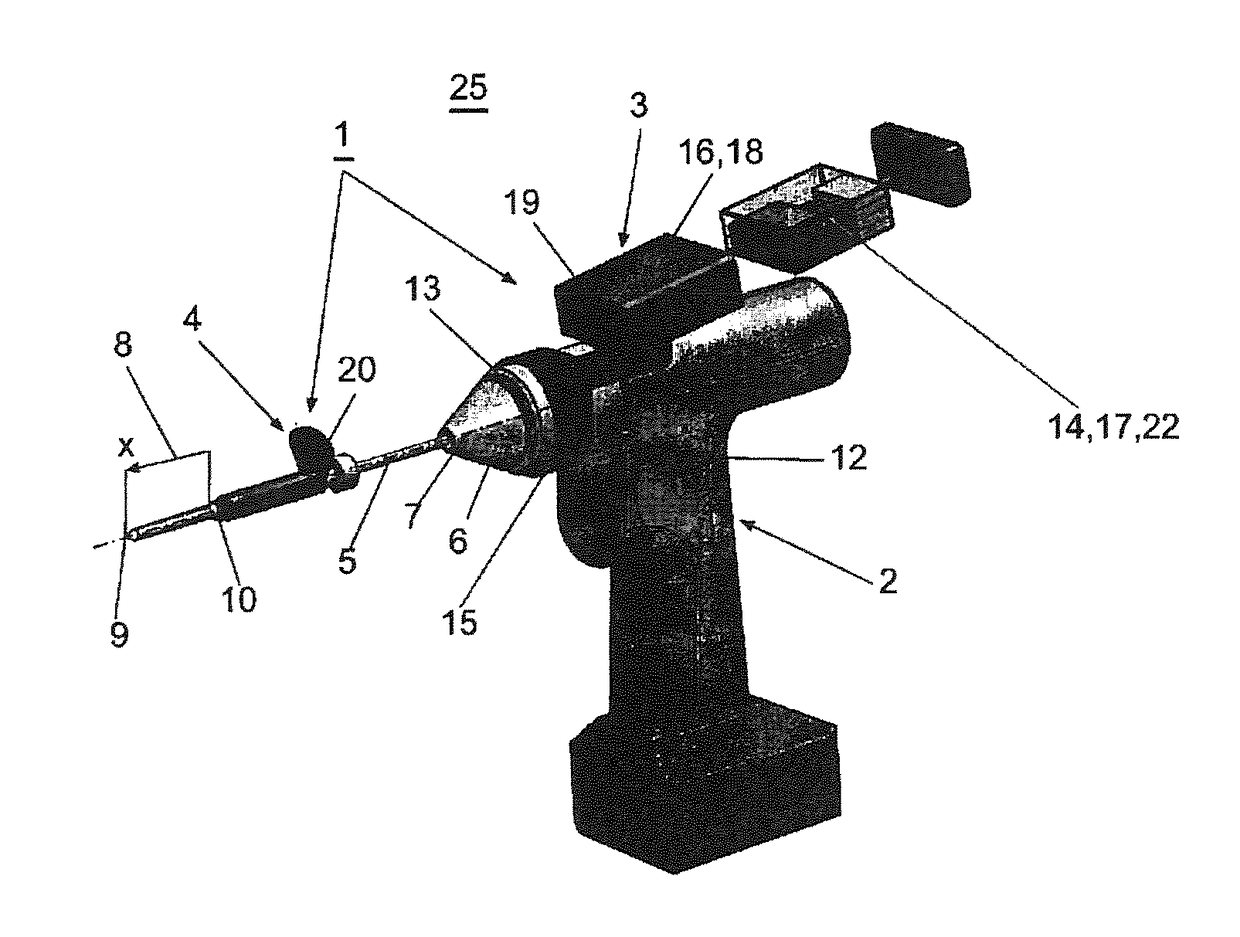
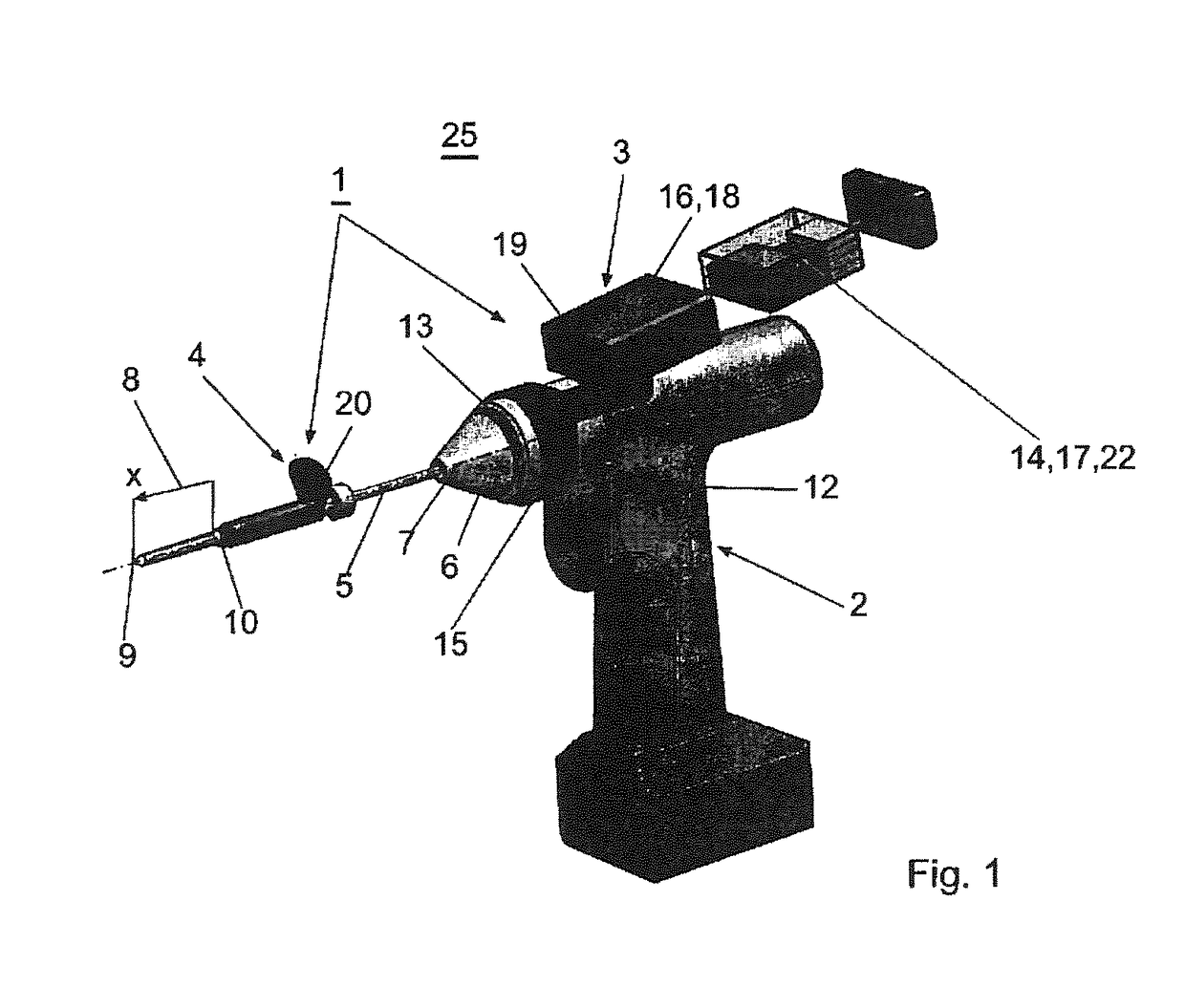
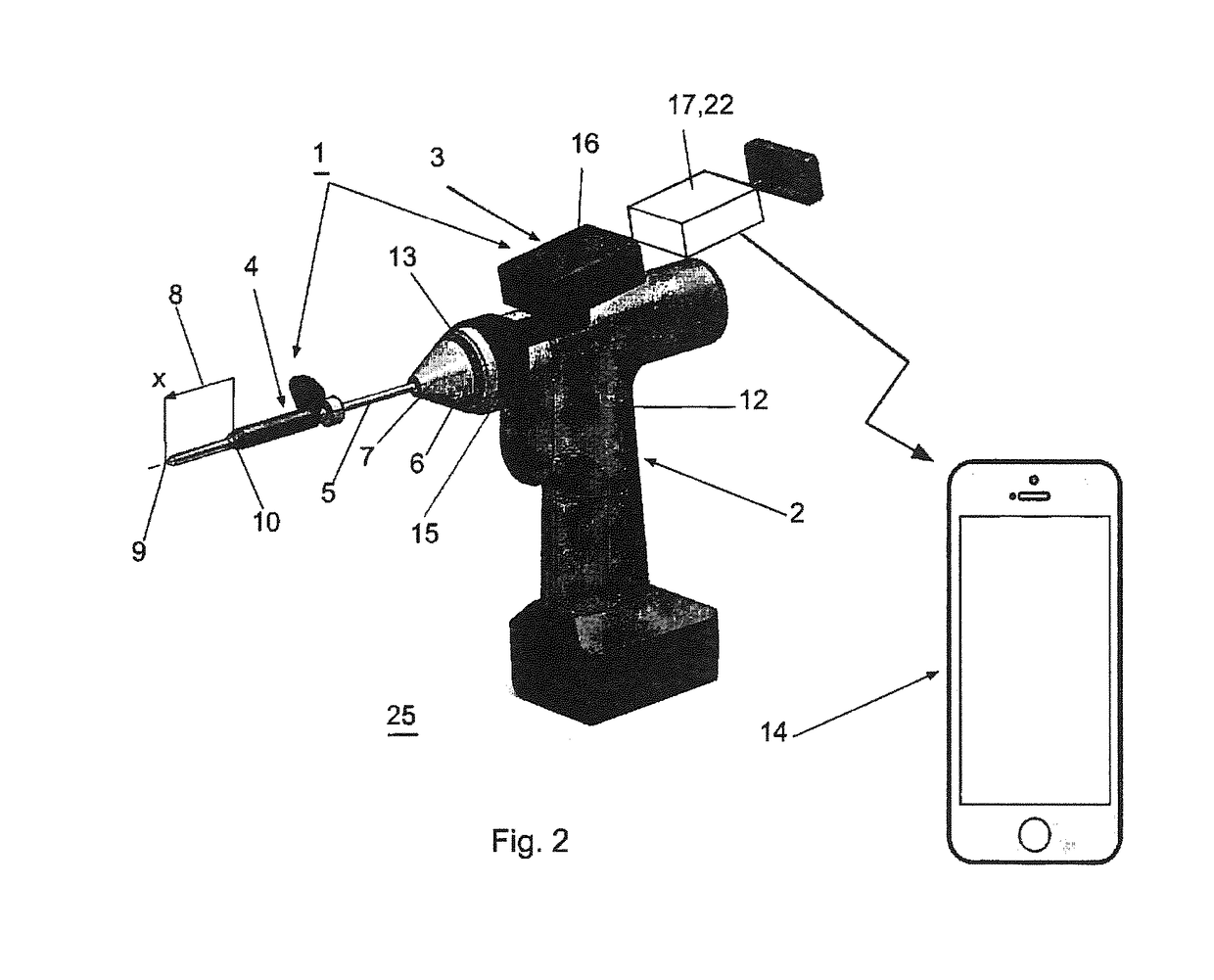
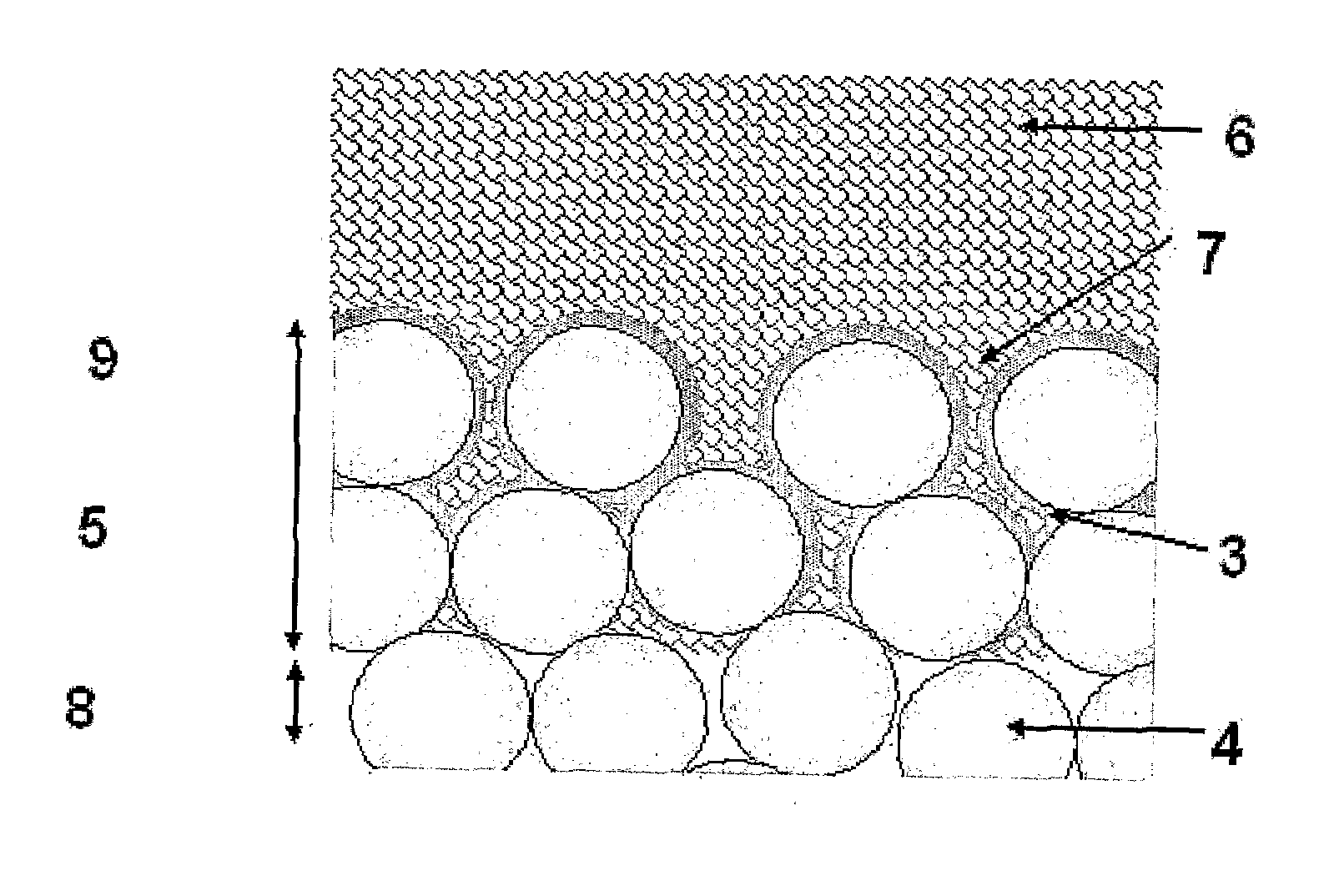
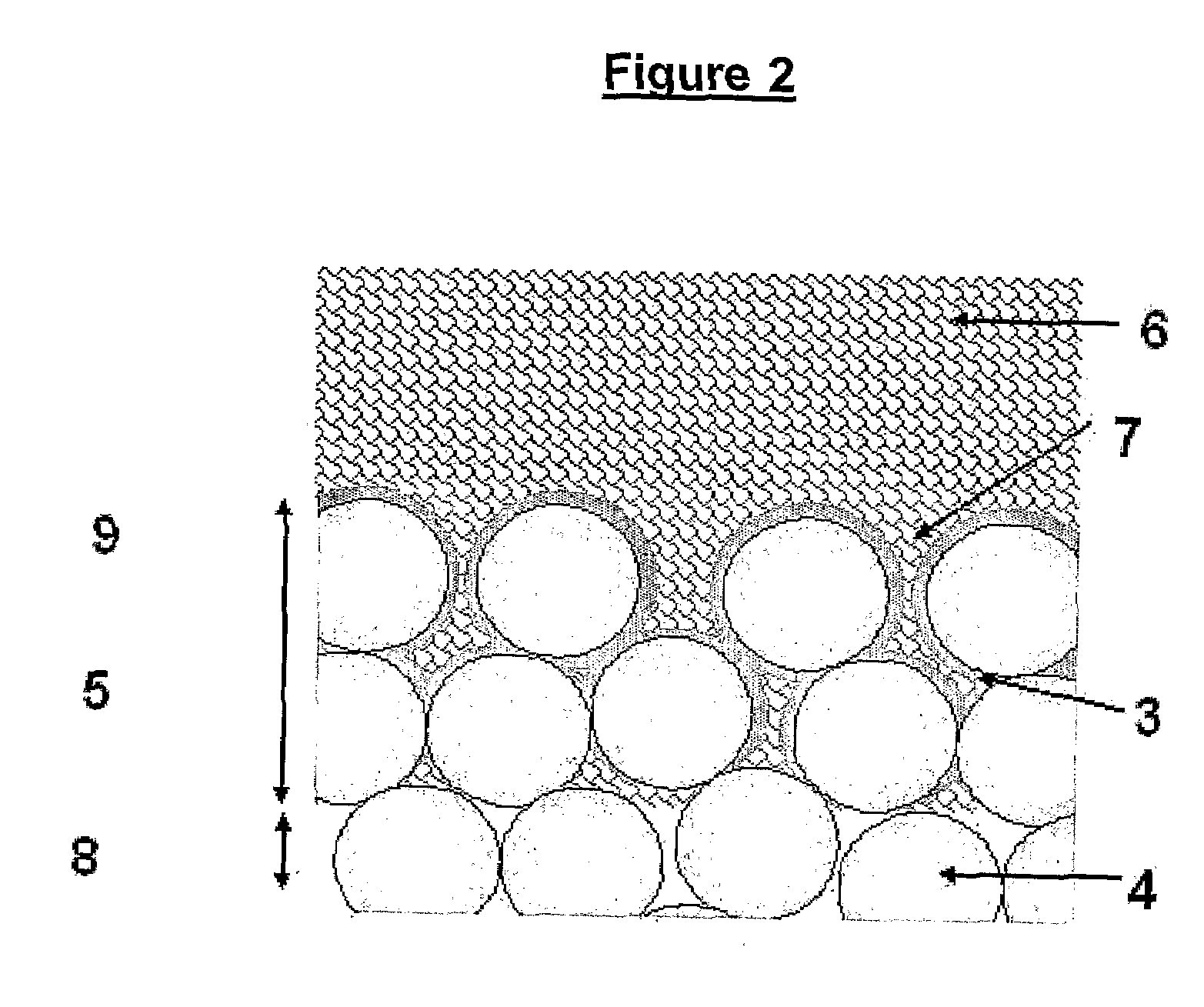
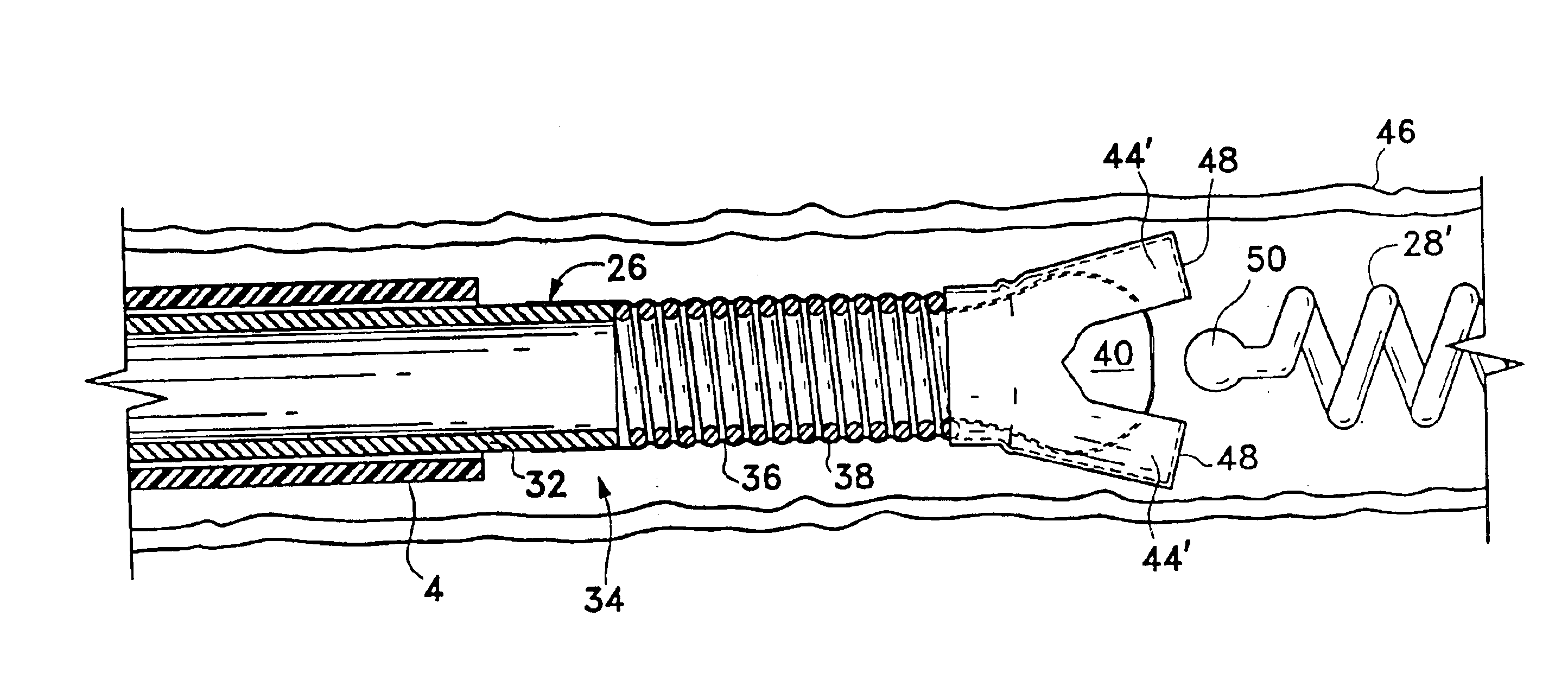
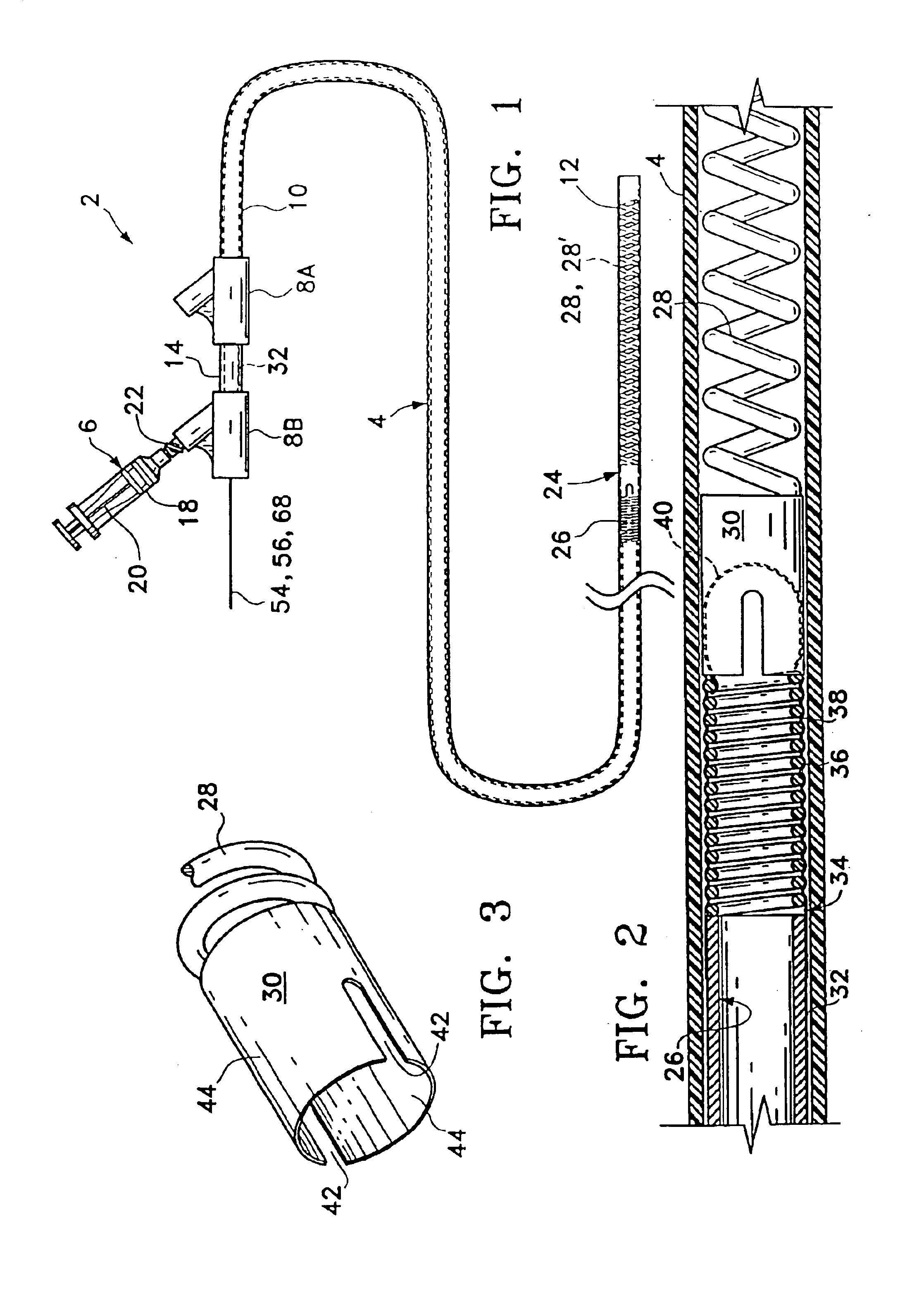
Surgical power drill including a measuring unit suitable for bone screw length determination
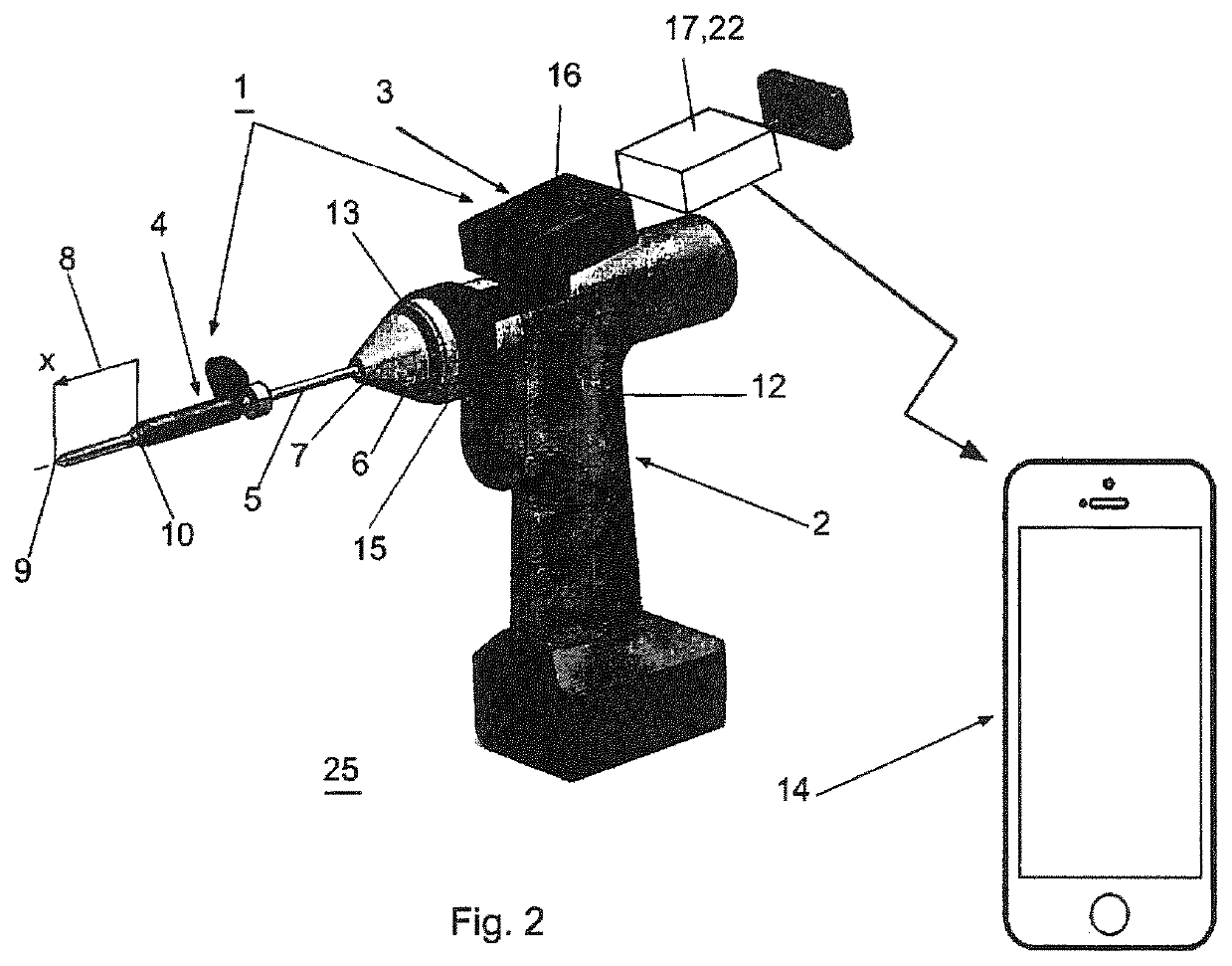
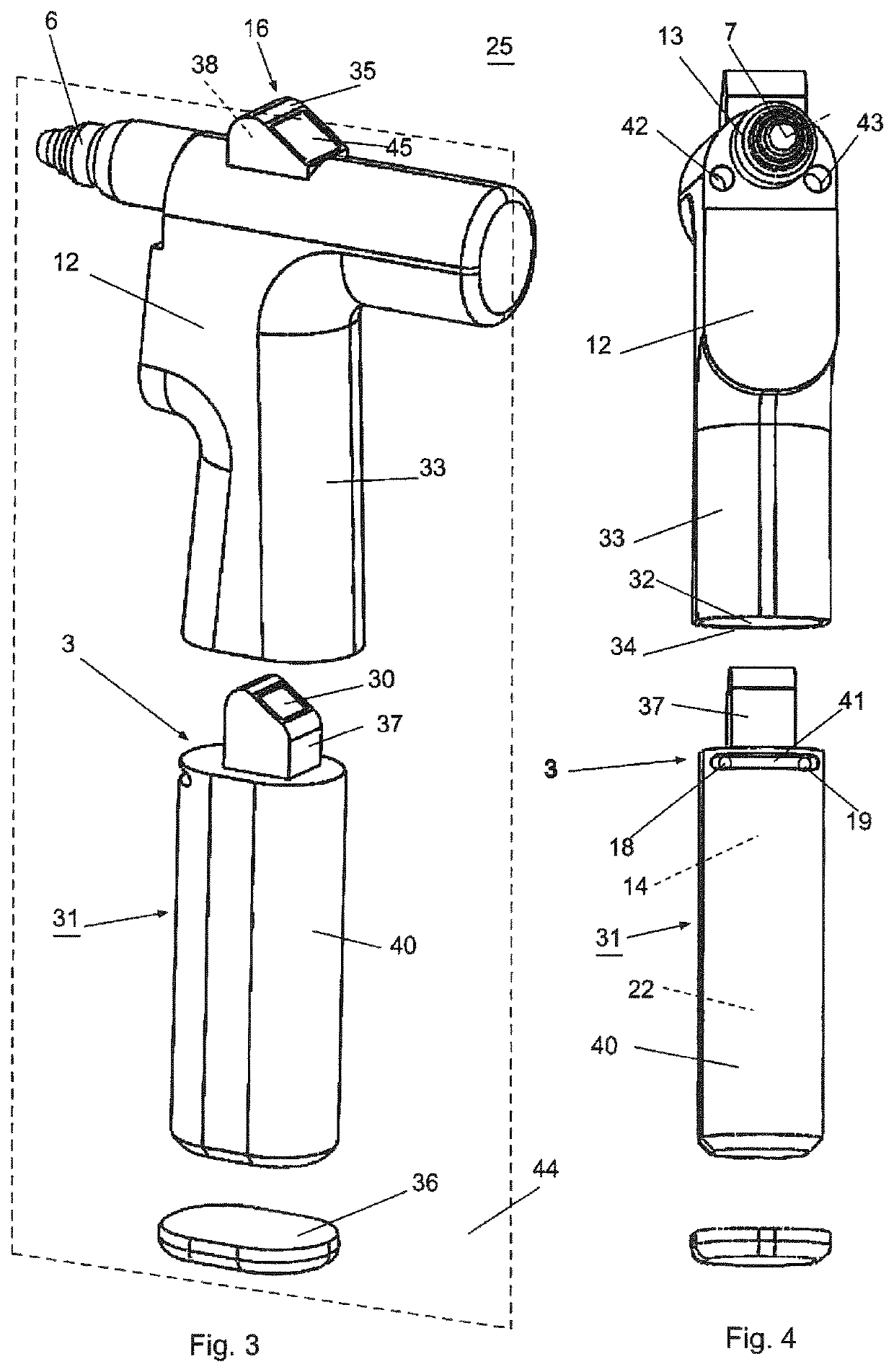
A device (25) for drilling holes in bone and configured to determine bone screw length, the device (25) including a surgical power drill (2) comprising: a) a housing (12) and; b) a measuring device (1) releasably attached or fixed to the housing (12), wherein the measuring device (1) is configured to measure the distance (x) covered by the housing (12) in the direction of the longitudinal axis (7) and relative to a surface of an implant (26) or a bone during a drilling process, wherein the measuring device (1) comprises a processing unit (14) to record the distance (x) covered with respect to time; the processing unit (14) comprises one or more differentiators to determine at least the first and second derivatives of the distance (x) covered with respect to time; and the processing unit (14) further comprises a peak detector to analyze one or more peaks occurring in the graph of the highest derivative with respect to time, and wherein the measuring device (1) comprises a laser device or an ultrasound position sensor for displacement assessment.
Owner:SYNTHES GMBH
System and method for all-inside suture fixation for implant attachment and soft tissue repair
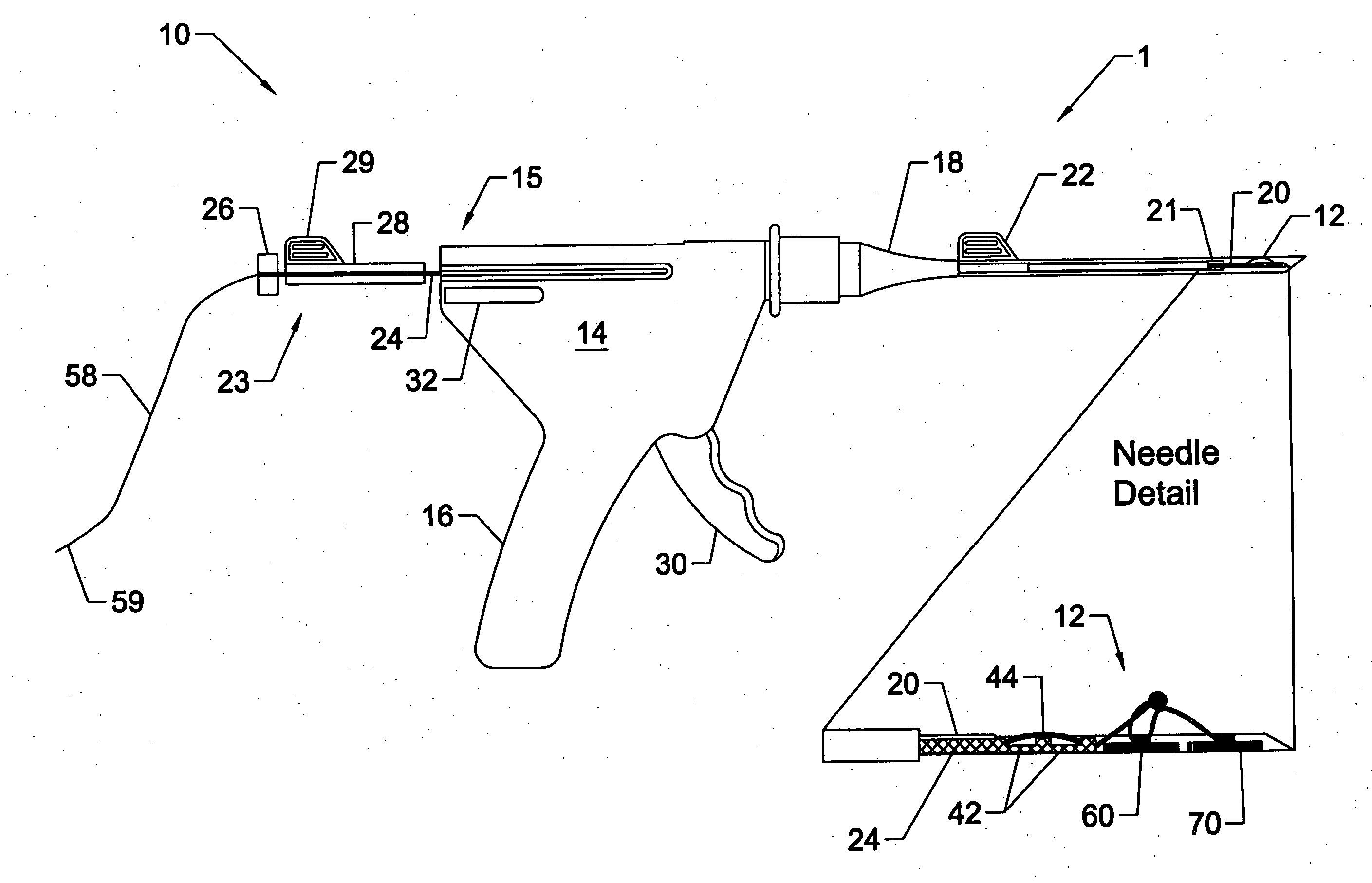
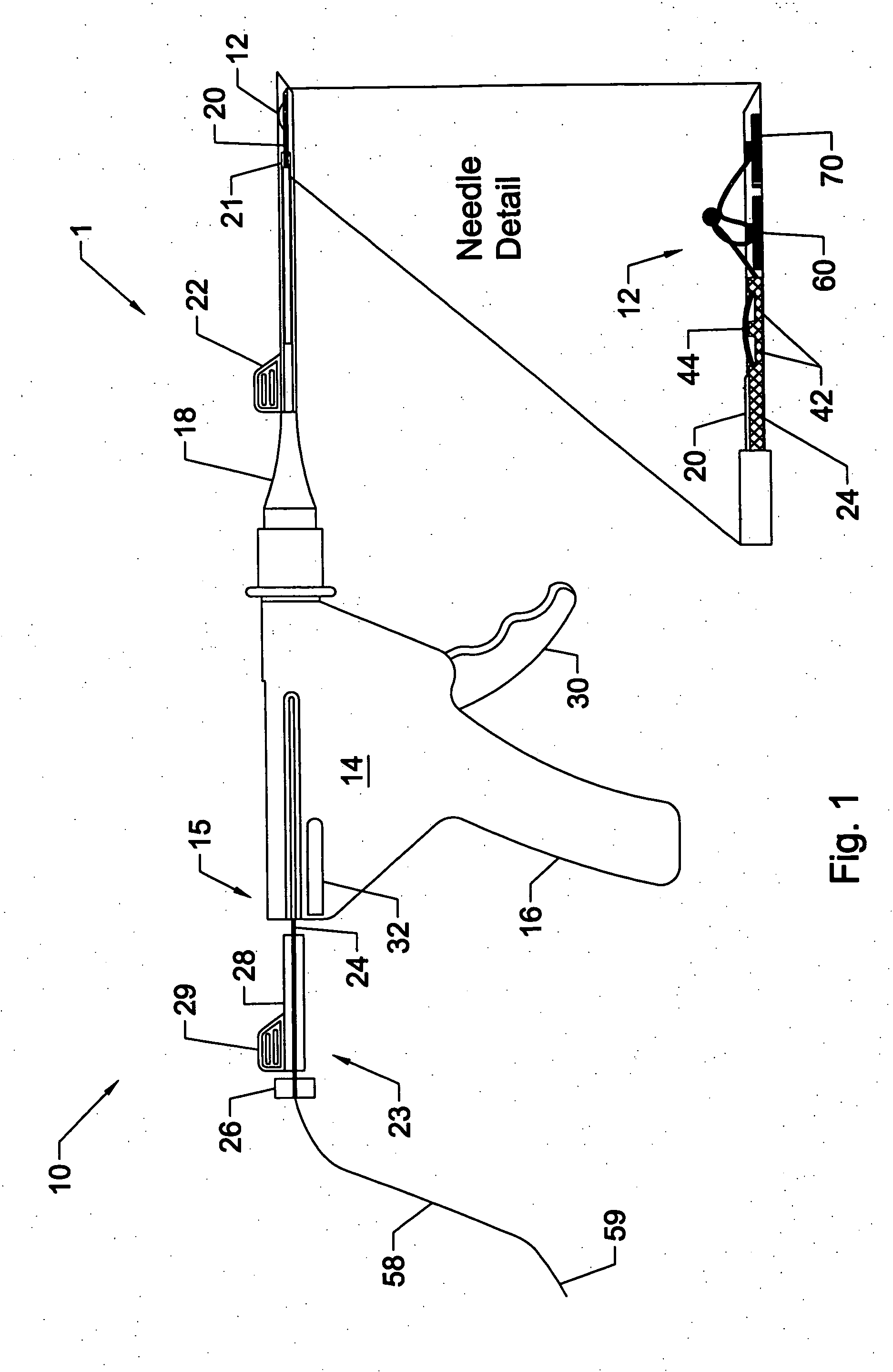
A system for repairing a meniscus includes a suture including a first anchor, a second anchor, and a flexible portion connecting the first anchor and the second anchor. The flexible portion includes a self-locking slide knot between the first anchor and the second anchor. The system also includes a needle having a longitudinal extending bore and an open end. The bore is configured to receive the first anchor and the second anchor. The system further includes a pusher configured to be movable within the bore of the needle. The pusher is configured to (1) discharge the first anchor and the second anchor, and (2) push the self-locking slide knot after the discharge of the second anchor.
Owner:STRYKER CORP
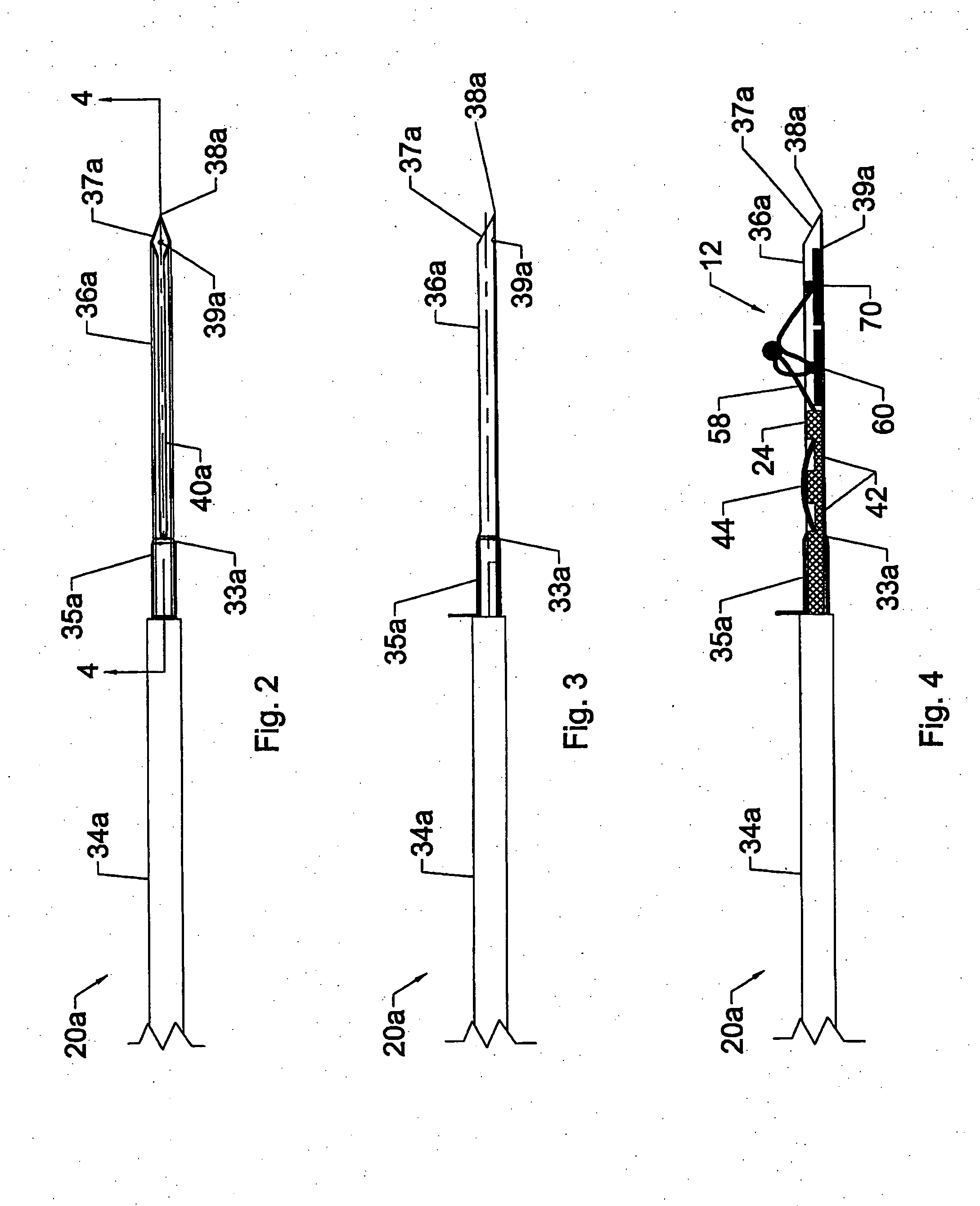
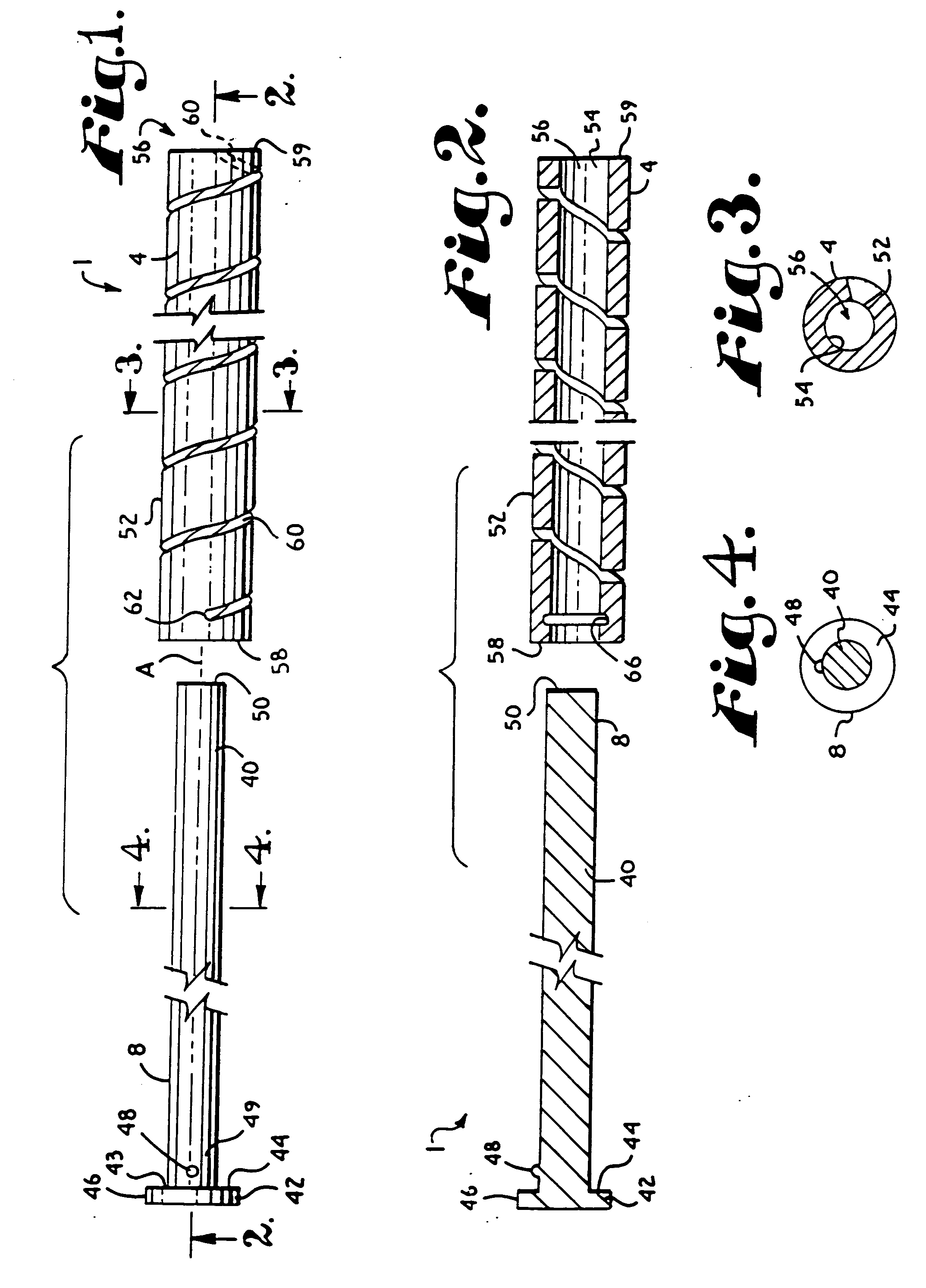
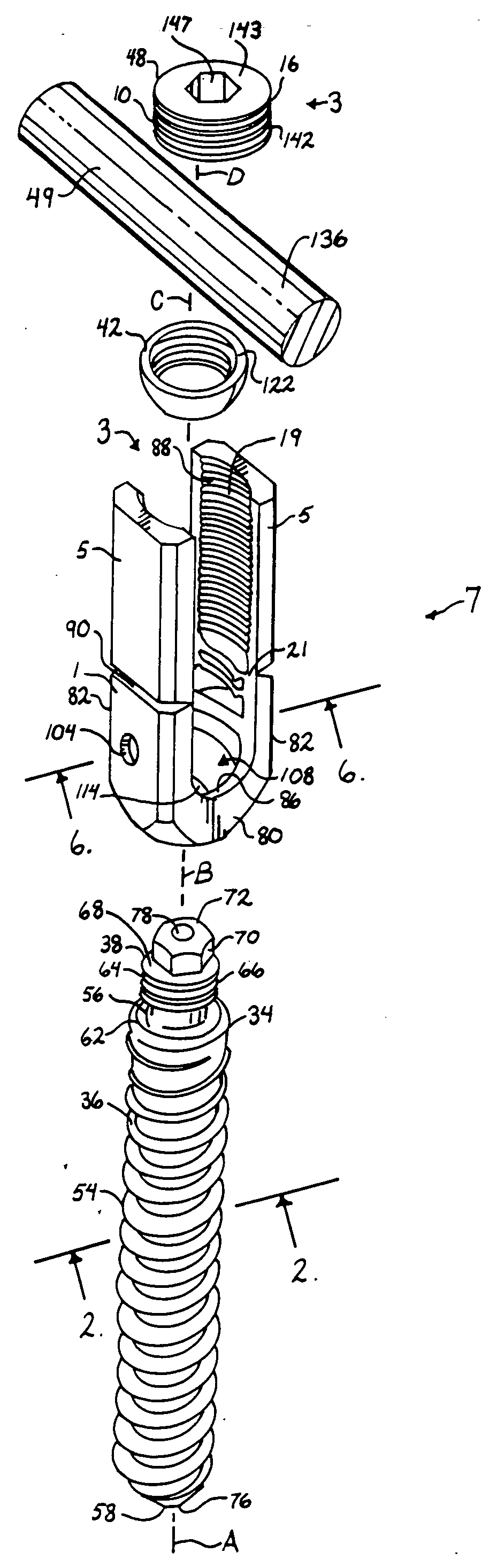
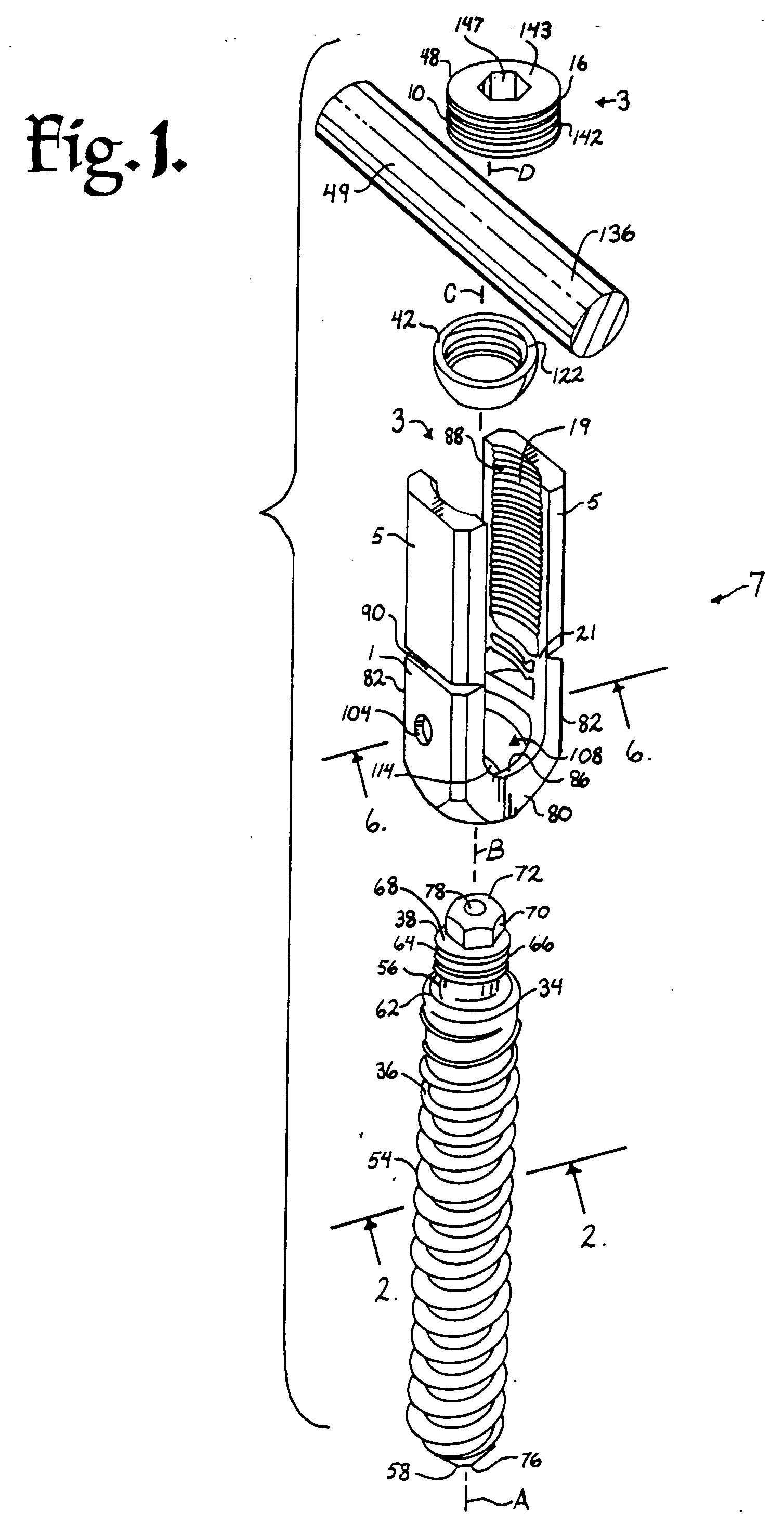
Dynamic fixation assemblies with inner core and outer coil-like member
A dynamic fixation medical implant includes a longitudinal connecting member assembly having an elongate coil-like outer member and an inner cylindrical core attached to the outer member at only one end thereof. Some assemblies include a second longitudinal connecting member in the form of a rod that is fixed to the inner core and extends outwardly from the assembly. Certain assemblies include a threaded core or threaded inserts that cooperate with a helical slit of the coil-like outer member. Two or more cooperating bone screw assemblies attach to the connecting member assembly. The bone screw assemblies may include upper and lower compression members for affixing to and cradling the coil-like outer member only, allowing relative movement between the outer member and the inner cylindrical core. Press fit or snap-on features attach one end of the coil-like outer member to one end of the inner cylindrical core.
Owner:JACKSON
Spinal fixation tool set and method for rod reduction and fastener insertion
A tool for implantation of a rod into a bone screw implanted in a human spine includes a guide member having a laterally opening channel disposed along an entire length thereof for side loading and receiving an implant fastener. A rod pushing member and a handle with a laterally opening channel are coaxial with the guide member, with the rod pushing member being rotatingly mateable to the guide member and the handle having a spring attachment mechanism for attaching the handle to the guide member. The guide member includes spring tabs for attachment to a bone screw, the tabs biased away from the bone screw. The rod pushing member includes a sleeve that extends substantially about the guide member, pressing the spring tabs toward the bone screw and into apertures on the bone screw arms. The rod pushing member sleeve also operatively functions as a rod pusher that abuts a rod as the sleeve is translated along the guide member and toward the bone screw. The handle lateral opening receives and supports a manipulation tool for inserting and installing an implant fastener for attaching the rod to the bone screw.
Owner:JACKSON
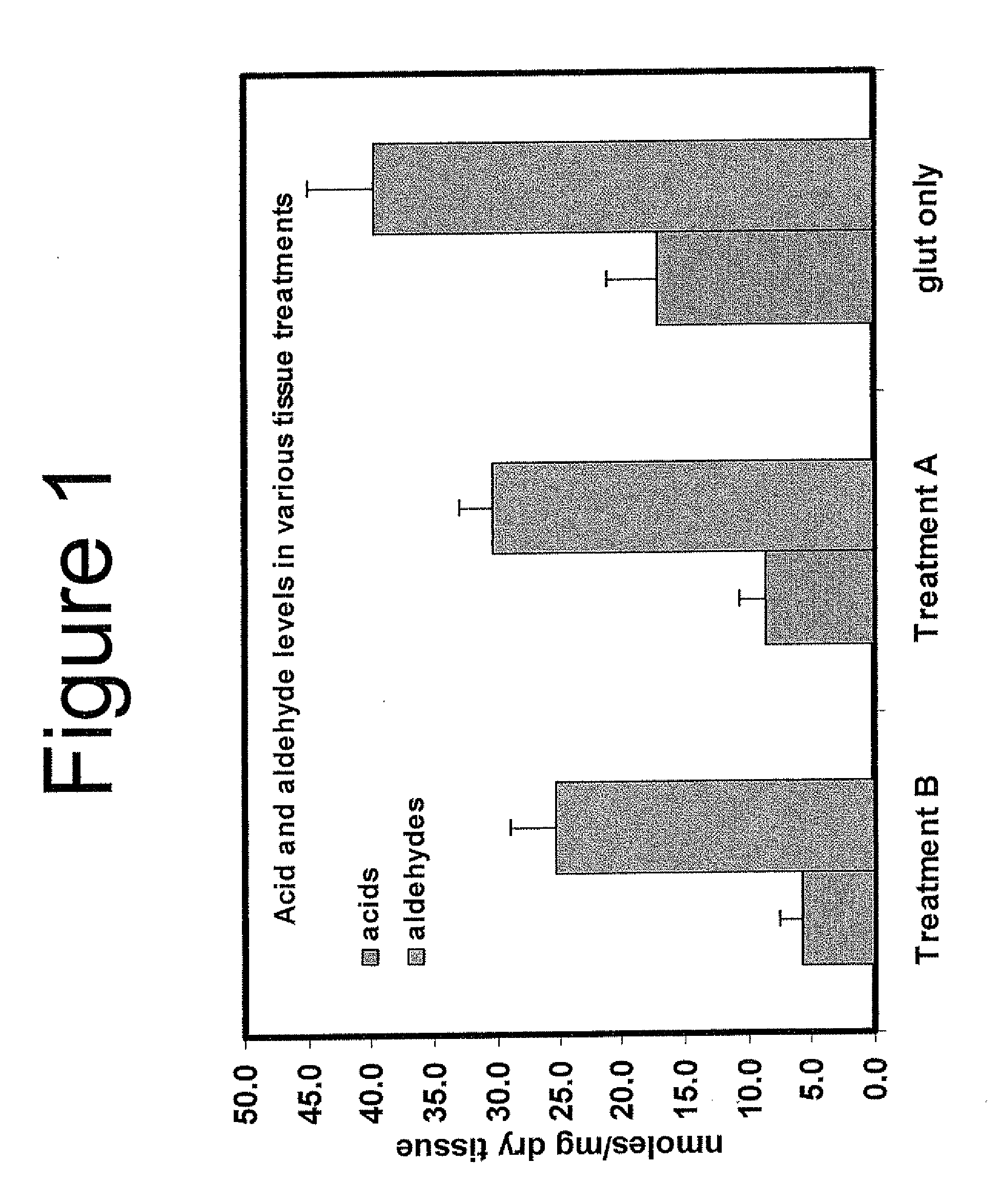
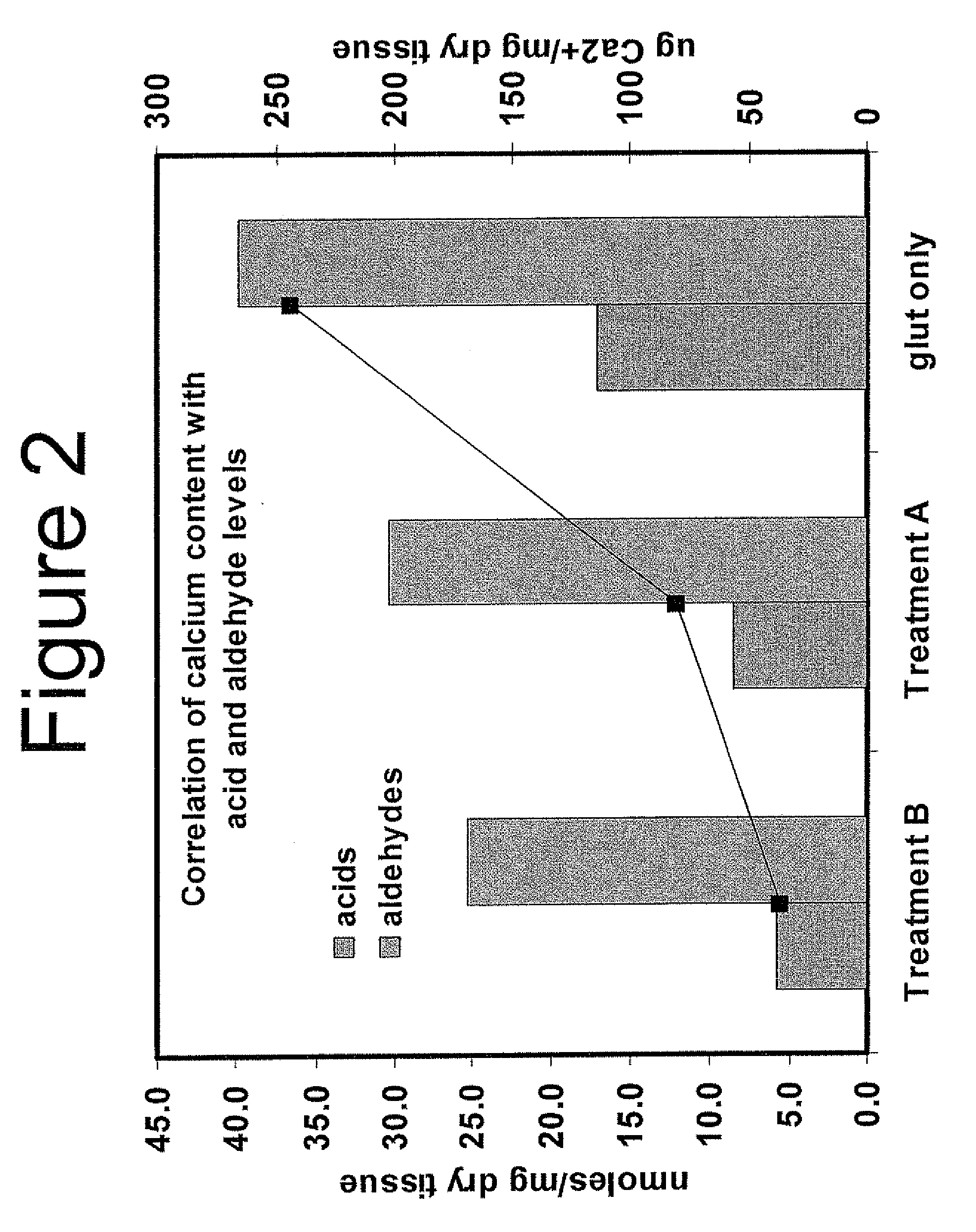
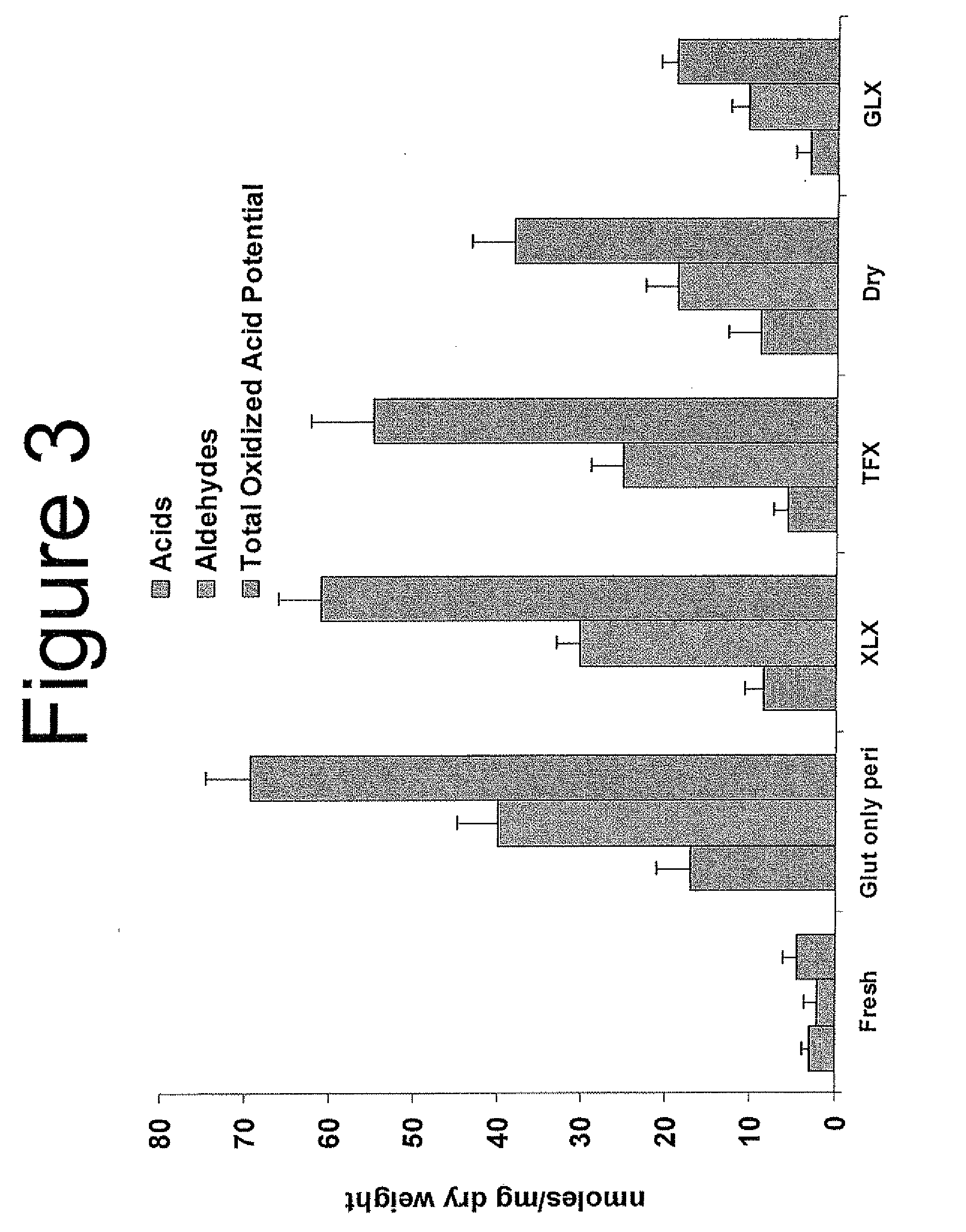
Capping Bioprosthetic Tissue to Reduce Calcification
A treatment for bioprosthetic tissue used in implants or for assembled bioprosthetic heart valves to reduce in vivo calcification. The method includes applying a calcification mitigant such as a capping agent or an antioxidant to the tissue to specifically inhibit oxidation in tissue. Also, the method can be used to inhibit oxidation in dehydrated tissue. The capping agent suppresses the formation of binding sites in the tissue that are exposed or generated by the oxidation and otherwise would, upon implant, attract calcium, phosphate, immunogenic factors, or other precursors to calcification. In one method, tissue leaflets in assembled bioprosthetic heart valves are pretreated with an aldehyde capping agent prior to dehydration and sterilization.
Owner:EDWARDS LIFESCIENCES CORP
Helical reverse angle guide and advancement structure with break-off extensions
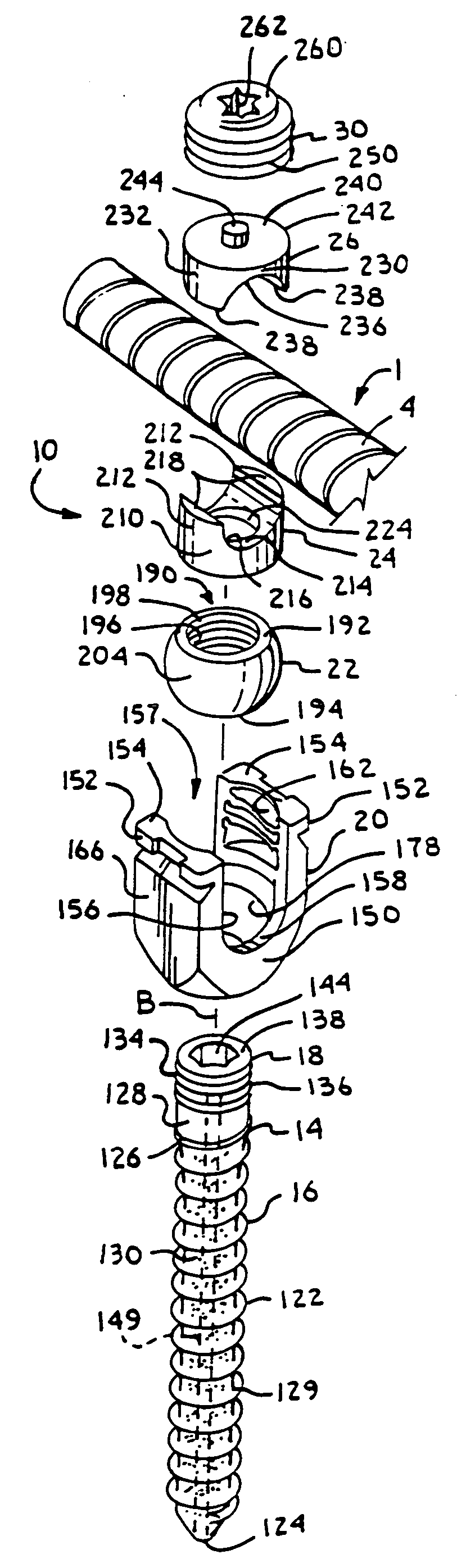
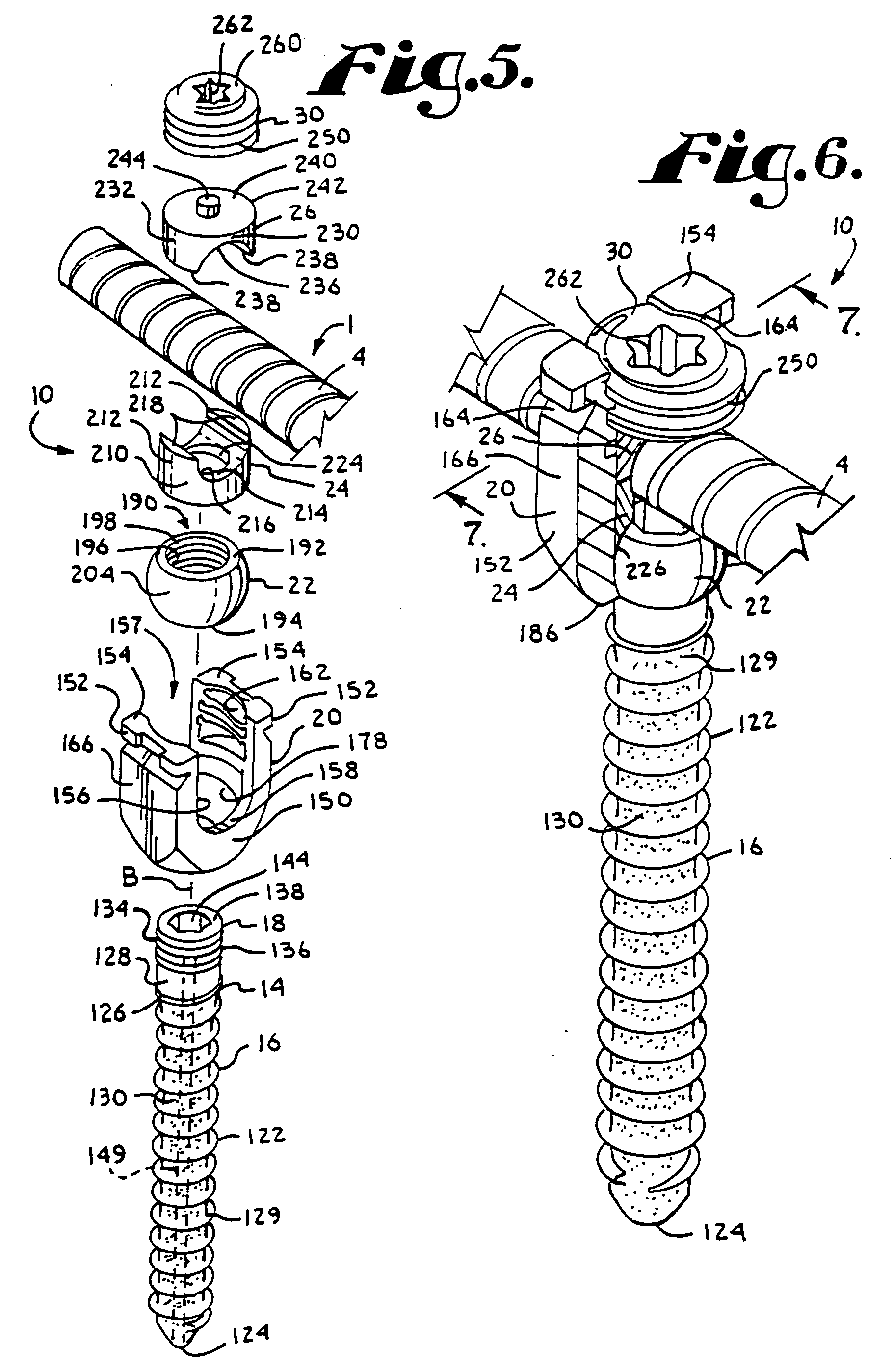
A spinal fixation device combines an anchor member with an open receiver, such as a polyaxial bone screw or a hook, with a rotatable closure that operably clamps a spinal fixation rod to the anchor member. The anchor member has spaced apart arms forming a rod receiving channel. The arms have arm extensions or tabs connected to main portions of the arms by weakened regions to enable the extensions to be broken off or separated after the rod is clamped. The closure and inner surfaces of the arms and tabs have mating helical, anti-splay, reverse angle guide and advancement structure formed thereon that mechanically cooperate to prevent splaying of the arms and the extensions as the closure is advanced into the rod receiving channel. The increased length of the arms with the extensions enables the rod to be captured at a greater distance from the seat of the channel and allows the rod to be urged toward the seat by helical advancement of the closure into the channel, starting between the extensions. Separation of the break-off extensions results in an implant with a desirable low profile.
Owner:JACKSON
Over-wire rotation tool
ActiveUS20100280604A1Avoid accessEasy accessSuture equipmentsAnnuloplasty ringsEngineeringMechanical engineering
Apparatus is provided that includes an implant structure, which includes a contracting mechanism, which includes a rotatable structure, arranged such that rotation of the rotatable structure contracts the implant structure. A longitudinal member is coupled to the contracting mechanism. A tool for rotating the rotatable structure is configured to be guided along the longitudinal member, to engage the rotatable structure, and to rotate the rotatable structure in response to a rotational force applied to the tool. Other embodiments are also described.
Owner:VALTECH CARDIO LTD
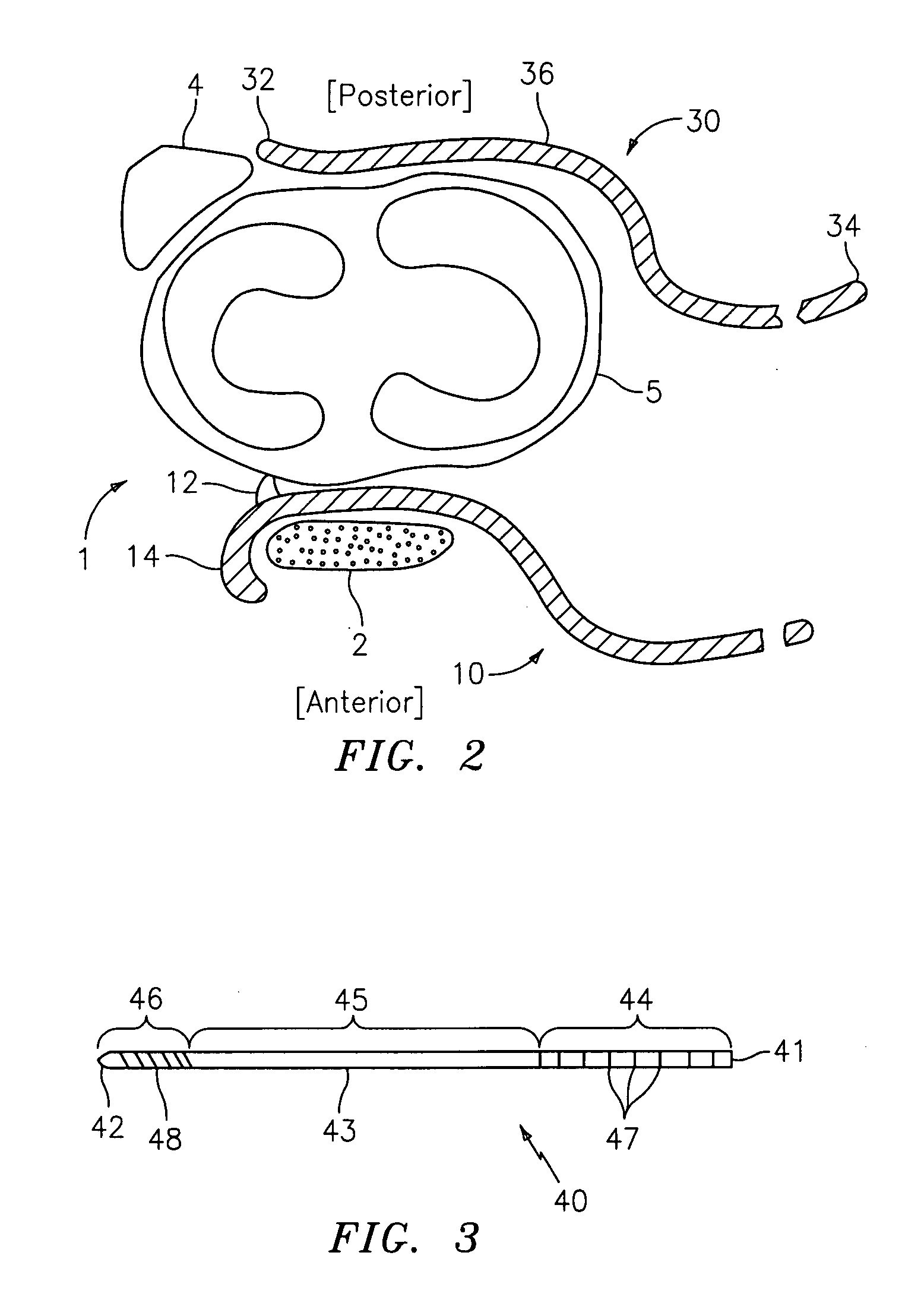
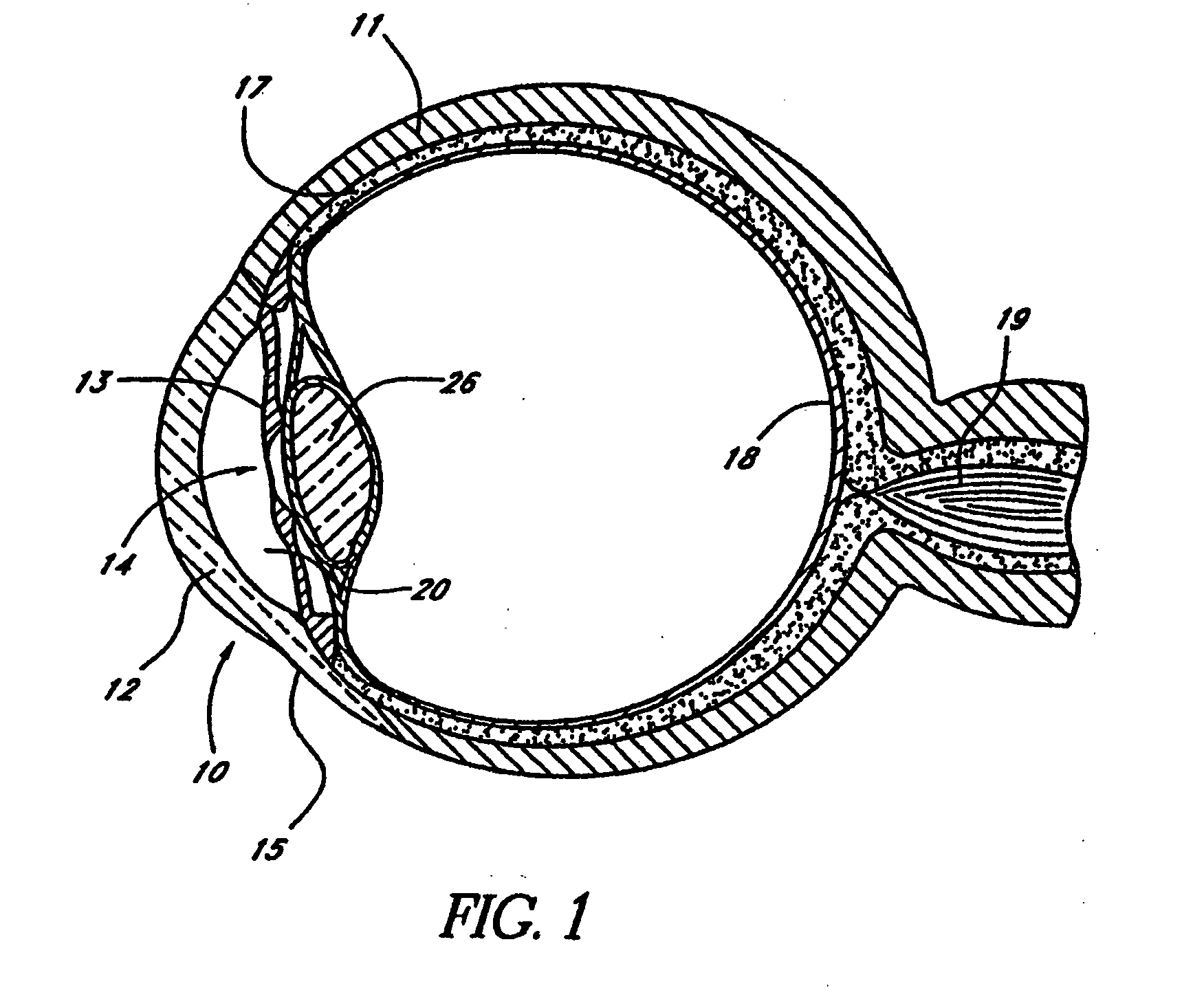
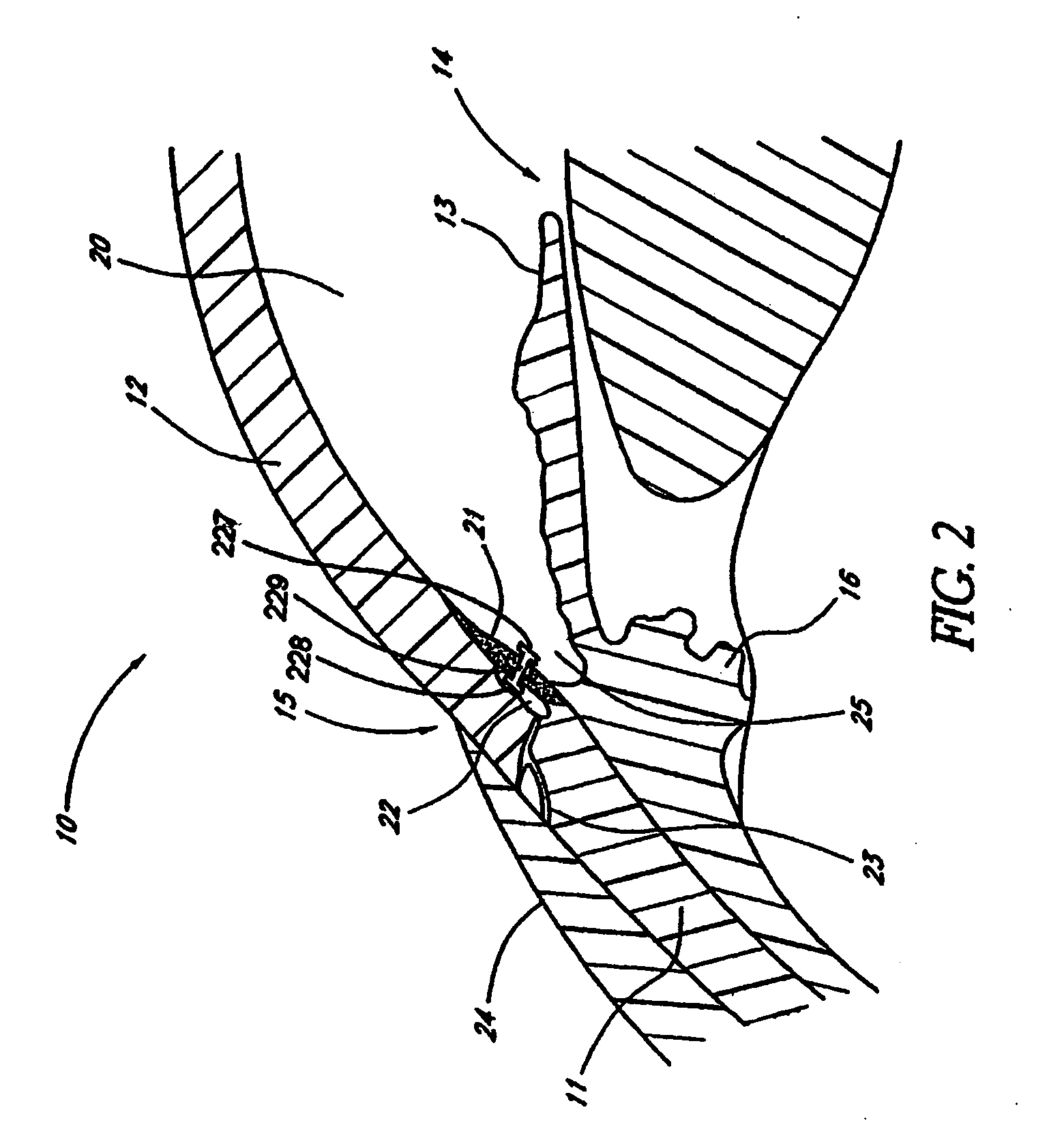
Expandable glaucoma implant and methods of use
Disclosed is an implant for use in an eye with glaucoma, the implant including an inlet section in fluid communication with an outlet section, the inlet section being sized and shaped to fit at least partially in the anterior chamber of the eye, and the outlet section being sized and shaped to fit at least partially in Schlemm's canal of the eye. The implant also includes an expandable substrate suitable for expansion in the eye to assist in retaining the implant in the eye.
Owner:GLAUKOS CORP
Cervical facet resurfacing implant
The present invention relates to prostheses for treating spinal pathologies, and more specifically to a system and method for treating articulating surfaces of cervical vertebrae facet joints. The system includes a superior implant for placement on a superior articulating surface and an inferior implant for placement on an inferior articulating surface. In addition, described is a method for providing articulating surfaces for cervical vertebrae facet joint articular facets.
Owner:LANTERNA MEDICAL TECH
Open wedge osteotomy system and surgical method
ActiveUS20050273114A1Reduce overall surgeon learning curveInternal osteosythesisDiagnosticsImplantSurgical methods
Owner:ARTHREX
Inserter instrument and implant clip
ActiveUS20050143749A1Amount of timeSafely insertedDiagnosticsJoint implantsCouplingBiomedical engineering
A method and apparatus assisting safe one-handed insertion of an implant. An implant implantation device has a frame which includes a trigger mechanism, an outer sleeve mechanically coupled to the frame, an inner shaft having a grabber for mechanically engaging an implant, the inner shaft slidably disposed within the outer sleeve, and a retaining element disposed over the inner shaft for directing the grabber toward a closed position. An implant clip has a first member, a second member pivotally coupled to the first member, a first implant holder pivotally coupled to the first member, the coupling causing the implant clip to have a closed position and an open position, and a second implant holder, the second implant holder pivotally coupled to the second member, a surface of the first implant holder and a surface of the second implant holder remaining substantially parallel to each other while the first member and the second member pivot between the closed position and the open position.
Owner:DEPUY SPINE INC (US)
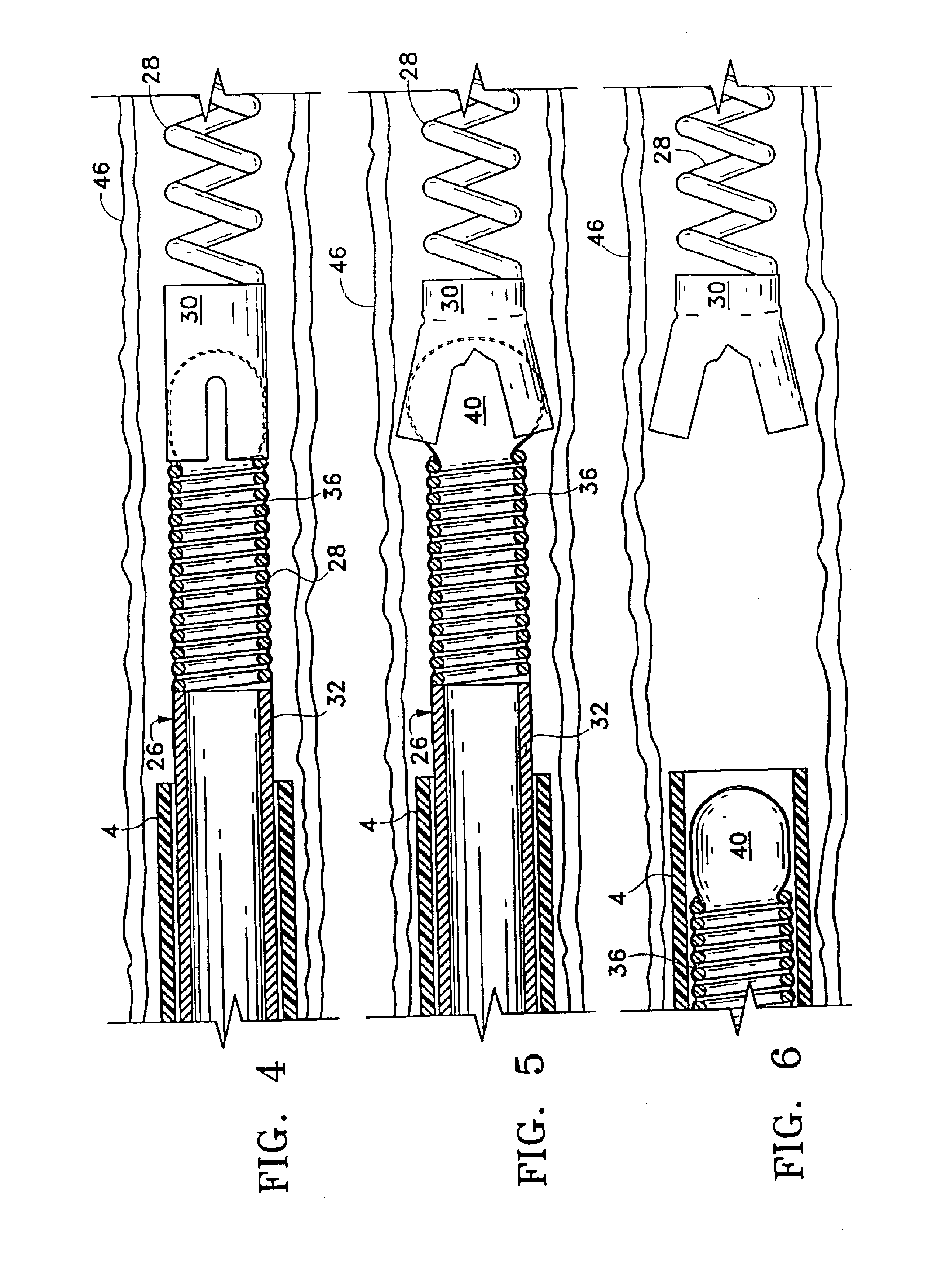
Surgical power drill including a measuring unit suitable for bone screw length determination
A device (25) for drilling holes in bone and configured to determine bone screw length, the device (25) including a surgical power drill (2) comprising: a) a housing (12) and; b) a measuring device (1) releasably attached or fixed to the housing (12), wherein the measuring device (1) is configured to measure the distance (x) covered by the housing (12) in the direction of the longitudinal axis (7) and relative to a surface of an implant (26) or a bone during a drilling process, wherein the measuring device (1) comprises a processing unit (14) to record the distance (x) covered with respect to time; the processing unit (14) comprises one or more differentiators to determine at least the first and second derivatives of the distance (x) covered with respect to time; and the processing unit (14) further comprises a peak detector to analyze one or more peaks occurring in the graph of the highest derivative with respect to time, and wherein the measuring device (1) comprises a laser device or an ultrasound position sensor for displacement assessment.
Owner:SYNTHES GMBH
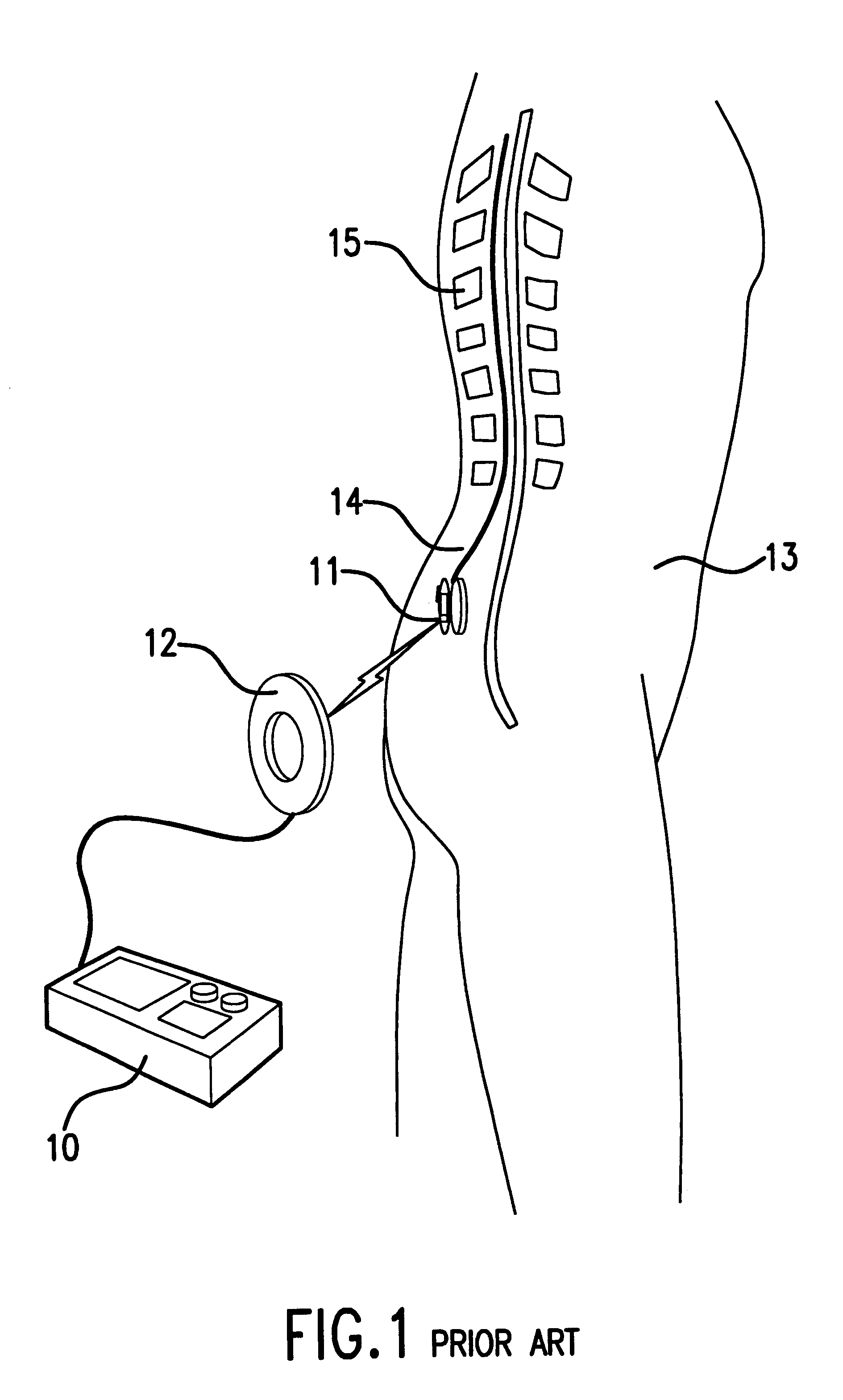
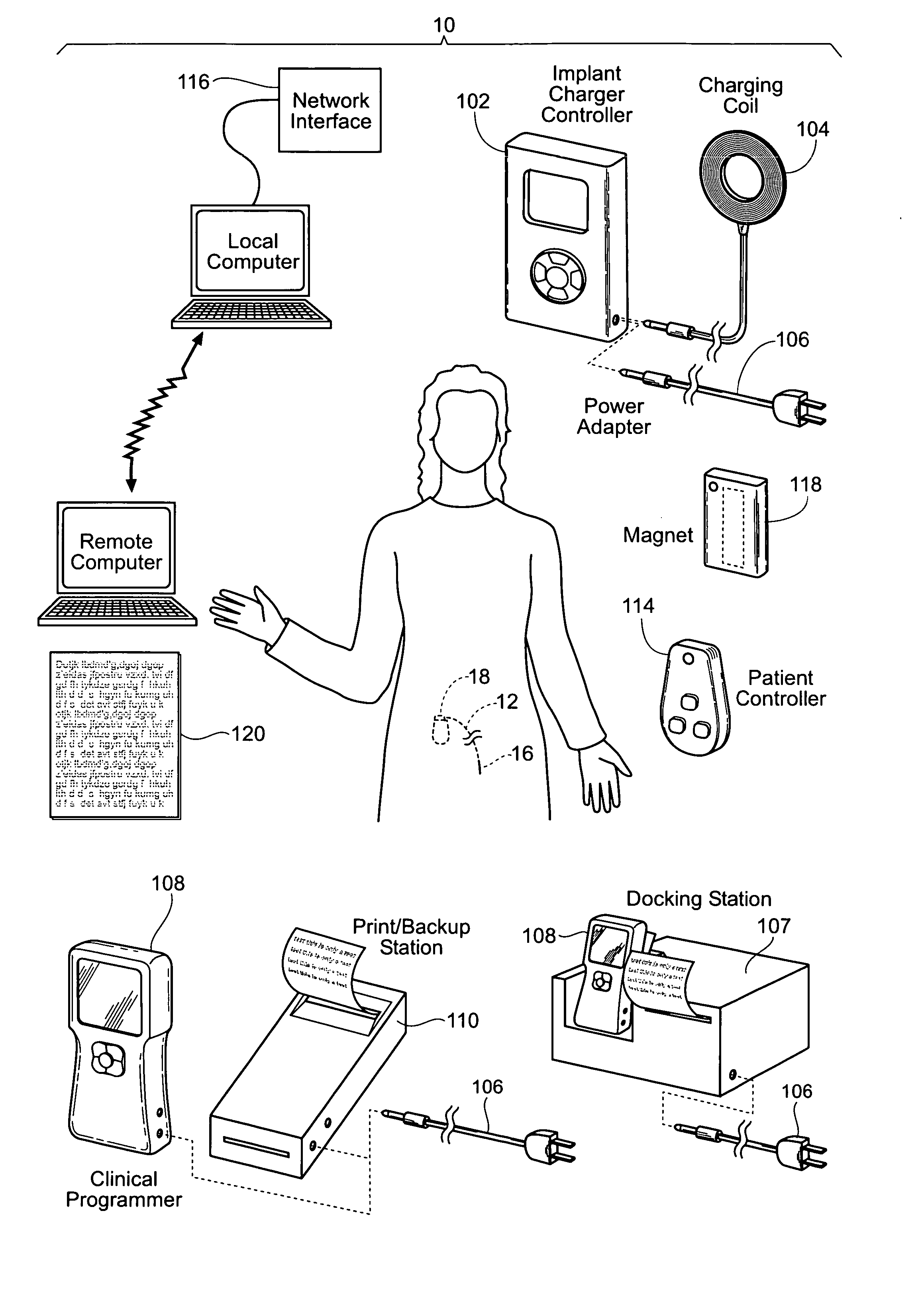
Implantable pulse generator systems and methods for providing functional and / or therapeutic stimulation of muscles and / or nerves and / or central nervous system tissue
InactiveUS20070060979A1Spinal electrodesImplantable neurostimulatorsRadio frequencyBiomedical engineering
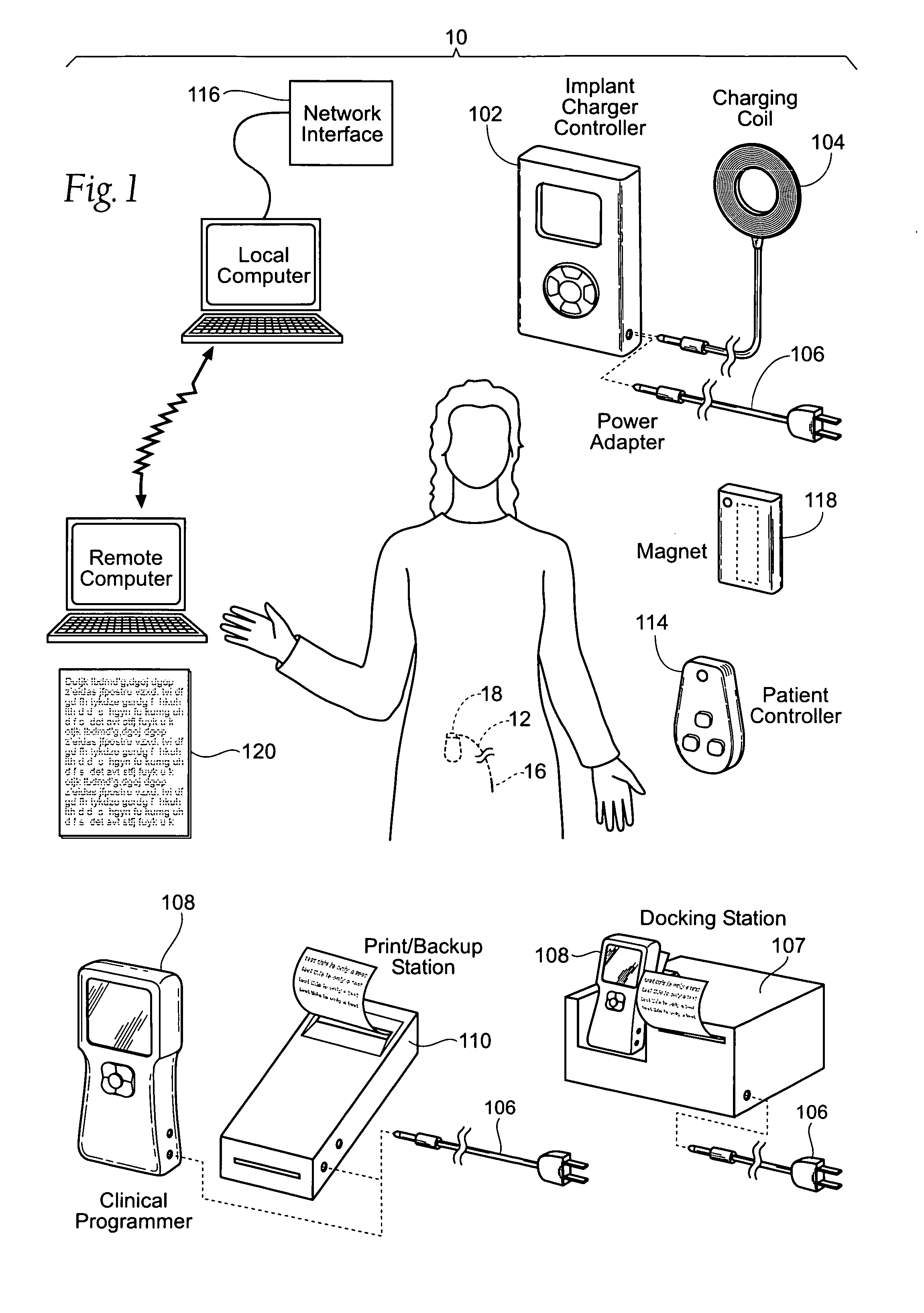
Improved assemblies, systems, and methods provide a stimulation system for prosthetic or therapeutic stimulation of muscles, nerves, or central nervous system tissue, or any combination. The stimulation system includes both implantable components and external components. The implantable components comprise a pulse generator including an operating system, a predefined set of stimulation parameters, a rechargeable battery, a power receiving coil, and a lead coupled to an electrode to provide electrical stimulation. An external clinical programmer system comprises a clinical programmer adapted for radio frequency wireless telemetry communication with the pulse generator, and a docking station, the docking station including a cradle to dock the hand held clinical programmer, the cradle adapted to provide access to a printer and / or non-volatile memory. An external patient controller system comprises a hand held implant charger controller adapted for radio frequency wireless telemetry communication with the pulse generator, the implant charger controller including a charging coil.
Owner:MEDTRONIC URINARY SOLUTIONS
Osteotomy system
InactiveUS20080195099A1Sufficient widthSuture equipmentsSurgical furniturePerformed ProcedureValgus deformity
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Injectable glaucoma implants with multiple openings
InactiveUS20050266047A1Faster and safe and less-expensive surgical procedureRapid visual recoveryOrganic active ingredientsEye surgerySchlemm's canalImplant
Intraocular stents and applicators are disclosed for treating glaucoma. The stents are configured to extend between the anterior chamber of the eye and Schlemm's canal for enhancing outflow of aqueous from the anterior chamber so as to reduce intraocular pressure. The stents can have features for anchoring the stent into Schlemm's canal as well as preventing the walls of Schlemm's canal from closing the outlet of the stents. The applicators can be steerable so as to make implantation easier. Additionally, the applicators can be configured to hold a plurality of stents so that multiple stents can be implanted through one incision without removing the applicator from the incision between serial implantations.
Owner:GLAUKOS CORP
Device
InactiveUS20100094430A1High porositySusceptible to corrosive attackBone implantPretreated surfacesBone replacementMaterials science
An implant for bone replacement and attachment in an animal's body including, a structural portion having an outer porous surface, a ceramic material applied to the porous surface of the structural portion, characterised in that the thickness of the ceramic material as applied utilizing pulsed pressure MOCVD is such that at least some of the pores of the porous surface are not completely closed.
Owner:CANTERPRISE LTD
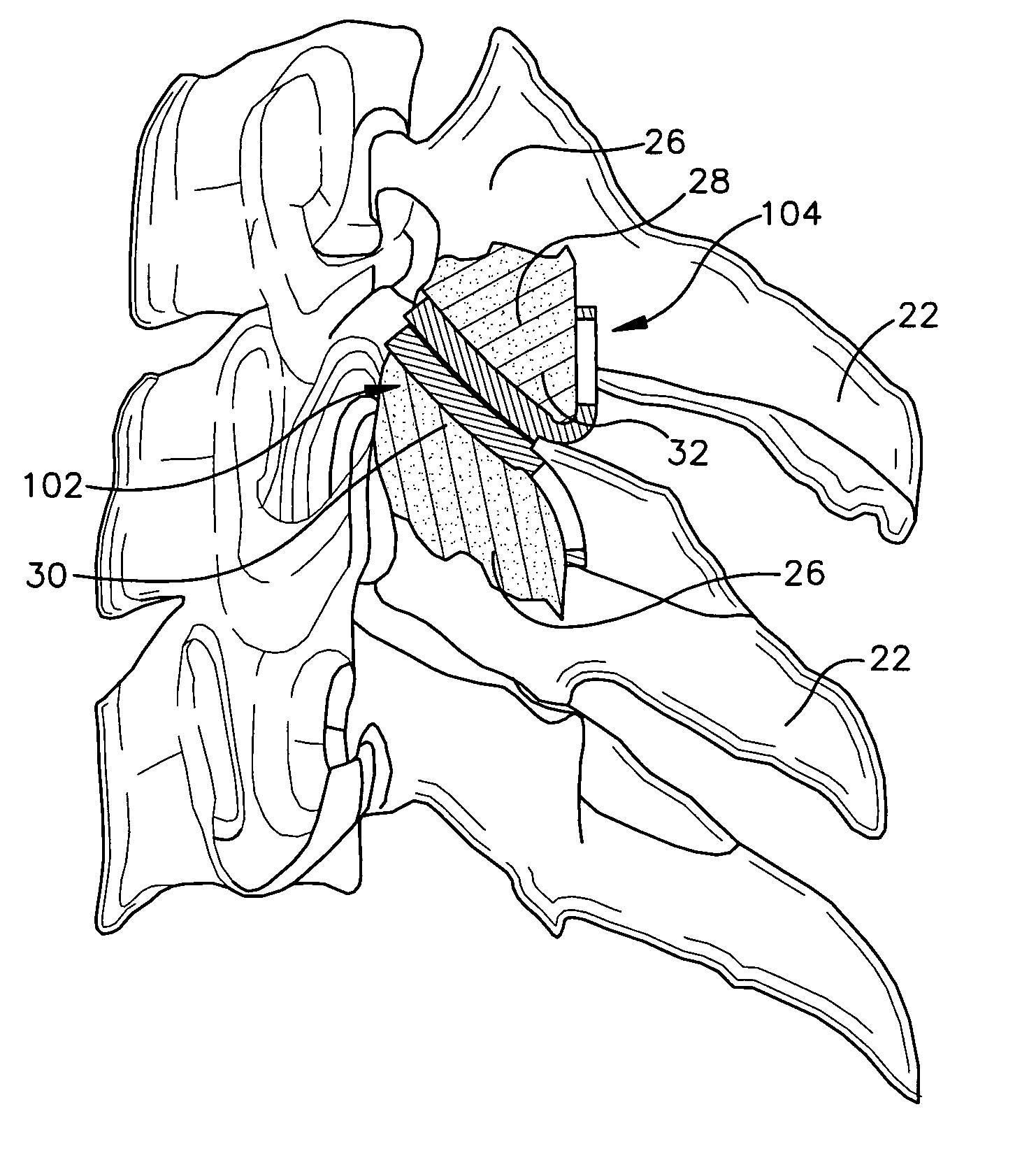
Implant delivery assembly with expandable coupling/decoupling mechanism
An occlusive implant delivery assembly includes a rapid response decoupling or detachment mechanism that does not effect significant migration of the implant during release. The assembly includes an occlusive implant device, such as an embolic coil, a pusher or device to carry the implant to the selected location, and an expandable coupling-decoupling mechanism for releasing the implant at the selected site. The mechanical construction provides rapid release times. In addition, the releasing mechanism generally operates without exerting any significant force on the implant, thereby avoiding any significant displacement of the implant during release.
Owner:STRYKER CORP +1
Implant for treating ailments of a joint or a bone
InactiveUS6436146B1Increase coefficient of frictionImprove stabilityFinger jointsWrist jointsDiseasePyrolytic carbon
The implant has at least one contact surface portion, made of pyrolytic carbon, designed to be in mobile contact with at least one bony surface when the implant is implanted in a patient. Furthermore, the implant is free from any attaching means, so that it remains free with respect to the at least one bony surface when implanted in the patient.
Owner:TORNIER SA SAINT ISMIER
Biodegradable injectable implants and related methods of manufacture and use
InactiveUS20030093157A1Broaden applicationSolution deliveryPharmaceutical non-active ingredientsGlycolic acidImplant
This invention is directed to the field of medical implants, and more specifically to biodegradable injectable implants and their methods of manufacture and use. The injectable implants disclosed herein comprise glycolic acid and bio-compatible / bio-absorbable polymeric particles containing a polymer of lactic acid. The particles are small enough to be injected through a needle but large enough to avoid engulfment by macrophages. The injectables of this invention may be in a pre-activated solid form or an activated form (e.g., injectable suspension or emulsion).
Owner:MEDGRAFT MICROTECH
Implant with composite coating
InactiveUS6261322B1High strengthCost effectiveImpression capsBone implantBiocompatible coatingBiocompatibility Testing
Systems and methods are described for implants with composite coatings to promote tissue in-growth and / or on-growth. An implant includes: a substrate; a structured surface formed on at least a portion of the substrate; and a biocompatible coating deposited on at least a fraction of the structured surface. The systems and methods provide advantages in that the implant has good biocompatibility while the biocompatible coating has good strength.
Owner:SHALBY ADVANCED TECH INC
Apparatus for Bone Restoration of the Spine and Methods of Use
ActiveUS20120071977A1Inhibition of contractionInternal osteosythesisBone implantMechanical resistanceBack pain
The subject disclosure is directed to systems, apparatuses, devices and methods for vertebral and spinal correction. In some embodiments, an expandable implant is provided which may be inserted inside the vertebral body and / or between two vertebrae, for instance, for maintenance and / or restoration of a space therein or there between. In certain embodiments, the implant includes a mechanical resistance that prevents the expandable implant from contracting once it has been expanded. Methods of treatment and methods of use of such implants for the alleviation of back pain (for example) are also provided herein.
Owner:STRYKER EUROPEAN OPERATIONS LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com