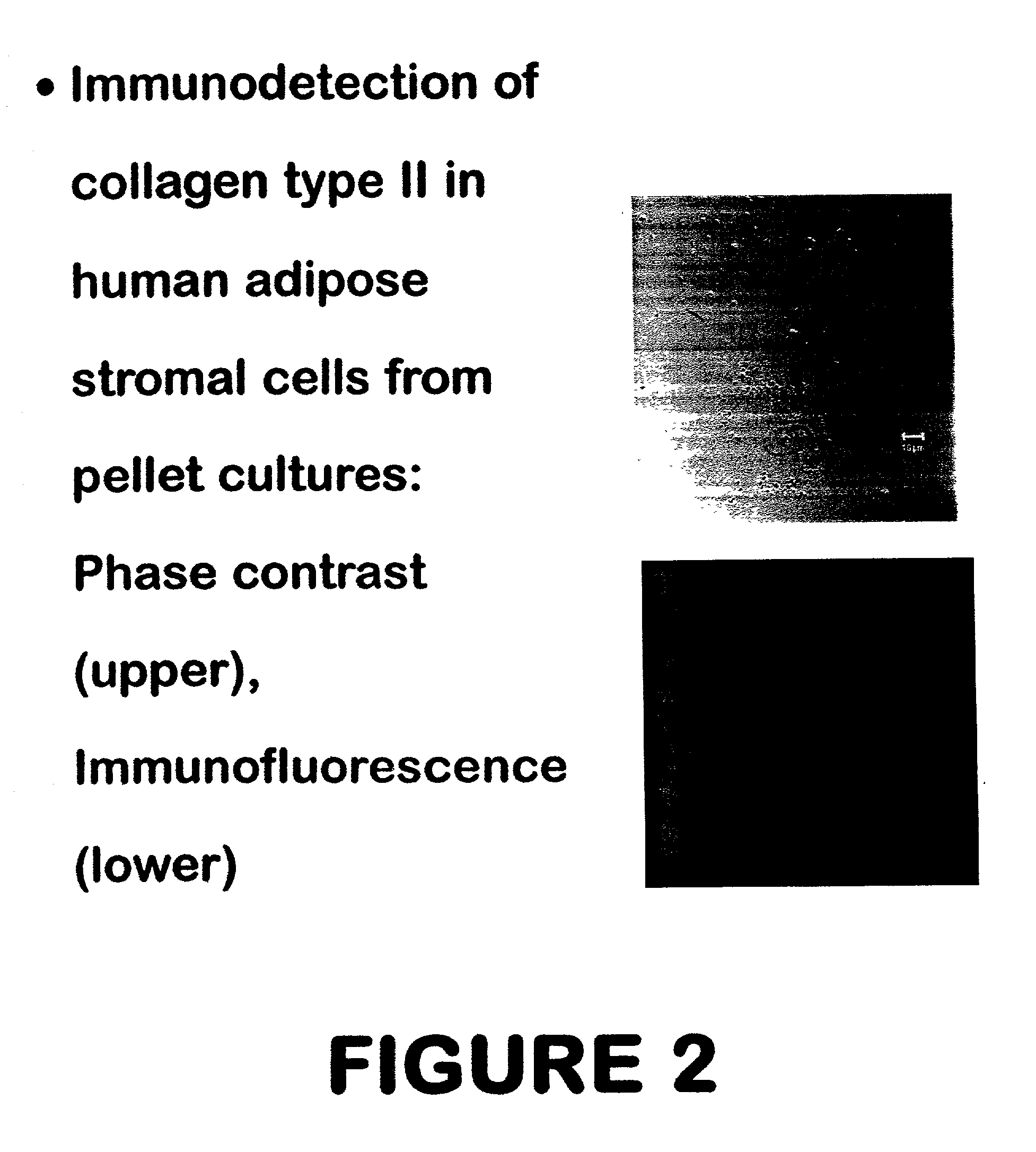
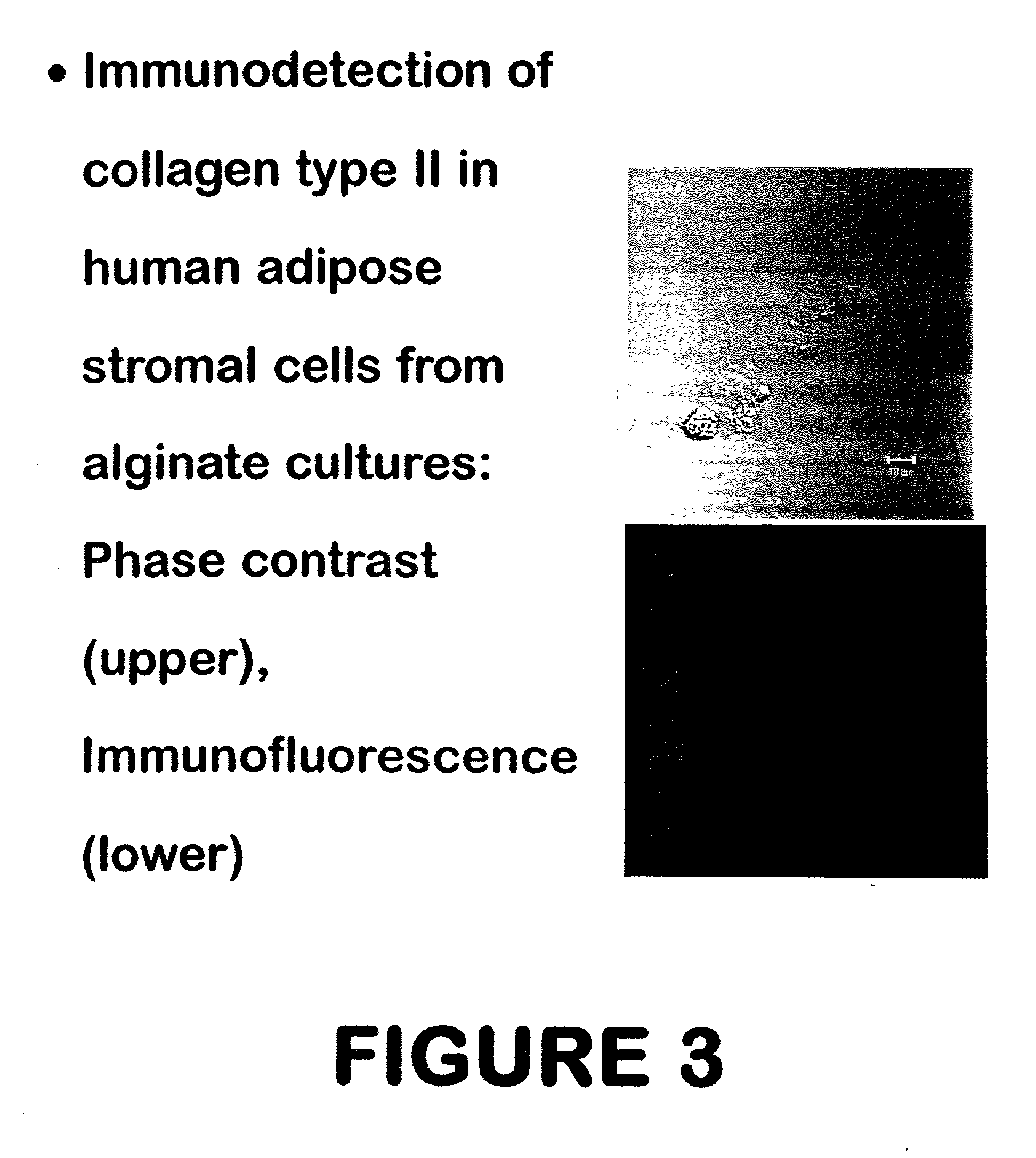
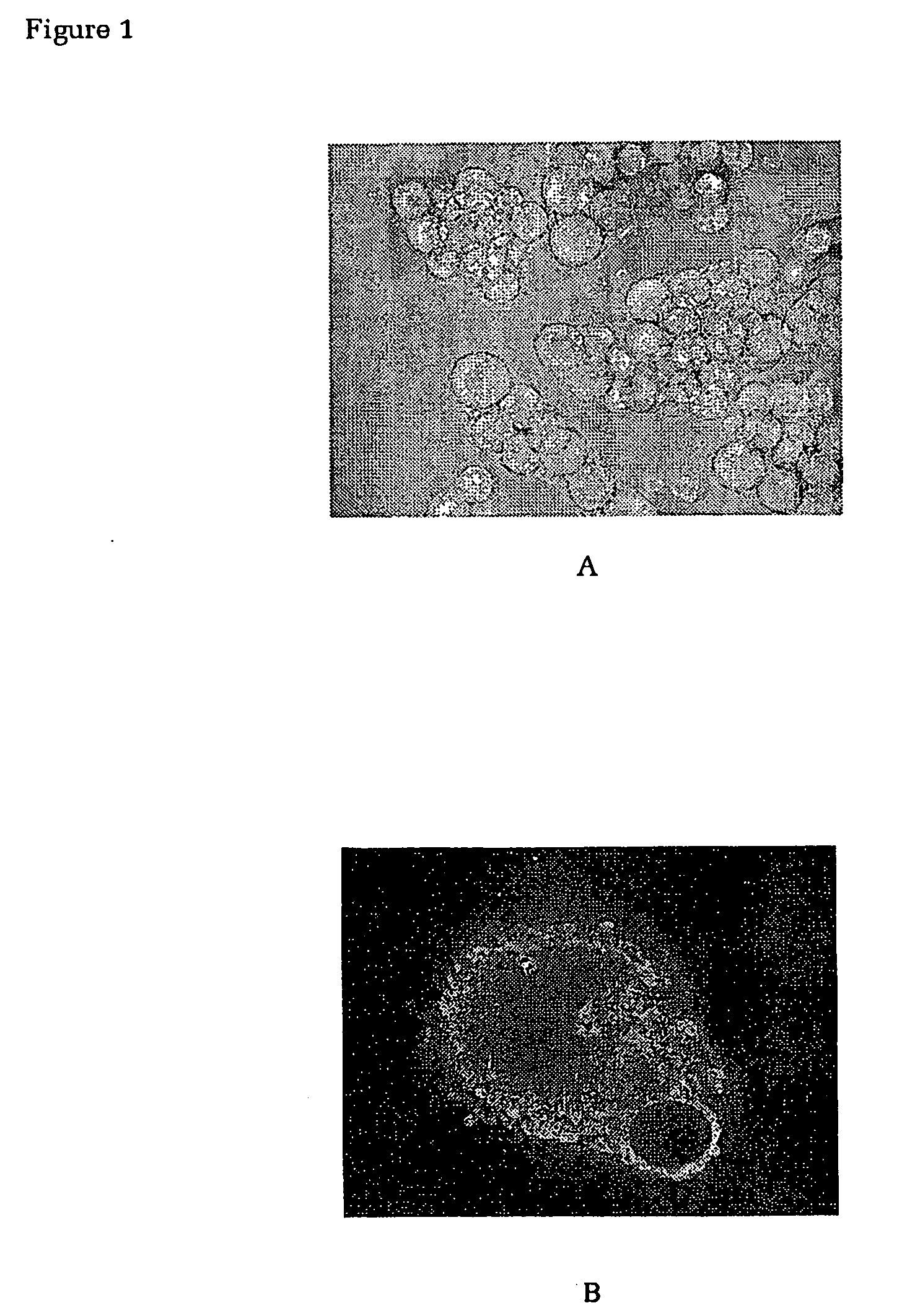
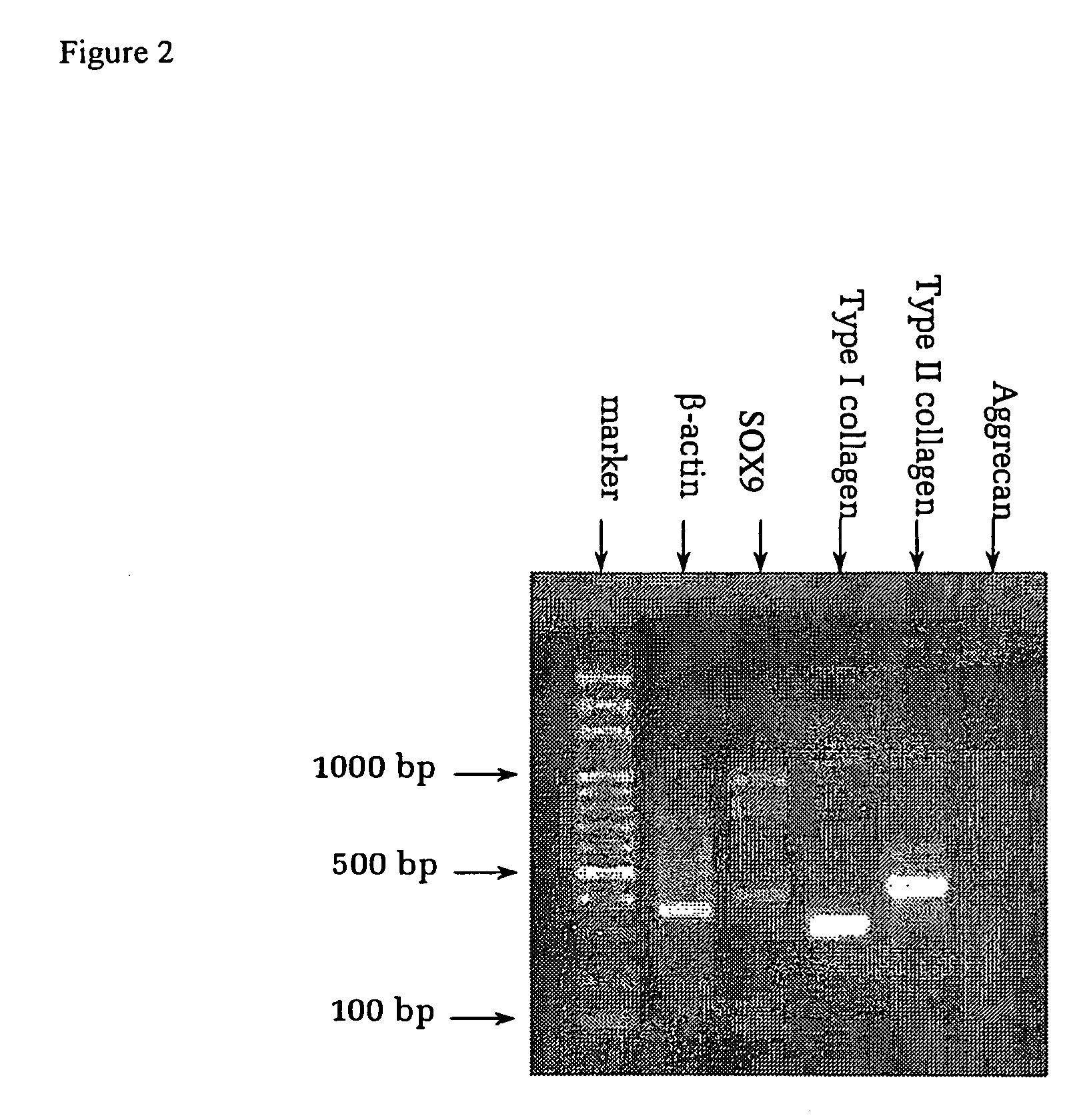
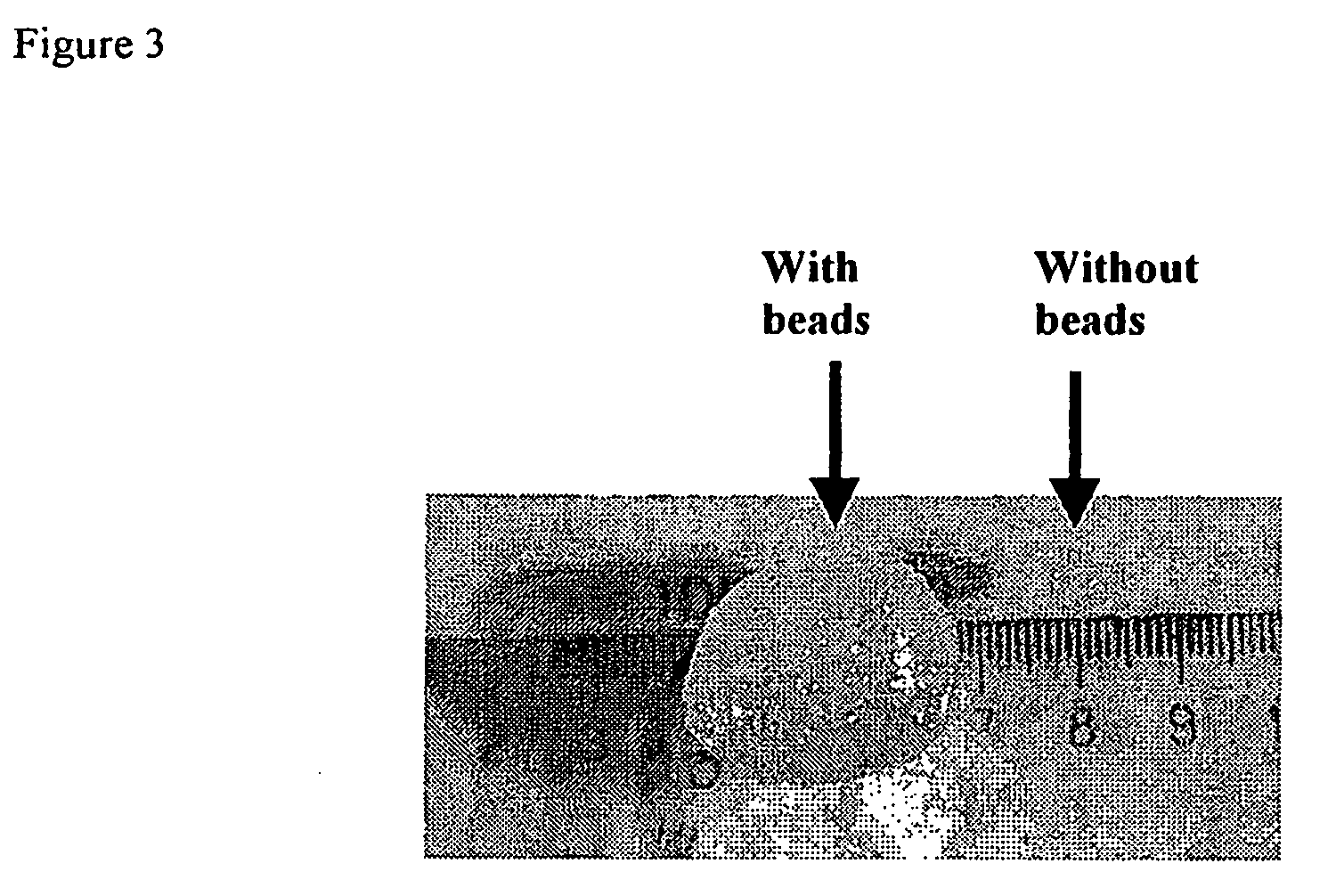
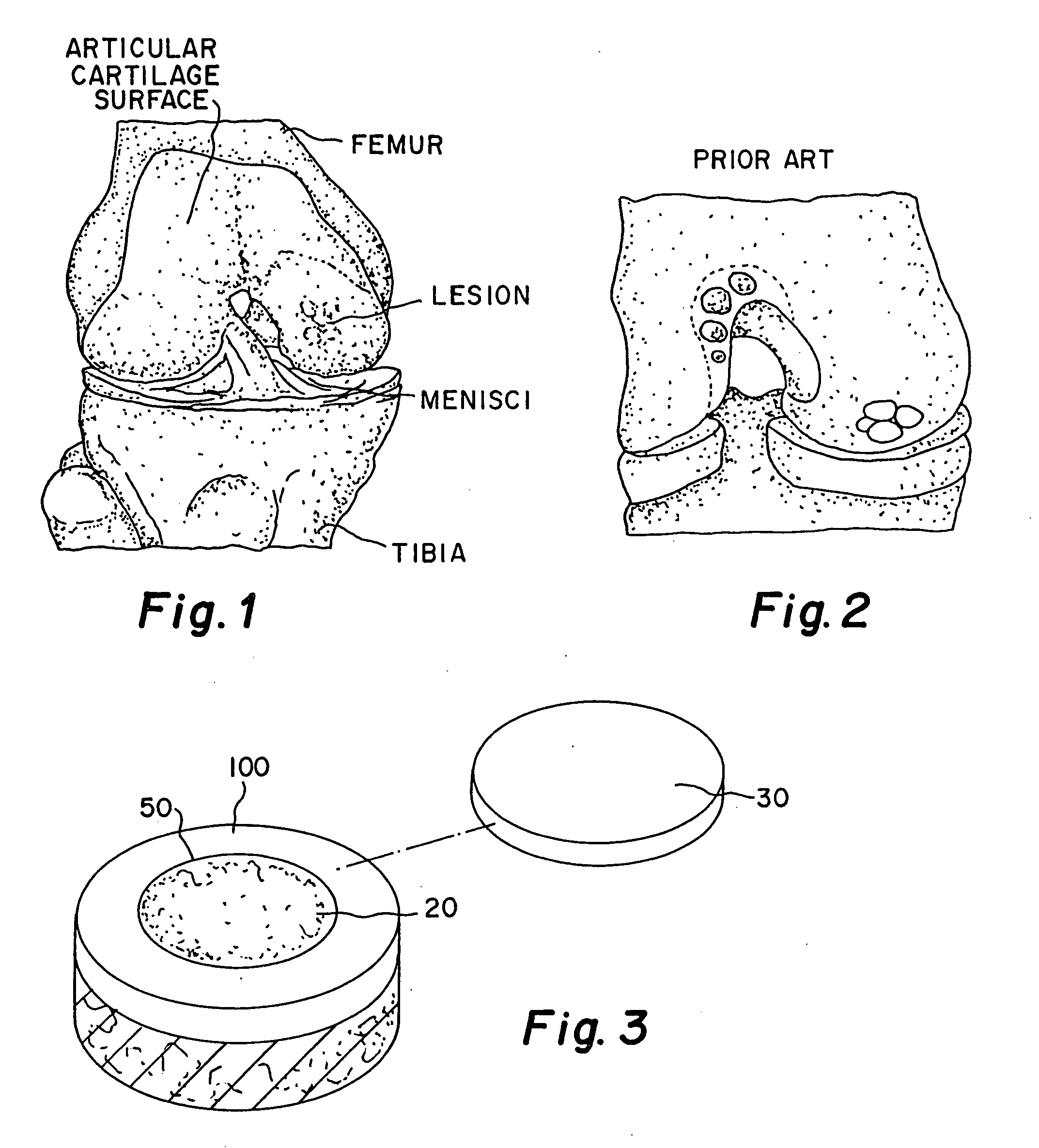
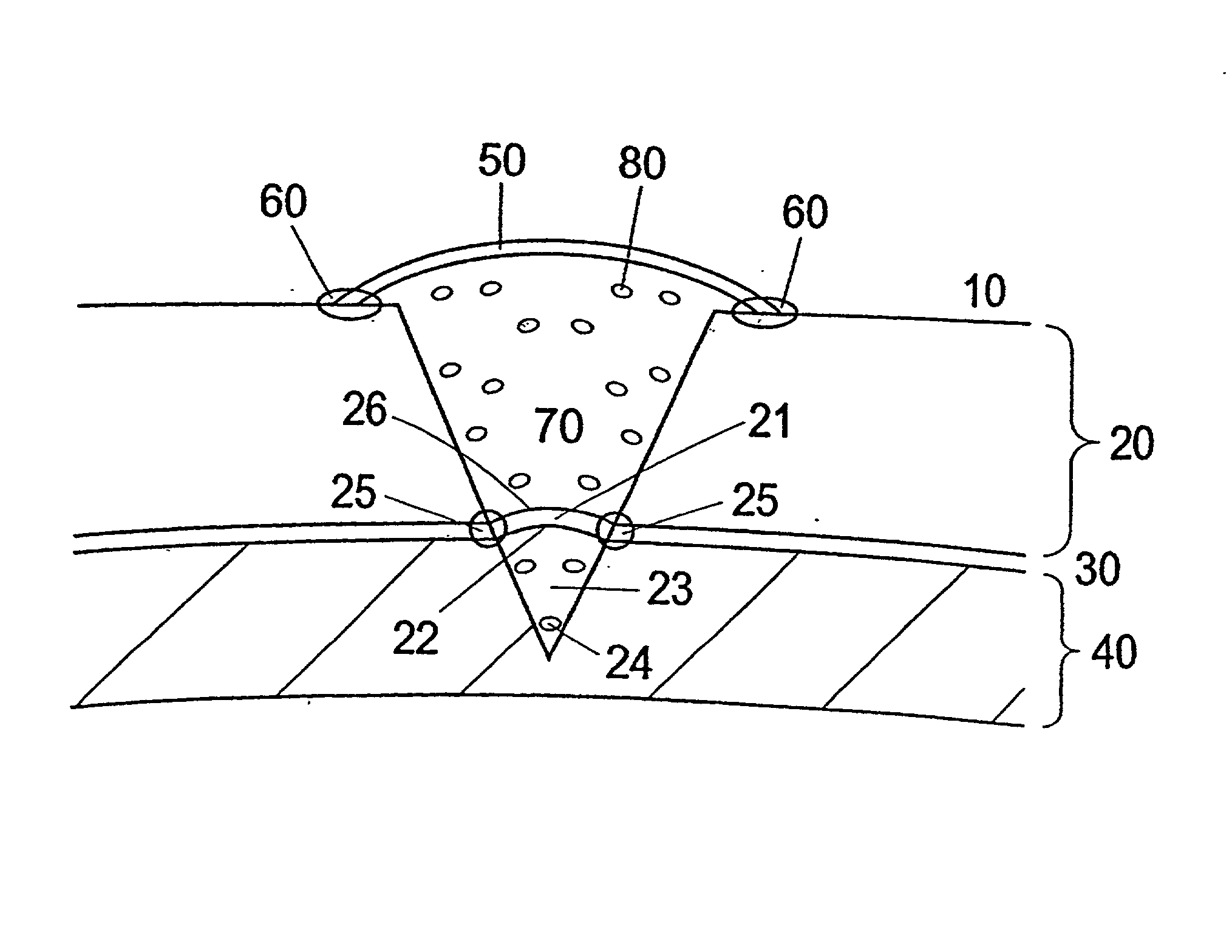
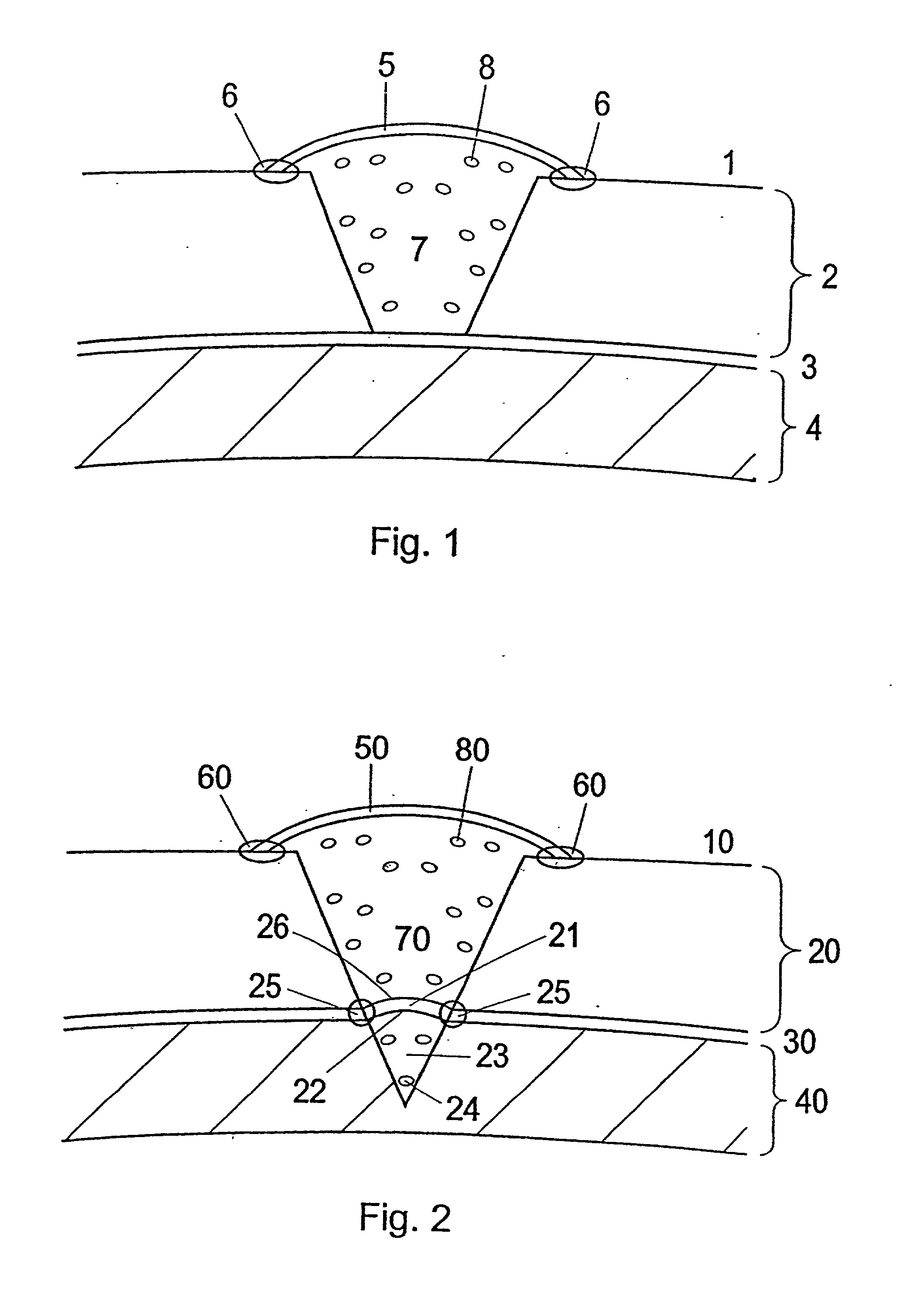
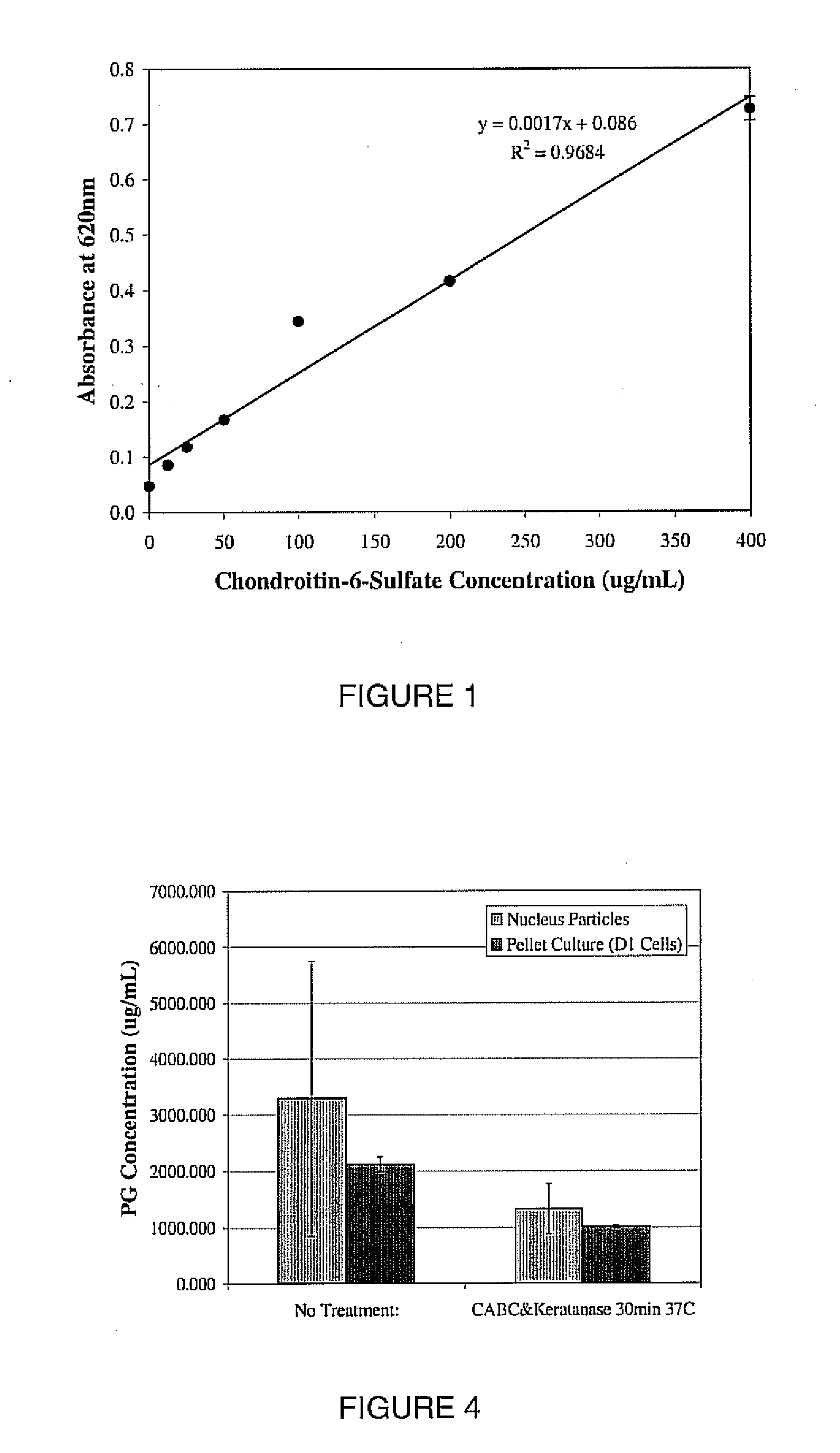
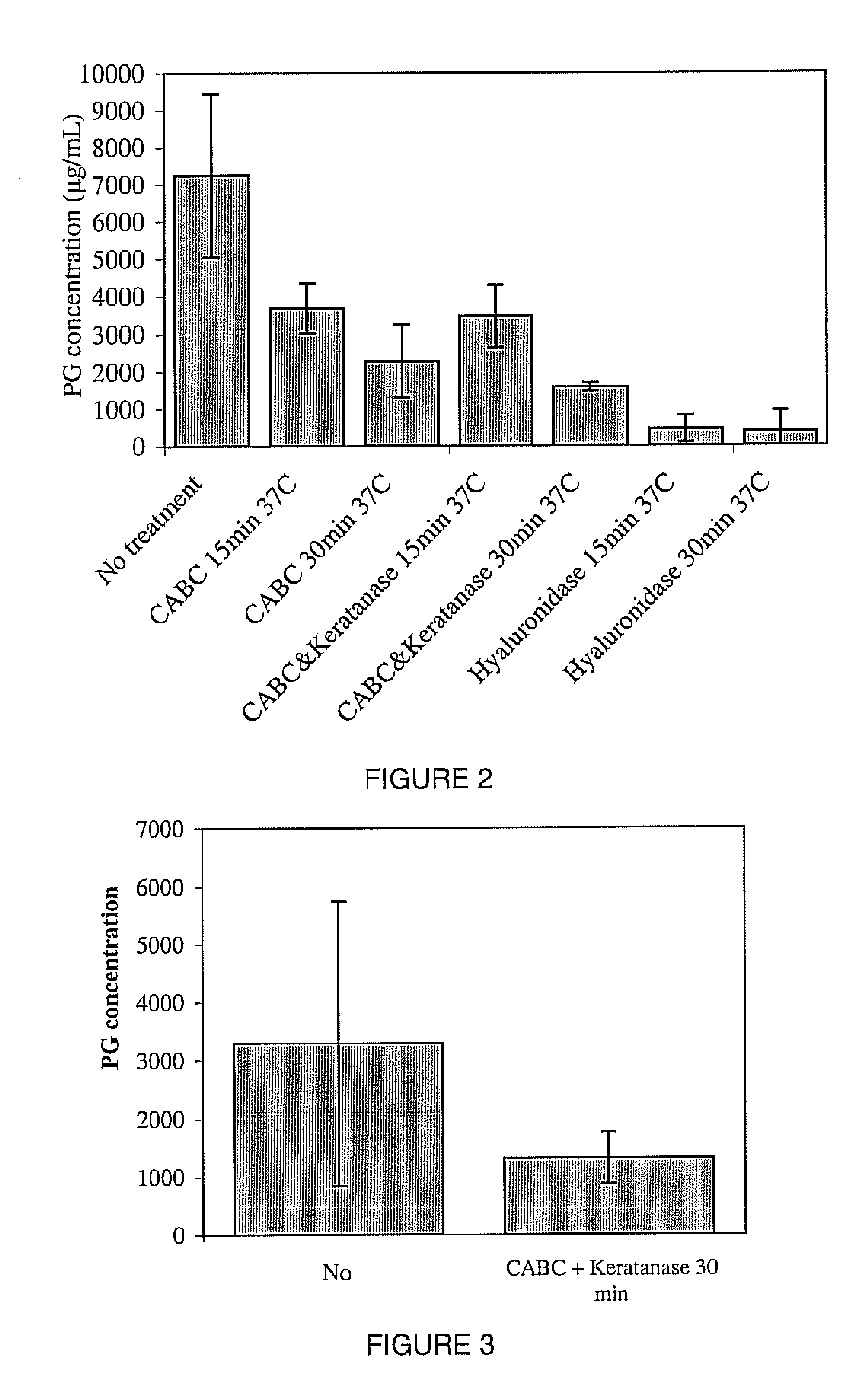
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
691 results about "Chondrocyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
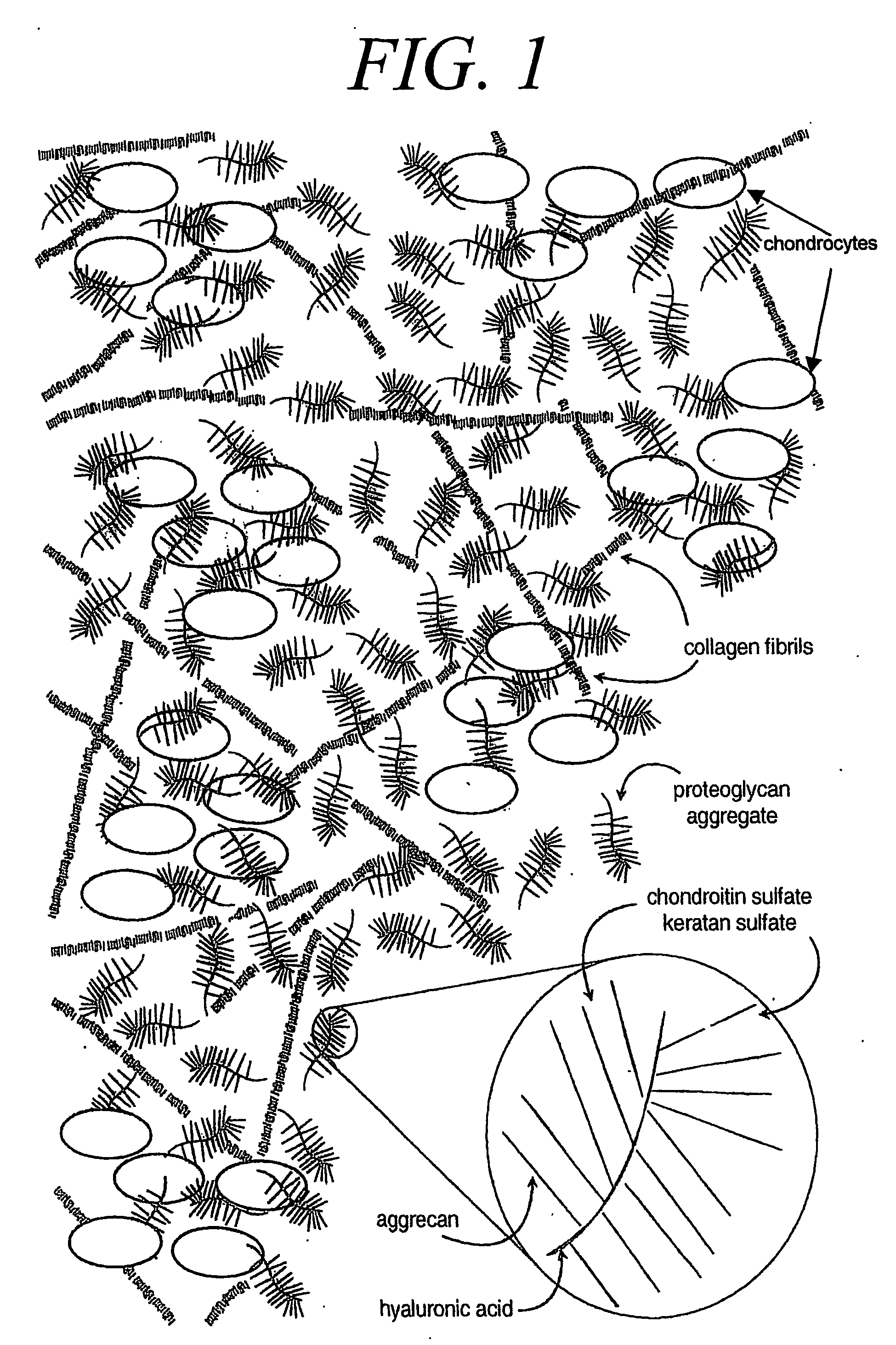
Chondrocytes (/ˈkɒndrəsaɪt, -droʊ-/, from Greek χόνδρος, chondros = cartilage + κύτος, kytos = cell) are the only cells found in healthy cartilage. They produce and maintain the cartilaginous matrix, which consists mainly of collagen and proteoglycans. Although the word chondroblast is commonly used to describe an immature chondrocyte, the term is imprecise, since the progenitor of chondrocytes (which are mesenchymal stem cells) can differentiate into various cell types, including osteoblasts.
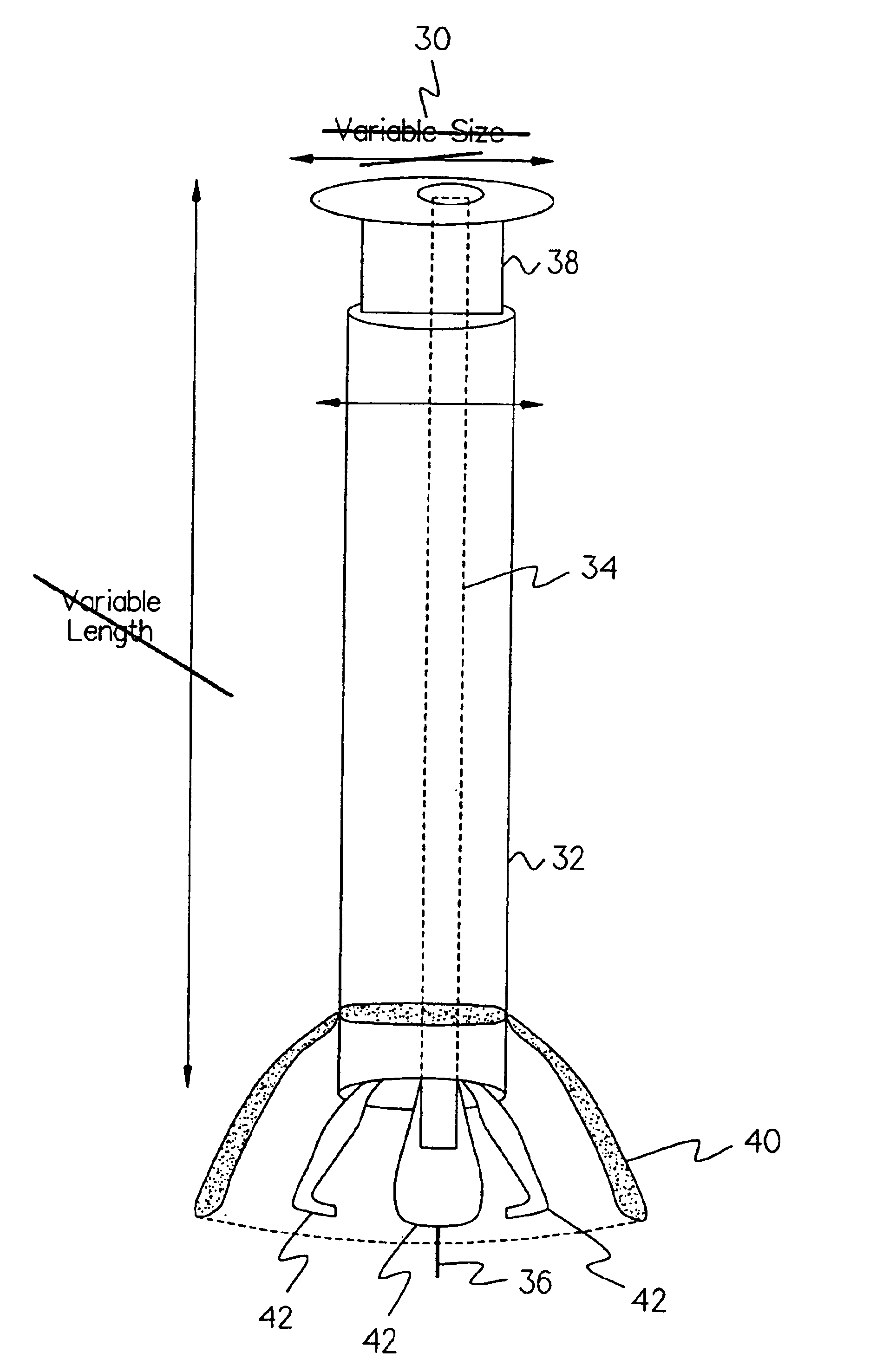
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
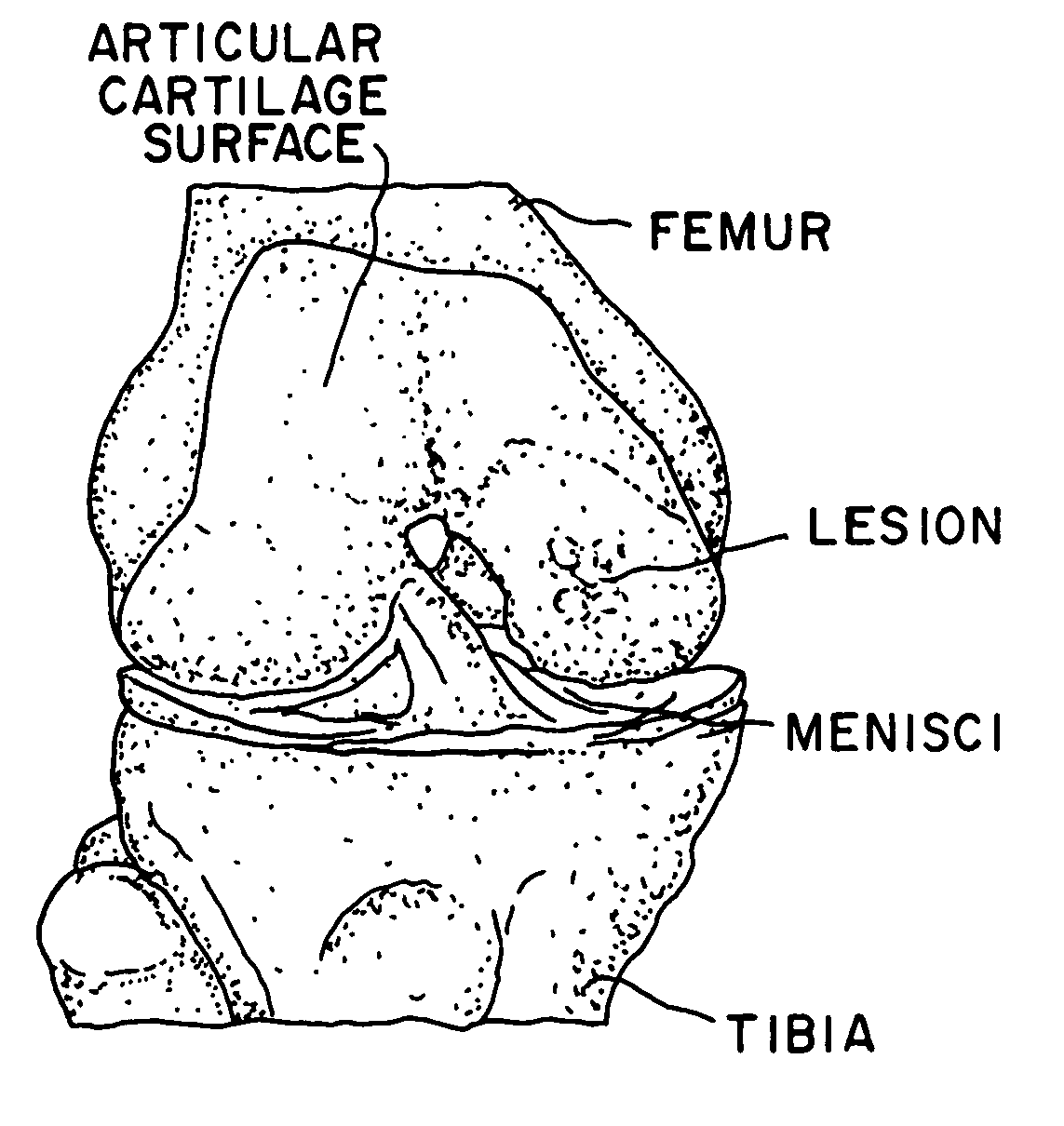
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
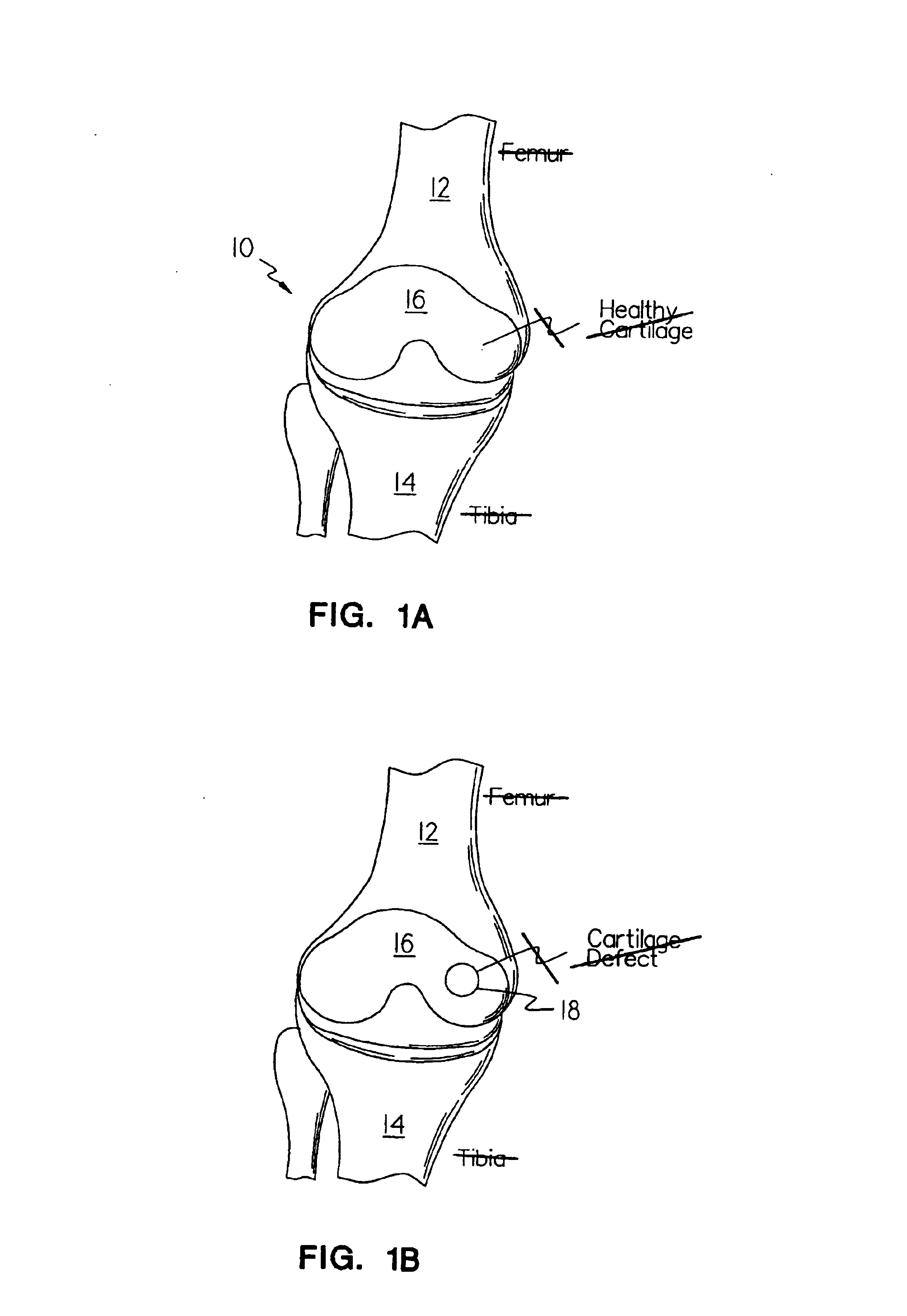
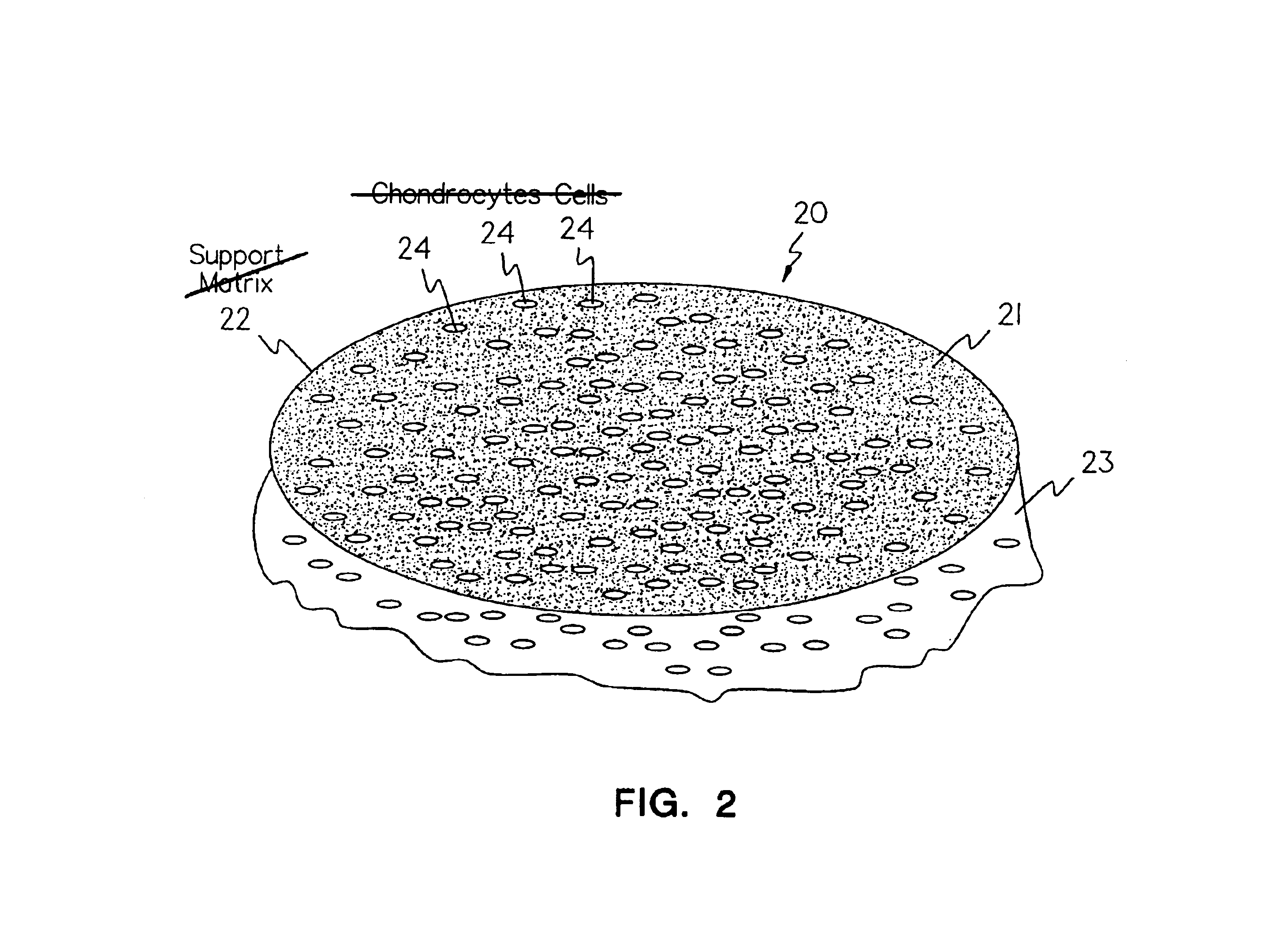
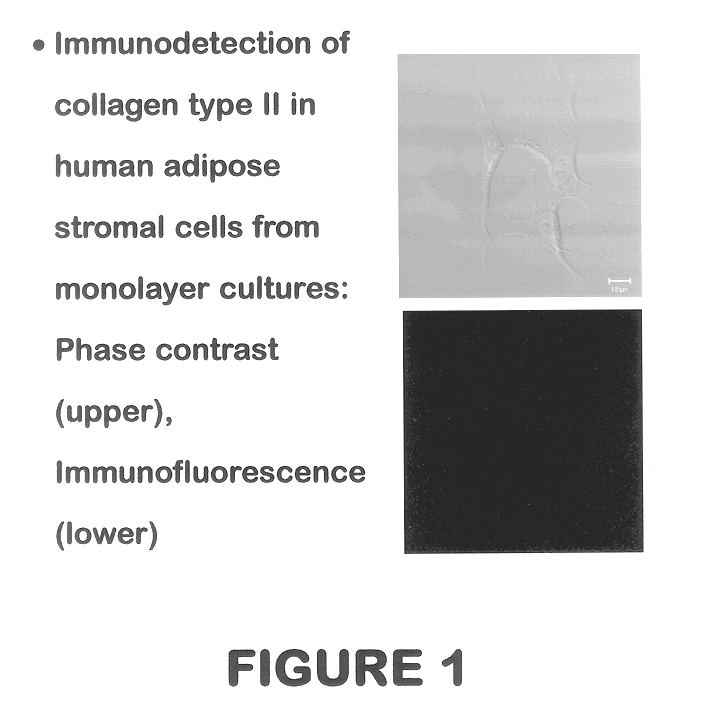
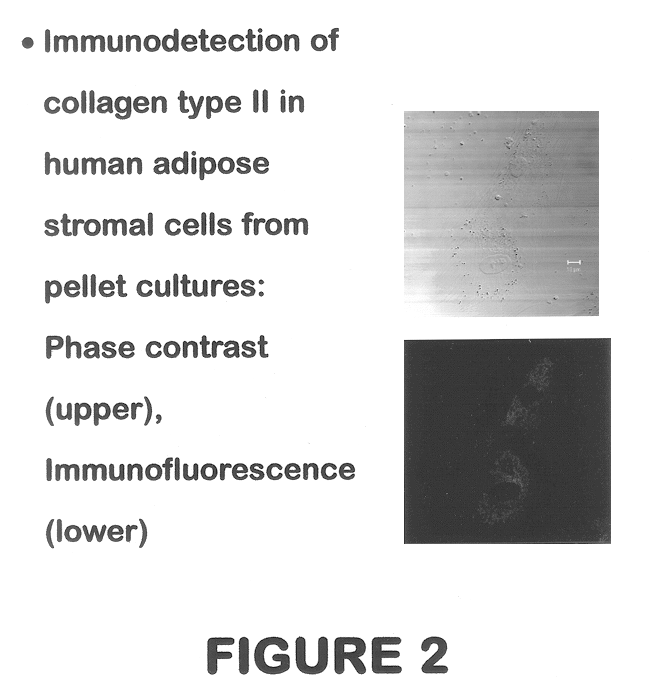
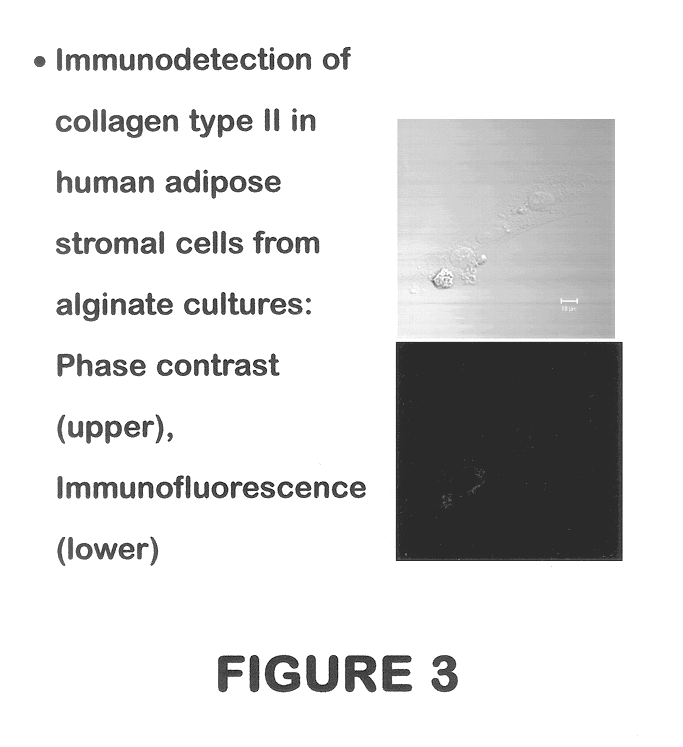
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Chondroprotective/restorative compositions and methods of use thereof
The instant invention provides a method of treating or preventing osteoarthritis, joint effusion, joint inflammation and pain, synovitis, lameness, post operative arthroscopic surgery, deterioration of proper joint function including joint mobility, the reduction or inhibition of metabolic activity of chondrocytes, the activity of enzymes that degrade cartilage, the reduction or inhibition of the production of Hyaluronic acid, said method comprising orally administering to a mammalian species a therapeutically effective amount of Hyaluronic Acid or pharmaceutically acceptable salts thereof. Additionally, compositions containing hyaluronic acid; chondroitin sulfate, and glucosamine sulfate in a paste formulation are also disclosed which can be administered on their own or can be used as a feed additive.
Owner:PIERCE SCOTT W
Cell-culture and polymer constructs
InactiveUS6378527B1Efficient disseminationPerfect cartilage repair surgeryDiagnosticsSurgeryImplanted deviceTissue replacement
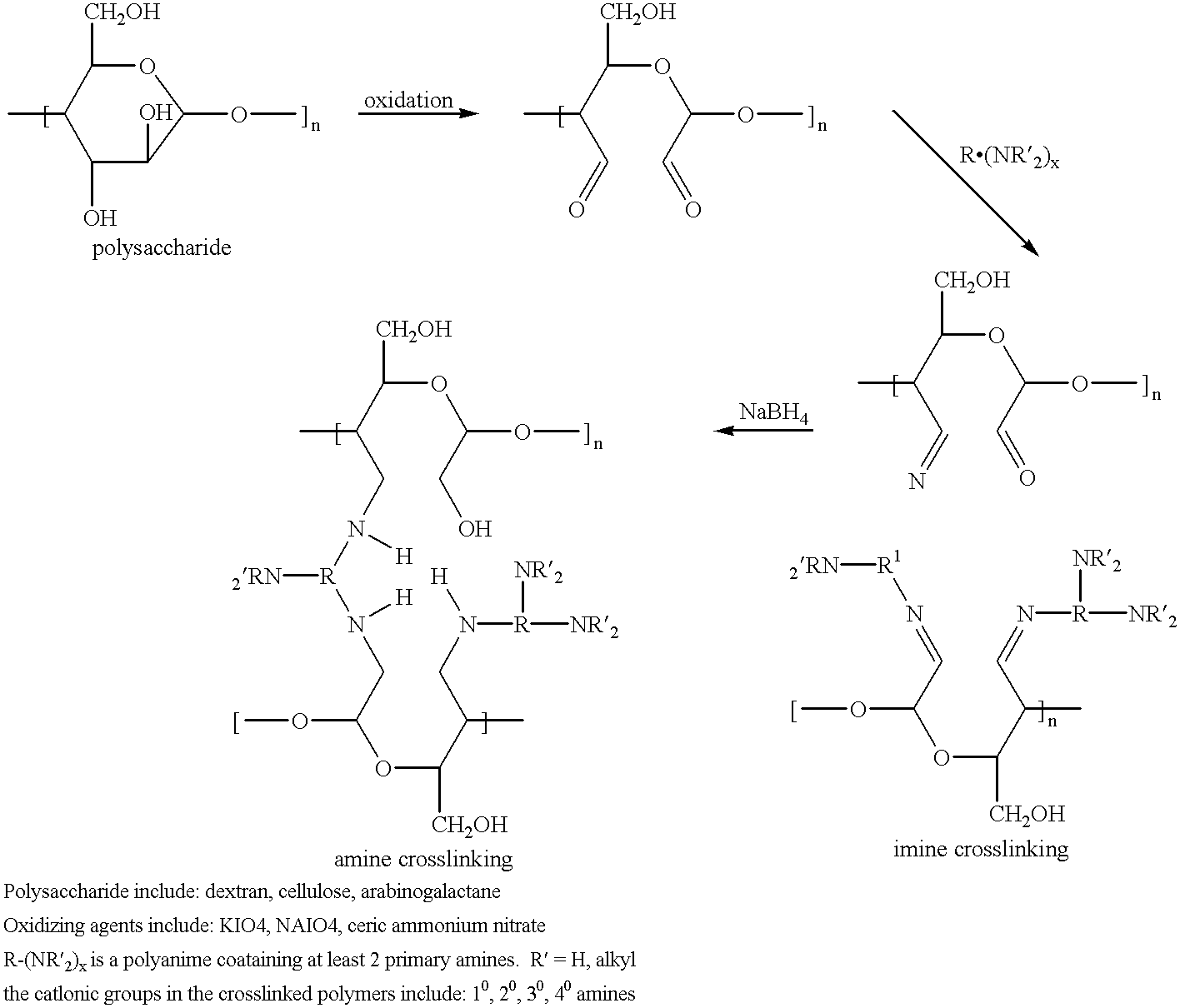
Cells grown on a microcarrier are separated from the microcarrier by enzymatically digesting the microcarrier. More specifically, chondrocytes may be grown on dextran microcarrier beadlets and then the beadlets digested using dextranase to separate the chondrocytes from the carrier. Cells can also be grown on chitosan microcarriers to be used for implantation. In addition, cells can be grown on polysaccharide polymers to be used as implant devices. Various polymers serve as scaffolds for cells to be used for implantation. The polymers can be used for cell culture as well as for preparing scaffolds useful for tissue replacement such as cartilage tissue.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Chondroprotective/restorative compositions and methods of use thereof
The instant invention provides a method of treating or preventing osteoarthritis, joint effusion, joint inflammation and pain, synovitis, lameness, post operative arthroscopic surgery, deterioration of proper joint function including joint mobility, the reduction or inhibition of metabolic activity of chondrocytes, the activity of enzymes that degrade cartilage, the reduction or inhibition of the production of Hyaluronic acid, said method comprising orally administering to a mammalian species a therapeutically effective amount of Hyaluronic Acid or pharmaceutically acceptable salts thereof. Additionally, compositions containing hyaluronic acid; chondroitin sulfate, and glucosamine sulfate in a paste formulation are also disclosed which can be administered on their own or can be used as a feed additive.
Owner:PIERCE SCOTT W
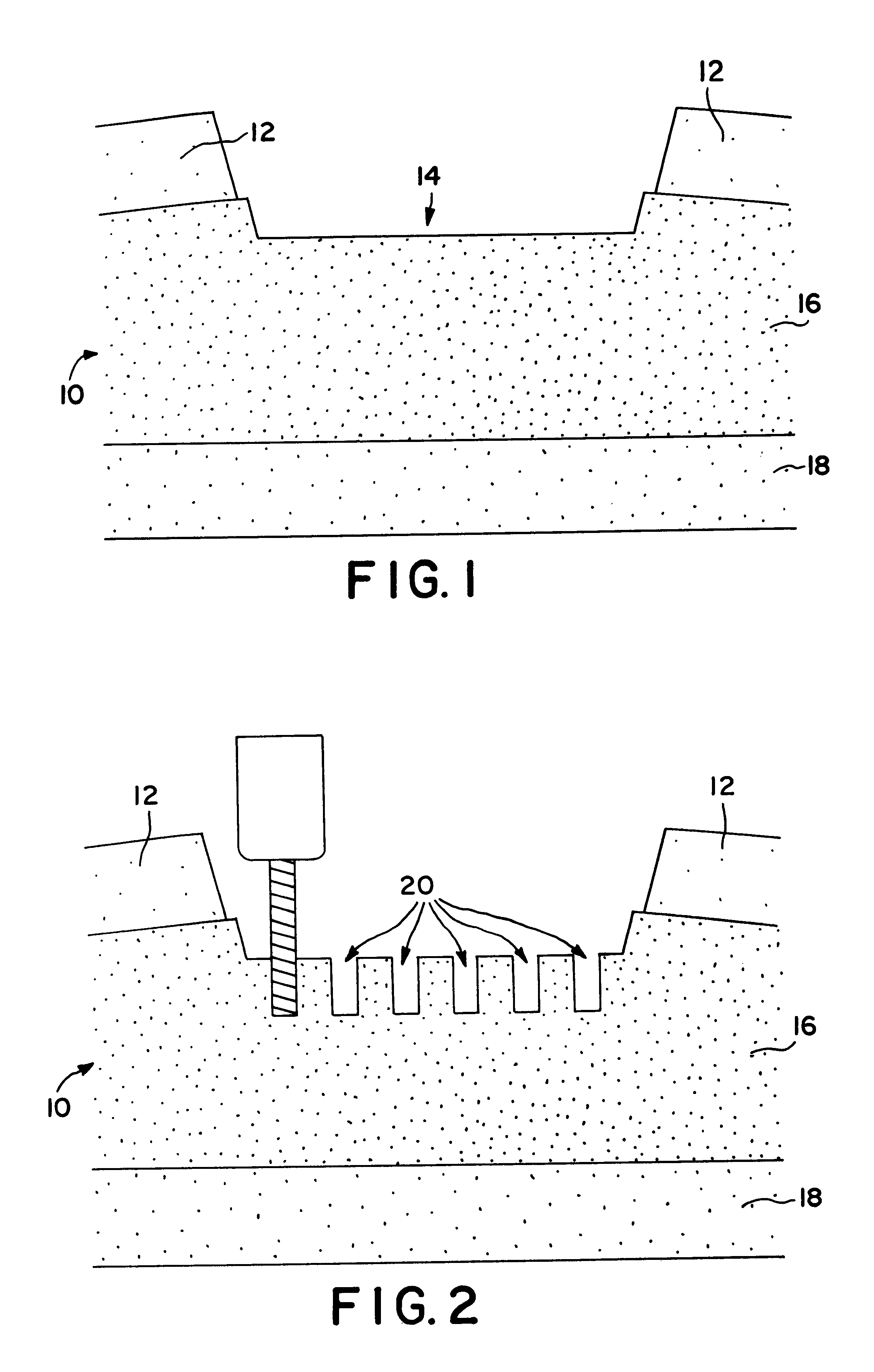
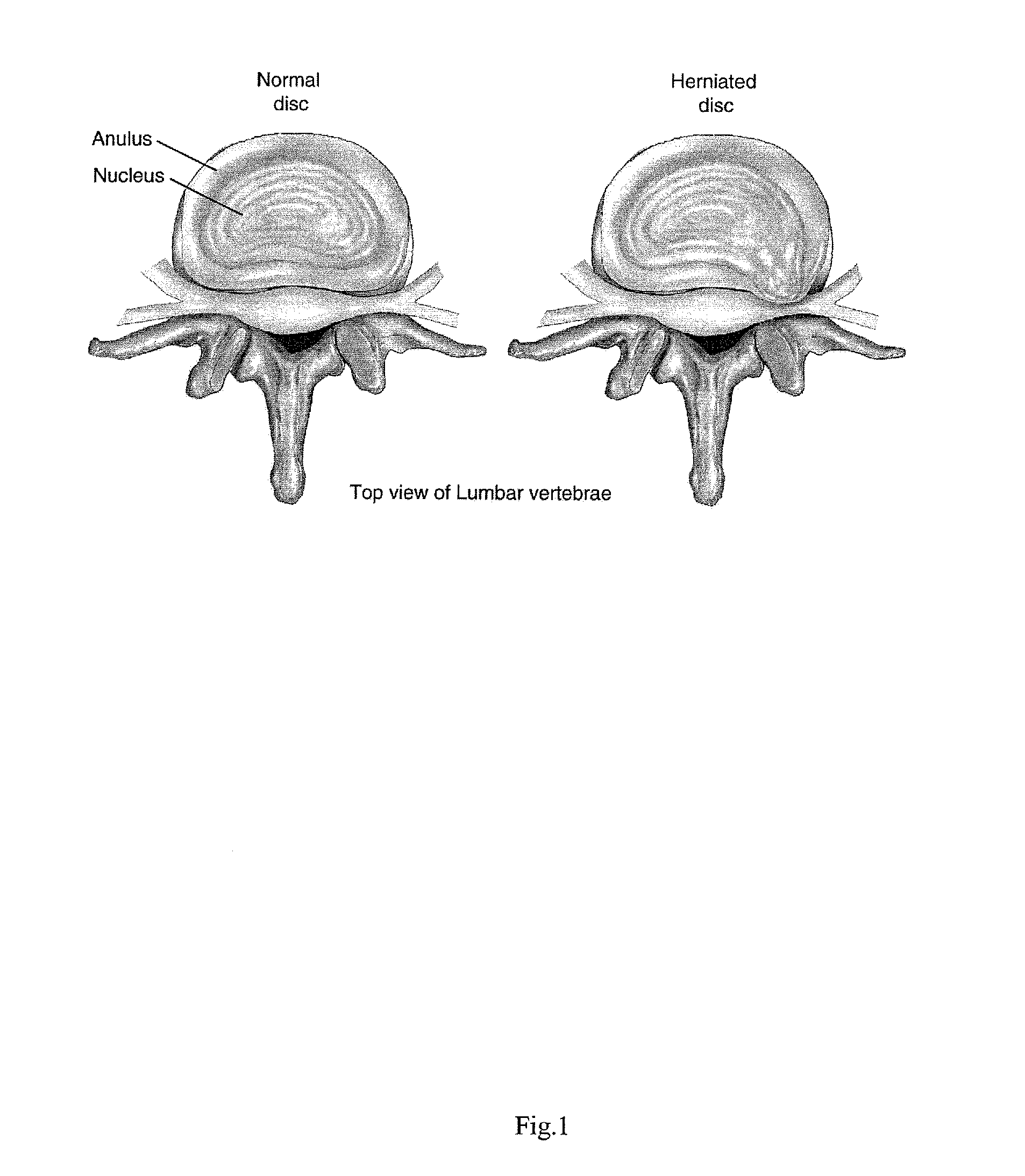
Compositions and methods for augmentation or repair of intervertebral discs
InactiveUS20050118228A1Enhance the imageGood treatment effectBiocideBone implantStem Cell IsolationIntervertebral disc
A method of augmenting and / or repairing an intervertebral disc by administering stem cell material into the disc. The stem cells may be undifferentiated cells, or they may be cells that have differentiated and have subsequently been dedifferentiated. The stem cells may be induced to express at least one characteristic of human intervertebral disc cells, such as fibroblast cells, chondrocyte cells, or notochordal cells, by exposing them to agents and / or environments calculated to induce the desired differentiation. In some embodiments, the stem cell material may be provided in conjunction with a collagen-based material, which may be a collagen-rich lattice or particles of collagen material. The stem cell material may be provided as a stem cell isolate, which may be substantially free of non-stem cell material. Other therapeutic agents may be administered with the stem cell material.
Owner:SDGI HLDG
Resorbable polymeric device for localized drug delivery
InactiveUS20050177118A1Good adhesionMinimizes procedural discomfortMedical devicesMammal material medical ingredientsOsteoblastHigh doses
An implantable device for facilitating the healing of voids in bone, cartilage and soft tissue is disclosed. A preferred embodiment includes a cartilage region comprising a polyelectrolytic complex joined with a subchondral bone region. The cartilage region, of this embodiment, enhances the environment for chondrocytes to grow articular cartilage; while the subchondral bone region enhances the environment for cells which migrate into that region's macrostructure and which differentiate into osteoblasts. Another embodiment is arranged for the local delivery of therapeutic agent. A preferred embodiment is a porous resorbable implant, wherein the therapy delivery may be localized in nature, rather than systemic, such that higher doses at the target site may be allowed than would be tolerable by the body systemically.
Owner:KENSEY NASH CORP
Method for fabricating cell-containing implants
InactiveUS6886568B2Negate needImprove fixation and localizationBiocideDiagnosticsMicrocarrierBody region
Owner:CHONDROS +1
Device for regeneration of articular cartilage and other tissue
InactiveUS20050074481A1Good adhesionMinimizes procedural discomfortBone implantMammal material medical ingredientsSubchondral boneOsteoblast
An implantable device for facilitating the healing of voids in bone, cartilage and soft tissue is disclosed. A preferred embodiment includes a cartilage region comprising a polyelectrolytic complex joined with a subchondral bone region. The cartilage region, of this embodiment, enhances the environment for chondrocytes to grow articular cartilage; while the subchondral bone region enhances the environment for cells which migrate into that region's macrostructure and which differentiate into osteoblasts. A hydrophobic barrier exists between the regions, of this embodiment. In one embodiment, the polyelectrolytic complex transforms to hydrogel, following the implant procedure.
Owner:KENSEY NASH CORP
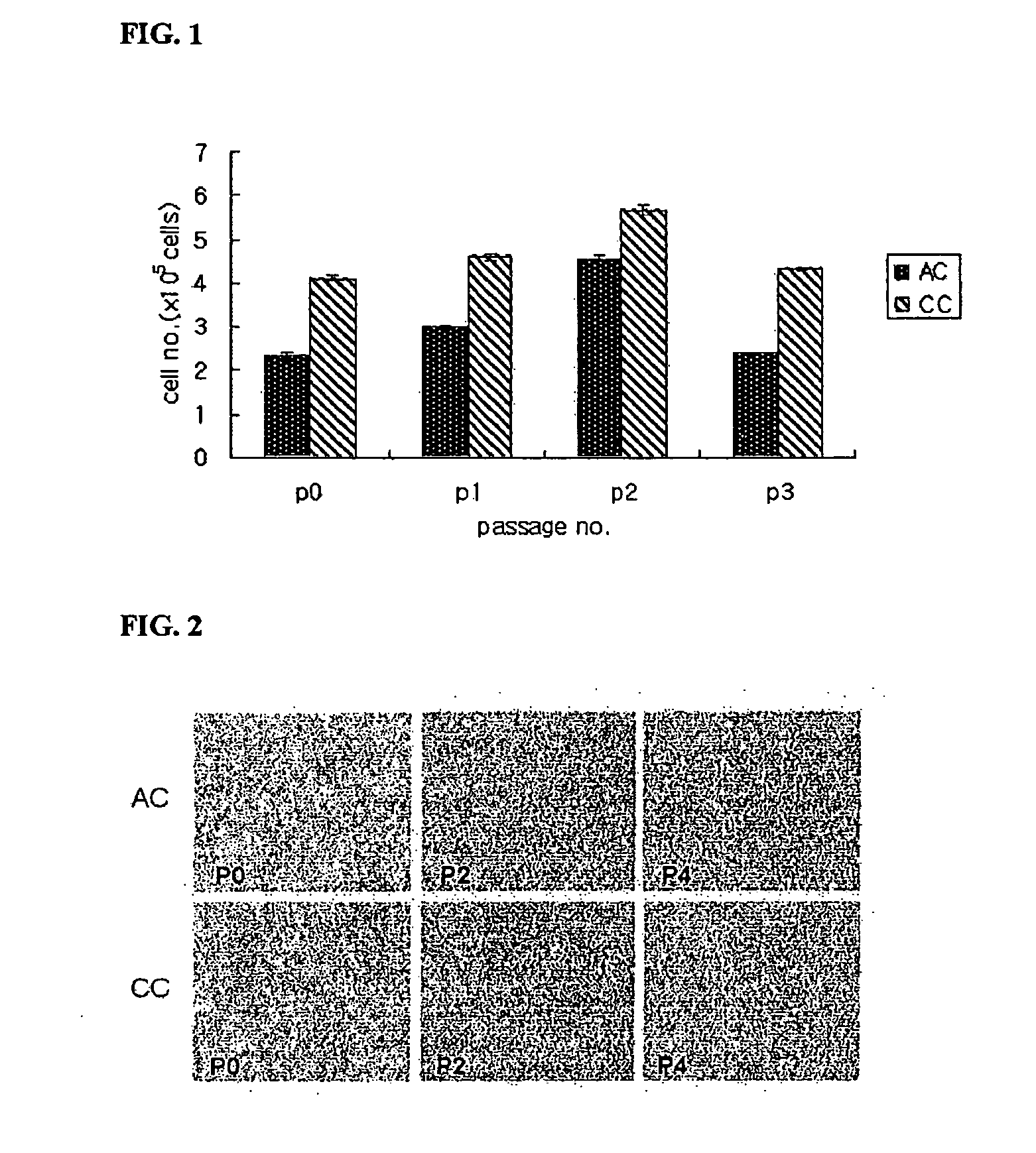
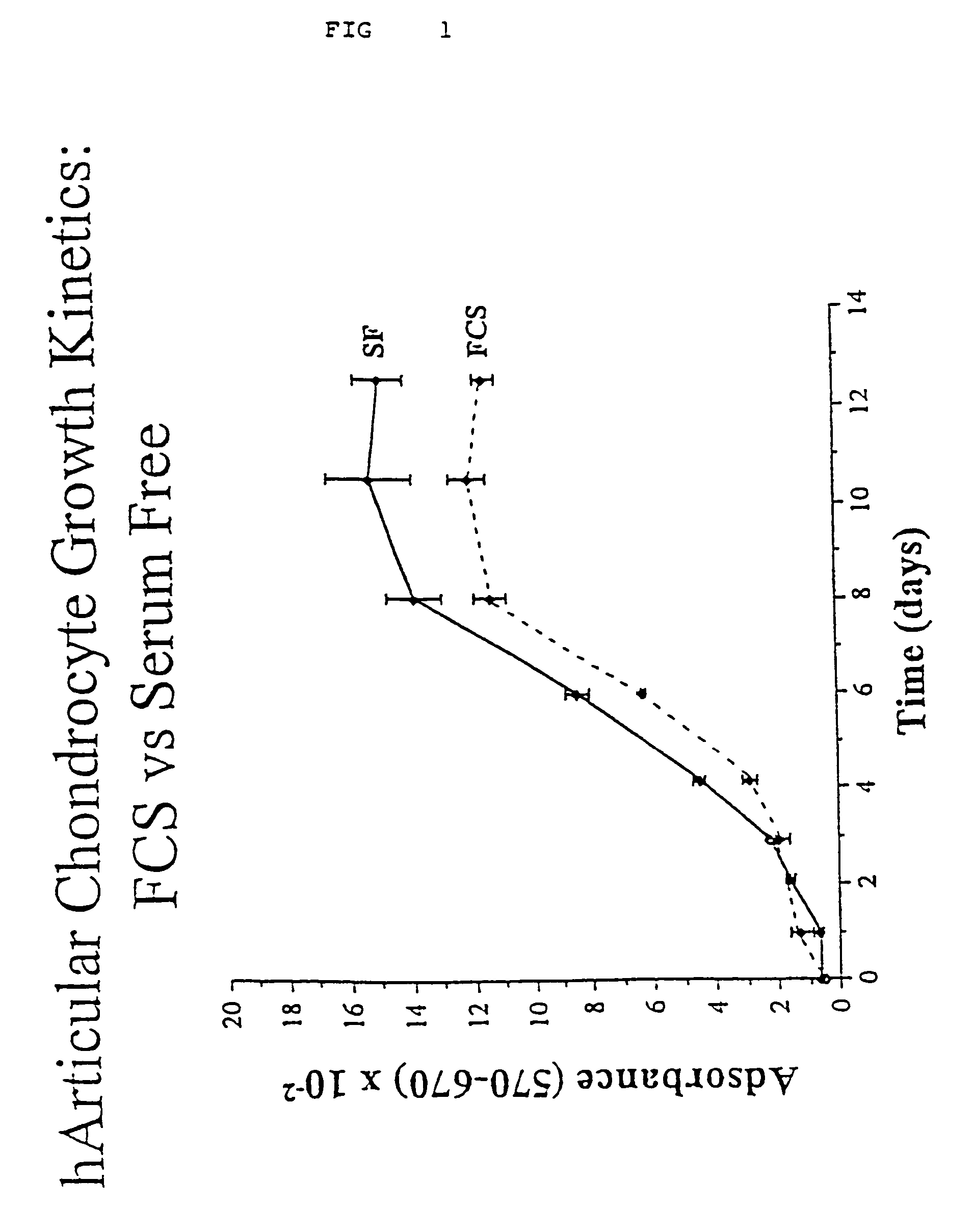
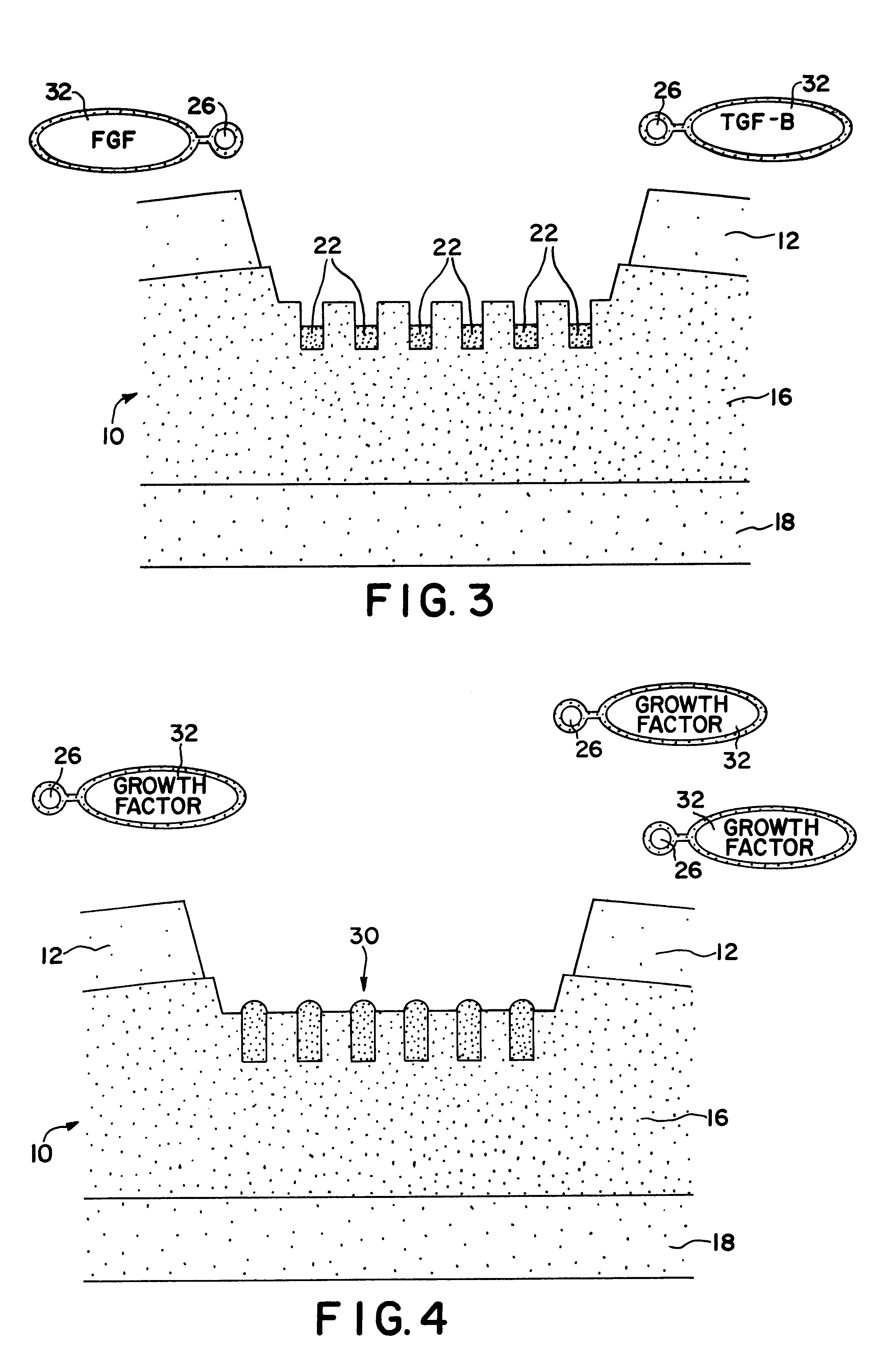
Serum-free media for chondrocytes and methods of use thereof
InactiveUS7169610B2Safe effective inexpensiveSafe and effective and inexpensiveCulture processArtificial cell constructsLipid formationSerum free media
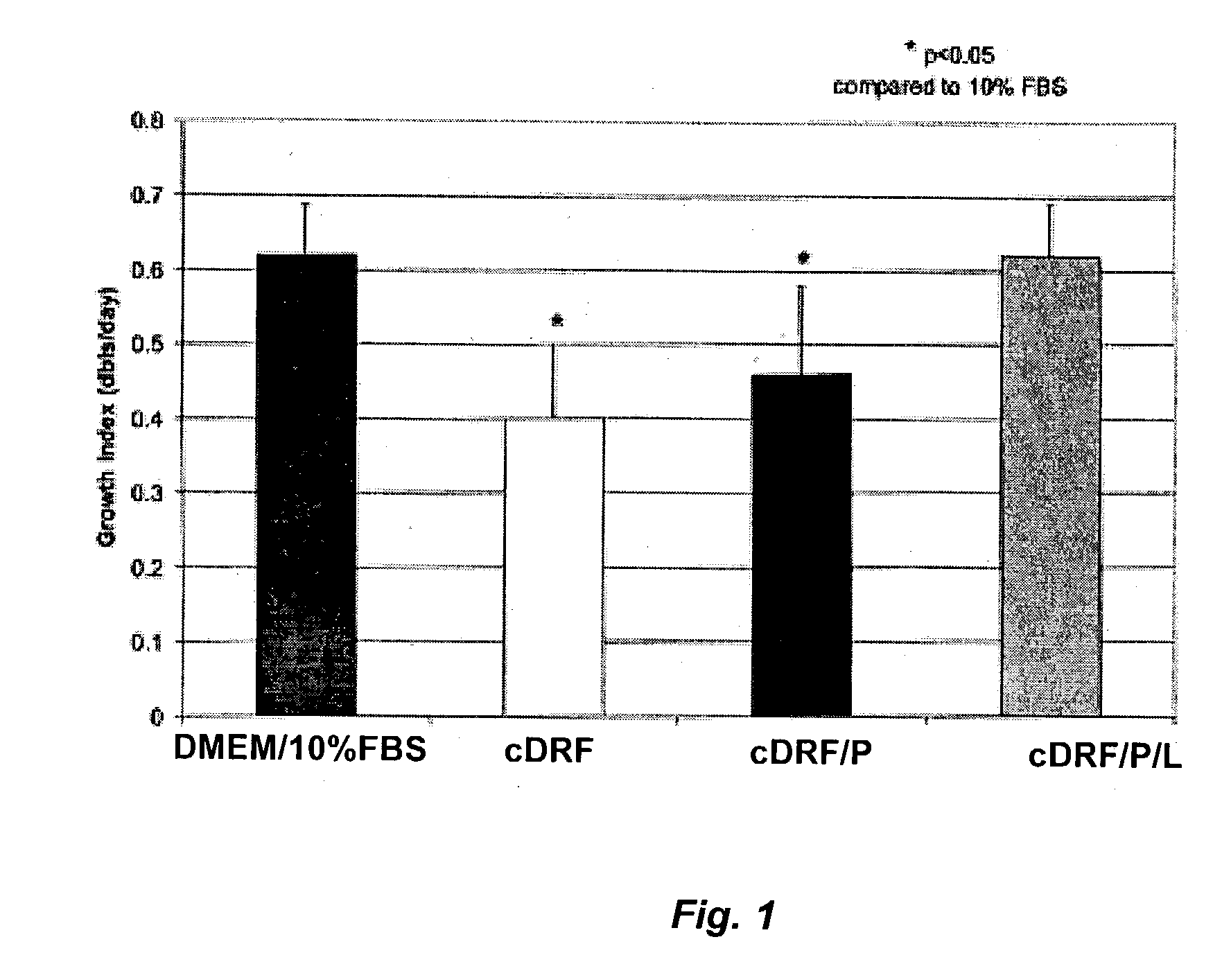
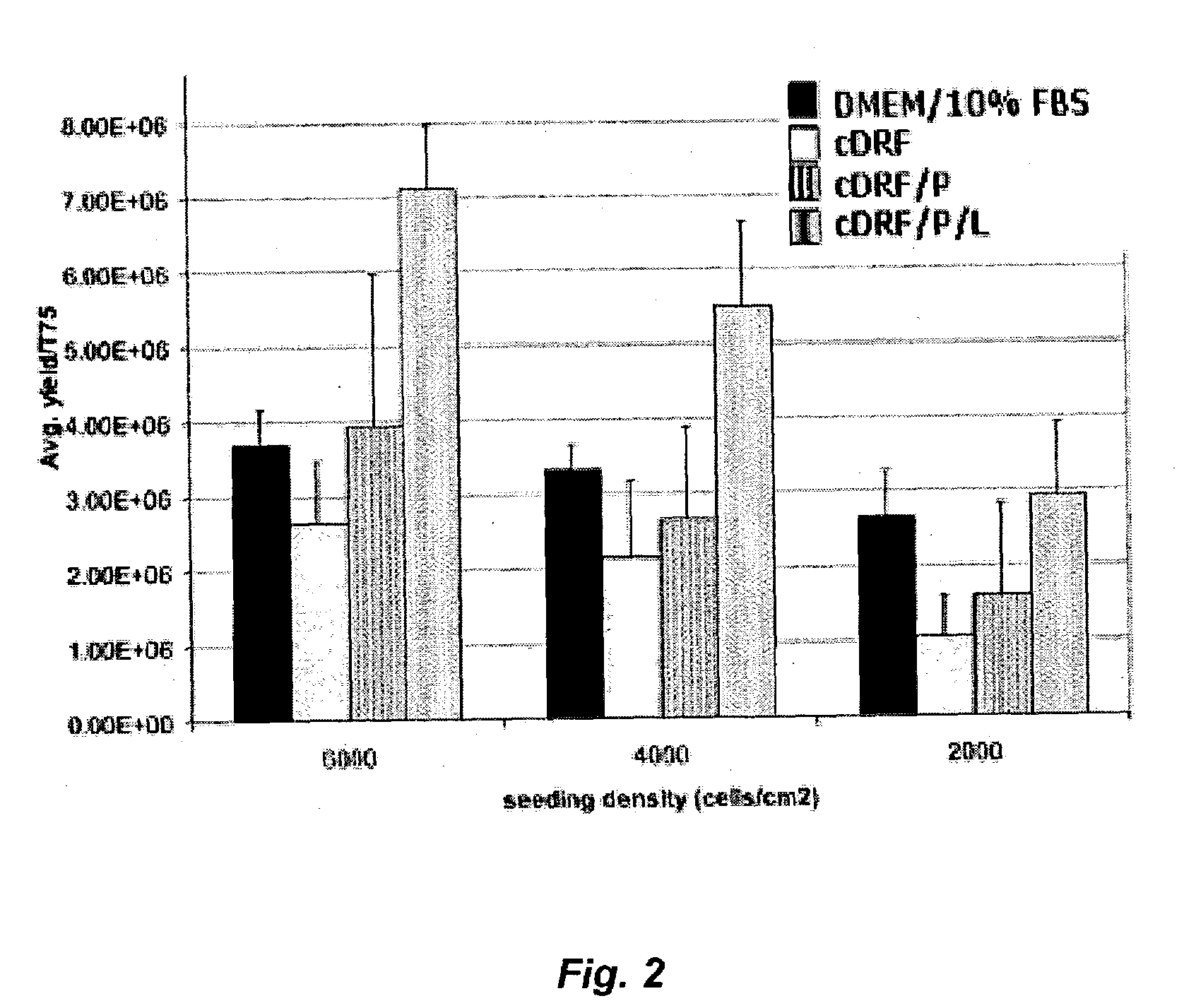
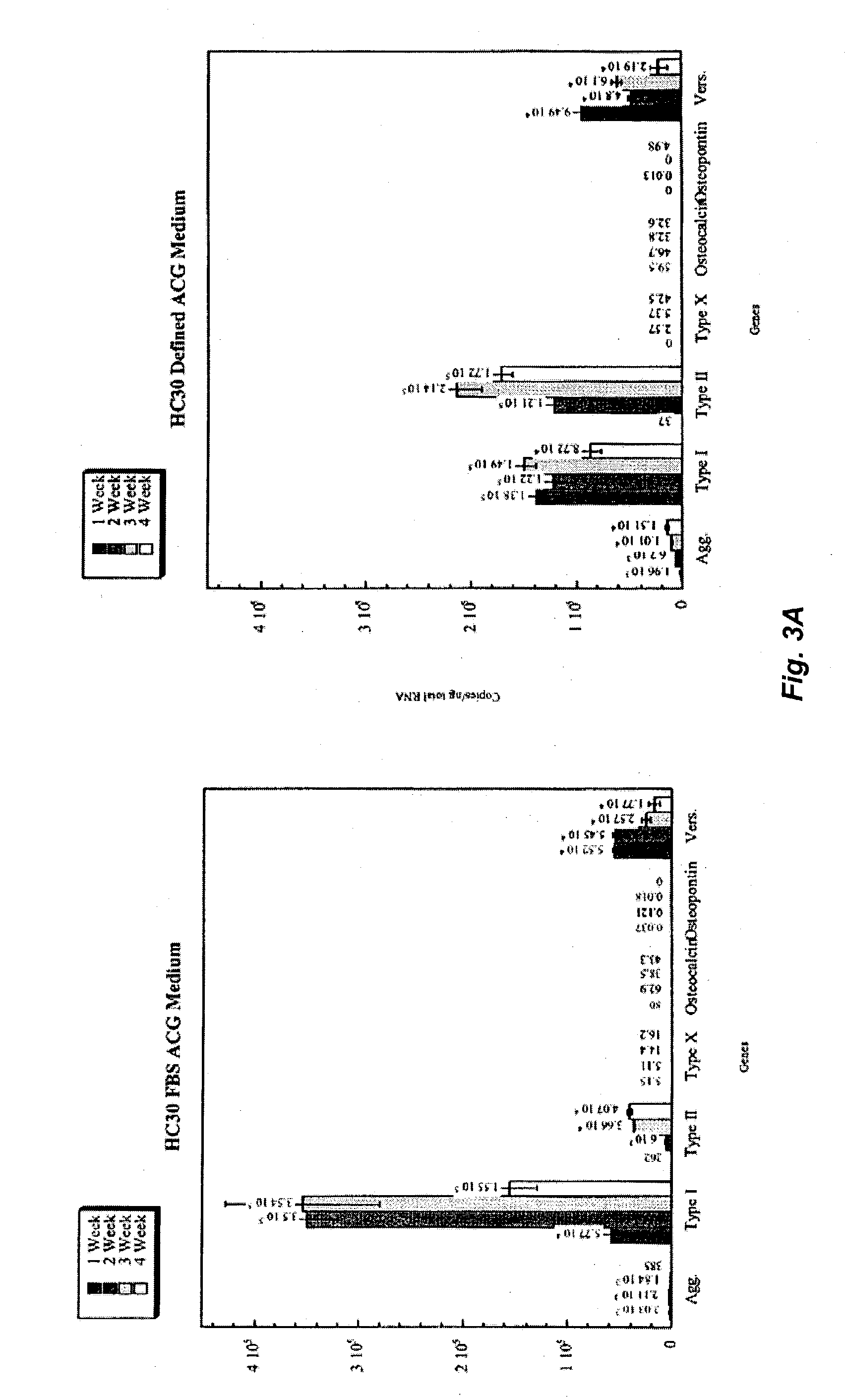
The present invention provides defined serum-free cell culture media useful in culturing fibroblasts, especially articular chondrocytes, that avoids problems inherent in the use of serum-containing media. The defined media comprise platelet-derived growth factor (PDGF), and chemically defined lipids, or combinations of these compounds. In another aspect, the present invention also provides tissue culture methods that comprise incubating chondrocytes in the defined serum free media. The methods enhance attachment and proliferative expansion of chondrocytes seeded at low density while maintaining their redifferentiation potential.
Owner:GENZYME CORP
Glue for cartilage repair
ActiveUS7067123B2Promote migrationIncreased proliferationBiocidePeptide/protein ingredientsMedicineBone marrow cell
The invention is directed toward a sterile cartilage defect implant material comprising milled lyophilized allograft cartilage pieces ranging from 0.01 mm to 1.0 mm in size in a bioabsorbable carrier taken from a group consisting of sodium hyaluronate, hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran or polymers with allogenic chondrocytes or bone marrow cells in an amount exceeding the natural occurrence of same in hyaline cartilage and adding a cell growth additive.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Graft collar and scaffold apparatuses for musculoskeletal tissue engineering and related methods
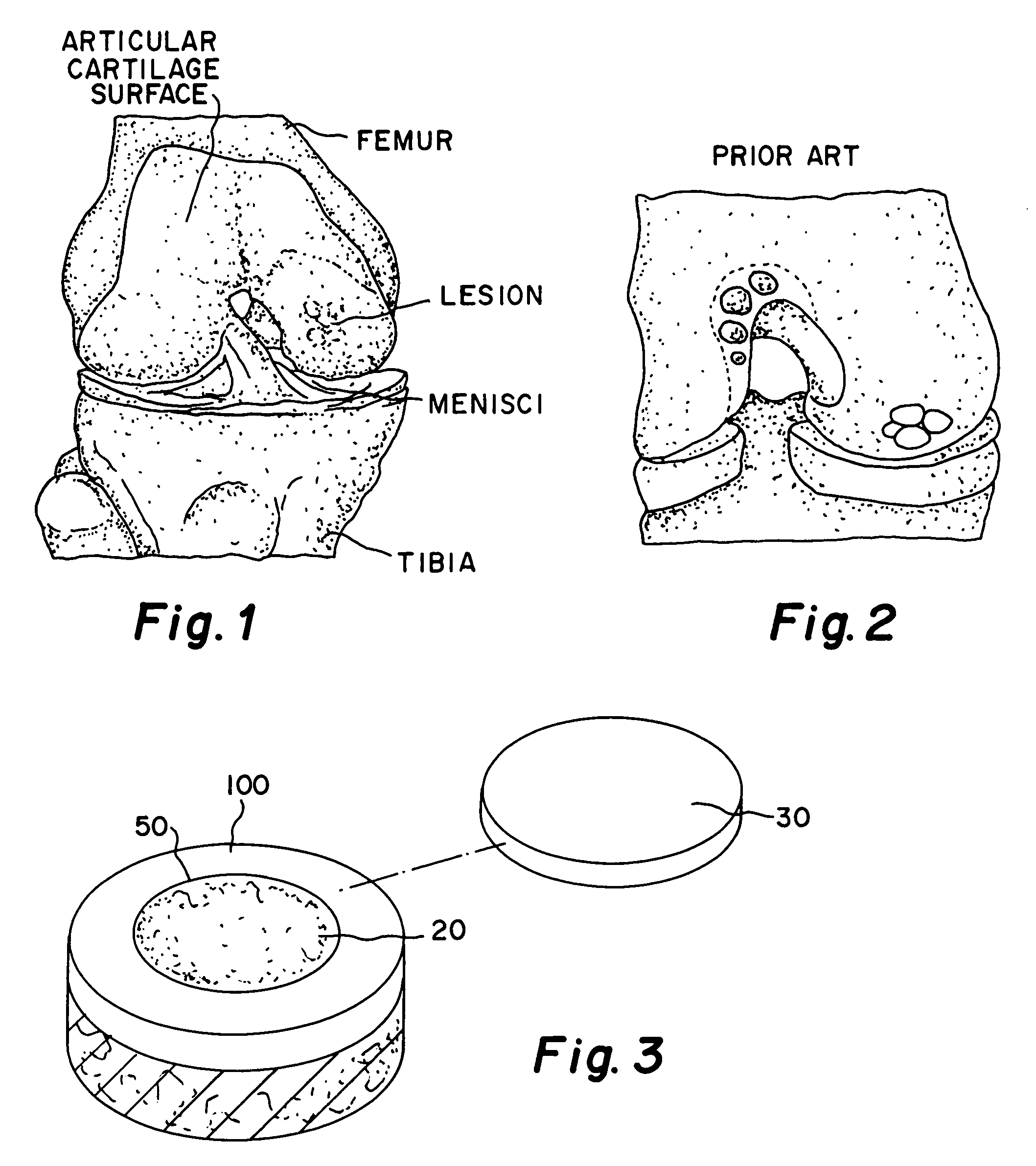
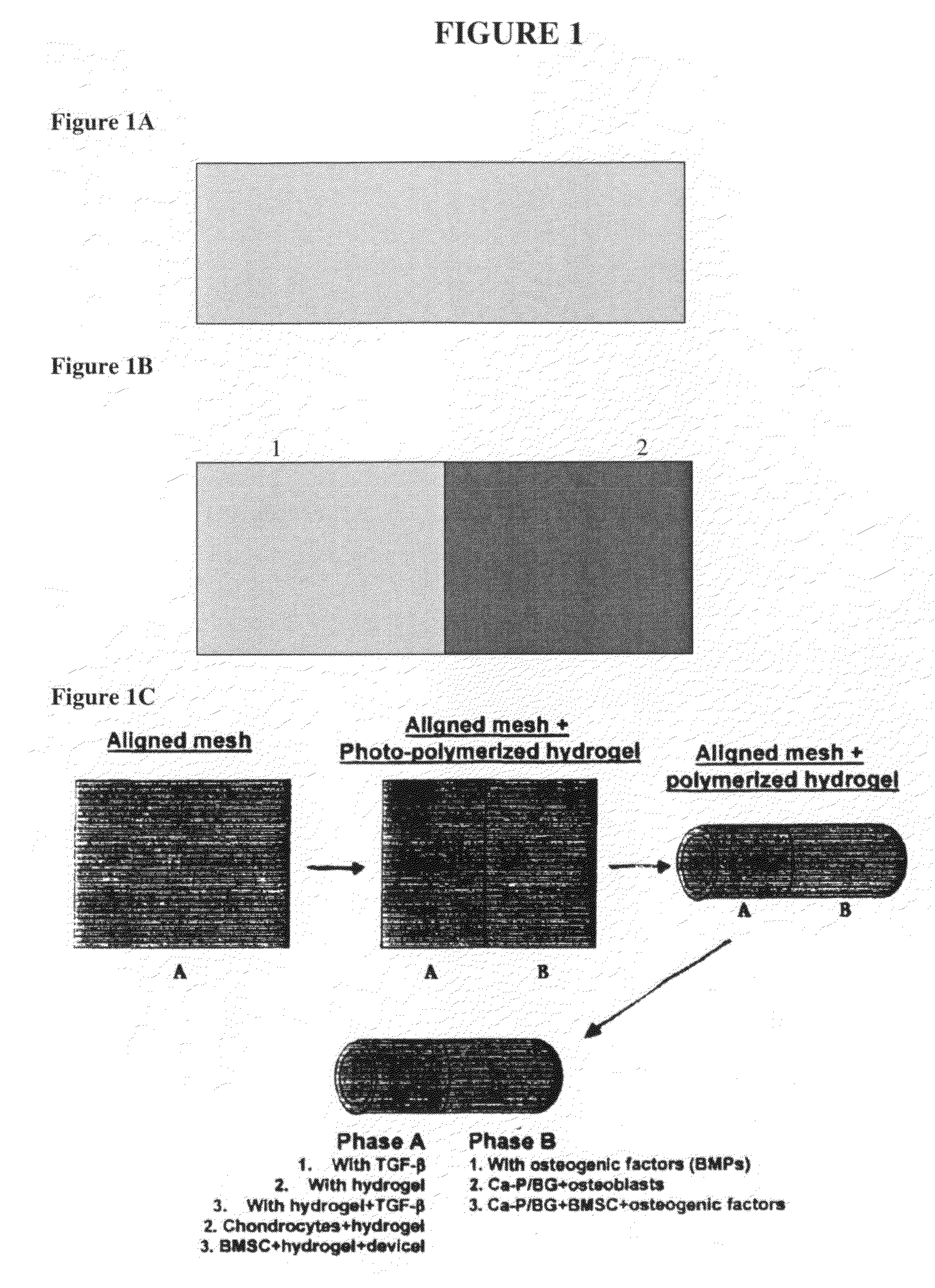
This application describes apparatuses and methods for musculoskeletal tissue engineering. Specifically, graft collar and scaffold apparatuses are provided for promoting fixation of musculoskeletal soft tissue to bone.This application provides for graft collars comprising biopolymer mesh and / or polymer-fiber mesh for fixing tendon to bone. In one aspect, the graft collar comprises more than one region, wherein the regions can comprise different materials configured to promote integration of and the regeneration of the interfacial region between tendon and bone.This application also provides for scaffold apparatuses and methods for fixing musculoskeletal soft tissue to bone. The scaffold apparatus is multiphasic, preferably triphasic, and each phase is configured promote growth and proliferation of a different cell and its associated tissue. In one aspect, the scaffold apparatus is triphasic, with phases comprising materials to promote growth and proliferation of fibroblasts, chondroblasts, and osteoblasts. In addition, an apparatus comprising two portions, each of said portion being the scaffold apparatus described above is provided, wherein each of said portion encases one end of a soft tissue graft. Further, a triphasic interference screw is provided.This application further provides apparatuses and methods for inducing formation of fibrocartilage comprising wrapping a graft collar with polymer-fiber mesh configured to apply compression to the graft collar. In another aspect, the polymer-fiber is applied directly to the graft to apply compression to the graft.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
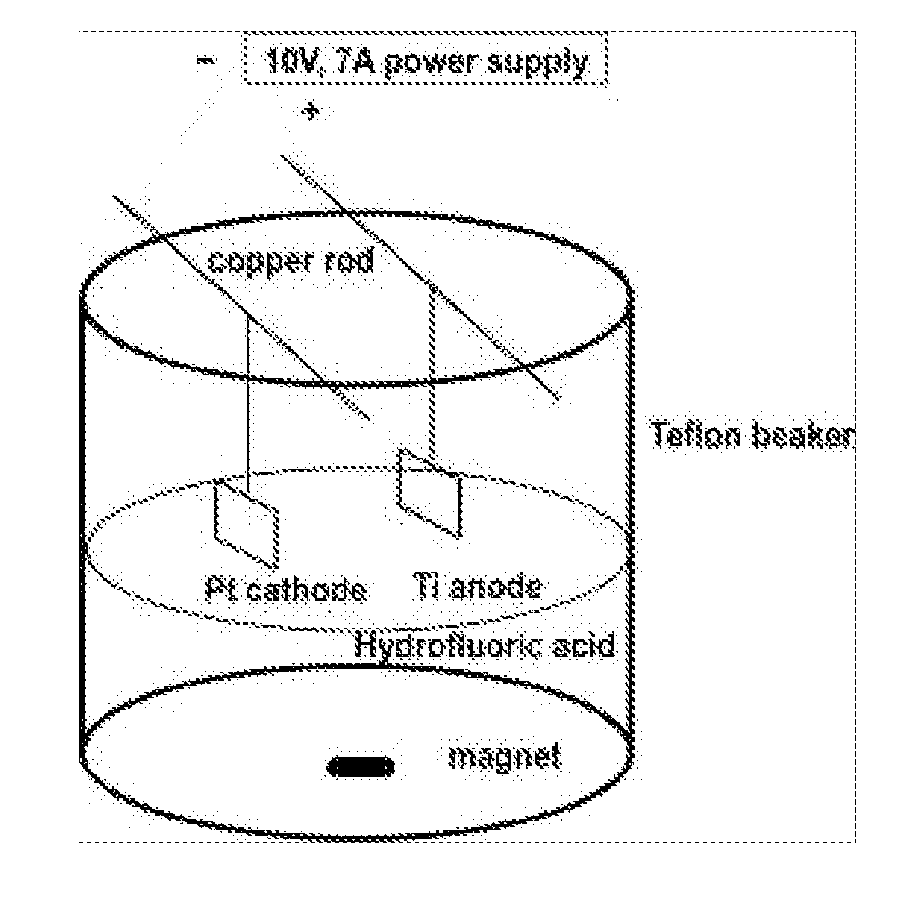
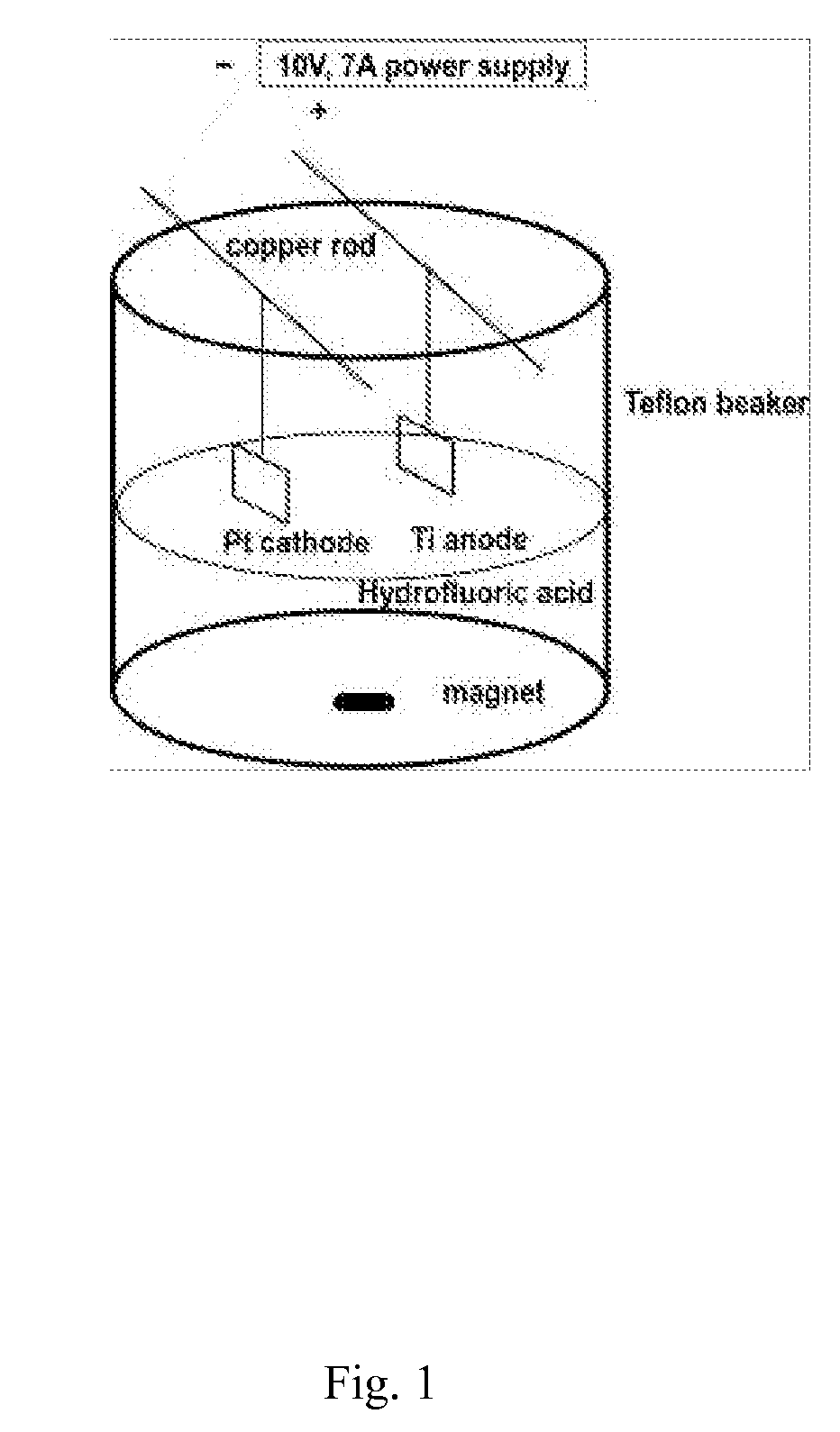
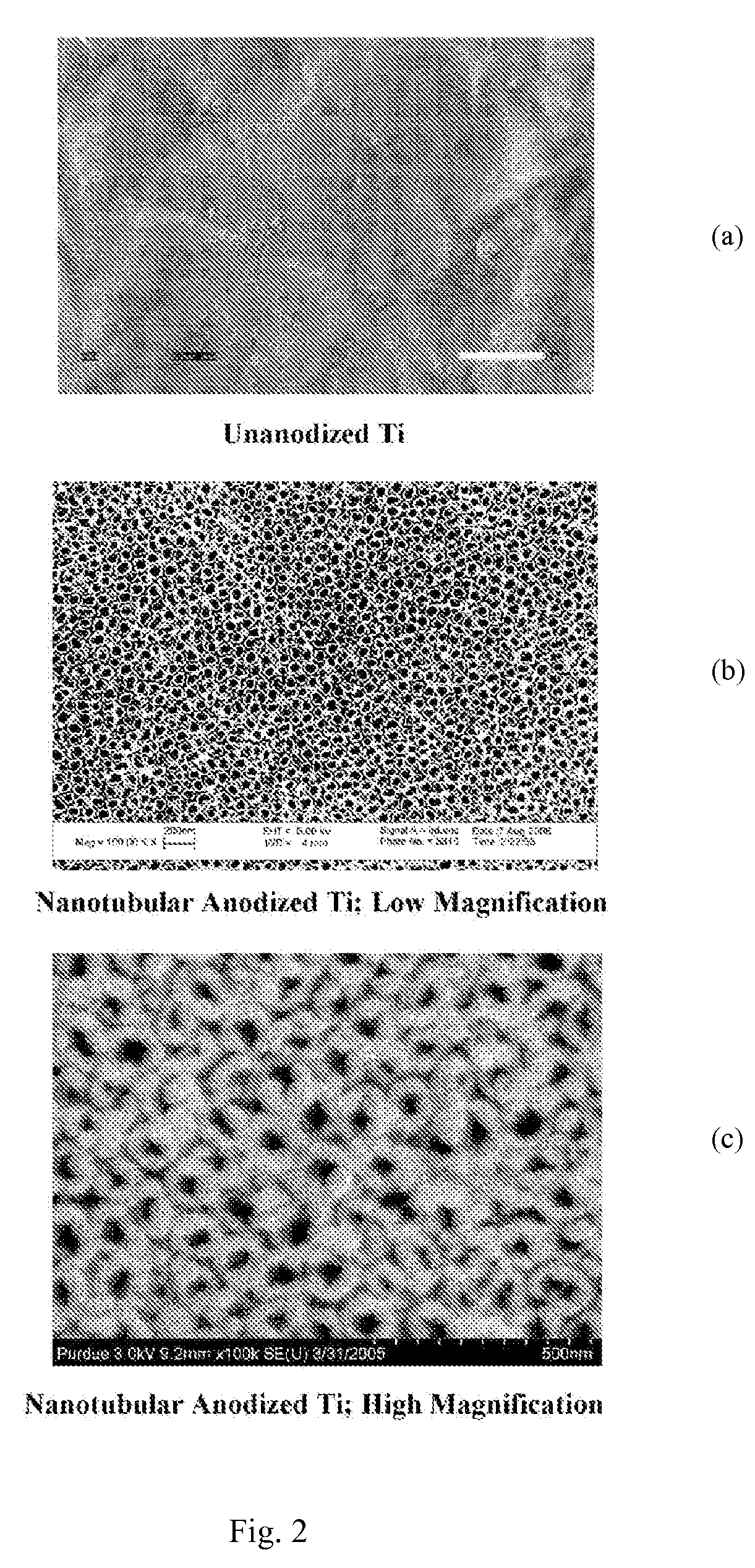
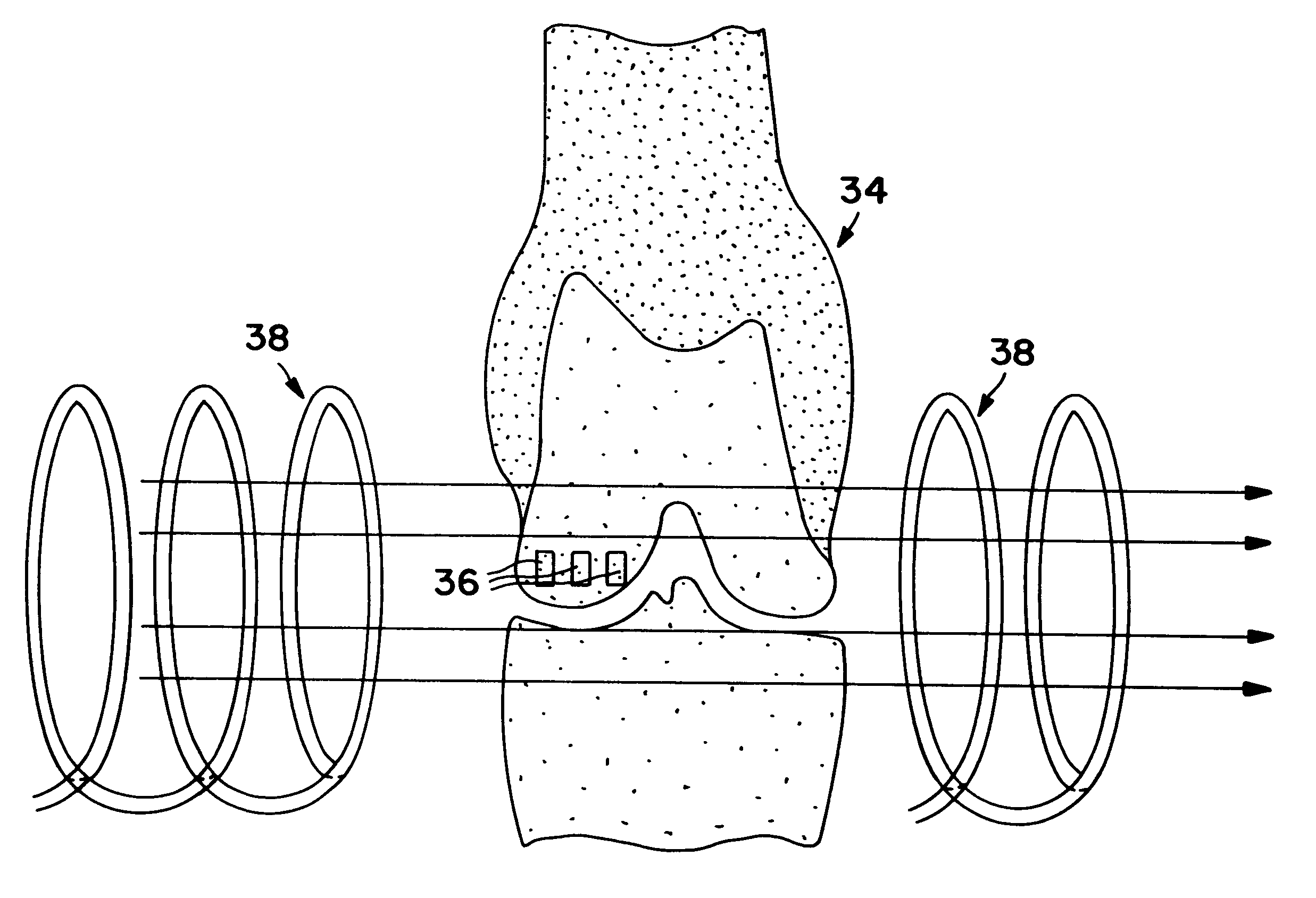
Method for producing nanostructures on a surface of a medical implant
InactiveUS20110125263A1Enhanced and increased in vivo chondrocyte functionalityImprove adhesionSurface reaction electrolytic coatingPharmaceutical delivery mechanismPorosityIn vivo
A method for treating a surface of a medical implant to create nanostructures on the surface that results in increased in-vivo chondrocyte adhesion to the surface. Further, disclosed is a method to fabricate a drug delivery system. The drug delivery system includes a medical implant that has undergone a surface treatment process that results in the modification of the surface configuration and topography. The modified surface acts as a depot or reservoir for loaded biological material, biologic agents or pharmaceutical products. Additionally, a device for delivering pharmaceutical products or other biological materials is disclosed. The device includes integrally attached nanostructures that retain or adsorb the loaded pharmaceutical products and / or biological materials. Further disclosed is a medical implant that includes a surface configured to allow for and regulate protein adsorption. The surface of the medical implant has a layer of nanostructures rigidly attached with varying porosity and orientation that allow for surface protein adsorption to be controlled.
Owner:BROWN UNIVERSITY
Human stem cells originating from human amniotic mesenchymal cell layer
InactiveUS20060281178A1Stable supplyEffective drug deliveryNervous system cellsArtificial cell constructsCell layerOsteocyte
Owner:SAKURAGAWA NORIO +1
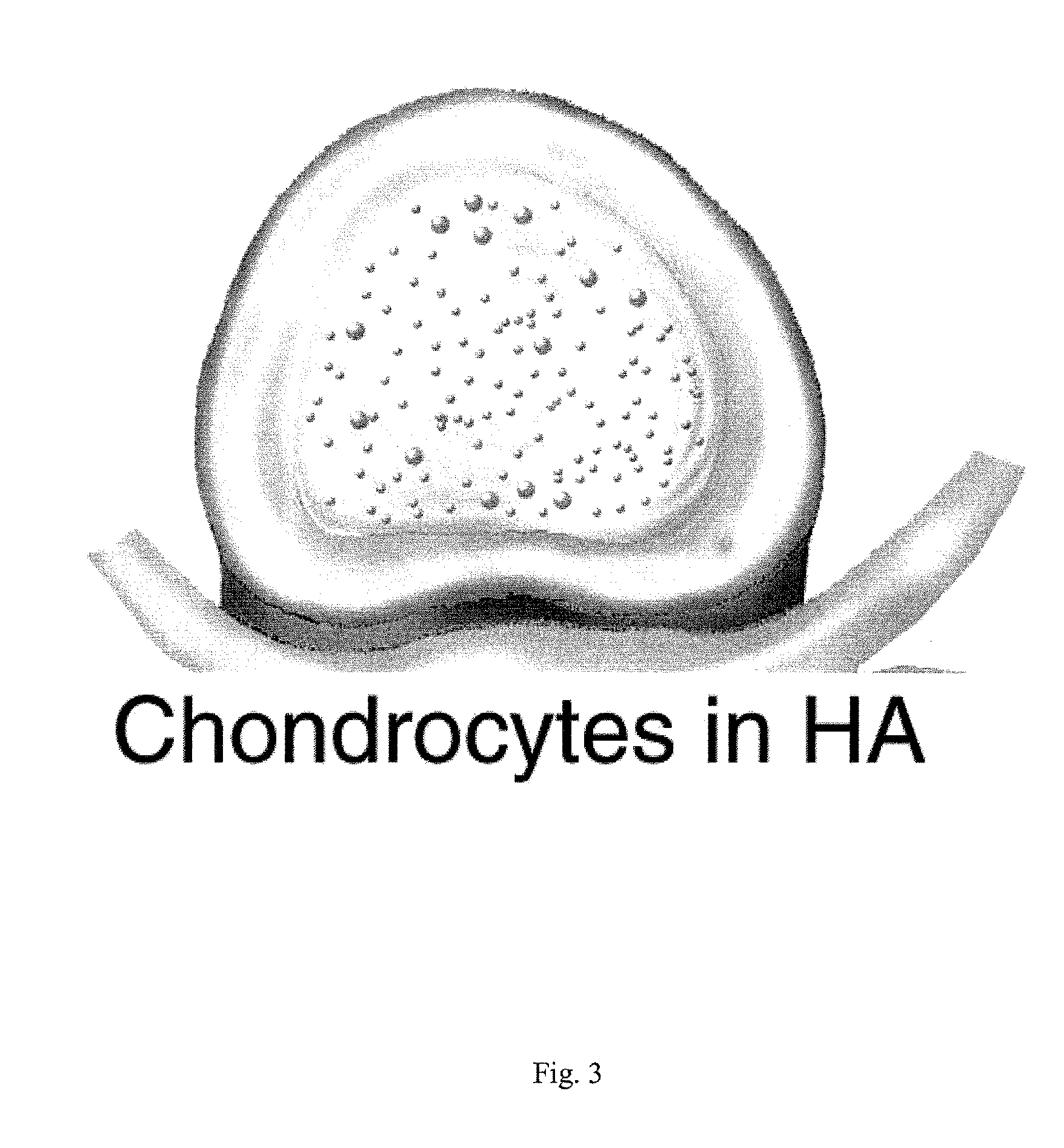
Artificial cartilage containing chondrocytes obtained from costal cartilage and preparation process thereof
ActiveUS20090228105A1Ability to useEfficient regenerationBone implantMammal material medical ingredientsMesenchymal stem cellCostal cartilage
The present invention relates to an artificial cartilage containing mesenchymal stem cell (MSC)-like dedifferentiated cells obtained by passage culturing costal chondrocytes, and a preparation process thereof.
Owner:BIO SOLUTION CO LTD +2
Serum-free medium for mesenchymal stem cells
Serum-free media for growth and proliferation of chondrocytes and mesenchymal stem cells in culture are provided. A serum-free medium for growth of chondrocytes includes a serum-free composition comprising FGF-2, linoleic acid, ascorbic acid, B-mercaptoethanol, transferrin and dexamethasone. Further, the composition comprises EGF, PDGFbb, insulin and albumin. A method for growing chondrocytes in a serum free medium comprising the compostion is also provided. Also provided for mesenchymal stem cell growth, is a serum-free medium which includes a composition comprising FGF-2, LIF, SCF, pantotenate, biotin and selenium and method, therefore.
Owner:CONSORZIO PER LA GESTIONE DEL CENT DI BIOTECNOLOGIA AVANZATA +1
Means for cartilage repair
InactiveUS6179871B1Minimize extraneous effectEvenly spacedPowder deliveryElectrotherapyChondral defectGrowth promoting
A method for repairing a defect in cartilage, comprising the provision of apertures in the cartilage by drilling holes at the base of the cartilage defect, which holes may enter the mesenchymal depot, introducing a porous scaffold material containing a plurality of magnetic particles into the apertures, and subsequently and sequentially injecting magnetically-tagged cartilage growth promoting materials such as various growth factors or chondrocytes into the area of the defect. The magnetically tagged growth promoting material is then drawn into the apertures by magnetic attraction of the magnetic particles contained in the porous scaffold material, either by virtue of the particles being permanently magnetic, or by the imposition of an external magnetic field. The present application claims the biodegradable porous scaffold material containing the plurality of magnetic particles.
Owner:HALPERN ALAN A
Intervertebral Disc Repair, Methods and Devices Therefor
The present application discloses compositions, methods and devices for treatment of a degenerative intervertebral disc. A composition can comprise chondrocytes expressing type II collagen. These chondrocytes can be obtained from human cadavers up to about two weeks following death, and can be grown in vitro. The compositions can further comprise one or more biocompatible molecules. Treatment of a degenerative disc can comprise injecting or implanting a composition comprising the chondrocytes into a degenerative disc through an aperture or incision. If the aperture or incision is closed with a suture or a glue after introduction of the chondrocytes, the closure can withstand over 400 N of compression force.
Owner:VELOCITY FINANCIAL GRP INC ITS SUCCESSORS & ASSIGNS
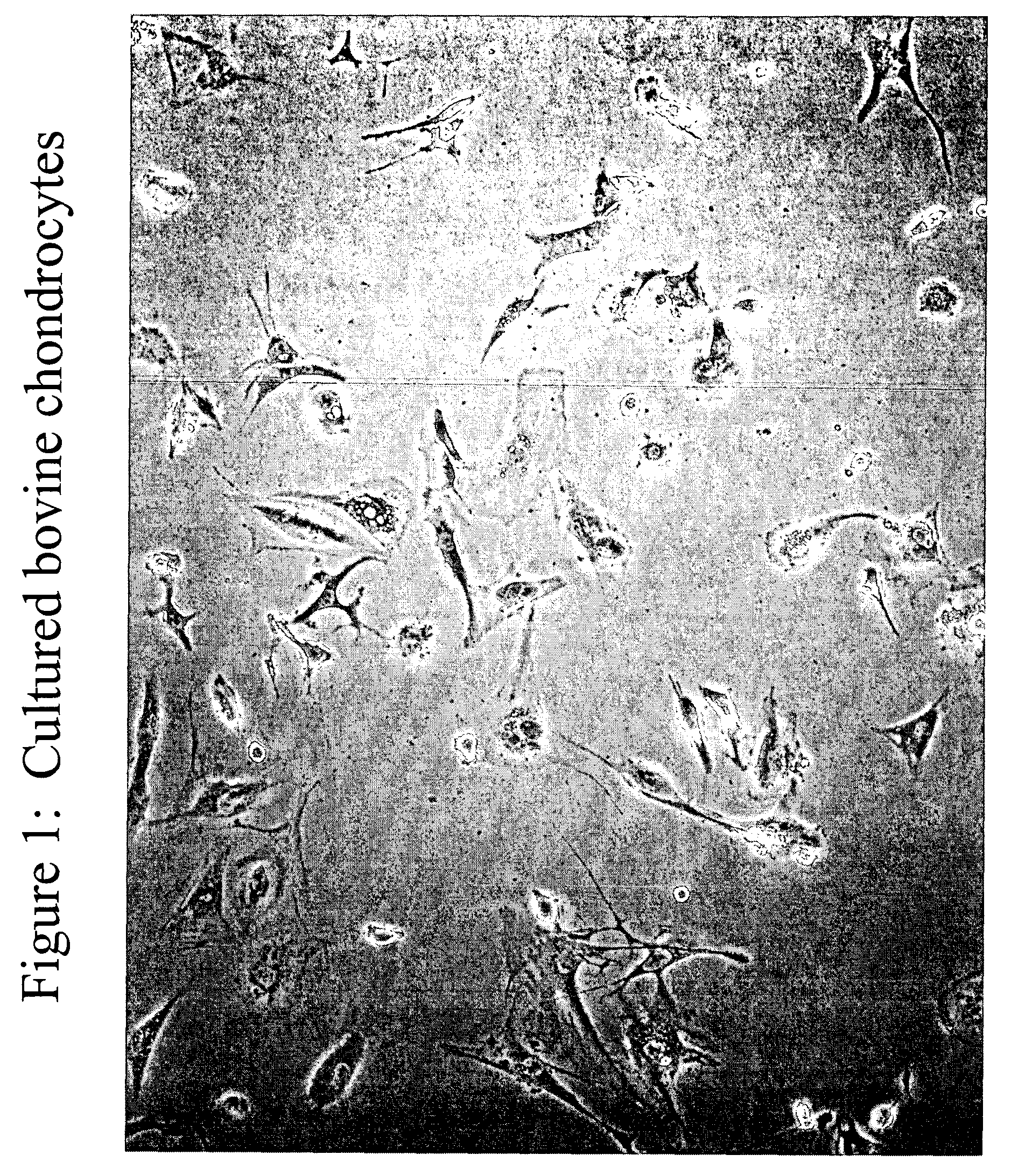
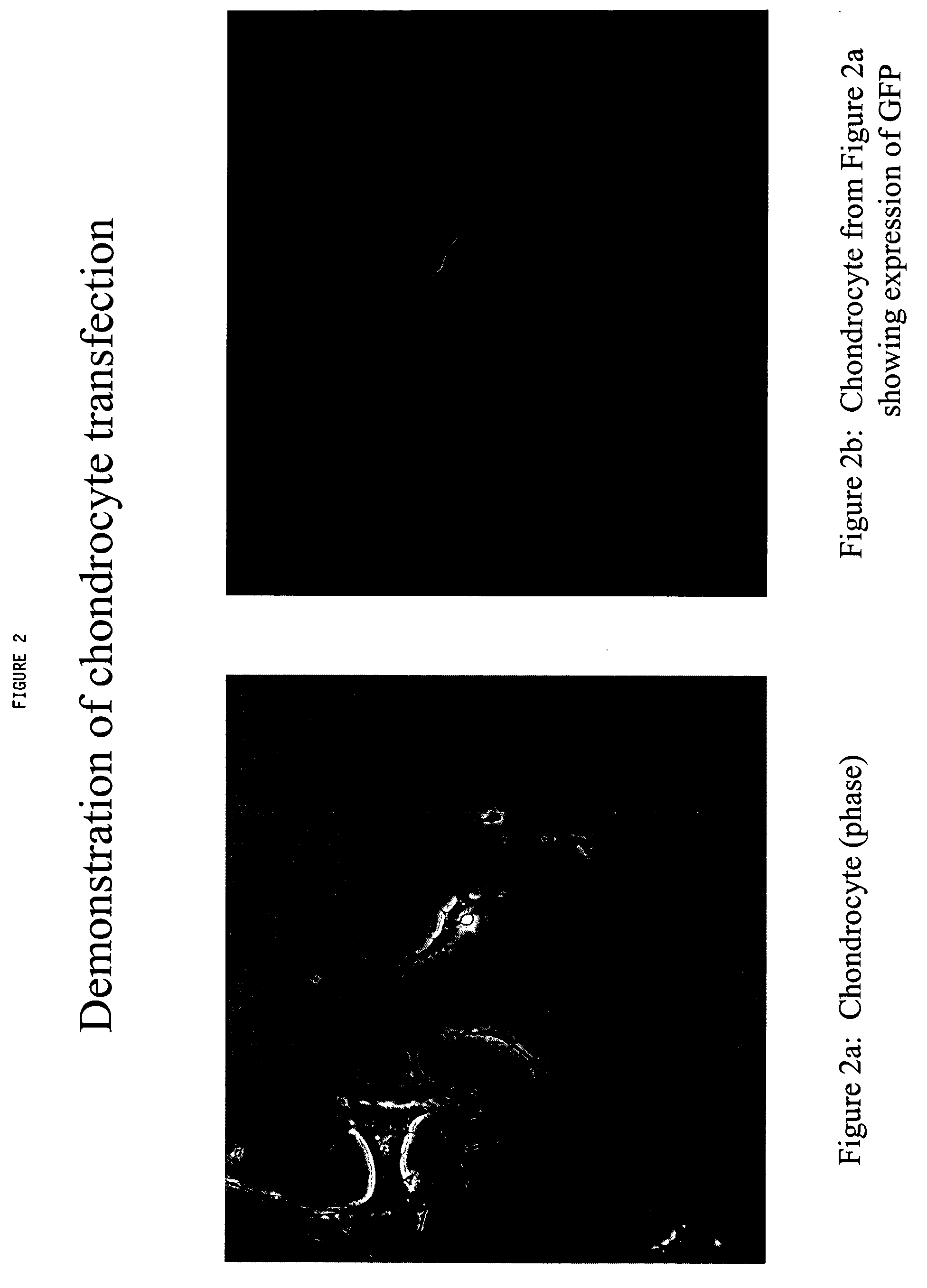
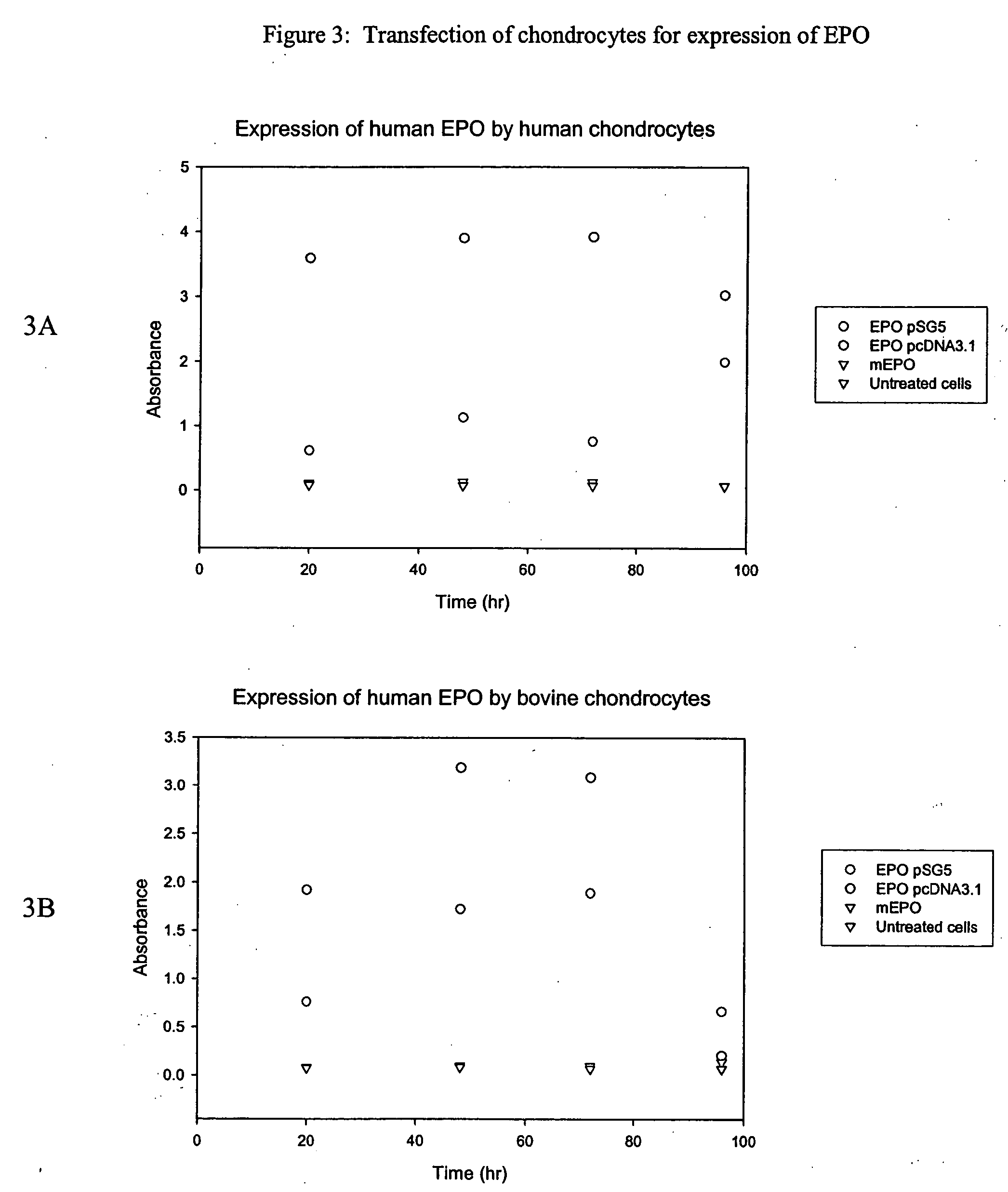
Chondrocyte therapeutic delivery system
ActiveUS20050054595A1Easy to optimizeAmeliorate disorderSenses disorderNervous disorderGenetically engineeredDelivery system
Methods and compositions for producing therapeutic agents using chondrocytes are provided. The genetically engineered chondrocytes can be used to express the therapeutic agent in a subject, including in an environment typically associated with chondrocytes and in an environment not typically associated with chondrocytes.
Owner:DEPUY SYNTHES PROD INC
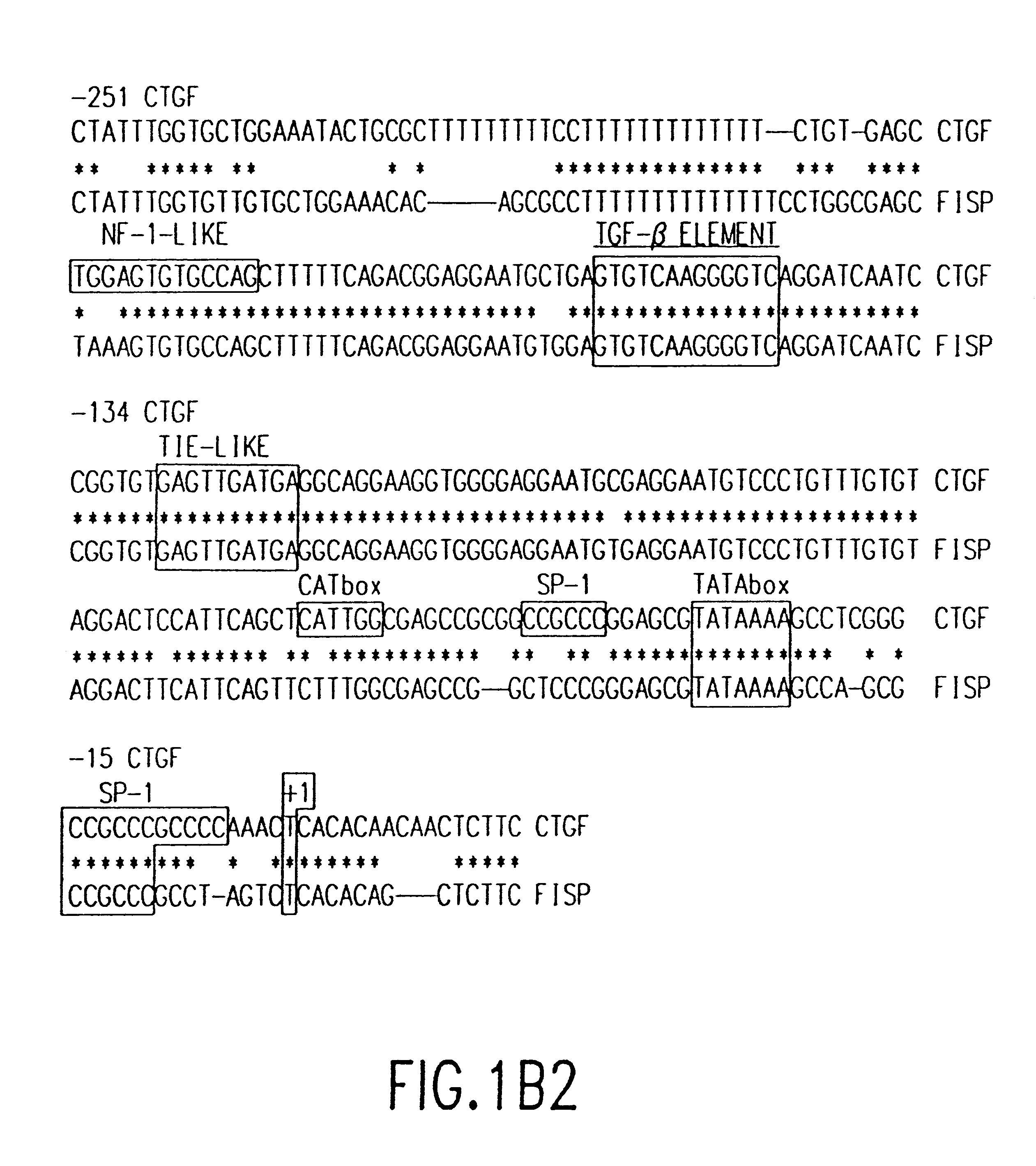
Methods and uses of connective tissue growth factor for differentiation of mesenchymal stem cells
The present invention relates to novel methods and compositions related to the administration of connective tissue growth factor, alone or in combination with other growth factors, compositions or compounds, to induce the differentiation of mesenchymal stem cells into chondrocytes or asteablasts.
Owner:UNIV OF SOUTH FLORIDA +1
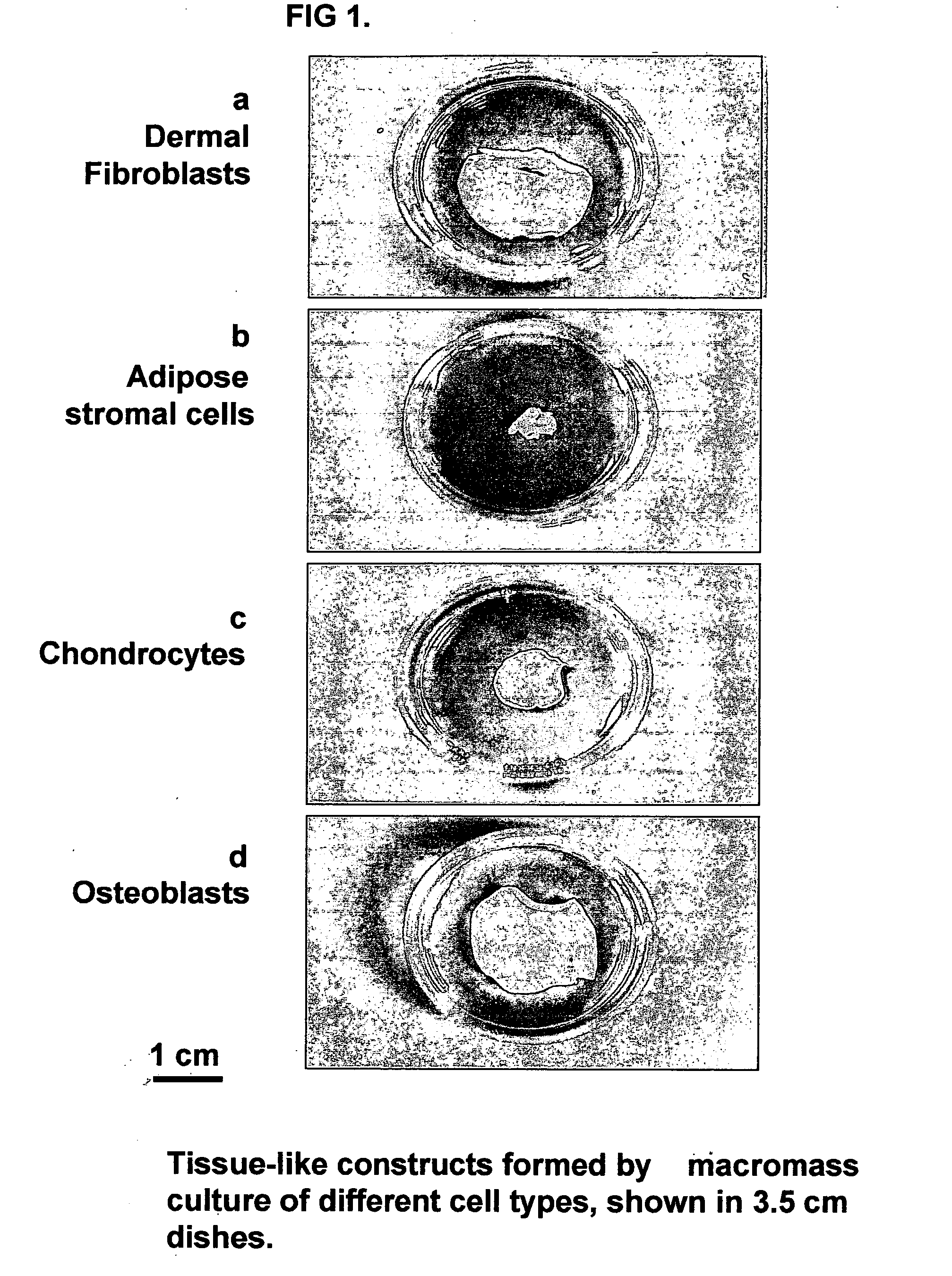
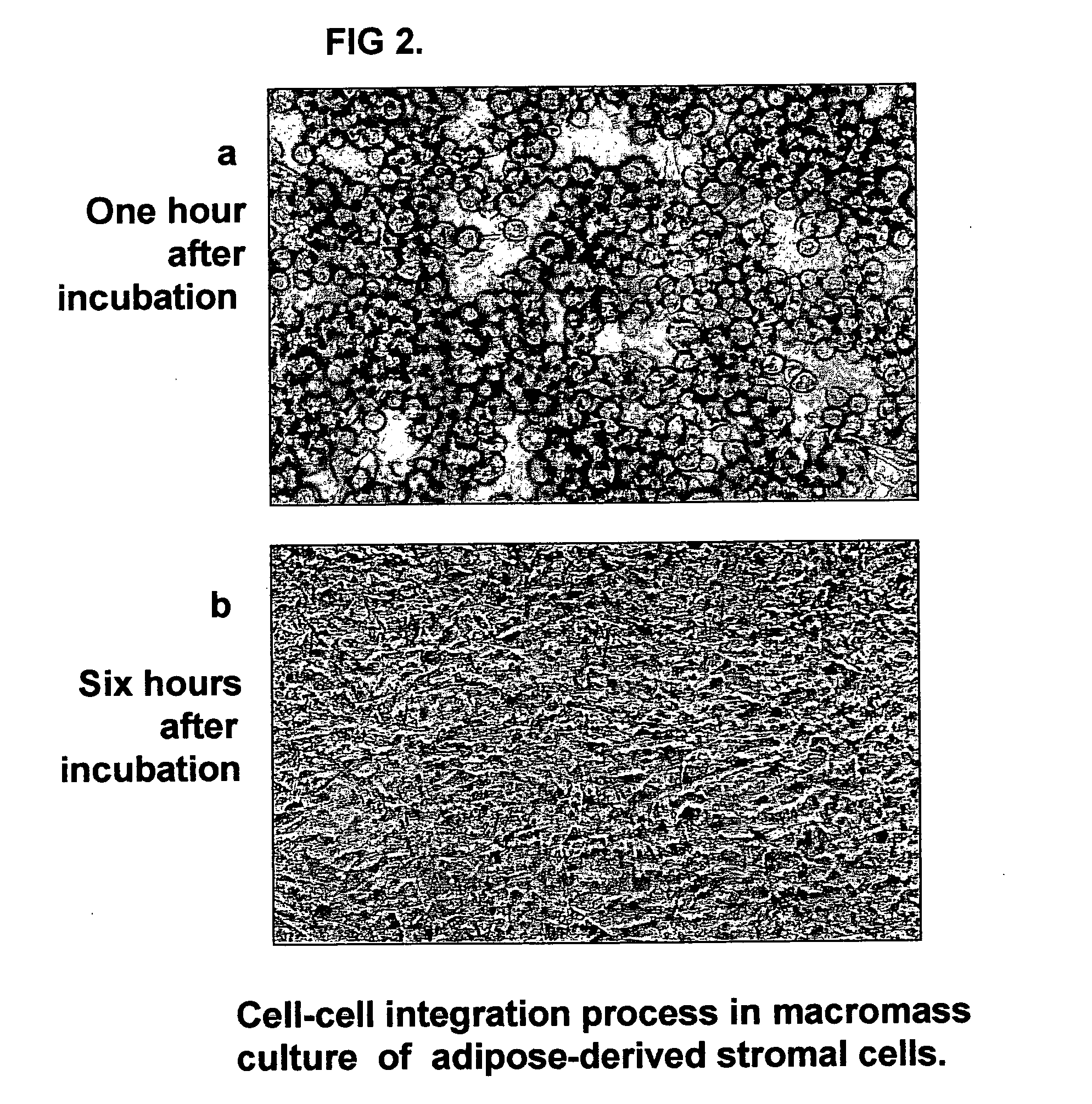
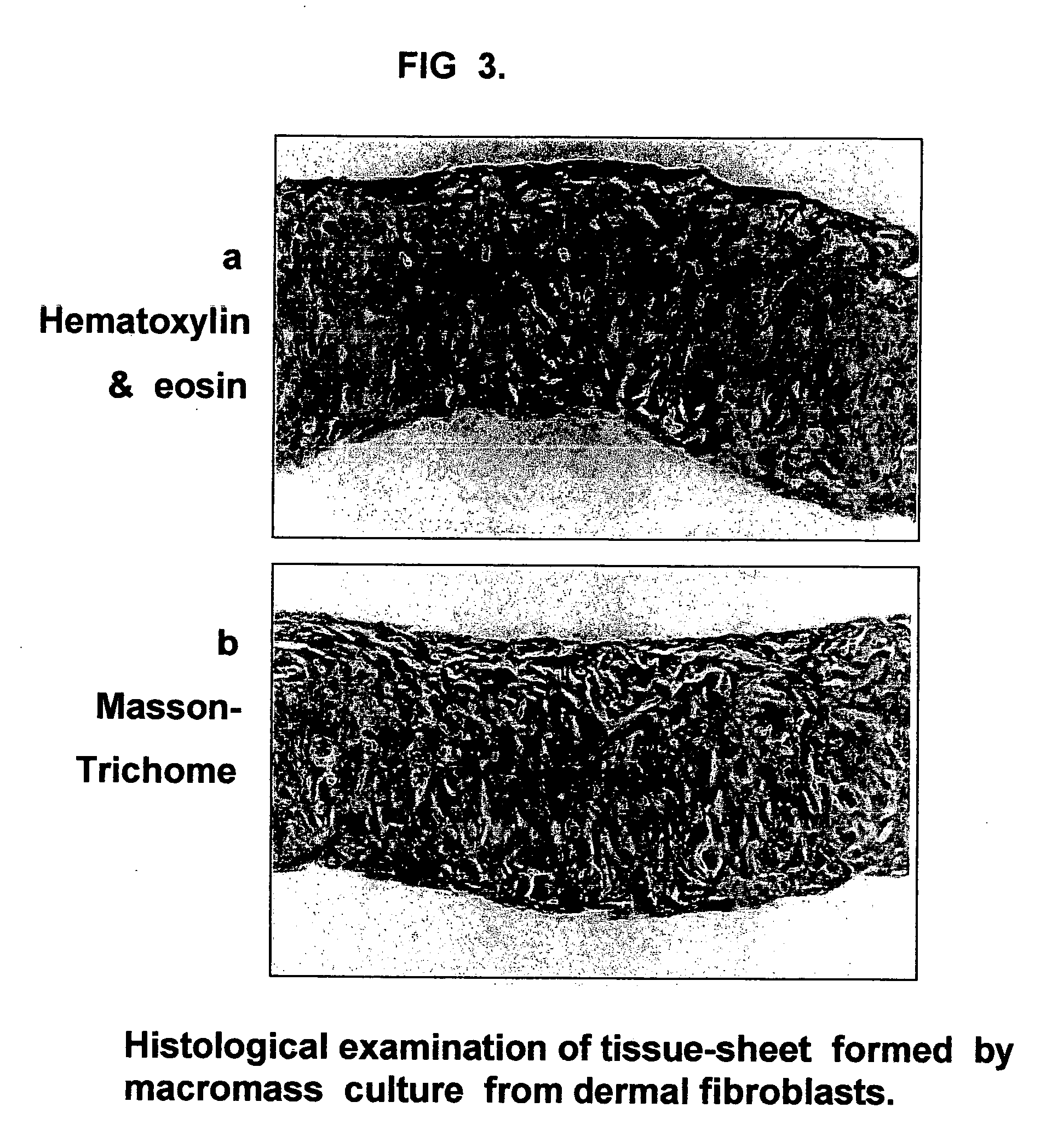
Tissue-like organization of cells and macroscopic tissue-like constructs, generated by macromass culture of cells, and the method of macromass culture
InactiveUS20040082063A1Sectioned easilySmall sizeEpidermal cells/skin cellsMammal material medical ingredientsHigh cellFiber
Three-dimensional tissue-like organization of cells by high cell-seeding-density culture termed as macromass culture is described. By macromass culture, cells can be made to organize themselves into a tissue-like form without the aid of a scaffold and three-dimensional macroscopic tissue-like constructs can be made wholly from cells. Tissue-like organization and macroscopic tissue-like constructs can be generated from fibroblastic cells of mesenchymal origin (at least), which can be either differentiated cells or multipotent adult stem cells. In this work, tissue-like organization and macroscopic tissue-like constructs have been generated from dermal fibroblasts, adipose stromal cells-derived osteogenic cells, chondrocytes, and from osteoblasts. The factor causing macroscopic tissue formation is large scale culture at high cell seeding density per unit area or three-dimensional space, that is, macromass culture done on a large scale. No scaffold or extraneous matrix is used for tissue generation, the tissues are of completely cellular origin. No other agents (except high cell-seeding-density) that aid in tissue formation such as tissue-inducing chemicals, tissue-inducing growth factors, substratum with special properties, rotational culture, etc, are employed for tissue formation. These tissue-like masses have the potential for use as tissue replacements in the human body. Tissue-like organization by high cell-seeding-density macromass culture can also be generated at the microscopic level.
Owner:RELIANCE LIFE SCI PVT
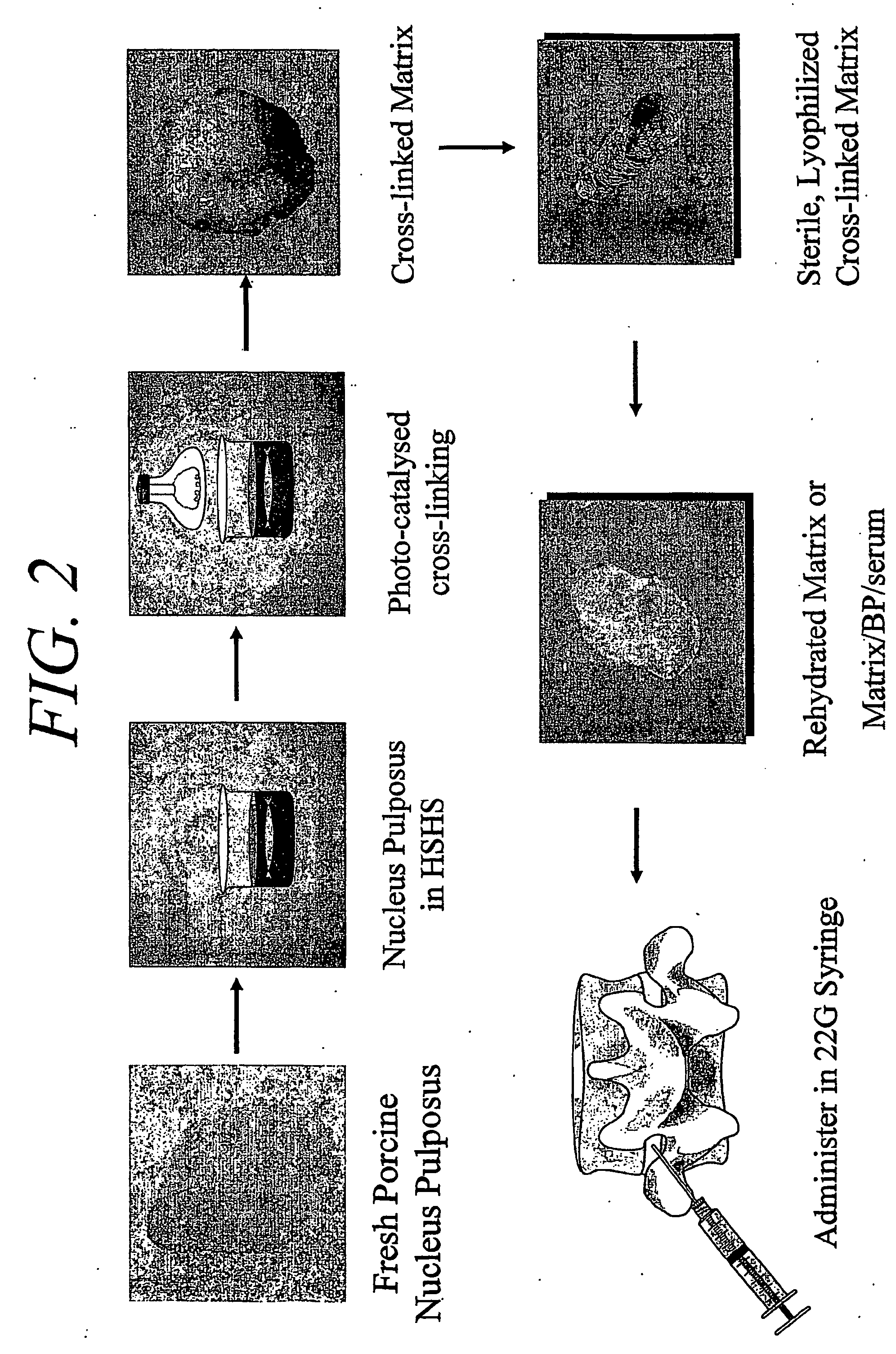
Hydrogel compositions comprising nucleus pulposus tissue
InactiveUS20070003525A1Particular utilityImprove featuresBiocideSkeletal disorderHard tissueKinetic function
Disclosed are methods and compositions useful in the treatment, augmentation and / or repair of soft and / or hard tissues of animals, and in particular, vertebrates such as humans. The invention provides hydrogel compositions for use in the preparation of medicaments for wound healing, cartilage and meniscus repair, dermal augmentation, and bone fusion, as well as methods for the treatment of intervertebral disc impairment. In particular embodiments, the invention provides compositions useful in restoring hydrodynamic function, increasing intervertebral disc height, and improving proliferation and survival of chondrocytes and other cells in intervertebral discs that have been compromised by injury, degenerative disease, congenital abnormalities, and / or the aging process.
Owner:ZIMMER ORTHOBIOLOGICS
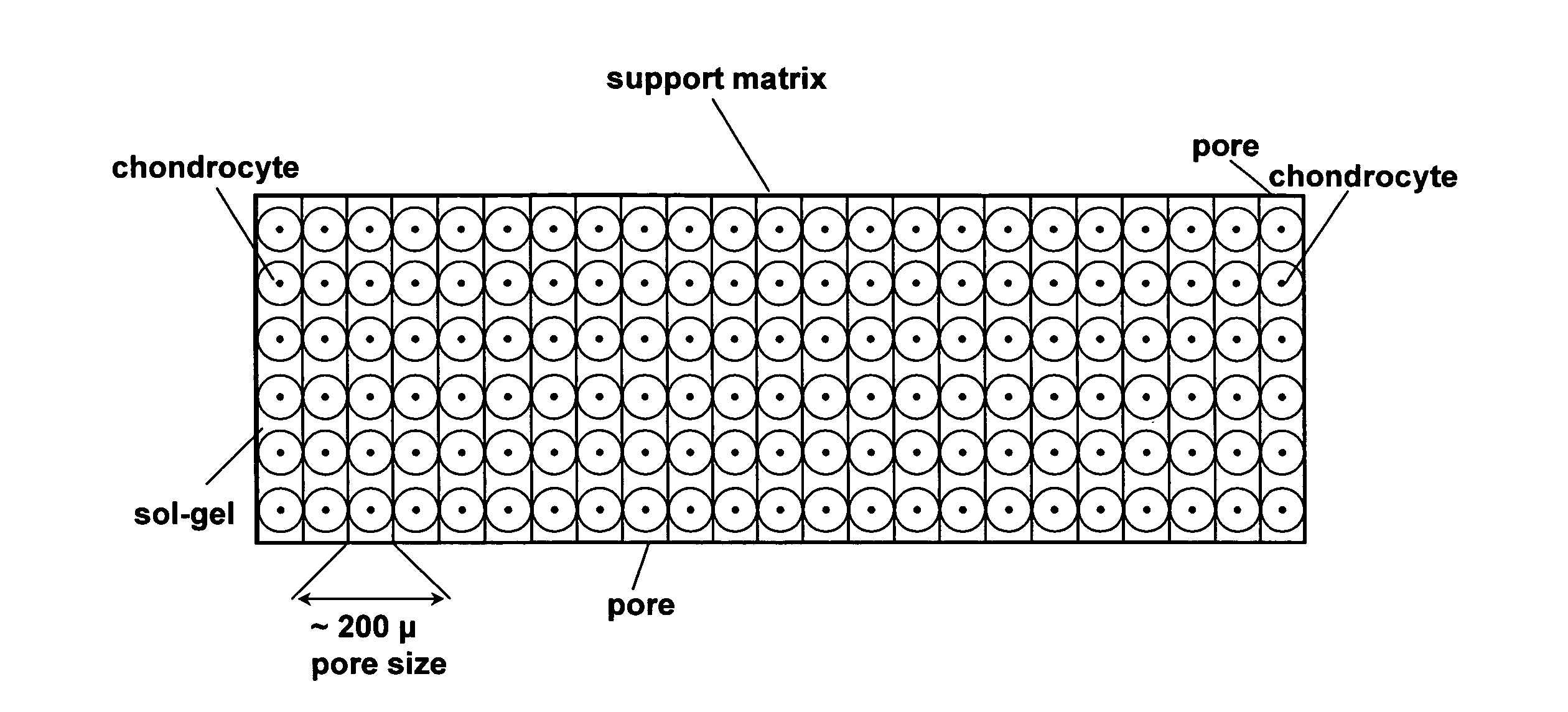
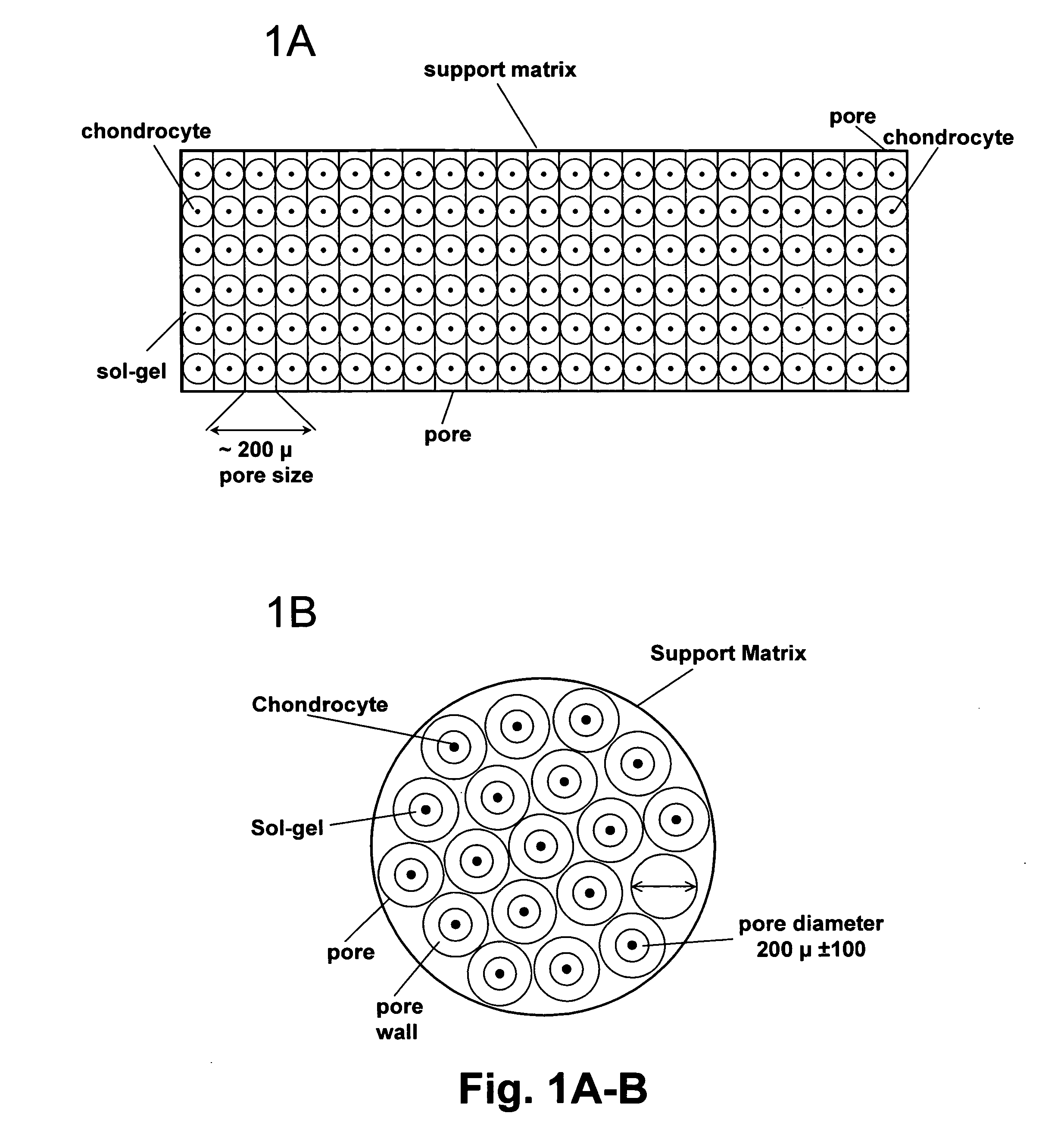
Cell-support matrix having narrowly defined uniformly vertically and non-randomly organized porosity and pore density and a method for preparation thereof
A cell-support matrix having narrowly defined uniformly vertically and non-randomly organized porosity and pore density and a method for preparation thereof. The matrix suitable for preparation of cellular or acellular implants for growth and de novo formation of an articular hyaline-like cartilage. A gel-matrix composite system comprising collagen-based matrix having a narrowly defined porosity capable of inducing hyaline-like cartilage production from chondrocytes in vivo and in vitro.
Owner:OCUGEN INC
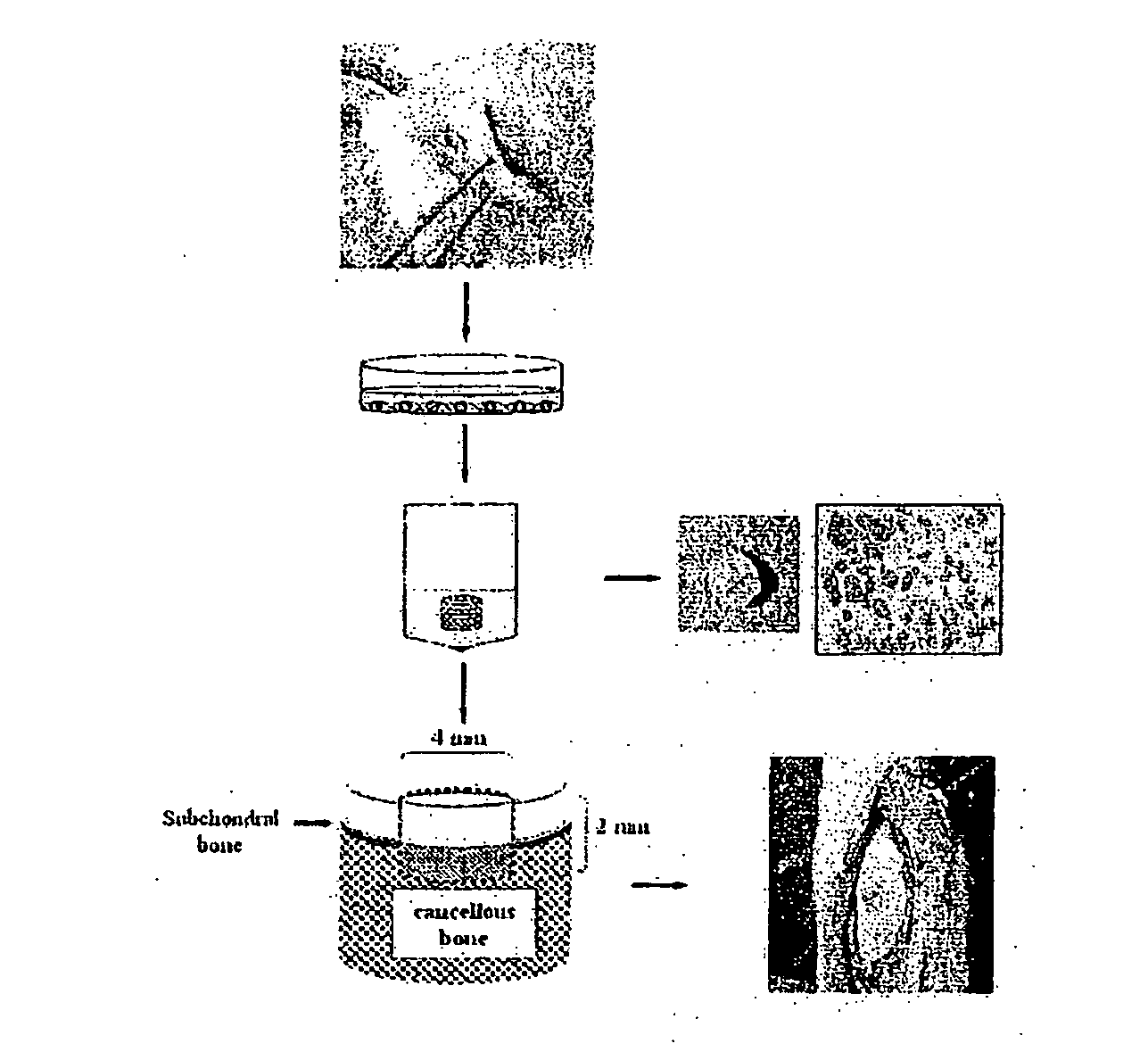
Implants and Methods for Repair, Replacement and Treatment of Disease
Implants comprising cartilage and trabecular metal, and methods of making the implants are disclosed. Further disclosed are therapeutic uses of the implants, which include methods of treatment or repair of an chondral or osteochondral defect, such as a chondral or osteochondral injury, lesion or disease. An implant comprises cartilage or chondrocytes and a subchondral base comprising trabecular metal. An implant can comprise a geometric shape such as a cylinder or an anatomical shape such as a condyle, and can be used in conjunction with a positioning structure.
Owner:ZIMMER INC
Methods and devices for tissue repair
InactiveUS20090098177A1Reduce movementReduce resistanceOrganic active ingredientsBiocideProgenitorDamages tissue
Methods for treating diseased or damaged tissue in a subject are disclosed, involving administering to said subject at a site wherein diseased or damaged tissue occurs, cells of a type(s) normally found in healthy tissue corresponding to the diseased or damaged tissue, and / or suitable progenitor cells thereof, in association with bioresorbable beads or particles and optionally a gel and / or gel-forming substance. Where the cells an / or suitable progenitor cells thereof are chondrocytes, embryonic stem cells and / or bone marrow stromal cells, the methods of the invention are suitable for treating, for example, articular cartilage degeneration associated with primary osteoarthritis. Also disclosed is a device having tissue-like characteristics for treating diseased or damaged tissue in a subject, wherein the device comprises cells of a type(s) normally found in healthy tissue corresponding to the diseased or damaged tissue, and / or suitable progenitor cells thereof, in association with bioresorbable beads or particles and optionally a gel and / or gel-forming substance.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Cartilage repair mixture containing allograft chondrocytes
InactiveUS20060210643A1Promote migrationIncreased proliferationPeptide/protein ingredientsSurgeryCartilage repairDextran
The invention is directed toward a sterile cartilage defect implant material comprising milled lyophilized allograft cartilage pieces ranging from 0.01 mm to 1.0 mm in size in a bioabsorbable carrier taken from a group consisting of sodium hyaluronate, hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran or mixed polymers with allograft chondrocytes added in an amount ranging from 2.5×105 to 2.5×107.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Vitro repair of bone and/or cartilage defects
The present invention relates to methods and materials for in vivo repair of cartilage or bone and cartilage defects in mammals. The invention relates to membranes carrying a composition comprising at least one stimulation molecule, which is capable of inducing signal transduction in chondroblasts / chondrocytes and / or osteoblasts / osteocytes. Furthermore the invention relates to a method for the preparation of chondroblasts / chondrocytes and / or osteoblasts / osteocytes suspensions.
Owner:INTERFACE BIOTECH
Tissue transplantation compositions and methods
A biomedical material for transplant to a subject is provided according to embodiments of the present invention which includes an isolated donor tissue enzyme-treated to reduce the amount of proteoglycans in the donor tissue compared to untreated tissue. Isolated cells are optionally added to the enzyme-treated donor tissue, including leukocytes, particularly monocytes; macrophages; platelets; cells derived from an intervertebral disc such as chondrocyte-like nucleus pulposus cells; fibrocytes; fibroblasts; mesenchymal stem cells; mesenchymal precursor cells; chondrocytes; or a combination of any of these. The isolated donor tissue is articular cartilage or an intervertebral disc tissue such as nucleus pulposus tissue and / or annulus fibrosis tissue enzyme-treated to remove proteoglycans normally present in these tissues. A biomedical material of the present invention is administered to a subject to treat a disorder or injury, such as a disorder or injury to connective tissue.
Owner:FERREE BRET
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com