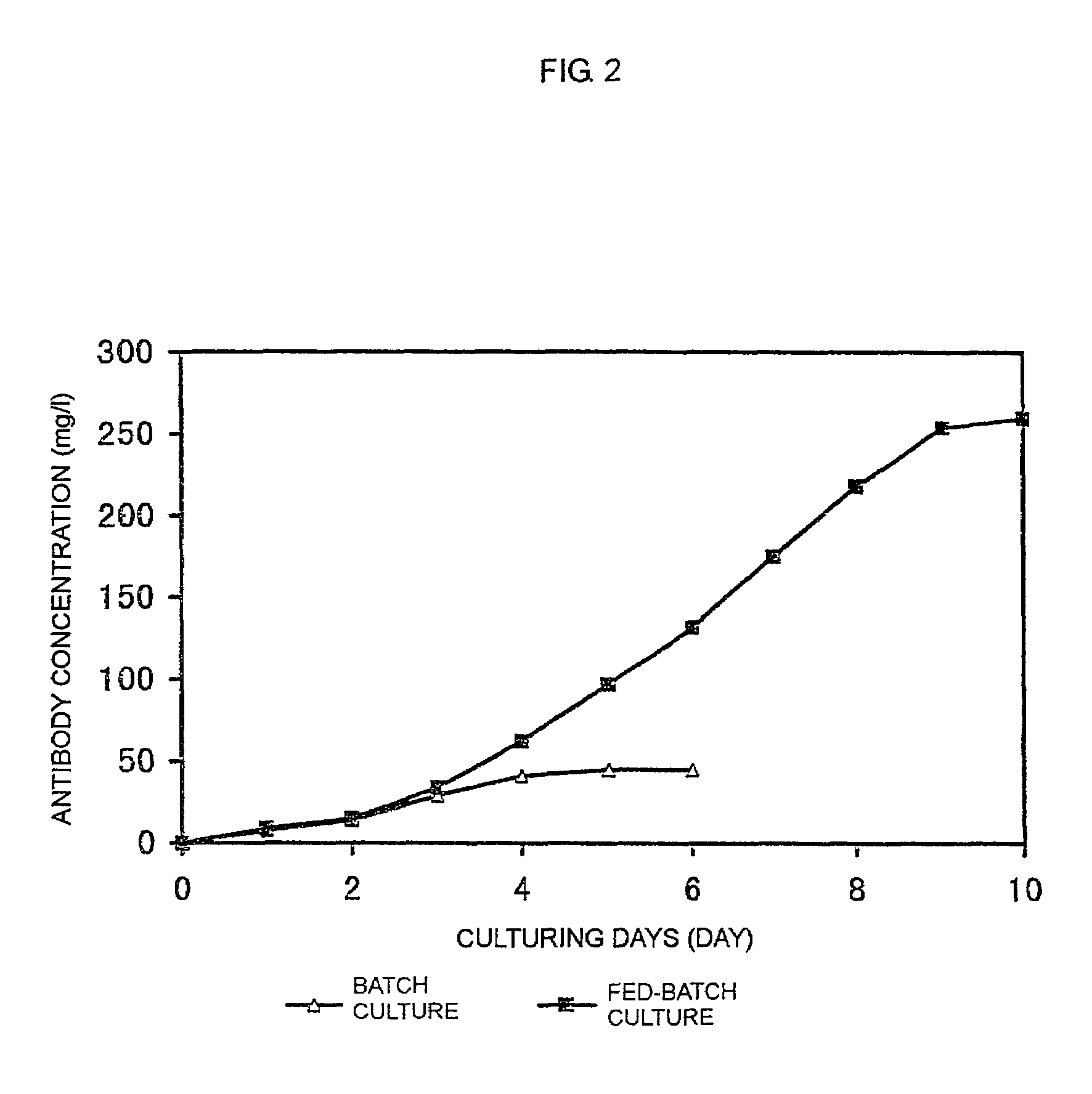
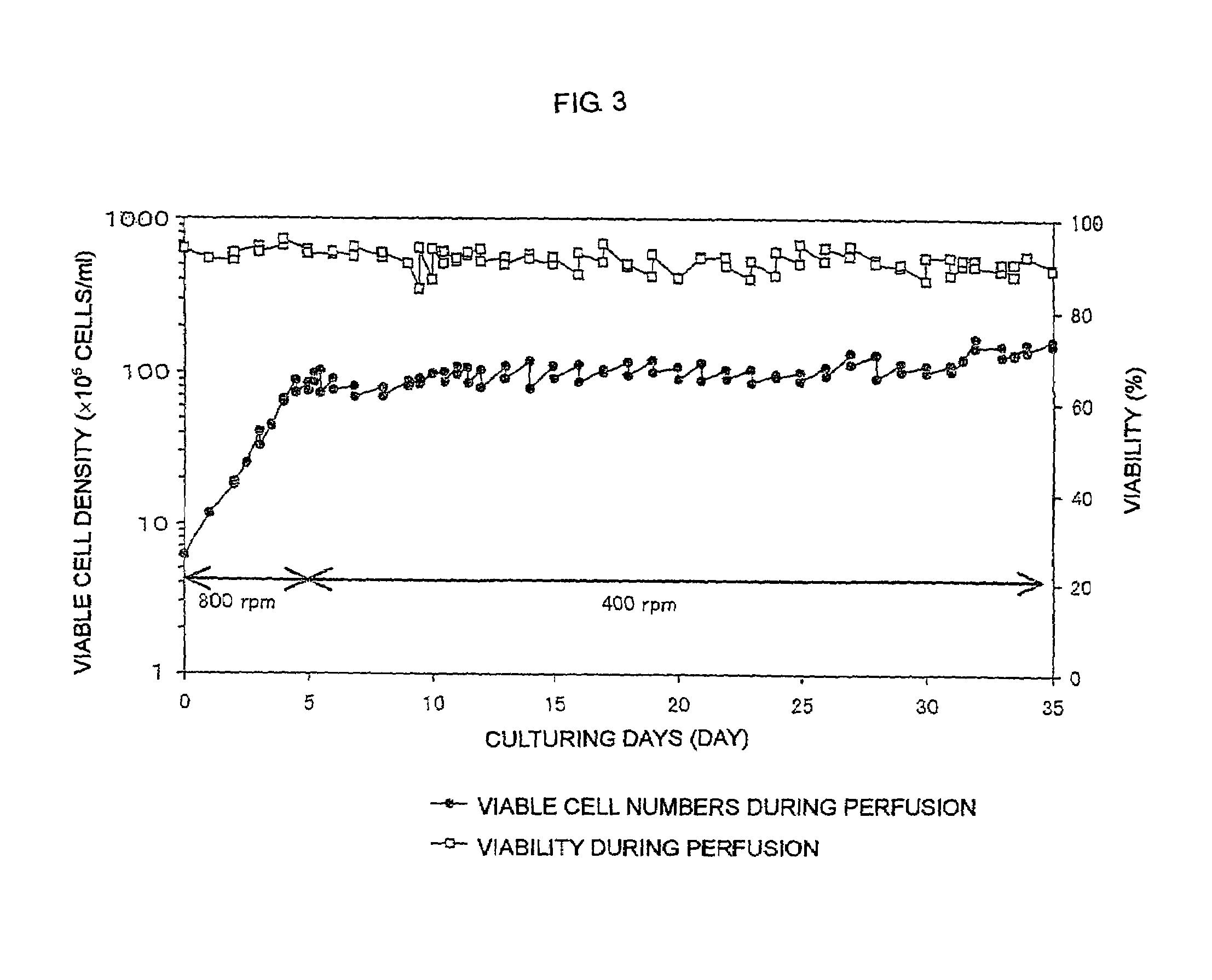
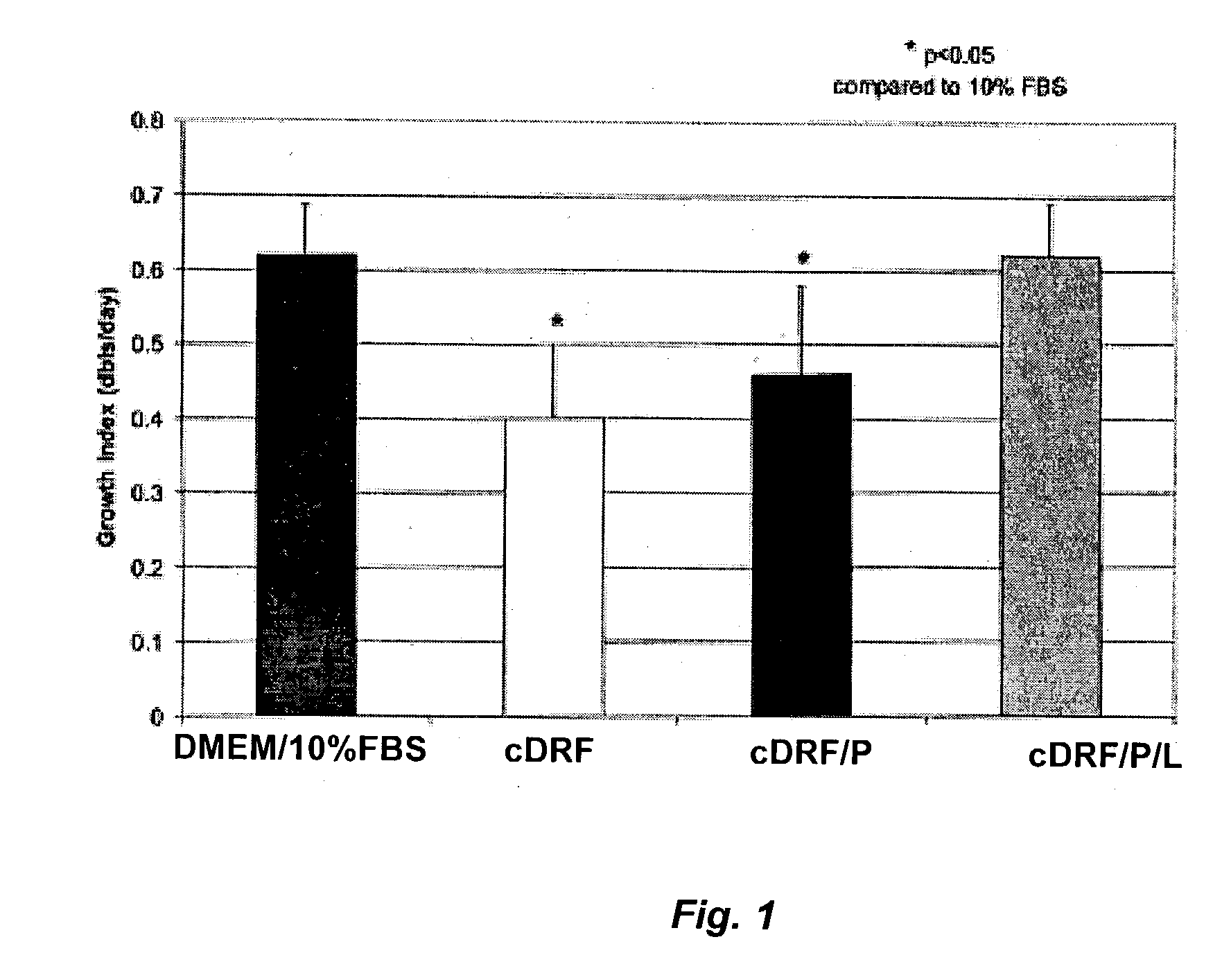
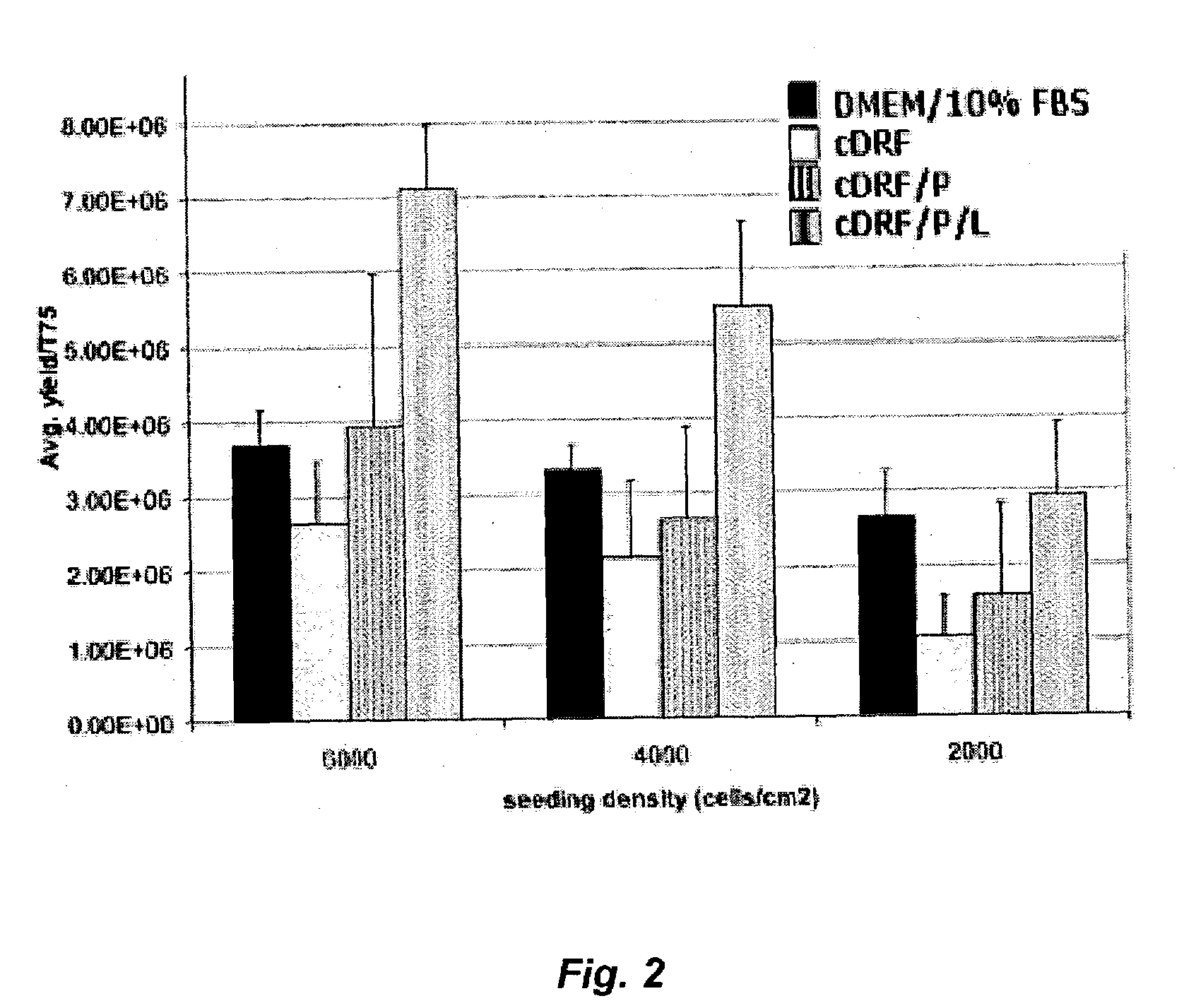
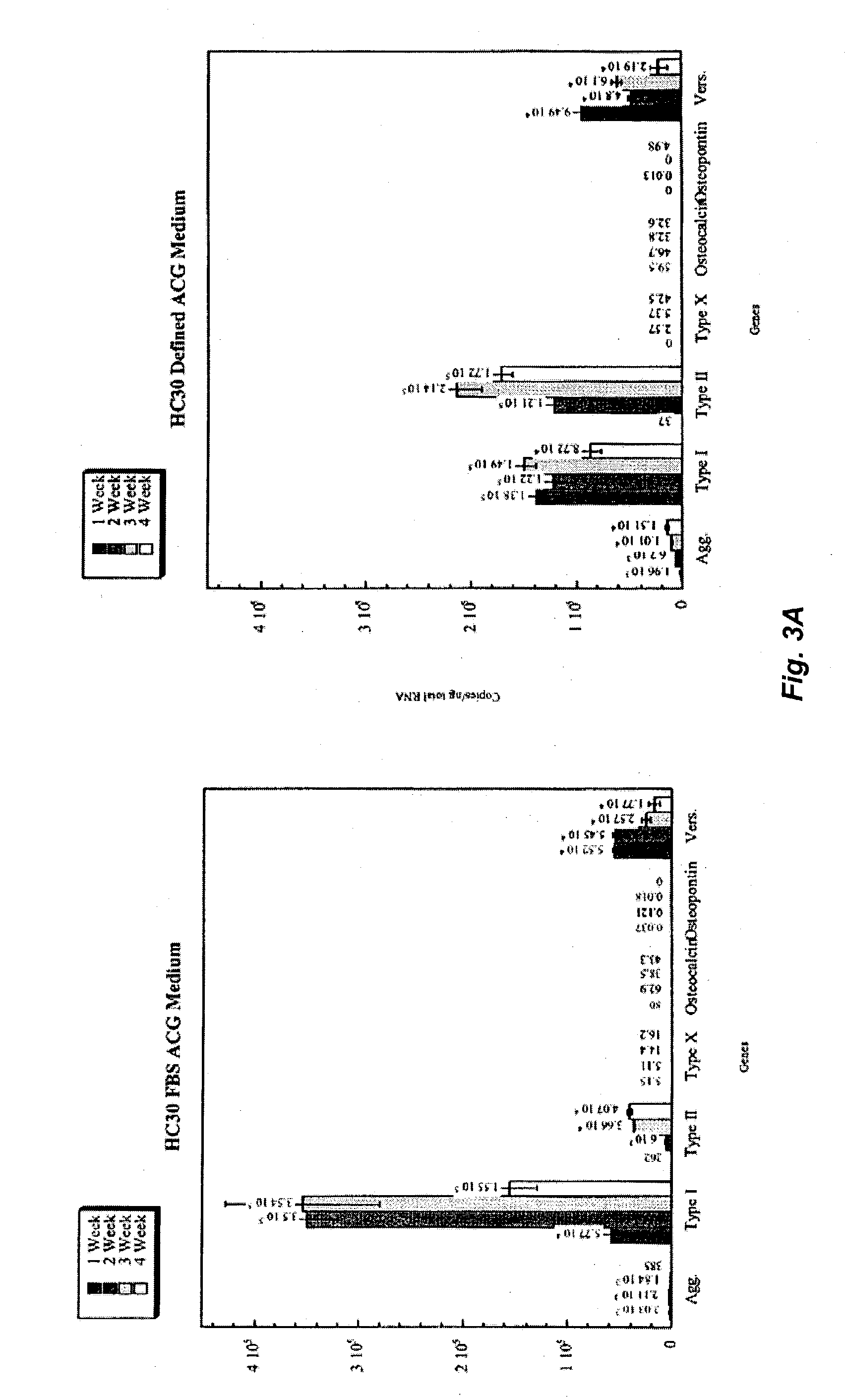
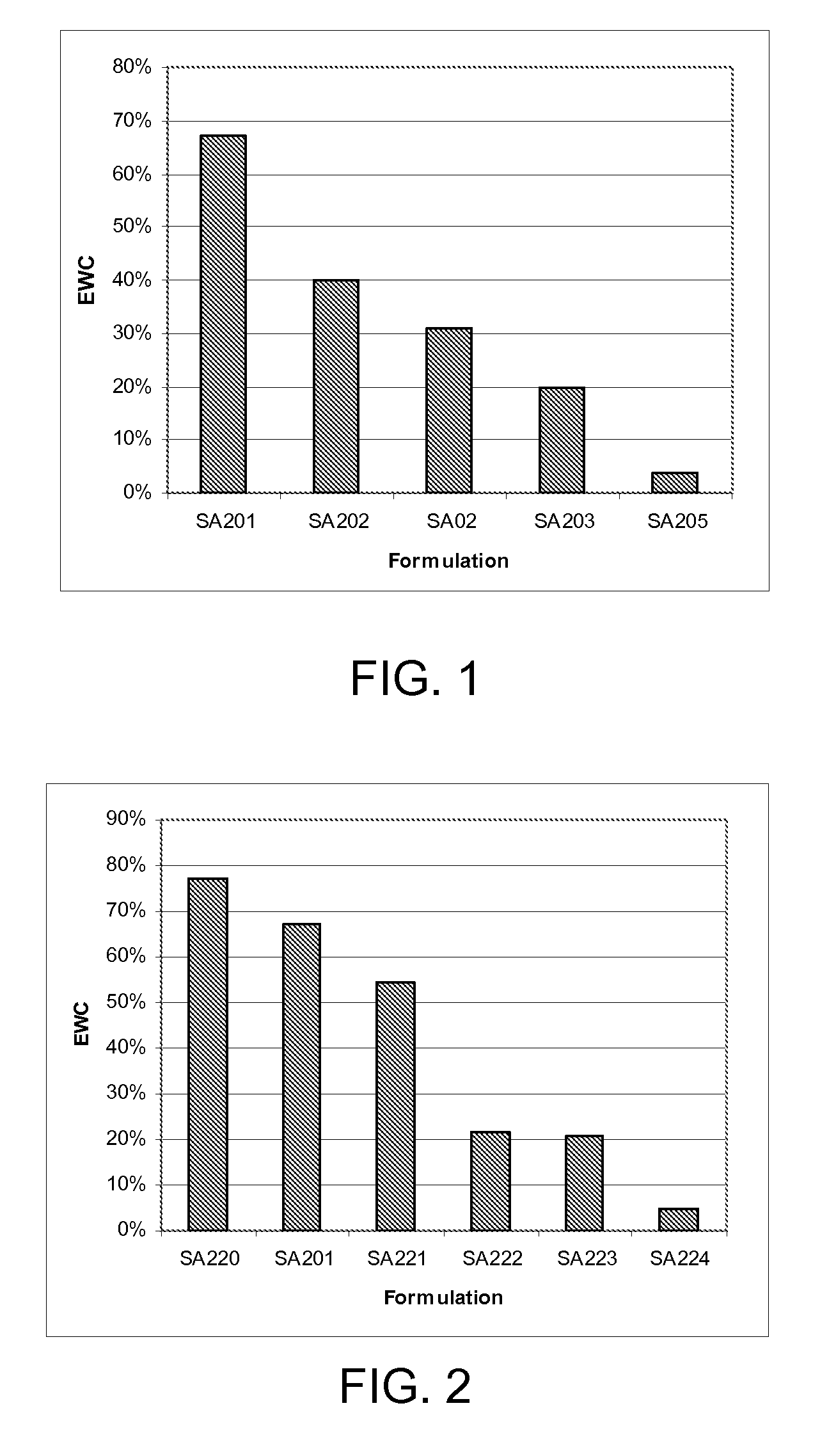
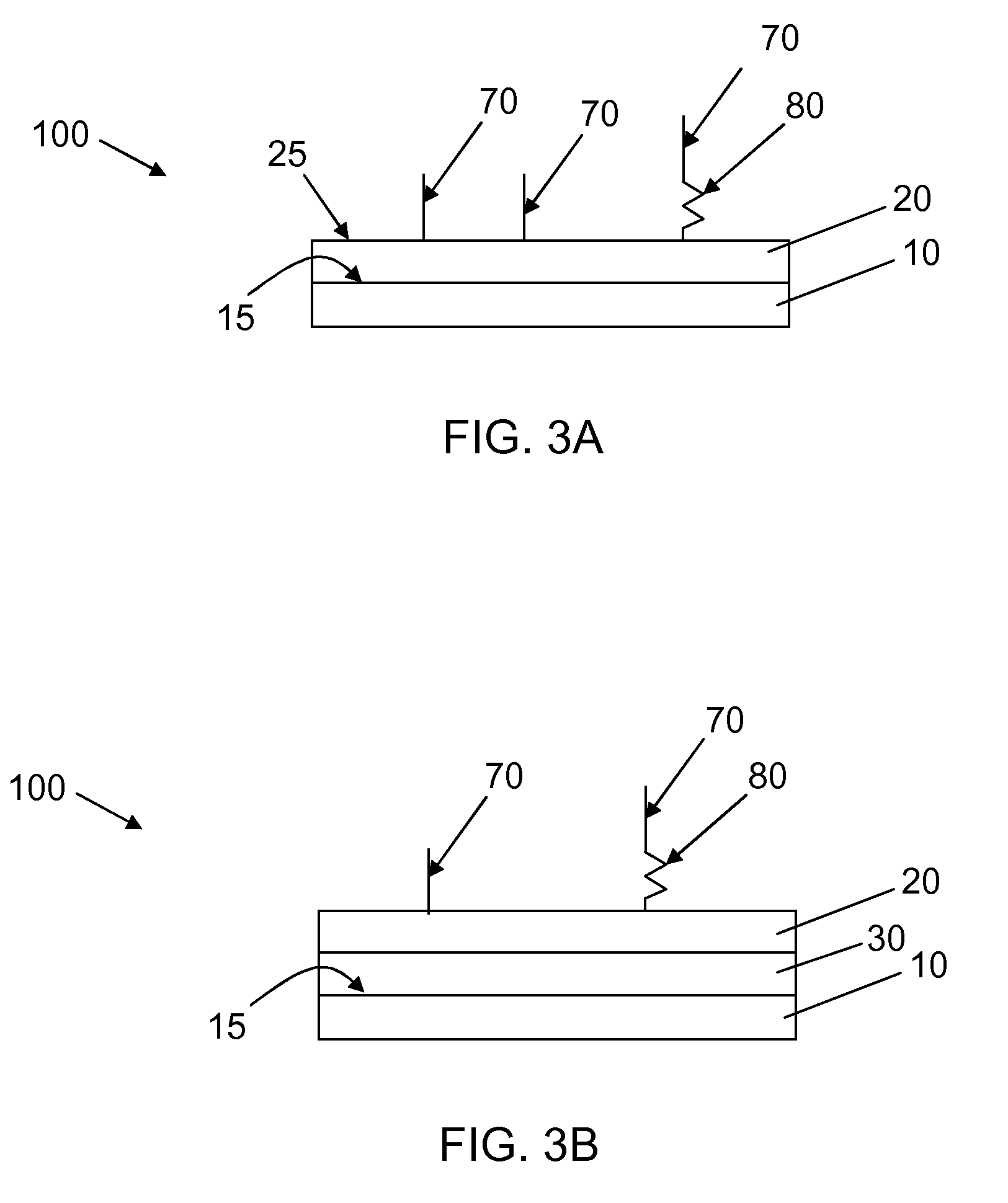
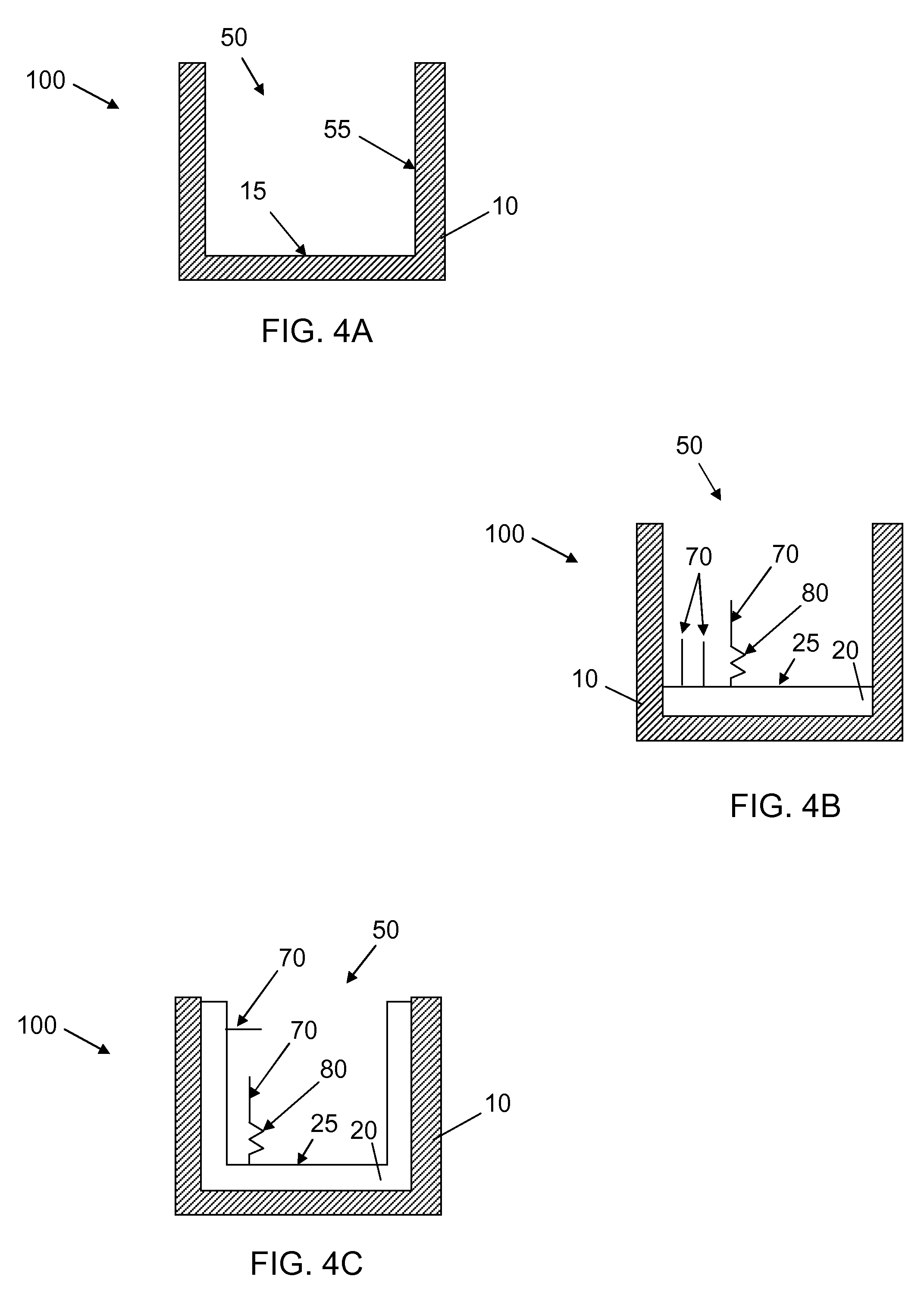
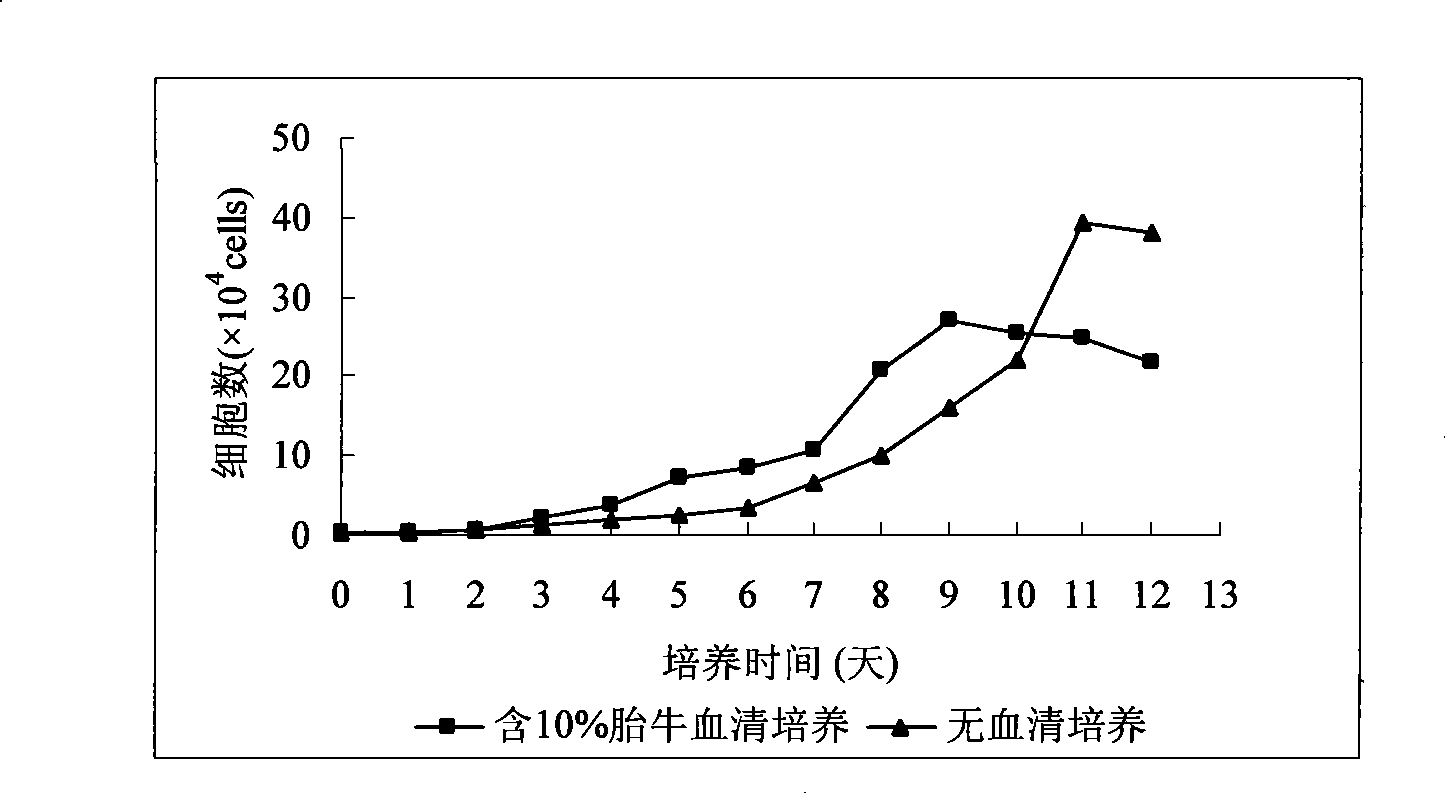
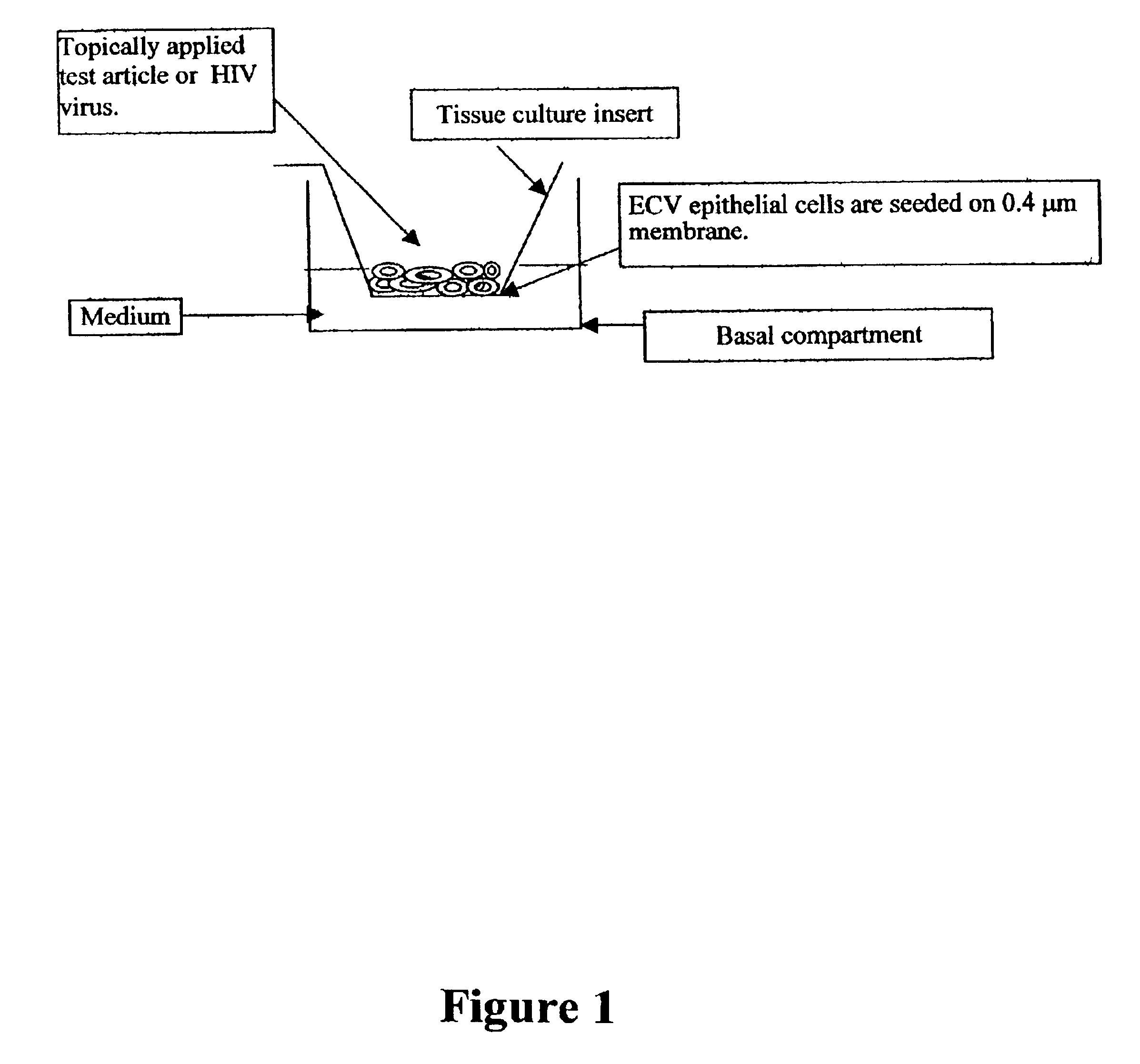
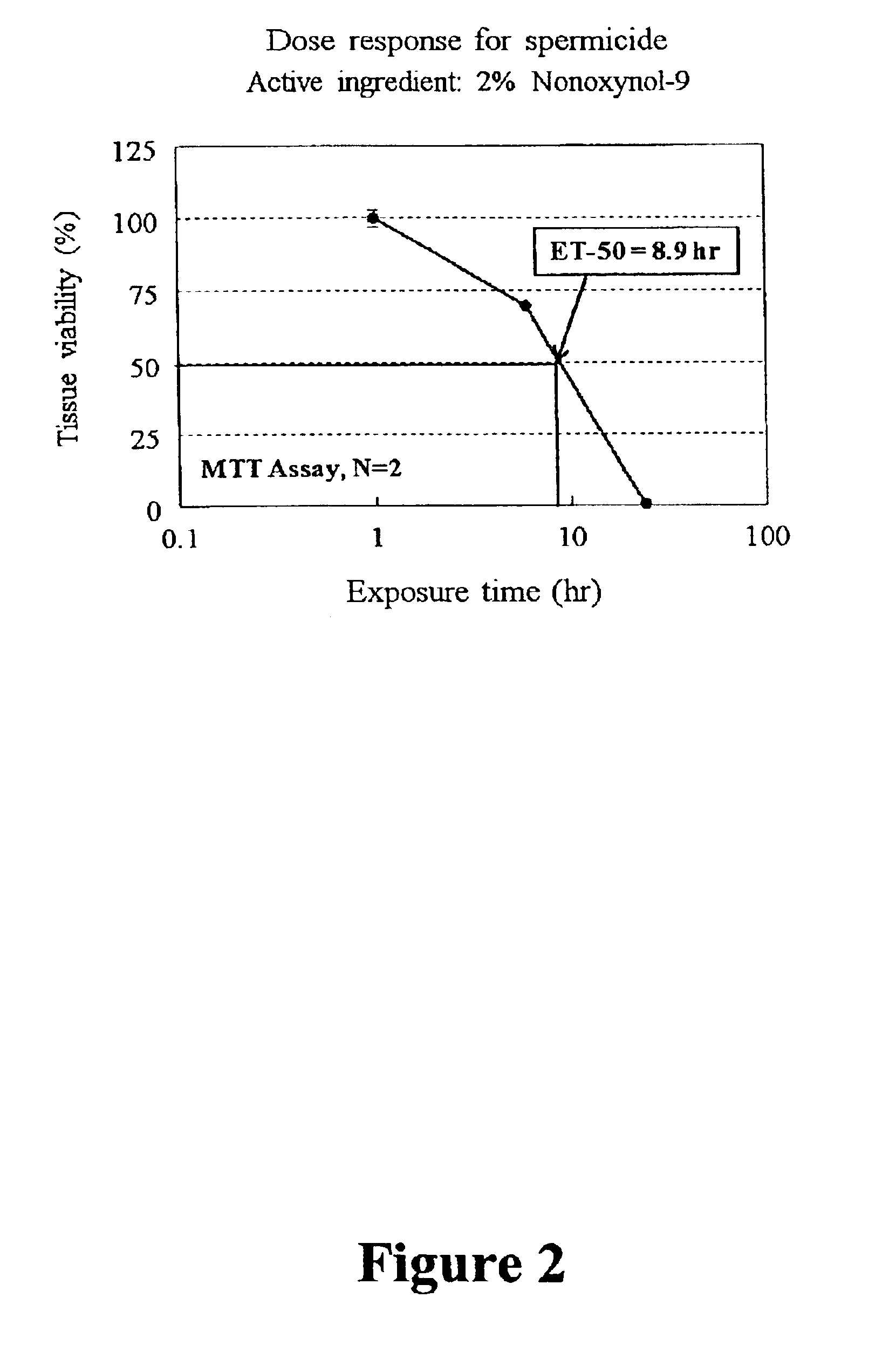
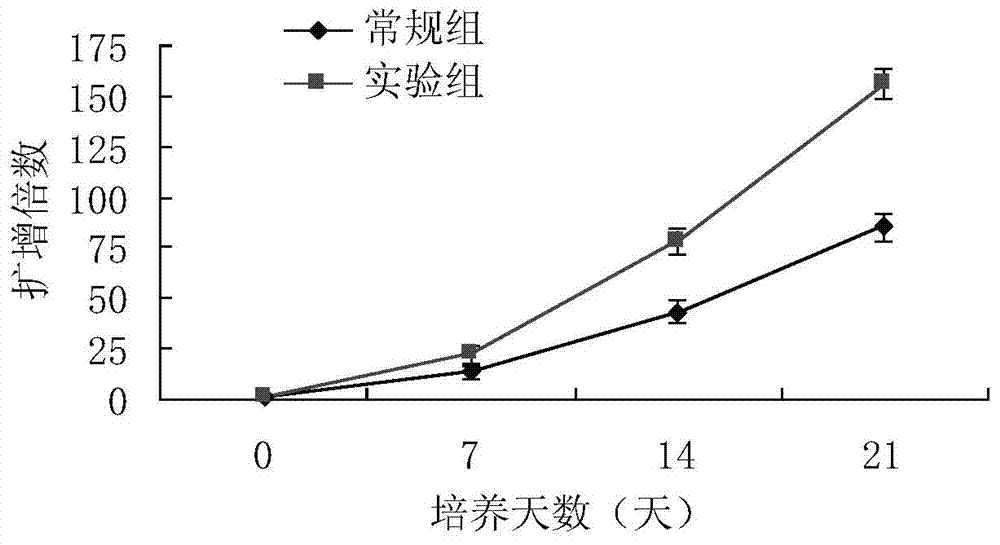
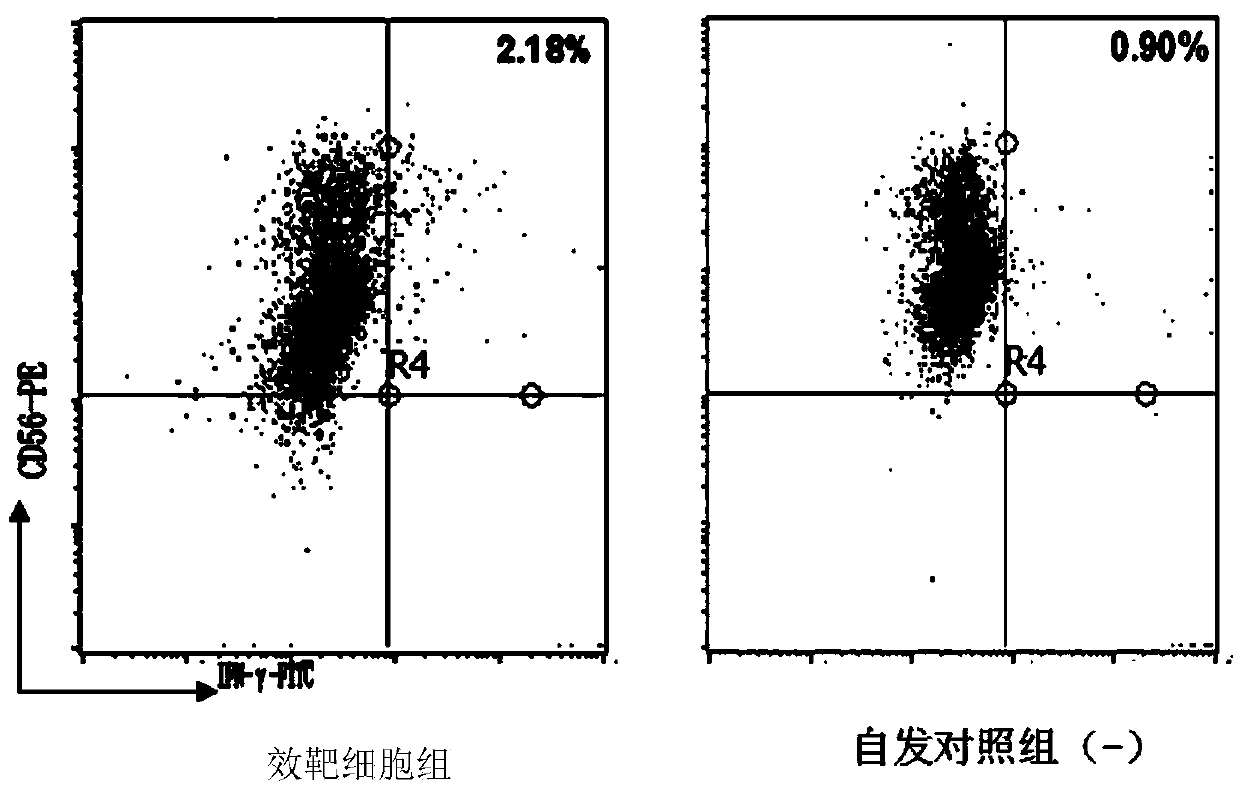
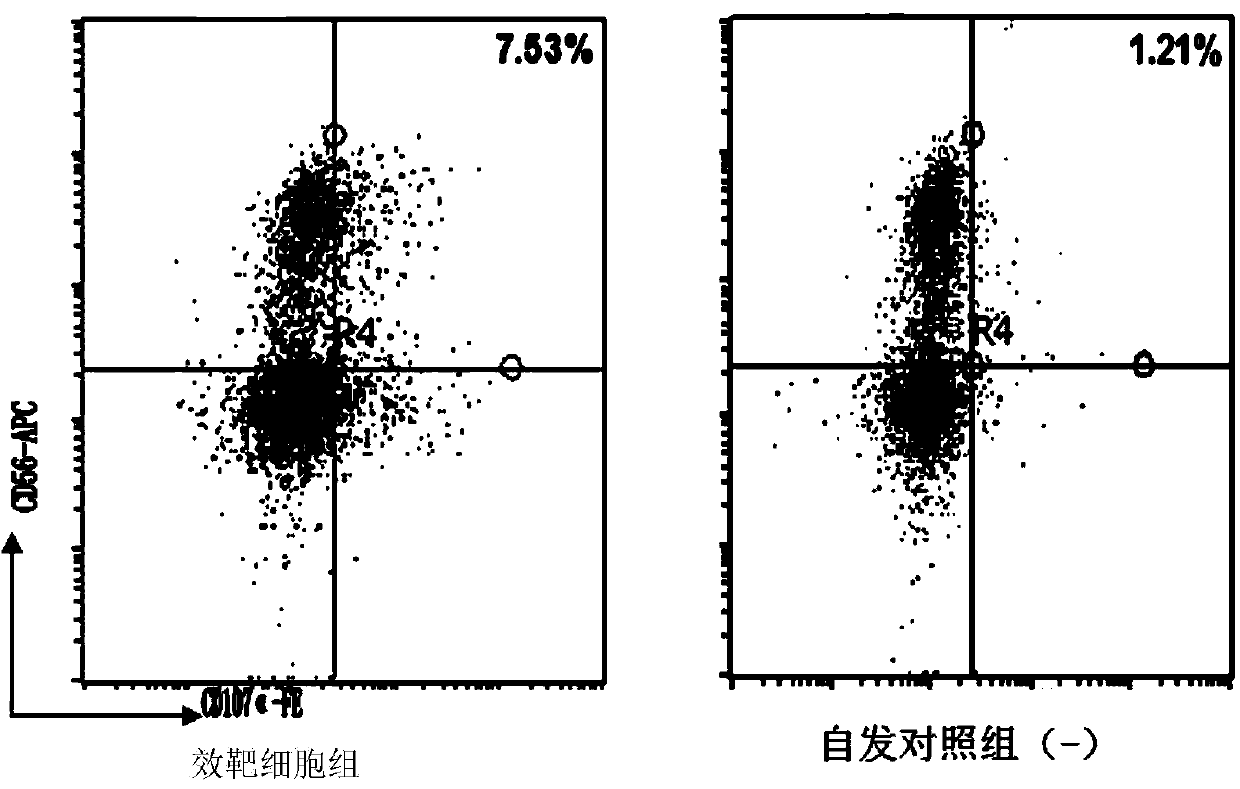
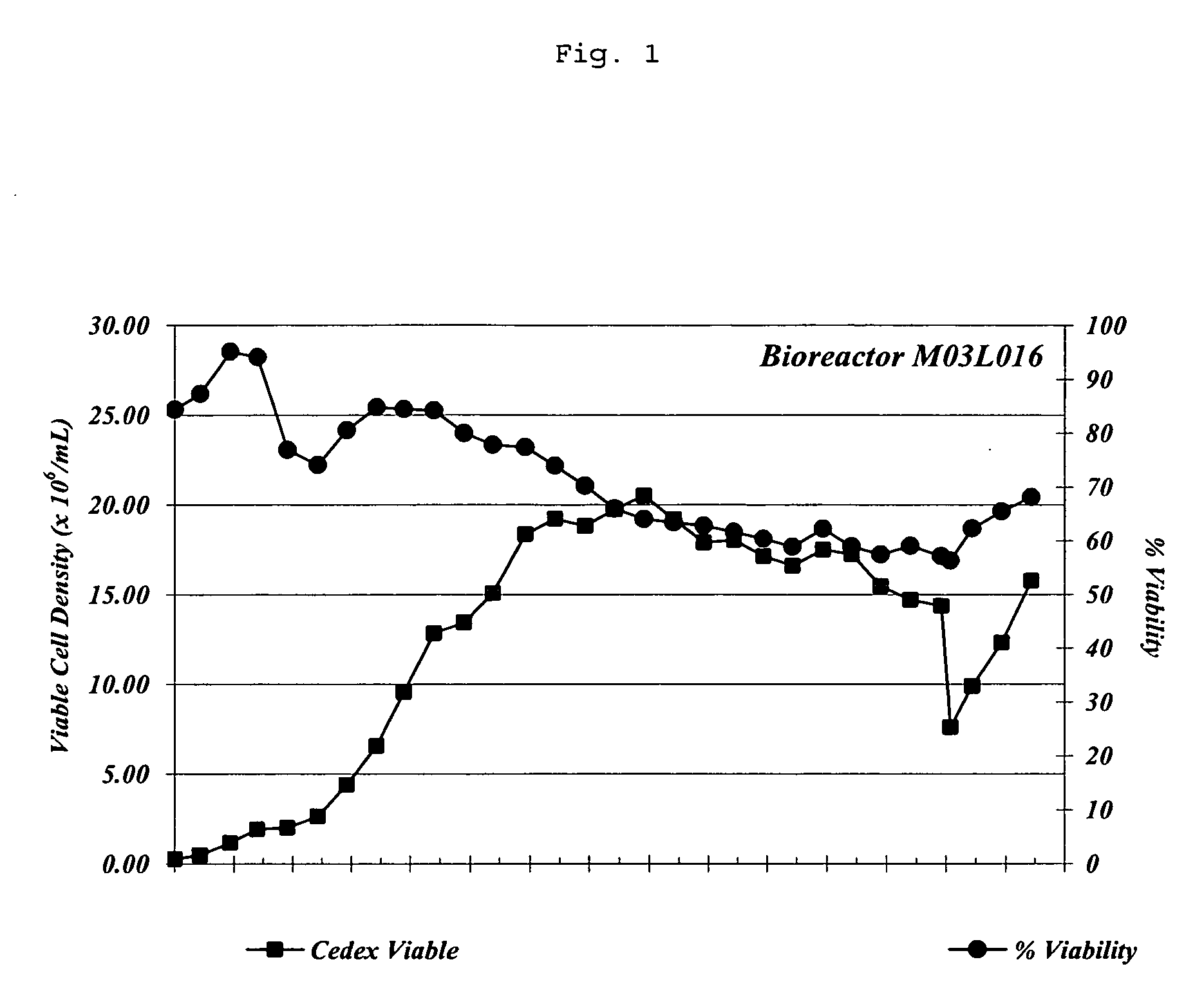
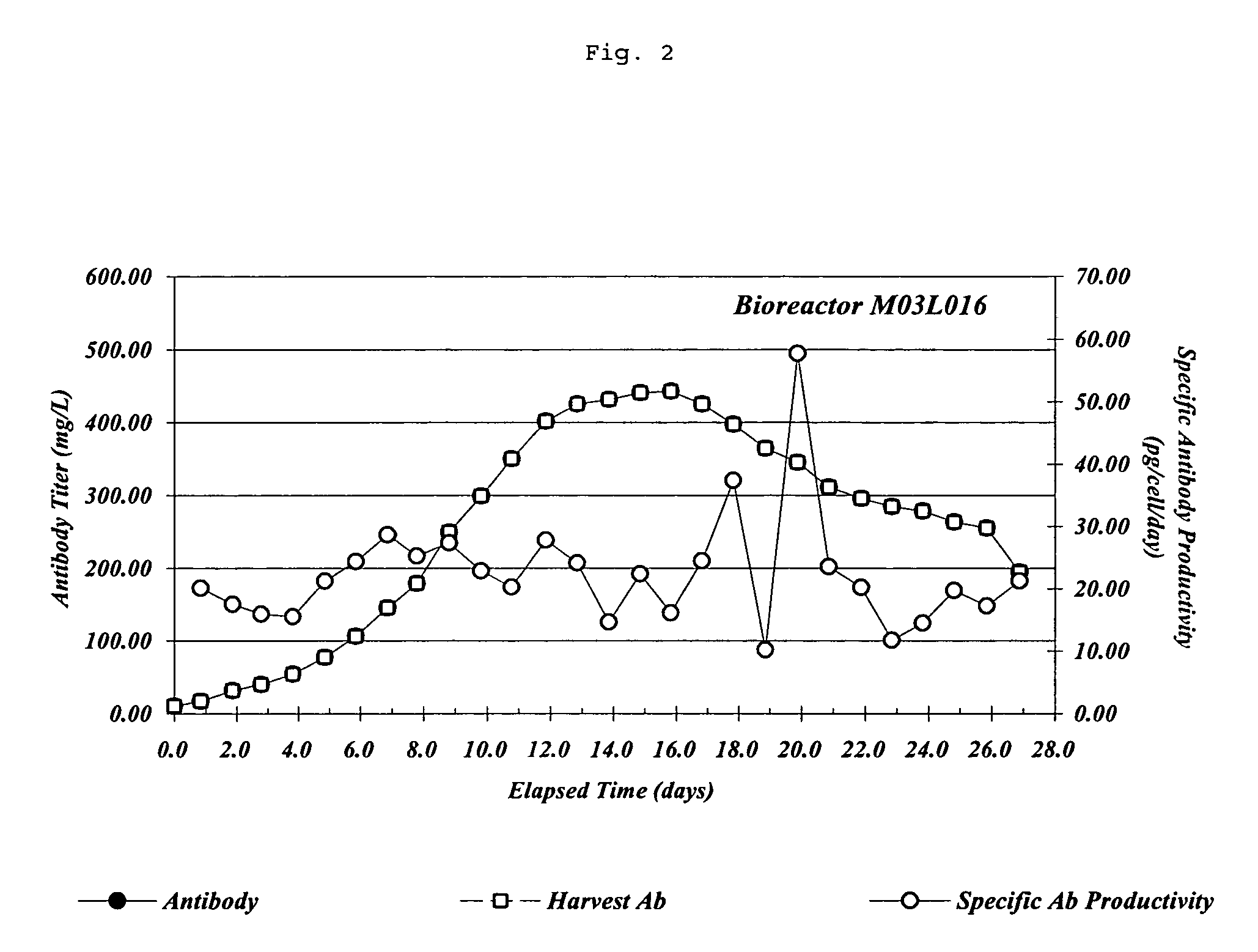
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
456 results about "Serum free media" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
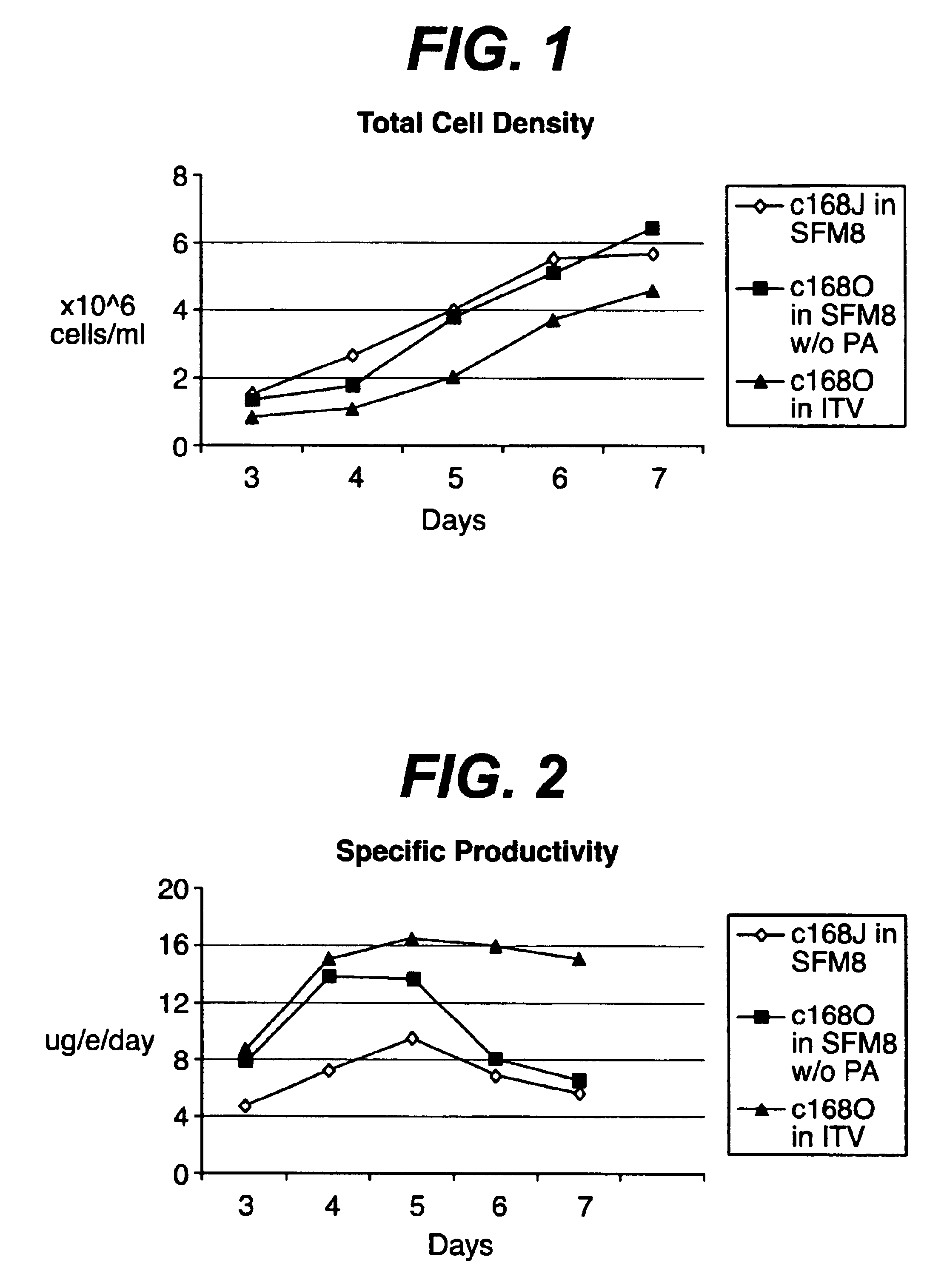
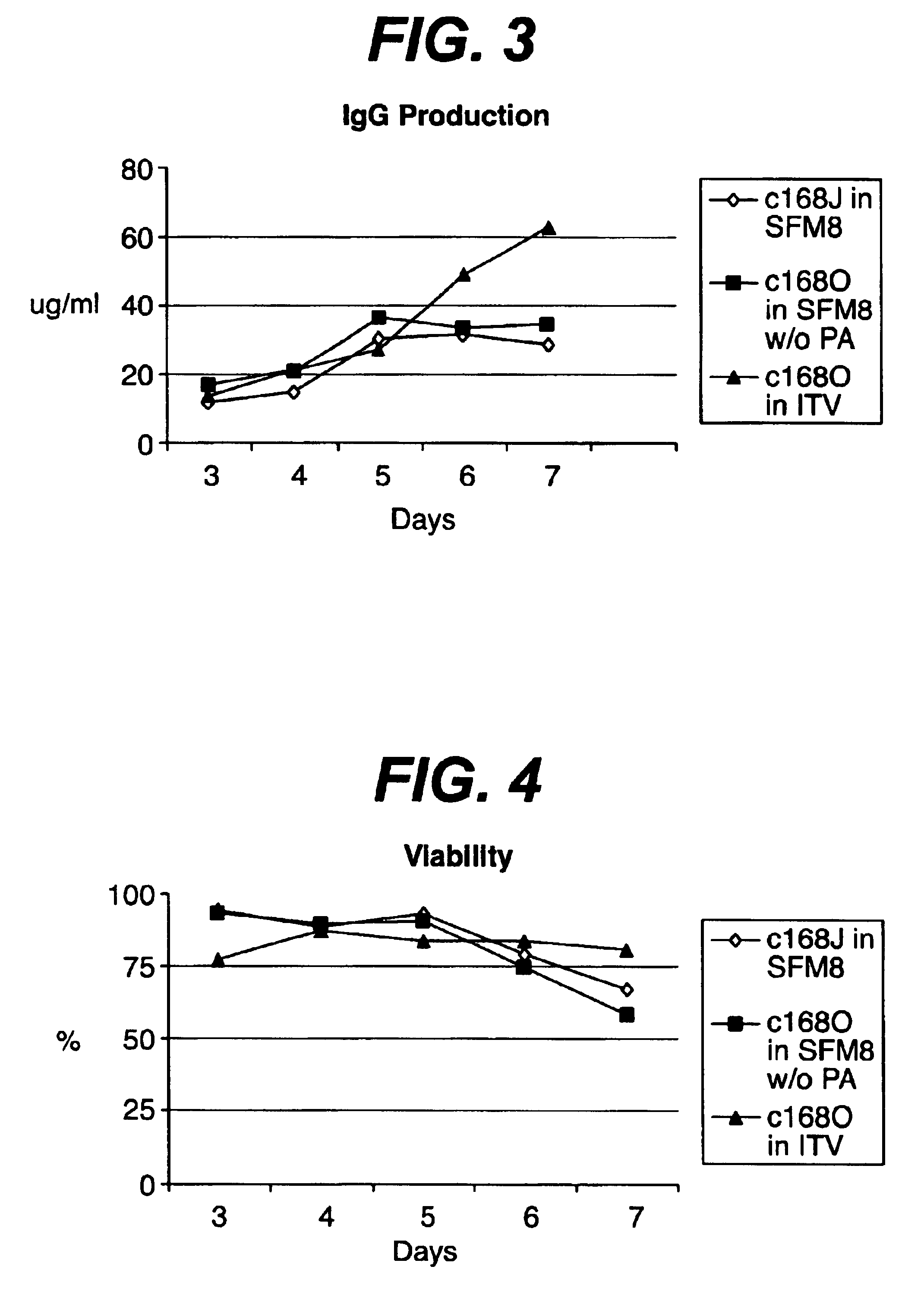
There is a clear distinction between serum-free media and chemically defined media. Serum-free media may contain undefined animal-derived products such as serum albumin (purified from blood), hydrolysates, growth factors, hormones, carrier proteins, and attachment factors.
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
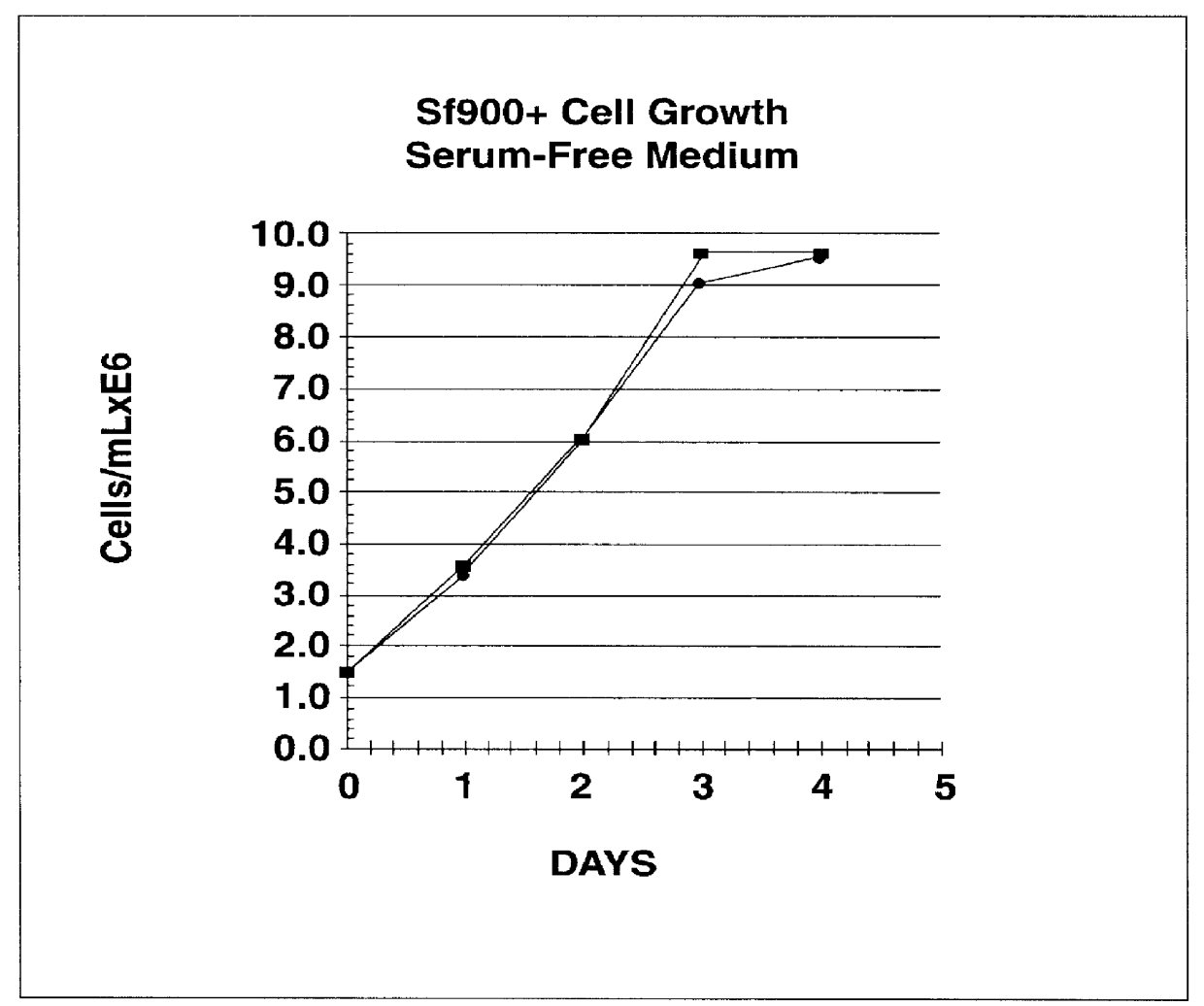
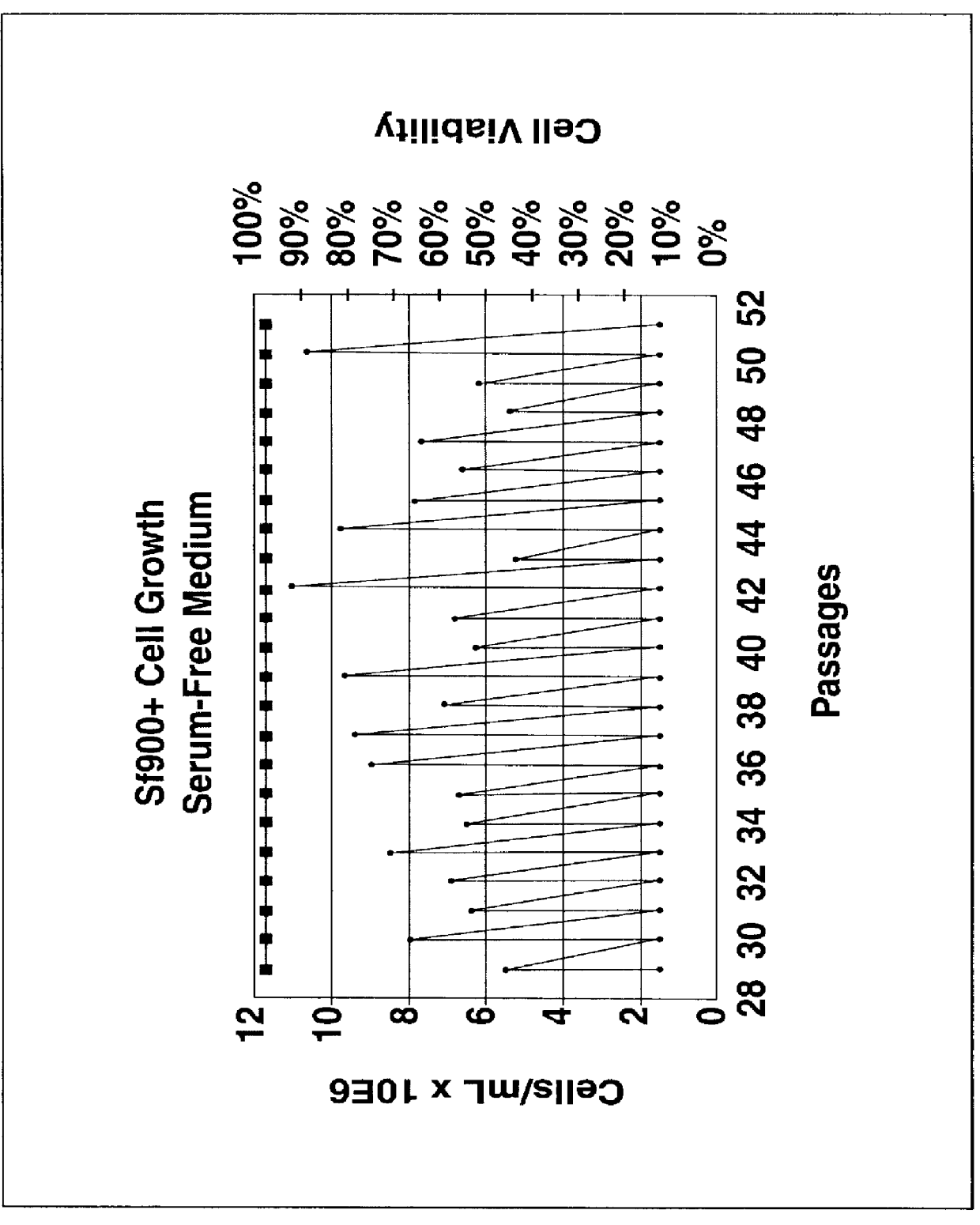
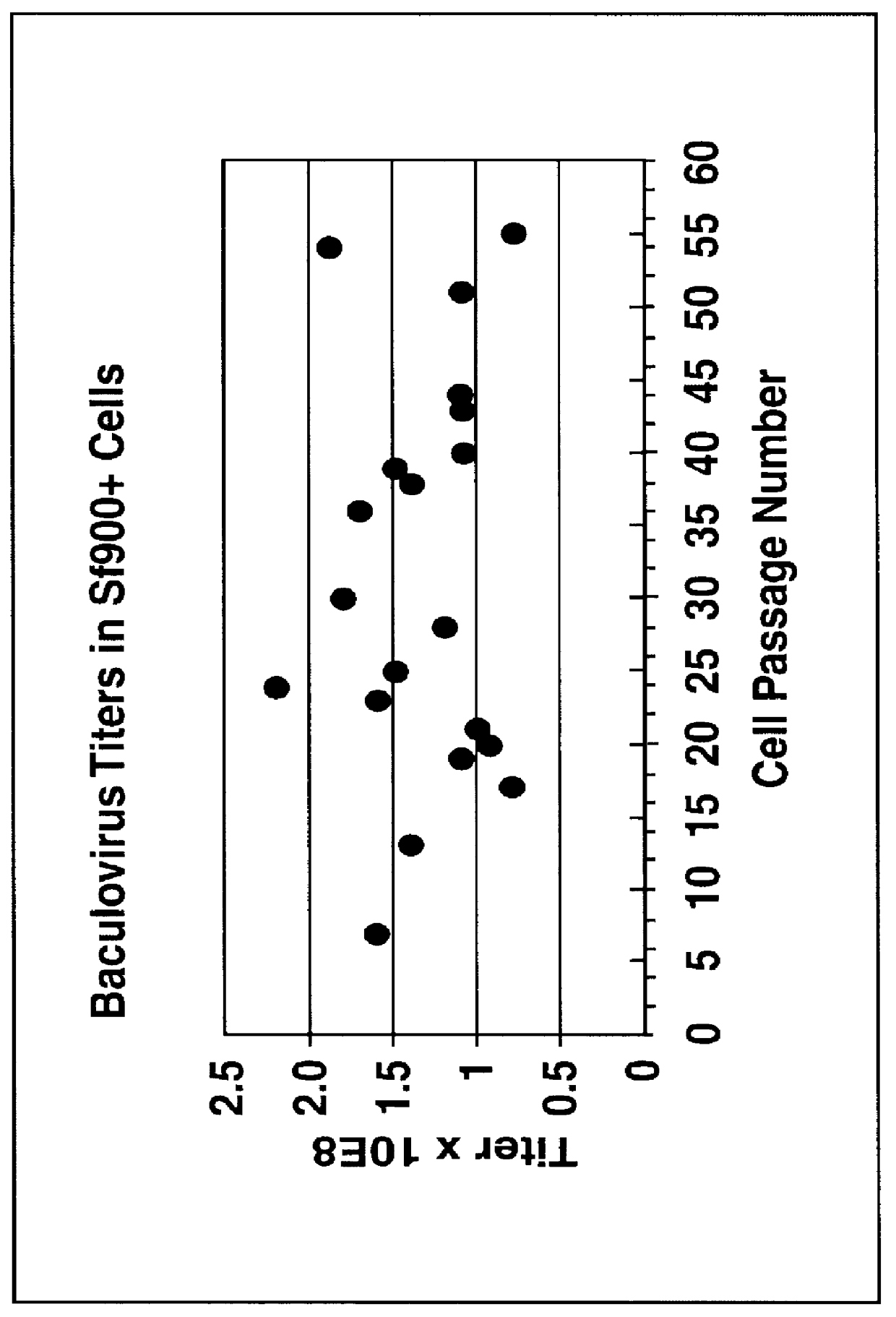
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Process for producing polypeptide
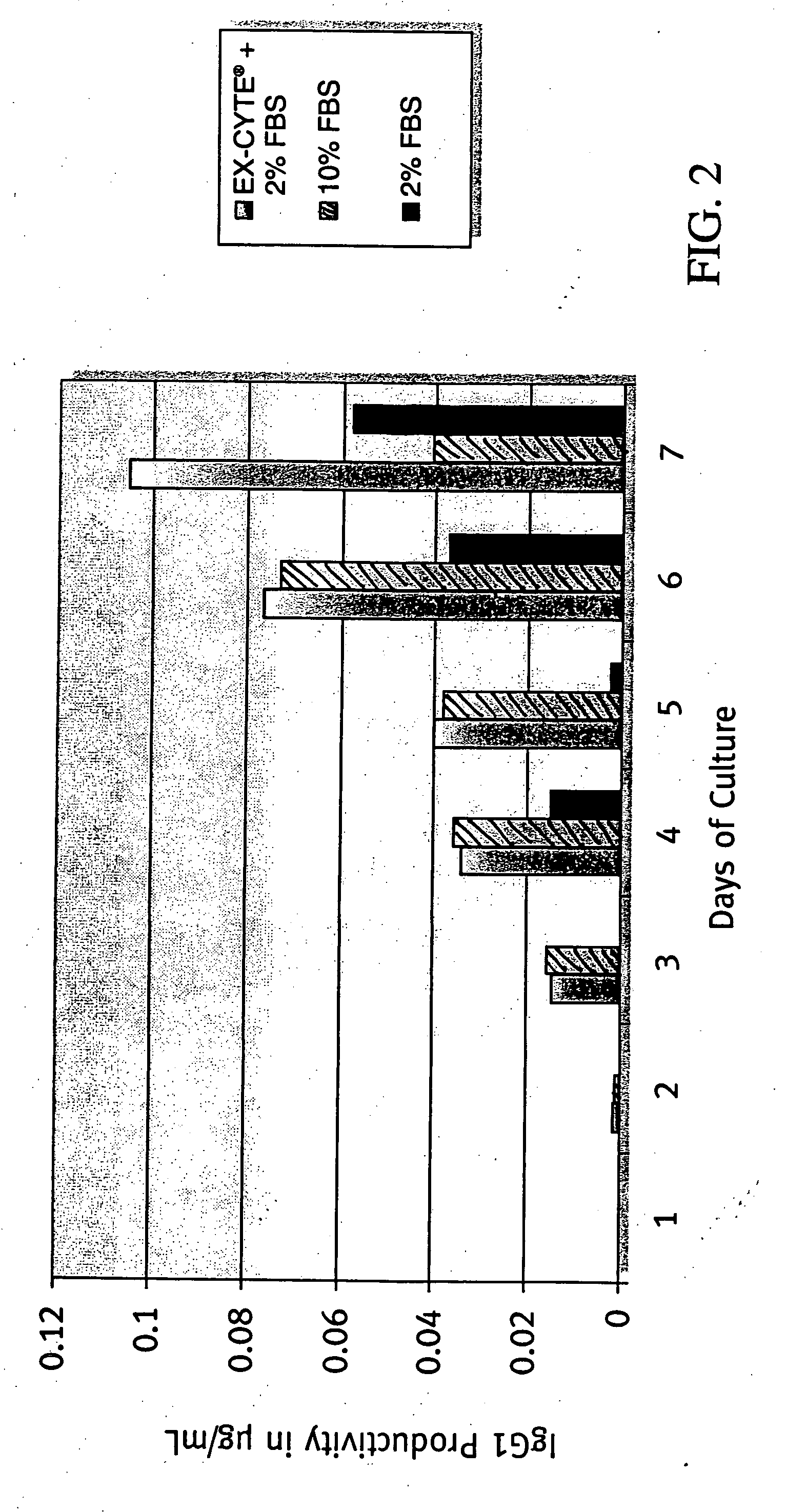
InactiveUS7504256B1Efficient productionImprove metabolic efficiencyFused cellsImmunoglobulinsSerum igeSerum free media
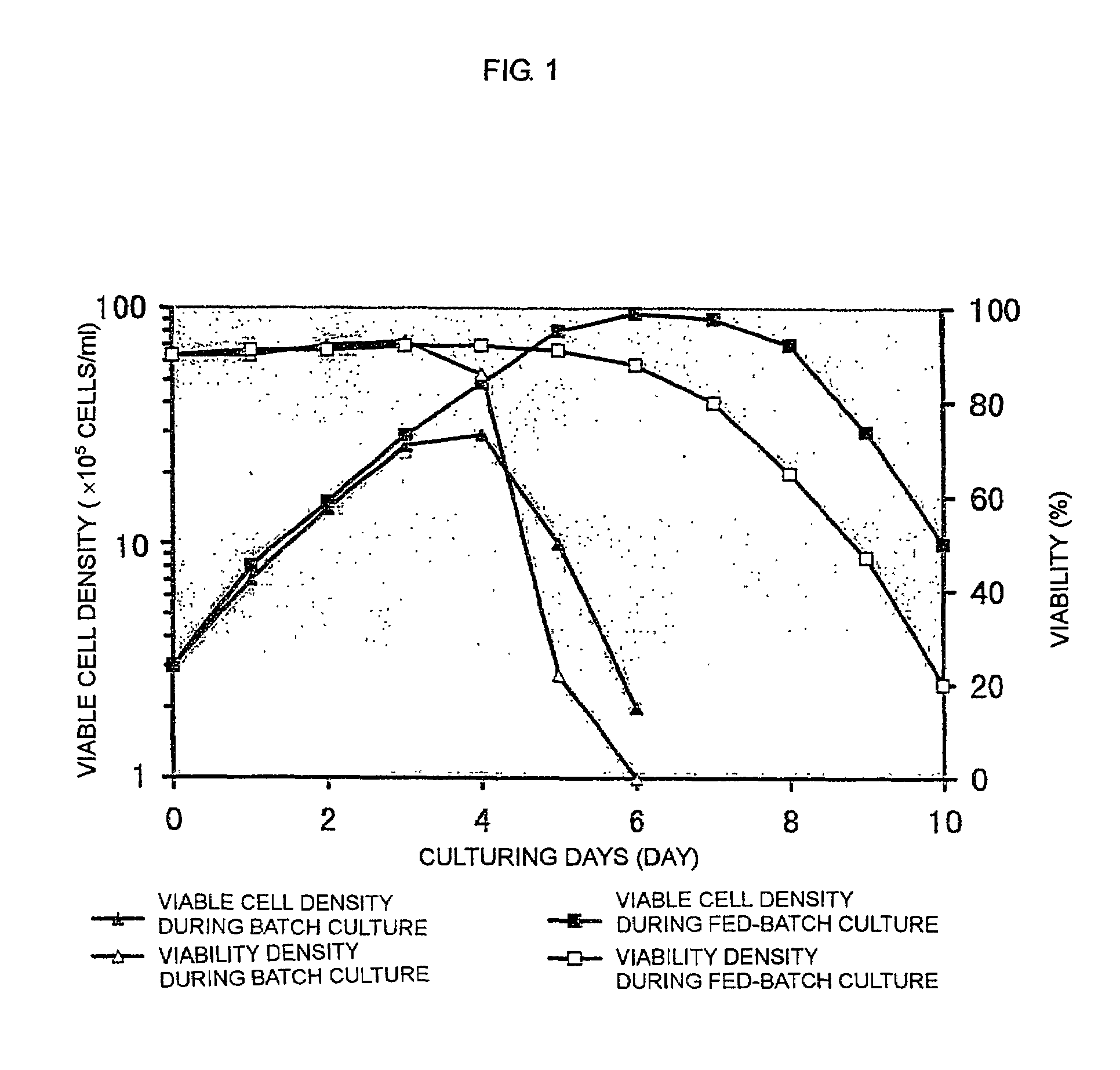
The present invention relates to a process for producing a desired polypeptide using rat cells. Specifically, the present invention relates to a process for producing the polypeptide which comprises culturing rat cells such as YB2 / 3HL.P2.G11.16Ag.20 (hereinafter referred to as YB2 / 0), preferably rat cells to which a recombinant DNA comprising DNA encoding a desired polypeptide such as an immunologically functional molecule is introduced, in a medium which does not contain serum (hereinafter referred to as a serum-free medium). Among the desired polypeptides obtained by the process of the present invention, an antibody obtained by using a transformant of YB2 / 0 has a high antibody-dependent cell-mediated cytotoxic activity (hereinafter sometimes referred to as ADCC activity) and is useful as a pharmaceutical agent.
Owner:KYOWA HAKKO KIRIN CO LTD
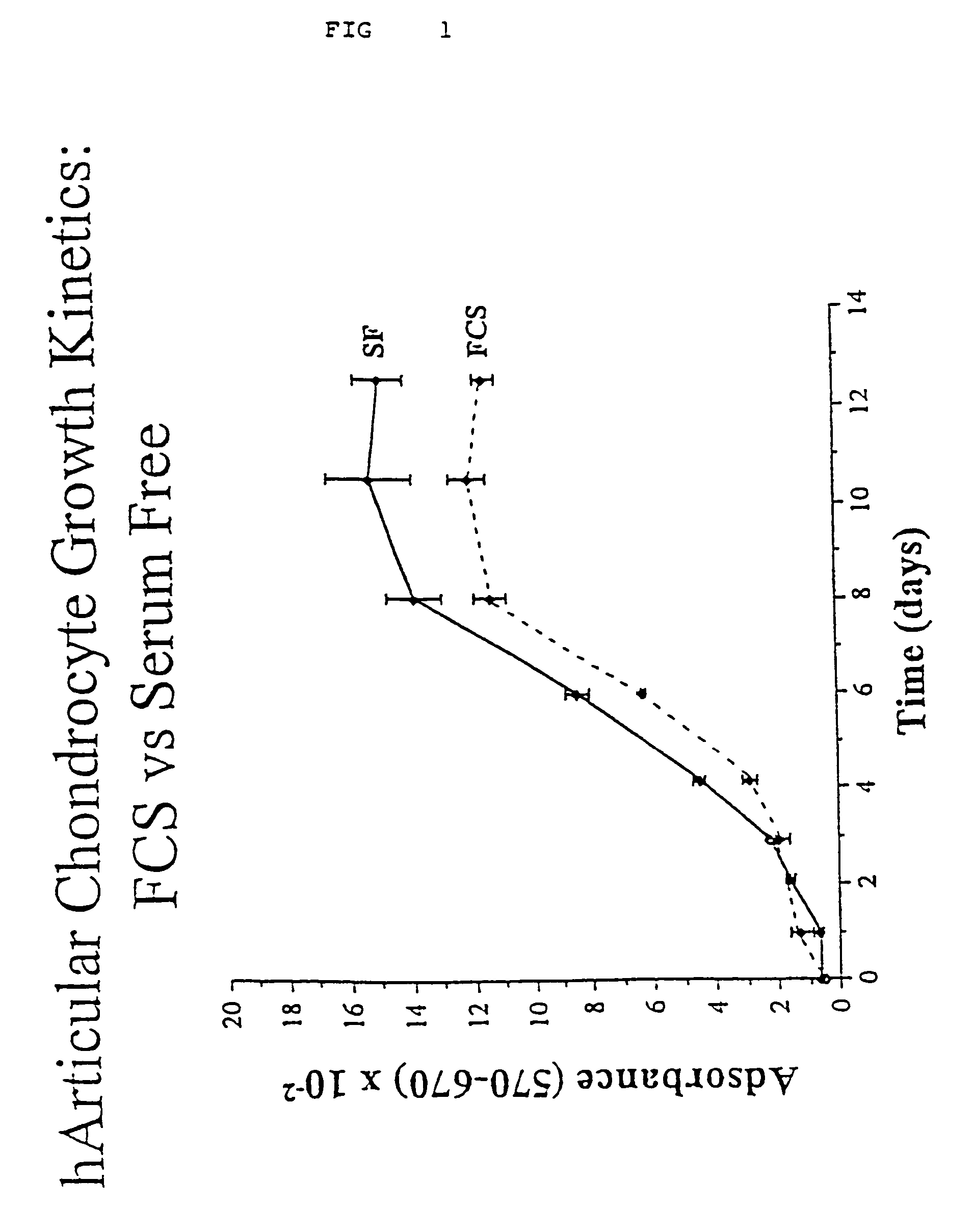
Serum-free media for chondrocytes and methods of use thereof
InactiveUS7169610B2Safe effective inexpensiveSafe and effective and inexpensiveCulture processArtificial cell constructsLipid formationSerum free media
The present invention provides defined serum-free cell culture media useful in culturing fibroblasts, especially articular chondrocytes, that avoids problems inherent in the use of serum-containing media. The defined media comprise platelet-derived growth factor (PDGF), and chemically defined lipids, or combinations of these compounds. In another aspect, the present invention also provides tissue culture methods that comprise incubating chondrocytes in the defined serum free media. The methods enhance attachment and proliferative expansion of chondrocytes seeded at low density while maintaining their redifferentiation potential.
Owner:GENZYME CORP
Mammalian cell lines for increasing longevity and protein yield from a cell culture
ActiveUS7537930B2Increase longevity and recombinant protein yieldIncreases lifespan and viabilityVirus peptidesApoptosis related proteinsSerum free mediaApoptosis
Owner:IMMUNOMEDICS INC
Modified Vaccinia Ankara virus variant and cultivation method
InactiveUS7445924B2Reduce riskGenetic material ingredientsVirus peptidesSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Methods for protein expression in mammalian cells in serum-free medium
ActiveUS7608425B2Increase longevity and recombinant protein yieldIncreases lifespan and viabilityPeptide/protein ingredientsApoptosis related proteinsSerum free mediaMammal
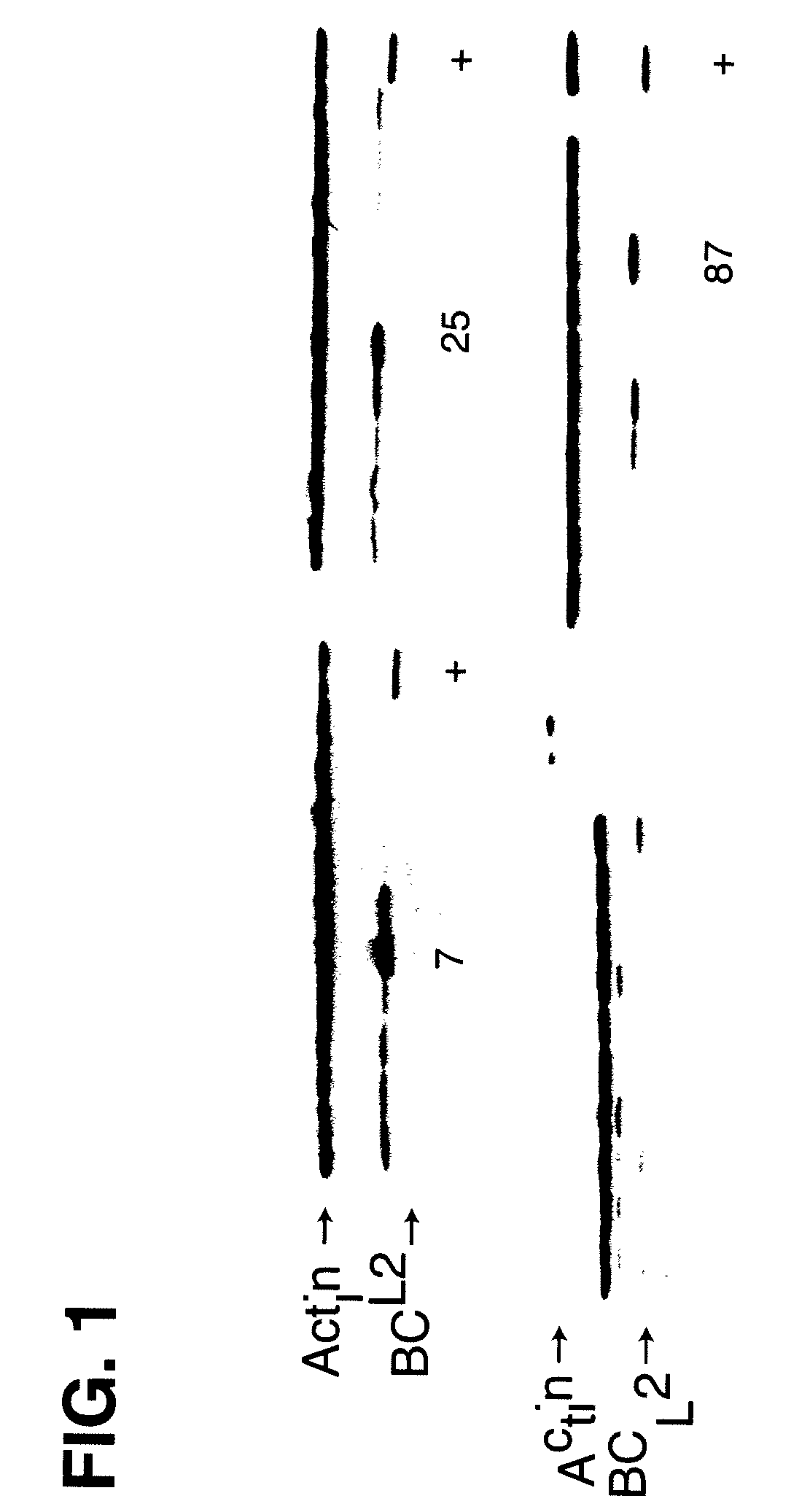
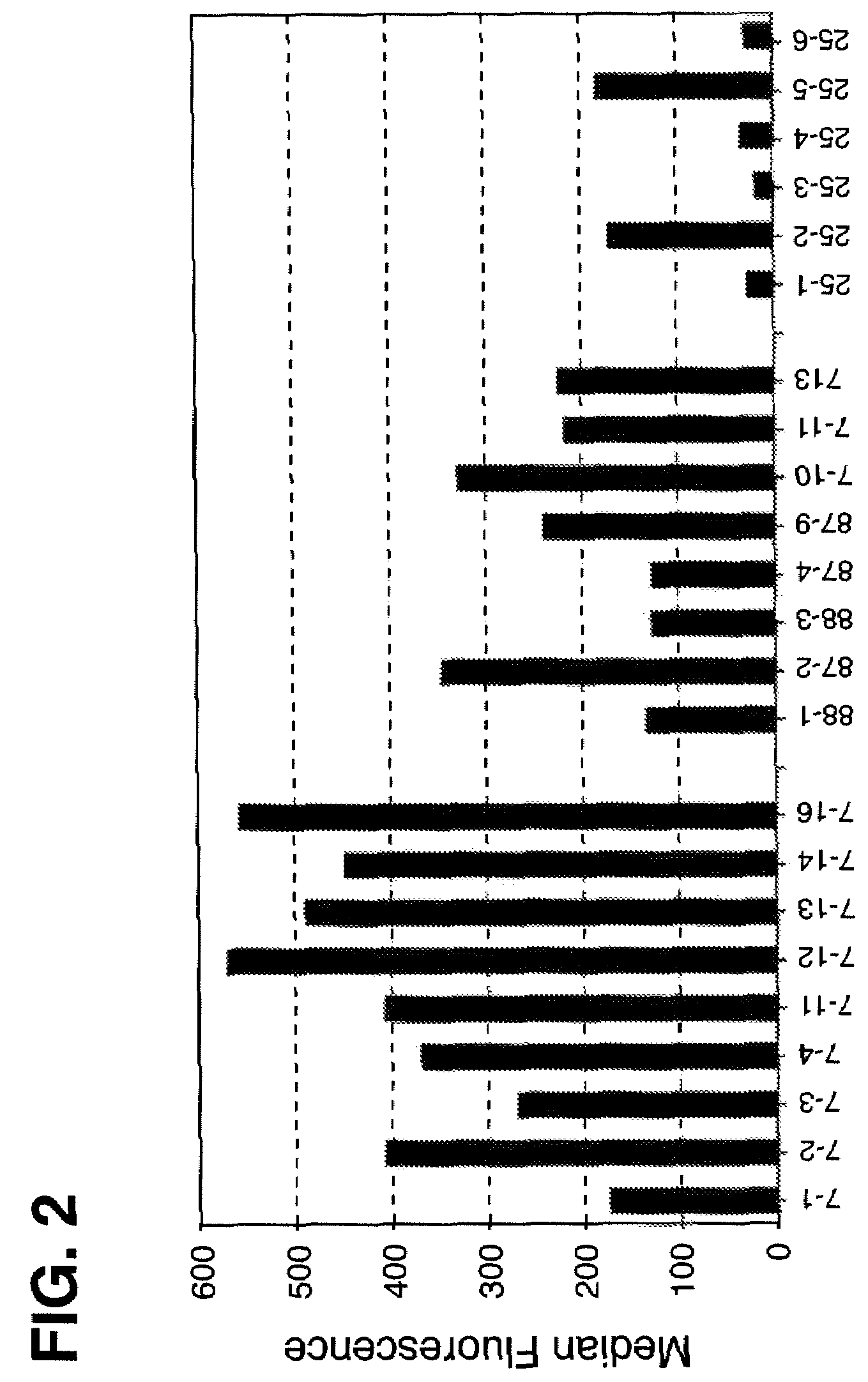
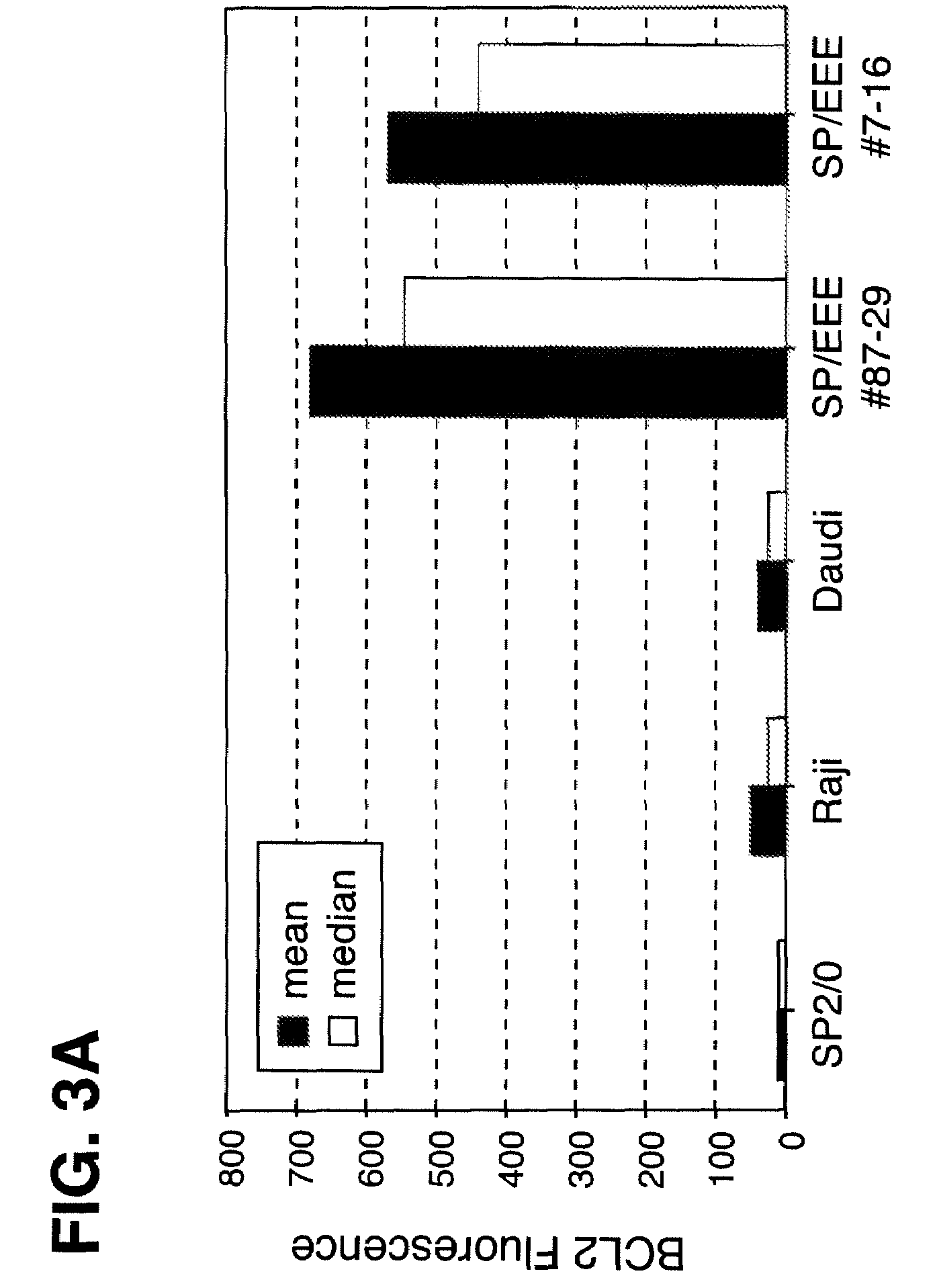
Disclosed are compositions and methods for increasing the longevity of a cell culture and permitting the increased production of proteins, preferably recombinant proteins, such as antibodies, peptides, enzymes, growth factors, interleukins, interferons, hormones, and vaccines. Cells transfected with an apoptosis-inhibiting gene or vector, such as a triple mutant Bcl-2 gene, can survive longer in culture, resulting in extension of the state and yield of protein biosynthesis. Such transfected cells exhibit maximal cell densities that equal or exceed the maximal density achieved by the parent cell lines. Transfected cells can also be pre-adapted for growth in serum-free medium, greatly decreasing the time required to obtain protein production in serum-free medium. In certain methods, the pre-adapted cells can be used for protein production following transfection under serum-free conditions. In preferred embodiments, the cells of use are SpESF or SpESF-X cells.
Owner:IMMUNOMEDICS INC
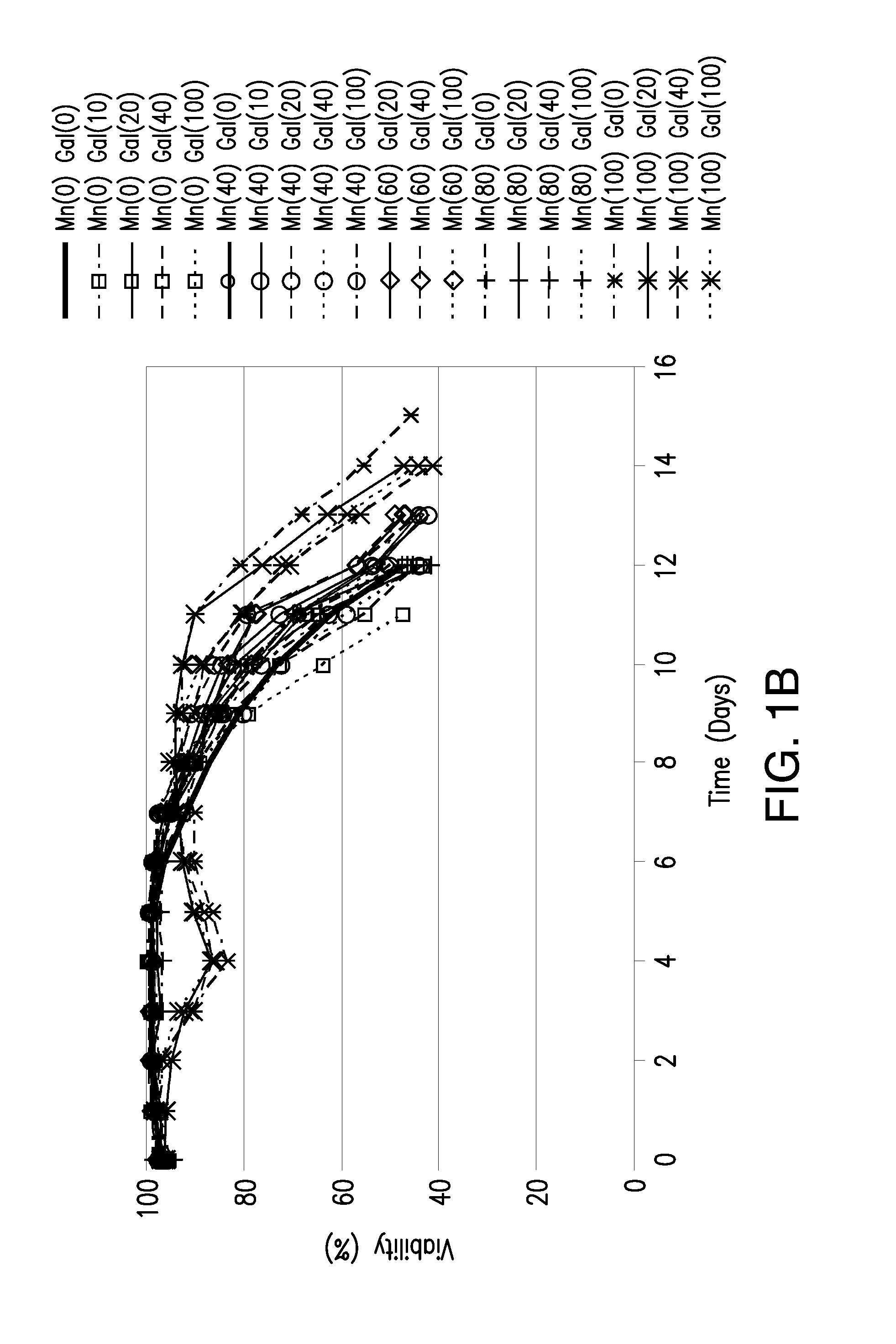
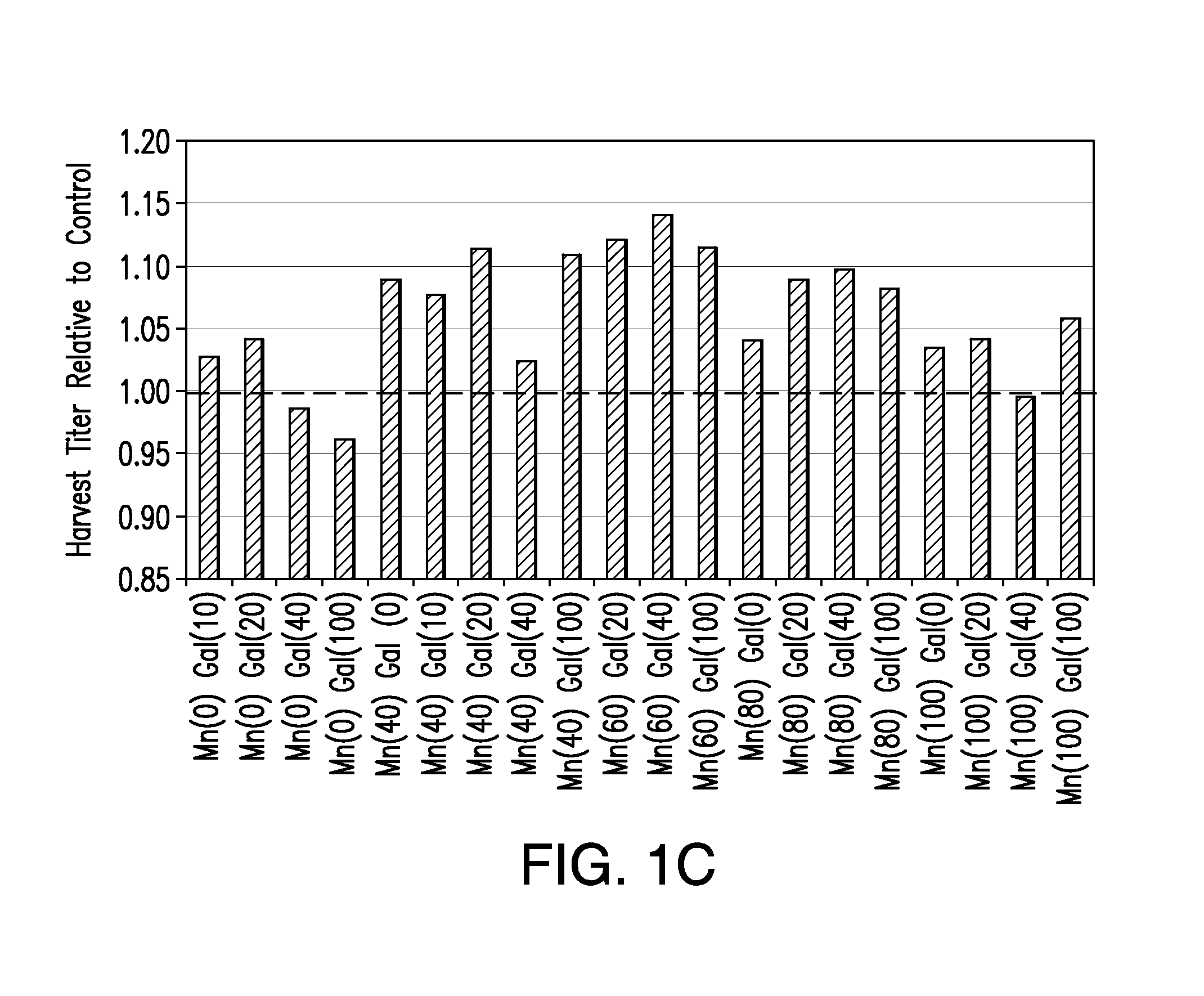
Methods for controlling the galactosylation profile of recombinantly-expressed proteins
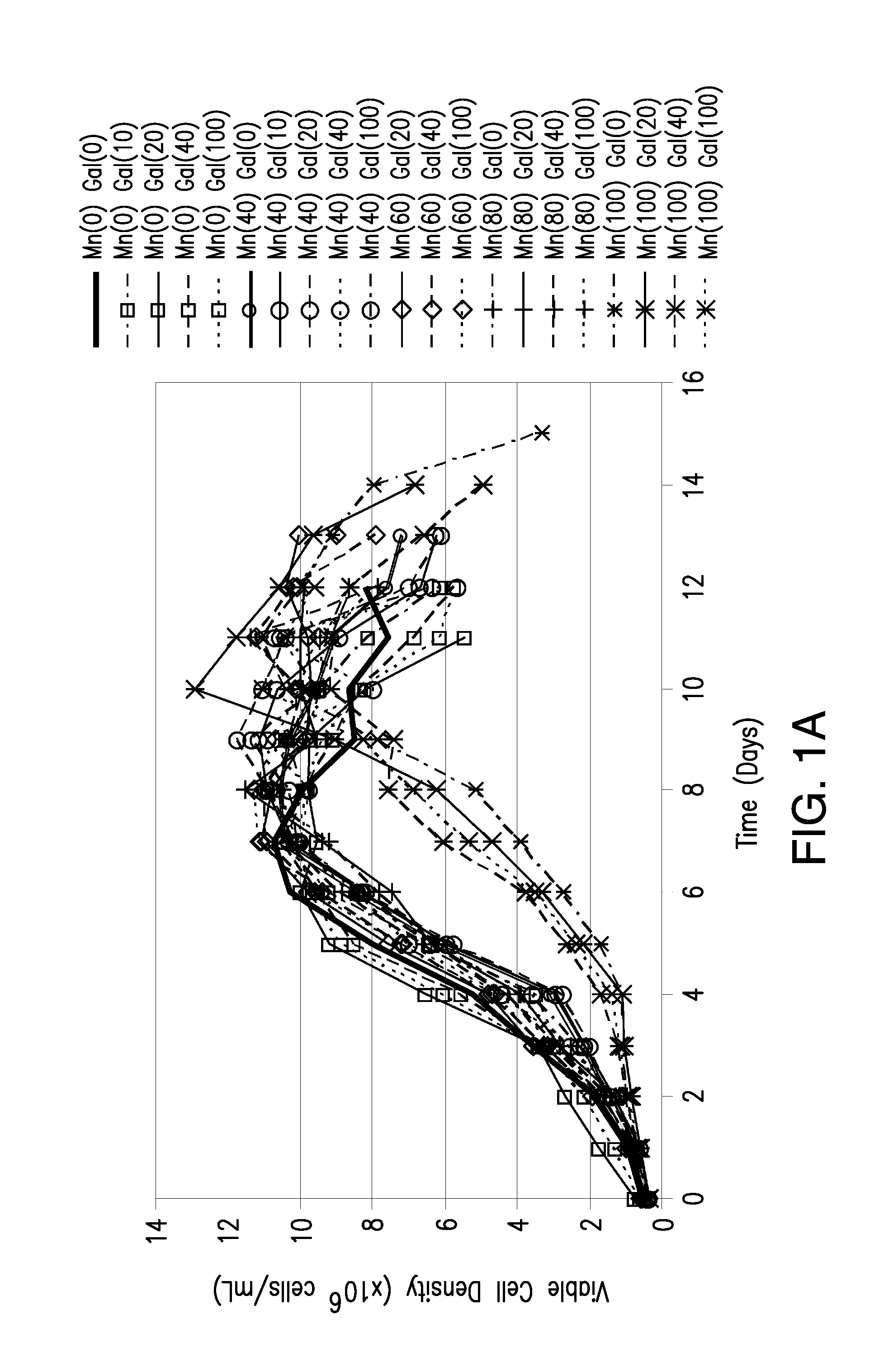
The present invention relates to methods for modulating the glycosylation profile of recombinantly-expressed proteins. In particular, the present invention relates to methods of controlling the galactosylation profile of recombinantly-expressed proteins by supplementing production medium, e.g., a hydrolysate-based or a chemically defined medium, with manganese and / or D-galactose.
Owner:ABBVIE INC
Chemically defined medium for cultured mammalian cells
InactiveUS6900056B2Reduced regulatory concerns for proteinsAdvantageously producedCell receptors/surface-antigens/surface-determinantsGenetically modified cellsSerum free mediaChemical composition
Owner:CENTOCOR
Serum-free medium for mesenchymal stem cells
Serum-free media for growth and proliferation of chondrocytes and mesenchymal stem cells in culture are provided. A serum-free medium for growth of chondrocytes includes a serum-free composition comprising FGF-2, linoleic acid, ascorbic acid, B-mercaptoethanol, transferrin and dexamethasone. Further, the composition comprises EGF, PDGFbb, insulin and albumin. A method for growing chondrocytes in a serum free medium comprising the compostion is also provided. Also provided for mesenchymal stem cell growth, is a serum-free medium which includes a composition comprising FGF-2, LIF, SCF, pantotenate, biotin and selenium and method, therefore.
Owner:CONSORZIO PER LA GESTIONE DEL CENT DI BIOTECNOLOGIA AVANZATA +1
Synthetic surfaces for culturing cells in chemically defined media
InactiveUS20090191627A1Reduce Potential ContaminationExtended shelf lifeArtificial cell constructsCell culture supports/coatingSerum free mediaCross-link
Synthetic surfaces capable of supporting culture of eukaryotic cells including stem cells and undifferentiated human embryonic stem cells in a chemically defined medium include a swellable (meth)acrylate layer and a polypeptide conjugated to the swellable (meth)acrylate layer. The swellable (meth)acrylate layer may be formed by polymerizing monomers in a composition that includes a carboxyl group-containing (meth)acrylate monomer, a cross-linking (di- or higher-functional) (meth)acrylate monomer, and a hydrophilic monomer capable of polymerizing with the carboxyl group-containing (meth)acrylate monomer and the cross-linking (meth)acrylate monomer. The swellable (meth)acrylate layer has an equilibrium water content in water of between about 5% and about 70%. The conjugated peptide may include an RGD amino acid sequence.
Owner:GERON CORPORATION
Swellable (METH)acrylate surfaces for culturing cells in chemically defined media
InactiveUS20090191632A1Reduce Potential ContaminationExtended shelf lifeCell culture supports/coatingEmbryonic cellsSerum free mediaPolymer science
Synthetic surfaces capable of supporting culture of undifferentiated human embryonic stem cells in a chemically defined medium include a swellable (meth)acrylate layer and a peptide conjugated to the swellable (meth)acrylate layer. The swellable (meth)acrylate layer may be formed by polymerizing monomers in a composition that includes hydroxyethyl methacrylate, 2-carboxyehylacrylate, and tetra(ethylene glycol) dimethacrylate. The conjugated peptide may include an amino acid sequence of XaanProGlnValThrArgGlyAspValPheThrMetPro, where n is an integer from 0 to 3 and where Xaa is any amino acid. Further, disclosed herein is a swellable (meth)acrylate synthetic surface which can be sterilized by gamma irradiation.
Owner:GERON CORPORATION
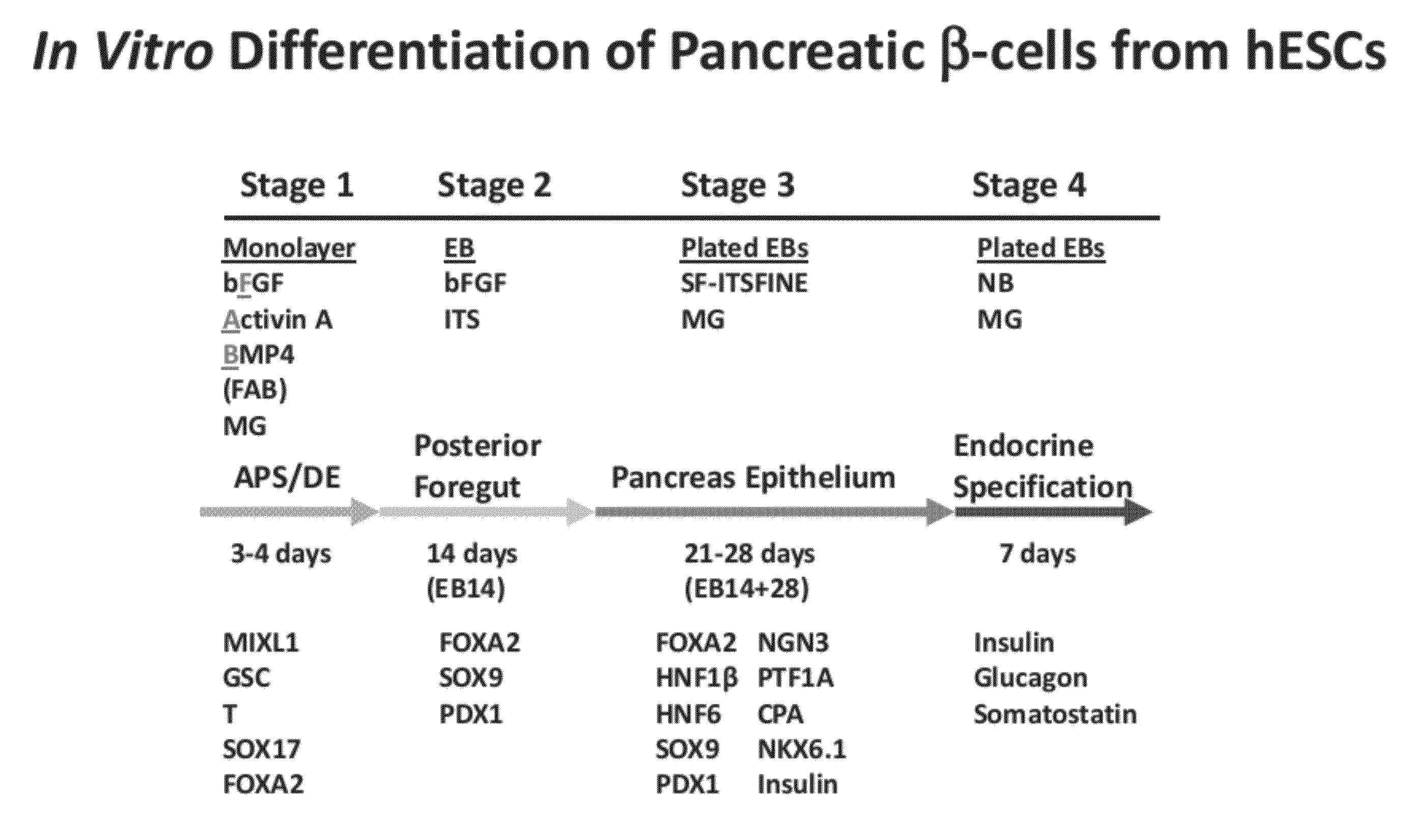
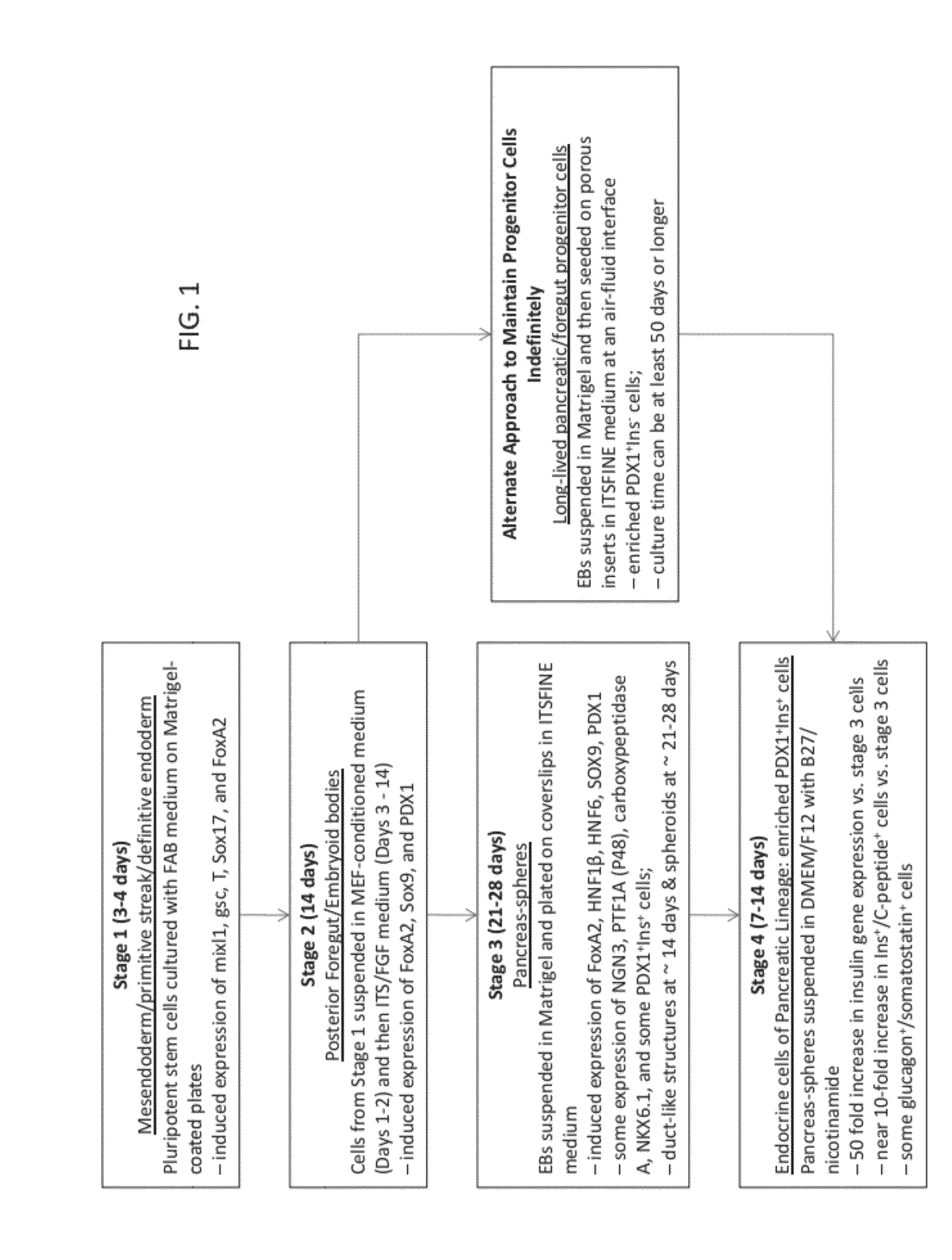
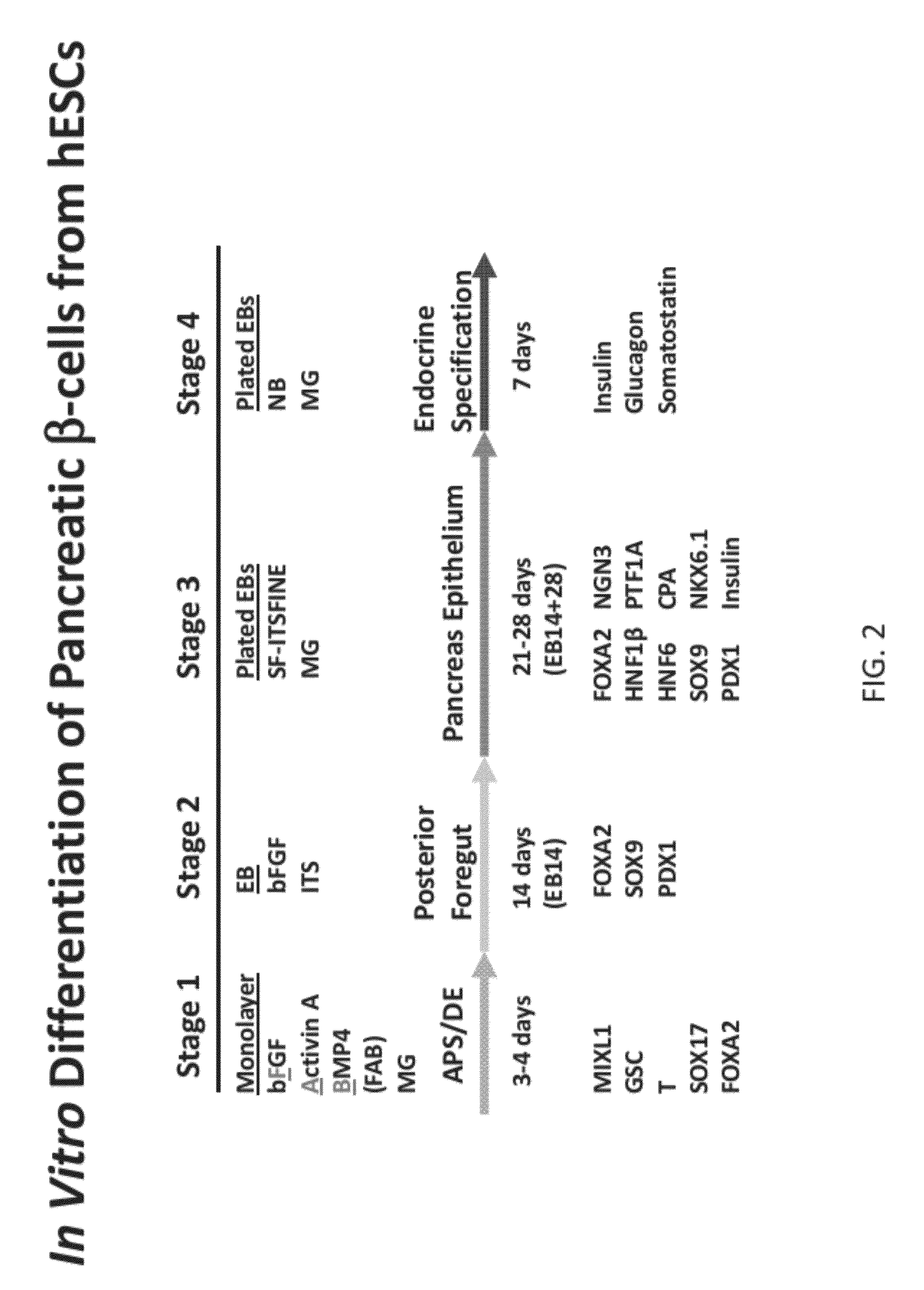
Methods and devices for differentiating pluripotent stem cells into cells of the pancreatic lineage
Methods and devices for culturing human pluripotent stem cells to produce cells of the pancreatic lineage are disclosed. The methods include steps of culturing the stem cells under conditions that induce the expression of mesendoderm / primitive streak and definitive endoderm markers in a chemically defined medium including an effective amount of i) fibroblast growth factor, ii) Activin A, and iii) bone morphogenetic protein. The methods further include the steps of culturing cells under conditions favoring the formation of at least one of intact embryoid bodies and pancreatic progenitor PDX1+ Ins− cells.
Owner:WISCONSIN ALUMNI RES FOUND
Cell culture media
InactiveUS20060073591A1Reduces and eliminates useImprove performanceCulture processCell culture mediaSerum free mediaCell culture media
The invention is a cell culture medium that can include reduced or no serum and that enhances the performance of serum-free media for cell culture. The medium supports the growth of cells for both small scale and large scale propagation of cells. The invention also includes a method of cultivating cells using the cell culture medium of the present invention.
Owner:MILLIPORE CORP
Serum-free medium for in vitro cultivation and amplification of mesenchymal stem cells
ActiveCN101412985AResidue reductionMaintain multilineage potentialSkeletal/connective tissue cellsSerum free mediaAntioxidant
The present invention belongs to the field of biotechnology, and discloses a serum-free culture medium with specific chemical compositions for in vitro culture and amplification of bone marrow mesenchymal stem cells. By adding insulin, transferrin, ethanolamine, sodium selenite, growth factors, adherent factors, hormone, putrescine, inorganic salt, vitamin, albumin and antioxidant into a basic culture medium, the bone marrow mesenchymal stem cells can attach to the culture medium under a serum free condition, so the in vitro culture and amplification are realized, the potential of multi-directional differentiation is maintained, and the amplified cells can be induced to be osteoblast and lipocyte in vitro. The serum-free culture medium has the advantages that the clinic level cell products for human produced by the serum free culture medium can effectively avoid the potential risk of producing cell products by serum culture medium. The drawing appended is a photo of the confluence of the bone marrow mesenchymal stem cells cultured by the serum-free culture medium.
Owner:EAST CHINA UNIV OF SCI & TECH
Three dimensional vaginal tissue model containing immune cells
InactiveUS6943021B2Improve survivabilityInduced proliferationBiocideEpidermal cells/skin cellsSerum free mediaAir liquid interface
Disclosed is a cervico-vaginal tissue equivalent comprised of vaginal epithelial cells and immune cells, cultured at the air-liquid interface. The tissue equivalent is capable of being infected with a sexually transmitted pathogen such as a virus (e.g., HIV), a bacteria, a helminthic parasite, or a fungus. The tissue equivalent is also capable of undergoing an allergic-type reaction or an irritant-type reaction. The tissue equivalent is characterized as having nucleated basal layer cells and nucleated suprabasal layer cells, and further as having cell layers external to the suprabasal layer progressively increasing in glycogen content and progressively decreasing in nuclei content. Immune cells of the tissue equivalent are primarily located in the basal and suprabasal layers. Also disclosed are methods for producing the tissue equivalent. The methods involve providing vaginal epithelial cells and immune cells, seeding the cells onto a porous support, and co culturing the seeded cells at the air-liquid interface under conditions appropriate for differentiation. One such method disclosed is for generation of the tissue equivalent in serum free medium. Specific cells from which the tissue equivalent is generated, and also specific preferred components of the medium in which the tissue equivalent is generated are provided. Also disclosed is a cervico-vaginal tissue equivalent produced by the methods disclosed herein.
Owner:MATTEK CORP
Modified vaccinia ankara virus variant and cultivation method
InactiveUS20050214323A1Reduce riskViral antigen ingredientsGenetic material ingredientsSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Method used for in vitro proliferation of NK cells
ActiveCN103756963AIncrease lethalityEasy to synthesizeBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
Owner:SHANGHAI CLAISON BIOTECH
Serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture
ActiveCN101760442ASupports adherent growthReduce the burden of separation and purification in the later stageVertebrate cellsArtificial cell constructsLipid formationSerum free media
The invention relates to the culture medium research and development technical field of modern biological technology and provides a serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture, which comprises 21 amino acids, 6 vitamins, 8 salts, 8 lipids, 4 trace elements, 2 buffers, 1 protein hydrolysate, 1 acid-base indicator and 6 other additives. The serum-free medium can be prepared by the conventional preparation method, and an application method thereof is the conventional method. The serum-free medium has the beneficial effects that: the serum-free medium does not contain serum, has clear components, is beneficial for separating and purifying the product and improves the product quality; the serum-free medium supports long-term subculture of MDCK cells and does not require long-term and complex adaptation process; and the serum-free medium can well support the adherent growth and single-cell suspension growth of the MDCK cells, has clear components and easy preparation and utilization, and is suitable for mass production of biological products.
Owner:EAST CHINA UNIV OF SCI & TECH
Method of Inducing the Differentiation of Embryonic Stem Cells Into Nerve by Serum-Free Suspension Culture
ActiveUS20080044901A1Effectively lead to differentiationEfficient inductionNervous system cellsEmbryonic cellsSerum free mediaNervous system
The present invention provides a clinically applicable method of inducing differentiation of embryonic stem cells, particularly a method of inducing differentiation of embryonic stem cells into forebrain neurons. More specifically, the present invention provides a method of inducing differentiation of embryonic stem cells, comprising culturing the embryonic stem cells as a floating aggregate in a serum-free medium, particularly a method of inducing differentiation of the embryonic stem cells into nervous system cells such as forebrain neurons and cerebellar neurons and sensory organ cells; a floating aggregate of embryonic stem cells obtained by culturing the embryonic stem cells as a floating aggregate in a serum-free medium; and cells derived from a floating aggregate of embryonic stem cells, particularly nervous system cells such as forebrain neurons and cerebellar neuron, sensory organ cells such as retinal precursor cells, and the like.
Owner:RIKEN
Clonal human embryonic stem cell lines and methods of generating same
InactiveUS20030073234A1Microbiological testing/measurementArtificial cell constructsSerum free mediaStem cell line
A method of establishing a clonal embryonic stem cell line capable of sustaining a phenotype of normal embryonic stem cells following at least eight months of in vitro culture is disclosed. The method is effected by culturing an individual embryonic stem cell for at least eight months in a serum-free medium, thereby establishing the clonal embryonic stem cell line capable of sustaining said phenotype of normal embryonic stem cells following at least eight months of in vitro culture.
Owner:WISCONSIN ALUMNI RES FOUND
Multipotent stem cells derived from human adipose tissue and cellular therapeutic agents comprising the same
ActiveUS20070110729A1High proliferation ratePositive immunological responsesBiocideNervous disorderCartilage cellsSerum free media
This invention relates to human adipose tissue-derived multipotent adult stem cells. More particularly, the invention relates to human adipose tissue-derived multipotent stem cells, which can be maintained in an undifferentiated state for a long period of time by forming spheres and have high proliferation rates, as well as methods for isolating and maintaining the adult stem cells, and methods for differentiating the multipotent adult stem cells into nerve cells, fat cells, cartilage cells, osteogenic cells and insulin-releasing pancreatic beta-cells. Also, the invention relates to cellular therapeutic agents for treating osteoarthritis, osteoporosis and diabetes and for forming breast tissue, which contain the differentiated cells or the adult stem cells. Although the multipotent stem cells are adult stem cells, they have the ability to differentiate into osteogenic cells, nerve cells, astrocytes, fat cells, chrondrogenic cells or insulin-releasing pancreatic beta-cells, and so are effective in treating osteoporosis, osteoarthritis, nerve disease, diabetes, etc. Also, the stem cells form spheres in a serum-free medium containing CORM-2, and thus can be maintained in an undifferentiated state for a long period of time. Also, the stem cells have very high proliferation rates. Accordingly, the stem cells are useful as cellular therapeutic agents.
Owner:RNL BIO
Method For In Vitro Amplification Of Adult Stem Cells
InactiveUS20080085555A1Effective expansionHigh expansion efficiencyCulture processArtificial cell constructsSerum free mediaMicrobiology
The present invention provides a method of efficiently expanding non-adhesive adult stem cells in vitro. More specifically, the present invention provides a method of expanding non-adhesive adult stem cells, comprising culturing non-adhesive adult stem cells in the co-presence of adhesive feeder cells and non-adhesive non-stem cells in serum-free medium, wherein the ratio of non-adhesive adult stem cell count to total cell count in the medium is kept low; non-adhesive adult stem cells obtained by the expansion method and differentiated cells thereof, and a composition comprising them; and a serum-free medium comprising a specified factor, and a kit for its preparation and the like.
Owner:FOUND FOR BIOMEDICAL RES & INNOVATION +1
Culture medium used for Vero cell and cultivation method thereof
InactiveCN101182490AFast growthArtificial cell constructsVertebrate cellsSerum free mediaMicrobiology
The invention relates to the preparation and application method of a medium, which is specifically related to a medium which is used for Vero cell and a culture method of the Vero cell. The invention belongs to the biological product area. The culture method of the invention is that the adhering Vero cells are suspend and cultured in SFM serum-free medium for the domestication and adaptation; the growing pattern of the Vero cell changes from adherence growth to suspension growth; the Vero cells are further amplified in Vero amplification CHEMICALLY DEFINED MEDIUM. The method of the invention has good effects; the growing pattern of the Vero cell is transformed from adherence growth to suspension growth, which changes the growth pattern of the cell and greatly increases the yield of the cell.
Owner:天津百若克医药生物技术有限责任公司
Multipotent stem cells derived from human adipose tissue and cellular therapeutic agents comprising the same
ActiveUS7807461B2High proliferation ratePositive immunological responsesNervous disorderSkeletal disorderSerum free mediaBrown adipose tissue
This invention relates to human adipose tissue-derived multipotent adult stem cells. More particularly, the invention relates to human adipose tissue-derived multipotent stem cells, which can be maintained in an undifferentiated state for a long period of time by forming spheres and have high proliferation rates, as well as methods for isolating and maintaining the adult stem cells, and methods for differentiating the multipotent adult stem cells into nerve cells, fat cells, cartilage cells, osteogenic cells and insulin-releasing pancreatic beta-cells. Also, the invention relates to cellular therapeutic agents for treating osteoarthritis, osteoporosis and diabetes and for forming breast tissue, which contain the differentiated cells or the adult stem cells. Although the multipotent stem cells are adult stem cells, they have the ability to differentiate into osteogenic cells, nerve cells, astrocytes, fat cells, chrondrogenic cells or insulin-releasing pancreatic beta-cells, and so are effective in treating osteoporosis, osteoarthritis, nerve disease, diabetes, etc. Also, the stem cells form spheres in a serum-free medium containing CORM-2, and thus can be maintained in an undifferentiated state for a long period of time. Also, the stem cells have very high proliferation rates. Accordingly, the stem cells are useful as cellular therapeutic agents.
Owner:RNL BIO
Compositions and methods for modular soft tissue repair
InactiveUS20100098739A1Superior in vivo engraftmentSuperior in tissue integrationBiocideMammal material medical ingredientsSerum free mediaTissue repair
Adipose tissue-derived stromal cells induced to form multicellular aggregates can be formed reliably and consistently, they can be maintained for prolonged periods in adherent or suspension culture, and they are able to survive, grow, and / or differentiate in serum-free media conditions. The present invention provides compositions and methods for the use of such aggregates in tissue repair. This culture platform provides a controlled and defined system in which to study and standardize adult stem cell biology, and begets an instinctive “modular” approach to the predictable replacement and regeneration of adipose tissue.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Method for in-vitro amplification of NK cells
InactiveCN102994449AEasy to synthesizeIncrease lethalityBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention relates to a method for in-vitro amplification of NK cells, and in particular relates to a method for massive in-vitro amplification of NK cells, wherein the method comprises the following steps of: a, inoculating a peripheral blood mononuclear cell in a CD3McAb and CD226McAb pre-coated culture bottle for coculture; b, adding 1L-2 and 1L-18, coculturing for 72hours to stimulate amplification of NK cells; c, transferring the NK cells, K562 cells after lethal treatment and a serum-free medium containing 1L-2 and 1L-18 in a cell culture bag for coculture; and d, collecting the NK cells. According to the method for in-vitro amplification of the NK cells, two antibodies CD3McAb and CD226McAb are simultaneously coated, so the cell factor synthesis and ADCC effect are promoted, and killing toxicity of the NK cells is remarkably improved; the activation and amplification on the NK cells are achieved just by the 1L-2 and 1L-18 cell factors, so the amplification multiple and cell toxicity of the NK cells are guaranteed, and the cost of cell culture is reduced.
Owner:SHANGHAI CLAISON BIOTECH
Method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells
ActiveCN104357390AHigh purityHigh activityBlood/immune system cellsAdoptive cellular immunotherapySerum free media
The invention discloses a method for simultaneous and efficient amplification of CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells. The method comprises the steps as follows: the concentration of separated PBMC (peripheral blood mononuclear cells) is adjusted by a serum-free medium containing autologous plasma, an Anti-CD16 antibody, IL-2 and IL-15 are added, and then the mixture is transferred into a T175 culture flask for culture; an Anti-CD3 antibody and an Anti-CD137 antibody are added; a serum-free medium containing the autologous plasma, IL-2 and IL-15 is supplemented every two days according to the cell growth condition; the cell concentration is controlled to be about 1.5*10<6> / ml; and after culture is performed for 14-21 days, large quantities of high-purity CD<3+>CD<56+>CIK cells and CD<3->CD<56+>NK cells can be obtained simultaneously, and the total cell quantity can reach an effective value of the cell quantity required for adoptive cellular immunotherapy clinically for tumor. The method for simultaneous and efficient amplification of the CD<3+>CD<56+>CIK cells and the CD<3->CD<56+>NK cells is simple, convenient, effective and high in cell killing activity.
Owner:HRYZ (SHENZHEN) BIOTECH CO +1
Efficient culture of stem cells for the production of hemoglobin
InactiveUS6361998B1Efficiently transfectedExtend your lifeBlood/immune system cellsFermentationSerum free mediaSerum ige
The present invention describes a serum-free medium that promotes the growth and differentiation of erythroid cells, cells that are highly transducible by a non-viral method and genes which increase the growth of erythroid cells and decrease their dependency on Epo. This invention can be used in the expansion of hematopoietic stem cells to produce cultures of erythroid cells as a source of erythroid-specific proteins such as hemoglobin. Hematopoietic stem cells are cultured ex vivo in a serum-free culture medium with the addition of IL-3, SCF and EPO. Cells transfected with the gene described in the present invention can be cultured in the serum-free culture medium with decreased dependency on Epo and other cytokines, thereby reducing the cost of the production of hemoglobin.
Owner:THERAPURE BIOPHARMA INC
Efficient multiplication CTL preparation method killing tumors in targeted mode
InactiveCN103923880AEfficient proliferative abilityInhibition of differentiationBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
The invention discloses an efficient multiplication CTL preparation method killing tumors in a targeted mode. The CTL preparation method comprises the following steps: (a) removing CD4+CD25+Treg cells through immunomagnetic bead negative sorting; (b) arranging mixed cells in a serum-free medium for cultivation, and obtaining suspension cells and adherent cells; (c) adding GM-SCF and IL-4 in the adherent cells, culturing the cells for five days; in the sixth day, adding a tumour cell holoantigen, and in the seventh day, adding TNF-alpha and IL-27; (d) transferring the suspension cells to a culture flask wrapped by a CD3 monoclonal antibody and recombinant human fibronectin, adding IFN-gamma, in the second day, adding IL-2, IL-12 and the IL-27, and culturing the mixture till the eighth day to obtain CIK cells; (e) mixing the CIK cells and mature DC cells, and adding the IL-12, IL-7 and an anti-CD 28 monoclonal antibody for cultivation; in the third day, adding an anti-CTLA-4 monoclonal antibody, and then culturing the mixture for four days. According to the efficient multiplication CTL preparation method killing tumors in the targeted mode, efficiency of in-vitro CTL cell proliferation is improved, activity of killing the tumor cells in the targeted mode is improved, transformation of peripheral blood mononuclear cells to the CD4+CD25+Treg cells is inhibited.
Owner:四川全组生命科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com