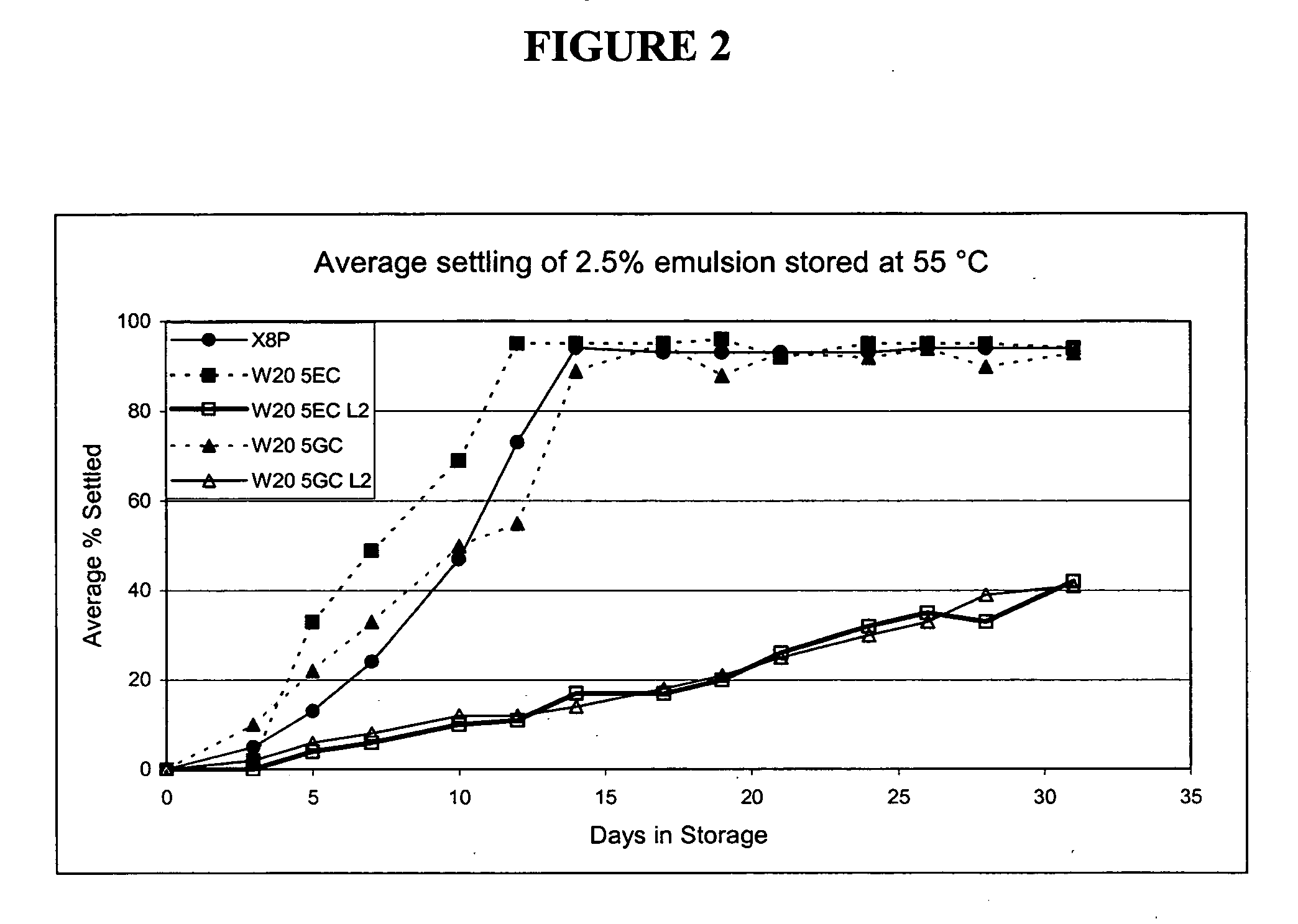
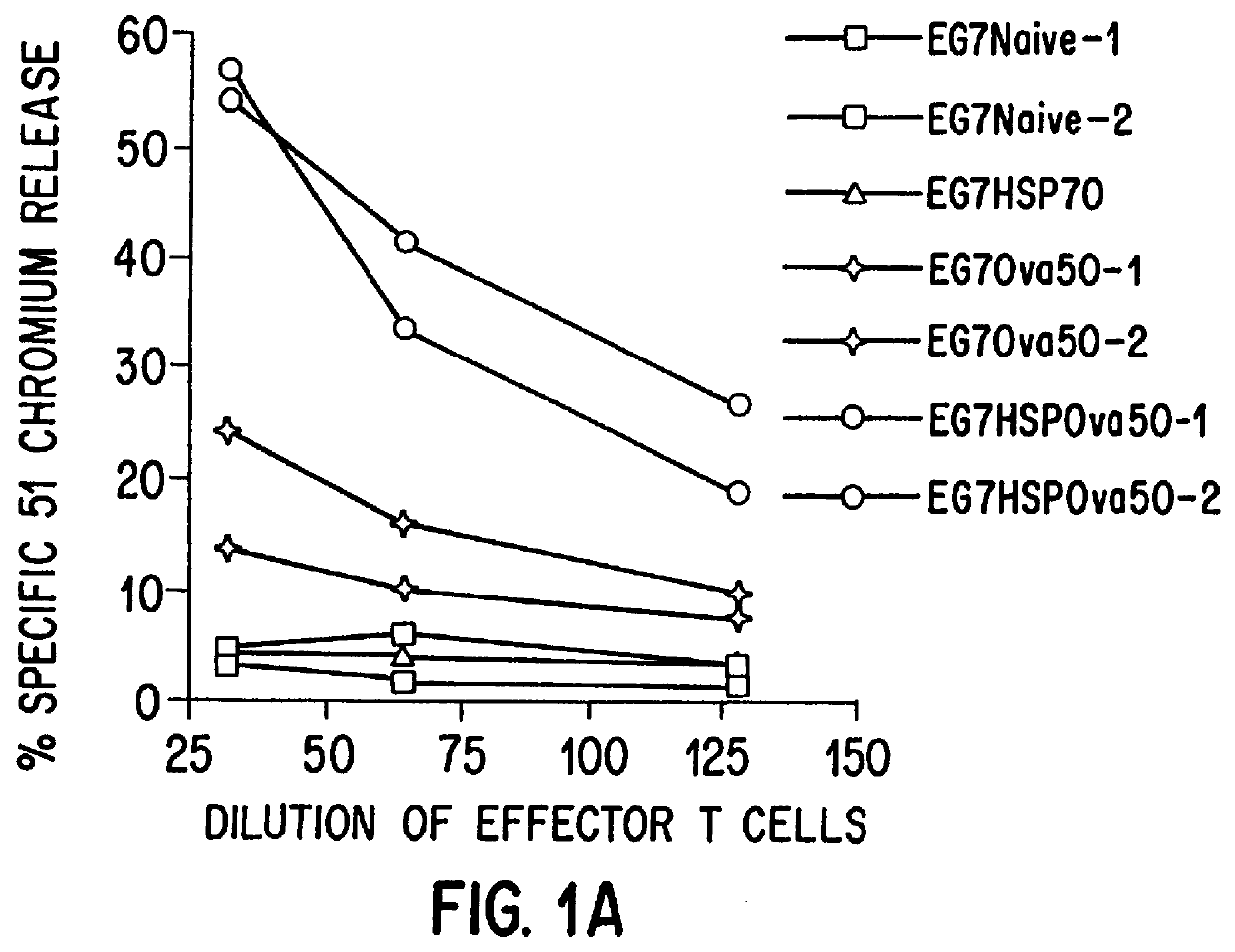
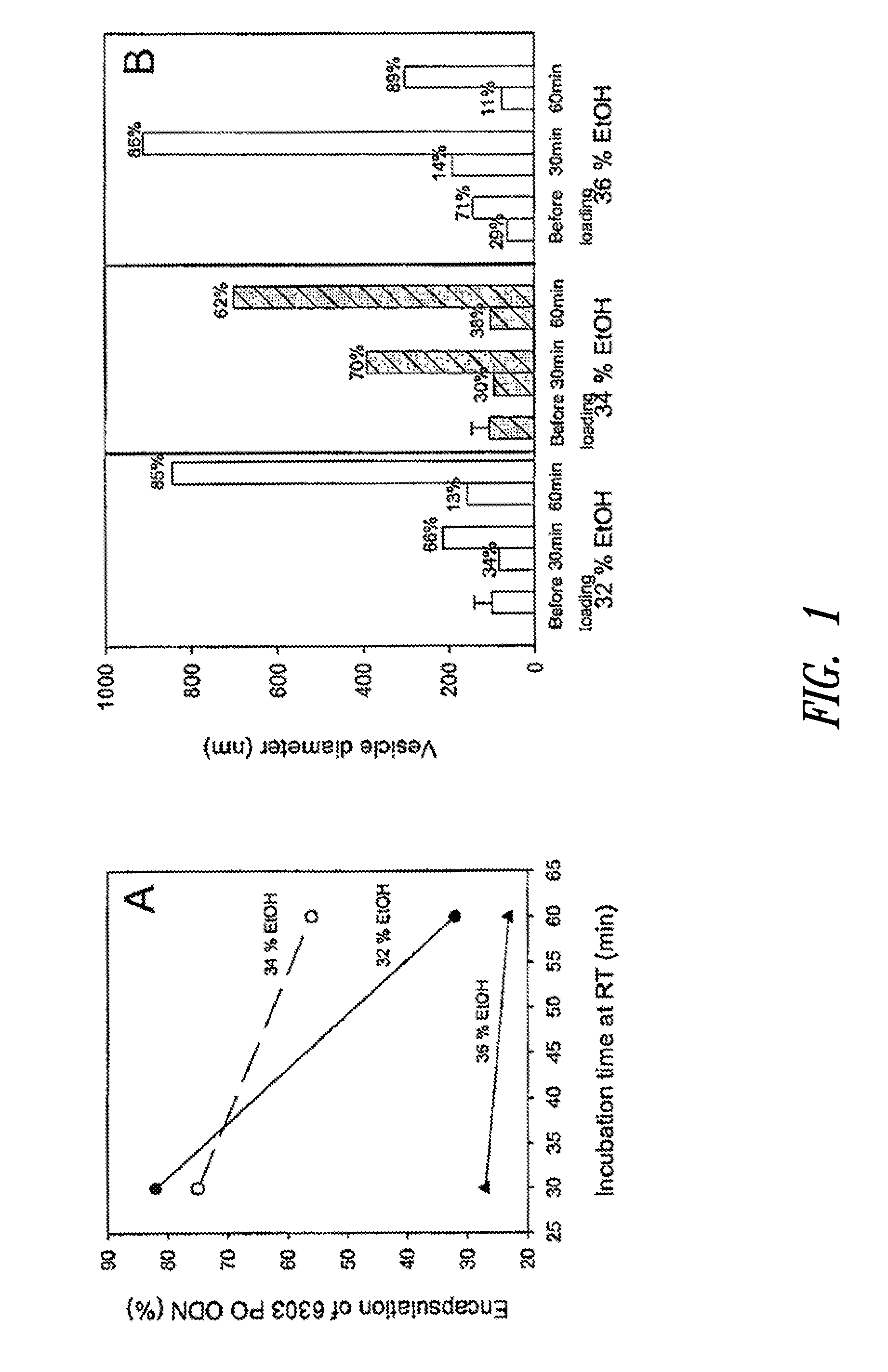
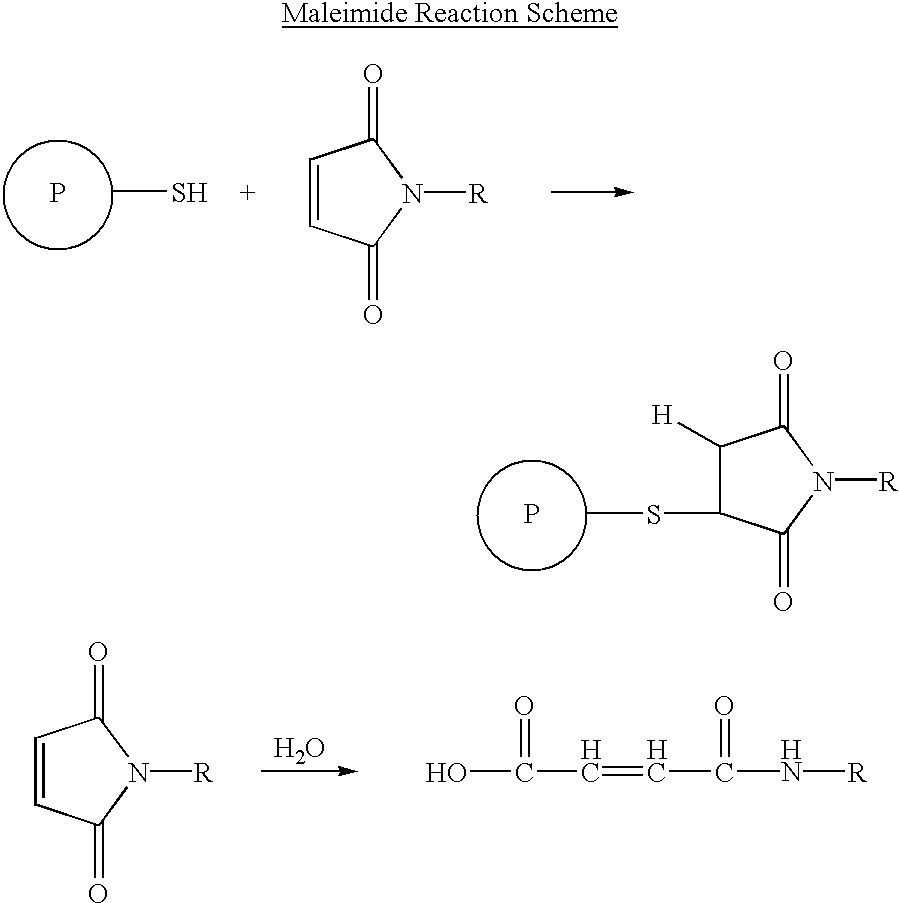
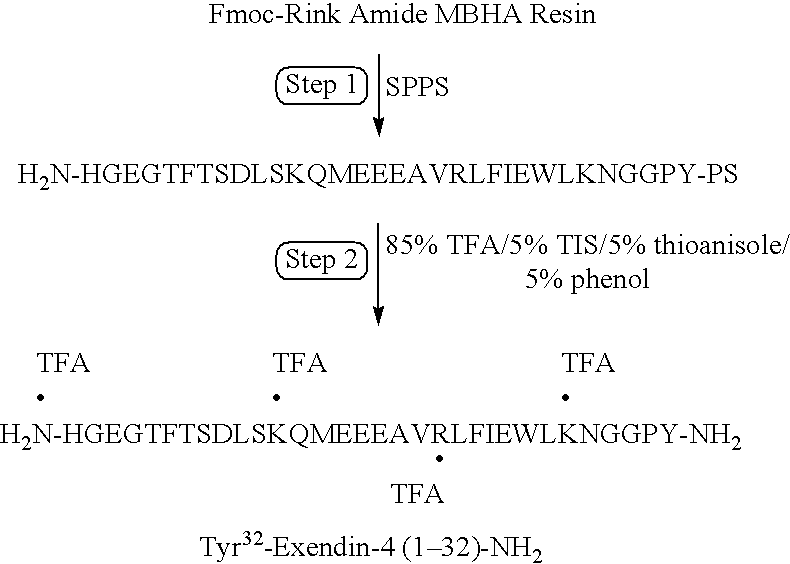
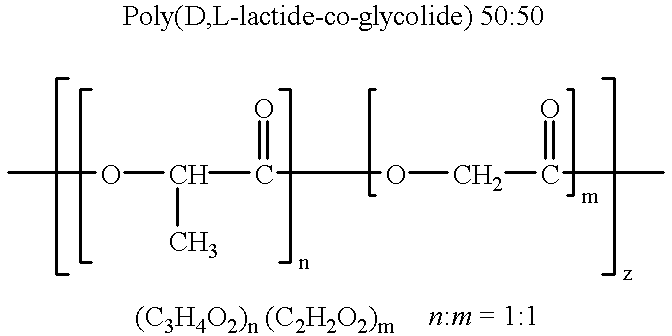Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
12790results about "Viral antigen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
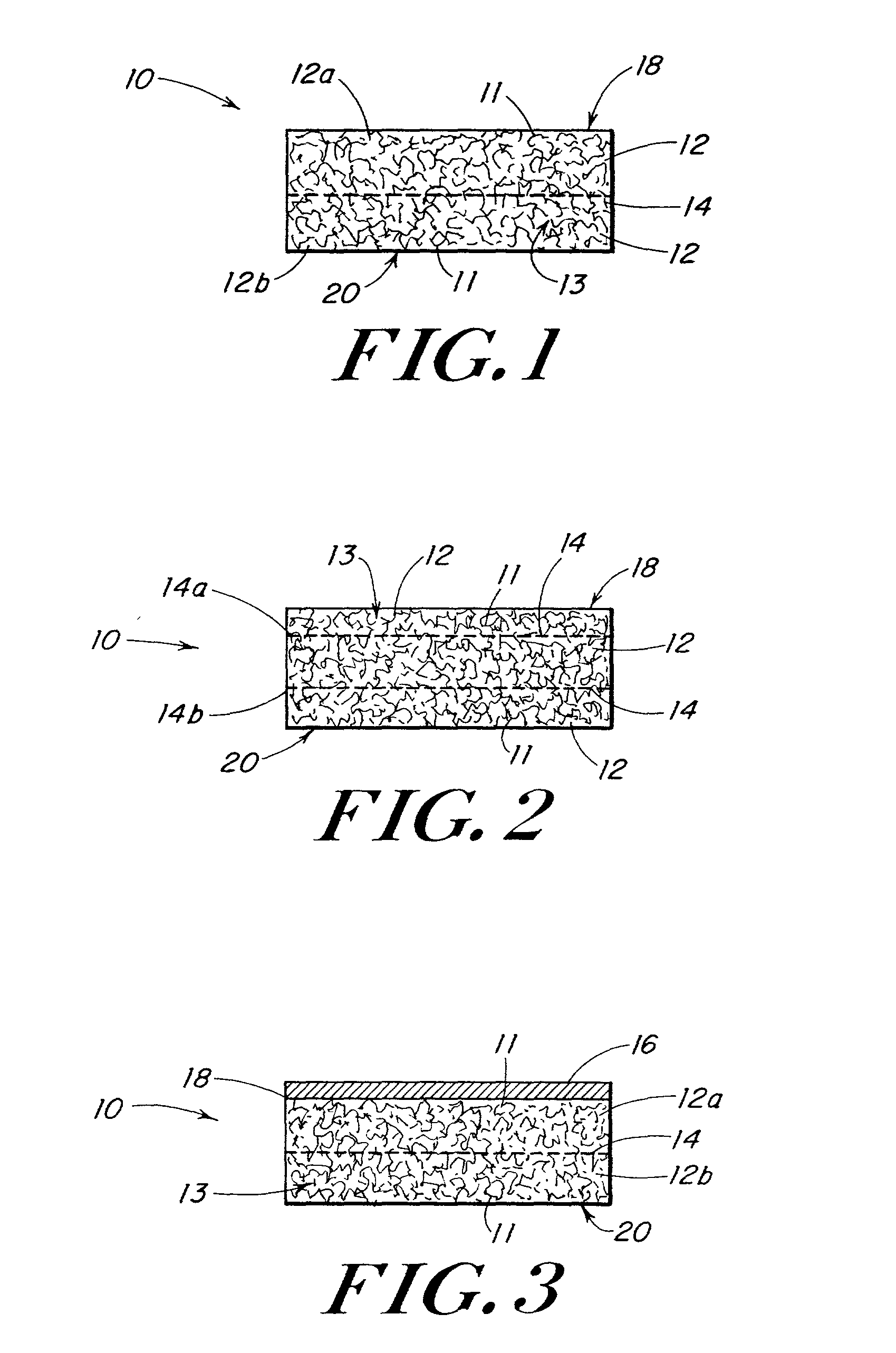
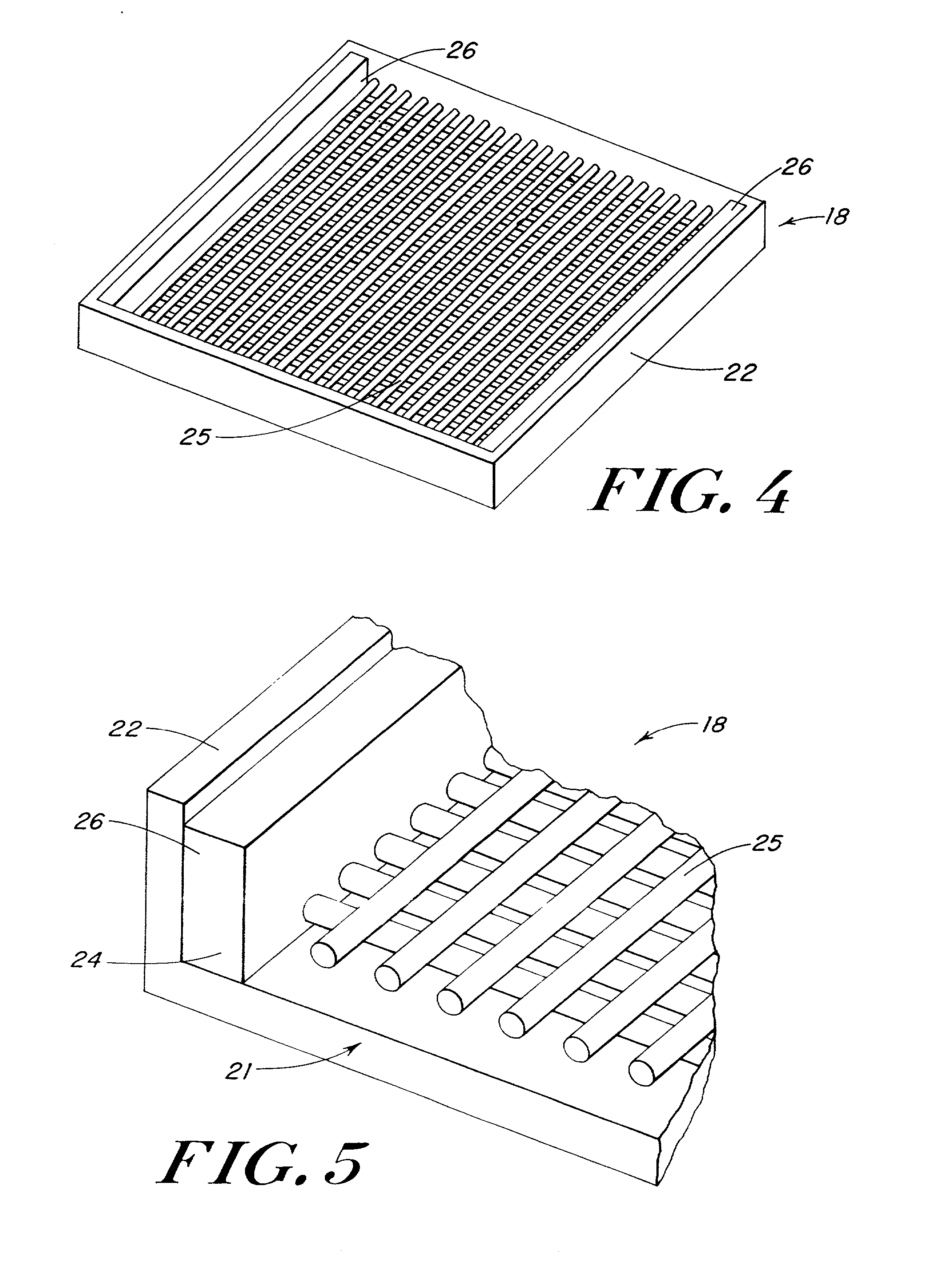
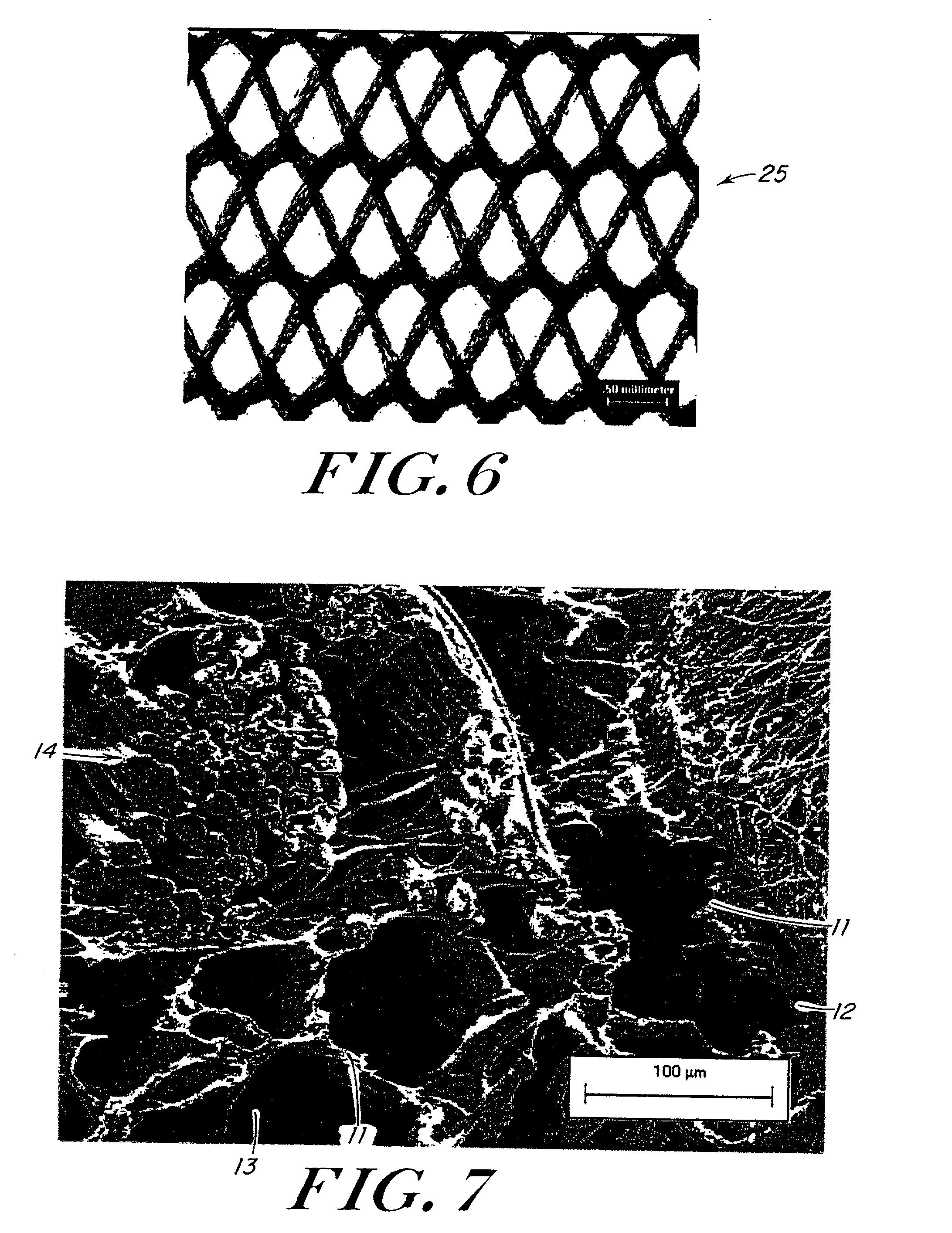
Use of reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
A biocompatible tissue repair stimulating implant or "scaffold" device is used to repair tissue injuries, particularly injuries to ligaments, tendons, and nerves. Such implants are especially useful in methods that involve surgical procedures to repair injuries to ligament, tendon, and nerve tissue in the hand and foot. The repair procedures may be conducted with implants that contain a biological component that assists in healing or tissue repair.
Owner:DEPUY MITEK INC
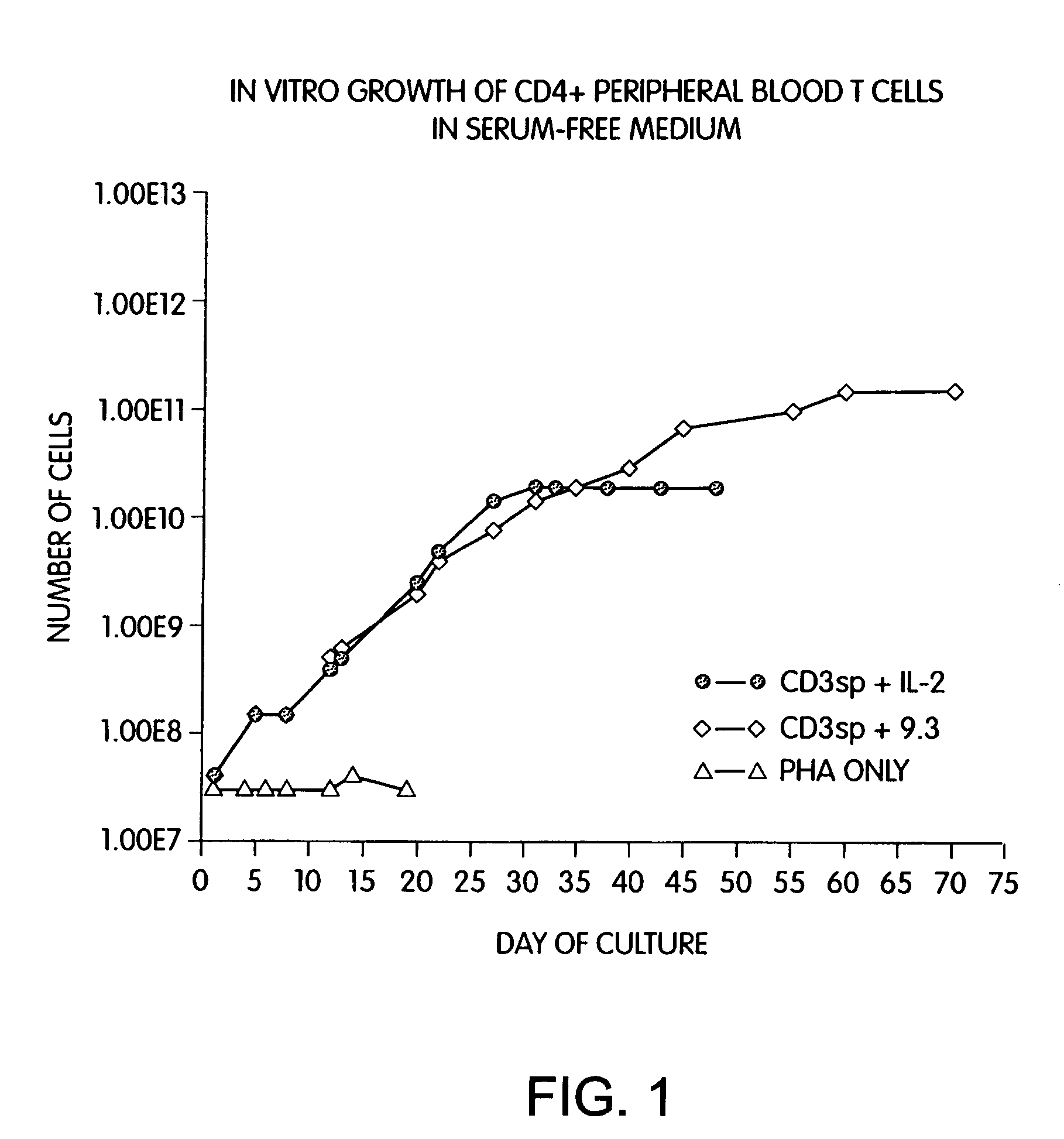
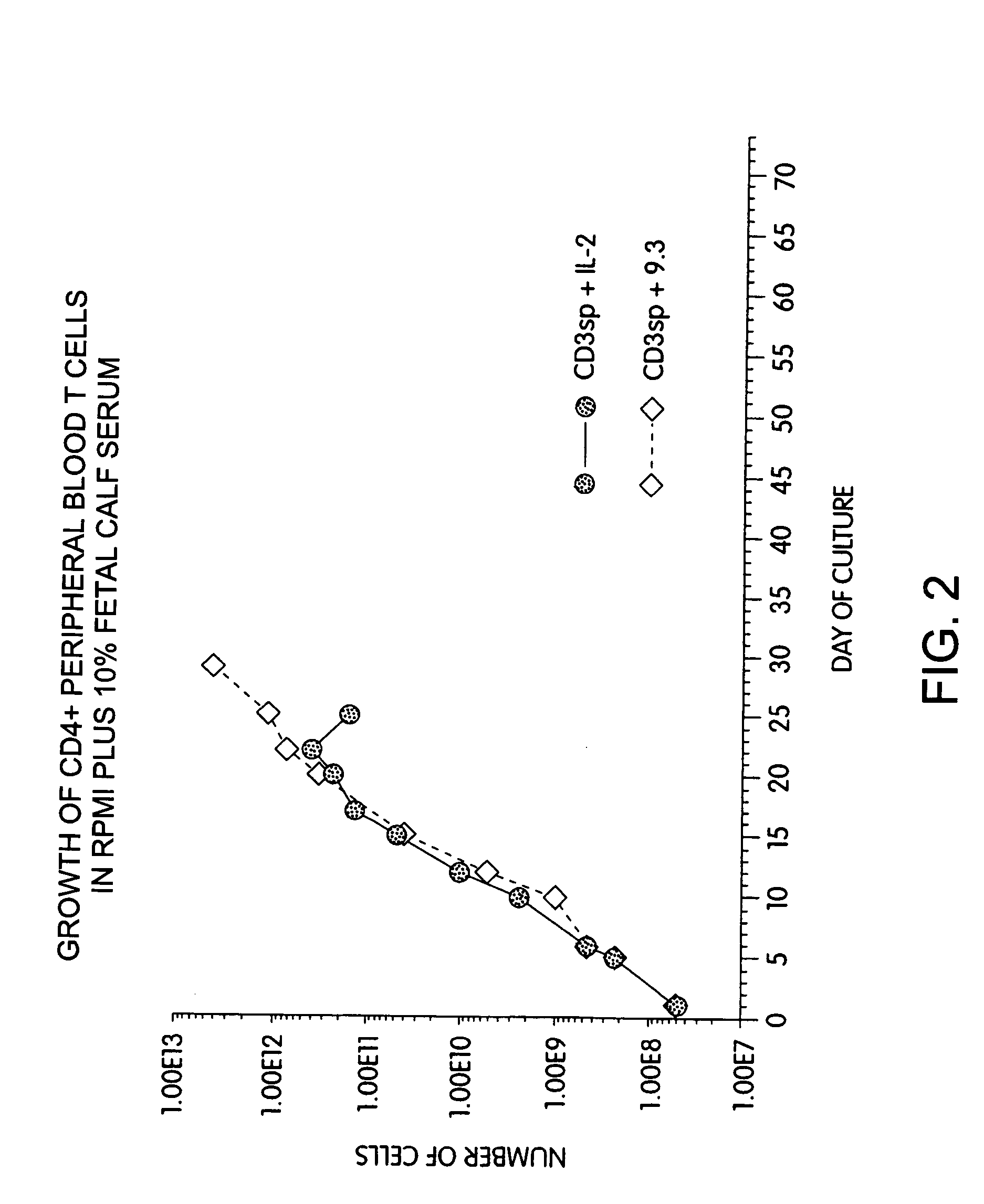
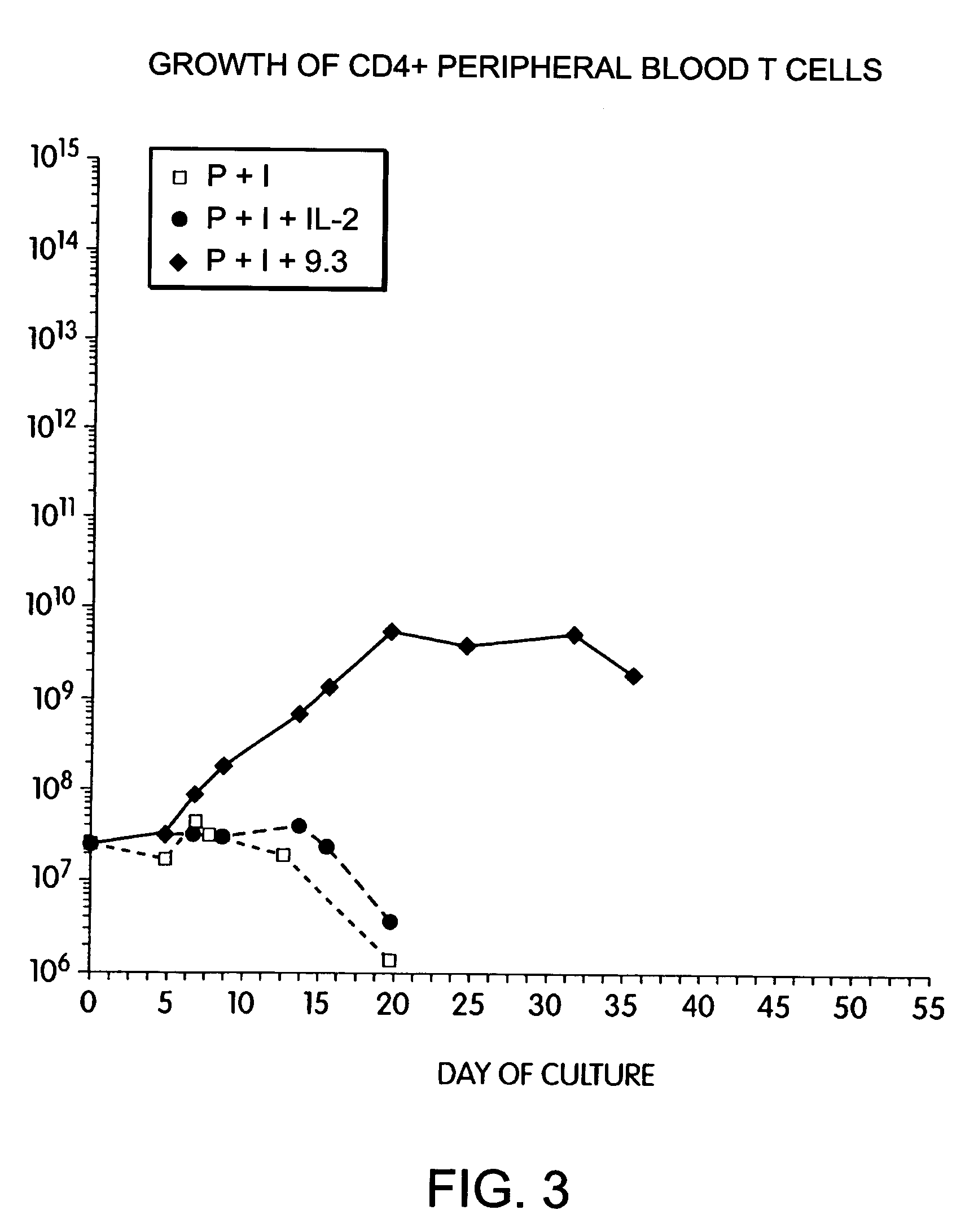
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
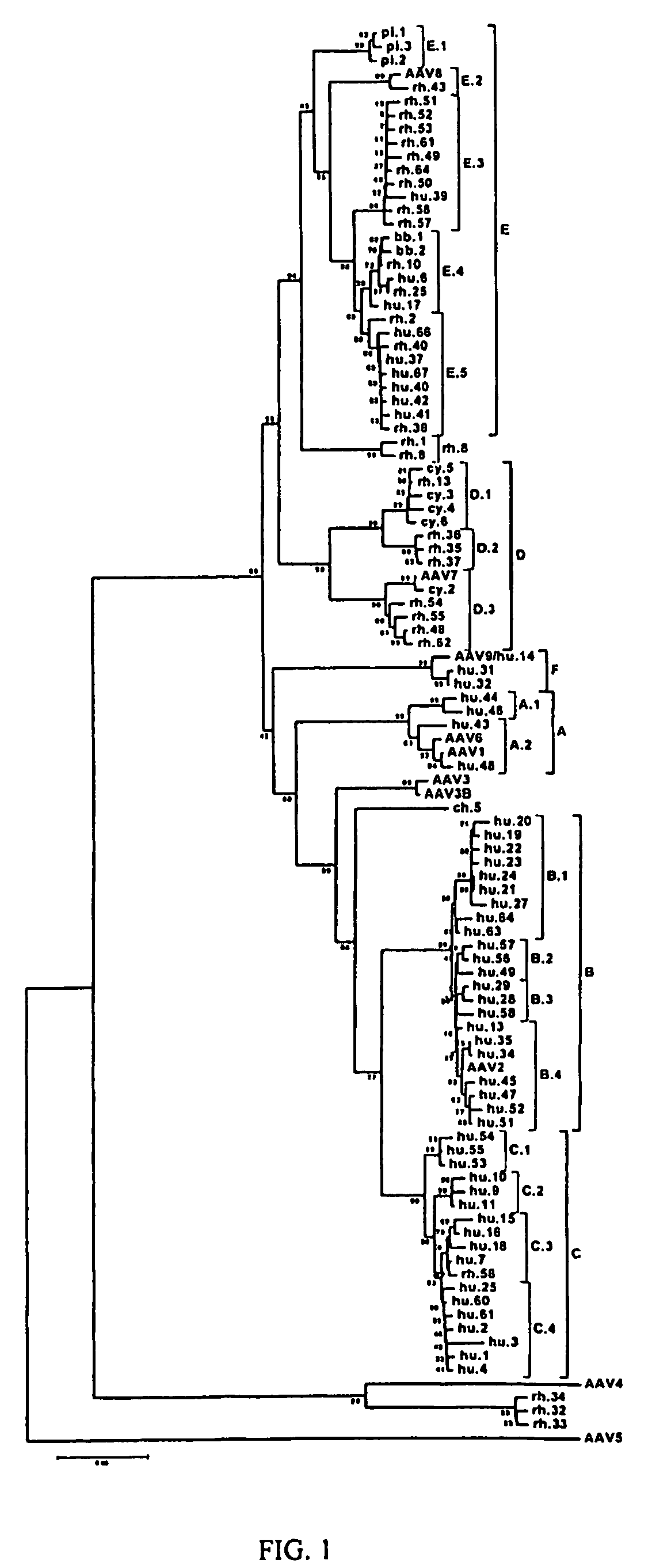
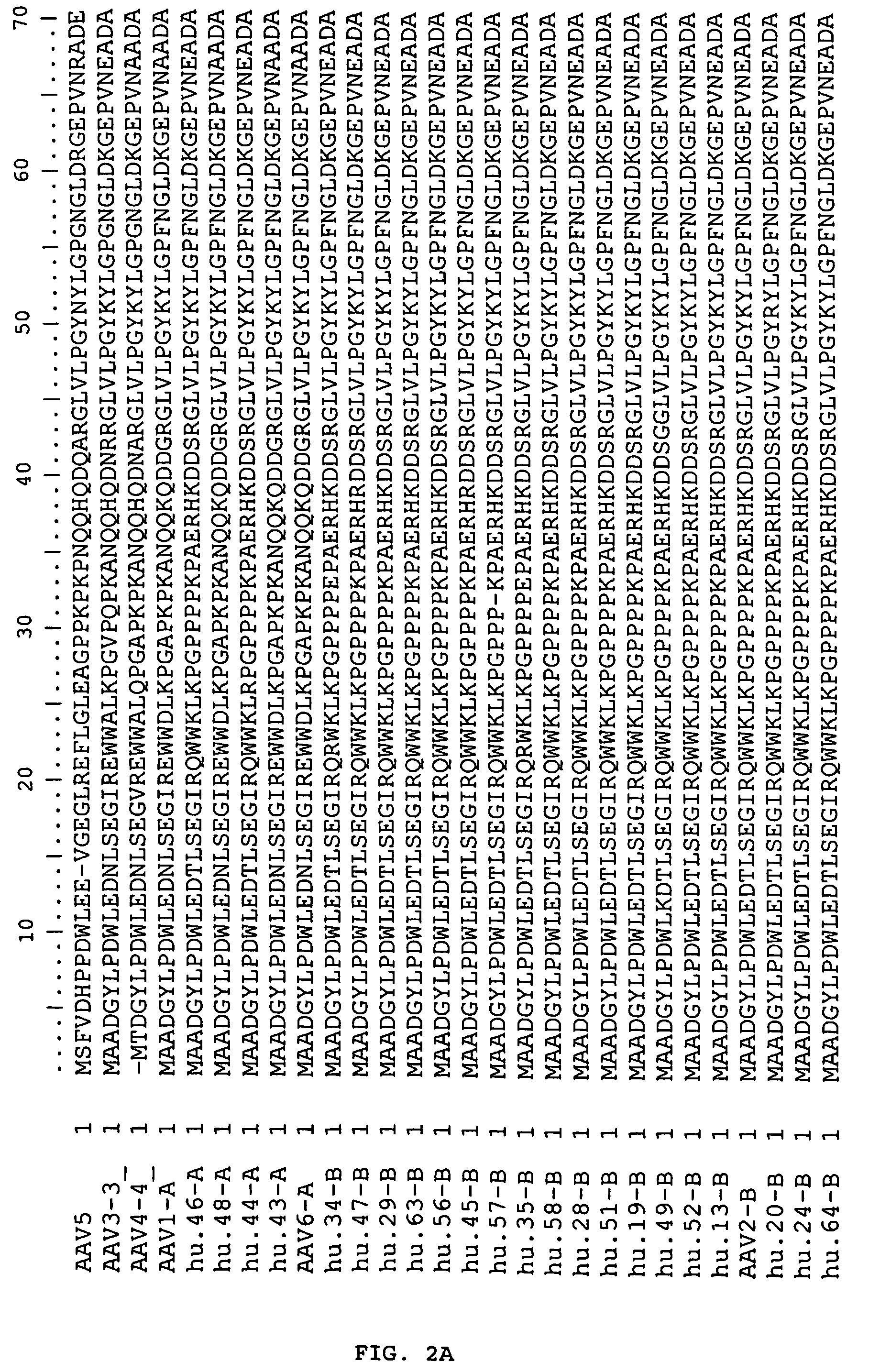
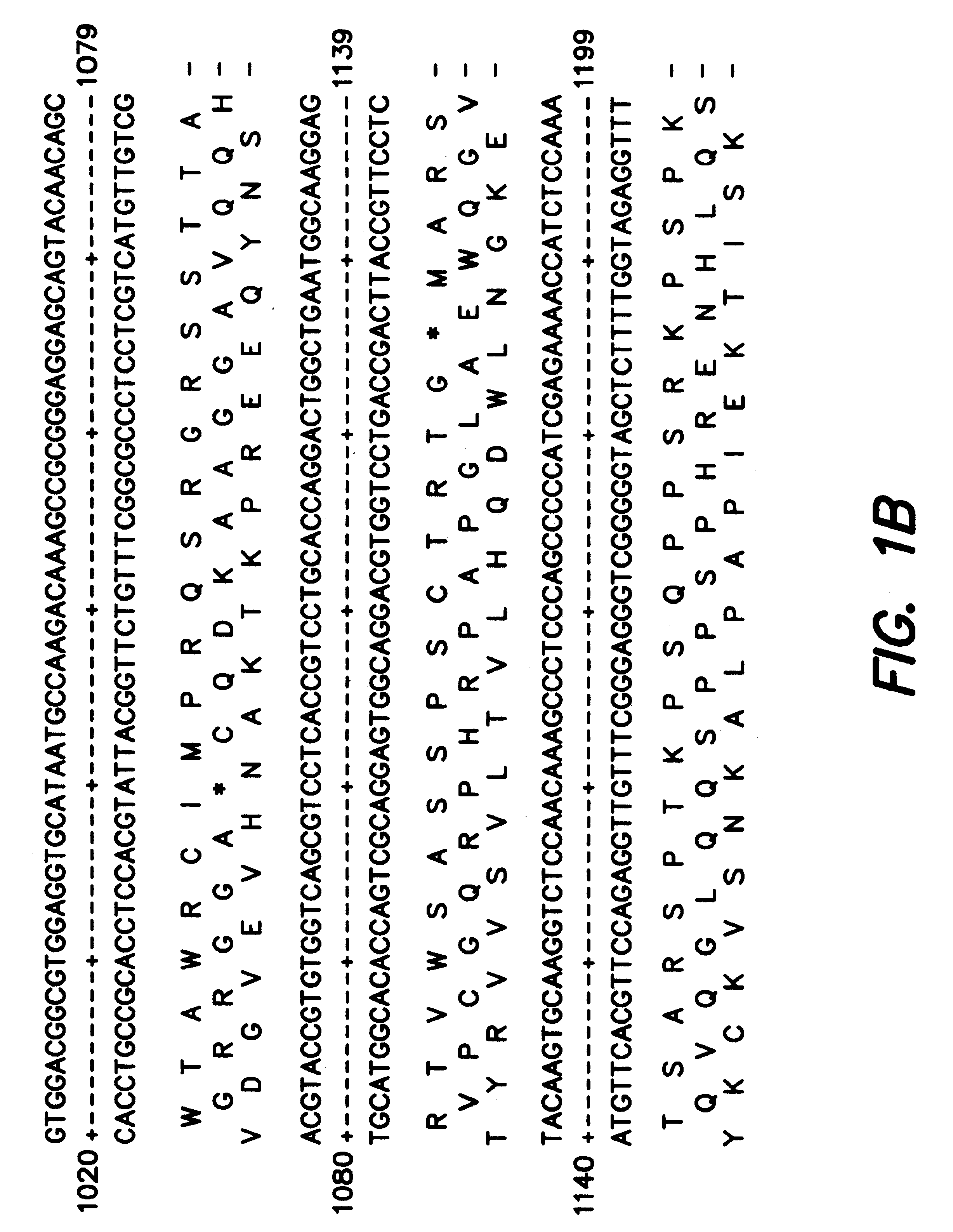
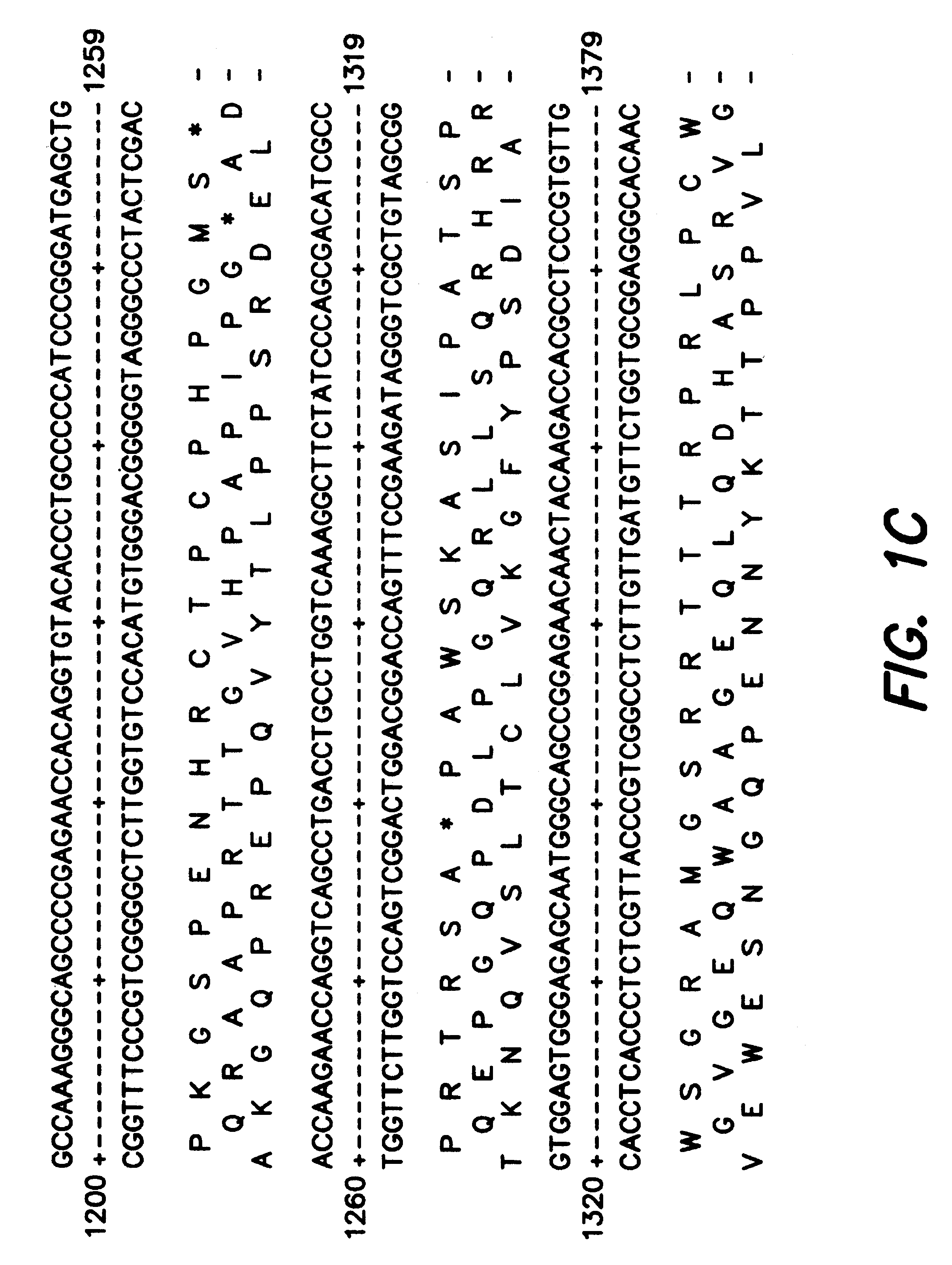
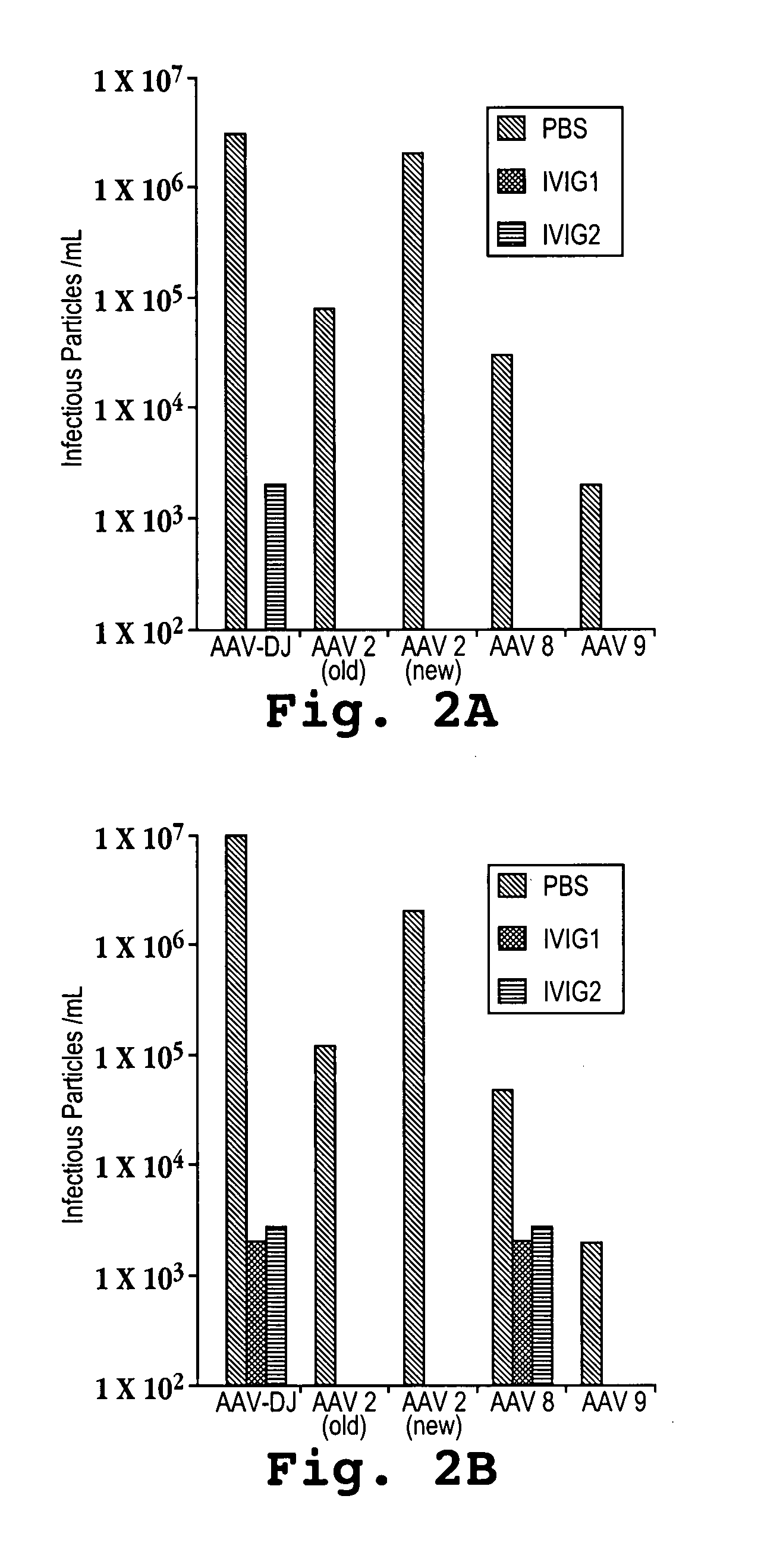
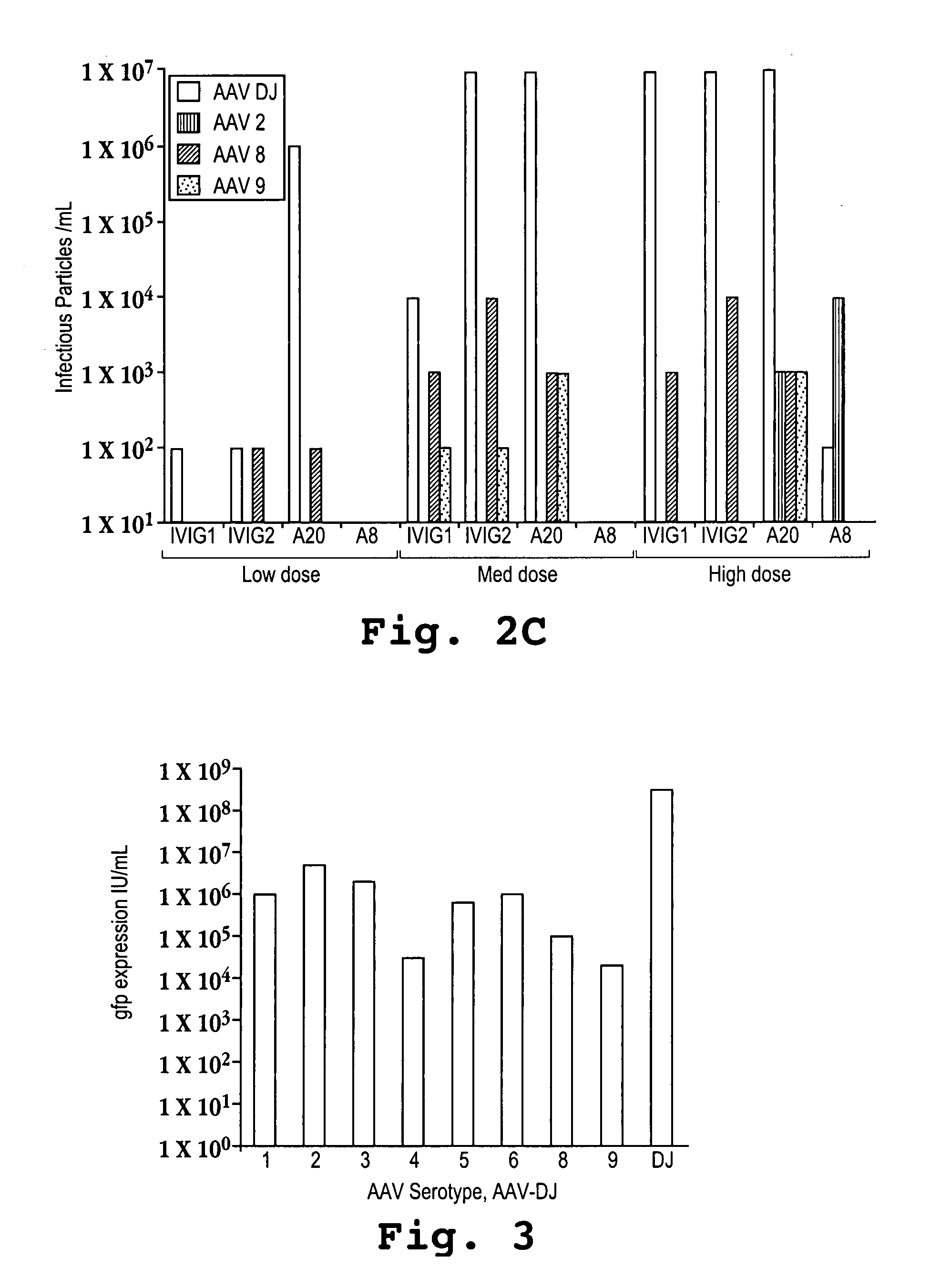
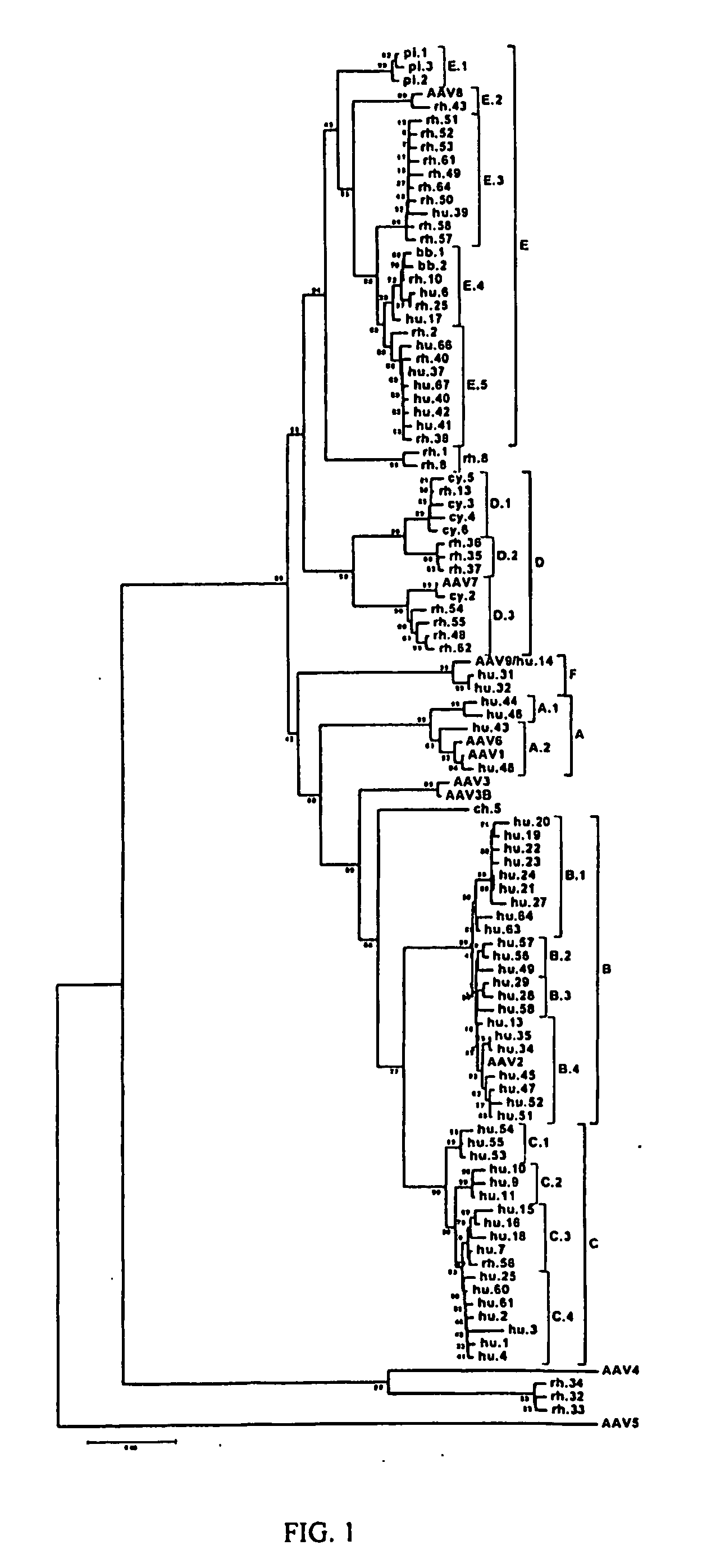
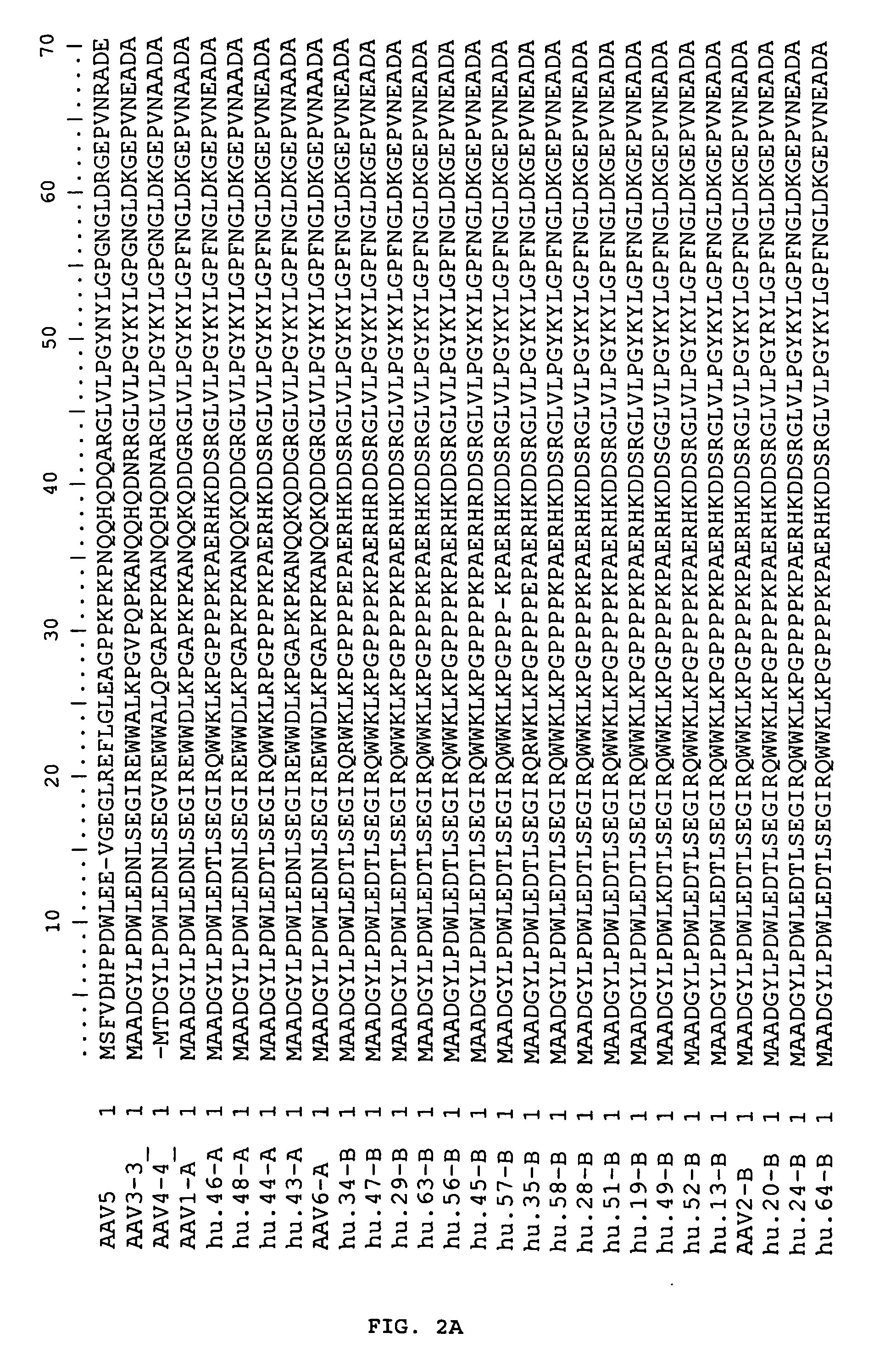
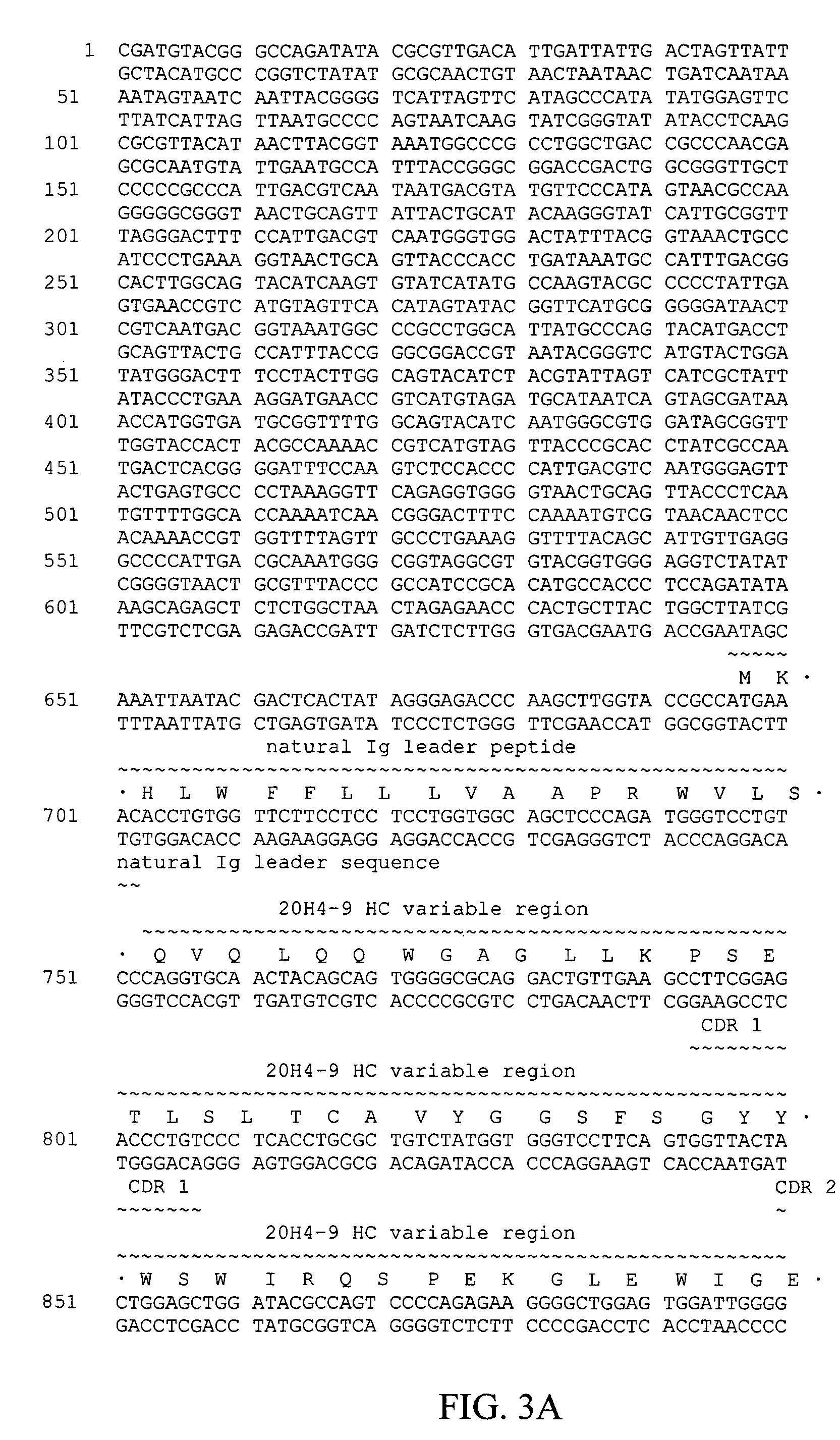
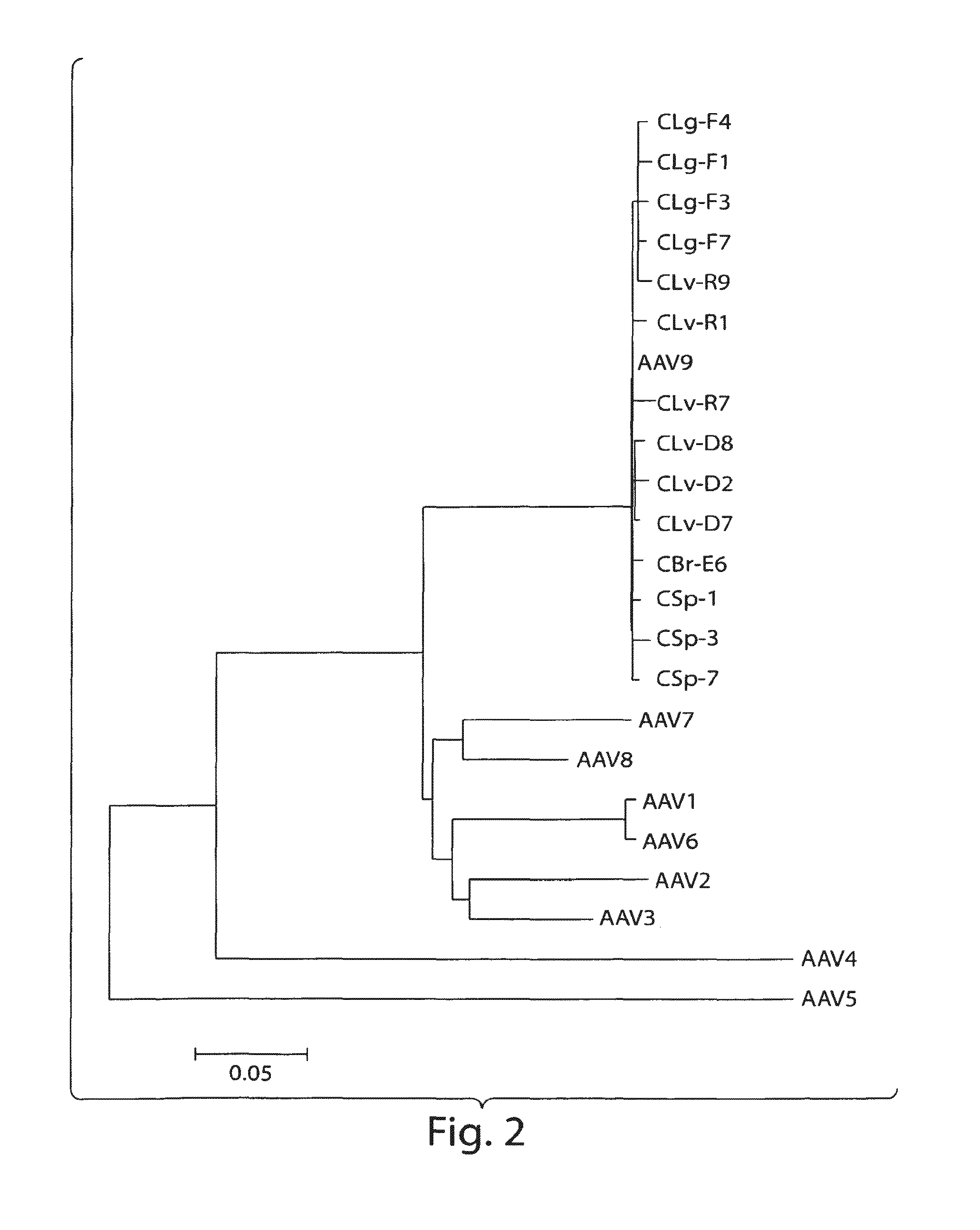
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Receptor specific transepithelial transport of therapeutics
InactiveUS6485726B1Effective strategyImprove abilitiesBacterial antigen ingredientsPeptide/protein ingredientsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
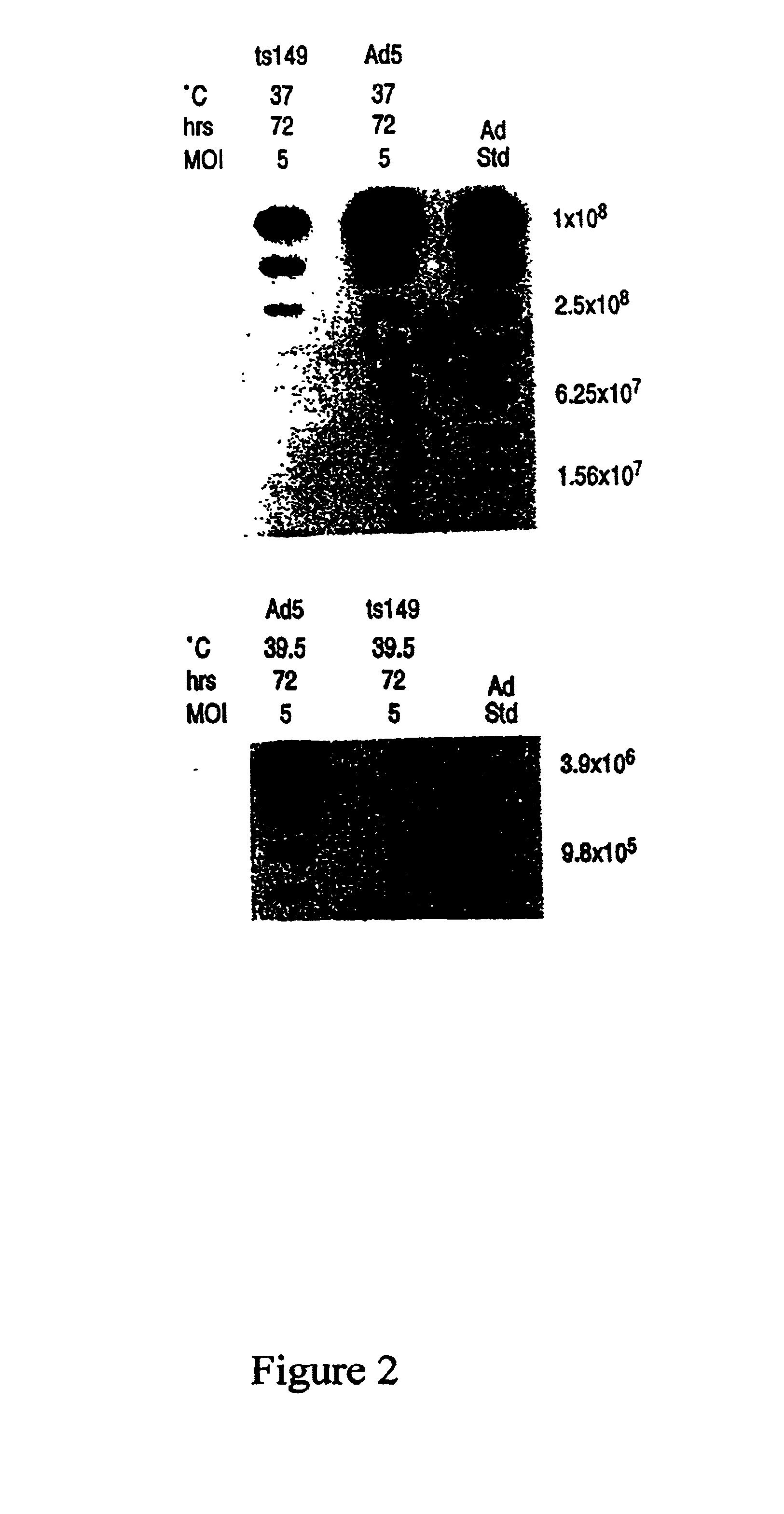
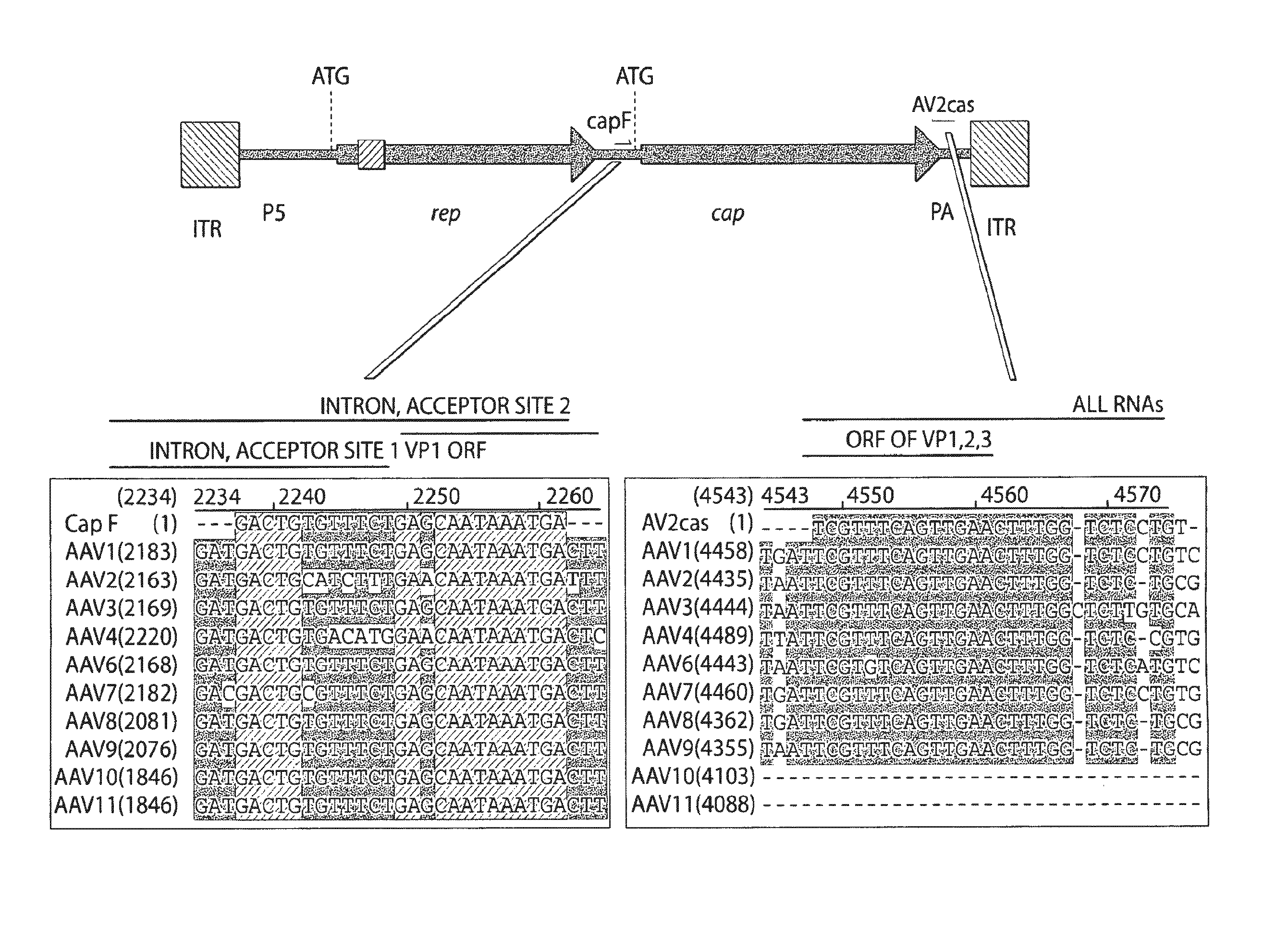
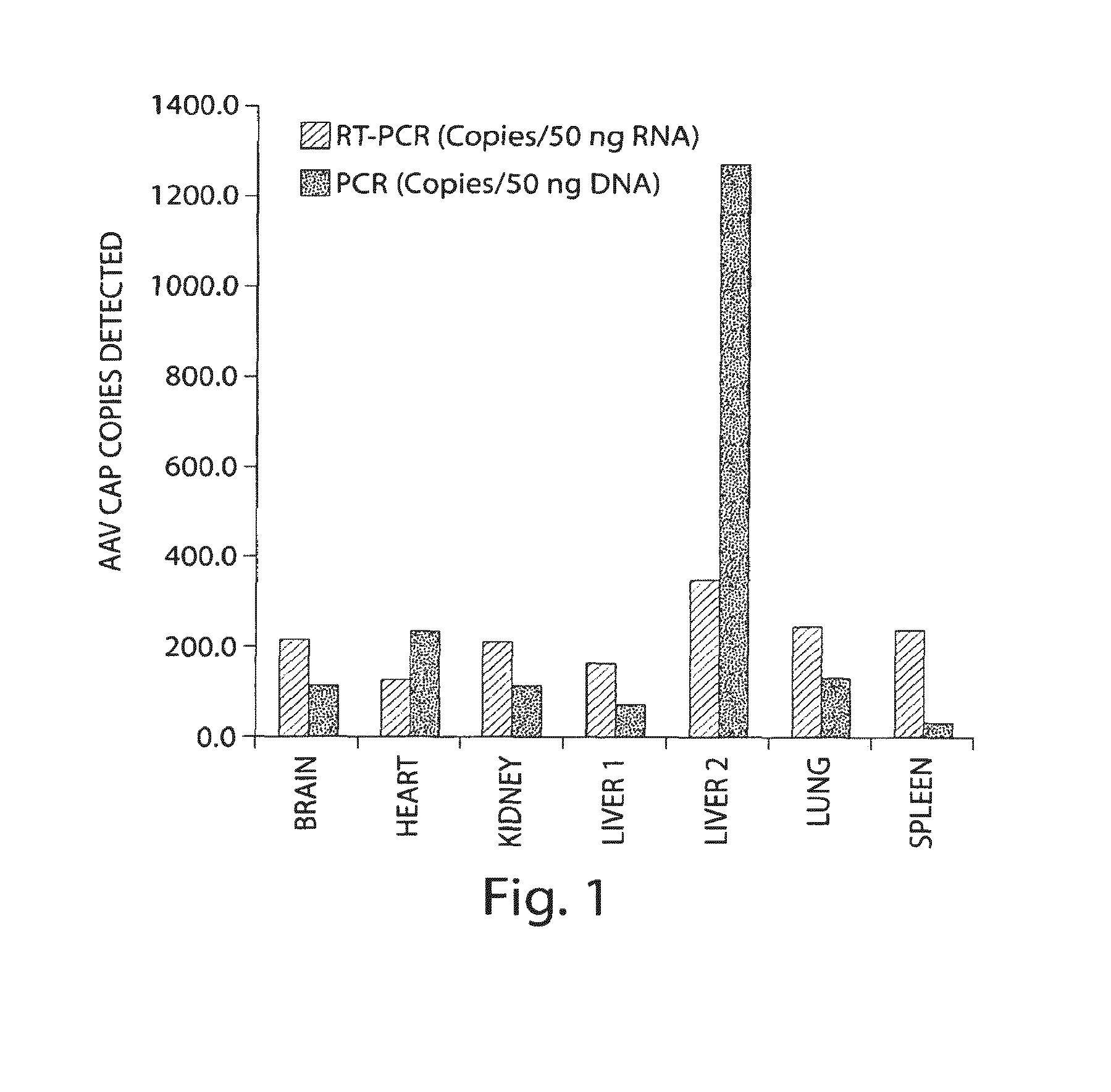
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
AAV capsid library and AAV capsid proteins
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Amino lipids and methods for the delivery of nucleic acids
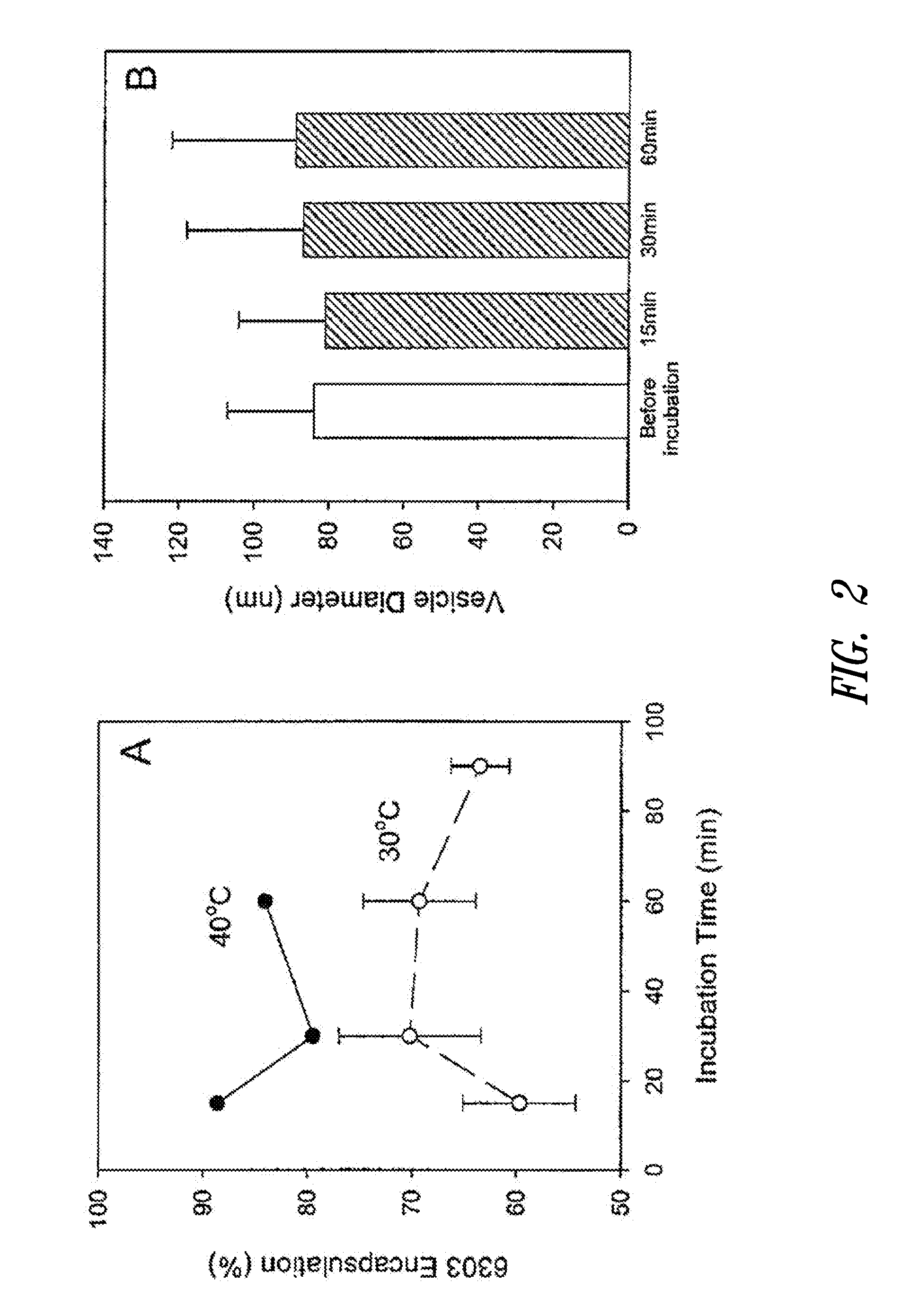
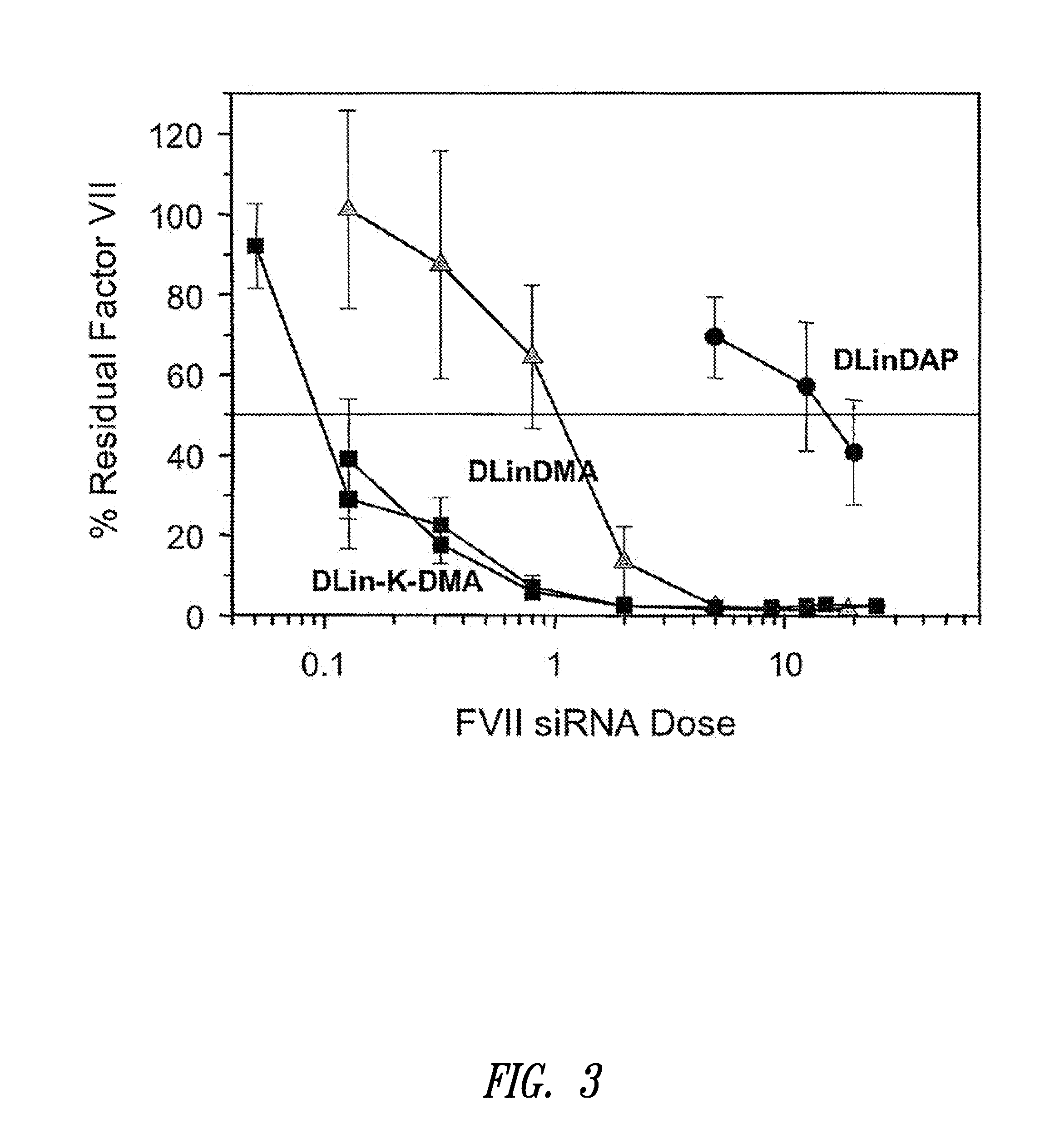
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
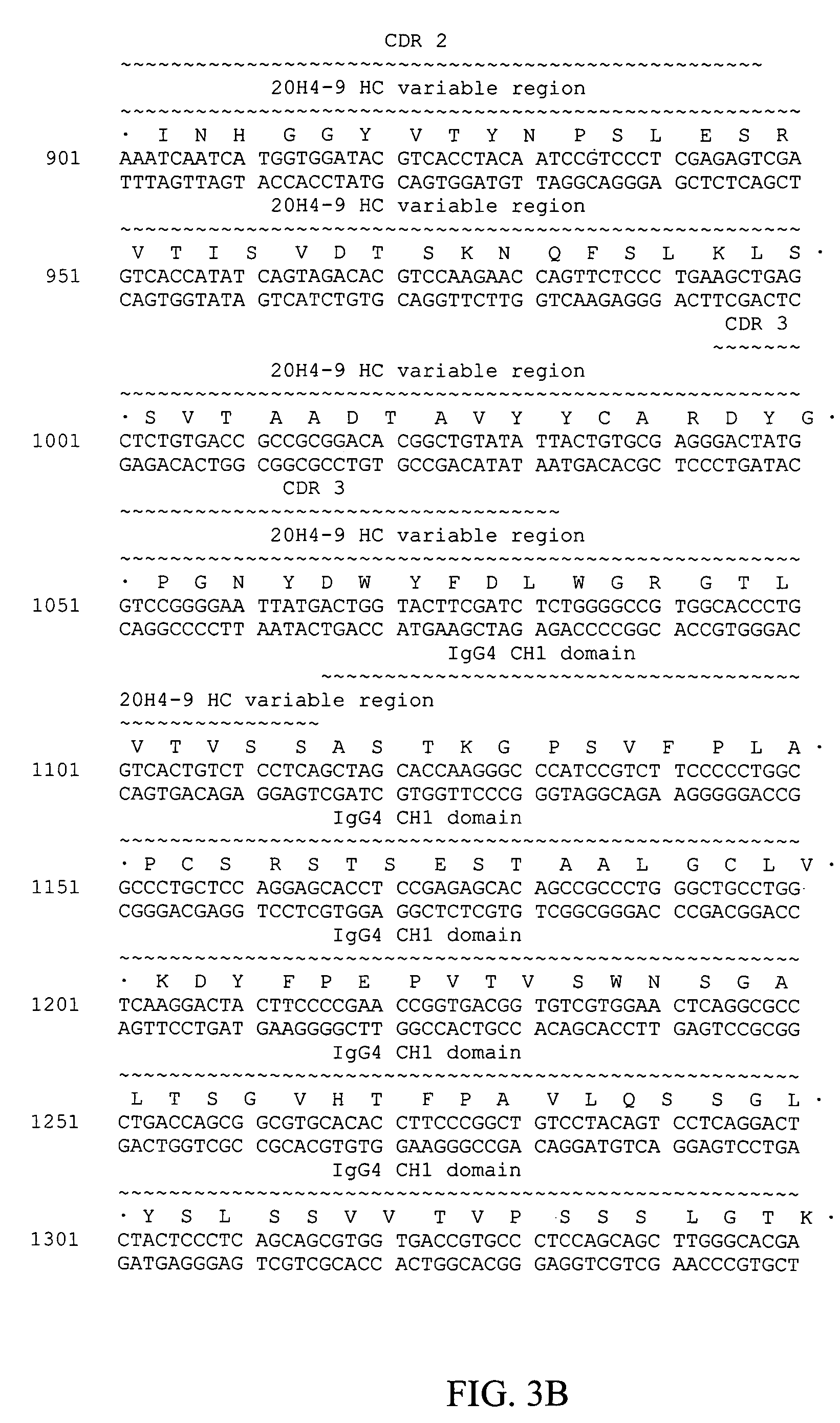
Fully human antibodies against human 4-1BB
Fully human antibodies and antigen-binding portions thereof that bind to human 4-1BB and that allow binding of human 4-1BB to a human 4-1BB ligand. In one aspect, the antibody is an IgG4 antibody. Also provided is a method for treating a disease in a subject comprising administering a therapeutically effective amount of the antibody to said subject.
Owner:BRISTOL MYERS SQUIBB CO
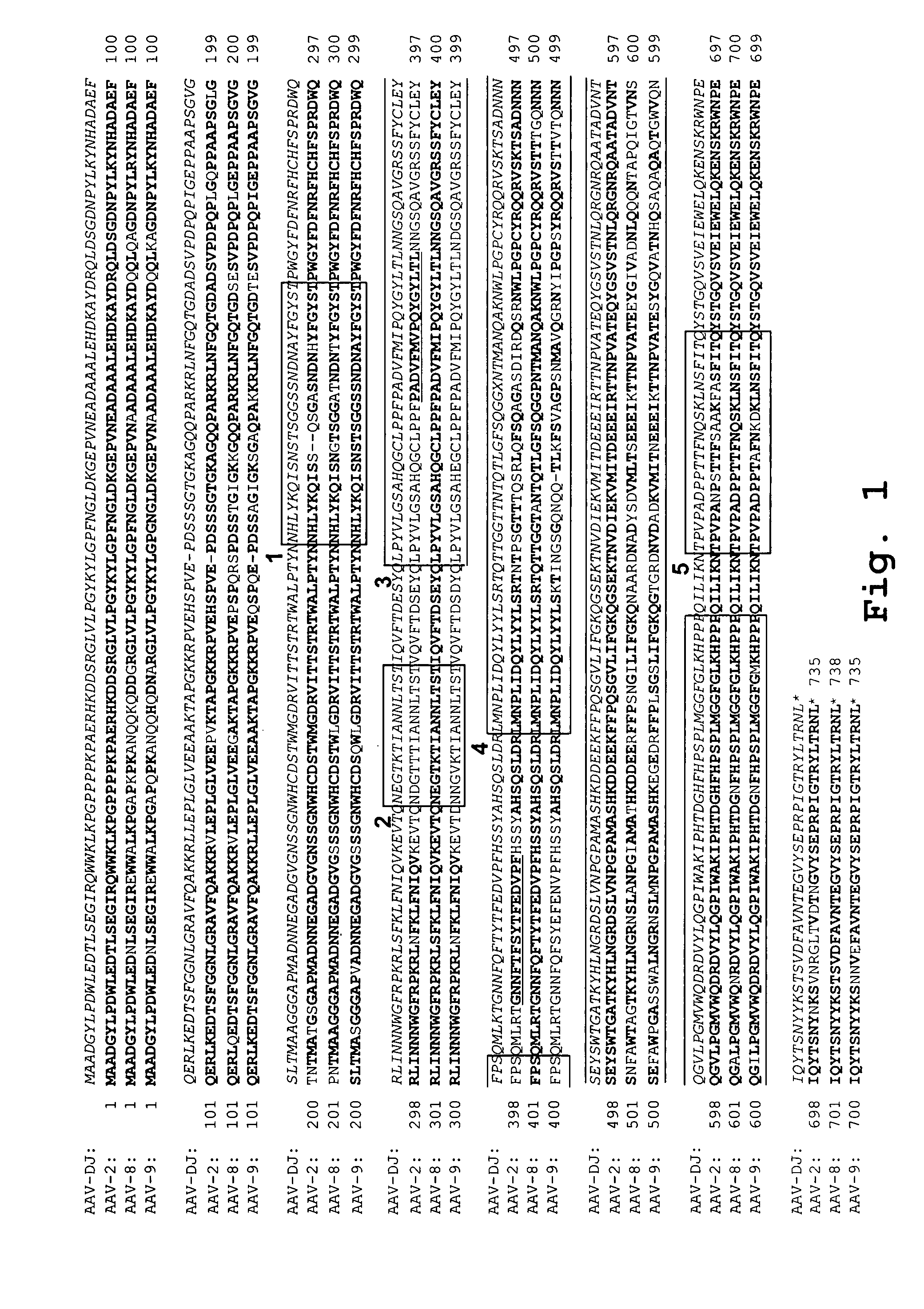
AAV's and uses thereof
The invention in some aspects relates to recombinant adeno-associated viruses having distinct tissue targeting capabilities. In some aspects, the invention relates to gene transfer methods using the recombinant adeno-associate viruses. In some aspects, the invention relates to isolated AAV capsid proteins and isolated nucleic acids encoding the same.
Owner:UNIV OF MASSACHUSETTS
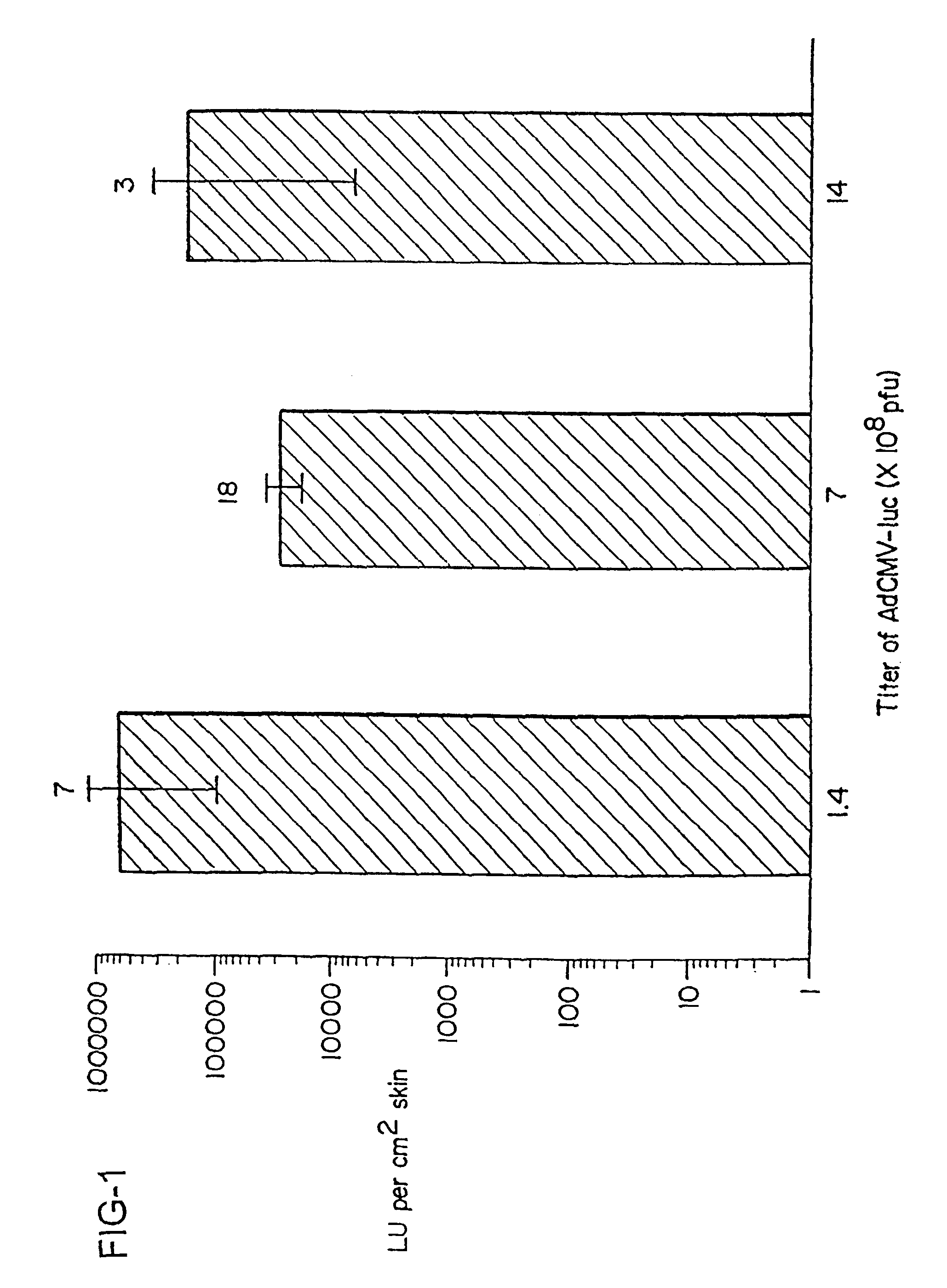
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
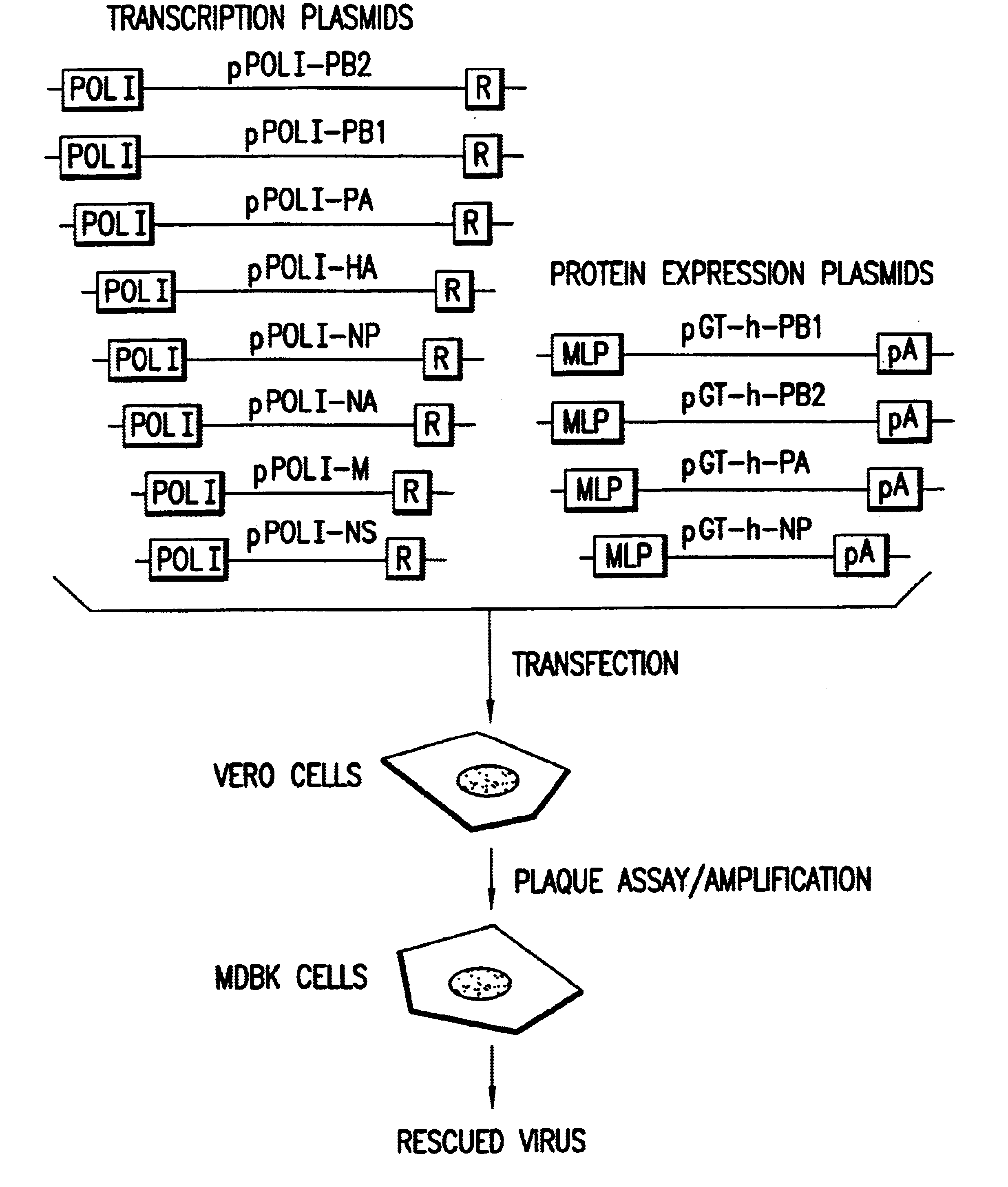
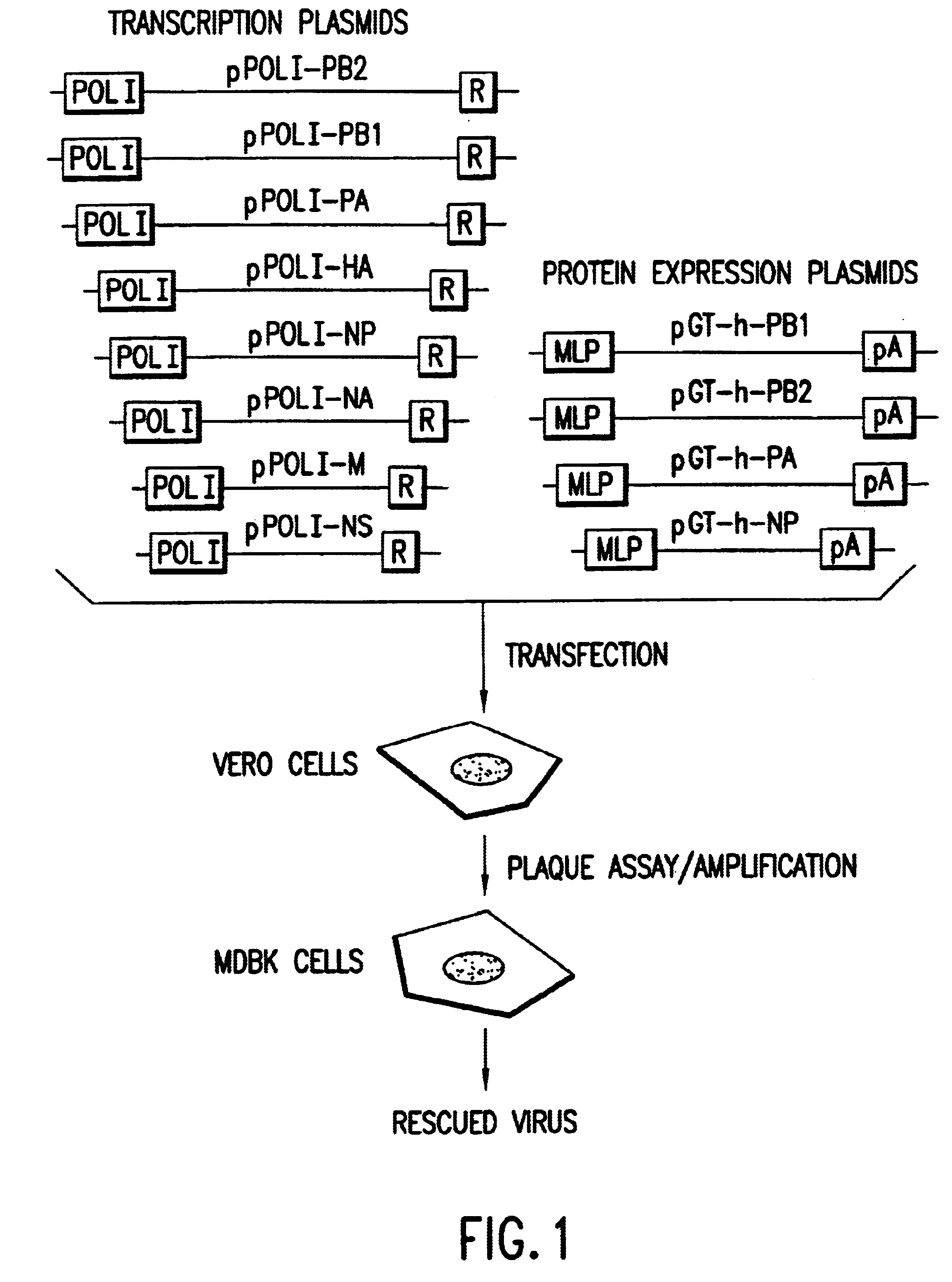
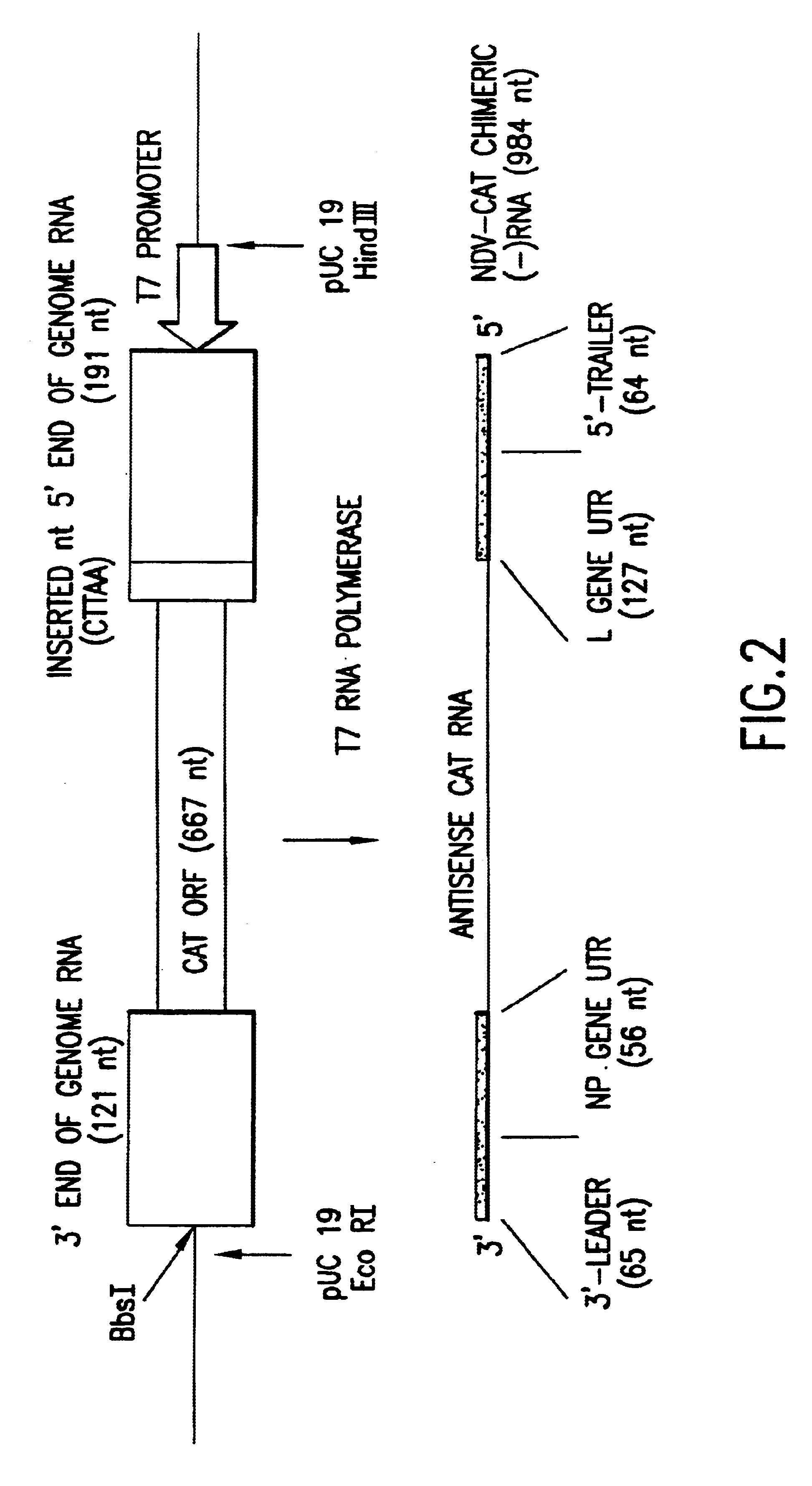
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
Microparticles with adsorbent surfaces, methods of making same, and uses thereof
InactiveUS6884435B1Stimulate immune responseEasy to usePowder deliverySsRNA viruses positive-senseAntigenDisease
The present invention is directed to microparticles, to microparticle compositions containing the same, to methods of forming the same, and to uses for the same, including use for a vaccine, for raising an immune response, for treatment of a disease and for diagnosis of a disease. The microparticles comprise a biodegradable polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, or a polycyanoacrylate, and a detergent selected from a cationic detergent and an anionic detergent. The microparticles further comprise an antigen adsorbed on the surface of the microparticle.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
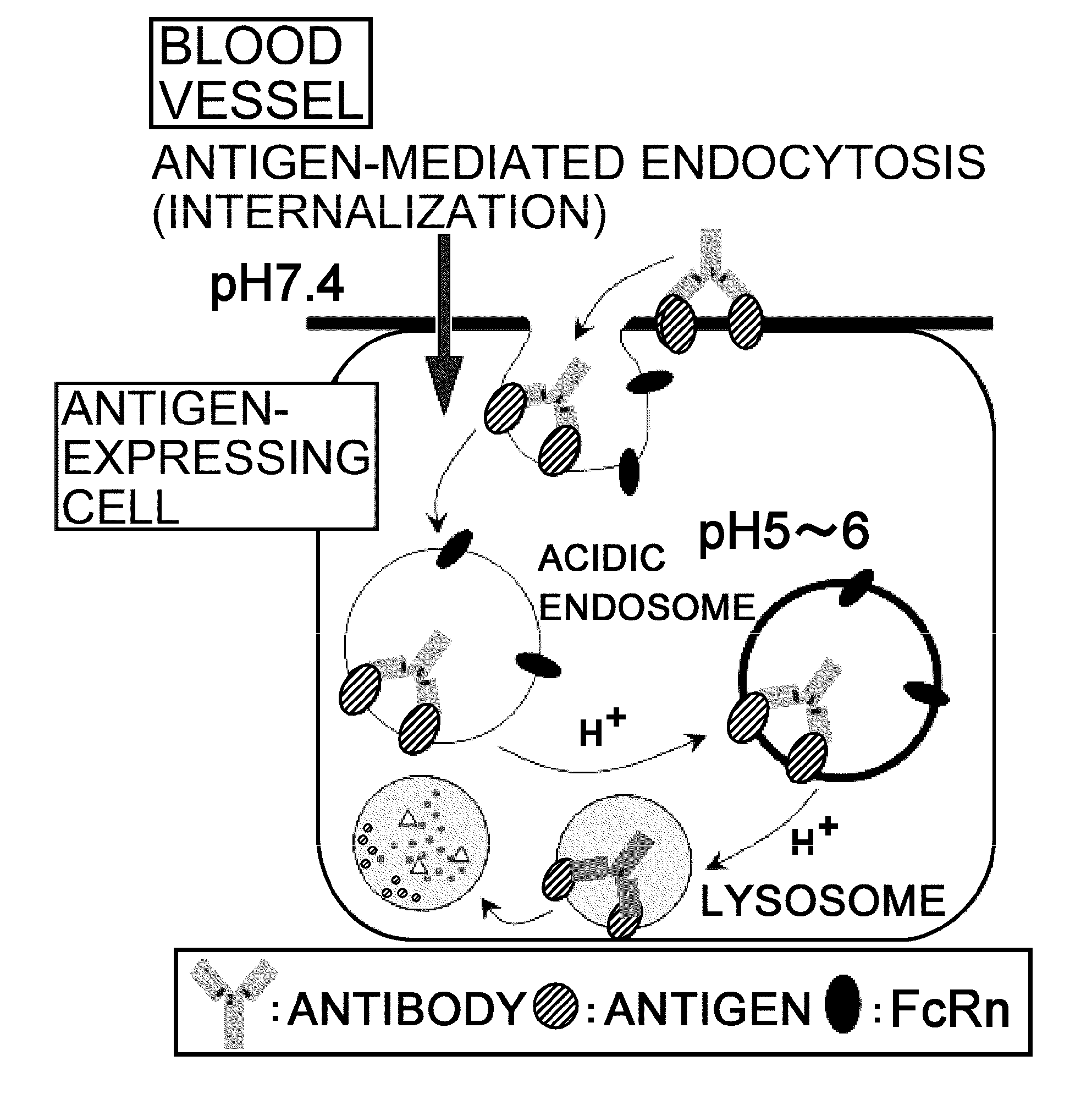
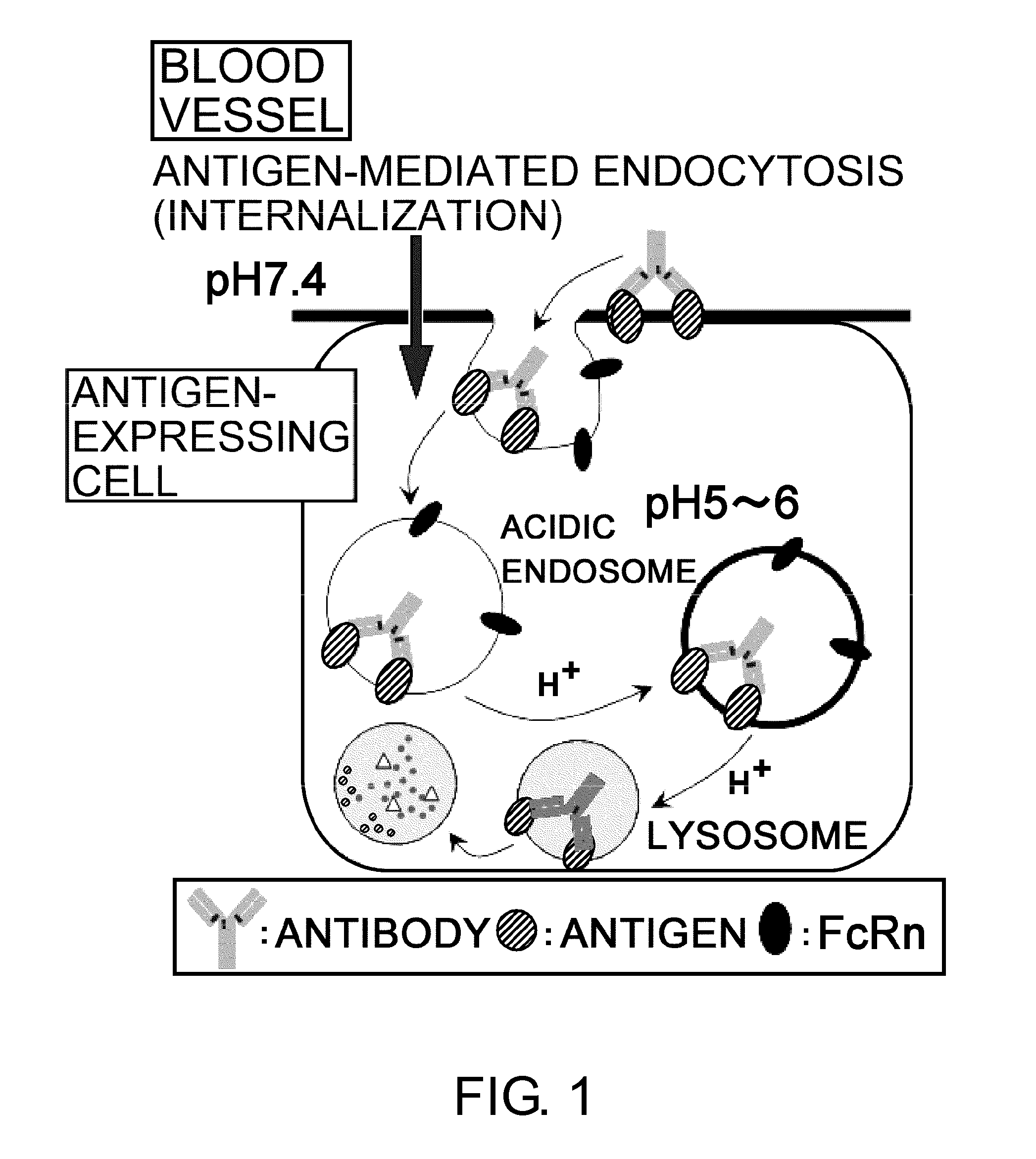
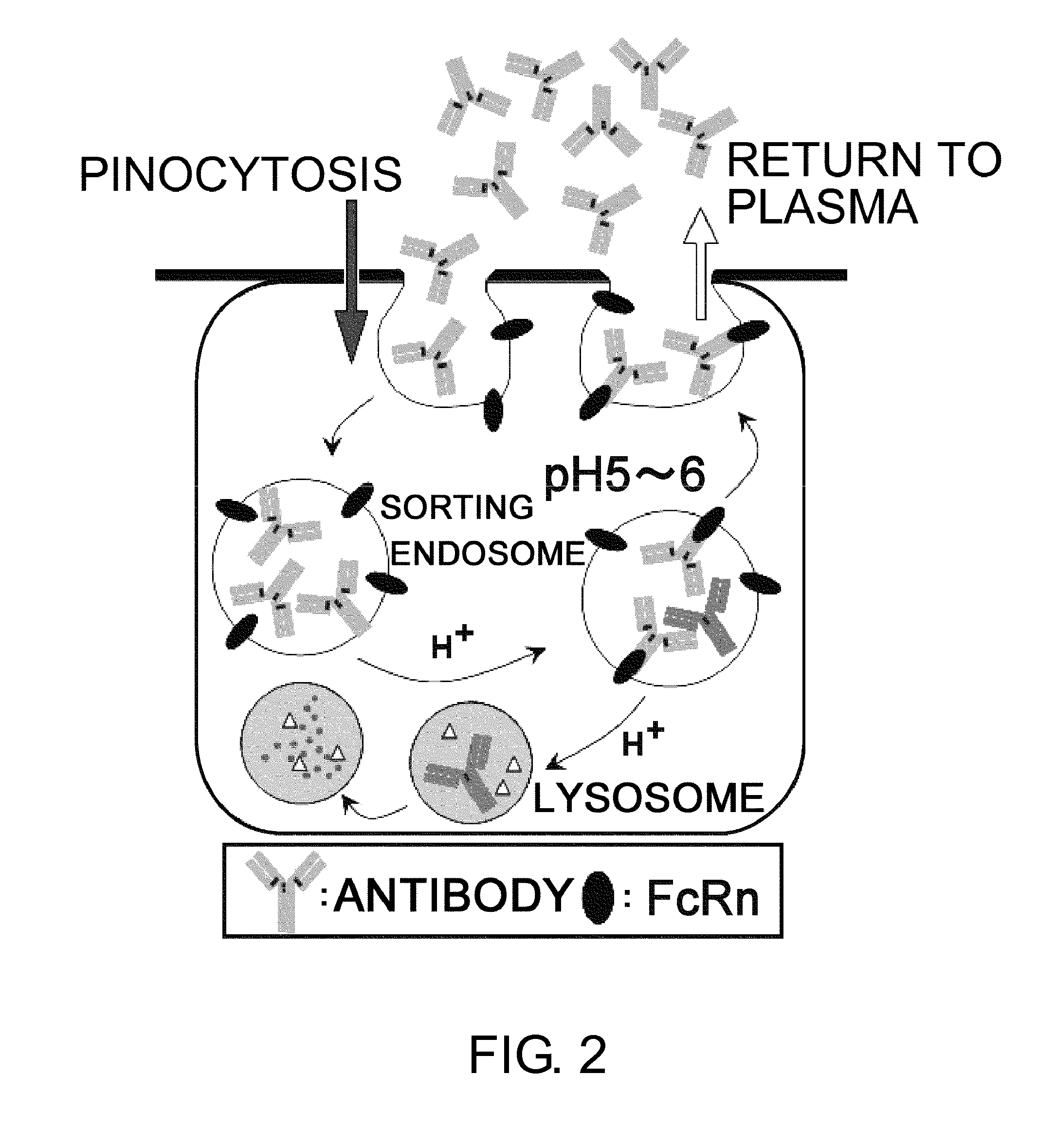
Antigen-binding molecule capable of binding to two or more antigen molecules repeatedly
InactiveUS20110111406A1Pharmacokinetics of the antigen-binding molecule can be improvedGood effectSsRNA viruses negative-senseCompound screeningHalf-lifeAntigen binding
The present inventors discovered that antibodies having weaker antigen-binding activity at the early endosomal pH in comparison with that at the pH of plasma are capable of binding to multiple antigen molecules with a single antibody molecule, have long half-lives in plasma, and have improved durations of time in which they can bind to antigen.
Owner:CHUGAI PHARMA CO LTD
Vaccine Nanotechnology
ActiveUS20100233251A1Modulating immune systemEnhance and suppress and direct and immune responseNervous disorderAntipyreticDiseaseNanocarriers
Owner:MASSACHUSETTS INST OF TECH +4
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
Vaccine formulations
ActiveUS7371395B2Improve stabilityStable and safe and easily administrableSsRNA viruses negative-senseAntibacterial agentsPlasmidBacilli
Owner:MERIAL INC
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
InactiveUS20060251684A1Reduce infectivityReduce morbiditySsRNA viruses negative-senseAntibacterial agentsPathogenic microorganismOrganic solvent
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Therapeutic and prophylactic methods using heat shock proteins
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Compositions and methods for the delivery of nucleic acids
InactiveUS20110117125A1Reduce particle aggregationReduce selection requirementsAntibacterial agentsOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:THE UNIV OF BRITISH COLUMBIA +2
Receptor specific transepithelial transport of therapeutics
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Synthetic glucopyranosyl lipid adjuvants
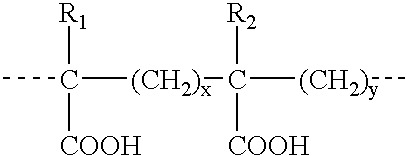
Compounds, particularly, glucopyranosyl lipid adjuvant (GLA) compounds, having the following structure (I) are provided:or a pharmaceutically acceptable salt thereof, wherein L1, L2, L3, L4, L5, L6, L7, L8, L9, L10, Y1, Y2, Y3, Y4, R1, R2, R3, R4, R5, R6, are as defined herein. Pharmaceutical compositions, vaccine compositions, and related methods for inducing or enhancing immune responses, are also provided.
Owner:ACCESS TO ADVANCED HEALTH INST
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
HIV envelope polypeptides
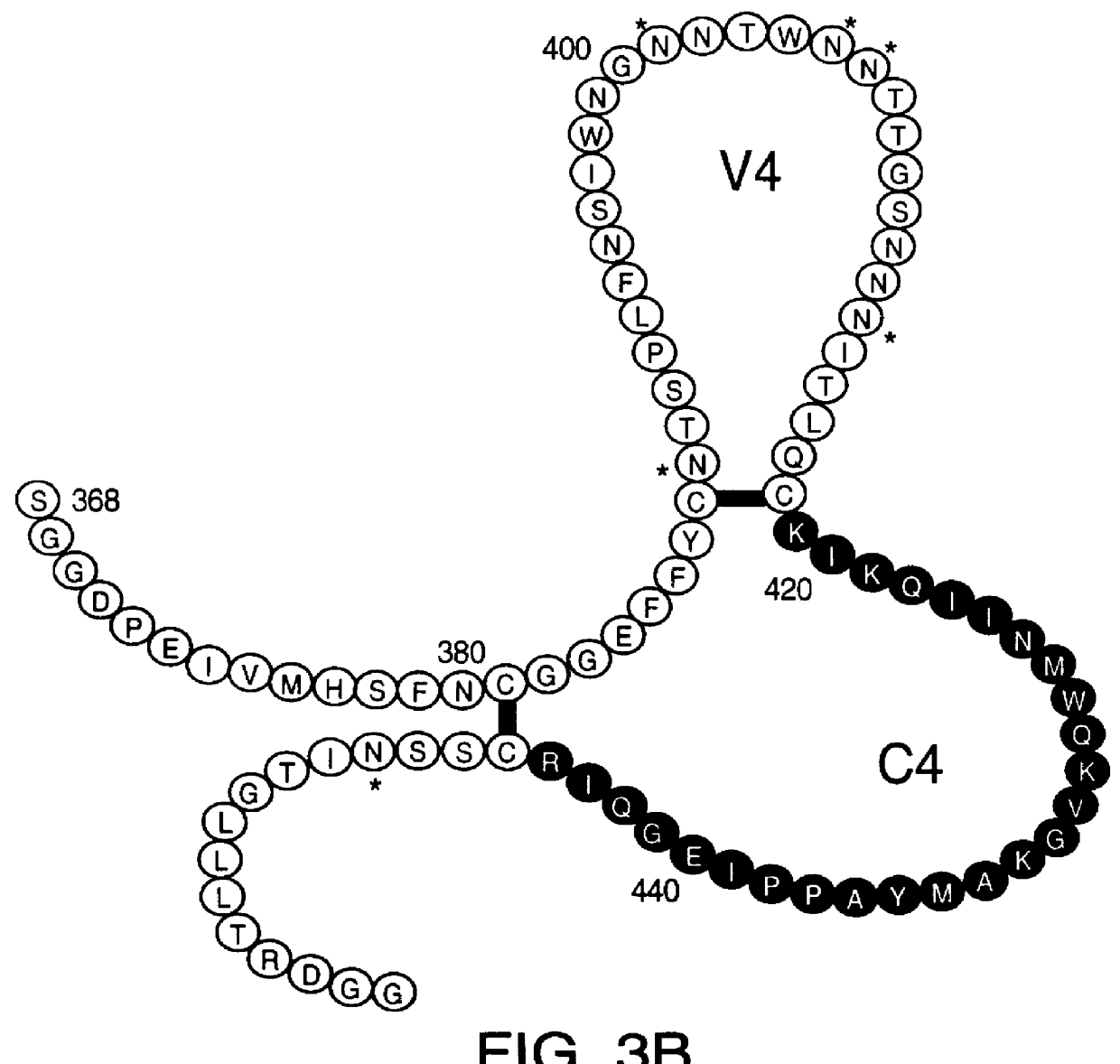
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
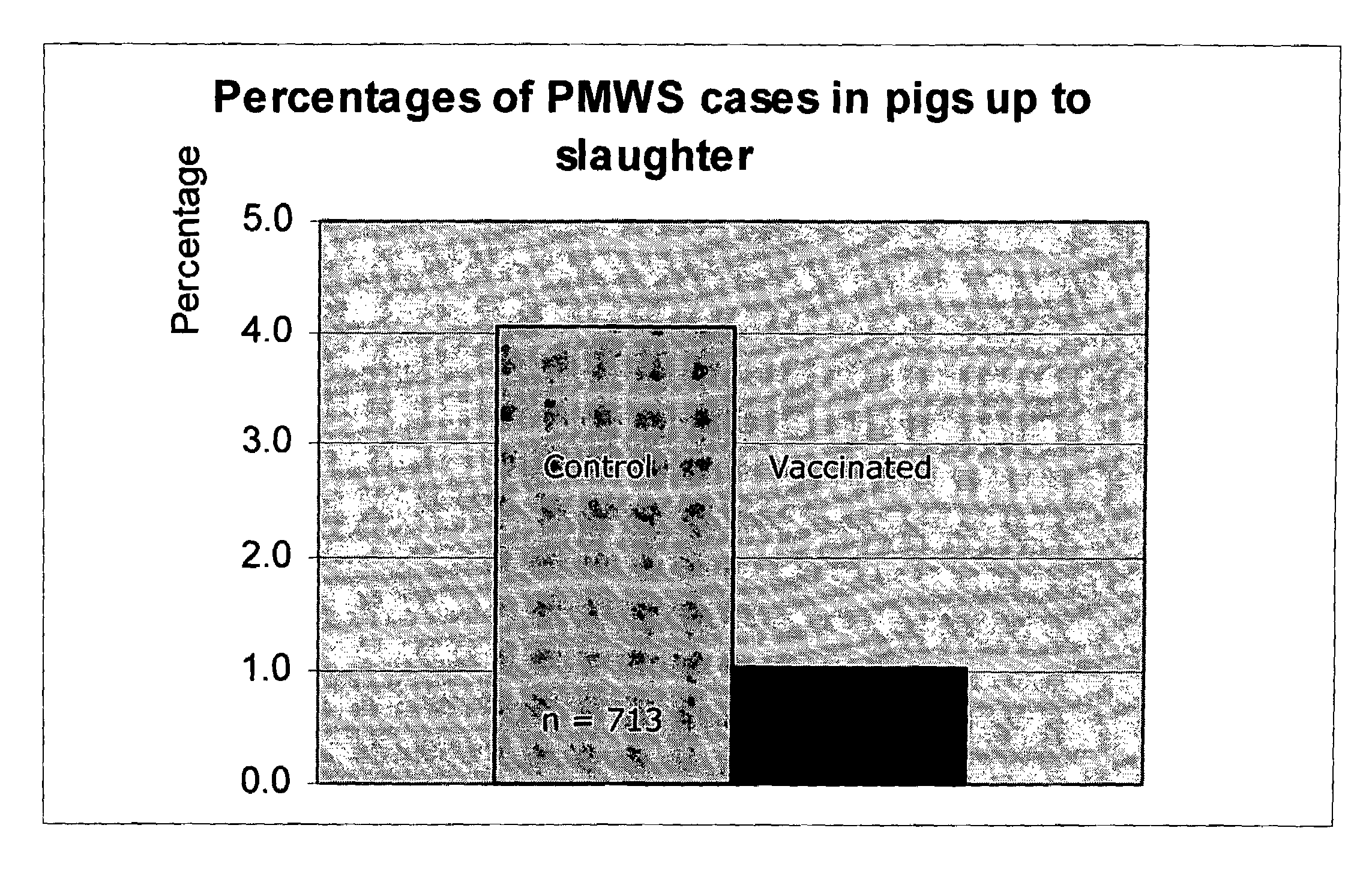
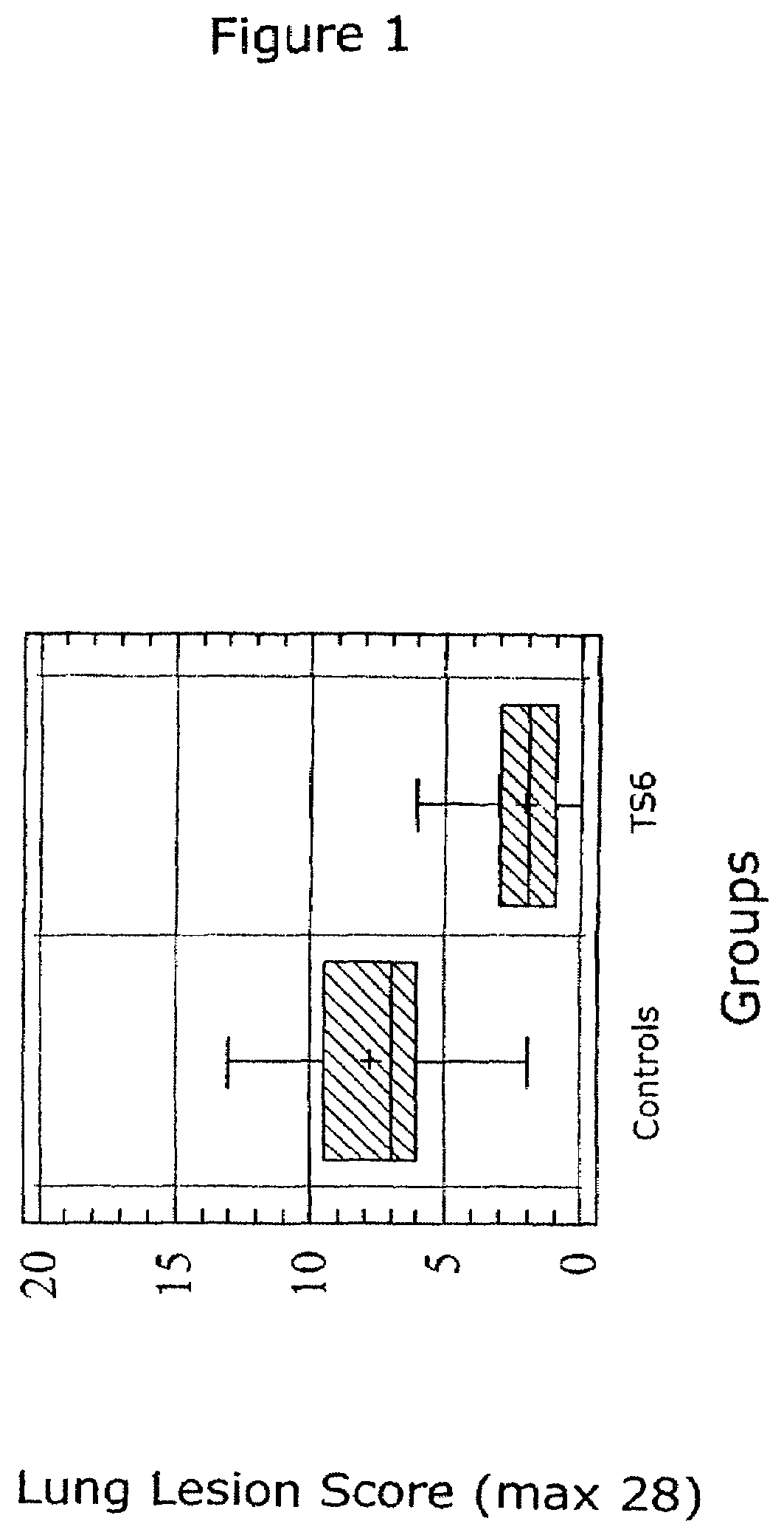
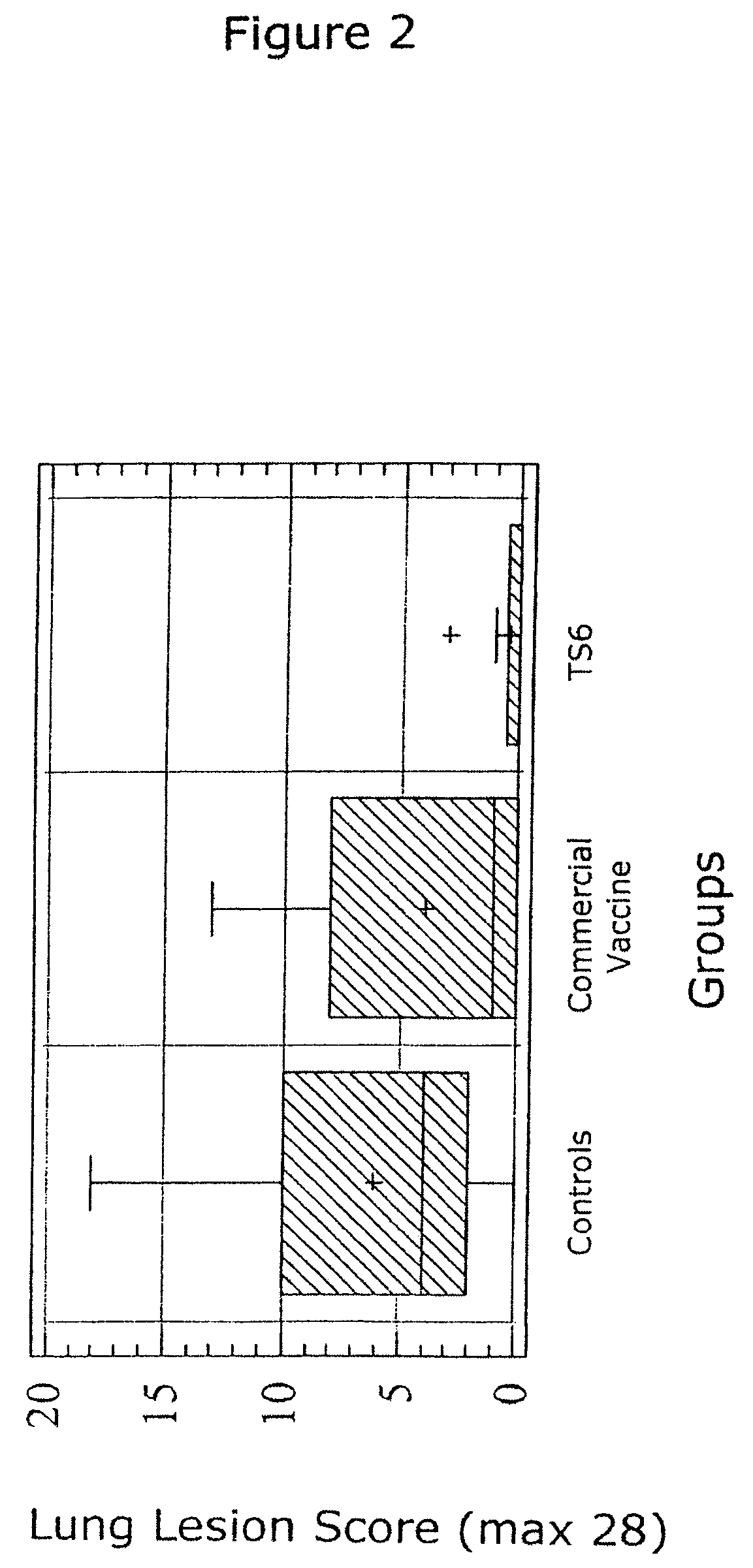
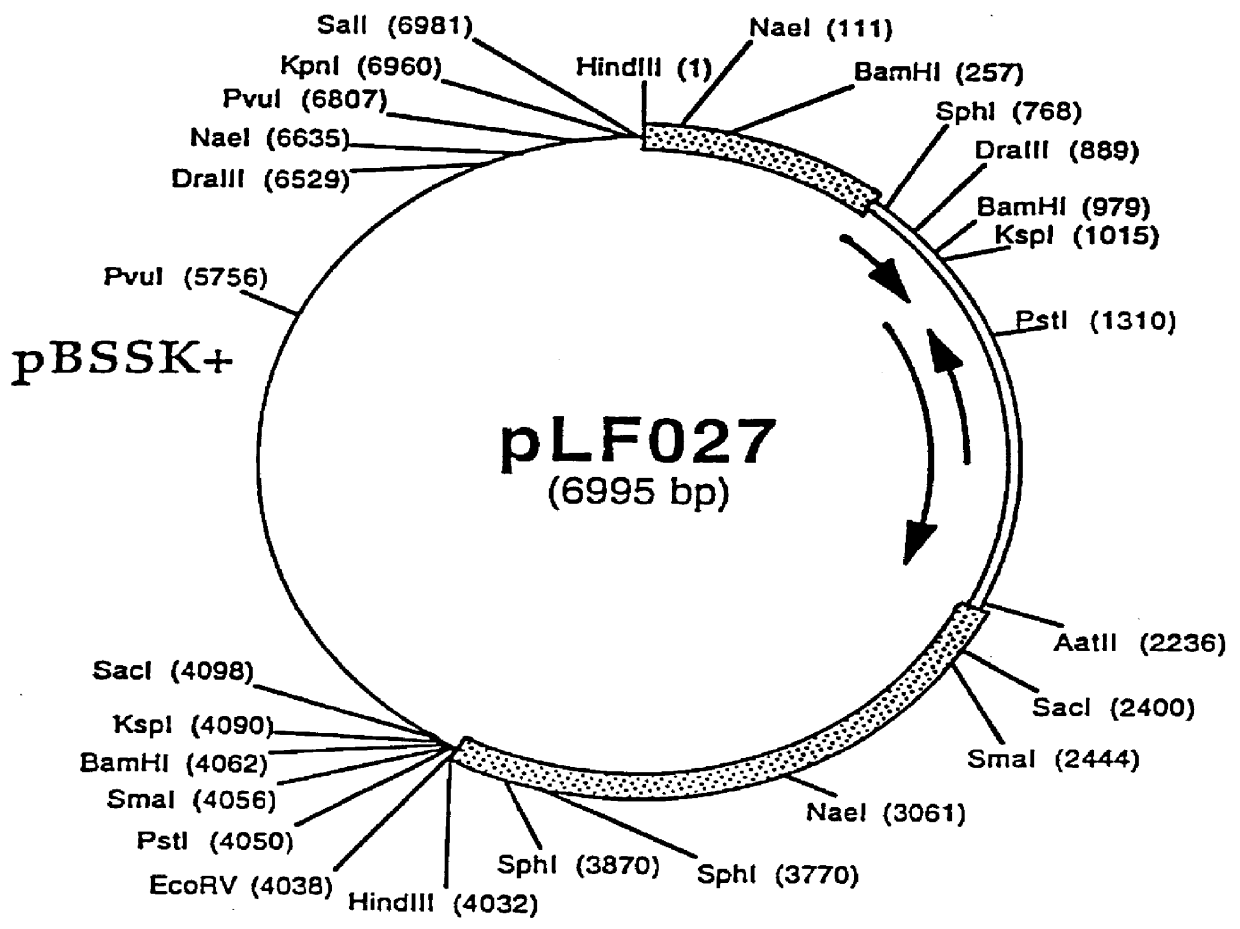
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com