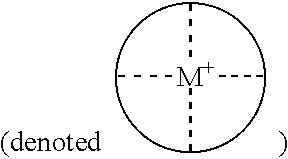
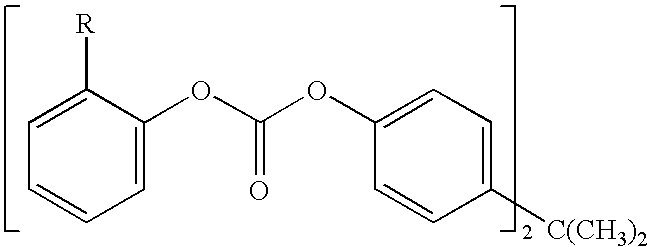
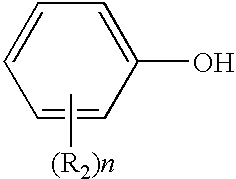
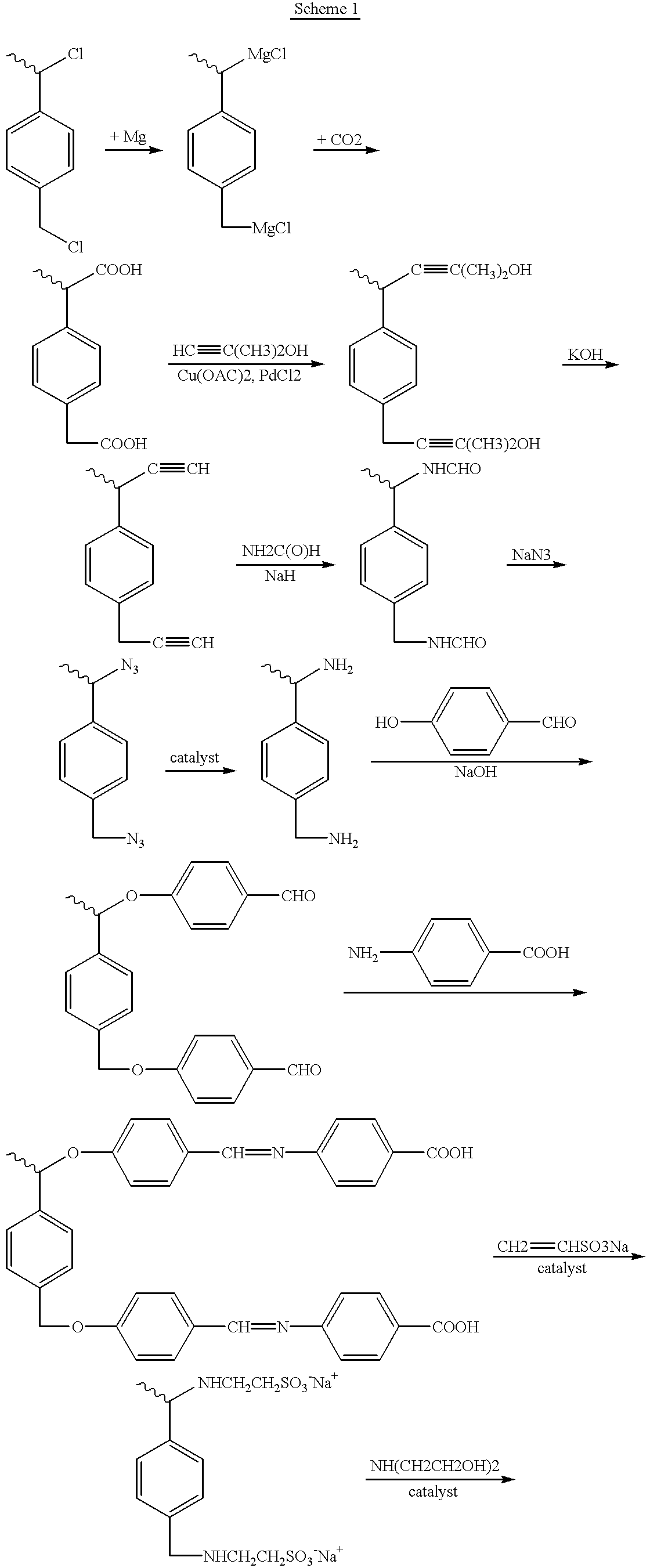
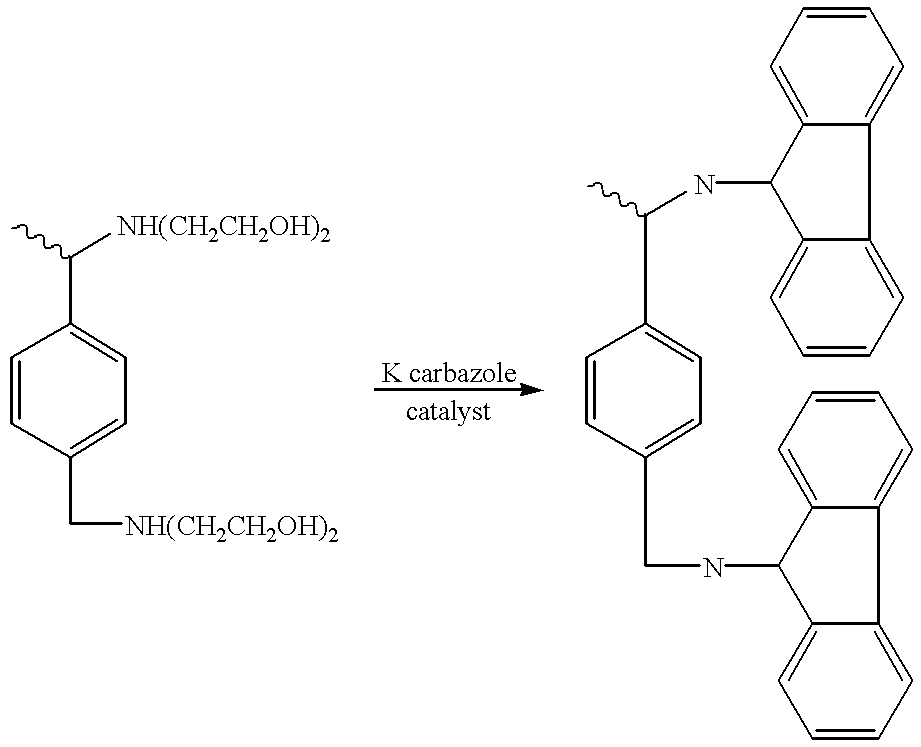
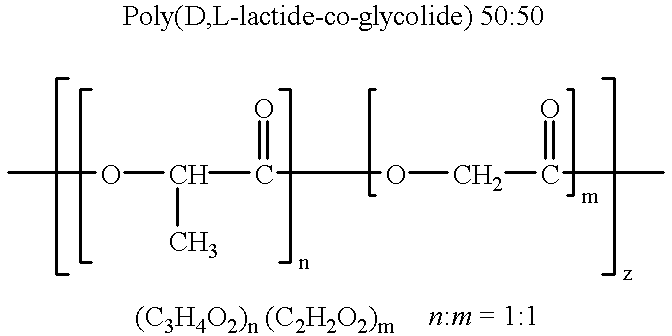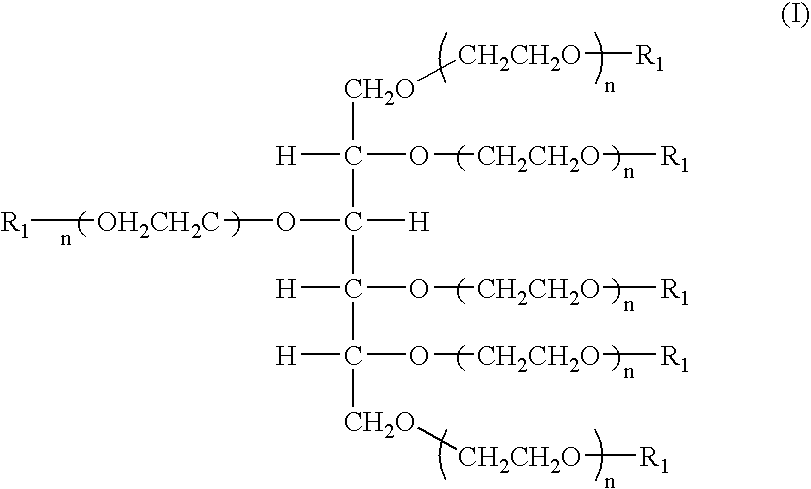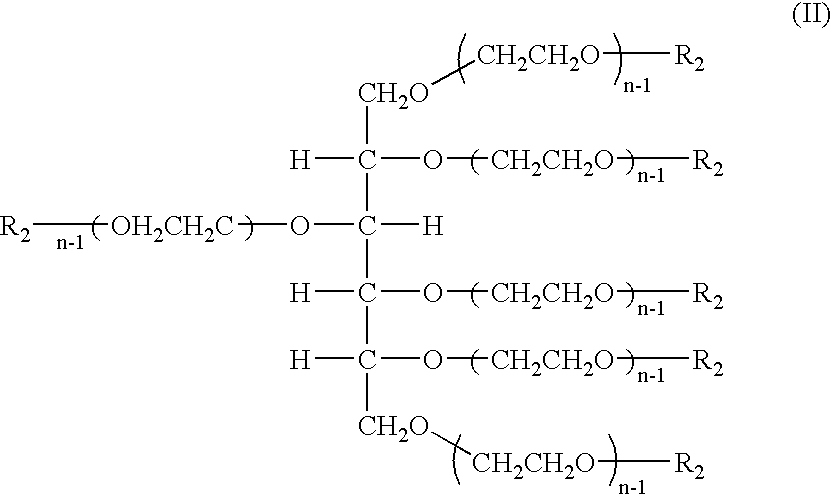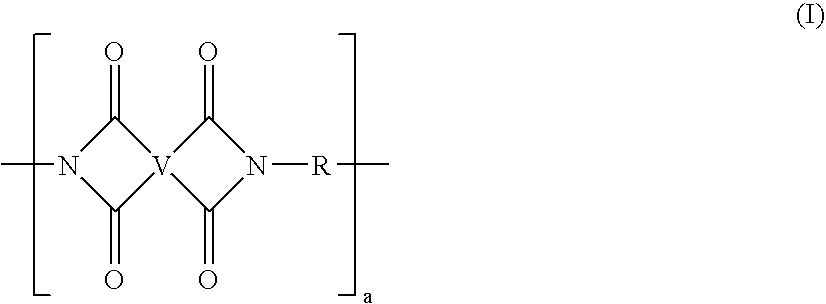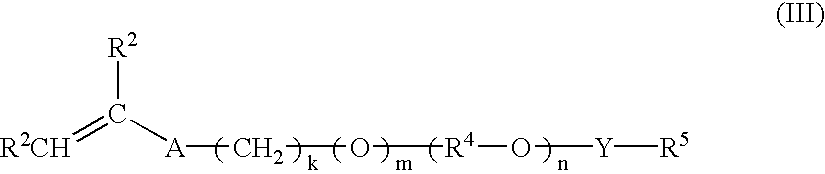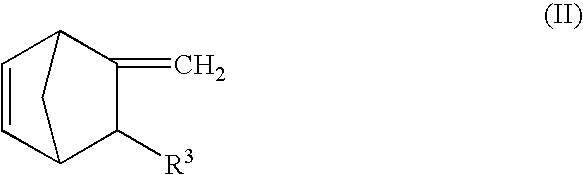Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3504 results about "End-group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
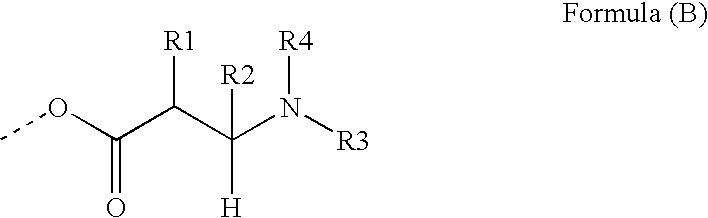
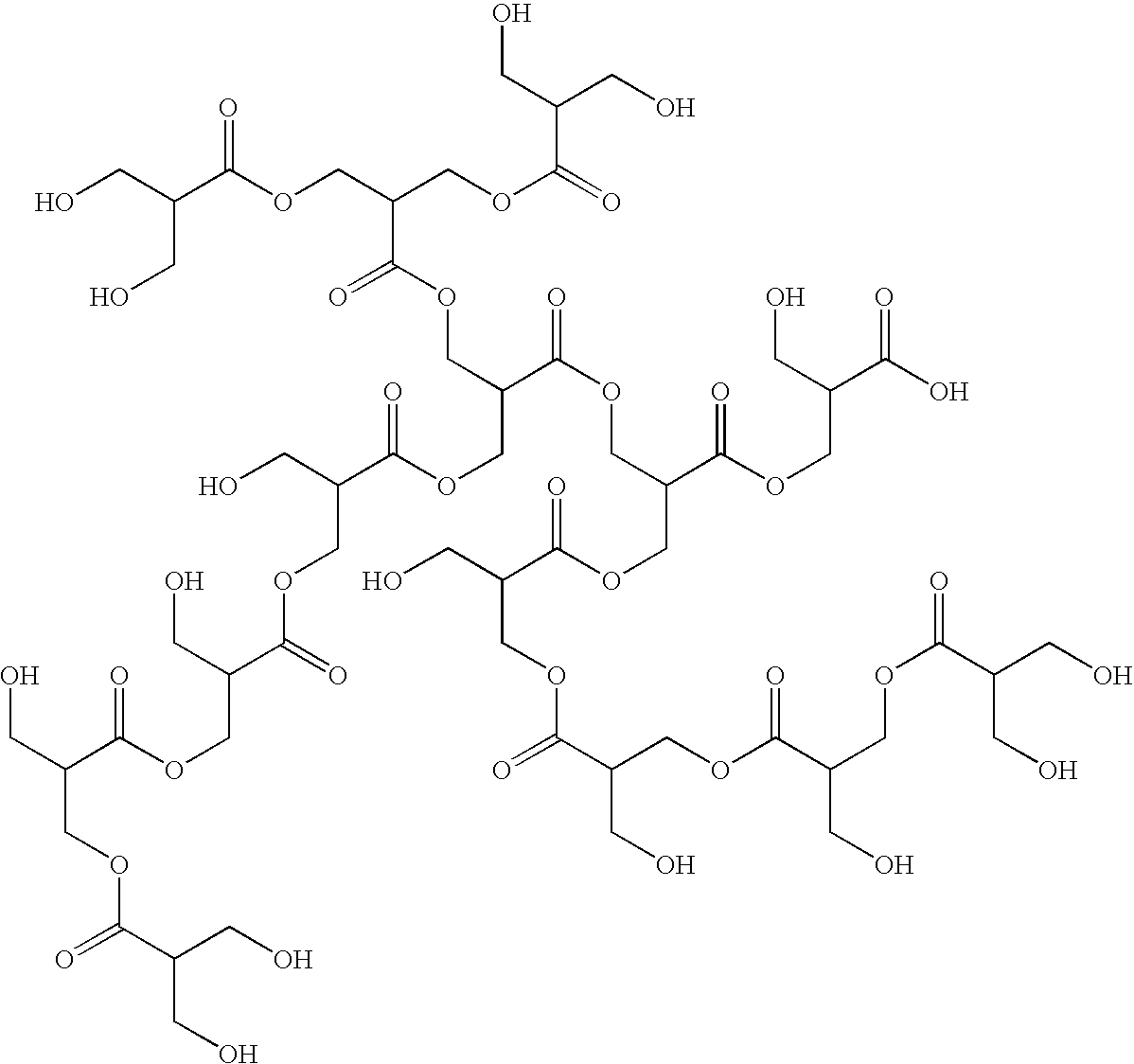
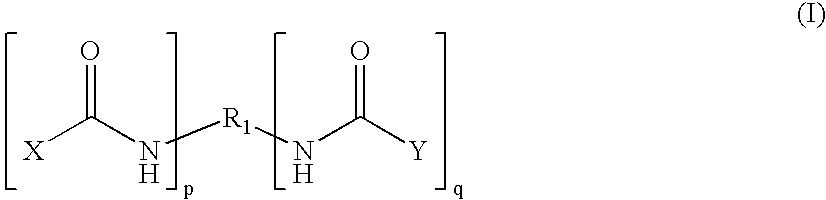
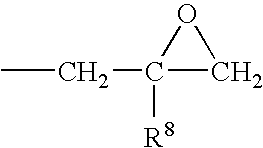
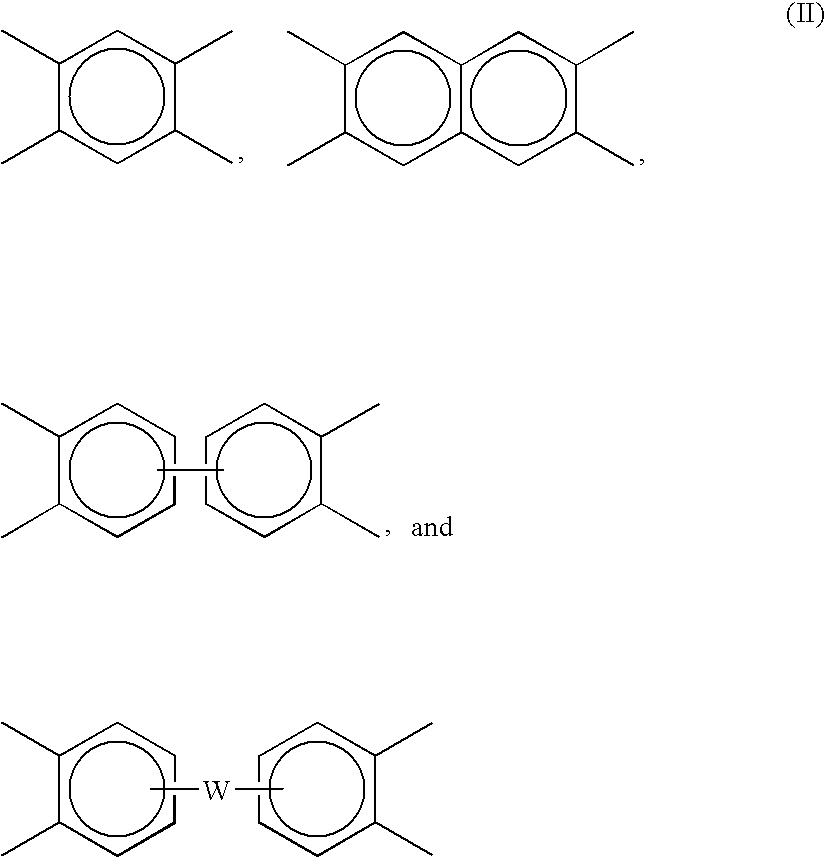
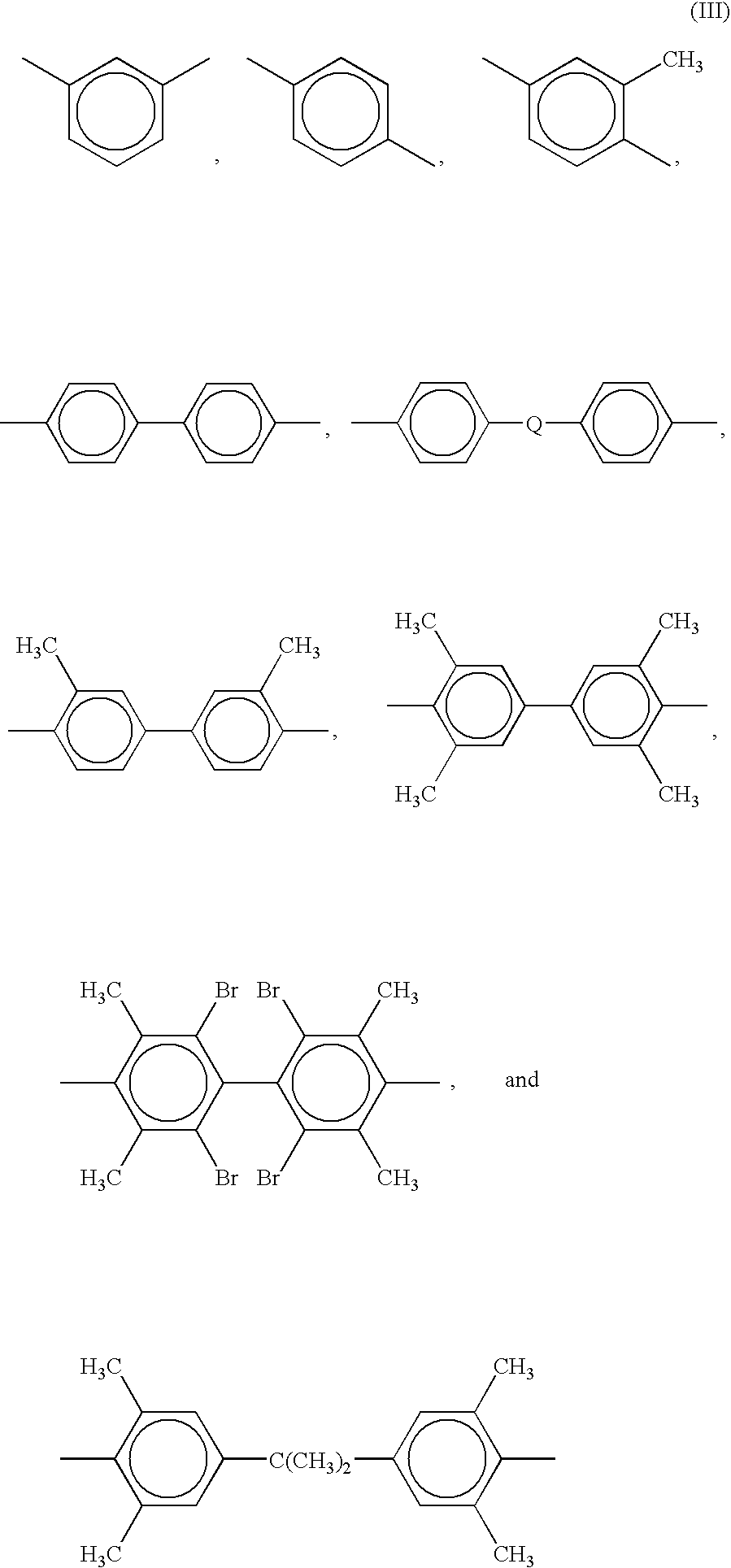
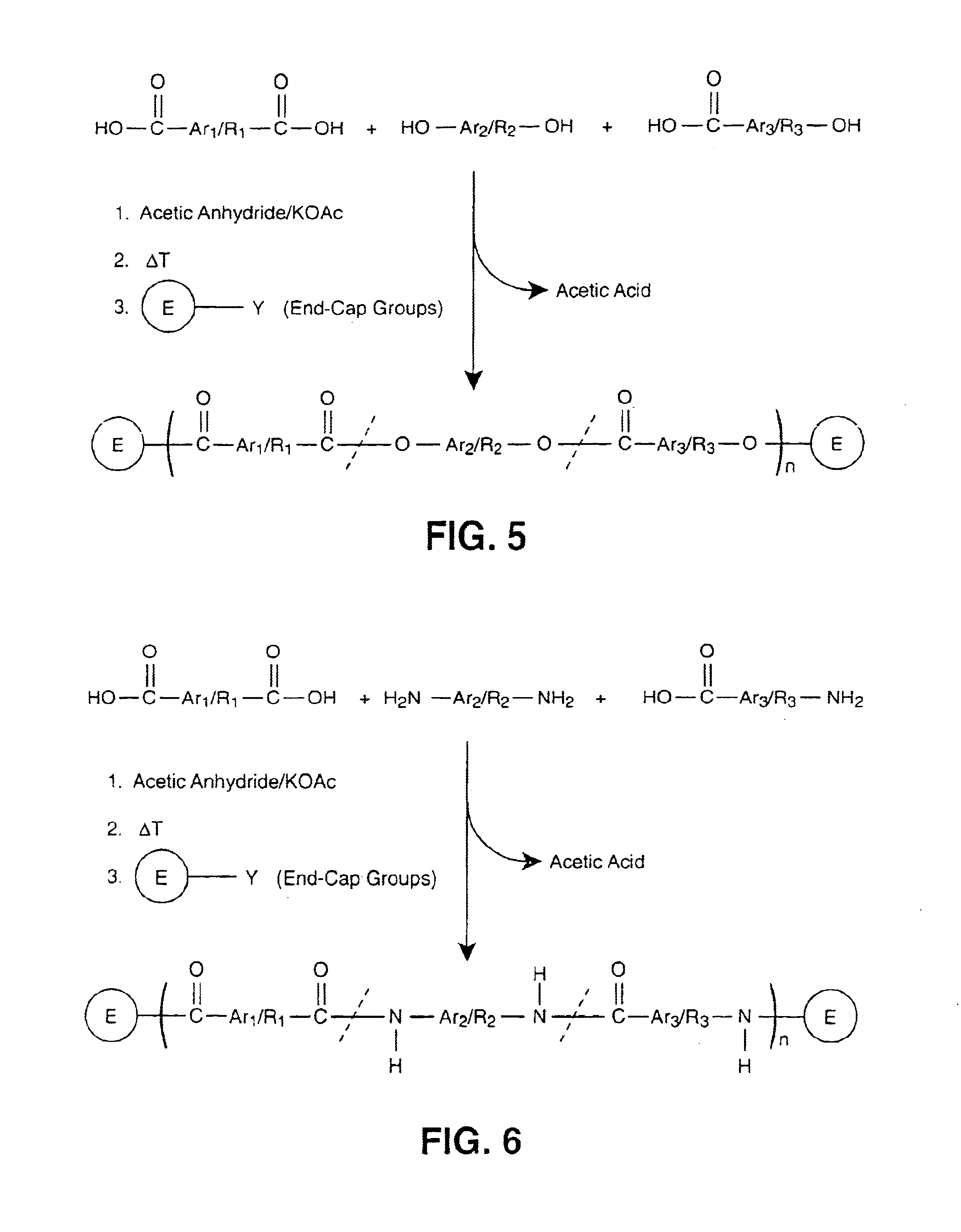
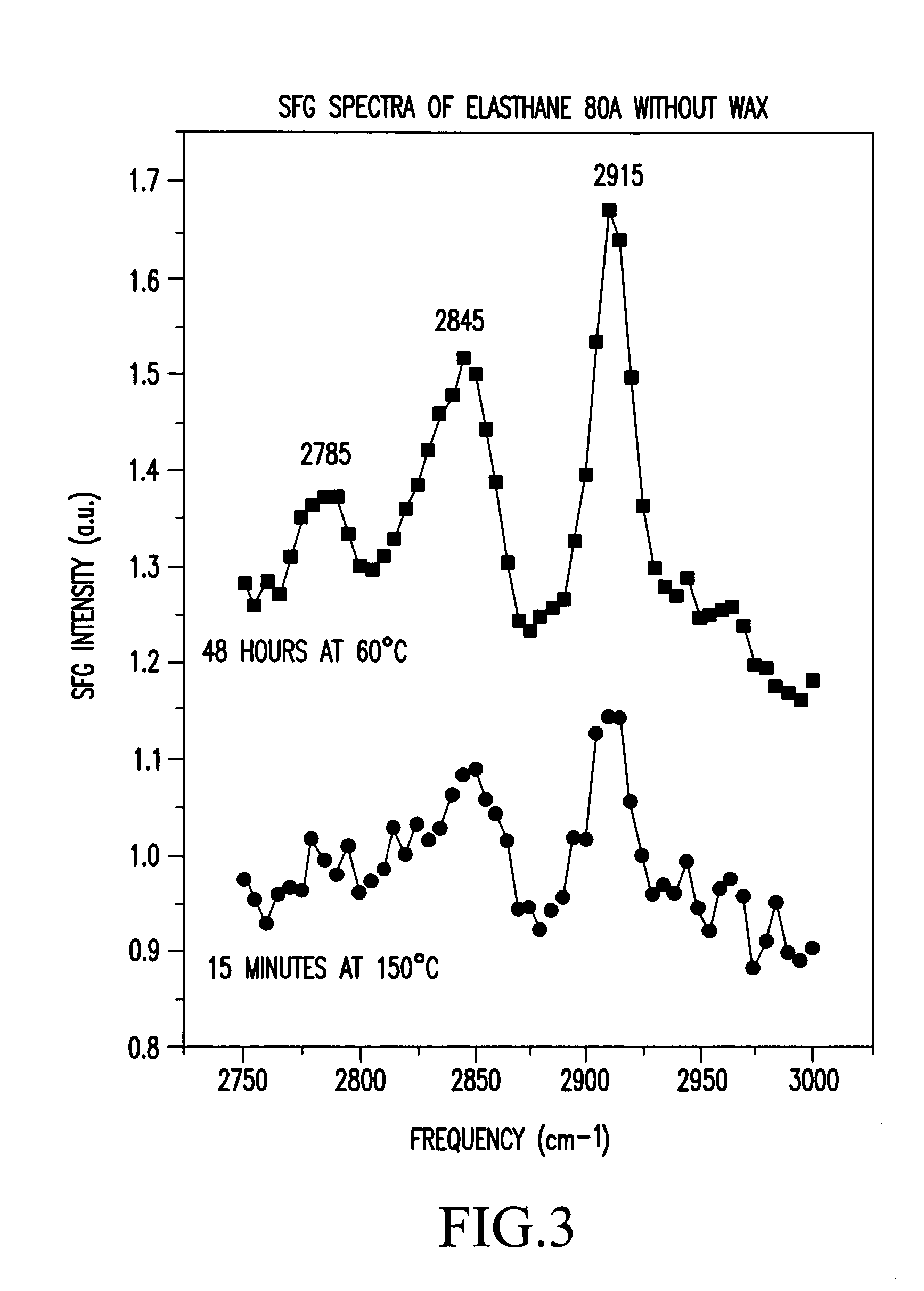
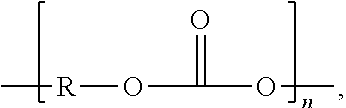
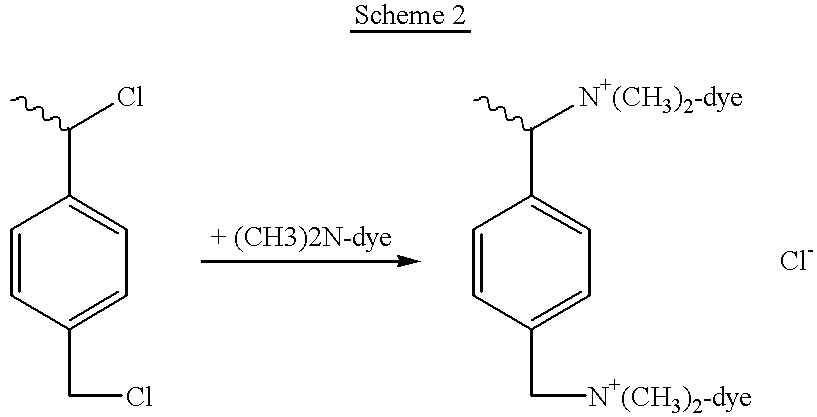
End groups are an important aspect of polymer synthesis and characterization. In polymer chemistry, end groups are functionalities or constitutional units that are at the extremity of a macromolecule or oligomer (IUPAC). In polymer synthesis, like condensation polymerization and free-radical types of polymerization, end-groups are commonly used and can be analyzed for example by nuclear magnetic resonance (NMR) to determine the average length of the polymer. Other methods for characterization of polymers where end-groups are used are mass spectrometry and vibrational spectrometry, like infrared and Raman spectrometry. Not only are these groups important for the analysis of the polymer, but they are also useful for grafting to and from a polymer chain to create a new copolymer. One example of an end group is in the polymer poly(ethylene glycol) diacrylate where the end-groups are circled.
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Compositions with Improved Adhesion to Low Surface Energy Substrates
InactiveUS20080113094A1Excellent low surface energyImprove adhesionSynthetic resin layered productsOrganic dyesOligomerEnd-group
Acrylic-based pressure sensitive adhesives are modified with a telechelic hydrocarbon oligomer. The oligomer comprises a hydrocarbon polymer chain or backbone and a functional end group, e.g., an oligomer prepared from a mono hydroxyl polybutadiene polymer and toluene diisocyanate. The oligomer attaches to the acrylic backbone of the polymer as a pendant group and in a preferred embodiment, the oligomer is mixed with the PSA shortly before the PSA is coated.
Owner:BRADY WORLDWIDE INC
Novel radiation curable compositions
Radiation curable compositions with a polymeric co-initiator are disclosed comprising a dendritic polymer core with at least one co-initiating functional group as an end group. The dendritic polymeric core is preferably a hyperbranched polymer. Industrial applications include varnishes, lacquers and printing inks. The polymeric co-initiator is especially useful in radiation curable inkjet ink.
Owner:AGFA NV
Toughened epoxy adhesive composition
ActiveUS20060276601A1Improved lap shear and impact peel strengthGood storage stabilityPolyureas/polyurethane adhesivesSynthetic resin layered productsElastomerEnd-group
The invention is an epoxy resin based adhesive composition comprising an epoxy resin and a compound comprising an elastomeric prepolymer residue selected from the group of a polyurethane, a polyurea and a polyurea polyurethane having isocyanate end groups, the isocyanate end groups of said prepolymer residue being capped by a capping compound selected from the group consisting of a primary aliphatic, cycloaliphatic, heteroaromatic and araliphatic amine, a secondary aliphatic, cycloaliphatic, aromatic, heteroaromatic and araliphatic amine, a thiol and an alkyl amide, said capping compound being bound to the end of the polymer chain of the elastomeric prepolymer in a manner such that the end to which it is bonded no longer has a reactive group. In addition to the capping compound defined above above, a capping compound selected from the group consisting of a phenol and a polyphenol can be used for capping the isocyanate end groups of the prepolymer residue
Owner:DOW GLOBAL TECH LLC
Hexa-arm polyethylene glycol and its derivatives and the methods of preparation thereof
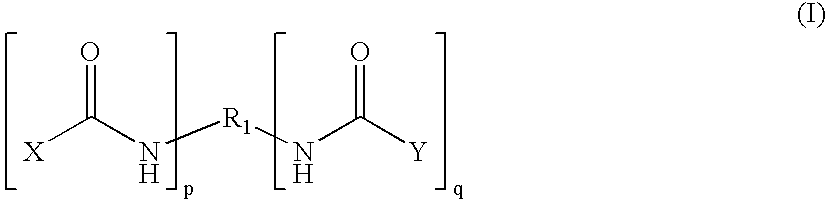
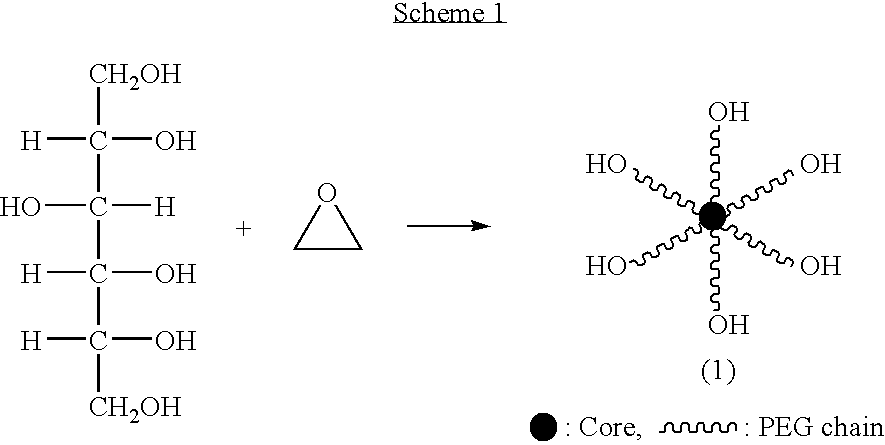
The present invention relates to novel hexa-arm polyethylene glycol (6-arm PEG) and its derivatives. The core of 6-arm PEG derivatives is sorbitol and the end groups can be derivatized into many different reactive functionalities that are useful in conjugating with many different targets. The present invention also provides a biodegradable polymeric hydrogel-forming composition comprising the 6-arm PEG and its derivatives, and methods of using such 6-arm PEG derivatives as surgical or biological implants or sealants.
Owner:SUN BIO INC
Polyimide sulfones, method and articles made therefrom
InactiveUS7041773B2Synthetic resin layered productsCeramic shaping apparatusVitrificationPolymer science
Polyimide sulfone resins are provided with a glass transition temperature of from 200–350° C., residual volatile species concentration of less than 500 ppm and a total reactive end group concentration of less than about 120 milliequivalents / kilogram resin. The resins have high heat capability and good melt stability. Methods to prepare the said resins and articles made from the resins are also provided.
Owner:SHPP GLOBAL TECH BV
Implantable biomedical devices including biocompatible polyurethanes
Disclosed are implantable devices that include biocompatible polyurethane materials. In particular, the disclosed polyurethane materials can maintain desired elastomeric characteristics while exhibiting thermoset-like behavior and can exhibit improved characteristics so as to be suitable in load-bearing applications. For example, the disclosed polyurethane materials can be suitable for use in artificial joints, including total joint replacement applications. The disclosed polyurethane materials include biocompatible cross-linking agents as chain extenders, more particularly chain extenders comprising a terminal group capable of side reactions and further comprising an electron withdrawing group immediately adjacent the terminal group. In addition, the reaction materials and conditions can be selected to encourage intermediate levels of cross-linking without the use of traditional cross-linking trifunctional reagents. In addition, the chain extenders can also include substantially inflexible moieties so as to increase the rigidity of the product polyurethanes.
Owner:CLEMSON UNIVERSITY +1
Liquid crystalline thermosets from ester, ester-imide, and ester-amide oligomers
InactiveUS6939940B2Low viscosityLow dielectric constantLiquid crystal compositionsLiquid crystallineEnd-group
Main chain thermotropic liquid crystal esters, ester-imides, and ester-amides were prepared from AA, BB, and AB type monomeric materials and were end-capped with phenylacetylene, phenylmaleimide, or nadimide reactive end-groups. The resulting reactive end-capped liquid crystal oligomers exhibit a variety of improved and preferred physical properties. The end-capped liquid crystal oligomers are thermotropic and have, preferably, molecular weights in the range of approximately 1000-15,000 grams per mole. The end-capped liquid crystal oligomers have broad liquid crystalline melting ranges and exhibit high melt stability and very low melt viscosities at accessible temperatures. The end-capped liquid crystal oligomers are stable for up to an hour in the melt phase. These properties make the end-capped liquid crystal oligomers highly processable by a variety of melt process shape forming and blending techniques including film extrusion, fiber spinning, reactive injection molding (RIM), resin transfer molding (RTM), resin film injection (RFI), powder molding, pultrusion, injection molding, blow molding, plasma spraying and thermo-forming. Once processed and shaped, the end-capped liquid crystal oligomers were heated to further polymerize and form liquid crystalline thermosets (LCT). The fully cured products are rubbers above their glass transition temperatures. The resulting thermosets display many properties that are superior to their non-end-capped high molecular weight analogs.
Owner:NASA
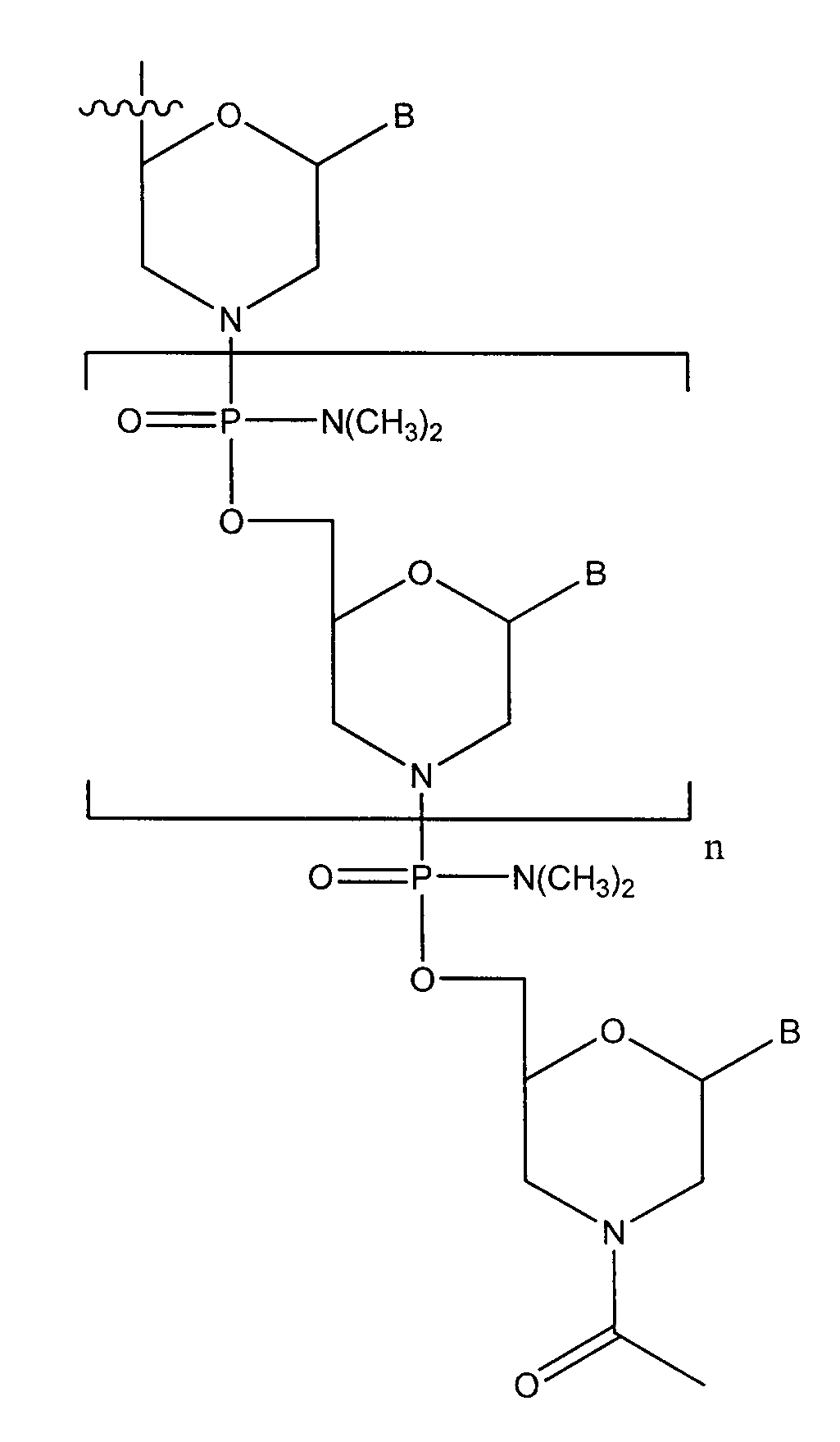
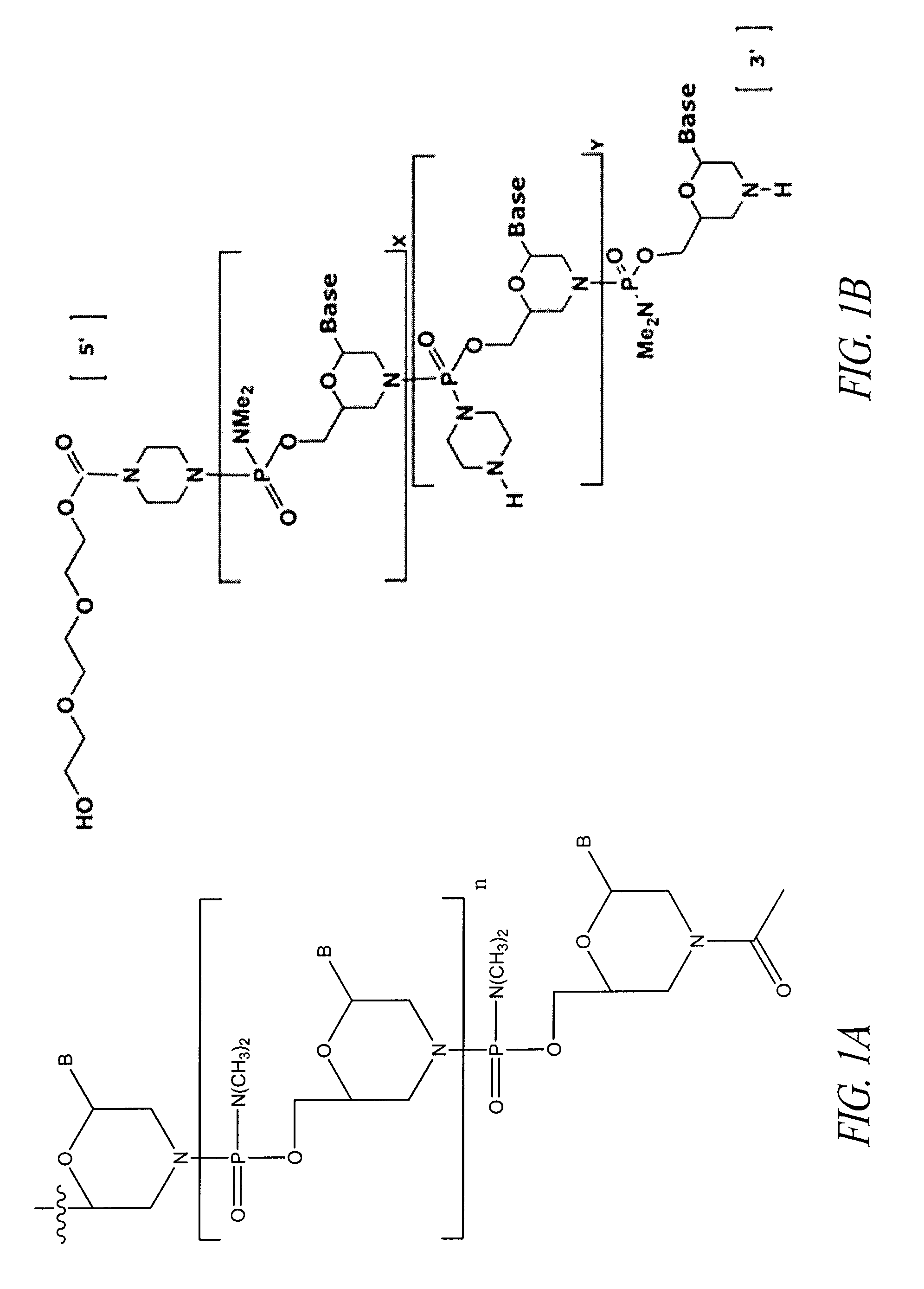
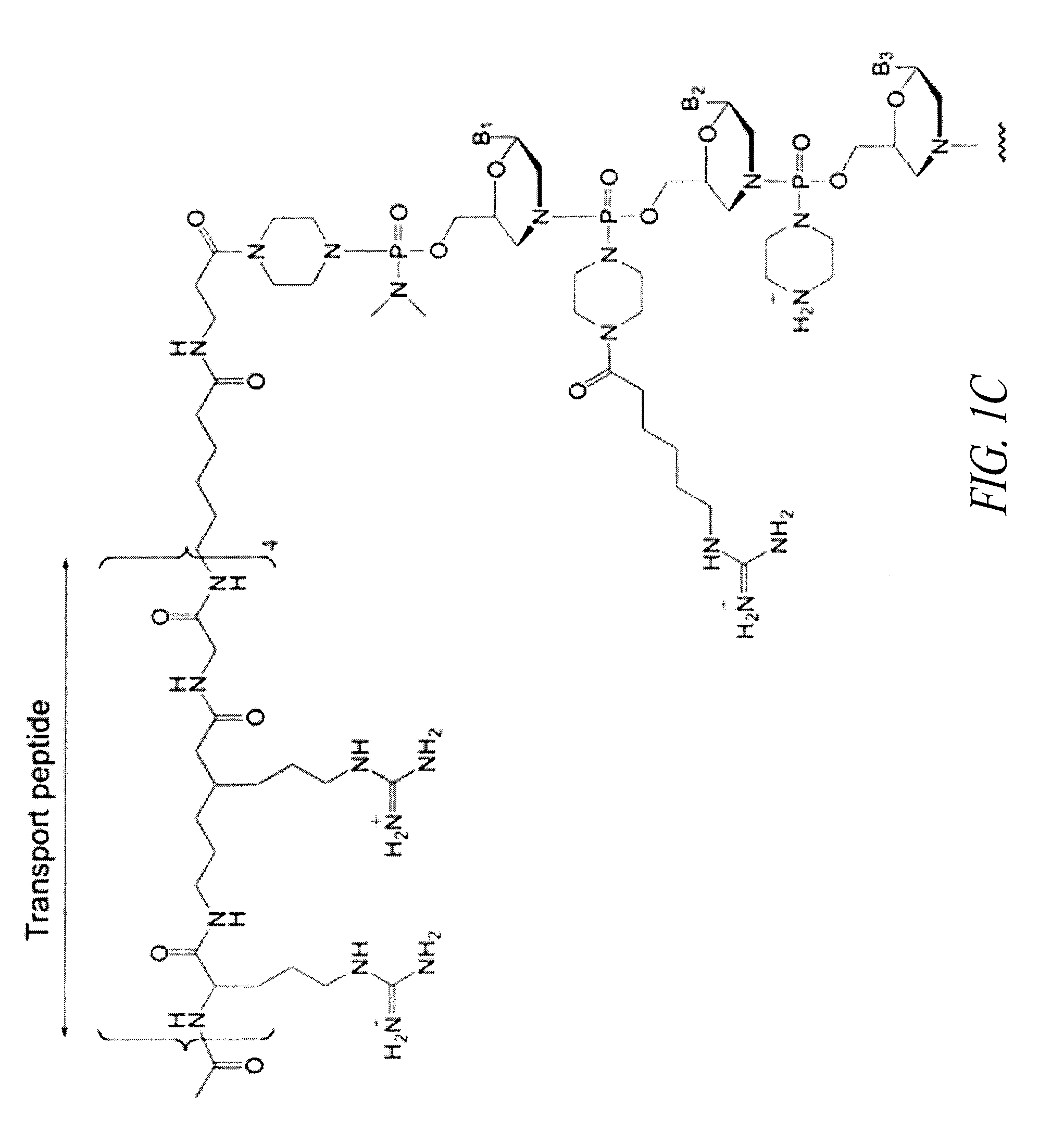
Oligonucleotide analogues having modified intersubunit linkages and/or terminal groups
ActiveUS20120065169A1Maintain good propertiesEnhanced cell deliveryAntibacterial agentsBiocideDiseaseEnd-group
Oligonucleotide analogues comprising modified intersubunit linkages and / or modified 3′ and / or 5′-end groups are provided. The disclosed compounds are useful for the treatment of diseases where inhibition of protein expression or correction of aberrant mRNA splice products produces beneficial therapeutic effects.
Owner:SAREPTA THERAPEUTICS INC
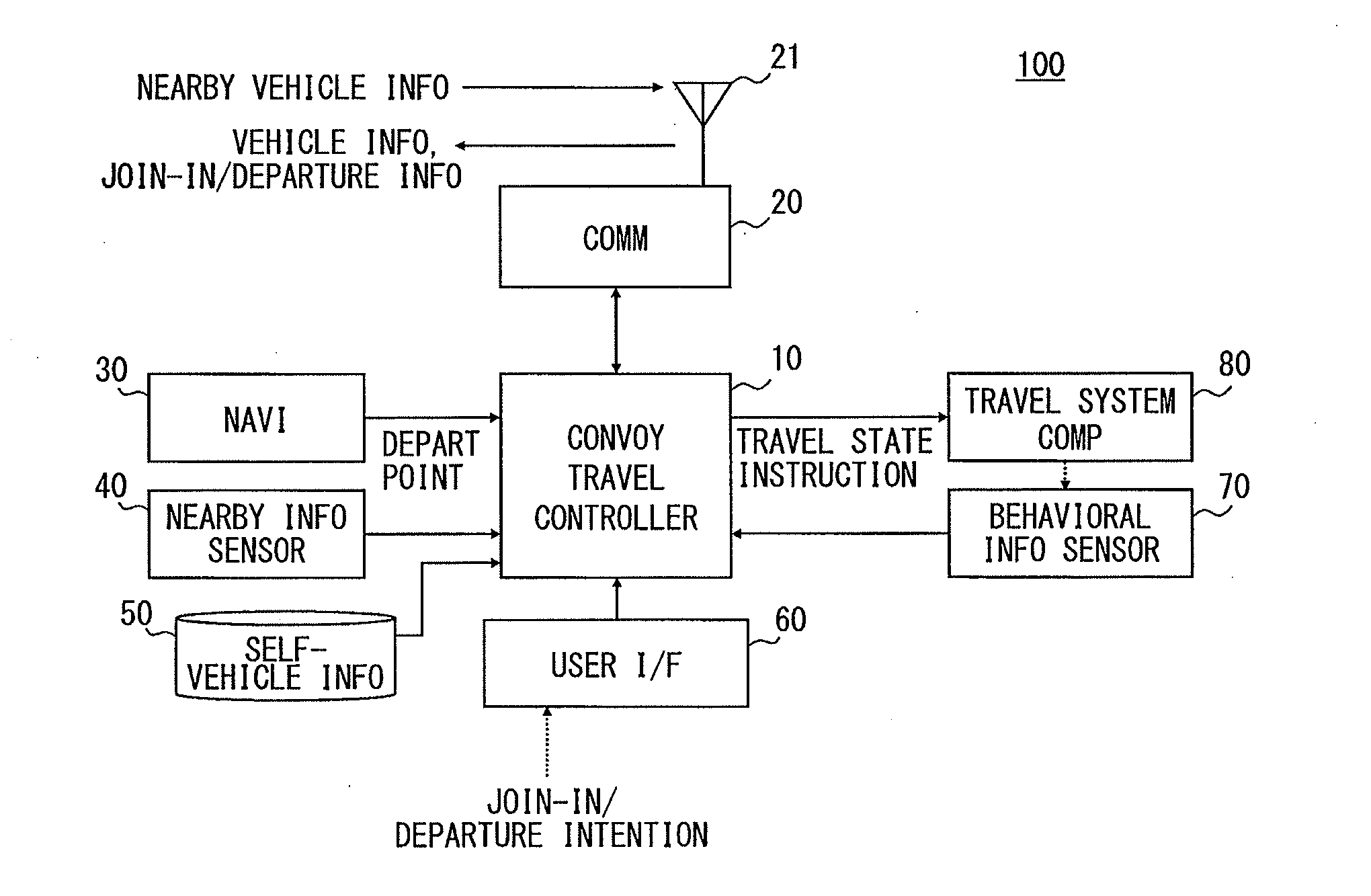
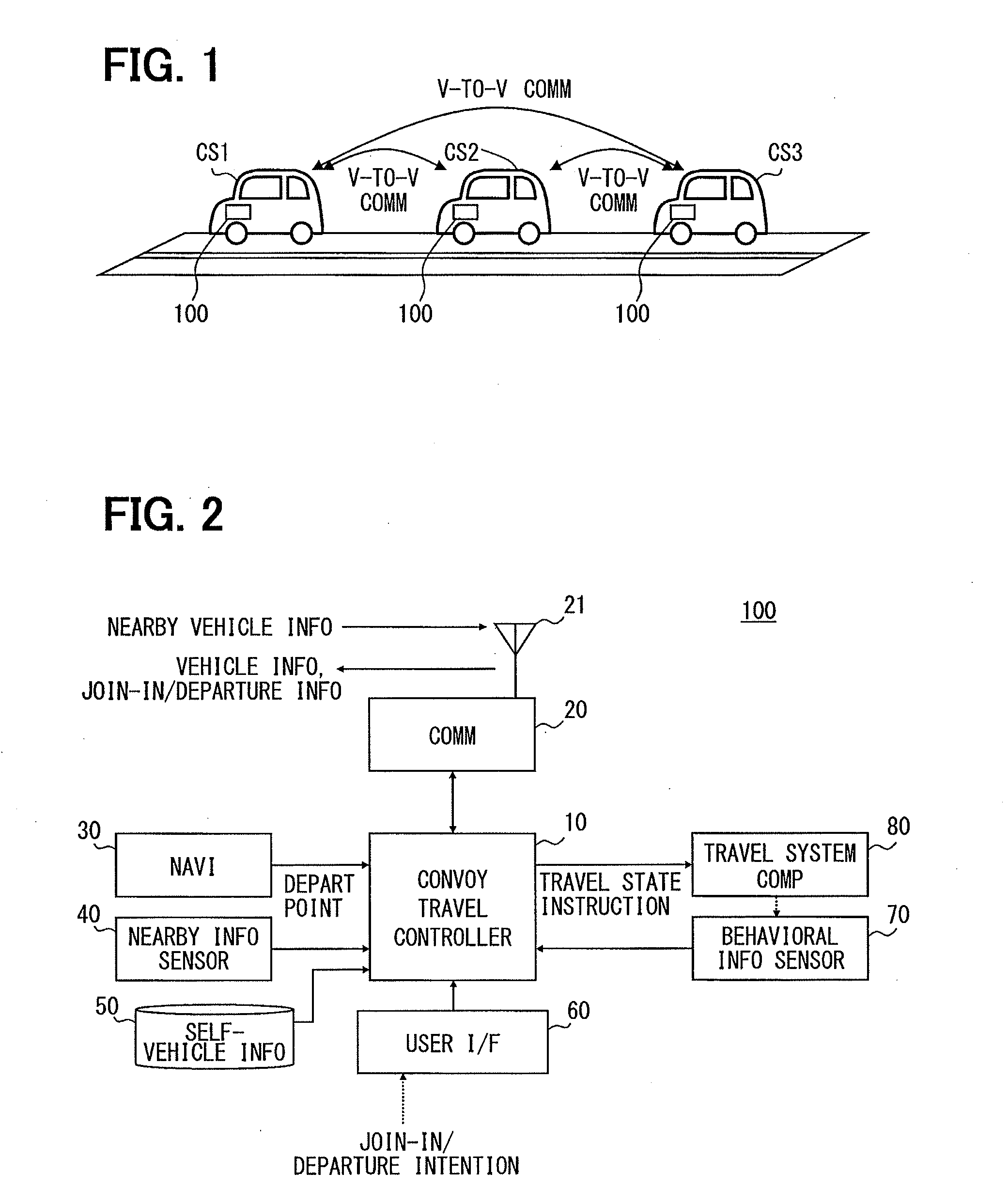
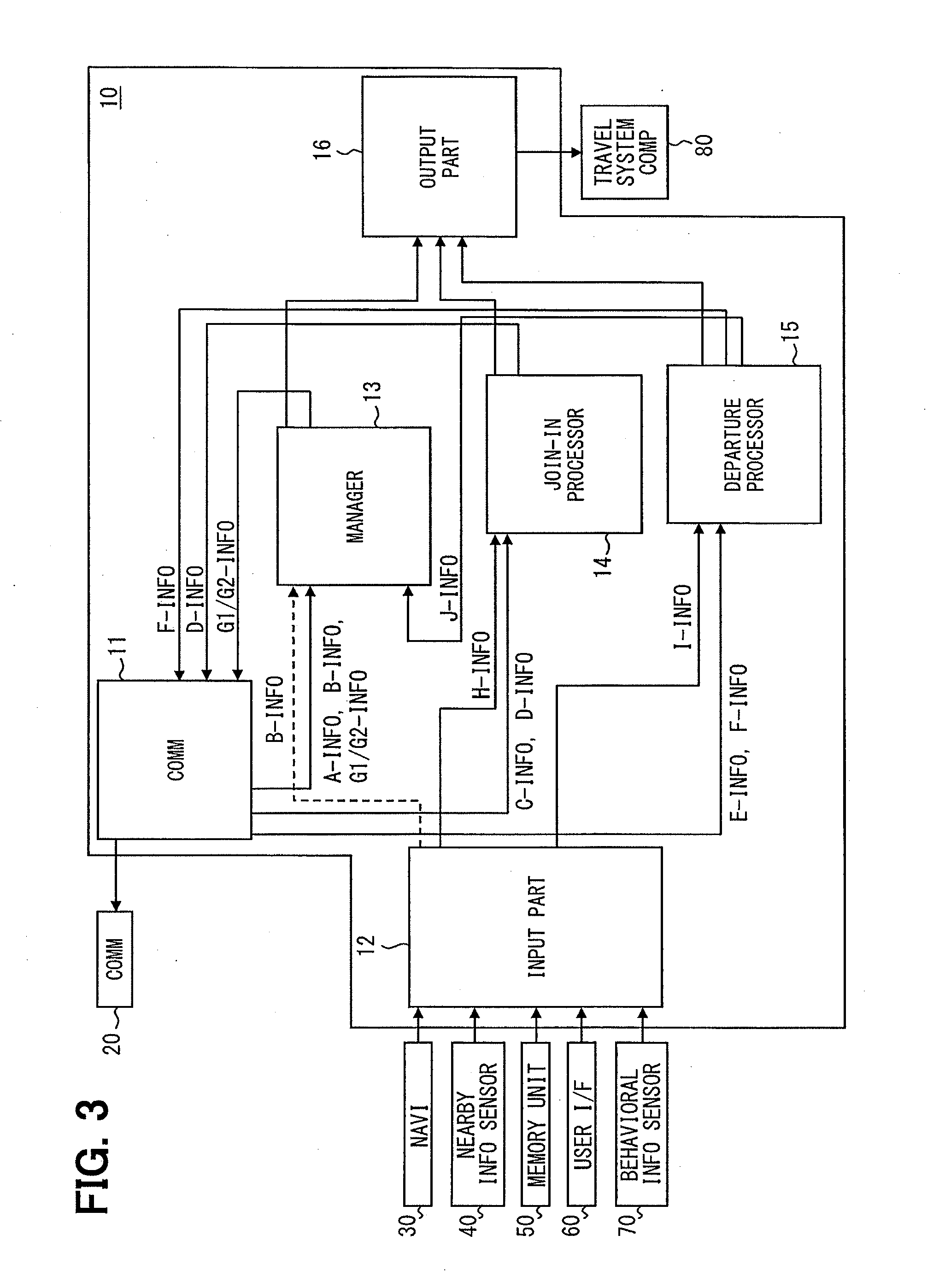
Platoon travel system
ActiveUS20140316865A1Reduce travel resistanceReduce energy consumptionAnalogue computers for vehiclesDiscounts/incentivesEnd-groupEngineering
A platoon travel system organizes and performs a platoon travel of plural vehicles along a preset travel route. The system has a grouping unit that divides the plural vehicles into a top group and a tail end group based on projection area information of the vehicles, and groups vehicles with a projection area in a first range to the top group and vehicles with a projection area in a second range to the tail end group, which is less than the first range. A final position determination unit determines a position of each of the plural vehicles in the vehicle groups based on the depart point information, positions the top group vehicles in an ascending order of depart point distances, and positions the tail end group vehicles in a descending order of depart point distances, thereby preventing deterioration of whole platoon energy consumption.
Owner:DENSO CORP
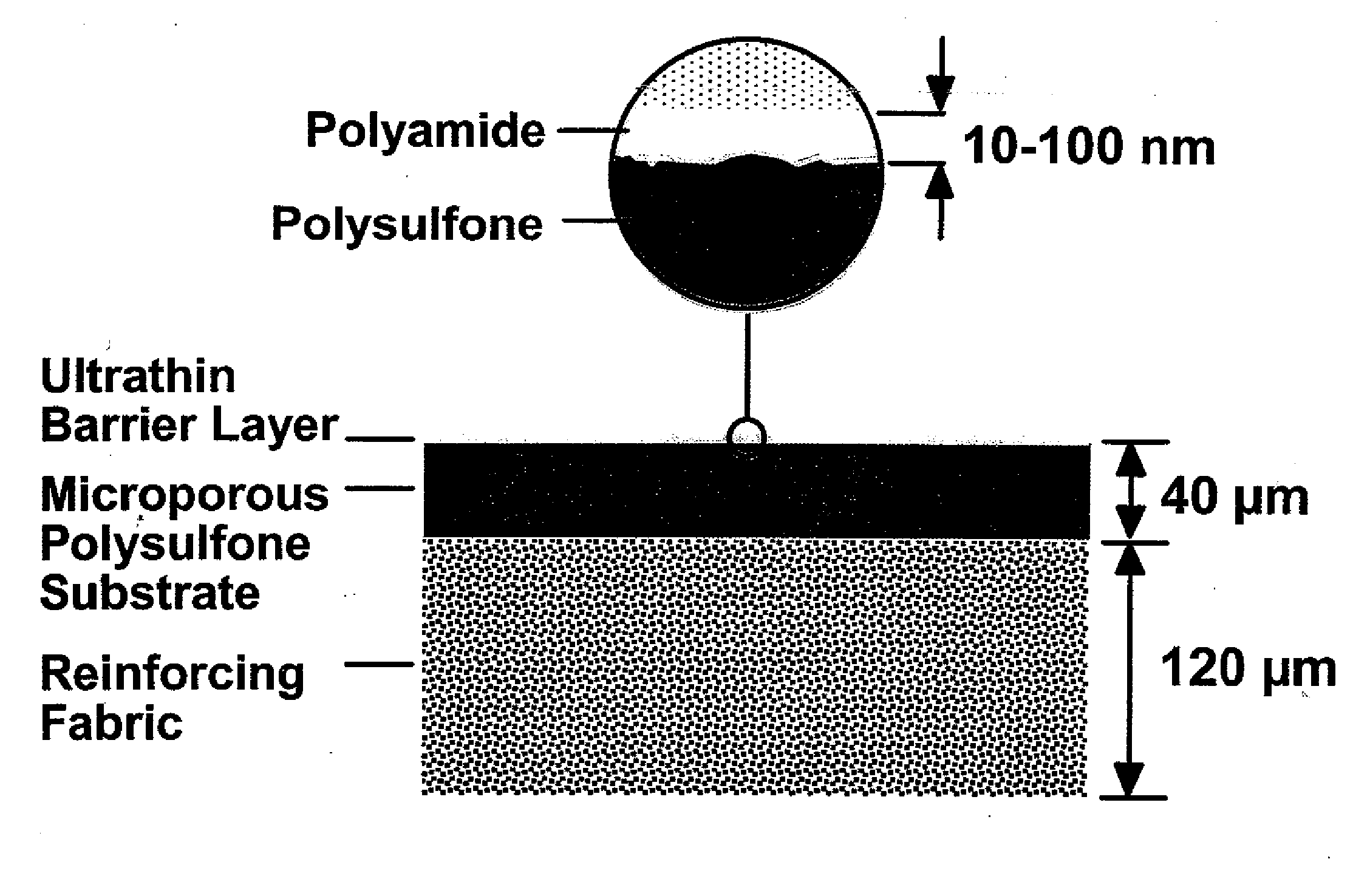
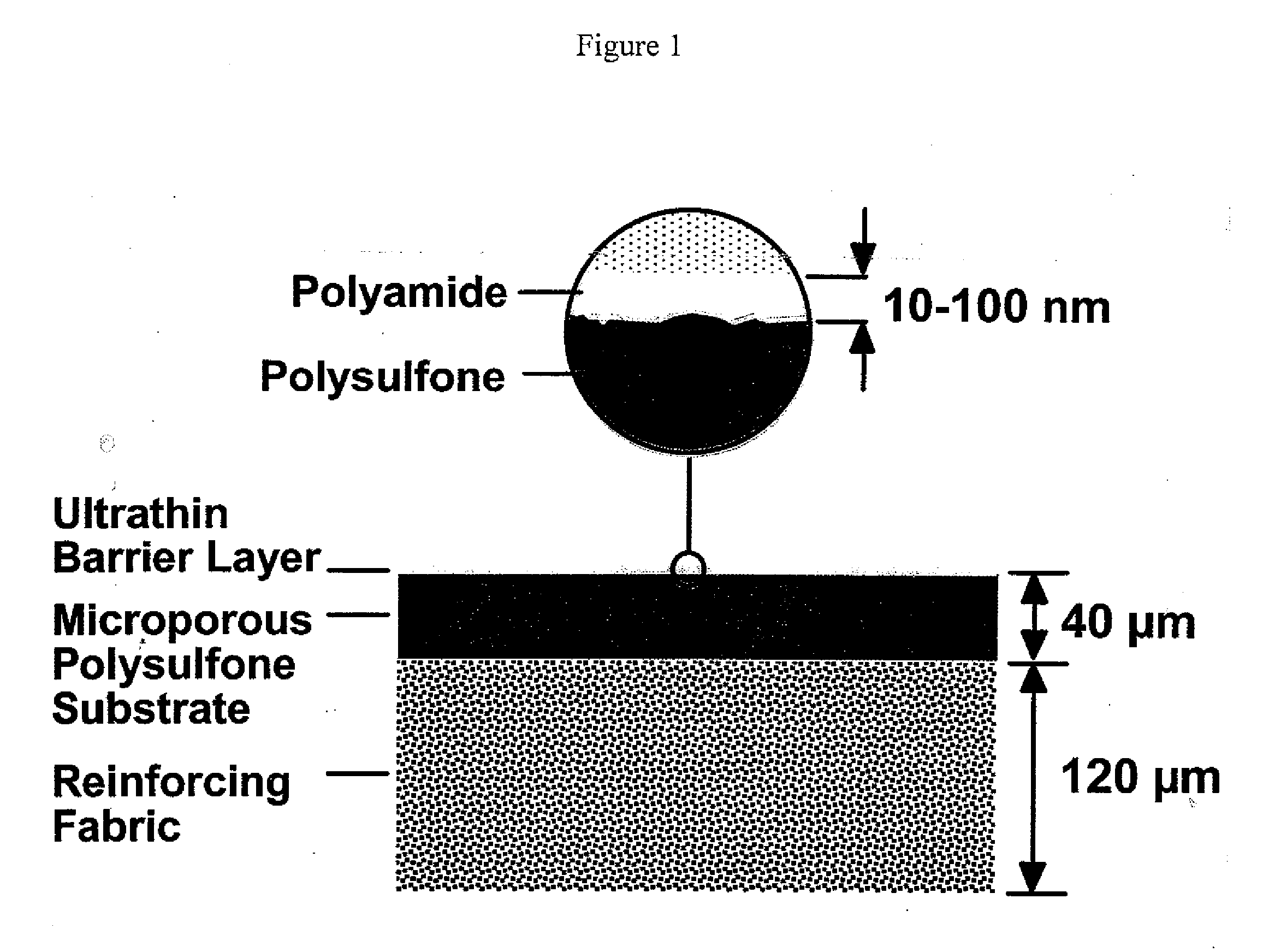
Reverse Osmosis Membrane with Branched Poly(Alkylene Oxide) Modified Antifouling Surface
InactiveUS20070251883A1Avoid problemsReduce dirtMembranesGeneral water supply conservationHydrogenEnd-group
Composite membranes that exhibit long-term resistance to biofouling comprise a porous support and a crosslinked polyamide discriminating layer having an external surface, the discriminating layer comprising a branched poly(alkylene oxide) (PAO) polymer attached to its external surface. The branched PAO polymer typically has the structure of a molecular comb or brush, and is made by polymerization of a PAO macromonomer of the following formula: RO—[(CHR′)n—O]m-V in which R is hydrogen or a C1-20 aliphatic or aromatic group, V is any group containing a polymerizable site, each R′ is independently hydrogen or a short chain alkyl group, n is an integer of 1-6, and m is an integer of 1 to about 200. The α end group can be either polymerized or copolymerized.
Owner:DOW GLOBAL TECH LLC
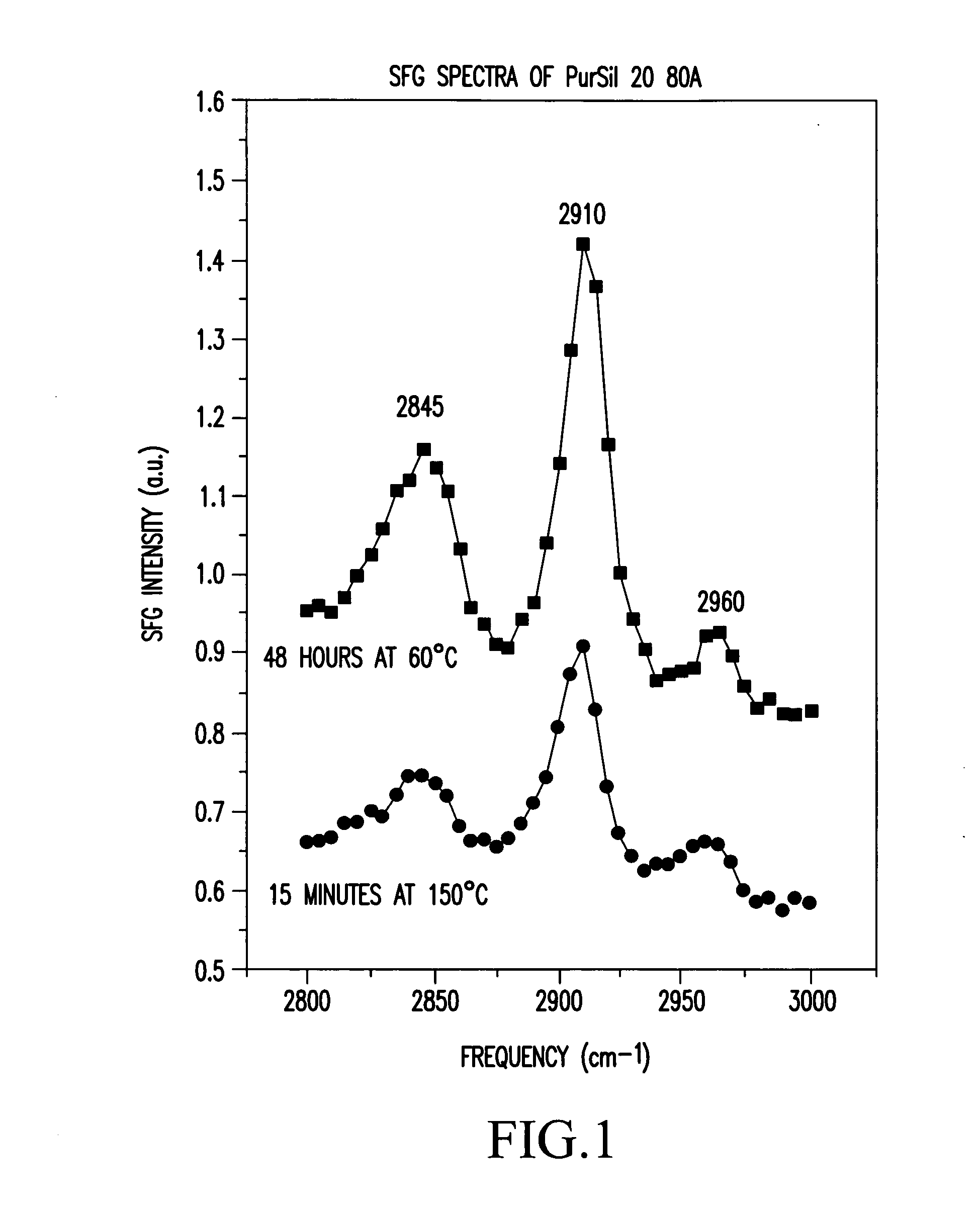
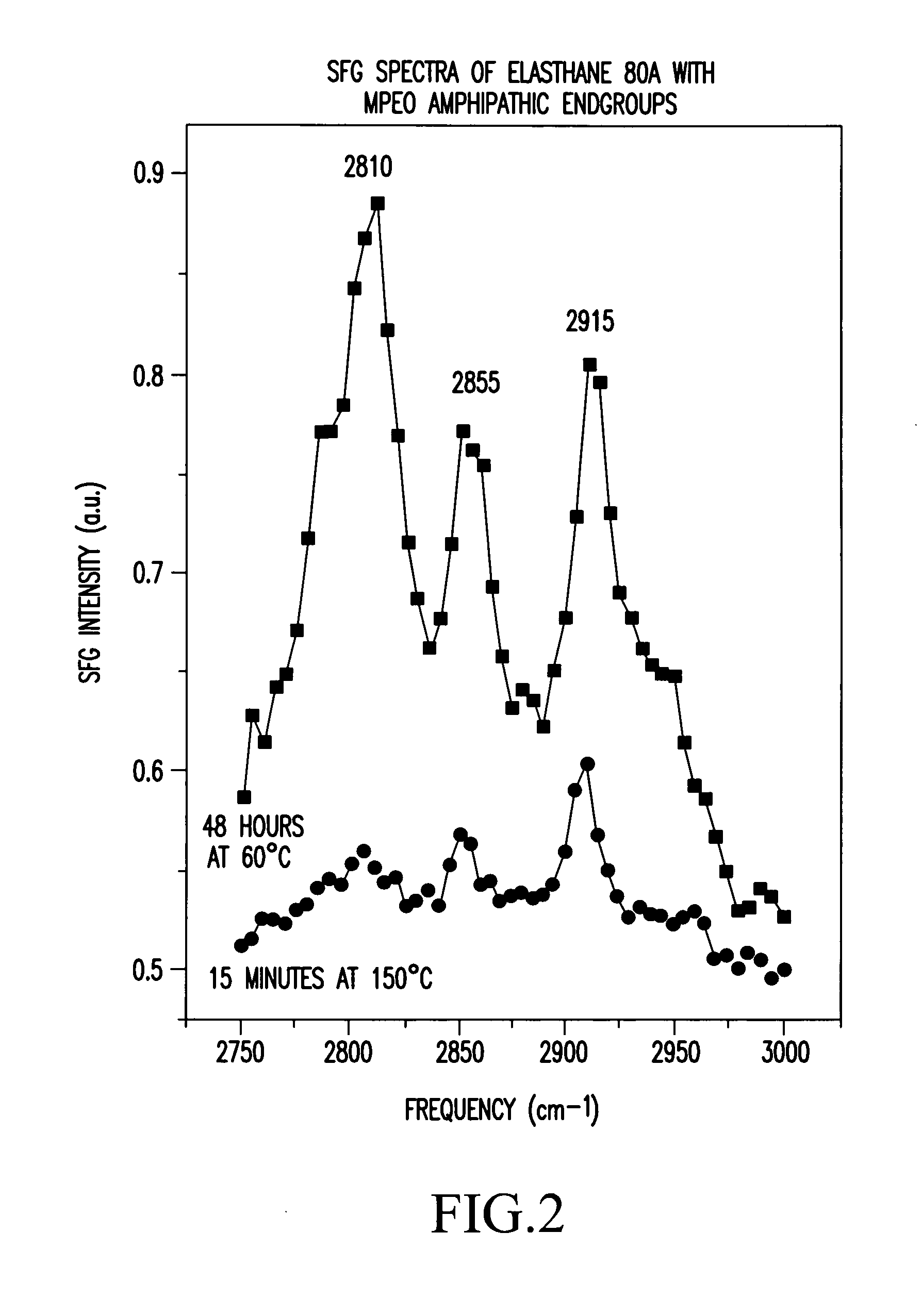
Control of polymer surface molecular architecture via amphipathic endgroups
ActiveUS20050282997A1Promote resultsReduce signal to noise ratioSurgeryCatheterPolymeric surfacePolyethylene oxide
Polymers whose surfaces are modified by endgroups that include amphipathic surface-modifying moieties. An amphipathic endgroup of a polymer molecule is an endgroup that contains at least two moieties of significantly differing composition, such that the amphipathic endgroup spontaneously rearranges its positioning in a polymer body to position the moiety on the surface of the body, depending upon the composition of the medium with which the body is in contact, when that re-positioning causes a reduction in interfacial energy. An example of an amphipathic surface-modifying endgroup is one that has both a hydrophobic moiety and a hydrophilic moiety in a single endgroup. For instance, a hydrophilic poly(ethylene oxide) terminated with a hydrophilic hydroxyl group is not surface active in air when the surface-modifying endgroup is bonded to a more hydrophobic base polymer. If the hydroxyl group on the oligomeric poly(ethylene oxide) is replaced by a hydrophobic methoxy ether terminus, the poly(ethylene oxide) becomes surface active in air, and allows the poly(ethylene oxide) groups to crystallize in the air-facing surface. In this example, immersion in water destroys the crystallinity as the poly(ethylene oxide) sorbs water and the hydrophobic methoxy group retreats below the surface of the polymer. Also disclosed are methods and articles of manufacture that make use of these polymers.
Owner:THE POLYMER TECH GROUP
Polymeric optical conductors
InactiveUS6766091B2Glass optical fibreOptical fibre with graded refractive index core/claddingFiberZero shear viscosity
An optical cable, which is flame-retardant, and has excellent adhesion of the protective covering to the fiber cladding and uniform thickness of the fiber cladding, contains a polymer optical conductor containing a fiber core; a single-layer or multi-layer fiber cladding; an inner external layer which adheres to the fiber cladding with a peel force of at least 50 N; and an outer external layer which adheres to the inner external layer with a peel force of not more than 30 N. The inner external layer contains a molding composition having a first polyamide selected from a) PA 11, b) PA 12, c) PA 1012, d) PA 1212, e) a copolyamide of at least two of PA 11, PA 12, PA 1012 and PA 1212, said copolyamide containing not more than 30 mol % of a comonomer, and f) mixtures thereof. The first polyamide contains at least 50 mueq / g of amino end groups. The first molding composition has a zero-shear viscosity of from 400 to 6000 Pas. The outer external layer contains a second molding composition which has the following i)-iii): i) from 20 to 95% by weight of a second polyamide selected from a) PA 11, b) PA 12, c) PA 1012, d) PA 1212, e) a copolyamide of at least two of PA 11, PA 12, PA 1012 and PA 1212, said copolyamide containing not more than 30 mol % of a comonomer, f) a polyetheramide of at least one of a)-e), and g) mixtures thereof, ii) from 5 to 45% by weight of a flame retardant, and iii) from 0 to 60% by weight of an impact modifier.
Owner:EVONIK DEGUSSA GMBH
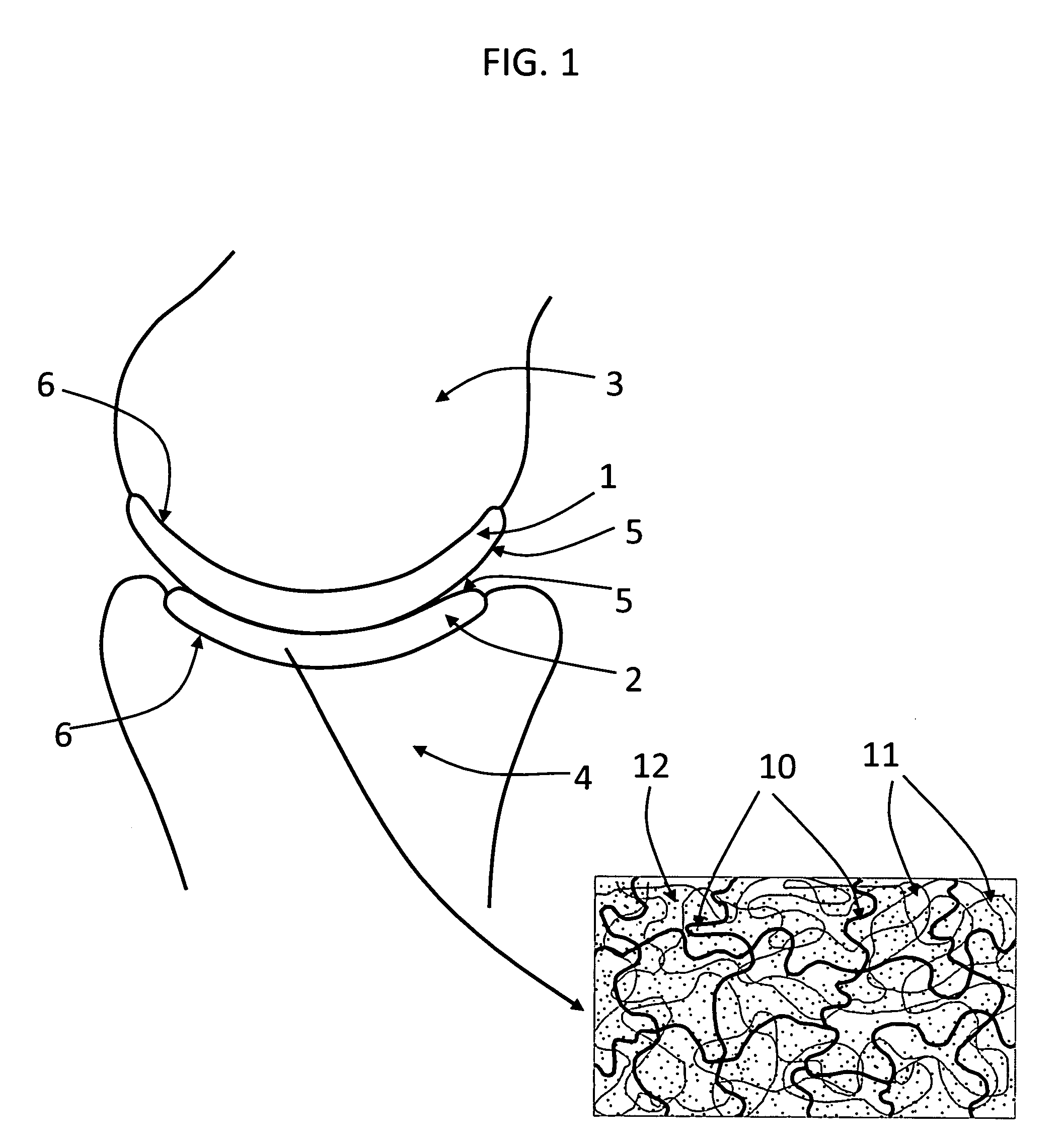
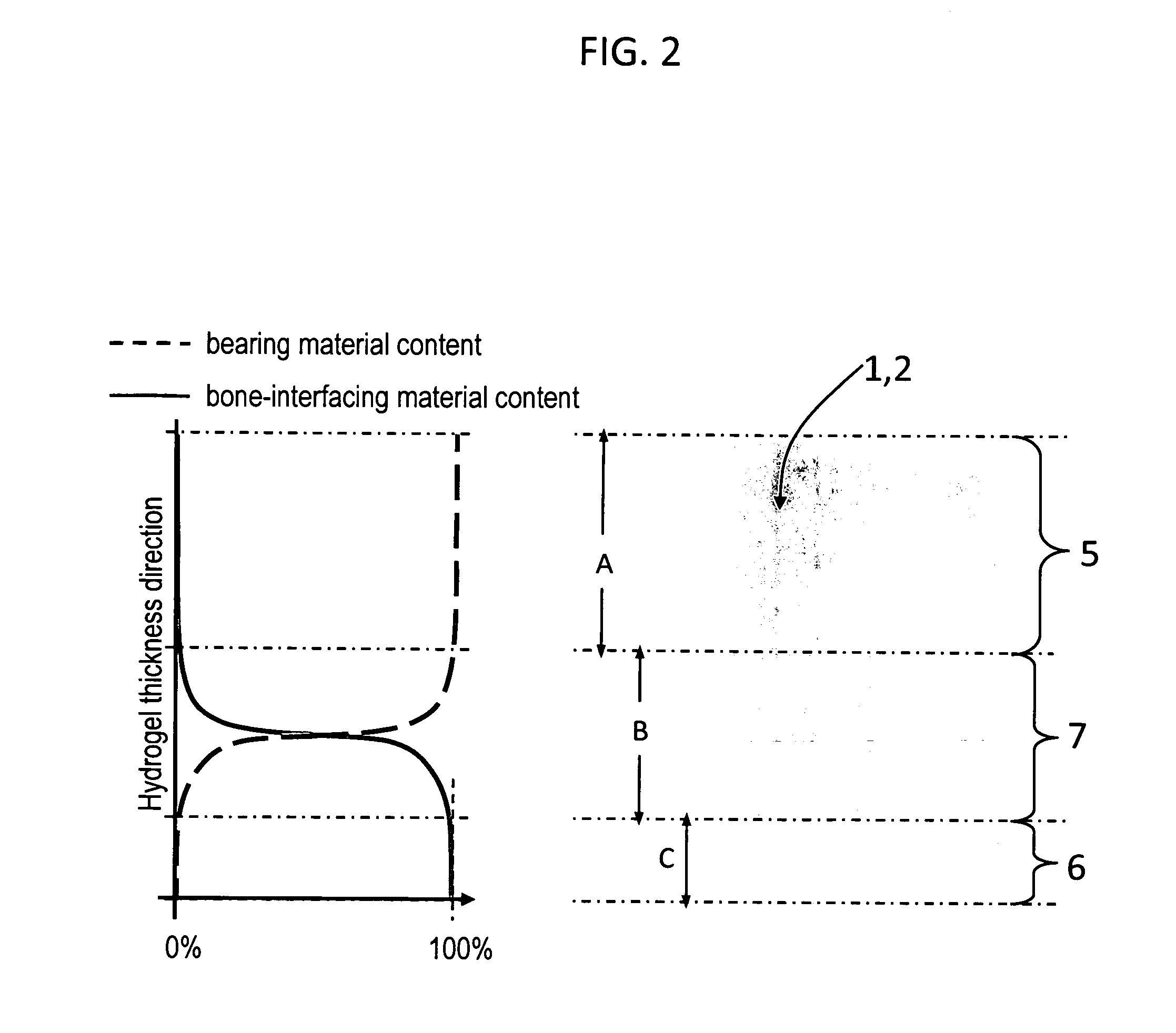
Hydrogel arthroplasty device
InactiveUS20090088846A1Stimulate bone cell growthEasy adhesionFinger jointsPowder deliveryCross-linkNeutral ph
An arthroplasty device is provided having an interpenetrating polymer network (IPN) hydrogel that is strain-hardened by swelling and adapted to be held in place in a joint by conforming to a bone geometry. The strain-hardened IPN hydrogel is based on two different networks: (1) a non-silicone network of preformed hydrophilic non-ionic telechelic macromonomers chemically cross-linked by polymerization of its end-groups, and (2) a non-silicone network of ionizable monomers. The second network was polymerized and chemically cross-linked in the presence of the first network and has formed physical cross-links with the first network. Within the IPN, the degree of chemical cross-linking in the second network is less than in the first network. An aqueous salt solution (neutral pH) is used to ionize and swell the second network. The swelling of the second network is constrained by the first network resulting in an increase in effective physical cross-links within the IPN.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Biodegradable polyesters
InactiveUS6120895AMonocomponent copolyesters artificial filamentPolyurea/polyurethane coatingsFiberPolymer science
PCT No. PCT / EP97 / 04908 Sec. 371 Date Mar. 17, 1999 Sec. 102(e) Date Mar. 17, 1999 PCT Filed Sep. 19, 1997 PCT Pub. No. WO98 / 12242 PCT Pub. Date Mar. 20, 1998Biodegradable polyesters based on A) 95-99.99 mol % of at least one polyester A containing as monomeric building blocks of an acid component comprising a11) 20-95 mol % of at least one aliphatic or cycloaliphatic dicarboxylic acid or its ester-forming derivative and a12) 5-80 mol % of at least one aromatic dicarboxylic acid or its ester-forming derivative and at least one dihydroxy compound or at least one amino alcohol or their mixtures, and B) 0.01-5 mol % of a mixture comprising mono-, bi-, tri-, tetra- and higher-nuclear isocyanurates or corresponding compounds containing two, three or four functional groups capable of reacting with the end groups of polyester A, or mixtures of the isocyanurates and the corresponding compounds, as well as molding compositions comprising said polyesters, their manufacture and their use in the manufacture of moldings, filsm, fibers and coatings.
Owner:BASF AG
Multi-purpose polymers, methods and compositions
Disclosed are multi-purpose alkali-swellable and alkali soluble associative polymers, which are the polymerization product of a monomer mixture comprising: (a) at least one acidic vinyl monomer; (b) at least one nonionic vinyl monomer; (c) a first associative monomer having a first hydrophobic end group; (d) a monomer selected from the group consisting of a second associative monomer having a second hydrophobic end, a semihydrophobic monomer and a combination thereof; and, optionally, (e) one or more crosslinking monomers or chain transfer agents. When monomer (d) is an associative monomer, the first and second hydrophobic end groups of monomers (c) and (d) have significantly different hydrophobic and / or steric character from one another. The multi-purpose associative polymers surprisingly provide desirable Theological and aesthetic properties in aqueous media.
Owner:INTERUNIVERSITAIR MICRO ELECTRONICS CENT (IMEC VZW) +1
Polycarbonate polyol compositions and methods
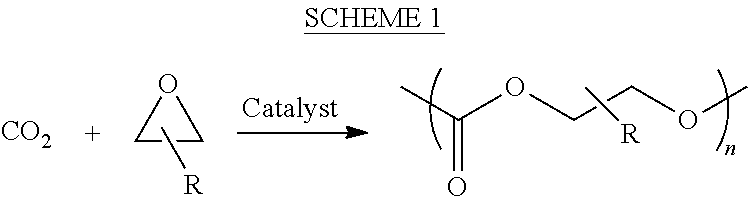
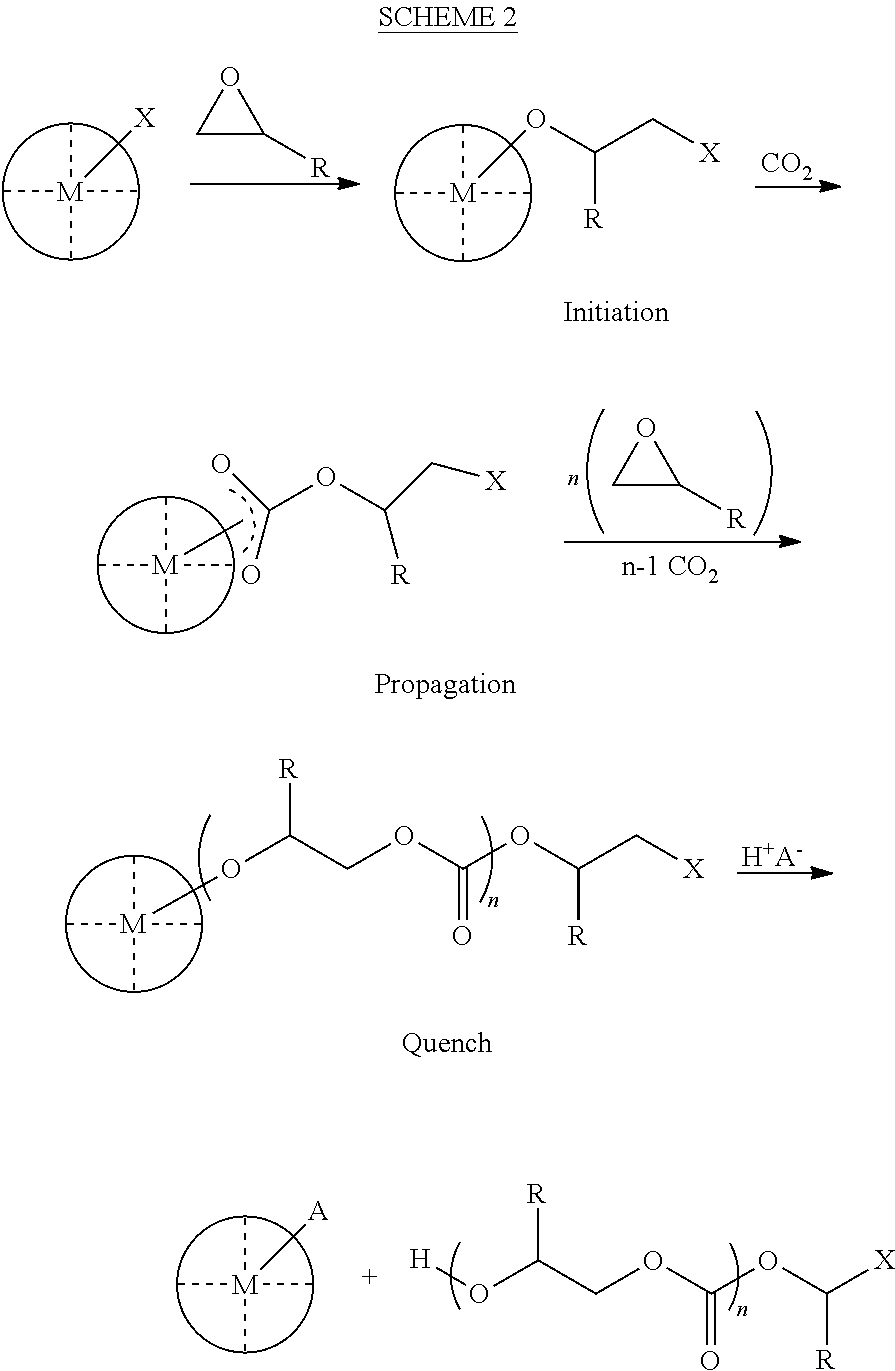
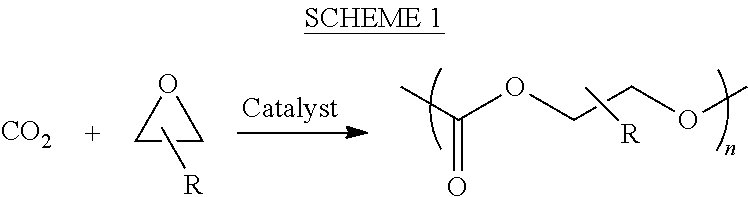
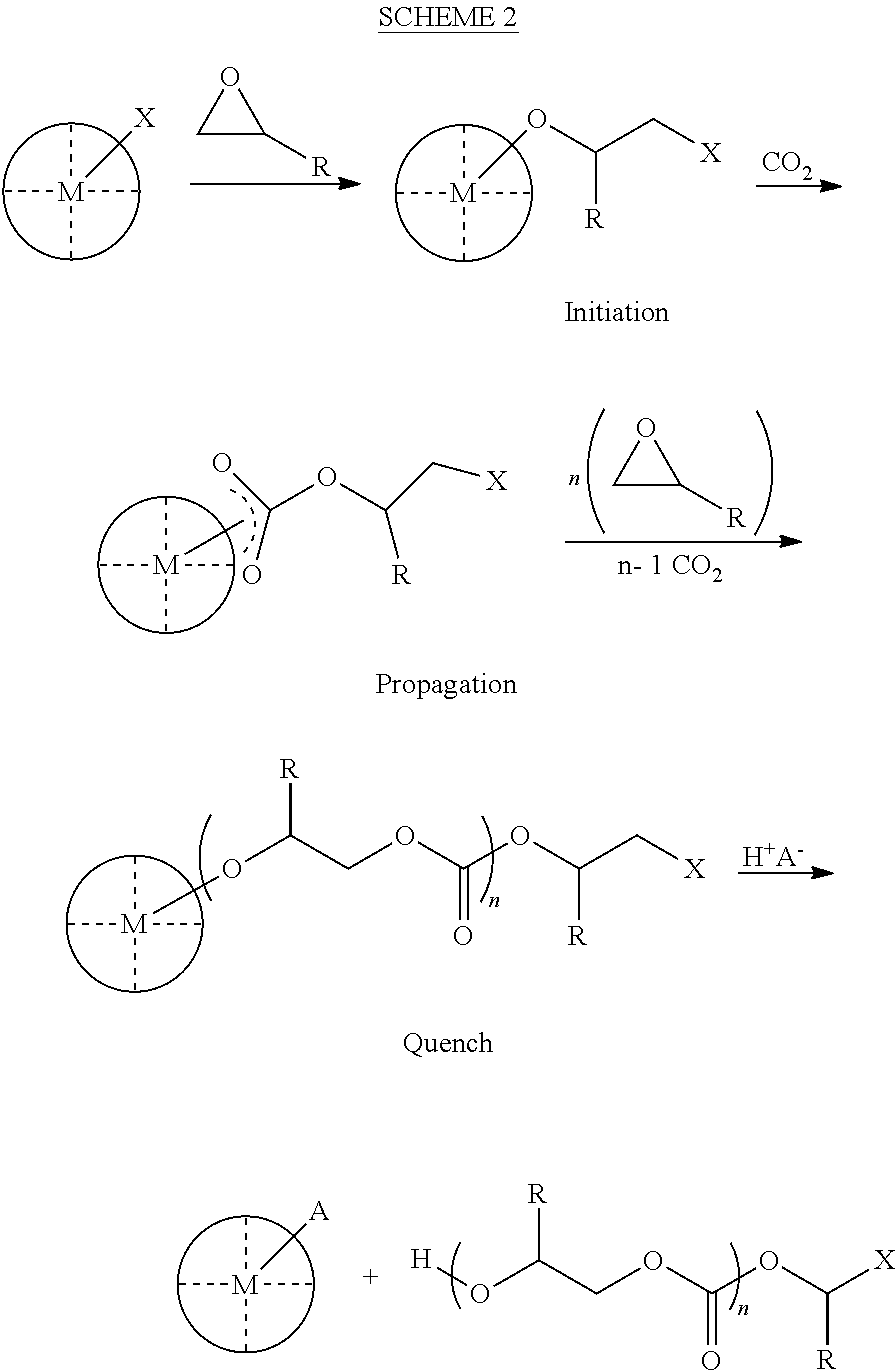
ActiveUS8247520B2Increase ratingsIncreases extentOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEnd-groupPolycarbonate
In one aspect, the present disclosure encompasses polymerization systems for the copolymerization of CO2 and epoxides comprising 1) a catalyst including a metal coordination compound having a permanent ligand set and at least one ligand that is a polymerization initiator, and 2) a chain transfer agent having two or more sites that can initiate polymerization. In a second aspect, the present disclosure encompasses methods for the synthesis of polycarbonate polyols using the inventive polymerization systems. In a third aspect, the present disclosure encompasses polycarbonate polyol compositions characterized in that the polymer chains have a high percentage of —OH end groups and a high percentage of carbonate linkages. The compositions are further characterized in that they contain polymer chains having an embedded polyfunctional moiety linked to a plurality of individual polycarbonate chains.
Owner:SAUDI ARAMCO TECH CO
Crosslinkable rubber compositions and use thereof
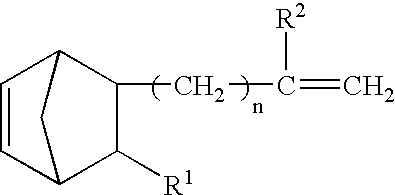
The crosslinkable rubber composition of the invention is crosslinkable by hot air, and a hot-air crosslinked rubber sheet thereof has no scratch on the surface in a hardness test using a pencil of HB and has a compression set of not more than 70% after a heat treatment at 150° C. for 22 hours. The rubber composition comprises an ethylene / α-olefin / non-conjugated polyene random copolymer rubber comprising a specific vinyl end group-containing norbornene compound, a SiH group-containing compound having at least two SiH groups in one molecule, and if necessary, an addition reaction catalyst comprising a platinum group element and a reaction inhibitor. The automobile weatherstrip, hose, rubber vibration insulator, belt, sealing material, expanded product, covered electric wire, electric wire joint, electric insulating part and household rubber product according to the invention comprise the above-mentioned rubber composition. The rubber composition has a high crosslinking rate and excellent productivity to produce crosslinked rubber molded products, is capable of undergoing hot-air crosslinking such as HAV or UHF and is capable of providing crosslinked rubber molded products having excellent compression set resistance, strength properties, heat resistance, weathering resistance and abrasion resistance.
Owner:MITSUI CHEM INC +1
Polycarbonate polyol compositions and methods
ActiveUS20110230580A1Efficient productionIncrease ratingsOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEnd-groupPolycarbonate
In one aspect, the present disclosure encompasses polymerization systems for the copolymerization of CO2 and epoxides comprising 1) a catalyst including a metal coordination compound having a permanent ligand set and at least one ligand that is a polymerization initiator, and 2) a chain transfer agent having two or more sites that can initiate polymerization. In a second aspect, the present disclosure encompasses methods for the synthesis of polycarbonate polyols using the inventive polymerization systems. In a third aspect, the present disclosure encompasses polycarbonate polyol compositions characterized in that the polymer chains have a high percentage of —OH end groups and a high percentage of carbonate linkages. The compositions are further characterized in that they contain polymer chains having an embedded polyfunctional moiety linked to a plurality of individual polycarbonate chains.
Owner:SAUDI ARAMCO TECH CO
Golf ball
ActiveUS20060135287A1Improved combination of characteristicEasy to compressGolf ballsSolid ballsElastomerEnd-group
An elastomeric composition for forming a golf ball or a component thereof is disclosed that includes the use of a non-conjugated diene monomer having two or more vinyl (CH2═CH—) terminal end groups. The composition produces a molded product exhibiting an enhanced combination of increased compression (i.e., softness) and resilience (C.O.R.).
Owner:TOPGOLF CALLAWAY BRANDS CORP
Photoresist polymer compositions
ActiveUS20060257781A1Overcomes drawbackPhotosensitive materialsPhotomechanical apparatusPolymer scienceEnd-group
The present invention is directed to the preparation of photoresist polymers via living free radical polymerization techniques. Sterically bulky ester monomers are utilized as the polymerization components. Use of chain transfer agents is included in polymerization processing conditions. Cleavage of polymer terminal end groups that include a heteroatom are described.
Owner:FREESLATE +1
Polyisobutylene urethane, urea and urethane/urea copolymers and medical devices containing the same
Owner:CARDIAC PACEMAKERS INC
Golf Ball
ActiveUS20070173607A1Improved combination of characteristicEasy to compressGolf ballsSolid ballsElastomerEnd-group
An elastomeric composition for forming a golf ball or a component thereof is disclosed that includes the use of a non-conjugated diene monomer having two or more vinyl (CH2═CH—) terminal end groups. The composition produces a molded product exhibiting an enhanced combination of increased compression (i.e., softness) and resilience (C.O.R.).
Owner:TOPGOLF CALLAWAY BRANDS CORP
Method for end-capping polycarbonate resins and composition for use in same
A method for end-capping polycarbonate resins, comprising the step of processing a mixture comprising a polycarbonate having free hydroxyl-end groups and an end-capping reagent in a melt transesterification reaction to produce a polycarbonate resin, wherein the end-capping reagent comprises a mixture of:(a) at least one species of a symmetrical activated aromatic carbonate, and (b) at least one species of a symmetrical non-activated aromatic carbonate, whereby said end-capping reagent reacts with at least some of the free hydroxyl end-groups of the polycarbonate to produce an end-capped polycarbonate resin.
Owner:SABIC GLOBAL TECH BV
Reinforced poly(arylene ether)/polyamide composition
A composition comprises greater than or equal to 20 weight percent glass fiber, based on the total weight of the composition, and a compatibilized blend of a poly(arylene ether) and an aliphatic-aromatic polyamide. The polyamide is composed of dicarboxylic acid units comprising 60 to 100 mol % of terephthalic acid units and diamine units comprising 60 to 100 mol % of 1,9-nonanediamine units and / or 2-methyl-1,8-octanediamine units. The polyamide has an amine end group content greater than 45 micromoles per gram of polyamide. The composition has a heat distortion temperature (HDT) greater than or equal to 230° C. when measured according to ASTM D 648 at 1.8 megapascals (MPa)
Owner:SABIC INNOVATIVE PLASTICS IP BV
Method for end-capping polycarbonate resins and composition for use in same
A method for end-capping polycarbonate resins, comprising the step of processing a mixture comprising a polycarbonate having free hydroxyl-end groups and an end-capping reagent in a melt transesterification reaction to produce a polycarbonate resin, wherein the end-capping reagent comprises a mixture of: (a) at least one species of a symmetrical activated aromatic carbonate, and (b) at least one species of an optionally-substituted phenol, whereby said end-capping reagent reacts with at least some of the free hydroxyl end-groups of the polycarbonate to produce an end-capped polycarbonate resin.
Owner:SABIC GLOBAL TECH BV
Vinyl hyperbranched polymer with photographically useful end groups
InactiveUS6252025B1Photoprinting processesMulticolor photographic processingDendrimerPolymer science
Photographically useful materials are disclosed comprising a hyperbranched polymer segment and multiple pendant photographically useful groups. Such materials may be prepared by forming an active hyperbranched polymer segment with multiple functionalized end group sites, and reacting the active hyperbranched polymer segment with an active compound comprising a photographically useful group to form a hyperbranched polymer ended with photographically useful groups. The hyperbranched segment may comprise any kind of polymer segment with hyperbranched architecture, and the active end groups may comprise any kind of reactive site. The active hyperbranched polymer may comprise any kind of other functional groups which are located in either backbone or the ends. The hyperbranched polymers containing photographically useful groups obtained in accordance with the invention are particularly advantageous in that they enable polymer structures comprising components exhibiting different photographically useful properties, while maintaining relatively low intrinsic viscosities compared to non-hyperbranched polymers containing photographically useful groups of similar chemical compositions. Additionally, the hyperbranched polymers are advantageous with respect to dendrimer type polymers in that a wide variety of hyperbranched polymer compositions may be synthesized in accordance with commercially acceptable processes.
Owner:EASTMAN KODAK CO
Method of preventing or reducing aluminosilicate scale in a bayer process
ActiveUS20050010008A2Reduce and eliminate aluminosilicate scalingReduce and even completely prevent formationSilicon organic compoundsAluminium compoundsPlate heat exchangerEnd-group
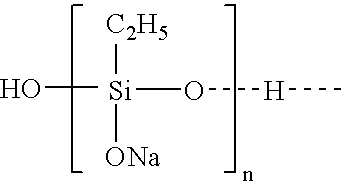
Abstract of the DisclosureMaterials and a process are provided whereby polymers with the pendant group or end group containing --Si(OR")3 (where R" is H, an alkyl group, Na, K, or NH4) are used to control aluminosilicate scaling in a Bayer process. When materials of the present invention are added to the Bayer liquor before the heat exchangers, they reduce and even completely prevent formation of aluminosilicate scale on heat exchanger walls. The present materials are effective at treatment concentrations that make them economically practical.
Owner:CYTEC TECH CORP
Silica-reinforced rubber compounded with blocked mercaptosilanes and alkyl alkoxysilanes
The invention provides a sulfur vulcanizable silica-reinforced elastomeric compound having improved tensile mechanical and dynamic viscoelastic properties. The compounds are formed by mixing an elastomer optionally having an alkoxysilane terminal group, with silica in the presence of an alkyl alkoxysilane and a blocked mercaptosilane. In particular, the mercaptosilane moiety of the blocked mercaptosilane and the alkyl alkoxysilane are present in a weight ratio of about 0.001:1 to about 0.20:1. Preferably, the blocked mercaptosilane and the alkyl alkoxysilane are compounded with the elastomer and the silica at high temperature to facilitate the silica-silane reaction. A deblocking agent is added at any desired mixing stage, optionally with the cure package, to allow binding of the mercaptosilane to the polymer.
Owner:BRIDGESTONE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com