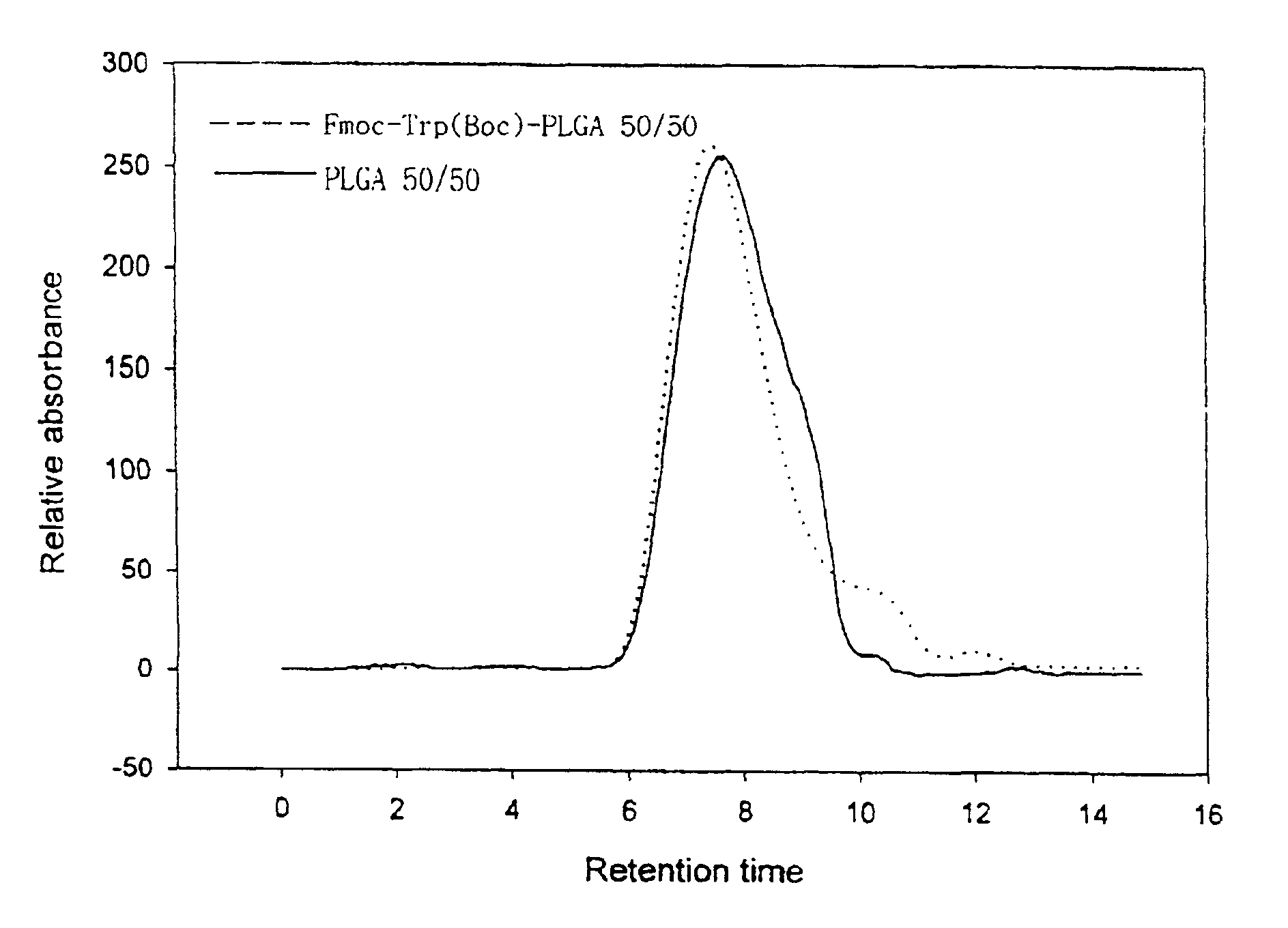
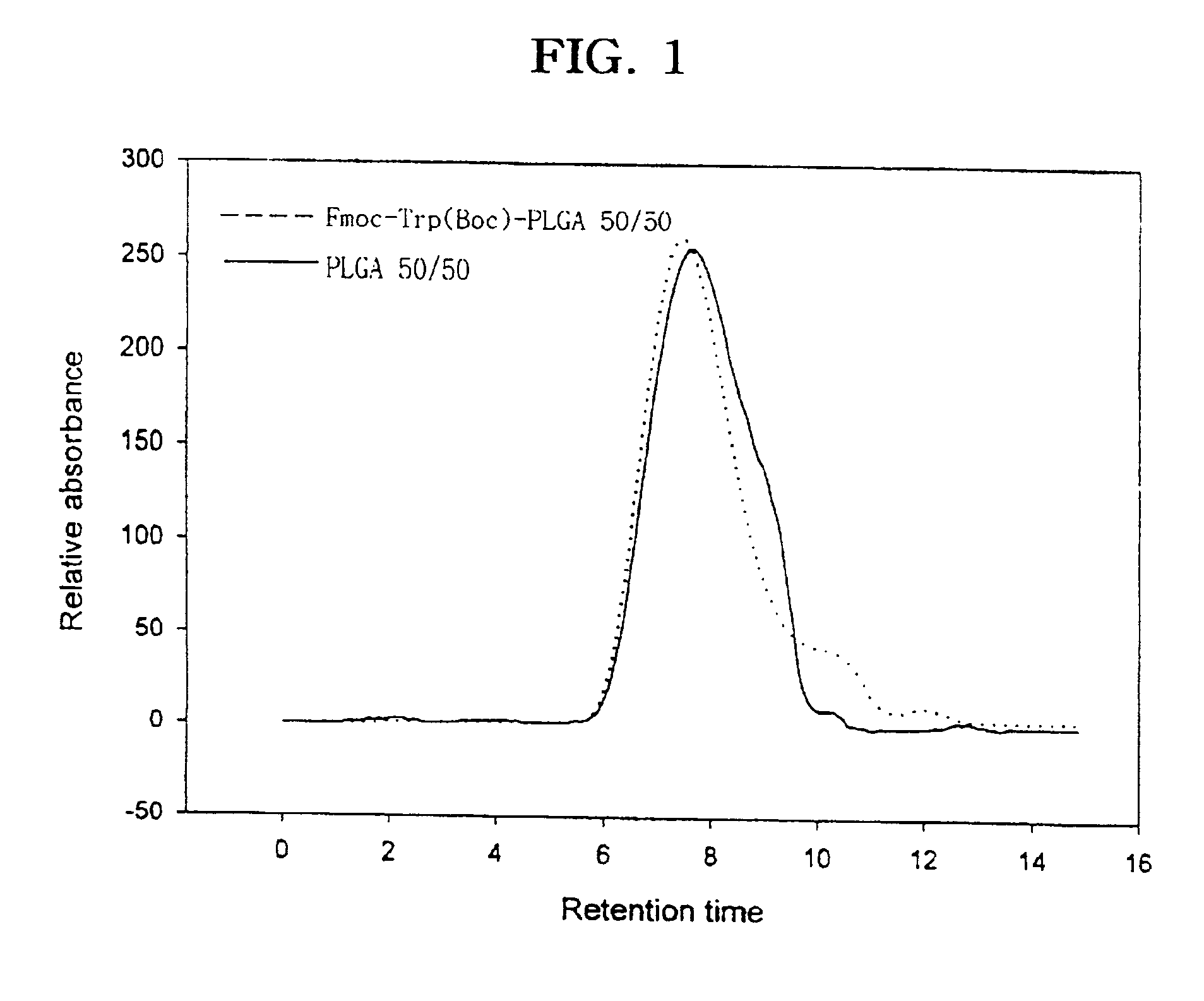
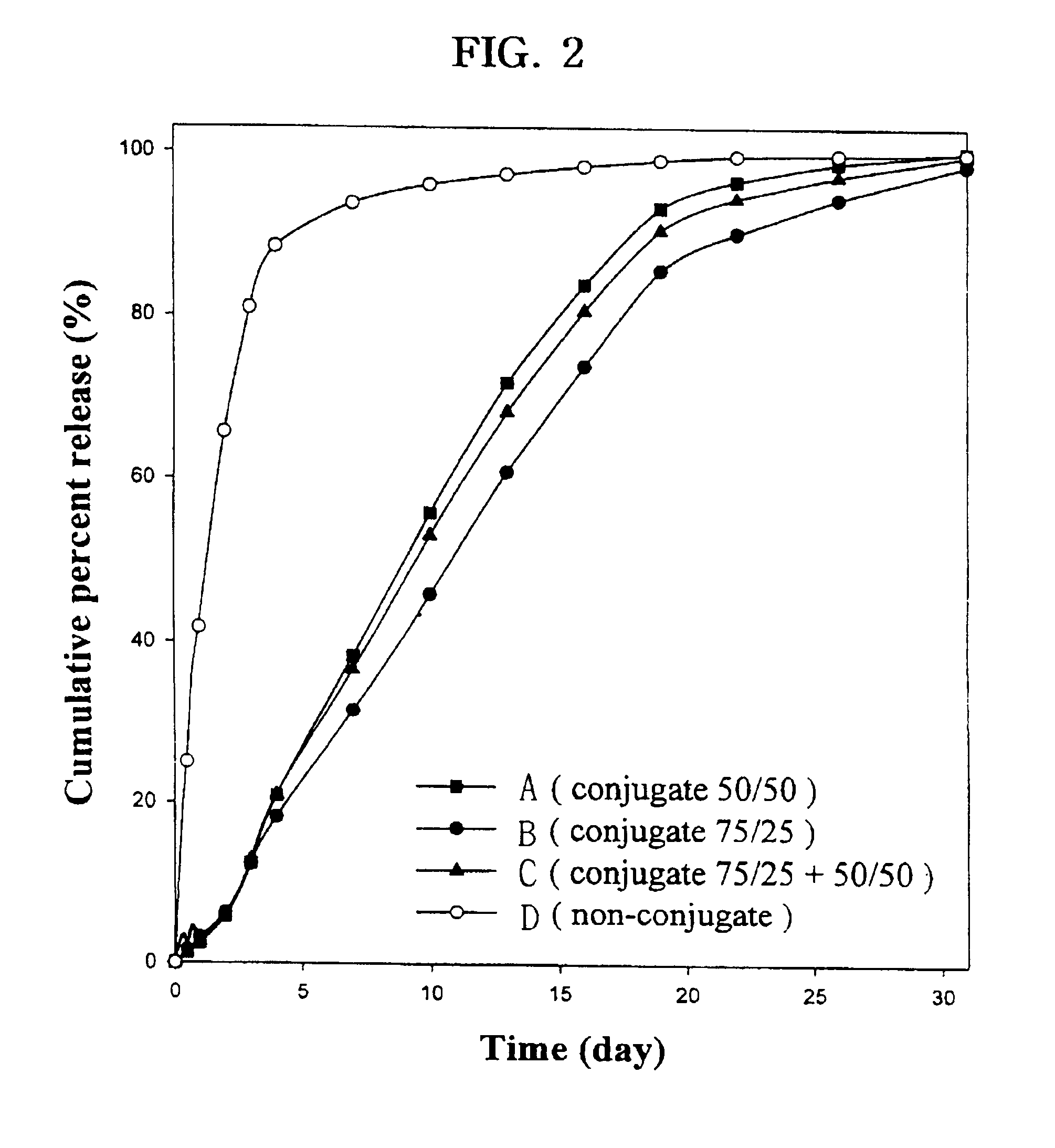
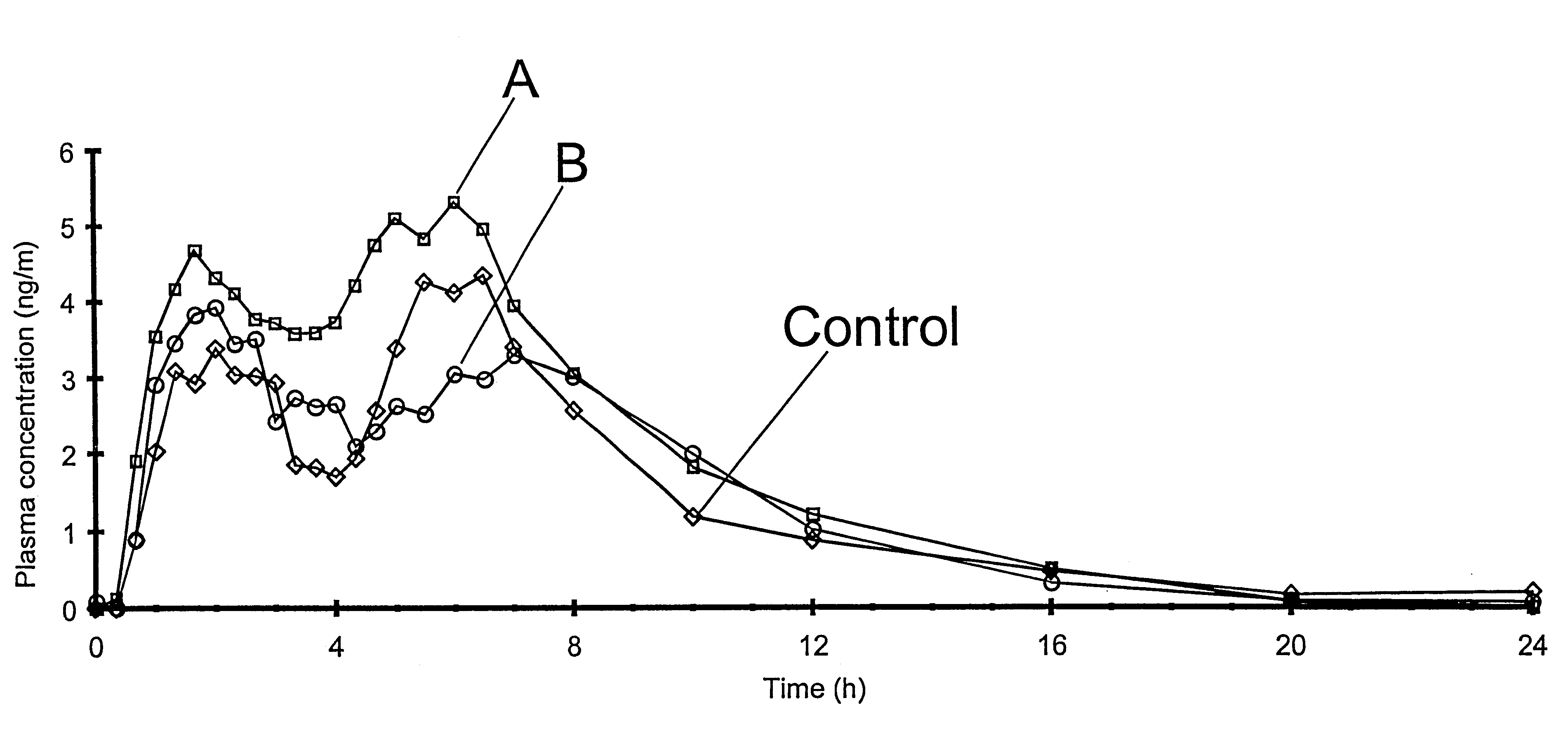
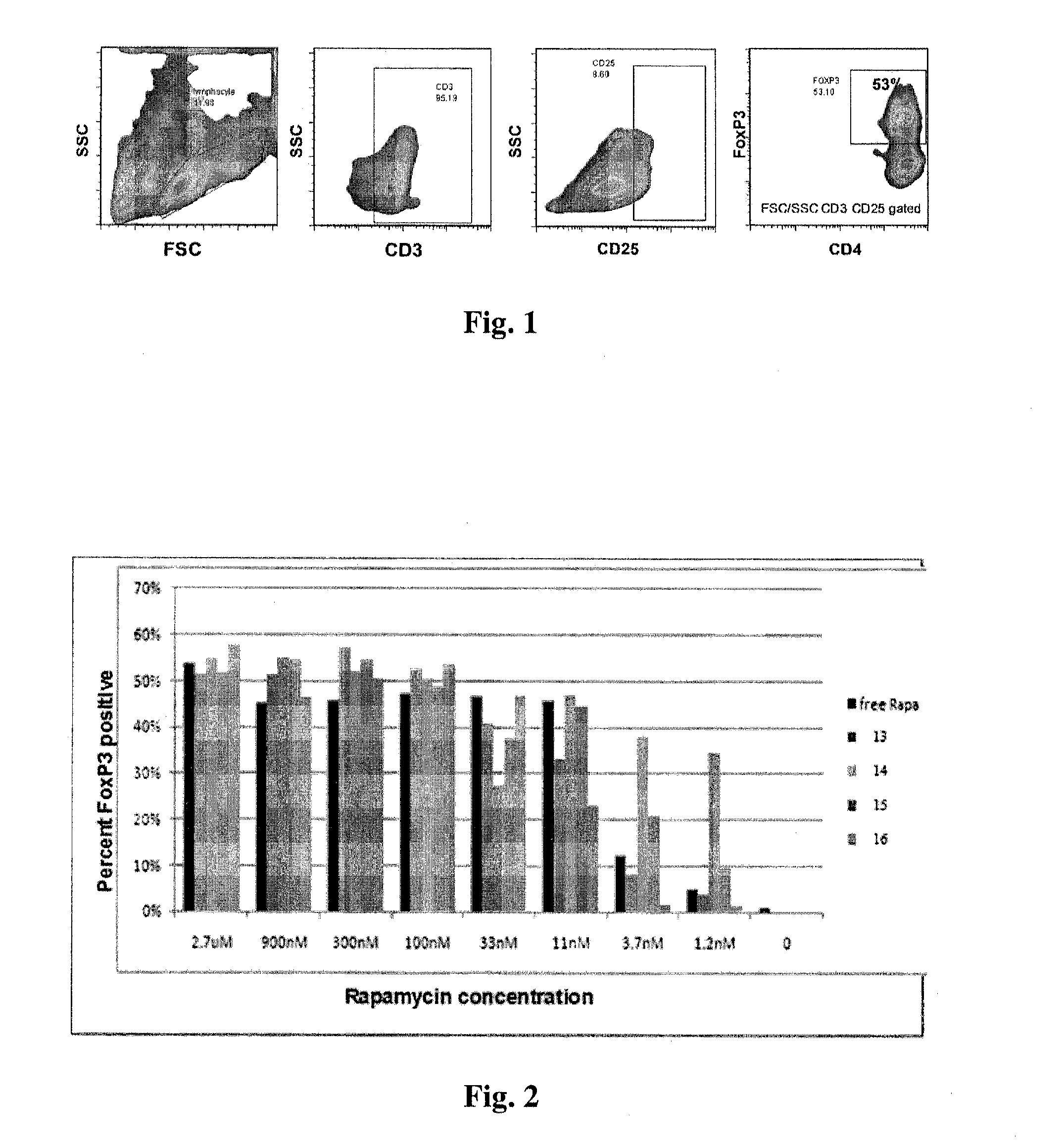
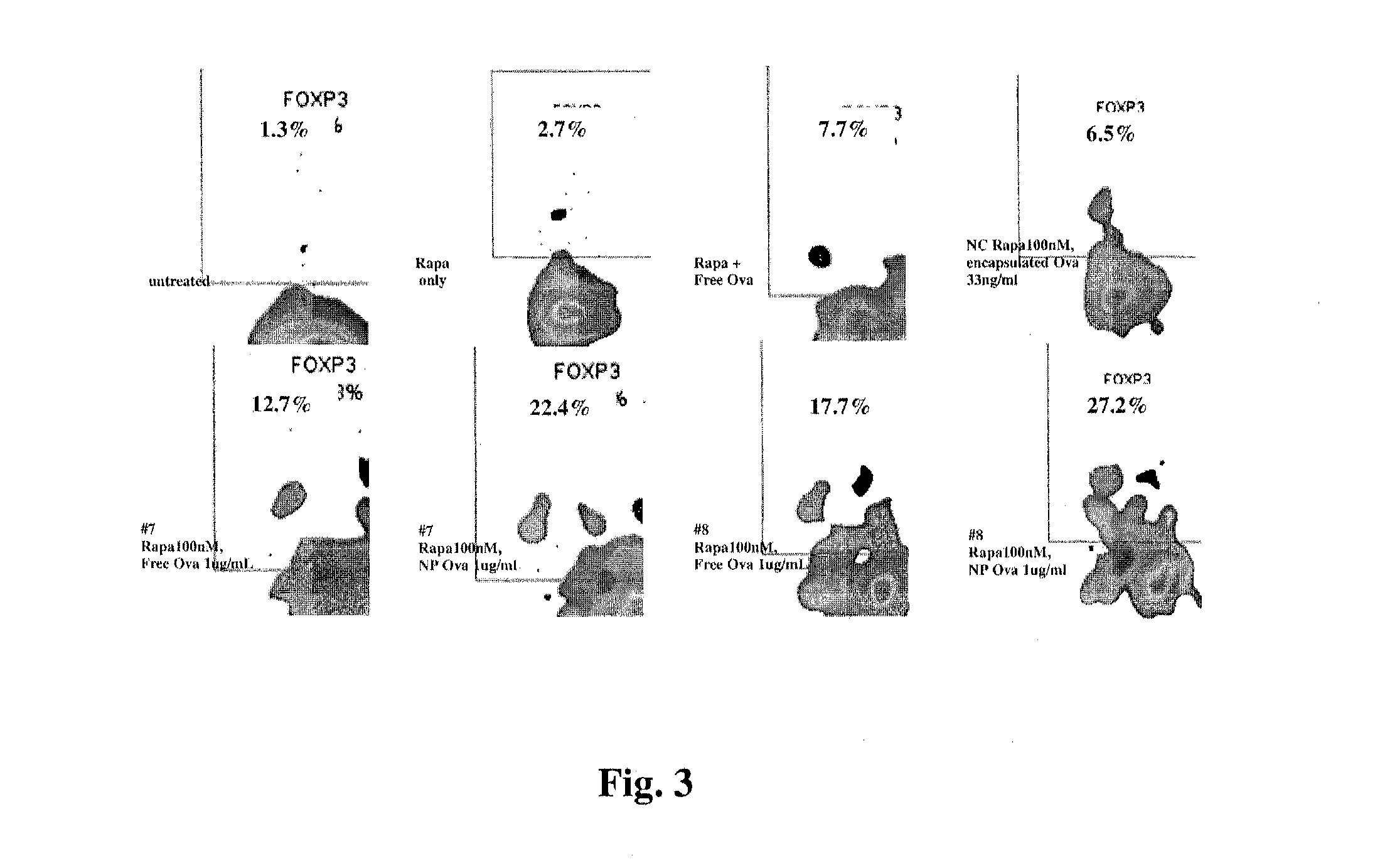
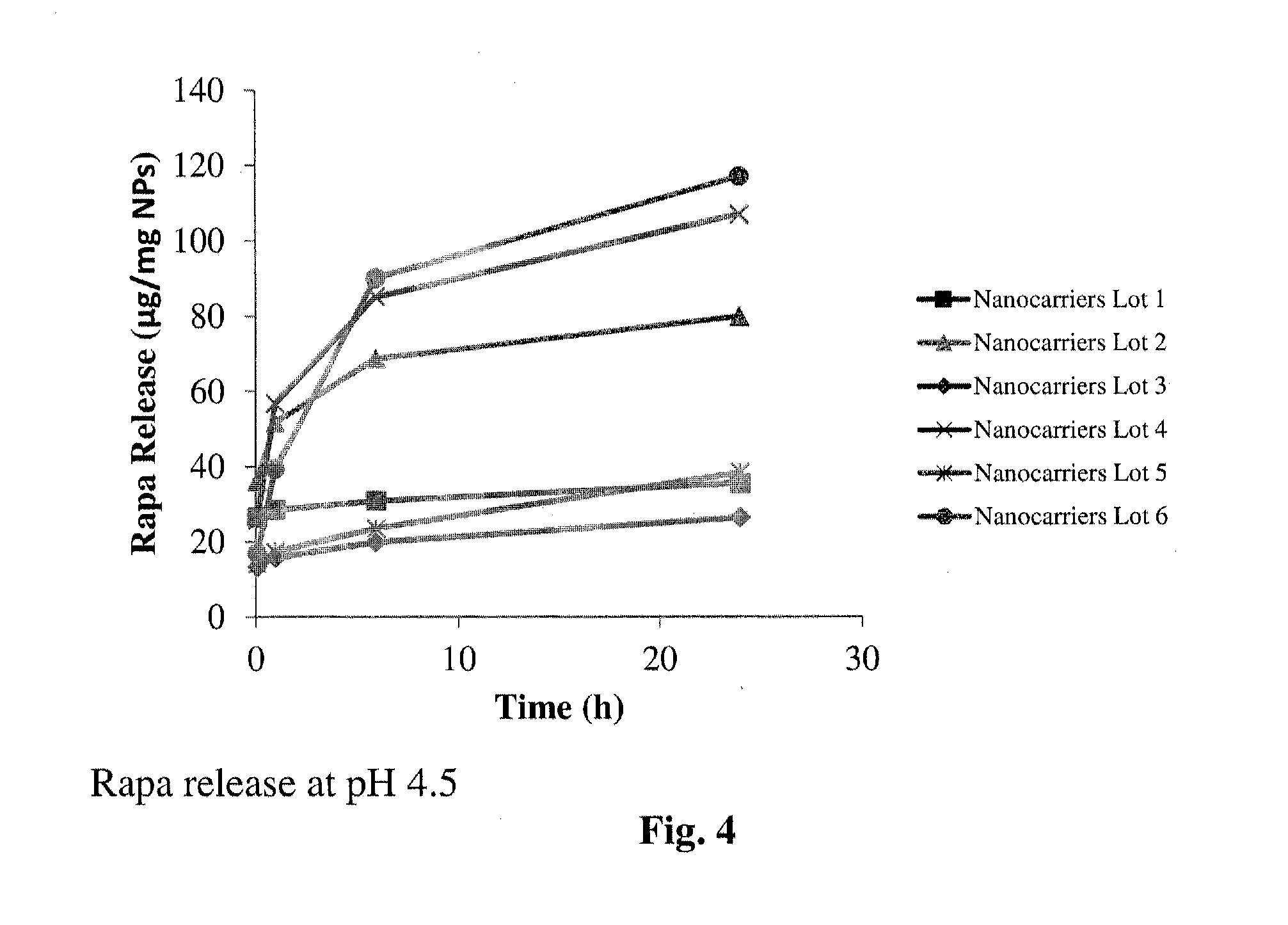
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41744results about "Powder delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
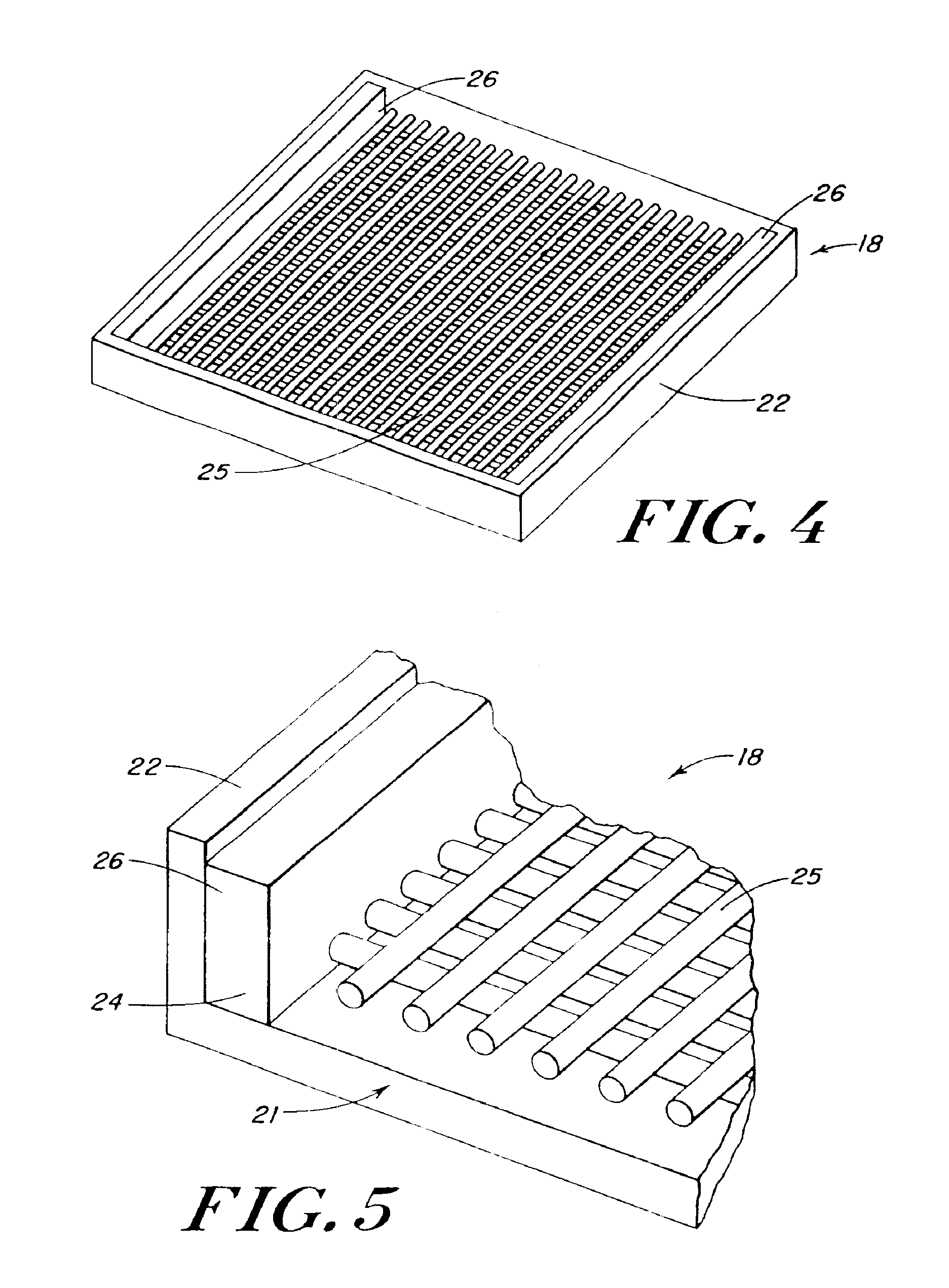
Foam composite for the repair or regeneration of tissue
InactiveUS6306424B1Variable mechanical strengthFacilitate cell migrationPowder deliveryPeptide/protein ingredientsTissue engineeringFibrous layer
The present patent describes a biocompatible composite made of a first fibrous layer attached to a three-dimensional inter-connected open cell porous foams that have a gradient in composition and / or microstructure through one or more directions. These composites can be made from blends of absorbable and biocompatible polymers. These biocompatible composites are particularly well suited to tissue engineering applications and can be designed to mimic tissue transition or interface zones.
Owner:ETHICON ENDO SURGERY INC
Biointerface membranes incorporating bioactive agents
InactiveUS20050031689A1Improve performancePowder deliveryAdditive manufacturing apparatusBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM
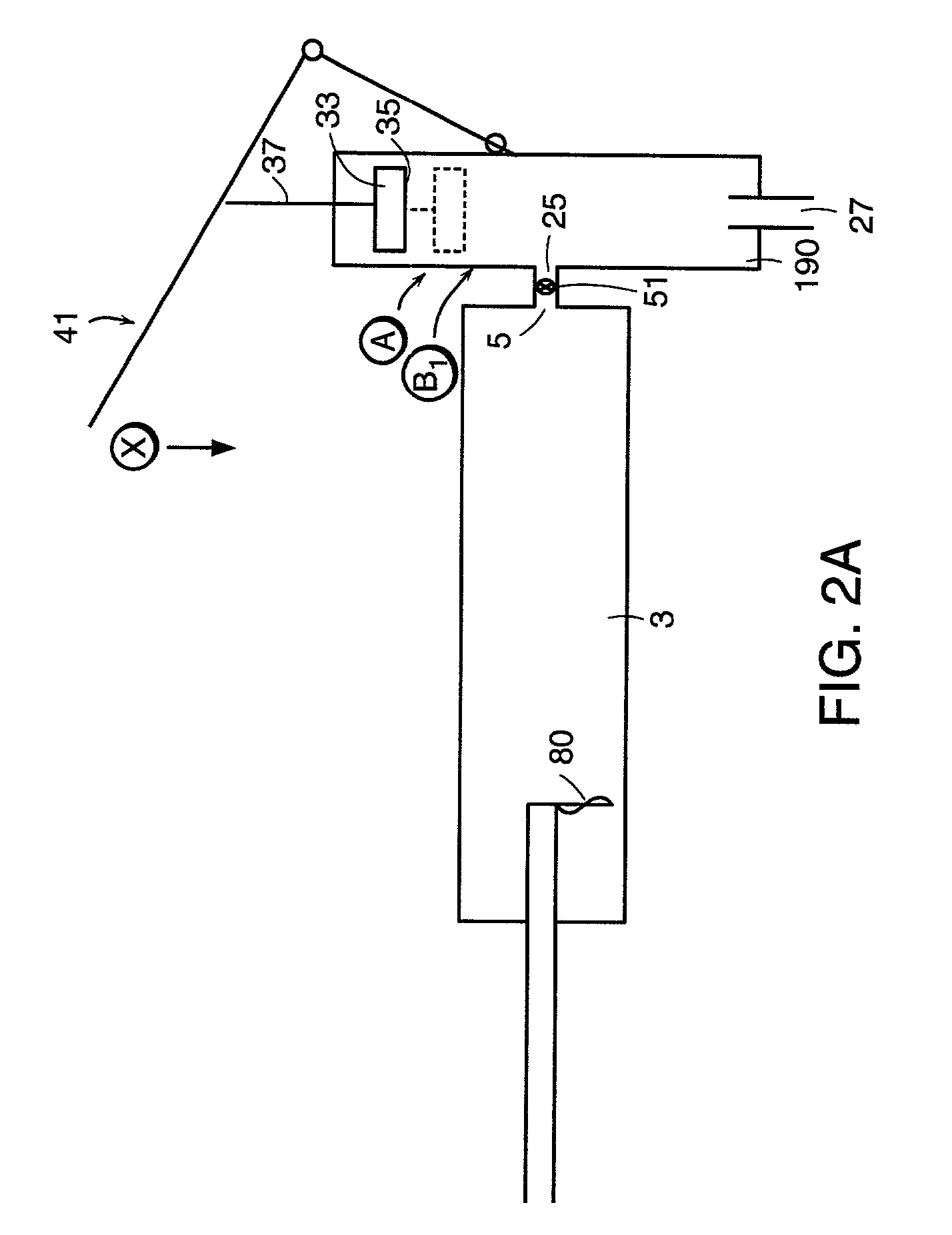
Vertebroplasty injection device
InactiveUS7008433B2Small sizeIncrease pressurePowder deliveryShaking/oscillating/vibrating mixersInjectable biomaterialBone cement
This invention relates to a mixing and delivery device suitable for delivering injectable biomaterials, and to preferred bone cement formulations.
Owner:DEPUY ACROMED INC
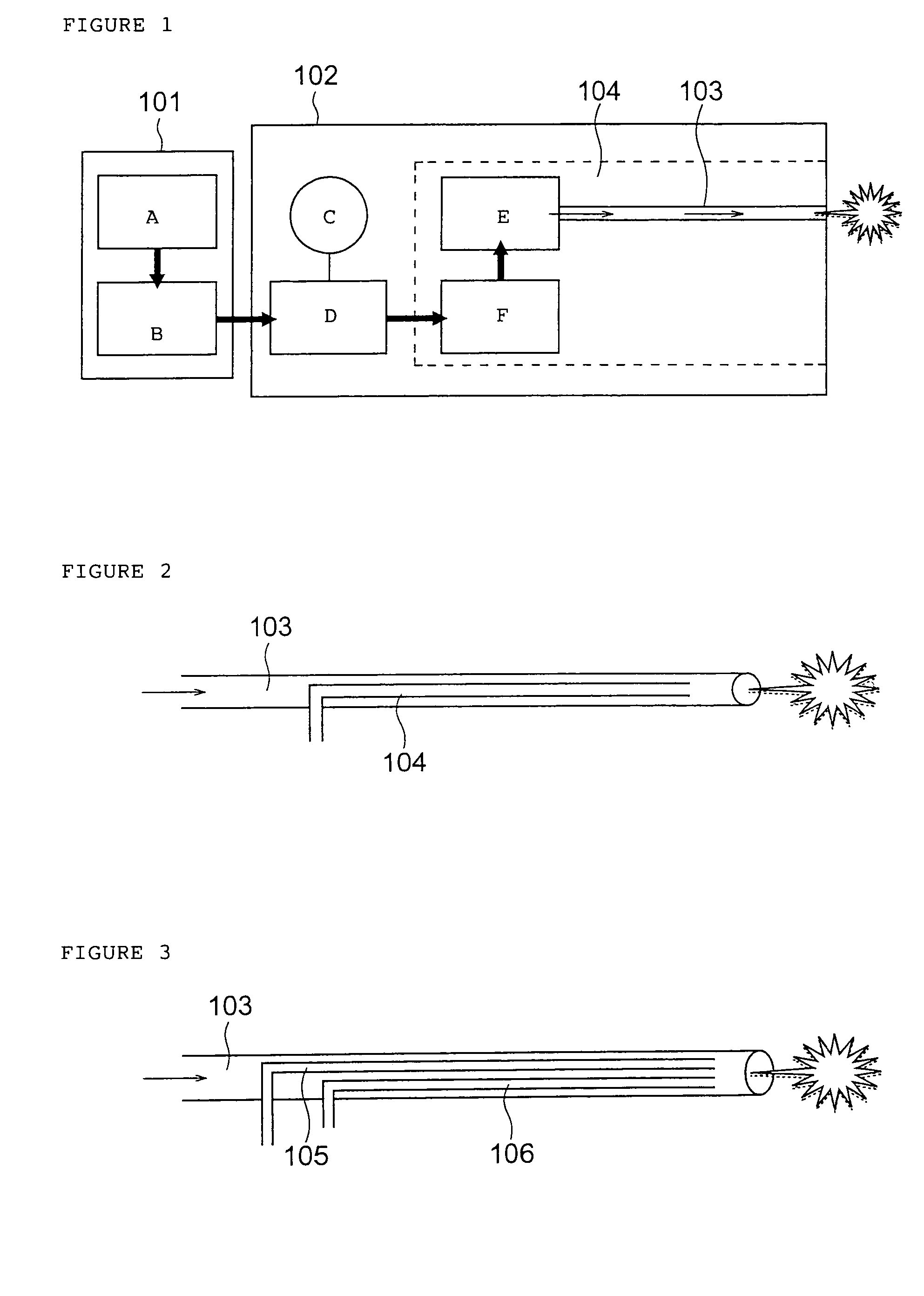
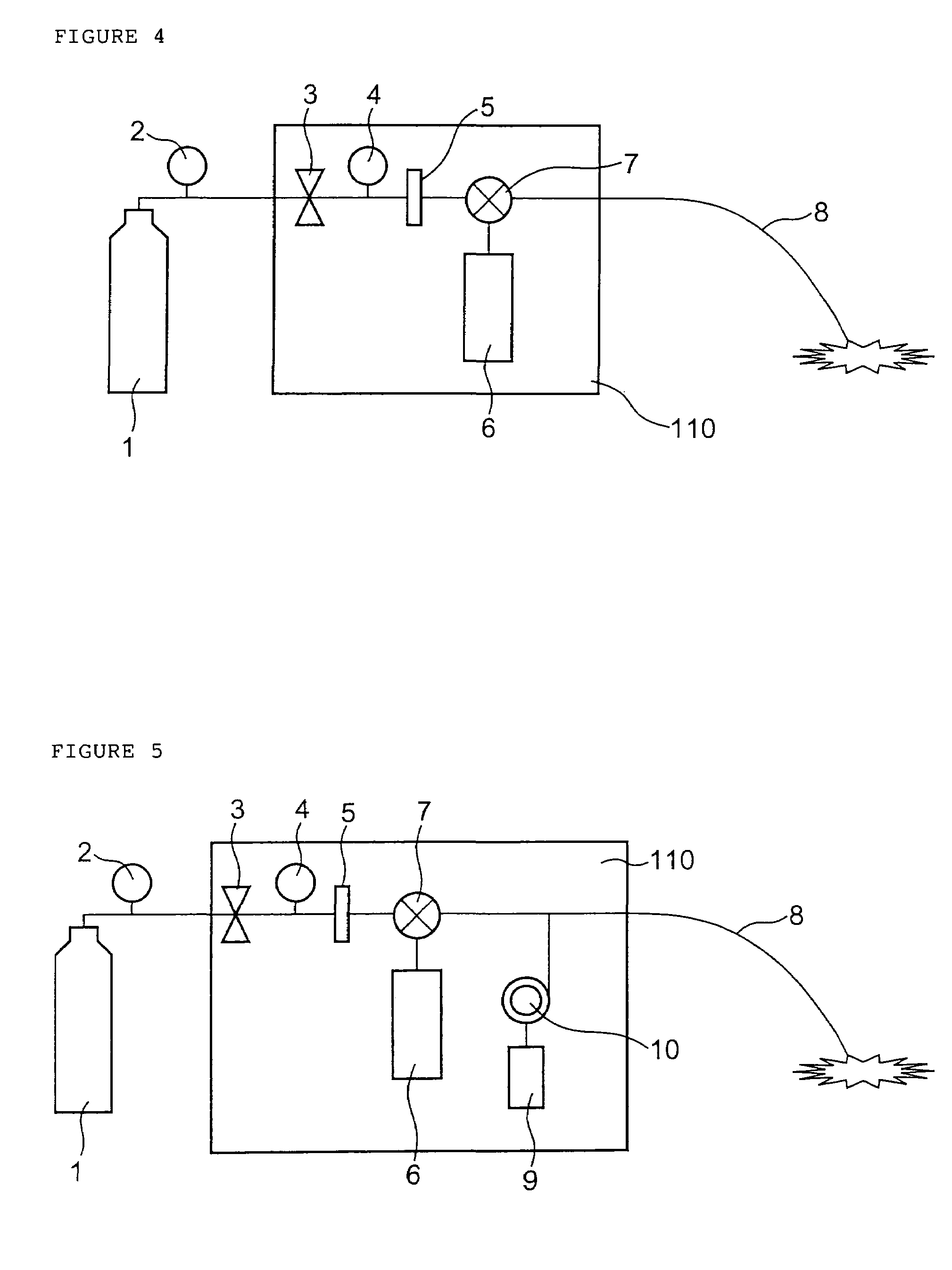
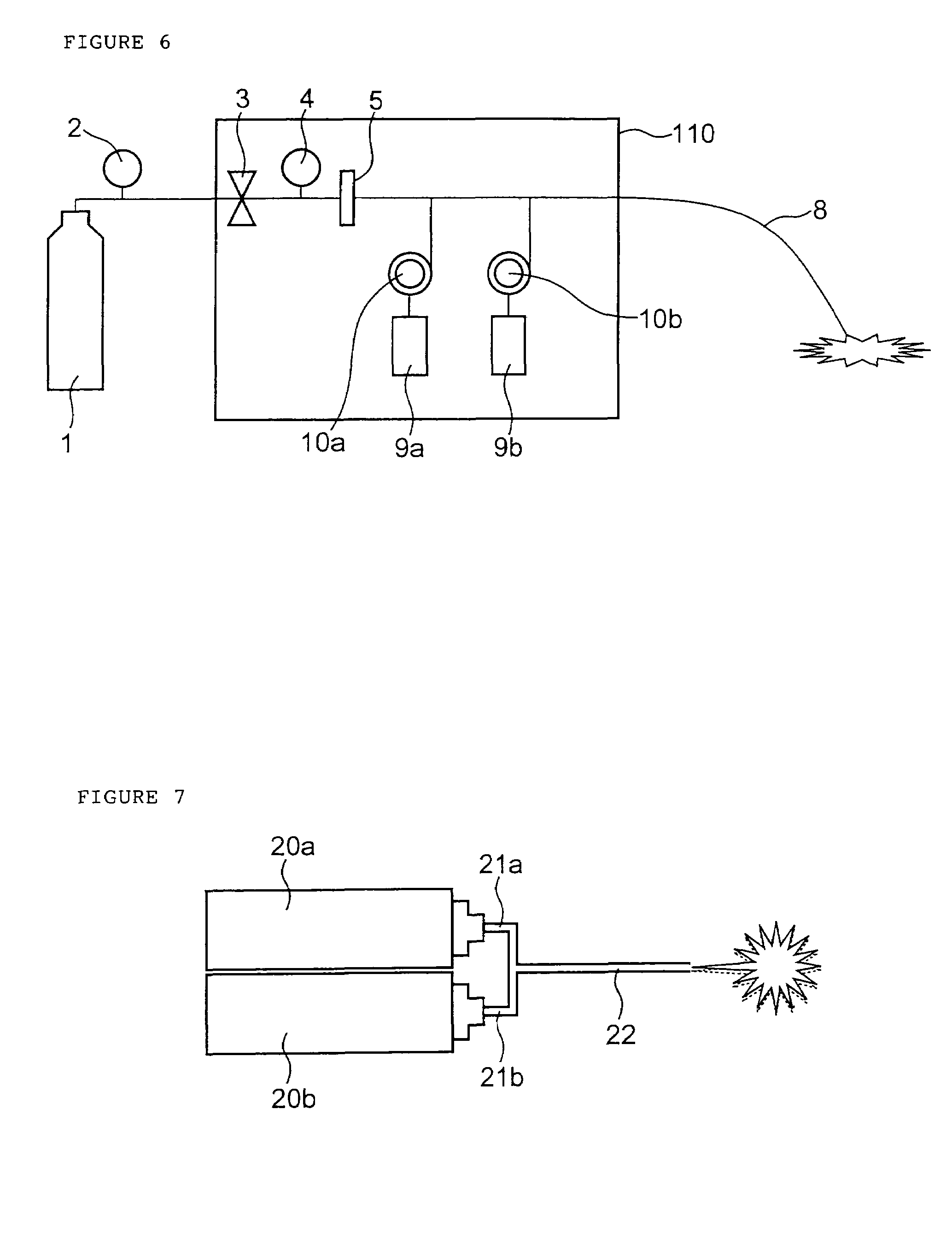
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
Extraction of solvents from drug containing polymer reservoirs
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Hemostatic compositions for arresting blood flow from an open wound or surgical site
A hemostatic composition for stopping or decreasing blood flow from an open wound or medical or surgical procedure. Compositions of the invention comprise a mixture of a cationic polymer and a cation exchange material. In one embodiment, the composition comprises a mixture: (1) a high molecular weight copolymer of diallyl dimethyl ammonium chloride (DADMAC) and acrylamide [DADMAC copolymer], and (2) the hydrogen form of a crosslinked, sulfonated polystyrene (hydrogen resin). In an exemplified embodiment, a composition of the invention comprises the mixture of DADMAC copolymer and hydrogen resin provided in a dry powdered form. The compositions of the invention may be applied directly to a wound or treatment site, or they may be incorporated into a wound dressing, such as a bandage. The seal formed at a wound or treatment site treated with the present invention is adhesive and exhibits considerable toughness.
Owner:BIOLIFE
Reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
InactiveUS6852330B2Sufficient structural integritySufficient propertyPowder deliveryOrganic active ingredientsTissue repairSoft tissue repair
A biocompatible tissue repair stimulating implant or “scaffold” device, and methods for making and using such a device, are provided. The implant includes one or more layers of a bioabsorbable polymeric foam having pores with an open cell pore structure. A reinforcement component is also present within the implant to contribute enhanced mechanical and handling properties. The implant houses a biological component that may be released to tissue adjacent the location in which the implant is implanted to faciliate and / or expedite the healing of tissue. This biological component resides primarily within the foam component of the implant, being incorporated within pores formed within the foam.
Owner:DEPUY SYNTHES PROD INC
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
Fiber strand and implantable supporting body having a fiber strand
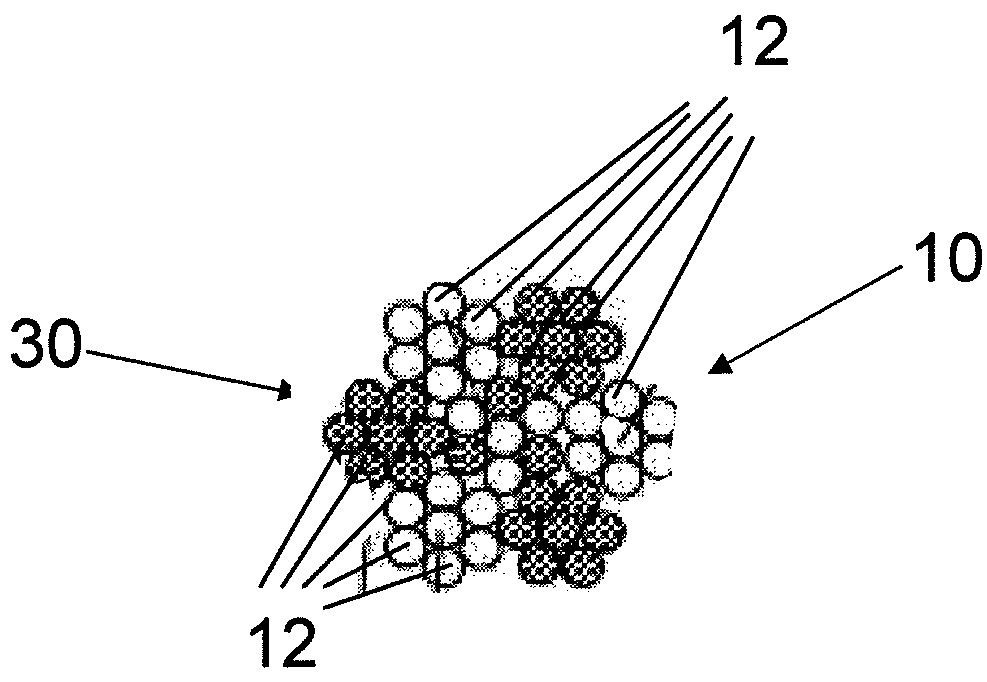
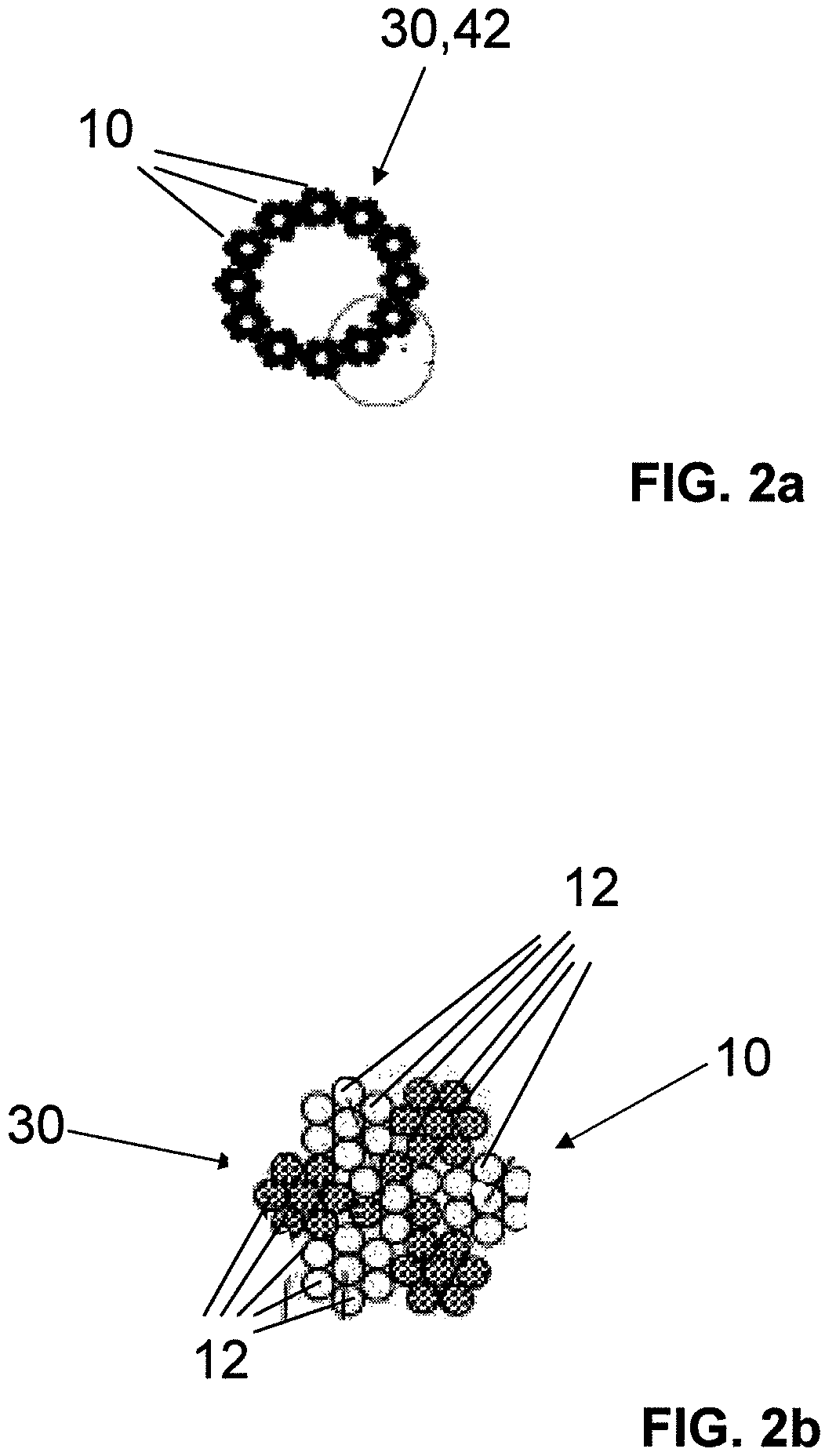
InactiveUS7997054B2Desired mechanical properties can be adjusted especially easilySignificant positive effectPowder deliveryStentsFiberBiomedical engineering
The invention relates to a fiber strand (10) for an implantable supporting body (100) comprising at least two individual fibers (12). The at least two individual fibers (12) are each shorter in their longitudinal extent than the longitudinal extent (14) of the fiber strand, and in their transverse extent they are each thinner than the transverse extent (16) of the fiber strand.
Owner:BIOTRONIK AG
Method of producing a sustained-release preparation
InactiveUS6197350B1Reduce the number of stepsSuitable for industrializationPowder deliveryPeptide/protein ingredientsBlood concentrationOrganic solvent
A method of producing sustained-release microcapsules which comprises dispersing a physiologically active polypeptide into a solution of a biodegradable polymer and zinc oxide in an organic solvent, followed by removing the organic solvent; which provides a sustained-release preparation showing a high entrapment ratio of the physiologically active polypeptide and its constant high blood concentration levels over a long period of time.
Owner:TAKEDA PHARMA CO LTD
Gel suitable for implantation and delivery system
InactiveUS20050186240A1Readily and quickly rehydratableFacilitates cellular ingrowthPowder deliveryProsthesisHigh concentrationSolvation
The invention concerns a dried form of a porous polymer gel material which may be rehydrated and placed under pressure or compression to induce solvation, thereby forming a high concentration gel, in the form of an injectable viscous putty or dough, which may be implantated in the body.
Owner:KENSEY NASH CORP
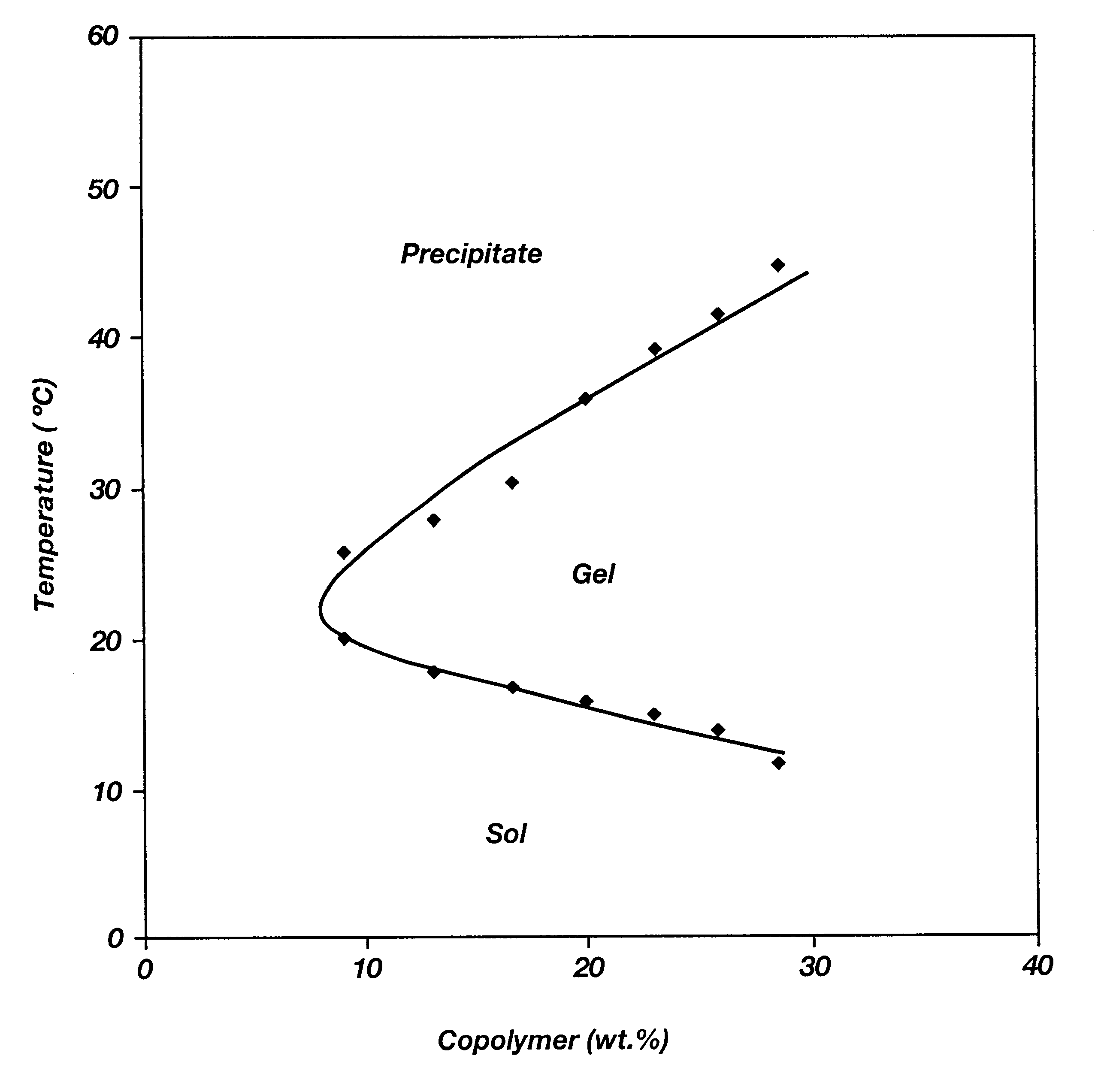
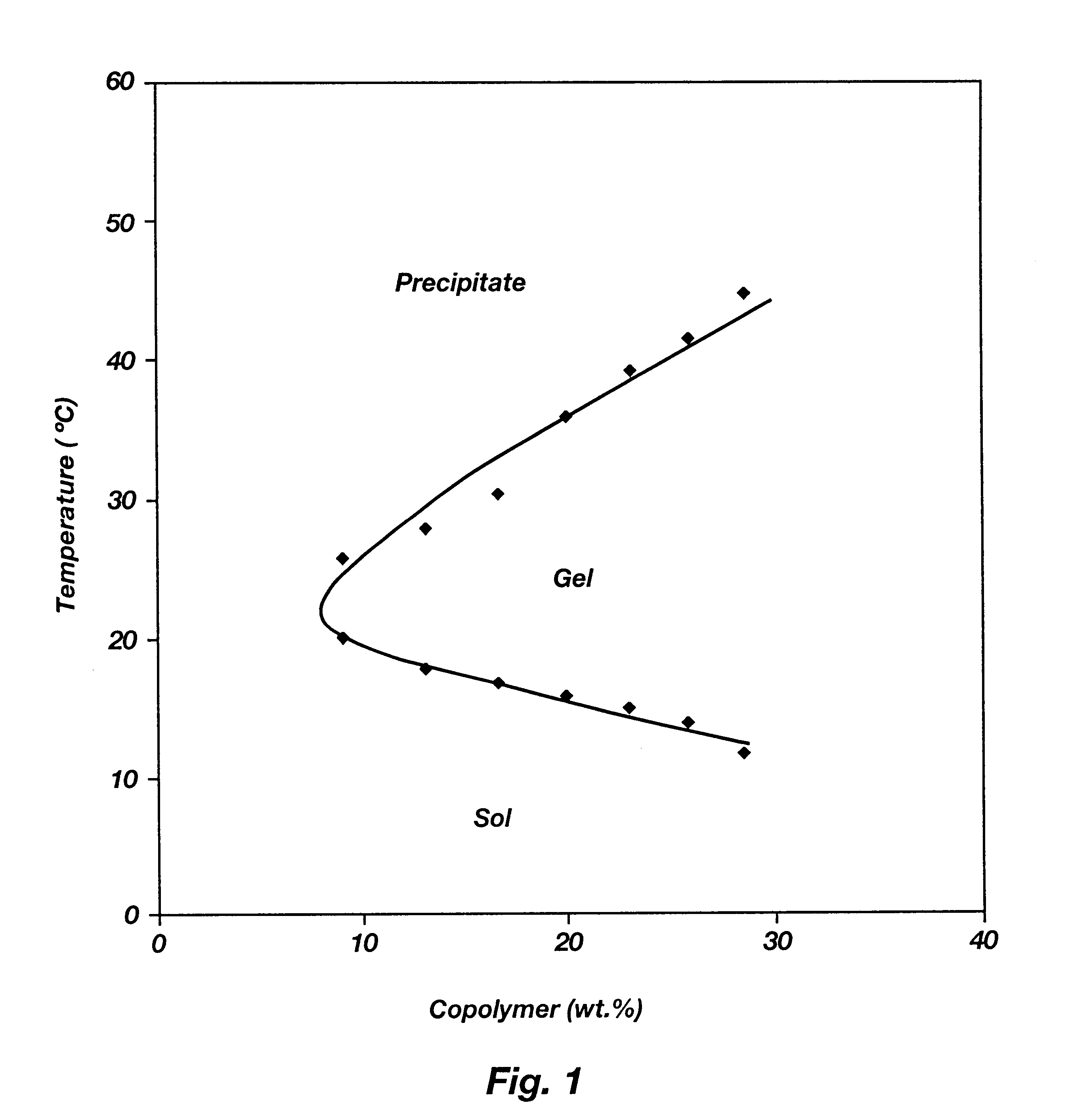
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Tissue electroperforation for enhanced drug delivery
The present invention relates to a method and a device for transporting a molecule through a mammalian barrier membrane of at least one layer of cells comprising the steps of: ablating the membrane with an electric current from a treatment electrode; and utilizing a driving force to move the molecule through the perforated membrane.
Owner:LIFESCAN INC
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
Multiparticulate modified release composition
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
Methods and compositions for therapeutic use of RNA interference
InactiveUS20030157030A1Effective amountImprove angiogenesisOrganic active ingredientsPowder deliveryGene expressionSmall interfering RNA
Abstract of Disclosure The present invention provides methods and compositions for attenuating expression of a target gene in vivo. In general, the method includes administering RNAi constructs (such as small-interfering RNAs (i.e., siRNAs) that are targeted to particular mRNA sequences, or nucleic acid material that can produce siRNAs in a cell), in an amount sufficient to attenuate expression of a target gene by an RNA interference mechanism, e.g., in a sequence-dependent, PKR-independent manner. In particular, the subject method can be used to alter the growth, survival or differentiation of cells for therapeutic and cosmetic purposes.
Owner:INSERT THERAPEUTICS INC
Method for producing microparticles loaded with proteins
InactiveUS7335401B2Improve bindingLow degreePowder deliveryLiquid surface applicatorsMicroparticleChemistry
The present invention concerns a method for producing microparticles loaded with proteins which is characterized in that the microparticles are loaded in suspension under strongly alkaline conditions. The invention also concerns microparticles which can be produced using this method and their use in a binding test e.g. in an immunoassay.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Three dimensional cell protector/pore architecture formation for bone and tissue constructs
InactiveUS7713542B2Greatly multiplied in vitroProtection from damagePowder deliveryBiocideIn vivoLiving cell
Living cellular material is encapsulated or placed in a protective material (cell protector) which is biocompatible, biodegradable and has a three-dimensional form. The three dimensional form is incorporated into a matrix that maybe implanted in vivo, ultimately degrade and thereby by replaced by living cell generated material.
Owner:ADA FOUND
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
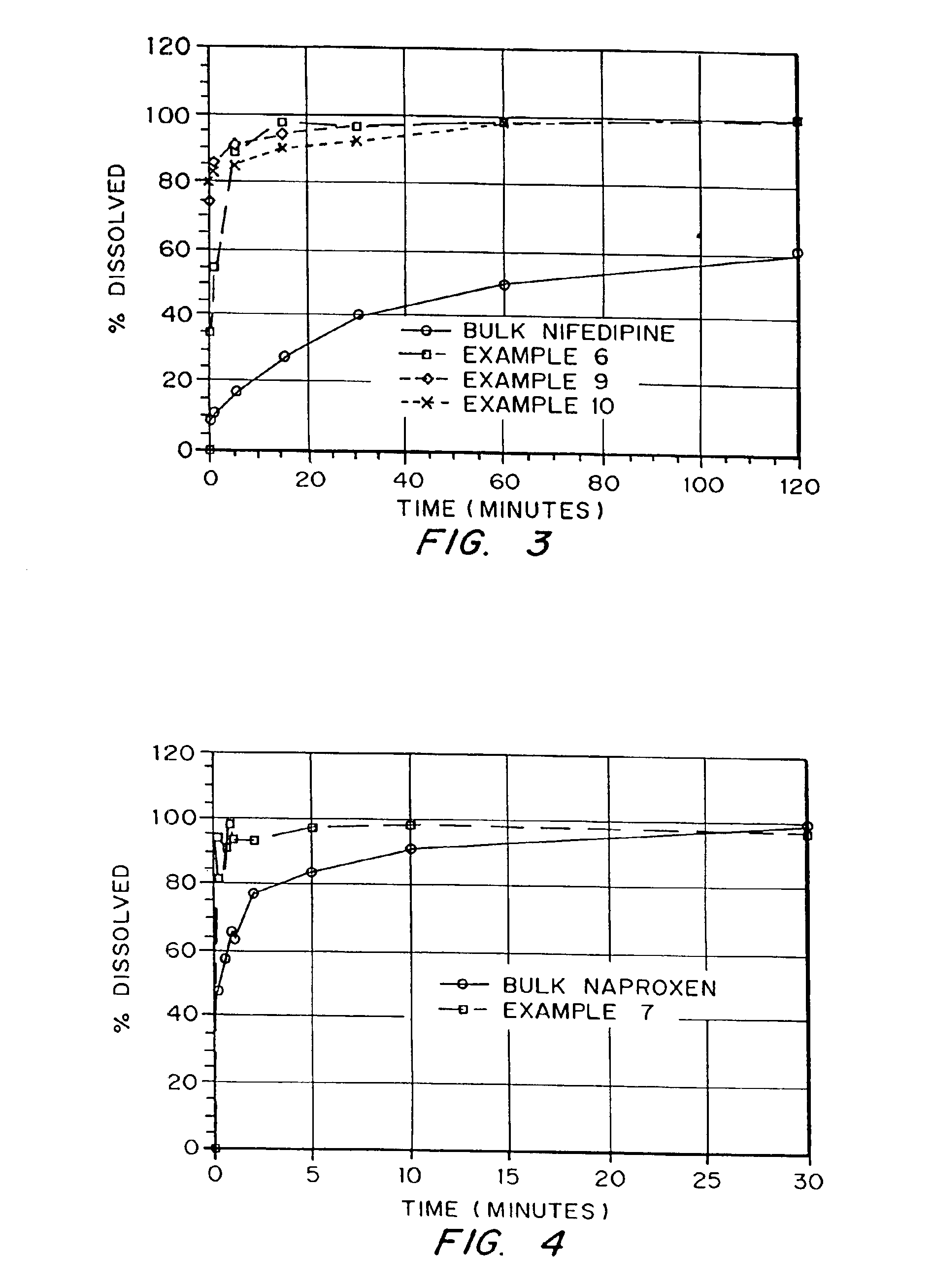
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Shaped load-bearing osteoimplant and methods of making same
InactiveUS20030039676A1Promotes new host bone tissue formationPermit of mechanical propertySuture equipmentsDental implantsMedicineHard tissue
A load-bearing osteoimplant, methods of making the osteoimplant and method for repairing hard tissue such as bone and teeth employing the osteoimplant are provided. The osteoimplant comprises a shaped, coherent mass of bone particles which may exhibit osteogenic properties. In addition, the osteoimplant may possess one or more optional components which modify its mechanical and / or bioactive properties, e.g., binders, fillers, reinforcing components, etc.
Owner:WARSAW ORTHOPEDIC INC
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com