Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
21603 results about "Delivery system" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
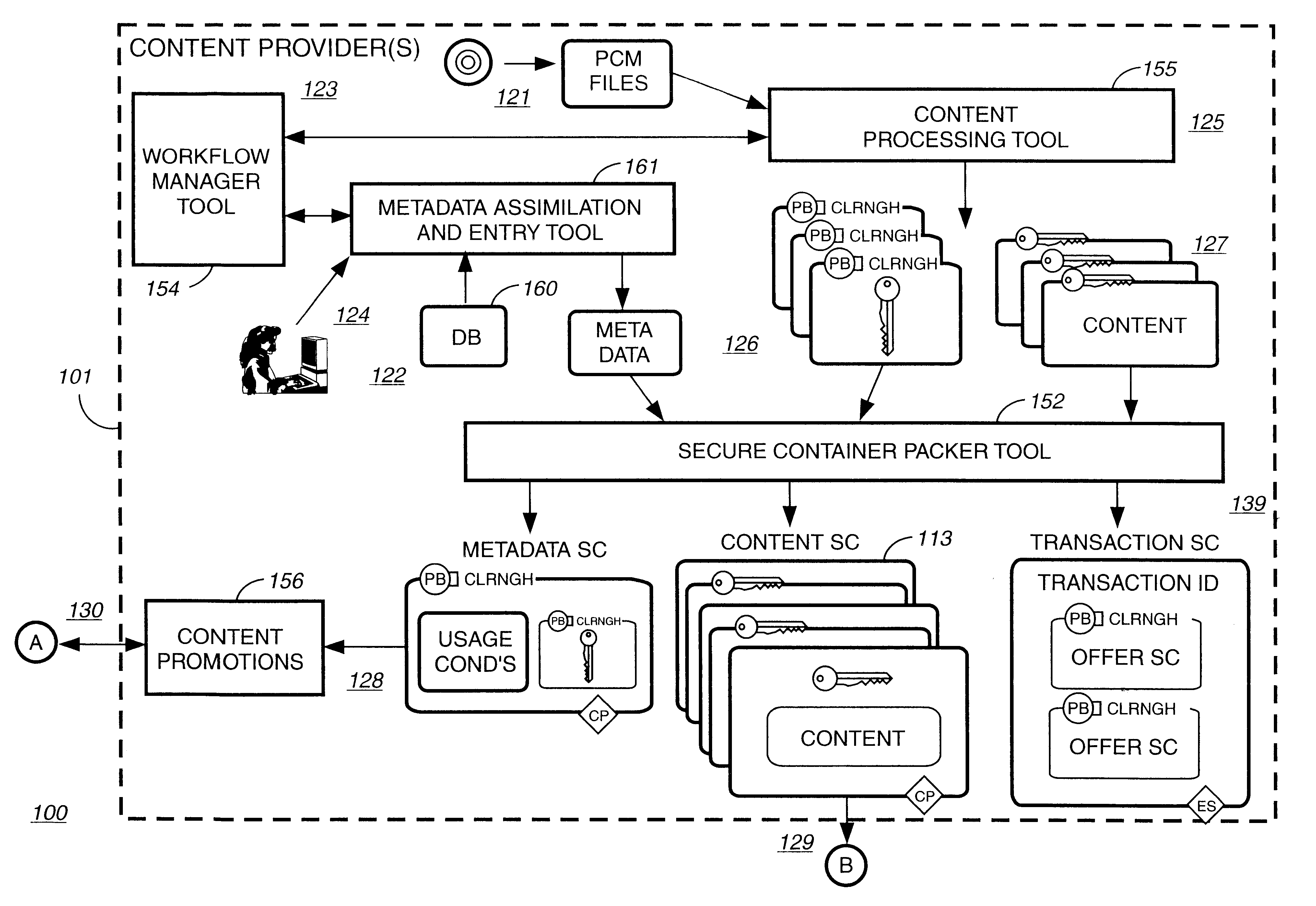
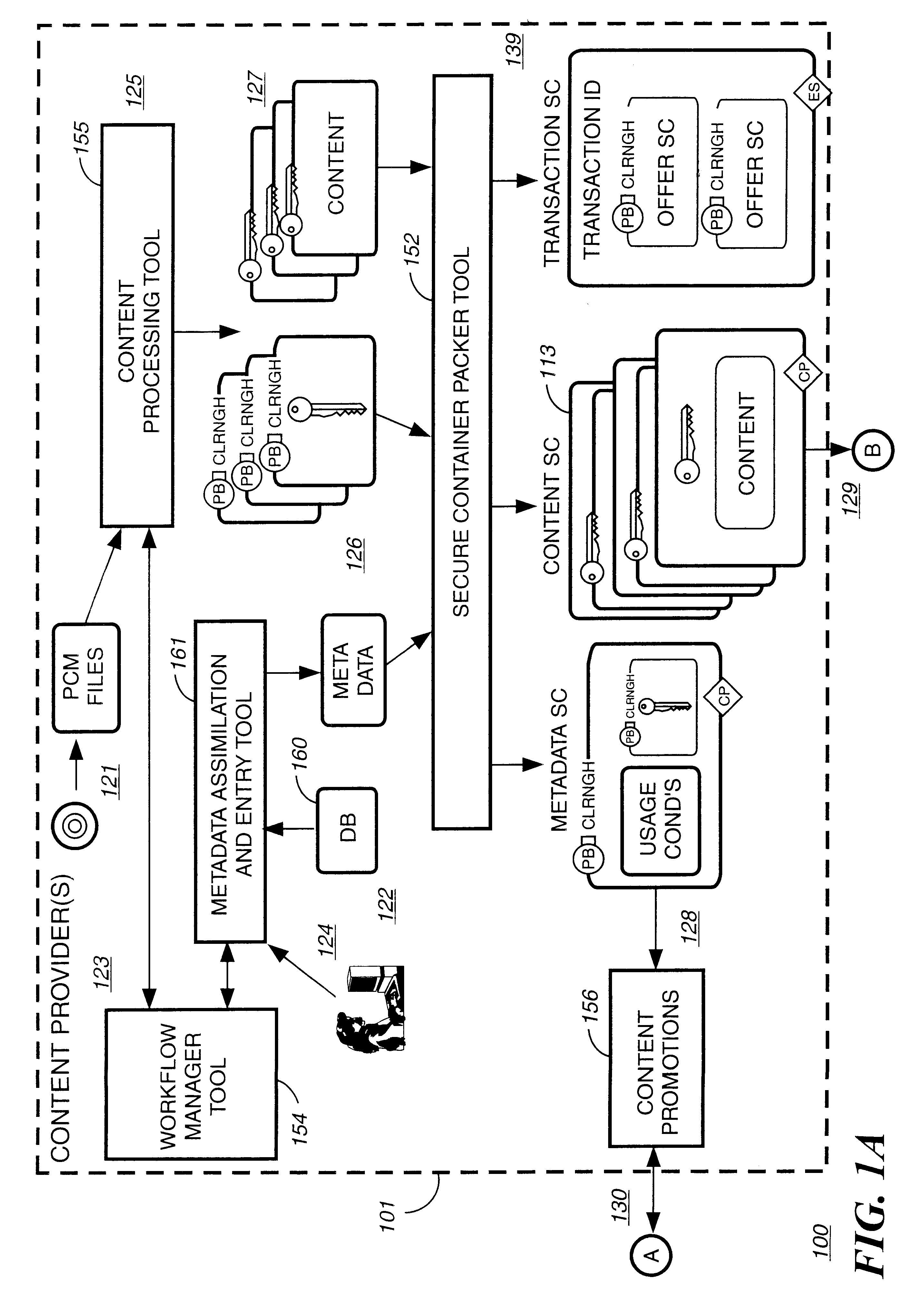
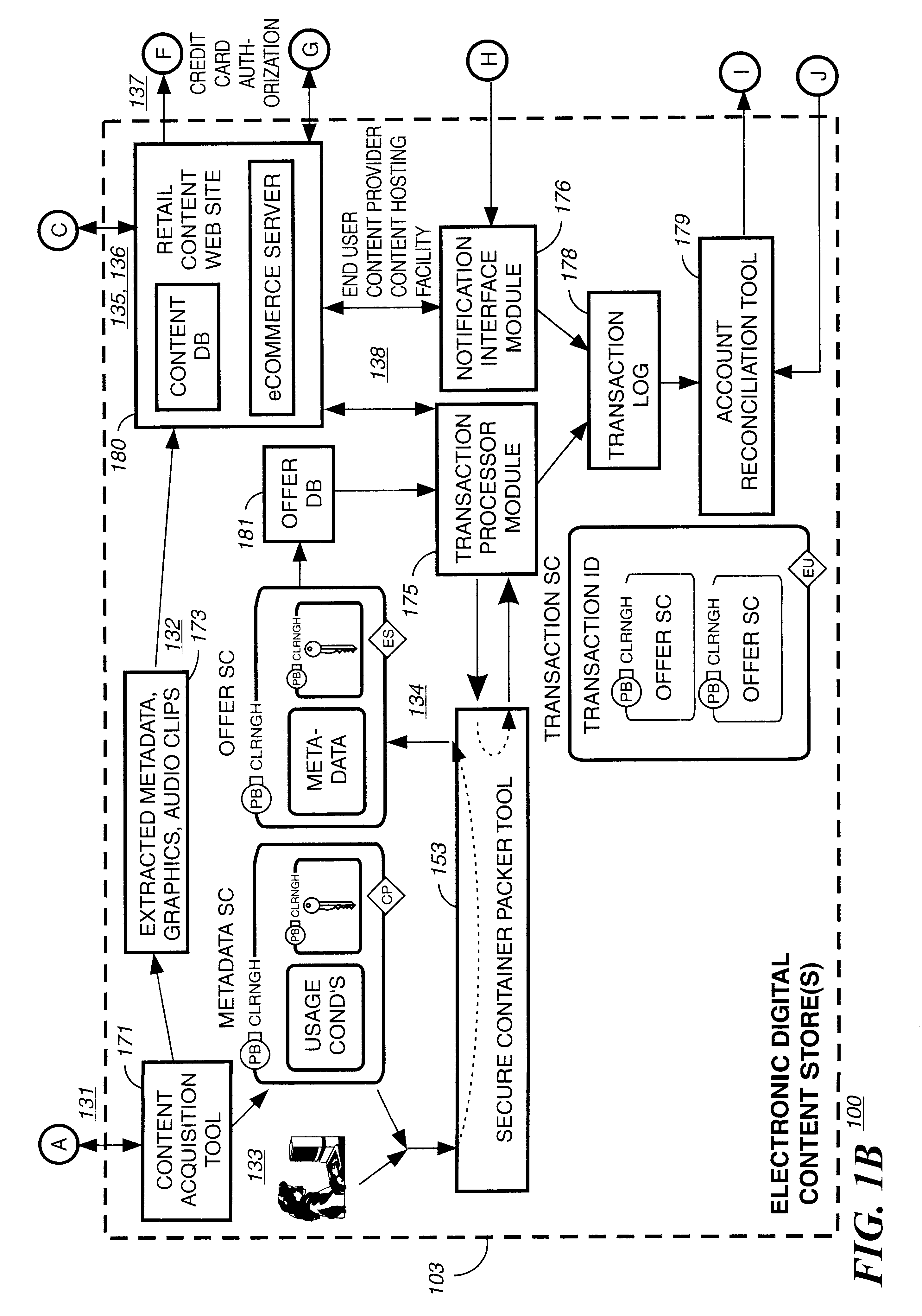
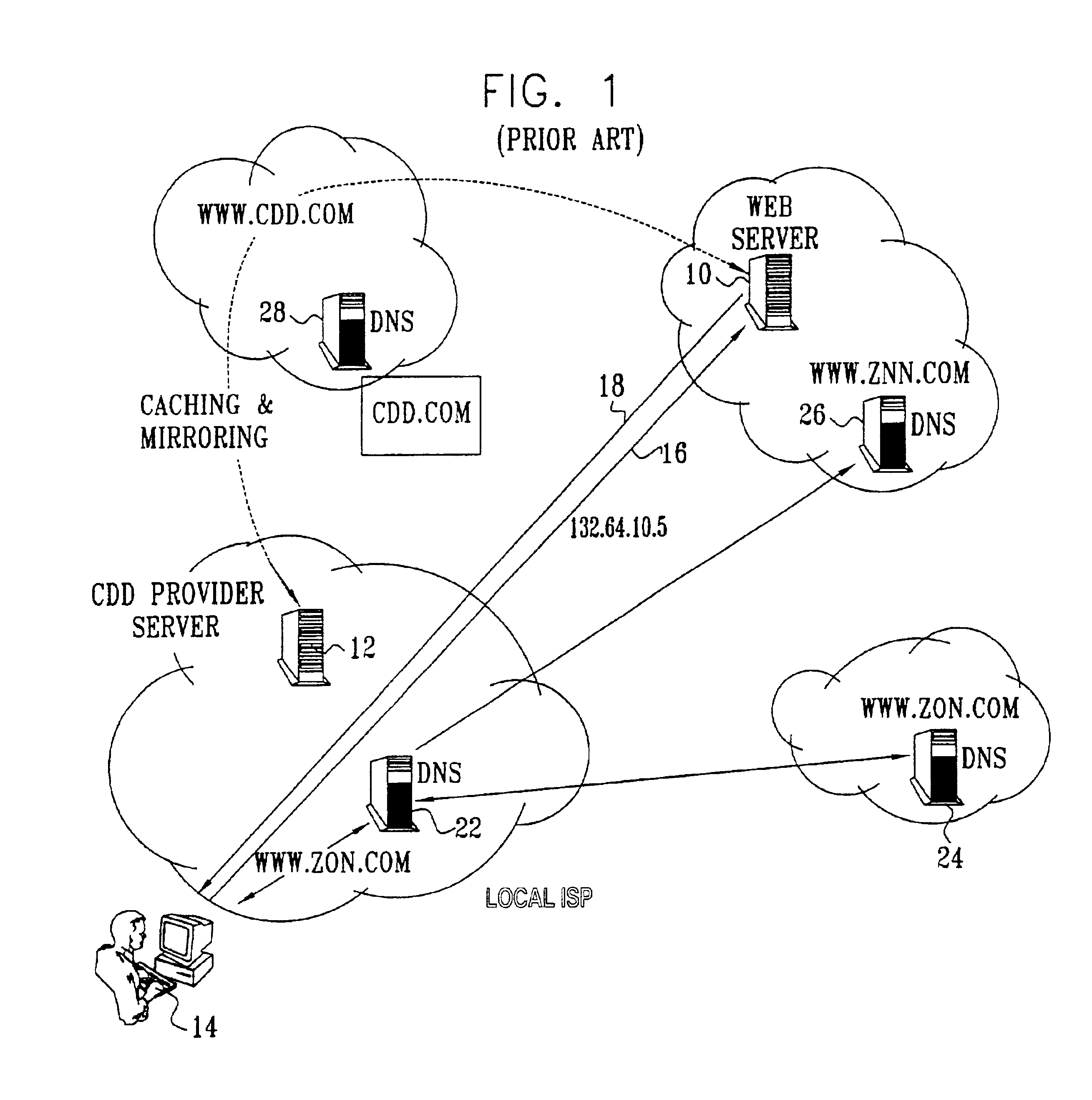
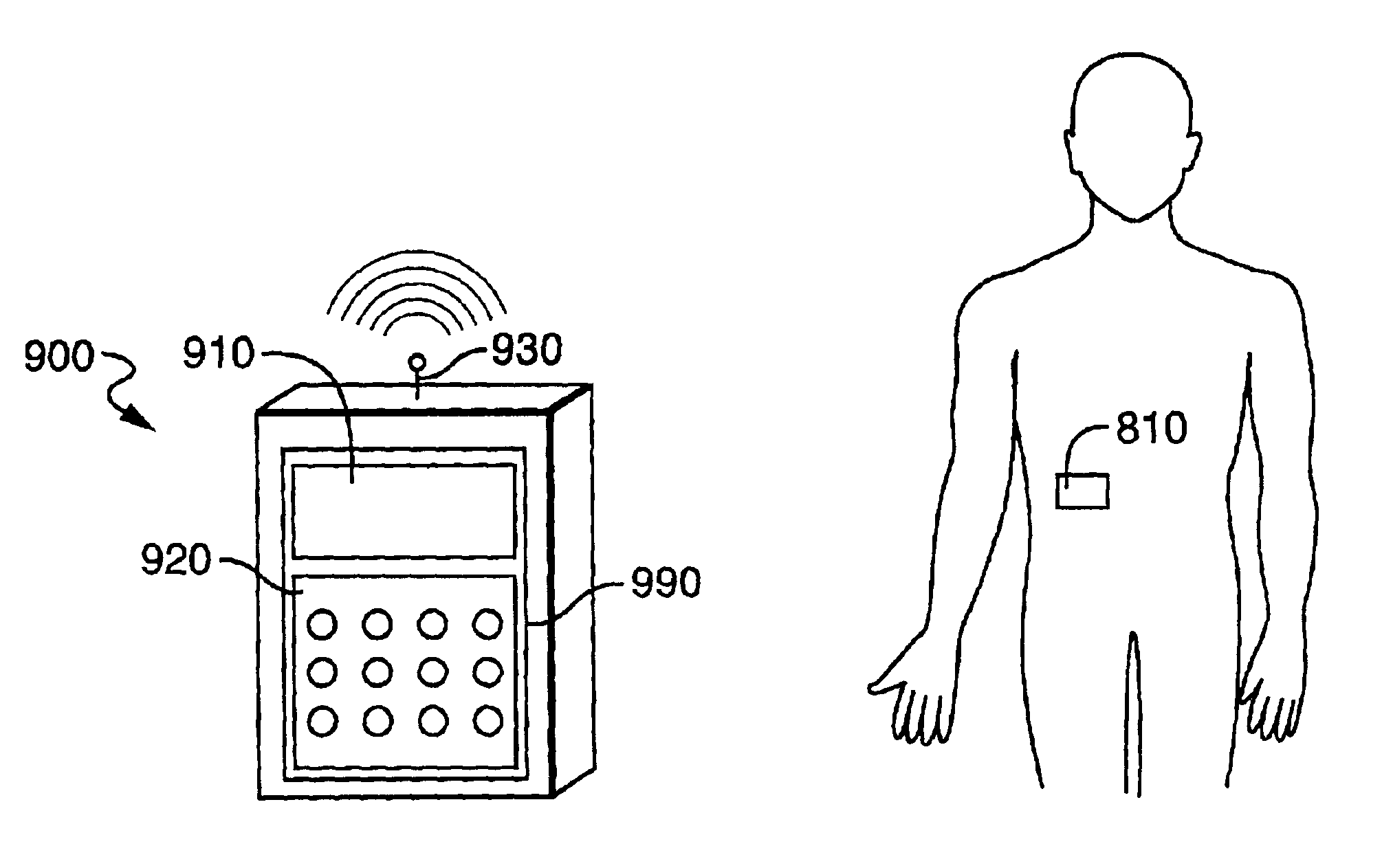
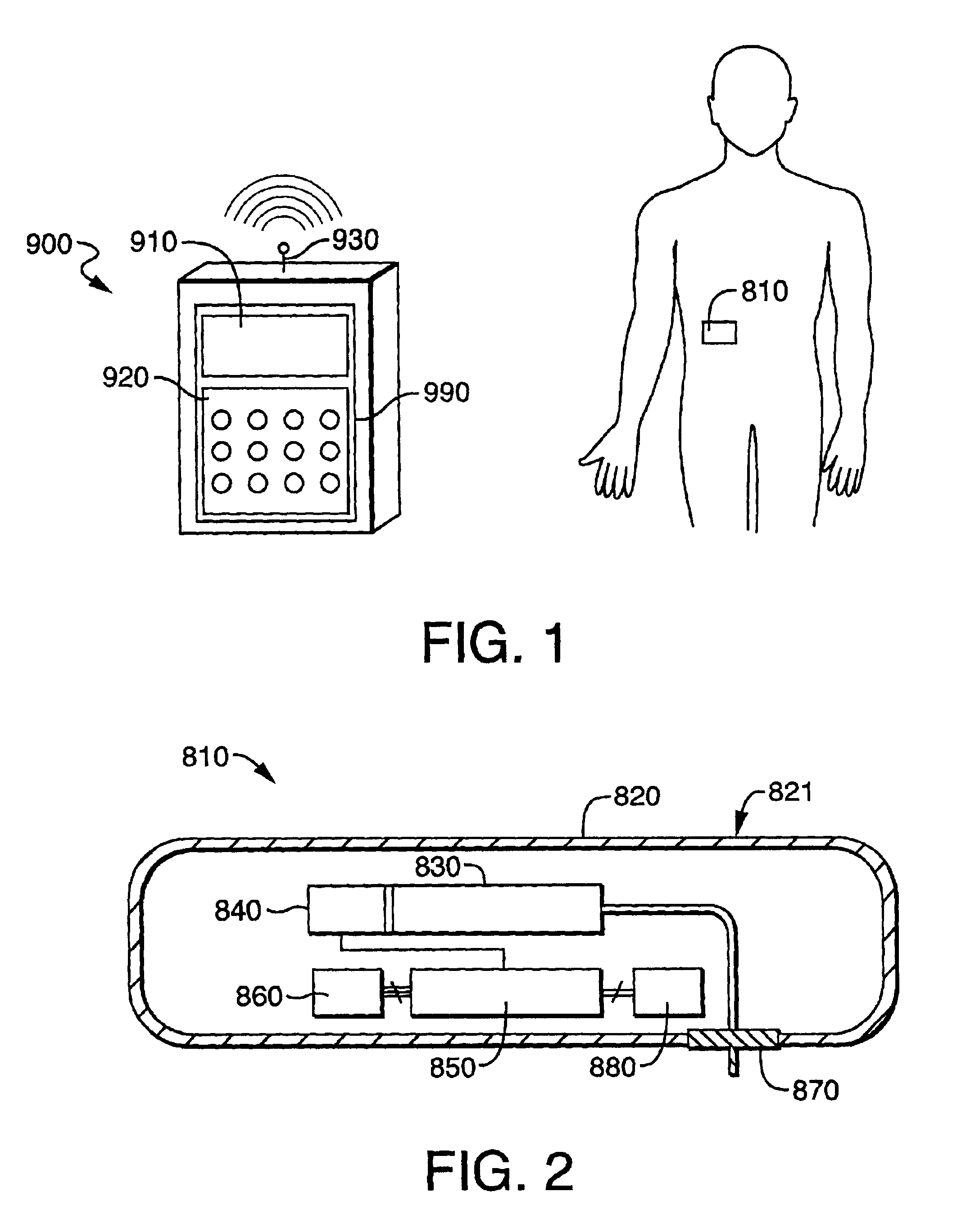
Electronic content delivery system
InactiveUS6226618B1Key distribution for secure communicationDigital data processing detailsDelivery systemSystem safety
Disclosed is a method and apparatus of securely providing data to a user's system. The data is encrypted so as to only be decryptable by a data decrypting key, the data decrypting key being encrypted using a first public key, and the encrypted data being accessible to the user's system, the method comprising the steps of: transferring the encrypted data decrypting key to a clearing house that possesses a first private key, which corresponds to the first public key; decrypting the data decrypting key using the first private key; re-encrypting the data decrypting key using a second public key; transferring the re-encrypted data decrypting key to the user's system, the user's system possessing a second private key, which corresponds to the second public key; and decrypting the re-encrypted data decrypting key using the second private key.
Owner:LEVEL 3 COMM LLC
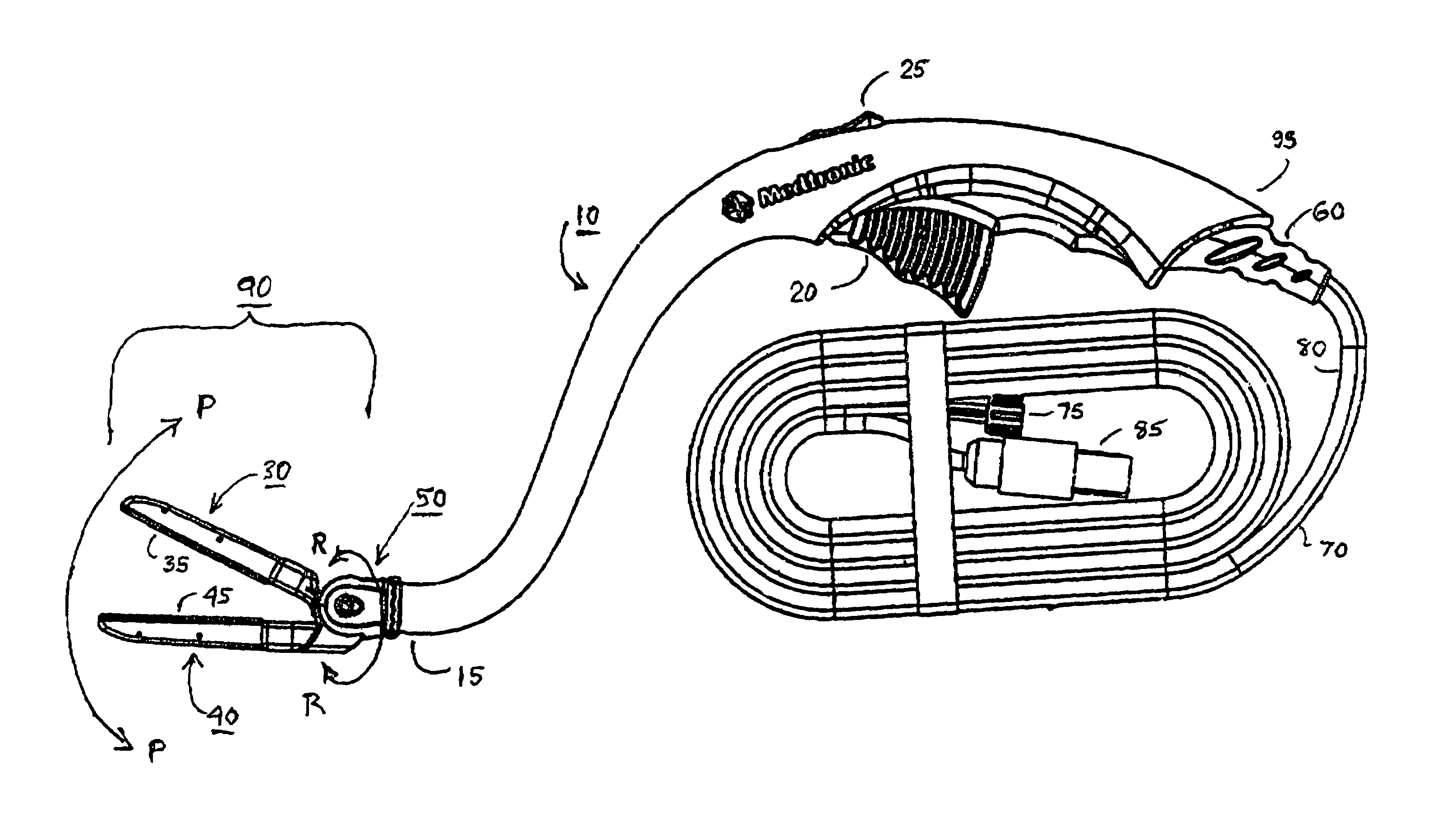
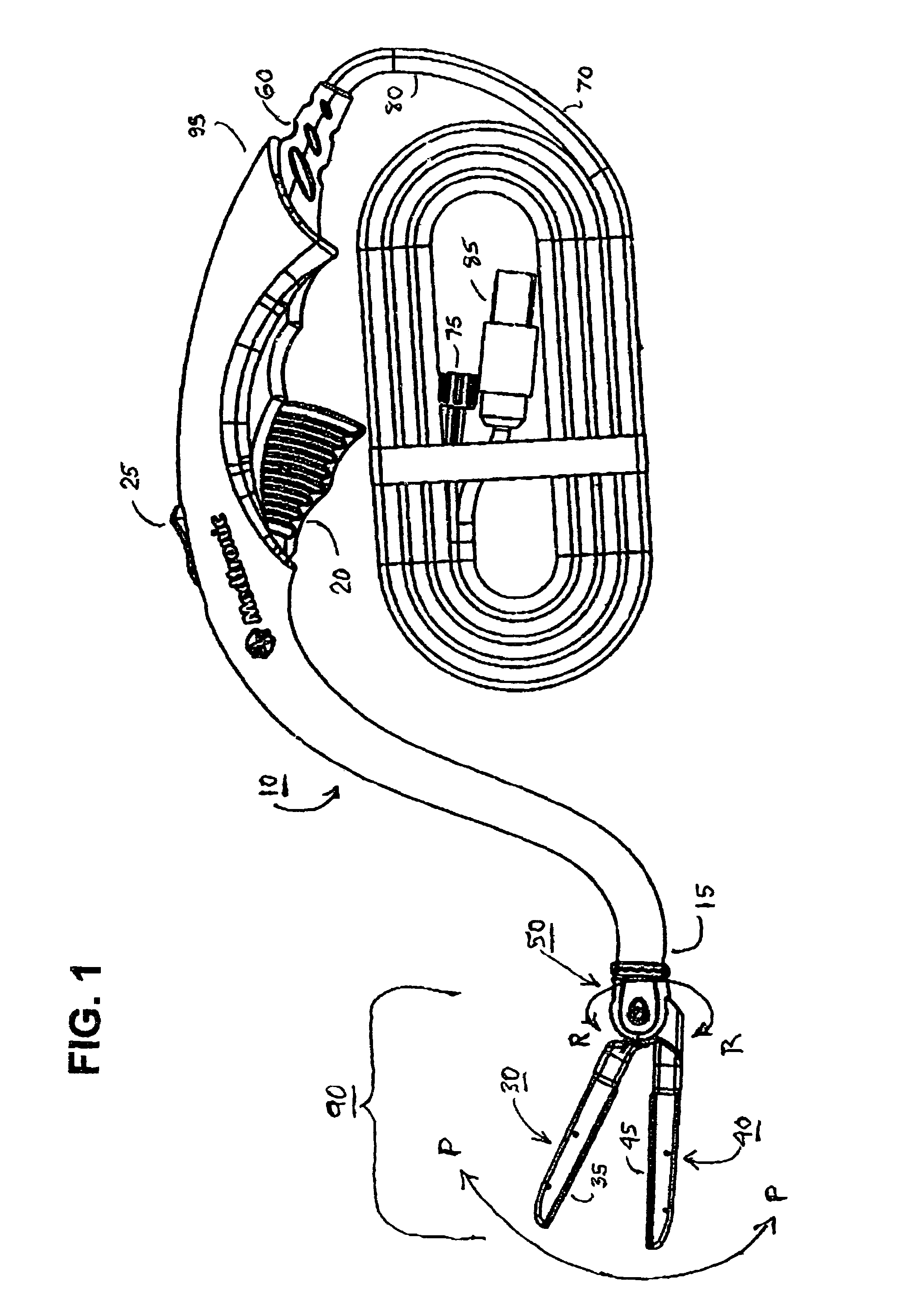
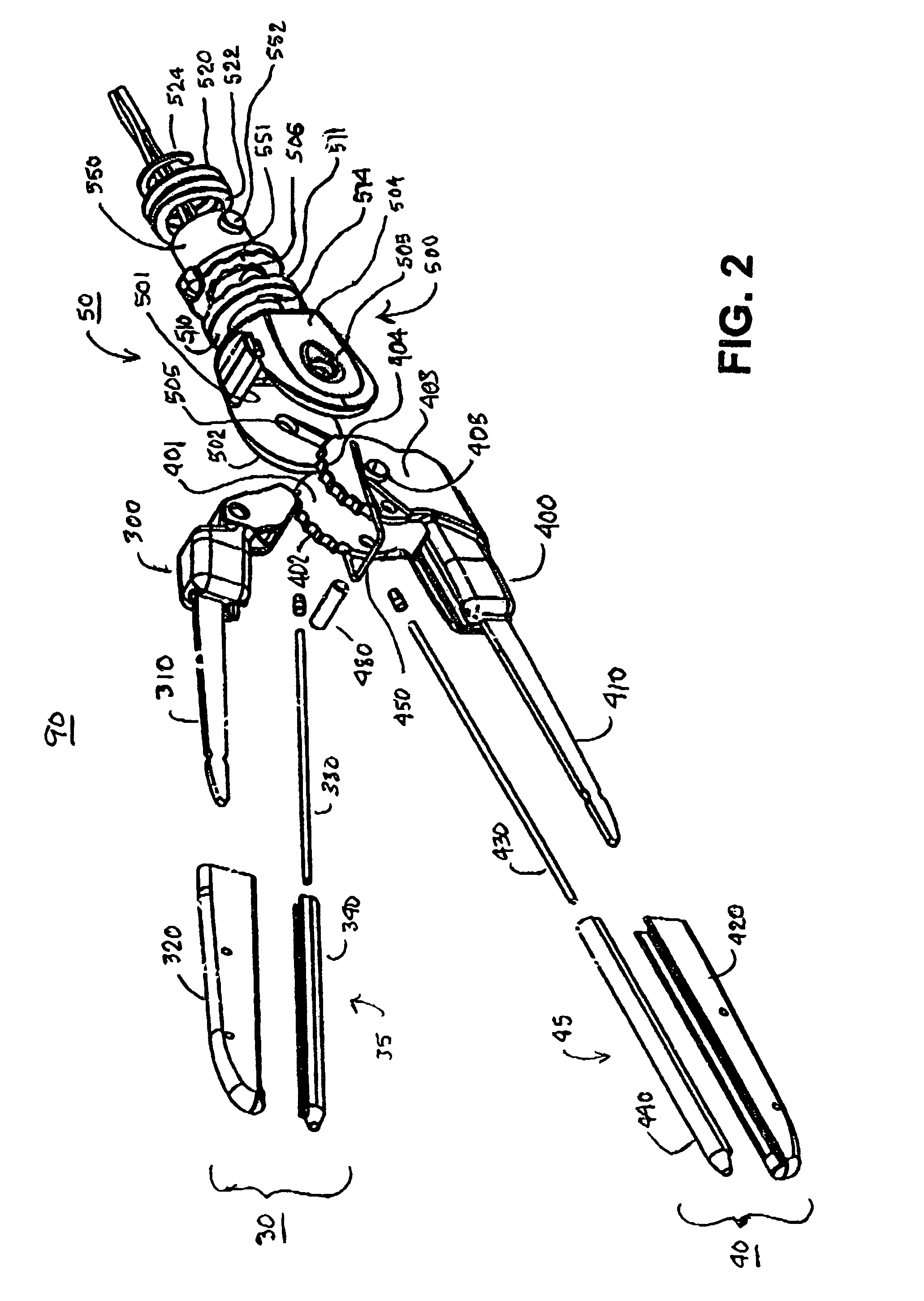
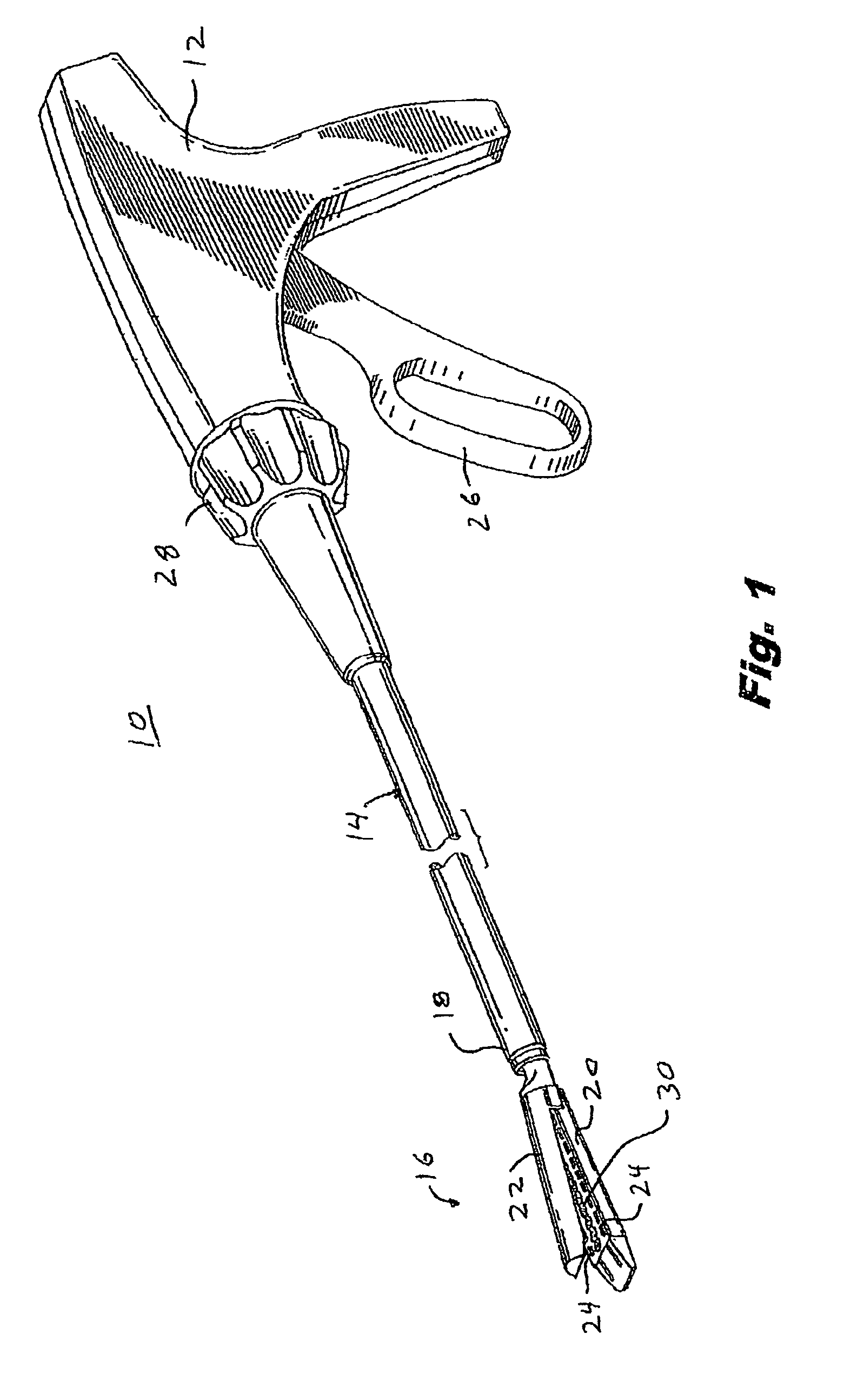
Electrosurgical hemostat
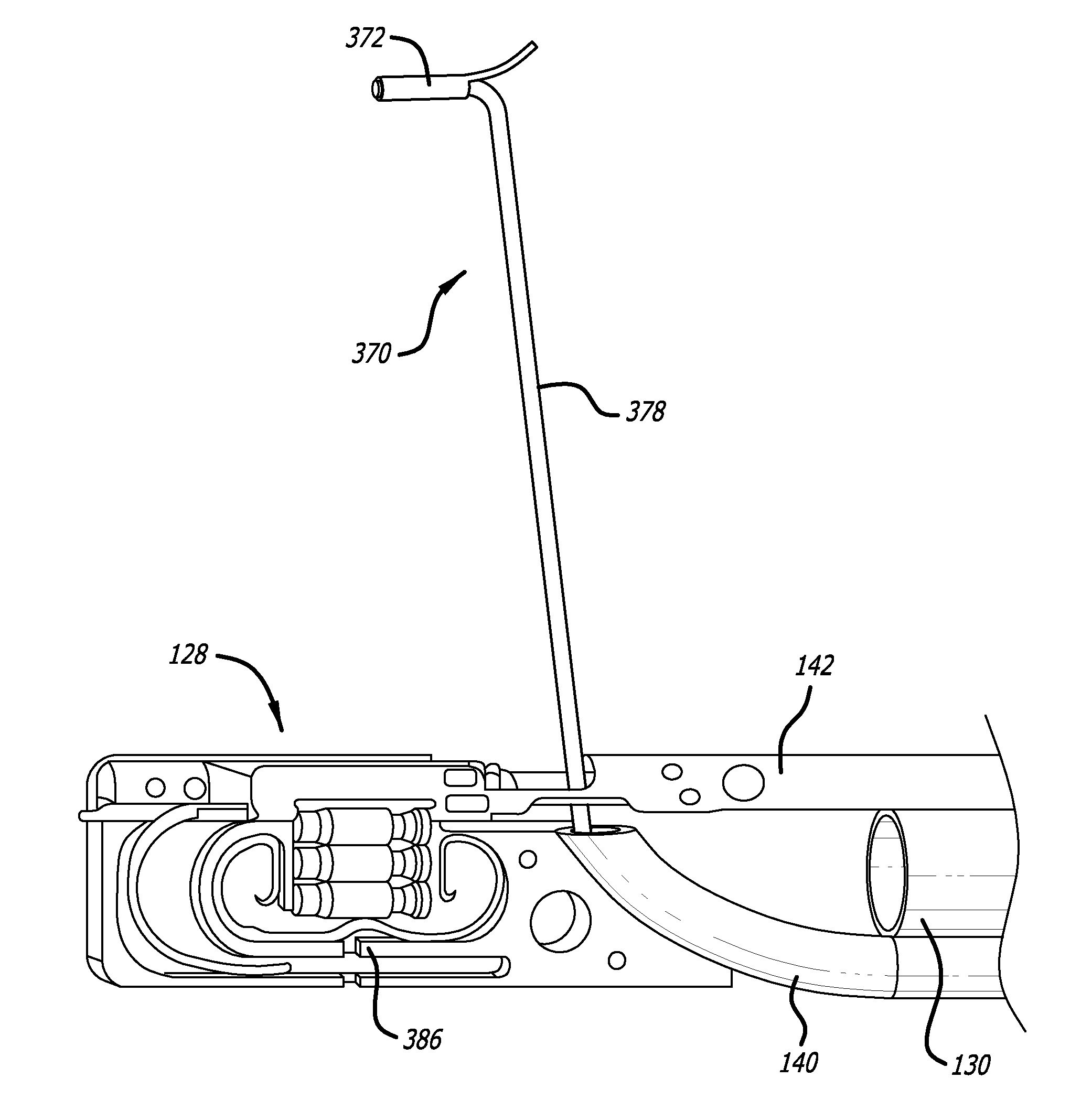
InactiveUS7083620B2Reduce usageImprove performanceCatheterSurgical instruments for heatingDetentEffective treatment
A hemostat-type device for ablative treatment of tissue, particularly for treatment of atrial fibrillation, is constructed with features that provide easy and effective treatment. A swiveling head assembly can allow the jaws to be adjusted in pitch and roll. Malleable jaws can permit curved lesion shapes. A locking detent can secure the jaws in a closed position during the procedure. An illuminated indicator provides confirmation that the device is operating. A fluid delivery system simplifies irrigated ablation procedures.
Owner:MEDTRONIC INC
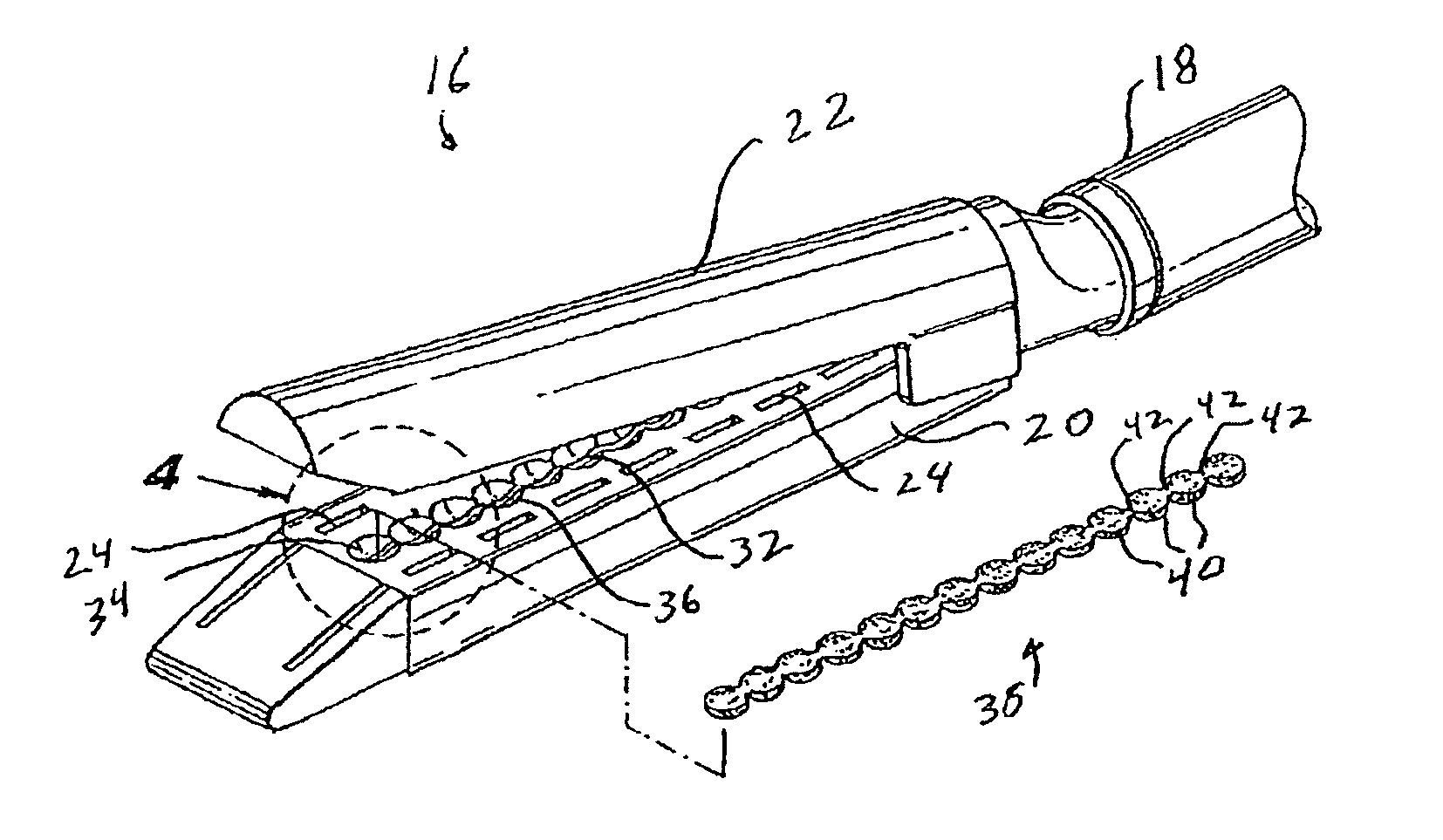
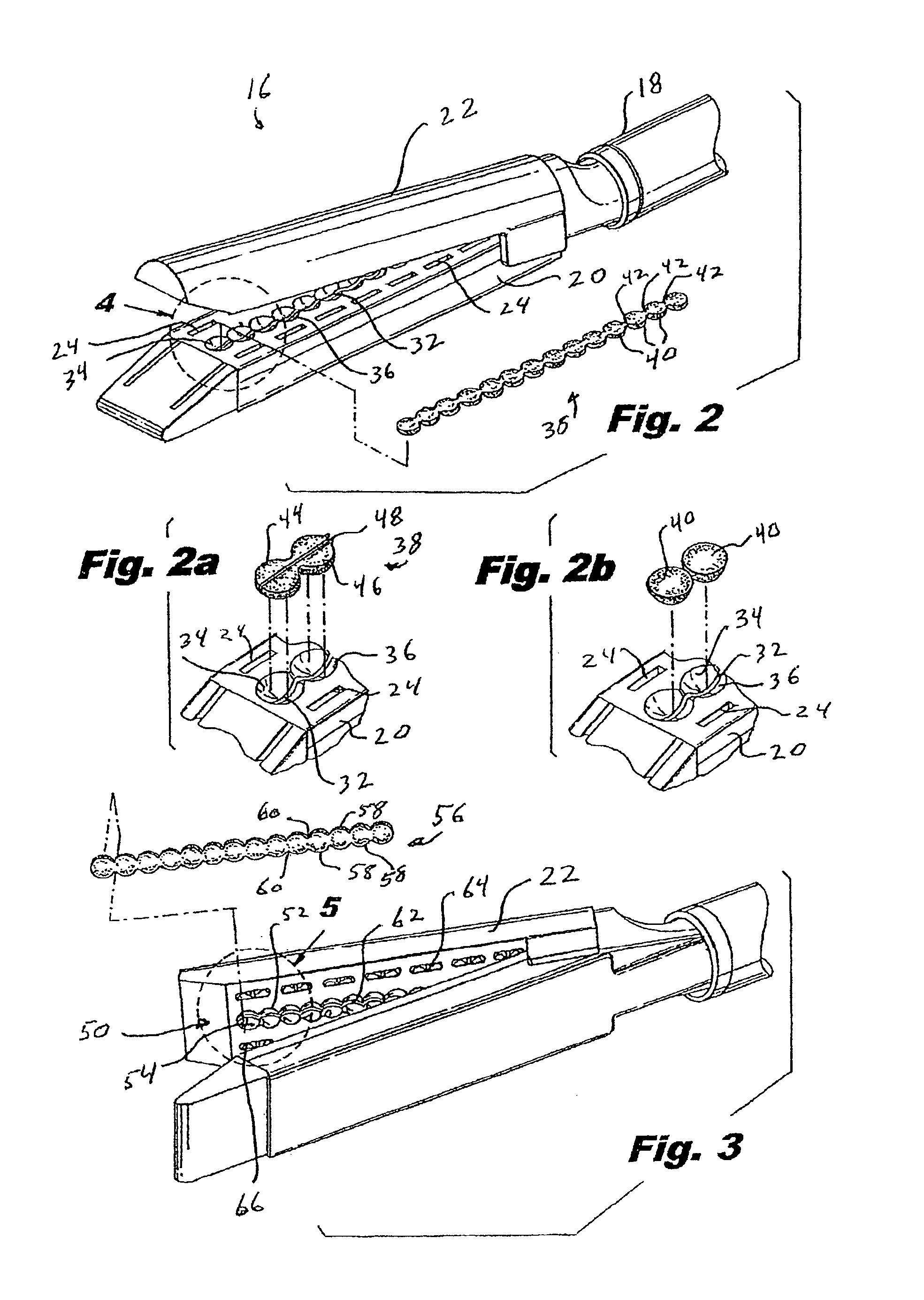
Materials delivery system for stapling device
There is disclosed a materials delivery system for use with a surgical stapling instrument having at least one jaw including a knife slot. The materials delivery system includes a series of treatment material receiving pockets positioned adjacent the knife slot such that a portion of each pocket is open to the knife slot. A source of treatment material is positioned within the pockets such that passage of the knife blade adjacent to the pockets repeatedly coats the knife blade with the treatment material.
Owner:COVIDIEN LP
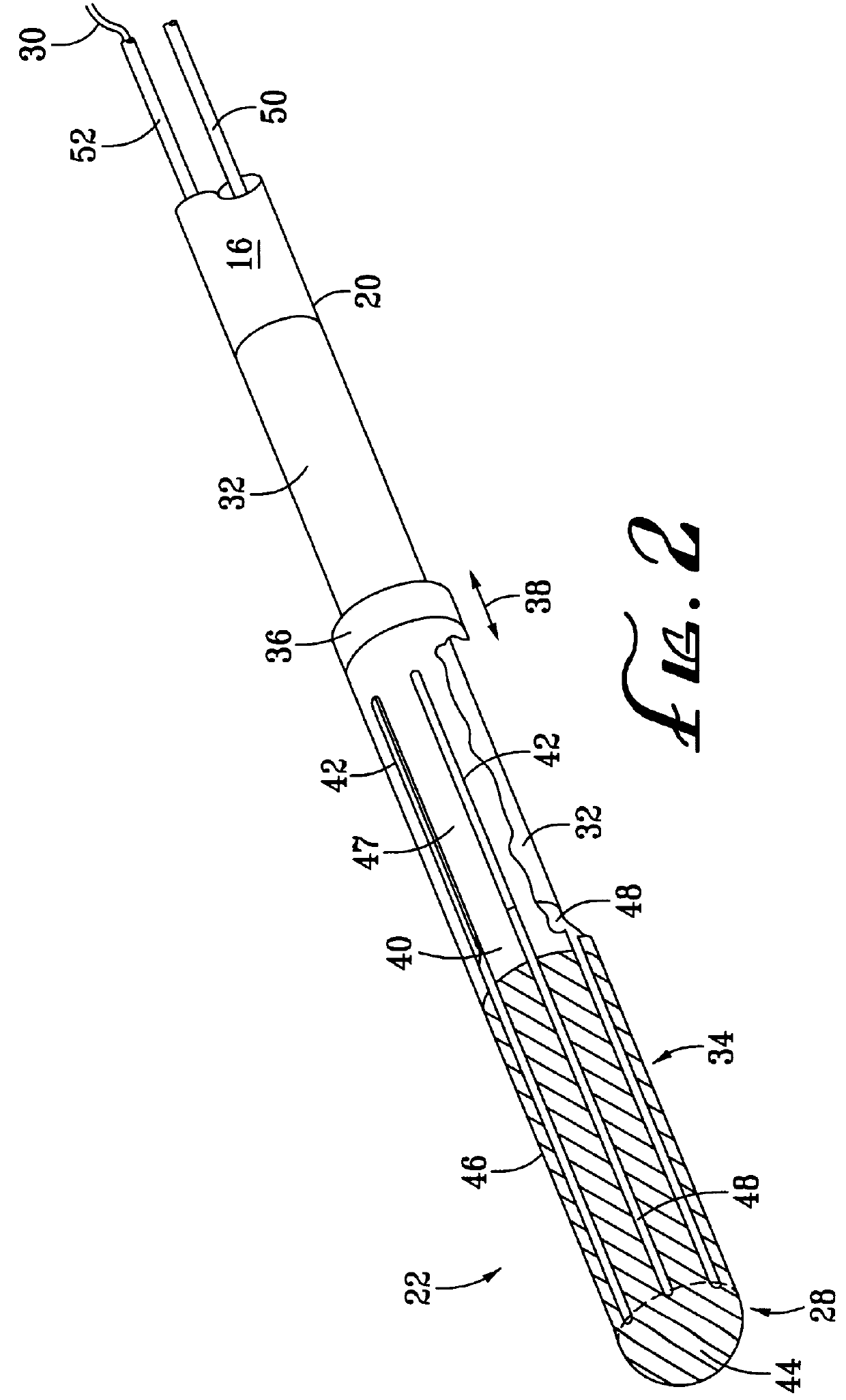
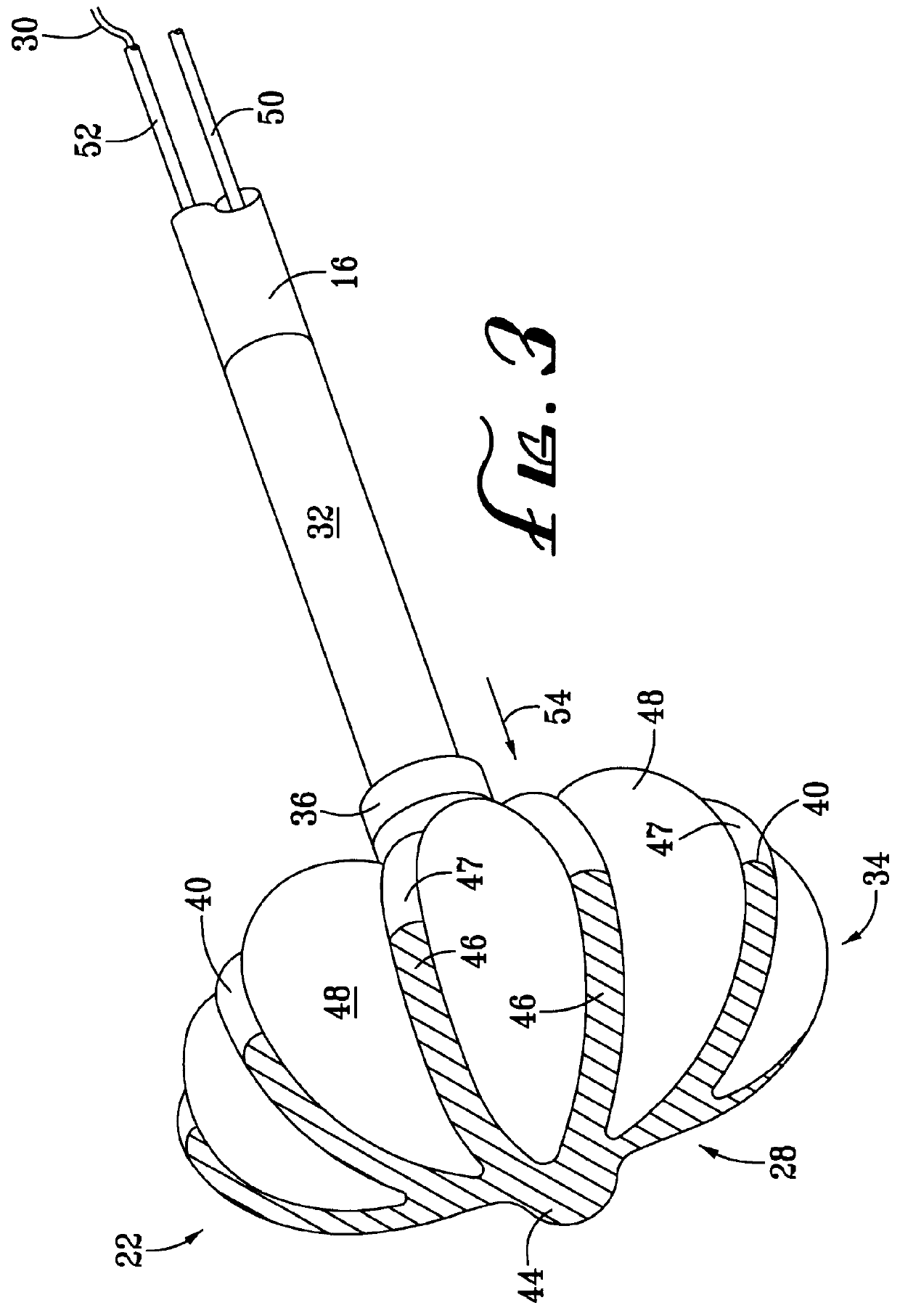
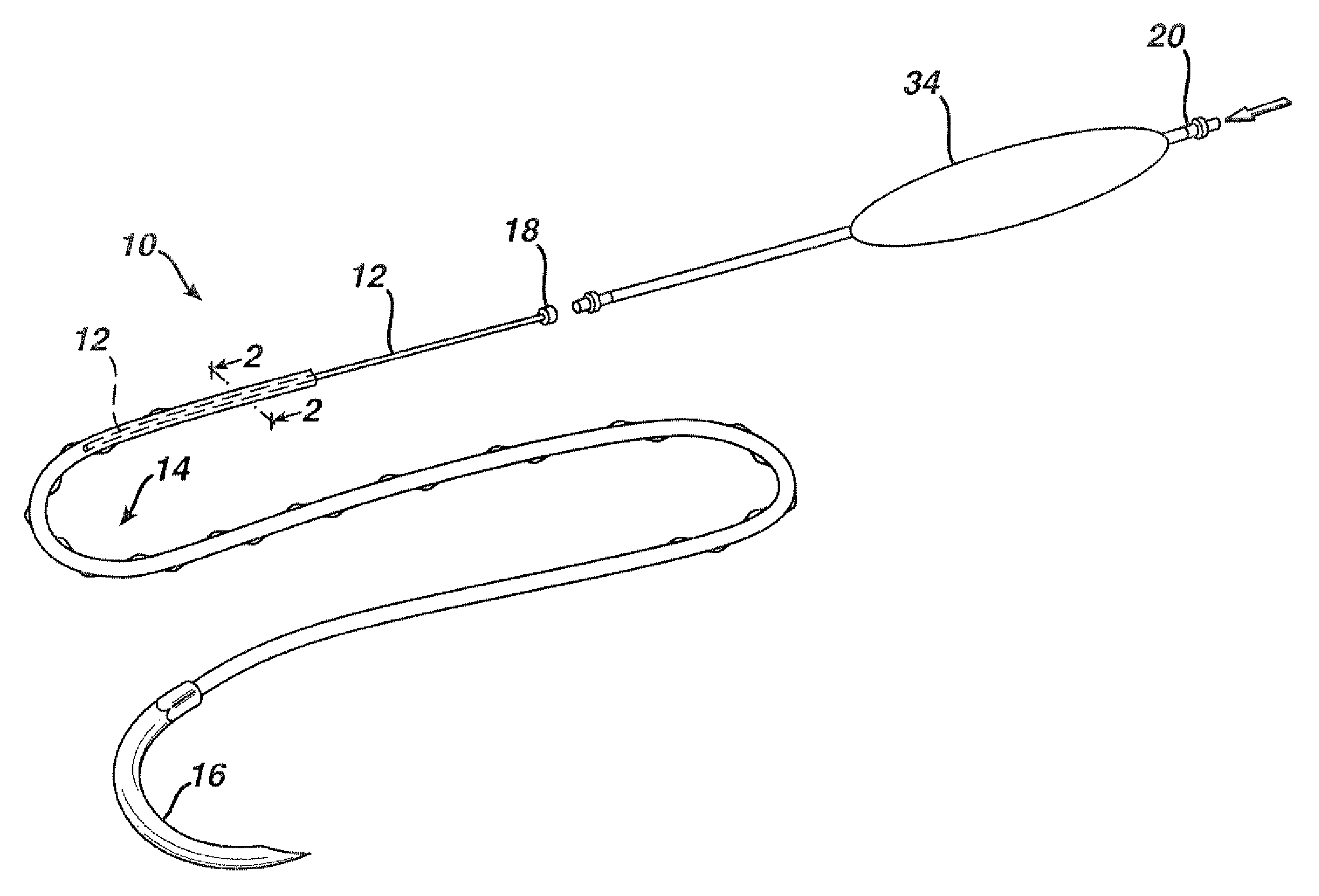
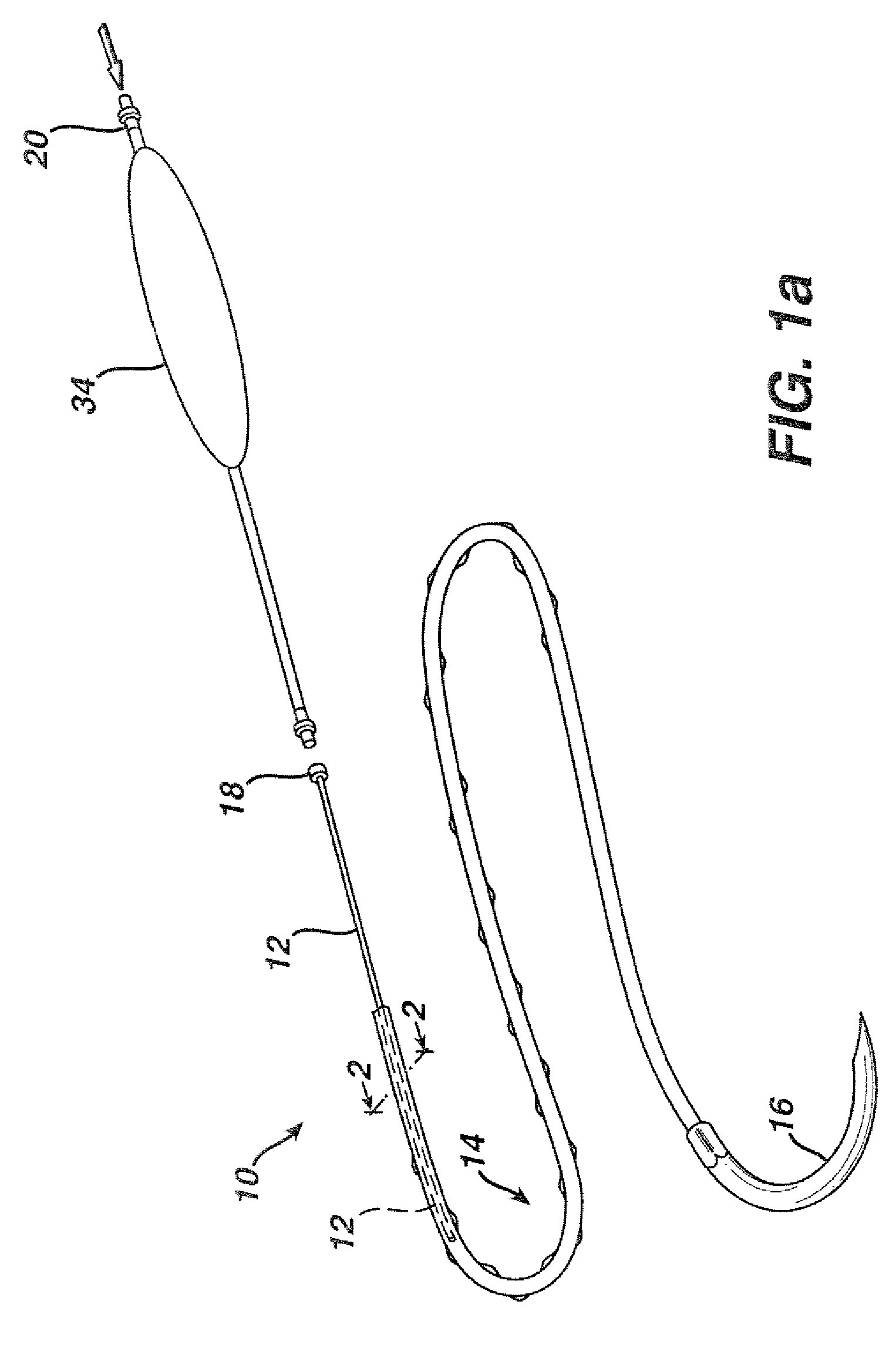
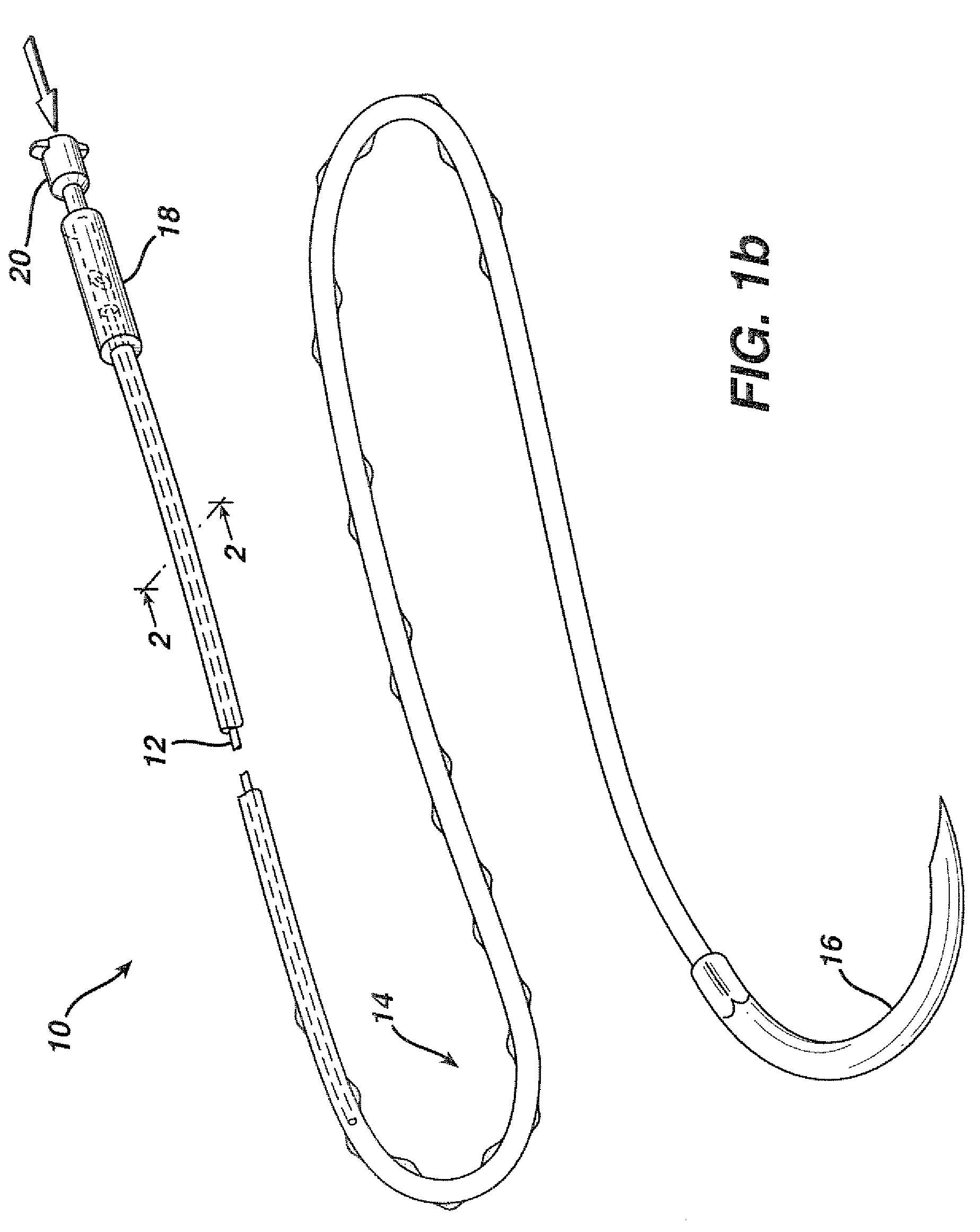
Flexible delivery device
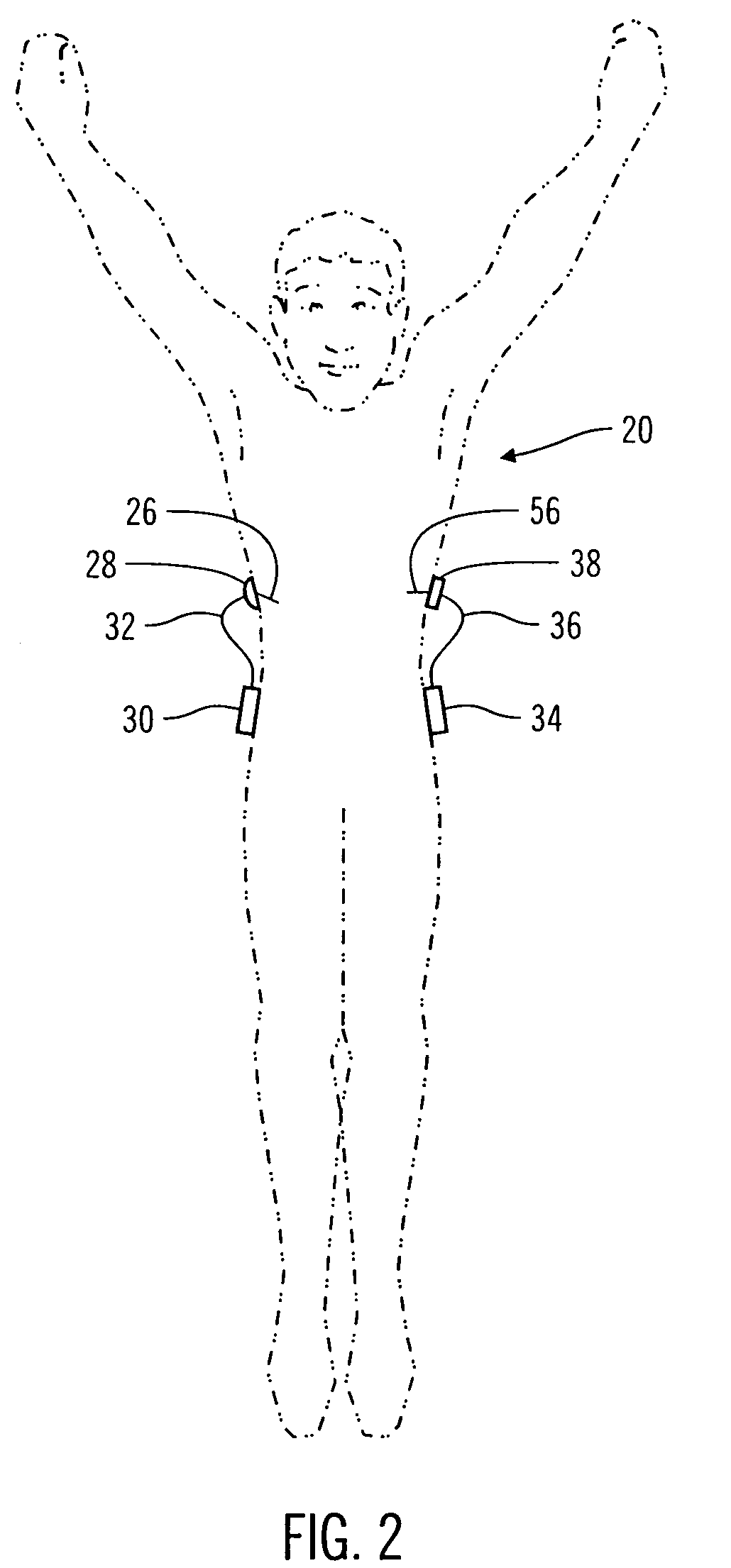
InactiveUS6921397B2Improved torque and flexure characteristicYielding couplingEar treatmentEngineeringDelivery system
This invention relates to catheter delivery systems, and more specifically, to a tubular device with improved torque and flexure characteristics. The present invention is a tubular device having improved torque and flexure characteristics which uses a series of permanently interlocking independent segments to provide the necessary torque and flexure characteristics.
Owner:ATRIAL SOLUTIONS INC
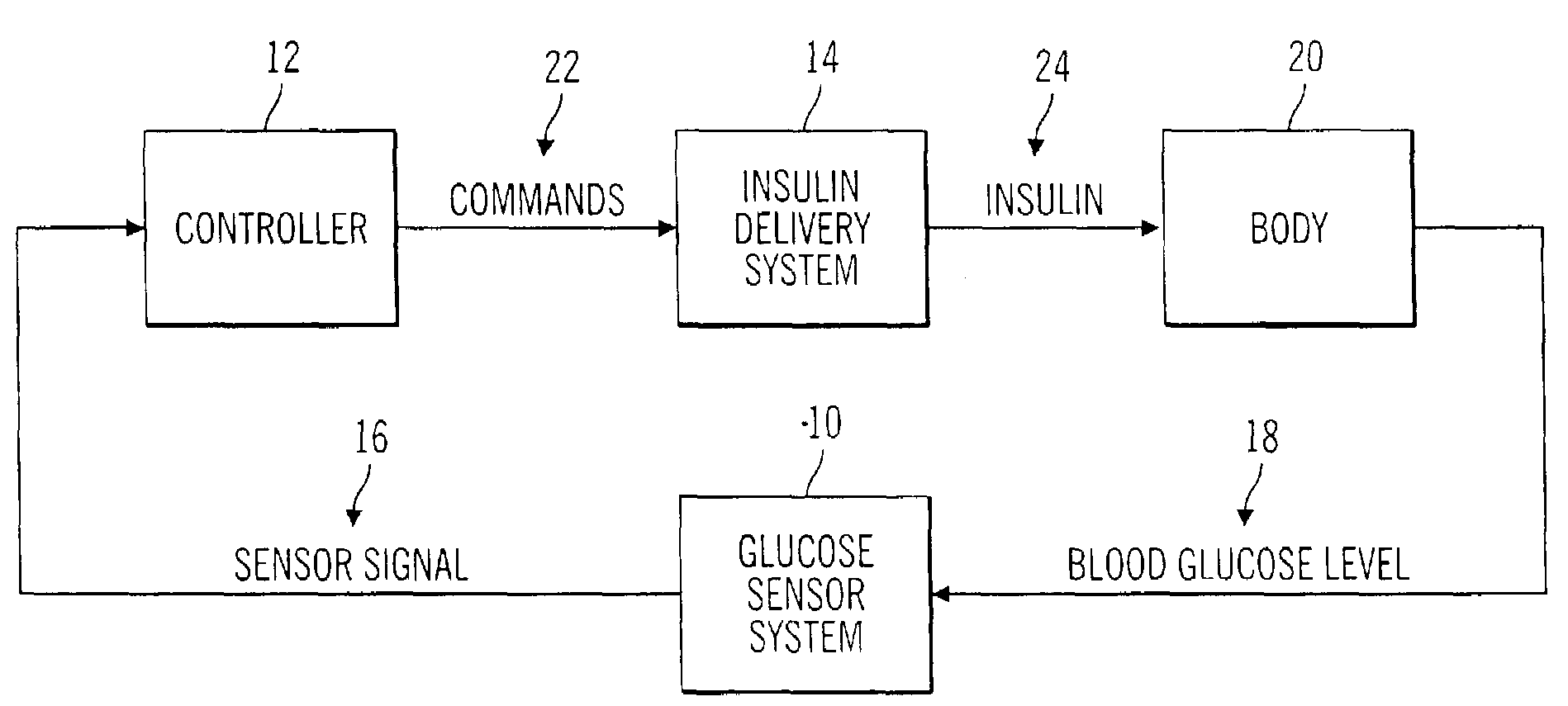
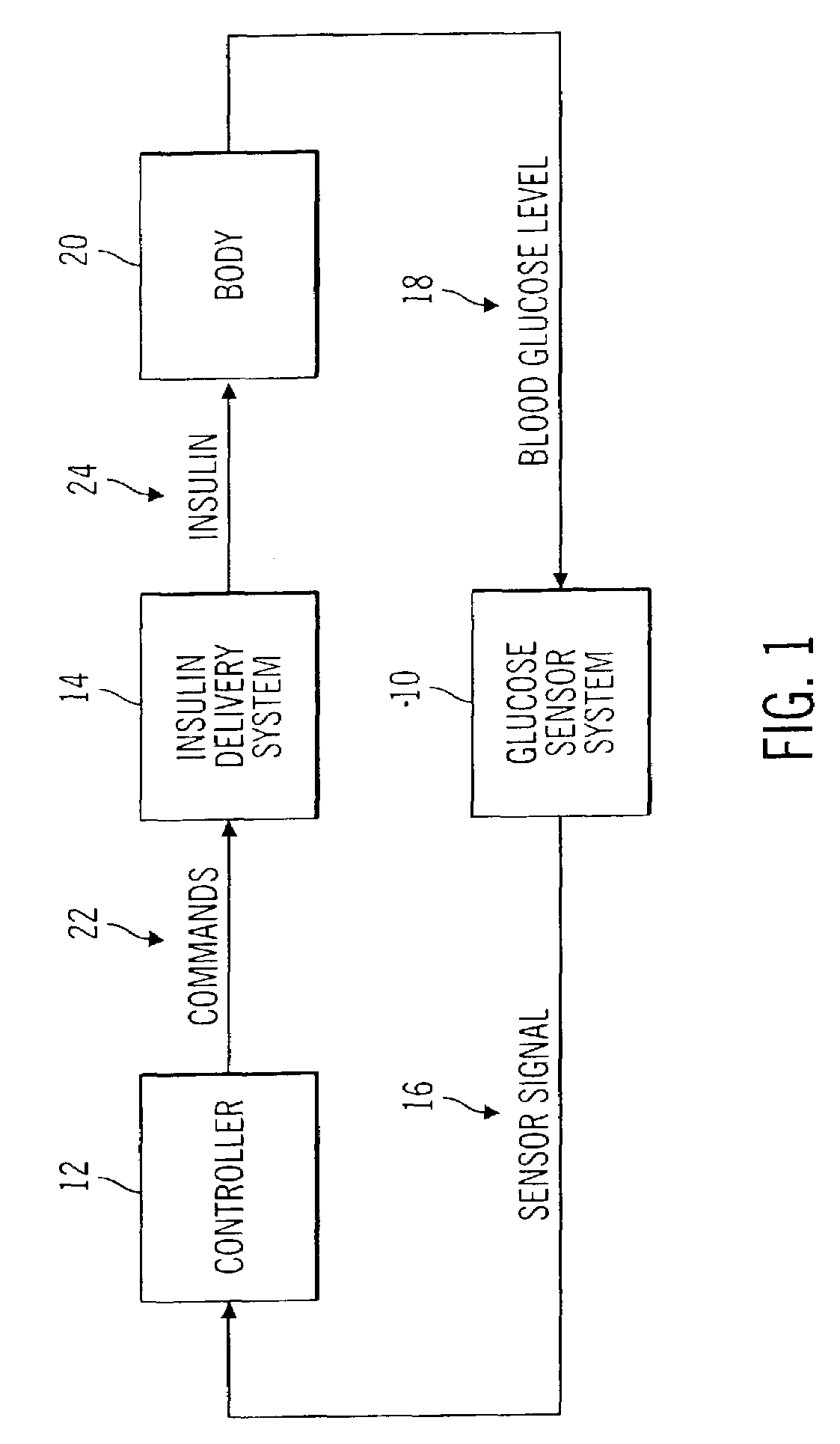
Closed loop system for controlling insulin infusion
InactiveUS7267665B2Other blood circulation devicesMedical devicesInsulin infusionConcentrations glucose
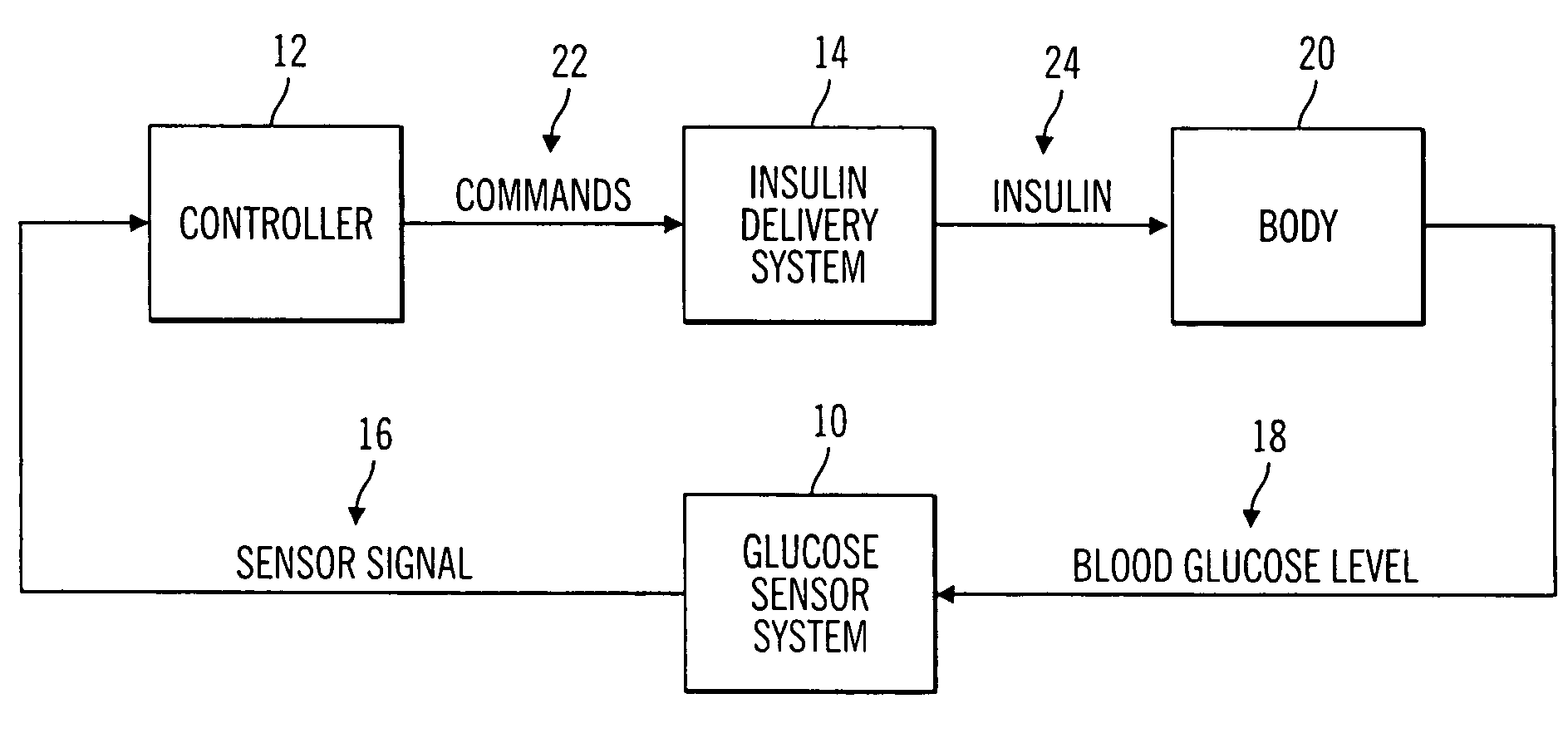
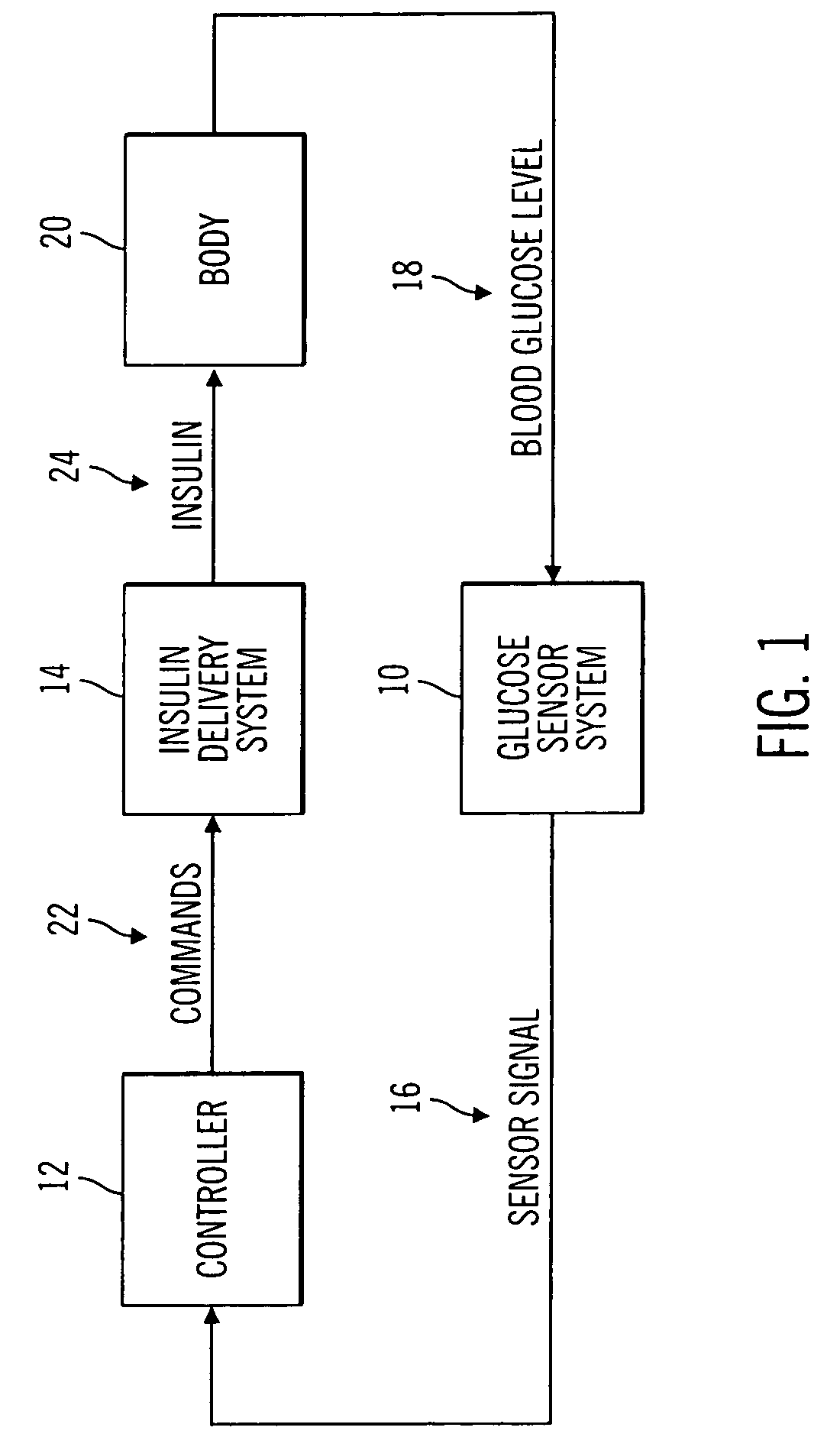
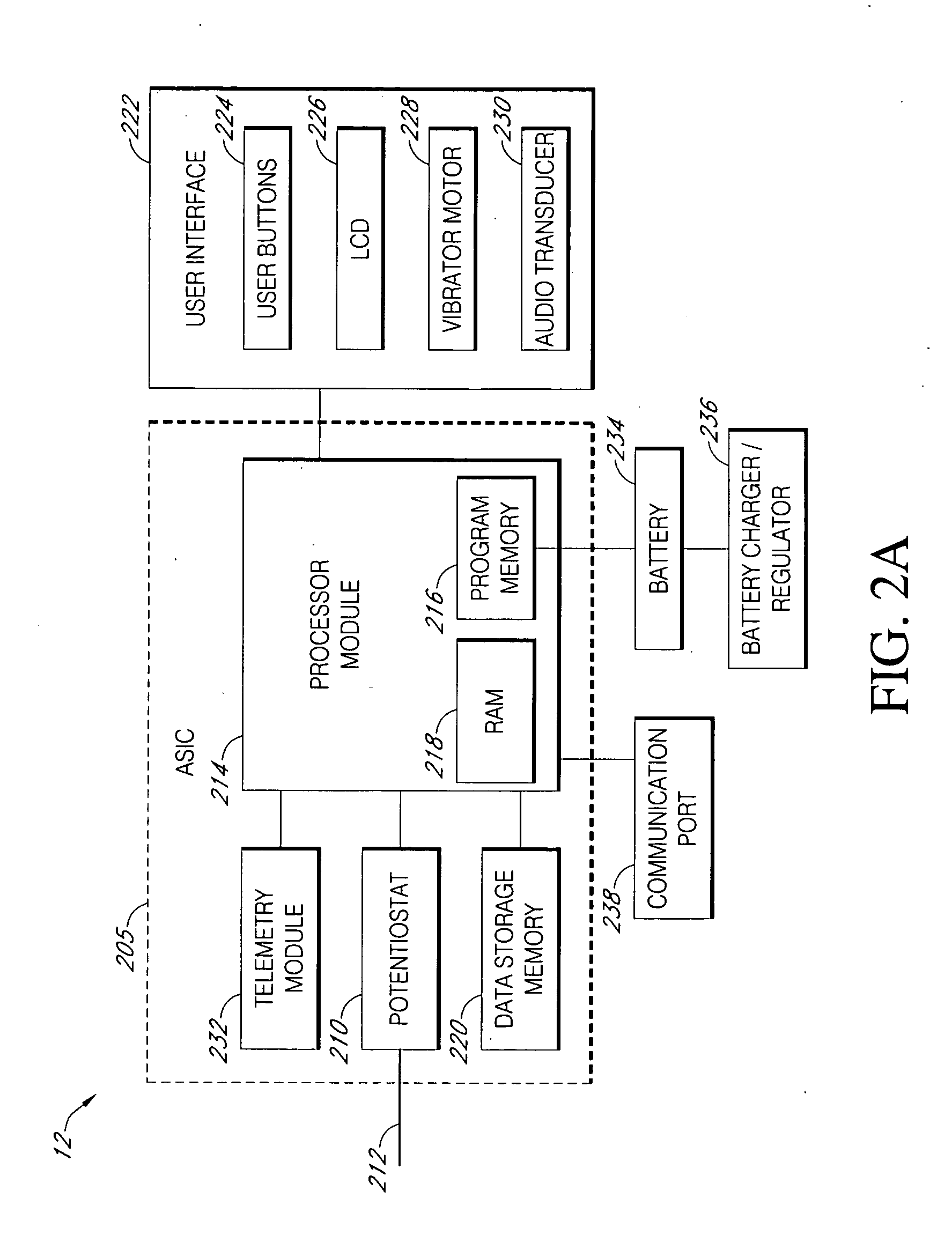
A closed loop infusion system controls the rate that fluid is infused into the body of a user. The closed loop infusion system includes a sensor system, a controller, and a delivery system. The sensor system includes a sensor for monitoring a condition of the user. The sensor produces a sensor signal, which is representative of the condition of the user. The sensor signal is used to generate a controller input. The controller uses the controller input to generate commands to operate the delivery system. The delivery system infuses a liquid into the user at a rate dictated by the commands from the controller. Preferably, the sensor system monitors the glucose concentration in the body of the user, and the liquid infused by the delivery system into the body of the user includes insulin.
Owner:MEDTRONIC MIMIMED INC
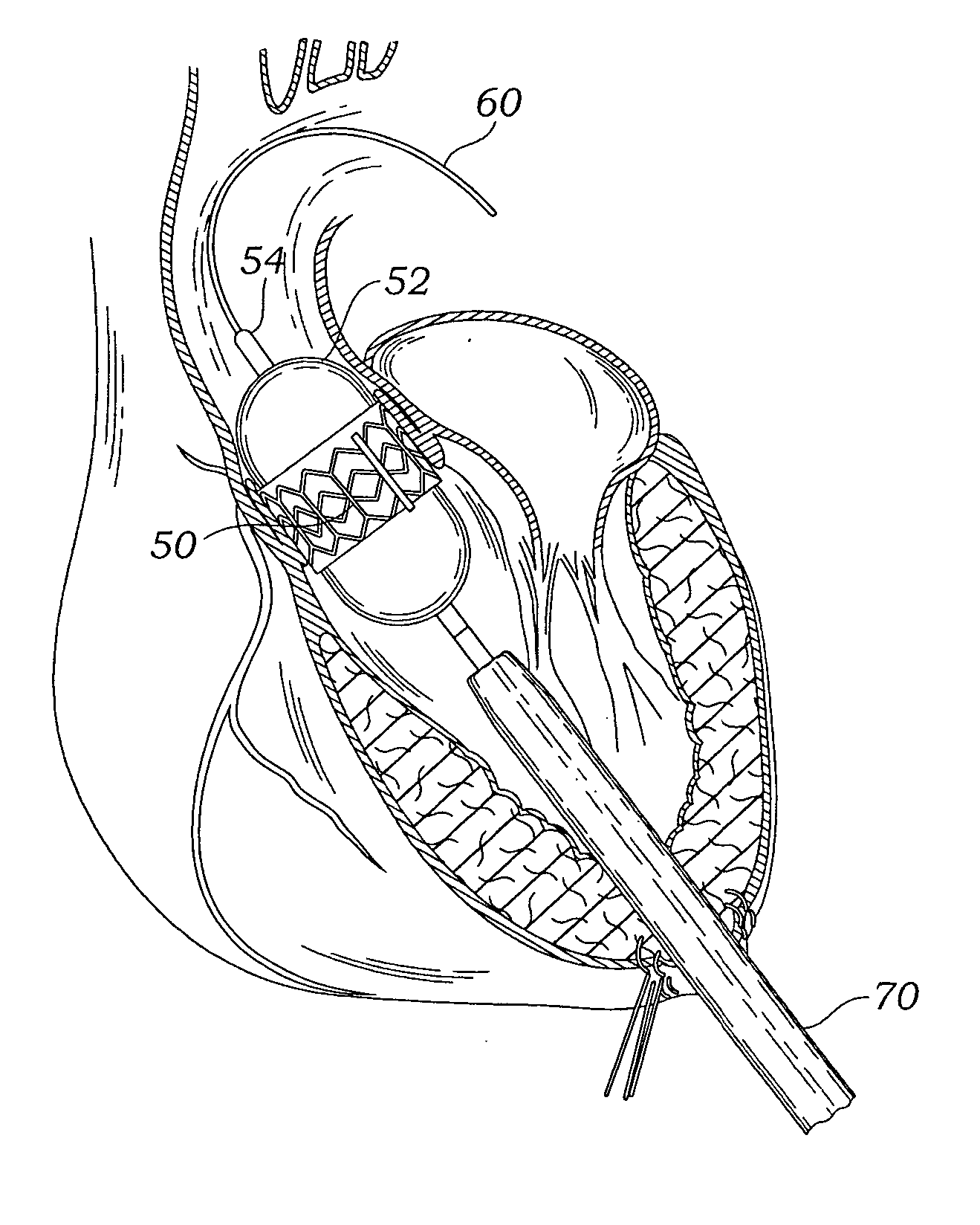
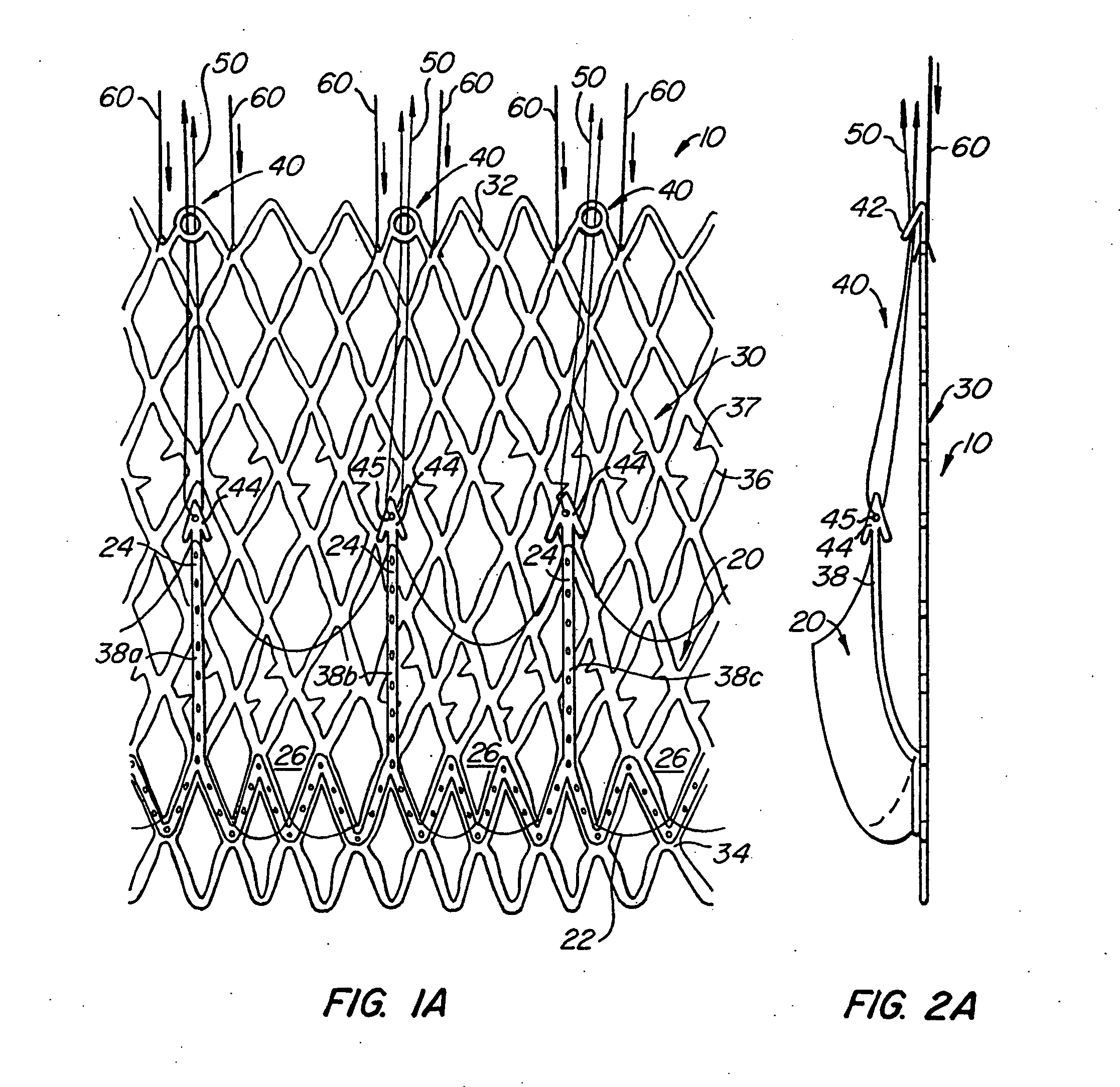
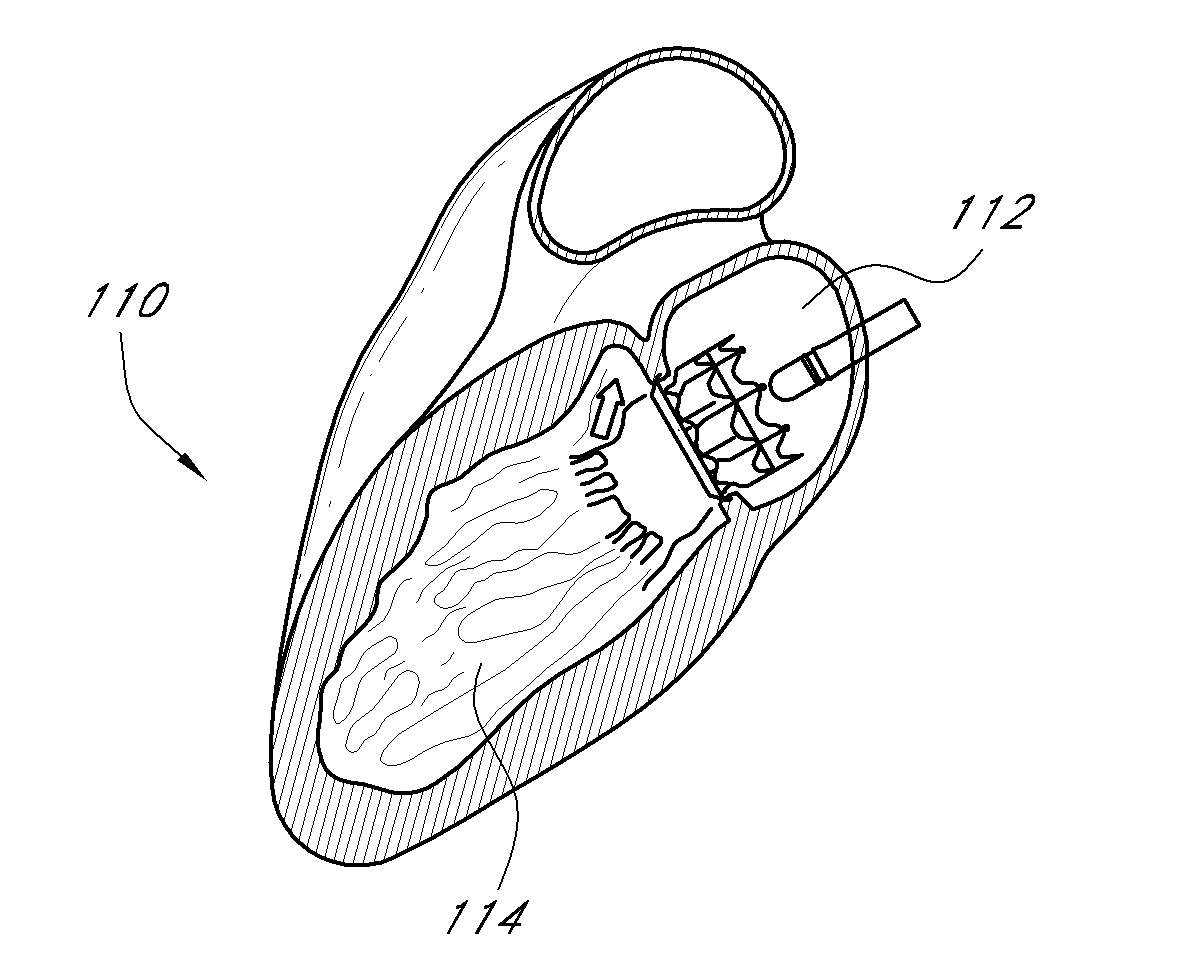
Transapical heart valve delivery system and method
ActiveUS20070112422A1Facilitate positioning of valveHelp positioningStentsBalloon catheterProsthetic heartLeft ventricular apex
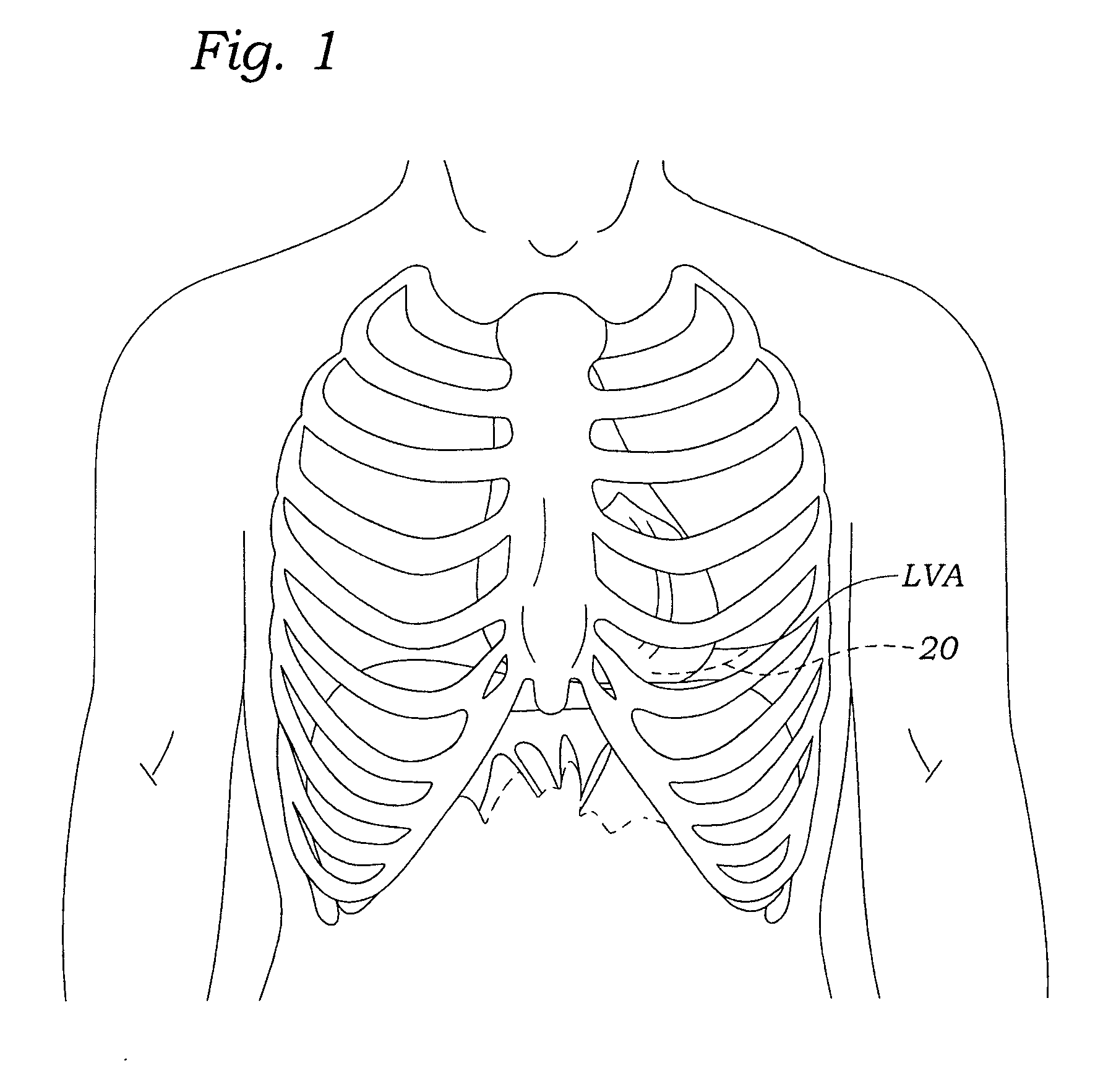
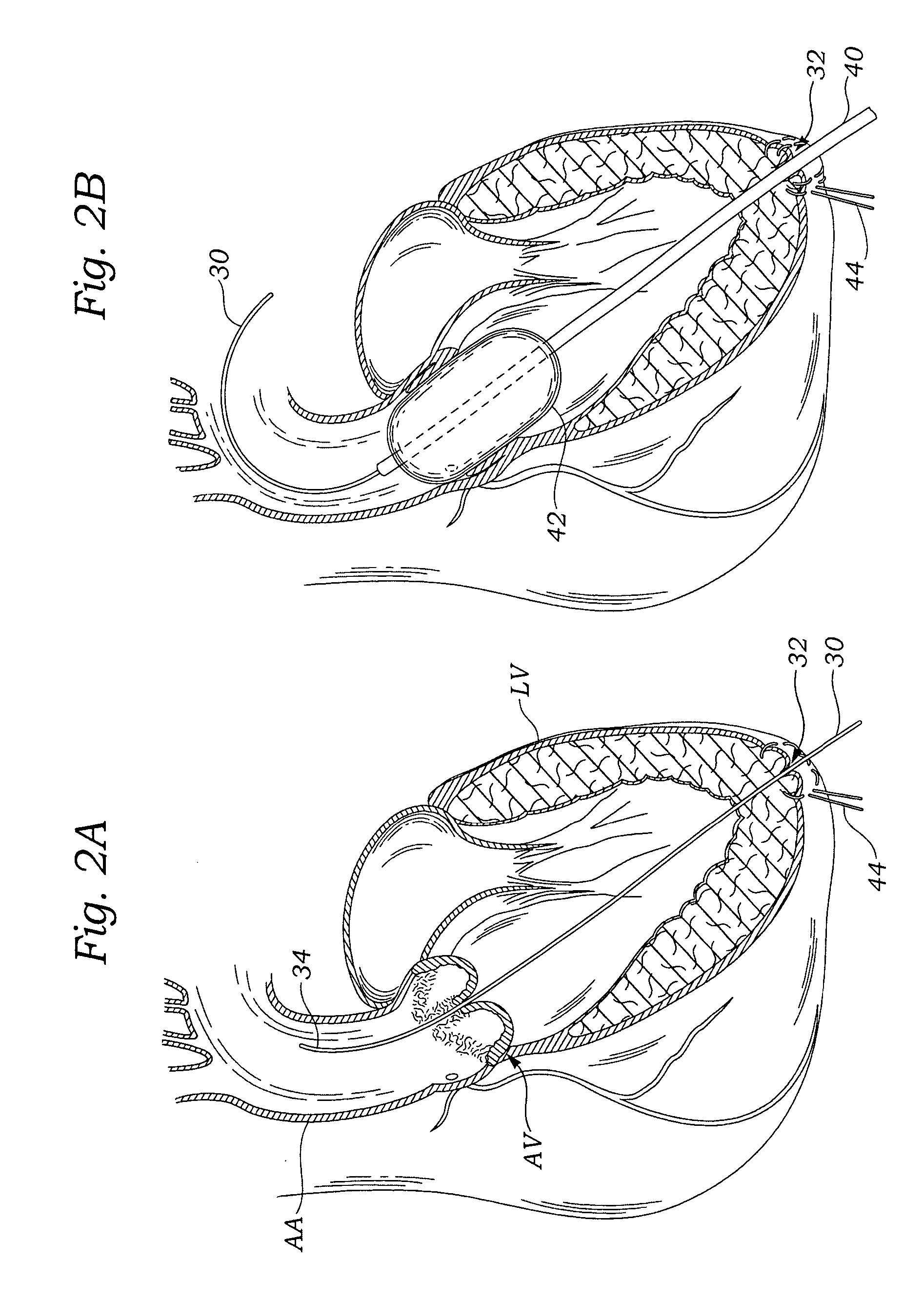
A delivery system and method for delivering a prosthetic heart valve to the aortic valve annulus. The system includes a balloon catheter having a steering mechanism thereon for delivering a balloon-expandable prosthetic heart valve through an introducer in an antegrade fashion to the aortic annulus. The balloon catheter passes through an introducer that accesses the left ventricle through its apex and a small incision in the chest wall. The balloon catheter includes a deflecting segment just proximal to the distal balloon to facilitate positioning of the prosthetic heart valve in the proper orientation within the aortic annulus. A slider in a deflection handle may be coupled to a deflection wire that actuates the deflecting segment. The method includes using two concentric rings of purse-string sutures around the puncture in the left ventricular apex to maintain a good seal around instruments passed therethrough. The prosthetic heart valve may be installed over the existing calcified leaflets, and a pre-dilation valvuloplasty procedure may also be utilized.
Owner:EDWARDS LIFESCIENCES CORP
Closed-loop method for controlling insulin infusion
A closed loop infusion system controls the rate that fluid is infused into the body of a user. The closed loop infusion system includes a sensor system, a controller, and a delivery system. The sensor system includes a sensor for monitoring a condition of the user. The sensor produces a sensor signal, which is representative of the condition of the user. The sensor signal is used to generate a controller input. The controller uses the controller input to generate commands to operate the delivery system. The delivery system infuses a liquid into the user at a rate dictated by the commands from the controller. Preferably, the sensor system monitors the glucose concentration in the body of the user, and the liquid infused by the delivery system into the body of the user includes insulin.
Owner:MEDTRONIC MIMIMED INC
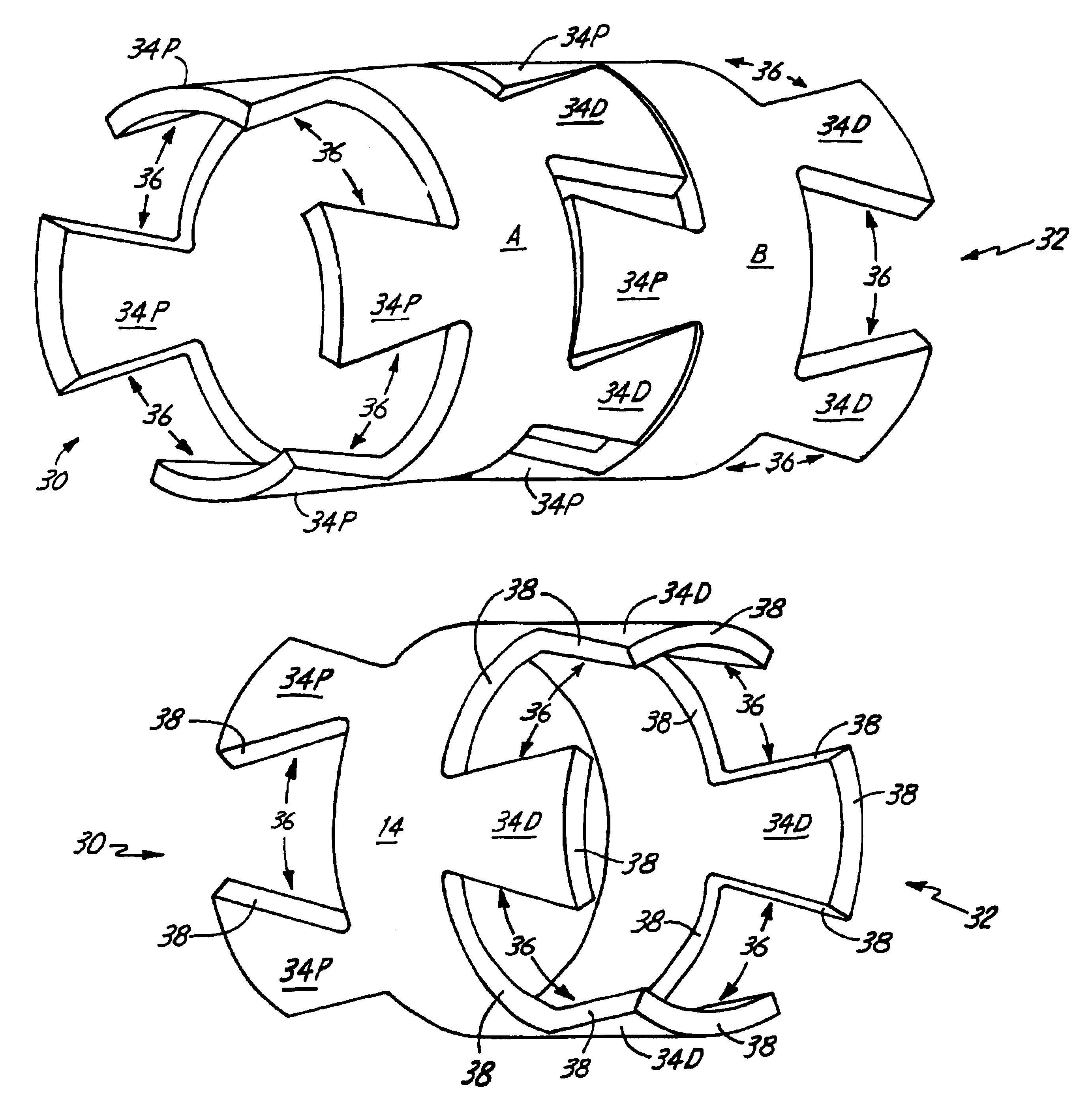
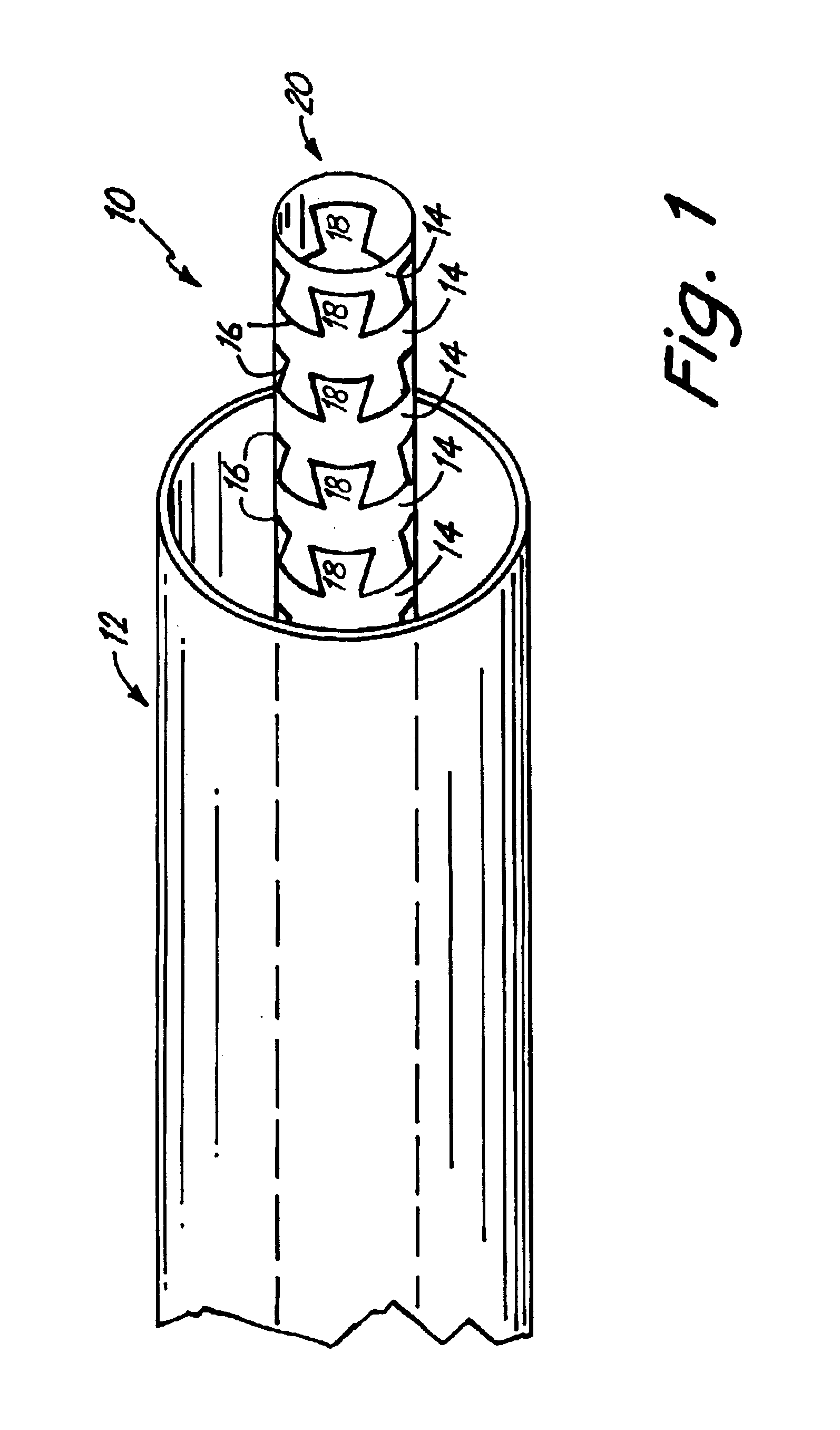
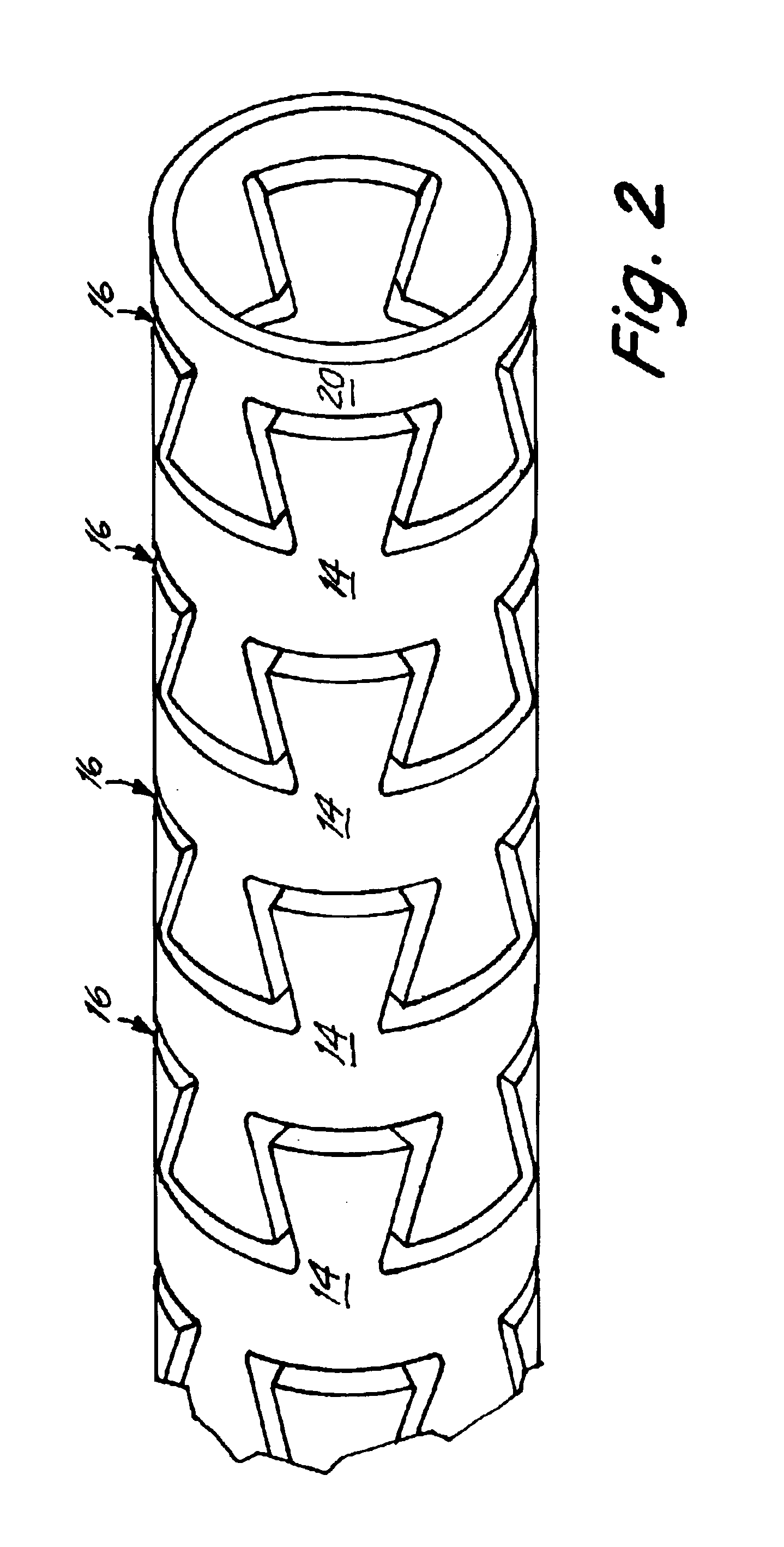
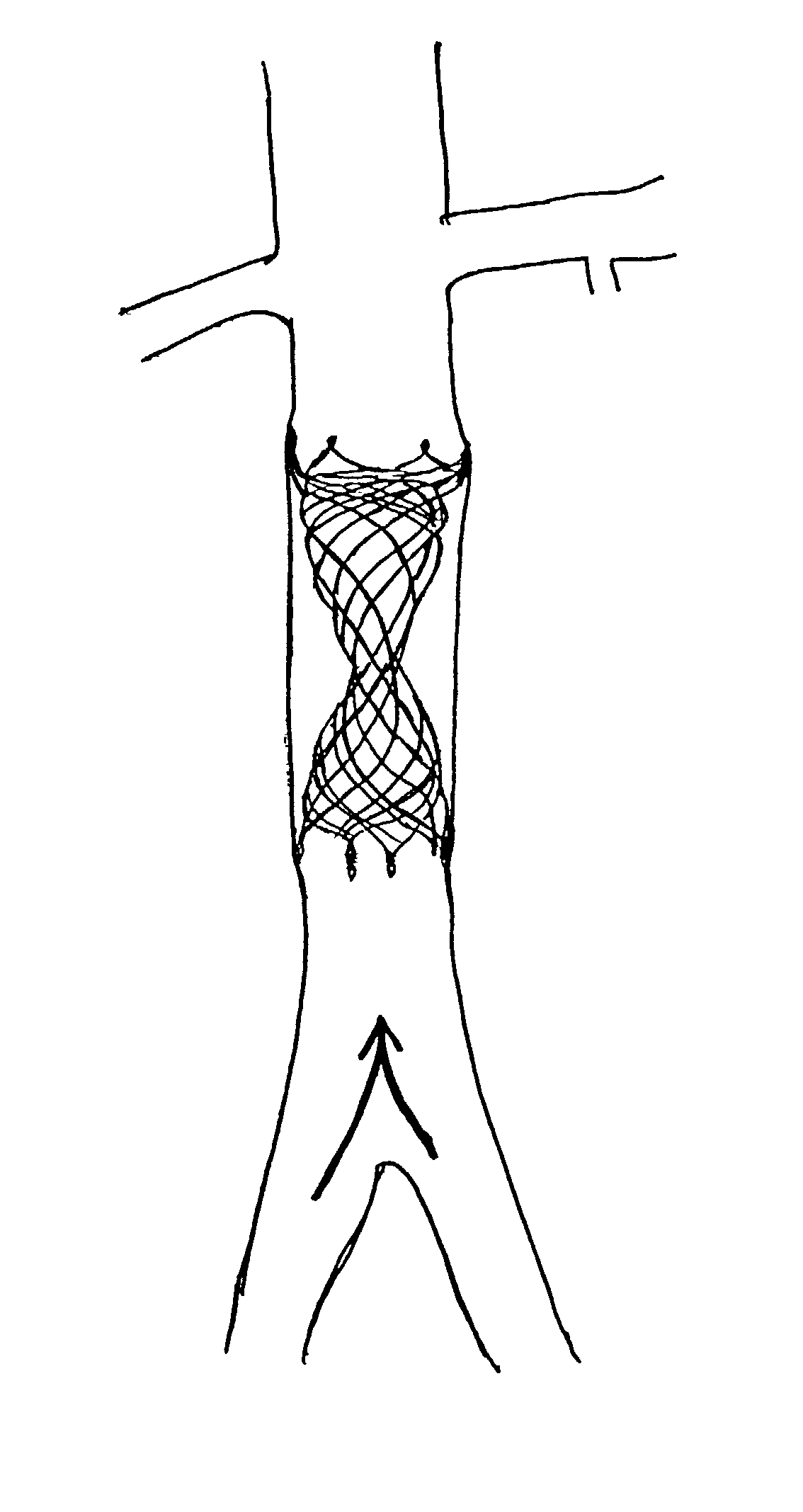
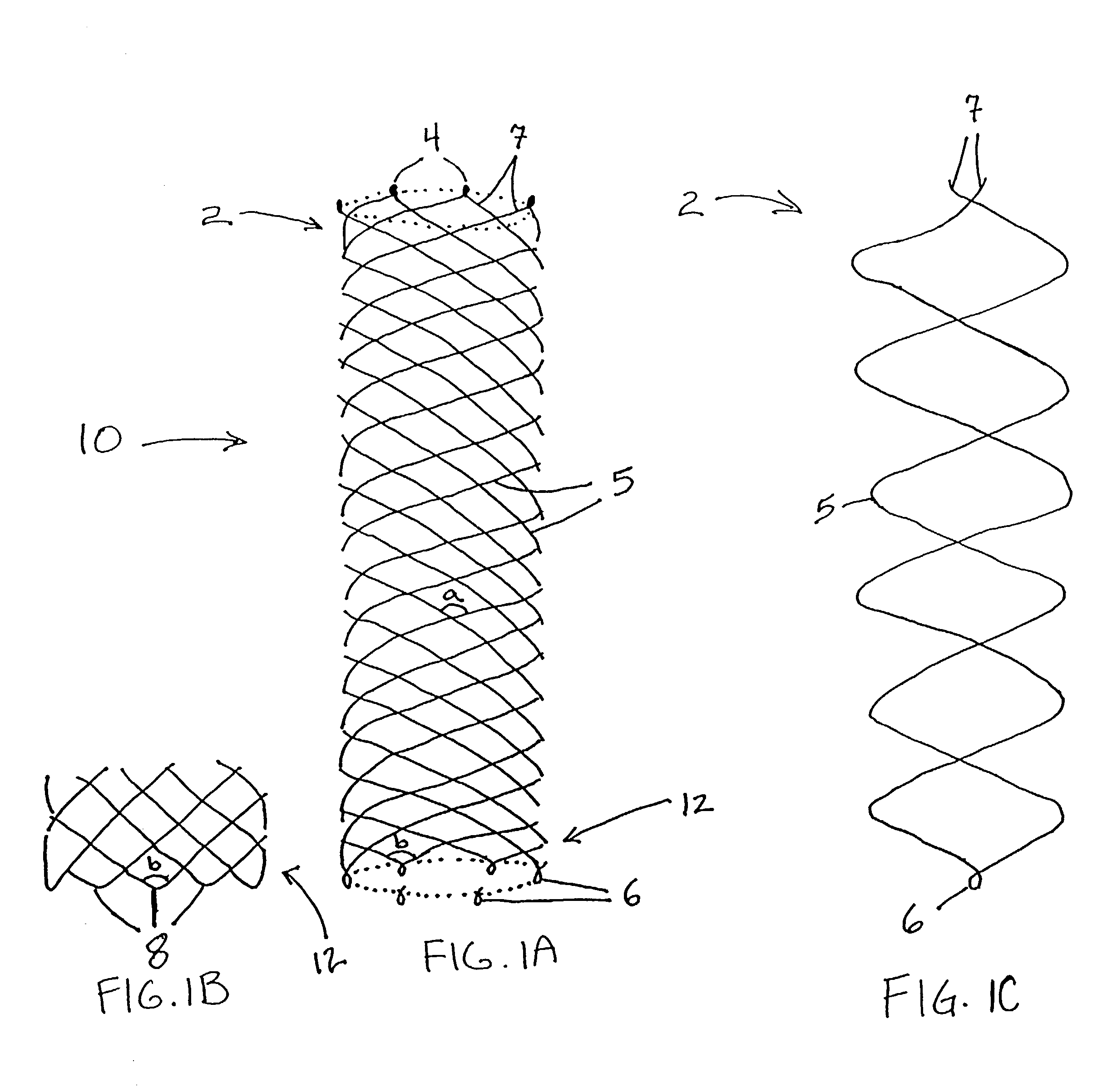
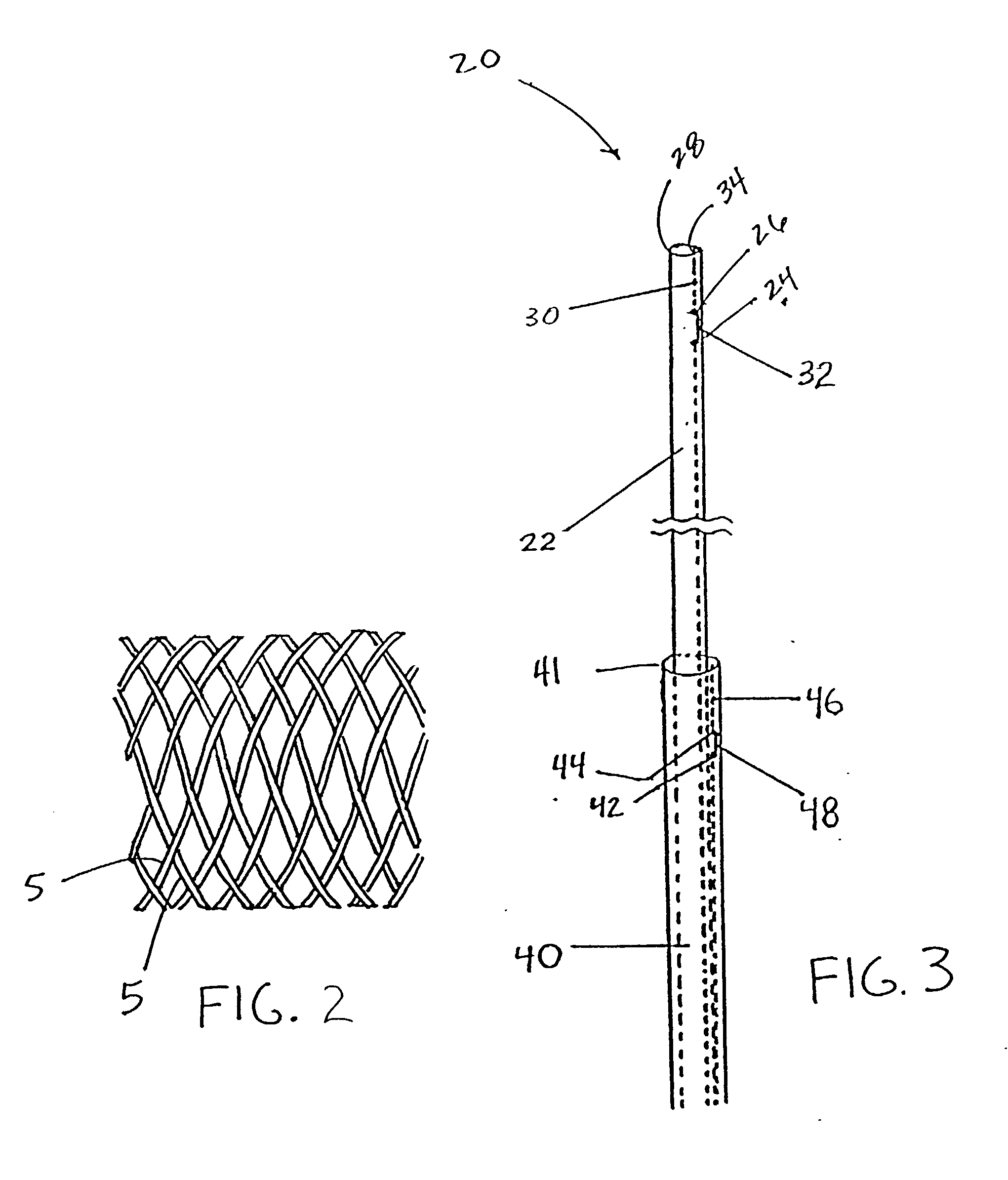
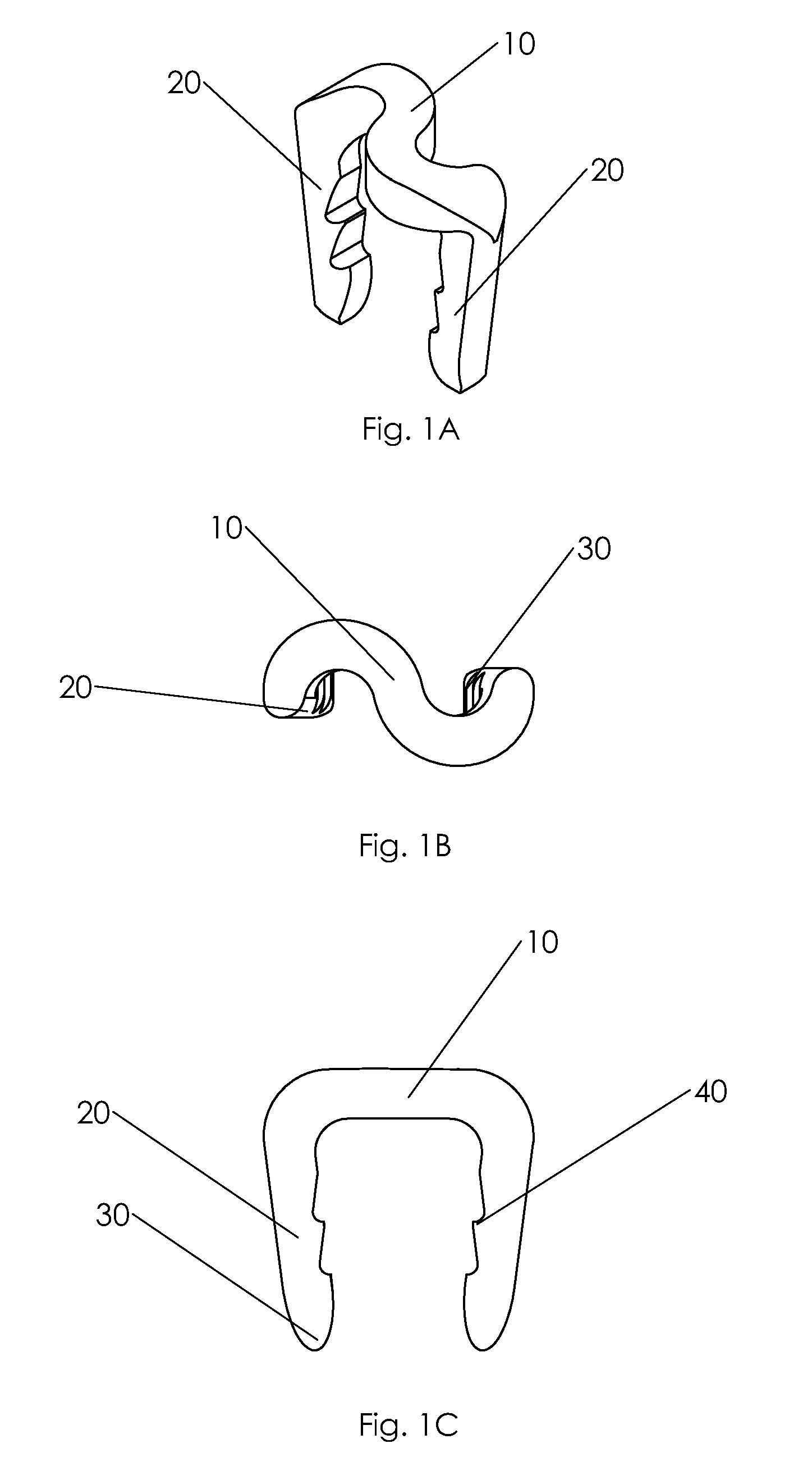
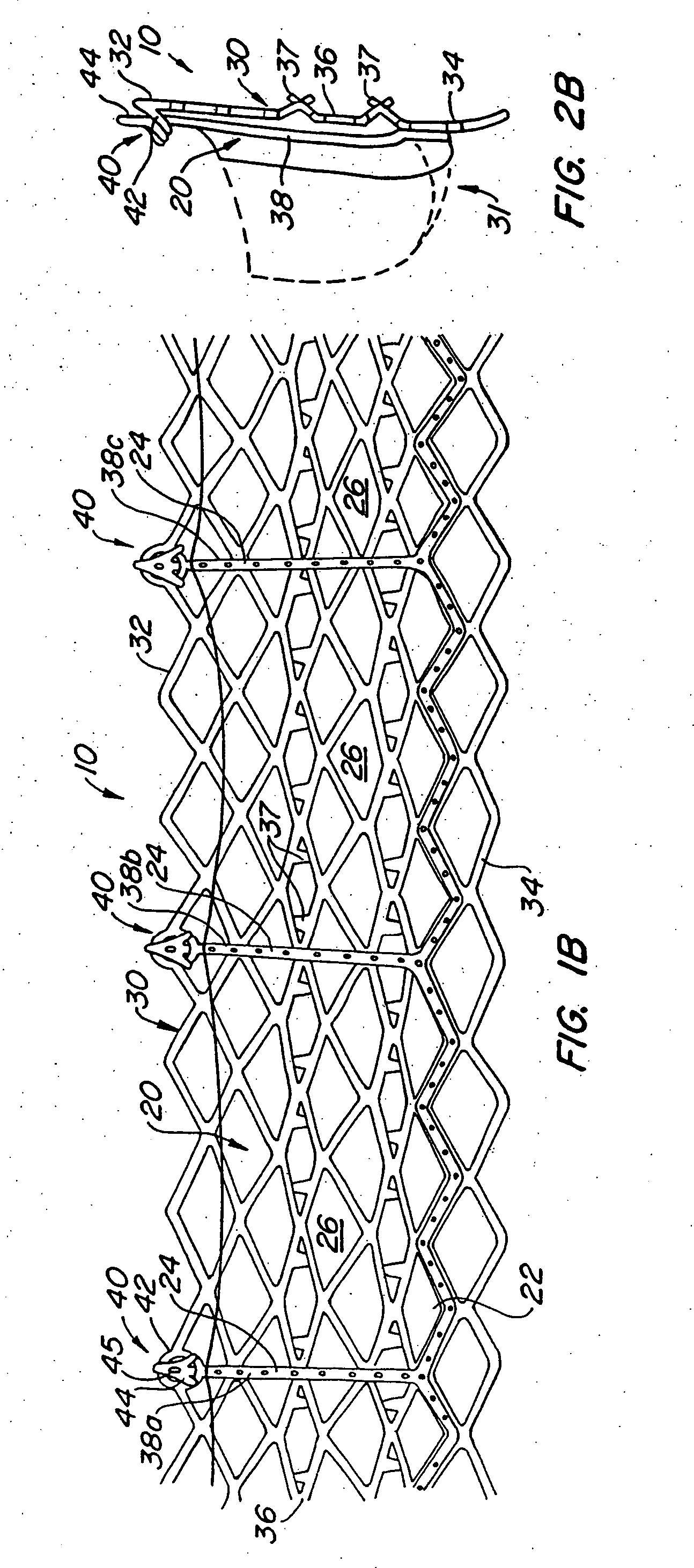
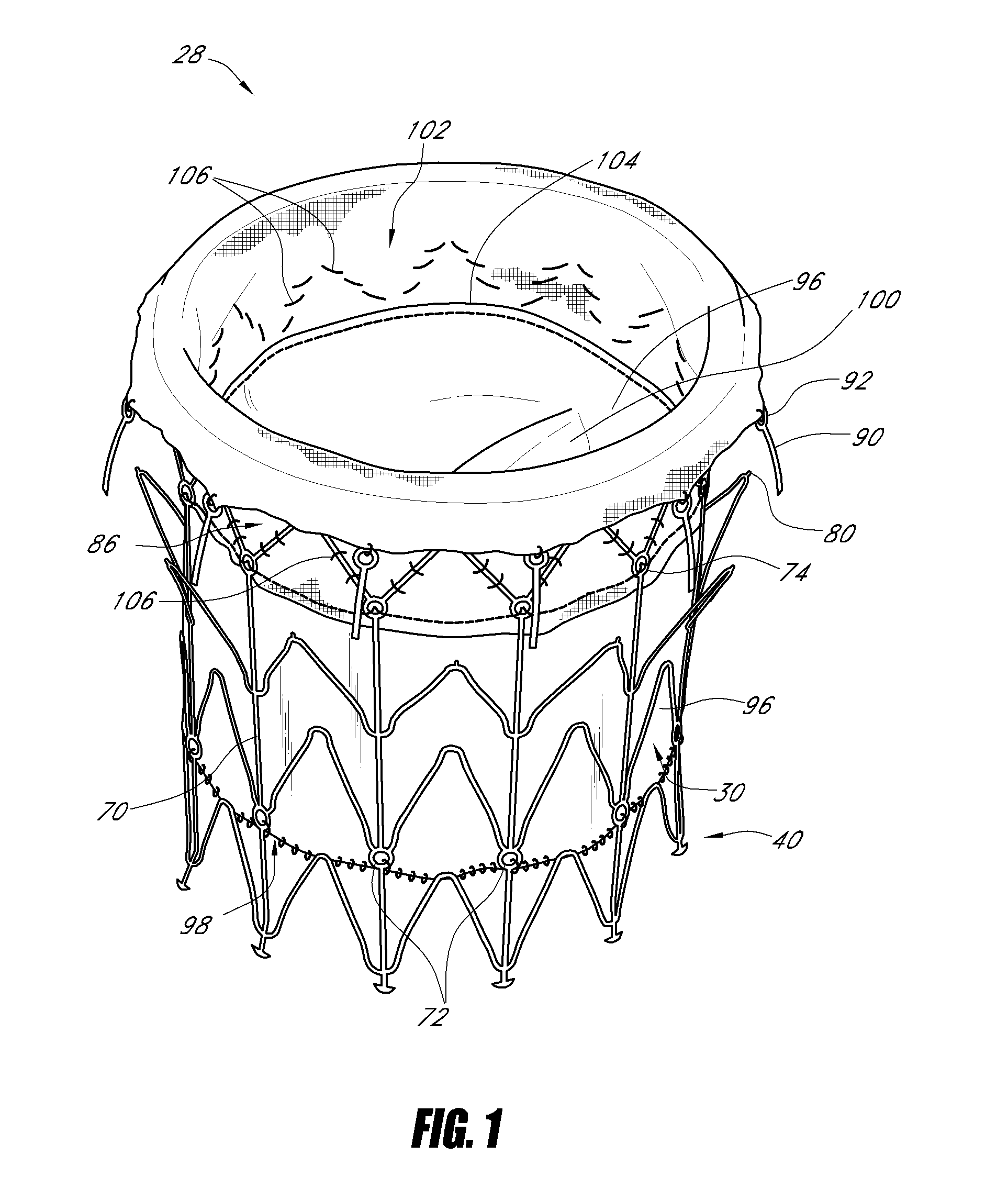
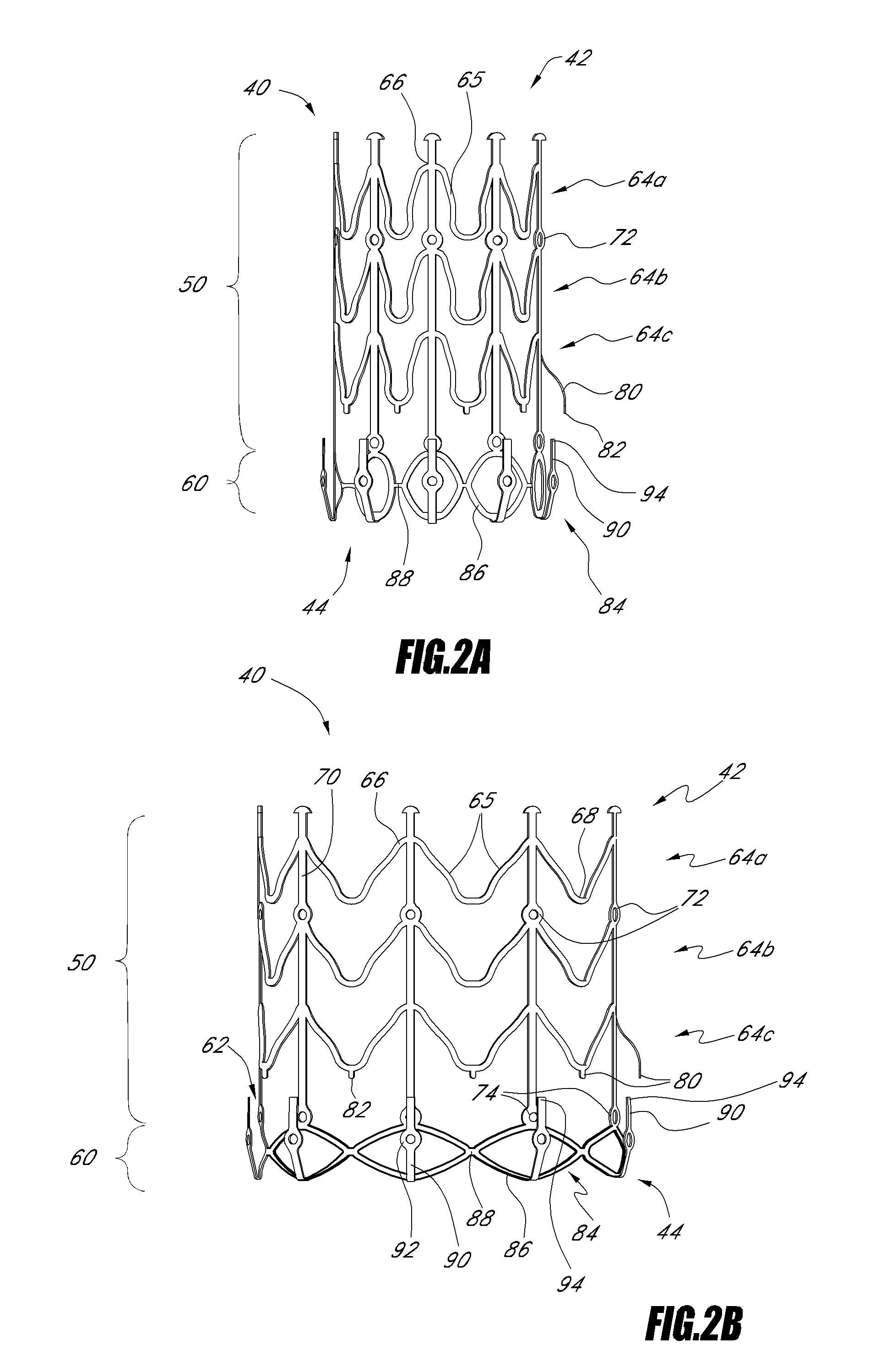
Delivery devices
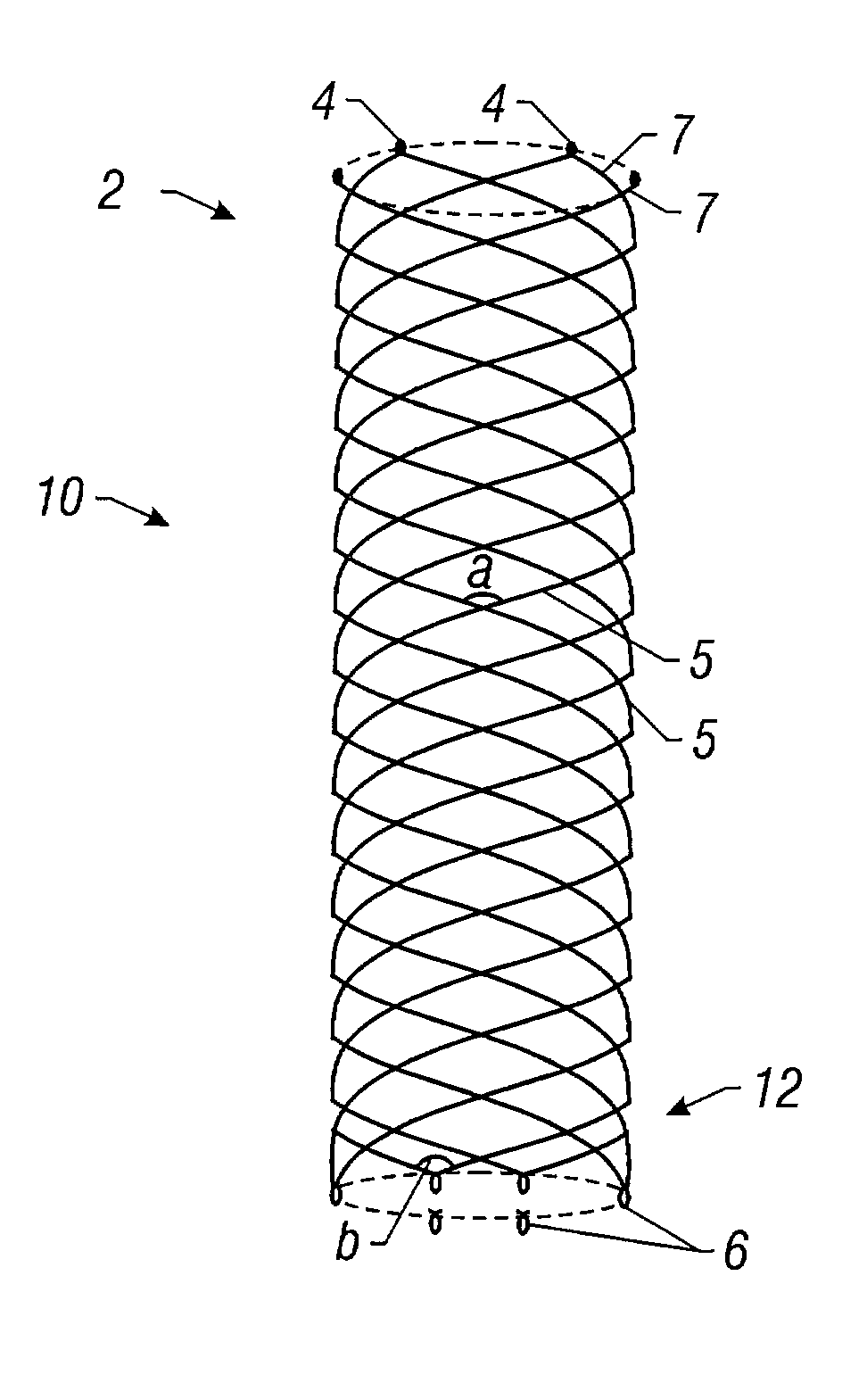
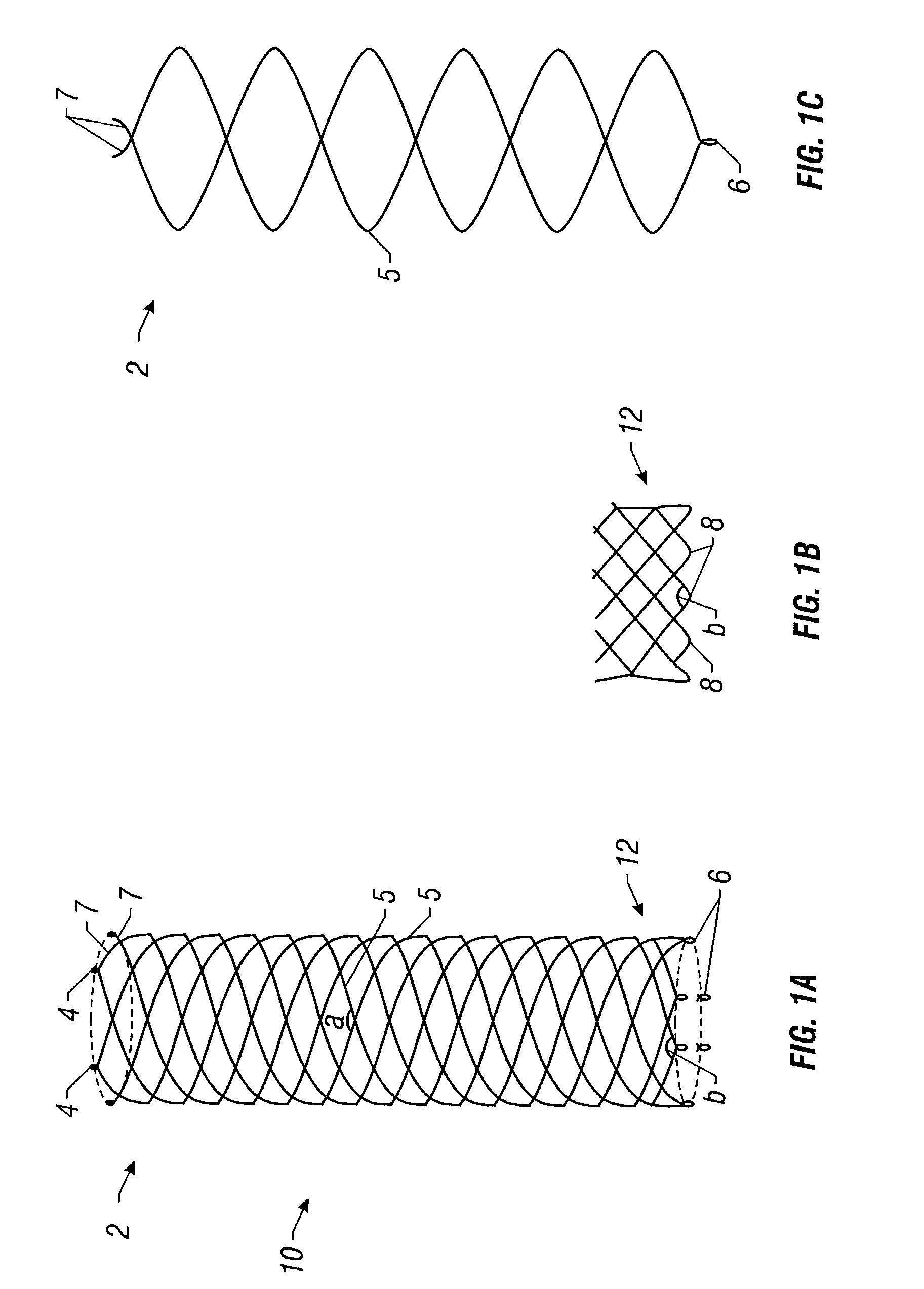
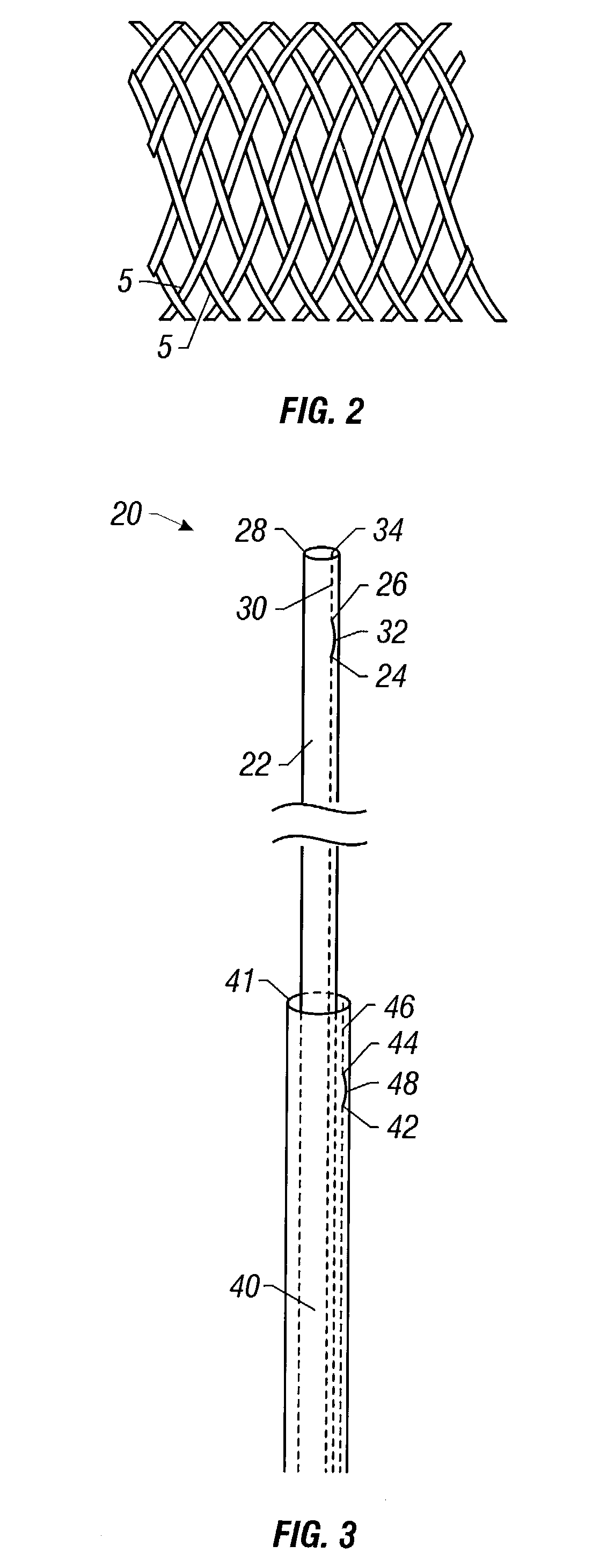
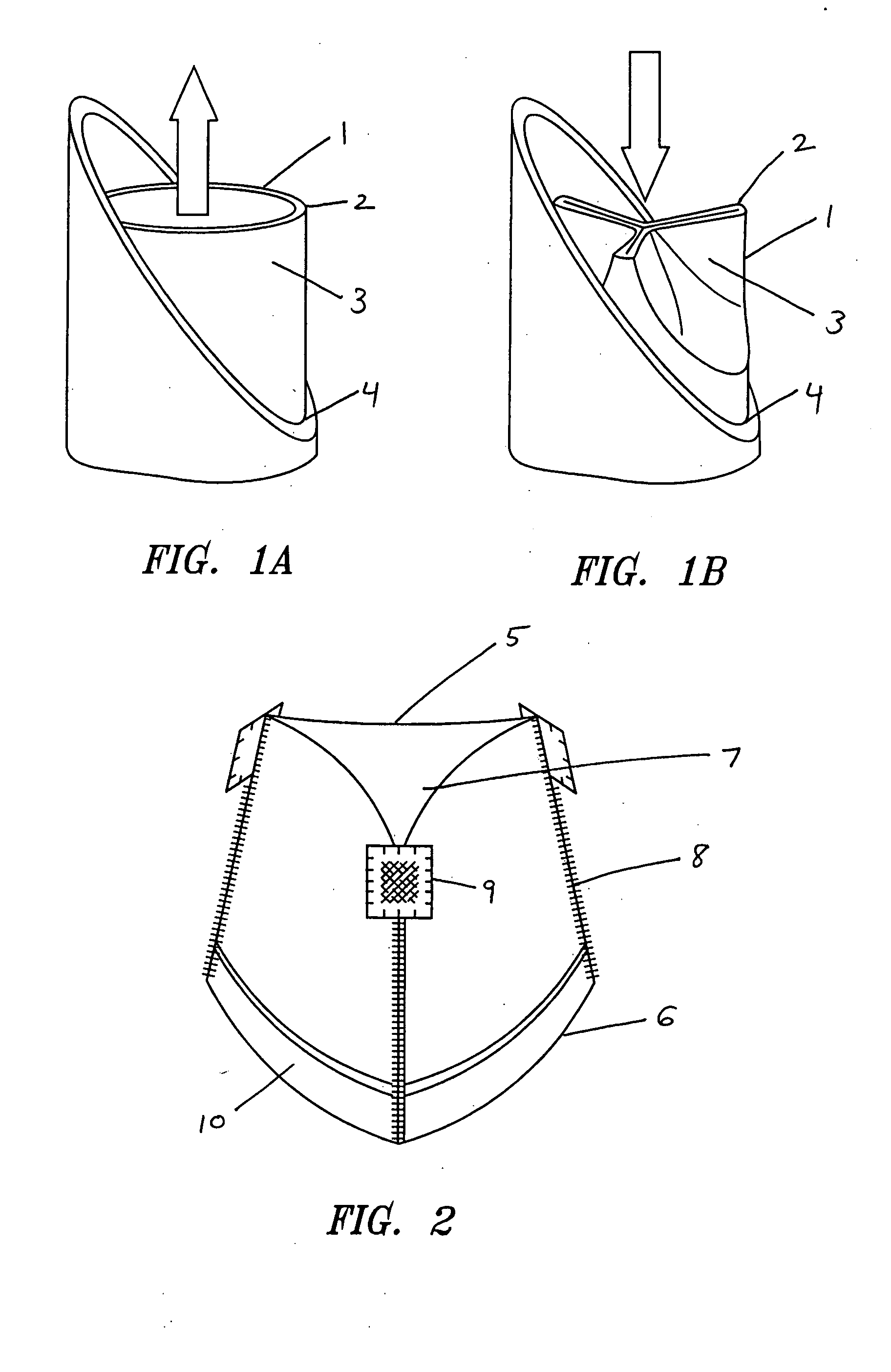
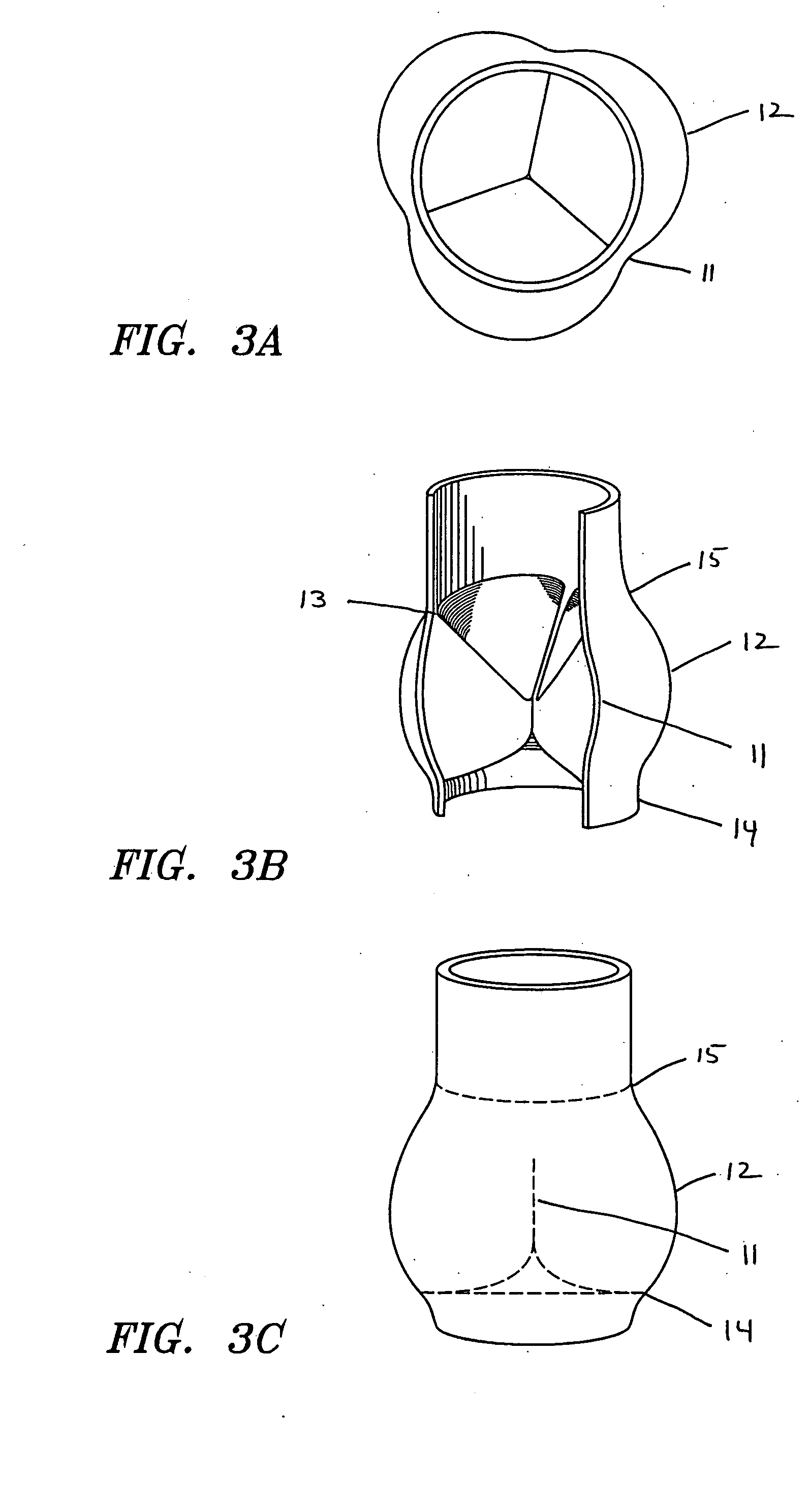
Self-expandable, woven intravascular devices for use as stents (both straight and tapered), filters (both temporary and permanent) and occluders for insertion and implantation into a variety of anatomical structures. The devices may be formed from shape memory metals such as nitinol. The devices may also be formed from biodegradable materials. Delivery systems for the devices include two hollow tubes that operate coaxially. A device is secured to the tubes prior to the implantation and delivery of the device by securing one end of the device to the outside of the inner tube and by securing the other end of the device to the outside of the outer tube. The stents may be partially or completely covered by graft materials, but may also be bare. The devices may be formed from a single wire. The devices may be formed by either hand or machine weaving. The devices may be created by bending shape memory wires around tabs projecting from a template, and weaving the ends of the wires to create the body of the device such that the wires cross each other to form a plurality of angles, at least one of the angles being obtuse. The value of the obtuse angle may be increased by axially compressing the body.
Owner:HYODOH HIDEKI +2
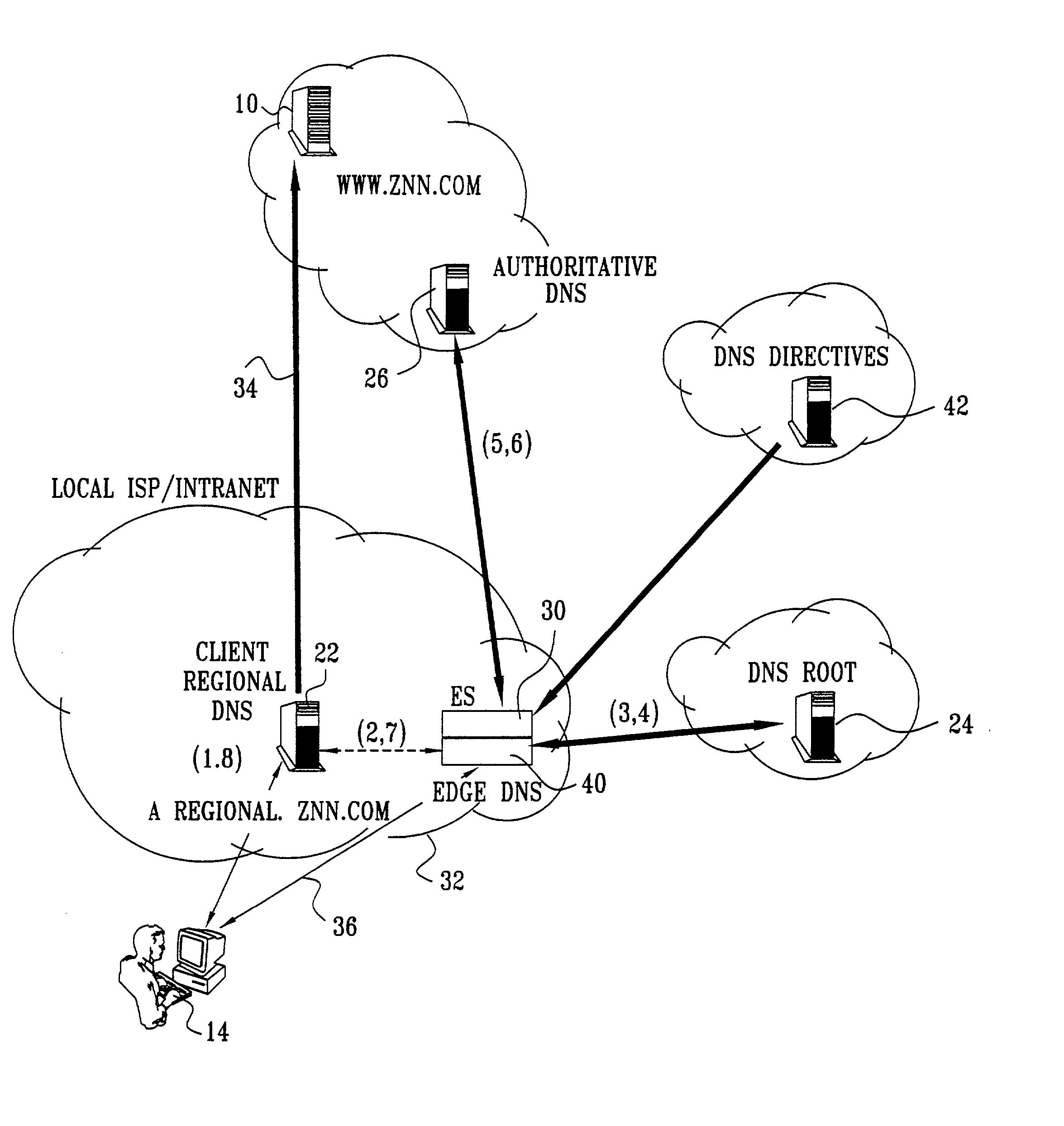
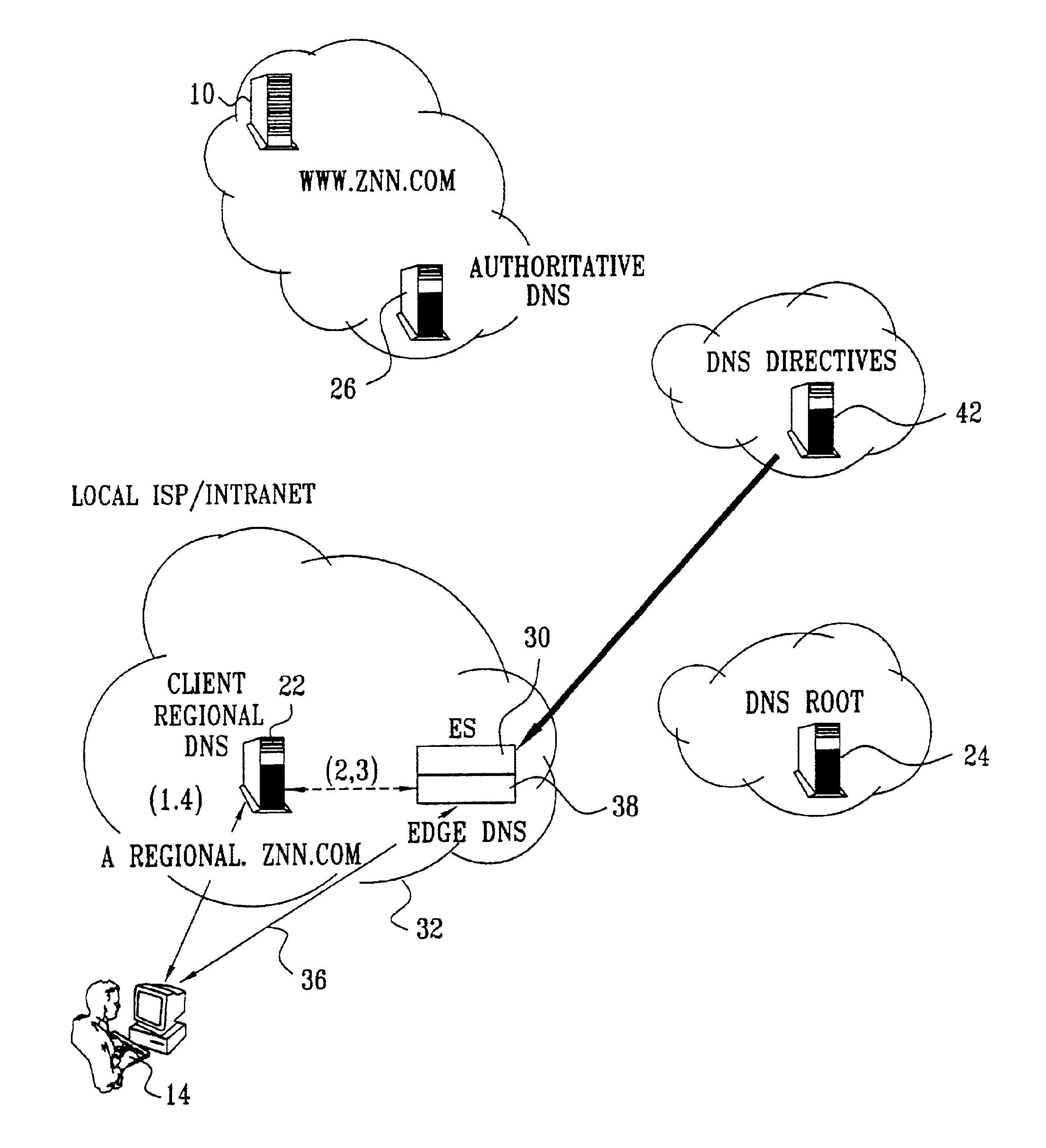
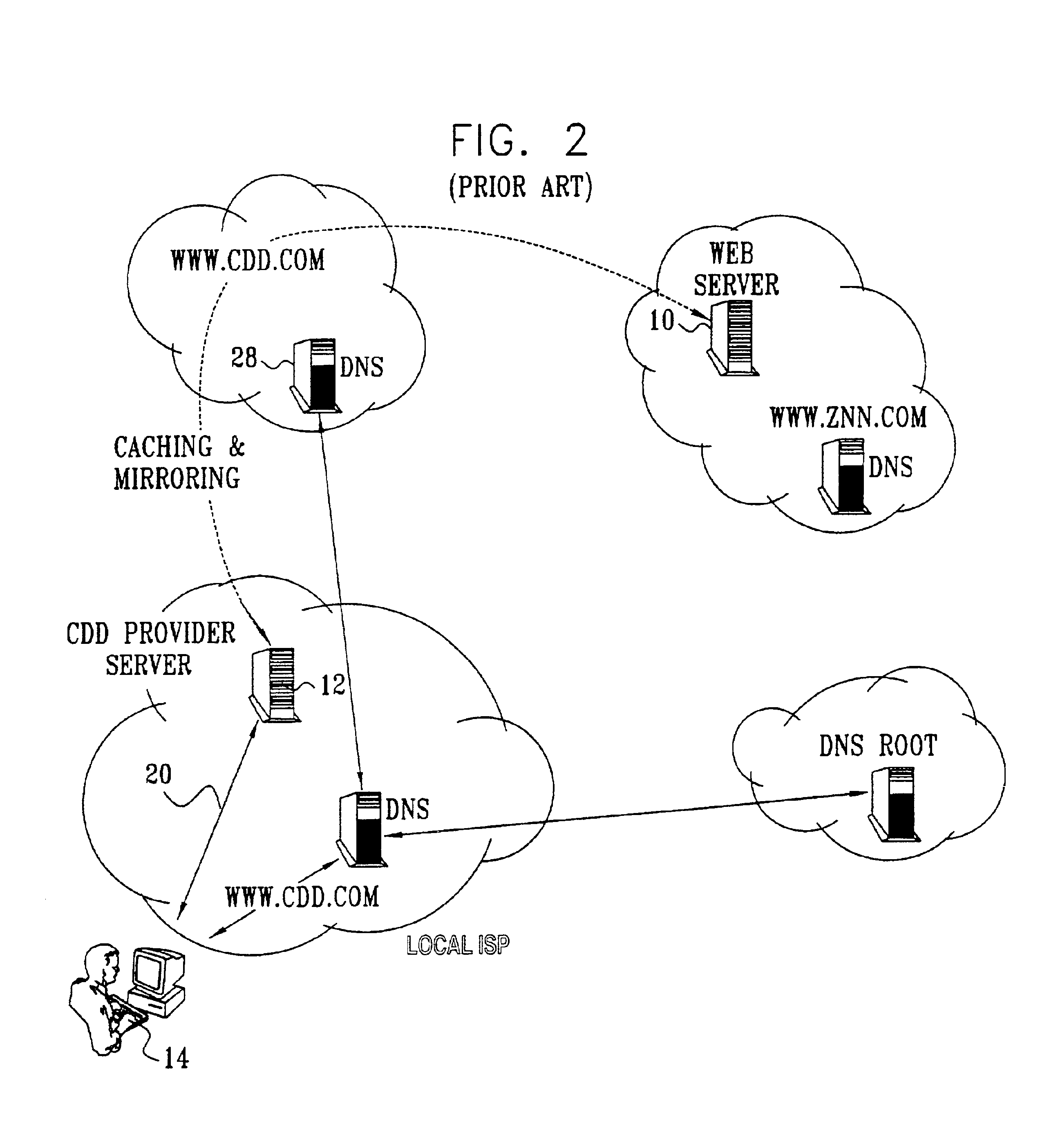
Differentiated content and application delivery via internet
InactiveUS20020010798A1Multiple digital computer combinationsWebsite content managementScalable systemEdge server
A technique for centralized and differentiated content and application delivery system allows content providers to directly control the delivery of content based on regional and temporal preferences, client identity and content priority. A scalable system is provided in an extensible framework for edge services, employing a combination of a flexible profile definition language and an open edge server architecture in order to add new and unforeseen services on demand. In one or more edge servers content providers are allocated dedicated resources, which are not affected by the demand or the delivery characteristics of other content providers. Each content provider can differentiate different local delivery resources within its global allocation. Since the per-site resources are guaranteed, intra-site differentiation can be guaranteed. Administrative resources are provided to dynamically adjust service policies of the edge servers.
Owner:CISCO TECH INC
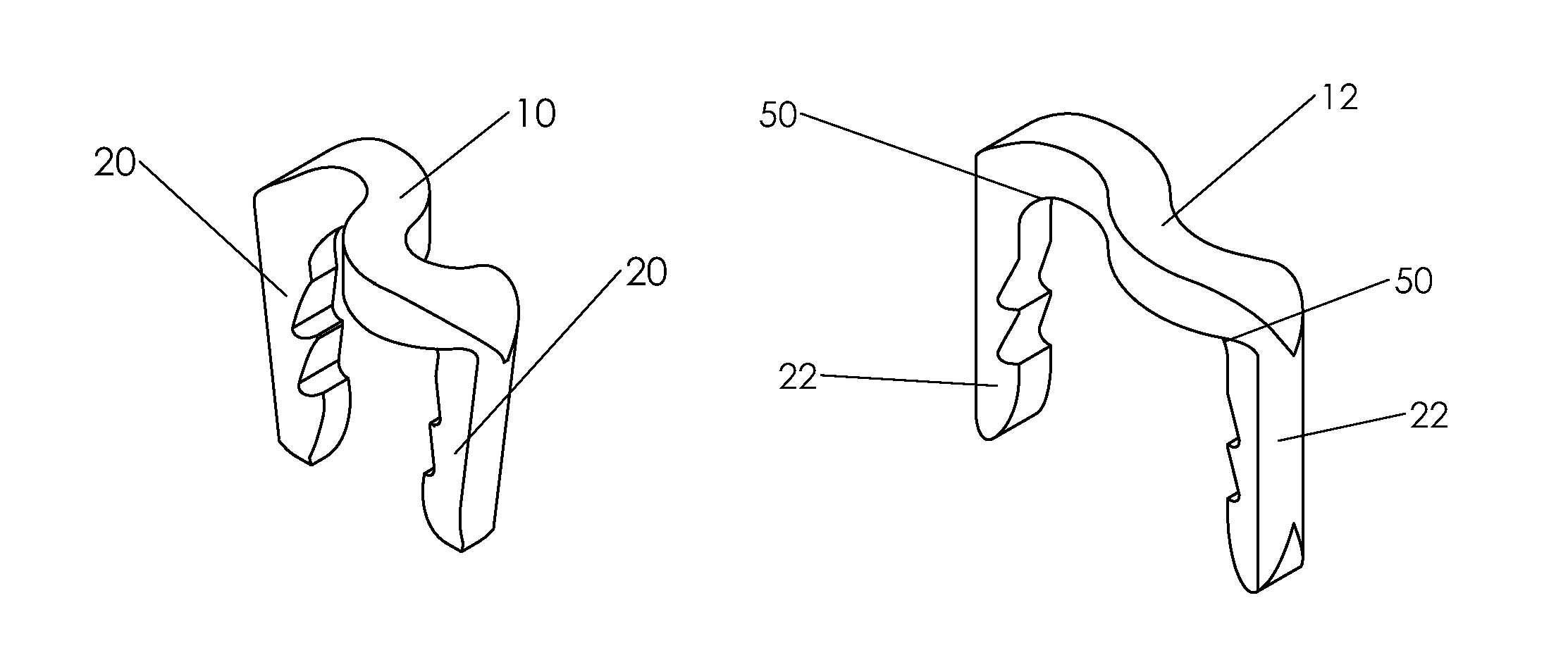
Bone staple, instrument and method of use and manufacturing
ActiveUS9017331B2Stores recoverable mechanical energyEasy to implantPinsInternal osteosythesisShape changeMechanical energy
Owner:FOX WILLIAM CASEY
Low profile heart valve and delivery system
Apparatus for endovascularly replacing a patient's heart valve, including: a delivery catheter having a diameter of 21 french or less; an expandable anchor disposed within the delivery catheter; and a replacement valve disposed within the delivery catheter. The invention also includes a method for endovascularly replacing a heart valve of a patient. In some embodiments the method includes the steps of: inserting a catheter having a diameter no more than 21 french into the patient; endovascularly delivering a replacement valve and an expandable anchor to a vicinity of the heart valve through the catheter; and deploying the anchor and the replacement valve.
Owner:BOSTON SCI SCIMED INC
Personalized content processing and delivery system and media
ActiveUS20060123053A1Improve economyImprove utilizationDigital data information retrievalDigital data processing detailsPersonalizationUser input
Owner:INSIGNIO TECH
Differentiated content and application delivery via internet
InactiveUS6976090B2Decentralized and differentiatedAdvanced technologyMultiple digital computer combinationsWebsite content managementScalable systemEdge server
Owner:CISCO TECH INC
Heart valve delivery system
ActiveUS20070005131A1Easy to navigateIncrease thrustBalloon catheterHeart valvesProsthetic heartBalloon catheter
A delivery system for delivering a prosthetic heart valve to a native valve site within the human vasculature. The prosthetic valve is disposed on a balloon at the end of a balloon catheter. The balloon catheter passes through a delivery sleeve assembly and handle. A pull wire travels from the handle to a distal end of the delivery sleeve assembly. Actuation of the handle pulls on the pull wire, which causes openings in a slotted tube of the delivery sleeve assembly to close, thus causing the delivery sleeve assembly to bend. A stretchable cover is placed over the slotted tube for biasing the steerable catheter toward a straight position. Once advanced to the native valve site, the prosthetic valve is deployed by inflating the balloon.
Owner:EDWARDS LIFESCIENCES CORP
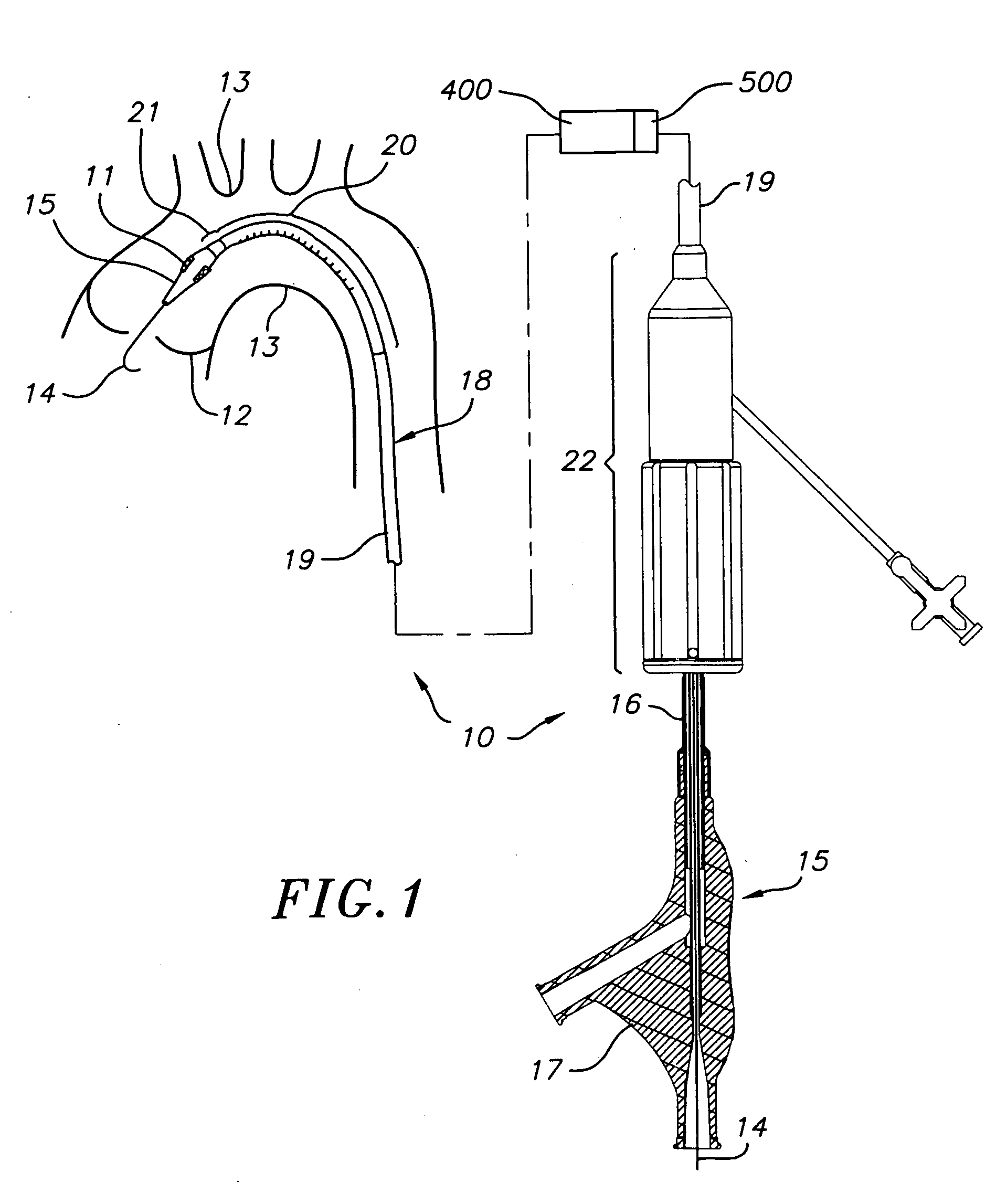
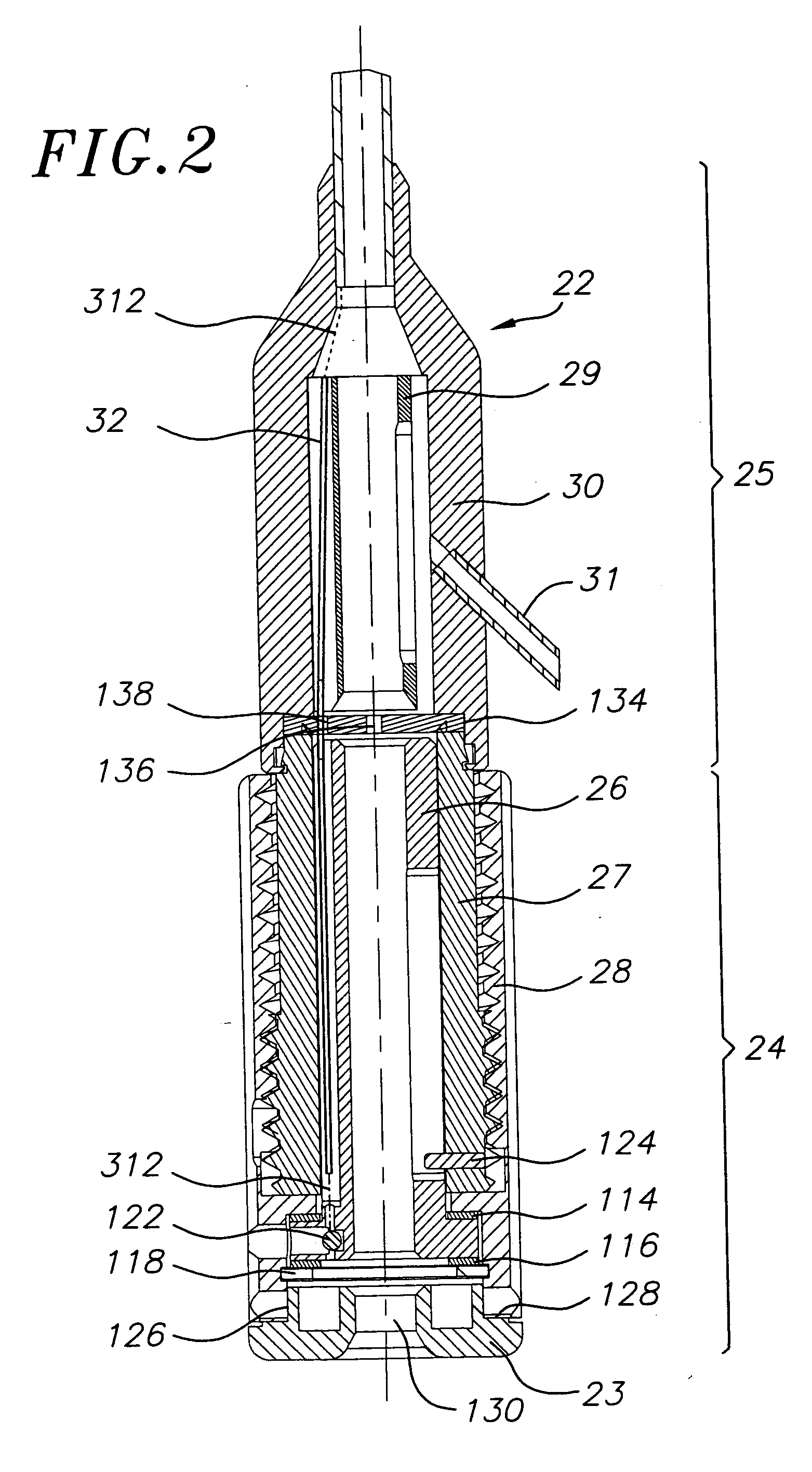
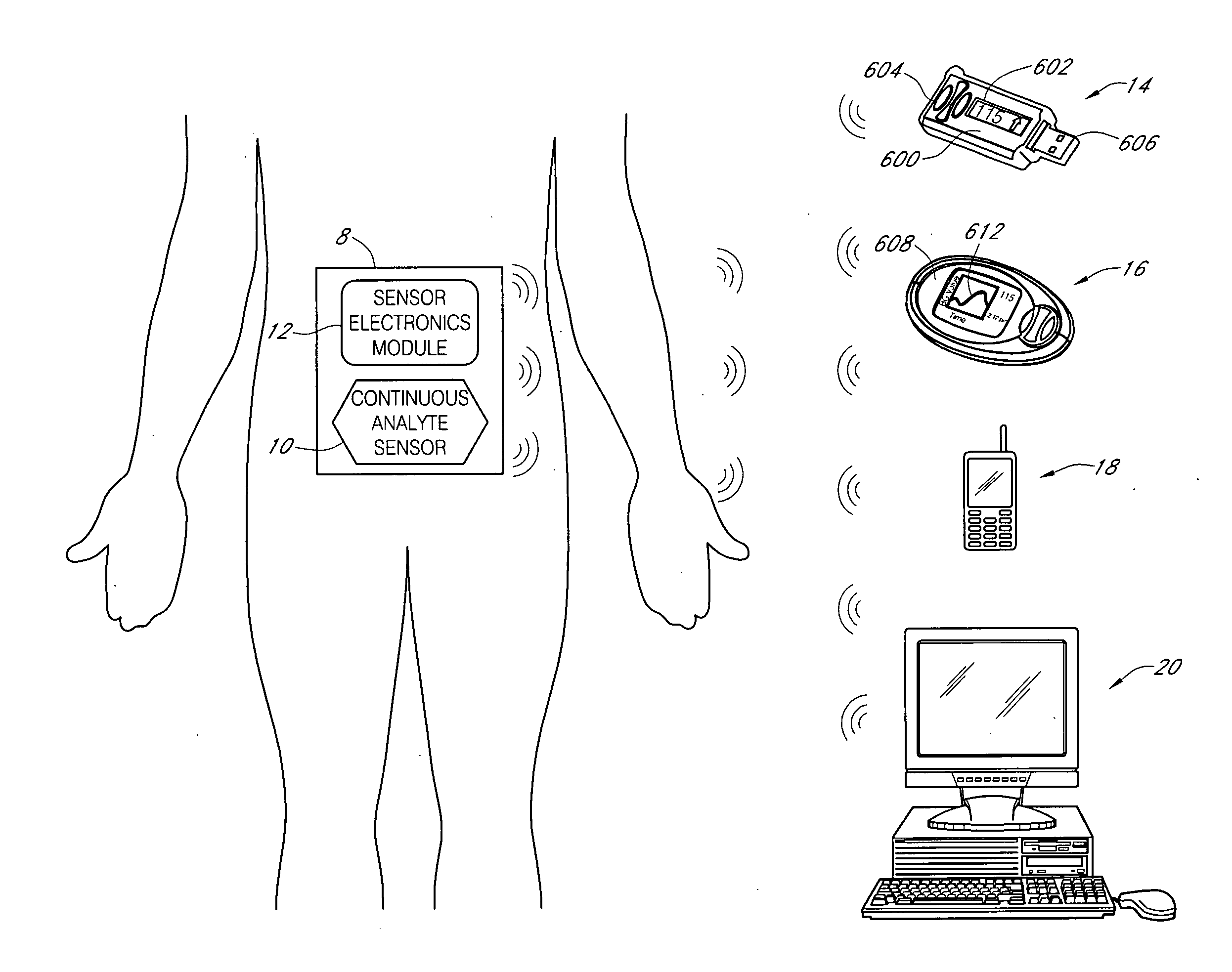
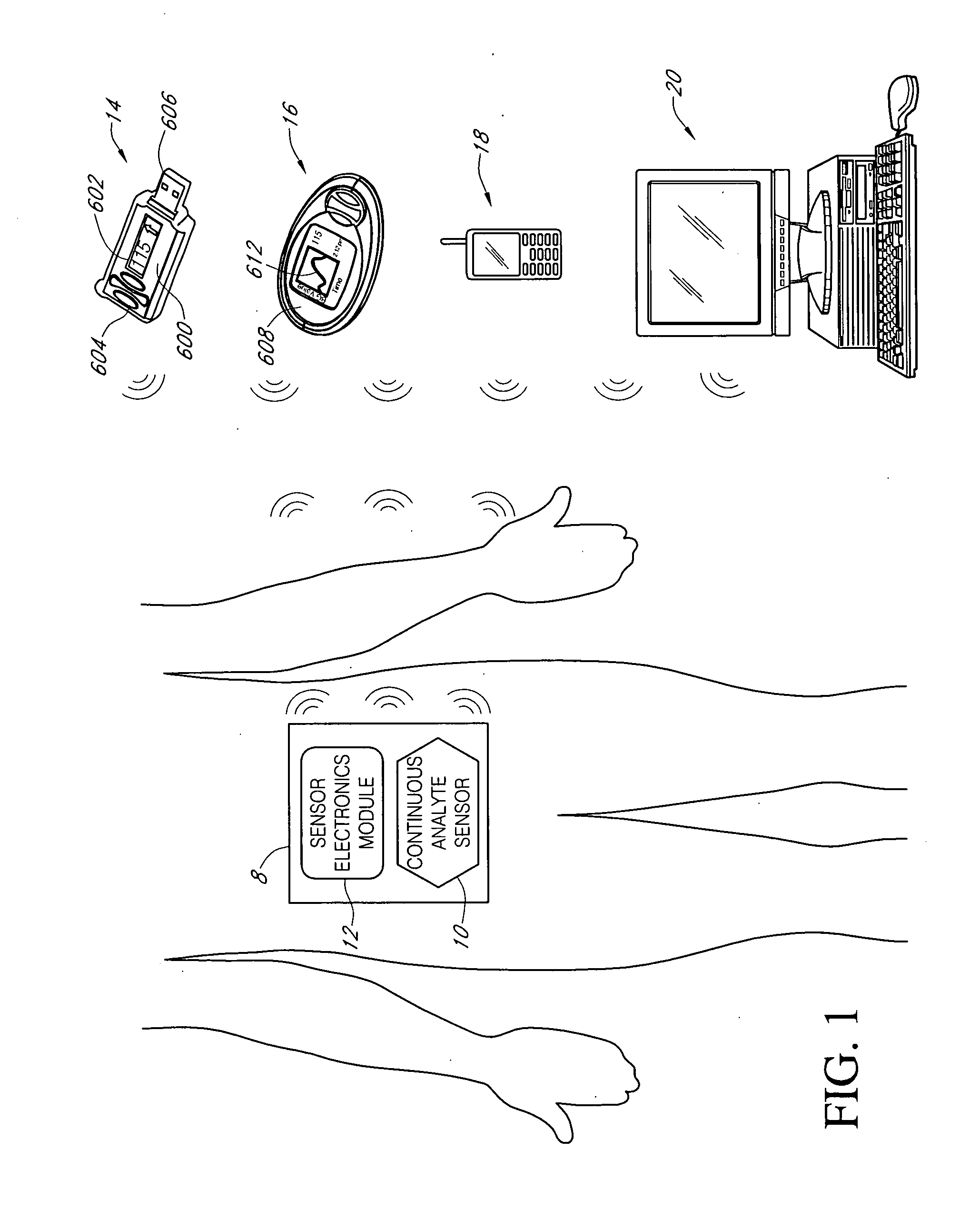
Systems and methods for blood glucose monitoring and alert delivery
Systems and methods for continuous measurement of an analyte in a host are provided. The system generally includes a continuous analyte sensor configured to continuously measure a concentration of analyte in a host and a sensor electronics module physically connected to the continuous analyte sensor during sensor use, wherein the sensor electronics module is further configured to directly wirelessly communicate displayable sensor information to a plurality of different types of display devices.
Owner:DEXCOM
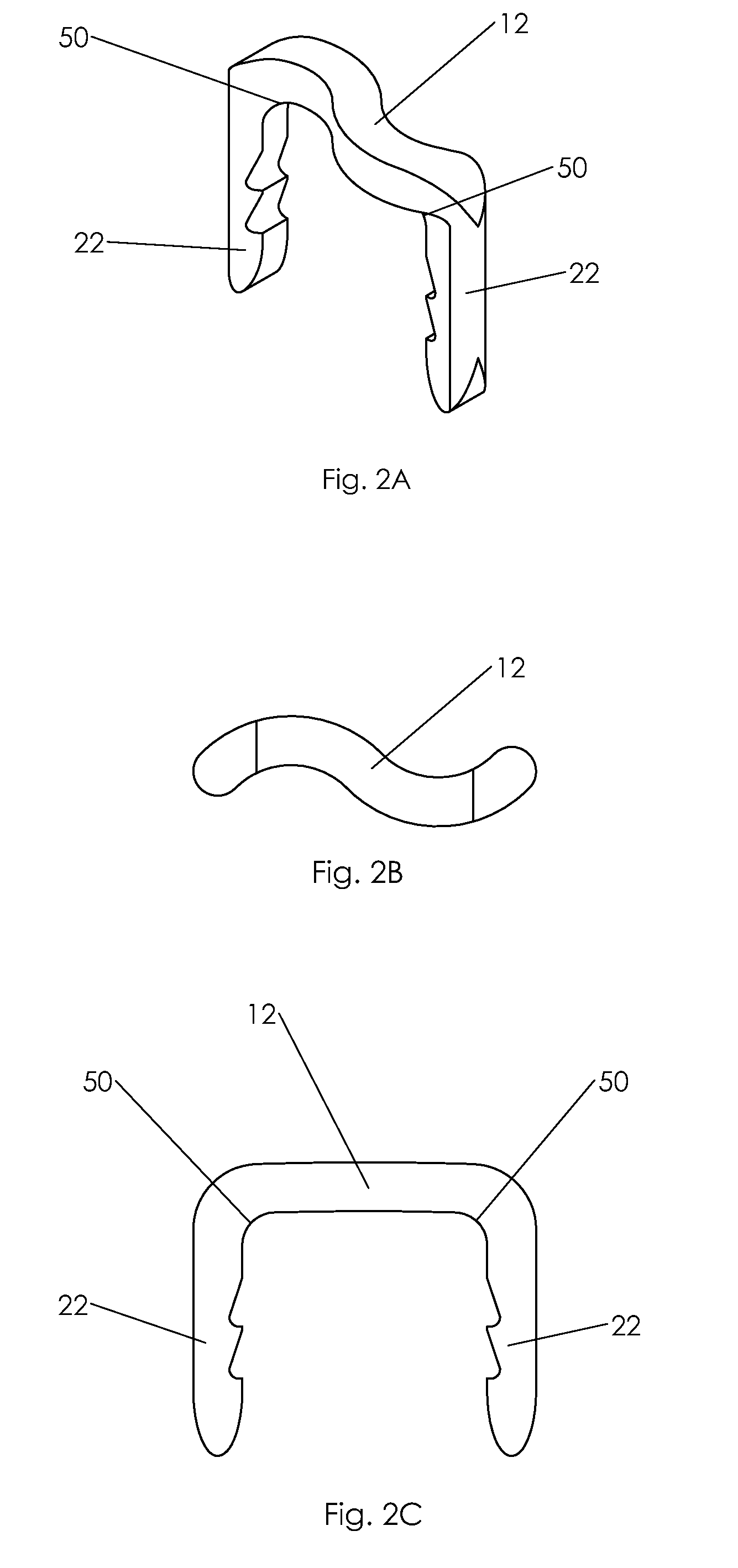
Methods for creating woven devices
Self-expandable, woven intravascular devices for use as stents (both straight and tapered), filters (both temporary and permanent) and occluders for insertion and implantation into a variety of anatomical structures. The devices may be formed from shape memory metals such as nitinol. The devices may also be formed from biodegradable materials. Delivery systems for the devices include two hollow tubes that operate coaxially. A device is secured to the tubes prior to the implantation and delivery of the device by securing one end of the device to the outside of the inner tube and by securing the other end of the device to the outside of the outer tube. The stents may be partially or completely covered by graft materials, but may also be bare. The devices may be formed from a single wire. The devices may be formed by either hand or machine weaving. The devices may be created by bending shape memory wires around tabs projecting from a template, and weaving the ends of the wires to create the body of the device such that the wires cross each other to form a plurality of angles, at least one of the angles being obtuse. The value of the obtuse angle may be increased by axially compressing the body.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
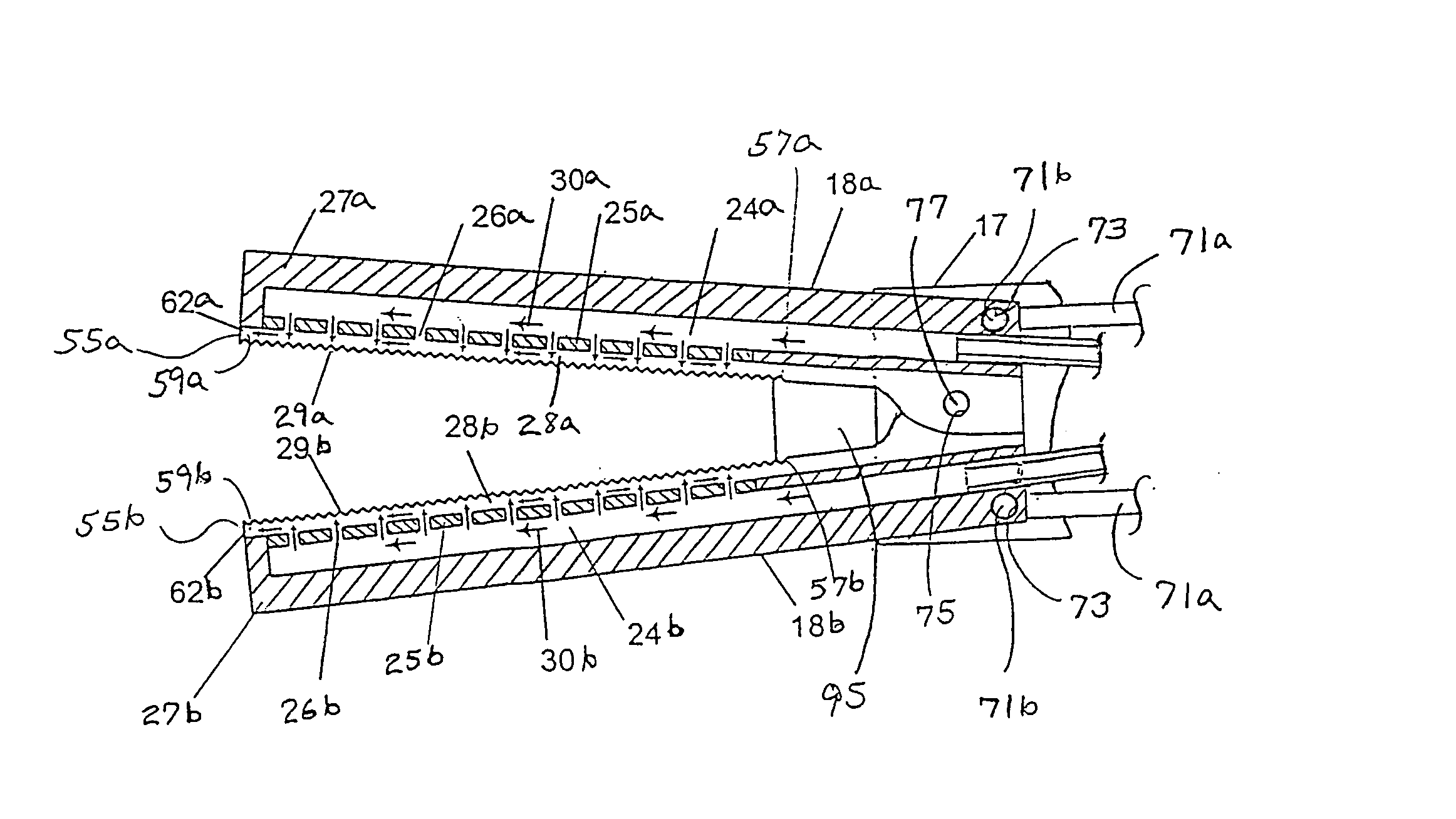
Multi-actuating trigger anchor delivery system
ActiveUS8758366B2Sufficient energyEasy to withdrawSuture equipmentsWound clampsDiseaseBiomedical engineering
A single trigger system and associated method for manipulating tissues and anatomical or other structures in medical applications for the purpose of treating diseases or disorders or other purposes. In one aspect, the system includes a delivery device configured to deploy and implant anchor devices for such purposes.
Owner:TELEFLEX LIFE SCI LTD
Colon targeted delivery system
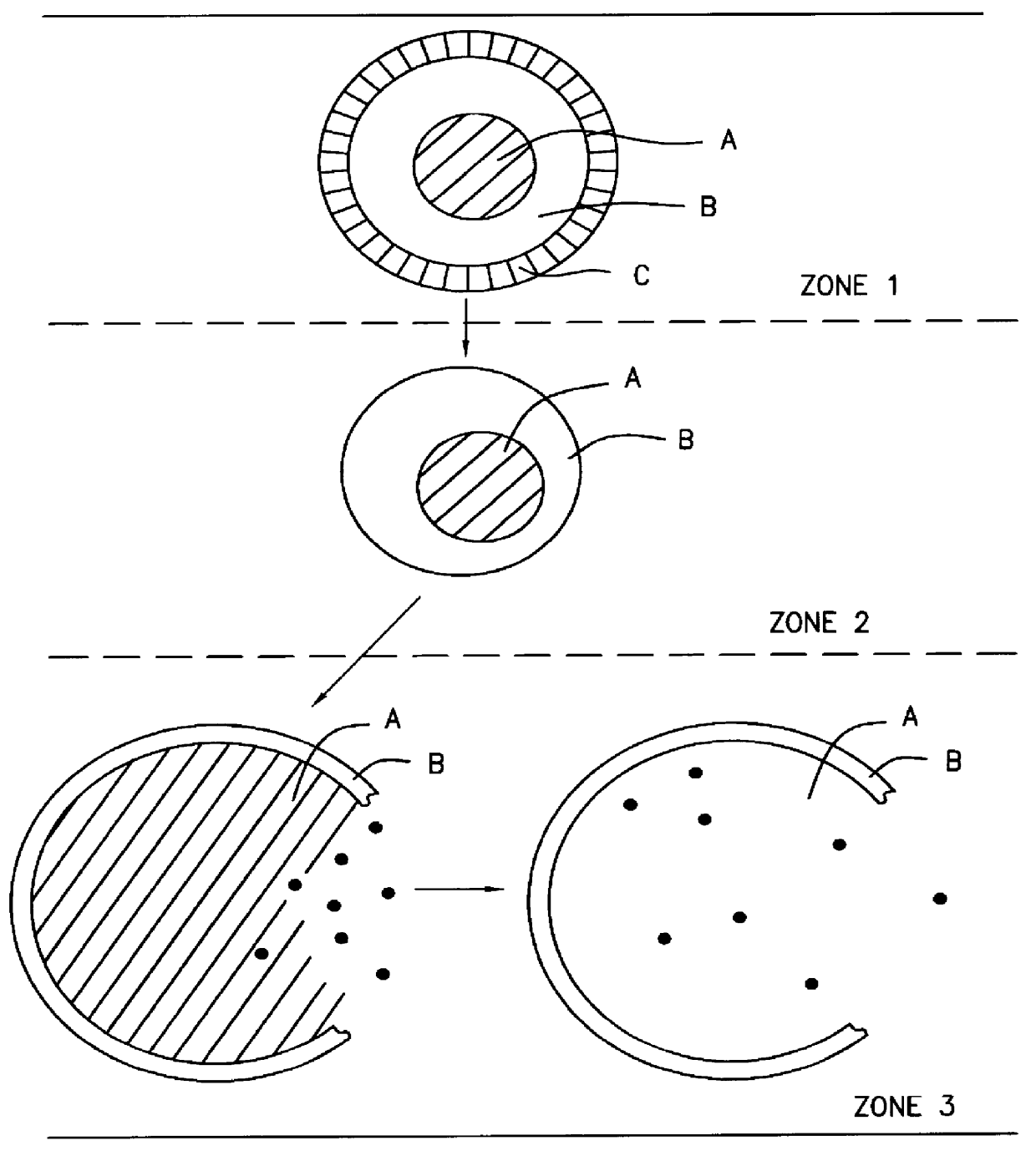
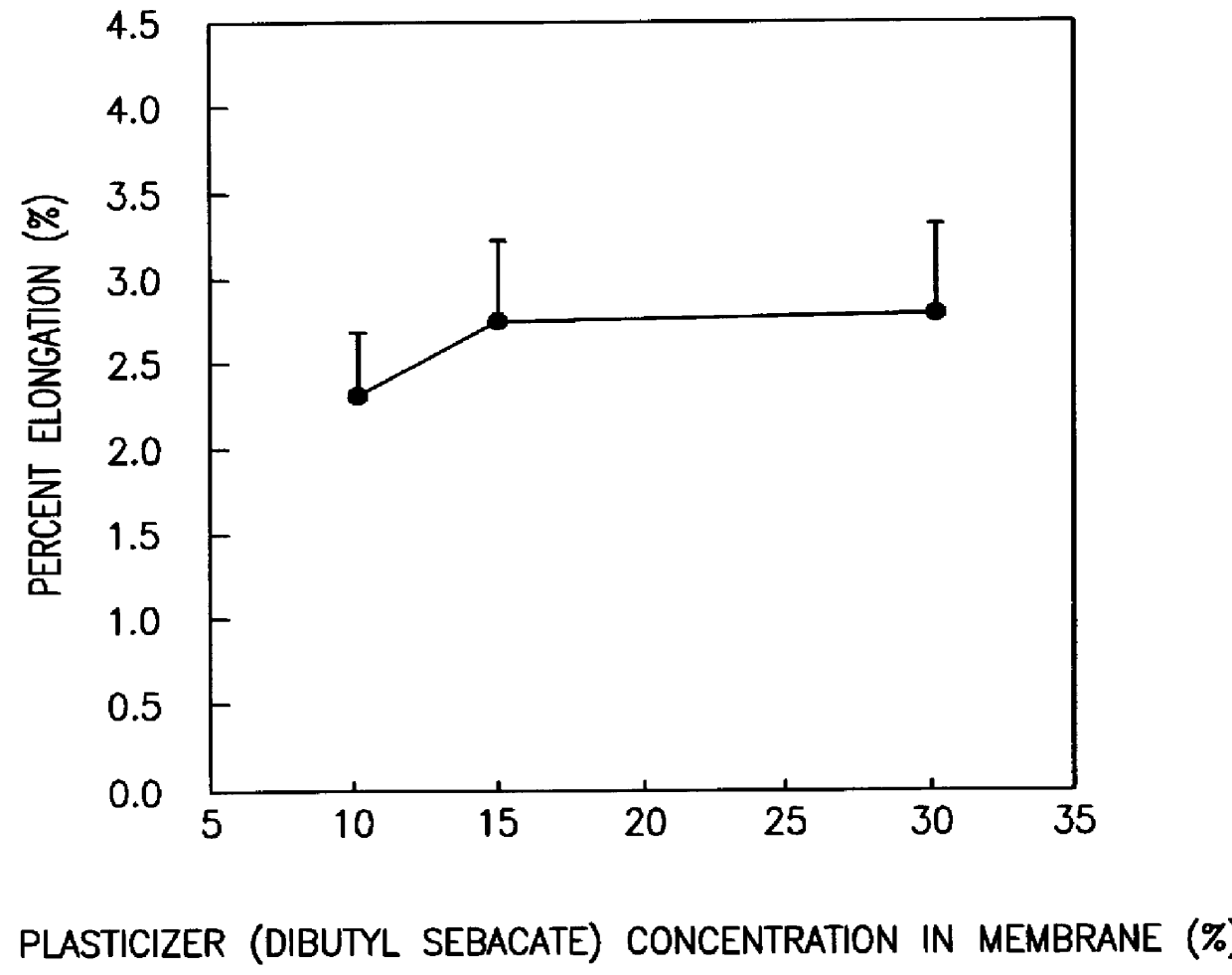
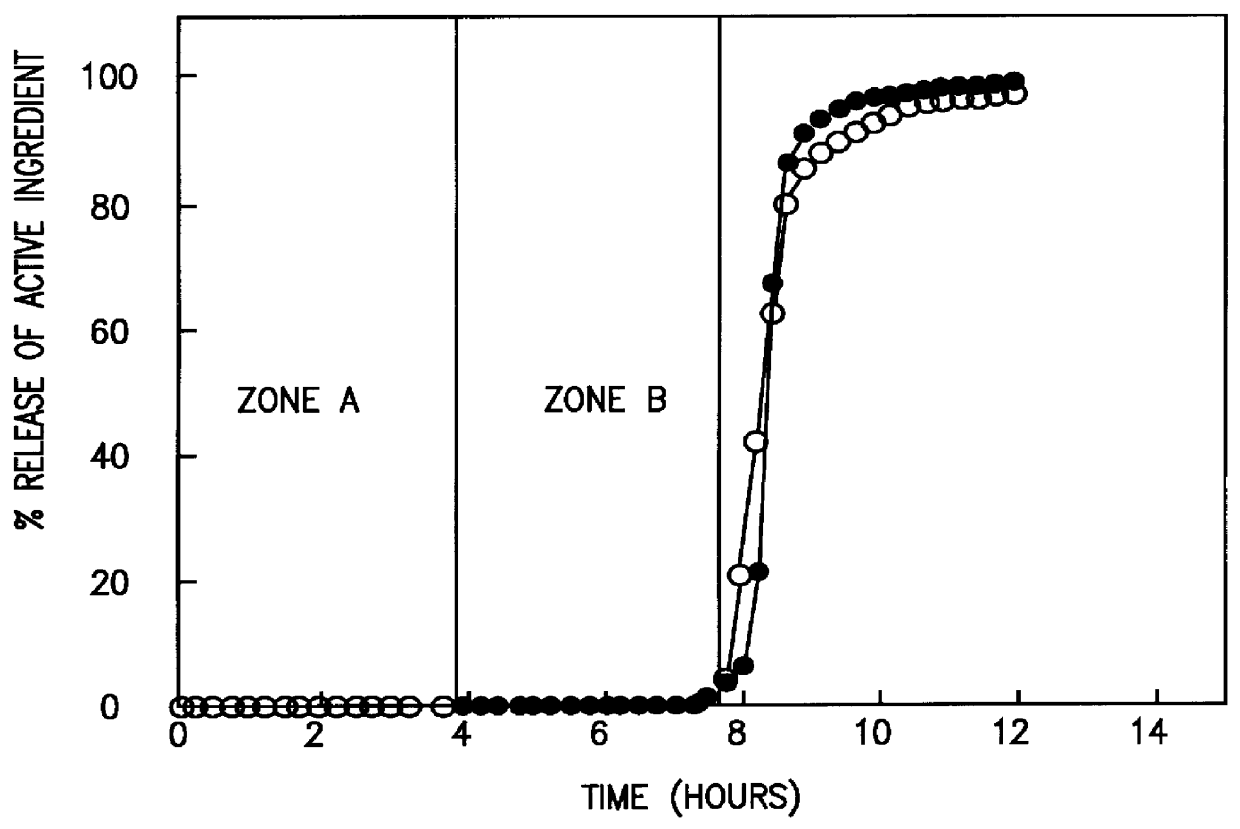
A novel delivery system for targeting drugs to the colon is herein described. The system is a tablet comprised of three parts: 1) an outer enteric coating, 2) an inner semi-permeable polymer membrane containing a plasticizer and 3) a central core comprising swelling excipients and an active ingredient. The novel dosage form described herein will release the drug consistently in the colon by a time-dependent explosion mechanism. This delivery system is particularly suitable for delivering viral protease inhibitors to the colon.
Owner:F HOFFMANN LA ROCHE & CO AG
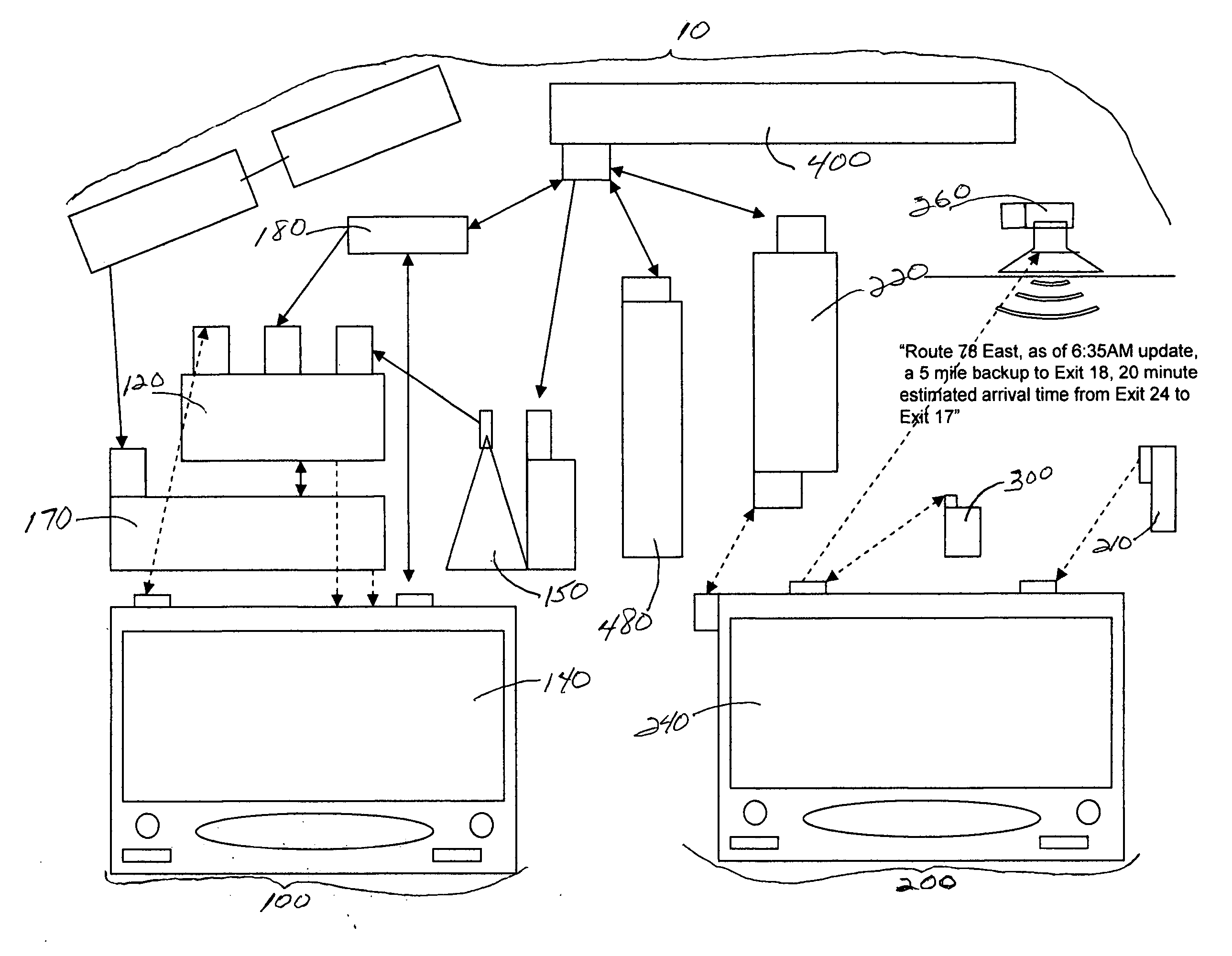
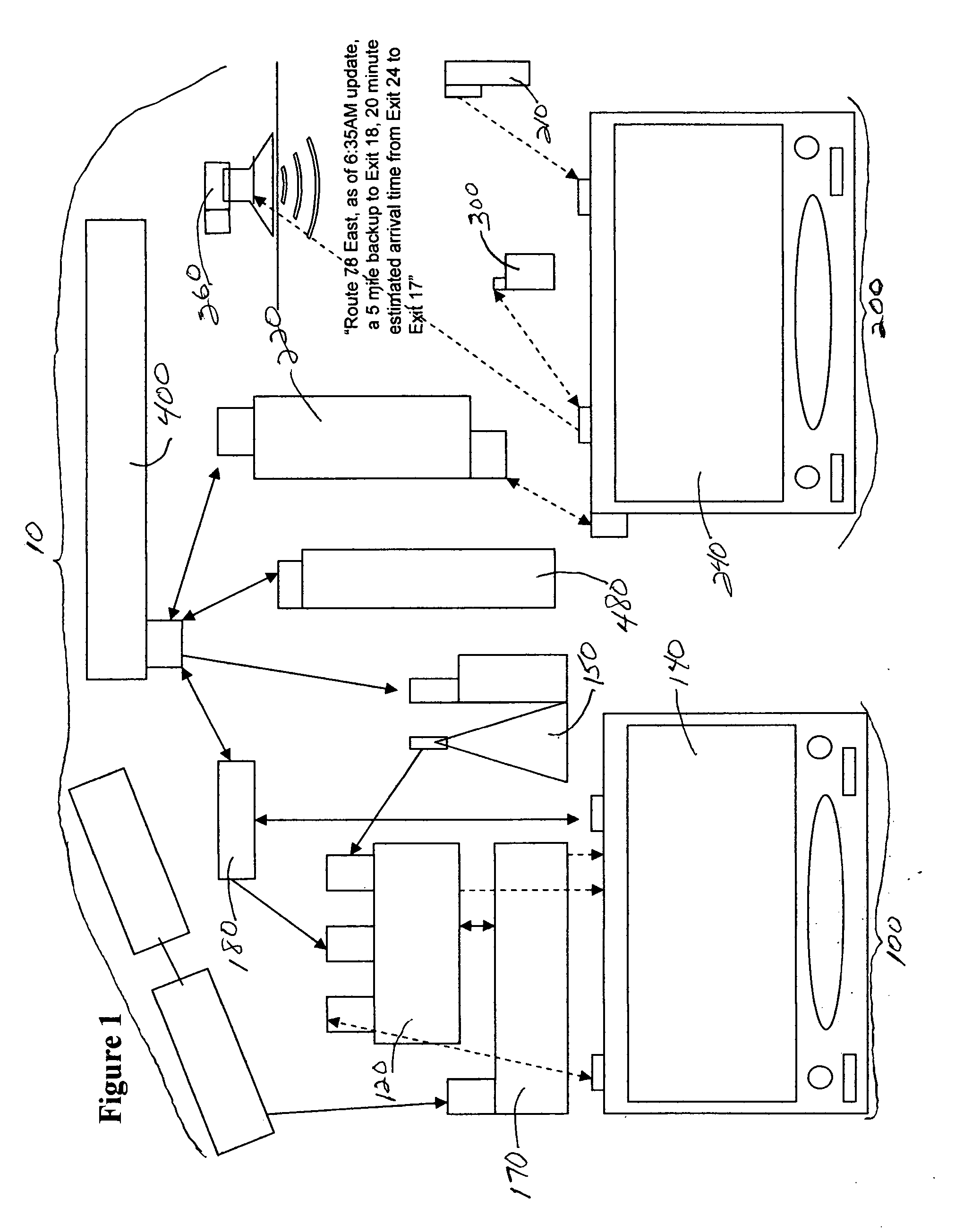
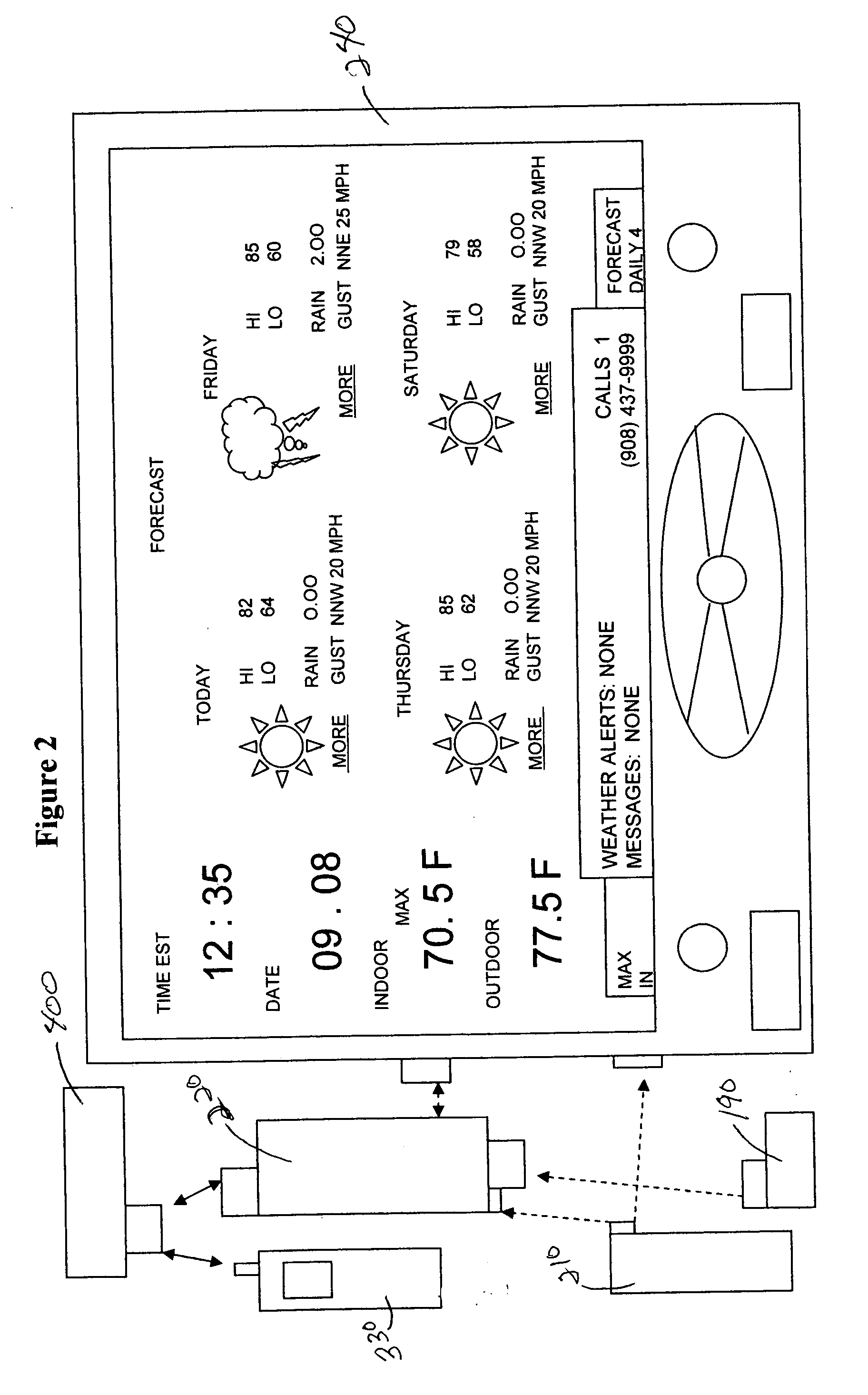
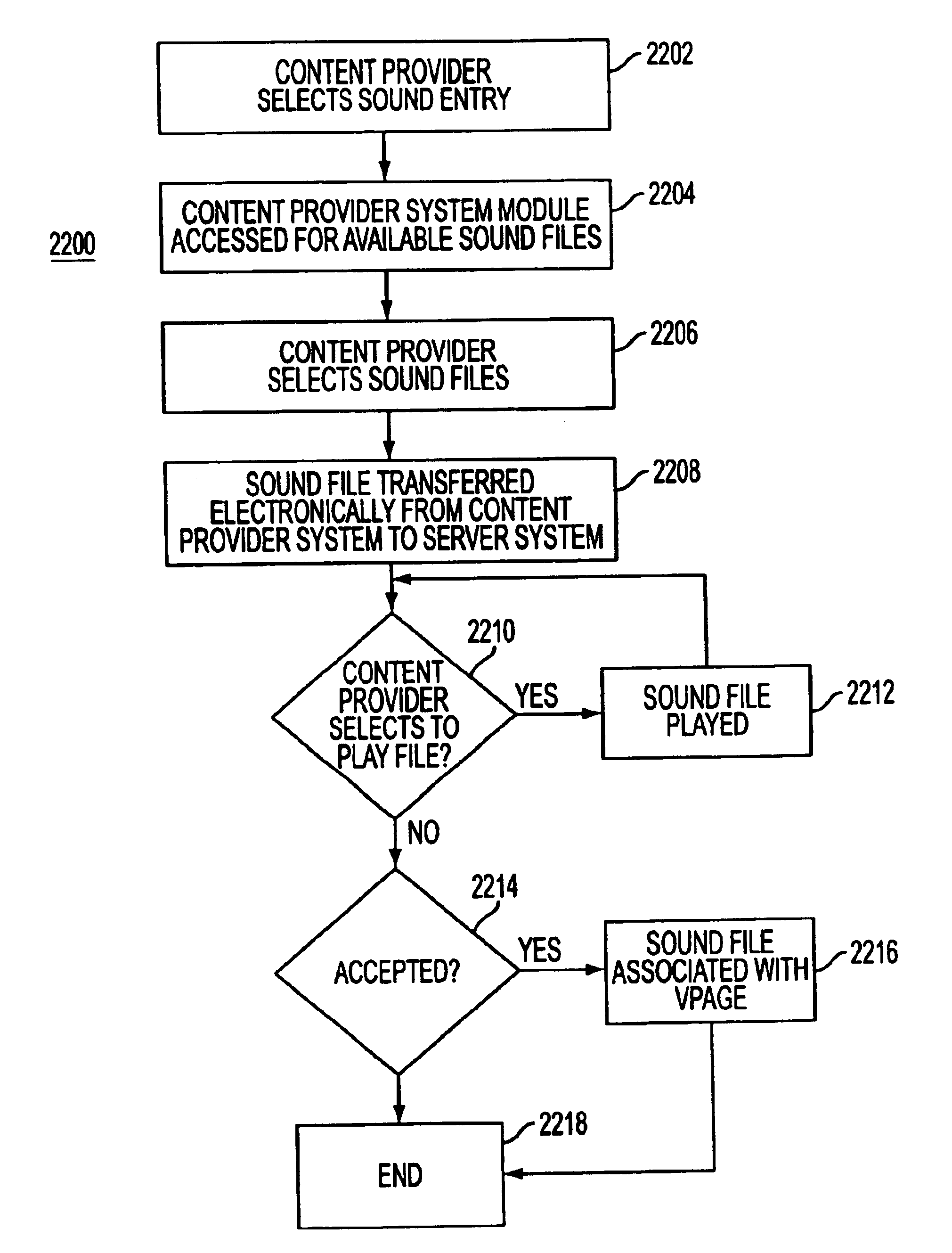
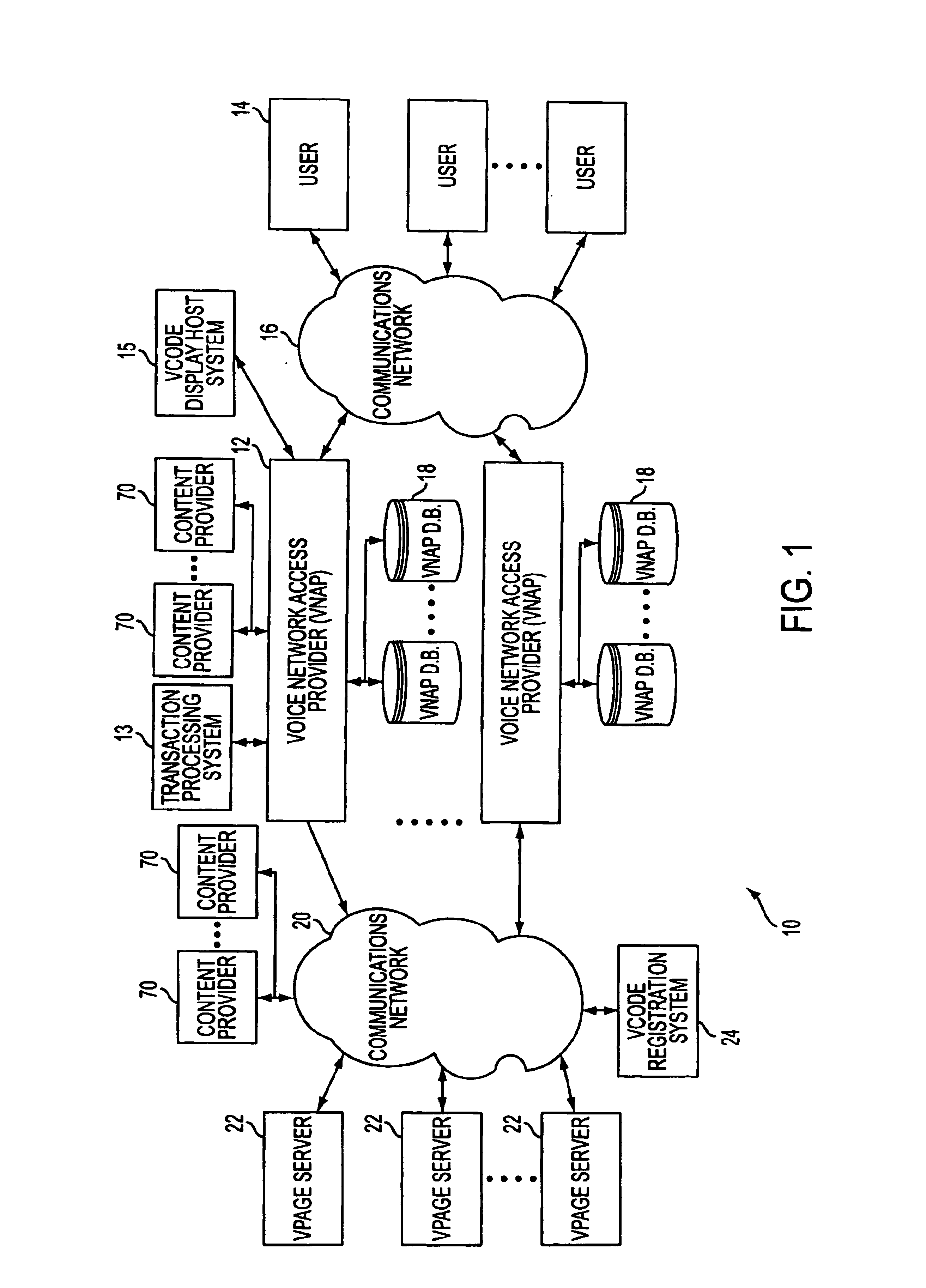
System and method for generating voice pages with included audio files for use in a voice page delivery system
InactiveUS6895084B1Require lotSimplify creationAutomatic call-answering/message-recording/conversation-recordingRecord information storageUser deviceSpeech sound
A content provider system for enabling content providers to create voice pages with audio files included for use in a network for voice page delivery through which subscribers request a voice page and a voice page server system delivers the voice page audibly to the subscriber. A content provider selects a voice page into which the audio file is to be incorporated, selects the audio file and the content provider system then transfers the audio file to a voice page server system which generates a voice page with the audio file included using XML-based tags designated for audio files. The audio files are uploaded from a number of user devices including a telephony device, a web-based system and a PDA.
Owner:GENESYS TELECOMMUNICATIONS LABORATORIES INC
Intravascular delivery system for therapeutic agents
Described herein is a system for intravascular drug delivery system, which includes a reservoir implantable a blood vessel, an intravascular pump fluidly coupled to the reservoir and an anchor expandable into contact with a wall of the blood vessel to retain the system within the vasculature. Delivery conduits may be extend from the reservoir and are positionable at select locations within the vasculature for target drug delivery to select organs or tissues.
Owner:WILLIAMS MICHAEL S +2
Transcutaneous fluid delivery system
InactiveUS6960192B1Avoiding general upkeep and maintenanceEasy to carryAutomatic syringesMedical devicesFluid transportBiological activation
A device for delivering fluid to a person includes a reservoir for containing a fluid to be delivered to the person; a fluid transport device for dispensing fluid from the reservoir to the person, the fluid transport device including a proximal end in fluid communication with the reservoir and a distal end having a penetrating member for piercing the skin of the person to facilitate the delivery of fluid to the person through the fluid transport device; a housing containing the reservoir and the fluid transport device, the housing including an exit port for receiving the distal end of the fluid transport device upon injection of the distal end into the person and means for securing a first wall of the housing to the skin of the person; and an injection activation device including a driving mechanism contacting the fluid transport device for driving the penetrating member from a first position within the housing, through the exit port to a second position, external to the housing and into the skin of the person.
Owner:INSULET CORP
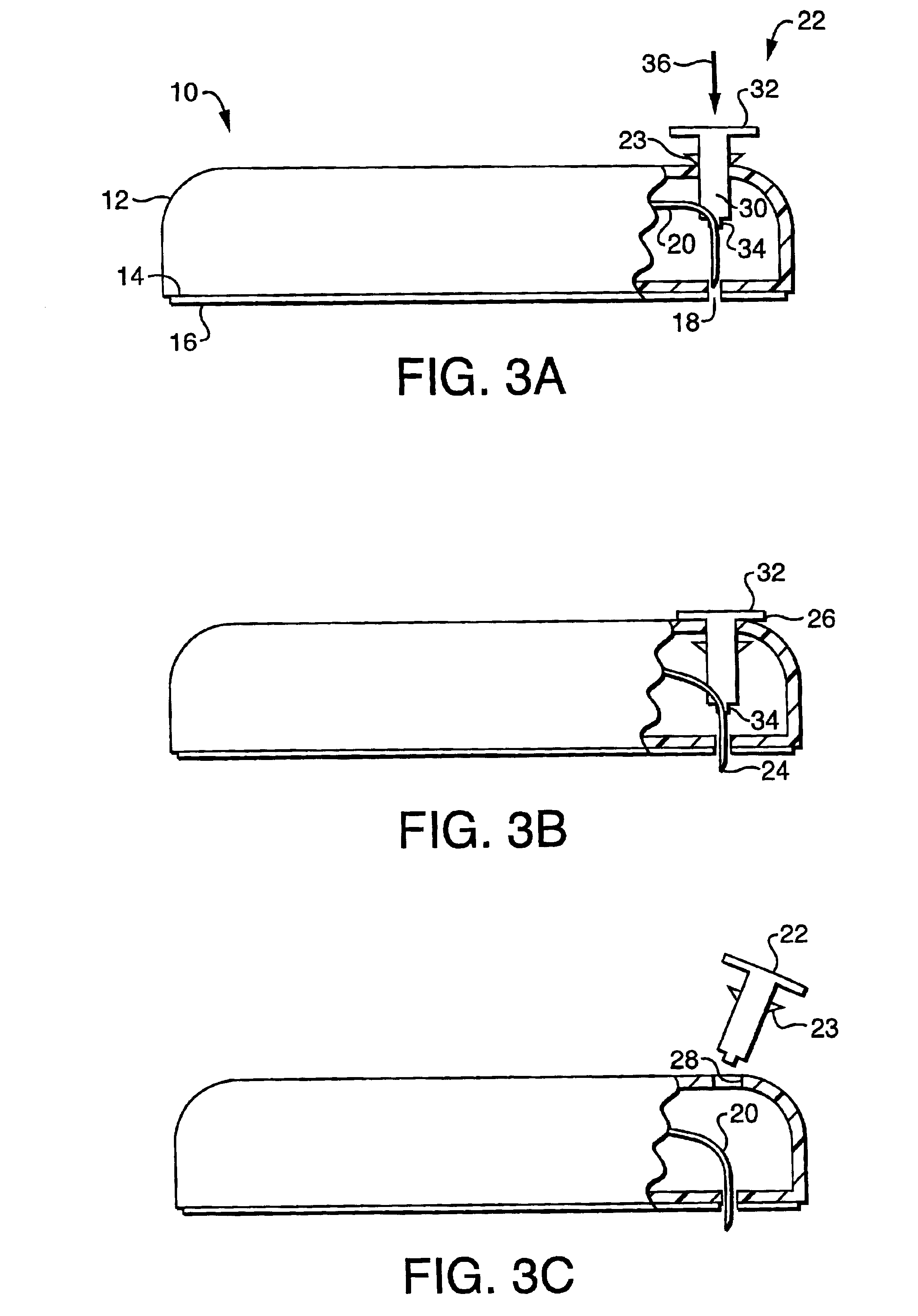
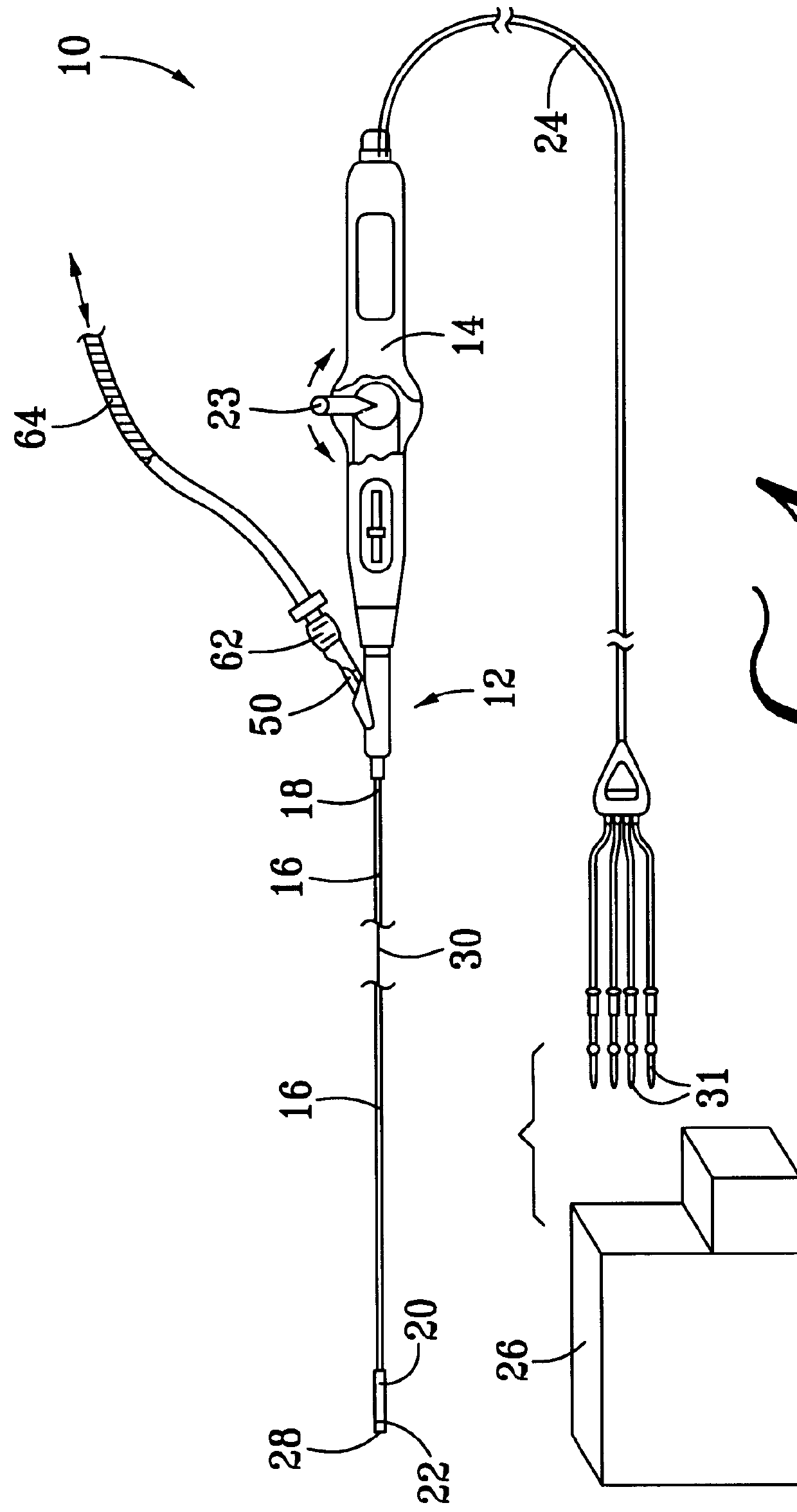
Collapsible spline structure using a balloon as an expanding actuator
InactiveUS6142993AAvoid smallLarge electrodeElectrotherapyDiagnostic recording/measuringActuatorGuide tube
A collapsible electrode catheter assembly (10) having a delivery system (12) for controlling a catheter guide tube (16) having a catheter distal end assembly (22) thereon. The catheter distal end assembly (22) has an electrode structure (28) on an expanding assembly (34) for enlarging the electrode structure (28) in an expanded condition. A balloon structure (48) is used to expand and contract the expanding assembly (34) when an inflation medium (64) is alternately introduced into and withdrawn from the balloon structure (48).
Owner:EP TECH
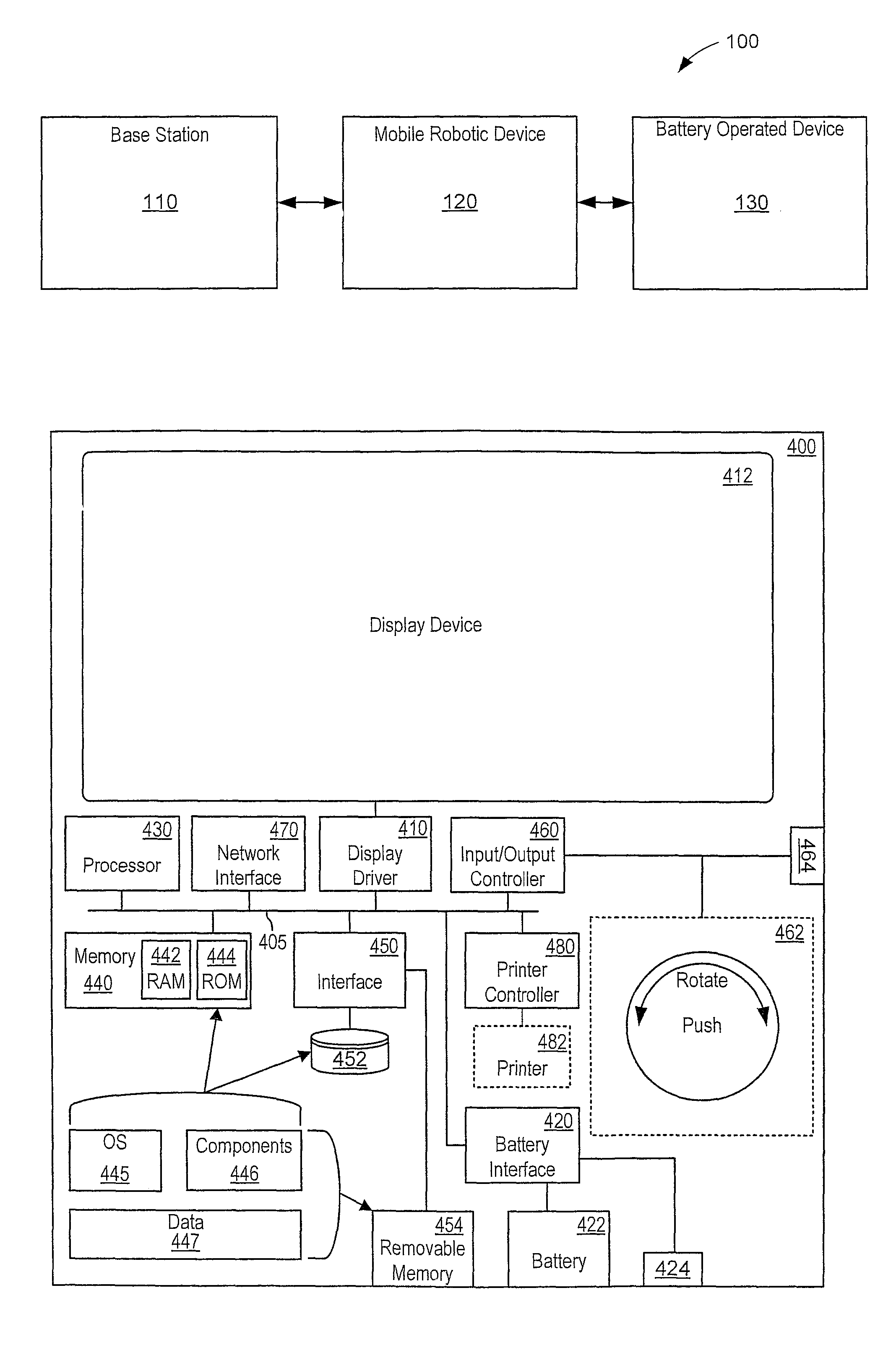
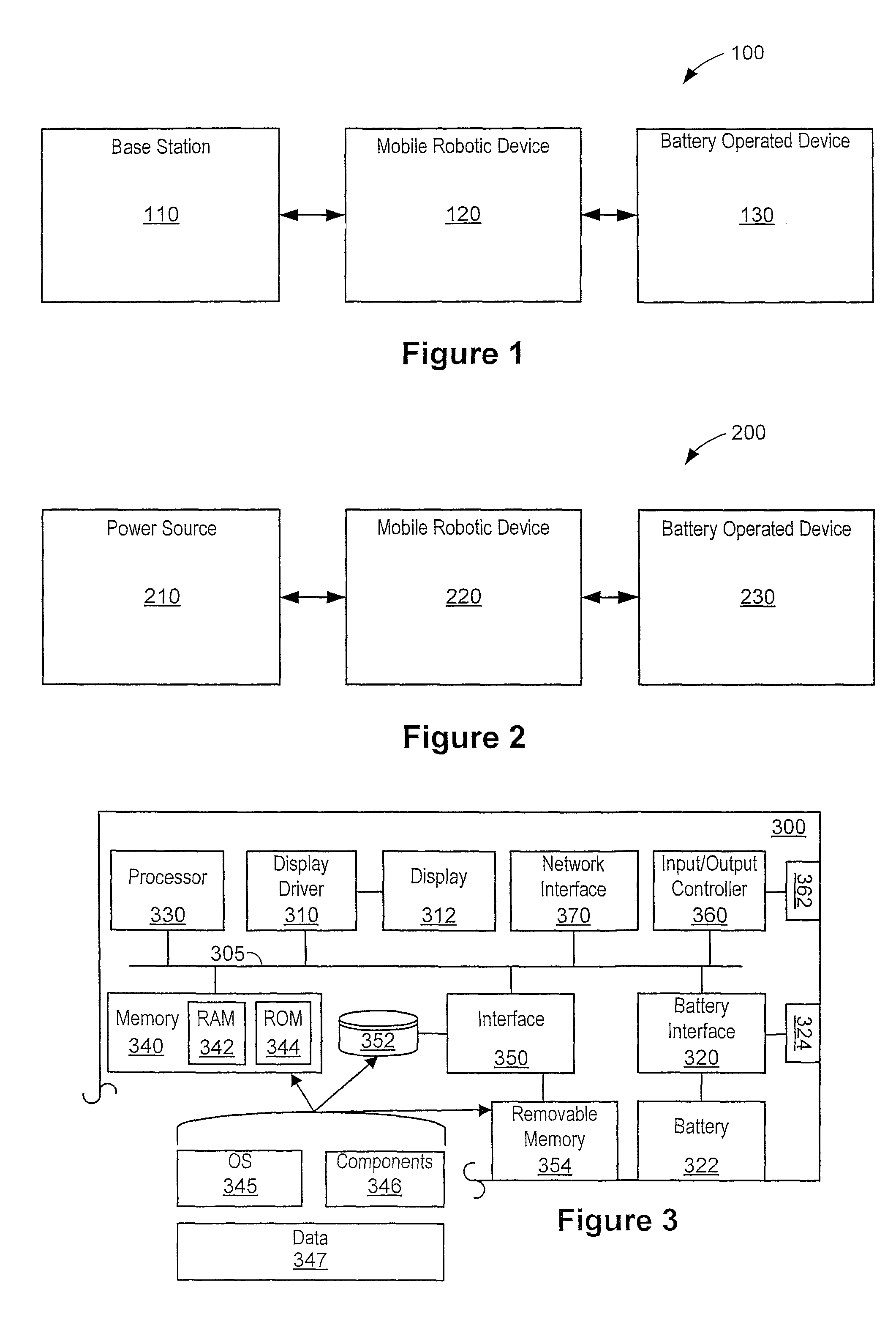
Automated battery and data delivery system
Systems and methods are provided that use a mobile robotic device to transport rechargeable batteries between a base station, which charges the batteries, and a battery operated device, such as battery powered kiosk or signage system, which uses a charged battery as a power source. After traveling to a battery operated device, the mobile robotic device removes any discharged batteries from the battery operated device and installs a charged battery. The mobile robotic device then travels to the base station and attaches the discharged battery to the base station for recharging. The mobile robotic device may be configured to perform other transfers, such as data transfers and paper transfers. In addition, the mobile robotic device may be configured to perform a photographic and spatial survey of the isles, retail shelves, and surrounding environment for various purposes, such as generating three-dimensional store models and remote viewing.
Owner:MACDONALD MURRAY
Gel suitable for implantation and delivery system
InactiveUS20050186240A1Readily and quickly rehydratableFacilitates cellular ingrowthPowder deliveryProsthesisHigh concentrationSolvation
The invention concerns a dried form of a porous polymer gel material which may be rehydrated and placed under pressure or compression to induce solvation, thereby forming a high concentration gel, in the form of an injectable viscous putty or dough, which may be implantated in the body.
Owner:KENSEY NASH CORP
Active suture for the delivery of therapeutic fluids
An active suture that can be used for the delivery of therapeutic fluids to the tissue surrounding a wound is disclosed. The active suture may include a connector designed to join a fluid source, such as a syringe, conventional IV delivery system, or infusion pump to an internal passageway that is embedded within a braided suture. The internal passageway may be comprised of a fine polymeric tube and is capable of conducting and emitting a fluid into at least a portion of the braided suture and surrounding tissue. The invention enables delivery of an efficacious volume of drug bearing solution on the order of milliliters per day, provides a high level of fluid delivery rate control enabling the physician to start or stop drug administration at his / her discretion, and offers a means of providing more than one type of medication that may be selected post-surgically in accord with unexpected patient symptoms that may arise.
Owner:ETHICON INC
Fluid assisted medical devices, fluid delivery systems and controllers for such devices, and methods
InactiveUS20050033278A1Increase speedWide sizeCatheterSurgical instruments for heatingRate changeMedical device
Medical devices, methods and systems for treating tissue are provided. An exemplary system comprises a fluid from a fluid from a fluid source at a fluid flow rate, a surgical device which provides power and the fluid to the tissue and a control mechanism which changes a fluid flow rate provided from the surgical device and changes a power level provided from the surgical device. The fluid flow rate changes between at least two-zero flow rates and the power level changes between at least two non-zero levels. An exemplary method comprises providing a fluid from a fluid source at a fluid flow rate, providing a surgical device which provides power and the fluid to the tissue, and changing the fluid flow rate of fluid provided from the surgical device with a change in power level provided from the surgical device.
Owner:SALIENT SURGICAL TECH
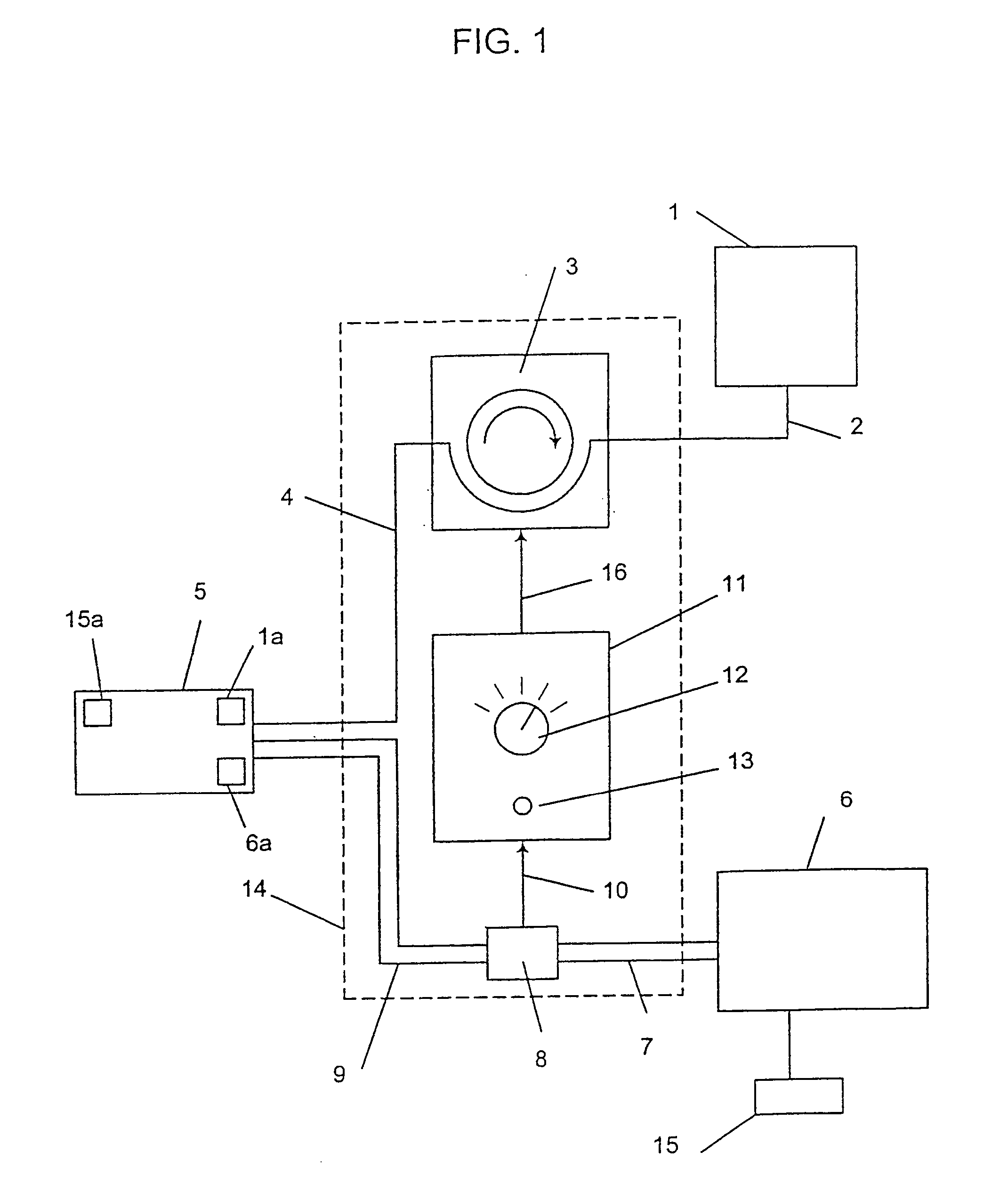
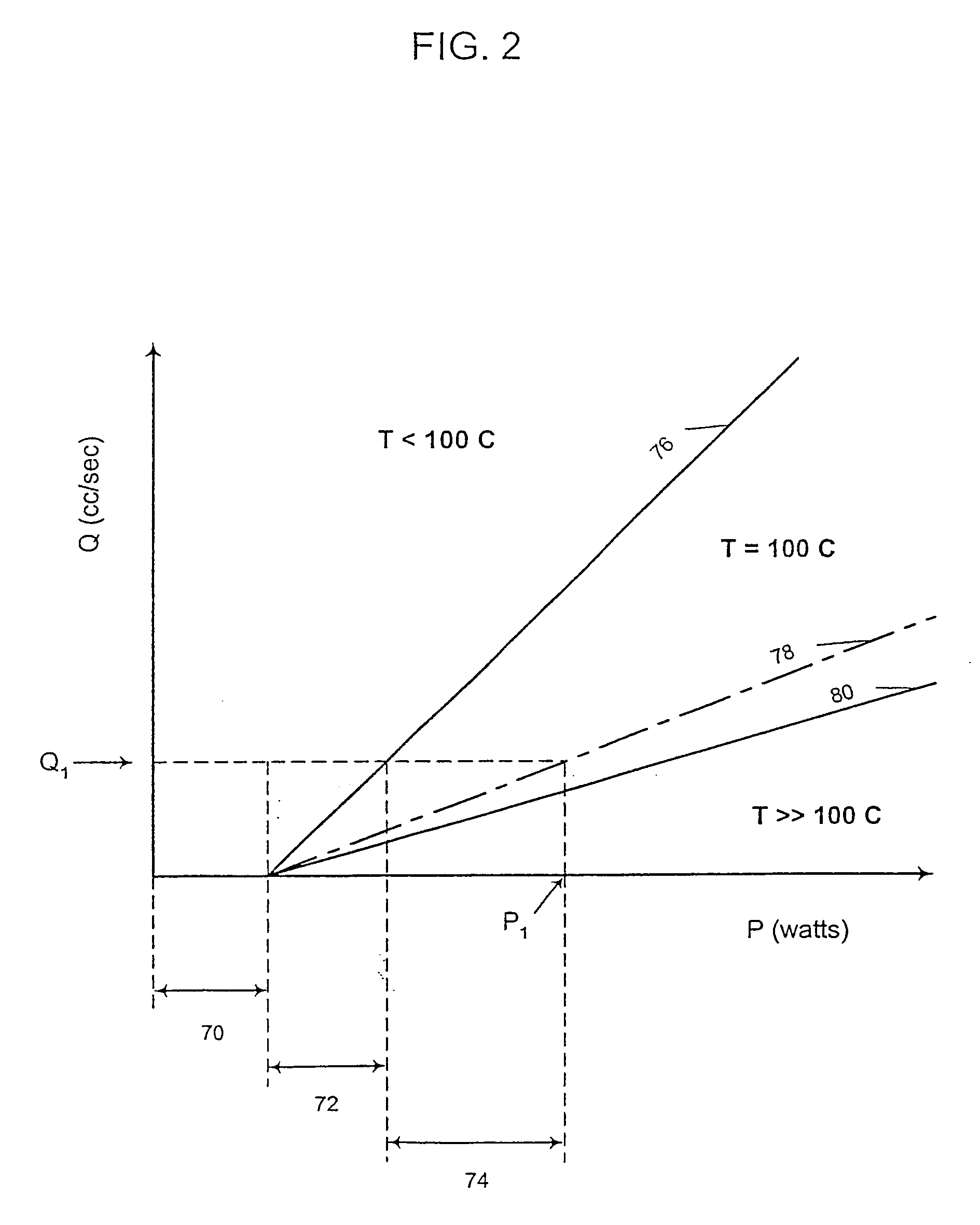
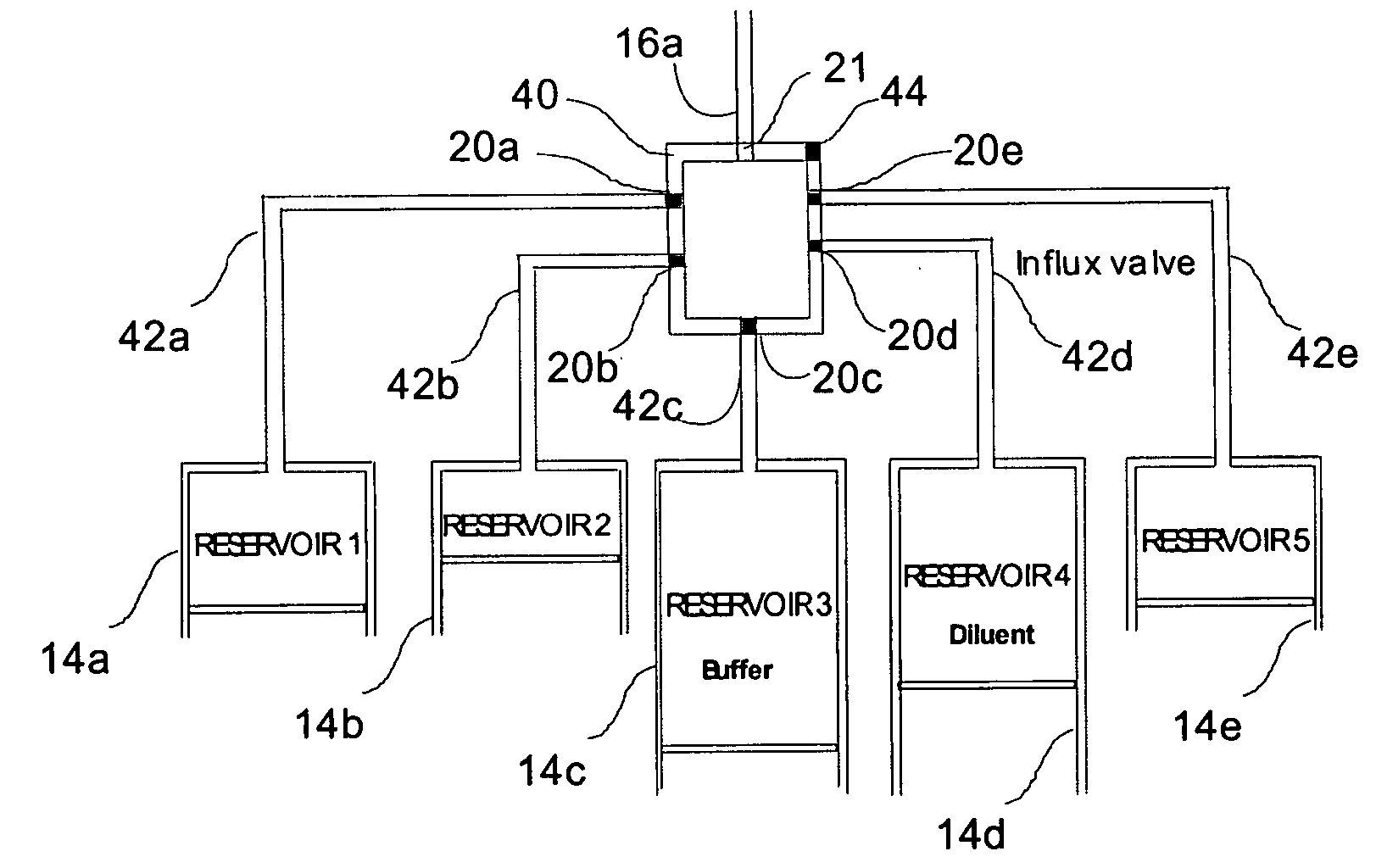
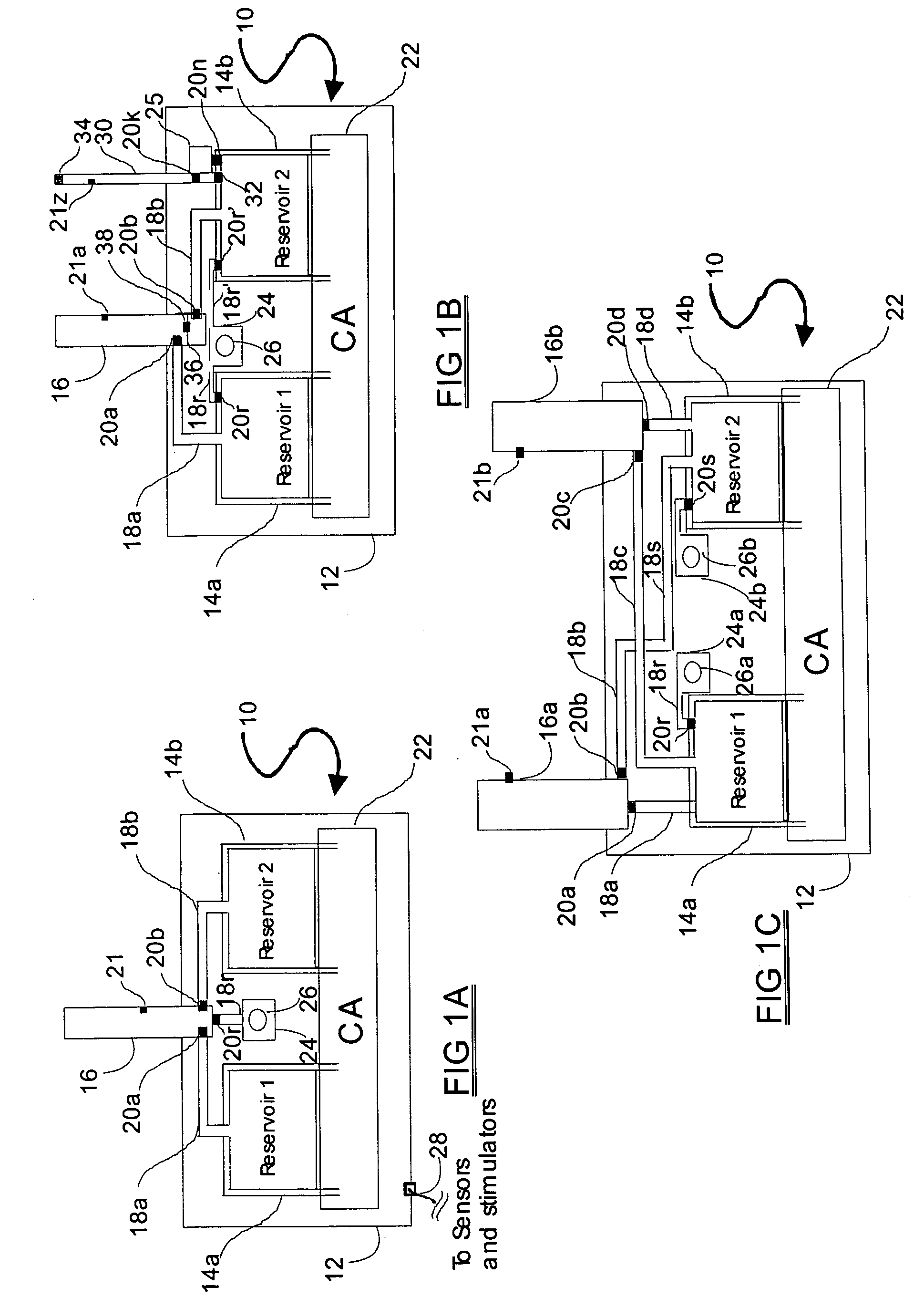
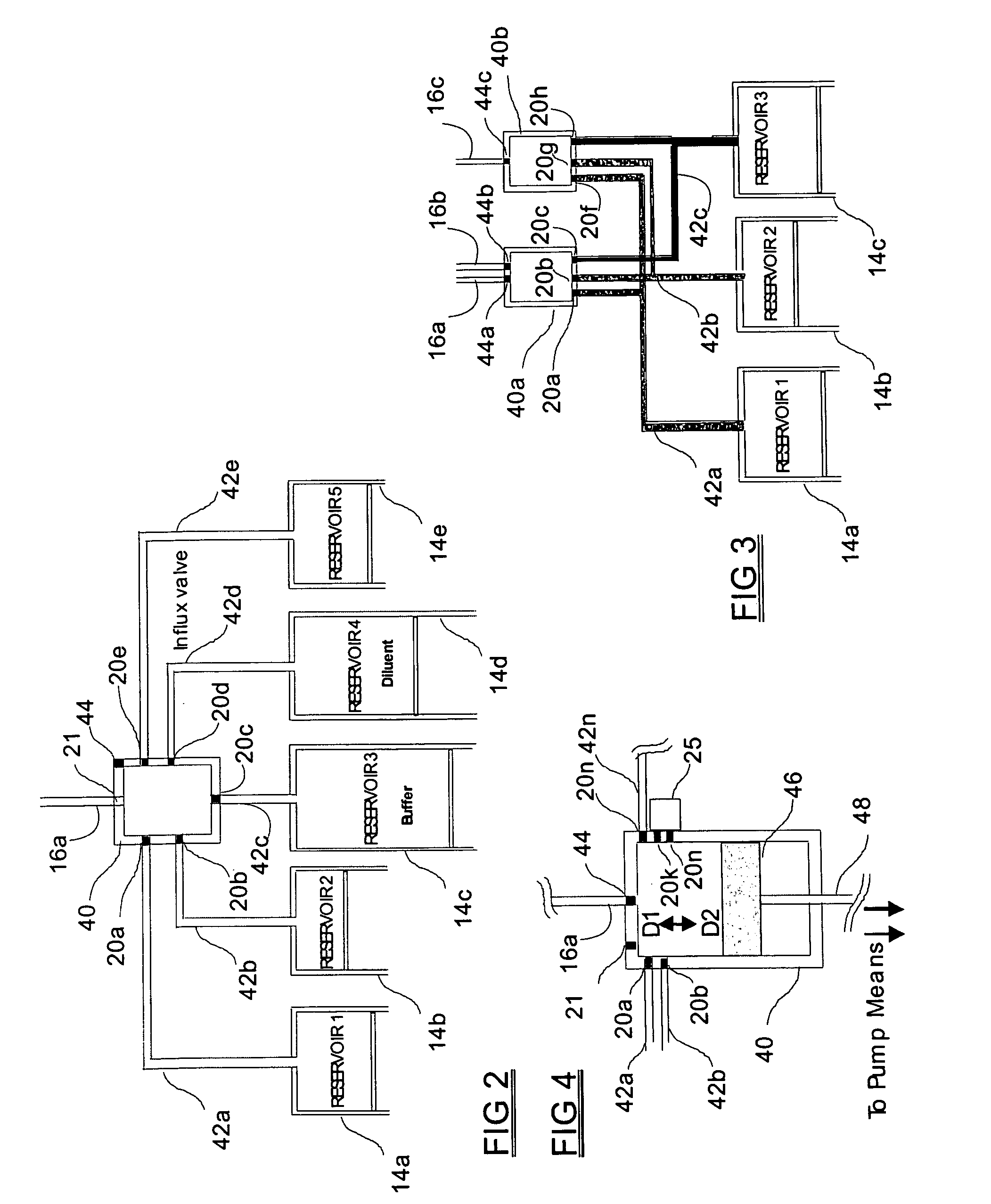
Programmable medical drug delivery systems and methods for delivery of multiple fluids and concentrations
InactiveUS20050277912A1Extension of timeEasy to fillMulti-lumen catheterDrug and medicationsControl mannerDelivery system
A drug delivery system provides for mixing various drugs in an optimally controlled manner, for using flow controllers to guide multiple drugs into a single or into multiple catheters, for enabling a single lumen catheter to treat a specific region with several drugs, for allowing for dilution of a concentrated drug in order to both increase the time between refilling and also for providing any concentration of a drug that might be desired, for using a buffer fluid to deliver exact amounts of several drugs from the same catheter or to separate several drugs within a single catheter, for using external fluid present in the human body either as a diluent or buffer fluid, and for providing for a drug testing / filler apparatus to be used prior to implant to ensure proper function and easy means of filling multiple reservoirs with different fluids, and also after implant for refilling operations. The drug delivery system (DDS) can perform both bolus and continuous delivery of substances, and enable the measured delivery of any one of several drugs to one or more distal locations at independently programmable rates. New types of catheter systems and uses therefore are also described. Catheter hub assemblies allowing for easy replacement of drug delivery systems offer advantages when replacing drug delivery systems. New methods for using the DDS in the promotion of healthy pregnancy and treatment of a developing fetus are also possible.
Owner:JOHN SASHA
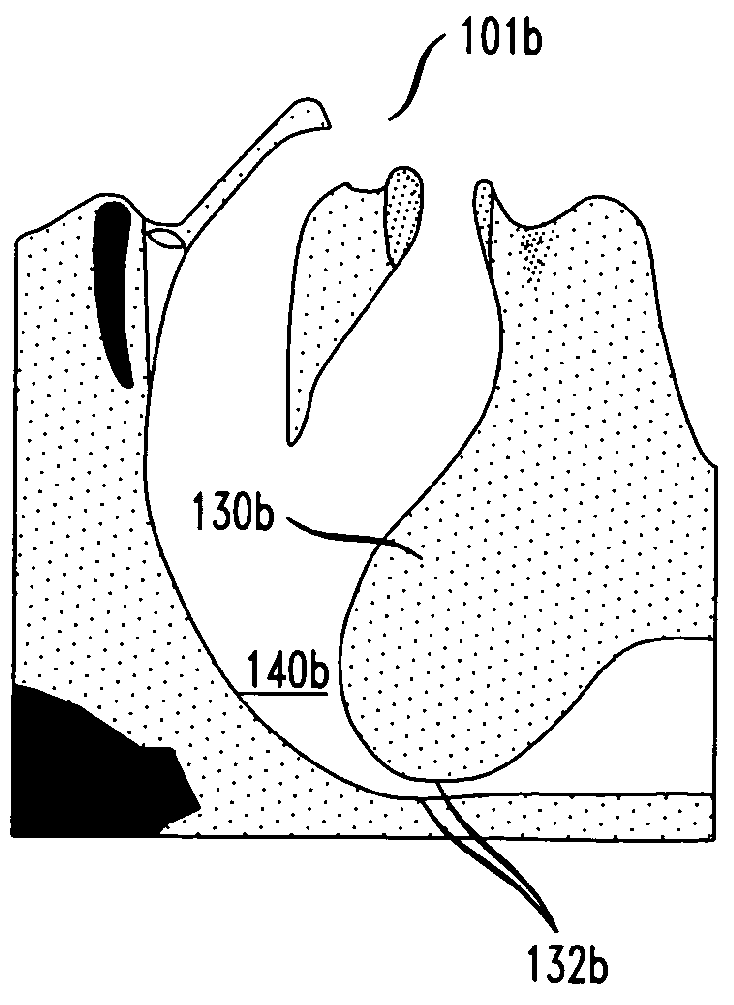
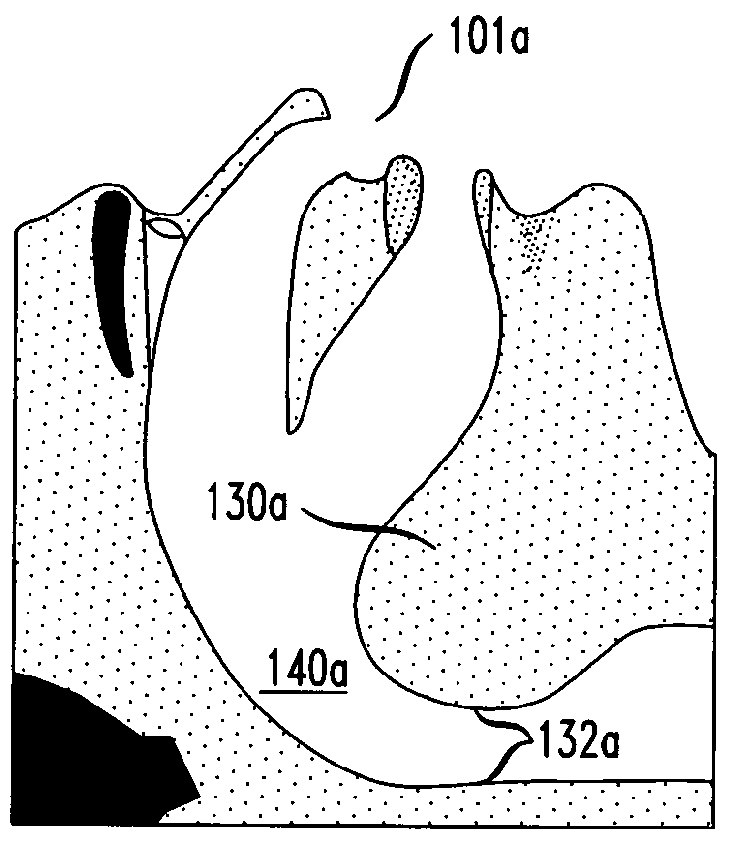
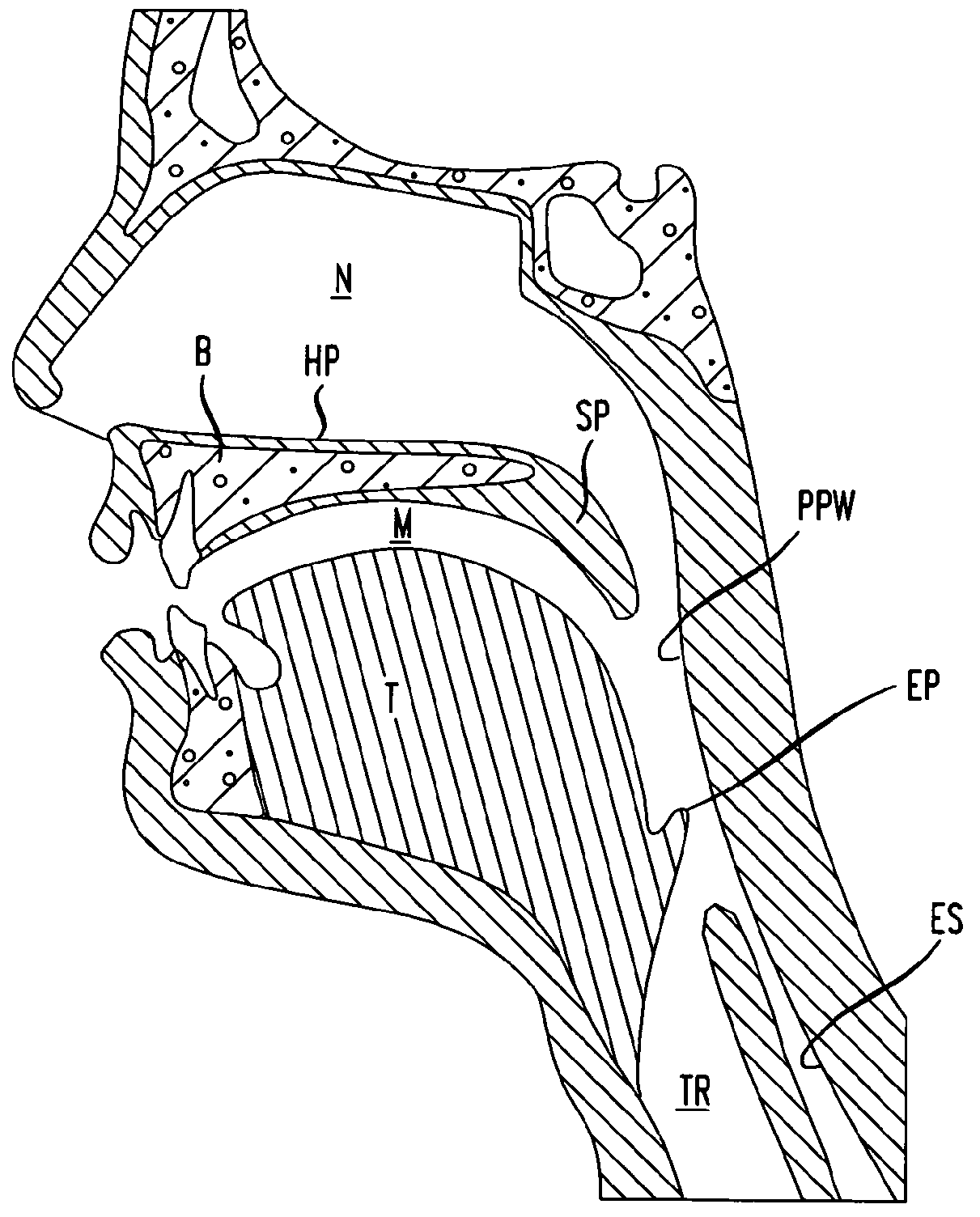
Methods and devices for treatment of obstructive sleep apnea
ActiveUS8413661B2Avoid blockingAlters the geometry of the airwayElectrotherapyDiagnosticsHand heldNeck of pancreas
Owner:ETHICON INC
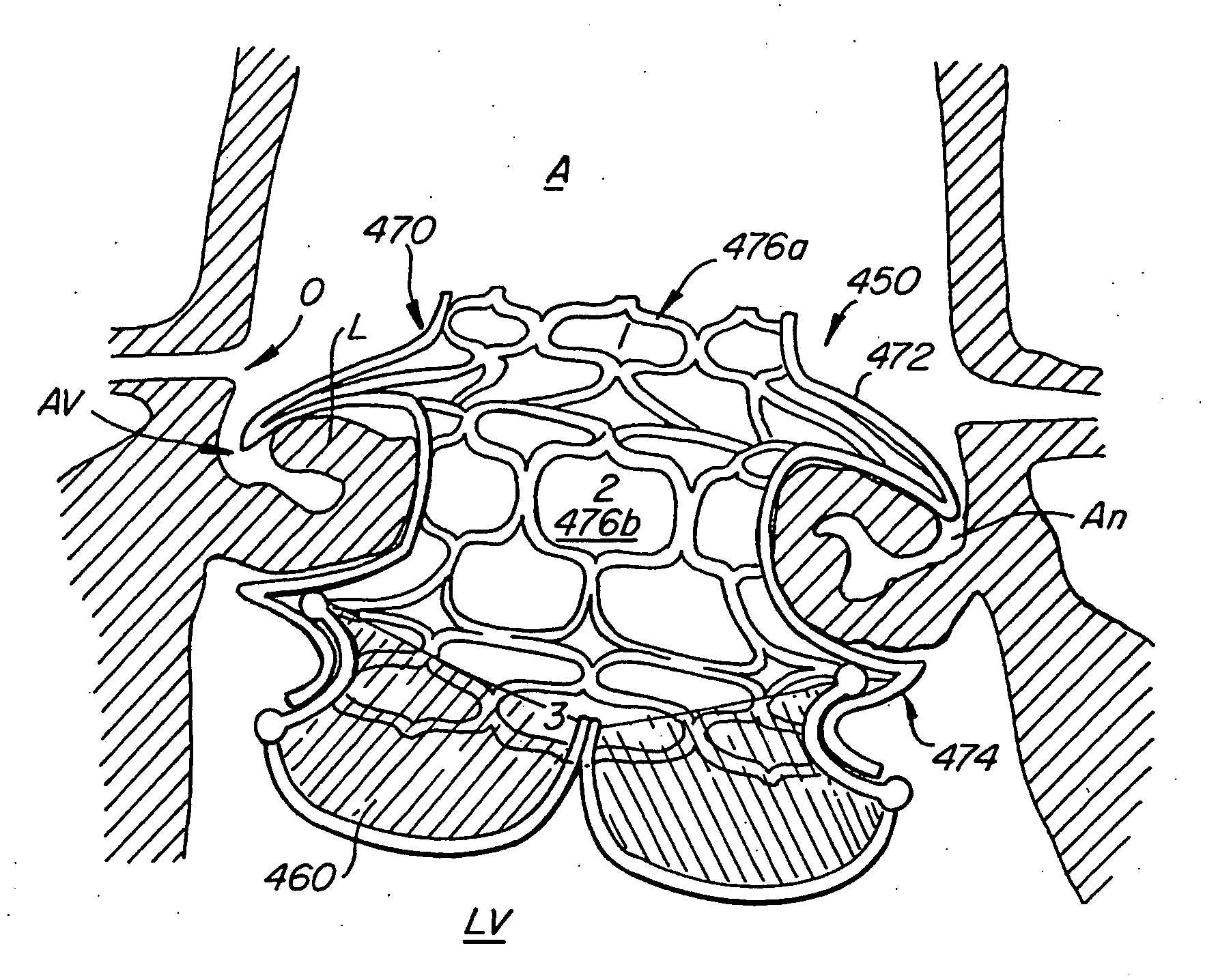
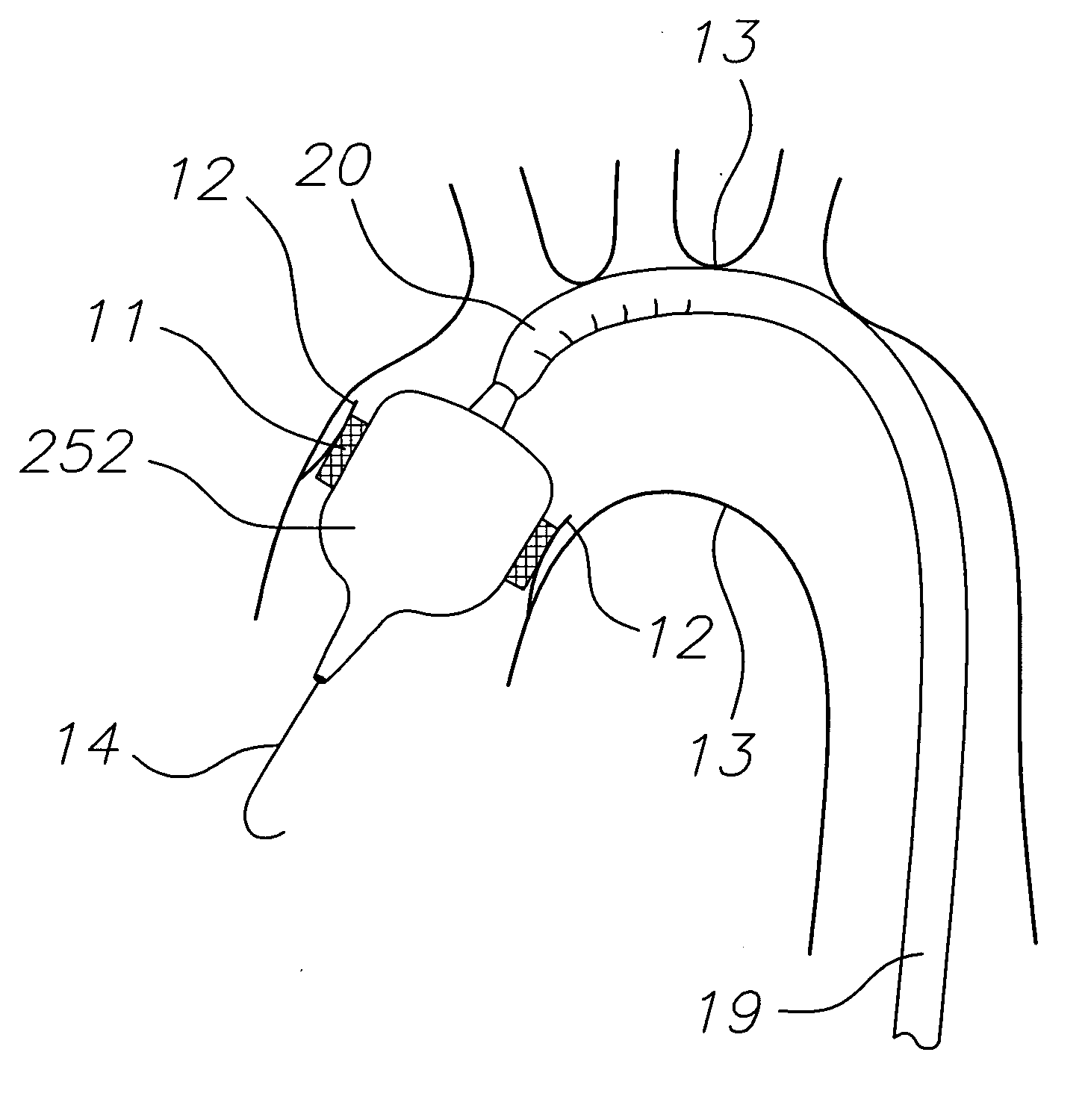
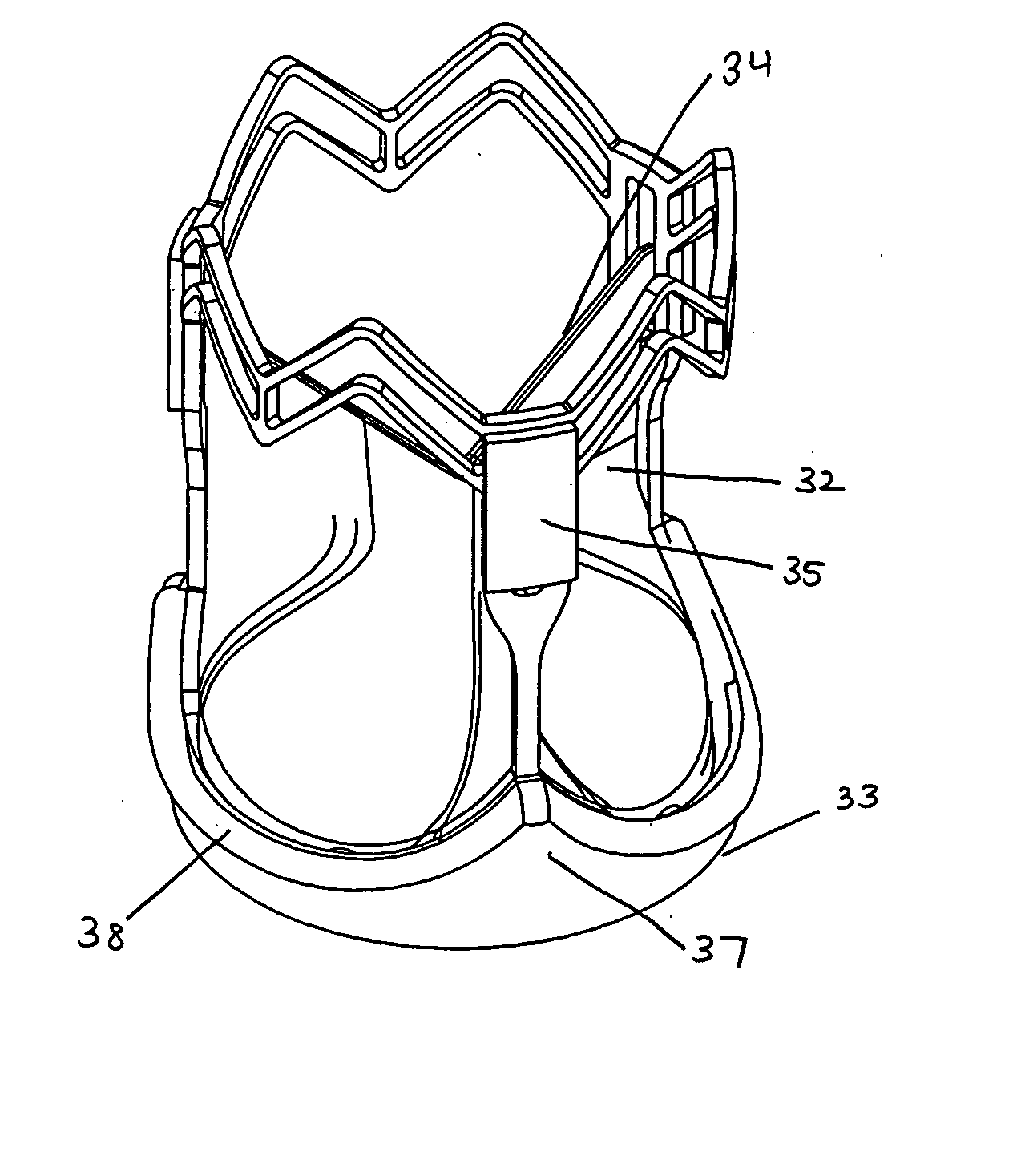
Vascular implant and delivery system
ActiveUS20100298931A1Reliable and controlled mannerStentsHeart valvesVascular implantInsertion stent
A vascular implant for replacing a native heart valve comprises a self expanding stent supporting a valve body having leaflets. The stent preferably comprises an anchoring structure configured to prevent the implant from passing through the valve annulus. For delivery, the implant is compacted within a delivery device and secured at one end. During delivery the implant is partially released from the delivery device, and positioning of the implant is verified prior to full release. The implant can be at least partially resheathed and repositioned if desired.
Owner:EDWARDS LIFESCI CARDIAQ
Minimally invasive valve replacement system
InactiveUS20050096738A1Reduce and eliminate needShorten the timeStentsHeart valvesEngineeringDelivery system
Methods and systems for minimally invasive replacement of a valve. The system includes a collapsible valve and anchoring structure, devices and methods for expanding the valve anchoring structure, adhesive means to seal the valve to the surrounding tissue, a catheter-based valve sizing and delivery system, native valve removal means, and a temporary valve and filter assembly to facilitate removal of debris material. The valve assembly comprises a valve and anchoring structure for the valve, dimensioned to fit substantially within the valve sinus.
Owner:CALI DOUGLAS S +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com