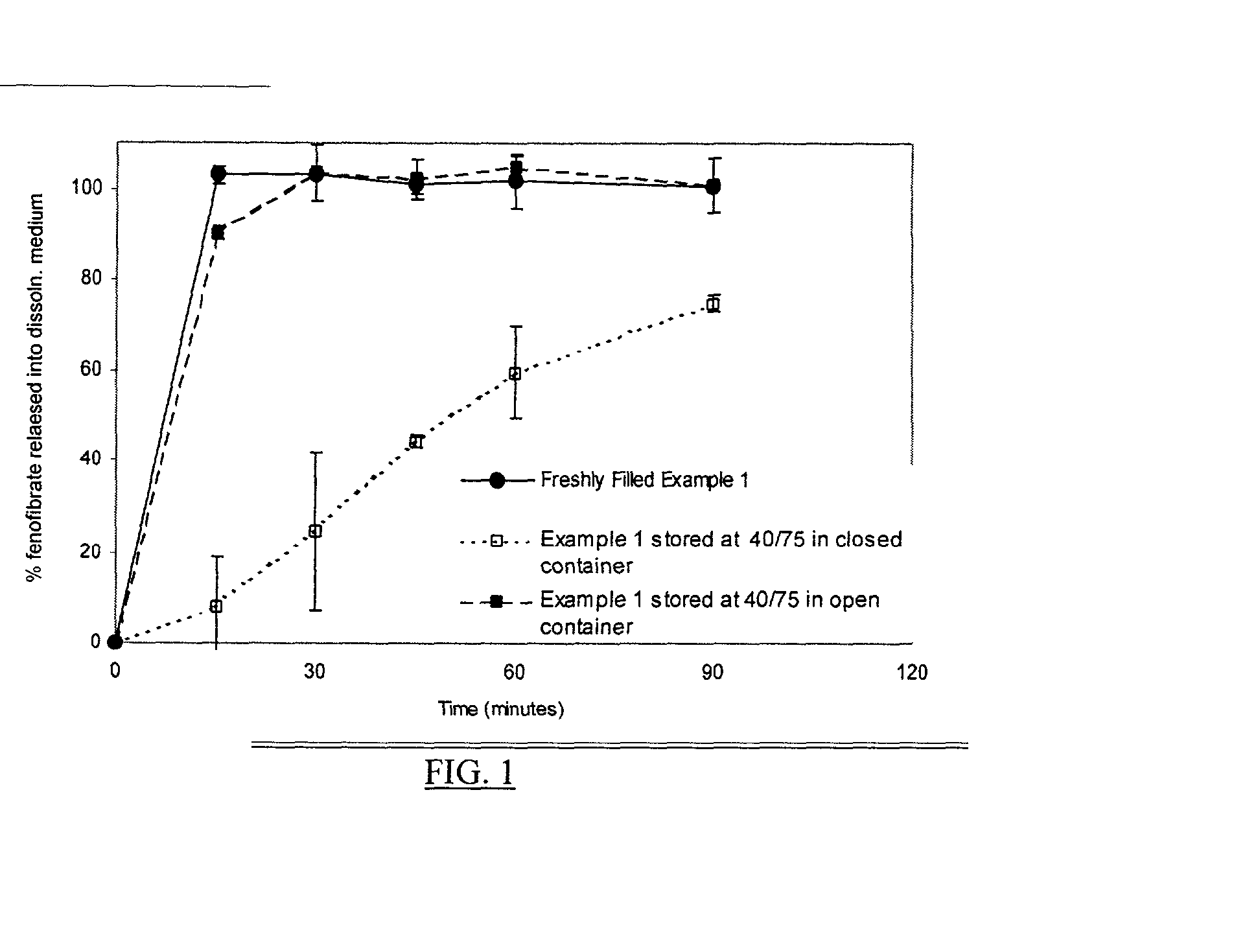
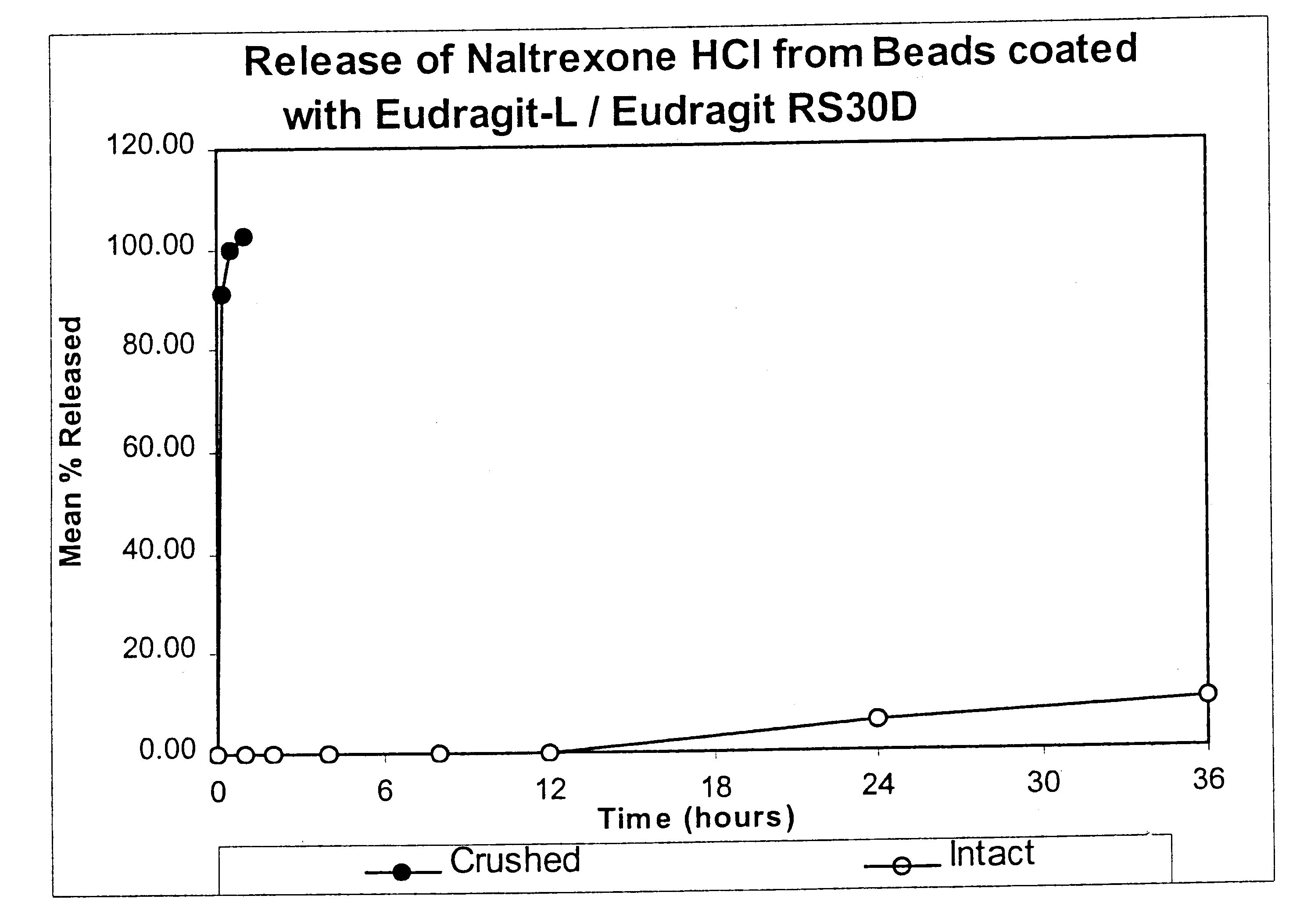
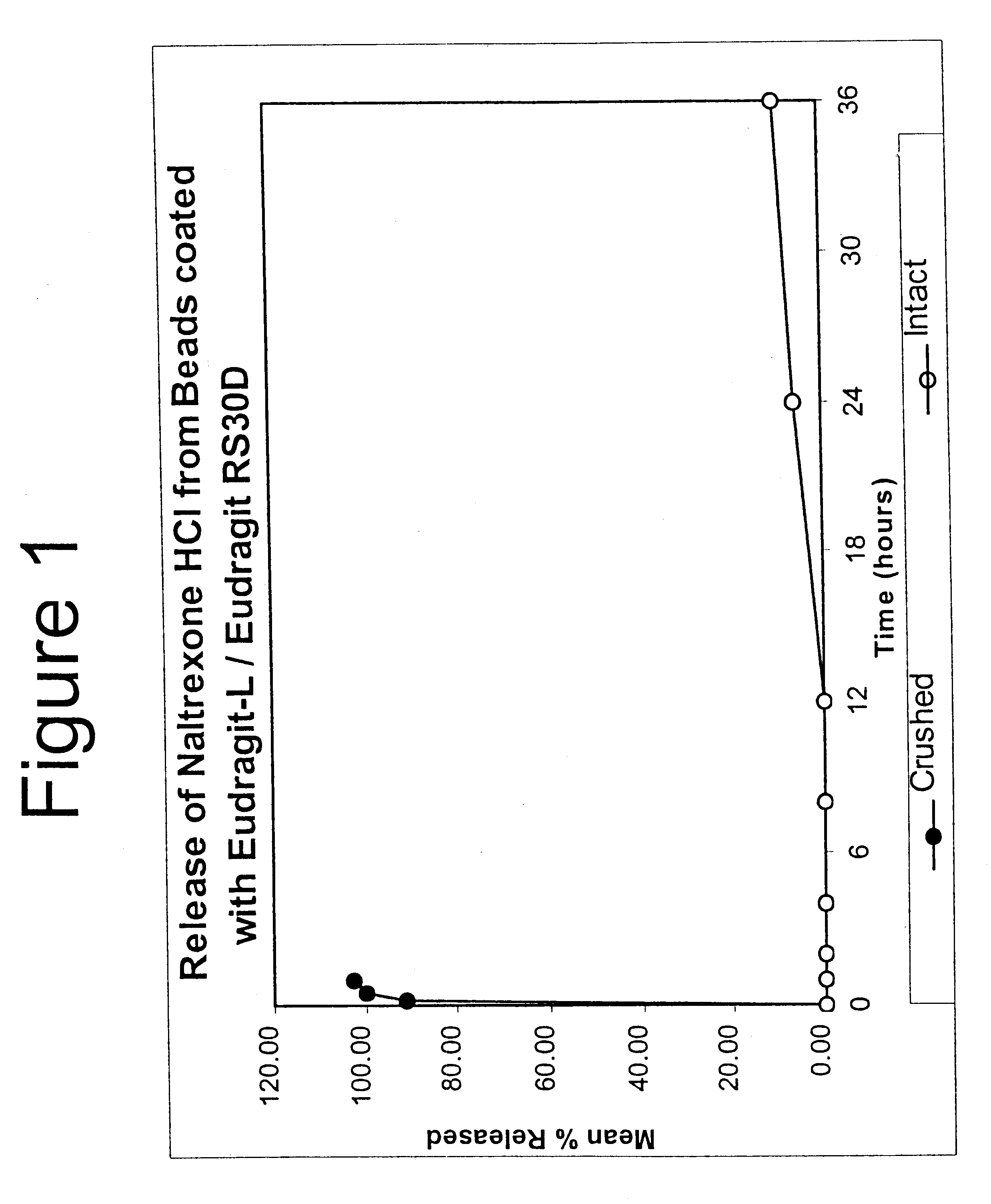
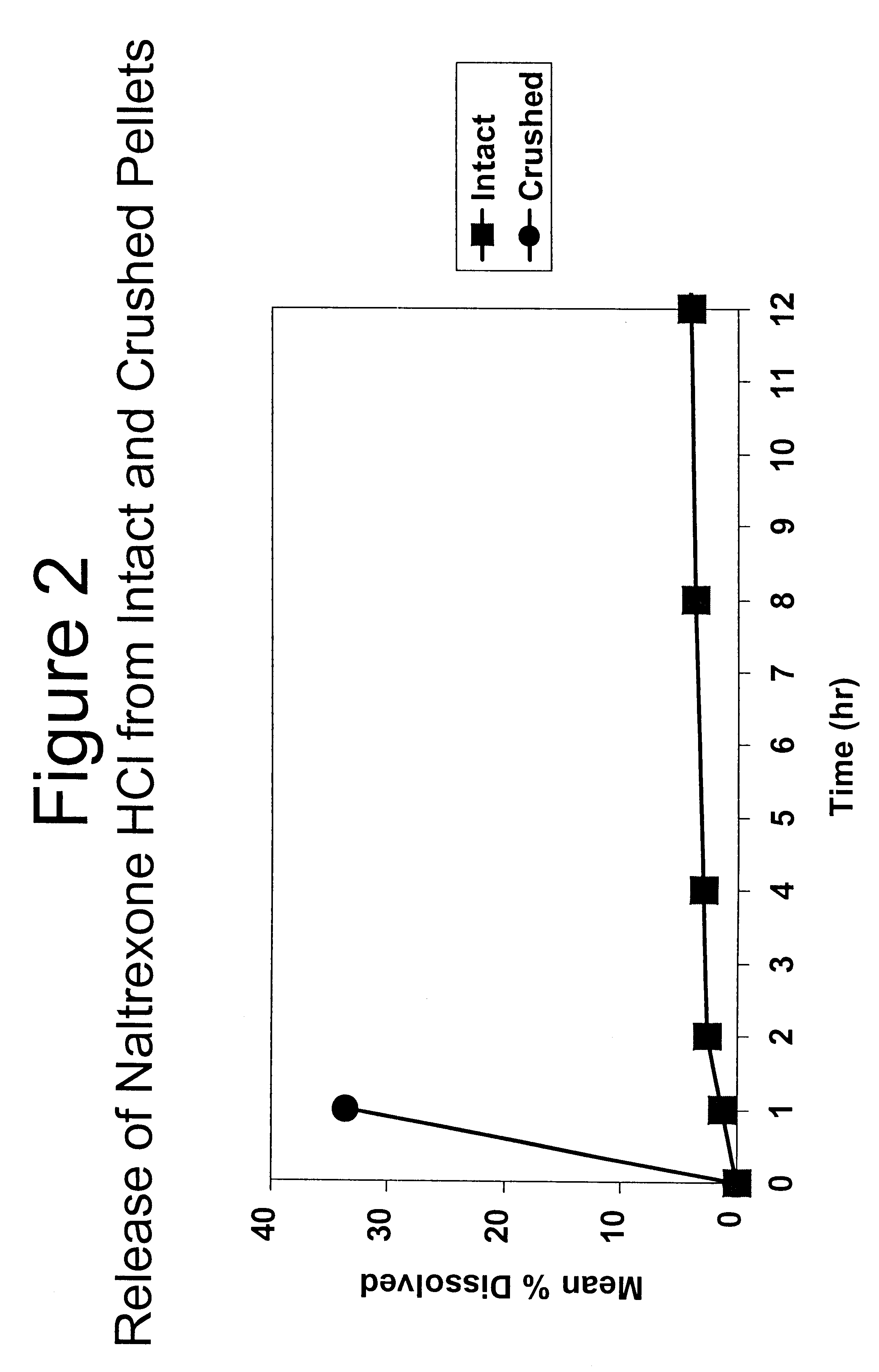
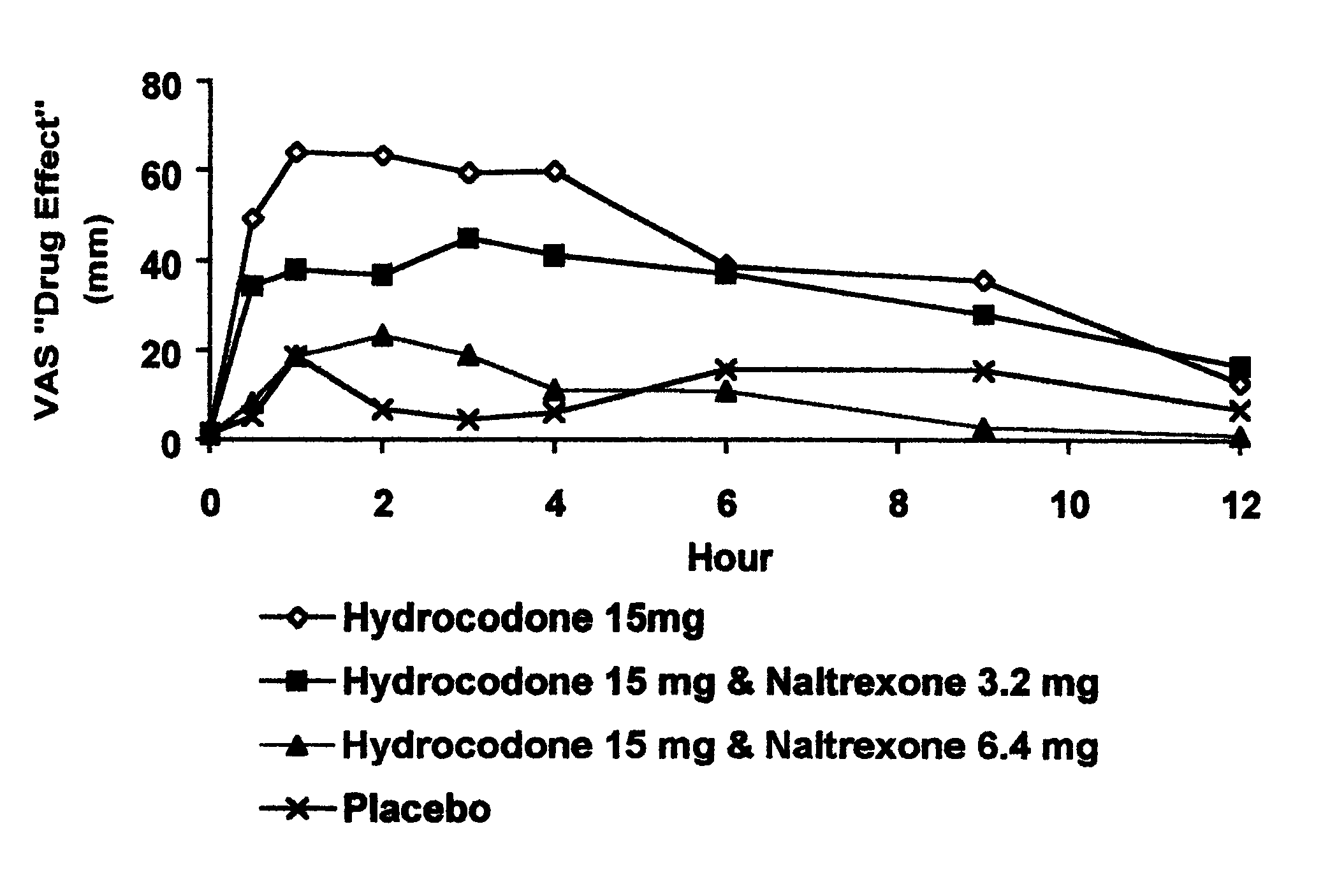
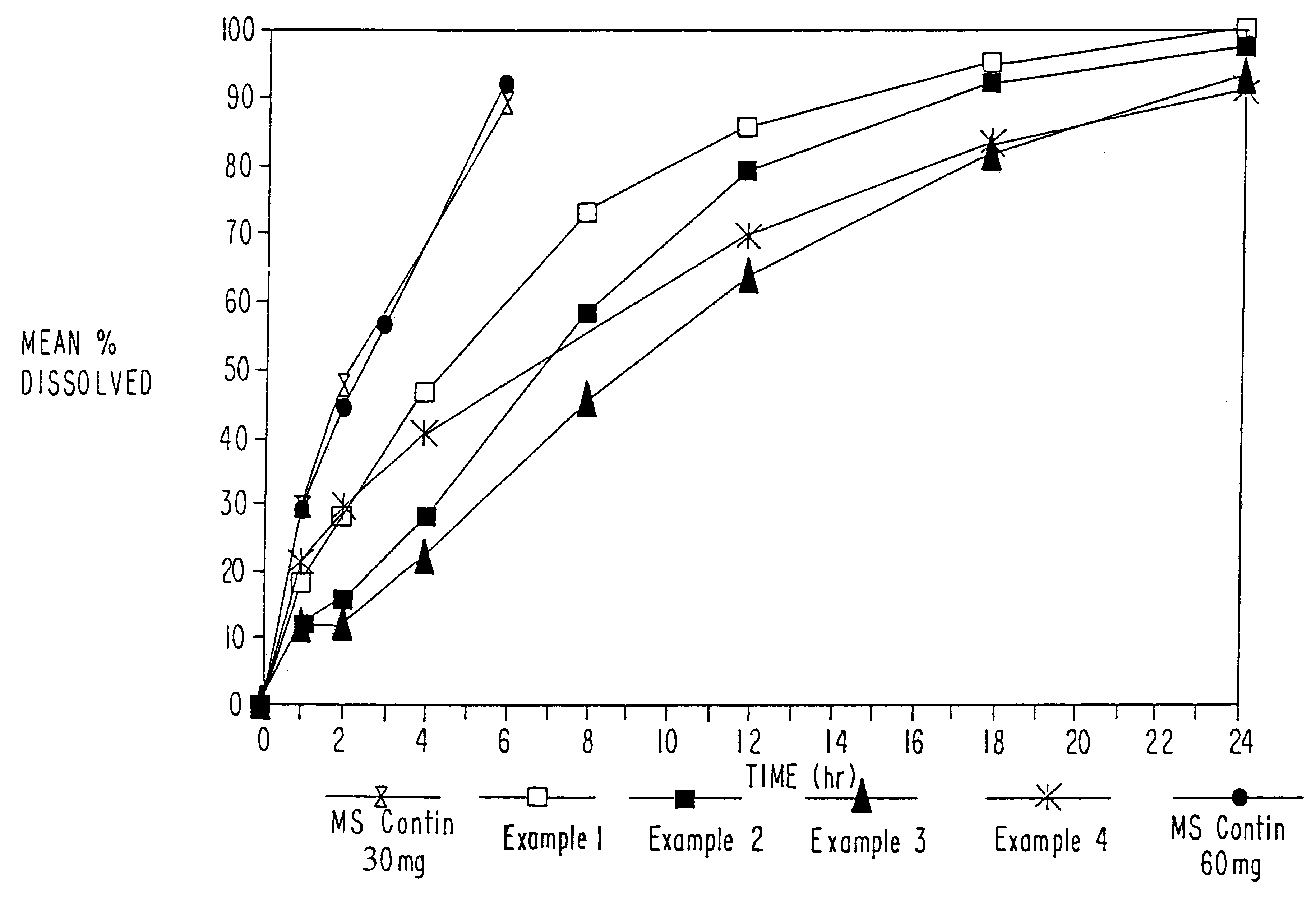
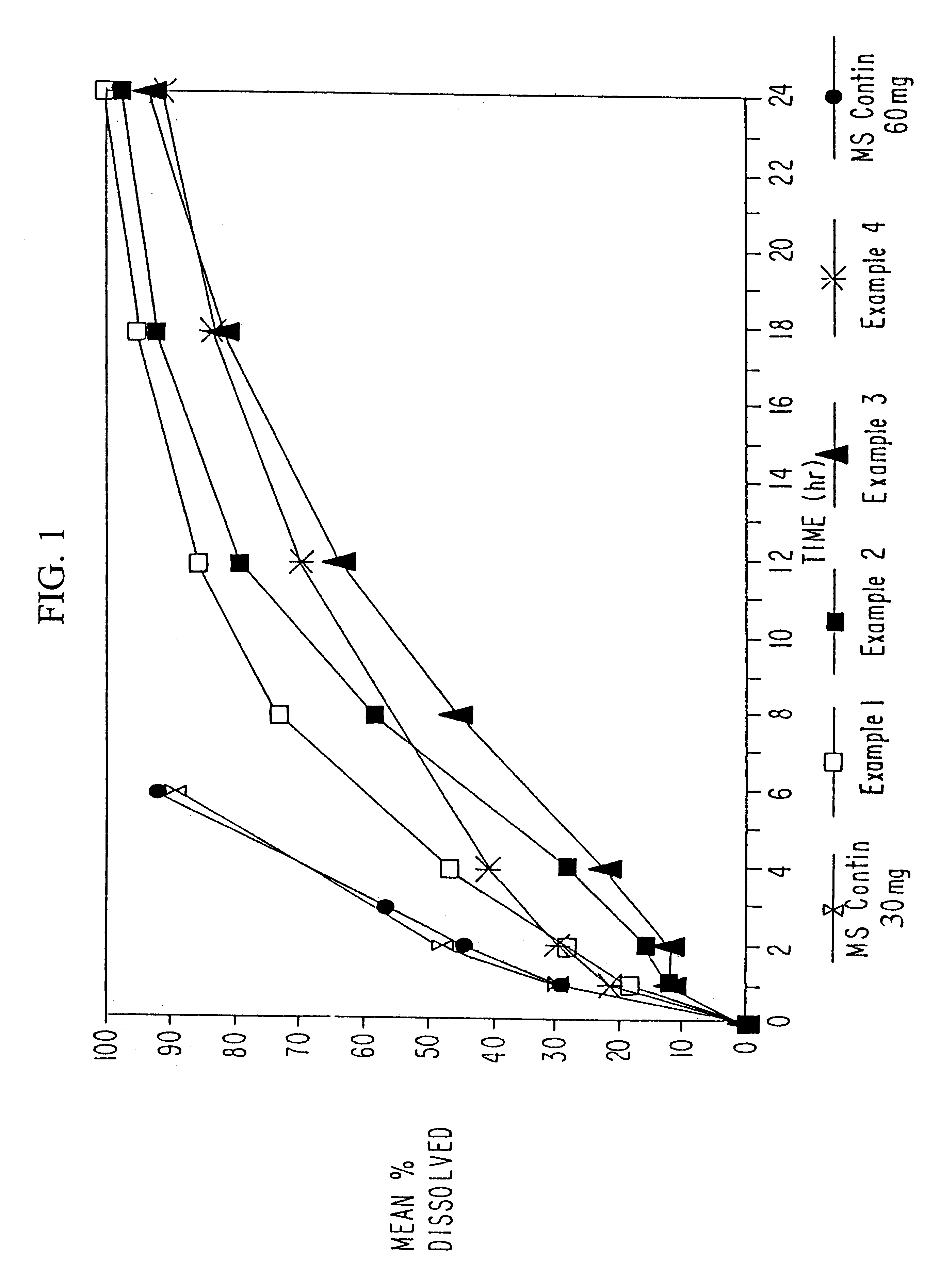
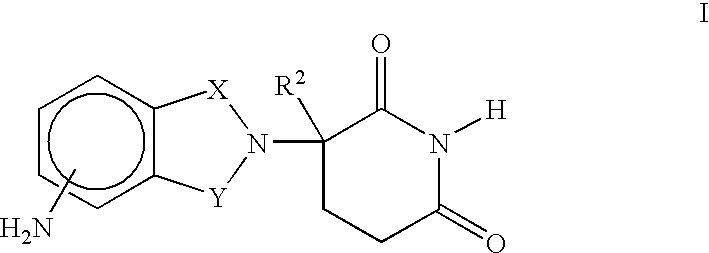
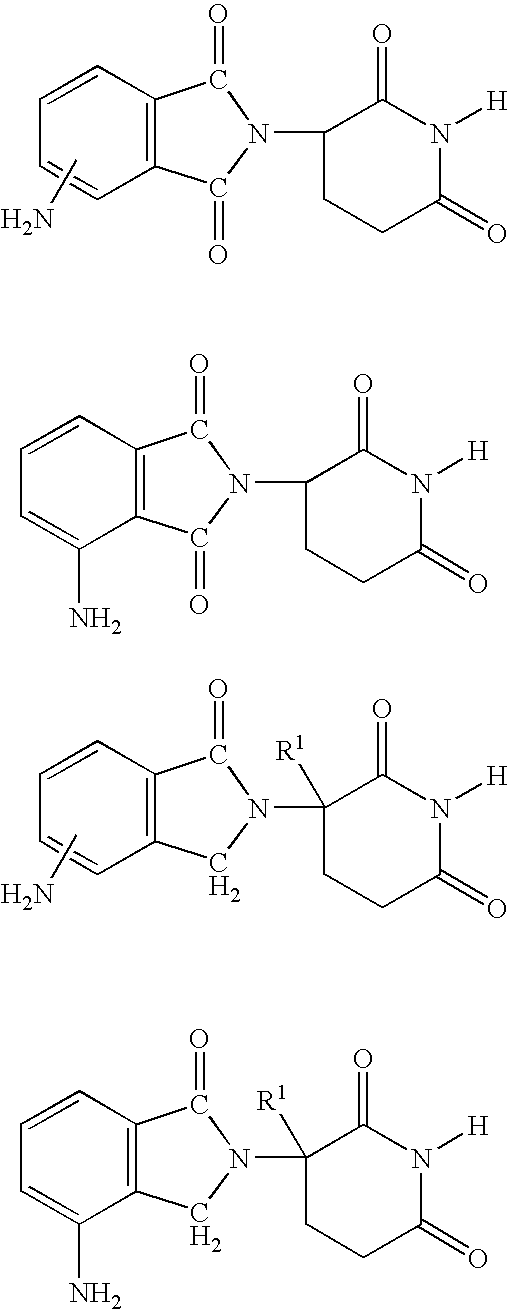
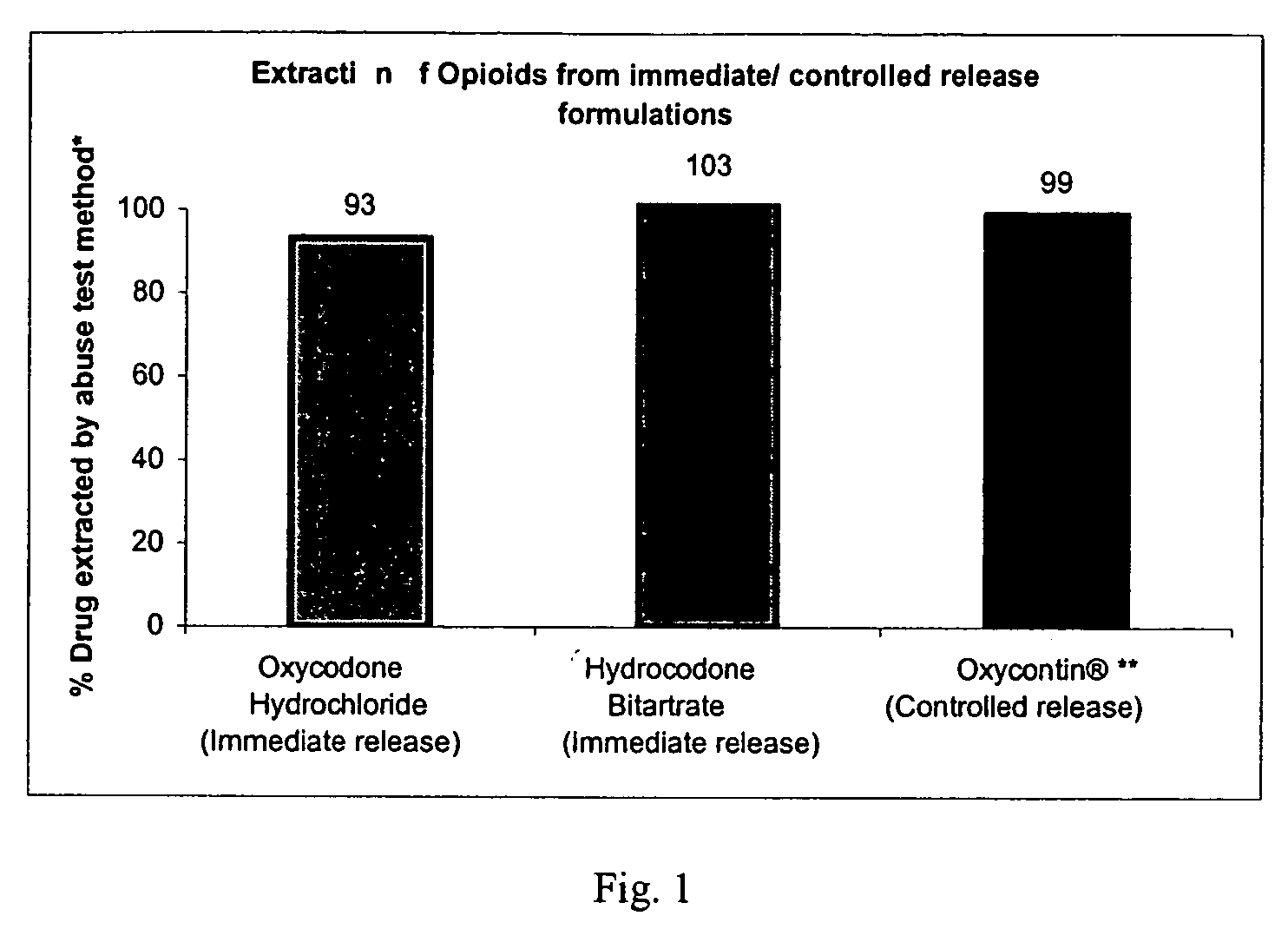
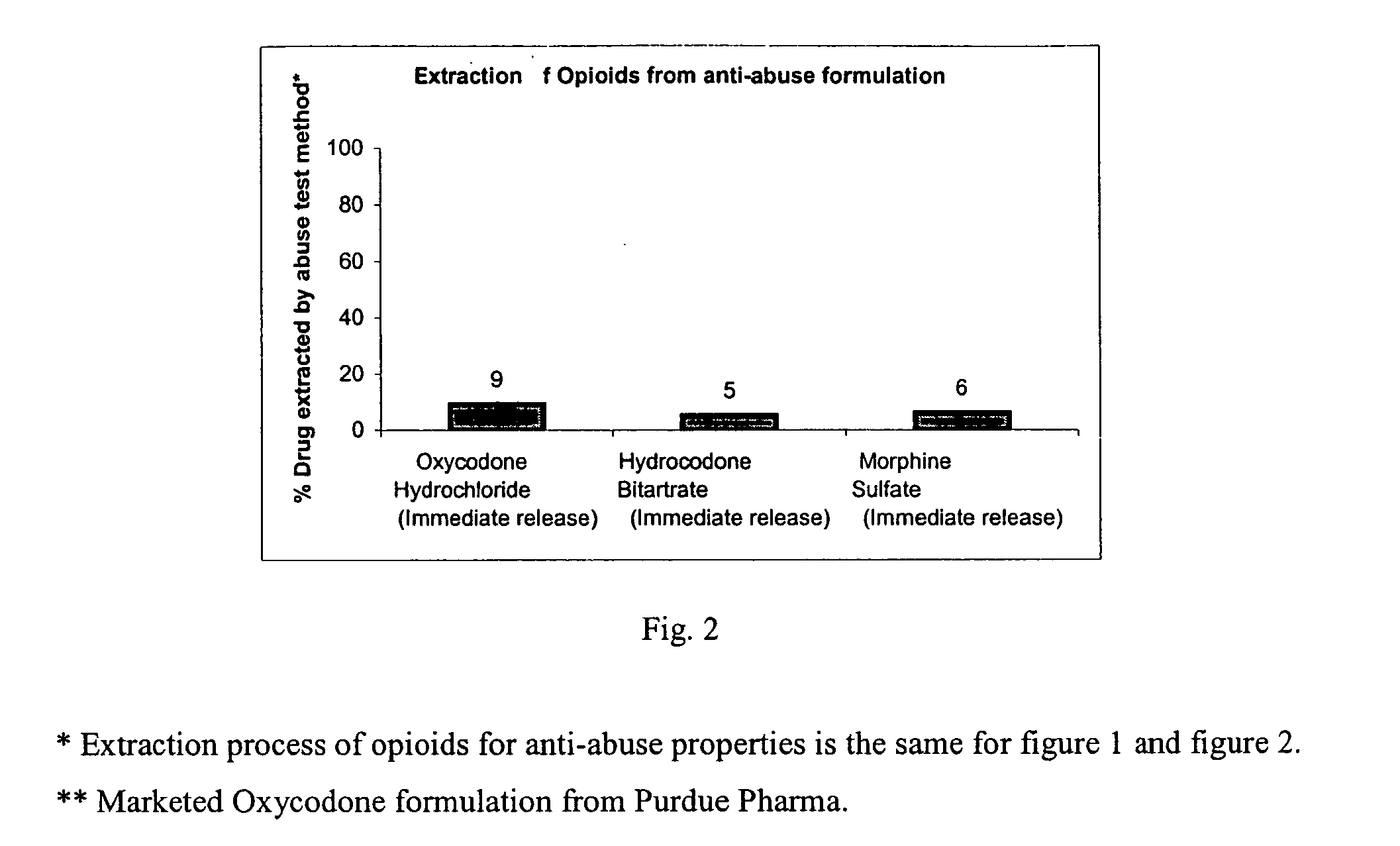
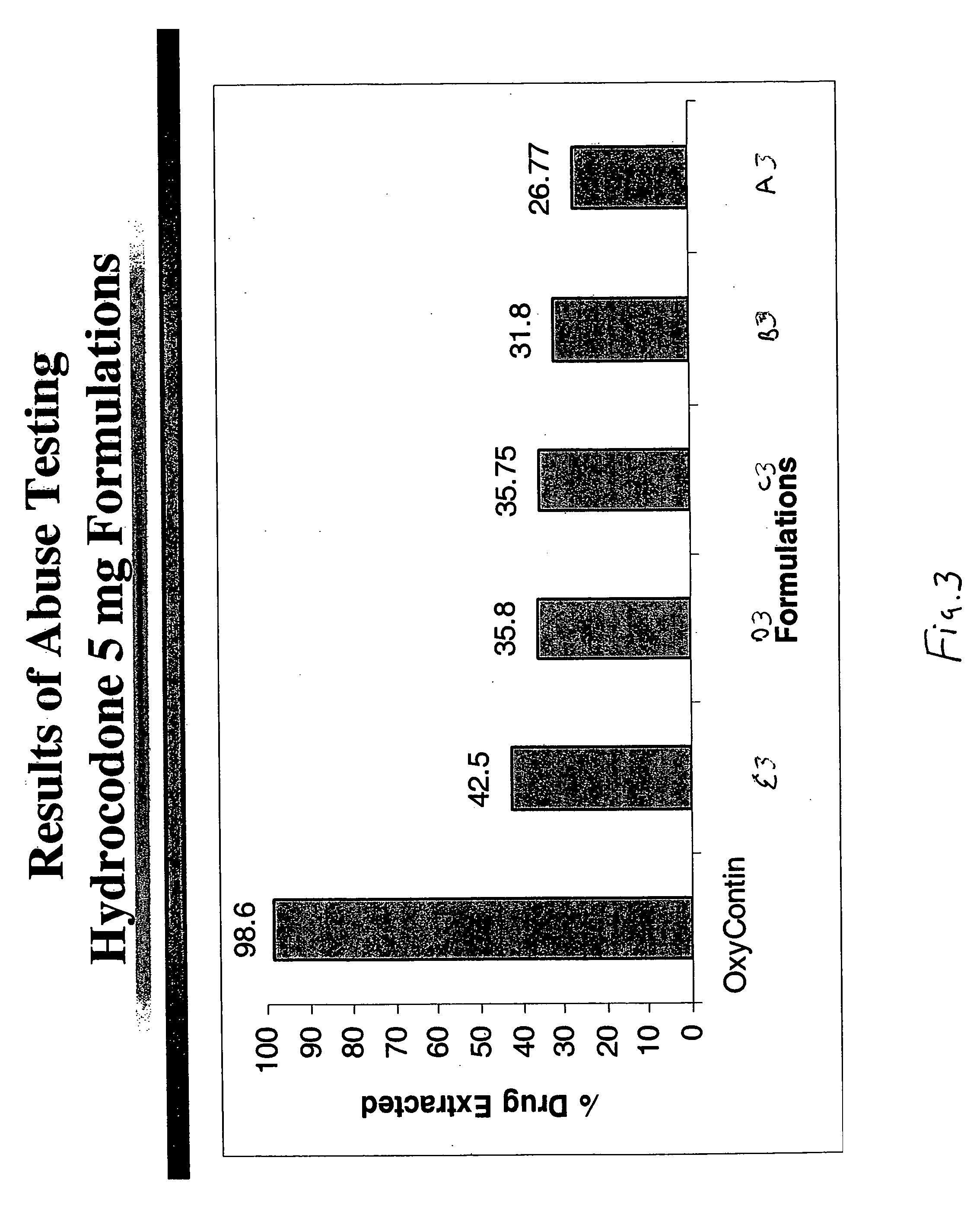
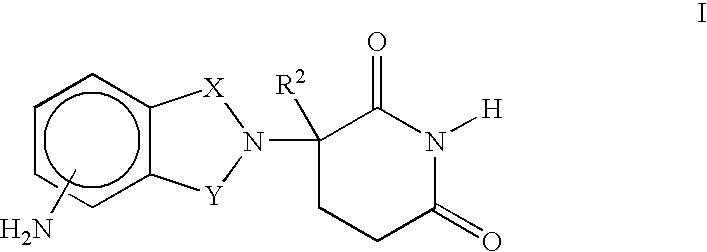
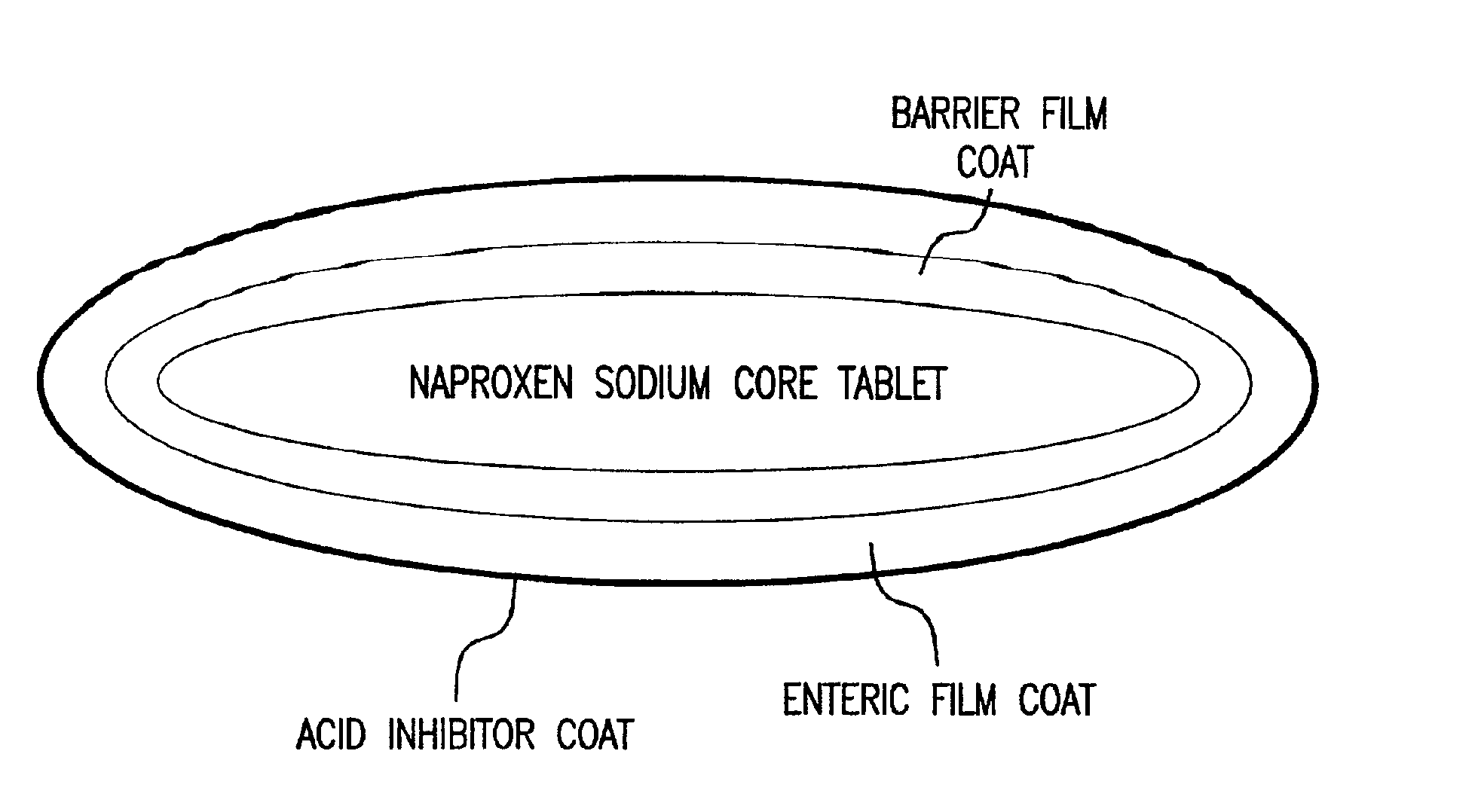
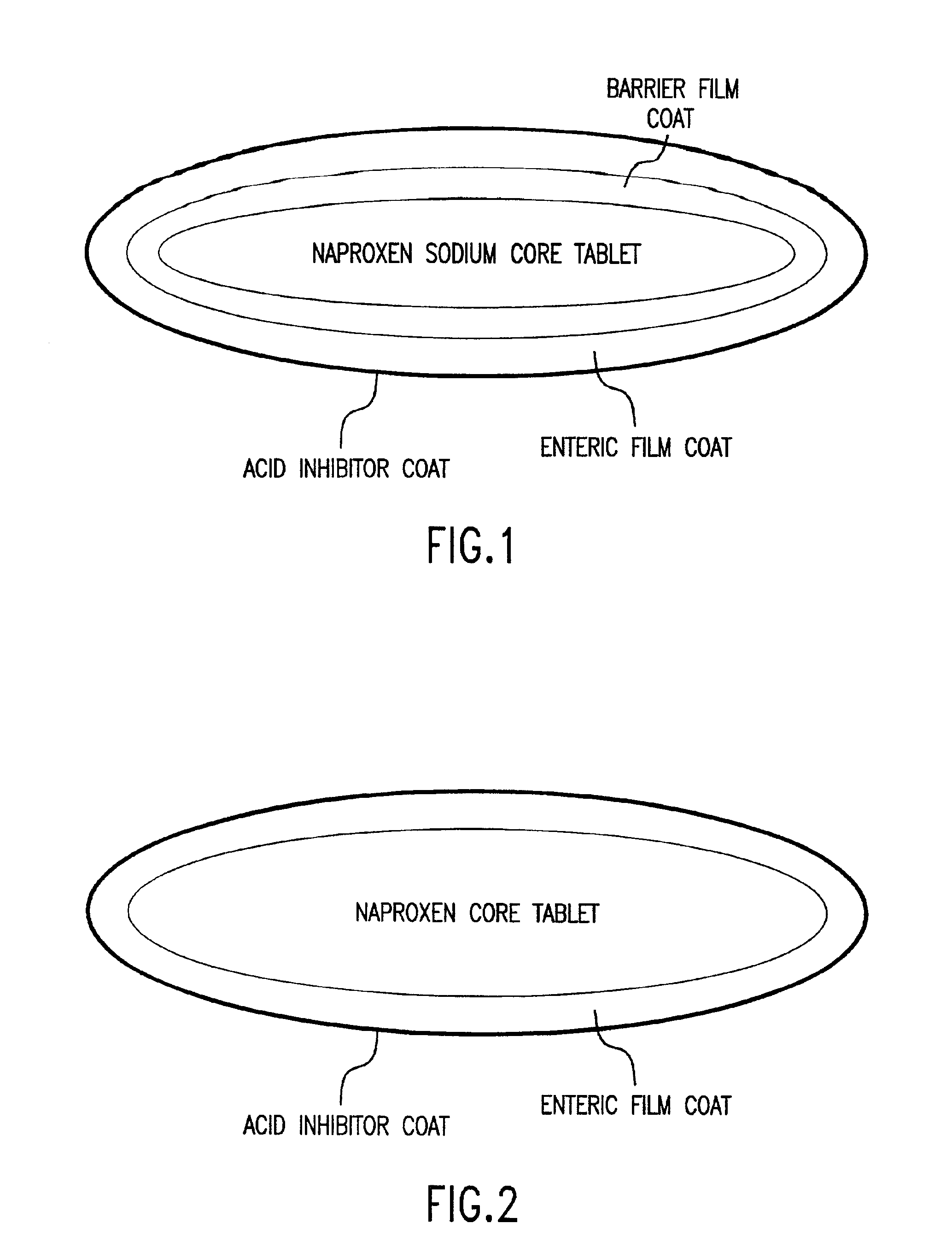
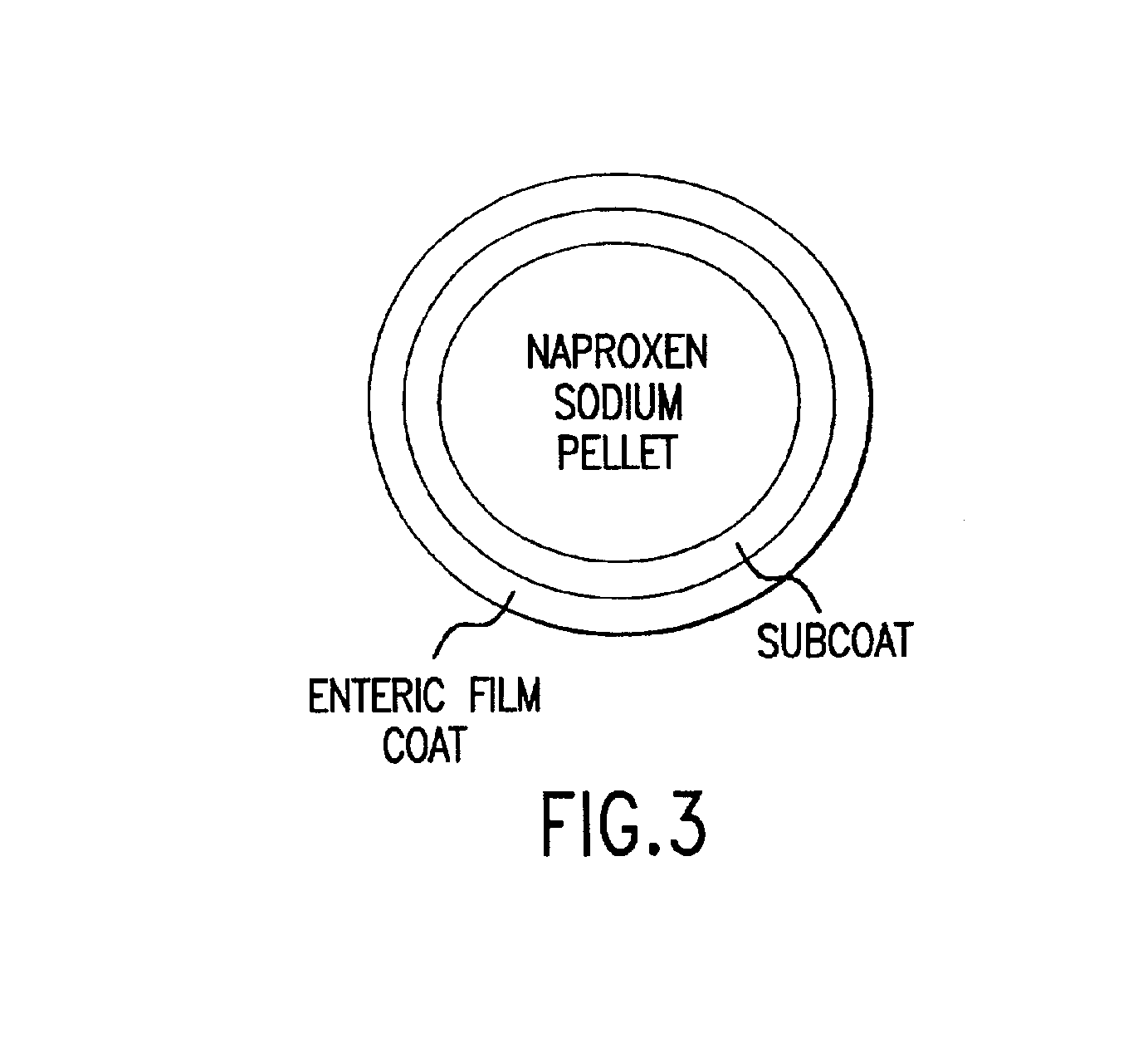
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
18416 results about "Dosage form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
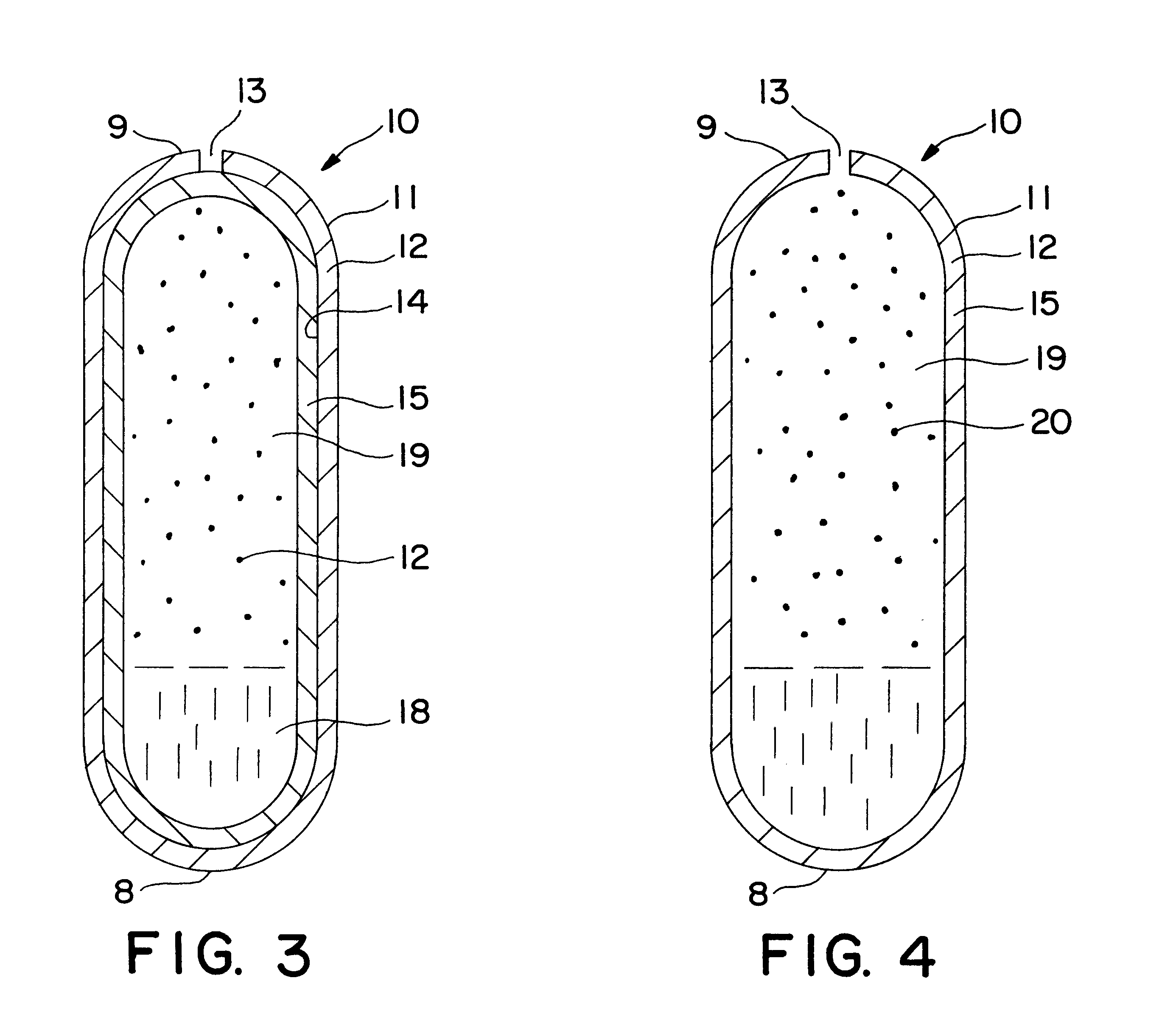
Dosage forms (also called unit doses) are pharmaceutical drug products in the form in which they are marketed for use, with a specific mixture of active ingredients and inactive components (excipients), in a particular configuration (such as a capsule shell, for example), and apportioned into a particular dose. For example, two products may both be amoxicillin, but one is in 500 mg capsules and another is in 250 mg chewable tablets. The term unit dose can also sometimes encompass non-reusable packaging as well (especially when each drug product is individually packaged), although the FDA distinguishes that by unit-dose "packaging" or "dispensing". Depending on the context, multi(ple) unit dose can refer to distinct drug products packaged together, or to a single drug product containing multiple drugs and/or doses. The term dosage form can also sometimes refer only to the pharmaceutical formulation of a drug product's constituent drug substance(s) and any blends involved, without considering matters beyond that (like how it is ultimately configured as a consumable product such as a capsule, patch, etc.). Because of the somewhat vague boundaries and unclear overlap of these terms and certain variants and qualifiers within the pharmaceutical industry, caution is often advisable when conversing with someone who may be unfamiliar with another person's use of the term.
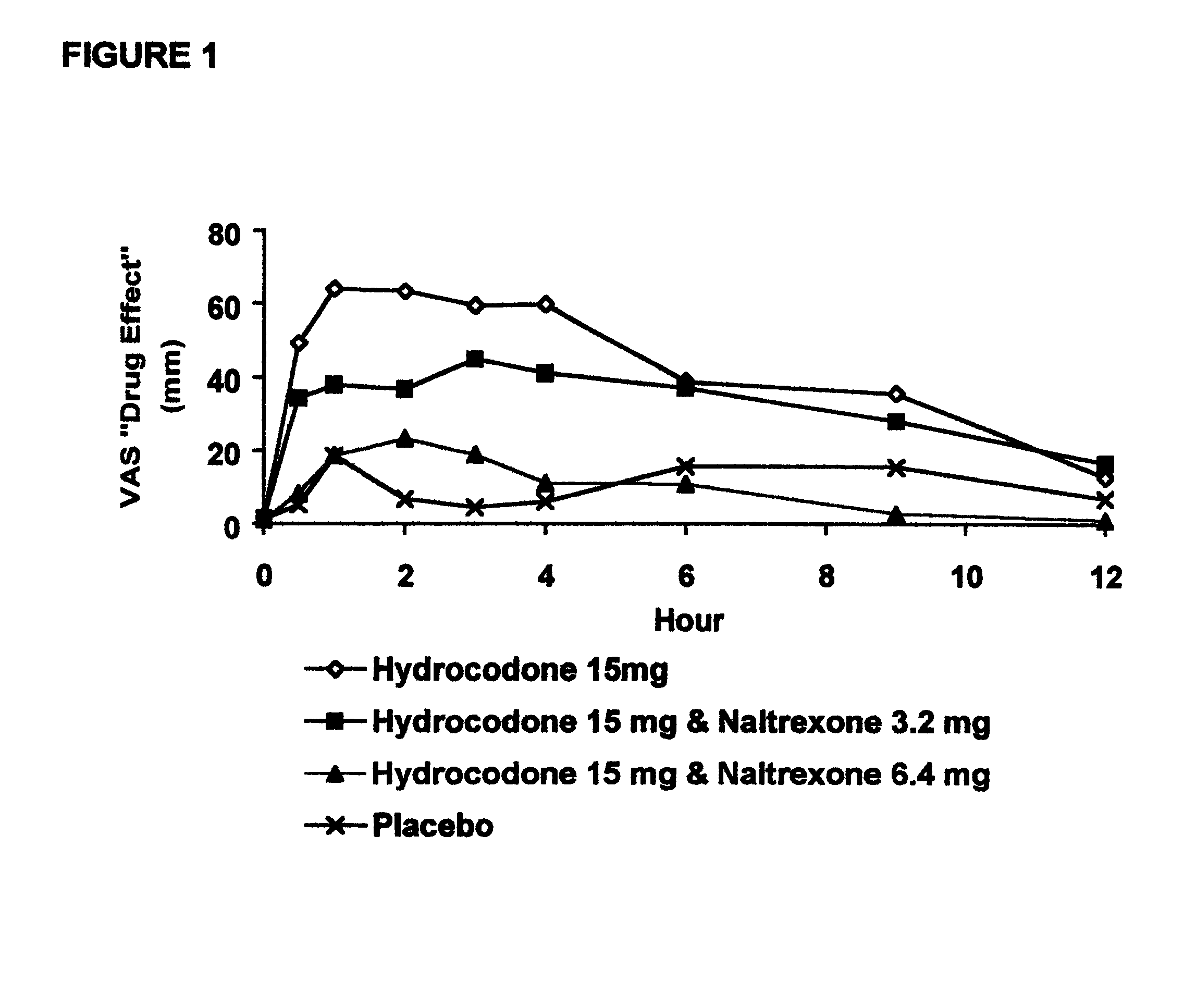
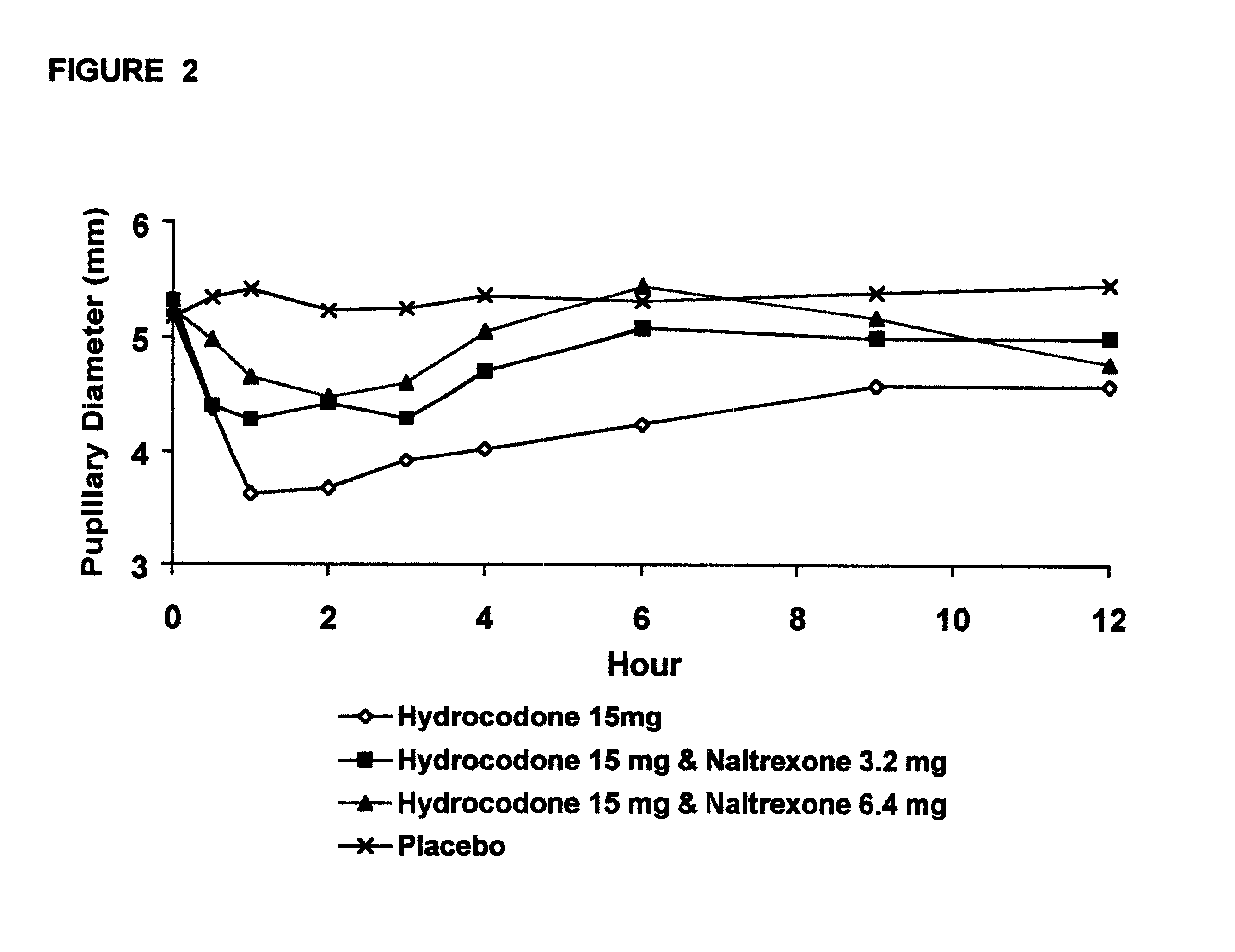
Method of preventing abuse of opioid dosage forms
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Pharmaceutical dosage forms for highly hydrophilic materials
Pharmaceutical dosage forms having a highly hydrophilic fill material and a shell encapsulating the fill material are disclosed and described. Generally, the shell has at least one plasticizing agent therein in order to provide the shell with an effective plasticity. In one aspect, the shell may have included therein an amount of plasticizing agent that is sufficient to provide the shell with an effective plasticity upon migration of a portion of the plasticizing agent into the fill material. In another aspect, the plasticizing agent may have a solubility in the fill material of less than about 10% w / w. In yet another aspect, a combination of a plasticizing agent, and a plasticizing agent having a solubility in the fill material of less than about 10% w / w, may be presented in a total amount sufficient to provide the shell with an effective plasticity upon migration of plasticizing agent into the fill material.
Owner:LIPOCINE
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Drug storage and dispensing devices and systems comprising the same
ActiveUS20070186923A1Minimizing saliva influxPowdered material dispensingDrug and medicationsDrug StorageBiomedical engineering
Drug storage and dispensing devices for dispensing a drug dosage form to a patient are disclosed. The dispensing device has a programmable lock-out feature for locking the dispensing device and is capable of detecting the identity of a user. The invention further provides a method for the treatment of subject, by administering to the subject a drug dosage form using a dispensing device of the invention.
Owner:ACEIRX PHARM INC
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Melt-extruded orally administrable opioid formulations
InactiveUS6261599B1Sustained effectSlow and control releaseBiocideOrganic active ingredientsMelt extrusionDosage form
Bioavailable sustained release oral opioid analgesic dosage forms, comprising a plurality of multiparticulates produced via melt extrusion techniques disclosed.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
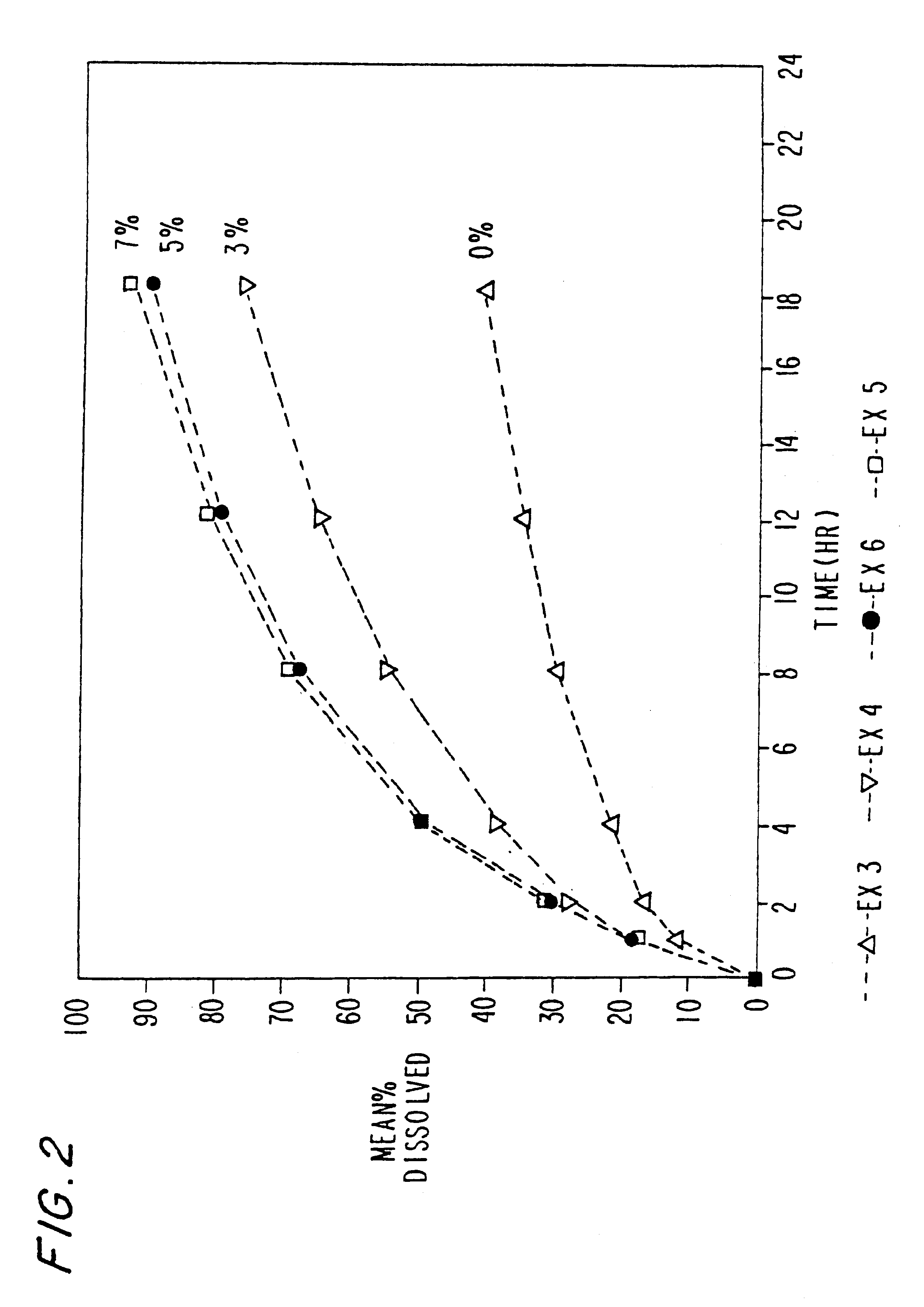
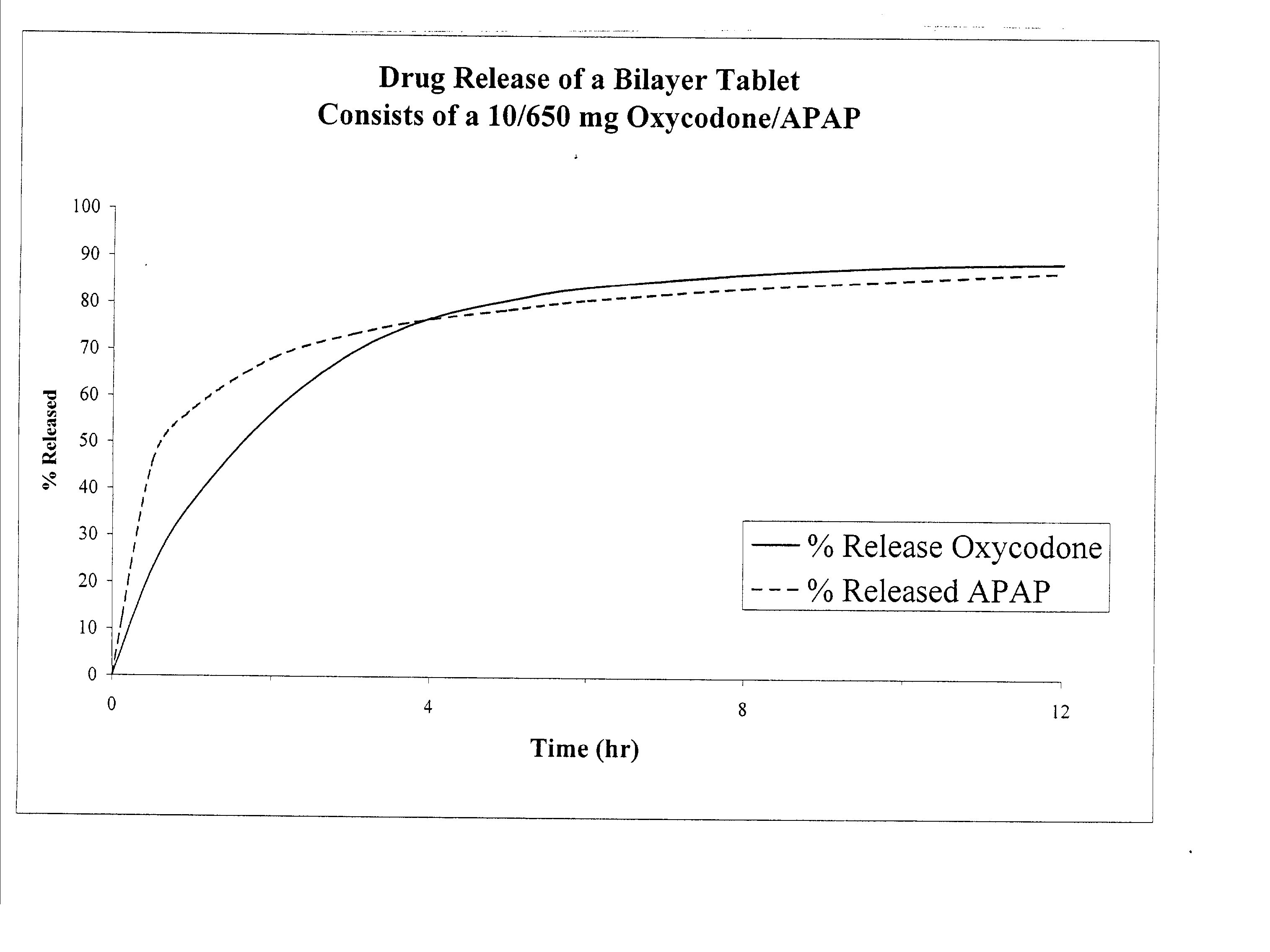
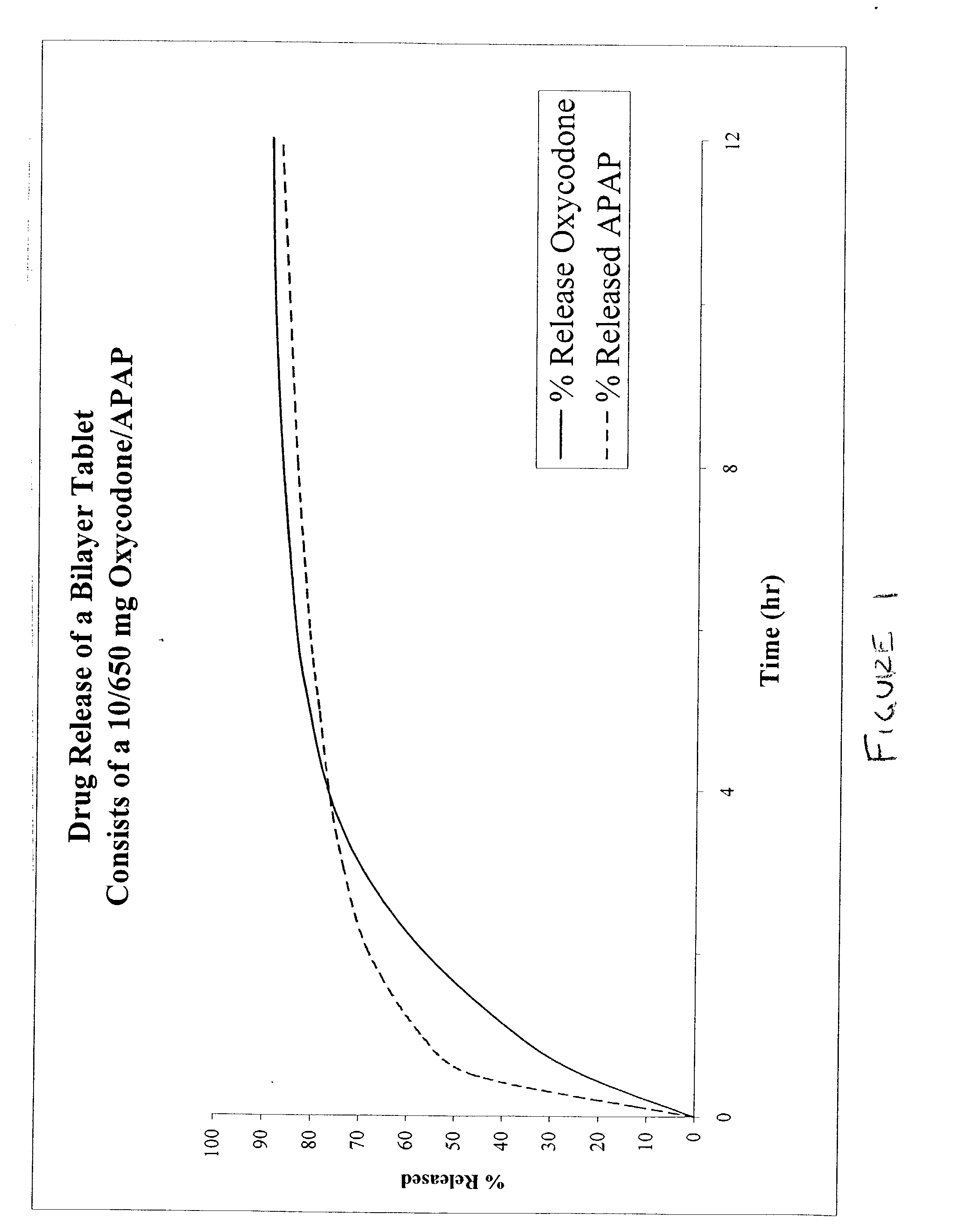
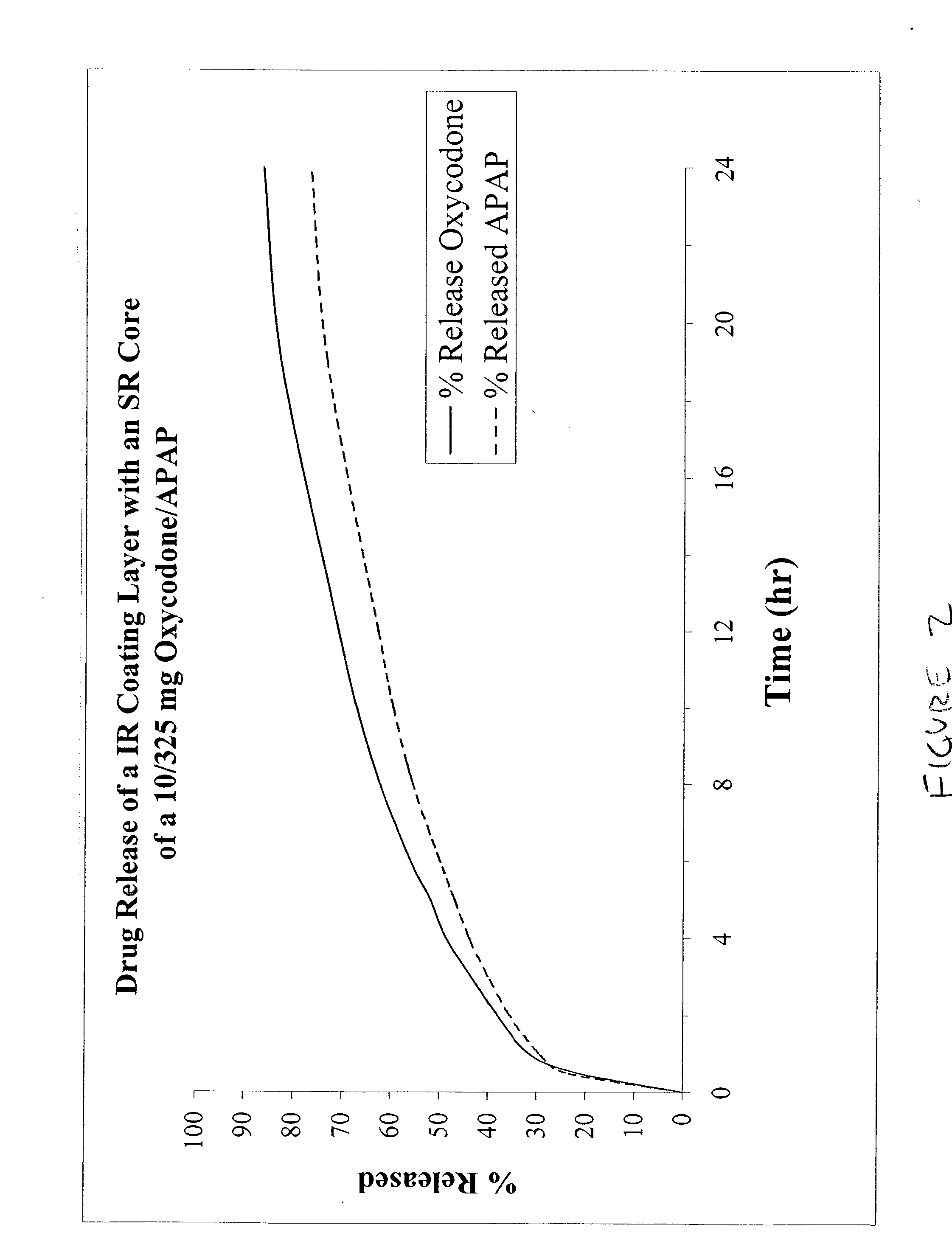
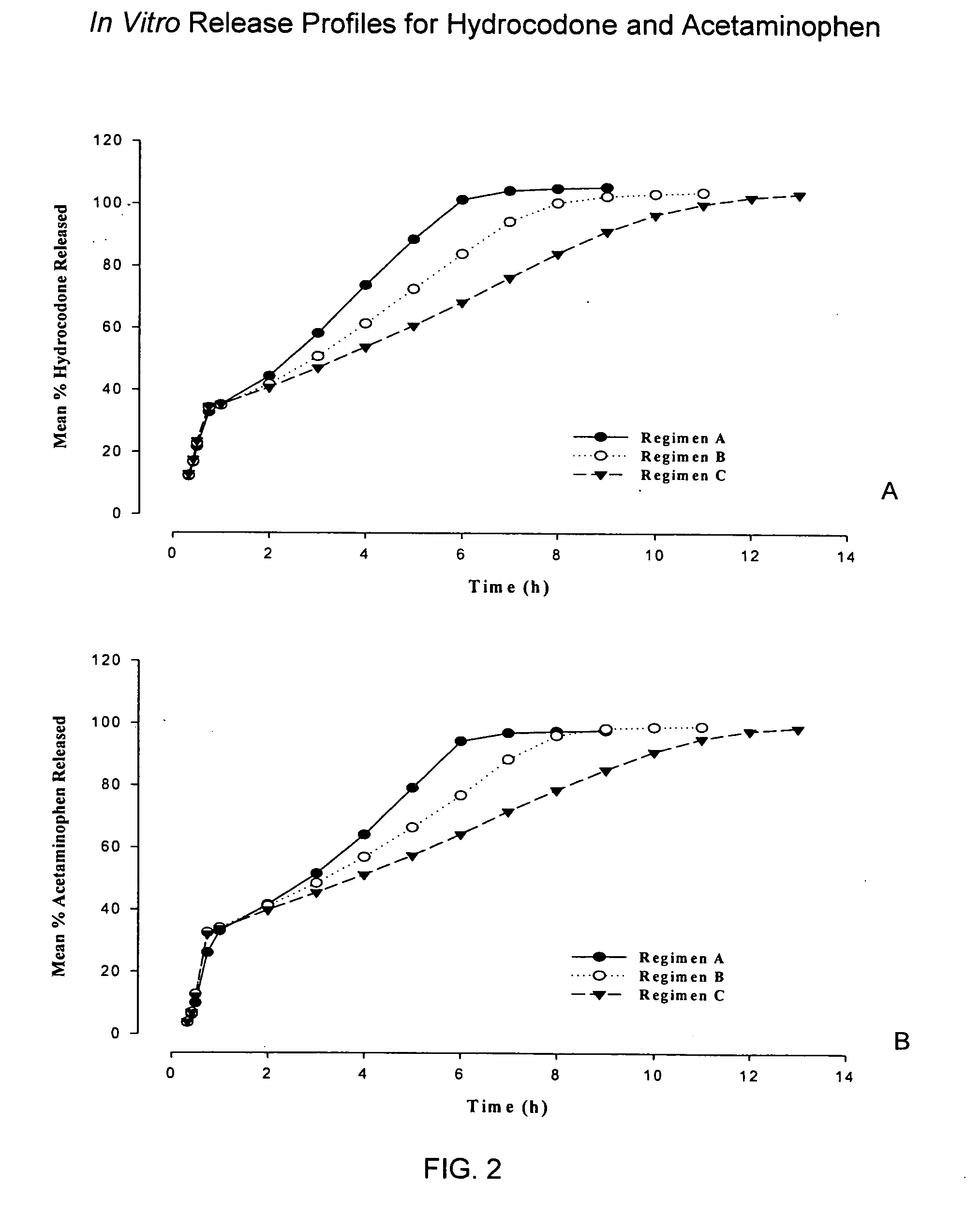
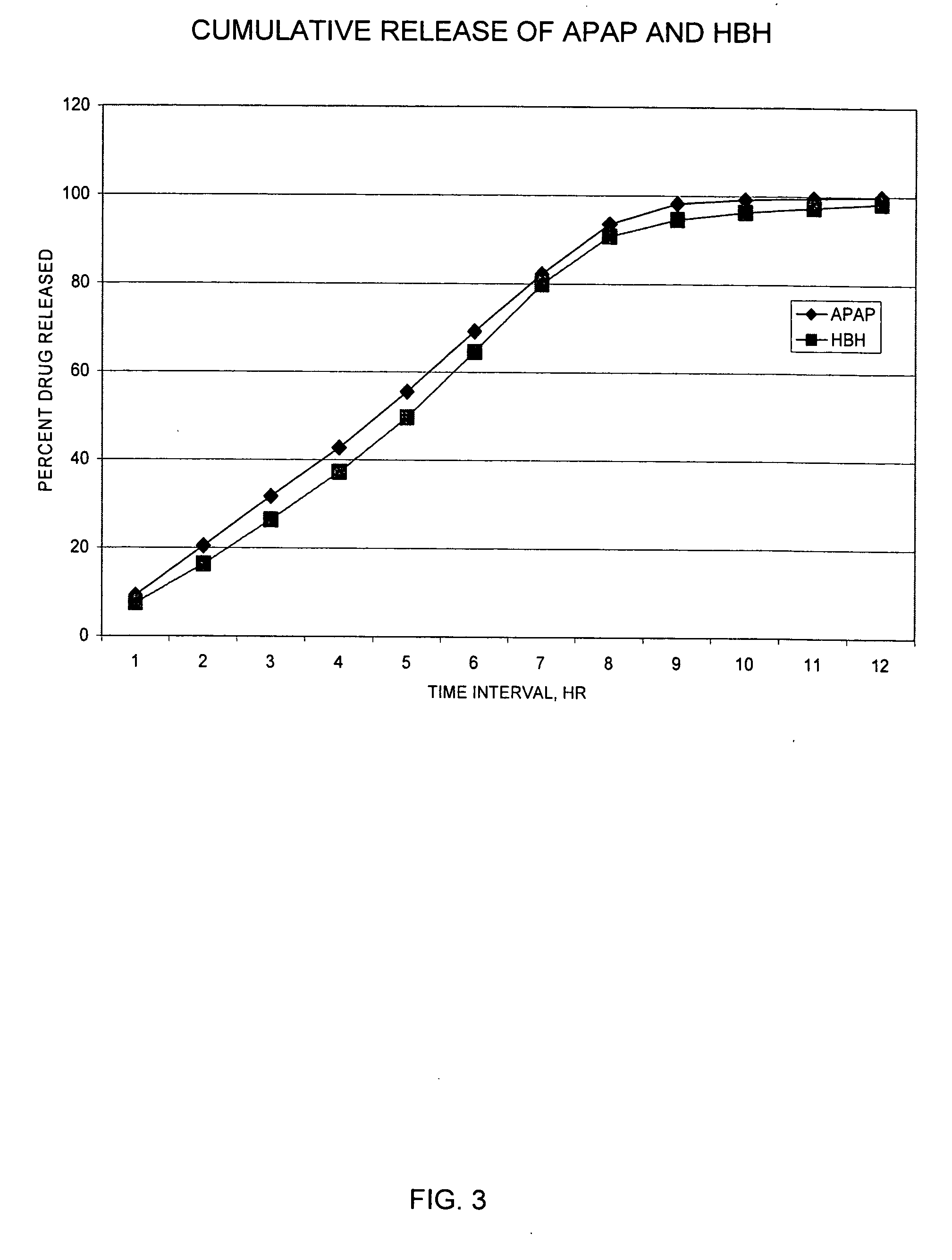
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
Orally administrable opioid formulations having extended duration of effect
InactiveUS6294195B1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Sustained release oral solid dosage forms of opioid analgesics are provided as multiparticulate systems which are bioavailable and which provide effective blood levels of the opioid analgesic for at least about 24 hours. A unit dose of the opioid analgesic contains a plurality of substrates including the opioid analgesic in sustained release form. The substrates have a diameter from about 0.1 mm to about 3 mm.
Owner:PURDUE PHARMA LP
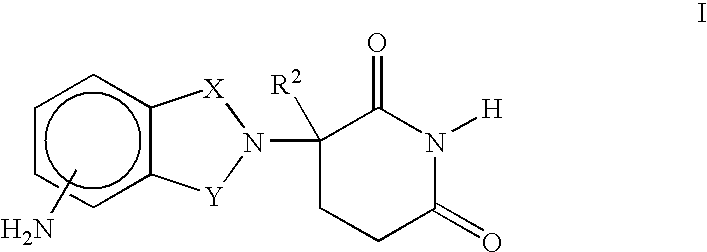
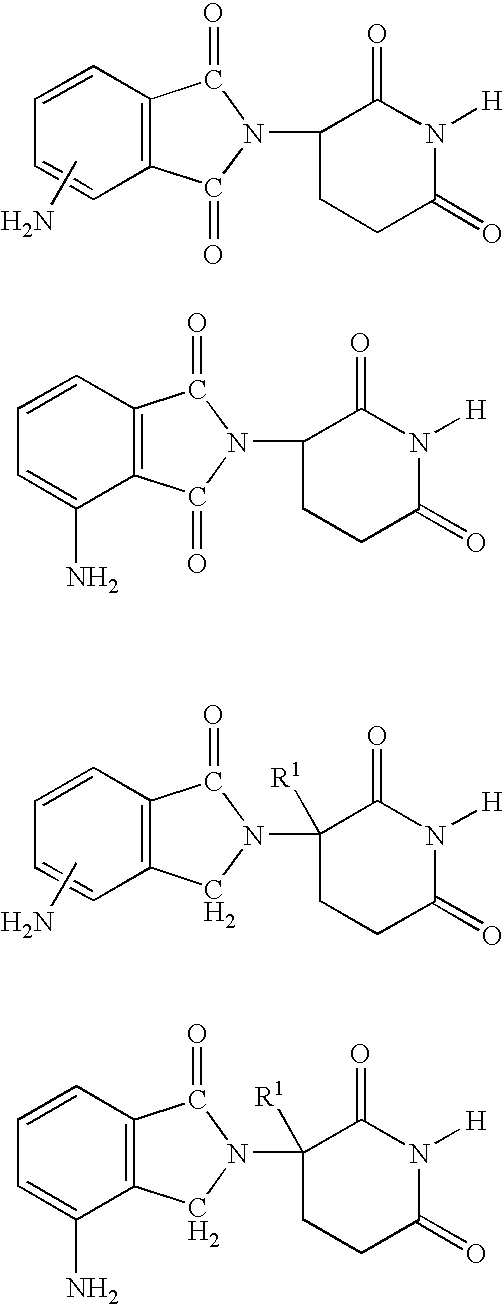
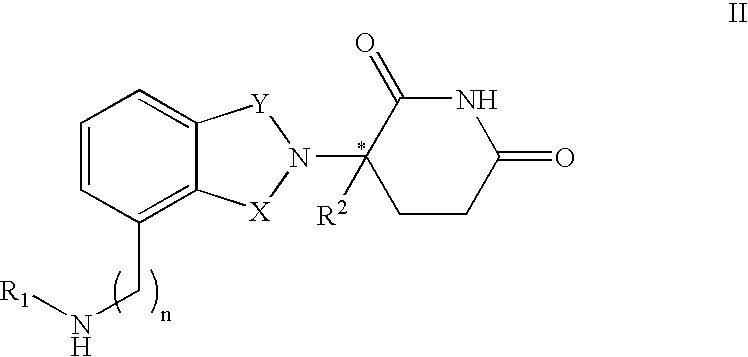
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myeloproliferative diseases
InactiveUS20040087546A1Reduce adverse effectsImprove toleranceBiocideNervous disorderActive agentMyeloproliferative disease
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, and / or the transplantation of blood or cells. Particular second active agents are capable of suppressing the overproduction of hematopoietic stem cells or ameliorating one or more of the symptoms of a myeloproliferative disease. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Methods and compositions using immunomodulatory compounds for treatment and management of cancers and other diseases
ActiveUS20040029832A1Prevent proliferationAntibacterial agentsBiocideSide effectBiologically-Based Therapy
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Methods and compositions for deterring abuse of opioid containing dosage forms
This invention relates to an abuse deterrent dosage form of opioid analgesics, wherein an analgesically effective amount of opioid analgesic is combined with a polymer to form a matrix.
Owner:HALSEY DRUG
Methods of using and compositions comprising immunomodulatory compounds for treatment and management of macular degeneration
InactiveUS20040091455A1Extension of timeUseful in treatingBiocideSenses disorderActive agentSurgical procedures
Methods of treating, preventing and / or managing macular degeneration are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or surgery. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Once-a-day, oral, controlled-release, oxycodone dosage forms
Oxycodone formulations are provided which produce substantially flat in vivo steady state plasma profiles. Tolerance levels associated with such profiles and tolerance levels associated with biphasic profiles are shown not to be statistically different. The substantially flat in vivo steady state plasma profiles are produced by dosage forms having substantially zero order in vitro release profiles. Such release profiles produce low single dose in vivo Cmax levels which can reduce the probability of adverse side effects.
Owner:ALZA CORP
Tamper resistant dosage forms
ActiveUS20090081290A1Reduces and prevents stickingBiocidePowder deliveryOpioid analgesicsDosage form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Pharmaceutical compositions for the coordinated delivery of NSAIDs
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO
Methods of using and compositions comprising (+) sibutramine optionally in combination with other pharmacologically active compounds
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (+) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
Abuse-proofed dosage form
ActiveUS8114383B2Pulverisation of the dosage form is considerably more difficultComplicating or preventing the subsequent abuseOrganic active ingredientsPowder deliveryBreaking strengthPhysiology
The present invention relates to an abuse-proofed, thermoformed dosage form containing, in addition to one or more active ingredients with abuse potential optionally together with physiologically acceptable auxiliary substances, at least one synthetic or natural polymer with a breaking strength of at least 500 N and to a process for the production thereof.
Owner:GRUNENTHAL GMBH
Delivery of oral drugs
InactiveUS20010020147A1Comfortable and convenient motionComfortable and convenient feelPowder deliveryLiquid surface applicatorsMean diameterHuman patient
Disclosed is a system for delivery of a drug comprising a multiple unit dosing device comprising a housing and an actuator, said device containing multiple doses of multiparticulates comprising drug particles, said device upon actuation delivering a unit dose of said multiparticulates, said drug particles having a mean diameter of greater than 10 mum to about 1 mm such that an effective dose of said drug cannot be delivered into the lower lung of a human patient. Also disclosed are novel methods, devices and dosage forms for delivering a drug.
Owner:PHARMAKODEX LTD
High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same
InactiveUS7014866B2Satisfactory bioavailabilitySatisfactory dissolutionPowder deliveryBiocideHigh dosesNelfinavir mesylate
A solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate is provided comprising amorphous nelfinavir mesylate in an amount of from about 400 mg to about 700 mg calculated as nelfinavir base, and a pharmaceutically acceptable water soluble, non-ionic synthetic block copolymer of ethylene oxide and propylene oxide, the copolymer having a melting point of at least about 45° C. and an HLB value at 25° C. of from about 18 to about 29, wherein the copolymer is present from about 40% to about 65% by weight of the nelfinavir mesylate. A hot melt granulation process for making the dosage form is provided.
Owner:F HOFFMANN LA ROCHE & CO AG
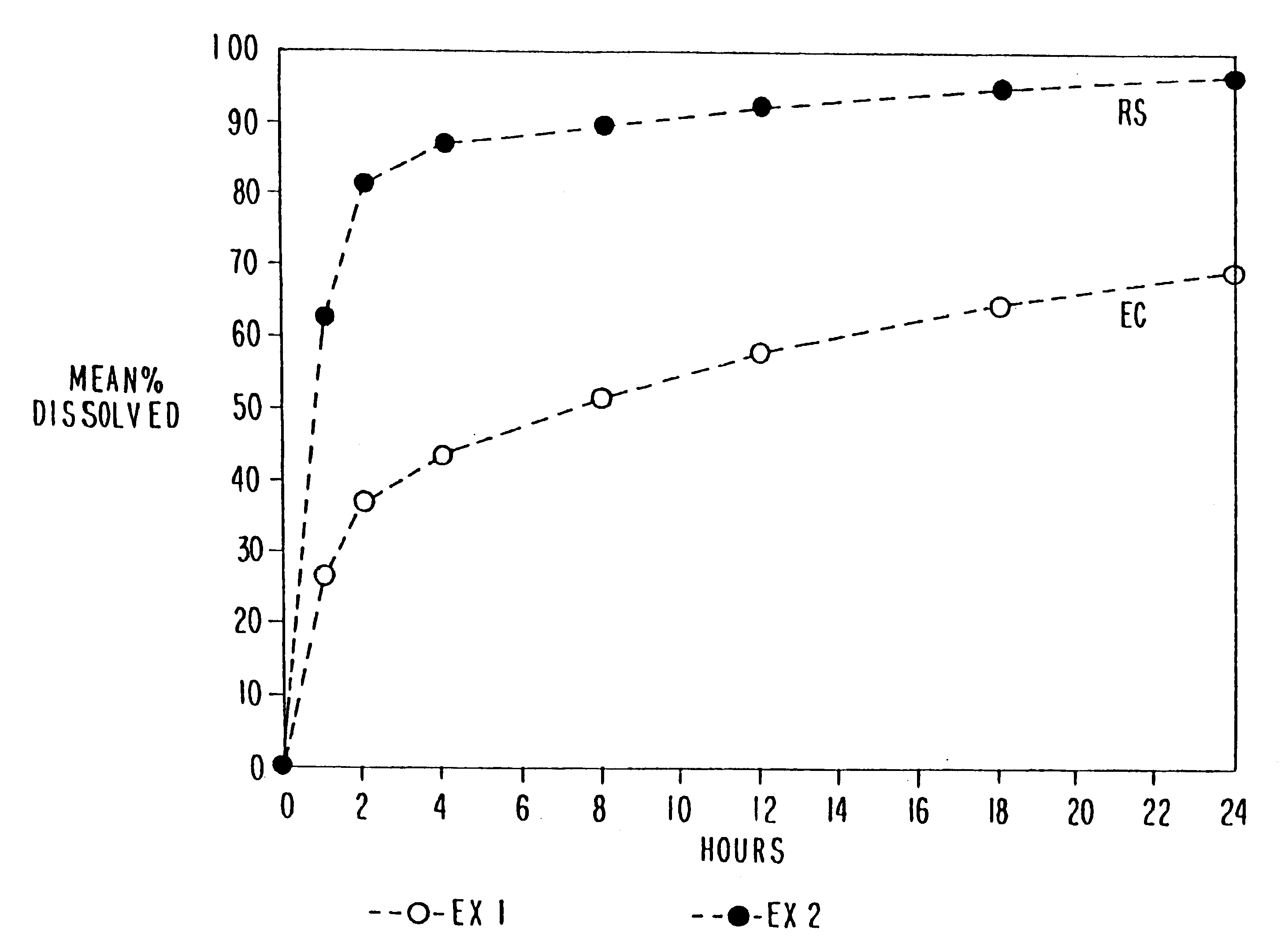
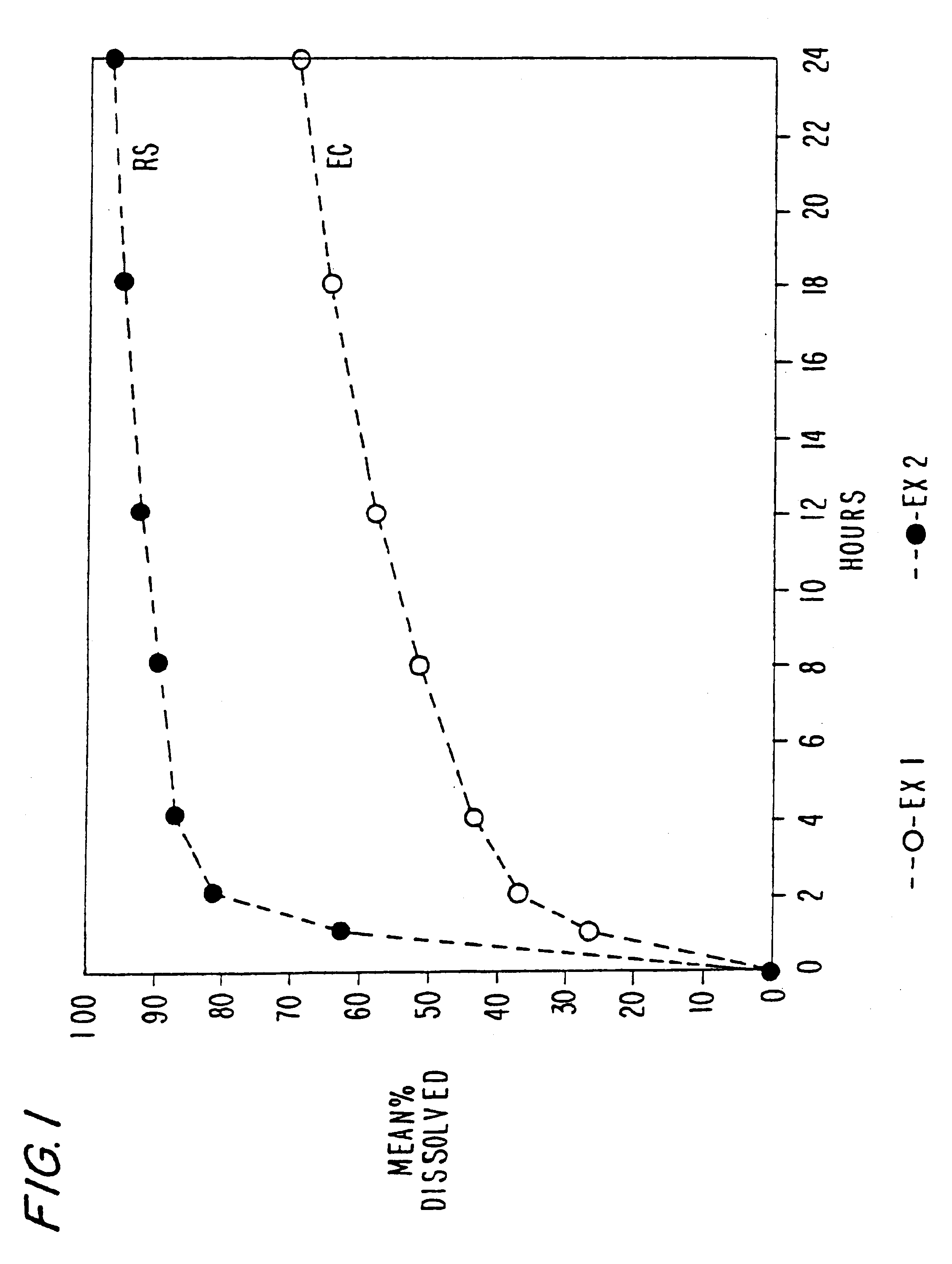
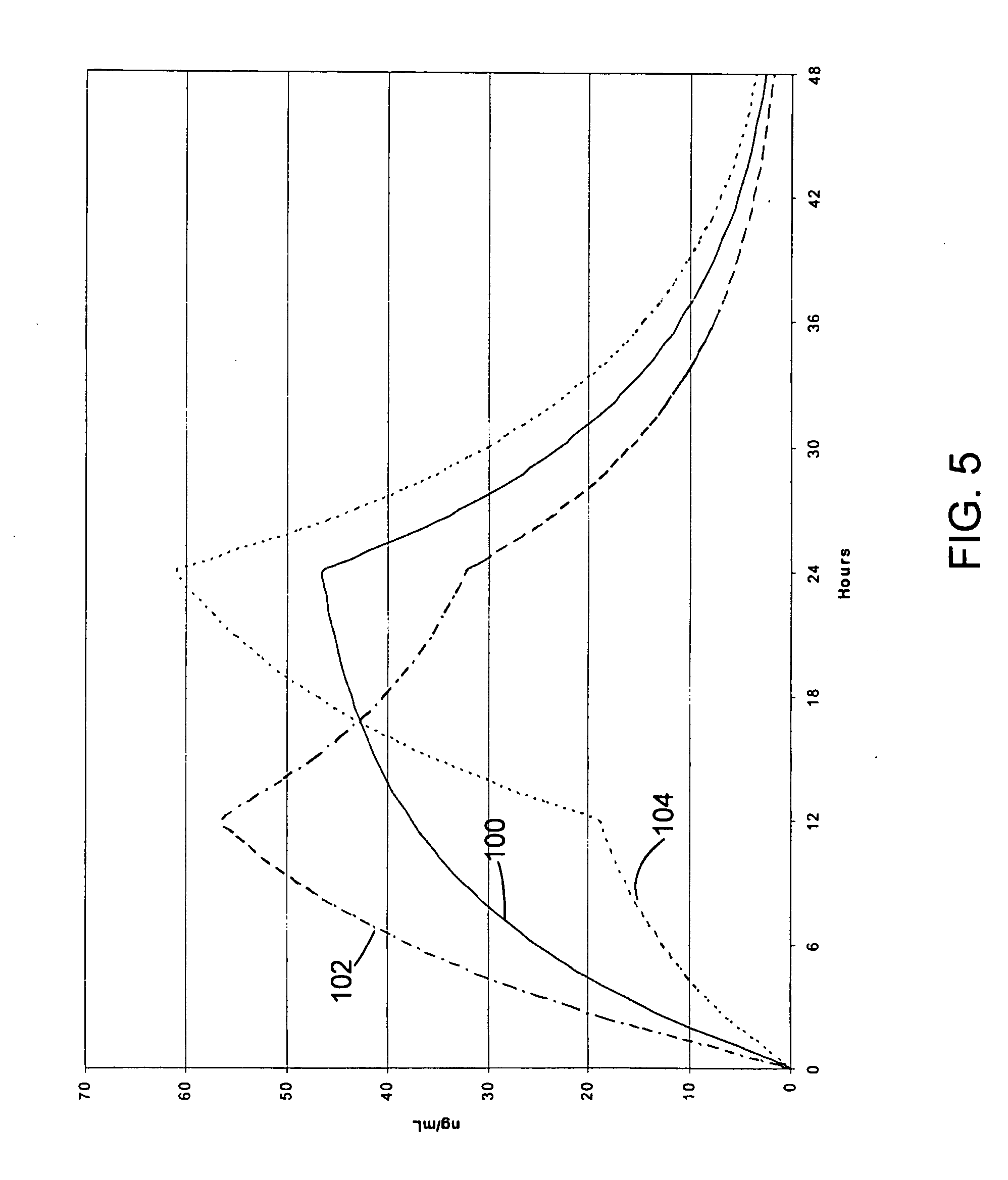
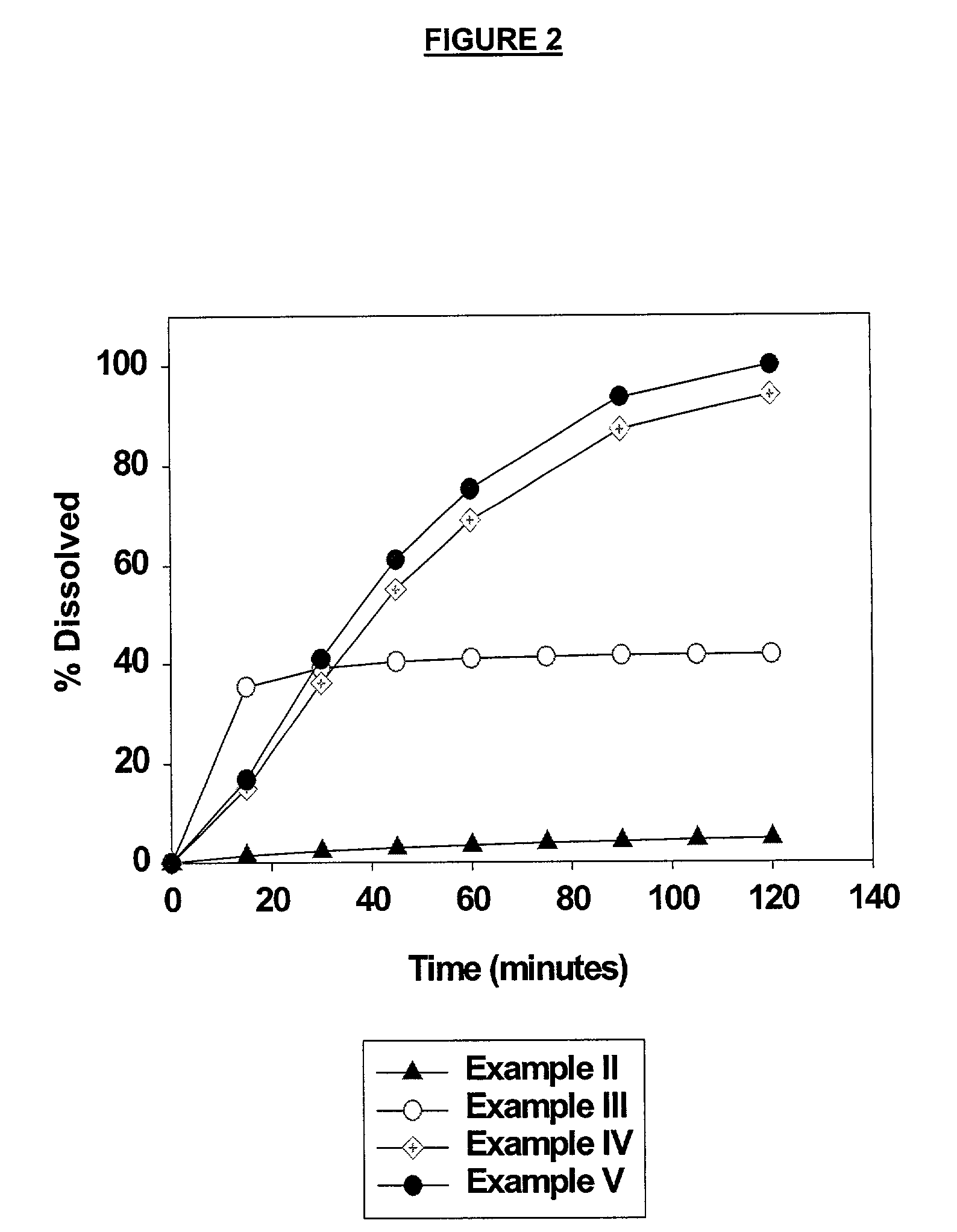
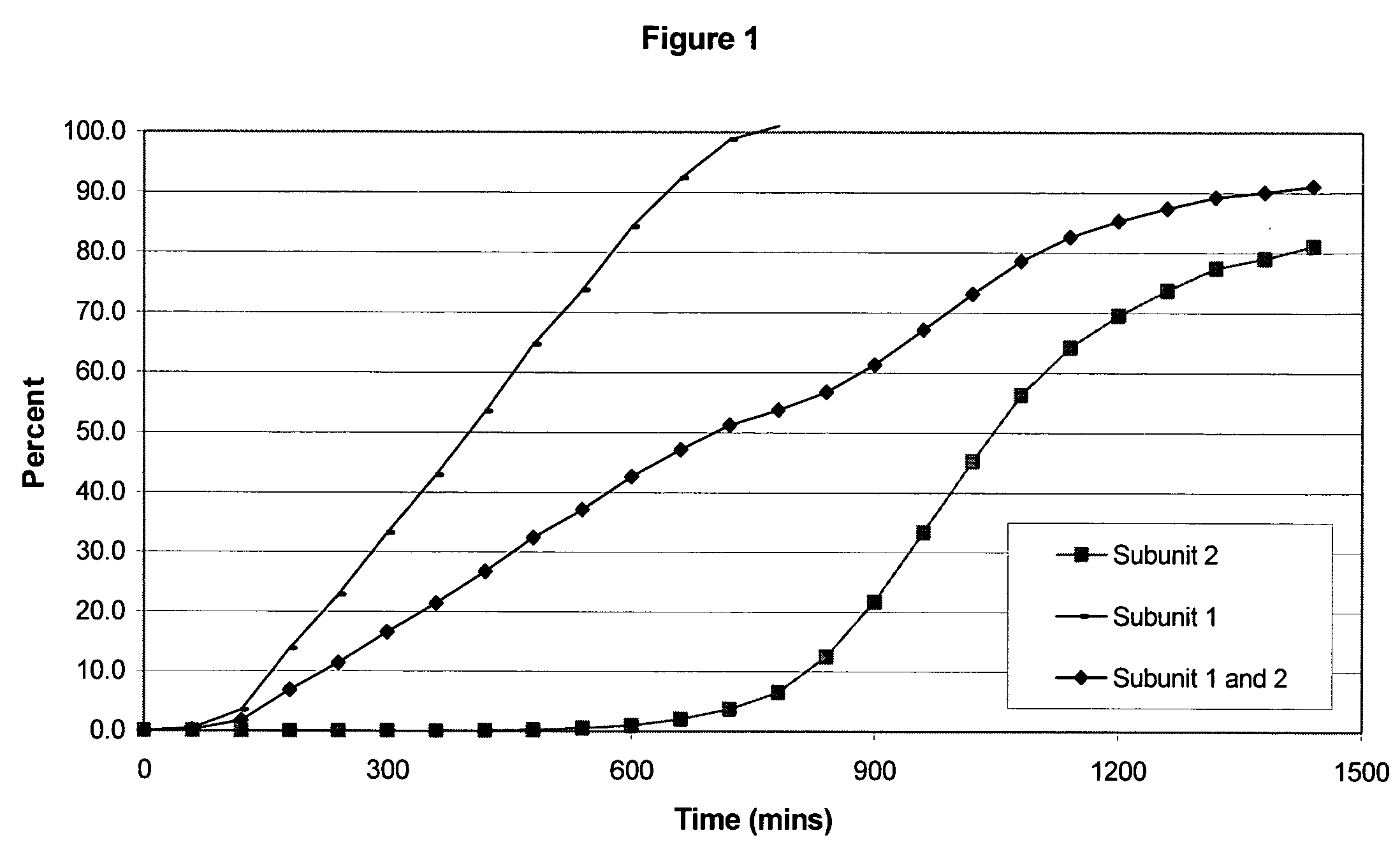
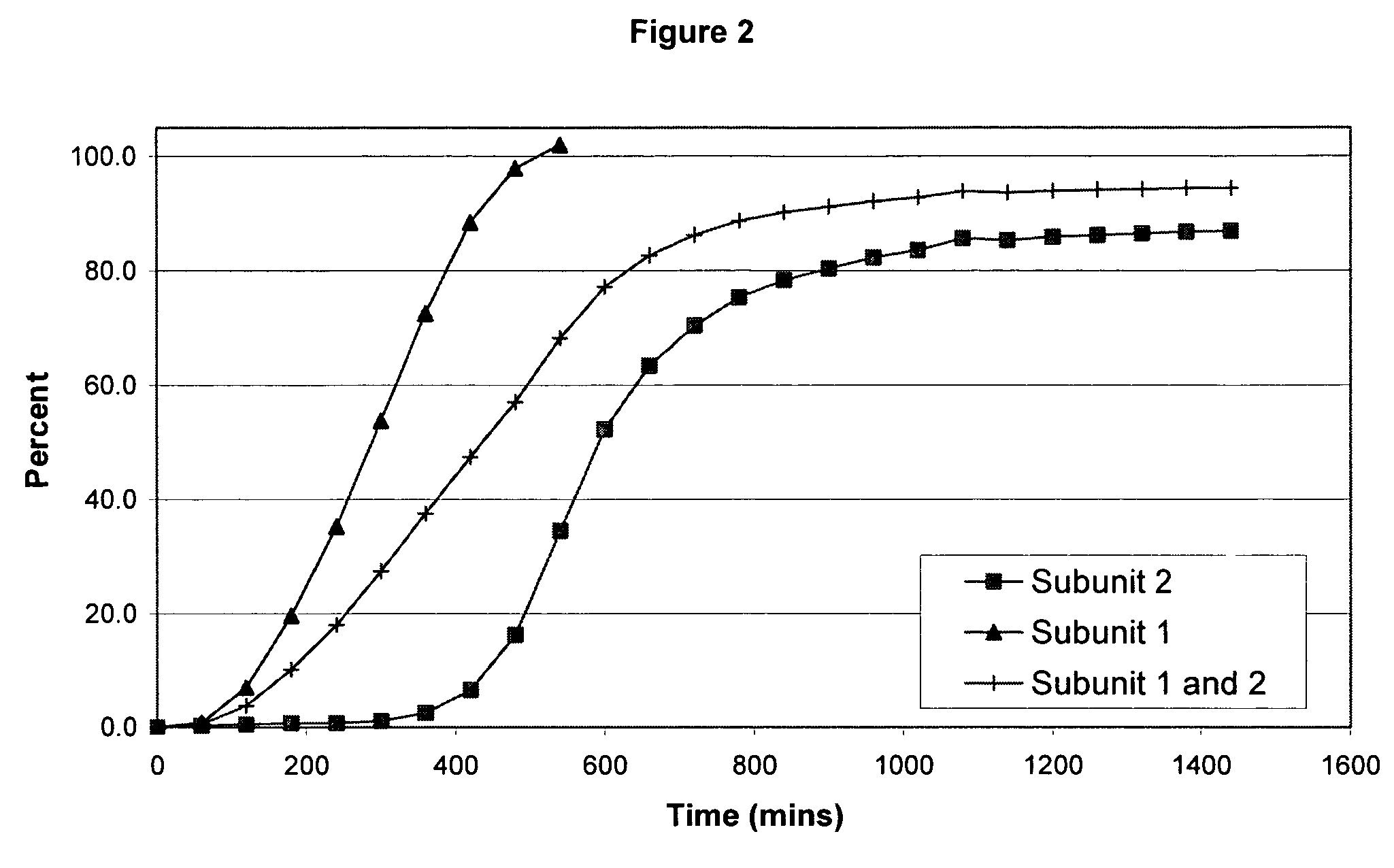
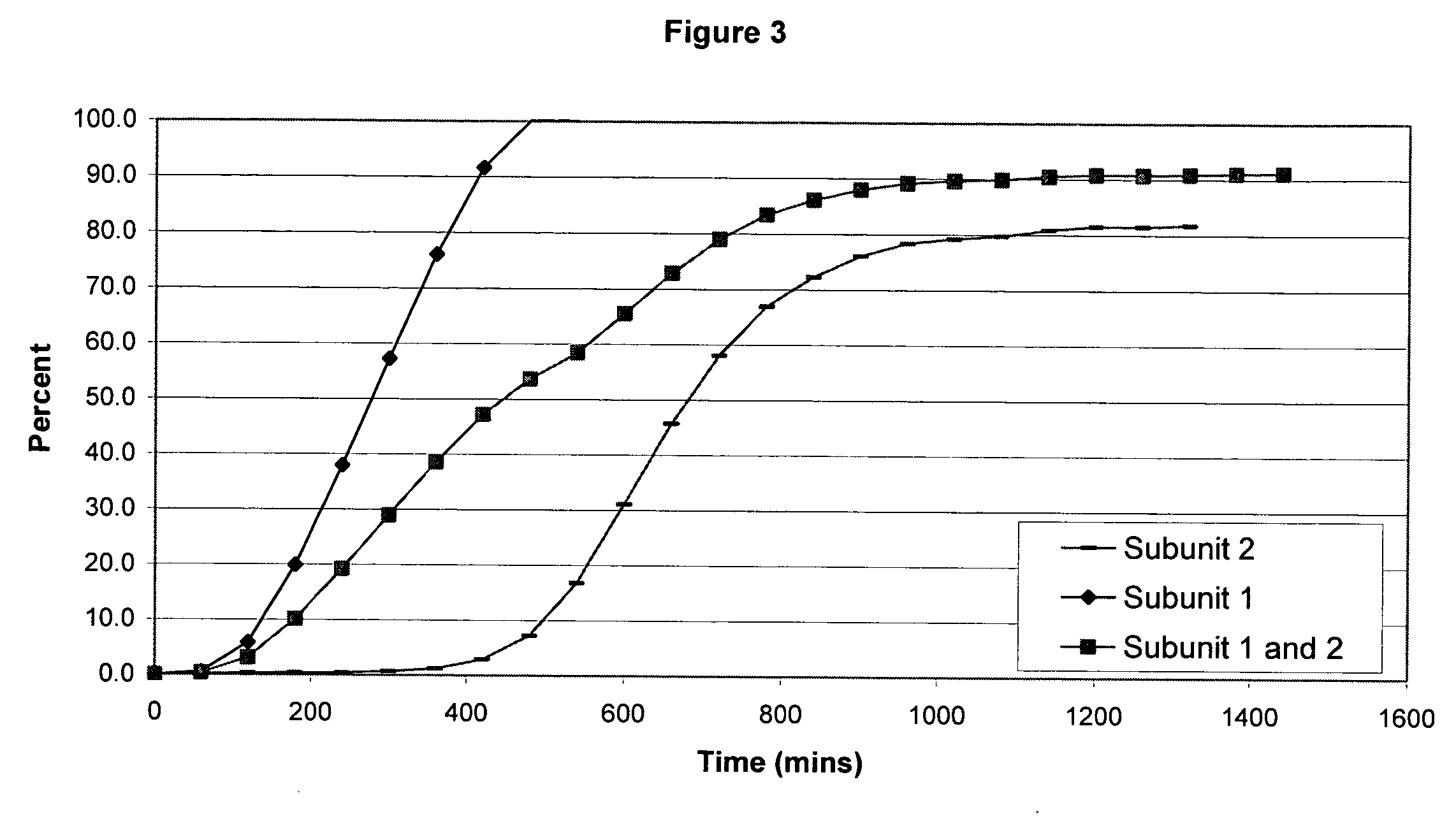
Sustained release opioid formulations and method of use
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
Oral devices and methods for controlled drug release
Drug dosage forms, which are housed in oral devices, and methods for controlled drug release are provided. The oral devices are permanently or removably inserted in the oral cavity and refilled or replaced as needed. The controlled drug release may be passive, based on the dosage form, or electronically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled release may be any one of the following: release in accordance with a preprogrammed schedule, release at a controlled rate, delayed release, pulsatile release, chronotherapeutic release, closed-loop release, responsive to a sensor's input, release on demand from a personal extracorporeal system, release in accordance with a schedule specified by a personal extracorporeal system, release on demand from a monitoring center, via a personal extracorporeal system, and release in accordance with a schedule specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted by an electrotransport mechanism. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen.
Owner:WOLFAF ANDY +1
Pharmaceutical compositions for lipophilic drugs
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Dosage form comprising liquid formulation
InactiveUS6174547B1Improve oral bioavailabilityImprove bioavailabilityCapsule deliveryEmulsion deliveryPharmaceutical formulationDosage form
Owner:ENCINAL PHARMA INVESTMENTS
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myelodysplastic syndromes
InactiveUS20040220144A1Extension of timeBiocidePeptide/protein ingredientsBULK ACTIVE INGREDIENTActive ingredient
Methods of treating, preventing and / or managing myclodysplastic syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient, and / or the transplantation of blood or cells. Specific second active ingredients are capable of affecting or blood cell production. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Omega 3 fatty acid formulations
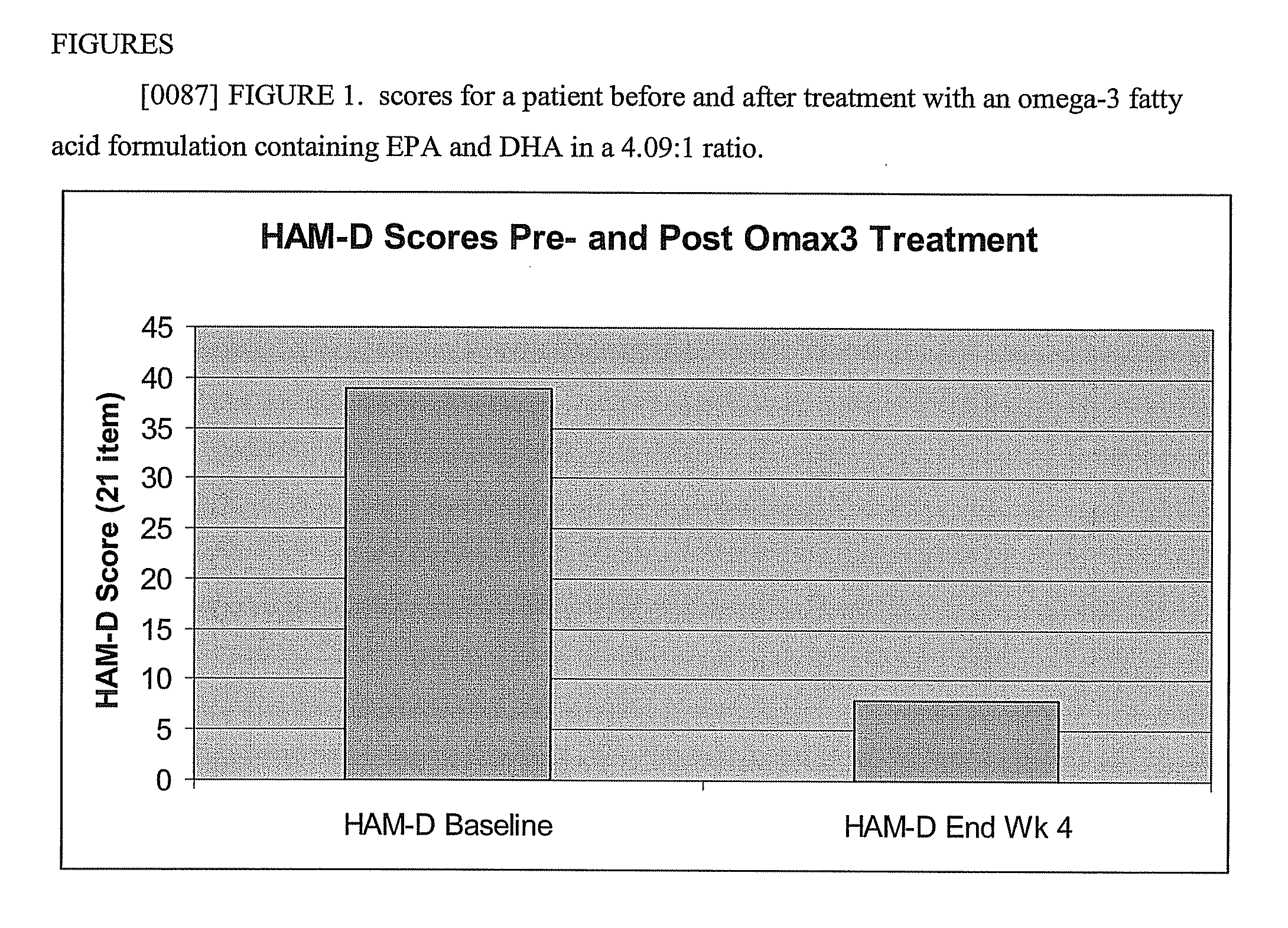
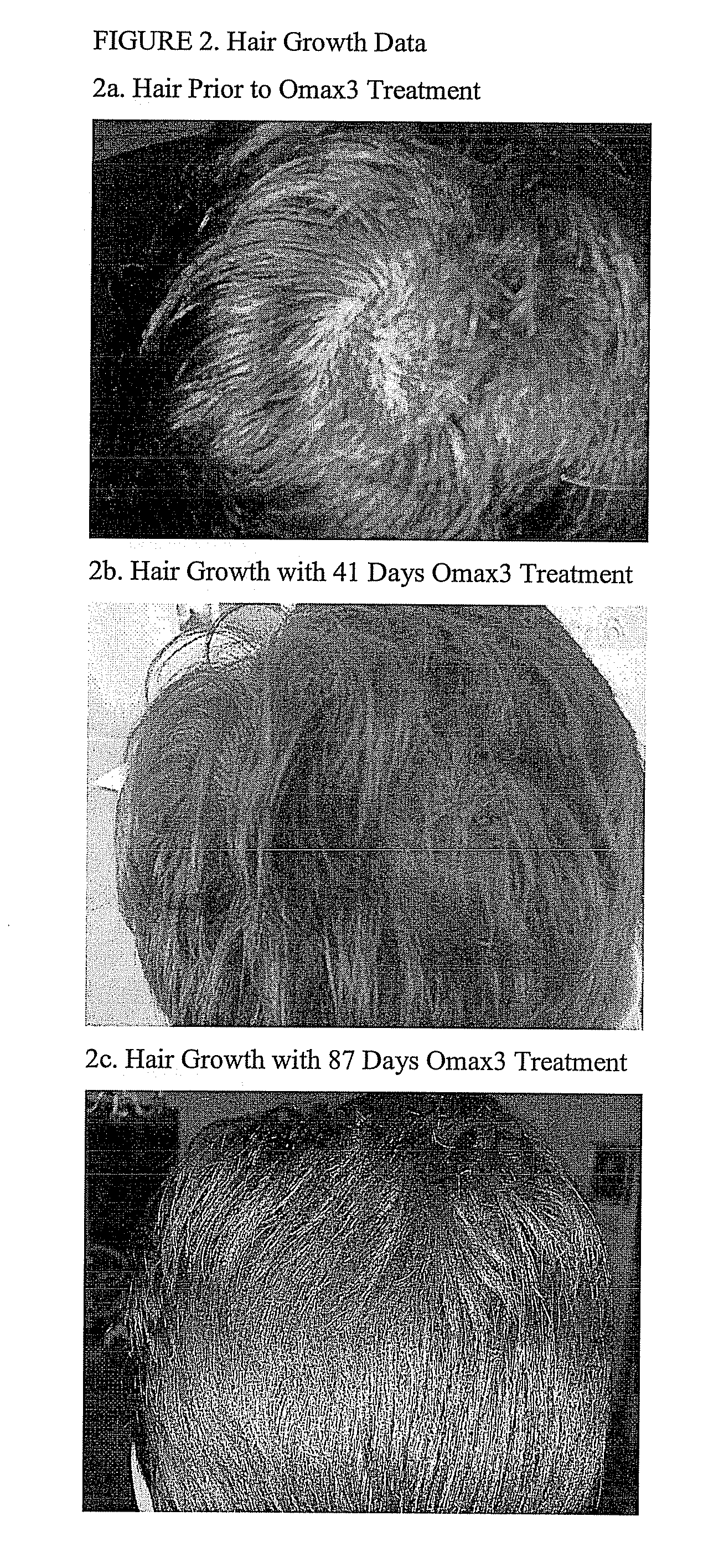
The present invention provides highly purified omega-3 fatty acid formulations. Certain formulations provided herein have contain greater than 85% omega-3 fatty acids by weight. Certain other formulations provided herein contain EPA and DHA in a ratio of from about 4.01:1 to about 5:1. The invention also provides methods of using the dosage forms to treat a variety of cardiovascular, autoimmune, inflammatory, and central nervous system disorders by administering a formulation of the invention to a patient in need thereof.
Owner:CENESTRA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com