Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
234 results about "Blood level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
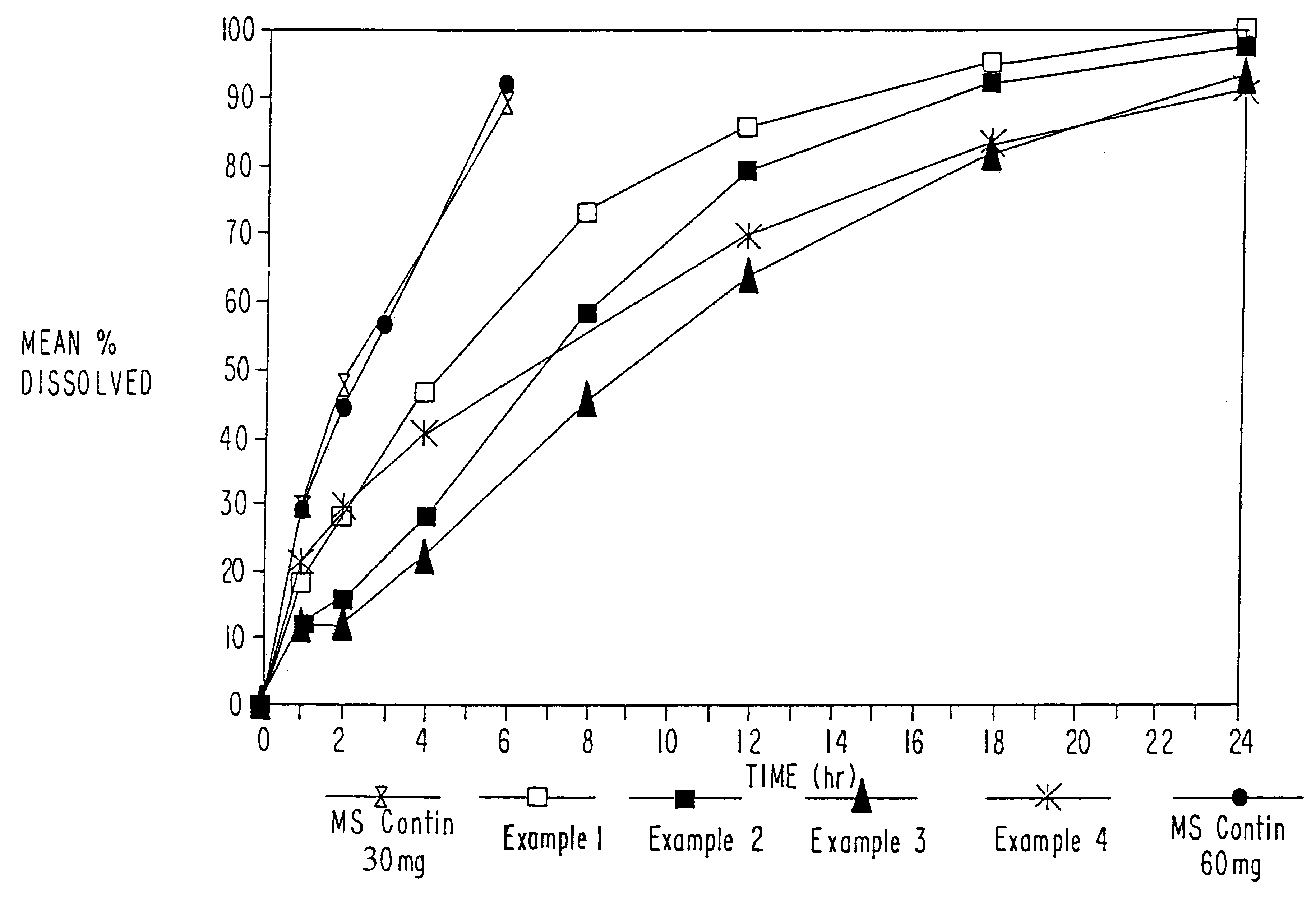
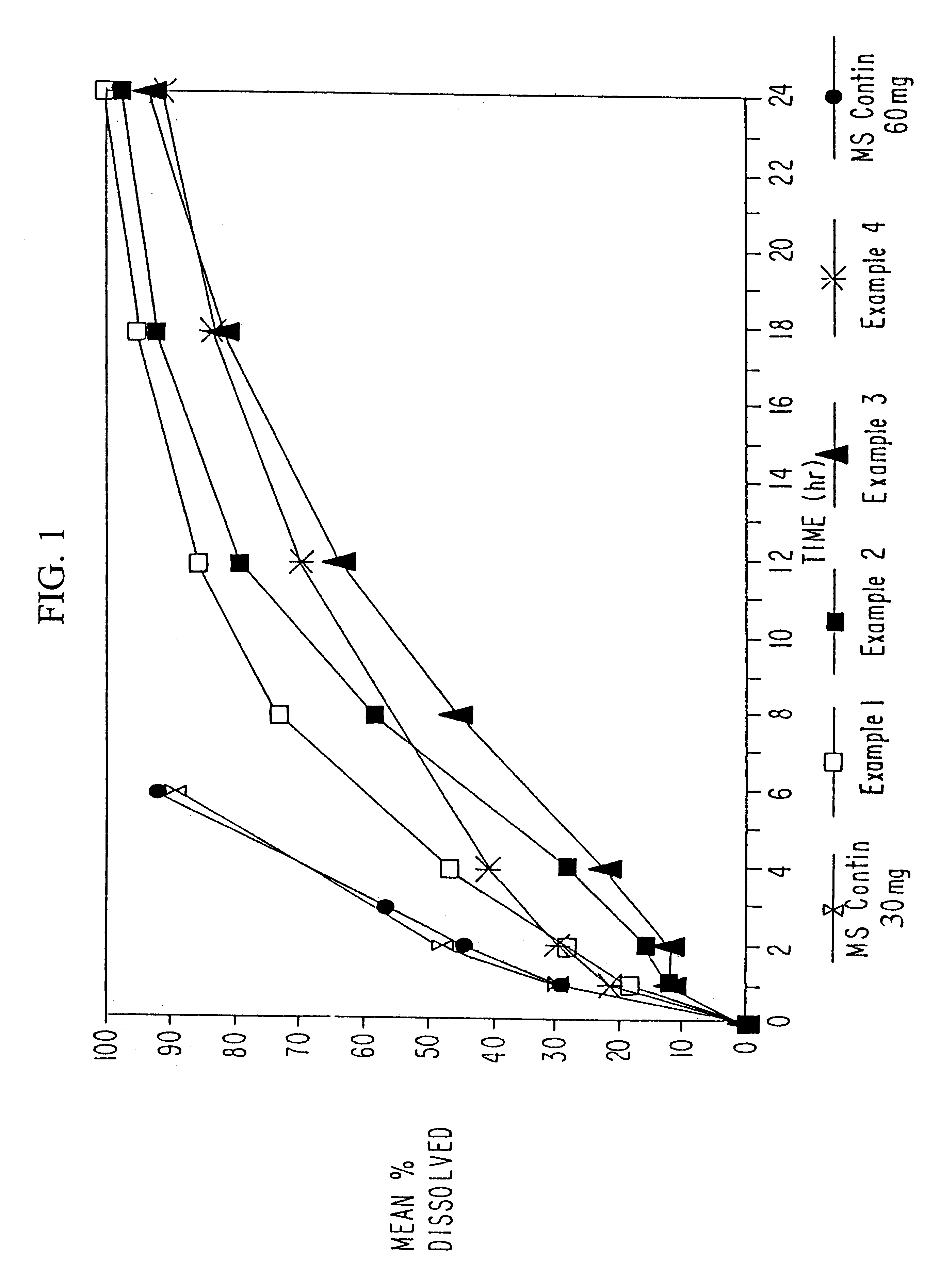
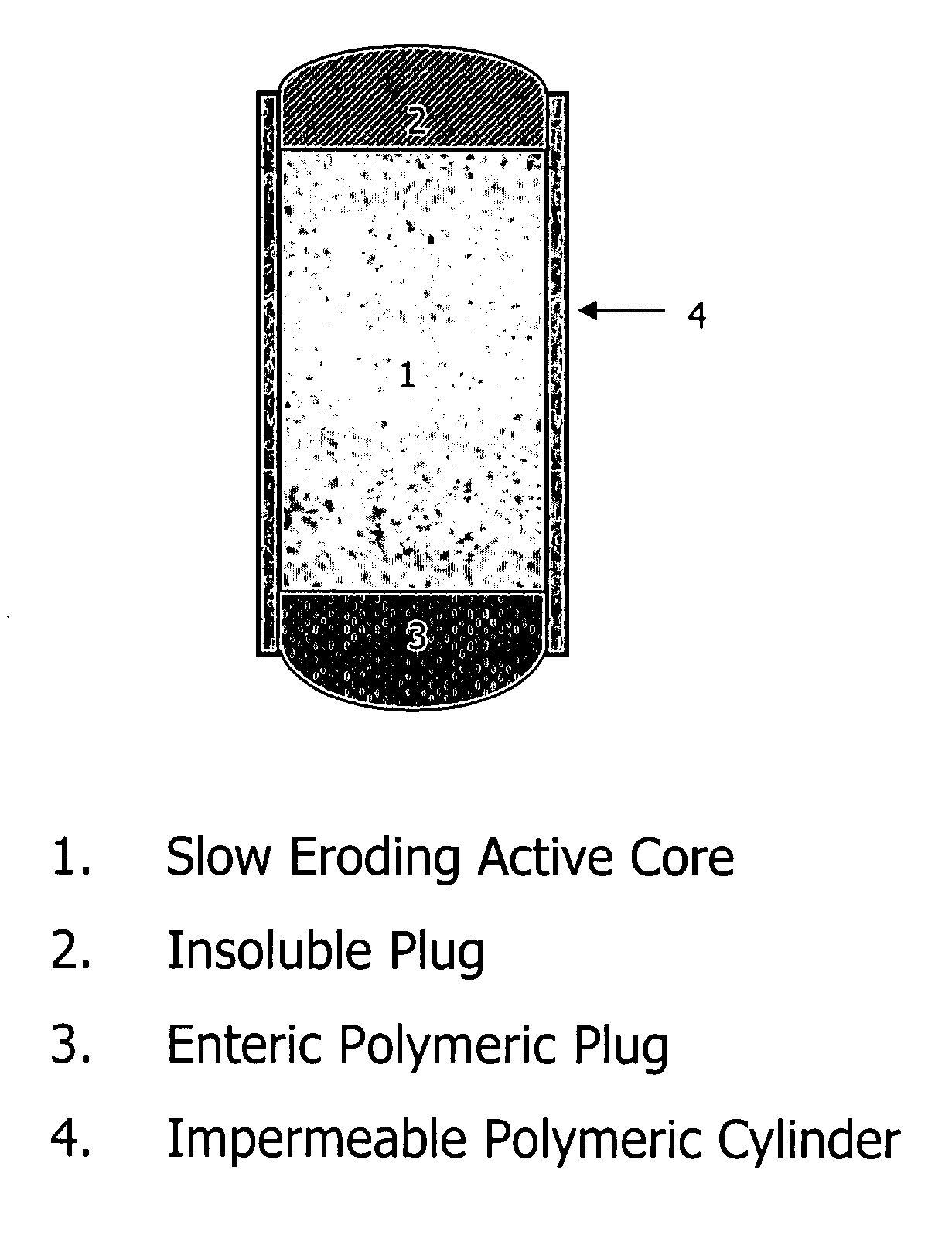
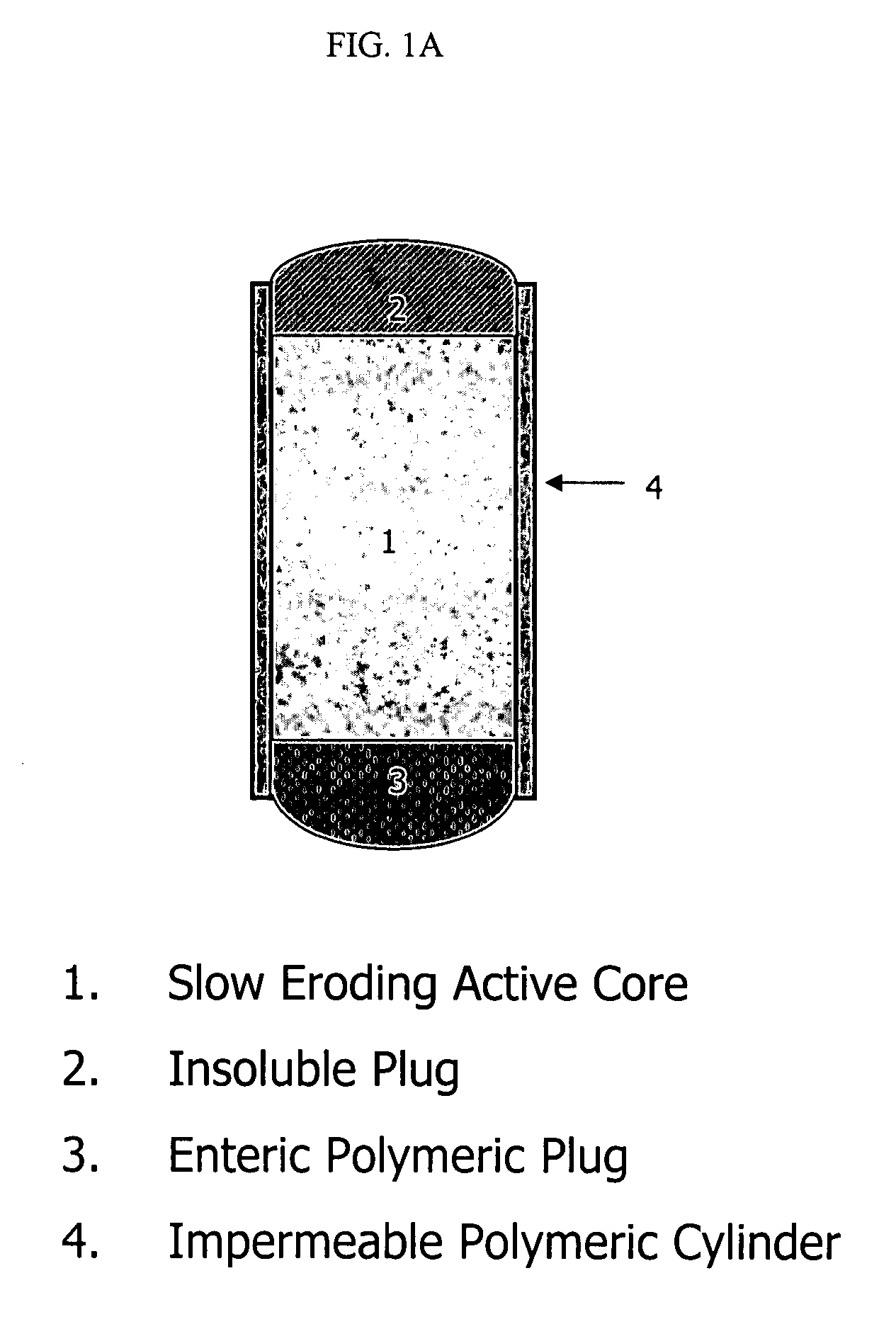
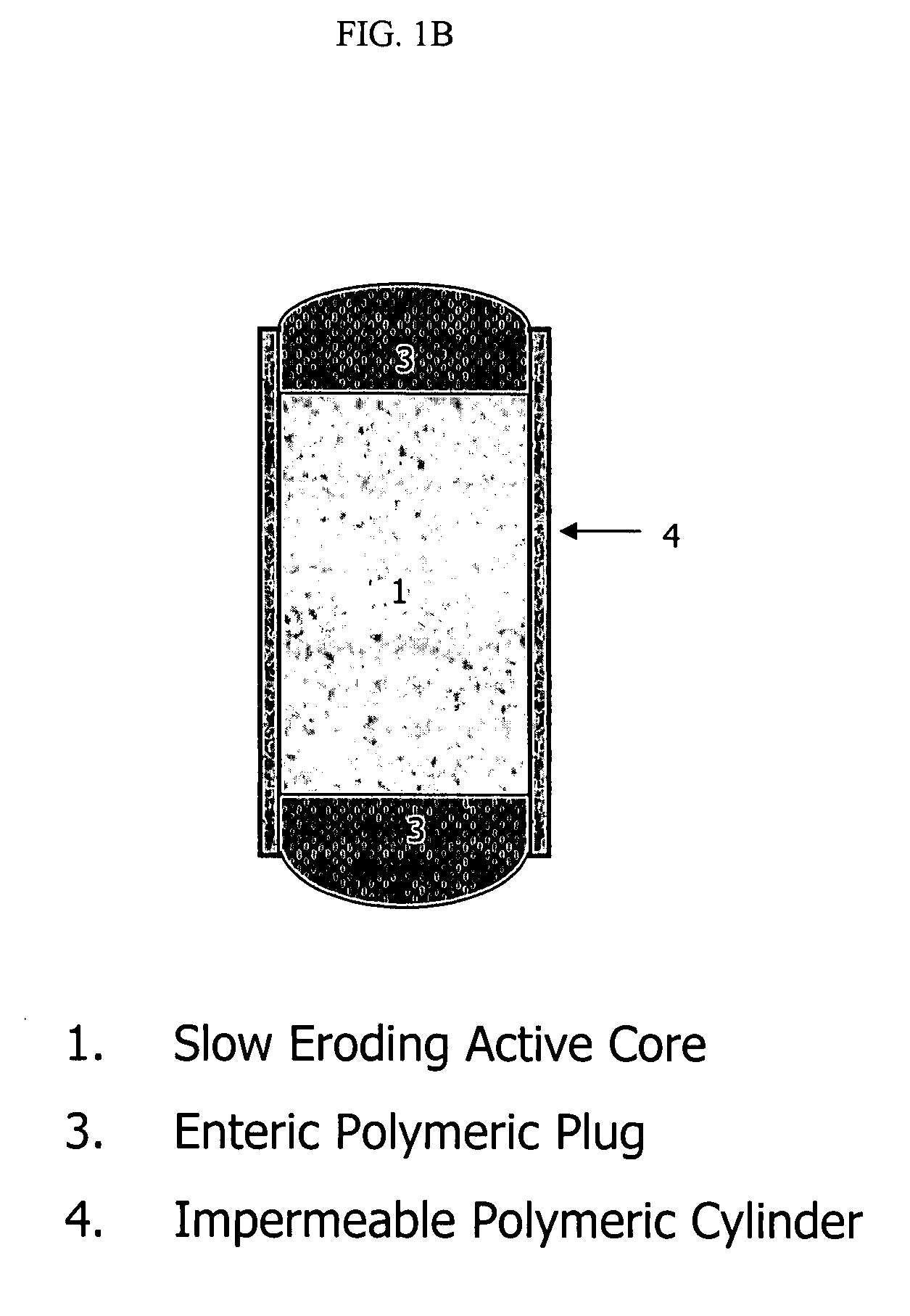
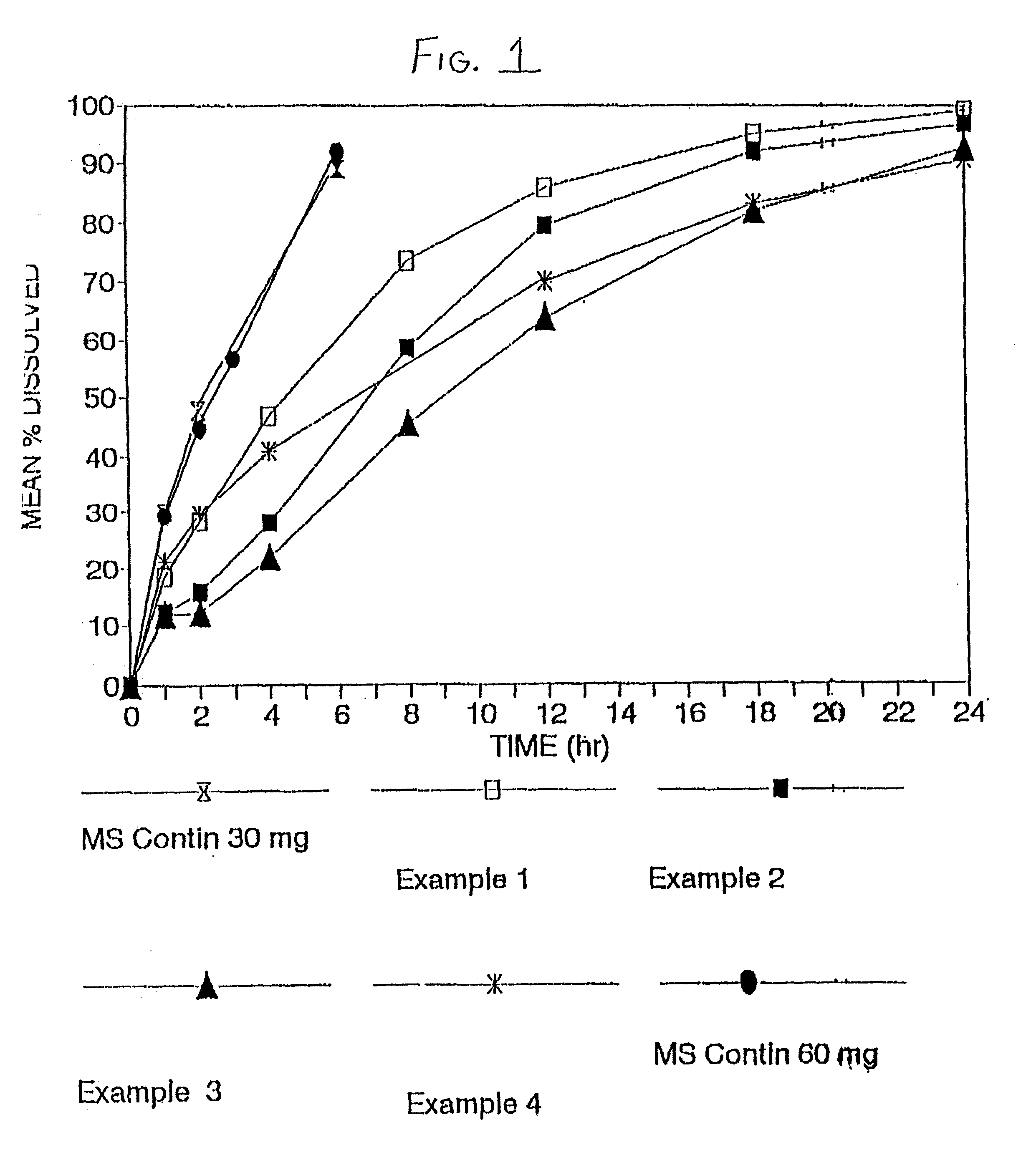
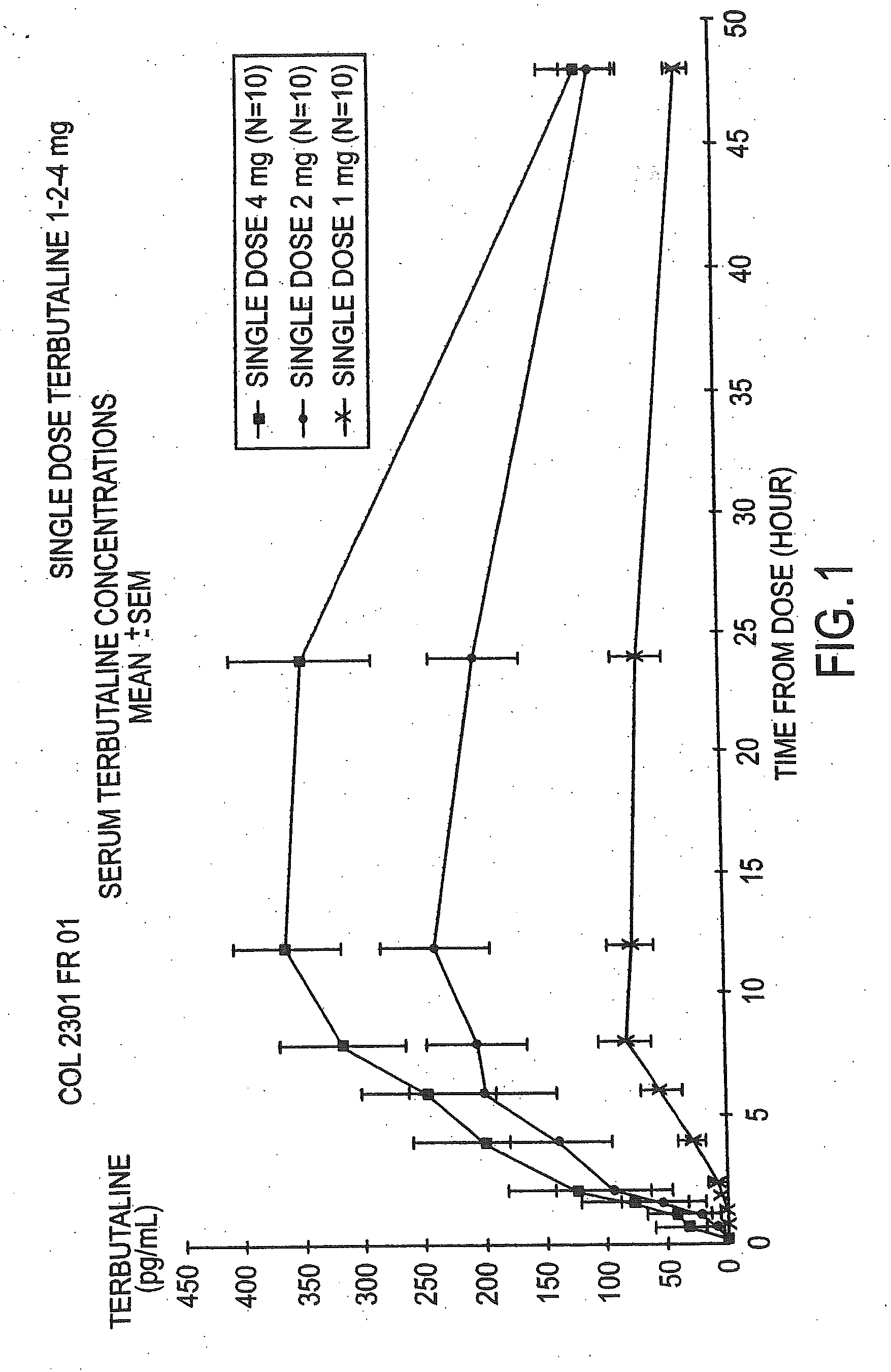
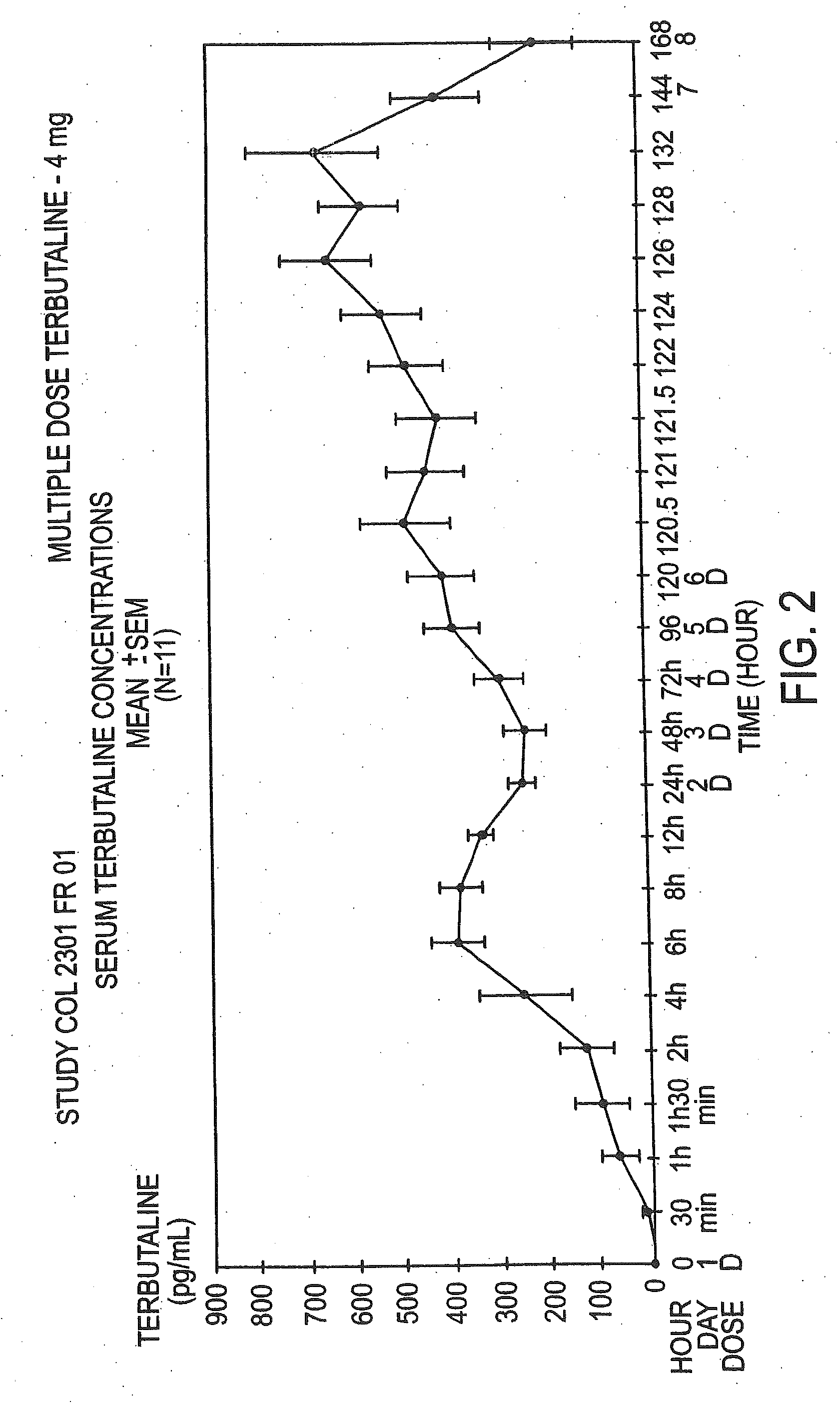
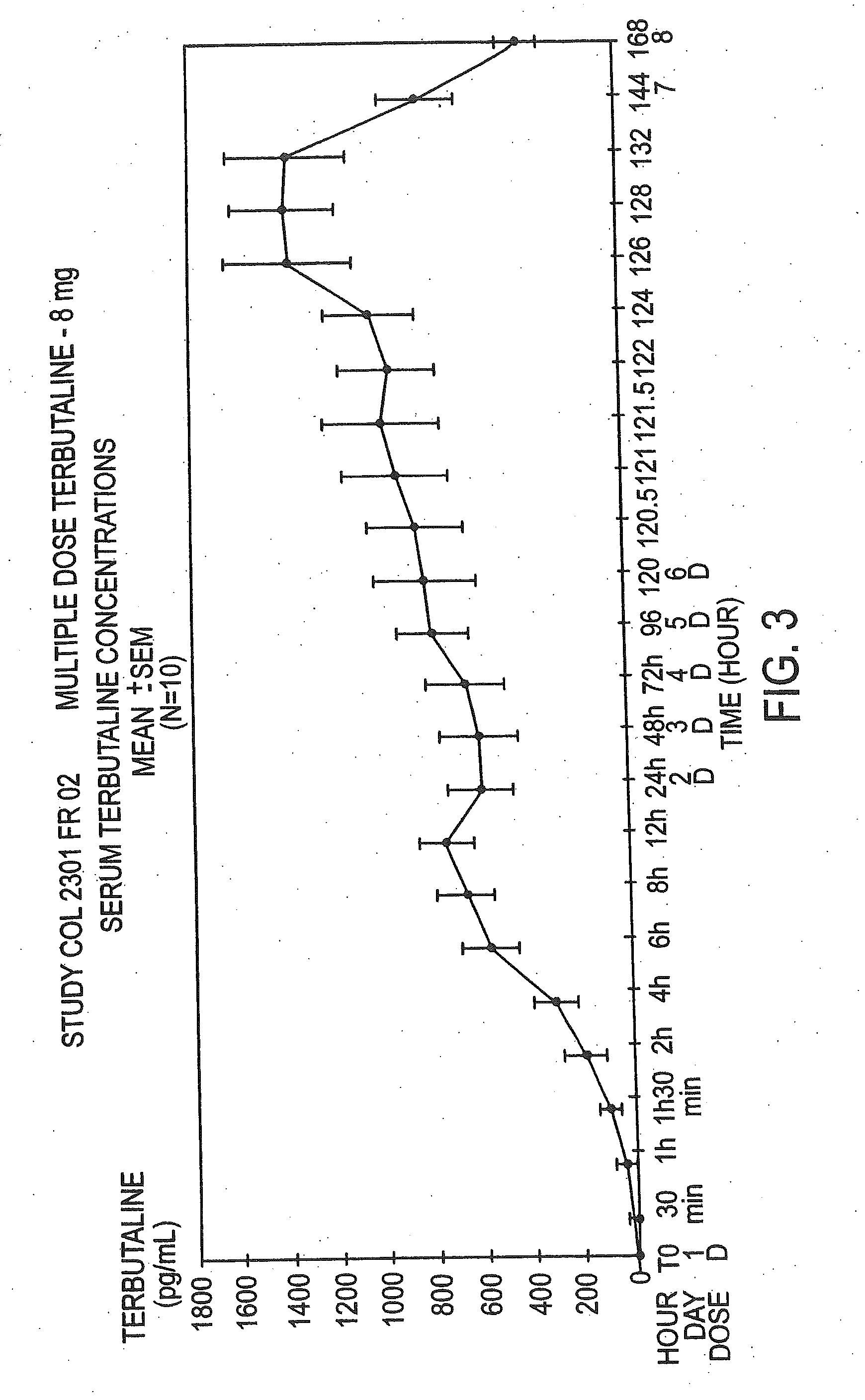
Orally administrable opioid formulations having extended duration of effect
InactiveUS6294195B1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Sustained release oral solid dosage forms of opioid analgesics are provided as multiparticulate systems which are bioavailable and which provide effective blood levels of the opioid analgesic for at least about 24 hours. A unit dose of the opioid analgesic contains a plurality of substrates including the opioid analgesic in sustained release form. The substrates have a diameter from about 0.1 mm to about 3 mm.
Owner:PURDUE PHARMA LP
Method and apparatus for non-invasive measurement of blood analytes
InactiveUS20060063993A1Shorten the timeReduce the amount requiredDiagnostics using spectroscopyRaman scatteringBlood levelAnalyte
The present invention discloses a method and apparatus and method for achieving non-invasive measurement of analytes from human and animal blood through the skin using Raman lightwave technology. The apparatus includes a hydraulic tissue permeation unit, which controls the amount of blood in the laser tissue interaction region. Two or more spectra are obtained at different blood levels. These spectra are used to improve the measurements.
Owner:YU DEJIN +1
Antibody profiling for determination of patient responsiveness
InactiveUS20080026485A1Increase probabilityConvenient careDisease diagnosisDiseaseAutoimmune disease
Compositions and methods are provided for prognostic classification of autoimmune disease patients into subtypes, which subtypes are informative of the patient's need for therapy and responsiveness to a therapy of interest. The patterns of circulating blood levels of serum autoantibodies and / or cytokines provides for a signature pattern that can identify patients likely to benefit from therapeutic intervention as well as discriminate patients that have a high probability of responsiveness to a therapy from those that have a low probability of responsiveness. Additionally, serum autoantibody and / or cytokine signature patterns can be utilized to monitor responses to therapy. Assessment of this signature pattern of autoantibodies and / or cytokines in a patient thus allows improved methods of care. In one embodiment of the invention, the autoimmune disease is rheumatoid arthritis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Assessing blood brain barrier dynamics or identifying or measuring selected substances or toxins in a subject by analyzing Raman spectrum signals of selected regions in the eye
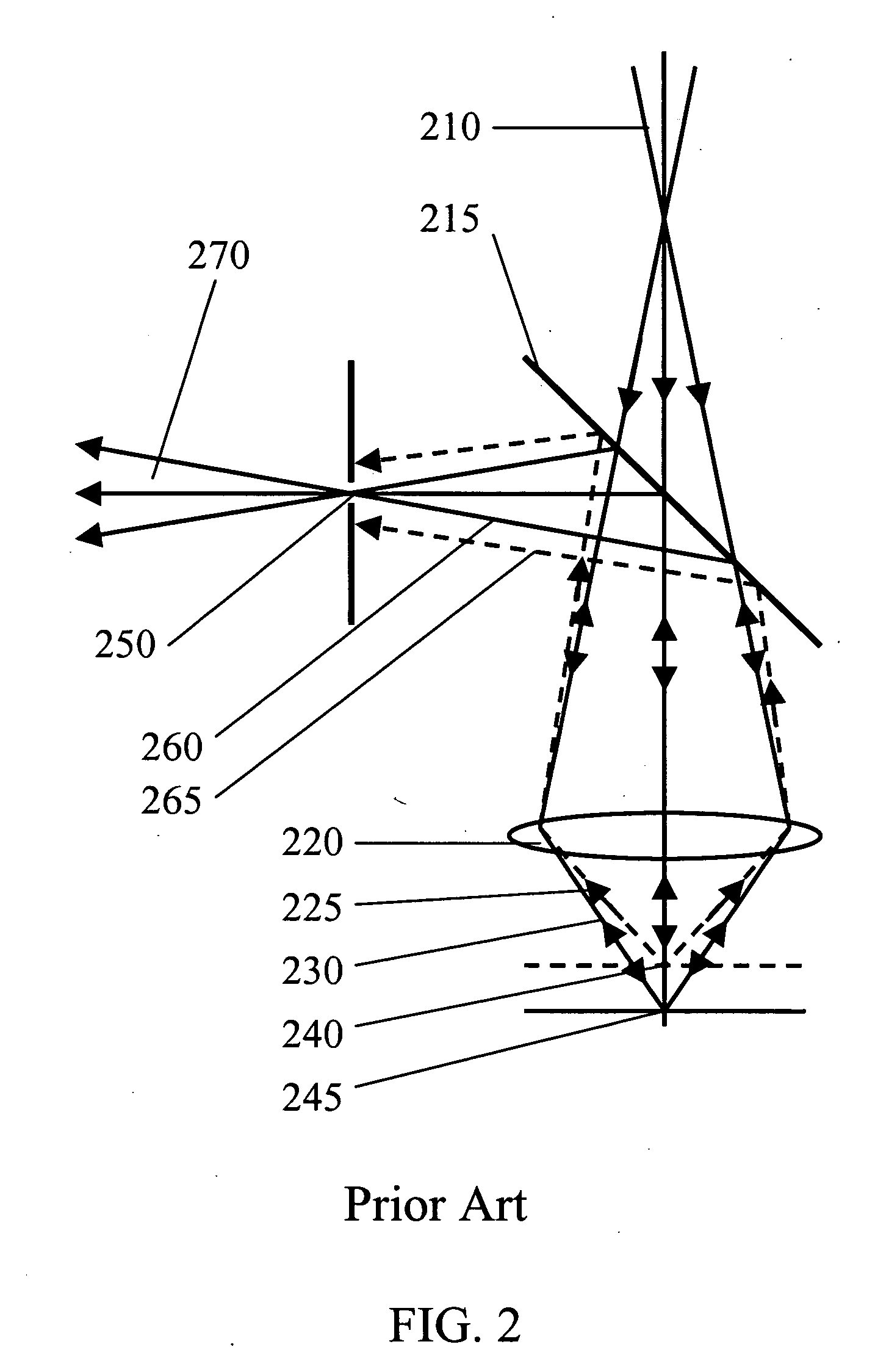
InactiveUS6574501B2Reduced energy/density exposure ratingImproved margin of safetyRaman scatteringDiagnostic recording/measuringConjunctivaNon invasive
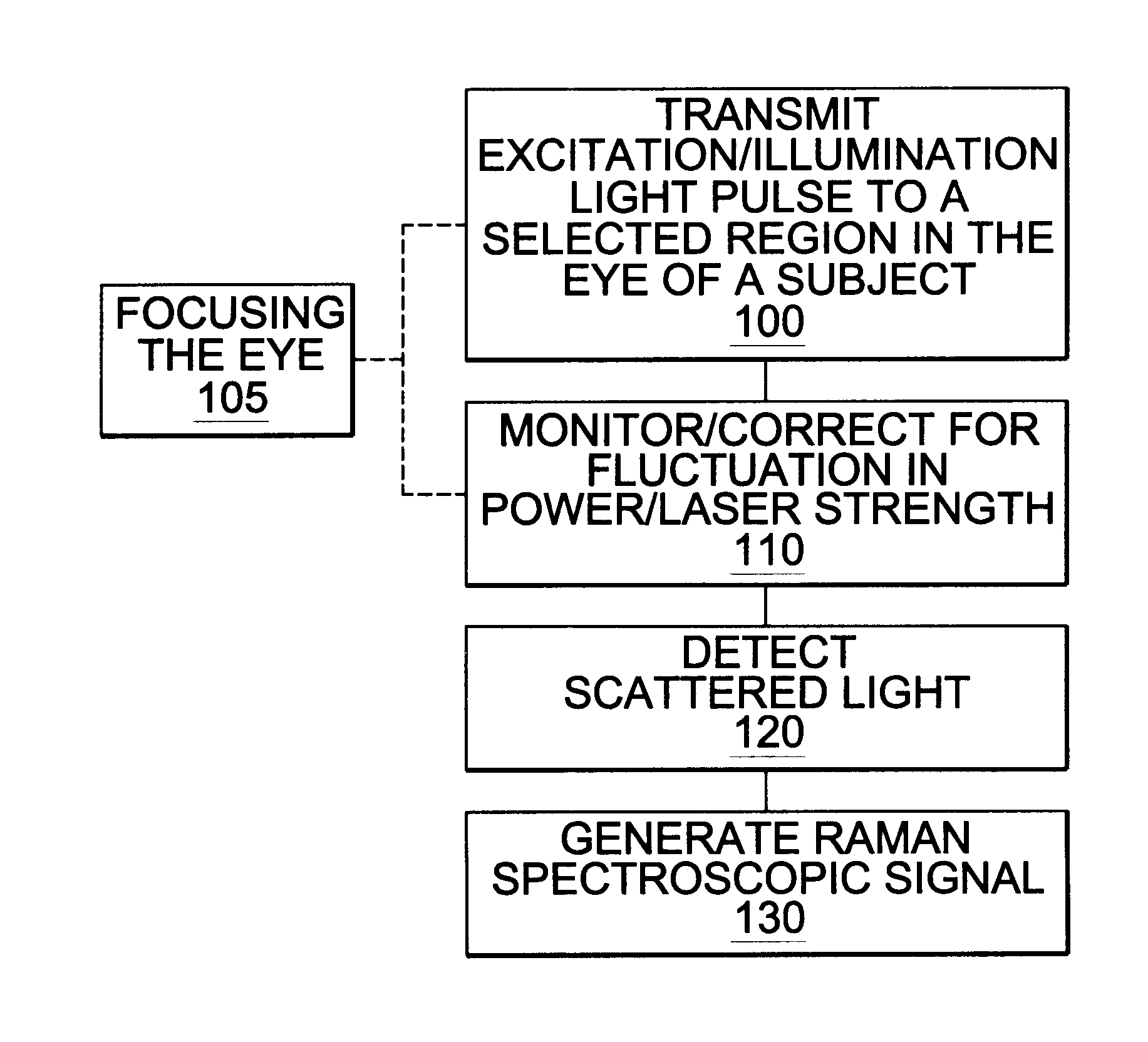
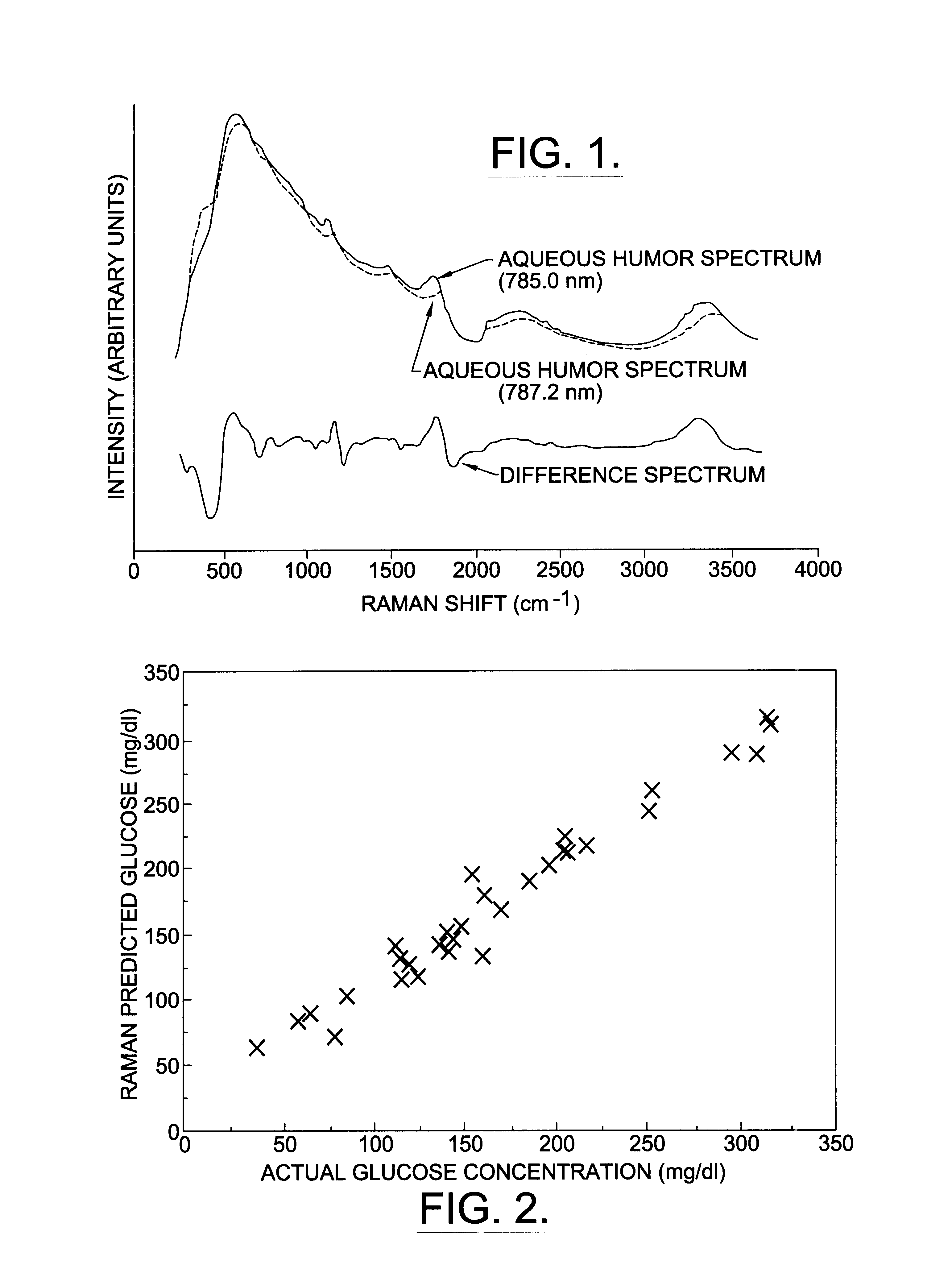
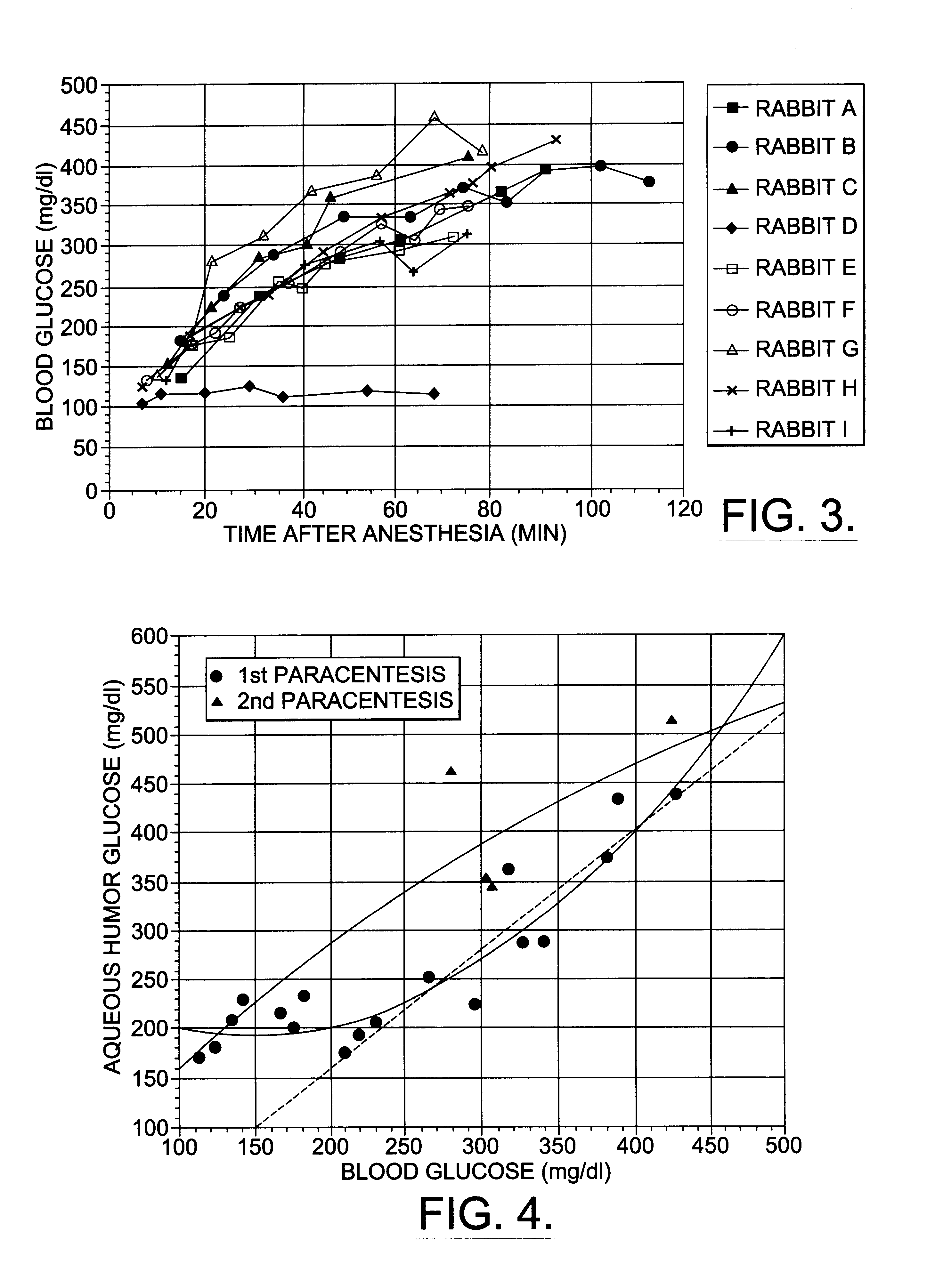
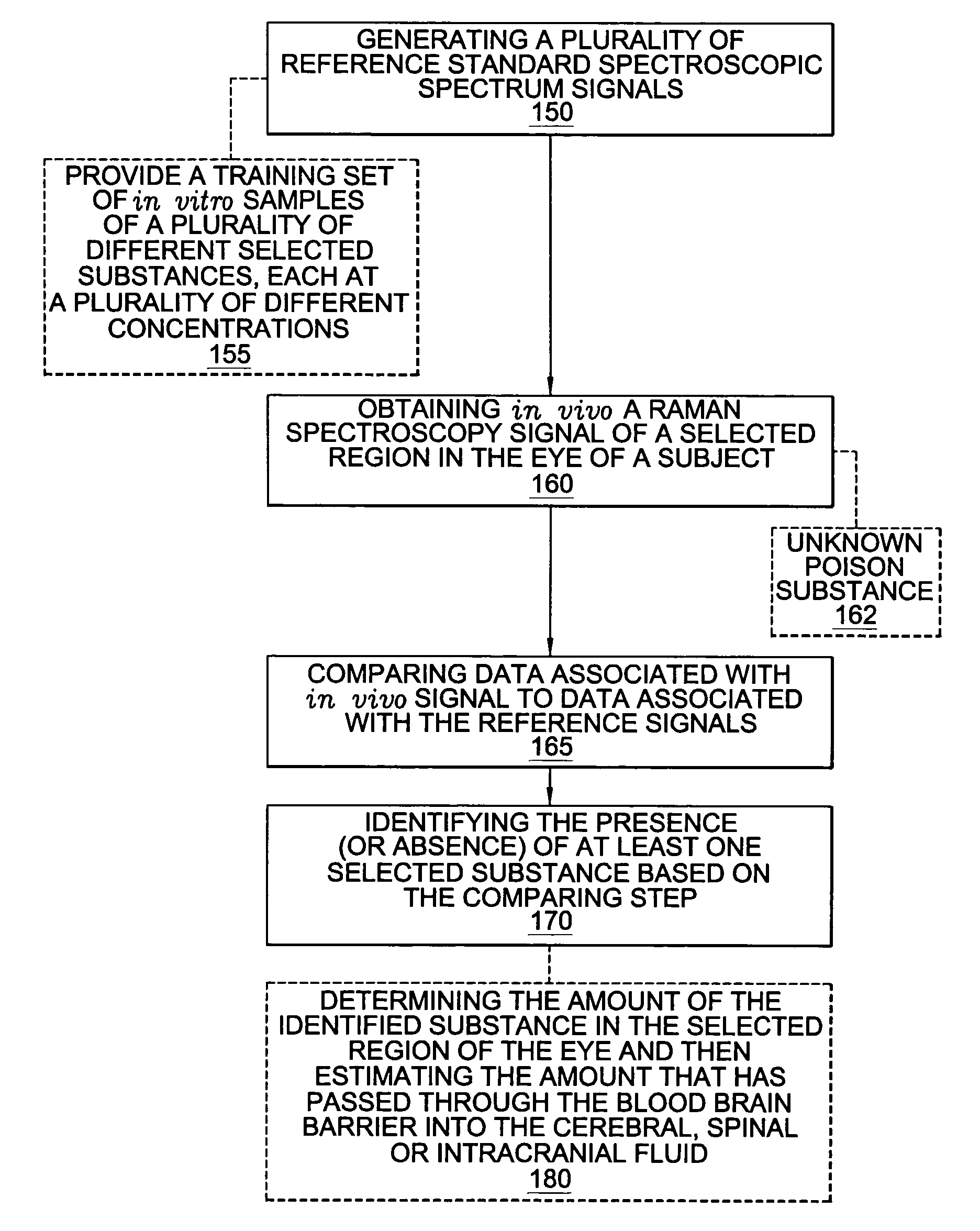
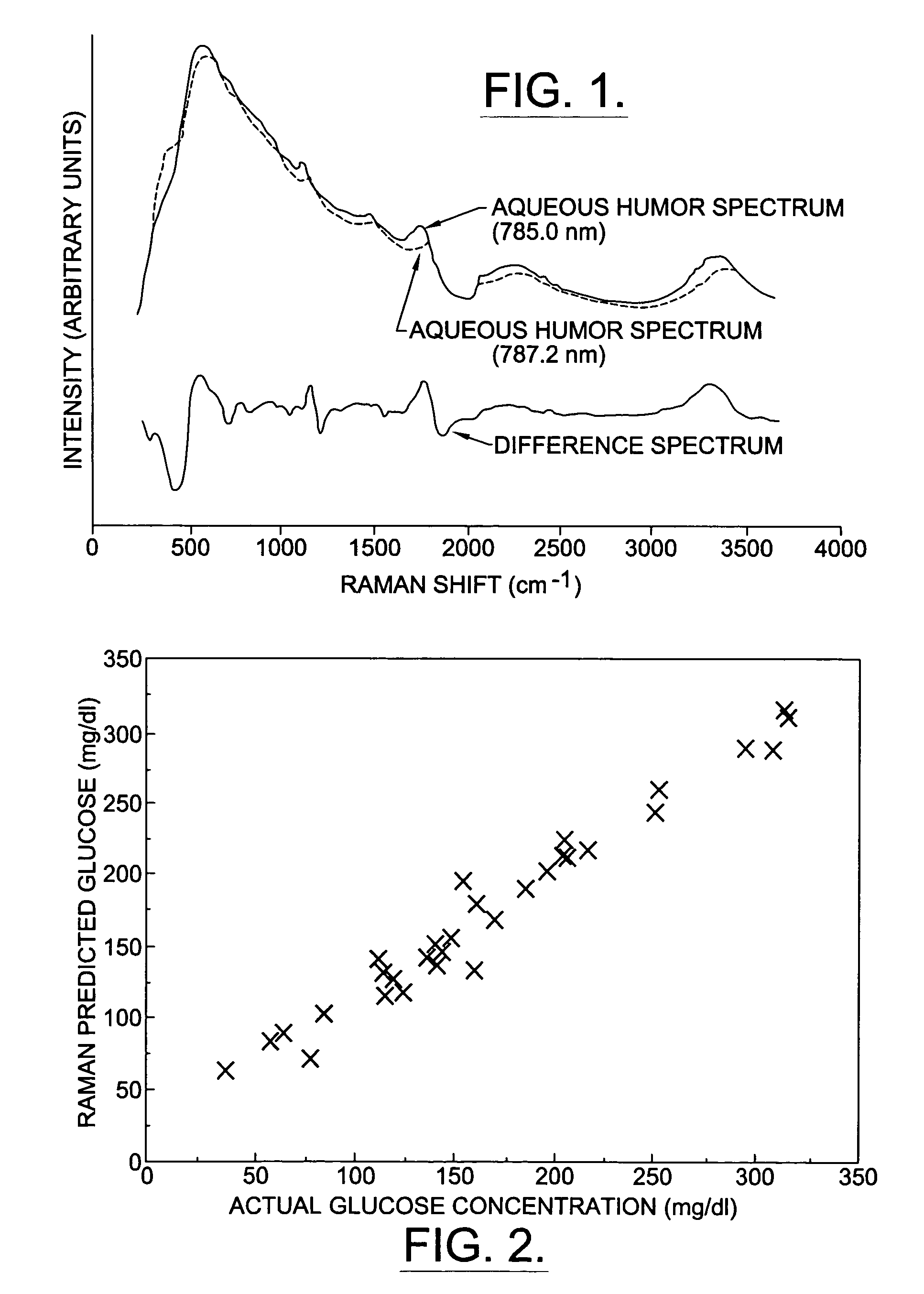
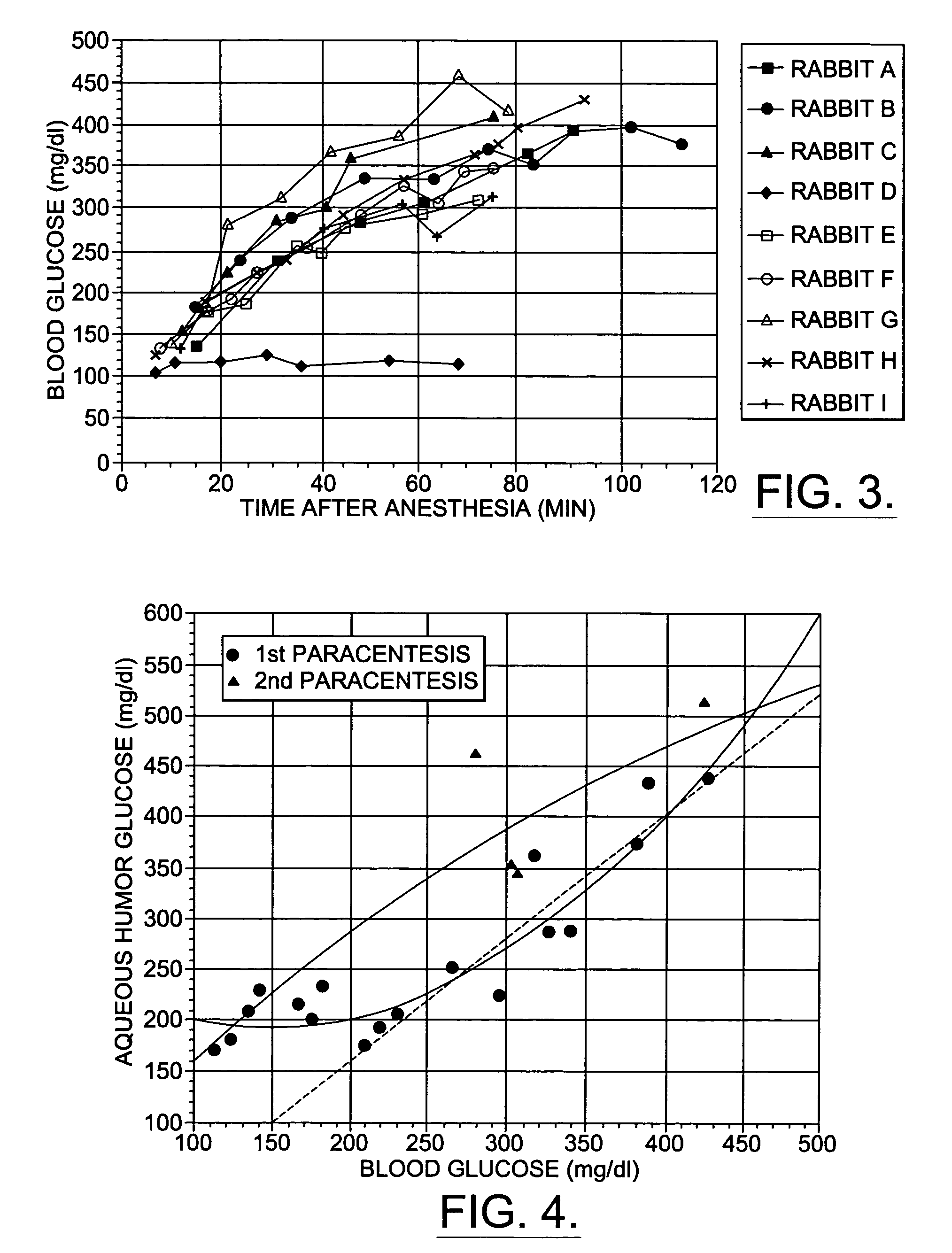
A non-invasive method for analyzing the blood-brain barrier includes obtaining a Raman spectrum of a selected portion of the eye and monitoring the Raman spectrum to ascertain a change to the dynamics of the blood brain barrier. Also, non-invasive methods for determining the brain or blood level of an analyte of interest, such as glucose, drugs, alcohol, poisons, and the like, comprises: generating an excitation laser beam (e.g., at a wavelength of 600 to 900 nanometers); focusing the excitation laser beam into the anterior chamber of an eye of the subject so that aqueous humor, vitreous humor, or one or more conjunctiva vessels in the eye is illuminated; detecting (preferably confocally detecting) a Raman spectrum from the illuminated portion of the eye; and then determining the blood level or brain level (intracranial or cerebral spinal fluid level) of an analyte of interest for the subject from the Raman spectrum. In certain embodiments, the detecting step may be followed by the step of subtracting a confounding fluorescence spectrum from the Raman spectrum to produce a difference spectrum; and determining the blood level and / or brain level of the analyte of interest for the subject from that difference spectrum, preferably using linear or nonlinear multivariate analysis such as partial least squares analysis. Apparatus for carrying out the foregoing methods are also disclosed.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Assessing blood brain barrier dynamics or identifying or measuring selected substances, including ethanol or toxins, in a subject by analyzing Raman spectrum signals
InactiveUS7398119B2Fast “ triage ” assessmentReliable and faster treatment decisionRadiation pyrometrySpectrum investigationNon invasivePhysics
A non-invasive method for analyzing the blood-brain barrier includes obtaining a Raman spectrum of a selected portion of the eye and monitoring the Raman spectrum to ascertain a change to the dynamics of the blood brain barrier.Also, non-invasive methods for determining the brain or blood level of an analyte of interest, such as glucose, drugs, alcohol, poisons, and the like, comprises: generating an excitation laser beam at a selected wavelength (e.g., at a wavelength of about 400 to 900 nanometers); focusing the excitation laser beam into the anterior chamber of an eye of the subject so that aqueous humor, vitreous humor, or one or more conjunctiva vessels in the eye is illuminated; detecting (preferably confocally detecting) a Raman spectrum from the illuminated portion of the eye; and then determining the blood level or brain level (intracranial or cerebral spinal fluid level) of an analyte of interest for the subject from the Raman spectrum. In certain embodiments, the detecting step may be followed by the step of subtracting a confounding fluorescence spectrum from the Raman spectrum to produce a difference spectrum; and determining the blood level and / or brain level of the analyte of interest for the subject from that difference spectrum, preferably using linear or nonlinear multivariate analysis such as partial least squares analysis. Apparatus for carrying out the foregoing methods are also disclosed.
Owner:CALIFORNIA INST OF TECH +1
Topiramate compositions and methods of enhancing its bioavailability
ActiveUS20080085306A1Reduced adverse eventConvenient treatmentBiocideNervous disorderRegimenImmediate release
Owner:SUPERNUS PHARM INC
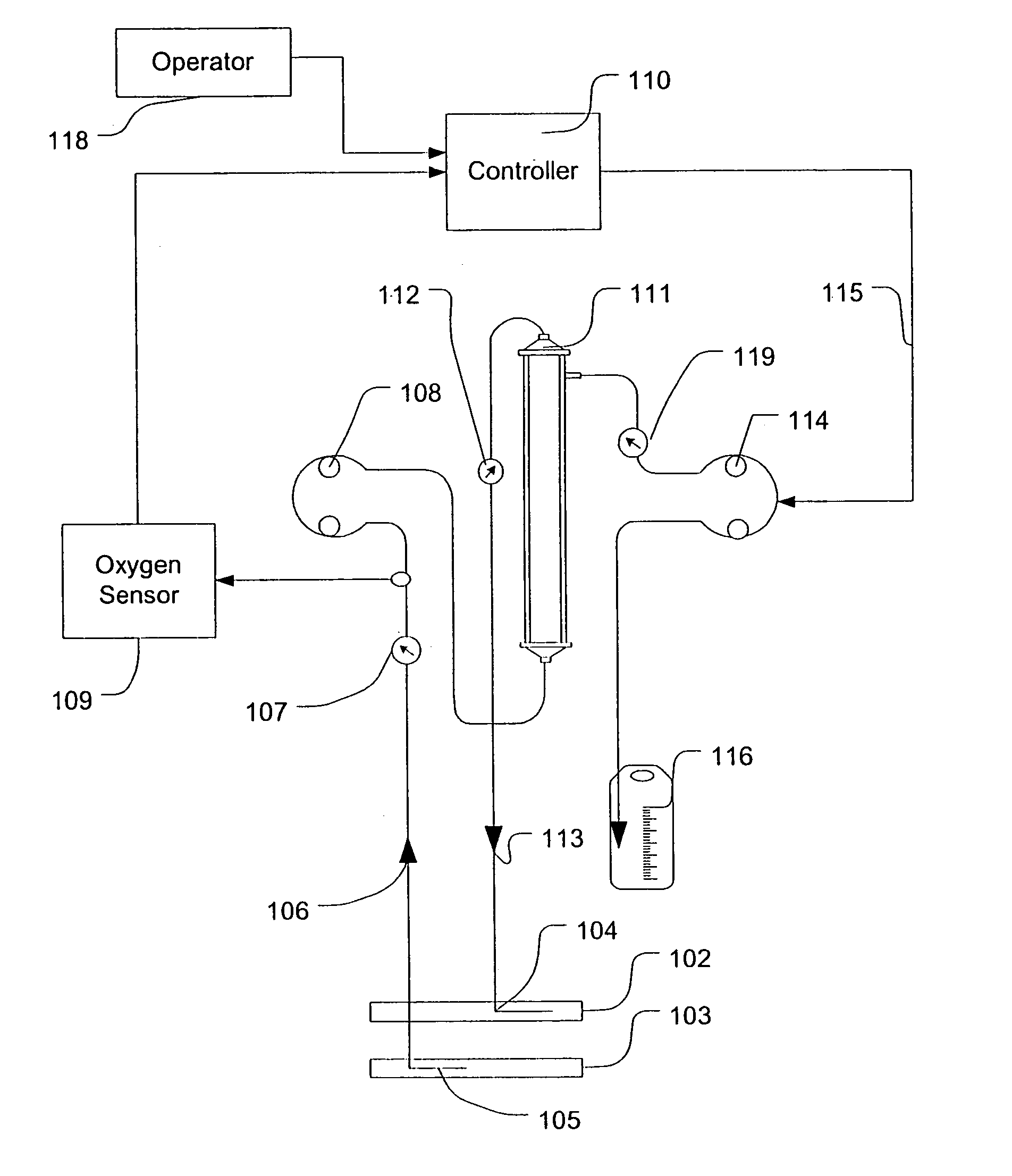
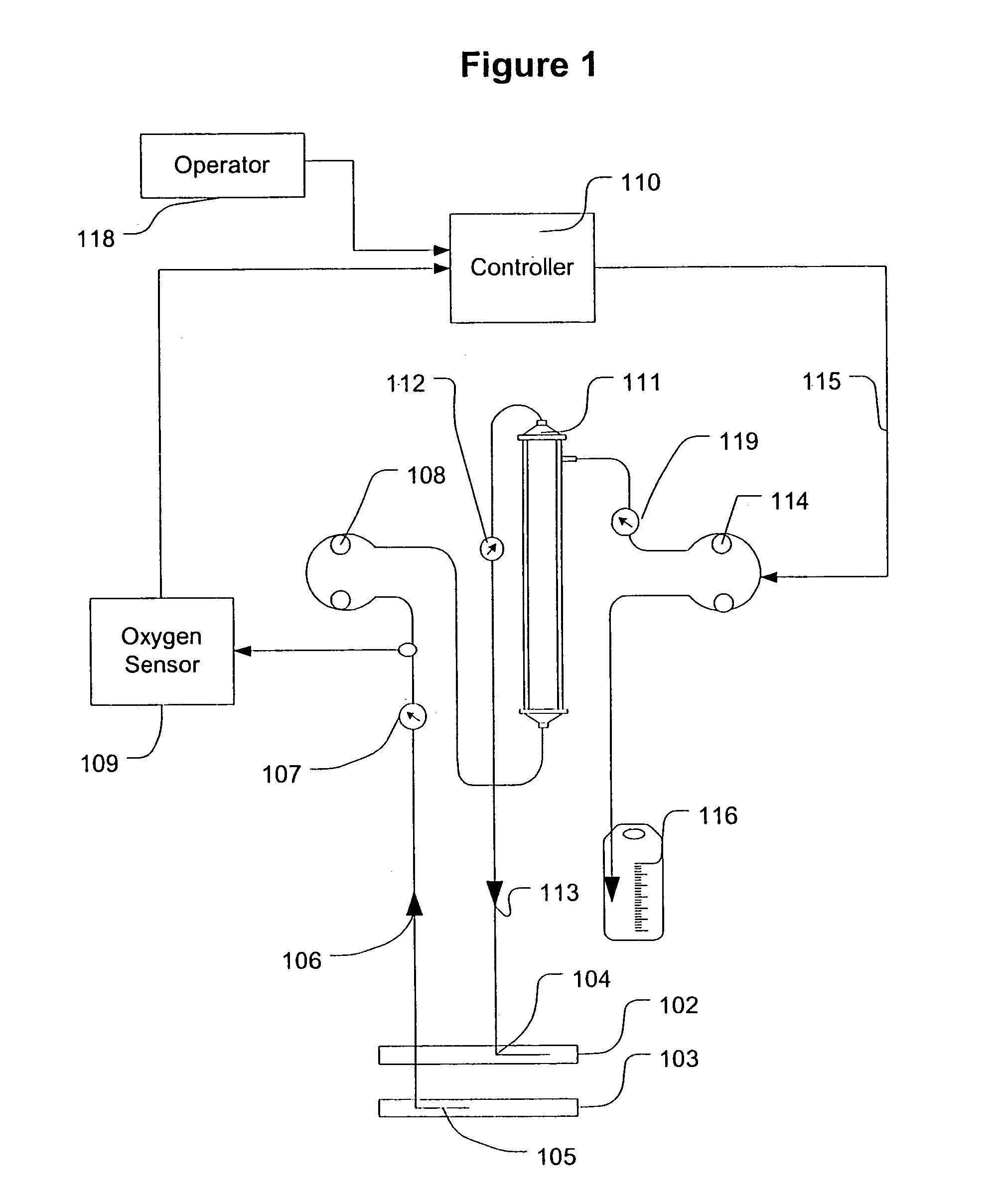
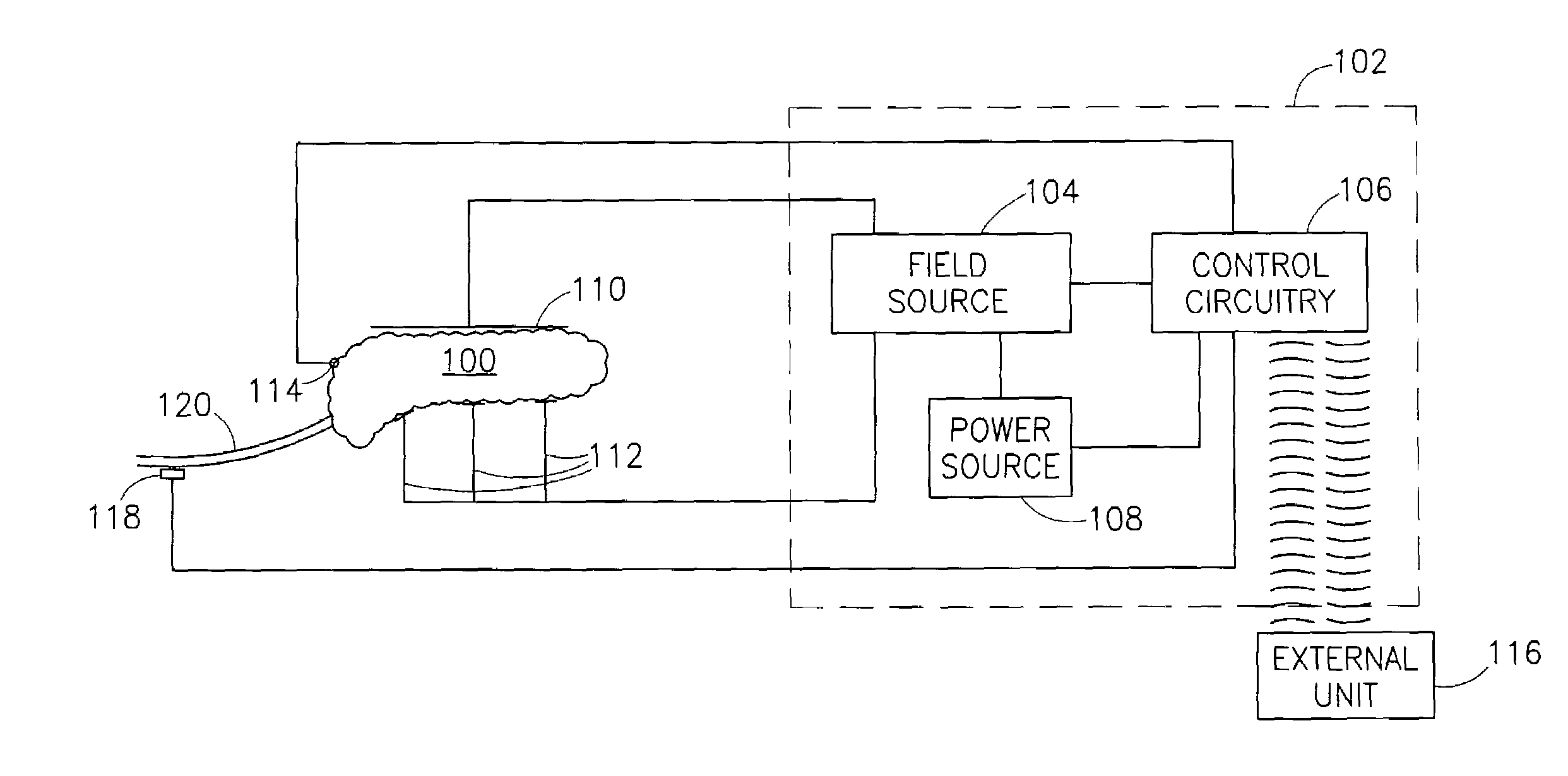
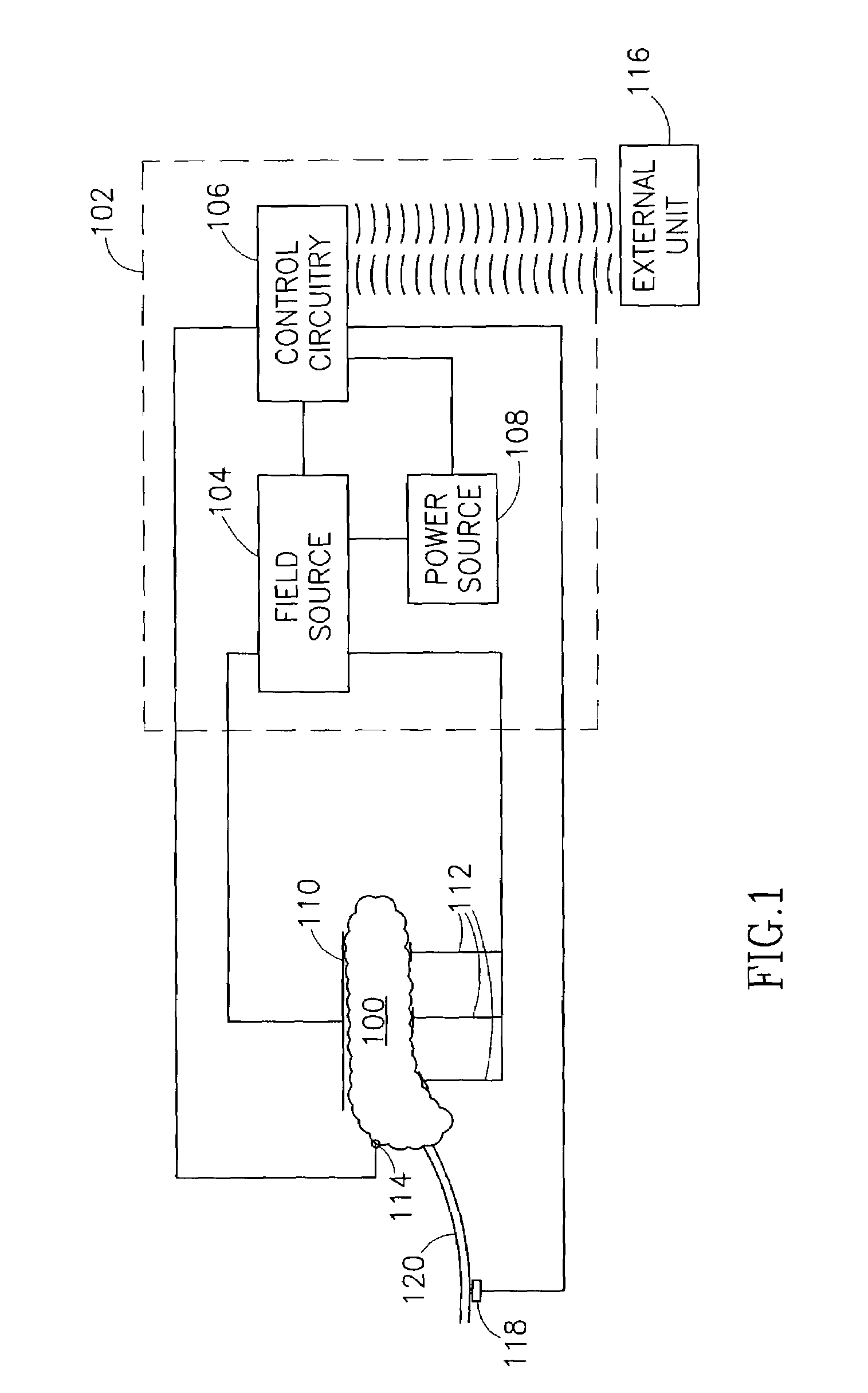
Feedback control of ultrafiltration to prevent hypotension
InactiveUS7175809B2Easy to adaptAvoid hypotensionSemi-permeable membranesOther blood circulation devicesBlood levelVein
A method and system for the extracorporeal treatment of blood to remove fluid from the fluid overloaded patient is disclosed that non-invasively measures an oxygen level in the venous blood. The oxygen blood level is used to detect when hypotension is about to occur in a patient. The oxygen level measurements are used as feedback signals. These feedback signals are applied to automatically control the rate of fluid extraction to achieve the desired clinical outcome and avoid precipitating a hypotensive crisis in the patient.
Owner:GAMBRO LUNDIA AB
Immune enhancing compositions and methods of use thereof
InactiveUS20050271726A1Easy to synthesizeEffective absorptionPowder deliveryOrganic active ingredientsGlycineBlood level
A method of administering parenterally, particularly intramuscularly, glutamine and cystine and glycine plus selenium; or lactalbumin plus selenium; or lactalbumin and glutamine and cystine and glycine plus selenium, through a long-acting pharmaceutically acceptable carrier to a patient. The method comprises injecting a mixture of glutamine, cystine, glycine, lactalbumin and selenium in order to maintain the mixture systemically or locally for a sufficient time period so as to maintain blood levels of glutathione within an improved therapeutic range.
Owner:CRUM ALBERT
Blood glucose level control
InactiveUS8019421B2Reduced effectivenessOverworking pancreasInternal electrodesSurgical instrument detailsBlood levelBlood insulin
A pancreatic controller, comprising:at least one electrode adapted for electrifying at least a portion of a pancreas; anda controller programmed to electrify said electrode so as to positively control at least the effect of at least two members of a group consisting of blood glucose level, blood insulin level and blood level of another pancreatic hormone. In one example, the controller controls insulin, glucagon and / or glucose blood levels.
Owner:TYLERTON INT INC
Controlled release compositions of estradiol metabolites
The present invention provides improved sustained release formulations of estradiol metabolites, including 2-hydroxyestradiol, 2-methoxyestradiol, 4-hydroxyestradiol and 4-methoxyestradiol, useful for therapeutic treatments. The invention also provides methods of producing sustained release forms of estradiol metabolites. The compositions of the present invention include microparticles, nanoparticles, patches, crystals, gels, rods, stints, pallets, discs, lozenges, wafers, capsules, films, microcapsules nanocapsules, hydrogels, liposomes, implants and vaginal rings. Compositions also include formulations for transdermal and intravenous delivery of estradiol metabolites. The present invention provides numerous improvements over previous forms of estradiol metabolites, such advantages including the sustained release of normally short half-life compounds to maintain therapeutic blood levels.
Owner:PR PHARMA
Dietary regimen of nutritional supplements for relief of symptoms of arthritis
InactiveUS6136795AReduce inflammationPermits healingBiocideAcidic food ingredientsDocosahexaenoic acidRegimen
This invention is directed to a dietary regimen and a unique combination of nutritional supplements and a method. More specifically, this invention is directed to a unique combination of nutritional supplements which provides symptomatic relief from arthritis. The unique combination of nutritional supplements of this invention is believed to function by both increasing the available (effective blood level) of anti-inflammatory agents and promotion of the healing / regenerative process in the effected joints, thus, producing unexpected and lasting symptomatic relief from the debilitating effects of both osteoarthritis and rheumatoid arthritis. The essential nutritional supplements of the dietary regimen of this invention are as follows: (a) gamma linolenic acid (unrefined), hereinafter "GLA"(b) a mixture of eicosapentaenoic acid and docosahexaneoic acid, hereinafter collectively "EPA"(c) a mixture of chondroitin sulfate, N-acetyl glucosamine sulfate, glucosamine sulfate and manganese aspartate, hereinafter collectively "CHONDROX"The regimen is adjusted based upon the weight of the individual, and once symptomatic relief is achieved, the individual remains essentially free from the debilitating effects of arthritis so as long the daily regimen is faithfully followed.
Owner:IRWIN NATURALS 4HEALTH INC +1
N-acetylcysteine compositions and methods for the treatment and prevention of cysteine/glutathione deficiency in diseases and conditions
InactiveUS20050070607A1Low toxicityAllow administrationBiocideOrganic active ingredientsCysteine thiolateClinical settings
Life-threatening hepatotoxicity in the setting of acetaminophen overdose is due to depletion of glutathione (GSH), a vital cysteine-containing tripeptide that protects cells and organs against oxidant injury. Rapid administration of N-acetylcysteine (NAC), which provides the cysteine necessary to replenish the depleted GSH, is the standard of care for preventing injury in acetaminophen overdose. Beneficial effects of NAC treatment have also been demonstrated in respiratory, cardiovascular, endocrine and infectious and other diseases. In fact, over fifty randomized placebo-controlled trials conducted in diverse clinical settings document positive responses to NAC treatment. The present invention relates to cysteine / glutathione (GSH) deficiency as a previously unrecognized clinical entity that can complicate the course of commonly encountered diseases and methods of treatment of this generalized deficiency involving administering N-acetylcysteine (NAC) or a pharmaceutically acceptable salt or derivative to a subject in need thereof and monitoring the subjects appropriate glutathione blood levels as needed.
Owner:ANDRUS JAMES +4
Orally administrable opioid formulations having extended duration of effect
InactiveUS20020081333A1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Owner:PURDUE PHARMA LP
Dosing regimen
Methods and kits are provided enabling a twice daily dosing regimen that achieves daily patient blood levels of active pharmaceutical ingredient comparable to a dosing regimen requiring the same active ingredient to be administered three times a day.
Owner:ELAN PHRMA INT LTD
Method and apparatus for non-invasive measurement of blood analytes
InactiveUS20060063992A1Shorten the timeReduce the amount requiredRaman scatteringDiagnostic recording/measuringBlood levelAnalyte
The present invention discloses a method and apparatus and method for achieving non-invasive measurement of analytes from human and animal blood through the skin using Raman lightwave technology. The apparatus includes a hydraulic tissue permeation unit, which controls the amount of blood in the laser tissue interaction region. Two or more spectra are obtained at different blood levels. These spectra are used to improve the measurements.
Owner:YU DEJIN +1
Cardiopulmonary bypass extracorporeal blood circuit apparatus and method
InactiveUS20050118059A1Implement extensionsSignificant improvementOther blood circulation devicesHaemofiltrationBlood levelVein
An extracorporeal blood circuit for use with a venous return line and an arterial line coupled to a patient. The extracorporeal blood circuit can include a venous air removal device coupled to the venous return line. The venous air removal device can perform an active air removal function. The extracorporeal blood circuit can include a sensor that determines a blood level in the venous air removal device, a purge line coupled to the venous air removal device, and a controller connected to the sensor. The controller can cause the venous air removal device to perform the active air removal function through the purge line when the blood level is less than a threshold. The extracorporeal blood circuit can further include a pump coupled to the venous air removal device, an oxygenator coupled to the pump, and a blood filter coupled to the oxygenator and the arterial line.
Owner:MEDTRONIC INC
Localized vaginal delivery without detrimental blood levels
The invention relates to a pharmaceutical composition for vaginal administration of a treating agent normally associated with undesired side effects at detrimental blood levels. The composition releases the treating agent at a rate to achieve local tissue concentrations without such detrimental blood levels by using a therapeutically effective amount of the treating agent and a bioadhesive, cross-linked water swellable, but water-insoluble polycarboxylic acid polymer. Using this composition and the method of treatment provides sufficient local levels of the drug to provide therapeutic efficacy, but avoids many untoward adverse events. The invention also relates to a pharmaceutical composition for use during menses that includes a treating agent and a bioadhesive, cross-linked water swellable, but water-insoluble polycarboxylic acid polymer.
Owner:COLUMBIA LABORATORIES INC
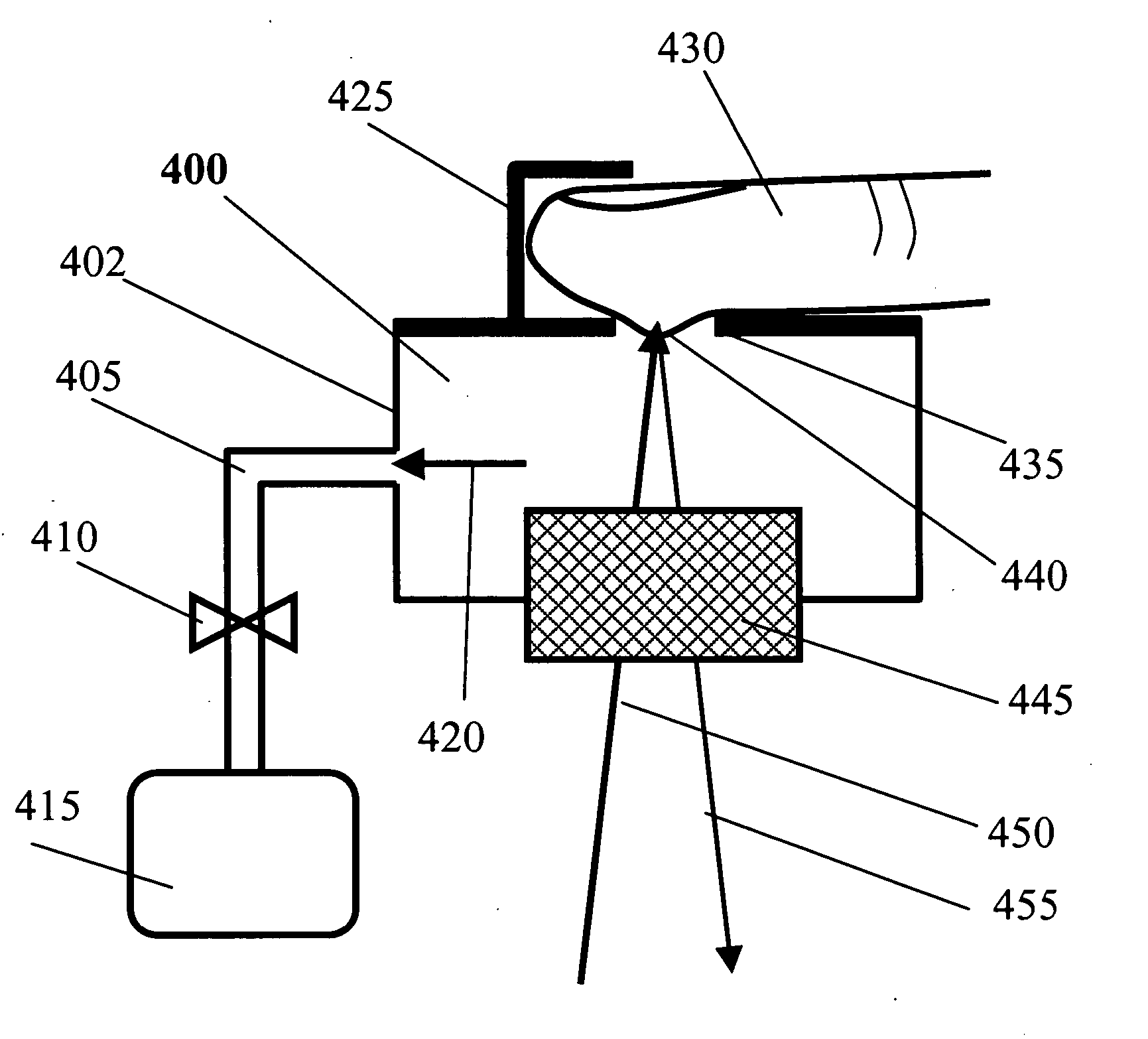
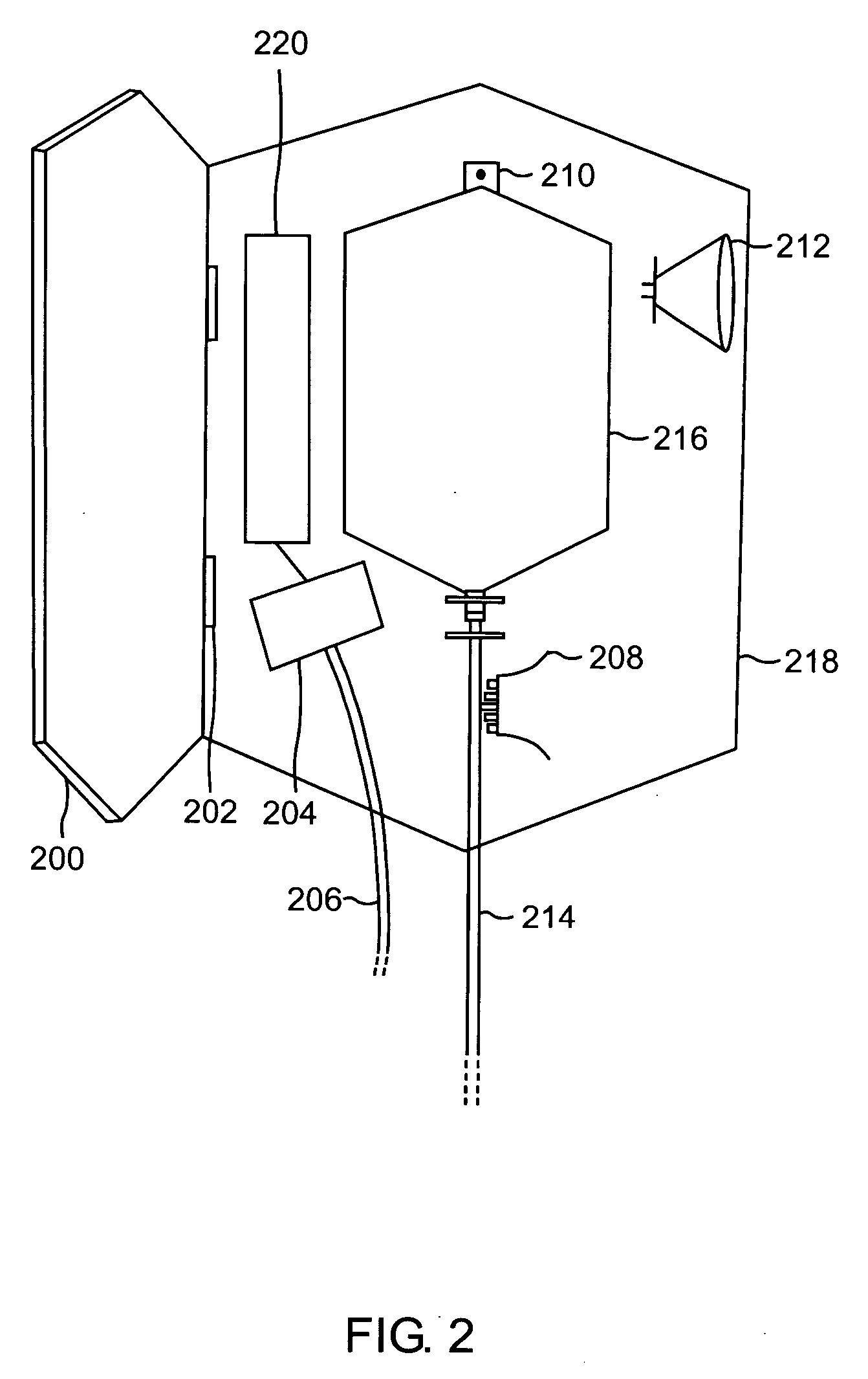
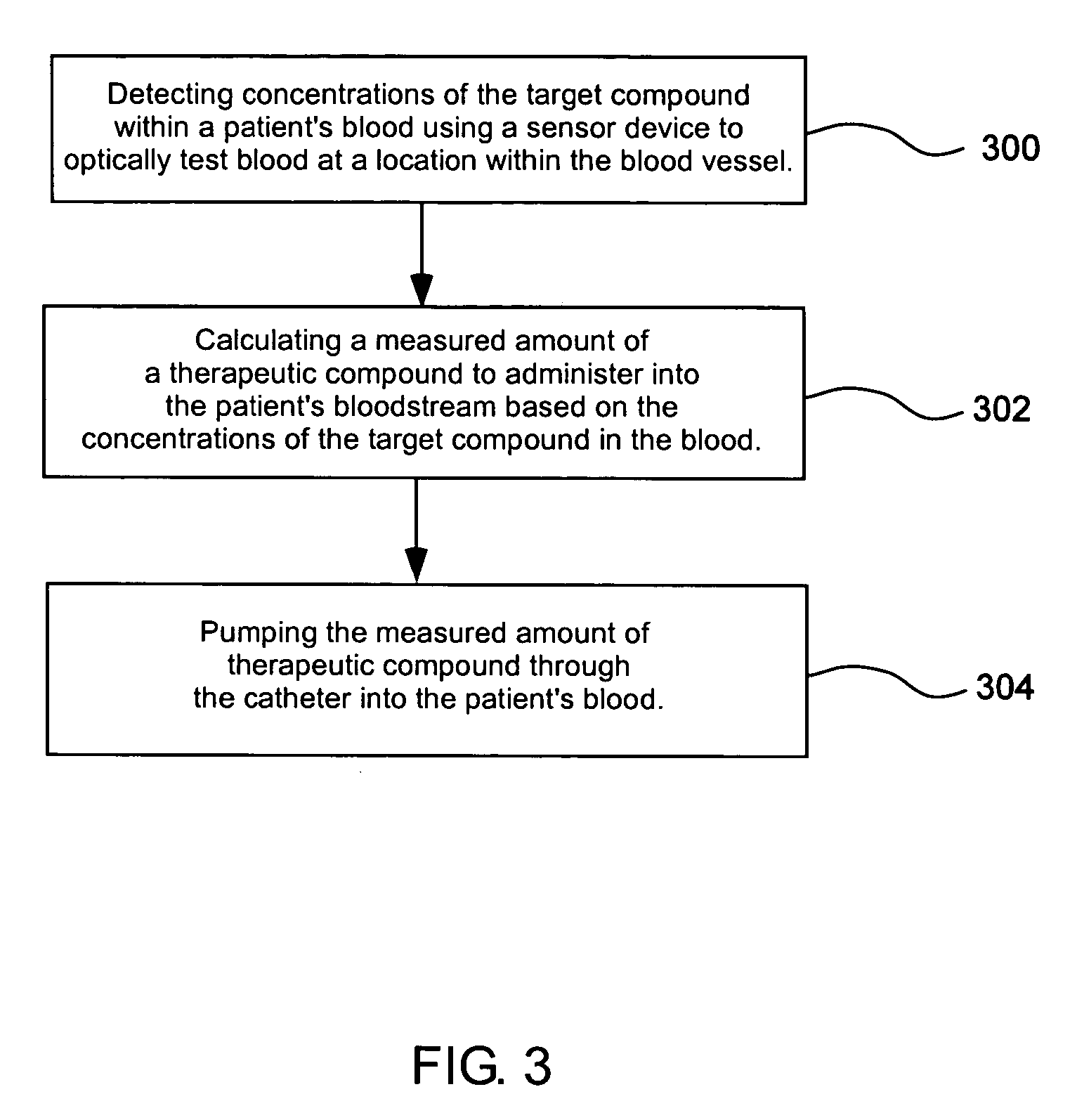
Blood testing and therapeutic compound delivery system
A system and method are provided for determining intravenous blood levels of a target compound contained in a blood vessel of a patient. The method includes the operation of detecting concentrations of the target compound within a patient's blood using a sensor device configured to optically test blood at a location within the blood vessel. Another operation is calculating a measured amount of a therapeutic compound to administer into the patient's bloodstream based on the concentrations of the target compound in the blood. The measured amount of therapeutic compound may then be pumped through the catheter into the patient's blood.
Owner:JONES CHRISTOPHER W
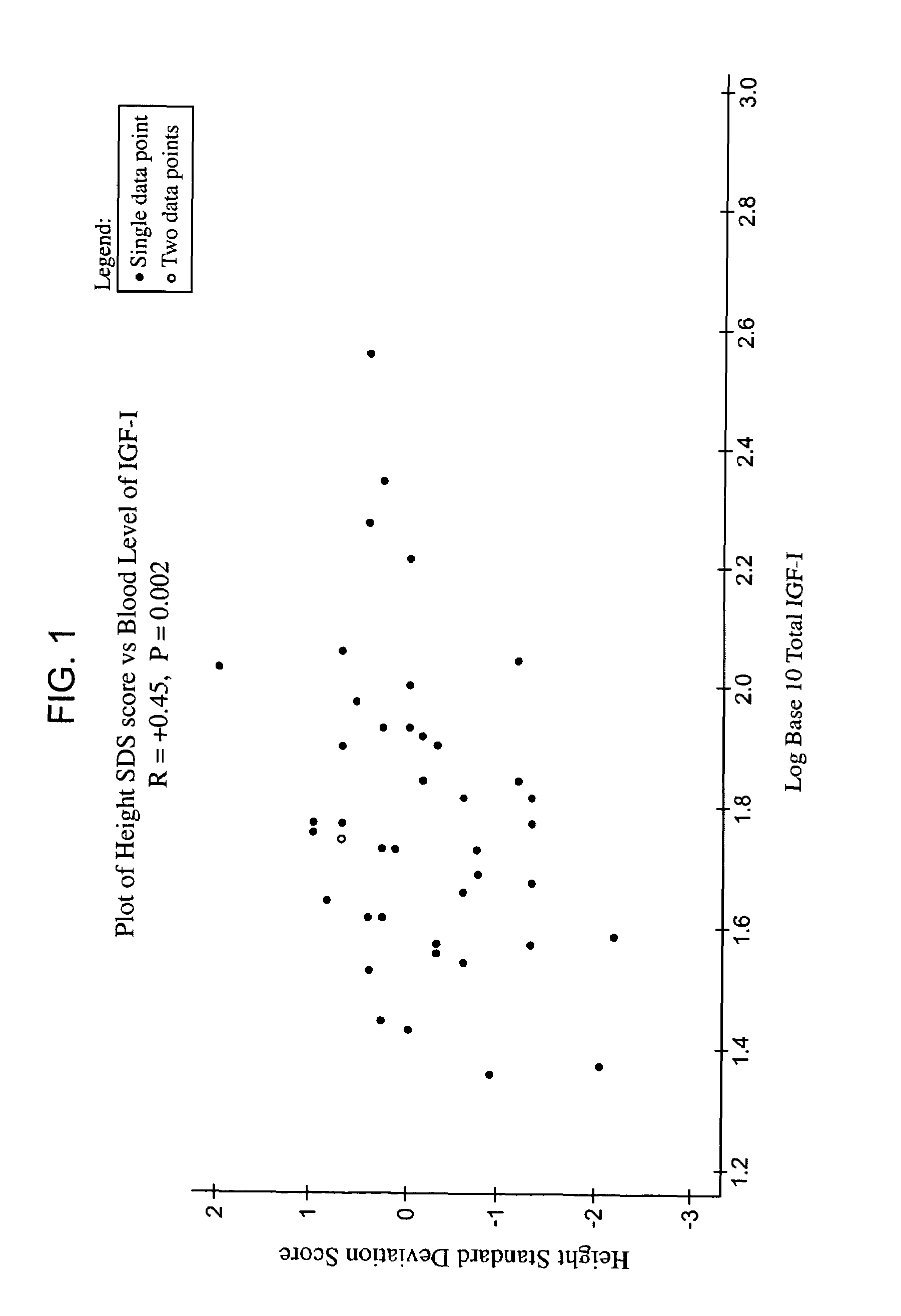
Methods for treatment of insulin-like growth factor-1 (IGF-1) deficiency
ActiveUS7258864B2To promote metabolismIncrease probabilityPeptide/protein ingredientsPeptide preparation methodsInitial treatmentBlood level
Owner:IPSEN BIOPHARMACEUTICALS INC
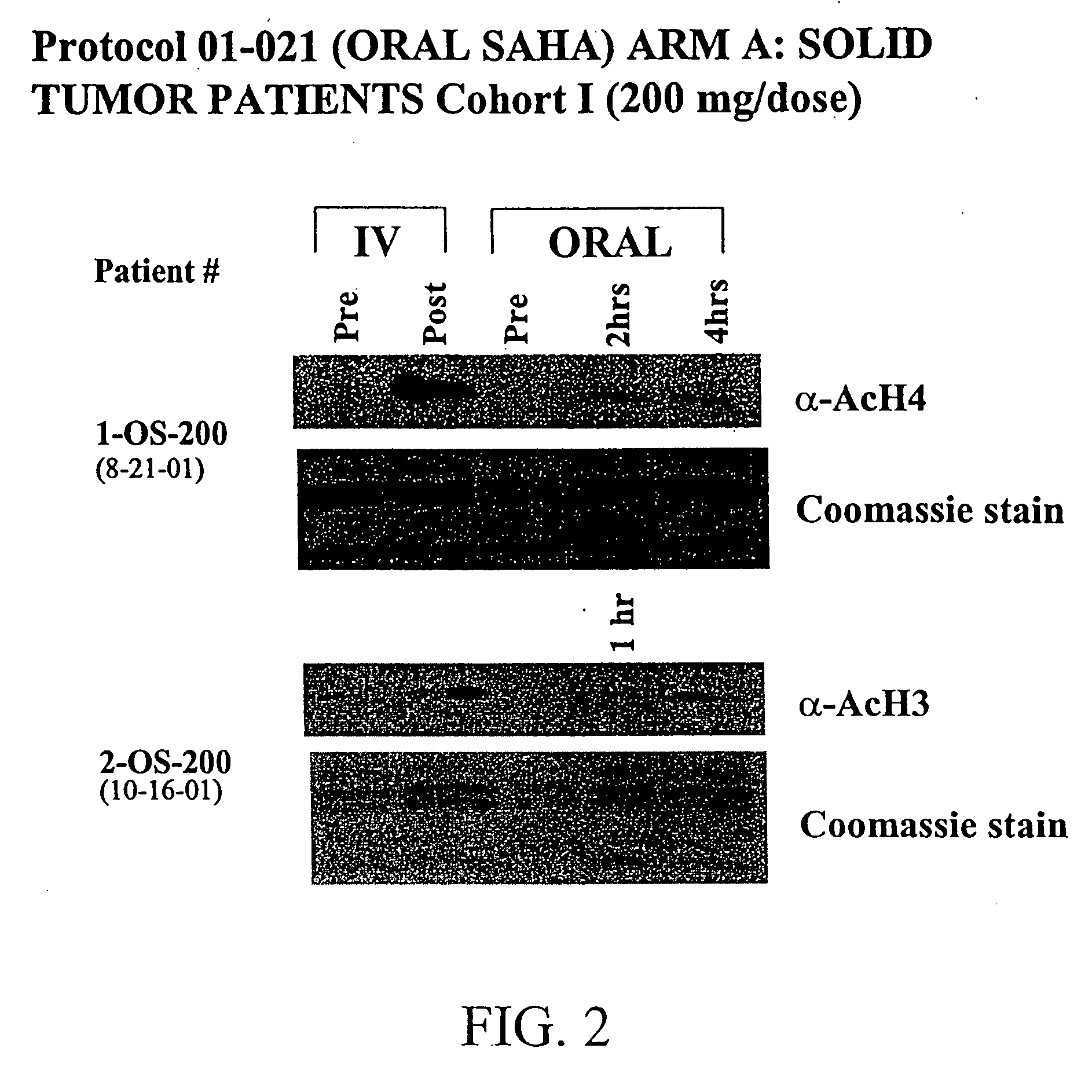
Methods of treating cancer with HDAC inhibitors
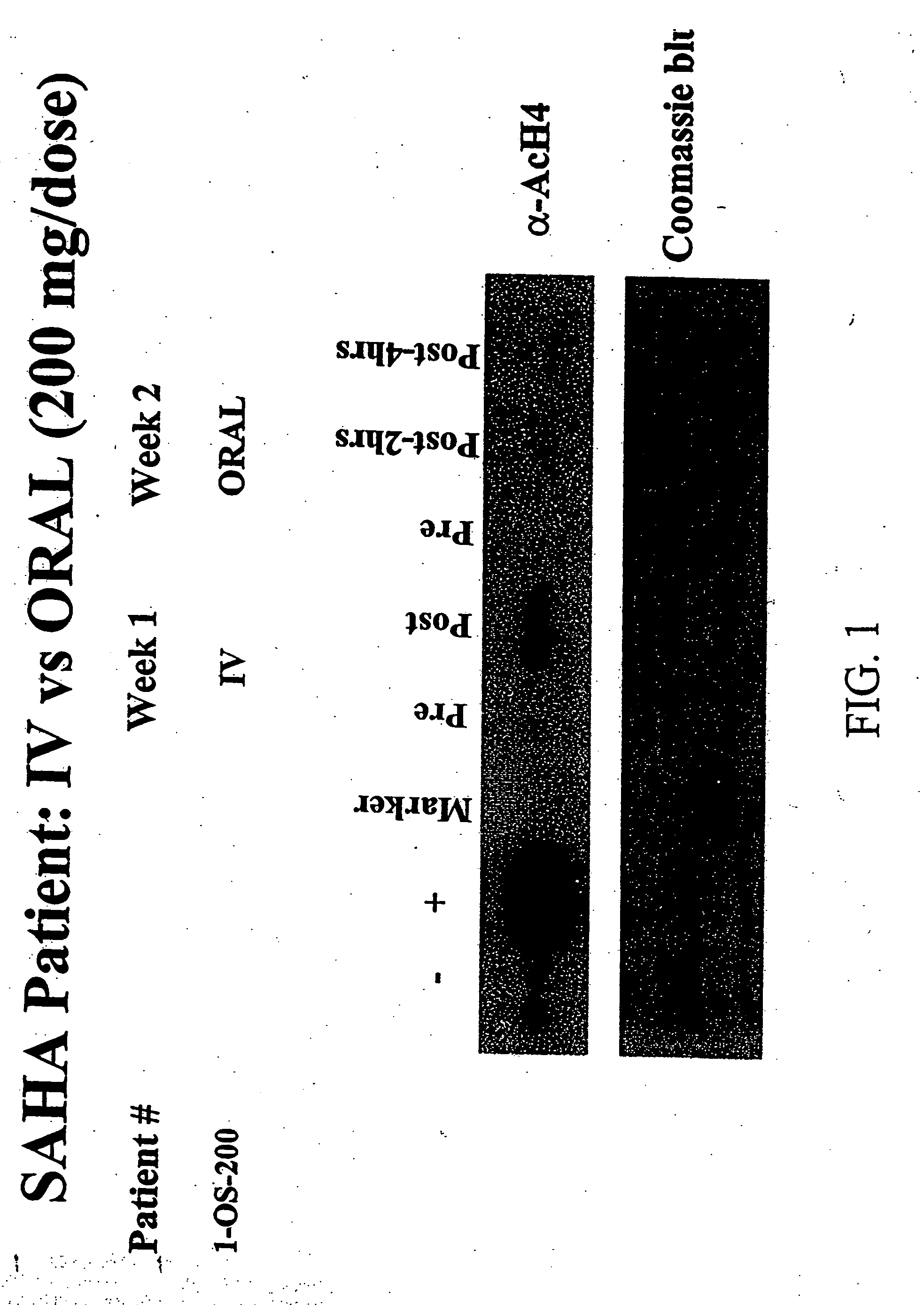
InactiveUS20060276547A1Better pharmacokinetic profileImprove bioavailabilityBiocideCyclic peptide ingredientsDosing regimenIn vivo
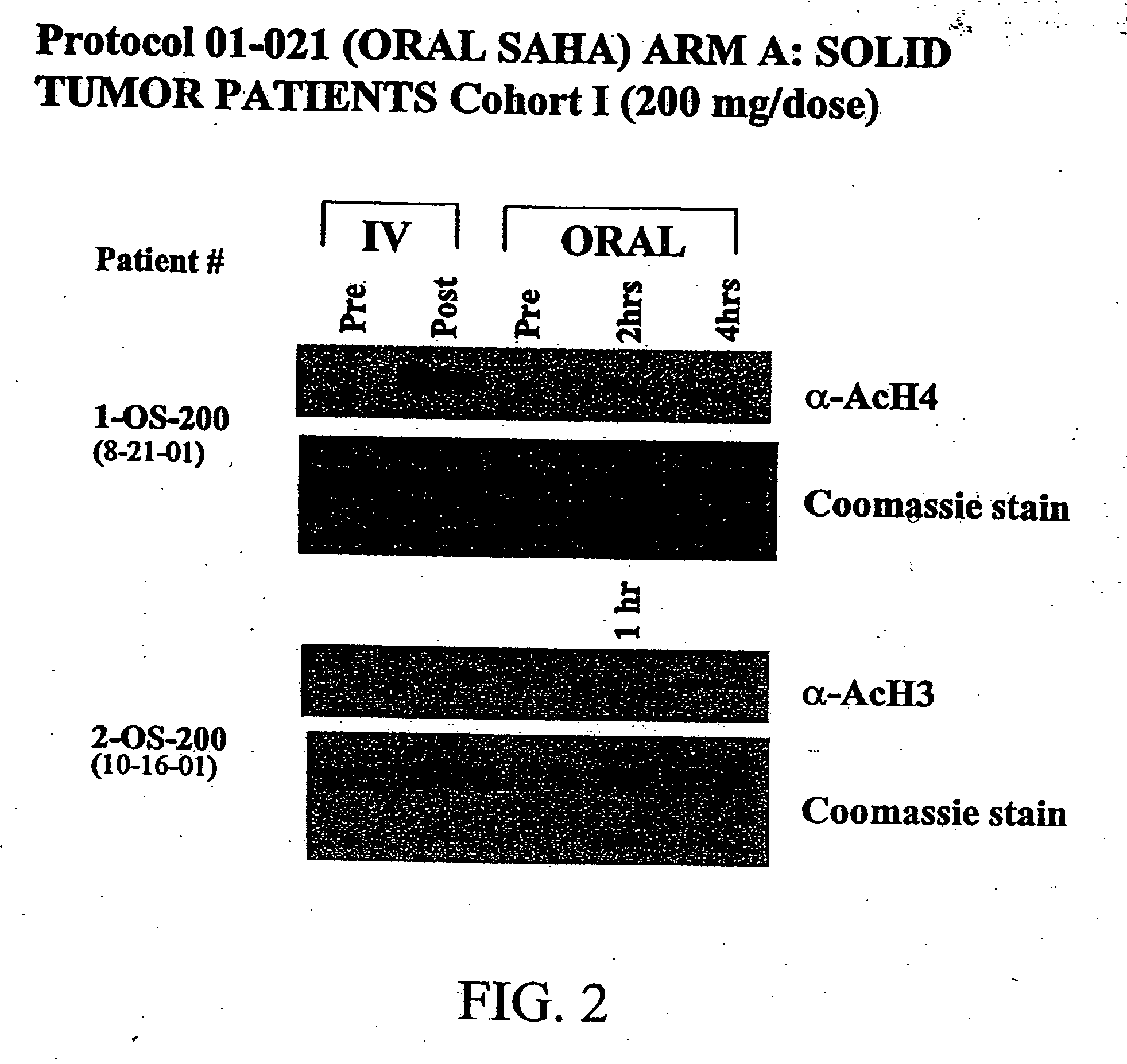
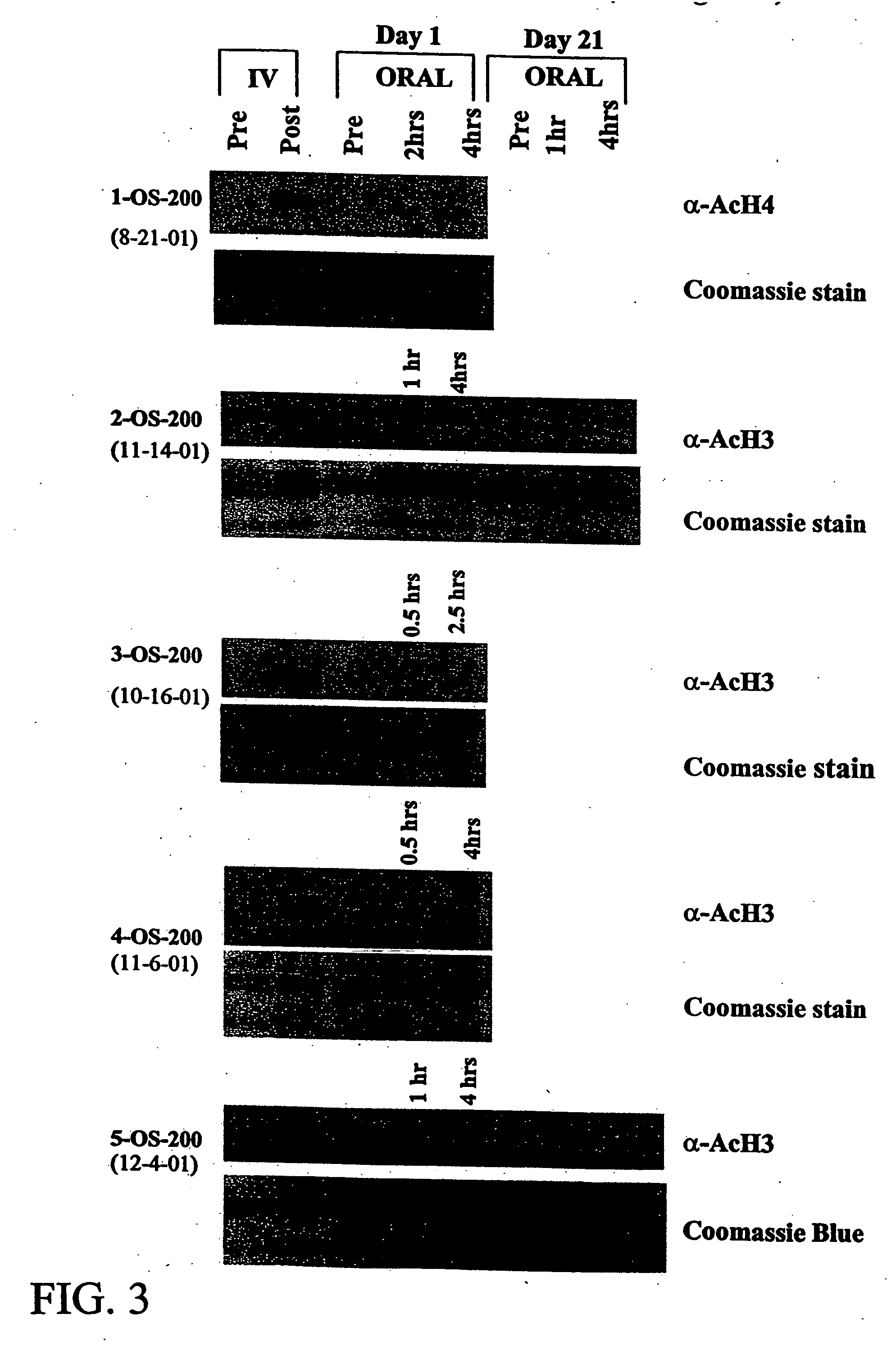
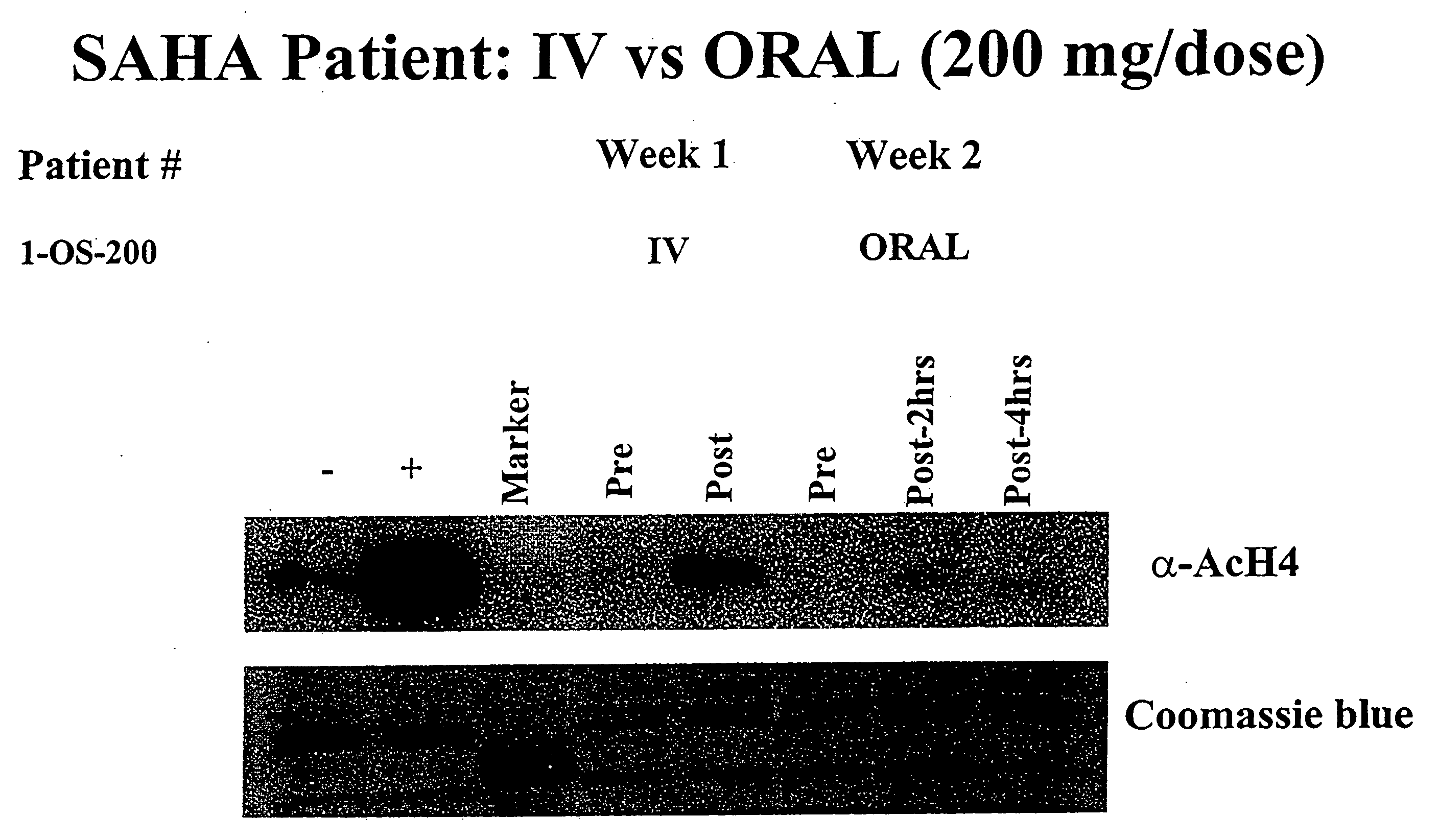
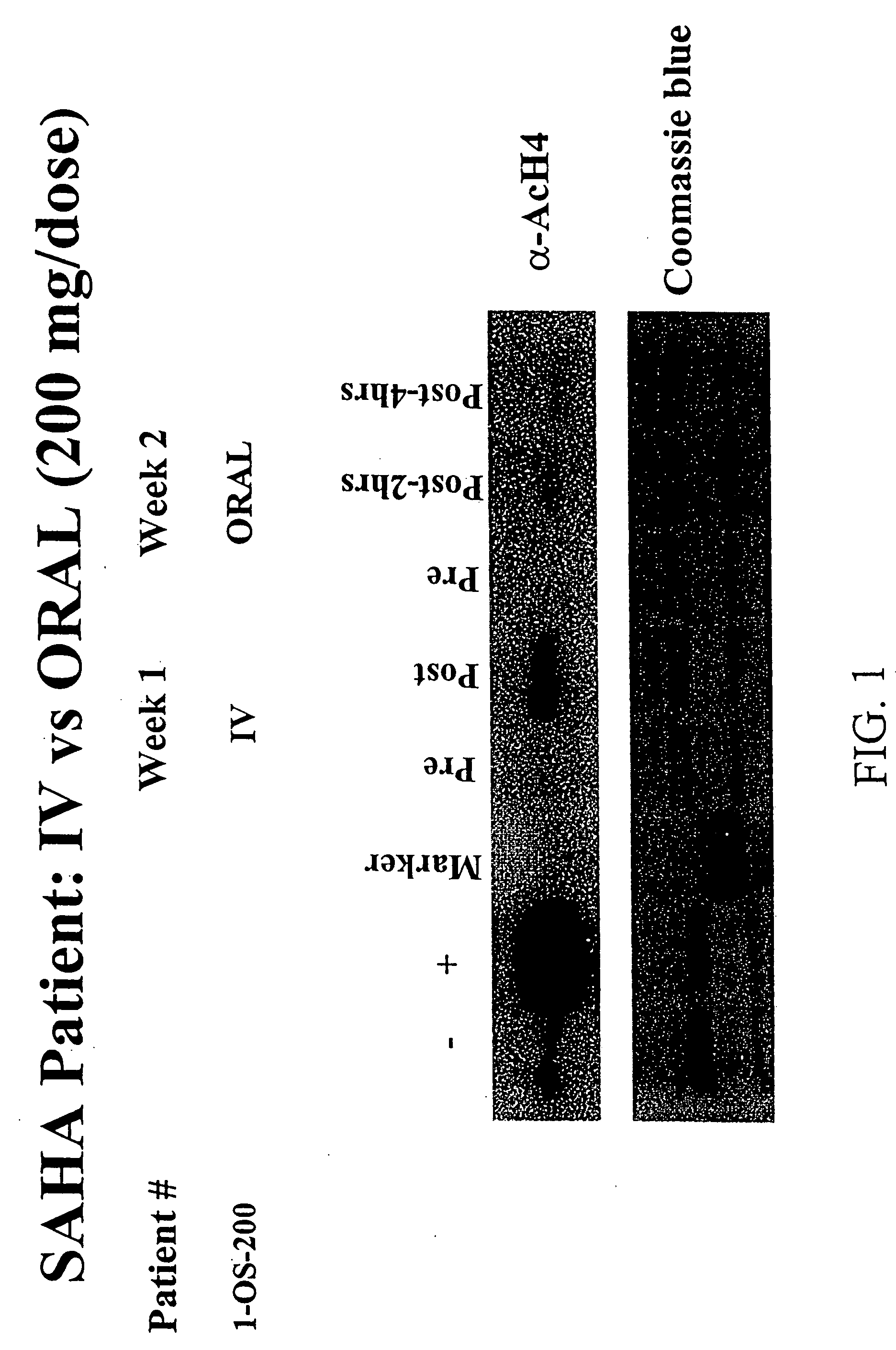
The present invention relates to methods of treating cancers, e.g., lymphoma. More specifically, the present invention relates to methods of treating diffuse large B-cell lymphoma (DLBCL), by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC +1
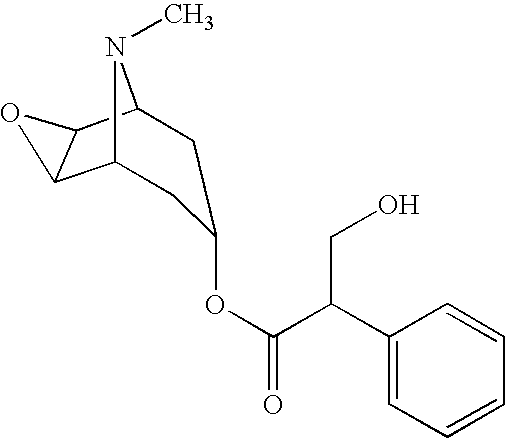
Multi-Phase Release Methscopolamine Compositions
Formulations have been developed administering methscopolamine in multi-phases. In a preferred embodiment, the formulation contains methscopolamine in an immediate release. (“IR”) form and a sustained or delayed release (“DR”) form and / or poised release (“PR”) form. In another embodiment, the methscopolamine is released in a gradient, decreasing the side effects associated with rapidly elevated blood levels. In another embodiment the drug is bound to an ion-exchange resin, which can be suspended in a liquid or incorporated into a matrix for delayed, sustained and / or pulsed release. Dosage unit forms may be tablets, gels, liquids, capsules, beads, microparticles, films or lozenges. Multi-phase delivery can also be achieved through the use of a kit that provides for dosage escalation. This kit can be a blister pack or equivalent, wherein the drug is packaged so that a first dosage is taken, then sequentially larger dosages. The dosages can be the same in each unit, and instructions provided so that the correct dosage is obtained through the number of units and the time of administration or the dosages may be different, and the units ordered so dial the desired dosage administration profile is obtained when the patient takes the units in order as instructed.
Owner:AURIGA LAB
Method of Cancer Treatment with 2-(1H-Indole-3-Carbonyl)-Thiazole-4-Carboxylic Acid Methyl Ester
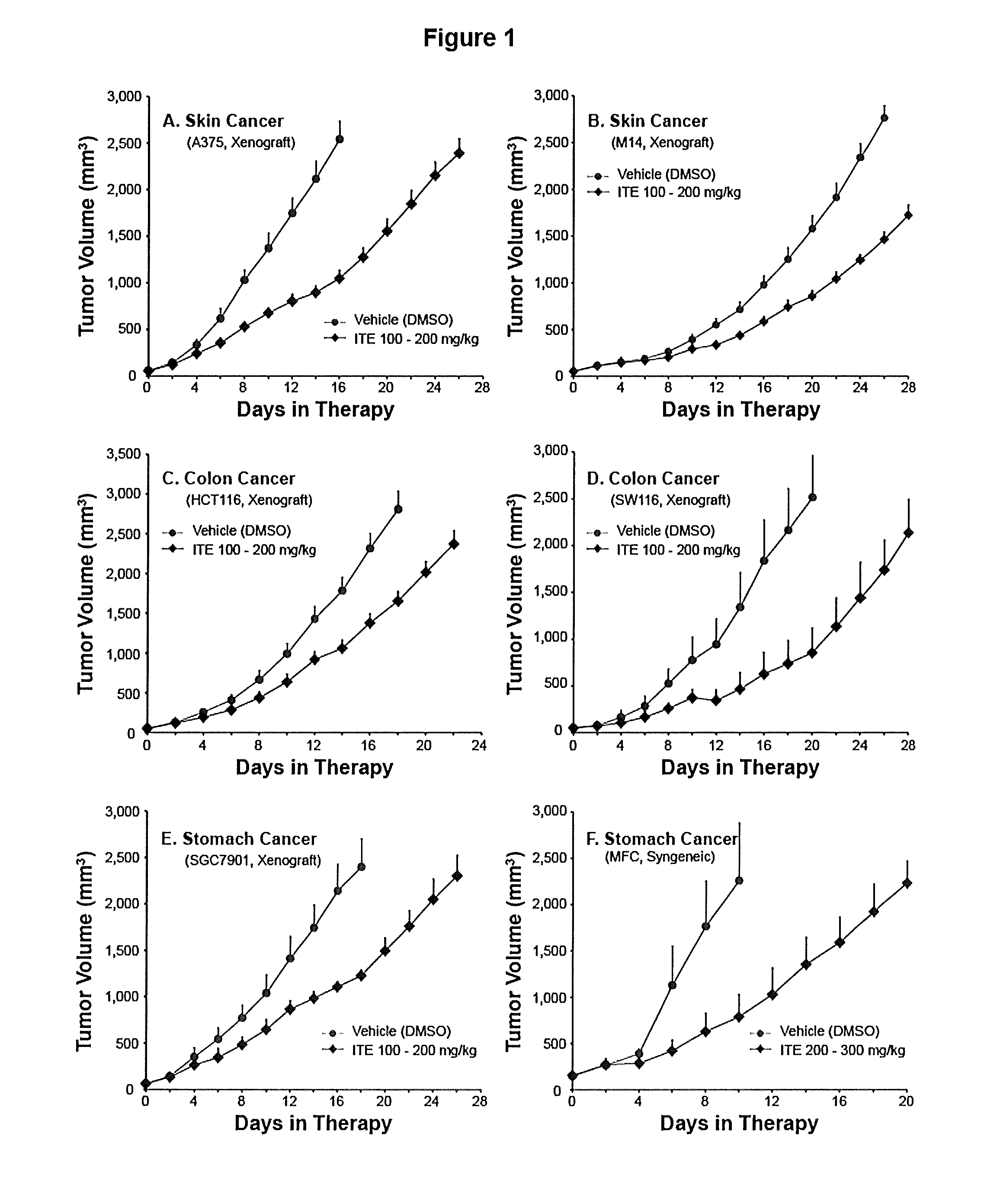
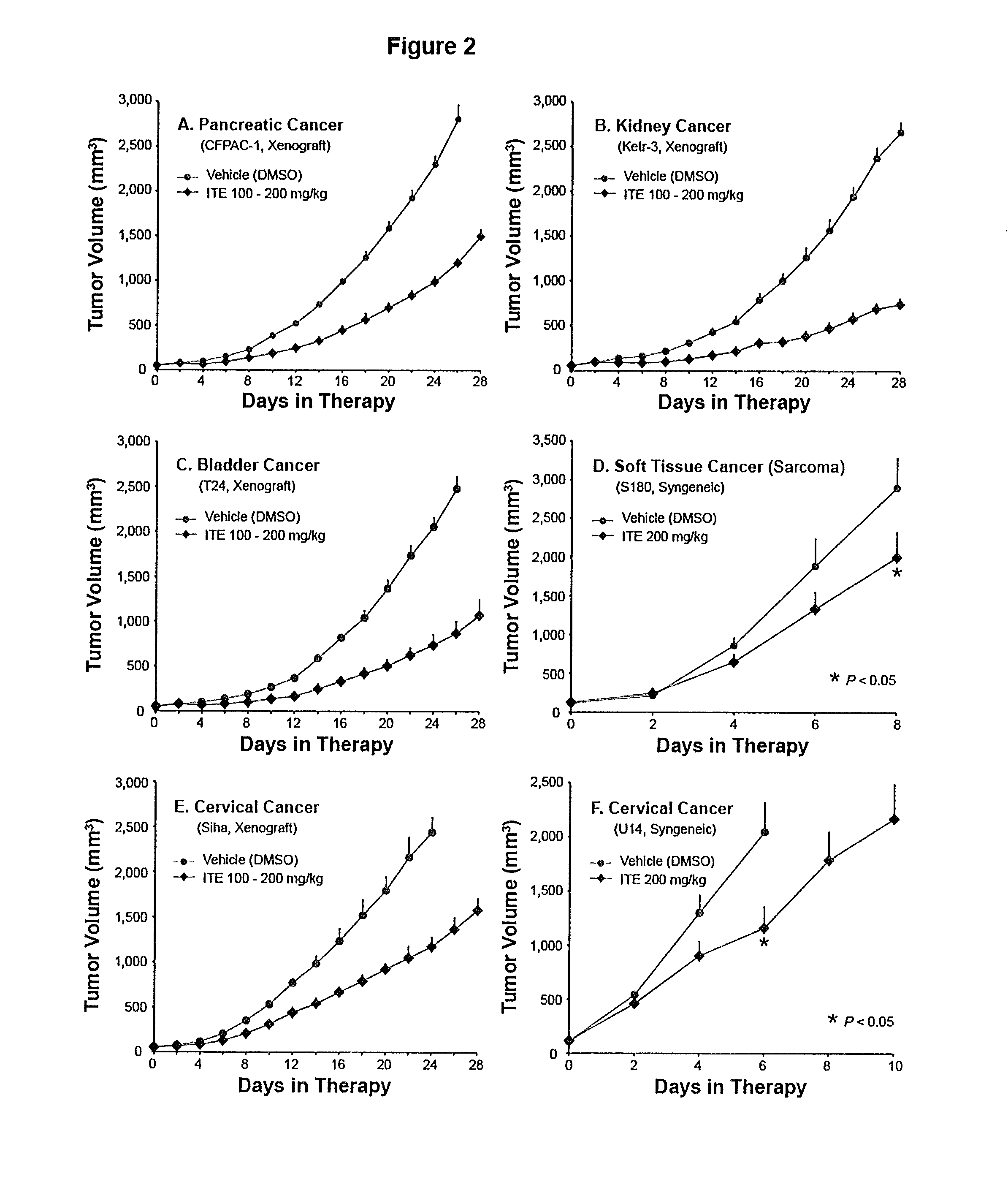
A method of cancer treatment is provided that includes administering an effective amount of an endogenous ligand for the aryl hydrocarbon (Ah) receptor (AhR) named ITE or one of its structural analogs (the active ingredient) to a subject with cancer is disclosed. An effective dose and dosing frequency of the active ingredient are determined by measuring its blood levels of the subject after dosing. The active ingredient formulated with a carrier system is applied topically, enterally, or parenterally to the subject. An oral dose of water, in addition to normal water drinking, is administered to help alleviate feces hardening, a complication of ITE dosing. Subjects with cancers of skin, colon (or rectum), stomach, pancreas, kidney, bladder, soft tissue, and cervix, are preferably accepted for treatment or intervention.
Owner:ARIAGEN INC
Methods of treating cancer with HDAC inhibitors
InactiveUS20080249179A1Better pharmacokinetic profileImprove bioavailabilityBiocideOrganic chemistryDosing regimenIn vivo
The present invention relates to methods of treating cancers, e.g., leukemia. More specifically, the present invention relates to methods of treating acute and chronic leukemias including Acute Lymphocytic Leukemia (ALL), Acute Myeloid Leukemia (AML), Chronic Lymphocytic leukemia (CLL), Chronic myeloid leukemia (CML) and Hairy Cell Leukemia, by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC +1
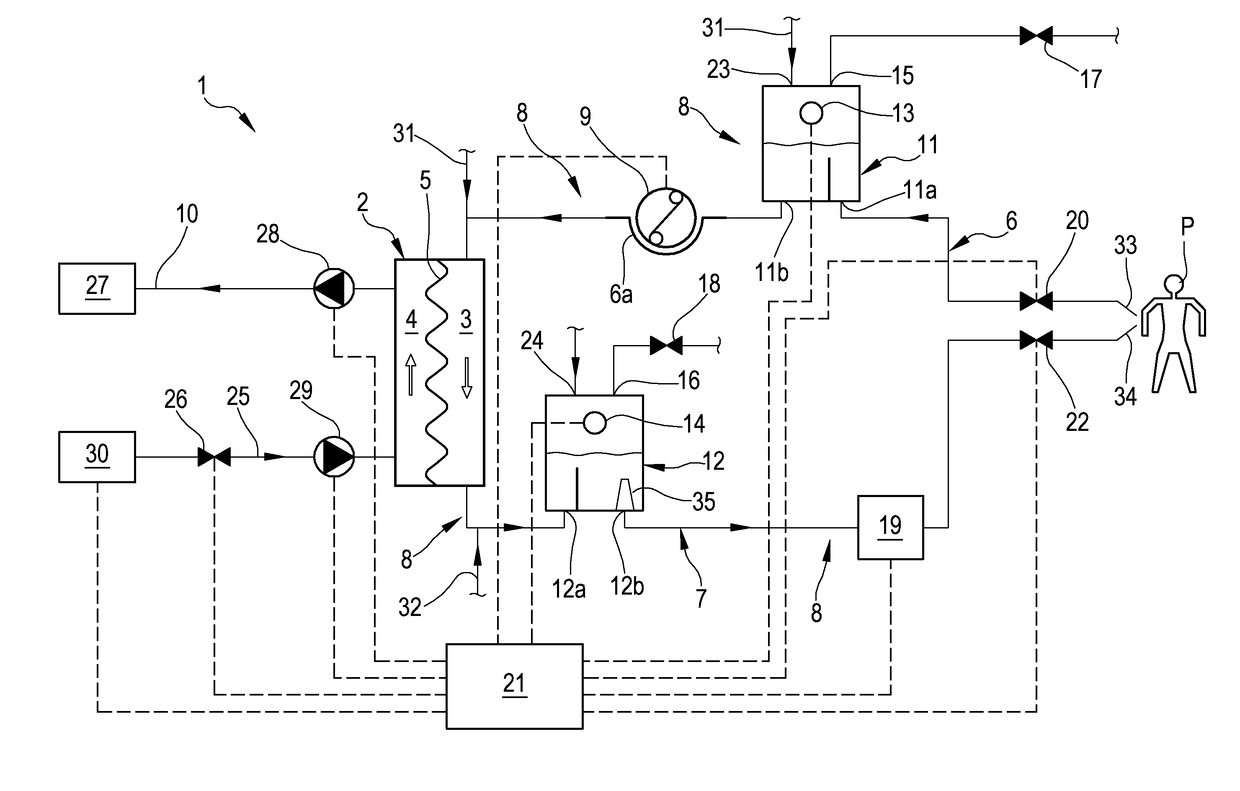
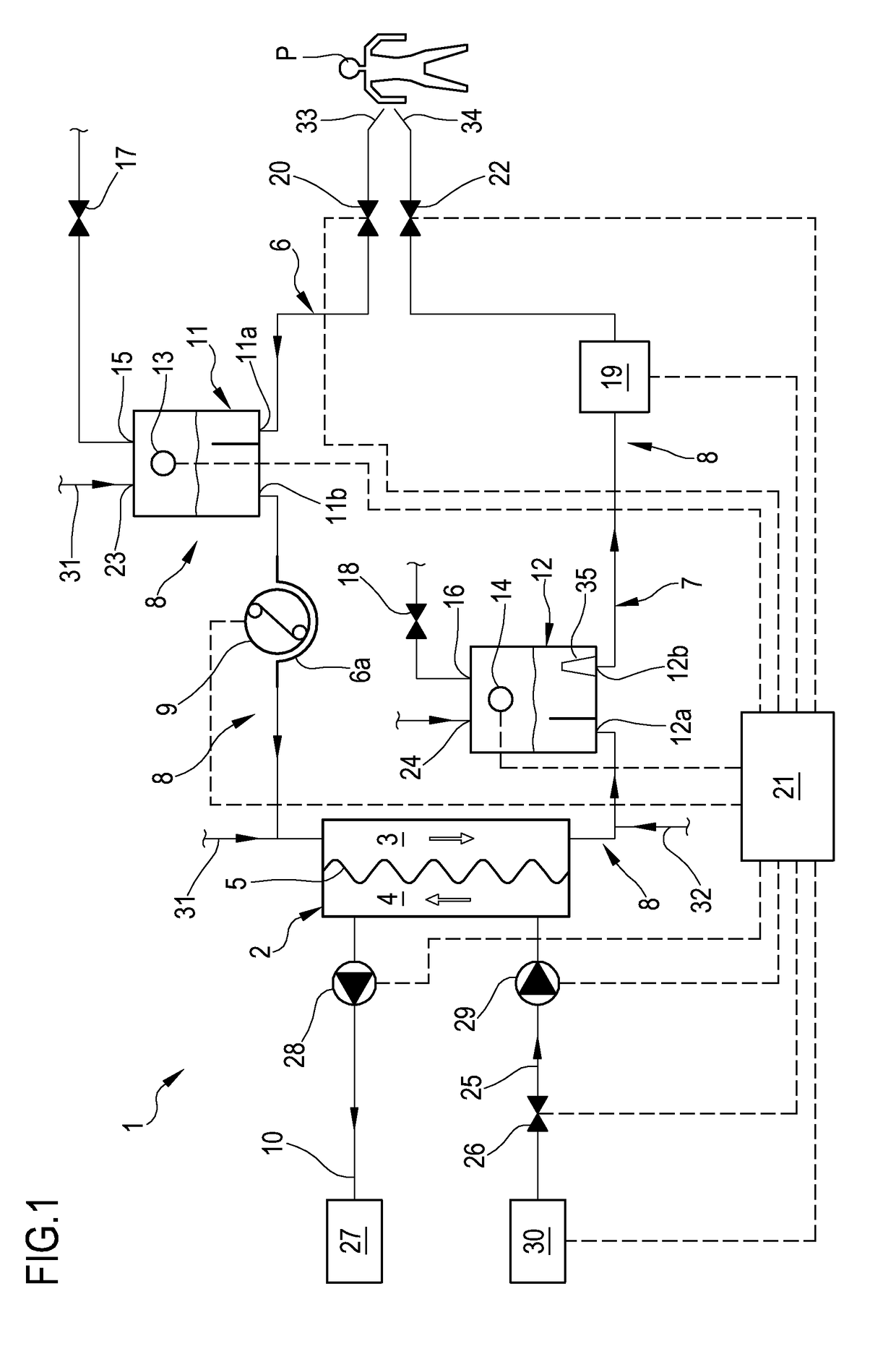
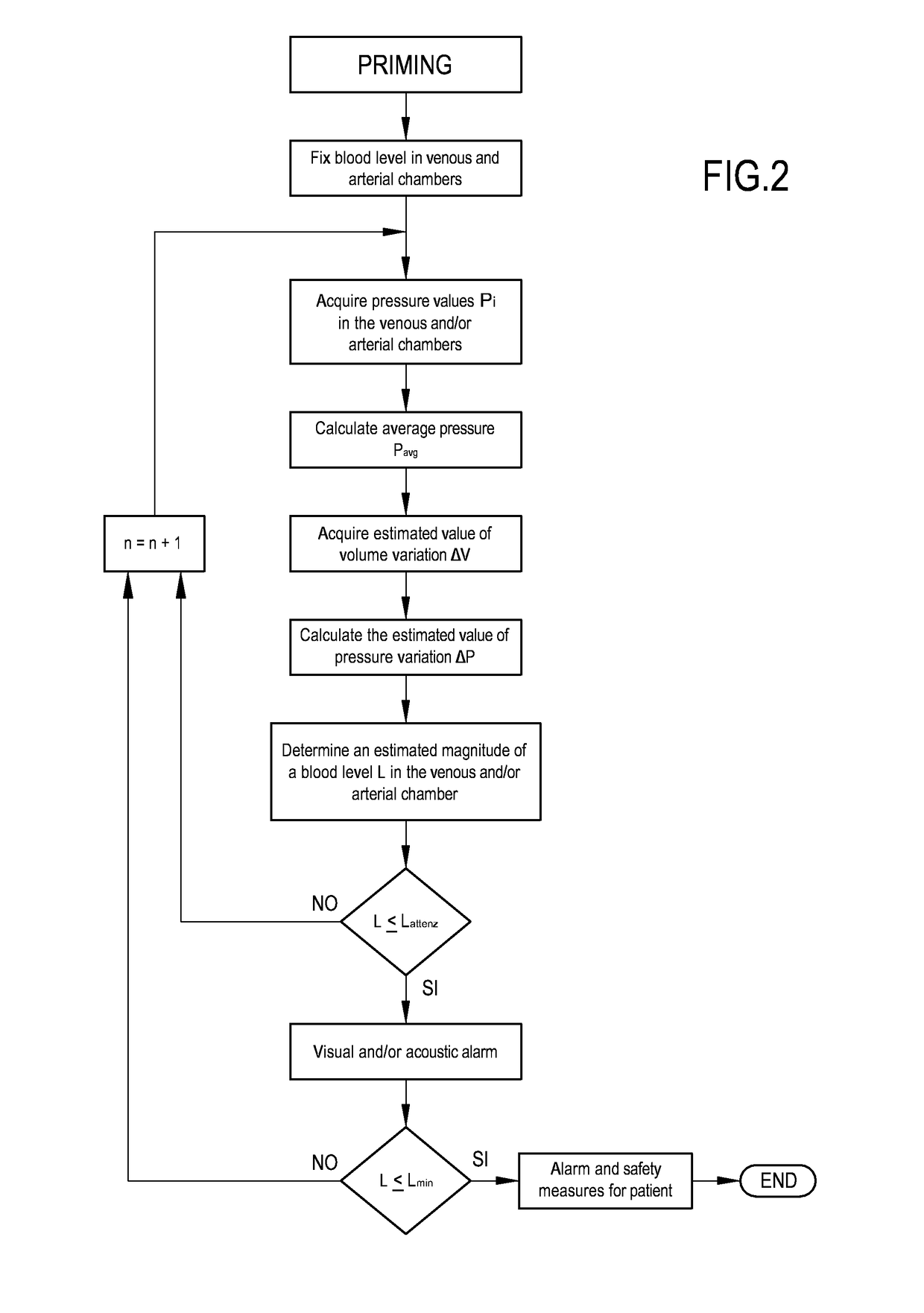
Apparatus and method of controlling an extracorporeal blood treatment
ActiveUS9770546B2Easy to detectReducing the false positivesOther blood circulation devicesHaemofiltrationBlood treatmentsBlood level
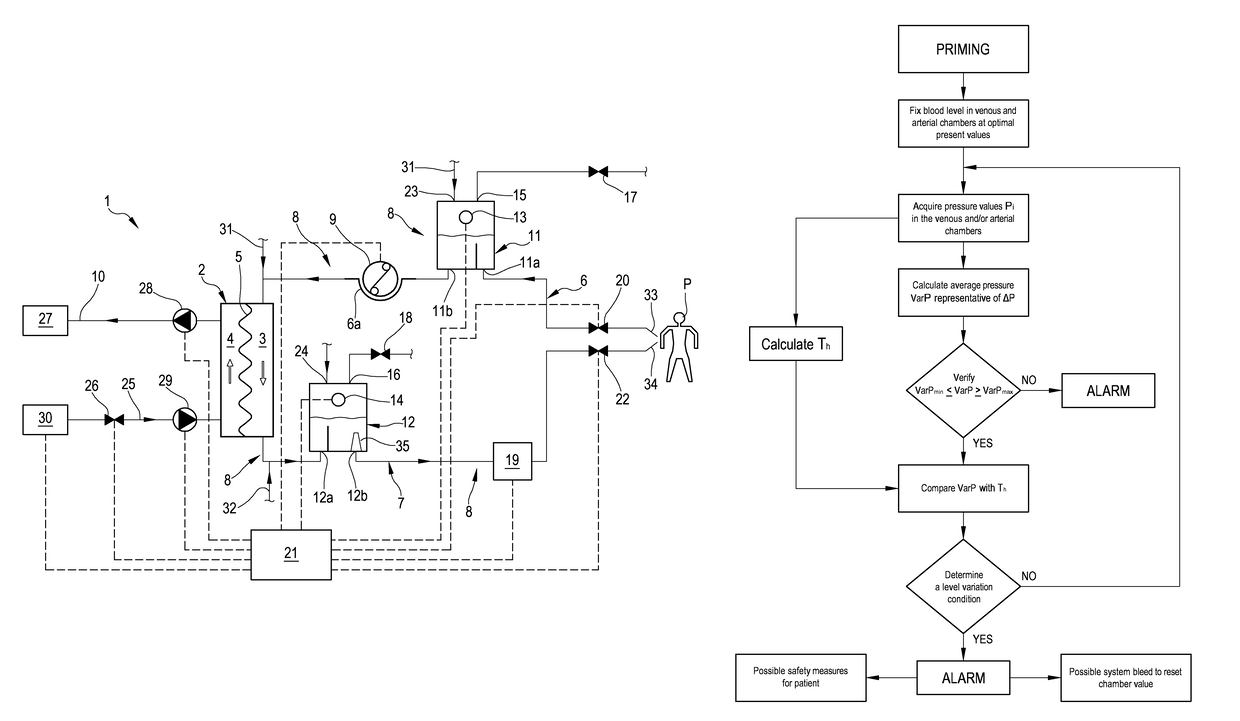
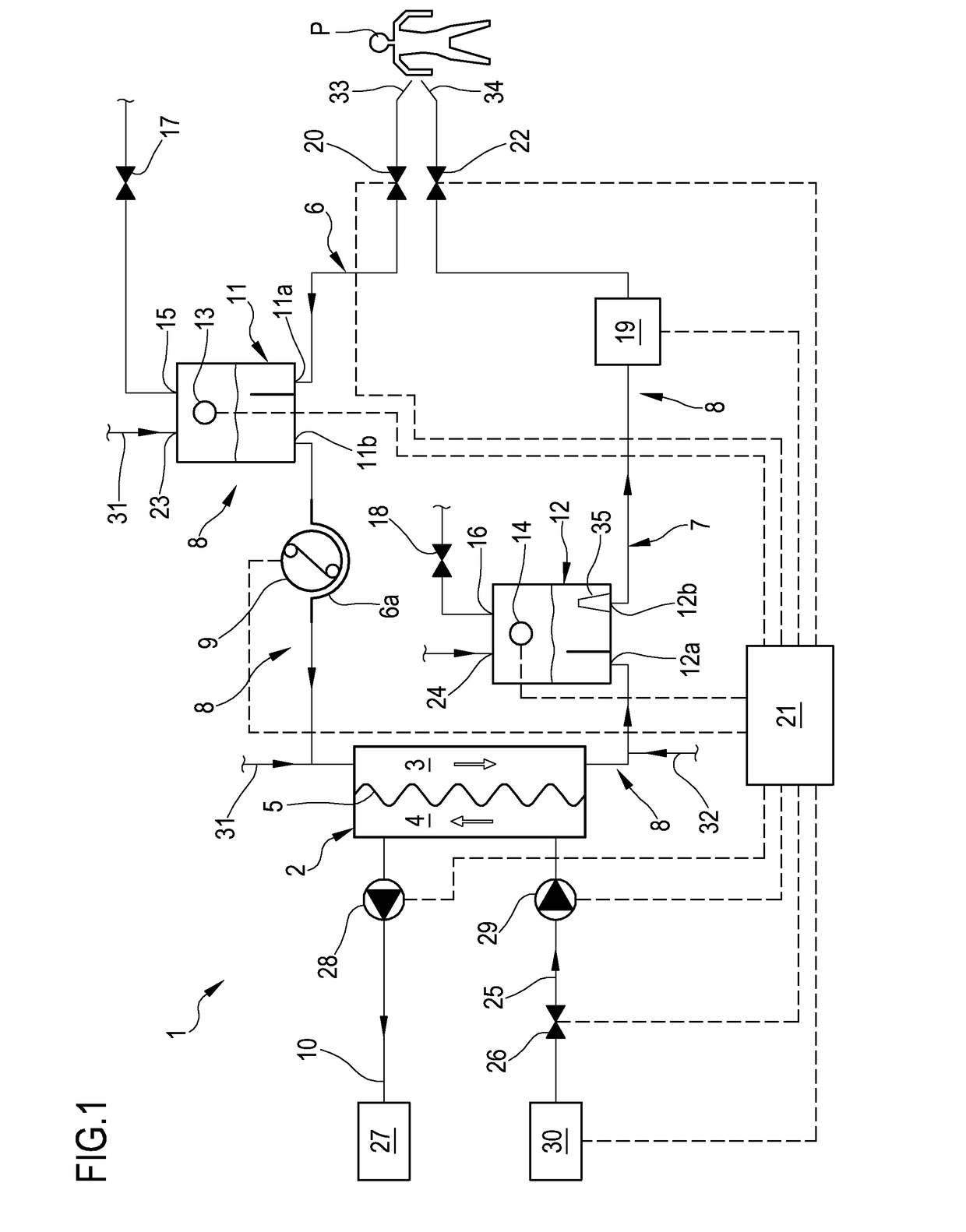
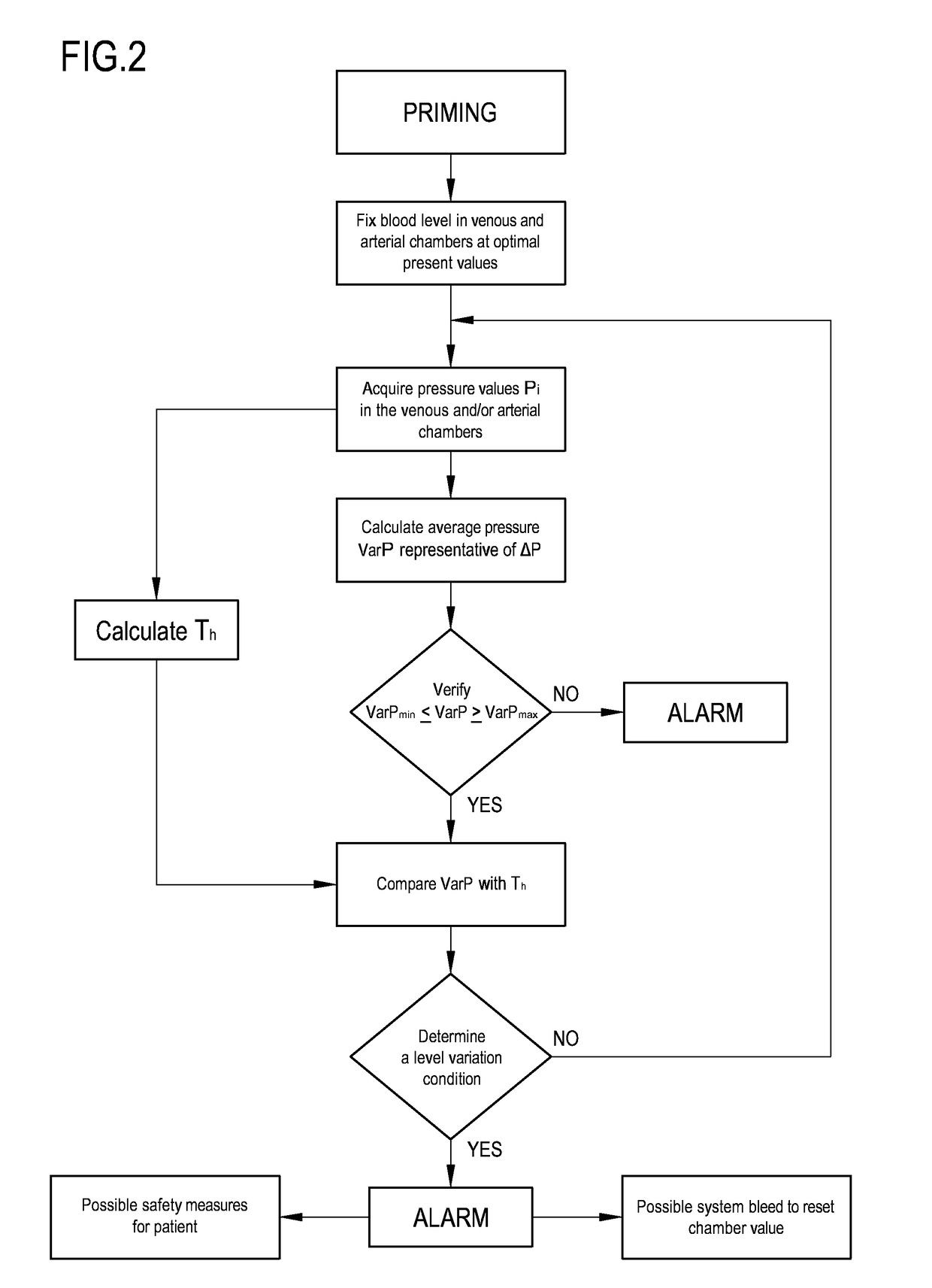
An apparatus is described for extracorporeal blood treatment (1), comprising a treatment unit (2), an extracorporeal blood circuit (8) and a fluid evacuation line (10). The apparatus comprises a control unit (21) connected with a pressure sensor (13, 14) and with a blood pump (9) and configured to move the blood pump (9), generating a variable flow (Q(t)) with a constant component (Qb) and a variable component (Qvar(t)) having a nil average value; the variable flow generates, in the expansion chamber (11, 12), a progression of the pressure that is variable over time (P(t)) with a pressure component (Pvar(t)) oscillating about an average value (Pavg). The control unit receives, from the sensor, a plurality of values (Pj) and calculates the average value of the pressure (Pavg), acquires an estimated value of volume variation (AP) in the expansion chamber (11, 12) connected to the variable flow component (Qvar(t)), calculates, as a function of the pressure values (Pj), an estimated value of pressure variation (AP) in the expansion chamber (11; 12) that is representative of the oscillating pressure component (Pvar(t)) and determines a representative magnitude of a blood level (L) in the expansion chamber (11, 12) as a function of the average value (Pavg) of the pressure (P(t)), of the estimated value of volume variation (AV) and of the estimated pressure variation (AP) in the expansion chamber.
Owner:GAMBRO LUNDIA AB
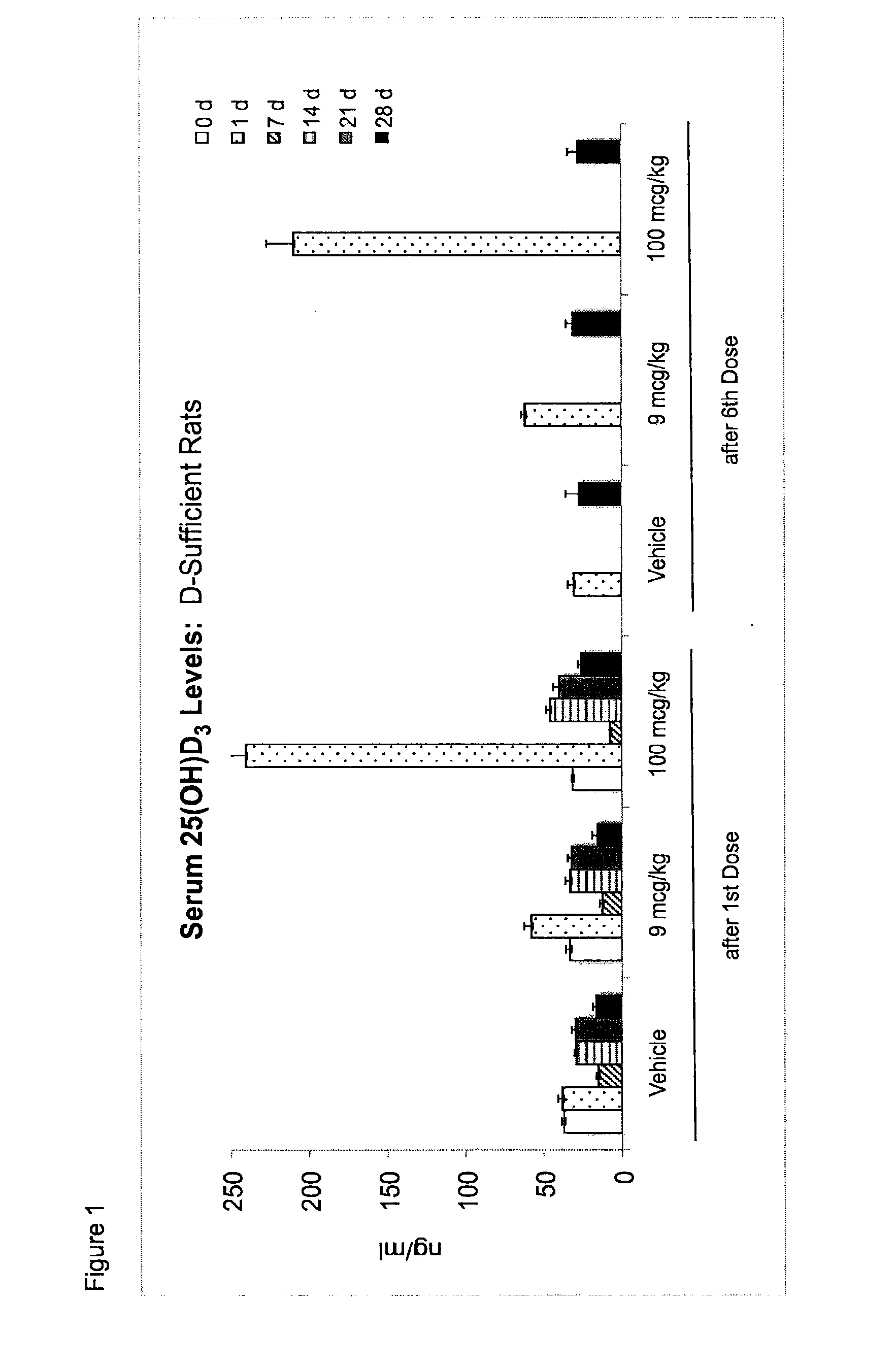
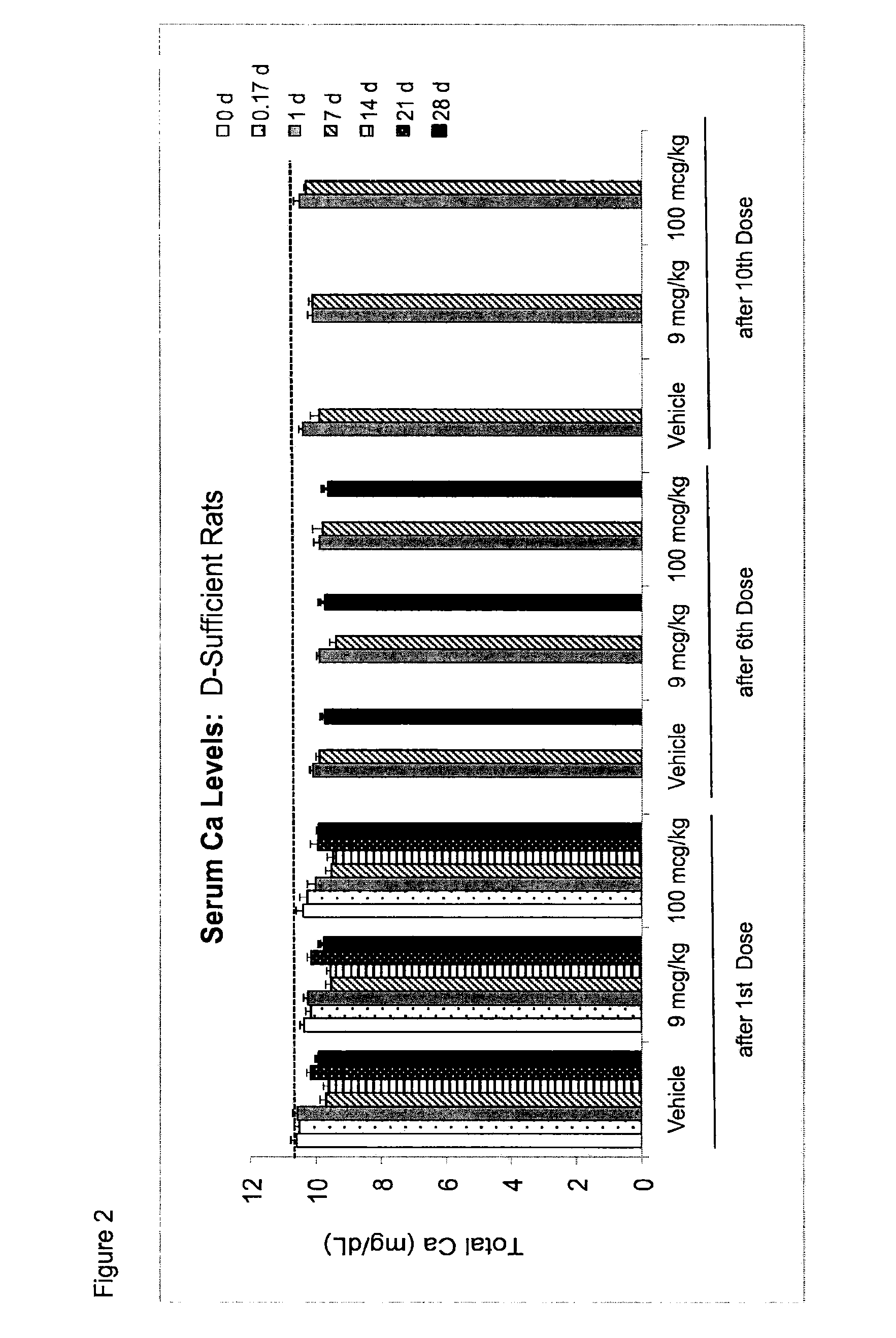
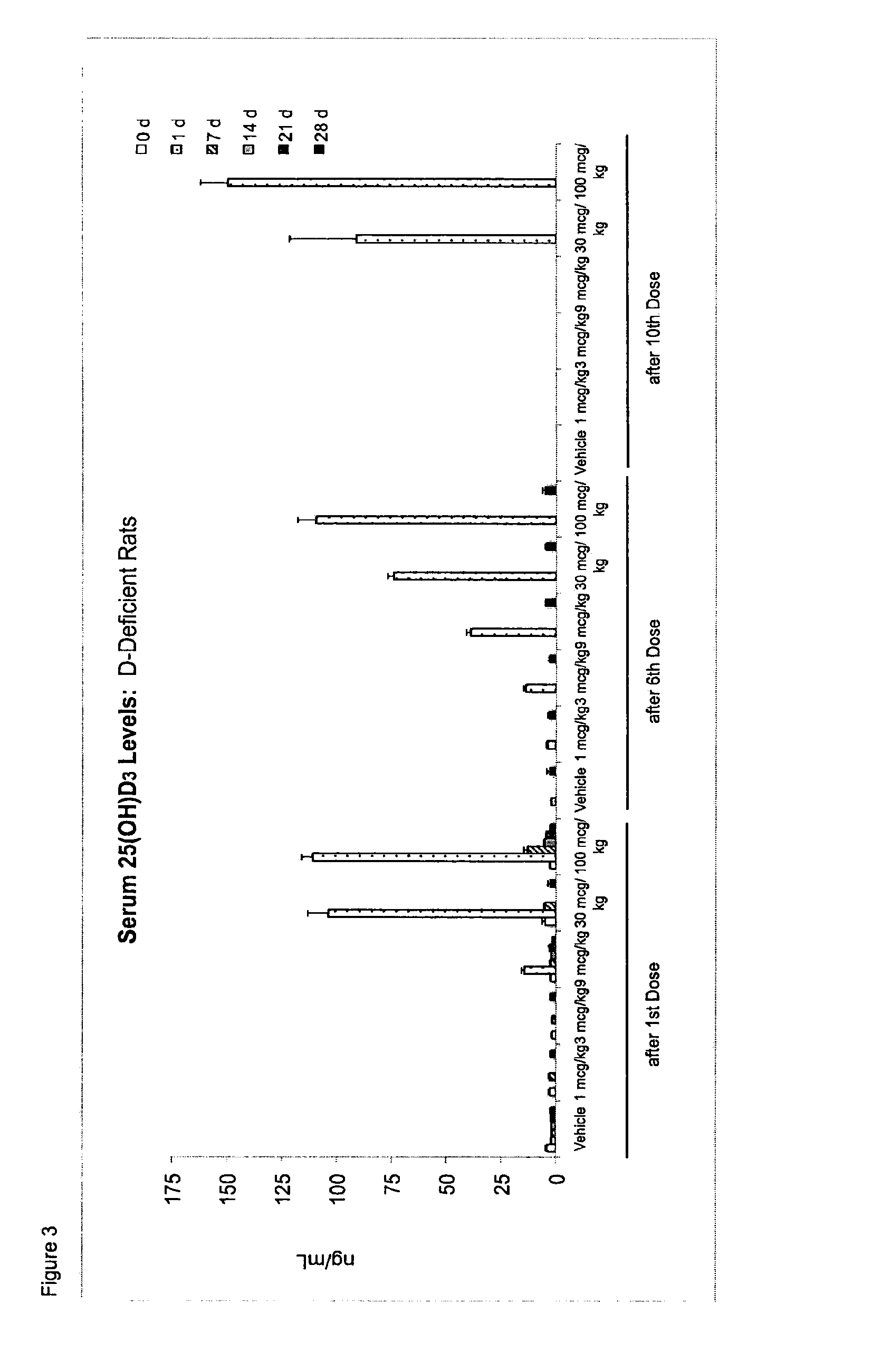
Once-a-week administration of 25-hydroxy vitamin d3 to sustain elevated steady-state pharmacokinetic blood concentration
An oral dosage form comprising a single dose of 25-hydroxy-vitamin D3 sufficient to elevate the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml for at least 7 days and a pharmaceutically suitable oral carrier system, wherein subsequent single doses at least every 7 days are sufficient to sustain the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml at steady-state pharmacokinetics is disclosed. A method of elevating and sustaining the blood level concentration of 25-hydroxy-vitamin D3 in a human in need thereof comprising orally administering or parenterally administering by injection or infusion, at least once every 7 days, a single dose of 25-hydroxy-vitamin D3 sufficient to elevate the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml for at least 7 days, wherein the single doses orally administered at least every 7 days are sufficient to sustain the serum level in a human to a concentration in the range of 30 ng / ml to 200 ng / ml at steady-state pharmacokinetics is disclosed. The human in need thereof may be a human deficient in vitamin D having a serum level concentration of 25-hydroxy-vitamin D3 less than 30 ng / ml.
Owner:WISCONSIN ALUMNI RES FOUND
Apparatus for extracorporeal blood treatment and a control method therefor
ActiveUS9750865B2Easy to detectLowering the false positivesOther blood circulation devicesHaemofiltrationBlood levelBlood treatments
Owner:GAMBRO LUNDIA AB
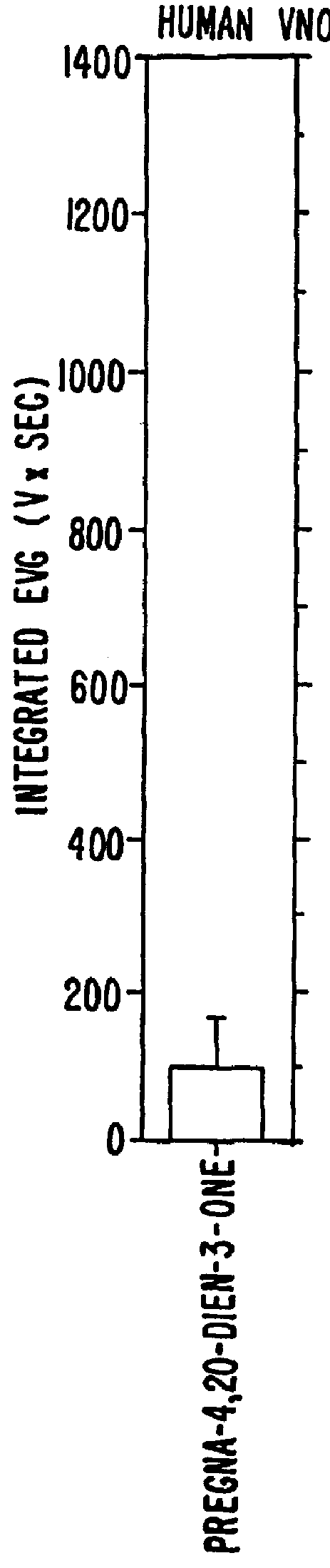
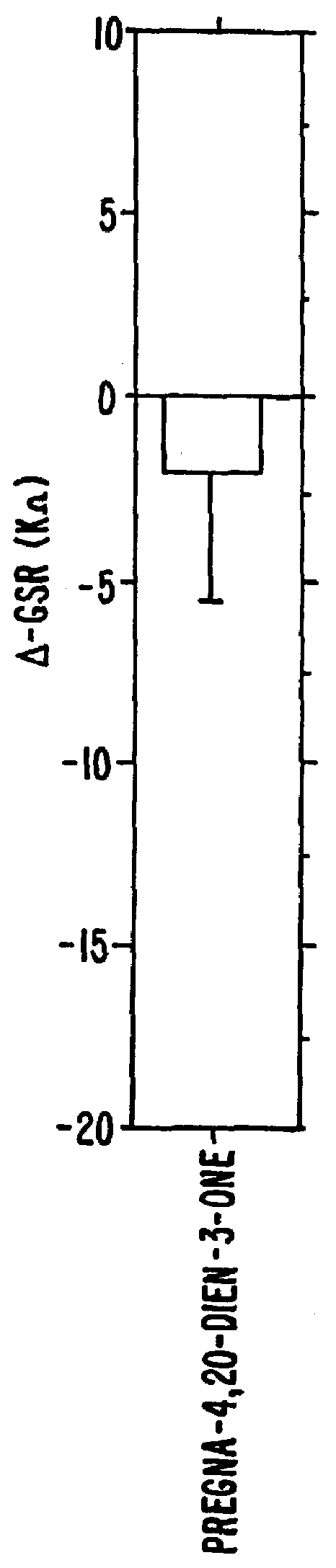
Steroids as neurochemical initiators of change in human blood levels of LH
The invention relates to a method of altering the blood levels of LH or FSH in an individual. The method comprises nasally administering a steroid which is a human vomeropherin, such that the vomeropherin binds to a specific neuroepithelial receptor. The steroid or steroids is / are preferably administered in the form of a pharmaceutical composition containing one or more pharmaceutically acceptable carriers.
Owner:PHERIN PHARMA INC
Biomarkers for the diagnosis of autoimmune disease
InactiveUS20090142792A1Convenient careMicrobiological testing/measurementDisease diagnosisAutoimmune conditionAutoimmune disease
Compositions and methods are provided for prognostic classification of autoimmune disease patients into subtypes, which subtypes are informative of the patient's probability of developing clinical symptoms or severe disease. The patterns of circulating blood levels of serum cytokines provides for a signature pattern that can discriminate patients that have a high probability of developing disease from those that have a low probability of developing disease. Assessment of this signature pattern in a patient thus allows improved methods of care and intervention. In one embodiment of the invention, the autoimmune disease is rheumatoid arthritis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Controlled release topical testosterone formulations and methods
InactiveUS20150297733A1Constant effective testosterone blood levelEasy to useOrganic active ingredientsInorganic non-active ingredientsBlood levelControlled release
The present invention relates to testosterone topical formulations, especially high testosterone concentration formulations, such as between about 6% to about 15% w / w or higher, for the controlled release of testosterone into the systemic circulation of males and females for providing constant effective testosterone blood levels, without inducing undesired testosterone spike in blood levels or testosterone transference, following topical administration. The present invention also relates to methods and pre-filled multi-dose airless applicator systems for pernasal administration of the nasal testosterone gels of the present invention
Owner:ACERUS BIOPHARMA INC
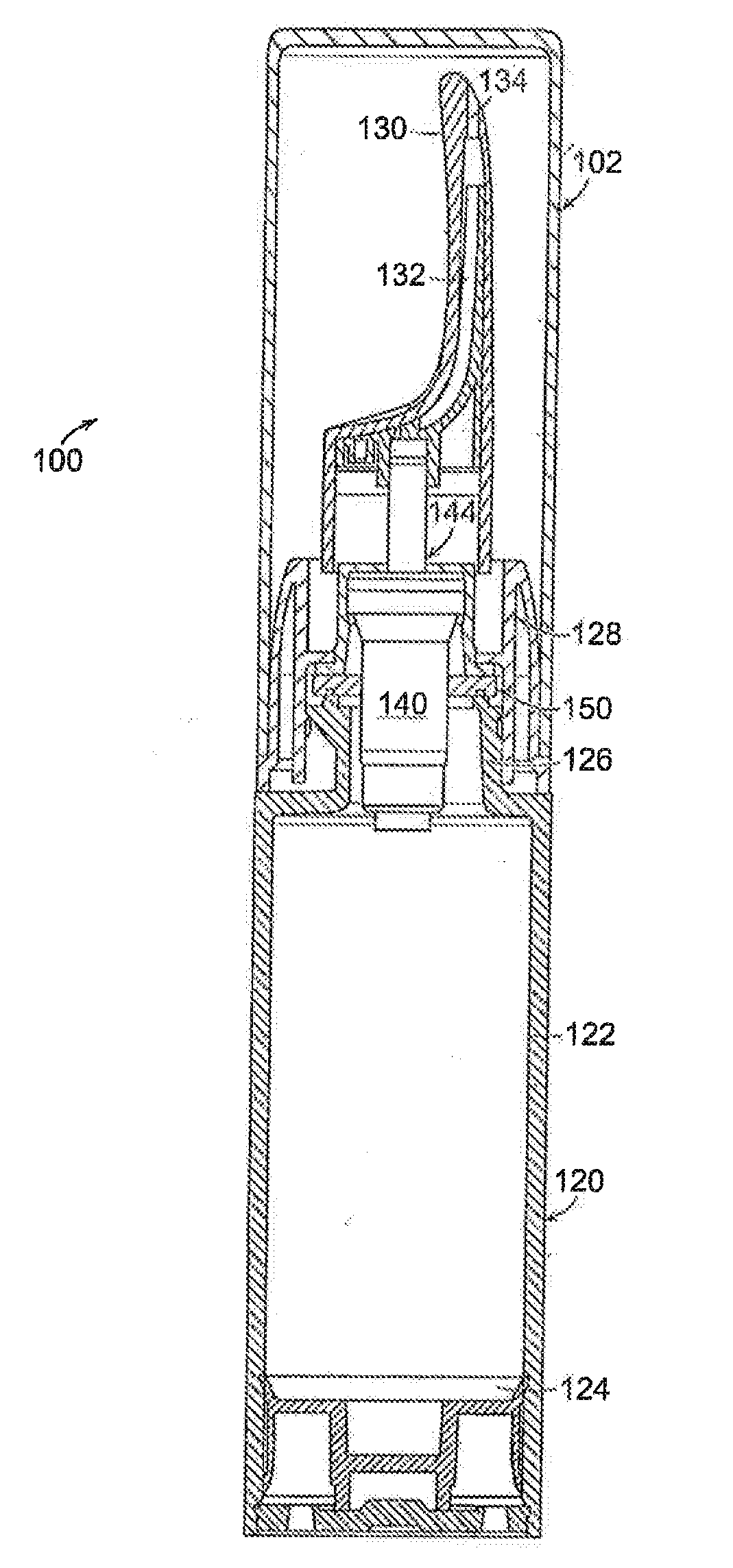
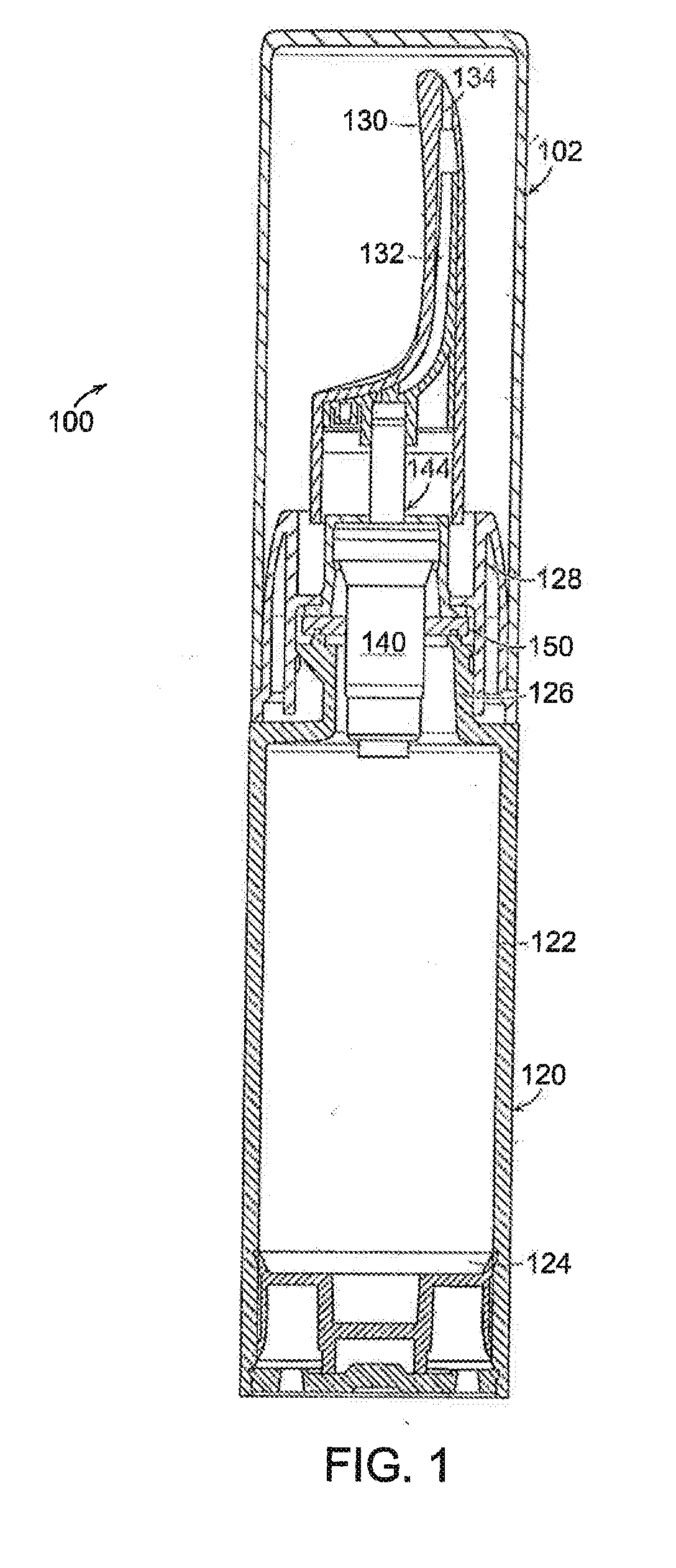
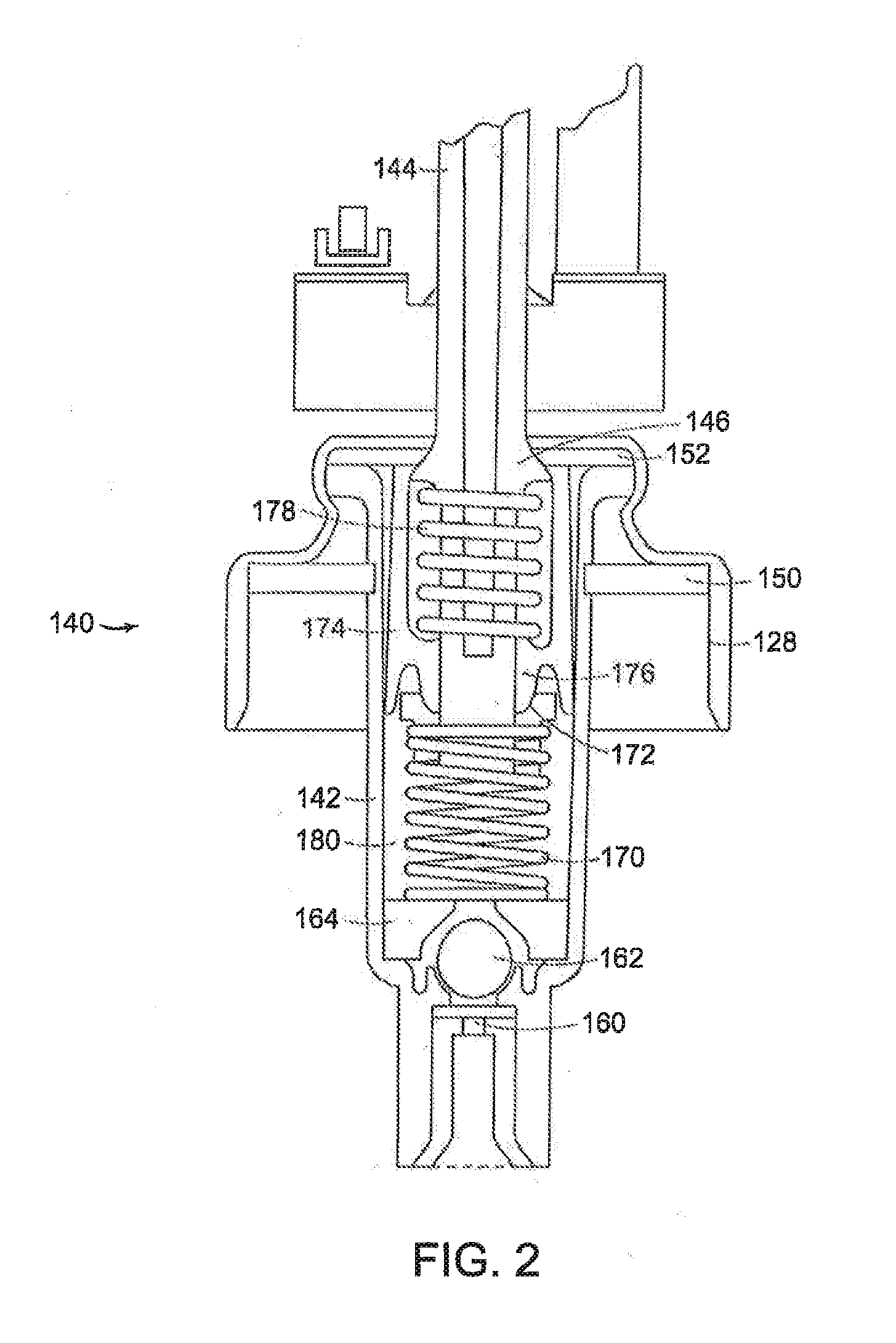
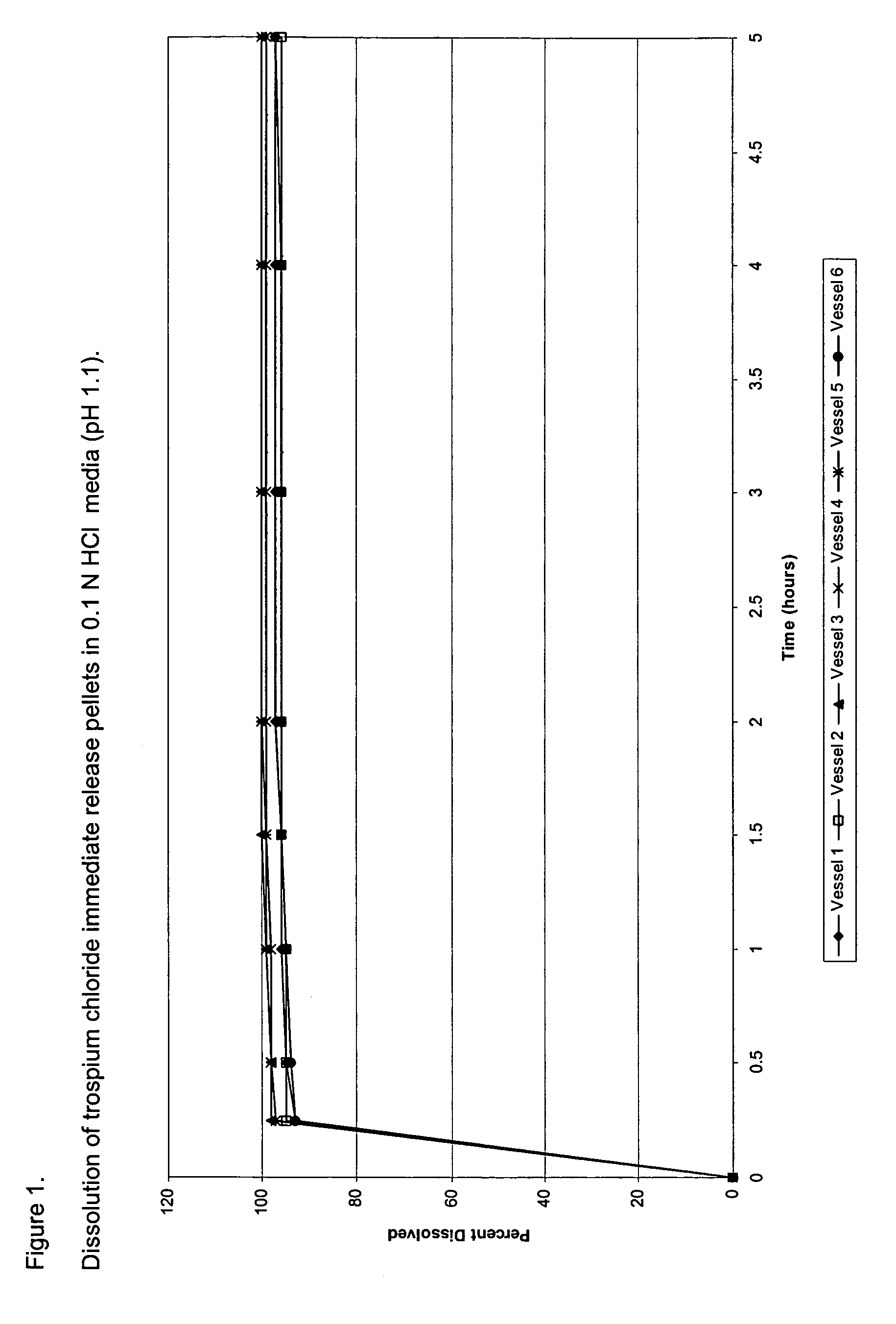
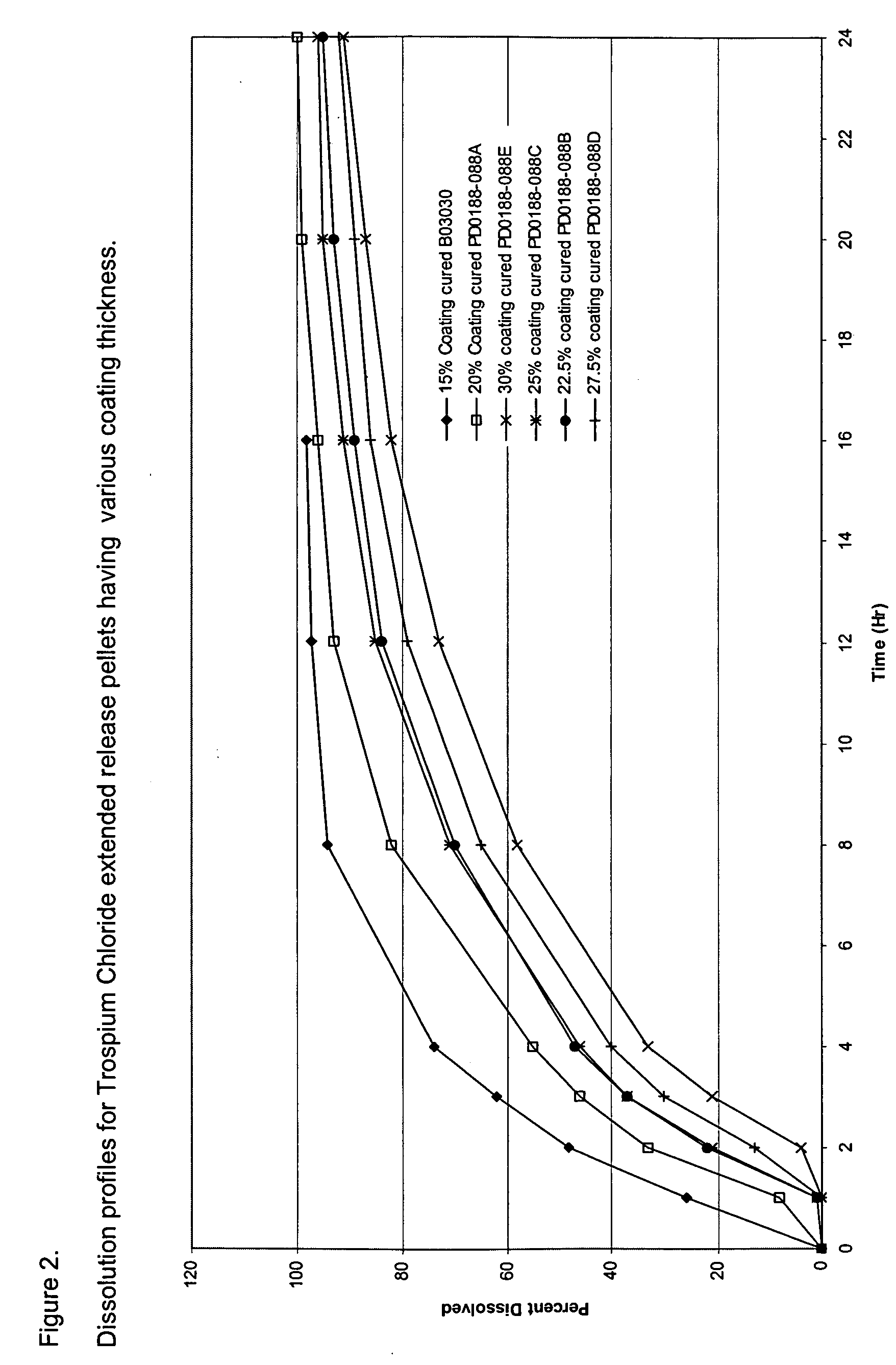
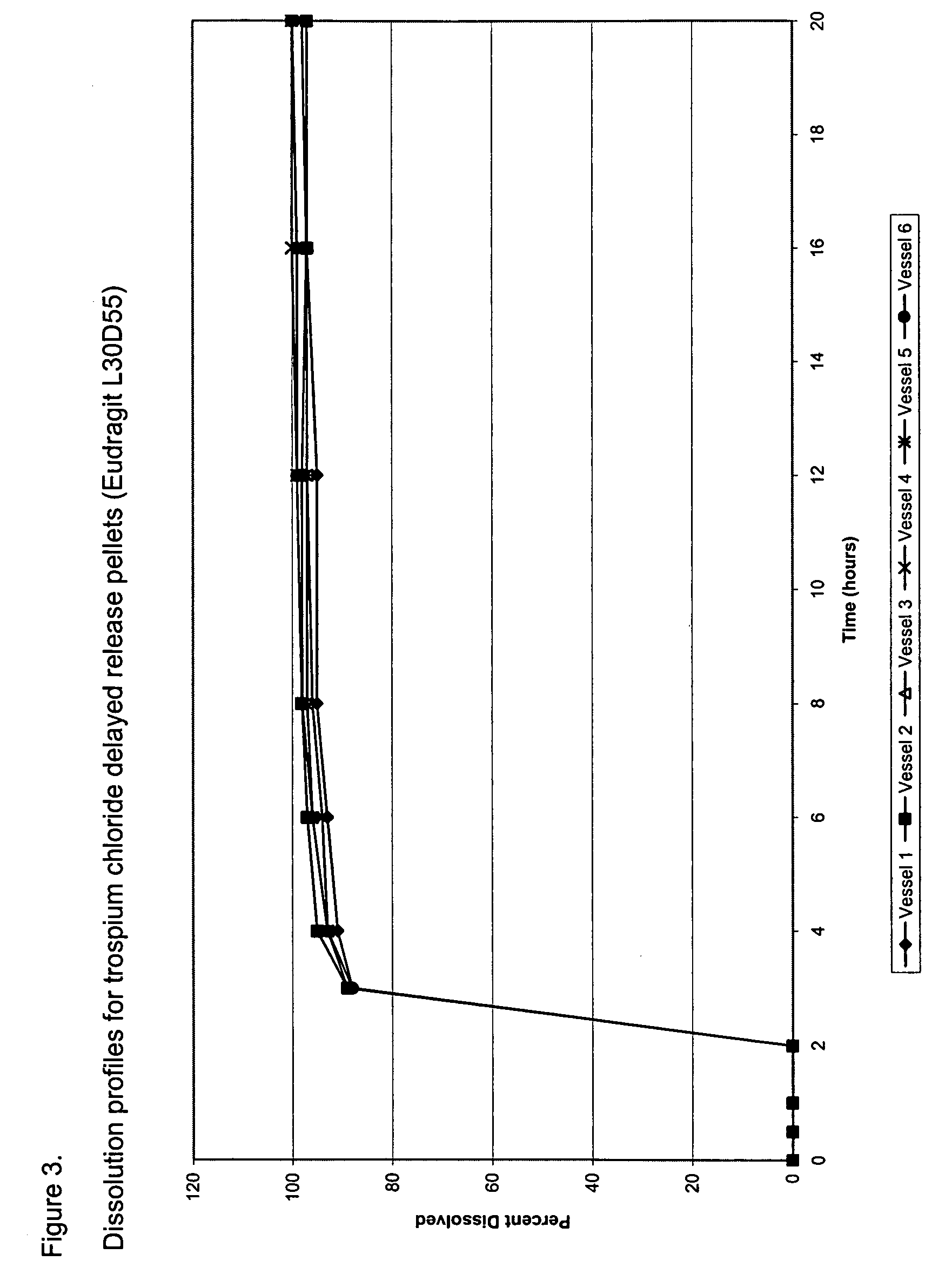
Once daily dosage forms of trospium
A pharmaceutical composition of a pharmaceutically acceptable trospium salt, with upon administration to a human patient generates an average steady state blood levels of trospium with a minimum (Cmin) and maximum (Cmax) blood levels of about 0.5-2.5 ng / ml and about 2.0-6.0 ng / ml, respectively.
Owner:TCD ROYALTY SUB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com