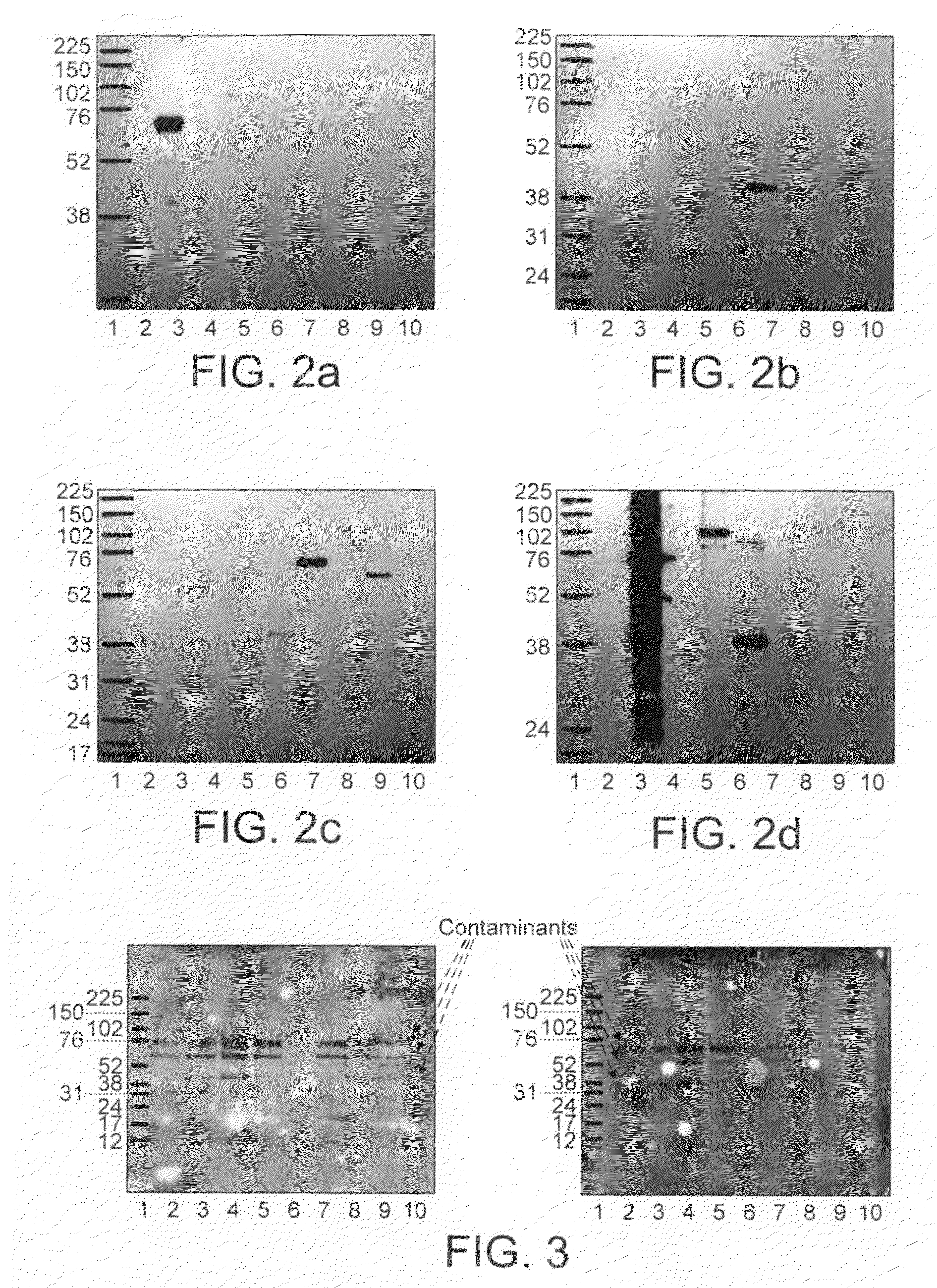
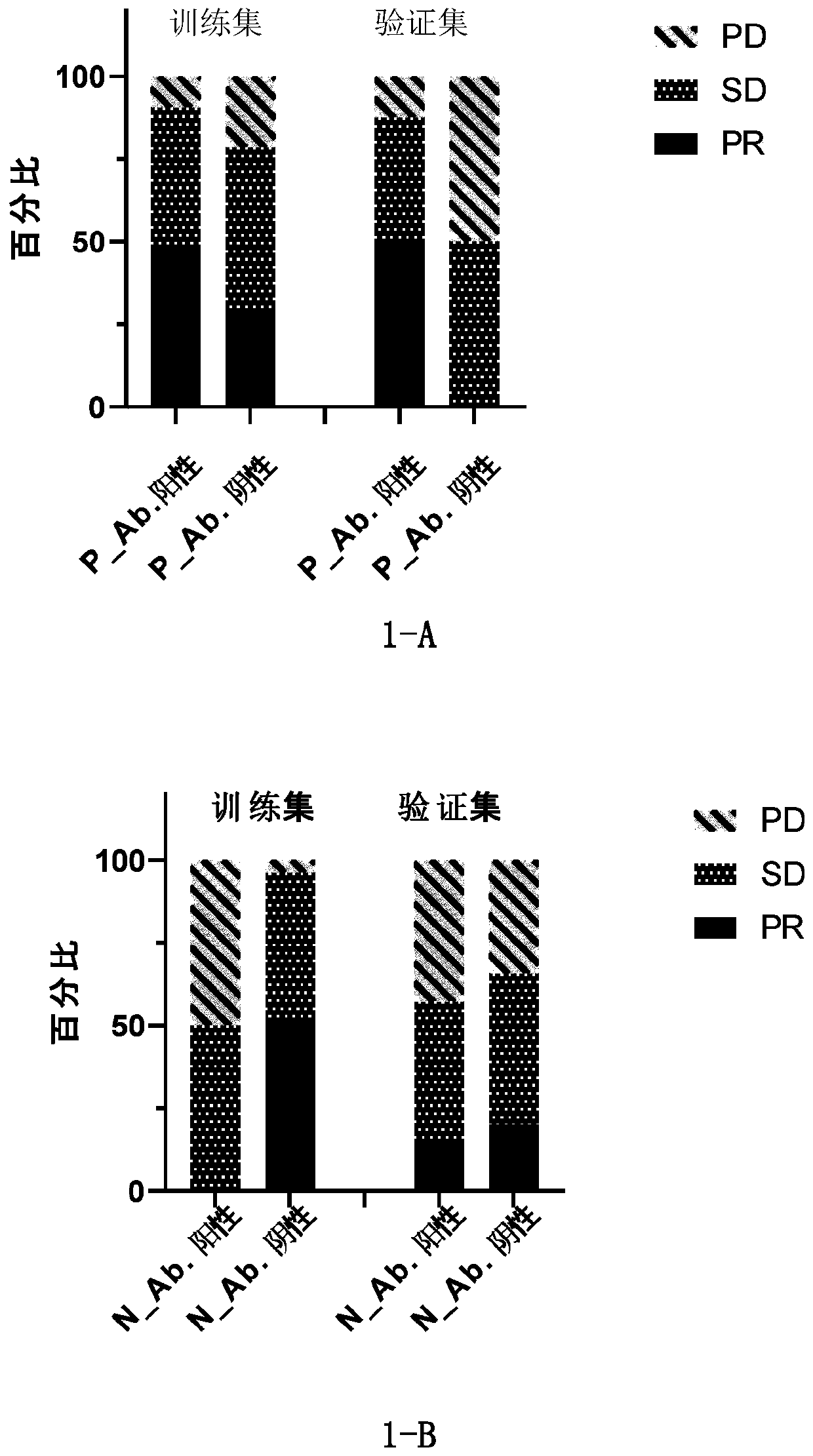
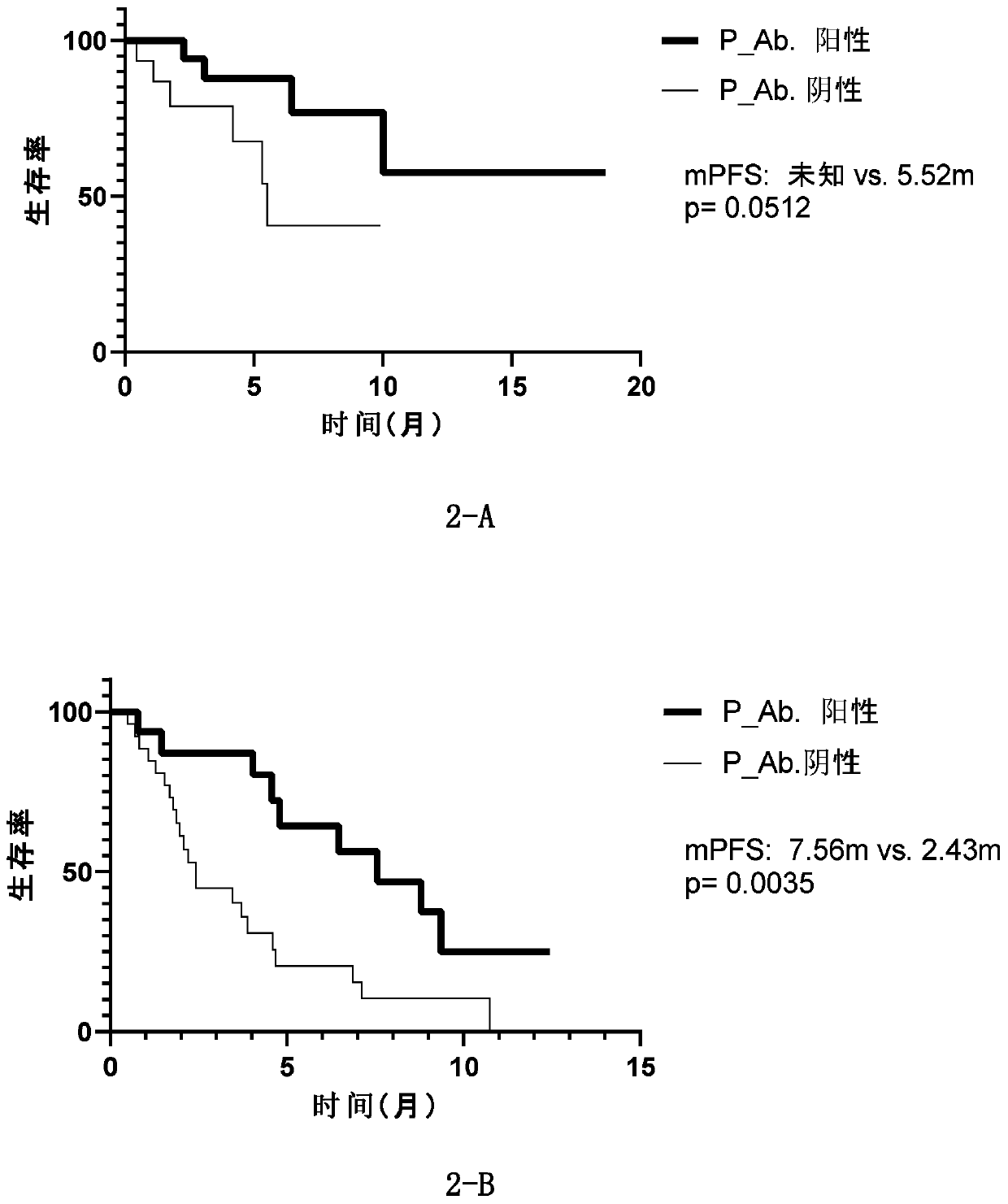
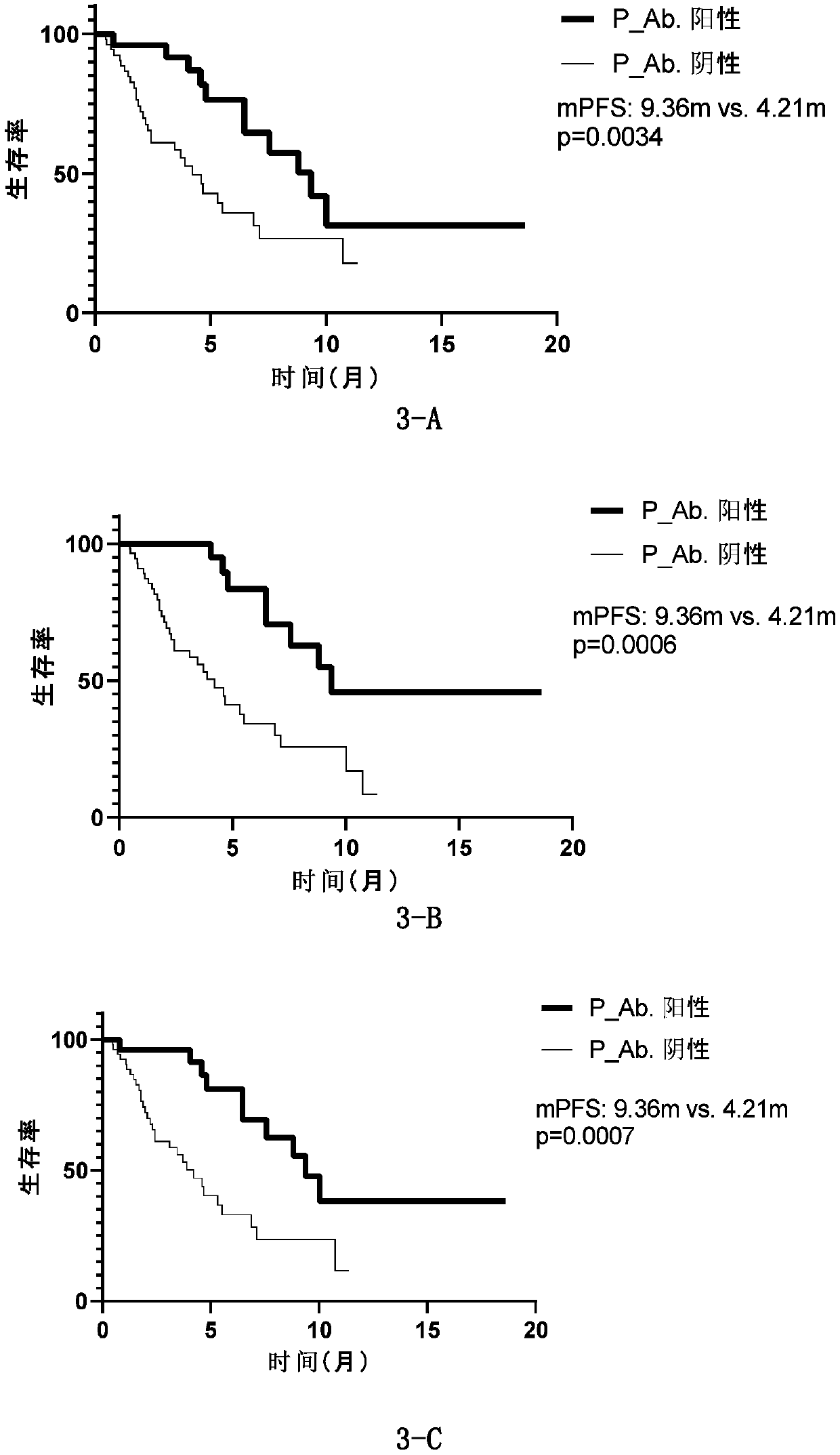
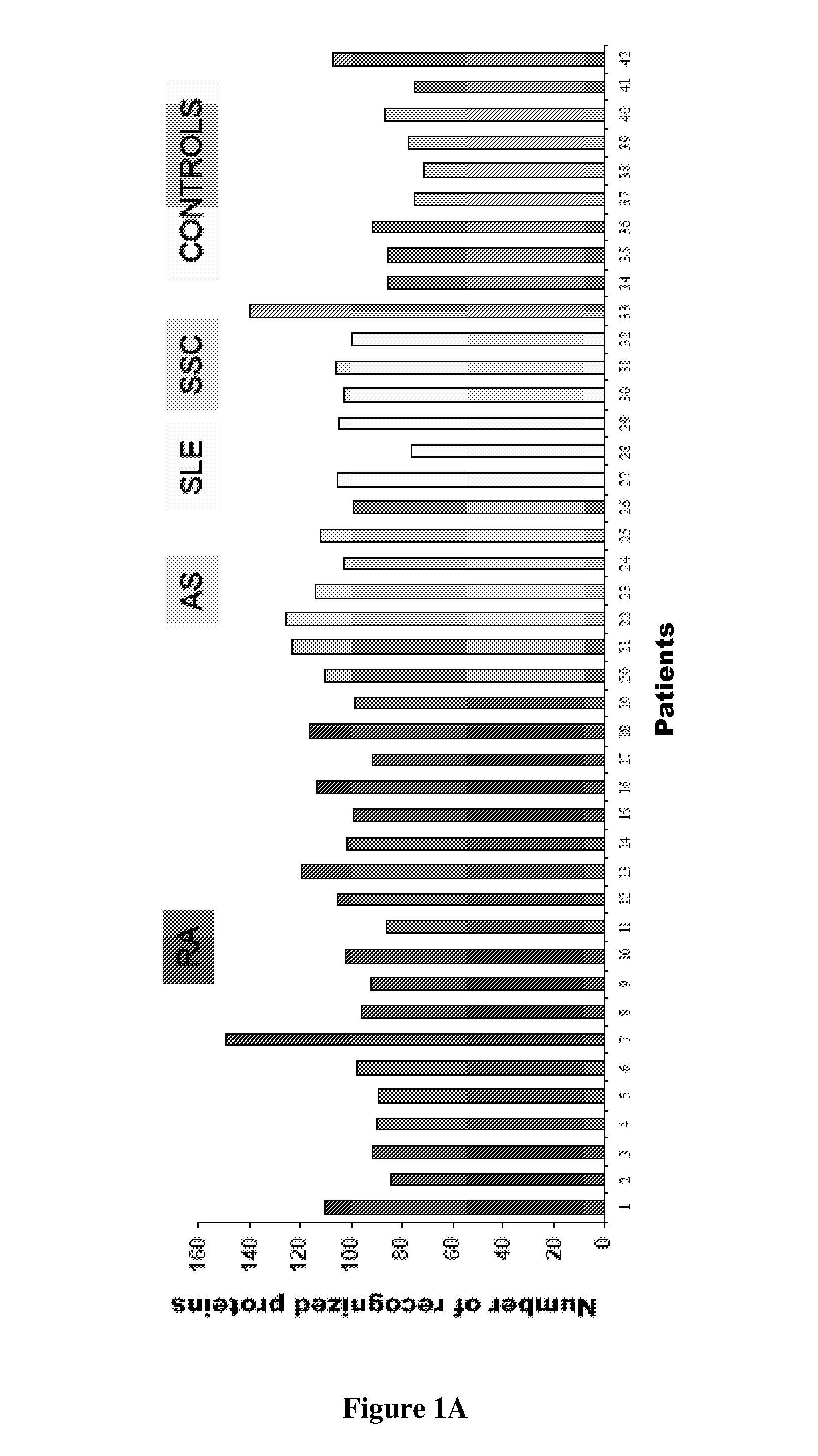
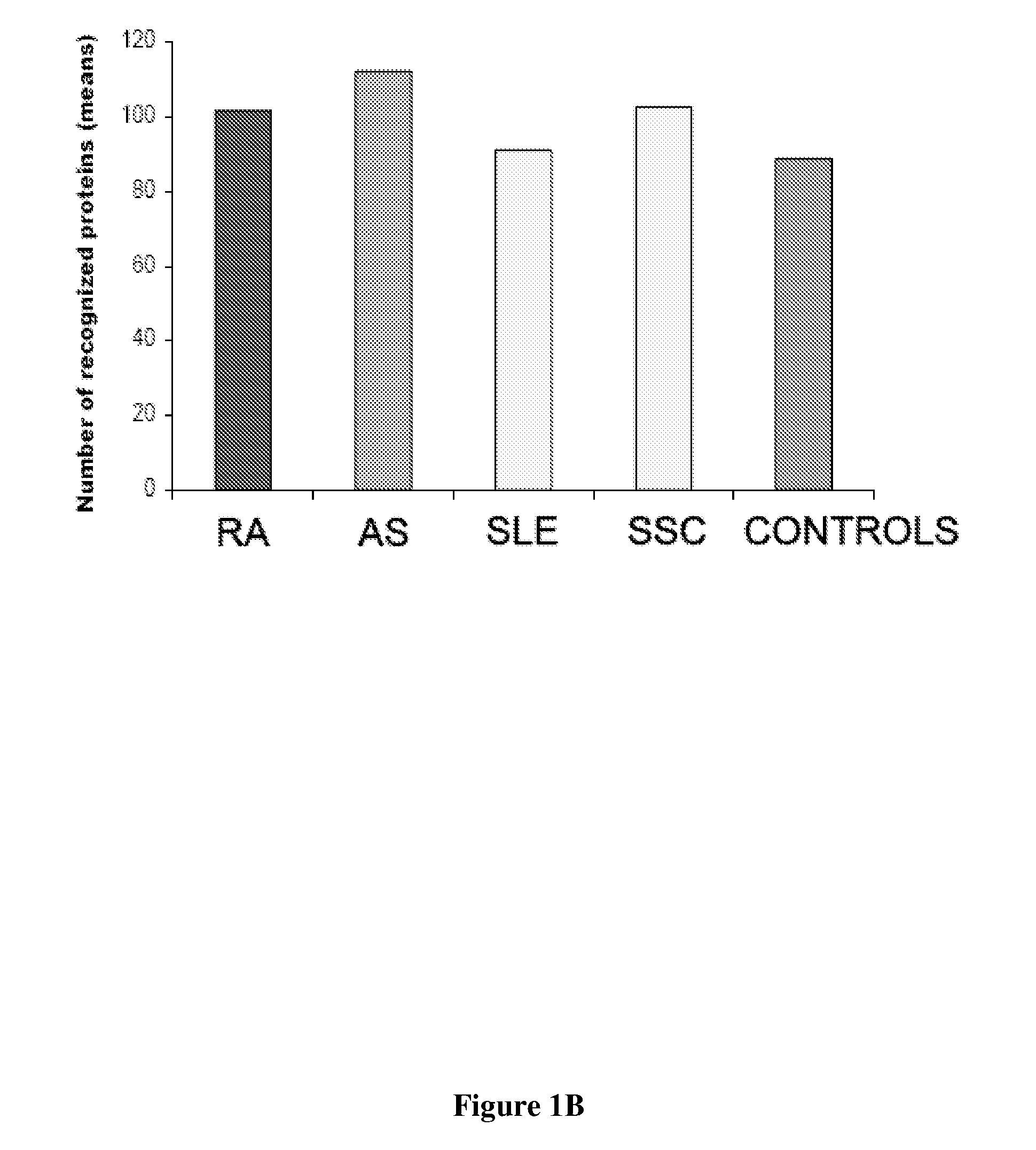
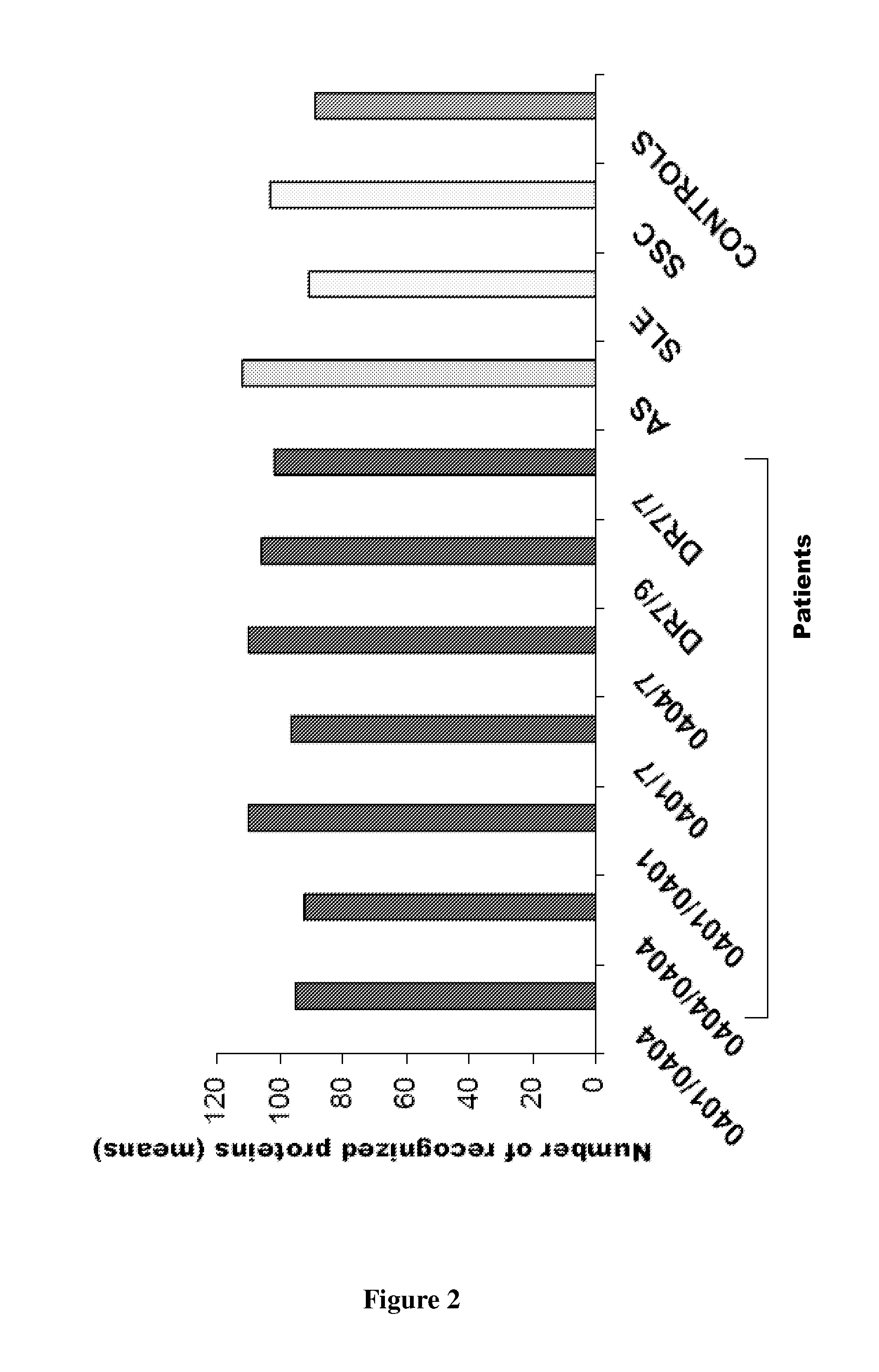
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
447 results about "Autoantibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An autoantibody is an antibody (a type of protein) produced by the immune system that is directed against one or more of the individual's own proteins. Many autoimmune diseases (notably lupus erythematosus) are caused by such autoantibodies.
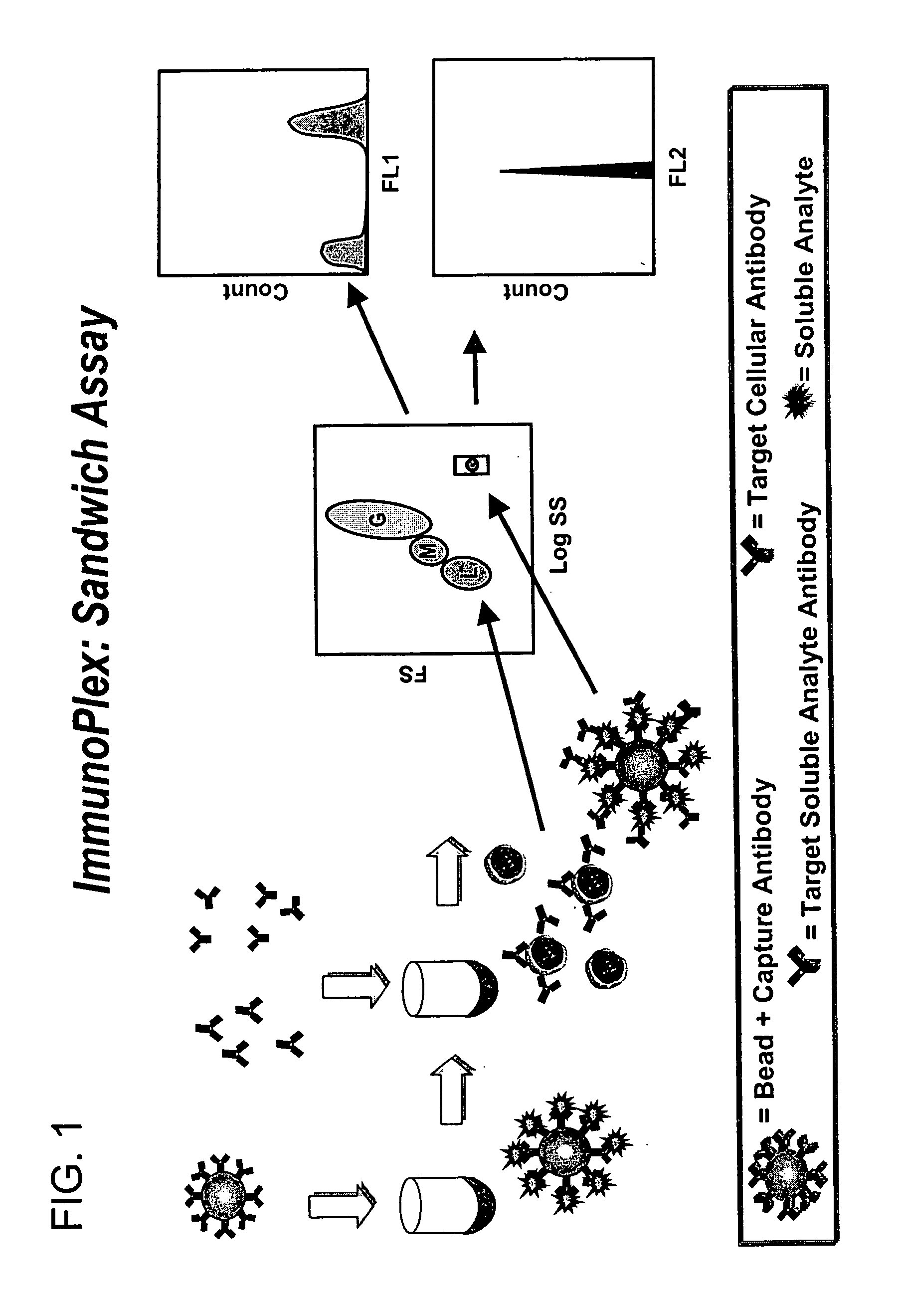
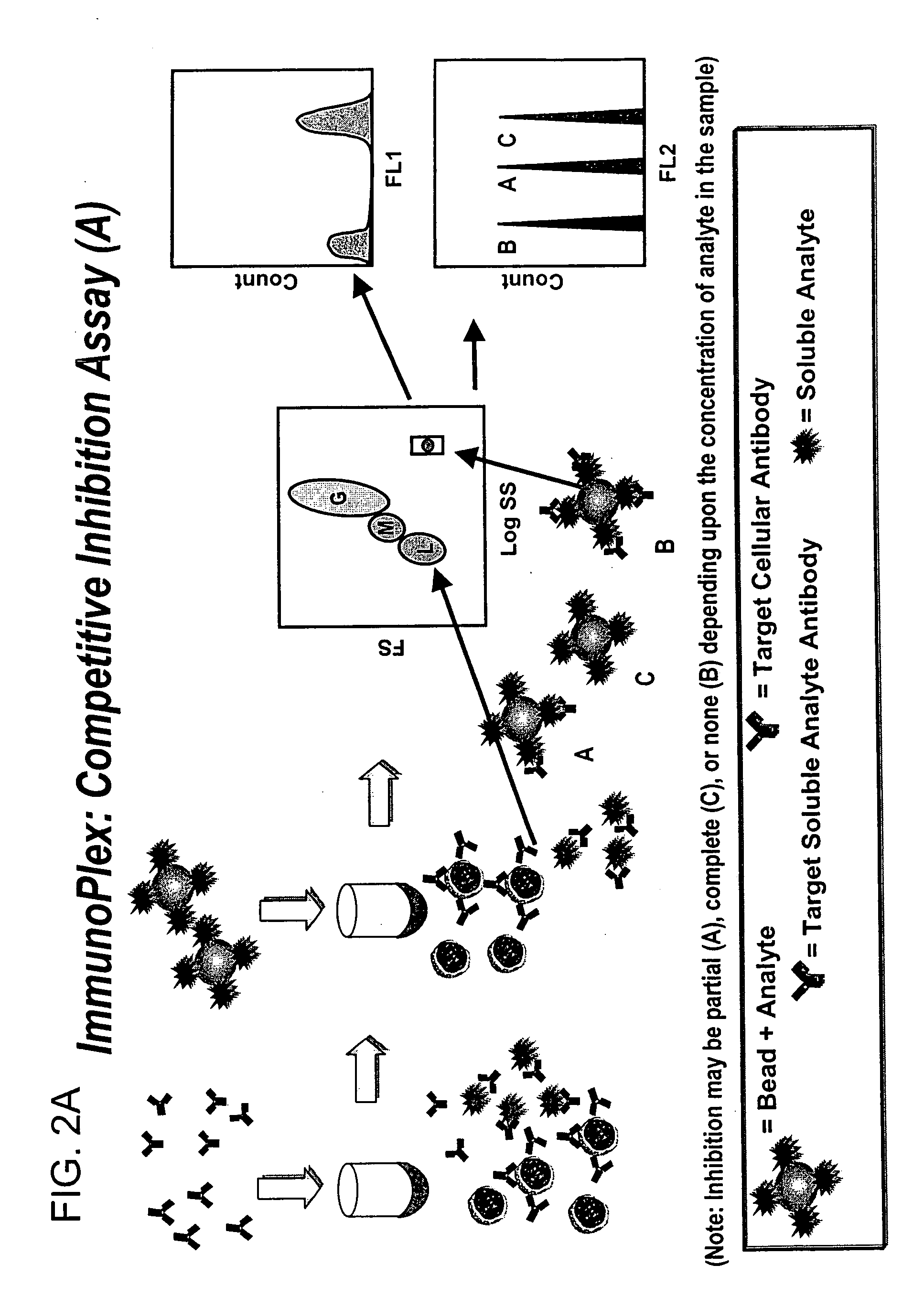
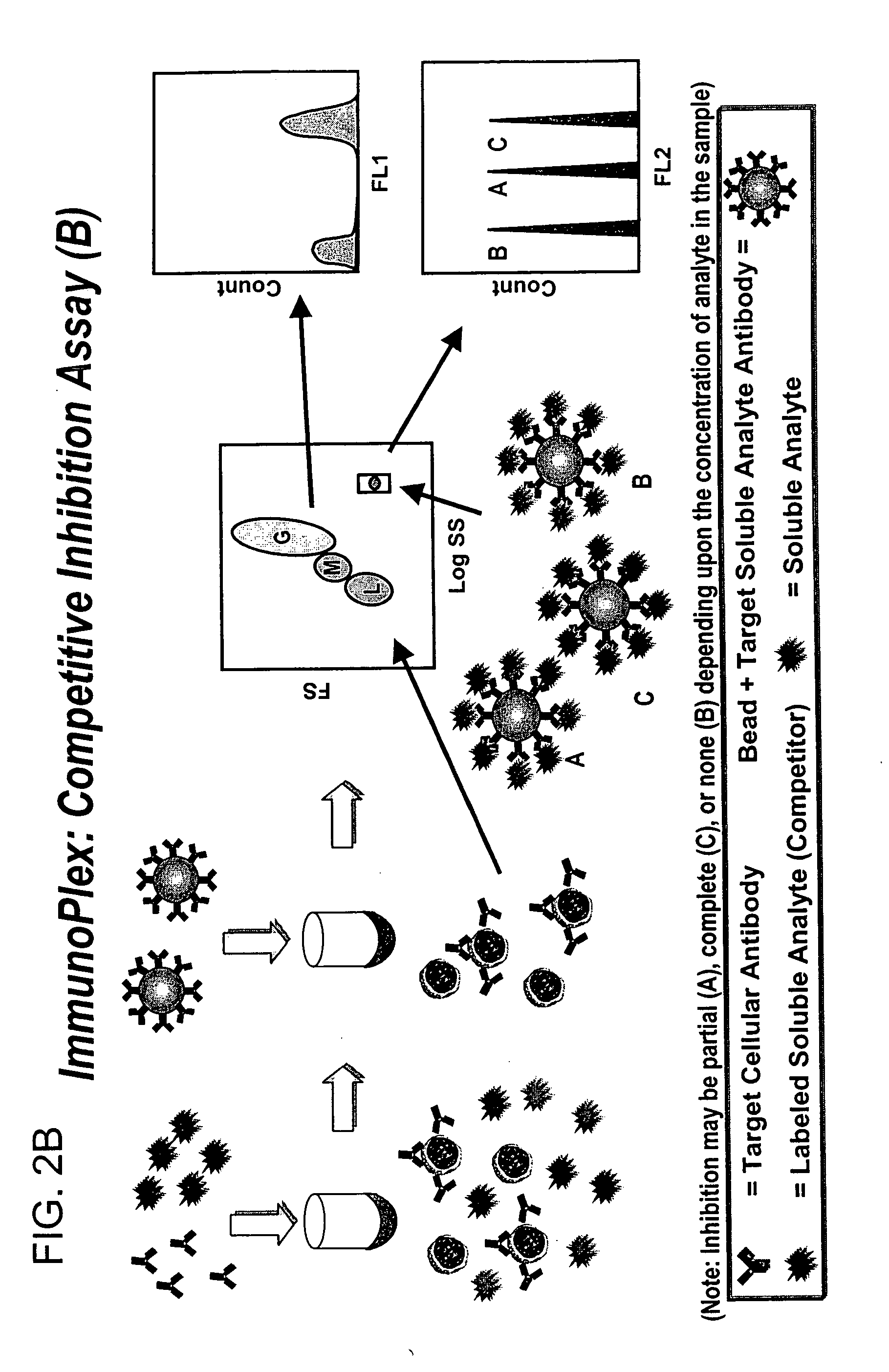
Methods for substantially simultaneous evaluation of a sample containing a cellular target and a soluble analyte
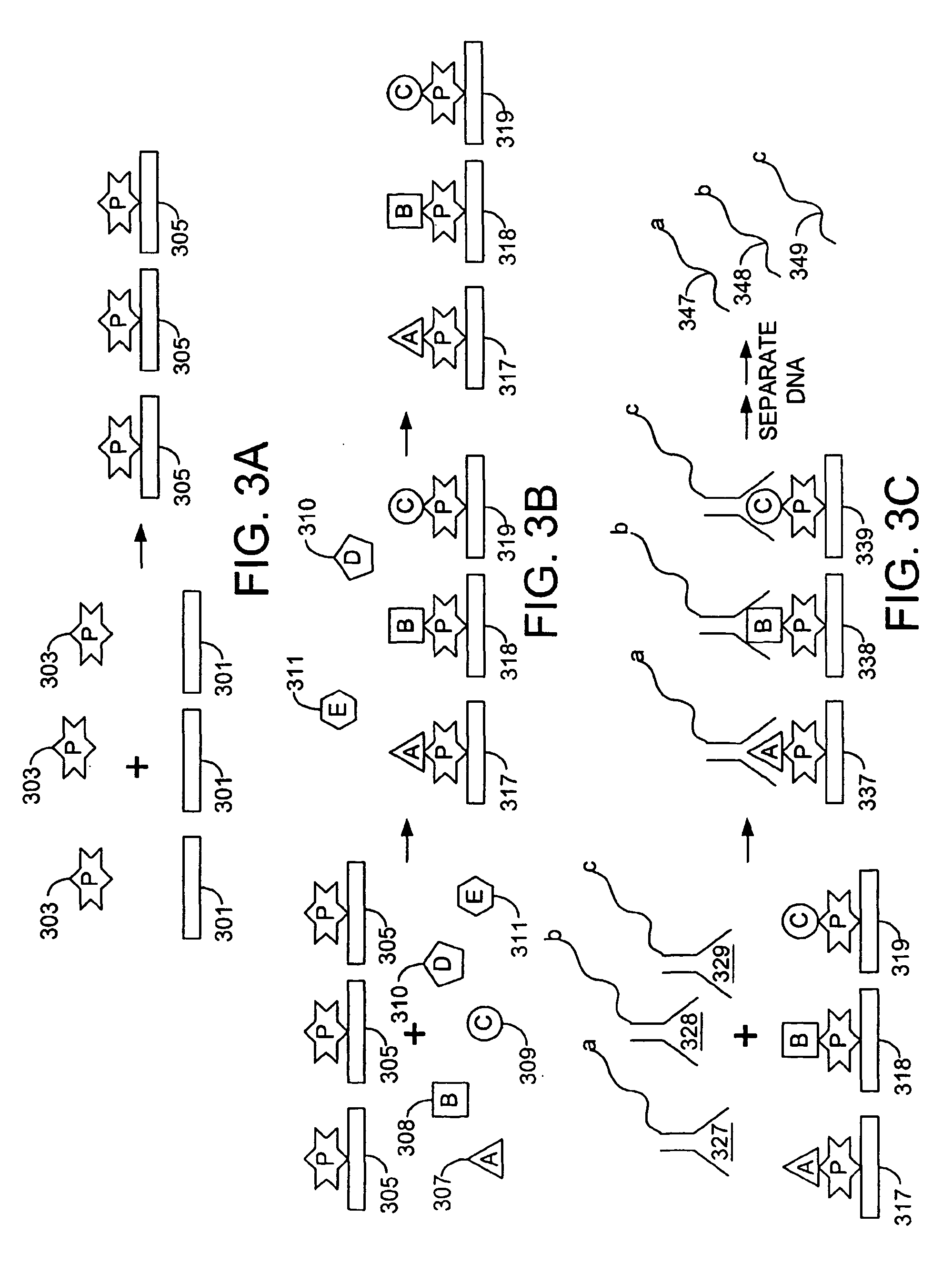
Methods are provided for monitoring treatment of a subject using heparin therapy or a ligand, such as an CD20 or CD52 antibody, that binds to a cellular marker and to which the subject may develop masking reactions or auto-antibodies that inhibit evaluation of treatment. The methods involve adding to a single container a sample containing cells that express the cellular target with (i) a soluble ligand that binds the cellular target, (ii) a soluble ligand that binds the soluble analyte or a competing soluble analyte; and (iii) a capture medium that binds directly to the soluble analyte, indirectly to the soluble analyte, or to the soluble ligand that binds to the soluble analyte. Complexes formed in the container by interaction of these components are substantially simultaneously analyzed and quantitated without physically separating the complexes. Kits for performing the assays are also provided.
Owner:BECKMAN COULTER INC
Cancer Detection Methods and Reagents
InactiveUS20110086061A1High sensitivityViral antigen ingredientsSnake antigen ingredientsAntigenAutoantibody
The present invention comprises methods and compositions for detecting cancer in an individual comprising autoantibodies to cancer-associated antigens. Specifically, the present invention comprises methods and compositions for detecting autoantibodies to cancer-associated antigen in a bodily fluid as well as use of said autoantibodies as a means to detect the presence of cancer-associated antigens.
Owner:ONCIMMUNE
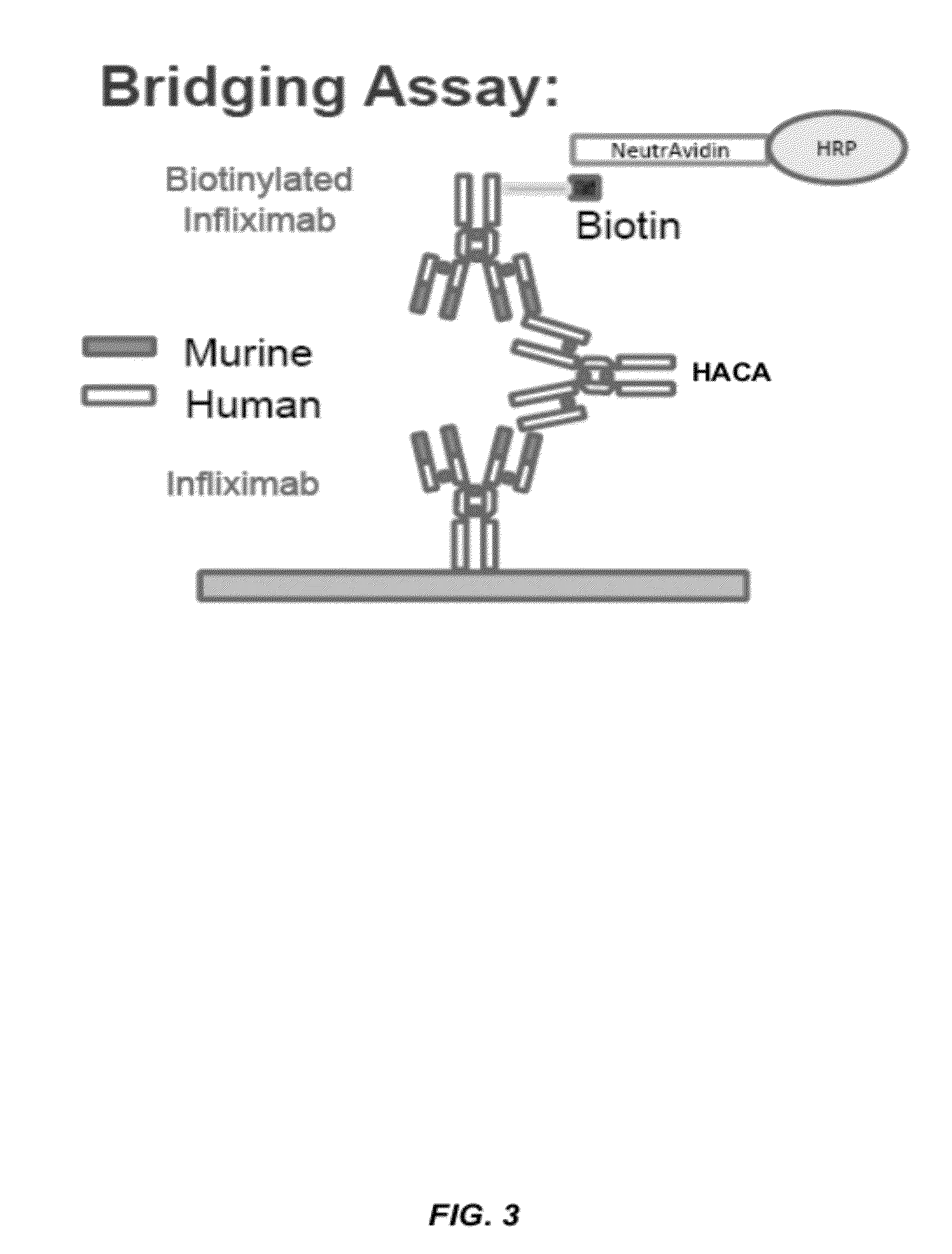
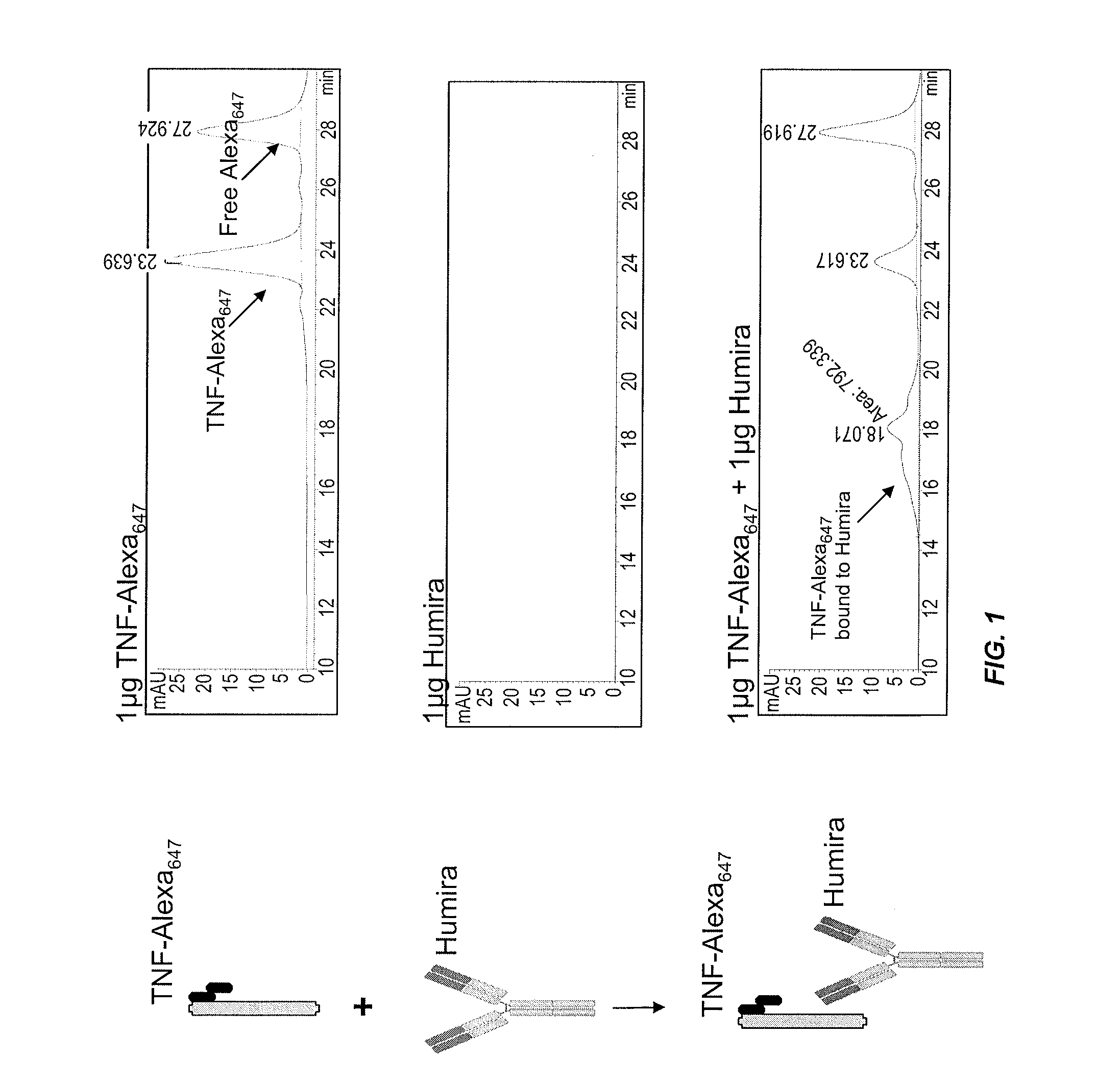
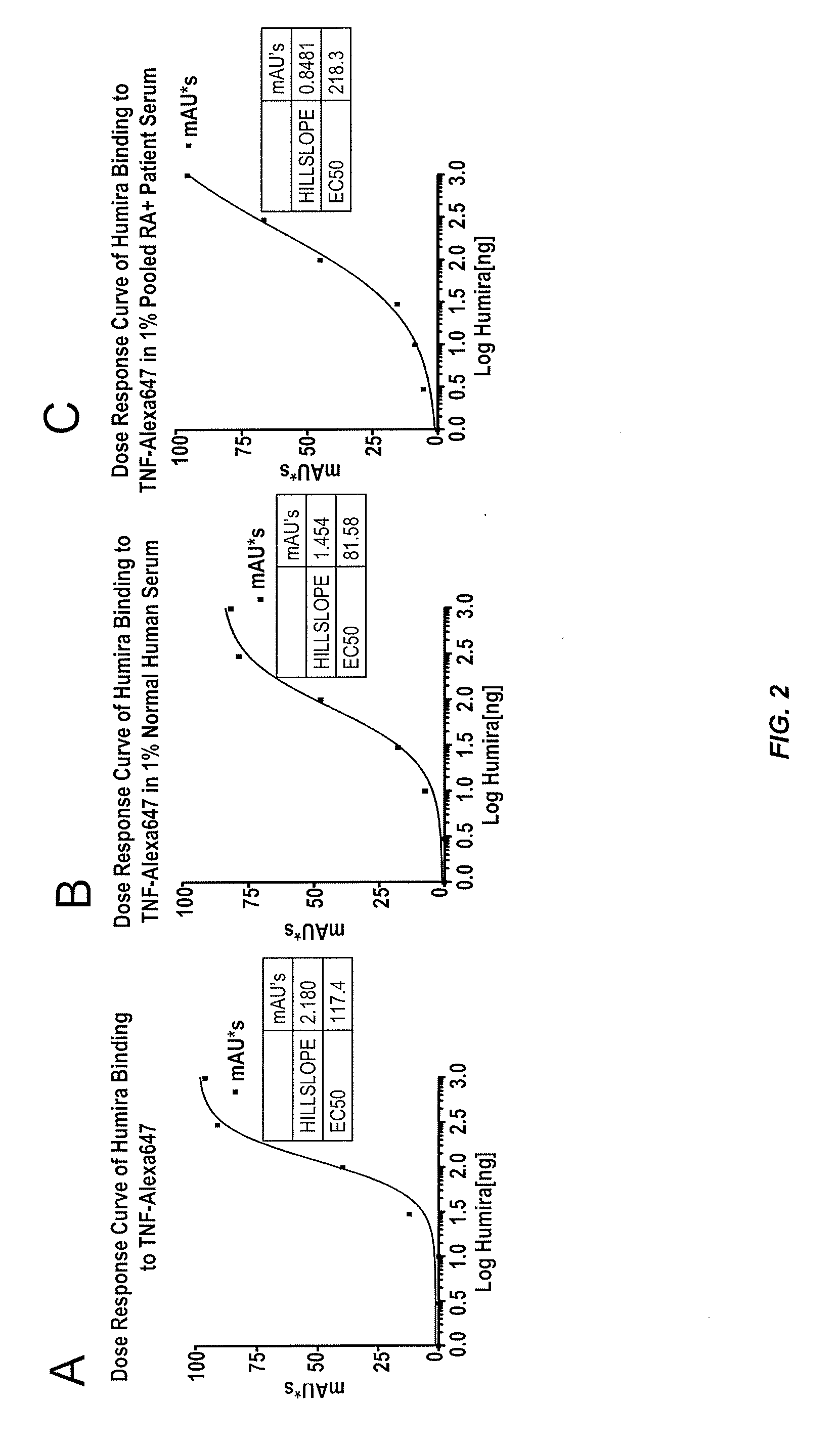
Assays for the detection of anti-TNF drugs and autoantibodies
ActiveUS8574855B2Convenient treatmentLow toxicityAntibacterial agentsSenses disorderAutoantibodyDrug treatment
Owner:PROMETHEUS LAB
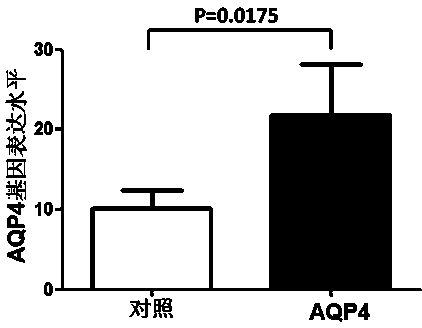
Detection method of aquaporin-4 autoantibody, fusion expression virus vector and application thereof
InactiveCN103937836ATime consumingGreat loss of manpower and material resourcesIndividual particle analysisBiological testingAutoantibodyViral vector
The invention discloses a detection method of an aquaporin-4 autoantibody, a fusion expression virus vector and application thereof. The virus vector is pCDH-MCS-AQP4-EF1-copGFP, the nucleotide sequence of which is shown as a sequence table SEQIDNO.2. The invention further discloses application of the fusion expression virus vector in improving the clinical diagnosis and treatment level of ophthalmoneuromyelitis and multiple sclerosis. By adopting immunofluorescent cytochemical staining with higher sensitivity and specificity combined with a flow cytometric detection technology, the AQP-4 antibody is quantitively and qualitatively detection clinically, so that the detection result is more stable, and the labor and the time cost are extremely saved. The project is established to extremely improve the diagnosis and treatment level of ophthalmoneuromyelitis and multiple sclerosis, so that the labor capacity and the living quality of the patient are improved, and huge social and economic benefits are generated.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
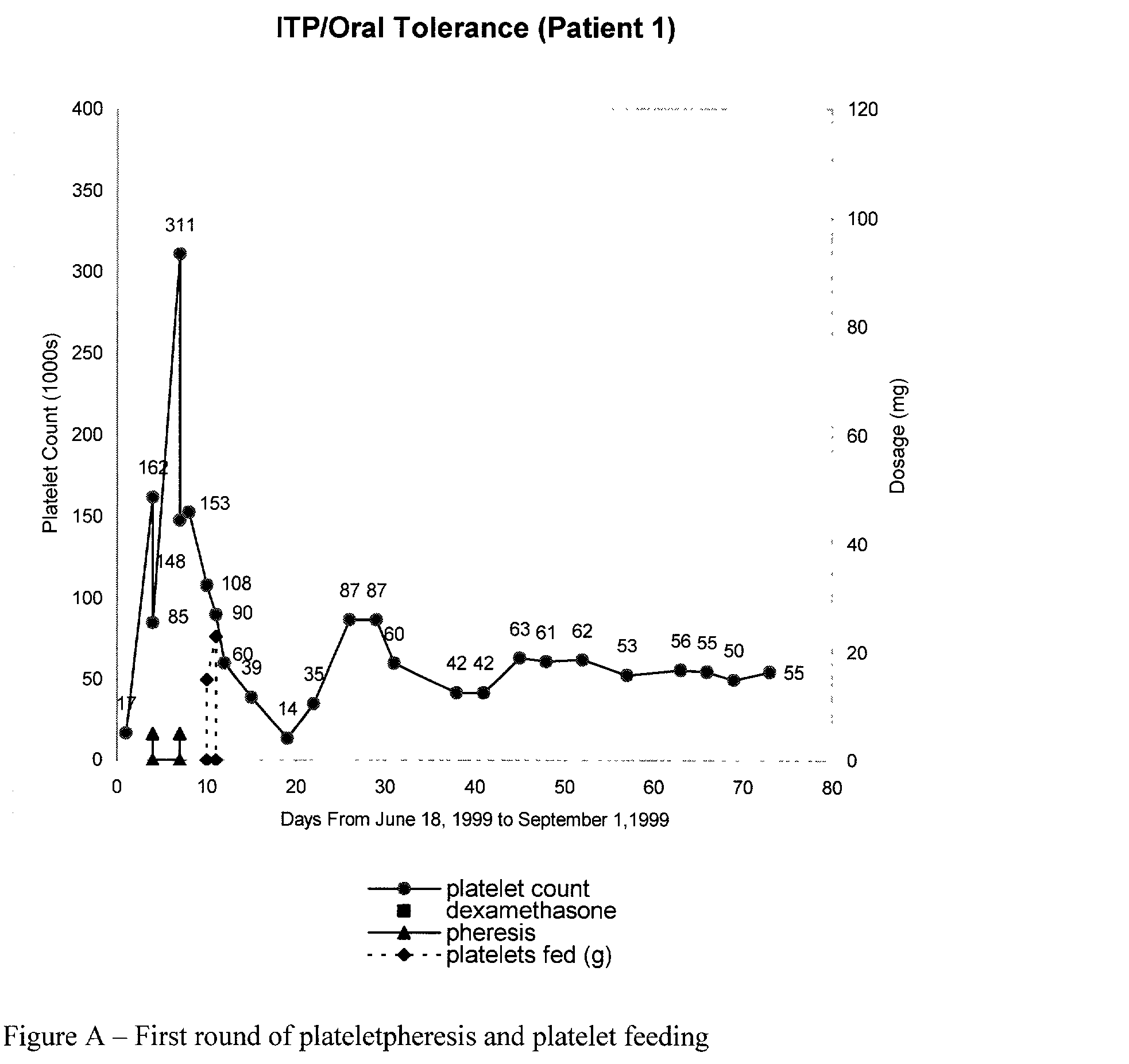
Oral tolerance using allogeneic platelets in ITP
ITP may be treated using a method that involves identifying the autoimmune response, collecting allogeneic platelets, sterilizing allogeneic platelets and feeding them orally. Auto-antigens contained on the surface of allogeneic platelets administered to the intestines deactivate or delete lymphocytes responsible for auto-antibody production. This treatment can be used for ITP, specifically targets the cause of the disease and provides the possibility of a sustained response without further medication.
Owner:KASHA JOHN R JR
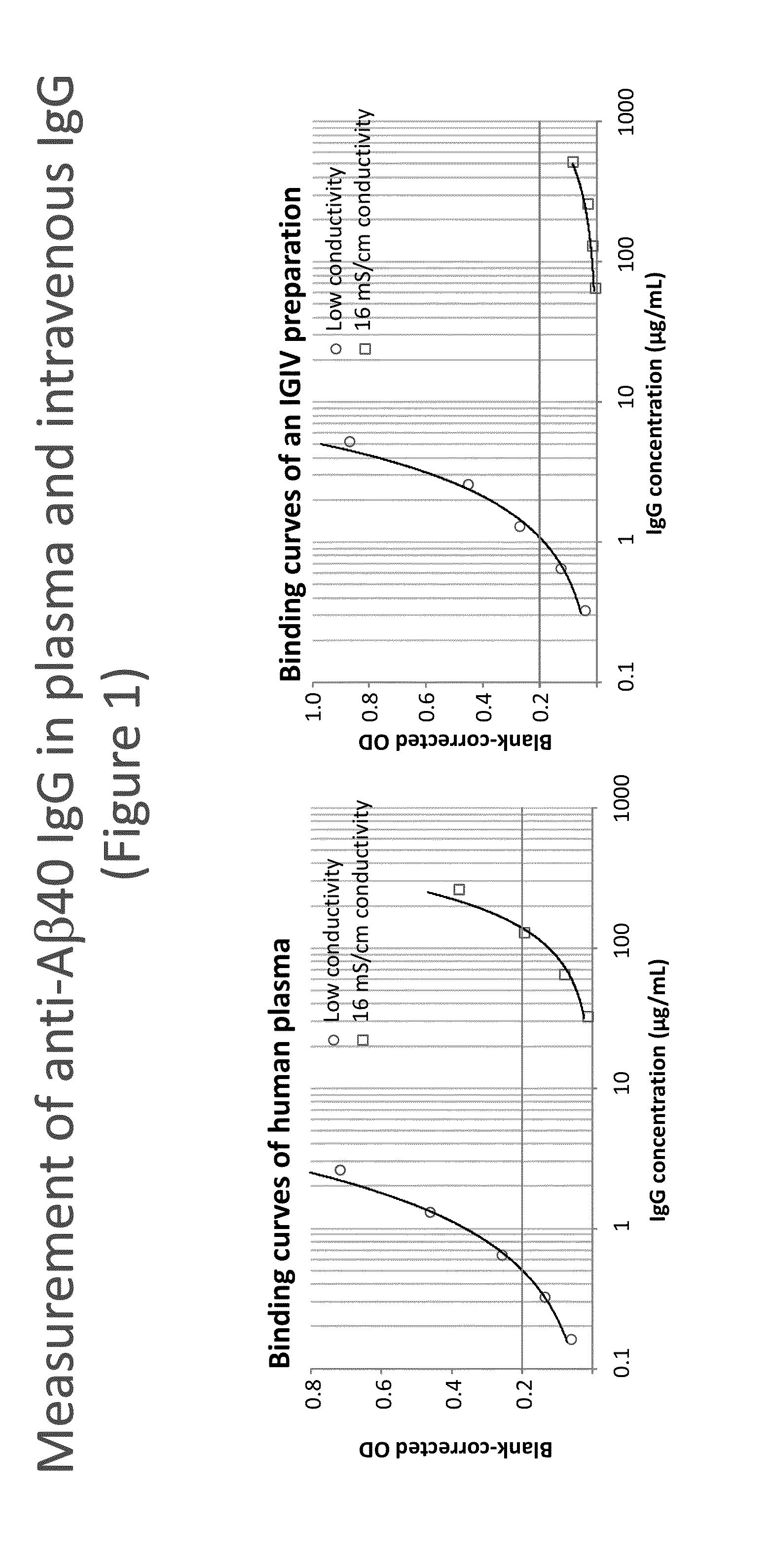
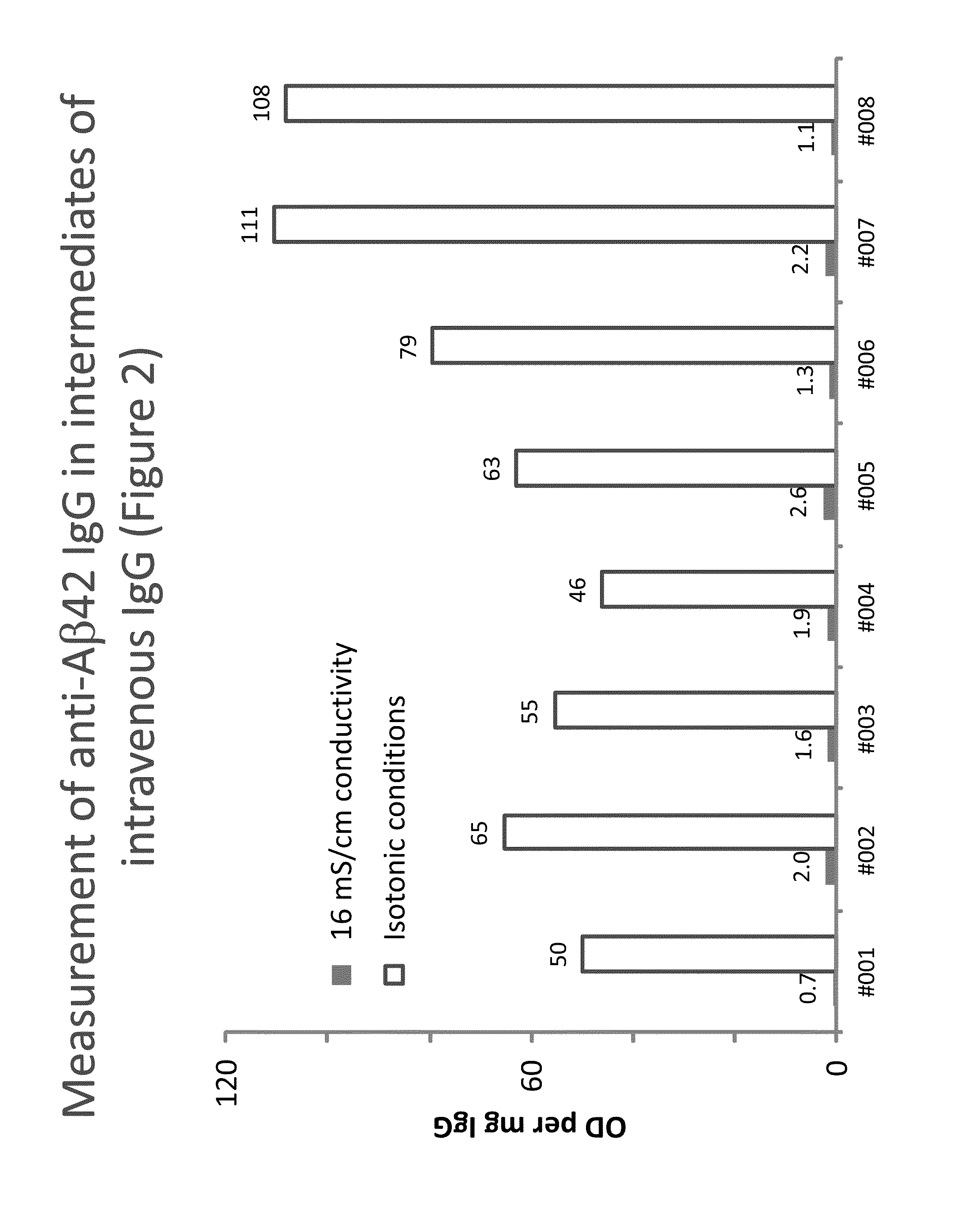
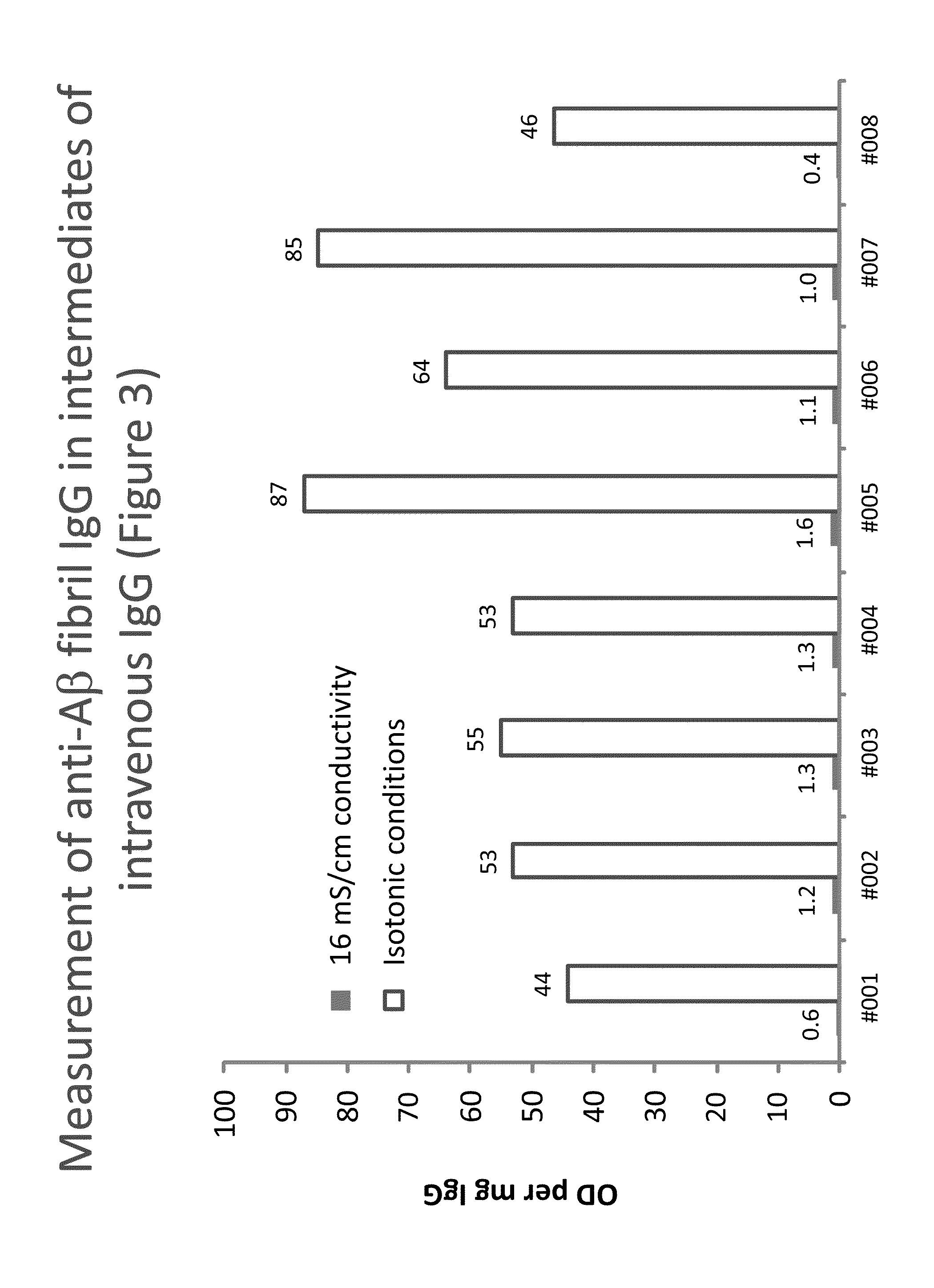
Measurement of autoantibodies at low conductivity with increased sensitivity
ActiveUS20130149700A1High sensitivityReduce conductivityMicrobiological testing/measurementBiological material analysisAutoantibodyBiochemistry
Methods for detecting or capturing low-avidity autoantibodies in a biological sample are provided. Target antigen used to assay for the low-avidity autoantibodies of interest is immobilized on a solid phase. The biological sample is contacted under low conductivity condition with the target antigen for which the autoantibodies has specific binding affinity. Binding of the target antigen to the autoantibodies of interest in the biological sample is then detected to ascertain the presence or concentration of the autoantibodies of interest.
Owner:TAKEDA PHARMA CO LTD
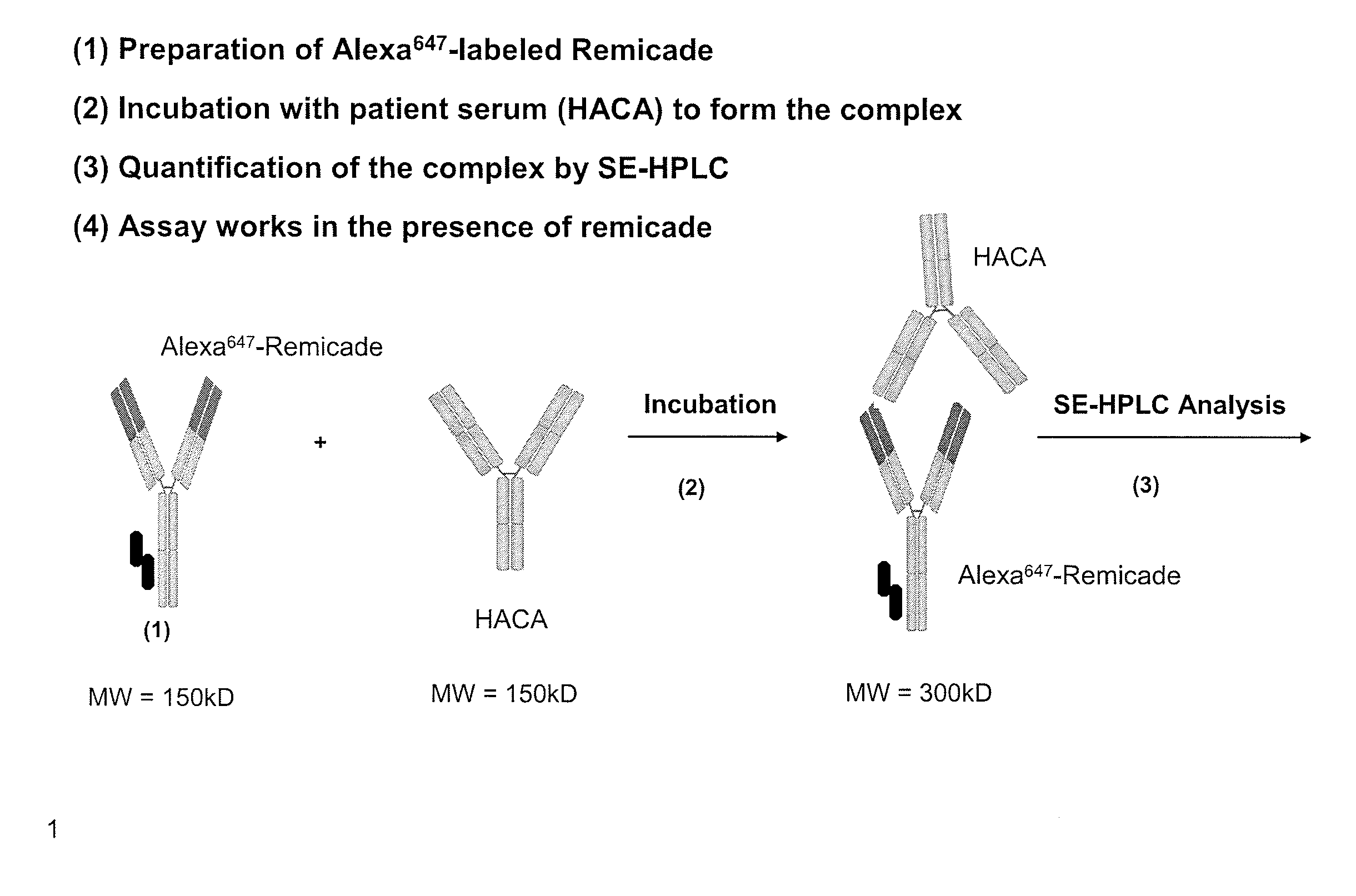
Methods and kits for monitoring resistance to therapeutic agents
InactiveUS20050059023A1Reduces and eliminates therapeutic efficacy of therapeuticAvoid adverse reactionsMicrobiological testing/measurementBiological testingAntibodyNatural substance
The present invention relates to novel methods and kits for monitoring the therapeutic inactivating capacity of a subject. Moreover, the present invention further relates to methods and kits for determining and / or monitoring a therapeutic protocol for a subject afflicted with auto antibodies specific for a natural substance, wherein these auto antibodies develop as a result of therapeutic administration of the natural substance or an analog thereof. These methods and kits can be used, for example, to initiate, terminate, or adjust the level of administration of any of a variety of therapeutic agents.
Owner:SCANTIBODIES LAB
Assays for detecting autoantibodies to Anti-tnfalpha drugs
InactiveUS20140045276A1Low toxicityConvenient treatmentDisease diagnosisBiological testingDiseaseMedicine
The present invention provides assays for detecting and measuring the presence or level of autoantibodies to anti-TNFα drug therapeutics in a sample. The present invention is useful for optimizing therapy and monitoring patients receiving anti-TNFα drug therapeutics to detect the presence or level of autoantibodies against the drug. The present invention also provides methods for selecting therapy, optimizing therapy, and / or reducing toxicity in subjects receiving anti-TNFα drugs for the treatment of TNFα-mediated disease or disorders.
Owner:NESTEC SA +1
Calibrator For Immunoassays
Owner:ONCIMMUNE
Antigen protein combination for detection, diagnosis or risk prediction of Alzheimer, and kit containing antigen protein combination
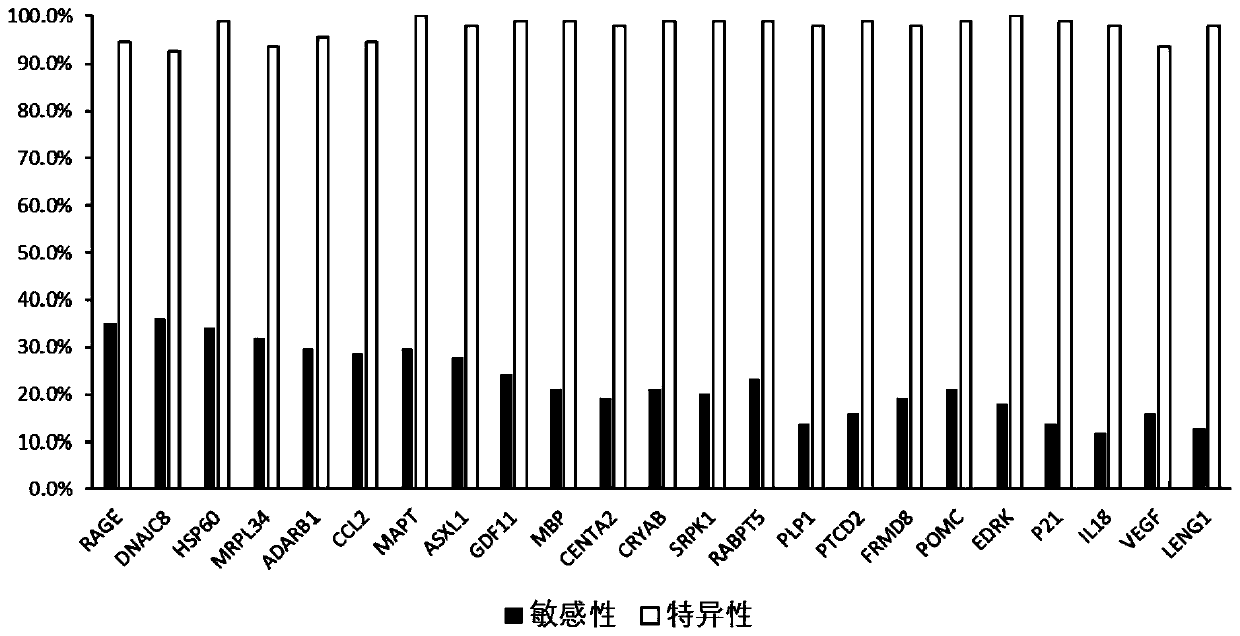
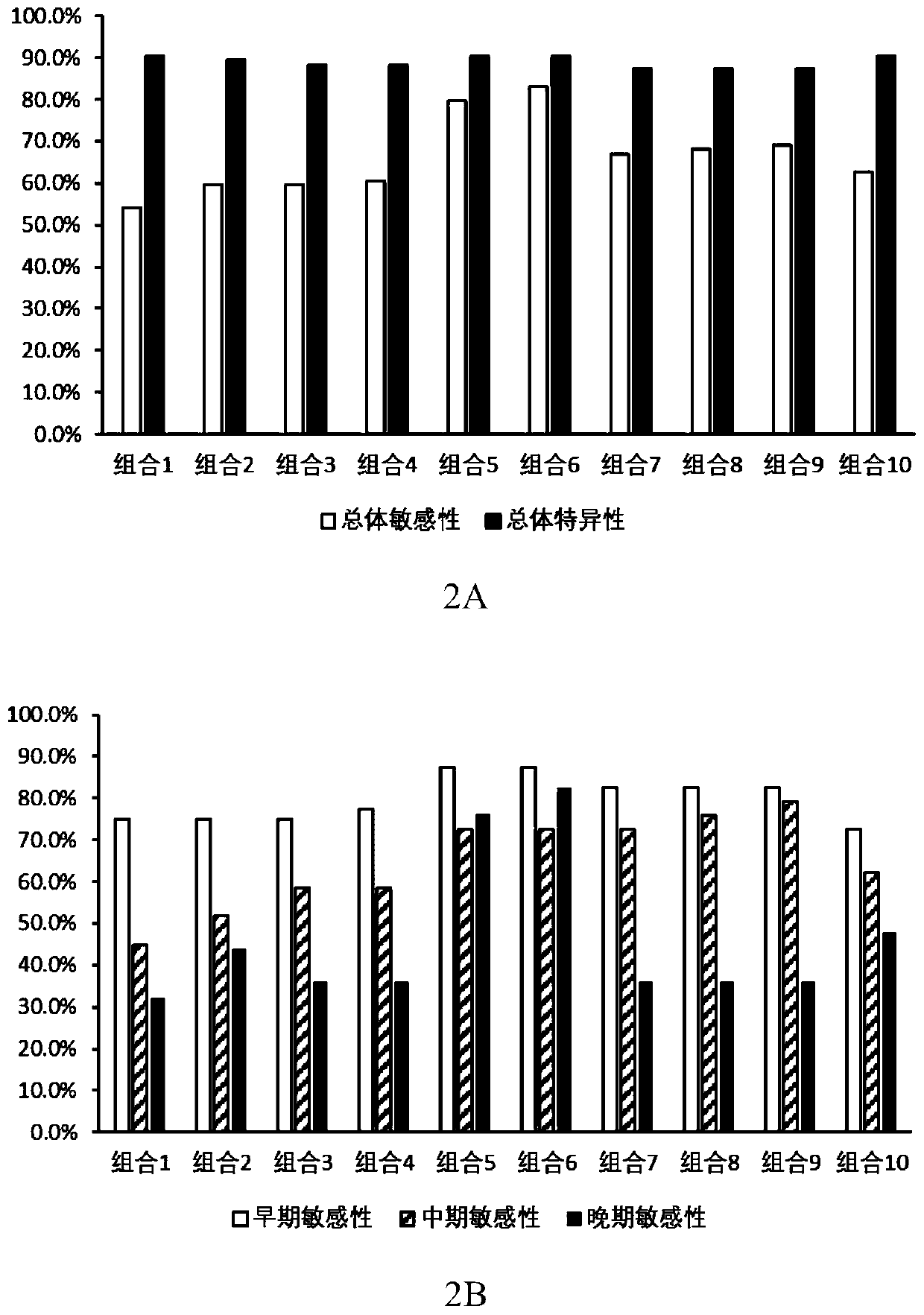
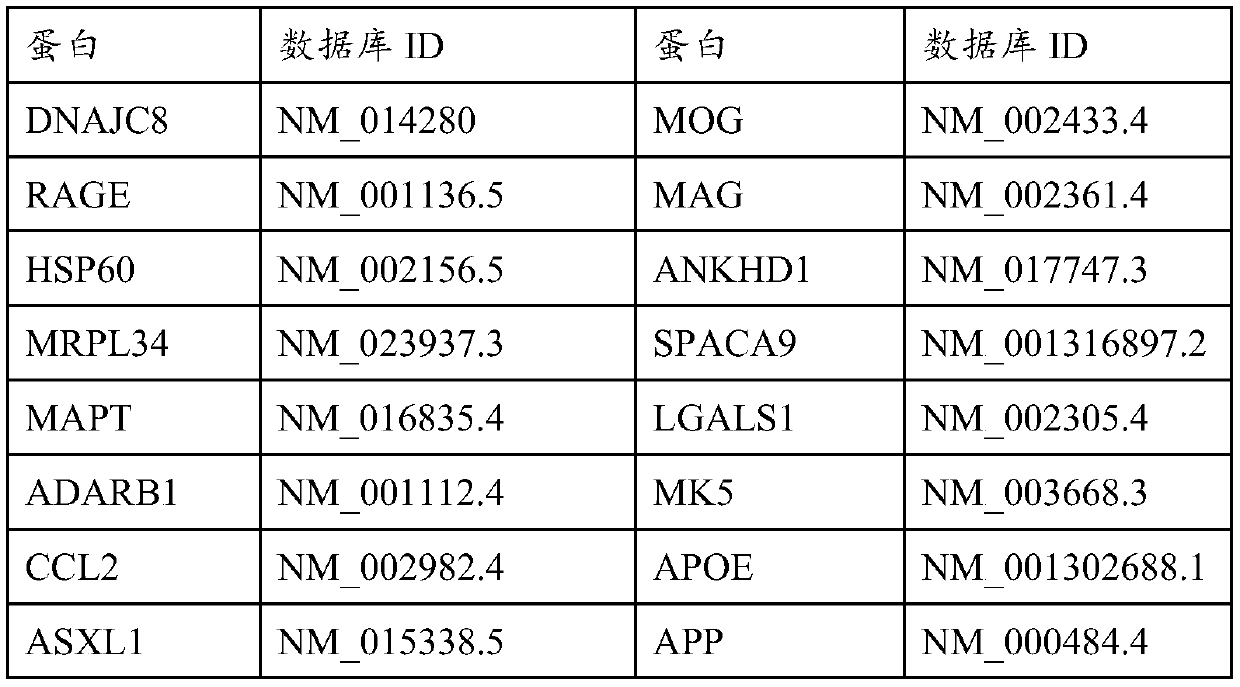
The invention provides a kit for detecting an Alzheimer autoantibody in serum. The kit comprises an antigen protein combination, the antigen protein combination contains at least four proteins, and the proteins are selected from RABPT5, RAGE, CRYAB, SRPK1, MAPT, MBP, PLP1, PTCD2, FRMD8, POMC, DNAJC8, CENTA2, HSP60, ADARB1, ASXL1, EDRK, GDF11, P21, CCL2, IL18, VEGF, LENG1 and MRPL34. The inventionfurther provides application of the limited antigen protein combination to preparation of a reagent for detection, diagnosis or risk prediction of Alzheimer, and methods for detection, diagnosis or risk prediction of Alzheimer.
Owner:湖南诺琪生物科技有限公司
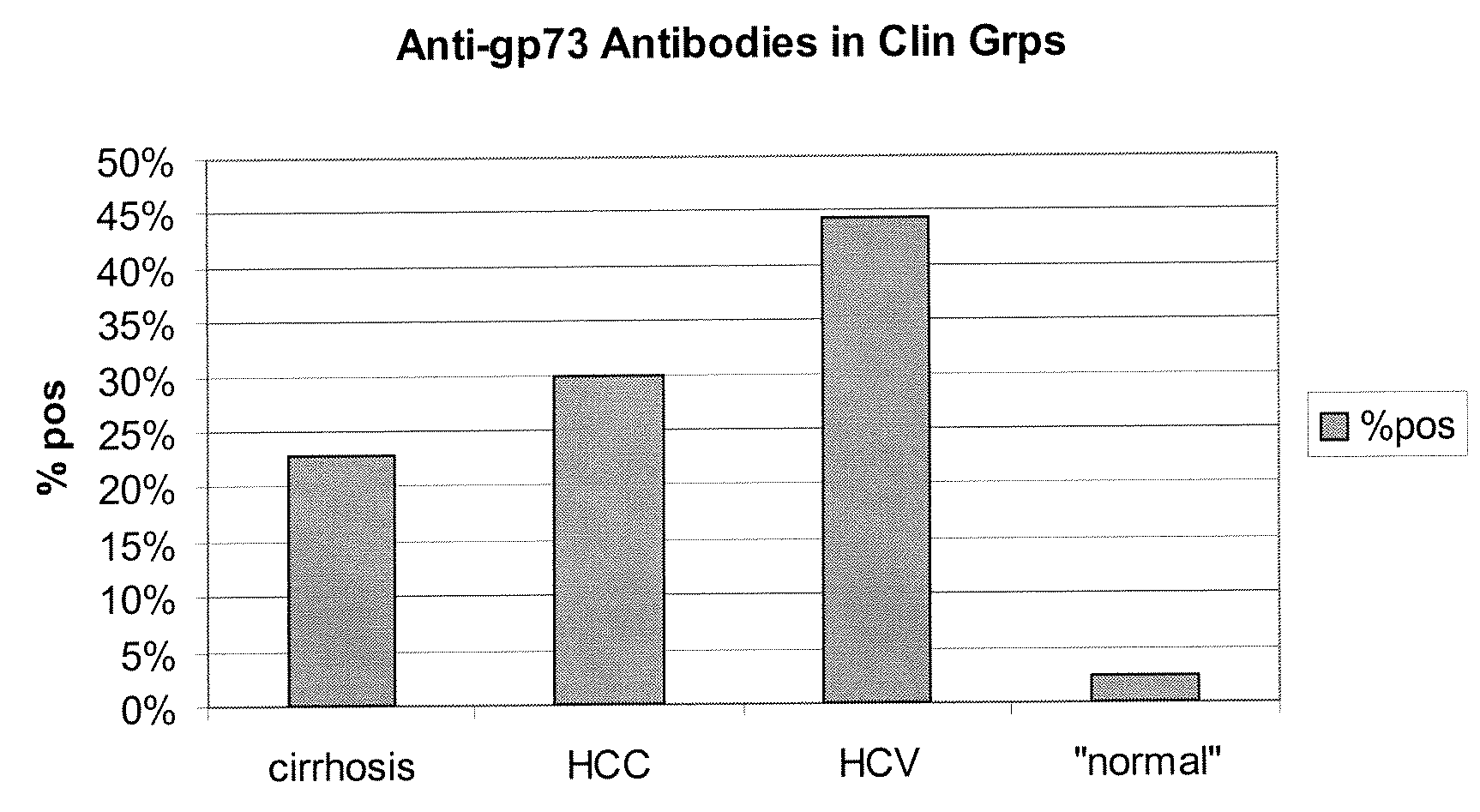
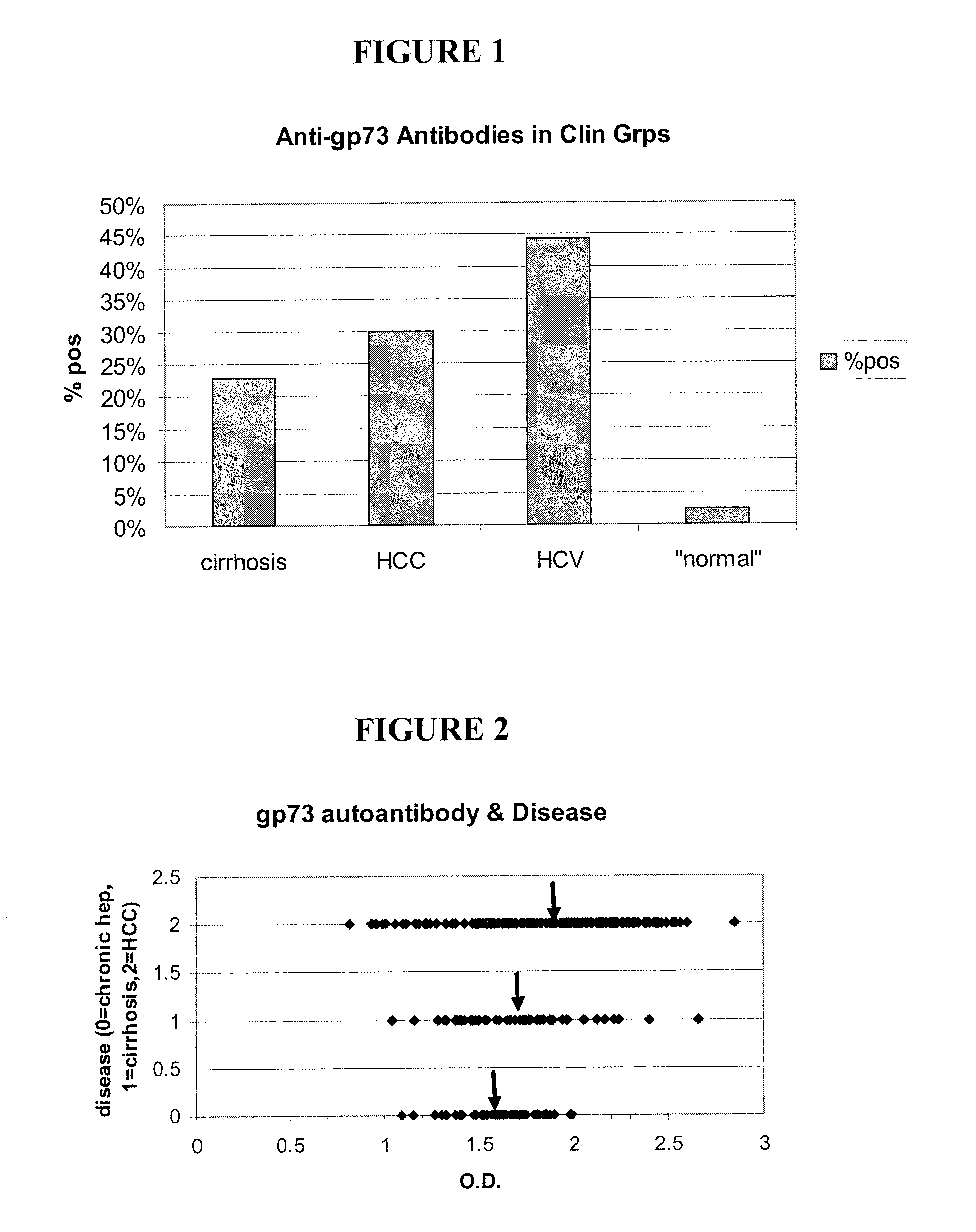
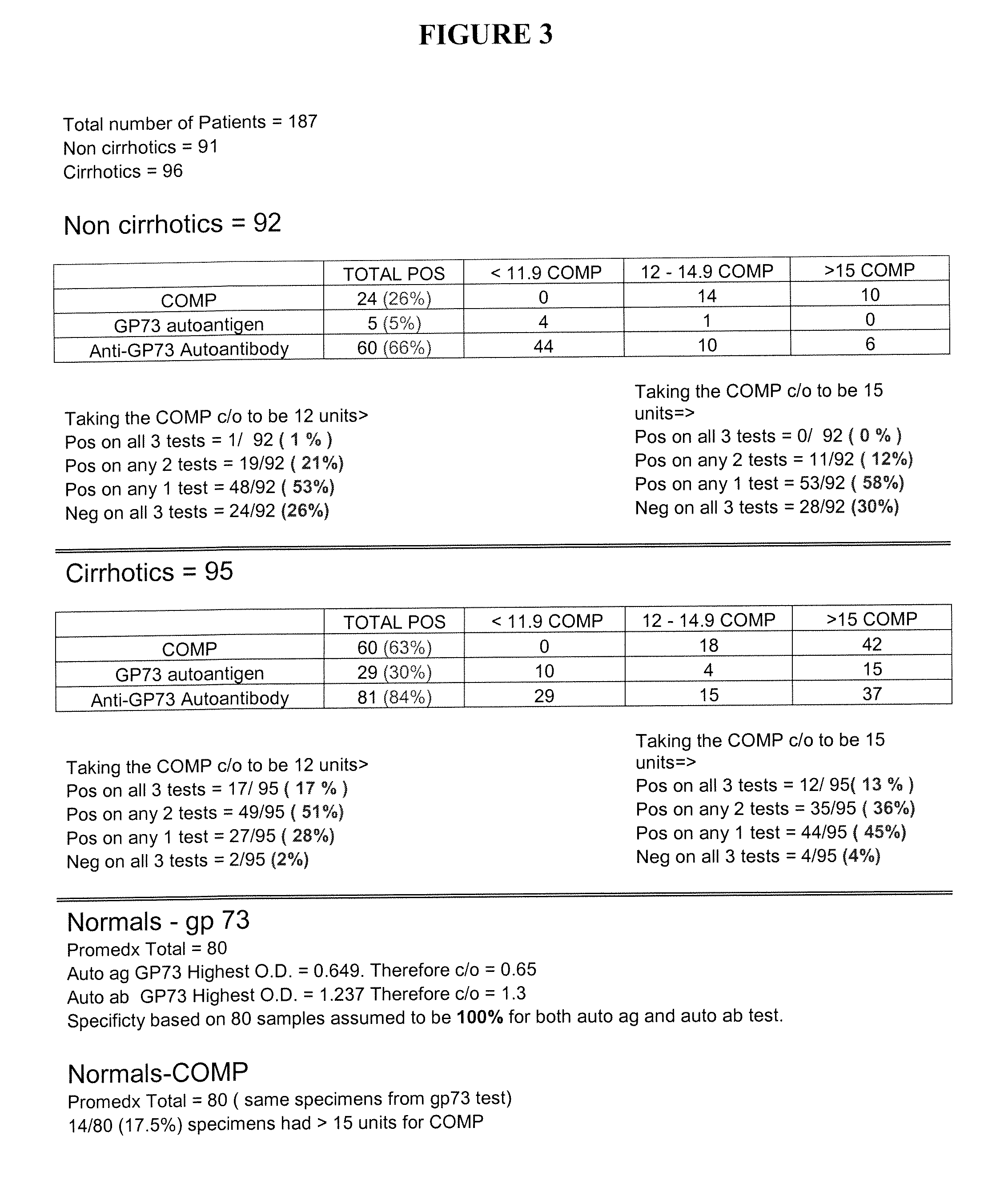
Methods and assays for detecting GP73-specific autoantibodies
The present invention provides a method for detecting autoantibodies in a subject which reacts with a GP73 antigen. Increased levels of GP73-specific autoantibodies in a sample from the subject which bind to GP73 antigen are indicative of liver disease in the subject.
Owner:INOVA DIAGNOSTICS INC
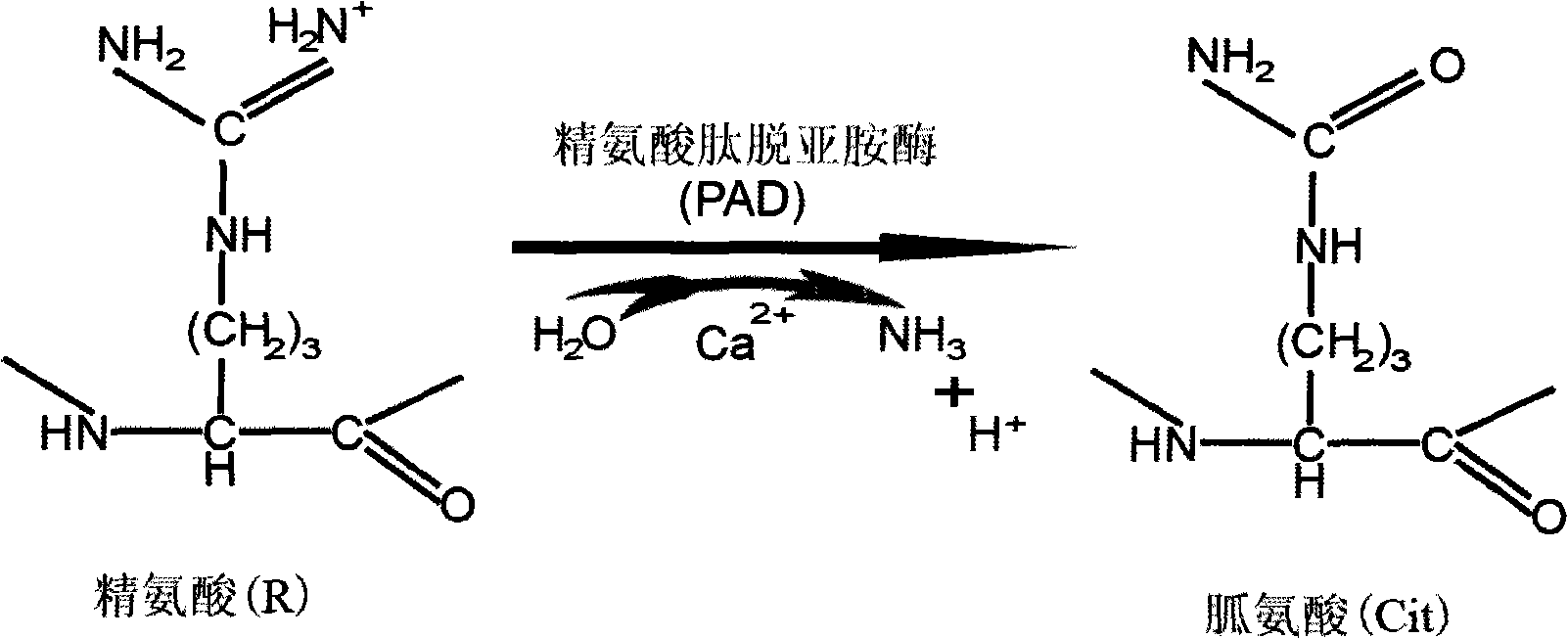
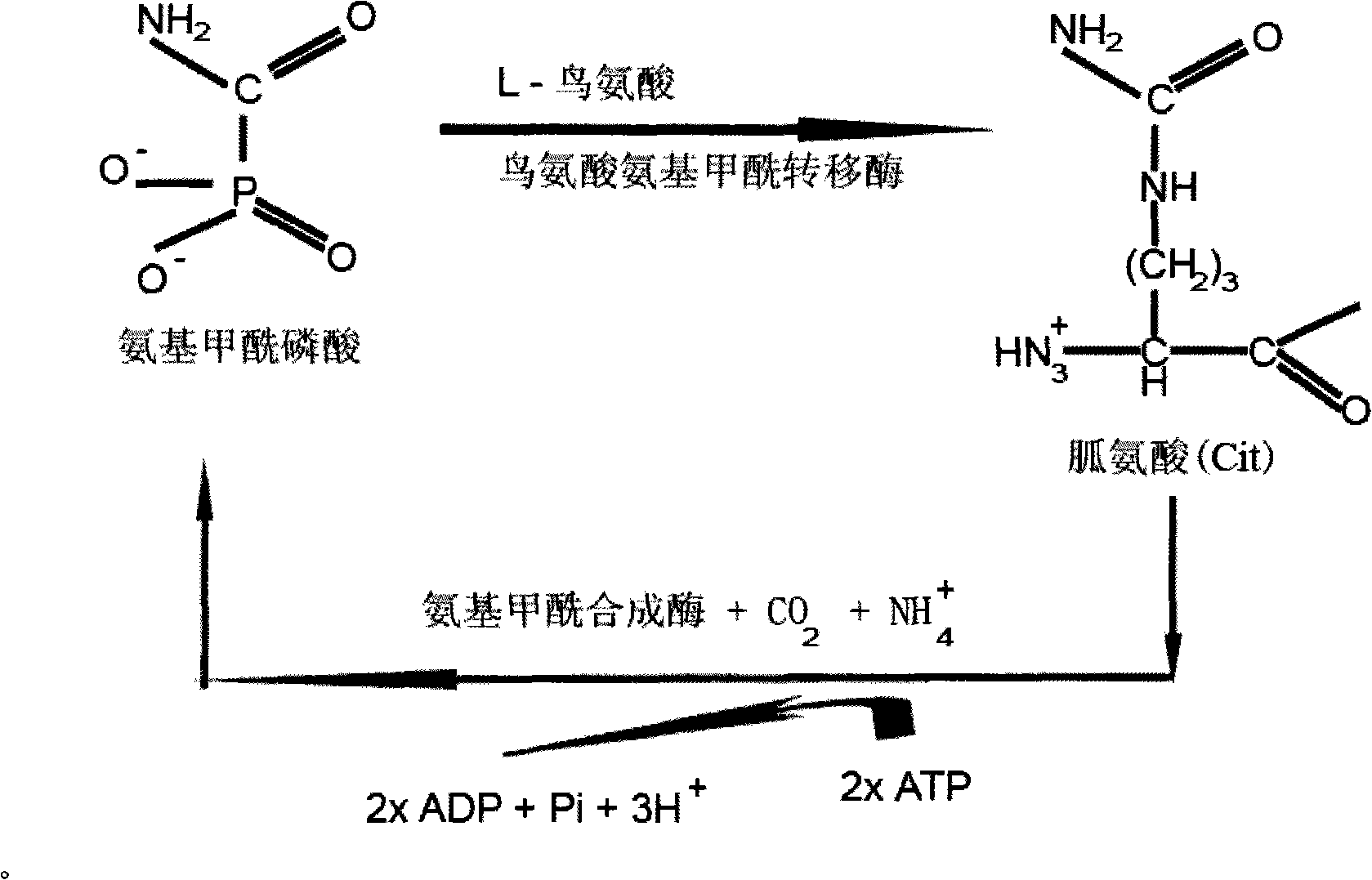
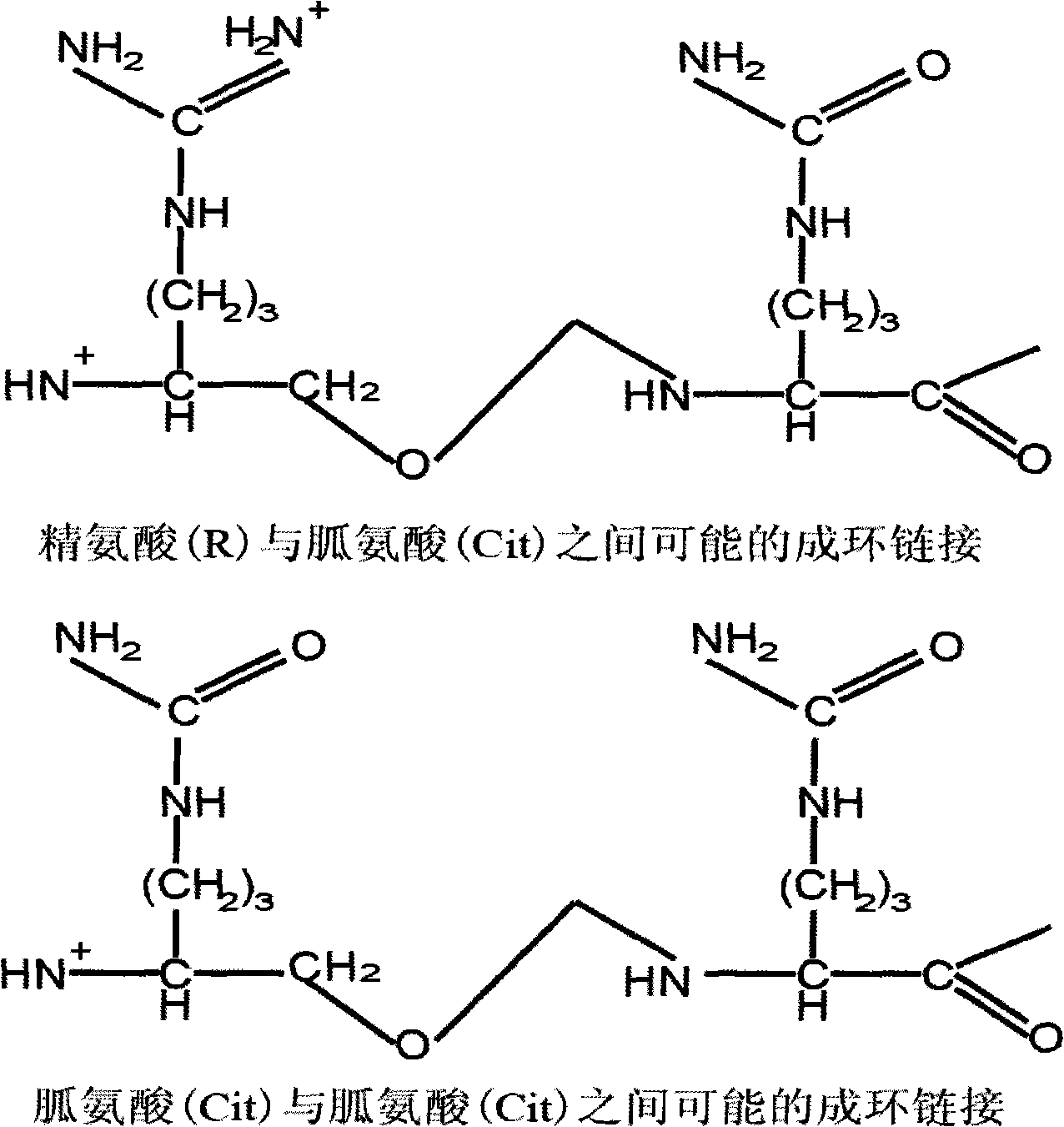
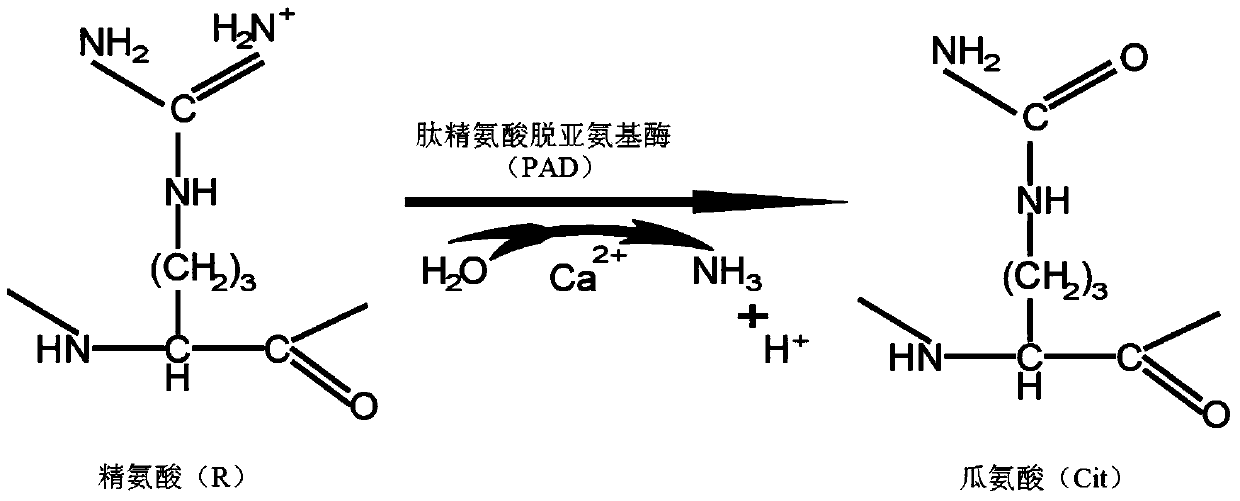
Modified allosteric type cyclic citrulline polypeptide, and fusion protein, antibody and reagent kit thereof
ActiveCN101407541ABuild specificityImmunoglobulins against animals/humansBiological testingAntigenAutoantibody
The invention discloses five categories of denaturizing allosteric cyclic citrulline polypeptide, fusion protein, antibodies and a kit thereof, which are formed by synthesizing related antigens that antibodies of rheumatoid arthritis patients can specifically recognize, independent designing amino acid sequences and structures with special derivations, and tests prove that the invention can combine with antibodies in RA blood serum and have better immunity reaction compared with the existing cyclic citrulline polypeptide detections, such as CCP and the like. The polypeptides carry out denaturizing and allosteric design and synthesis according to metabolism of arginine residues, and compared with projects of CCP, C-CRP and RF and the like, the five categories of denaturizing allosteric cyclic citrulline polypeptides are characterized by good specificity, high relevance ratio and high primitive diagnosis rate, etc.
Owner:上海精臻生物科技有限公司
Human body anti-AQP4 autoantibodies detection material and preparation method thereof
ActiveCN107022548AEasy to useLower requirementDisease diagnosisBiological testingHuman bodyAutoantibody
The invention provides a human body anti-AQP4 autoantibodies detection material and a preparation method thereof. The preparation method comprises the steps of firstly acquiring M1 and M23 subtype AQP4 overall length target genes, dyeing into an HEK293 cell, and building an HEK293 / pCI-neo-M1-AQP4 cell and an HEK293 / pCI-neo-M23-AQP4 cell expressing M1 and M23 subtype AQP4; extracting an AQP4-cell membrane complex of the cells; finally curing the AQP4-cell membrane complex on a carrier, and obtaining the human body anti-AQP4 autoantibodies detection material. According to the human body anti-AQP4 autoantibodies detection material and the preparation method thereof provided by the invention, the extracted AQP4-cell membrane complex is used as a detection material, so that the intrinsic protein amount causing background coloration can be reduced; compared with a method for using the cell as the detection material for carrying out immunofluorescence detection, the detection sensitivity and the specificity are further increased, a detection result is easier and clear to judge, and the AQP4 autoantibodies of a patient can be simply, conveniently and quickly detected.
Owner:SHAANXI MYBIOTECH CO LTD
Prostate Cancer Glycan Markers and Autoantibody Signatures
ActiveUS20090258792A1High degreeSaccharide librariesLibrary screeningProstate cancer cellGriffonia simplicifolia
Disclosed are methods for probing the immunogenic sugar moieties of prostate cancer cells. The methods detect a number of glyco-epitopes that are highly and differentially expressed among prostate cancers of various Gleason grades. The glyco-epitopes exist on the surfaces of prostate cells. The methods also comprise the detection of autoantibodies in prostate cancer subjects. The antibodies bound to a glyco-motif of N-glycans that is normally “cryptic.” This target is highly expressed in prostate cancers. Lectins and antibodies that detect these glyco-epitopes that expressed in prostate cancer tissues include Euonymus europaeus lectin (EEL); Psophocarpus Tetragonolobus Lectin-I (PTL-I); Griffonia Simplicifolia Lectin-I-A4 (GSL-I-A4); Griffonia Simplicifolia Lectin-I-B4 (GSL-I-B4); Sambucus nigra I agglutinin (SNA-I; Phaseolus vulgaris-L (PHA-L; Galanthus nivalis agglutinin (GNA); Narcissus pseudonarcissus agglutinin (NPA); Artocarpus integrifolia agglutinin (Jacalin); and mAb TM10 (IgM).
Owner:SRI INTERNATIONAL
Biomarker related to tumor immunotherapy effect and application of biomarker
The invention relates to a biomarker related to a tumor immunotherapy effect and an application thereof, and discloses a biomarker for predicting the tumor immunotherapy effect, and the biomarker is an autoantibody of a group of tumor-associated antigens. The biomarker can be detected in a sample from a tumor patient to predict the clinical effect of administration of immunotherapy, thereby facilitating the determining of whether the tumor patient can benefit from immunotherapy. The invention also provides an antigen protein combination for detecting the biomarker, a kit containing the antigenprotein combination, and a corresponding detection or diagnosis method.
Owner:HANGZHOU KAIBAOLUO BIOLOGICAL SCI & TECH
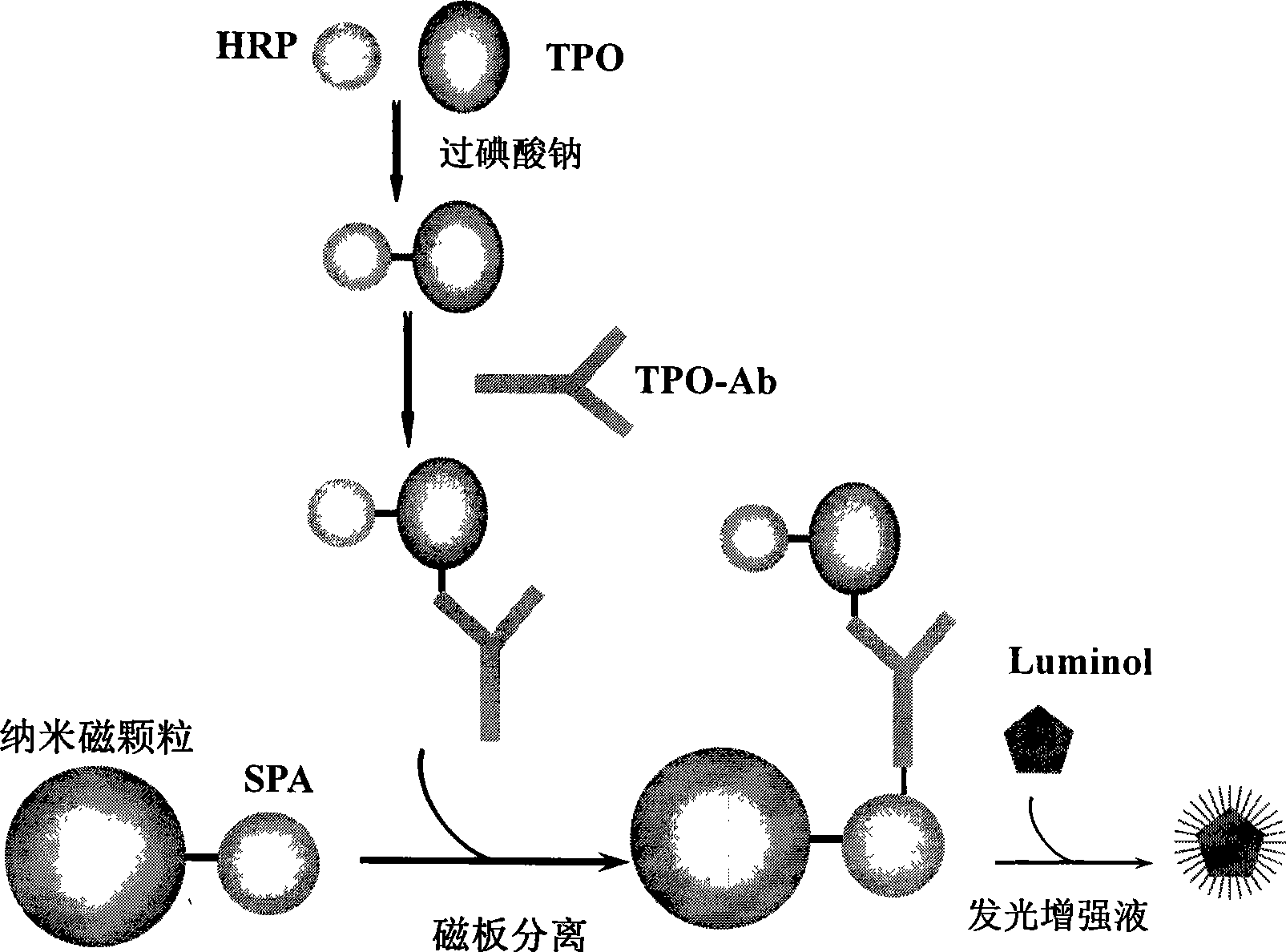
Chemiluminescent ligand analysis method for quantitative detection of human auto-antibody
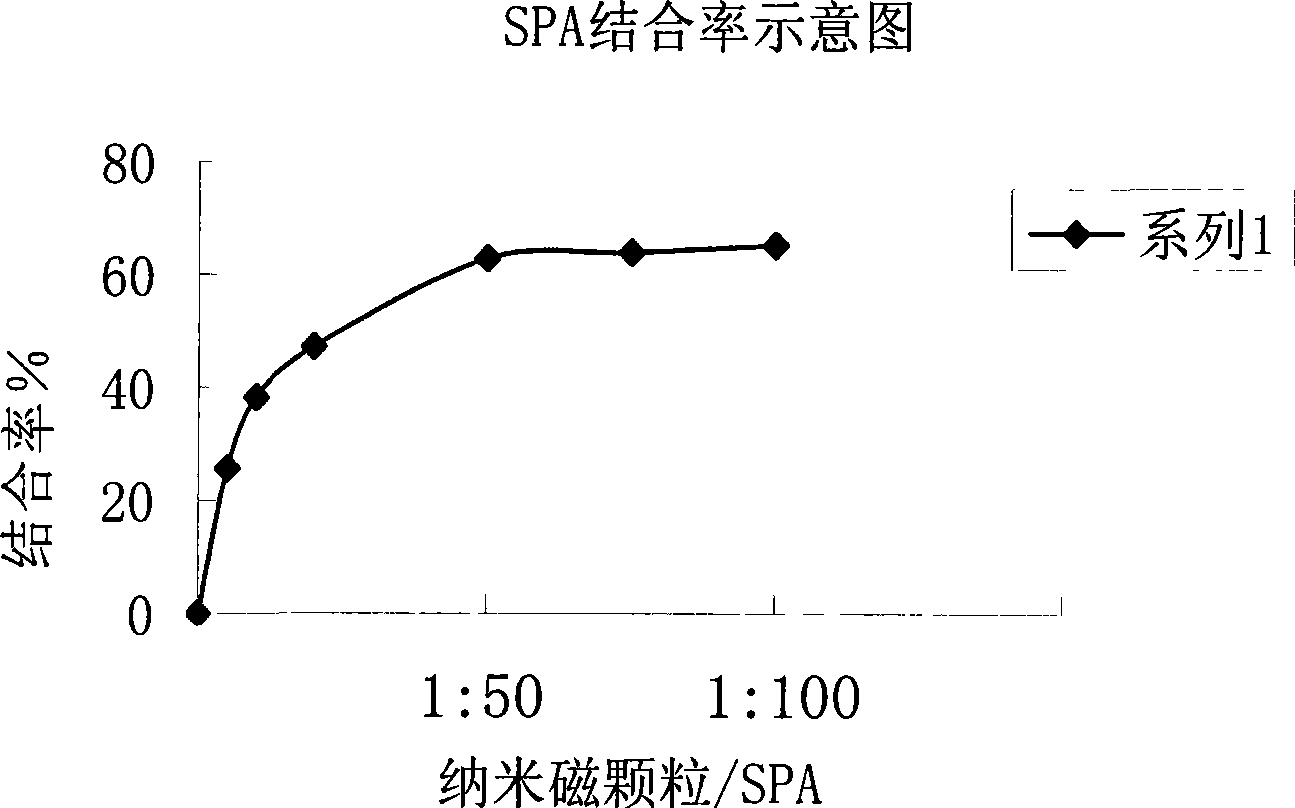
InactiveCN101470117ASolve the problem of inaccurate quantitative detectionNo cross-reactivityBiological testingAutoantibodyBiomedical technology
A chemical luminous ligand analysis method for quantitatively checking human antibodies belongs to the biomedical technical field, which comprises: using the generality that staphylococcal protein A (SPA) can react with Fc point of IgG in mice; using the monoclonal antibody of high affinity and specificity to prepare a standard product to establish a standard curve; respectively reacting the monoclonal antibody and the antibody in the sample with enzyme-labeled antigens; using the nanometer magnetic particles coated by SPA as ligand to separate solid and liquid; and using enzymatic chemical illumination reaction catalyzed by horseradish peroxidase (HRP) to process illumination check; thereby establishing a chemical luminous ligand quantitative analysis on human antibodies. The chemical luminous ligand analysis method is suitable for quantitatively checking all human antibodies, for resolving the problem of prior art while most human antibodies can not be accurately and quantitatively checked, avoiding cross reaction and avoiding false negative result or false positive result.
Owner:天津市协和医药科技集团有限公司
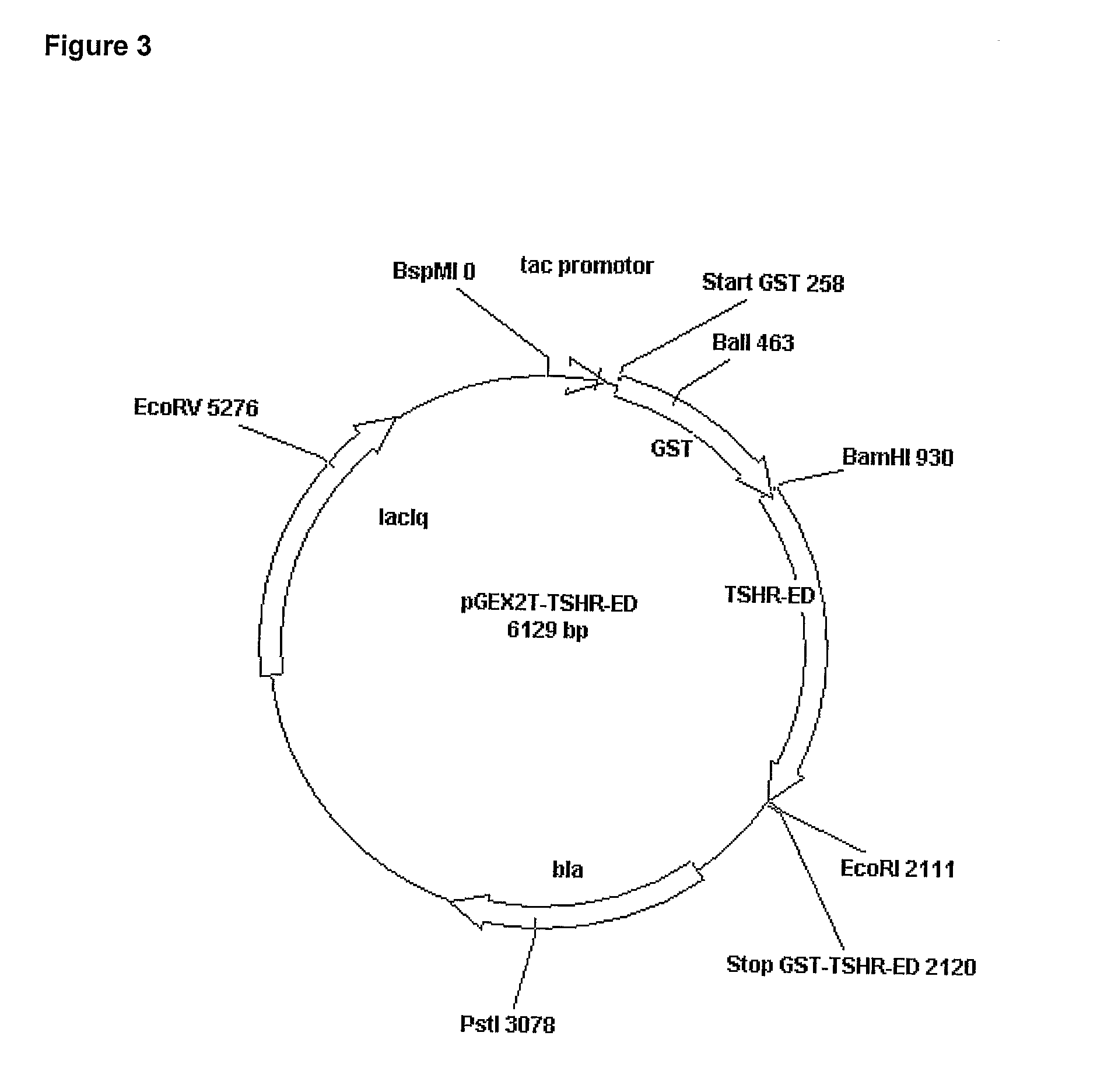
Recombinant Polypeptides and Methods for Detecting and/or Quantifying Autoantibodies Against Tsh Receptor
InactiveUS20080305098A1Facilitate detection and isolation and identificationHigh binding activitySugar derivativesBacteriaAutoantibodyAutoimmune condition
The present invention relates to unglycosylated isolated and purified recombinant polypeptides comprising a fusion protein able to bind to autoantibodies produced in response to an autoimmune disease associated with an immune reaction to a TSH-receptor (TSHR). Also disclosed are methods of detecting and / or quantifying such autoantibodies using the isolated and purified recombinant polypeptides and respective kits.
Owner:DR FENNING BIOMED +1
Array systems and methods
Methods, kits, arrays, and biosensors for detecting proteins, modified-proteins, protein-protein interactions, protein-DNA interactions, autoantibodies, and protein-small molecule interactions, are disclosed. A representative method of detecting proteins of the present invention includes exposing a solid support to a solution containing proteins; conjugating proteins to the solid support; exposing the solide support to a plurality of types of DNA-conjugated antibodies, wherein each type of DNA-conjugated antibody has an affinity for a specified protein; forming a complex between a protein conjugated with the solid support and a type of DNA-conjugated antibody when the protein is the specified protein for which the DNA-conjugated antibody has an affinity; separating the complex from the solution of proteins and the DNA-conjugated antibodies; releasing DNA from the DNA-conjugated antibodies; and detecting the DNA, wherein each DNA indicates the presence of the specified proteins.
Owner:HUANG RUO PANG
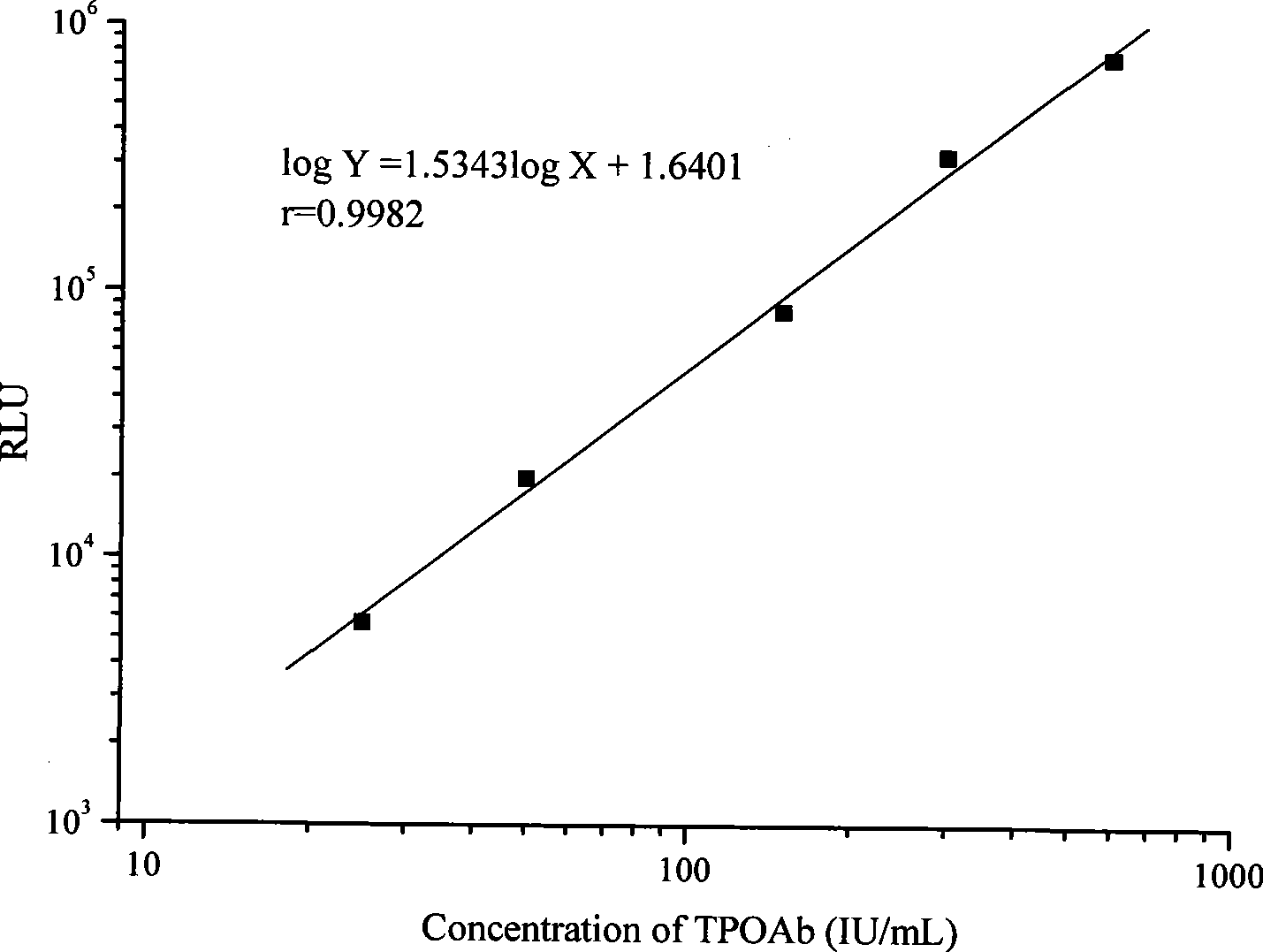
Chemiluminescence immune analytic reagent kit for detecting thyroid peroxidase autoantibody
InactiveCN101377496AGuaranteed SensitivityHigh sensitivityChemiluminescene/bioluminescenceAntigenChemiluminescent immunoassay
The invention provides a chemiluminescent immunoassay kit for thyroid peroxidase autoantibodies and a preparation method thereof. The invention has the advantages that the reaction pattern of the double antigen sandwich method is adopted, the chemiluminescent technology and the biotin-avidin immune amplifying technological principle are effectively utilized, horse radish peroxidase is adopted to be as the marker enzyme, the content of the thyroid peroxidase autoantibodies in human serum samples is quantitatively detected, and the detection sensitivity can be ensured. The kit of the invention has the advantages of high sensitivity, good repeatability, good stability and high detection efficiency, and can provide a timely and reliable experimental basis for the clinical diagnosis and the treatment of thyroid disorders.
Owner:北京科美东雅生物技术有限公司
Method of screening remedy for heart disease and medicinal composition for treating heart disease
ActiveUS20060246525A1Improve solubilityImprove stabilityPeptide/protein ingredientsMicrobiological testing/measurementI antibodyHeart disease
A method for screening a substance that inhibits the onset of anti-cardiac troponin I autoantibody-related disease, a pharmaceutical composition and a base material for therapy of cardiac disease that contains the substance obtained by aforesaid method thereof, a therapeutic apparatus that removes anti-troponin I autoantibody for aforesaid antibody related disease, a method of making an animal model for evaluating cardiac disease characterized by administrating anti-cardiac troponin I antibody, a method of selection of a therapeutic substance for cardiac disease characterized by using aforesaid animals, and a diagnosis of dilated cardiomyopathy characterized by measuring anti-cardiac troponin I autoantibody. An apparatus of the present invention that removes anti-cardiac troponin I antibody and a pharmaceutical composition for therapy of the antibody related disease may be useful for a therapy and / or prevention of cardiac disease.
Owner:ONO PHARMA CO LTD +1
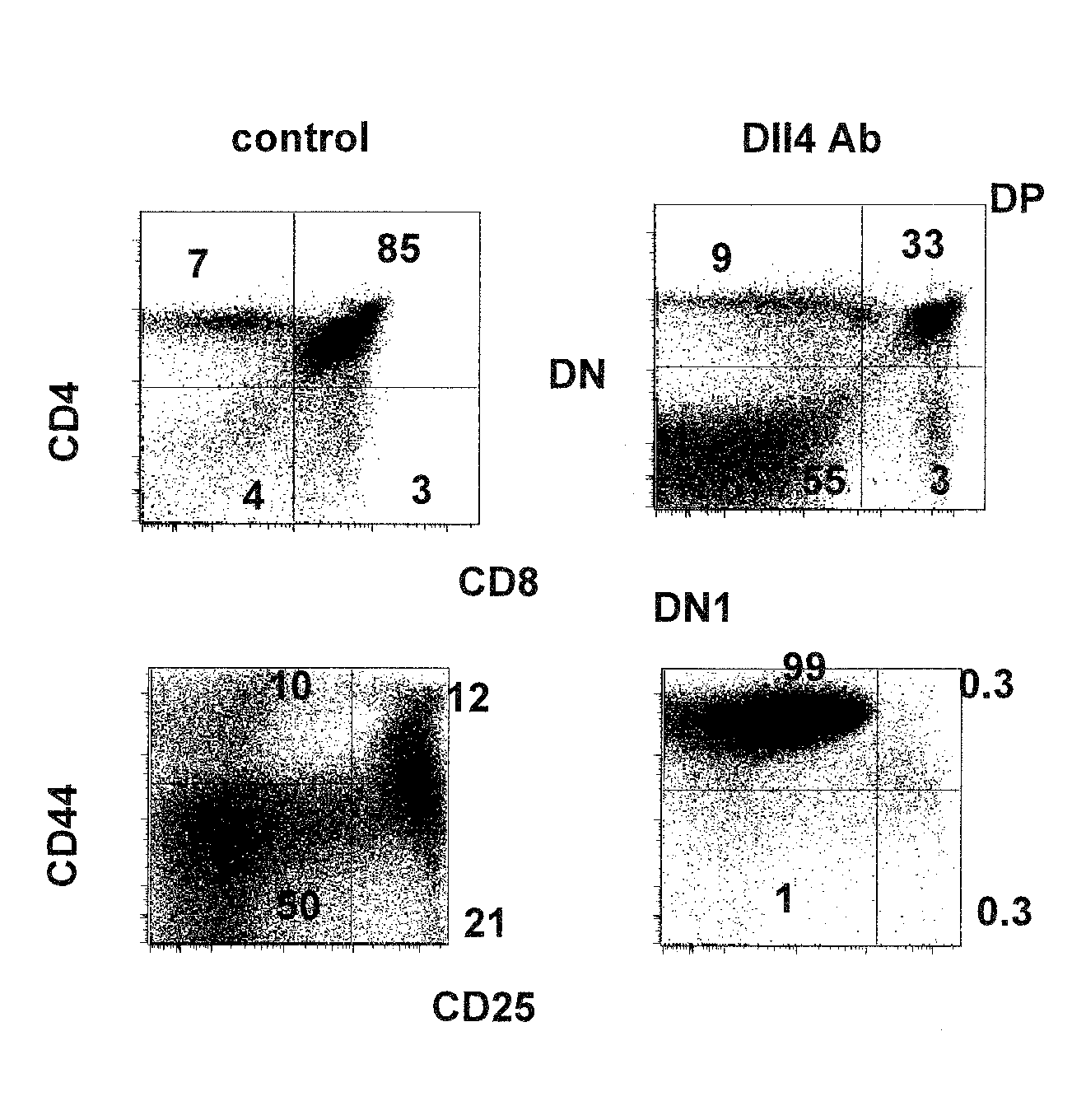
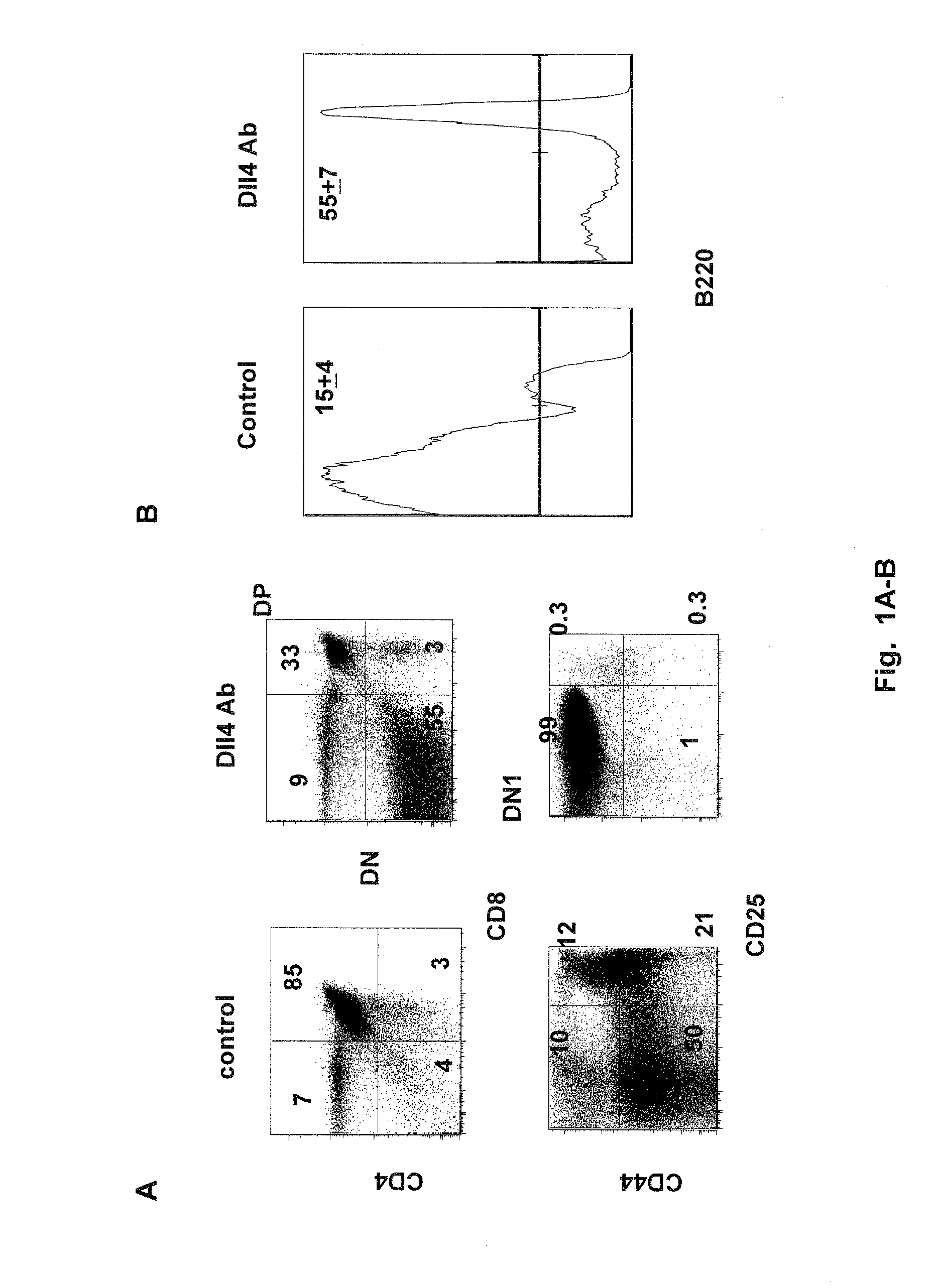
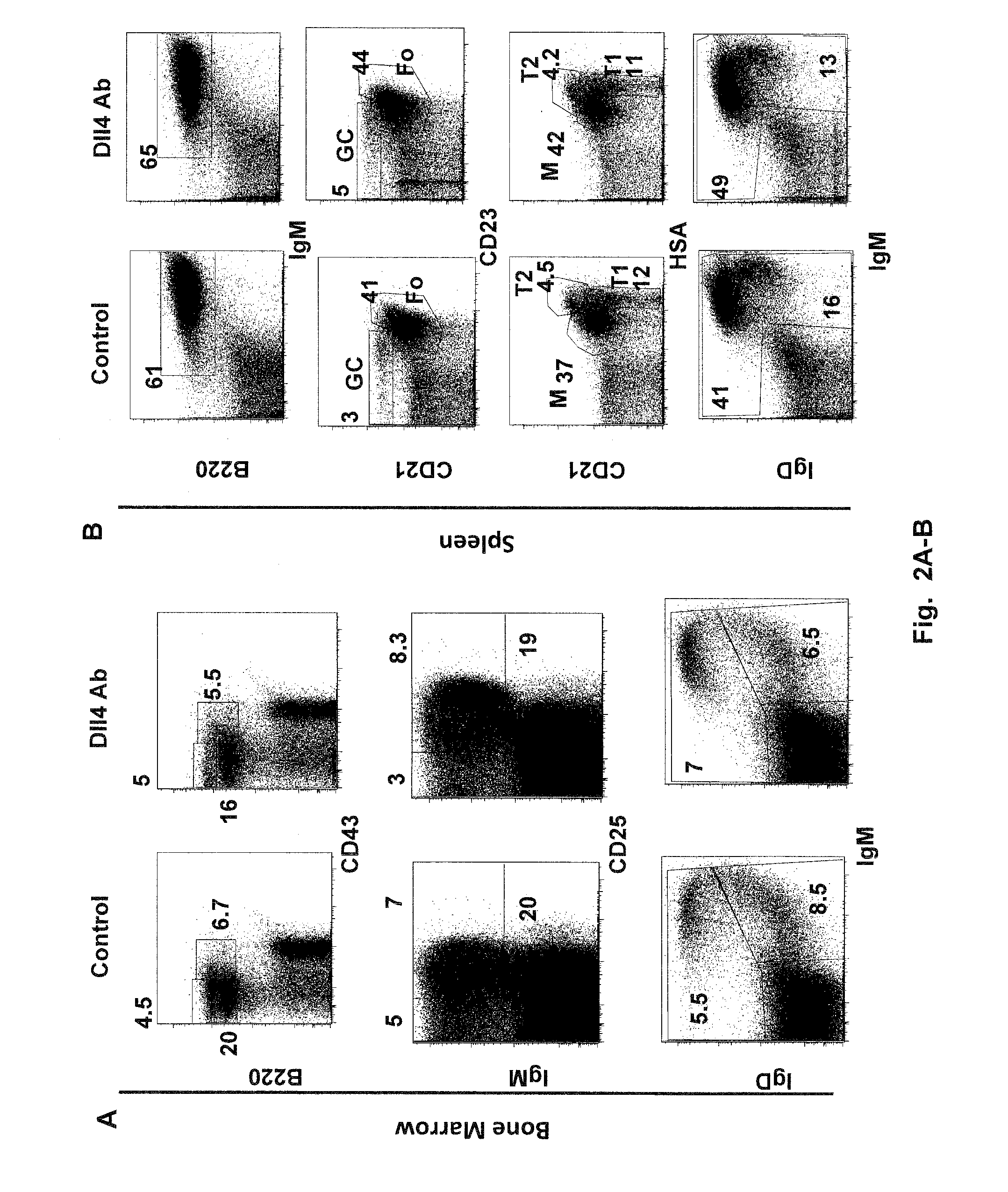
Methods of treating diseases with dll4 antagonists
ActiveUS20110189176A1Increase the number ofImprove the level ofNervous disorderAntipyreticDiseaseRegulatory T cell
The present invention provides methods of preventing, treating or ameliorating diabetes by administering to a subject in need thereof a therapeutically effective amount of Dll4 antagonists that block Dll4-Notch signal pathways. As observed in a mouse model of diabetes, Dll4 antagonists exhibit protective effects on pancreatic islets, lower blood glucose levels, and block the production of auto-antibodies, including those against insulin and glutamic acid decarboxylase 65 (GAD65), via the expansion of regulatory T cells (Tregs). Thus, the present invention further provides methods of lowering the levels of blood glucose, and / or reducing or blocking the production of auto-antibodies, by administering to a subject in need thereof a therapeutically effective amount of Dll4 antagonists. Suitable Dll4 antagonists for the invention include antibodies or antibody fragments that specifically bind Dll4 and block Dll4-Notch interactions, the extracellular domain of Dll4, and the like.
Owner:REGENERON PHARM INC
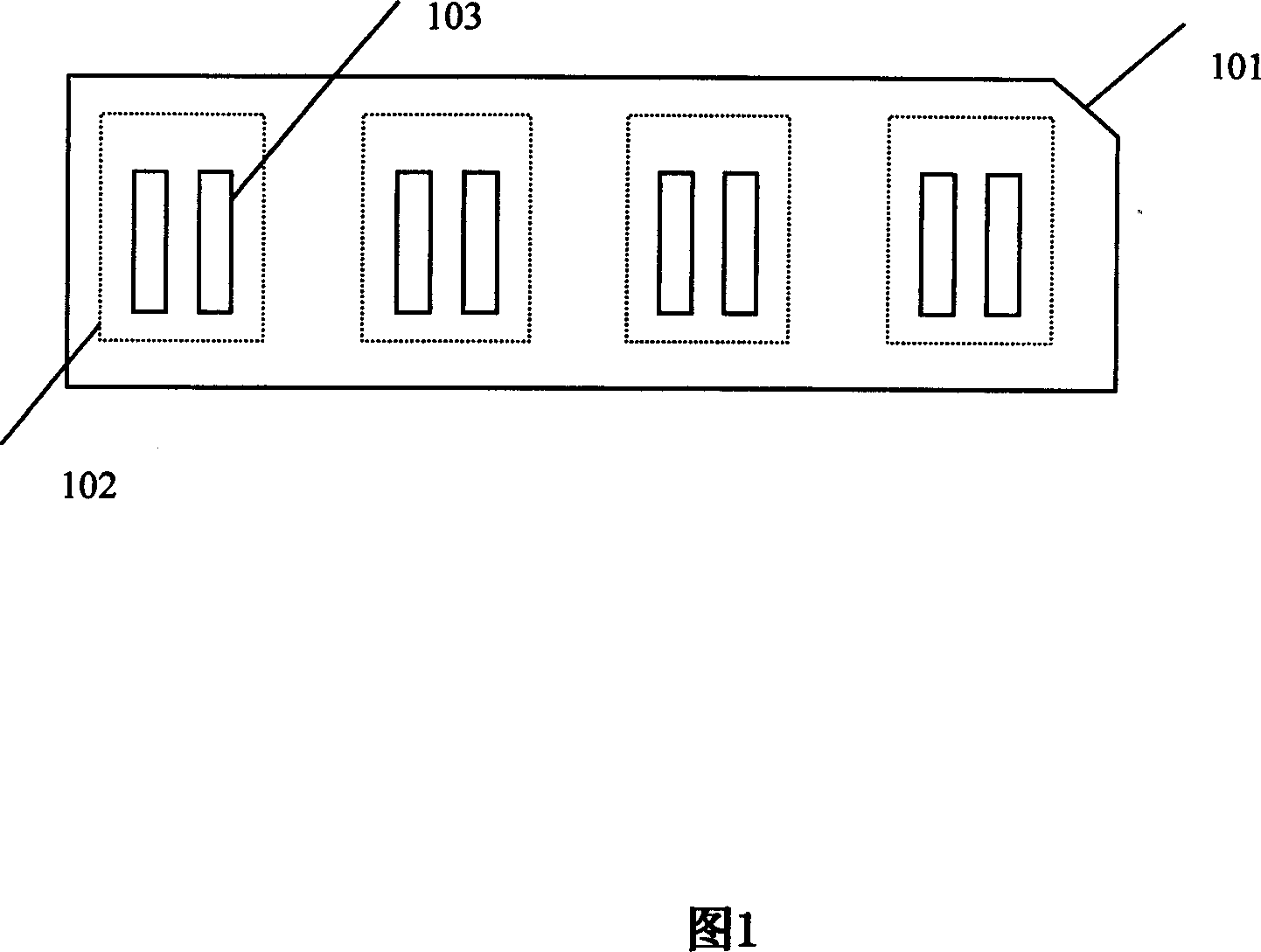
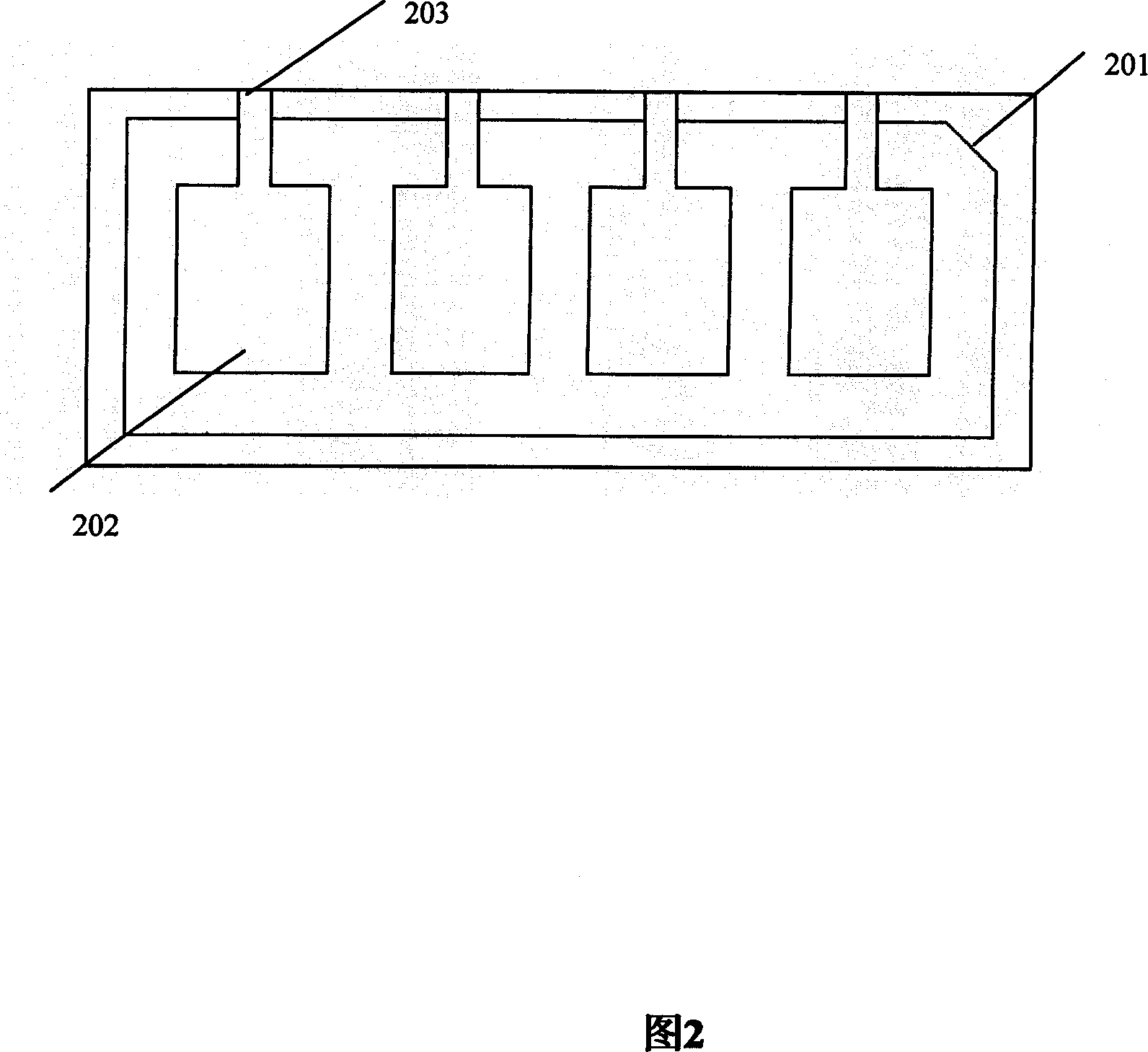
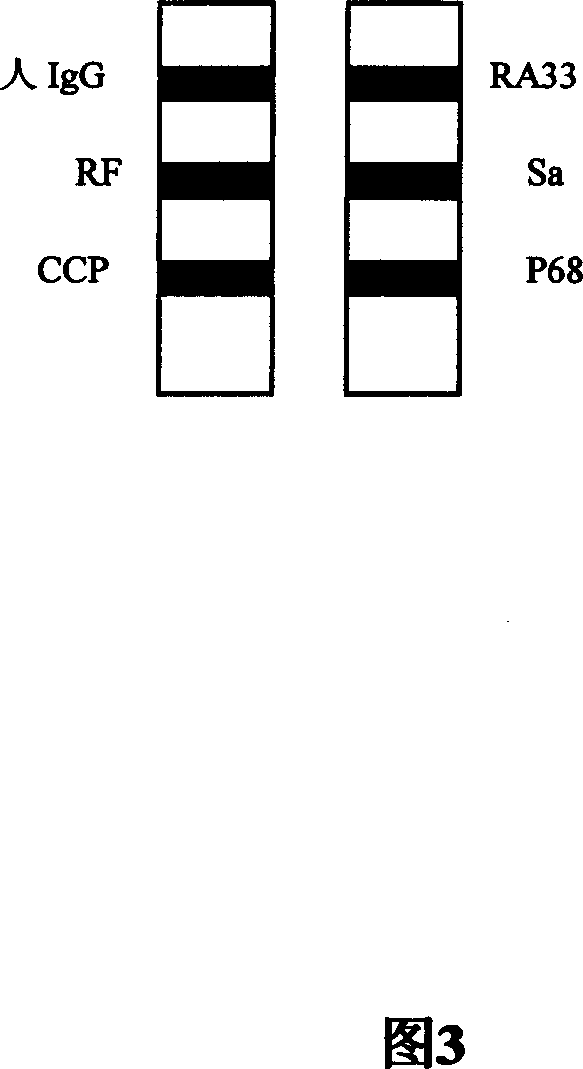
Concatenate antigen membrane detecting kit and its preparing method
InactiveCN101025418AImprove accuracy and convenienceEasy to operateMaterial analysisChemistryResponse control
The invention relates to a test reagent box of multiple antigen membrane method which is used for diagnosing RA, it includes tablets and response groove, the tablets hold on the response groove; the tablets gap corresponding to the response groove gap; there is multi-personal reaction zone on the tablets which correspond with the multi-groove of reaction groove, there is a gap on the top of each groove; membrane bar test paper adhere to each reaction zone and with RA correlate antigen and response control belt; the RA related antigen is the corresponding antigen of RF, anti-CCP, anti-RA-33 antibody, anti-Sa antibody, anti-P68 or more than four kinds of antigen in antigen epitope polypeptide or antigen epitope polypeptide, the response control belt is a mixture of man IgG and man IgM. The reagent box in this invention overcome the lack of detection sensitivity and Specificity of the single autoantibody and the red tape of several autoantibody detection in a single operation, it provide the accuracy and convenience of RA diagnosis. The invention also disclosed the method of how to make the test box.
Owner:SHANGHAI KEXIN BIOTECH
Methods and Kits for the Diagnosis of Rheumatoid Arthritis
InactiveUS20110065609A1Peptide librariesBiological testingAutoantibody productionRheumatoid arthritis
The present invention relates to the identification and use of proteins with clinical relevance to rheumatoid arthritis (RA). In particular, the invention provides the identity of marker proteins that specifically react with RA-associated autoantibodies. Also provided are methods, arrays and kits for using these proteins in the diagnosis of RA, and in the selection and / or monitoring of treatment regimens.
Owner:LUNIV D AIX MARSEILLE +1
Peptides for Treatment and Diagnosis of Autoimmune Disease
ActiveUS20090304577A1Increase productionInhibit bindingAntibacterial agentsOrganic active ingredientsEpitopeAutoantibody
There are provided peptides derived from antibodies with reactivity against a GPI linkage epitope and functionally equivalent ligands. These peptides can be used in the therapy and diagnosis of a variety of diseases, all of which are considered to be caused by the inappropriate presence in the body of autoantibodies which are reactive with GPI linkage epitopes. There is also described a mechanism of action of these autoantibodies which compromises the organism, so causing disease, and a method of prevention of disease and detection of the autoantibody.
Owner:MATOSSIAN ROGERS ARPI
Assays for autoantibodies
A method and kit for screening a sample of body fluid for at least one autoantibody to at least one antigen. A source of at least one antigen to the autoantibody is provided. A substrate having immobilized thereto at least one antibody to the antigen is also provided. The antigen source is contacted with the sample of body fluid, so as to obtain a mixture wherein the antigen is allowed to substantially bind with the autoantibody, when the latter is present in the sample of body fluid. The mixture is allowed to flow relative to the substrate so as to allow the mixture to contact the antibody immobilized to the substrate. Labeling means are provided to permit monitoring of binding of the autoanitbody and the antigen present in the mixture, so as to provide an indication of the presence of the autoanitbody in the sample of body fluid.
Owner:R S R
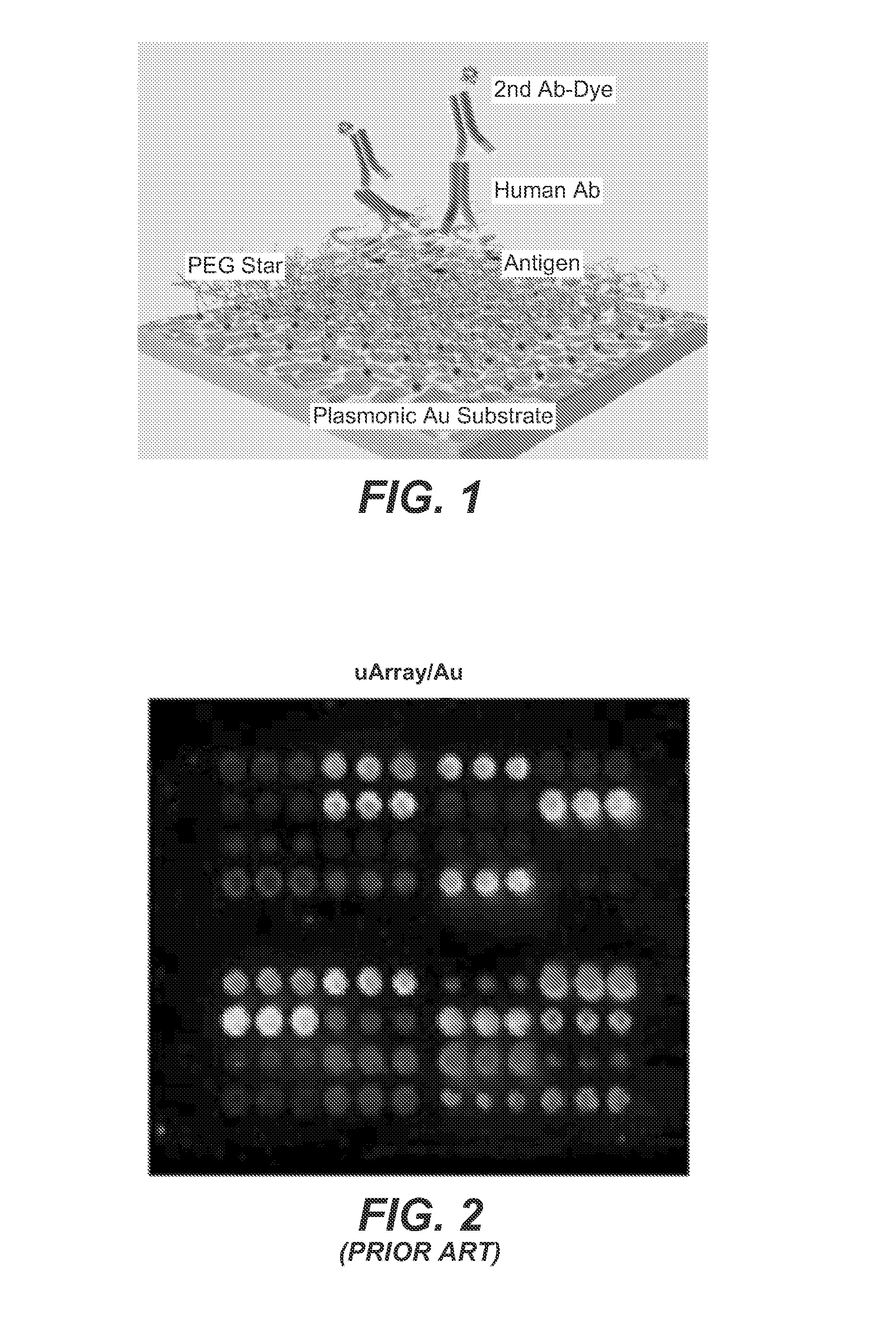
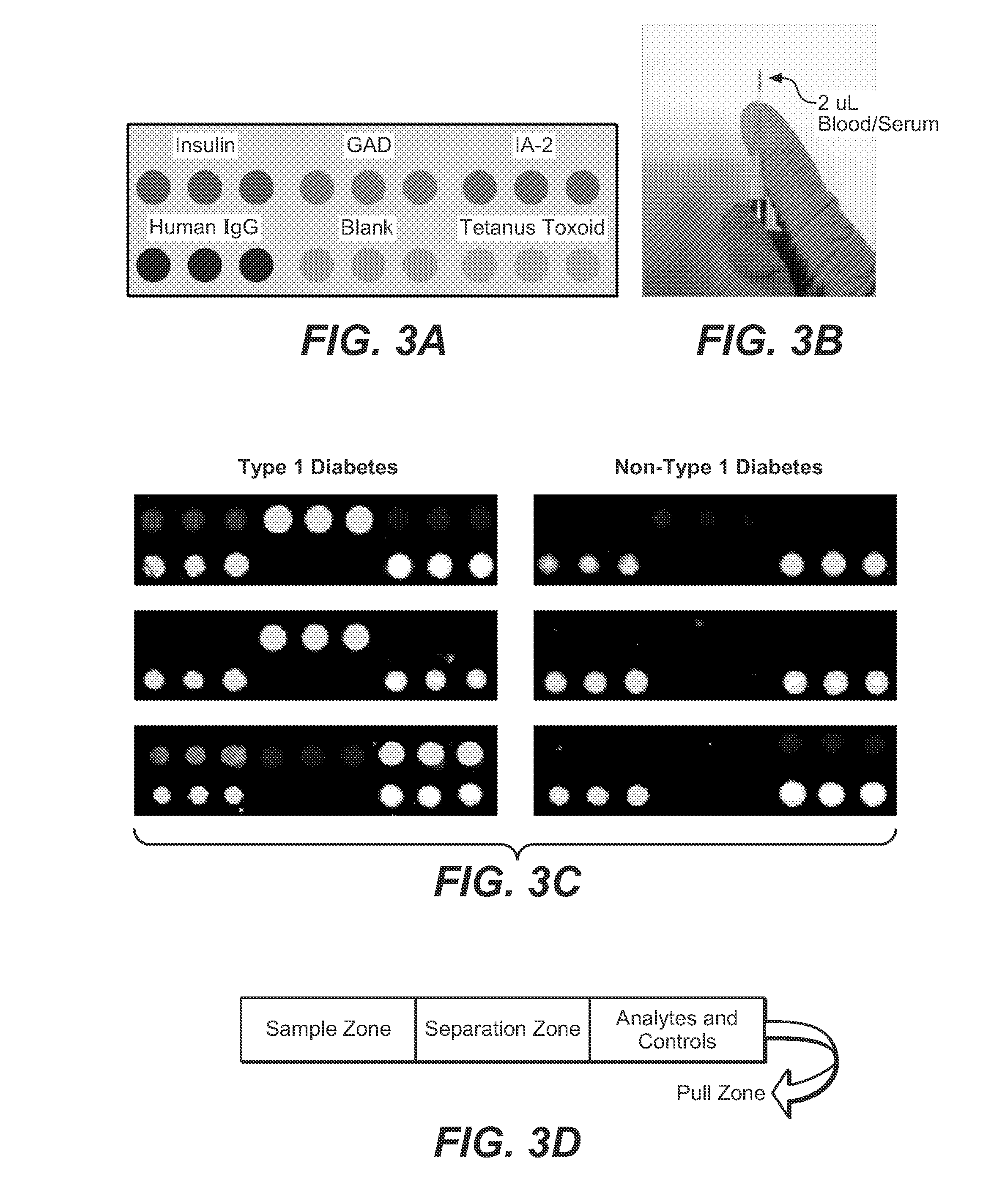
Plasmonic substrate for multiplex assessment of type 1 diabetes
InactiveUS20160025744A1Rapid and sensitive and specific diagnosisReliable detectionPeptide librariesLibrary screeningFluorophoreType 1 diabetes
Disclosed are methods and materials providing fluorescence detection of autoantibodies present in individuals who have developed or are at risk for type 1 diabetes. Provided is a plasmonic chip capable of fluorescence-enhancement of >100-fold. The fluorescent signal is generated by an anti-human antibody antibody, such as an anti-IgG antibody that is coupled to a fluorophore selected to emit at a wavelength enhanced by the plasmonic chip, for example in the NIR.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Rheumatoid arthritis autoantibody conjugated antigen and applications thereof
The present invention relates to a rheumatoid arthritis autoantibody conjugated antigen and applications thereof. The present invention discloses a novel detection antigen of rheumatoid arthritis antibody, wherein particularly the detection antigen can be used as the RA diagnosis and early diagnosis indicator, and is superior to the clinically developed detections of CCP, CRP, IgG-RF, IgM-RF and the like, such that the detection antigen can be used as the valuable reference indicator for clinical early treatment or prevention of RA, and can further be used as the RA pathogenesis development indicator.
Owner:SHANGHAI EAST HOSPITAL
Kit for detecting specificity platelet antoantibody by combination of monoclonal antibody and nano-microspheres
InactiveCN101713782ASmall saturation capacity changeUse less bloodFluorescence/phosphorescenceMicrosphereMean fluorescence intensity
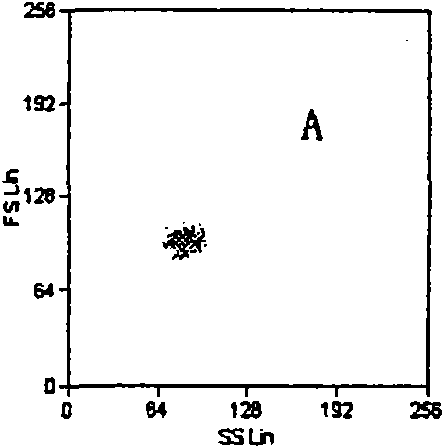
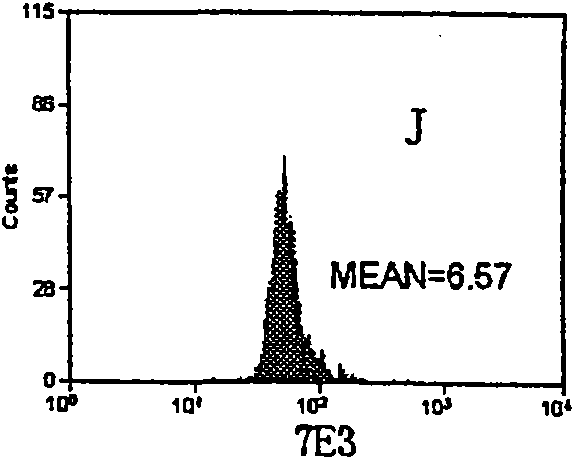
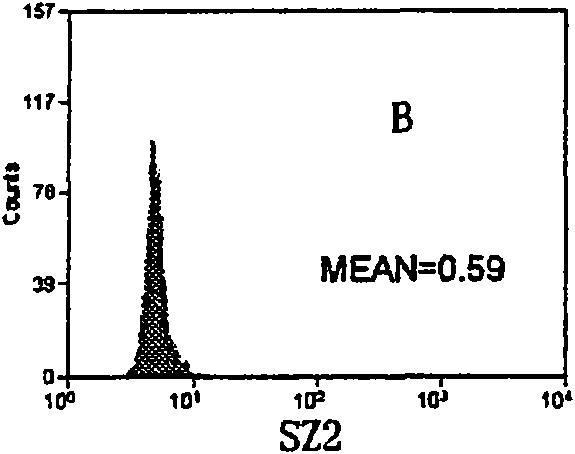
The invention relates to a set of a kit for detecting specificity platelet antoantibody by the combination of monoclonal antibody and nano-microspheres. The kit comprises each 3 ml of four types of monoclonal antibody-polystyrene nano-microspheres PBS solution, 6 ml goat-anti-human polyclonal antibody and PBS solution, wherein the four types of the monoclonal antibody-polystyrene nano-microspheres PBS solution are sealed by bovine serum albumin and are respectively connected with a monoclonal antibody SZ-2, a monoclonal antibody SZ-22, a monoclonal antibody SZ-21, a monoclonal antibody 7E3, and the concentration of polystyrene nano-microspheres is 2 mg / ml; the goat-anti-human polyclonal antibody is connected with fluorescein isothiocyanate and with the concentration of the goat-anti-human polyclonal antibody is 15 mug / ml. A detection process of the specificity platelet antoantibody comprises the following steps of: oscillating monoclonal antibody--microspheres and platelet lysis buffer sample for incubation; washing by PBS; adding the goat-anti-human polyclonal antibody connected with the fluorescein isothiocyanate; washing, re-suspending by the PBS; detecting on a flow cytometry to obtain average fluorescence intensity values of the four types of the nano-microspheres respectively; and confirming whether the specificity platelet antoantibody is positive or not according to a ratio of the obtained values to a basic contrast value of that of a healthy person, in order to provide foundation for early diagnosis of ITP. The detection has the advantages of simple process, high sensitivity and good specificity.
Owner:苏州苏大赛尔免疫生物技术有限公司 +1
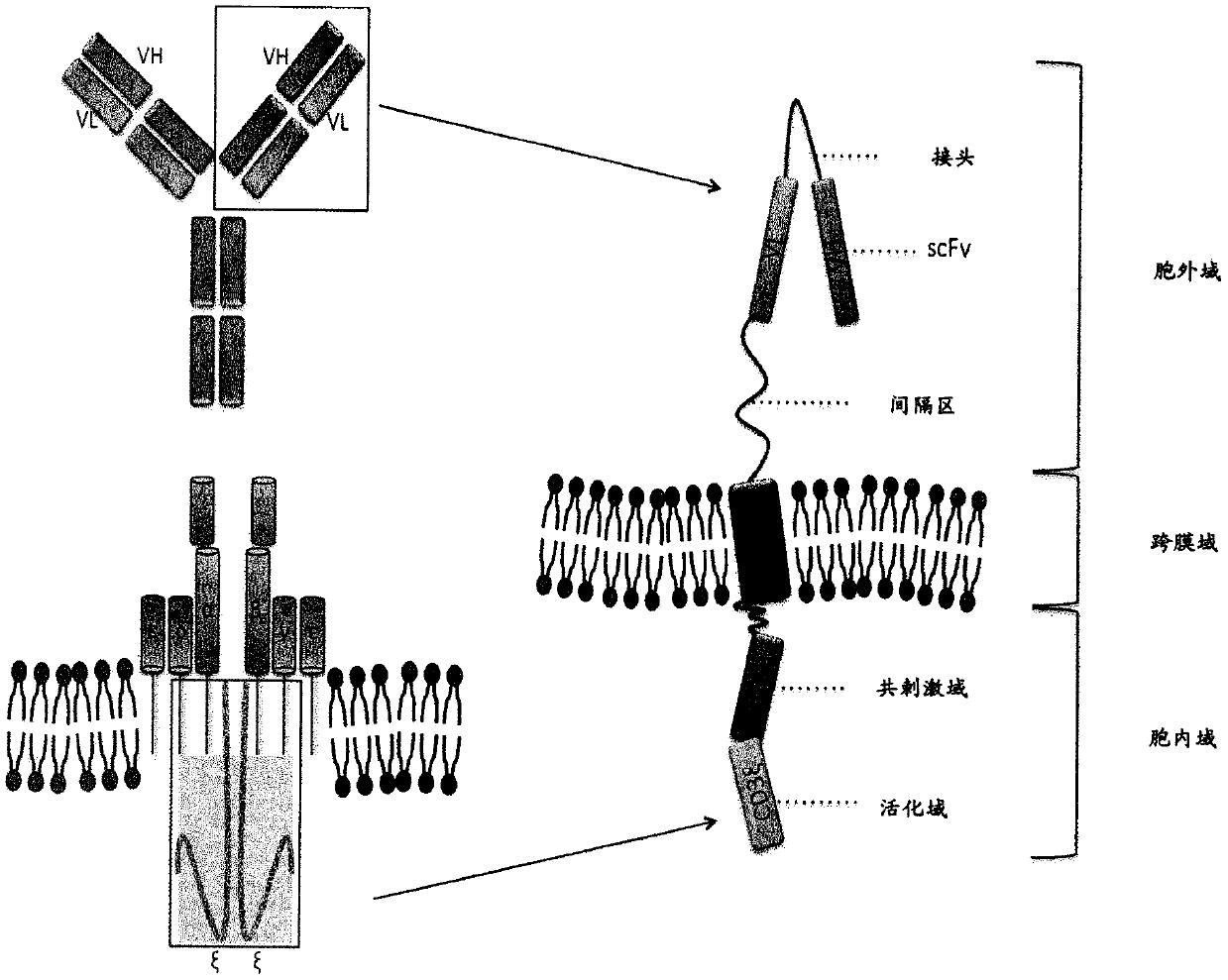
Chimeric antigen receptor and car-t cells that bind bcma
PendingCN109641012AHigh biosecurityNo off-target reactivityAntipyreticAnalgesicsAutoimmune conditionAutoantibody
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
Vaccine
The present invention relates to an isolated polypeptide useful for immunisation against self-antigens. In particular the invention relates to a self-protein that is capable of raising auto-antibodies when administered in vivo. The invention particularly relates to rendering human cytokines immunogenic in humans. The invention further relates to pharmaceutical compositions comprising such compounds and their use in medicine and to methods for their production.
Owner:GLAXO GRP LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com