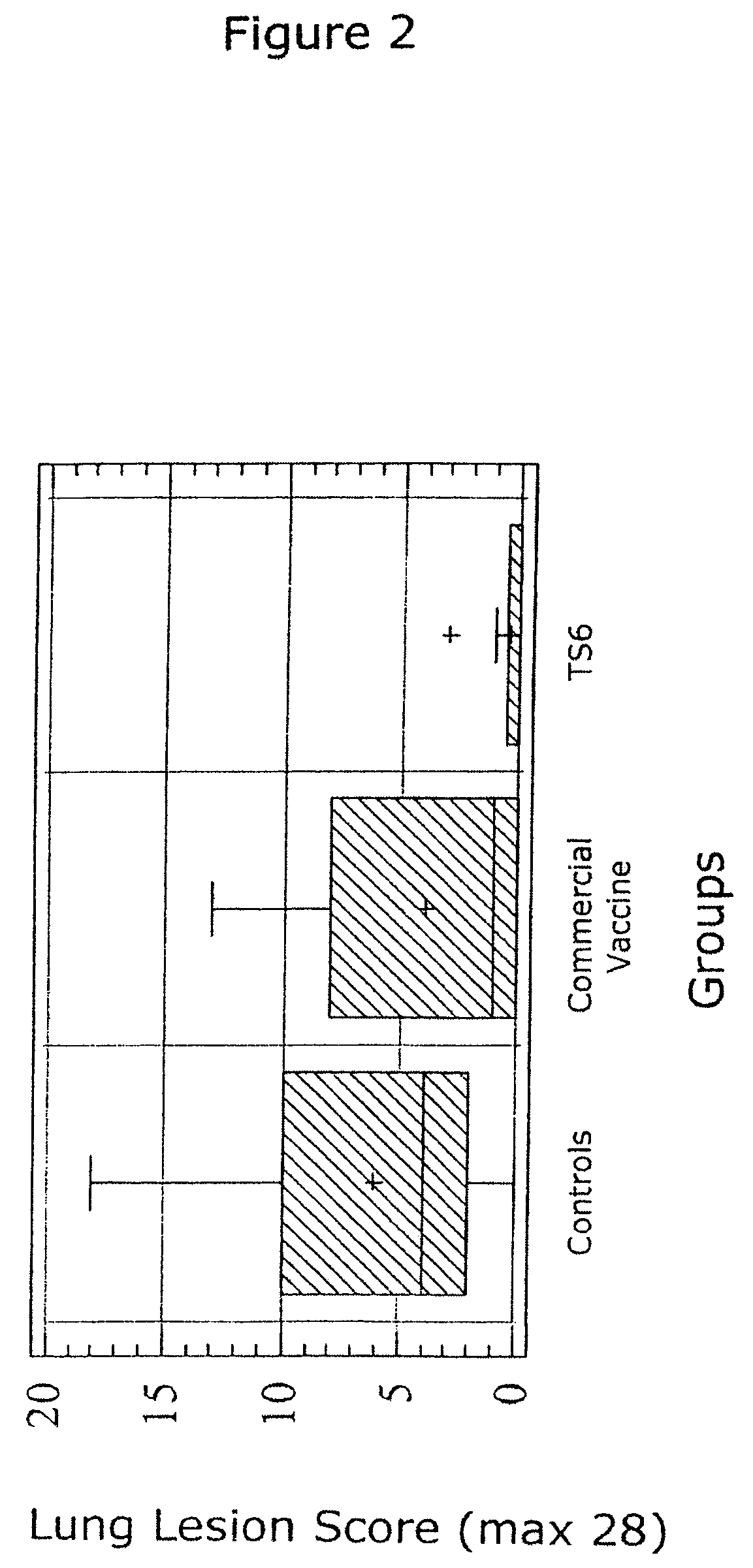
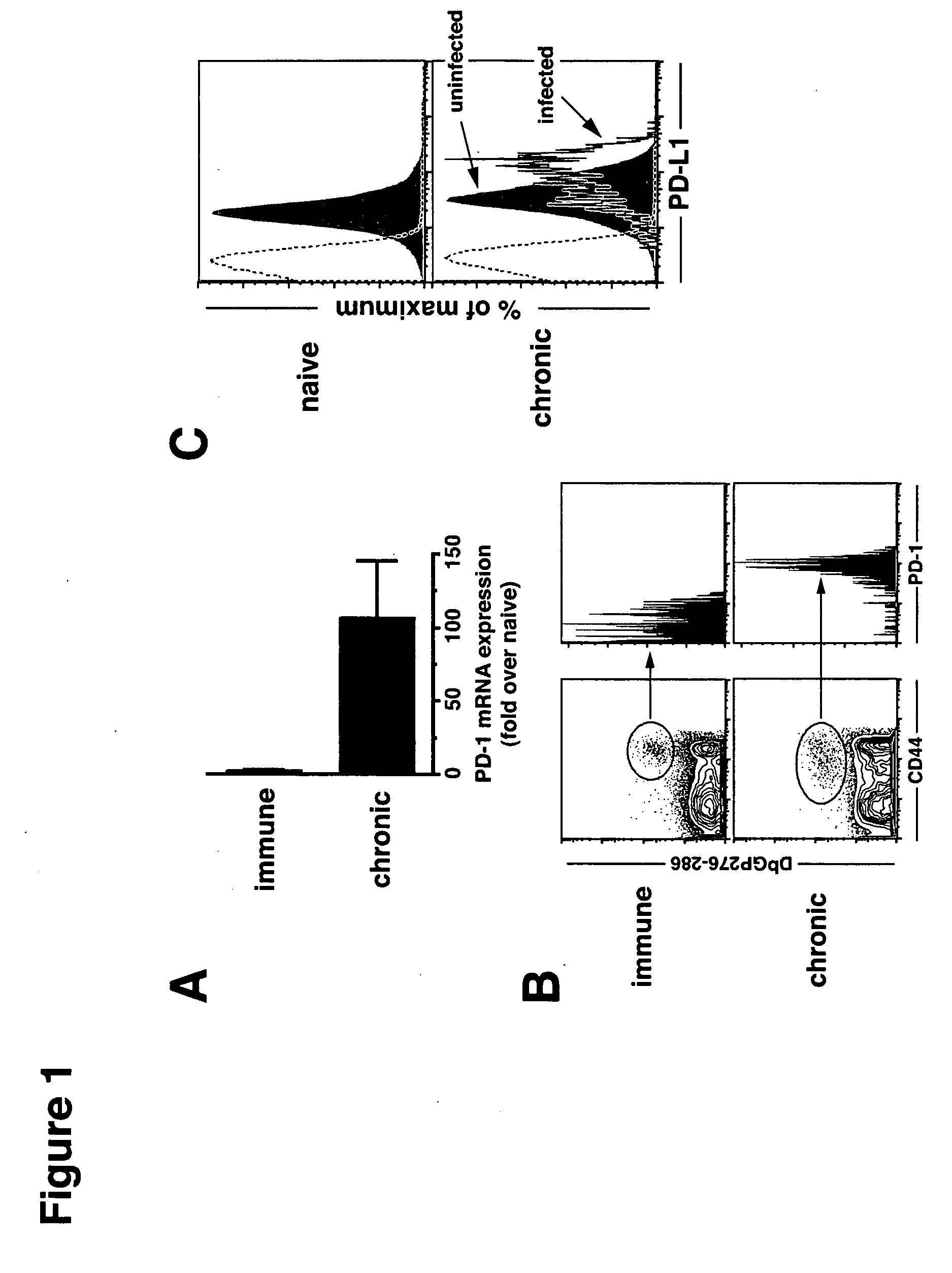
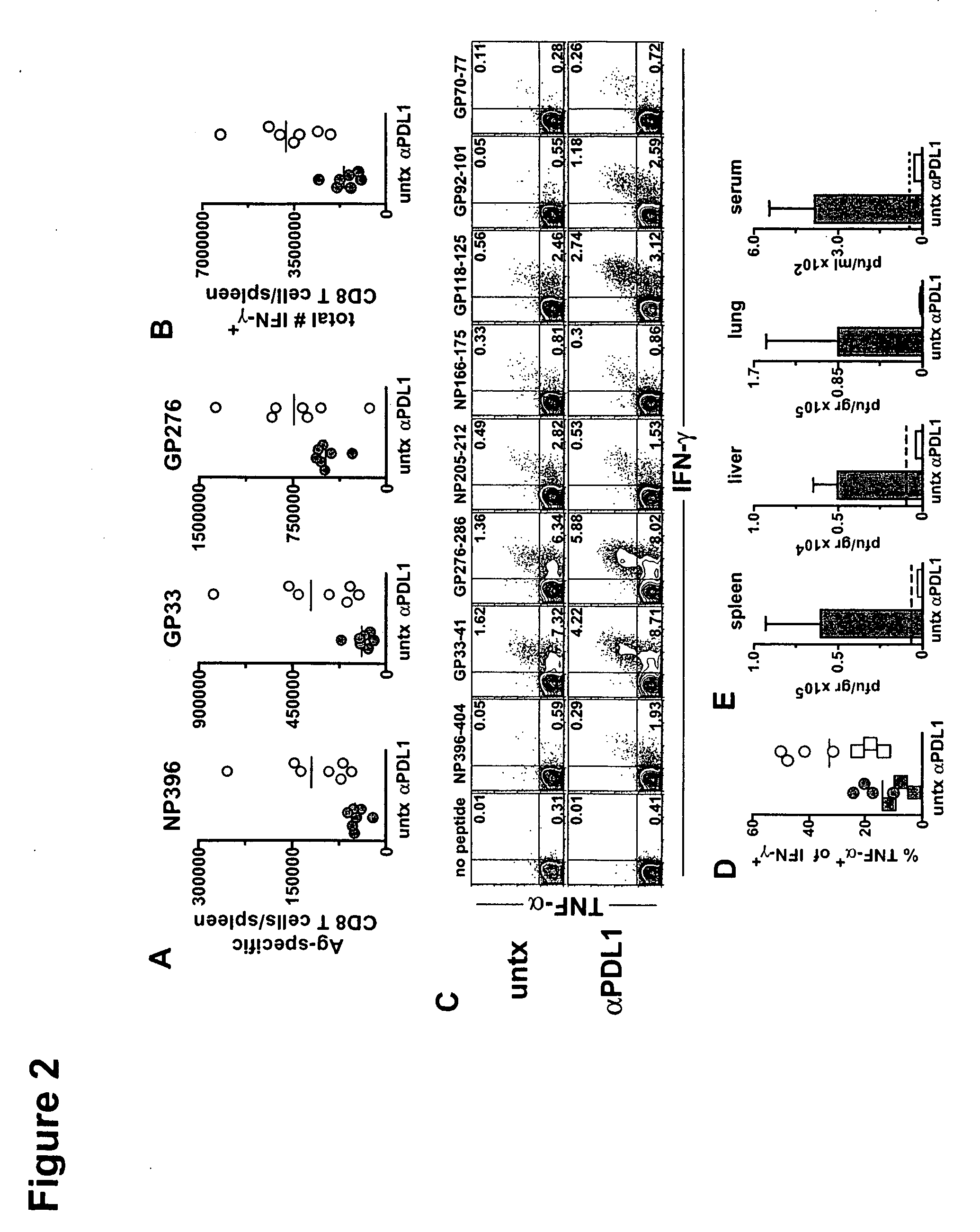
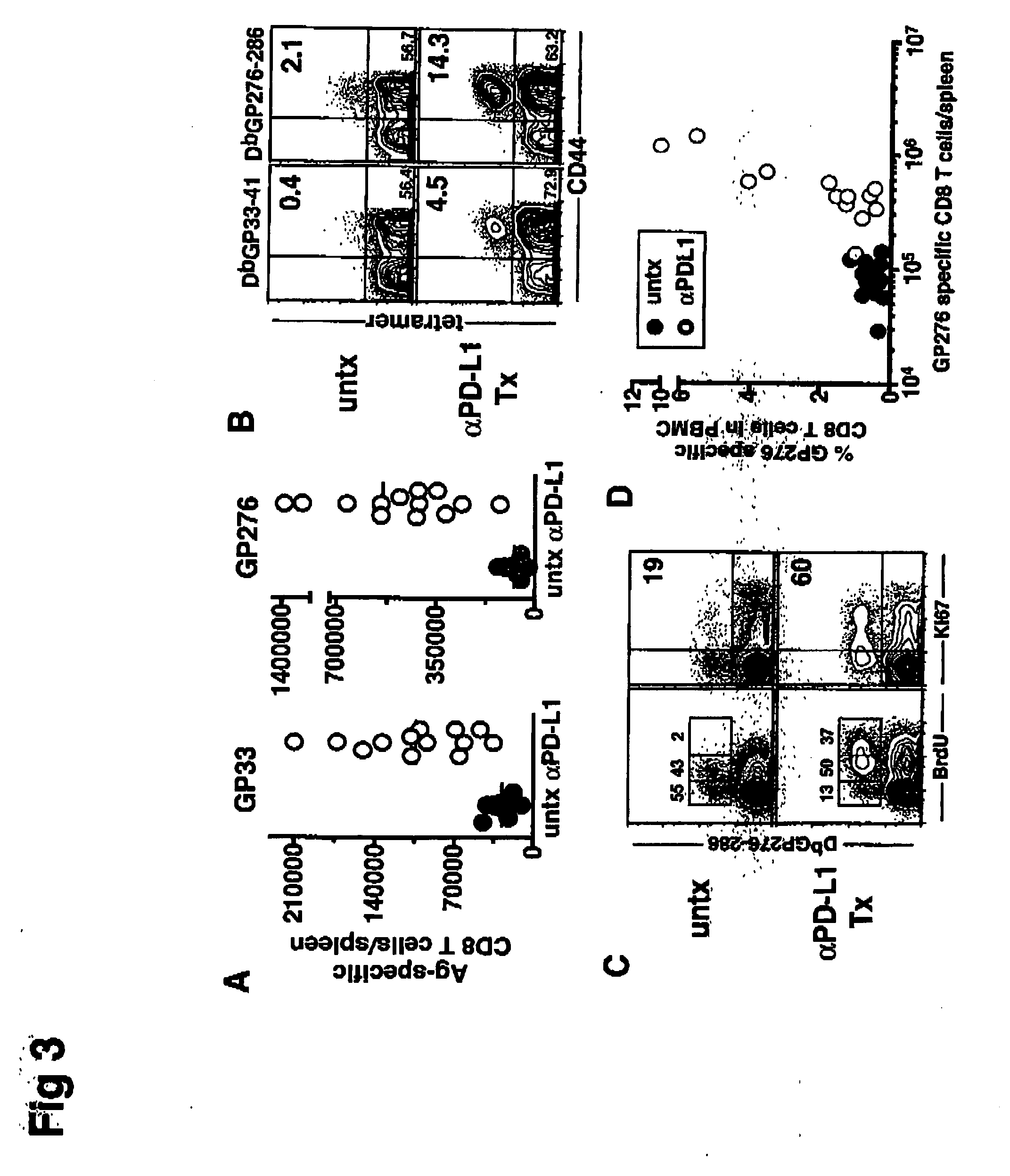
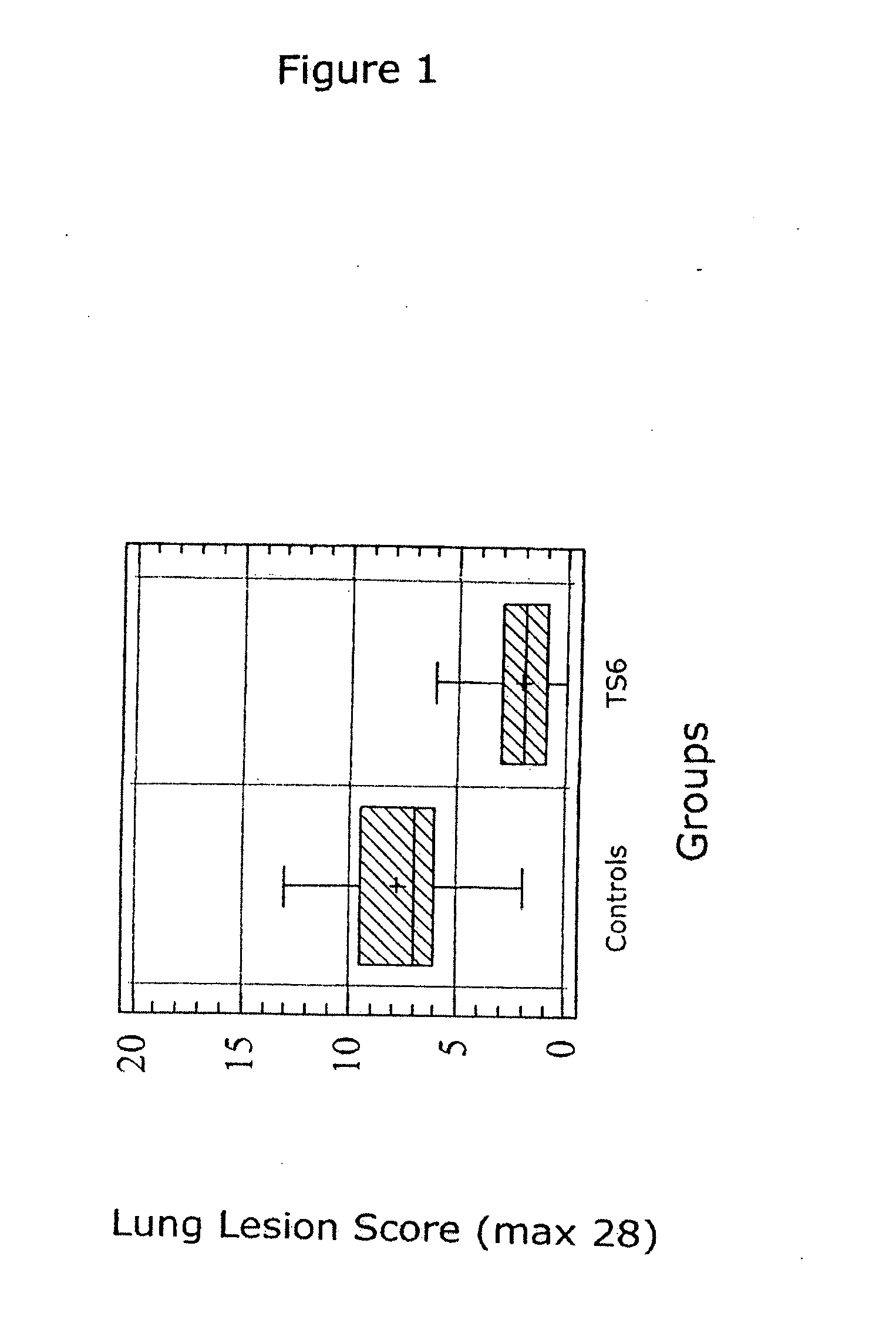
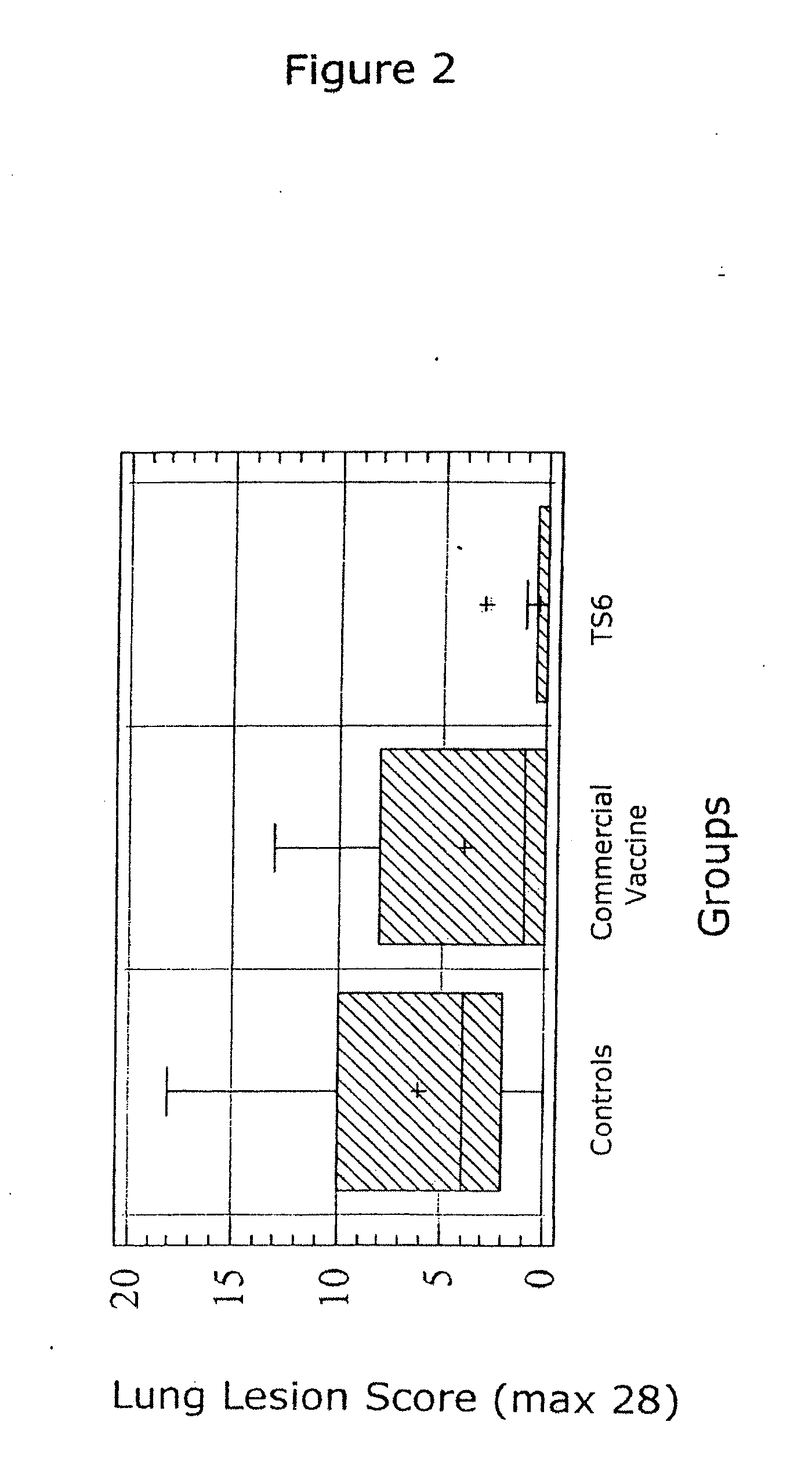
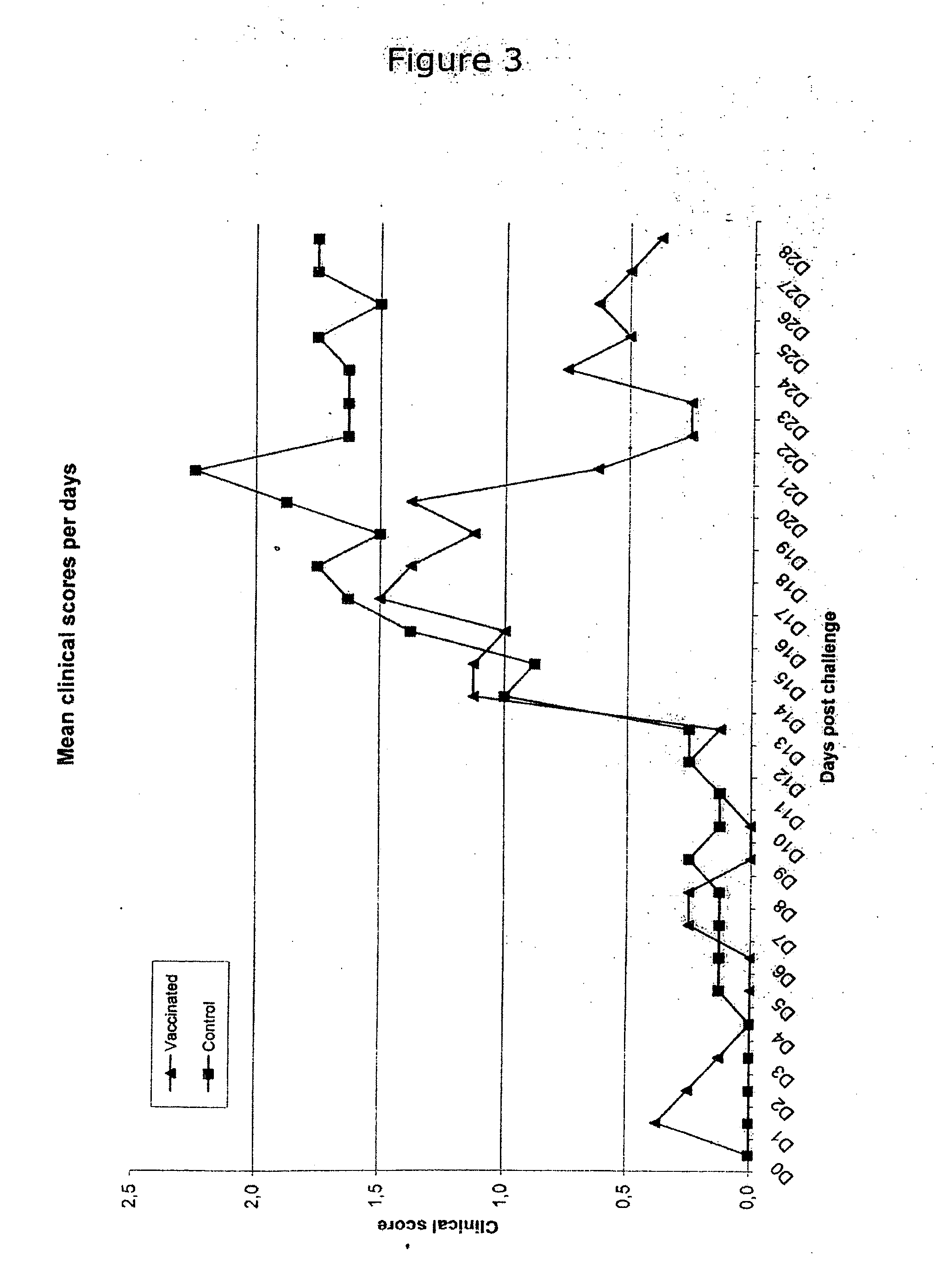
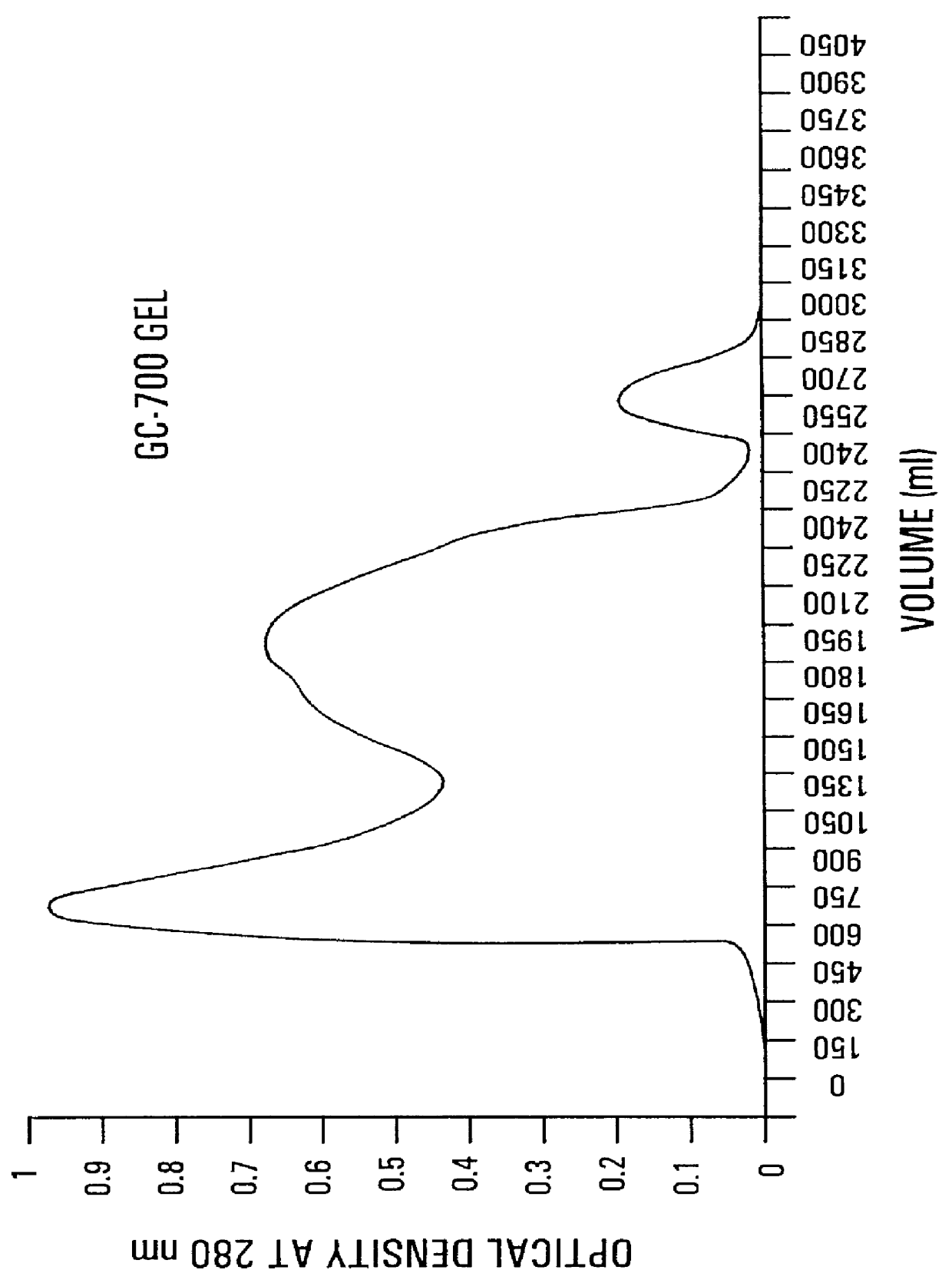
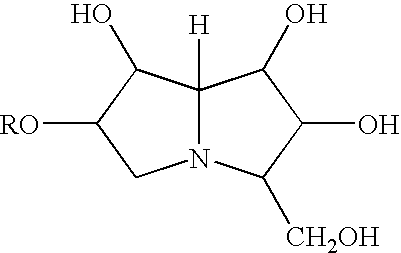
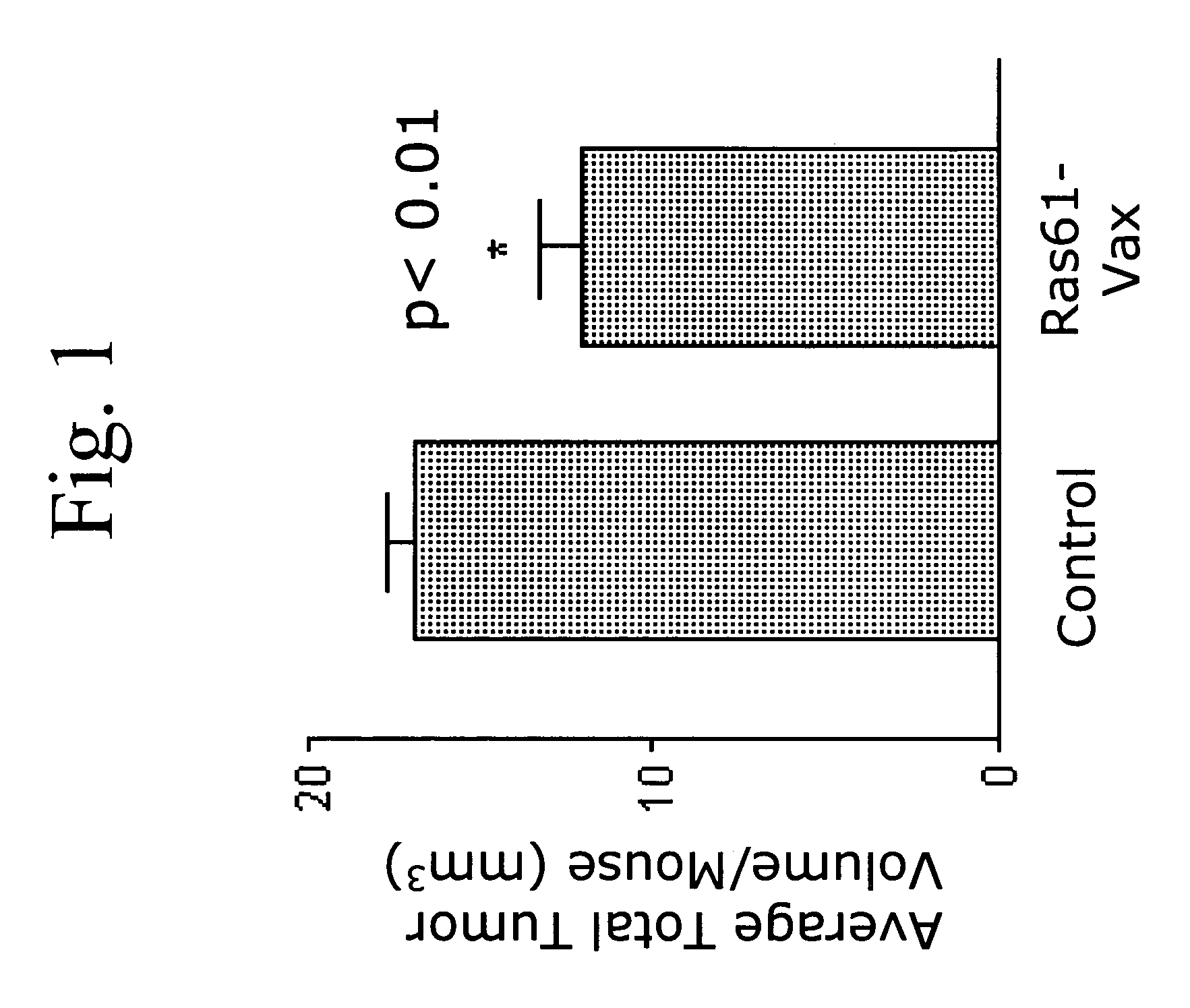
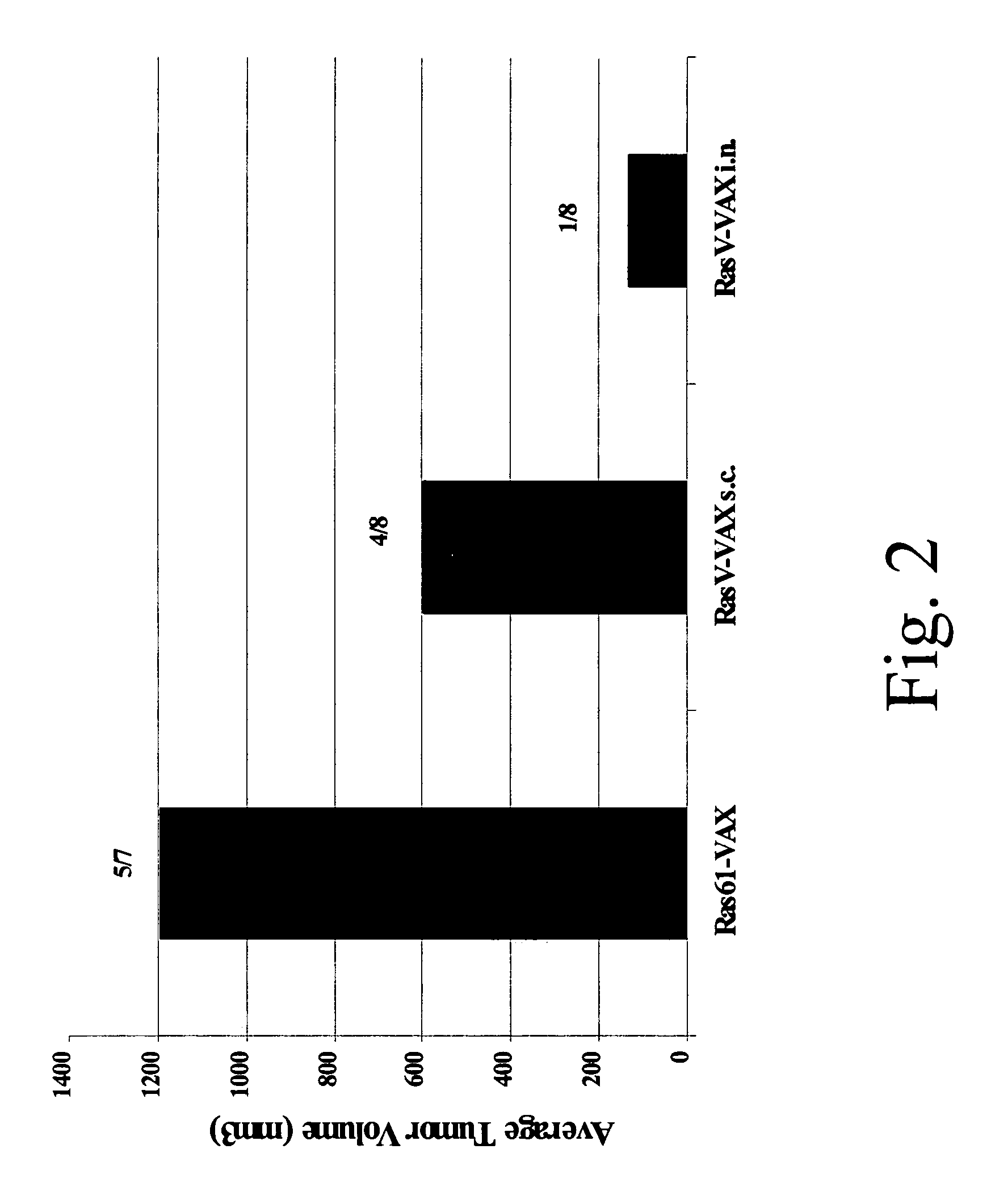
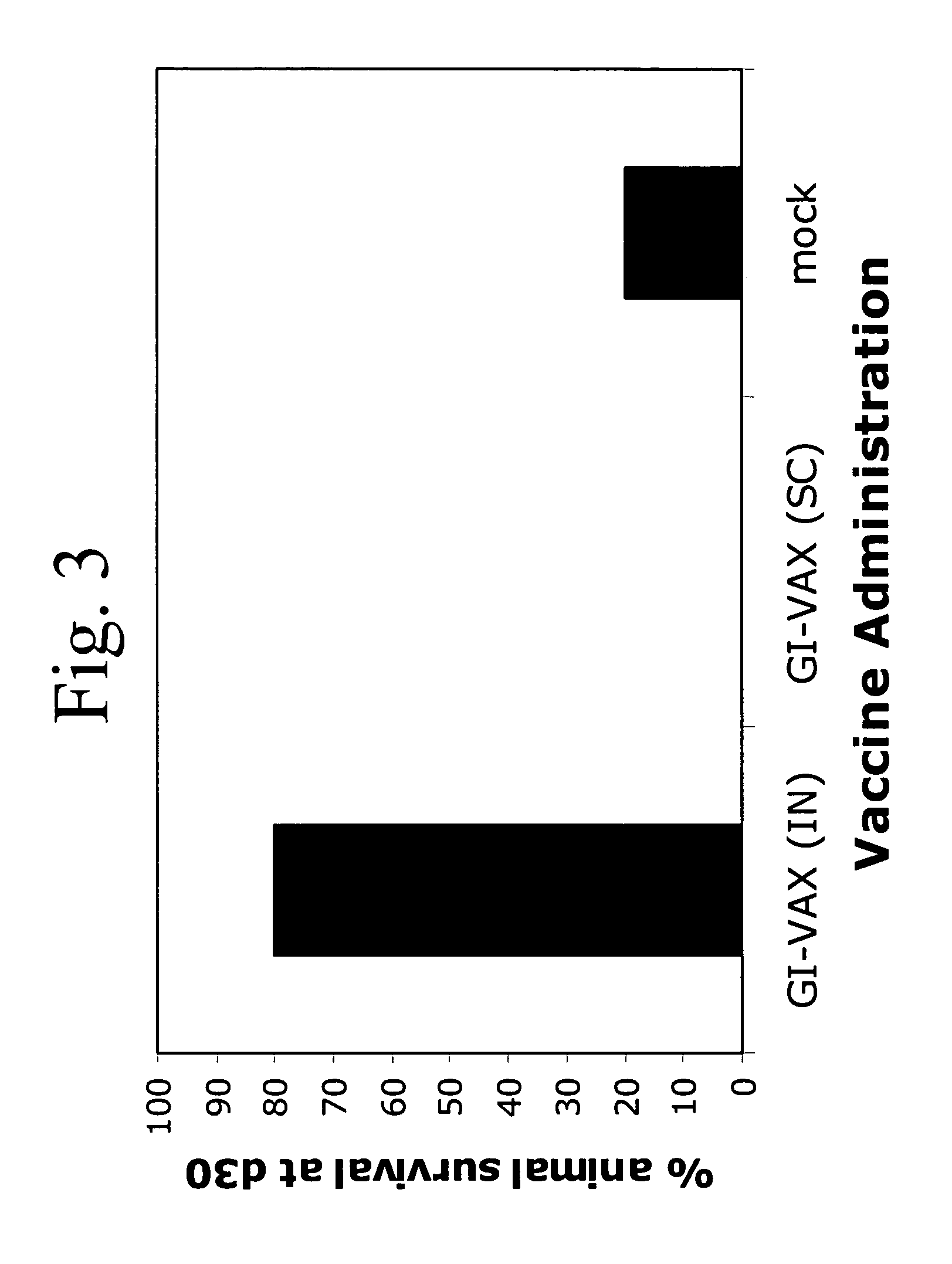
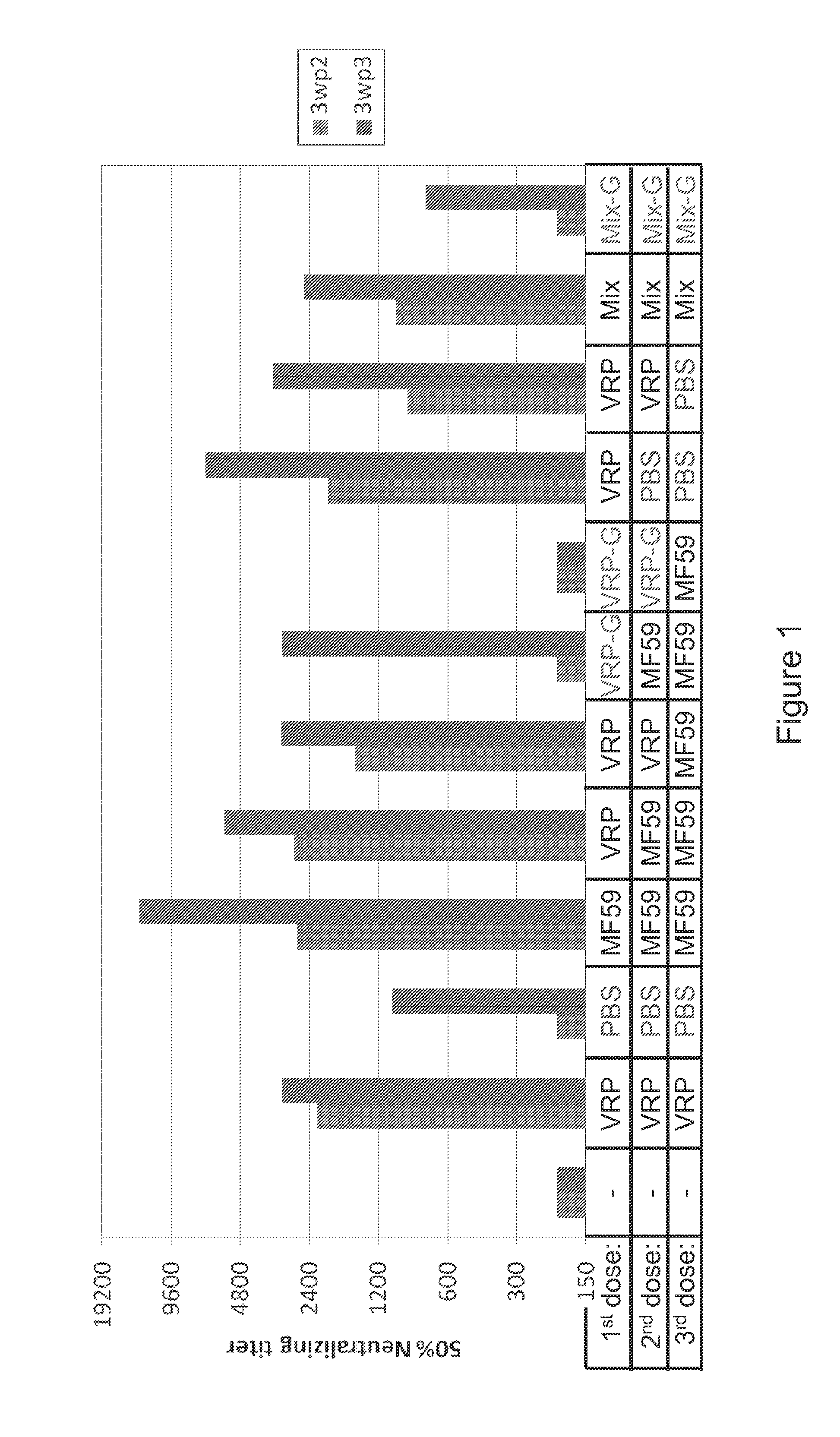
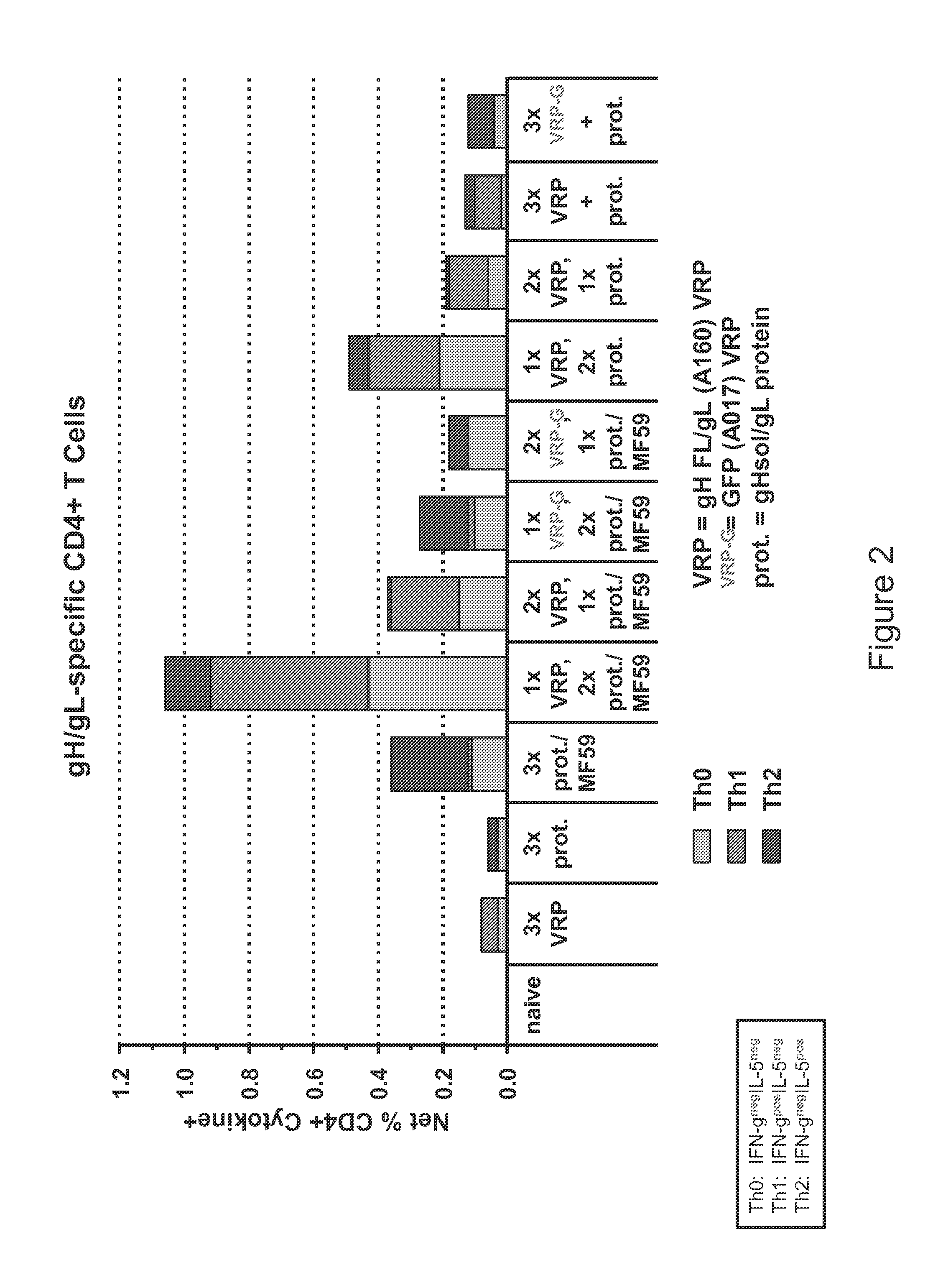
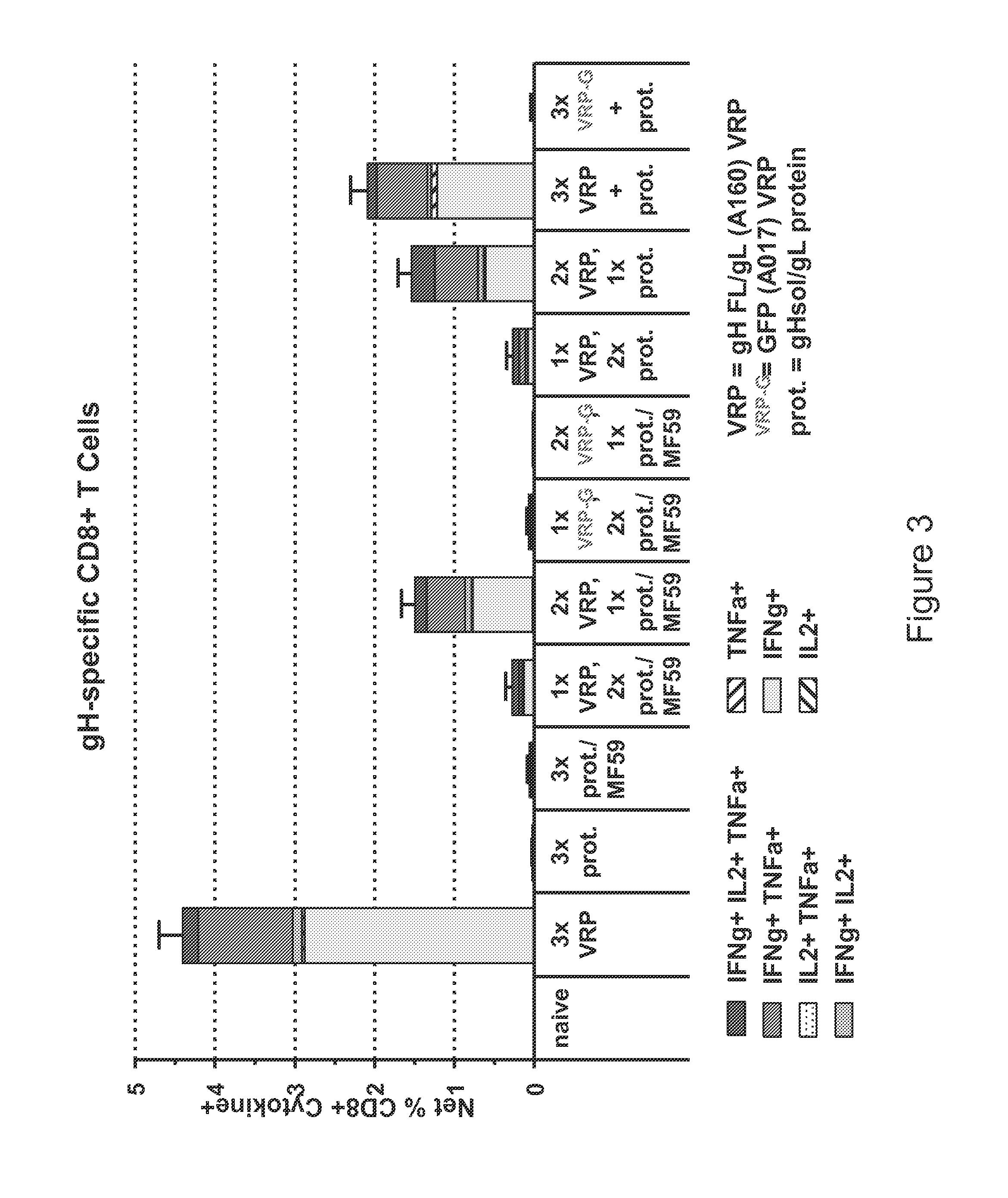
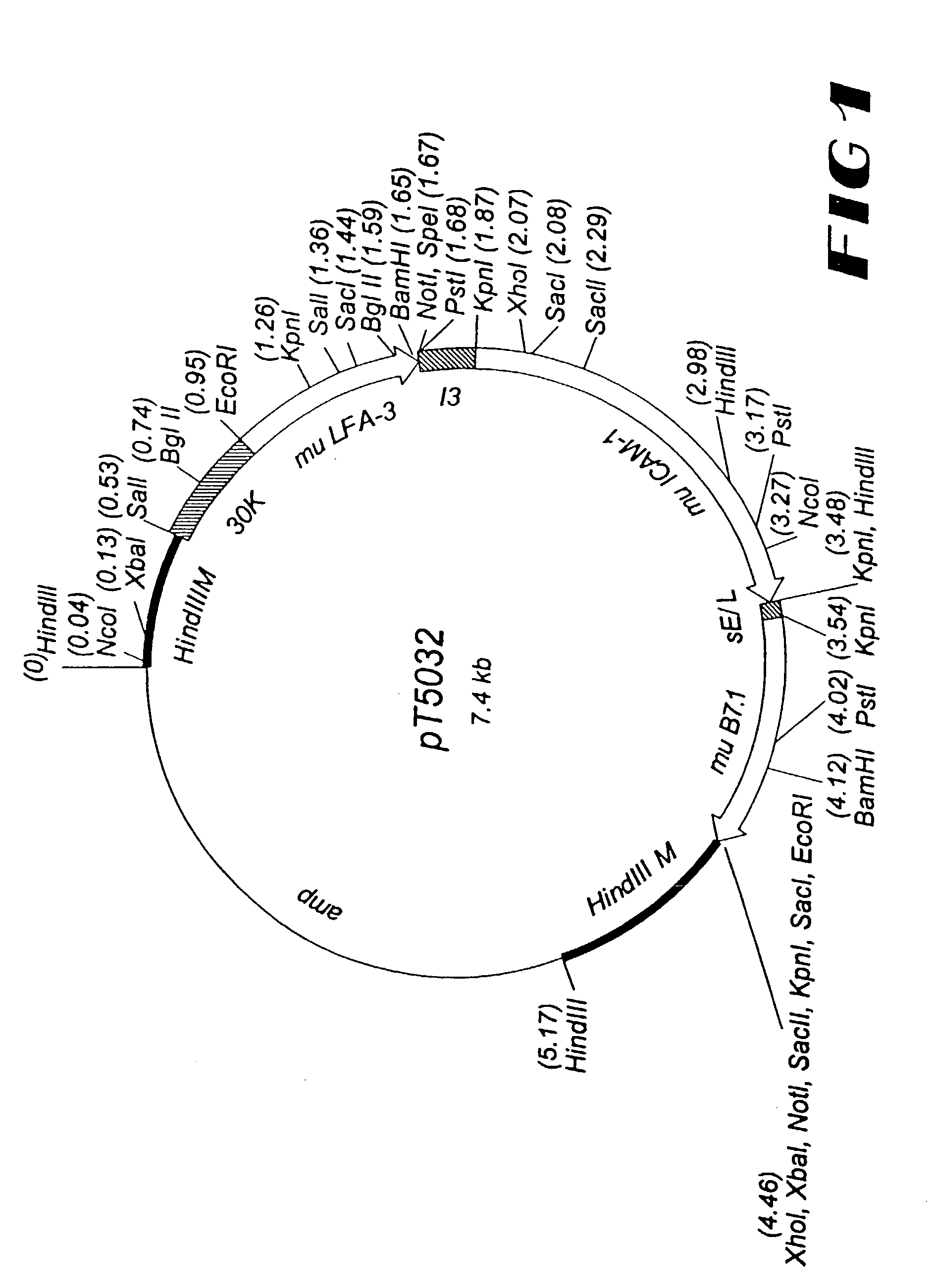
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1878results about How to "Enhance immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
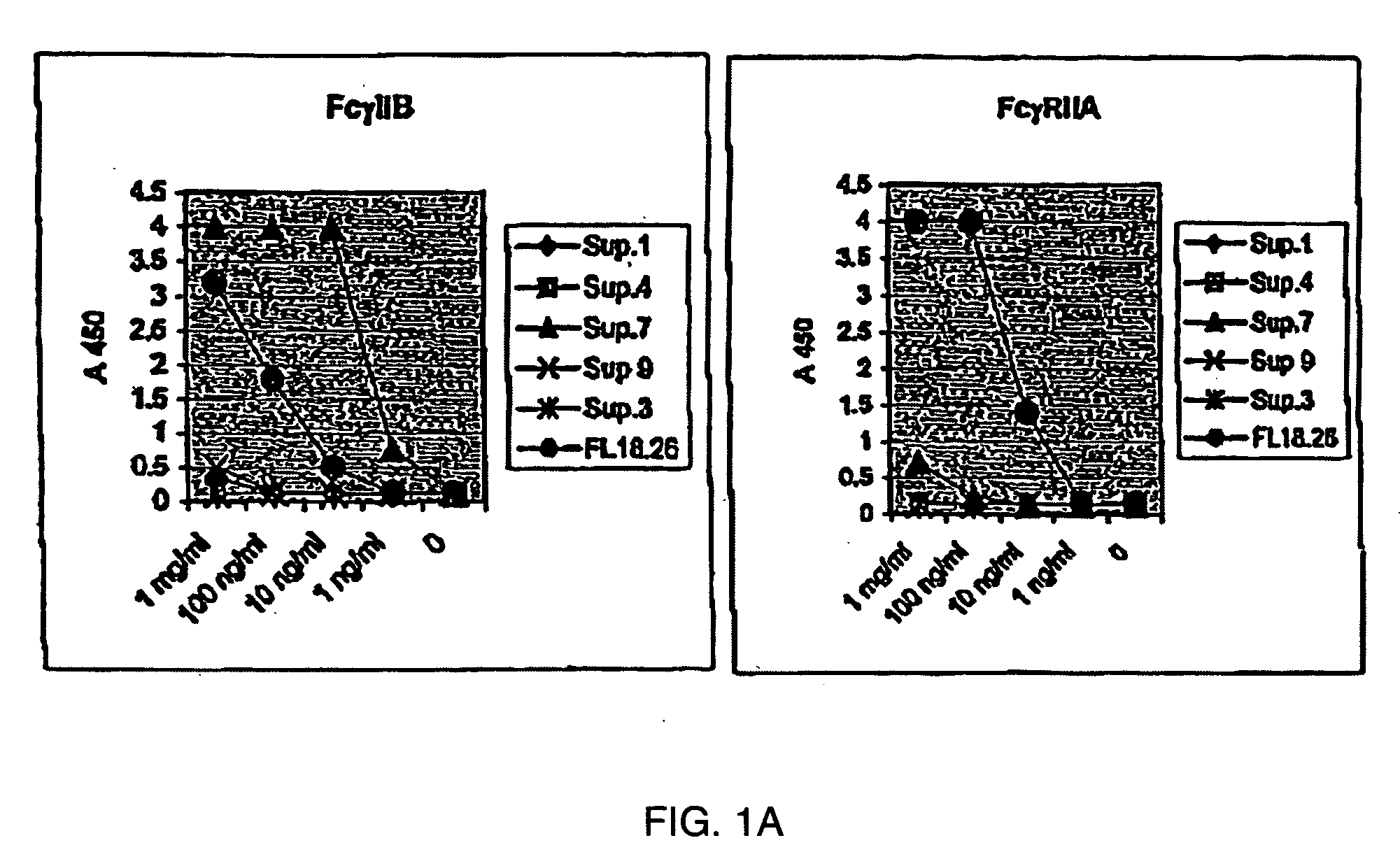
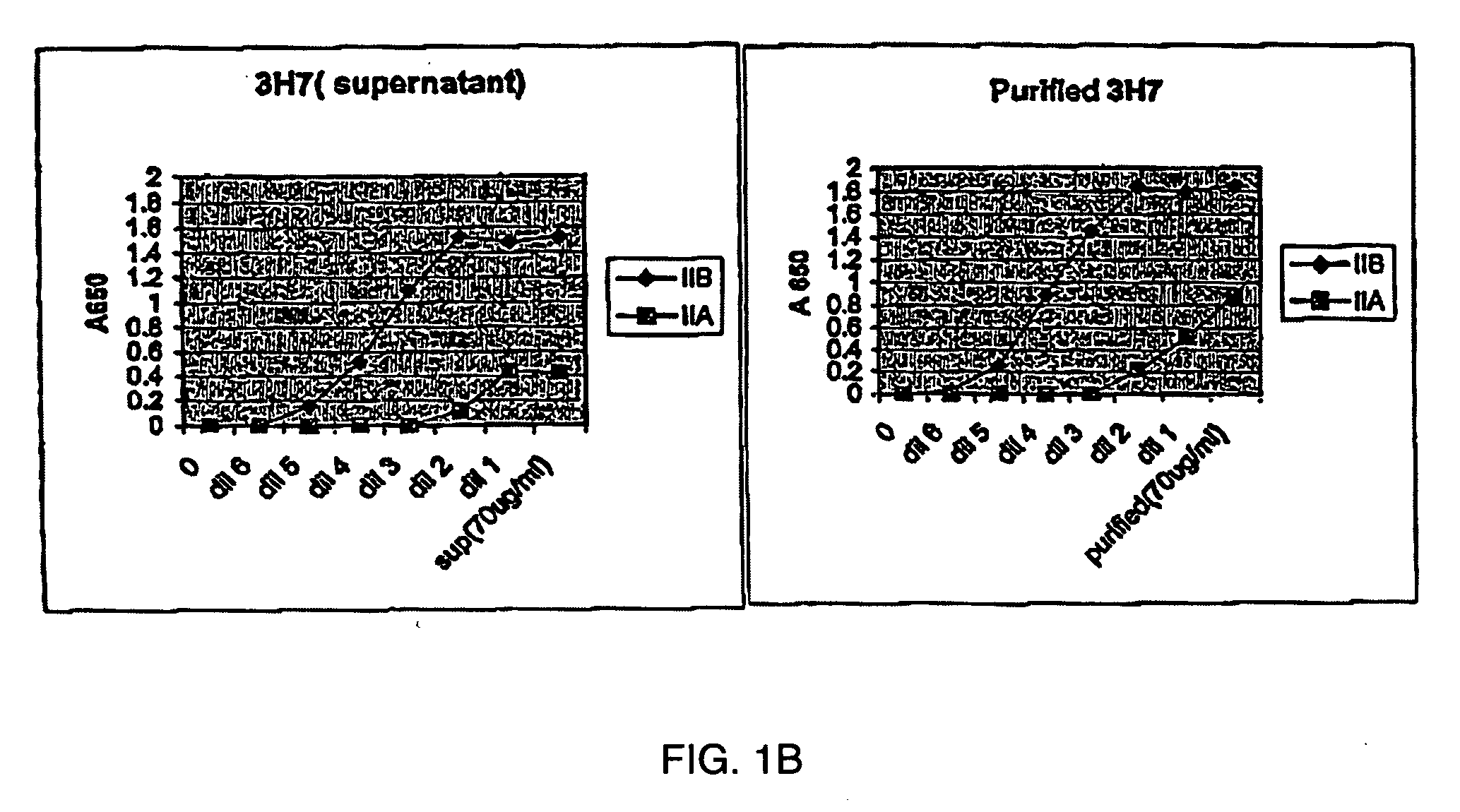
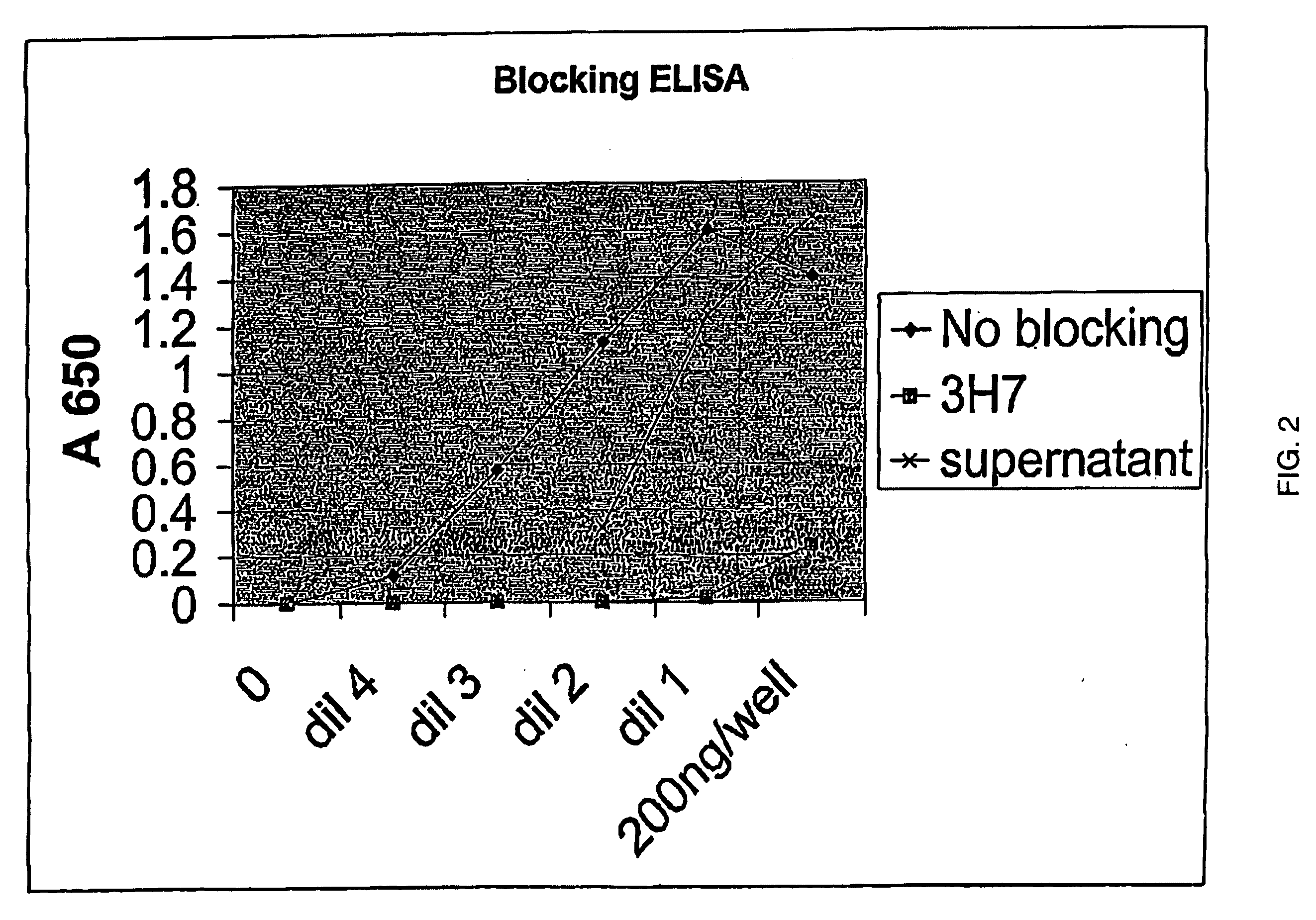
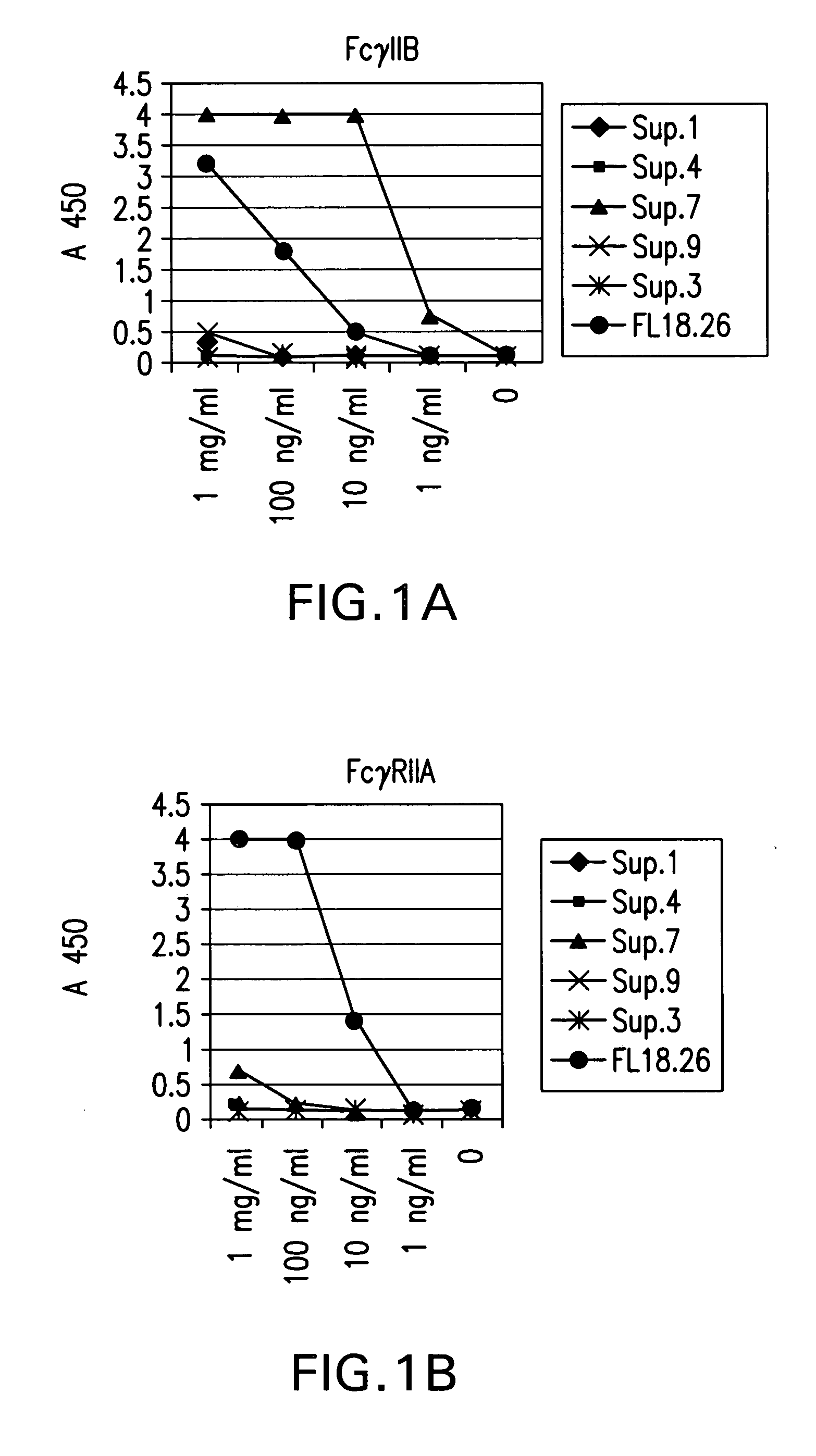
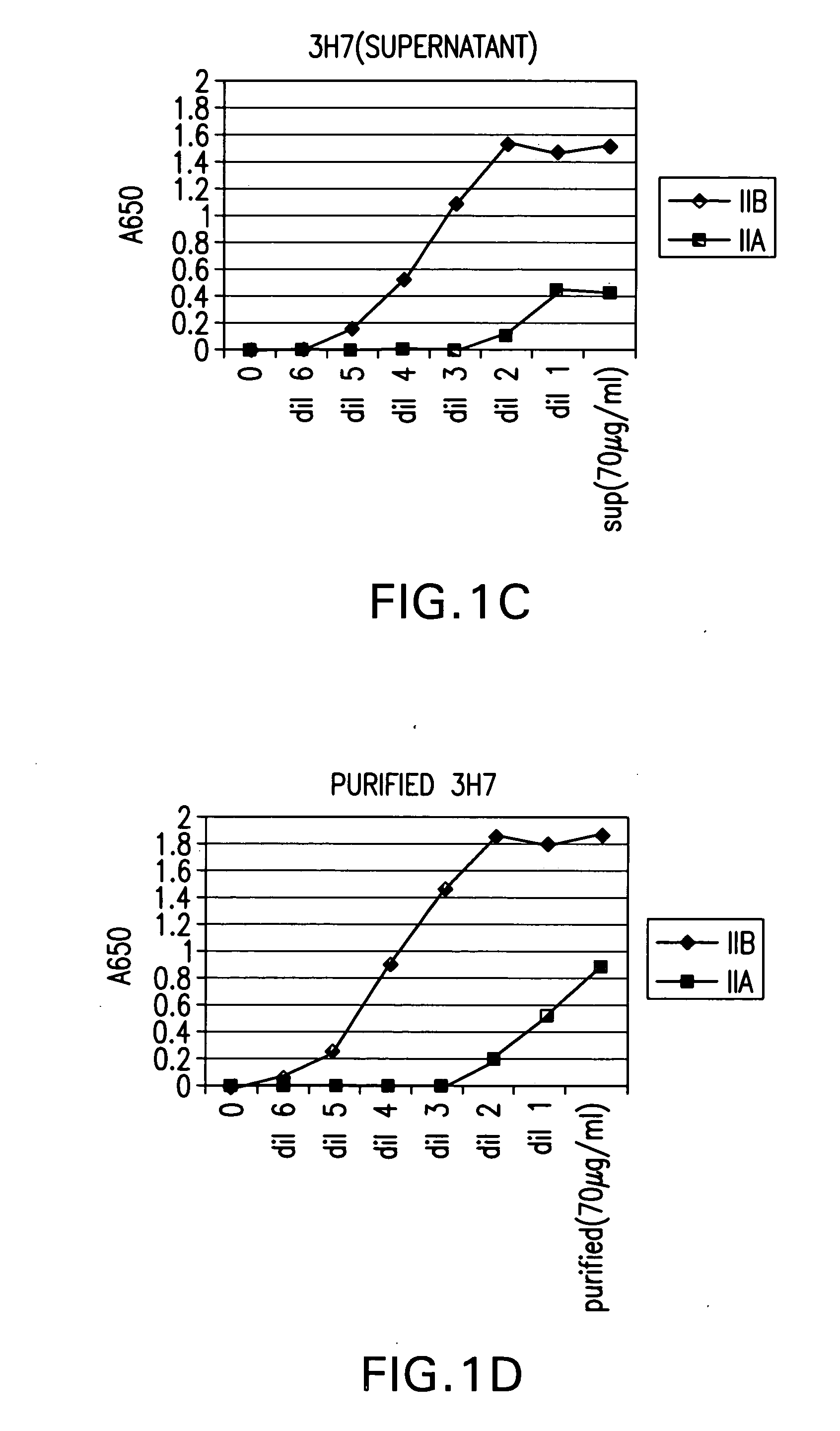
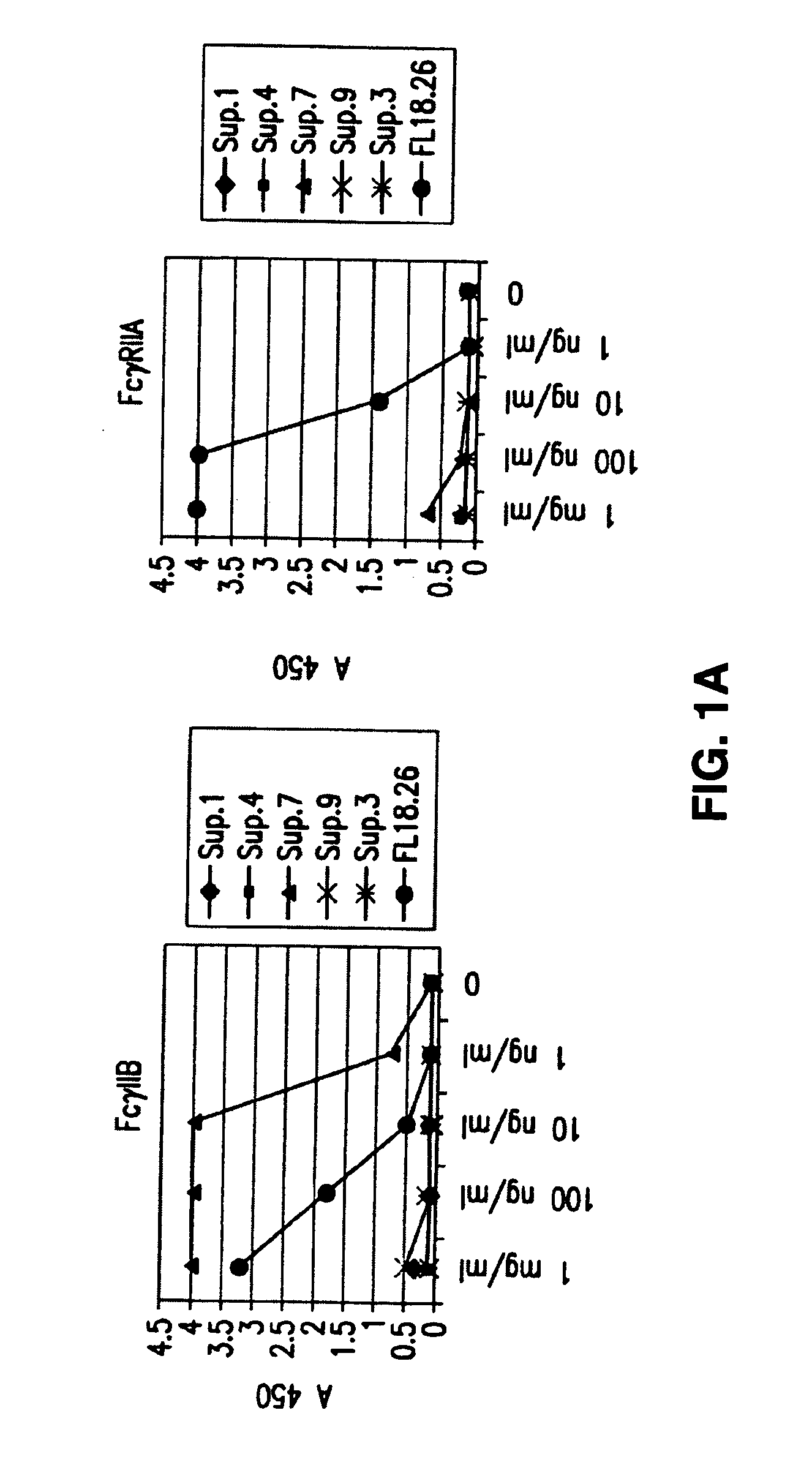
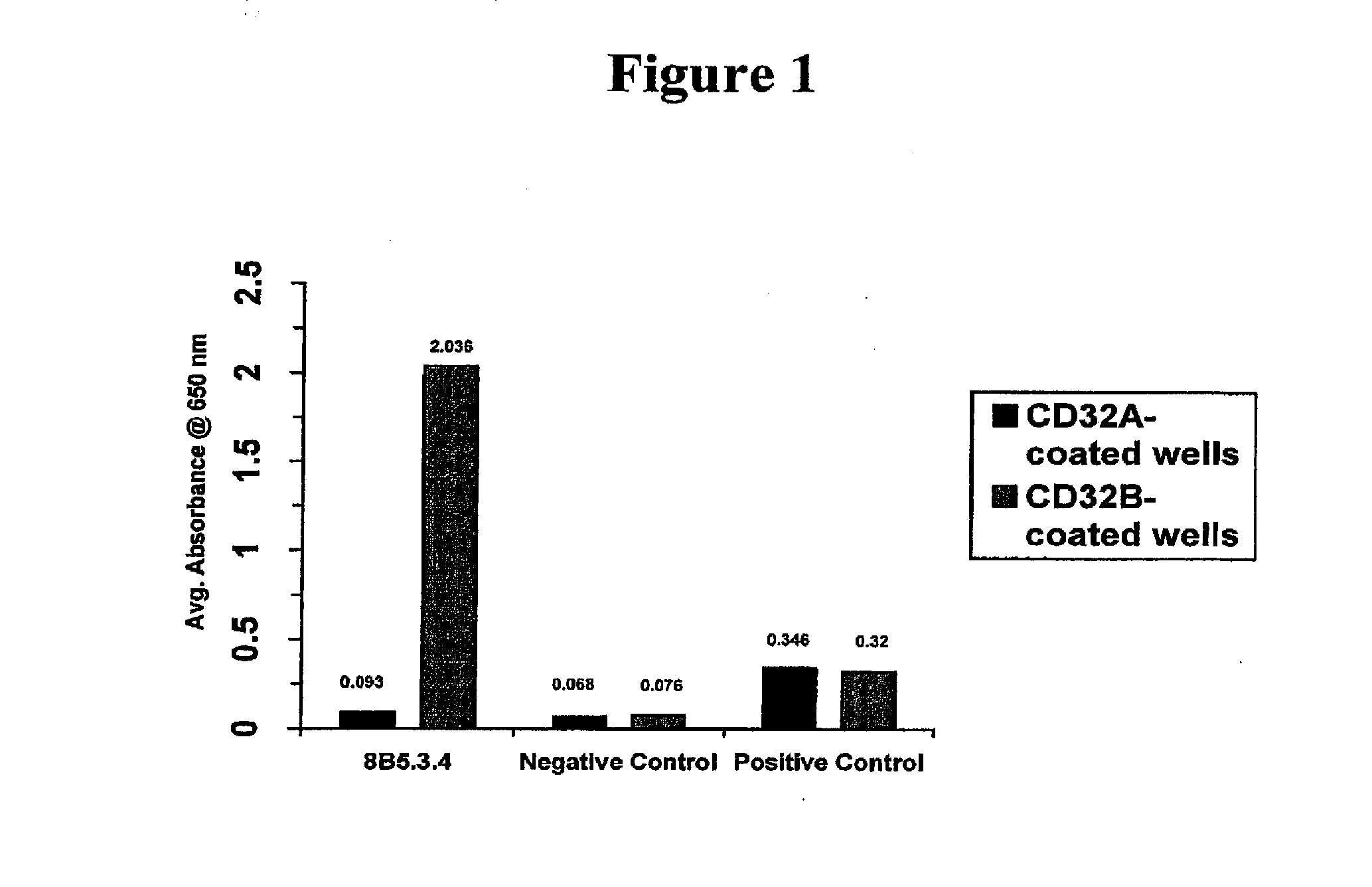
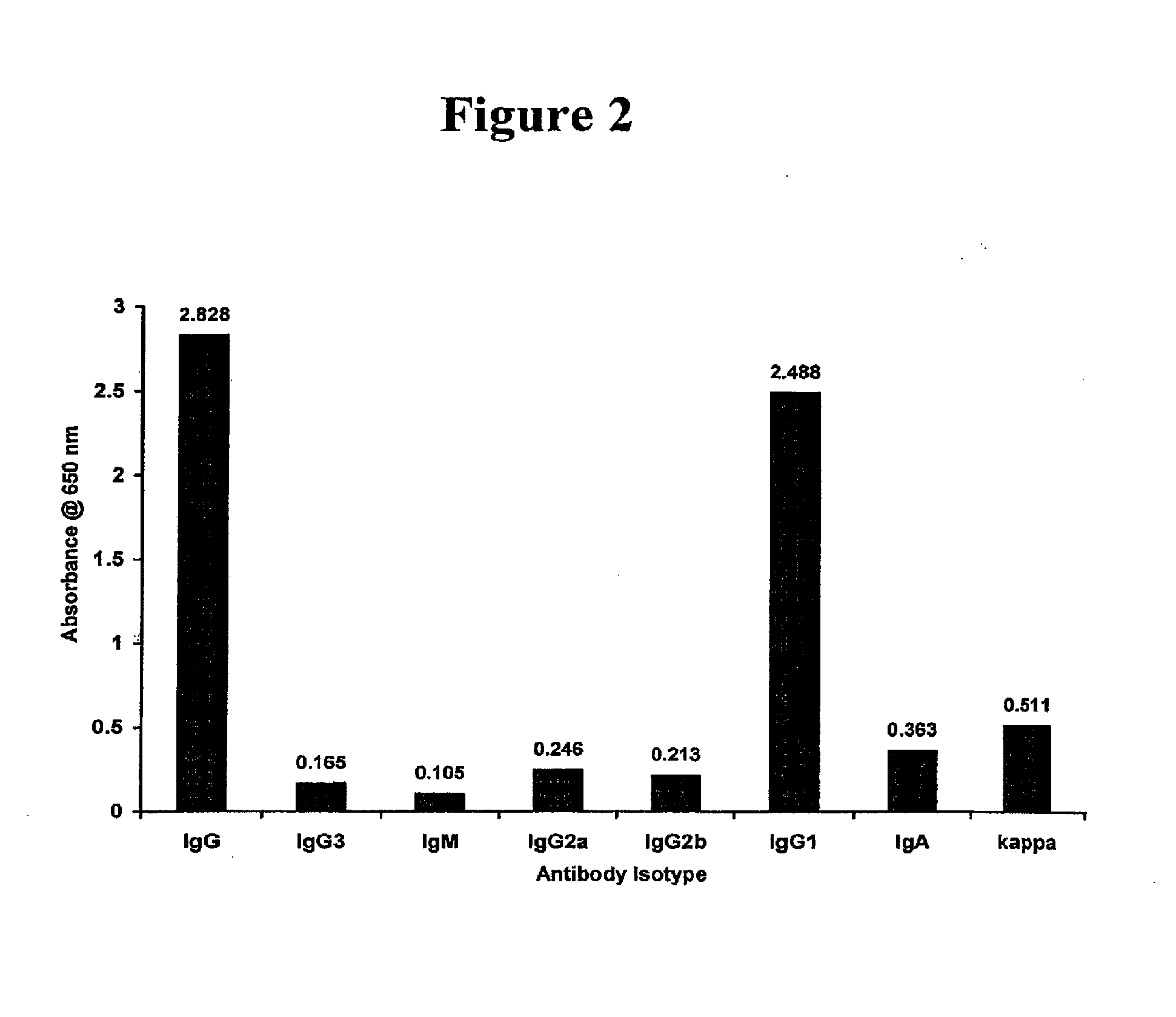
Fcgamma-RIIB-specific antibodies and methods of use thereof
InactiveUS20050260213A1Good curative effectAvoid managementHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen Binding FragmentTherapeutic effect
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Fcgamma riib specific antibodies and methods of use thereof
InactiveUS20050215767A1Enhance immune responseImprove responseSenses disorderAntipyreticTherapeutic antibodyAntiendomysial antibodies
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Vaccine formulations
ActiveUS7371395B2Improve stabilityStable and safe and easily administrableSsRNA viruses negative-senseAntibacterial agentsPlasmidBacilli
Owner:MERIAL INC
Methods and compositions for the treatment of persistent infections
ActiveUS20070122378A1Reduced activityReduce expressionAntibacterial agentsOrganic active ingredientsMicrobiologyPathology
The present invention provides methods and compositions for the treatment, prevention, or reduction of persistent infections, such as chronic infections, latent infections, and slow infections and cancer. The methods and compositions of the invention are also useful for the alleviation of one or more symptoms associated with such infections and cancer.
Owner:DANA FARBER CANCER INST INC +3
Variant IgG3 Rituxan and therapeutic use thereof
InactiveUS20020128448A1Prevent and reduce proliferation of cellReduce and prevent proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Antigen binding
Monoclonal anti-human CD20 antigen binding antibodies containing human IgG3 constant domains are provided. These antibodies possess effector functions that render them well suited for use in therapeutic methods, especially treatments wherein inhibition of B cell function or B cell number is therapeutically desirable.
Owner:BIOGEN INC
Yeast-dendritic cell vaccines and uses thereof
Owner:UNIV OF COLORADO THE REGENTS OF +1
DNA vaccines encoding antigen linked to a domain that binds CD40
InactiveUS7118751B1Improve abilitiesEasy to demonstrateAntibody mimetics/scaffoldsVirus peptidesPeptide antigenEukaryotic plasmids
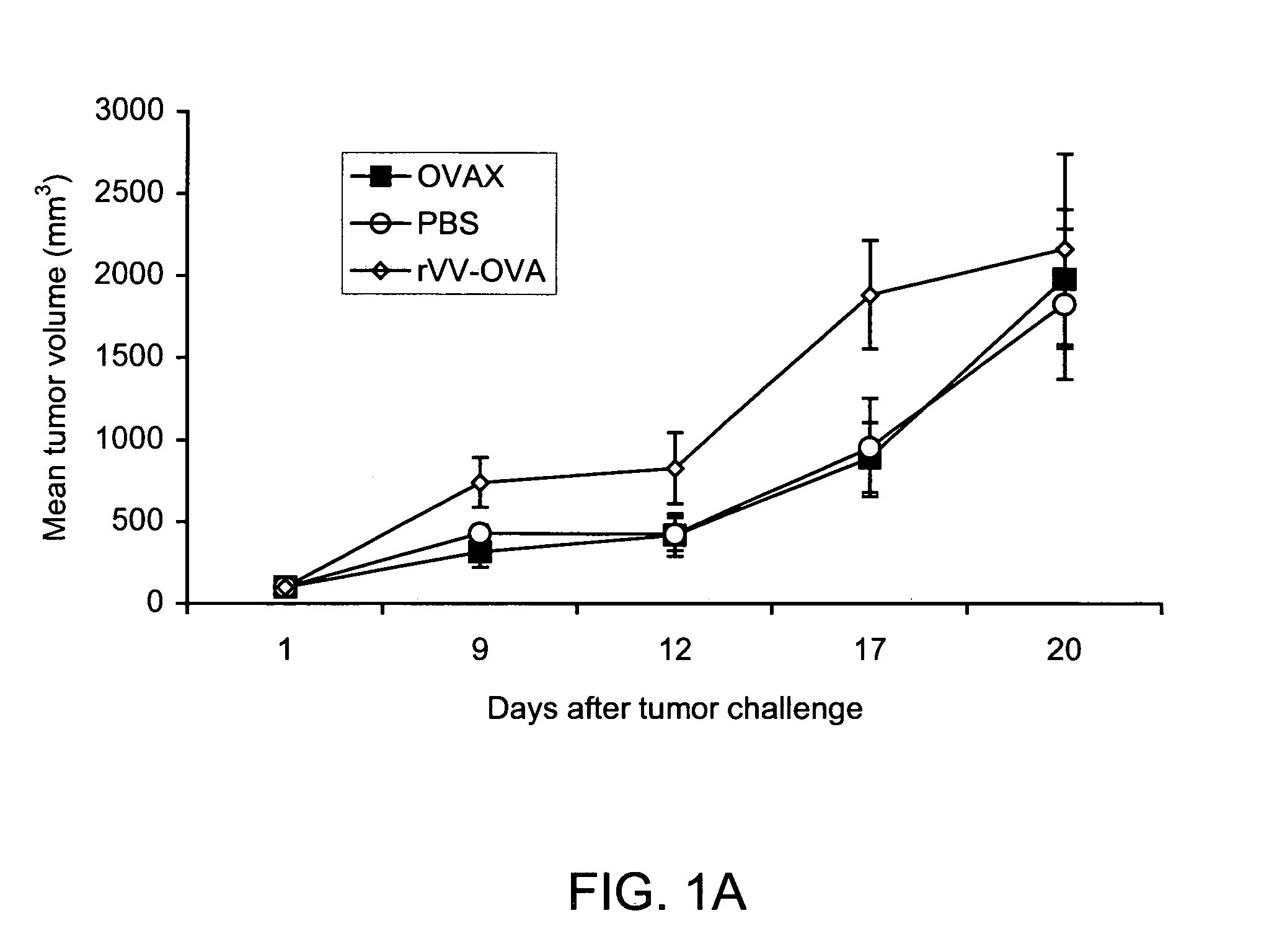
Vaccines that target one or more antigens to a cell surface receptor improve the antigen-specific humoral and cellular immune response. Antigen(s) linked to a domain that binds to a cell surface receptor are internalized, carrying antigen(s) into an intracellular compartment where the antigen(s) are digested into peptides and loaded onto MHC molecules. T cells specific for the peptide antigens are activated, leading to an enhanced immune response. The vaccine may comprise antigen(s) linked to a domain that binds at least one receptor or a DNA plasmid encoding antigen(s) linked to a domain that binds at least one receptor. A preferred embodiment of the invention targets HIV-1 env antigen to the CD40 receptor, resulting in delivery of antigen to CD40 positive cells, and selective activation of the CD40 receptor on cells presenting HIV-1 env antigens to T cells.
Owner:HAYDEN LEDBETTER MARTHA S +1
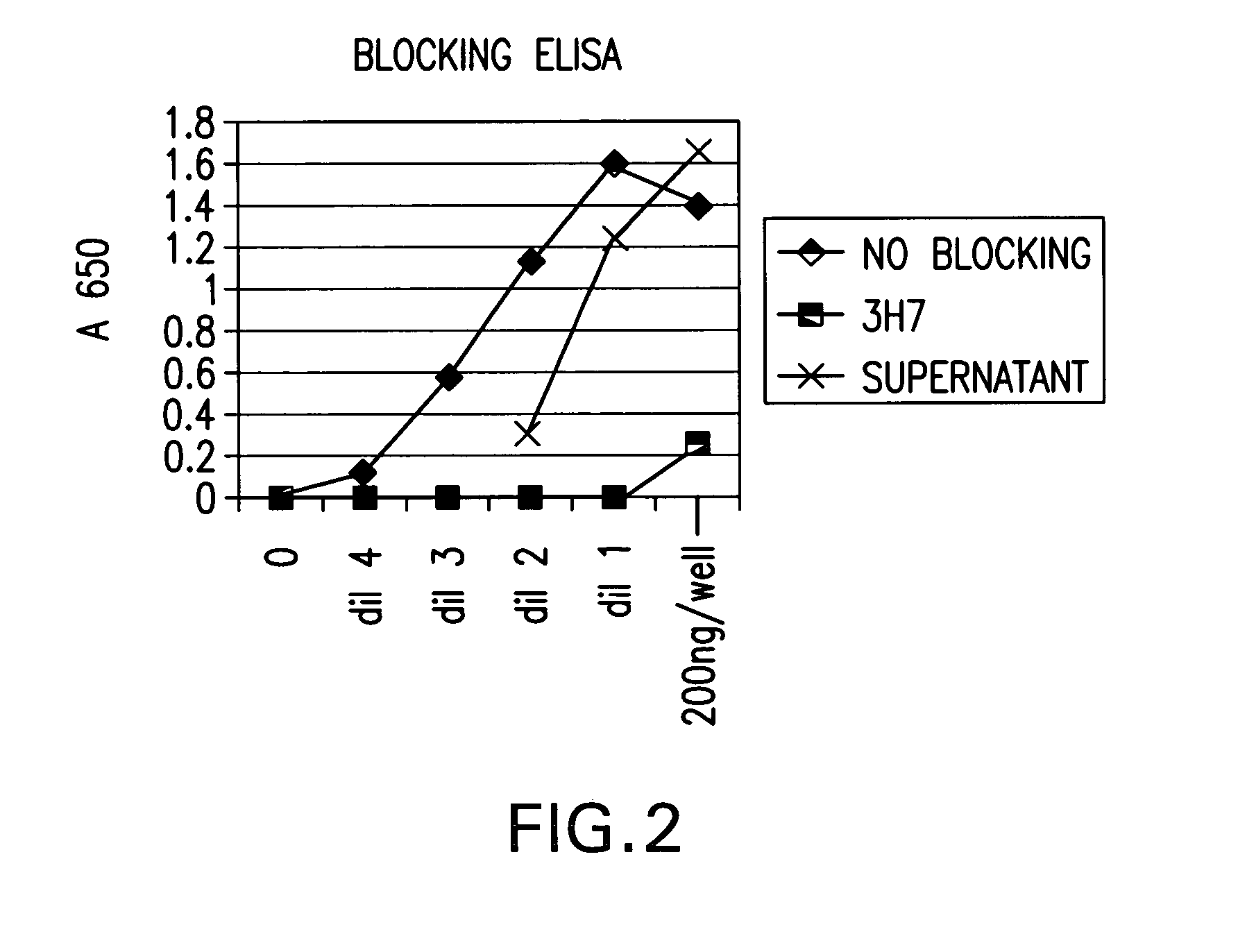
FcgammaRIIB-specific antibodies and methods of use thereof
InactiveUS20060177439A1Good curative effectConvenient treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCell activationImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind the extracellular domain of FcγRIIB, particularly human FcγRIIB, and block the Fc binding site of human FcγRIIB. The invention provides methods of treating cancer and / or regulating immune complex mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Vaccine Nanotechnology
ActiveUS20130236533A1Facilitate acquisitionModulating the immune systemNervous disorderAntipyreticDiseaseNanocarriers
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +3
Human IL-2 as a vaccine adjuvant
InactiveUS6060068AEnhance immune responseBacterial antigen ingredientsPeptide/protein ingredientsInterleukin IIInterleukin 2
Methods for enhancing the immune response to vaccination in animals, including humans, comprise administering interleukin-2 (IL-2) as part of the vaccination regimen, preferably for 5 to 14 days post-vaccination. In addition, compositions for enhancing the immune response of an animal to a vaccine employ IL-2 as an active ingredient, preferably human IL-2.
Owner:CHIRON CORP
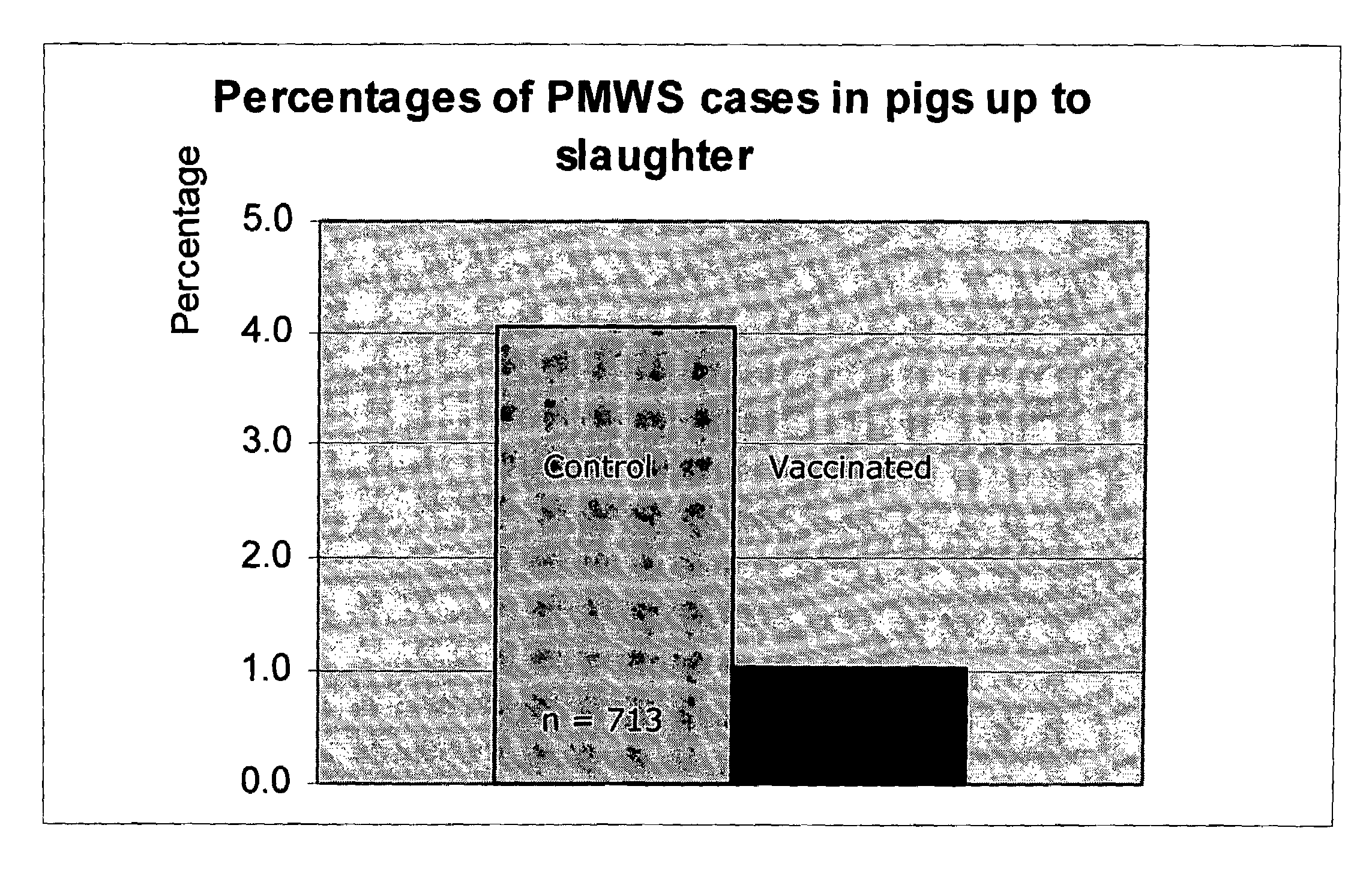
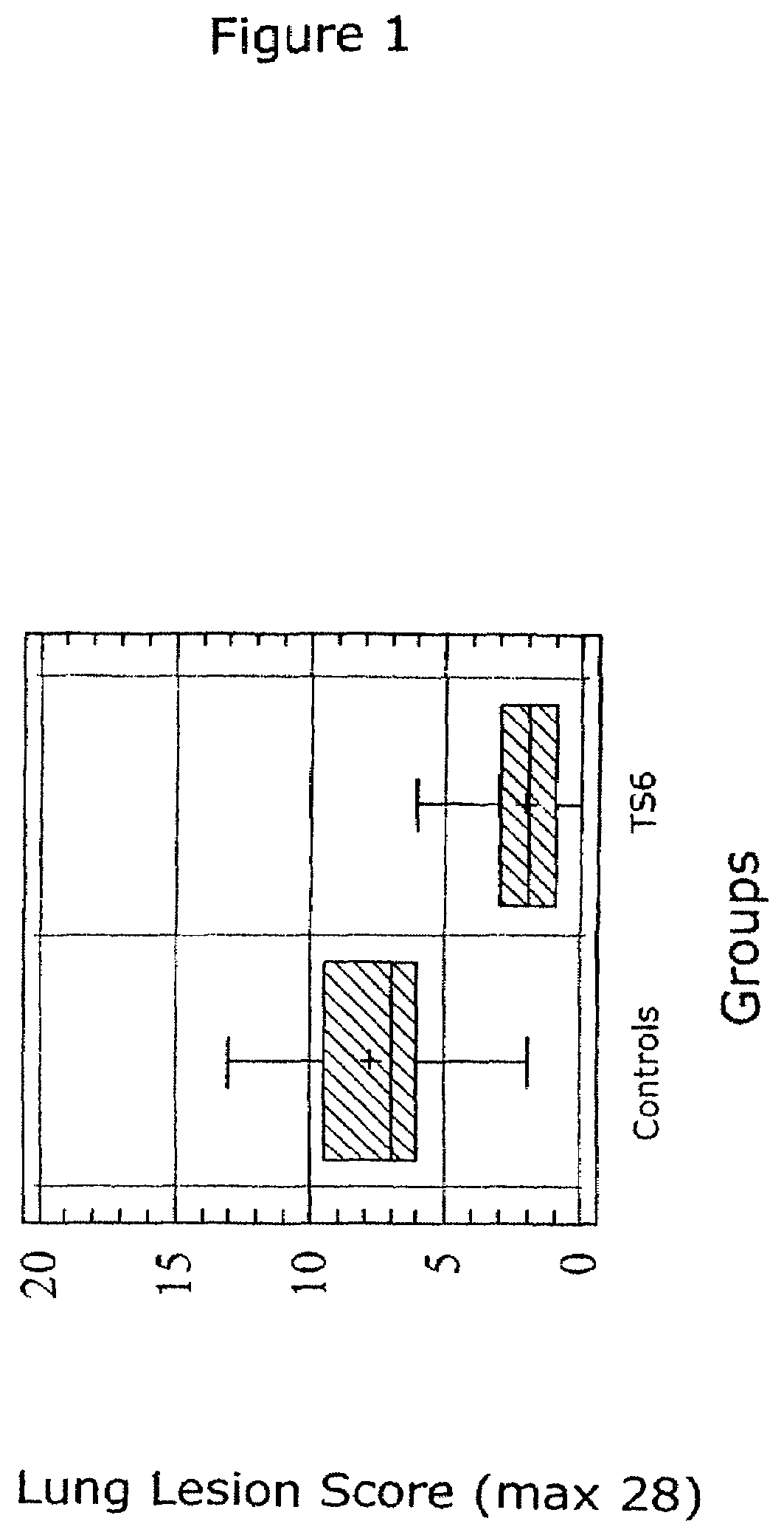
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
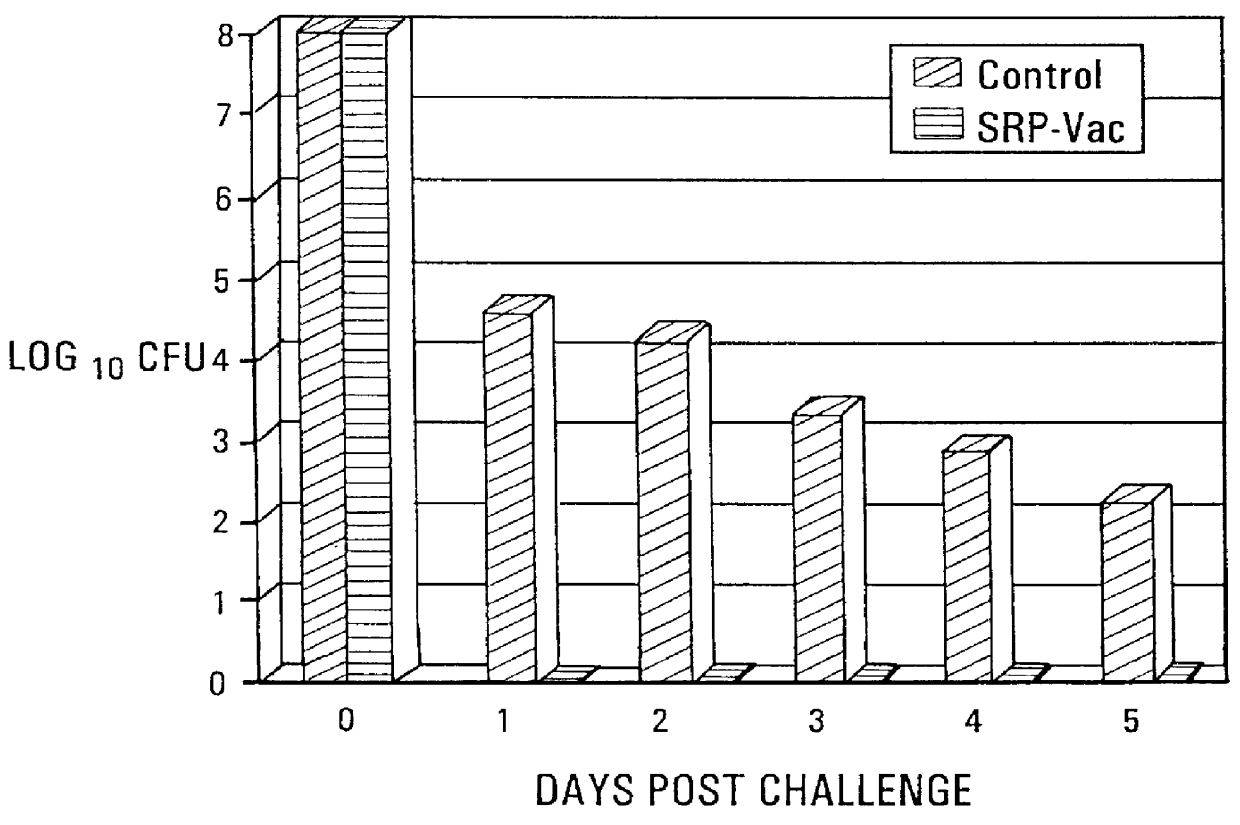
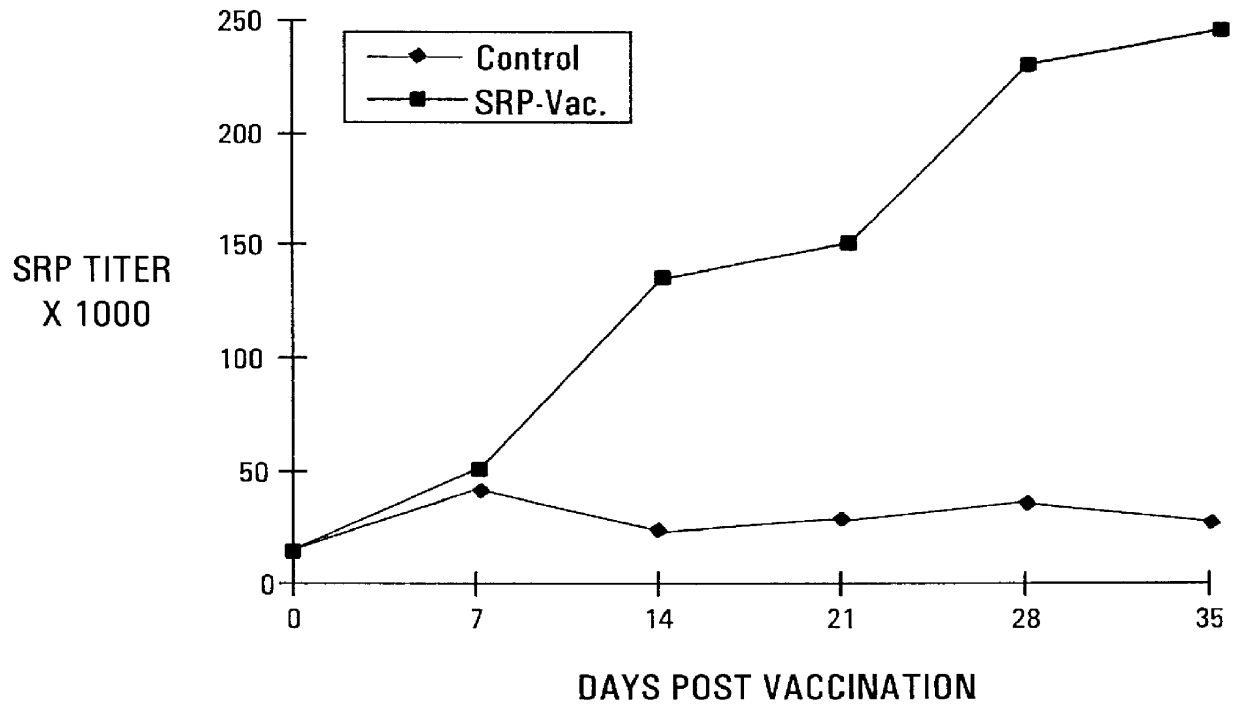
Active immunization using a siderophore receptor protein
InactiveUS6027736AEasy to produceInhibition capacityAntibacterial agentsBacterial antigen ingredientsADAMTS ProteinsMicrobiology
The invention provides a vaccine for immunizing poultry and other animals against infection by a gram-negative bacteria, and a method of immunizing an animal using the vaccine. The vaccine may contain purified siderophore receptor proteins derived from a single strain or species of gram-negative bacteria or other organism, which are cross-reactive with siderophores produced by two or more strains, species or genera of gram-negative bacteria. The invention further provides a process for isolating and purifying the siderophore receptor proteins, and for preparing a vaccine containing the proteins. Also provided is a method for diagnosing gram-negative sepsis.
Owner:EPITOPIX LLC
Biodegradable immunomodulatory formulations and methods for use thereof
InactiveUS7250403B2Increasing interferon-gammaImprove the situationAntibacterial agentsOrganic active ingredientsImmunomodulationsPolynucleotide
The invention provides new compositions and methods for immunomodulation of individuals. Immunomodulation is accomplished by administration of immunomodulatory polynucleotide / microcarrier (IMP / MC) complexes. The IMP / MC complexes may be covalently or non-covalently bound, and feature a polynucleotide comprising at least one immunostimulatory sequence bound to a biodegradable microcarrier or noncarrier.
Owner:DYNAVAX TECH CORP
Adjuvanted influenza vaccines for pediatric use
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Yeast-based vaccines as immunotherapy
InactiveUS7465454B2Enhance immune responseExtended half-lifeBiocideAntibody mimetics/scaffoldsYeastDisease
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
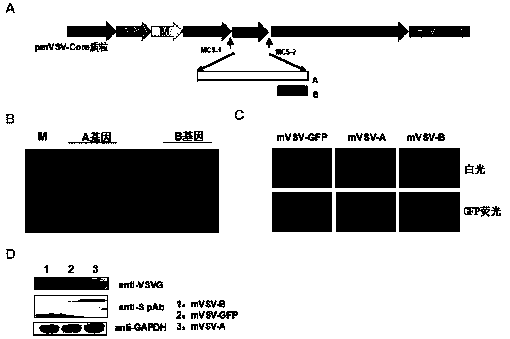
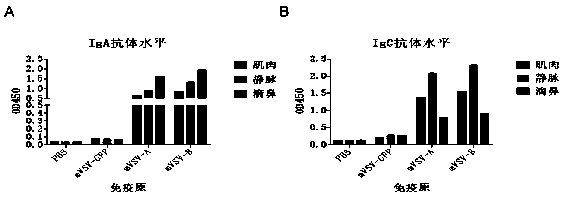
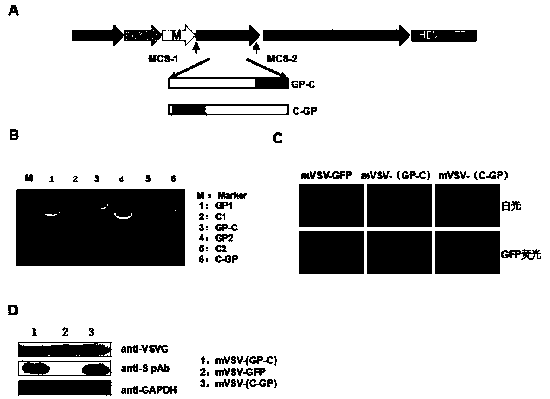
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Method for inducing mucosal humoral and cell-mediated immune responses by sublingual administration of antigens
InactiveUS20080112974A1Enhance immune responseInduce immune responseBacterial antigen ingredientsViral antigen ingredientsCell-mediated immune responsePathogen
Described are methods for inducing both a mucosal and a systemic immune response in the respiratory, digestive or urogenital tracts of a mammal to a microbial pathogen. The methods comprise topically administering onto the sublingual mucosa of the mammal an amount of an antigen effective to induce the mucosal and systemic immune responses and a pharmaceutically acceptable carrier or diluent. Pharmaceutical formulations and dosage forms for immunizing a mammal against a microbial pathogen to elicit a mucosal and systemic immune response in the respiratory, digestive or urogenital tracts are also described.
Owner:DUOTOL
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS7425620B2Enhance immune responseImprove responseSenses disorderAntipyreticTherapeutic antibodyAntiendomysial antibodies
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Adjuvanted influenza vaccines for pediatric use
InactiveUS20120027813A1Raise useful immune responseEnhance immune responseSsRNA viruses negative-senseViral antigen ingredientsInfluenza B virusesAdjuvant
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Controlled Delivery of TLR Agonists in Structural Polymeric Devices
ActiveUS20130202707A1Increase successStimulate immune responsePowder deliveryOrganic active ingredientsTlr agonistsDendritic cell
The present invention comprises compositions, methods, and devices for creating an stimulating an antigen-specific dendritic cell immune response. Devices and methods provide prophylactic and therapeutic immunity to subjects against cancer and infectious agents.
Owner:DANA FARBER CANCER INST INC +1
Novel vaccine formulations
ActiveUS20060233831A1Improve stabilityStable and safe and easily administrableAntibacterial agentsBiocideAdjuvantNon ionic
The present invention relates to oil-in-water emulsions, their use as adjuvants, and pharmaceutical, immunologic, or vaccine compositions that may comprise the same. In one embodiment, the oil-in-water (O / W) emulsion may comprise an aqueous solution containing an immunogen, a mineral oil, a non-ionic lipophilic ethoxylated fatty alcohol and a non-ionic hydrophilic surfactant. In another embodiment, the oil-in-water (O / W) emulsion may comprise an aqueous solution containing an immunogen, a non-ionic lipophilic surfactant, a mineral oil and a non-ionic hydrophilic ethoxylated fatty alcohol. The present invention also encompasses a method of making a vaccine composition using the adjuvant of the instant invention, the vaccine composition so obtained and methods of use.
Owner:MERIAL INC
Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
ActiveUS20080044429A1Good curative effectEnhanced effector functionSugar derivativesPeptide/protein ingredientsTreatment effectAntigen Binding Fragment
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
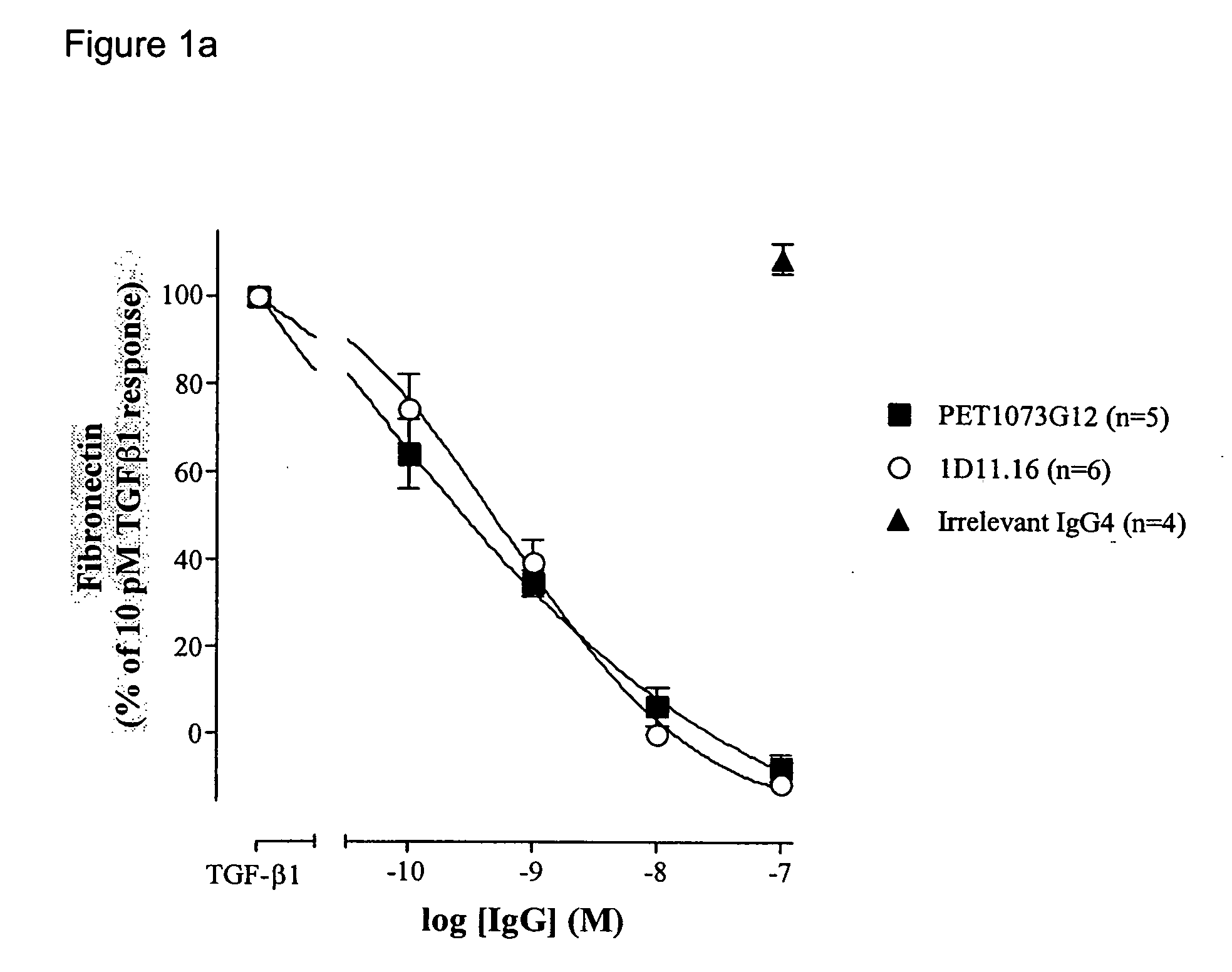
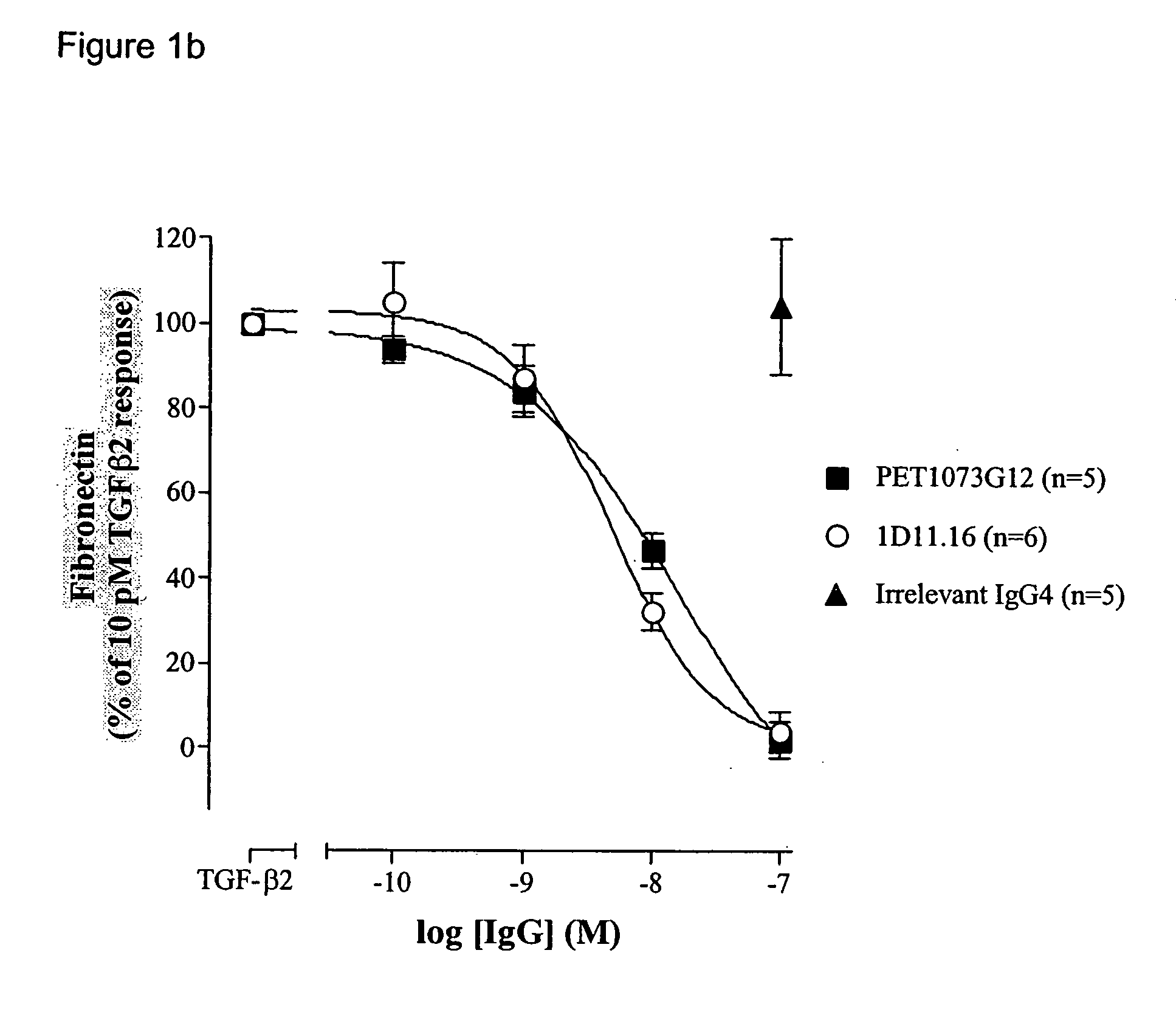
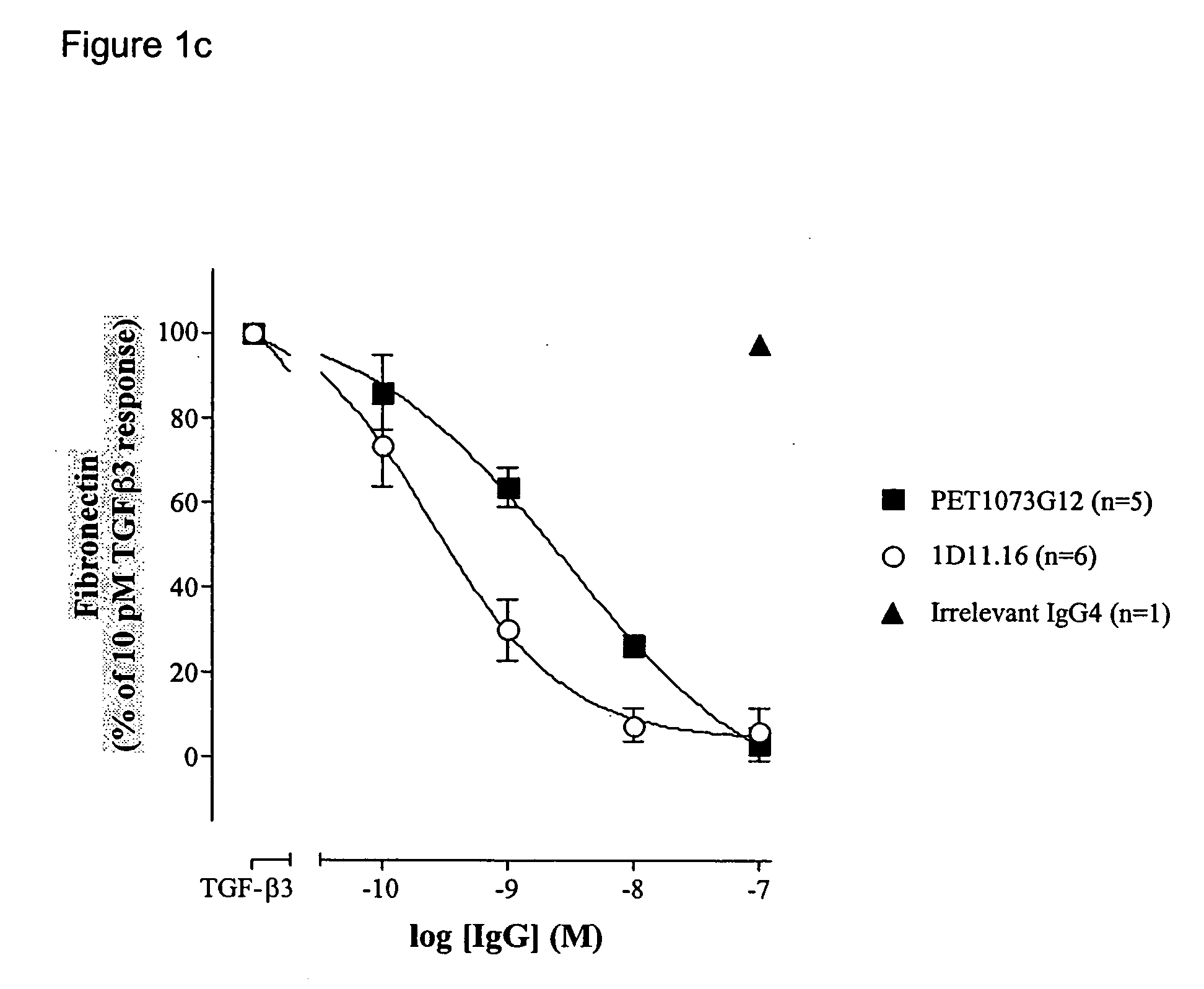
Antibodies to TGF-beta
ActiveUS20060251658A1Improve effectivenessEasy to detectAnimal cellsSugar derivativesAntibody fragmentsTransforming growth factor beta
The present invention relates to antibody molecules, in particular antibody molecules that bind Transforming Growth Factor beta (TGFβ), and uses thereof. More particularly, the invention relates to antibody molecules that bind and preferably neutralise TGFβ1, TGFβ2 and TGFβ3, so-called “pan-specific” antibody molecules, and uses of such antibody molecules. Preferred embodiments within the present invention are antibody molecules, whether whole antibody (e.g. IgG, such as IgG1 or IgG4) or antibody fragments (e.g. scFv, Fab, dAb).
Owner:GENZYME CORP +1
Novel methods for therapeutic vaccination
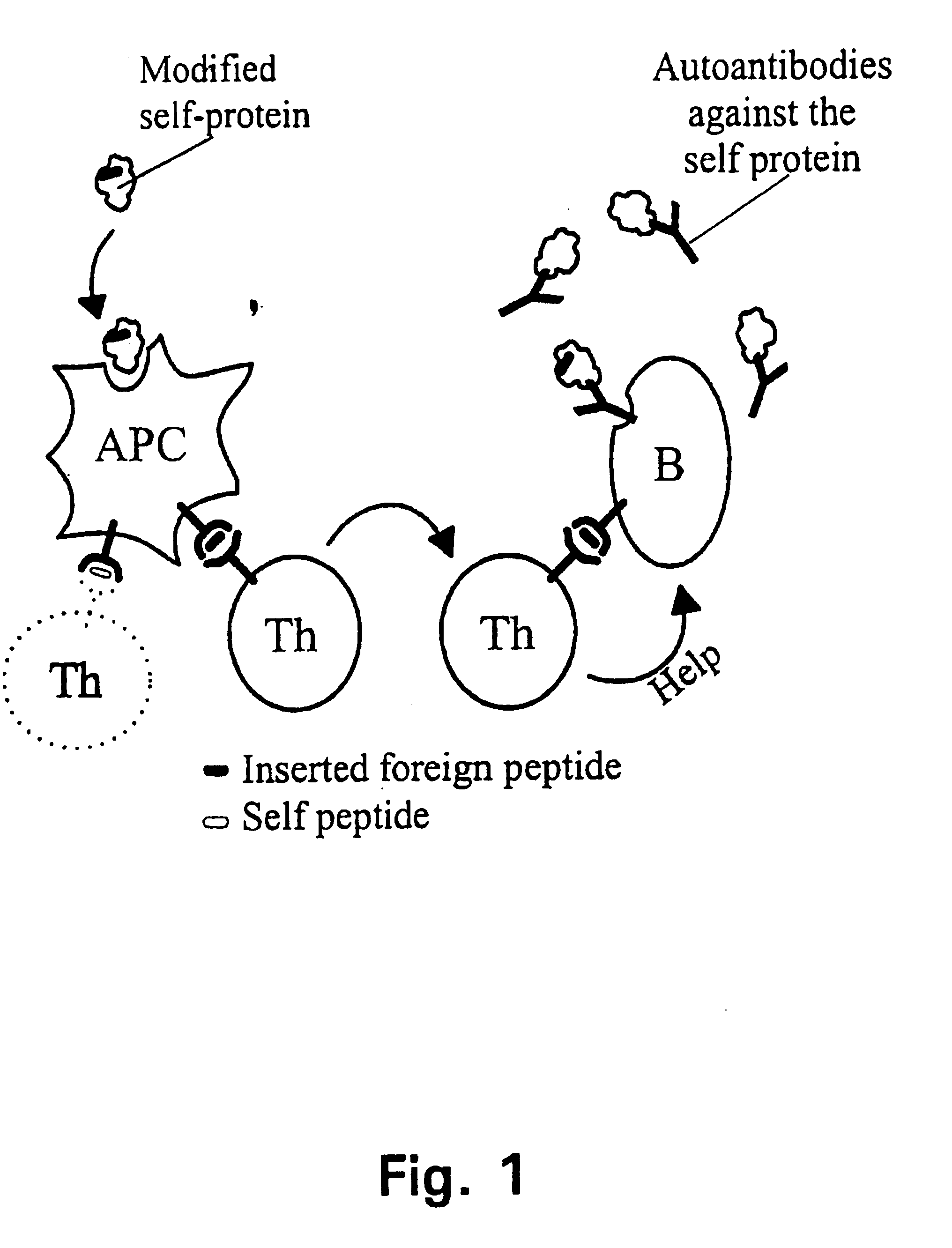
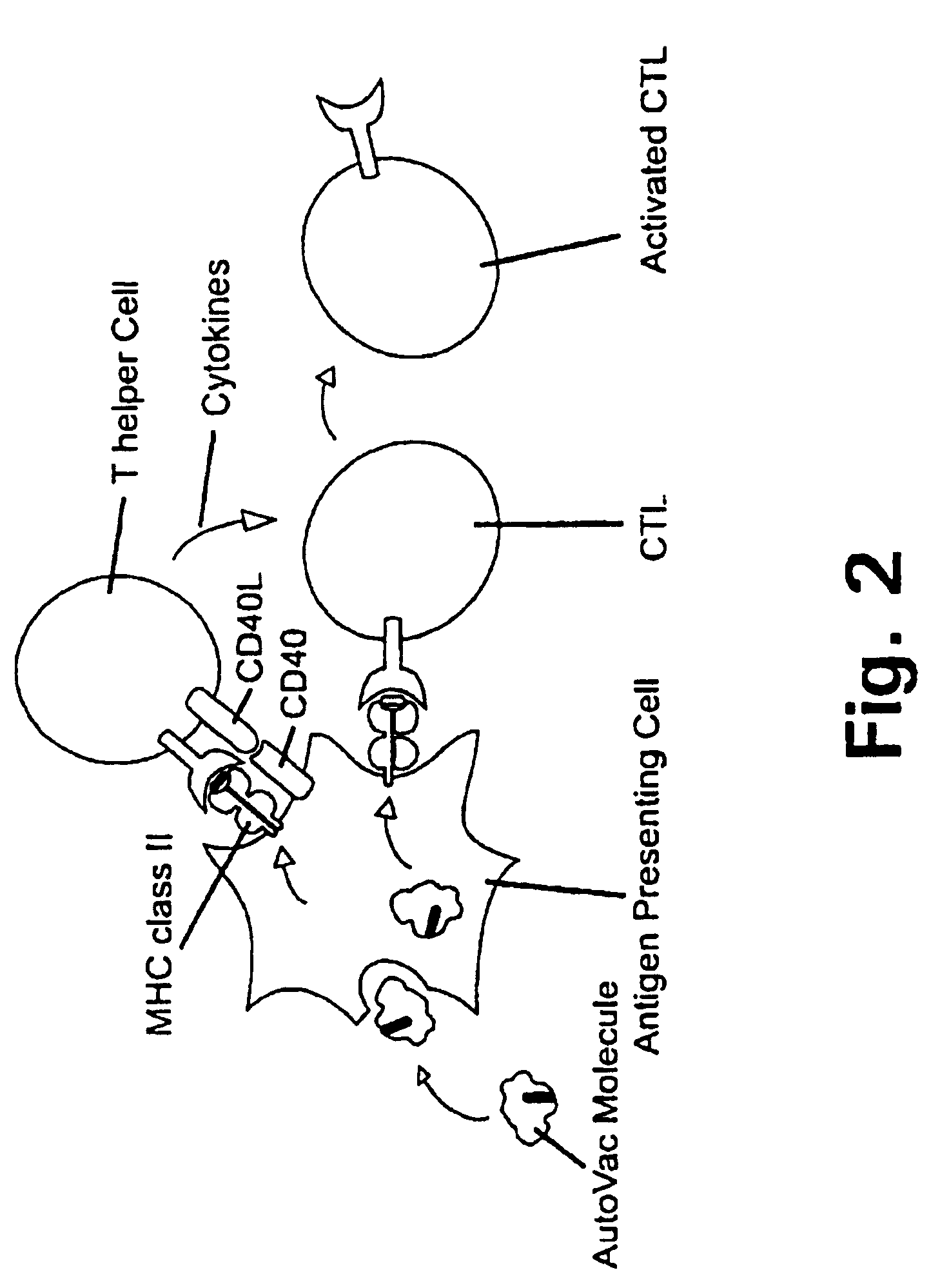
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Immunogenic compositions and uses thereof
ActiveUS20140242152A1Enhance immune responseSsRNA viruses negative-sensePowder deliveryEpitopeImmunogenicity
This invention generally relates to immunogenic compositions that comprise an RNA component and a polypeptide component. Immunogenic compositions that deliver antigenic epitopes in two different forms—a first epitope from a pathogen, in RNA-coded form; and a second epitope from the same pathogen, in polypeptide form—are effective in inducing immune response to the pathogen. The invention also relates to a kit comprising an RNA-based priming composition and a polypeptide-based boosting composition. The kit may be used for sequential administration of the priming and the boosting compositions.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS7211432B2Enhance immune responseFacilitated DiffusionBiocideGenetic material ingredientsDendritic cellBiological activation
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of these costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of T-cell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater than the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+ and CD8+ T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced antigen-presenting functions.
Owner:UNITED STATES OF AMERICA
Immunomodulatory formulations and methods for use thereof
InactiveUS7129222B2Increasing interferon-gammaImprove the situationAntibacterial agentsOrganic active ingredientsNanocarriersImmunomodulations
The invention provides new compositions and methods for immunomodulation of individuals. Immunomodulation is accomplished by administration of immunomodulatory polynucleotide / microcarrier (IMP / MC) complexes. The IMP / MC complexes may be covalently or non-covalently bound, and feature a polynucleotide comprising at least one immunostimulatory sequence bound to a nonbiodegradable microcarrier or nanocarrier.
Owner:DYNAVAX TECH CORP
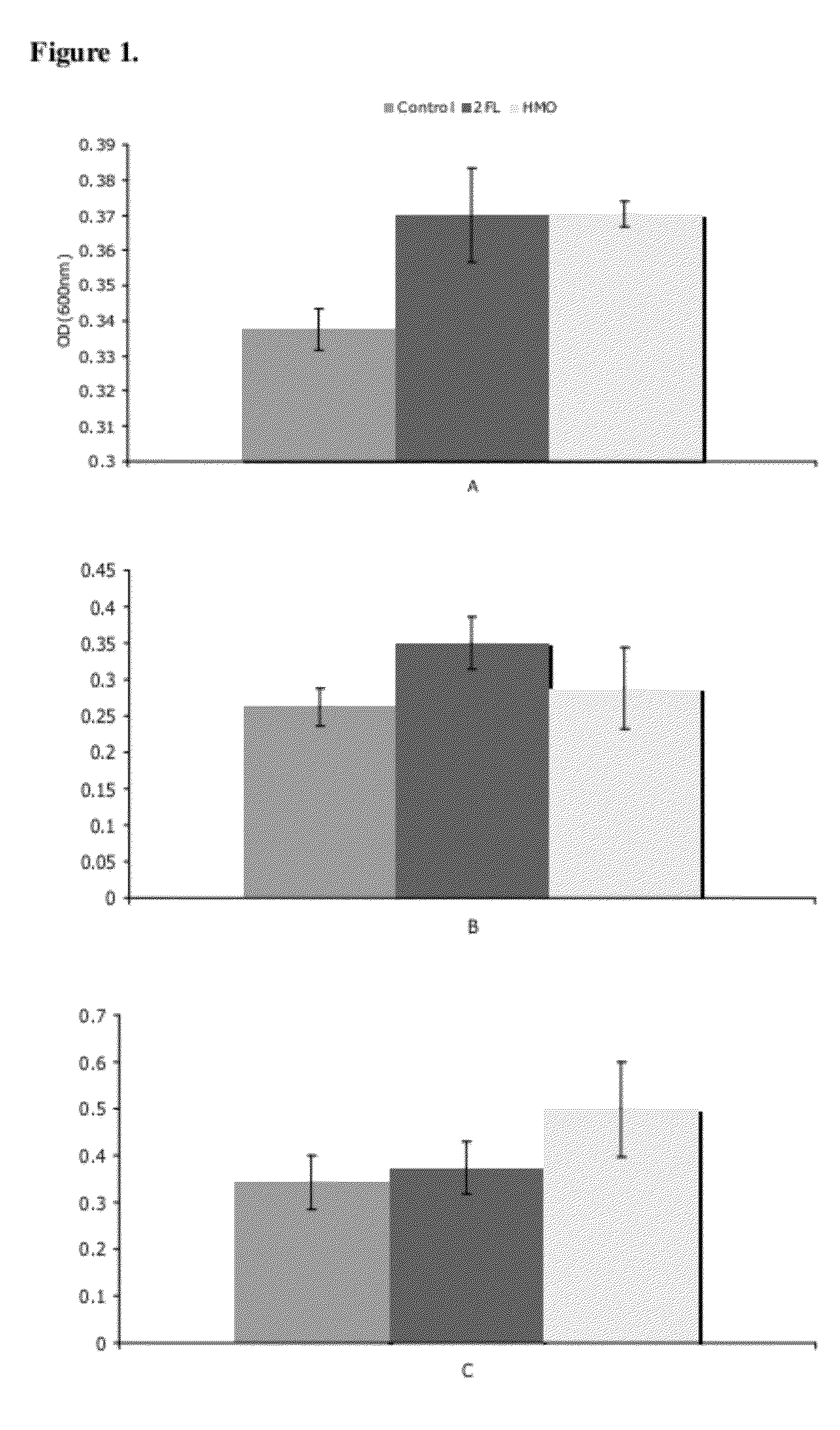
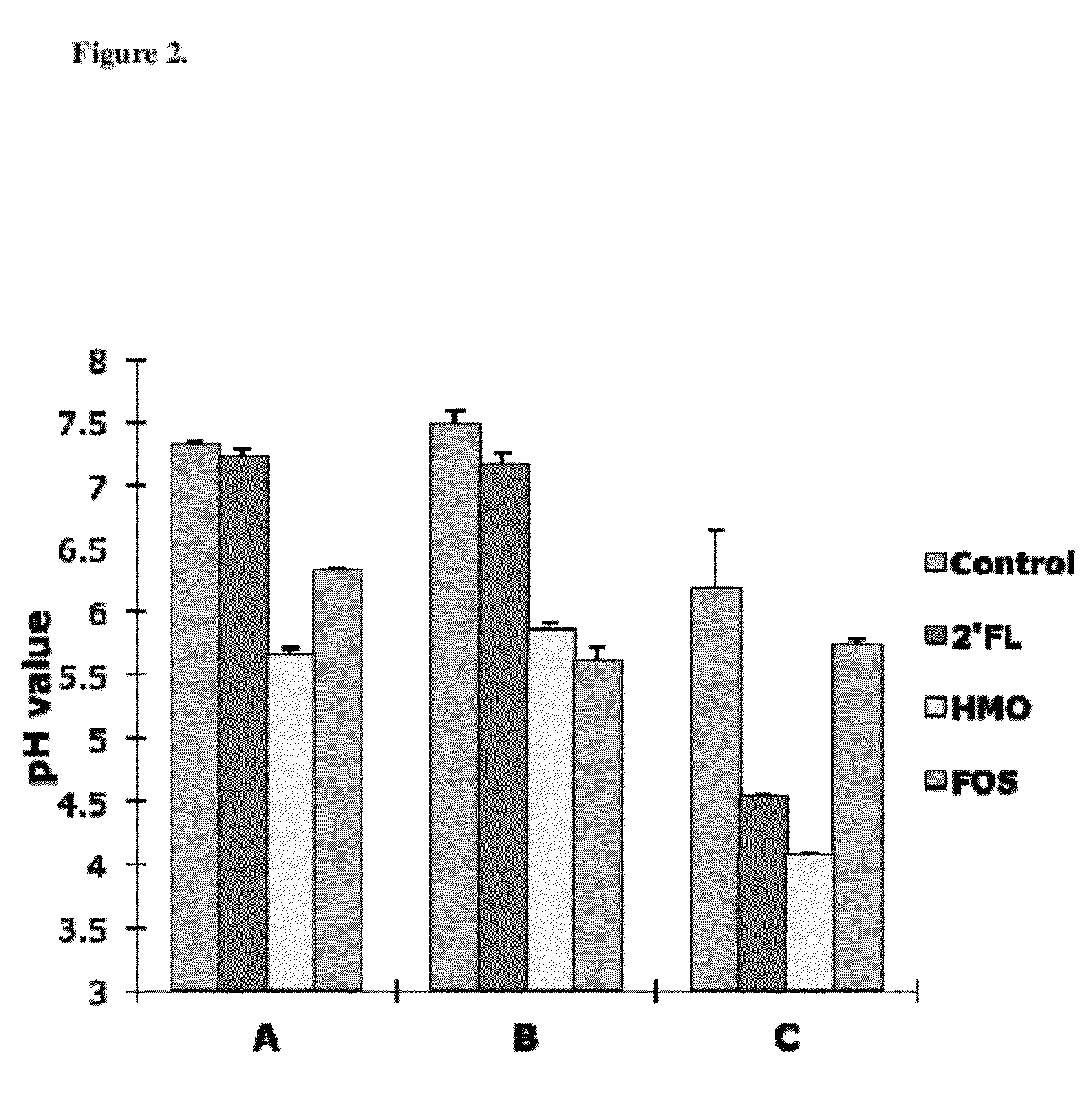
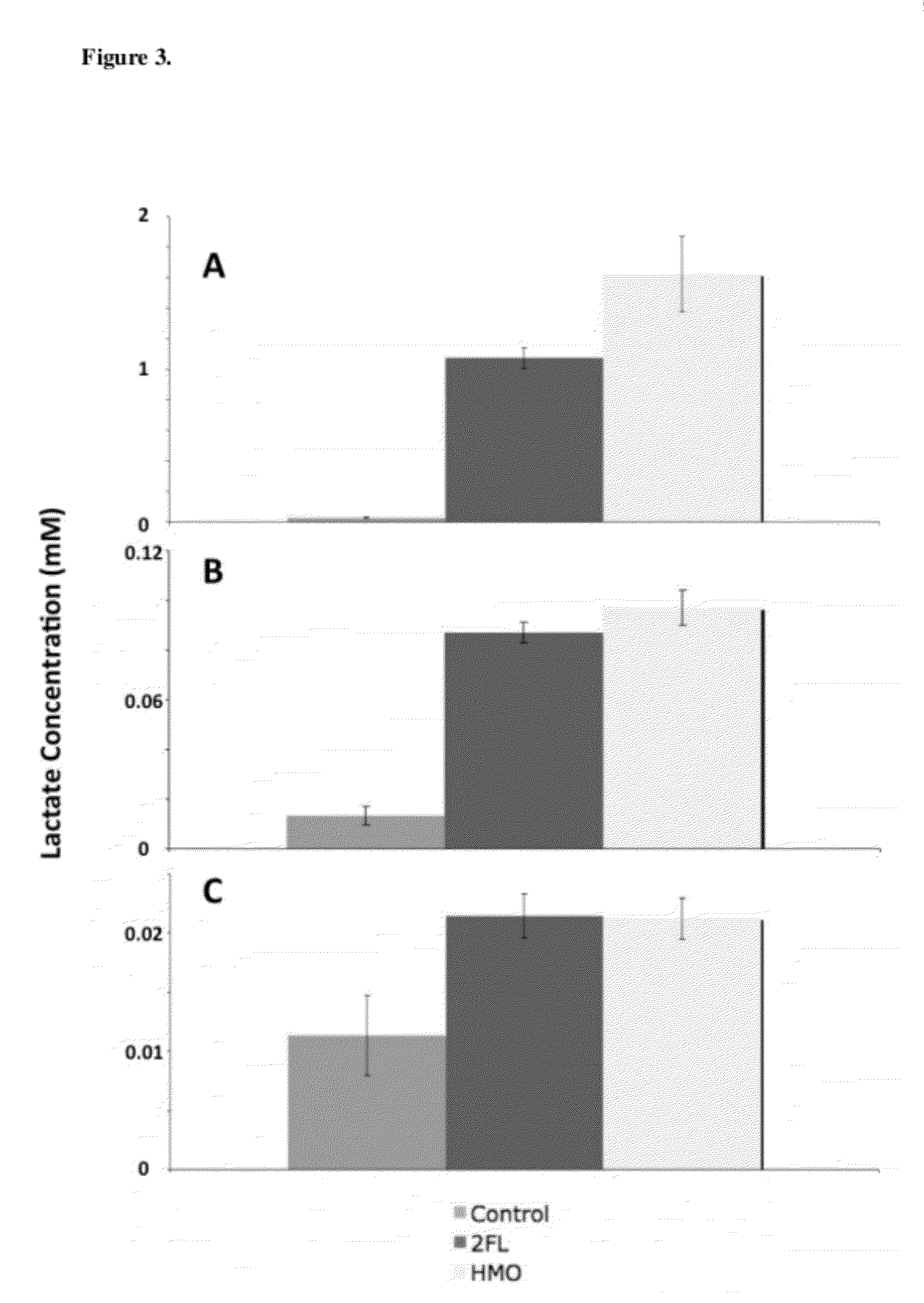
Use Of Purified 2'-Fucosyllactose, 3-Fucosyllactose and Lactodifucotetraose as Prebiotics
ActiveUS20120294840A1Maximize beneficial effectImprove survival and effectivenessAntibacterial agentsOrganic active ingredientsBiology2'-Fucosyllactose
Owner:BOSTON COLLEGE +1
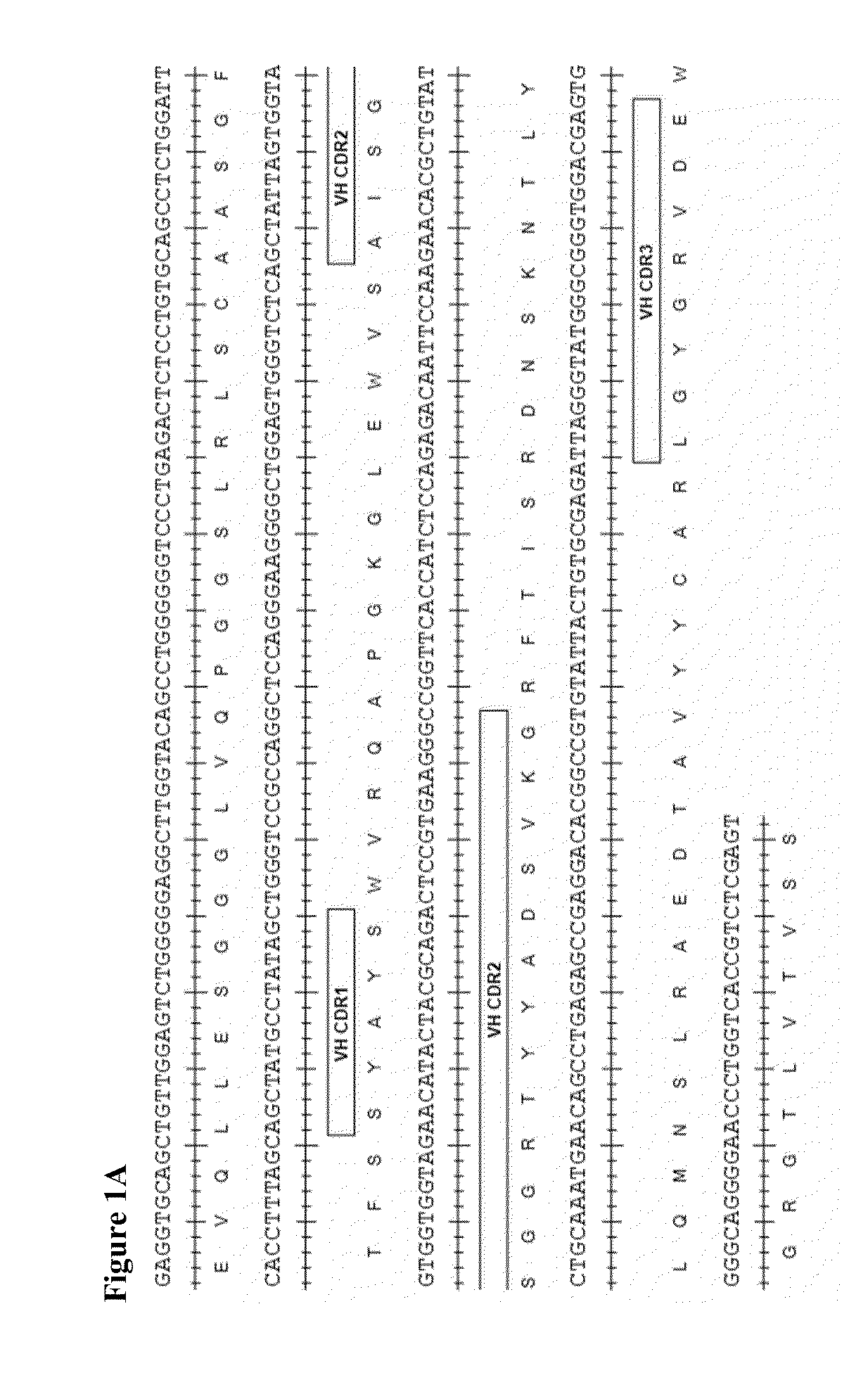
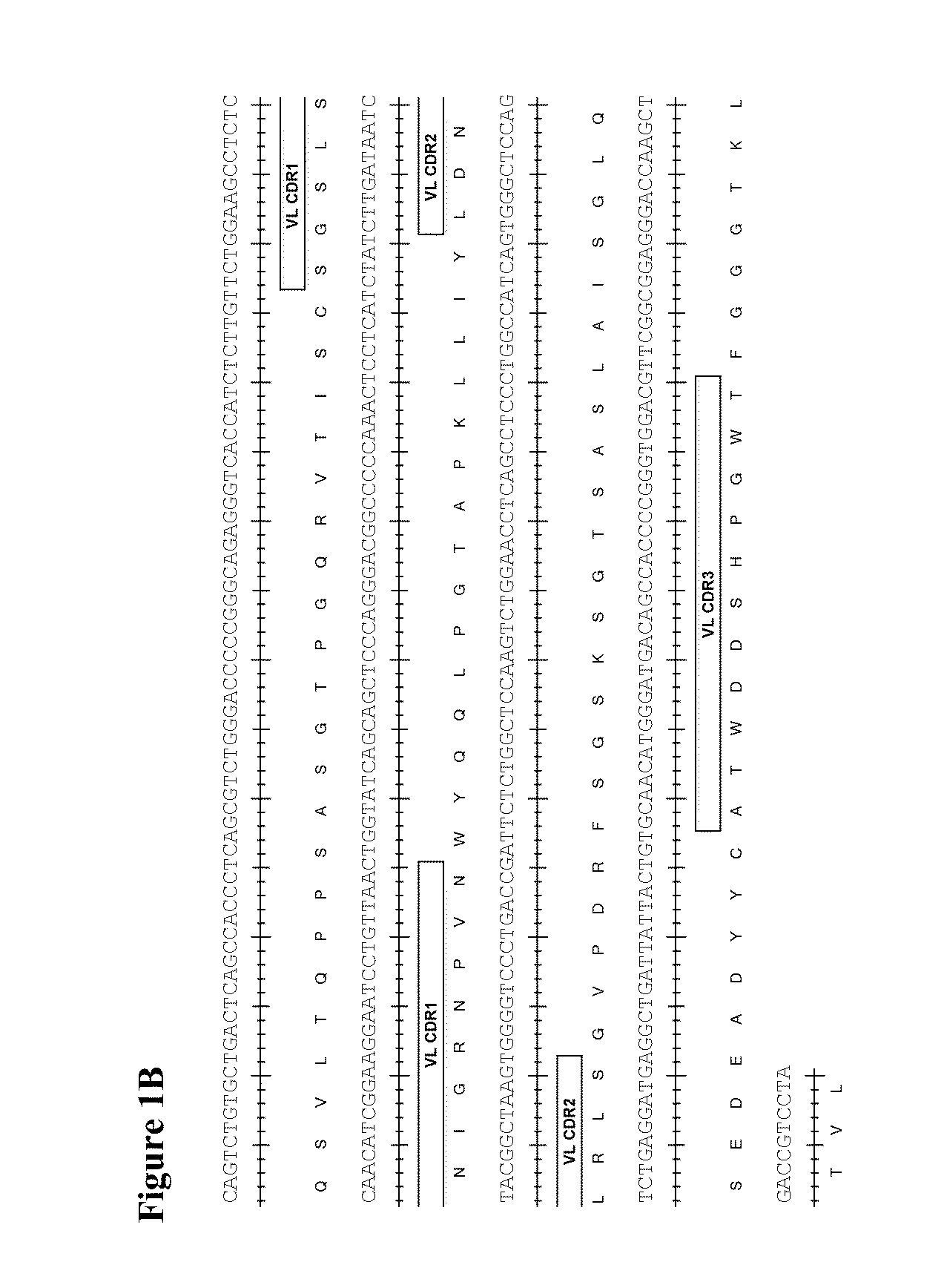
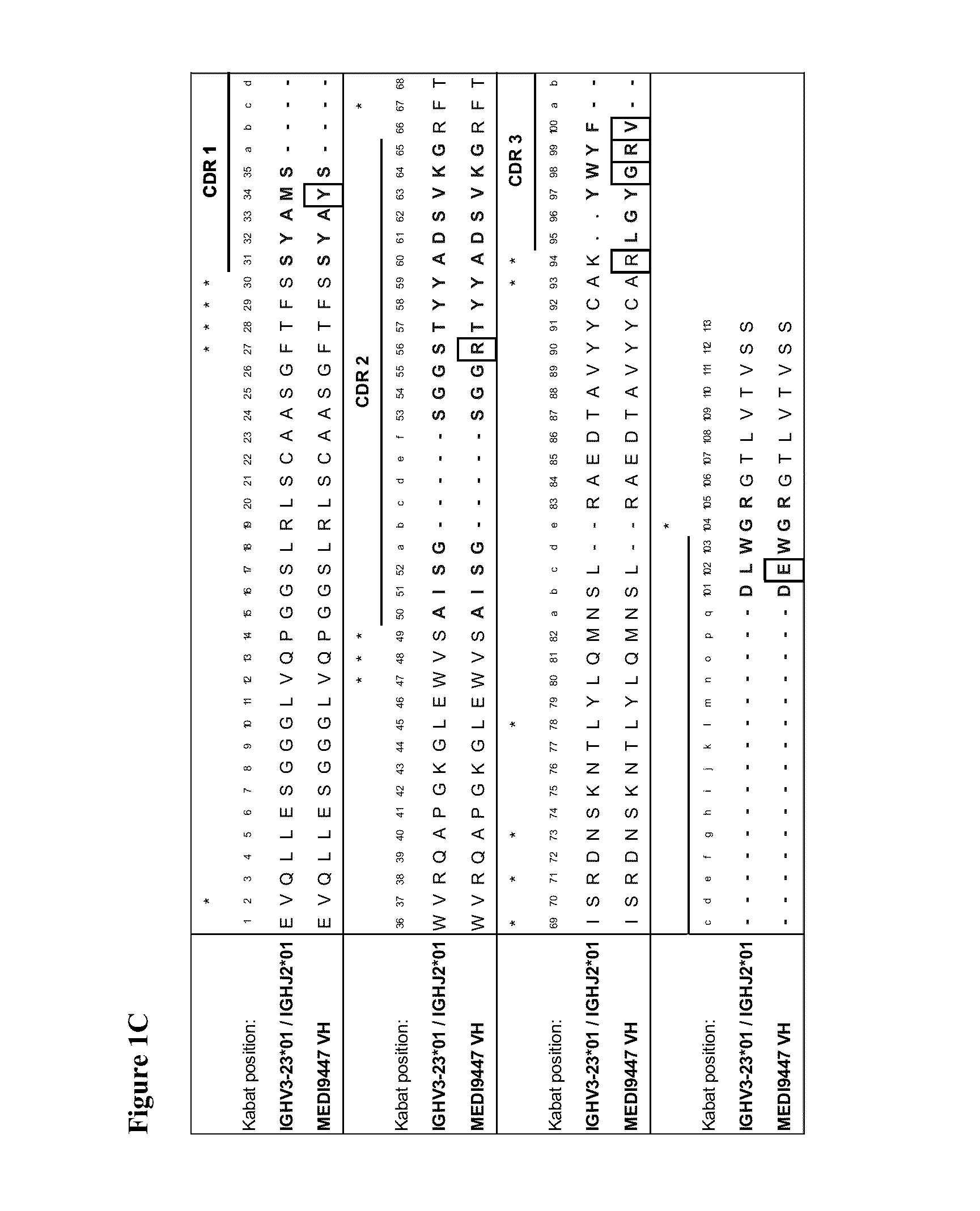
Therapeutic combinations comprising Anti-cd73 antibodies and uses thereof
InactiveUS20160129108A1Increase survivalIncrease immune responseOrganic active ingredientsAntibody ingredientsWilms' tumorAntibody
The present invention provides therapeutic combinations featuring anti-CD73 antibodies (e.g., MEDI9447) and A2A receptor inhibitors and methods of using such combinations for reducing tumor-mediated immunosuppression.
Owner:MEDIMMUNE LTD
Synergistic Liposomal Adjuvants
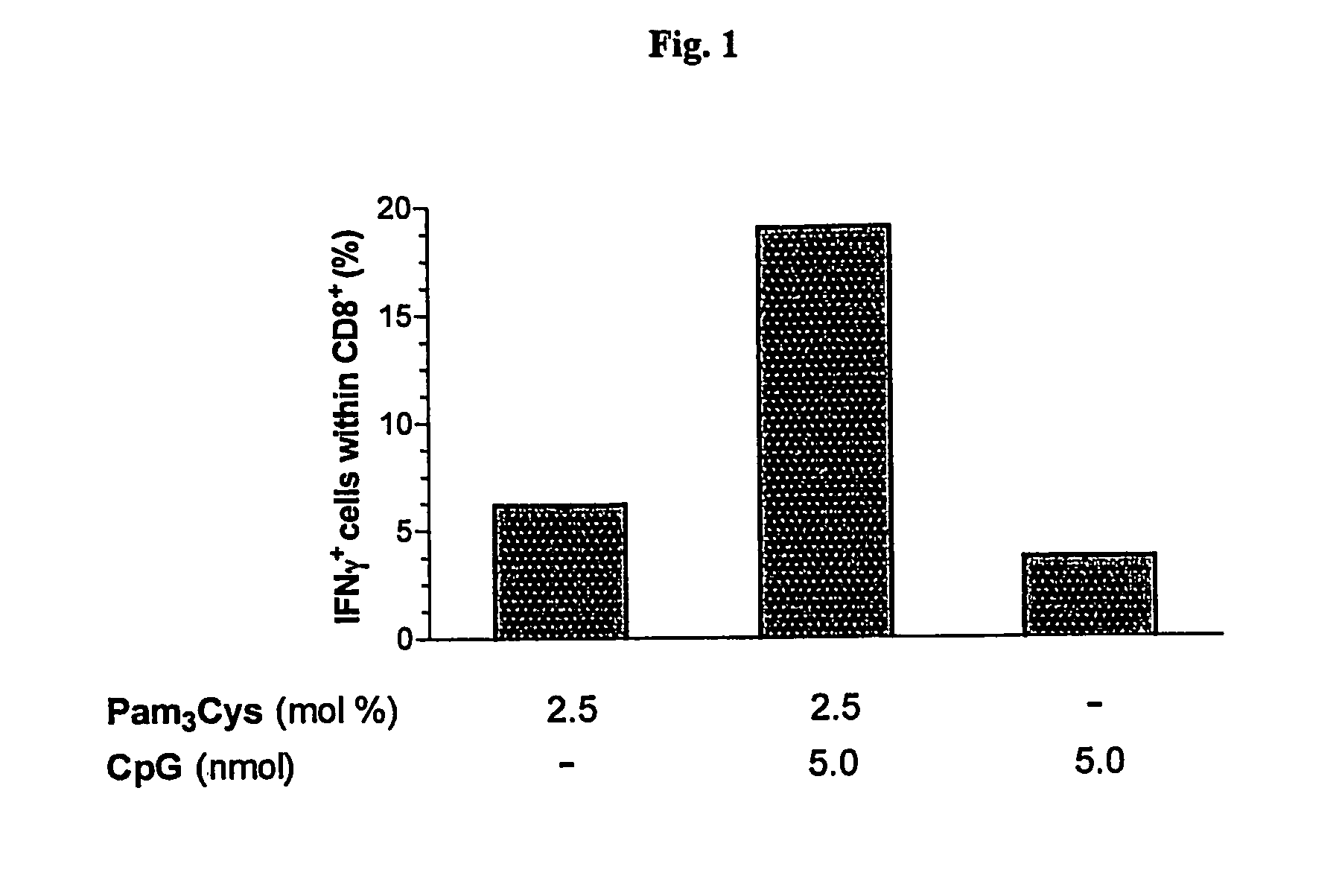
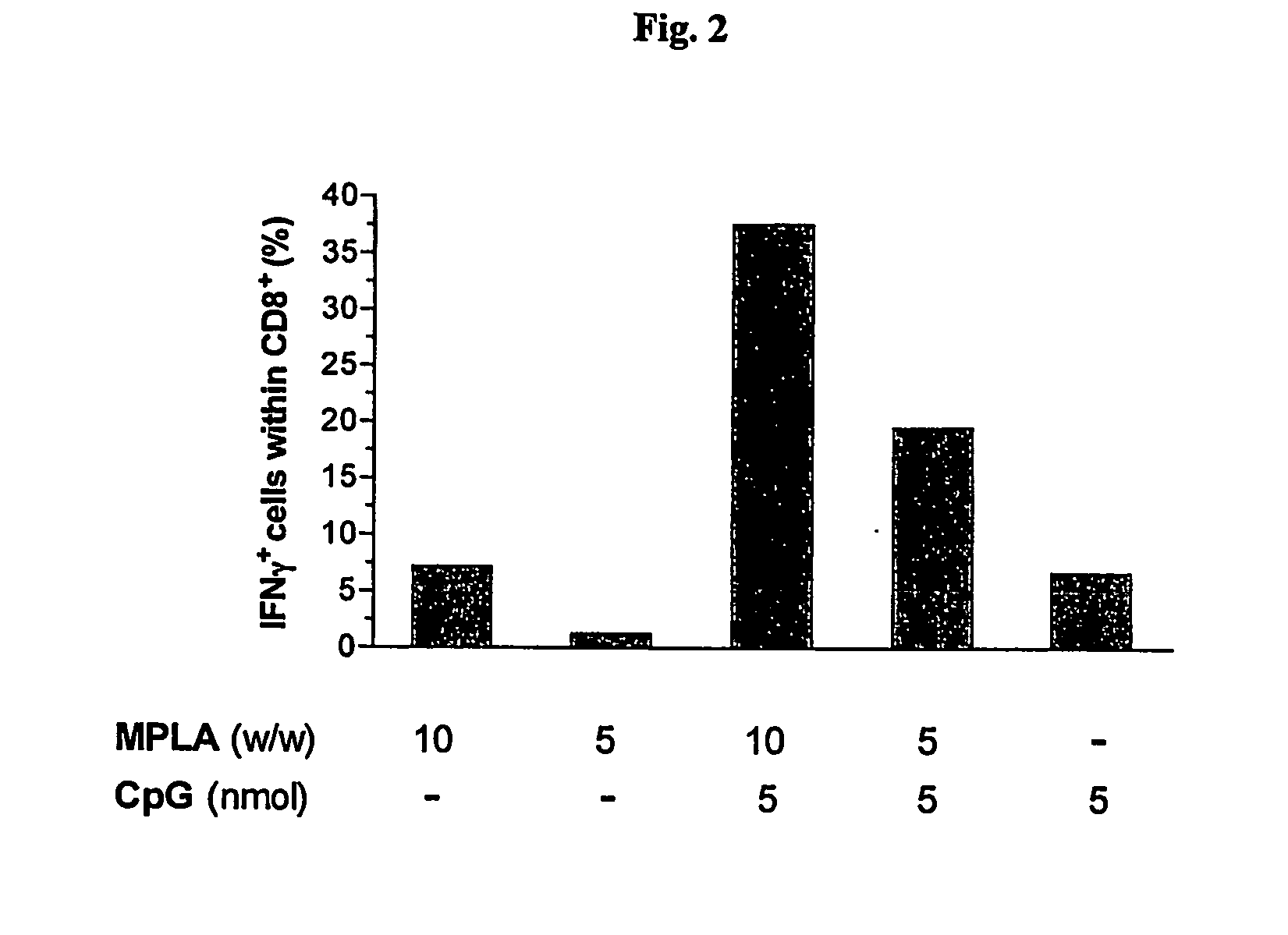
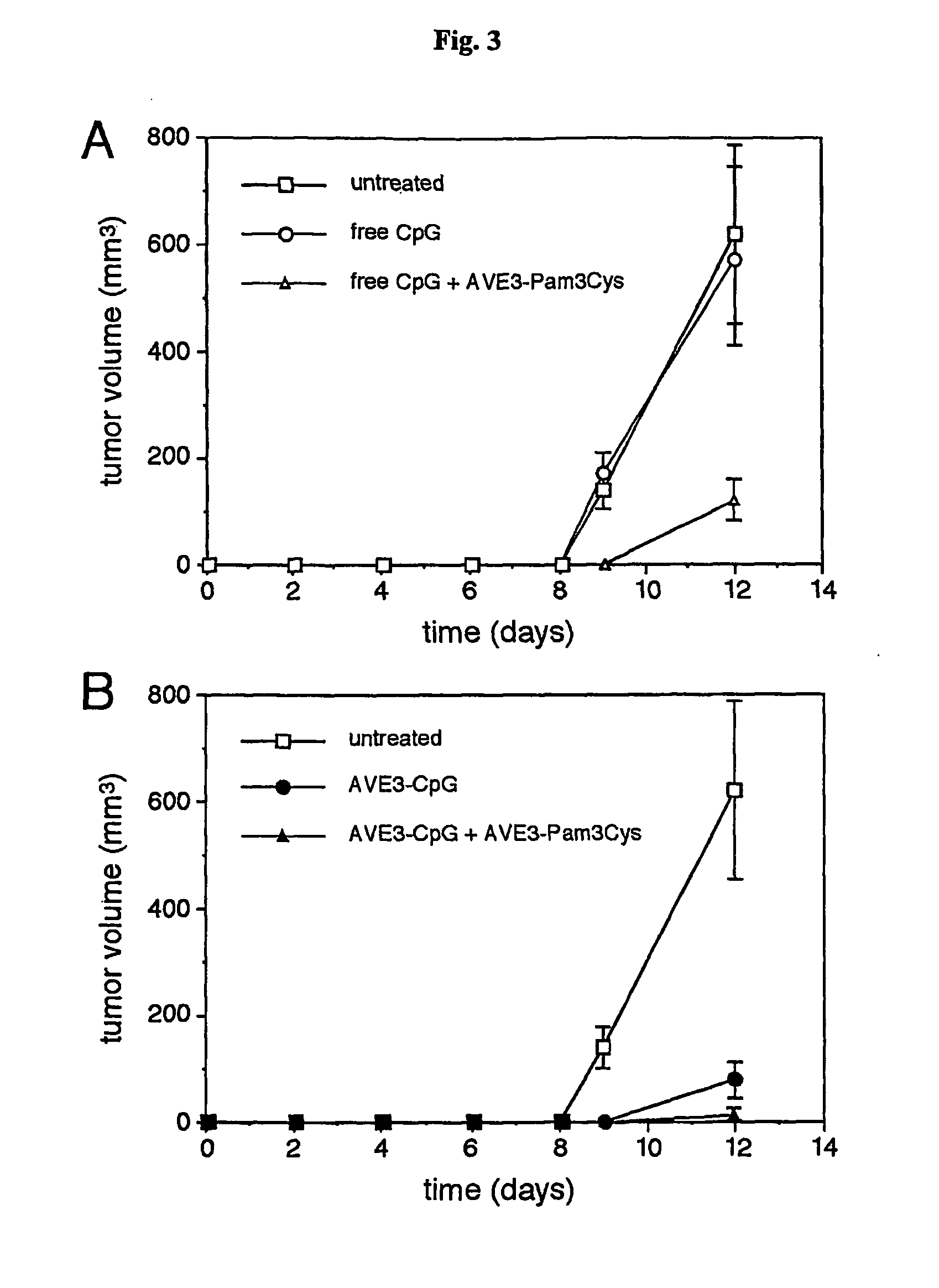
InactiveUS20070298093A1Improve actionEnhance immune responseAntibacterial agentsAntimycoticsVascular diseaseAdjuvant
The present invention relates to liposome, mixtures or liposomes and liposomal compositions comprising at least two different adjuvants and a therapeutic agent, their production and use for the prevention and therapy of proliferative diseases, infectious diseases, vascular diseases, rheumatoid diseases, inflammatory diseases, immune diseases, in particular autoimmune diseases and allergies.
Owner:PHARMEXA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com