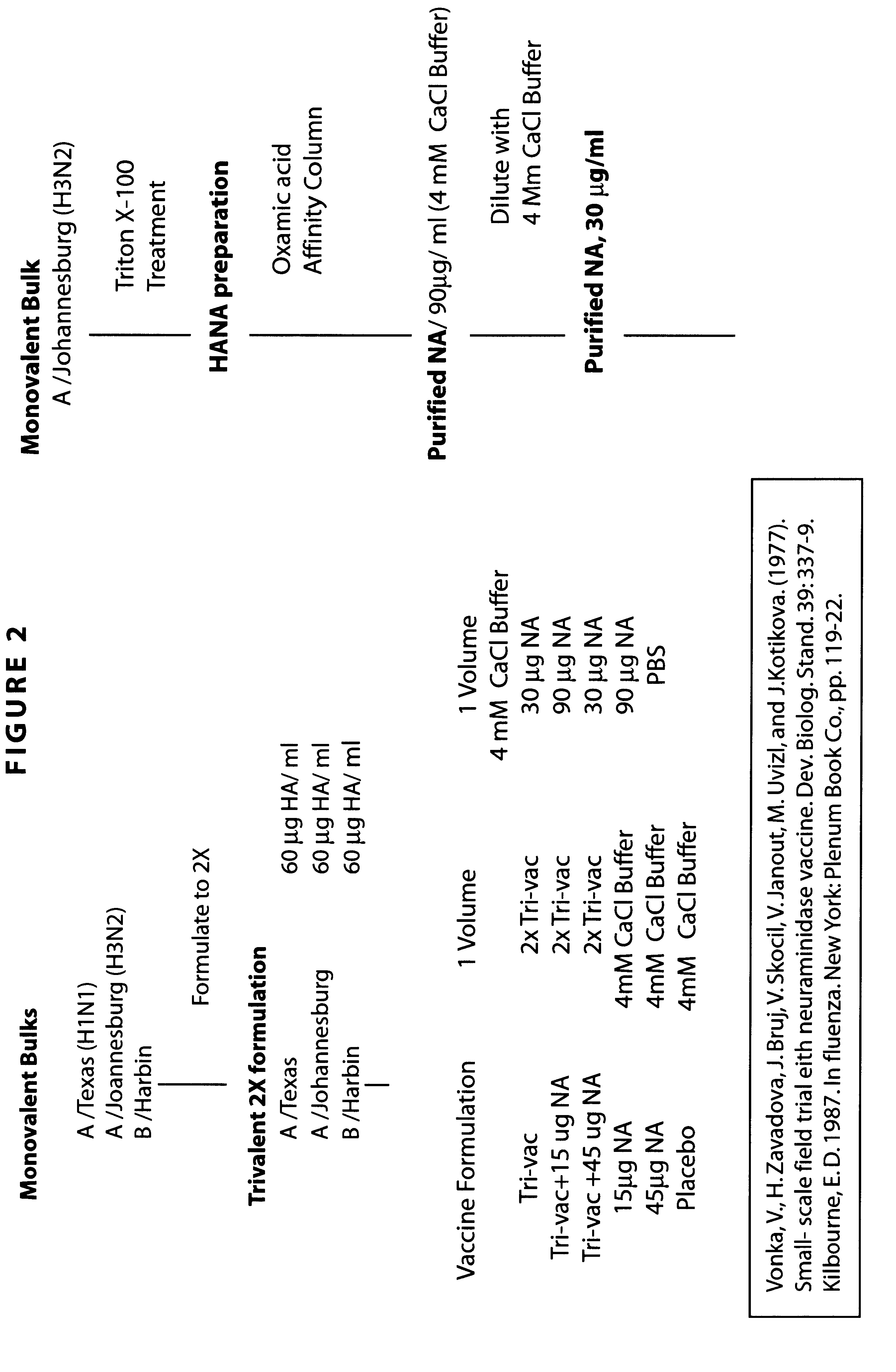
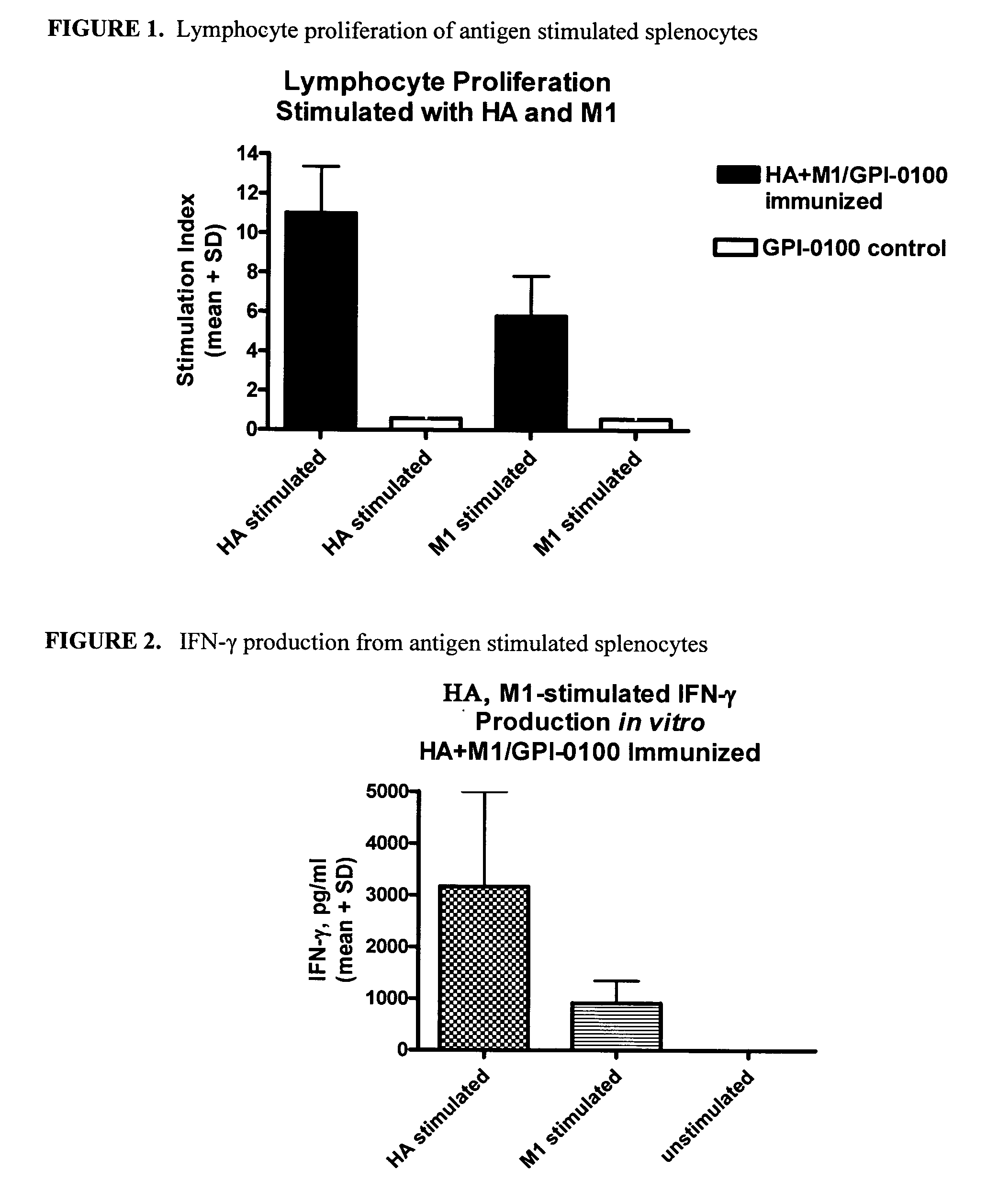
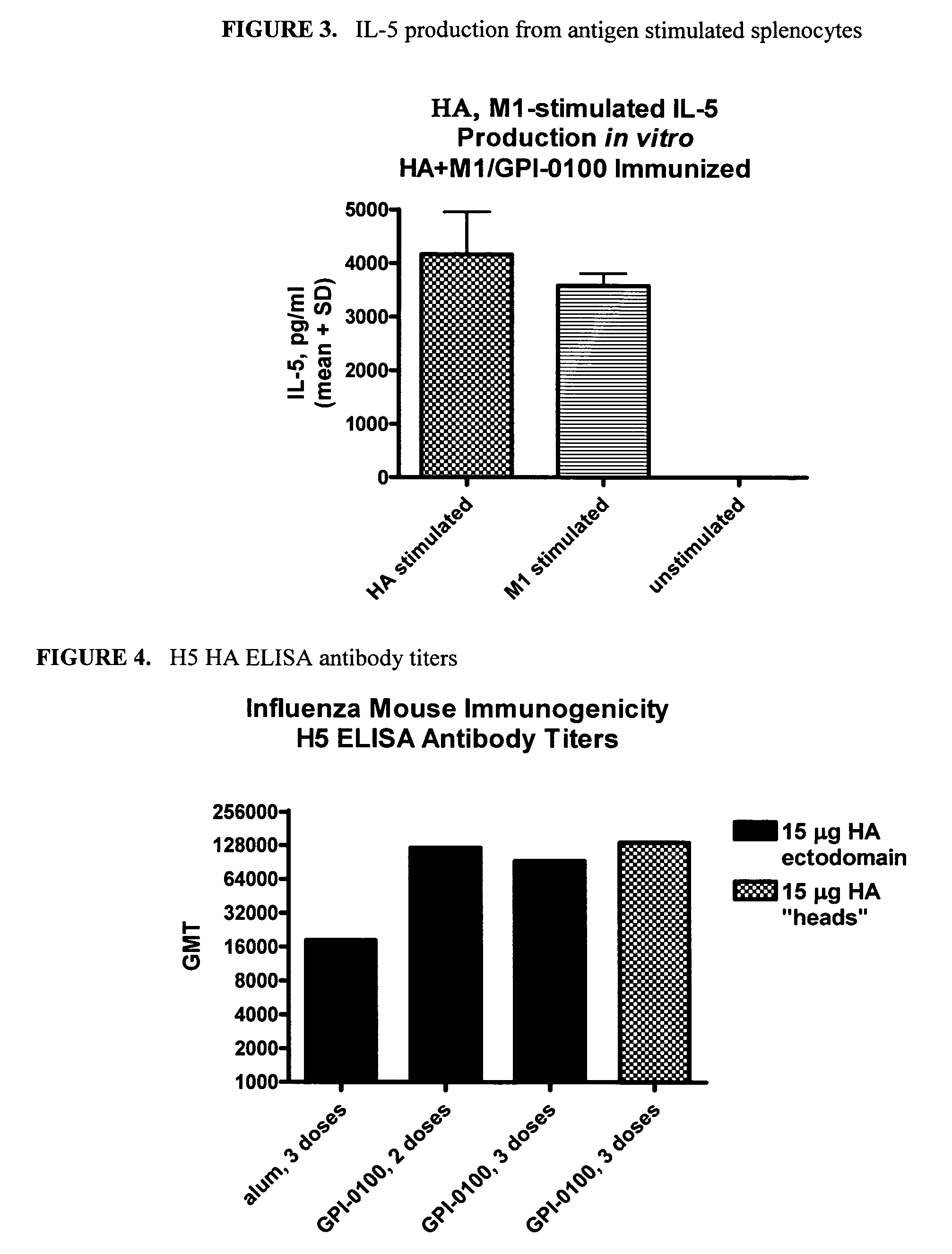
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1897 results about "Influenza a" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
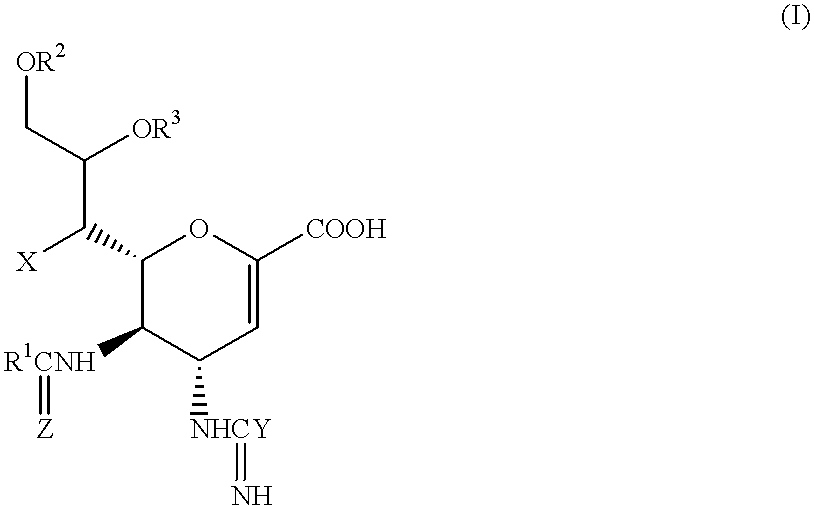
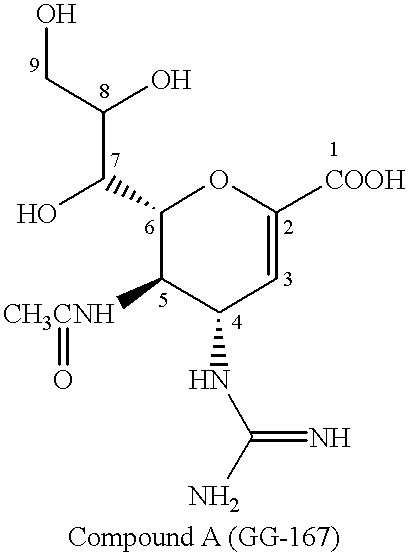
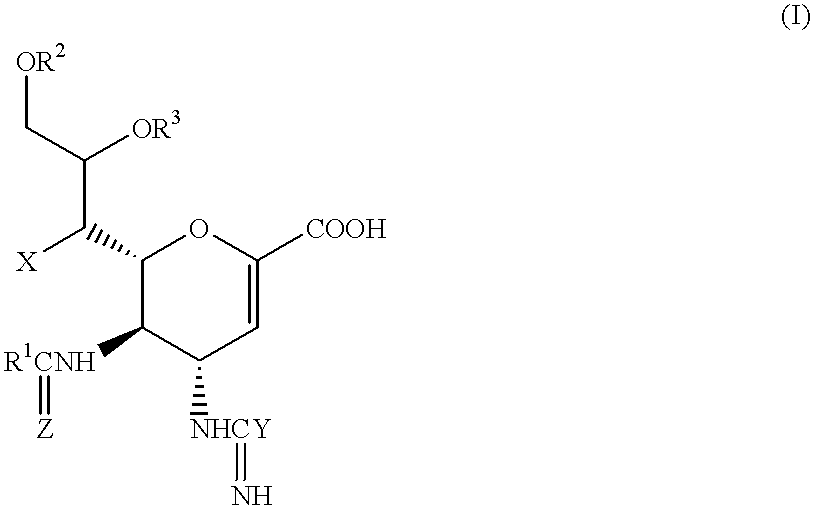
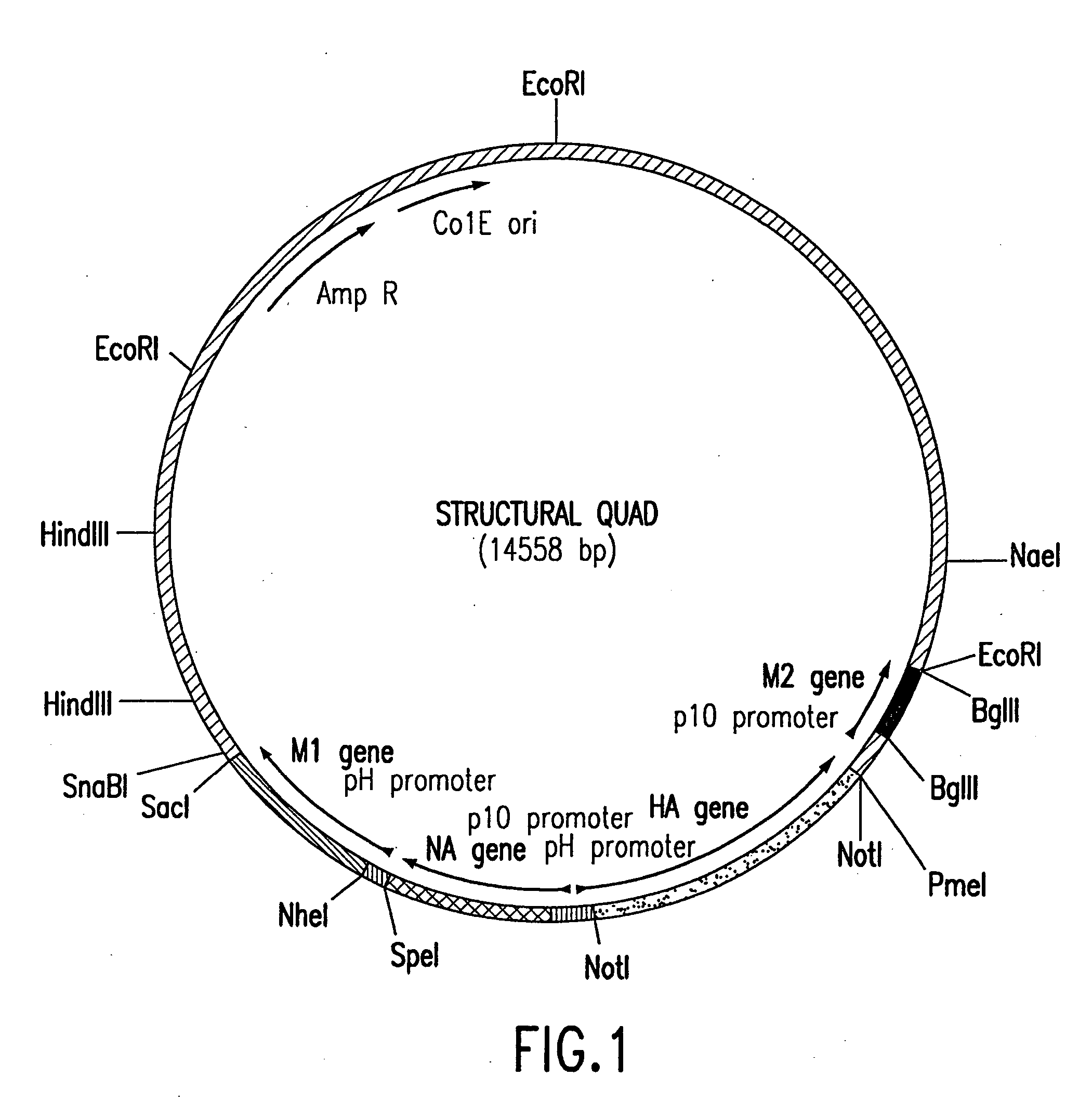
Functional influenza virus-like particles (VLPs)
ActiveUS20050009008A1SsRNA viruses negative-senseVirus peptidesMultiple copyVirus Structural Proteins
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Dry powder compositions for RNA influenza therapeutics
InactiveUS20070172430A1Reduce deliveryIncrease the amount of waterSsRNA viruses negative-sensePowder deliveryInhalationInfluenza treatment
A dry powder formulation for mucosal, intranasal, inhalation or pulmonary delivery which may include one or more siRNAs or dicer-active precursors thereof targeted to a transcript involved in infection by, or replication or production of an influenza virus.
Owner:MARINA BIOTECH INC
Neuraminidase-supplemented compositions
InactiveUS6485729B1Less importancePrevents and lessens HA immunodominanceSsRNA viruses negative-senseAntibody mimetics/scaffoldsNasal cavityAdjuvant
An anti-influenza vaccine composition wherein the improvement is that the vaccine includes, as an additive, neuraminidase (NA). The base anti-influenza vaccine can be any commercially available anti-influenza vaccine. The composition can include and be administered with an adjuvant. The vaccine composition provides protection in a host, animal or human, against influenza infection, including viral replication and systemic infection. Oral, nasal or other mucosal or per needle administration, including intracutaneous, intradermal, intramuscular, intravascular, and intravenous, are included.
Owner:PROTEIN SCI
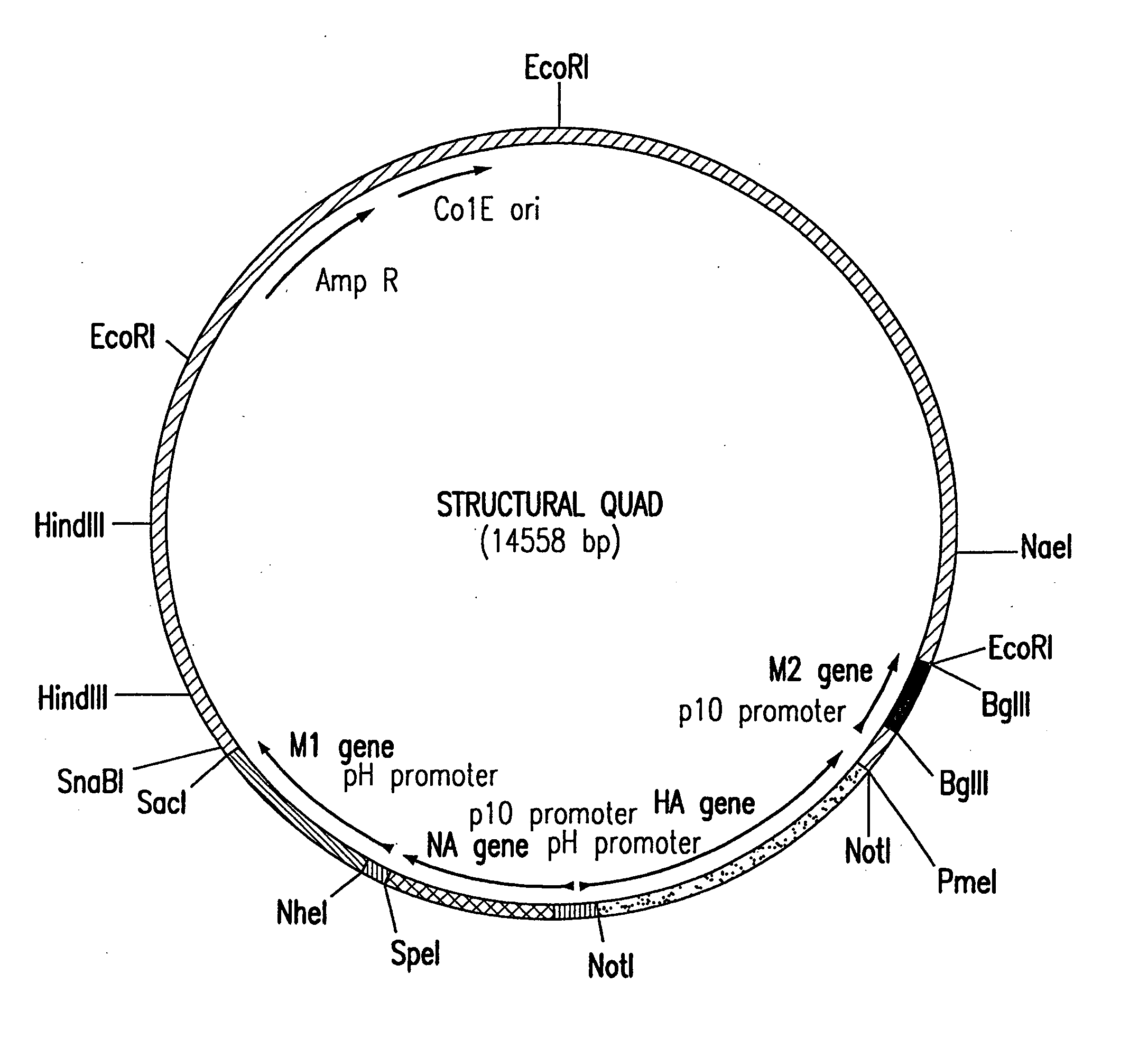
Assembly of wild-type and chimeric influenza virus-like particles (VLPs)
InactiveUS20050186621A1Minimal numberSsRNA viruses negative-senseFungiHeterologousVirus-like particle
Influenza virus-like particles (VLPs) comprising the structural proteins HA, NA, M1 and M2 are described. VLPs are also generated containing M1 alone, as are VLPs with M1 and any one or two of HA, NA and M2. VLPs with HA from one influenza subtype and NA from a different influenza subtype are also described, as are VLPs in which a portion or all of HA or NA is replaced by a heterologous moiety not produced by influenza virus, so as to comprise chimeric VLPs.
Owner:WYETH HOLDINGS LLC
Combination vaccine
ActiveUS20160166678A1SsRNA viruses negative-senseSugar derivativesHemagglutininFamily Orthomyxoviridae
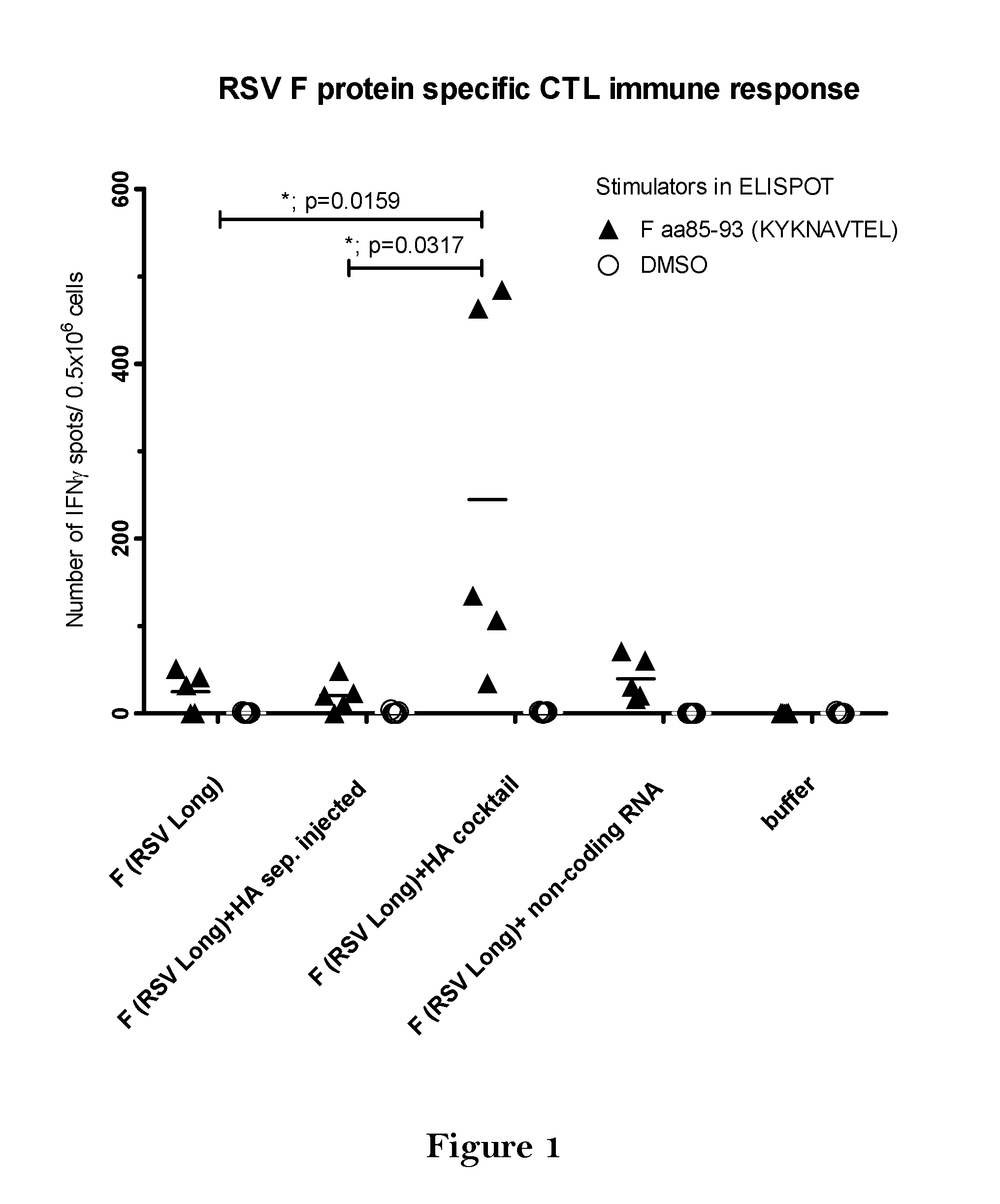
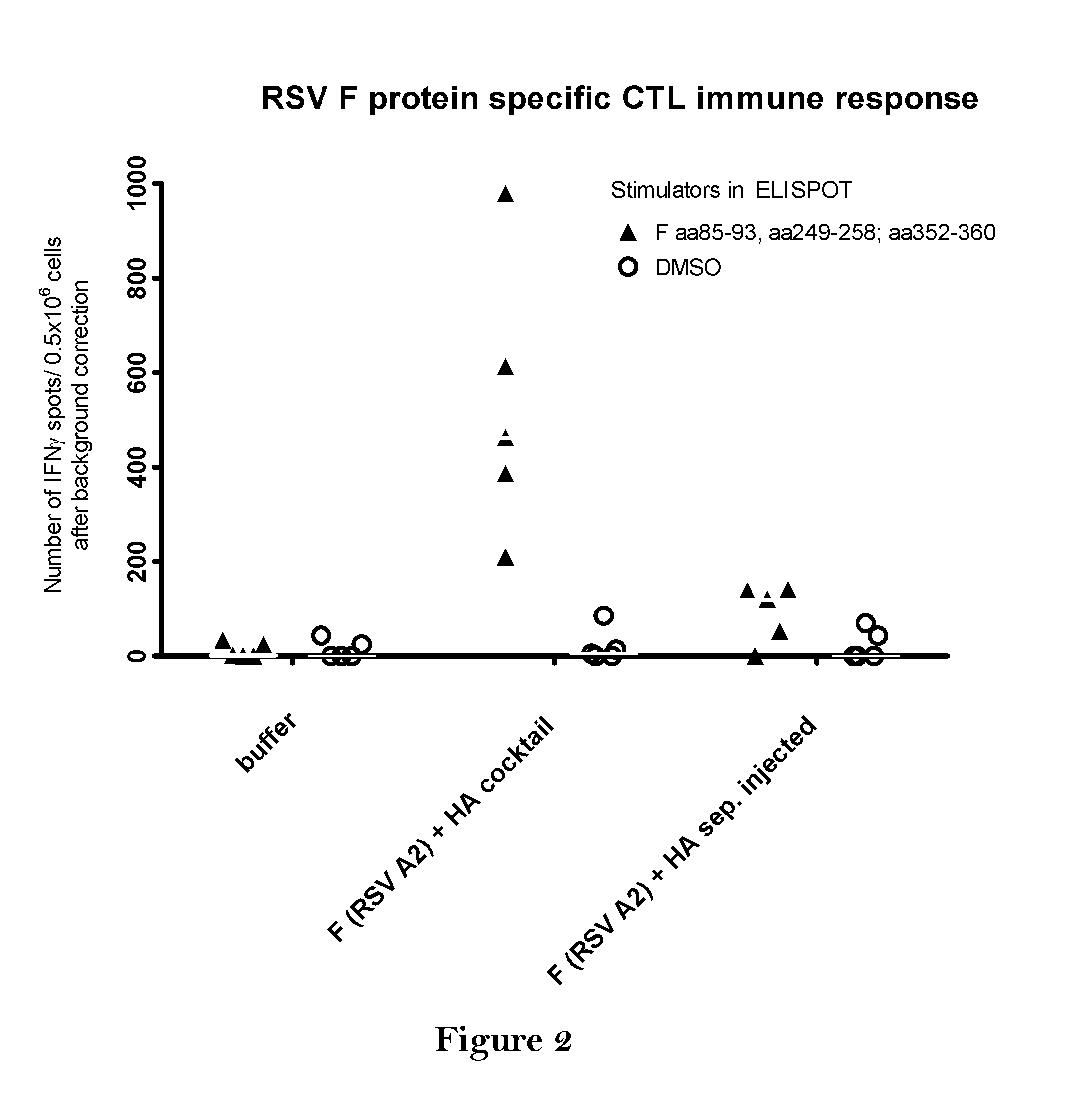
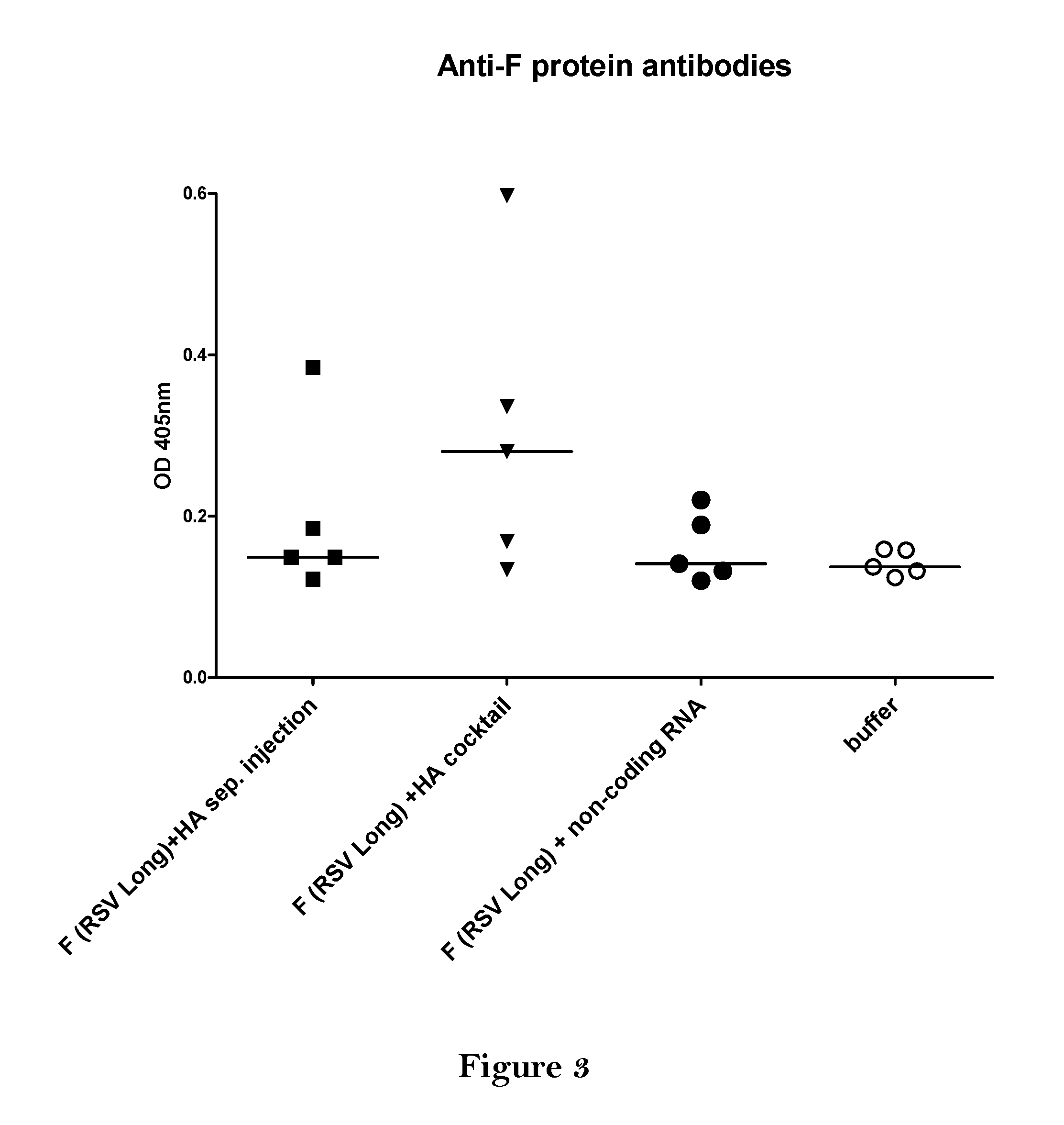
The present invention relates to a vaccine, especially a combination vaccine providing at least a first and a second antigenic function, the combination vaccine comprising at least one RNA encoding at least one or more proteins or fragments, variants or derivatives of proteins awarding antigenic function, wherein the first antigenic function being a Fusion (F) protein or a fragment, variant or derivative of a Fusion (F) protein derived from the virus family Paramyxoviridae and the second antigenic function being an Hemagglutinin (HA) protein or a fragment, variant or derivative of an Hemagglutinin (HA) protein derived from the virus family Orthomyxoviridae. Furthermore, the present invention is directed to a kit or kit of parts comprising the components of said combination vaccine and to said combination vaccine for use in a method of prophylactic or therapeutic treatment of diseases, particularly in the prevention or treatment of infectious diseases like RSV and influenza.
Owner:CUREVAC SE
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
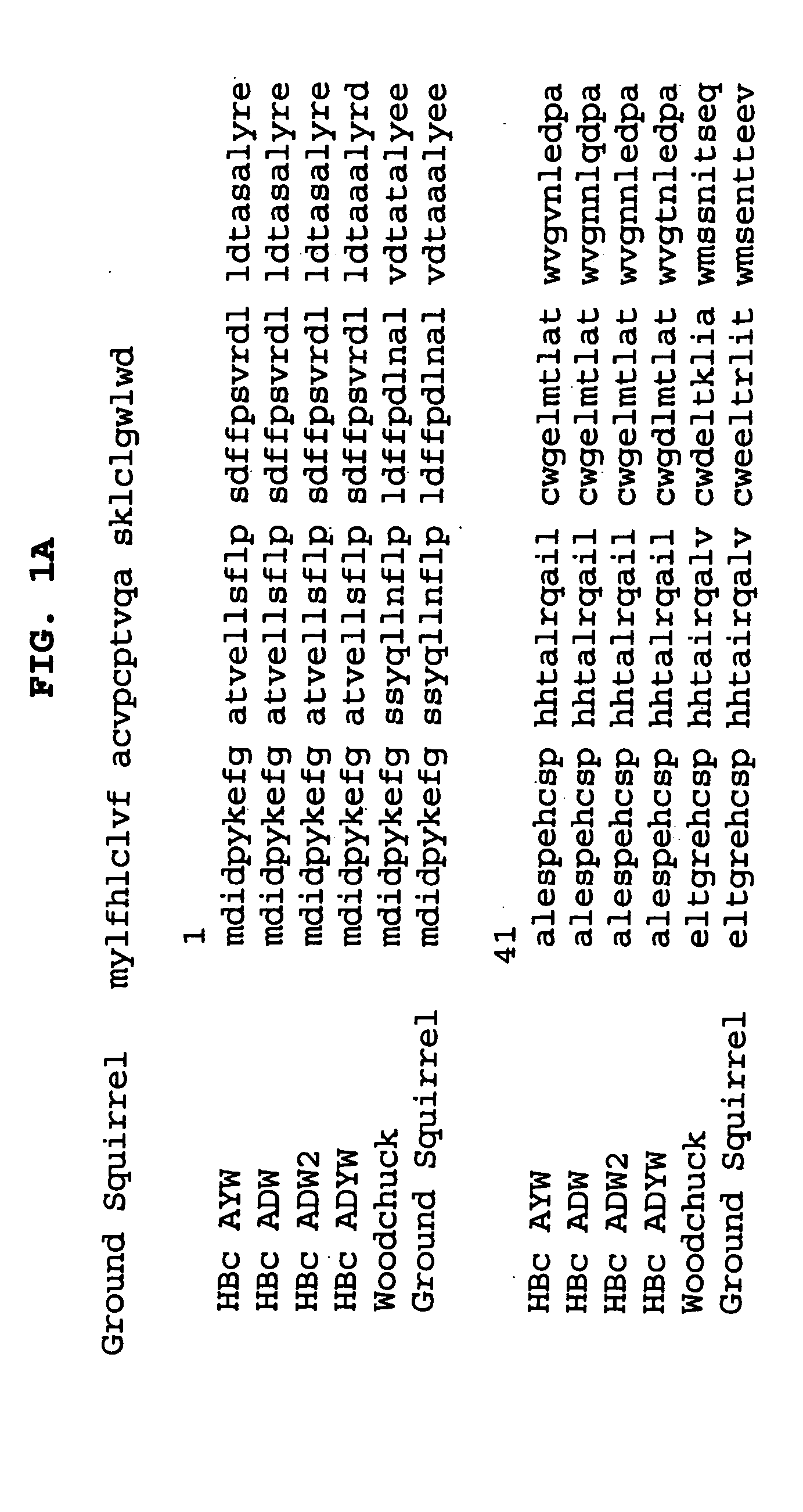
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
Influenza virus vaccines and uses thereof
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
Lipid nanoparticle mRNA vaccines
PendingUS20200163878A1Promote localizationEasy translationSsRNA viruses negative-sensePowder deliveryAntigenRabies vaccination
The invention relates to mRNA comprising lipid nanoparticles and their medical uses. The lipid nanoparticles of the present invention comprise a cationic lipid according to formula (I), (II) or (III) and / or a PEG lipid according to formula (IV), as well as an mRNA compound comprising an mRNA sequence encoding an antigenic peptide or protein. The invention further relates to the use of said lipid nanoparticles as vaccines or medicaments, in particular with respect to influenza or rabies vaccination.
Owner:ACUITAS THERAPEUTICS INC +1
Influenza Hemagglutinin And Neuraminidase Variants
InactiveUS20080069821A1Extended half-lifeReducing/increasing polypeptide antigenicityFungiVirusesHemagglutininNeuraminidase
Owner:MEDIMMUNE LLC +1
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Functional influenza virus like particles (VLPs)
ActiveUS20070184526A1Protective responseSsRNA viruses negative-senseViral antigen ingredientsSeasonal influenzaAvian influenza virus
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
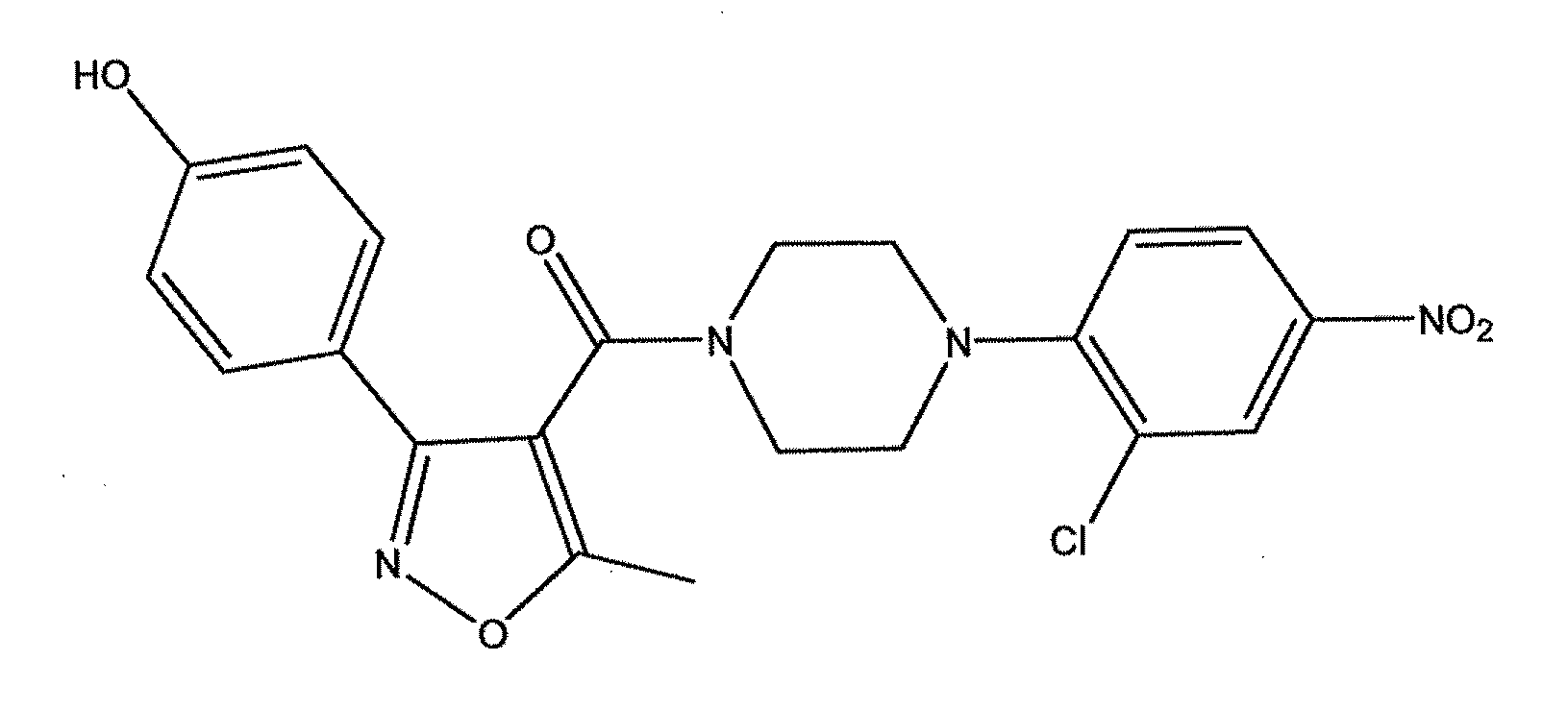
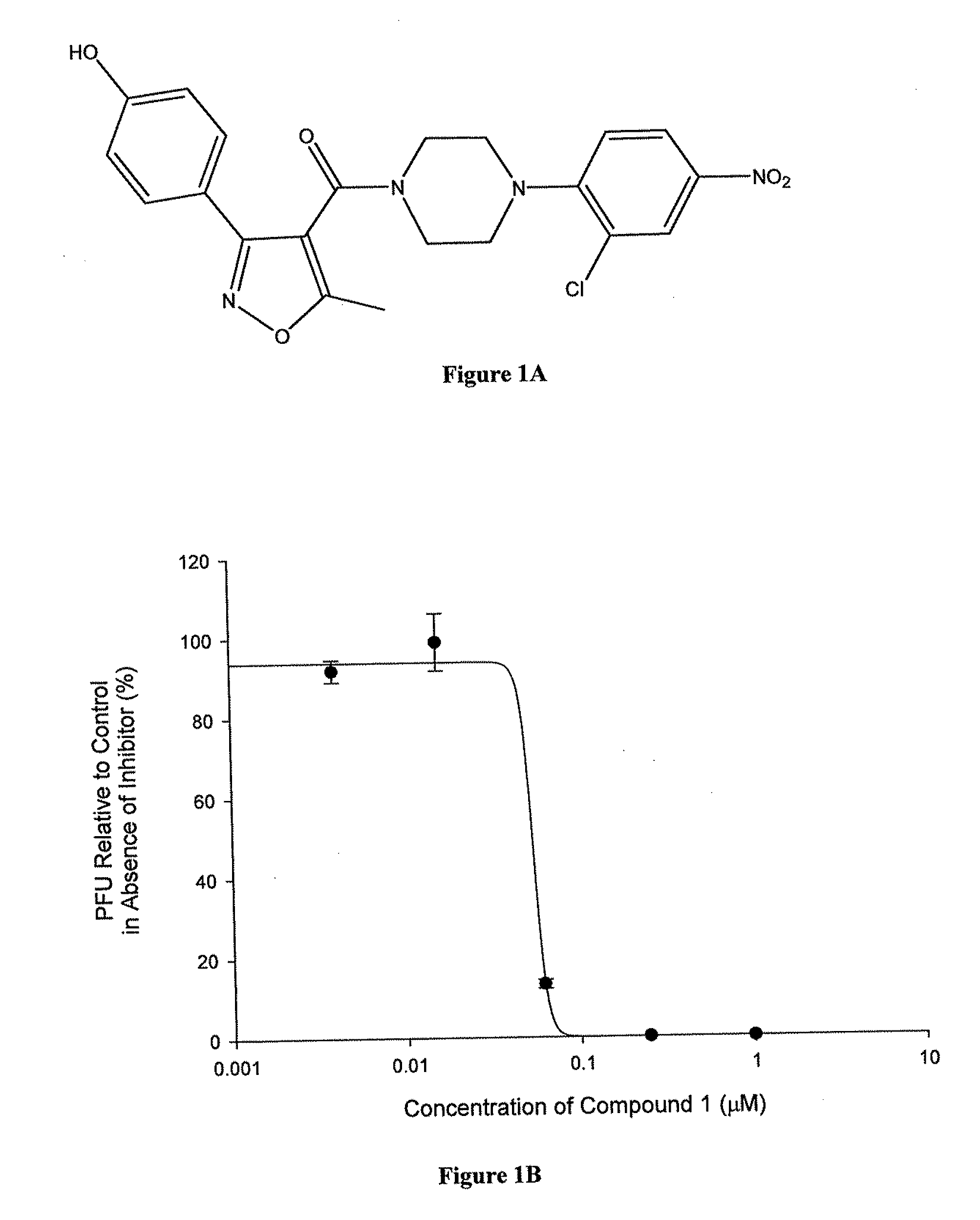
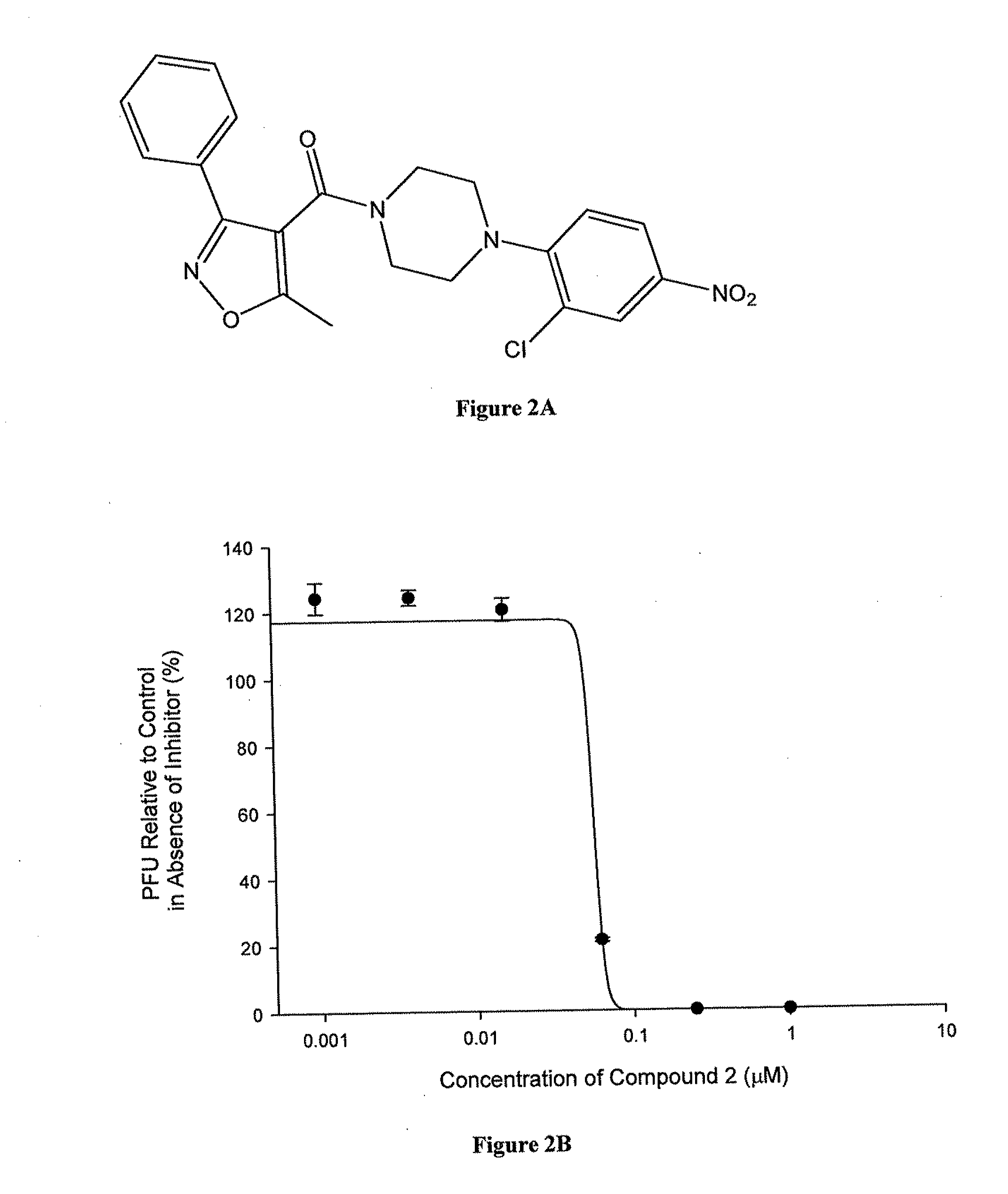
Antiviral compounds and methods of making and using thereof
ActiveUS20110212975A1Organic active ingredientsOrganic chemistryPiperazinePharmaceutical preservatives
Compounds which exhibit antiviral activity, particularly against influenza virus, and methods of making and using thereof are described herein. In one embodiment, the compounds are heterocyclic amides containing piperazine and isozazole rings and optionally substituted with one or more substituents. The compounds can be formulated with one or more pharmaceutically acceptable excipients to form compositions suitable for enteral or parenteral administration. The compounds are preferably used to treat or prevent Influenza A infections, such as H1N1, H2N2, H3N2, H5N1, H7N7, H1N2, H9N2, H7N2, H7N3, and H10N7.
Owner:VERSITECH LTD
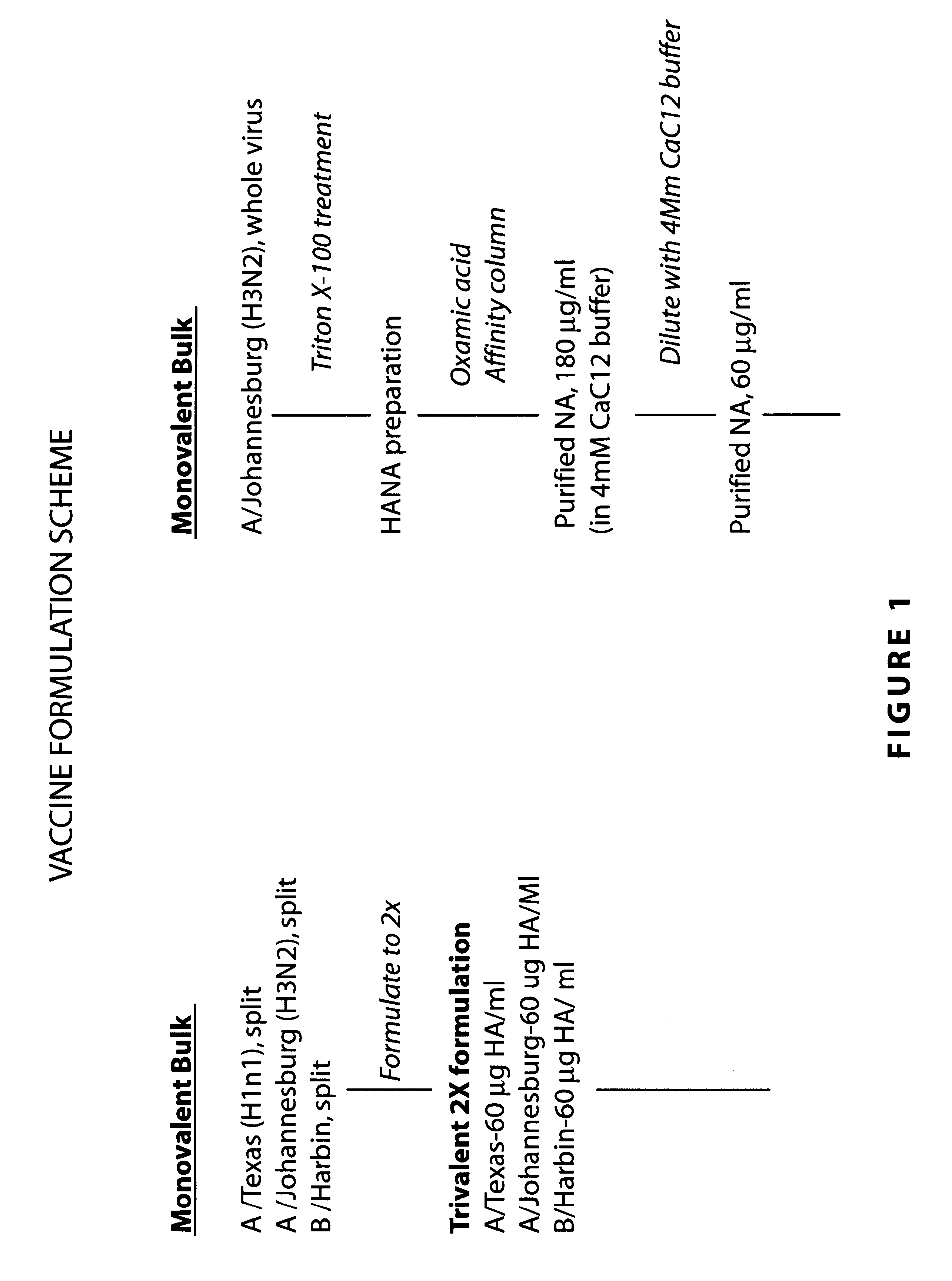
Influenza vaccine
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:SMITHKLINE BEECHAM PHARMA GMBH +1
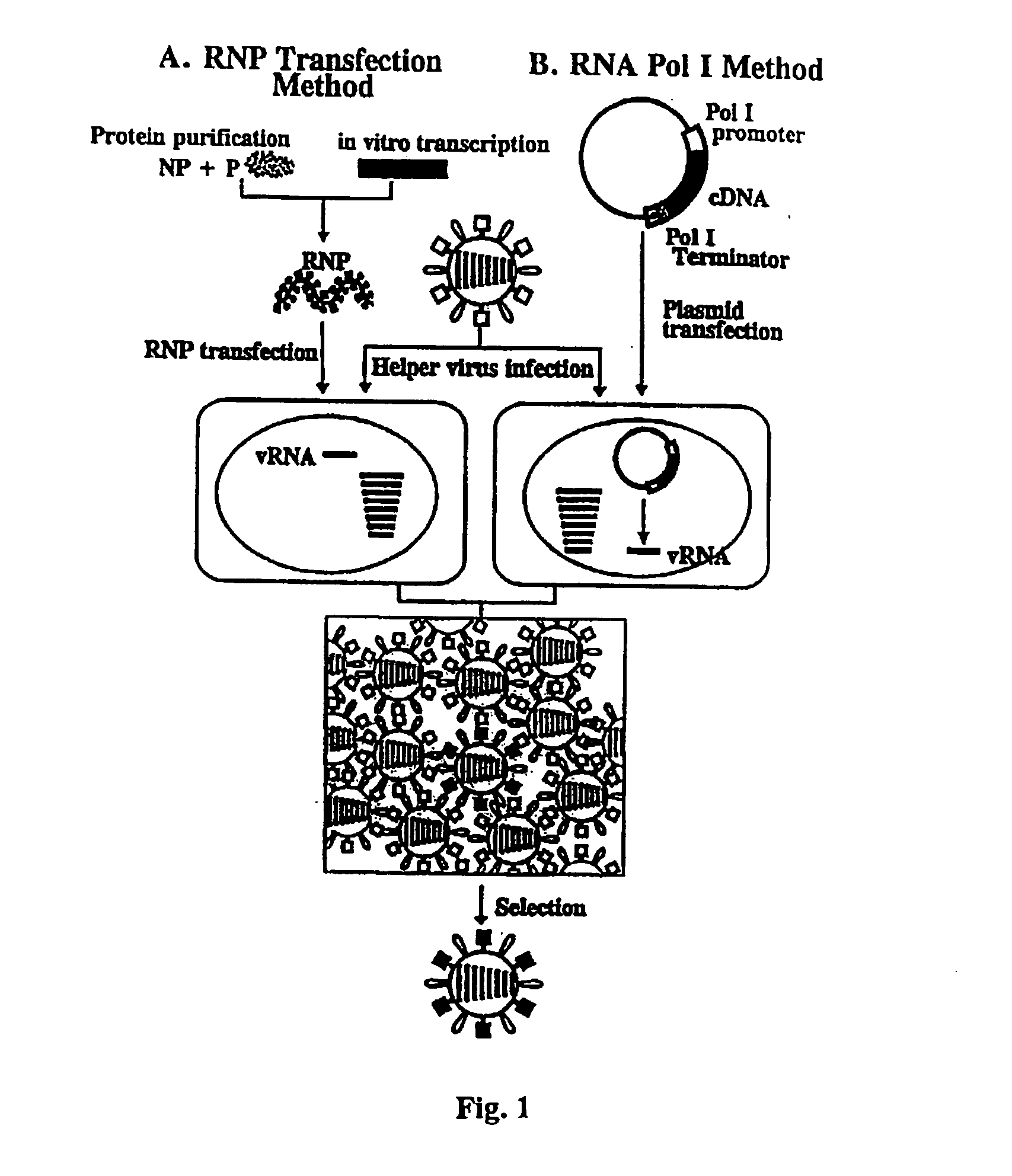
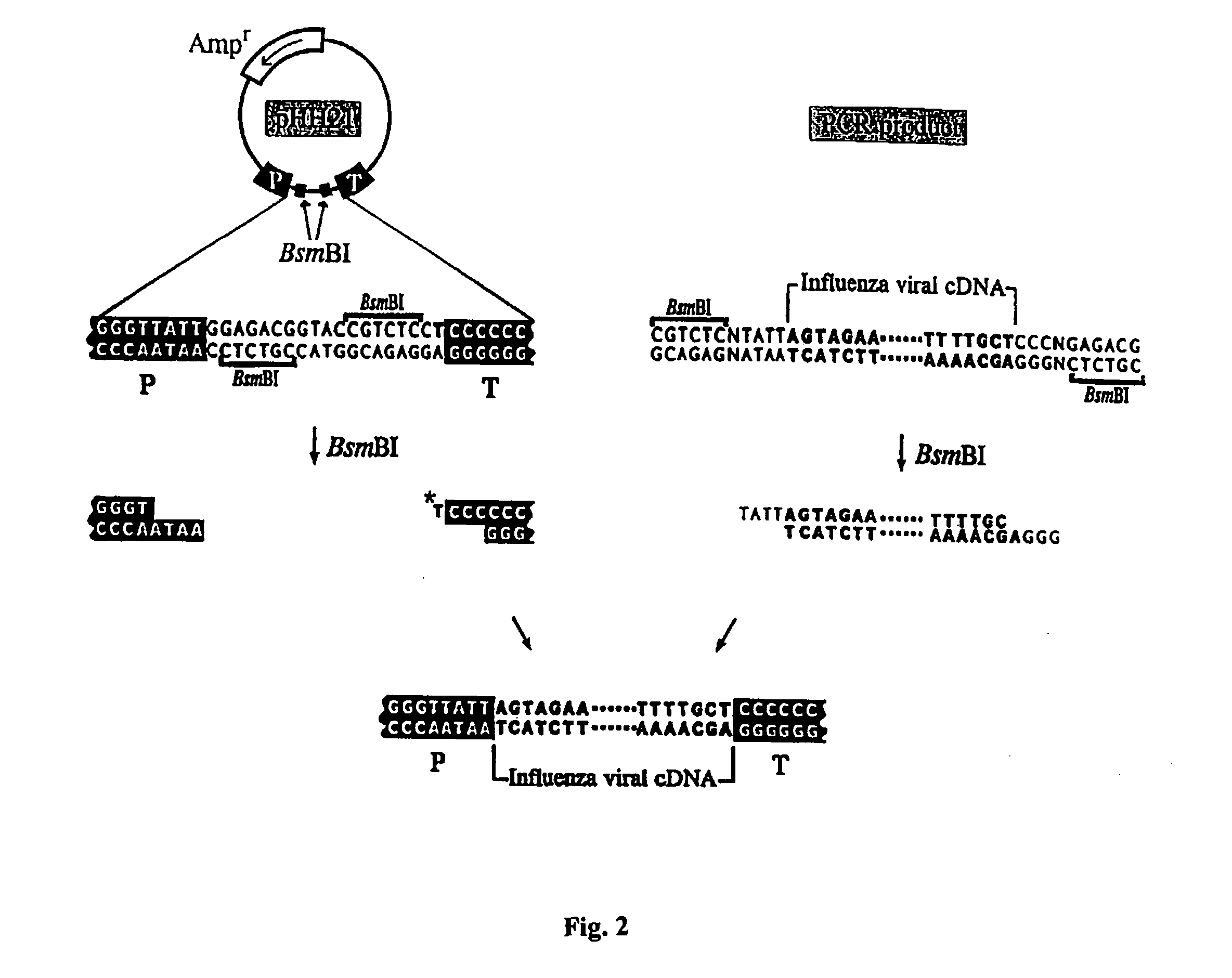
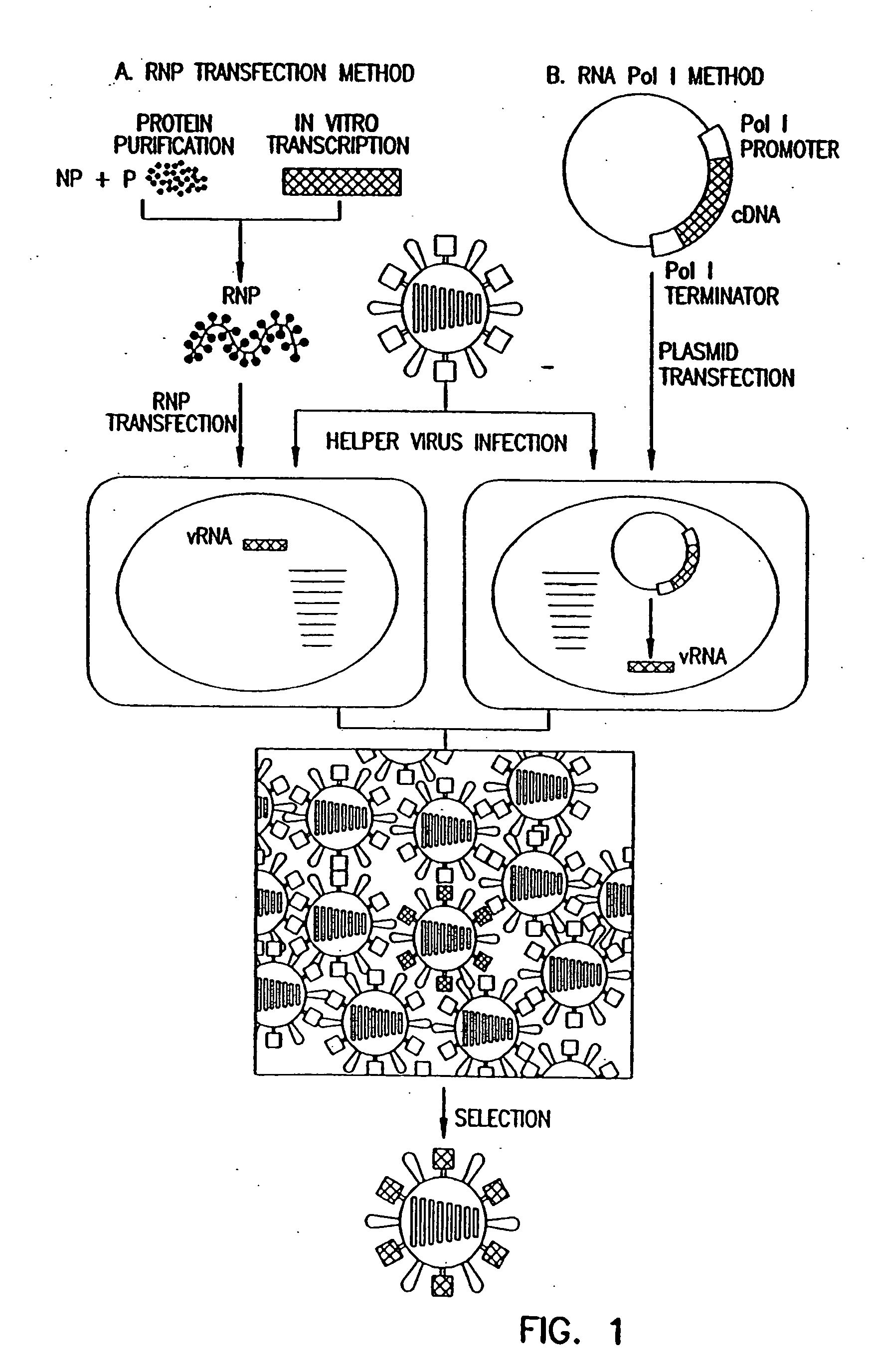
Recombinant influenza vectors with a PolII promoter and ribozymes for vaccines and gene therapy
InactiveUS20050037487A1Easy to operateEnhances these viruses as vaccine vectorsSsRNA viruses negative-senseSugar derivativesHelper virusViral vector
The invention provides a composition useful to prepare influenza viruses, e.g., in the absence of helper virus, using vectors which include Po1II promoters and multiple ribozyme sequences.
Owner:WISCONSIN ALUMNI RES FOUND
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050123550A1Enhances therapeutic efficacy and protective immune responseGood curative effectSsRNA viruses negative-senseOrganic active ingredientsInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
Influenza antigens, vaccine compositions, and related methods
ActiveUS20070275014A1Reduces and eliminates riskLess considerationSsRNA viruses negative-senseAntibody mimetics/scaffoldsInfluenza aImmunologic function
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by influenza virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:IBIO
Broad spectrum anti-viral therapeutics and prophylaxis
The present invention provides new compositions and methods for preventing and treating pathogen infection. In particular, the present invention provides compounds having an anchoring domain that anchors the compound to the surface of a target cell, and a therapeutic domain that can act extracellularly to prevent infection of the target cell by a pathogen, such as a virus. Preferred target cells are epithelial cells. The invention provides compositions and methods for preventing viral diseases, such as influenza, using compounds having anchoring domains that can bind target cells linked to enzymatic activities that can act extracellularly to interfere with viral infection of target cells. The invention also provides compositions and methods for preventing viral diseases such as influenza using compounds having anchoring domains that can bind target cells linked to protease inhibitors that can act extracellularly to interfere with viral infection of target cells.
Owner:ANSUN BIOPHARMA
Influenza recombinant subunit vaccine
InactiveUS20070042002A1Improving immunogenicityImprove efficacySsRNA viruses negative-senseVirus peptidesAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:HAWAII BIOTECH INC
Methods and compounds for treating paramyxoviridae virus infections
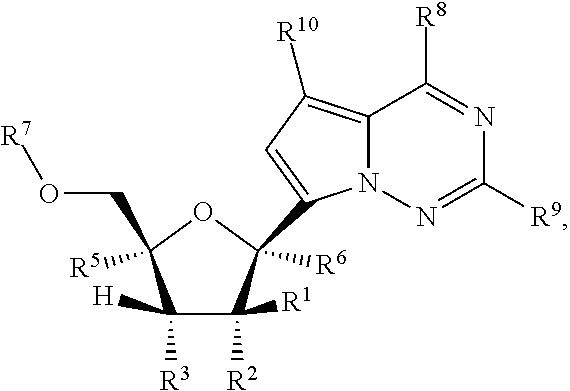
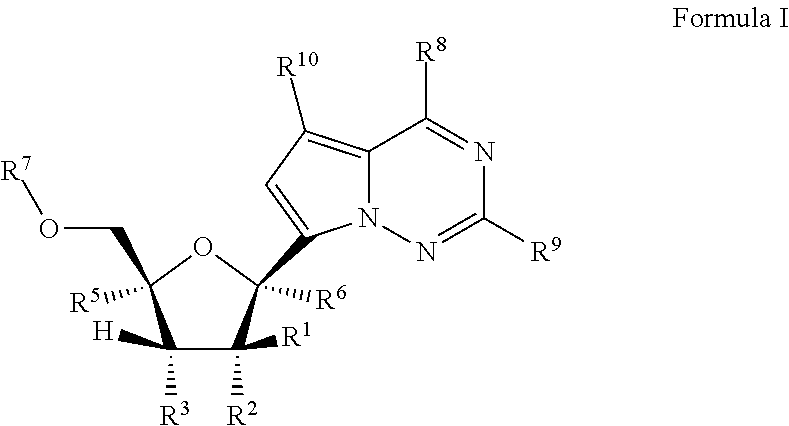
Provided are methods for treating Paramyxoviridae virus infections by administering ribosides, riboside phosphates and prodrugs thereof, of Formula I:wherein the 1′ position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Human parainfluenza and Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
Recombinant influenza viruses expressing tumor-associated antigens as antitumor agents
InactiveUS6884414B1Quick changeAvoid problemsSsRNA viruses negative-senseBiocideTumor reductionIn vivo
The present invention relates to the engineering of recombinant influenza viruses that express tumor-associated antigens. Expression of tumor-associated antigens by these viruses can be achieved by engineering specific epitopes into influenza virus proteins, or by engineering viral genes that encode a viral protein and the specific antigen as independent polypeptides. Tumor-bearing patients can be immunized with the recombinant influenza viruses alone, or in combination with another treatment, to induce an immune response that leads to tumor reduction. The recombinant viruses can also be used to vaccinate high risk tumor-free patients to prevent tumor formation in vivo.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Peptide-based vaccine for influenza
A human synthetic peptide-based influenza vaccine for intranasal administration comprises a mixture of flagella containing at least four epitopes of influenza virus reactive with human cells, each expressed individually in Salmonella flagellin, said influenza virus epitopes being selected from the group consisting of: (i) one B-cell hemagglutinin (HA) epitope; (ii) one T-helper hemagglutinin (HA) or nucleo-protein (NP) epitope that can bind to many HLA molecules; and (iii) at least two cytotoxic lymphocyte (CTL) nucleoprotein (NP) or matrix protein (M) epitopes that are restricted to the most prevalent HLA molecules in different human populations.
Owner:YEDA RES & DEV CO LTD
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050255121A1Good antigenicityEnhance immune responseSsRNA viruses negative-senseBiocideInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
Method for Producing the Flu Virus
The invention relates to a method for producing flu virus according to which:a) immunizing a hen by administering a flu vaccine to the hen,b) triggering embryogenesis in one or more eggs of the immunized hen,c) infecting the one or more embryonated eggs by inoculating a flu virus into the allantoic cavity of the eggs,d) incubating the one or more infected embryonated eggs under temperature and humidity conditions that allow replication of the virus, ande) harvesting the allantoic fluid of the one or more incubated eggs containing the virus.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC +1
Influenza virus-like particles (VLPS) comprising hemagglutinin produced within a plant
InactiveUS20100239610A1Enhance immune responseEasy to captureSsRNA viruses negative-senseVirus peptidesHemagglutininLipid formation
A method for synthesizing influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of influenza HA in plants and the purification by size exclusion chromatography. The invention is also directed towards a VLP comprising influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
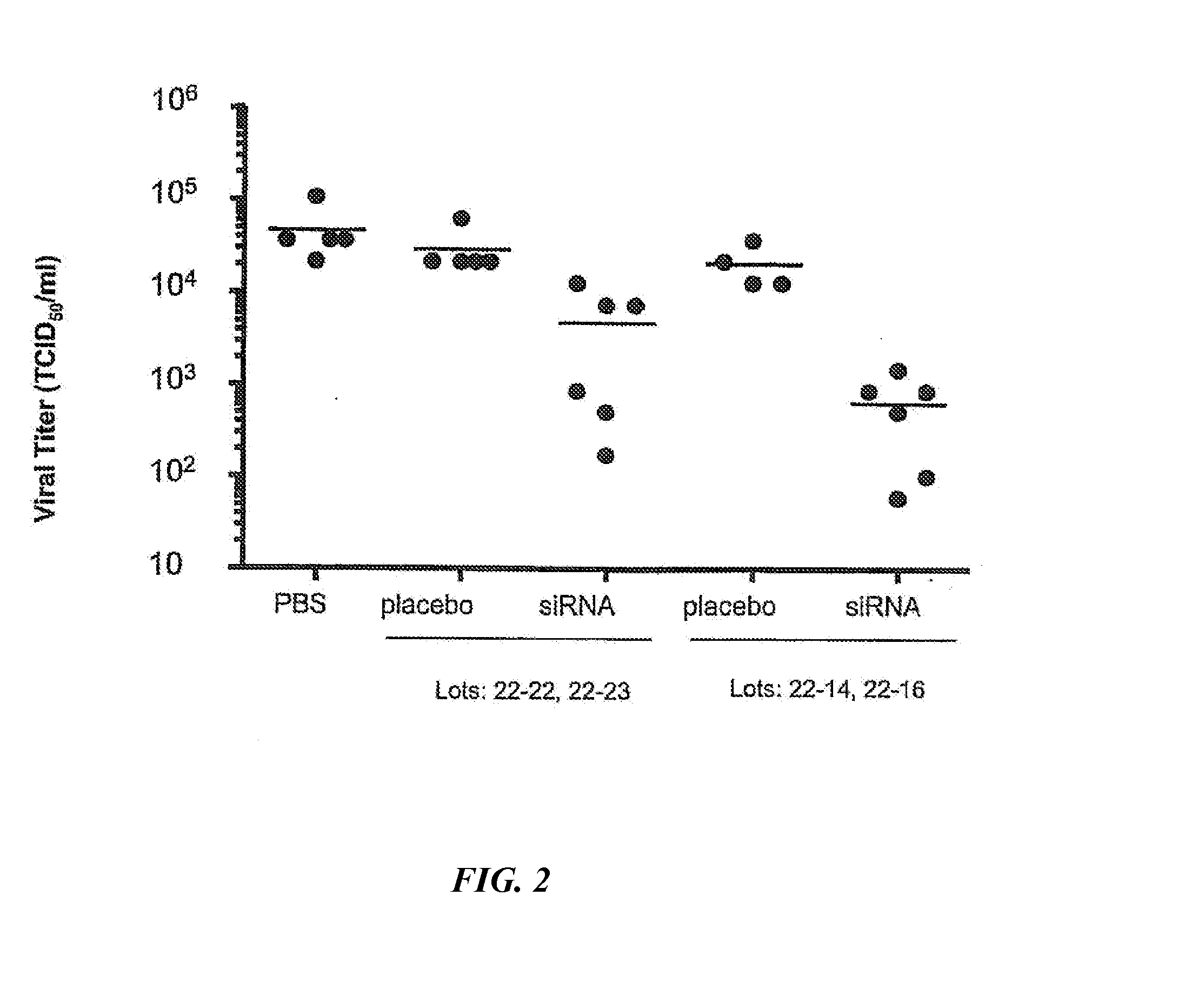
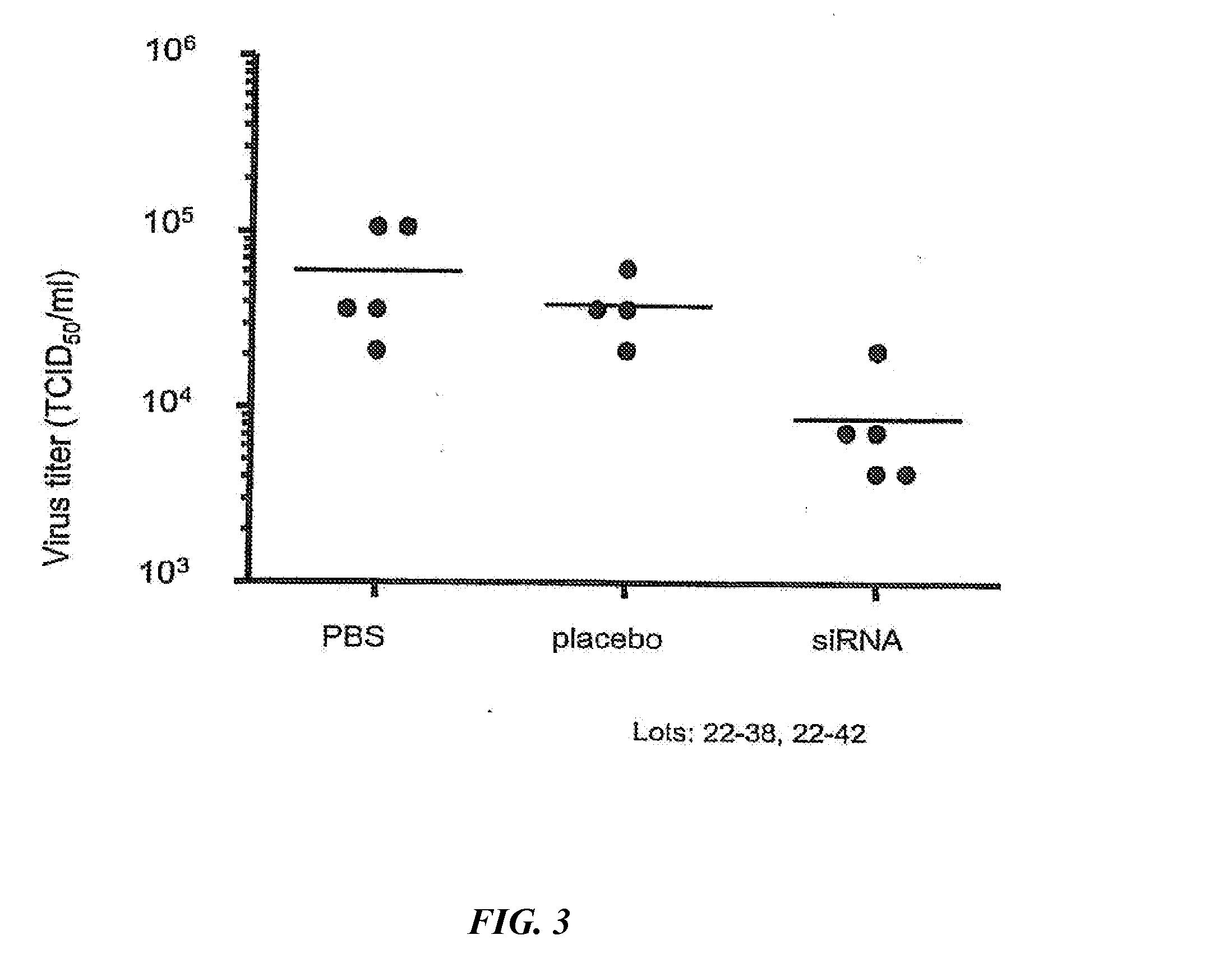
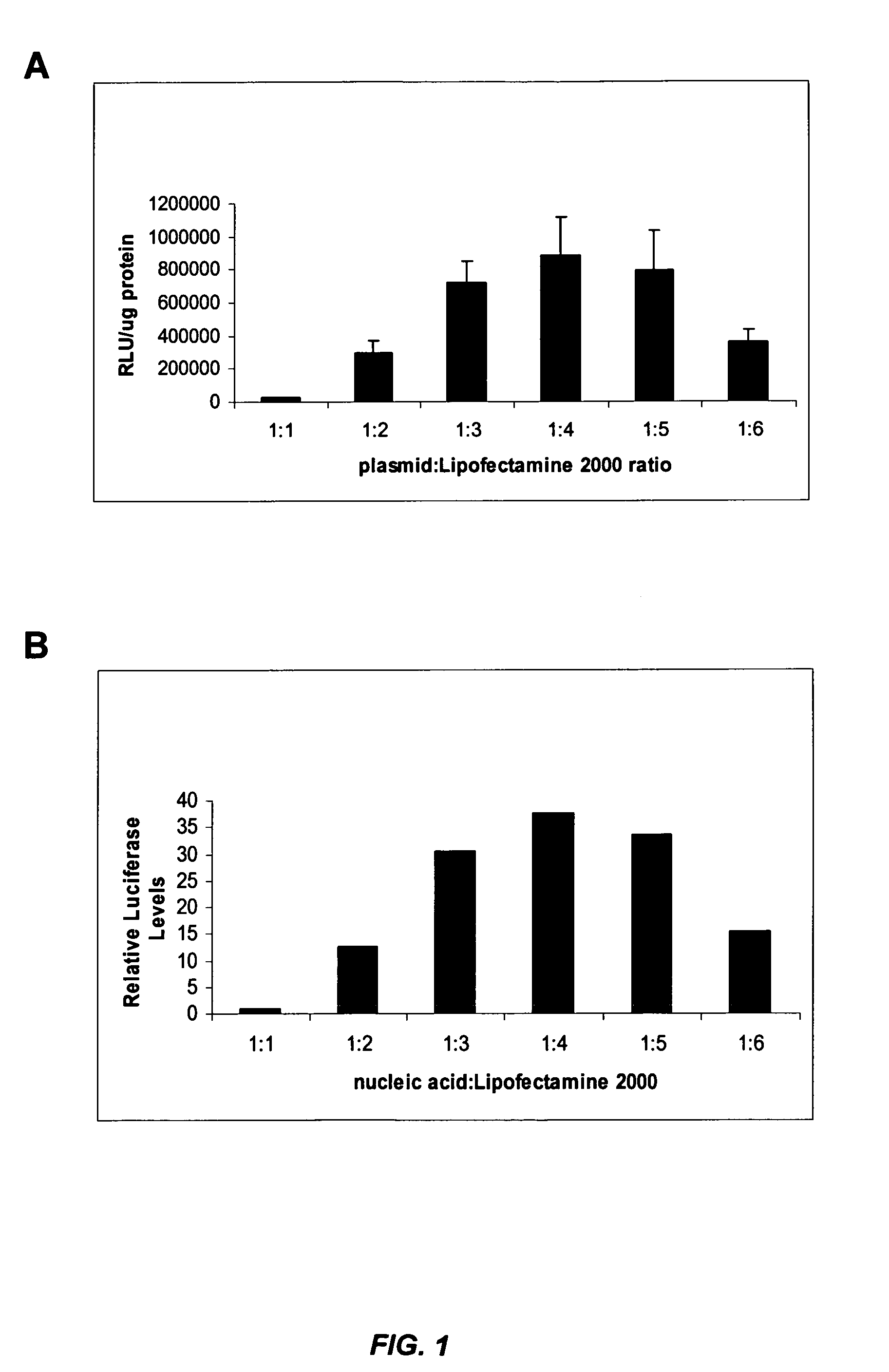
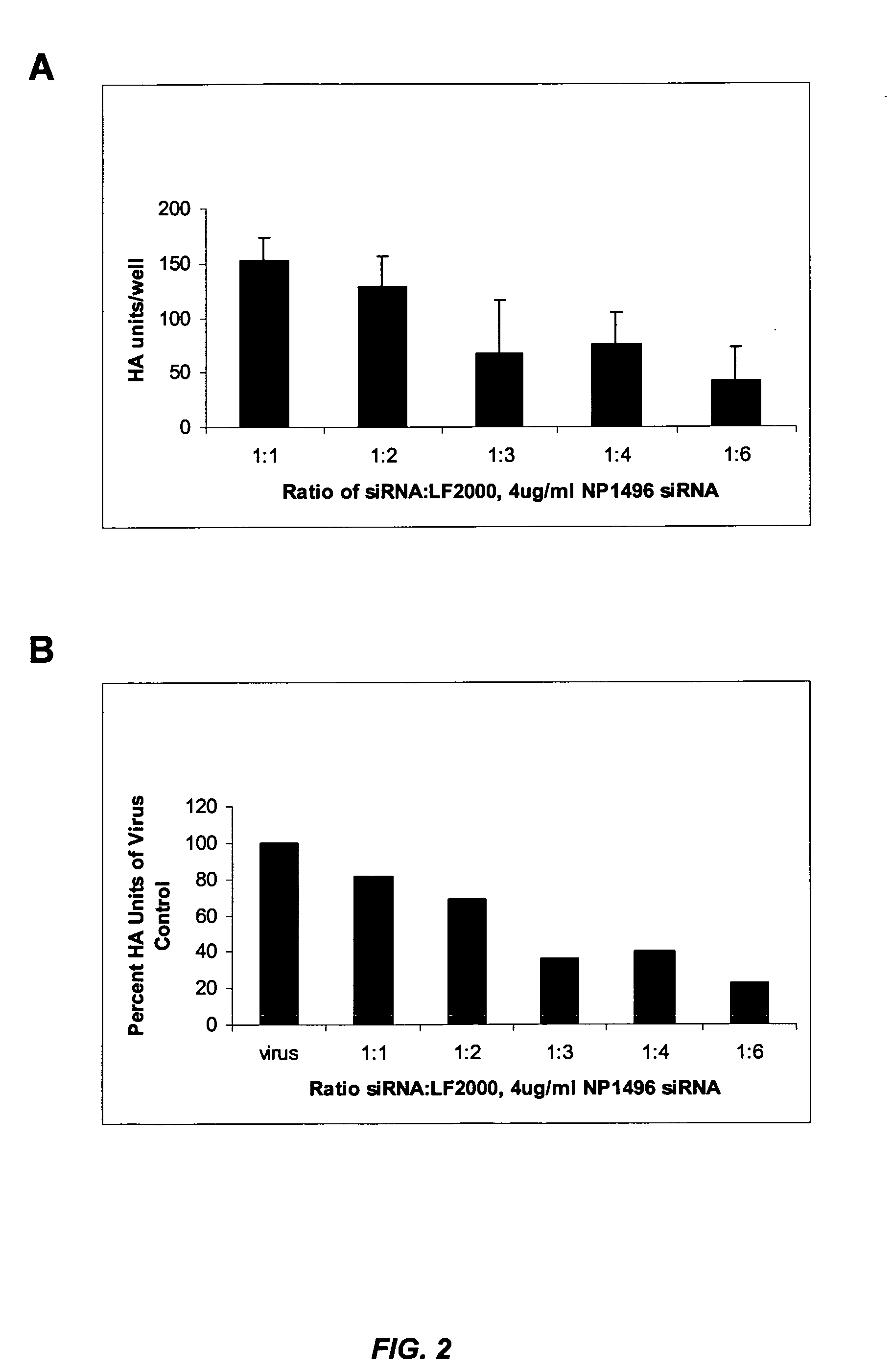
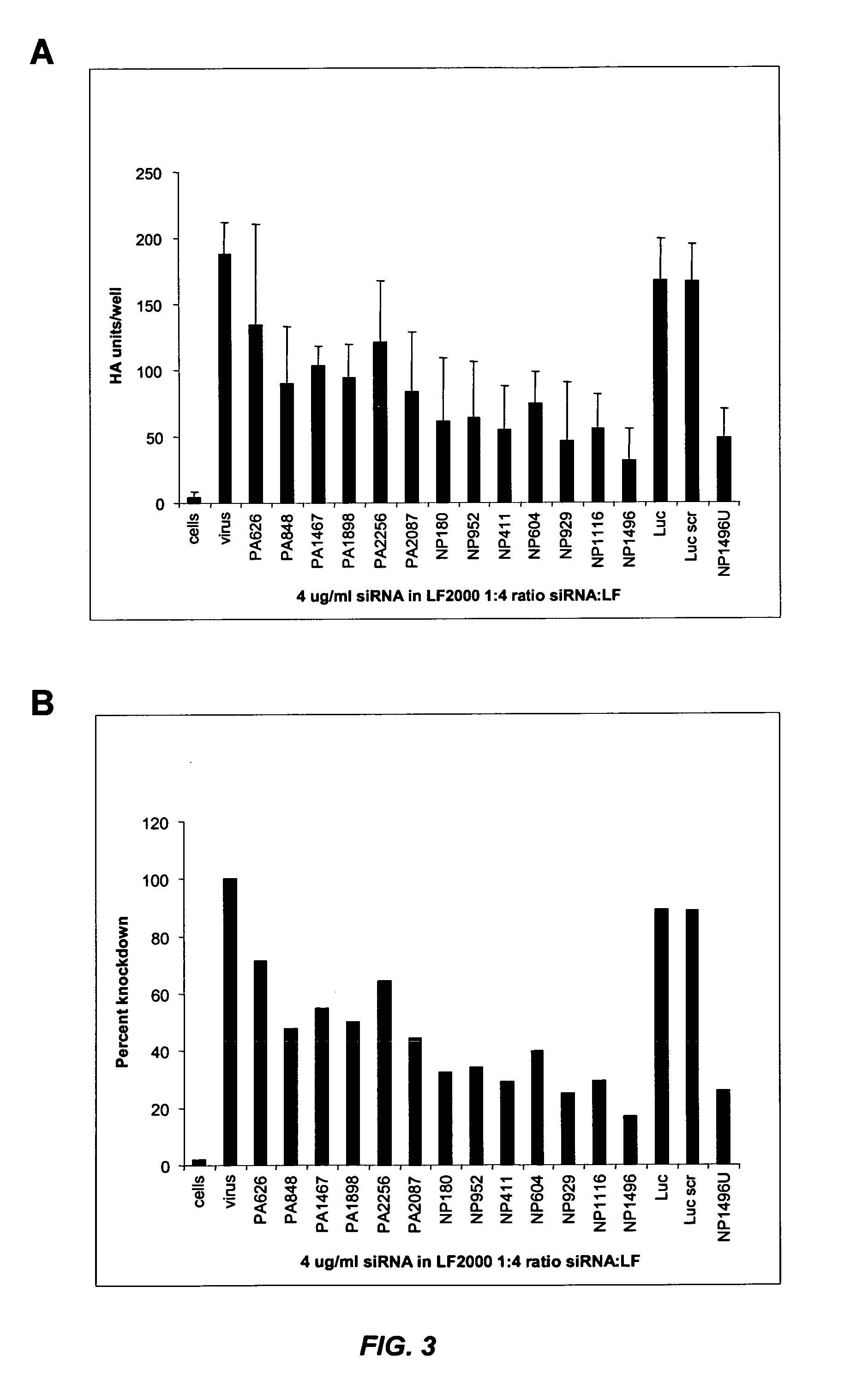
siRNA silencing of influenza virus gene expression
InactiveUS20070218122A1Reduce the amount requiredSsRNA viruses negative-sensePowder deliveryLipid formationSirna silencing
The present invention provides siRNA molecules that target influenza virus gene expression and methods of using such siRNA molecules to silence influenza virus gene expression. The present invention also provides nucleic acid-lipid particles that target influenza virus gene expression comprising an siRNA that silences influenza virus gene expression, a cationic lipid, and a non-cationic lipid.
Owner:PROTIVA BIOTHERAPEUTICS
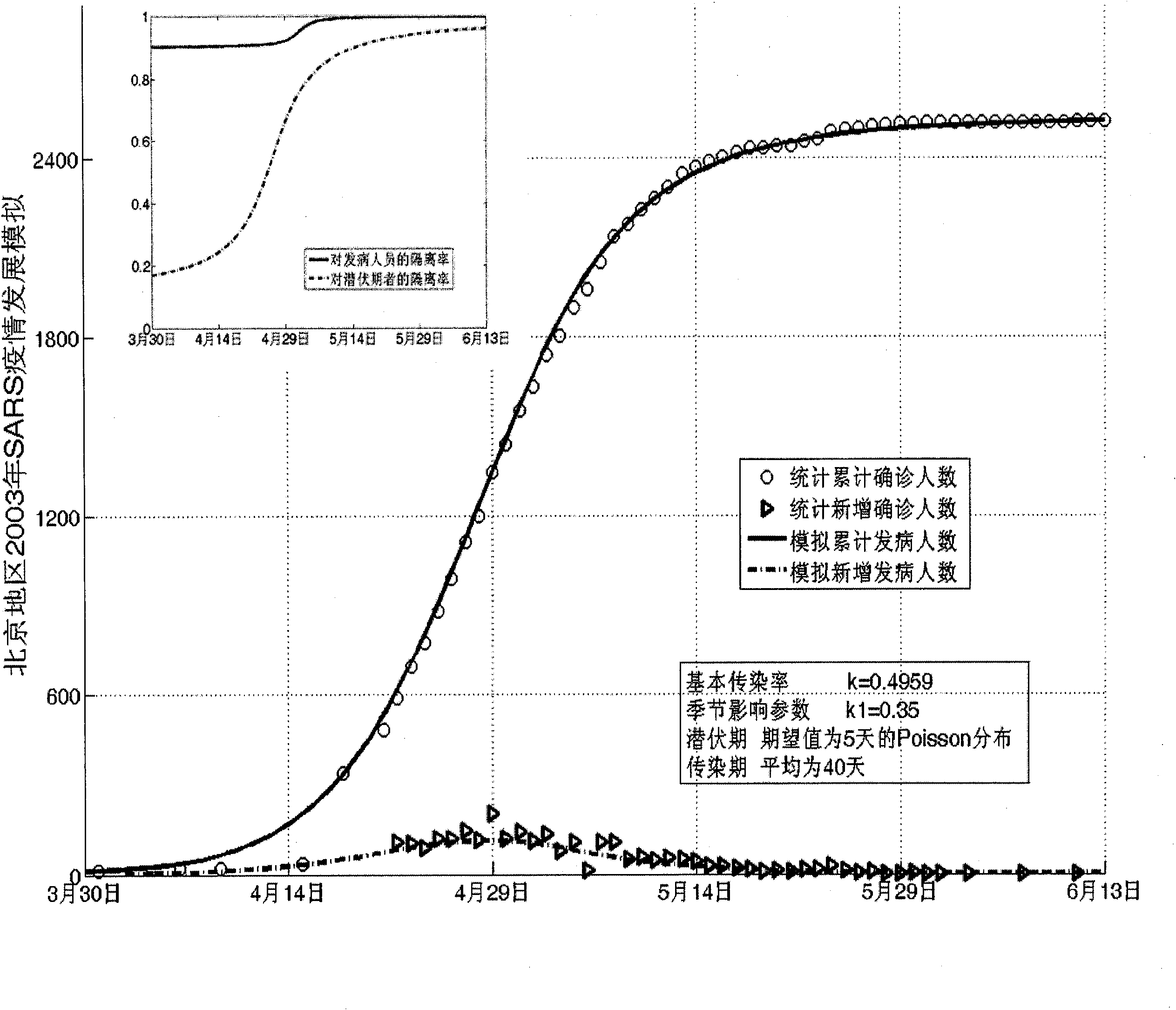
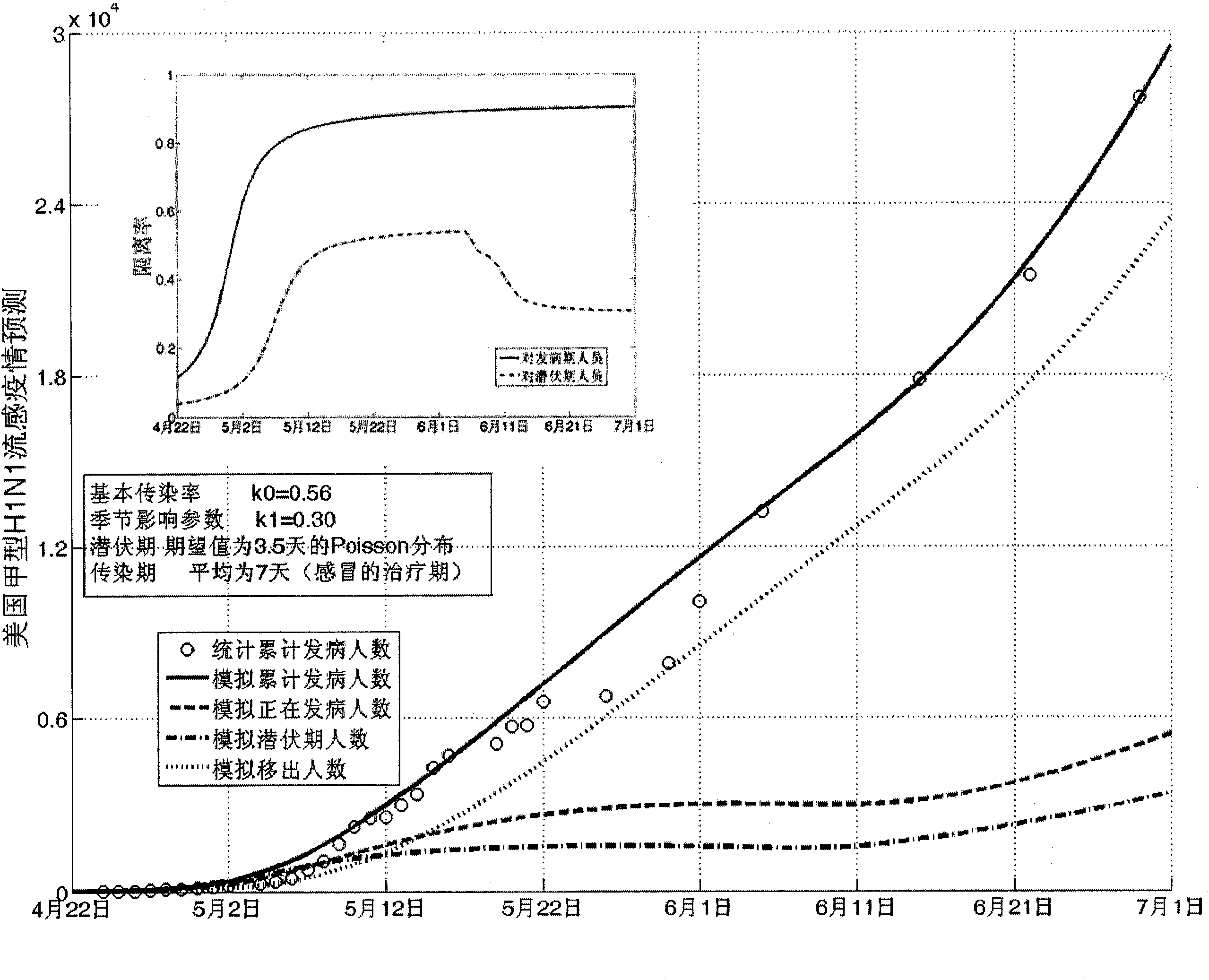
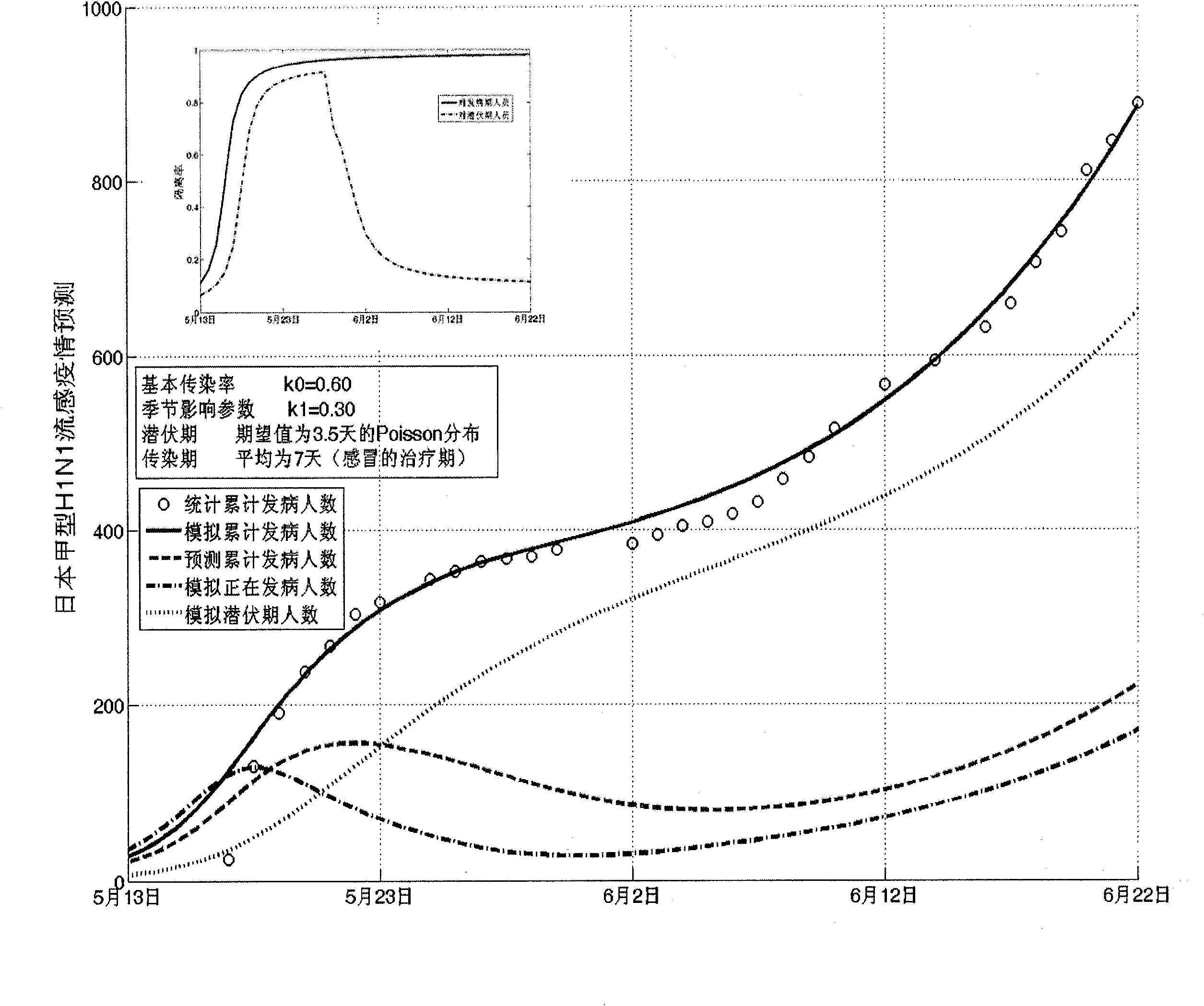
Infectious disease epidemic situation predicative analysis method based on nonlinear and coefficient variation predictive model
InactiveCN101794342ASpecial data processing applicationsSusceptible populationSevere acute respiratory syndrome
The invention establishes a nonlinear and coefficient variation infectious disease predictive model aiming at epidemic diseases with viruses which have infectivity at a latent period and a period of onset, provides an epidemic situation control function directly related to the model, simulating and predicting effects of different control measures and different control degrees on the basis of prediction in consideration of the control measures, considers the epidemic situation control as a continuous change process, integrally simulating and predicting development and control of the epidemic situation and provides crucial quantitative information for decision-making departments to optimally decide and control the epidemic situation with the smallest cost. By adopting the invention, a relative error for simulating SARS (Severe Acute Respiratory Syndromes) in Beijing areas in 2003 is 0.98% and predictive results for influenza A virus subtype H1N1 in US and Japan are well matched with the actual epidemic situation development, a quantified control factor for preventing the influenza A virus subtype H1N1 at an initial development stage and controlling the spread of the epidemic situation is obtained and epidemic situation development conditions of different control intensities and different susceptible people are predicted.
Owner:中国人民解放军防化指挥工程学院
Influenza vaccine
InactiveUS20090263422A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alphatocopherol, and an emulsifying agent.
Owner:HANON EMMANUEL JULES +1
Neutralizing Antibodies to Influenza Viruses
The present invention concerns methods and means for identifying, producing, and engineering neutralizing antibodies against influenza A viruses, and to the neutralizing antibodies produced. In particular, the invention concerns neutralizing antibodies against various influenza A virus subtypes, including neutralizing antibodies against two or more of H1, H2, H3, H5, H7 and H9, such as, for example all of H1, H2, H3, and H5 subtypes, and methods and means for making such antibodies. More specifically, the invention concerns antibodies capable of neutralizing more than one, preferably all, isolates of an influenza A virus subtype.
Owner:I2 PHARMA INC
Recombinant influenza viruses for vaccines and gene therapy
InactiveUS20060057116A1Improve efficiencyIncrease productionSsRNA viruses negative-senseBiocideHelper virusGene
The invention provides a composition useful to prepare influenza A viruses, e.g., in the absence of helper virus.
Owner:KAWAOKA YOSHIHIRO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com