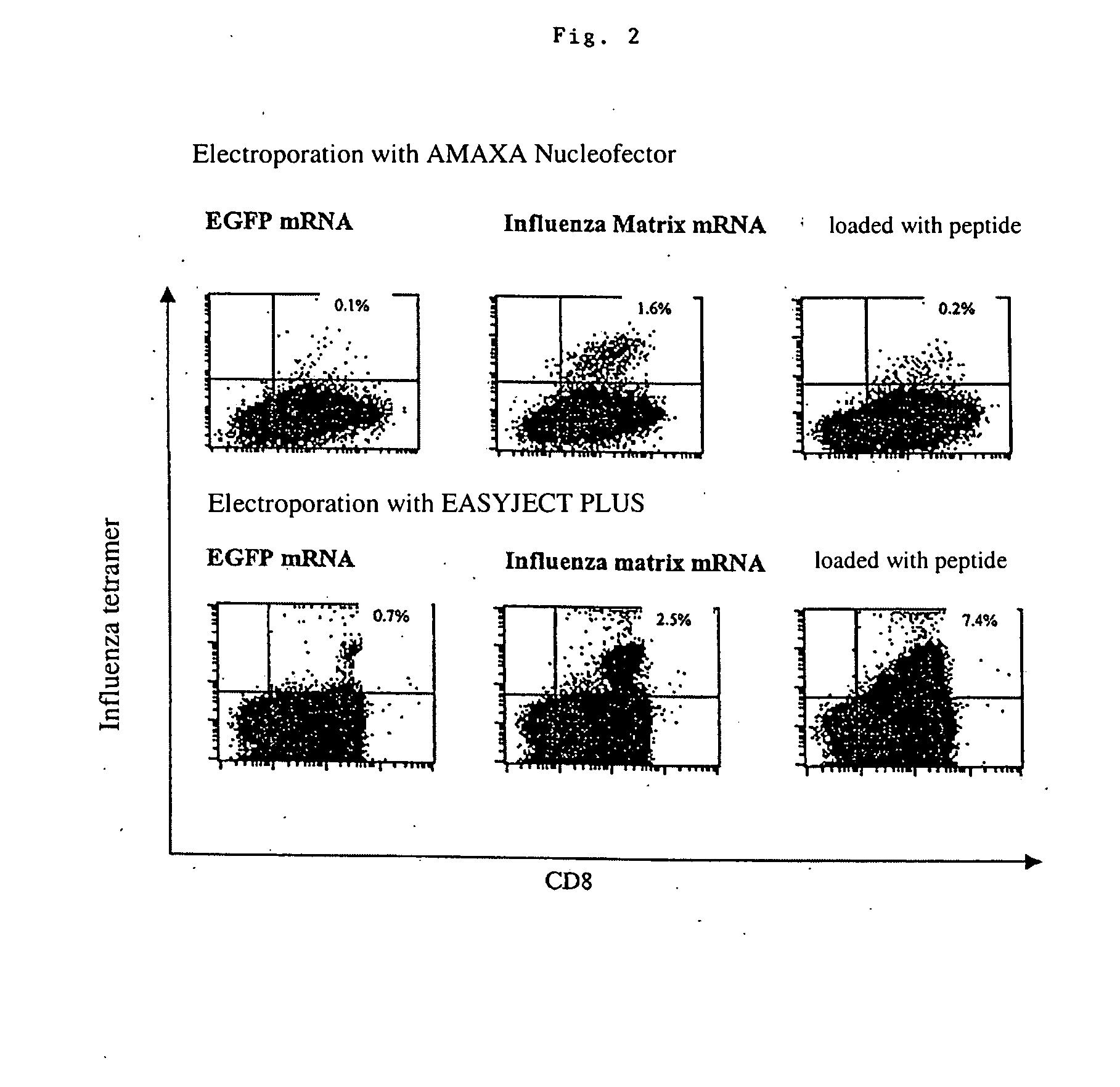
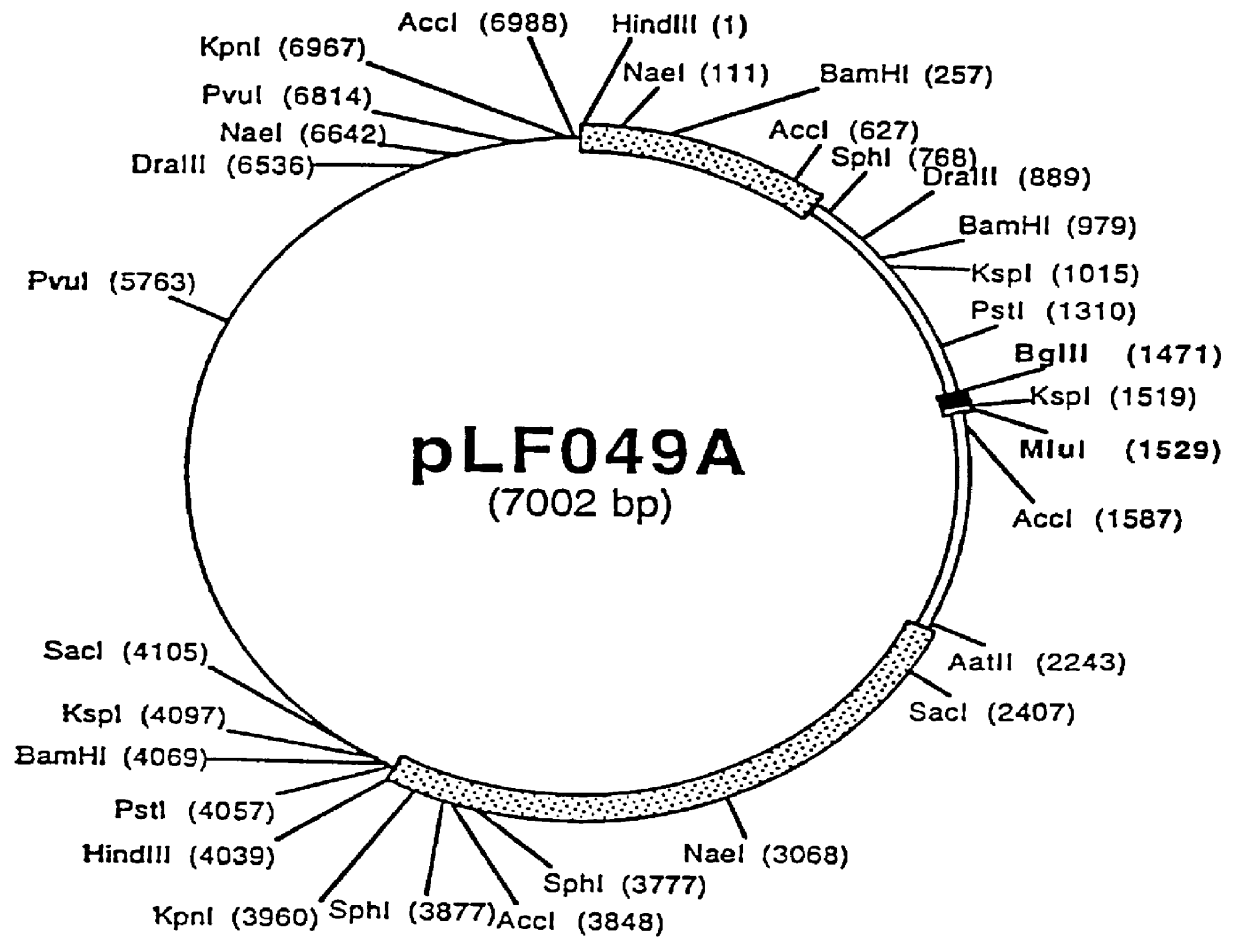
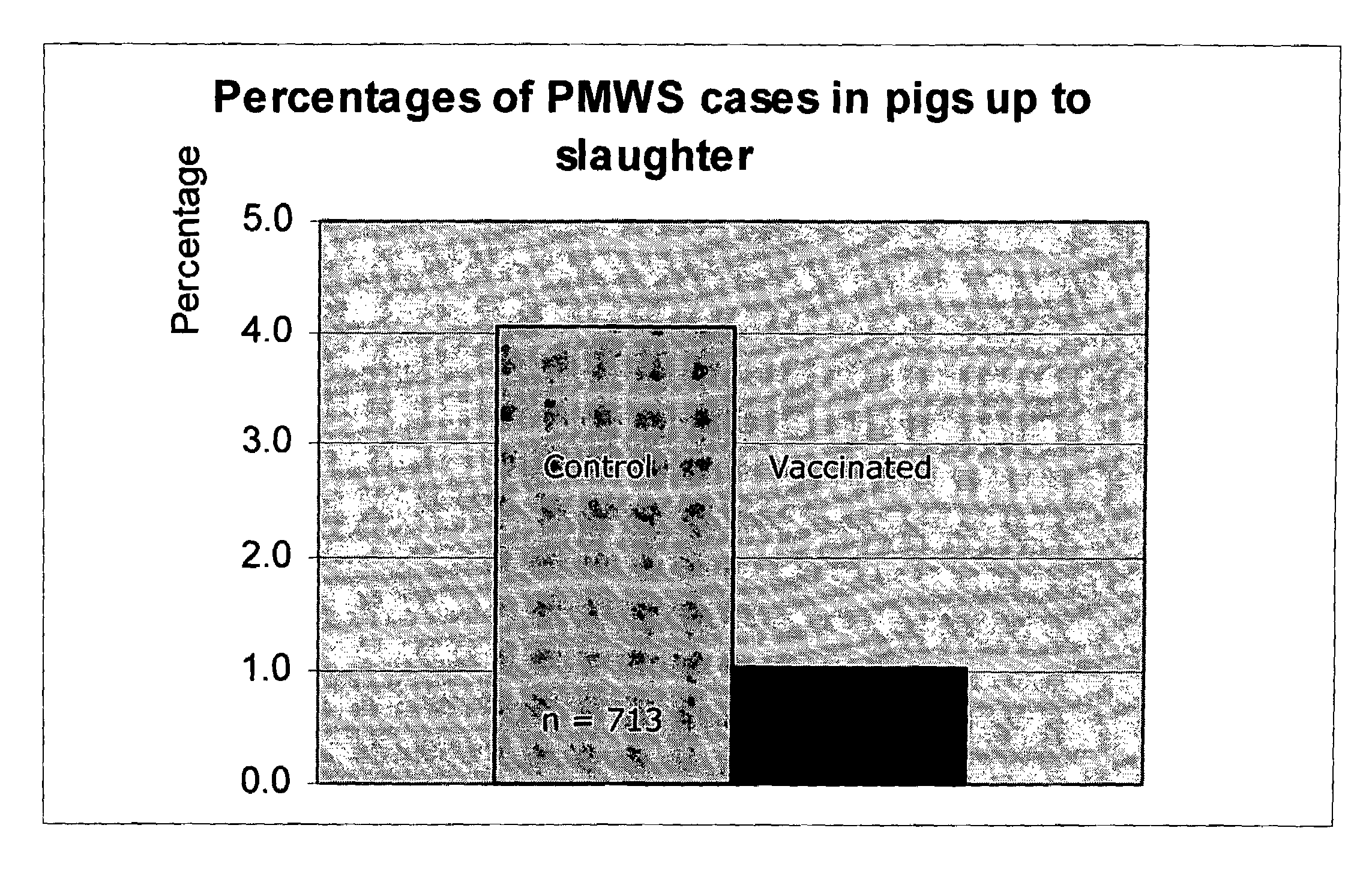
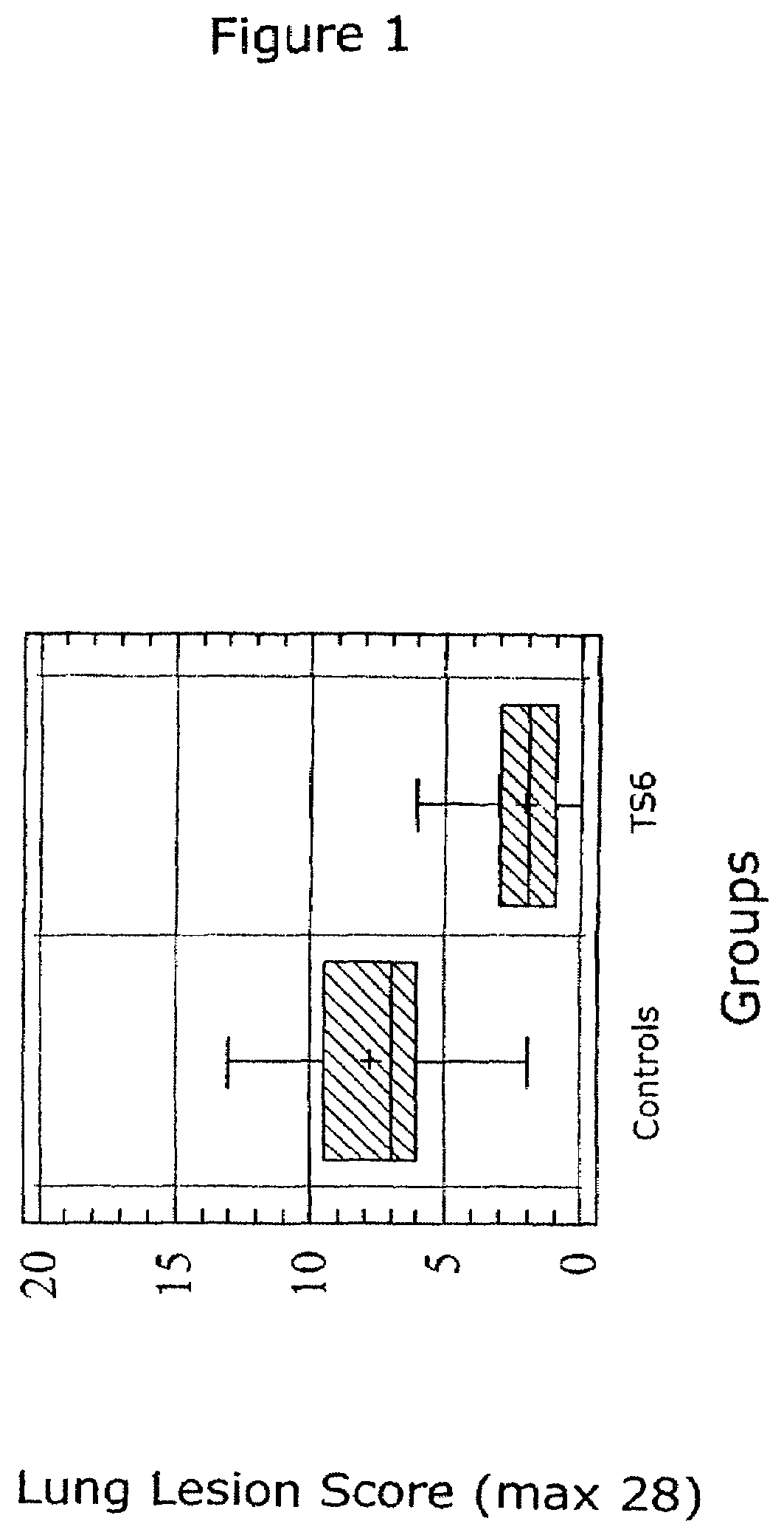
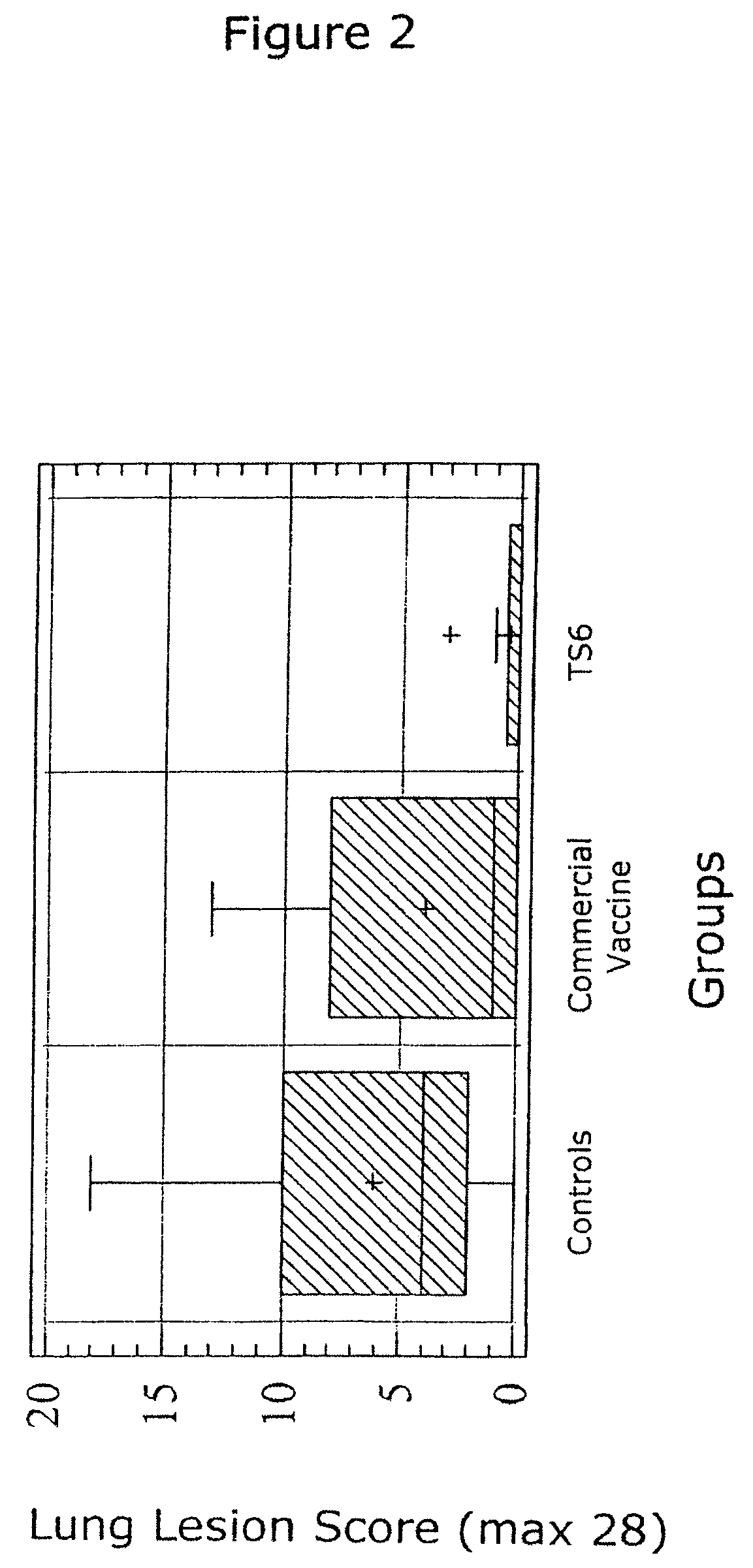
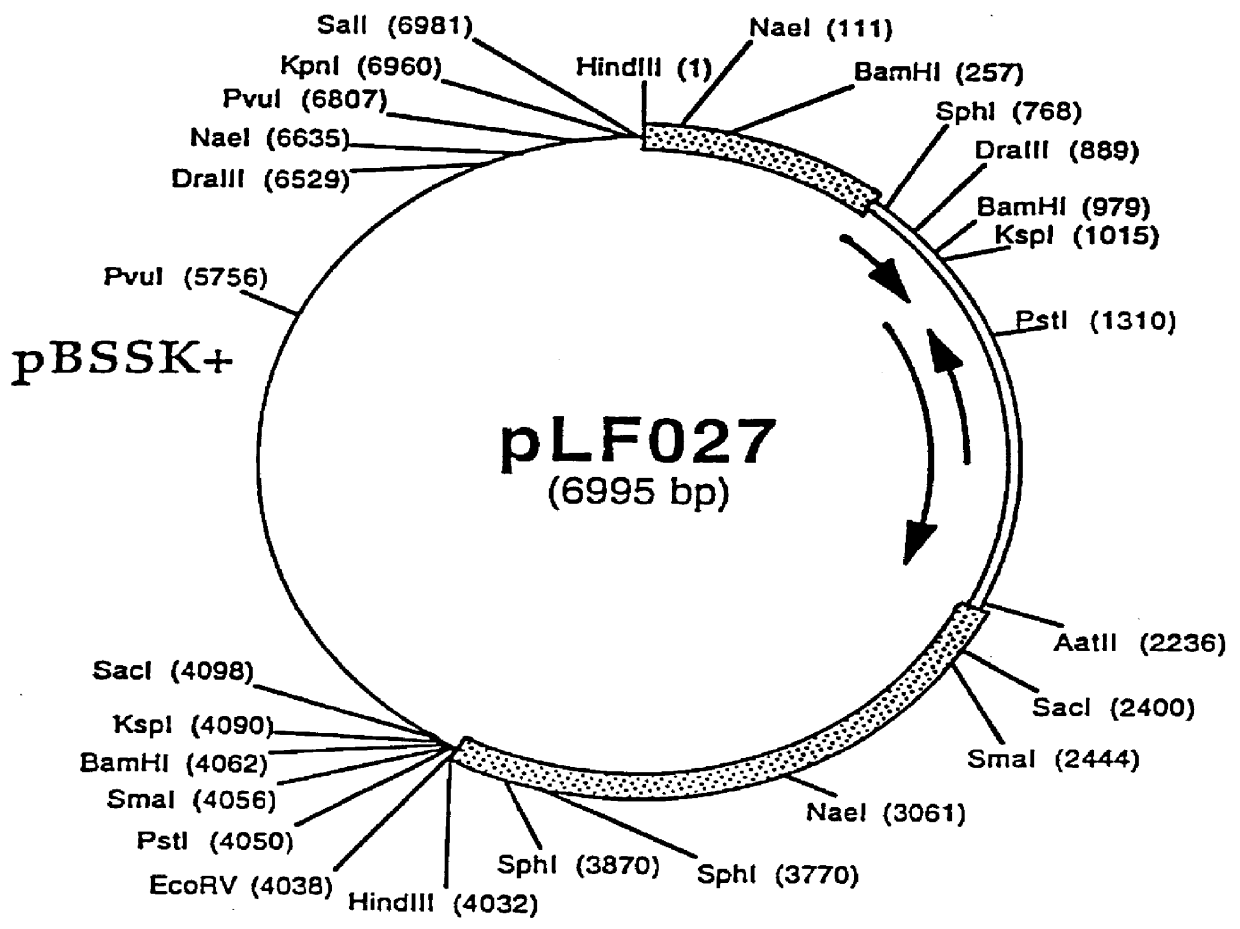
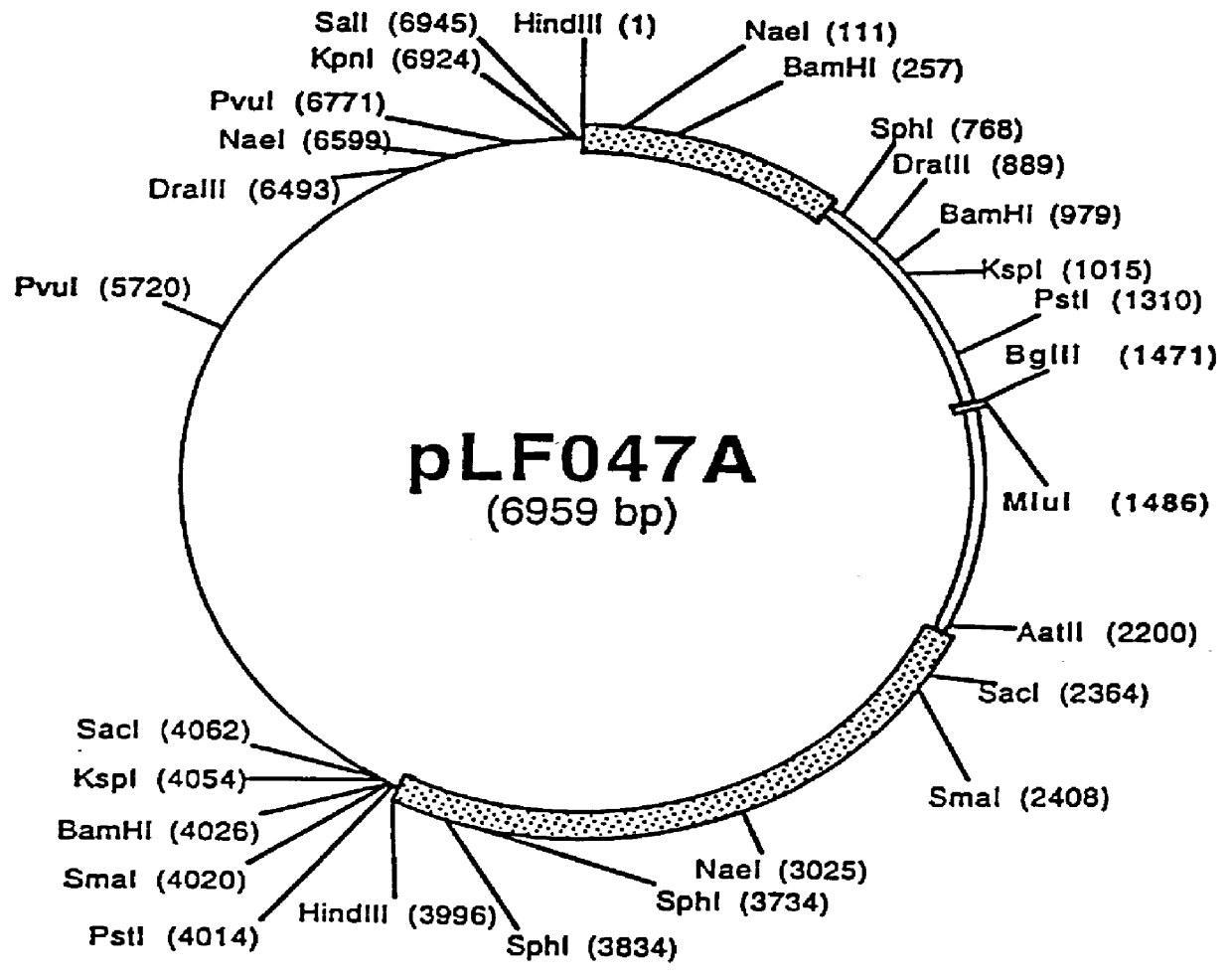
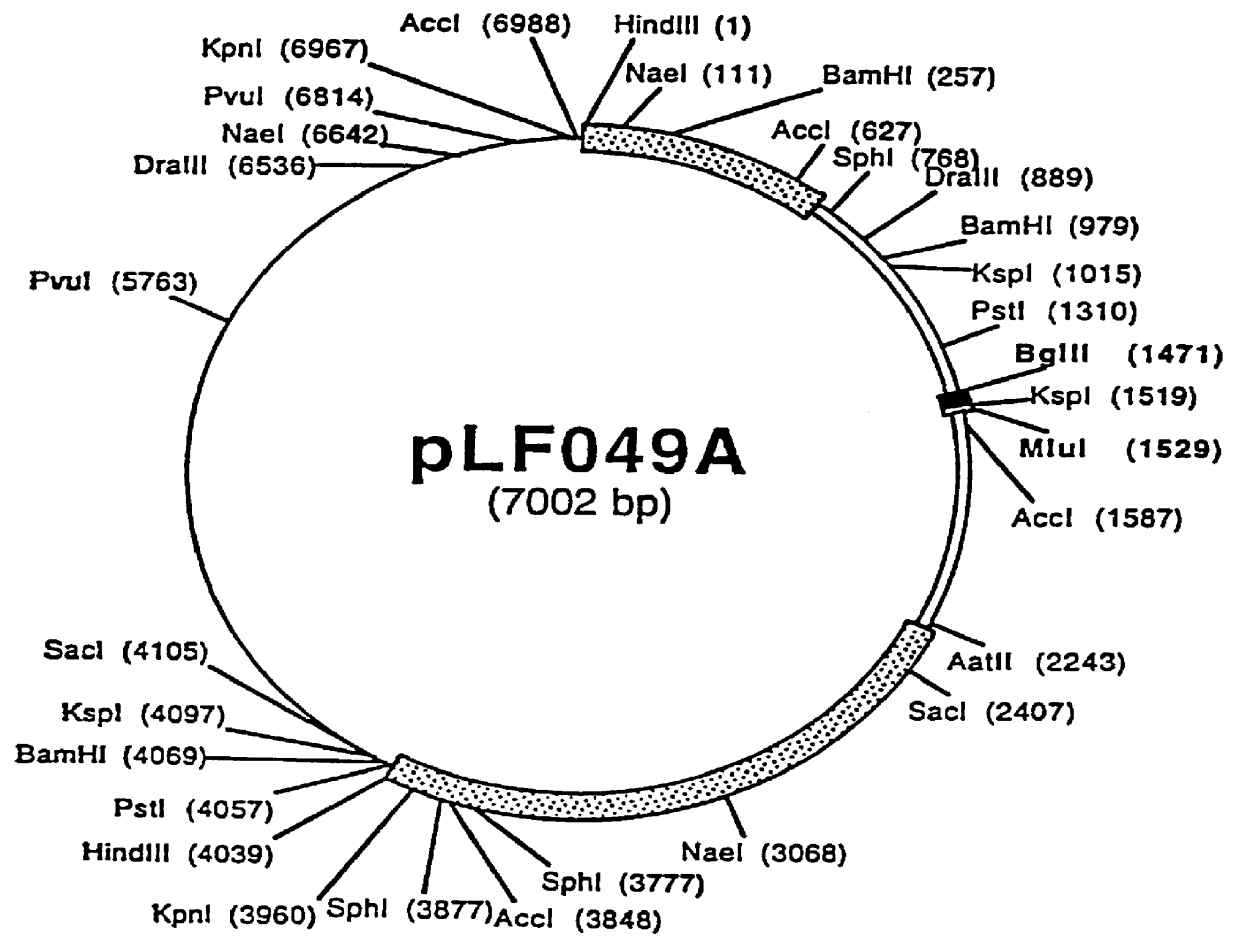
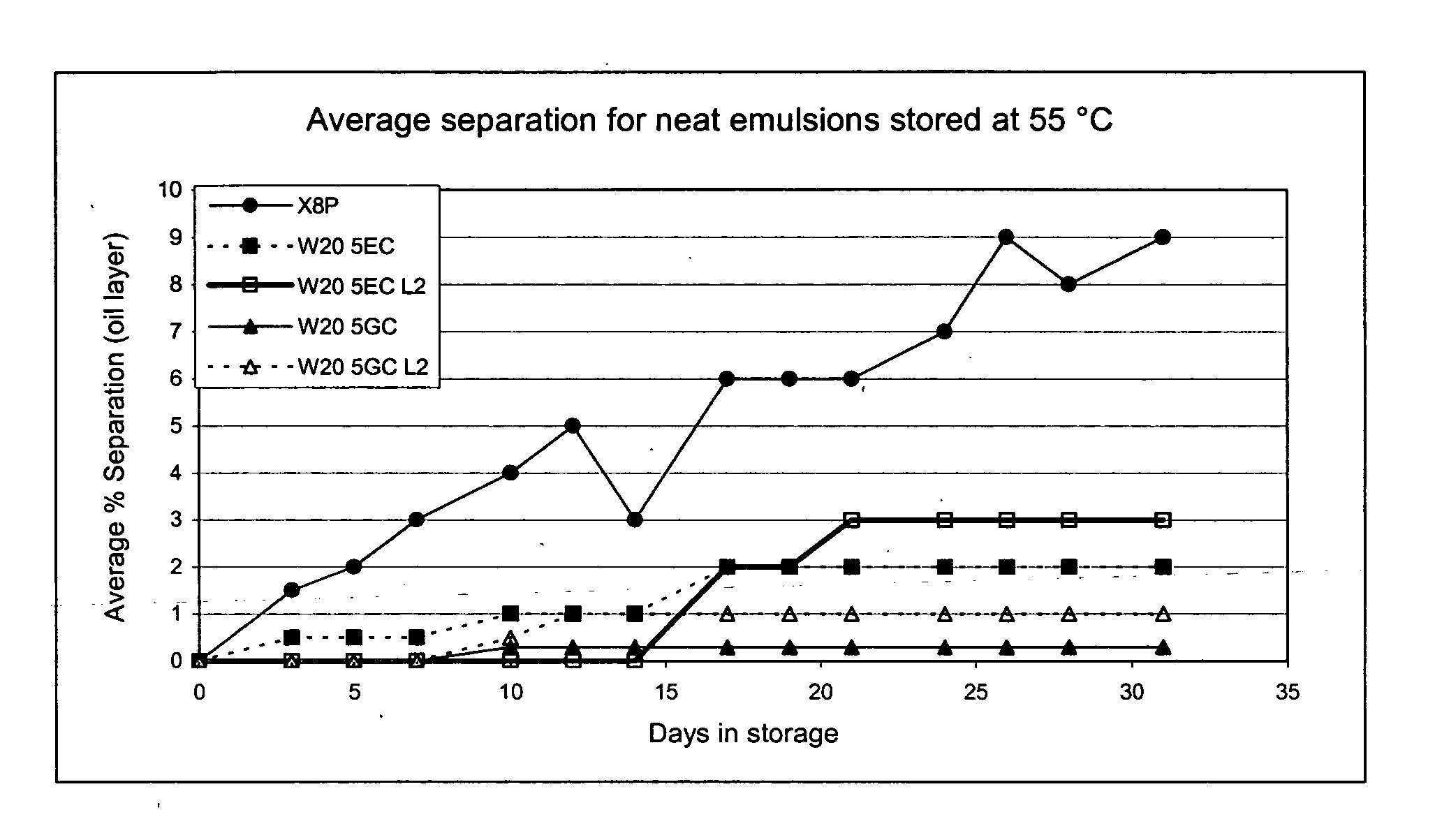
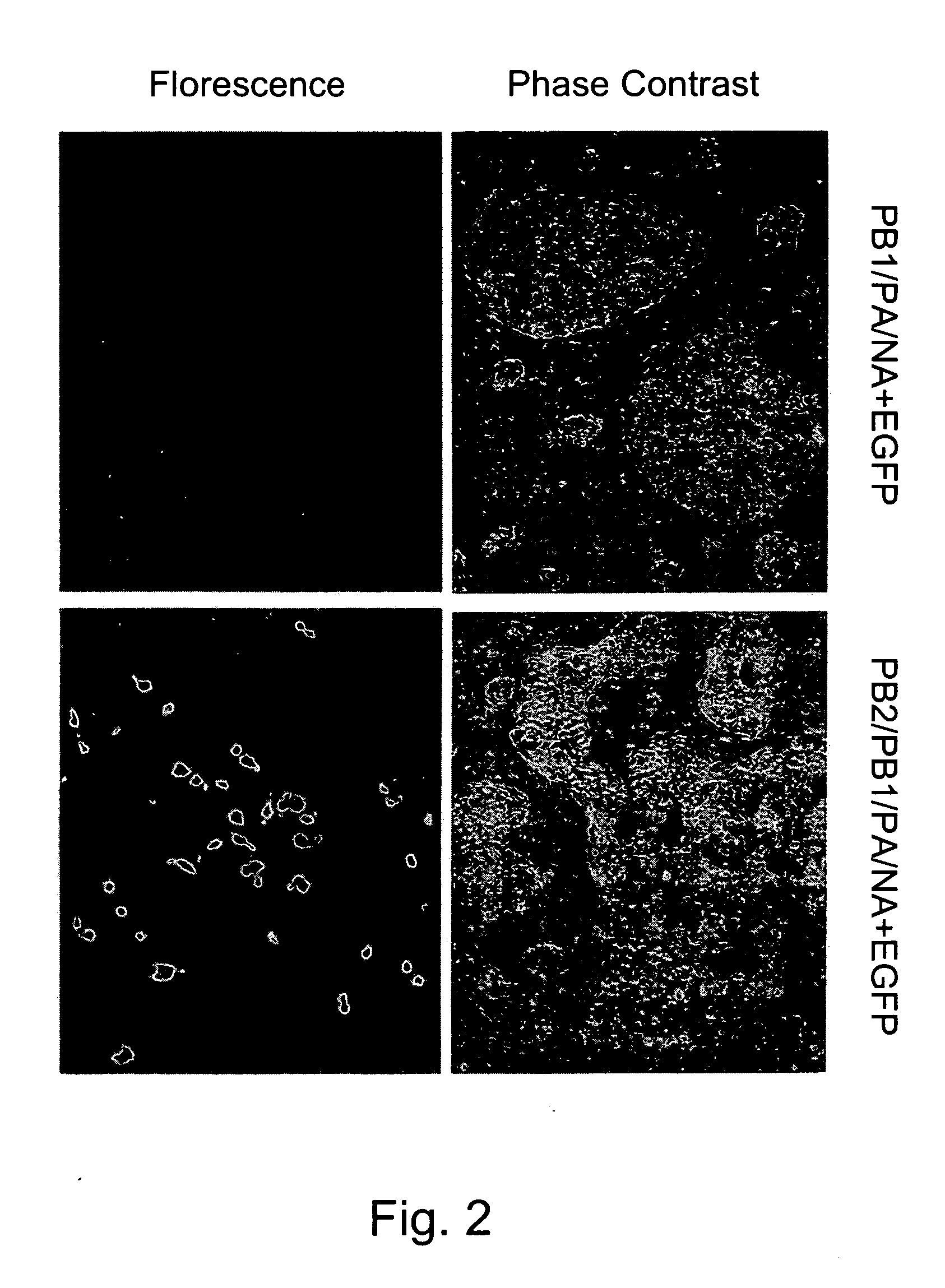
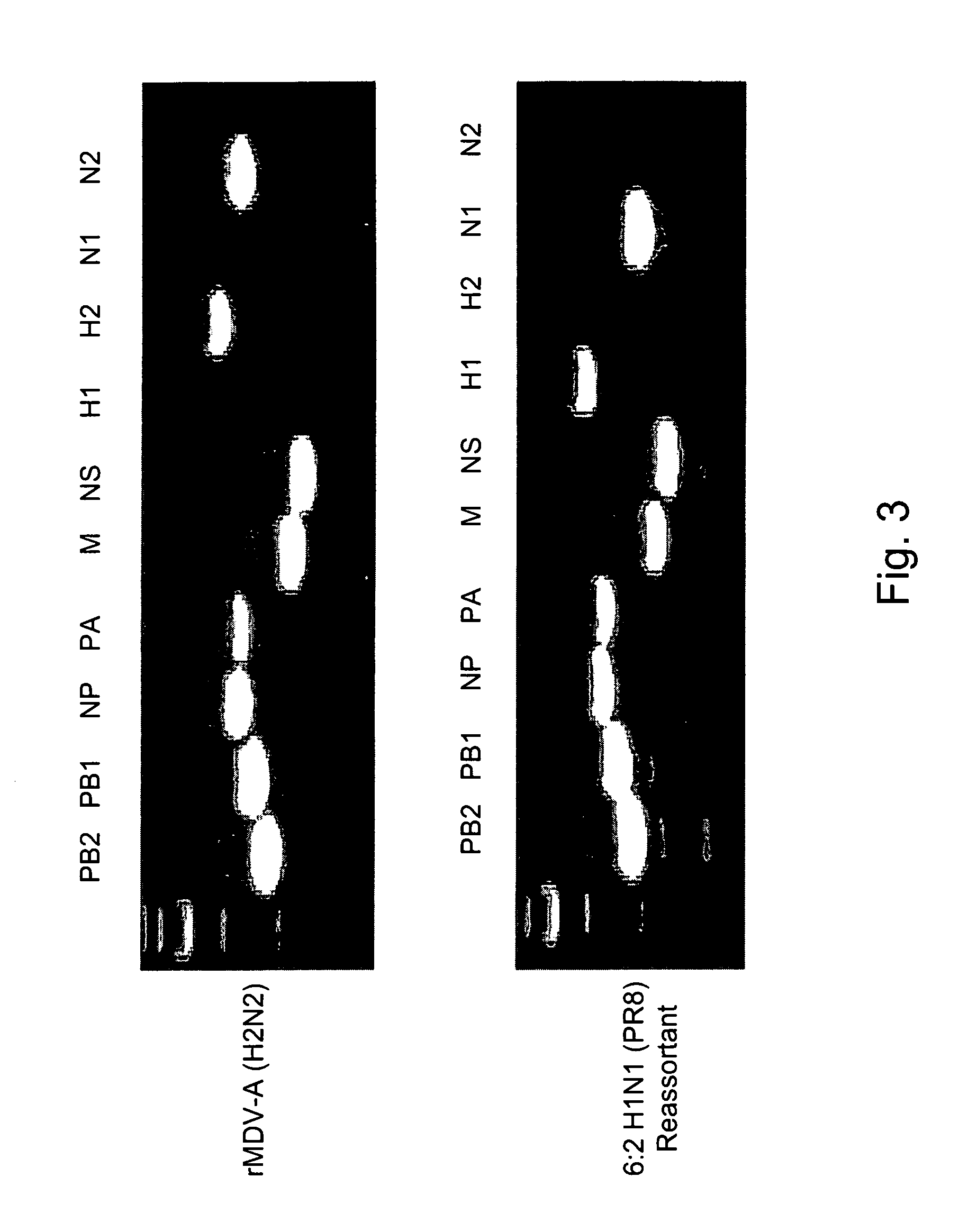
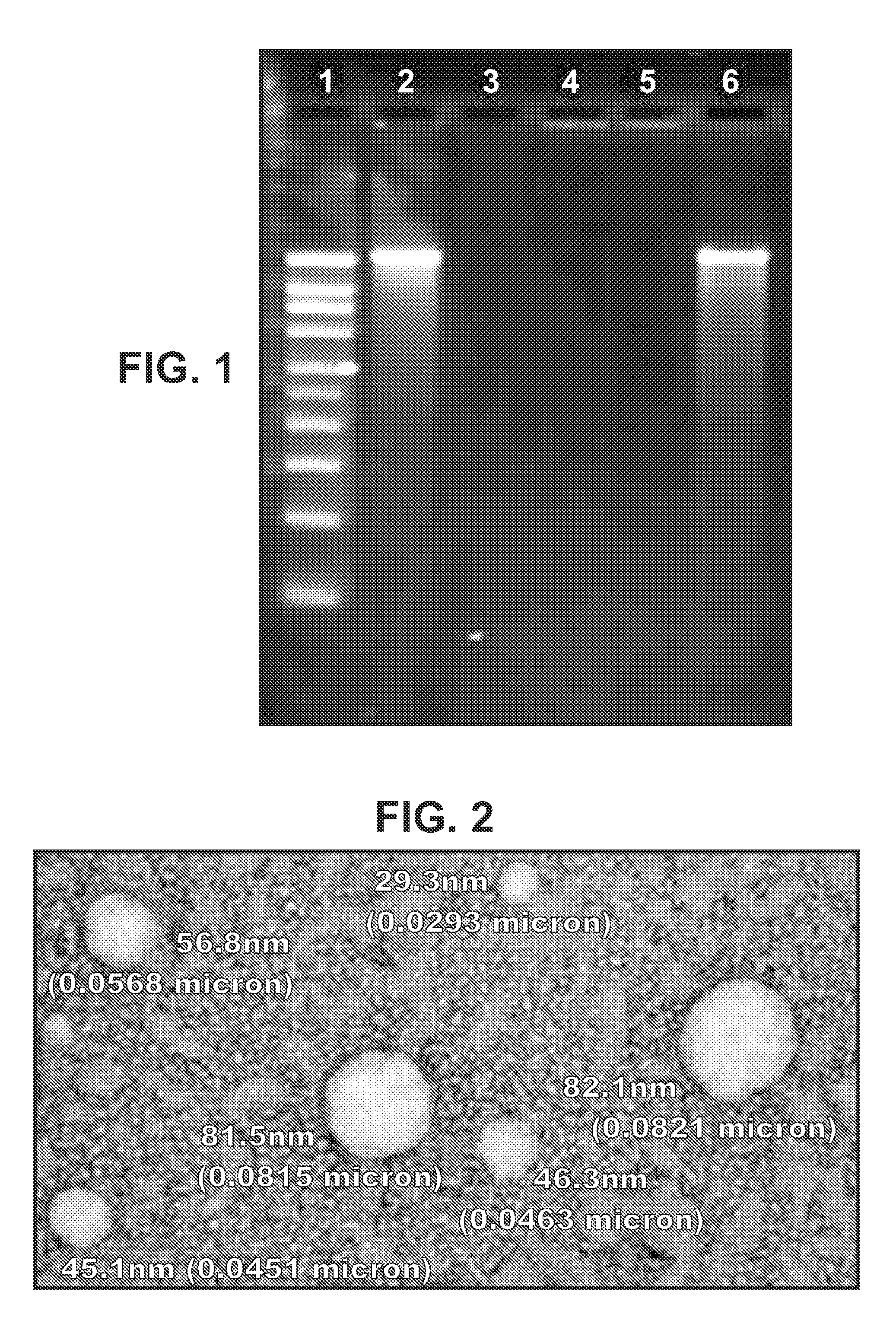
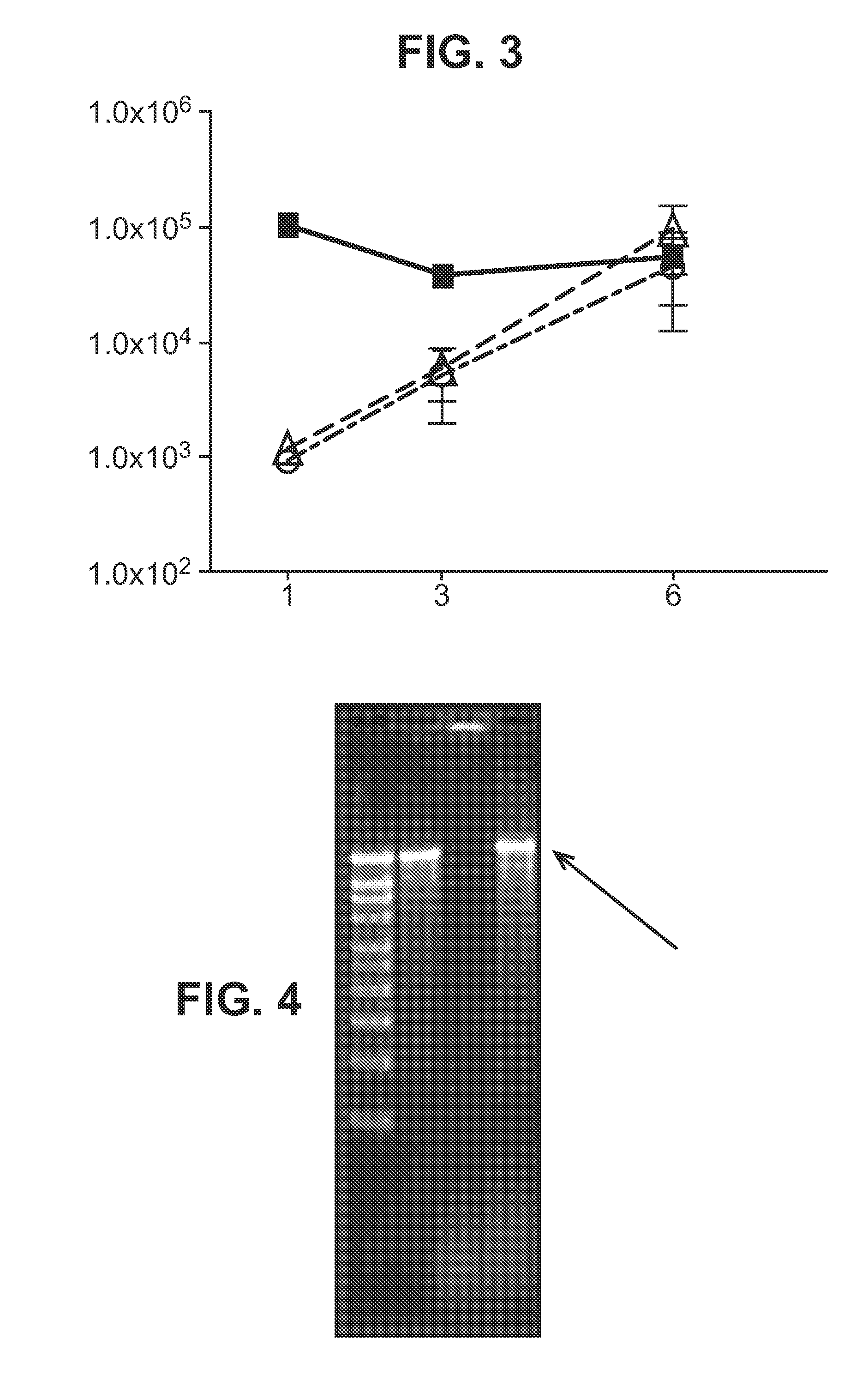
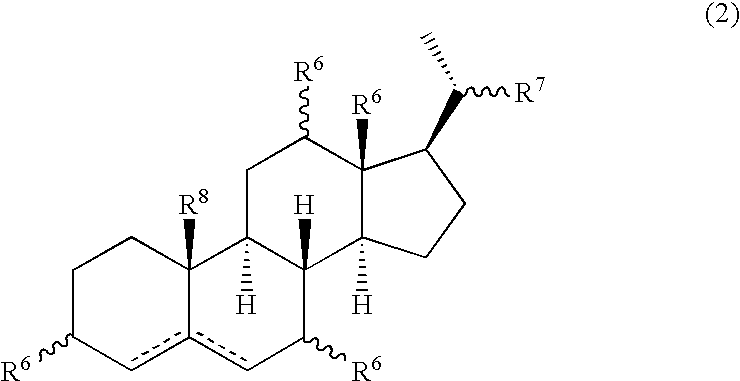
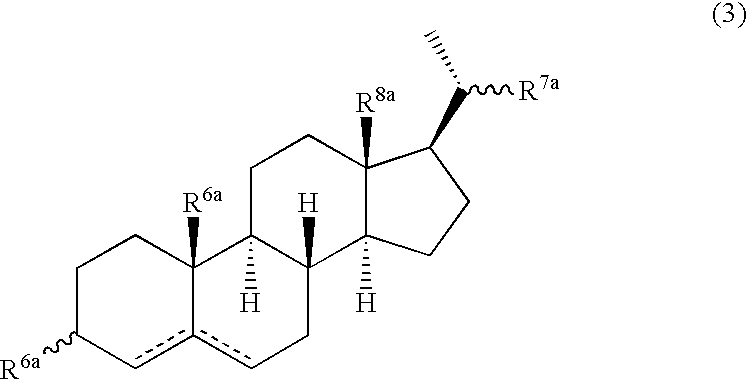
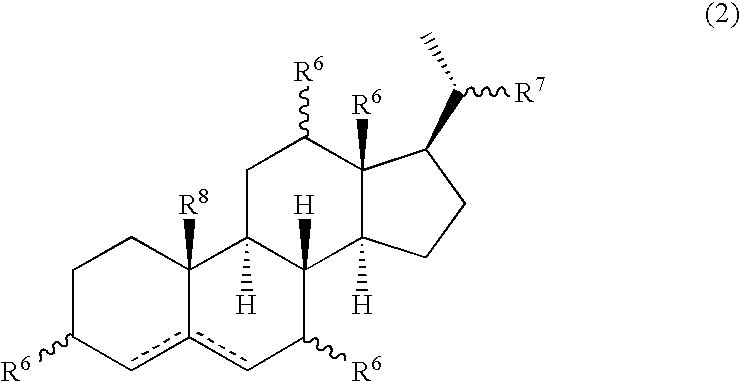
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5039results about "SsRNA viruses negative-sense" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
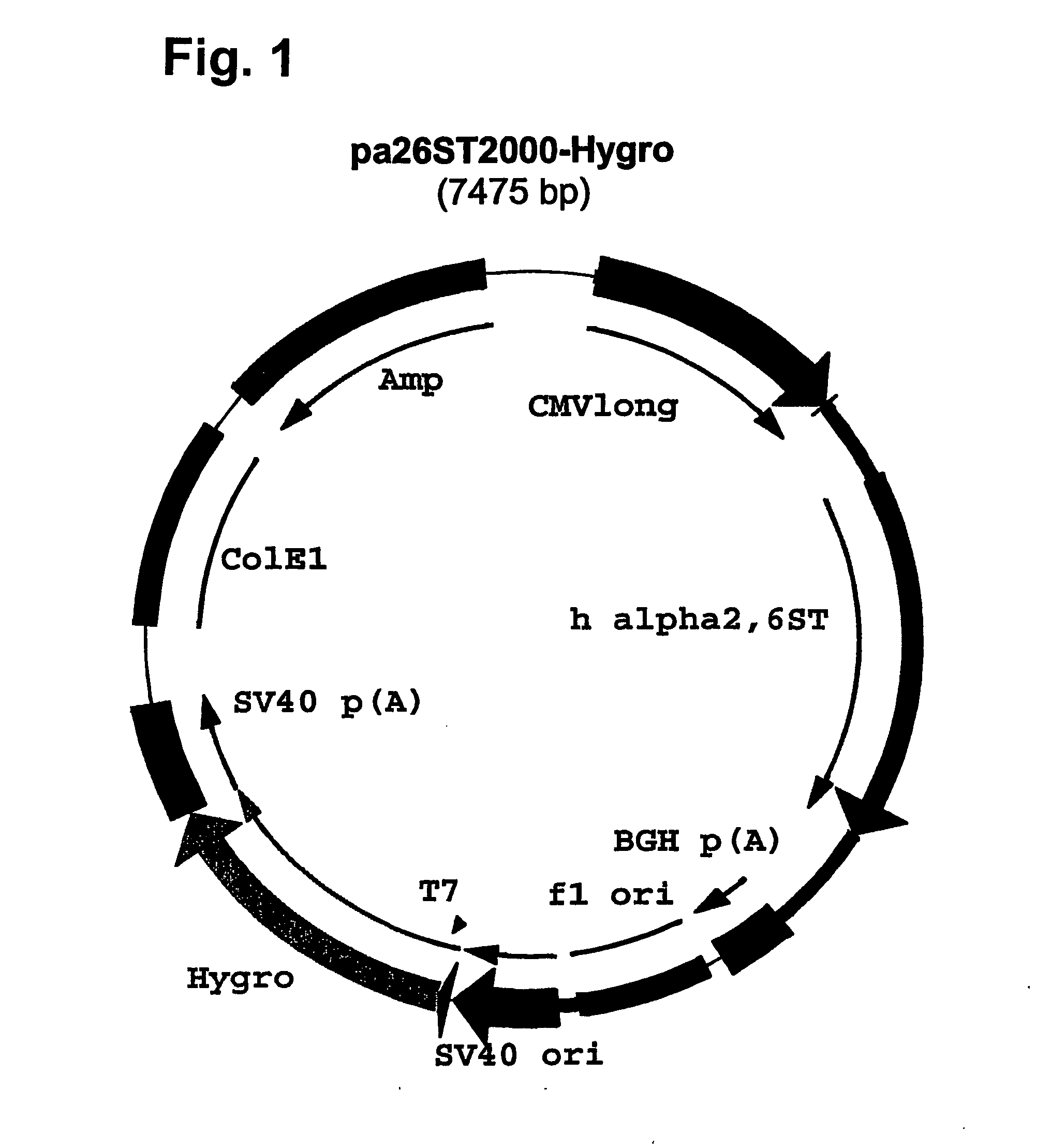
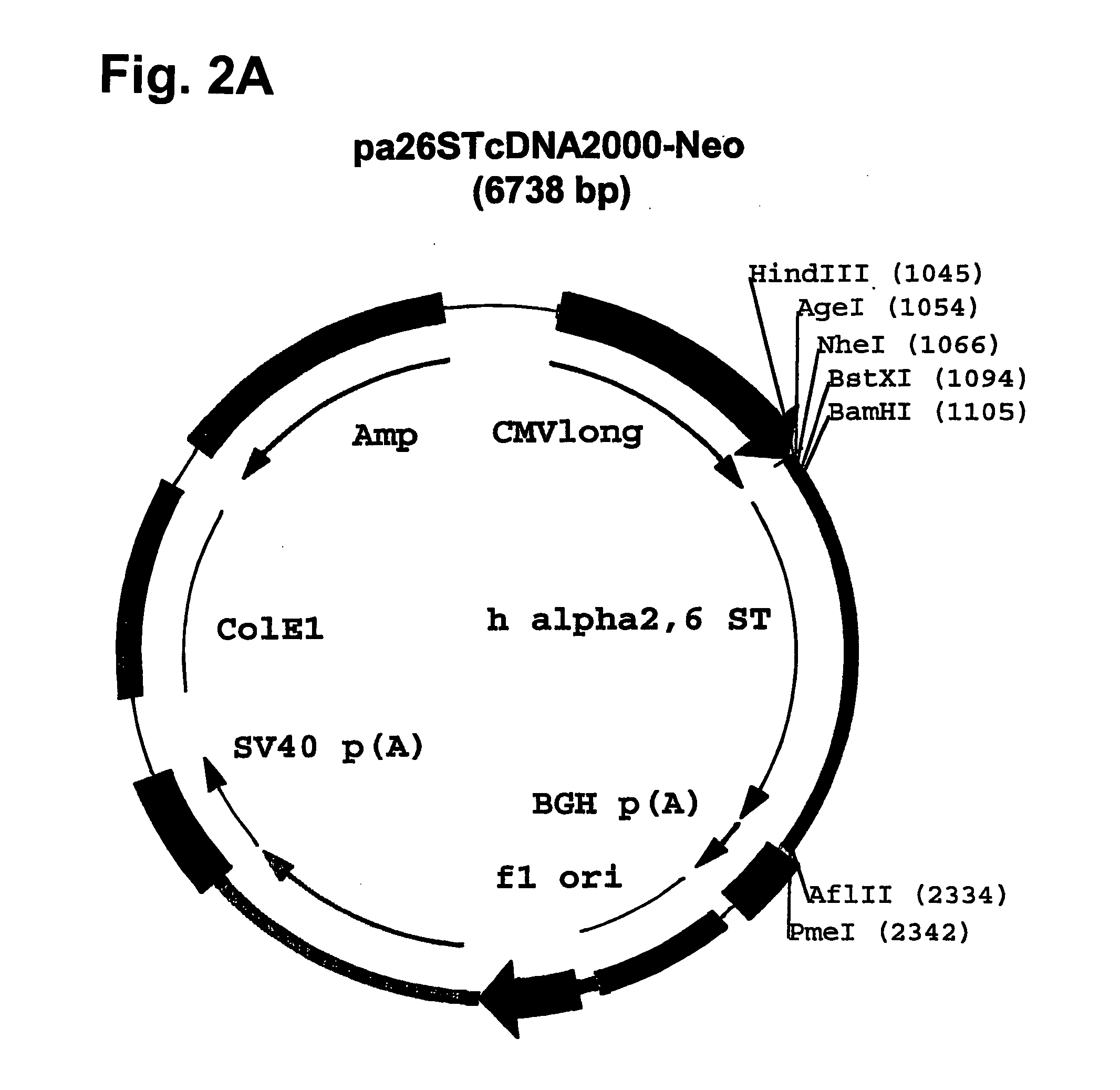
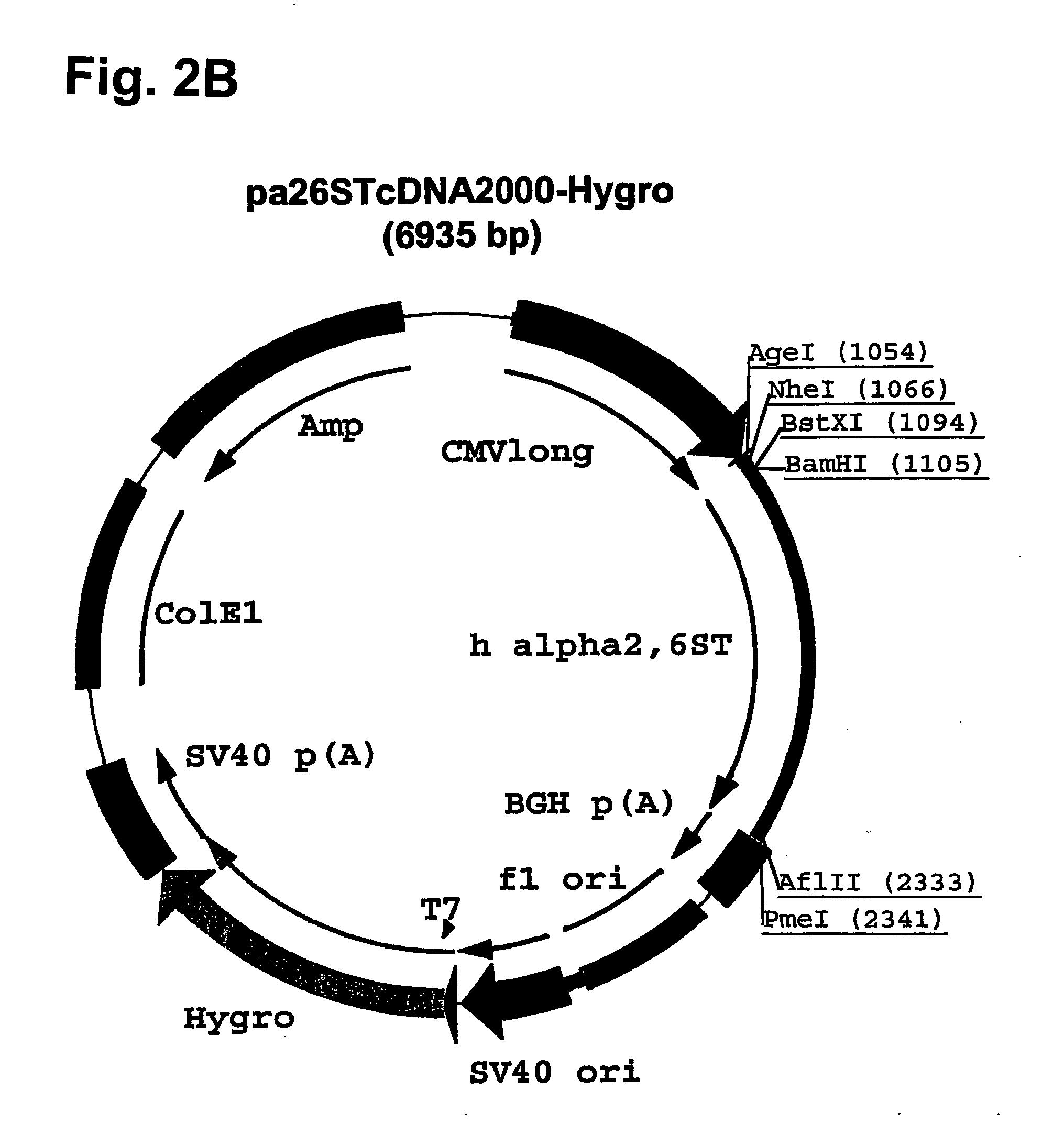
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
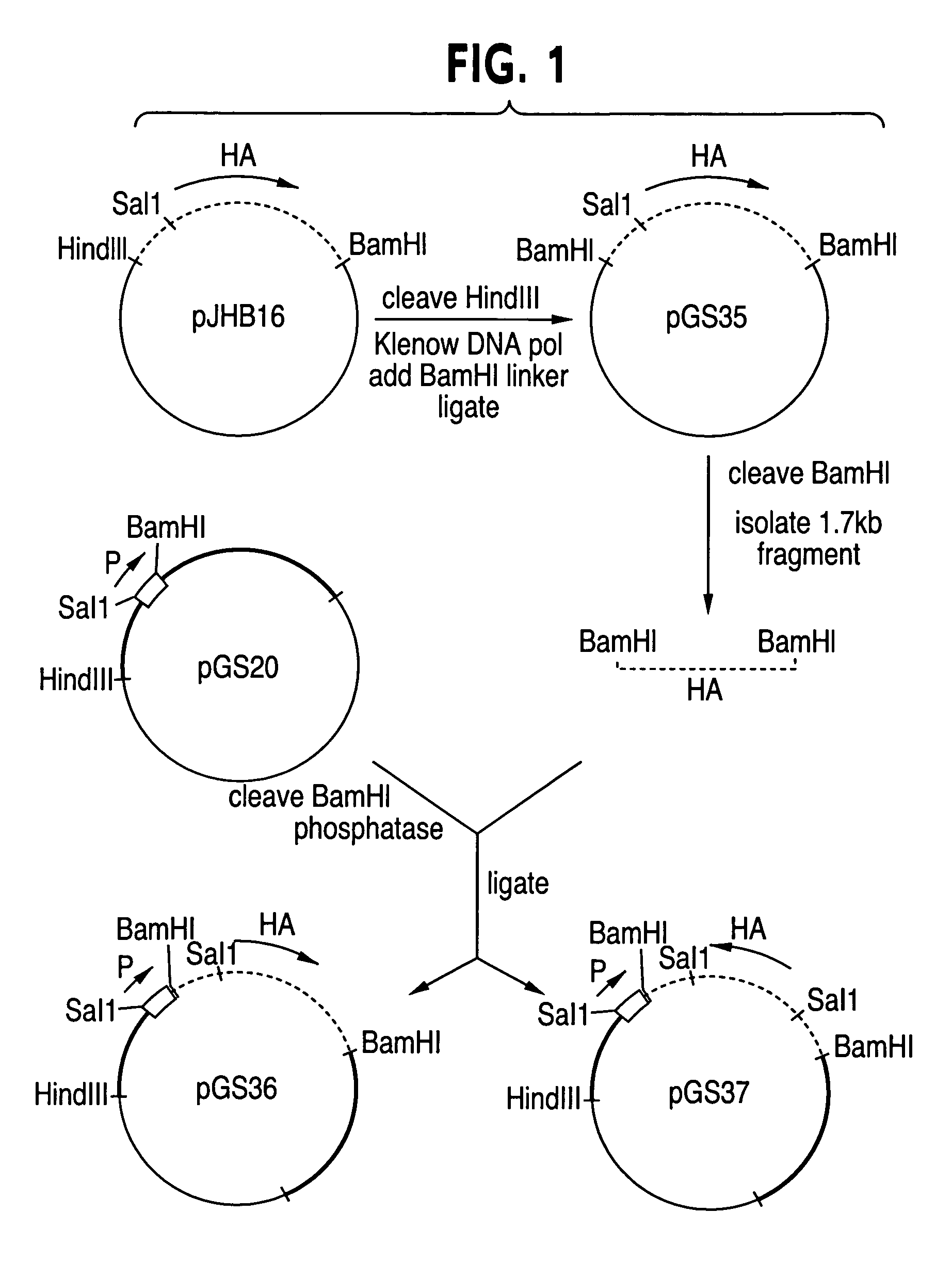
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
Antigen-binding molecule capable of binding to two or more antigen molecules repeatedly
InactiveUS20110111406A1Pharmacokinetics of the antigen-binding molecule can be improvedGood effectSsRNA viruses negative-senseCompound screeningHalf-lifeAntigen binding
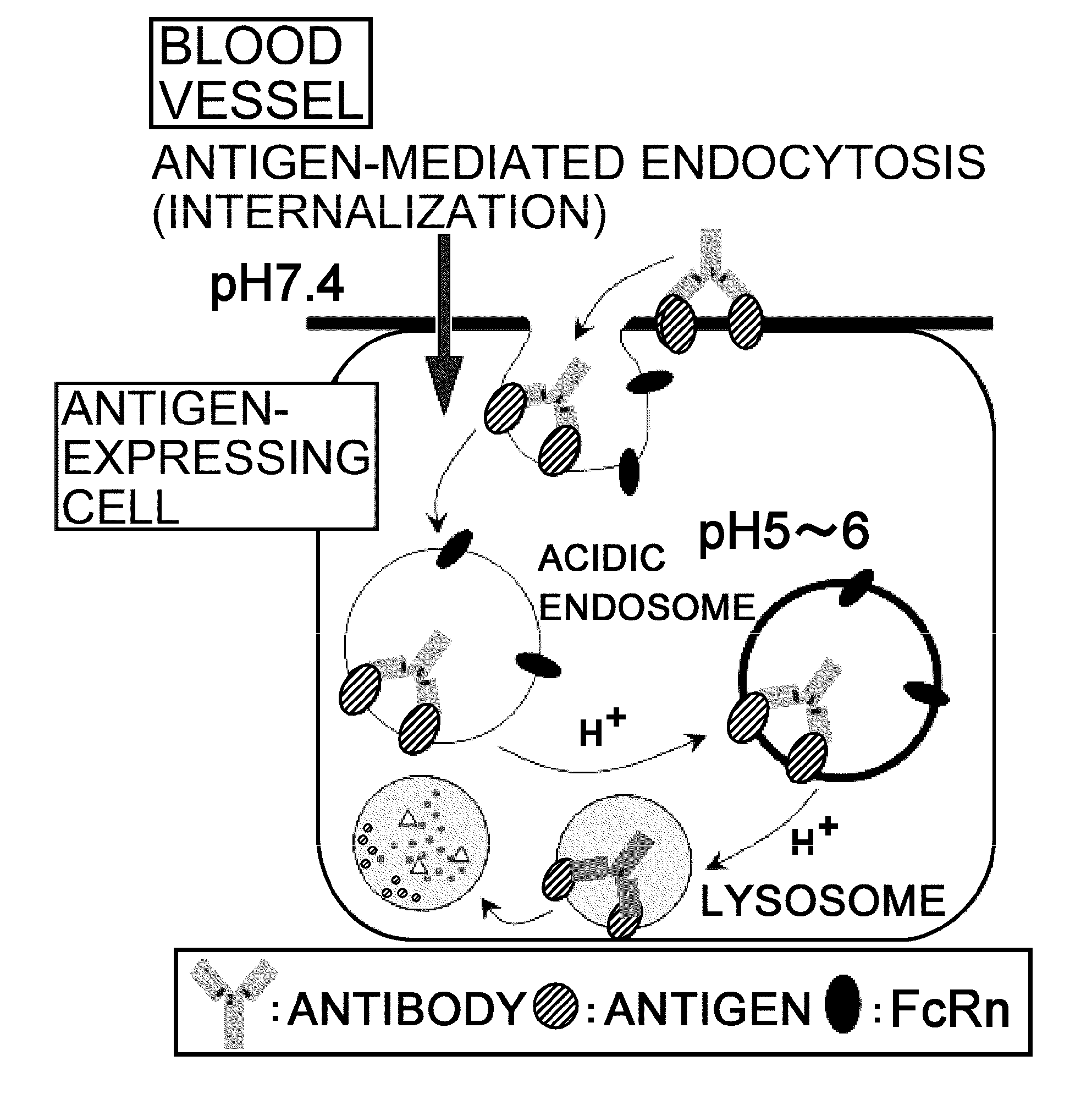
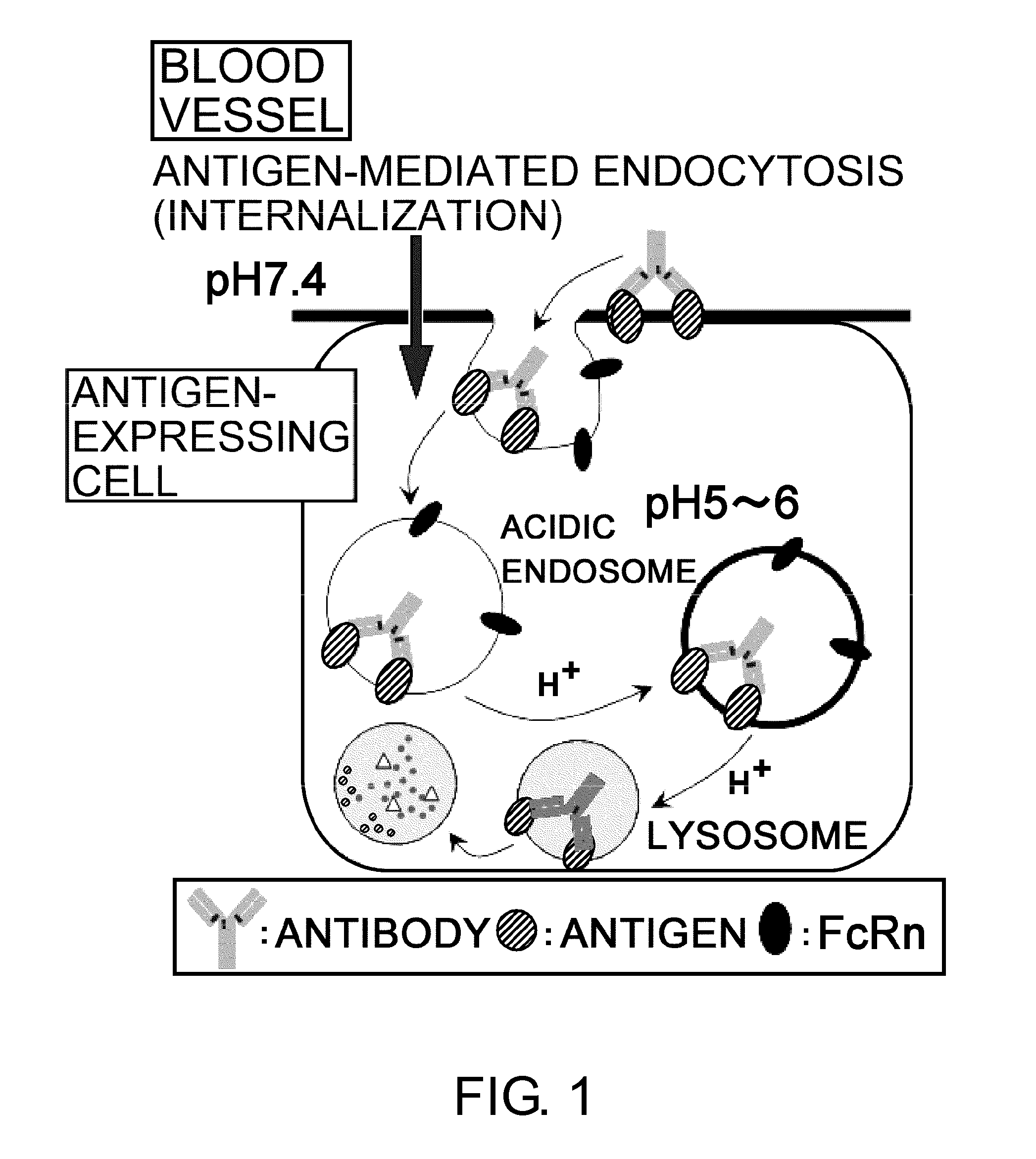
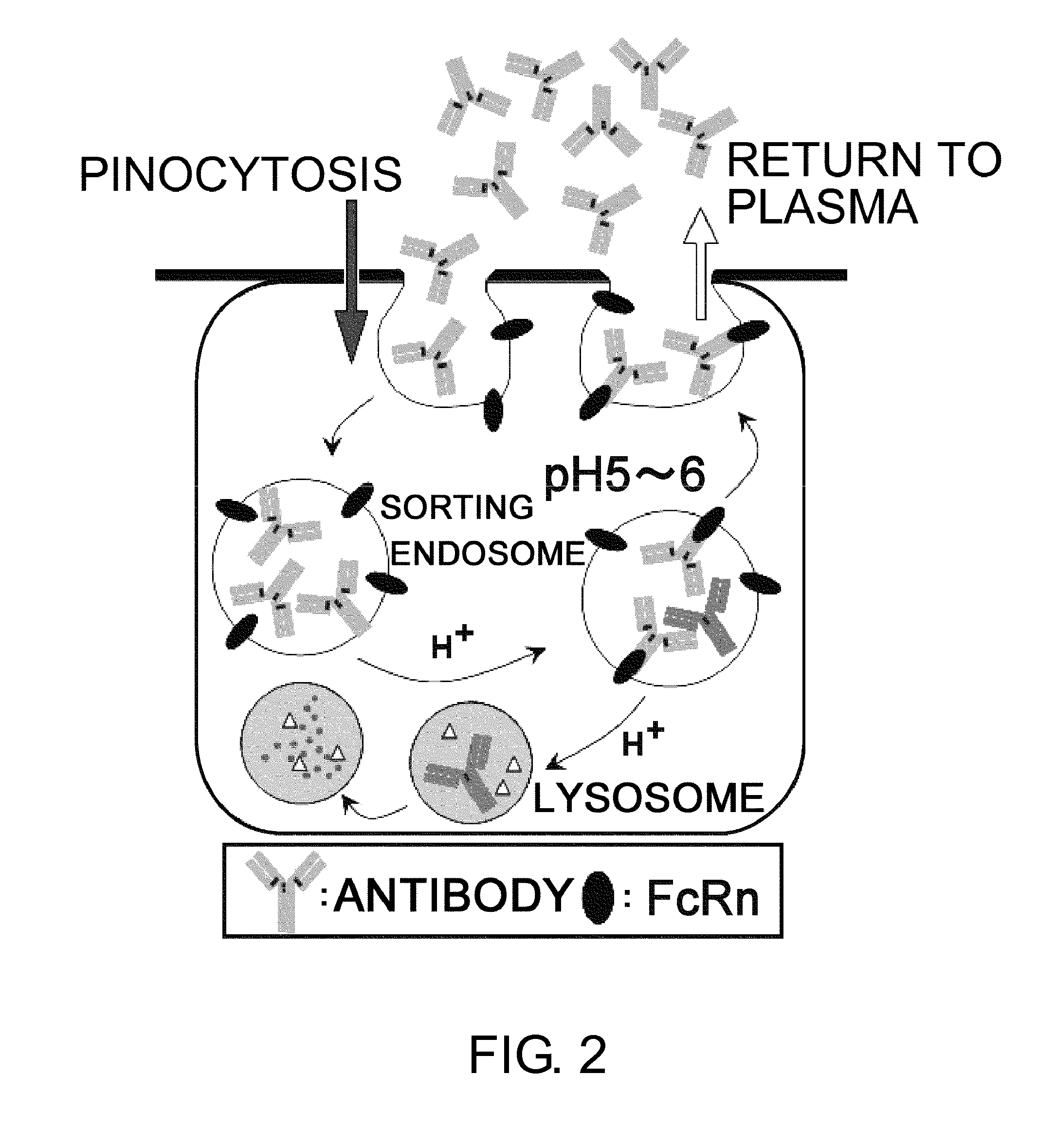
The present inventors discovered that antibodies having weaker antigen-binding activity at the early endosomal pH in comparison with that at the pH of plasma are capable of binding to multiple antigen molecules with a single antibody molecule, have long half-lives in plasma, and have improved durations of time in which they can bind to antigen.
Owner:CHUGAI PHARMA CO LTD
Uses and compositions for treatment of rheumatoid arthritis
ActiveUS20080166348A1Relieve symptomsInhibit progressSsRNA viruses negative-senseBiocideAntigen bindingRheumatoid arthritis
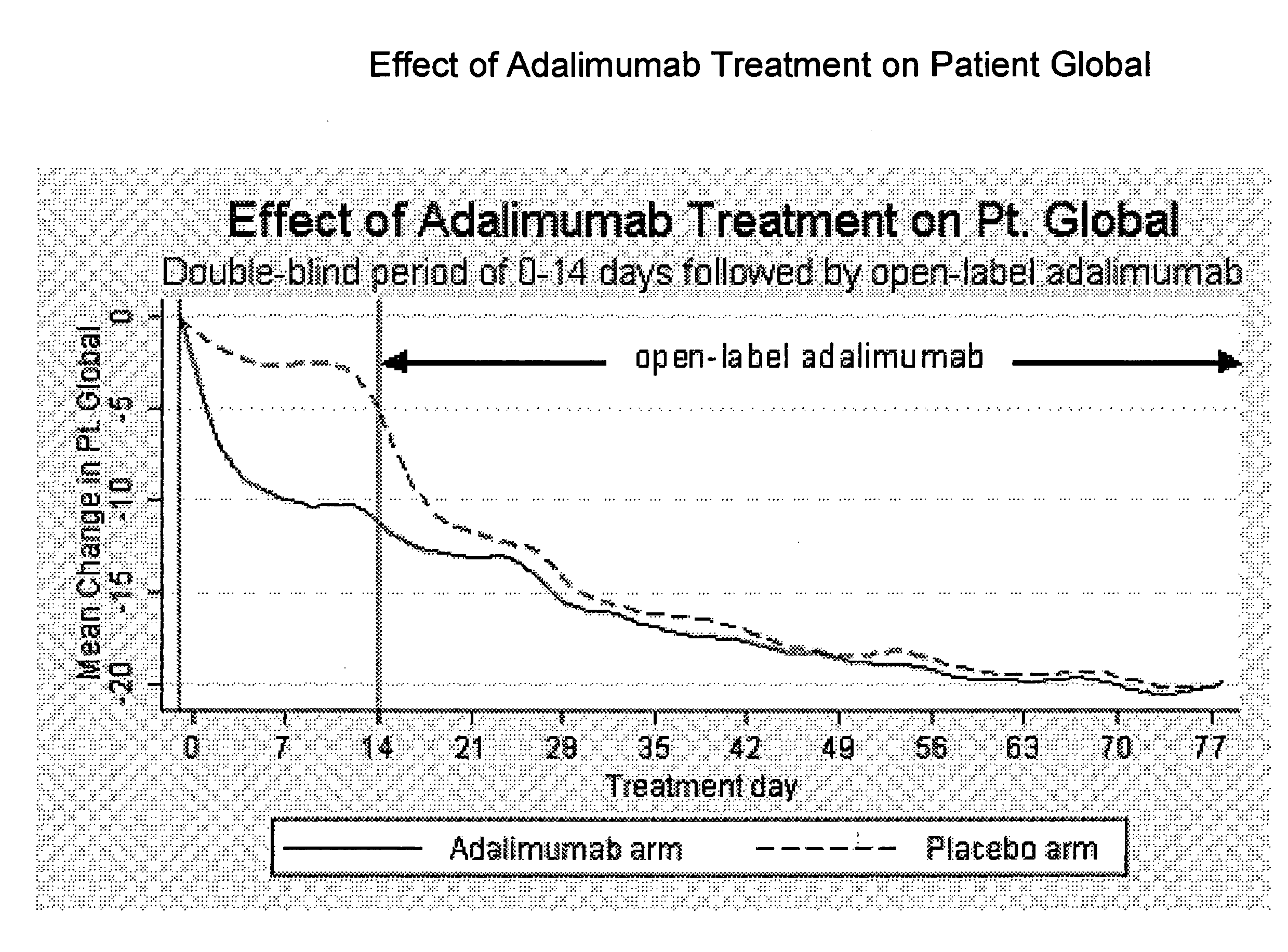
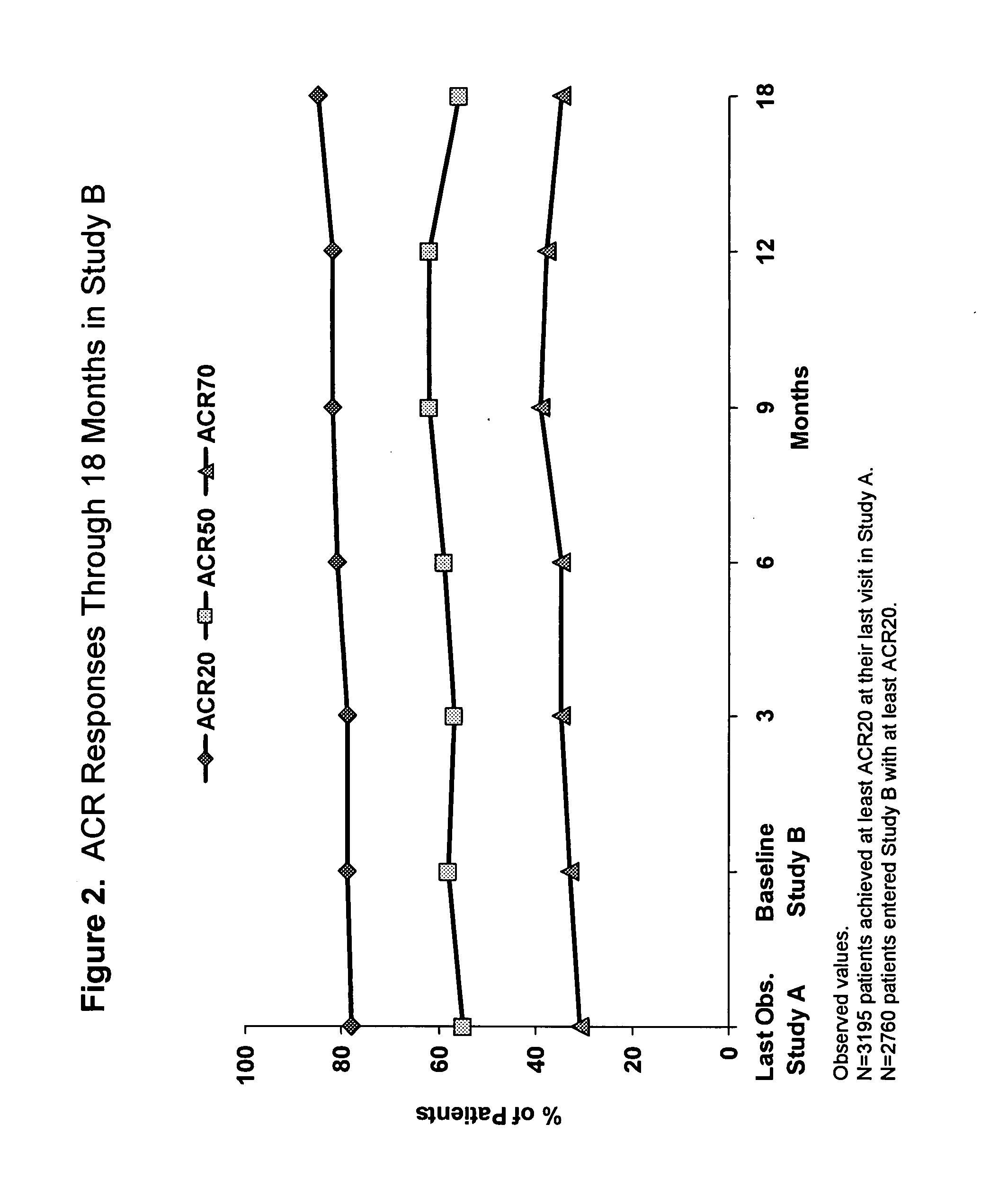
The invention provides methods, uses and compositions for the treatment of rheumatoid arthritis. The invention describes methods and uses for treating rheumatoid arthritis wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of rheumatoid arthritis in a subject.
Owner:ABBVIE BIOTECHNOLOGY LTD
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
Vaccine formulations
ActiveUS7371395B2Improve stabilityStable and safe and easily administrableSsRNA viruses negative-senseAntibacterial agentsPlasmidBacilli
Owner:MERIAL INC
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
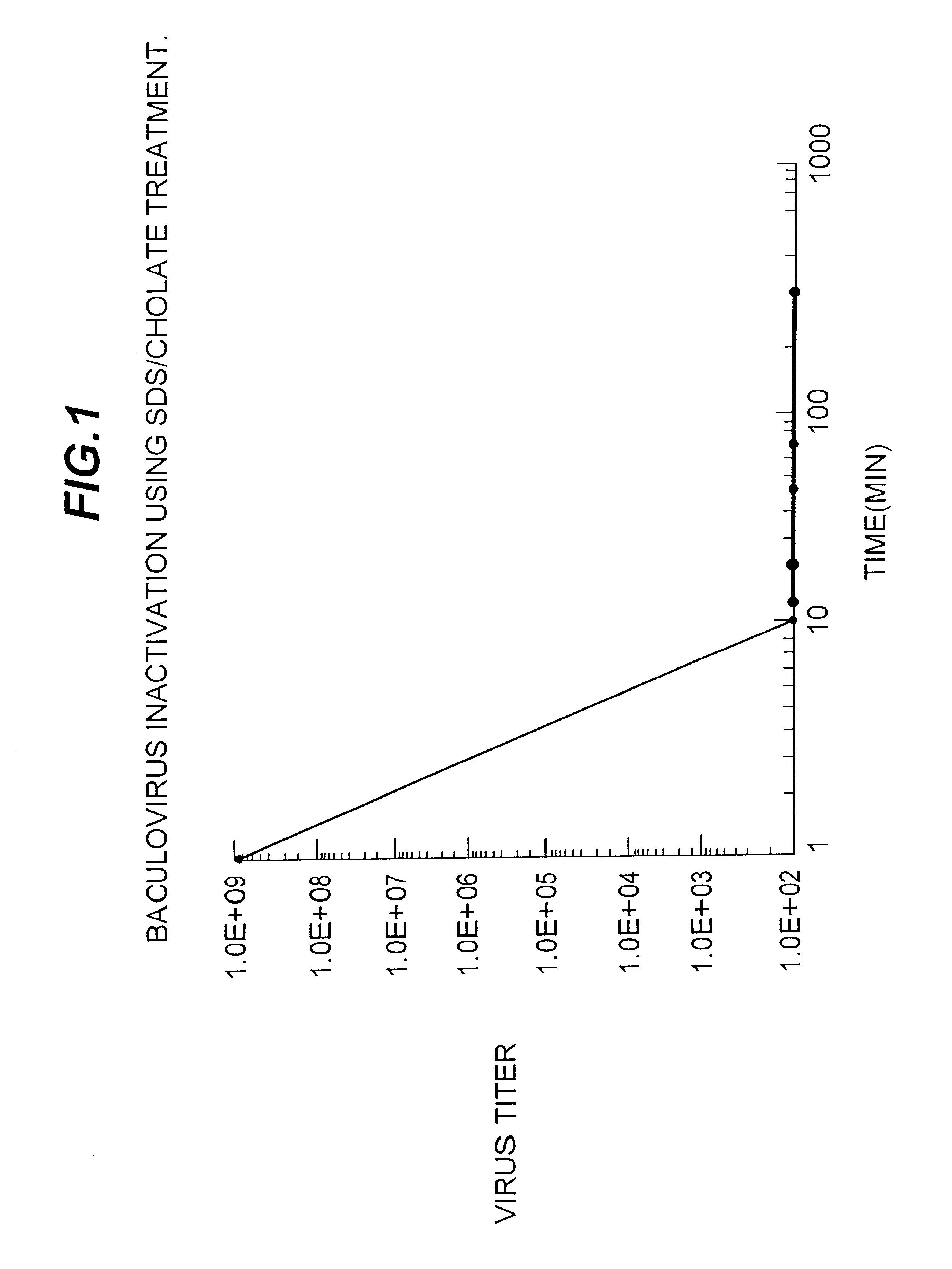
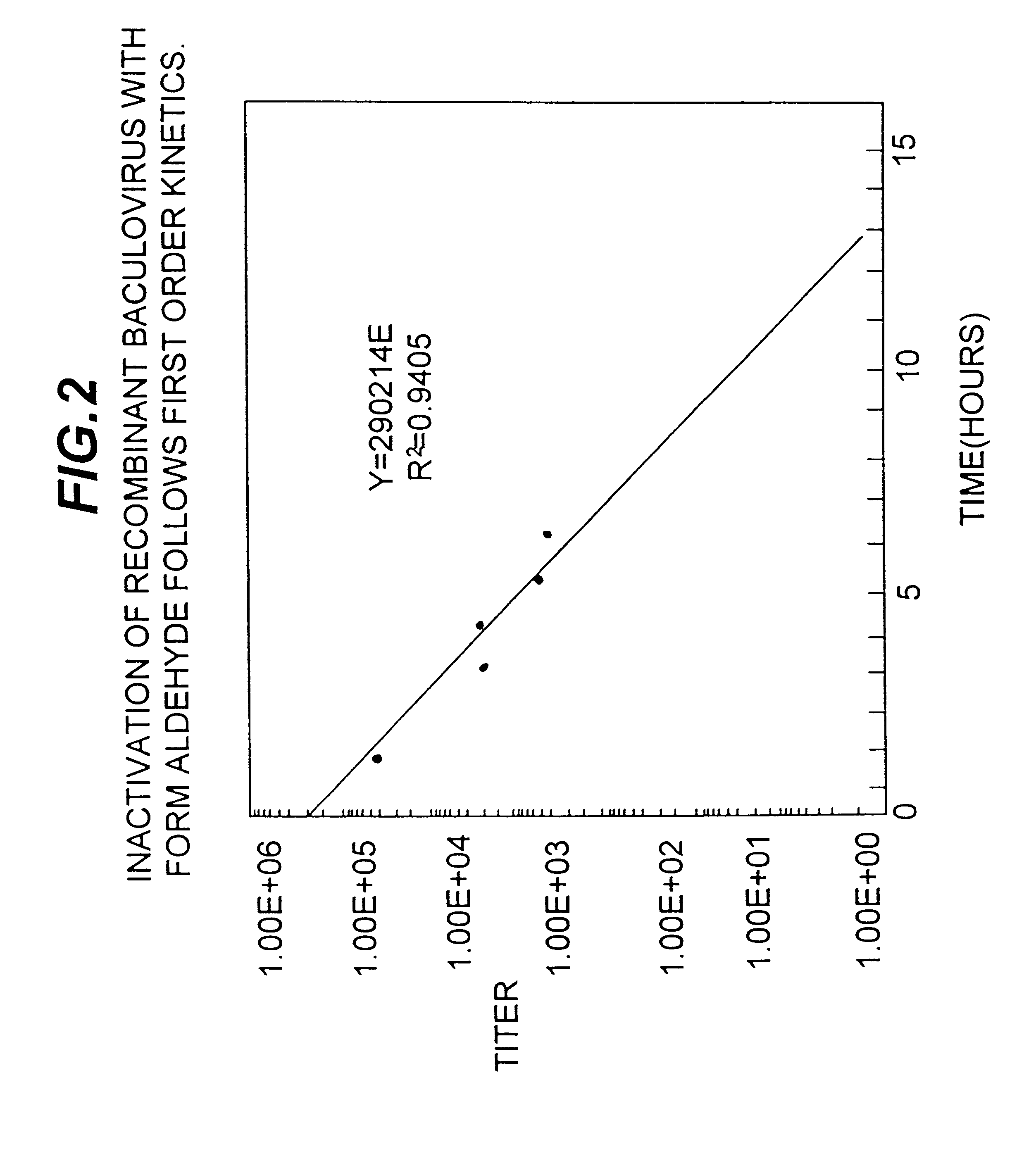
Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
InactiveUS20060251684A1Reduce infectivityReduce morbiditySsRNA viruses negative-senseAntibacterial agentsPathogenic microorganismOrganic solvent
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
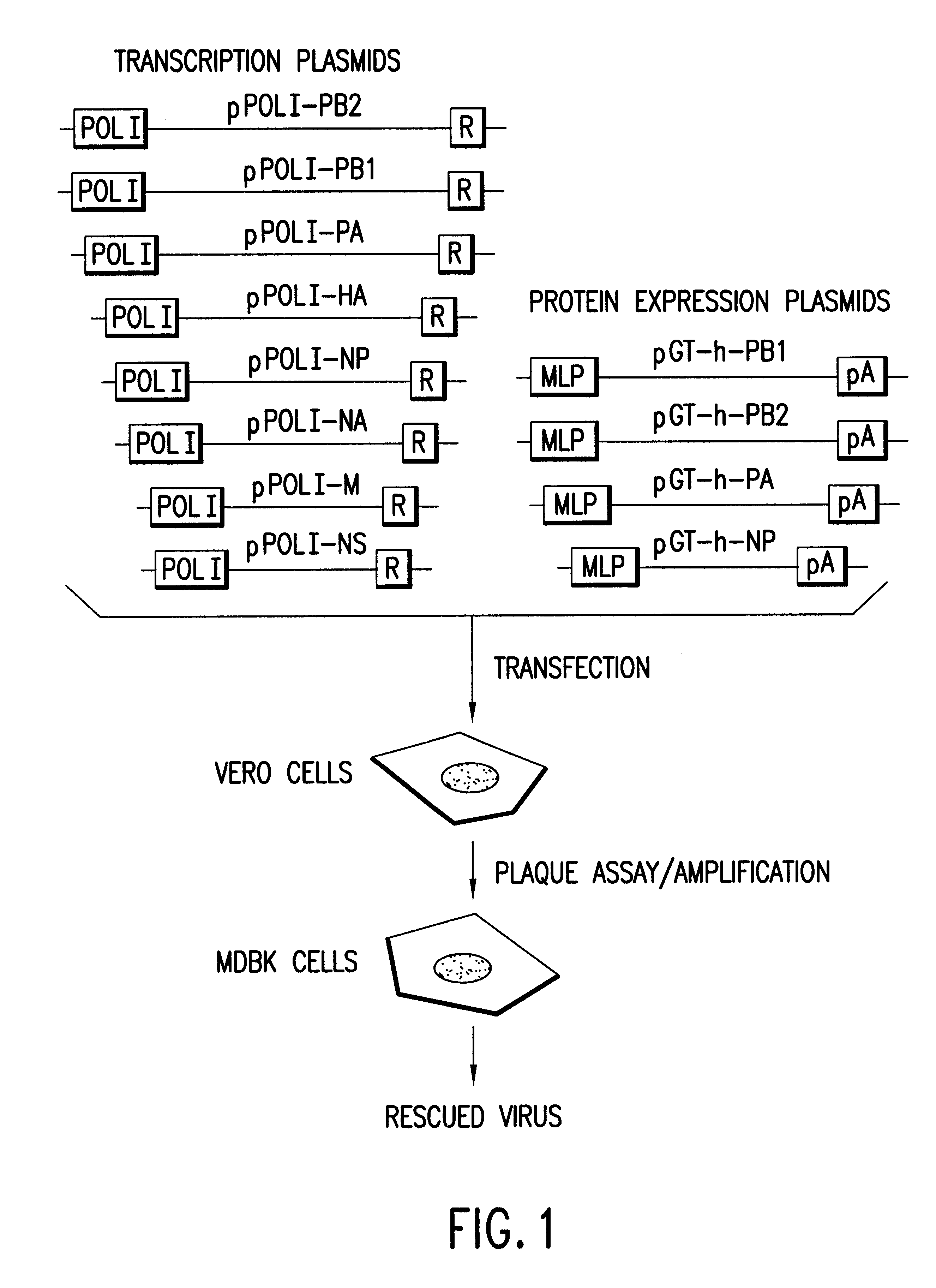
DNA transfection system for the generation of infectious influenza virus
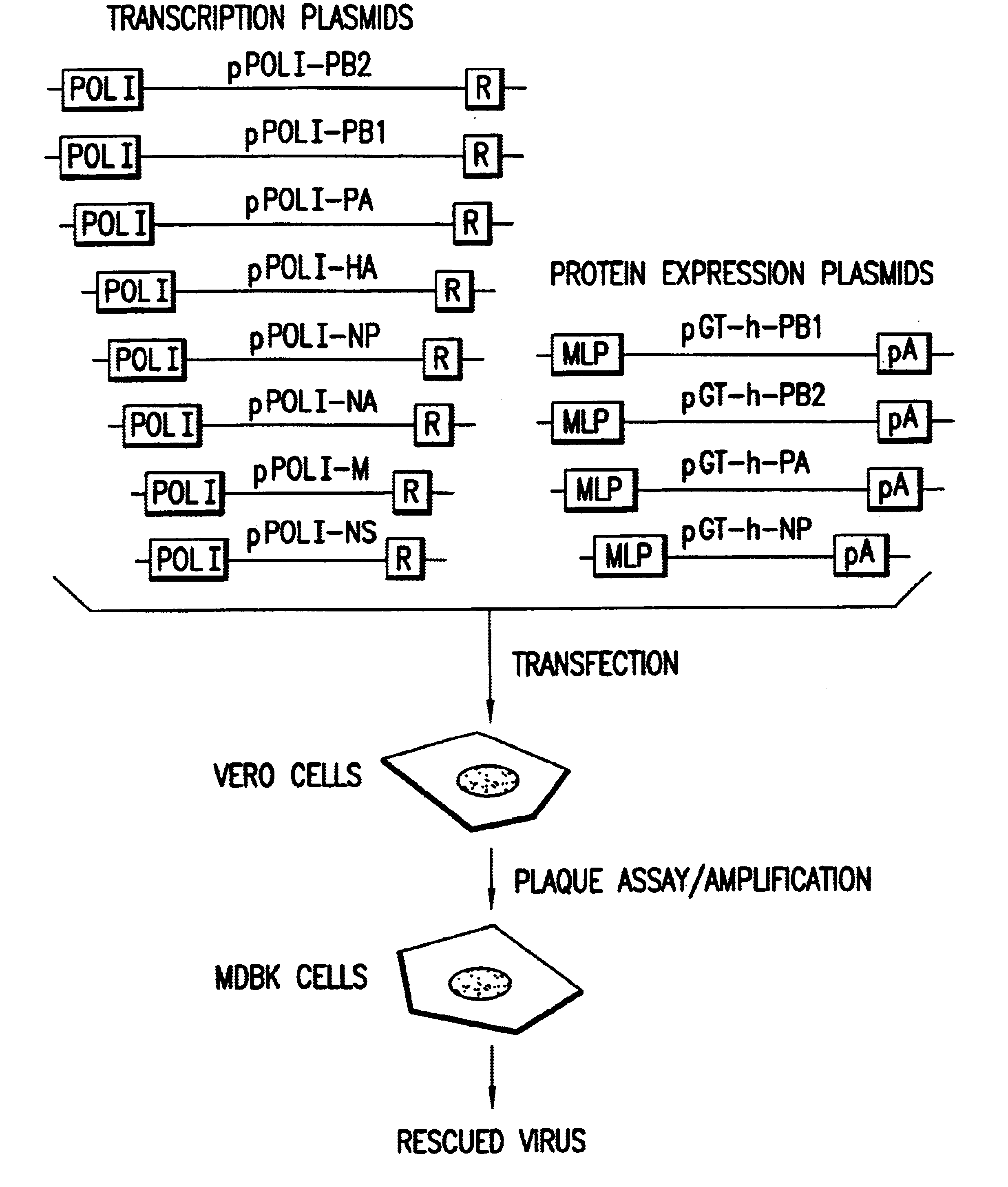
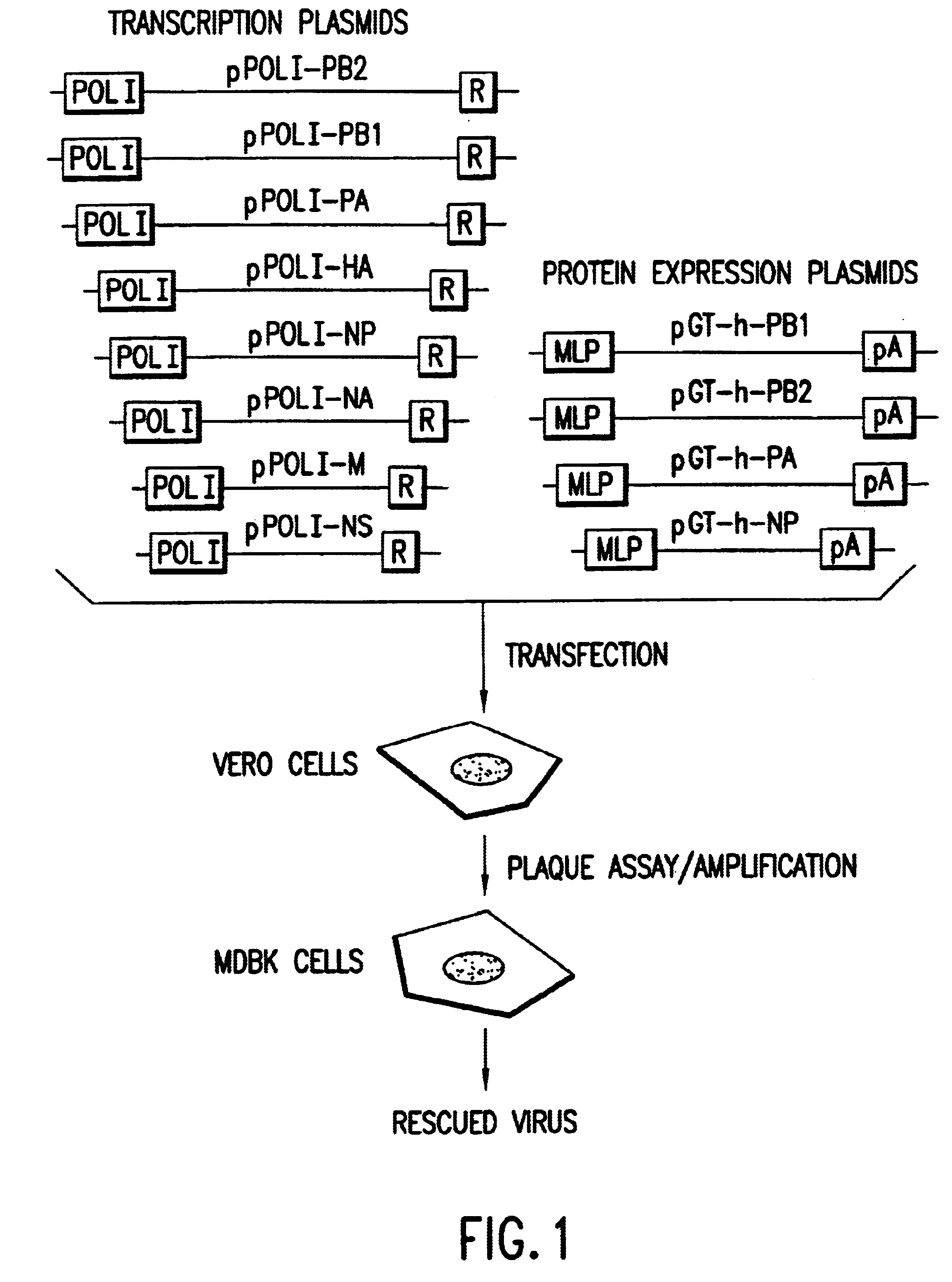
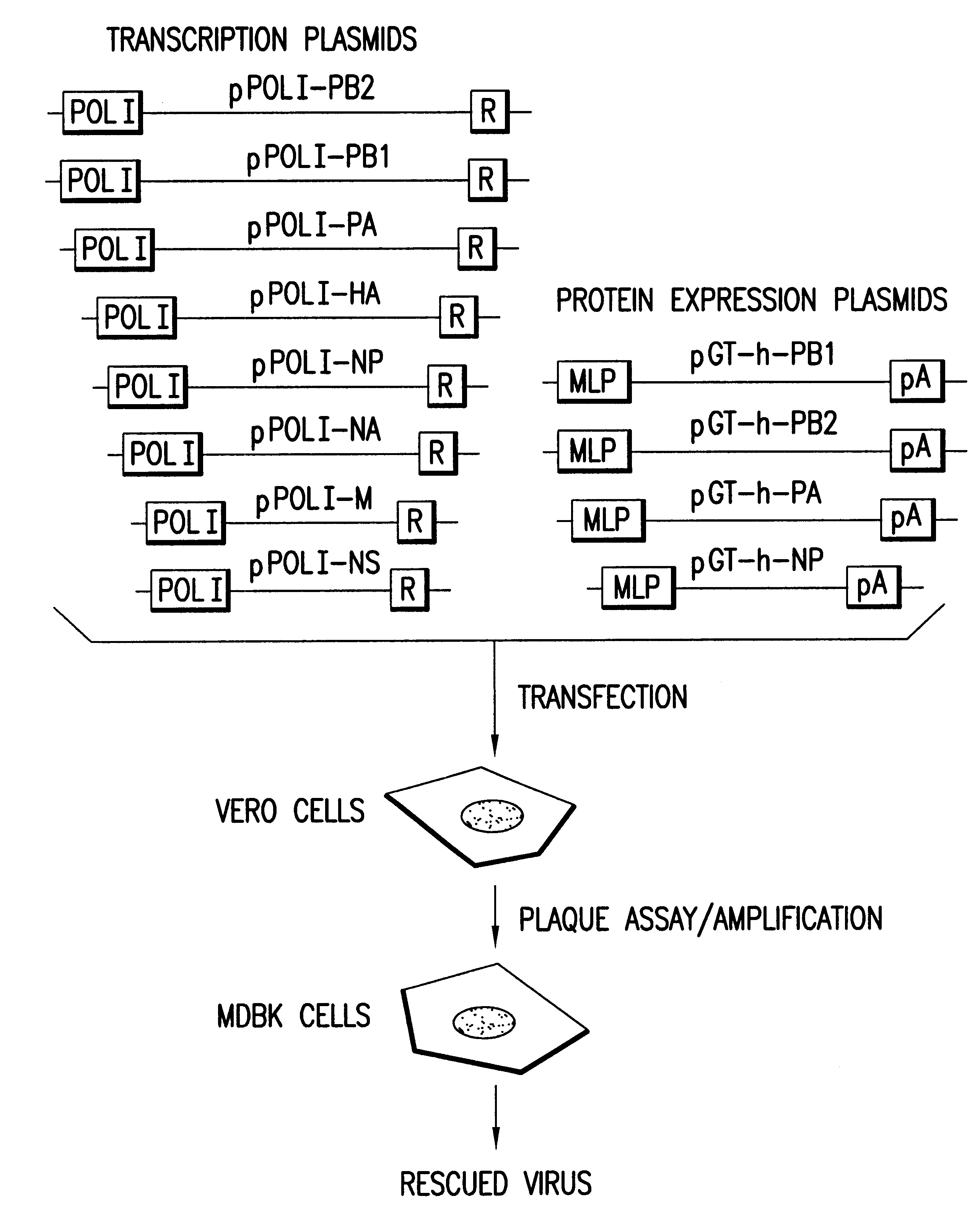
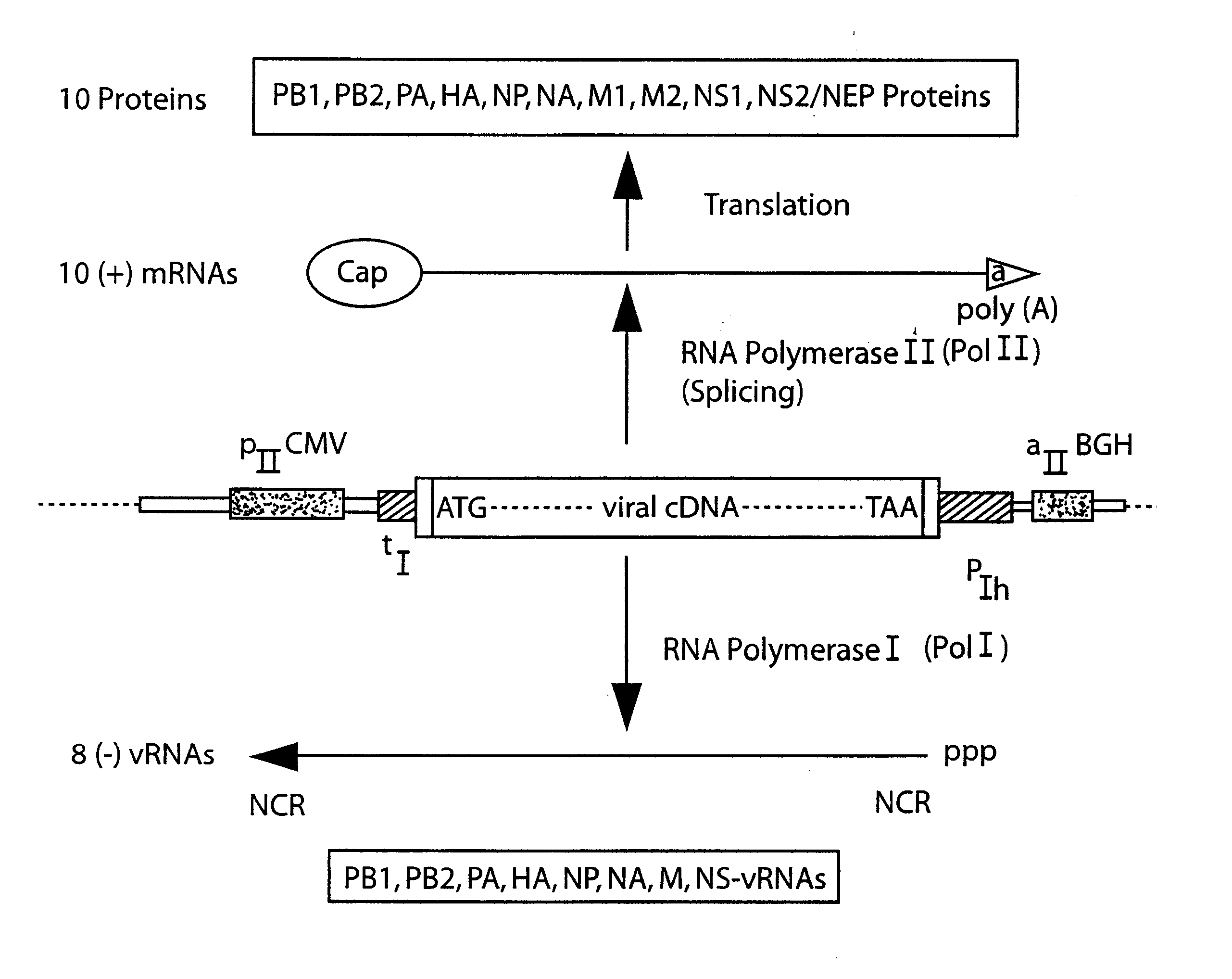
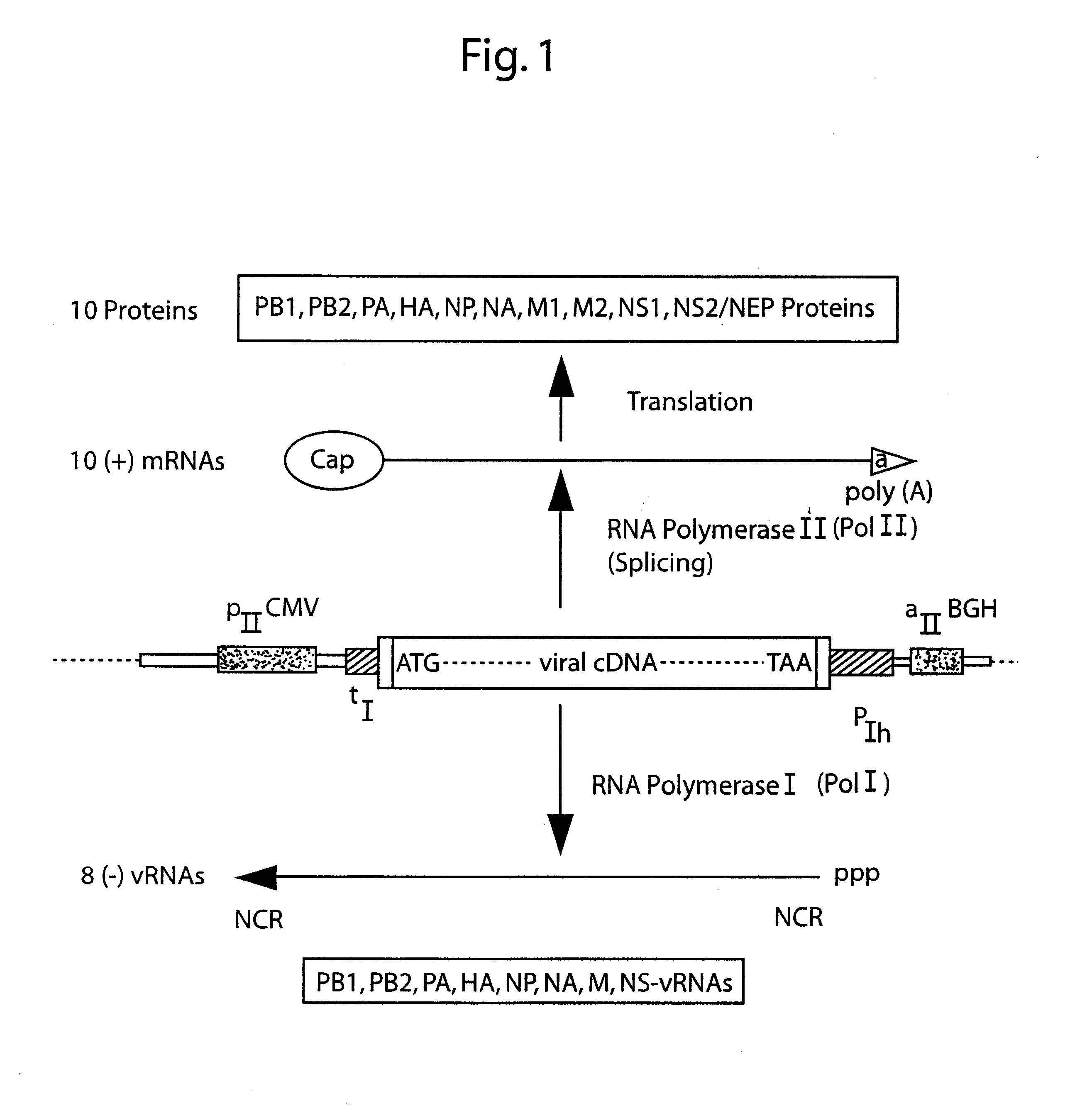
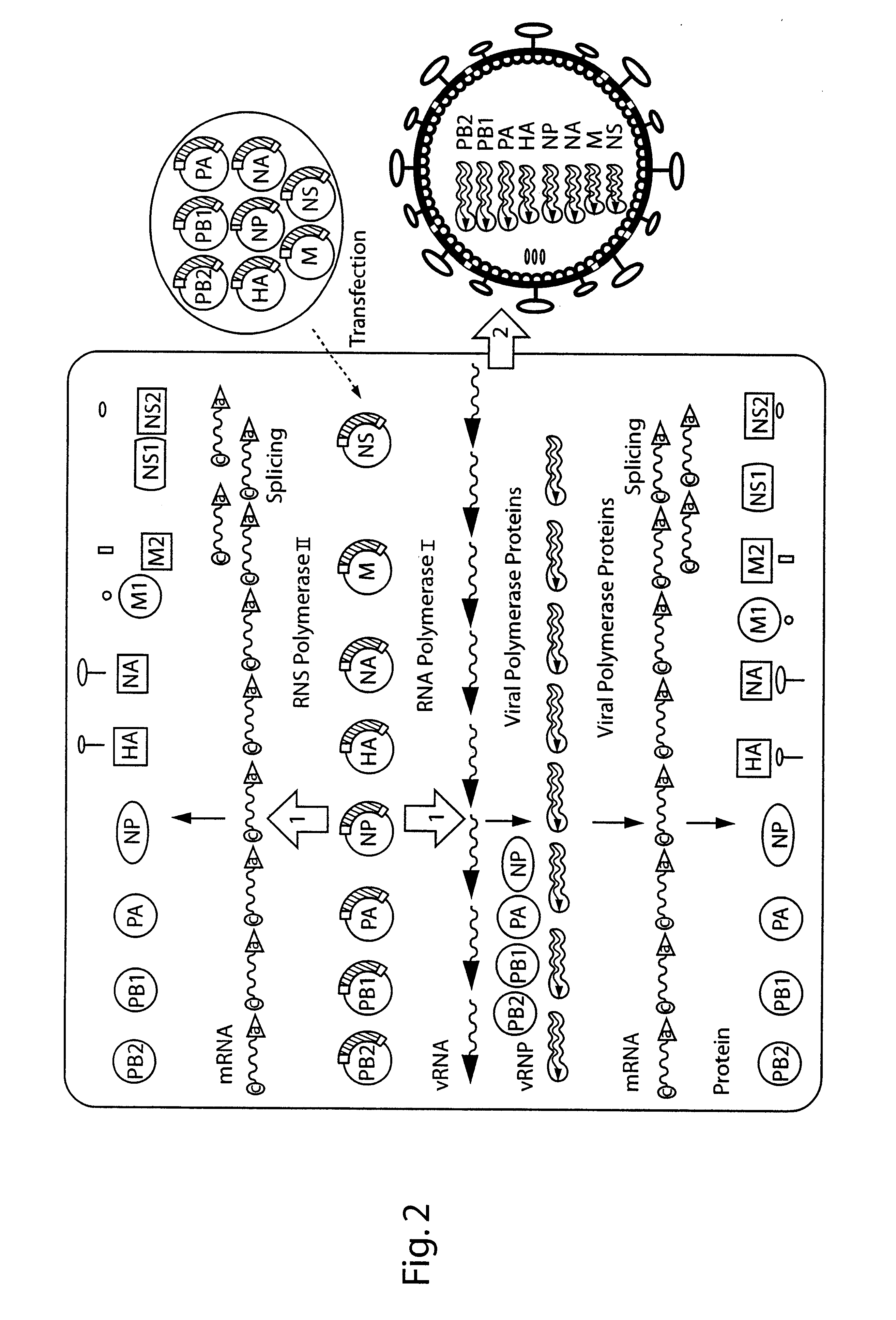
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Simian immunodeficiency virus peptides with antifusogenic and antiviral activities
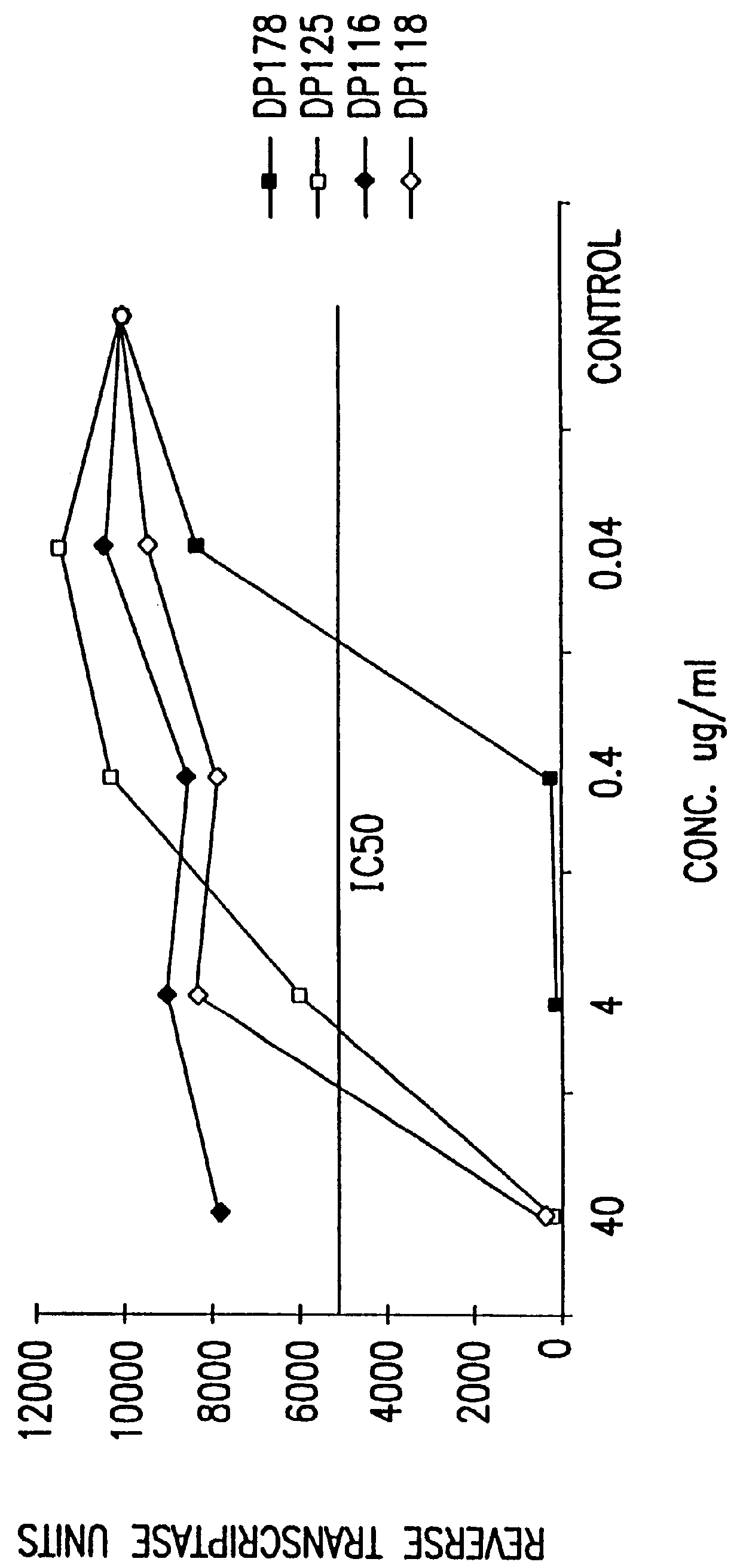
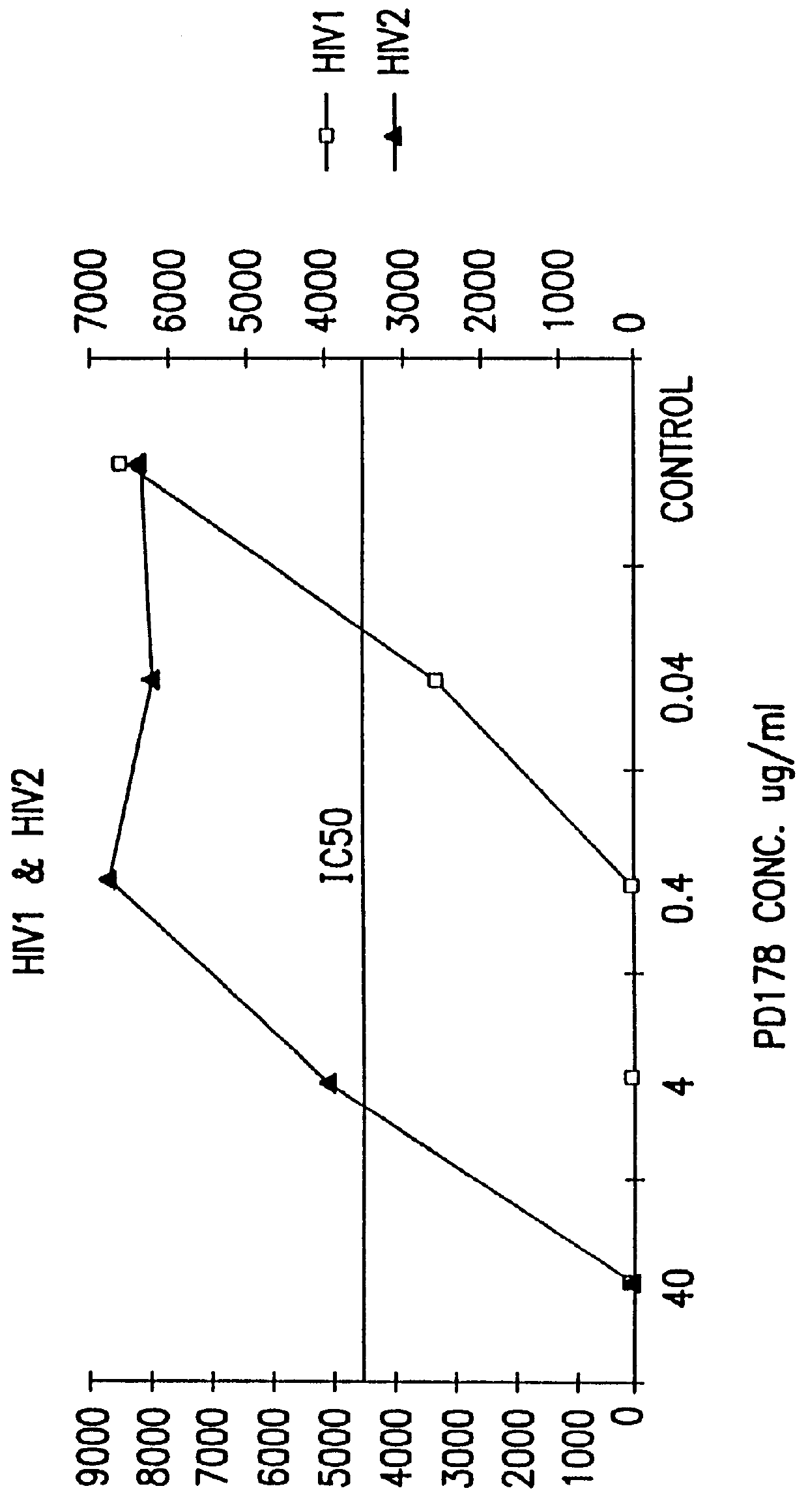
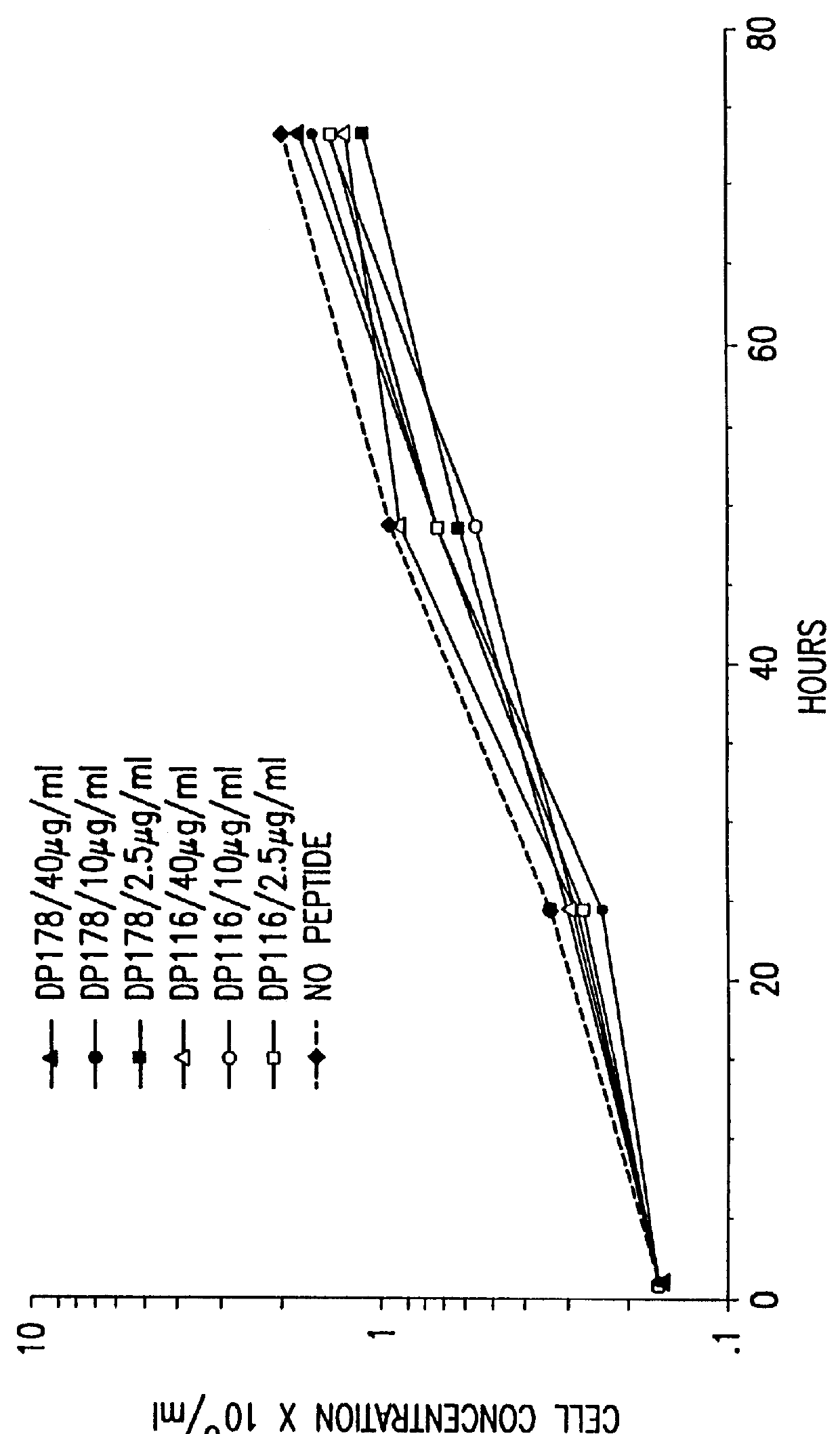
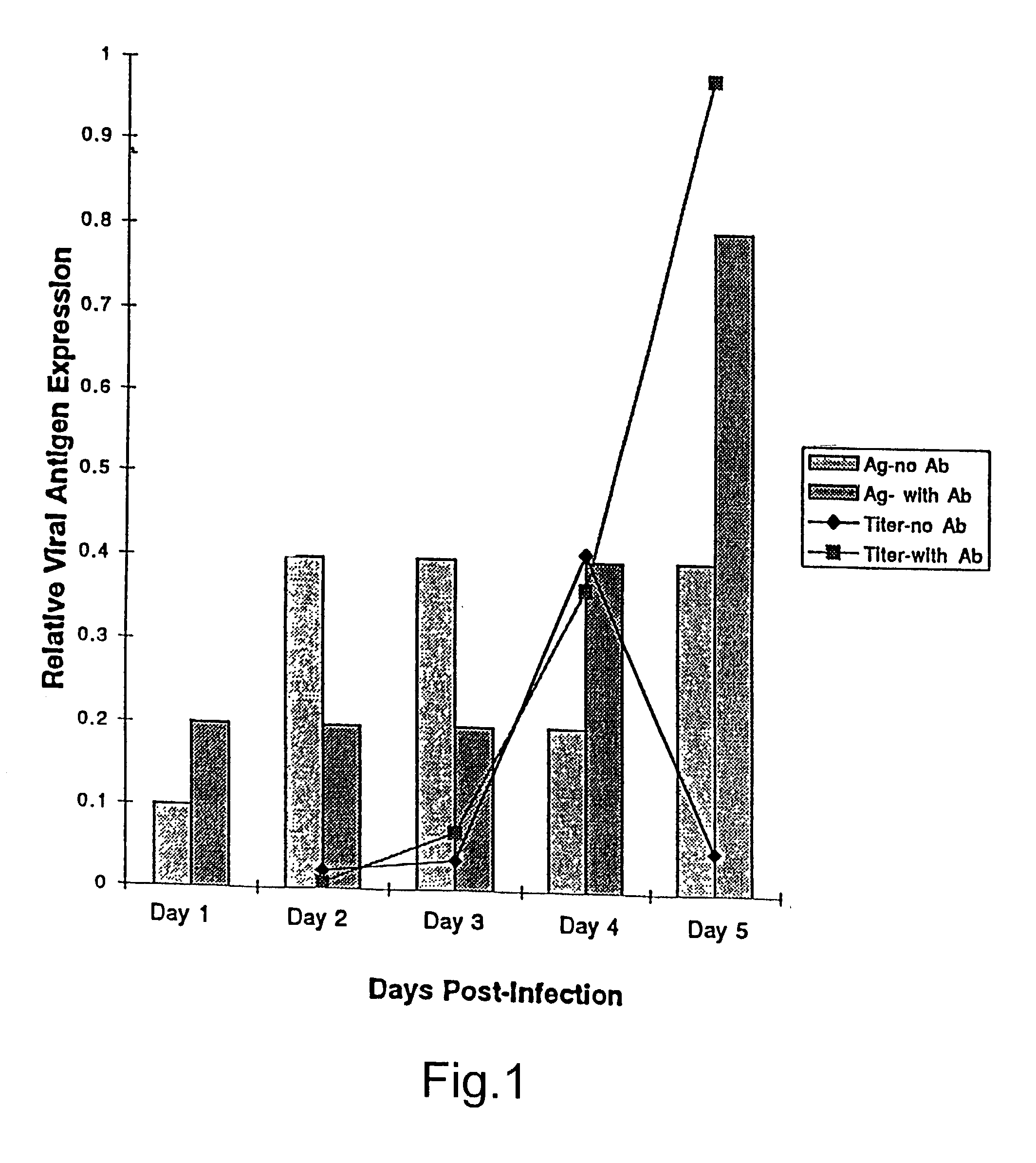
InactiveUS6017536ASsRNA viruses negative-sensePeptide/protein ingredientsSimian immunodeficiency viruses SIVDefective virus
The present invention relates to peptides which exhibit antifusogenic and antiviral activities. The peptides of the invention consist of a 16 to 39 amino acid region of a simian immunodeficiency virus (SIV) protein. These regions were identified through computer algorithms capable of recognizing the ALLMOTI5, 107x178x4, or PLZIP amino acid motifs. These motifs are associated with the antifusogenic and antiviral activities of the claimed peptides.
Owner:TRIMERIS +1
Treatment of neoplasms with viruses
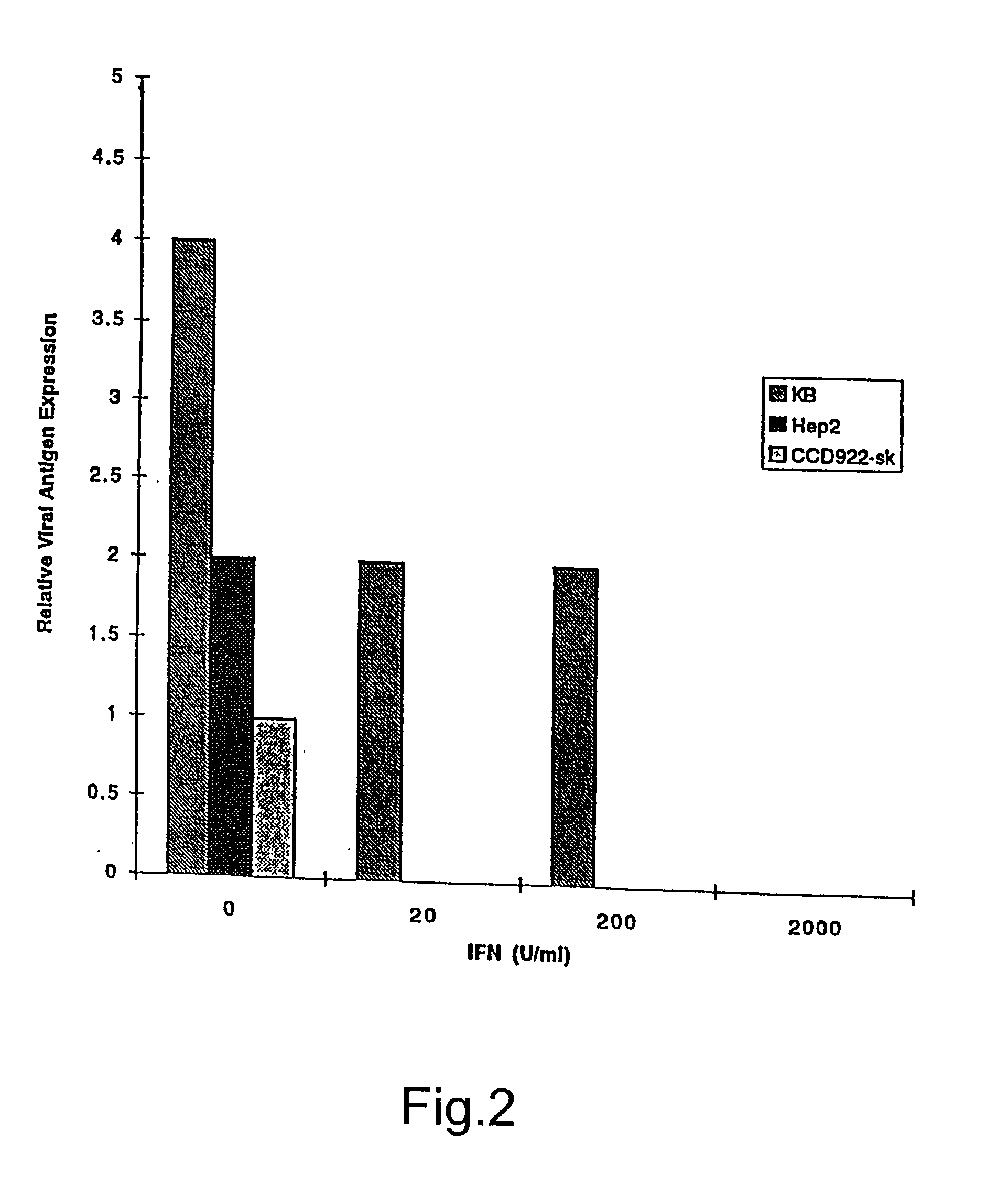
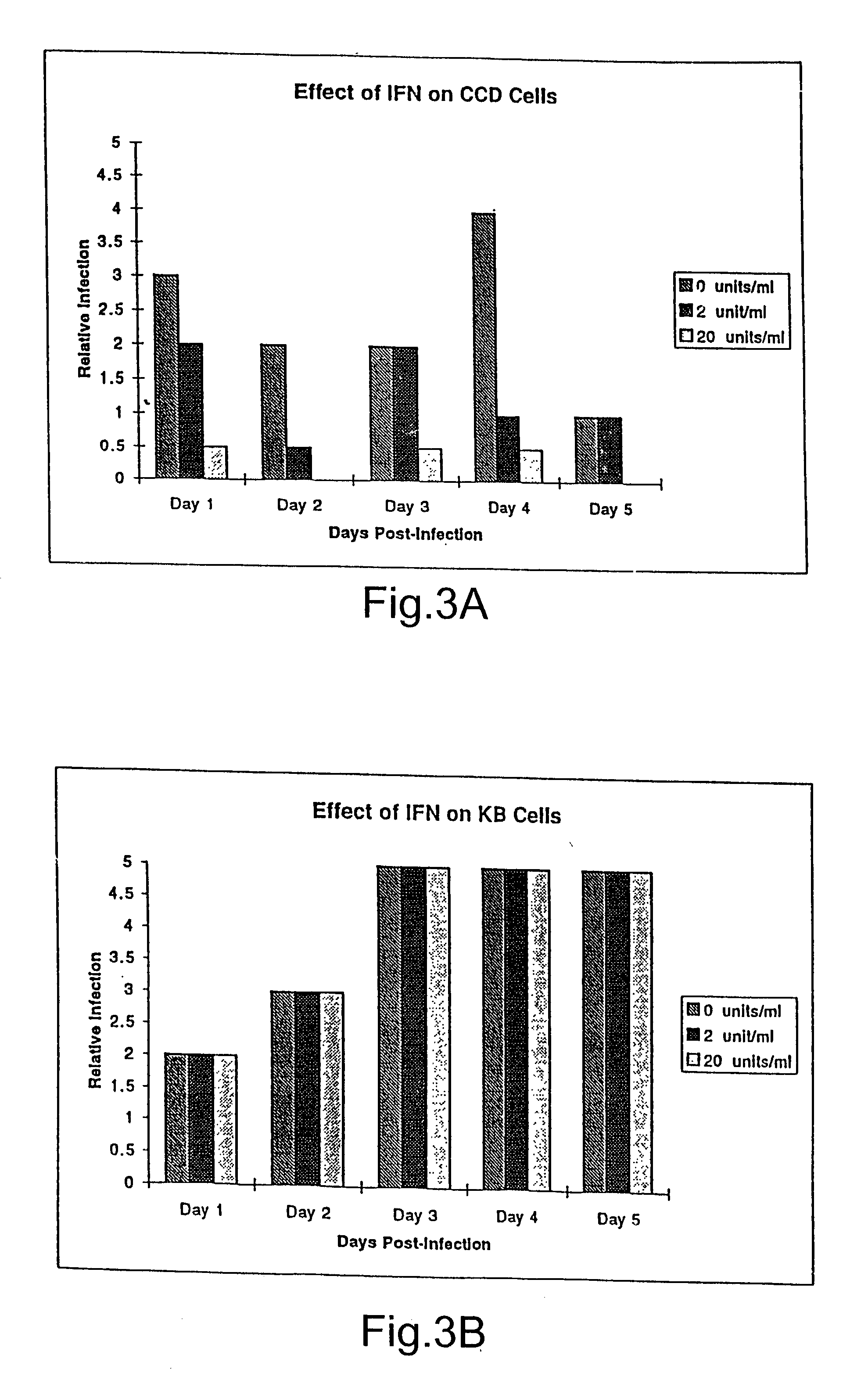
InactiveUS20030044384A1Convenient treatmentReduce productionSsRNA viruses negative-senseBiocideDiseaseAnti viral response
The subject invention relates to viruses that are able to replicate and thereby kill neoplastic cells with a deficiency in the IFN-mediated antiviral response, and their use in treating neoplastic disease including cancer and large tumors. RNA and DNA viruses are useful in this regard. The invention also relates to methods for the selection, design, purification and use of such viruses for cancer therapy.
Owner:PRO VIRUS
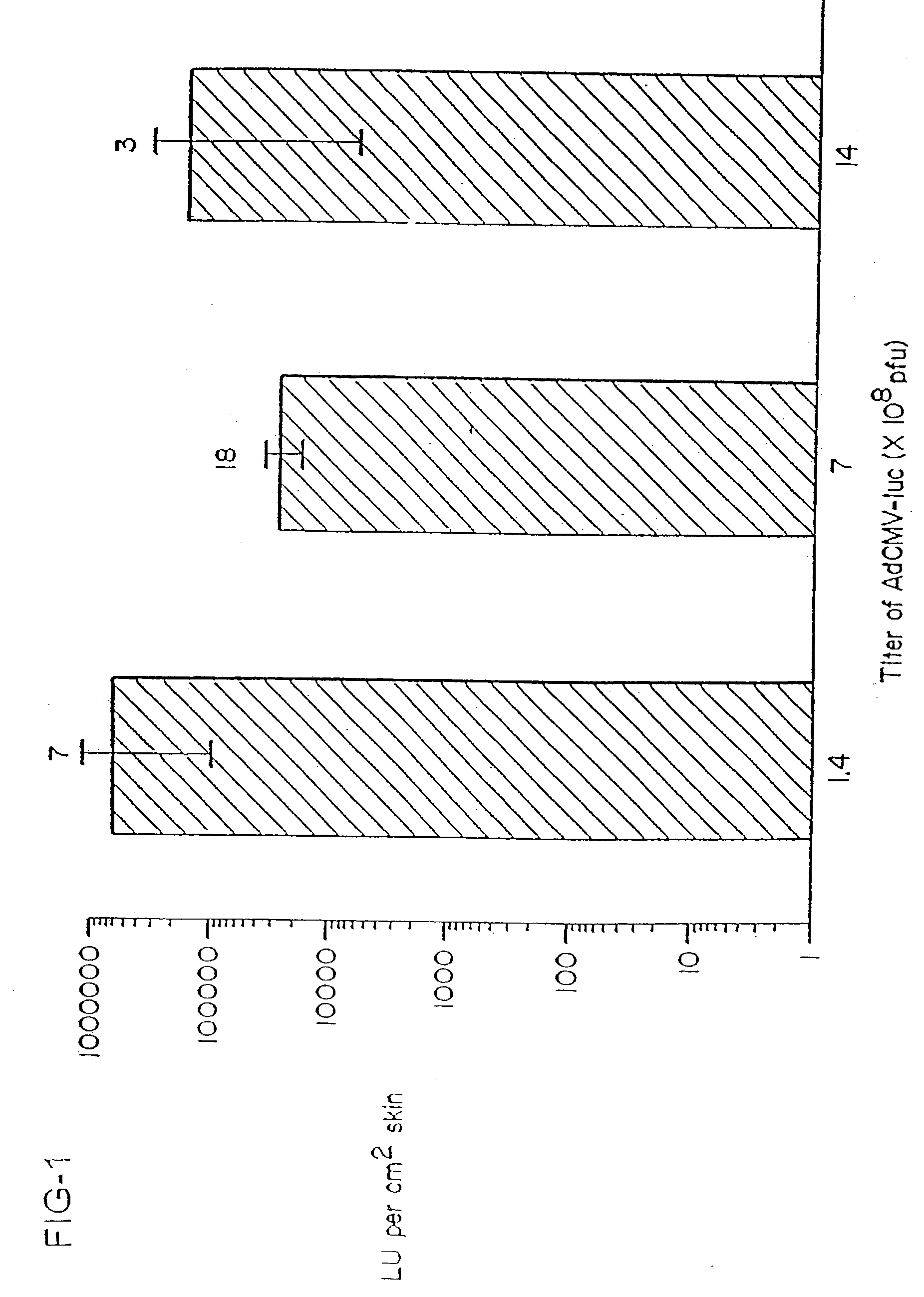
Vaccine and drug delivery by topical application of vectors and vector extracts
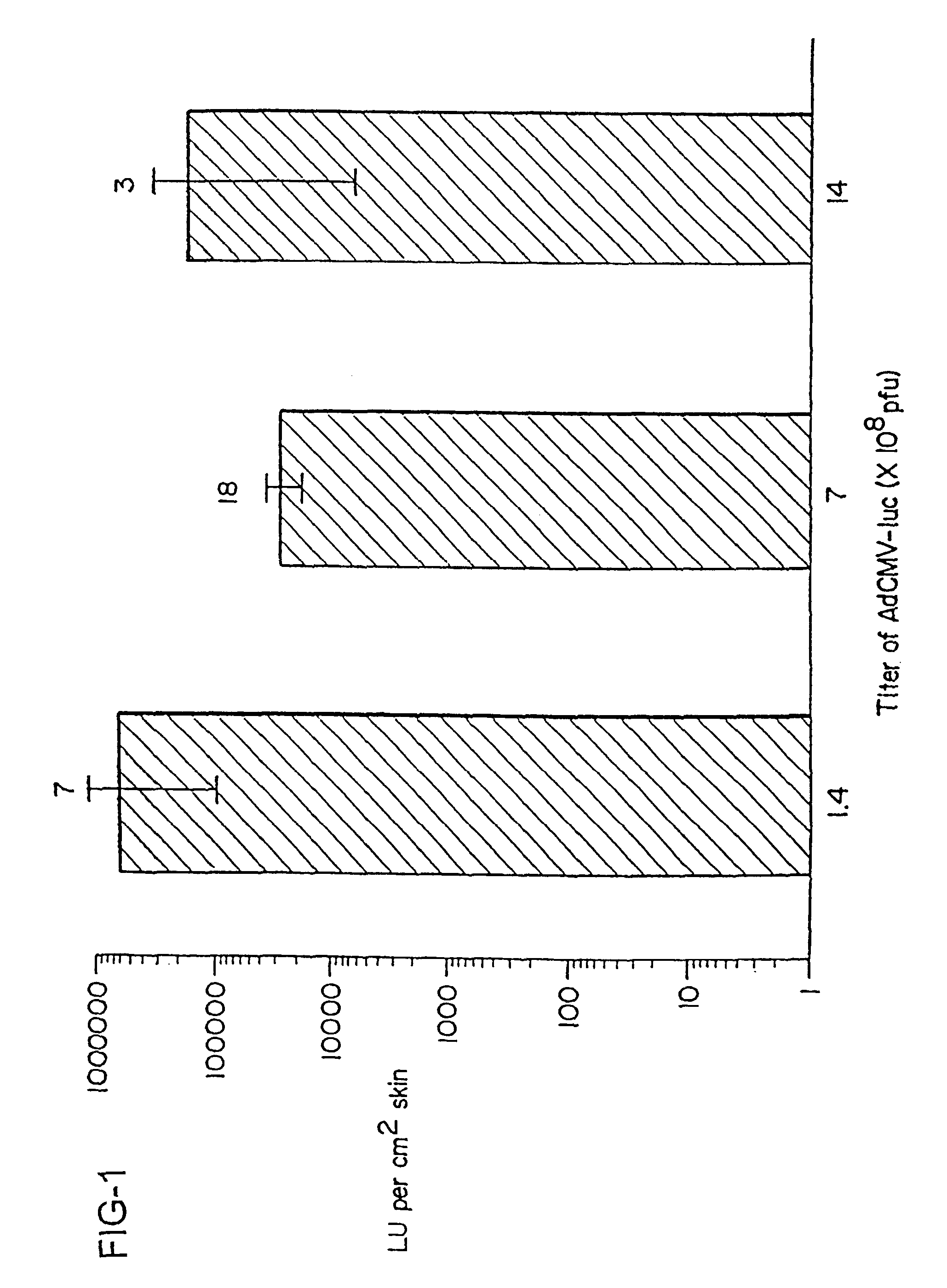
InactiveUS20040009936A1Improve vaccination schemeEfficient deliverySsRNA viruses negative-senseBiocideNeoplasmGlycoprotein
Disclosed and claimed are methods of non-invasive immunization and drug delivery in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of non-replicative vectors, products therefrom and uses for the methods and products therefrom. Also disclosed and claimed are methods of non-invasive immunization and drug delivery in an animal and / or a method of inducing a systemic immune response or systemic therapeutic response to a gene product comprising contacting skin of the animal with cell-free extracts in an amount effective to induce the response, wherein the extracts are prepared by filtration of disrupted cells, wherein the cell comprises and expresses a nucleic acid molecule. Preferably, the cell is temporarily disrupted by sonication, remaining intact and viable after the sonication. Also, methods are disclosed and claimed for enhancing the immunogenicity and efficacy of an epicutaneous vaccine for inducing a systemic immune response to an antigen, in an animal comprising contacting skin of the animal with vaccines admixed with heat-shock protein 27, in an amount effective to induce the response. The methods include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope or antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, anthrax germination factors, rabies glycoprotein, HBV surface antigen, HIV gp120, HIV gp160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, botulinum toxin A, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co- stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells including epidermal cells. The immune response can also be induced by antigens expressed from the nucleic acid molecule within the vector. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s) and / or all adenoviral genes.
Owner:UAB RES FOUND
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20120009221A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseViral antigen ingredientsAntigenPharmaceutical medicine
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
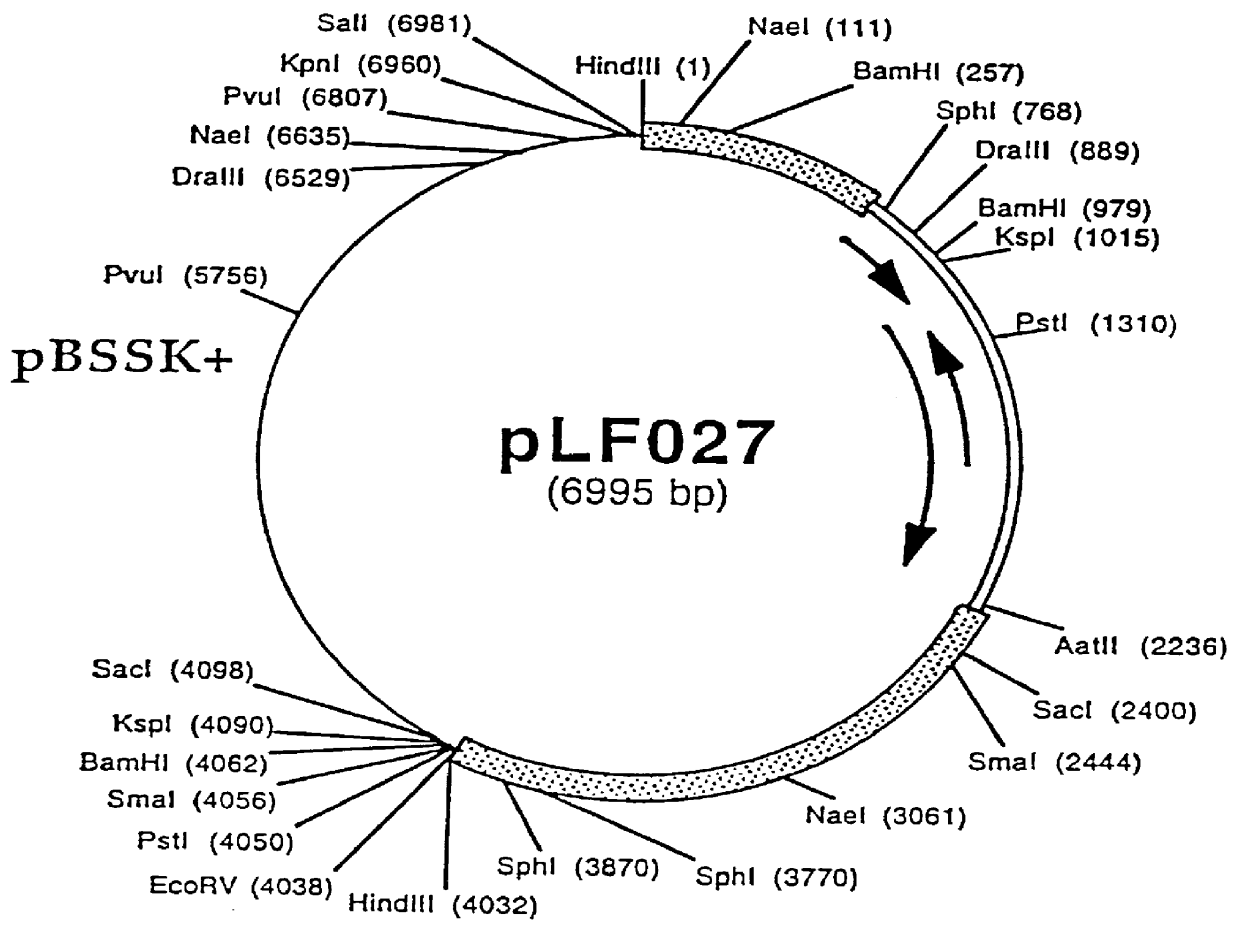
Retroviral vectors comprising a functional splice donor site and a functional splice acceptor site
InactiveUS7303910B2Efficient expressionIncrease rangeSsRNA viruses negative-senseFungiNucleotideNucleotide sequencing
A retroviral vector is described. The retroviral vector comprises a functional splice donor site and a functional splice acceptor site; wherein the functional splice donor site and the functional splice acceptor site flank a first nucleotide sequence of interest (“NOI”); wherein the functional splice donor site is upstream of the functional splice acceptor site; wherein the retroviral vector is derived from a retroviral pro-vector; wherein the retroviral pro-vector comprises a first nucleotide sequence (“NS”) capable of yielding the functional splice donor site and a second NS capable of yielding the functional splice acceptor site; wherein the first NS is downstream of the second NS; such that the retroviral vector is formed as a result of reverse transcription of the retroviral pro-vector.
Owner:OXFORD BIOMEDICA (UK) LTD
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
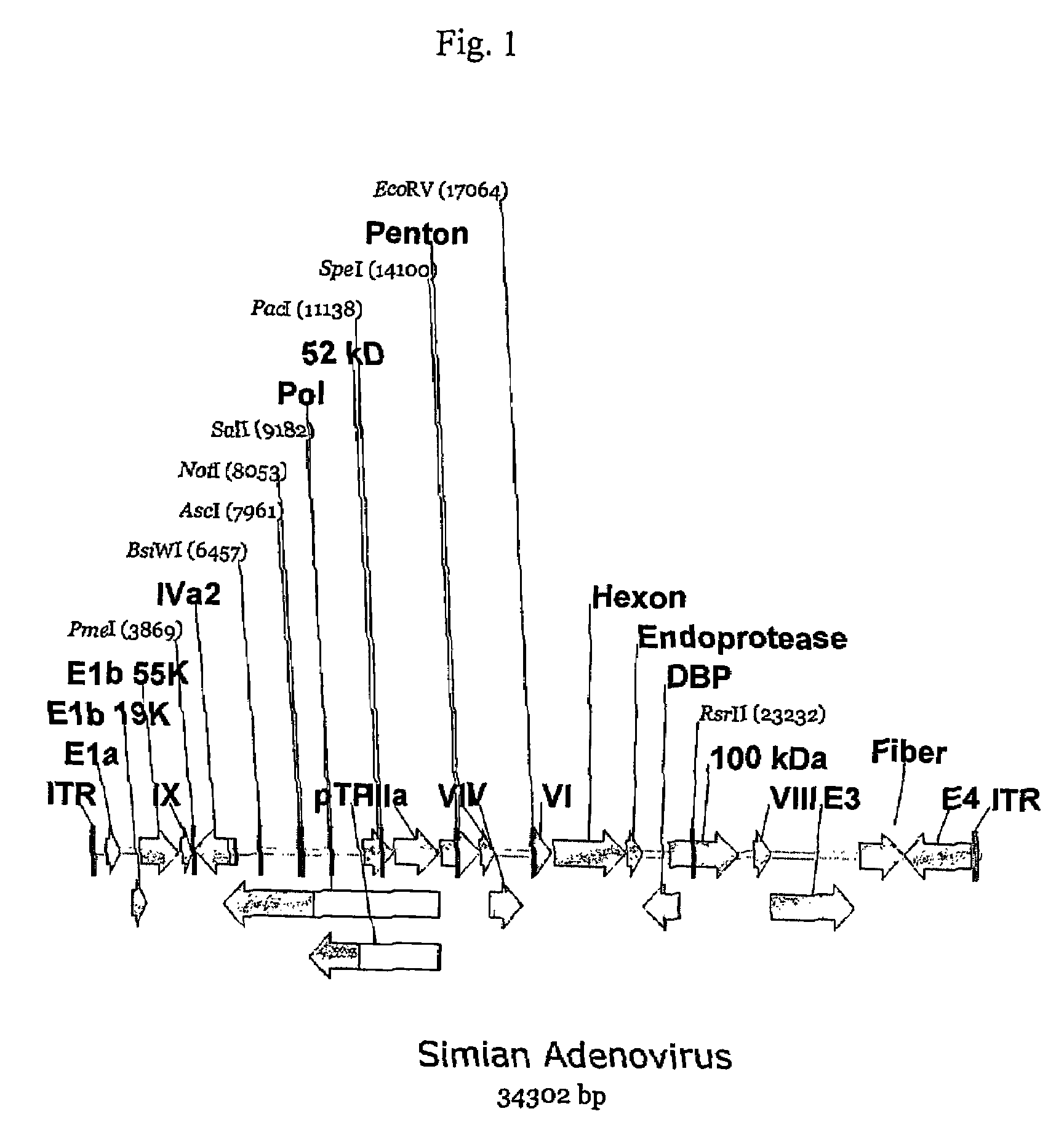
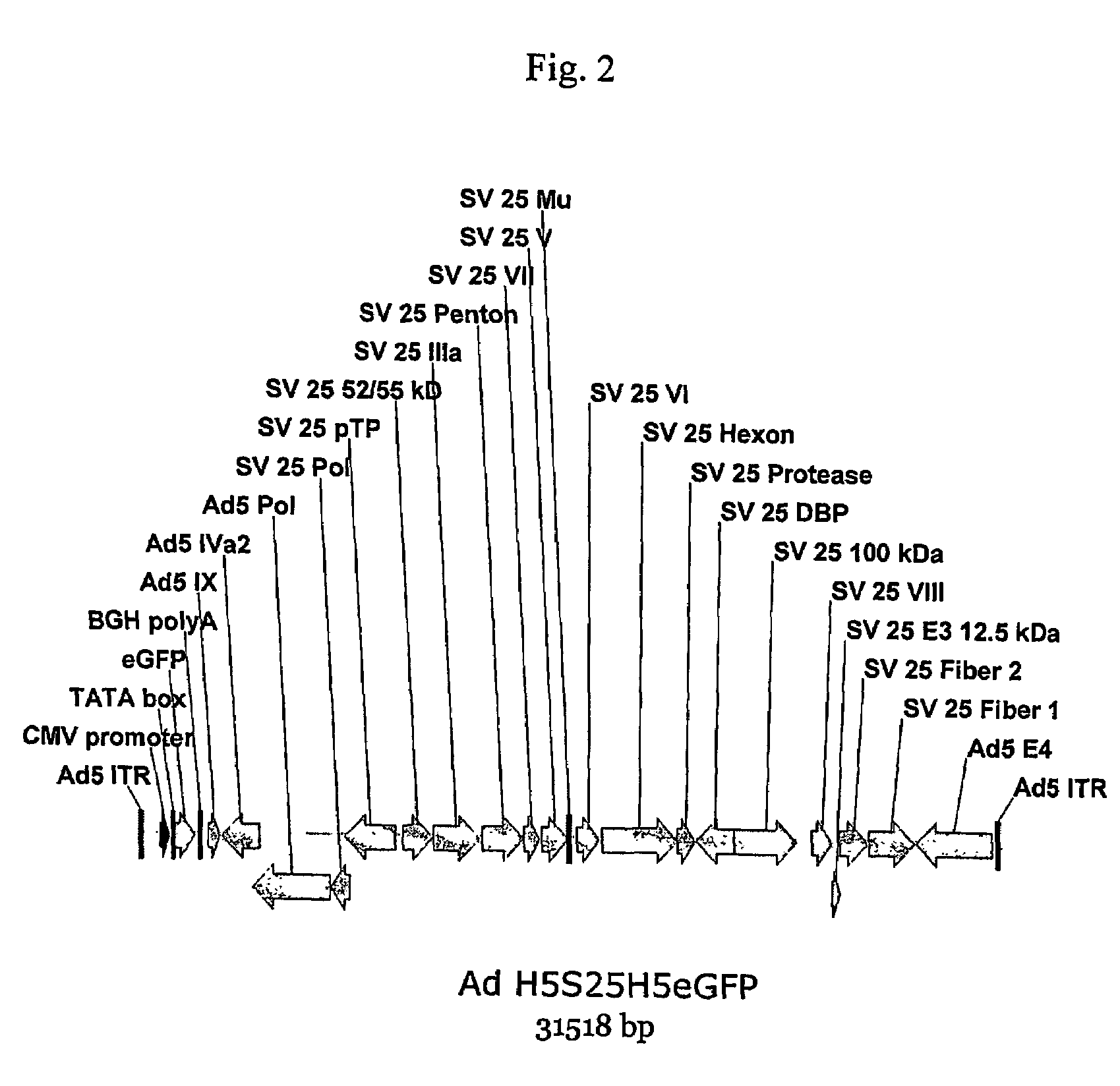
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the (5′) inverted terminal repeat, the left terminus spans the E4 region and the (3′) inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
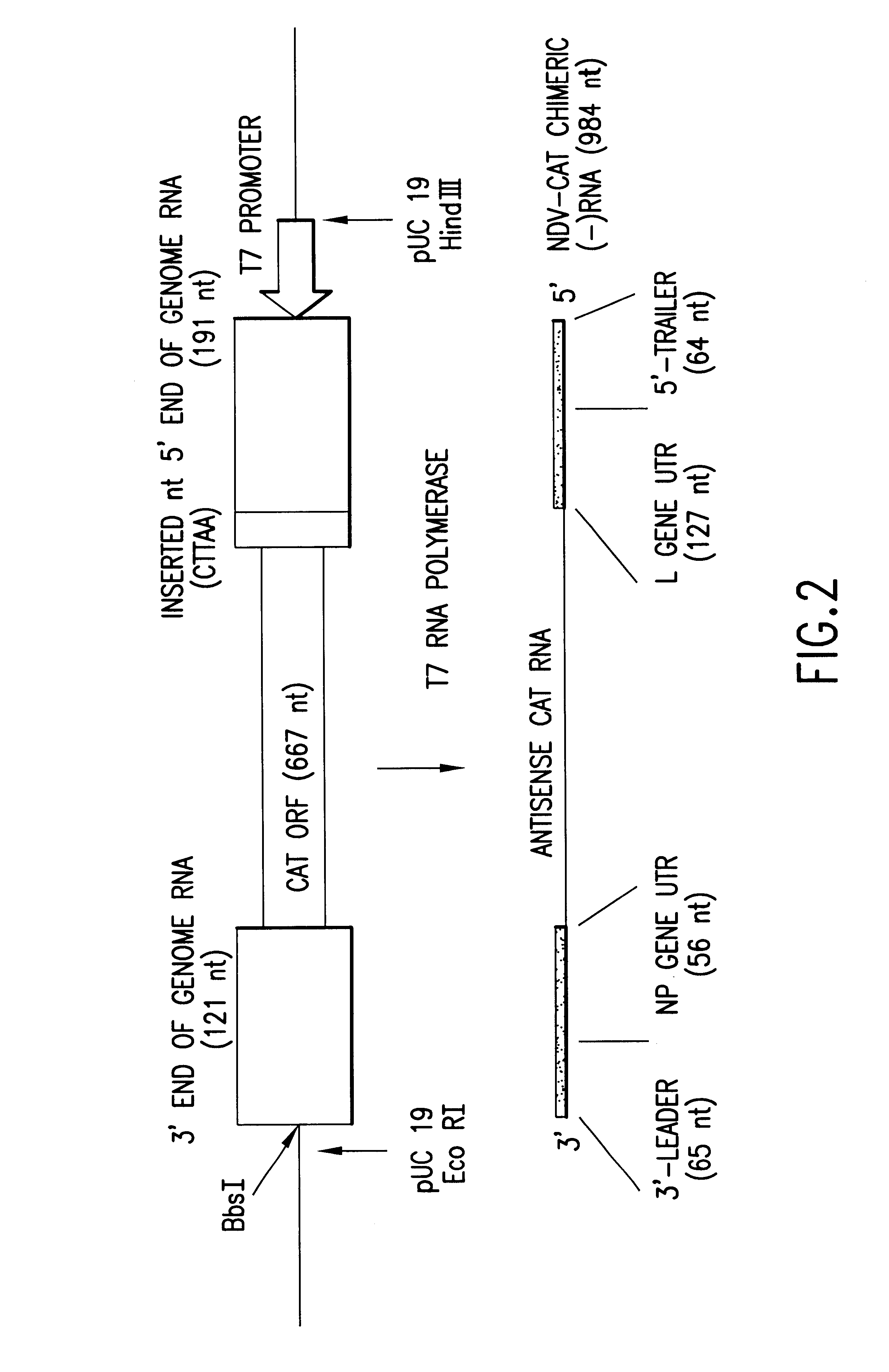
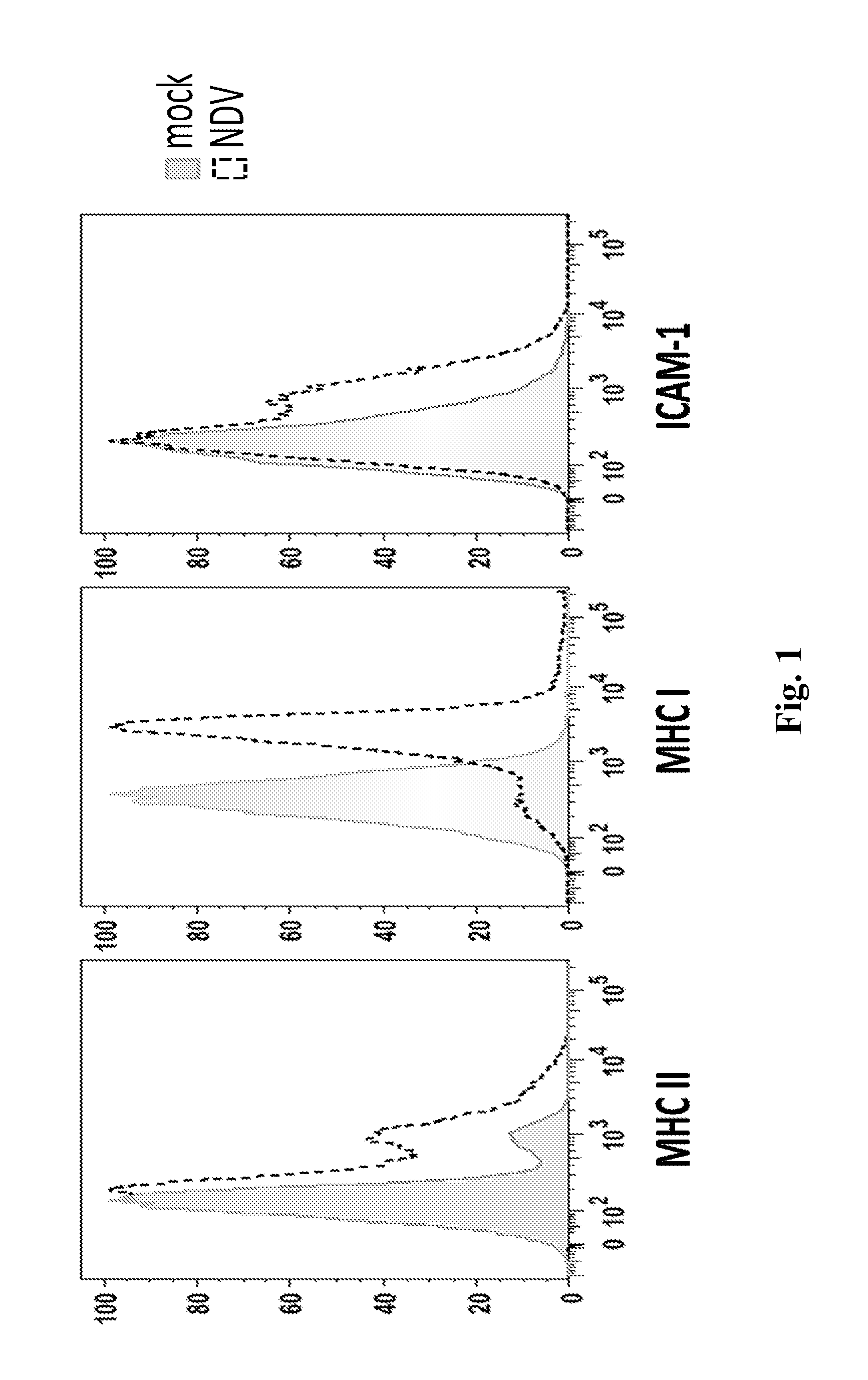
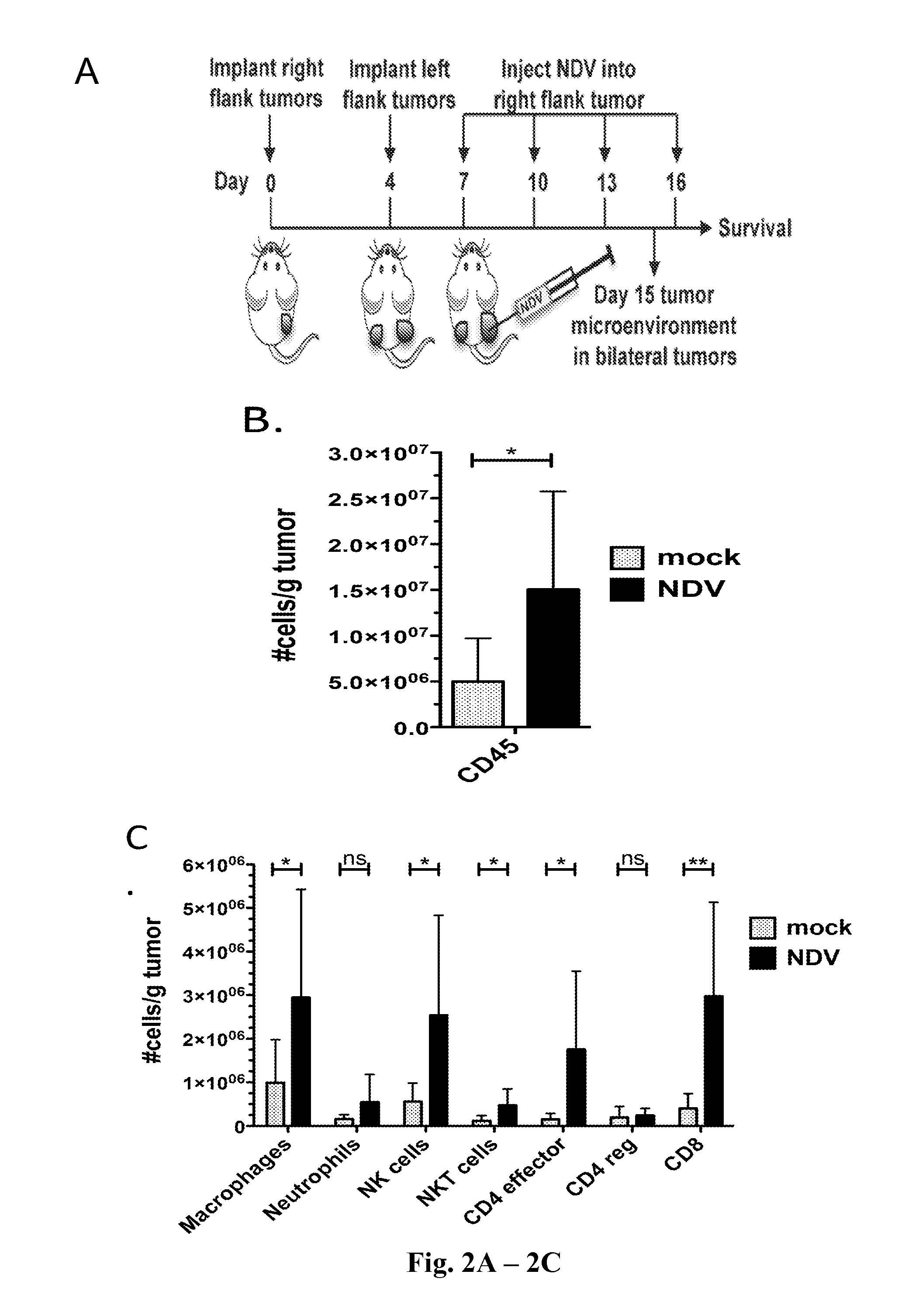
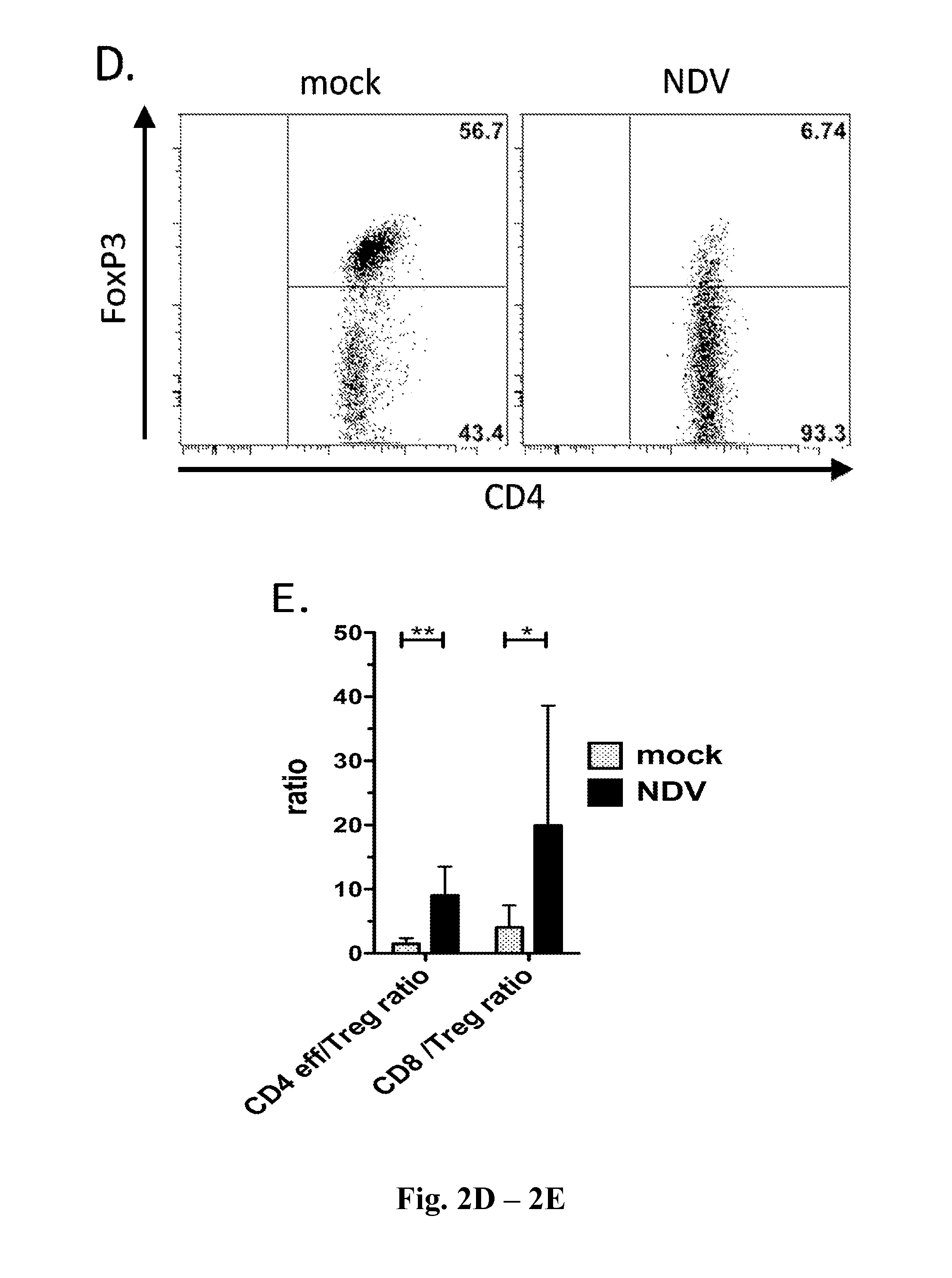
Newcastle Disease Viruses and Uses Thereof
InactiveUS20140271677A1Reduce severityPrevent relapseSsRNA viruses negative-senseViral antigen ingredientsNewcastle disease virus NDVAgonist
Described herein are chimeric Newcastle disease viruses engineered to express an agonist of a co-stimulatory signal of an immune cell and compositions comprising such viruses. Also described herein are chimeric Newcastle disease viruses engineered to express an antagonist of an inhibitory signal of an immune cell and compositions comprising such viruses. The chimeric Newcastle disease viruses and compositions are useful in the treatment of cancer. In addition, described herein are methods for treating cancer comprising administering Newcastle disease viruses in combination with an agonist of a co-stimulatory signal of an immune and / or an antagonist of an inhibitory signal of an immune cell.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Multi plasmid system for the production of influenza virus
InactiveUS20050266026A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggCold adapted
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. A method of producing a cold adapted (ca) influenza virus that replicates efficiently at, e.g., 25° C. (and immunogenic compositions comprising the same) is also provided.
Owner:MEDIMMUNE LLC
Immunisation of large mammals with low doses of RNA
ActiveUS20130149375A1Conveniently preparedImprove stabilityAntibacterial agentsSsRNA viruses negative-senseMammalImmunity response
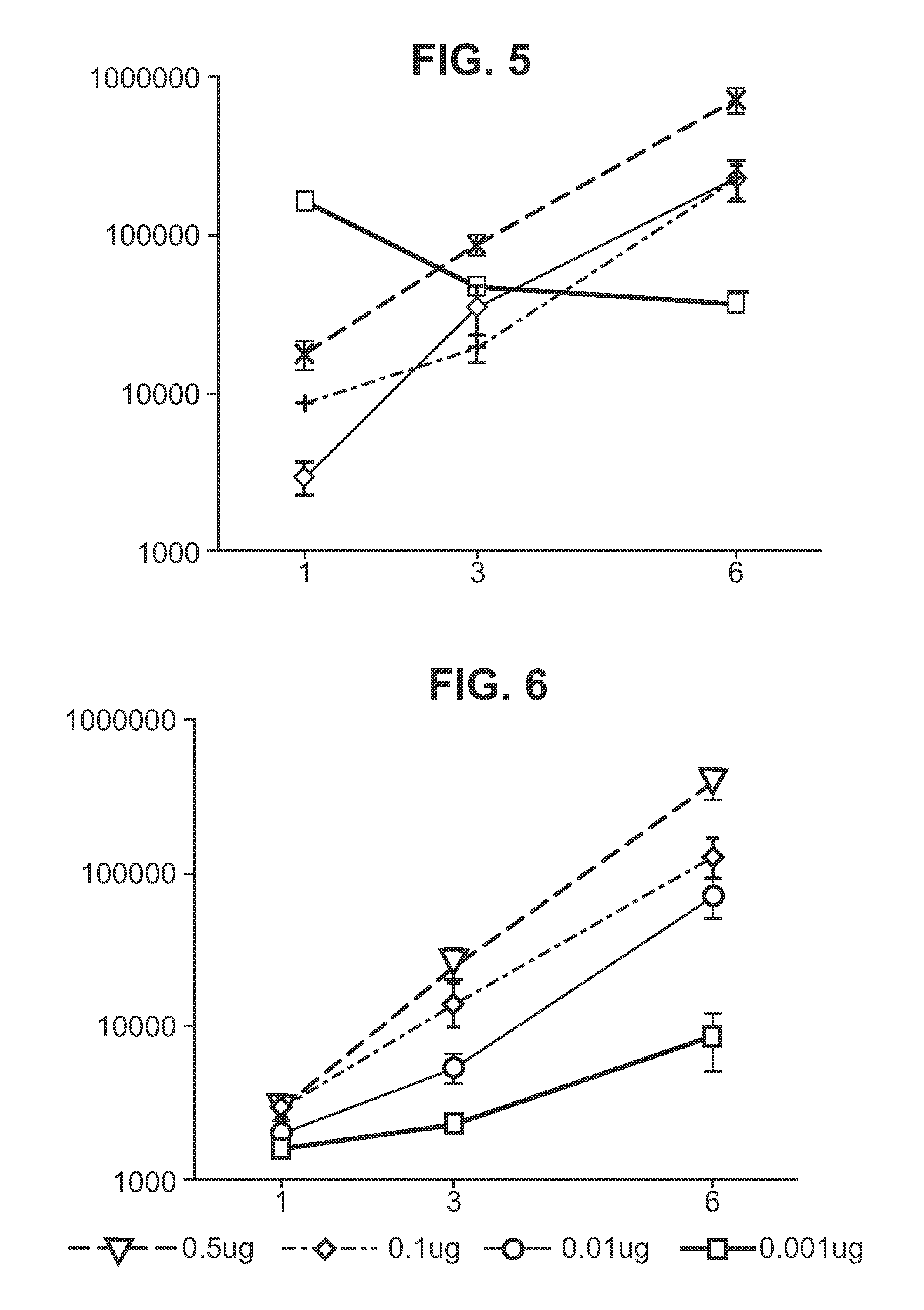
RNA encoding an immunogen is delivered to a large mammal at a dose of between 2 μg and 100 μg. Thus the invention provides a method of raising an immune response in a large mammal, comprising administering to the mammal a dose of between 2 μg and 100 μg of immunogen-encoding RNA. Similarly, RNA encoding an immunogen can be delivered to a large mammal at a dose of 3 ng / kg to 150 ng / kg. The delivered RNA can elicit an immune response in the large mammal
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Insect cells or fractions as adjuvant for antigens
Disclosed and claimed is an adjuvant for immunogenic, immunological, antigenic or vaccine compositions. The adjuvant is composed of insect cells or fractions thereof. Disclosed and claimed are also methods for preparing and using the adjuvant and compositions containing the adjuvant. Advantageously, a recombinant baculovirus containing DNA encoding and expressing an epitope of interest or antigen can be infected into insect cells such as insect cells derived from a Lepidopteran species such as S. frugiperda for expression, and the infected insect cells or a fraction thereof can be used with the expressed epitope of interest or antigen as an inventive antigen or in an inventive immunological, antigen or vaccine composition.
Owner:MERIAL LTD
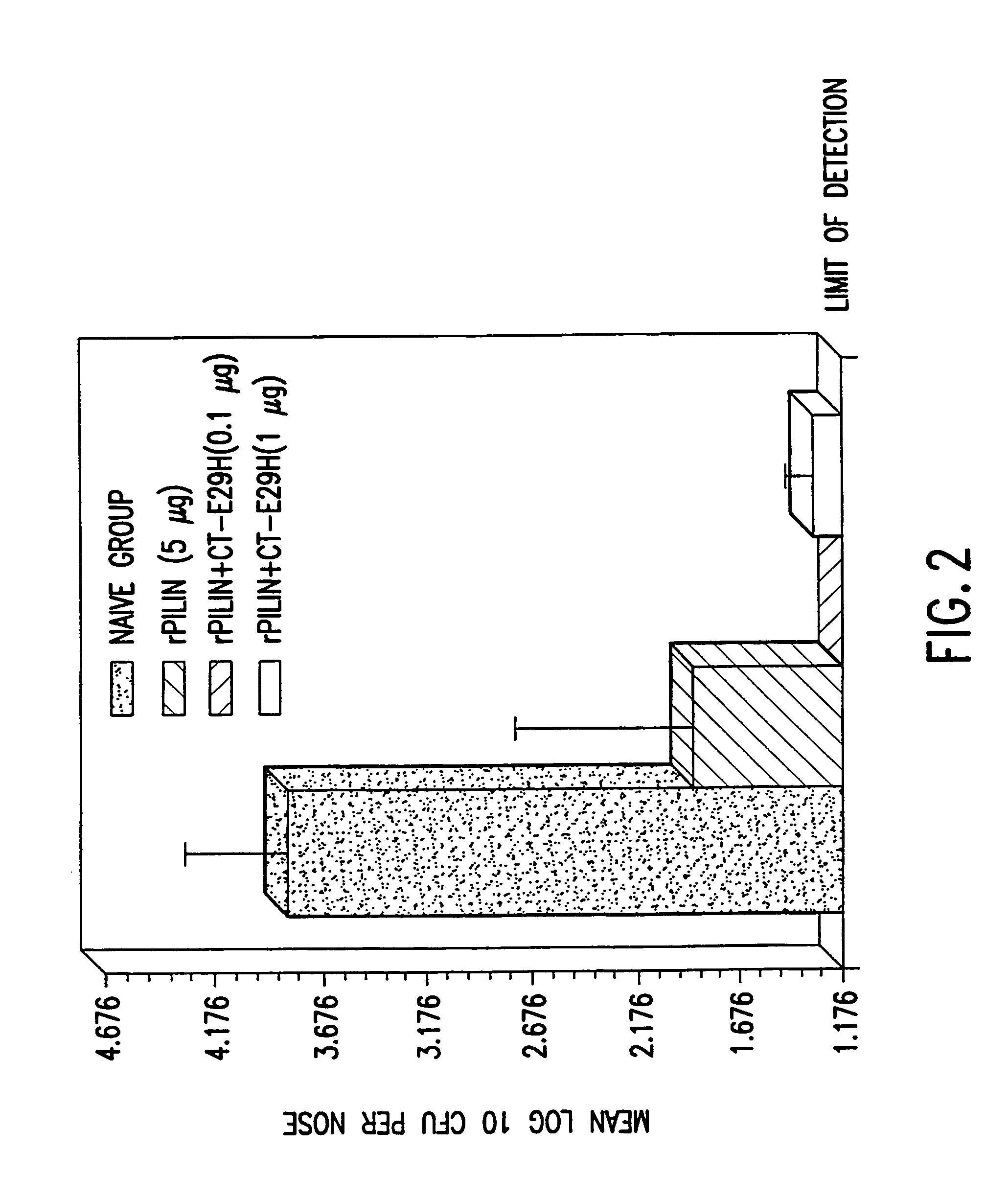
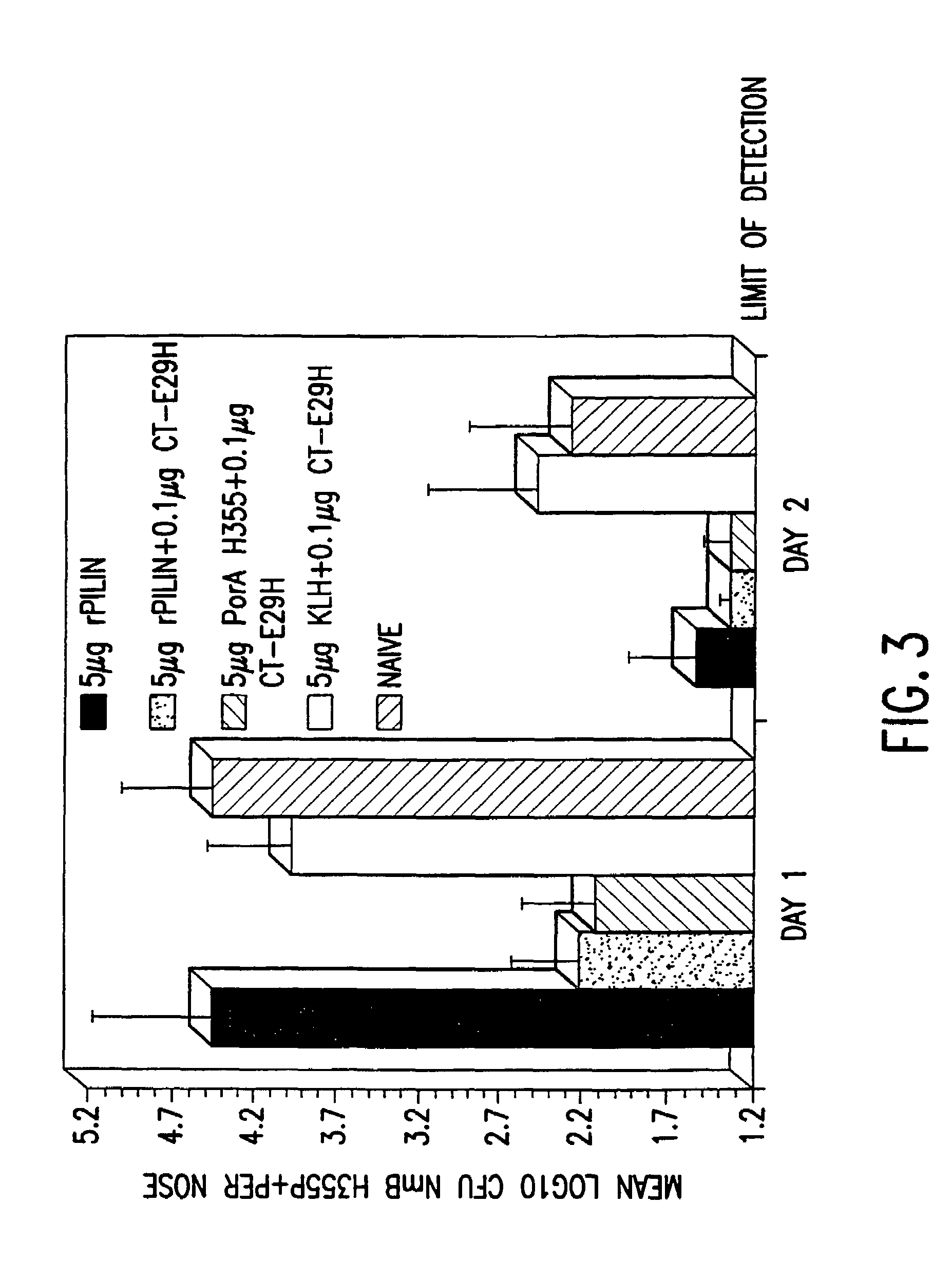
Mutant cholera holotoxin as an adjuvant
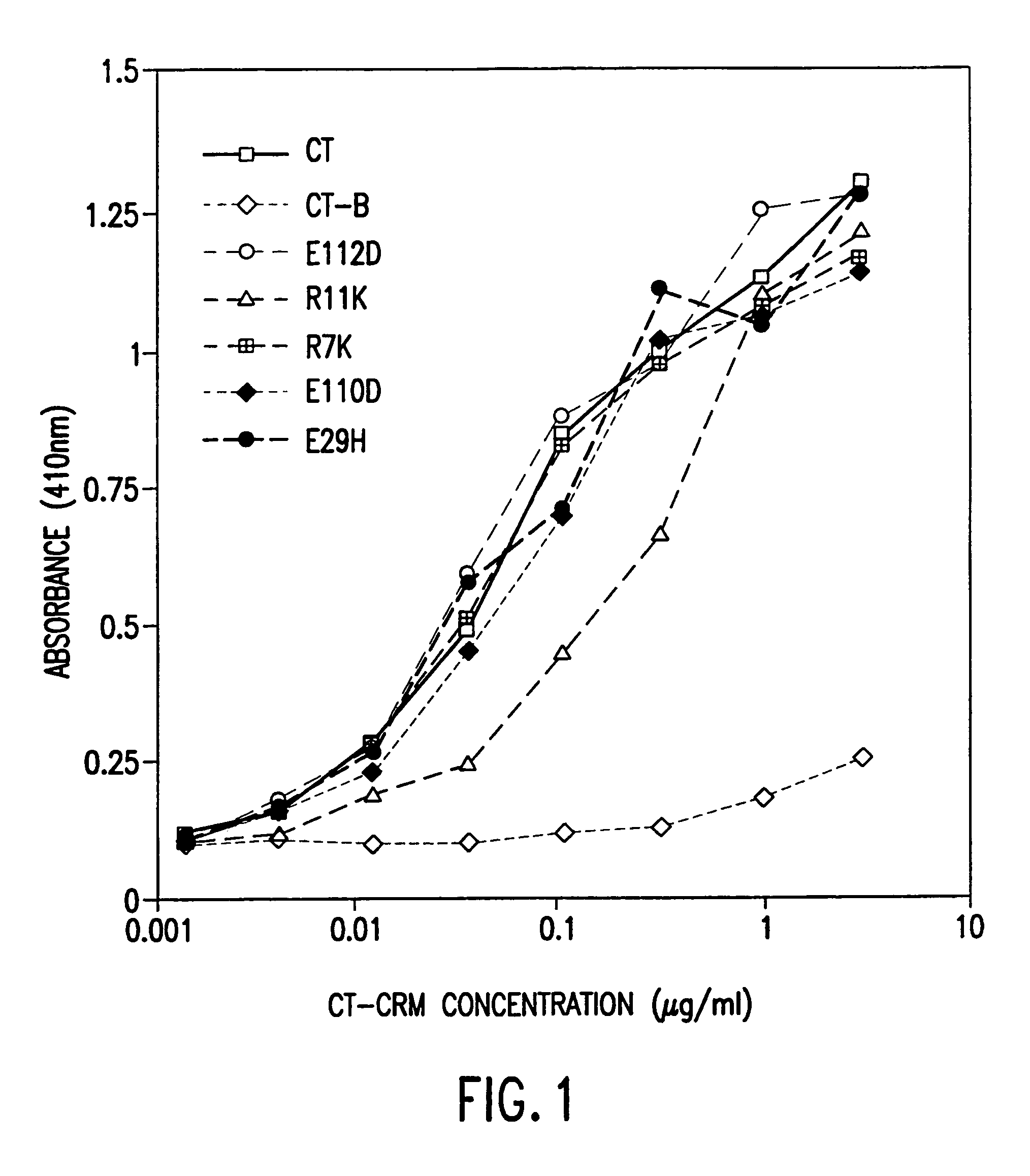
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
Vaccine delivery compositions and methods of use
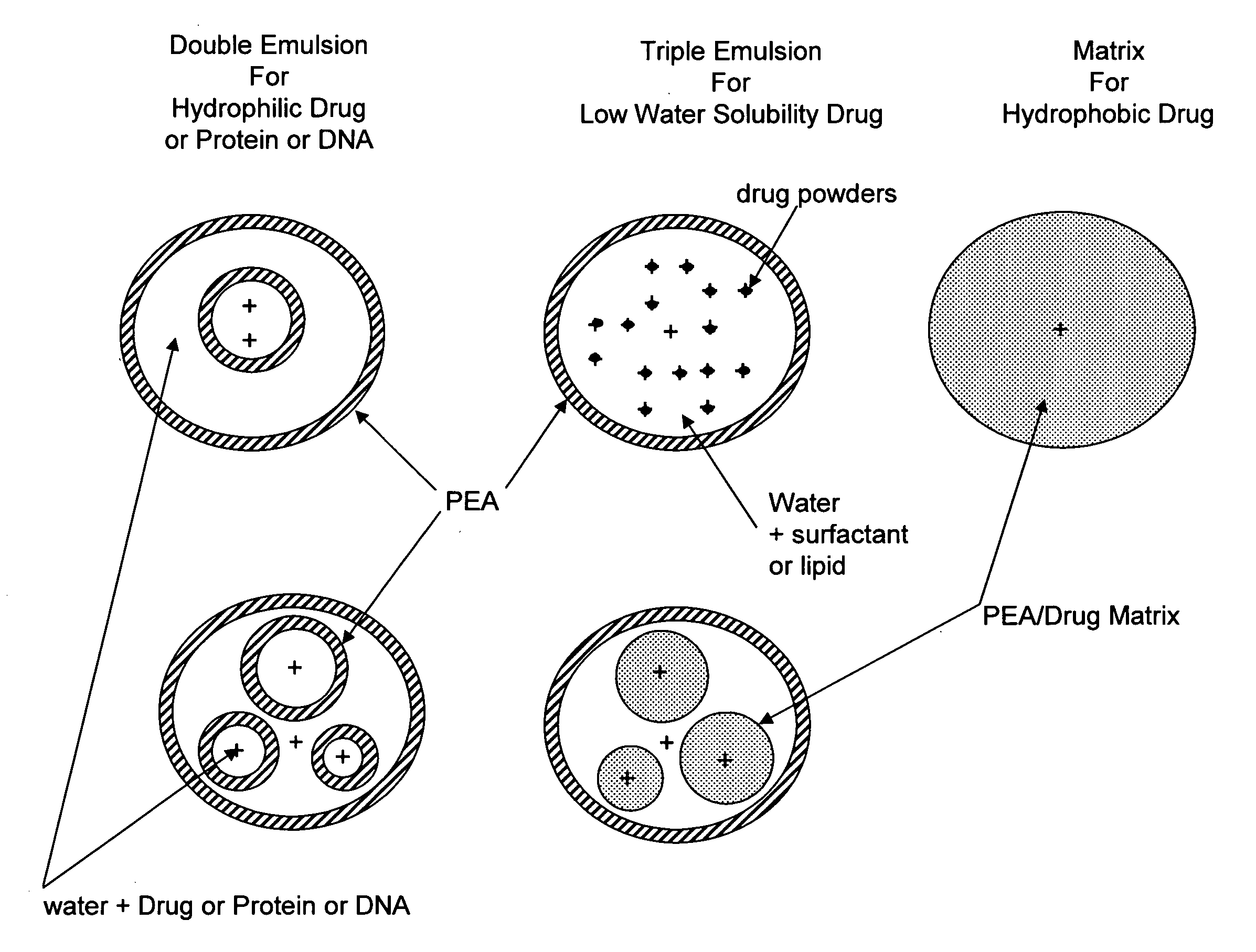
InactiveUS20080160089A1Easy to produceImprove efficiencySsRNA viruses negative-sensePowder deliveryPolyesterMHC class I
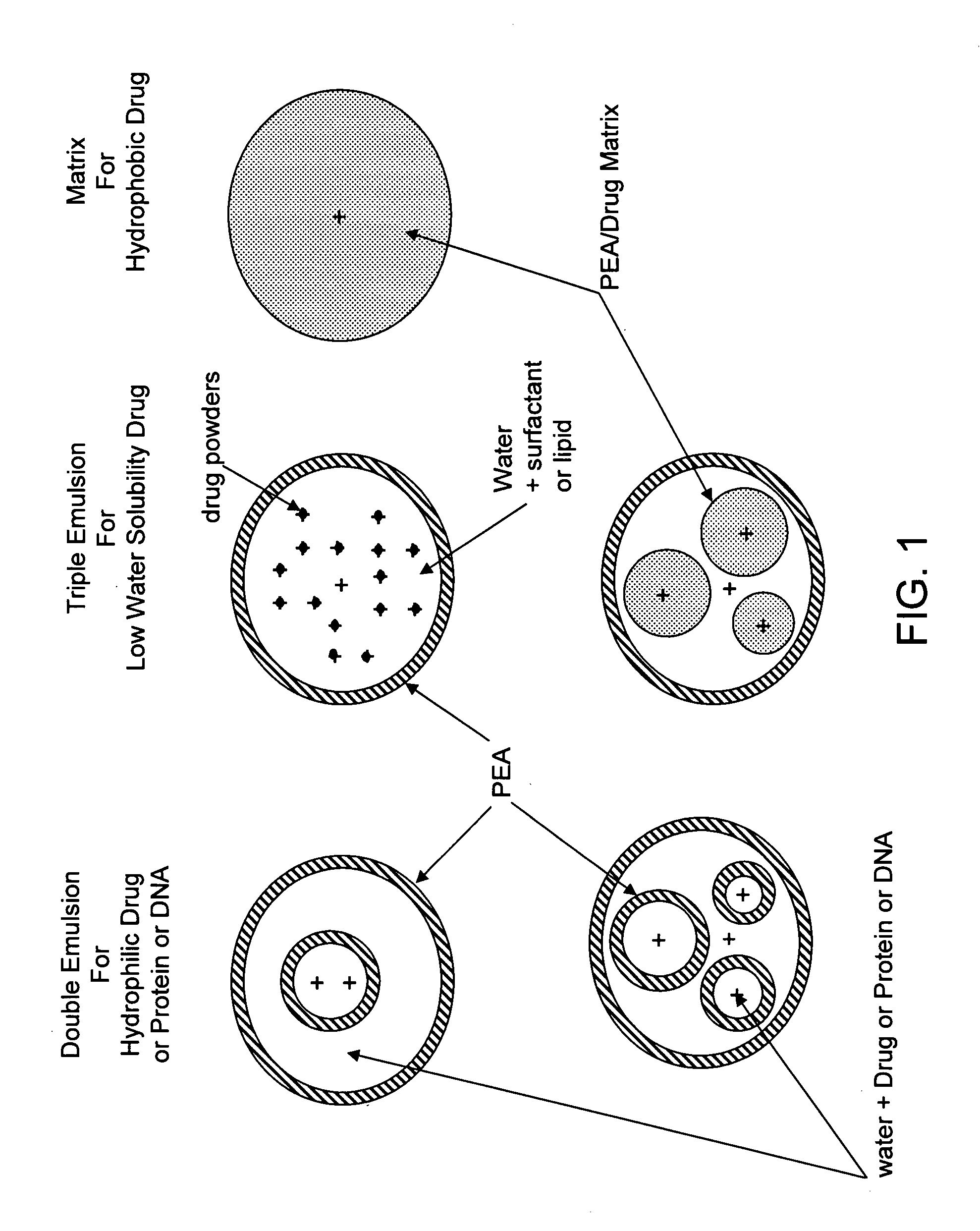
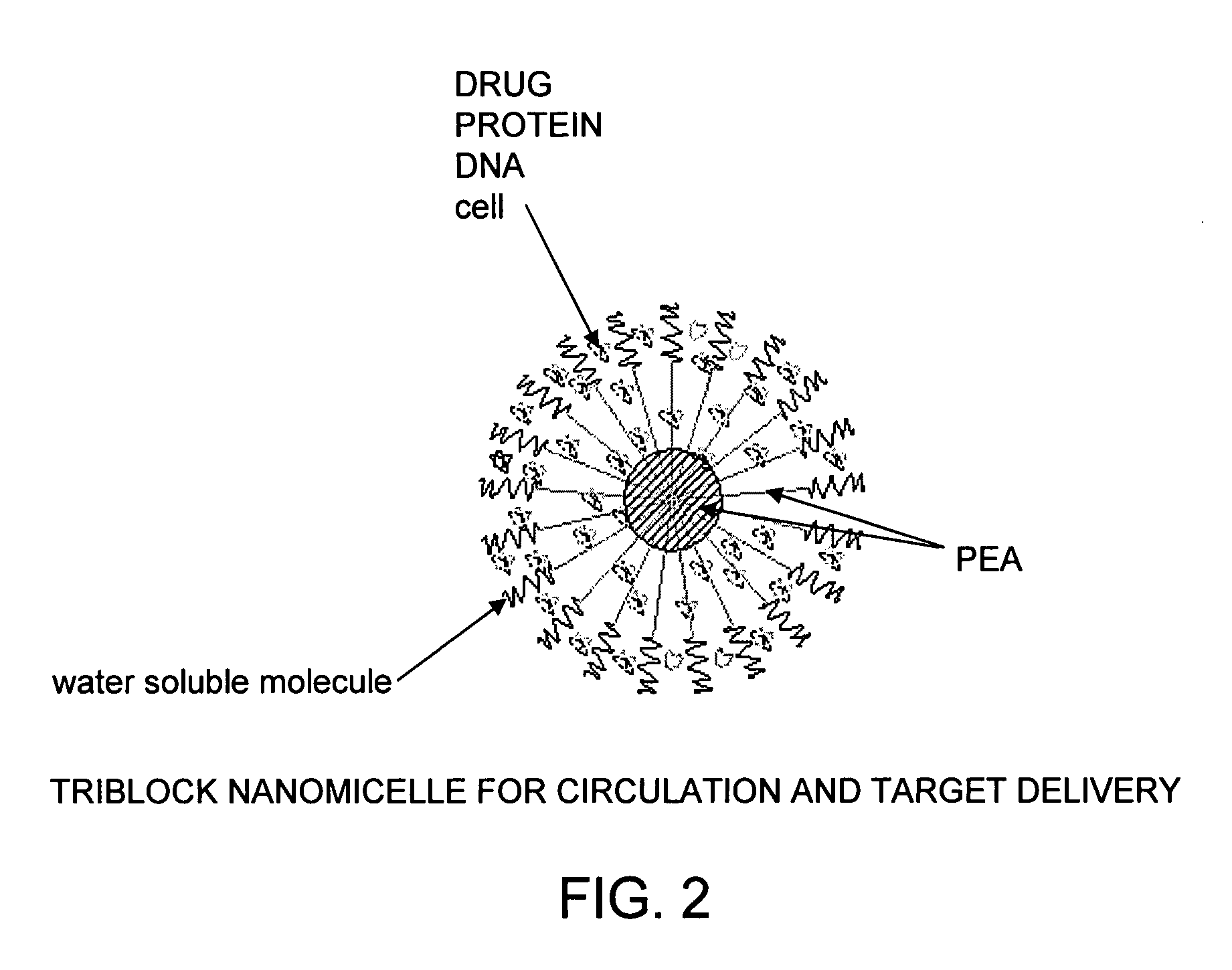
The present invention provides synthetic vaccines against a variety of pathogenic organisms and tumor cells in humans and other mammals based on biodegradable polymers containing polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) and immunostimulatory adjuvants. The vaccines can be formulated as a liquid dispersion of polymer particles or molecules in which are dispersed an immunostimulatory adjuvant, such as a TLR agonist, and whole protein or peptidic antigens containing MHC class I or class II epitopes derived from organism or tumor cell proteins. Methods of inducing an immune response via intracellular mechanisms to the pathogenic organism or tumor cells specific for the antigen in the invention compositions are also included.
Owner:MEDIVAS LLC
Recombinant protein production in a human cell
InactiveUS6855544B1Easy to handleLarge-scale (continuous) productionSsRNA viruses negative-senseSugar derivativesHamsterHuman cell
Methods and compositions for the production of recombinant proteins in a human cell line. The methods and positions are particularly useful for generating stable expression of human recombinant proteins of interest that are modified post-translationally, for example, by glycosylation. Such proteins may have advantageous properties in comparison with their counterparts produced in non-human systems such as Chinese Hamster Ovary cells.
Owner:JANSSEN VACCINES & PREVENTION BV
Multifunctional molecular complexes for gene transfer to cells
A multifunctional molecular complex for the transfer of a nucleic acid composition to a target cell is provided The complex is comprised of A) said nucleic acid composition and B) a transfer moiety comprising 1) one or more cationic polyamines bound to said nucleic acid composition, 2) one or more endosome membrane disrupting components attached to at least one nitrogen of the polyamine and 3) one or more receptor specific binding components.
Owner:WYETH LLC
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
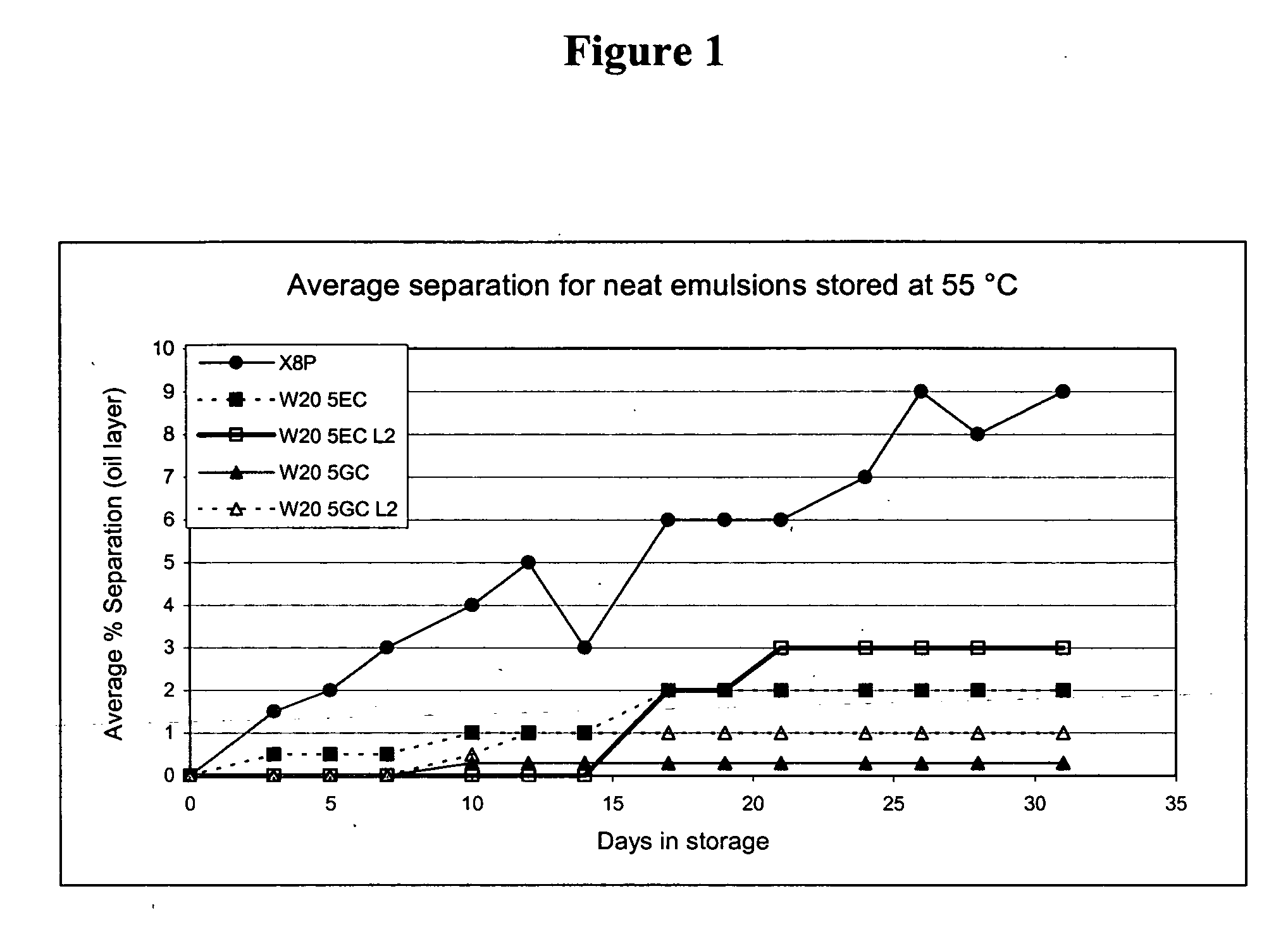
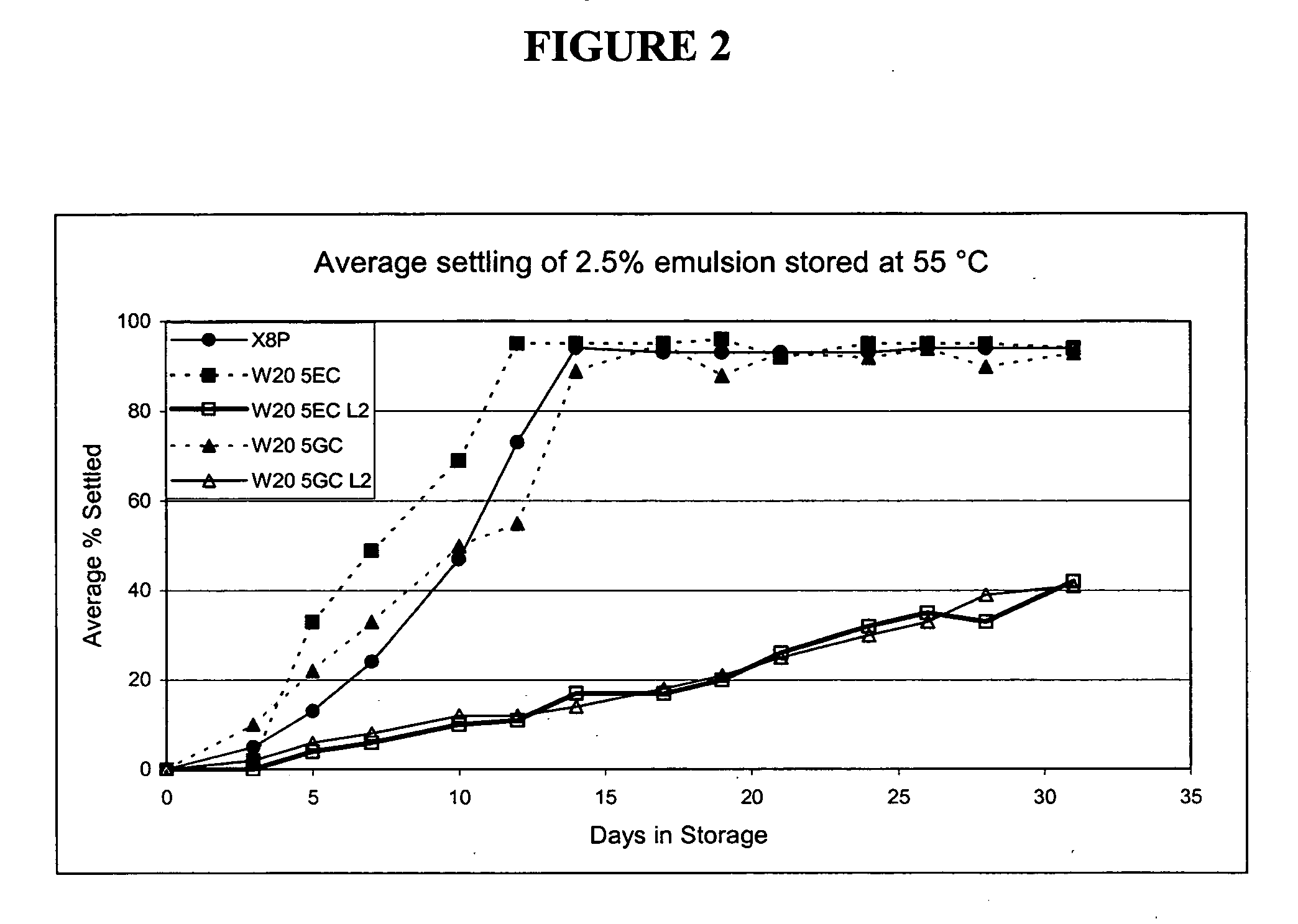
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com