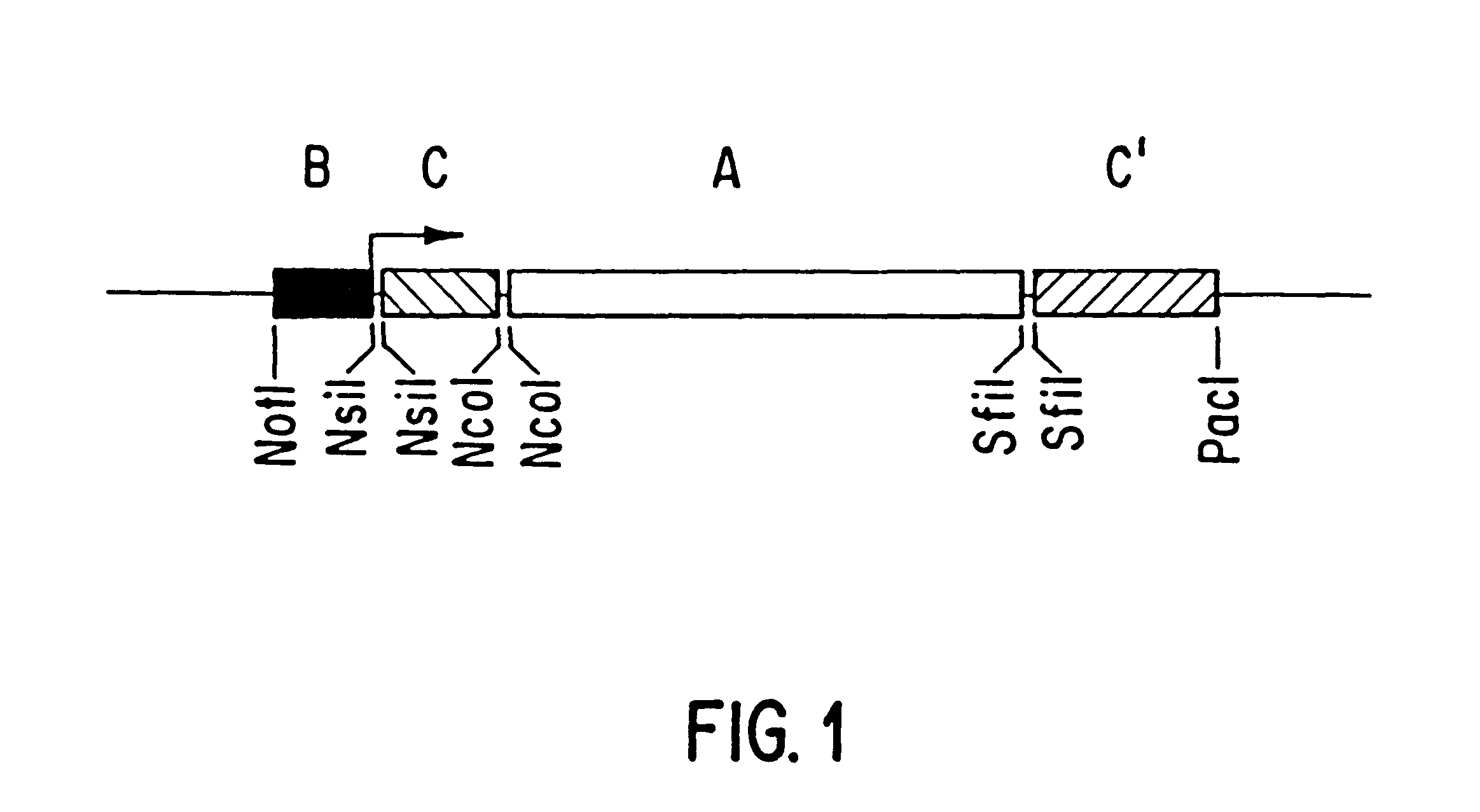
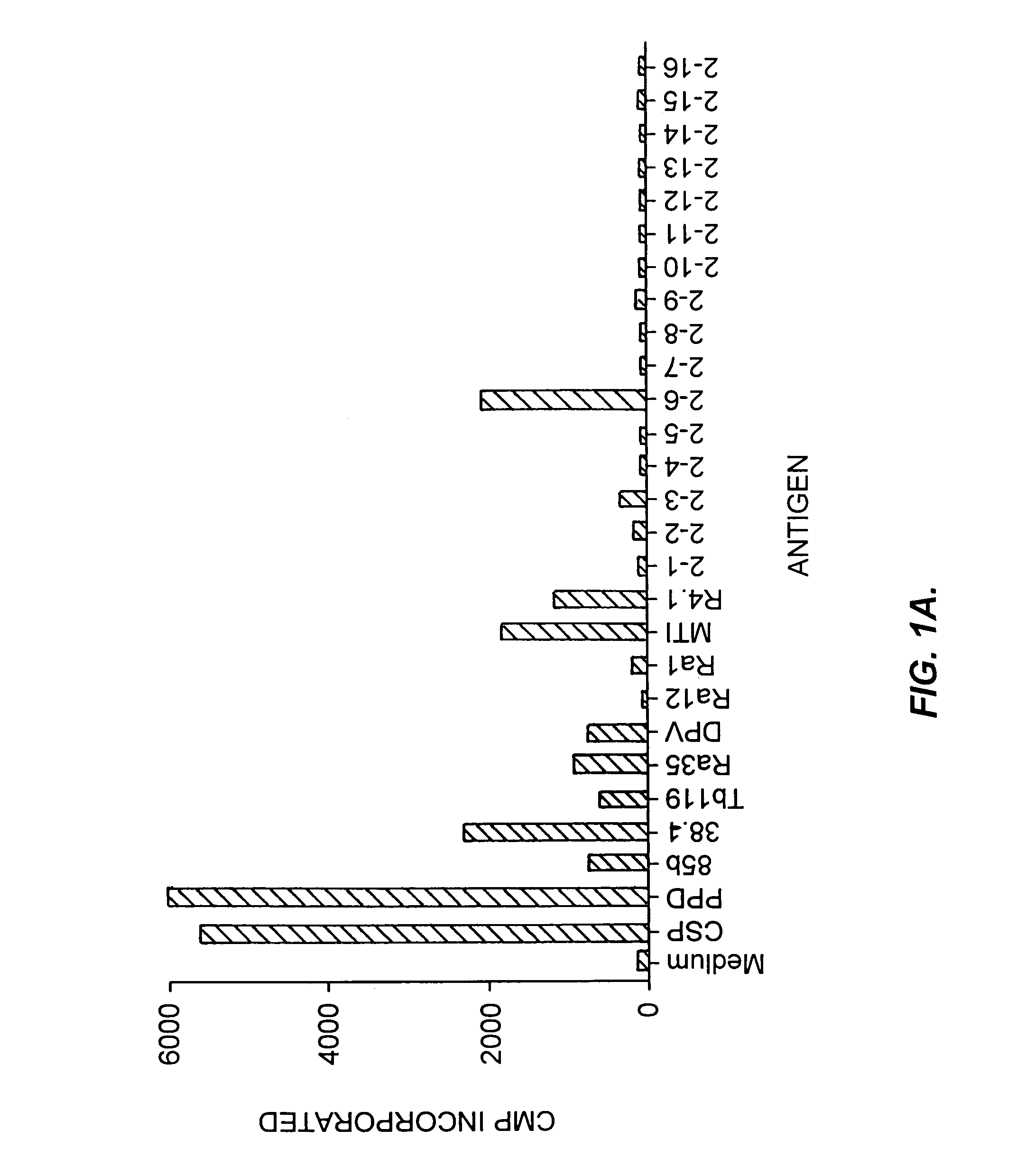
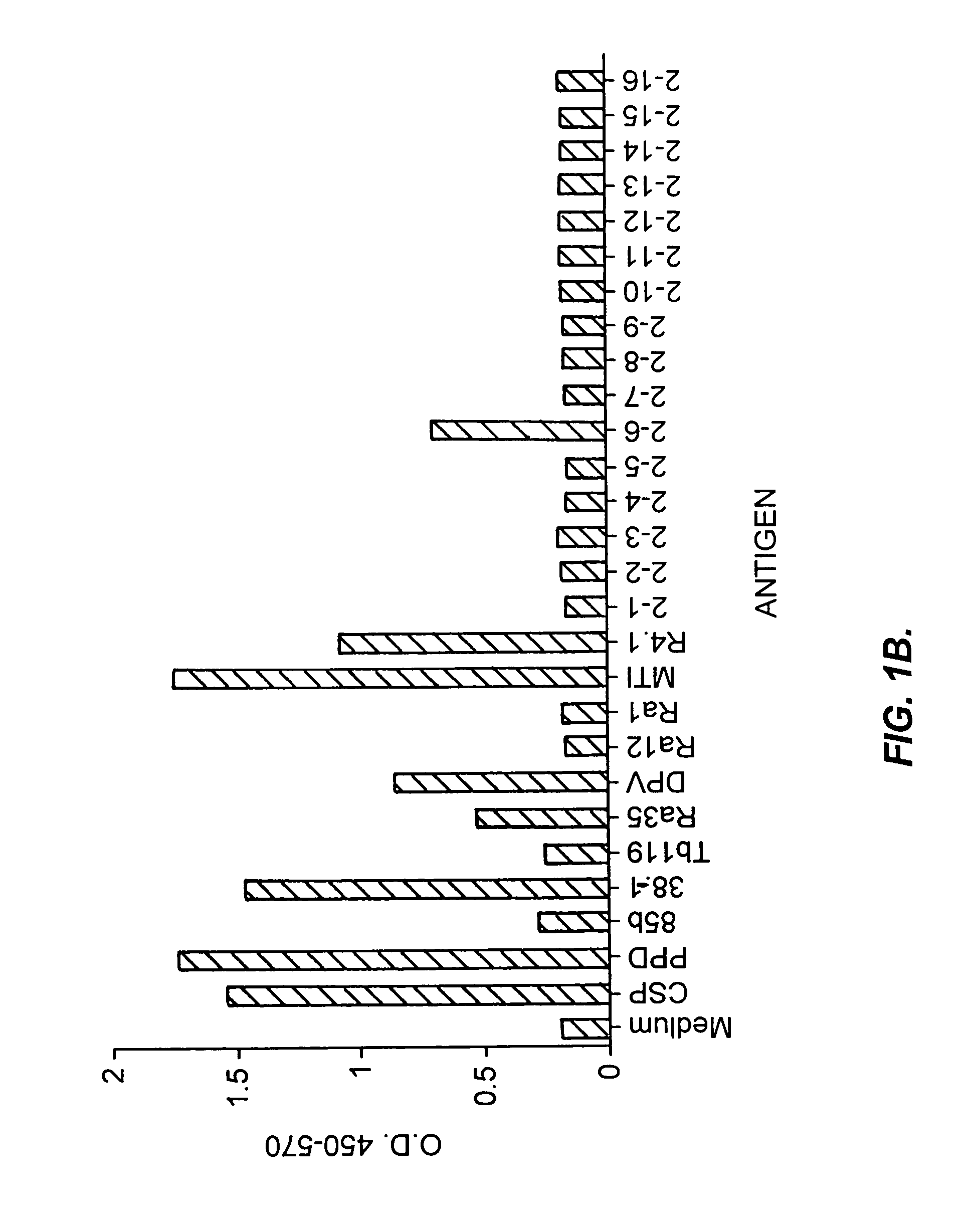
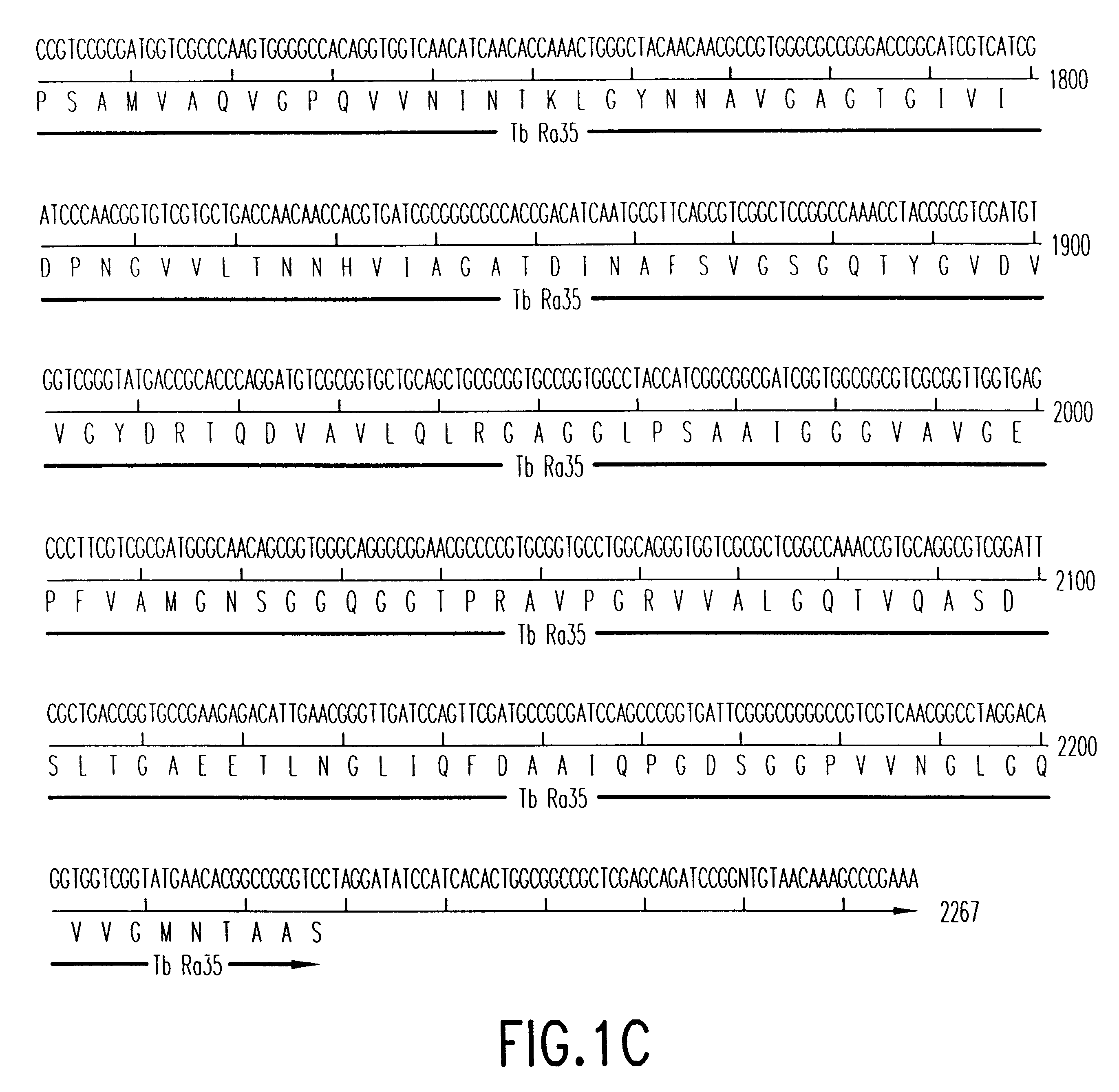
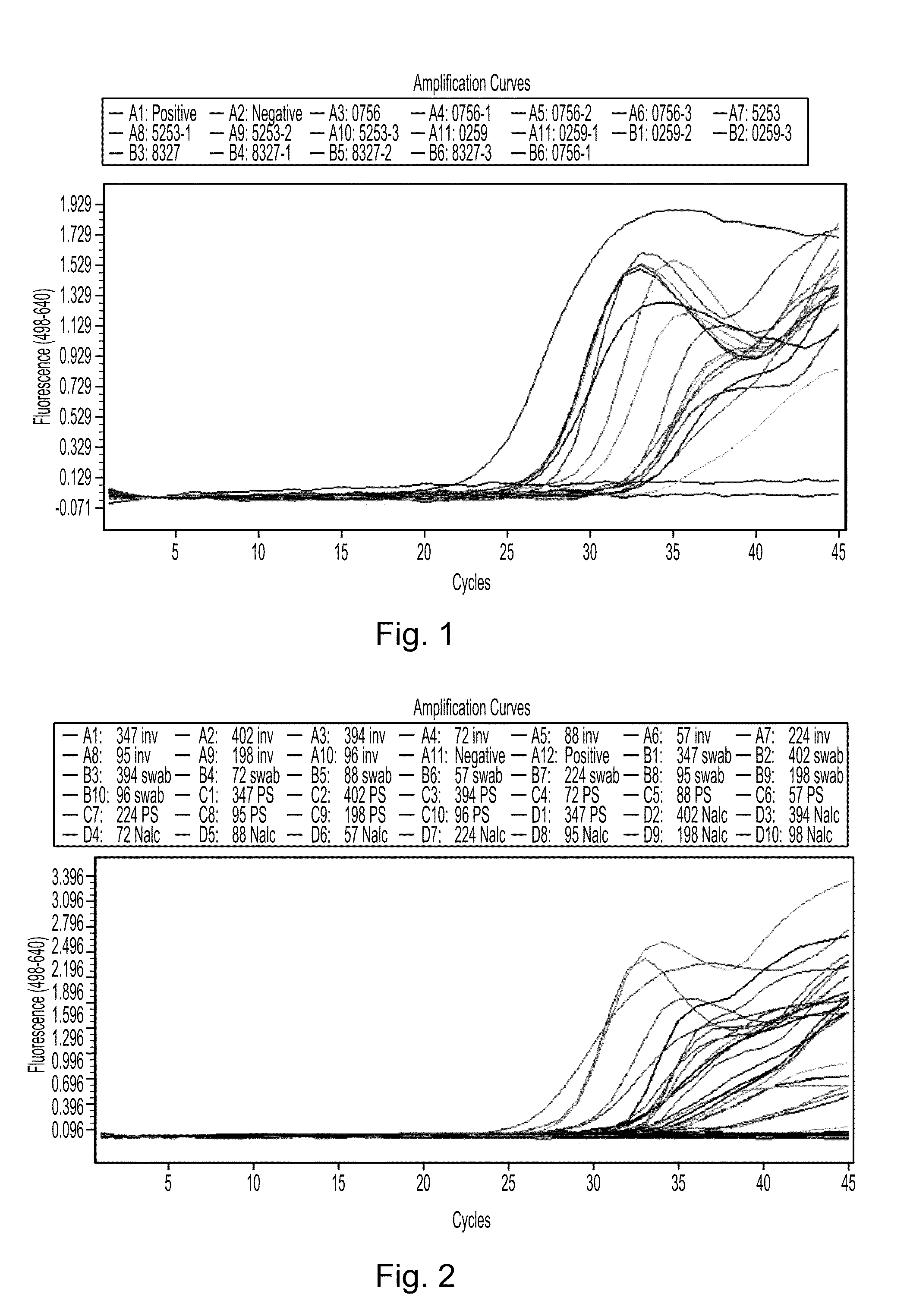
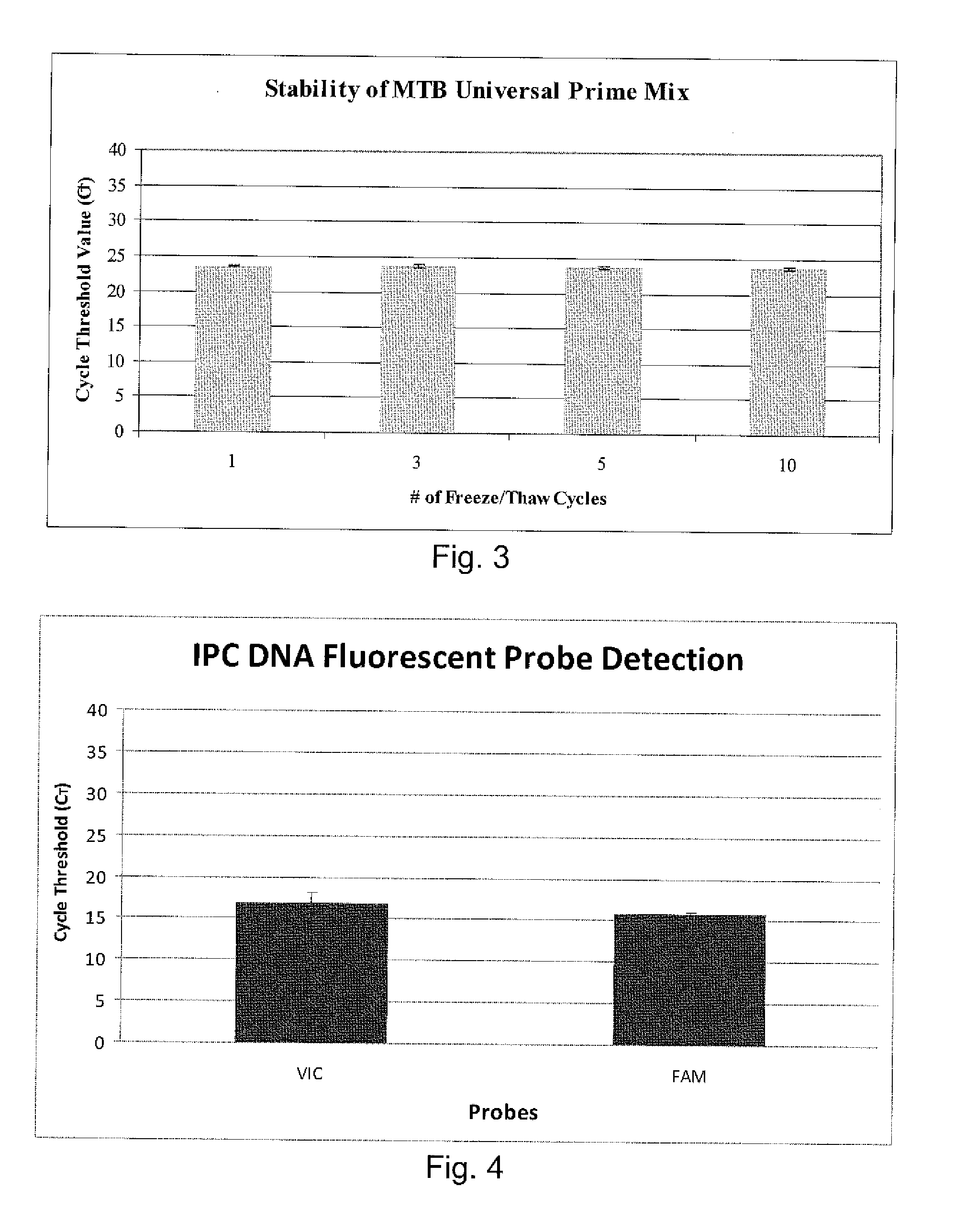
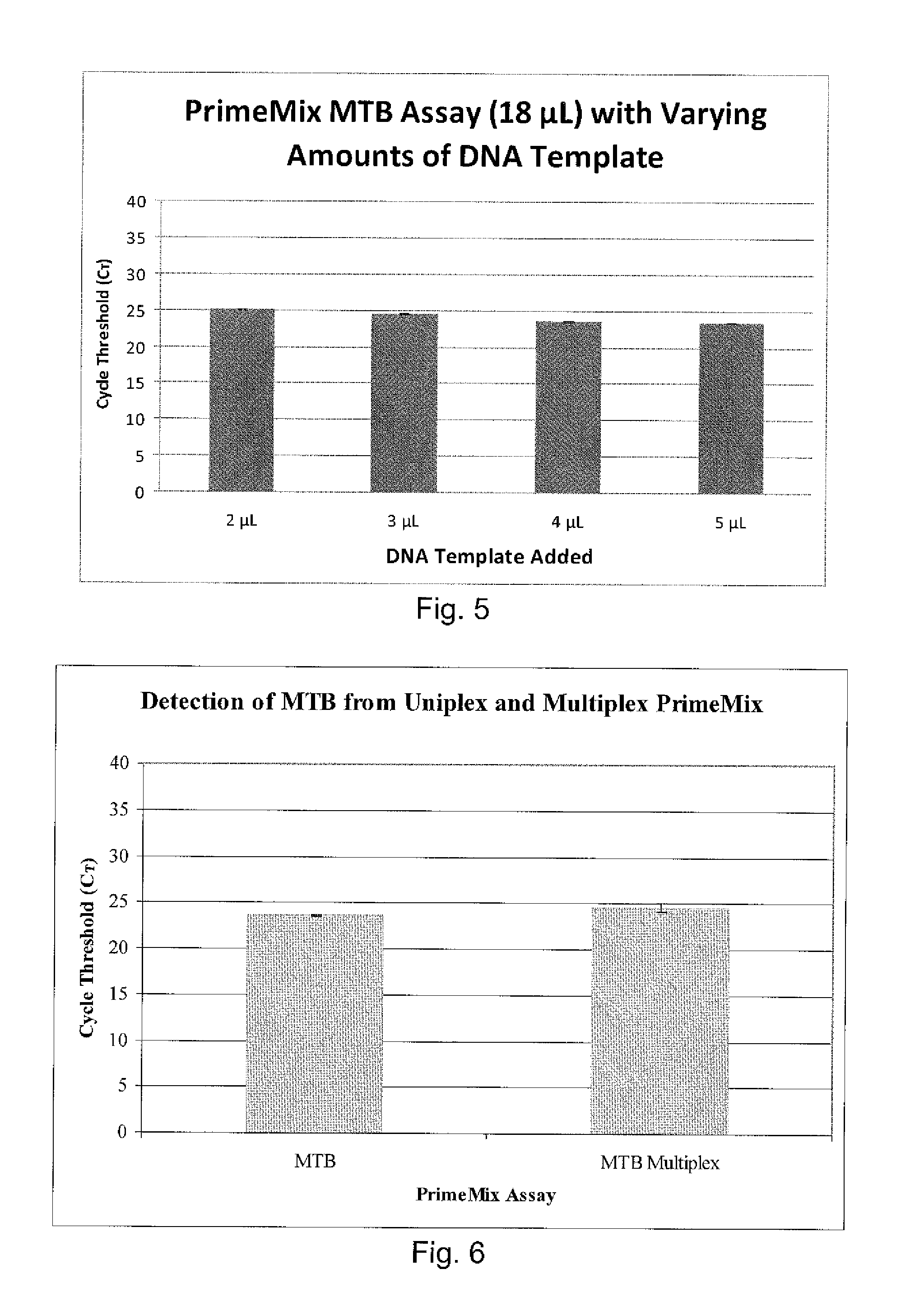
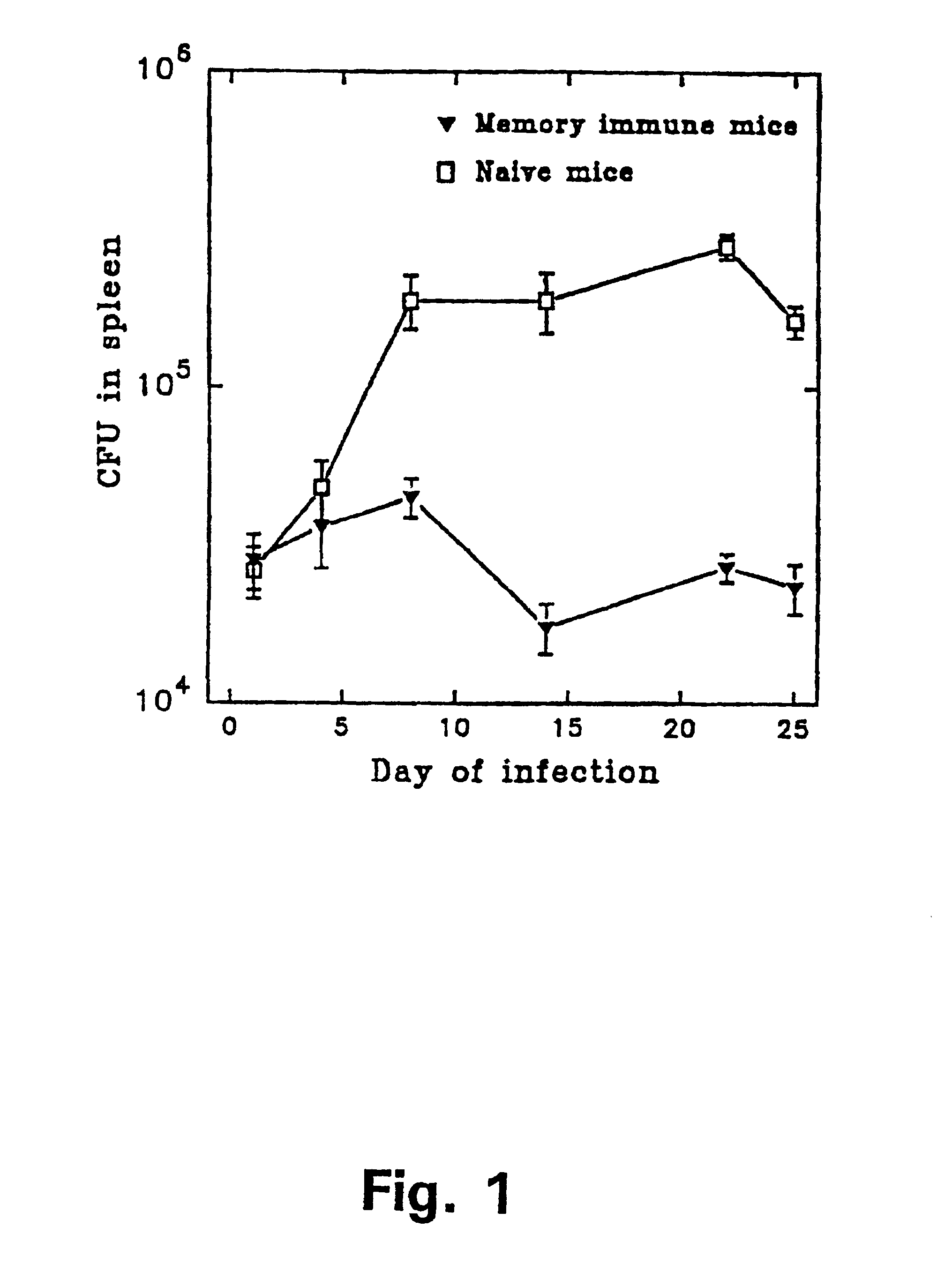
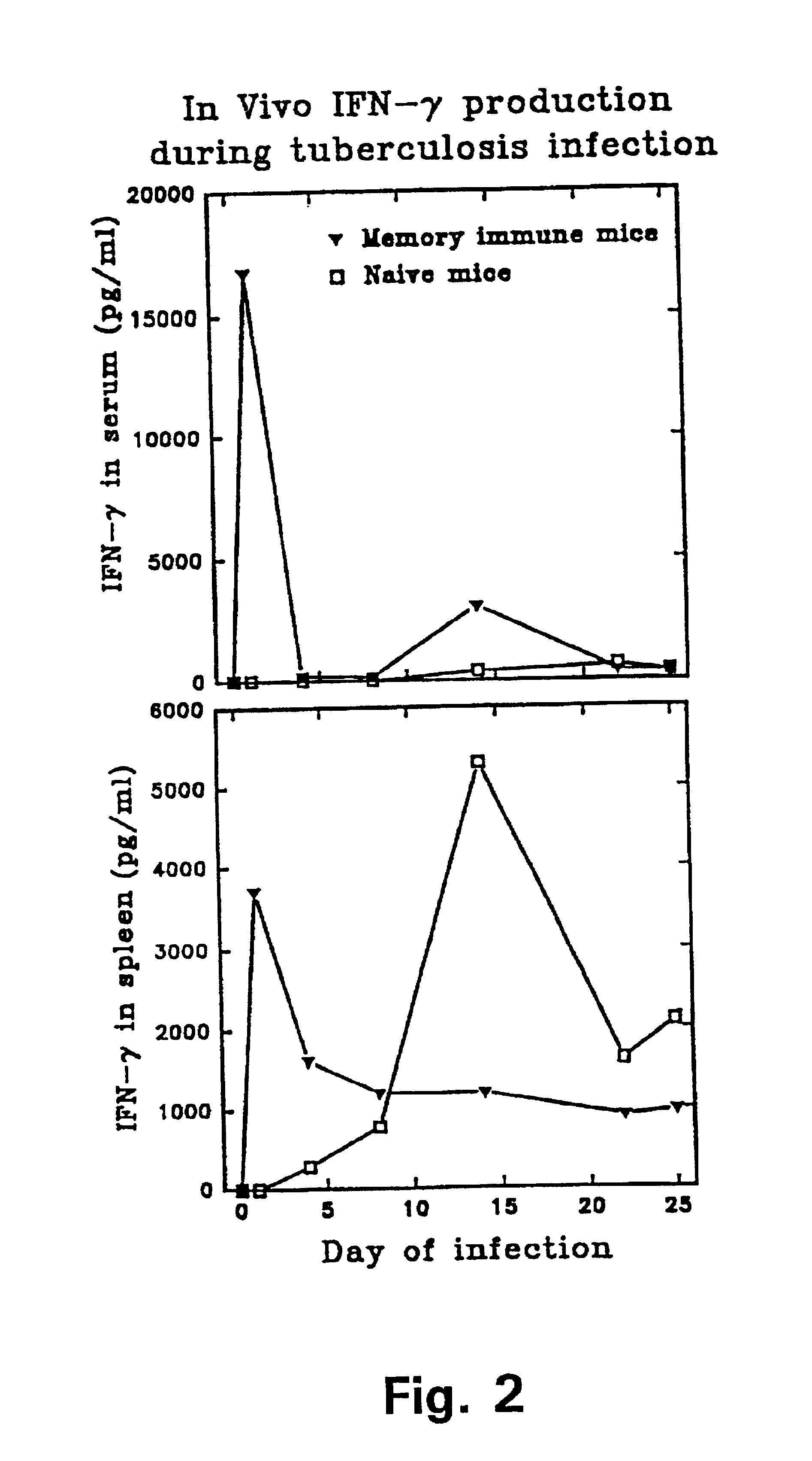
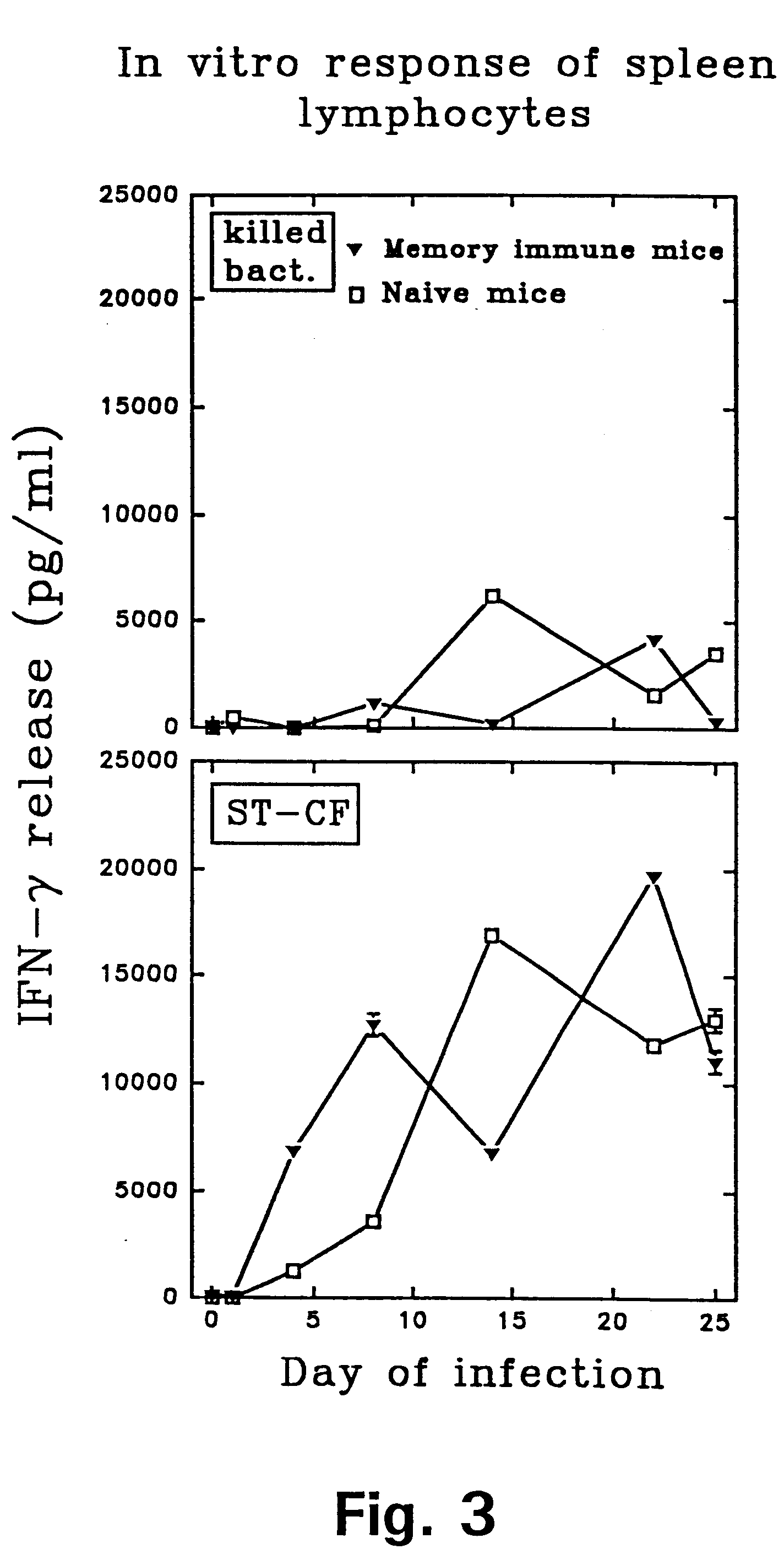
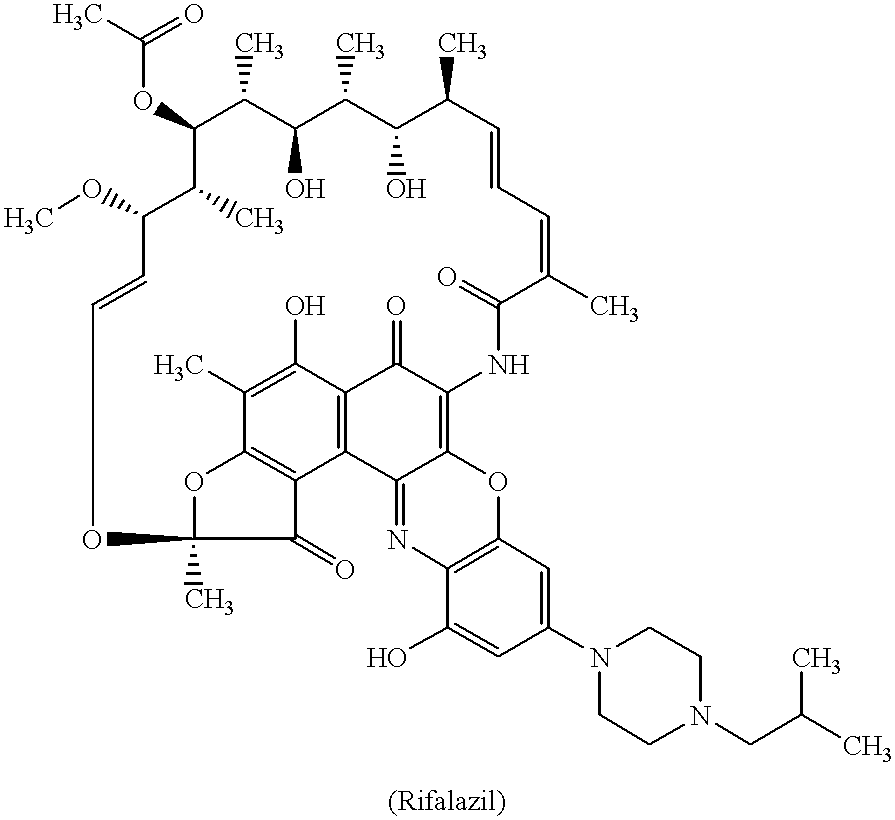
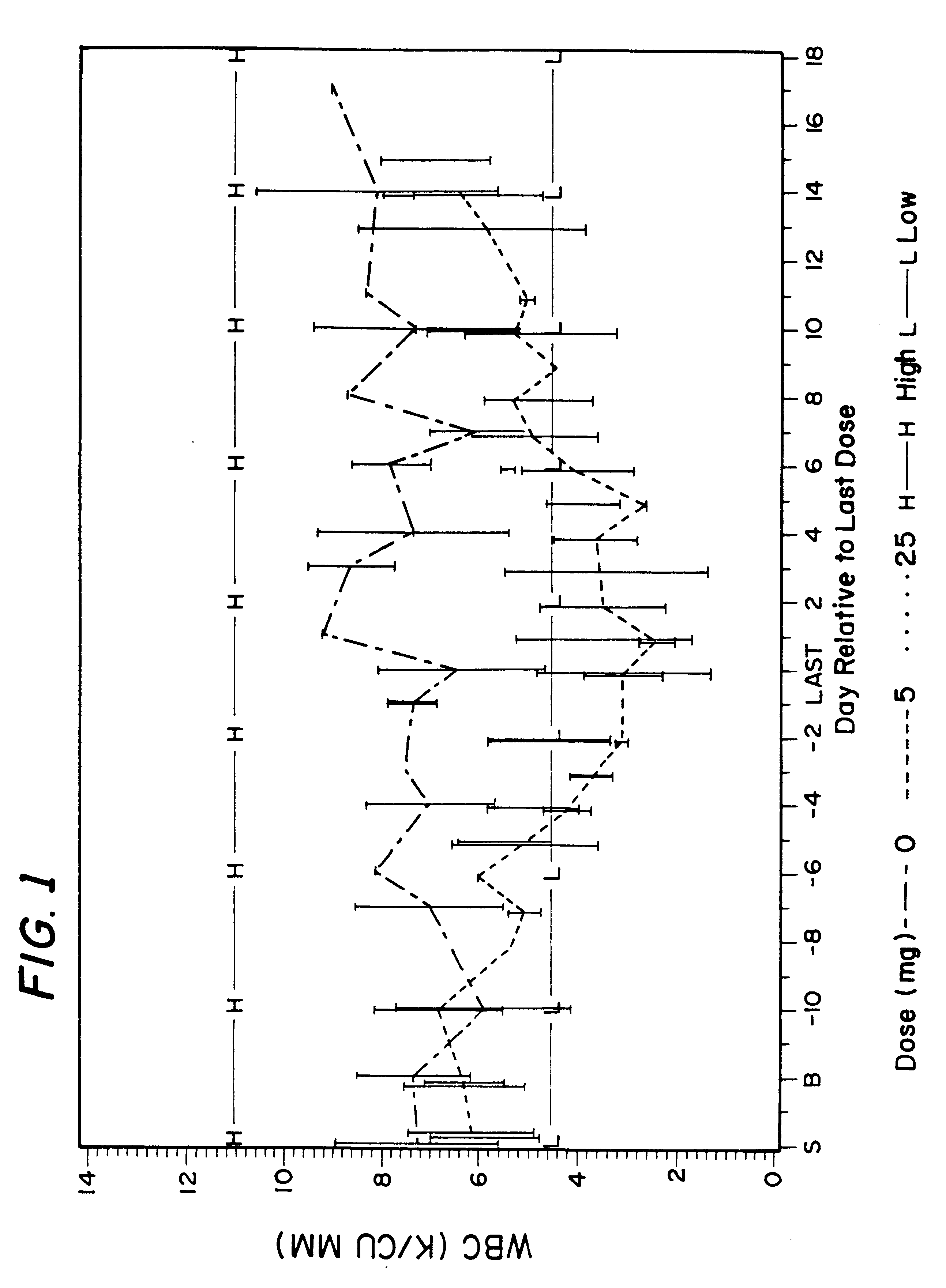
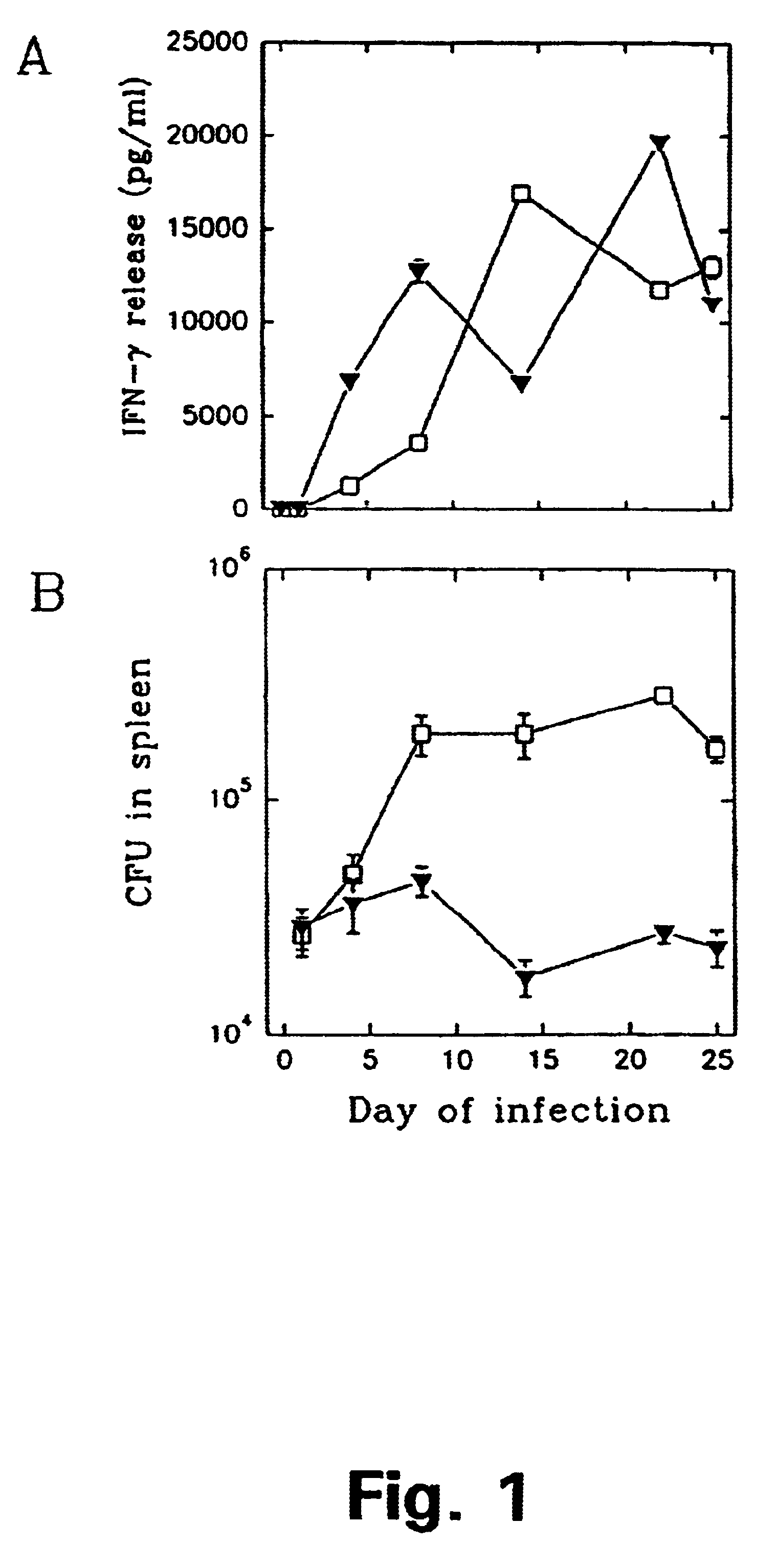
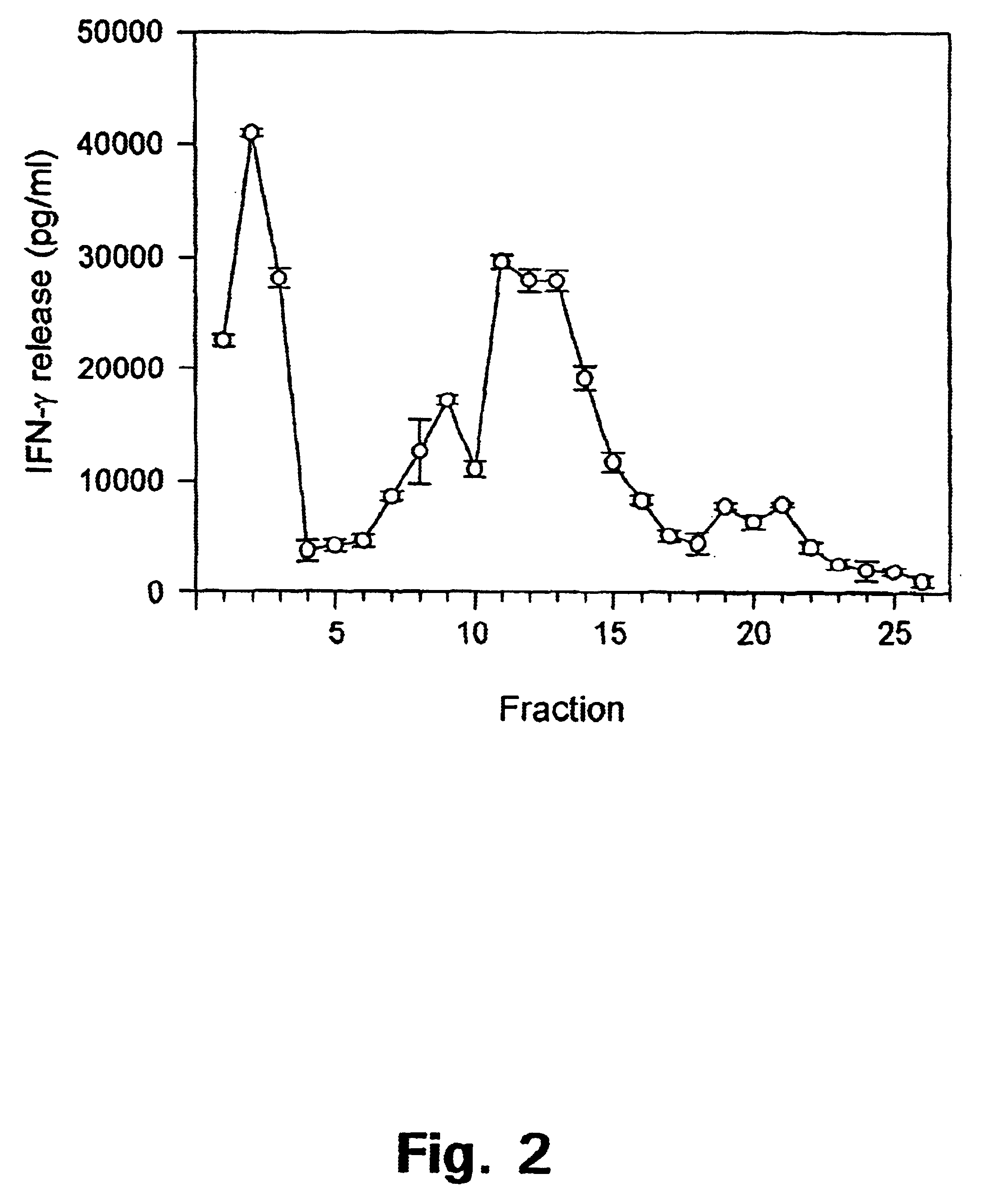
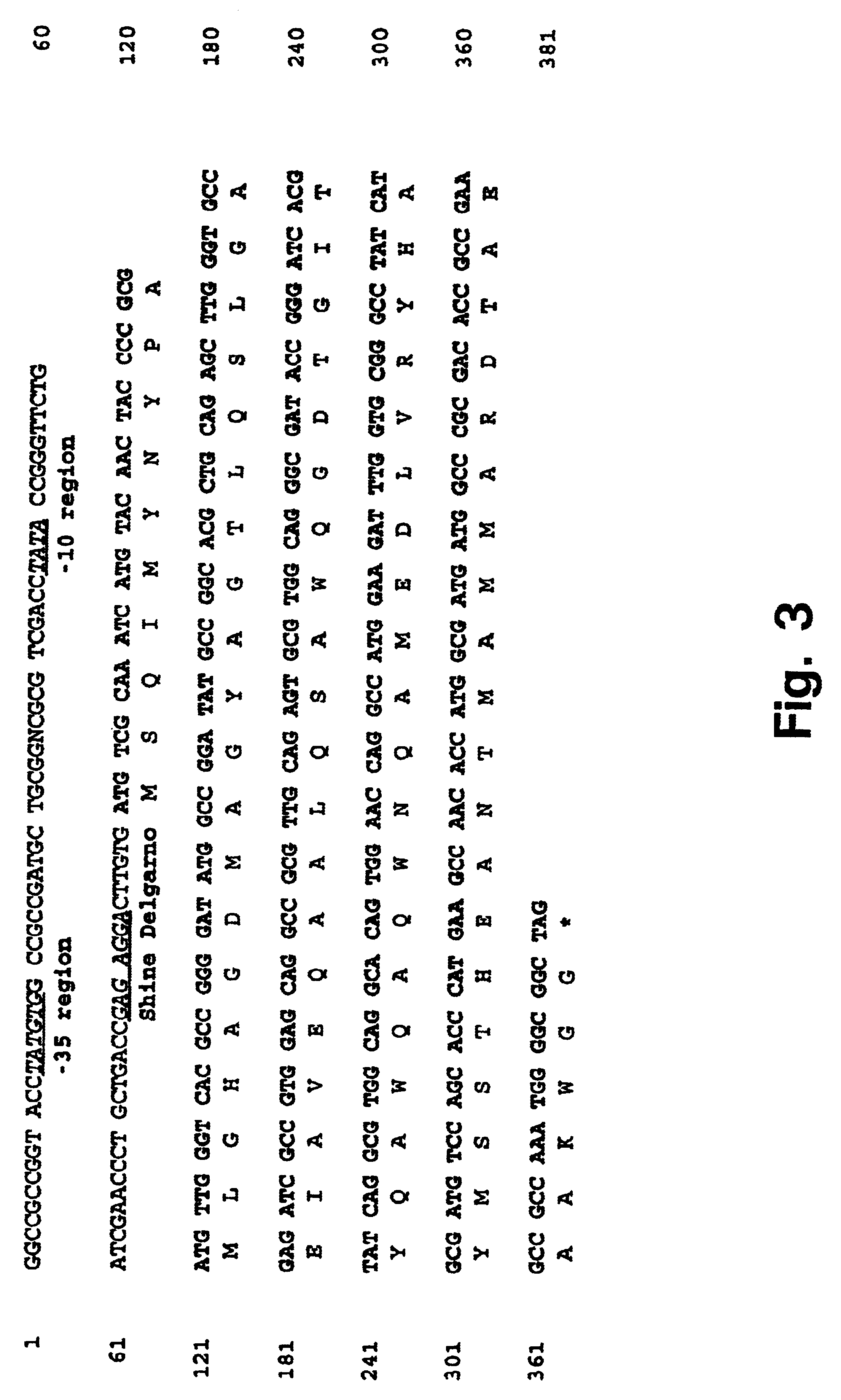
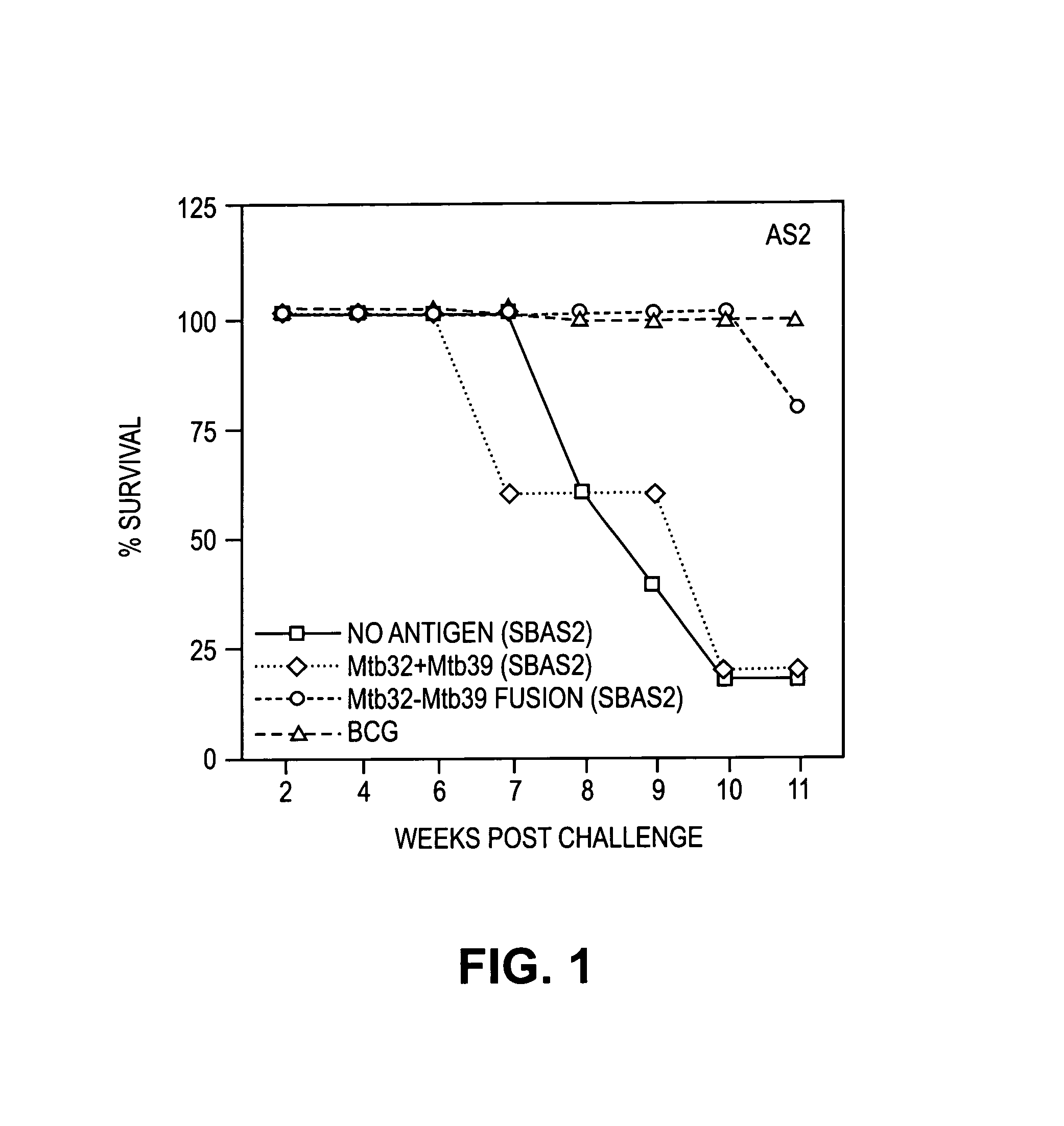
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1972 results about "Mycobacterium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycobacterium is a genus of Actinobacteria, given its own family, the Mycobacteriaceae. Over 190 species are recognized in this genus. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis (Mycobacterium tuberculosis) and leprosy (Mycobacterium leprae) in humans. The Greek prefix myco- means "fungus," alluding to the way mycobacteria have been observed to grow in a mold-like fashion on the surface of cultures. It is acid fast and cannot be stained by the Gram stain procedure.
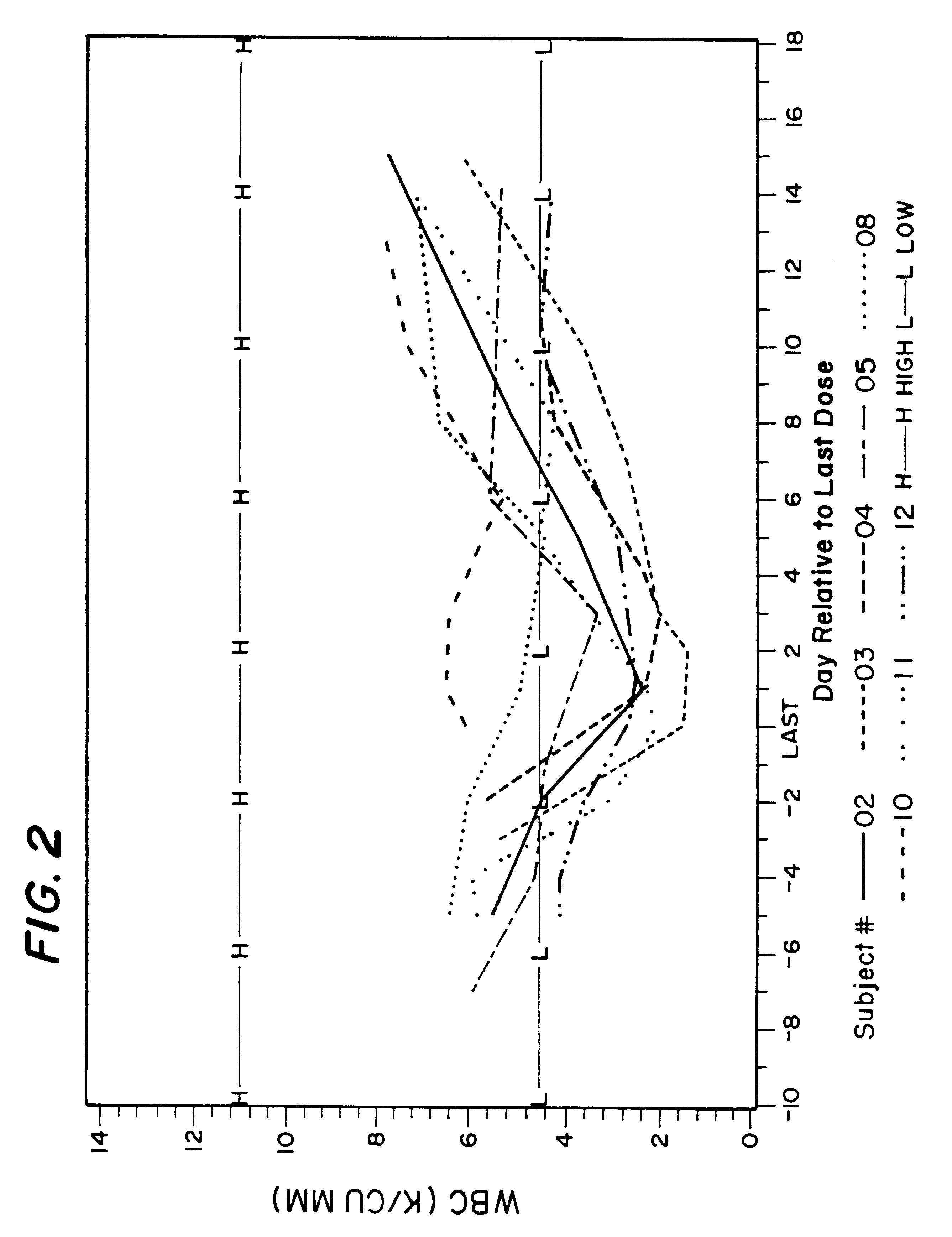
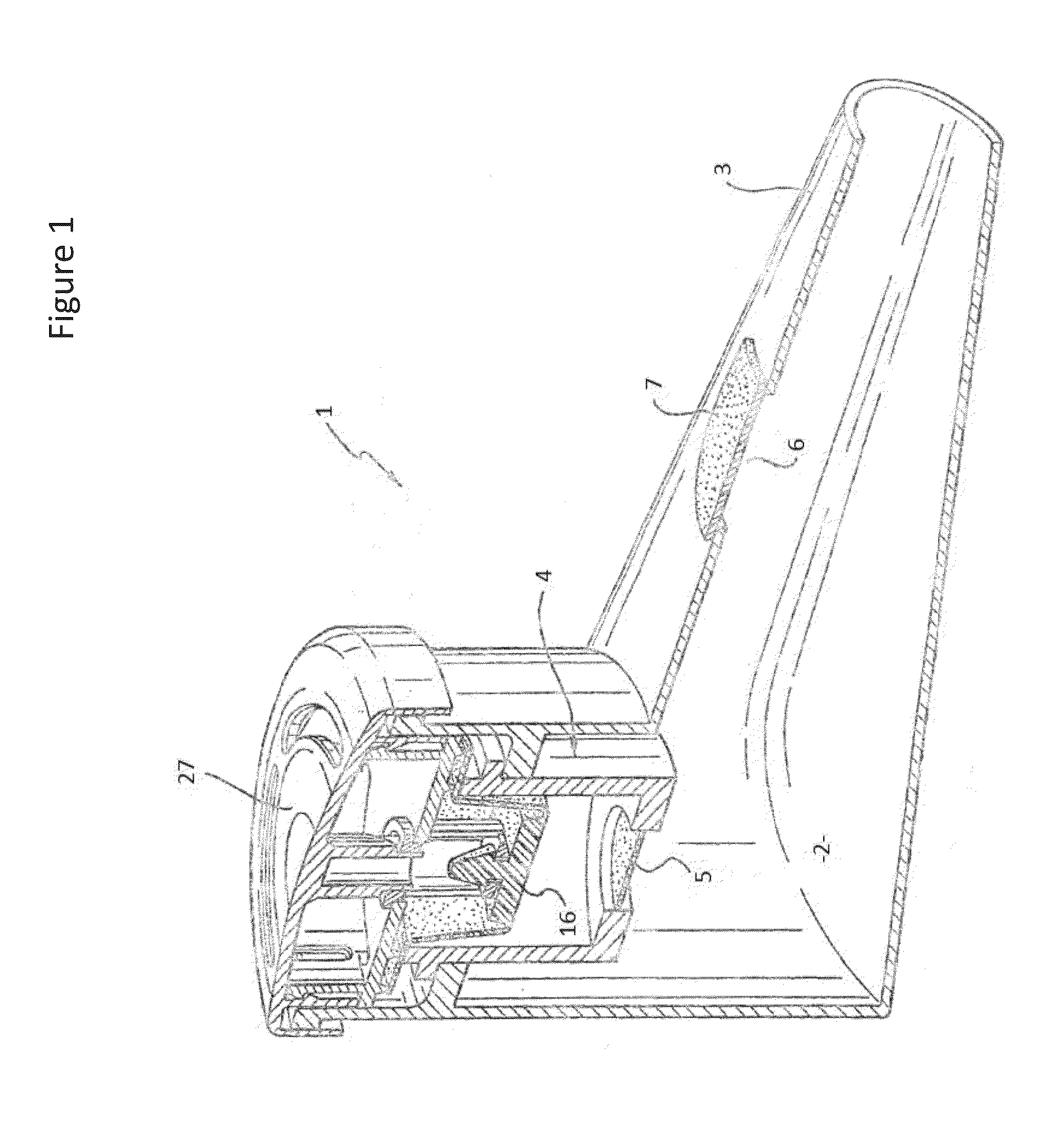
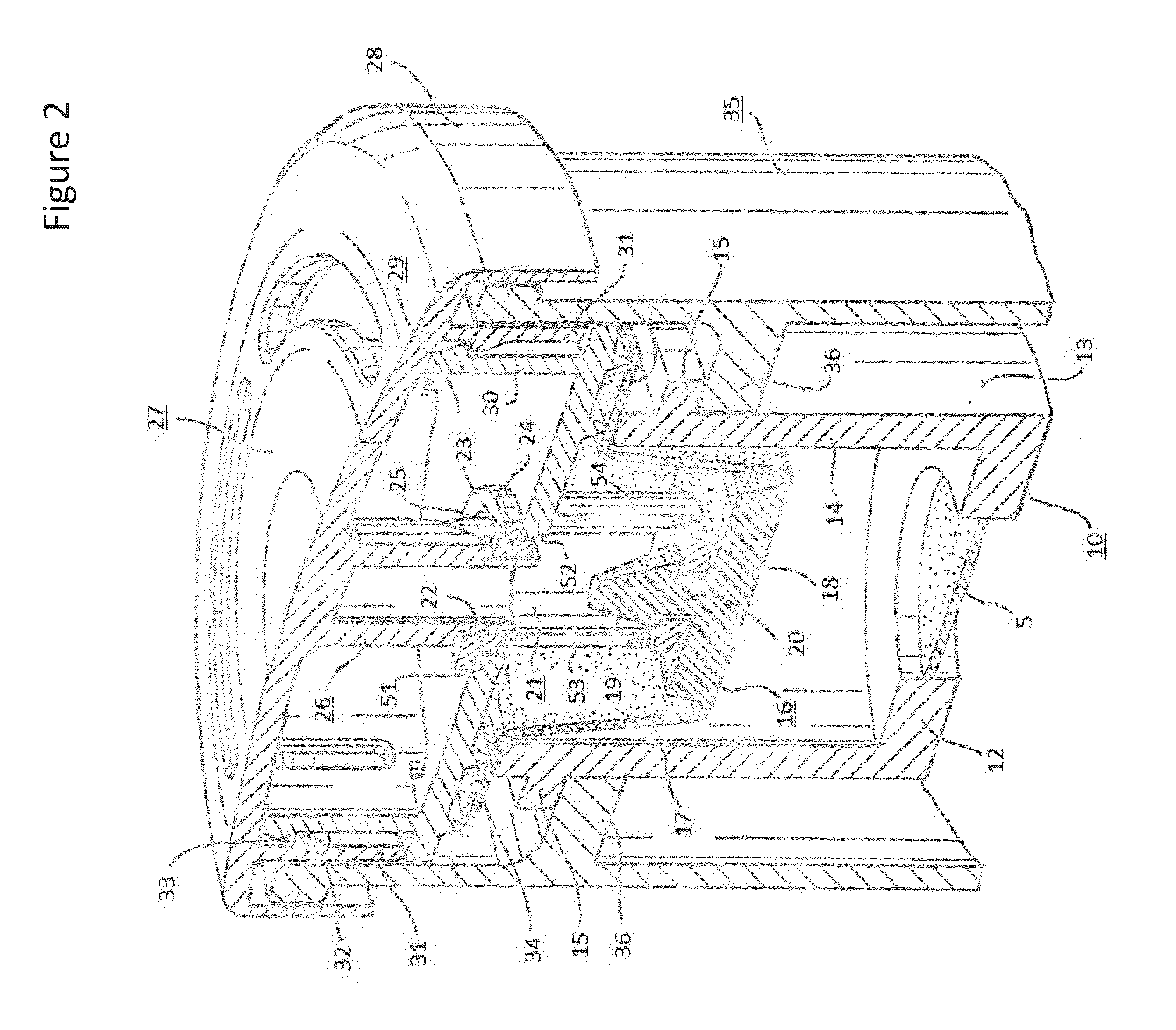
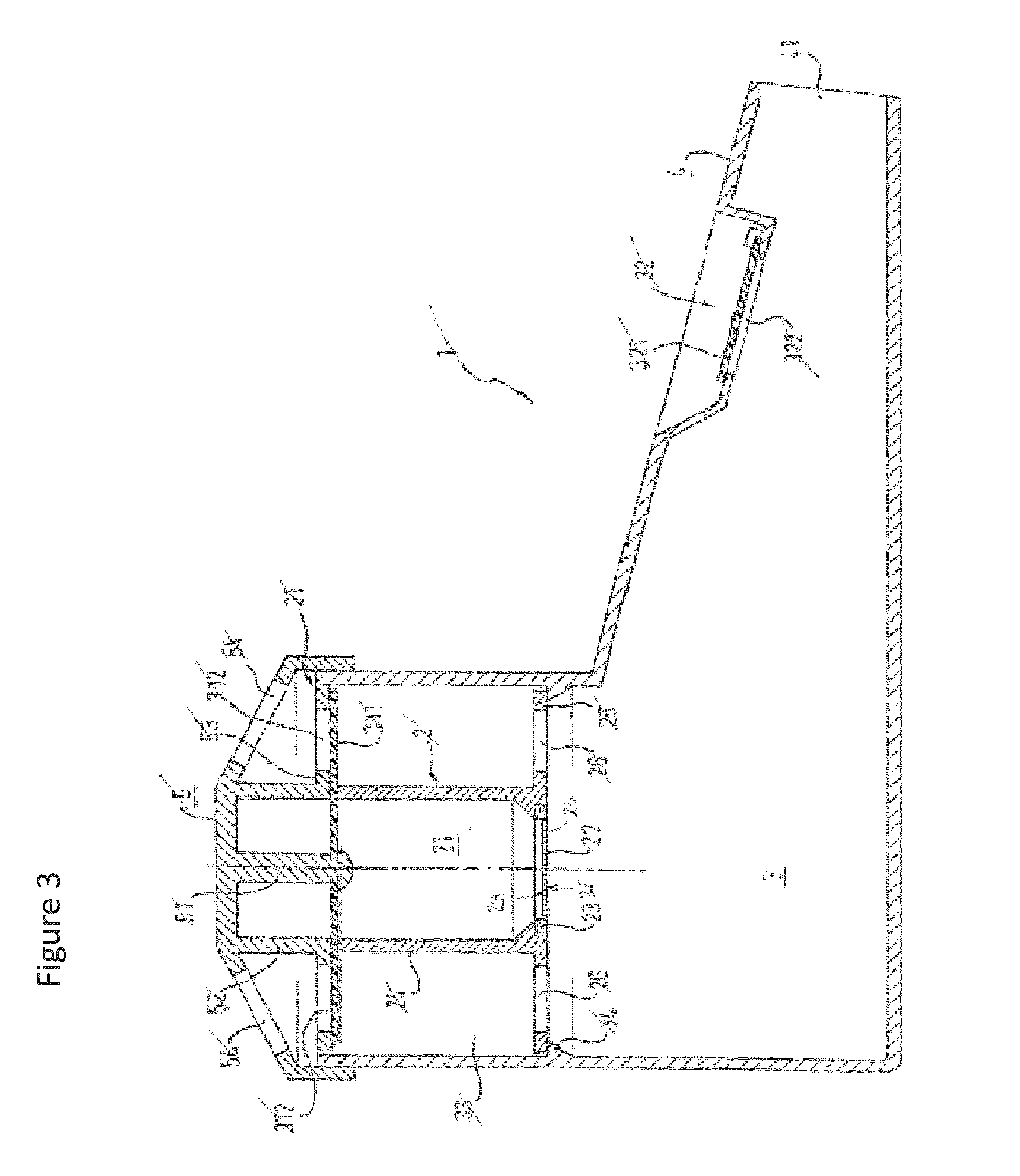
Method and a tobramycin aerosol formulation for treatment prevention and containment of tuberculosis
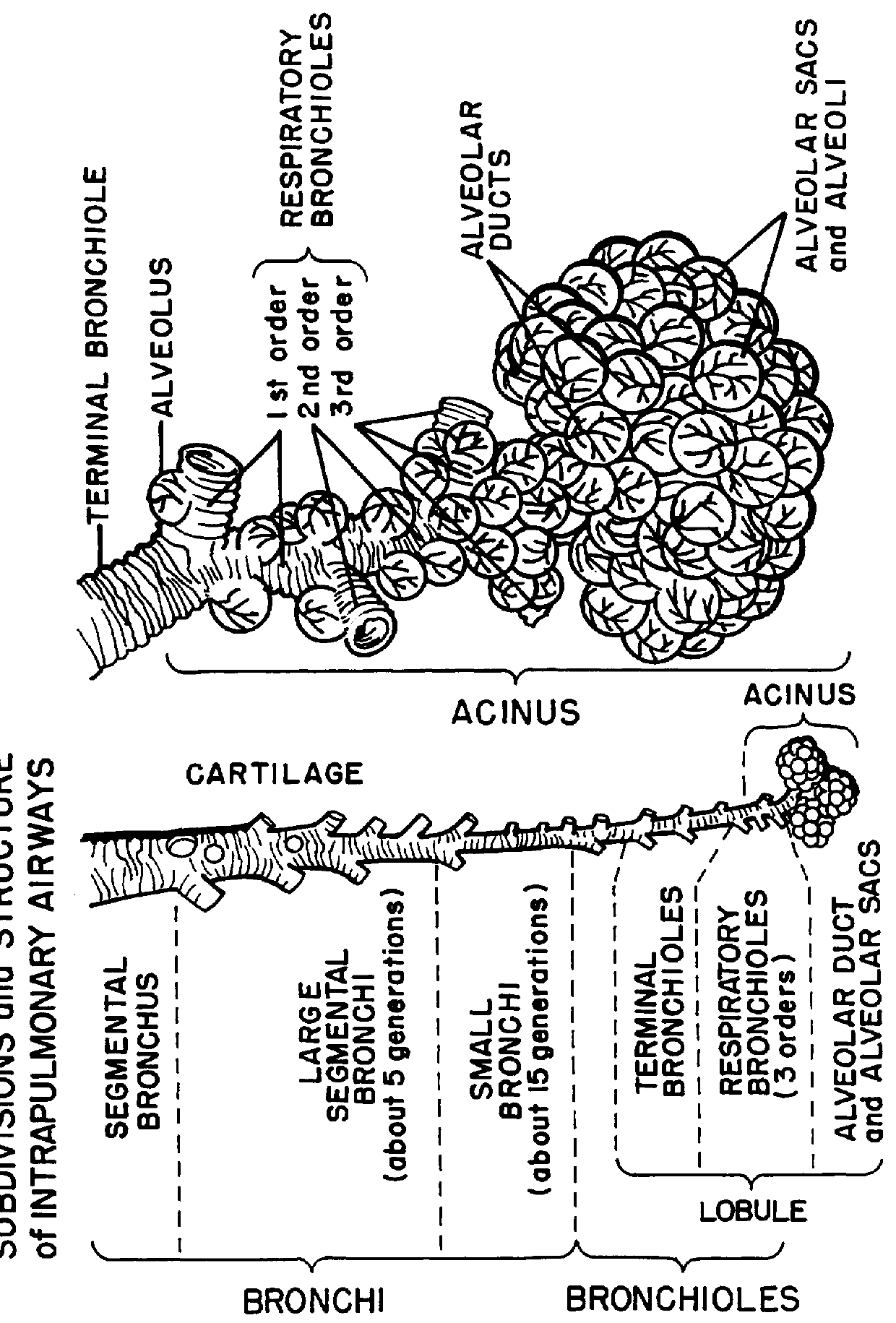
A method for treatment, prevention and containment of acute and chronic tuberculosis using a preservative-free concentrated tobramycin aerosol formulation delivering tobramycin to the lung endobronchial space including alveoli in an aerosol having mass medium average diameter predominantly between 1 to 5 mu . The method comprises administration of tobramycin in concentration one to ten thousand times higher than the minimal inhibitory concentration of Mycobacterium tuberculosis. A method for containment of and decreasing infectivity periods of tuberculosis patients to shorter periods of time.
Owner:CHIRON CORP
Vectors for the diagnosis and treatment of solid tumors including melanoma
The present invention is directed to the isolation and use of super-infective, tumor-specific vectors that are strains of parasites including, but not limited to bacteria, fungi and protists. In certain embodiments the parasites include, but are not limited to, the bacterium Salmonella spp., such as Salmonella typhimurium, the bacterium Mycobacterium avium and the protozoan Leishmania amazonensis. In other embodiments, the present invention is concerned with the isolation of super-infective, tumor-specific, suicide gene-containing strains of parasites for use in treatment of solid tumors.
Owner:YALE UNIV
Vectors for the diagnosis and treatment of solid tumors including melanoma
The present invention is directed to the isolation and use of super-infective, tumor-specific vectors that are strains of parasites including, but not limited to bacteria, fungi and protists. In certain embodiments the parasites include, but are not limited to, the bacterium Salmonella spp., such as Salmonella typhimurium, the bacterium Mycobacterium avium and the protozoan Leishmania amazonensis. In other embodiments, the present invention is concerned with the isolation of super-infective, tumor-specific, suicide gene-containing strains of parasites for use in treatment of solid tumors.
Owner:YALE UNIV
Compounds and methods for diagnosis and immunotherapy of tuberculosis
InactiveUS7087713B2Preventing and diagnosing tuberculosisAntibacterial agentsBacteriaProtective immunityImmunotherapy
Compounds and methods for diagnosing tuberculosis or for inducing protective immunity against tuberculosis are disclosed. The compounds provided include polypeptides that contain at least one immunogenic portion of one or more Mycobacterium proteins and DNA molecules encoding such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Mycobacterium infection in patients and biological samples. Antibodies directed against such polypeptides are also provided. In addition, such compounds may be formulated into vaccines and / or pharmaceutical compositions for immunization against Mycobacterium infection.
Owner:CORIXA CORP
One-tube nested PCR for detecting Mycobacterium tuberculosis
The present invention relates to a kit and method for detecting M. tuberculosis of suspected patient. The present invention also relates to primers and probe used to detect M. tuberculosis by performing one tube nested PCR.
Owner:ASIAGEN CORP
Fusion proteins of Mycobacterium tuberculosis antigens and their uses
InactiveUS6627198B2Improving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenMycobacterial antigen
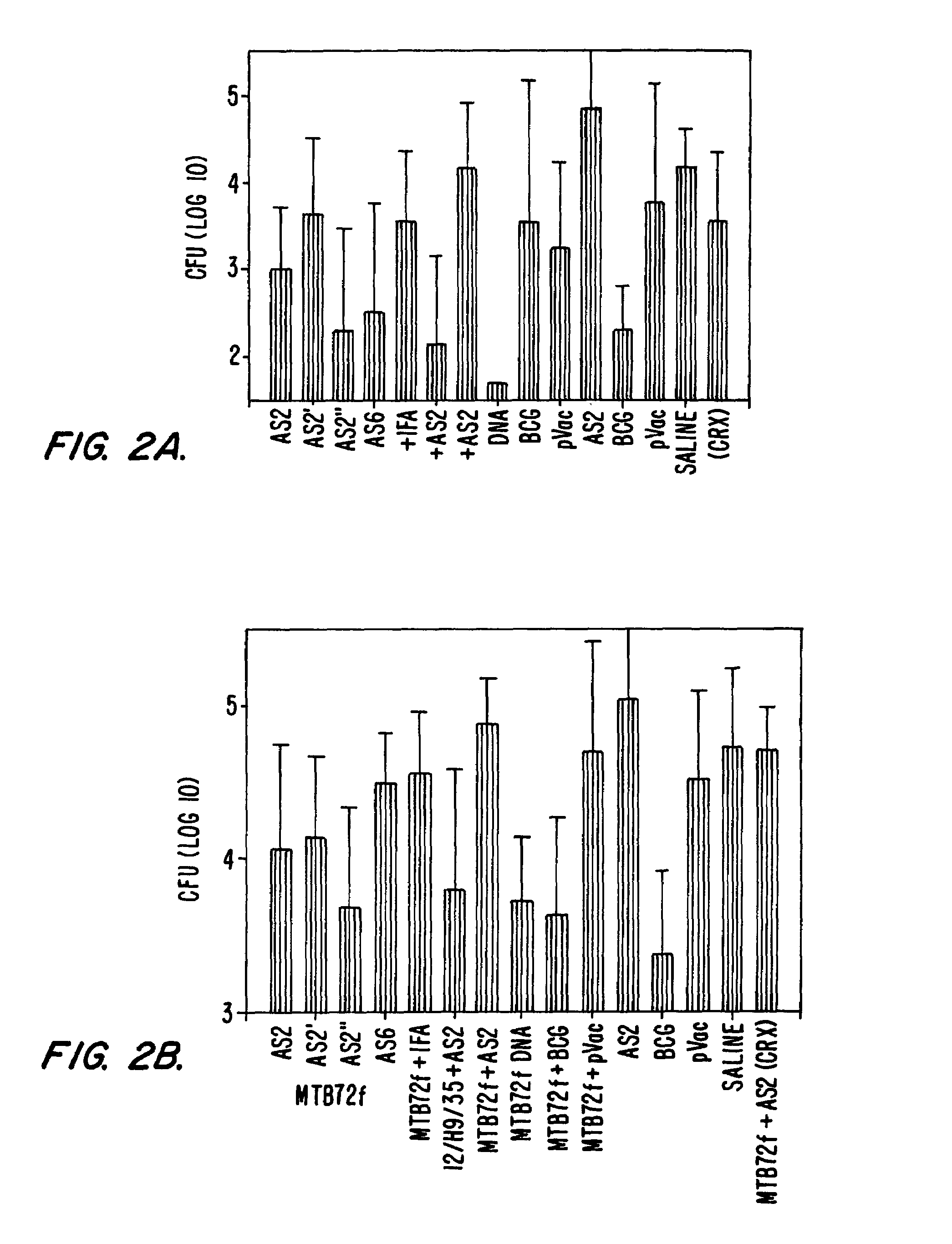
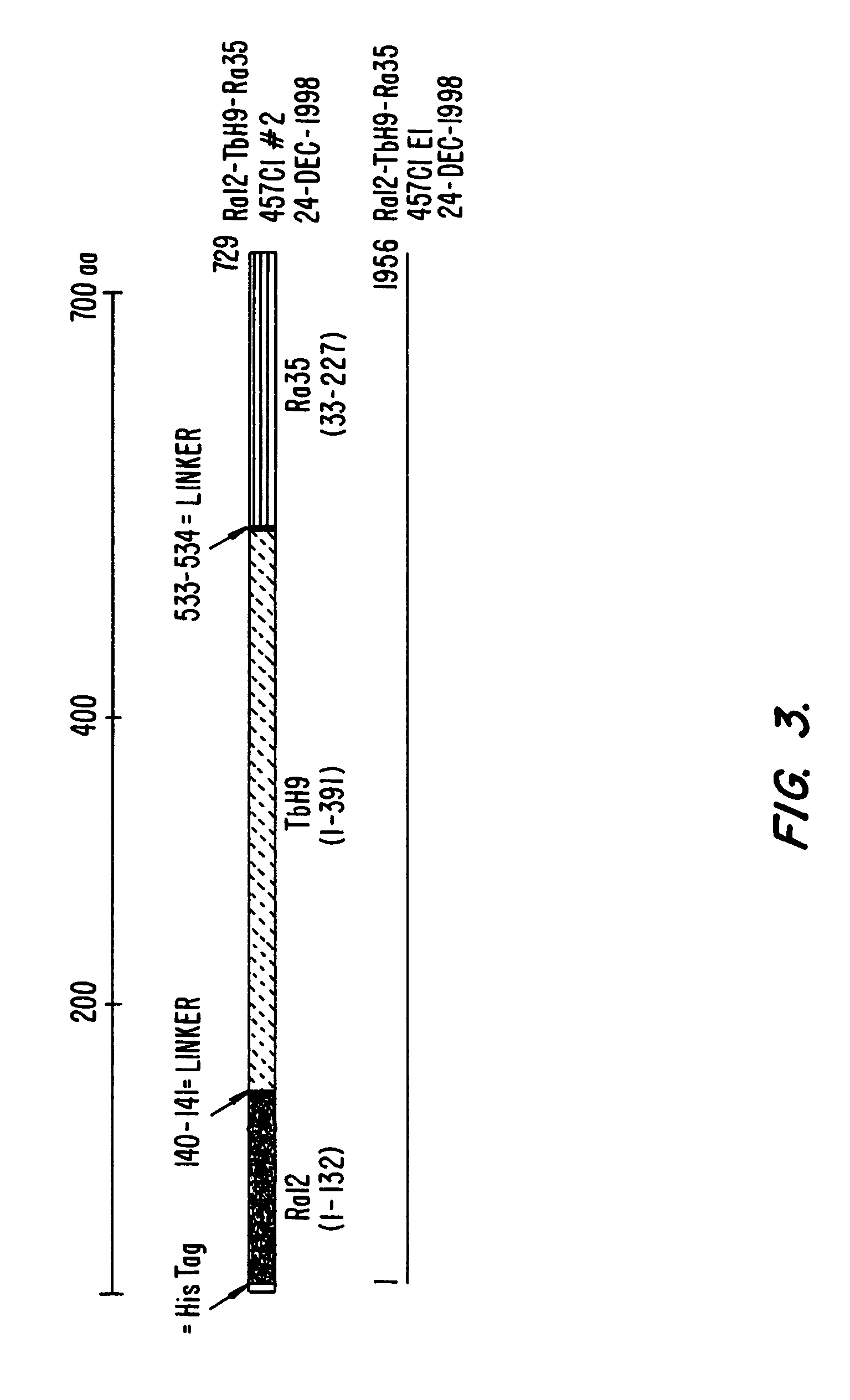
The present invention relates to fusion proteins containing at least two Mycobacterium tuberculosis antigens. In particular, it relates to bi-fusion proteins which contain two individual M. tuberculosis antigens, tri-fusion proteins which contain three M. tuberculosis antigens, tetra-fusion proteins which contain four M. tuberculosis antigens, and penta-fusion proteins which contain five M. tuberculosis antigens, and methods for their use in the diagnosis, treatment and prevention of tuberculosis infection.
Owner:CORIXA CORP
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS6544522B1Improving immunogenicityAntibacterial agentsPeptide/protein ingredientsAntigenMycobacterial antigen
The present invention relates to fusion proteins of Mycobacterium tuberclosis antigens. In particular, it relates to two fusion proteins, each of which contains three individual M. tuberculosis antigens, and a fusion protein of two M. tuberculosis antigens, their coding sequences, and methods for their use in the treatment and prevention of tuberculosis.
Owner:CORIXA CORP
Disinfecting compositions and methods of making and using same
InactiveUS20050019421A1High kill rateUse of compositionBiocidePeroxide active ingredientsSolventBiology
The present invention provides a composition, comprising: greater than about 0.1% by weight hydrogen peroxide; an aromatic acid component; surfactant; optionally, a solvent; and a carrier. The composition of the invention is useful as a disinfecting composition for killing microorganisms such as bacterium (including Mycobacterium), spores and fungi. The composition provides a pathogenic bacteria kill rate of 99.9% in about 30 seconds when bacteria are exposed to the composition and is effective in providing a Mycobacterium kill of 106 with two minutes or less. Moreover, the compositions of the invention are generally more resistant to catalase deactivation than, for example, an aqueous solution of hydrogen peroxide. The concentration of hydrogen peroxide within the composition may range from about 1% by weight to about 7% by weight and the concentration of aromatic acid component may range from about 0.1% by weight to about 5% by weight. The invention also provides a method for disinfection of a substrate utilizing the composition. The composition of the invention may be used in the foregoing method on a medical instrument, such as an endoscope or the like. Applying the compositions to a substrate may be accomplished in any of a variety of application methods such as by roll coating, dipping, spraying, or rotational tumbling. The composition may be applied to the substrate for a period of time ranging from about 30 seconds to about ten minutes. In this aspect, the invention can further comprise drying the substrate after removing the composition.
Owner:3M INNOVATIVE PROPERTIES CO
Compositions and methods for detecting, identifying and quantitating mycobacterial-specific nucleic acids
ActiveUS20110281754A1Inherent limitationAuxiliary diagnosisBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyTuberculosis mycobacterium
Disclosed are compositions and methods for isolating, detecting, amplifying, and quantitating Mycobacterium-specific nucleic acids in a sample. Also disclosed are compositions and diagnostic kits comprising Mycobacterium IS6110-specific oligonucleotide amplification primers and labeled oligonucleotide detection probes that specifically bind to the amplification products obtained therefrom. Also disclosed are compositions and methods for the isolation and characterization of nucleic acids that are specific to one or more tubercular pathogens, including Mycobacterium tuberculosis, in particular, from a wide variety of samples including those of biological, environmental, clinical and / or veterinary origin.
Owner:LONGHORN VACCINES & DIAGNOSTICS LLC
M. tuberculosis antigens
InactiveUS6991797B2High expressionEnhance immune responseBacteriaPeptide/protein ingredientsAntigenTuberculosis mycobacterium
The present invention is based on the identification and characterization of a number of novel M. tuberculosis derived proteins and protein fragments. The invention is directed to the polypeptides and immunologically active fragments thereof, the genes encoding them, immunological compositions such as vaccines and skin test reagents containing the polypeptides.
Owner:STATENS SERUM INST
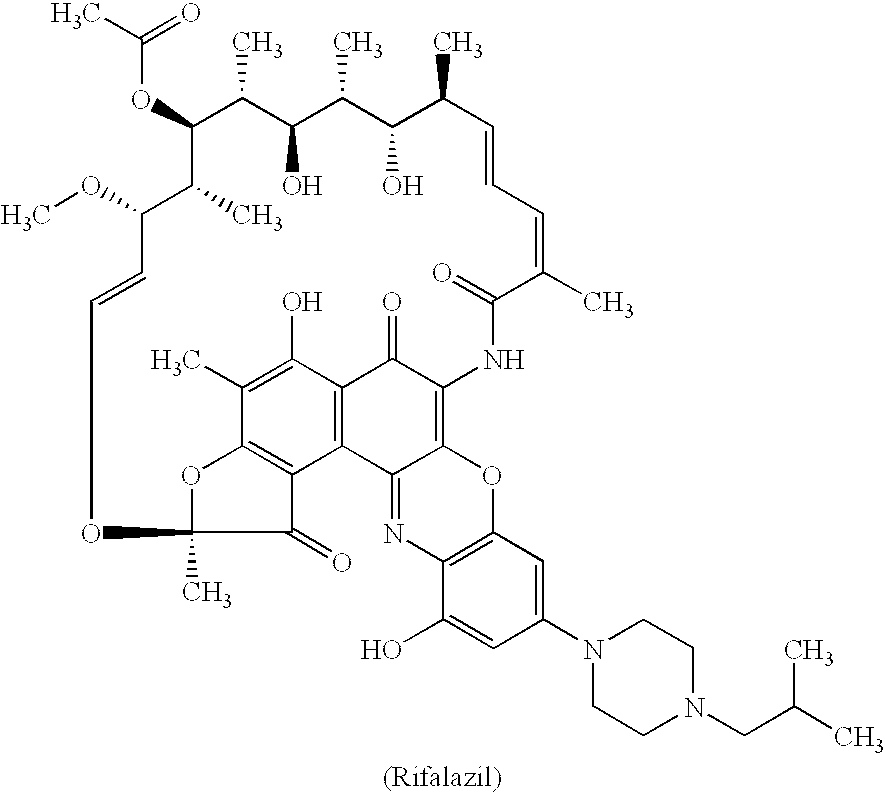
Method for treatment of bacterial infections with once or twice-weekly administered rifalazil
A method for treatment of bacterial infections with rifalazil administered once-weekly or twice-weekly. A method for treatment of tuberculosis caused by Mycobacterium tuberculosis, infections caused by Mycobacterium avium complex, infections caused by Chlamydia pneumoniae and infections caused by Helicobacter pylori by administering to a patient suffering from the bacterial infection 1-100 mg of rifalazil once or twice a week. In this dose regimen, the treatment is fast, efficacious and eliminates undesirable secondary symptoms observed with daily doses of 1-50 mg of rifalazil.
Owner:KANEKA CORP
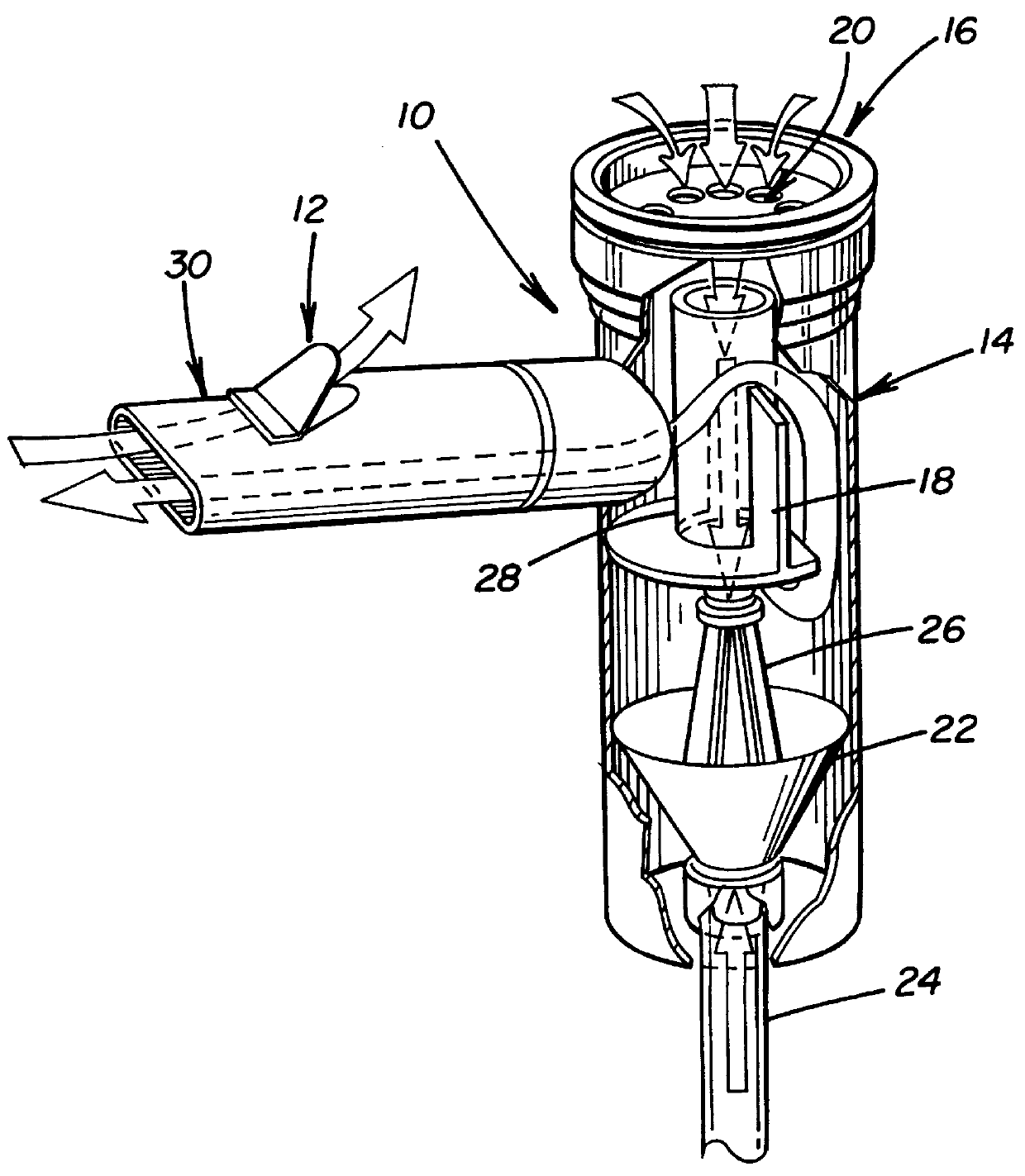
Systems for treating pulmonary infections
Provided herein are systems for treating a subject with a pulmonary infection, for example, a nontuberculous mycobacterial pulmonary infection, a Burkholderia pulmonary infection, a pulmonary infection associated with bronchiectasis, or a Pseudomonas pulmonary infection. The system includes a pharmaceutical formulation comprising a liposomal aminoglycoside dispersion, and the lipid component of the liposomes consist essentially of electrically neutral lipids. The system also includes a nebulizer which generates an aerosol of the pharmaceutical formulation at a rate greater than about 0.53 gram per minute. The aerosol is delivered to the subject via inhalation for the treatment of the pulmonary infection.
Owner:INSMED INC
Immunogenic compositions comprising mycobacterium tuberculosis polypeptides and fusions thereof
ActiveUS20100129391A1Diagnosing and preventing and treating tuberculosisImprove the level ofAntibacterial agentsBacterial antigen ingredientsNucleotideImmunogenicity
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and polynucleotides encoding such compositions and fusion proteins. The invention also relates to methods for their use in the treatment, prevention and / or diagnosis of tuberculosis infection.
Owner:ACCESS TO ADVANCED HEALTH INST
Novel method of inducing antigen-specific t cells
InactiveUS20050002951A1Efficient inductionBacterial antigen ingredientsCancer antigen ingredientsPharmaceutical drugCell Wall Skeleton
The present invention provides a novel method for inducing antigen-specific T cells. A method for inducing antigen-specific T cells in a patient comprising administering to said patient in need thereof composition (a) which comprises a therapeutically effective amount of an antigen protein or an antigen peptide as an active ingredient, and composition (b) which comprises a therapeutically effective amount of a cell wall skeleton integrant of the BCG strain of Mycobacterium bovis as an active ingredient, wherein composition (b) is administered in advance and then composition (a) is administered, and related pharmaceutical compositions are provided.
Owner:SUGIYAMA HARUO +1
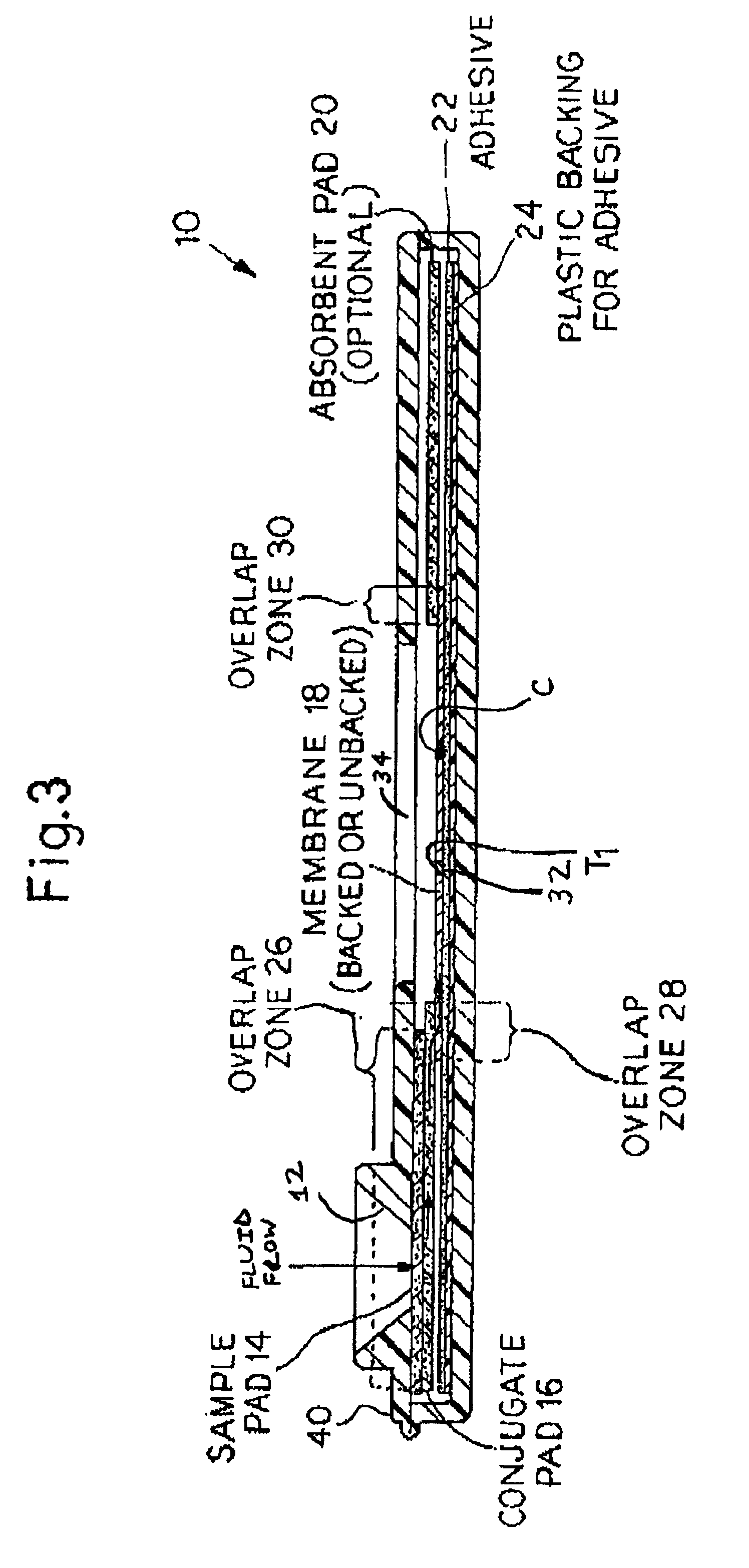
Rapid lateral flow assay for determining exposure to Mycobacterium tuberculosis and other mycobacteria
InactiveUS6841159B2Auxiliary diagnosisAuxiliary judgmentBacterial antigen ingredientsMicrobiological testing/measurementMycobacterial antigenImmunization status
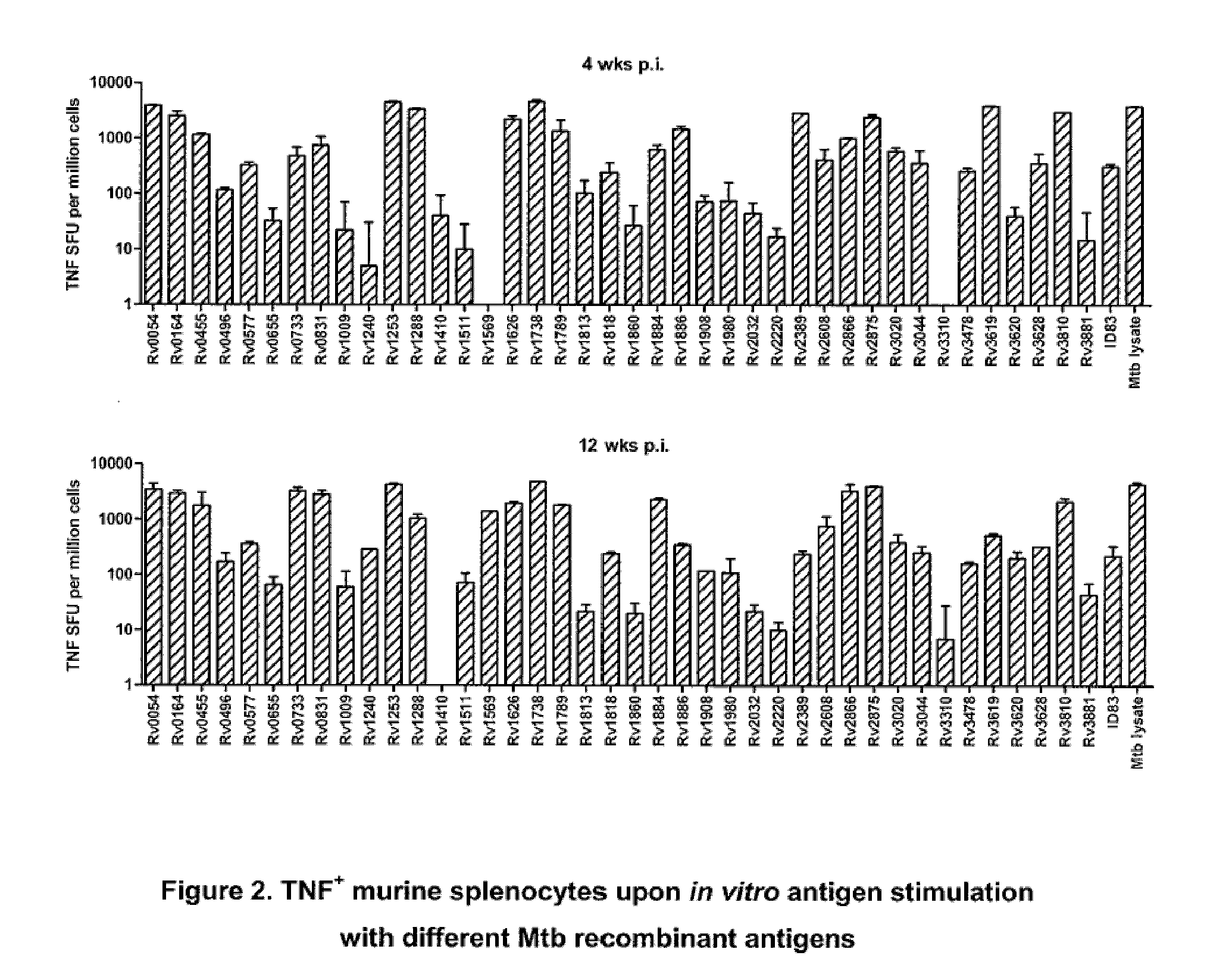
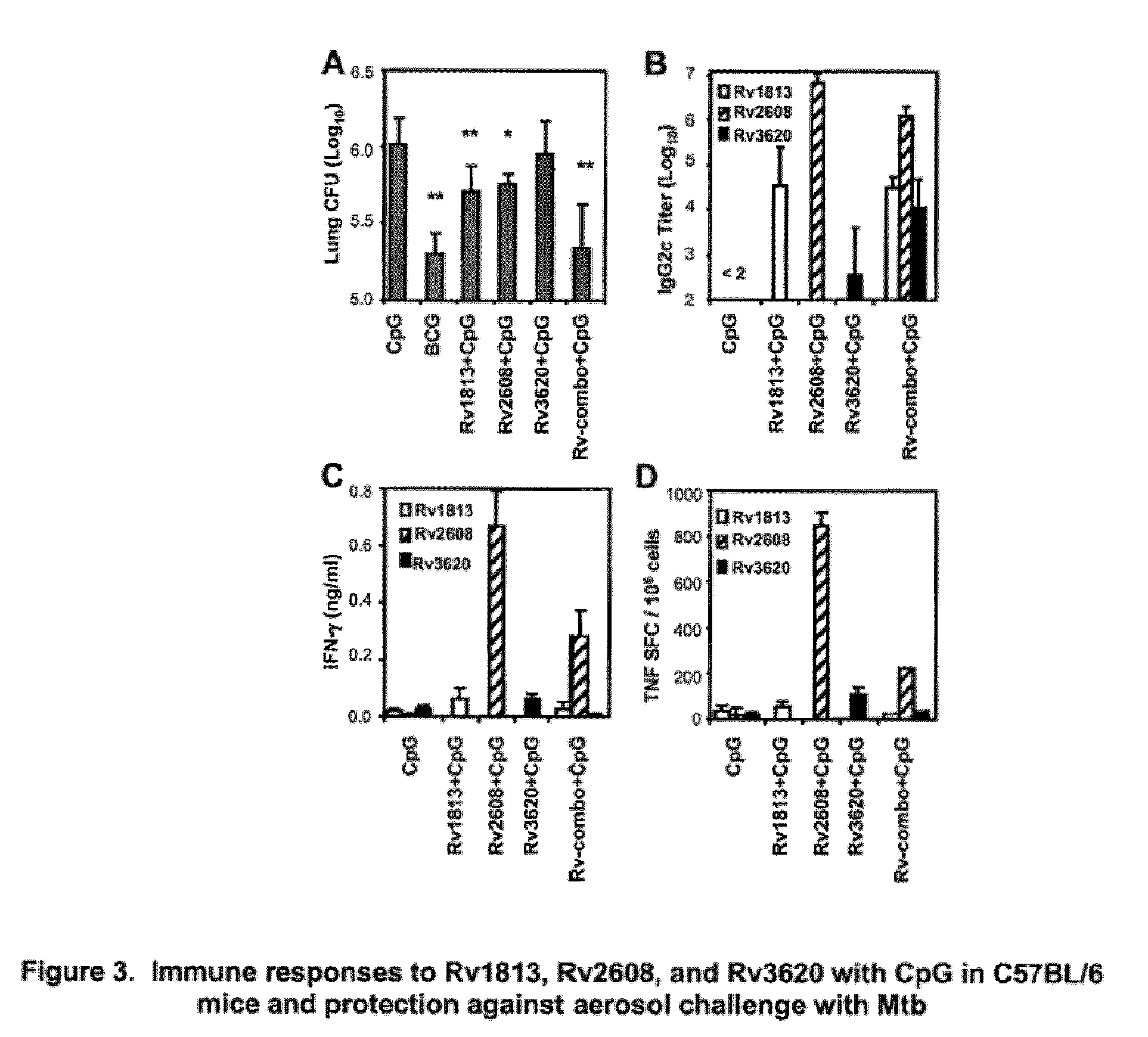
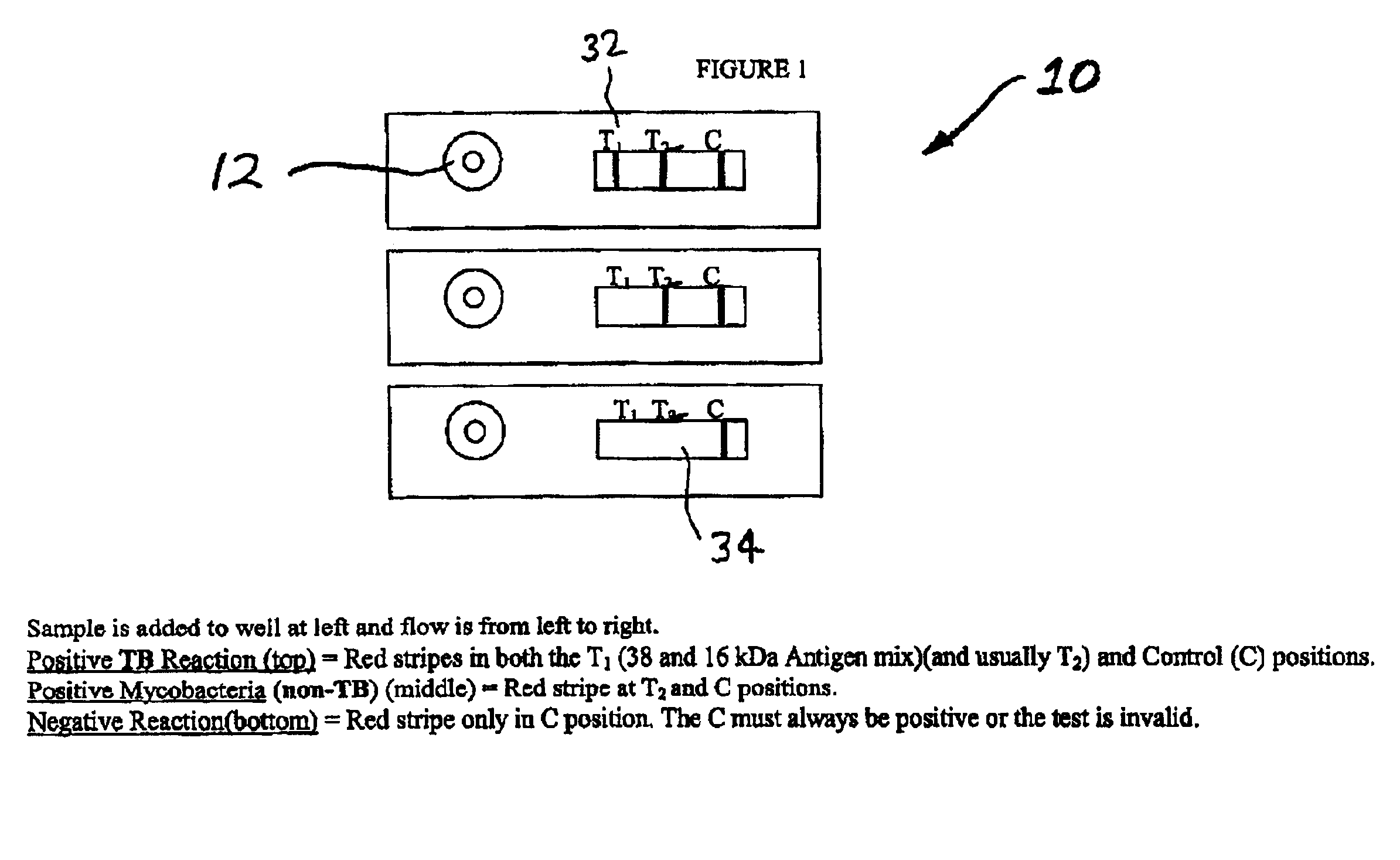
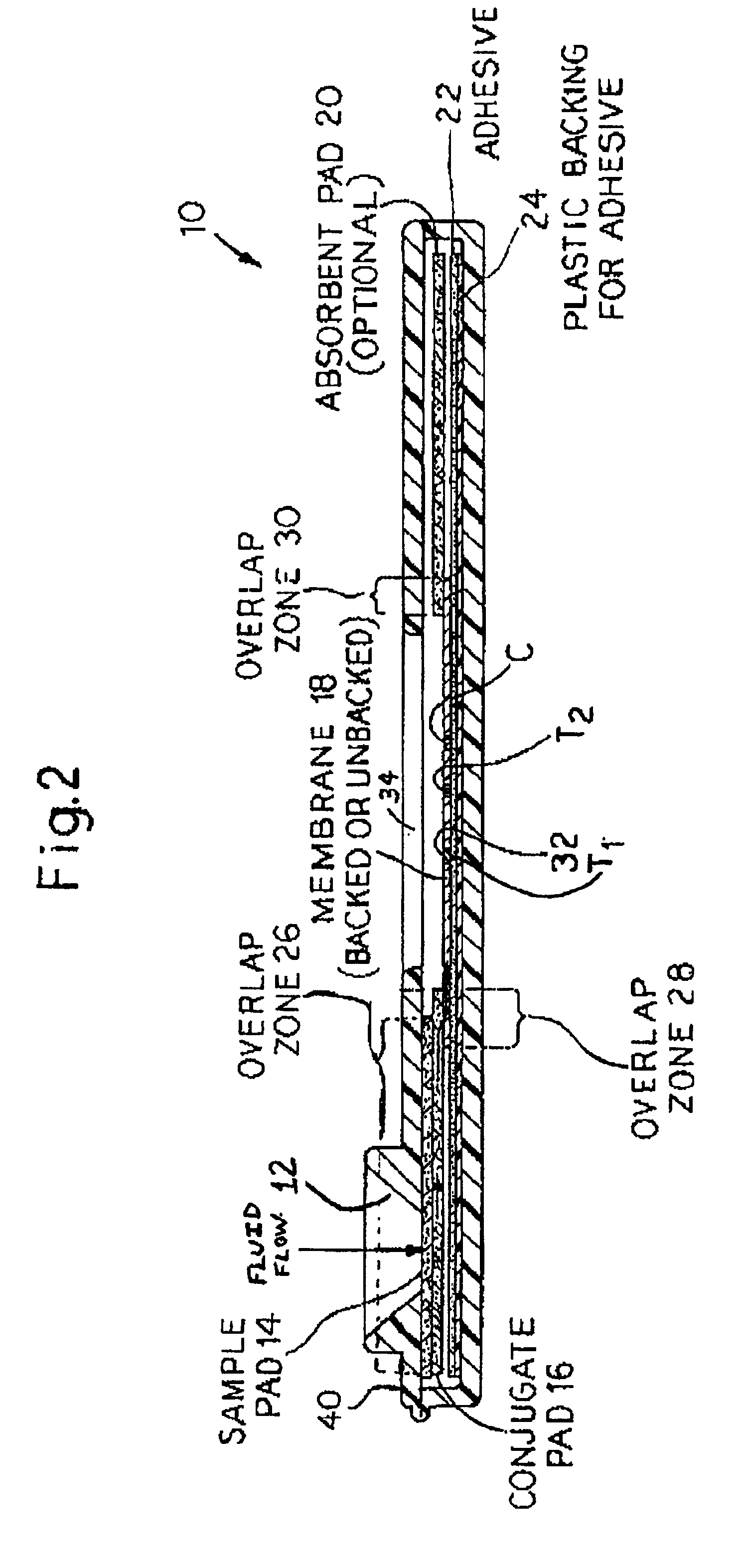
An assay method and kit is disclosed for detecting the presence of at least one predesignated, target antibody to a mycobacterium in a sample selected from one or more patient bodily fluids. The method comprises the following steps: (a) contacting the sample of one or more patient bodily fluids with at least one mycobacterium antigen on a lateral-flow assay membrane to bind to the target antibody in the sample; (b) previously, simultaneously or subsequently to step (a), binding the at least one mycobacterium antigen with a conjugated label producing a detectable signal; and (c) detecting the signal whereby the presence of the target antibody is determined in the sample by the intensity or presence of the signal. The method can further comprise the step of evaluating immunization status of the patient from whom the sample came by comparing the signal or lack thereof with immunizations previously received by the patient and in comparison to a known standard control. In a preferred embodiment, the mycobacterium antigen specifically binds to Mycobacterium tuberculosis specific antibodies. Preferably, the immunoassay of the present invention comprises a lateral-flow assay comprising a membrane, a conjugated label pad, and at least one mycobacterium antigen bound to the membrane. In a preferred embodiment, the at least one mycobacterium antigen is selected from the group consisting of 38 kDa and 16 kDa antigens.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +1
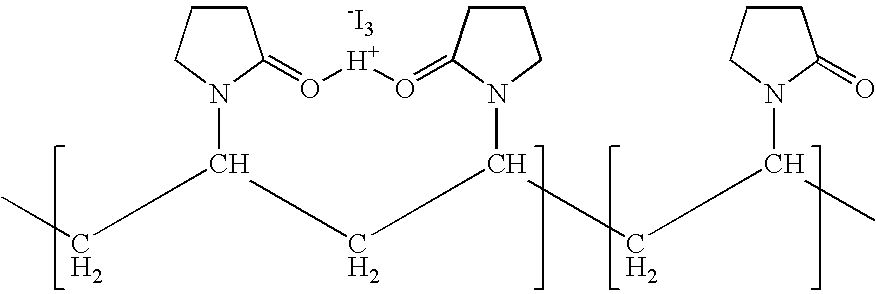
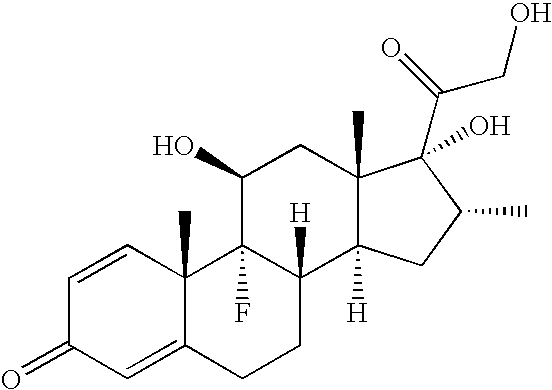
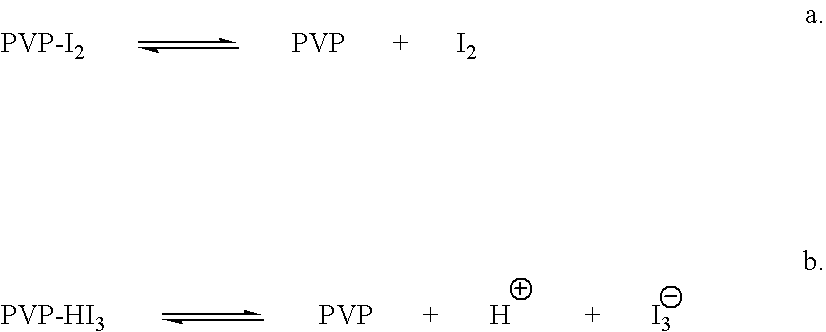
Ophthalmic compositions comprising povidone-iodine
ActiveUS7767217B2Increase the amount of lightBetter seeAntibacterial agentsBiocideConjunctivaClinical settings
A topical ophthalmic composition comprised of povidone-iodine 0.01% to 10.0% combined with a steroid or non-steroidal anti-inflammatory drug. This solution is useful in the treatment of active infections of at least one tissue of the eye (e.g., conjunctiva and cornea) from bacterial, mycobacterial, viral, fungal, or amoebic causes, as well as treatment to prevent such infections in appropriate clinical settings (e.g. corneal abrasion, postoperative prophylaxis, post-LASIK / LASEK prophylaxis). Additionally the solution is effective in the prevention of infection and inflammation in the post-operative ophthalmic patient.
Owner:CLARUS CLS HLDG LLC
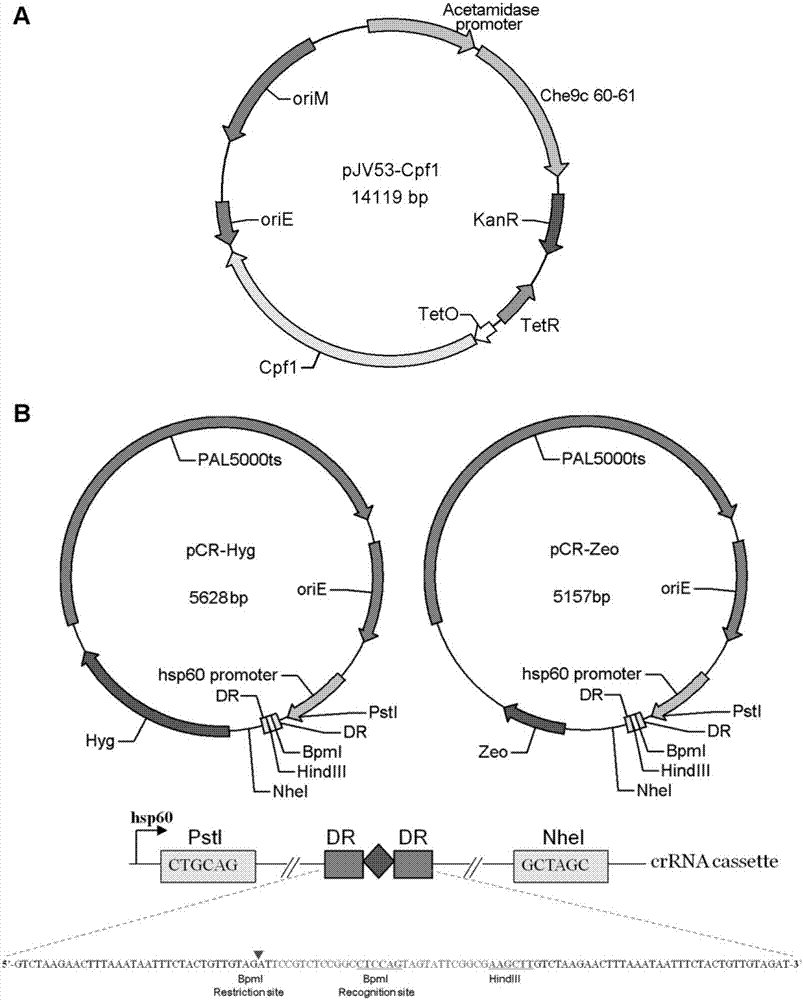
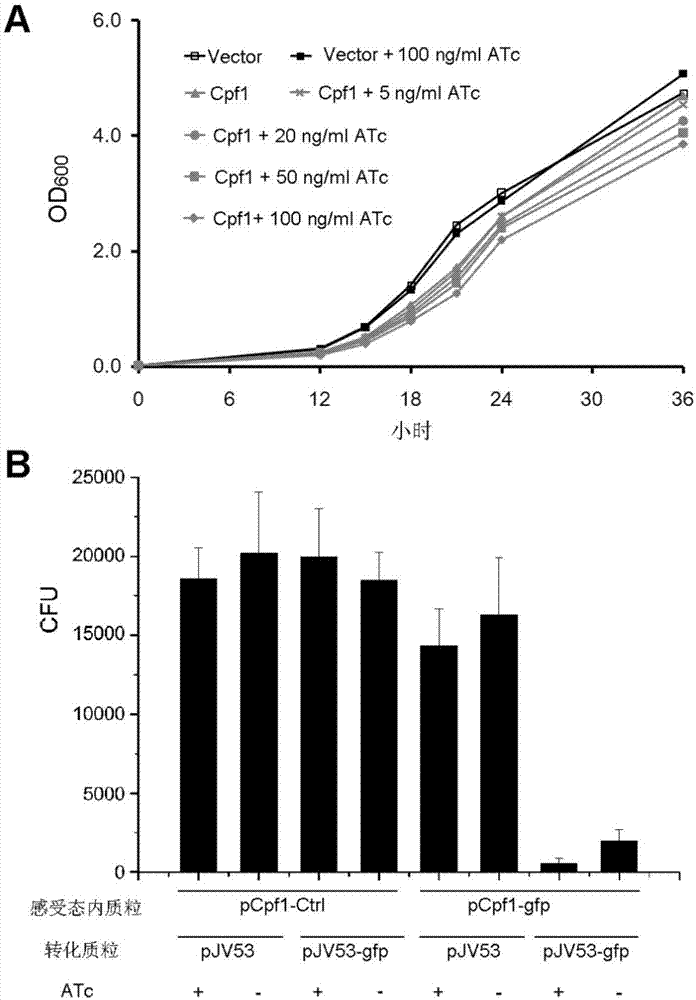
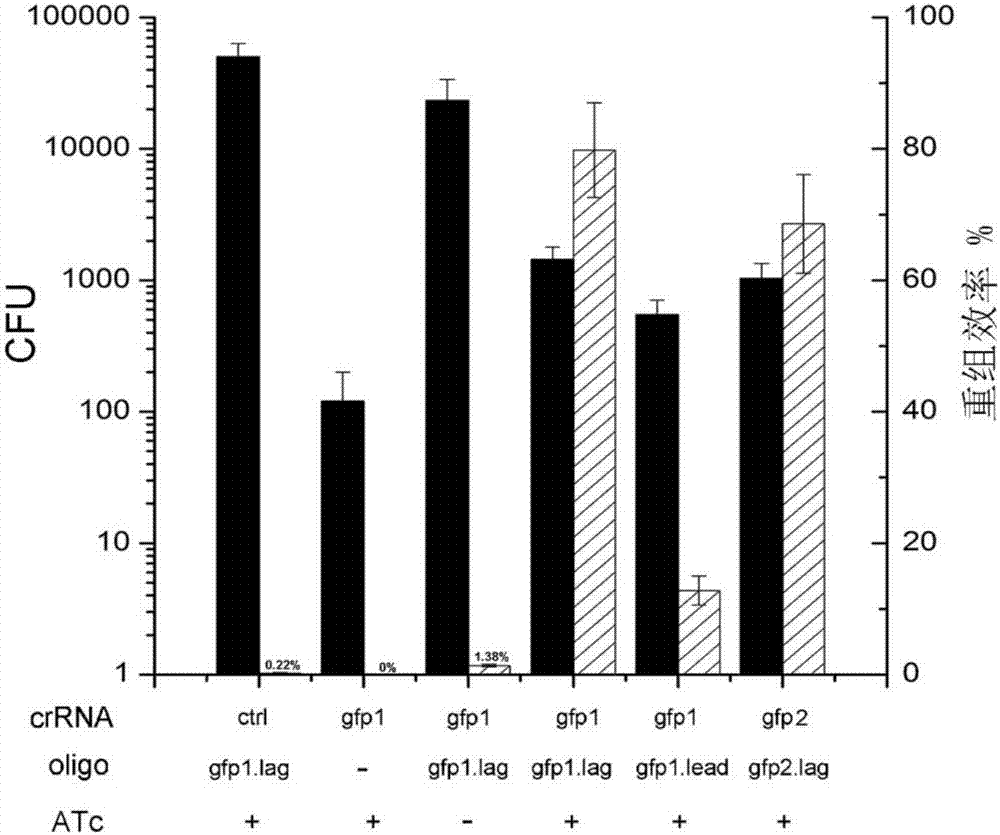
CRISPR/Cpf1 gene editing system and application of system in mycobacteria
ActiveCN107083392AAchieve mutationWork lessHydrolasesStable introduction of DNAEscherichia coliShuttle vector
The invention discloses a CRISPR / Cpf1 gene editing system and application of the system in mycobacteria. The CRISPR / Cpf1 gene editing system comprises a recombinant vector carrying optimized FnCpf1 genes and pCR plasmids, wherein the sequence of the optimized FnCpf1 genes is shown in SEQ ID No:1, and the recombinant vector is a colibacillus-mycobacterium shuttle vector containing recombinant enzyme gp60 and gp61 genes; the pCR plasmids contain promoter-driven direct repeat sequence-spacer sequence-direct repeat sequence units, wherein the spacer sequence contains target sequence connection sites. When the CRISPR / Cpf1system is used in the mycobacteria for CRISPR / Cpf1 assisted homologous recombination, high recombination efficiency can be obtained, and gene editing of the mycobacteria can be achieved.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
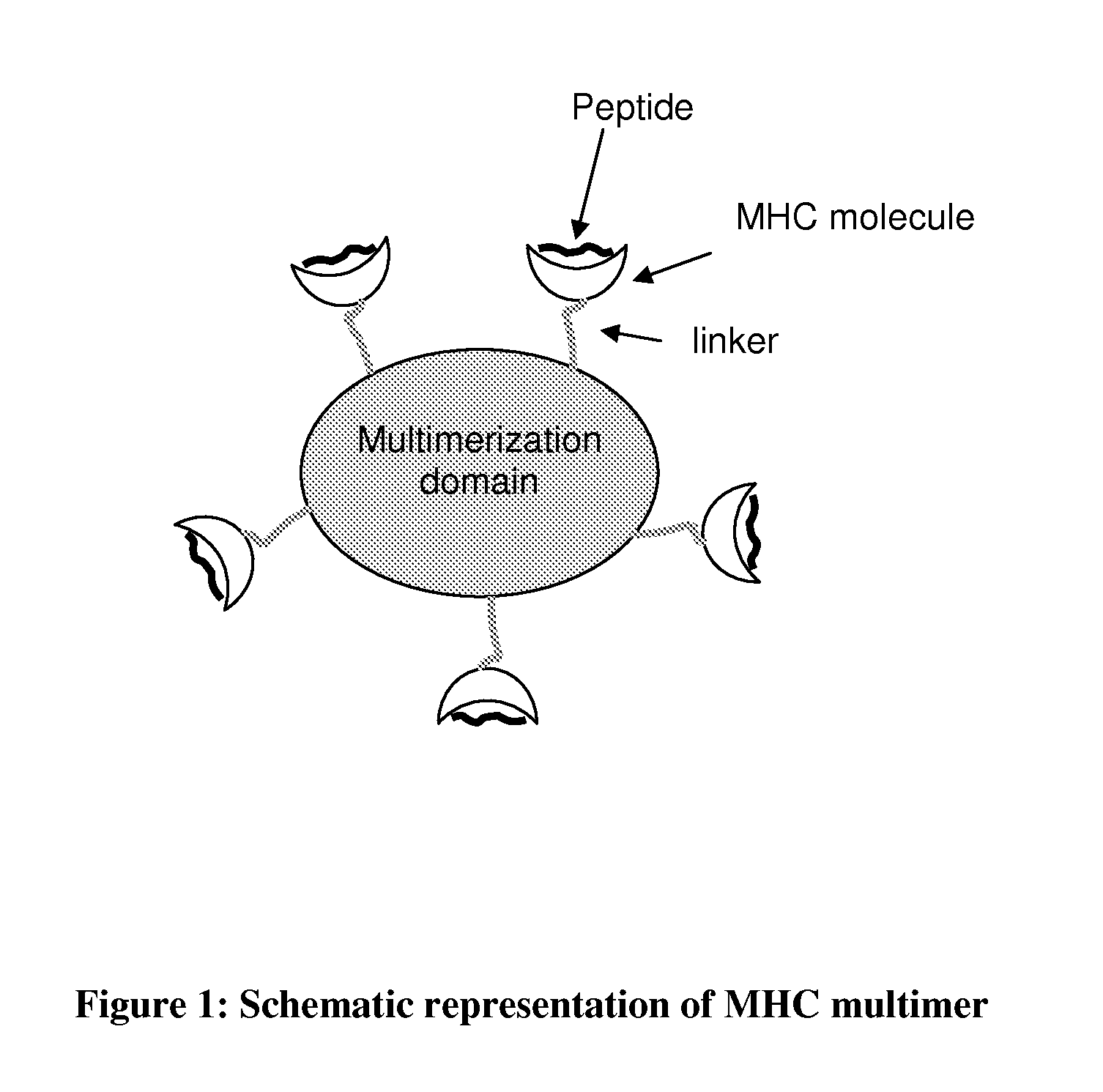
MHC Multimers in Tuberculosis Diagnostics, Vaccine and Therapeutics
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising Mycobacterium tuberculosis antigenic peptides and uses there of.
Owner:AGILENT TECH INC
Nucleic acids fragments and polypeptide fragments derived from M. tuberculosis
InactiveUS6641814B1Bioreactor/fermenter combinationsBiological substance pretreatmentsSkin testADAMTS Proteins
The present invention is based on the identification and characterization of a number of M. tuberculosis derived novel proteins and protein fragments (SEQ ID NOs: 2, 4, 6, 8, 10, 12, 14, 16, 17-23, 42, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72-86, 88, 90, 92, 94, 141, 143, 145, 147, 149, 151, 153, and 168-171). The invention is directed to the polypeptides and immunologically active fragments thereof, the genes encoding them, immunological compositions such as vaccines and skin test reagents containing the polypeptides. Another part of the invention is based on the surprising discovery that fusions between ESAT-6 and MPT59 are superior immunogens compared to each of the unfused proteins, respectively.
Owner:STATENS SERUM INST
Fusion proteins of Mycobacterium tuberculosis
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Hybrids of M. tuberculosis antigens
InactiveUS7037510B2Improving immunogenicityImprove propertiesBacteriaPeptide/protein ingredientsImmunological memoryImmunodominant Antigens
The present invention discloses fusion proteins of the immunodominant antigens ESAT-6 and Ag85B from Mycobacterium tuberculosis or homologues thereof, and a tuberculosis vaccine based on the fusion proteins, which vaccine induces efficient immunological memory.
Owner:STATENS SERUM INST
Fusion proteins of mycobacterium tuberculosis
InactiveUS7311922B1Good antigenicityHigh sensitivityAntibacterial agentsPeptide/protein ingredientsAntigenSerum ige
The present invention relates to fusion proteins containing at least two Mycobacterium species antigens. In particular, it relates to nucleic acids encoding fusion proteins that include two or more individual M. tuberculosis antigens, which increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Probiotics with methods for growth and use separately and in combination
InactiveUS20150246081A1Strengthening the immune systemBiocideUnknown materialsDietary supplementDiverticulitis
A dietary supplement that may contain probiotics from the genera Akkermansia, Bacteriodes, Faecalibacterium, Eubacterium, Escherichia, Collinsella, Desulfovibrio, Clostridium, Mycobacterium, Pediococcus, and Bifidobacterium. The dietary supplement may provide a variety of benefits including weight management, blood sugar management, treatment of irritable bowel syndrome, treatment of Crohn's disease, treatment of diverticulitis, treatment for inflammatory bowel, treatment for dysbiosis, and strengthening the immune system.
Owner:MORRIS SHAYNE KENNETH
Method for treatment of bacterial infections with once or twice-weekly administered rifalazil
A method for treatment of bacterial infections with rifalazil administered once-weekly or twice-weekly. A method for treatment of tuberculosis caused by Mycobacterium tuberculosis, infections caused by Mycobacterium avium complex, infections caused by Chlamydia pneumoniae and infections caused by Helicobacter pylori by administering to a patient suffering from the bacterial infection 1-100 mg of rifalazil once or twice a week. In this dose regimen, the treatment is fast, efficacious and eliminates undesirable secondary symptoms observed with daily doses of 1-50 mg of rifalazil.
Owner:KANEKA CORP
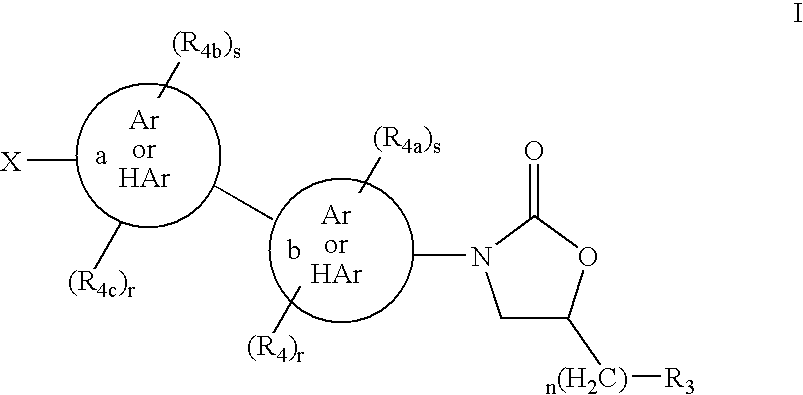
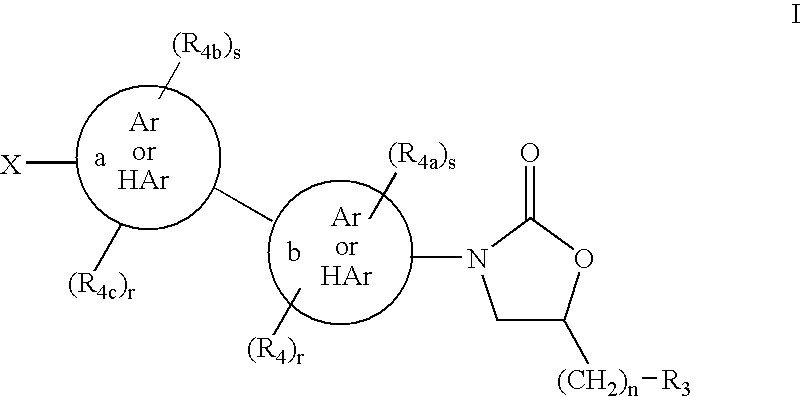
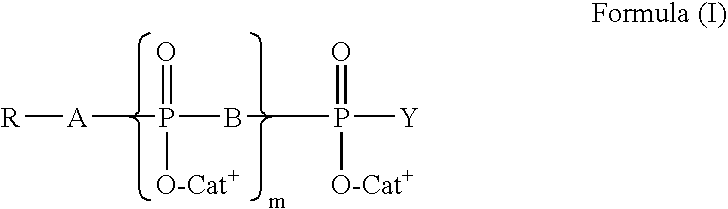
Cyclopropyl group substituted oxazolidinone antibiotics and derivatives thereof
This invention relates to new oxazolidinones having a cyclopropyl moiety, which are effective against aerobic and anerobic pathogens such as multi-resistant staphylococci, streptococci and enterococci, Bacteroides spp., Clostridia spp. species, as well as acid-fast organisms such as Mycobacterium tuberculosis and other mycobacterial species. The compounds are represented by structural formula I: its enantiomer, diastereomer, or pharmaceutically acceptable salt or ester thereof.
Owner:KYORIN PHARMA CO LTD +1
Fusion proteins of mycobacterium tuberculosis
InactiveUS7083796B2Good antigenicityHigh sensitivityAntibacterial agentsPeptide/protein ingredientsSerum igeBiology
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
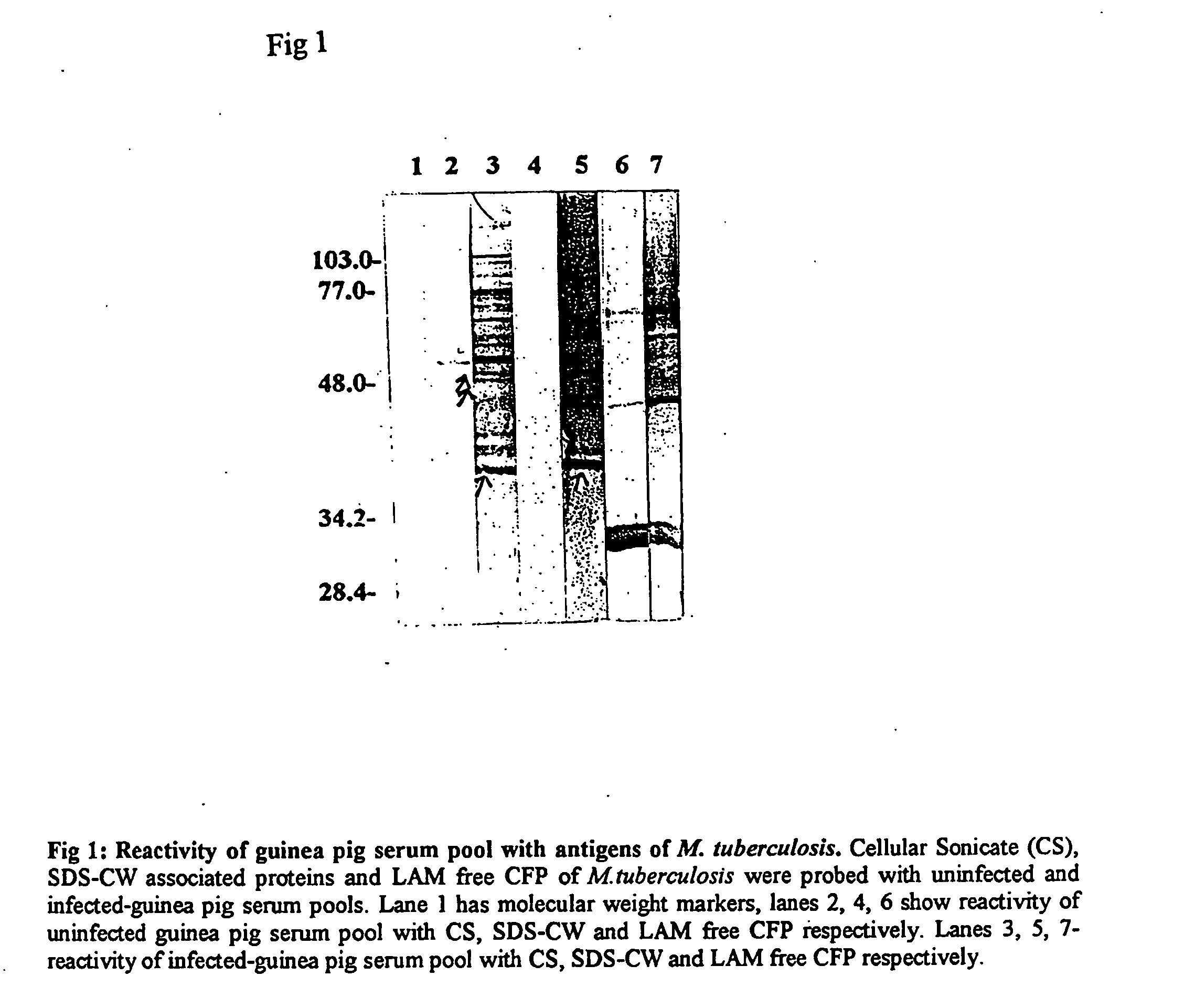
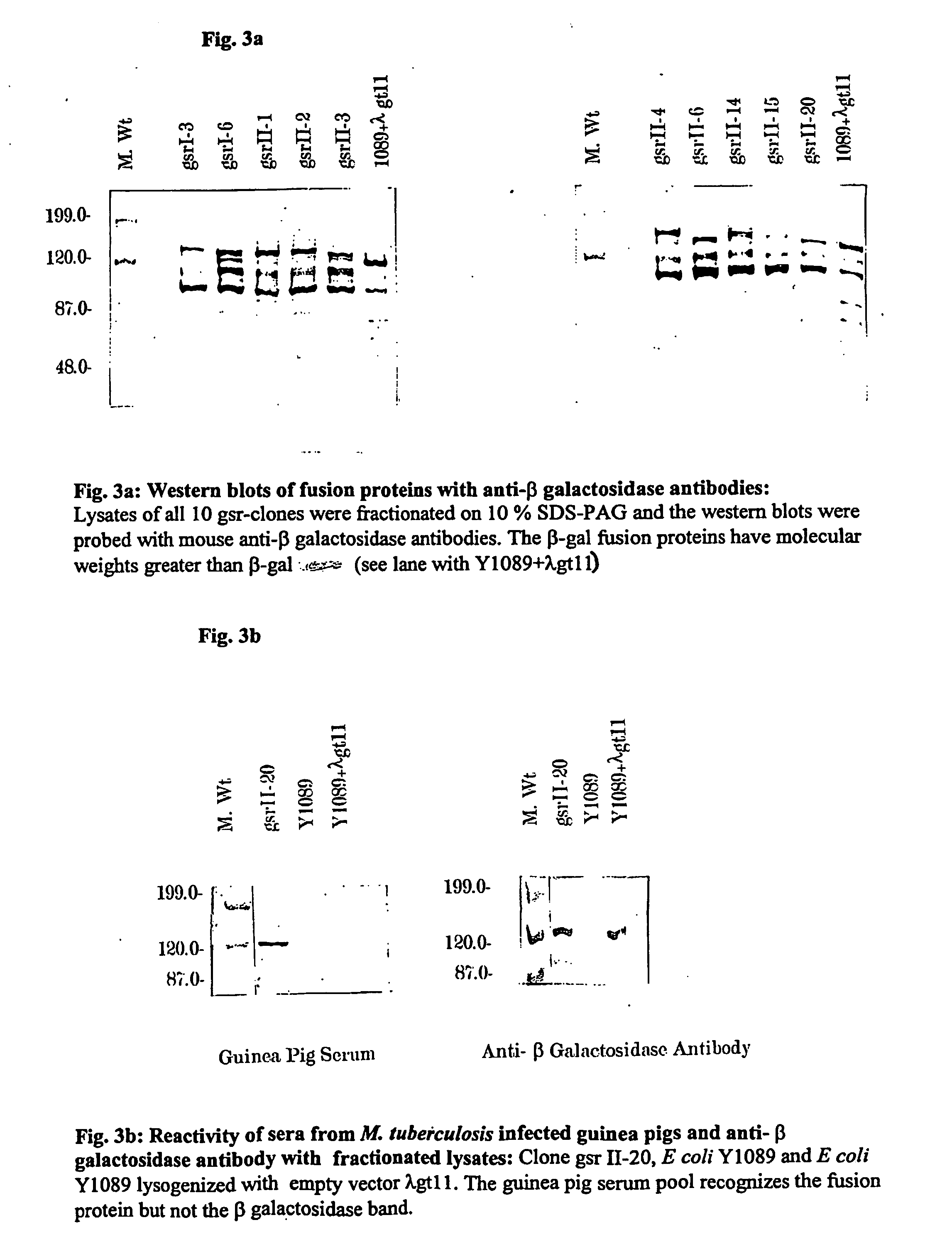
Mycobacterial proteins as early antigens for serodiagnosis and vaccines
In view of the paucity of human material available to study the immunological events occurring after inhalation of virulent bacilli, but prior to development of clinical TB, the present invention is based in part on studies of aerosol infected rabbits. The present inventors reasoned that by 3-5 weeks post-infection, the sera from infected rabbits would contain antibodies to the antigens being expressed by the in vivo bacteria.
Owner:NEW YORK UNIV
Antimicrobial hexapeptides
ActiveUS20060229252A1Growth inhibitionPrevent microbial infectionAntibacterial agentsPowder deliveryAcid-fastMammal
The invention encompasses hexapeptides consisting of alternating hydrophobic residues (B) at positions 2, 4, and 6, hydrophilic, hydrophilic, charged residues (X) at positions 1 and 3, and a naphthylalanine (Nal), an aliphatic or aromatic residue (O) at position five, represented generally by the formula XBXBOB, which exhibit antimicrobial activity against infections caused by a variety of pathogens. These pathogens may include gram positive or negative bacteria, acid-fast bacteria such a mycobacteria, parasites, dermatophytes, or fungal pathogens. Typical fungal pathogens include Candida albicans and typical dermatophytes include Trichophyton rubrum and Trichophyton mentagrophytes. The hexapeptides of the present invention exhibit antifungal activity, antibacterial activity, desirable stability, and lack toxicity to the mammal receiving treatment.
Owner:HELIX BIOMEDIX INC
Composition and method for the treatment of carcinoma
InactiveUS20070134273A1Good effectConvenient treatmentAntibacterial agentsBiocideMycobacterial antigenCompound (substance)
The present invention relates to compositions and methods useful for treating a carcinoma or viral infection in mammals, including humans. The methods and compositions typically comprise use of an immunogenic or immunomodulatory compound, and a gamma delta T cell activator, such that the composition is effective for treating a carcinoma or viral infection. In a preferred aspect of the invention, the methods comprise use of a gamma delta T cell activator and a Mycobacterium antigen, which for example is an attenuated strain of Mycobacterium bovis (Bacillus Calmette-Guerin (BCG)).
Owner:ROMAGNE FRANCOIS +1
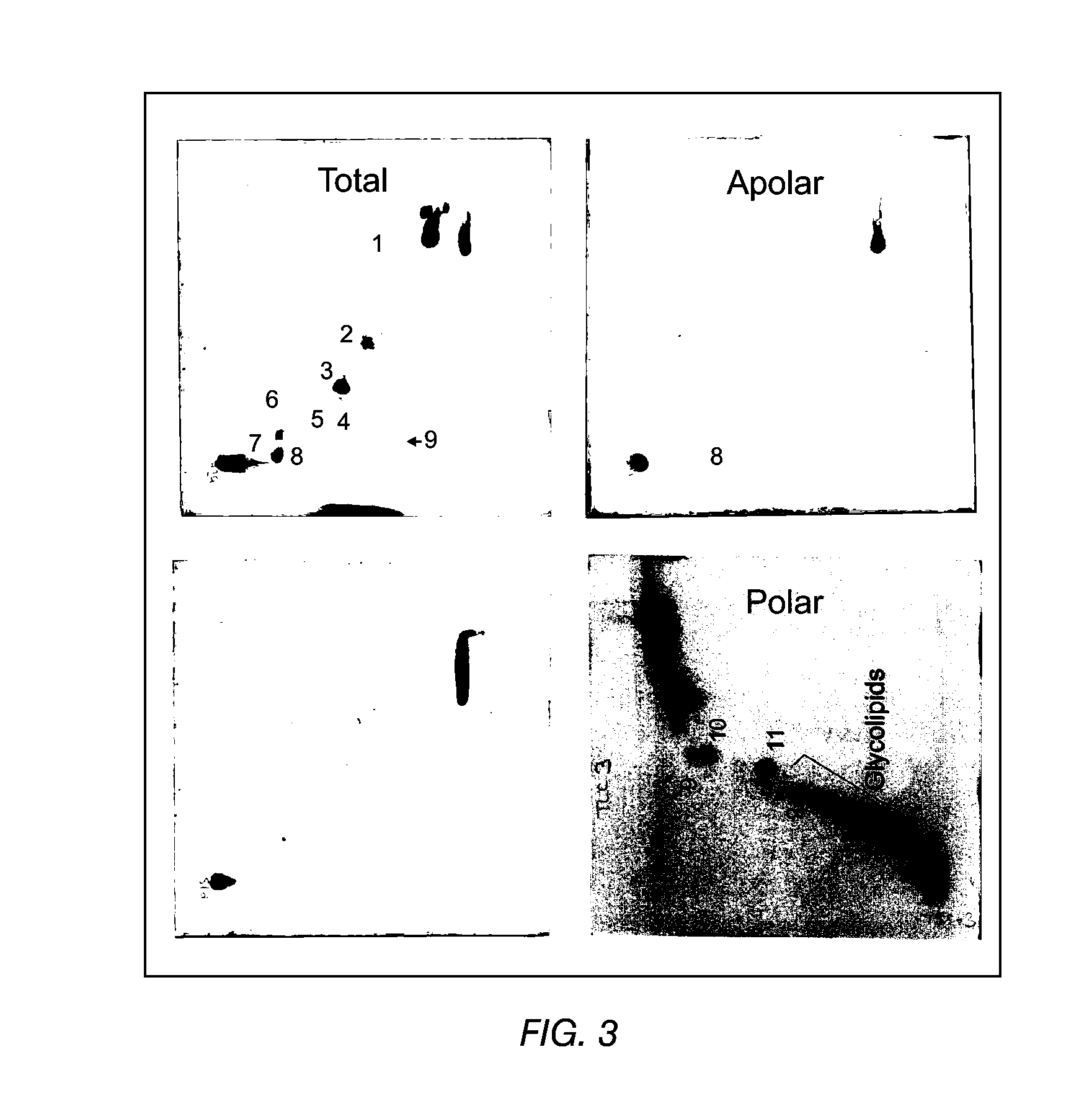
Adjuvant combinations of liposomes and mycobacterial lipids for immunization compositions and vaccines
ActiveUS8241610B2Good auxiliary effectEnhance immune responseAntibacterial agentsBiocideLiposomeMycobacterium
The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG. The total lipid extract contains both apolar lipids, polar lipids, and lipids of intermediate polarity of which the apolar lipids were found to induce the most powerful immune responses. The total lipids may be extracted with chloroform / methanol and re-dissolved in water before the addition of surfactant. This preparation may be used to induce prominent cell-mediated immune responses in a mammal in order to combat pathogens, or as a treatment for cancer.
Owner:STATENS SERUM INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com