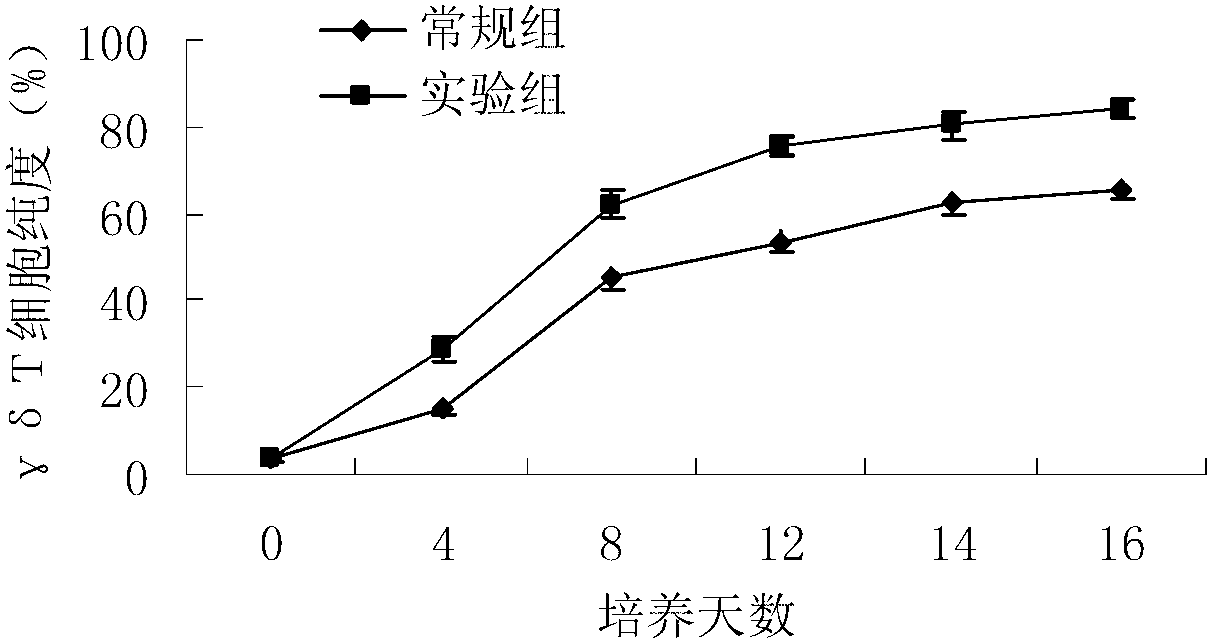
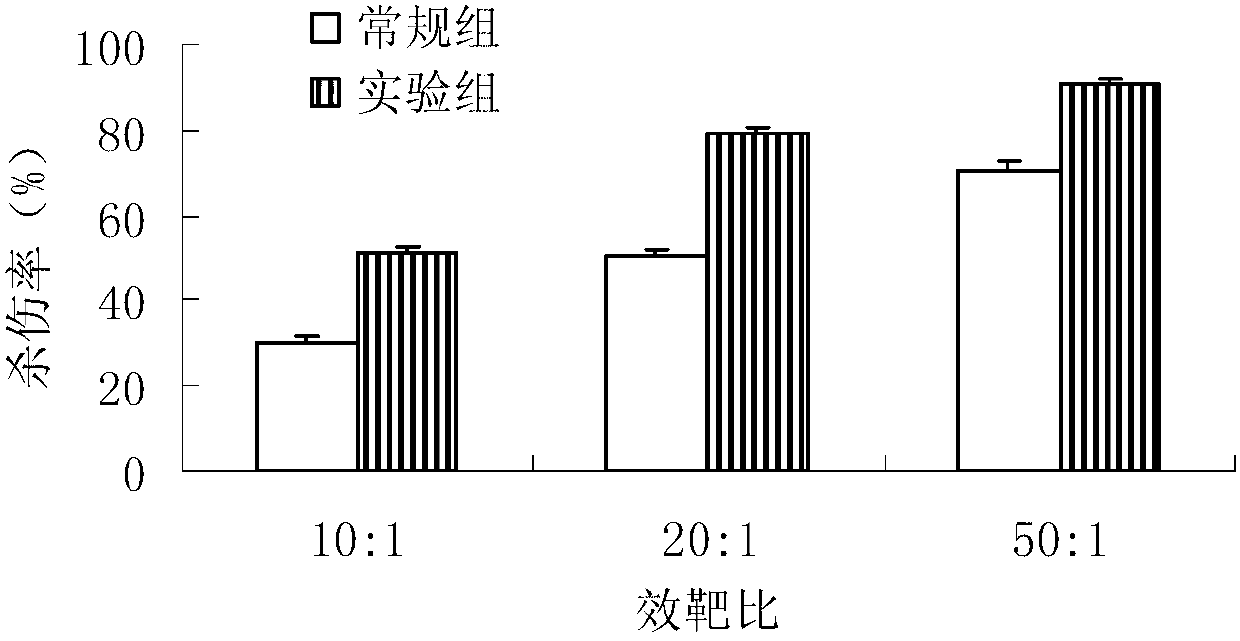
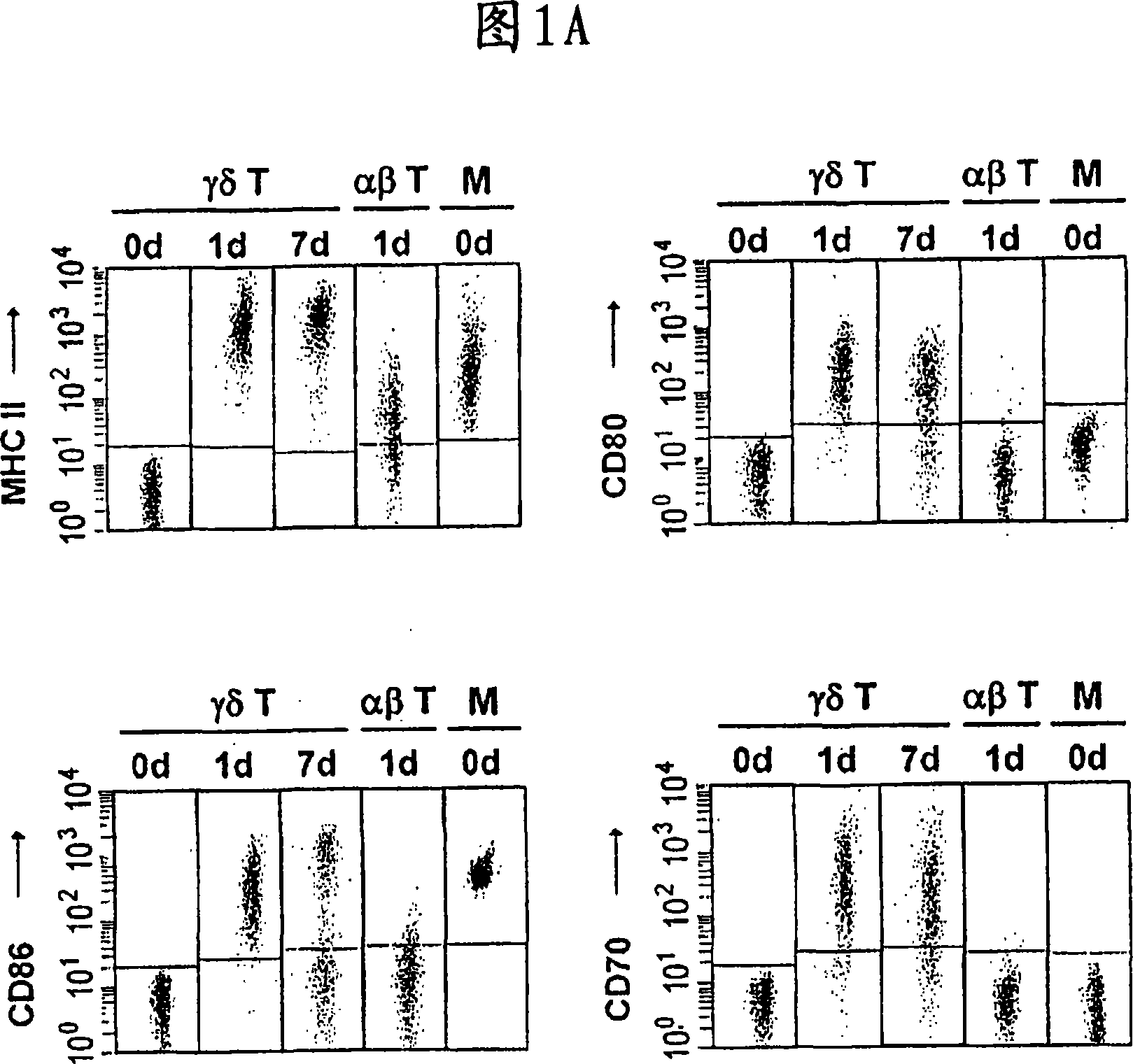
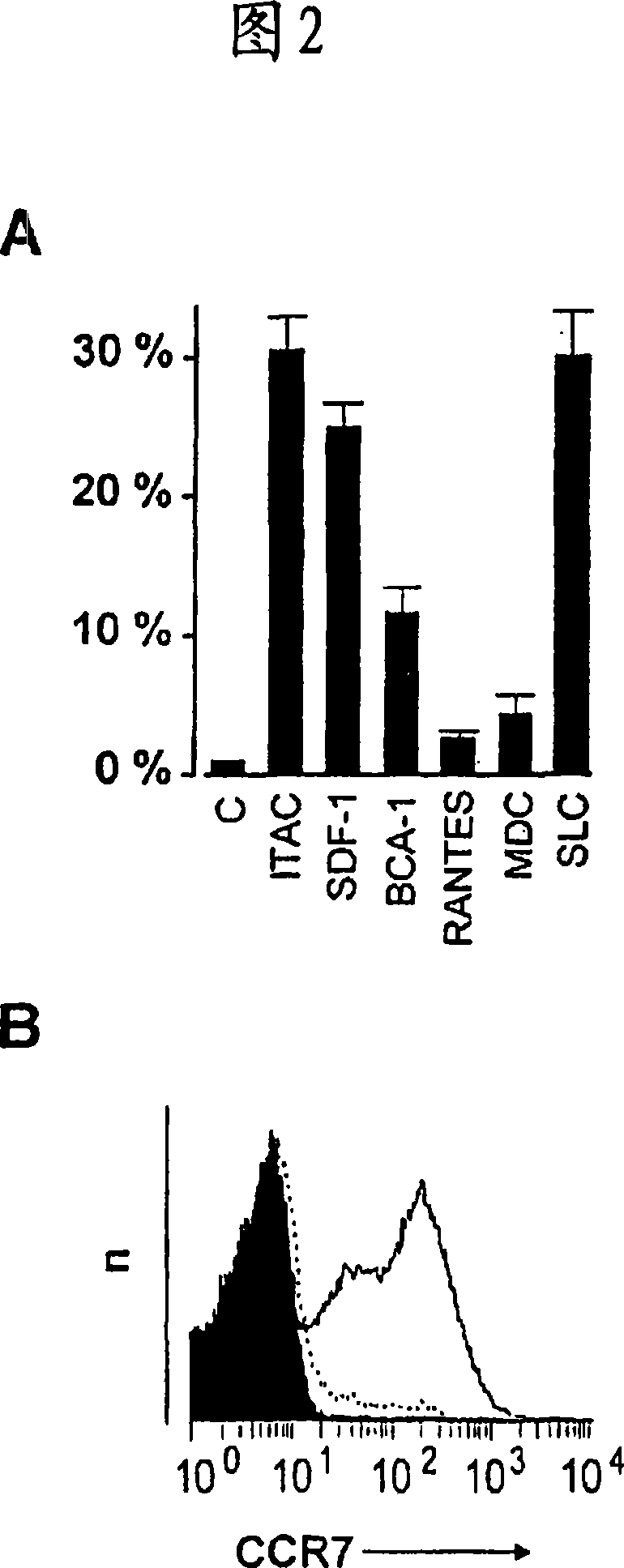
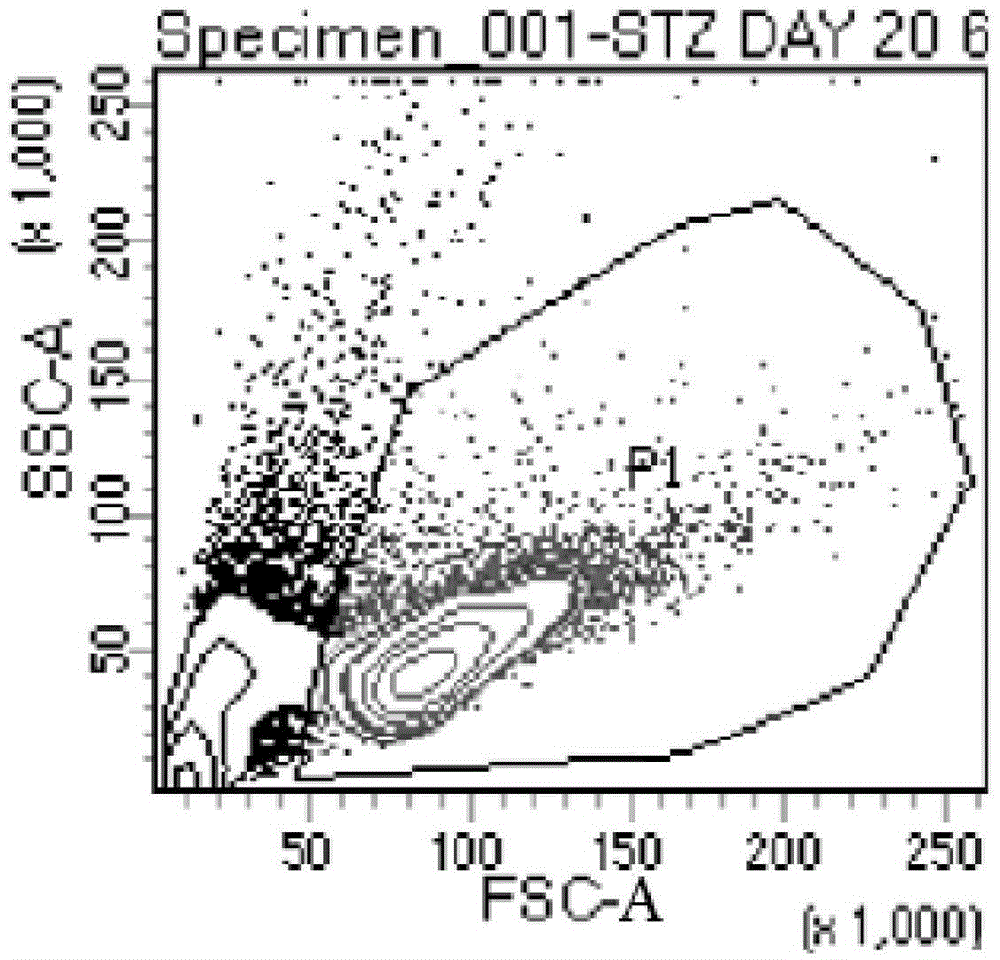
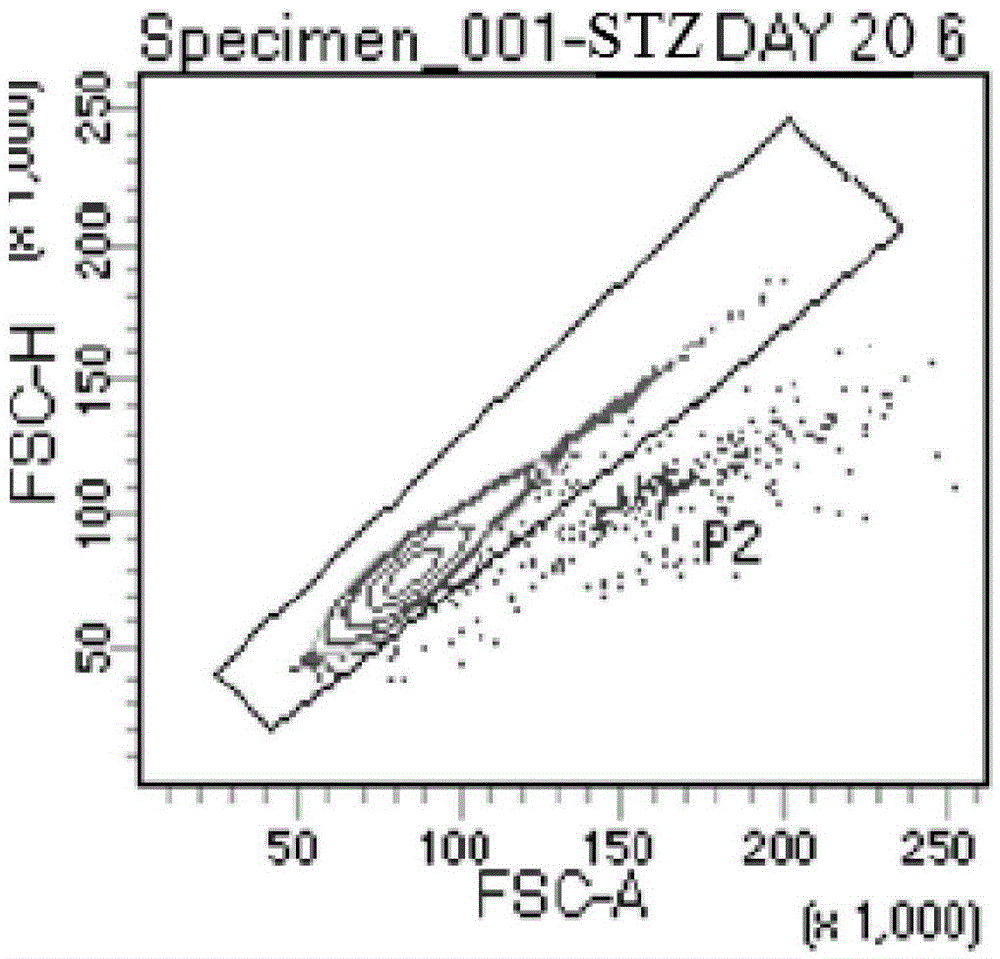
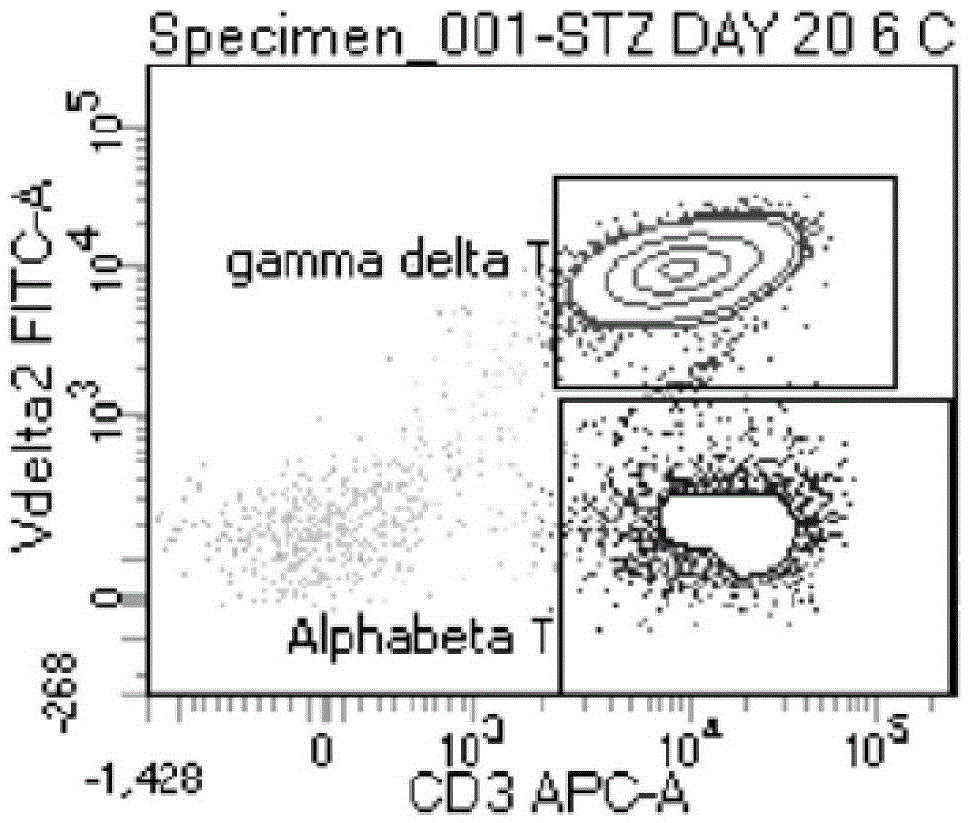
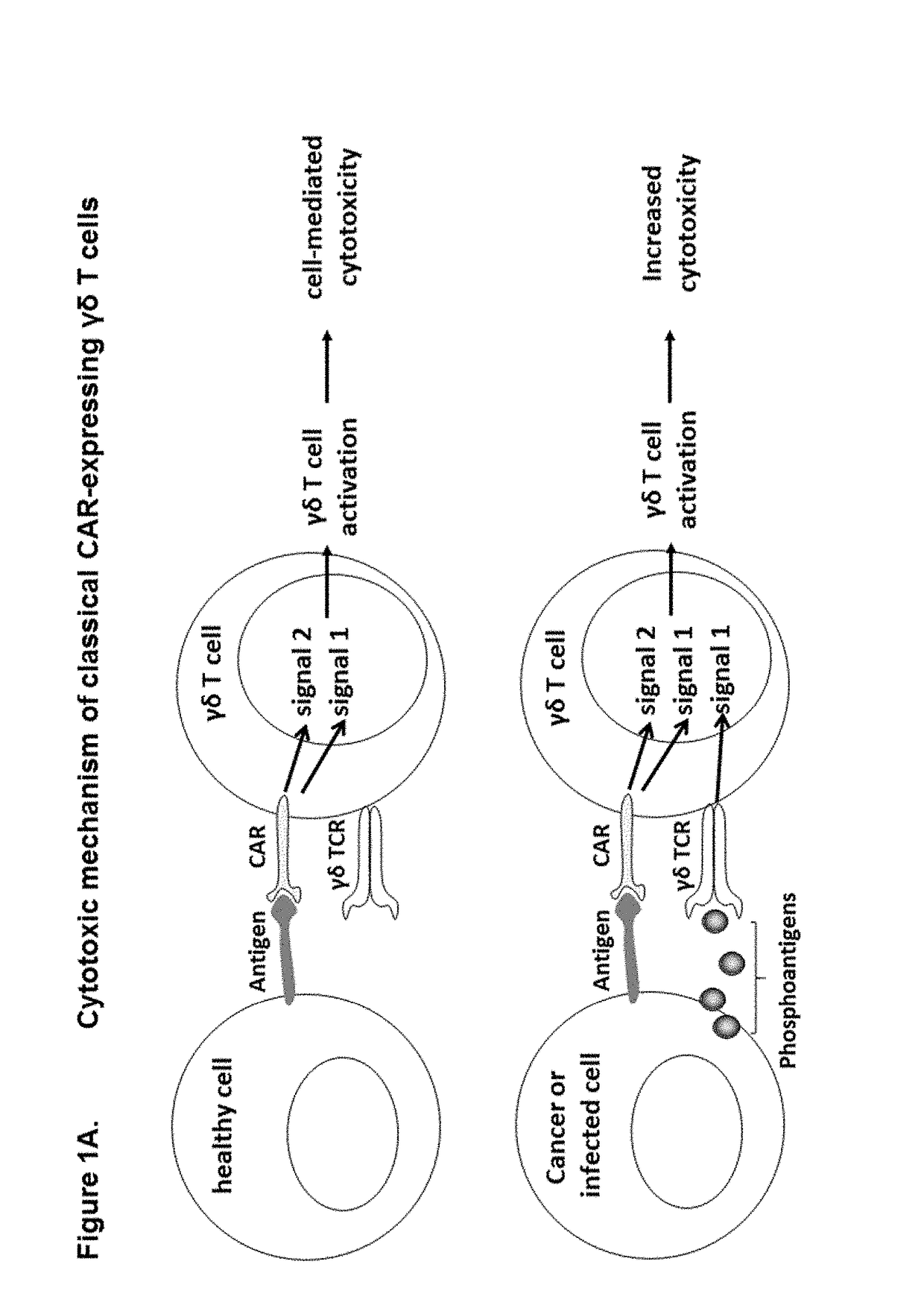
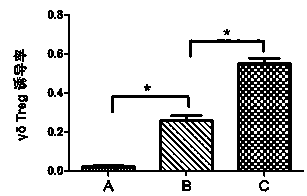
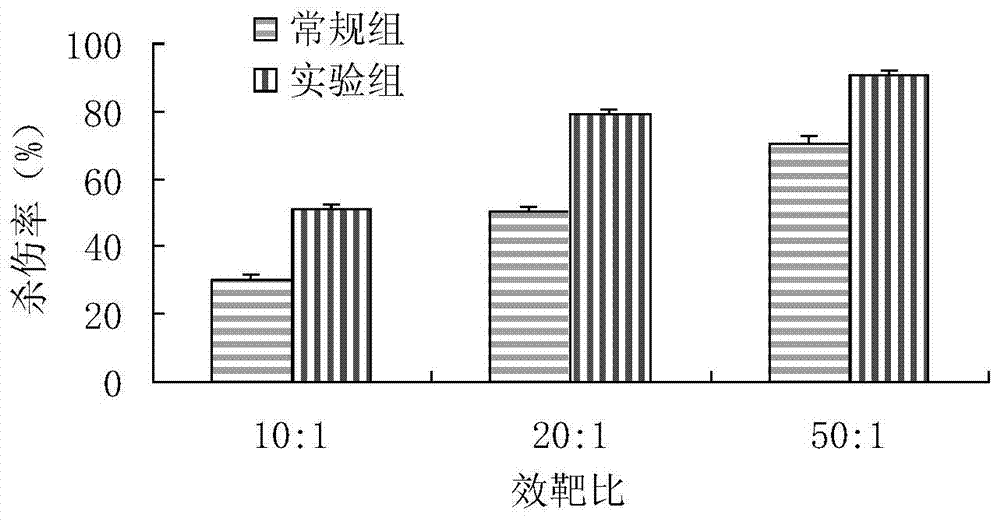
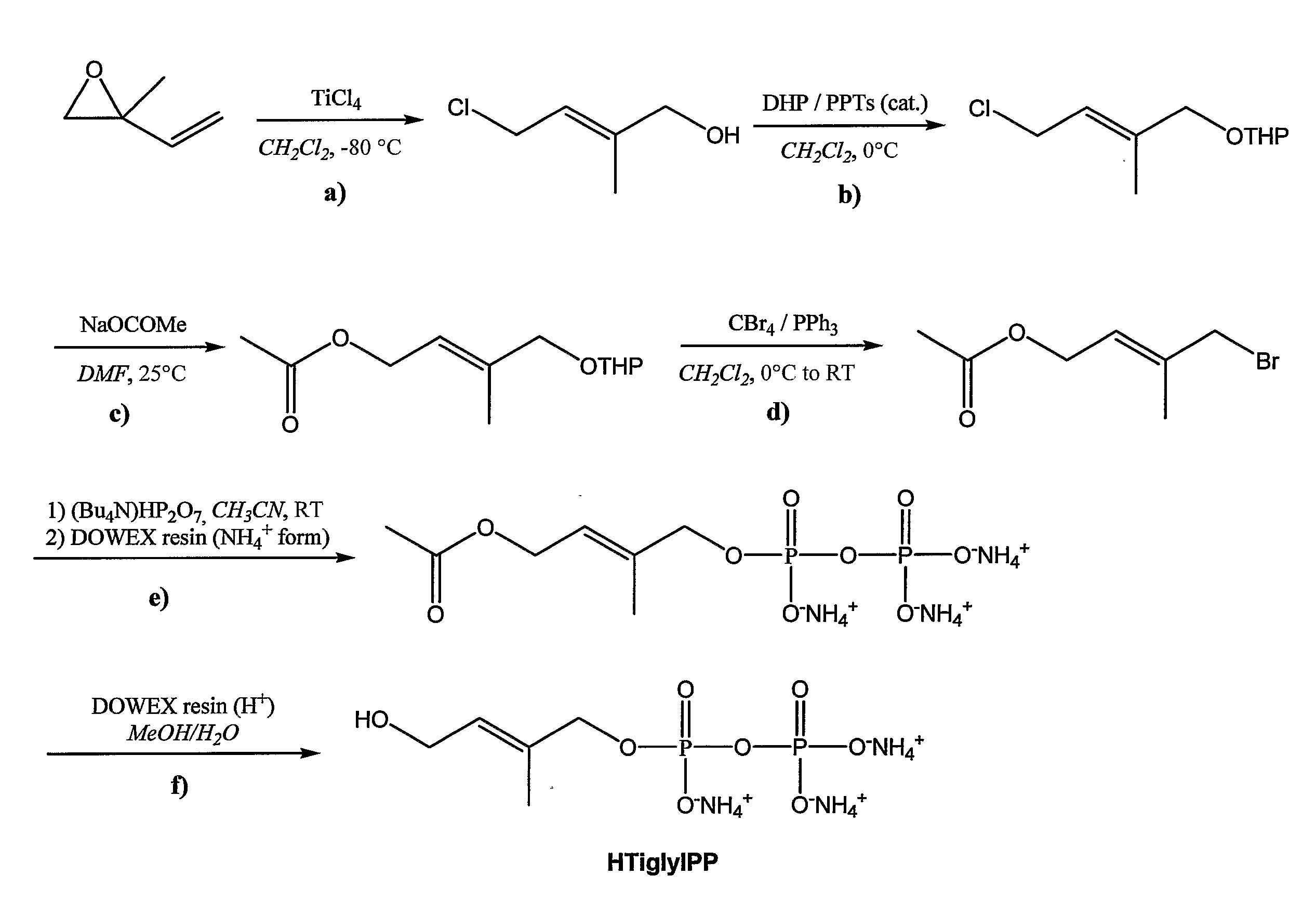
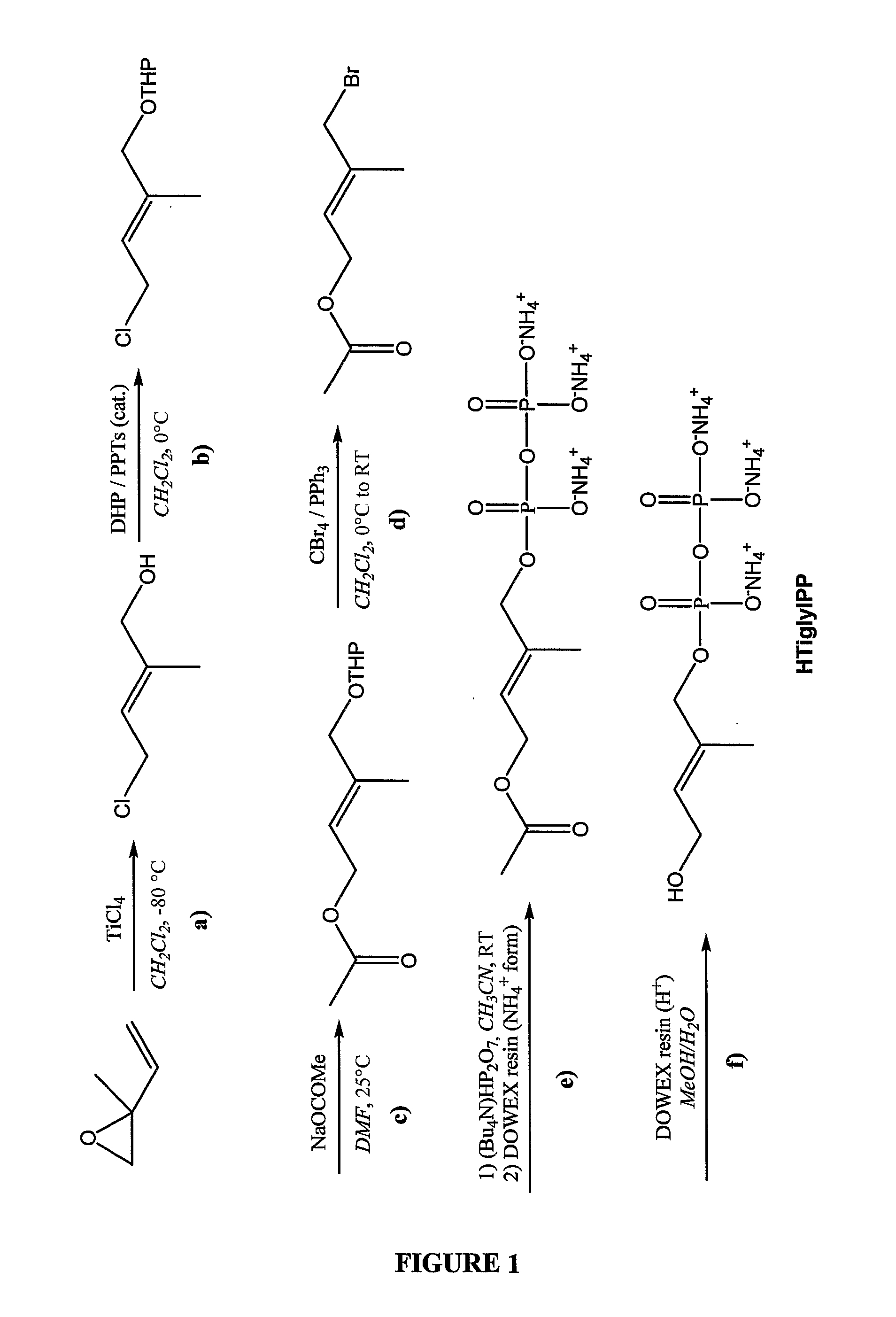
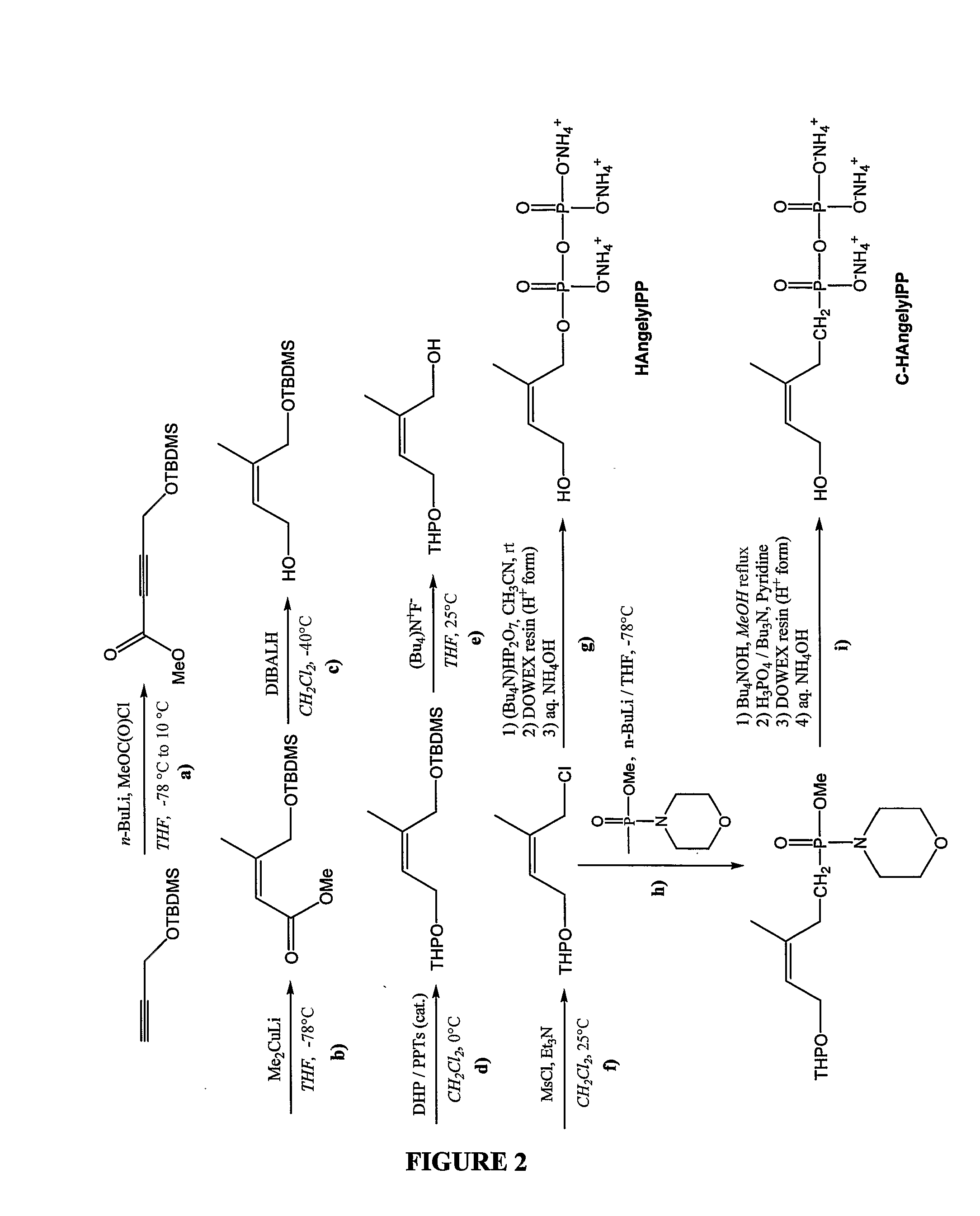
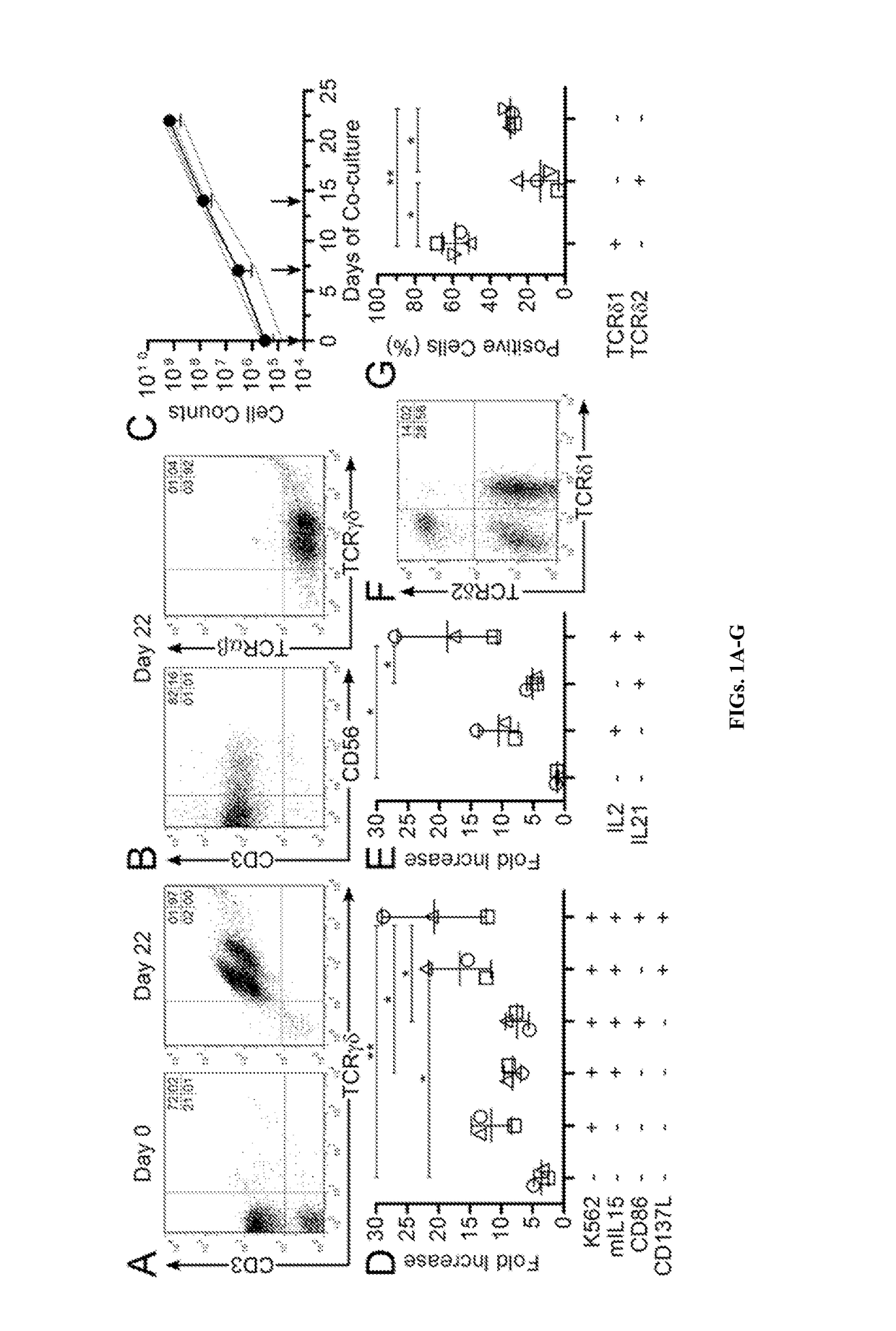
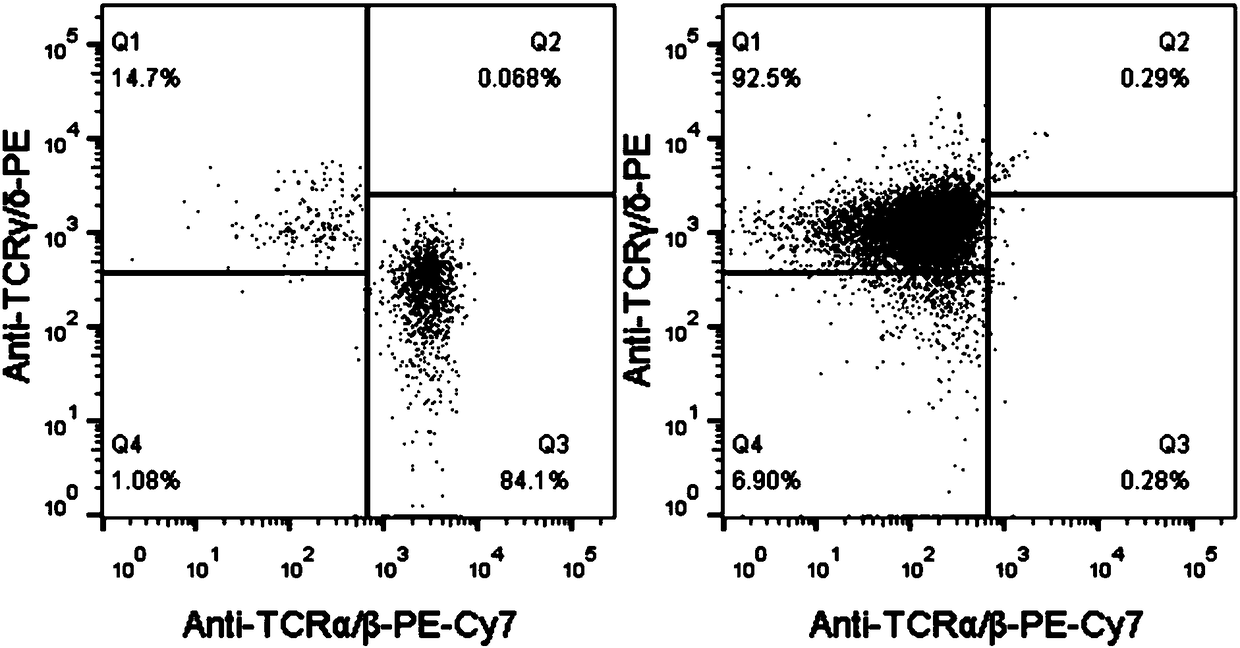
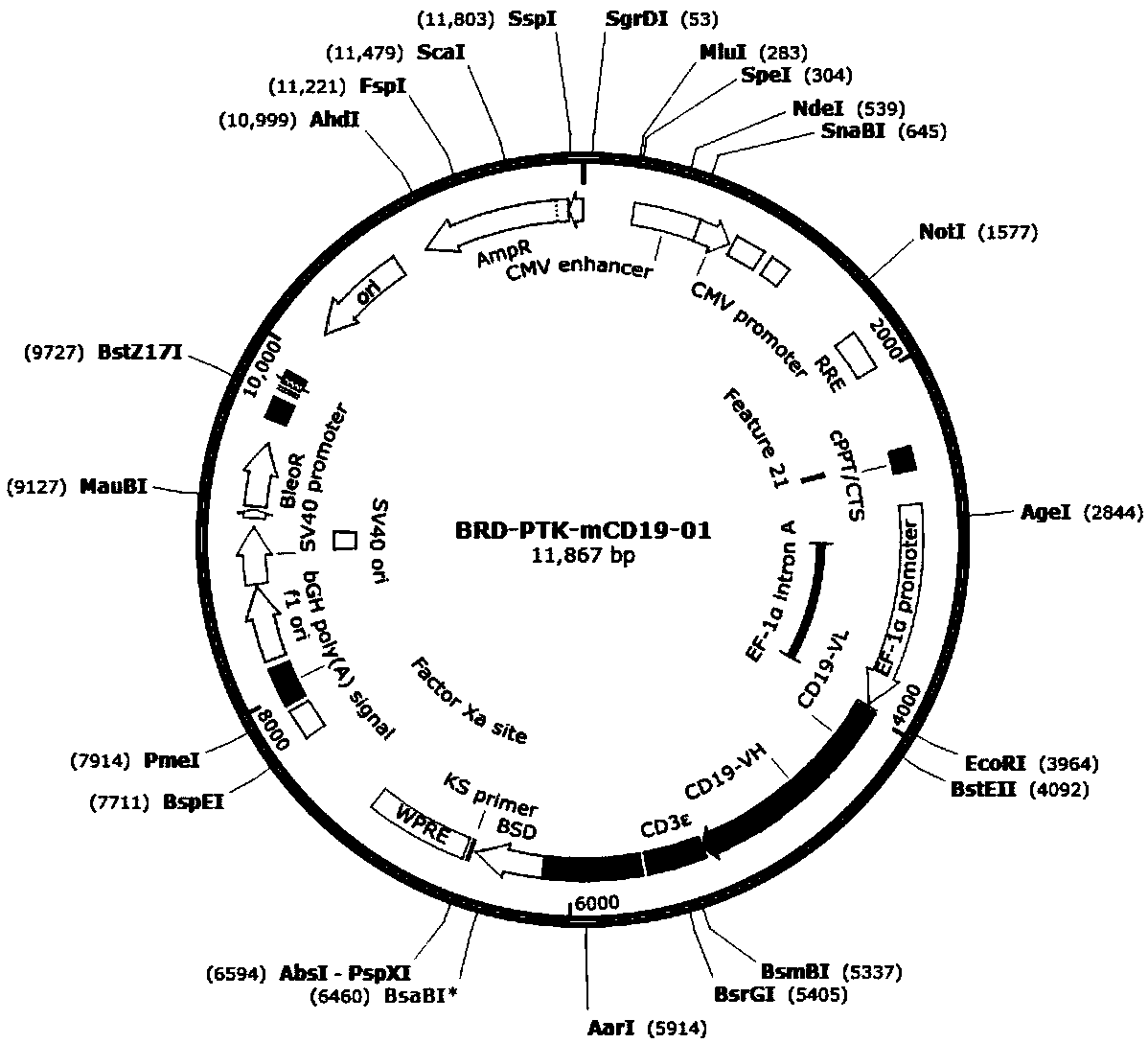
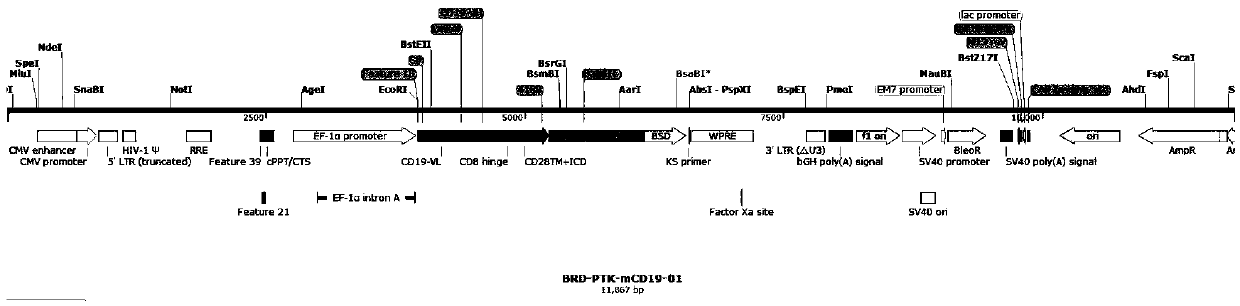
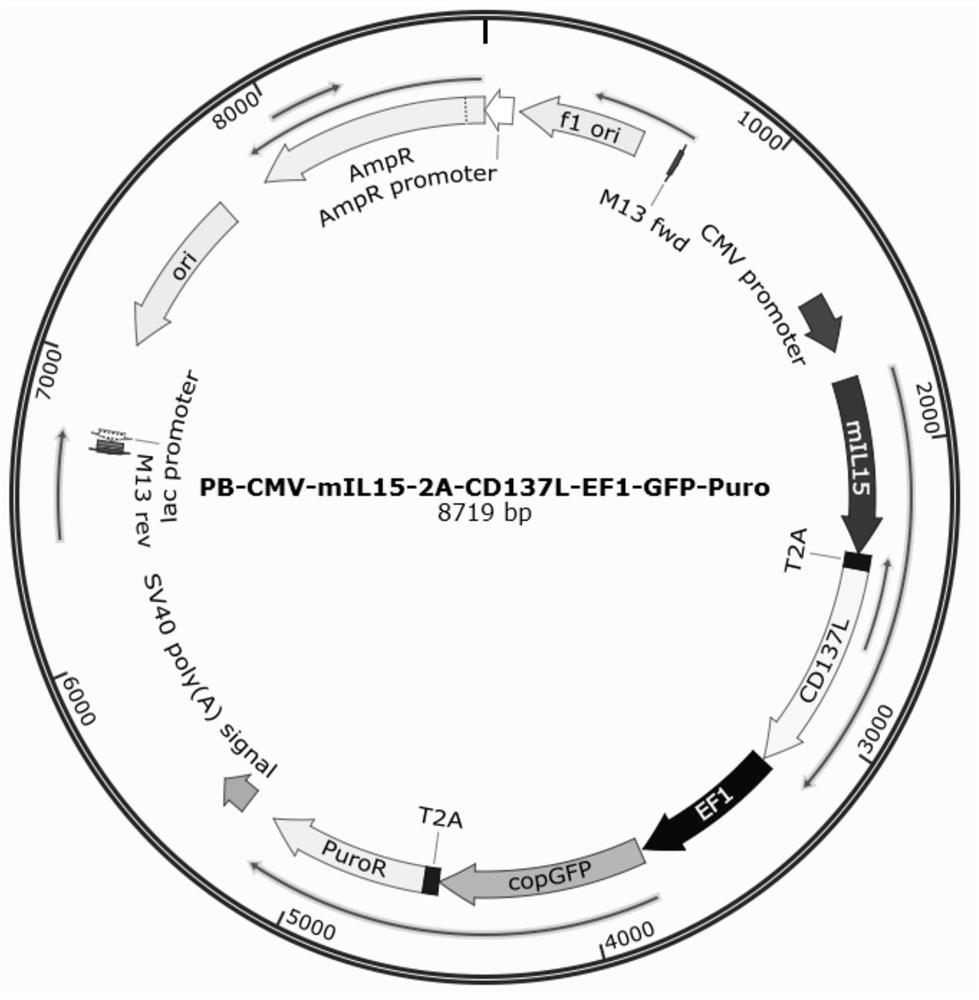
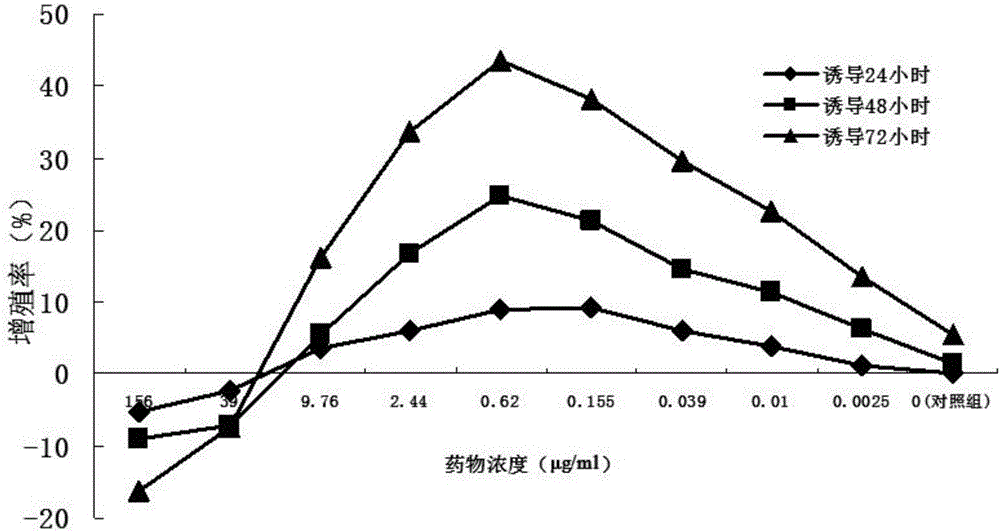
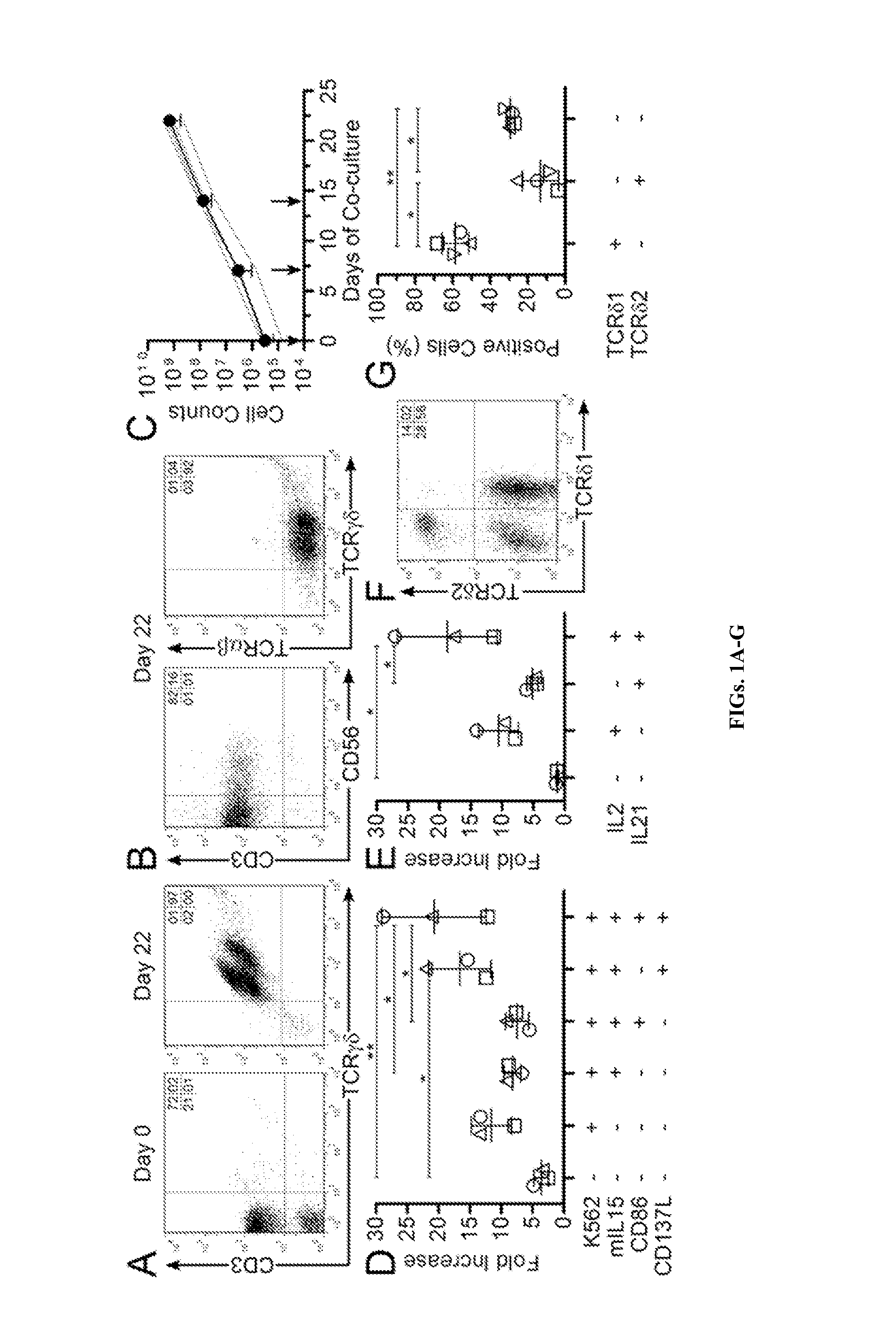
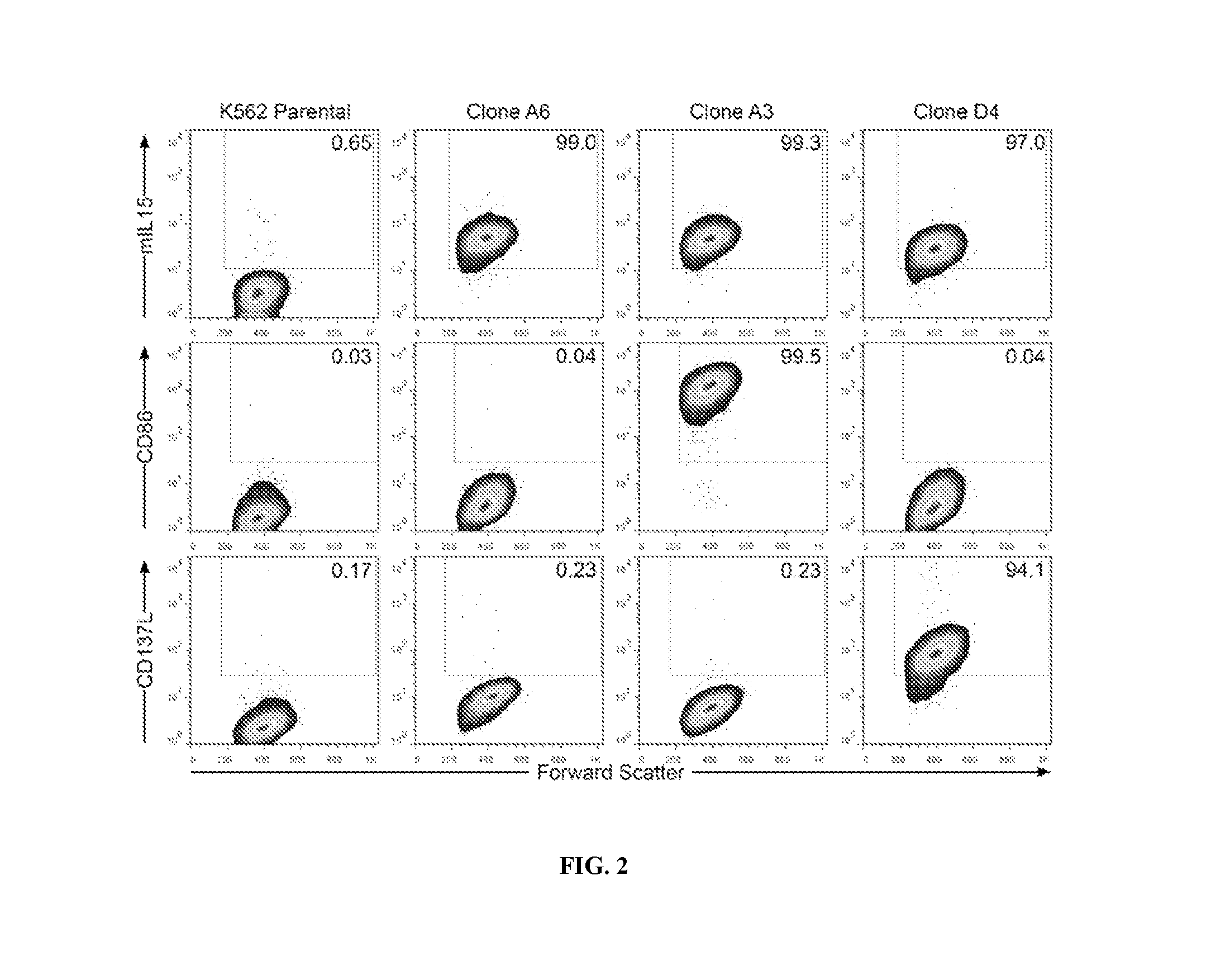
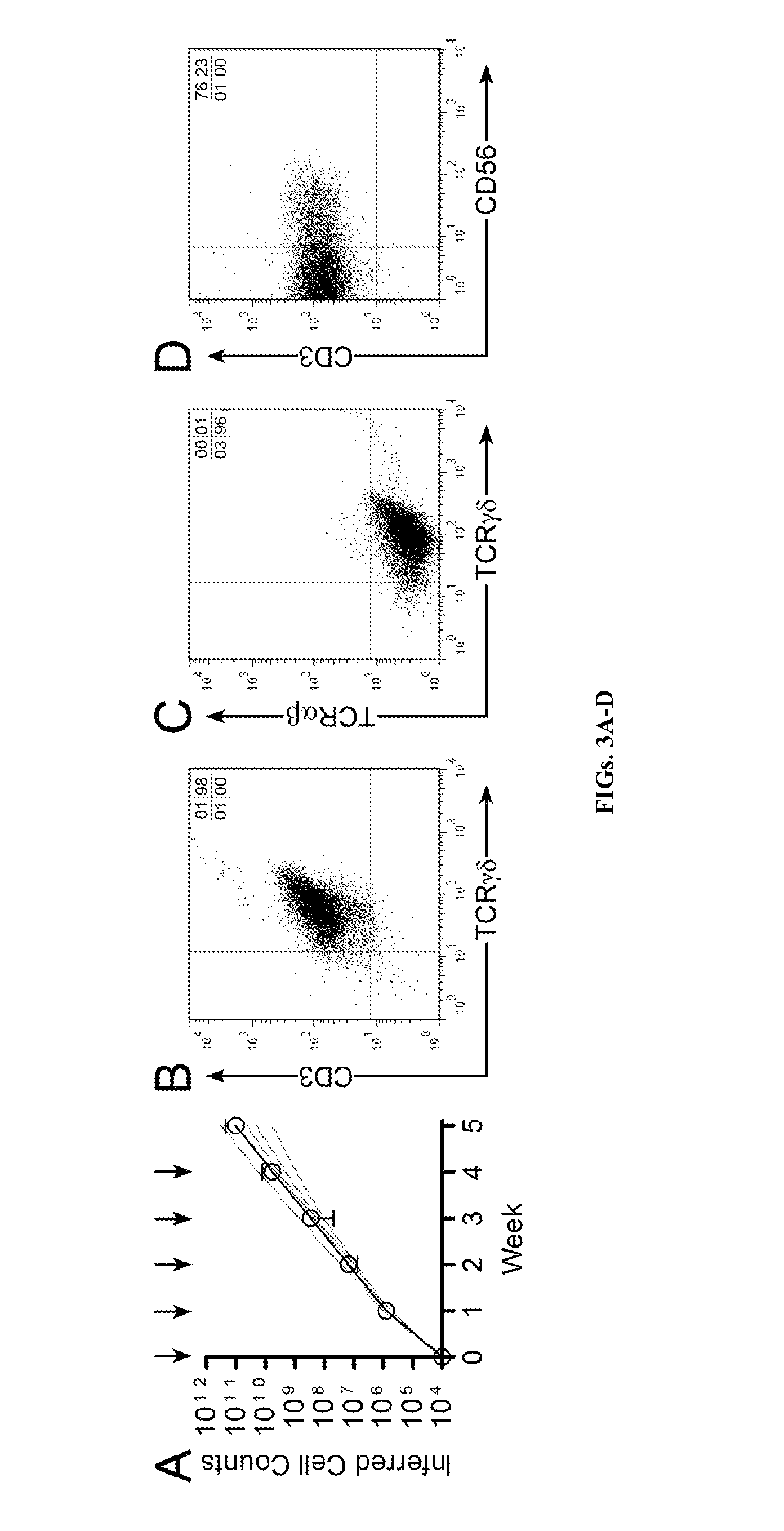
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Gamma delta T cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
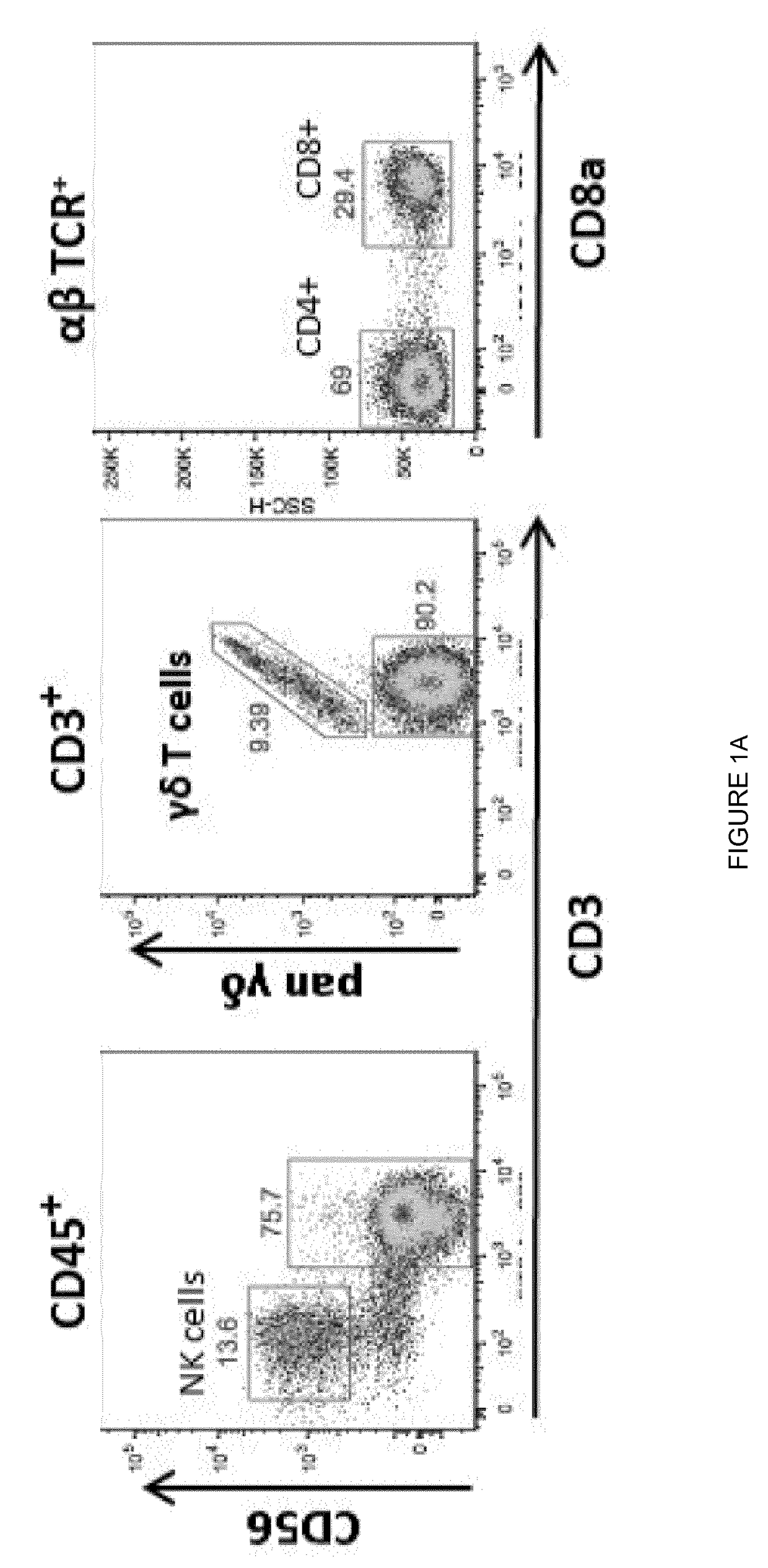
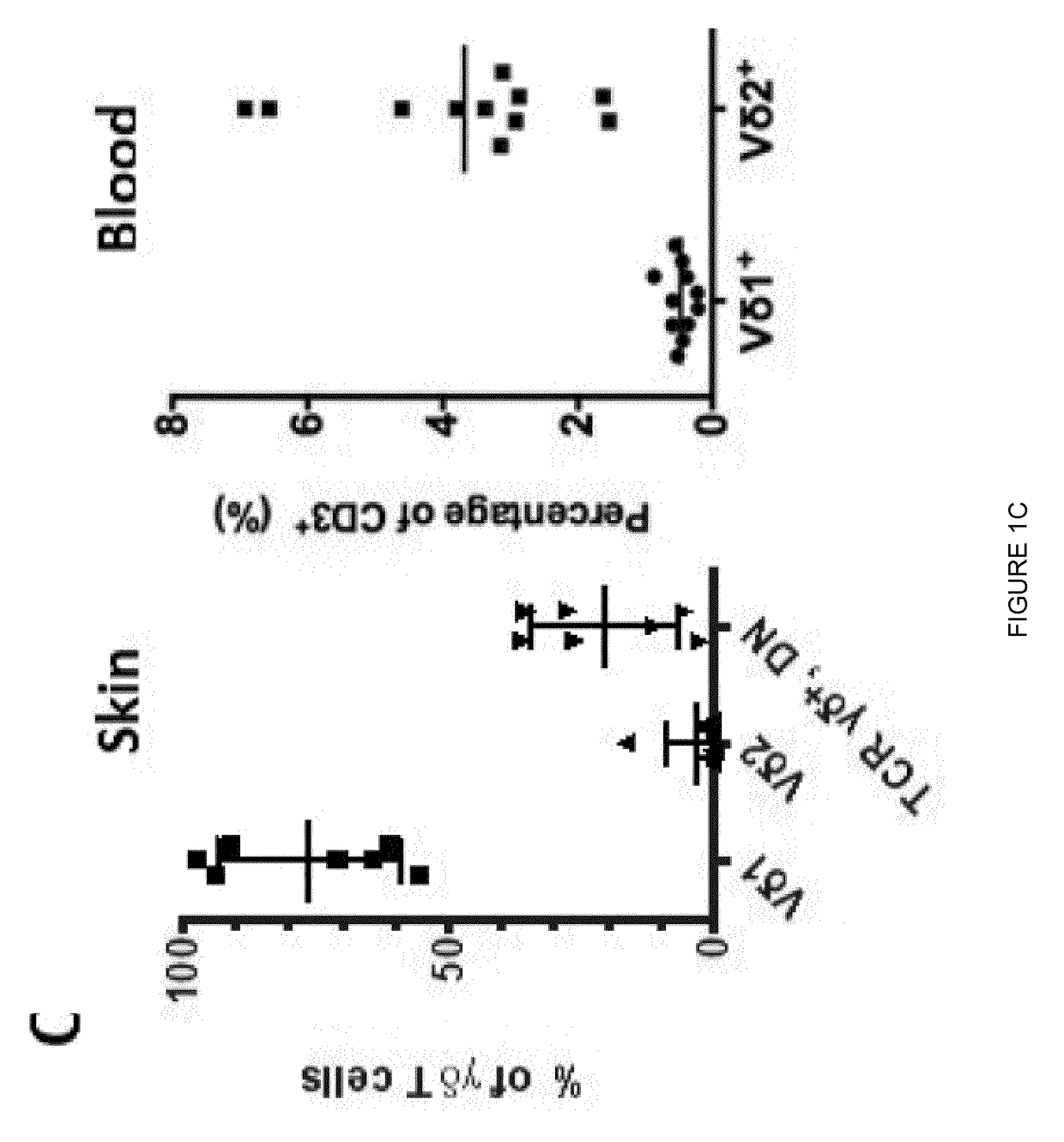
Gamma delta T cells (γδ T cells) are T cells that have a distinctive T-cell receptor (TCR) on their surface. Most T cells are αβ (alpha beta) T cells with TCR composed of two glycoprotein chains called α (alpha) and β (beta) TCR chains. In contrast, gamma delta (γδ) T cells have a TCR that is made up of one γ (gamma) chain and one δ (delta) chain. This group of T cells is usually less common than αβ T cells, but are at their highest abundance in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs).
Method for efficiently multiplying gamma delta T cells by stimulating peripheral blood in vitro and application of method
ActiveCN105112370AIntact Antitumor CytotoxicityMammal material medical ingredientsBlood/immune system cellsWhite blood cellAntigen receptors
The invention belongs to the field of medical biology engineering, and particularly relates to a method for effectively multiplying gamma delta T cells by stimulating peripheral blood in vitro and application of the method. The method comprises the step of using feeder cells, an OKT3 (ornithine ketoacid transaminase) antibody, interleukin-2 and zoledronic acid. The feeder cells are formed by specifically inserting CD64, CD86 and CD137L genes in a target site of a genome of the feeder cells. After the zoledronic acid and the nterleukin-2 are used for increasing the proportion of the gamma delta T cells of the peripheral blood, protein products of genes, the OKT3 antibody and the interleukin-2 act in a combined manner, and the gamma delta T cells can be stimulated so that a large amount of gamma delta T cells can be multiplied. The multiplied gamma delta T cells can be used for killing tumor cells which are pretreated by the zoledronic acid, or the tumor cells can be directly killed by modifying and expressing chimeric antigen receptors (CAR) via a genetic engineering means. The gamma delta T cells which are obtained by the method have complete anti-tumor cytotoxicity, and can kill solid tumor cells and non-solid tumor cells.
Owner:杭州朔溪生物医药有限公司
Composition and method for the treatment of carcinoma
InactiveUS20070134273A1Good effectConvenient treatmentAntibacterial agentsBiocideMycobacterial antigenCompound (substance)
The present invention relates to compositions and methods useful for treating a carcinoma or viral infection in mammals, including humans. The methods and compositions typically comprise use of an immunogenic or immunomodulatory compound, and a gamma delta T cell activator, such that the composition is effective for treating a carcinoma or viral infection. In a preferred aspect of the invention, the methods comprise use of a gamma delta T cell activator and a Mycobacterium antigen, which for example is an attenuated strain of Mycobacterium bovis (Bacillus Calmette-Guerin (BCG)).
Owner:ROMAGNE FRANCOIS +1
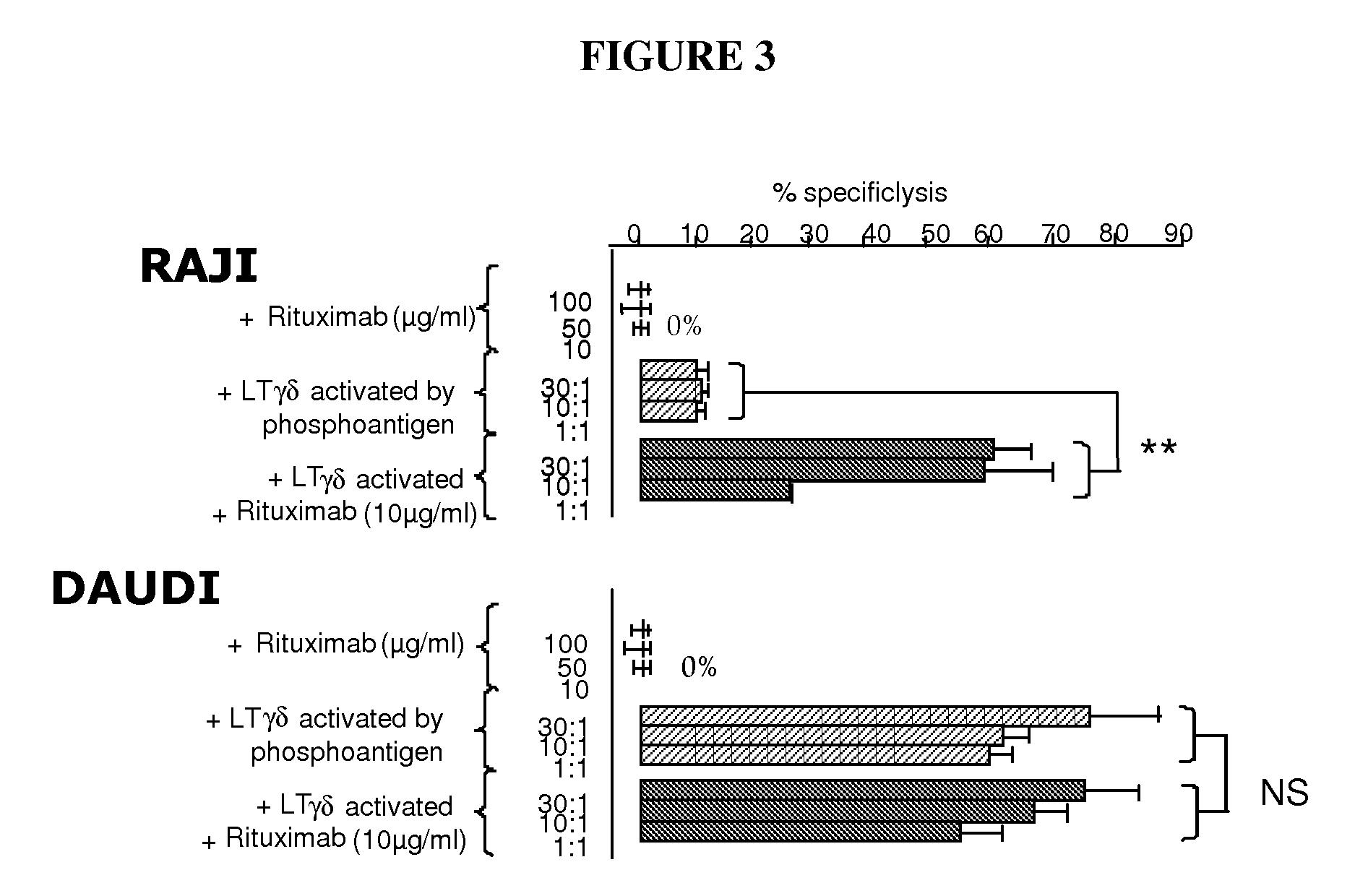
Methods and Compositions for Increasing the Efficiency of Therapeutic Antibodies Using Gamma Delta T Cell Activators
InactiveUS20090304688A1Good curative effectGreater target cell lysisOrganic active ingredientsAntiviralsTherapeutic antibodyT cell
The present invention relates to methods and compositions for increasing the efficiency of therapeutic antibodies. More particularly, the invention relates to the use of a therapeutic antibody in combination with a γδ T cell activating compound or activated γδ T cells. thereby allowing a potentiation of γδ T cell cytotoxicity in mammalian subjects in order to enhance the efficiency of the treatment in human subjects, particularly through an increase of the depletion of targeted cells.
Owner:INNATE PHARMA SA +1
Method for in-vitro amplification of gamma-delta-T cells
InactiveCN102994448AFully stimulatedInhibit apoptosisBlood/immune system cellsPeripheral blood mononuclear cellMicrobiology
The invention relates to a method for culturing gamma-delta-T cells, and in particular relates to a method for in-vitro amplification of gamma-delta-T cells, wherein the method comprises the following operating steps of: pre-coating a T75 culture bottle by a TCR-gamma-delta resisting antibody and CD28McAb for later use use; isolating the peripheral blood mononuclear cell (PBMC) of a patient; regulating the PBMC concentration to 1*10<6> 6 / ml by a serum-free culture medium which contains 5% of autologous plasma, and transferring PBMC cell suspension into the T75 culture bottle; adding an initial culture medium which contains proper concentrations of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; culturing in a saturated humid environment containing 5% of CO2 at 37 DEG C; depending on growth situation of the cell, changing the culture medium every 2-3days, to control the cell concentration at about 2.5*10<6> / ml; meanwhile, compensating full doses of Zoledronat, HSP70, 1L-2, 1L-7 and 1L-15; and continuously culturing for 12-16days, to obtain a great amount of gamma-delta-T cells which are comparatively high in purity.
Owner:SHANGHAI CLAISON BIOTECH
Preparation of antigen-presenting human gamma delta t cells and use in immunotherapy
InactiveCN101031641AGood effectEffective antigen uptakeTissue cultureVaccinationEpidermal Dendritic Cells
The invention relates to a method for the preparation of efficient antigen-presenting human Gamma Delta T cells, to the Gamma Delta T cells prepared by such a method, and to their use in immunotherapy, vaccination, vaccine development and diagnostics. Similar to dendritic cells (DCs) in potency and efficacy, these human Gamma Delta T cells process antigens and present antigenic peptides to Alpha Beta T cells and induce antigen-specific responses (proliferation and differentiation) in nave Alpha Beta T cells. Gamma Delta T cells are easily purified from peripheral blood, acquire''maturation'' status (expression of essential adhesion, co-stimulatory and major histocompatibility complex molecules) within 1 day of in vitro culture under stimulation and induce strong primary and secondary T helper cell and cytotoxic T cell responses. The Gamma Delta T cells may be used in a method of treatment of tumors or chronic or recurrent infectious diseases, in identification of novel tumor or pathogen-derived antigens, and in the diagnosis of the immune competence of a patient.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
Expansion method of various lymphocyte subpopulations and application of expansion method
ActiveCN105624107ASuppress deathImprove developmentGenetic material ingredientsMammal material medical ingredientsWhite blood cellNatural Killer Cell Inhibitory Receptors
The invention discloses an expansion method of various lymphocyte subpopulations and application of the expansion method to preparation of an adoptive immunotherapy medicine for cancers. The expansion method comprises the following steps: S1, adding IFN gamma and zoledronic acid into a culture medium of PBMC collected in the blood of a tumor patient, and re-adding an OKT3 antibody and recombinant human interleukin-2 after culture to stimulate PBMC so as to finish preliminary expansion; S2, after finish of preliminary expansion, mixing immune lymphocytes with feeder layer cells, and after adding zoledronic acid, an OKT3 antibody and recombinant human interleukin-2, continually performing expansion. By adoption of the expansion method, after twice expansion, the sum of the immune cells can be 10,000 to 80,000 times by expansion, the expanded immune cells include alpha beta T cells, gamma delta T cells, NKT cells and NK cells, and tumor cells can be directly killed, or a cellular immunotherapy drug is prepared to kill the tumor cells.
Owner:杭州朔溪生物医药有限公司
Modified gamma delta t cells and uses thereof
ActiveUS20180125889A1Antibacterial agentsOrganic active ingredientsGamma-delta T-Cell ReceptorAntigen receptors
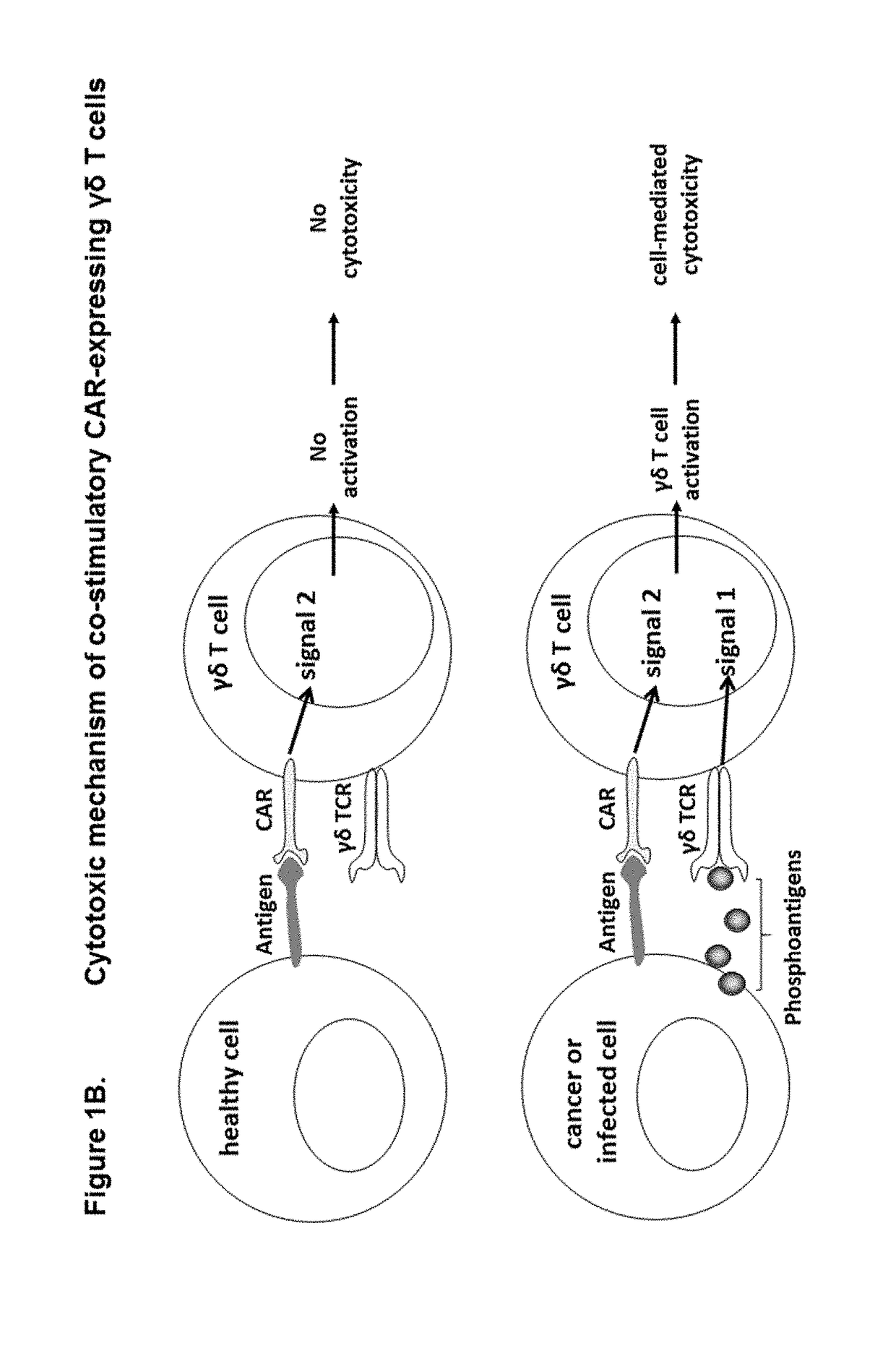
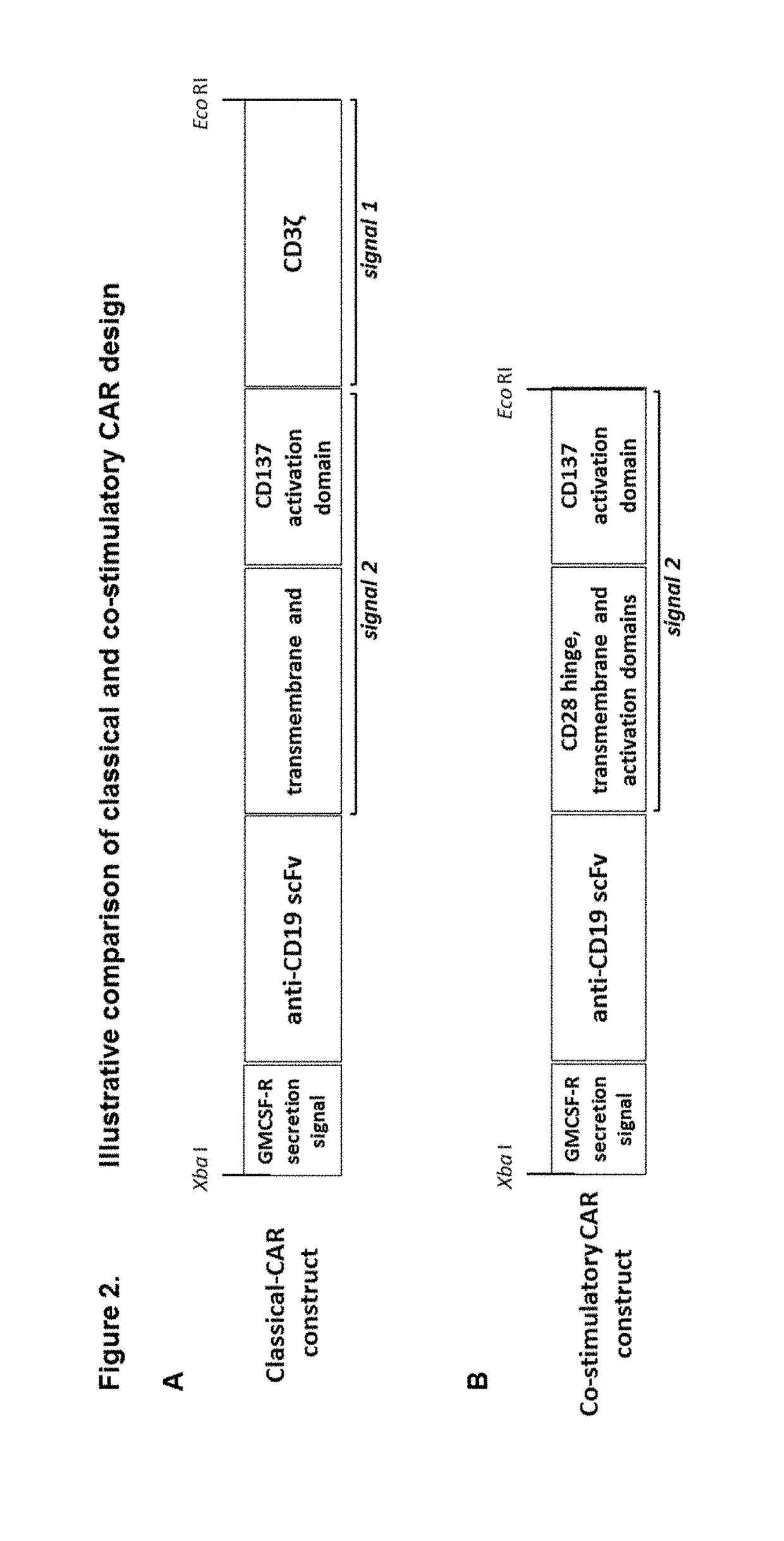
The present invention provides composition and methods for the treatment of cancer or infectious diseases in a human. The invention includes the generation and administration of gamma delta T cells that express chimeric antigen receptors (CARs) comprising an antigen binding domain, a hinge domain, a transmembrane domain, a costimulatory signalling domain with the inclusion or not of a CD3 zeta signalling domain. Expression of CAR sequence omitting the CD3 zeta signalling domain in gamma delta T cells, provides for a CAR-T therapy in vivo, which will effect cytolysis only on target cells providing ligands for activation of the gamma delta T cell receptor (TCR).
Owner:TC BIOPHARM LTD
Inducing culture method for regulatory T cell
ActiveCN102168067AGood energyShort cycleBlood/immune system cellsIndividual particle analysisCell sorterRegulatory T cell
The invention provides an inducing culture method for regulatory T cells and relates to an inducing culture method for regulatory gamma delta T cells. In the method, cell factors are applied and deoxyribonucleic acid (DNA) demethylated medicament decitabine is combined so as to cooperatively induce the generation of the regulatory gamma delta T cells, and an enrichment process of magnetic cell sorter (MACS) positive sorting is adopted; after enrichment, detections of cell growth state and activity are carried out so as to ensure the next experience. The method has the advantages of simple and practicable inducing and enrichment processes, short period, low cost, high inducing efficiency and good repeatability; and after enrichment, cell activity is good, the quantity and activity of cells can ensure the next in vivo and in vitro studies, thereby providing a good study platform for defining the biological characteristics of the gamma delta T cells. Thus, the method has a good popularization value.
Owner:ZHEJIANG UNIV
Composition for stimulating and inducing single karyocyte to be amplified to gamma deltaT cell and application of composition
ActiveCN108220239AImprove proliferative abilityHigh purityBlood/immune system cellsCell culture active agentsHigh cellPeripheral blood mononuclear cell
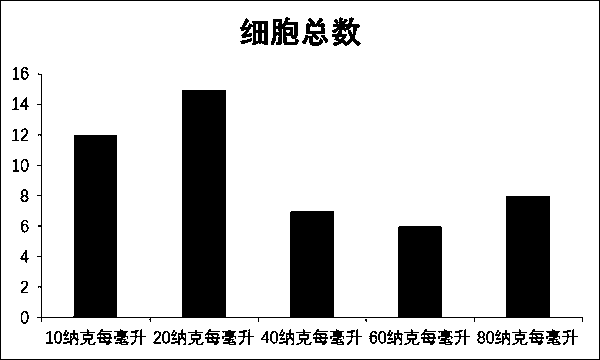
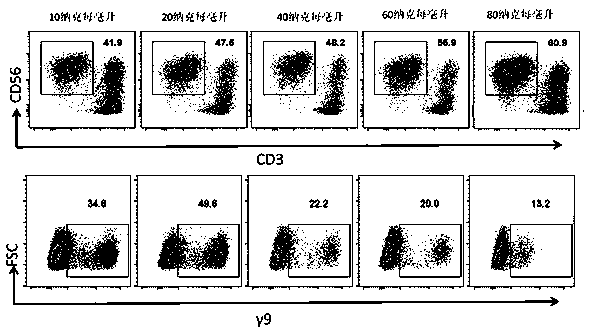
The invention relates to a composition for stimulating and inducing a single aryocyte to be amplified to a gamma delta T cell. The composition is prepared from zoledronic acid, anti-human CD3Ab, anti-human CD28Ab, IL-15, IL-21 and IL-2. The invention also provides a method for the in-vitro mass amplification of gamma deltaT cells by utilizing the composition, and particularly relates to a method for in-vitro inducing the mass amplification of gamma deltaT cells by utilizing PBMCs. The zoledronic acid is used for induction and activation, the anti-human CD3Ab, the anti-human CD28Ab, IL-15, IL-21 and IL-2 are used for inducing and activating the proliferation, and various amplification factor combinations to jointly stimulate and induce the amplification of gamma deltaT cells, and the obtained gamma deltaT cell has the characteristics of large quantity, high purity, high cell toxicity and the like and has good clinical application value.
Owner:安徽瑞达健康产业有限公司
Culture method of rapamycin induced regulatory gamma delta T cells
ActiveCN103436493AVigorousStrong immunosuppressive functionBlood/immune system cellsBiochemistryCytokine
The invention provides a culture method of rapamycin induced regulatory gamma delta T cells. The culture method is characterized in that mononuclear cells derived from human peripheral blood are amplified by uniting multiple cell factors with macrolides immunosuppressor-rapamycin through induction in vitro, so that the lots of regulatory gamma delta T cells are cultured. The culture method is simple in the whole process of induction, amplification and enrichment, short in cell culture period and low in cost, has strong feasibility, high induction efficiency and good repeatability and can be applied to the culture of the regulatory gamma delta T cells. The regulatory gamma delta T cells induced by the method have good activity, a relatively strong immunosuppression function and very high popularization value and can be used for ensuring the further research.
Owner:ZHEJIANG UNIV
Method used for in vitro amplification of gamma-delta-T cells
InactiveCN103756962AFully stimulatedInhibit apoptosisBlood/immune system cellsPeripheral blood mononuclear cellLevamisole
The invention relates to a method for culturing gamma-delta-T cells, and more specifically relates to a method for in-vitro amplification of gamma-delta-T cells. The method comprises the following operating steps: pre-coating a T75 culture bottle with a TCR-gamma-delta resisting antibody and CD28McAb for later use; isolating peripheral blood mononuclear cells (PBMC) of patients; regulating PBMC concentration to 1*10<6> / ml with a serum-free culture medium which contains 5% of autologous plasma, and transferring the PBMC cell suspension into the T75 culture bottle; adding an initial culture medium which contains proper concentrations of Zoledronat, HSP70, Toll-like Receptors7 (TLR7) ligand, Levamisole (LMS), IL-2, IL-7 and IL-15; culturing in a saturated humid environment containing 5% of CO2 at 37 DEG C; according to growth situation of the cell, changing the culture medium every 2 to 3 days so as to control the cell concentration at about 2.5*10<6> / ml; meanwhile, compensating full doses of Zoledronat, HSP70, Toll-like Receptors7 (TLR7) ligand, Levamisole (LMS), IL-2, IL-7 and IL-15;; and continuously culturing for 12to 16 days so as to obtain a great amount of gamma-delta-T cells which are comparatively high in purity.
Owner:SHANGHAI CLAISON BIOTECH
Class of Gamma Delta T Cells Activators and Use Thereof
Owner:INNATE PHARMA SA
Polyclonal gamma delta T cells for immunotherapy
ActiveUS9907820B2Improve effectivenessConvenient treatmentAntibacterial agentsGenetically modified cellsAbnormal tissue growthT cell
Provided herein is a method of expanding clinically-relevant quantities of polyclonal γδ T cells that have anti-tumor, anti-viral, and anti-bacterial reactivity. Polyclonal γδ T cells can target a variety of tumors, including solid tumors as well as other conditions, such as viral and bacterial infections.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
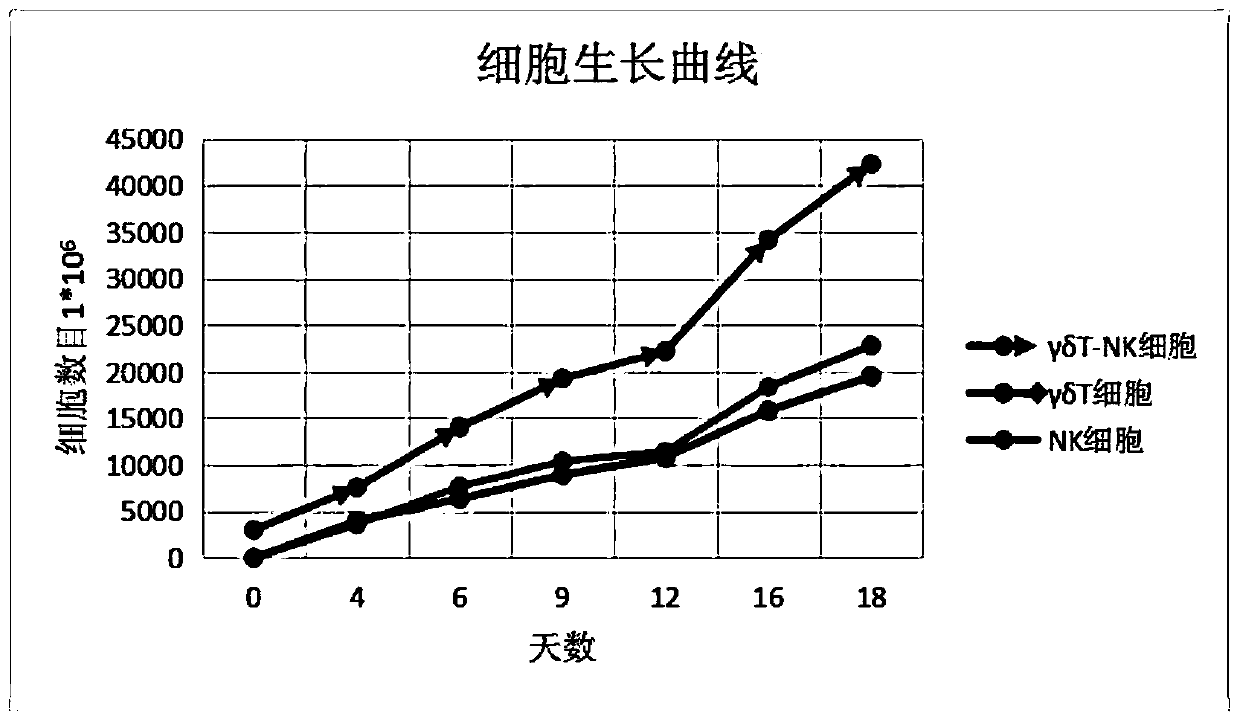
Method for co-culturing, inducing and amplifying gamma delta T cells and NK cells
InactiveCN110564683AImprove cultivation efficiencyToxic/activeCulture processMammal material medical ingredientsCytotoxicityMicrobiology
The invention discloses a method for co-culturing, inducing and amplifying gamma delta T cells and NK cells. The gamma delta T cells and the NK cells are obtained in a co-culture manner; peripheral blood is collected to separate and screen mononuclear cells and stimulate and induce the mononuclear cells to induce the gamma delta T cells and the NK cells, the gamma delta T cells and the NK cells are subjected to activation amplification culture to amplify a gamma delta T cell and NK cell composite cell substance having characteristics of large number, high amplification multiple, strong cytotoxicity and the like so as to form the co-culture cell composition through compounding, and the co-culture cell composition has good clinical application value. The co-culture induced amplification method improves the cell culture efficiency, greatly reduces the culture cost, reduces the difficulty of a culture technology, simplifies the clinical treatment process, reduces the treatment cost, and simplifies the treatment means.
Owner:安徽瑞达健康产业有限公司
Expansion of non-haematopoietic tissue-resident gamma delta t cells and uses of these cells
ActiveUS20180312808A1Quick upgradeUnique propertyMammal material medical ingredientsAntiviralsWhite blood cellBiology
This invention relates to the expansion of non-haematopoietic tissue-resident γδ T cells in vitro by culturing lymphocytes obtained from non-haematopoietic tissue of humans or non-human animals in the presence of interleukin-2 (IL-2) and / or interleukin-15 (IL-15) and the absence of TCR activation or co-stimulation signals, without any direct contact with stromal or epithelial cells. Methods of non-haematopoietic tissue-resident γδ T cell expansion are provided, as well as populations of non-haematopoietic tissue-resident γδ T cells and uses thereof.
Owner:THE FRANCIS CRICK INST LTD
Method for inducing il-2-free proliferation of gamma delta t cells
InactiveUS20150259645A1High proliferation rateIncreased toxicityOrganic active ingredientsPeptide/protein ingredientsDiseaseInducer Cells
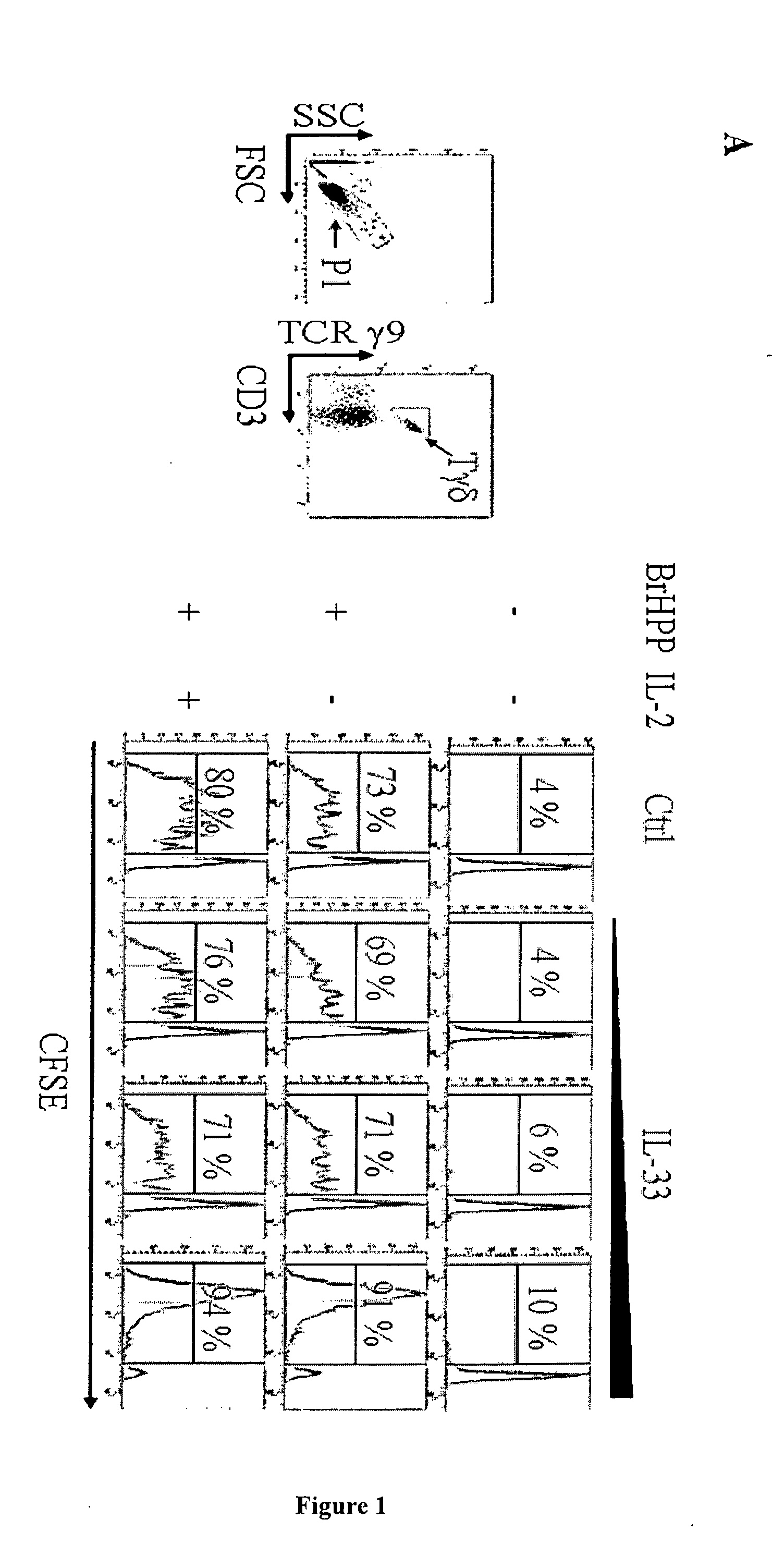
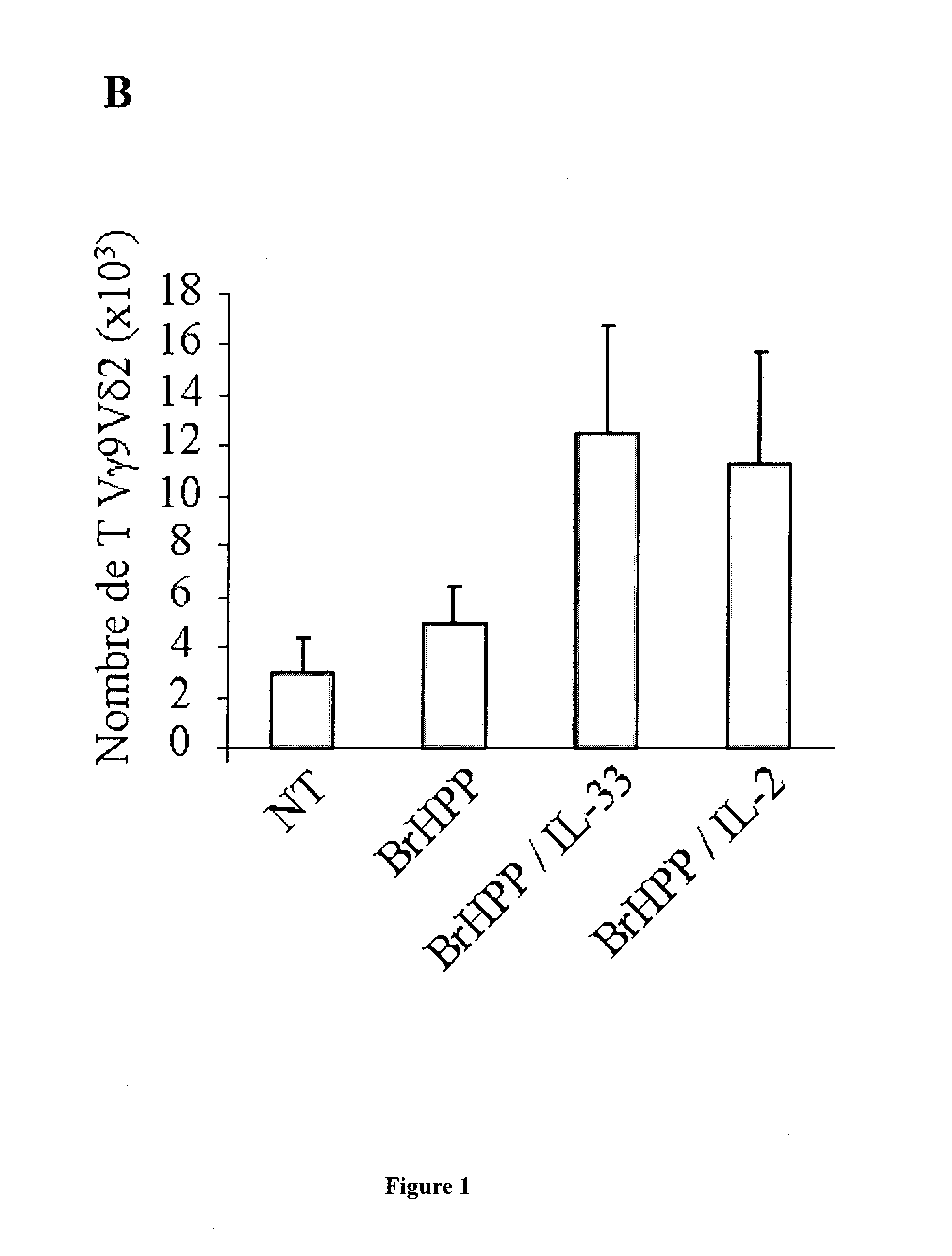
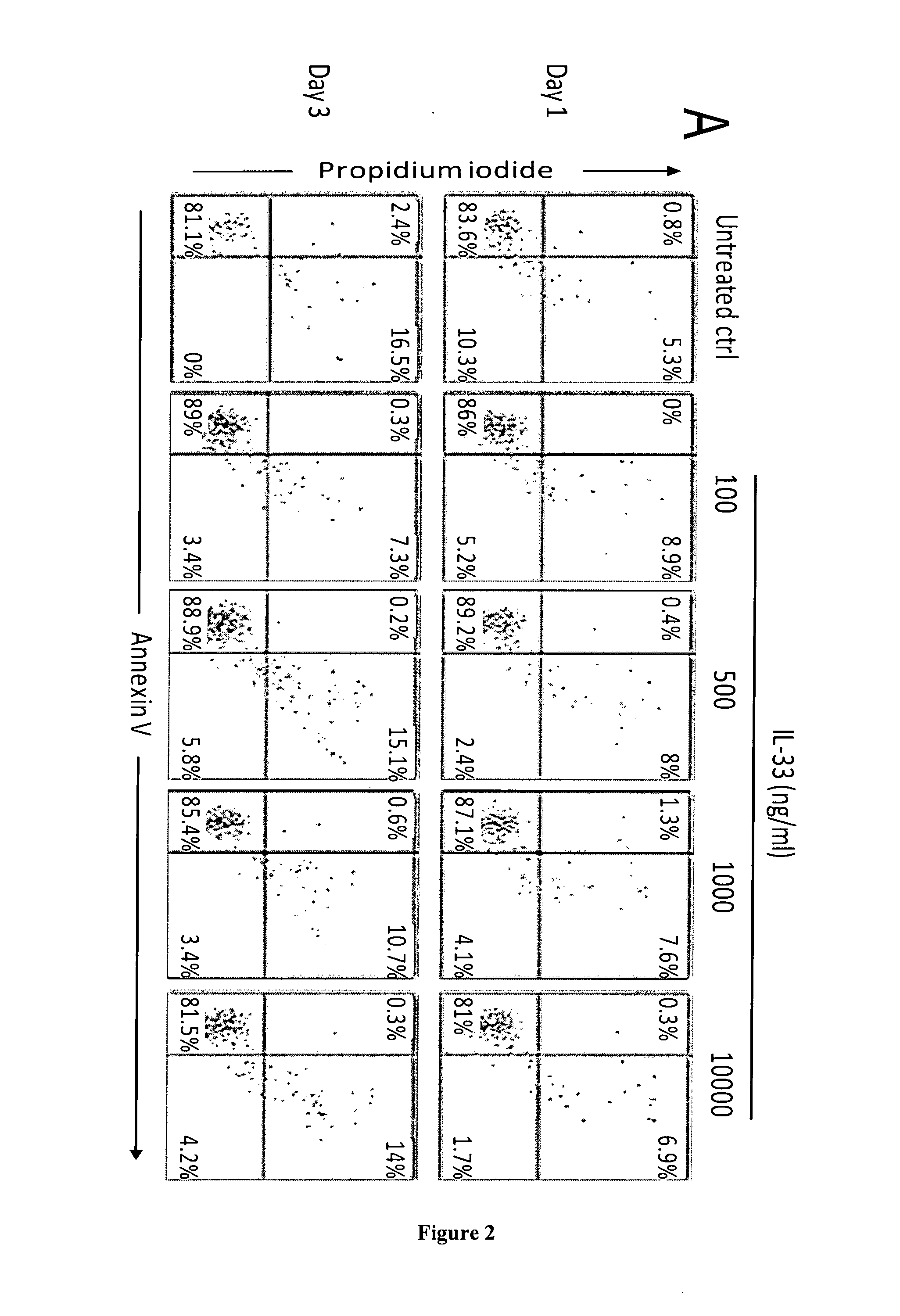
The present invention concerns a method of inducing IL-2-free proliferation of γδ T cells using a combination of a γδ T cell activator and IL-33 for use in therapy of infection, cancer, autoimmunity as well as other diseases.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Building method of NK (natural killer) cell and gamma delta T cell co-culture
ActiveCN109517793APromote amplificationImprove cultivation efficiencyBlood/immune system cellsCell culture active agentsNatural Killer Cell Inhibitory ReceptorsCentrifugation
The invention relates to the technical field of cell culture, in particular to a building method of NK (natural killer) cell and gamma delta T cell co-culture capable of realizing the in vitro commonmultiplication culture on NK cells and gamma delta T cells. The method mainly comprises the following steps of S1, reagent selection; S2, performing PBMC culture by a culture medium for 3 days; S3, supplementing the culture medium; maintaining the concentration; performing culture for 4 days; S4, after the seventh day, removing supernatant through centrifugation; transferring the cells into a T25cell culture bottle; then, replacing an EX culture medium; S5, after the tenth day, supplementing the culture medium for maintaining the concentration; S6, after the twelfth day, continuously supplementing the culture medium for maintaining the concentration; S7, after the fourteenth day, detecting the NK cell and gamma delta T cell proportion and the cell total number. The co-culture building method has the advantages that two kinds of anti-tumor immune cells with similar properties are cultured in one step; the cell culture efficiency is improved; the culture cost can be greatly reduced through being compared with that of independent culture of various immune cells for obtaining NK cells and gamma delta T cells; the culture technical difficulty is also reduced.
Owner:广州长峰生物技术有限公司
Application of novel gamma delta T cell to preparation of kit for evaluating curative effect of AML (acute myeloid leukemia)
ActiveCN107860924APredict clinical responsePredictive prognostic evaluationDisease diagnosisMyeloid leukemiaClinical efficacy
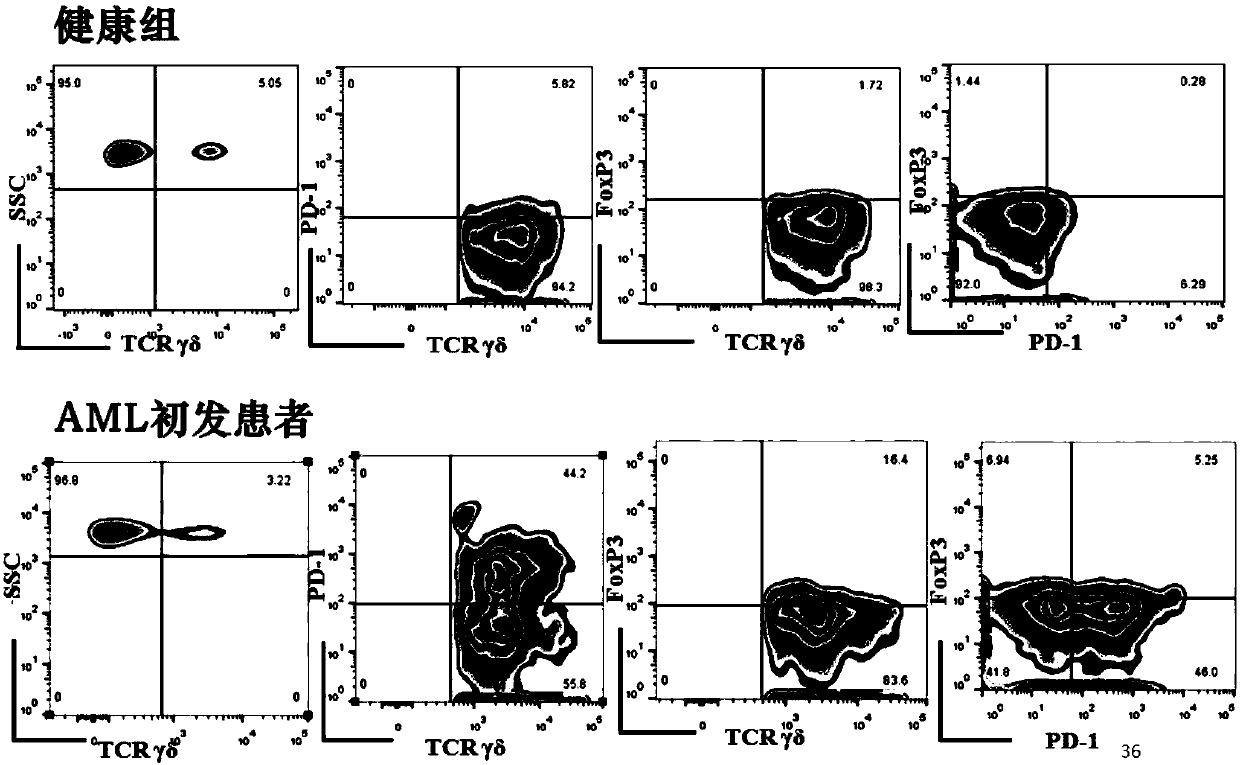
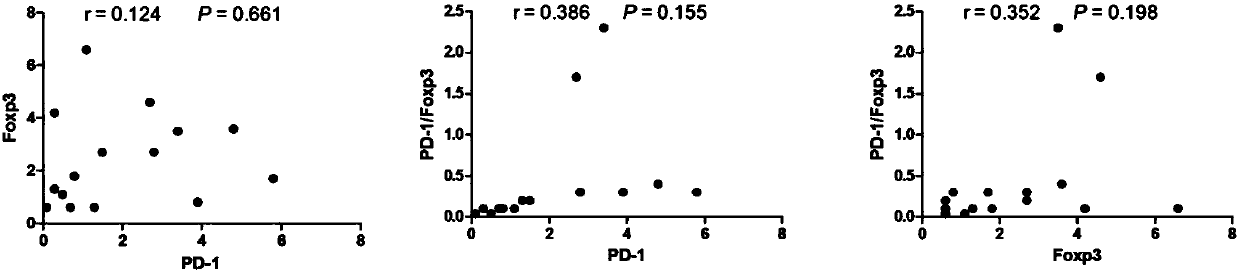
The invention provides application of a novel gamma delta T cell subpopulation to preparation of a kit for predicting the curative effect and prognosis of AML (acute myeloid leukemia). The inventor based on the invention discovers the expression condition of the novel gamma delta T cell subpopulation in peripheral blood of patients suffering from AML is associated with the curative effect and theprognosis of the patients suffering from AML for the first time. When the expression ratio of the novel PD1+Foxp3+gamma delta T cell subpopulation is high, the possibility of bad clinical curative effect of the patients suffering from AML is high. The expression ratio of the novel gamma delta T cell subpopulation has important guide significance in prognosis judgment of the patients suffering fromAML and formulation of a clinical treatment scheme. More base research data can be provided for individual treatment of the patients suffering from AML, and wide application prospect in clinical curative effect and prognosis evaluation of the patients suffering from AML is achieved.
Owner:上海普锐暨医学检验实验室有限公司
Serum-free medium suitable for culturing gamma delta T cells
The invention discloses a serum-free medium suitable for culturing gamma delta T cells. The serum-free medium consists of a basal culture medium component I, a gamma delta T cell specific culture component II and water. Experiments show that by adopting the serum-free medium suitable for culturing the gamma delta T cells, the gamma delta T cells obtained is greater in quantity, higher in purity and better in killing effect. The serum-free medium suitable disclosed by the invention has the advantages that 1) the risk of animal components which are used in animal serums in the cell culture with the gamma delta T cells on patients with cell therapy and uncertain impact of uncertain components in the animal serums on the result of the cell culture are avoided; 2) the yield of the gamma delta T cells is greatly improved; and 3) the final density of the gamma delta T cell culture is increased so as to lower the cost of cell culture.
Owner:SHANGHAI CLAISON BIOTECH
Method for producing chimeric antigen receptor modified gamma delta T cell
ActiveCN108588023AHigh transfection efficiencyImprove production efficiencyAntibody mimetics/scaffoldsTransferasesAntigen receptorsChimeric antigen receptor
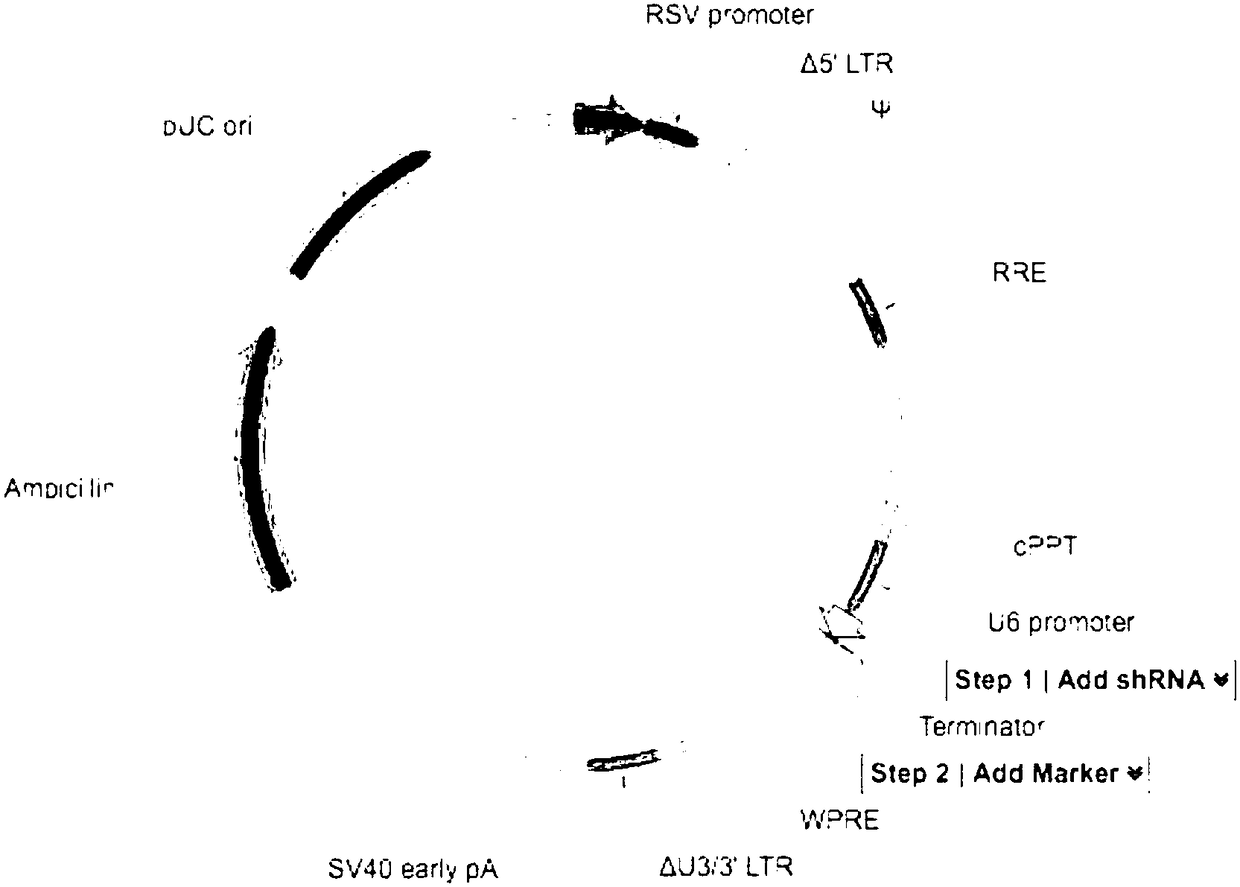
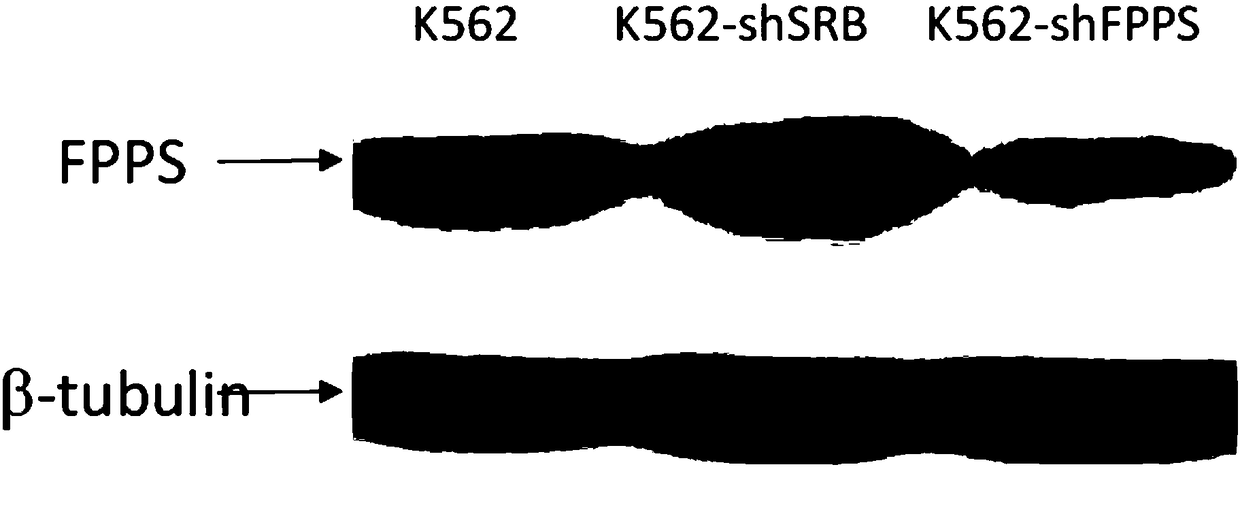
The invention discloses a method for producing a chimeric antigen receptor modified gamma delta T cell. The method ensures that shFPPS targeted to FPP synthetase transfers a K562 cell through a lentiviral vector, the expression quantity of FPPS in the K562 cell is lowered, and a K562-shFPPS cell line with lowed FPPS expression quantity is constructed. According to the method, a K562-shFPPS cell line is added into a gamma delta T cell culture system to be co-cultured with gamma delta T cell, and the fact that the K562-shFPPS cell line can promote the in vitro differentiation and expansion of the gamma delta T cell is found. According to the method, the lentiviral vector expressing CAR is further added into the gamma delta T cell culture system containing the cell line, so that co-culturingis performed, and the fact that the K562-shFPPS cell line can further effectively improve the transfection efficiency of CAR gene is found. The method effectively solves the technical bottleneck of mass production of CAR-gamma delta T cell, and has an excellent application prospect.
Owner:HEBEI SENLANG BIOTECH CO LTD
Method for preparing CAR-T cell from gamma delta T cell derived from umbilical cord blood, CAR-T cell and application
PendingCN109609465AEfficient killingEliminate exclusionPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenVirus type
The invention discloses a method for preparing a CAR-T cell from gamma delta T cell derived from umbilical cord blood, the CAR-T cell and an application and also relates to nucleic acid coding a chimeric antigen receptor and a recombinant expression vector expressing the chimeric antigen receptor. The umbilical cord blood is used as a source of the gamma delta T cell, the gamma delta T cell is separated from the umbilical cord blood, after efficient amplification, the T cell is transduced by a lentiviral vector loaded with the chimeric antigen receptor, and the CAR-T cell can be obtained, so that positive tumor cells or other infectious cells which can efficiently or specifically kill related antigens are obtained. The CAR-T cell will play roles in tumor cell therapy and immunotherapy.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Culture method for simultaneously amplifying gamma delta T and NK
PendingCN113430167AQuality assuranceGuaranteed quantityGenetically modified cellsBlood/immune system cellsAntigenNatural Killer Cell Inhibitory Receptors
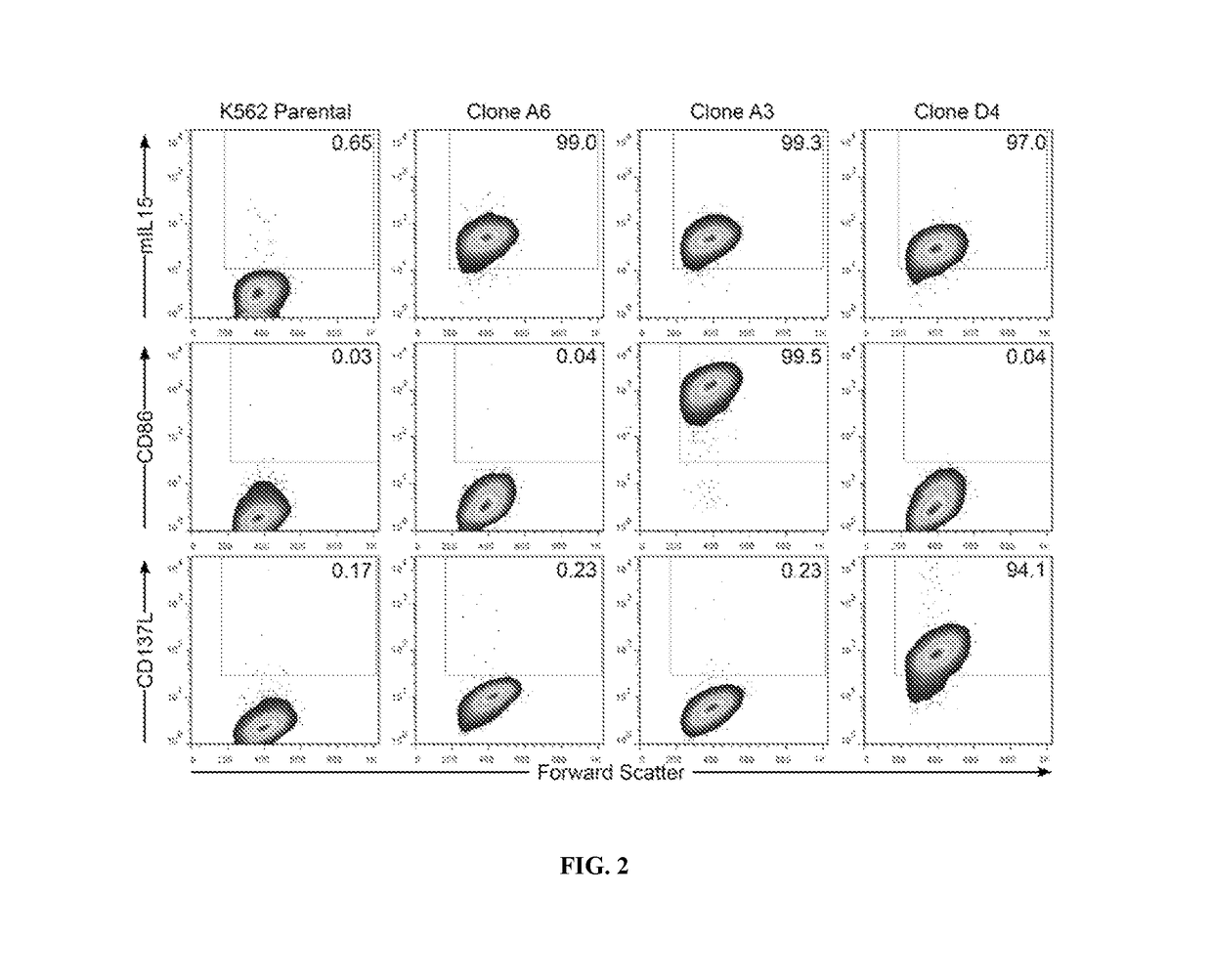
The invention relates to the field of immune medicine, in particular to a cell culture method which comprises the following steps: co-culturing an inactivated artificial antigen presenting cell with high expression of mIL15 and CD137L and PBMC in a culture medium, wherein the culture medium comprises a basic culture medium and additional components, the additional components comprise IL2 with the concentration of 100 IU / mL to 1200 IU / mL and zoledronic acid with the concentration of 3 mu M to 7 mu M. The ratio of NK cells to gamma delta T cells cultured by the method is high, and the cells have a more excellent anti-tumor effect.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Preparation Of Antigen-Presenting Human Gamma-Delta T Cells And Use In Immunotherapy
InactiveUS20090208517A1Improve purification effectEfficient workSnake antigen ingredientsDead animal preservationDendritic cellVaccination
The invention relates to a method for the preparation of efficient antigen-presenting human γδ T cells, to the γδ T cells prepared by such a method, and to their use in immunotherapy, vaccination, vaccine development and diagnostics. Similar to dendritic cells (DCs) in potency and efficacy, these human γδ T cells process antigens and present antigenic peptides to αβ T cells and induce antigen-specific responses (proliferation and differentiation) in naïve αβ T cells. γδ T cells are easily purified from peripheral blood, acquire “maturation” status (expression of essential adhesion, co-stimulatory and major histocompatibility complex molecules) within 1 day of in vitro culture under stimulation and induce strong primary and secondary T helper cell responses. The γδ T cells may be used in a method of treatment of tumors or chronic or recurrent infectious diseases, in identification of novel tumor or pathogen-derived antigens, and in the diagnosis of the immune competence of a patient.
Owner:MOSER BERNHARD +1
Culture method for gamma delta T cell
InactiveCN106399245AIncrease the amplification factorIncrease secretionBlood/immune system cellsCell culture active agentsMicrobiologyT cell
The invention relates to the medical field and discloses a culture method for a gamma delta T cell. The culture method comprises the steps of carrying out induced culture on a mononuclear cell containing IL-15, OKT-3 and IL-2 in a serum-free culture medium until the gamma delta T cell is induced, then adding a tumor cell lysis solution, continuing to carry out culture and multiplication, then adding TNF-alpha, continuing to carry out culture to promote the maturity and further multiplication of gamma delta T cell, and culturing for 14 days to obtain the gamma delta T cell. Cell factors of an induced culture medium are regulated in the earlier stage, and stimulation and sensitization are carried out by virtue of the tumor cell lysis solution and TNF-alpha in the middle and later stage of the culture, so that the amplification multiple of the cell is increased, the killing activity is enhanced, and the secretion amounts of IFN-gamma and IL-4 are increased.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Application of hemsley rockvine root extract in preparing tumor immunity therapeutic drug and human gamma delta T cell proliferation agent
InactiveCN106421184APromote proliferationEnhance killing activityBlood/immune system cellsAntineoplastic agentsHigh concentrationT cell
The invention relates to application of a hemsley rockvine root extract in preparing a tumor immunity therapeutic drug and a human gamma delta T cell proliferation agent, belonging to the technical field of medicine and biology. The hemsley rockvine root extract can notably accelerate proliferation of human gamma delta T cells, improves the expressions of porforin, CD107a and granzyme B of the gamma delta T cells, and has certain dose dependence; and the gamma delta T cells treated by the hemsley rockvine root extract can apparently improve the tumor-cytotoxicity activity of the gamma delta T cells and have certain dose dependence. A high-concentration hemsley rockvine root extract can inhibit the growth of tumor cells. The invention initially discovers that a low-concentration hemsley rockvine root extract can notably promote proliferation of the human gamma delta T cells; the low-concentration hemsley rockvine root extract can promote expressions of the porforin, granzyme B and CD107a of the gamma delta T cells; the cytotoxicity activity of the gamma delta T cells is apparently improved; and different extracted components of the hemsley rockvine root are apparently different in proliferation of the gamma delta T cells.
Owner:浙江博纳生物科技有限公司
Polyclonal gamma delta t cells for immunotherapy
ActiveUS20160256487A1Improve therapeutic potentialEnhances cytokine productionAntibacterial agentsGenetically modified cellsBacilliGamma delta T cell
Provided herein is a method of expanding clinically-relevant quantities of polyclonal γδ T cells that have anti-tumor, anti-viral, and anti-bacterial reactivity. Polyclonal γδ T cells can target a variety of tumors, including solid tumors as well as other conditions, such as viral and bacterial infections.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Preparation of Antigen-Presenting Human Gamma-Delta T Cells and Use in Immunotherapy
InactiveUS20080075732A1Improve purification effectEfficient workBiocideLibrary screeningAbnormal tissue growthDendritic cell
The invention relates to a method for the preparation of efficient antigen-presenting human γδ T cells, to the γδ T cells prepared by such a method, and to their use in immunotherapy, vaccination, vaccine development and diagnostics. Similar to dendritic cells (DCs) in potency and efficacy, these human γδ T cells process antigens and present antigenic peptides to αβ T cells and induce antigen-specific responses (proliferation and differentiation) in naïve αβ T cells. γδ T cells are easily purified from peripheral blood, acquire “maturation” status (expression of essential adhesion, co-stimulatory and major histocompatibility complex molecules) within 1 day of in vitro culture under stimulation and induce strong primary and secondary T helper cell and cytotoxic T cell responses. The γδ T cells may be used in a method of treatment of tumors or chronic or recurrent infectious diseases, in identification of novel tumor or pathogen-derived antigens, and in the diagnosis of the immune competence of a patient.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
Method for amplifying killing activity gamma-delta T cell by induction in vitro
ActiveCN108949685AGood antitumor activityA large amountCulture processBlood/immune system cellsSerum free mediaLymphocyte
The invention relates to a method for amplifying a gamma-delta T cell by induction in vitro, and belongs to the technical field of biology. The method comprises the following steps: acquiring peripheral blood mononuclear cells (PBMCs) through a lymphocyte separation medium; performing stimulation culturing for 3 days by utilizing a serum-free medium containing zoledronic acid, IL-2, IL-7 and IL-15; performing stimulation amplifying culturing with a serum-free medium containing IL-2, IL-7 and IL-15; and adding nicotinamide when culturing is performed for 7-9 days to improve the anti-tumor activity of the gamma-delta T cells. According to the method, the PBMCs do not needed to be purified, and the gamma-delta T cells having the purity of 90 percent or more can be obtained when culturing is performed for 14 days; and the anti-tumor activity of the gamma-delta T cells can be remarkably improved by adding the nicotinamide. The gamma-delta T cells can be amplified for 1500 times or more, which can meet the requirement for the number of gamma-delta T cells in clinical application. The method is simple in operation steps and strong in operability, and has a good popularization value.
Owner:吉林省吉恩致合生物治疗技术有限公司
Gamma delta-T cell exosome used for tumor immunotherapy and preparation method of gamma delta-T cell exosome
ActiveCN108103026AHas antigen presenting functionChemotaxisOrganic active ingredientsGenetically modified cellsAbnormal tissue growthAntitumor immunity
The invention provides a gamma delta-T cell exosome used for tumor immunotherapy. The gamma delta-T cell exosome is loaded with miR-138. The exosome (gamma delta TDE) derived from gamma delta-T cellscarries exogenous miR-138 for realizing direct targeted recognition and killing on tumor cells, meanwhile, PD-1 / PD-L1 is inhibited, the antitumor immunity activity of T lymphocytes / NK cells is enhanced, and the effect of inhibiting tumor growth / invasion / metastasis is achieved through direct and indirect paths.
Owner:SICHUAN CANCER HOSPITAL
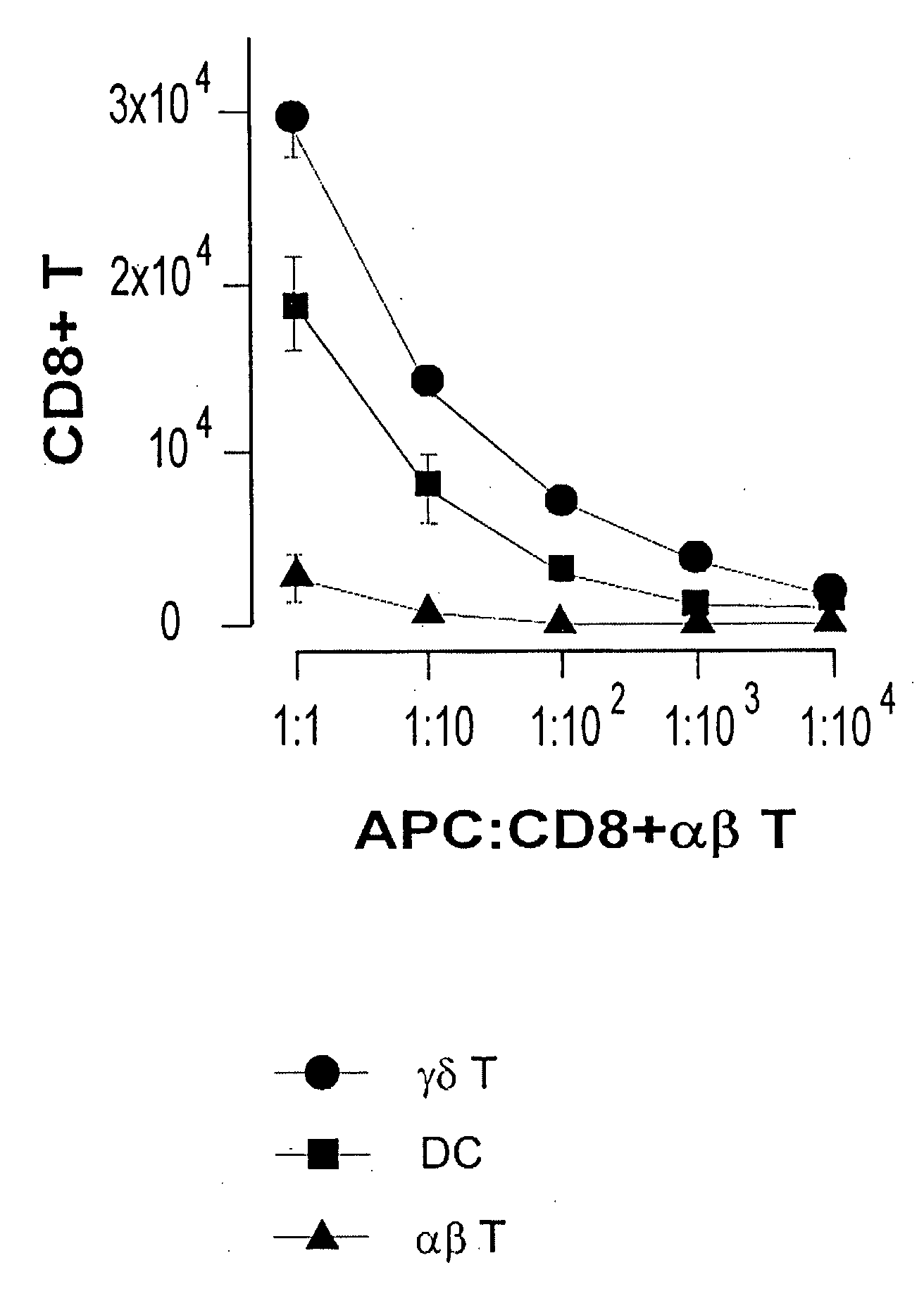
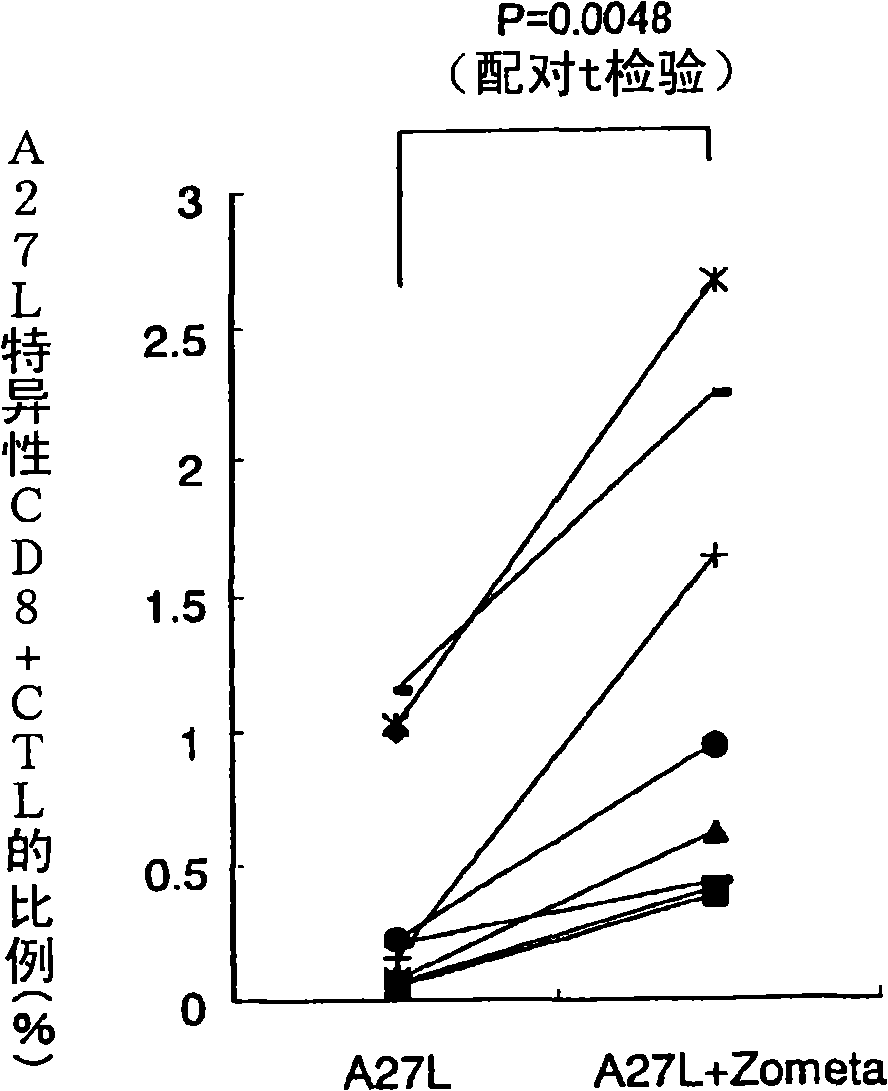
Method for activation treatment of antigen-presenting cell
An activated antigen-presenting cell capable of inducing an immunocompetent cell including a disease antigen-specific CD8+CTL and / or a gamma delta T cell in vivo and / or in vitro with good efficiency; a pharmaceutical comprising the activated antigen-presenting cell; a therapeutic / prophylactic method using the activated antigen-presenting cell; a method for induction of an immunocompetent cell including a disease antigen-specific CD8+CTL and / or a gamma delta T cell which is induced by using the activated antigen-presenting cell; an immunocompetent cell induced by the method; a pharmaceutical comprising the immunocompetent cell; and a therapeutic / prophylactic method using the immunocompetent cell. An antigen-presenting cell is sensitized with a disease antigen and also co-sensitized with bisphosphonate, thereby producing an increased number of a disease antigen-specific CD8+CTL and / or a gamma delta T cell at a higher ratio compared to a method without co-sensitization with bisphosphonate.
Owner:迈世耐特股份公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com