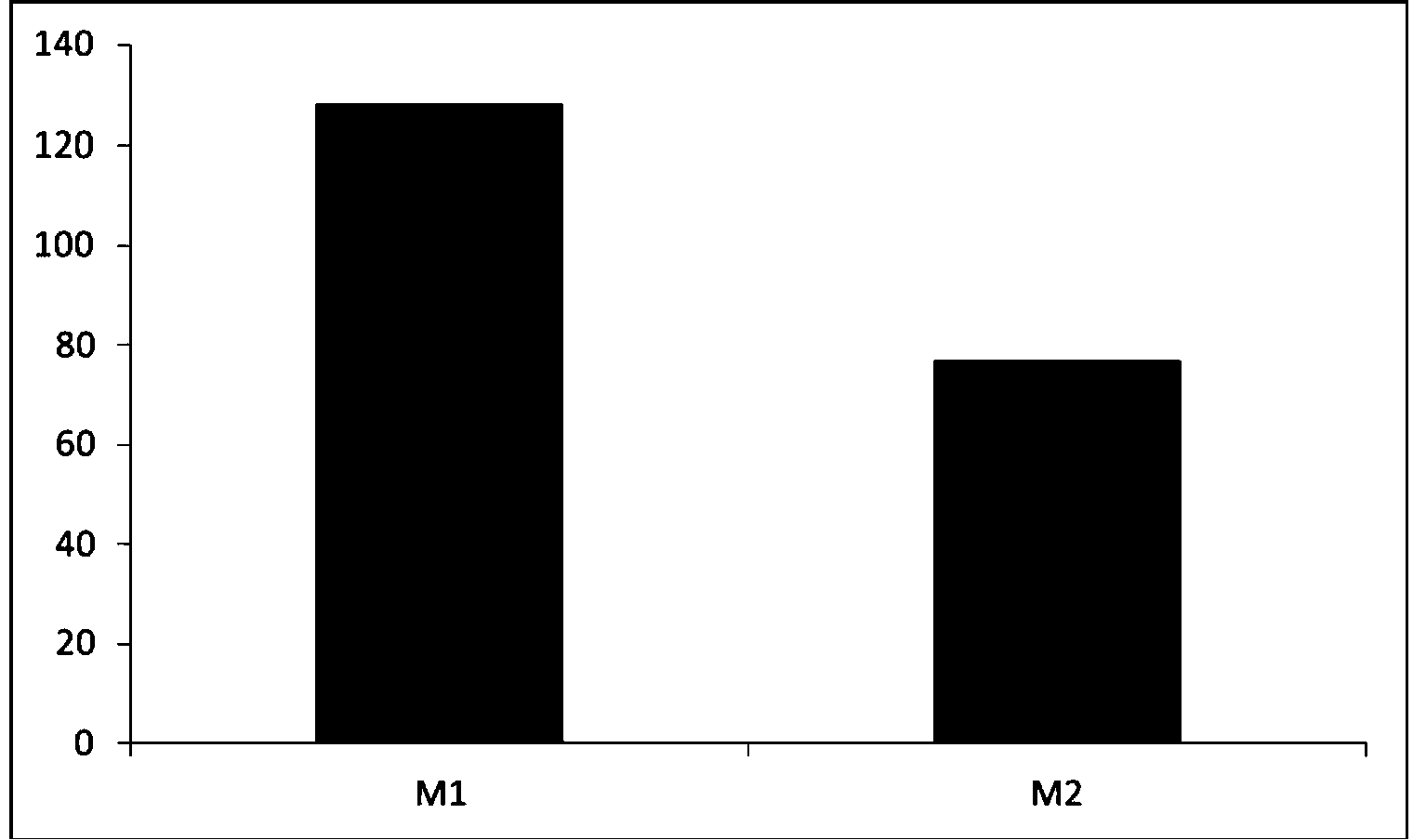Serum-free medium suitable for culturing gamma delta T cells
A serum-free medium and basal medium technology, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve the problems of different serum origins, batch numbers, influence on cell growth, and high price, so as to avoid inconvenience. Determining effect, good killing effect, effect of increasing final density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of serum-free medium suitable for culturing γδT cells
[0032] Prepare 100L of culture medium, calculated according to Table 3, see Table 4 for the quality of each component required to prepare 100L of γδT cell serum-free culture medium.
[0033] Table 4
[0034]
[0035]
[0036] The preparation method is:
[0037] (1) Take the raw materials of component I, mix them evenly, dry them under vacuum at 60°C for 15 hours, and set aside; take the raw materials of component II, dry them under vacuum at 30°C for 18 hours, mix them well, and set them aside;
[0038] (2) Mix the above dried components I and II, pin mill for 3 hours, and pass through a 30-mesh sieve to obtain a solid powder. The temperature in the production process is controlled at 20-25°C, and the humidity is controlled at 15-20%. The nitrogen pressure was controlled at 150-300 psi; water was added to the solid powder, mixed evenly, and filtered through a 0.2 μm filter membrane to ...
Embodiment 2
[0039] Example 2 Using γδT cell serum-free medium to cultivate γδT cells and comparing the culture effect with the culture medium of the control group
[0040] Proceed as follows:
[0041] (1) Machine mining at least 1×10 9 Mononuclear cells (PBMC) of patients (blood cell separator model COM.TEC, manufacturer Fresenius Kabi, set the program to autoMNC in Leukocyte-Aphersis);
[0042] (2) Transfer the collected blood sample into a 50ml centrifuge tube, centrifuge at 400g for 10min; take the supernatant plasma and freeze it, and dilute the remaining blood with PBS;
[0043] (3) Slowly add 30ml of diluted blood to 15ml of Ficoll-Hypaque, set the deceleration process to brake off, and then centrifuge at 900g for 20min;
[0044] (4) After centrifugation, gently insert a flat-mouth Pasteur pipette into the mononuclear cell layer, carefully and accurately draw the layer of cells along the tube wall and transfer to another new 50ml centrifuge tube, add physiological 50ml of saline,...
Embodiment 3
[0050] Example 3 γδT Cell Flow Cytometry Detection
[0051] Proceed as follows:
[0052] (1) will contain approximately 1x10 6 Add the cell suspension to be tested into the corresponding numbered flow tube, 1500rpm, 5min, discard the supernatant;
[0053] (2) Add 2mL flow staining buffer, resuspend the cells, 1500rpm, 5min, discard the supernatant;
[0054] (3) Add 300 μL of flow staining buffer, resuspend the cells, and divide them into three parts. One part is set as a negative control (no antibody), and the other two tubes are added with fluorescently labeled monoclonal antibodies (note that the corresponding quantity);
[0055] (4) Incubate at room temperature in the dark for 30 minutes; add 1 mL flow staining buffer, 1500 rpm, 5 minutes, discard the supernatant; repeat washing once;
[0056] (5) Resuspend the cells with 300 μL flow staining buffer and wait for analysis.
[0057] Results: The γδT cells obtained on the 15th day of culture in two different media were de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com