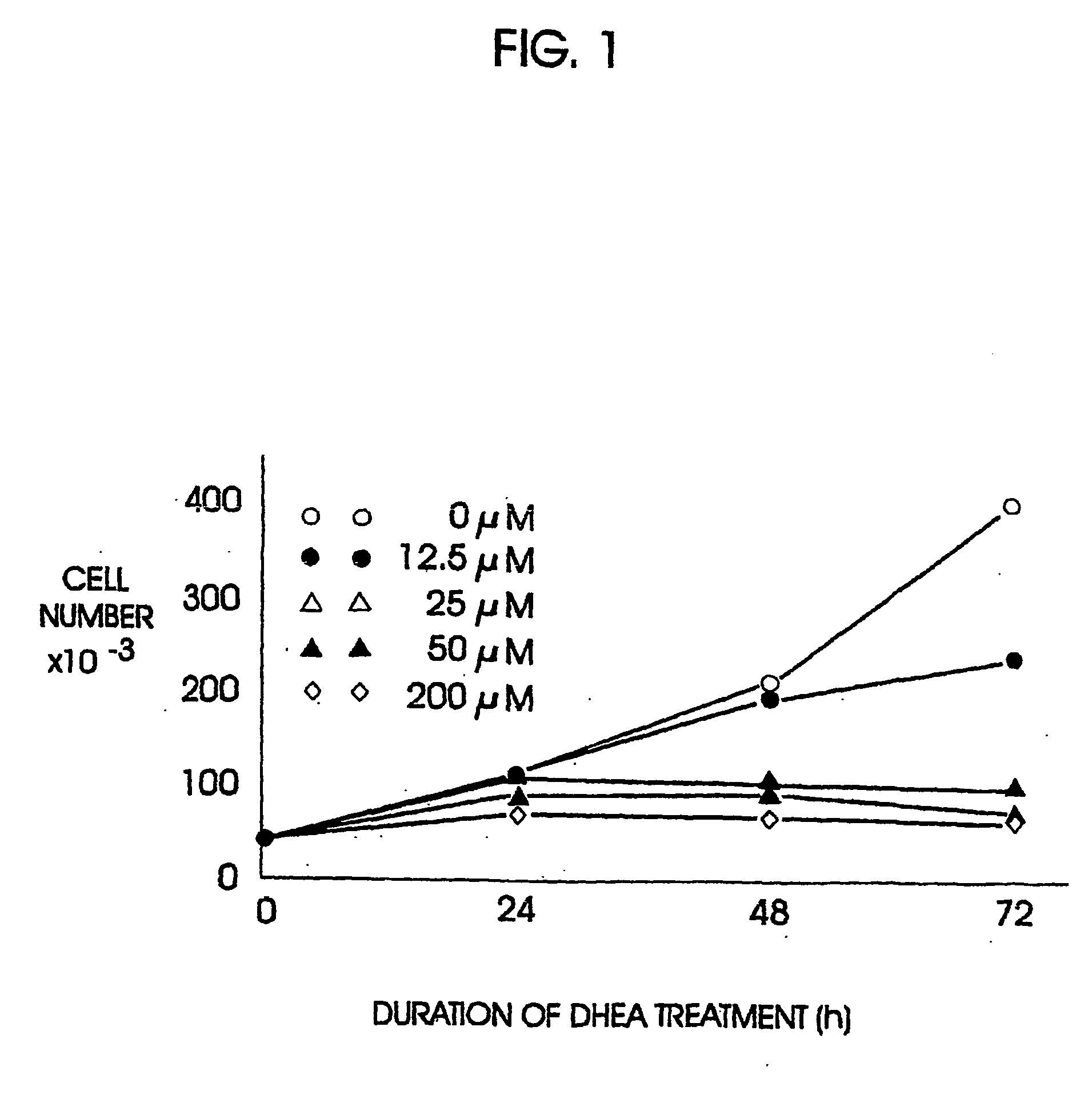
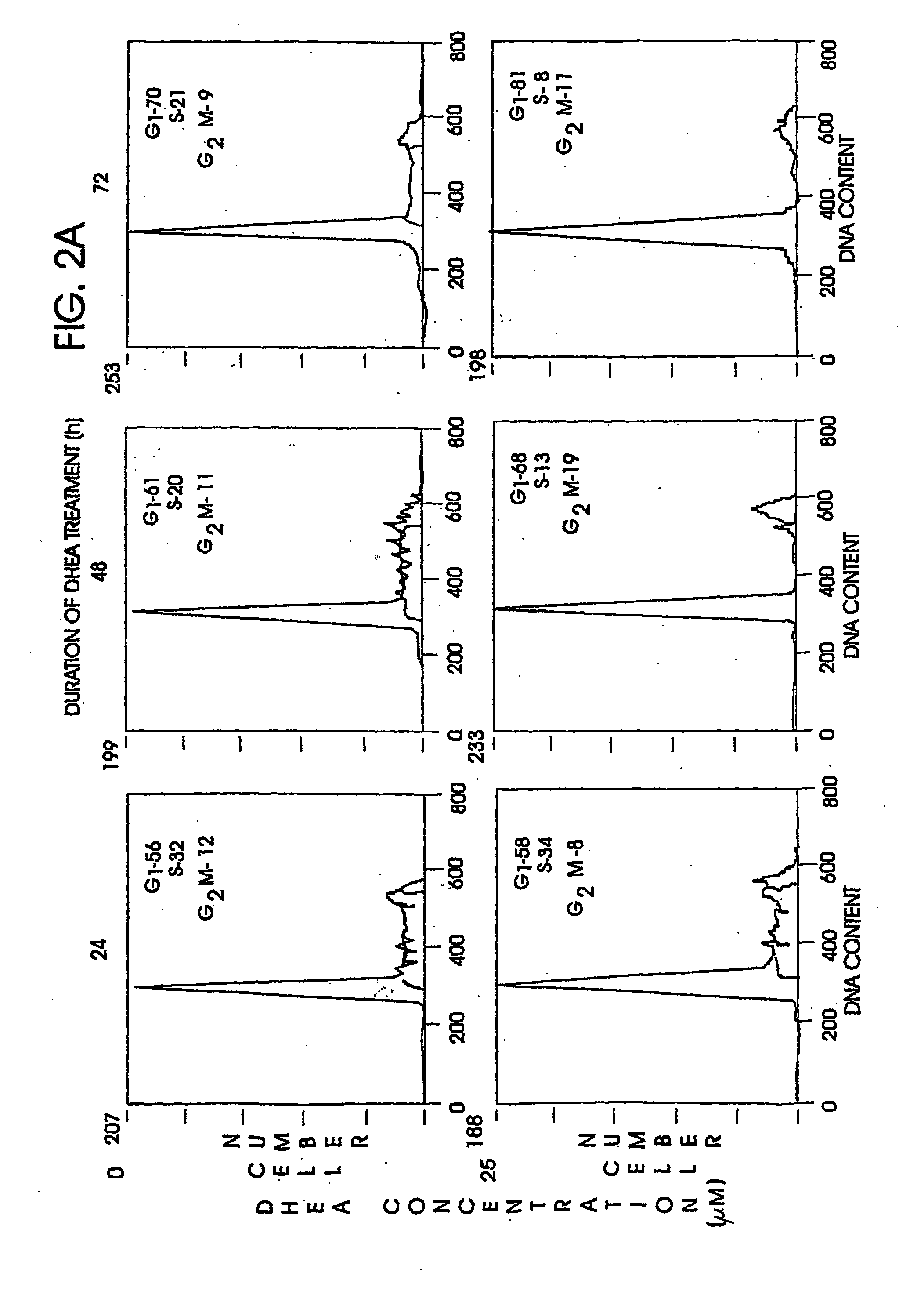
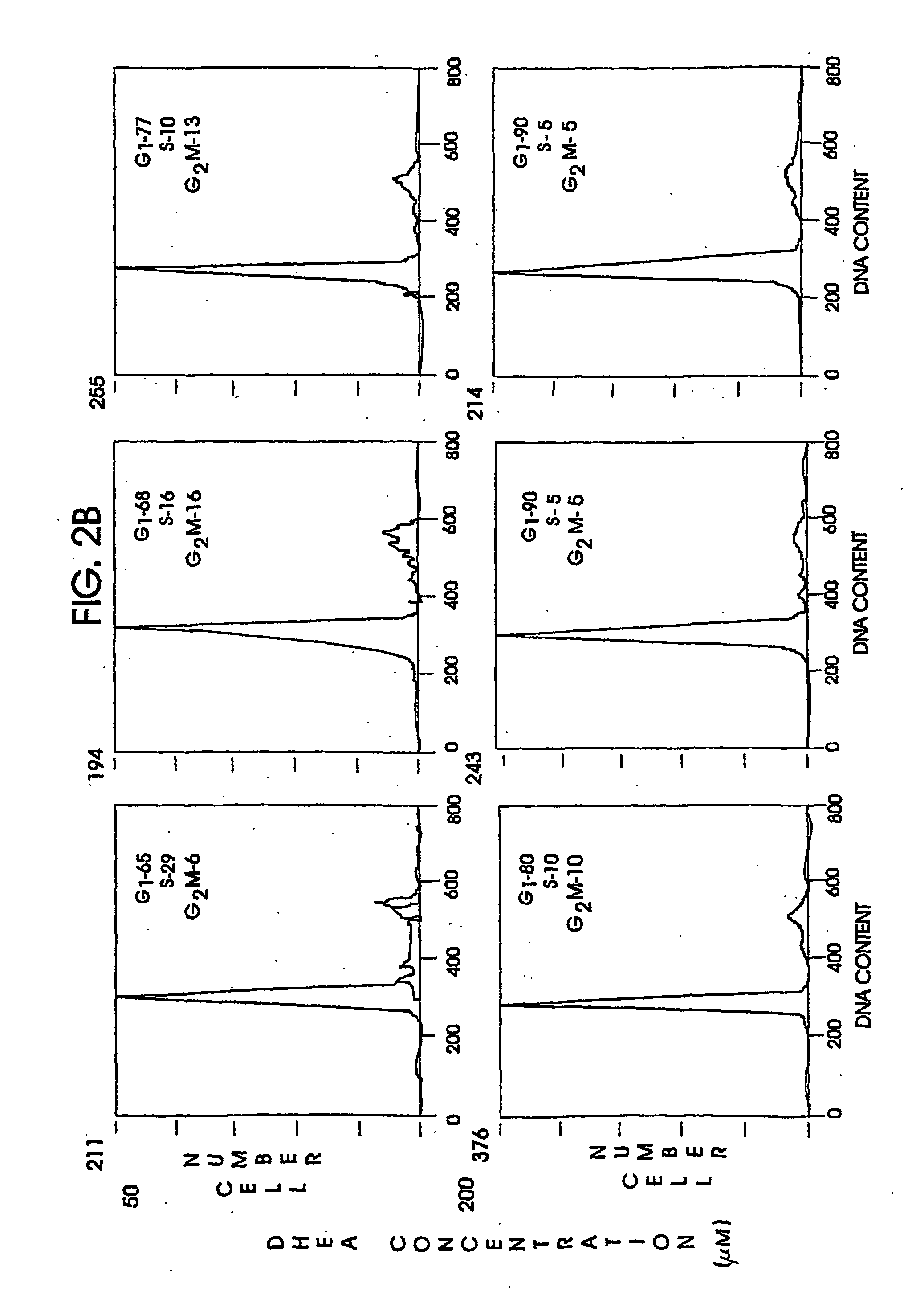
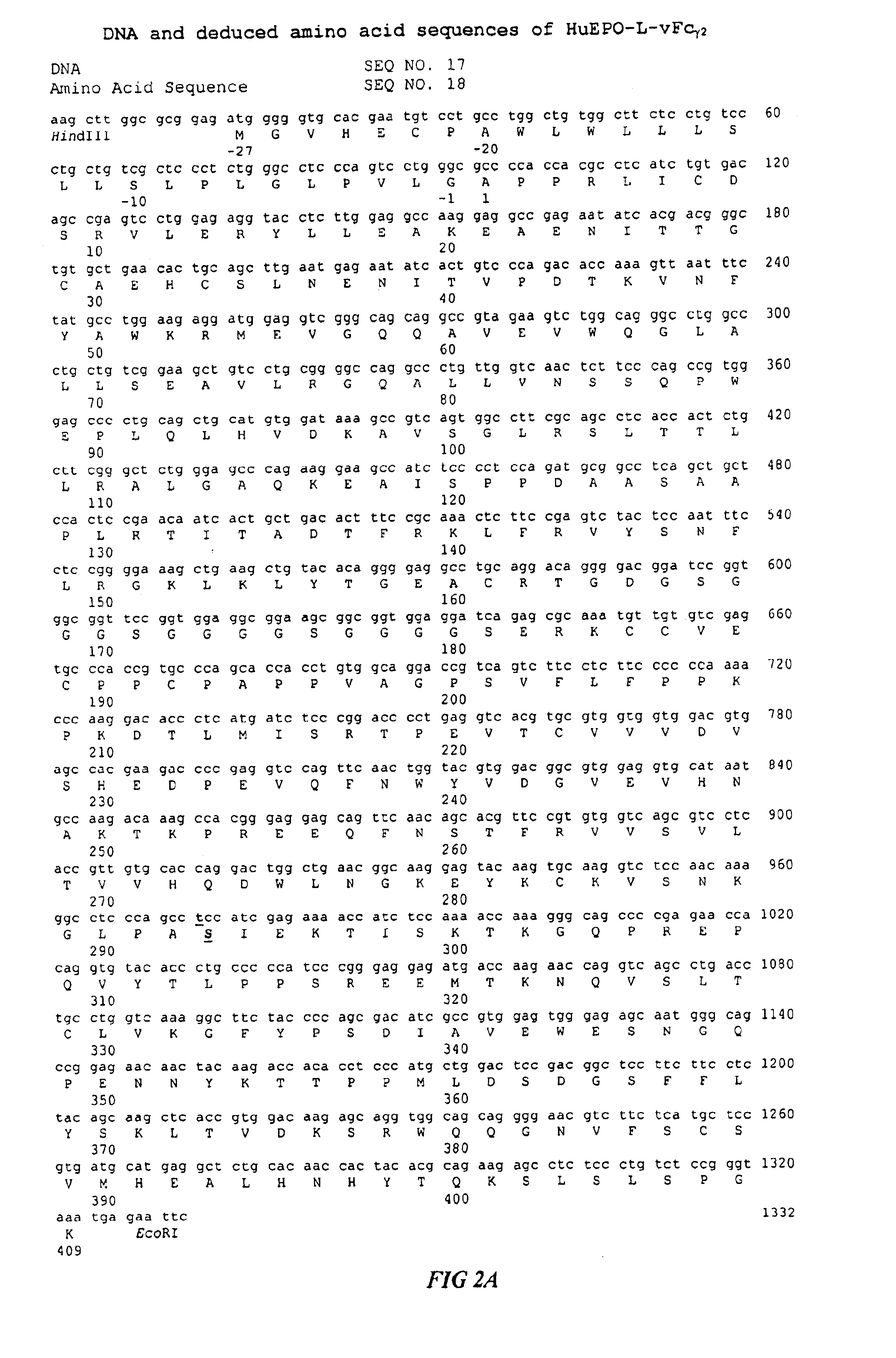Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76153 results about "Side effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequences of the use of a drug. Developing drugs is a complicated process, because no two people are exactly the same, so even drugs that have virtually no side effects, might be difficult for some people. Also, it is difficult to make a drug that targets one part of the body but that doesn’t affect other parts, the fact that increases the risk of side effects in the untargeted parts.
Apparatus for blocking activation of tissue or conduction of action potentials while other tissue is being therapeutically activated
InactiveUS6928320B2Avoid actionInhibition of activationHeart defibrillatorsArtificial respirationSide effectSphincter
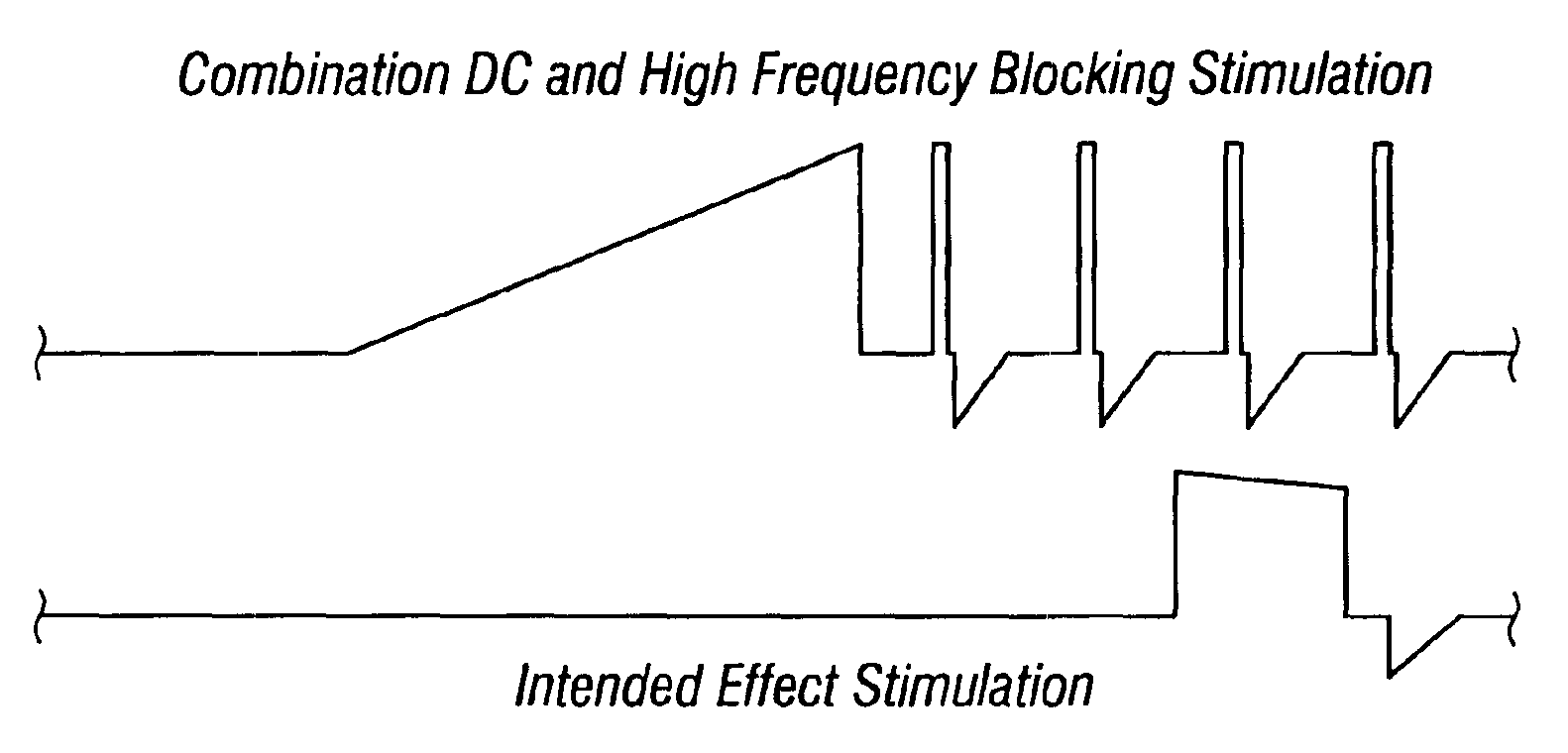
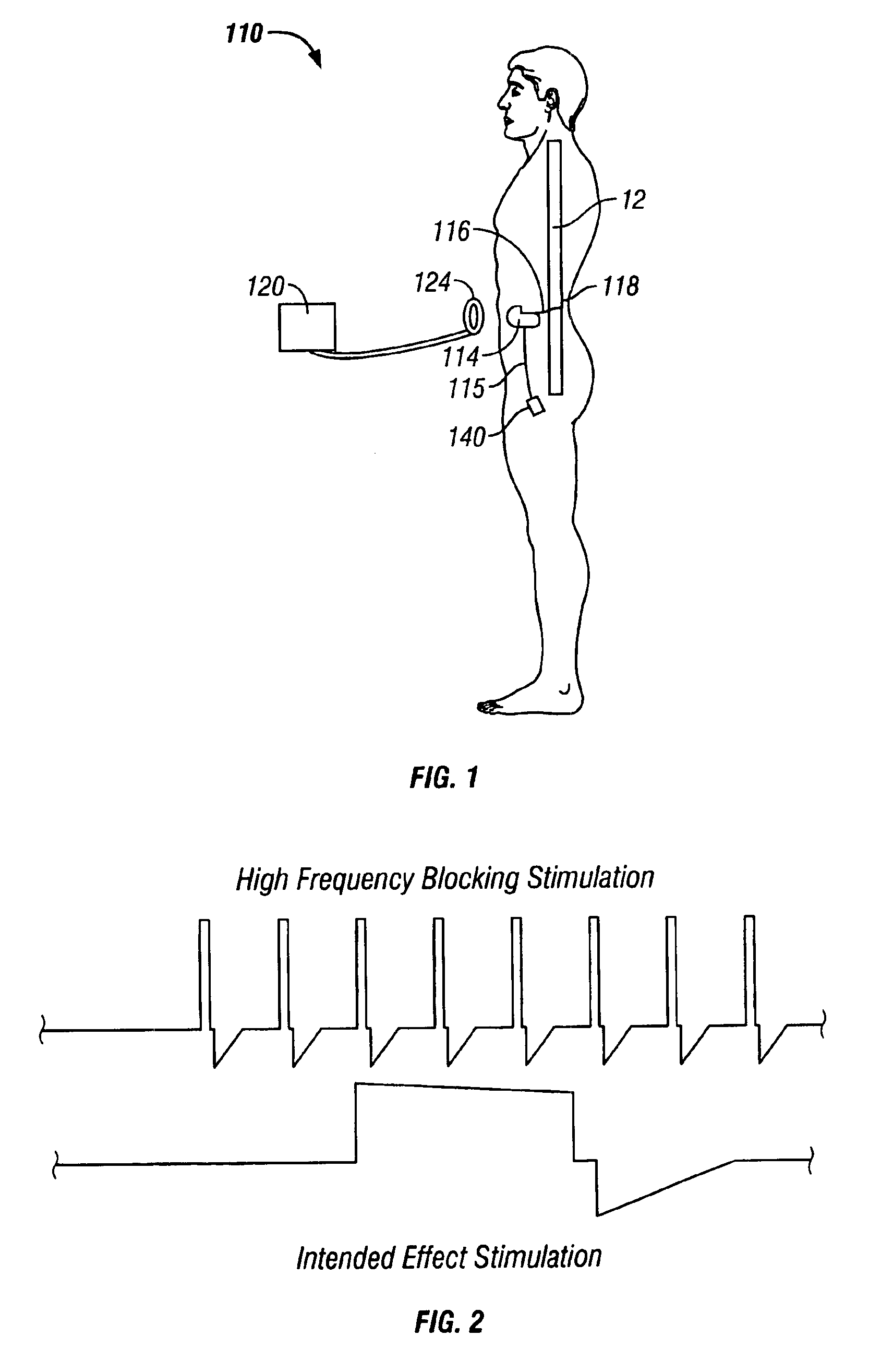
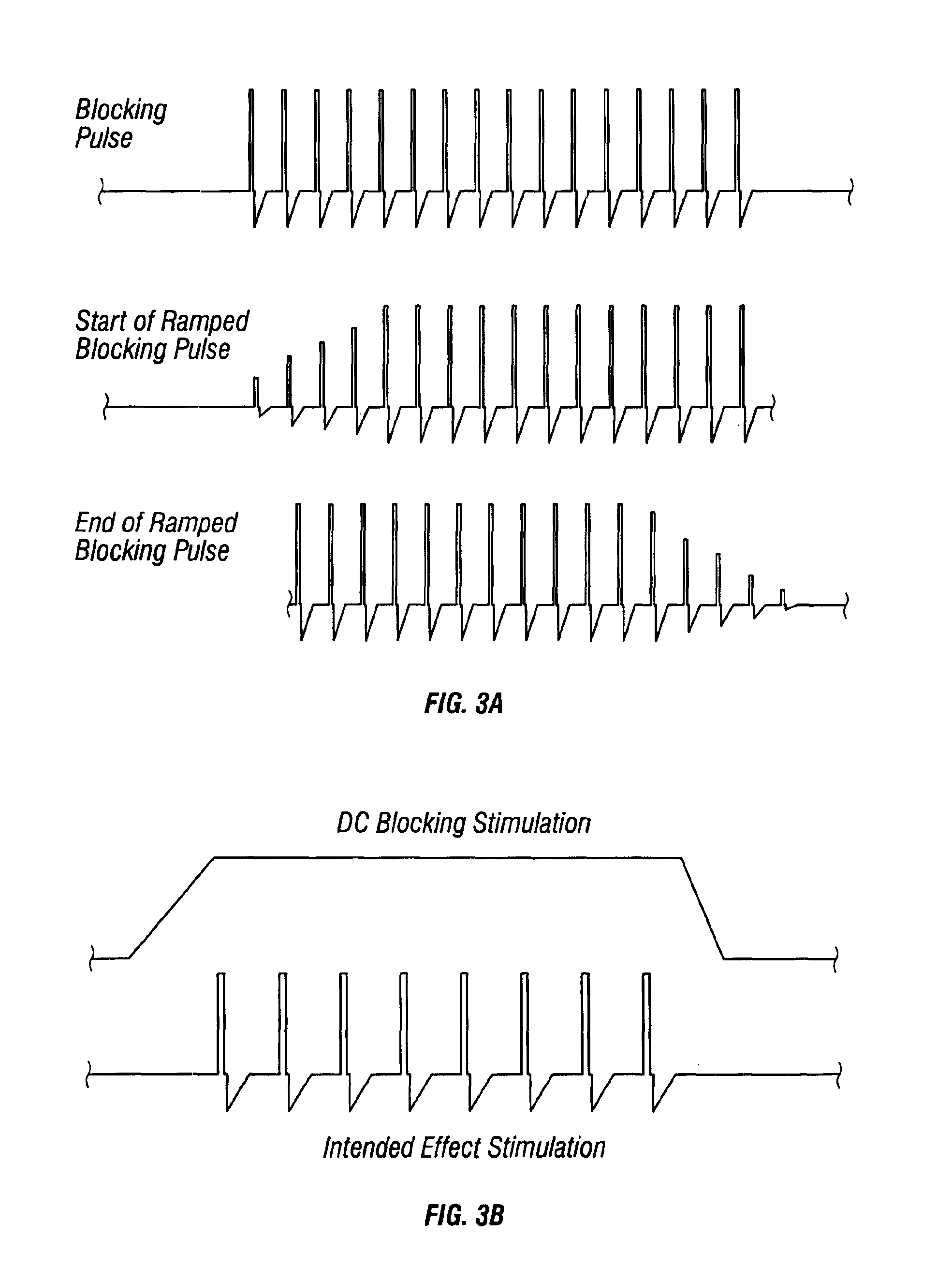
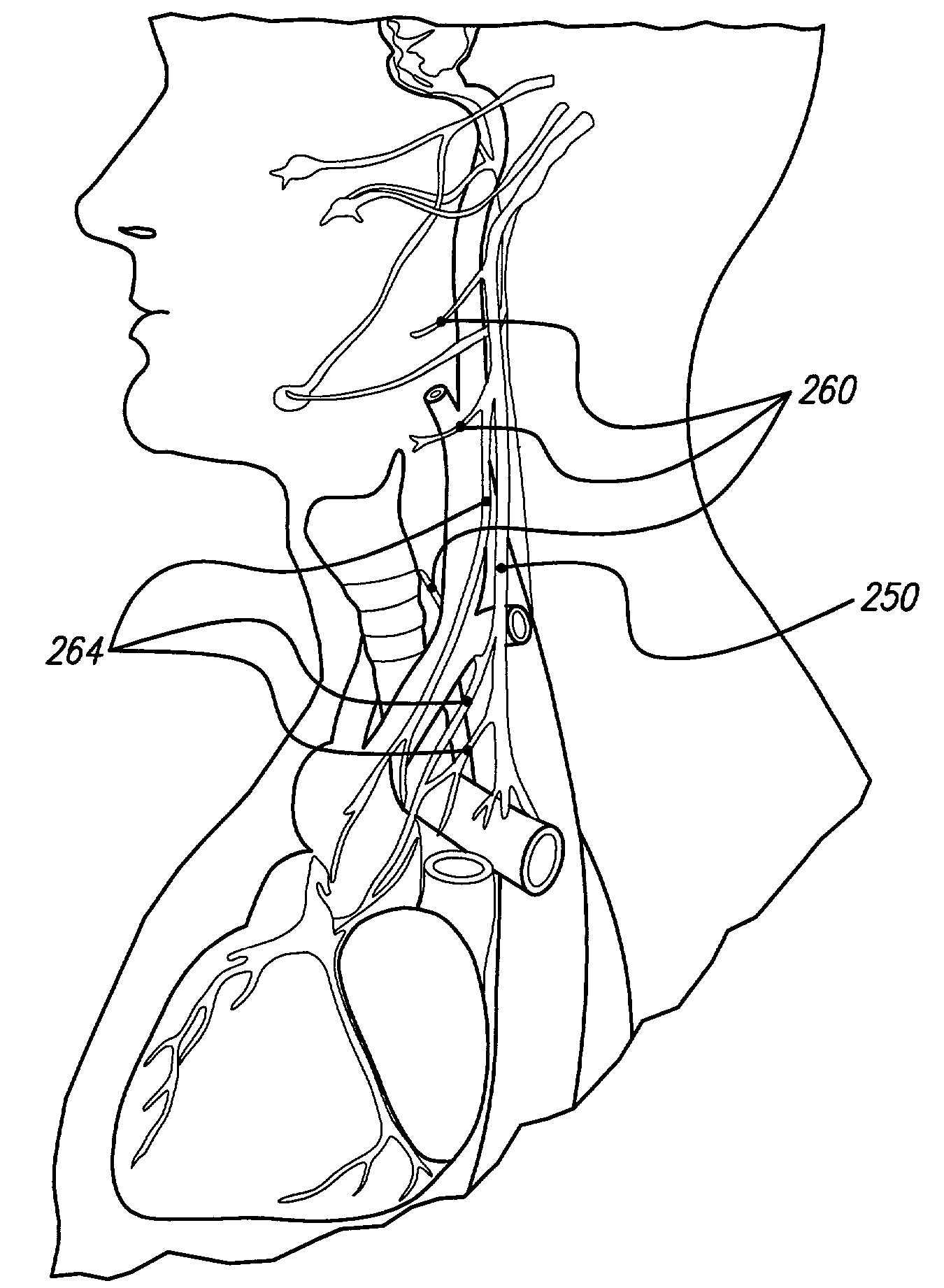
A desired effect is produced by therapeutically activating tissue at a first site within a patient's body and a corresponding undesired side effect is reduced by blocking activation of tissue or conduction of action potentials at a second site within the patient's body by applying high frequency stimulation and / or direct current pulses at or near the second site. Time-varying DC pulses may be used before or after a high frequency blocking signal. The high frequency stimulation may begin before and continue during the therapeutic activation. The high frequency stimulation may begin with a relatively low amplitude, and the amplitude may be gradually increased. The desired effect may be promotion of micturition or defecation and the undesired side effect may be sphincter contraction. The desired effect may be defibrillation of the patient's atria or defibrillation of the patient's ventricles, and the undesired side effect may be pain.
Owner:MEDTRONIC INC
Vagus nerve stimulation via unidirectional propagation of action potentials
ActiveUS7292890B2Less power consumptionExcessive stimulationSpinal electrodesSurgerySide effectMedicine
Methods of stimulating a vagus nerve include providing at least one implantable stimulator with at least two electrodes, configuring the electrodes to apply stimulation that unidirectionally propagates action potentials along a vagus nerve, and applying the stimulation to the vagus nerve to effectively select afferent fibers, thereby treating at least one of epilepsy and depression while limiting side effects of bidirectional stimulation. At least one of the electrodes comprises a leadsless electrode.
Owner:BOSTON SCI NEUROMODULATION CORP
System and methods for treatment of alzheimer's and other deposition-related disorders of the brain
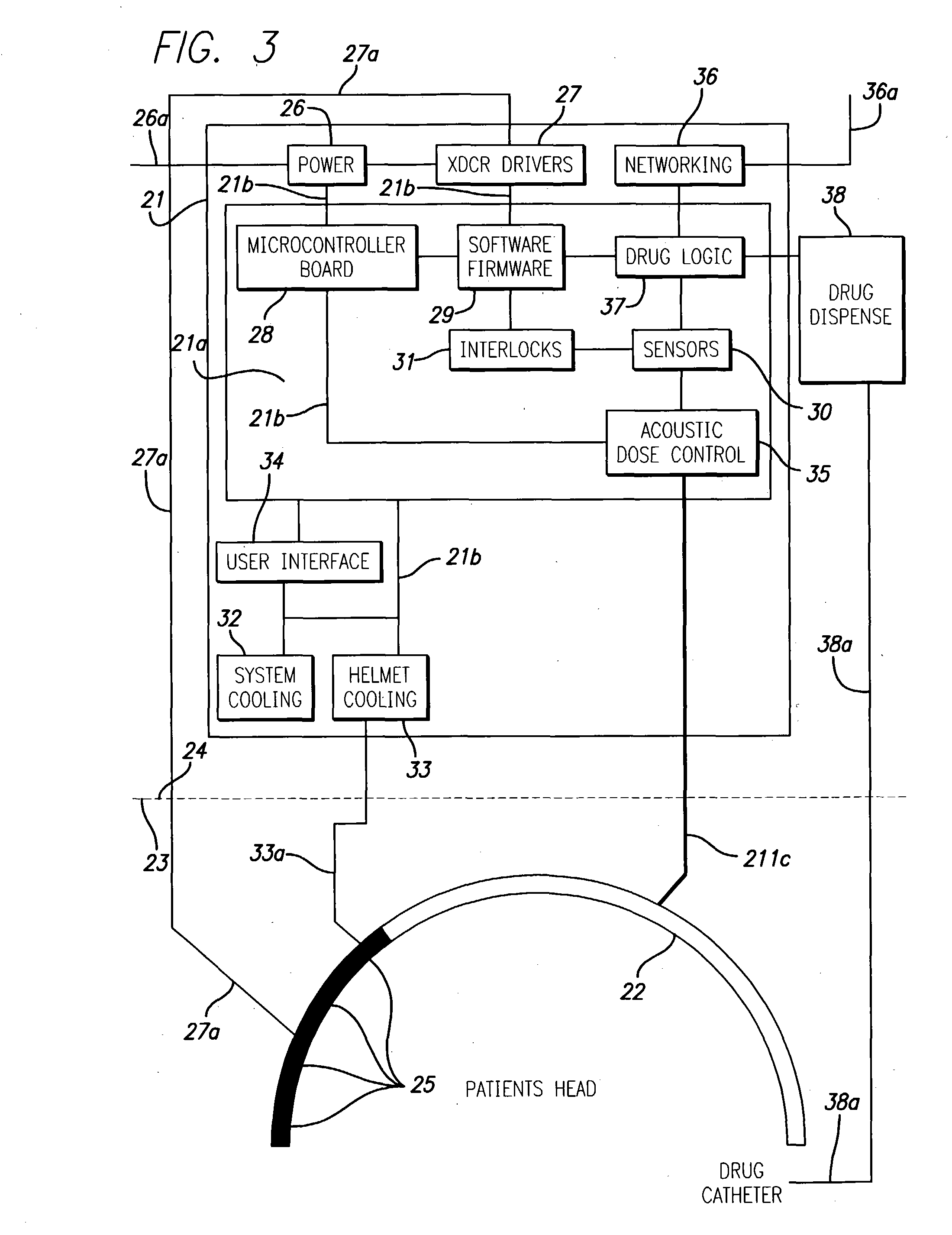
InactiveUS20040049134A1Slowing, stopping or avoiding a patient's cognitive lossesMinimal adverse side effectUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseSide effect
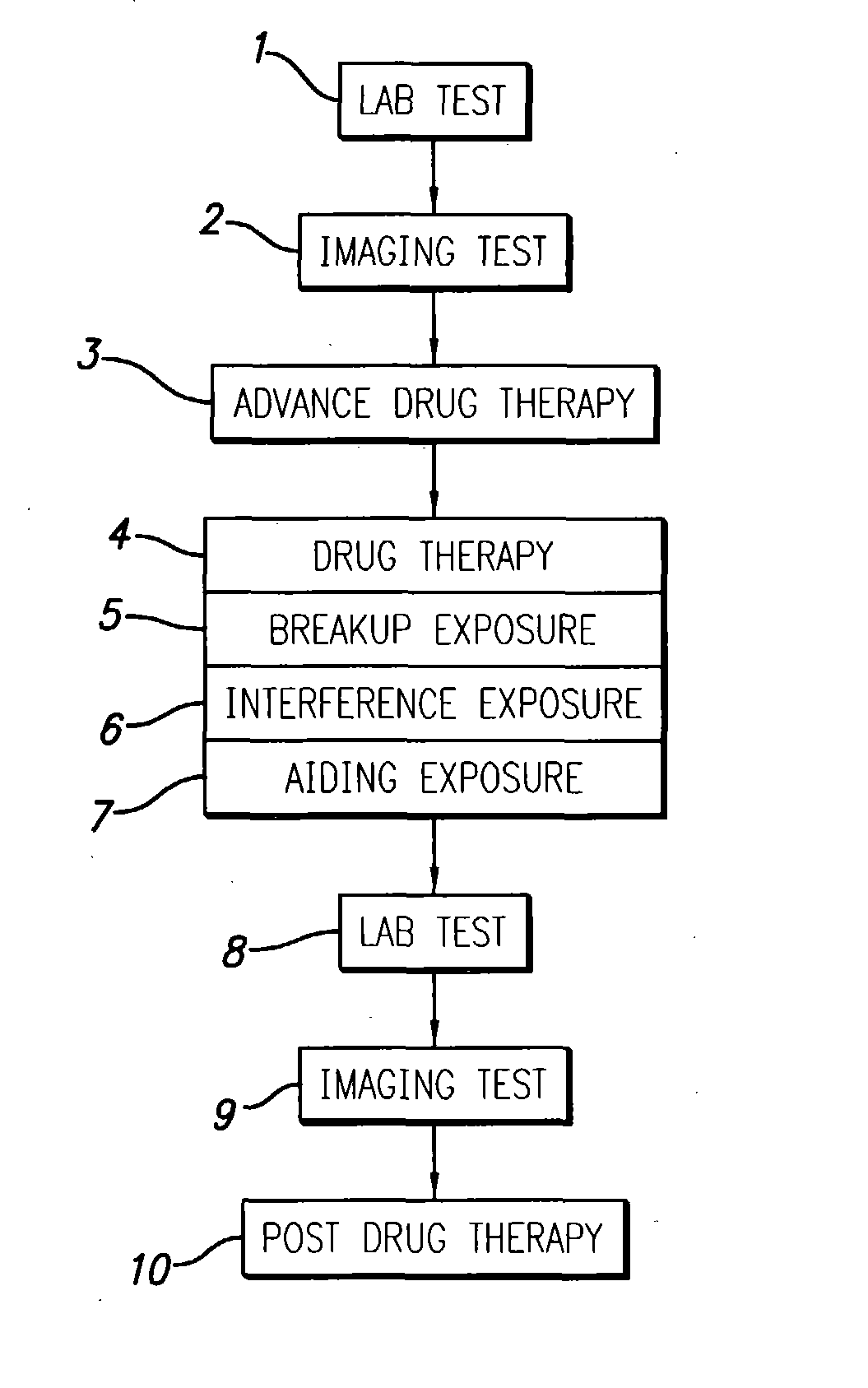
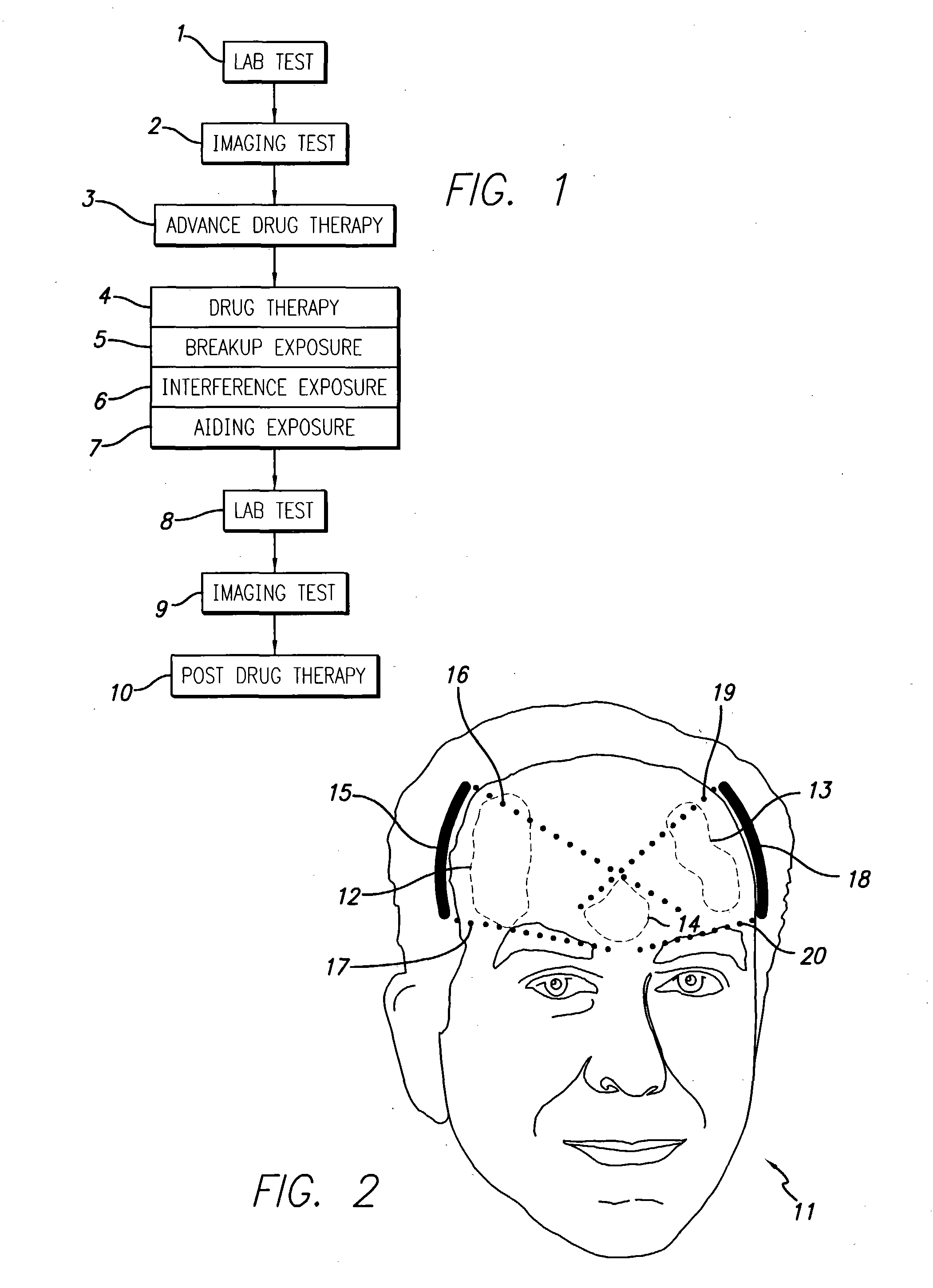
A system and methods are provided for the therapeutic treatment of brain-plaques, fibrils, abnormal-protein related or aggregation-prone protein related deposition-diseases. The system employs acoustic exposure therapy means for delivering therapeutic energy to at least one brain region. The therapy supports at least one of the following processes: (i) physical breakup, erosion, disentanglement, de-aggregation, dissolution, de-agglomeration, de-amalgamation or permeation of the deposits, (ii) interference in at least one deposit formation process, deposition related chemical reaction or biological or genetic pathway contributing to the deposits or deposition-related processes, and (iii) aiding the recovery, growth, regrowth or improved functionality of brain-related cells or functional pathways negatively impacted by, stressed by or disposed to the deposits, deposition-processes or deposition disease state, or supporting the growth of newly transplanted cells anywhere in the brain-related anatomy. The system and methods treat Alzheimer's and other deposition-related disorders of the brain, with minimal adverse side effects to the patient and may be used in cooperation with a drug.
Owner:TOSAYA CAROL A +1
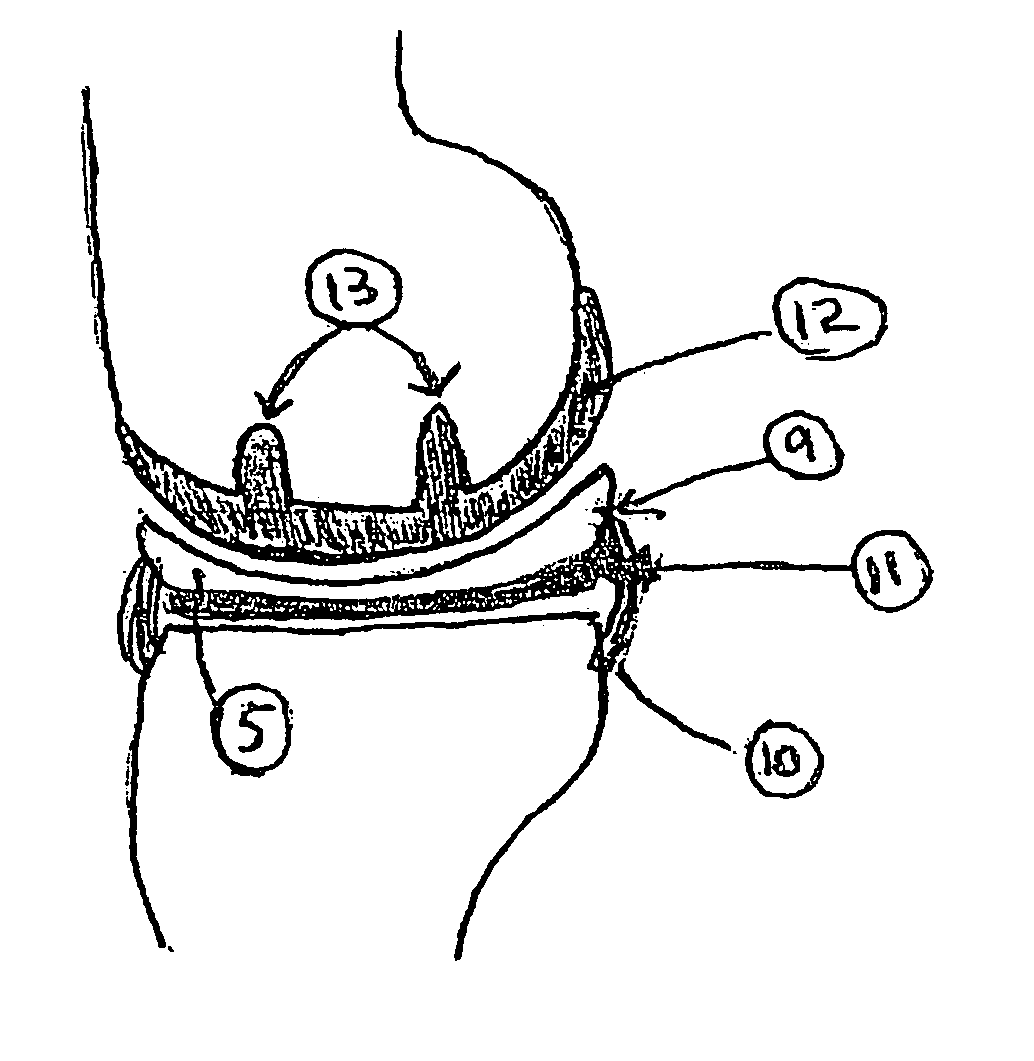
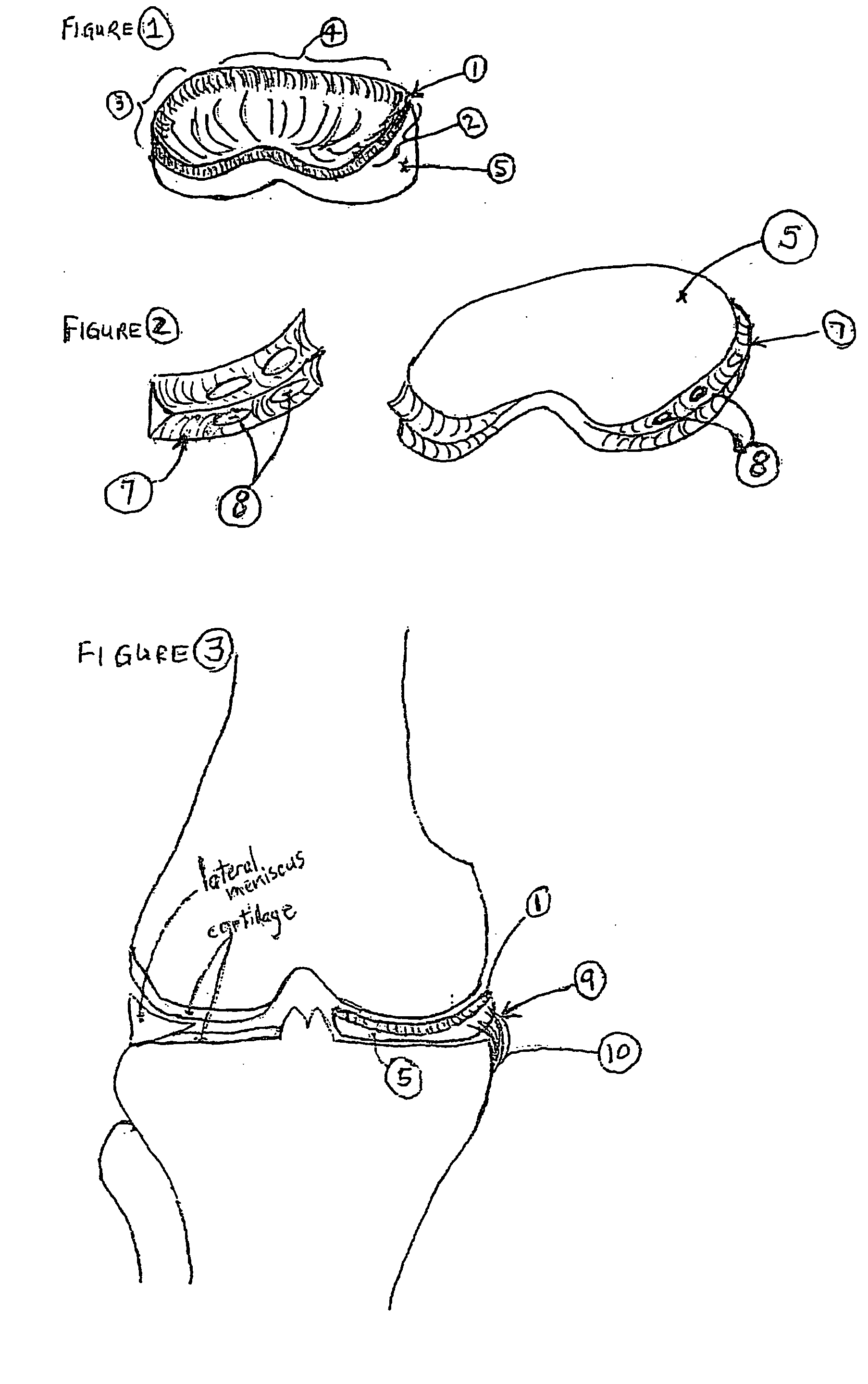
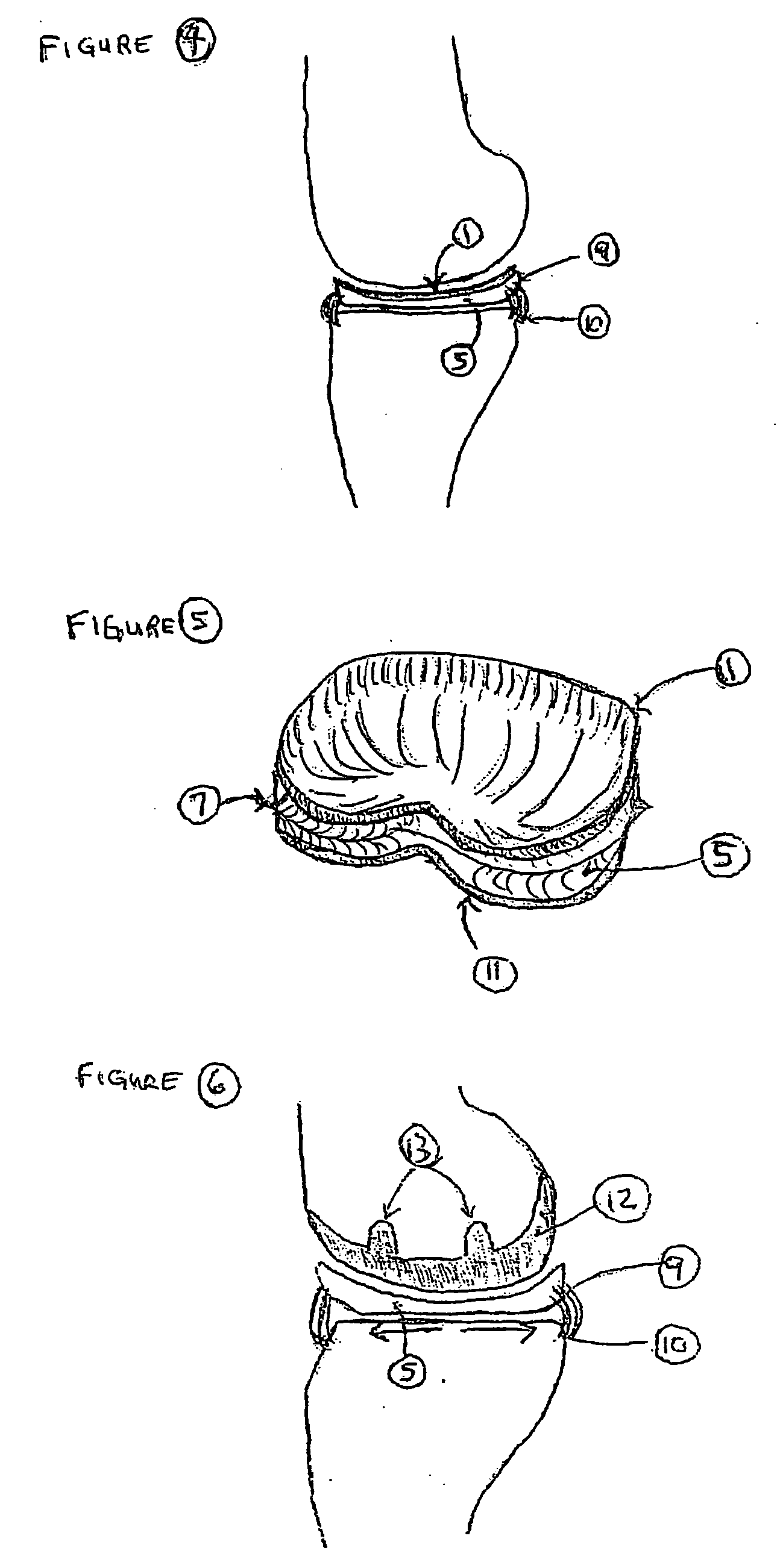
Unicondylar knee implant
InactiveUS20050171604A1Reduce frictionMinimal incisionSuture equipmentsSurgical needlesFiberSide effect
A knee prosthesis, methods of implanting the prosthesis, method of treating arthritis of the knee, and a kit therefore are provided. The prosthesis answers many of the limitations of current knee prosthetic devices by providing a two-component (or alternatively, an optional three-component) device, as either a single structure, or as separate pieces. One of the components is constructed of low friction material, while the second is composed of a weight-dissipating cushioning material; the optional third component is constructed of low friction material. The prosthesis is initially attached to surrounding soft tissue in the knee by biodegradable sutures; it is held permanently in place by fibrous ingrowth into a porous collagen rim in the cushioning component. Major improvements provided by the present invention over currently available prostheses include minimal incisions, minimal or no bone cuts, minimal overall dissection (these improvements lead to shorter hospital stays and rapid rehabilitation and fewer potential for side effects), less prosthetic wear, greater longevity, fewer activity restrictions, able to be used on young, large, active patients, ease of revision, ease of conversion into a total knee arthroplasty if needed.
Owner:MICHALOW ALEXANDER
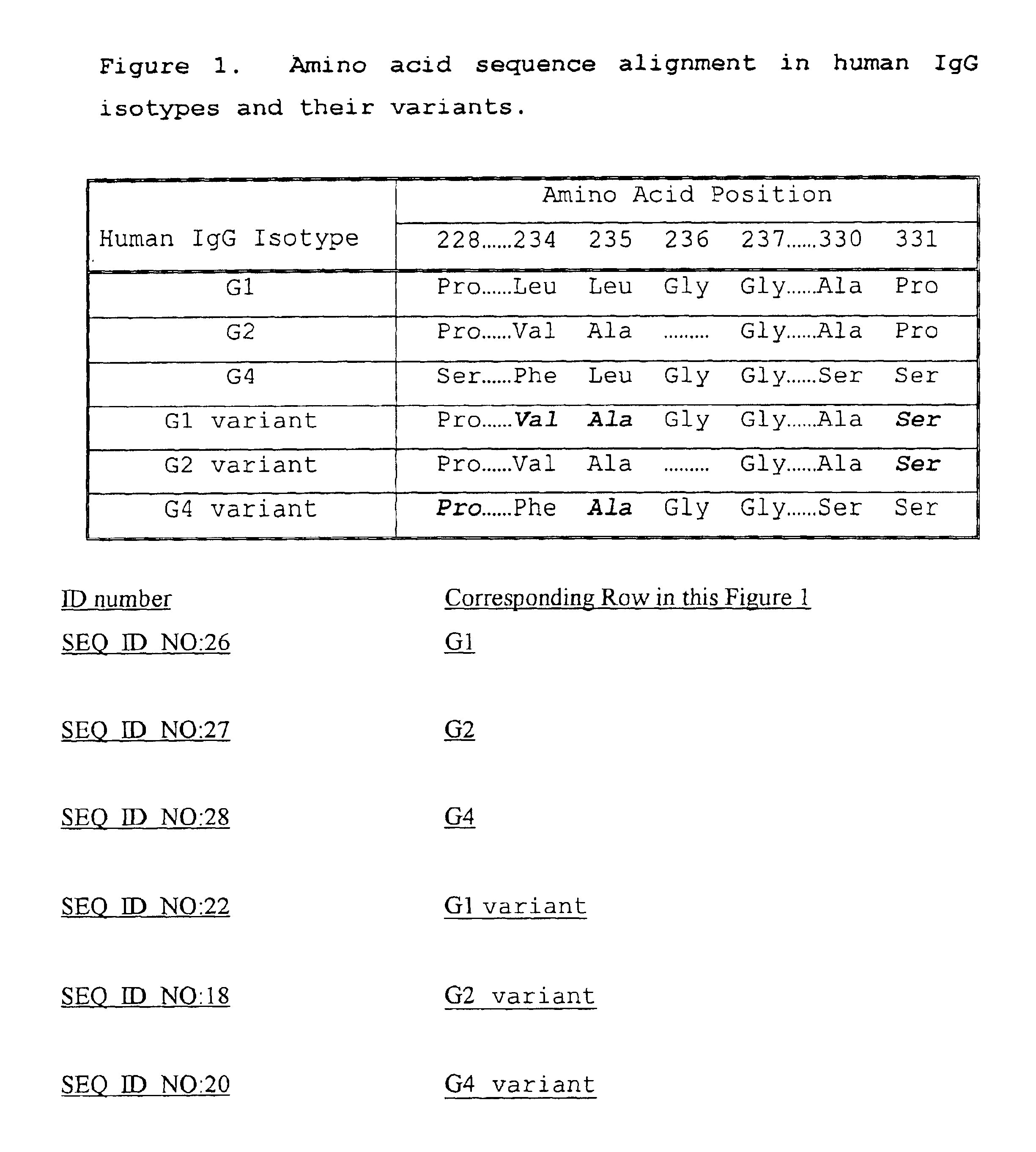
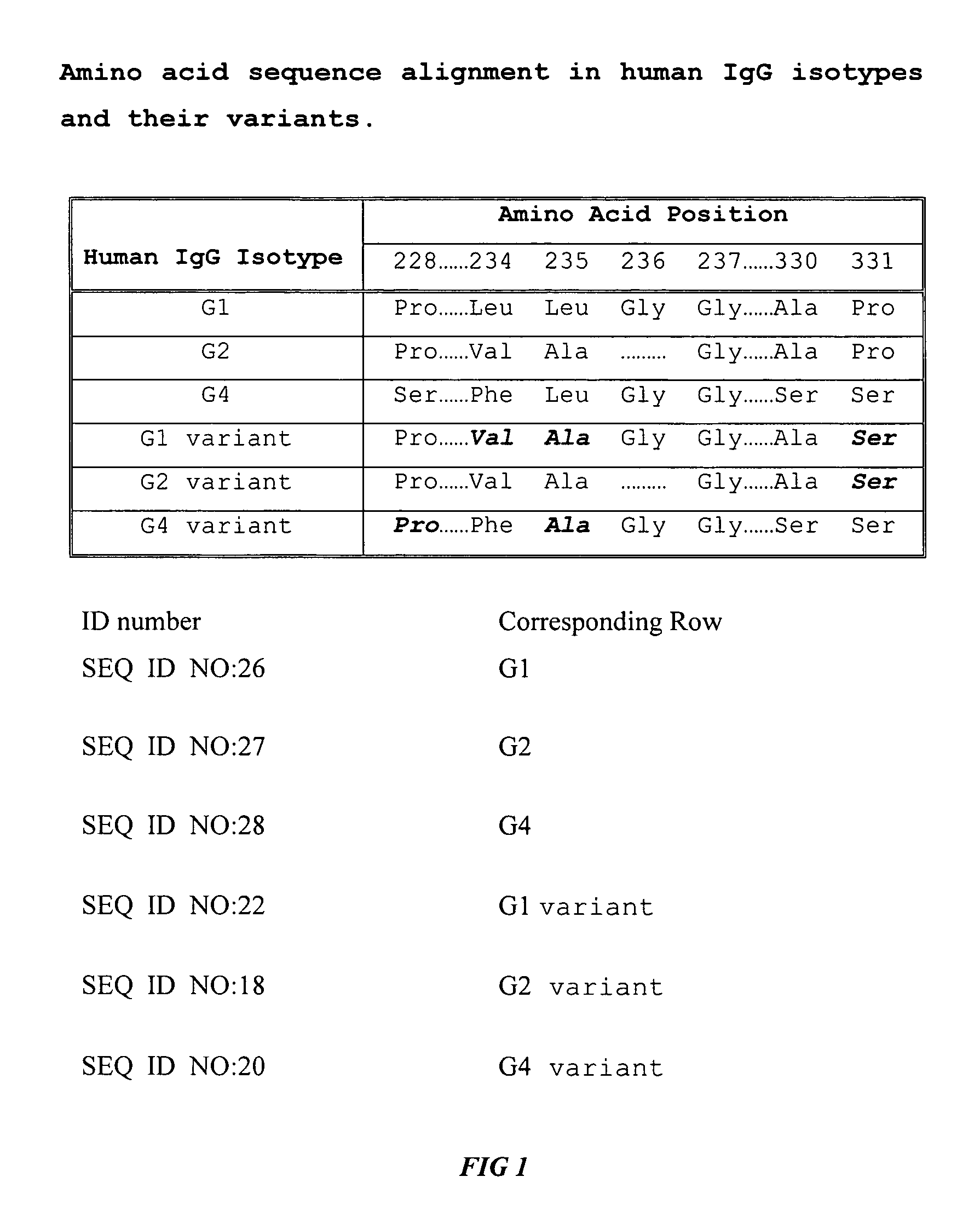
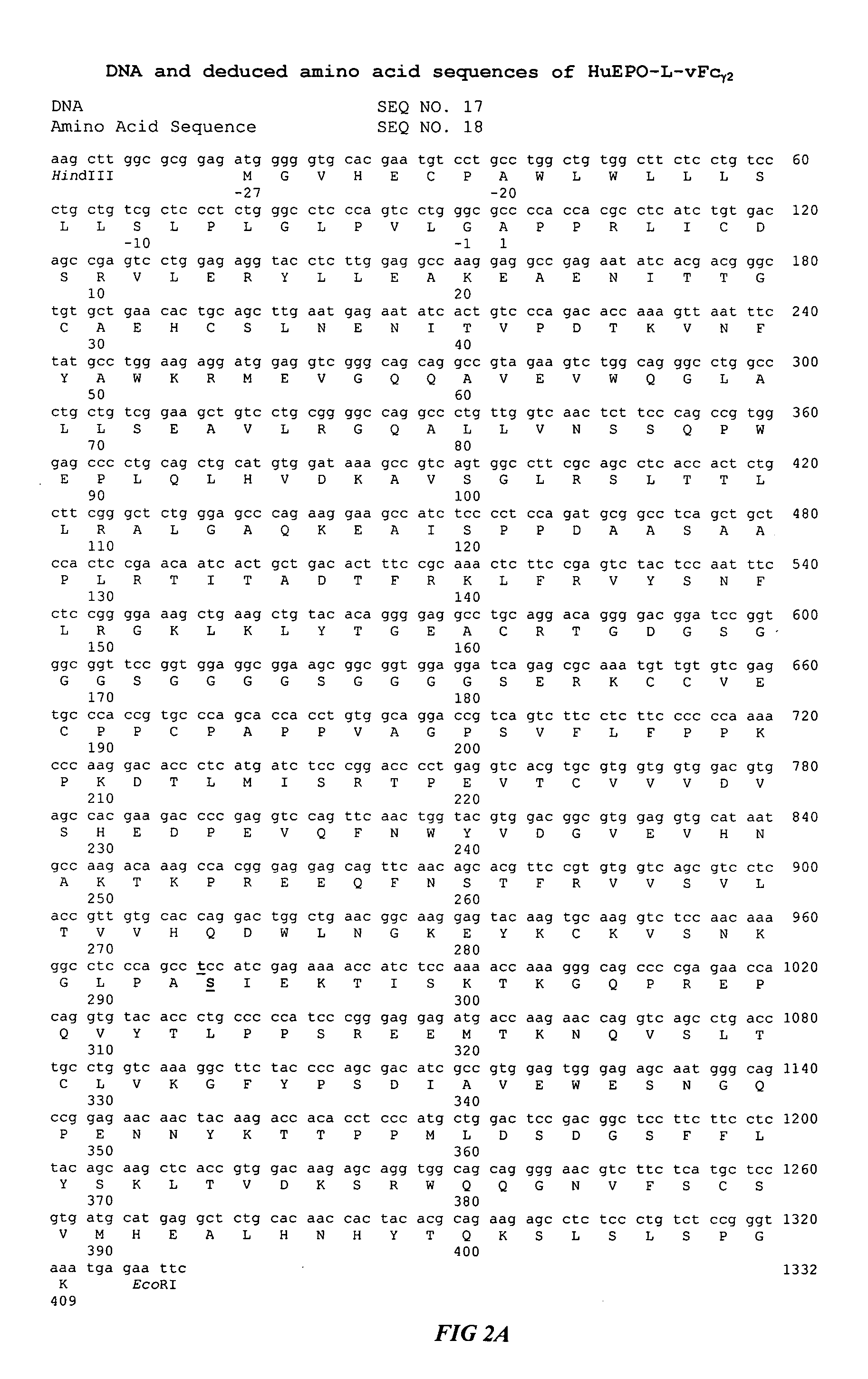
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Methods and compositions using immunomodulatory compounds for treatment and management of cancers and other diseases
ActiveUS20040029832A1Prevent proliferationAntibacterial agentsBiocideSide effectBiologically-Based Therapy
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Methods and compositions for the treatment of pain and other alpha 2 adrenergic-mediated conditions
InactiveUS20050059744A1Improve effectivenessHigh activityBiocideOrganic active ingredientsSedative EffectsSide effect
Owner:ALLERGAN INC
Implantable microstimulators and methods for unidirectional propagation of action potentials
InactiveUS7860570B2Increase stimulationLess power consumptionSpinal electrodesArtificial respirationNerve fiber bundleSide effect
Miniature implantable stimulators (i.e., microstimulators) are capable of producing unidirectionally propagating action potentials (UPAPs). The methods and configurations described may, for instance, arrest action potentials traveling in one direction, arrest action potentials of small diameters nerve fibers, arrest action potentials of large diameter nerve fibers. These methods and systems may limit side effects of bidirectional and / or less targeted stimulation.
Owner:BOSTON SCI NEUROMODULATION CORP
Cavernous nerve stimulation via unidirectional propagation of action potentials
InactiveUS7203548B2Less power consumptionExcessive stimulationSpinal electrodesExternal electrodesDiseaseSide effect
Methods of using unidirectionally propagating action potentials (UPAPs) for cavernous nerve stimulation and for certain disorders are provided. Stimulators capable of creating such UPAPs include, but are not limited to, miniature implantable stimulators (i.e., microstimulators), possibly with programmably configurable electrodes. In one aspect, a method includes providing at least one implantable stimulator with at least two electrodes, disposing the electrodes to apply stimulation that unidirectionally propagates action potentials along a cavernous nerve; and applying the stimulation to the cavernous nerve, thereby treating erectile dysfunction while limiting side effects of bidirectional stimulation.
Owner:BOSTON SCI NEUROMODULATION CORP
Once-a-day, oral, controlled-release, oxycodone dosage forms
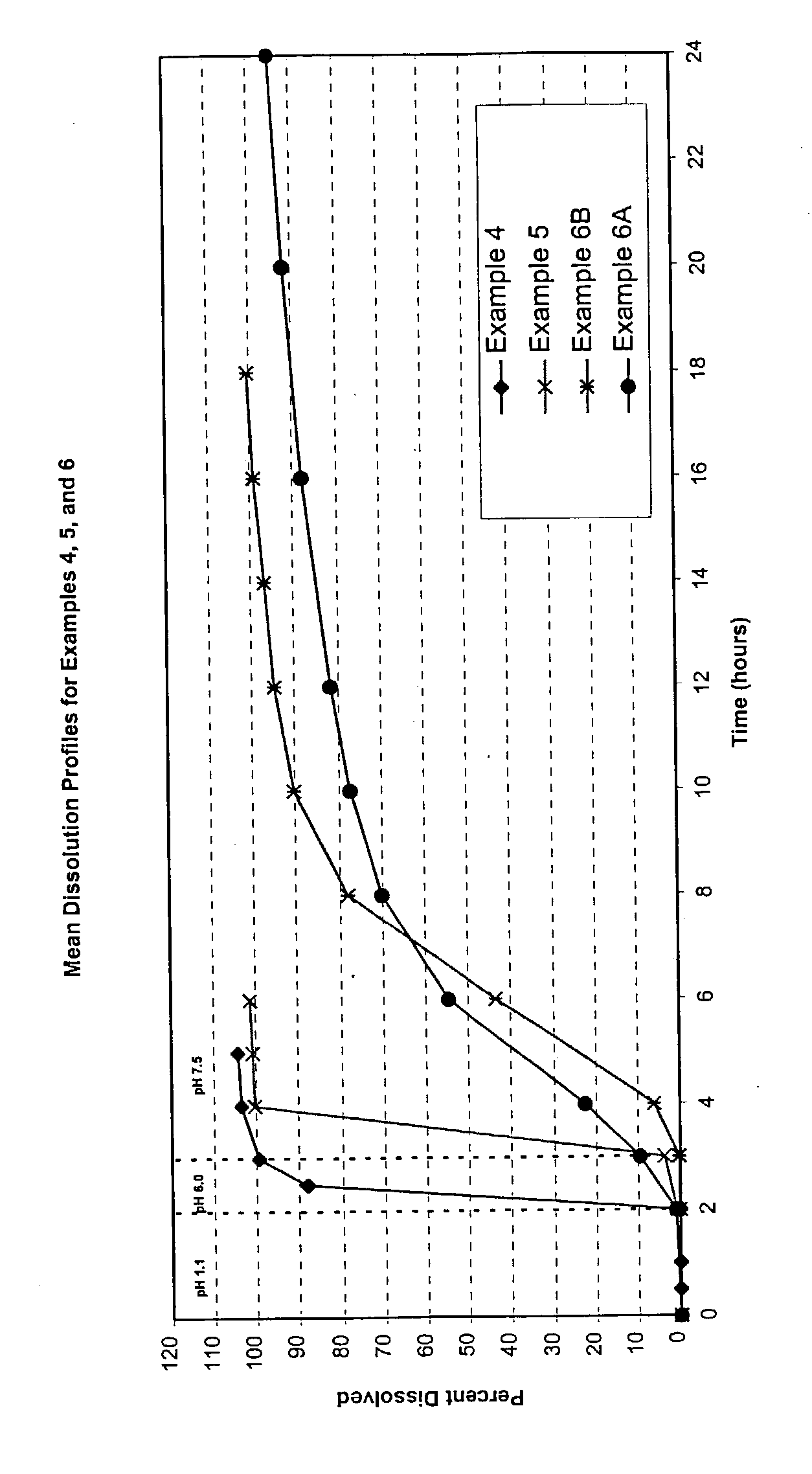
Oxycodone formulations are provided which produce substantially flat in vivo steady state plasma profiles. Tolerance levels associated with such profiles and tolerance levels associated with biphasic profiles are shown not to be statistically different. The substantially flat in vivo steady state plasma profiles are produced by dosage forms having substantially zero order in vitro release profiles. Such release profiles produce low single dose in vivo Cmax levels which can reduce the probability of adverse side effects.
Owner:ALZA CORP
Tobacco and/or tobacco substitute composition for use as a snuff in the oral cavity
InactiveUS20050061339A1Reduce releaseAvoid spreadingTobacco treatmentSide effectEnvironmental health
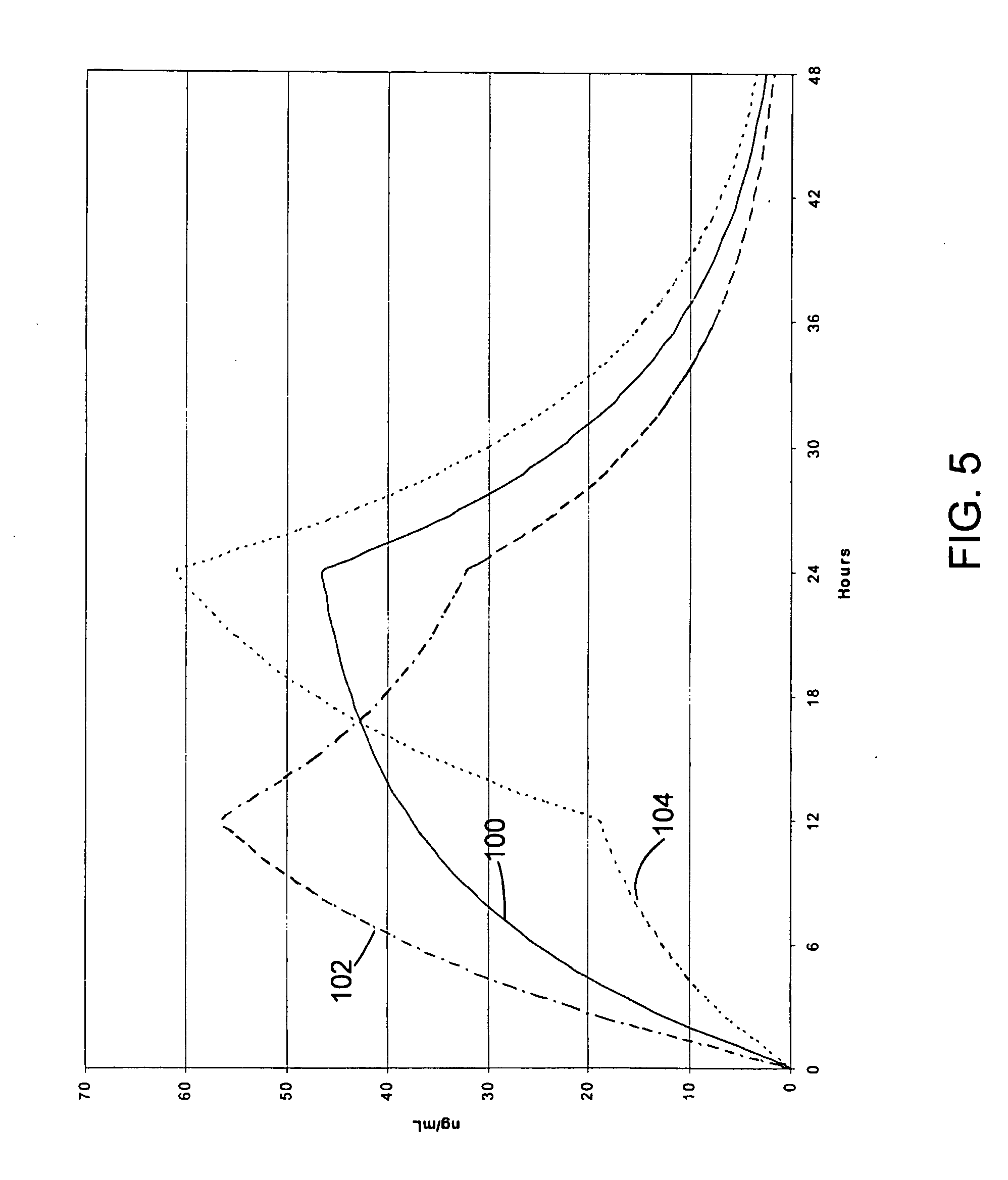
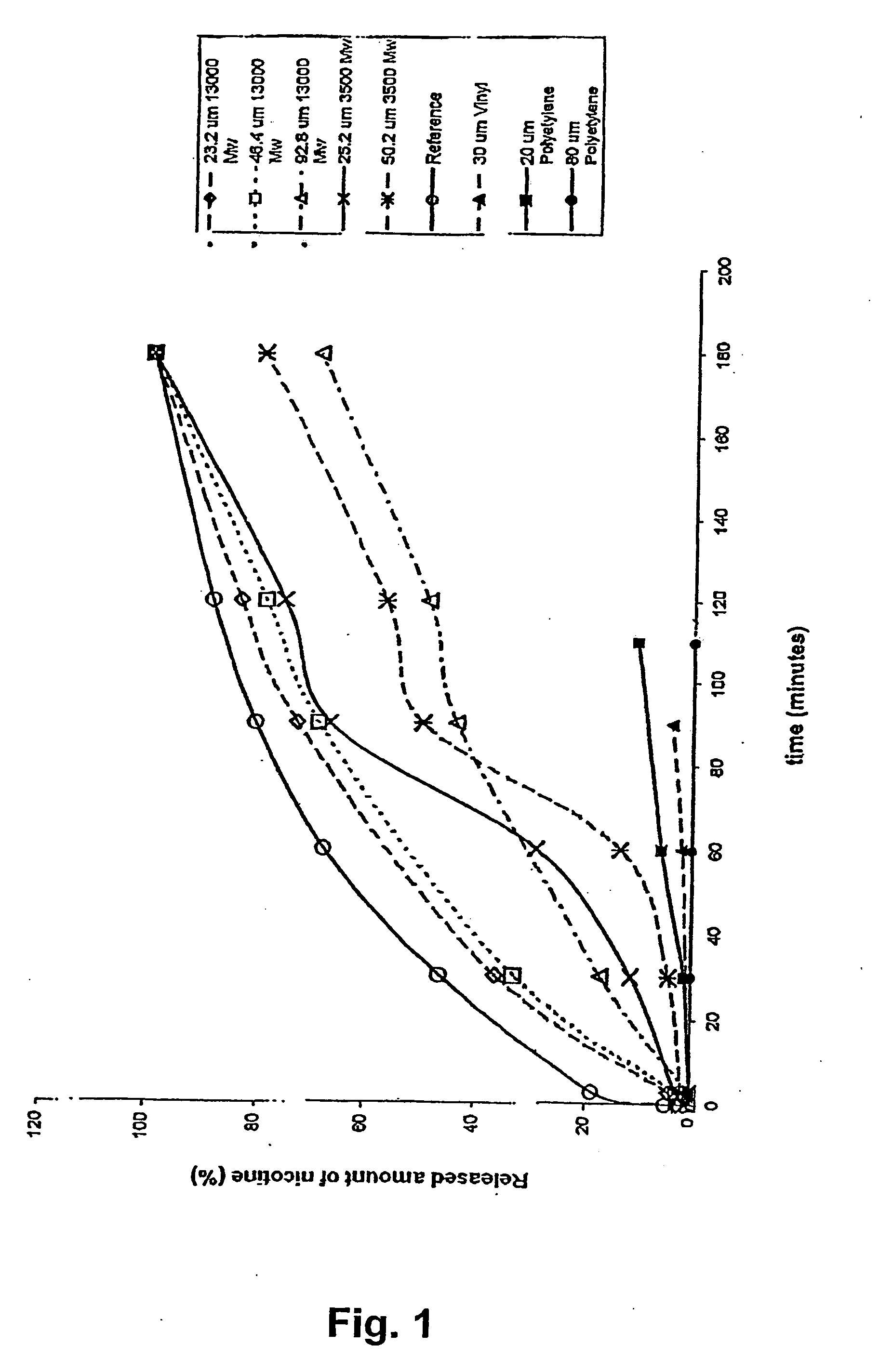
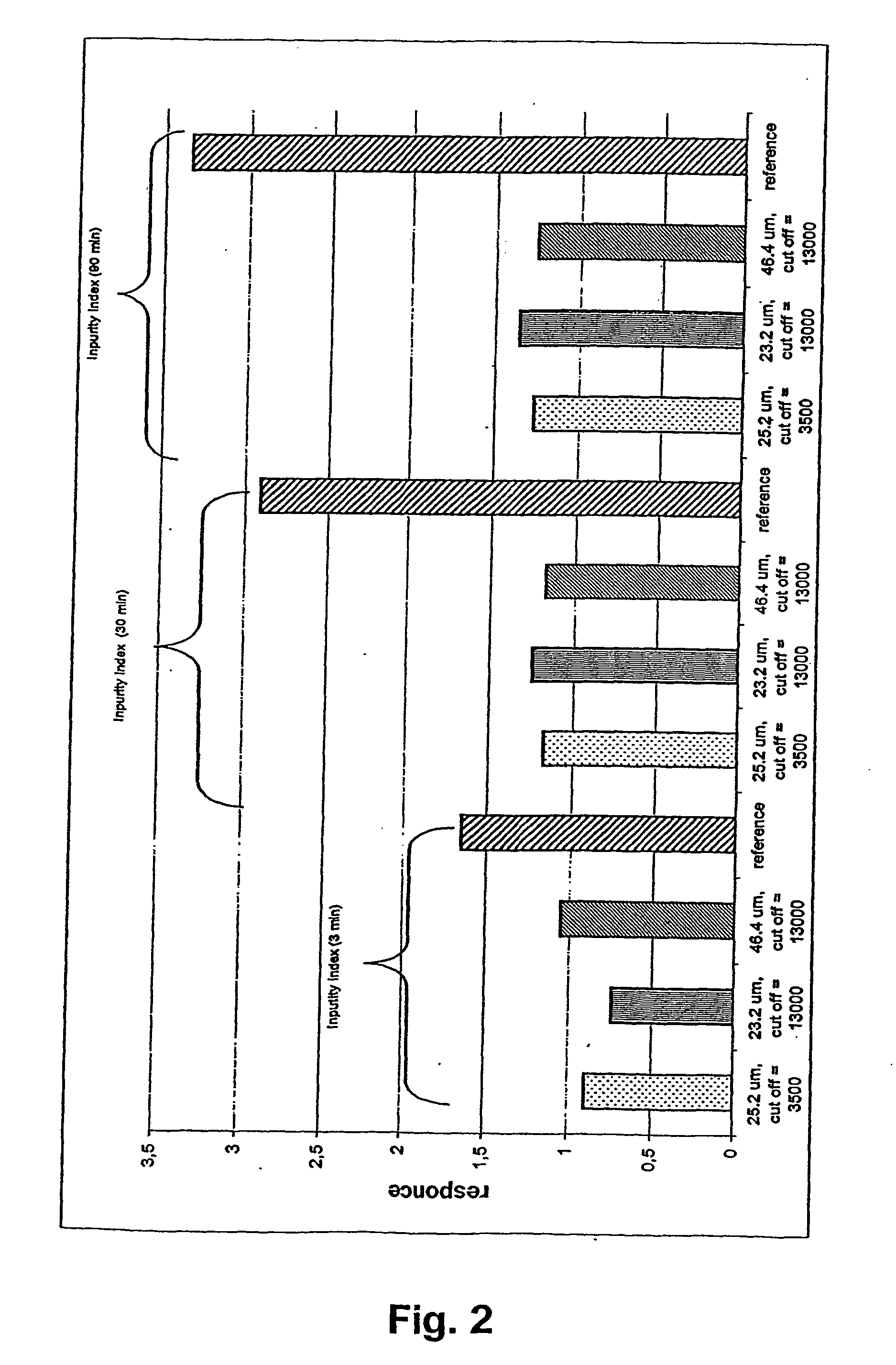
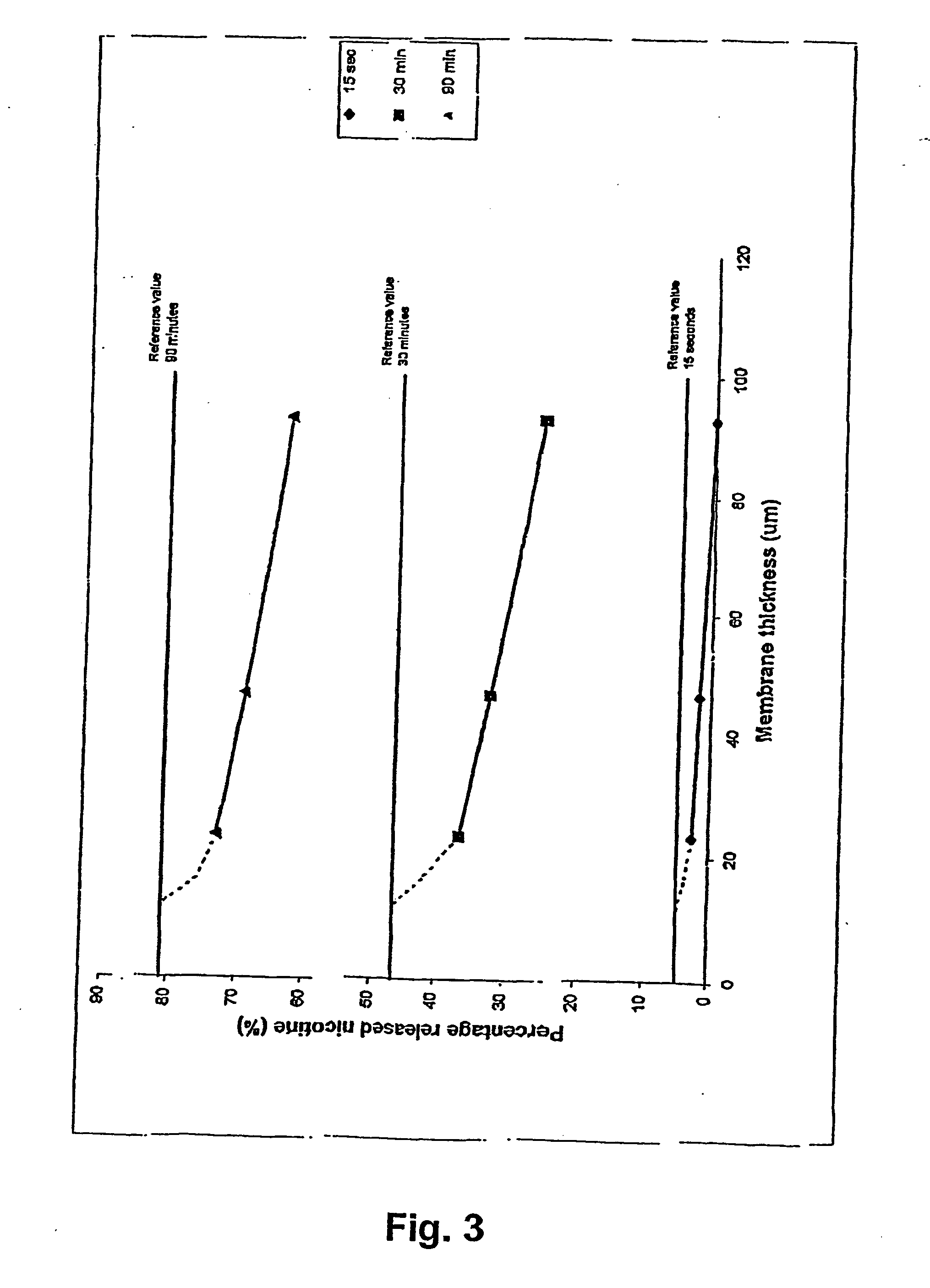
A novel composition for the use as snuff in the oral cavity, the composition comprising tobacco and / or a tobacco substitute encapsulated in a membrane material comprising one or more membranes at least one of which being water permeable and water-insoluble. A novel composition enables a selective release of e.g. nicotine while it at the same time reduces the release of substances, which normally lead to unwanted side effects. The novel compositions may be used as a healthier alternative to snuff and other tobacco products such as, e.g., cigarettes, cigars and pipe. Methods for giving up smoking, reducing nicotine craving, reducing side effects normally related to smoking and snuffing of tobacco as welt a method for the preparation of a composition according to the invention.
Owner:GALENICA
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
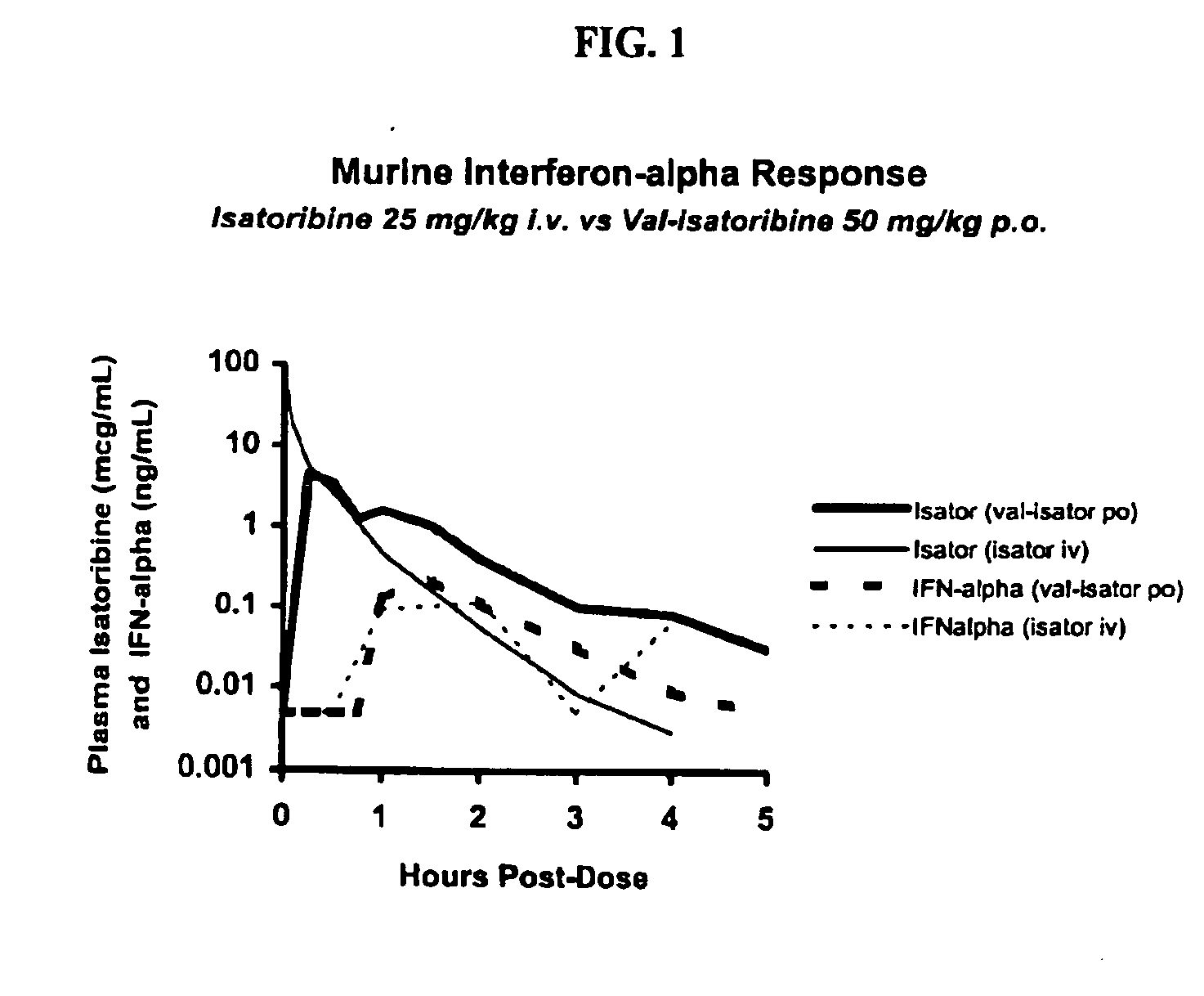
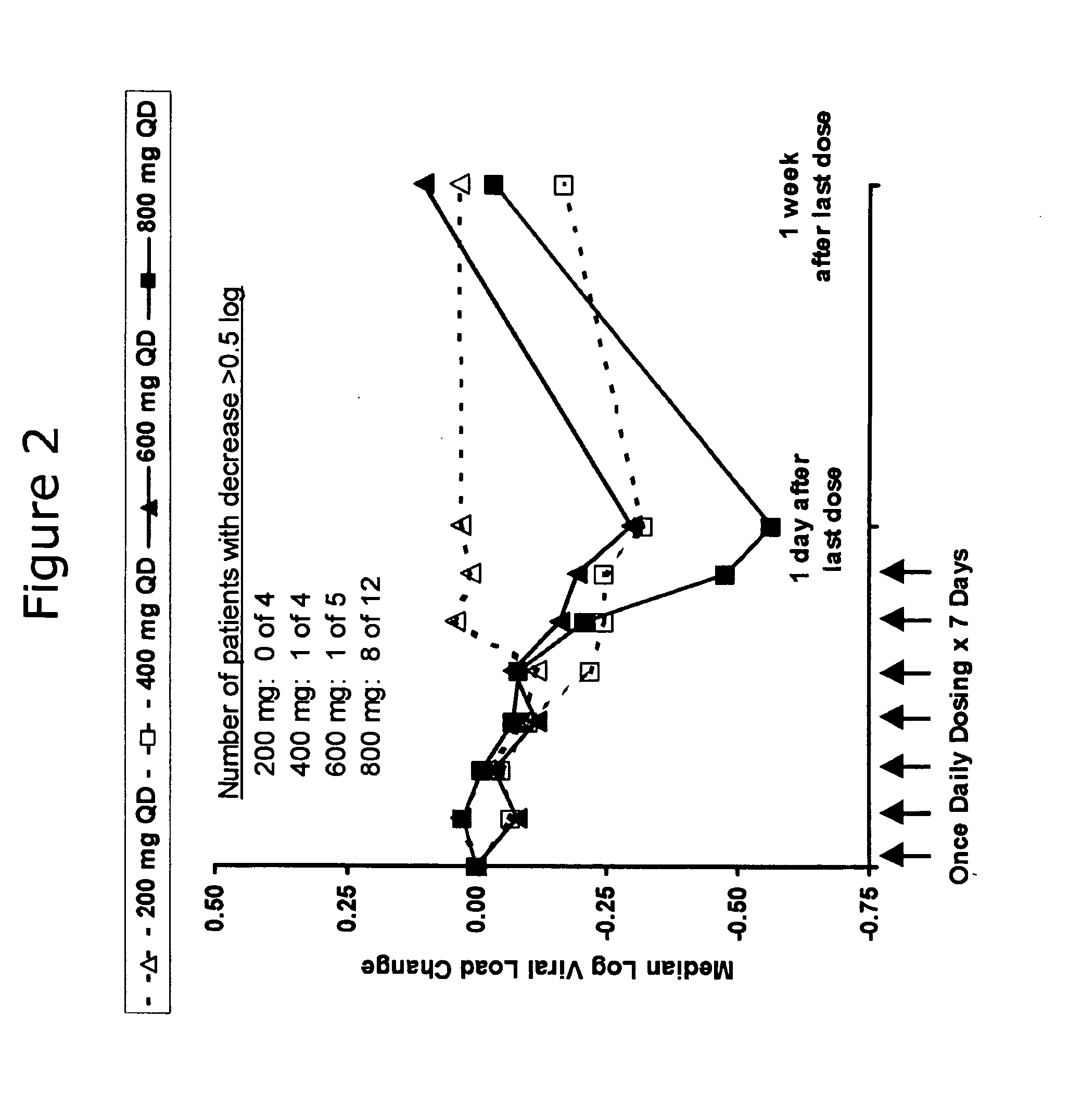
Administration of TLR7 ligands and prodrugs thereof for treatment of infection by hepatitis C virus
InactiveUS20050054590A1Reduce sensitivityAvoid spreadingBiocideDigestive systemHepatitis c viralSide effect
This invention relates to methods for treating or preventing hepatitis C virus infections in mammals using Toll-Like Receptor (TLR)7 ligands and prodrugs thereof. More particularly, this invention relates to methods of orally administering a therapeutically effective amount of one or more prodrugs of TLR7 ligands for the treatment or prevention of hepatitis C viral infection. Oral administration of these TLR7 immunomodulating ligands and prodrugs thereof to a mammal provides therapeutically effective amounts and reduced undesirable side effects.
Owner:ANDADYS PHARMA INC
Selective stimulation systems and signal parameters for medical conditions
Devices, systems and methods are provided for targeted treatment of a variety of conditions, particularly conditions that are associated with or influenced by the nervous system, such as pain. Targeted treatment of such conditions is provided with minimal deleterious side effects, such as undesired motor responses or undesired stimulation of unaffected body regions. This is achieved by directly neuromodulating a target anatomy associated with the condition while minimizing or excluding undesired neuromodulation of other anatomies.
Owner:TC1 LLC
Pain management with stimulation subthreshold to paresthesia
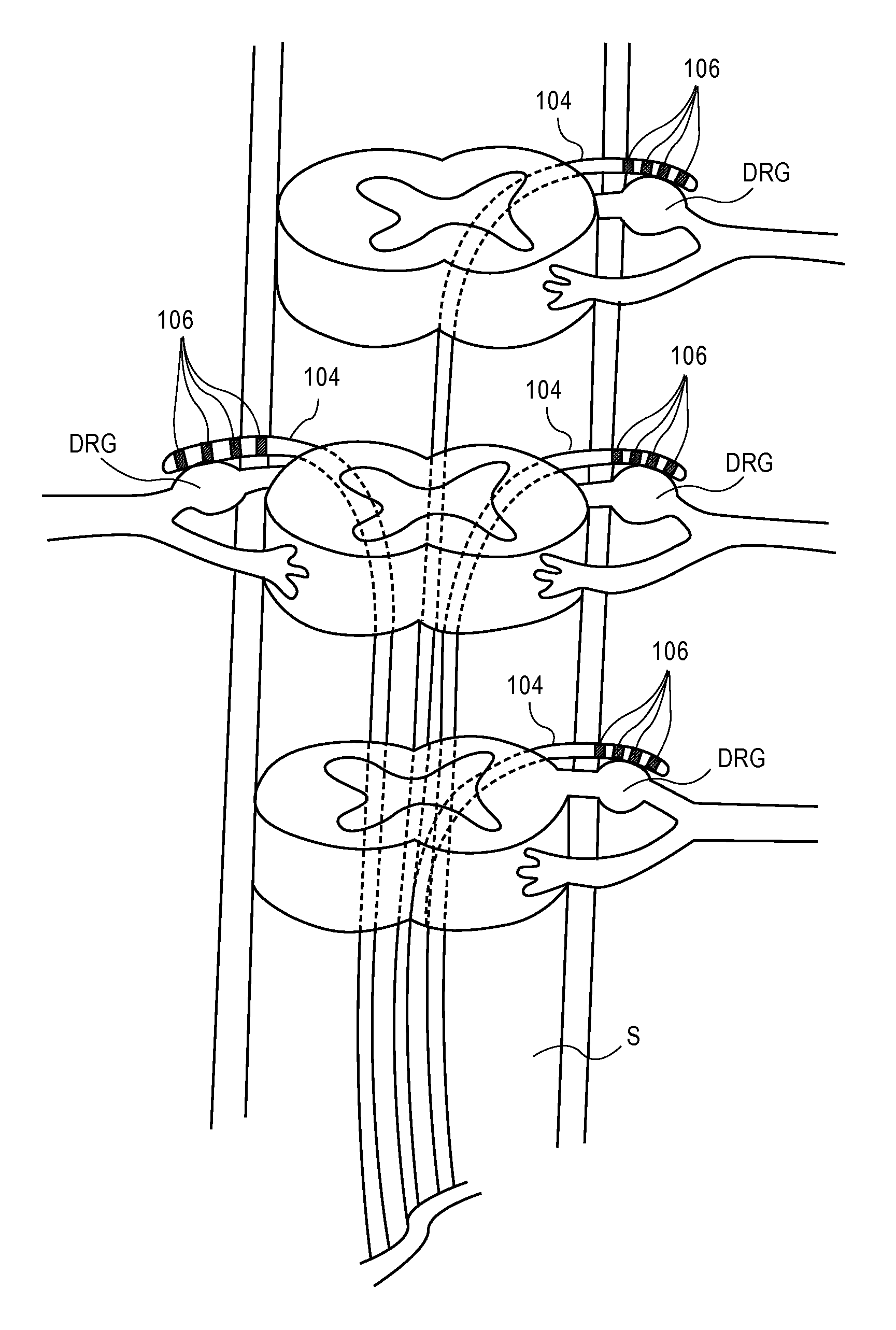
ActiveUS20100249875A1Minimizing complicationsMinimizing effectsSpinal electrodesArtificial respirationHypesthesiaSide effect
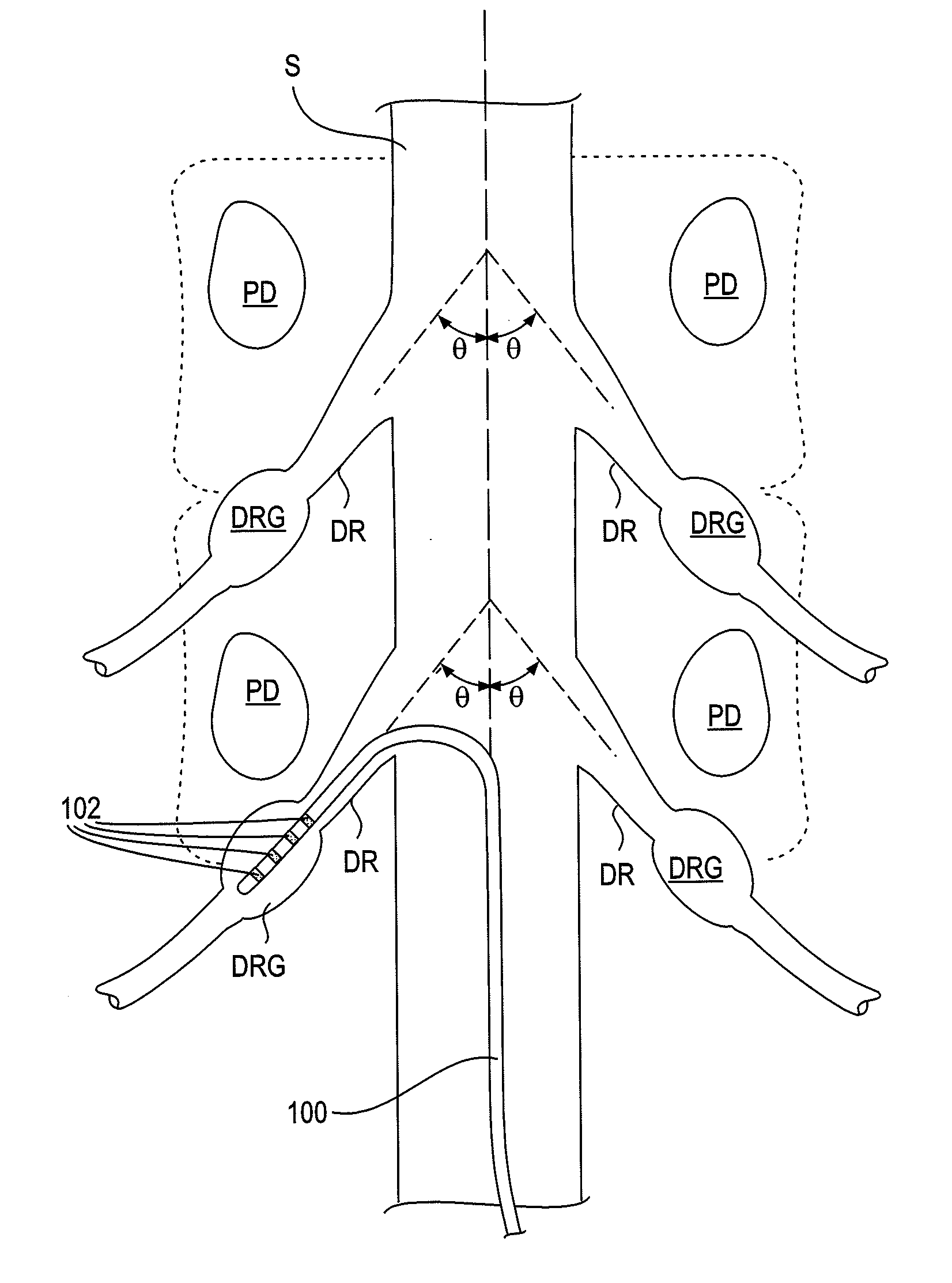
Devices, systems and methods are provided for treating pain while minimizing or eliminating possible complications and undesired side effects, particularly the sensation of paresthesia. This is achieved by stimulating in proximity to a dorsal root ganglion with stimulation energy in a manner that will affect pain sensations without generating substantial sensations of paresthesia. In some embodiments, such neurostimulation takes advantage of anatomical features and functions particular to the dorsal root ganglion.
Owner:TC1 LLC
Drug formulations having reduced abuse potential
InactiveUS20040228802A1Prevent extractionReduce potential for abuseBiocidePowder deliverySide effectDrug formulations
Drug formulations having reduced abuse potential which contain one or more of (1) a bittering agent, (2) a bright deterrent / indicator dye and (3) fine insoluble particulate matter. The bittering agent and dye are in a form which does not affect proper administration of the drug, but the bittering agent creates a bitter side effect when the dosage form is crushed or chemically extracted and nasally, orally, buccally or sublingually administered and the dye produces a bright color when crushed and contacted. The fine insoluble particulate matter hinders extraction of the drug from the dosage form and, when crushed, can deter intravenous injection because of the presence of the insoluble particles or hinder injection by blocking an intravenous needle. The bright color of the dye, when extracted, also has a psychologically deterrent effect on intravenous abusers.
Owner:SUPERNUS PHARM INC
Sling delivery system and method of use
InactiveUS6971986B2Structural damageReduce the amount requiredSuture equipmentsIncision instrumentsDiseaseSide effect
An apparatus and method of use are disclosed to treat urological disorders. The biocompatible device includes a handle, needle, dilator and sling assembly configured to be minimally invasive and provide sufficient support to the target site. In addition, the configuration of the sling assembly also allows the sling to be adjusted during and / or after implantation. The device and treatment procedure are highly effective and produce little to no side effects or complications. Further, operative risks, pain, infections and post operative stays are reduced, thereby improving patient quality of life.
Owner:STASKIN DAVID MD DR
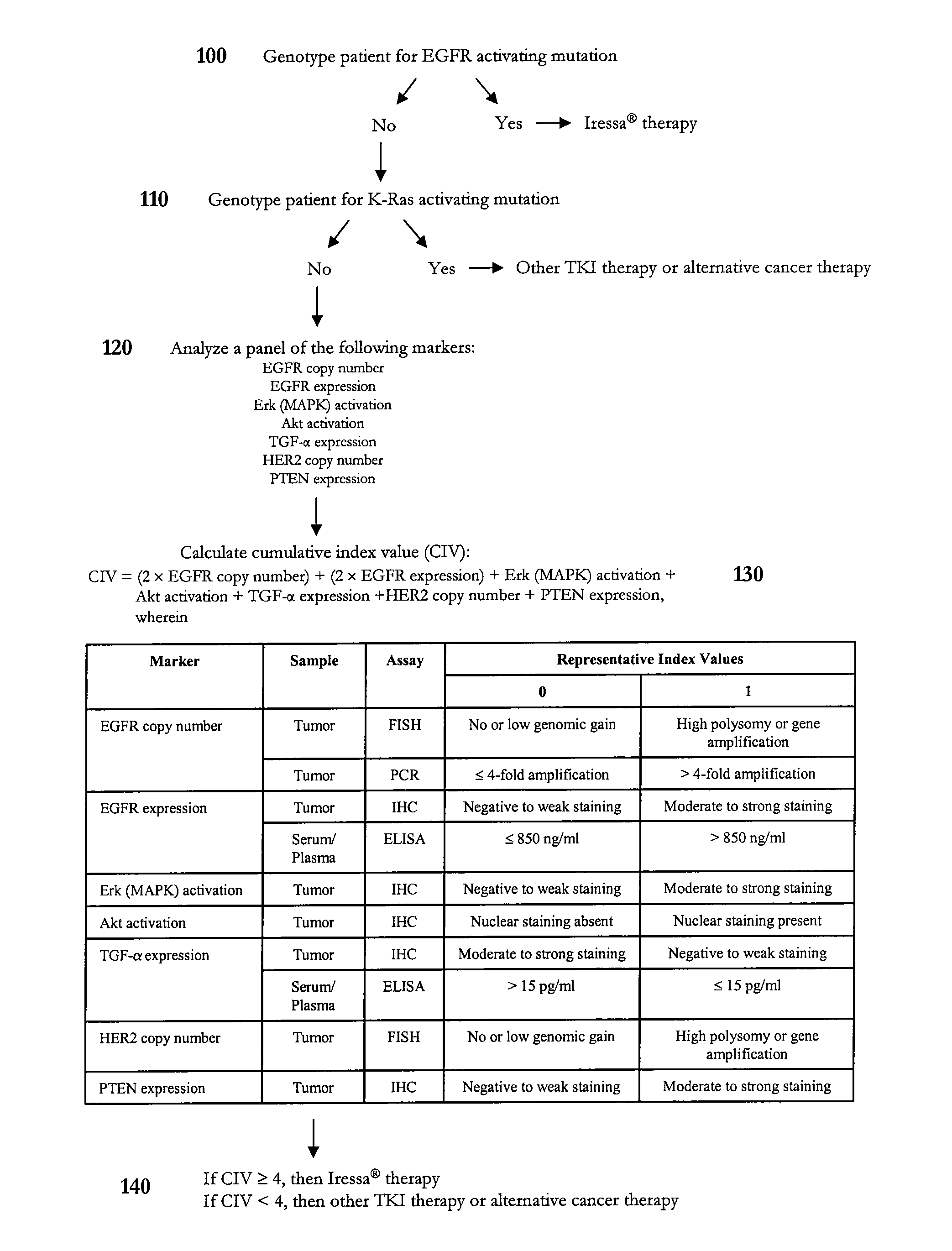
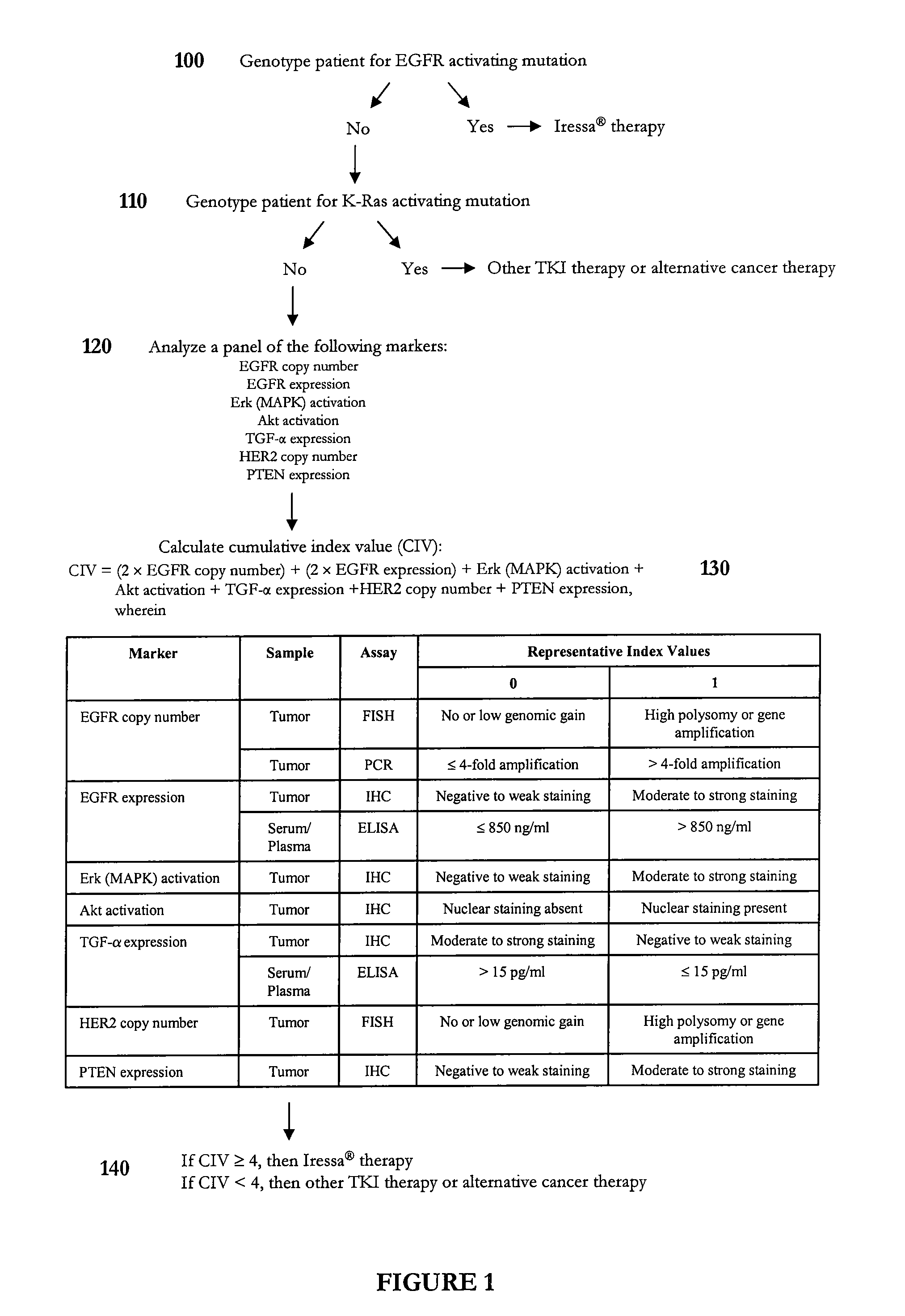
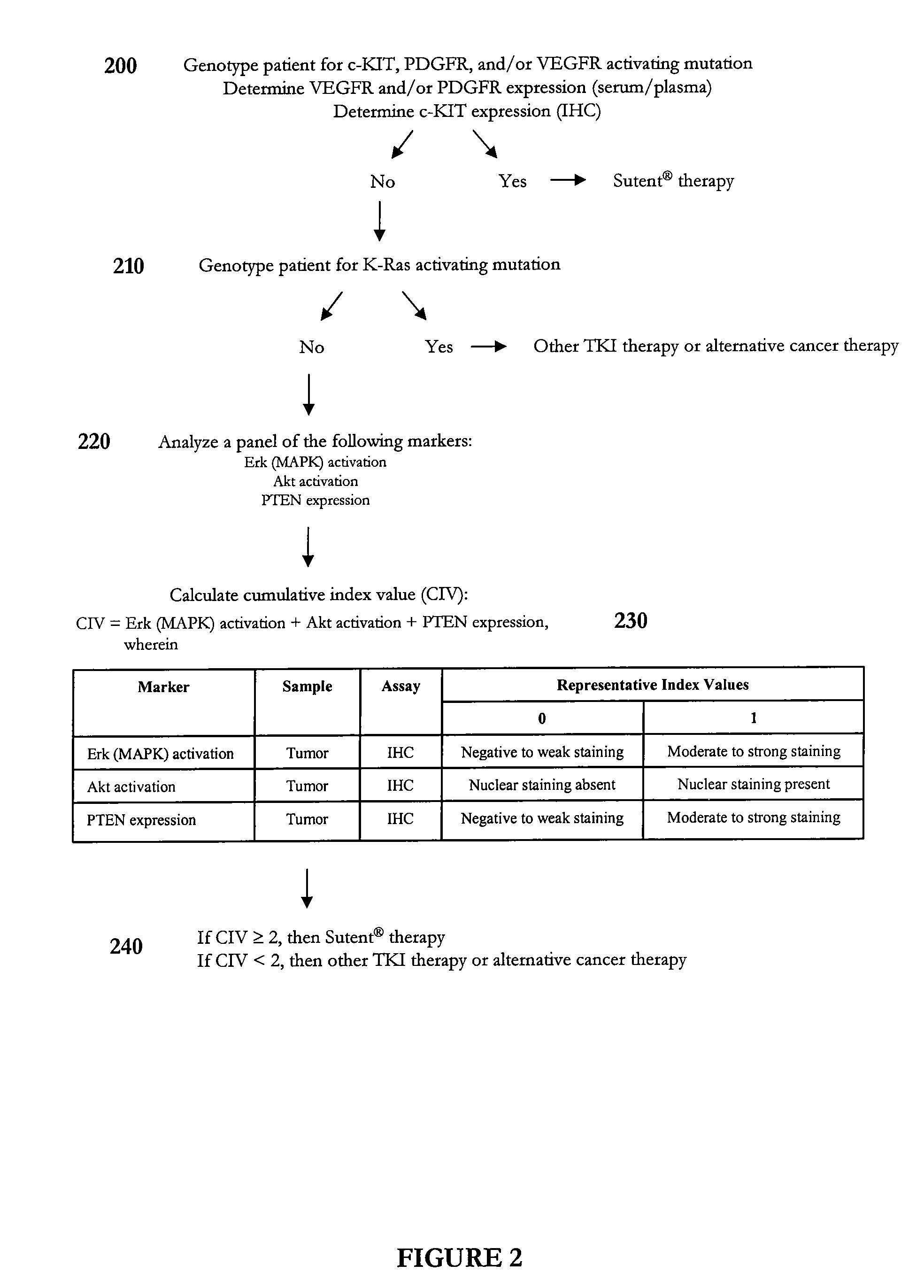
Methods of predicting and monitoring tyrosine kinase inhibitor therapy
InactiveUS20070254295A1Eliminate side effectsCompound screeningApoptosis detectionAbnormal tissue growthSide effect
Owner:SOC DES PROD NESTLE SA
Methods related to immunostimulatory nucleic acid-induced interferon
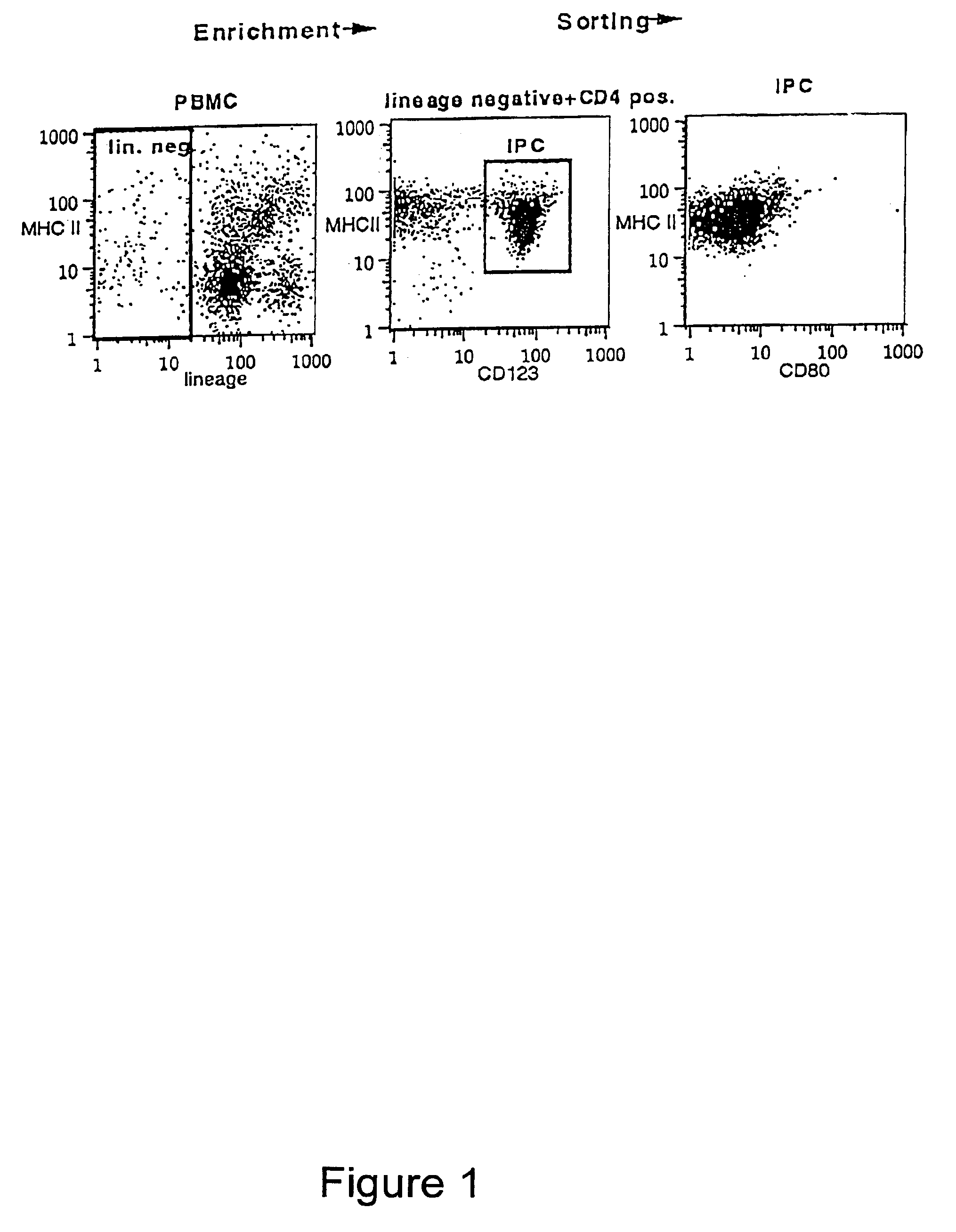
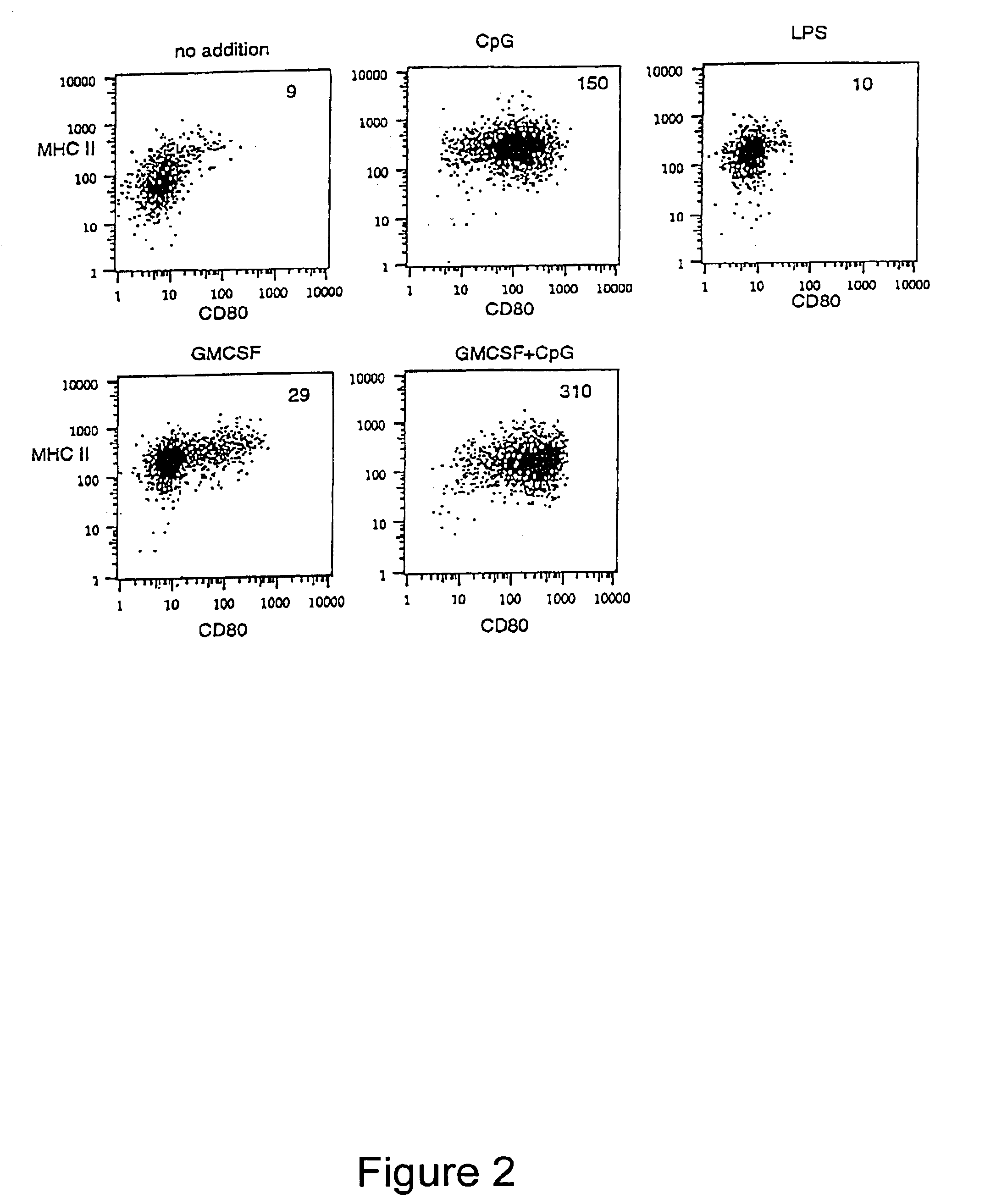
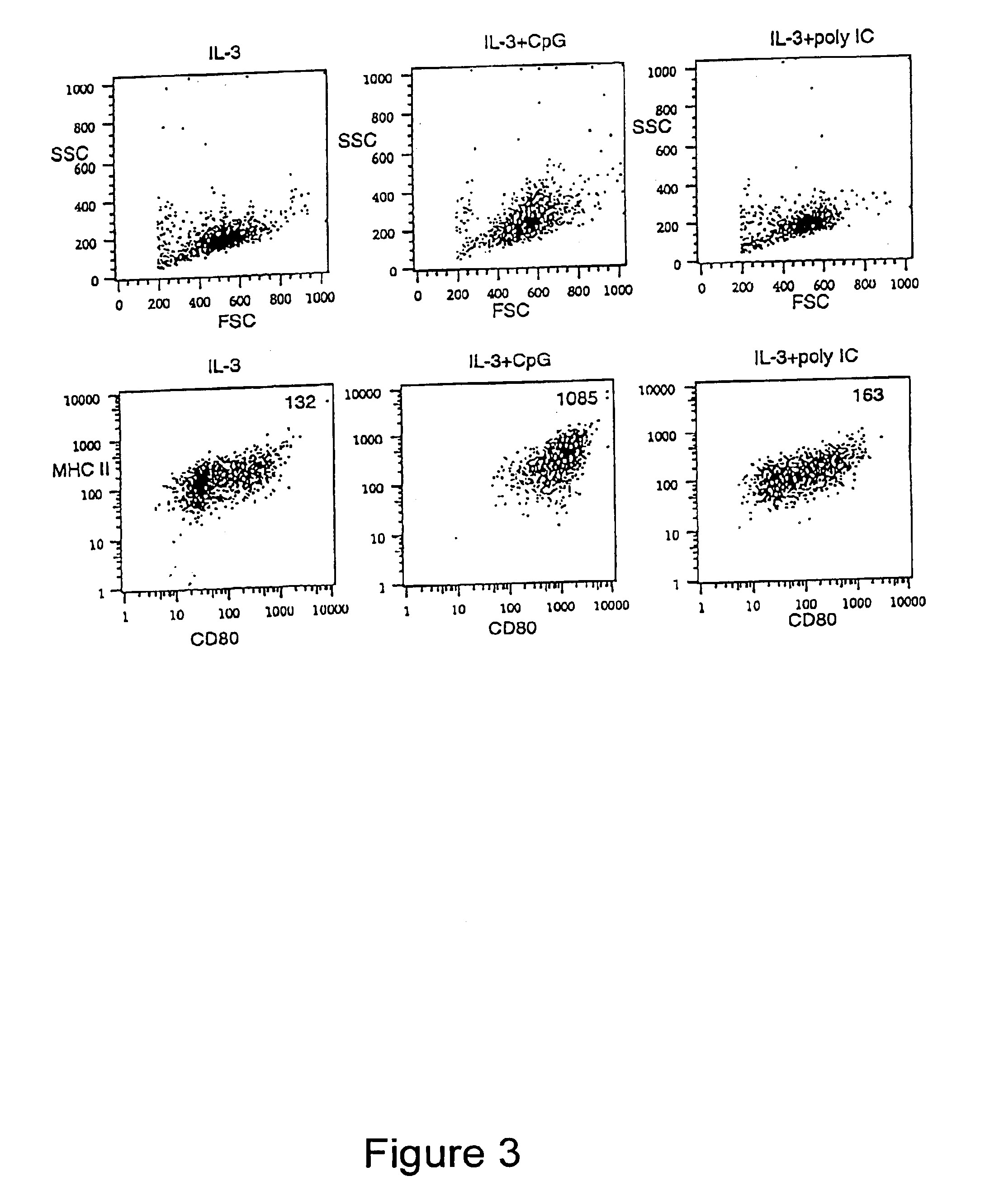
InactiveUS6949520B1Increased proliferationIncrease productionPeptide/protein ingredientsGenetic material ingredientsSide effectMedicine
Methods and compositions are provided for extending the clinical utility of IFN-α in the treatment of a variety of viral and proliferative disorders. Among other aspects, the invention provides methods which increase the efficacy of IFN-α treatment and reduce IFN-α treatment-related side effects. In addition, methods are provided for supporting the survival and for activating natural interferon producing cells (IPCs) in vitro without exogenous IL-3 or GM-CSF. The invention is based on the discovery that certain CpG and non-CpG ISNAs promote survival and stimulation of IPCs.
Owner:COLEY PHARMA GMBH +2
Directional brain stimulation and recording leads
InactiveUS7212867B2Improve abilitiesLess interferenceHead electrodesExternal electrodesElectricitySide effect
A directional brain stimulation lead assembly provides a lead body and an insulating member defining one or more windows that selectively expose portions of electrodes carried by the lead body to produce a directional stimulation current field. The lead assembly can achieve more effective localization of electrical stimulation to very small brain targets, and thereby reduce the incidence of material side effects caused by collateral stimulation of brain tissue adjoining a desired brain target. In addition, the directional lead can sense brain activity on a more localized basis.
Owner:MEDTRONIC INC
Compositions and methods for the treatment of cancer
InactiveUS20020035090A1Improve toleranceReducing and avoiding adverse effectBiocideCarbohydrate active ingredientsCancer preventionSide effect
This invention relates to compositions comprising thalidomide and another anti-cancer drug which can be used in the treatment or prevention of cancer. Preferred anti-cancer drugs are topoisomerase inhibitors. A particular composition comprises thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and irinotecan. The invention also relates to methods of treating or preventing cancer which comprise the administration of a thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of chemotherapy or radiation therapy which comprise the administration of thalidomide to a patient in need of such reduction or avoidance.
Owner:CELGENE CORP
Stimulation leads, delivery systems and methods of use
InactiveUS20100179562A1Minimizing complicationMinimize side effectsSpinal electrodesDiagnosticsSide effectMedicine
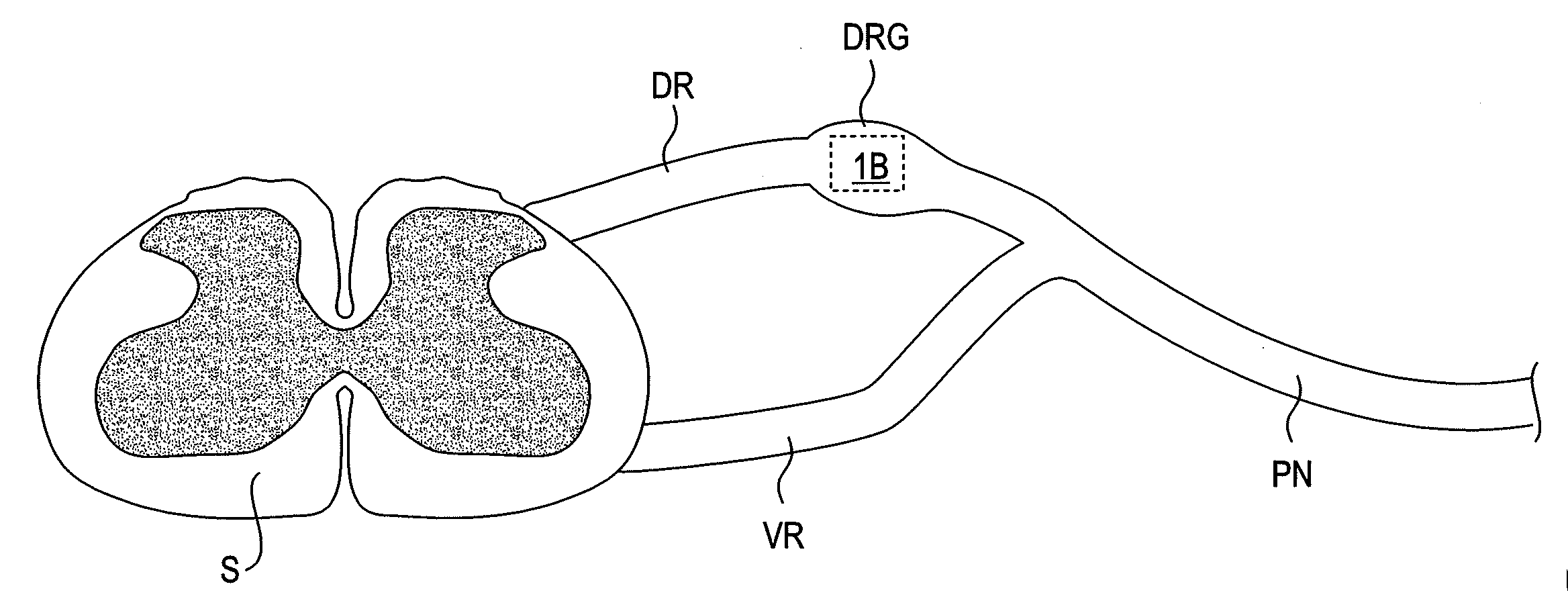
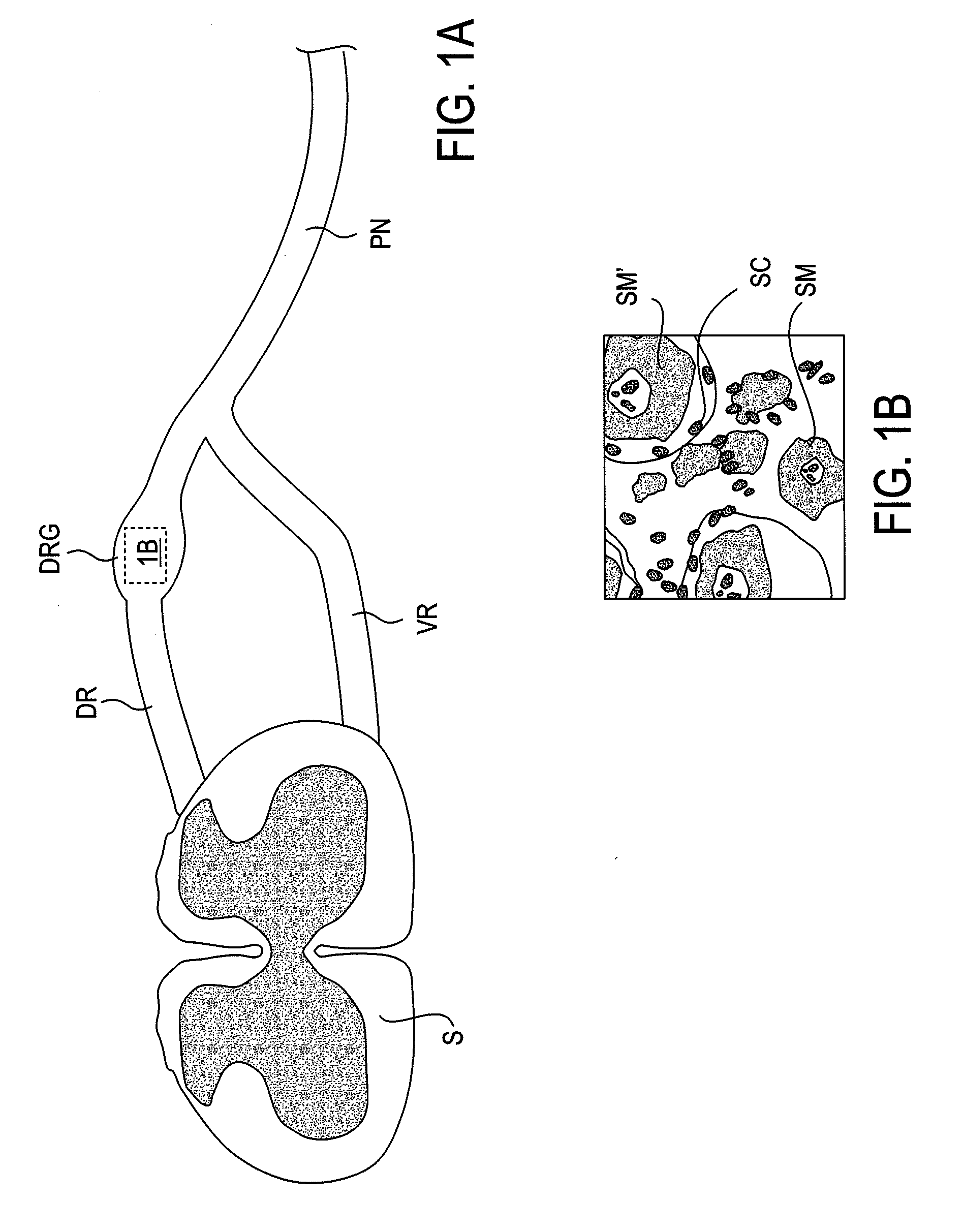
Devices, systems and methods are provided for accessing and treating anatomies associated with a variety of conditions while minimizing possible complications and side effects. This is achieved by directly neuromodulating a target anatomy associated with the condition while minimizing or excluding undesired neuromodulation of other anatomies. Typically, this involves stimulating portions of neural tissue of the central nervous system, wherein the central nervous system includes the spinal cord and the pairs of nerves along the spinal cord which are known as spinal nerves. In particular, some embodiments of the present invention are used to selectively stimulate portions of the spinal nerves, particularly one or more dorsal root ganglions (DRGs), to treat chronic pain while causing minimal deleterious side effects such as undesired motor responses.
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG SMI S A R L SJM LUX SMI
Short duration pre-pulsing to reduce stimulation-evoked side-effects
A method and neurostimulation system of providing therapy to a patient is provided. At least one electrode is placed in contact with tissue of a patient. A sub-threshold, hyperpolarizing, conditioning pre-pulse (e.g., an anodic pulse) is conveyed from the electrode(s) to render a first region of the tissue (e.g., dorsal root fibers) less excitable to stimulation, and a depolarizing stimulation pulse (e.g., a cathodic pulse) is conveyed from the electrode(s) to stimulate a second different region of the tissue (e.g., dorsal column fibers). The conditioning pre-pulse has a relatively short duration (e.g., less than 200 μs).
Owner:BOSTON SCI NEUROMODULATION CORP
Treatment regimen for parkinson's disease
InactiveUS20120295960A1Reduce potential side effectsReduce maintenanceNervous disorderNucleic acid vectorSide effectCombination therapy
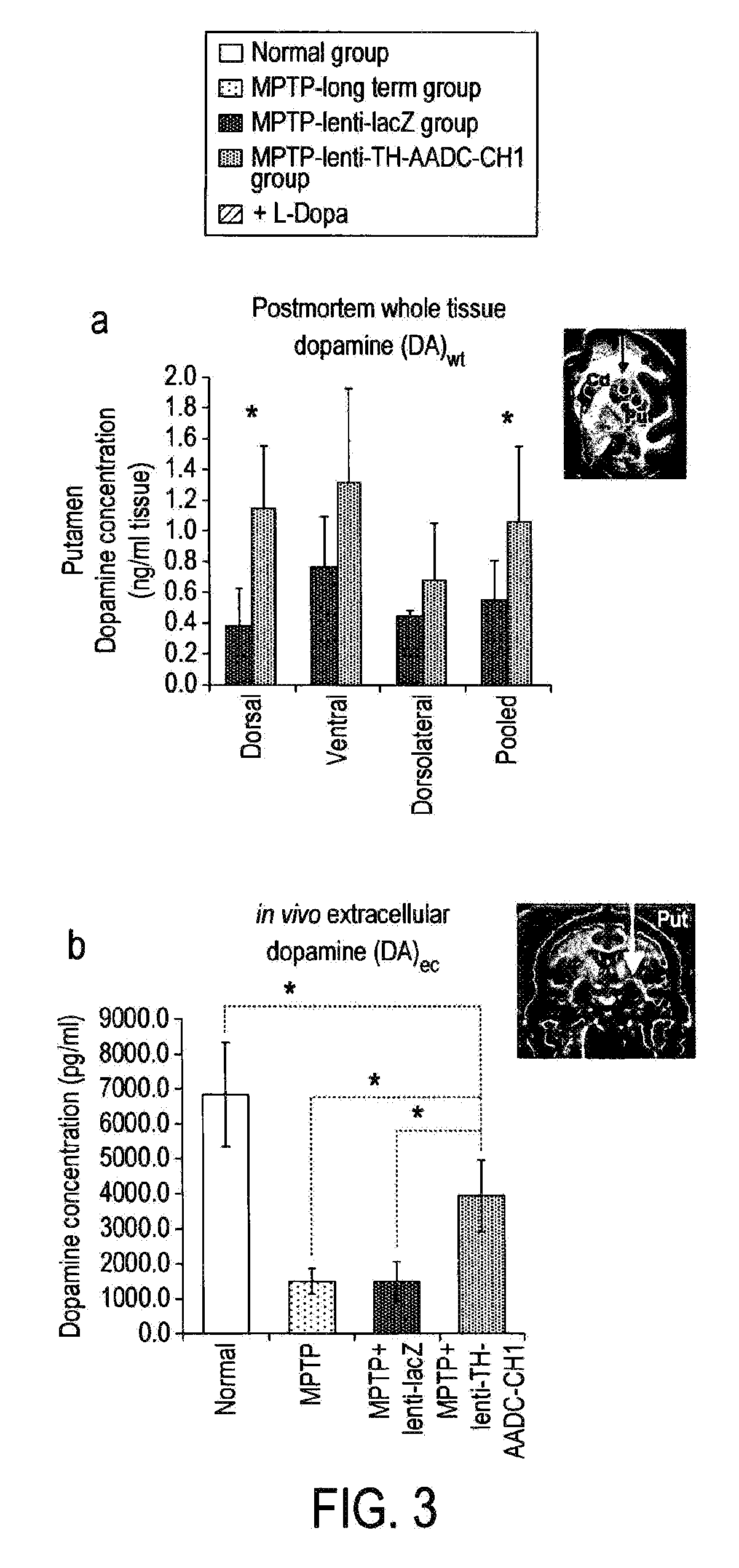
Provided is an improved treatment for Parkinson's Disease where the efficacy of L-Dopa treatment is increased by including gene therapy in the treatment regimen. The combination therapy results in long-term improvements in response to L-Dopa and diminished side effects caused by L-Dopa.
Owner:OXFORD BIOMEDICA (UK) LTD
Oral transmucosal delivery of drugs or any other ingredients via the inner buccal cavity
InactiveUS6210699B1Avoid irritationAvoid the tasteAdhesive dressingsPill deliverySide effectAdditive ingredient
A device and method for the oral transmucosal delivery of active substances to the oral cavity utilizing an unplasticized polyvinyl pyrrolidone polymer (PVP) as the primary mucoadhesive. The device is applied and adheres to the mucosa of the oral cavity without causing side effects or leaving an unpleasant taste. Preferably the device is a bilayer tablet having a mucoadhesive layer and an overlying active substance containing layer. The mucoadhesive layer may contain PVP as the only adhesive or may be combined with other hydrophilic polymeric substances. The active layer also contains a hydrophilic polymer carrier. The layers in the device dissolve and release the active substance to the oral cavity and is particularly adapted for the delivery of substances active in the oral cavity such as breath fresheners and substances to combat dry mouth. It is also useful for the delivery of ionic drugs such as peptides.
Owner:WATSON PHARMA INC +1
Individualized cancer treatments
InactiveUS20070172844A1Increase probabilityLow toxicityBioreactor/fermenter combinationsBiological substance pretreatmentsDNA microarrayPlatinum
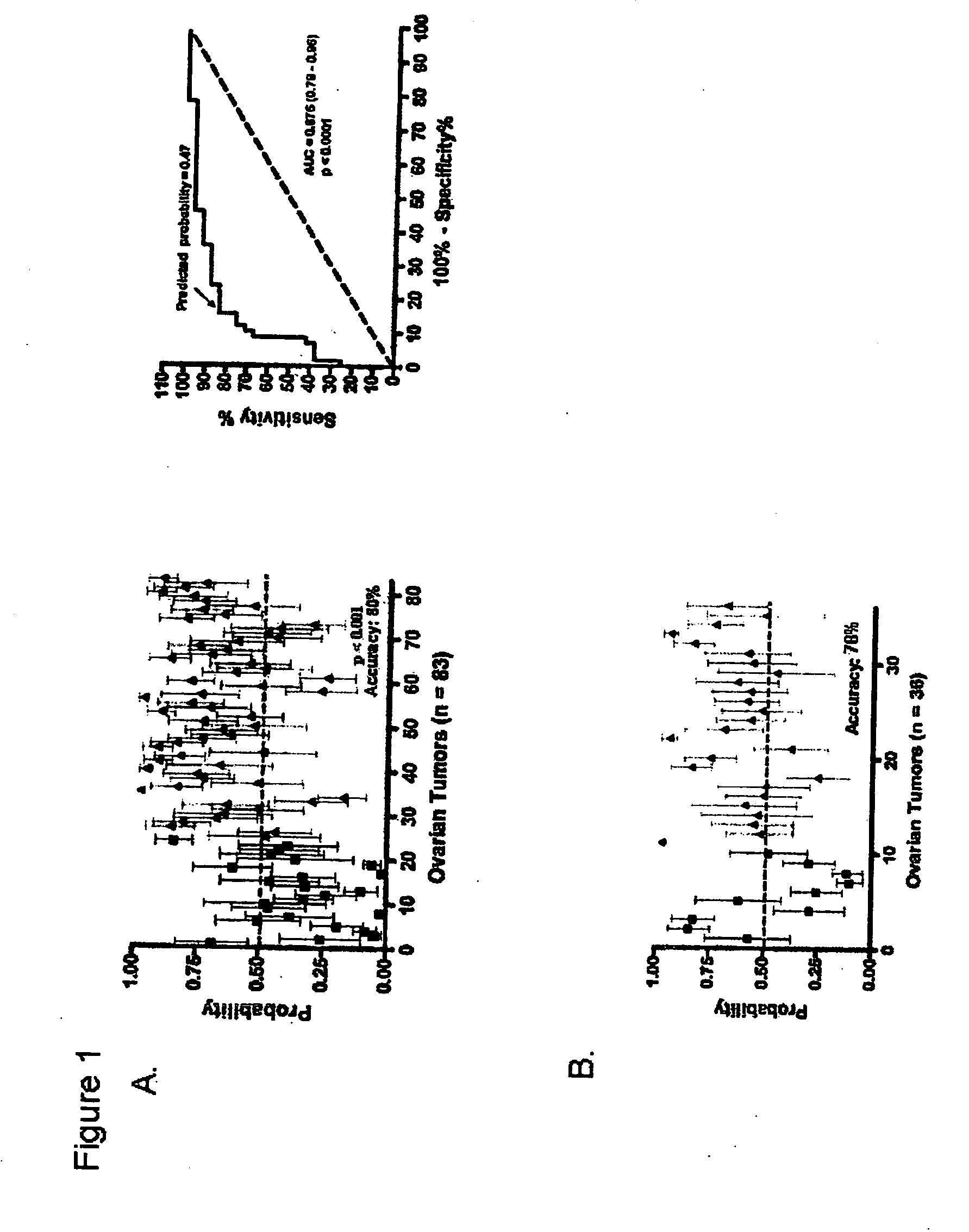
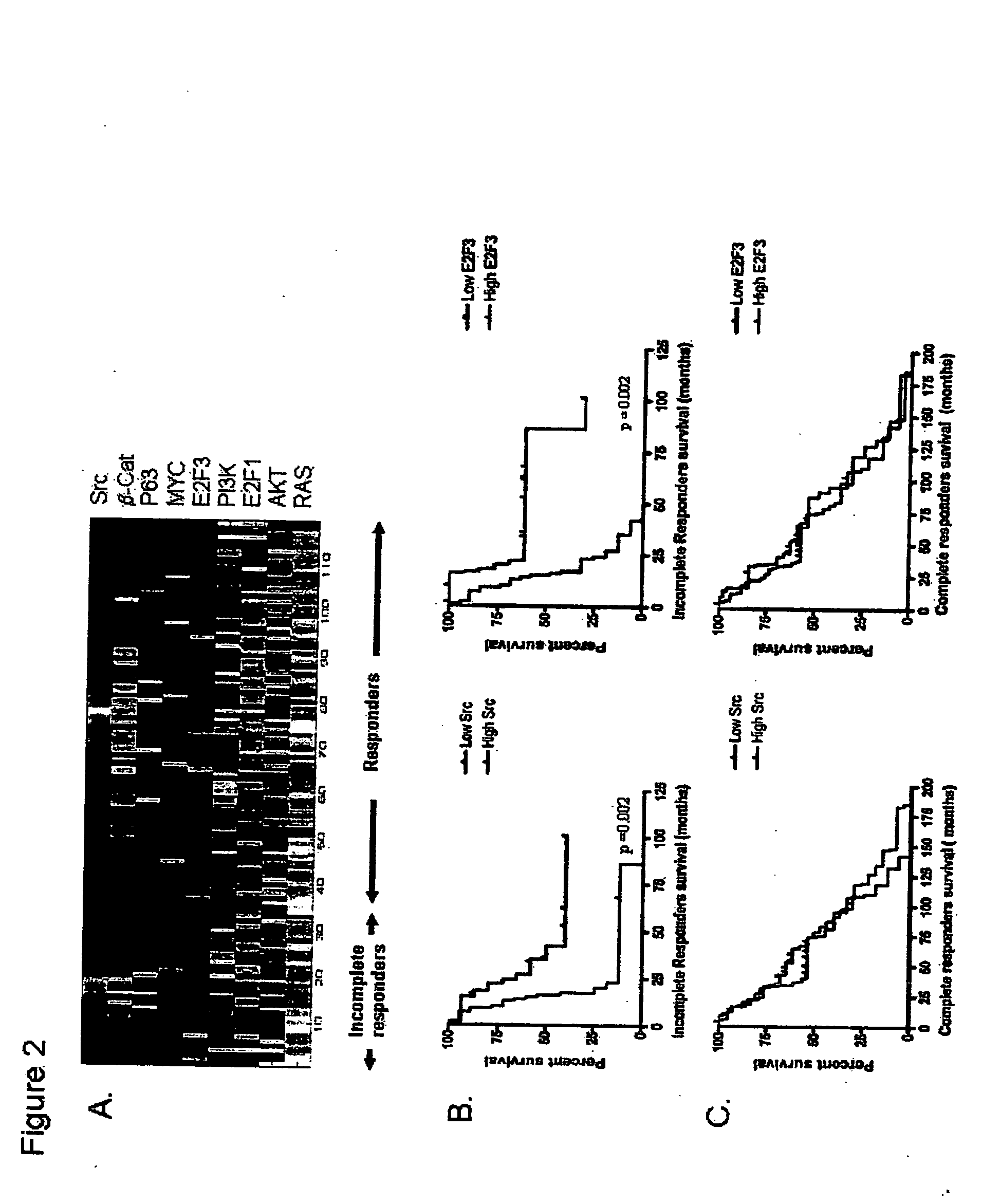
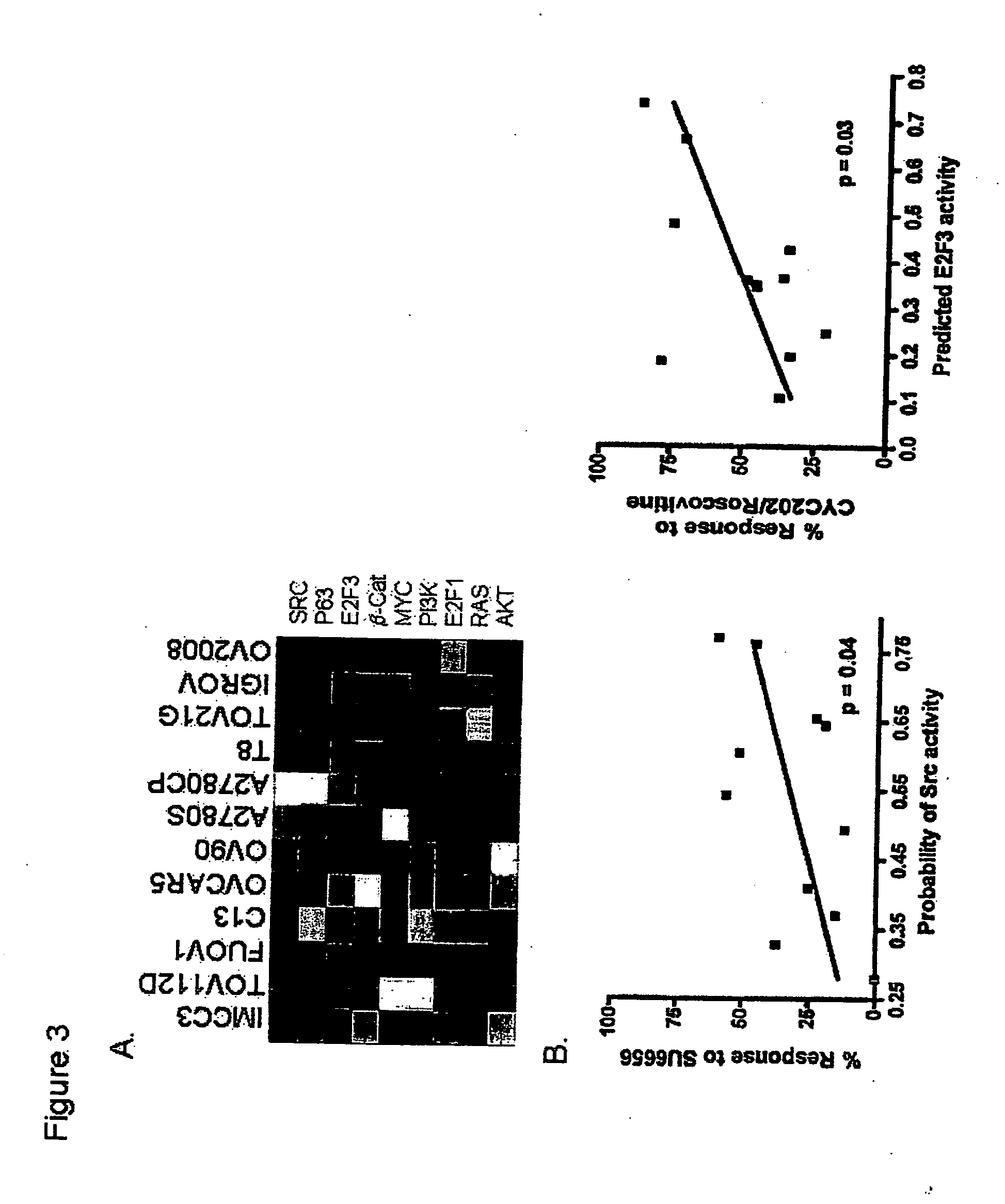
The invention provides for compositions and methods for predicting an individual's responsitivity to cancer treatments and methods of treating cancer. In certain embodiments, the invention provides compositions and methods for predicting an individual's responsitivity to chemotherapeutics, including platinum-based chemotherapeutics, to treat cancers such as ovarian cancer. Furthermore, the invention provides for compositions and methods for predicting an individual's responsivity to salvage therapeutic agents. By predicting if an individual will or will not respond to platinum-based chemotherapeutics, a physician can reduce side effects and toxicity by administering a particular additional salvage therapeutic agent. This type of personalized medical treatment for ovarian cancer allows for more efficient treatment of individuals suffering from ovarian cancer. The invention also provides reagents, such as DNA microarrays, software and computer systems useful for personalizing cancer treatments, and provides methods of conducting a diagnostic business for personalizing cancer treatments.
Owner:UNIV OF SOUTH FLORIDA +1
Compositions, formulations and kit with anti-sense oligonucleotide and anti-inflammatory steroid and/or obiquinone for treatment of respiratory and lung disesase
InactiveUS20070021360A1Decreased airwayOrganic active ingredientsBiocideDiseaseAntiendomysial antibodies
A pharmaceutical composition and formulations comprise preventative, prophylactic or therapeutic amounts of an oligo(s) anti-sense to a specific gene(s) or its corresponding mRNA(s), and a glucocorticoid and / or non-glucocorticoid steroid or a ubiquinone or their salts. The agents, composition and formulations are used for treatment of ailments associated with impaired respiration, bronchoconstriction, lung allergy(ies) or inflammation, and abnormal levels of adenosine, adenosine receptors, sensitivity to adenosine, lung surfactant and ubiquinone, such as pulmonary fibrosis, vasoconstriction, inflammation, allergies, allergic rhinitis, asthma, impeded respiration, lung pain, cystic fibrosis, bronchoconstriction, COPD, RDS, ARDS, cancer, and others. The present treatment is effectively administered by itself for conditions without known therapies, as a substitute for therapies exhibiting undesirable side effects, or in combination with other treatments, e.g. before, during and after other respiratory system therapies, radiation, chemotherapy, antibody therapy and surgery, among others. Each of the agents of this invention may be administered directly into the respiratory system so that they gain direct access to the lungs, or by other effective routes of administration. A kit comprises a delivery device, the agents and instructions for its use.
Owner:EPIGENESIS PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com