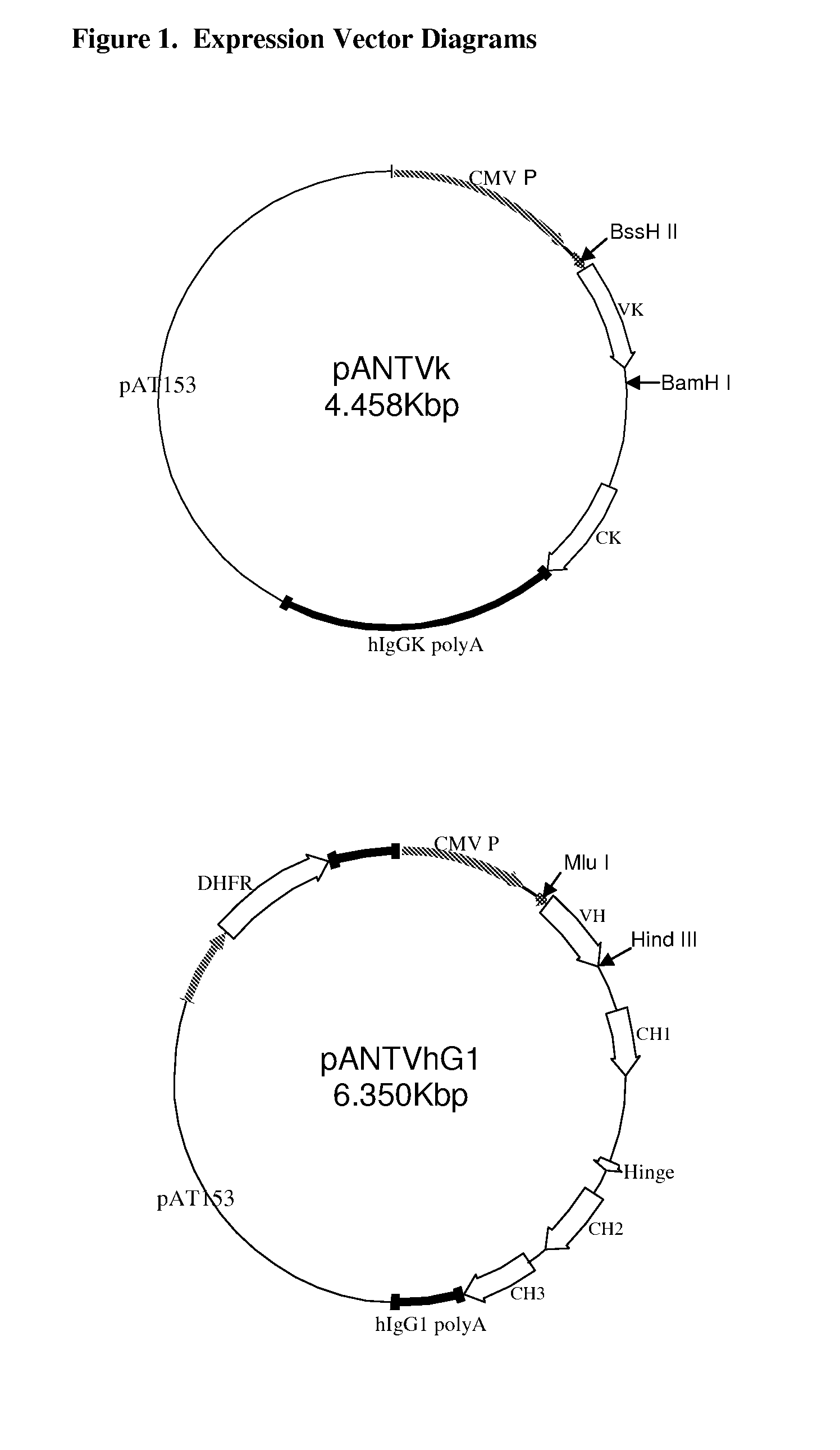
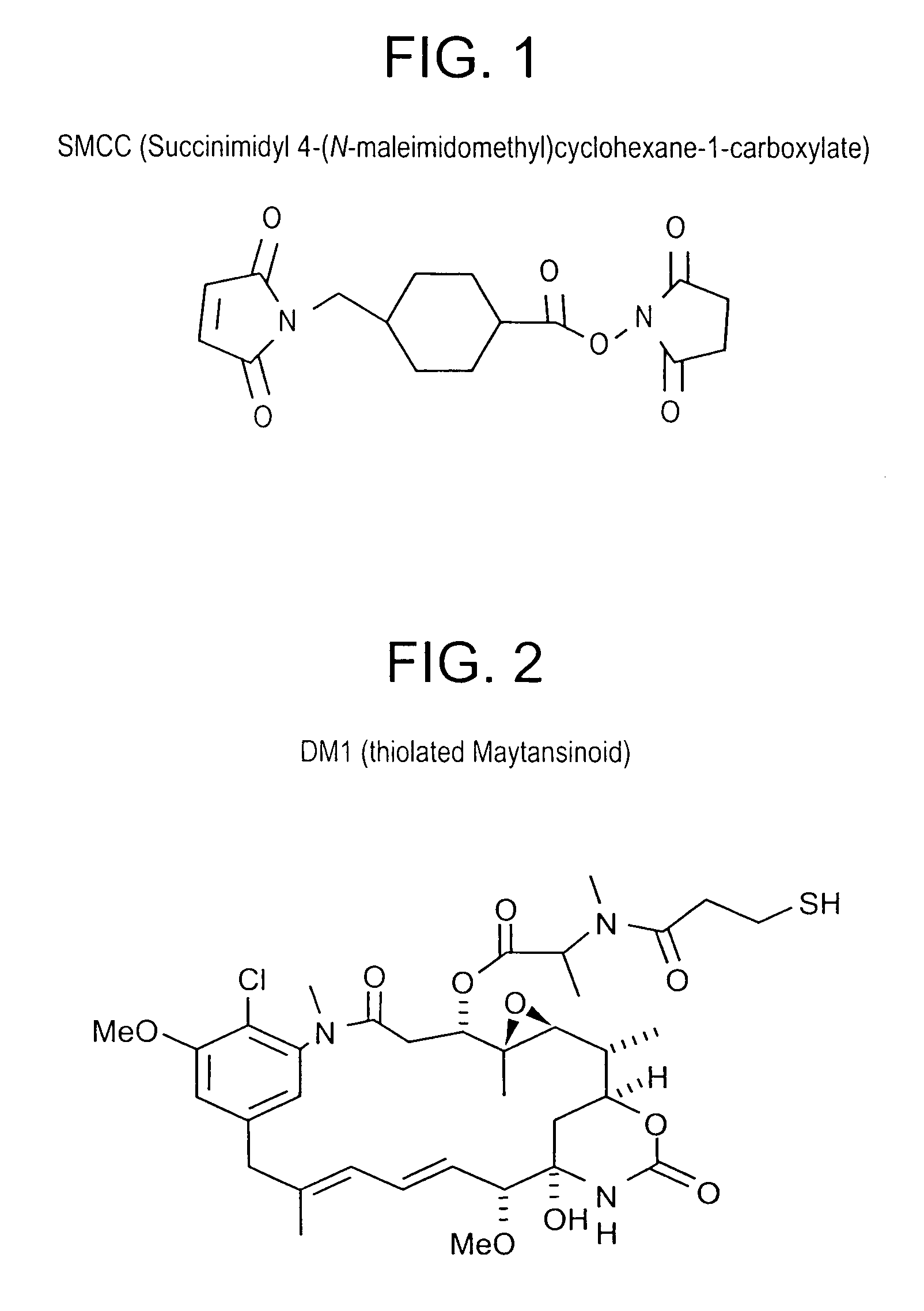
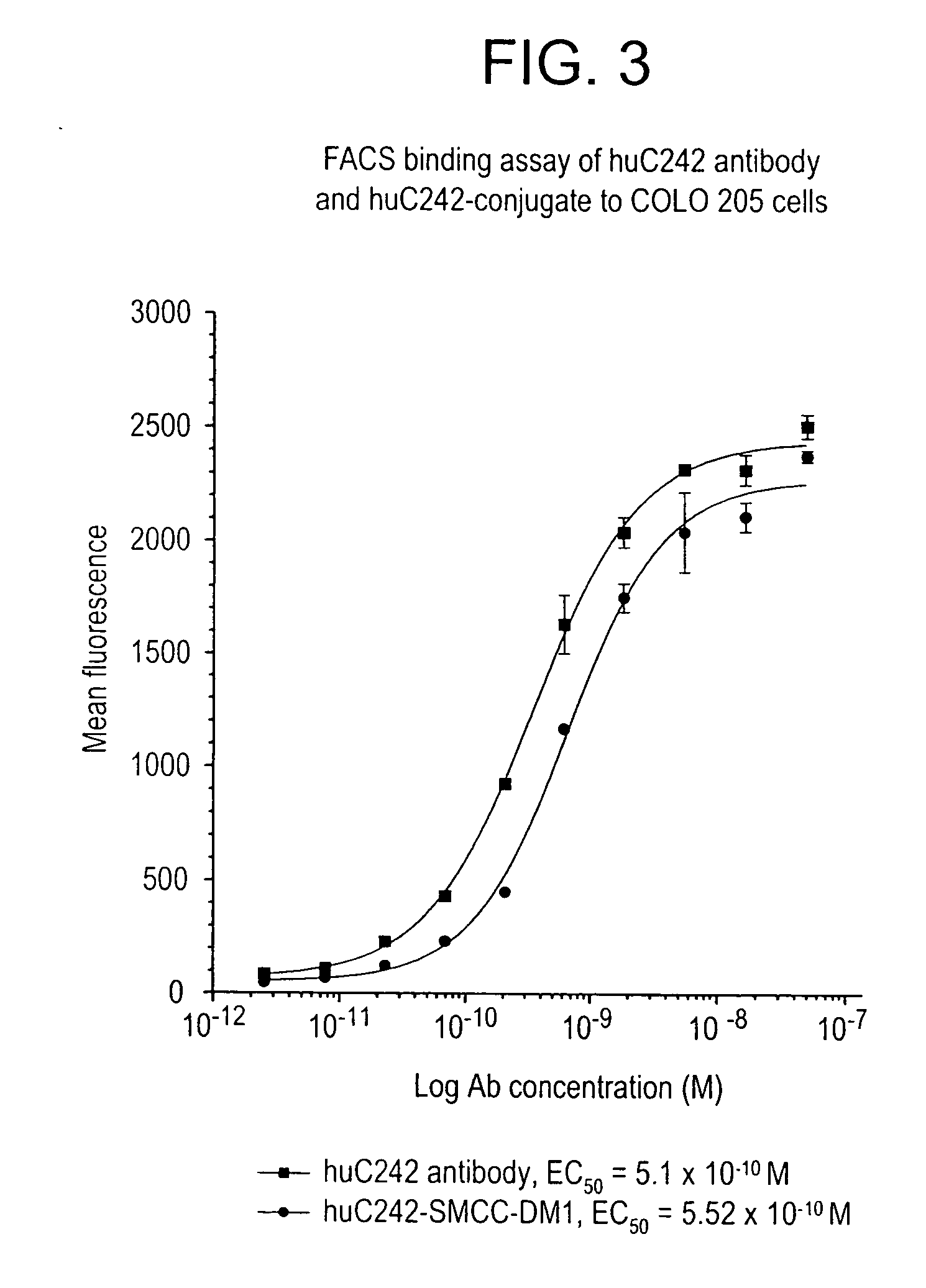
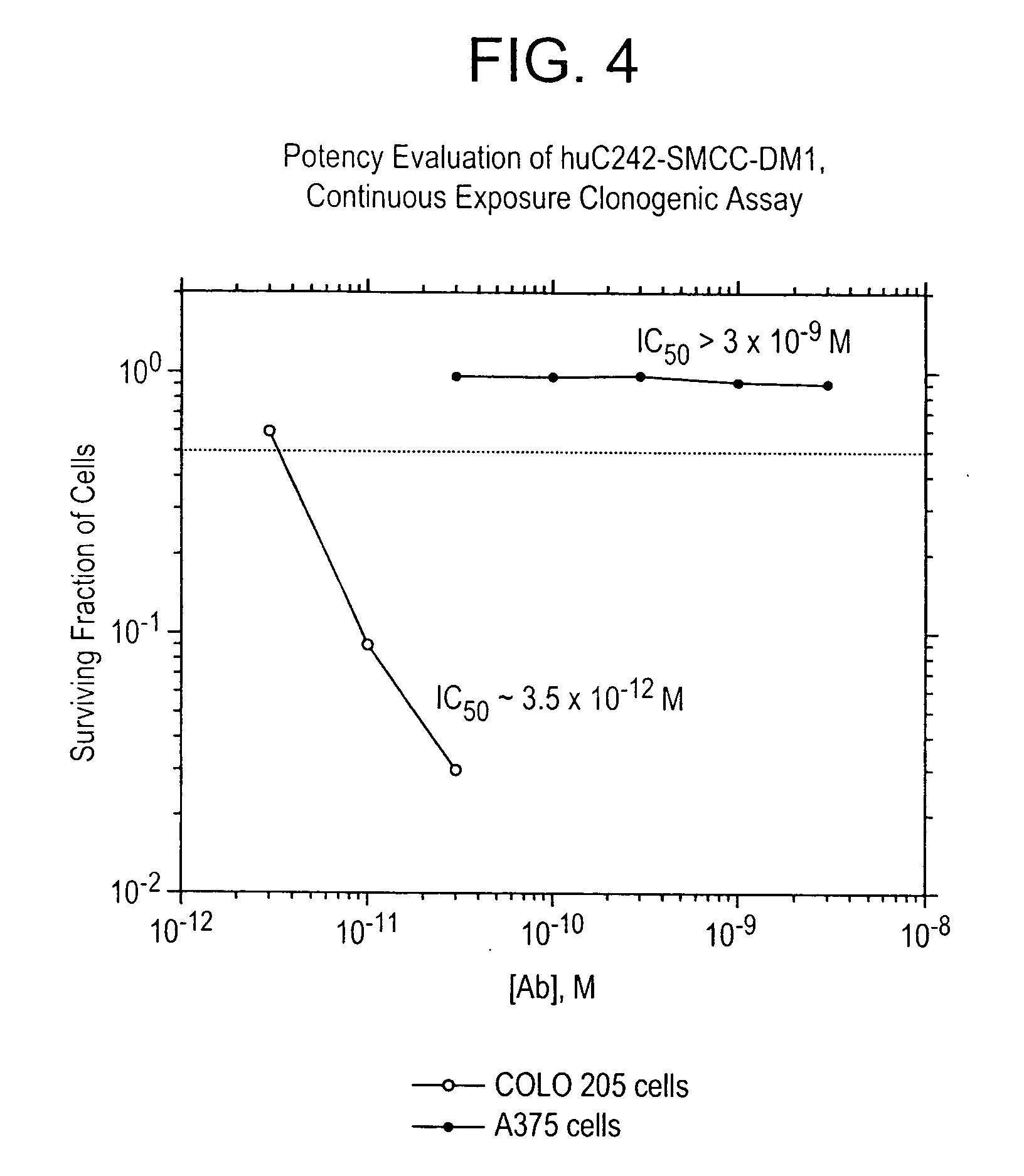
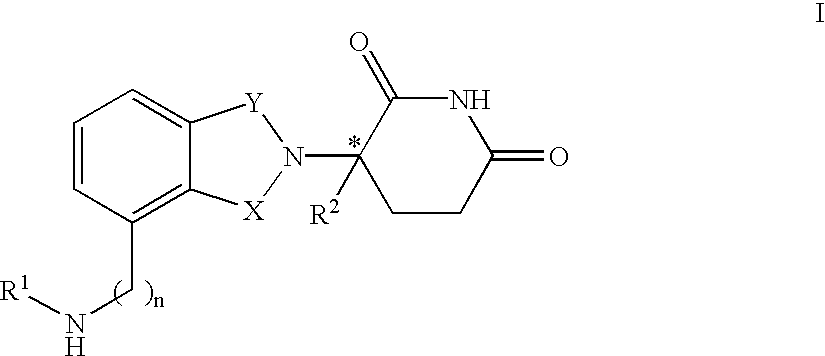
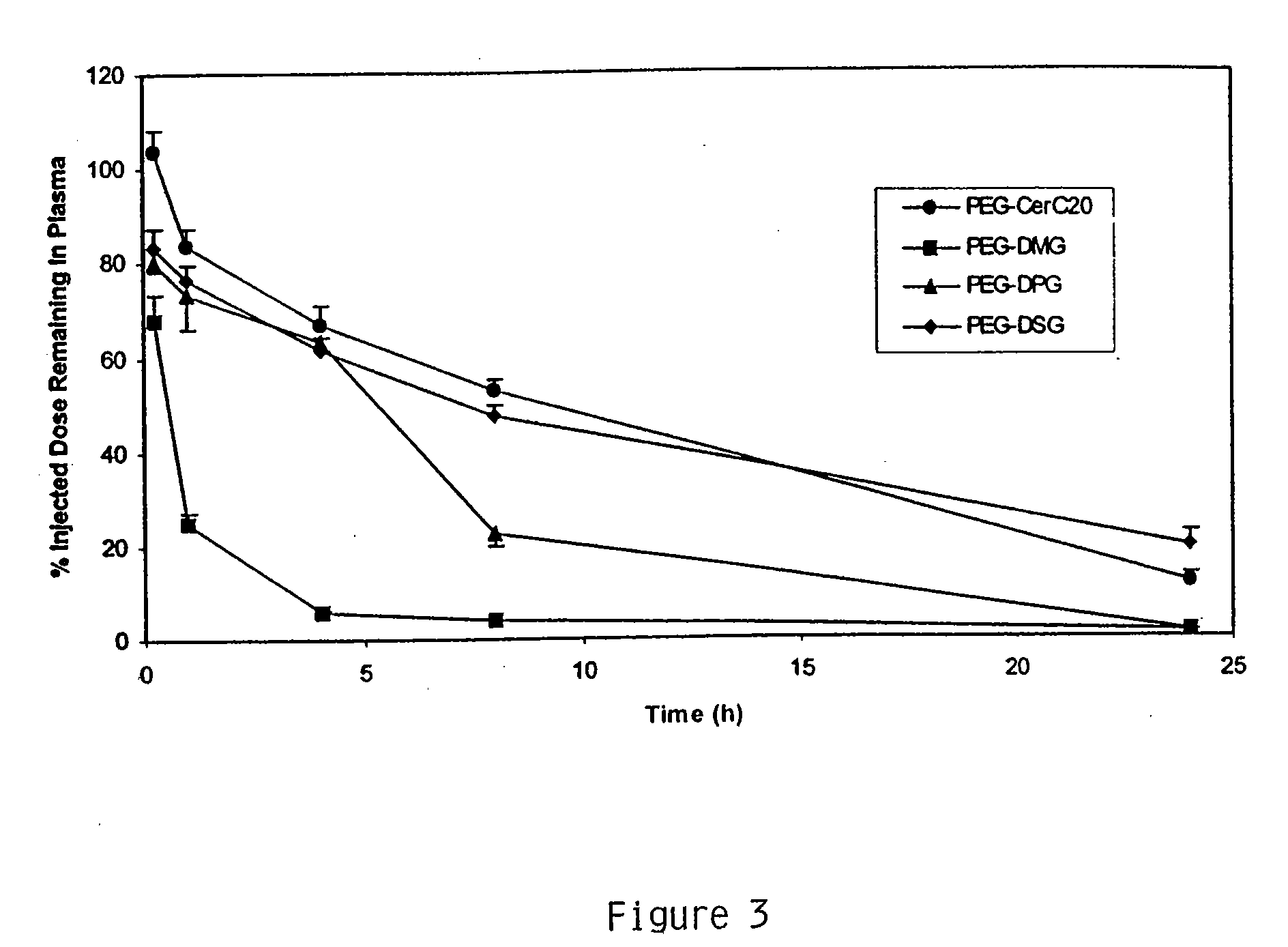
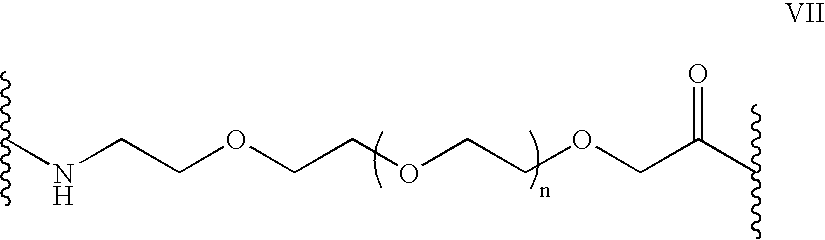
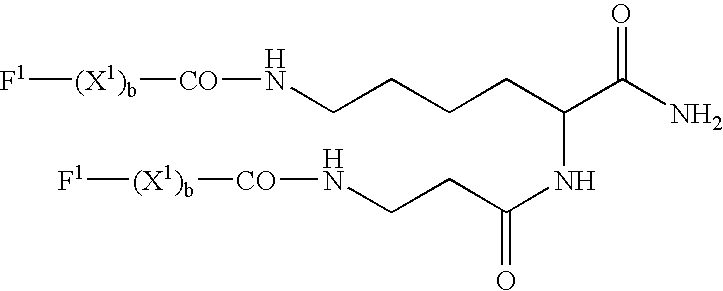
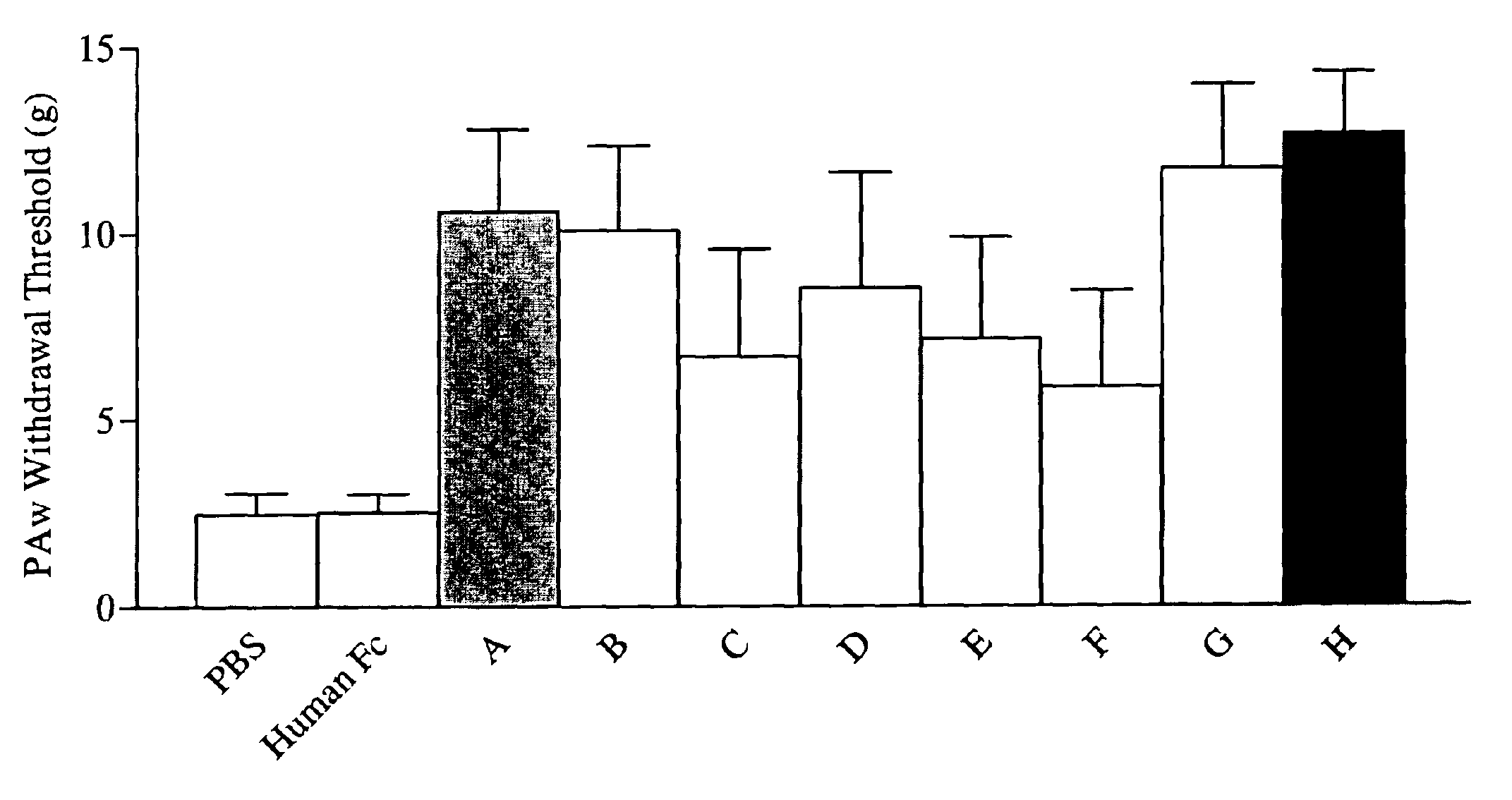
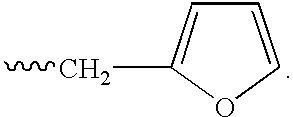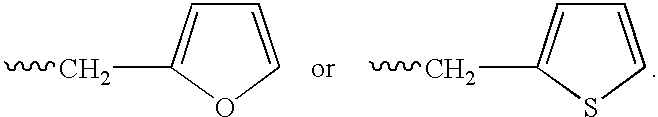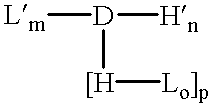Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
105273results about "Digestive system" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
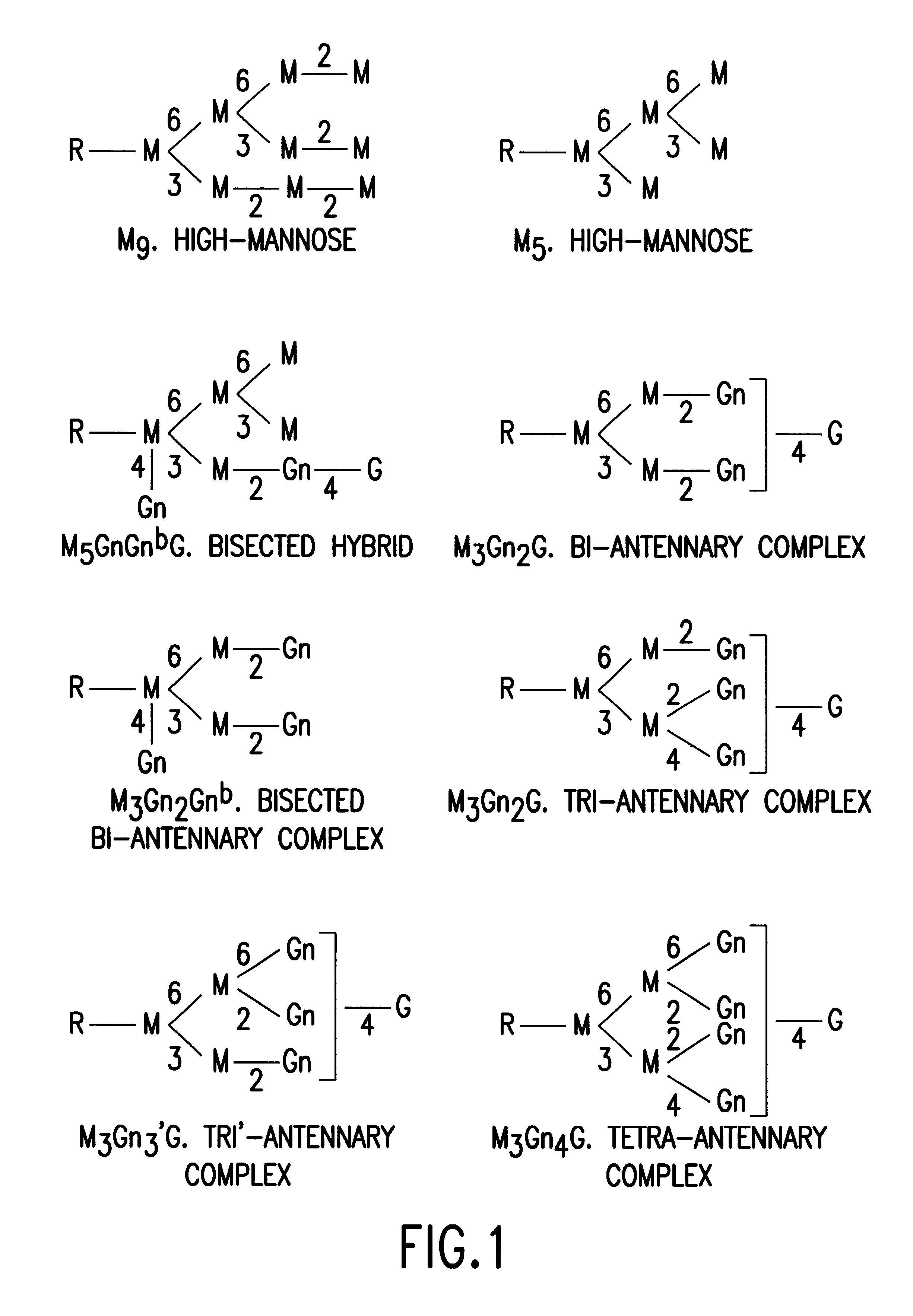
Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity
InactiveUS6602684B1Increase healing valueEnhanced Fc-mediated cellular cytotoxicityNanotechFungiAntibody fragmentsADAMTS Proteins
The present invention relates to the field glycosylation engineering of proteins. More particular, the present invention is directed to the glycosylation engineering of proteins to provide proteins with improved therapeutic properties, e.g., antibodies, antibody fragments, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin, with enhanced Fc-mediated cellular cytotoxicity.
Owner:ROCHE GLYCART AG
Antigen binding molecules with increased Fc receptor binding affinity and effector function
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies specific for human CD20. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
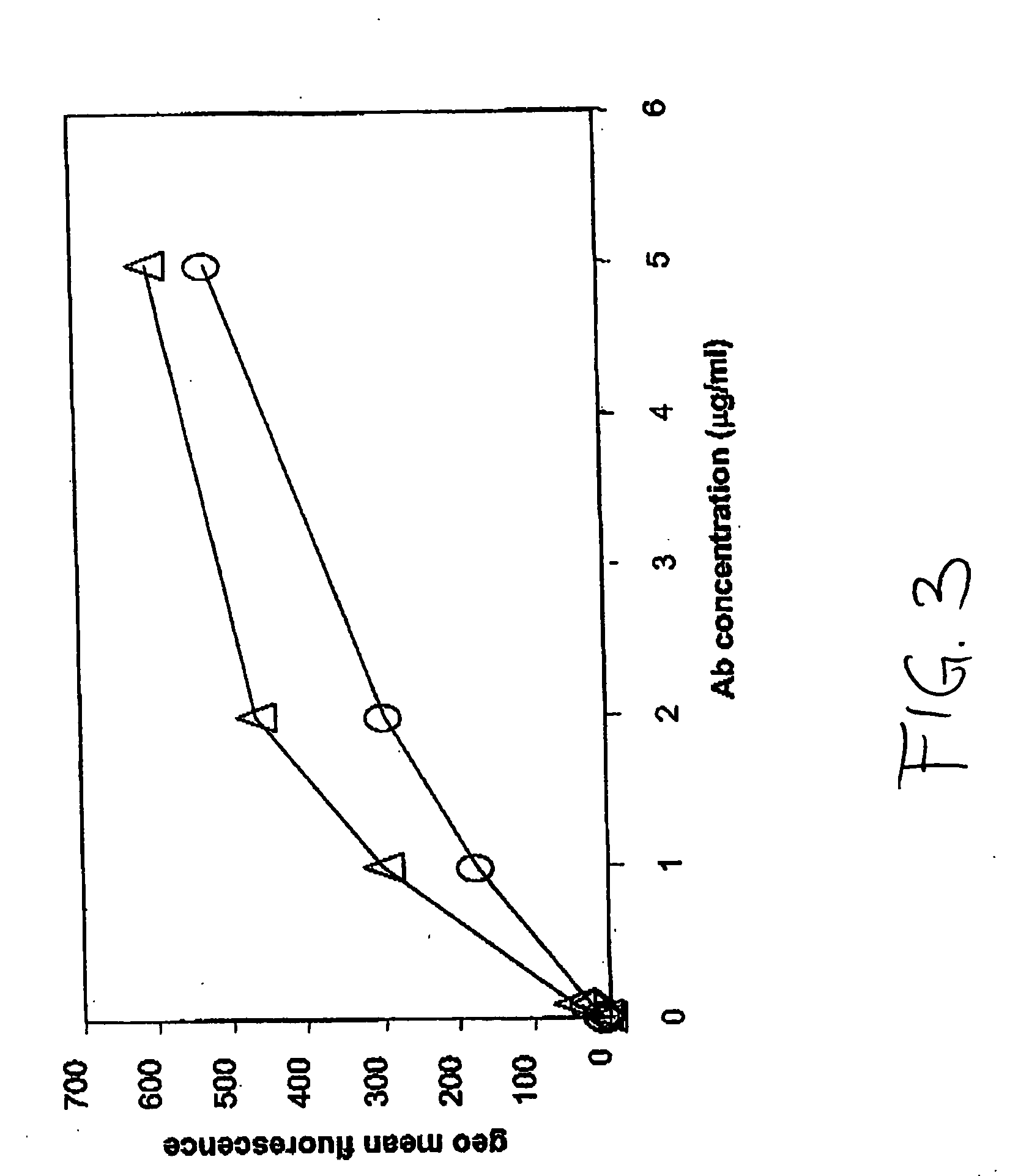
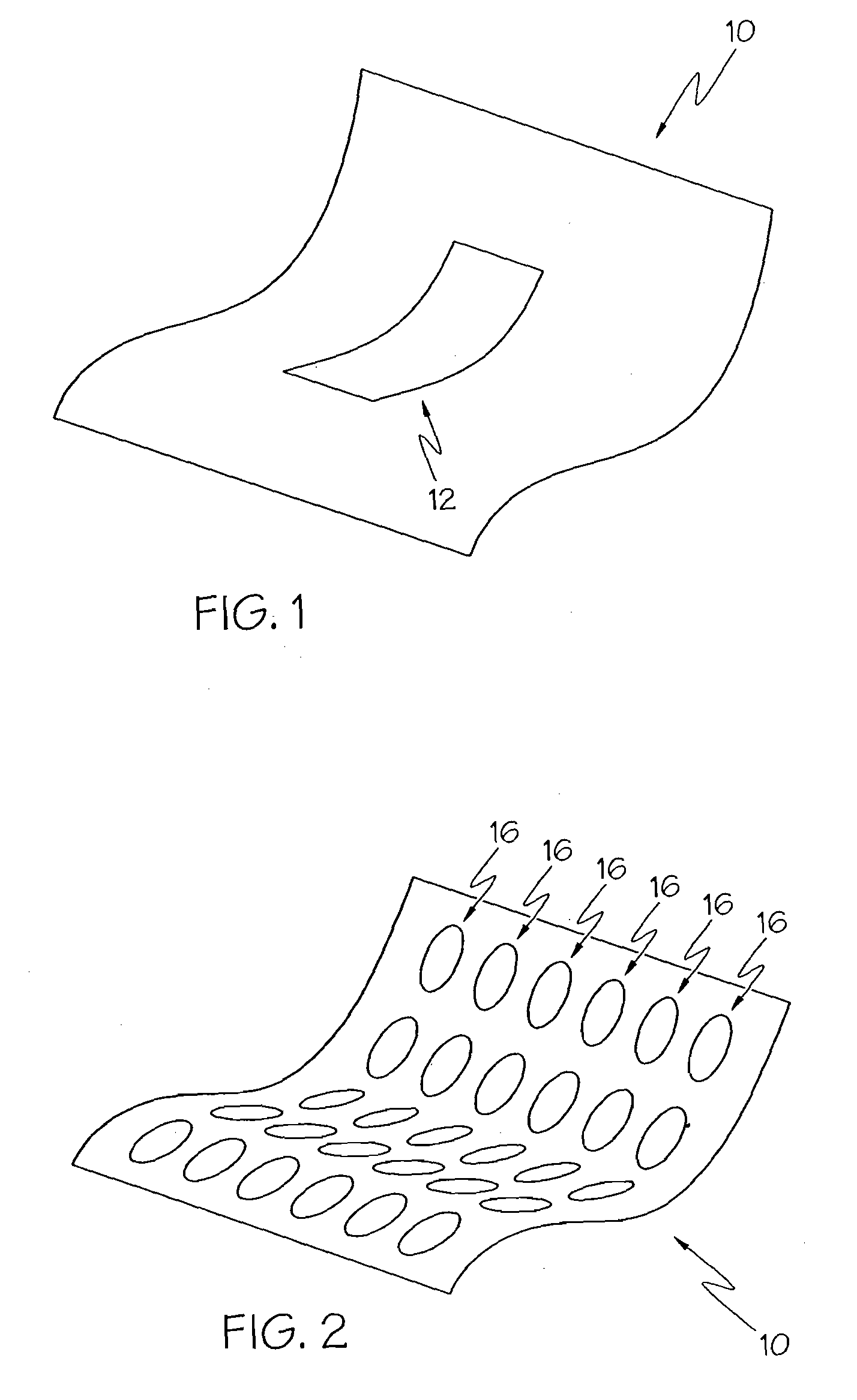
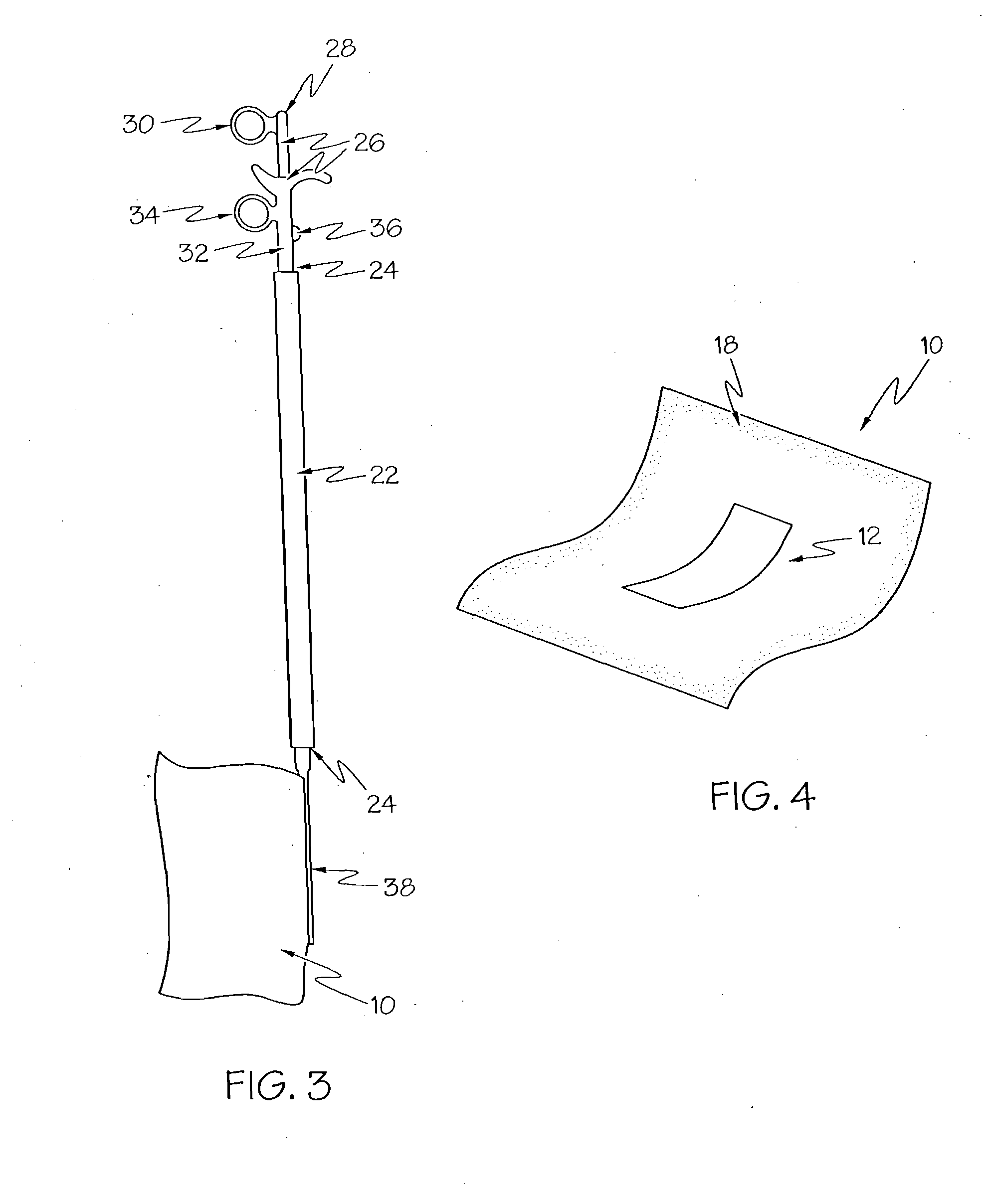
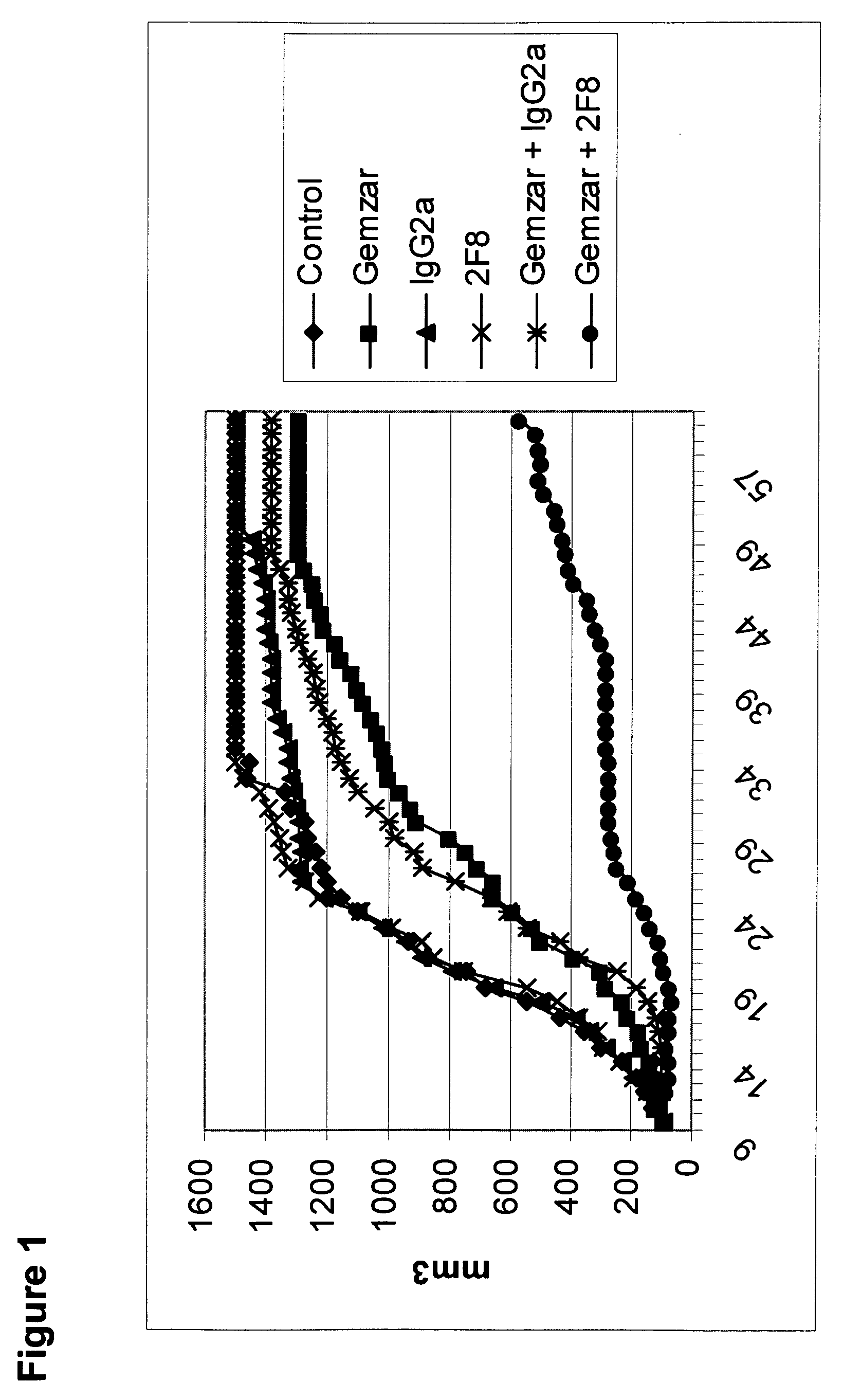
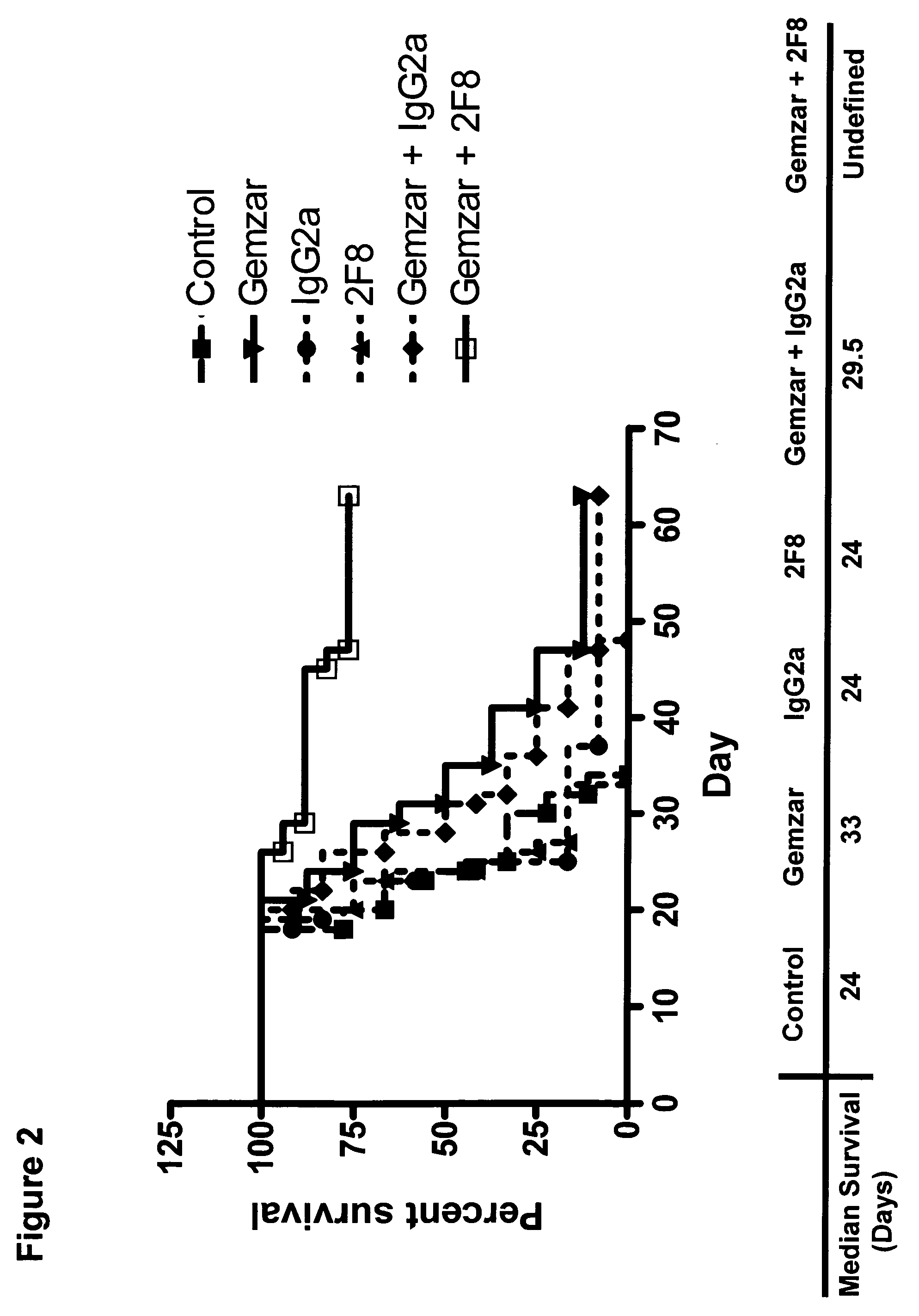
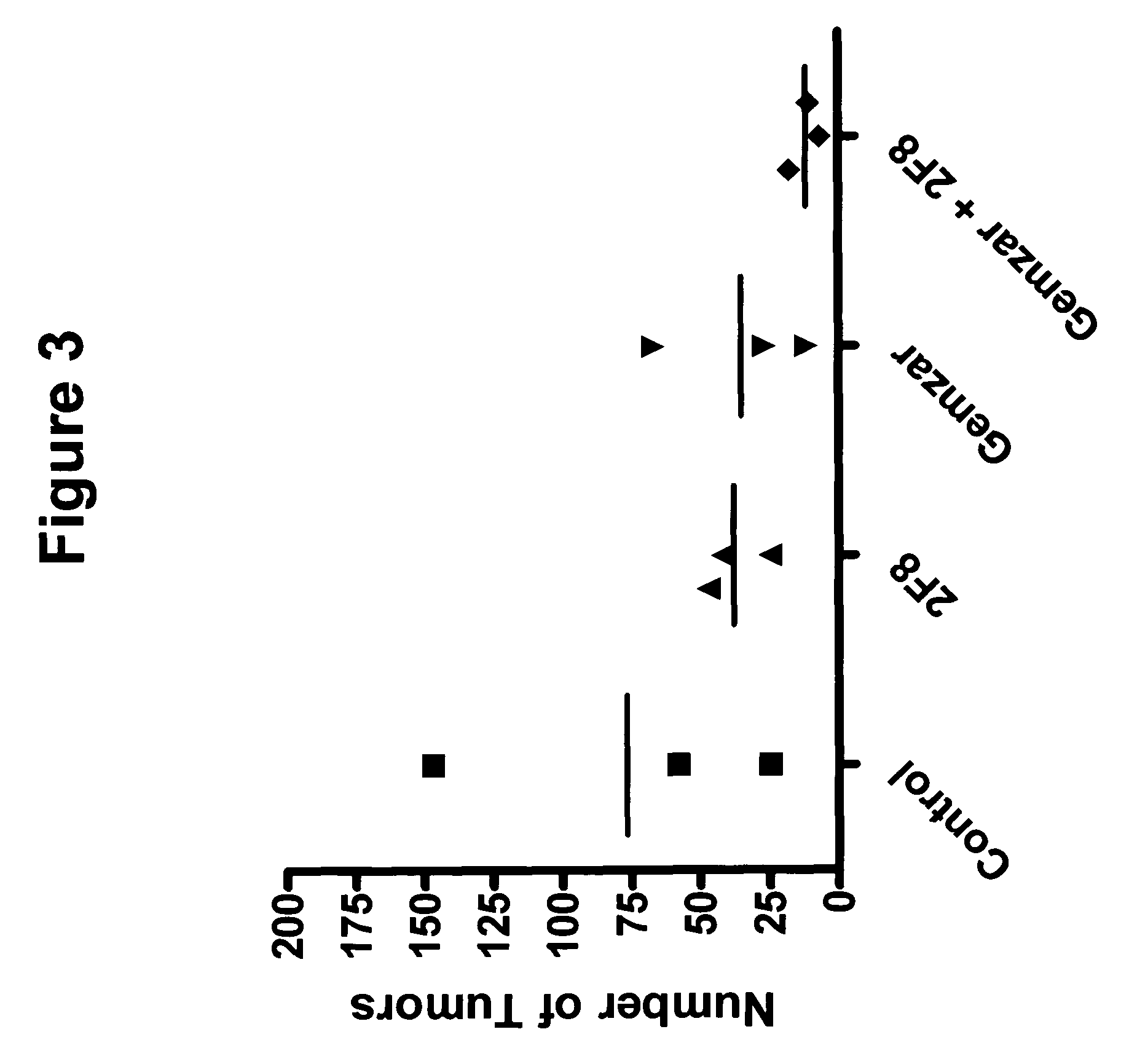
Methods for reduction of post operative ileus.
InactiveUS20080085296A1Reducing post-operative ileus and/or gastric stasisReducing post-operative ileusBiocideDigestive systemButtressSurgical department
An apparatus and method for reducing post-operative ileus and / or gastric stasis is described. The method can include applying to the intestine a therapeutically effective amount of a composition comprising a drug that is effective in reducing post-operative ileus and / or gastric stasis, such as by introducing the composition through a surgical access device, such as a trocar or endoscope. The apparatus can include a sheet of material, a surgical fastener, or a buttress comprising the composition.
Owner:ETHICON ENDO SURGERY INC
Human antibodies that bind human IL-12 and methods for producing
InactiveUS6914128B1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
Drug-eluting medical devices
Owner:ZILBERMAN MEITAL
Drug-eluting medical devices
Composite structures composed of a device as a core structure, being a medical device or article, and a porous polymeric coat and designed capable of encapsulating bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures.
Owner:RAMOT AT TEL AVIV UNIV LTD
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Combination therapies employing GITR binding molecules
ActiveUS8591886B2Small sizeImprove securityOrganic active ingredientsNervous disorderInternal medicine
Owner:GITR
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
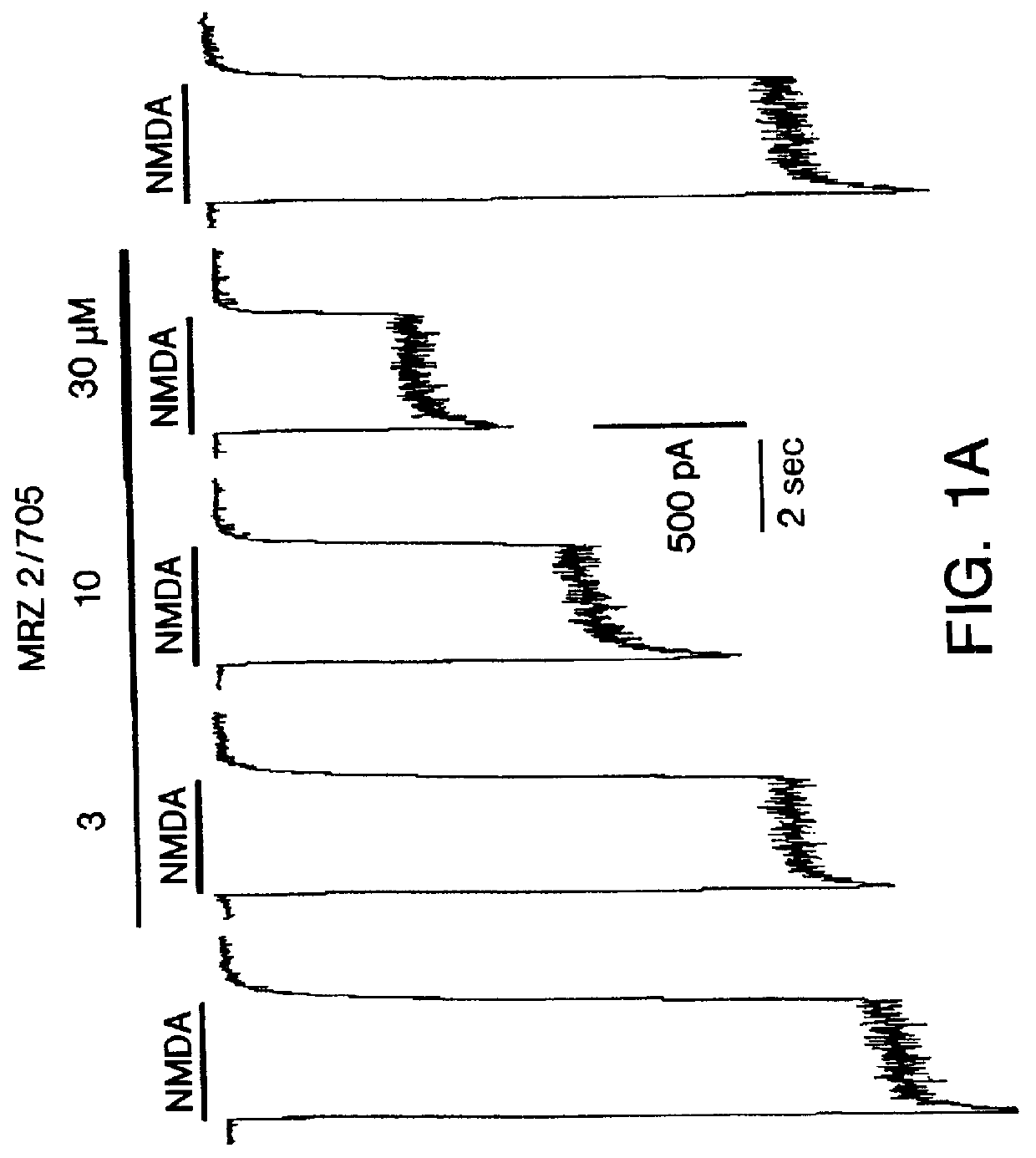
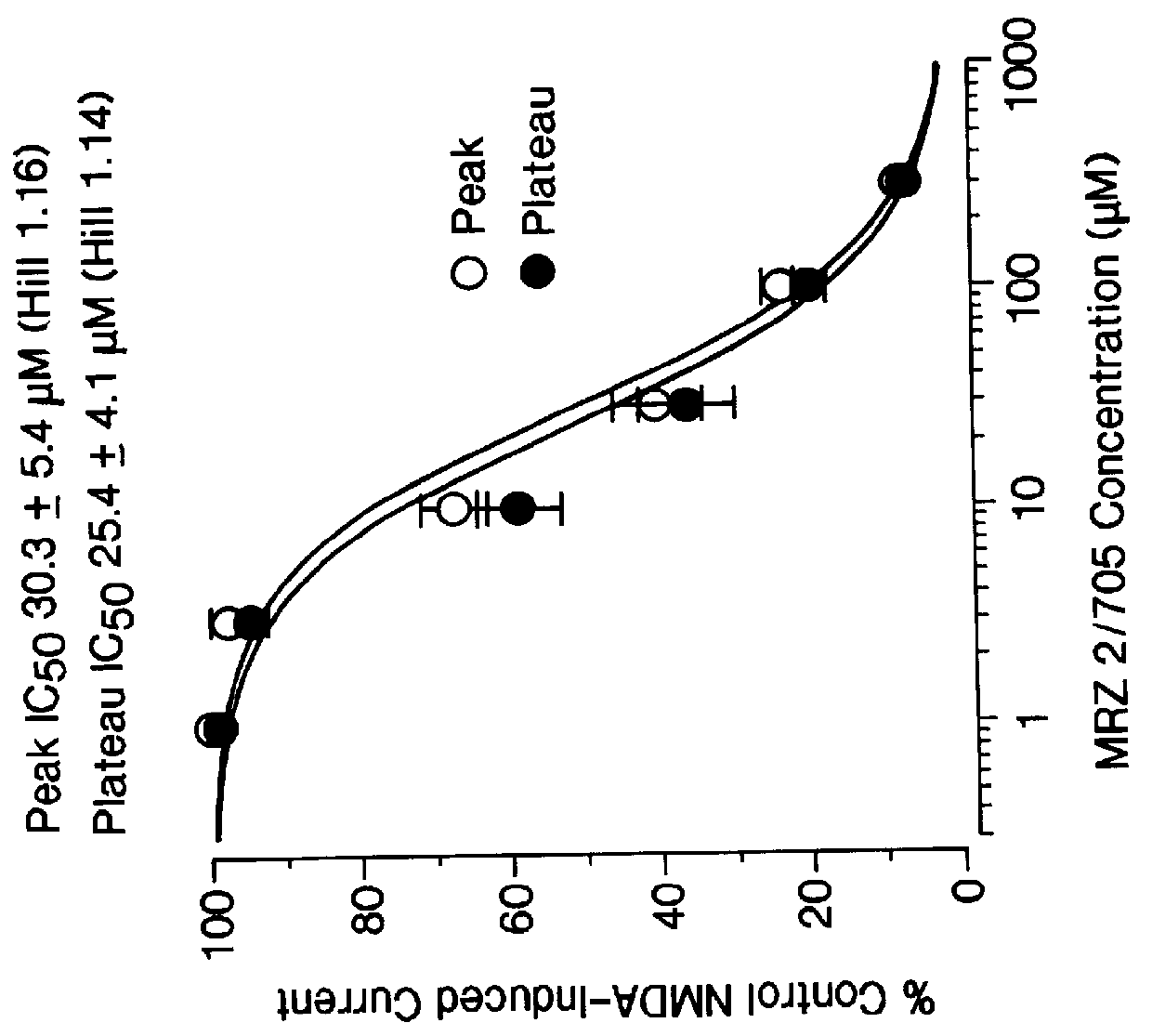
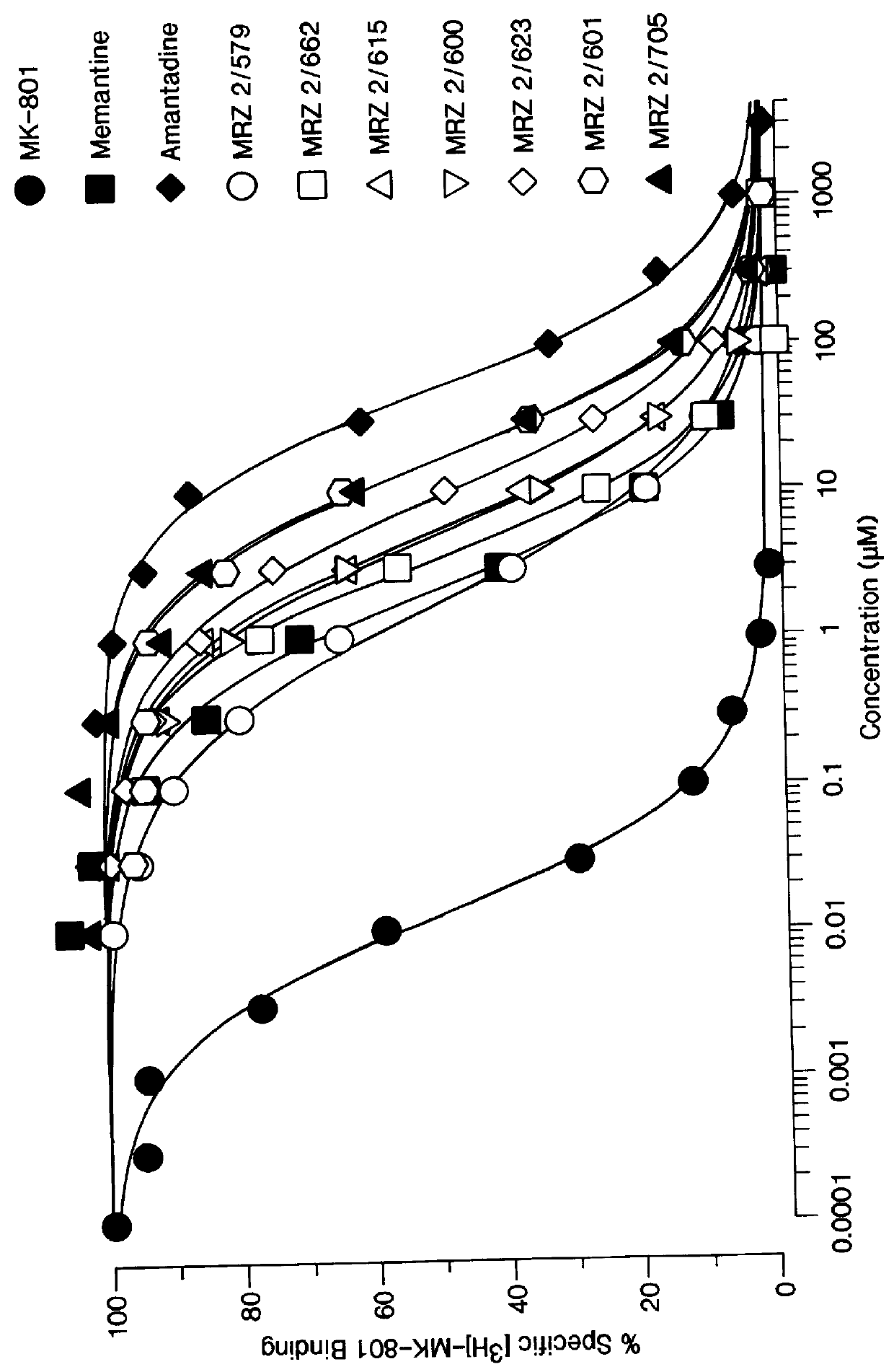
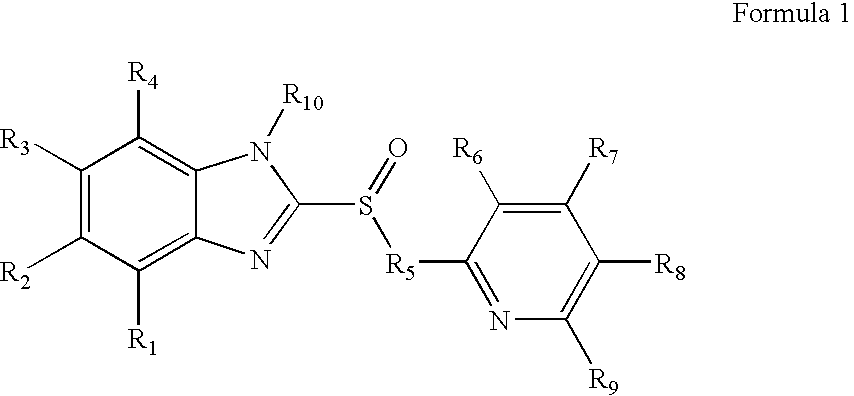
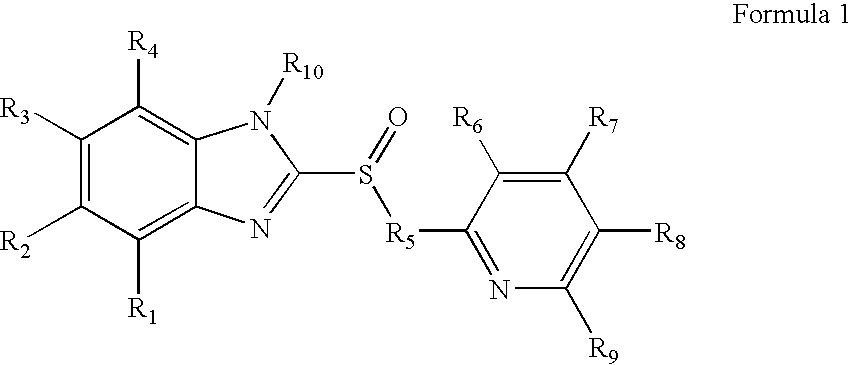
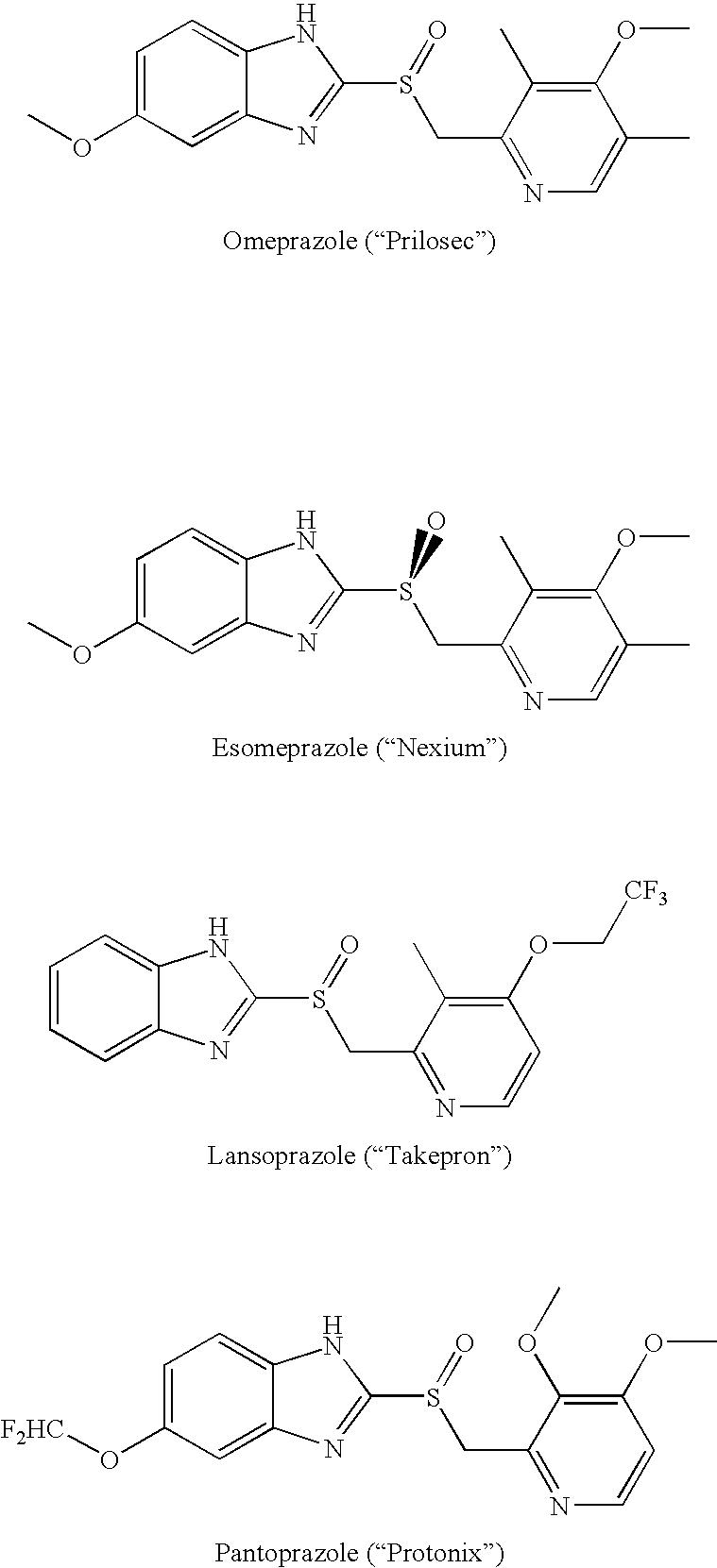
1-Amino-alkylcyclohexane NMDA receptor antagonists
Certain 1-aminoalkylcyclohexanes are systemically-active uncompetitive NMDA receptor antagonists having rapid blocking / unblocking kinetics and strong voltage-dependency and are therefore useful in the alleviation of conditions resulting from disturbances of glutamatergic transmission giving them a wide range of utility in the treatment of CNS disorders involving the same, as well as in non-NMDA indications, due to their immunomodulatory, antimalarial, anti-Borna virus, and anti-Hepatitis C activities and utilities. Pharmaceutical compositions thereof and a method-of-treating conditions which are alleviated by the employment of an NMDA receptor antagonist, as well as the aforementioned non-NMDA indications, and a method for the preparation of the active 1-aminoalkylcyclohexane compounds involved.
Owner:MERZ PHARMA GMBH & CO KGAA
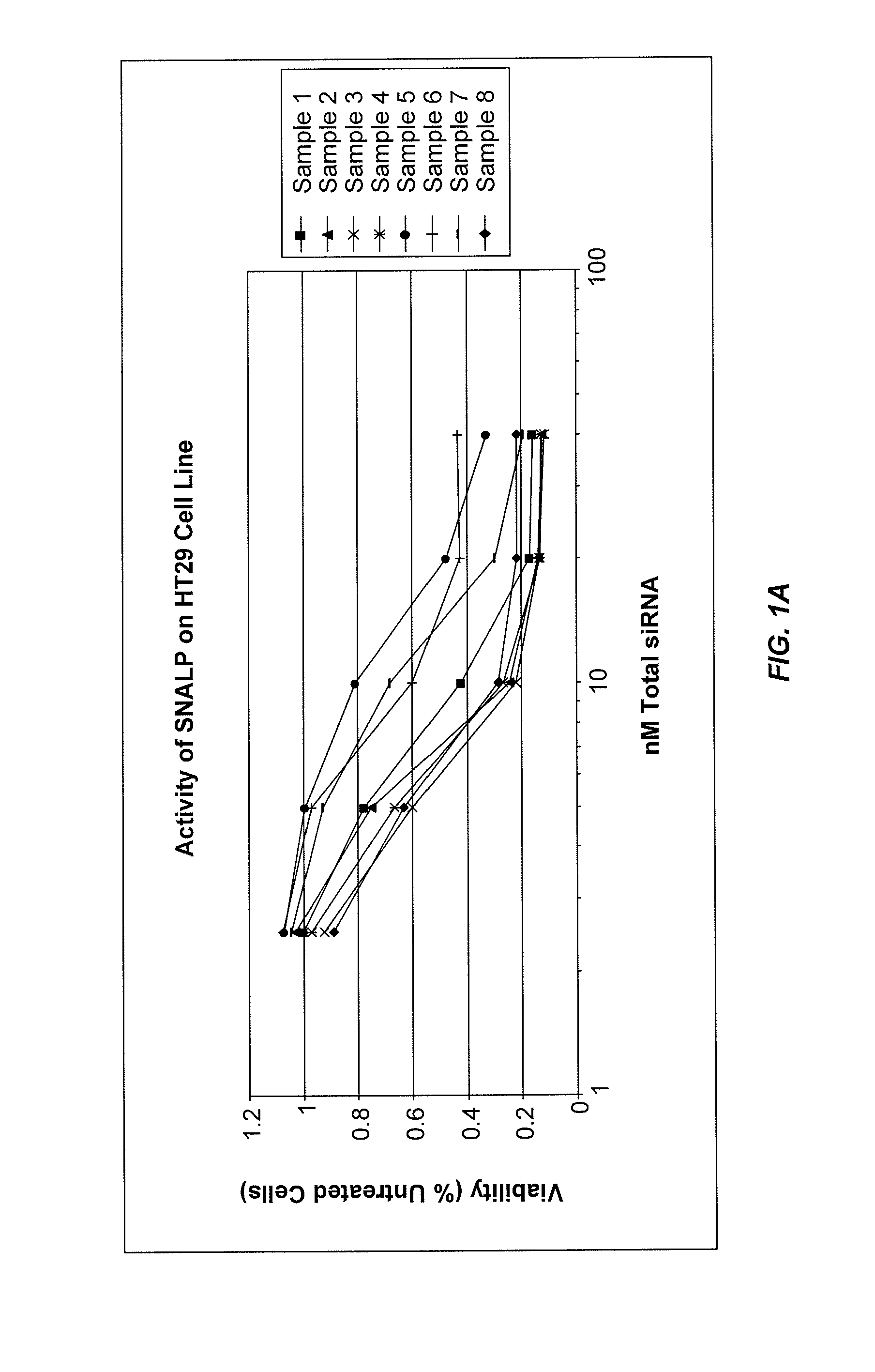
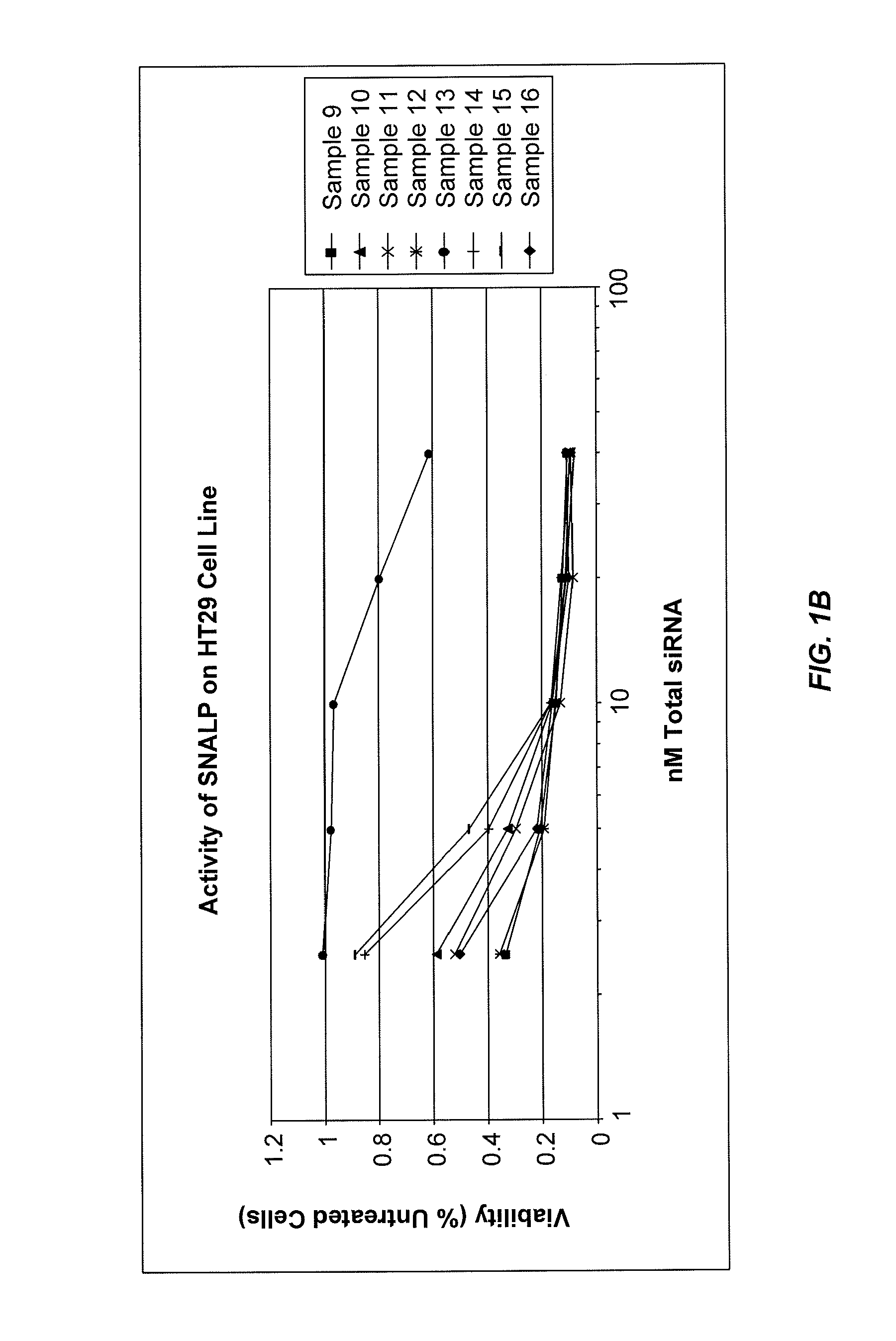
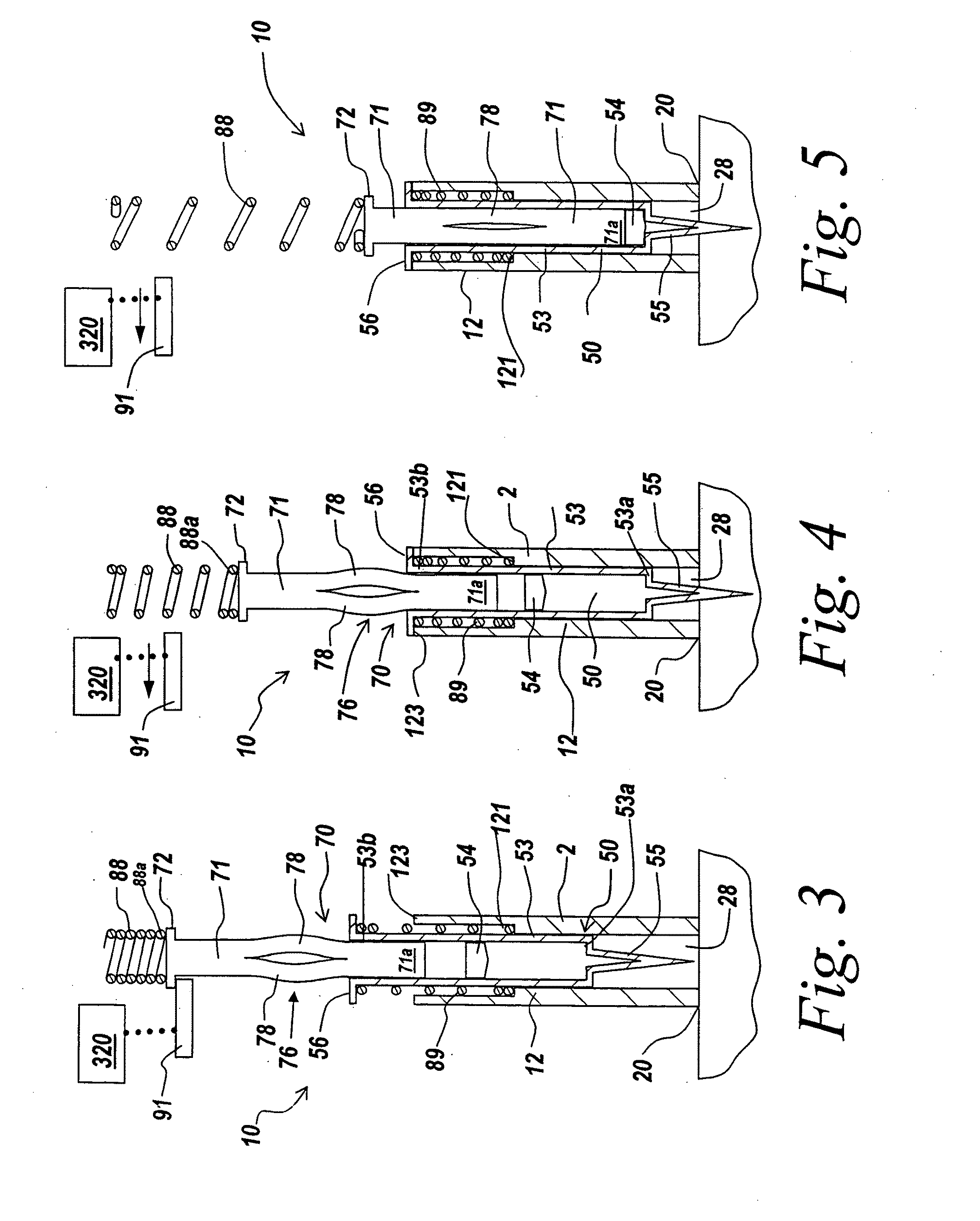
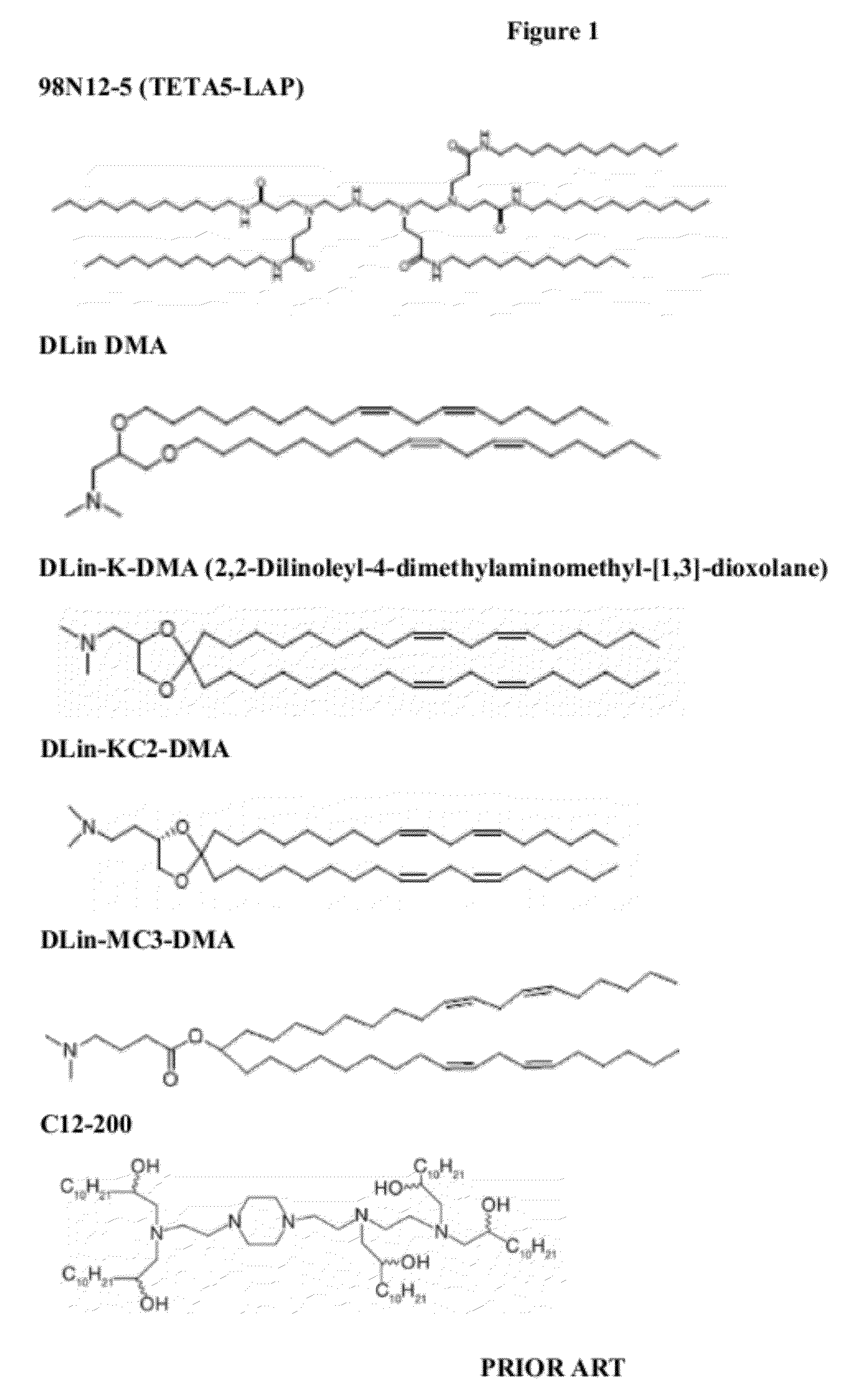
Lipid formulations for nucleic acid delivery
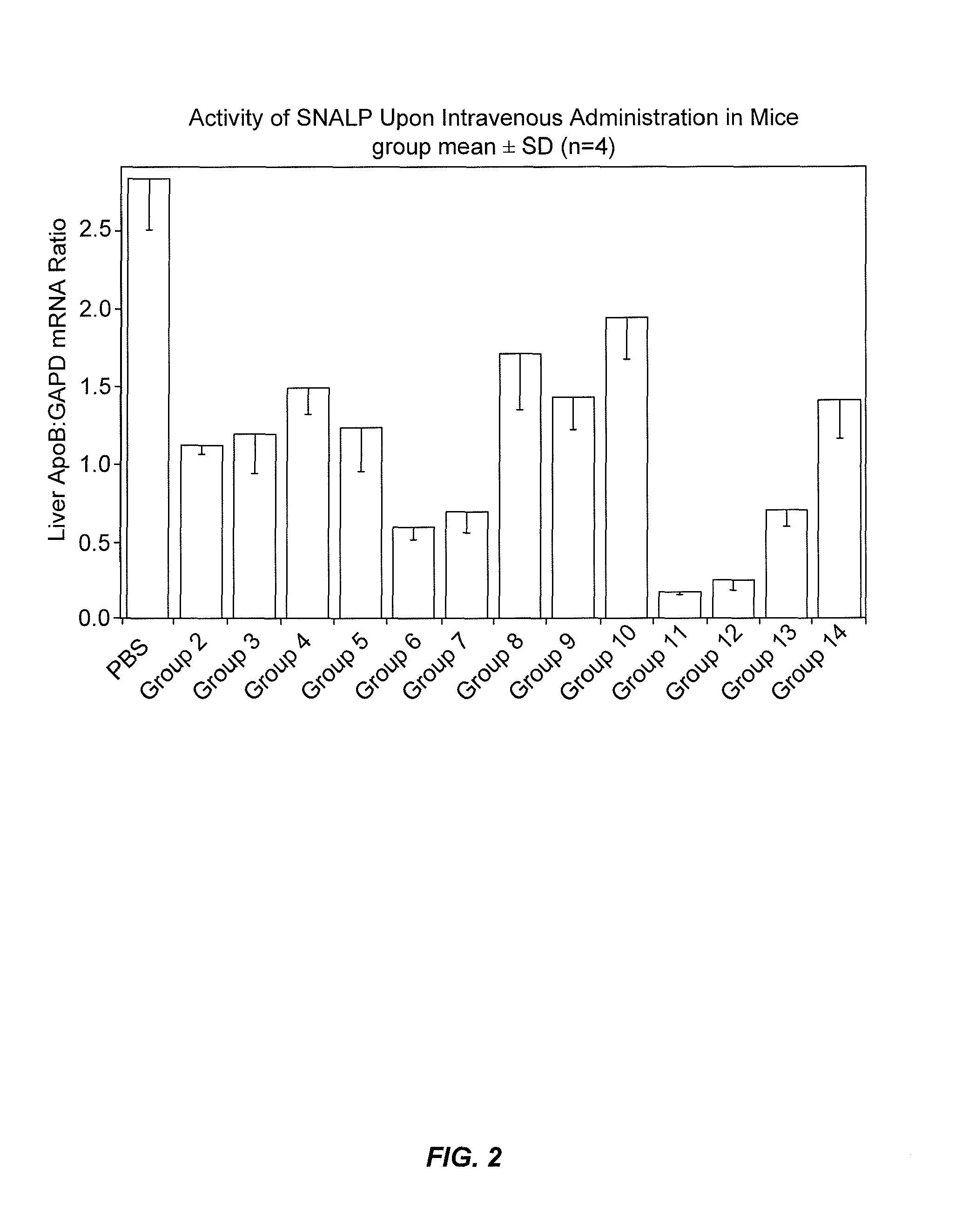
The present invention provides novel, stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (such as one or more interfering RNA), methods of making the SNALP, and methods of delivering and / or administering the SNALP.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
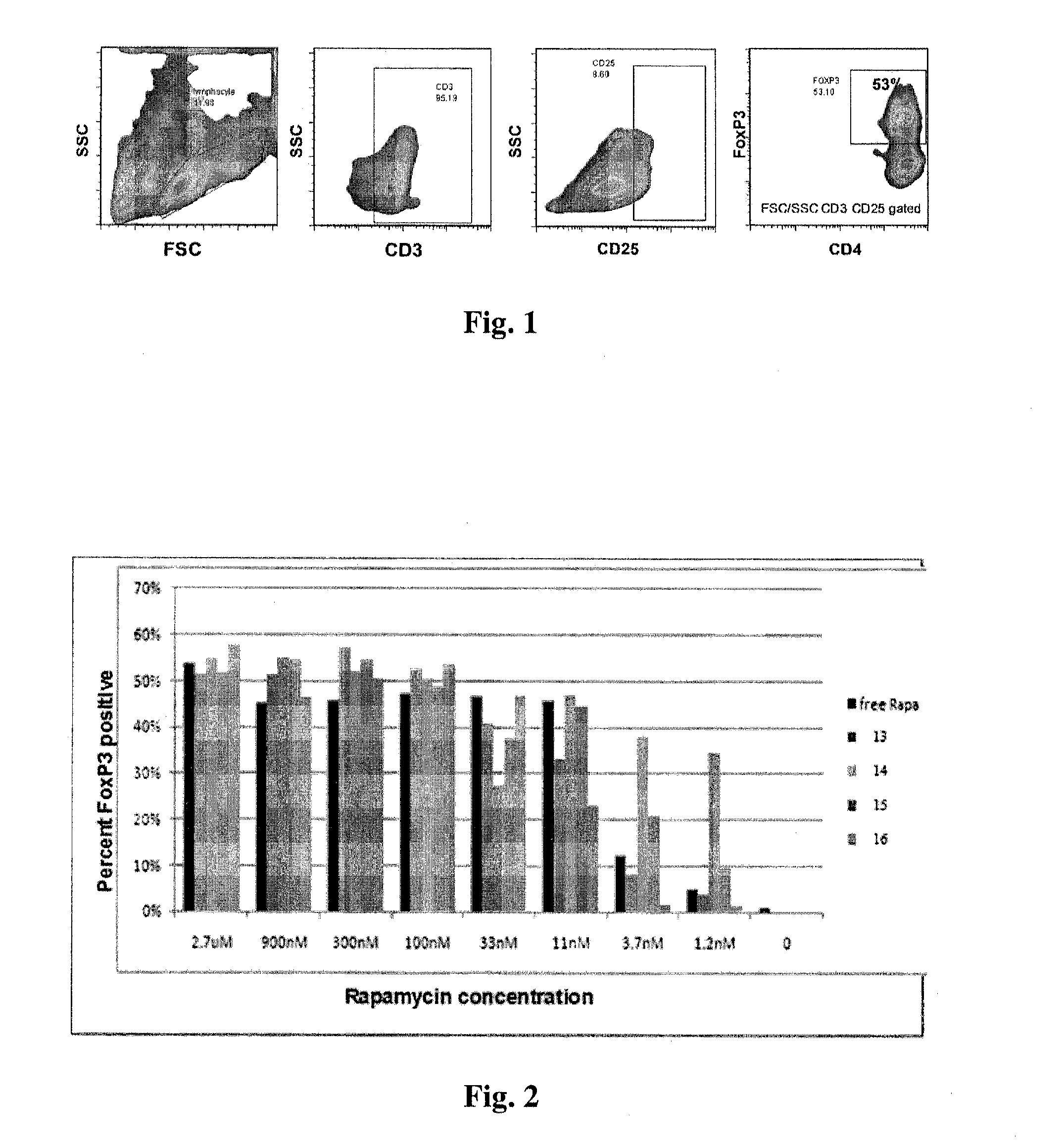
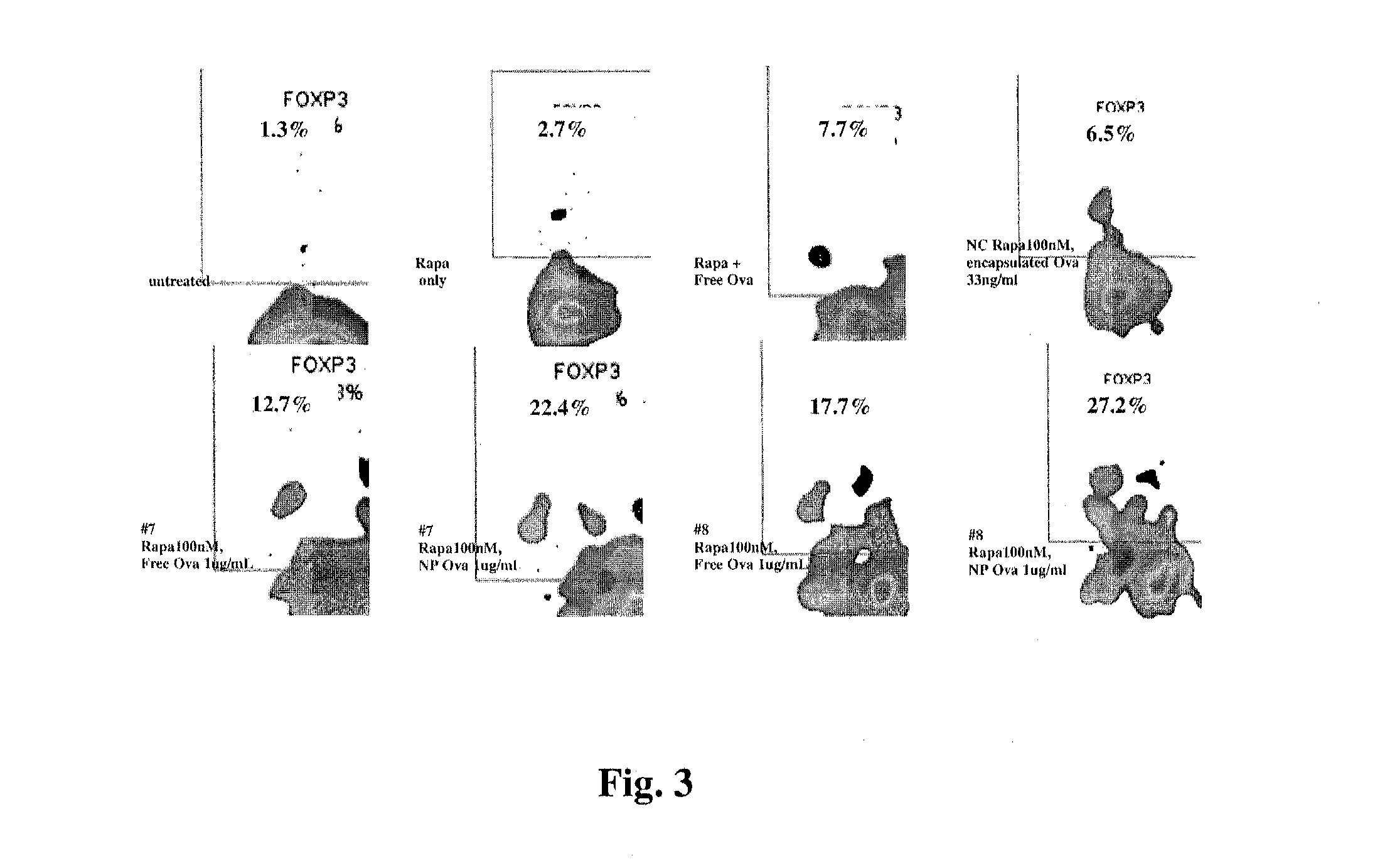
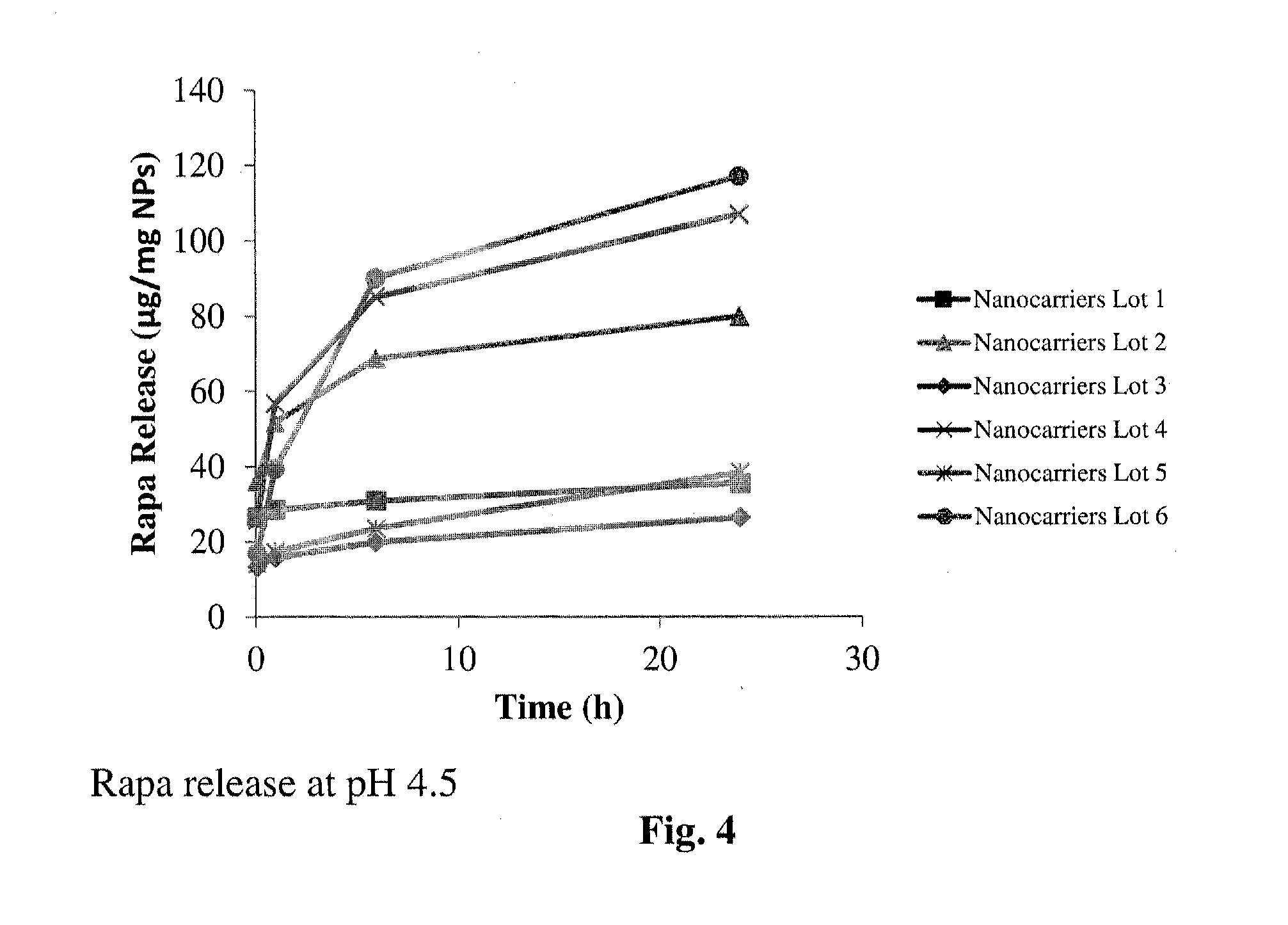
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS7355008B2Function increaseGood curative effectAntibacterial agentsSenses disorderTherapeutic antibodyEffector cell
Owner:MARCOGENICS INC +1
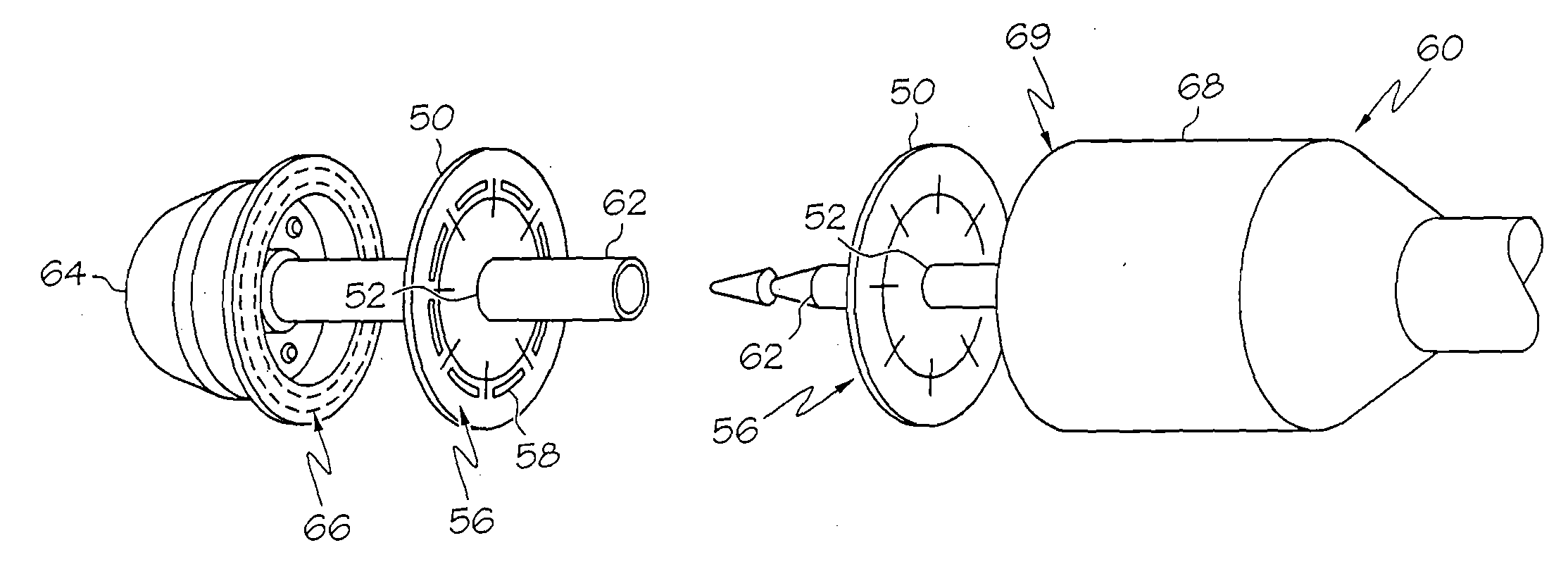
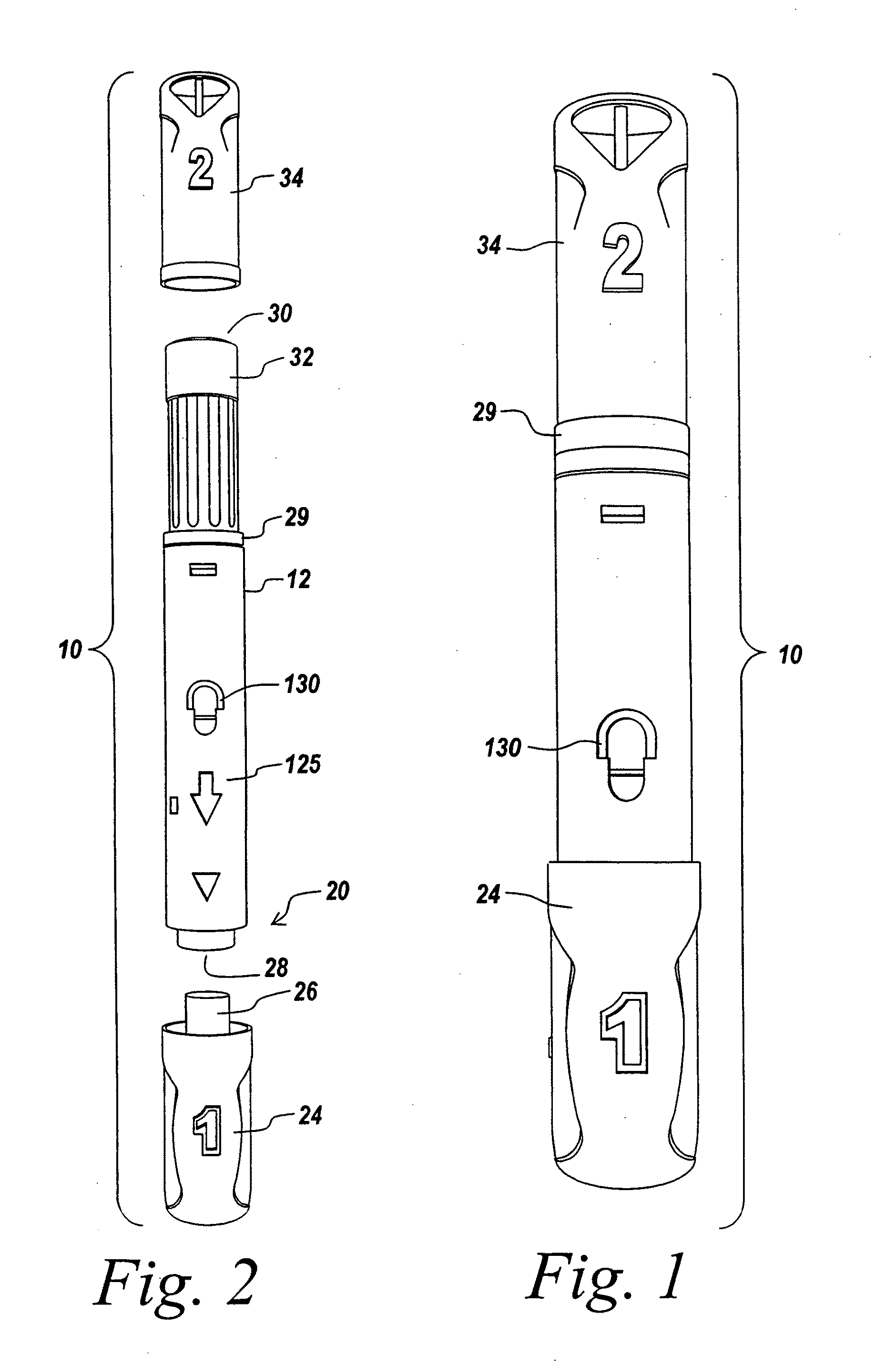
Automatic injection device
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
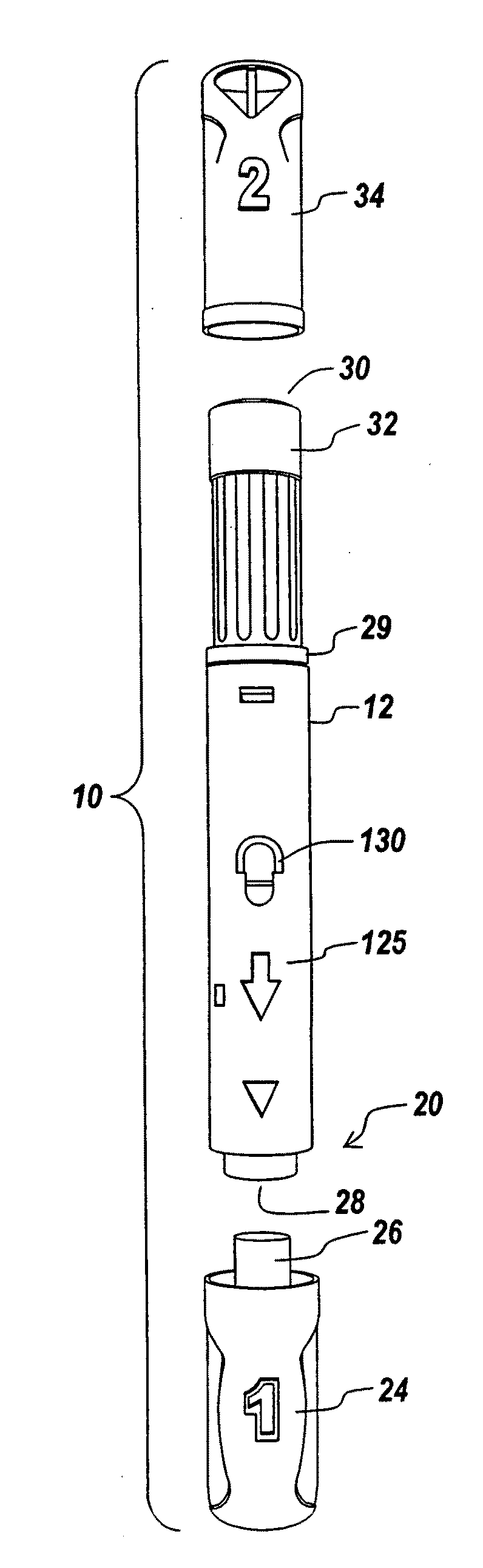
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
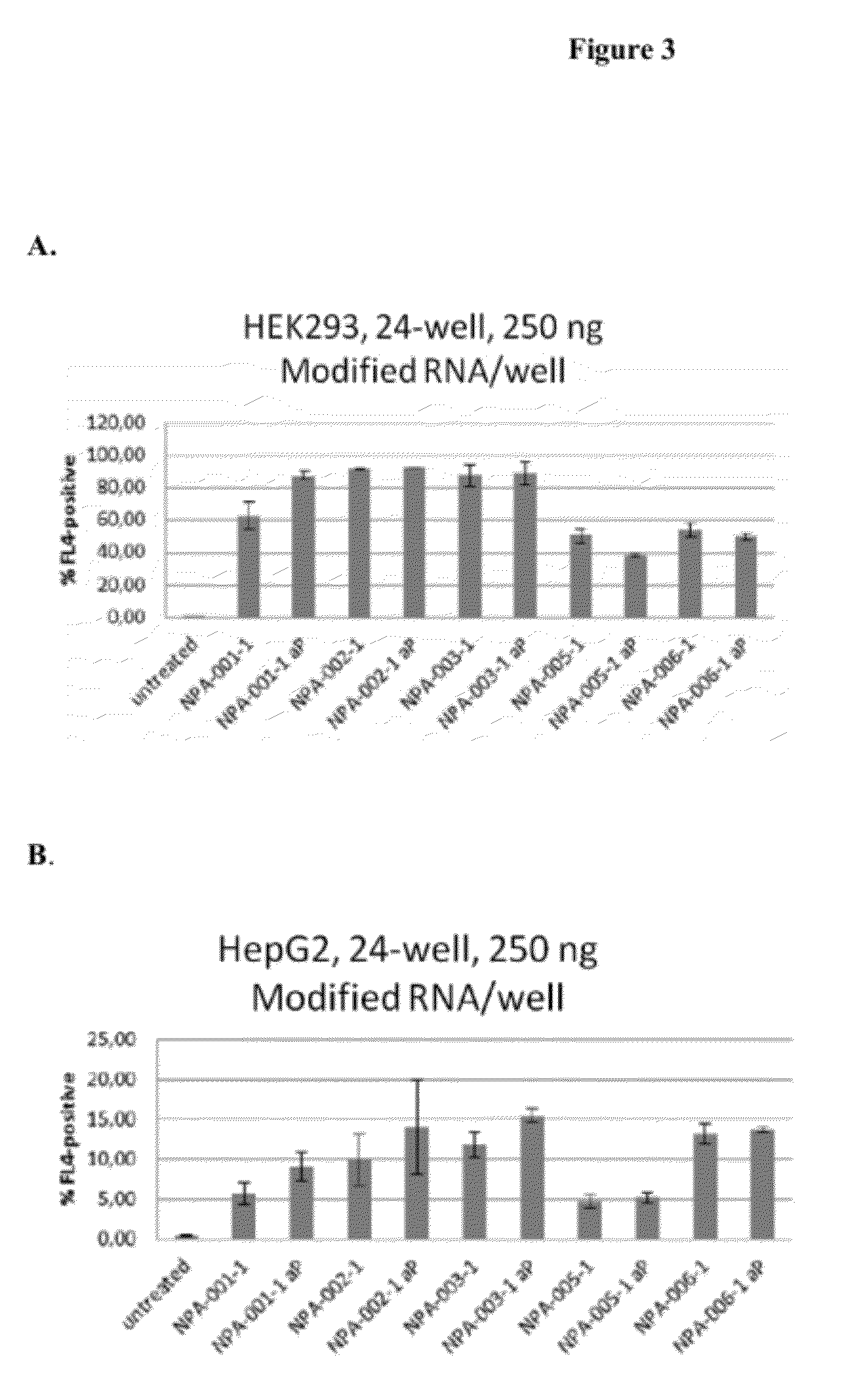
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
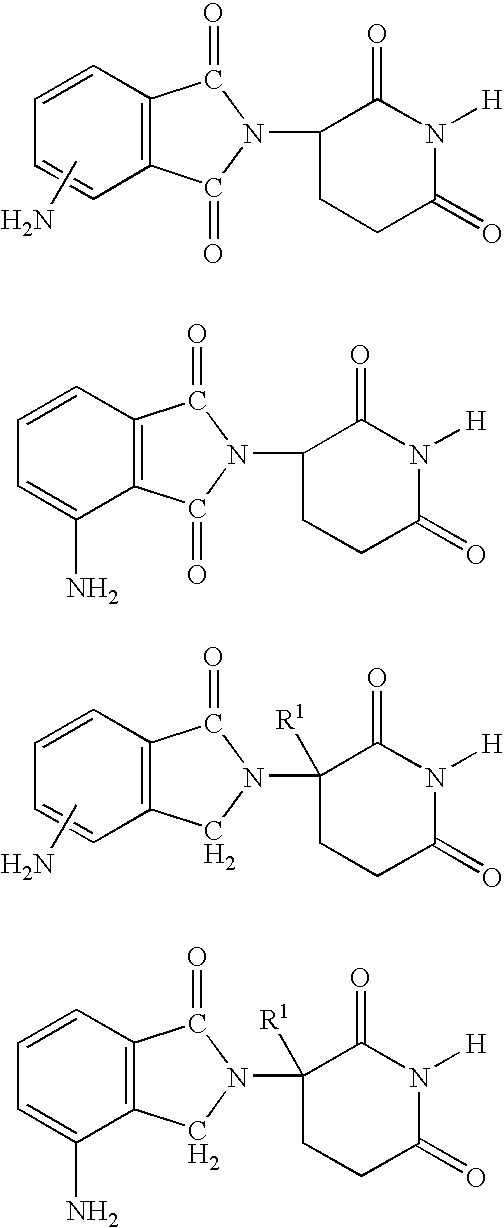
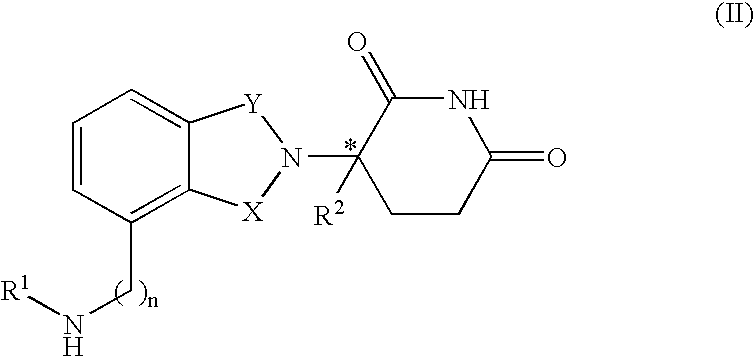
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Modulators of toll-like receptors
Owner:GILEAD SCI INC
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
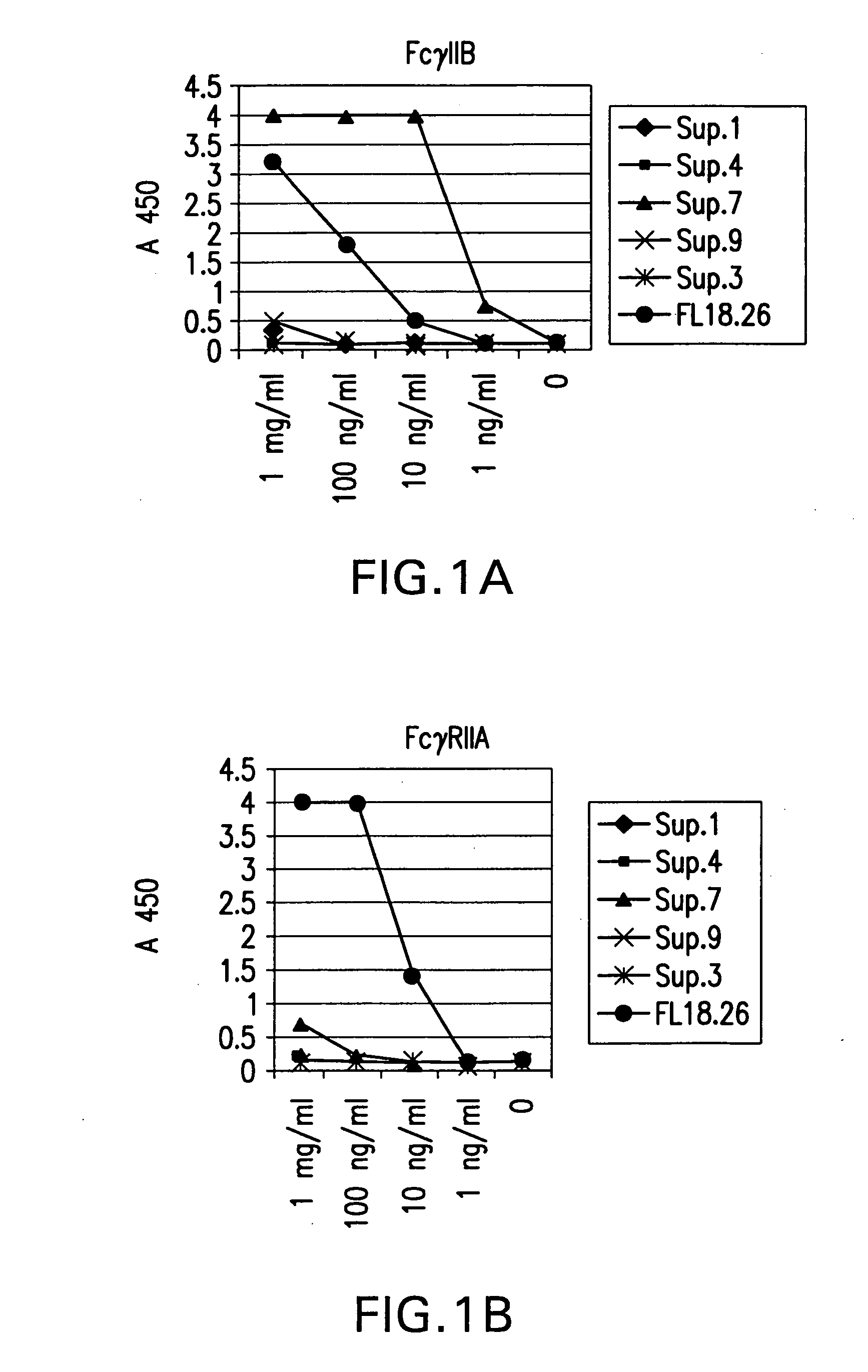
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Lipid encapsulated interfering RNA
ActiveUS20060240093A1Inhibit aggregationMetabolism disorderMicroencapsulation basedLipid formationLipid particle
The present invention provides compositions and methods for silencing gene expression by delivering nucleic acid-lipid particles comprising a siRNA molecule to a cell.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Cross-linkers and their uses
ActiveUS20090274713A1Good water solubilityHigh sensitivityOrganic active ingredientsTripeptide ingredientsCell bindingCross-link
Charged or pro-charged cross-linking moieties and conjugates of cell binding agents and drugs comprising the charged or pro-charged cross-linking moieties and method of making the same.
Owner:IMMUNOGEN INC
Modulation of stem and progenitor cell differentiation, assays, and uses thereof
InactiveUS20030235909A1Modulate their differentiationIncrease speedOrganic active ingredientsSenses disorderAssayPlacenta
The present invention relates to methods of modulating mammalian stem cell and progenitor cell differentiation. The methods of the invention can be employed to regulate and control the differentiation and maturation of mammalian, particularly human stem cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem or progenitor cell populations along specific cell and tissue lineages, and in particular, to the differentiation of embryonic-like stem cells originating from a postpartum placenta or for the differentiation of early progenitor cells to a granulocytic lineage. Finally, the invention relates to the use of such differentiated stem or progenitor cells in transplantation and other medical treatments.
Owner:SIGNAL PHARMA LLC +2
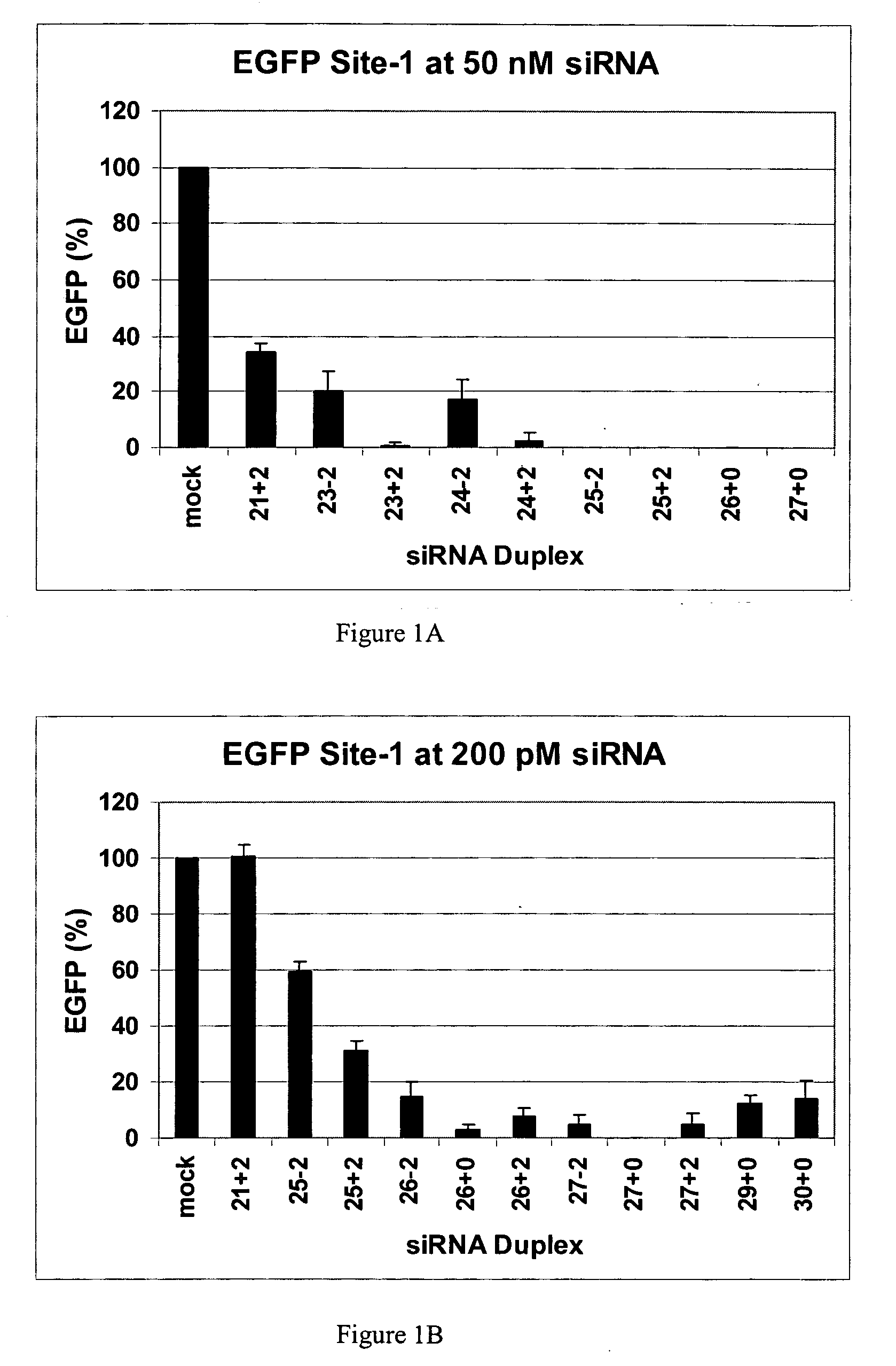
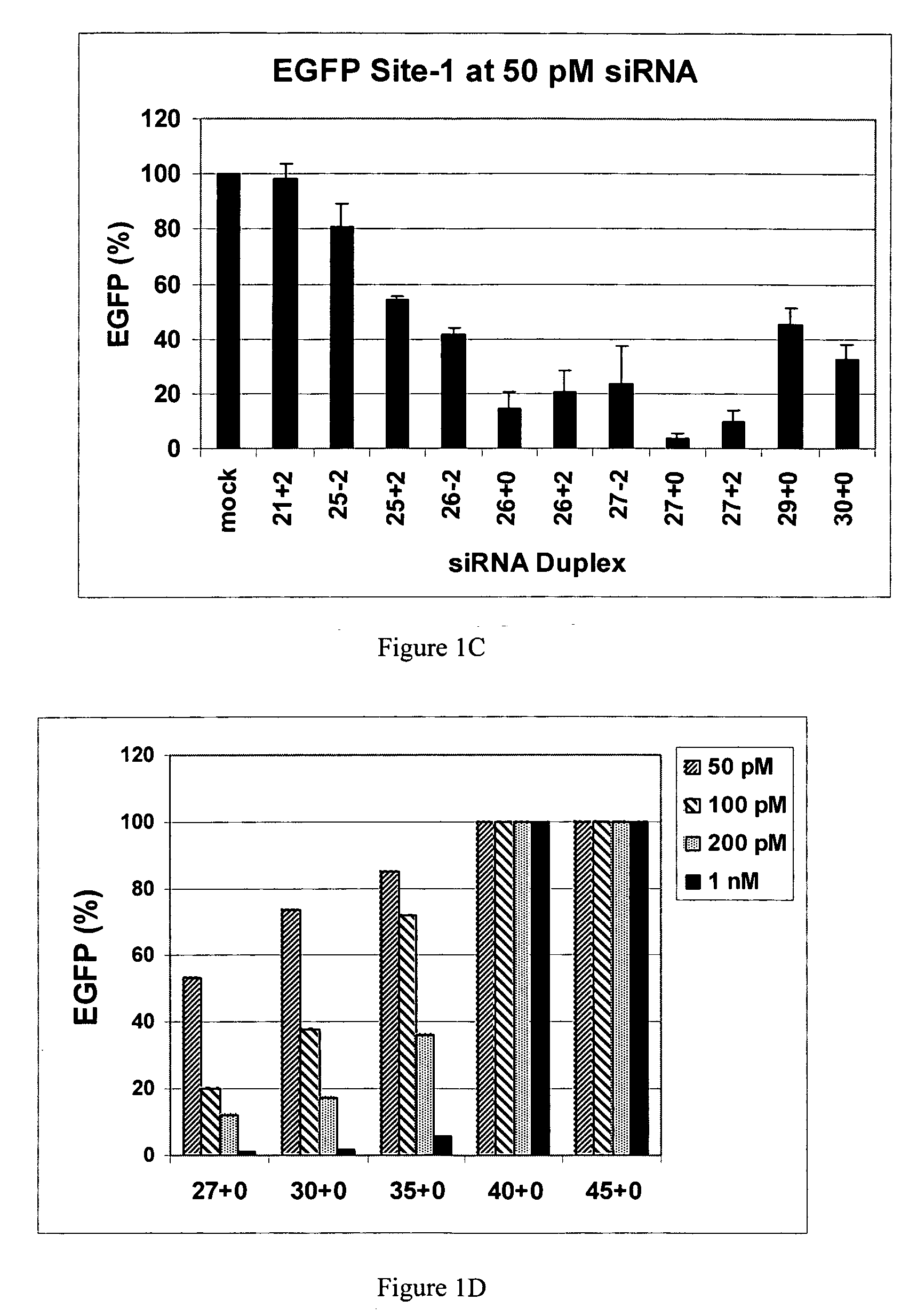
Methods and compositions for the specific inhibition of gene expression by double-stranded RNA
The invention is directed to compositions and methods for selectively reducing the expression of a gene product from a desired target gene in a cell, as well as for treating diseases caused by the expression of the gene. More particularly, the invention is directed to compositions that contain double stranded RNA (“dsRNA”), and methods for preparing them, that are capable of reducing the expression of target genes in eukaryotic cells. The dsRNA has a first oligonucleotide sequence that is between 25 and about 30 nucleotides in length and a second oligonucleotide sequence that anneals to the first sequence under biological conditions. In addition, a region of one of the sequences of the dsRNA having a sequence length of at least 19 nucleotides is sufficiently complementary to a nucleotide sequence of the RNA produced from the target gene to trigger the destruction of the target RNA by the RNAi machinery.
Owner:CITY OF HOPE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com