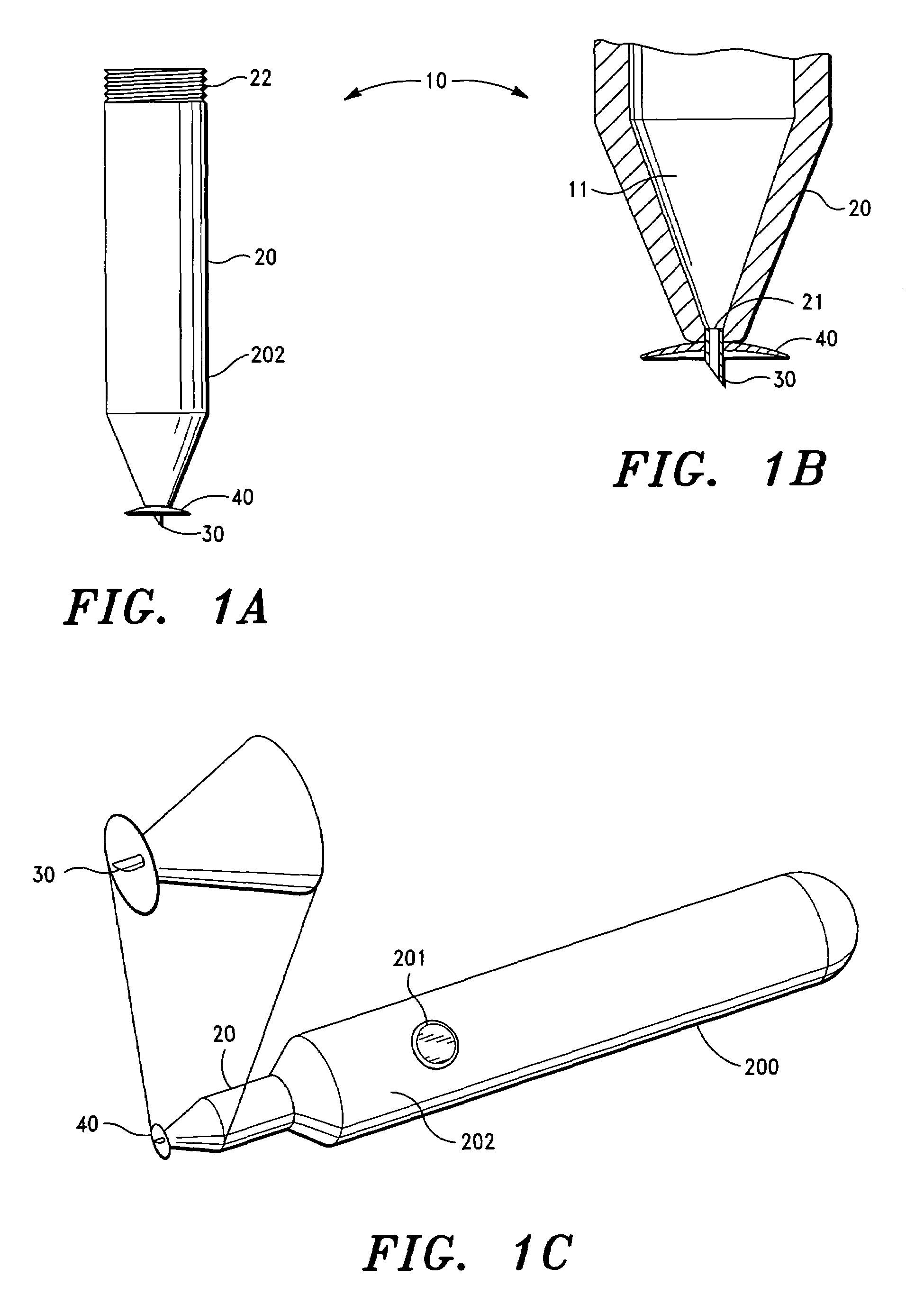
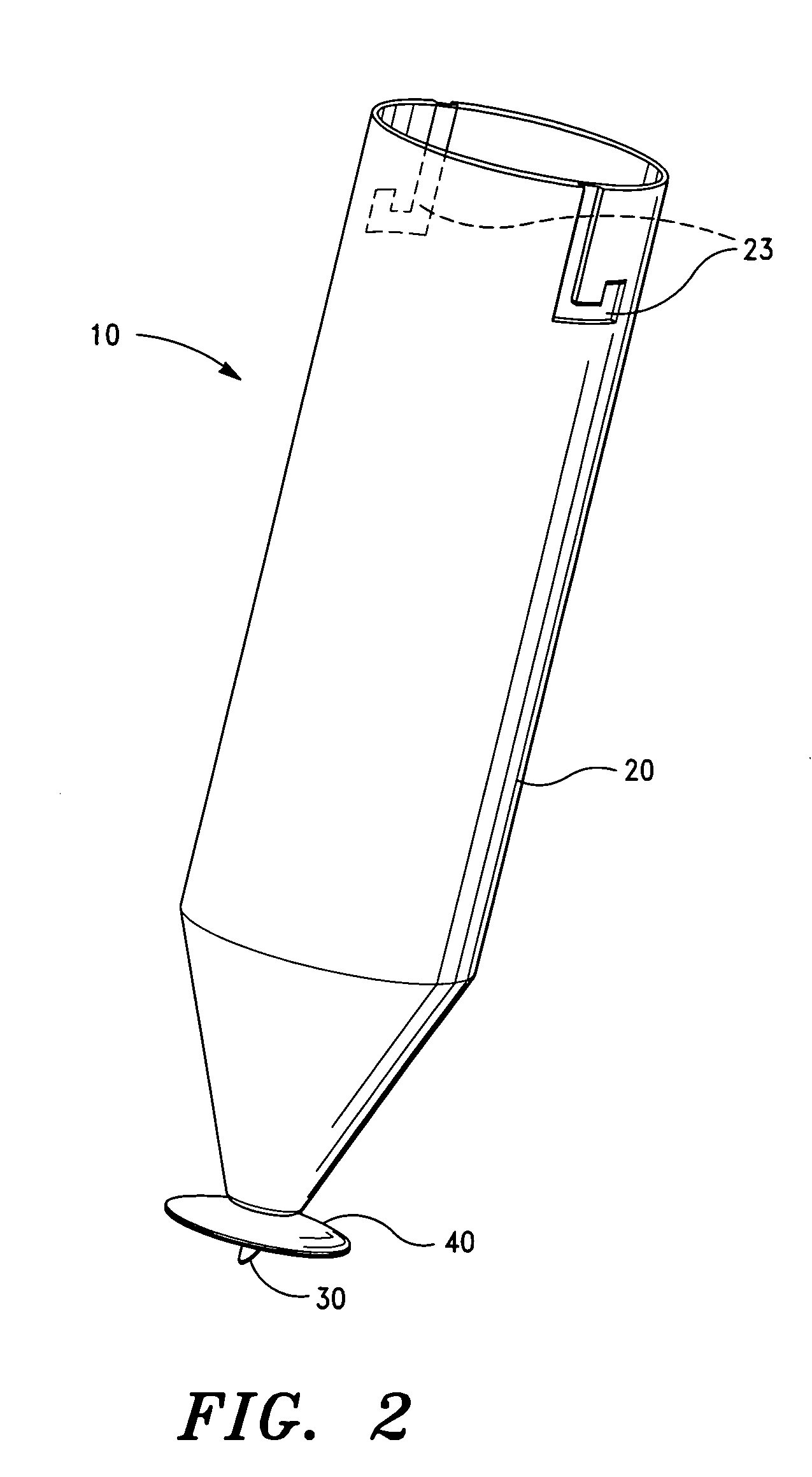
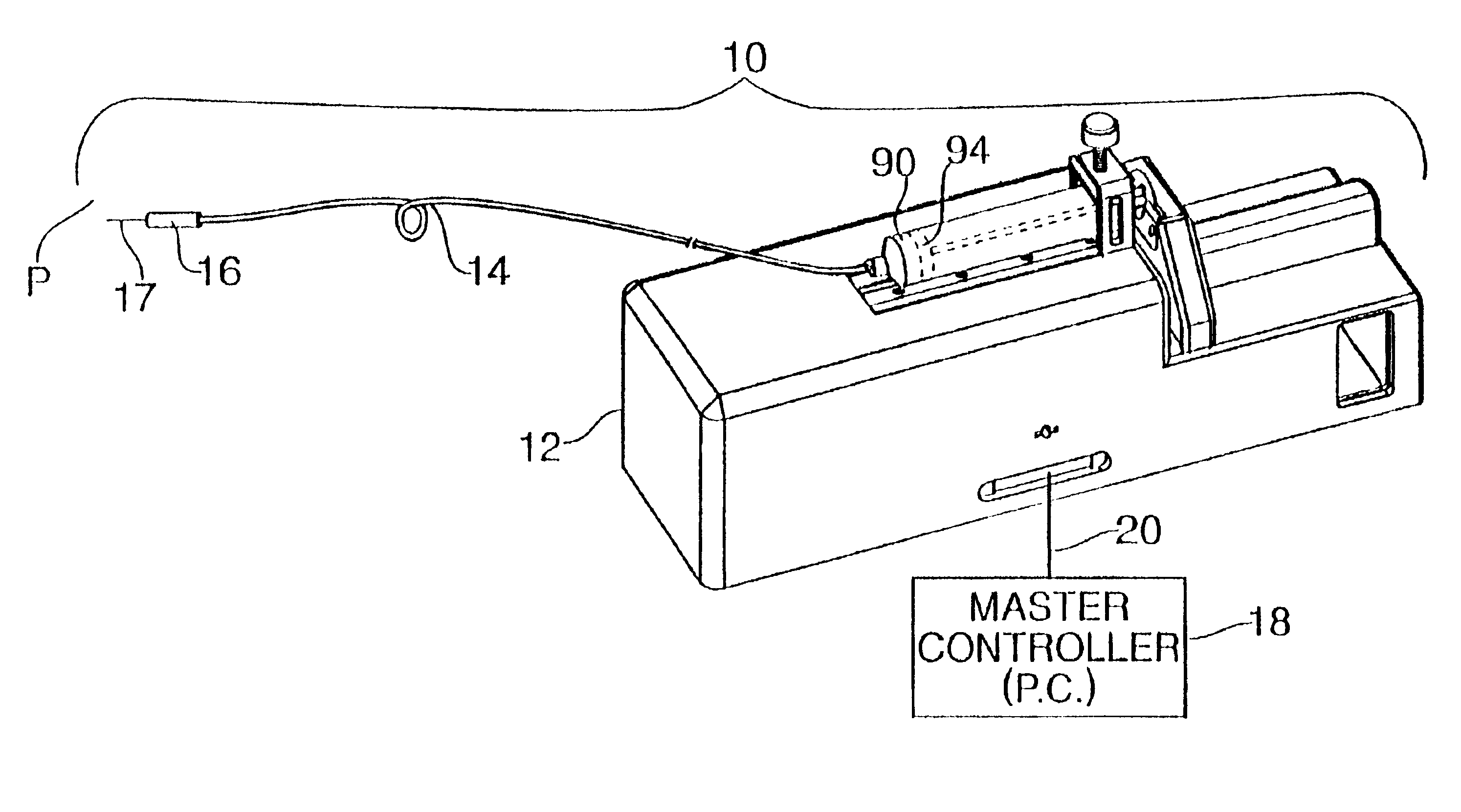
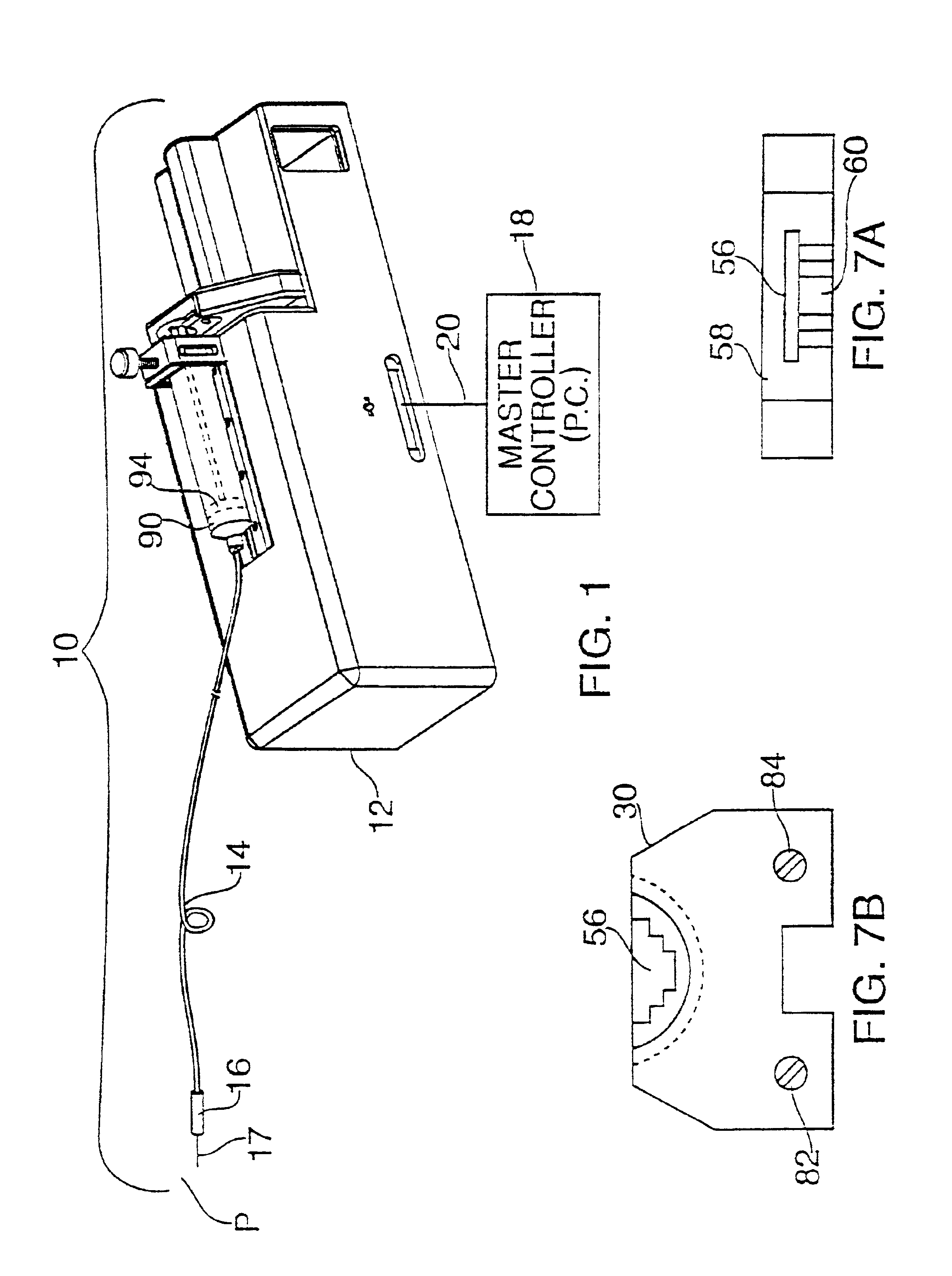
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7806 results about "Injection device" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
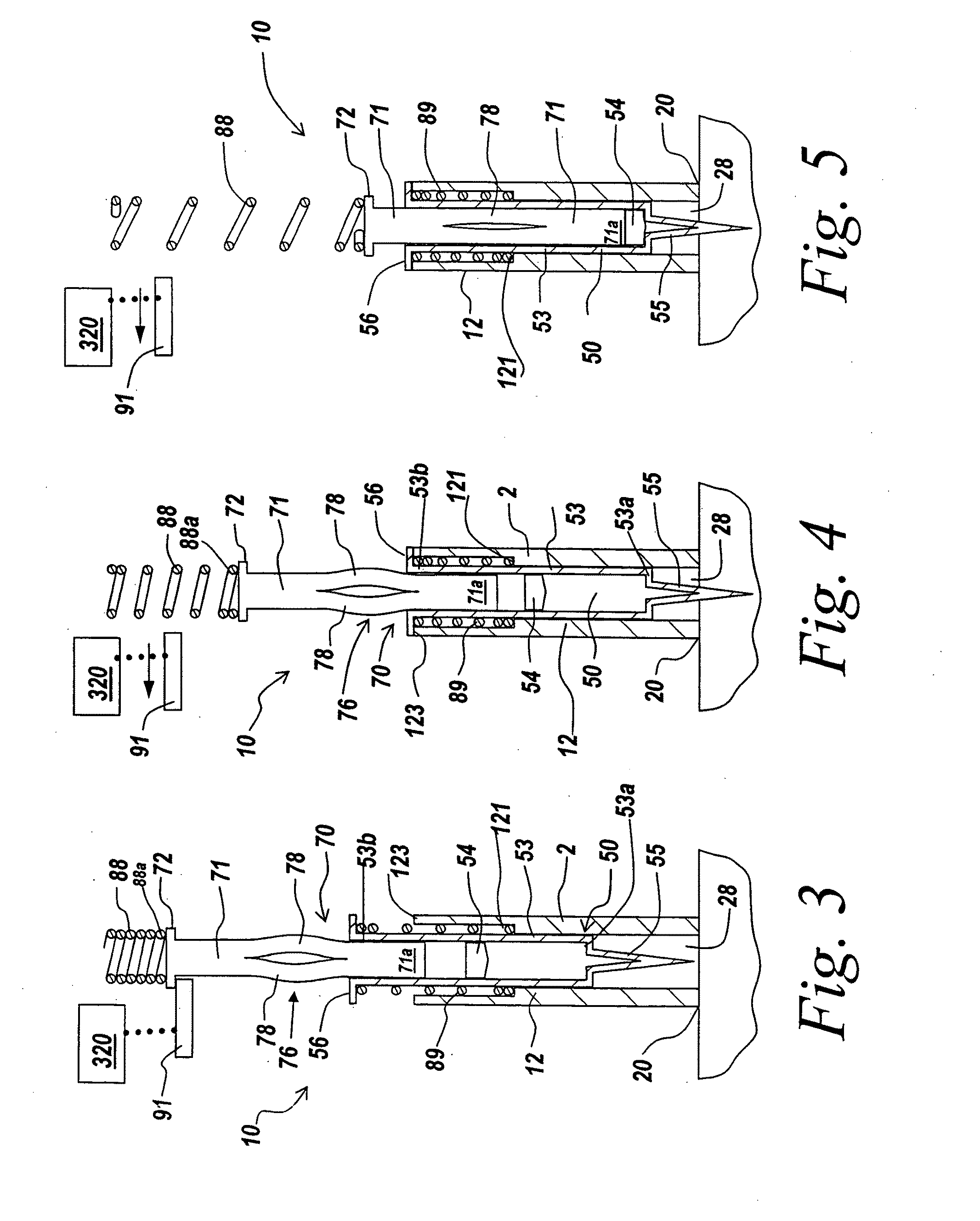
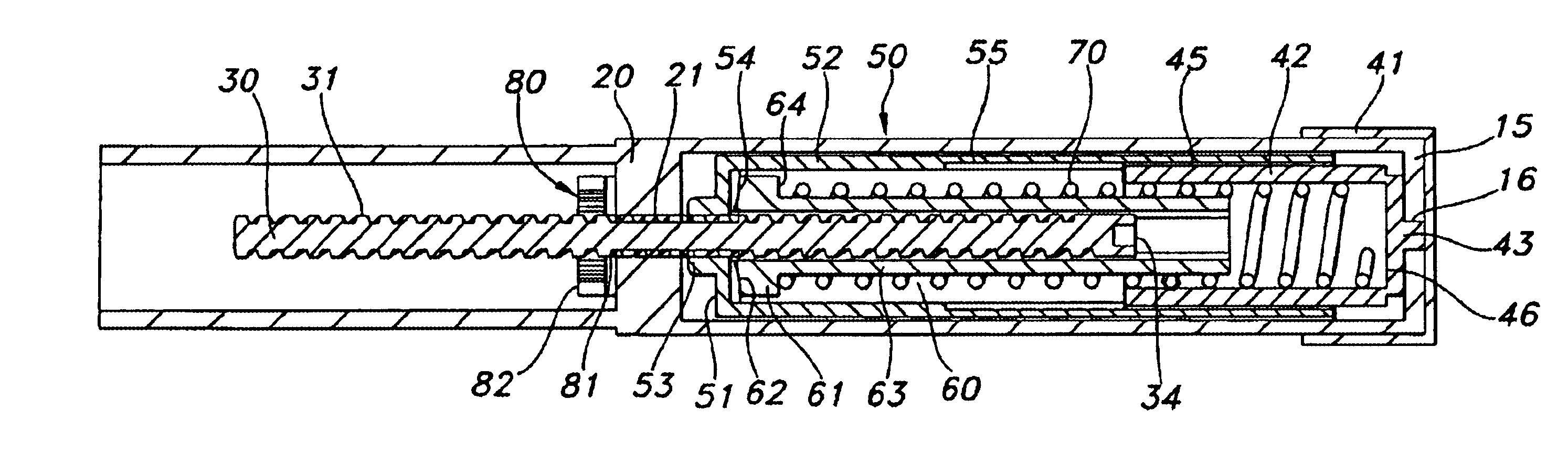
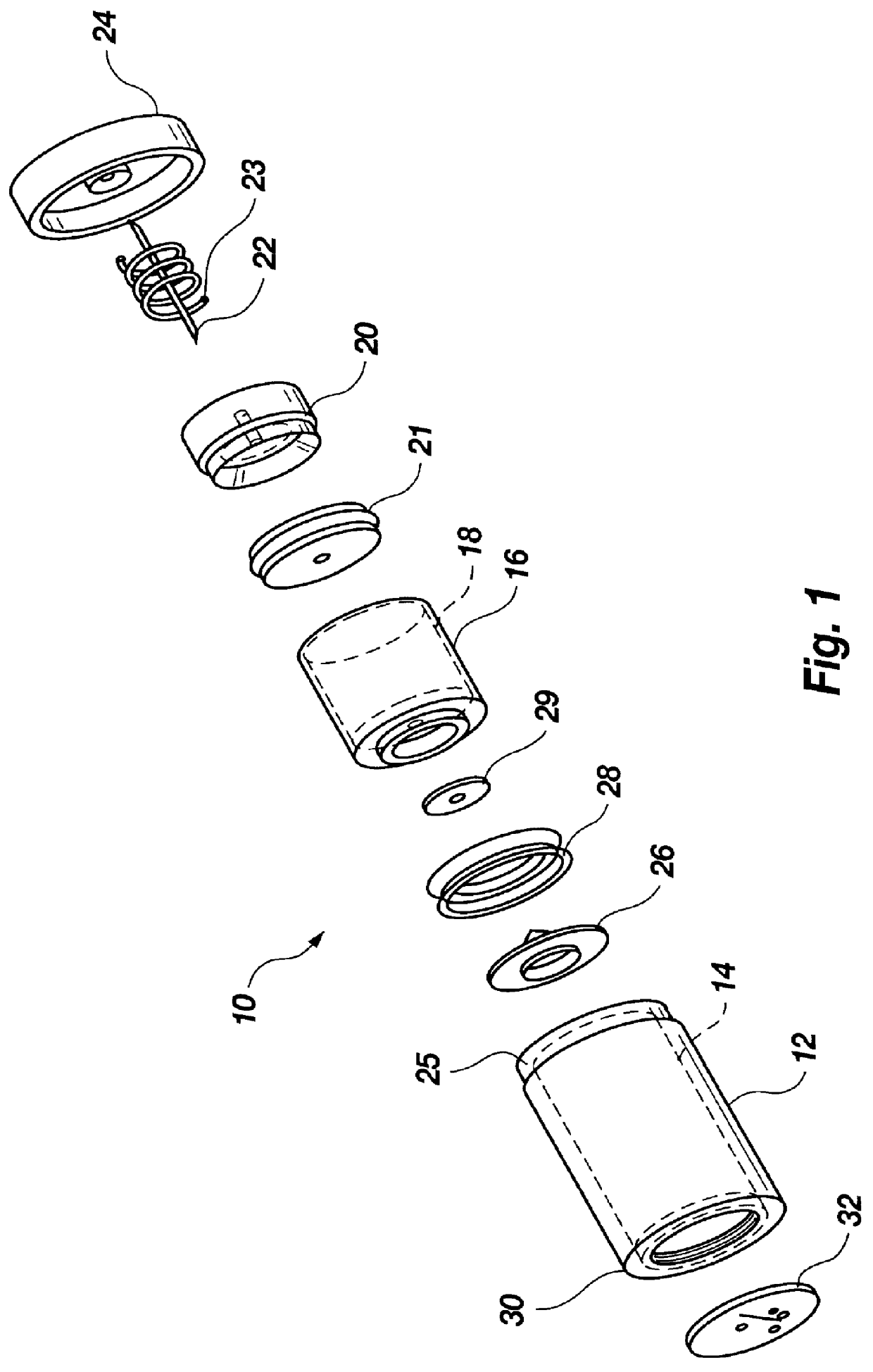
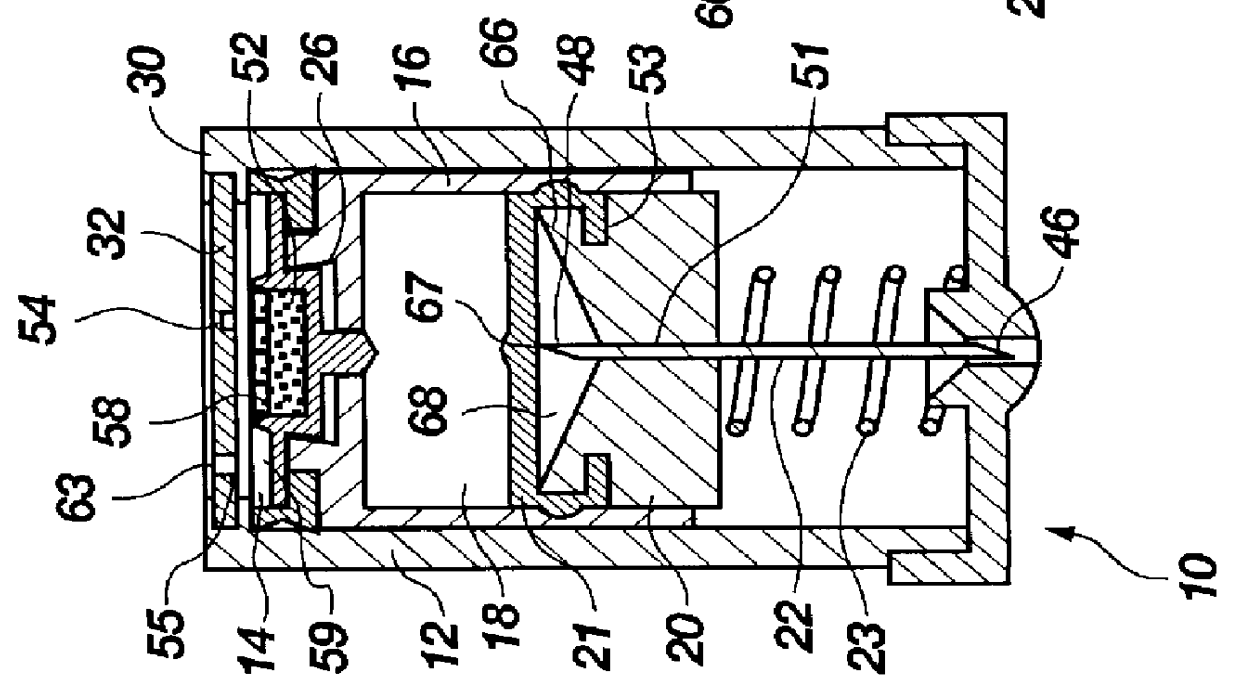
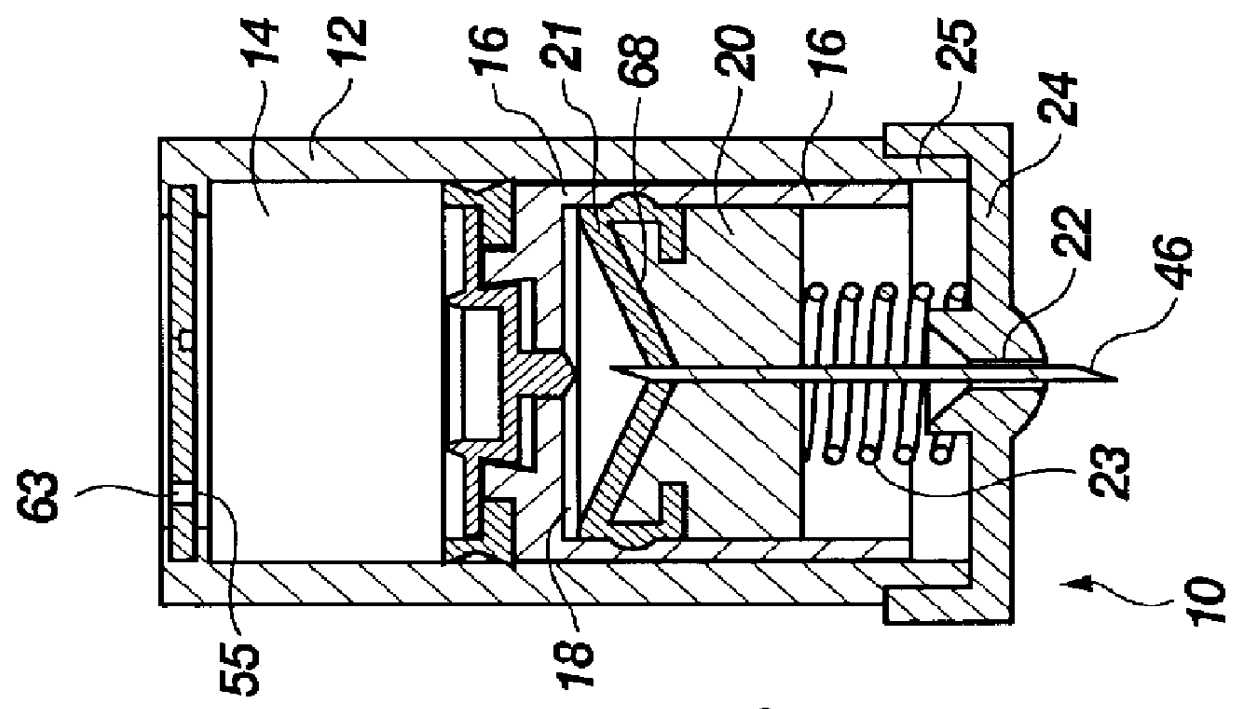
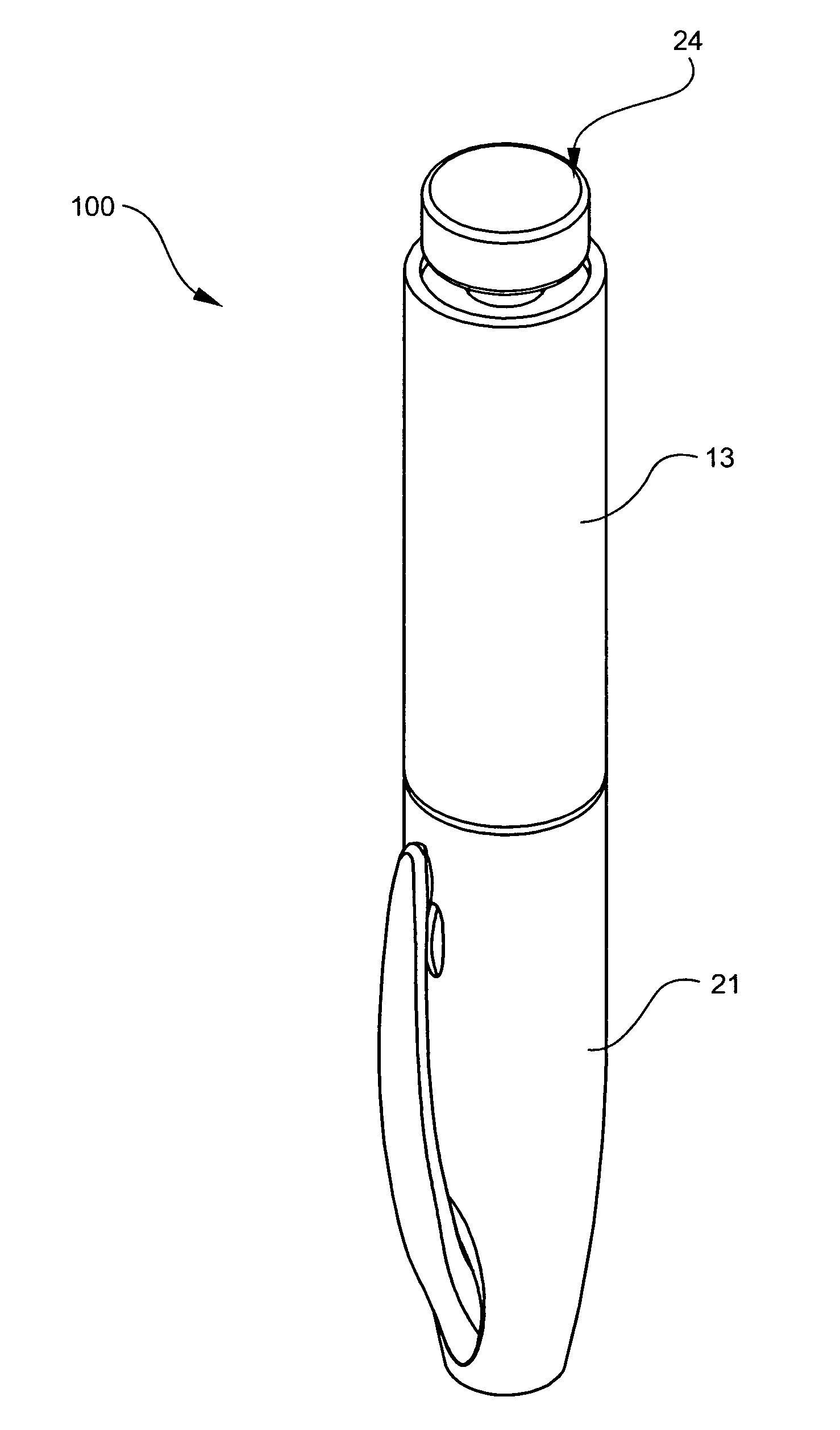
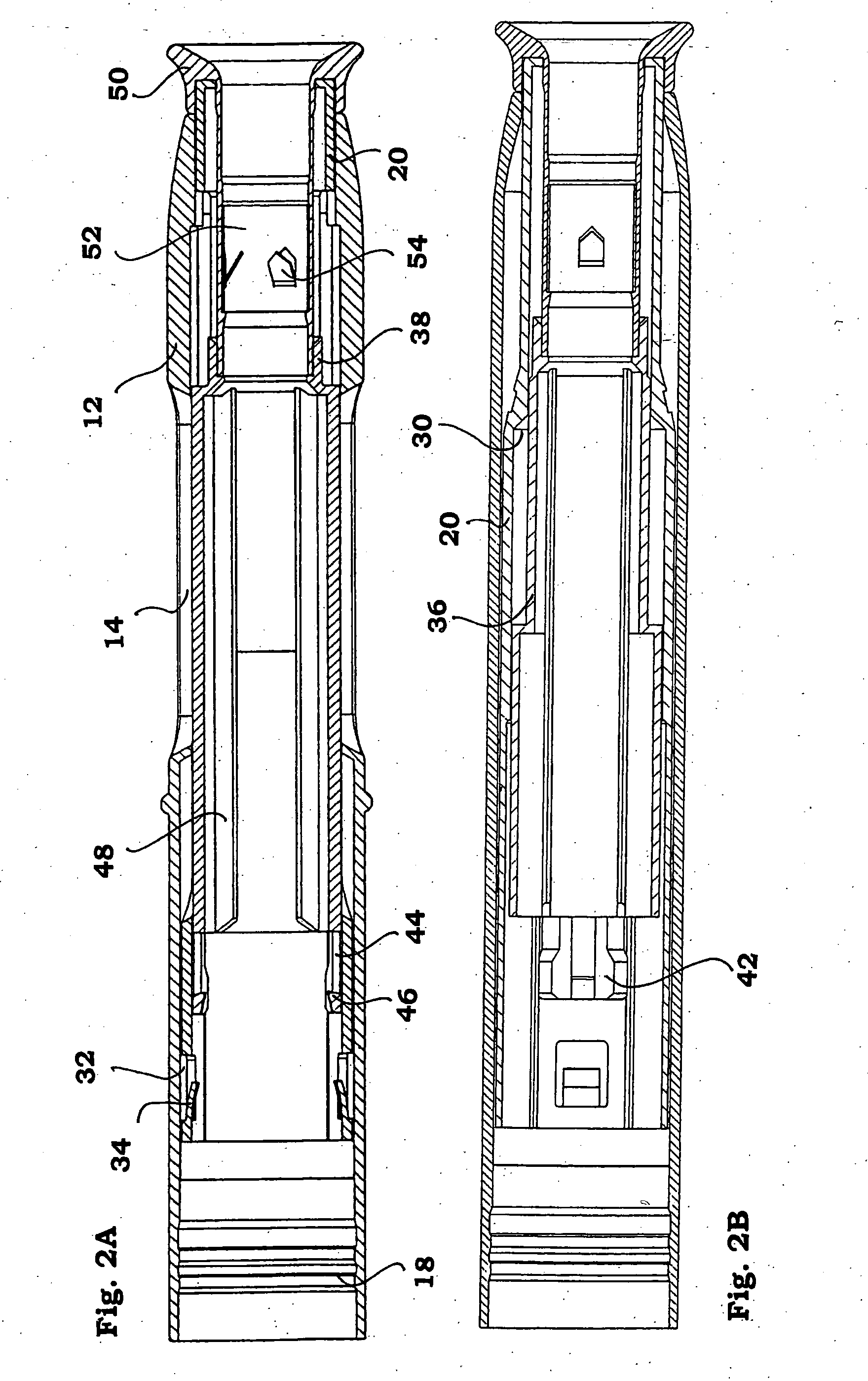
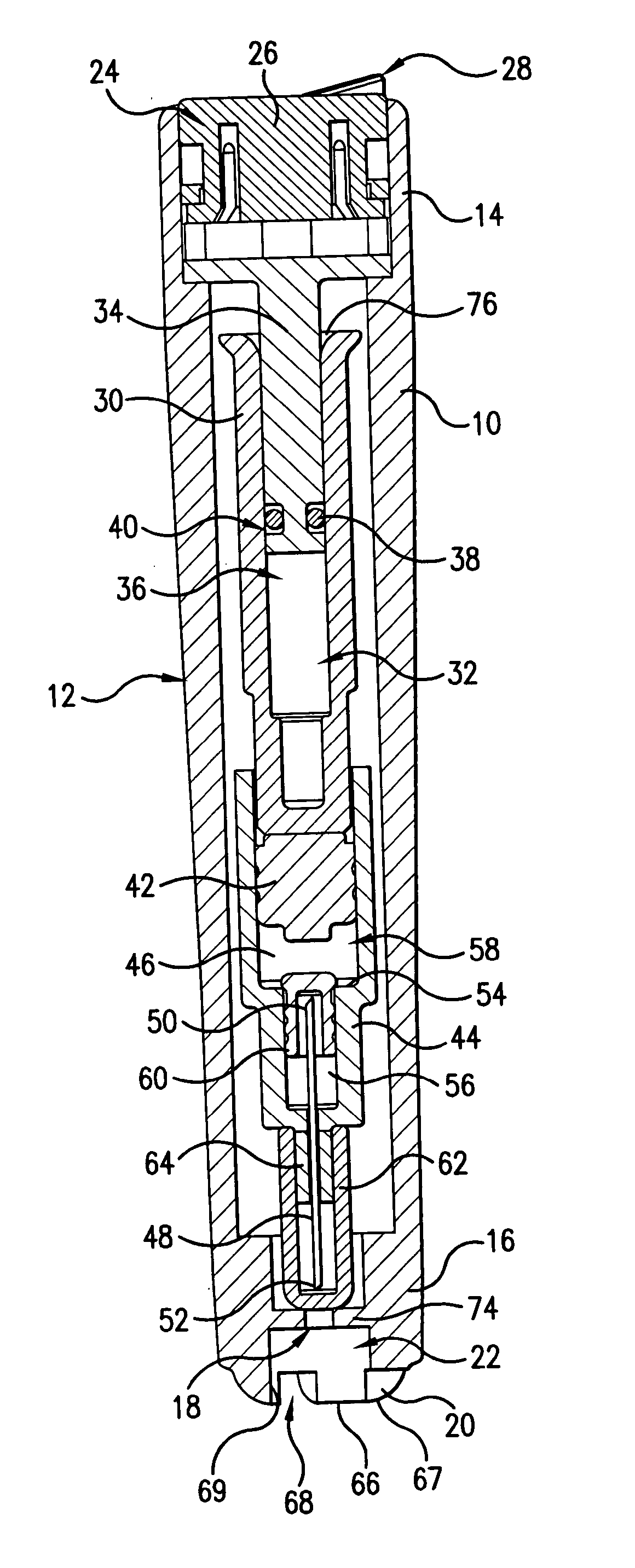
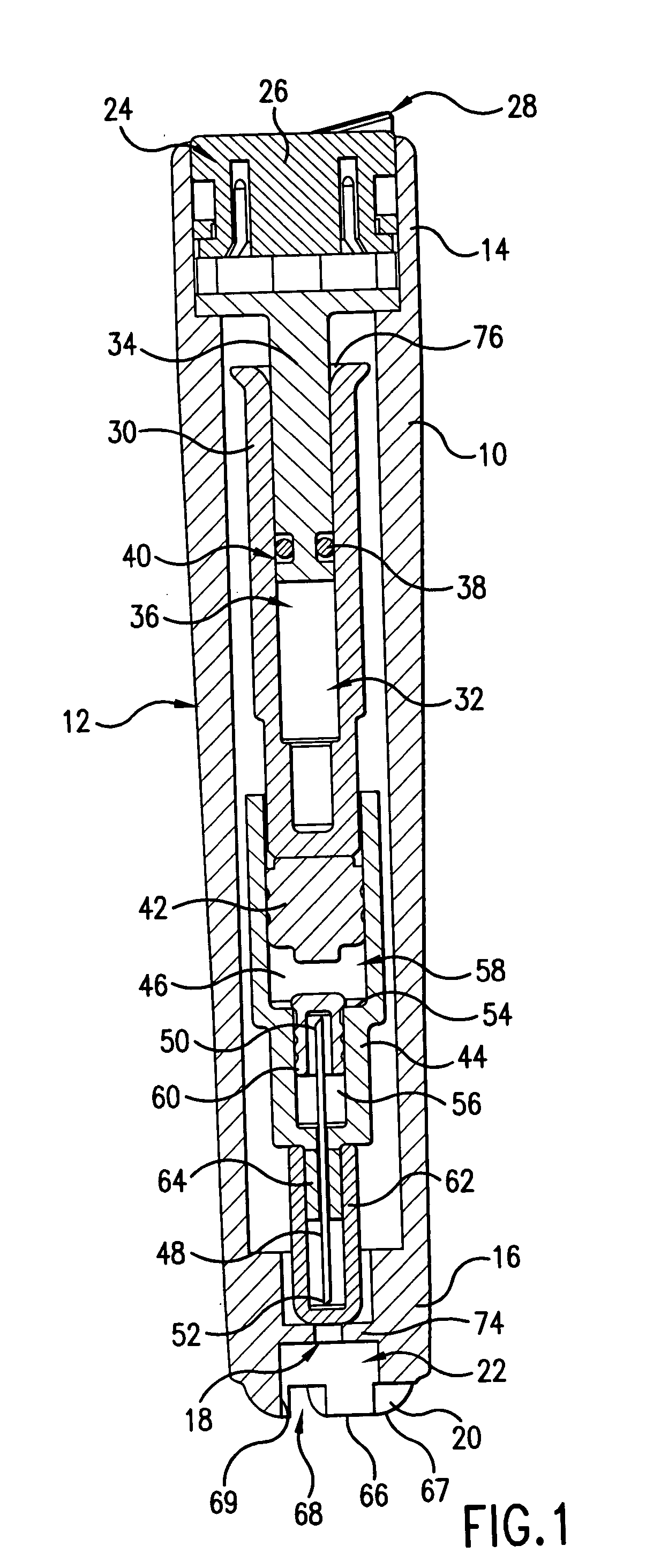
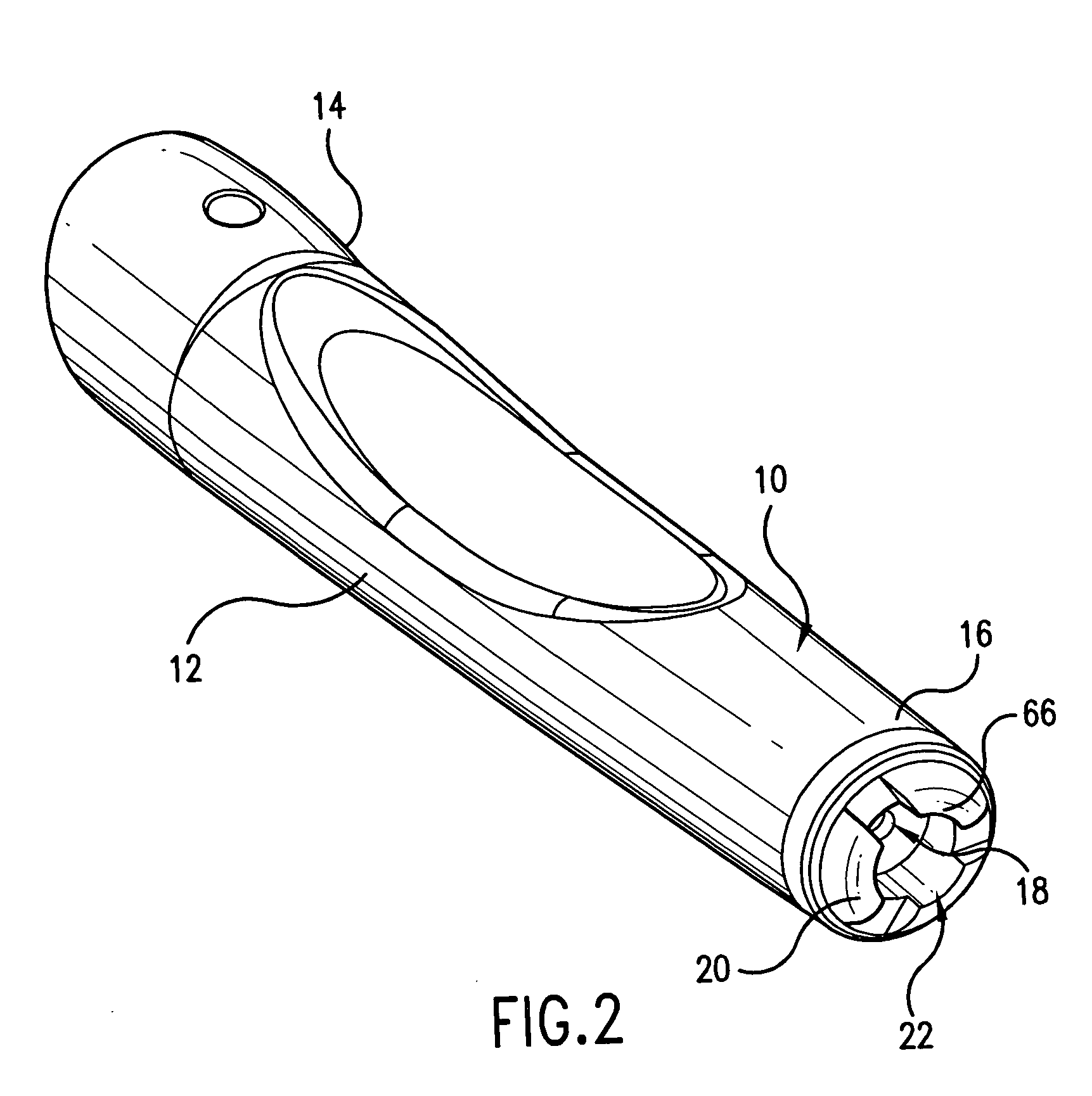
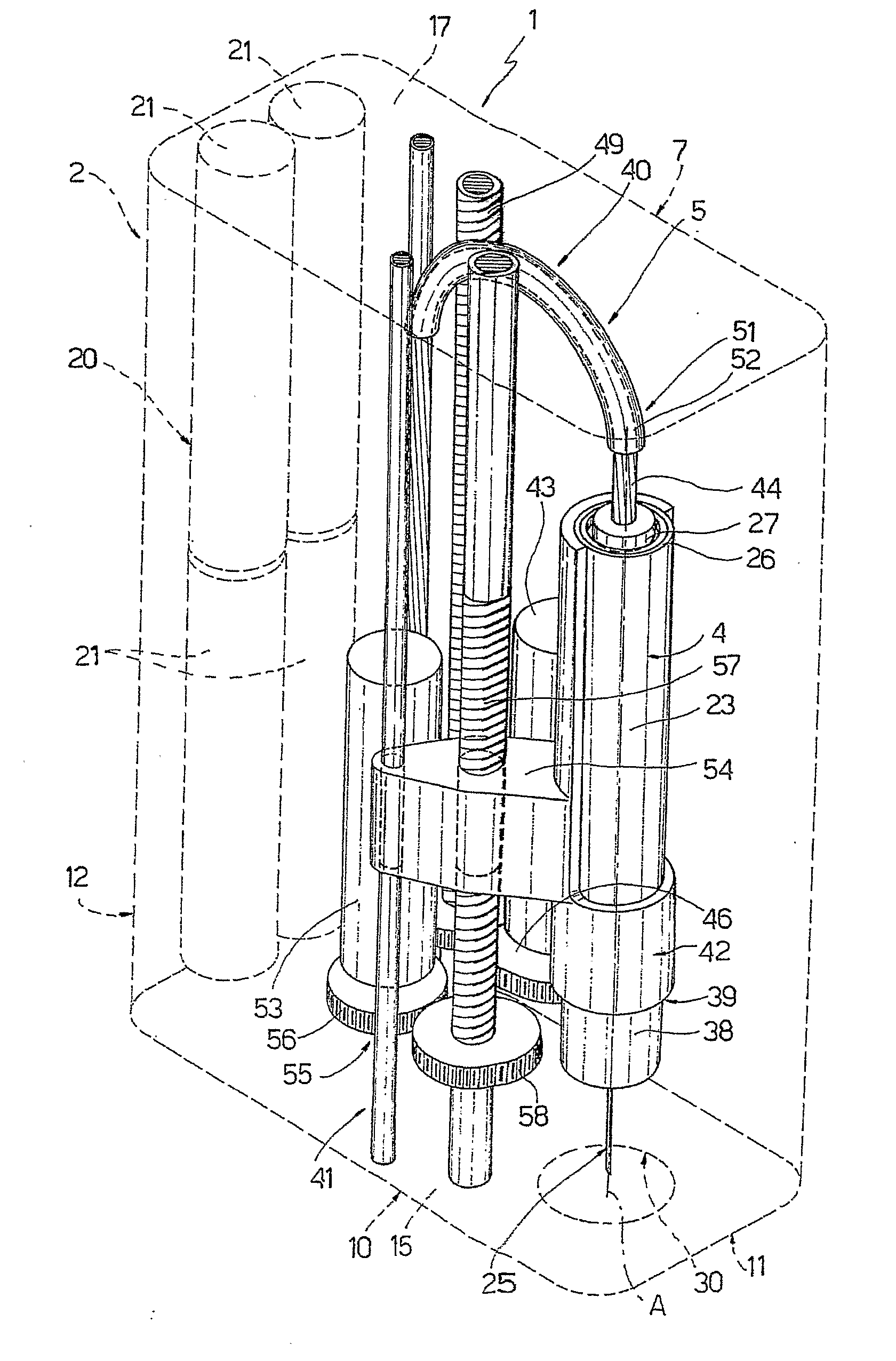
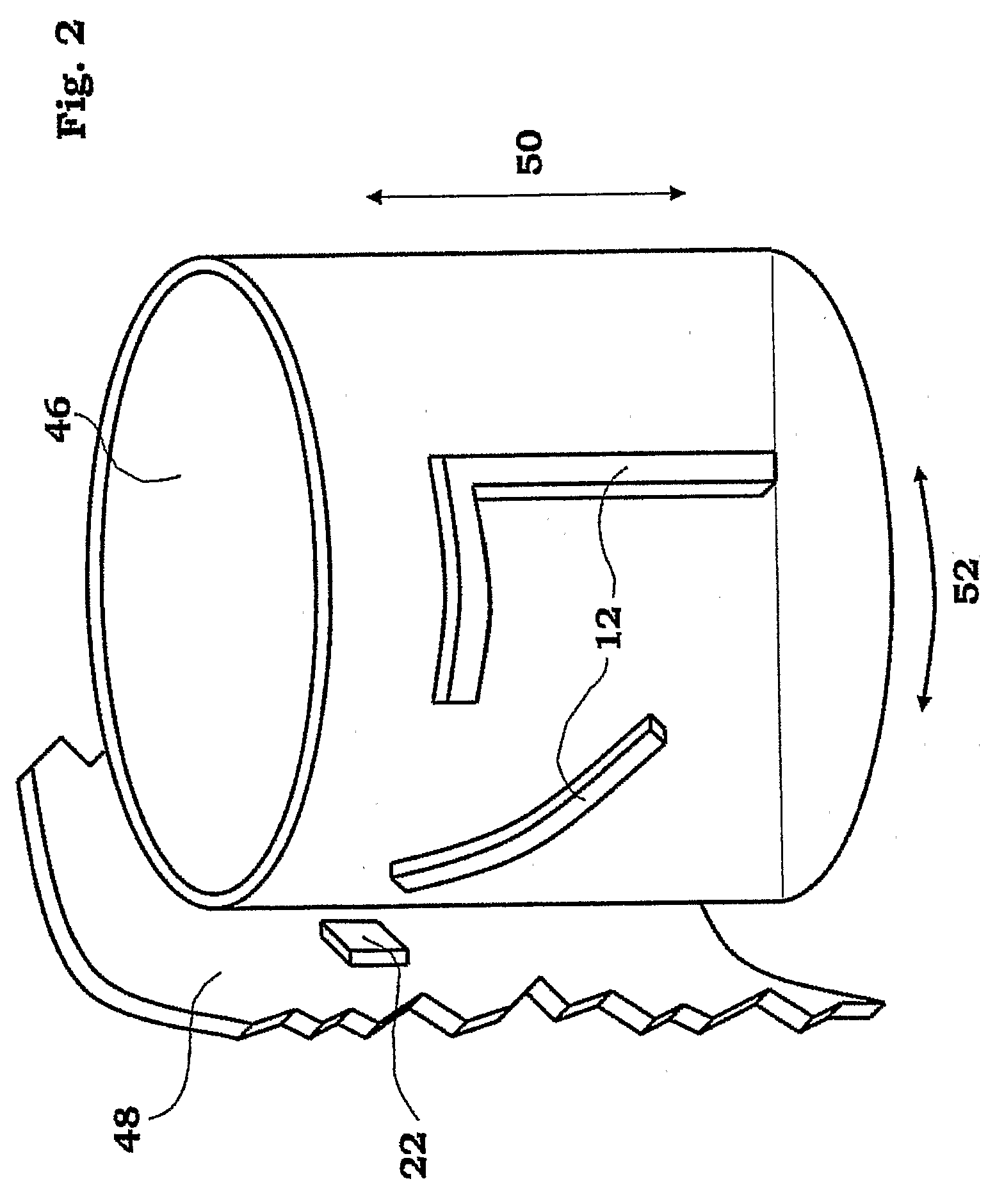
The injection device is a device that causes the resin material to be melted by heat and then injected into the mold. The resin is extruded from the head into the barrel as shown, and the melt is conveyed to the front end of the barrel by the rotation of the screw. In that process, the resin material in the barrel is heated by the action...
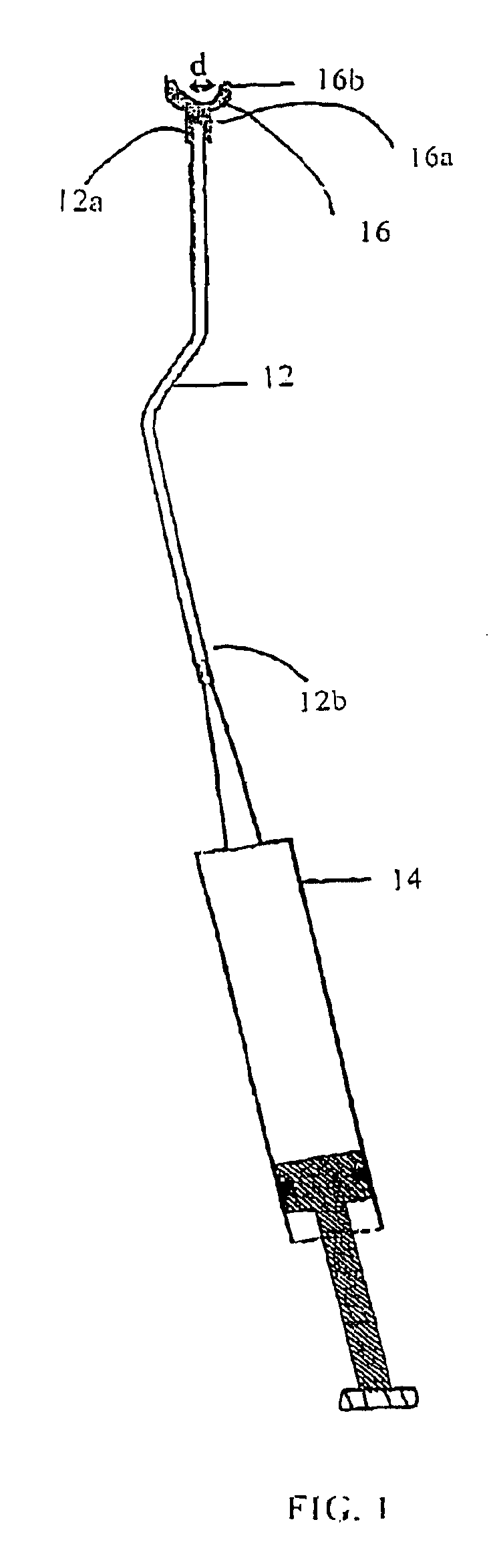
Vertebroplasty injection device
InactiveUS7008433B2Small sizeIncrease pressurePowder deliveryShaking/oscillating/vibrating mixersInjectable biomaterialBone cement
This invention relates to a mixing and delivery device suitable for delivering injectable biomaterials, and to preferred bone cement formulations.
Owner:DEPUY ACROMED INC
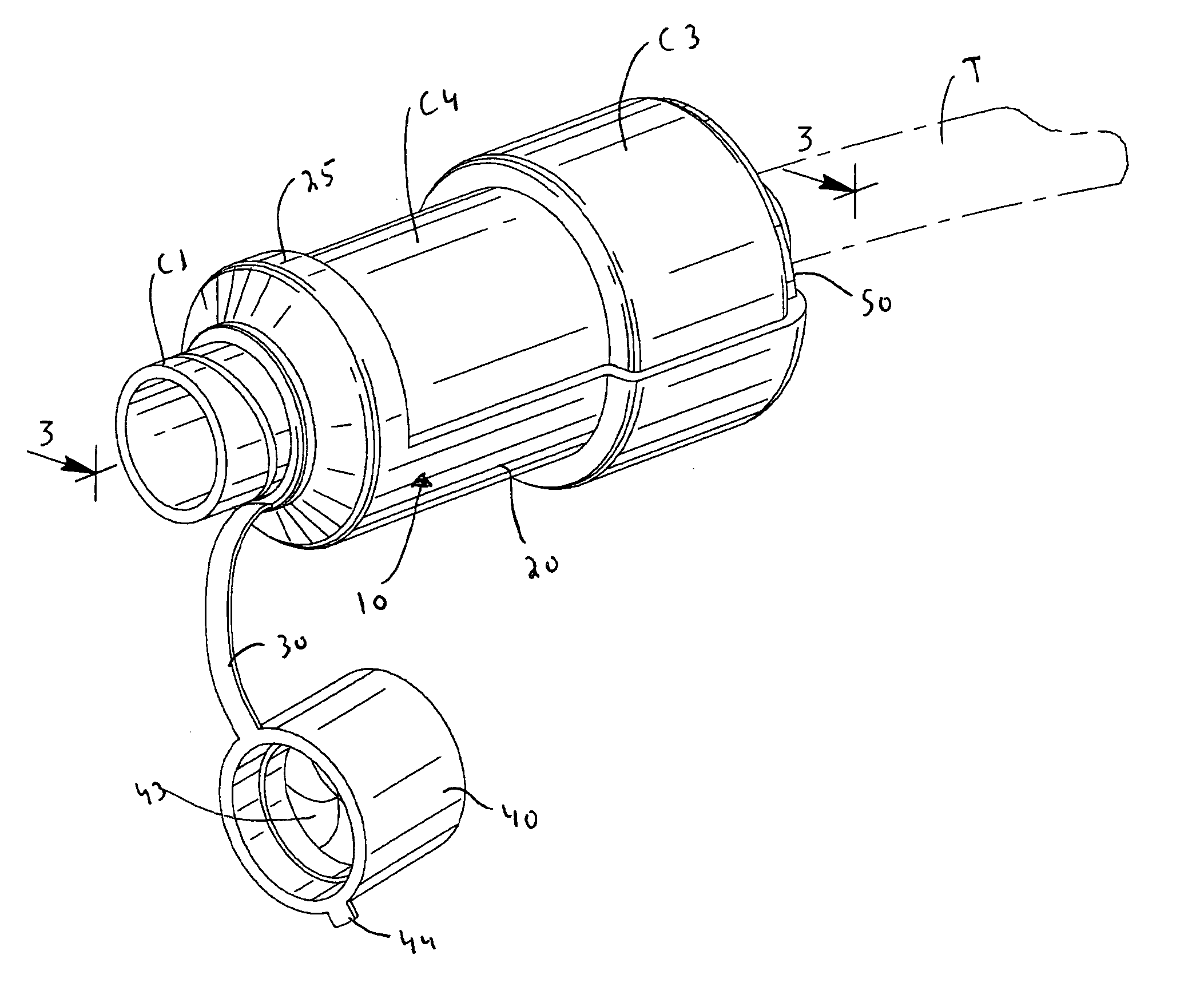
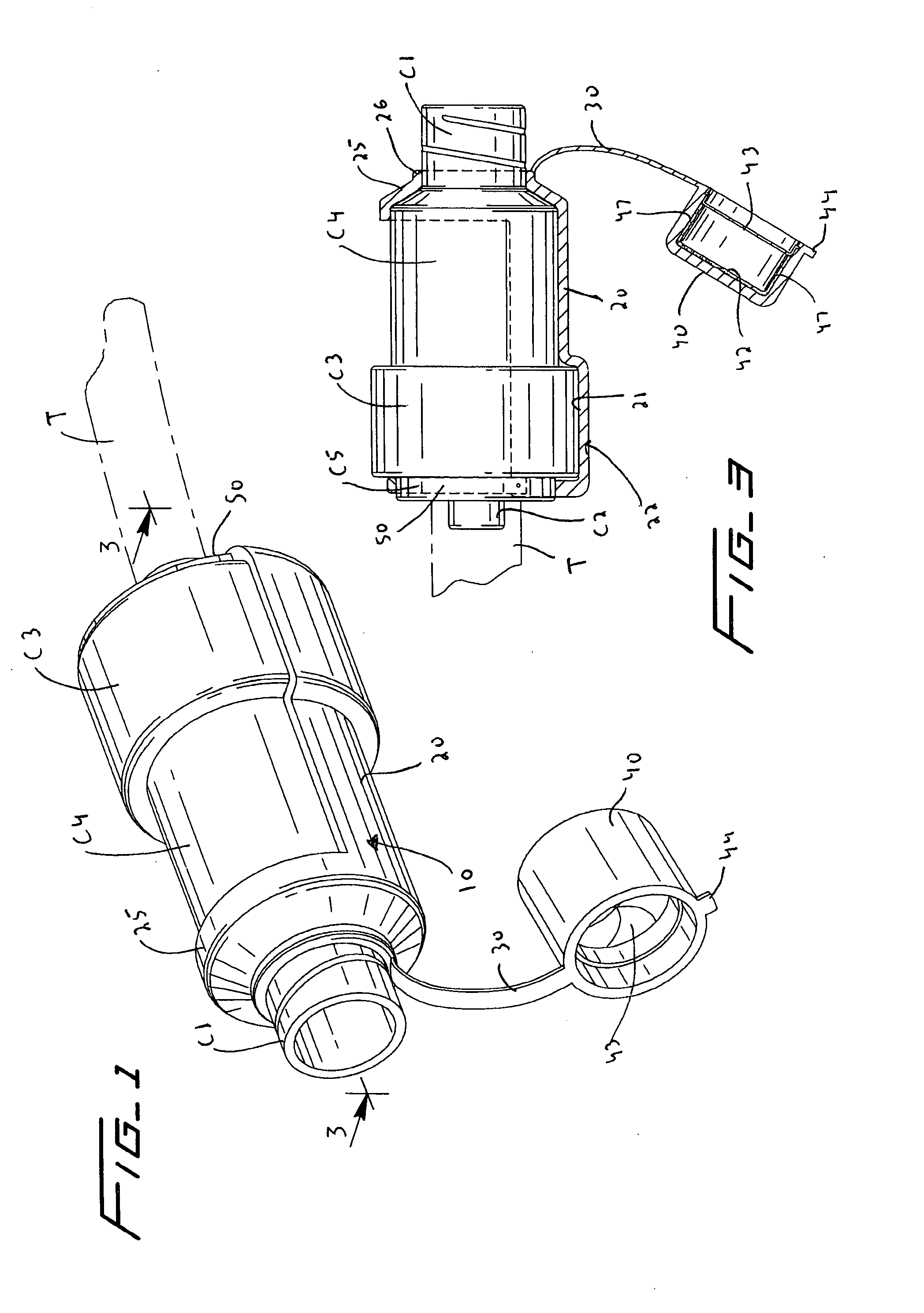
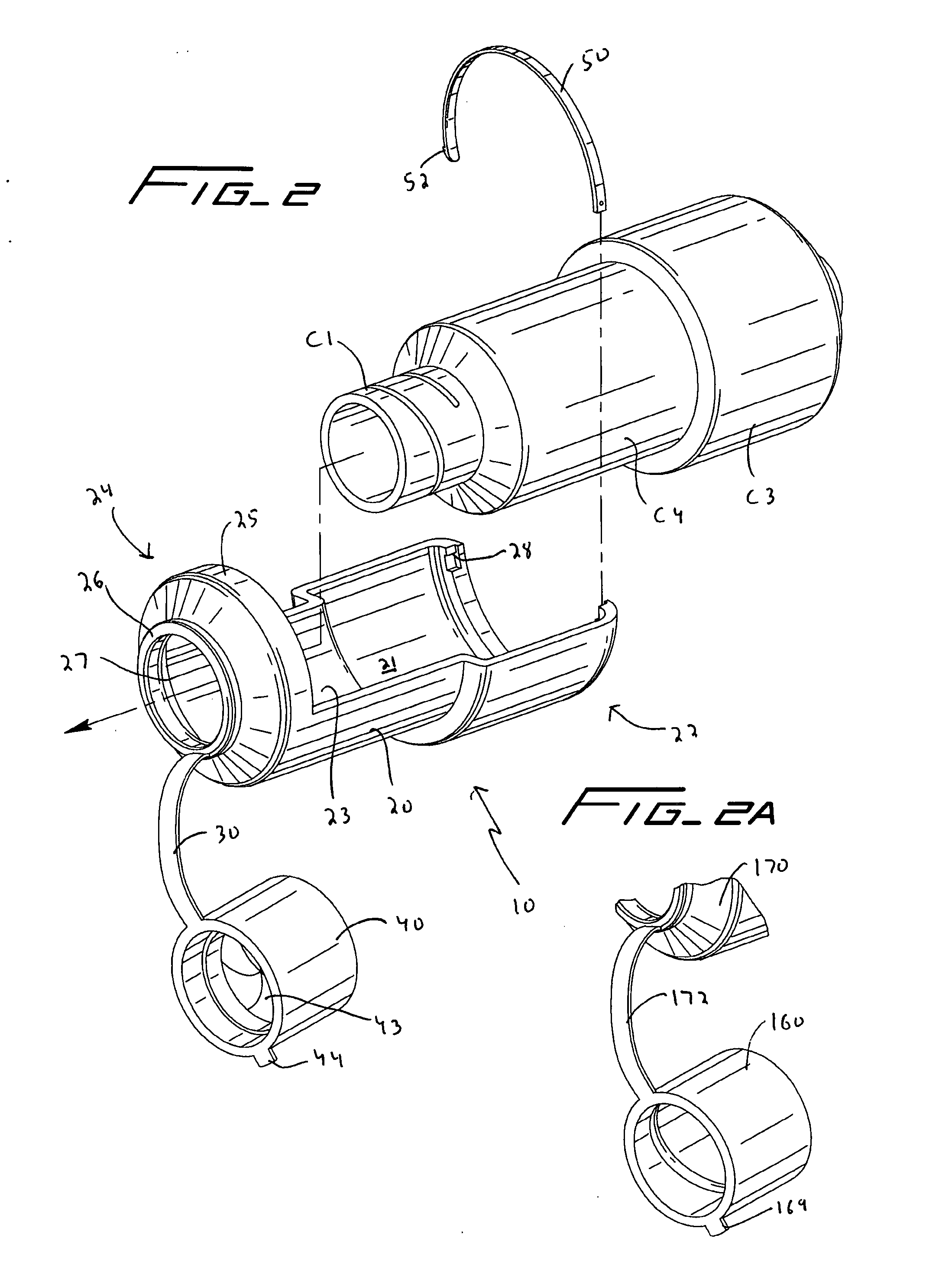
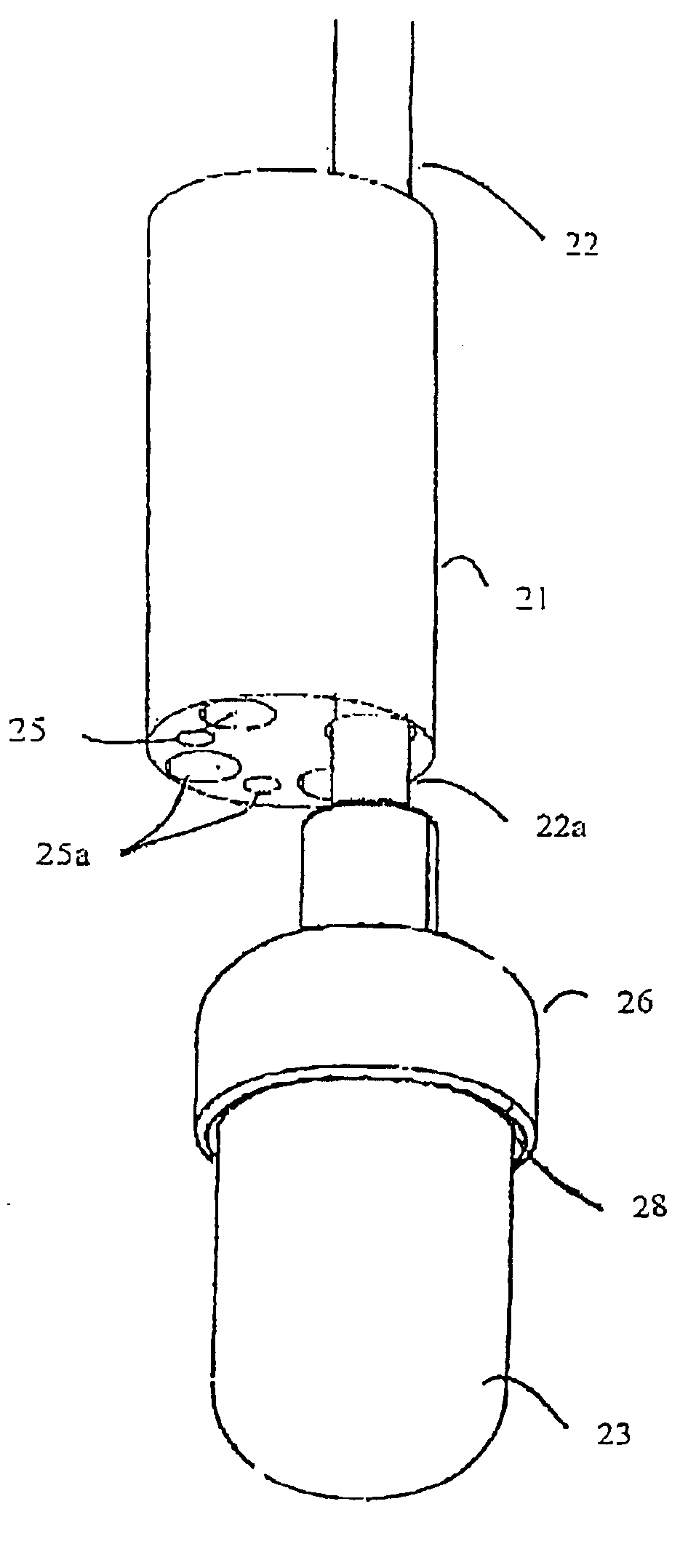
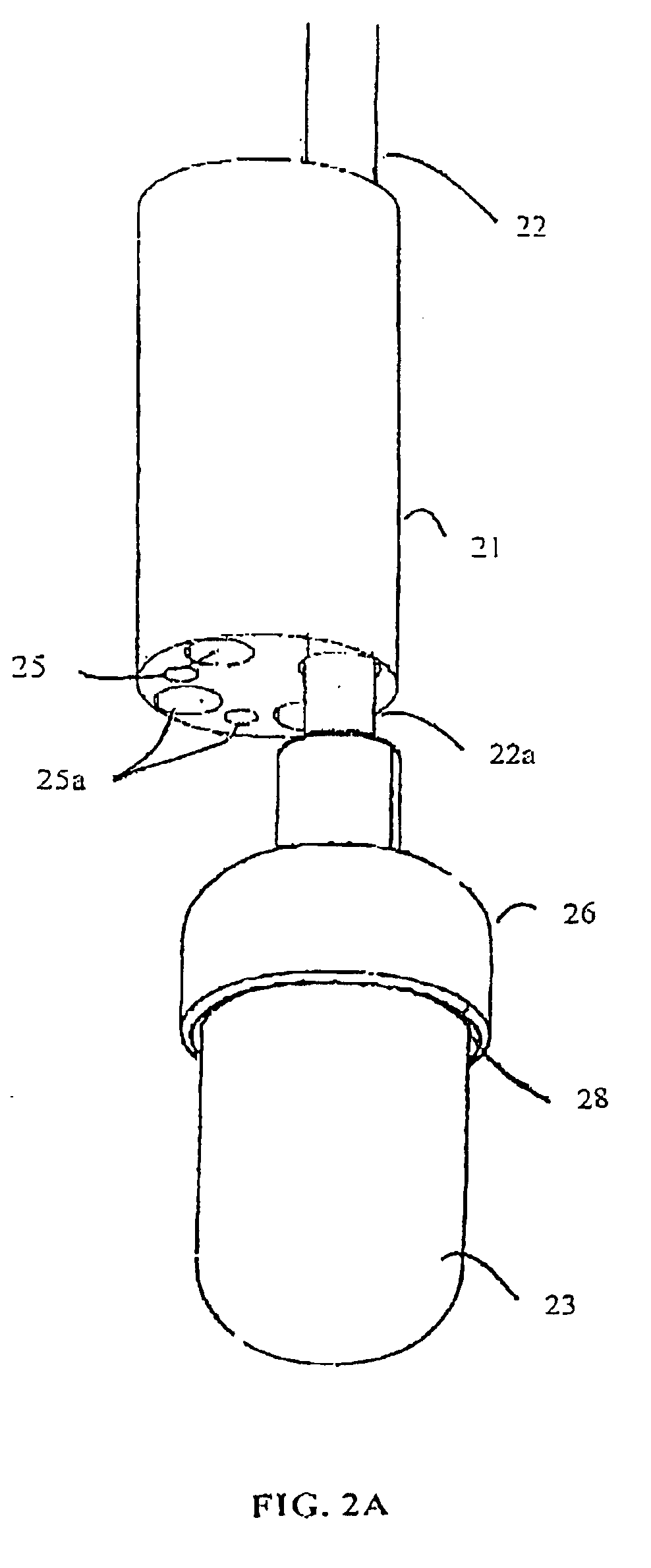
Coupling for injection devices
InactiveUS8574199B2Reduce the slack occurringAmpoule syringesMedical devicesCouplingInjection device
Owner:NOVO NORDISK AS
Automatic injection device
ActiveUS20100160894A1Easy to useReduce anxietyPeptide/protein ingredientsAntipyreticHypodermoclysisSubcutaneous injection
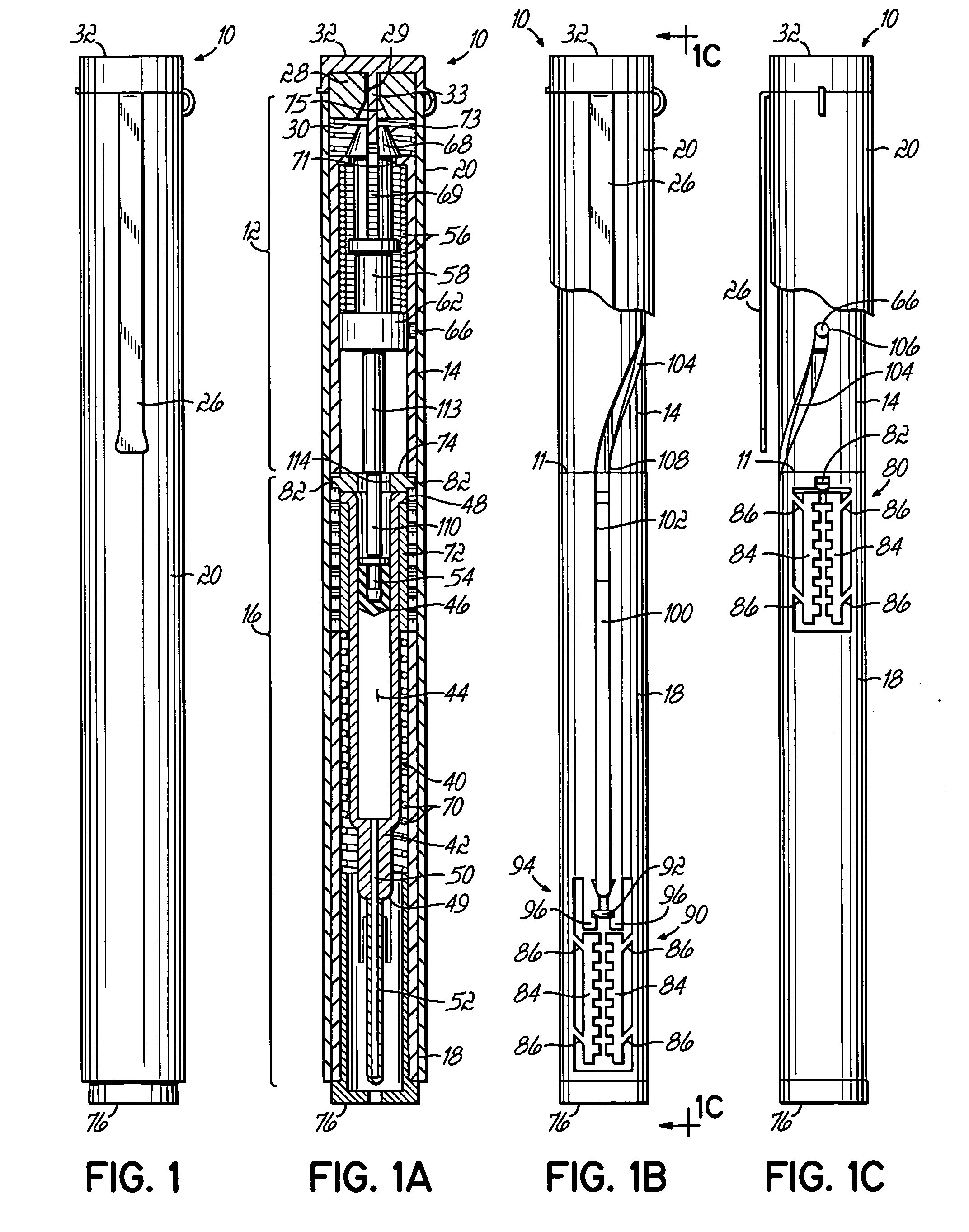
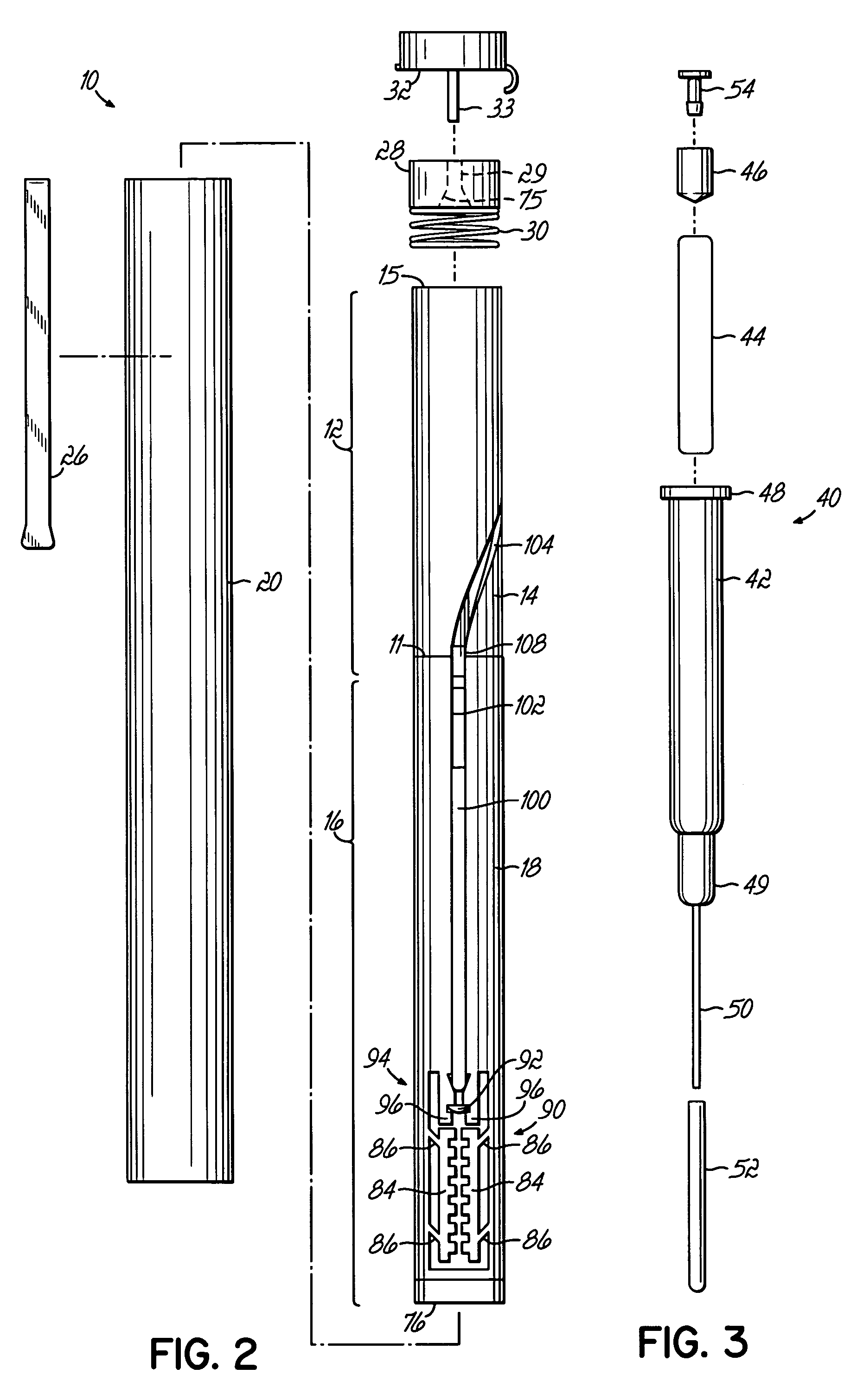
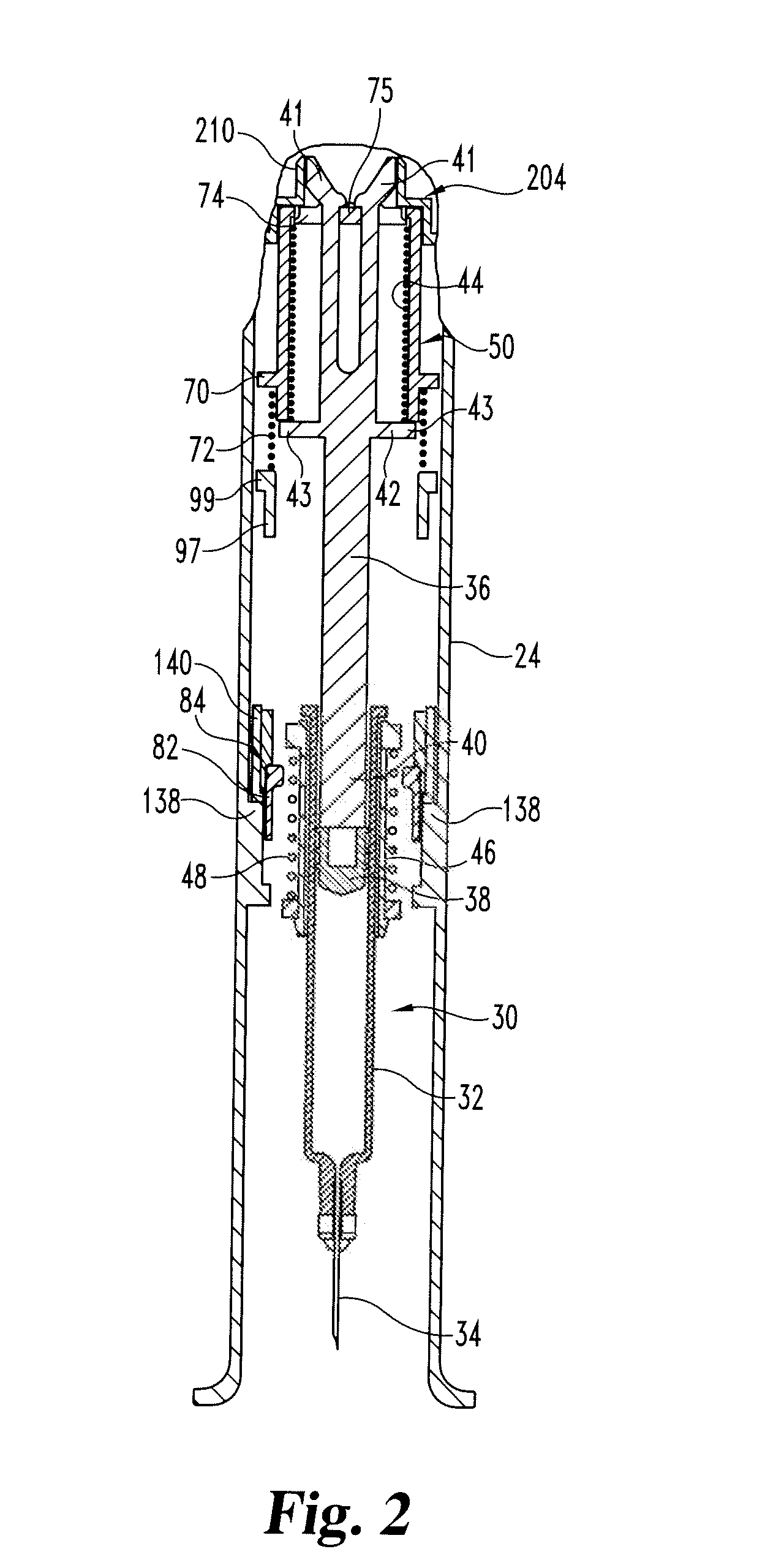
The invention provides an automatic injection device for providing a subcutaneous injection of a substance into a user, comprising: a housing having an open first end and a second end; a syringe movably disposed in the housing, the syringe including a barrel portion for holding the substance, a hollow needle in fluid communication with the barrel portion for ejecting the substance from the syringe, and a bung for sealing the barrel portion and selectively applying pressure to the substance to force the substance through the hollow needle; a plunger for first moving the syringe towards the first end such that the needle projects from the first end and subsequently applying pressure to the bung, the plunger including a rod connected at a first end to the bung, a compressible expanded central portion and a flange between a second end of the rod and the compressible expanded central portion; and a biasing mechanism for biasing the plunger towards the first open end of the housing, the biasing mechanism disposed about the second end of the rod between the flange and the second end of the housing. The present invention also provides methods and kits for using an automatic injection device, and methods and kits for promoting an automatic injection device comprising a medication based on advantageous properties of the device as compared to a pre-filled syringe. The invention also provides methods and kits for training a recipient on use of the automatic injection device.
Owner:ABBVIE BIOTECHNOLOGY LTD
Automatic injection device with reset feature
InactiveUS6899699B2Solve the lack of resistanceEasy to solveAmpoule syringesAutomatic syringesInjection deviceBiomedical engineering
The present invention relates to a dose setting and expelling device comprising a drive member and a dose setting mechanism which simultaneously sets a given dose and stores the energy necessary for a subsequently driving the drive member in order to expel a dose of medicine from an injection device. According to the invention the dose setting mechanism allows adjustment in both directions, such that a given set dose can be reduced or cancelled by reversing the input motion, typically by rotating a setting member backwardly, this in contrast to the known devices which either requires an additional release mechanism or which cannot be reversed at all.
Owner:NOVO NORDISK AS
Disposable fluid injection module
An automated injection module is comprised of a housing, a piston drug capsule disposed within the housing, a piston core including a puncture seal membrane defining a reservoir for holding a drug between the puncture seal membrane and the piston drug capsule, an injection device having at least one sharp end for puncturing the puncture seal upon activation of the device, an end cap on a distal end of the housing, and a pressure source on a proximal end of the housing. The pressure source is preferably a propellant that ignites and forces the piston toward the distal end. Substantially simultaneously, the injection device pierces the puncture seal membrane, the piston core is forced into the piston drug capsule, and the drug is evacuated from the reservoir through the injection device and into a patient.
Owner:SARCOS LC
Injection device with secondary reservoir
A method and apparatus for injecting fluid into areas having high density tissue that creates a high backpressure resistance on the injection device is disclosed. The high backpressure resistance is overcome through a mechanical advantage achieved by using a secondary reservoir having a cross-sectional area smaller than the cross-sectional area of a primary reservoir. Exemplary injection device reservoir housings may comprise a primary reservoir, a secondary reservoir, a check valve, a septum penetrating cannula, travel limits, a pen needle connecting portion, sliding seal guide ribs, a sliding seal, a pen needle assembly, a needle stop, and a patient needle.
Owner:EMBECTA CORP
Device for an injector
InactiveUS20050101919A1Improve functionalityEasy to useAutomatic syringesInfusion needlesInjection siteEngineering
The present invention relates to an injection device comprising a generally elongated tubular housing, a syringe containing medicament and having a needle, a needle shield slidably arranged to the housing and protruding a distance outside the front end of the housing, a plunger arranged to act on the syringe and a pre-tensioned drive member arranged the drive the plunger, characterized in that it comprises radially acting holding elements capable of releasably holding the plunger, axially acting actuator elements connected to the needle shield and capable of releasably locking the holding elements and axially acting activator elements capable of releasing the holding elements from the actuator elements have when the needle shield and the actuator elements has moved axially a certain distance due to that injection device has been placed against an injection site.
Owner:SHL MEDICAL
Intradermal injector
InactiveUS20050033234A1Avoid back pressureAvoid insufficient lengthAmpoule syringesAutomatic syringesInjection siteSkin contact
An injection device that comprises a chamber configured for containing a substance to be injected and a needle operatively associated with the chamber and having a length sufficient to deliver the substance to an intradermal injection site. A collar surrounds the needle, defining a collar cavity. The collar also has a peripheral forward skin-contacting surface that surrounds and is radially spaced from the needle and injection site by an area that is sufficiently large to allow a patient's skin to move into the collar cavity to properly position the needle for intradermal delivery of the substance to the injection site to allow spread of the injected substance under the skin while inhibiting or preventing backpressure within the skin from forcing the substance out through the injection site.
Owner:ANTARES PHARMA
Secure, networked and wireless access, storage and retrival system and method utilizing tags and modular nodes
InactiveUS20050062603A1Quick and seamless handlingHigh levelIndividual entry/exit registersElectric signalling detailsComputer hardwareMedical equipment
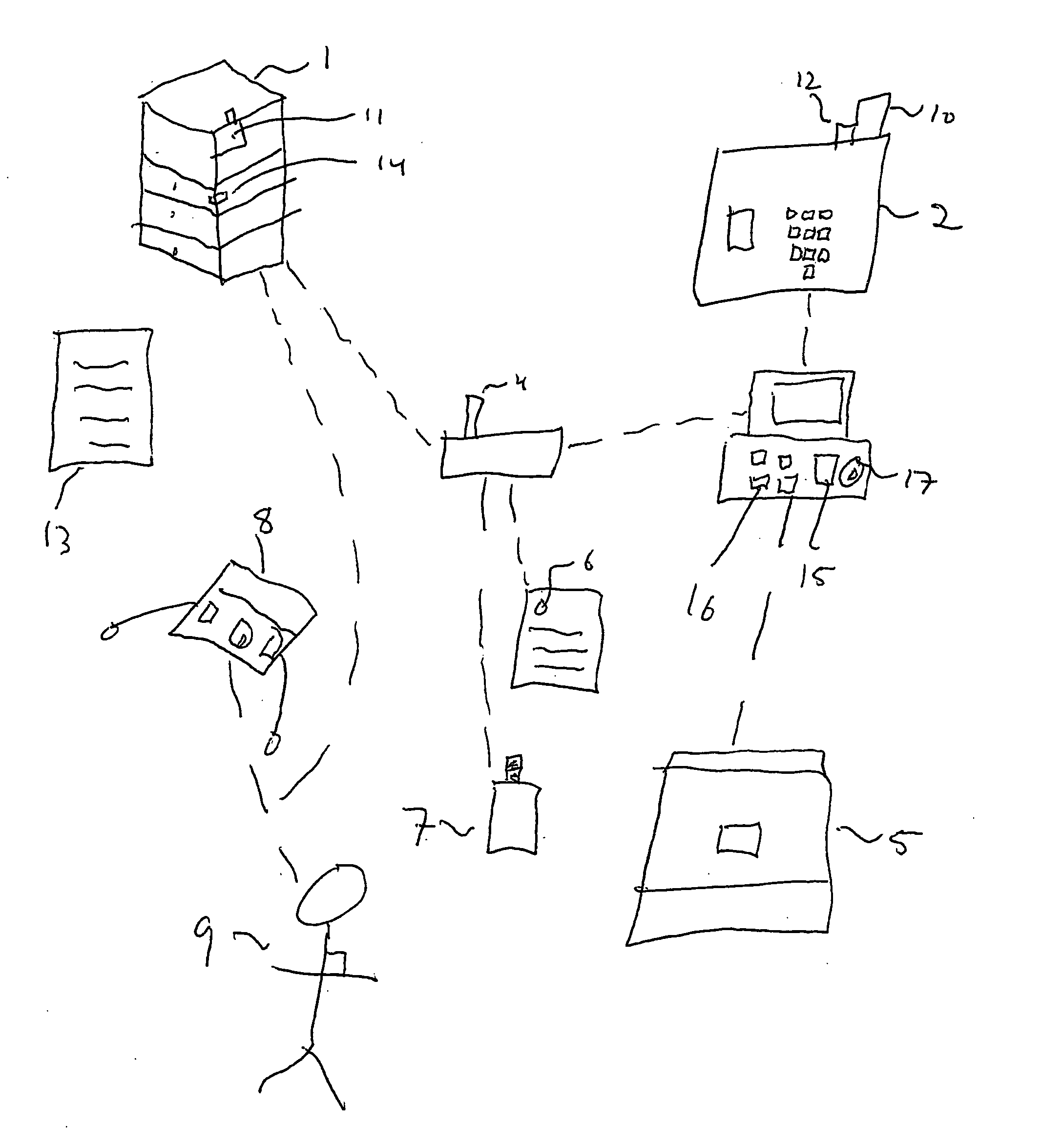
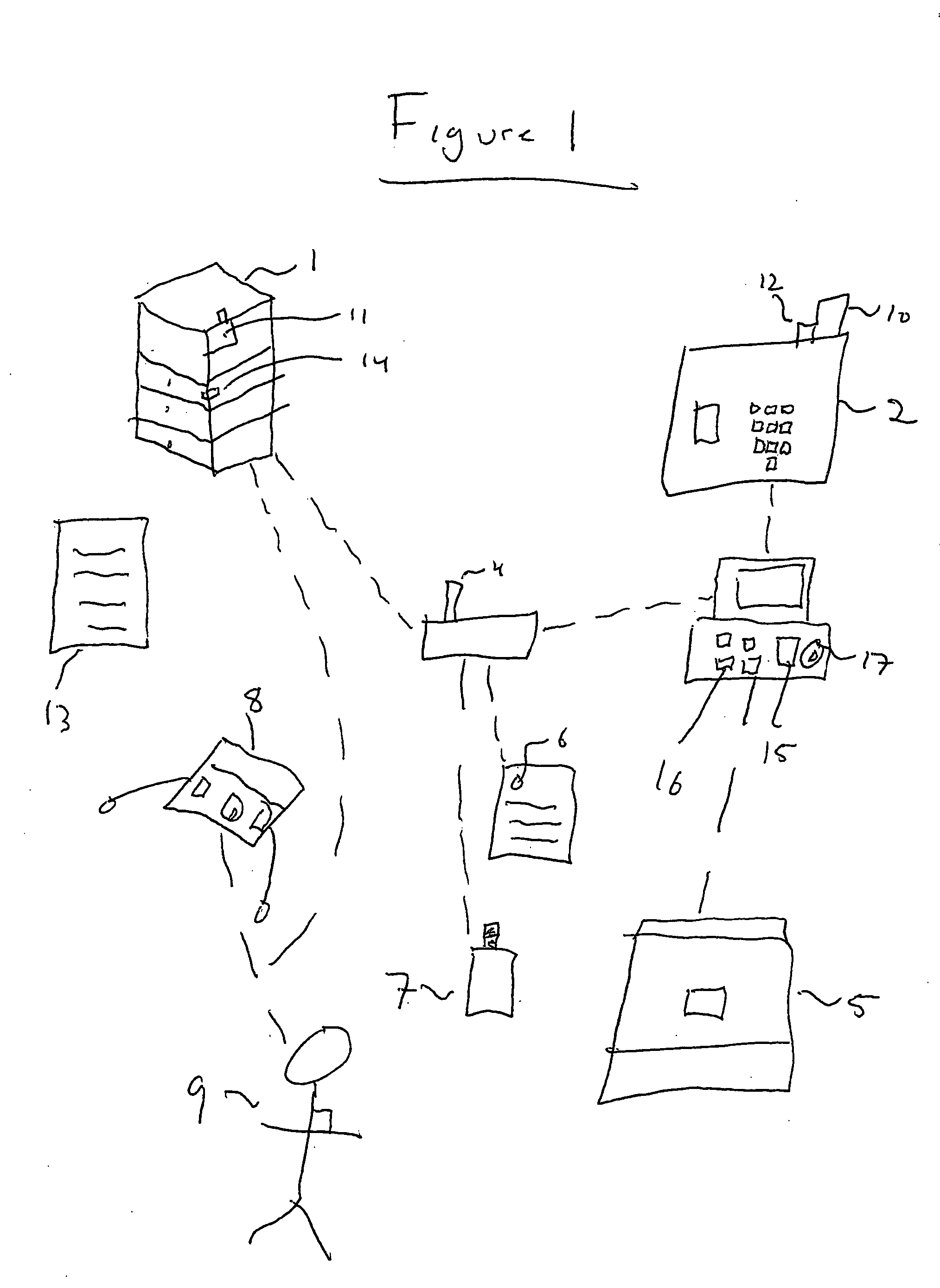
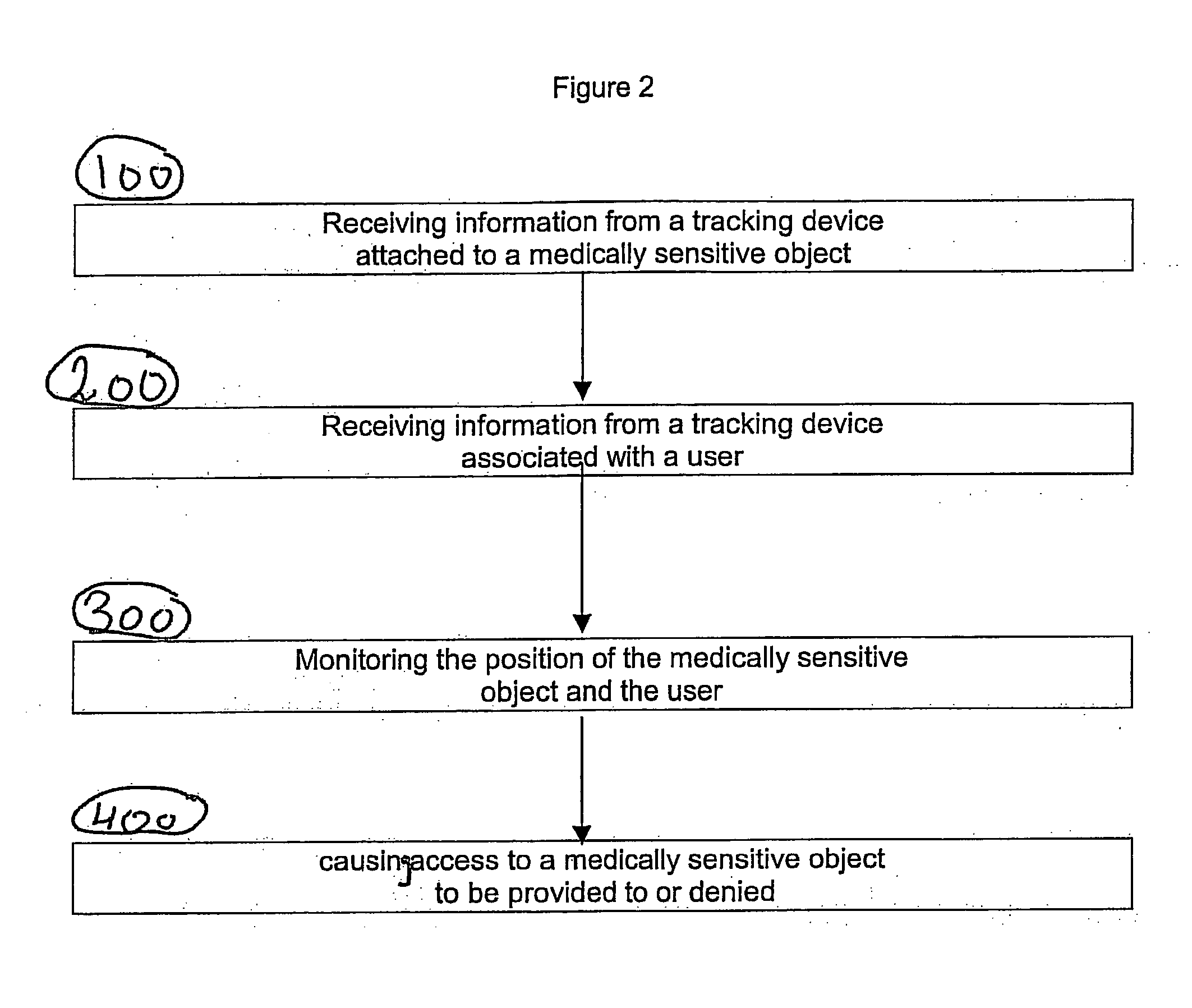
A system apparatus and method of monitoring in a secured fashion the access, storage and retrieval of information, using a networked modular wireless device. The system may include a network of wireless, Wi-Fi devices (or any other wireless communication mechanism such as GPRS, GSM, iDen), or Nodes, each one of them possibly controlling the access to a medically sensitive object, such as a drawer (or cabinet) or to a medical device, or to another information source, item of equipment, drug, etc as well as tracking via RFid readers the access to the records or information contained in it. In the case of a physical file, each file has an RFid tag on it that is being read when removed or returned to the cabinet. Access to the cabinet and physical records, or to the medical device is monitored by reading the RFiD identity card of personnel accessing the cabinet or medical devices. In addition to controlling the access to the cabinet or medical device by controlling the cabinet lock (or in the case of a small medical device, the lock of an IV, injection device, specimen collection unit, or of a large medical device such as a defibrillator), the node can alert electronically by sending a message to the controlling unit, or by sending a physical alert (such as an alarm signal), when unauthorized personnel is attempting to access the cabinet, the files or devices. The system is useful in the context of monitoring the information contained in physical files, such as medical information, and can be used for access to medical devices, in order to better monitor the authorization rights of personnel participating in processes such as drug delivery or specimen collection. A control unit monitors activity at a plurality of nodes, and assists in storing the list of authorized personnel and files, and can store electronically captured information regarding the physical files (for example, the reason for accessing the file and reasons for changes in it) or medical device. The system can communicate with other information management systems.
Owner:G D H
Injecting device
ActiveUS20050261634A1Efficient use ofMinimum of operating stepAmpoule syringesAutomatic syringesEngineeringBiological activation
An injection device having an elongated body, including a container with medicament, elements for connecting a needle to the container, actuating elements capable of injecting a dose of medicament upon activation, activating elements capable of activating the actuating elements, a needle shield arranged to the body and slidable between an extended and a retracted position in relation to the body. The needle shield is designed and arranged such that, upon penetration of the needle into a patient, when moved to its retracted position, acts on the activating elements, which in turn activates the actuating elements and injects a dose.
Owner:SHL MEDICAL AG
Tissue augmentation methods using a medical injection apparatus
InactiveUS20070078435A1Simple processReduce effortInfusion syringesMedical devicesHand heldInjection device
An injection apparatus that includes components that facilitate injection of relatively viscous materials into a subject is provided as well as methods of use. An injection apparatus may include a transition-bore needle apparatus, which has a proximal end, a distal end, and a lumen extending from the proximal end to the distal end, in which the diameter of the proximal end is greater than the diameter of the distal end. An injection apparatus may include a hand-held injection facilitation apparatus, which may be coupled to a syringe. The hand-held injection facilitation apparatus can include a pivot arm and a body with a rod disposed within the body and coupled to the pivot arm. Movement of the pivot arm results in a proximal or distal movement of the rod within the body to effectively cause material to be expelled from the syringe. An injection apparatus may include a transition-bore needle apparatus and a hand-held injection facilitation apparatus in combination.
Owner:ARTES MEDICAL USA
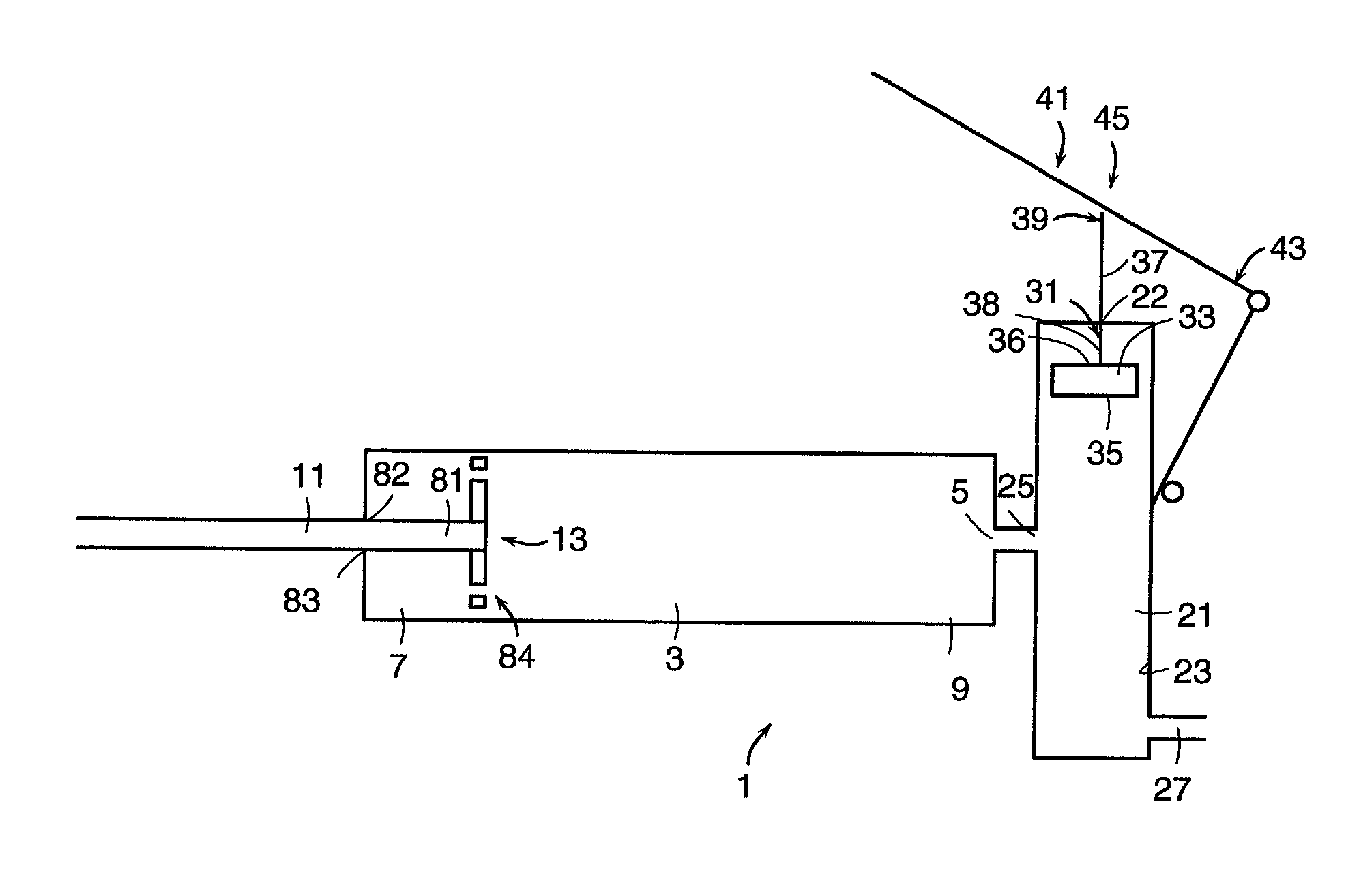
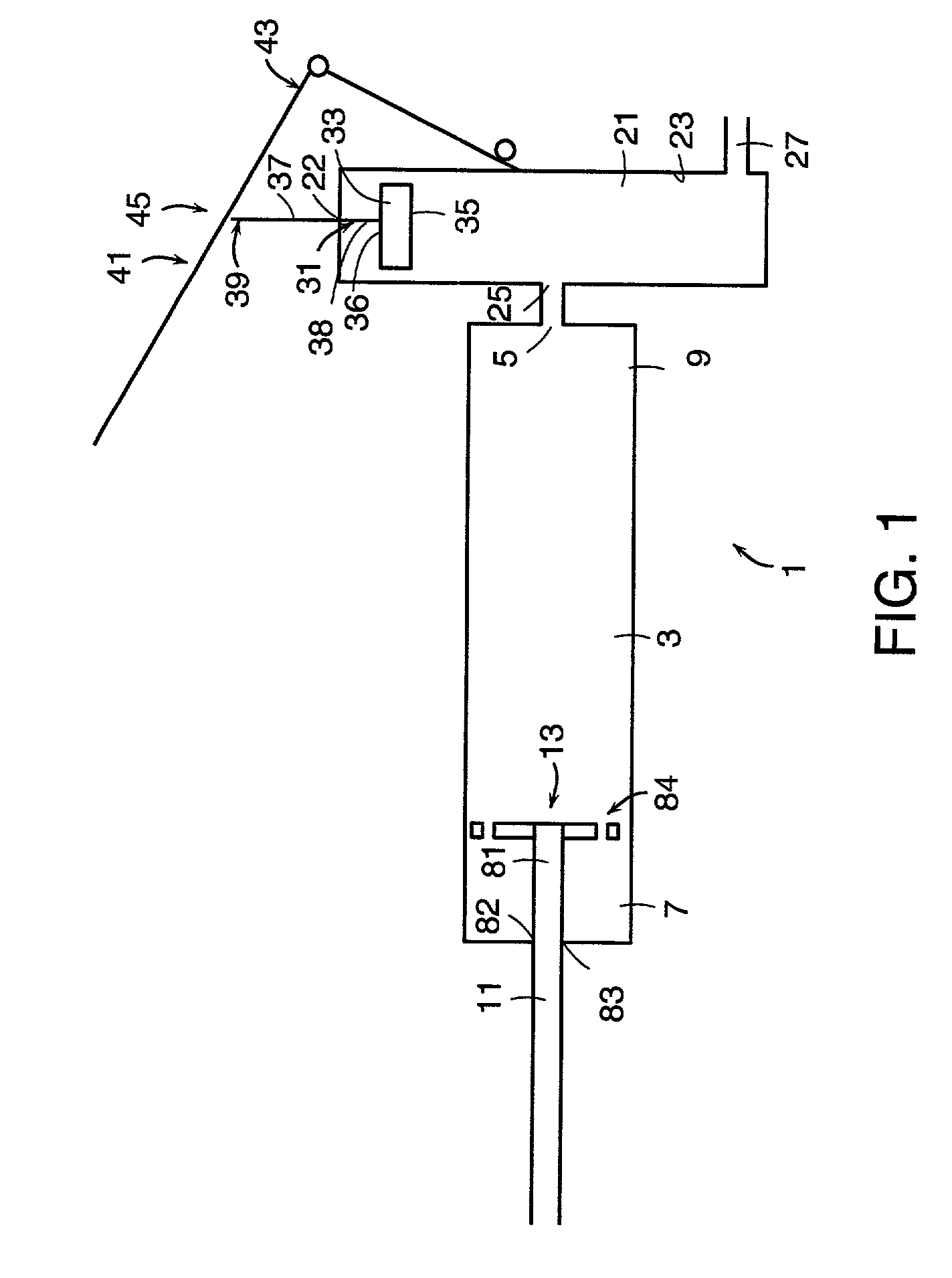
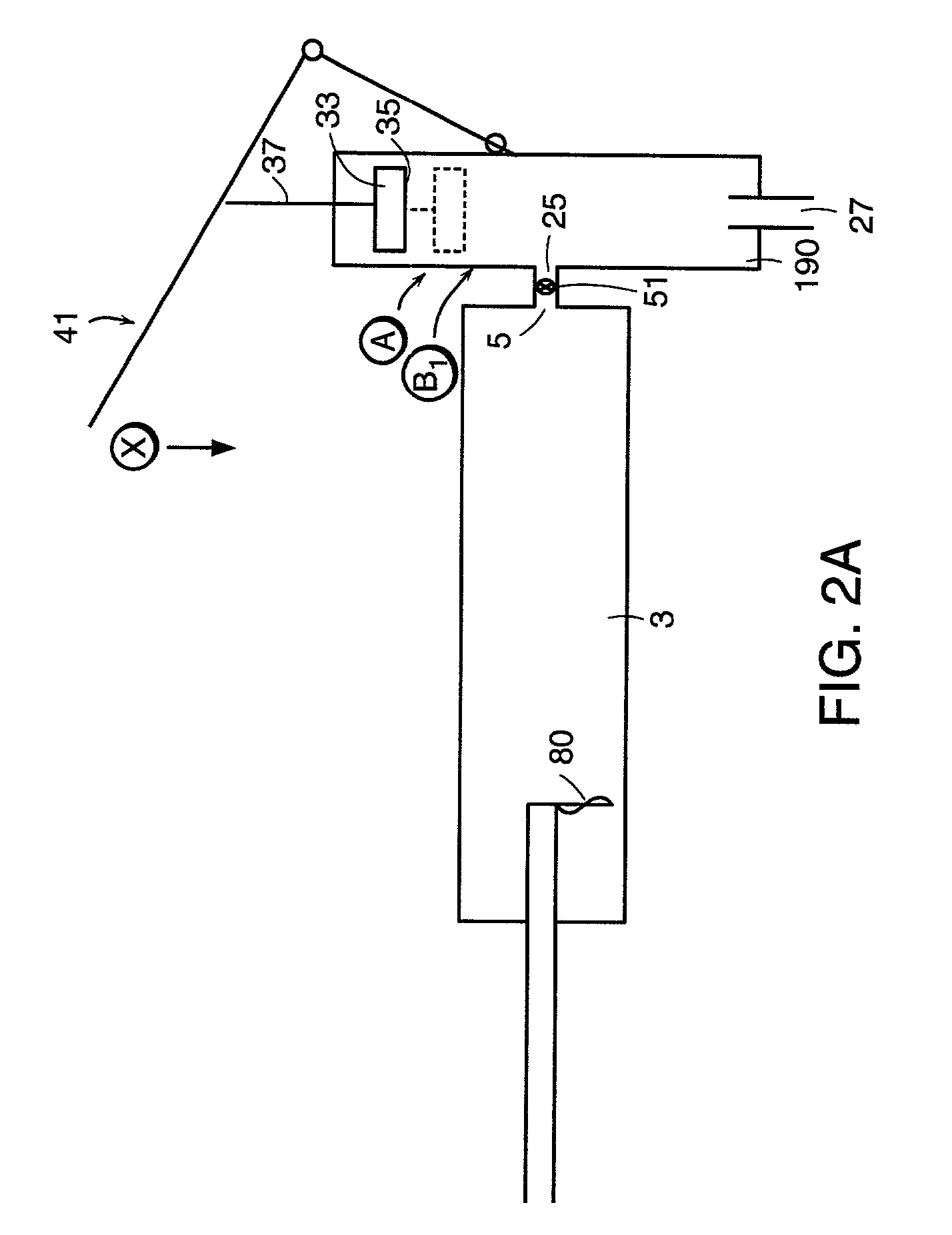
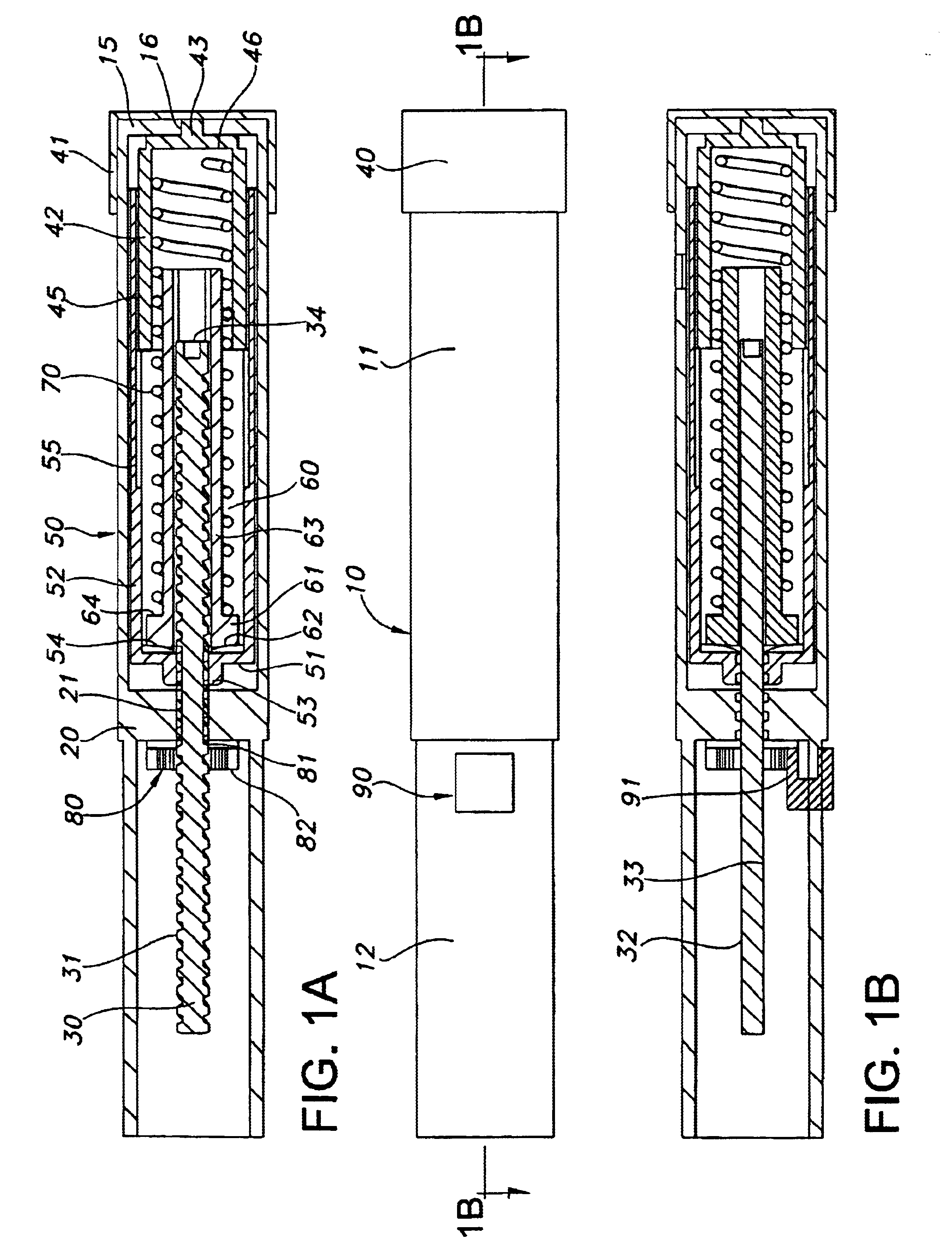
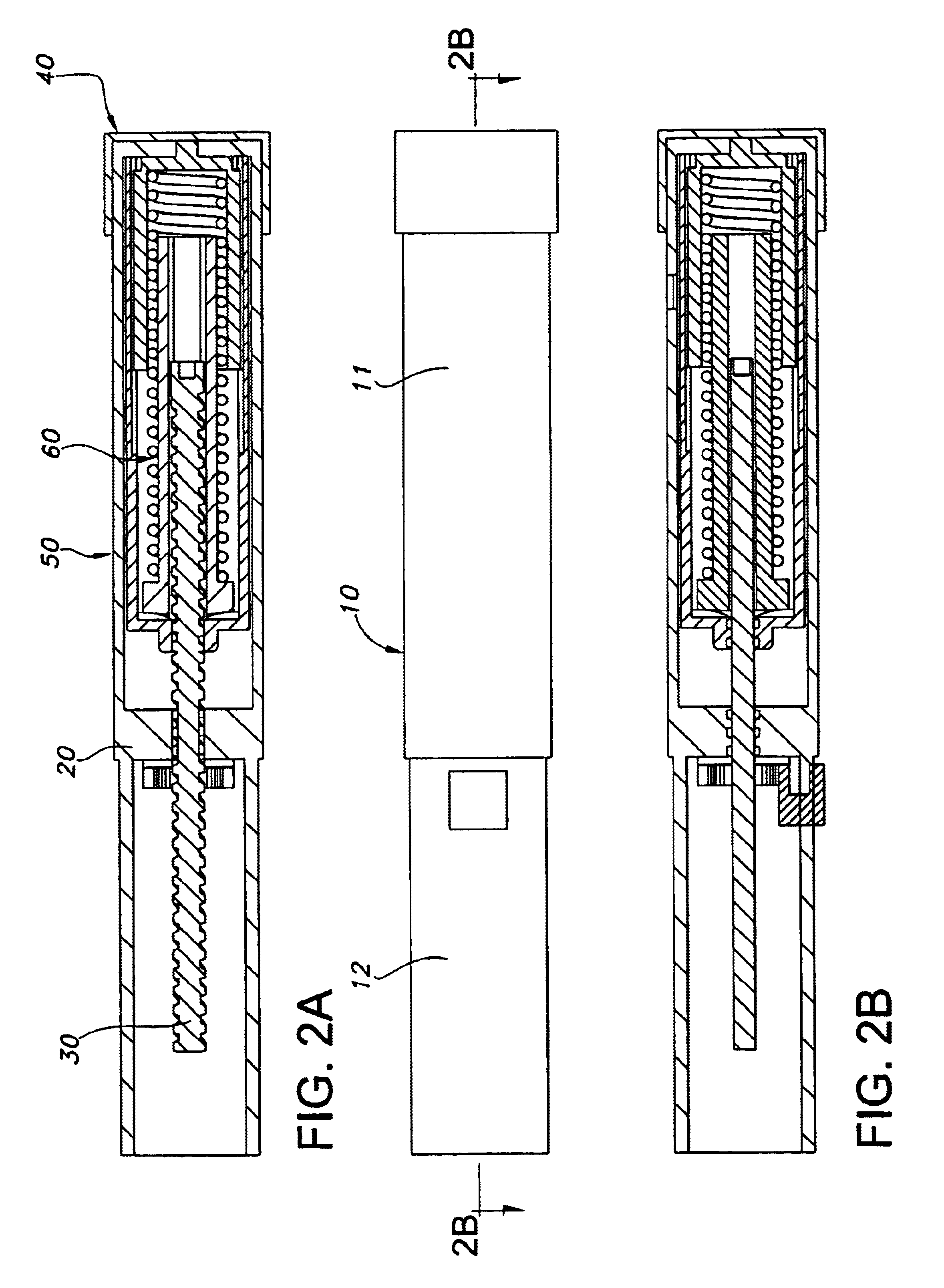
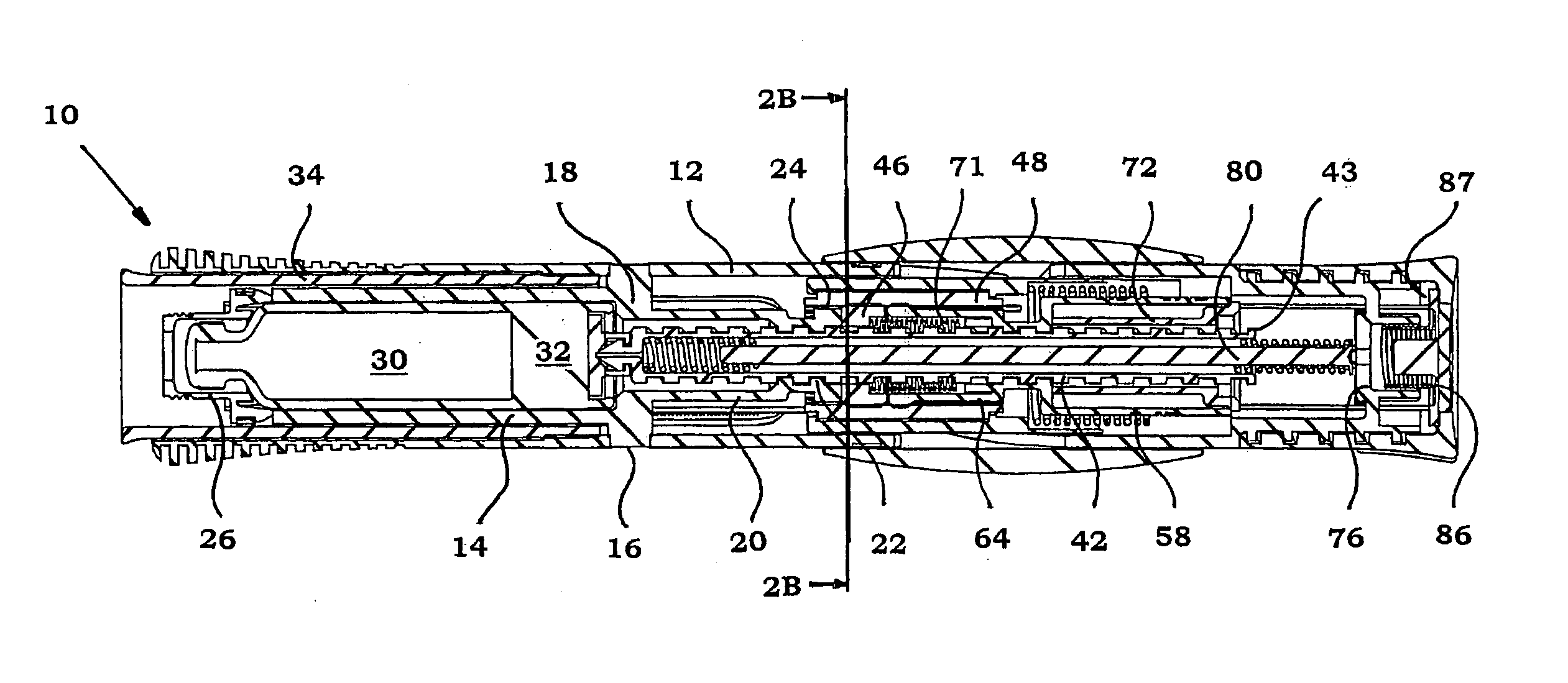
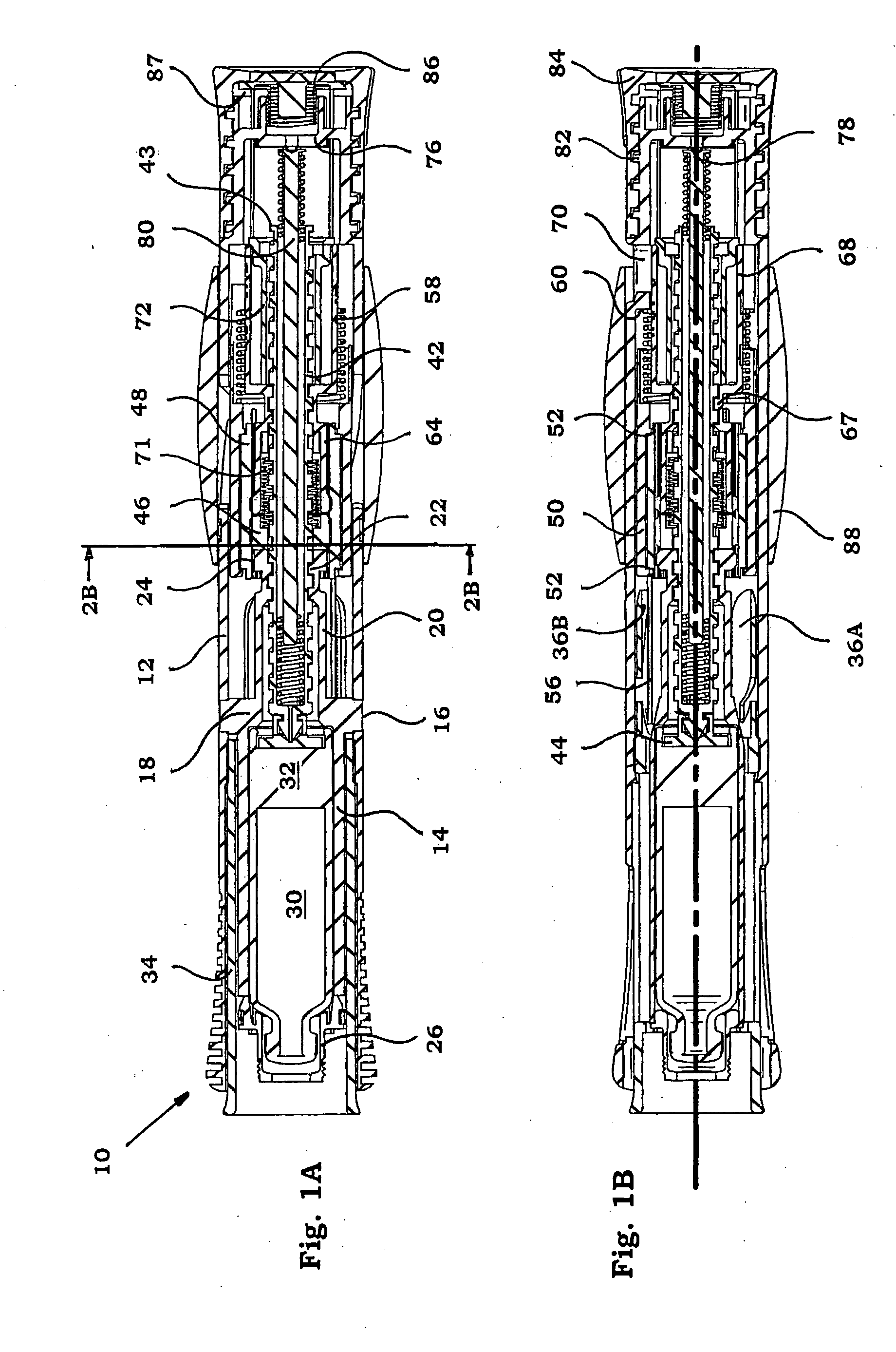
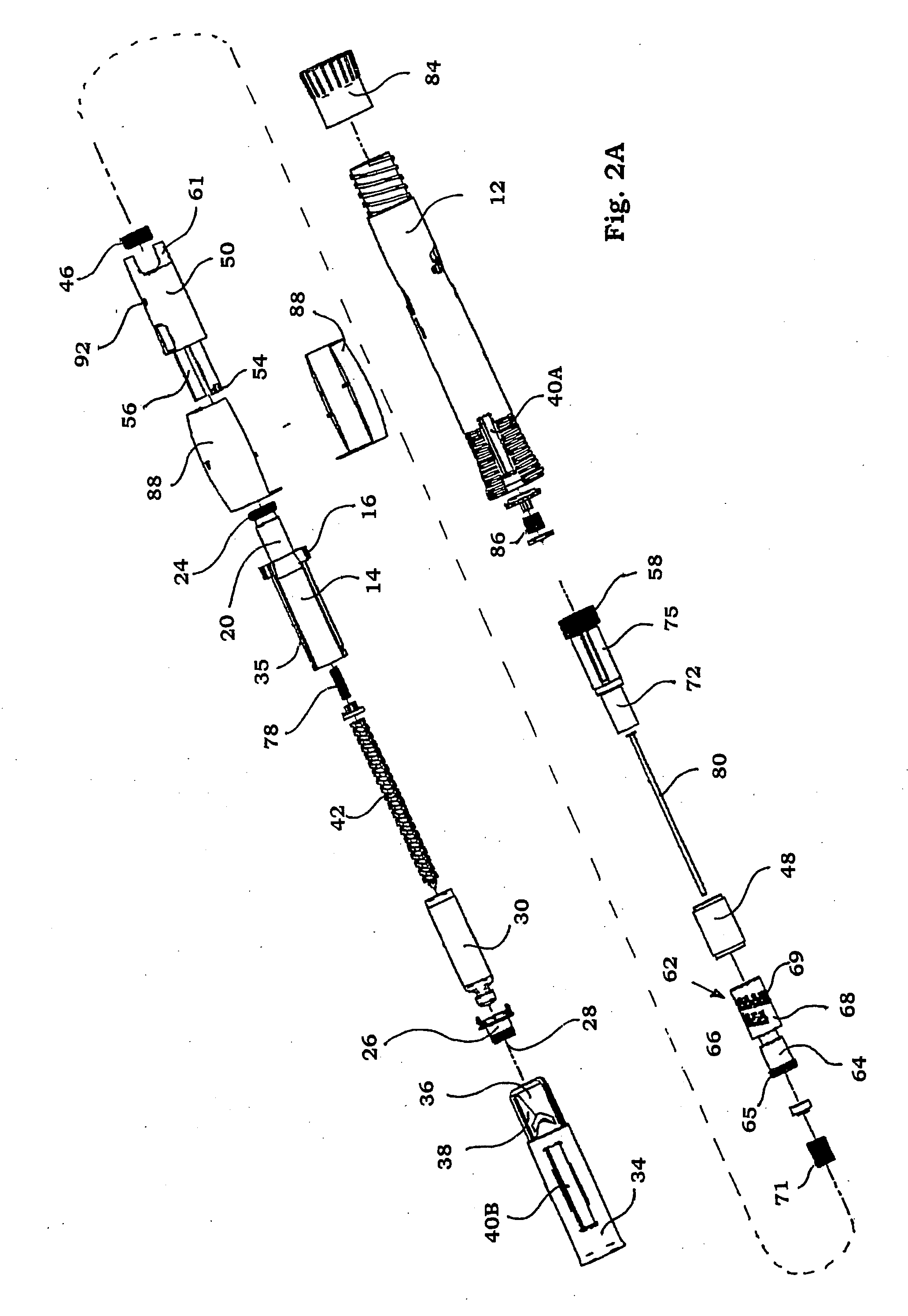
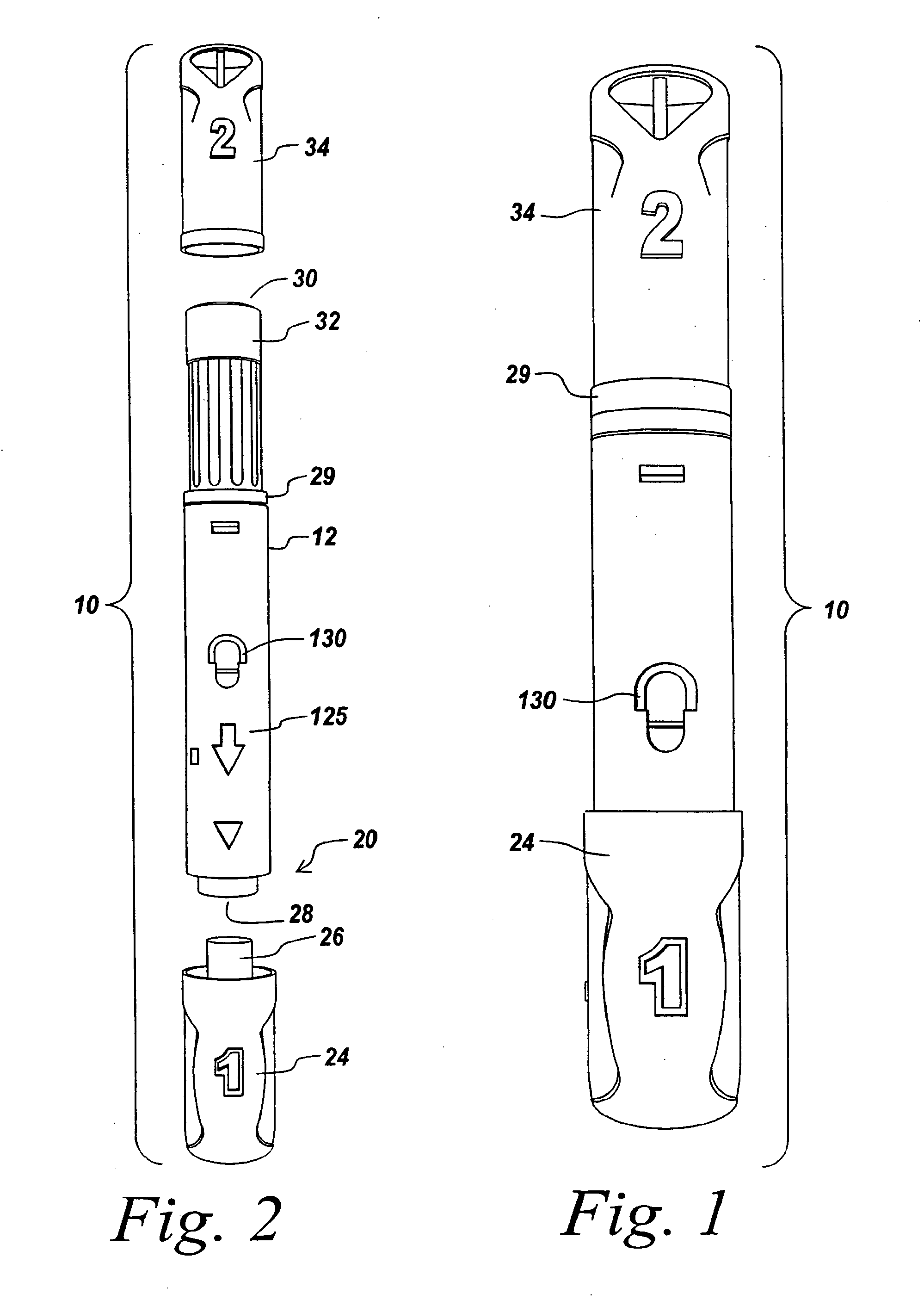
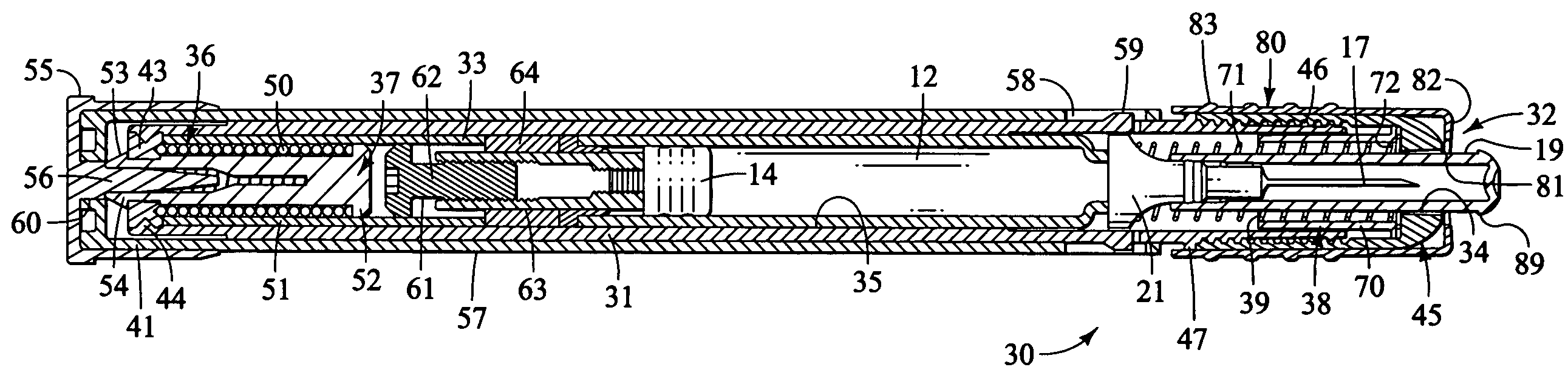
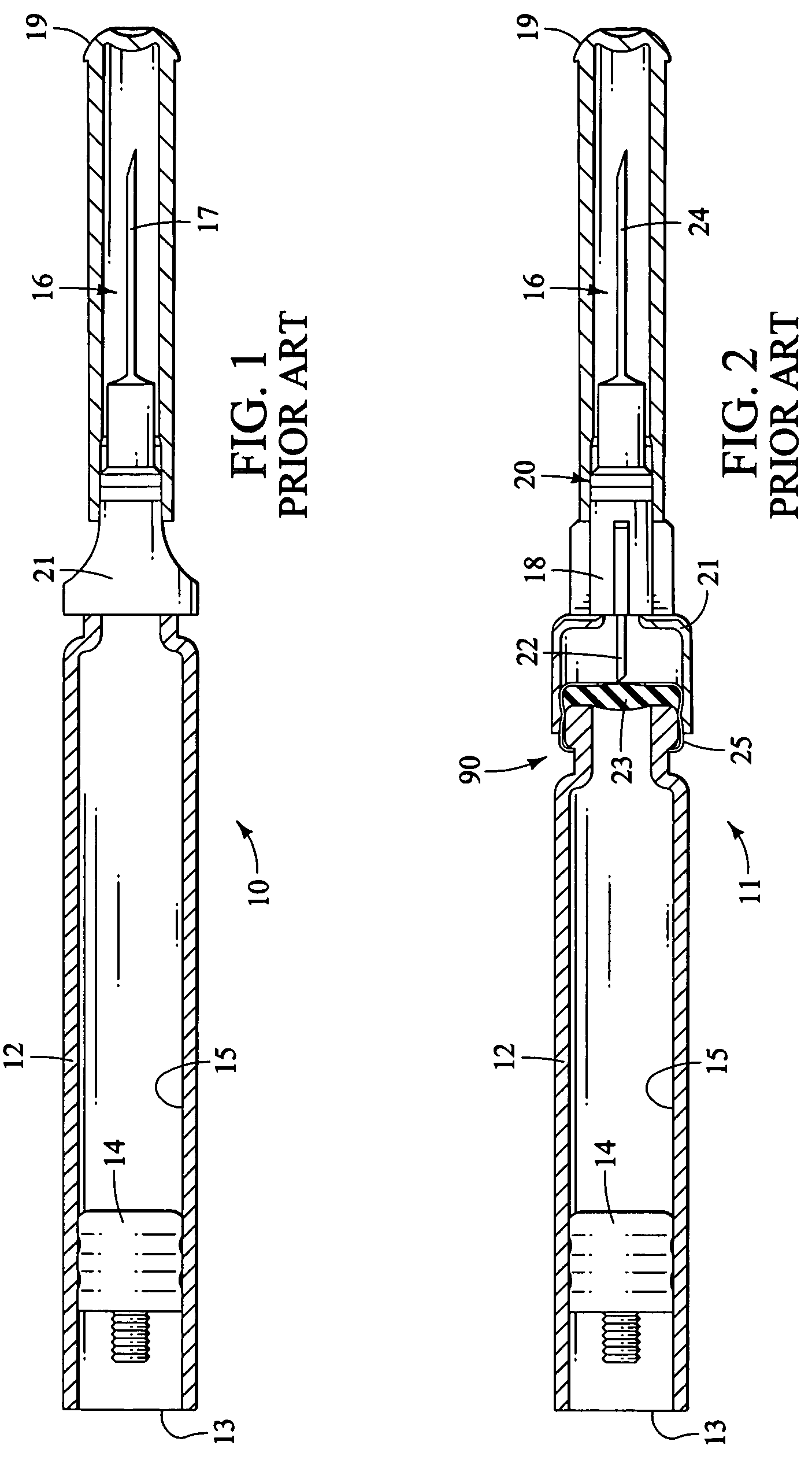
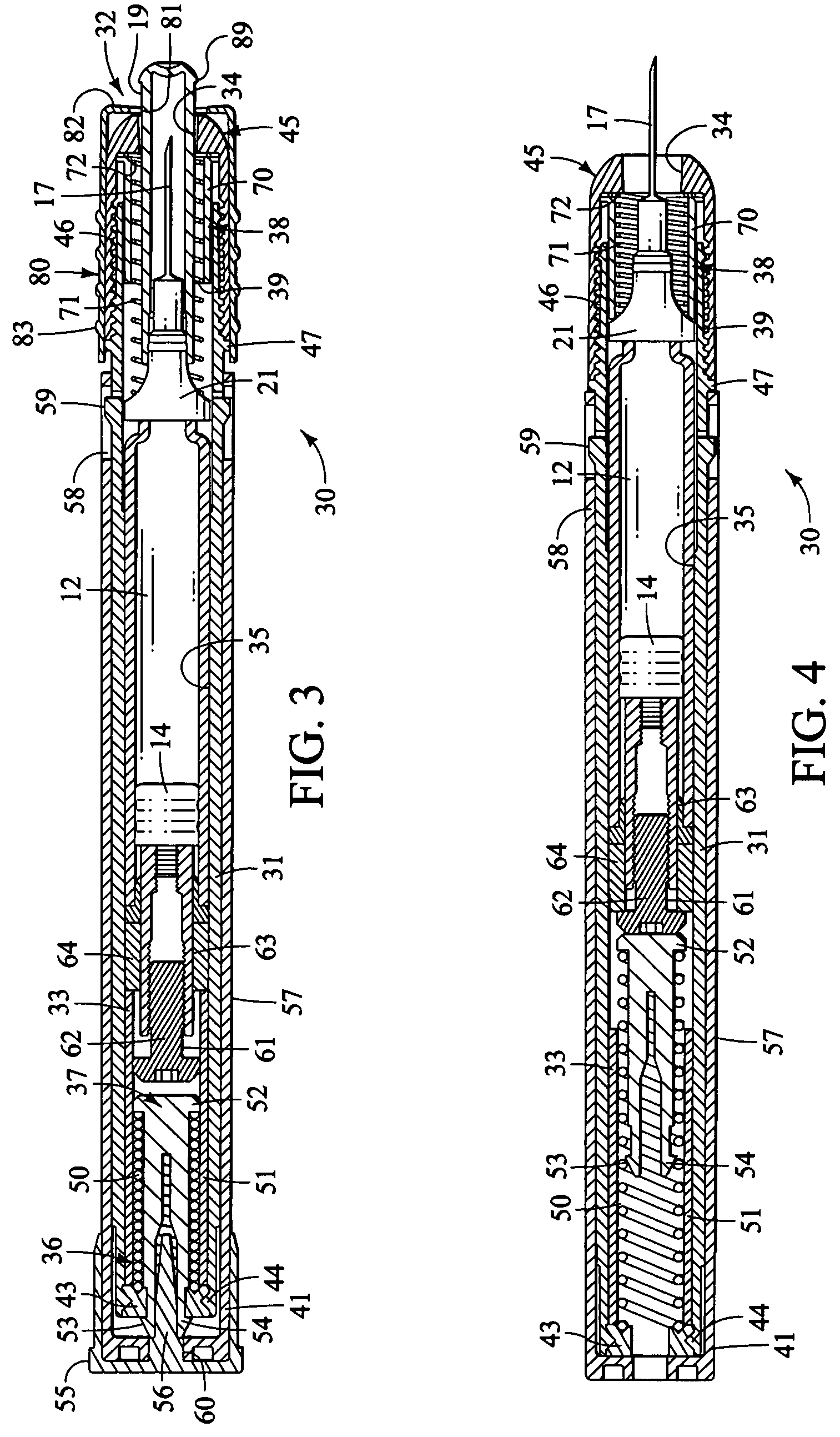
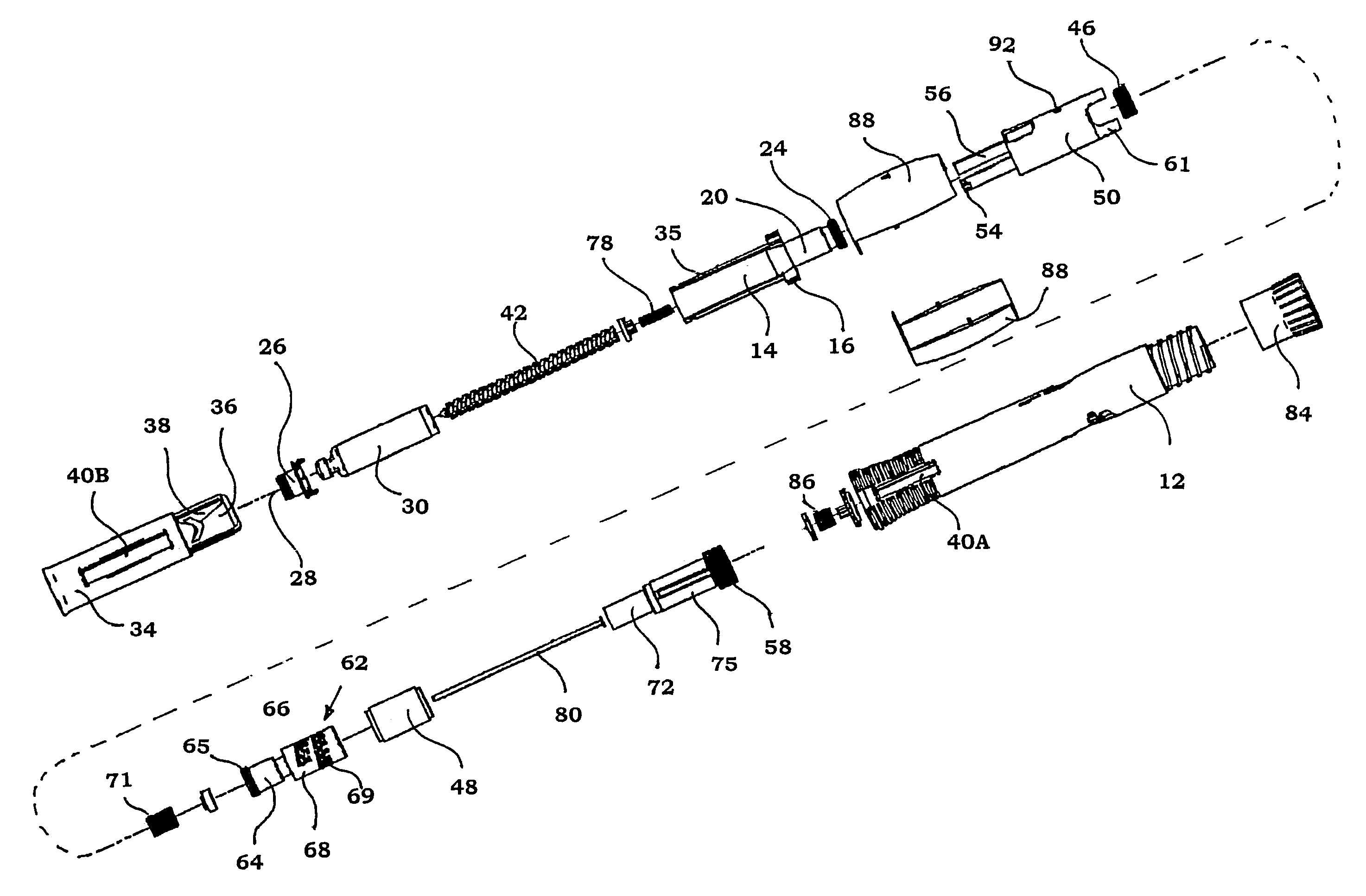
Injection device
InactiveUS6454743B1Simplify mechanical designMinimal requirementAutomatic syringesMedical devicesEngineeringBiomedical engineering
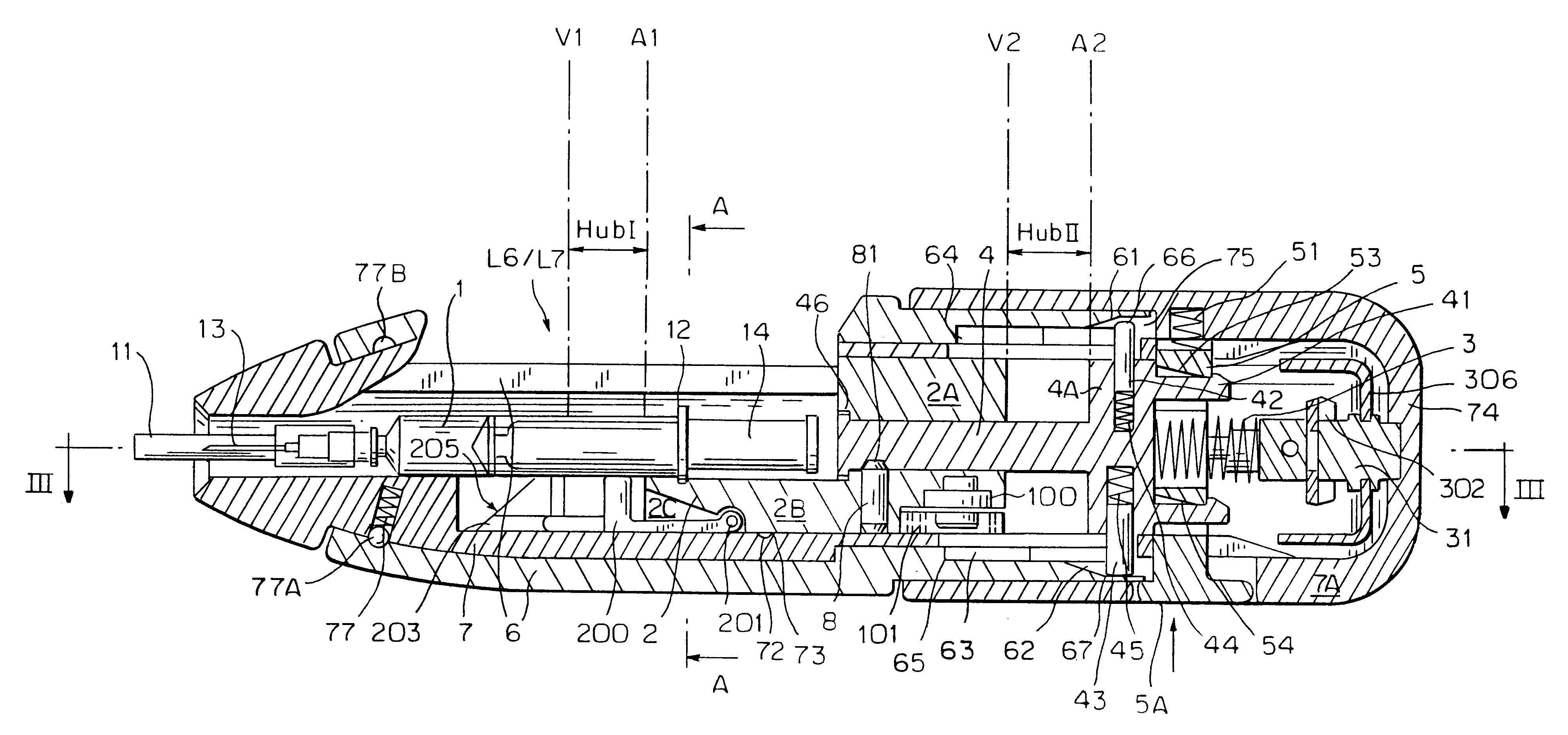
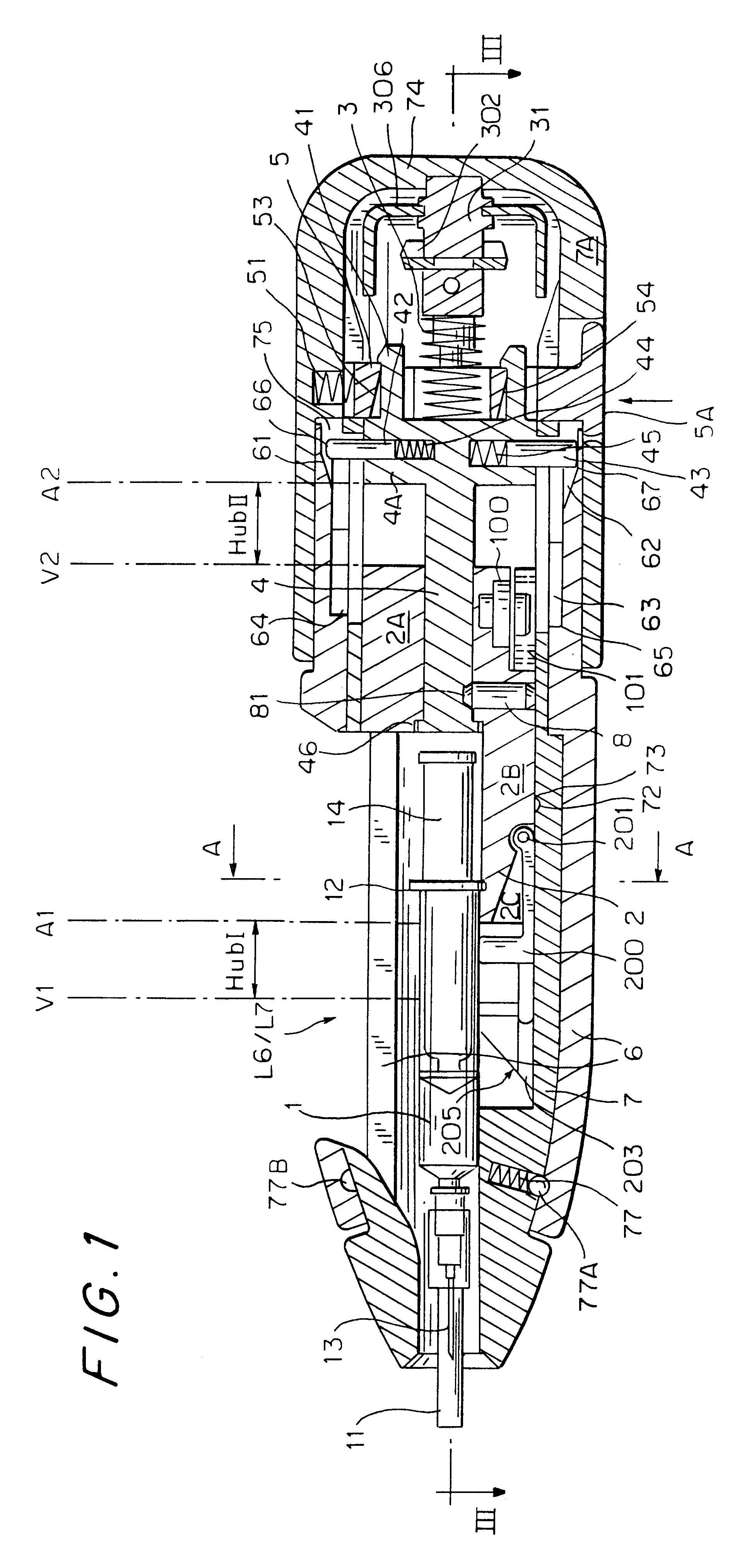
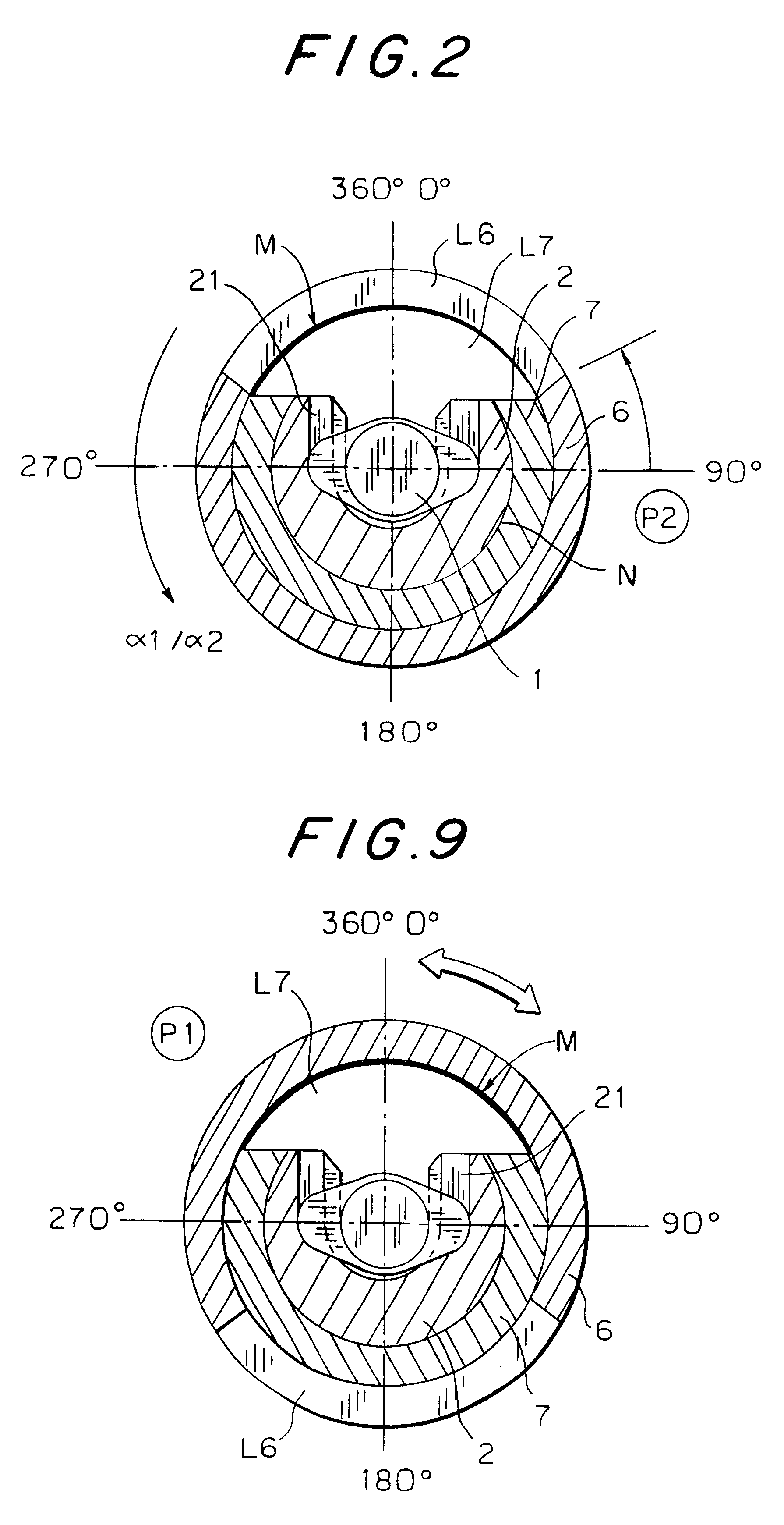
An injection device that is used with a syringe, wherein the injection needle of the syringe is initially introduced into the skin and the injection fluid is injected afterwards. The injection device is essentially driven and controlled by a control sleeve (6) which can be displaced and / or rotated in relation to the housing (7) and which can be moved between a closing and functional position (P1) and an open and safety position (P2). In the closing and functional position, the control sleeve prevents access to the syringe and activates a release device for the injection process. In the open and safety position, a syringe (1) can be removed or inserted. A plurality of components carrying out the injection process (a slide (2) in which the syringe (1) is placed and a plunger (4) that impinges upon the syringe piston) are moved or controlled depending on the movement and position of the control sleeve or supported (for example, an ejection device for the syringe or a signaling device informing that injection has been completed). The device enables full-automatic injection that can be reliably carried out by patients themselves with few handling procedures.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Automatic injection device
An injector device comprising a body containing a syringe with a needle and plunger, a drive spring coupled with the syringe and operable, when released, to drive the syringe forward to inject the needle and subsequently to dispense a dosage from the syringe, a housing containing the body and drive spring, and a release apparatus coupled with the housing. The drive spring is initially locked in an unreleased position, and the body is slidable with respect to the housing and configured for sliding upward in the housing when the injector device is pressed down at an injection site to engage the release apparatus and release the drive spring for delivering a dosage.
Owner:PA2008
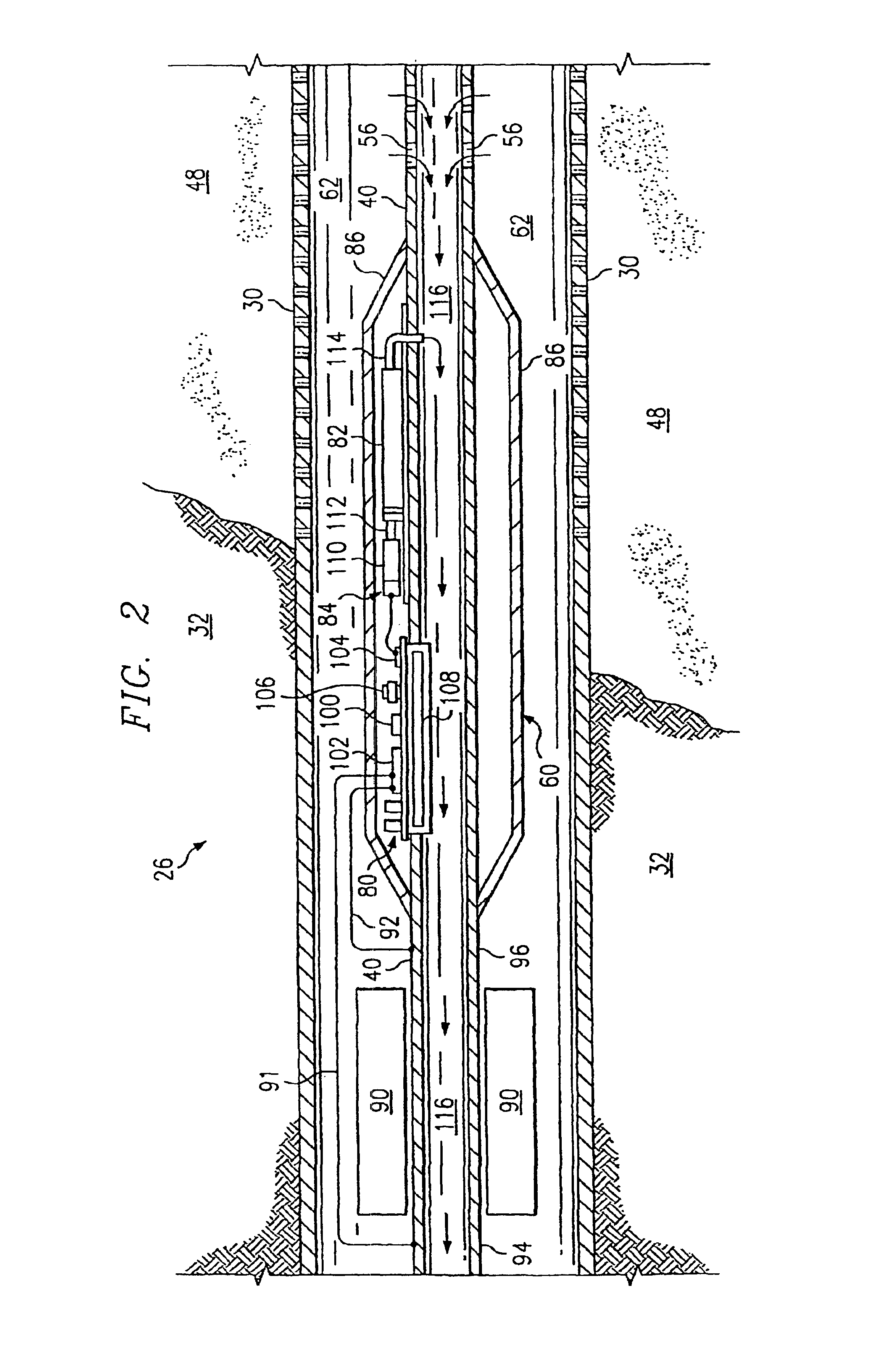
Controlled downhole chemical injection
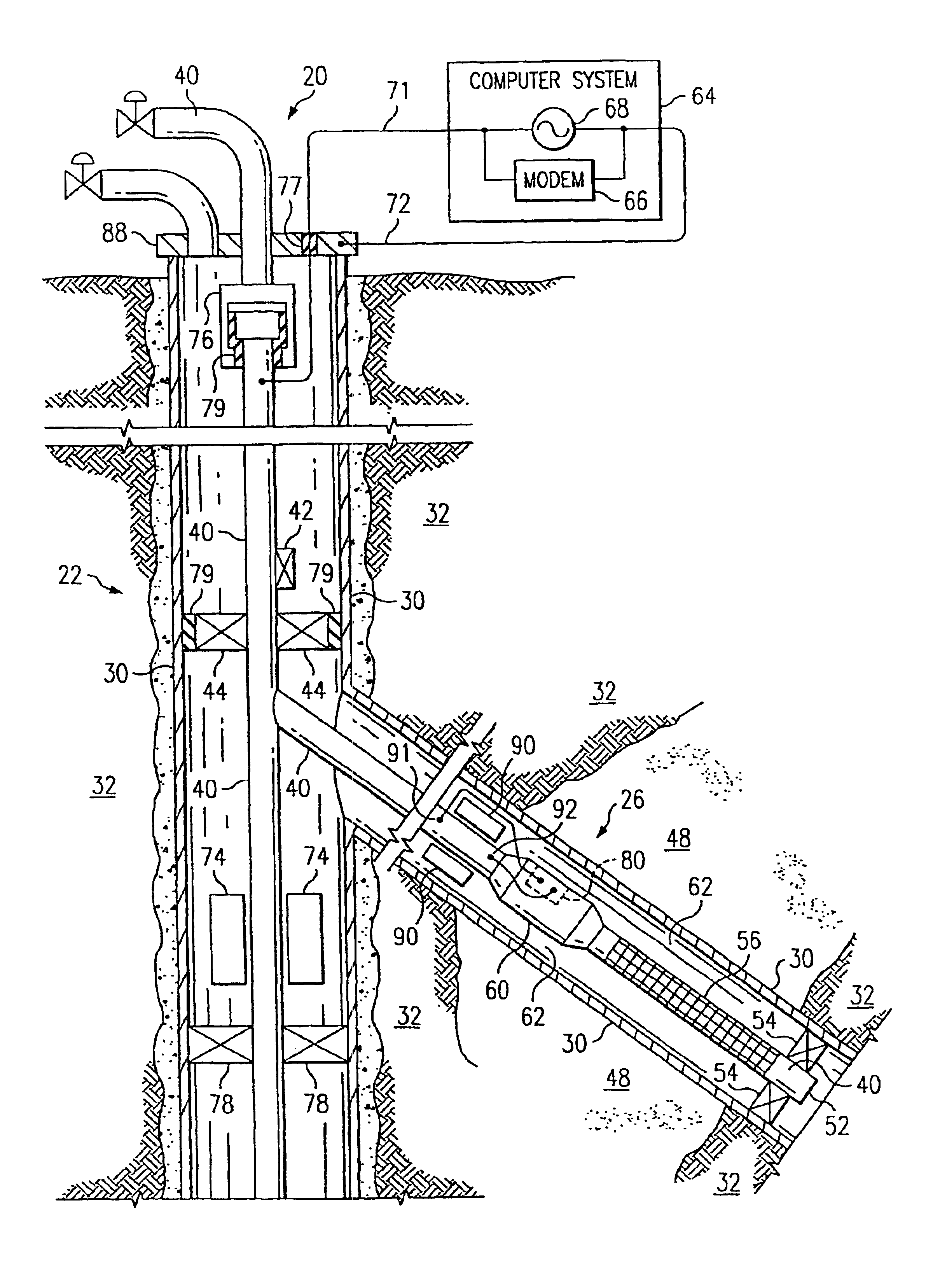
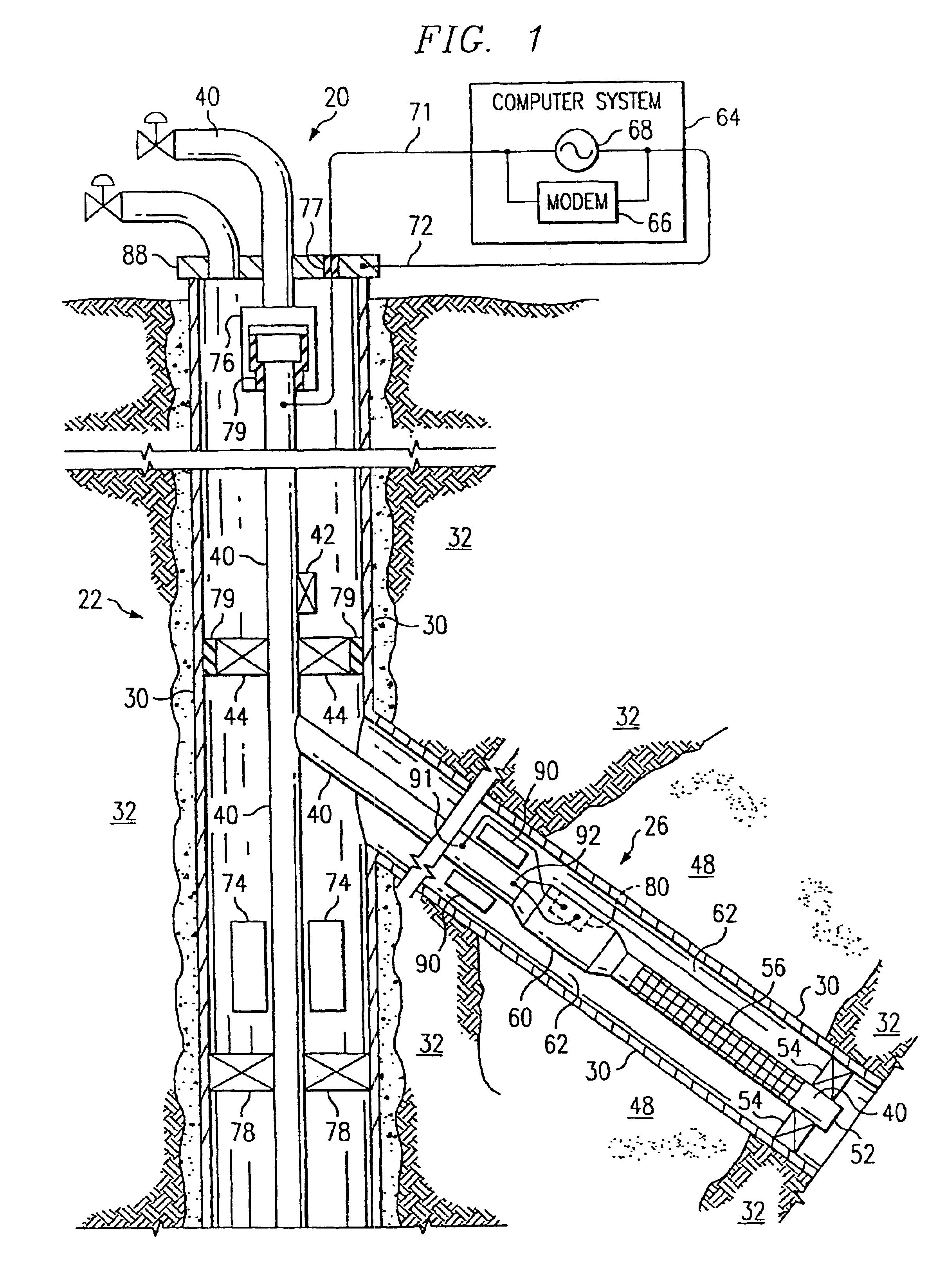
InactiveUS6981553B2Improve efficiencyImprove flow characteristicsNear-field transmissionSurveyElectrical conductorPetroleum
A petroleum well having a well casing, a production tubing, a source of time-varying current, a downhole chemical injection device, and a downhole induction choke. The casing extends within a wellbore of the well. The tubing extends within the casing. The current source is located at the surface. The current source is electrically connected to, and adapted to output a time-varying current into, the tubing and / or the casing, which act as electrical conductors for providing downhole power and / or communications. The injection device having a communications and control module, a chemical container, and an electrically controllable chemical injector. The communications and control module is electrically connected to the tubing and / or the casing. The chemical injector is electrically connected to the communications and control module, and is in fluid communication with the chemical container. The downhole induction choke is located about a portion of the tubing and / or the casing. The chemical injector is electrically connected to the communications and control module, and is in fluid communication with the chemical container. The downhole induction choke is located about a portion of the tubing and / or the casing. The induction choke is adapted to route part of the electrical current through the communications and control module by creating a voltage potential between one side of the induction choke and another side of the induction choke. The communications and control module is electrically connected across the voltage potential. Also, a method is provided for controllably injecting a chemical into the well downhole, which may be used to: improve lift efficiency with a foaming agent, prevent deposition of solids with a paraffin solvent, improve a flow characteristic of the flow stream with a surfactant, prevent corrosion with a corrosion inhibitor, and / or prevent scaling with scale preventers.
Owner:SHELL OIL CO
Automatic injection device
ActiveUS20120107783A1Easy to useReduce anxietyAntipyreticAutomatic syringesSubcutaneous injectionInjection device
Owner:ABBVIE BIOTECHNOLOGY LTD
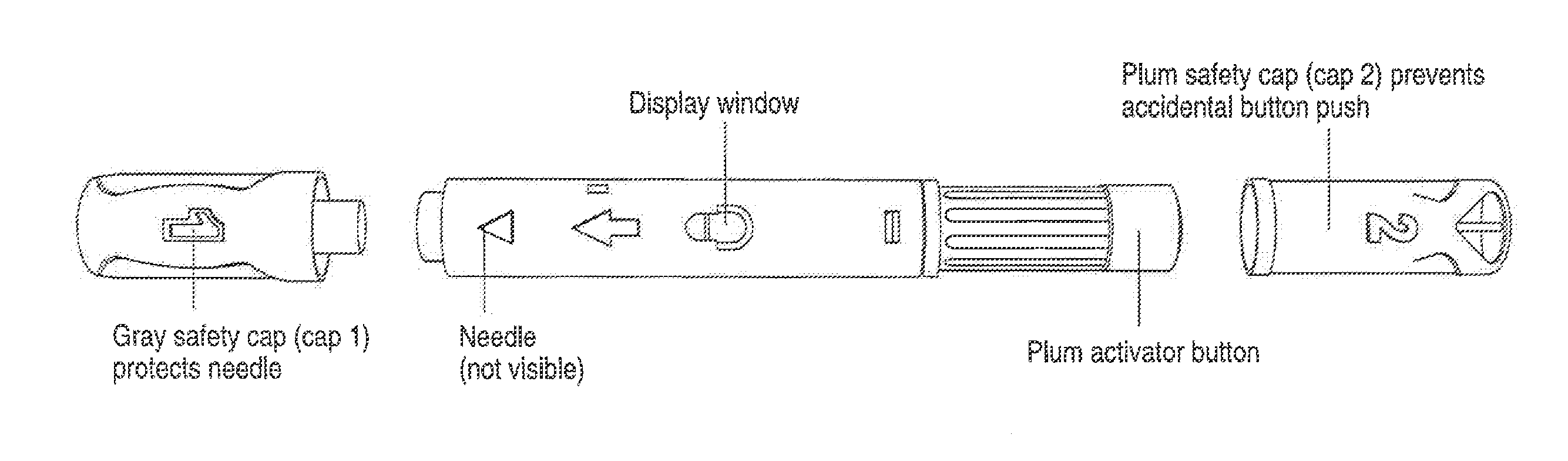
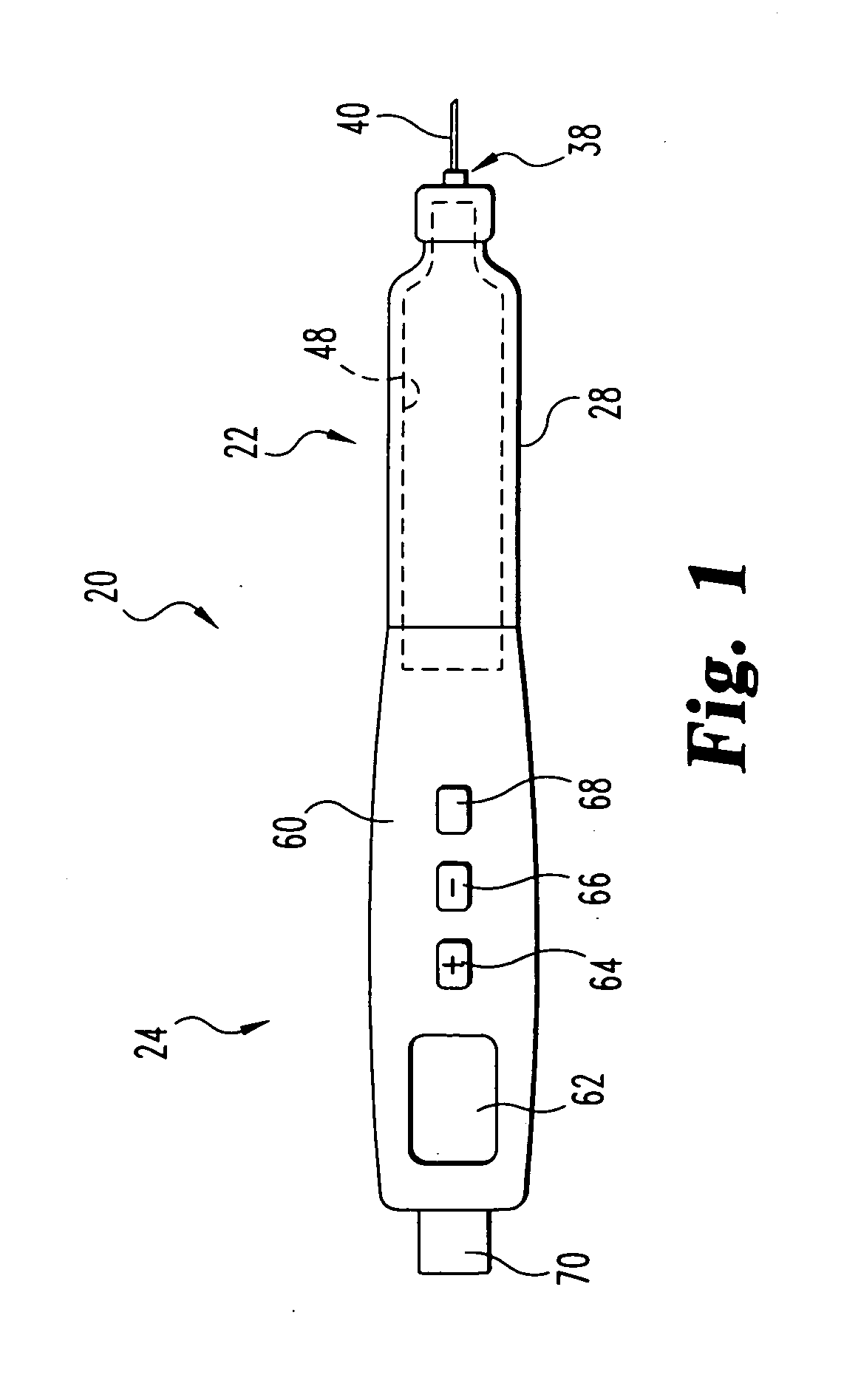
Hand-held electronically controlled injection device for injecting liquid medications
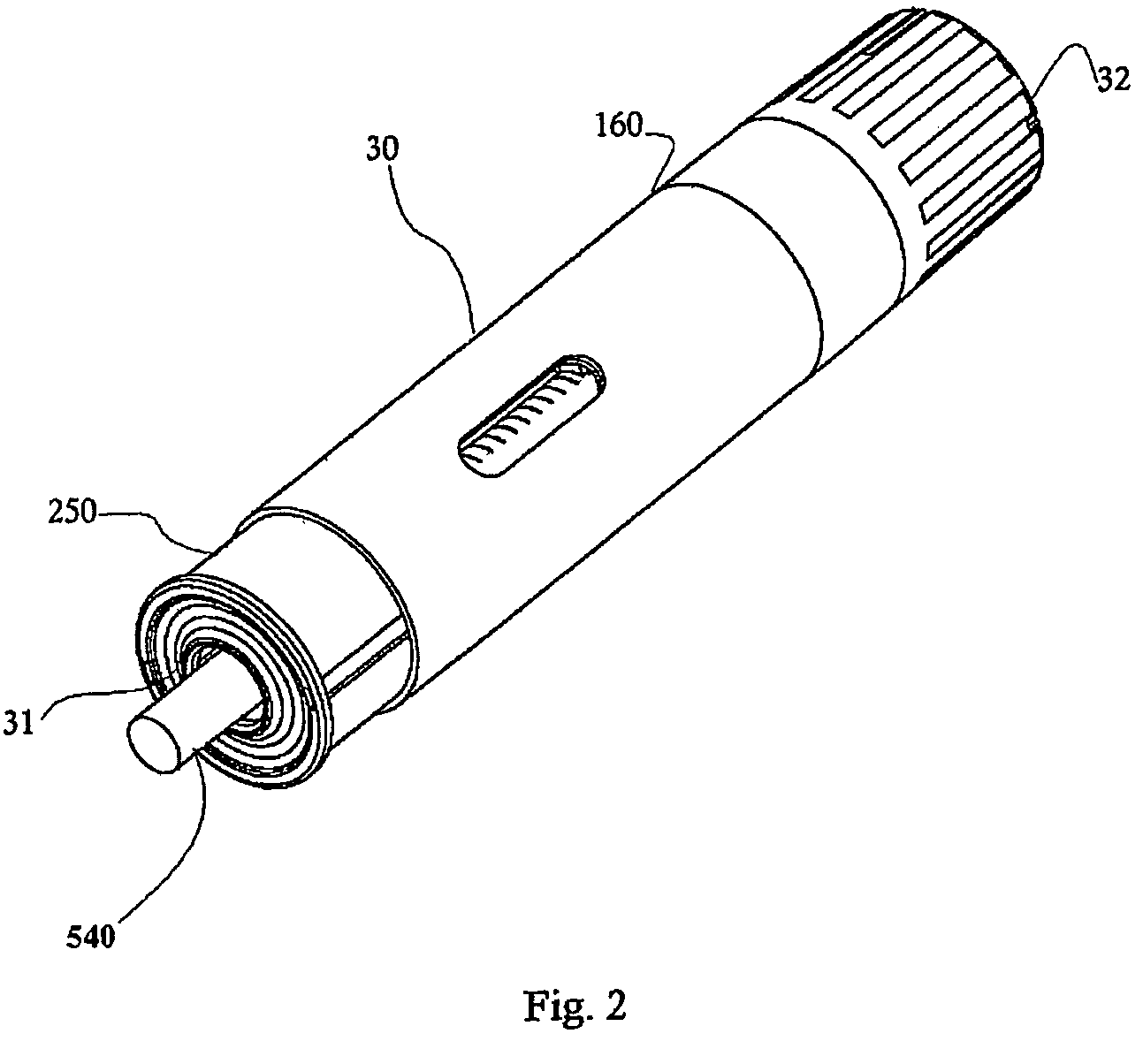
A hand-held, electronically controlled injection device (1) for injecting preset doses of liquid medications, having a housing (2) for receiving a cartridge (4) containing the liquid medication and having a contact surface (16) for contacting a patient's skin; and actuator elements (41) for moving the cartridge (4) within the housing (2) to and from the contact surface (16).
Owner:ARES TRADING SA
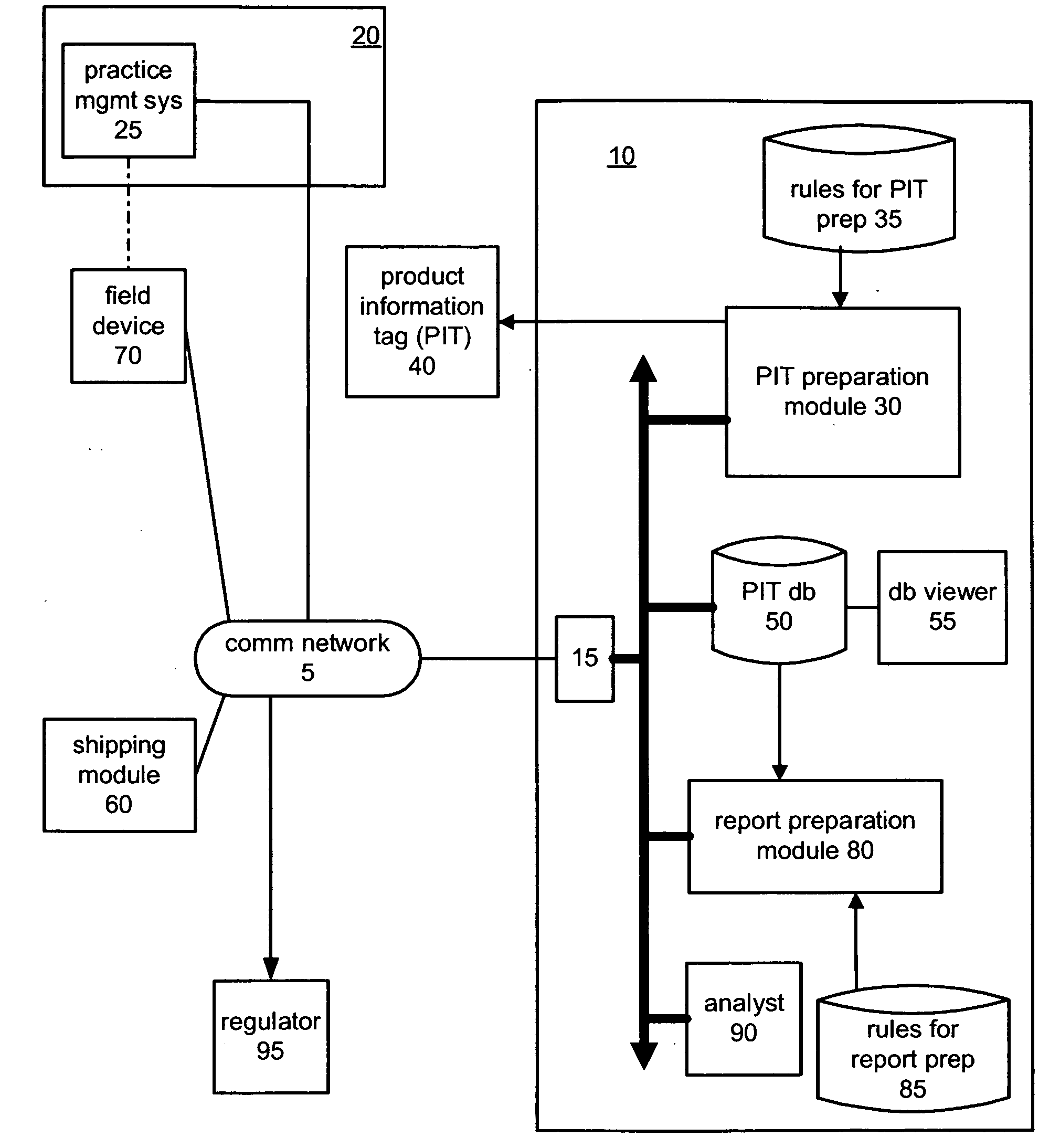
Injection device and case with reporting ability
ActiveUS20090128330A1Drug and medicationsDigital data processing detailsCommunication interfaceData acquisition
A container for a product uses a location circuit for determining the location of the container, a storage element for storing a container identification code, a plurality of data acquisition components for acquiring status of at least two characteristics of at least one of the container, the product and a user of the product, and a communication interface for generating and sending a communication signal including (a) the container identification code from the storage element, (b) the location of the container from the location circuit, and (c) the status of the at least two characteristics from the data acquisition components. The container may be an auto-injector for containing a medicament, or a case for containing an item such as an auto-injector. Generally, the location circuit uses the global positioning system (GPS). The data acquisition components are chosen from a camera, and at least one sensor for sensing at least one of a thermal image, vibration, temperature, humidity, a chemical and an audio signal. The characteristic may be use of the product, or lack of use of at least one of the container and the product.
Owner:NEW DIRECTIONS TECH CONSULTING LLC
Delay mechanism for automatic injection device
ActiveUS20100049125A1Suitable for useMechanism is slowInternal osteosythesisAutomatic syringesEngineeringCam
A delay mechanism for staging the operation of an automatic injection apparatus (20) to ensure medication contents are properly delivered prior to the needled syringe (32) of the apparatus being retracted. In one form, the delay mechanism includes a shuttle (50), a follower (110), a locking member, a damping compound, and a driver and a driver biasing element (44). The shuttle is for a needled syringe of the apparatus and includes a first latching element. The follower includes a second latching element and a cammable surface, which second latching element is for cooperating with the first latching element to limit motion of the shuttle relative to the follower in a second direction opposite the first direction. The locking member is movable from a locking position to a release position by engagement with the syringe plunger during an injection, the locking member, when in the locking position, preventing rotation of the follower relative to the shuttle, the locking member, when in the release position, allowing rotation of the follower relative to the shuttle. The damping compound is between the follower and a supporting surface to dampen rotation of the follower relative to the shuttle. The driver is rotatably fixed relative to the shuttle and includes a camming surface. The shuttle allowing retracting of the syringe needle into the housing of the automatic injection apparatus after injection.
Owner:ELI LILLY & CO
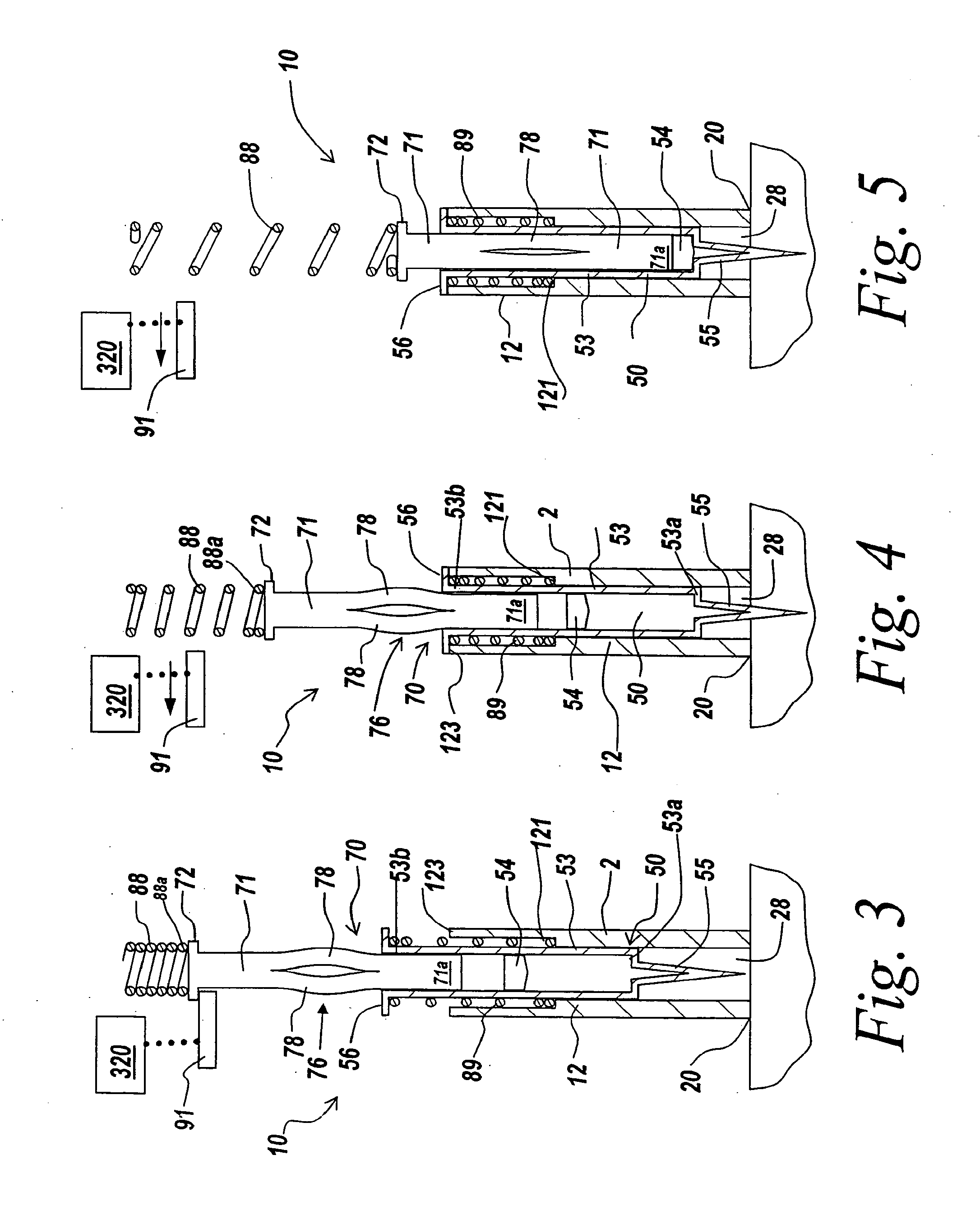
Injecting apparatus
ActiveUS7717877B2Facilitates automatic insertionAmpoule syringesAutomatic syringesCaregiver personInjection site
An injector is automatic in that the needle is inserted into the injection site (e.g., a patient's skin) with user or caregiver assistance, the delivery is automatically initiated upon needle insertion, and the needle is retracted automatically after the end of delivery. Preferably the needle is not seen by the user prior to, during or after injection. Prior to and after injection, the needle is hidden in the device so as to avoid any potential injury or health risk to the user or health care provider. The injector includes a housing and a shield arranged to slide relative to the housing and a driver moving during drug delivery. The housing and shield form a cartridge enclosure. The cartridge is shielded and locked after delivery is completed. A needle-locking mechanism can be used in any number of pen-like injectors or safety needles.
Owner:WEST PHARMA SERVICES OF DELAWARE
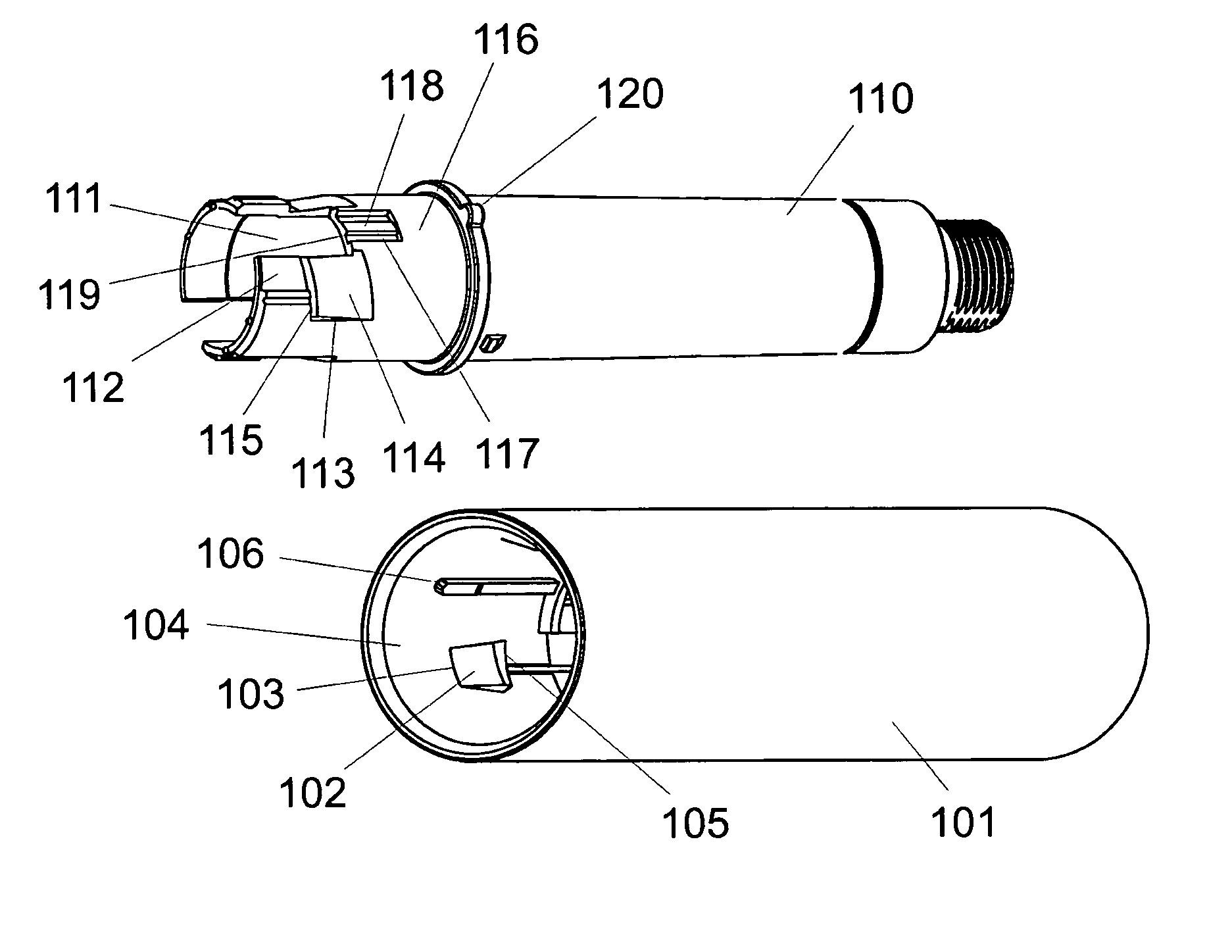
Injection device
InactiveUS20060258990A1HandlingSimple and safe operationAutomatic syringesMedical devicesRisk strokeSubcutaneous tissue
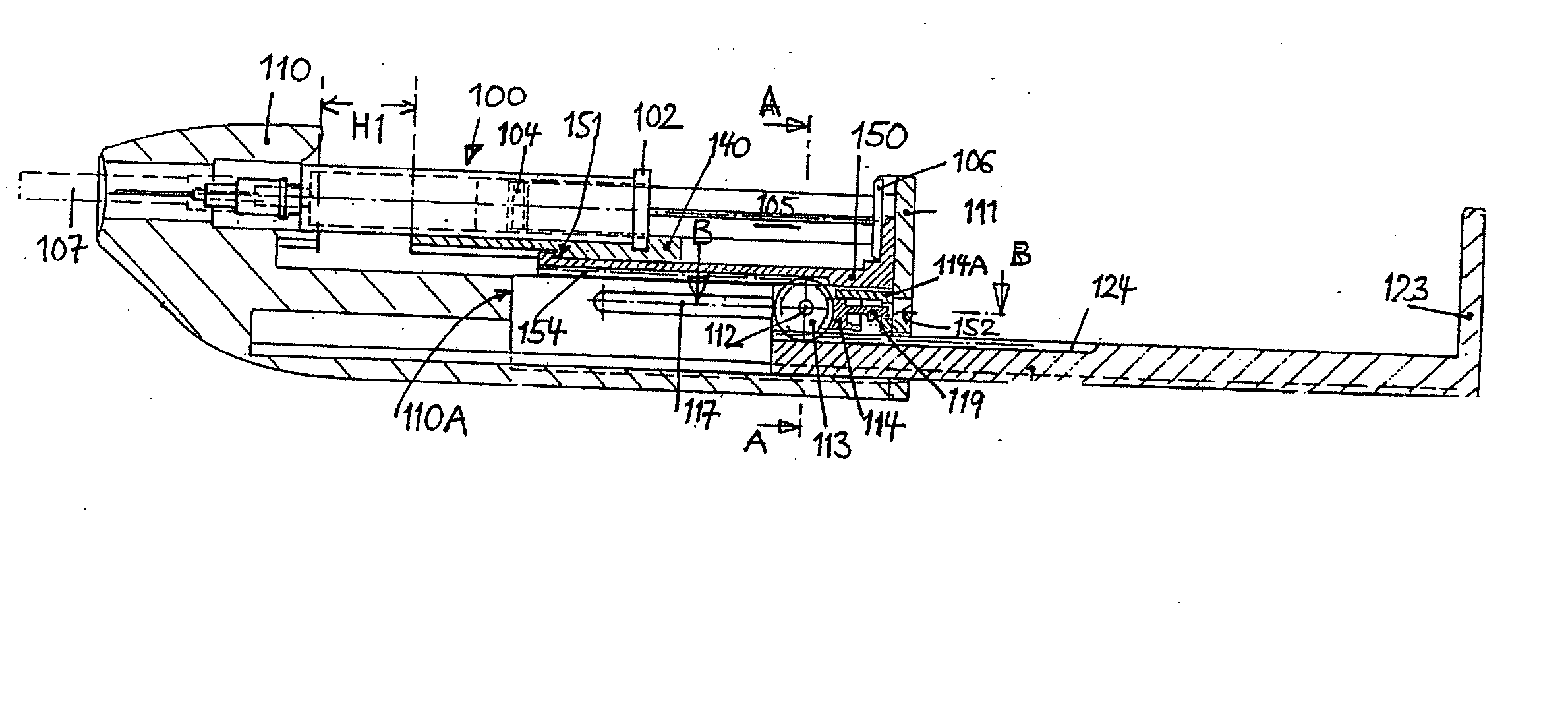
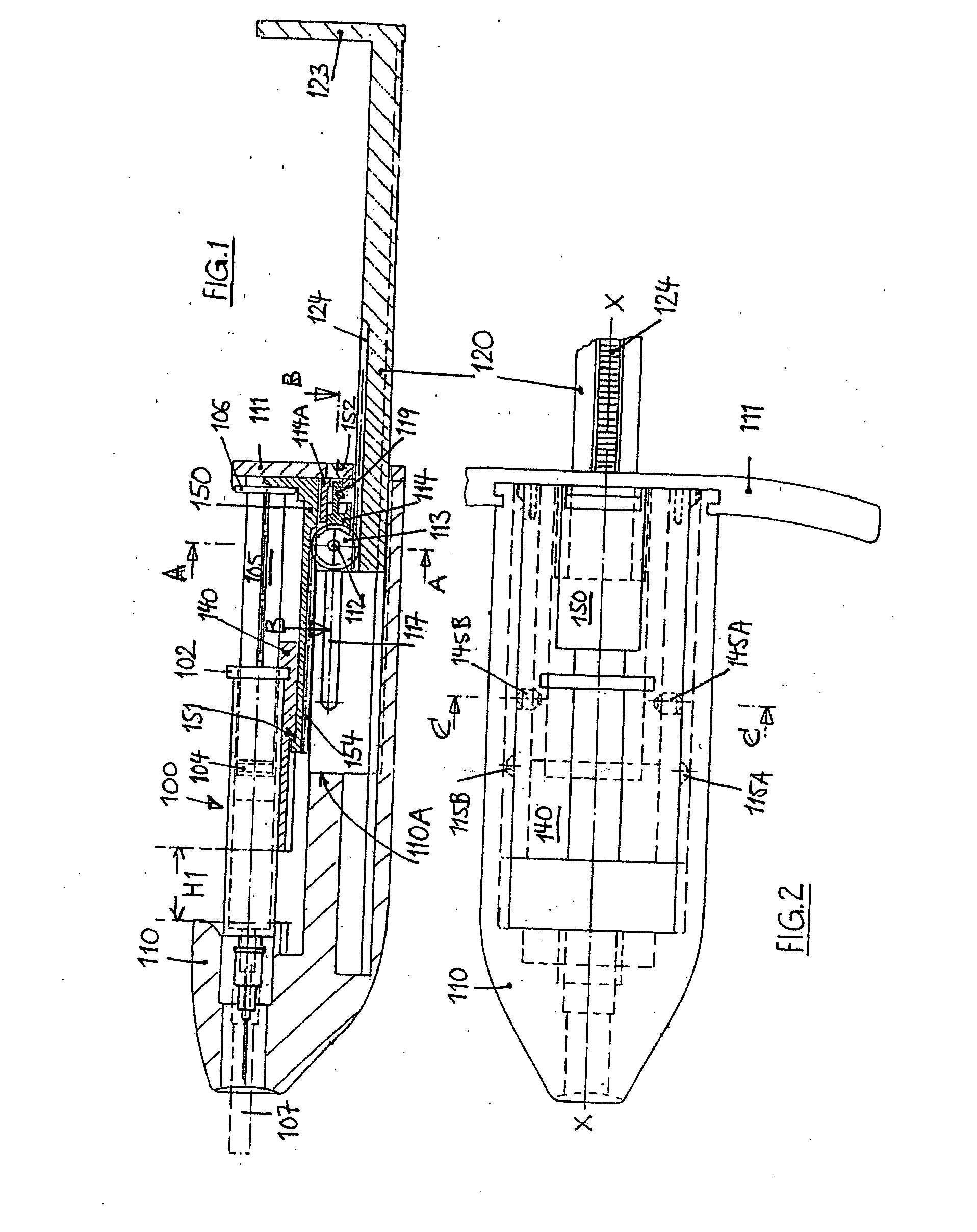
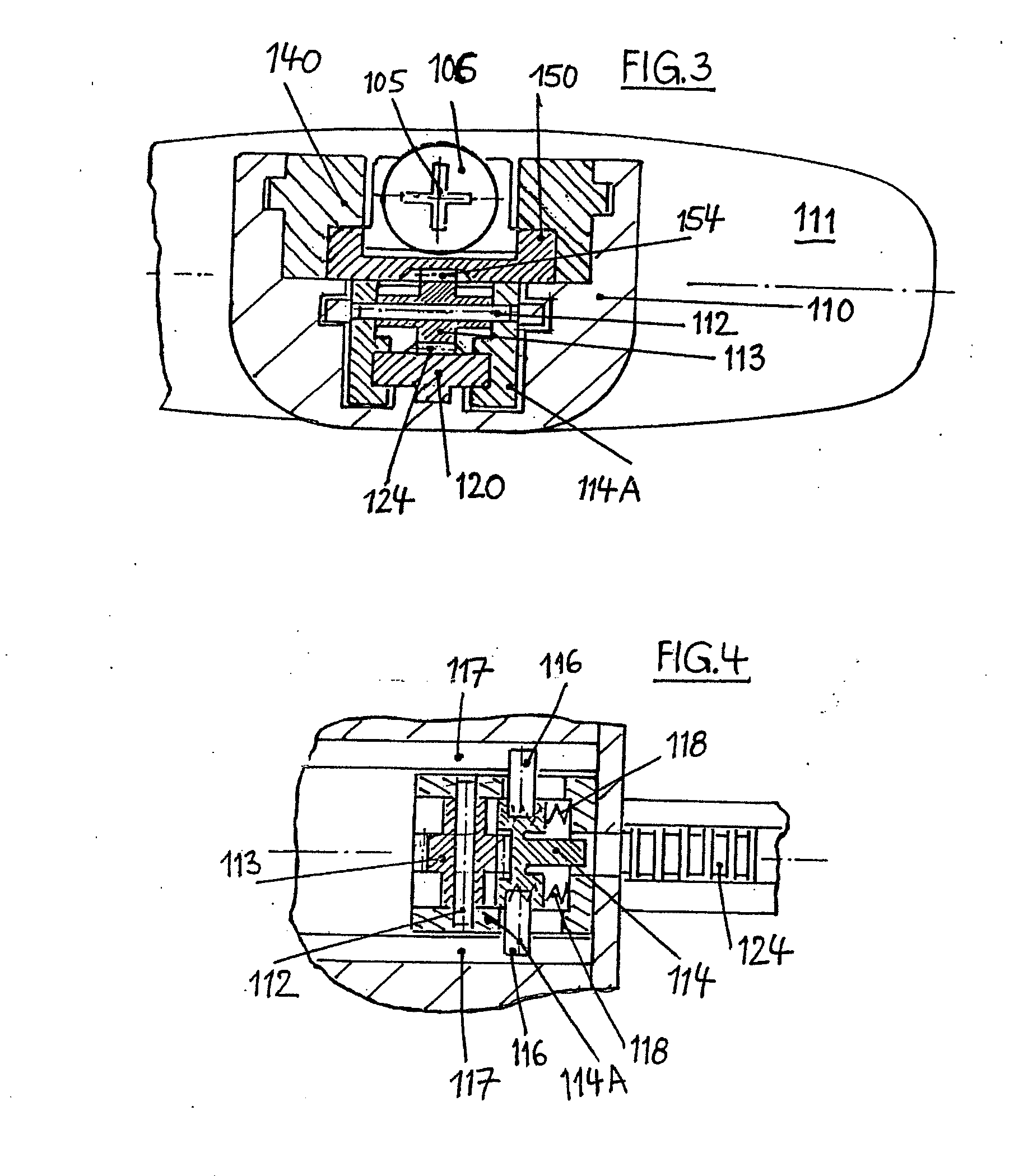
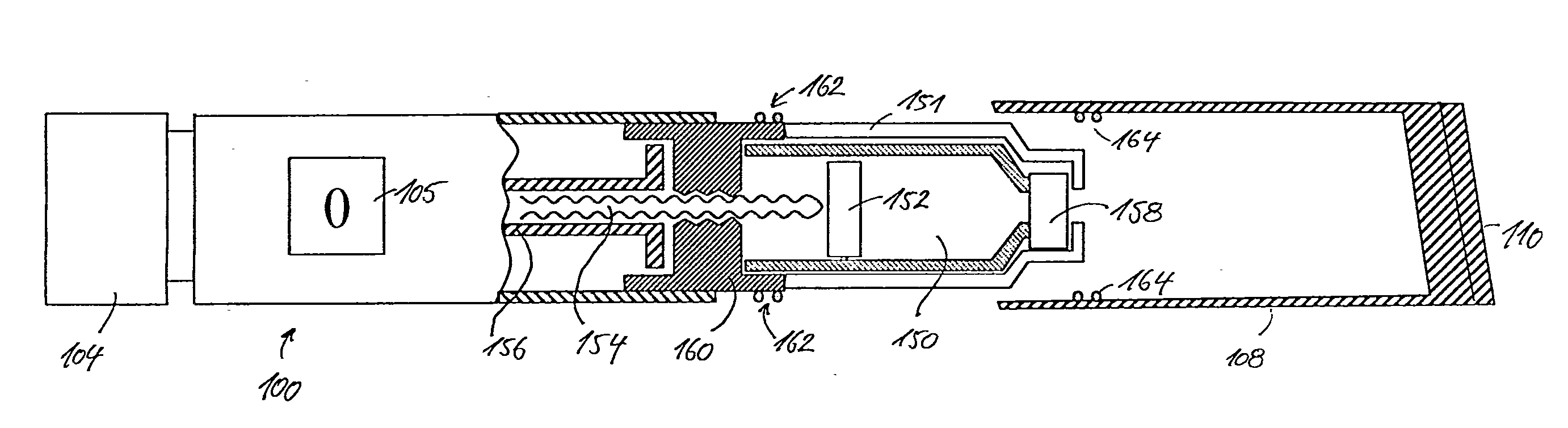
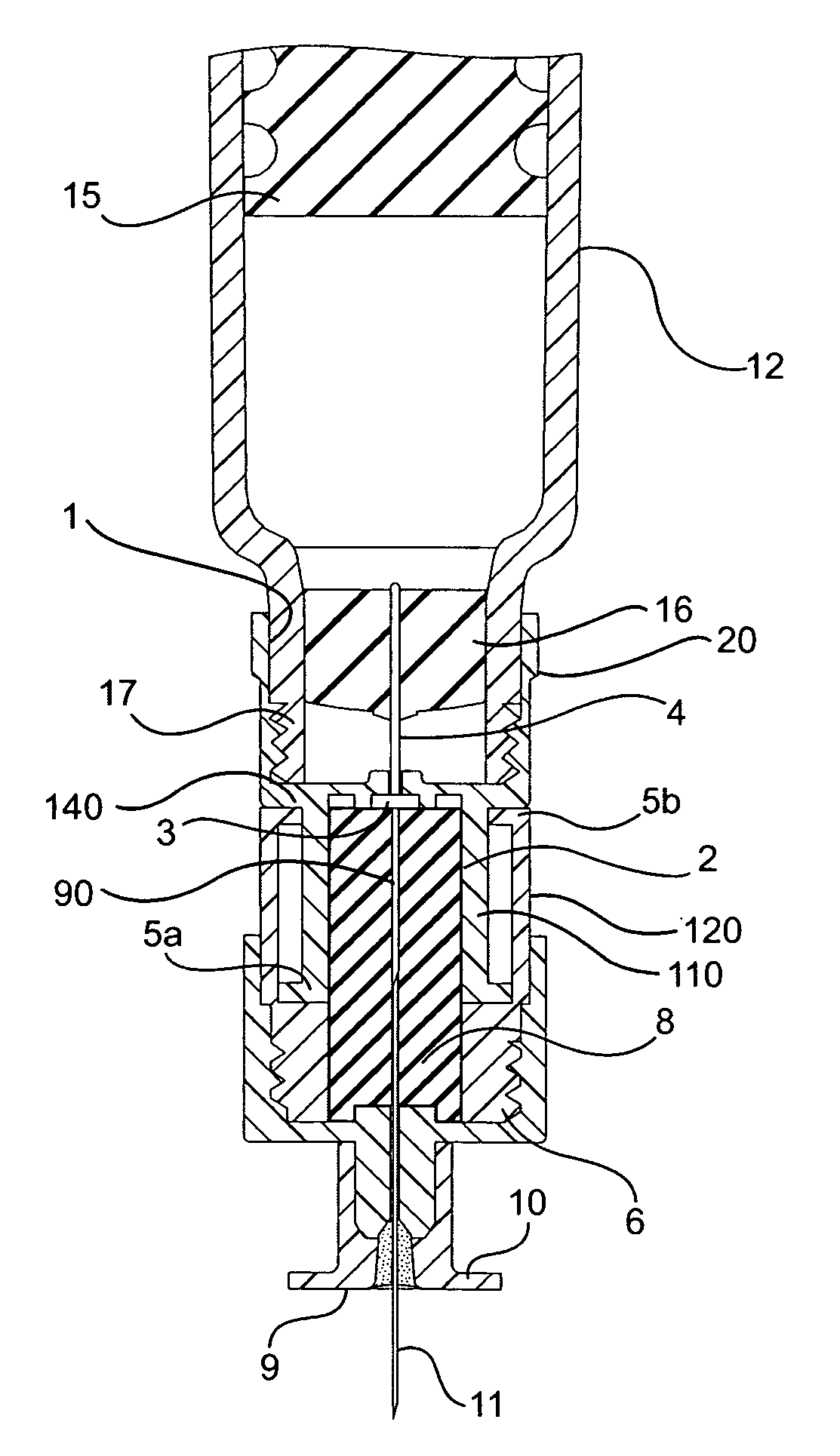
An injection device for a syringe, having a syringe body, a cannula with a needle, a plunger with a plunger rod, and an injection carriage for displacing the syringe body and the plunger, comprises at least one actuating element that acts on the injection carriage to carry out the injection procedure. The actuating element (120, 220, 320) cooperates with components which withdraw the needle (108, 208, 308) from the puncture site once the injection procedure has been completed, using a return stroke (H3) that is applied to the injection carriage. A single, targeted linear movement inserts the needle to a defined depth, injects the medicament and, once the injection has been completed, produces a return stroke which allows the needle to be withdrawn into the housing and thus out from the puncture site. The injection device is advantageously equipped with additional components which produce a delay (TV) between the completion of the injection stroke (H2) and the start of the return stroke (H3). The advantage of said delay is that the pressure that has been produced in the subcutaneous tissue by the injection of the medicament can subside before the needle is withdrawn, thus preventing to a great extent the penetration of the medicament into the insertion channel of the needle. A volume adapter (410) can advantageously be used to predetermine the injection stroke (H2) and thus the quantity of a medicament that is administered during the course of the injection stroke (H2).
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Injection Device with a Processor for Collecting Ejection Information
InactiveUS20080188813A1Improve patient safetyHigh dexterityAmpoule syringesAutomatic syringesBiomedical engineeringInjection device
A medication delivery device comprises an injection device having a reservoir comprising a medicament to be ejected, and a sensor arranged to detect an ejection of the medicament from the injection device, the sensor being arranged to output a signal comprising ejecting information, and a processor for collecting and storing the ejection information.
Owner:NOVO NORDISK AS
Medicine injection devices and methods
A reloadable medicine injector and methods are described in which a barrel with a receiving cavity is adapted to slidably receive a syringe subassembly for axial movement therein. Upon removal of a safety and release of a syringe driver, the syringe driver moves forward and injects the syringe needle. A plurality of penetration controls are shown for controlling injection needle penetration depth. The penetration controls have an abutment and various lengths to provide different needle penetration depth positions. In one form of penetration control a sleeve is used against which the syringe or related parts contact. In another form the front return spring is used as a penetration control. A cushioning ring may be used to reduce syringe breakage. A load distribution and guide ring may be used to distribute loading applied to the syringe and help guide the moving syringe.
Owner:WASHINGTON BIOTECH CORP +1
Injecting device
ActiveUS7112187B2Small sizeEasy to useAmpoule syringesAutomatic syringesNeedle punctureBiological activation
An injection device having an elongated body, including a container with medicament, elements for connecting a needle to the container, actuating elements capable of injecting a dose of medicament upon activation, activating elements capable of activating the actuating elements, a needle shield arranged to the body and slidable between an extended and a retracted position in relation to the body. The needle shield is designed and arranged such that, upon penetration of the needle into a patient, when moved to its retracted position, acts on the activating elements, which in turn activates the actuating elements and injects a dose.
Owner:SHL MEDICAL AG
Injection Device
ActiveUS20080262436A1Easy to handleLow costAmpoule syringesAutomatic syringesSyringe needleInjection device
Injection device comprising a tubular elongated main body, a needle shield slidably arranged in said main body, a needle shield link slidably connected to said needle shield, a enclosure containing medicament arranged in said main body, a needle connected to said enclosure, a plunger operatively arranged to said enclosure for ejecting said medicament through said needle and arranged on its upper part with a number of outwardly extending stop members, spring means arranged to said plunger for operating said plunger, a dose activating means, a needle shield spring surrounding the needle shield link. The invention is characterised in that said injection device further comprises a first tubular member rotationally and slidably arranged inside said needle shield link, said tubular member comprises a number or ridges and protrusions on both its outer and inner surfaces, said ridges and protrusions on the outer surface of the tubular member co-operate with guide members arranged on the inner surface of said needle shield link, said ridges and protrusions on the inner surface of the tubular member co-operate with the outwardly extending stop members of the plunger that said injection device further comprises a second tubular member arranged inside said housing, arranged and designed with a number of ridges and protrusions on its inner and outer surfaces capable of setting and delivering a certain preset dose.
Owner:SHL MEDICAL AG
Injection device with secondary reservoir
A method and apparatus for injecting fluid into areas having high density tissue that creates a high backpressure resistance on the injection device is disclosed. The high backpressure resistance is overcome through a mechanical advantage achieved by using a secondary reservoir having a cross-sectional area smaller than the cross-sectional area of a primary reservoir. Exemplary injection device reservoir housings may comprise a primary reservoir, a secondary reservoir, a check valve, a septum penetrating cannula, travel limits, a pen needle connecting portion, sliding seal guide ribs, a sliding seal, a pen needle assembly, a needle stop, and a patient needle.
Owner:EMBECTA CORP
Cover for catheter assembly
A cover for a proximal end of a connector attached to a catheter to receive an injection device. The cover comprises a body portion configured and dimensioned to receive at least a portion of an outer surface of the connector, a securement portion providing an engagement force on the connector for securing the cover to the connector, a removable cap having an internal portion with an anti-microbial agent, and a flexible member connecting the removable cap to the body portion.
Owner:VALLEY DEVICES
Device and method for positioning an object in a body lumen
InactiveUS6884213B2Precise positioningPrecision releaseSurgeryMedical devicesMedicineInjection device
It is an object of the present invention to provide a device and method for controlled positioning and / or releasing an object in a body lumen.The device of the invention comprises a liquid filled tube detachably connected to an injecting apparatus at its proximal end and to a holding and releasing unit having a bore configured to hold and release the object, at its distal end. The holding and releasing unit is connected to the tube such that liquid can pass from the tube into the holding and releasing unit bore.The distal end of the tube is capable of being inserted and maneuvered through a body lumen.The object is retained in the device during its manipulation through the body lumen due to frictional force exerted by the holding and releasing unit on the object. The object is released by activating the injecting apparatus to achieve an hydraulic pressure in the holding and releasing unit bore which is higher than the frictional force.
Owner:GIVEN IMAGING LTD
Medication injecting apparatus with fluid container piston-engaging drive member having internal hollow for accommodating drive member shifting mechanism
A medication injecting apparatus having a motor-driven drive member that when advanced inserts into a fluid container for forcing fluid therefrom. The drive member includes an internal hollow in which fits at least a portion of the motorized driver assembly when the drive member is retracted to allow a compact apparatus to be provided.
Owner:ELI LILLY & CO
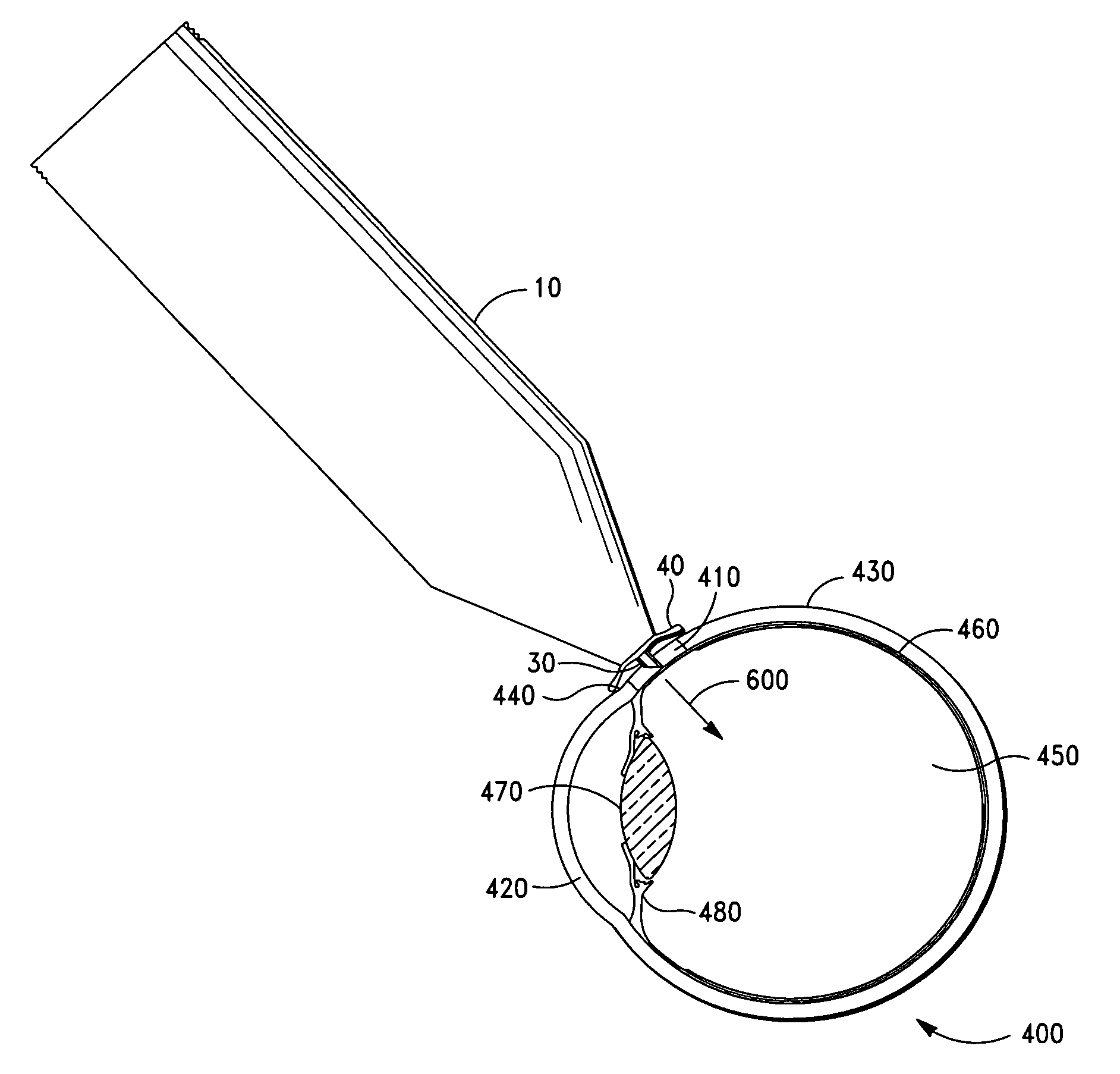
Intravitreal injection device, system and method
An intravitreal injection device for administering a pharmacological agent formulation to an intravitreal compartment of an eye, comprising (i) a nozzle member having an internal formulation chamber that is adapted to receive and contain the pharmacological agent formulation therein, (ii) a microneedle having a first end that is in communication with the nozzle member and a second ejection end, (iii) and piercing depth limiter means for limiting the penetration depth of the microneedle into the eye, the microneedle piercing depth limiter means including guide means for positioning the limiter means and guiding the microneedle.
Owner:KMG PHARMA
Drug delivery system with profiles
InactiveUS6945954B2Minimize damageMinimize responseSurgeryMedical devicesInternal pressureEngineering
Owner:MILESTONE SCIENTIFIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com