Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4996 results about "Propellant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A propellant or propellent is a chemical substance used in the production of energy or pressurized gas that is subsequently used to create movement of a fluid or to generate propulsion of a vehicle, projectile, or other object. Common propellants are energetic materials and consist of a fuel like gasoline, jet fuel, rocket fuel, and an oxidizer. Propellants are burned or otherwise decomposed to produce the propellant gas. Other propellants are simply liquids that can readily be vaporized.
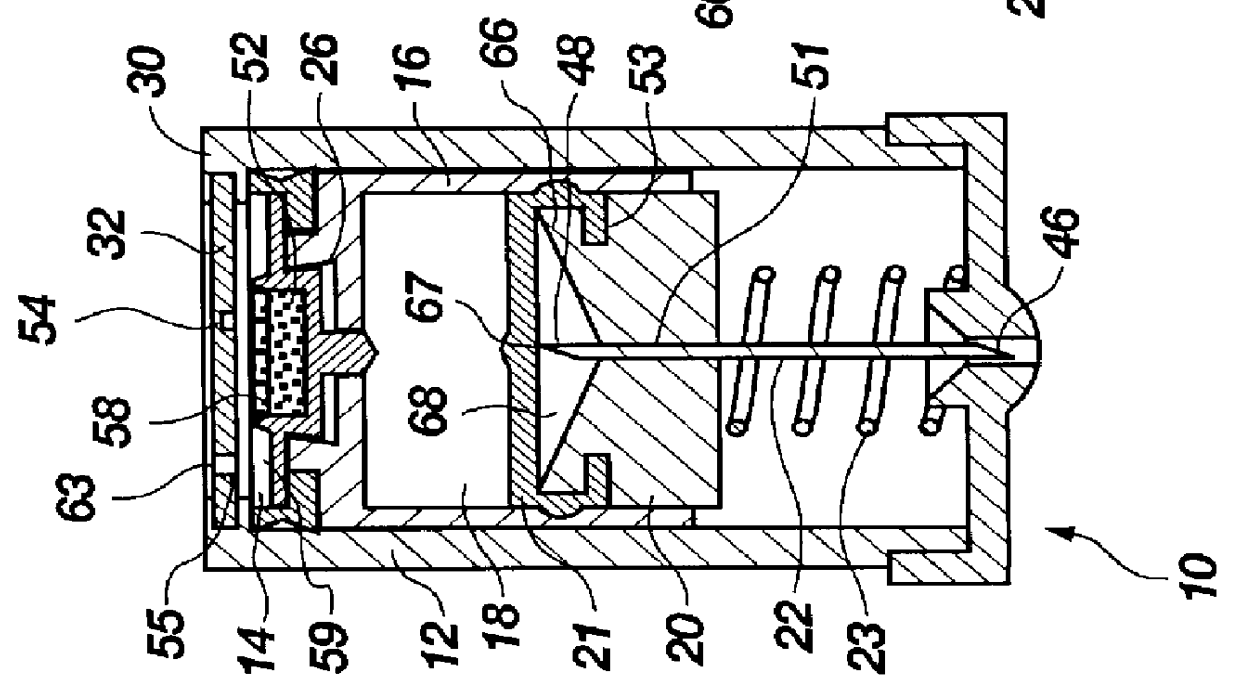
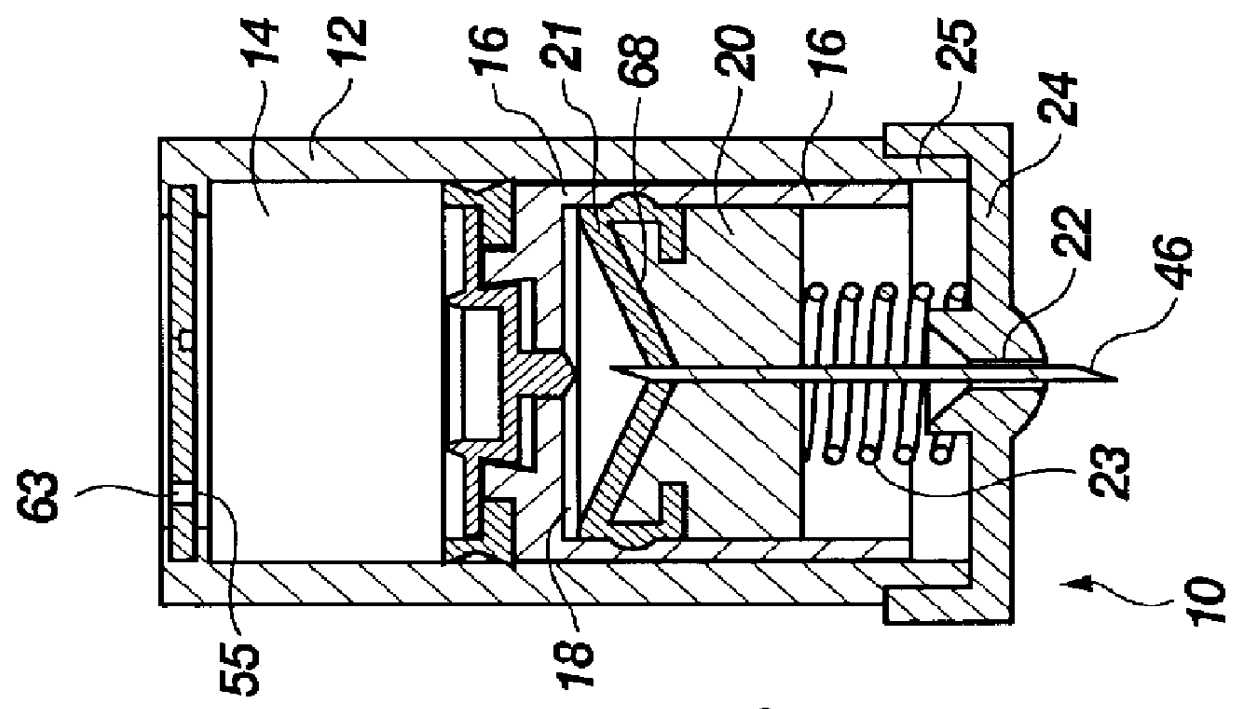
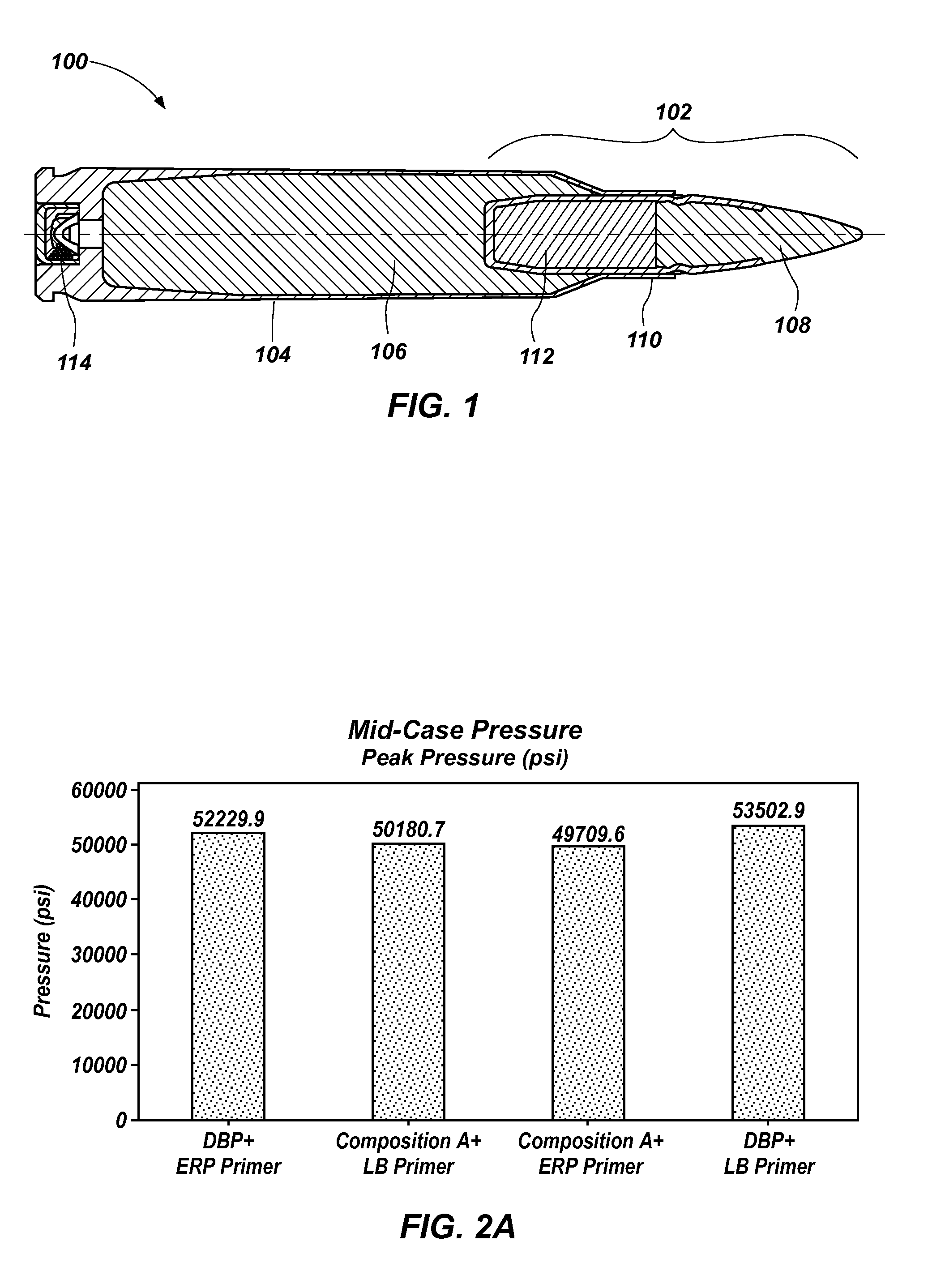
Projectile firing device using liquified gas propellant
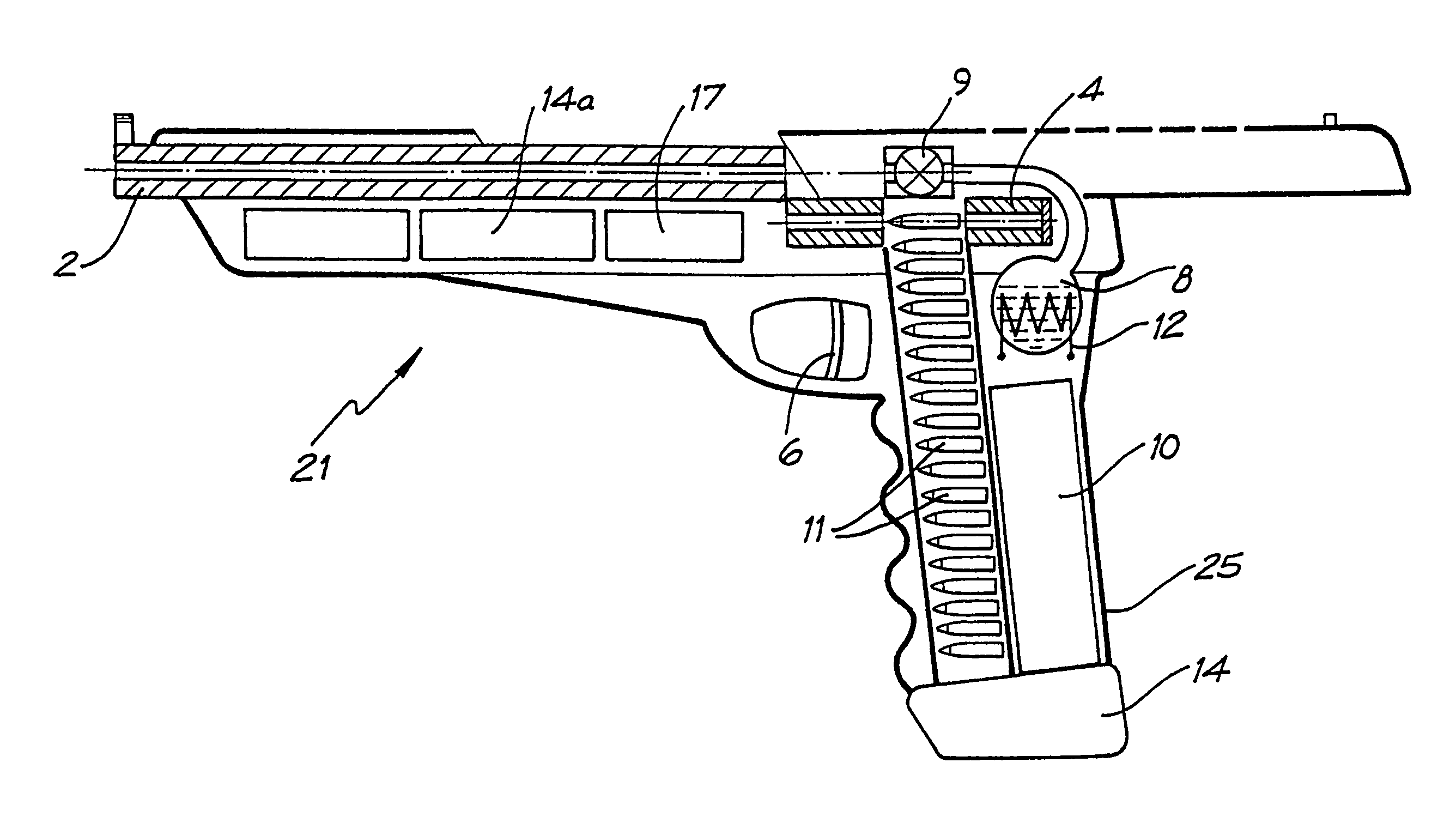
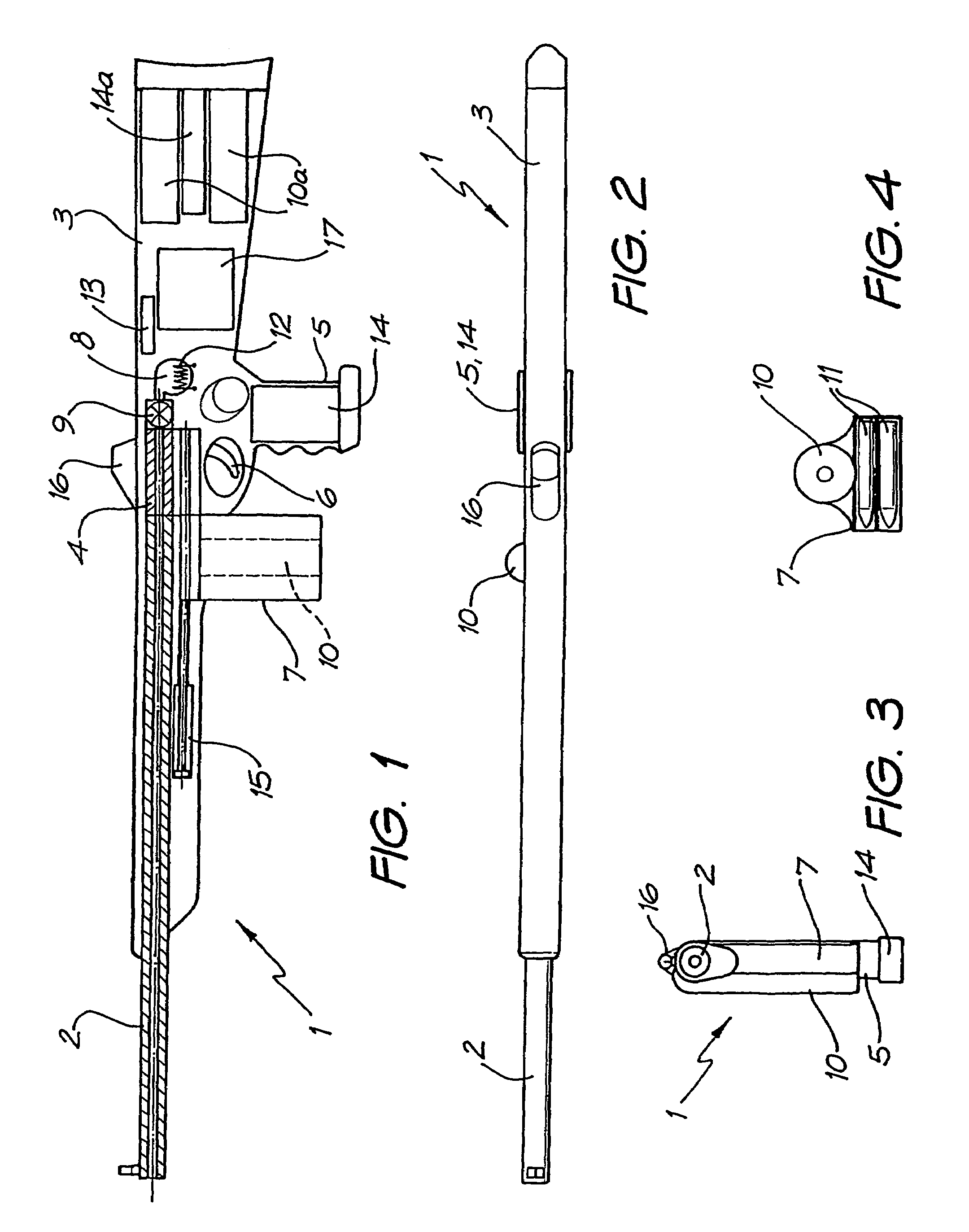
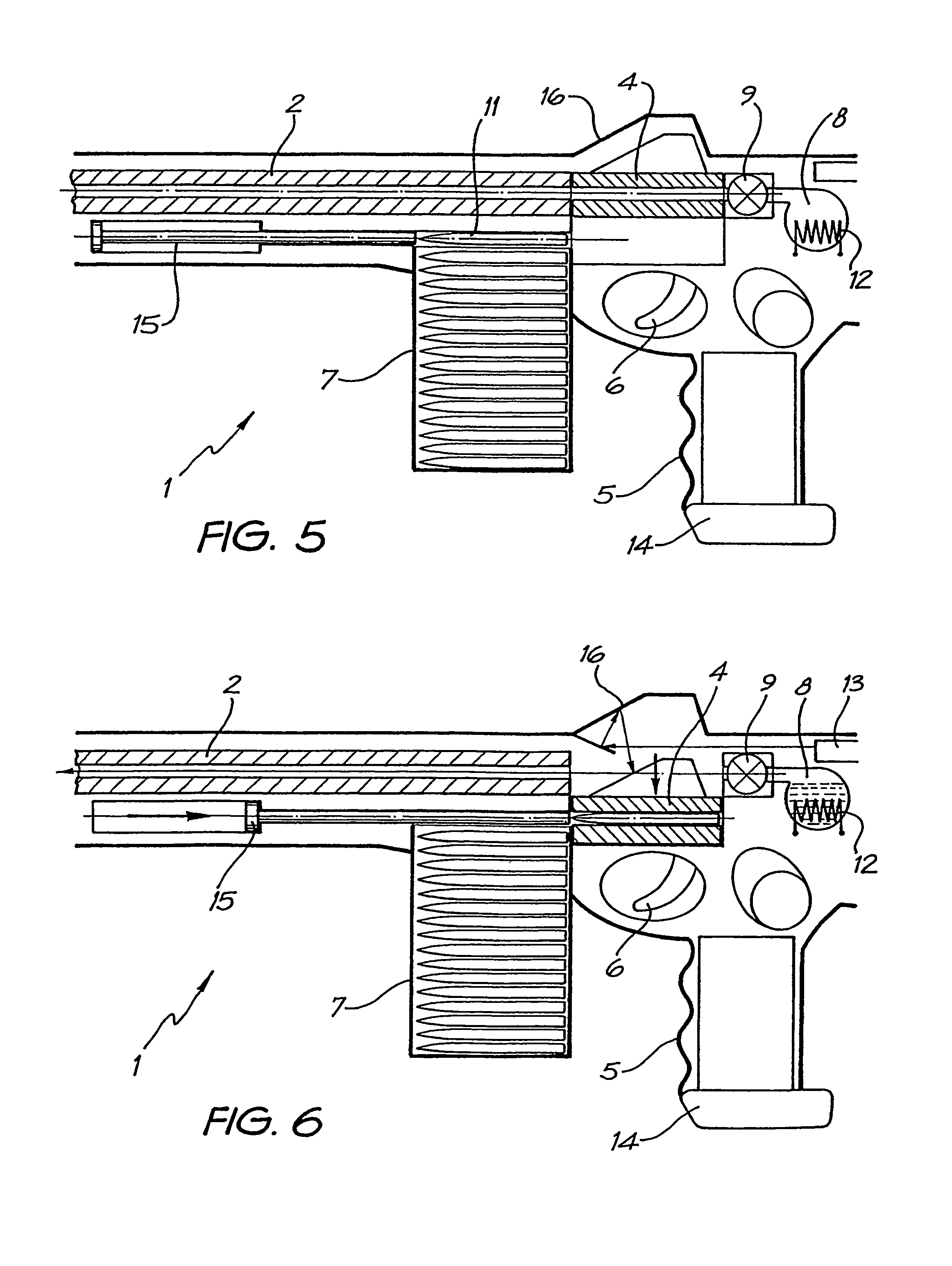
Rifle (1) comprises barrel (2) and loading means (15) for introducing a projectile from magazine (7) into breech (4). The projectile is propelled by a compressed gas propellant initially stored as a liquid in canister (10). The liquid is heated to a super critical state in chamber (8) by heating element (12) to induce a phase change such that the liquid becomes a highly dense gas. The phase change from liquid to gas provides the energy required to expel the projectile at high velocity from rifle (1), regardless of the ambient temperature. The propellant is preferably CO2 which is heated to 31.06° C. Rifle (1) produces minimal noise and no heat signature, making it suitable for military and stealth purposes. A pistol and launchers for grenades or mortar bombs are also disclosed. Another version can launch low earth orbit satellites or payloads.
Owner:POLY SYST PTY LTD
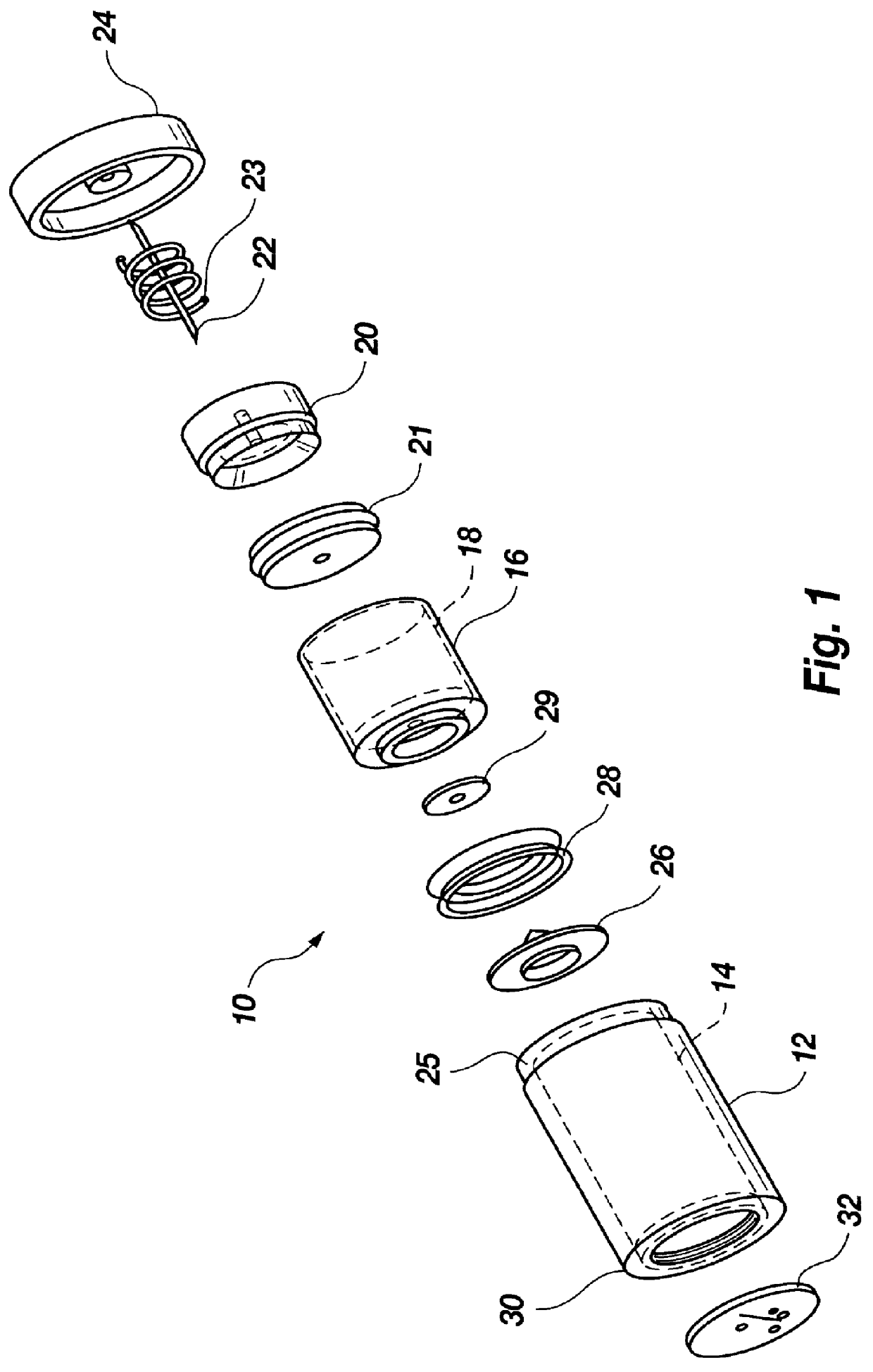
Disposable fluid injection module
An automated injection module is comprised of a housing, a piston drug capsule disposed within the housing, a piston core including a puncture seal membrane defining a reservoir for holding a drug between the puncture seal membrane and the piston drug capsule, an injection device having at least one sharp end for puncturing the puncture seal upon activation of the device, an end cap on a distal end of the housing, and a pressure source on a proximal end of the housing. The pressure source is preferably a propellant that ignites and forces the piston toward the distal end. Substantially simultaneously, the injection device pierces the puncture seal membrane, the piston core is forced into the piston drug capsule, and the drug is evacuated from the reservoir through the injection device and into a patient.
Owner:SARCOS LC
Antibiotic kit and composition and uses thereof
The present invention relates to a therapeutic kit to provide a safe and effective dosage of an antibiotic agent, including an aerosol packaging assembly including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam, wherein the pressurized product comprises a foamable composition including: an antibiotic agent; at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, an organic polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight, a surface-active agent, about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent, water; and liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Non-flammable insecticide composition and uses thereof
ActiveUS20070020304A1Prevent intrusionSafe and effective compositionCosmetic preparationsBiocideArthropod infestationFilm-forming agent
The present invention provides a safe and effective insecticide composition suitable for treating a subject infested with a parasitic anthropode or to prevent infestation by an arthropod. The insecticide composition is a foamable composition, including a first insecticide; at least one organic carrier selected from a hydrophobic organic carrier, a polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 5%, or about 5% to about 10%; or about 10% to about 20%; or about 20% to about 50% by weight; about 0.1% to about 5% by weight of a surface-active agent; about 0.01% to about 5% by weight of at least one polymeric agent selected from a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; and (5) a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Film forming foamable composition
InactiveUS20060193789A1Improve solubilityReduce deliveryCosmetic preparationsBiocideAlcohol freeFilm-forming agent
A foamable composition, includes (1) about 6% to about 70% by weight of at least one organic carrier; (2) about 0.1% to about 5% by weight of at least one surface-active agent; (3) about 0.01% to about 5% by weight of at least one film forming agent; (4) water; and (5) about 3% to about 25% by weight of the total composition of at least one liquefied or compressed gas propellant. The composition is substantially alcohol free and is used in treating, alleviating or preventing a disorder.
Owner:FOAMIX PHARMACEUTICALS LIMITED
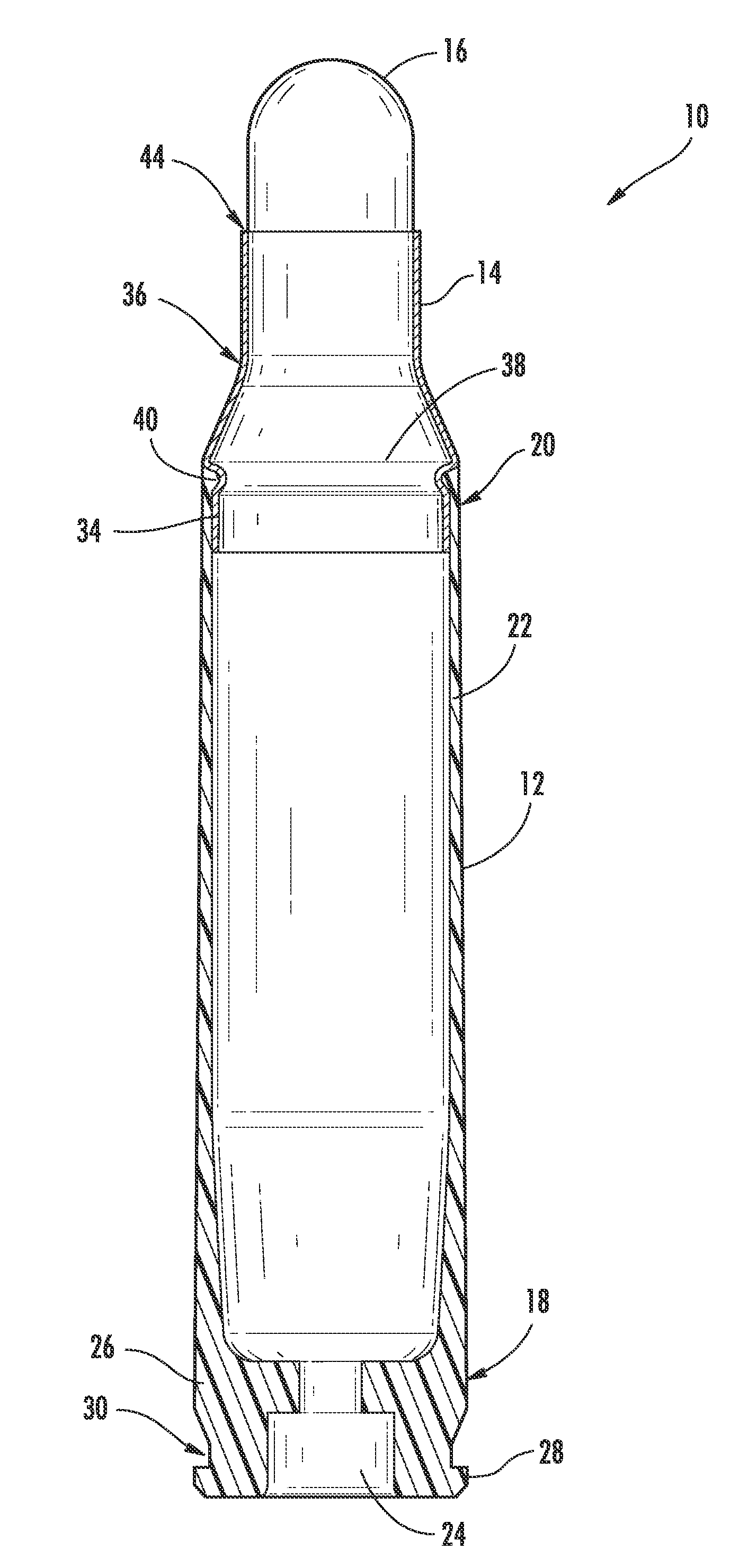
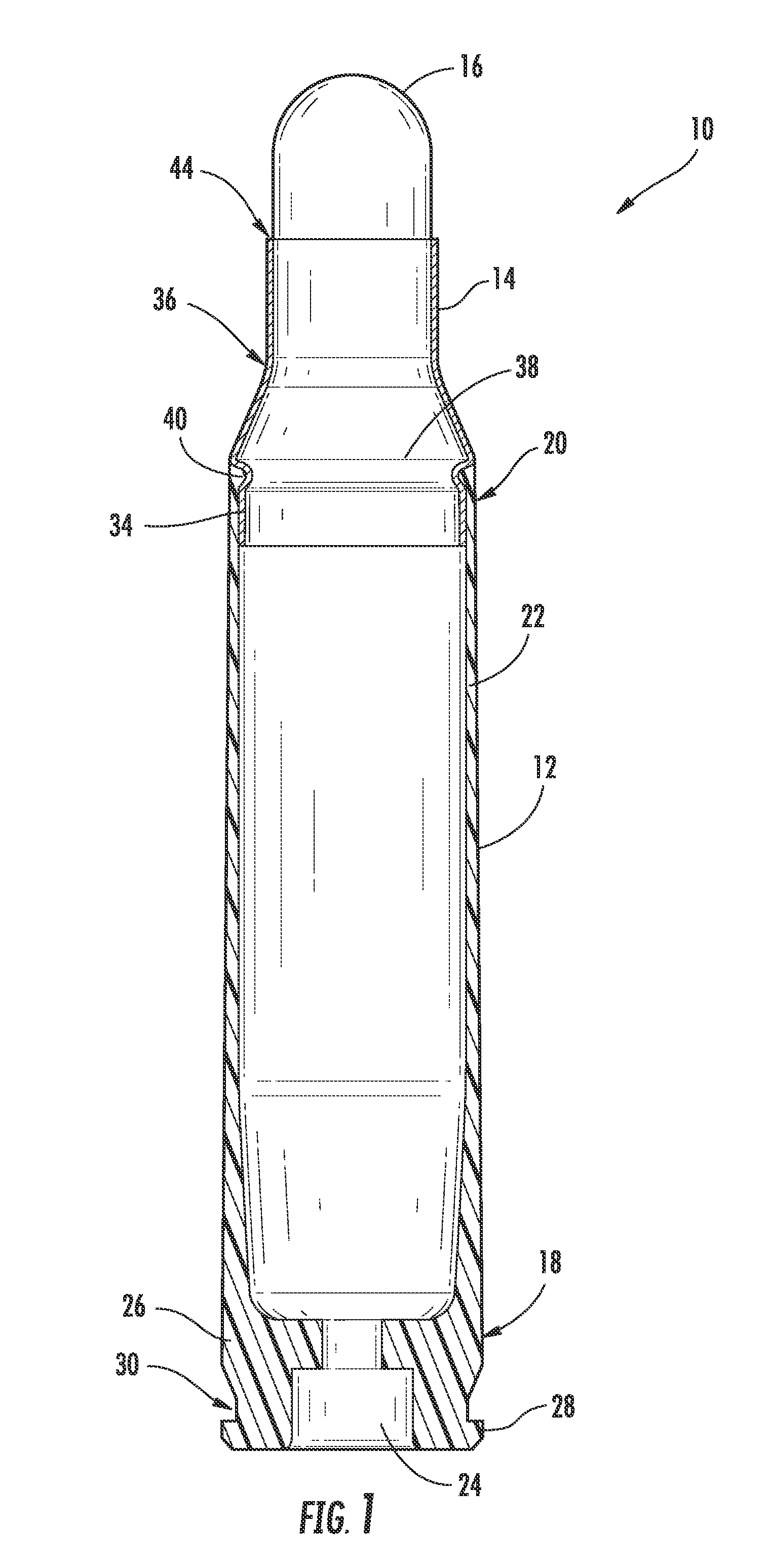
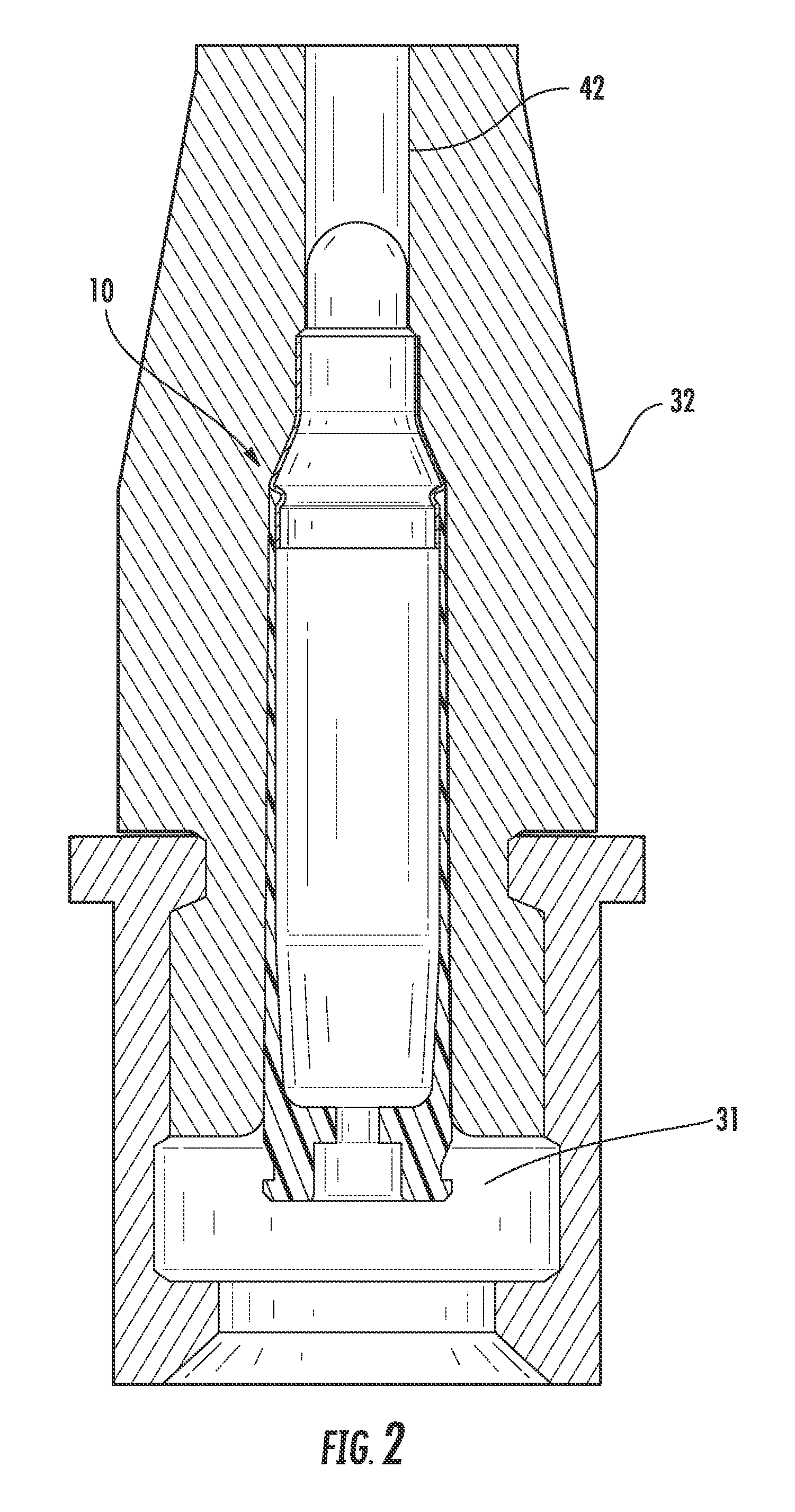
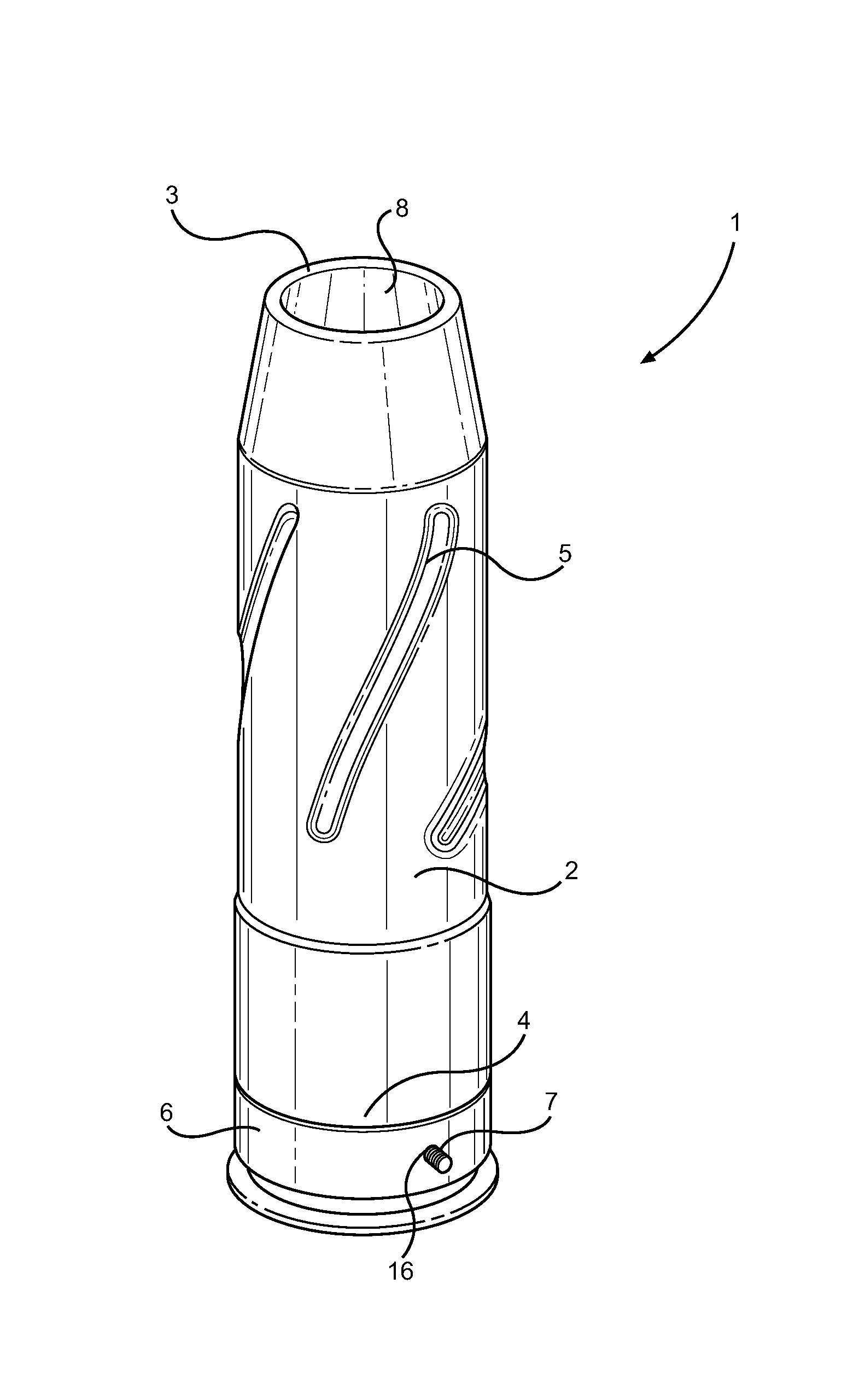
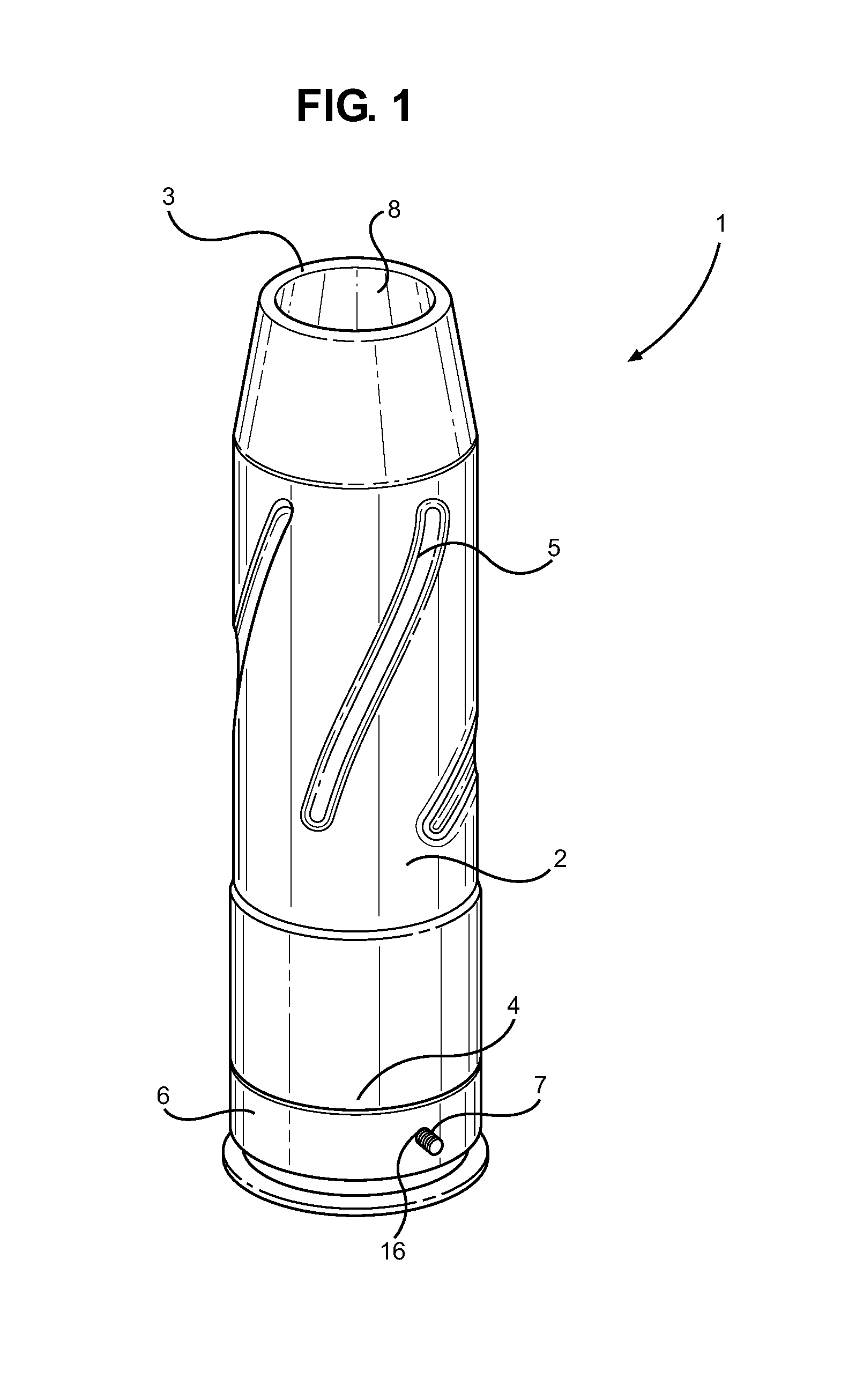
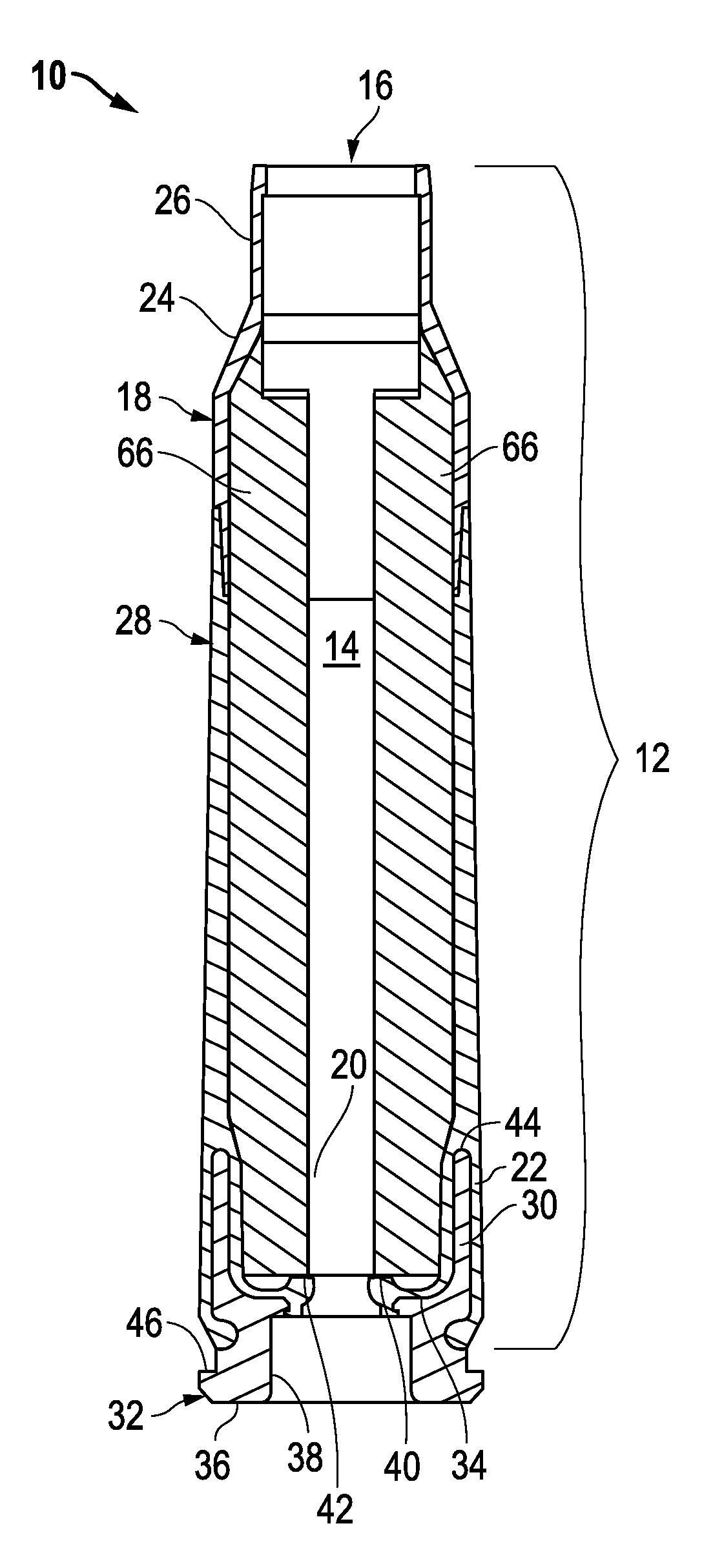
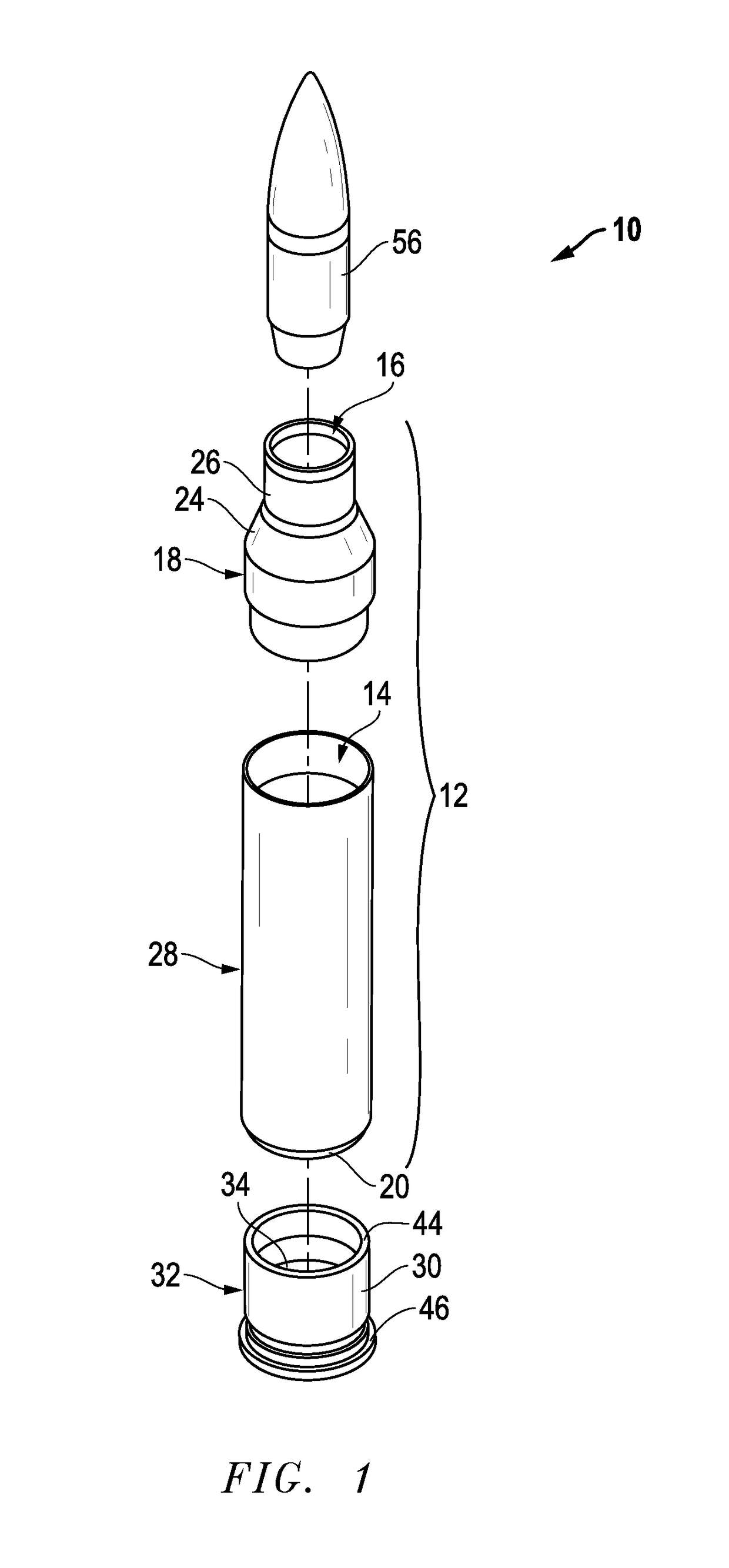
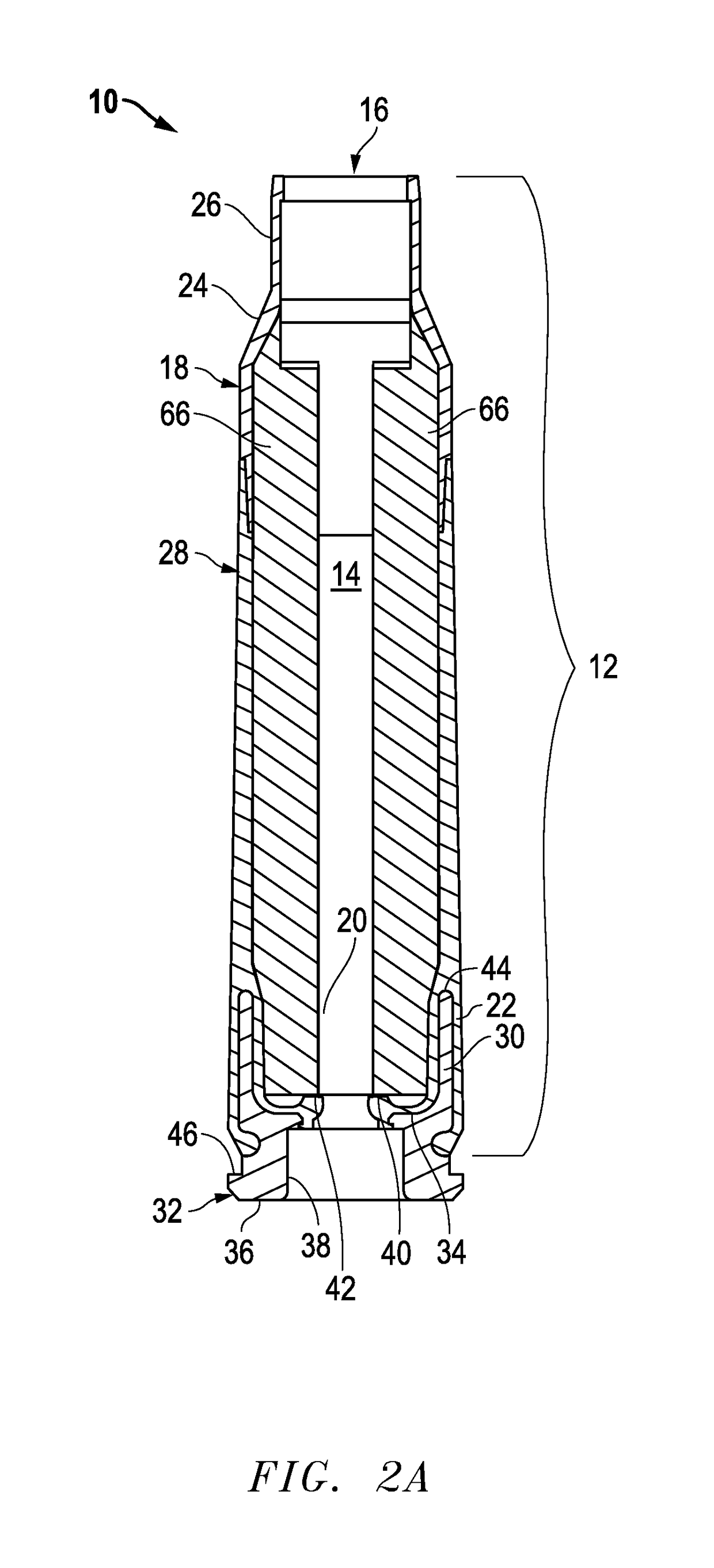
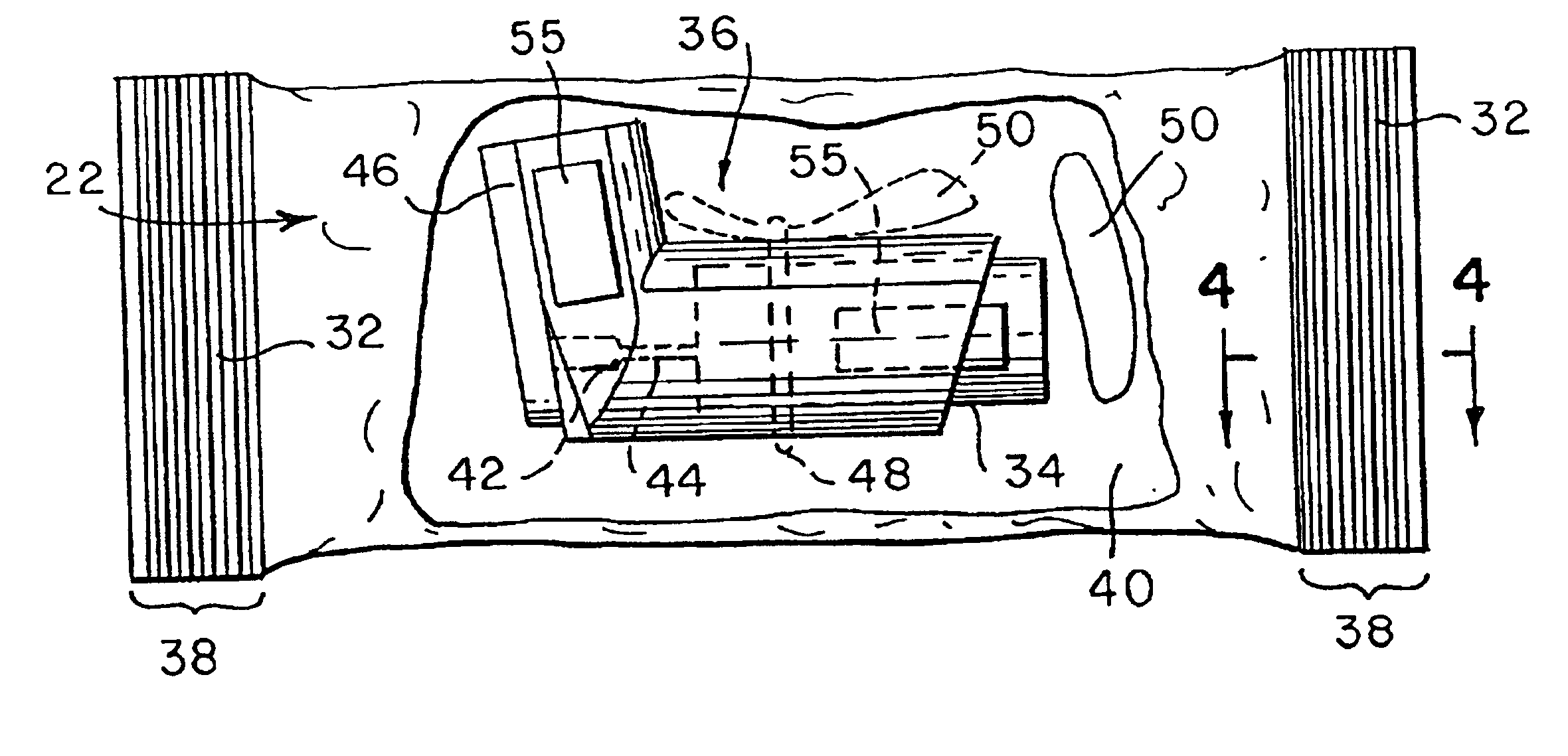
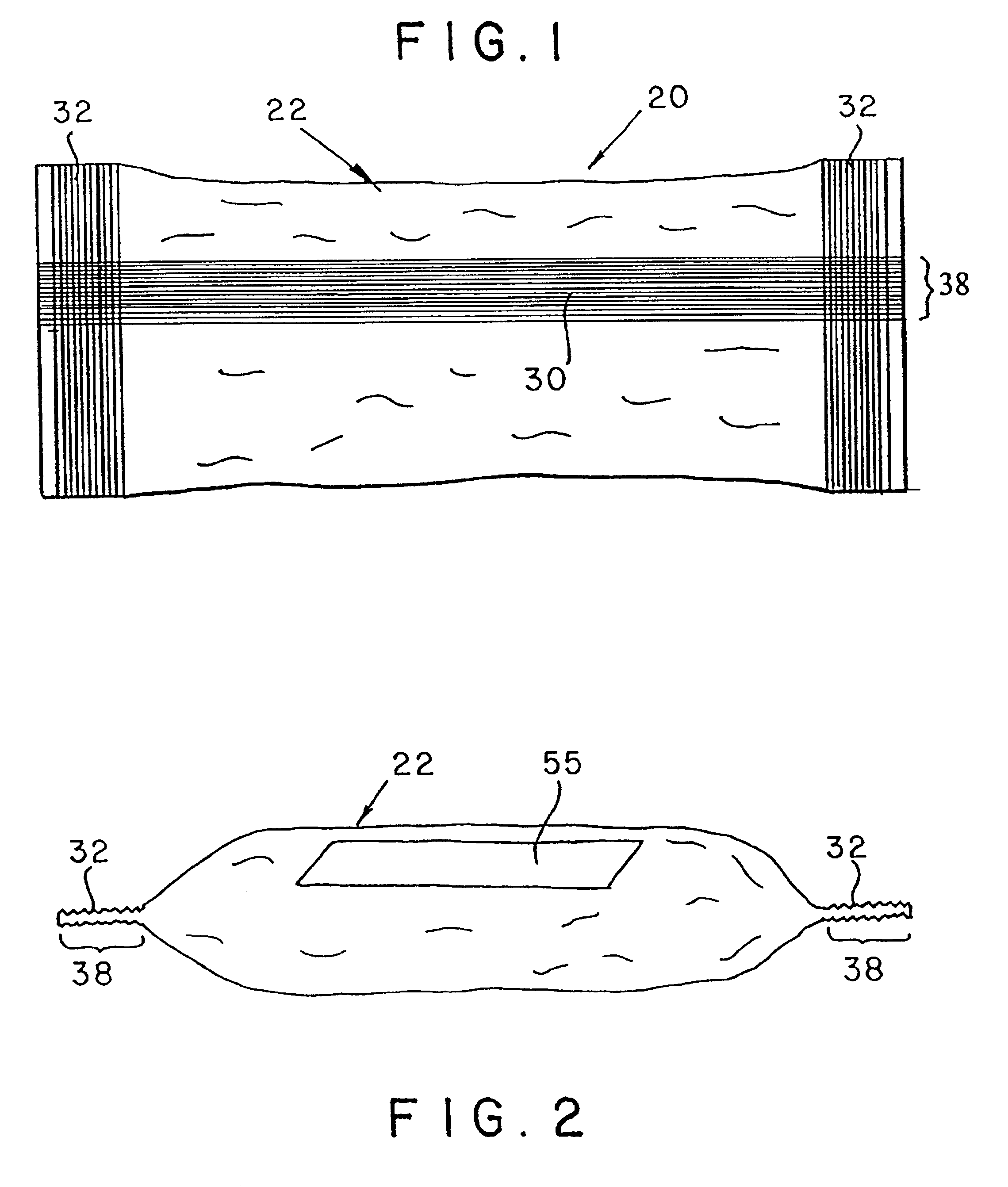
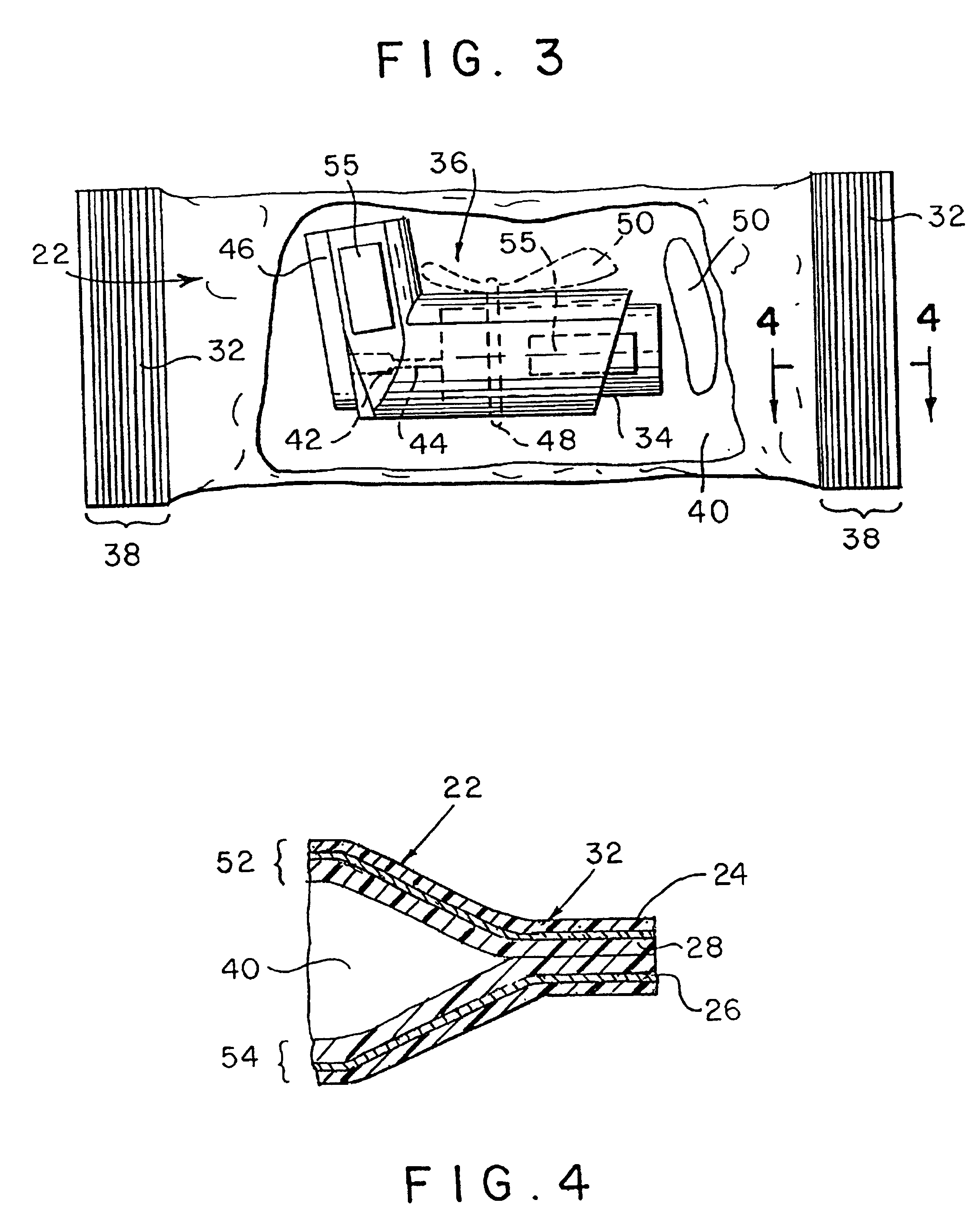
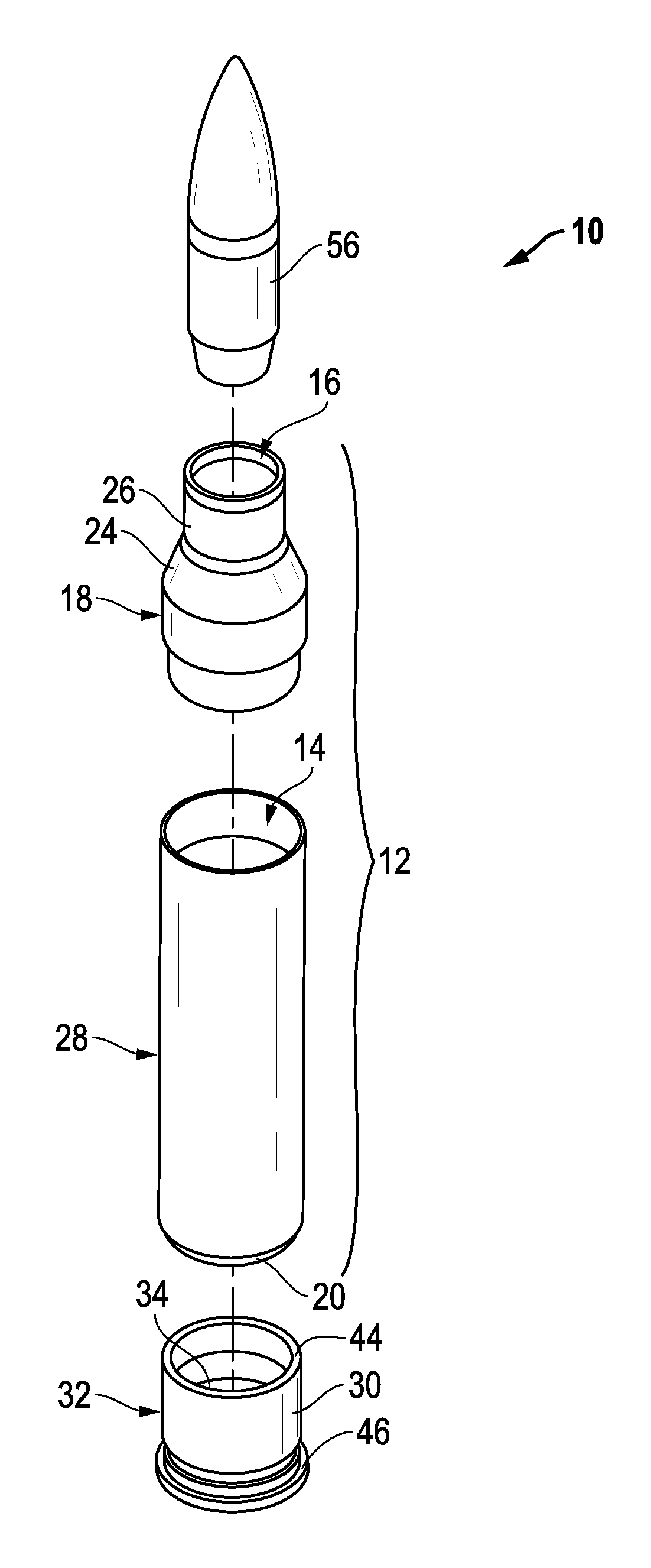
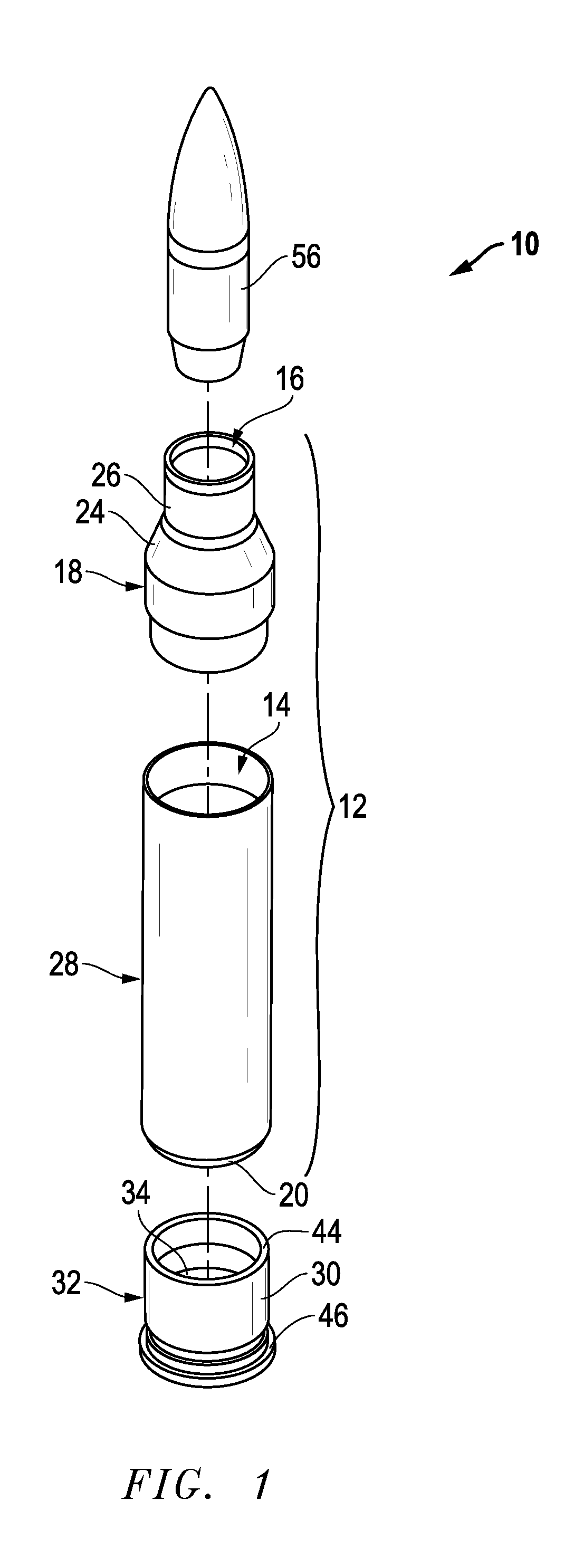
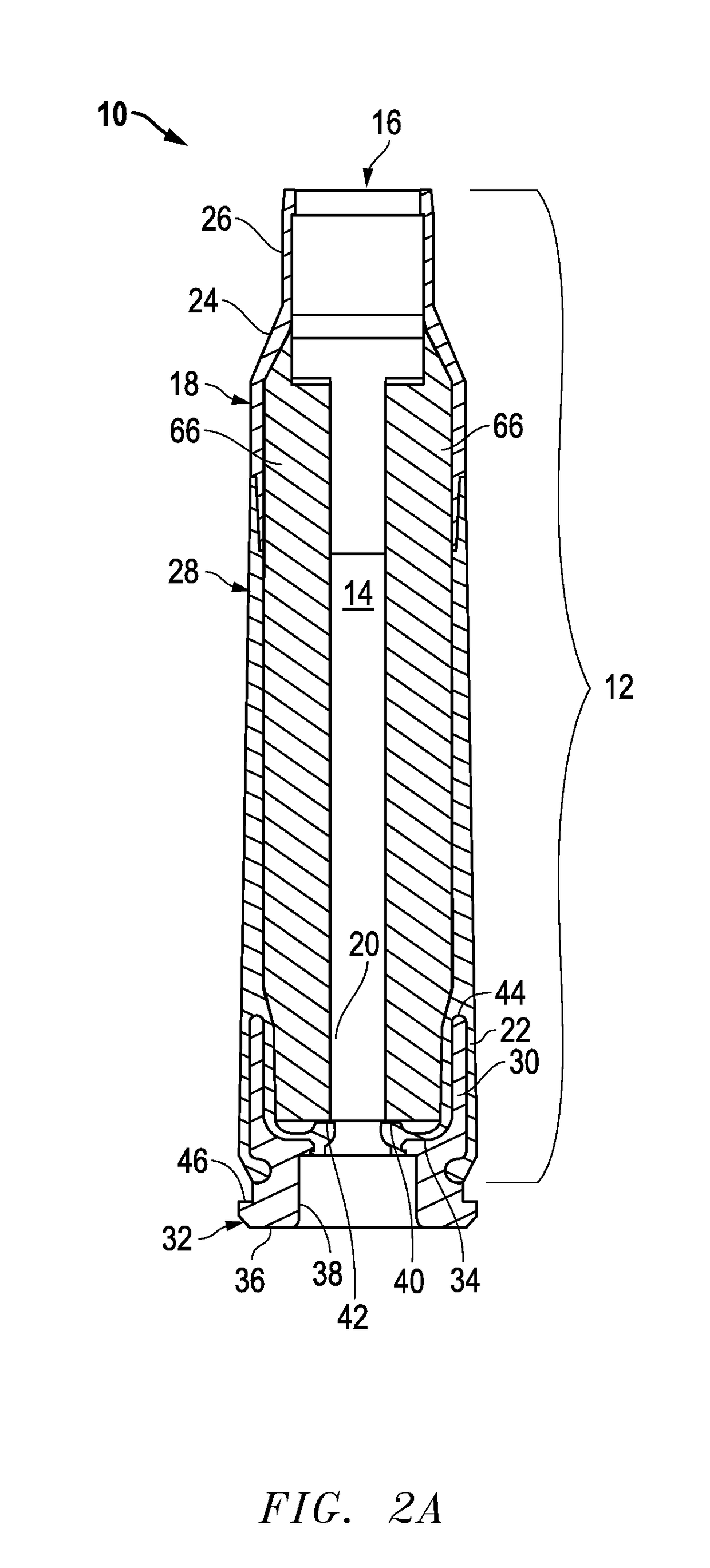
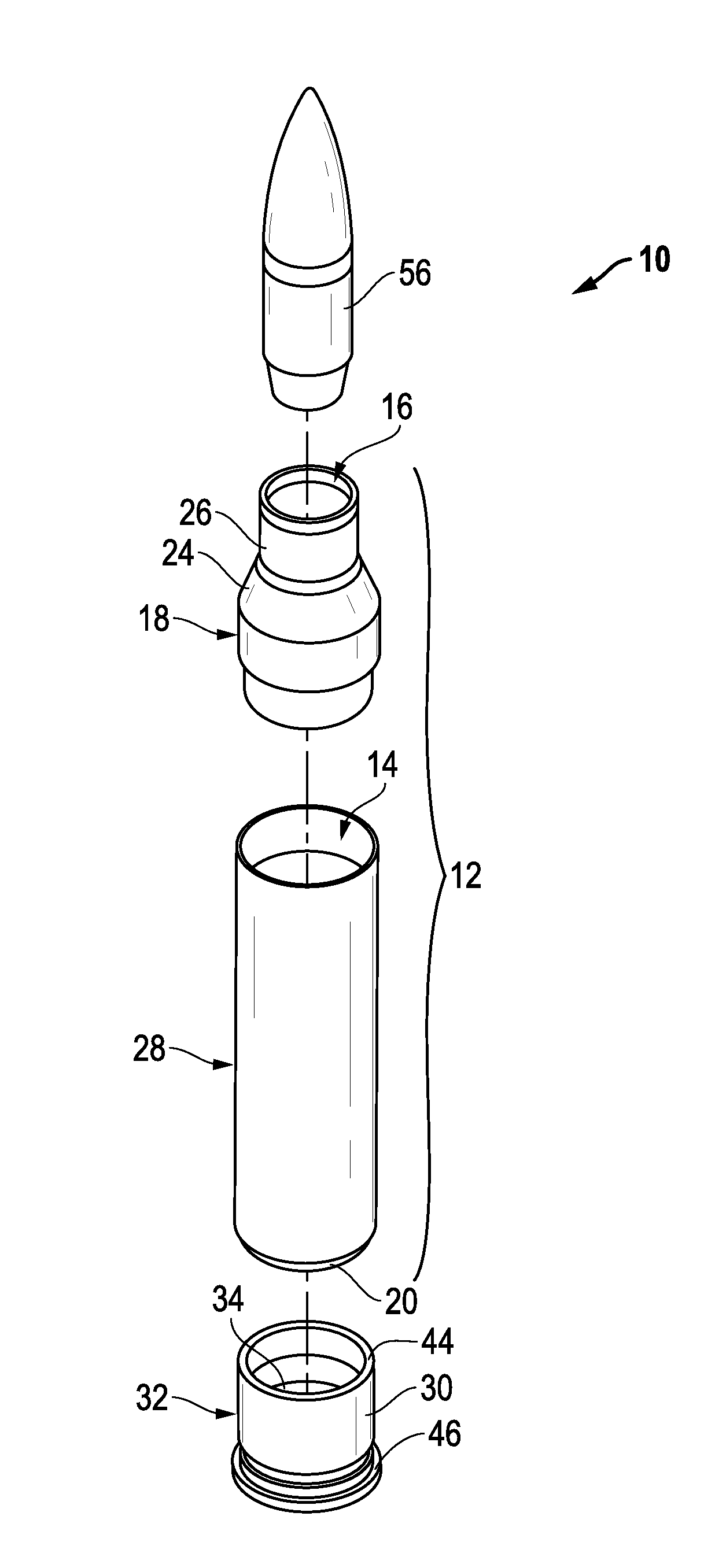
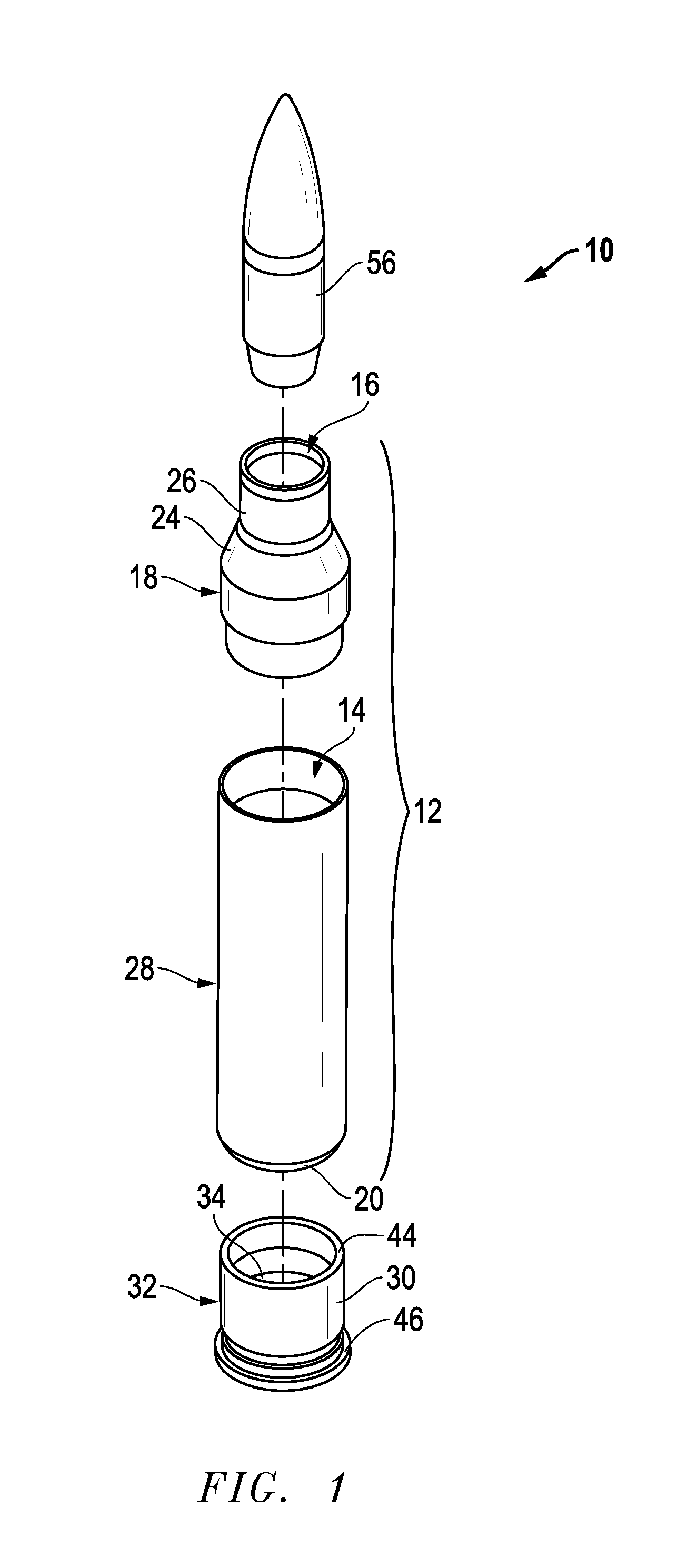
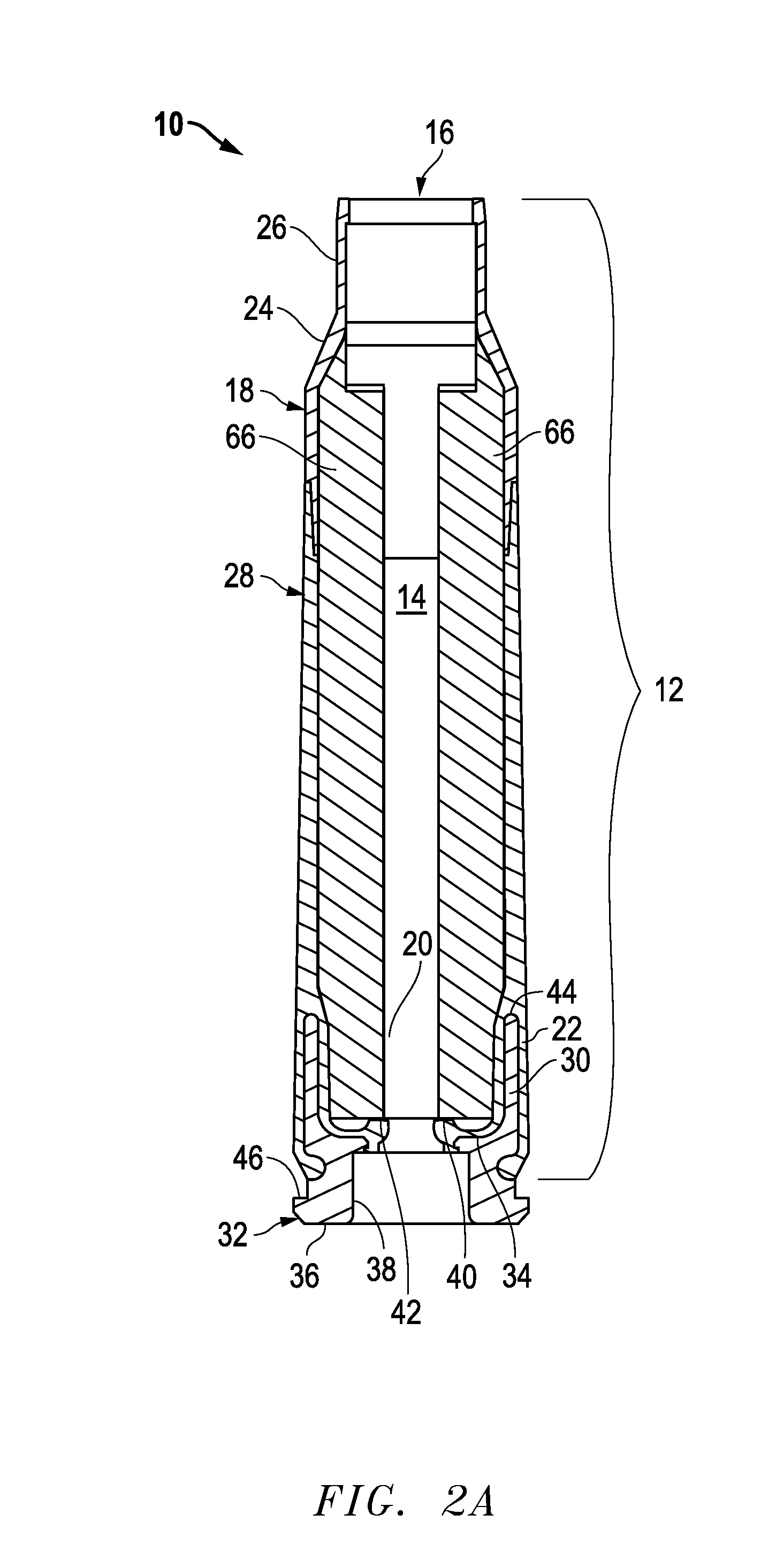
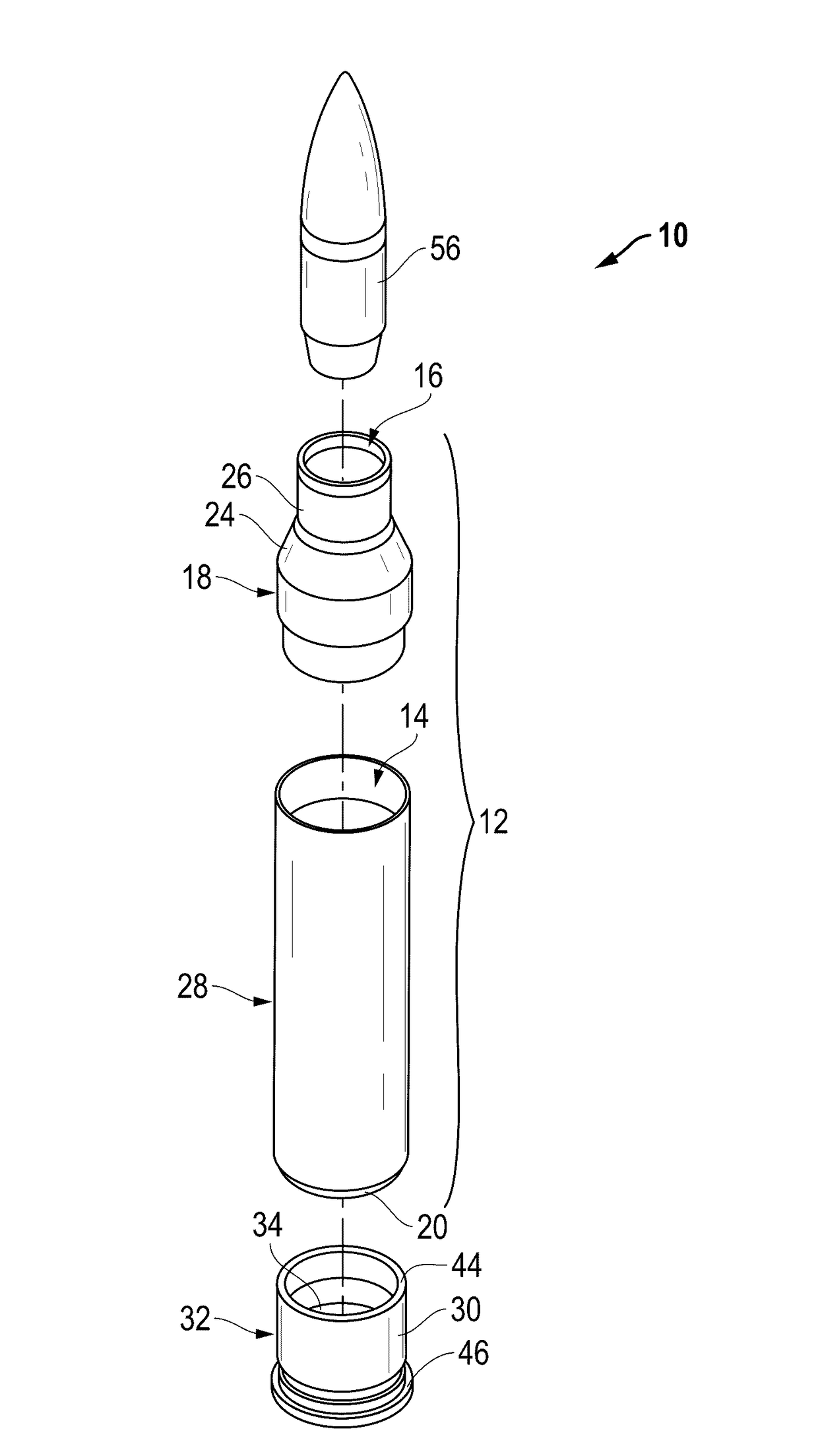
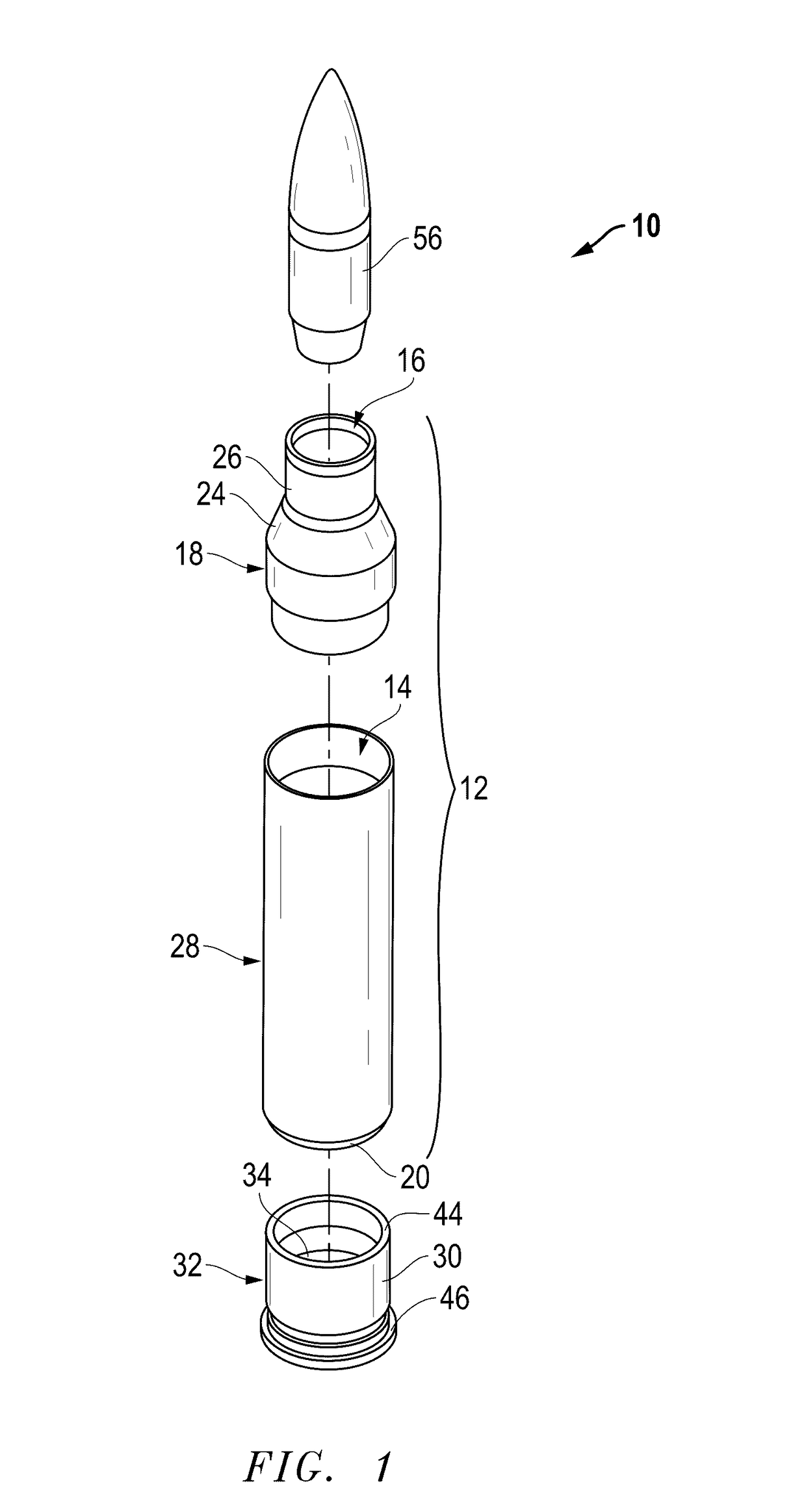
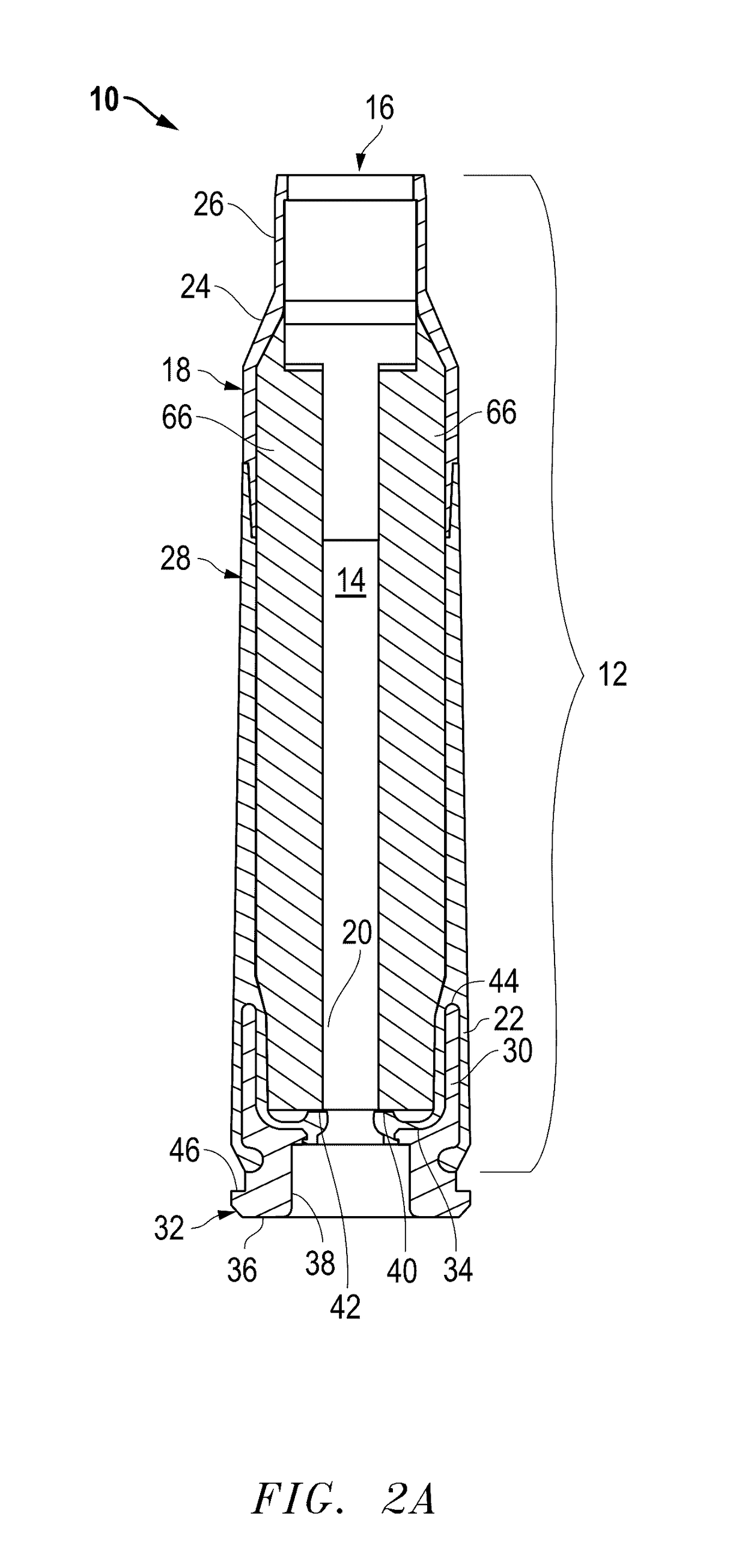
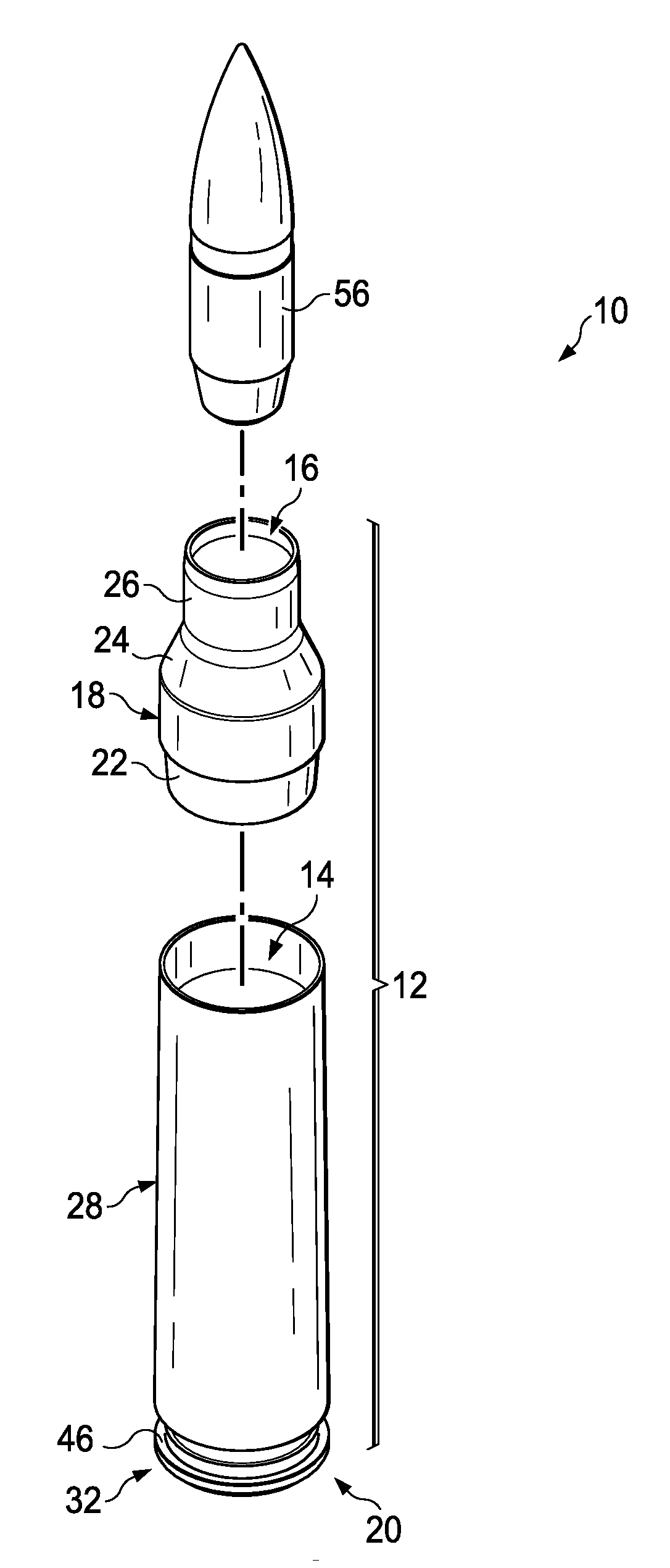
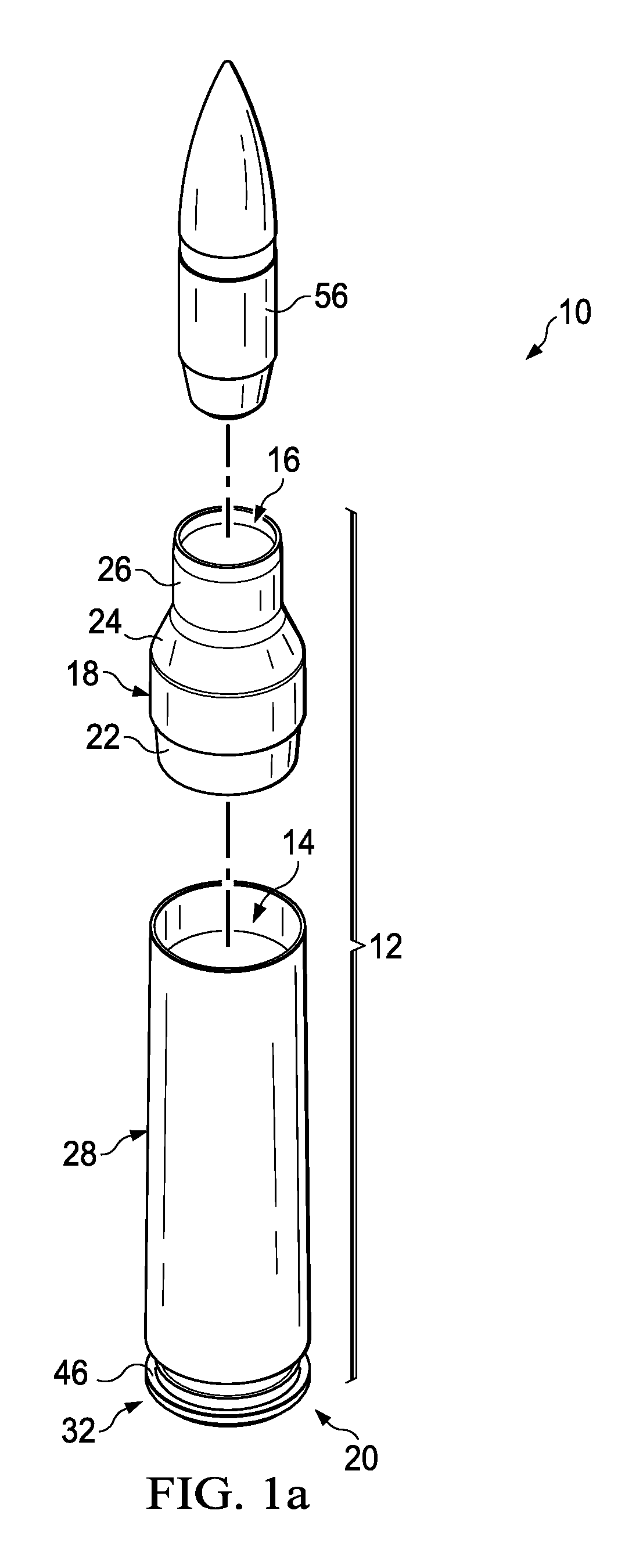
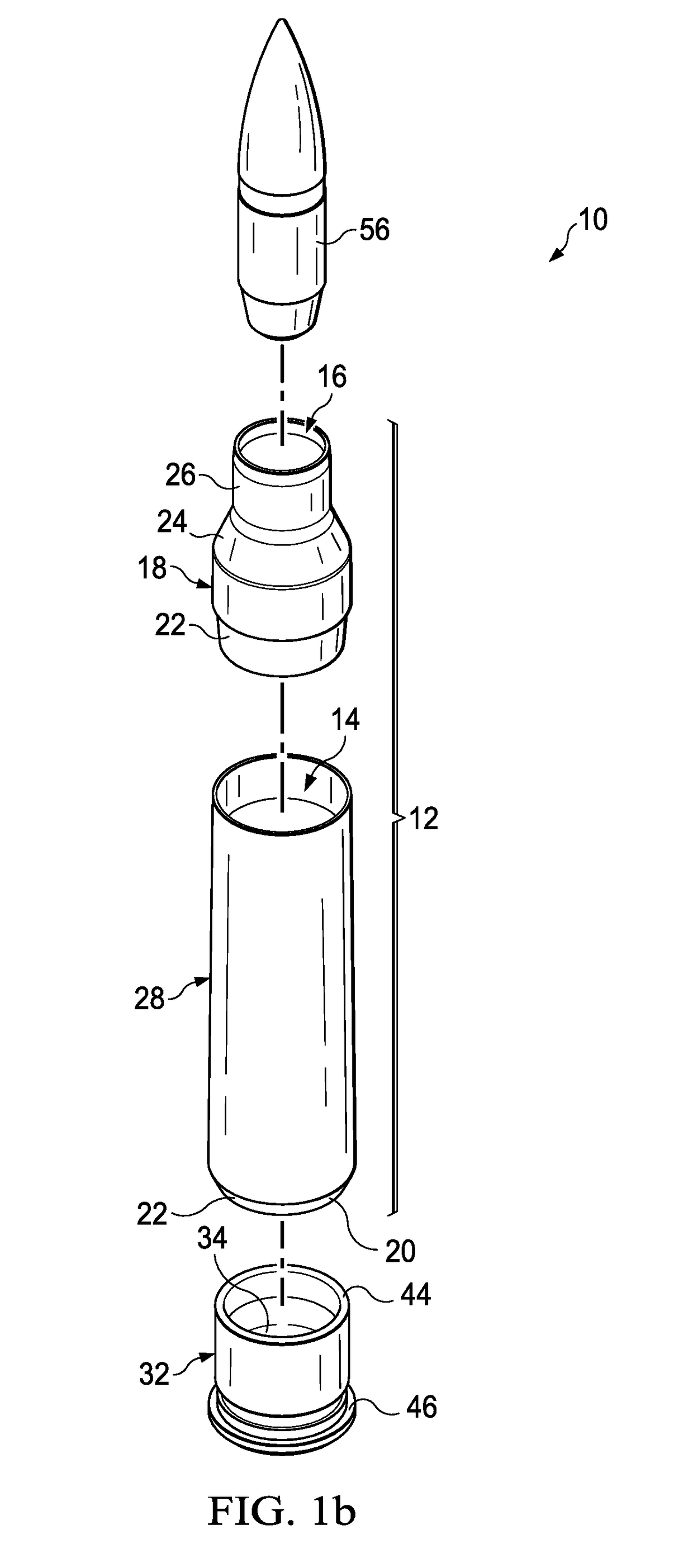
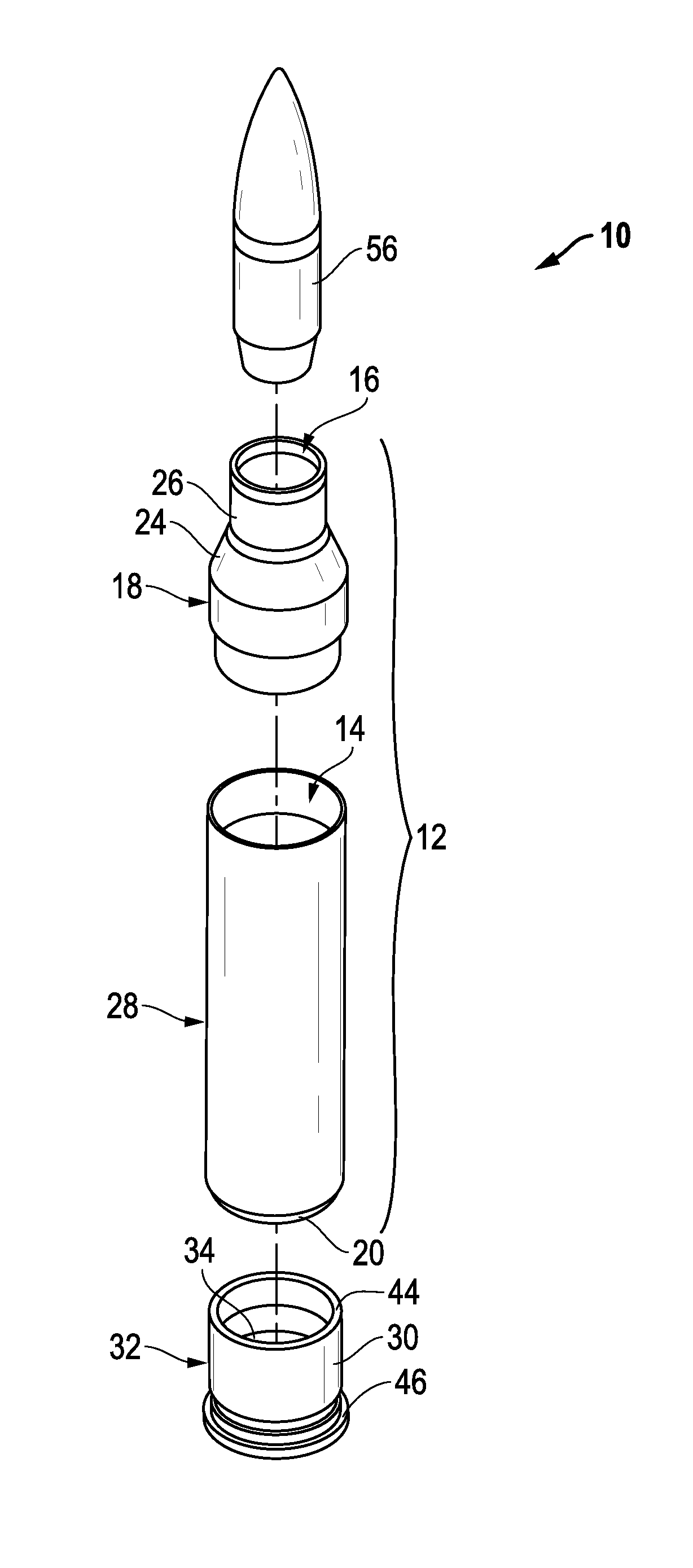
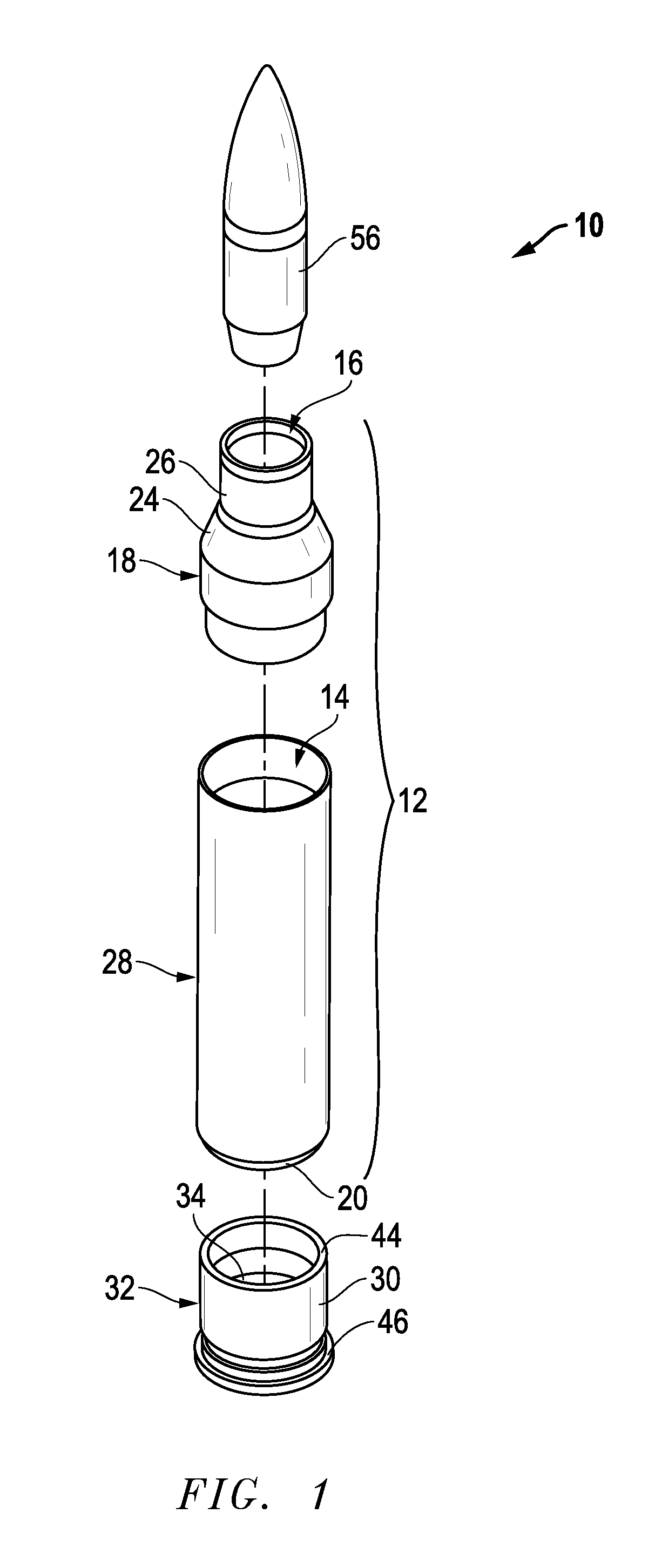
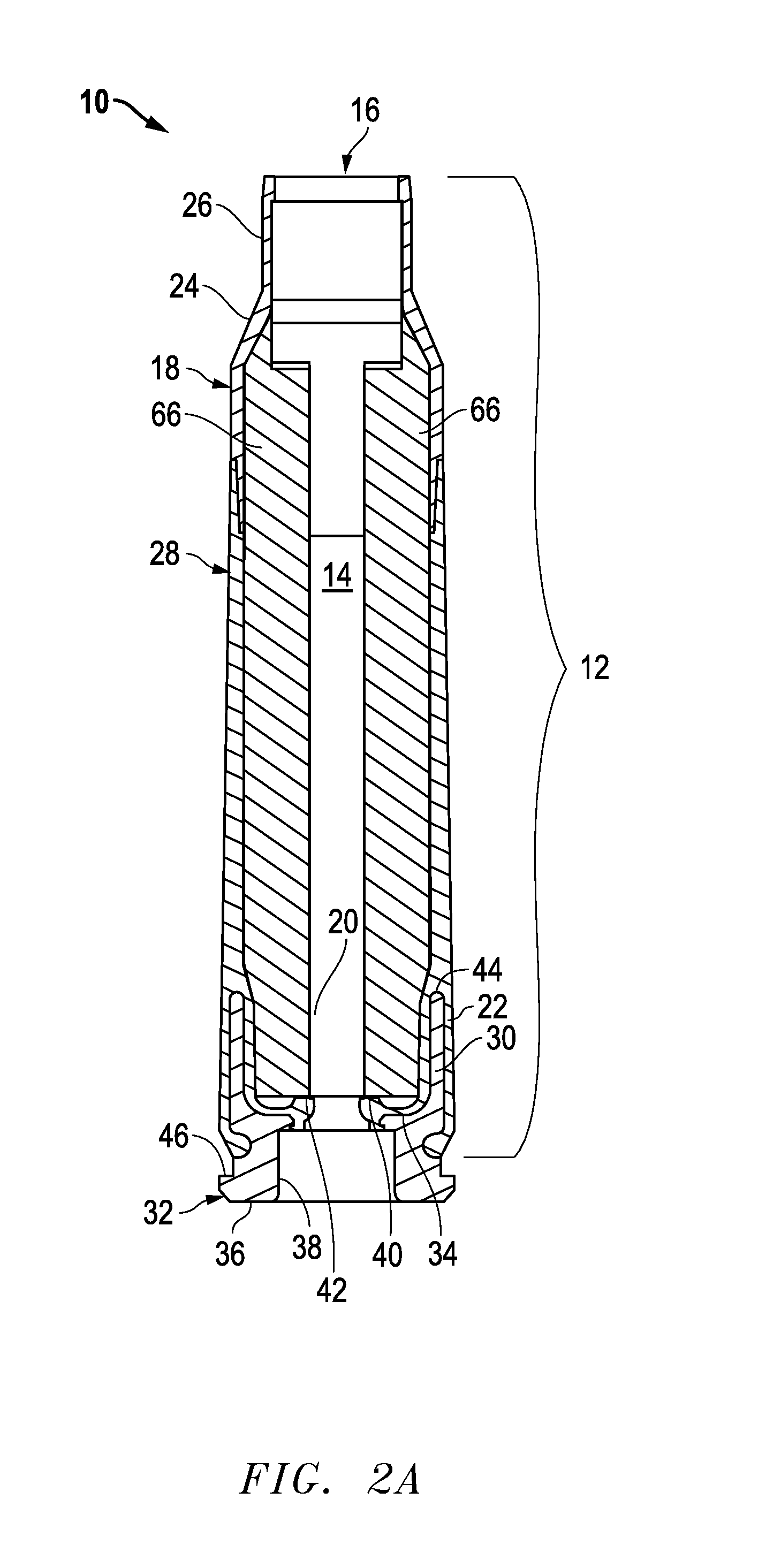
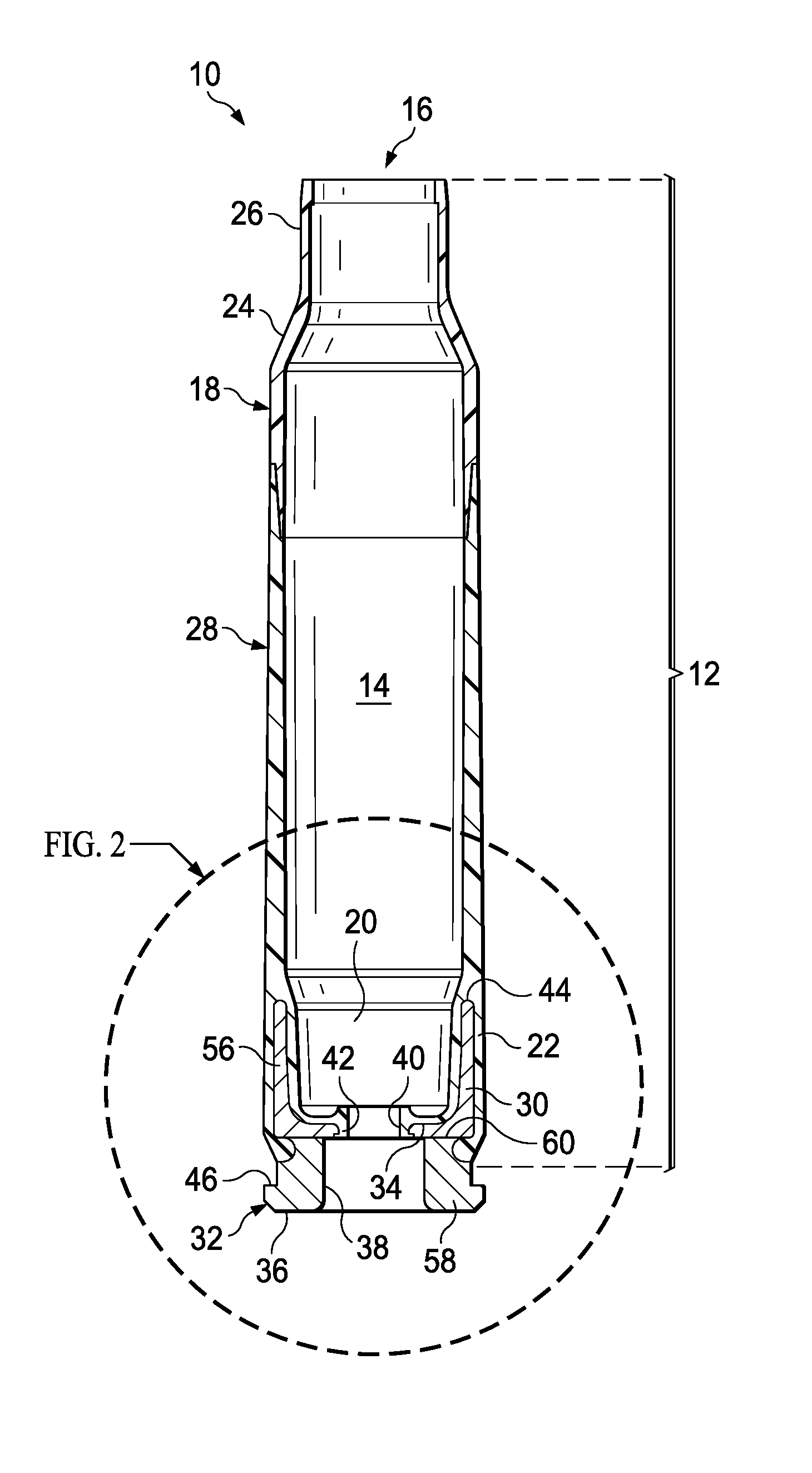
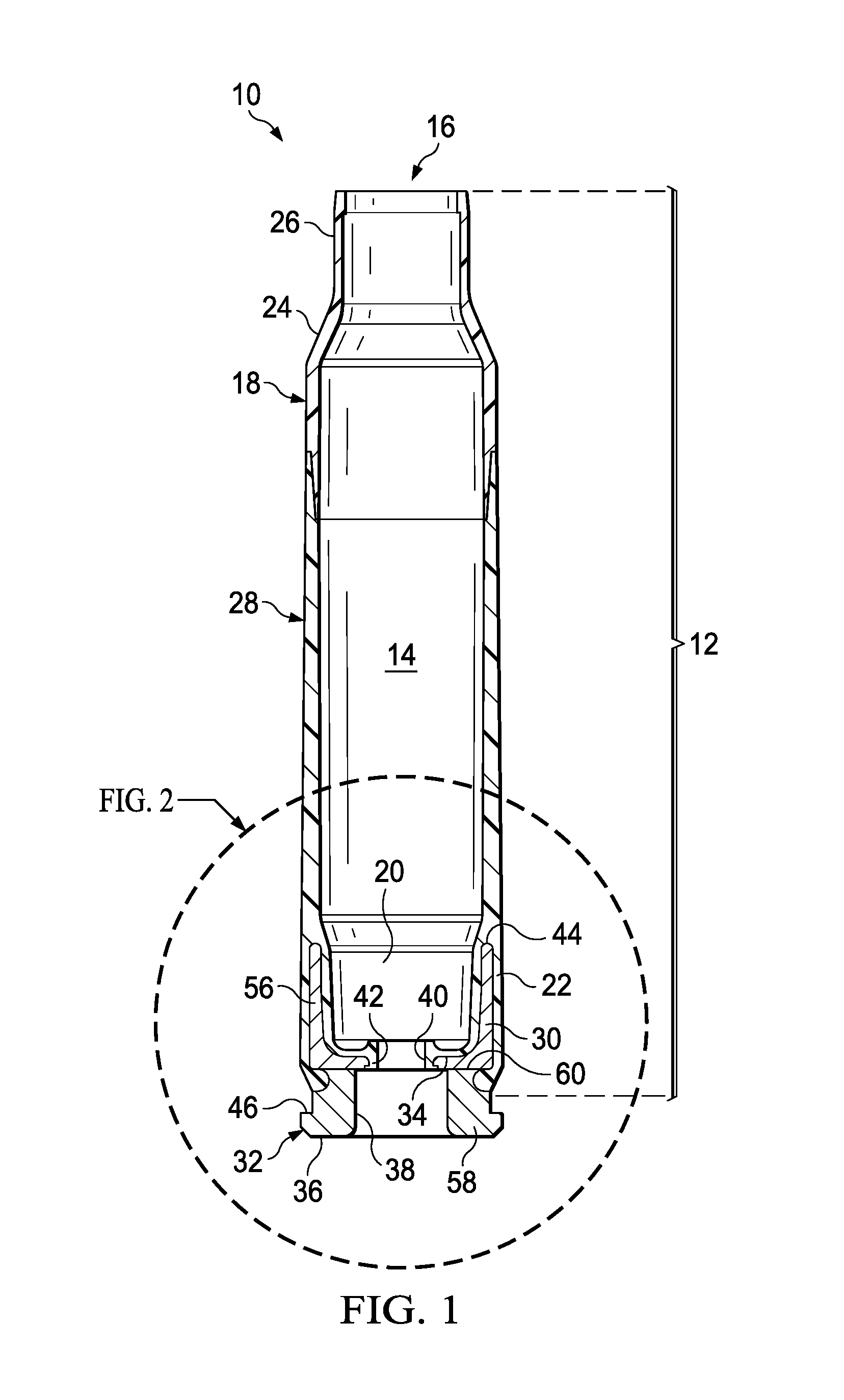
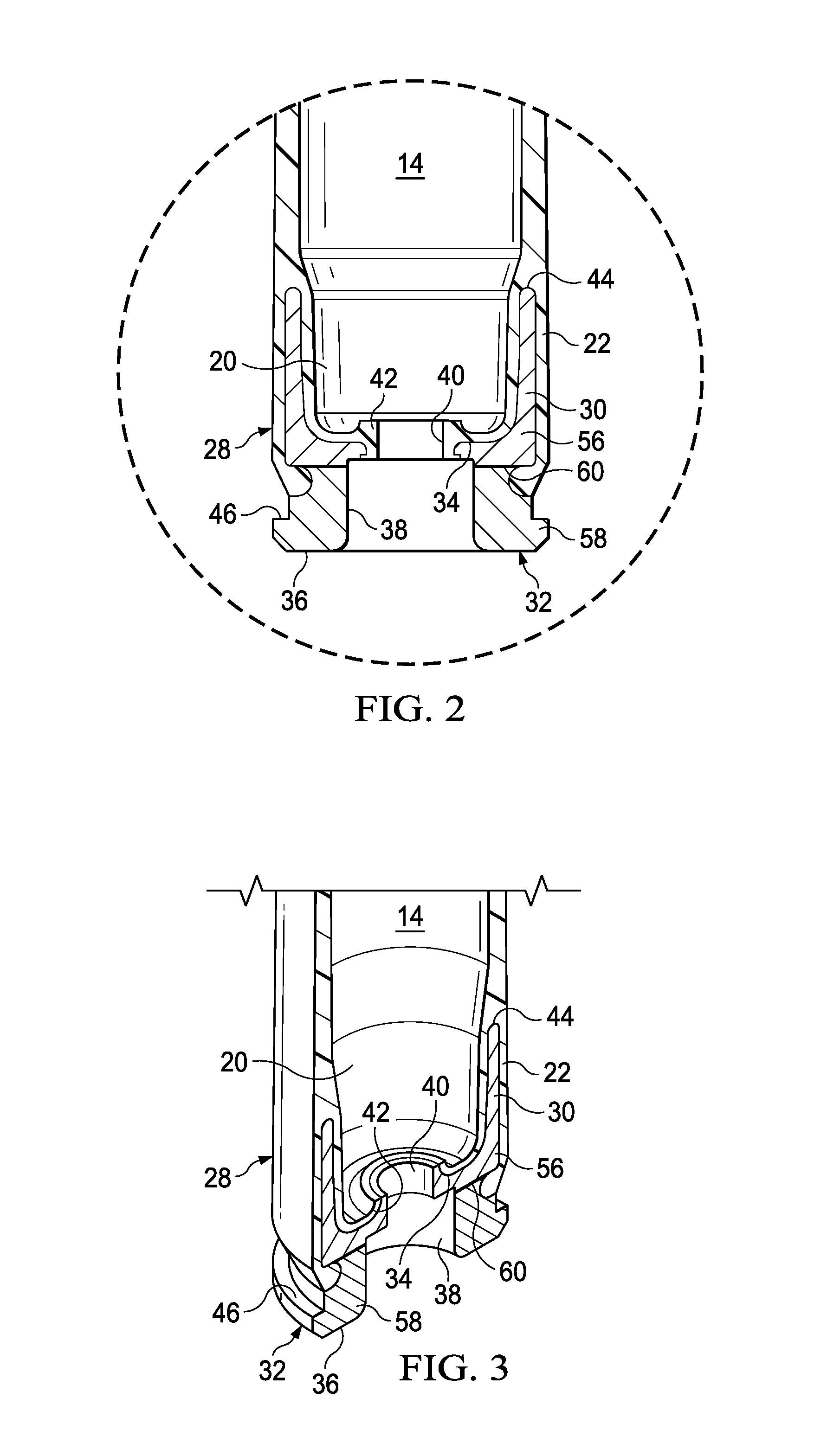
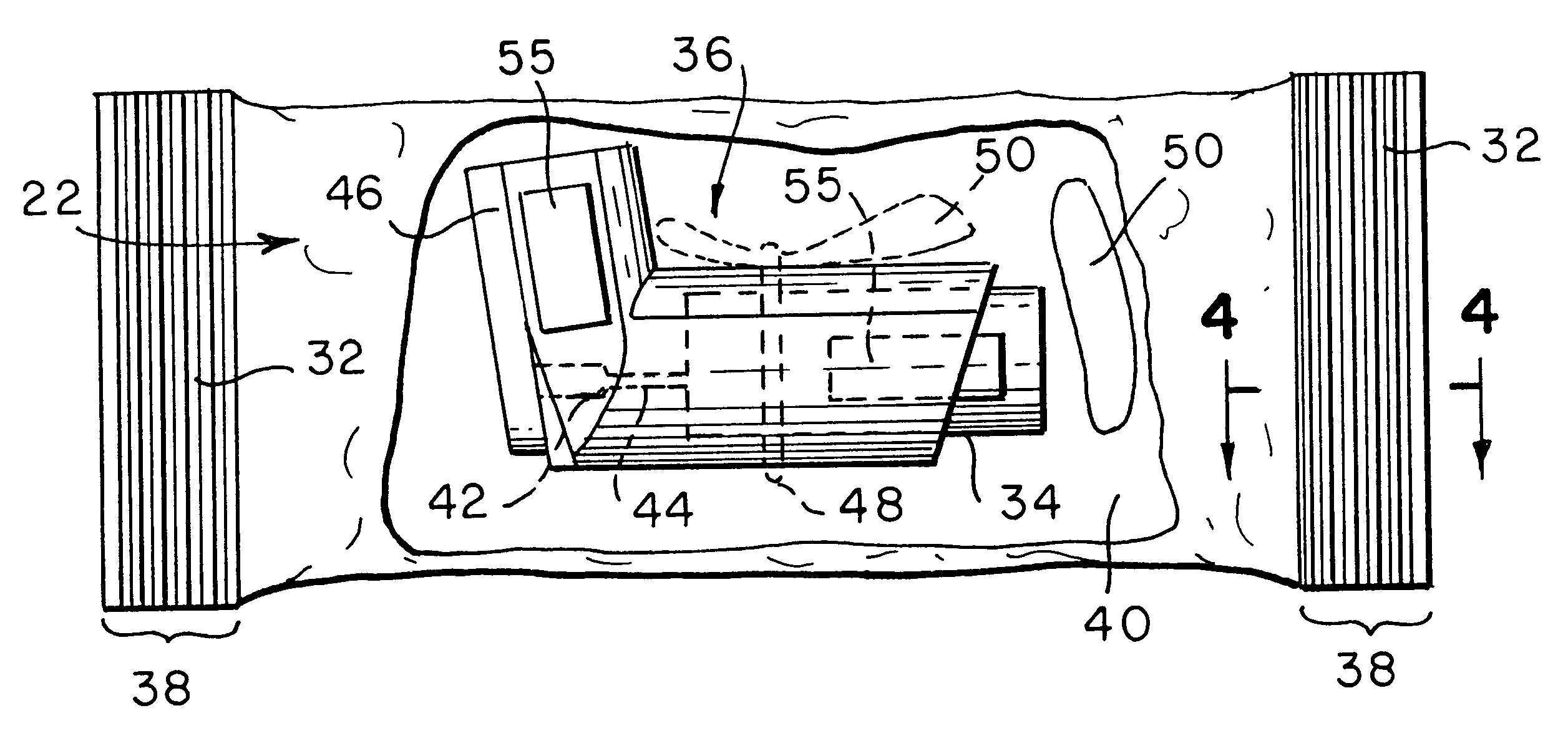
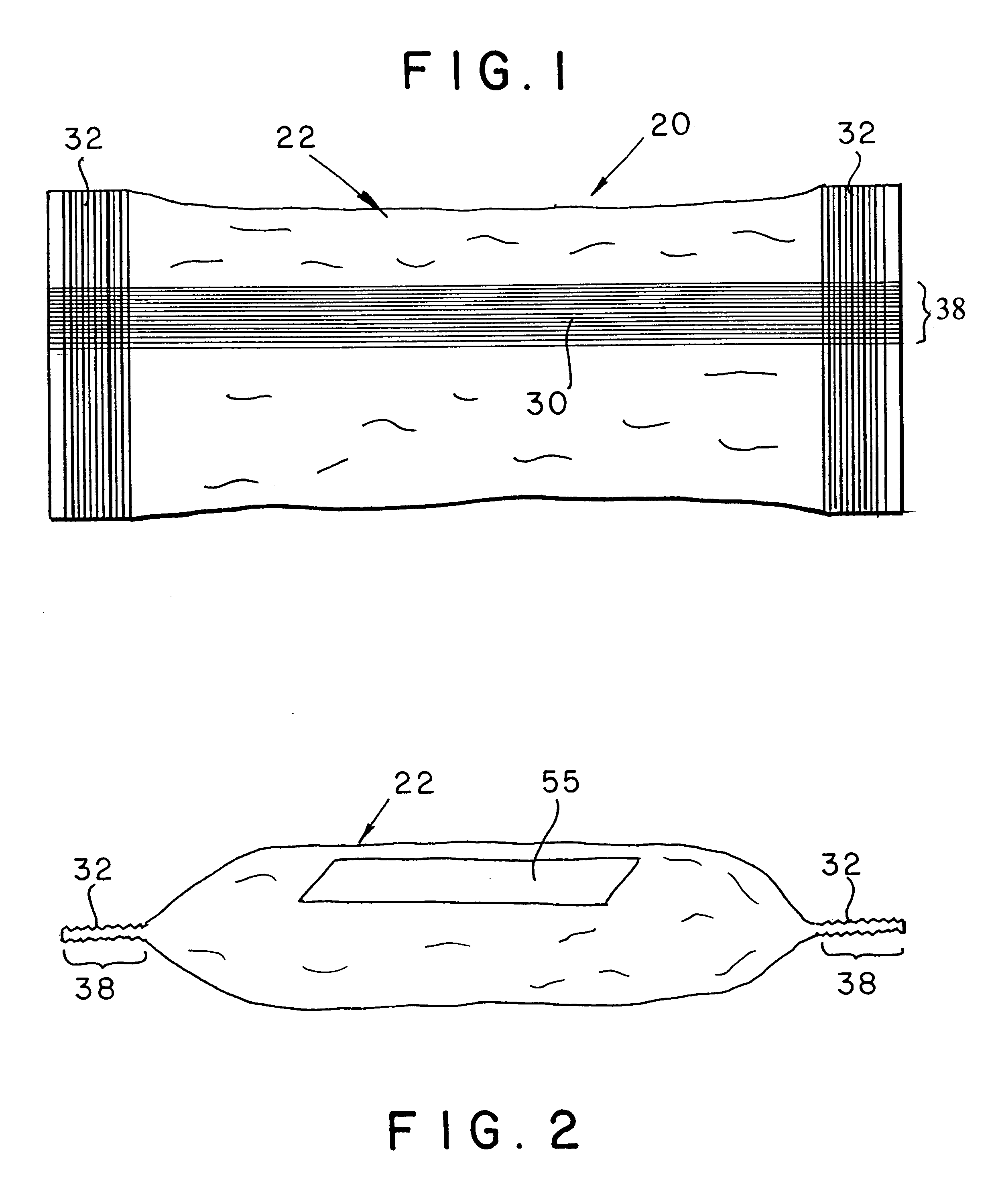
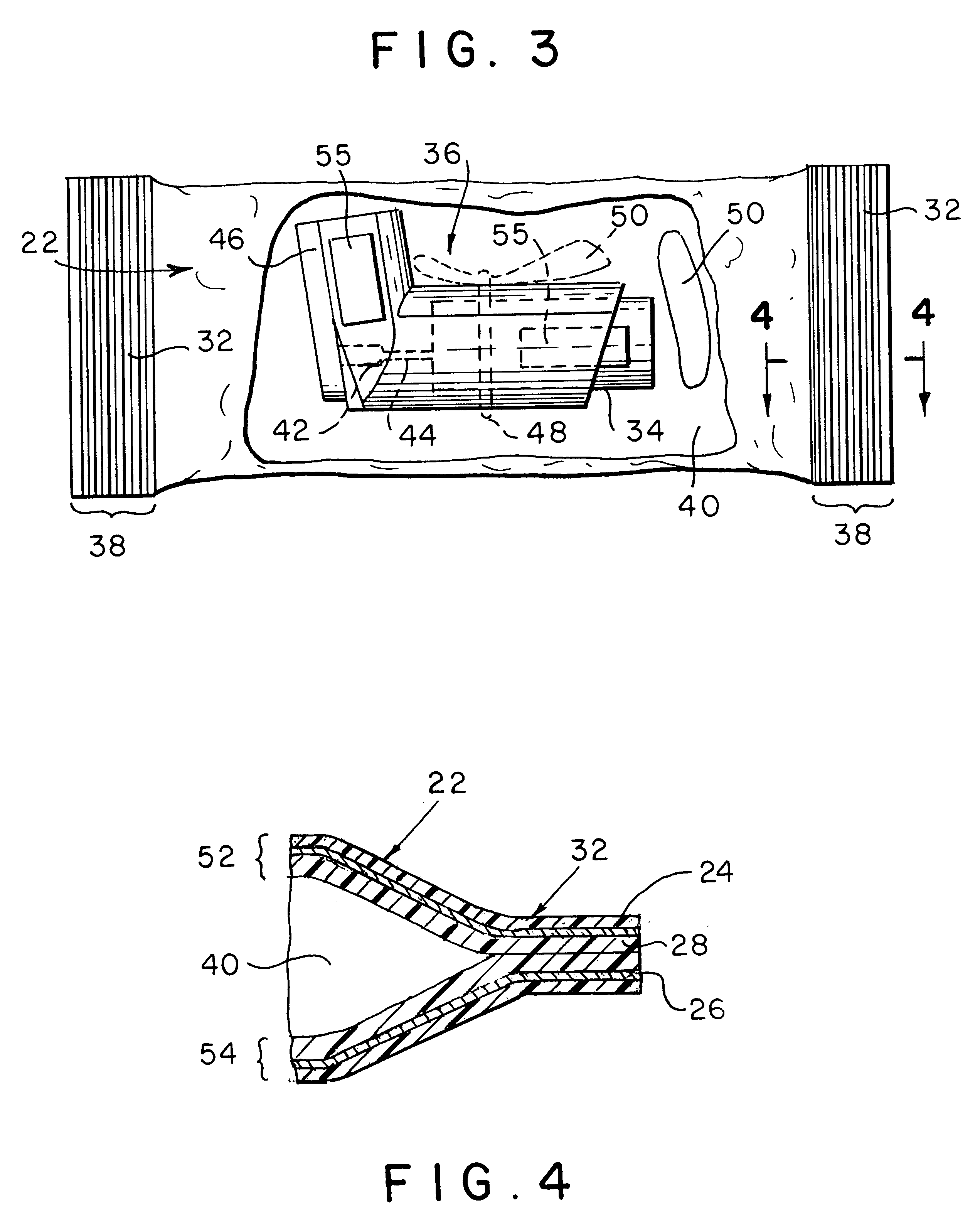
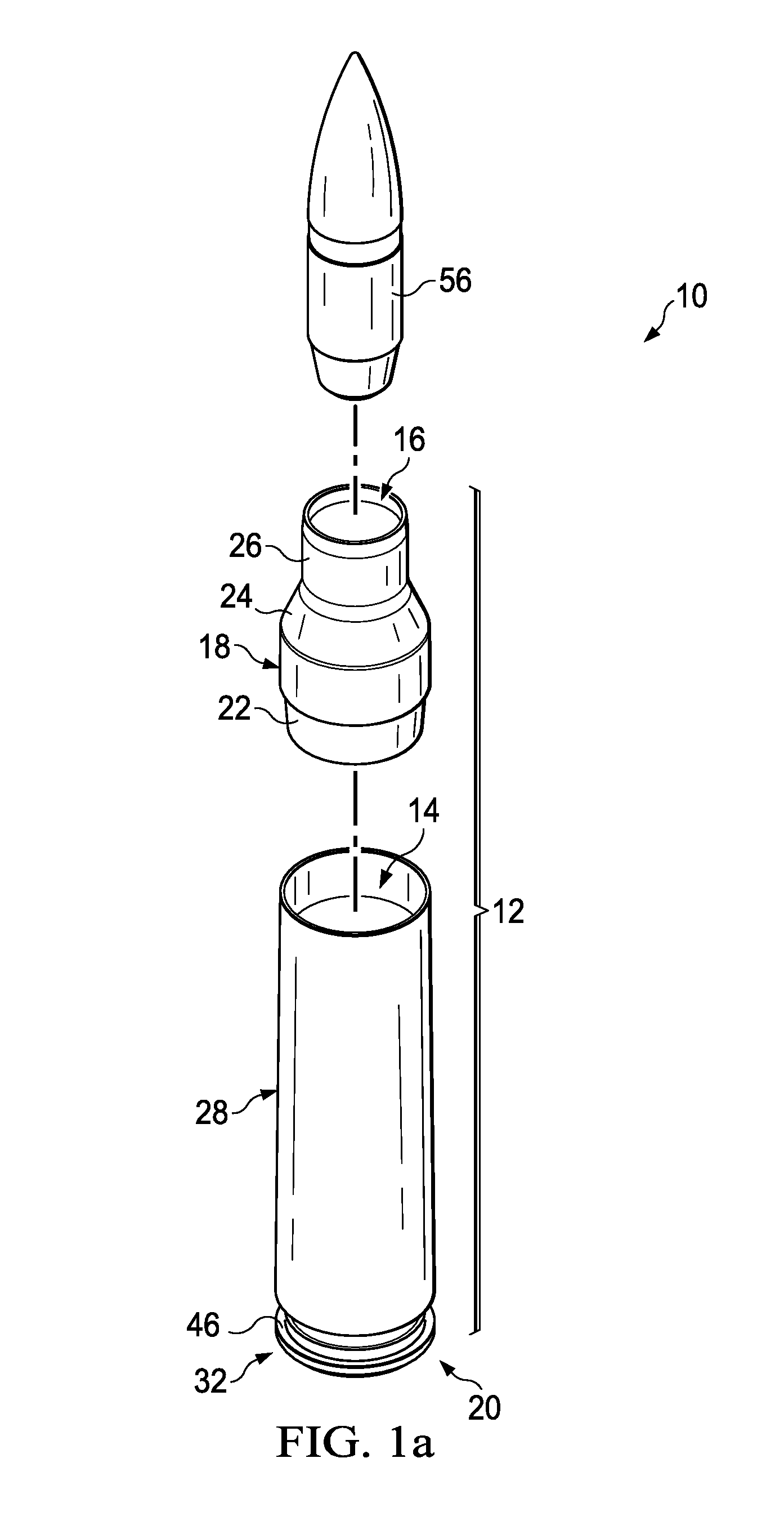
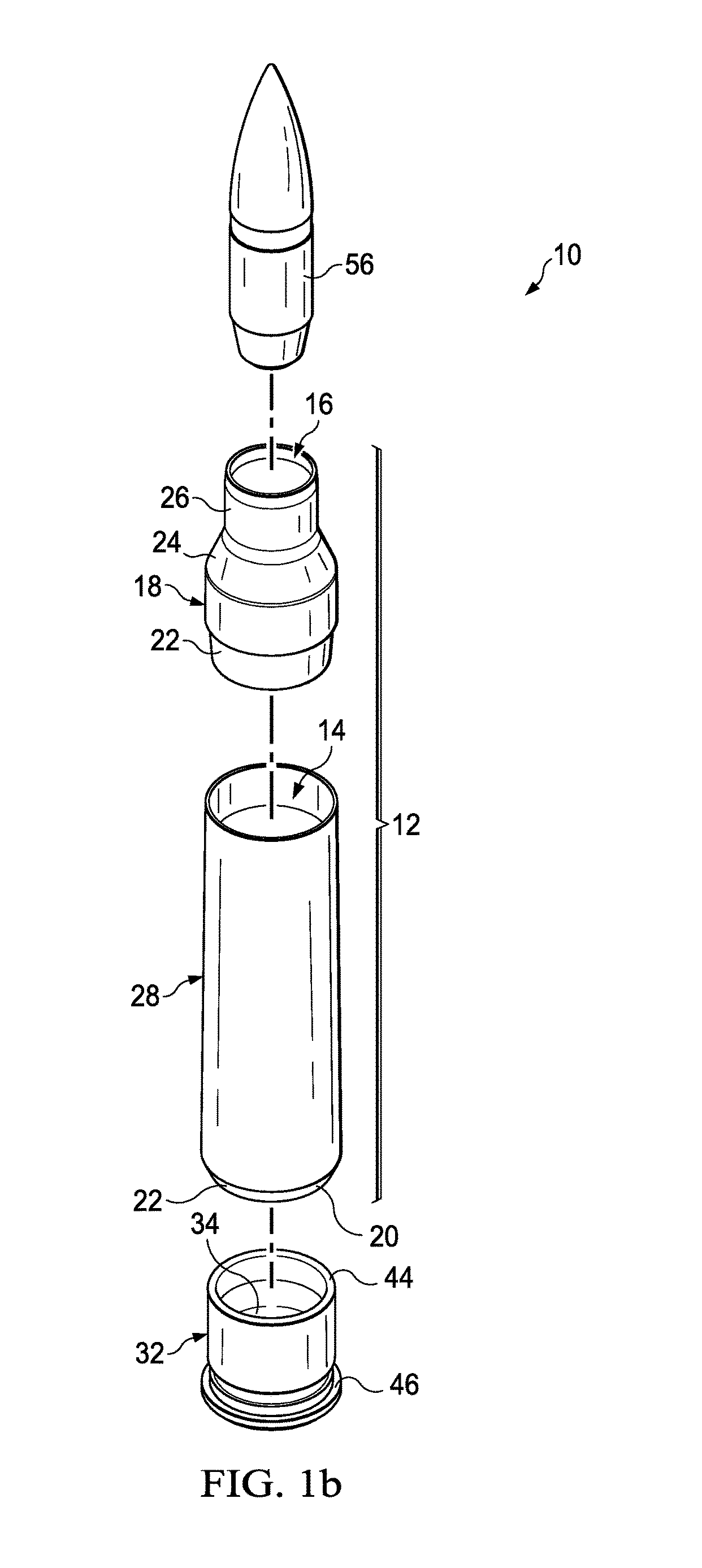
Ammunition assembly
Ammunition assemblies including ammunition cartridges are provided herein. According to some embodiments, ammunition cartridges may include a tubular casing having a first and a second end, wherein the tubular casing is at least partially constructed from a polymeric material, and wherein the tubular casing is adapted to receive and retain a propellant, a cap releaseably associable with the first end of the tubular casing, and a rim extending from the second end of the tubular casing, the rim and second end of the tubular casing cooperating together to form an extraction groove for receiving at least a portion of an extractor of a firearm.
Owner:MASON MARK
Oleaginous pharmaceutical and cosmetic foam
ActiveUS20050031547A1Pleasant and easy to spreadPatient compliance is goodAntibacterial agentsCosmetic preparationsActive agentNon ionic
The invention relates to stable oleaginous cosmetic or therapeutic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent selected from a hydrophobic solvent, a silicone oil, an emollient, a co-solvent, and mixtures thereof, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition; at least one gelling agent at a concentration of about 0.1% to about 5% by weight of the total composition; a therapeutically effective amount of at least one active agent; and at least one liquefied or compressed gas propellant, at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
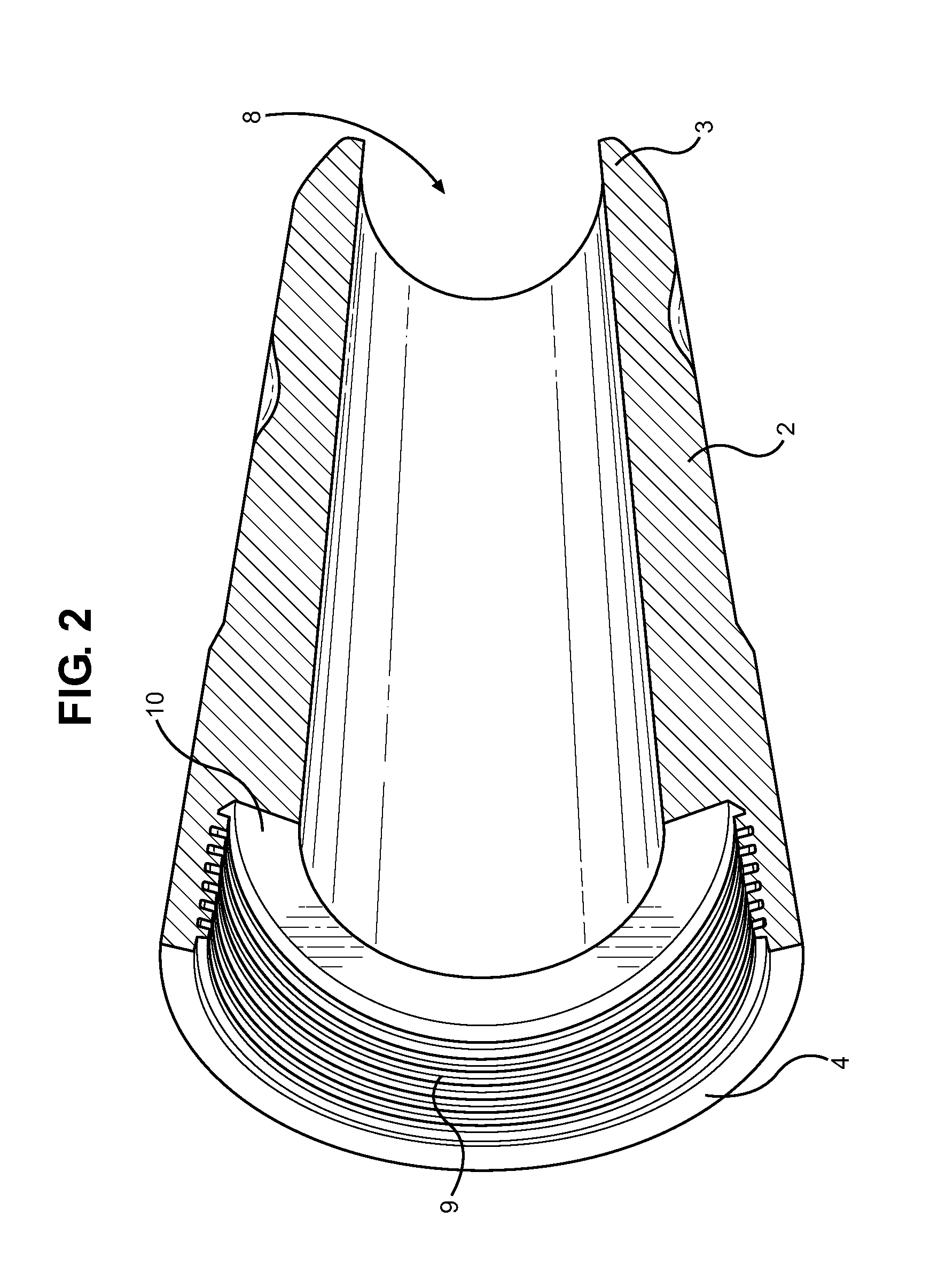
Munition having a reusable housing assembly and a removable powder chamber
A reusable shell casing having a housing with exterior grooves having a substantially central bore with a tapered end capable of receiving a payload and an opposite threaded end adapted to receive a novel reusable high pressure chamber assembly containing a removable powder chamber, a burst disk, and a threaded insert. The chamber assembly has a central bore having an upper bore portion that is cylindrically shaped having a threaded surface adapted to secure to the threaded portion of a threaded insert. The central bore also has a lower bore portion that is cylindrically shaped having a surface that is adapted to closely contour the circumference of the removable powder chamber. The powder chamber has a primary end having a primary opening adapted to receive a propellant located opposite a secondary end having a protrusion. The protrusion has a secondary opening adapted to receive a primer.
Owner:PACE SCOT M
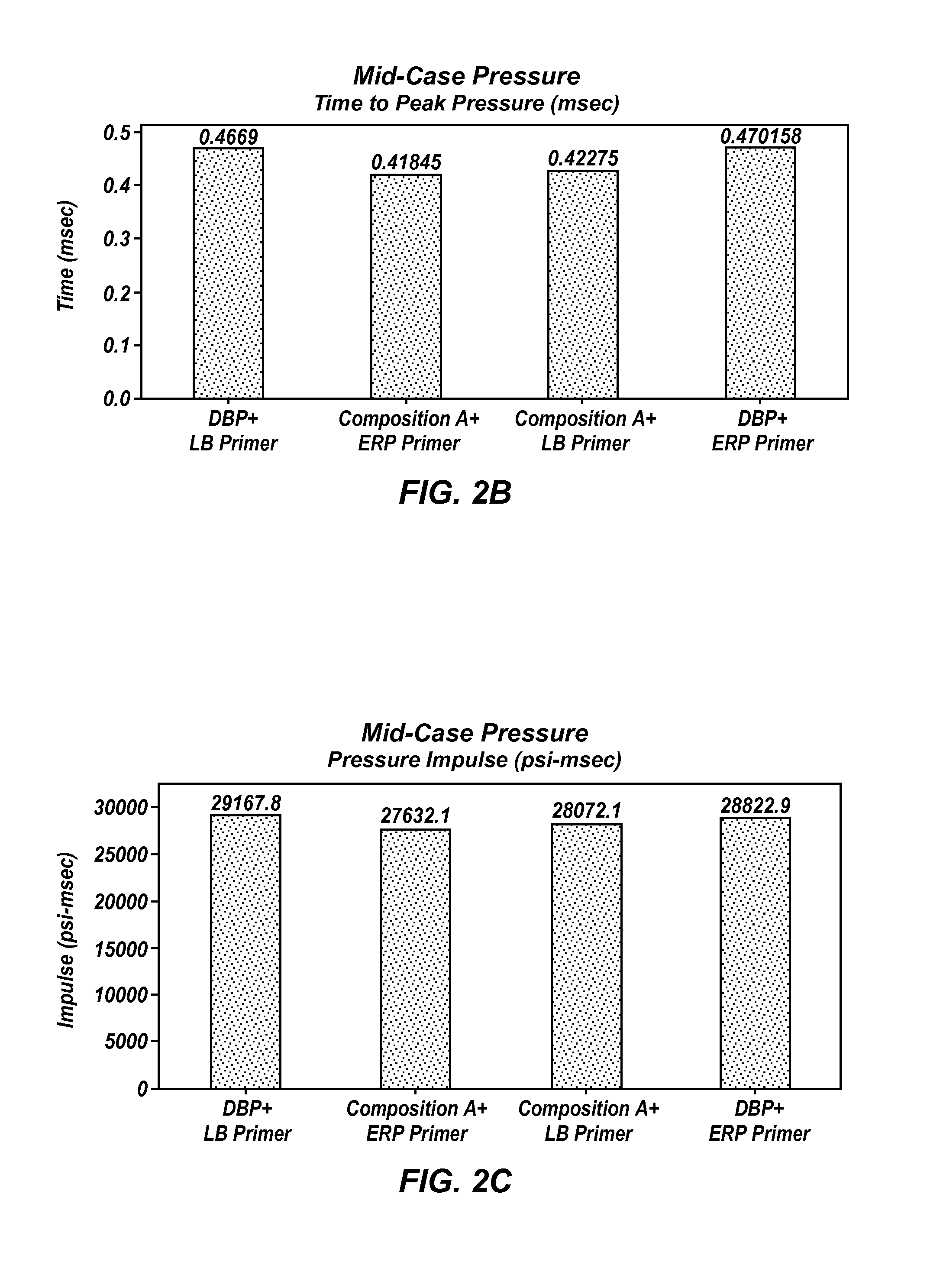
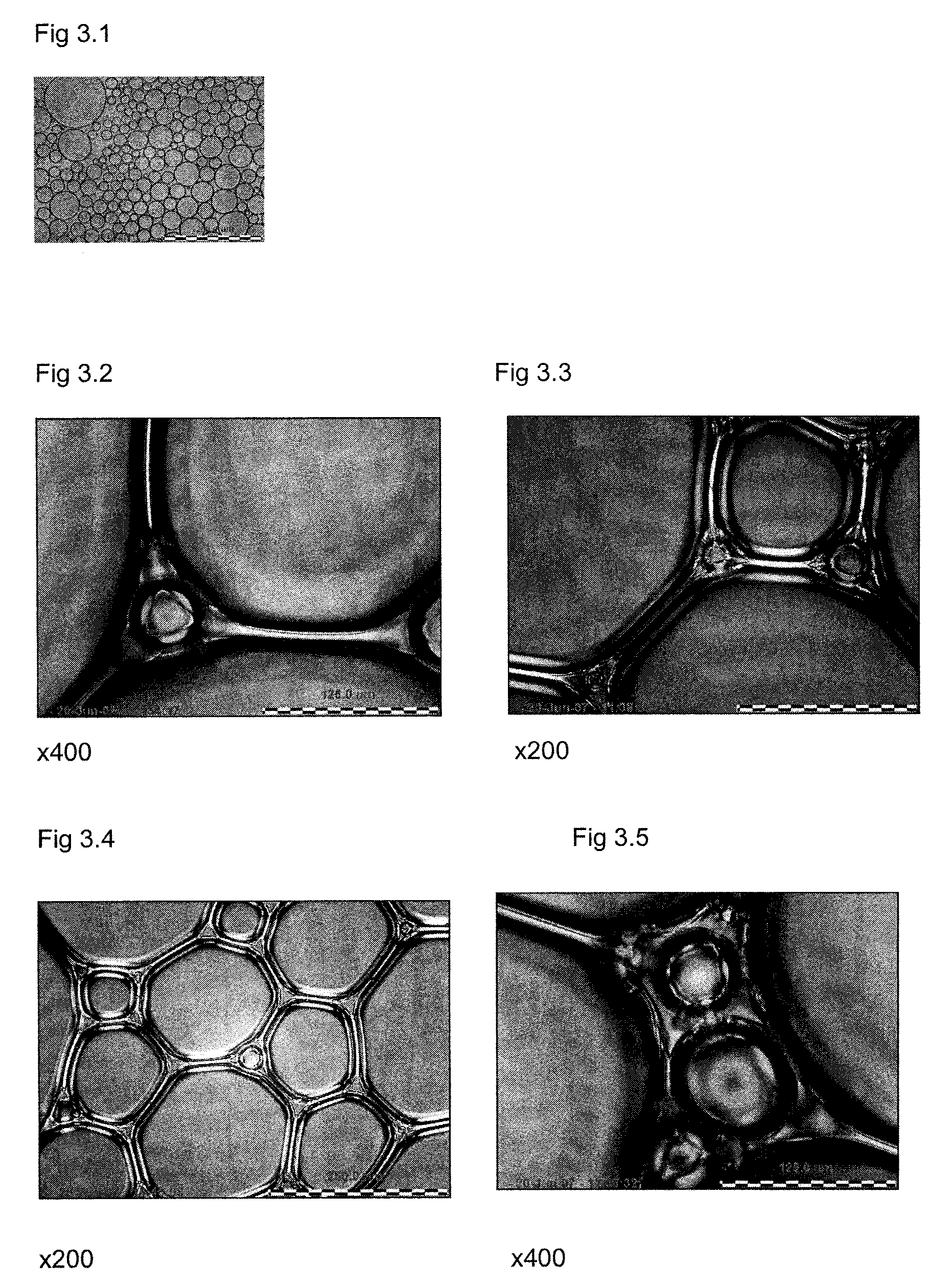
Propellant compositions including stabilized red phosphorus, a method of forming same, and an ordnance element including the same
Propellant compositions include an energetic binder, such as nitrocellulose, and a stabilized, encapsulated red phosphorous as a ballistic modifier. The propellant composition may additionally include an energetic plasticizer, such as nitroglycerine. For example, the propellant composition may be formed by mixing a double or multi base propellant that includes nitrocellulose plasticized with nitroglycerine with the stabilized, encapsulated red phosphorus. The propellant compositions may be substantially lead-free and may exhibit improved ballistic properties. Methods of forming such propellant compositions and an ordnance device including such propellant compositions are also disclosed.
Owner:NORTHROP GRUMMAN SYST CORP
Anti-infection augmentation foamable compositions and kit and uses thereof
Anti-infective foamable composition and kits include a foamable carrier; a therapeutically safe and effective concentration of an anti-infective agent; an augmenting agent selected from the group consisting of a keratolytic agent and a skin penetration enhancer; and a propellant. The composition is housed in a container and upon release is expandable to form a breakable foam. The foamable carrier is selected to generate a foam of good or excellent quality in the presence of the augmenting agent and anti-infective agent. Methods for treating, alleviating or preventing a disorder of the skin, a body cavity or mucosal surface, wherein the disorder involves a fungal, bacterial or viral infection as one of its etiological factors, is described.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Polypropylene glycol foamable vehicle and pharmaceutical compositions thereof
The present invention teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent water and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof. The present invention further teaches a foamable pharmaceutical carrier comprising polypropylene glycol (PPG) alkyl ether, a surface-active agent, and a liquefied hydrocarbon gas propellant; and pharmaceutical compositions thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
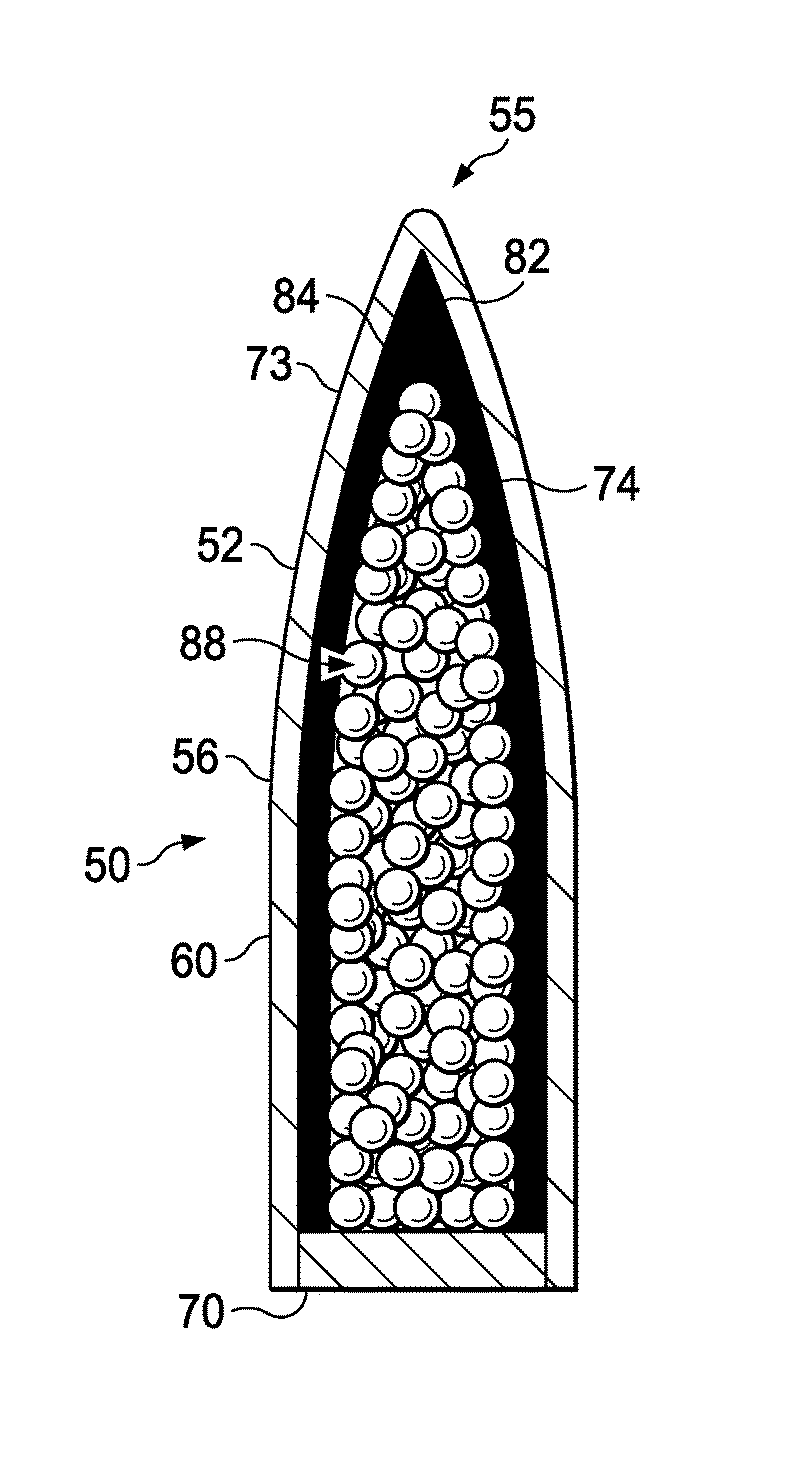
Subsonic polymeric ammunition
The present invention provides a subsonic ammunition including a polymeric casing body comprising a generally cylindrical hollow polymer body having a body base at a first end thereof and a mouth at a second end to define a propellant chamber; a propellant insert positioned in the propellant chamber to reduce the internal volume of the propellant chamber, wherein the propellant chamber has an internal volume that is at least 10% less than the open internal volume of a standard casing of equivalent caliber; a propellant disposed and confined within the propellant chamber; a primer insert positioned at the body base and in communication with the propellant chamber; a primer disposed in the primer insert in combustible communication with the propellant; and a projectile frictionally fitted in the mouth in combustible communication with the propellant.
Owner:TRUE VELOCITY IP HLDG LLC
Method and package for storing a pressurized container containing a drug
Owner:GLAXO SMITHKLINE LLC
Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof
ActiveUS20080044444A1Convenient vehicle for topical deliveryGood treatment effectPowder deliveryBiocideDicarboxylic acidCarboxylic acid
The present invention teaches a foamable pharmaceutical carrier comprising a benefit agent, selected from the group consisting of a dicarboxylic acid and a dicarboxylic acid ester; a stabilizer selected from the group consisting of at least one surface-active agent; at least one polymeric agent and mixtures thereof; a solvent selected from the group consisting of water, a hydrophilic solvent, a hydrophobic solvent, a potent solvent, a polar solvent, a silicone, an emollient, and mixtures thereof, wherein the benefit agent, stabilizer and solvent are selected to provide a composition that is substantially resistant to aging and to phase separation and or can substantially stabilize other active ingredients. The invention further relates to a foamable composition further containing a liquefied hydrocarbon gas propellant.
Owner:VYNE THERAPEUTICS INC
Foamable composition combining a polar solvent and a hydrophobic carrier
The present invention relates to a foamable vehicle or cosmetic or pharmaceutical composition, comprising: (1) an organic carrier, at a concentration of 10% to 70% by weight, wherein said organic carrier concurrently comprises: (i) at least one hydrophobic organic carrier, and (ii) at least one polar solvent; (2) at least one surface-active agent; (3) water; and (4) at least one liquefied or compressed gas propellant at a concentration of 3% to 25% by weight of the total composition. The present invention further provides a method of treating, alleviating or preventing a disorder of mammalian subject, comprising administering the above-mentioned compositions to an afflicted target site.
Owner:VYNE THERAPEUTICS INC
Subsonic polymeric ammunition
The present invention provides a subsonic ammunition including a polymeric casing body comprising a generally cylindrical hollow polymer body having a body base at a first end thereof and a mouth at a second end to define a propellant chamber; a propellant insert positioned in the propellant chamber to reduce the internal volume of the propellant chamber, wherein the propellant chamber has an internal volume that is between 25 and 80% less than the open internal volume of a standard casing of equivalent caliber; a propellant disposed and confined within the propellant chamber; a primer insert positioned at the body base and in communication with the propellant chamber; a primer disposed in the primer insert in combustible communication with the propellant; and a projectile frictionally fitted in the mouth in combustible communication with the propellant.
Owner:TRUE VELOCITY IP HLDG LLC
Subsonic polymeric ammunition cartridge
ActiveUS20160349023A1Reduce internal volumeCombustible communicationCartridge ammunitionPolymerPropellant
The present invention provides a subsonic ammunition cartridge including a polymeric casing body comprising a generally cylindrical hollow polymer body having a body base at a first end thereof and a mouth at a second end to define a propellant chamber; a propellant insert positioned in the propellant chamber to reduce the internal volume of the propellant chamber, wherein the propellant chamber has an internal volume that is at least 10% less than the open internal volume of a standard casing of equivalent caliber; and a primer insert positioned at the body base and in communication with the propellant chamber.
Owner:TRUE VELOCITY IP HLDG LLC
Subsonic polymeric ammunition cartridge
ActiveUS20170089675A1Reduce internal volumeCombustible communicationAmmunition fuzesCartridge ammunitionPolymerPropellant
The present invention provides a subsonic ammunition cartridge including a polymeric casing body comprising a generally cylindrical hollow polymer body having a body base at a first end thereof and a mouth at a second end to define a propellant chamber; a propellant insert positioned in the propellant chamber to reduce the internal volume of the propellant chamber, wherein the propellant chamber has an internal volume that is between 25 and 80% less than the open internal volume of a standard casing of equivalent caliber; and a primer insert positioned at the body base and in communication with the propellant chamber.
Owner:TRUE VELOCITY IP HLDG LLC
Polymer ammunition having a projectile made by metal injection molding
The present invention provides an ammunition having a metal injection molded projectile and a polymer cartridge case comprising a polymer ammunition cartridge comprising a bottom portion and a top portion that enclose a propellant chamber, wherein the bottom portion comprises a primer recess in communication with a primer flash hole that extends into a propellant chamber and the top portion comprises a projectile aperture; a primer inserted into the primer flash hole aperture; a propellant at least partially filling the propellant chamber; and a metal injection molded projectile frictionally fitted in the projectile aperture, wherein the metal injection molded projectile comprises a nose extending essentially symmetrically to a shoulder, and an essentially cylindrical bearing surface extending from the shoulder to a base.
Owner:TRUE VELOCITY IP HLDG LLC
Method of making polymeric subsonic ammunition
The present invention provides a method of making a subsonic ammunition having a polymeric casing body having a generally cylindrical hollow polymer body having a body base at a first end thereof and a mouth at a second end to define a propellant chamber; a propellant insert positioned in the propellant chamber to reduce the internal volume of the propellant chamber, wherein the propellant chamber has an internal volume that is at least 10% less than the open internal volume of a standard casing of equivalent caliber; and a primer insert positioned in the body base and in communication with the propellant chamber.
Owner:TRUE VELOCITY IP HLDG LLC
Polymer ammunition having a two-piece primer insert
The present invention provides ammunition having a two piece primer insert with a flange, a polymeric middle body extending from the primer insert to a cylindrical middle body coupling region, a polymeric projectile end having a projectile aperture mated to the polymeric middle body, a primer inserted into the primer aperture, a propellant at least partially filling the propellant chamber, and a projectile frictionally fitted in the bullet-end aperture.
Owner:TRUE VELOCITY IP HLDG LLC
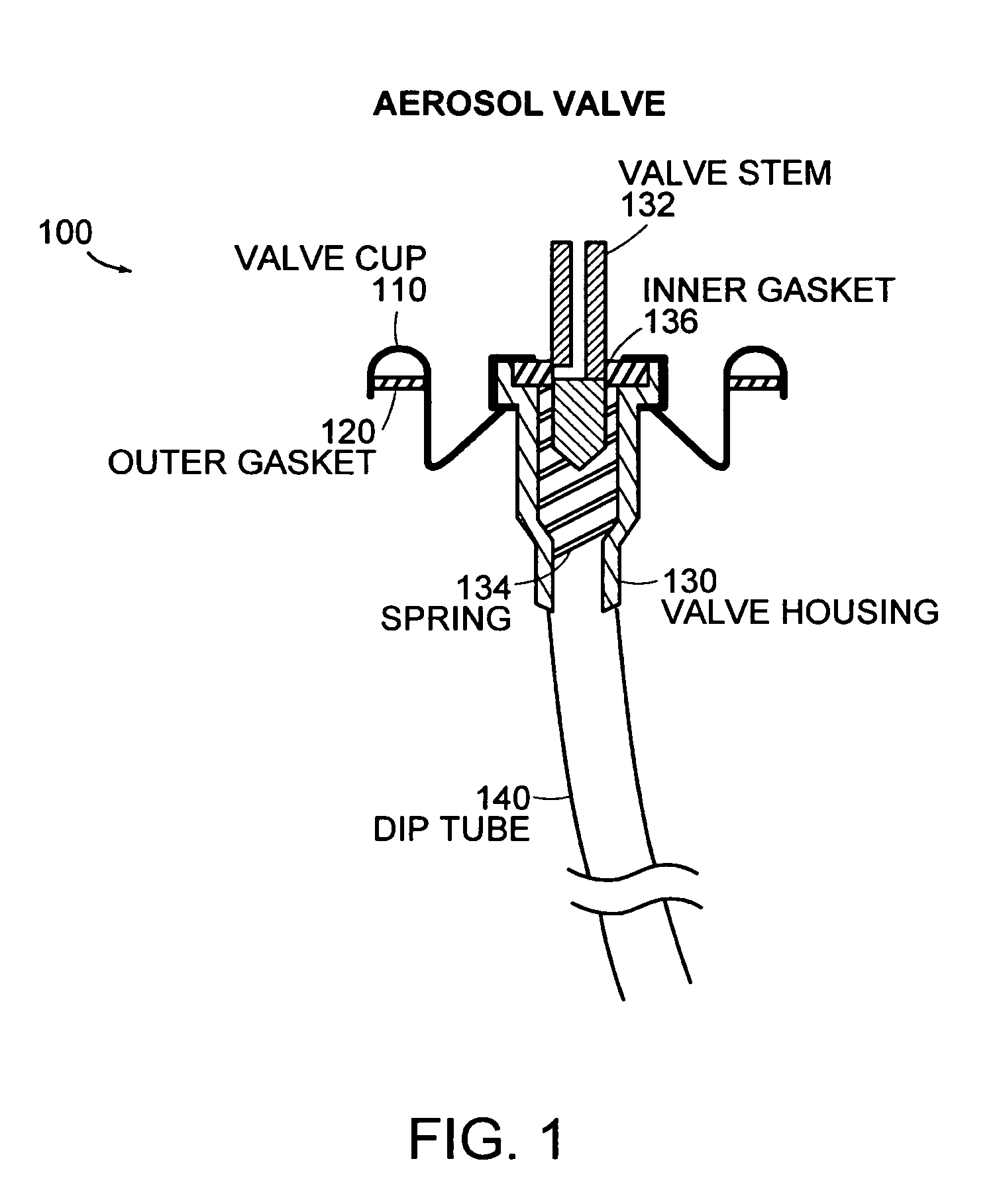
Method and package for storing a pressurized container containing a drug
InactiveUS6179118B1Reduce manufacturing complexityReduce manufacturing costWrappersDiagnosticsWater vaporWaste management
Owner:GLAXO SMITHKLINE LLC
Stabilized Iodocarbon Compositions
InactiveUS20080157022A1Reduce environmental damageLow propertyHalogenated hydrocarbon separation/purificationHeat-exchange elementsStabilizing AgentsSolvent composition
Disclosed are compositions comprising at least one iodocarbon compound and preferably at least one stabilization agent comprising a diene-based compound. These compositions are generally useful as refrigerants for heating and cooling, as blowing agents, as aerosol propellants, as solvent composition, and as fire extinguishing and suppressing agents.
Owner:HONEYWELL INT INC
Foamable compositions and kits comprising one or more of a channel agent, a cholinergic agent, a nitric oxide donor, and related agents and their uses
InactiveUS20080317679A1Efficient deliveryMinimizing systemic penetrationOrganic active ingredientsBiocideActive agentNitric oxide
The present invention relates to a foamable therapeutic composition comprising: (a) a therapeutically effective concentration of at least one active agent selected from the group consisting of a channel agent, a cholinergic agent, and a nitric oxide donor; and (b) a foamable carrier comprising:i. about 50% to about 98% of a solvent selected from the group consisting of water; a hydrophilic solvent; a hydrophobic solvent; a potent solvent; a polar solvent, a silicone, an emollient, and mixtures thereof;ii. 0% to about 48% of a secondary solvent selected from the group consisting of water; a hydrophilic solvent; a hydrophobic solvent; a potent solvent; a polar solvent, a silicone, an emollient, a co-solvent, a penetration enhancer and mixtures thereof;iii. a surface-active agent;iv. about 0% to about 5% by weight of at least one polymeric agent; andv. a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition;wherein the composition is housed in a container and is substantially flowable, andwhich upon release expands to form a breakable foam; andwherein the foamable carrier is selected to generate a foam of good to excellent quality.The invention further provides a method of treating, alleviating or preventing a disorder of mammalian subject, comprising administering such a composition to an afflicted target site.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Ammunition having a projectile made by metal injection molding
The present invention provides ammunition having a metal injection molded projectile and a metal cartridge case comprising a metal ammunition cartridge comprising a unitary body that enclose a propellant chamber, a primer recess in a bottom portion of the unitary body, a primer flash hole that connects the primer recess and the propellant chamber, and a projectile aperture in communication with the propellant chamber; a primer inserted into the primer flash hole aperture; a propellant at least partially filling the propellant chamber; and a metal injection molded projectile frictionally fitted in the projectile aperture, wherein the metal injection molded projectile comprises a nose extending essentially symmetrically to a shoulder, and an essentially cylindrical bearing surface extending from the shoulder to a base.
Owner:TRUE VELOCITY IP HLDG LLC
Metal Injection Molded Cased Telescoped Ammunition
ActiveUS20180066925A1Promote generationLight weightTransportation and packagingMetal-working apparatusMetal alloySlug
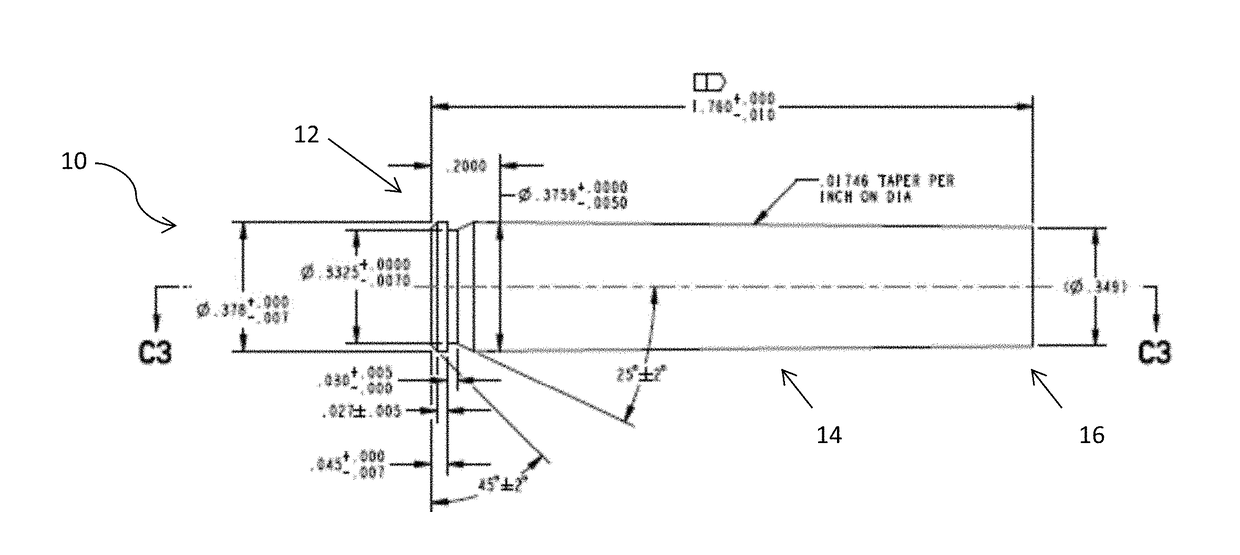
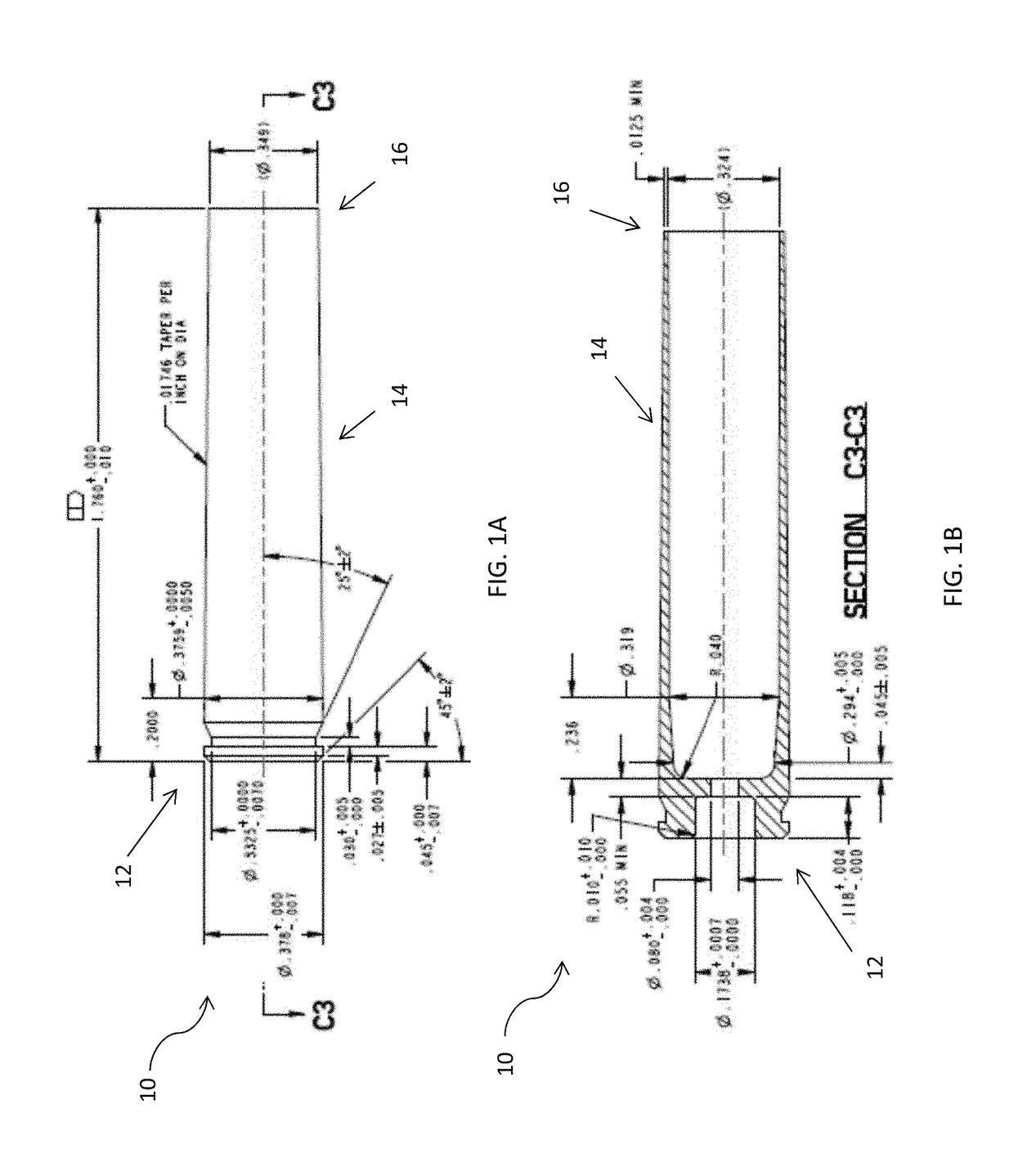
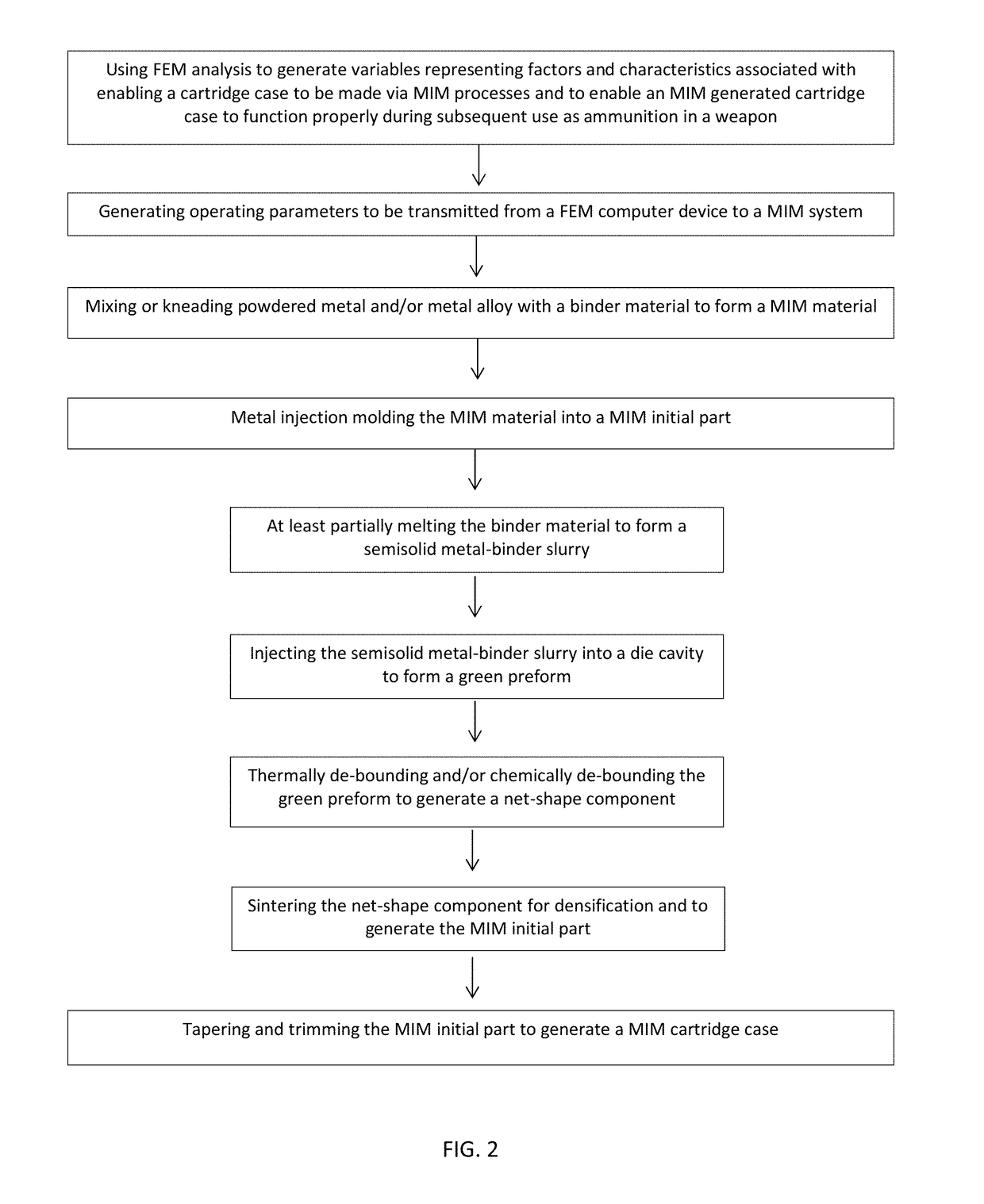
Disclosed is a cartridge case for various caliber ammunition that can consist essentially of a powdered metal and / or powdered metal alloy(s) that is formed into the cartridge case through an injection mold processing. Also disclosed is a method for forming a cartridge case, which may include use of Metal Injection Molding (“MIM”) processes to produce the cartridge case which retains a primer, propellant, and / or a bullet. Also disclosed are embodiments related to a case telescoped cartridge that may include a cap and a body. The body can consist essentially of or consists entirely of a powdered metal and / or powdered metal alloy(s) that has been formed through MIM. The cap can comprise plastic that has been formed through plastic molding or comprise powdered metal and / or powdered metal alloy(s) that has been formed through MIM.
Owner:CONCURRENT TECH
Cosmetic and pharmaceutical foam with solid matter
InactiveUS20050186147A1Good lookingLower yield strengthCosmetic preparationsAerosol deliveryMedicineSolvent
A foamable composition includes about 2 to about 30% by weight solid particles; about 2 to about 75% by weight hydrophobic solvent; about 10 to about 85% by weight water; about 0.1% to about 5% by weight surface-active agent; about 0.1% to about 5 wt % by weight stabilizer / gelling agent; and a liquefied or compressed gas propellant in a container, which upon release provides a breakable foam suitable for topical administration.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Compositions containing fluorine substituted olefins
The use to e of tetrafluoropropenes, particularly (HFO-1234) in a variety of applications, including refrigeration equipment, is disclosed. These materials are generally useful as refrigerants for heating and cooling, as blowing agents, as aerosol propellants, as solvent composition, and as fire extinguishing and suppressing agents.
Owner:HONEYWELL INT INC
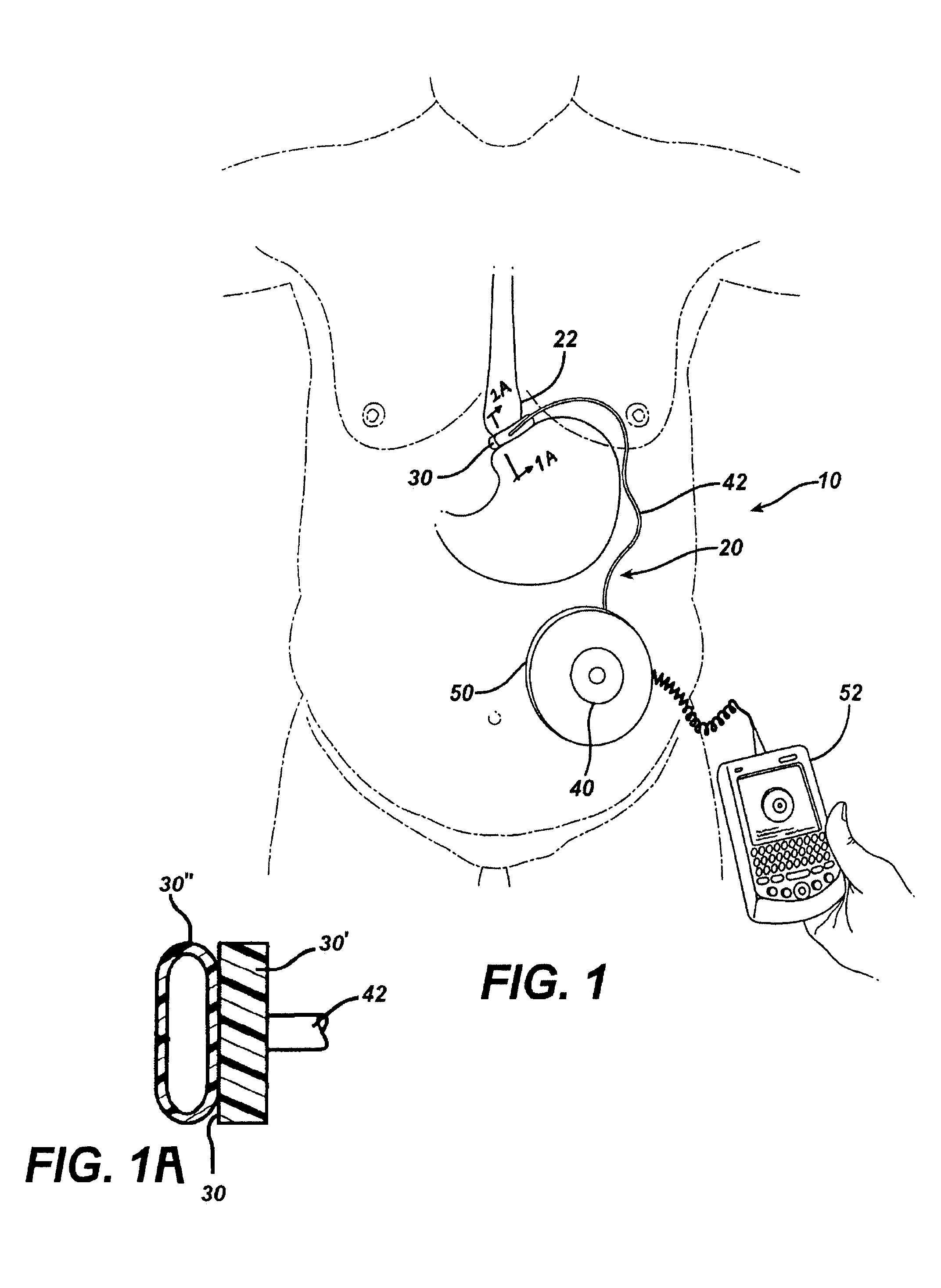
Thermodynamically driven reversible infuser pump for use as a remotely controlled gastric band
InactiveUS7351240B2Small volumeAvoid changeAnti-incontinence devicesFlexible member pumpsEngineeringPiezo electric
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band, by a combination of thermodynamic actuation and a piezo-electrically disengaged drum brake assembly that thereby achieves a desirable small volume device. A propellant within a propellant cavity surrounds a metal bellows accumulator biased at body temperature to either expand or collapse the bellows accumulator with the opposite direction of movement effected by a thermal element that heats in combination with a negatively-biased propellant or cools in combination with a positively-biased propellant. A drum brake assembly locks the metal bellows accumulator in place between adjustments by thermodynamic actuation by activating piezo-electric stack actuators that disengage calipers from a brake drum attached to the bellows accumulator.
Owner:ETHICON ENDO SURGERY INC
Sprayable formulations for the treatment of acute inflammatory skin conditions
A topical spray or foam, methods of making the formulation, and methods of use thereof, has been developed. In one preferred embodiment, the composition includes one or more active agents and exhibits both antibacterial activity and antifungal activity. Excipients such as chemical disinfectants, anti-pruritic agents to minimize itching, and skin protective compounds may be added. The composition may be formulated to be dispensed as a spray or foam and the spray or foam may be administered either by a hand pump or by an aerosolizing propellant. A second single phase formulation has also been developed. The formulation comprises a first drug which is water soluble or hydrophilic and a second drug which is lipid soluble or hydrophobic, wherein at least one of the drugs is bound to an ion-exchange resin. The use of binding resins, such as ion-exchange resins, allows drugs with incompatible solvent requirements to be prepared in a single-phase formulation.
Owner:COLLEGIUM PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com