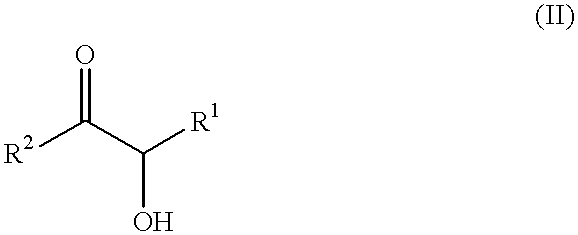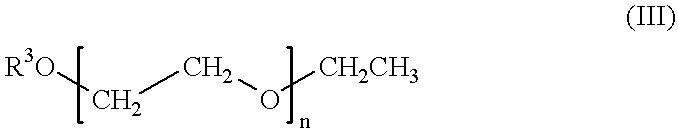Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51790 results about "Water soluble" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Water Soluble. Definition - What does Water Soluble mean? In horticulture, water soluble (often hyphenated: water-soluble) is an adjective used to describe something (often a fertilizer, powdered nutrients, amendments, or pesticides) that is highly capable of being dissolved in a solvent (usually water).
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
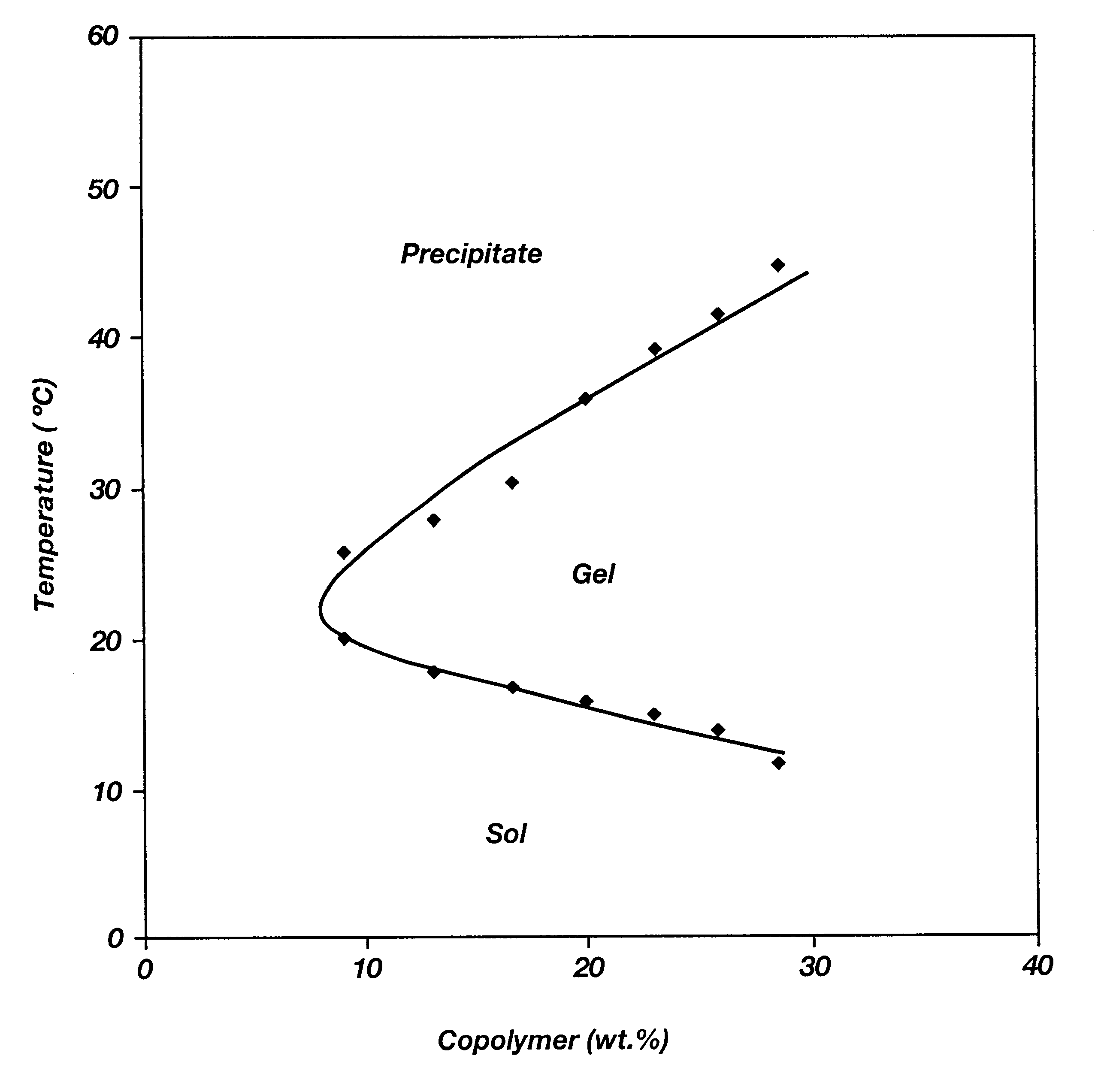
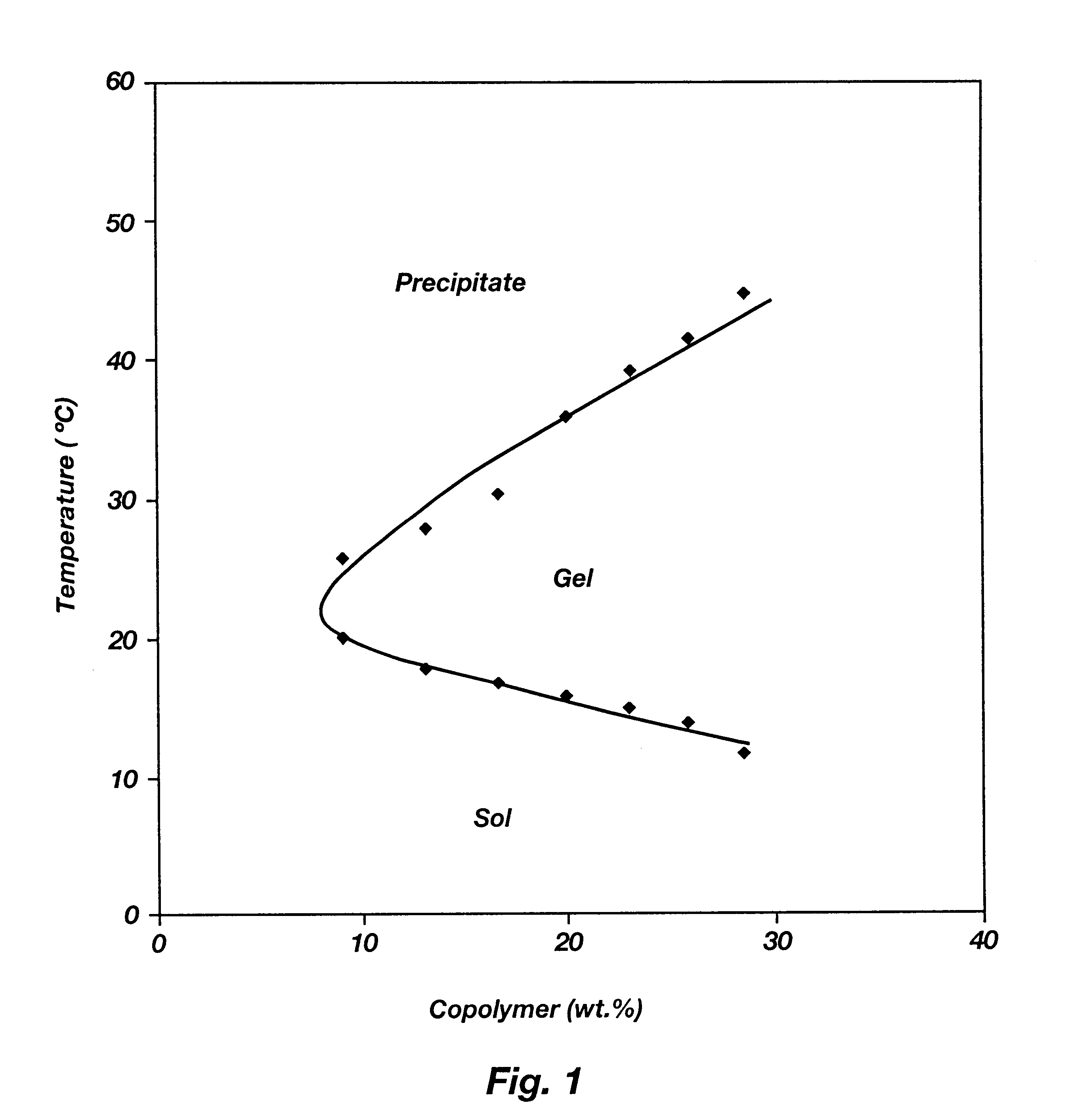
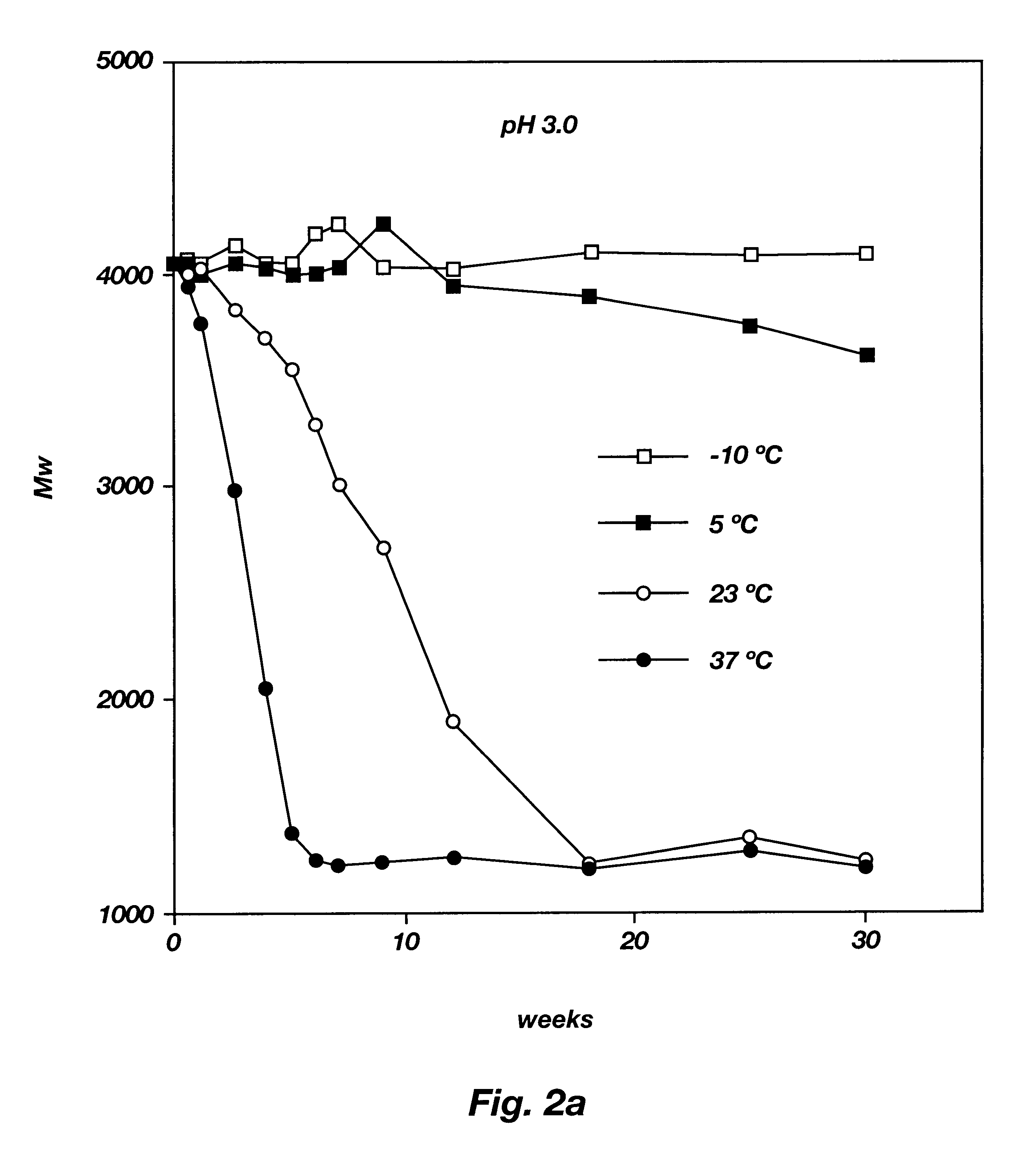
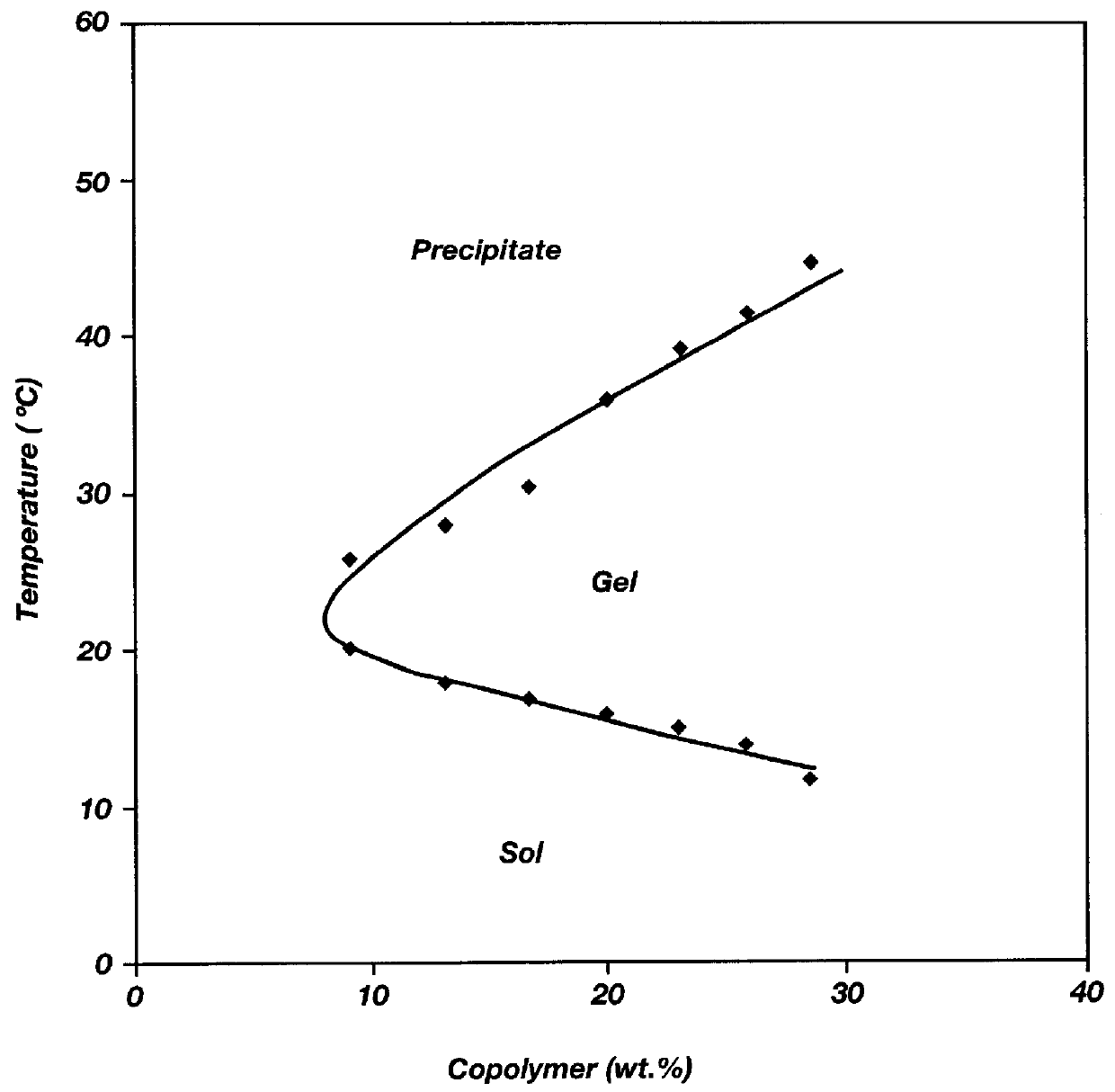
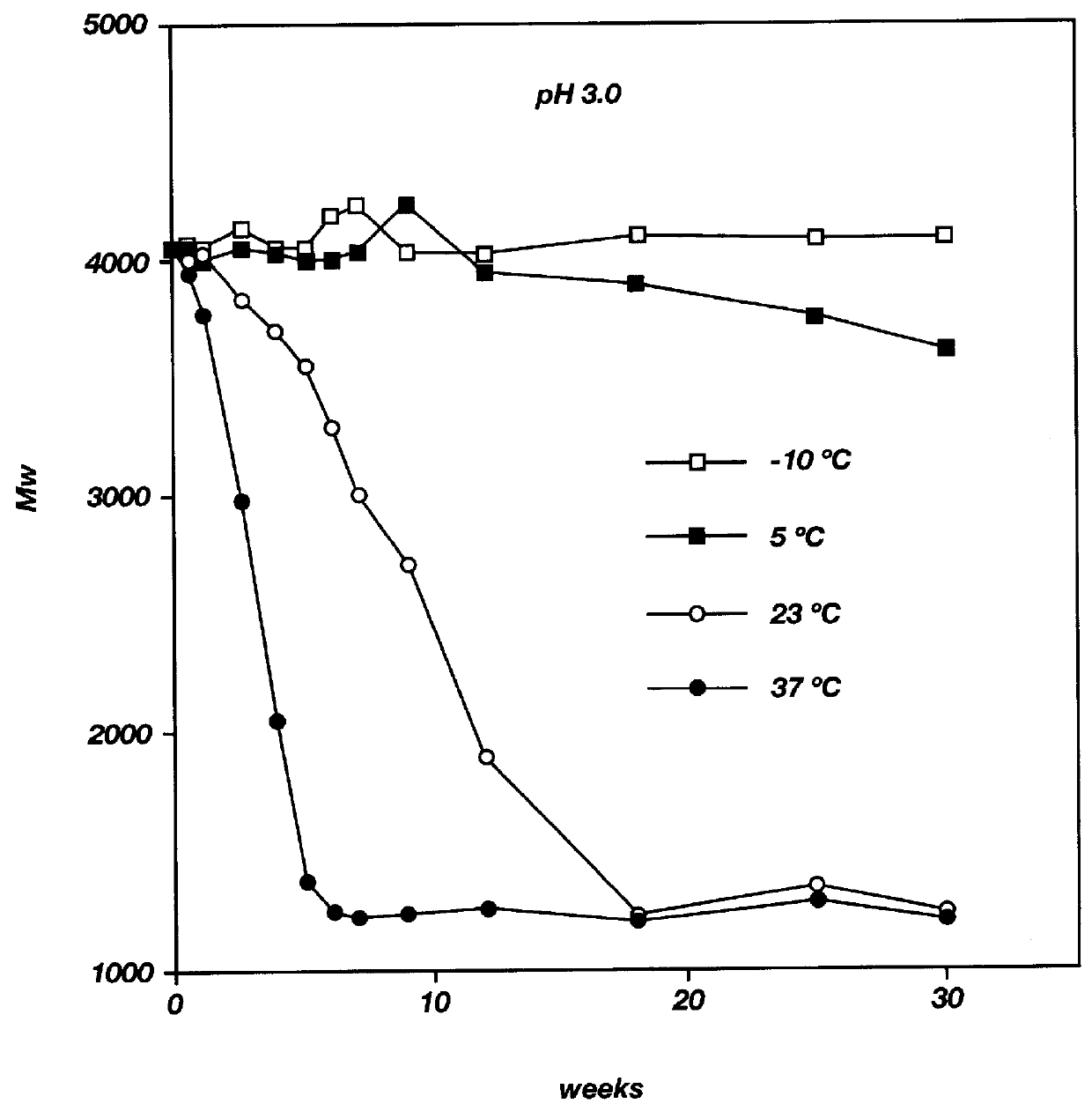
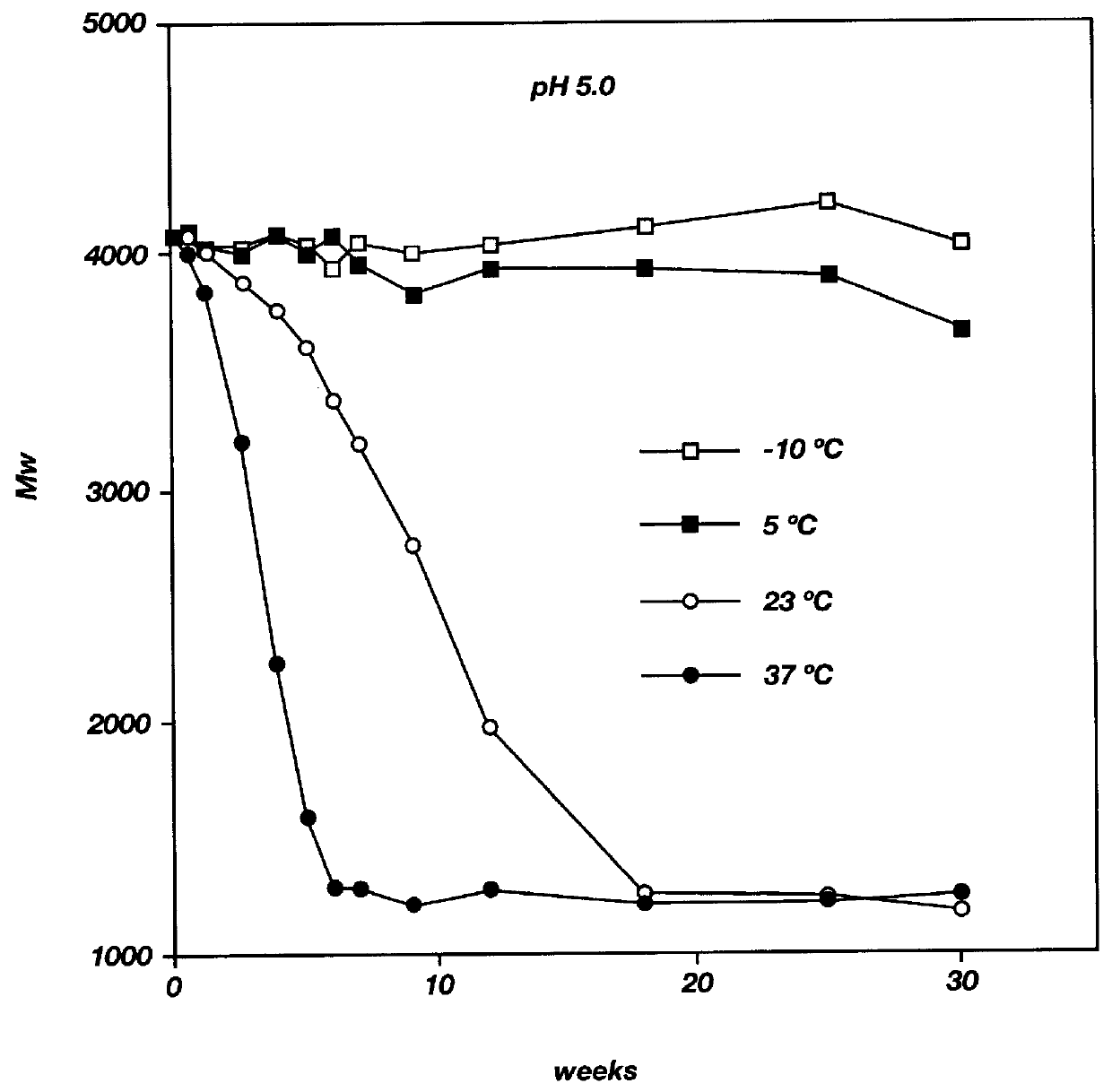
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Laundry multi-compartment pouch composition
InactiveUS20090011970A1Improve bleaching effectEasy to useDetergent materialsDetergent compounding agentsSolid componentWater soluble
This invention relates to a laundry pouch made from a water-soluble film and having at least two compartments, containing a solid component and a liquid component, wherein the solid phase contains a peroxide source and the liquid phase contains bleach activator. The present invention also relates to a method for treating laundry by placing the pouch, according to the present invention, in the drum of the washing machine.
Owner:THE PROCTER & GAMBLE COMPANY
Water-soluble copolymer film packet
Owner:MONOSOL LLC +1
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Water-soluble fluorescent nanocrystals
InactiveUS6444143B2High quantum yieldHigh spectral purityMaterial nanotechnologyNanosensorsQuantum yieldOrganic layer
A water soluble semiconductor nanocrystal capable of light emission is provided, including a quantum dot having a selected band gap energy, a layer overcoating the quantum dot, the overcoating layer comprised of a material having a band gap energy greater than that of the quantum dot, and an organic outer layer, the organic layer comprising a compound having a least one linking group for attachment of the compound to the overcoating layer and at least one hydrophilic group space apart from the linking group by a hydrophobic region sufficient to prevent electron charge transfer across the hydrophobic region. The particle size of the nanocrystal core is in the range of about 12.ANG. to about 150.ANG., with a deviation of less than 10% in the core. The coated nanocrystal exhibits photoluminescende having quantum yield of greater than 10% in water.
Owner:MASSACHUSETTS INST OF TECH
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
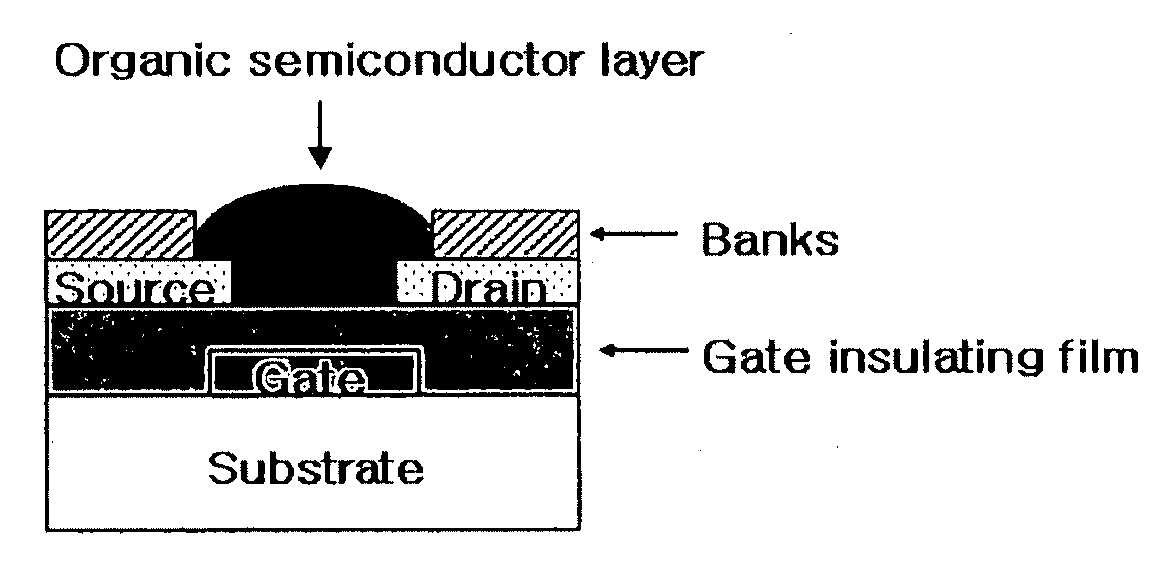
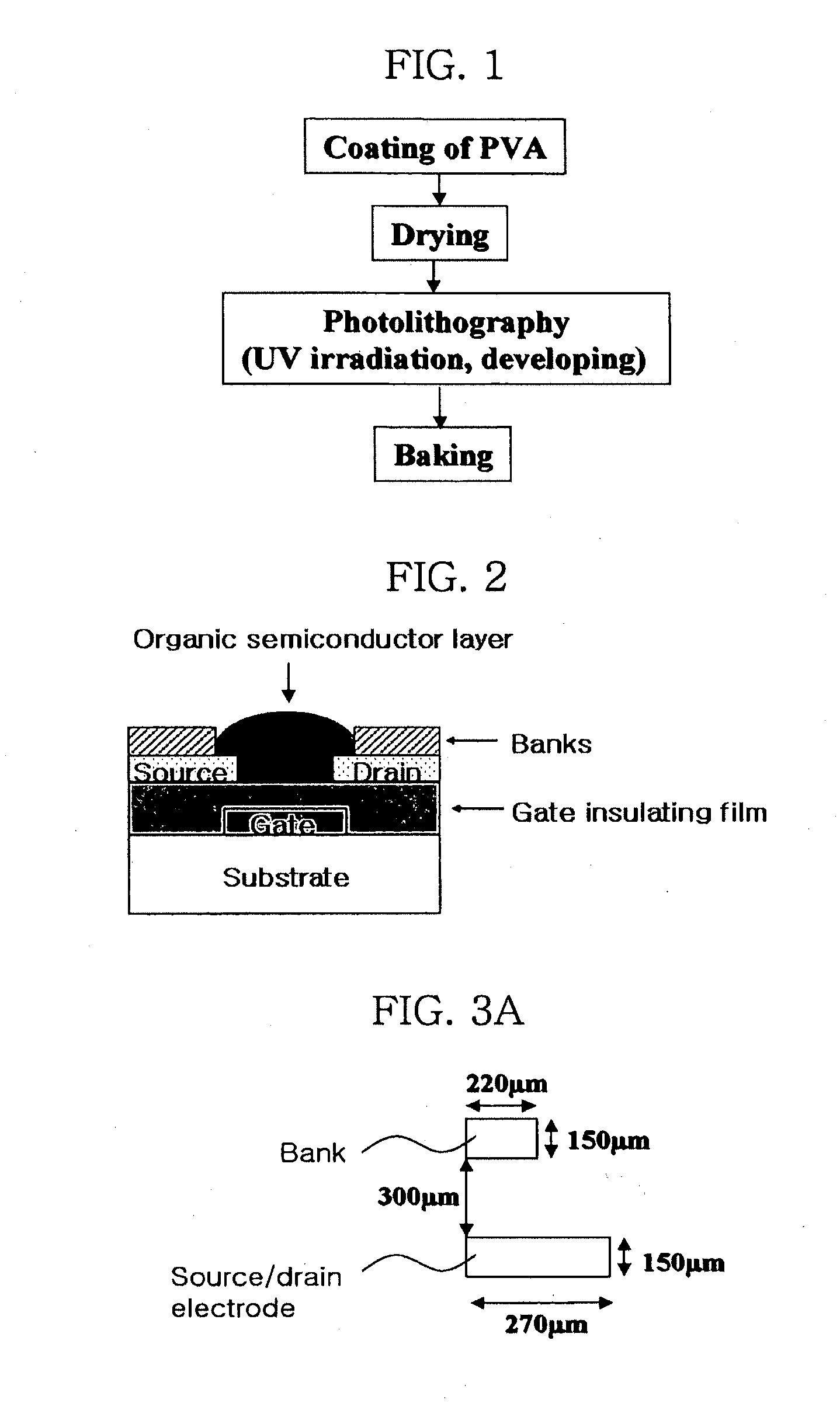
Methods of fabricating organic thin film transistors
InactiveUS20120122275A1Suppresses and eliminates adverse effectSuppress and eliminate degradationSolid-state devicesSemiconductor/solid-state device manufacturingOrganic structureSemiconductor materials
Disclosed is a method for forming banks during the fabrication of electronic devices incorporating an organic semiconductor material that includes preparing an aqueous coating composition having at least a water-soluble polymer, a UV curing agent and a water-soluble fluorine compound. This coating composition is applied to a substrate, exposed using UV radiation and then developed using an aqueous developing composition to form the bank pattern. Because the coating composition can be developed using an aqueous composition rather than an organic solvent or solvent system, the method tends to preserve the integrity of other organic structures present on the substrate. Further, the incorporation of the fluorine compound in the aqueous solution provides a degree of control over the contact angles exhibited on the surface of the bank pattern and thereby can avoid or reduce subsequent surface treatments.
Owner:SAMSUNG ELECTRONICS CO LTD
Water-dispersable materials
InactiveUS6211309B1Increase flexibilityGood dispersionMonocomponent polyesters artificial filamentMonocomponent polyamides artificial filamentThermoplasticFiber
The present invention relates to water-dispersible materials (e.g. fibers or films) and to a method of producing same. The materials of the invention comprise a water soluble component, for example, a sulfonated polycondensate thermoplastic, and a modifying auxiliary component, for example, a low melt temperature thermoplastic.
Owner:BASF CORP
Non-gelatin substitutes for oral delivery capsules, their composition and process of manufacture
Gelatin-free capsule for use in oral administration of medicines, cosmetic or bath applications, or dietary supplements can be prepared from compositions comprisinga) 8-50% by weight of water-dispersible or water-soluble plasticizer,b) 0.5 to 12% by weight kappa-carrageenan,c) 0 to 60% dextrins, andd) 1% to 95% by weight water,with the kappa-carrageenan comprising at least 50% by weight of all gums forming or contributing to formation of thermoreversible gels in the composition. A capsule for oral administration or cosmetic application may comprise a fill material to be administered to a patient or subject and a capsule, the capsule comprising an aqueous based film comprisinga) water-dispersible or water-soluble plasticizer, andb) carrageenan,with the carrageenan comprising at least 50% or 75% by weight of kappa-carrageenan, and the carrageenan comprising at least 50% or 75% by weight of all gums which form or contribute to the formation of thermoreversible gels. A process for forming the capsules may comprise heating the composition, casting or extruding the composition into a film, gelling the composition by cooling, associating a fill material with the gelled composition (usually as a film) and sealing the film about the fill material.
Owner:PATHEON SOFTGELS INC
Vater-absorbent resin composition and its production process
ActiveUS20060073969A1Little gel-blockingGood liquid permeabilityNon-fibrous pulp additionOther chemical processesLiquid diffusionCompound (substance)
There are disclosed a water-absorbent resin composition and its production process, wherein the water-absorbent resin composition causes little gel-blocking and is excellent in the liquid permeability and liquid diffusibility and is high also in the absorption performances and further is strong also against the physical damage; and there are further disclosed a water-absorbent resin composition and its production process, wherein the water-absorbent resin composition has the following further advantages, in addition to the above, of involving little segregation of the metal compound and further having a dust prevention effect. One of water-absorbent resin compositions according to the present invention is a water-absorbent resin composition comprising water-absorbent resin particles obtained by polymerizing a monomer including acrylic acid and / or its salt, with the composition having a mass-average particle diameter of 100 to 600 μm and comprising water-soluble polyvalent metal salt particles and the water-absorbent resin particles that have been surface-crosslinked.
Owner:NIPPON SHOKUBAI CO LTD
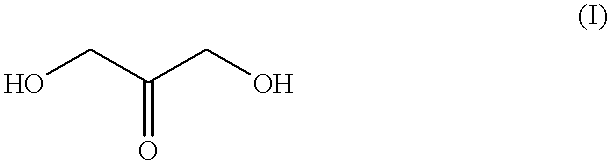
Self-tanning dihydroxyacetone formulations having improved stability and providing enhanced delivery
A composition is provided which is useful for self-tanning skin coloring and is characterized by improved stability, which comprises from about 0.5% to about 20.0% by weight, based on total weight of said composition, of a self-tanning skin coloring agent subject to chemical instability, which is preferably dihydroxyacetone; from about 2.0% to about 40.0% by weight of a polyethoxyglycol, which is preferably ethoxydiglycol; and from about 0.1% to about 15.0% by weight of a polyol comprising a polyhydric compound having at least three hydroxyl groups and at least three carbon atoms, which is preferably D-sorbitol. The self-tanning composition may further optionally contain from about 0.1% to about 8.0% by weight of a water soluble dihydroxyl compound having at least two, and up to eight carbon atoms, which is preferably ethylene glycol; and the self-tanning composition may still further optionally contain an acidifying agent in amount sufficient to maintain the pH of said total composition at from about 3.5 to about 4.5, which is preferably sorbic acid. Cosmetologic products and methods of tanning are also provided.
Owner:SCHERING PLOUGH HEALTHCARE PRODUCTS INC
Photobleach speckle and laundry detergent compositions containing it
InactiveUS20030087790A1Little and no stainingLittle or no stainingOrganic detergent compounding agentsDetergent dyesParticulatesBleach
A speckle composition for use in particulate laundry detergent compositions comprising a porous granular carrier, and at least 0.01 wt % photobleach, preferably at least 0.05 wt %, more preferably at least 0.1 wt %, based on the active ingredient the composition being layered with a finely divided high carrying capacity particulate material and / or a water-soluble material. The most preferred photobleach is a blend of Zn and Al sulphonated phthalocyanine.
Owner:HENKEL IP & HOLDING GMBH
Water-soluble rhodamine dyes and conjugates thereof
InactiveUS6191278B1Sugar derivativesDiaryl/thriaryl methane dyesEnergy transferNucleic acid sequencing
The present invention provides novel, water-soluble, red-emitting fluorescent rhodamine dyes and red-emitting fluorescent energy-transfer dye pairs, as well as labeled conjugates comprising the same and methods for their use. The dyes, energy-transfer dye pairs and labeled conjugates are useful in a variety of aqueous-based applications, particularly in assays involving staining of cells, protein binding, and / or analysis of nucleic acids, such as hybridization assays and nucleic acid sequencing.
Owner:APPL BIOSYSTEMS INC
High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same
InactiveUS7014866B2Satisfactory bioavailabilitySatisfactory dissolutionPowder deliveryBiocideHigh dosesNelfinavir mesylate
A solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate is provided comprising amorphous nelfinavir mesylate in an amount of from about 400 mg to about 700 mg calculated as nelfinavir base, and a pharmaceutically acceptable water soluble, non-ionic synthetic block copolymer of ethylene oxide and propylene oxide, the copolymer having a melting point of at least about 45° C. and an HLB value at 25° C. of from about 18 to about 29, wherein the copolymer is present from about 40% to about 65% by weight of the nelfinavir mesylate. A hot melt granulation process for making the dosage form is provided.
Owner:F HOFFMANN LA ROCHE & CO AG
High yield method of producing pure rebaudioside A
ActiveUS20060083838A1Reduction in yieldQuality improvementSugar derivativesMetabolism disorderSolubilityAdditive ingredient
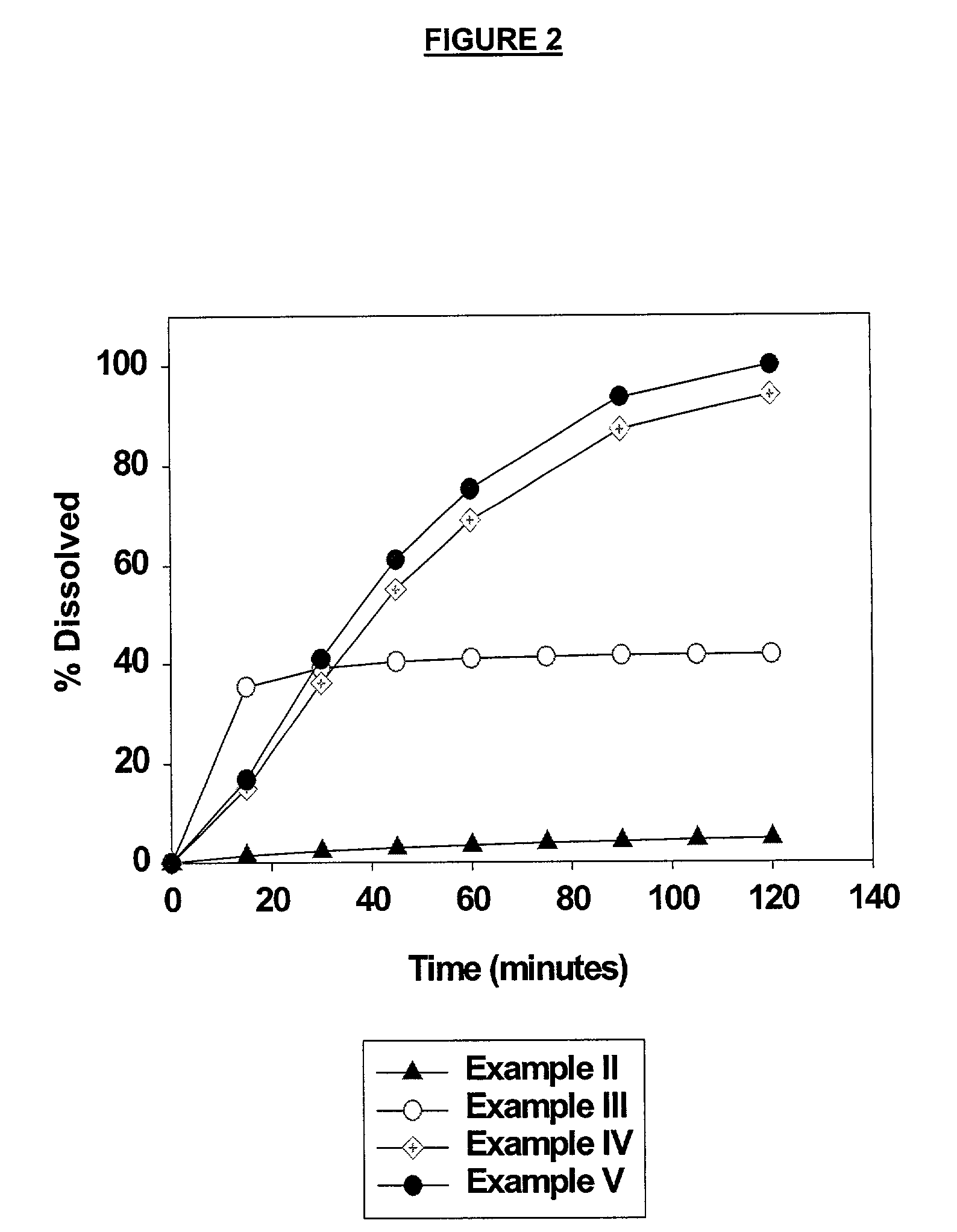
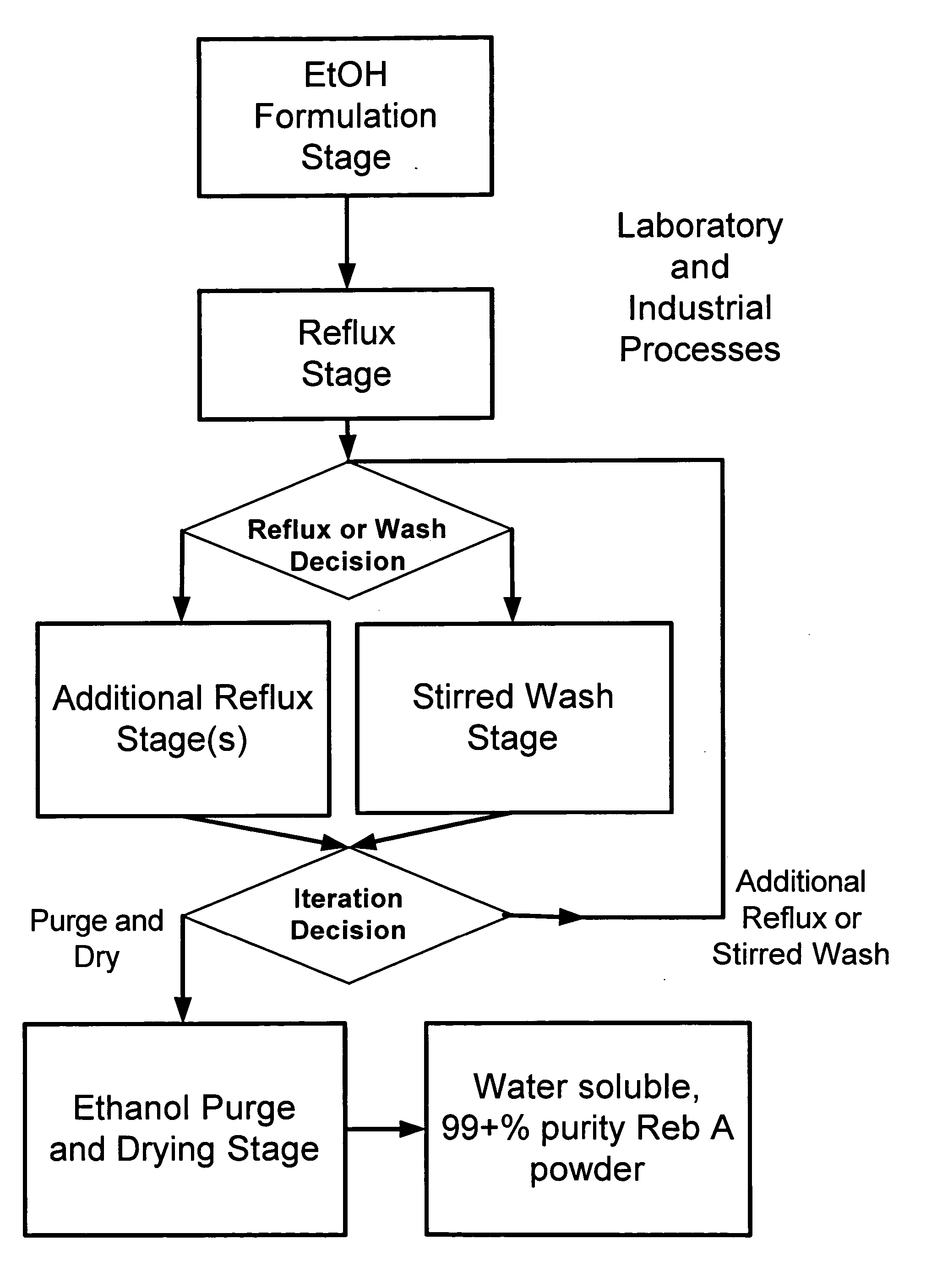
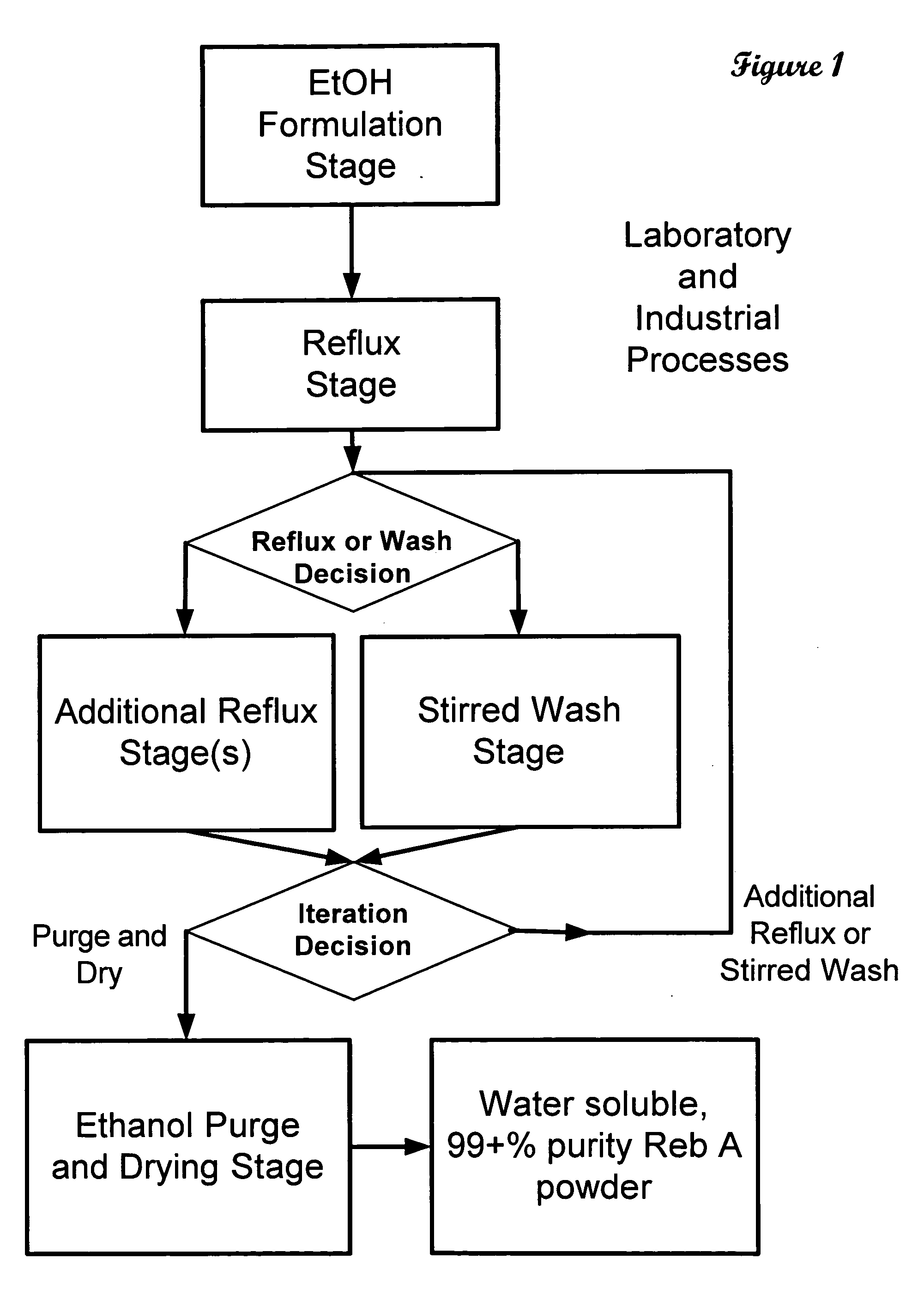
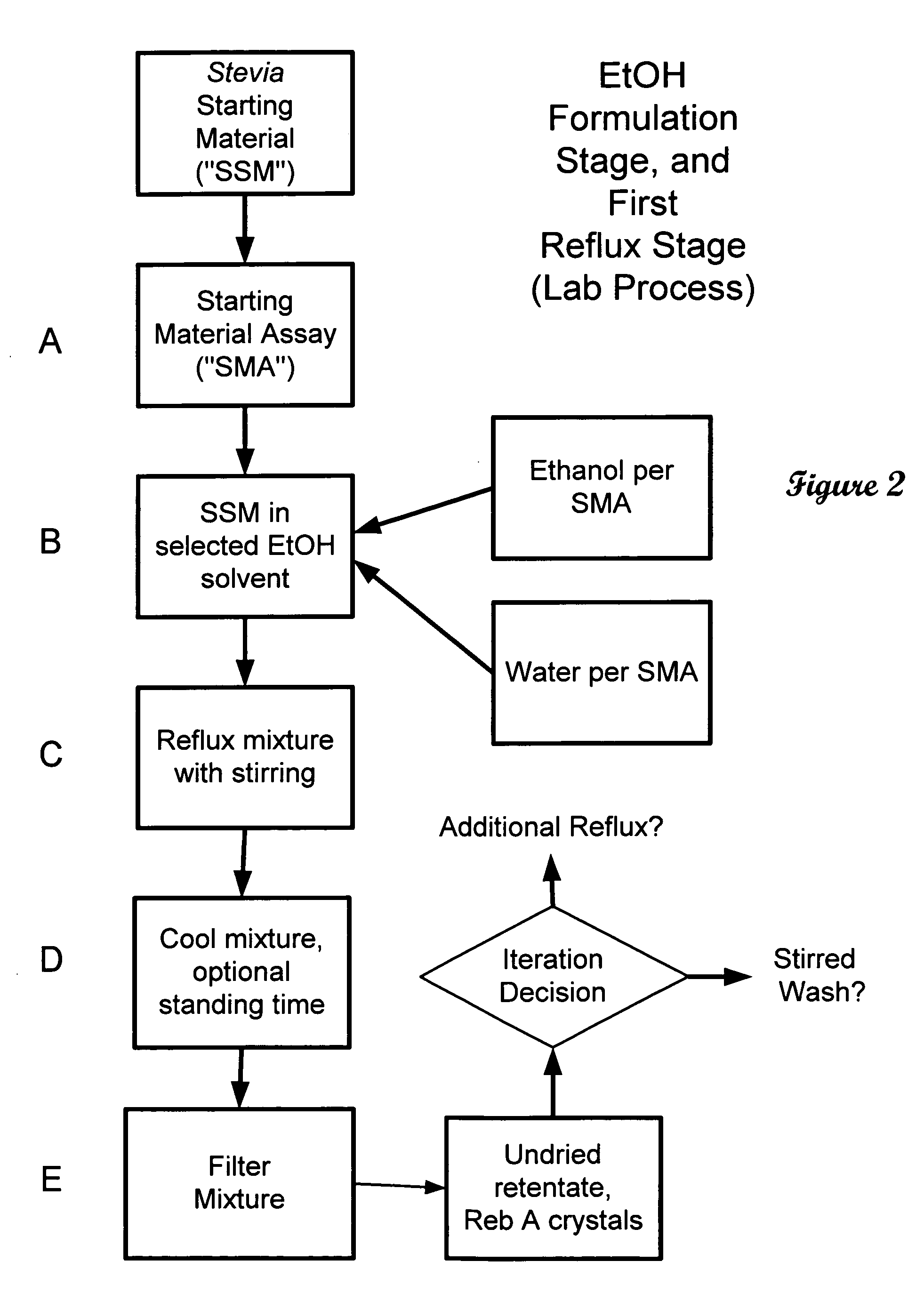
The invention provides a high throughput, high purity, high yield system and method of isolating and purifying rebaudioside A (“Reb A”), with acceptable water solubility for all commercial uses, from commercially available Stevia rebaudiana starting material. The invention also provides a means of maximizing yields of 99+% purity Reb A based on the attributes of a given batch of Stevia starting material. The Reb A produced by the invention is water soluble, devoid of bitterness heretofore associated with rebaudioside sweeteners, non-caloric, and suitable for use as a reagent and as an ingredient in orally consumed products, e.g., as a sweetener, flavor enhancer, and flavor modifier.
Owner:SWEET GREEN FIELDS INT CO LTD
Fire retardant compositions and methods for preserving wood products
Wood preservative compositions are disclosed. Treatment of lumber, plywood, and other wood products with a novel composition comprising the boron source composition, a melamine binder resin, and a urea casein activator resin protects lumber, plywood, and other wood products from attack by termites, fungi, fire and flame. The preservative can be formed by combining a source of boron such as boric acid and the water-soluble salts thereof, a melamine binder resin, and a urea casein resin. A wood preservative is characterized by a weight ratio of the urea casing activator resin to the melamine binder resin ranging from about 1:20 to 1:4 and a weight ratio of the boron source composition to the melamine binder resin ranging from about 1.3:1 to 9.6:1.
Owner:LOPEZ RICHARD A
Novel recombinant proteins with N-terminal free thiol
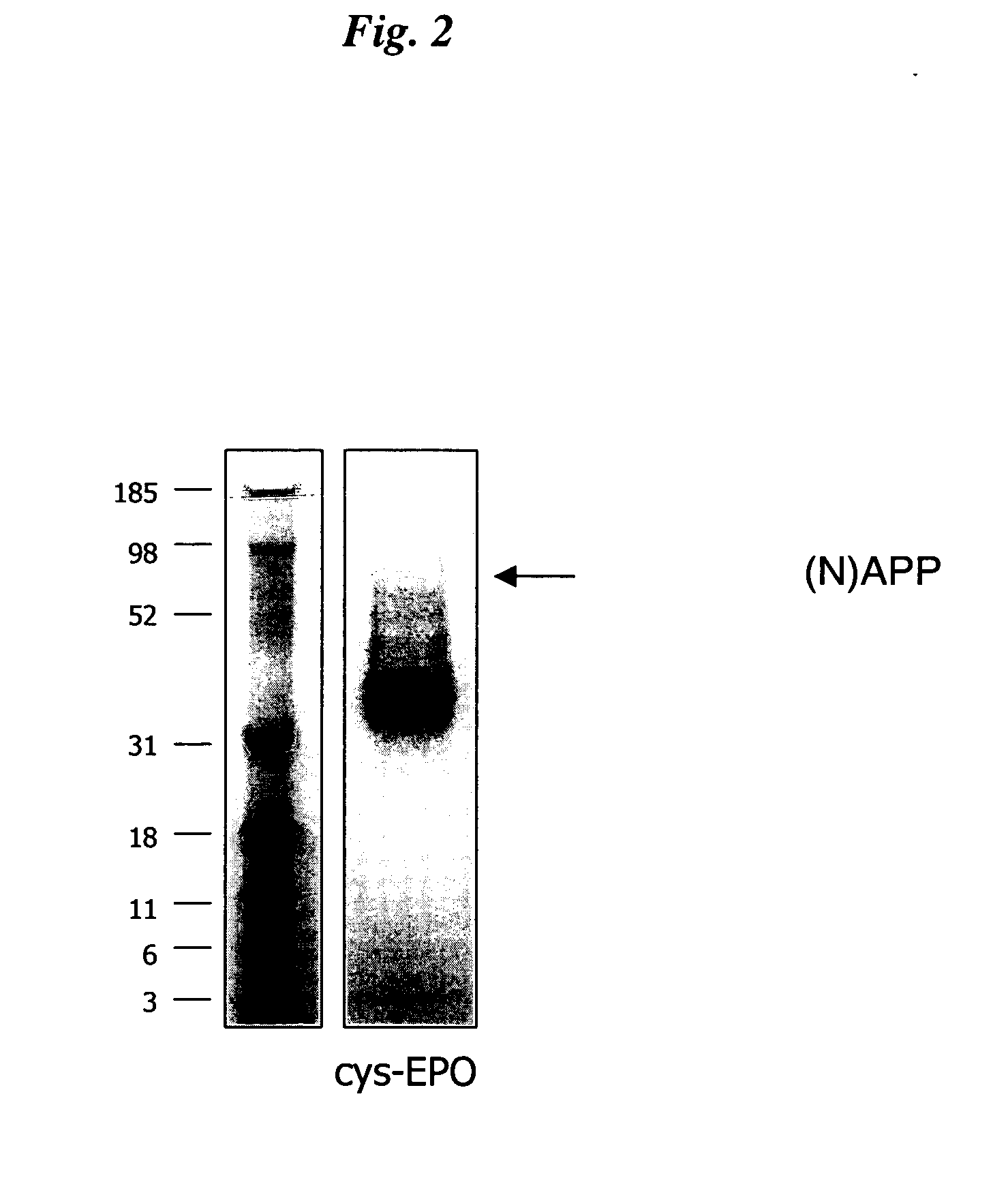
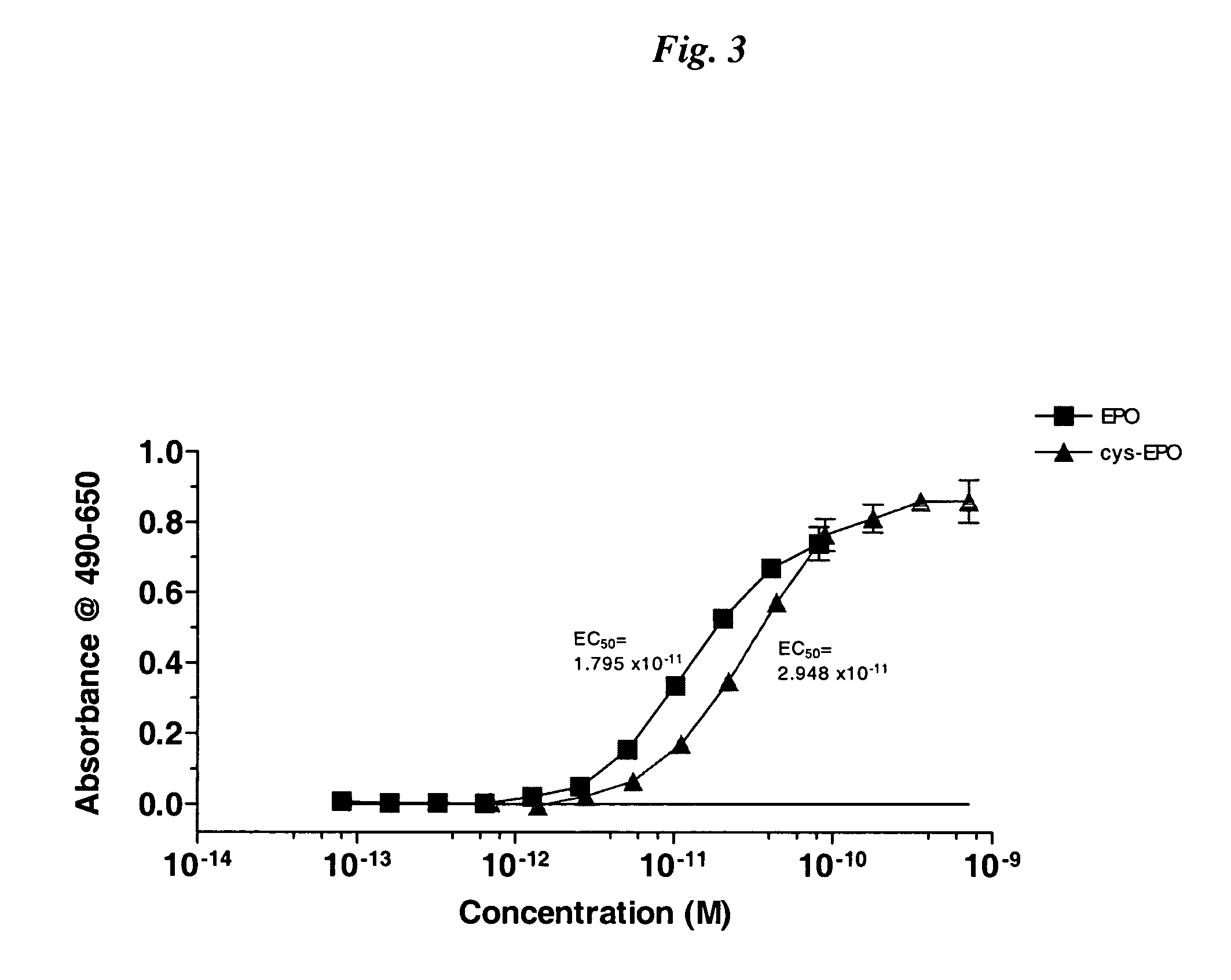
InactiveUS20050170457A1Extended half-lifeIncreases circulating serum half-lifePeptide/protein ingredientsTissue cultureCysteine thiolateHalf-life
The present invention relates to novel modified proteins having N-terminal free thiols that can be produced by recombinant methods and are ready for further chemical derivatization. In particular, the invention relates to erythropoietin conjugate compounds having altered biochemical, physiochemical and pharmacokinetic properties. More particularly, one embodiment of the invention relates to erythropoietin conjugate compounds of the formula: (M)n-X-A-cys-EPO (I) where EPO is an erythropoeitin moiety selected from erythropoietin or an erythropoietin variant having at least one amino acid different from the wild-type human EPO, or any pharmaceutical acceptable derivatives thereof having biological properties of causing bone marrow cells to increase production of red blood cells; cys represents the amino acid cysteine and occurs at position −1 relative to the amino acid sequence of the erythropoietin moiety; A indicates the structure of the residual moiety used to chemically attach X to the thiol group of −1Cys; X is a water soluble polymer such as a polyalkylene glycol or other polymer; M is an organic molecule (including peptides and proteins) that increases the circulating half-life of the construct; and N is an integer from 0 to 15.
Owner:CENTOCOR
Soluble deverting agents
Methods and compositions for stimulating single and multiple intervals in subterranean wells by diverting well treatment fluids into a particular direction or into multiple intervals using water soluble coated diverting agents are described. The water soluble coating of the diverting material is preferably a collagen, poly(alkylene) oxide, poly(lactic acid), polyvinylacetate, polyvinylalcohol, polyvinylacetate / polyvinylalcohol polymer or a mixture thereof applied as a coating on any number of proppants. The method allows for the diverting of the flow of fluids in a downhole formation during a well treatment, such as during a fracturing process. Following completion of a treatment such as a hydraulic stimulation, the soluble diverting agent can be dissolved and removed by the water component of the well production.
Owner:FAIRMOUNT SANTROL
Compositions and methods for mucosal delivery
Mucosal surface-coat-forming film dosage units containing a water-soluble hydrocolloid, an effective dose of an active agent and a mucosal adhesion enhancer; wherein the active agent is encapsulated within a polymer which is chemically or physically distinct from the hydocolloid; wherein the mucosal adhesion enhancer is a starch graft copolymer; wherein the film exhibits a dry tack value of less than 3.5 g, a wet tack of greater than 35 g, a gelation temperature that is greater than 70° C. for a 2% polymer solution, a dry film thickness of less than 20 mil, a water content of 0.5 to 10%, a tensile strength greater than 1500 psi, a modulus in the range of 35,000 to 300,000 psi, a % elongation of less than 20%, a tear probagation resistance of 0.001 to 1 N, and a dissolution time in the range of 1 to 600 seconds upon application to an oral mucosal surface.
Owner:THALLIUM HLDG CO LLC
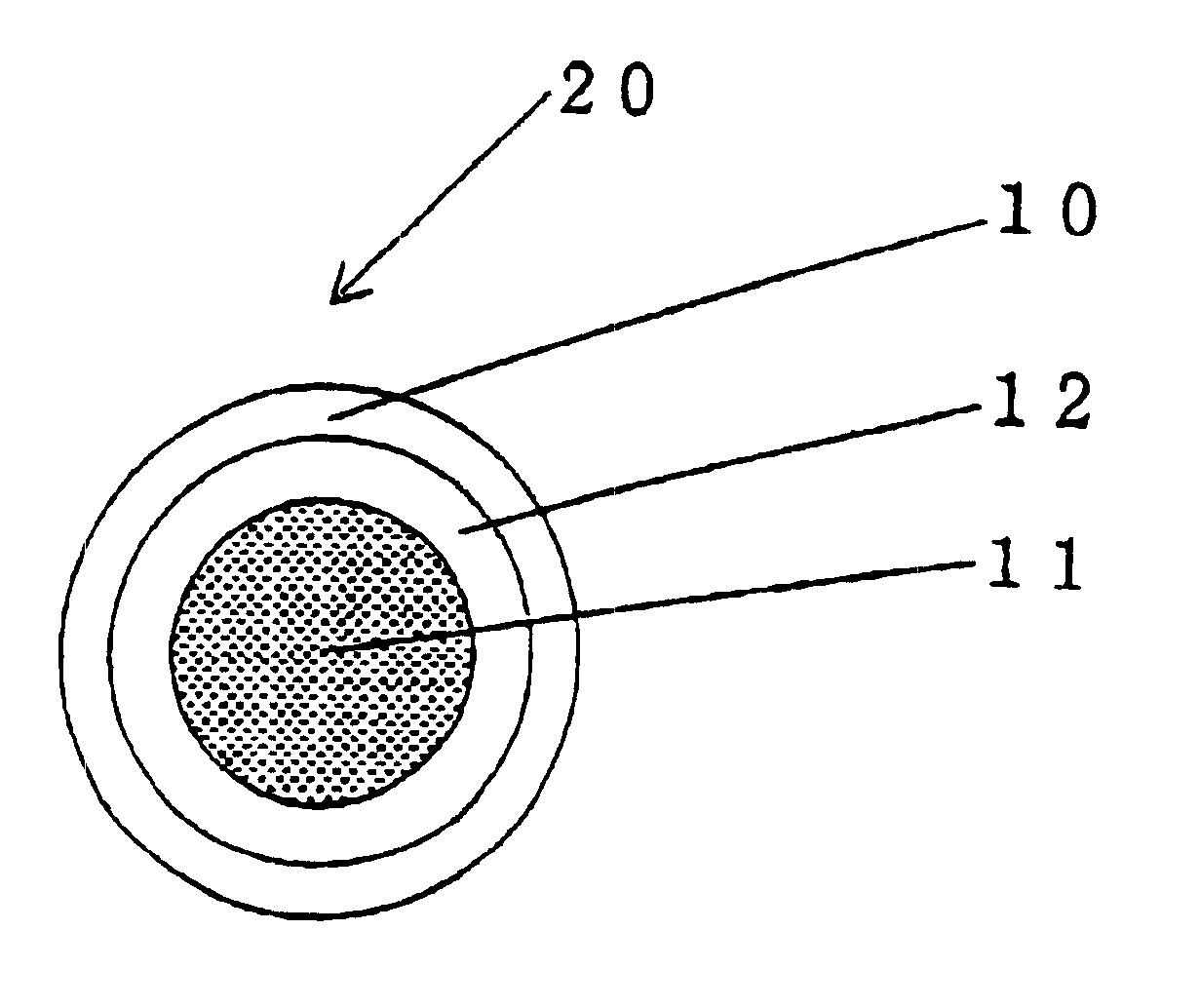
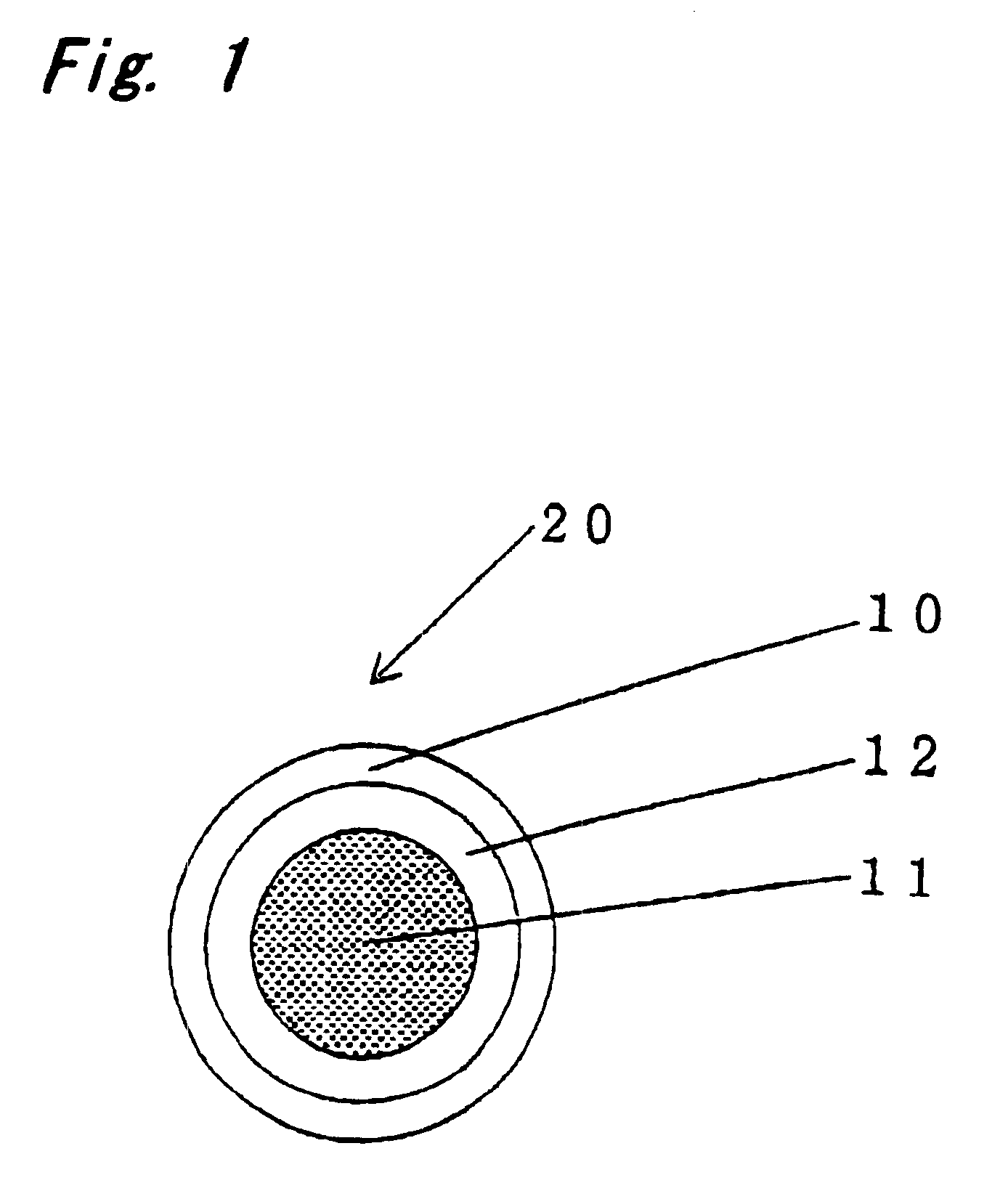
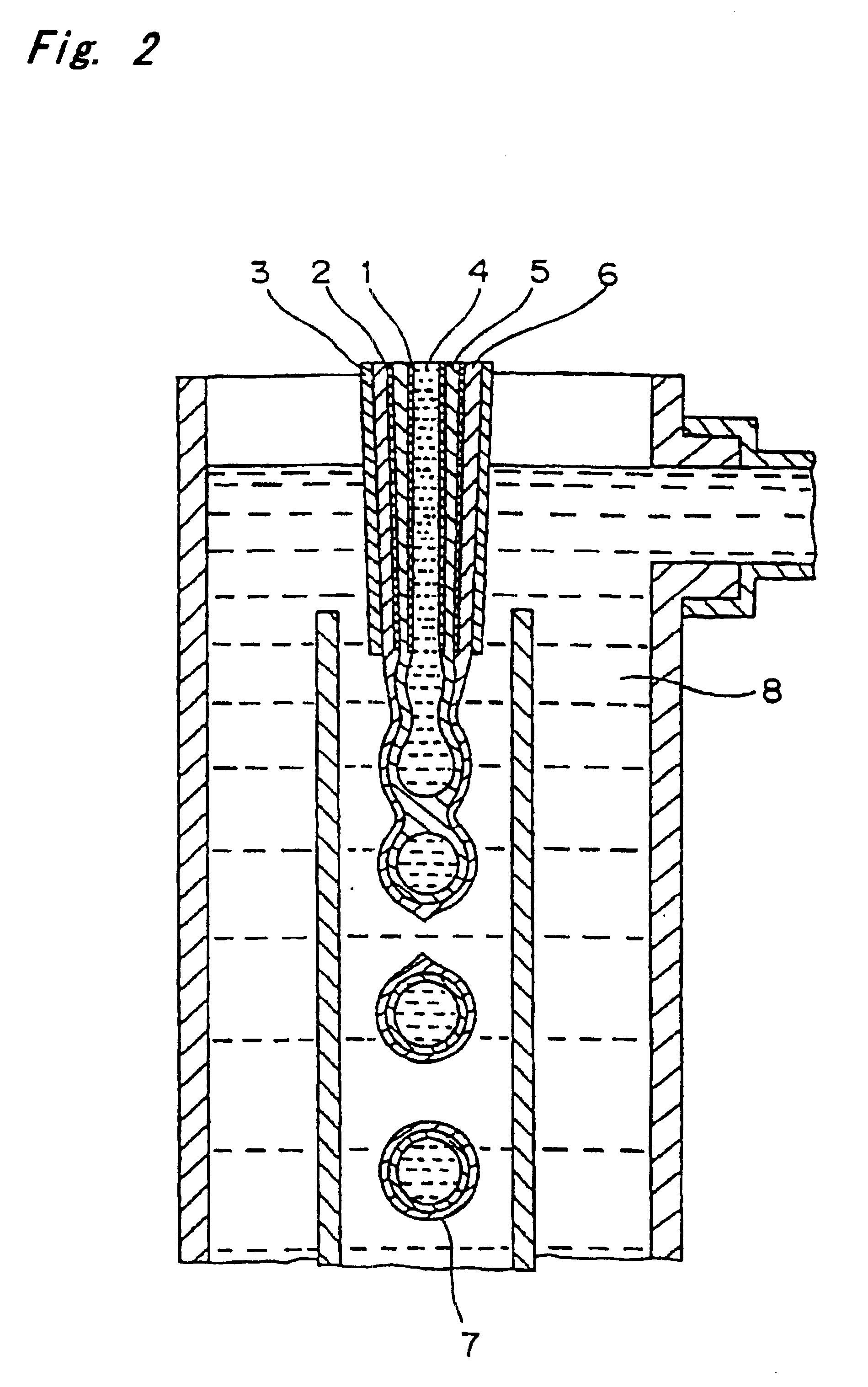
Encapsulated unsaturated fatty acid substance and method for producing the same
InactiveUS6531150B1Improve product qualityInhibit oxygen-permeabilityPowder deliveryGranular deliveryWater solubleGelatin
The present invention relates to an encapsulated unsaturated fatty acid substance in a form of a three-layered capsule, comprising an unsaturated fatty acid or a derivative thereof (11) as a content and a coating layer (10) mainly containing gelatin, encapsulating the content (11), wherein a water-soluble gel layer (12) containing an acid or an acid salt thereof is present between the coating layer (10) and the content (11). The encapsulated unsaturated fatty acid substance of the present invention is characterized by that it has neither insolubility nor deterioration with time, and that it is enteric.
Owner:MORISHITA JINTAN CO LTD
Bullet composition
Owner:HANSEN RICHARD D
Shaving aid material
A shaving aid material is provided that includes a water-soluble lubricious shaving aid in combination with a water-insoluble erodable medium. In some embodiments, the shaving aid material further includes a water-soluble thermoplastic polymer, and may also include a plasticizer. Optional additional ingredients include emulsifiers, surfactants, skin conditioners, fragrances, depilatory agents, cleaning agents, medicinal agents, etc.
Owner:EDGEWELL PERSONAL CARE BRANDS LLC
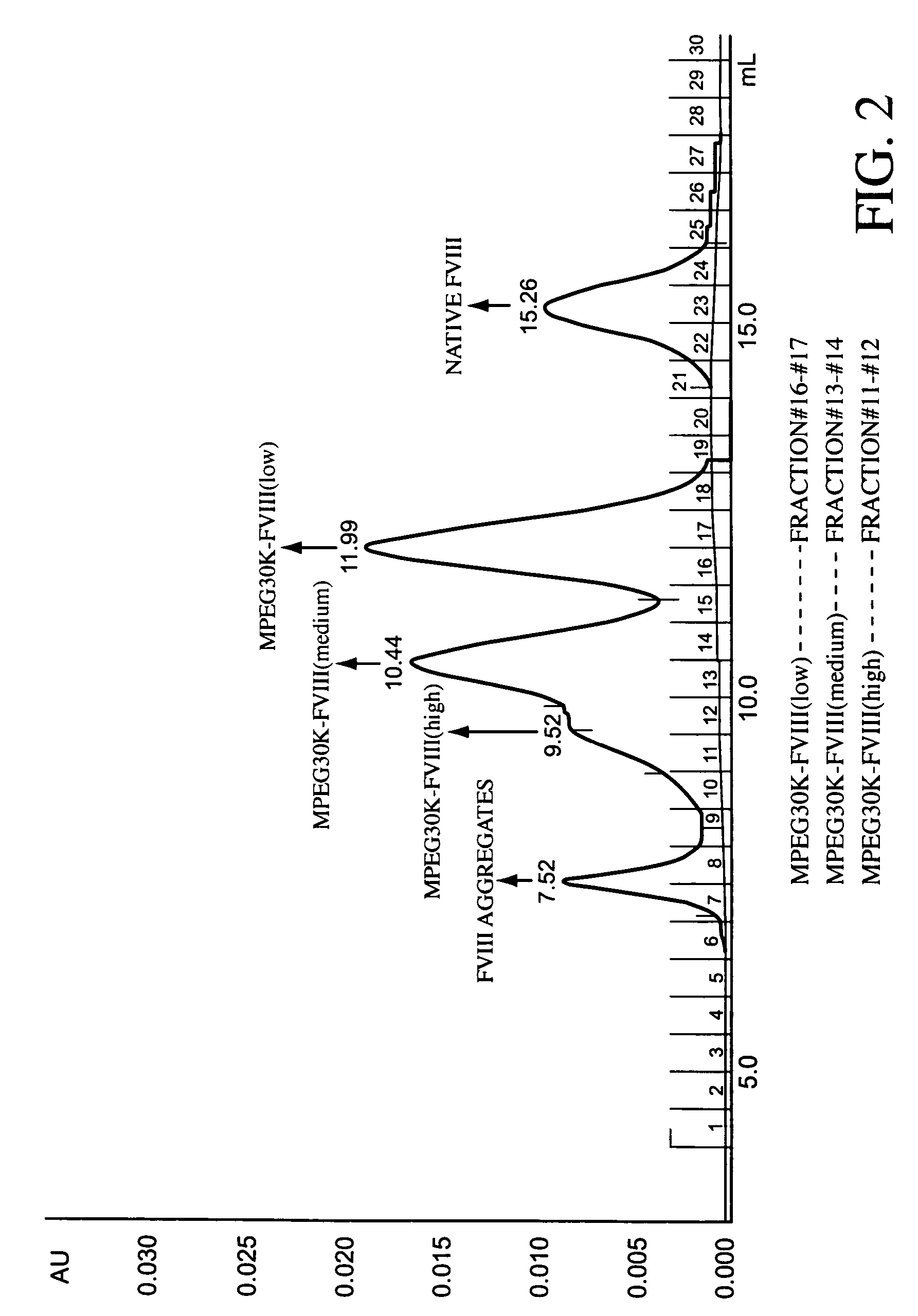
Polymer-factor VIII moiety conjugates
ActiveUS7199223B2Low immunogenicityReduce dosing frequencyPeptide/protein ingredientsMammal material medical ingredientsWater solubleEthylene glycol
Conjugates of a Factor VIII moiety and one or more water-soluble polymers are provided. Typically, the water-soluble polymer is poly(ethylene glycol) or a derivative thereof. Also provided are compositions comprising the conjugates, methods of making the conjugates, and methods of administering compositions comprising the conjugates to a patient.
Owner:NEKTAR THERAPEUTICS INC
Passivating solution and surface treatment method for galvanized material
InactiveCN101608306AImprove corrosion resistanceMeet the requirements of the RoHS directiveMetallic material coating processesChromium freeAlcohol
The invention relates to a passivating solution which is aqueous solution containing water-soluble molybdic compound, boric acid, water-soluble organic matter and silica sol, wherein the water-soluble organic matter is a mixture of alcohol and organic carboxylic acid. The invention also provides a surface treatment method for a galvanized material, which comprises the following step: enabling the passivating solution to be in contact with a galvanized material. A galvanized layer can be passivated by the contact between the passivating solution and the galvanized material so as to enable the galvanized material to have excellent corrosion resistance. In addition, the paint composition is chromium free and meets the requirements of a RoHS instruction.
Owner:PANGANG GROUP VANADIUM TITANIUM & RESOURCES +3
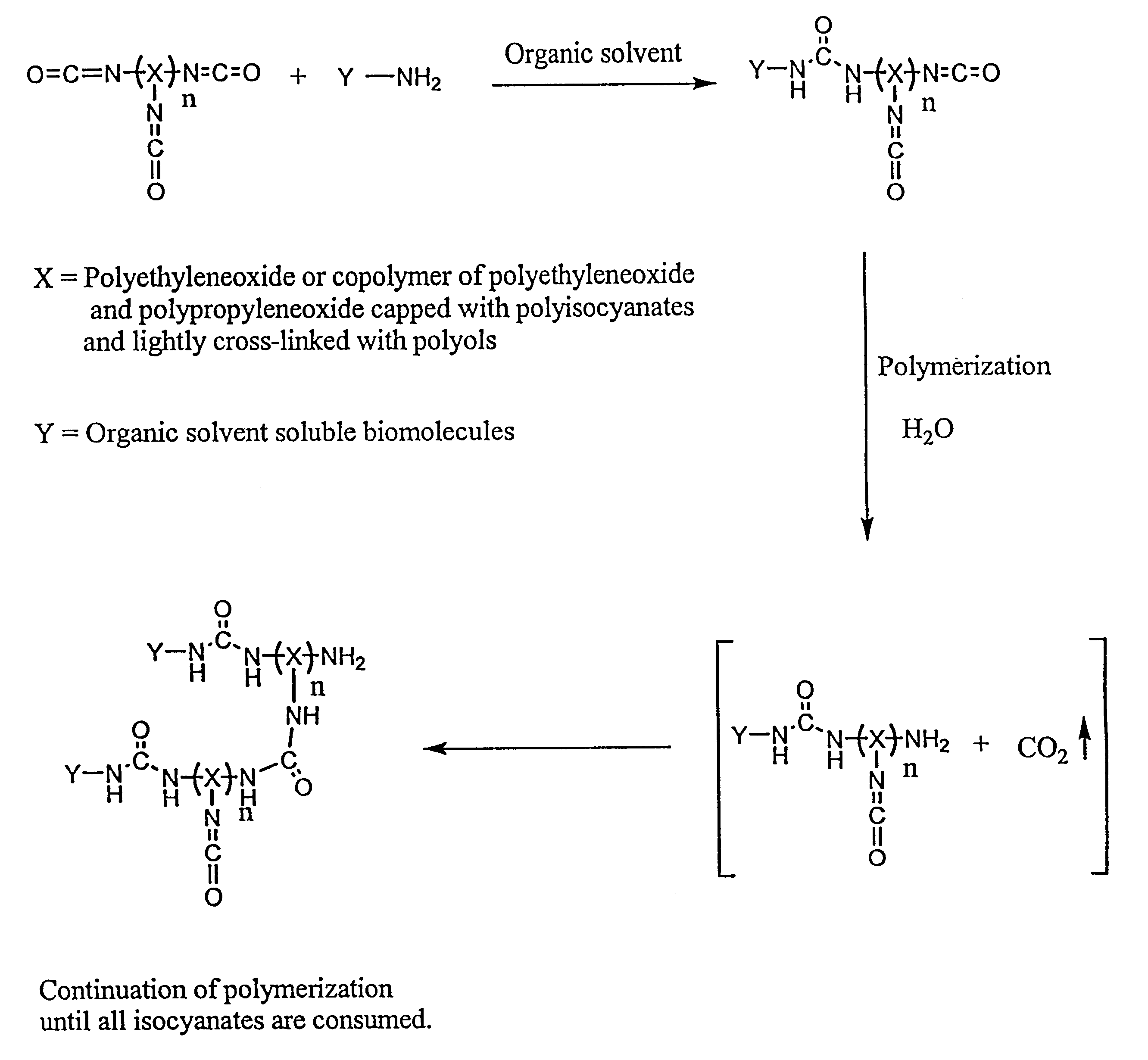
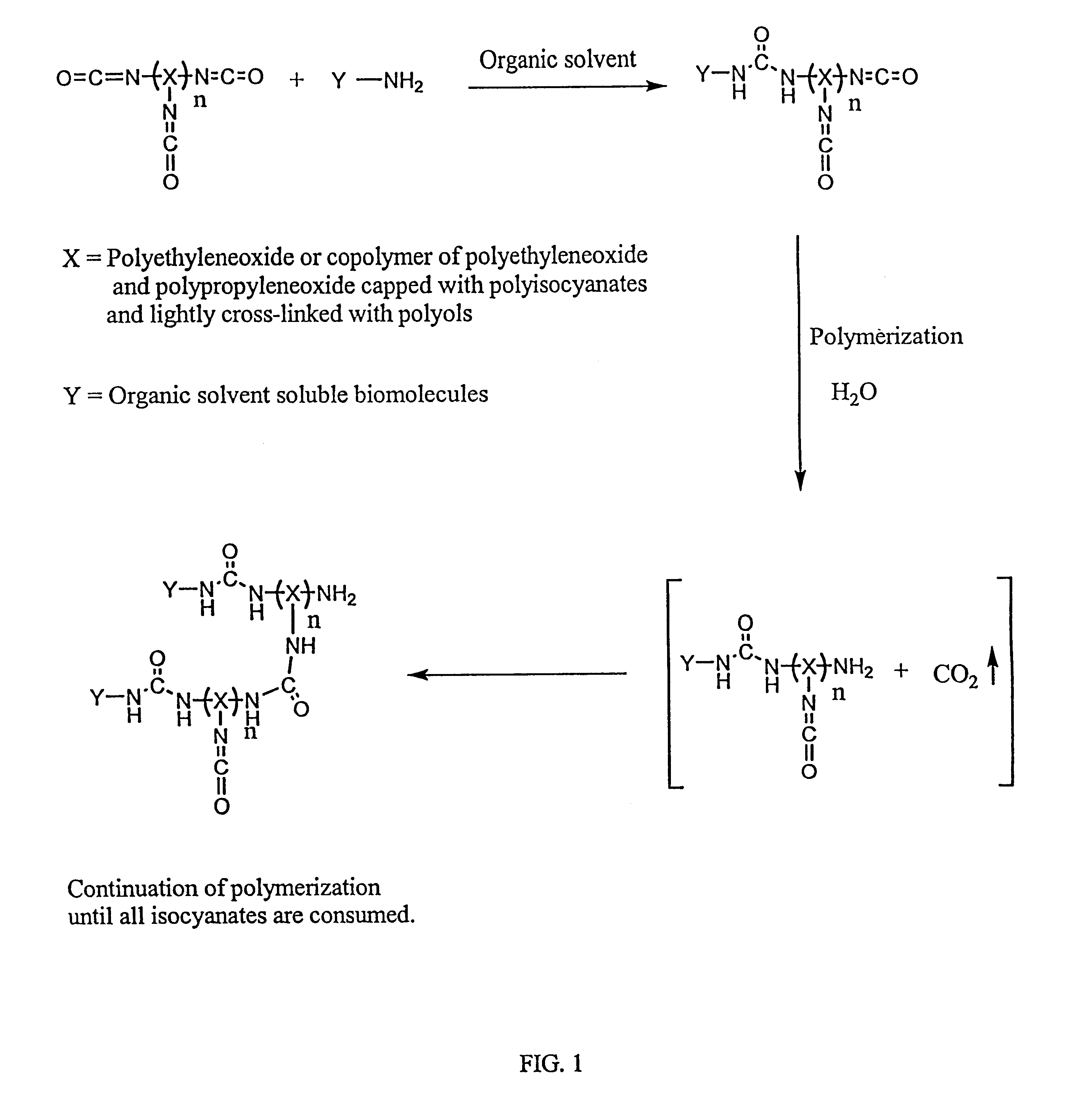
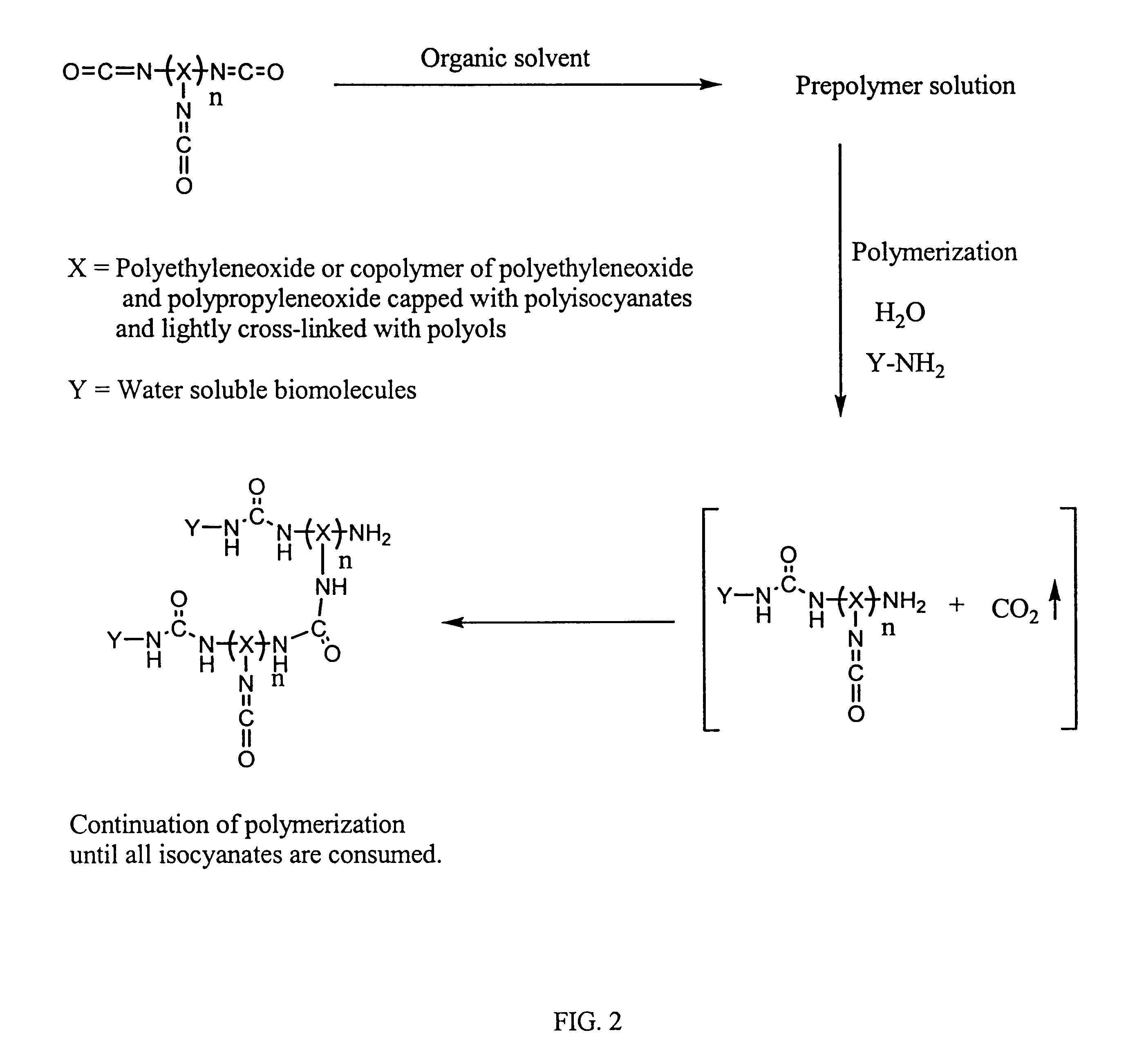
Method of making biochips and the biochips resulting therefrom
InactiveUS6174683B1Rapid and simple and cost-effective methodHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsSodium bicarbonateSolid substrate
Methods for preparing a biochip are provided herein wherein the biomolecular probe to be used with the biochip is alternatively bound to a hydrogel prepolymer prior to or simultaneously with polymerization of the prepolymer. In particularly preferred embodiments, a polyurethane-based hydrogel prepolymer is derivatized with an organic solvent soluble biomolecule, such as a peptide nucleic acid probe in aprotic, organic solvent. Following derivatization of the prepolymer, an aqueous solution, for example sodium bicarbonate, preferably buffered to a pH of about 7.2 to about 9.5, is added to the derivatized prepolymer solution to initiate polymerization of the hydrogel. Alternatively, a water soluble biomolecule, such as DNA or other oligonucleotide, is prepared in an aqueous solution and added to the polyurethane-based hydrogel prepolymer such that derivatization and polymerization occur, essentially, simultaneously. While the hydrogel is polymerizing, it is microspotted onto a solid substrate, preferably a silanated glass substrate, to which the hydrogel microdroplet becomes covalently bound. Most preferably the hydrogel microdroplets are at least about 30 mum thick, for example about 50 mum to about 100 mum thick. The resulting biochips are particularly useful for gene discovery, gene characterization, functional gene analysis and related studies.
Owner:BIOCEPT INC
Self-inflating intragastric volume-occupying device
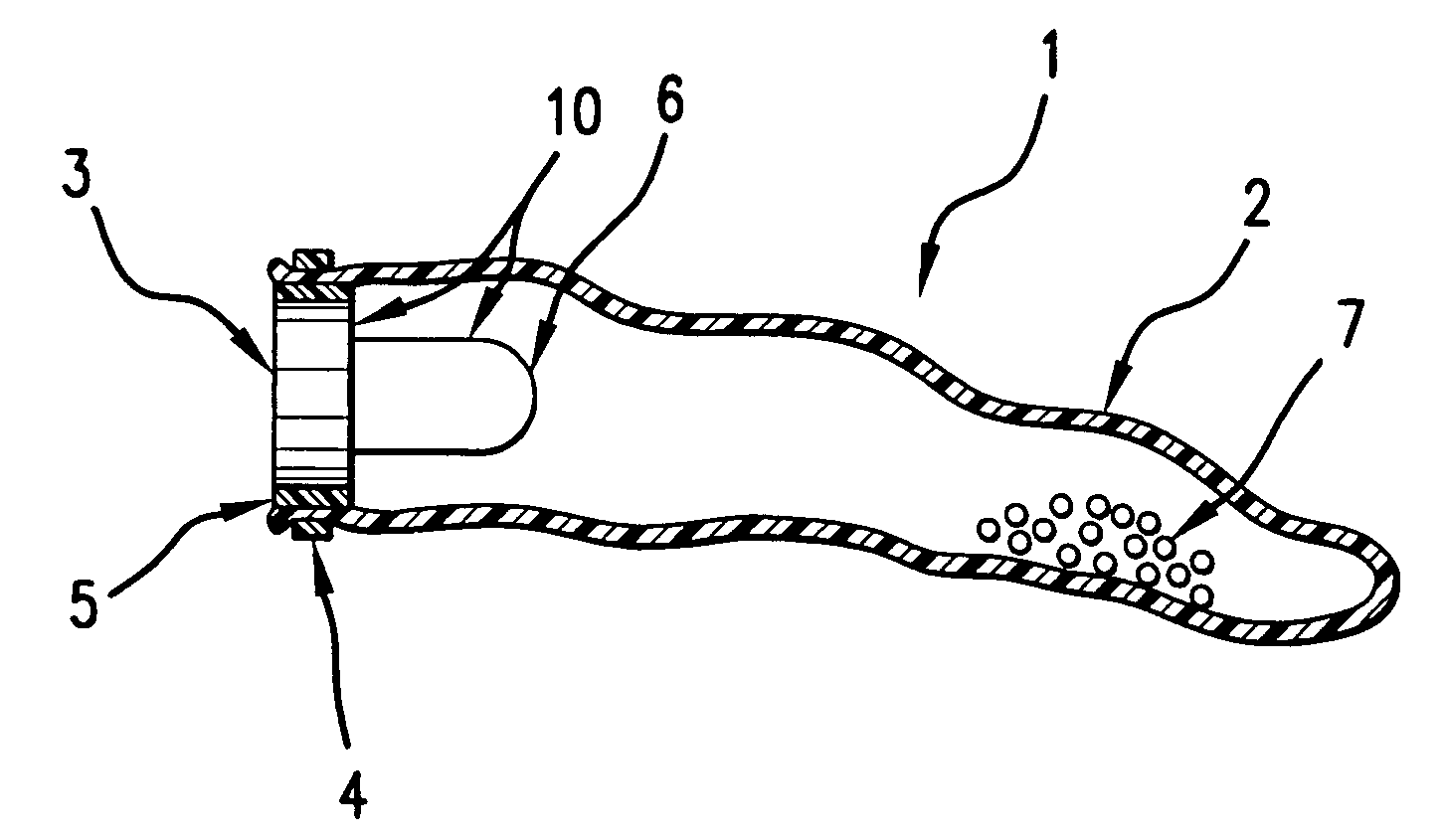
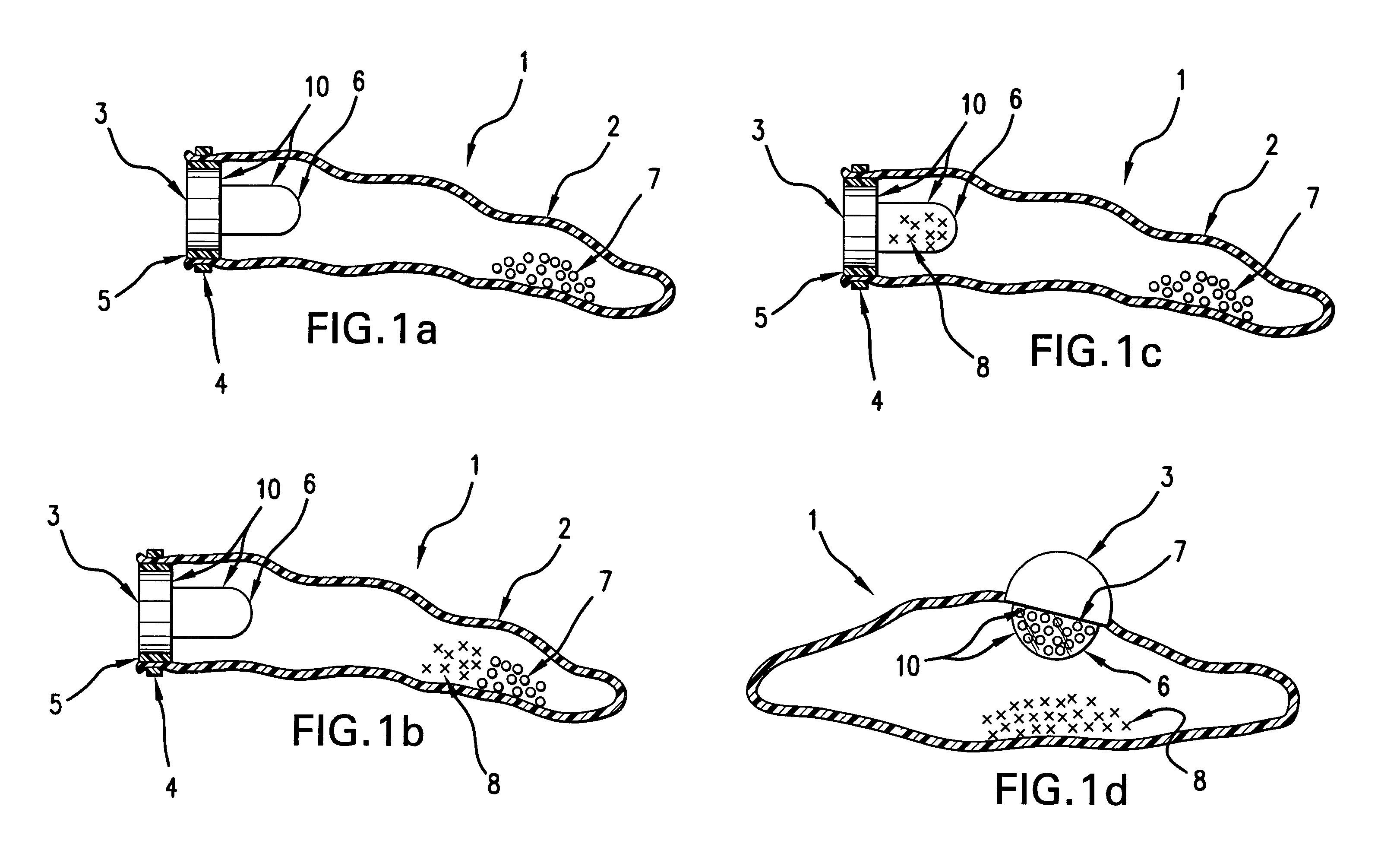
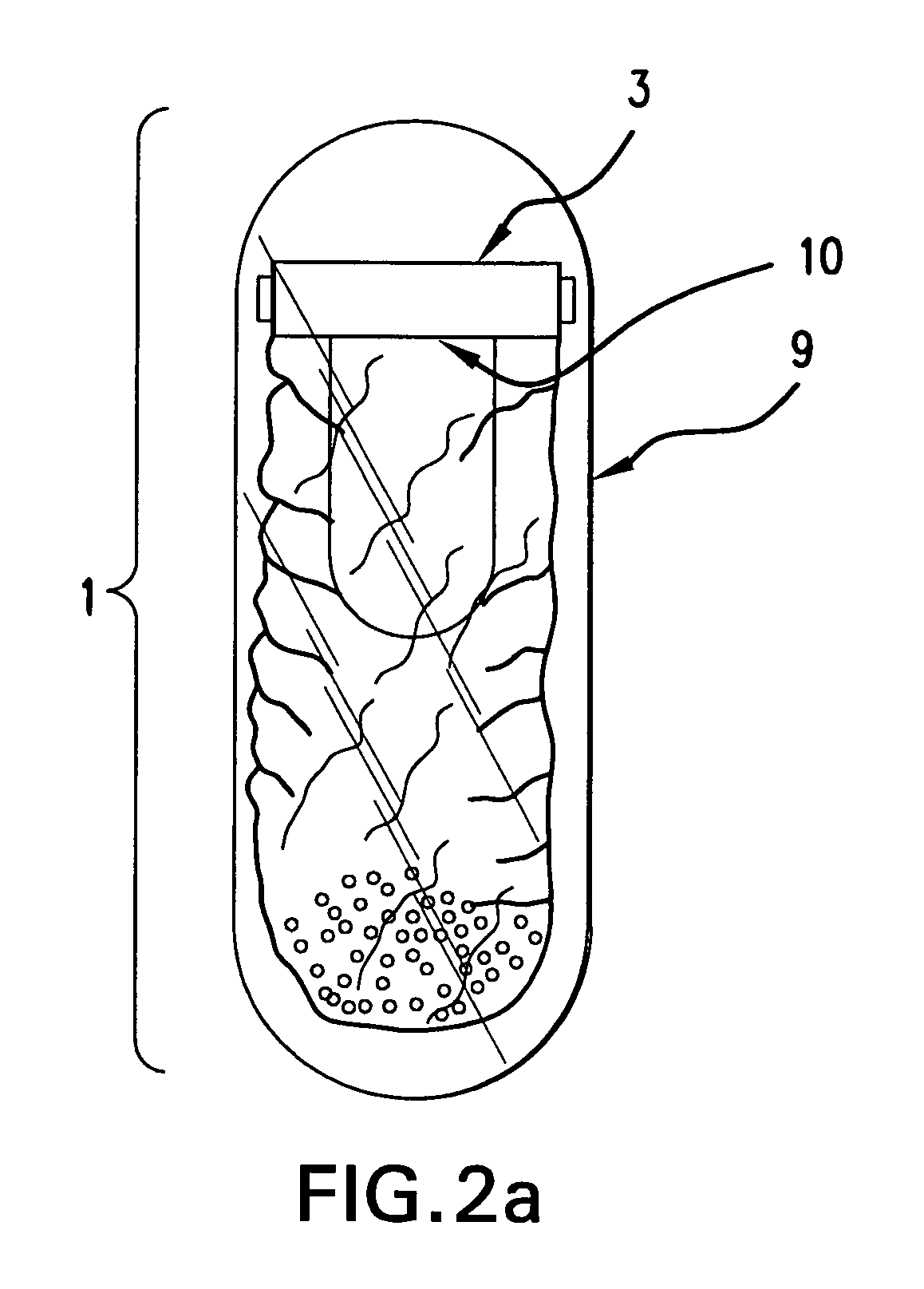
A self-inflating, intragastric balloon device is activated by addition of an activating liquid into an acid or water soluble vessel located within the balloon. After an approximately pre-determined time period post-activation, the activating liquid breaches the vessel wall and causes contact between an acid in liquid form and an emissive substance within the balloon, causing it to inflate. The device is conveniently provided to medical personnel as part of a kit that further contains a syringe and the activating liquid. The device also can have acid or pepsin degradable portions that cause it to deflate after residing in the stomach for a prolonged period of time during which it imparts a feeling of satiety in the patient.
Owner:OBALON THERAPEUTICS
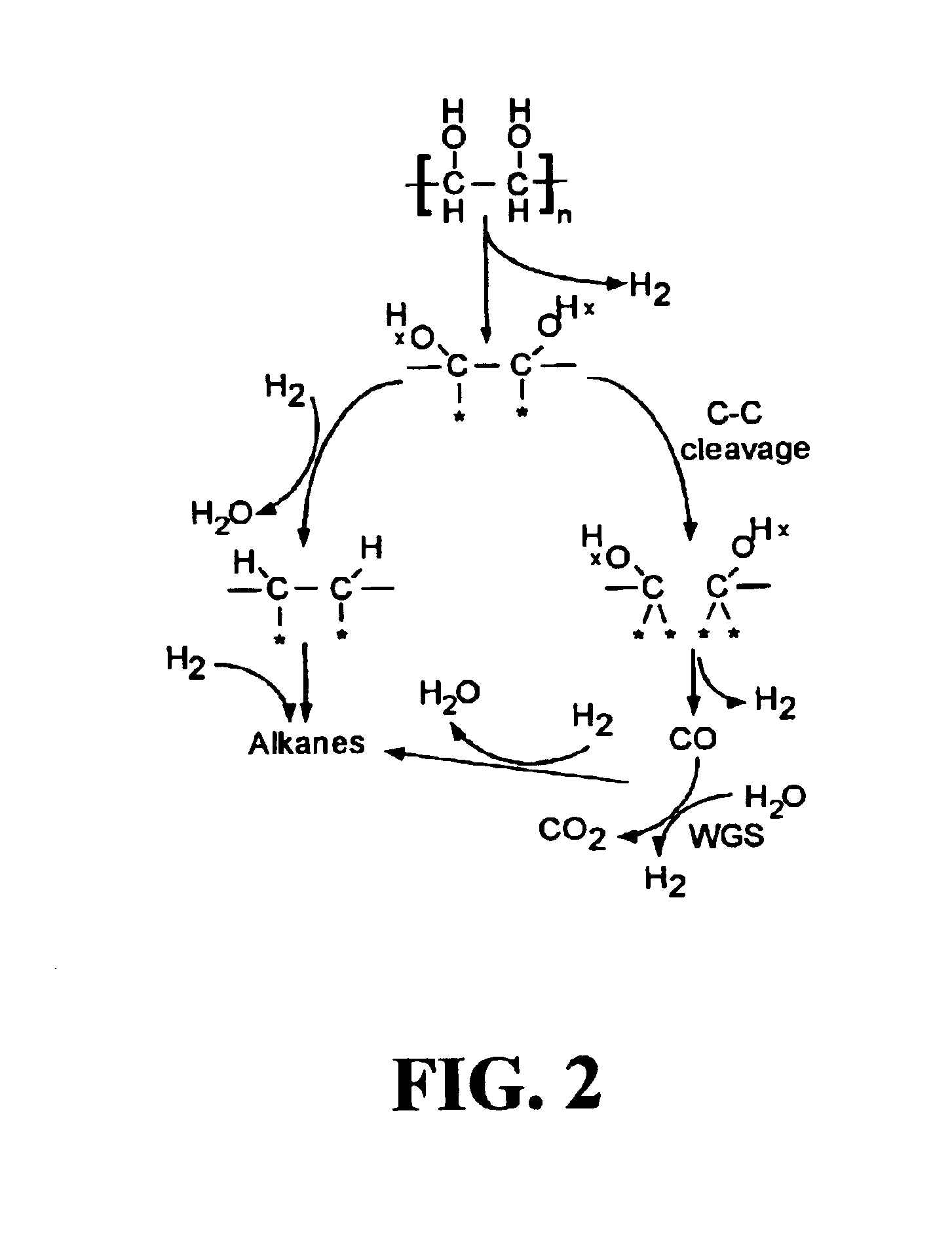
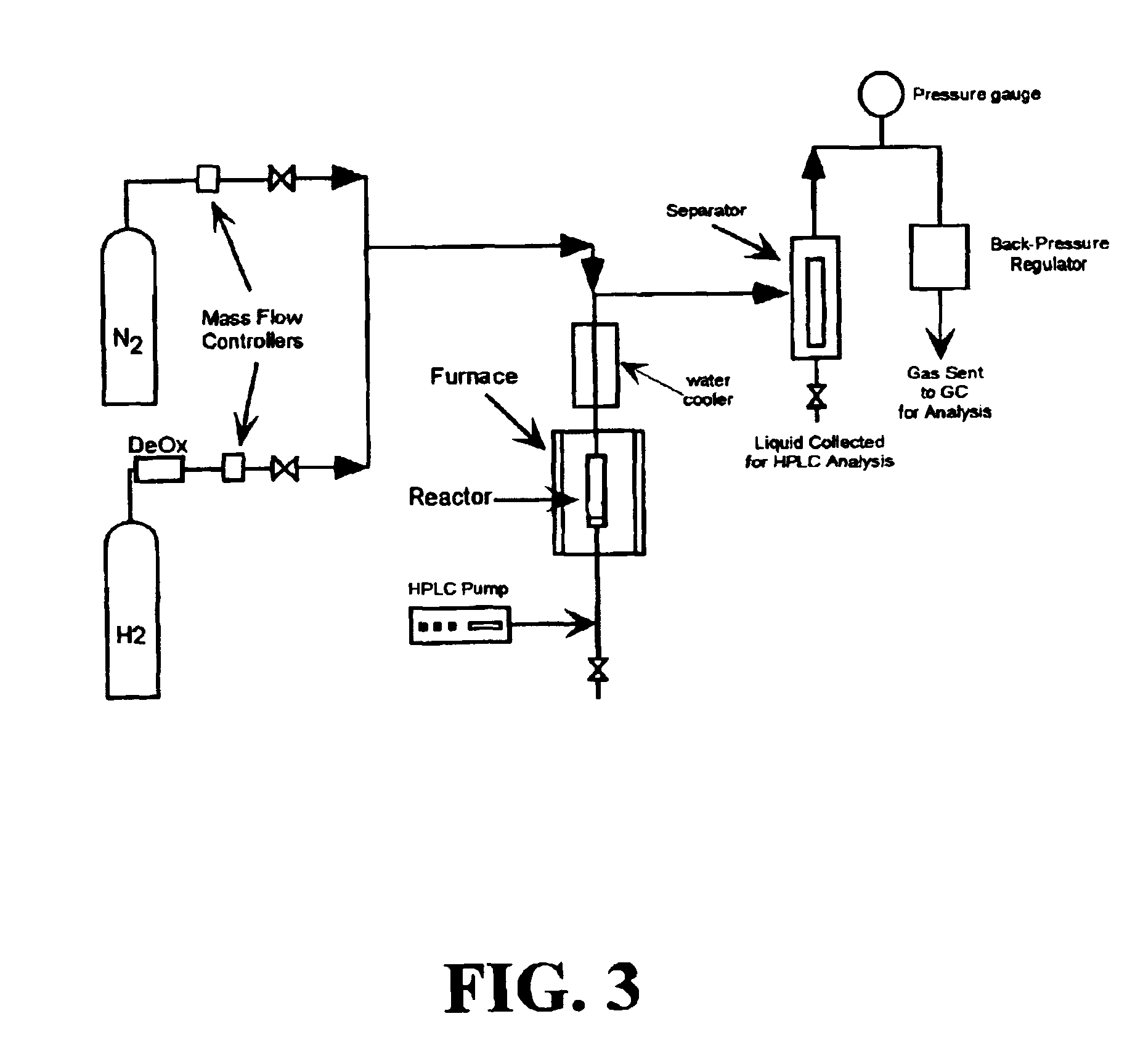
Low-temperature hydrocarbon production from oxygenated hydrocarbons
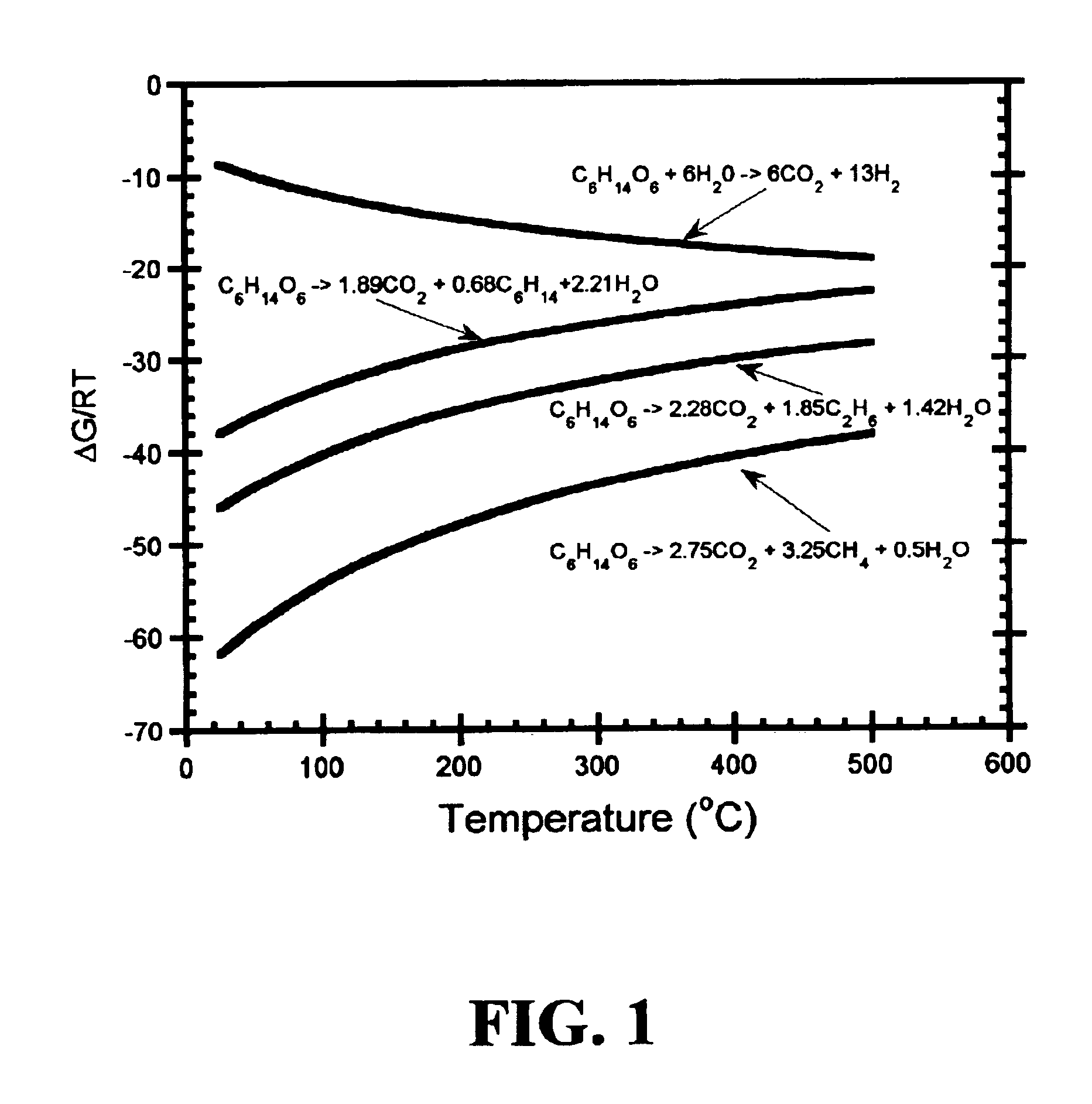
Disclosed is a method of producing hydrocarbons from oxygenated hydrocarbon reactants, such as glycerol, glucose, or sorbitol. The method can take place in the vapor phase or in the condensed liquid phase (preferably in the condensed liquid phase). The method includes the steps of reacting water and a water-soluble oxygenated hydrocarbon having at least two carbon atoms, in the presence of a metal-containing catalyst. The catalyst contains a metal selected from the group consisting of Group VIIIB transitional metals, alloys thereof, and mixtures thereof. These metals are supported on supports that exhibit acidity or the reaction is conducted under liquid-phase conditions at acidic pHs. The disclosed method allows the production of hydrocarbon by the liquid-phase reaction of water with biomass-derived oxygenated compounds.
Owner:WISCONSIN ALUMNI RES FOUND
Sustained-release microcapsule of amorphous water-soluble pharmaceutical active agent
InactiveUS6117455AHigh entrapment of a water-soluble drugSmall initial releaseNanotechGogglesEmulsionOrganic solvent
A sustained-release microcapsule contains an amorphous water-soluble pharmaceutical agent having a particle size of from 1 nm-10 mu m and a polymer. The microcapsule is produced by dispersing, in an aqueous phase, a dispersion of from 0.001-90% (w / w) of an amorphous water-soluble pharmaceutical agent in a solution of a polymer having a wt. avg. molecular weight of 2,000-800,000 in an organic solvent to prepare an s / o / w emulsion and subjecting the emulsion to in-water drying.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com