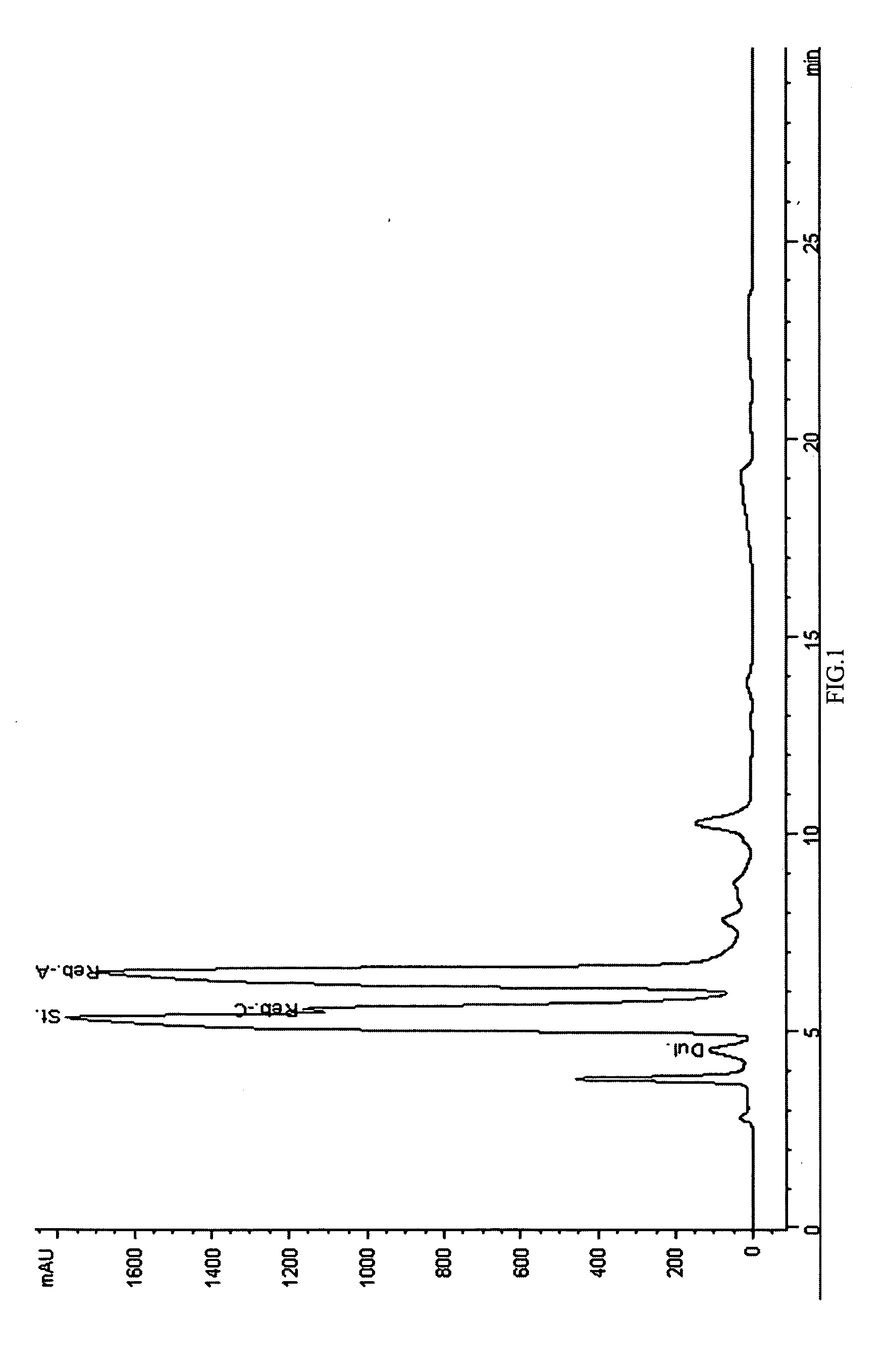
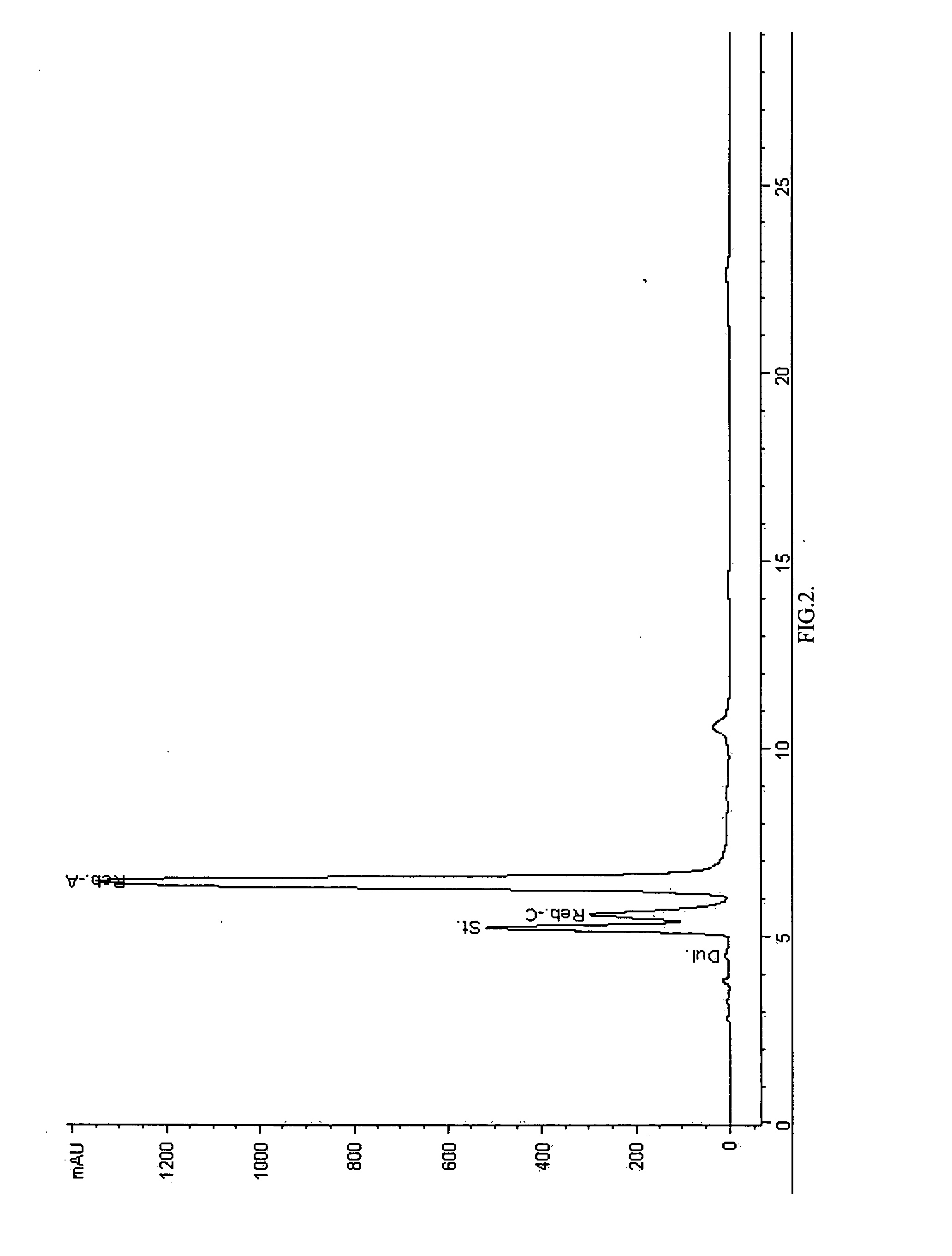
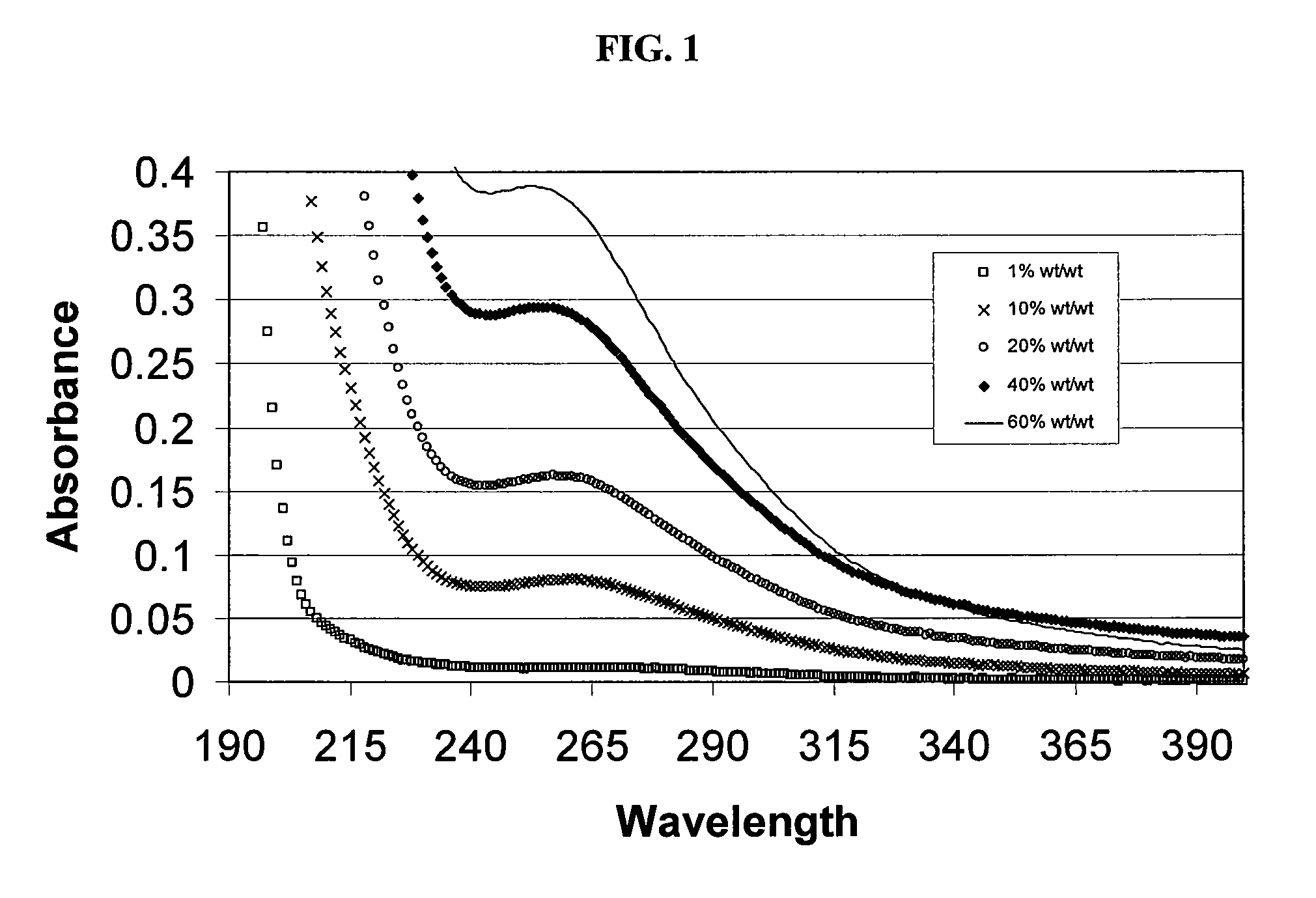
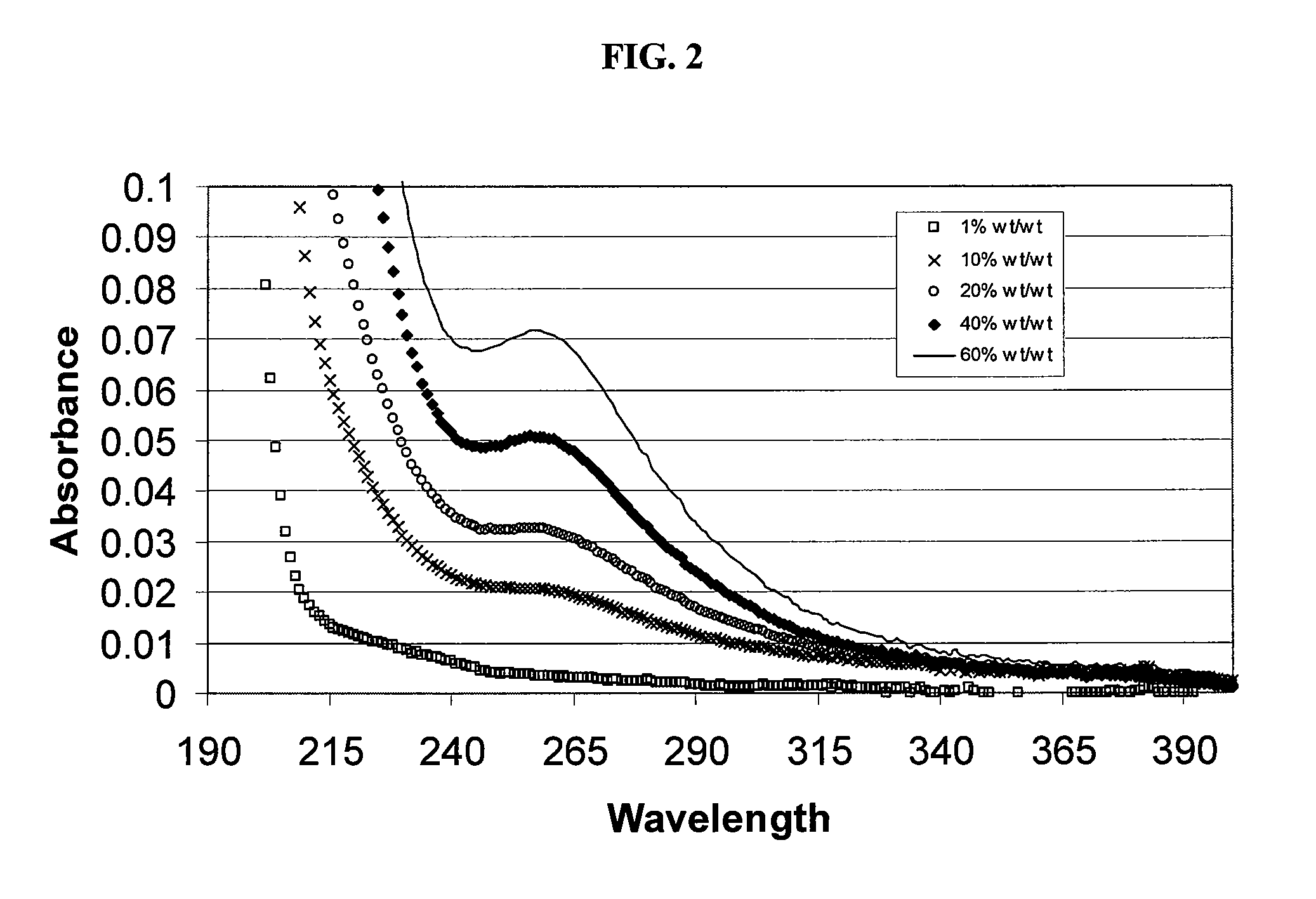
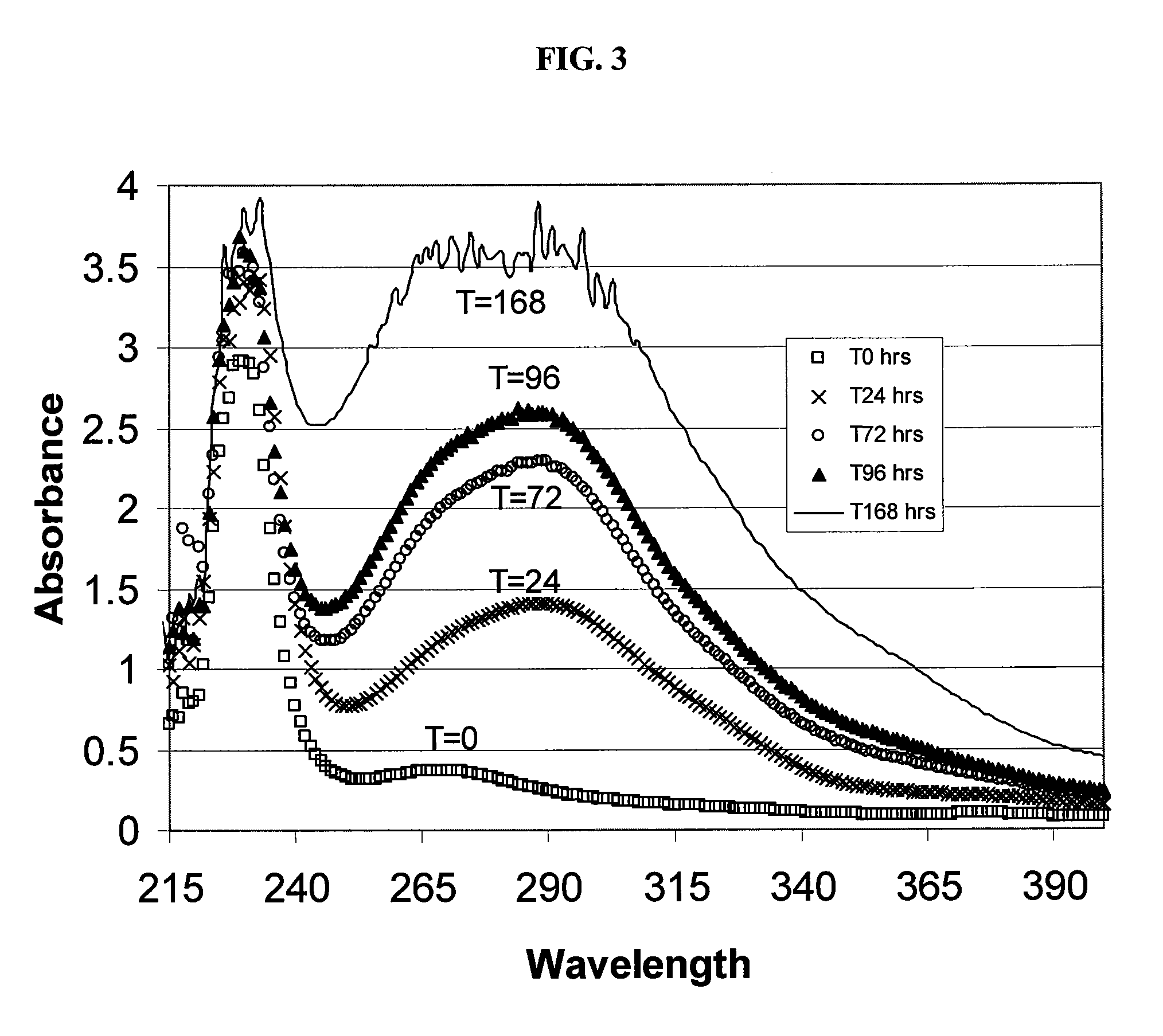
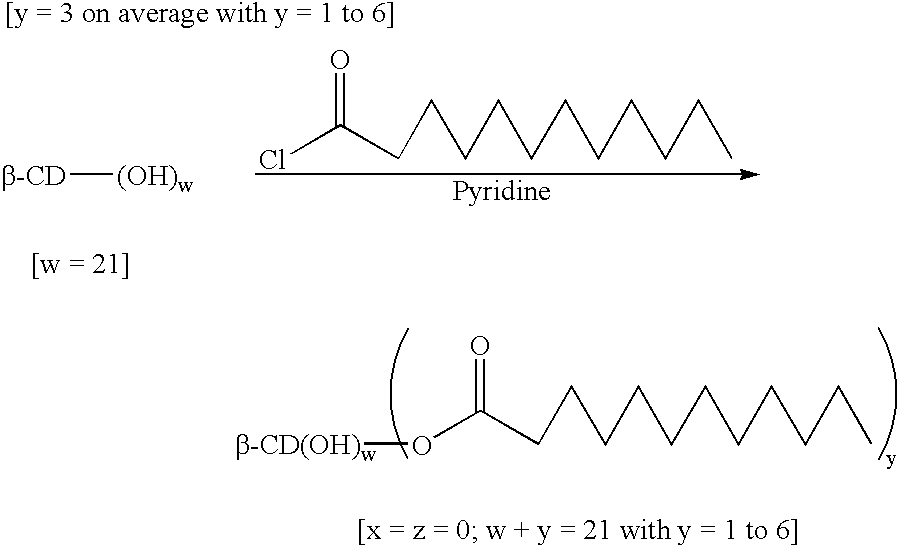
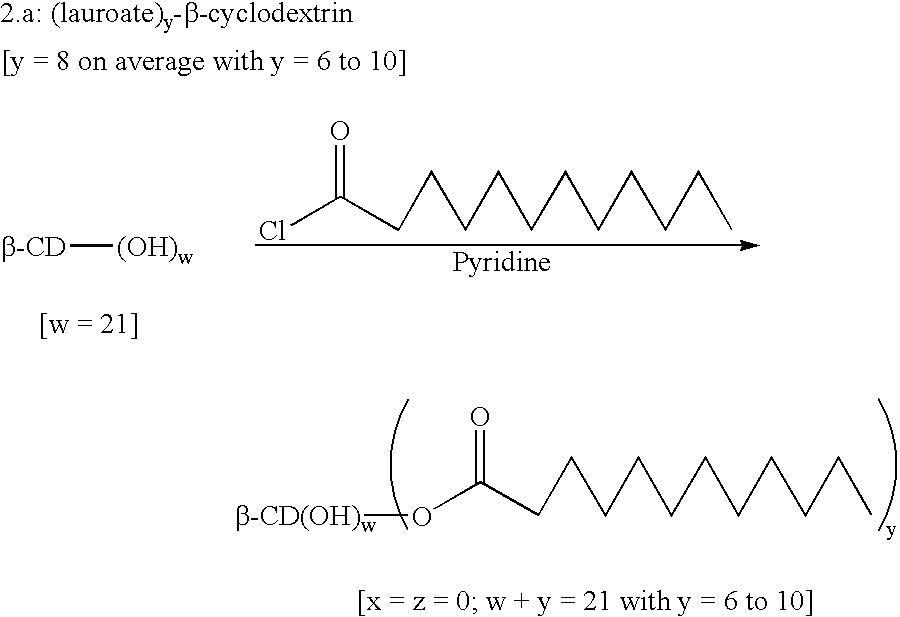
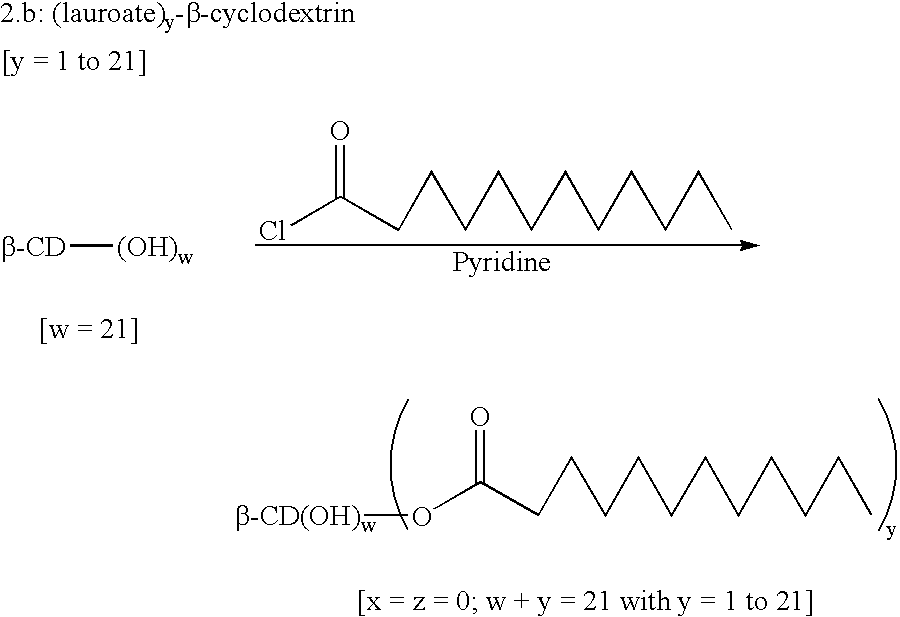
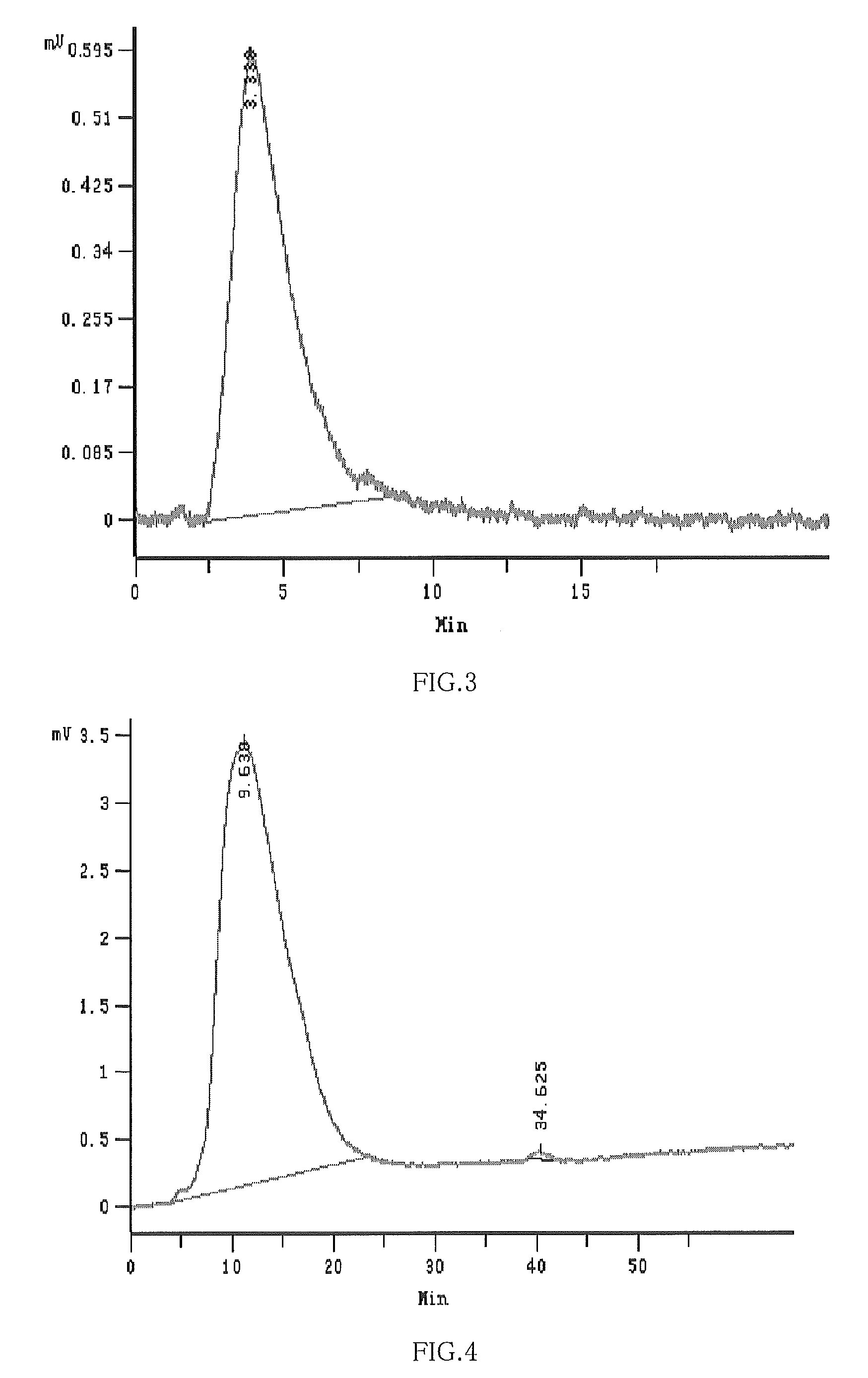
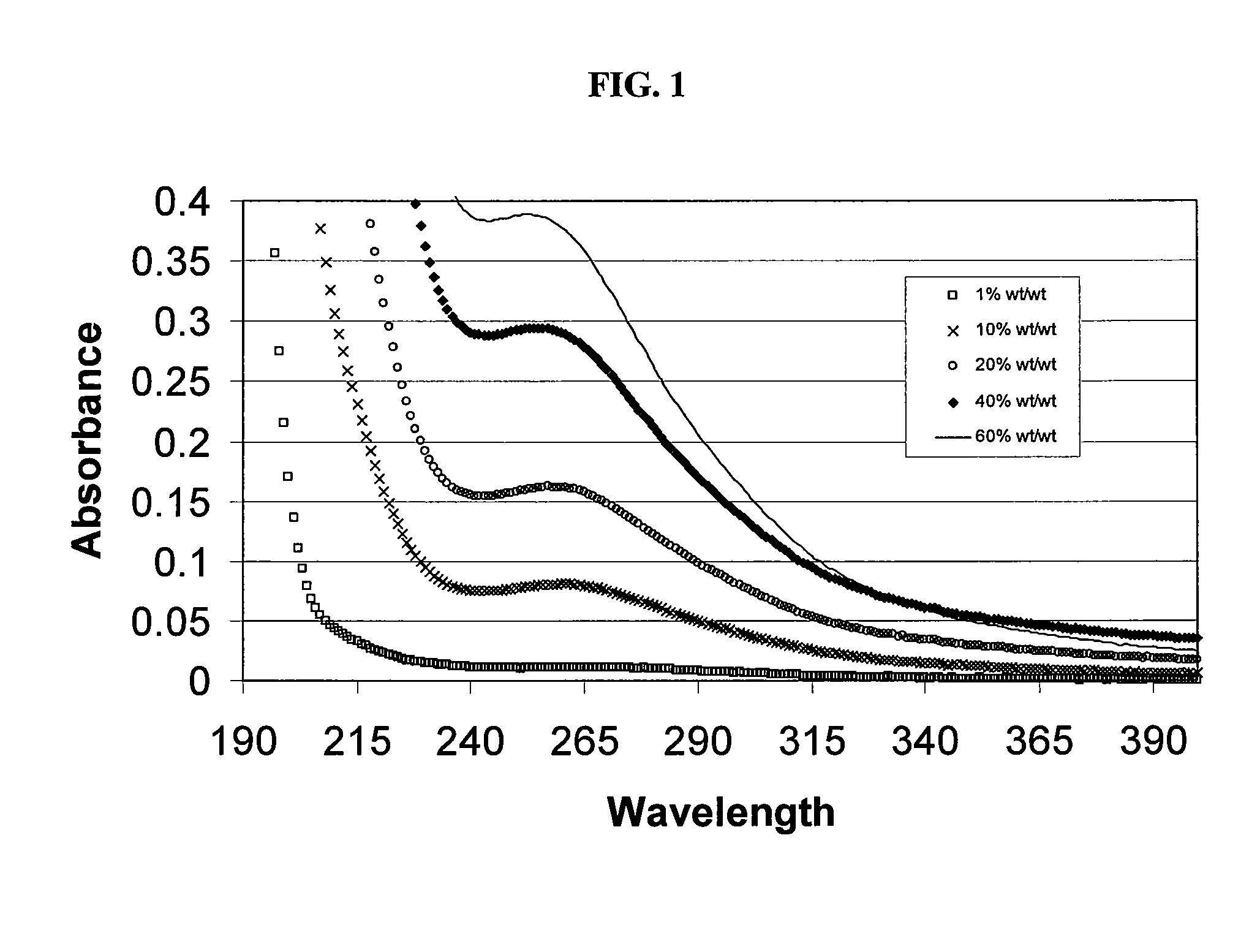
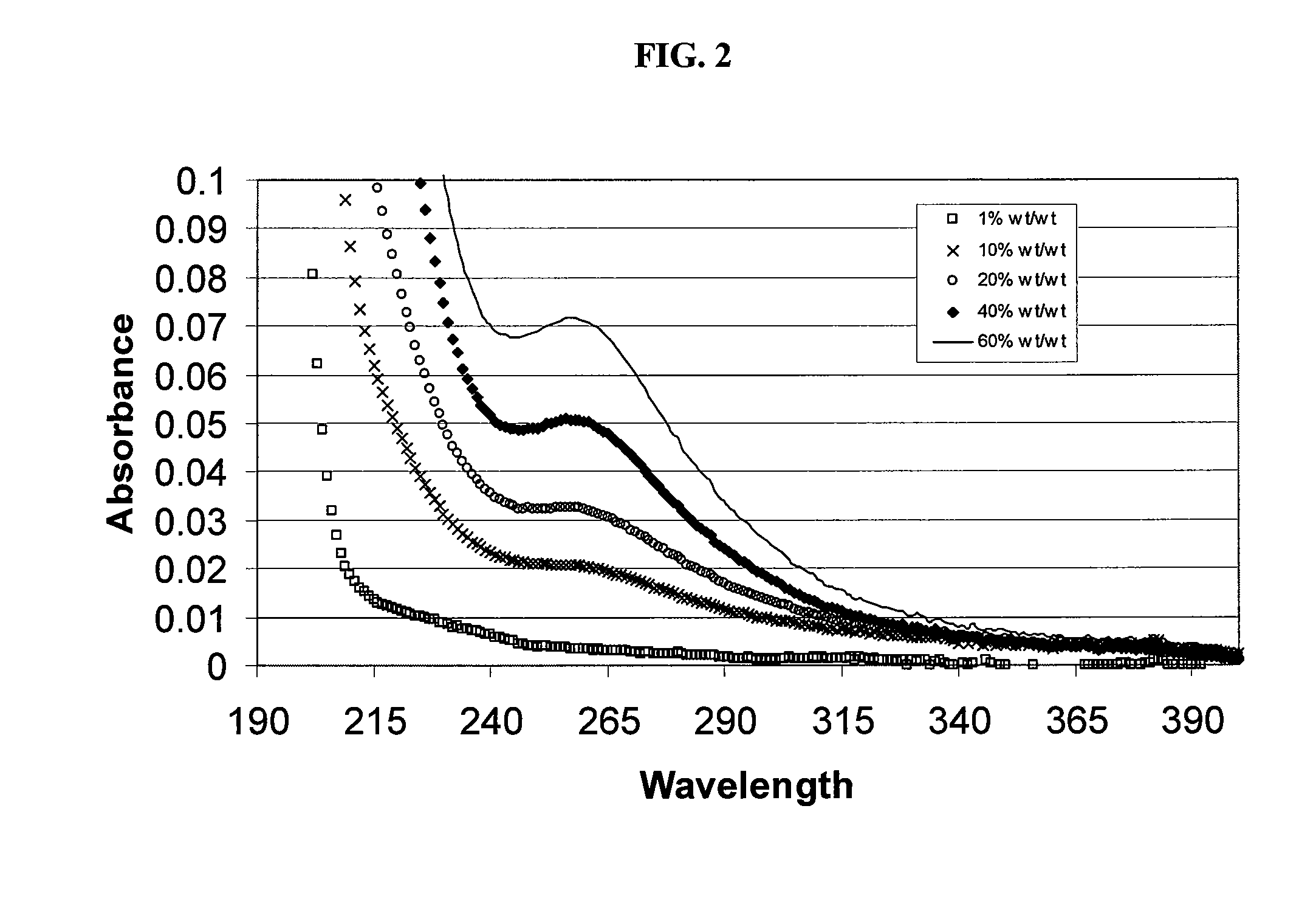
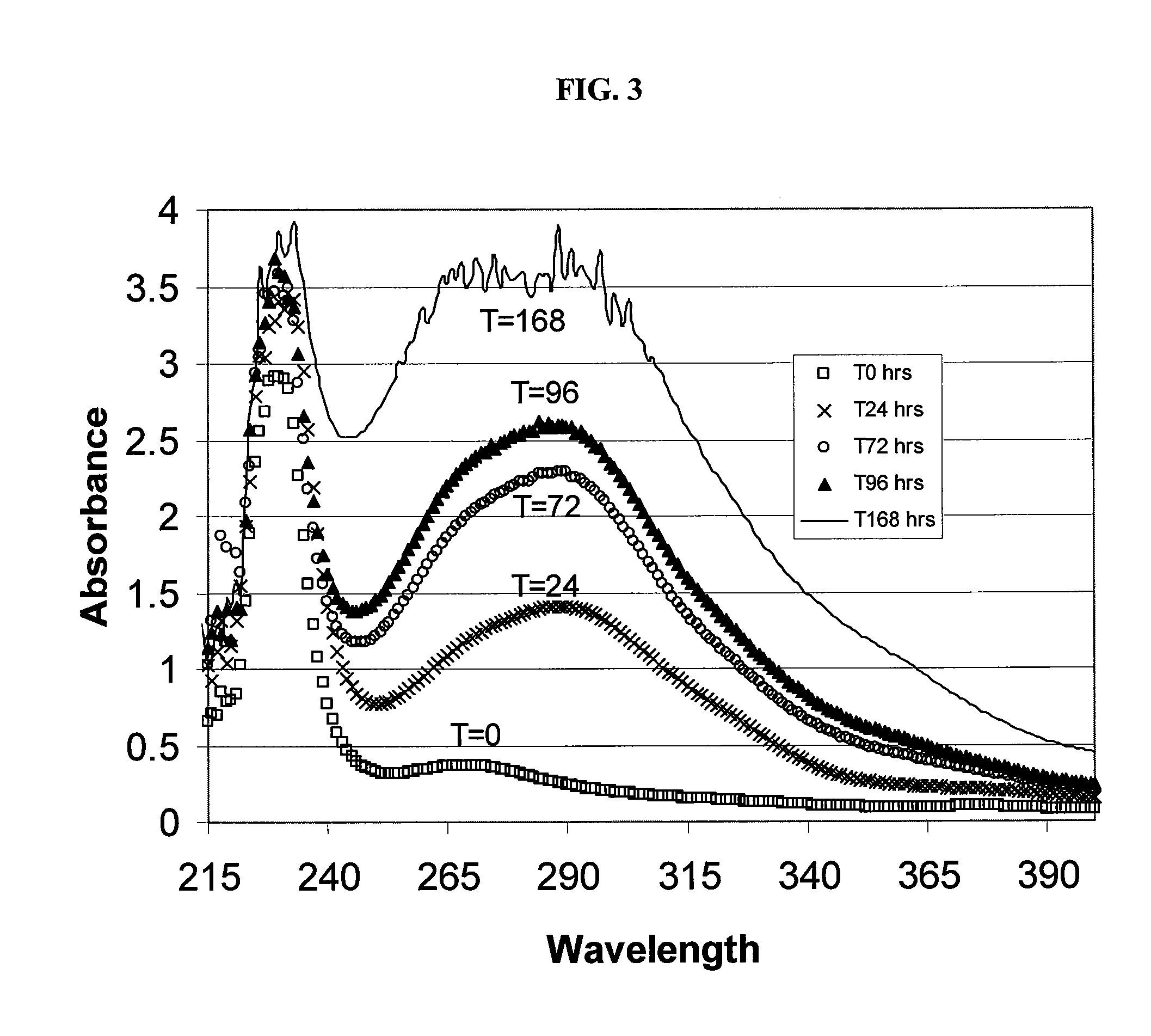
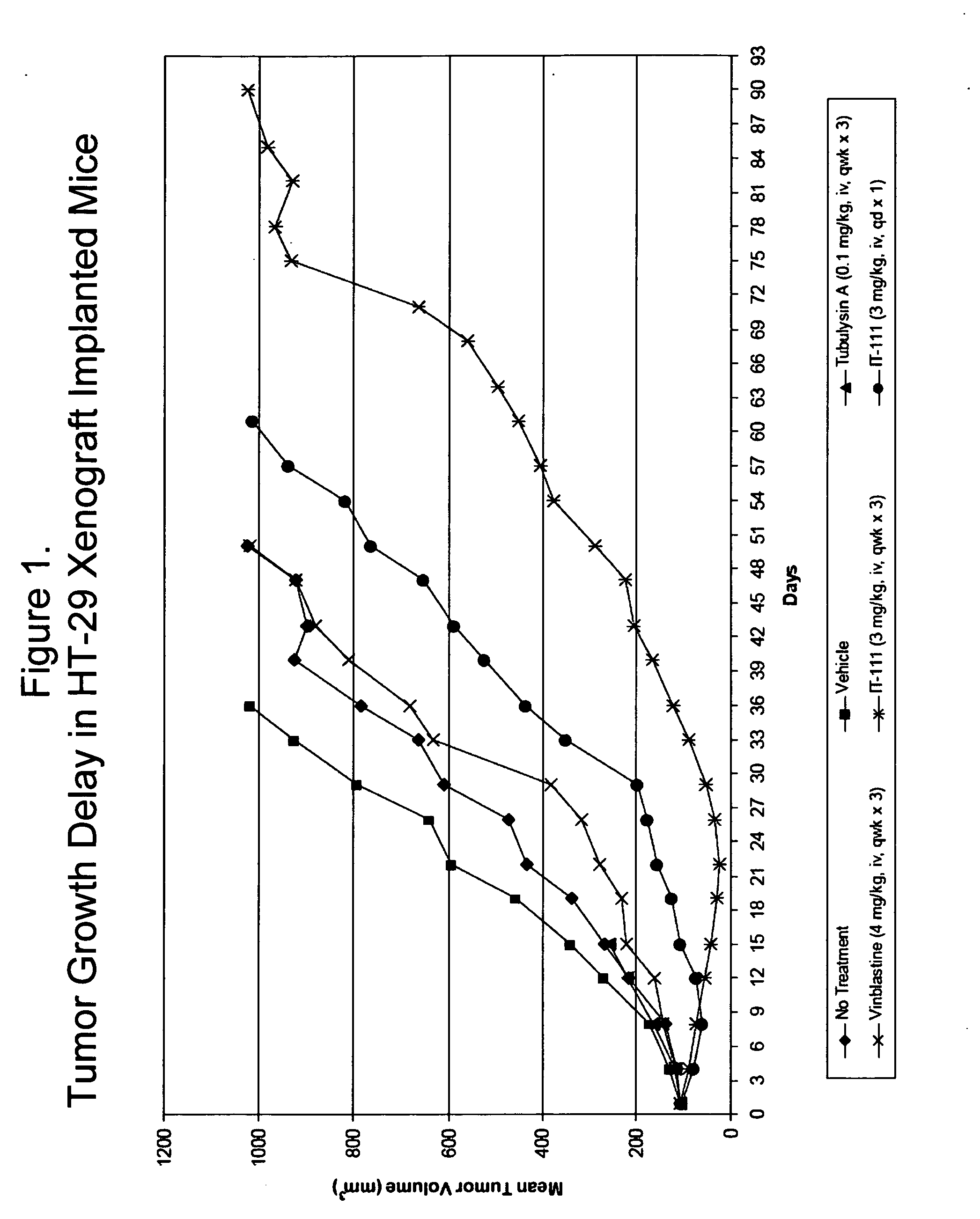
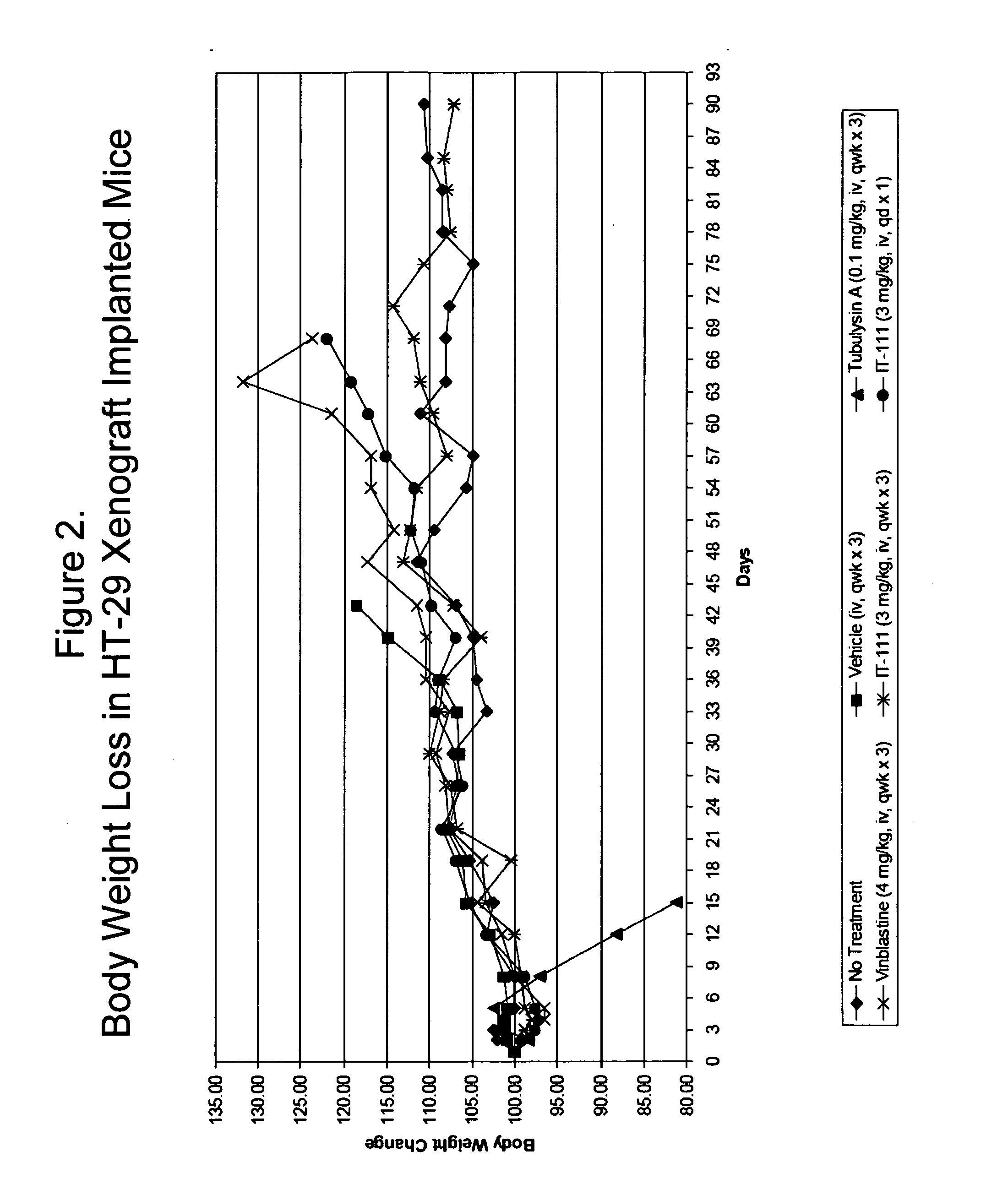
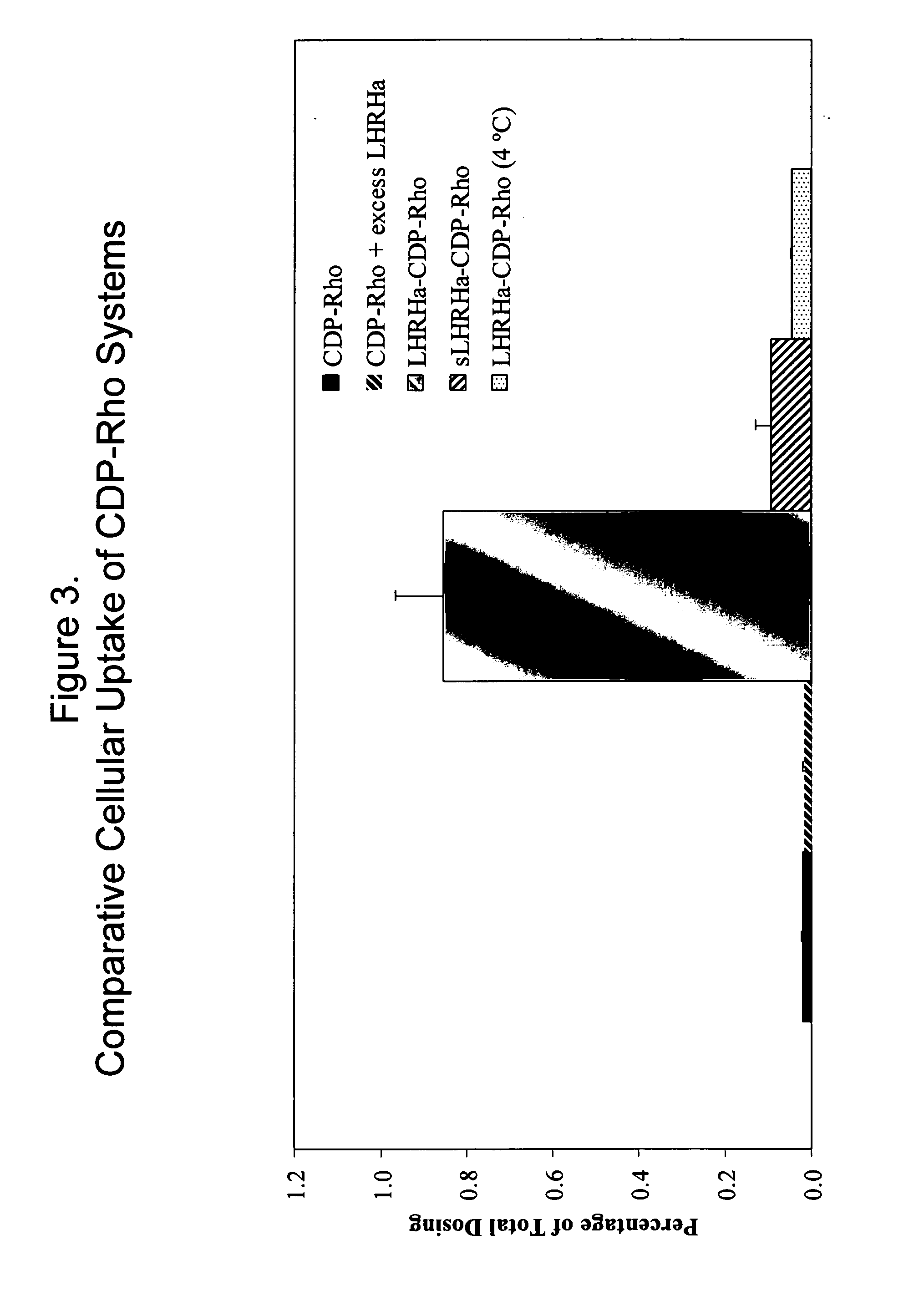
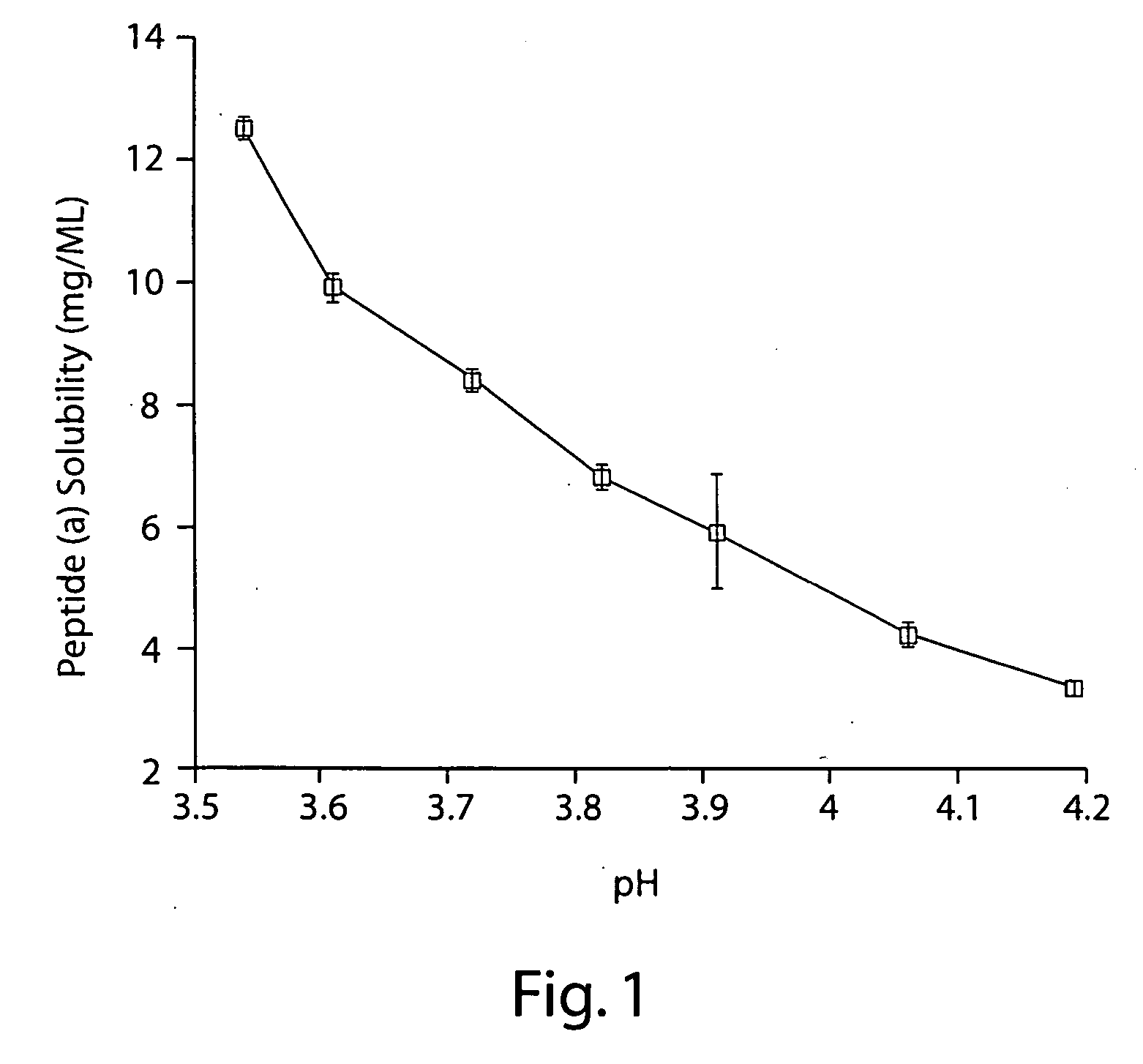
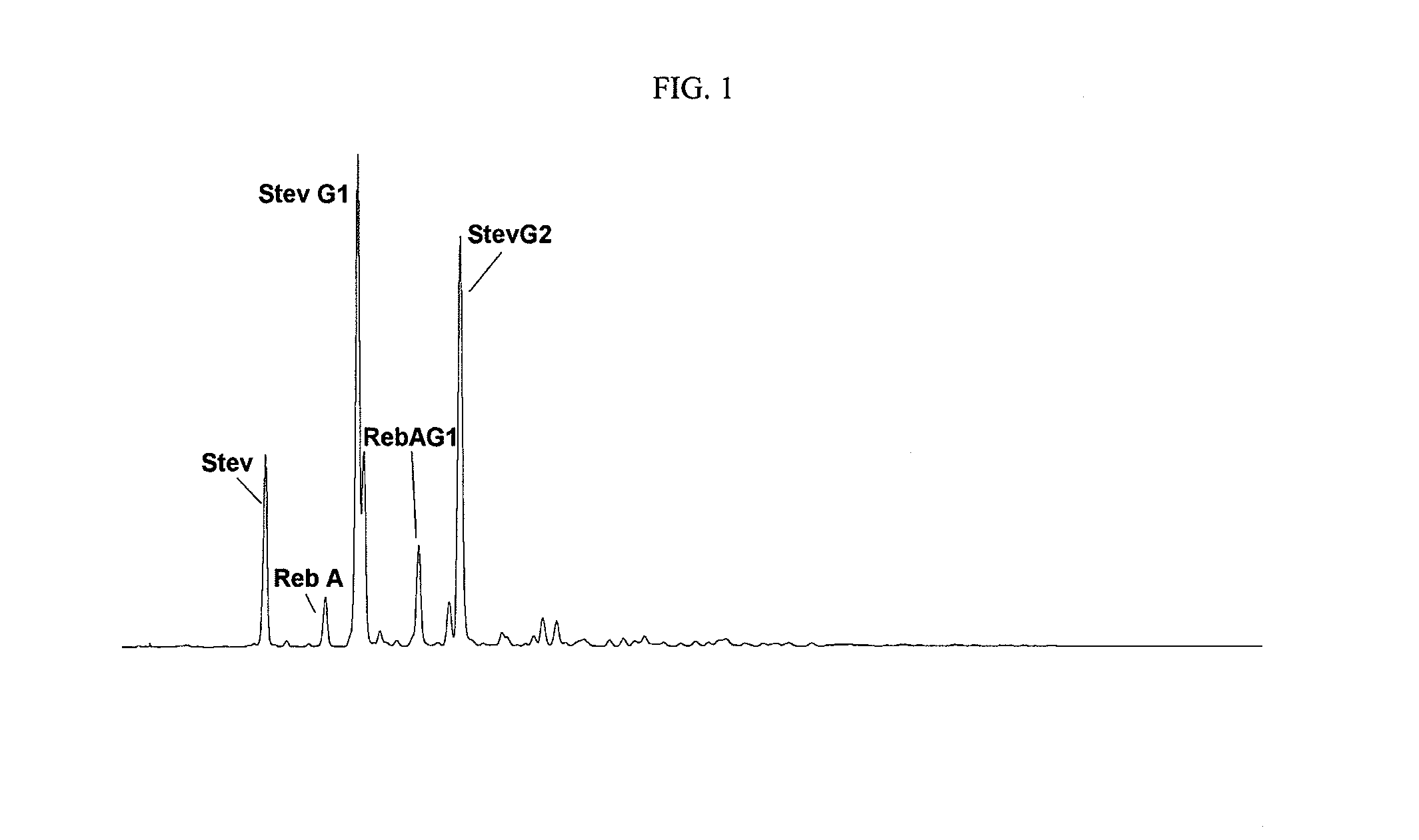
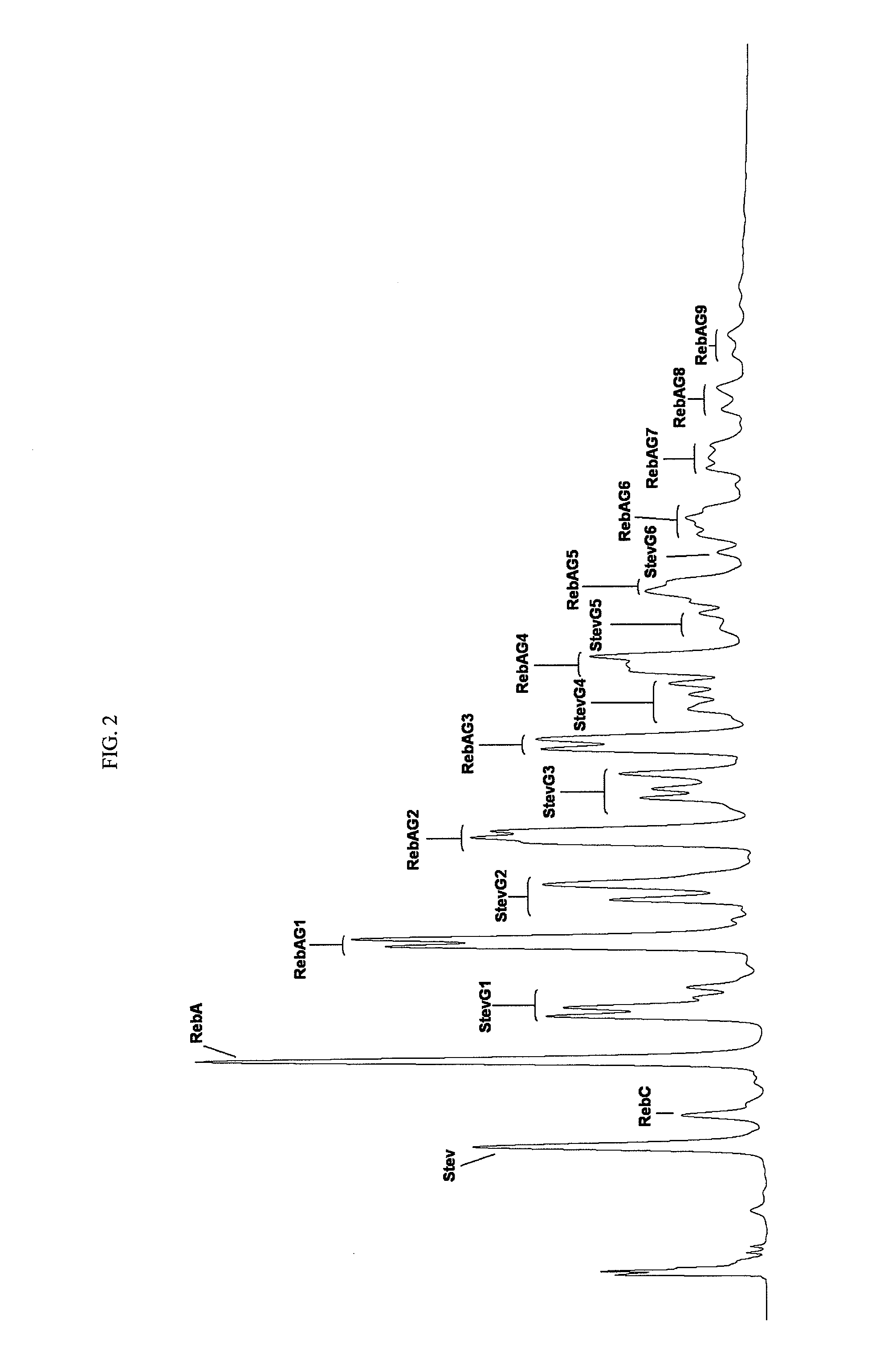
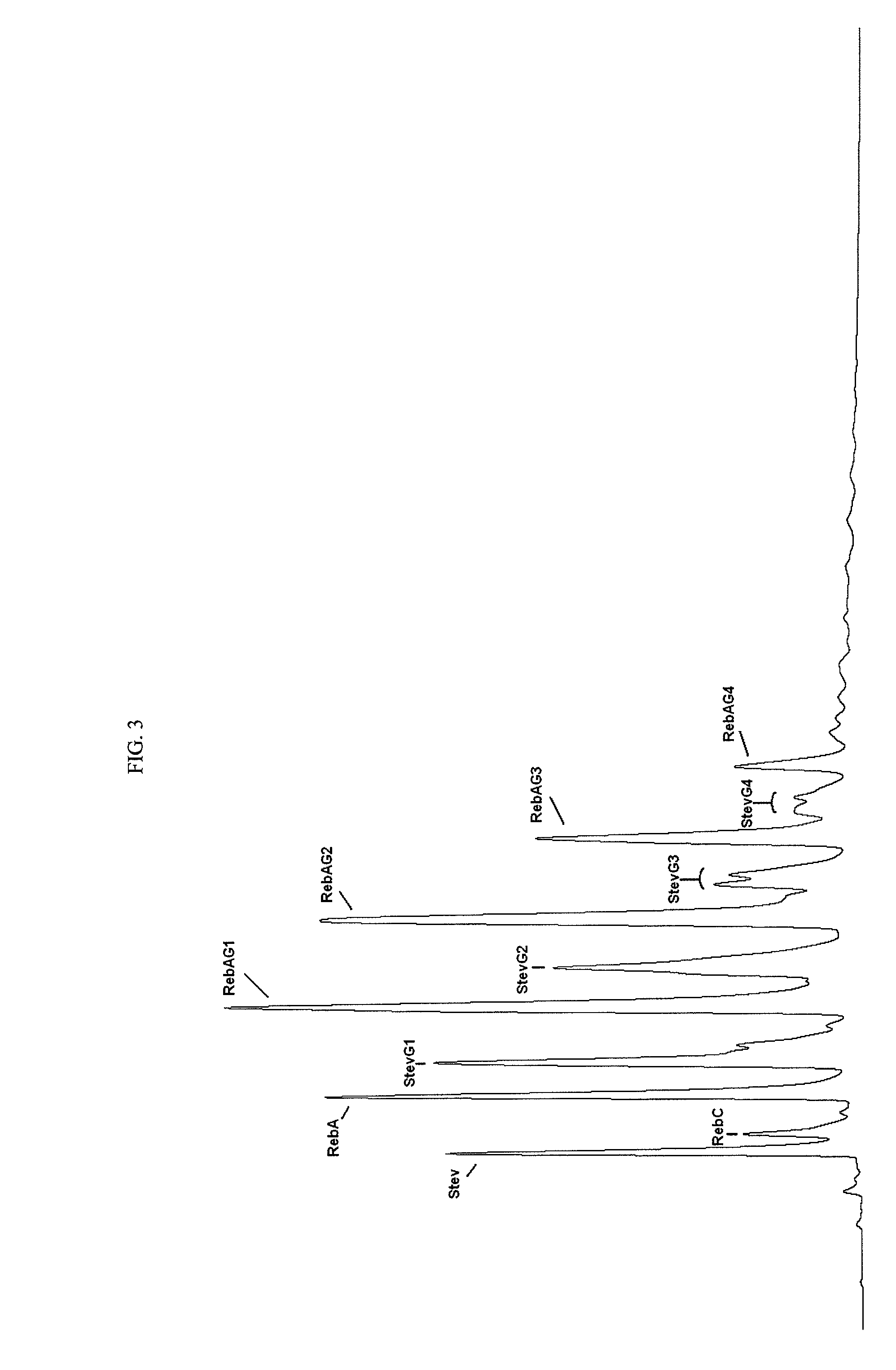
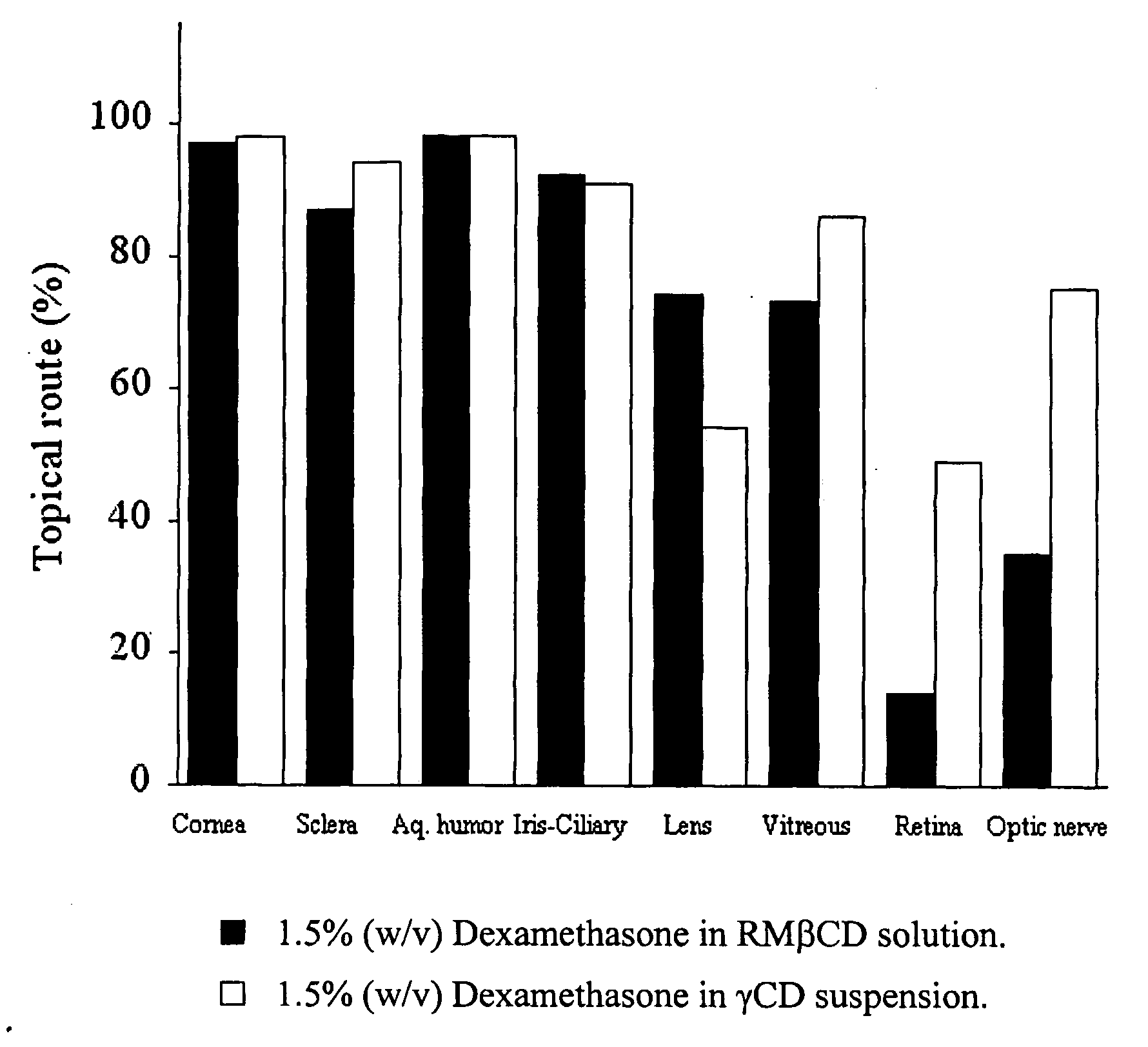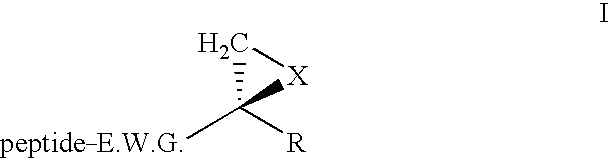Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7529 results about "Cyclodextrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
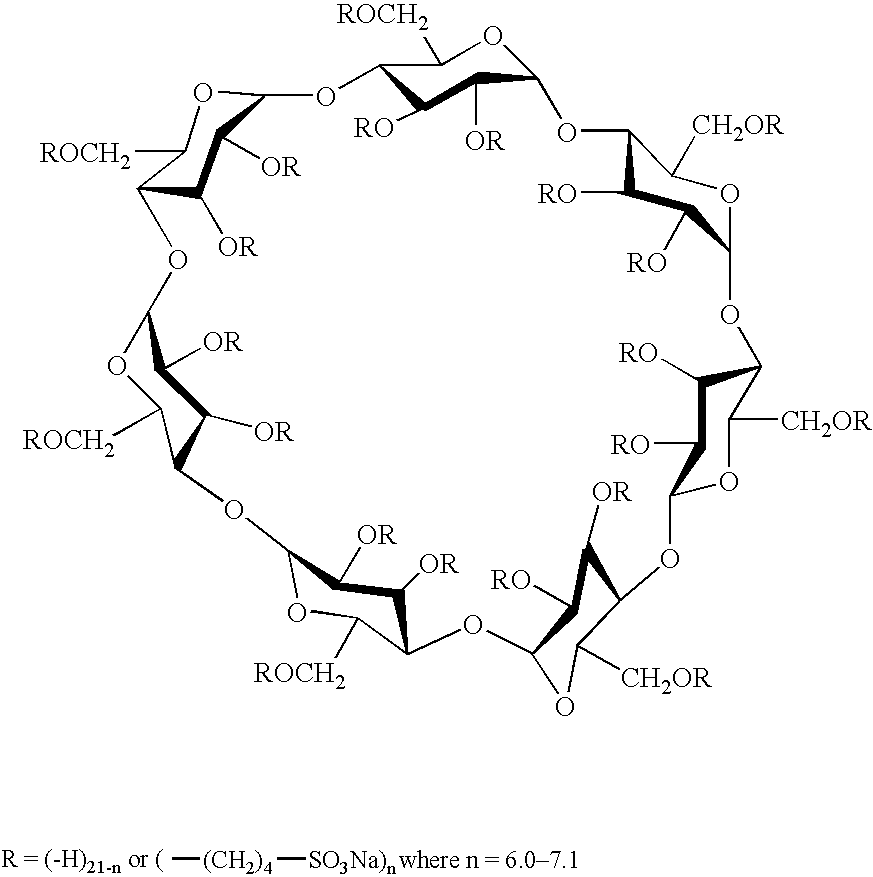
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical, drug delivery, and chemical industries, as well as agriculture and environmental engineering.
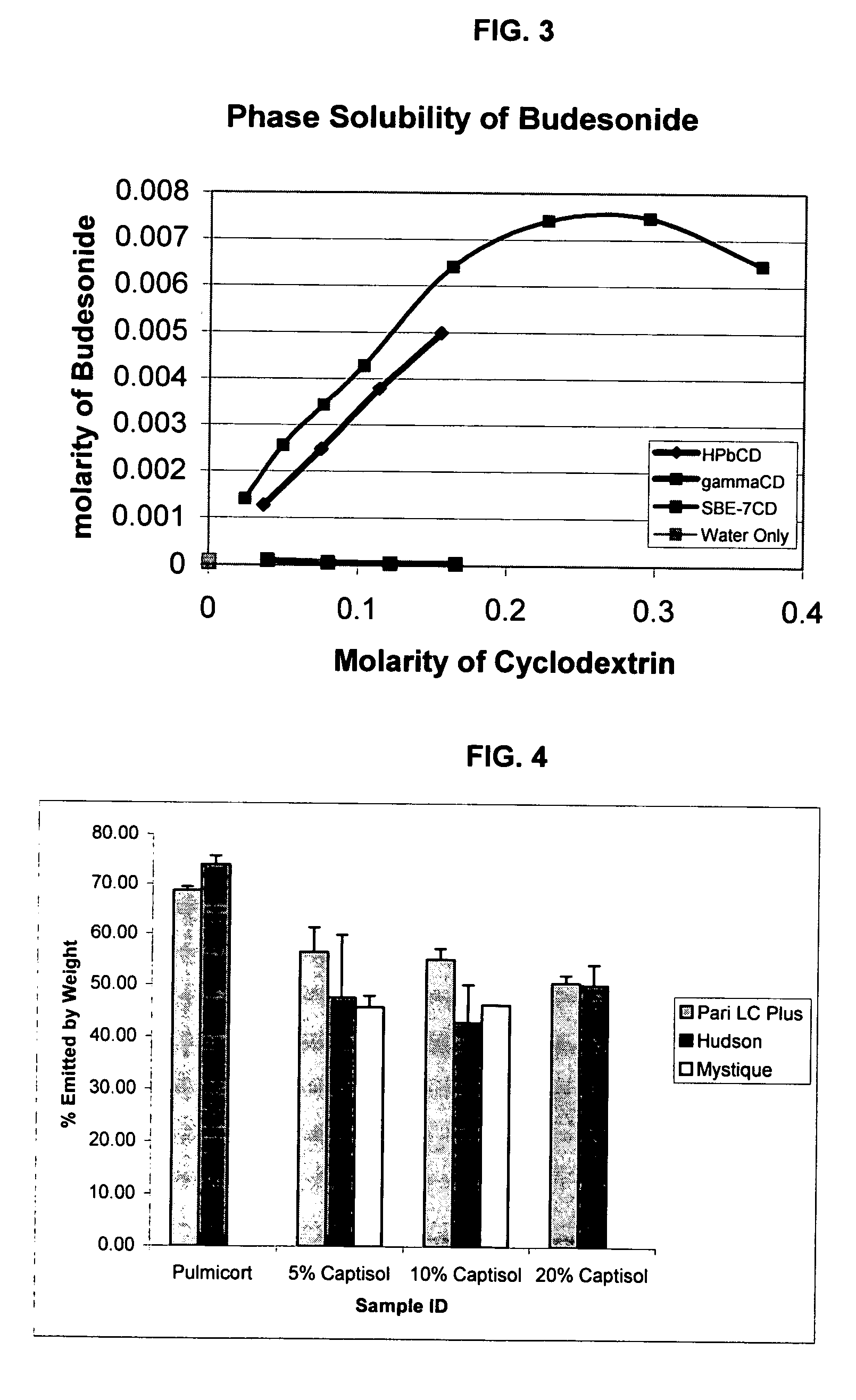
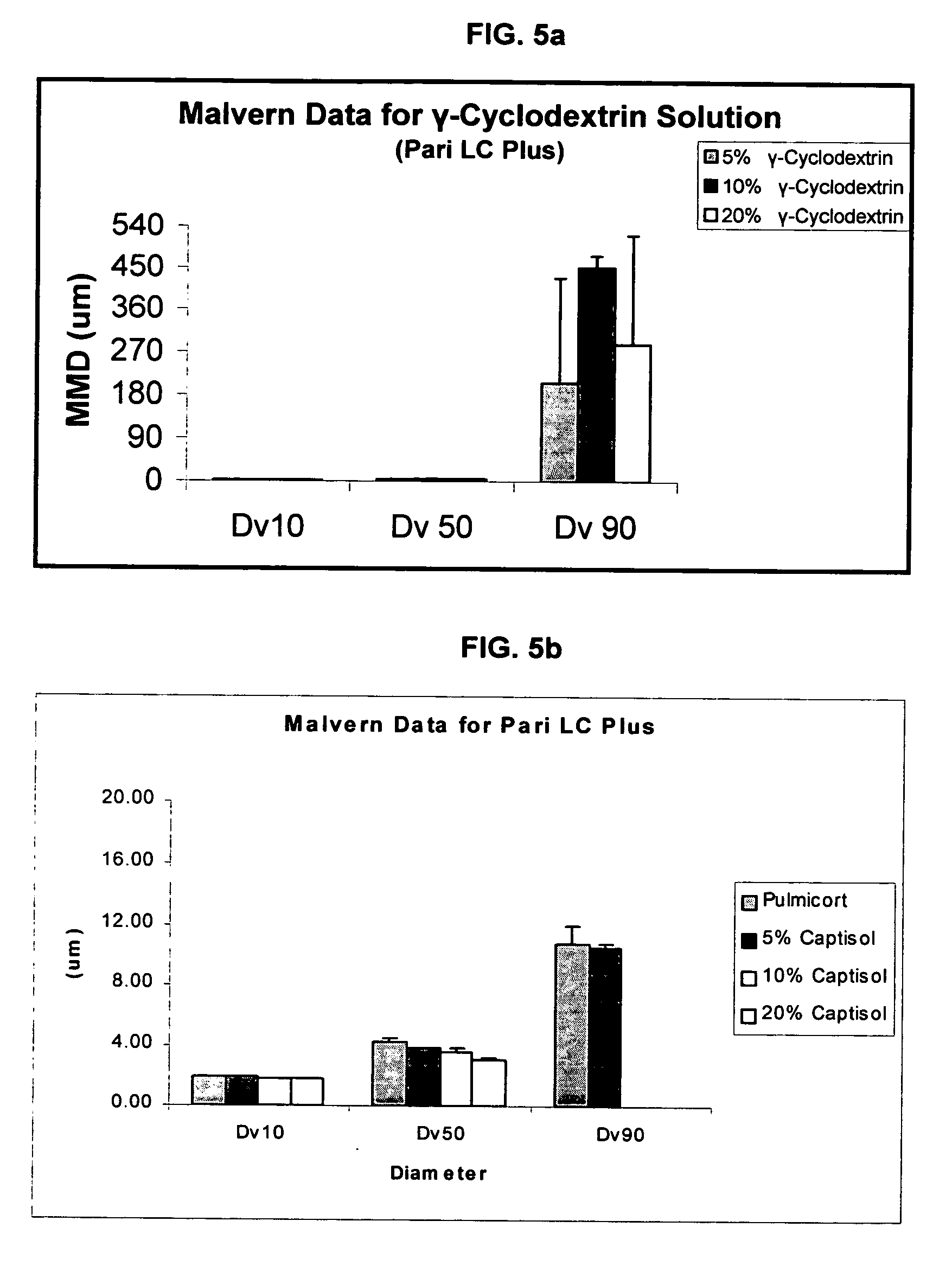
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
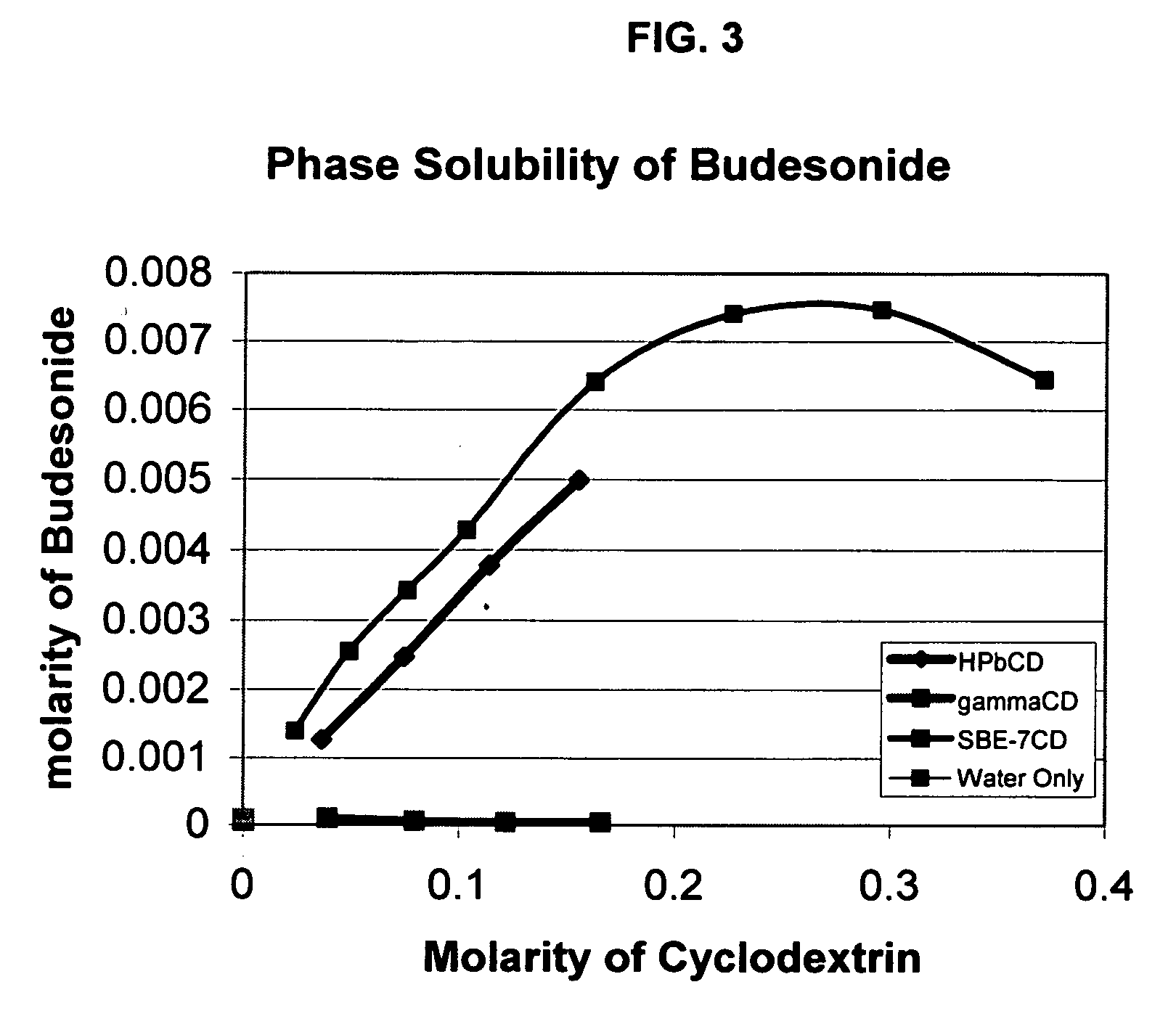
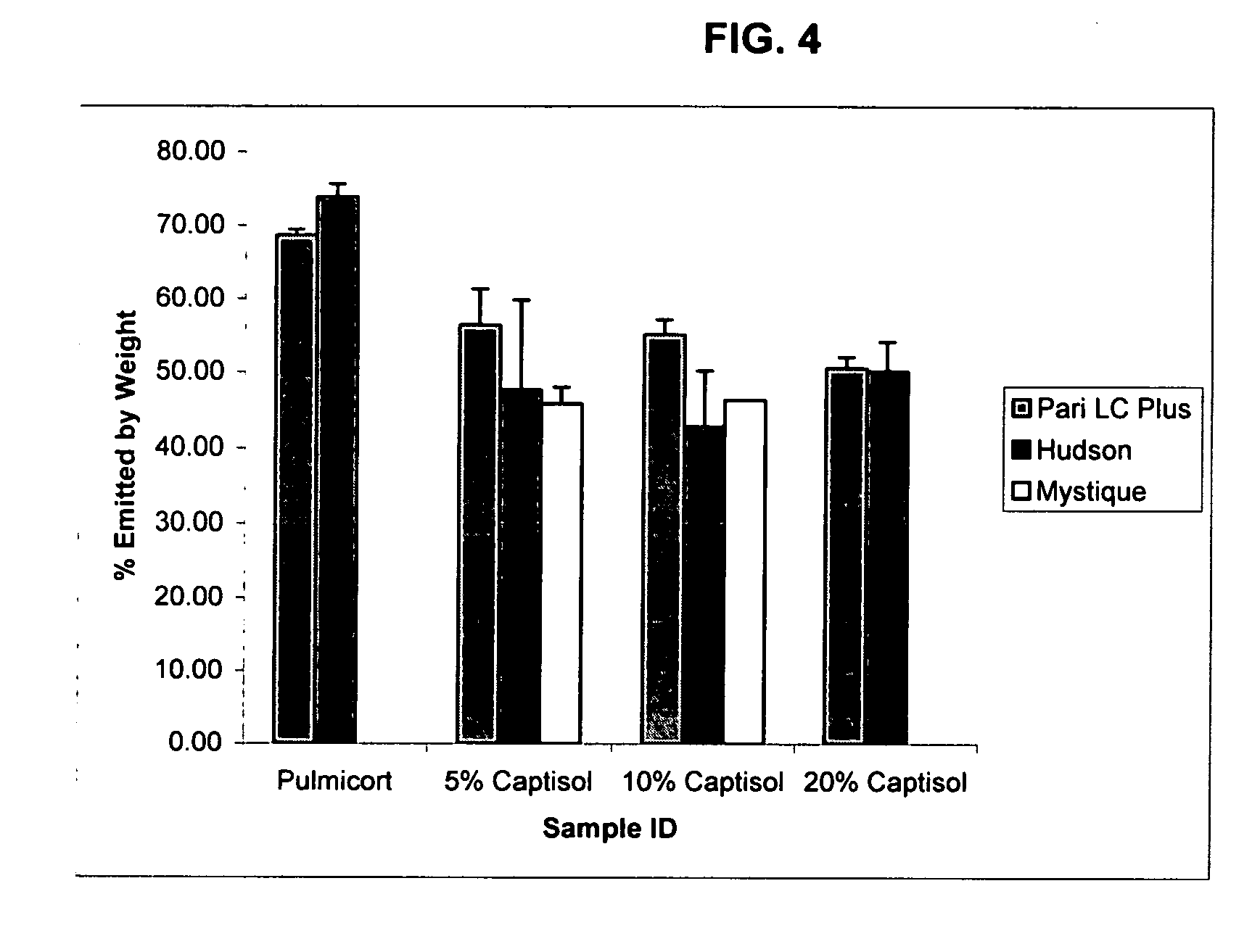
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
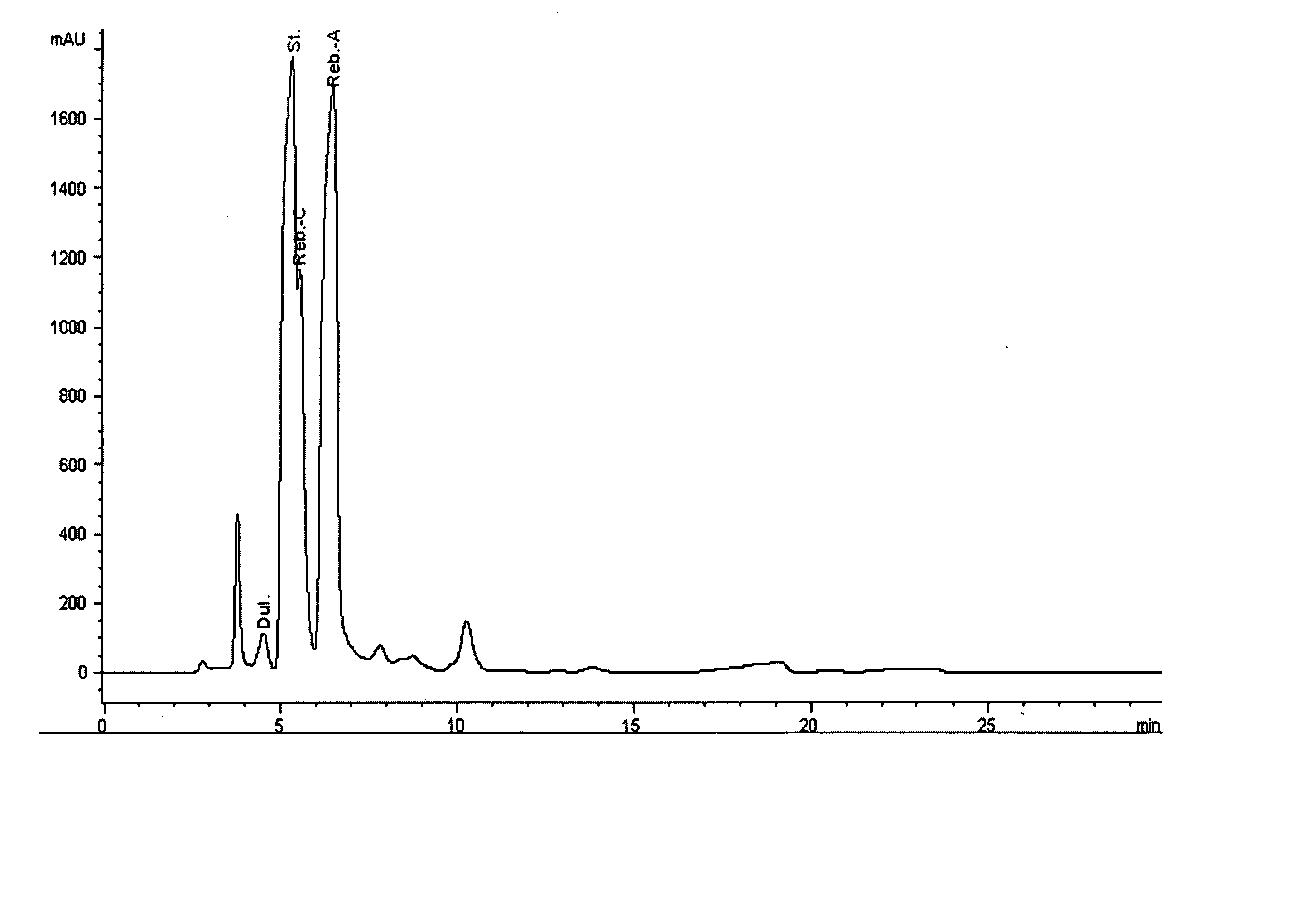
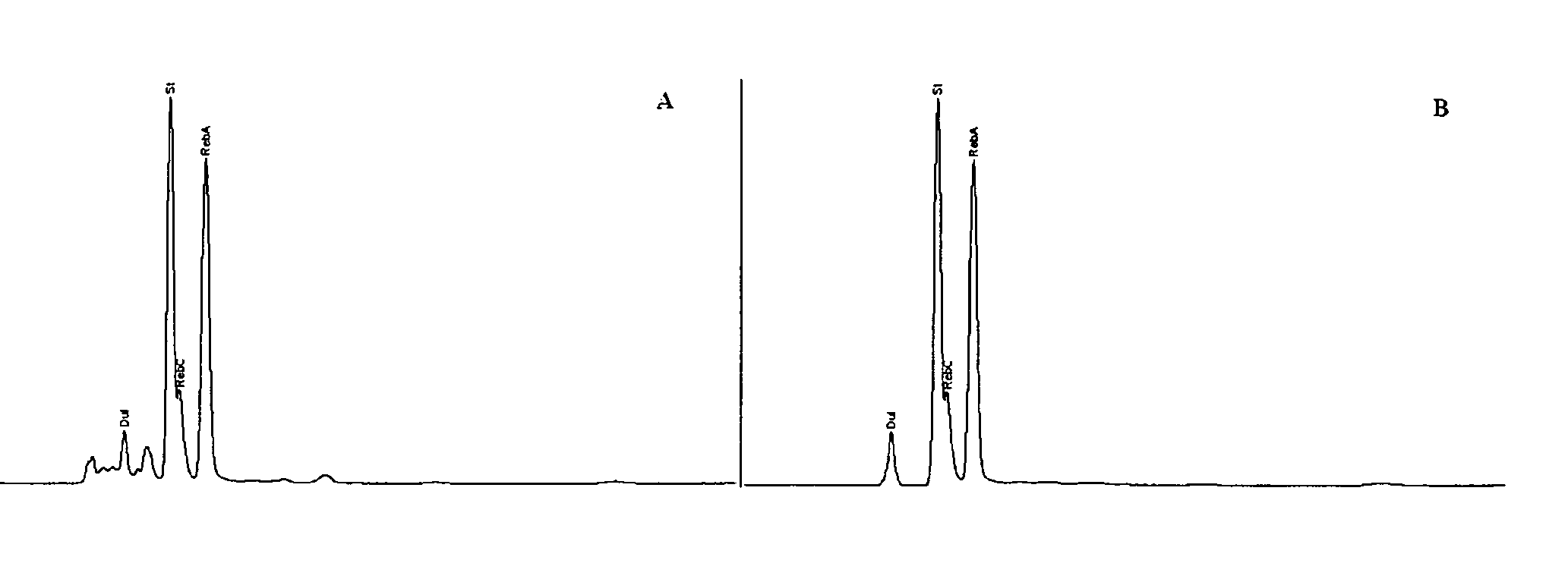
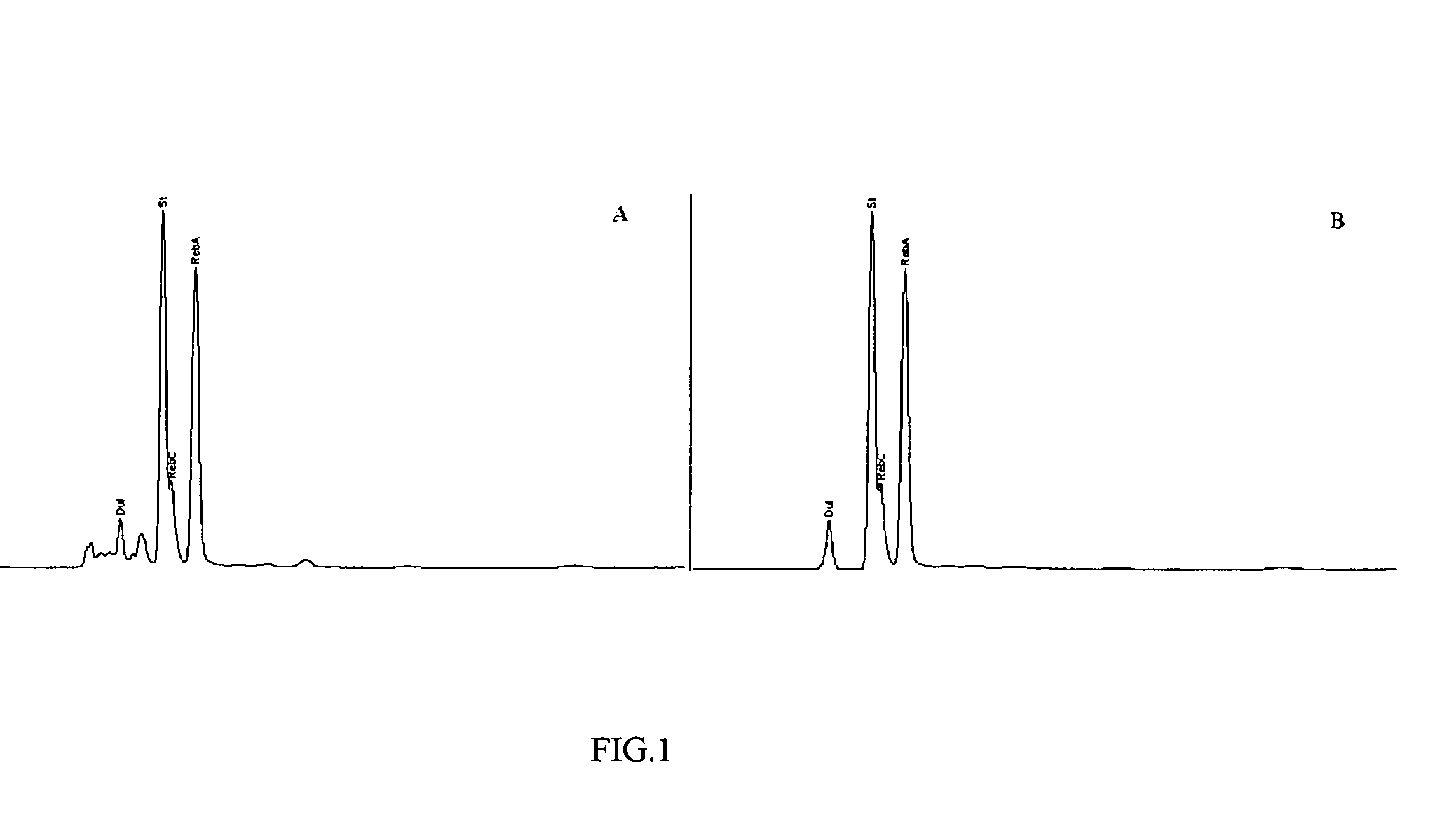
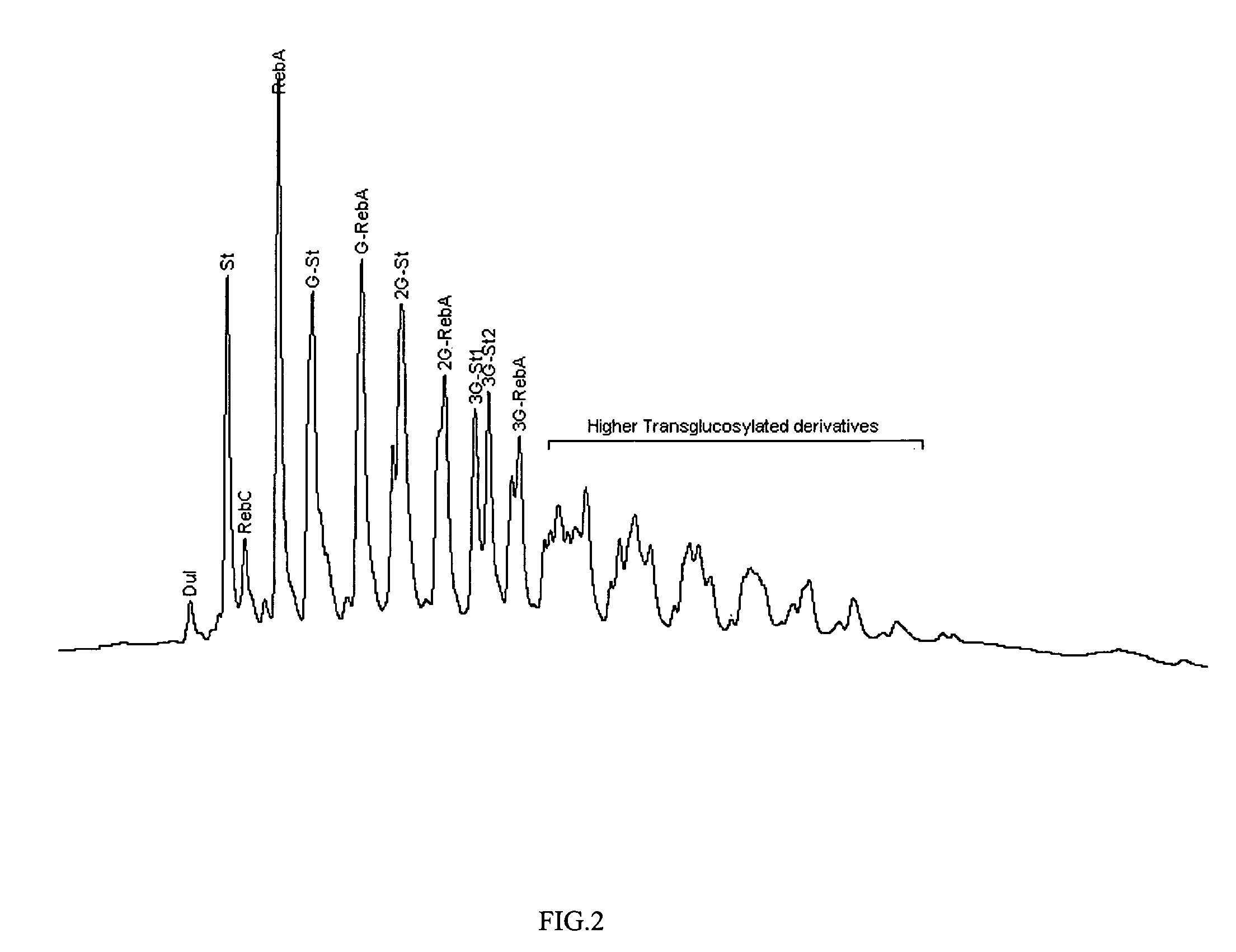
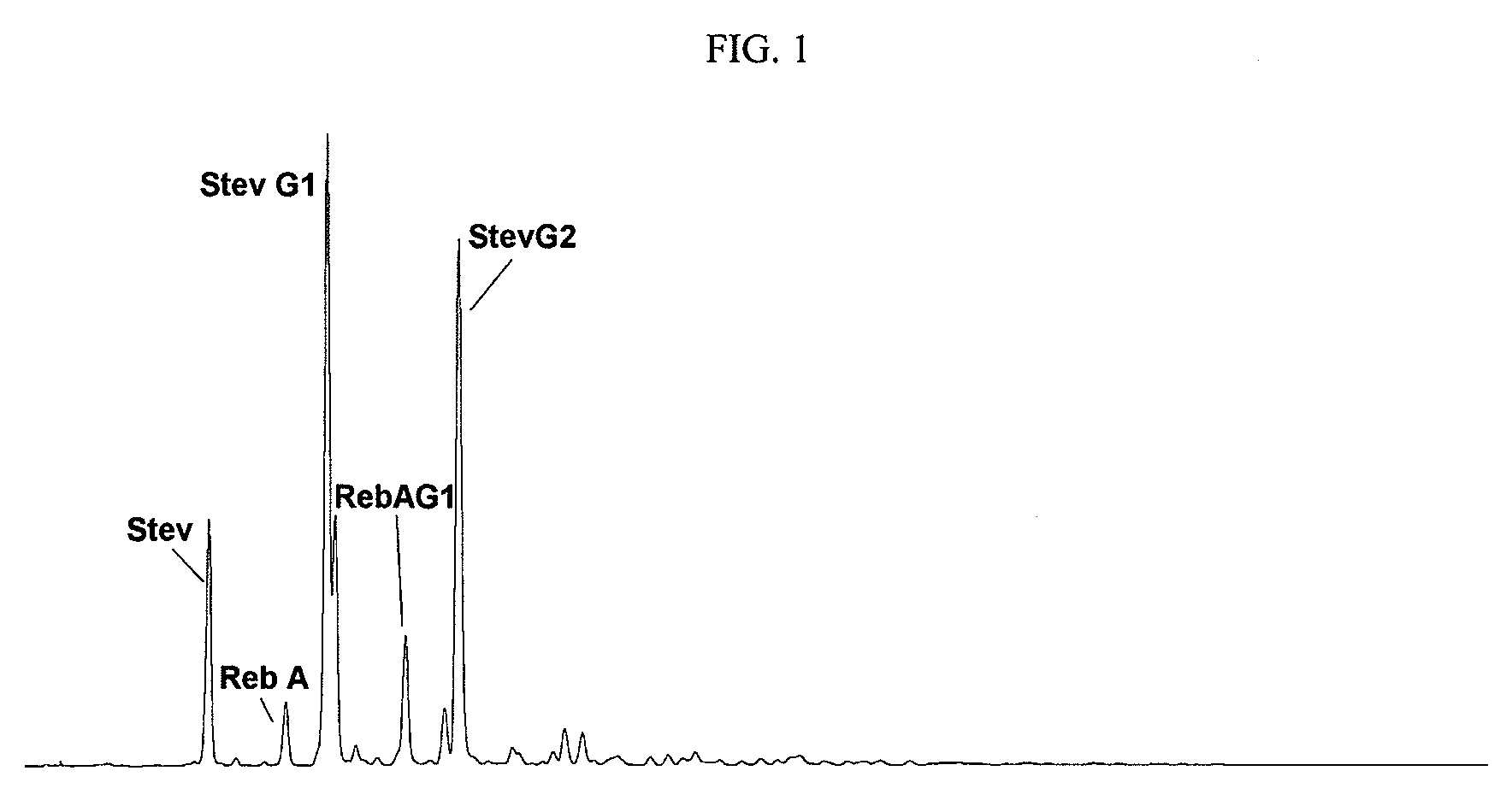
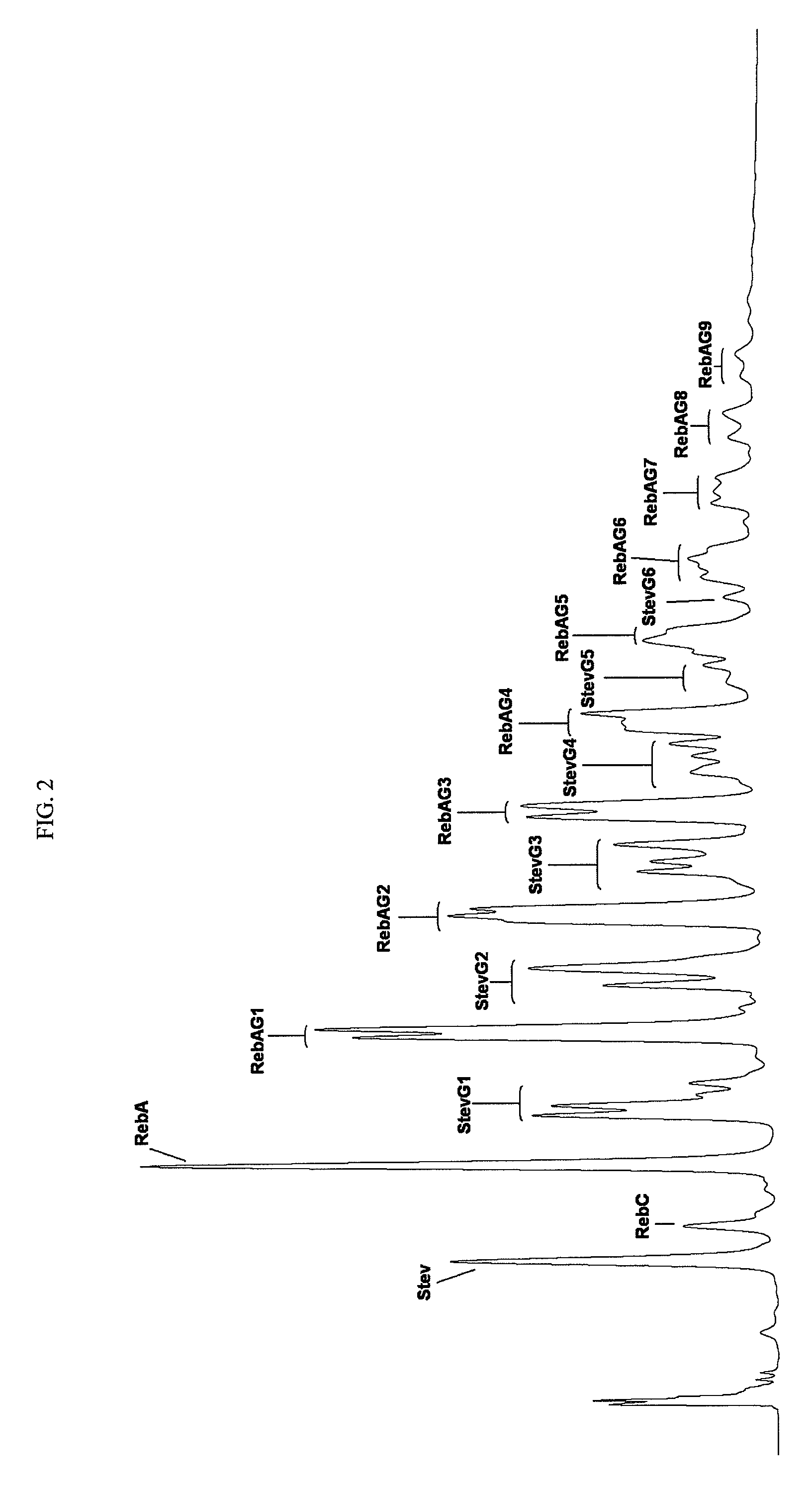
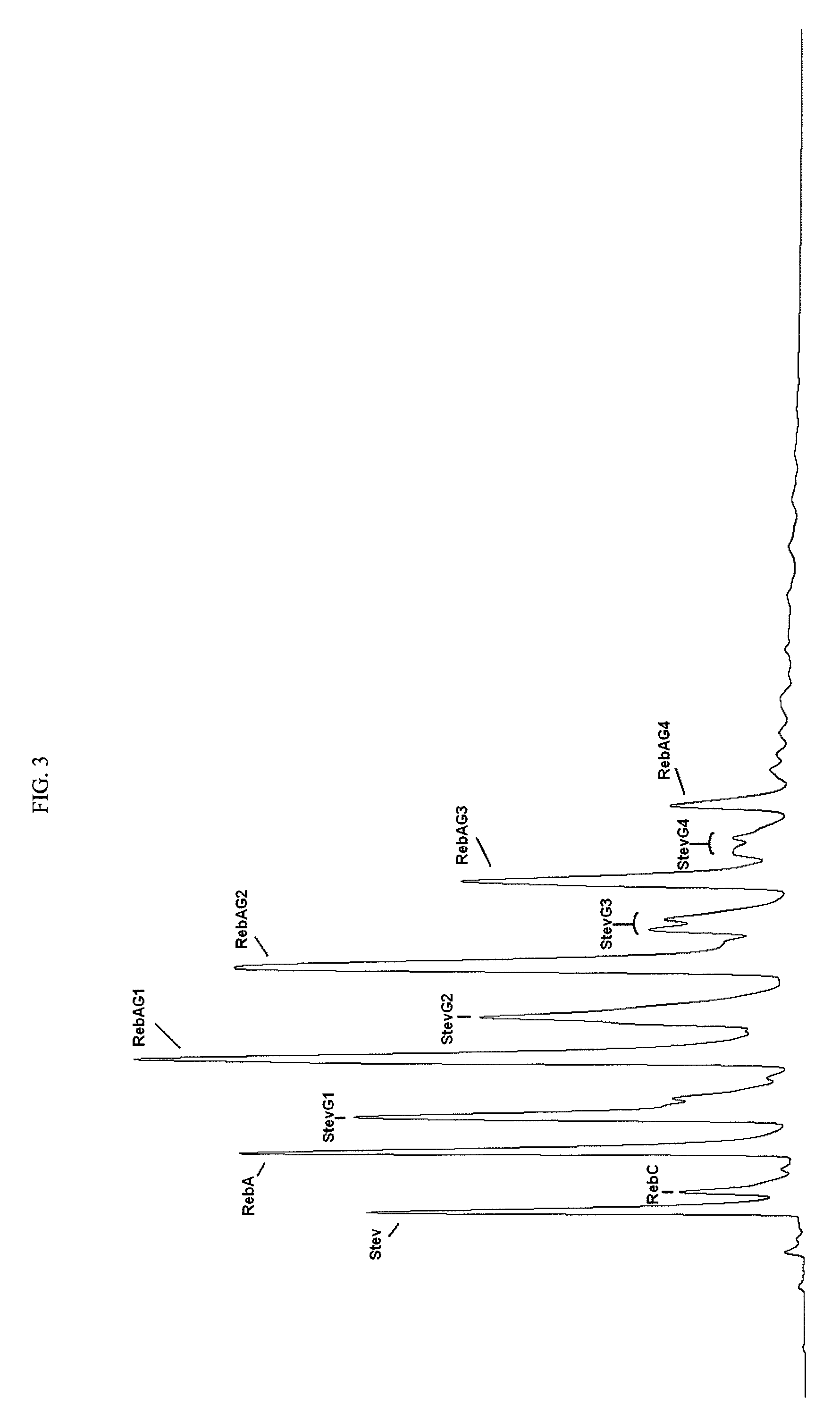
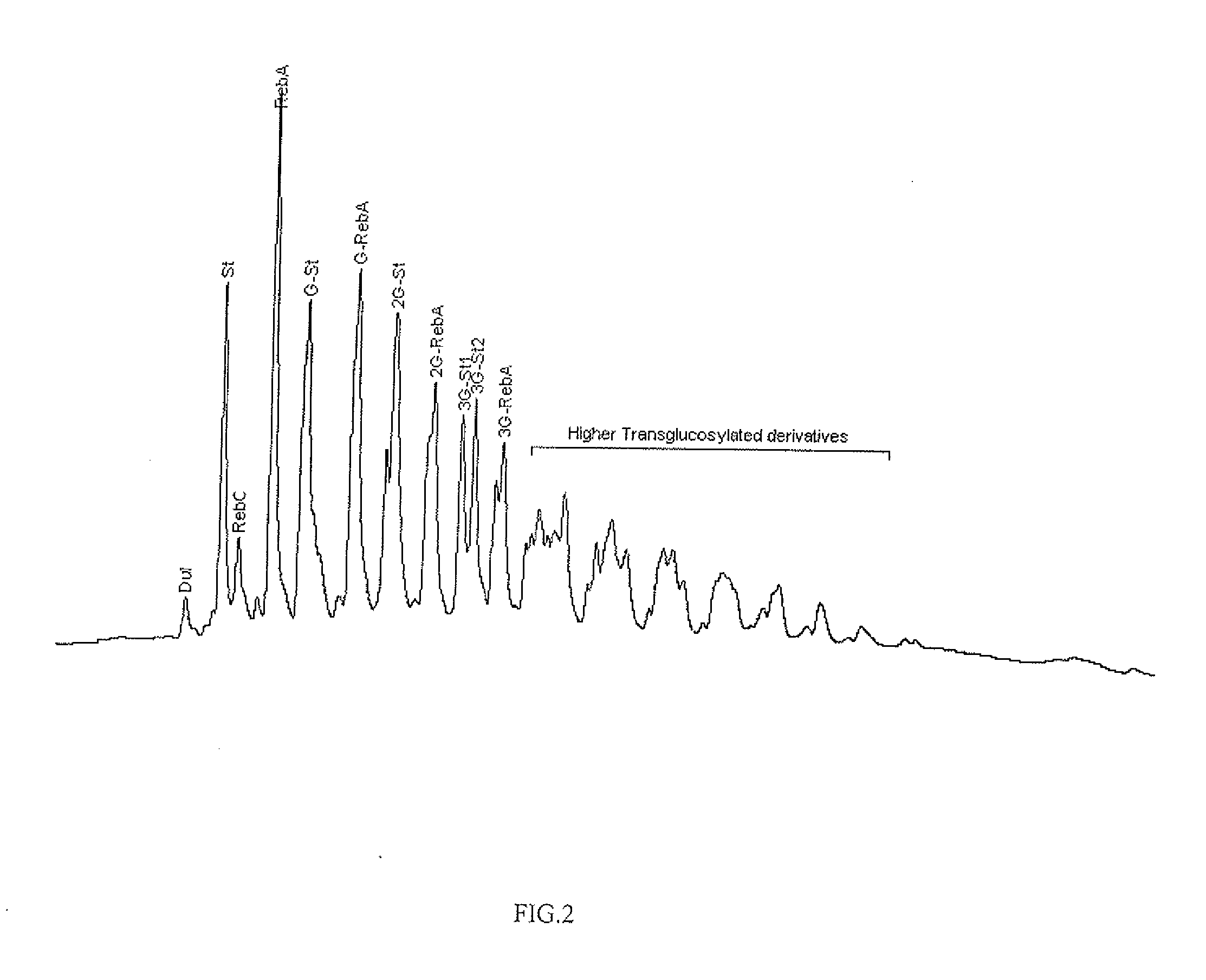
Extraction, separation and modification of sweet glycosides from the stevia rebaudiana plant
ActiveUS20060134292A1Increasing of degree of transglycosylationDelayed reaction timeBiocideAnimal repellantsPectinaseSodium Bentonite
The invention disclosed relates to a method for the extraction of sweet glycosides from the Stevia rebaudiana Bertoni plant and recovery of individual rebaudioside A and stevioside. The extraction is developed in the presence of pectinase, and the extract is purified using cyclodextrin and bentonite. High purity rebaudioside A is obtained by crystallization and recrystallization from ethanol. High purity stevioside is prepared from the filtrate by purification with cyclodextrin, bentonit, and ion exchange resins. The enzymatic modification of the rebaudioside A, stevioside and the purified extract is carried out using the transferring enzymes derived from Thermoactinomyces vilgaris and Bacillus halophilus.
Owner:PURECIRCLE SDN BHD
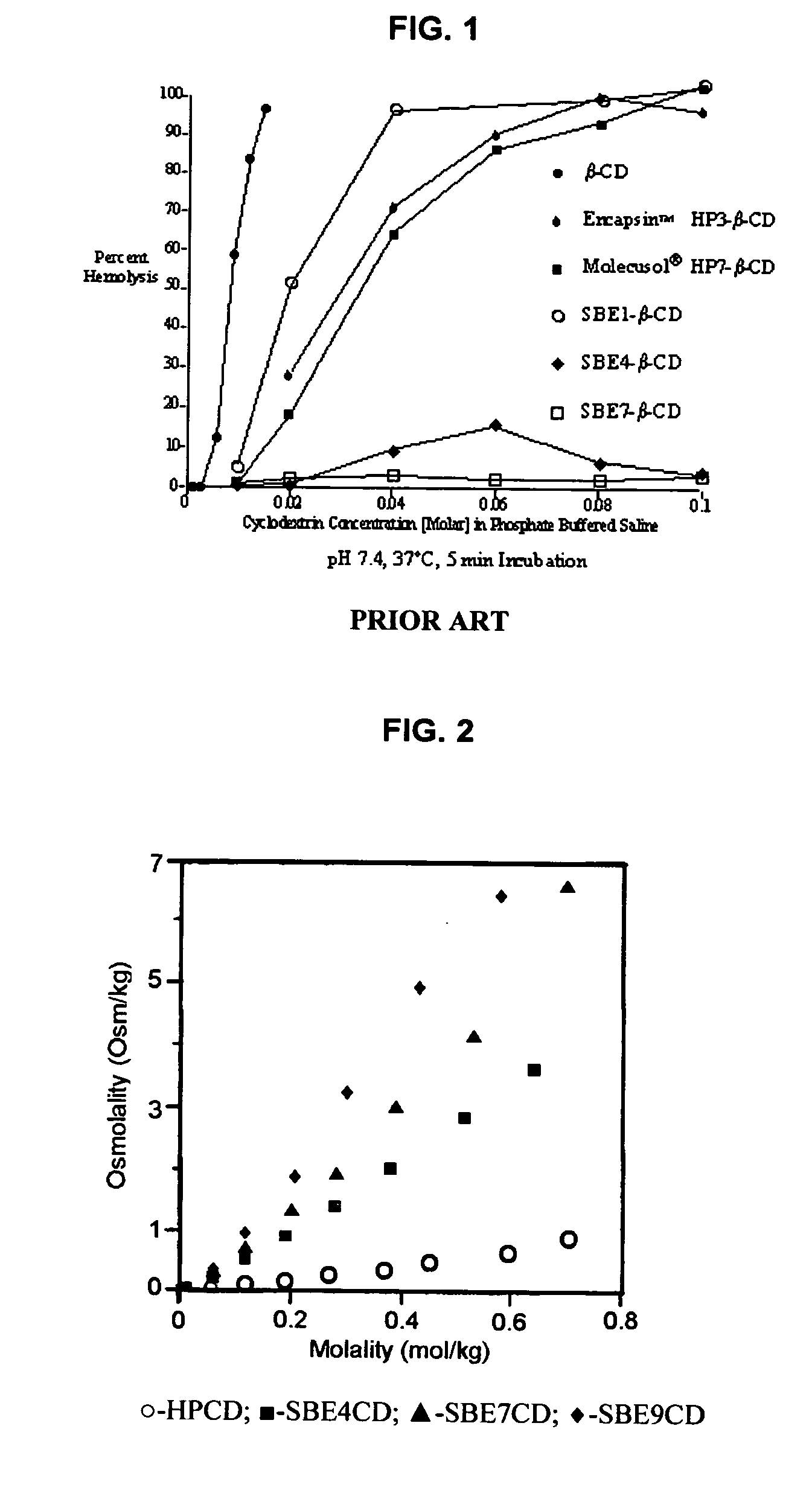
Sulfoalkyl ether cyclodextrin compositions
ActiveUS7635773B2Well mixedReduce the amount requiredOrganic active ingredientsSugar derivativesPhosphateEther
SAE-CD compositions are provided, along with methods of making and using the same. The SAE-CD composition comprises a sulfoalkyl ether cyclodextrin and less than 100 ppm of a phosphate, wherein the SAE-CD composition has an absorption of less than 0.5 A.U. due to a drug-degrading agent, as determined by UV / vis spectrophotometry at a wavelength of 245 nm to 270 nm for an aqueous solution containing 300 mg of the SAE-CD composition per mL of solution in a cell having a 1 cm path length.
Owner:CYDEX PHARMACEUTICALS INC
Cyclodextrins preferentially substituted on their primary face by acid or amine functions
InactiveUS6524595B1Improve bioavailabilityEasy to synthesizeBiocideOrganic active ingredientsCyclodextrinBULK ACTIVE INGREDIENT
Non-hydroxyalkylated cyclodextrins are disclosed wherein at least one primary alcohol function (CH2OH) is substituted, the -OH portion being replaced by a substituent with formula -O-CO-R or -NR1R2, where:R, R1 and R2 independently represent a linear or cyclic, saturated or unsaturated, hydroxylated or non-hydroxylated alkyl group containing 1 to 30 carbon atoms, preferably 1 to 22 carbon atoms, more preferably a fatty chain containing 2 to 22 carbon atoms. These cyclodextrins are used as vectors for at least one active ingredient, in particular to encourage tissue penetration, in a cosmetic application, or for the production of pharmaceutical compositions, in particular dermopharmaceuticals.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Sweetner and use
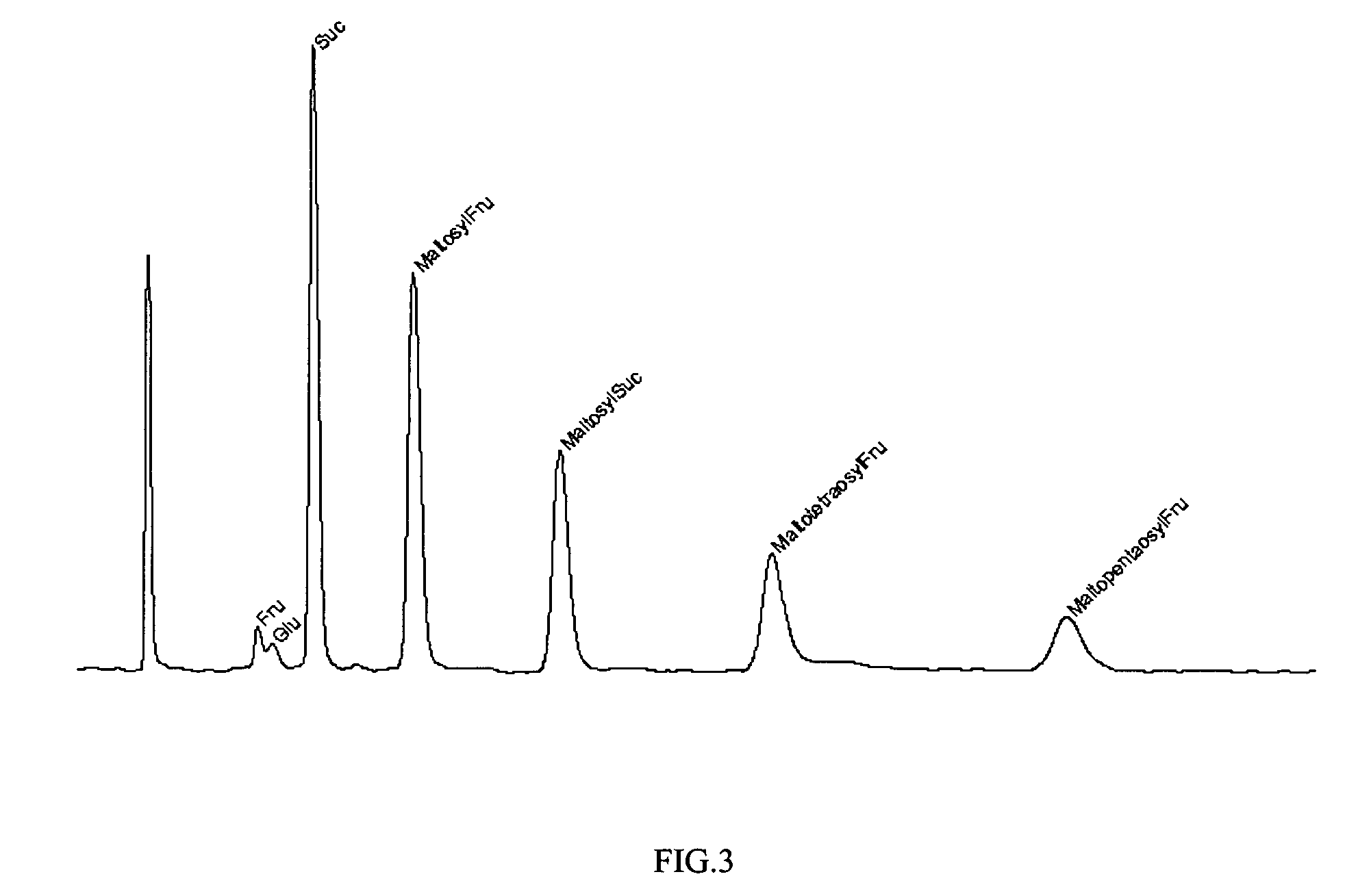
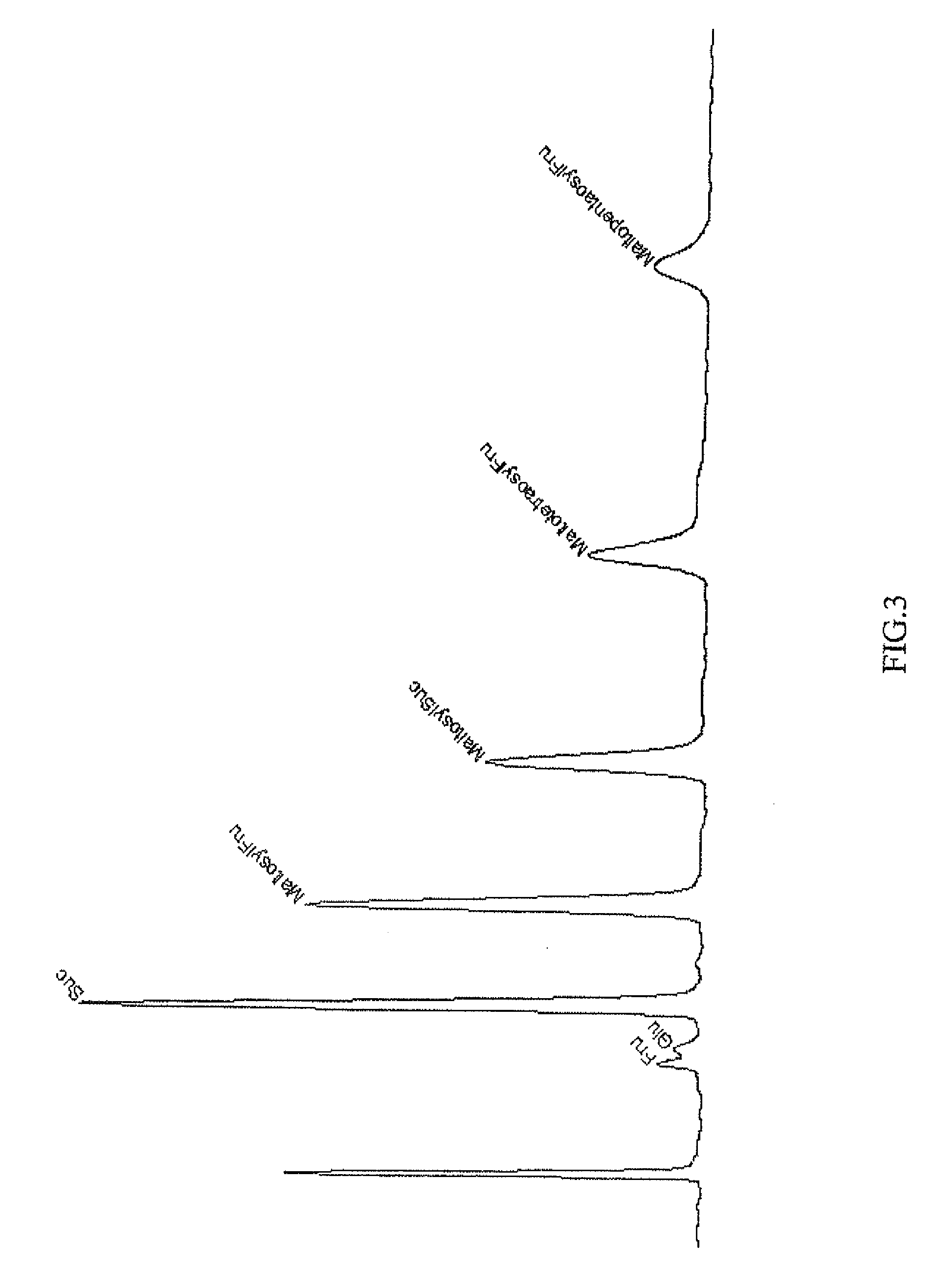
Sweeteners on the basis of a simultaneously transglucosylated sweet glycoside mixture of Stevia rebaudiana Bertoni are prepared. The transglycosylation was developed in the presence of starch under the action of cyclodextrin glucanotransferase. The remaining maltodextrins are transferred to the fructose-terminated oligosaccharides. The sweeteners are purified to not less than 98% content of sweet glycosides and derivatives. The preparations are almost non-caloric, non-cariogenic, non-bitter, non-lingering sweeteners, which may be advantageously applied in foods, beverages, cosmetics and milk products.
Owner:PURECIRCLE SDN BHD
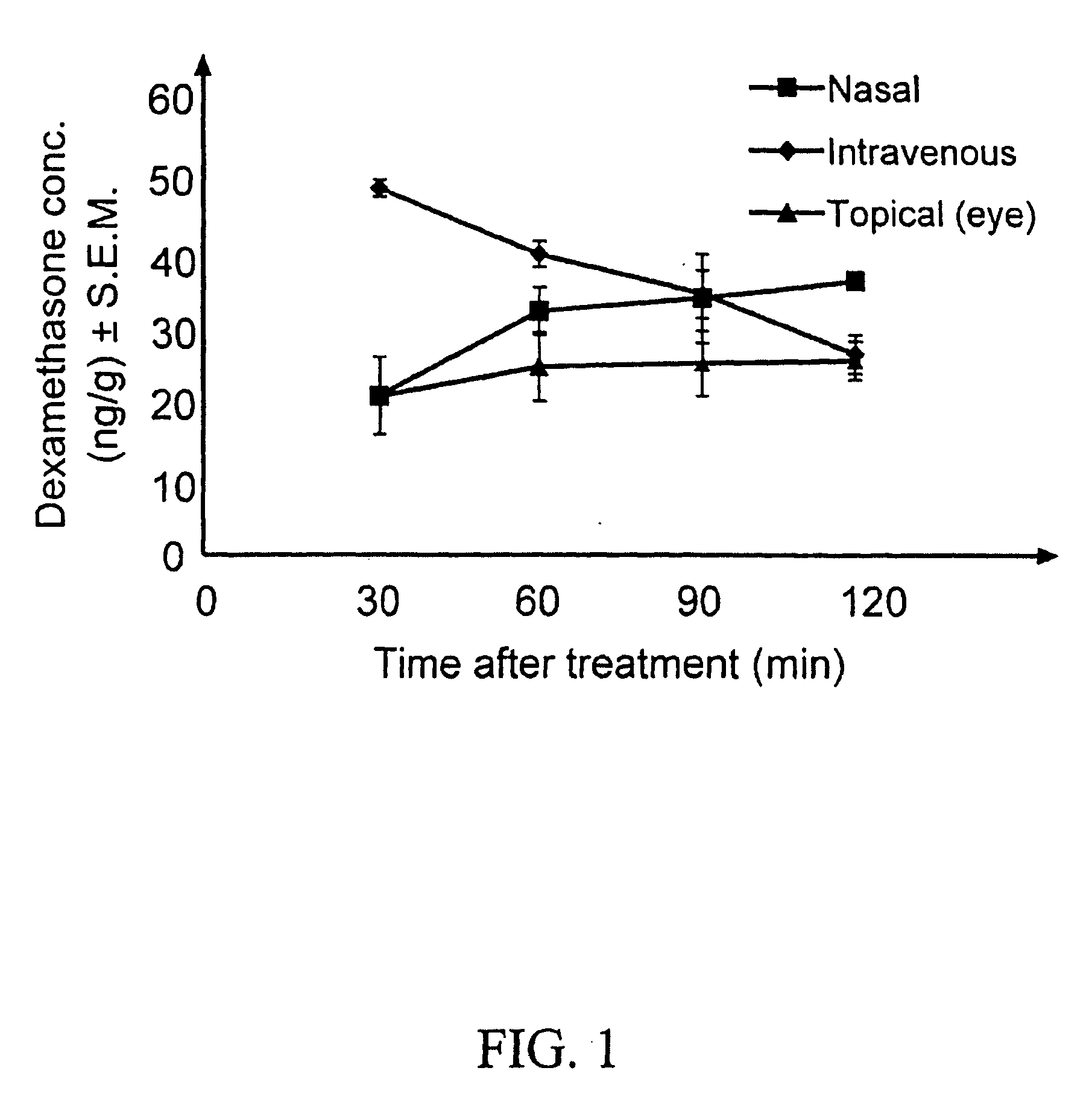
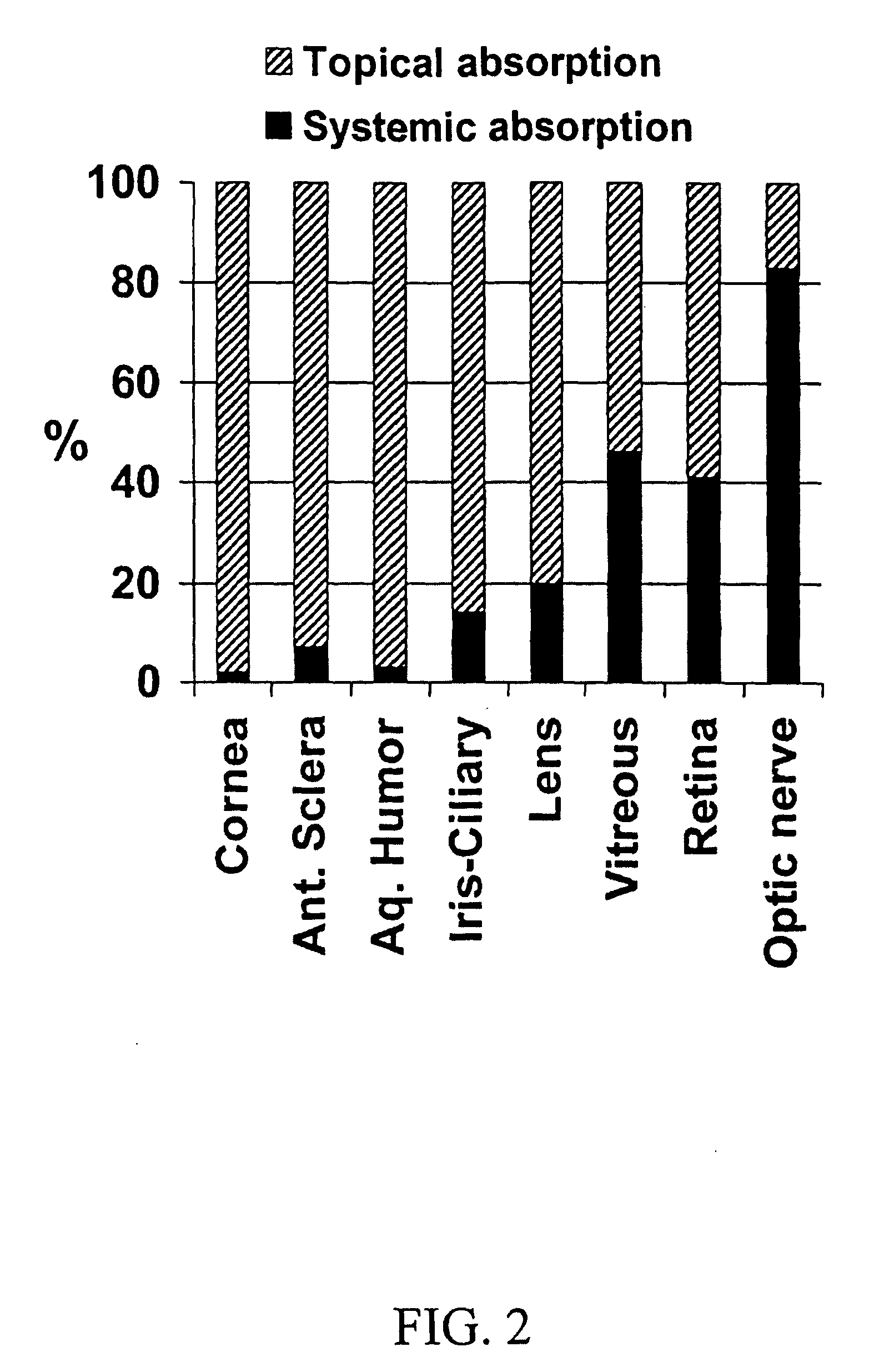
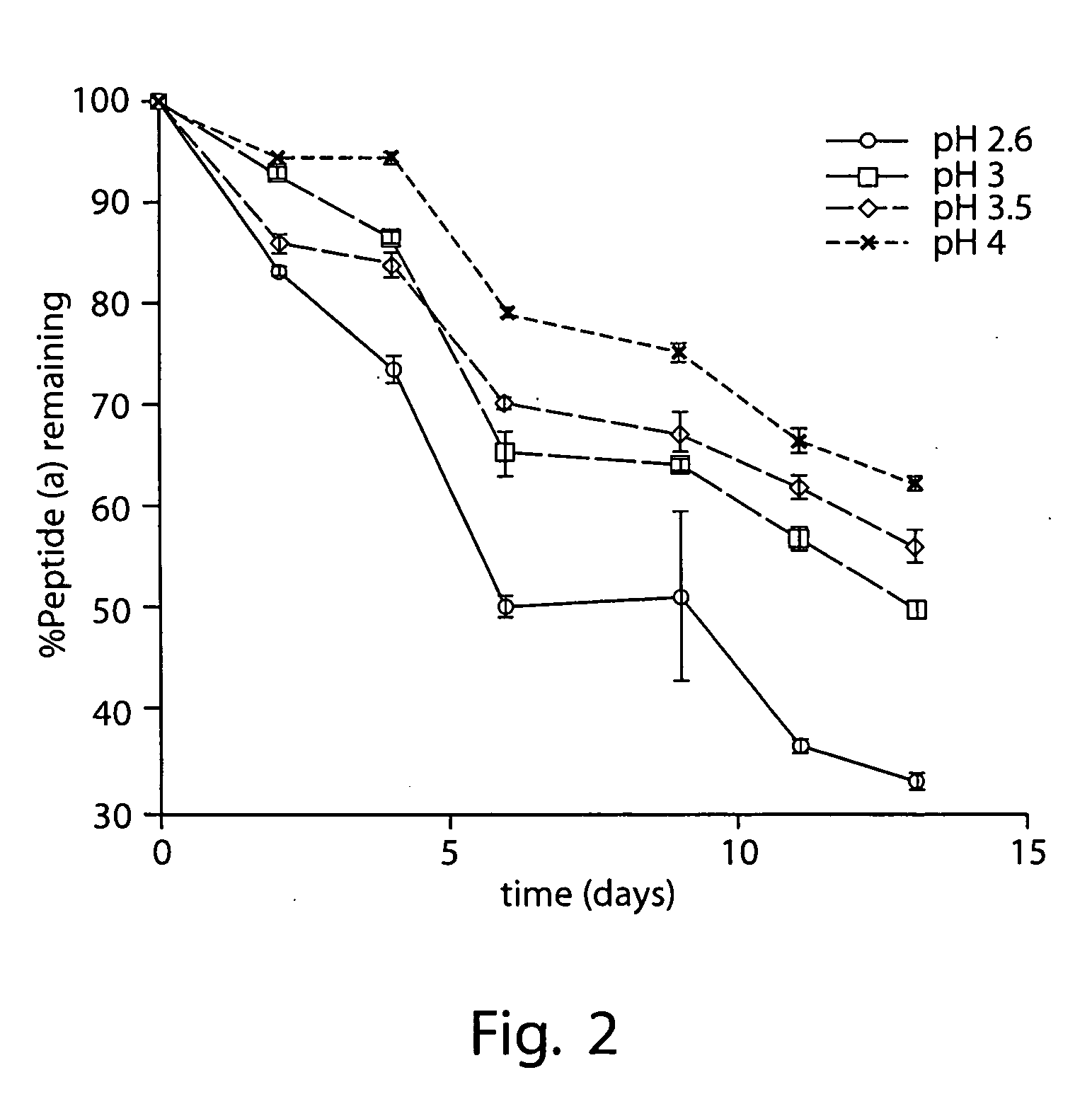
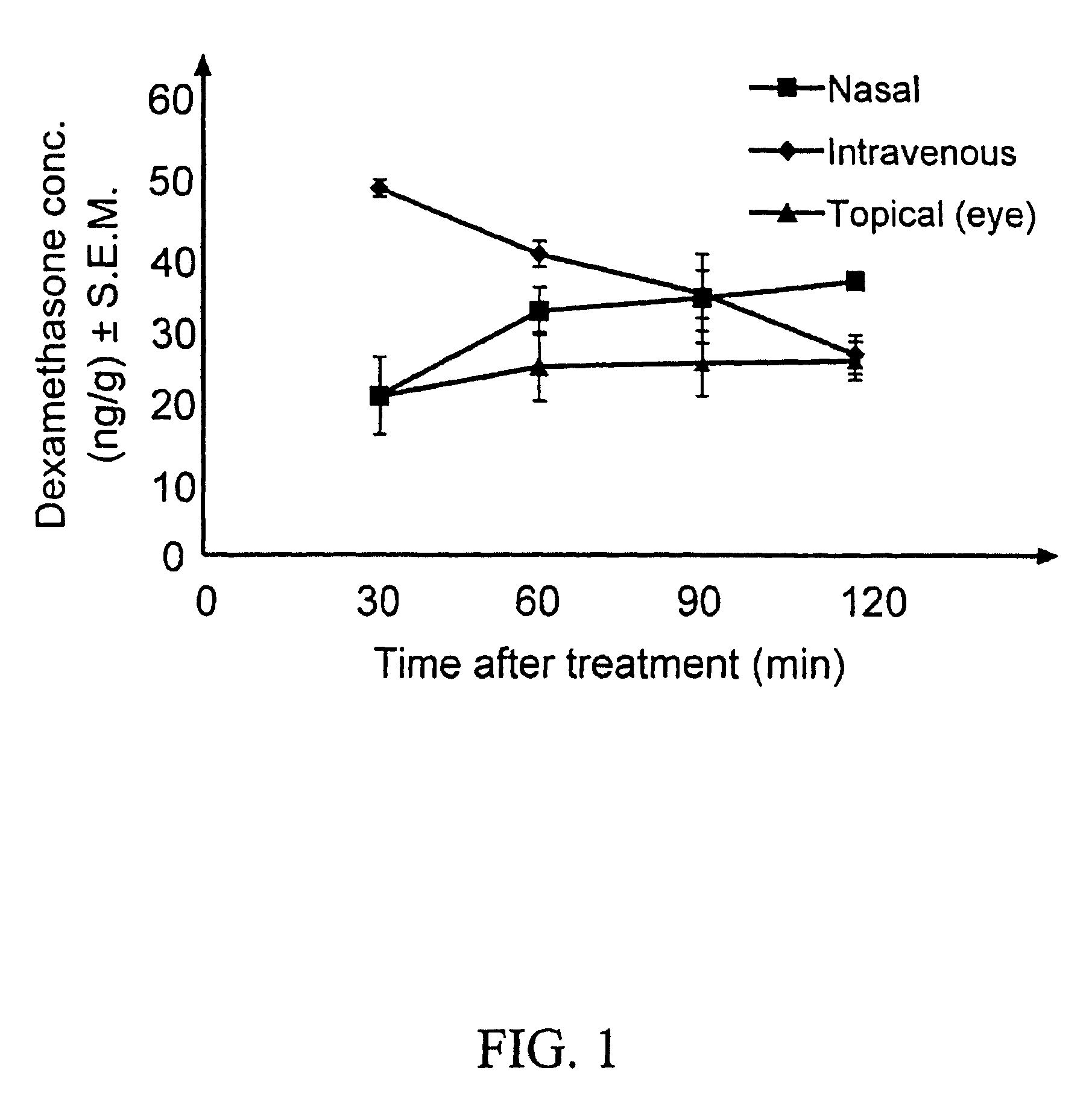
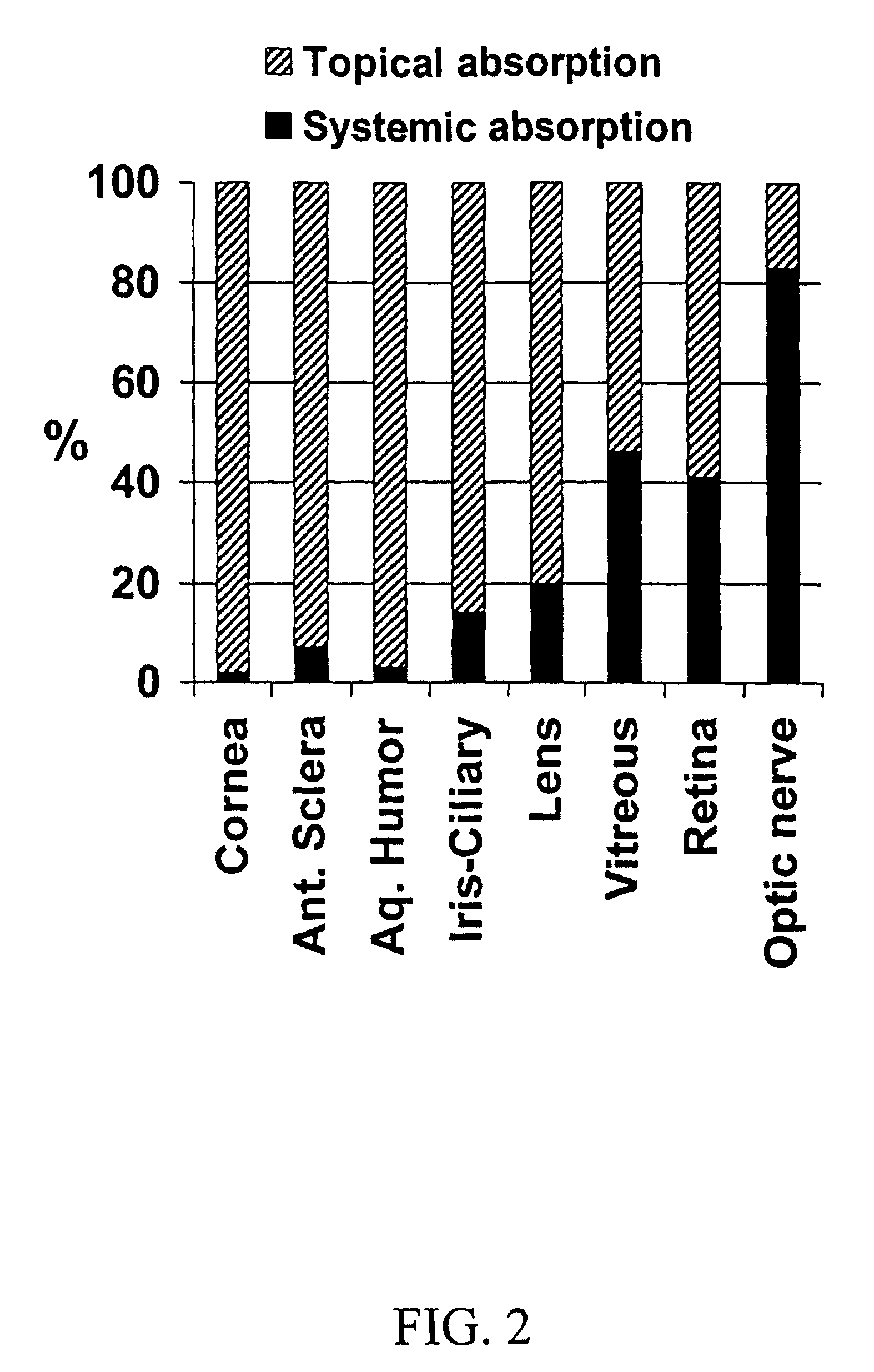
Cyclodextrin nanotechnology for ophthalmic drug delivery
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
Extraction, separation and modification of sweet glycosides from the Stevia rebaudiana plant
ActiveUS7838044B2High extraction rateSimple processBiocideAnimal repellantsPectinaseSodium Bentonite
The invention disclosed relates to a method for the extraction of sweet glycosides from the Stevia rebaudiana Bertoni plant and recovery of individual rebaudioside A and stevioside. The extraction is developed in the presence of pectinase, and the extract is purified using cyclodextrin and bentonite. High purity rebaudioside A is obtained by crystallization and recrystallization from ethanol. High purity stevioside is prepared from the filtrate by purification with cyclodextrin, bentonit, and ion exchange resins. The enzymatic modification of the rebaudioside A, stevioside and the purified extract is carried out using the transferring enzymes derived from Thermoactinomyces vulgaris and Bacillus halophilus.
Owner:PURECIRCLE SDN BHD
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Cyclodextrin-based polymers for therapeutics delivery
ActiveUS7270808B2Improve drug stabilityImprove solubilityAntibacterial agentsBiocideSolubilityPresent method
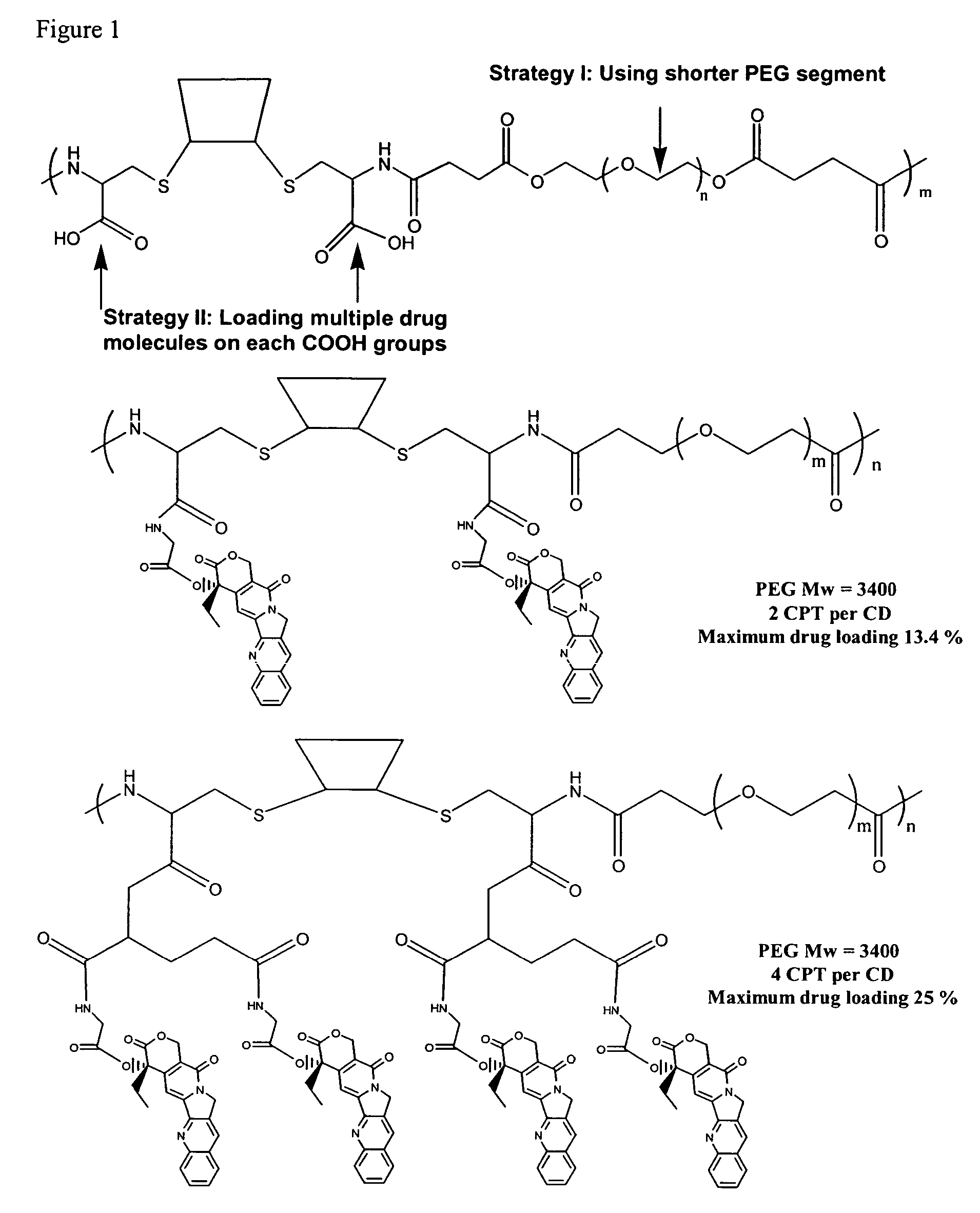
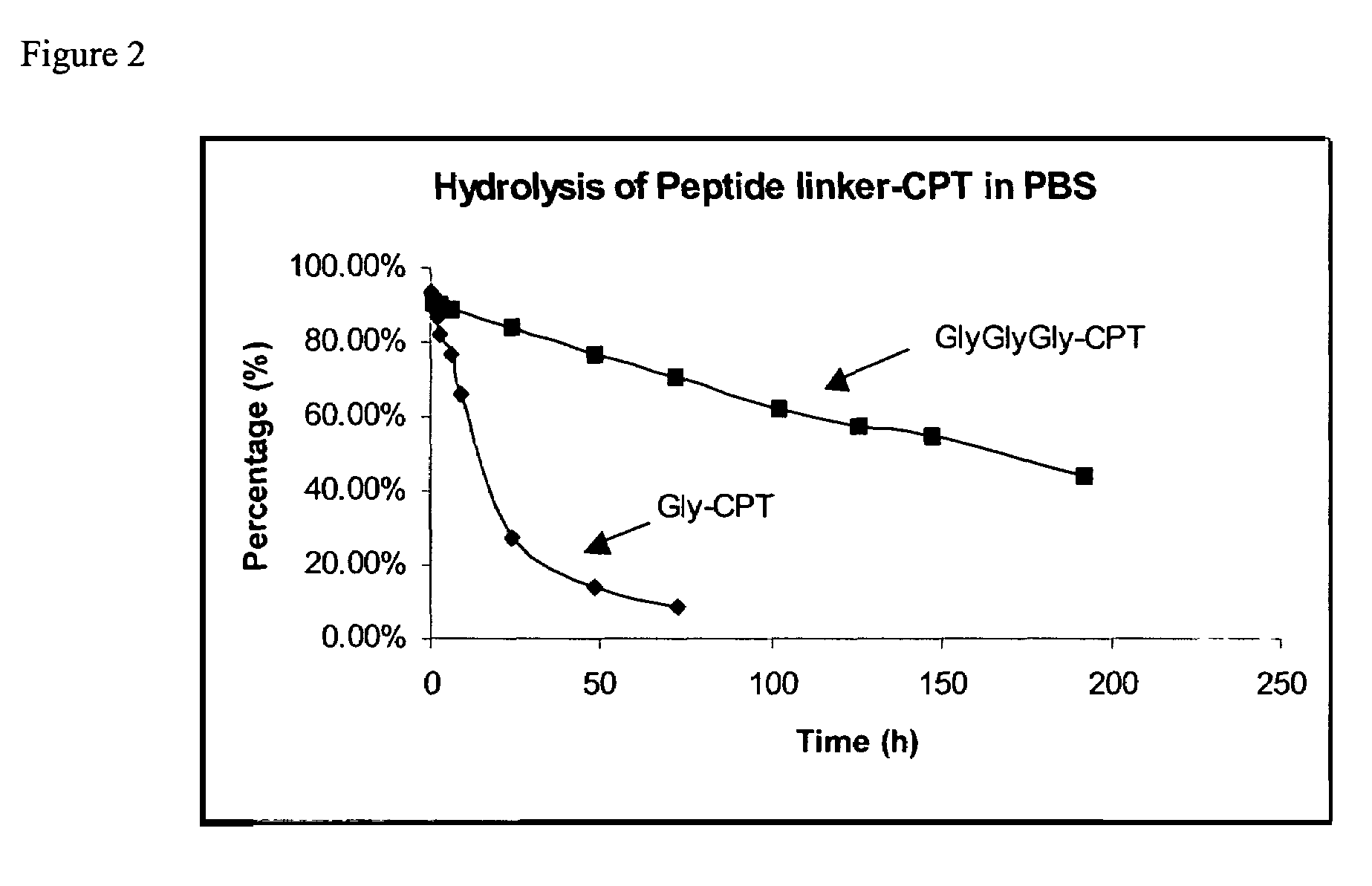
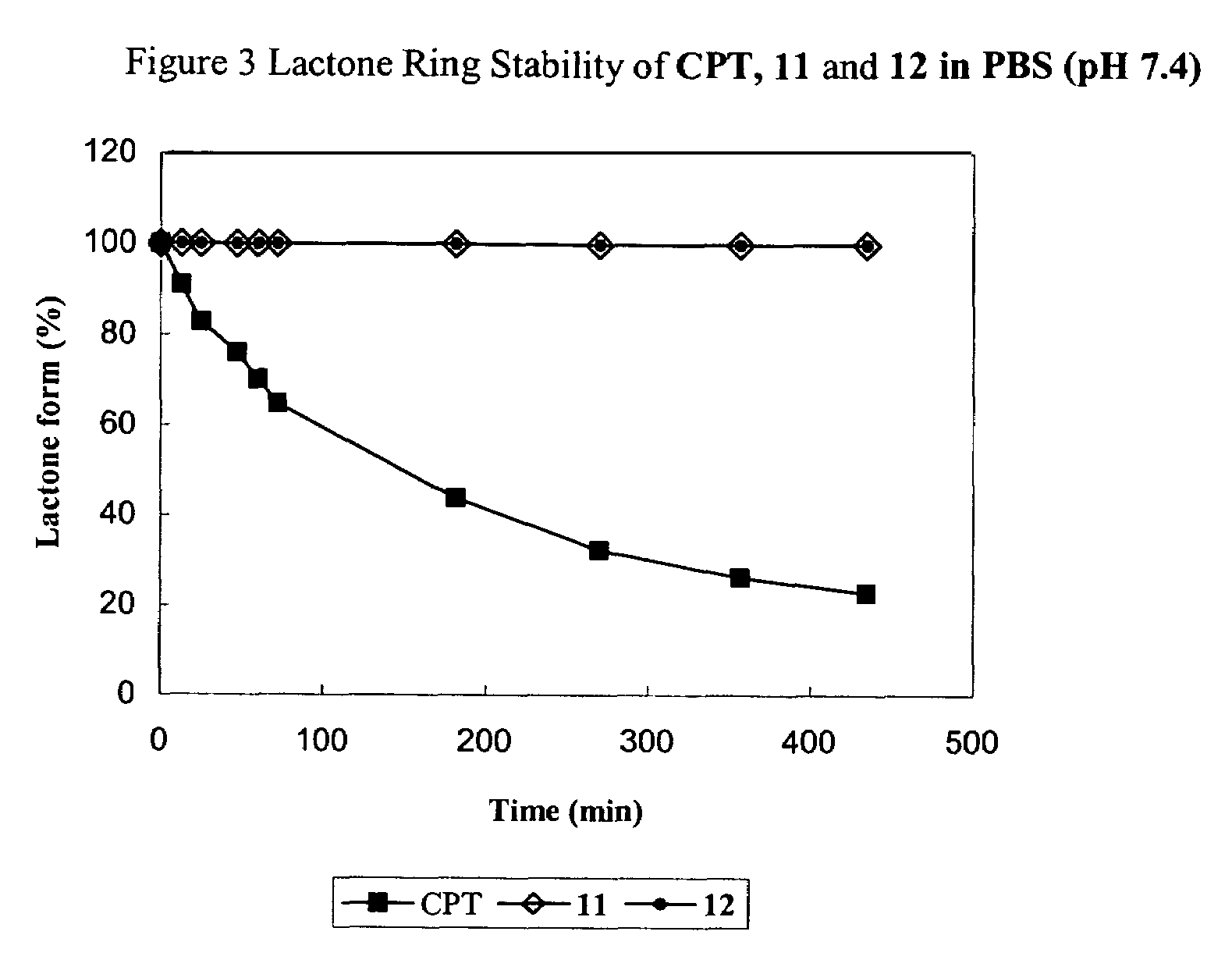
The present invention relates to novel compositions of therapeutic cyclodextrin containing polymeric compounds designed as a carrier for small molecule therapeutics delivery and pharmaceutical compositions thereof. These cyclodextrin-containing polymers improve drug stability and solubility, and reduce toxicity of the small molecule therapeutic when used in vivo. Furthermore, by selecting from a variety of linker groups and targeting ligands the polymers present methods for controlled delivery of the therapeutic agents. The invention also relates to methods of treating subjects with the therapeutic compositions described herein. The invention further relates to methods for conducting pharmaceutical business comprising manufacturing, licensing, or distributing kits containing or relating to the polymeric compounds described herein.
Owner:CERULEAN PHARMA
Sweetner and use
Sweeteners on the basis of a simultaneously transglucosylated sweet glycoside mixture of Stevia rebaudiana Bertoni are prepared. The transglycosylation was developed in the presence of starch under the action of cyclodextrin glucanotransferase. The remaining maltodextrins are transferred to the fructose-terminated oligosaccharides. The sweeteners are purified to not less than 98% content of sweet glycosides and derivatives. The preparations are almost non-caloric, non-cariogenic, non-bitter, non-lingering sweeteners, which may be advantageously applied in foods, beverages, cosmetics and milk products.
Owner:PURECIRCLE SDN BHD
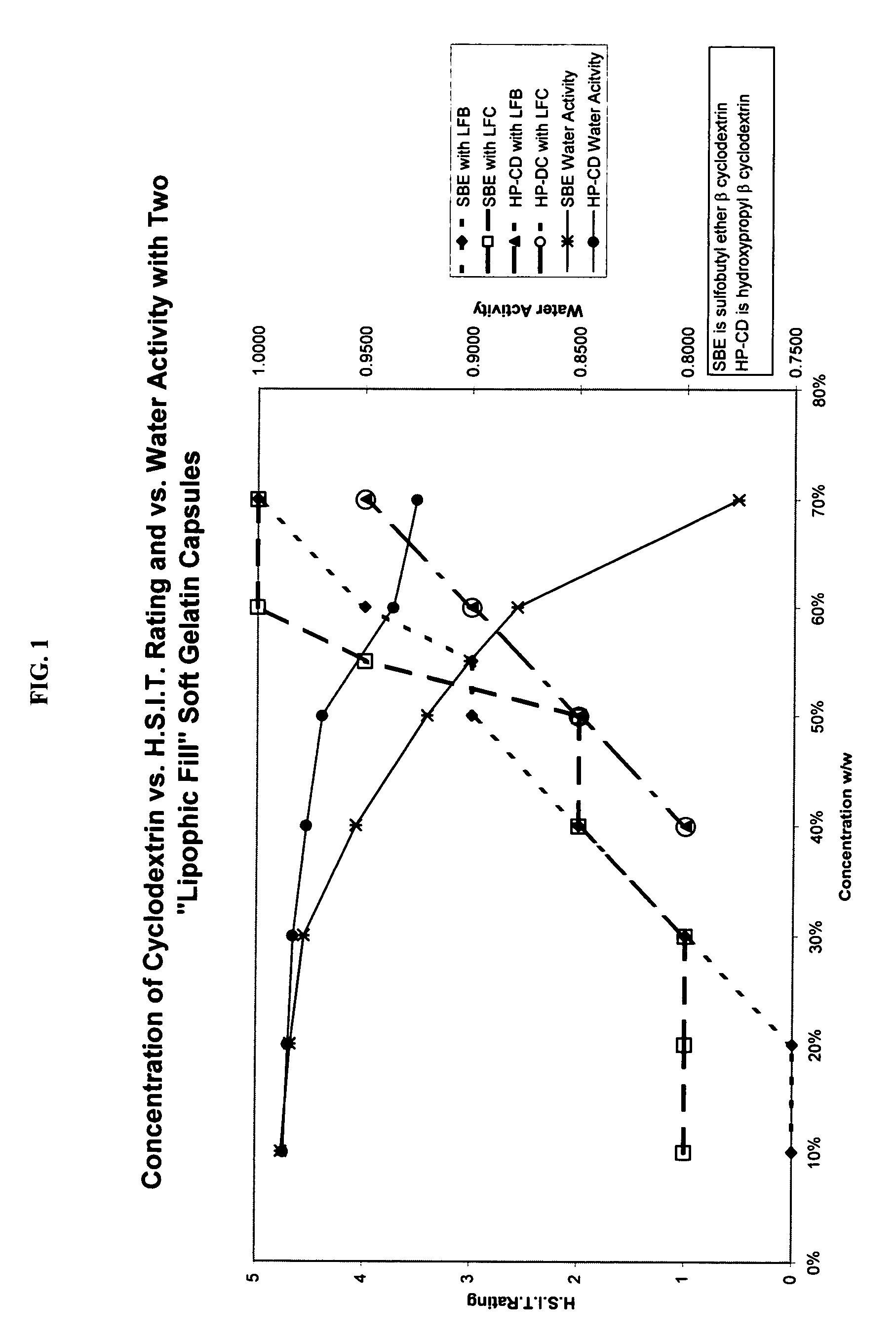
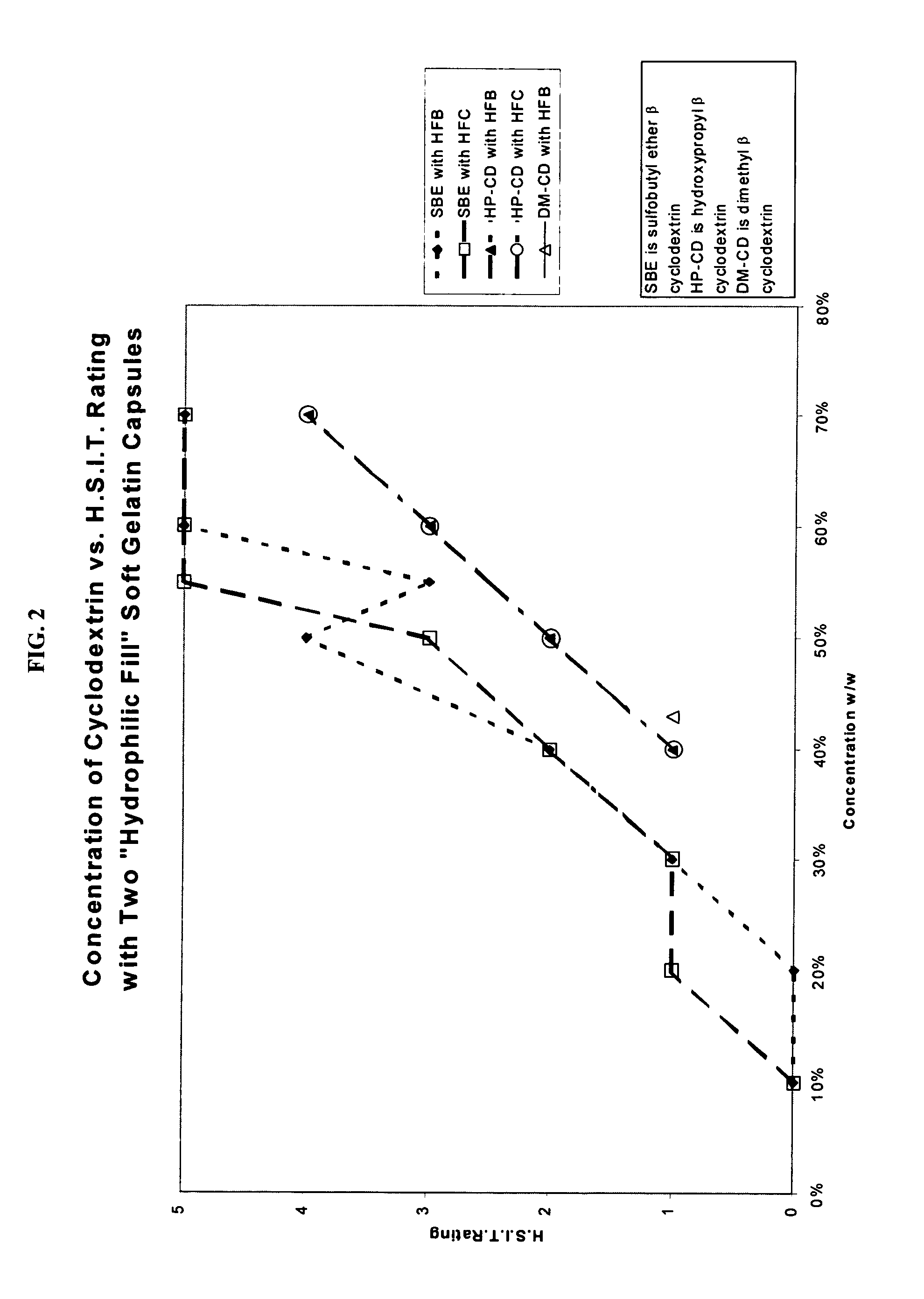
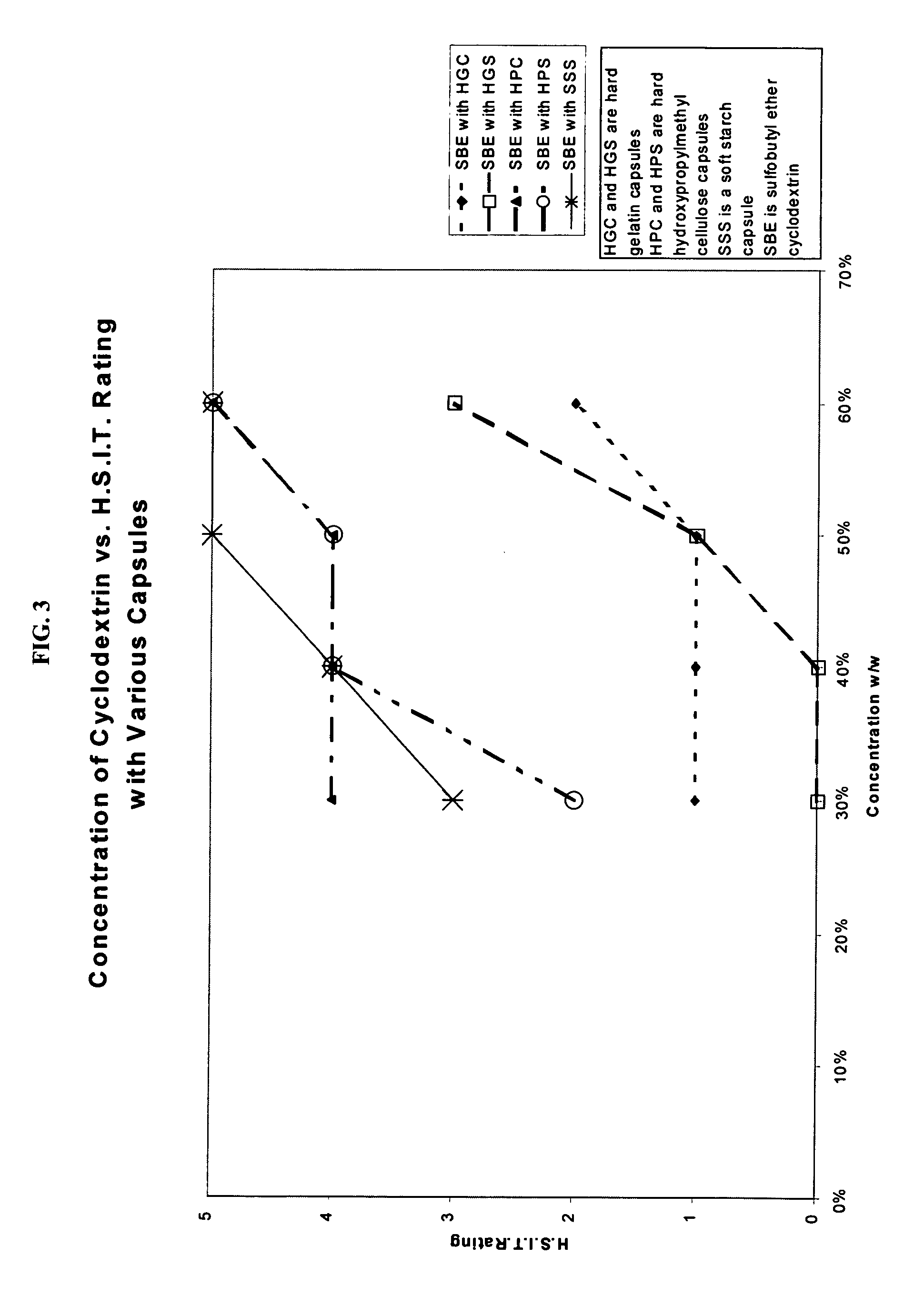
Capsules containing aqueous fill compositions stabilized with derivatized cyclodextrin
ActiveUS20050186267A1Reduce and stop dissolutionReduce and stop and erosionBiocideOrganic active ingredientsActive agentWater activity
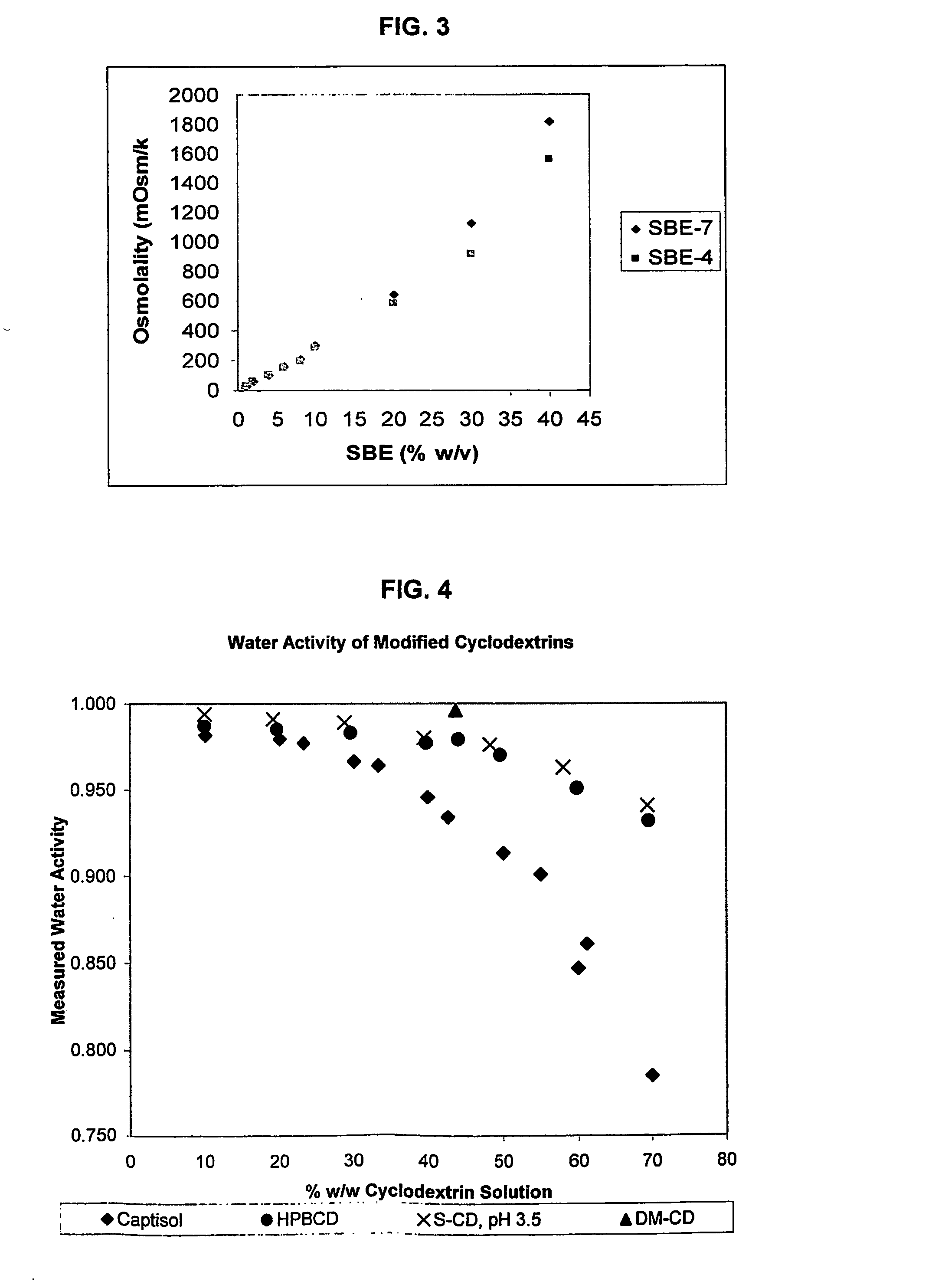
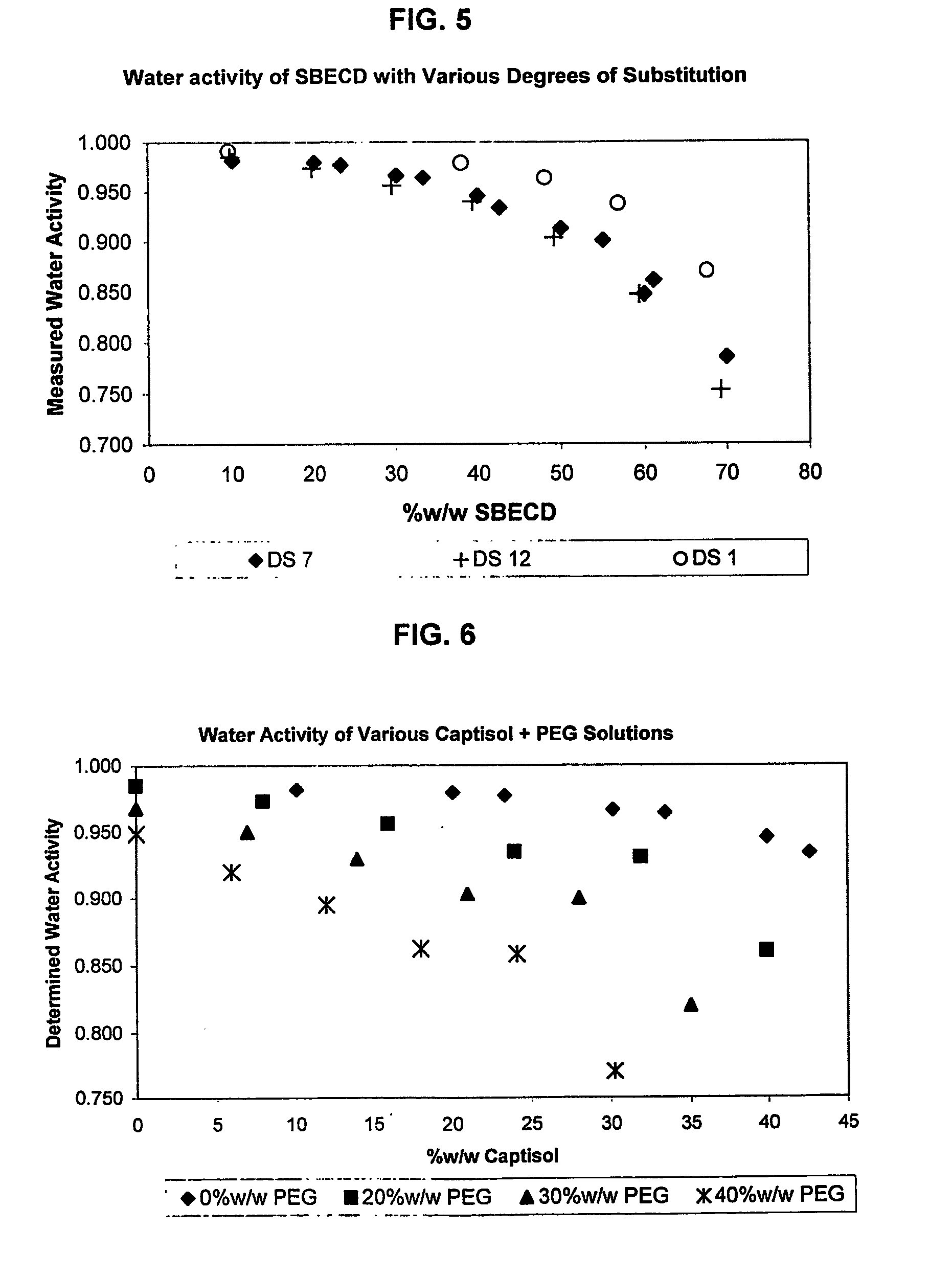
A capsule containing an aqueous fill composition that comprises water, a derivatized cyclodextrin, such as sulfoalkyl ether cyclodextrin (SAE-CD) or hydroxypropyl cyclodextrin (HPCD), optionally one or more active agents and optionally one or more excipients is stabilized from degradation, erosion, swelling or dissolution of its shell during storage. The derivatized cyclodextrin is present in an amount sufficient to reduce, eliminate or inhibit degradation, erosion, swelling and / or dissolution of the shell by water present in the fill composition. Alternatively, the derivatized cyclodextrin and another shell-stabilizing material together stabilize the shell from degradation, erosion, swelling and / or dissolution by water present in the fill composition. The derivatized cyclodextrin can reduce the water activity of the fill composition.
Owner:CYDEX PHARMACEUTICALS INC
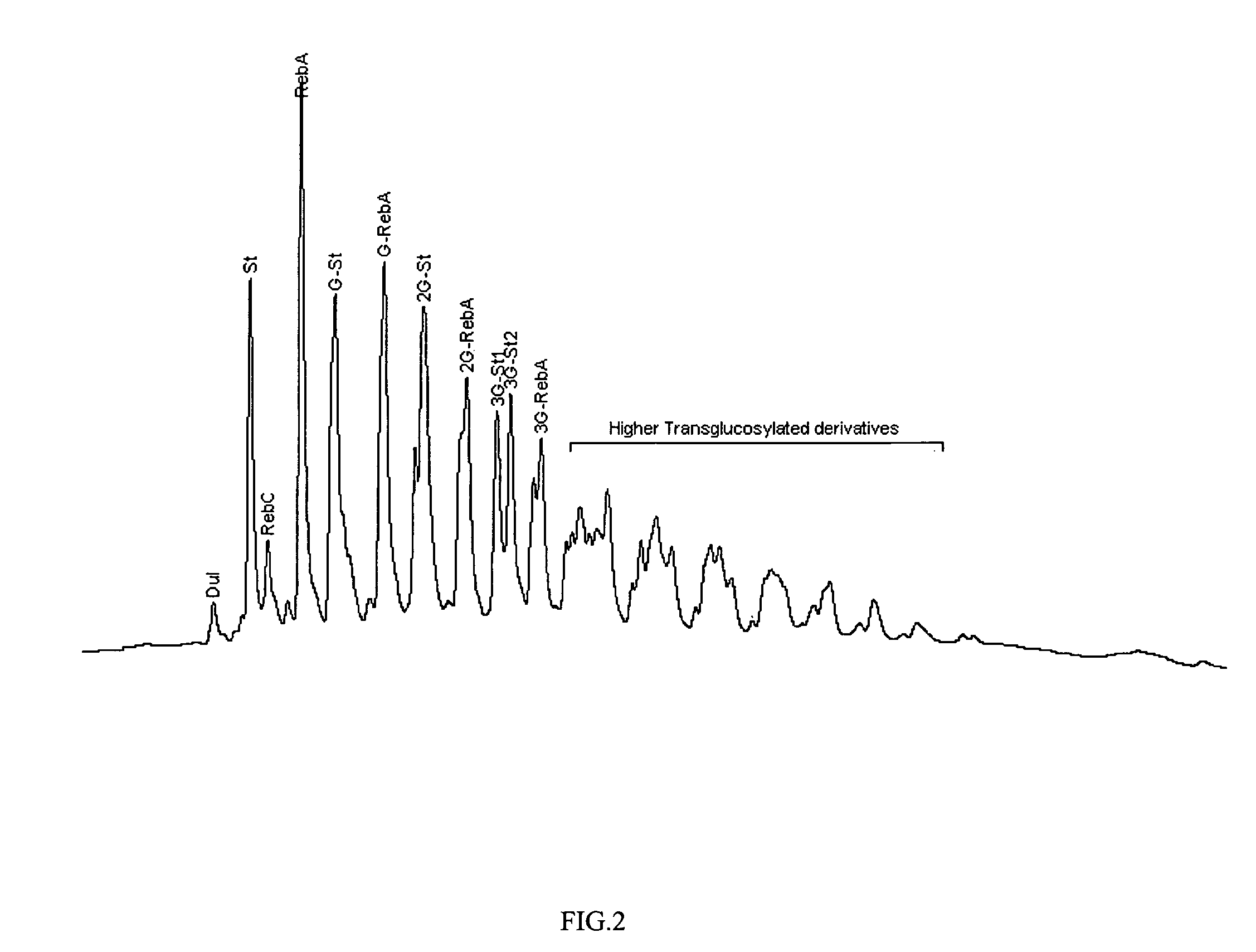
Method of preparing alpha-glucosyl Stevia composition
Glucosyl stevia compositions are prepared from steviol glycosides of Stevia rebaudiana Bertoni. The glucosylation was performed by cyclodextrin glucanotransferase using the starch as source of glucose residues. The short-chain glucosyl stevia compositions were purified to >95% content of total steviol glycosides. The compositions can be used as sweetness enhancers, flavor enhancers and sweeteners in foods, beverages, cosmetics and pharmaceuticals.
Owner:PURECIRCLE SDN BHD
Composition for enzyme inhibition
ActiveUS7737112B2Improve solubilityAntibacterial agentsOrganic active ingredientsSolubilityEnzyme inhibition
Compositions comprising one or more practically insoluble proteasome inhibitors and a cyclodextrin, particularly a substituted cyclodextrin, substantially increase the solubility of these proteasome inhibitors and facilitate their administration. Such compositions optionally comprise a buffer. Methods of treatment using such compositions are also disclosed.
Owner:ONYX THERAPEUTICS INC
Sweetner and use
Sweeteners on the basis of a simultaneously transglucosylated sweet glycoside mixture of Stevia rebaudiana Bertoni are prepared. The transglucosylation was developed in the presence of starch under the action of cyclodextrin glucanotransferase. The remaining maltodextrins are transformed to the fructose-terminated oligosaccharides by the addition of sucrose. The sweeteners are purified to not less than 98% content of sweet glycosides and derivatives. The preparations are almost non-caloric, non-cariogenic, non-bitter, non-lingering sweeteners, which may be advantageously applied in foods, beverages, cosmetics and milk products.
Owner:PURECIRCLE SDN BHD
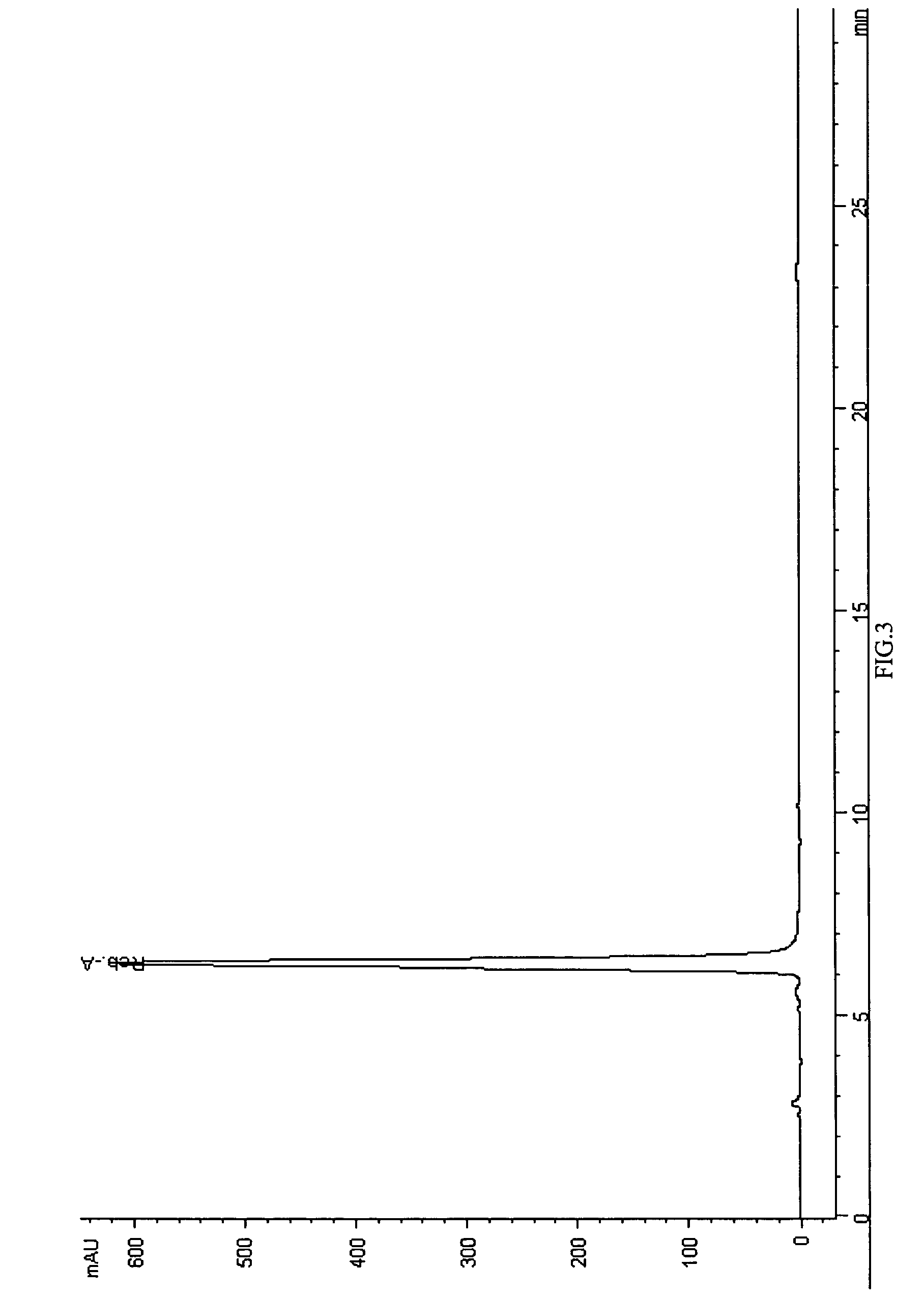
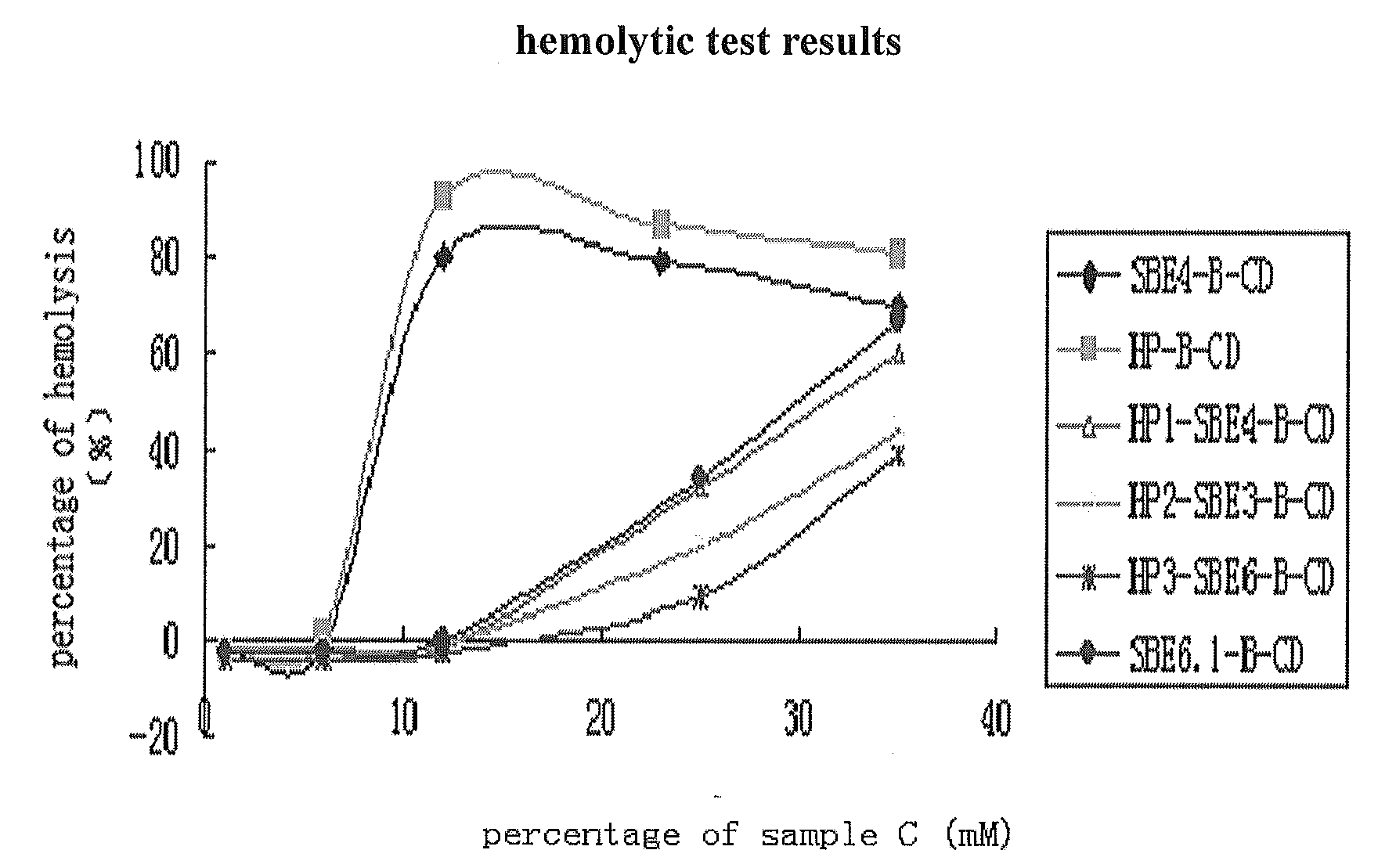
Hydroxypropyl-Sulfobutyl-Beta-Cyclodextrin, the Preparation Method, the Analytical Method, and the Pharmacutical Application Thereof
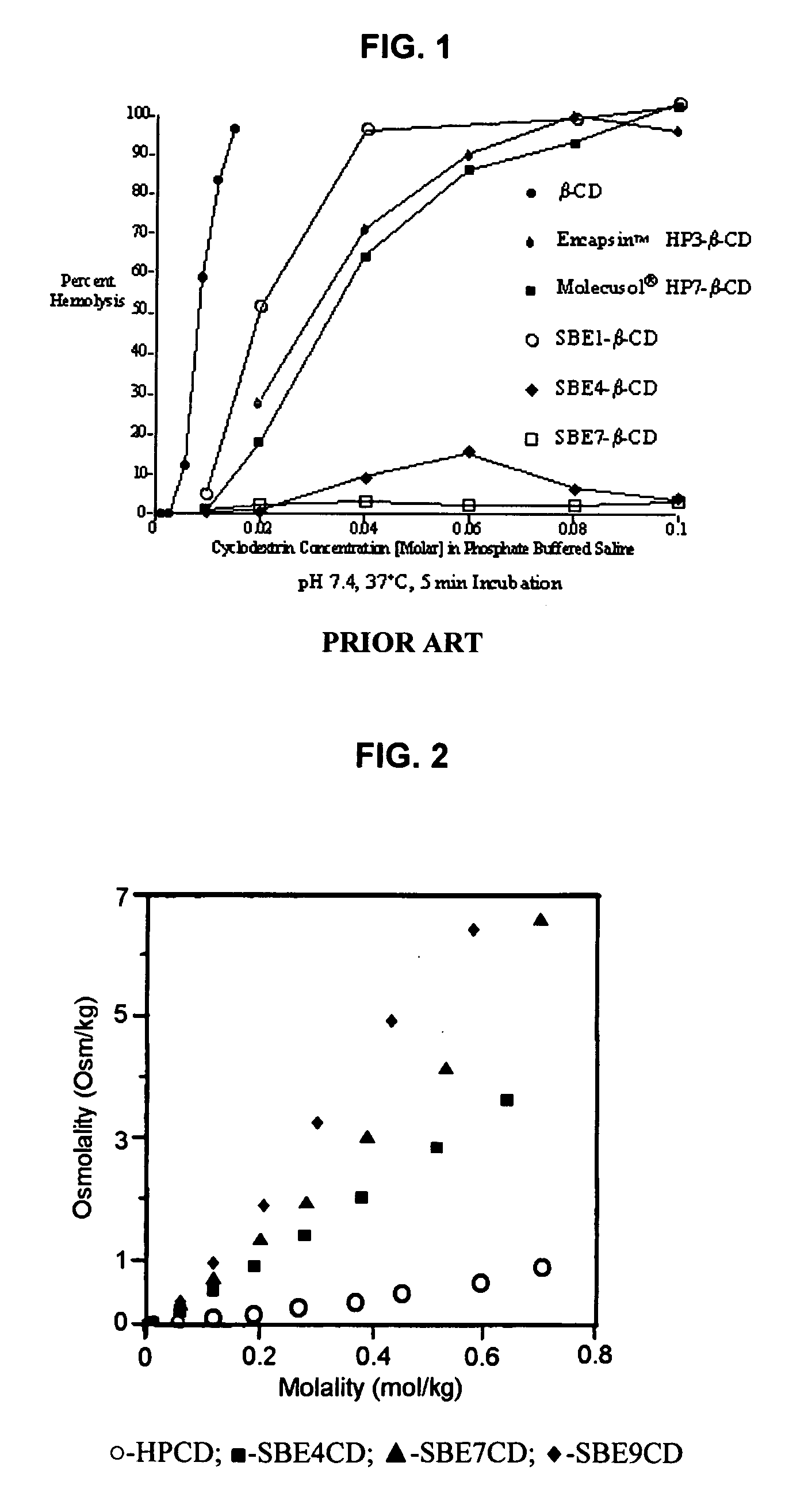
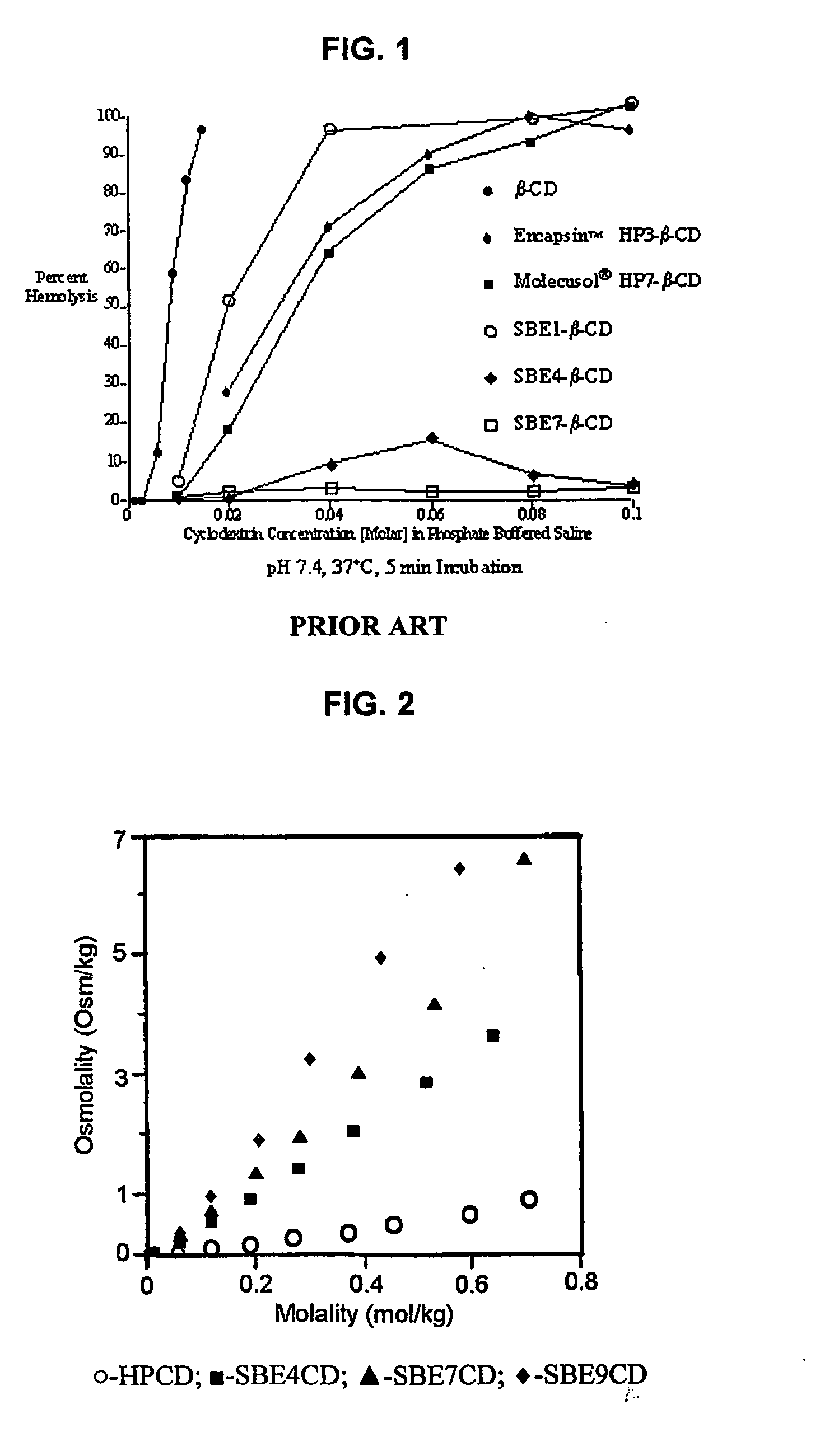
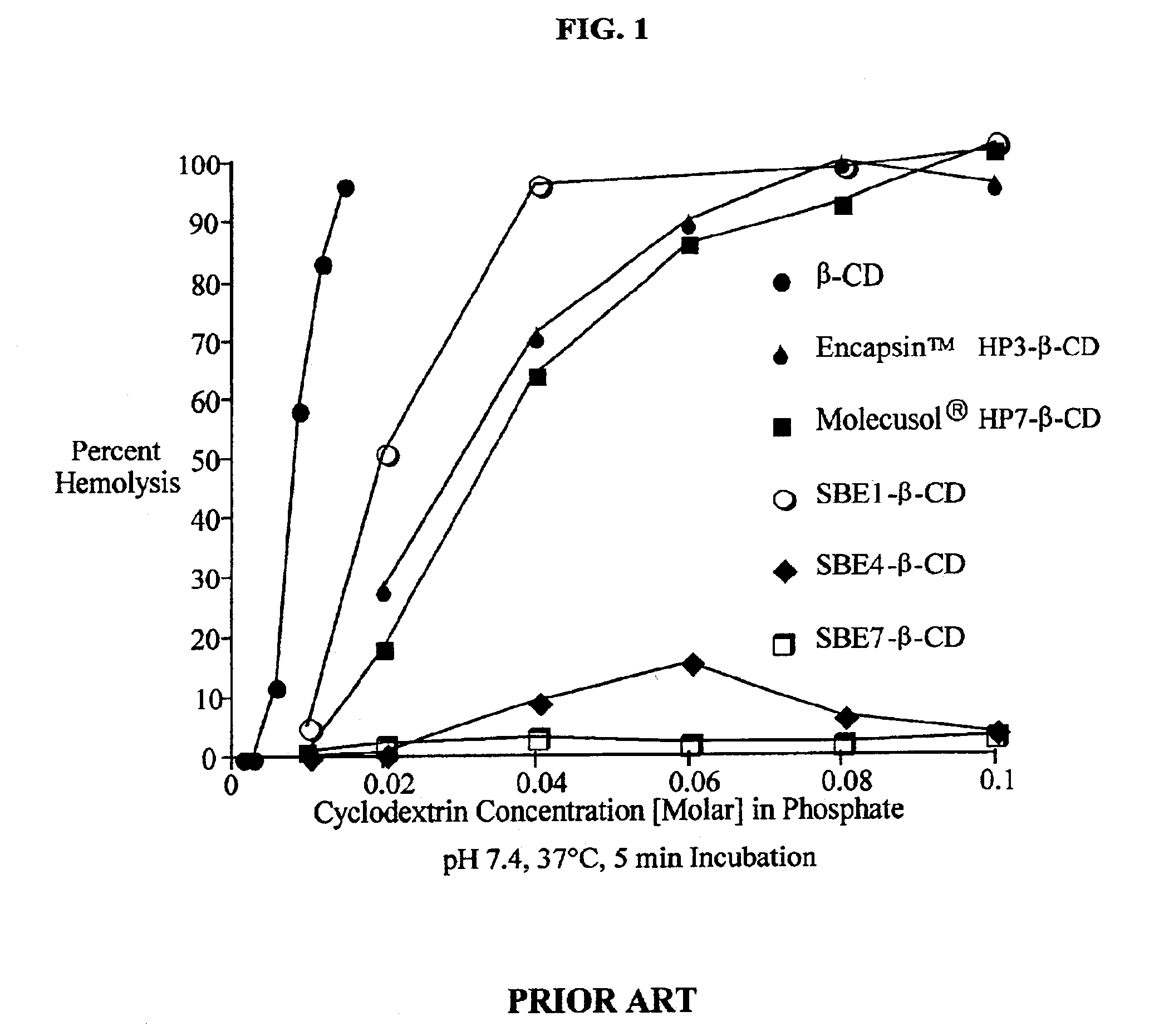
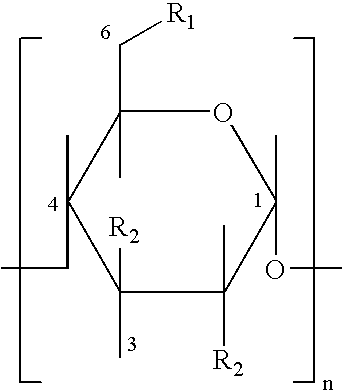
ActiveUS20090012042A1Little hemolysisLow toxicityBiocideOrganic active ingredientsHaemolysisCyclodextrin
Hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin, the preparation method, analytical method, and the pharmaceutical application thereof. The hydroxypropyl-sulfobutyl-&bgr;-cyclodextrin is a derivate of cyclodextrin which is substituted by hydroxypropyl group and sulfobutyl group: n-(2,3,6-O-2-hydroxypropyl)-m-(2,3,6-O-sulfobutyl)-&bgr;-cyclodextrin. The number of substituent groups per mole cyclodextrin is n hydroxypropyl groups and m sulfobutyl groups. “n” represents the average substituent degree of hydroxypropyl groups; “m” represents the average substituent degree of sulfobutyl groups; “n+m=z” is the gross average substituent degree, in which n is a random integer chosen from 1, 2, 3, 4, 5, 6, 7, 8, 9; m is a random integer chosen from 1, 2, 3, 4, 5, 6, 7, 8, 9; and the gross average substituent degree z is a random integer chosen from 2, 3, 4, 5, 6, 7, 8, 9, 10. The present invention shows low haemolysis and low toxicity.
Owner:SUN XIAODONG
Sweetener comprising a stevia-derived sweet substance
InactiveUS20070003679A1Bitter taste inherentEasy to useFood ingredientsFood preparationCyclodextrinSweetness
A sweetener containing a Stevia-derived sweet substance is provided that, while containing cyclodextrin only in small amounts, can mask the bitter taste inherent in the Stevia-derived sweet substance. Cyclodextrin with a particle size of less than or equal to 30 μm is blended into the Stevia-derived sweet substance at 0.5 to 20 mass % with respect to the mass of the Stevia-derived sweet substance.
Owner:SATO PHARMA
Sulfoalkyl Ether Cyclodextrin Compositions
ActiveUS20090270348A1Well mixedReduce the amount requiredOrganic active ingredientsBiocidePhosphateCyclodextrin
SAE-CD compositions are provided, along with methods of making and using the same. The SAE-CD composition comprises a sulfoalkyl ether cyclodextrin and less than 100 ppm of a phosphate, wherein the SAE-CD composition has an absorption of less than 0.5 A.U. due to a drug-degrading agent, as determined by UV / vis spectrophotometry at a wavelength of 245 nm to 270 nm for an aqueous solution containing 300 mg of the SAE-CD composition per mL of solution in a cell having a 1 cm path length.
Owner:CYDEX PHARMACEUTICALS INC
Sulfoalkyl ether cyclodextrin compositions and methods of preparation thereof
A particulate SAE-CD composition is provided. The SAE-CD composition has an advantageous combination of physical properties not found in known solid forms of SAECD. In particular, the SAE-CD composition possesses an advantageous physicochemical and morphological property profile such that it can be tailored to particular uses. The SAE-CD composition of the invention has improved flow and dissolution performance as compared to known compositions of SAE-CD.
Owner:CYDEX PHARMACEUTICALS INC
Use of sulfoalkyl ether cyclodextrin as a preservative
A method of preserving formulations is provided. The method includes the step of including a derivatized cyclodextrin in a formulation capable of sustaining microbial growth. One embodiment of the formulation employs a sulfoalkyl ether cyclodextrin as a preservative and optionally as a solubilizing and complexing agent. A suitable cyclodextrin is the CAPTISOL” brand cyclodextrin (sulfobutyl ether R-cyclodextrin). Whether or not the formulation includes a conventional preservative, the formulation will remain preserved for at least a minimum predetermined period. Specific embodiments of the invention include a carrier, a derivatized cyclodextrin and optionally one or more active agents, one or more water activity-reducing agents, and / or one or more complexation-enhancing agents. The derivatized cyclodextrin reduces the water activity of the formulation. A liquid formulation can be lyophilized or otherwise dried to yield a solid formulation that is optionally reconstitutable.
Owner:CYDEX INC
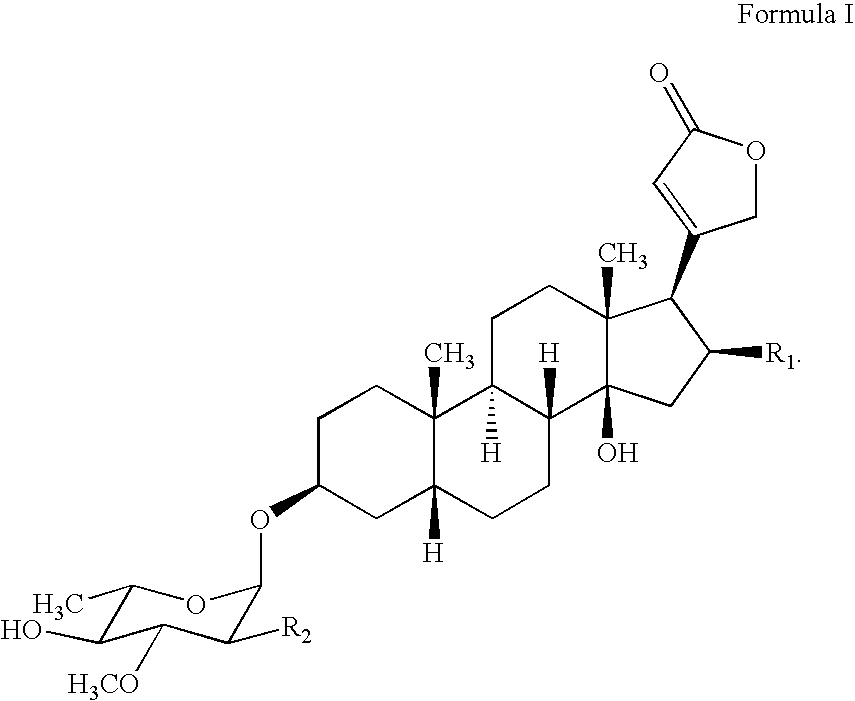
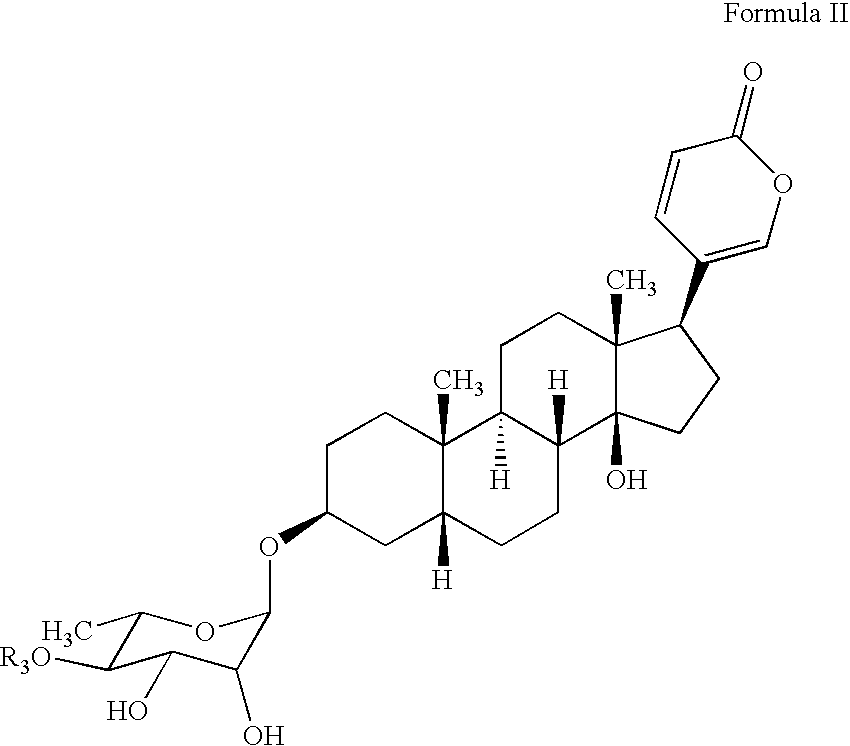
Water soluble formulations of digitalis glycosides for treating cell-proliferative and other diseases
The present invention provides method, preparation and use of a variety of pharmaceutical composition containing at least one digitalis glycosides such as oleandrin, odoroside-A, neriifolin, proscillaridin-A, methyl-proscillaridin-A, digitoxin, digoxin and amorphous cyclodextrins. In another aspect, the present invention provides an effective method to reduce the growth of cancers or reducing the incidence of metastases. In yet another aspect, the present invention provides an effective method for treating diseases in a warm-blooded animal.
Owner:TRINITY LAB INC
Cyclodextrin-based polymers for therapeutics delivery
InactiveUS20080176958A1Promote endocytosisAntibacterial agentsPowder deliveryPresent methodCyclodextrin
The present invention relates to novel compositions comprising polymeric moieties covalently attached to therapeutic agents, wherein the therapeutic agent is attached to the polymeric moiety through a tether. By selecting from a variety of tether groups and targeting ligands the polymers present methods for controlled delivery of the therapeutic agents. The invention also relates to methods of treating subjects with the therapeutic compositions described herein.
Owner:CERULEAN PHARMA
Composition for enzyme inhibition
ActiveUS20060128611A1SolubilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityProteasome degradation
Compositions comprising one or more practically insoluble proteasome inhibitors and a cyclodextrin, particularly a substituted cyclodextrin, substantially increase the solubility of these proteasome inhibitors and facilitate their administration. Such compositions optionally comprise a buffer. Methods of treatment using such compositions are also disclosed.
Owner:ONYX THERAPEUTICS
Cyclodextrin nanotechnology for ophthalmic drug delivery
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
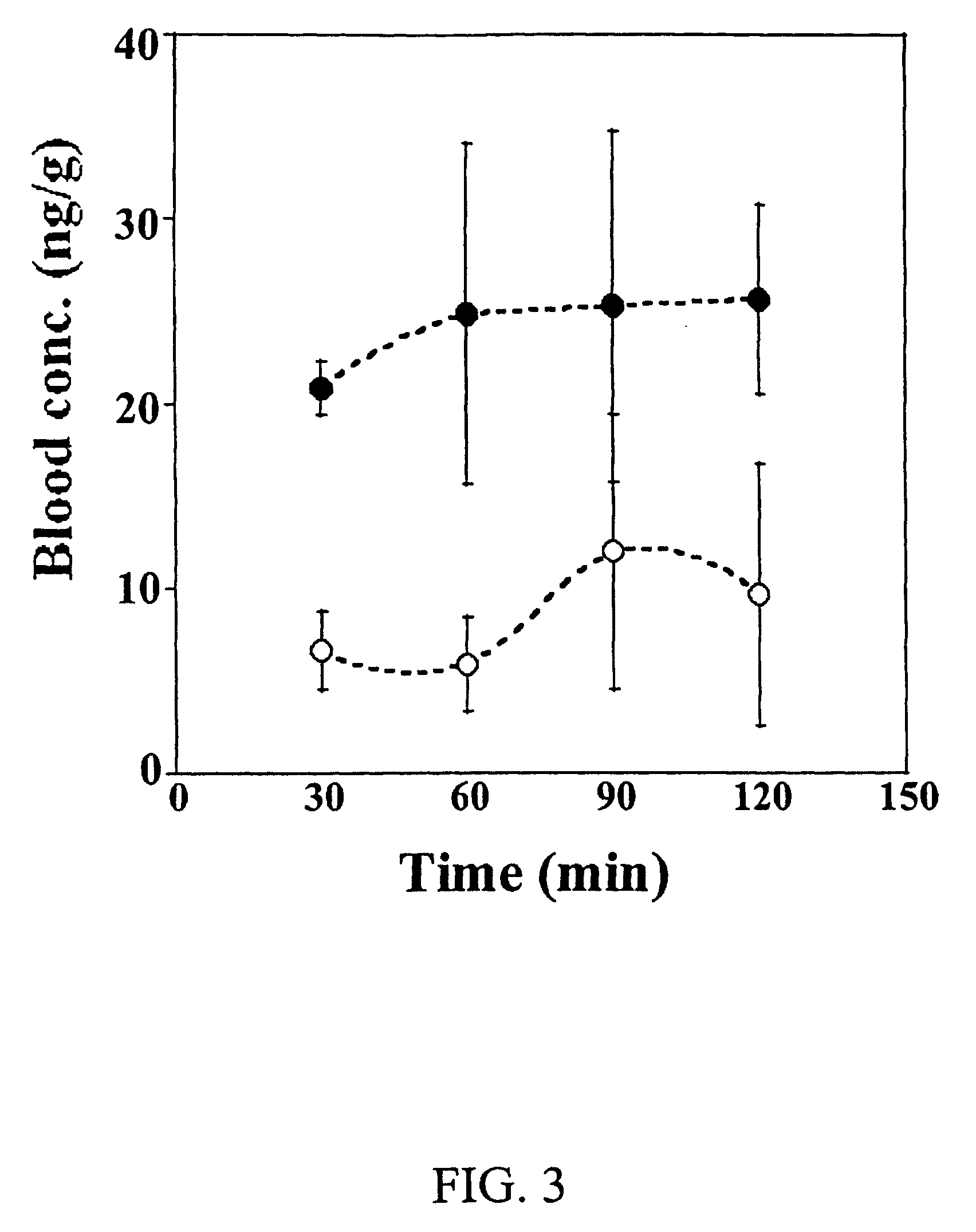
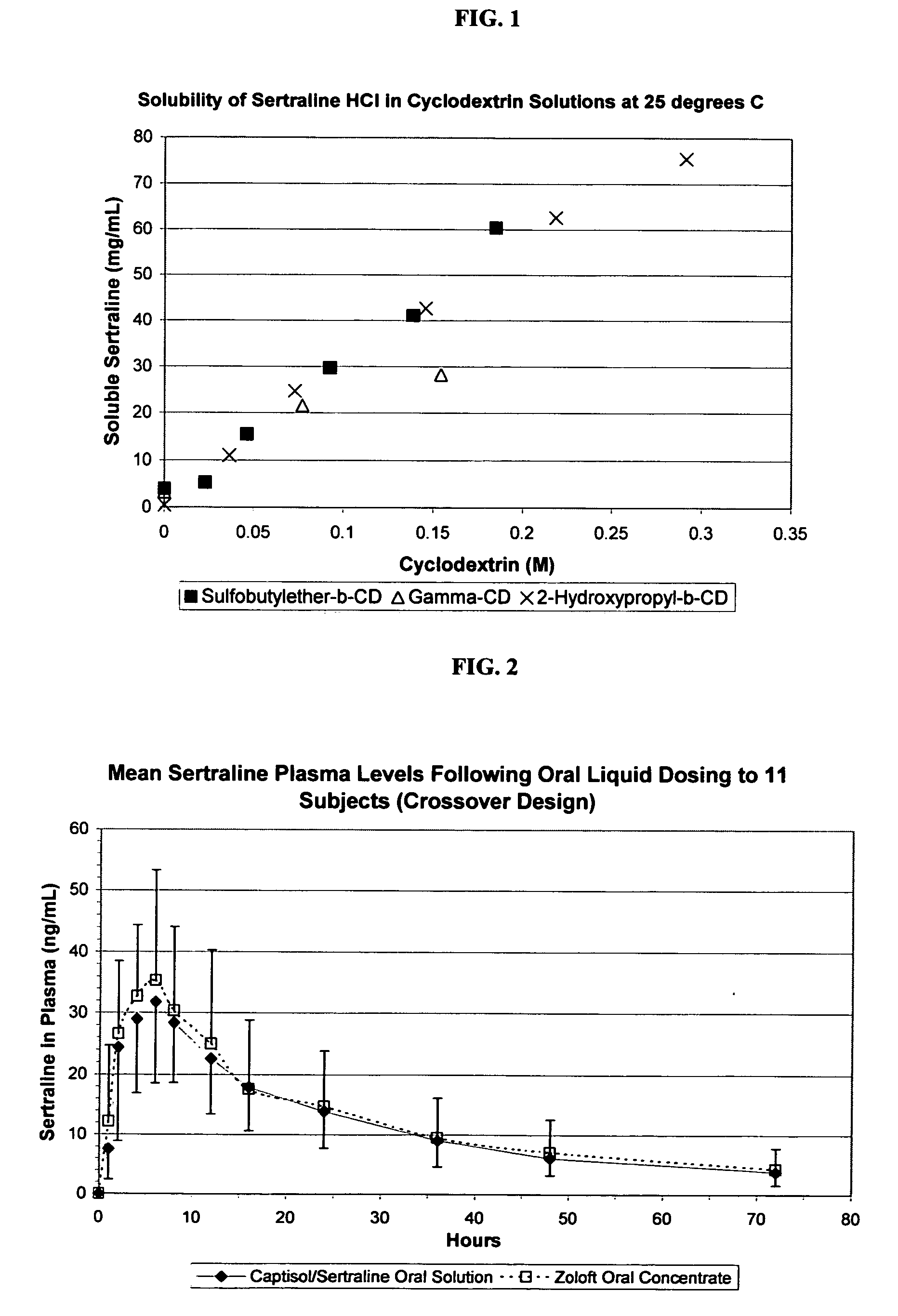
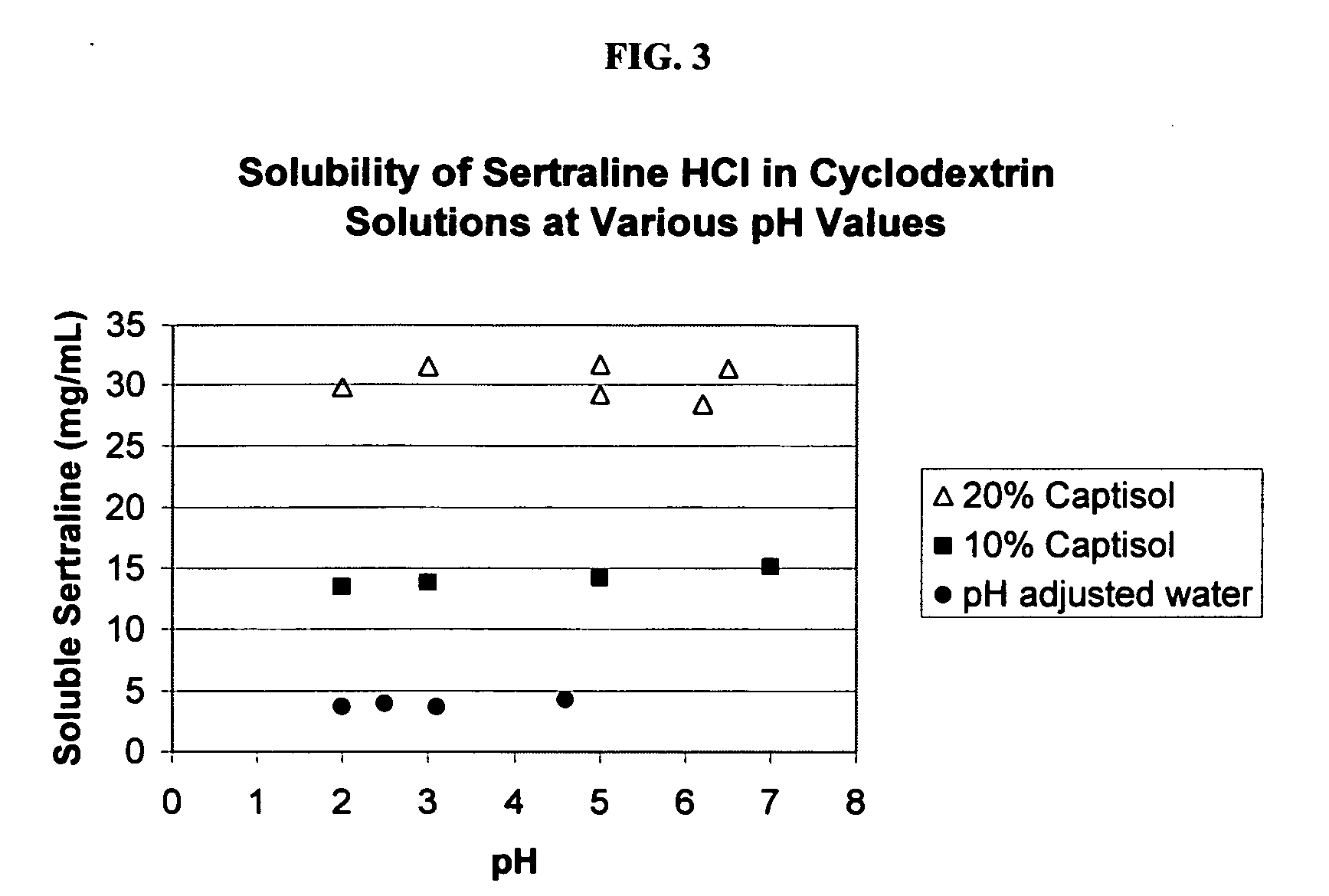
Taste-masked formulations containing sertraline and sulfoalkyl ether cyclodextrin
InactiveUS20050250738A1High photochemical stabilityReduce probabilityBiocideOrganic active ingredientsSertralineFruit juice
The present invention provides aqueous oral formulations containing sertraline, or a pharmaceutically acceptable salt thereof, and a sulfoalkyl ether cyclodextrin. The liquid formulations are pleasant tasting, convenient to use, and chemically and physically stable. The liquid formulations can be administered directly or diluted before administration. Unlike the commercially available ZOLOFT™ formulation, the liquid formulations herein do not precipitate upon dilution with water, fruit juices, sodas or other pharmaceutically acceptable oral liquid carriers. The sulfoalkyl ether cyclodextrin-containing formulation provides significant advantages over the marketed non-aqueous formulation and other cyclodextrin-containing formulations of sertraline. The formulation can be self-preserved against microbial growth. The SAE-CD-containing formulation of sertraline can be provided in liquid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid formulations can be prepared. The formulation is available as a clear solution or a suspension.
Owner:CYDEX PHARMACEUTICALS INC
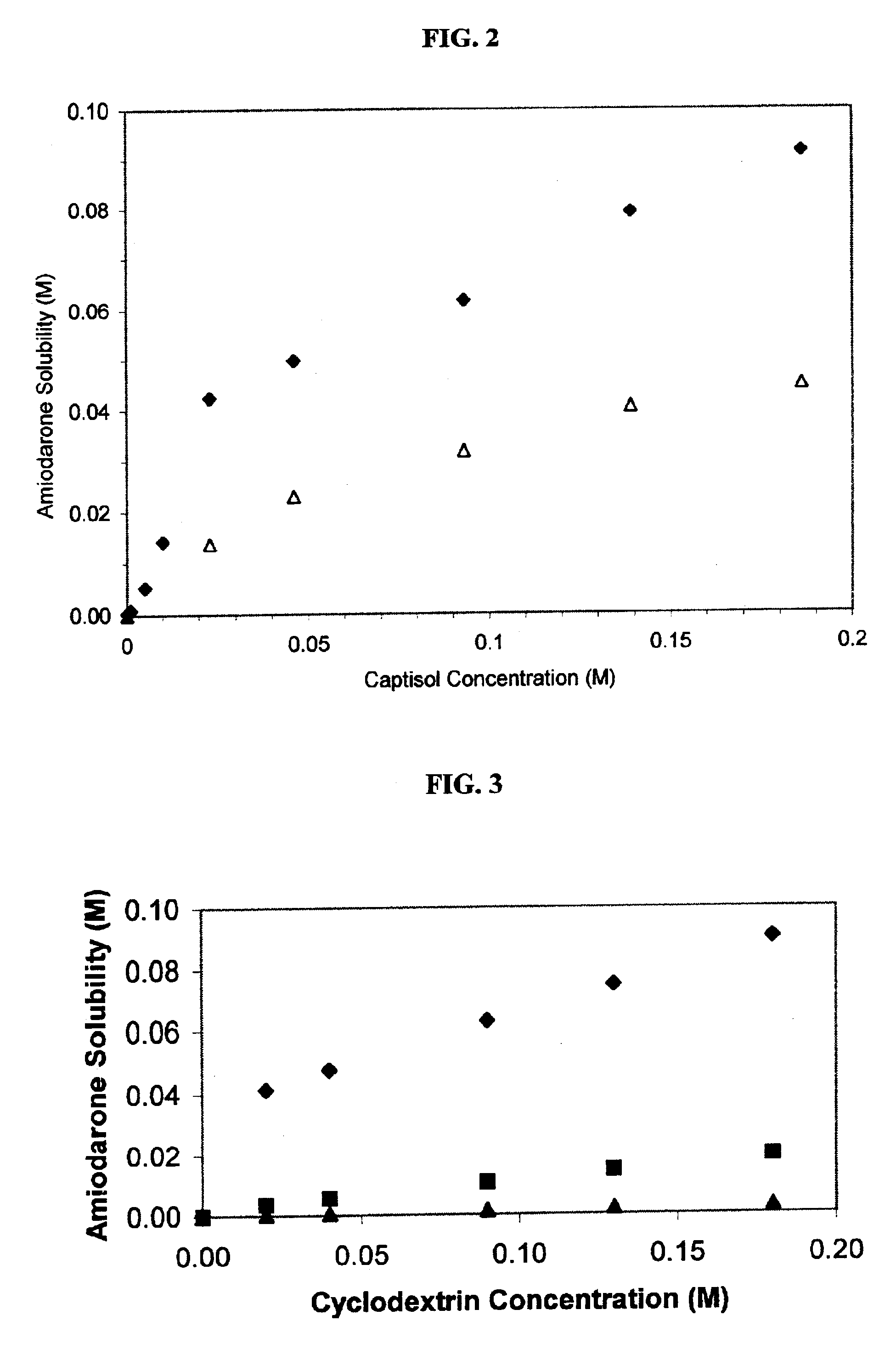
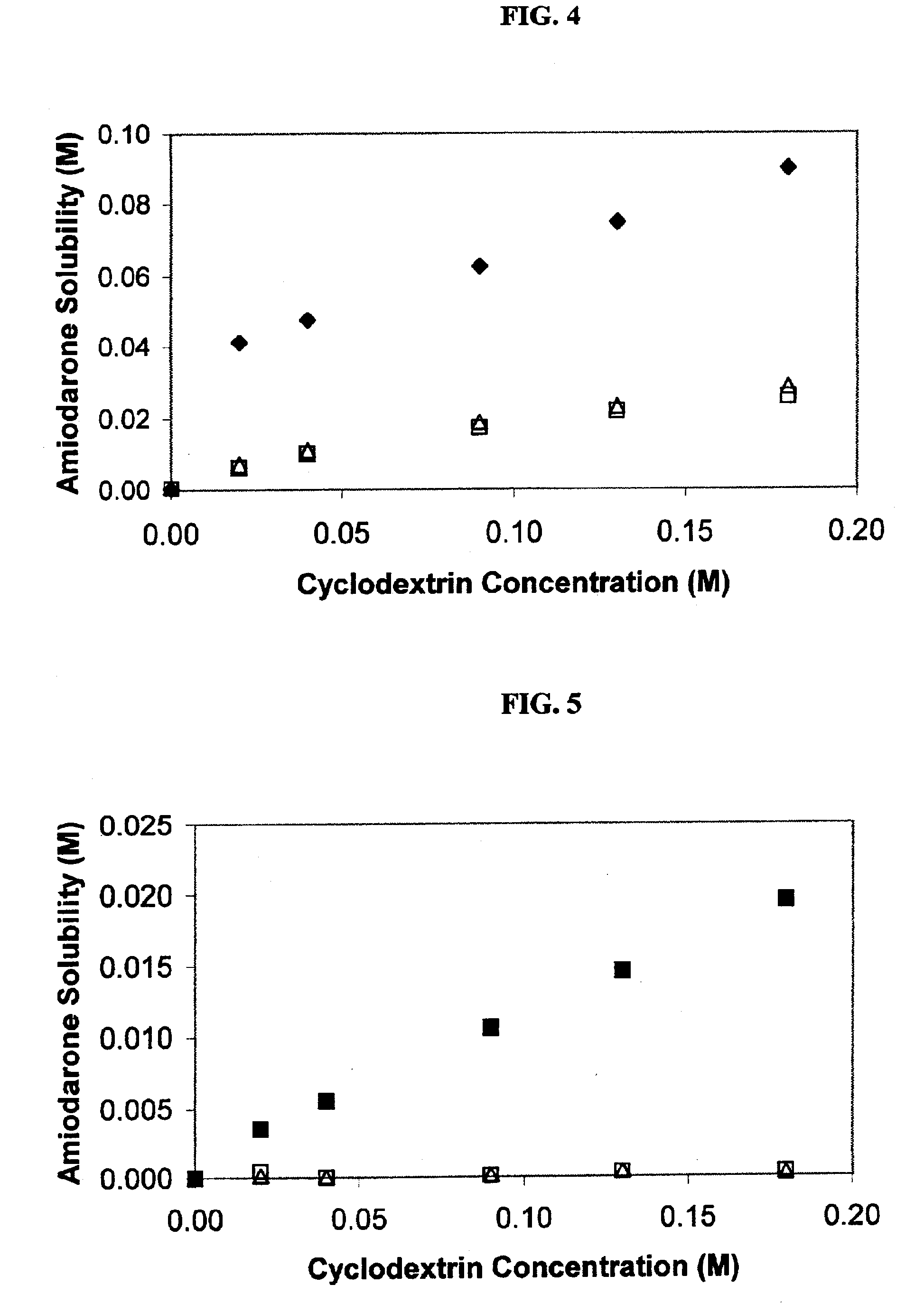
Formulations containing amiodarone and sulfoalkyl ether cyclodextrin
InactiveUS6869939B2Increase surface tensionAccurate doseCompounds screening/testingPowder deliveryCyclodextrinRoom temperature
The present invention provides aqueous parenteral formulations containing an antiarrhythmic agent, such as amiodarone, and a sulfoalkyl ether cyclodextrin. The liquid formulations are clear, sterilizable, and chemically and physically stable. The liquid formulations do not require a surfactant and do not precipitate upon dilution with distilled water or other pharmaceutically acceptable liquid carrier. The sulfoalkyl ether cyclodextrin-containing formulation provides significant advantages over other cyclodextrin-containing formulations of amiodarone. The formulation can be prepared in acidic, neutral and slightly basic medium while providing acceptable concentrations of amiodarone suitable for parenteral administration. An SAE-CD-containing formulation of amiodarone can be provided in liquid form or as a reconstitutable powder. Moreover, highly concentrated solutions exceeding 200 mg of amiodarone per mL can be prepared. Solutions can be made either dilutable or non-dilutable with water at room temperature or under conditions typically encountered in the clinic.
Owner:CYDEX PHARMACEUTICALS INC
Solution forms of cyclodextrins for nasal or throat delivery of essential oils
This invention further relates to a method for preventing or treating diseases or conditions of the oral cavity, throat or nose of warm-blooded animals including humans. More particularly, the invention pertains to a composition and method for spraying essential oils to the oral cavity, throat or nasal mucosa as cyclodextrin inclusion complexes. The spray composition includes a cyclodextrin in an amount of from about 0.1% w / v to about 20% w / v; at least one essential oil in an amount of from about 0.001% w / v to about 5.0% w / v; an effective amount of an antimicrobial preservative composition; and water. The composition may further comprise an alcohol co-solvent, a thickening agent, a sweetener, an antitussive, an anticholinergic, a decongestant, an antihistamine, an astringent, an anti-inflammatory steroid composition, a vitamin, a respiratory stimulant, a mucolytic agent, a bronchodilator, a beta-antagonist, an antidiarrheal agent, or combinations thereof.
Owner:QPHARMA
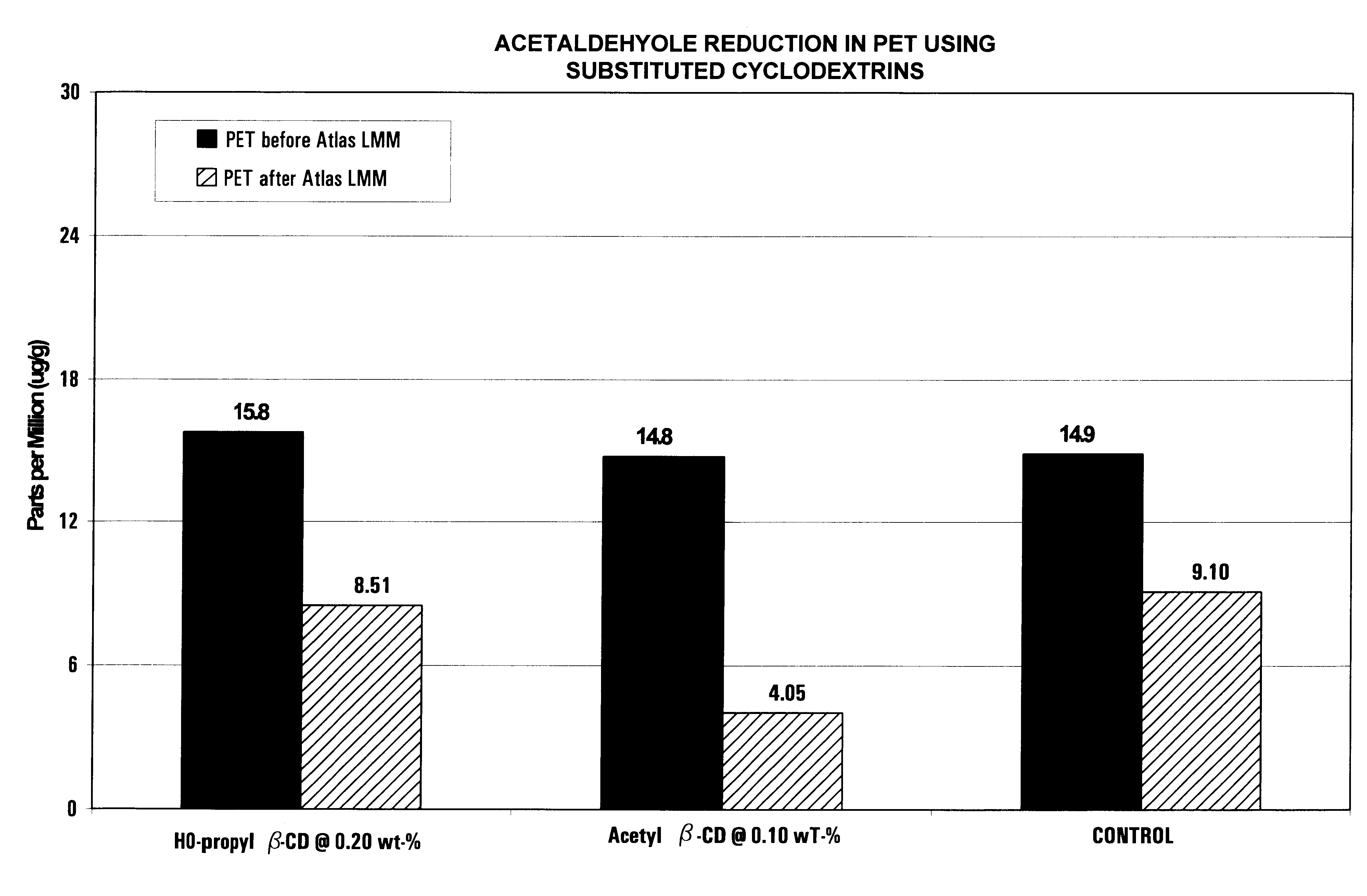
Reducing concentration of organic materials with substituted cyclodextrin compound in polyester packaging materials
InactiveUS6709746B2Inhibit productionImprove barrier propertiesNon-fibrous pulp additionWrappersScavengerCyclodextrin
Volatile organic compound or other materials are produced in the thermoplastic manufacture of thermoplastic polyester beverage containers. Such materials can be eluted into beverages such as carbonated beverages, sparkling or still water from the polyester. Such thermoplastic polyester resins can be manufactured with a substituted cyclodextrin material that can prevent the formation of, or react with, and absorb volatile by-products during the formation of thermoplastic preforms or containers from the thermoplastic pellet or chip. Further, as the preform is blown into a polyester container, the active materials of the invention prevent the generation of additional undesirable volatile materials. Lastly, the scavenger material can act as a barrier that prevents transport of materials from the exterior of the container into the container contents.
Owner:ARTEVA NORTH AMERICA SARL +1
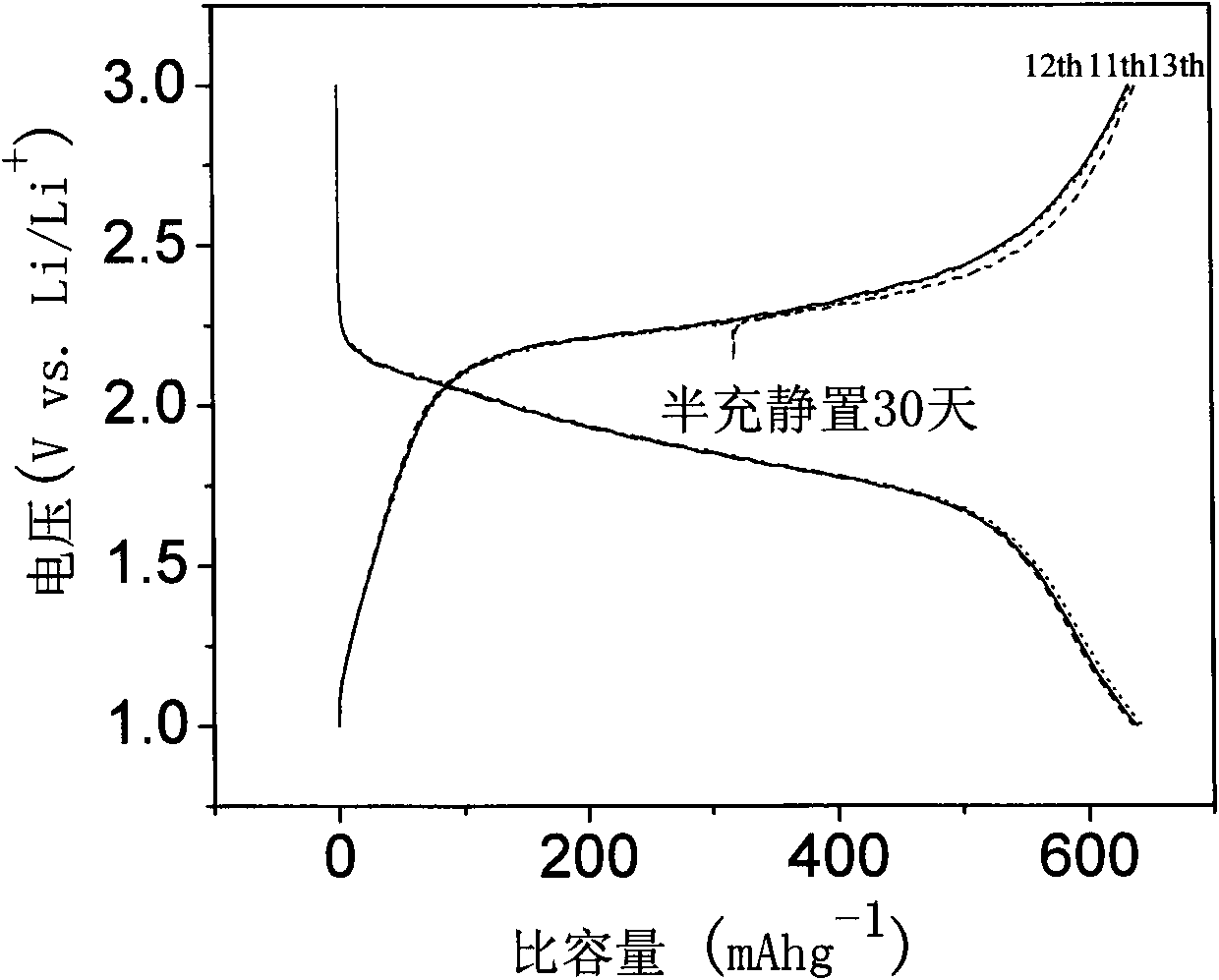
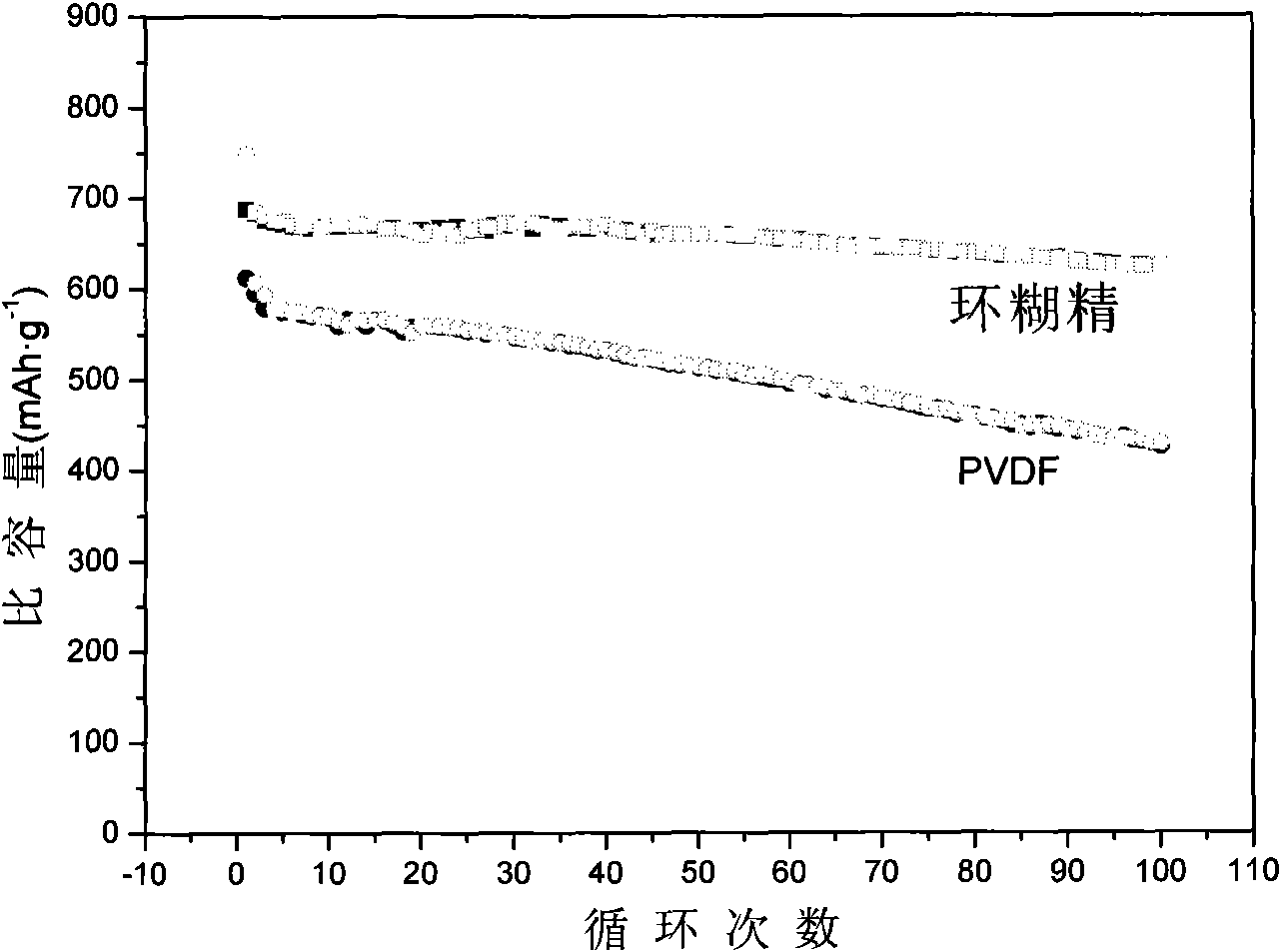
Sulfenyl anode of lithium-sulfur rechargeable battery and preparation method thereof
InactiveCN101577323APrevent self-dischargeImprove conductivityElectrode manufacturing processesActive material electrodesLithium metalMass ratio
The invention discloses sulfenyl anode of a lithium-sulfur rechargeable battery and a preparation method thereof. The sulfenyl anode is prepared by the steps of: equally mixing sulfenyl compound active material, cyclodextrin binder and carbon conductivity agent, coating the mixture on an aluminum foil current collector and obtaining the sulfenyl anode after drying and pressing. The coating thickness is 50 to 100 microns and the aluminum foil thickness is 20 to 30 microns; the mass ratio of the sulfenyl compound active material, the cyclodextrin binder and the carbon conductivity agent is 7 to 8:0.6 to 1:0.6 to 1.5, wherein the sulfenyl compound active material is formed by the steps of: equally mixing carbon nano tube, sulfur and polyacrylonitrile according to the mass ratio of 0.1 to 0.2:6 to 8:1 and sintering the mixture in protection of inert gas at the temperature of 300 to 320 DEG C for insulation for 6 to 8 hours. By using the lithium-sulfur rechargeable battery with the sulfenyl anode and lithium metal cathode, the reversible capacity of the sulfenyl compound active material reaches 680mAh.g under 0.1C multiplying power charge-discharge condition; and compared with the discharge capacity of second circulation, the discharge capacity after 100 times of circulation decreases less than 10 percent.
Owner:SHANGHAI JIAO TONG UNIV
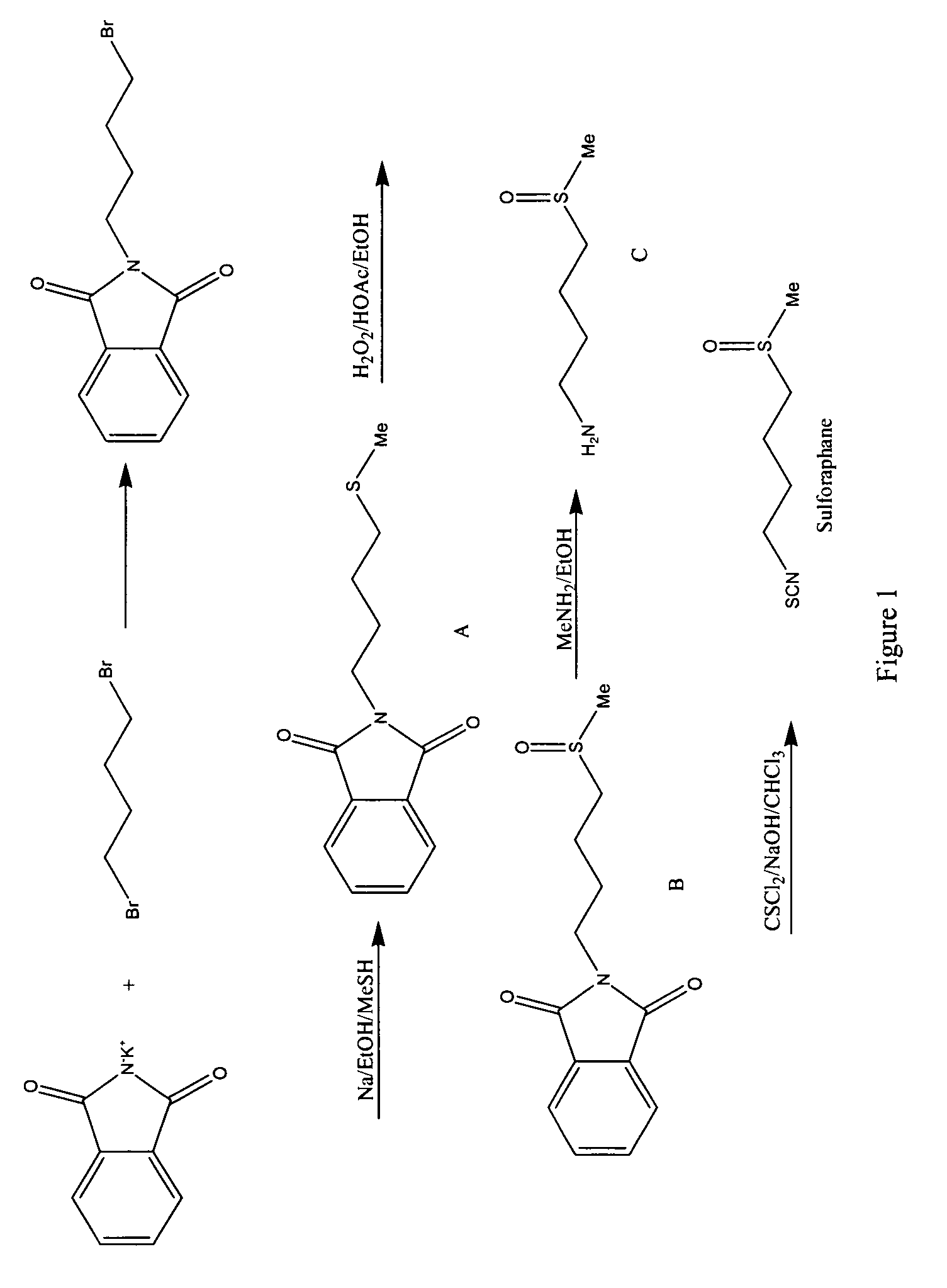
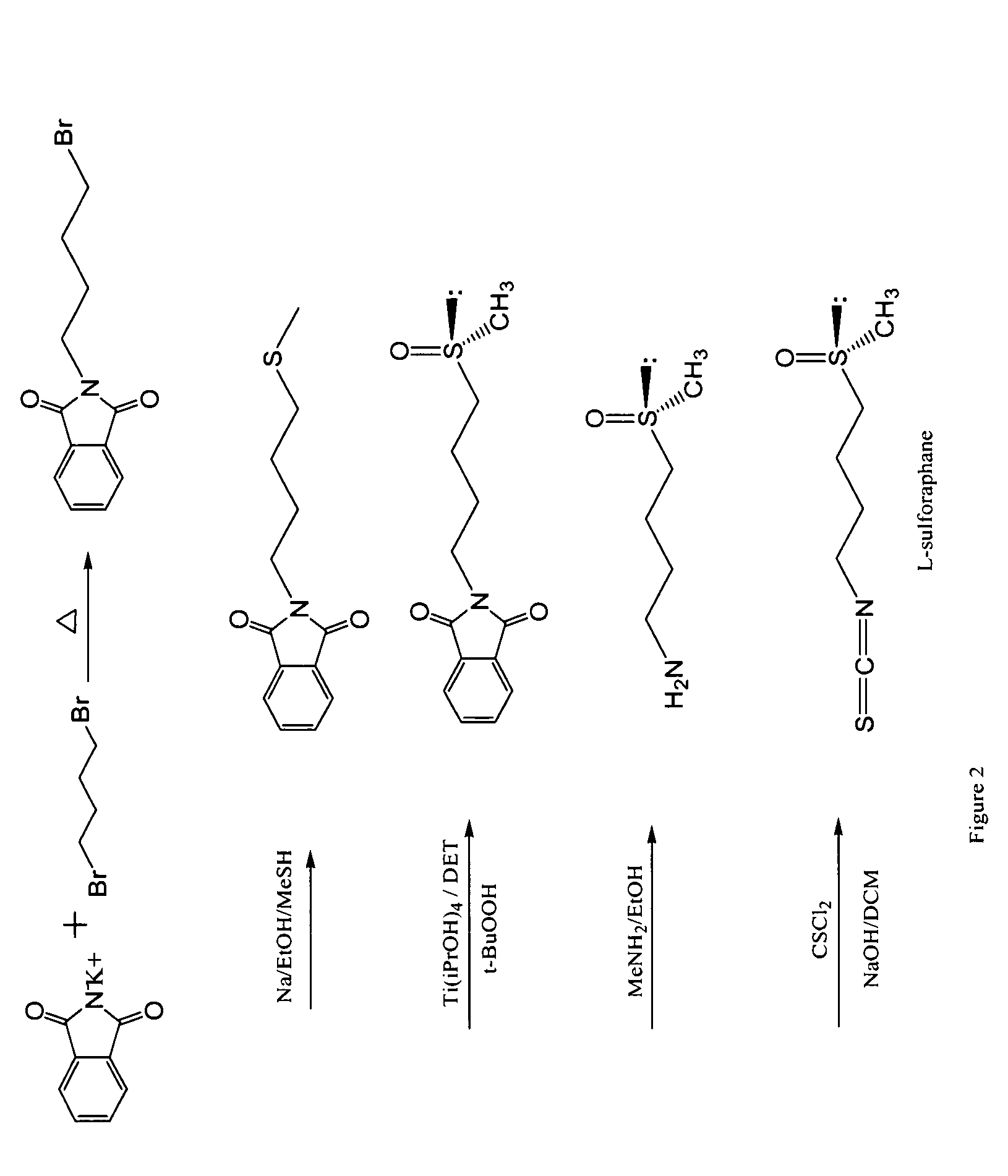
Stabilized sulforaphane
A method of stabilizing sulforaphane is provided. The method includes contacting sulforaphane, or an analog thereof, and a cyclodextrin to form a complex between the sulforaphane, or analog thereof, and the cyclodextrin.
Owner:PHARMAGRA LABS
Glucosyl Stevia Composition
ActiveUS20120214751A1High purityOvercome disadvantagesBiocideCosmetic preparationsCyclodextrinGlucose polymers
Glucosyl stevia compositions are prepared from steviol glycosides of Stevia rebaudiana Bertoni. The glucosylation was performed by cyclodextrin glucanotransferase using the starch as source of glucose residues. The short-chain glucosyl stevia compositions were purified to >95% content of total steviol glycosides. The compositions can be used as sweetness enhancers, flavor enhancers and sweeteners in foods, beverages, cosmetics and pharmaceuticals.
Owner:PURECIRCLE SDN BHD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com