Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6980results about "Electrode manufacturing processes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
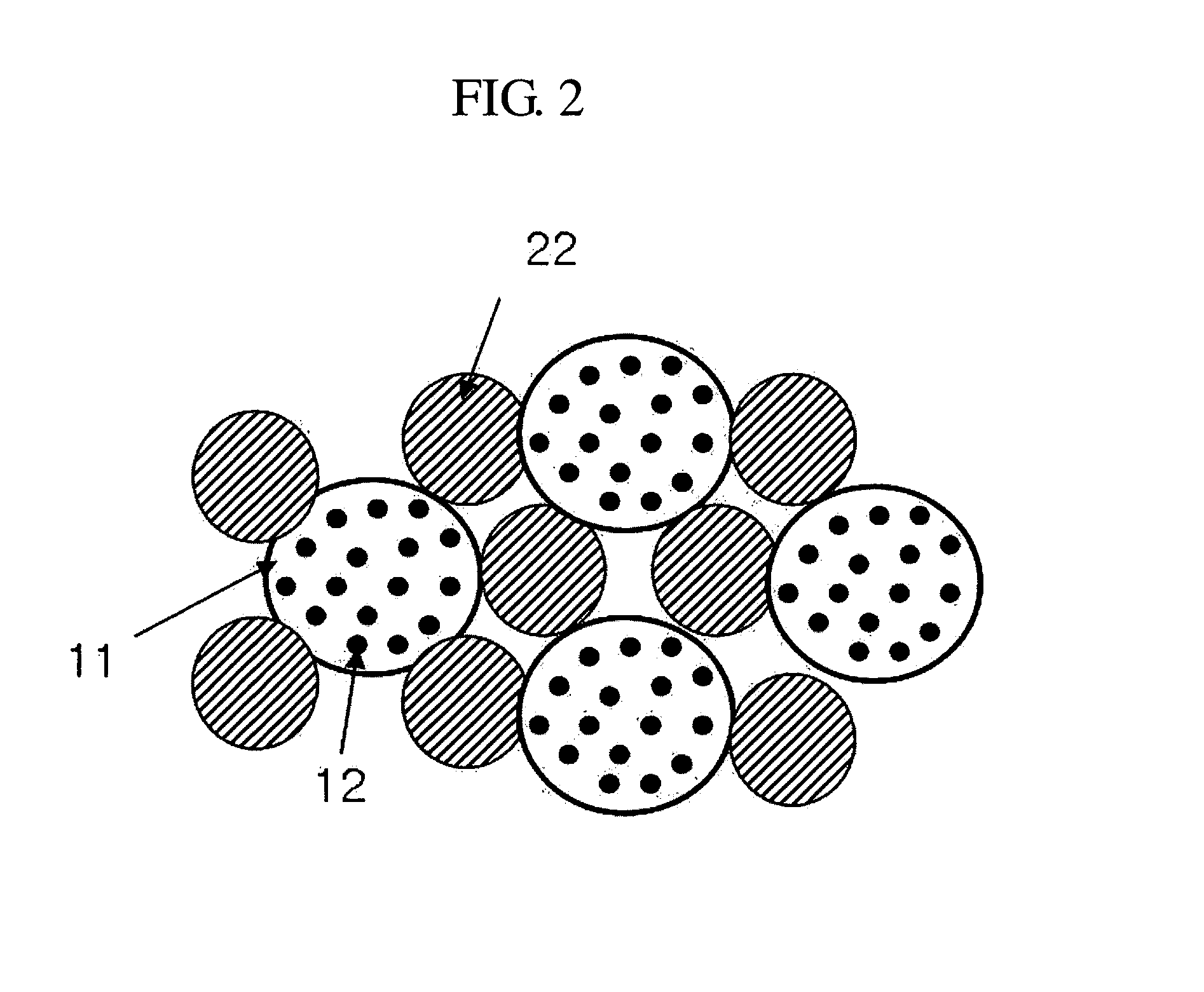
Electroactive high storage capacity polyacetylene-co-polysulfur materials and electrolytic cells containing same
InactiveUS6117590AHigh storage capacity per unit weightFacilitates electron transportElectrode manufacturing processesNon-aqueous electrolyte accumulatorsElectrochemical cellElectrode material
The present invention relates to novel electroactive energy storing polyacetylene-co-polysulfur (PAS) materials of general formula (C2Sx)n wherein x is greater than 1 to about 100, and n is equal to or greater than 2. This invention also relates to novel rechargeable electrochemical cells containing positive electrode materials comprised of said polyacetylene-co-polysulfur materials with improved storage capacity and cycle life at ambient and sub-ambient temperatures.
Owner:THE BANK OF NEW YORK +1
Mesoporous network electrode for electrochemical cell
InactiveUS20040131934A1Simplifies production of cellFull penetrationMaterial nanotechnologyElectrode manufacturing processesLithiumNanoparticle
A high kinetics rate electrochemical cell in which at least one of the electrodes is composed of a mesostructural electroactive material comprising nanoparticles forming a three-dimensional framework structure of mesoporous texture having a bicontinuous junction of large specific surface area with the electrolyte. A low temperature method of preparation of the electrodes employs a high-speed deposition of the electrically active material in the form of a thin film. The application of said electrodes in high power lithium ion insertion batteries, photovoltaic cells, supercapacitors and fast electrochromic devices is disclosed.
Owner:FRANCOIS SUGNAUX
Electrode materials with high surface conductivity
InactiveUS6855273B2Electrode manufacturing processesDouble layer capacitorsSurface conductivityIon exchange
The present invention concerns electrode materials capable of redox reactions by electrons and alkaline ions exchange with an electrolyte. The applications are in the field of primary (batteries) or secondary electrochemical generators, super capacitors and light modulating system of the super capacitor type.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Conductive lithium storage electrode
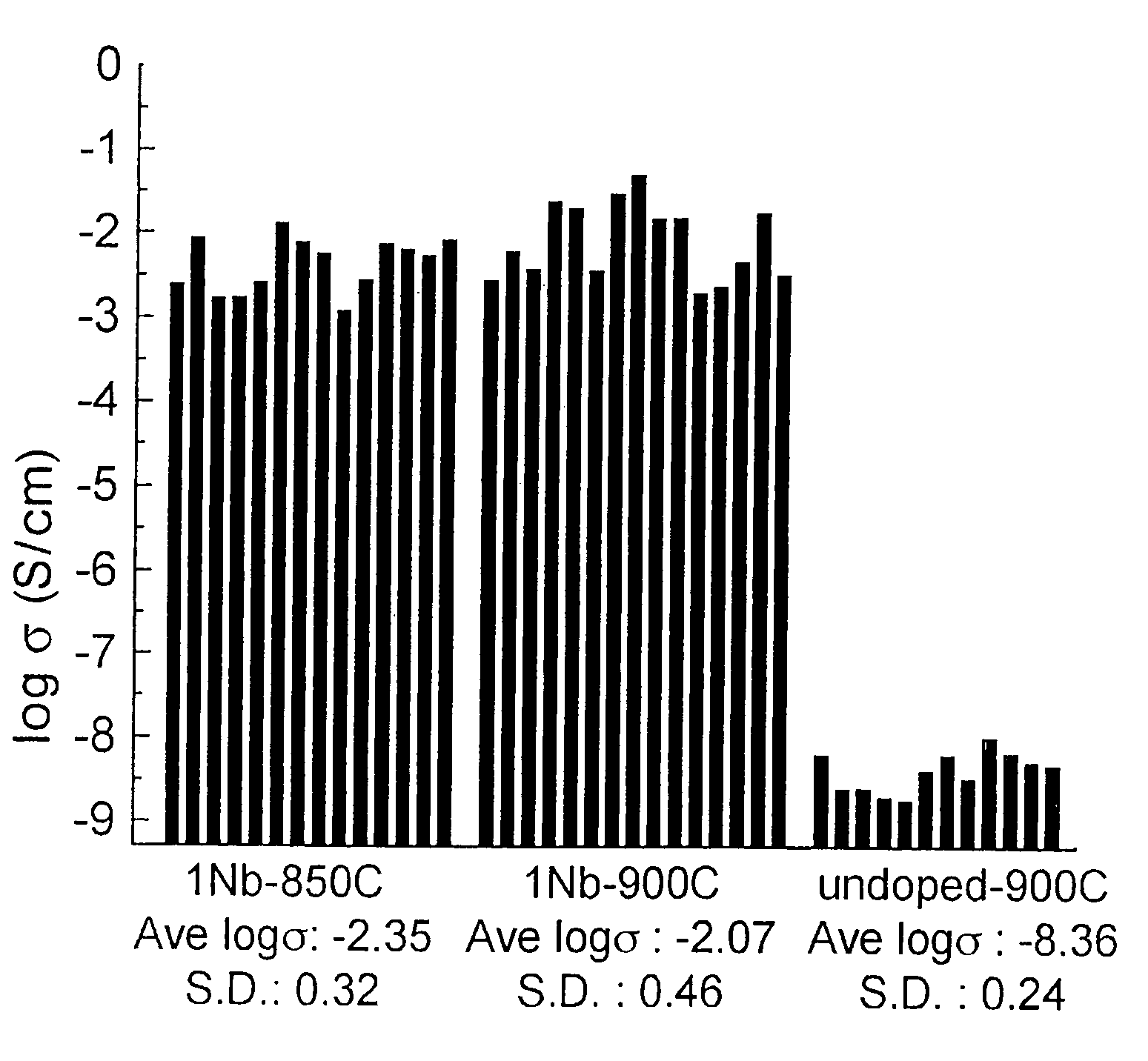
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
High energy lithium ion batteries with particular negative electrode compositions
ActiveUS20090305131A1Degree of crystallinity of will decreaseAlkaline accumulatorsElectrode manufacturing processesHigh energyMetal alloy
Combinations of materials are described in which high energy density active materials for negative electrodes of lithium ion batteries. In general, metal alloy / intermetallic compositions can provide the high energy density. These materials can have moderate volume changes upon cycling in a lithium ion battery. The volume changes can be accommodated with less degradation upon cycling through the combination with highly porous electrically conductive materials, such as highly porous carbon and / or foamed current collectors. Whether or not combined with a highly porous electrically conductive material, metal alloy / intermetallic compositions with an average particle size of no more than a micron can be advantageously used in the negative electrodes to improve cycling properties.
Owner:IONBLOX INC
Si/c composite, anode active materials, and lithium battery including the same
ActiveUS20090029256A1Improve initial Coulombic efficiencyGood capacity retentionLiquid surface applicatorsElectrode manufacturing processesLithium-ion batteryPorous silicon
An Si / C composite includes carbon (C) dispersed in porous silicon (Si) particles. The Si / C composite may be used to form an anode active material to provide a lithium battery having a high capacity and excellent capacity retention.
Owner:SAMSUNG SDI CO LTD
Powder material, electrode structure using the powder material, and energy storage device having the electrode structure
InactiveUS20080003503A1Fast chargingIncrease energy densityNon-metal conductorsElectrode manufacturing processesElectrical conductorHigh energy
A powder material which can electrochemically store and release lithium ions rapidly in a large amount is provided. In addition, an electrode structure for an energy storage device which can provide a high energy density and a high power density and has a long life, and an energy storage device using the electrode structure are provided. In a powder material which can electrochemically store and release lithium ions, the surface of particles of one of silicon metal and tin metal and an alloy of any thereof is coated by an oxide including a transition metal element selected from the group consisting of W, Ti, Mo, Nb, and V as a main component. The electrode structure includes the powder material. The battery device includes a negative electrode having the electrode structure, a lithium ion conductor, and a positive electrode, and utilizes an oxidation reaction of lithium and a reduction reaction of lithium ion.
Owner:CANON KK
Conductive graphene polymer binder for electrochemical cell electrodes
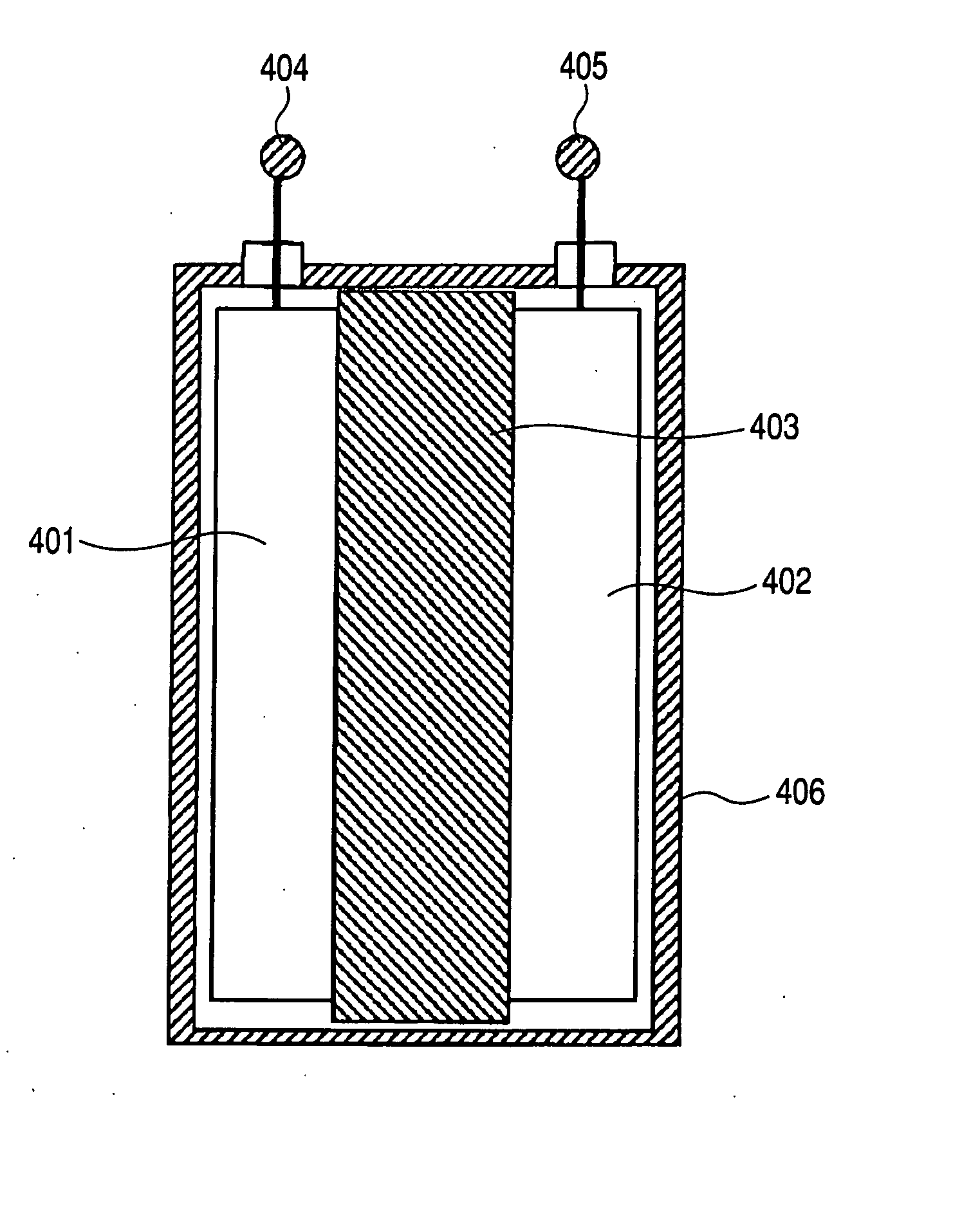
The present invention provides an electrically conductive electrode comprising particles of an electroactive material and a conductive graphene polymer binder that bonds multiple particles of the electroactive material together, wherein the binder is in an amount of from 0.01% to 90% by weight based on the total electrode weight. Also provided are (a) a precursor solution or suspension to the graphene polymer binder for the electrode; (b) a paste containing electroactive particles and a graphene polymer dispersed in a liquid; (c) a method of producing the electrode from the precursor paste; and (d) an electrochemical cell (a battery or supercapacitor) containing such an electrode.
Owner:GLOBAL GRAPHENE GRP INC
Electrode structure for lithium secondary battery and secondary battery having such electrode structure
InactiveUS20060127773A1Small capacity reductionLarge capacityElectrode manufacturing processesFinal product manufactureElectrochemical responseConductive polymer
In an electrode structure for a lithium secondary battery including: a main active material layer formed from a metal powder selected from silicon, tin and an alloy thereof that can store and discharge and capable of lithium by electrochemical reaction, and a binder of an organic polymer; and a current collector, wherein the main active material layer is formed at least by a powder of a support material for supporting the electron conduction of the main active material layer in addition to the metal powder and the powder of the support material are particles having a spherical, pseudo-spherical or pillar shape with an average particle size of 0.3 to 1.35 times the thickness of the main active material layer. The support material is one or more materials selected from a group consisting of graphite, oxides of transition metals and metals that do not electrochemically form alloy with lithium. Organic polymer compounded with a conductive polymer is used for the binder. There are provided an electrode structure for a lithium secondary battery having a high capacity and a long lifetime, and a lithium secondary battery using the electrode structure and having a high capacity, a high energy density and a long lifetime.
Owner:CANON KK
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS20100086853A1Electrode manufacturing processesAlkali metal oxidesDischarge rateLithium-ion battery
Owner:IONBLOX INC
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
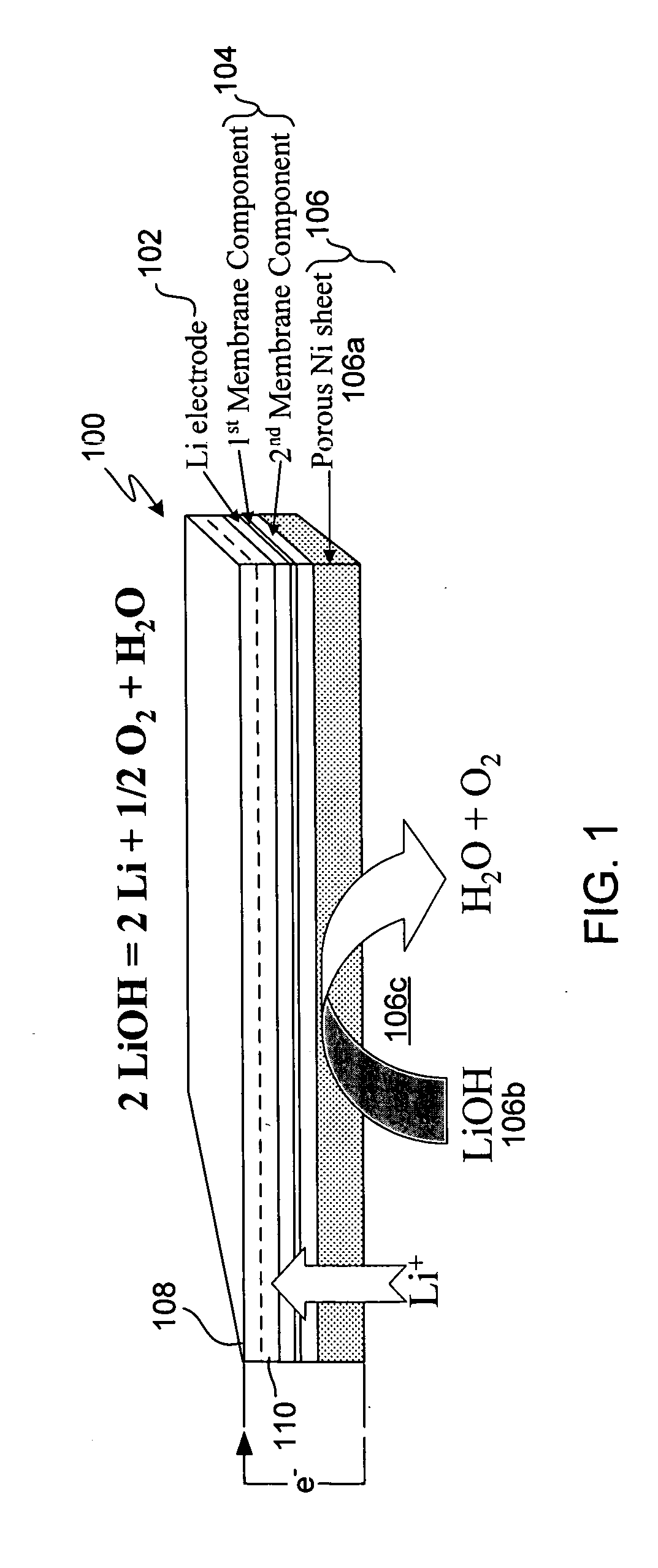
Compliant seal structures for protected active metal anodes
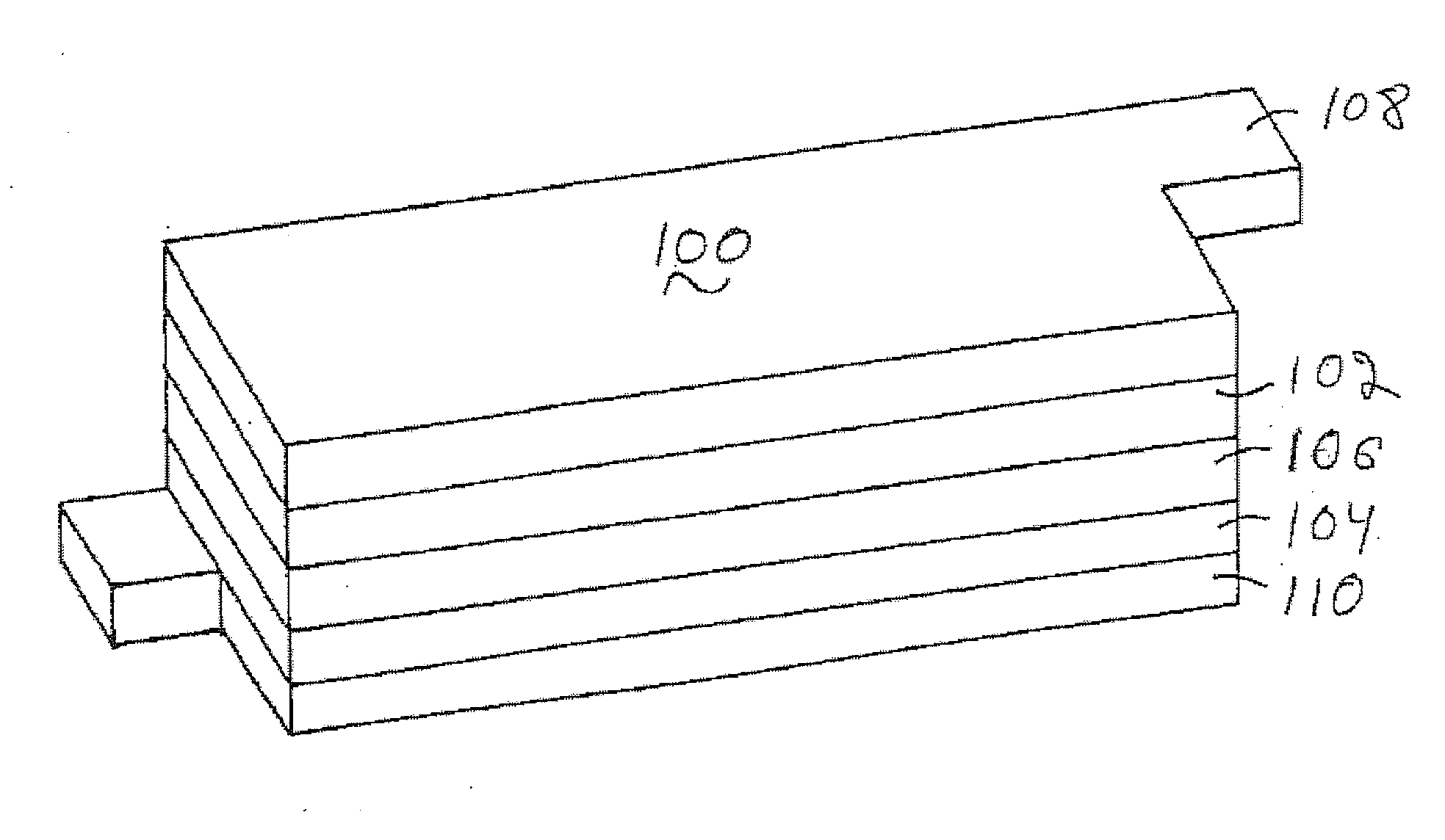
ActiveUS20070037058A1Reduced ionic contact areaReduce the total massPrimary cell to battery groupingElectrode manufacturing processesOptoelectronicsAnodic protection
Protected anode architectures have ionically conductive protective membrane architectures that, in conjunction with compliant seal structures and anode backplanes, effectively enclose an active metal anode inside the interior of an anode compartment. This enclosure prevents the active metal from deleterious reaction with the environment external to the anode compartment, which may include aqueous, ambient moisture, and / or other materials corrosive to the active metal. The compliant seal structures are substantially impervious to anolytes, catholyes, dissolved species in electrolytes, and moisture and compliant to changes in anode volume such that physical continuity between the anode protective architecture and backplane are maintained. The protected anode architectures can be used in arrays of protected anode architectures and battery cells of various configurations incorporating the protected anode architectures or arrays.
Owner:POLYPLUS BATTERY CO INC
Negative active material for a rechargeable lithium battery, a method of preparing the same, and a rechargeable lithium battery comprising the same
ActiveUS20050233213A1Improved cycle-life characteristic and charge and discharge characteristicIncrease chanceElectrode manufacturing processesNon-aqueous electrolyte accumulatorsHigh rateSilicon oxide
The present invention relates to a negative active material for a rechargeable lithium battery, which includes a silicon-based composite having a silicon oxide of the form SiOX where x≦1.5 and at least one element selected from the group consisting of B, P, Li, Ge, Al, and V, and a carbonaceous material. The negative active material of the present invention can improve the cycle-life and high-rate charge / discharge characteristics of a rechargeable lithium battery.
Owner:SAMSUNG SDI CO LTD
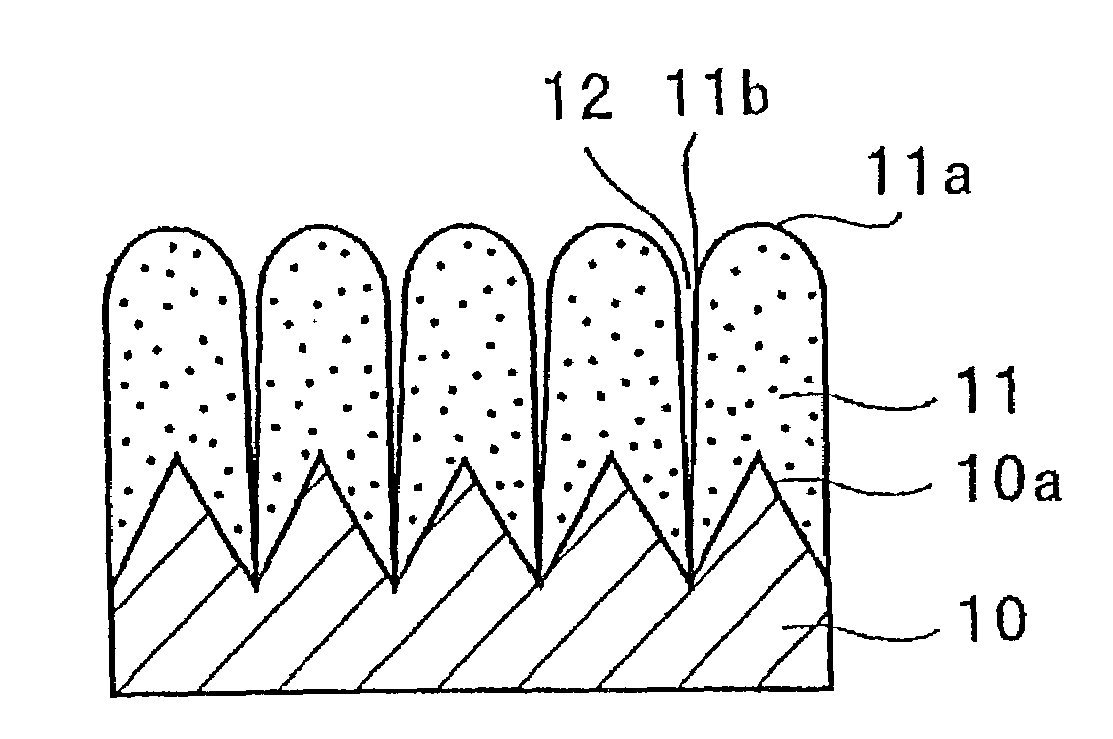
Electrode for rechargeable lithium battery and rechargeable lithium battery
InactiveUS7192673B1Improve charge and discharge cycle characteristicsInhibition formationElectrode manufacturing processesSmall-sized cells cases/jacketsAmorphous siliconMaterials science
An electrode for a rechargeable lithium battery which includes a thin film composed of active material that expands and shrinks as it stores and releases lithium, e.g., a microcrystalline or amorphous silicon thin film, deposited on a current collector, characterized in that said current collector exhibits a tensile strength (=tensile strength (N / mm2) per sectional area of the current collector material×thickness (mm) of the current collector) of not less than 3.82 N / mm.
Owner:SANYO ELECTRIC CO LTD
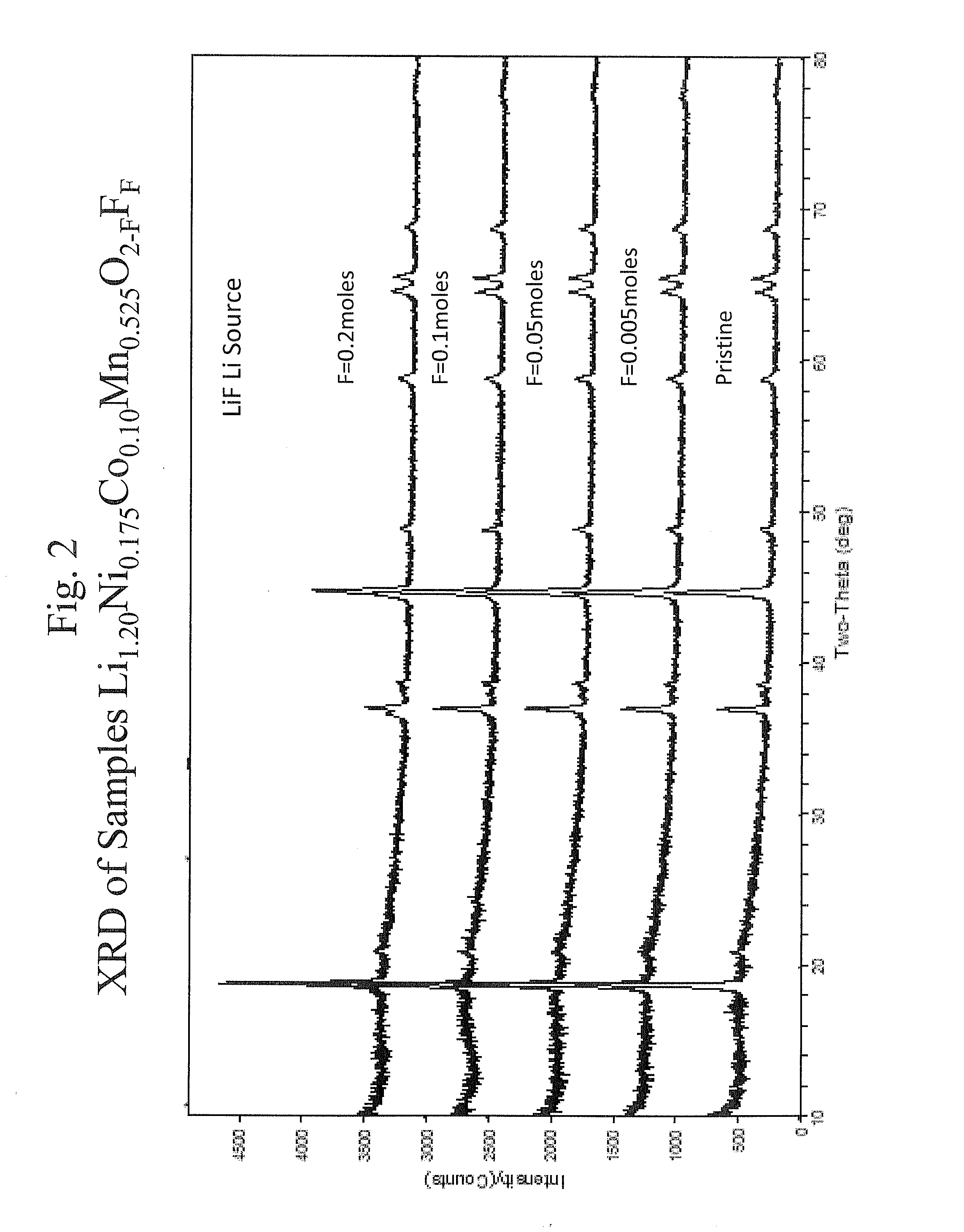
Fluorine doped lithium rich metal oxide positive electrode battery materials with high specific capacity and corresponding batteries
ActiveUS20100086854A1Desirable battery performanceElectrode manufacturing processesFinal product manufactureLithiumDopant
Lithium rich metal oxyfluorides are described with high specific capacity and, good cycling properties. The materials have particularly good high rate capabilities. The fluorine dopant can be introduced in a low temperature process to yield the materials with desirable cycling properties. In some embodiments, the positive electrode active materials have a composition represented approximately by the formula Li1+xNiαMnβCoγAδO2−zFz where:x is from about 0.02 to about 0.19,α is from about 0.1 to about 0.4,β is from about 0.35 to about 0.869,γ is from about 0.01 to about 0.2,δ is from 0.0 to about 0.1 andz is from about 0.01 to about 0.2,where A is Mg, Zn, Al, Ga, B, Zr, Ti, Ca, Ce, Y, Nb or combinations thereof.
Owner:IONBLOX INC
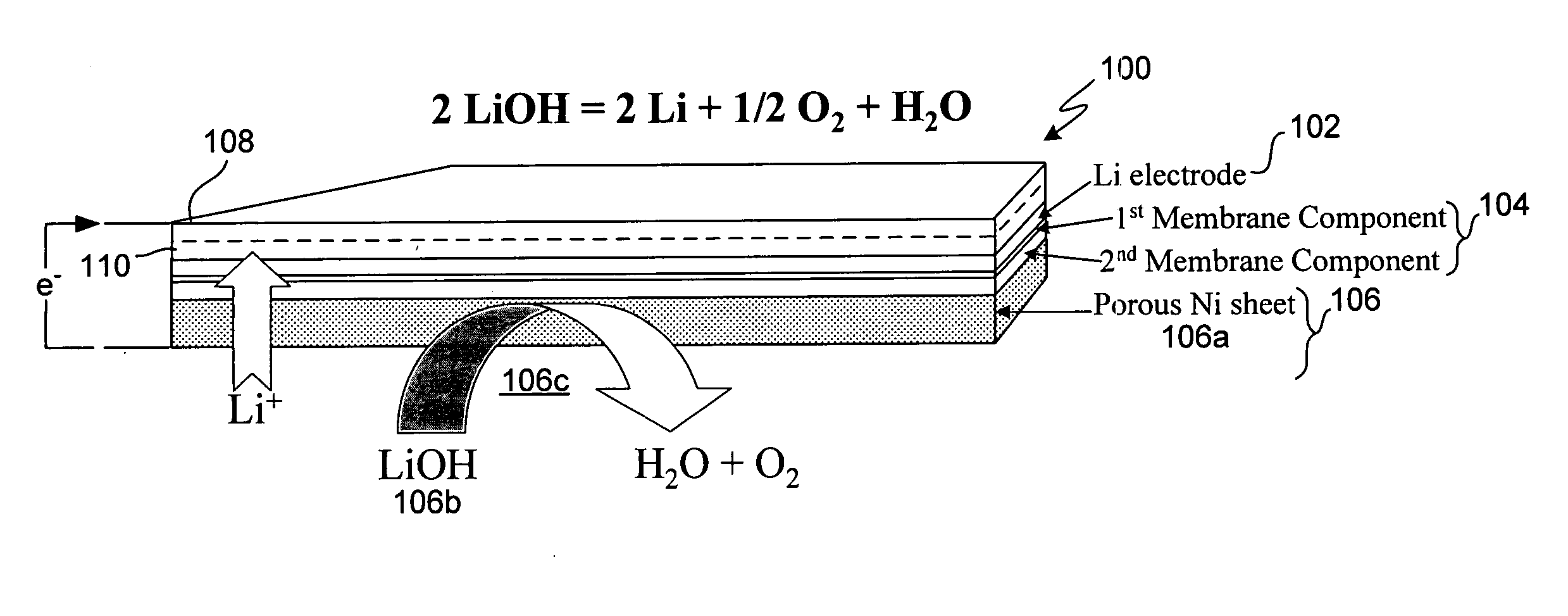
Active metal electrolyzer
InactiveUS20050100793A1Effective isolationReduce environmental pollutionPhotography auxillary processesElectrode manufacturing processesElectrolysisAqueous electrolyte
Electro-winning of active metal (e.g., lithium) ions from a variety of sources including industrial waste, and recycled lithium and lithium-ion batteries is accomplished with an electrolyzer having a protected cathode that is stable against aggressive solvents, including water, aqueous electrolytes, acid, base, and a broad range of protic and aprotic solvents. The electrolyzer has a highly ionically conductive protective membrane adjacent to the alkali metal cathode that effectively isolates (de-couples) the alkali metal electrode from solvent, electrolyte processing and / or cathode environments, and at the same time allows ion transport in and out of these environments. Isolation of the cathode from other components of a battery cell or other electrochemical cell in this way allows the use of virtually any solvent, electrolyte and / or anode material in conjunction with the cathode. The electrolyzer can be configured and operated to claim or reclaim lithium or other active metals from such sources.
Owner:POLYPLUS BATTERY CO INC
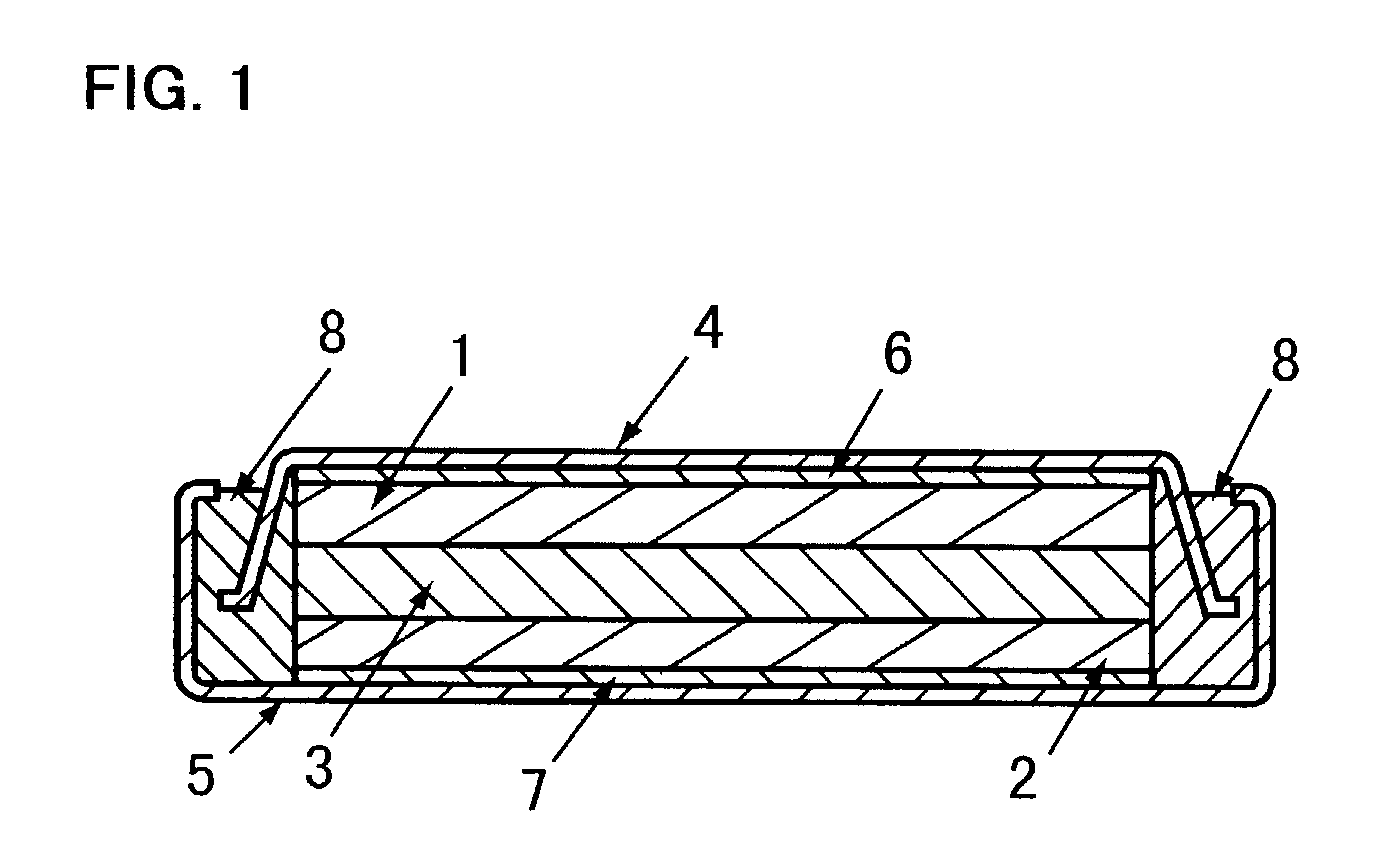
Three dimensional free form battery apparatus
A battery apparatus comprising a casing, at least two stacked lithium ion cells a member for maximizing the utilization of the casing and a member for precluding inadvertent deformation of the casing. The casing includes a non-uniform inner periphery. Each of at least two stacked lithium ion cells is positioned within the casing. The utilization maximizing member maximizes the utilization of the inner periphery of the casing by facilitating the independent shaping of each of the at least two stacked lithium ion cells to confirm to the inner periphery. As a result, the shape of one cell does not limit or dictate the shape of any other cell. The deformation precluding member is associated with each of the at least two lithium ion cells, and, substantially precludes inadvertent deformation of the casing by the at least two lithium ion cells, during cell cycling and storage. The invention further includes a process for fabricating a battery apparatus.
Owner:MITSUBISHI CHEM CORP
Electrode for use in lithium battery and rechargeable lithium battery
InactiveUS7235330B1High charge and discharge capacityImprove featuresElectrode manufacturing processesSmall-sized cells cases/jacketsAmorphous siliconSurface roughness
Owner:SANYO ELECTRIC CO LTD
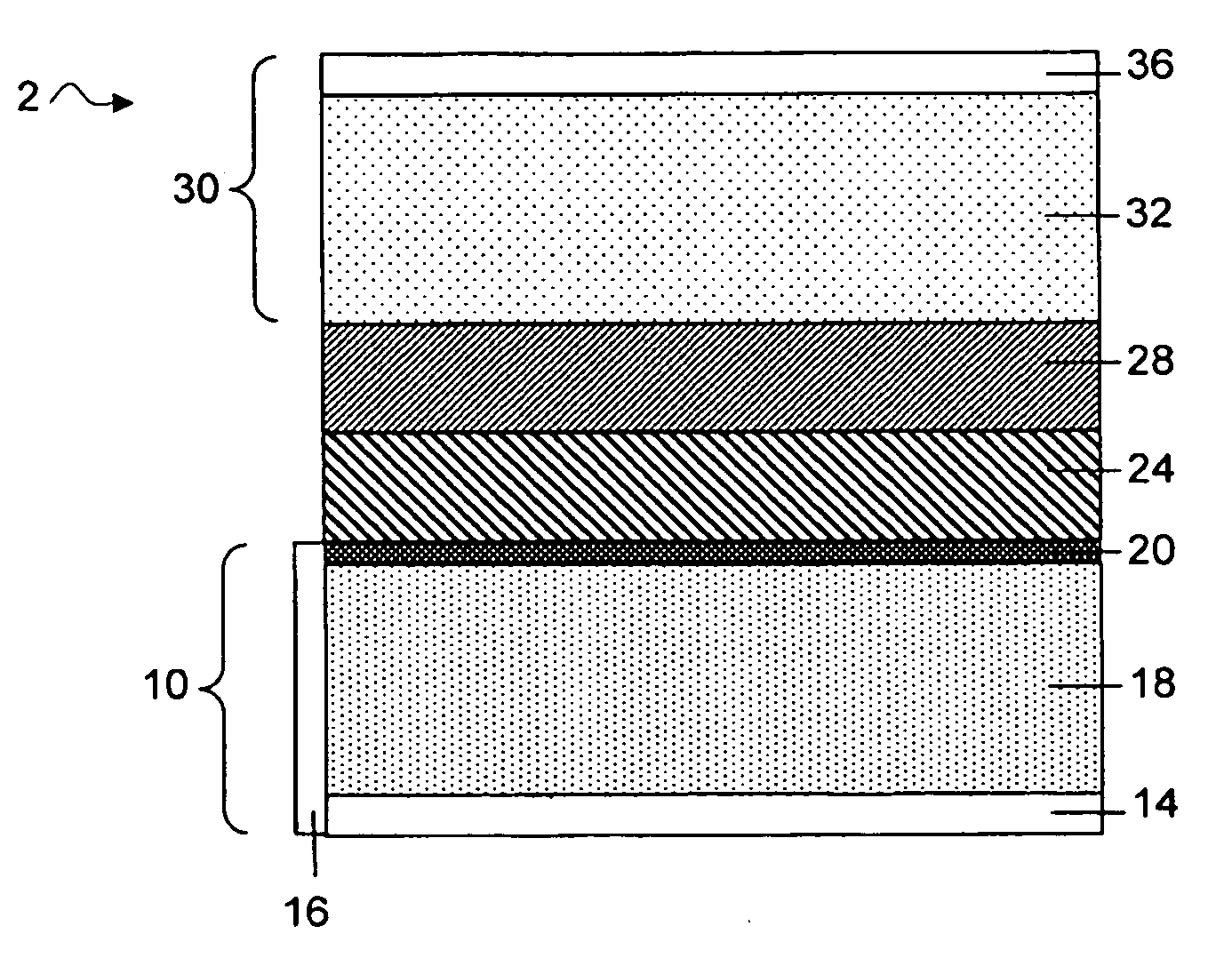
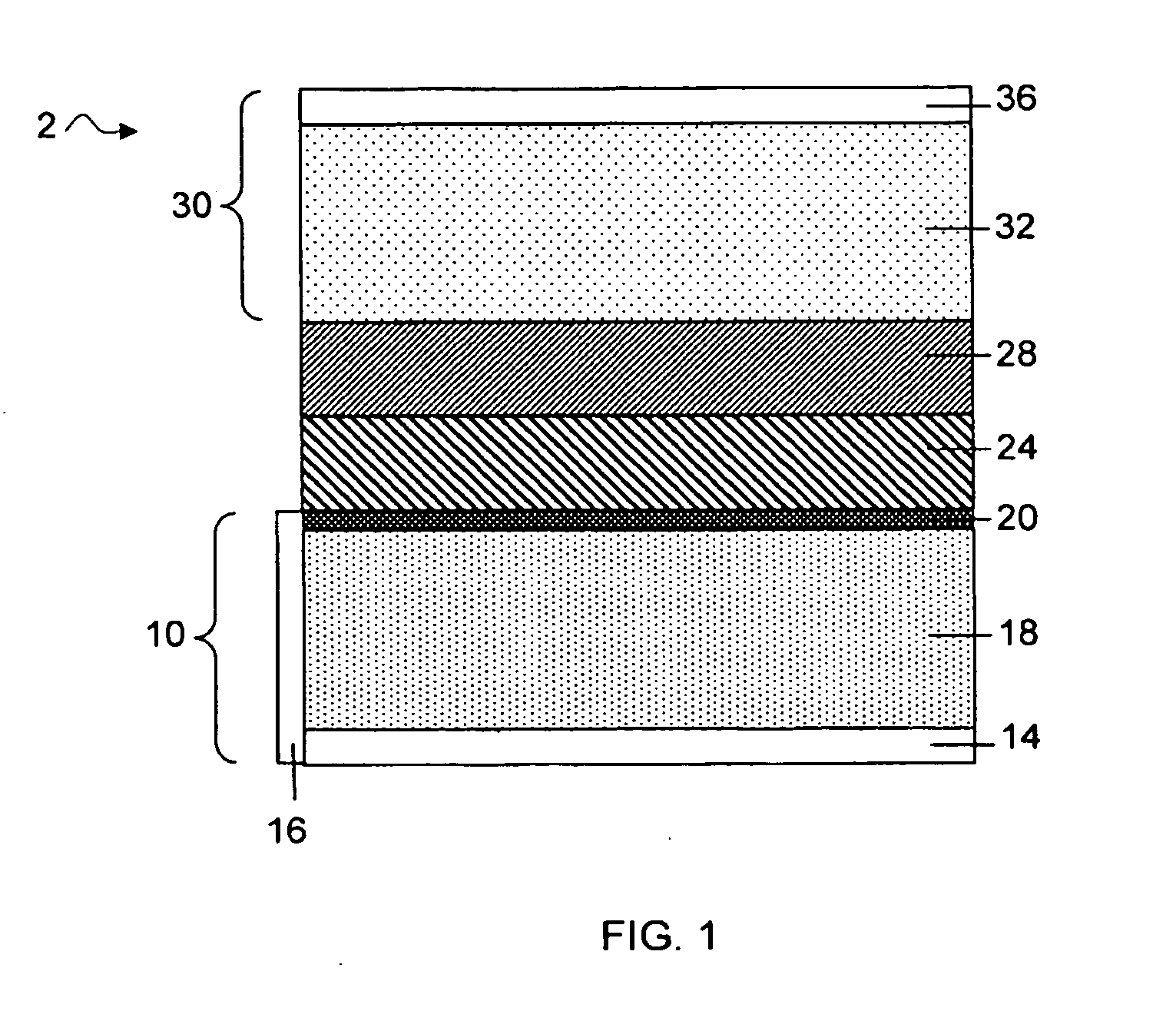
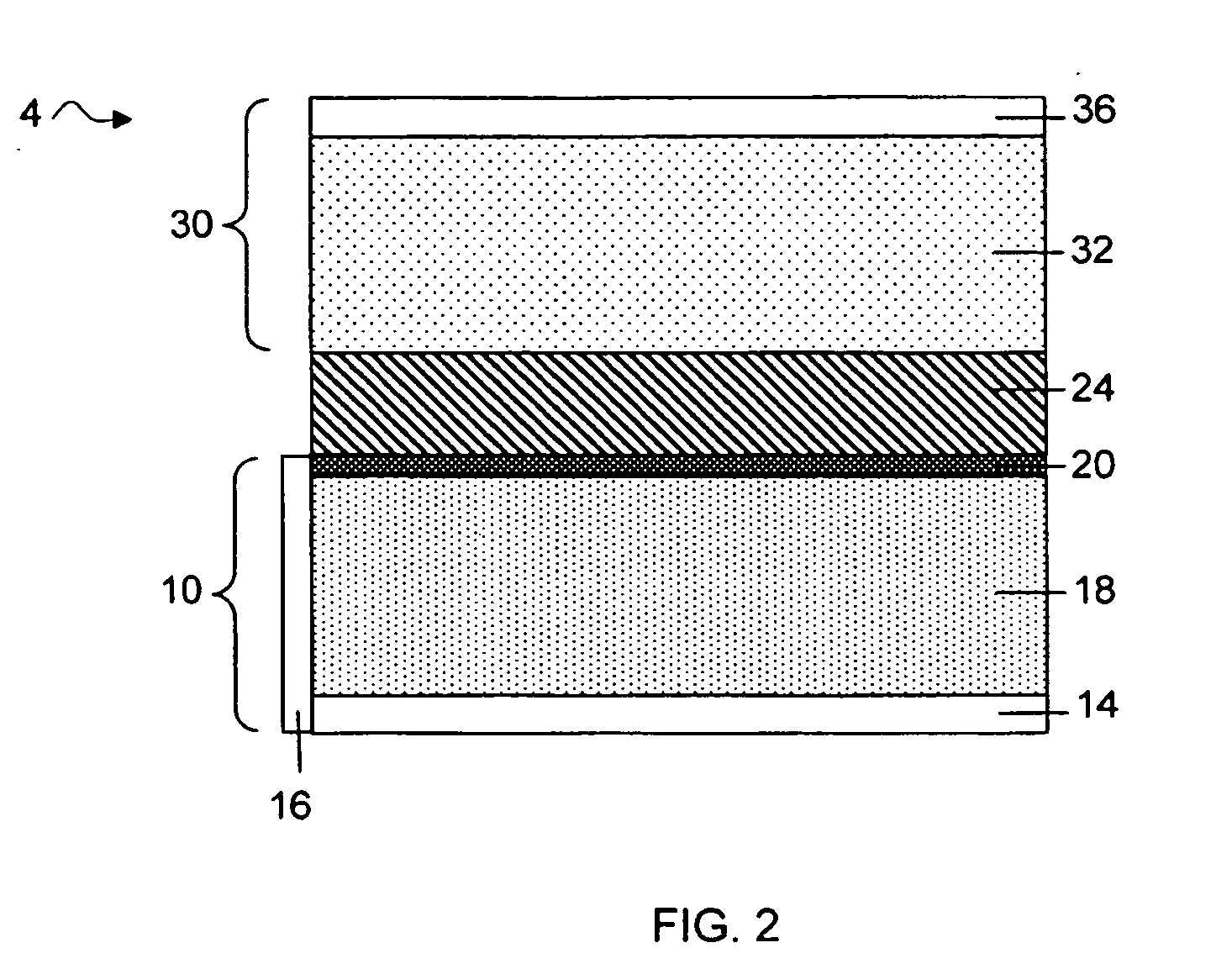
Methods and systems for making electrodes having at least one functional gradient therein and devices resulting therefrom
InactiveUS20110123866A1Improve performanceElectrode manufacturing processesElectric shock equipmentsHigh fluxEngineering
The invention disclosed herein provides for methods and apparatuses that yield electrodes having at least one functional gradient therein. In many embodiments, the electrodes comprise an electrode matrix having a plurality of layers, where at least two of the layers differs functionally, in composition, structure, or, organization. High-throughput electrode screening apparatuses are disclosed that include array formers and testers. Electrodes and battery cells arising from the methods and apparatuses disclosed herein are likewise disclosed. The methods, apparatuses, and resulting electrode and cell devices are, in some embodiments, ideally suited for use in lithium-ion batteries.
Owner:MOLECULAR NANOSYST
Lithium transition metal-based compound powder for positive electrode material in lithium rechargeable battery, method for manufacturing the powder, spray dried product of the powder, firing precursor of the powder, and positive electrode for lithium rechargeable battery and lithium rechargeable battery using the powder
ActiveUS20090104530A1Low costImprove security levelElectrode manufacturing processesNon-aqueous electrolyte accumulator electrodesMetalHigh voltage
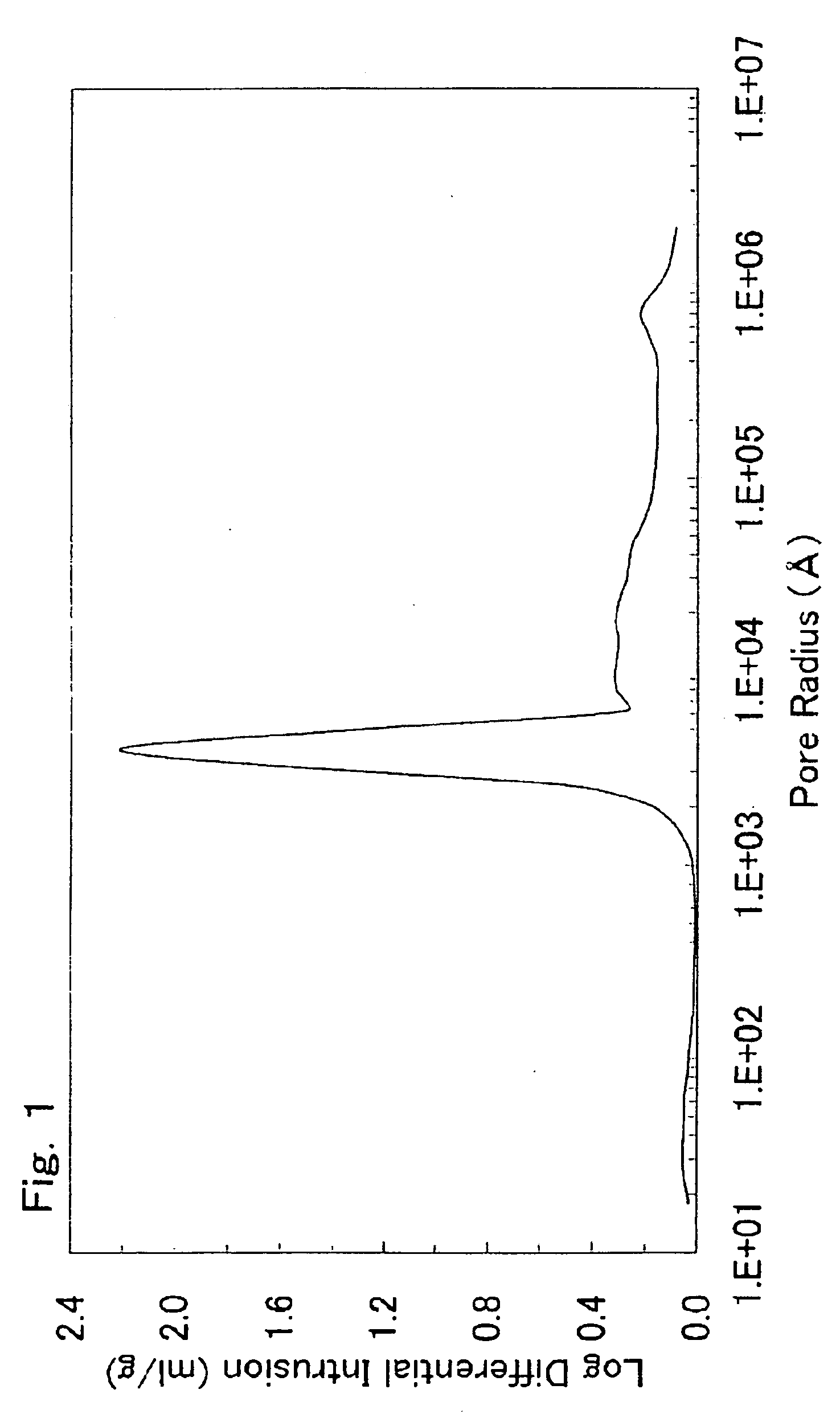
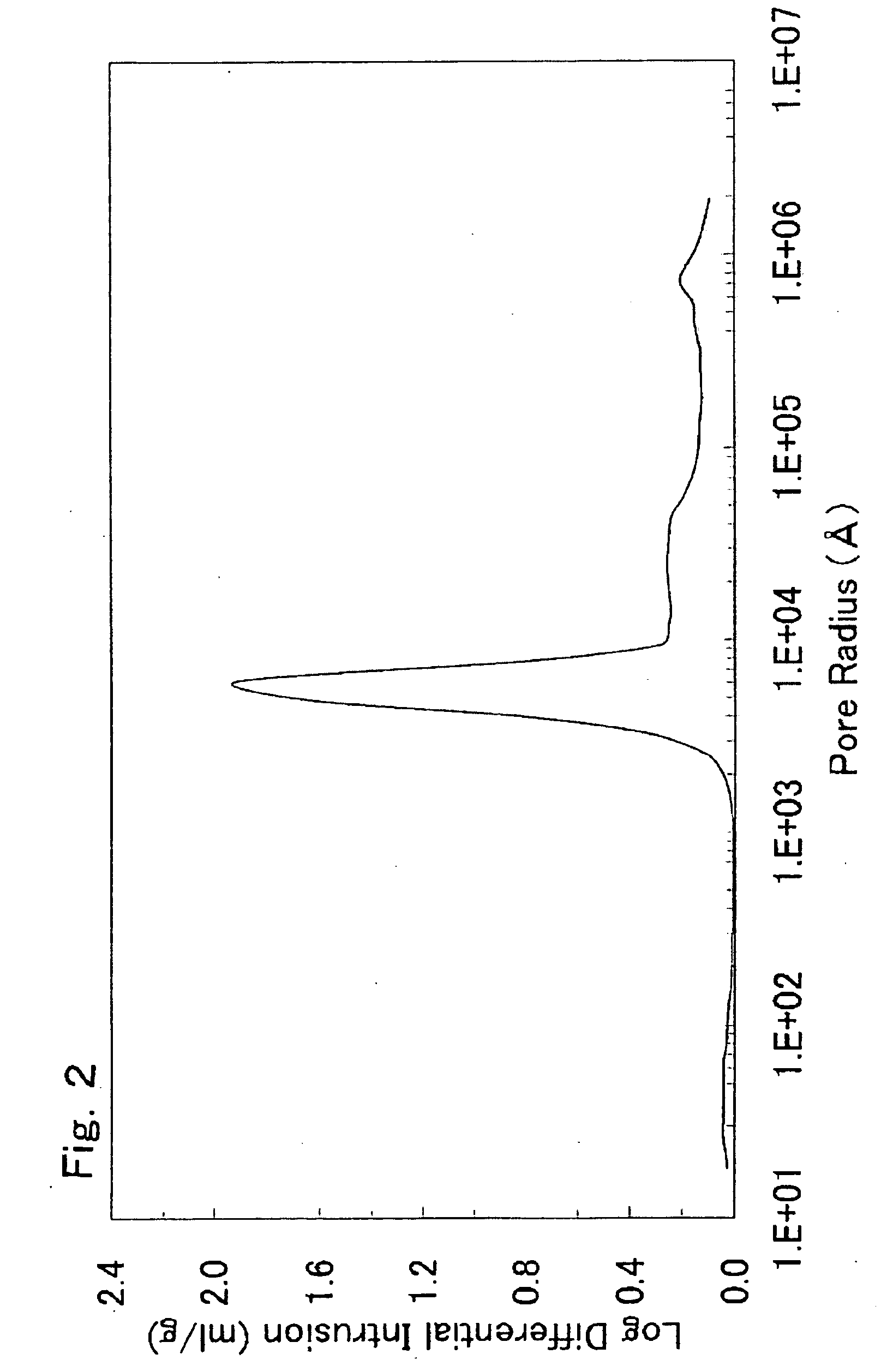
There is provided a powder of a lithium transition-metal compound for a positive-electrode material in a lithium secondary battery, in which the use of the powder as that of a positive-electrode material in a lithium secondary battery achieves a good balance among improvement in battery performance, cost reduction, resistance to a higher voltage, and a higher level of safety. The powder of the lithium transition-metal compound for a positive-electrode material in a lithium secondary battery is characterized in that in a mercury intrusion curve obtained by mercury intrusion porosimetry, the amount of mercury intruded is in the range of 0.8 cm3 / g to 3 cm3 / g when the pressure is increased from 3.86 kPa to 413 MPa.
Owner:MITSUBISHI CHEM CORP
Layered barrier structure having one or more definable layers and method
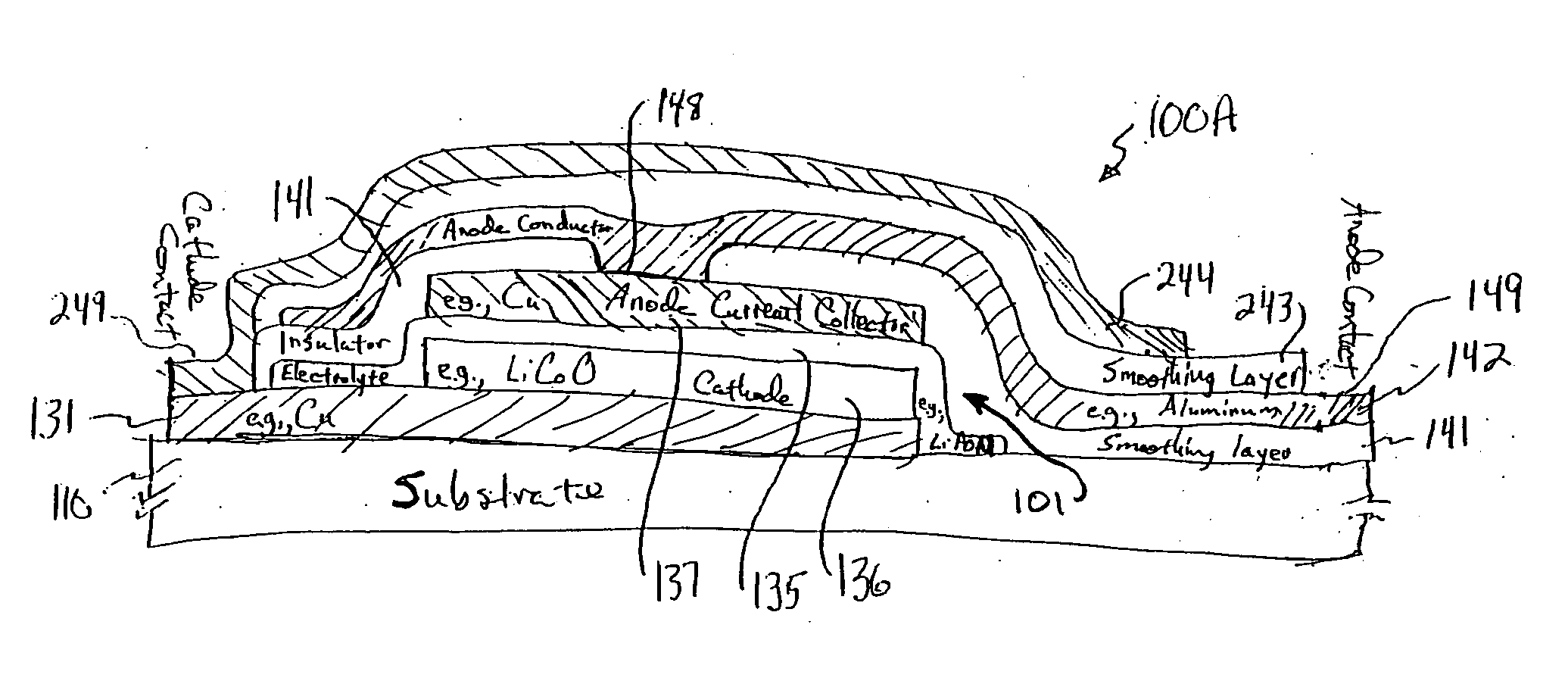
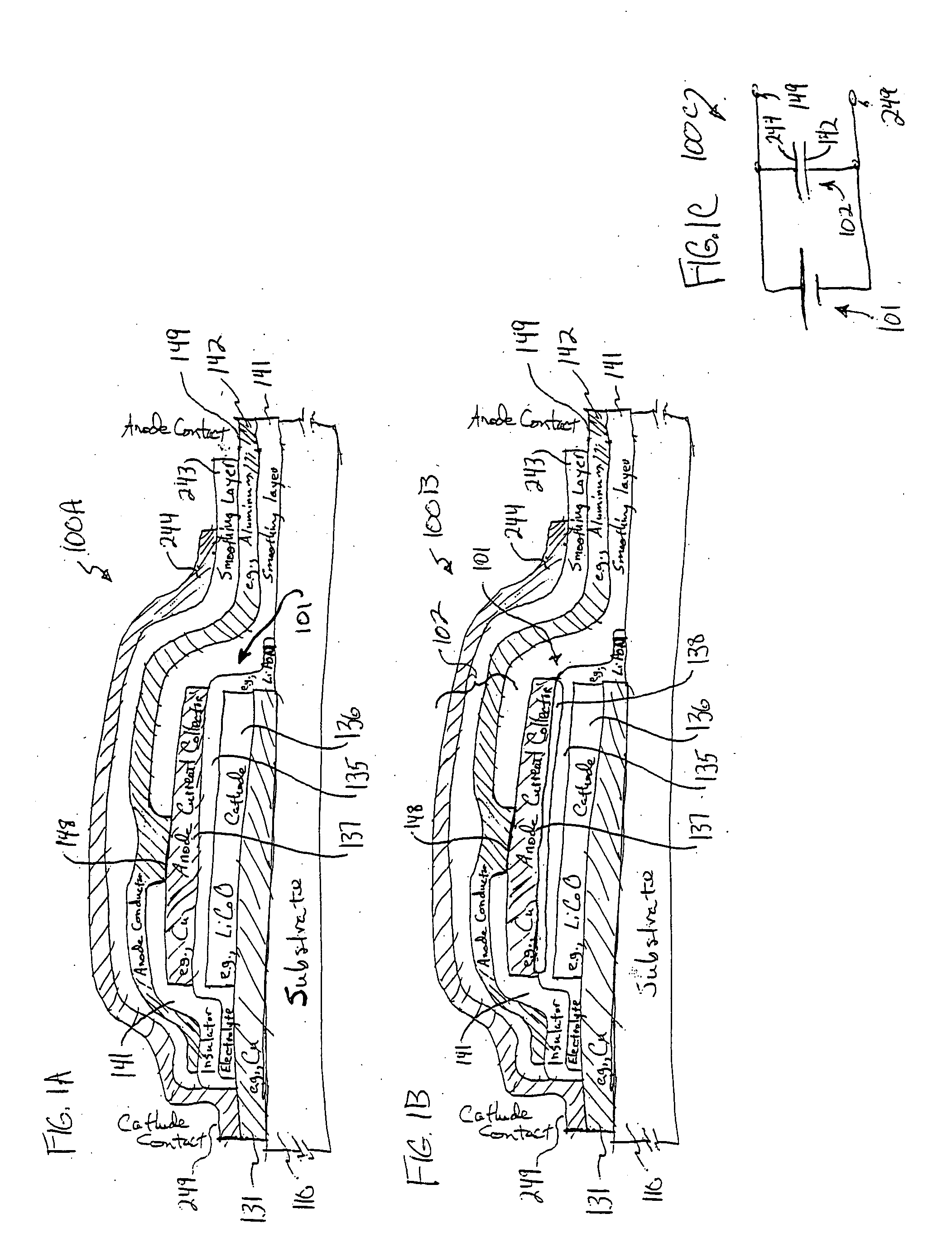
InactiveUS20050147877A1Reduce transmissionLow rateFuel and primary cellsElectrode manufacturing processesDielectricLithium compound
A system provides an environmental barrier also useful for providing a circuit, for example, one having a thin-film battery such as one that includes lithium or lithium compounds connected to an electronic circuit. An environmental barrier is deposited as alternating layers, at least one of the layers providing a smoothing, planarizing, and / or leveling physical-configuration function, and at least one other layer providing a diffusion-barrier function. The layer providing the physical-configuration function may include a photoresist, a photodefinable, an energy-definable, and / or a maskable layer. The physical-configuration layer may also be a dielectric. A layered structure, including a plurality of pairs of layers, each pair including a physical configuration layer and a barrier layer with low gas-transmission rates, may be used in reducing gas transmission rate to beyond currently detectable levels.
Owner:CYMBET CORP
Method for producing carbon coated nano stage lithium iron phosphate by precipitation
InactiveCN101393982AAvoid synthetic stepsEasy to controlElectrode manufacturing processesIron saltsPhosphate
The invention discloses a precipitation method for preparing nanometer level iron phosphate lithium coated with carbon. The method comprises the following steps: firstly, weighing iron salt, deionized water and a compound of metallic elements; after the stirring and the mixing are performed, adding a phosphorous compound and citric acid diluted with water to the mixture; after the stirring is performed again, adding a precipitation agent to the mixture and controlling to the neutrality; stirring to react in a container, and after the static placement, respectively adding the deionized water, a carbon source and lithium salt to mix uniformly after the precipitate is filtered and washed; stirring again to react, and drying the water at 30 to 160 DEG C and warming up at the heating rate under the protection of non-oxidized gas after a product is crashed; baking at a constant temperature of 450 to 850 DEG C, cooling down to a room temperature at a cooling rate or with a stove, and finally obtaining the nanometer level ferric phosphate lithium coated with the carbon after crashing is performed. The precipitation method has the advantage that the raw material cost and the processing cost are low because bivalent iron is taken as the raw material. The iron phosphate lithium prepared by using the process has the characteristics of good physical processing performance and good electrochemistry performance, and is suitable for industrialized production.
Owner:南京海泰纳米材料有限公司
Plating technique for electrode
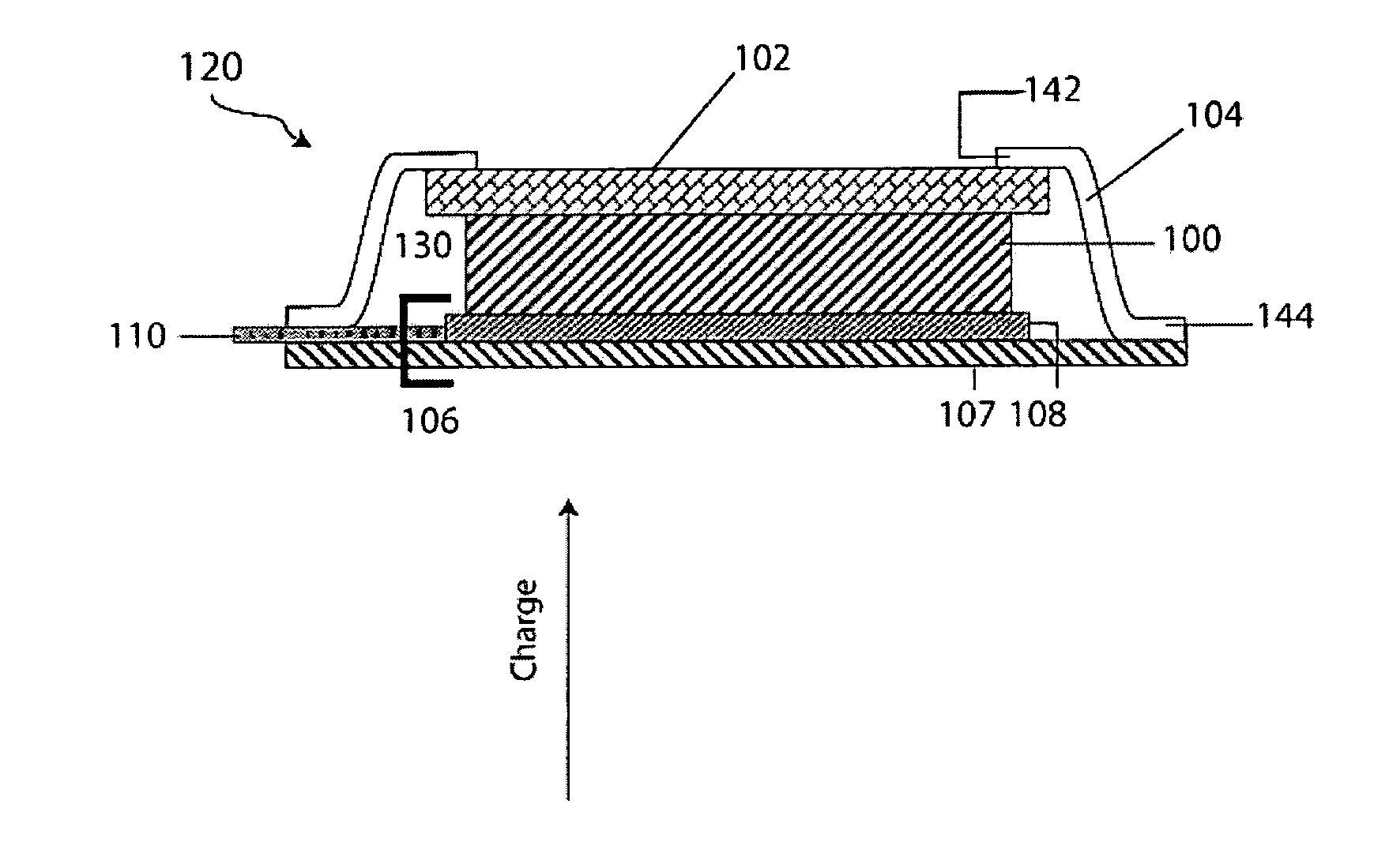
ActiveUS20130017441A1Electrode manufacturing processesFinal product manufactureElectrical batteryEngineering
Articles and methods for forming protected electrodes for use in electrochemical cells, including those for use in rechargeable lithium batteries, are provided. In some embodiments, the articles and methods involve an electrode that does not include an electroactive layer, but includes a current collector and a protective structure positioned directly adjacent the current collector, or separated from the current collector by one or more thin layers. Lithium ions may be transported across the protective structure to form an electroactive layer between the current collector and the protective structure. In some embodiments, an anisotropic force may be applied to the electrode to facilitate formation of the electroactive layer.
Owner:SION POWER CORP
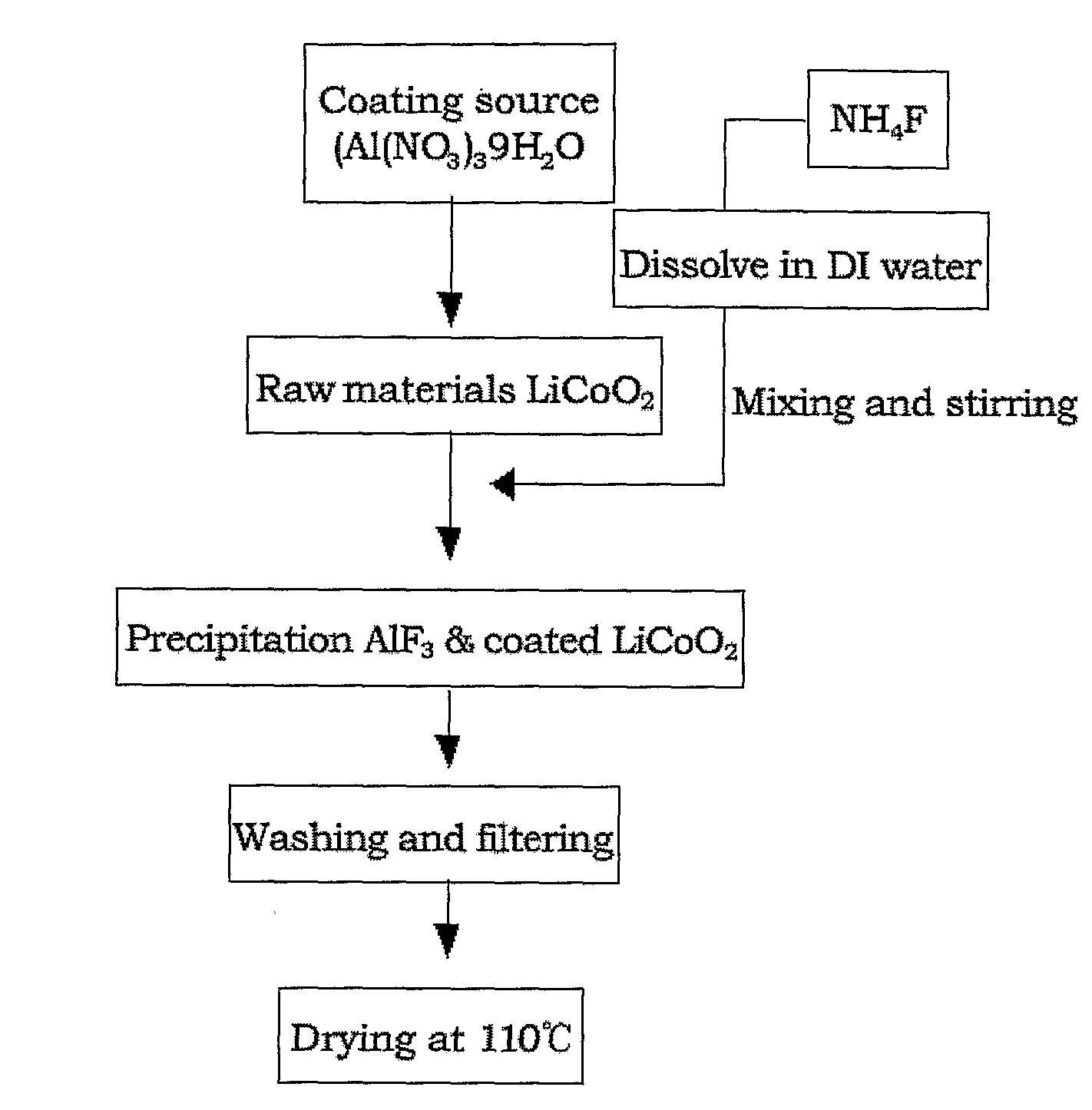
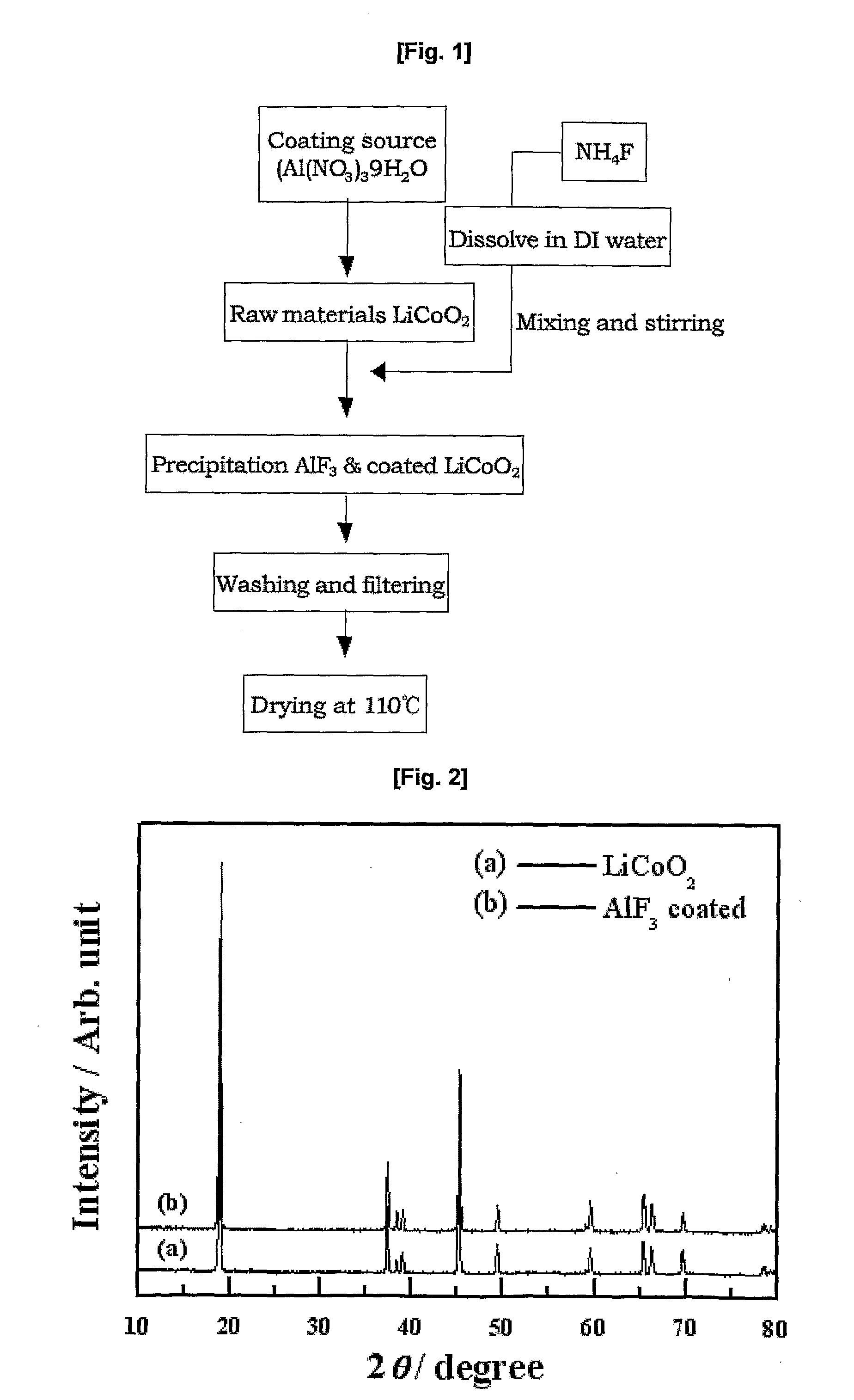
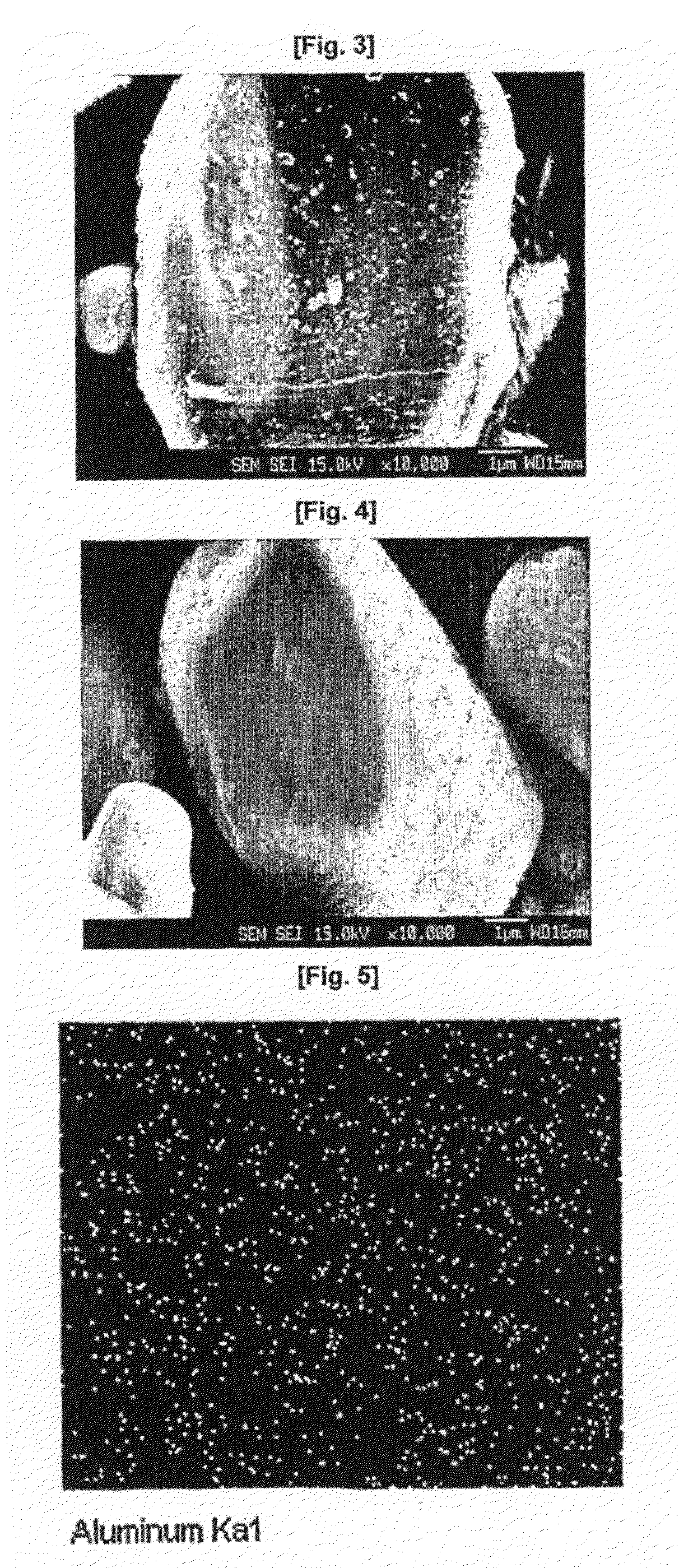
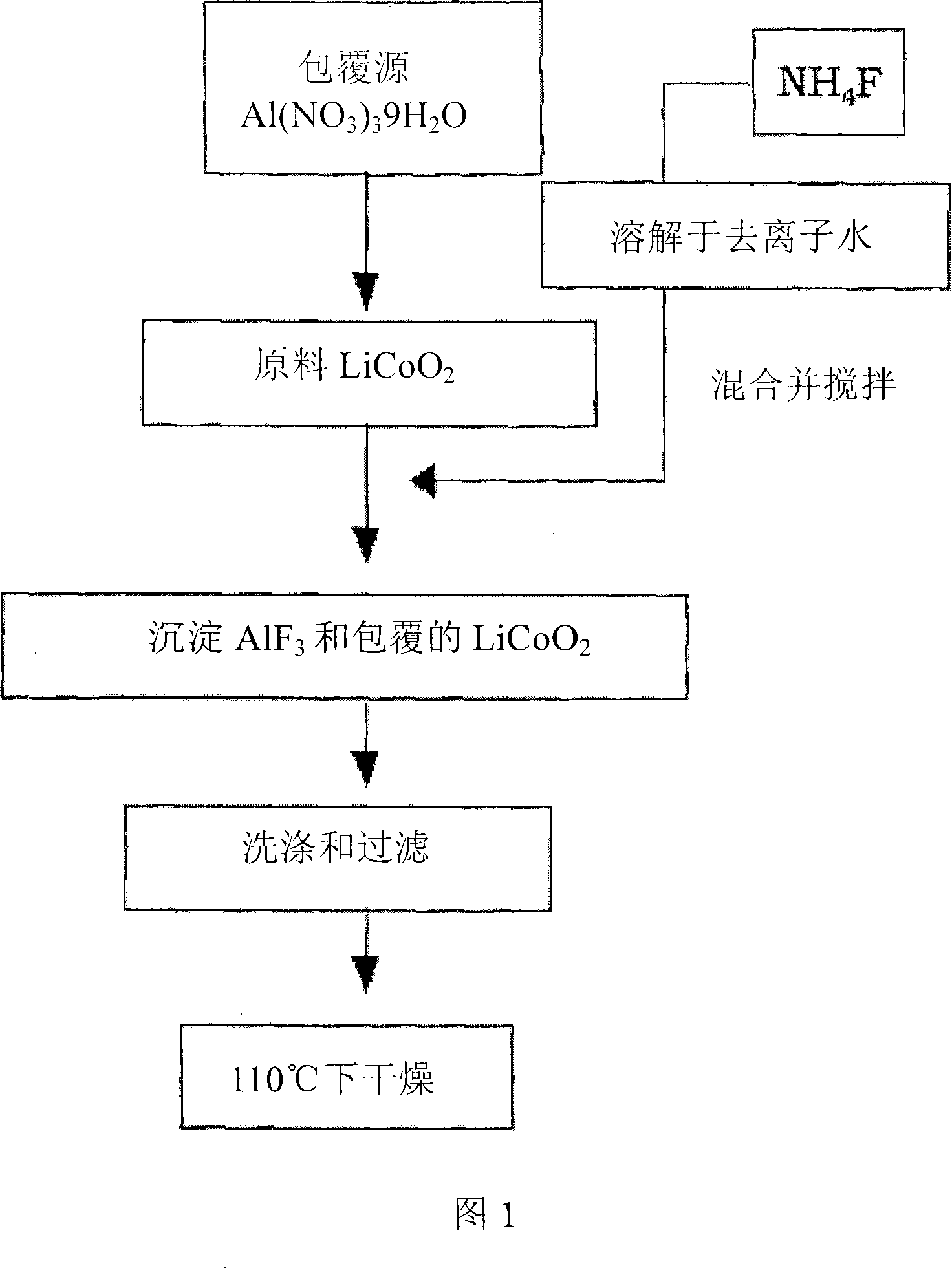
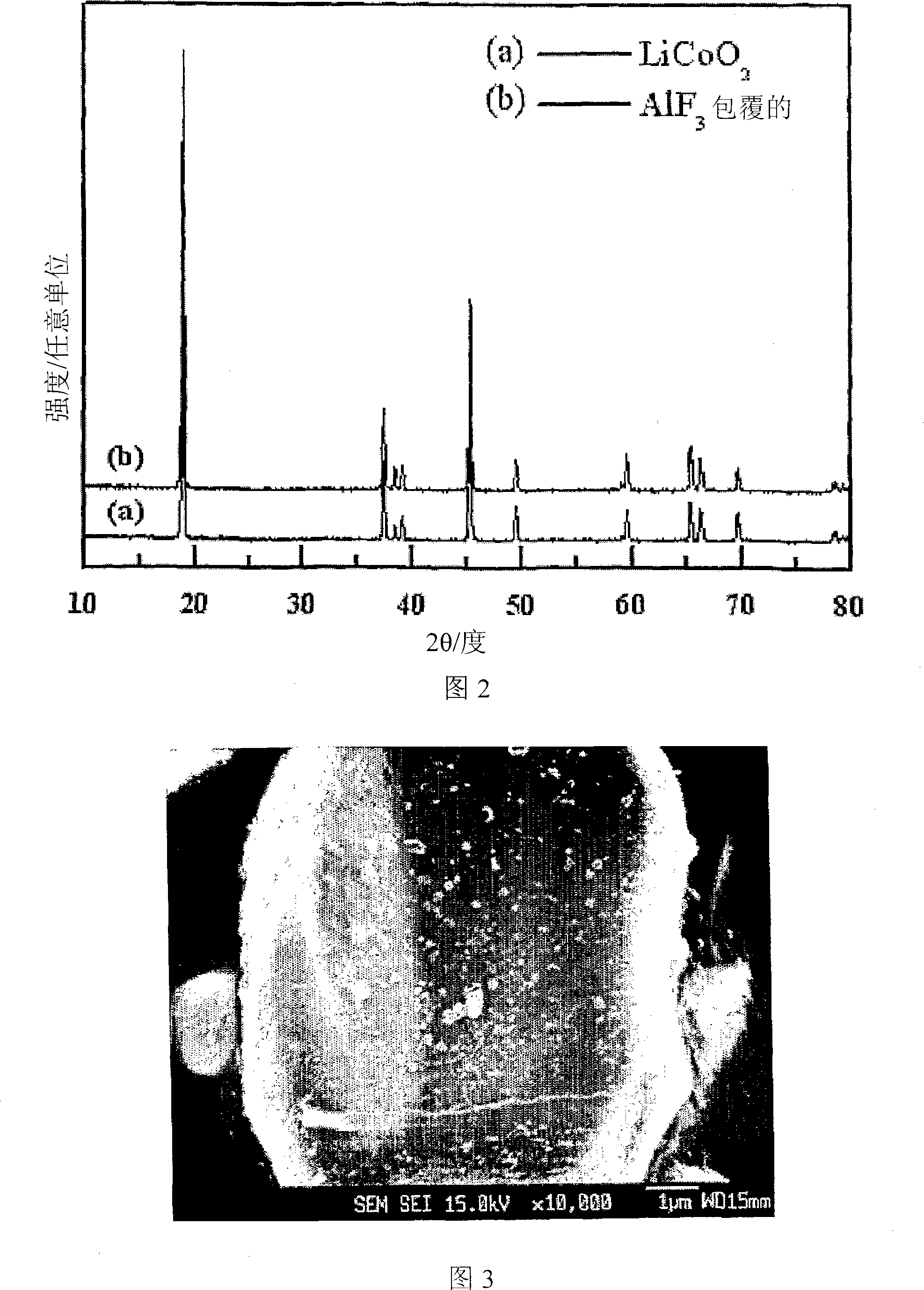
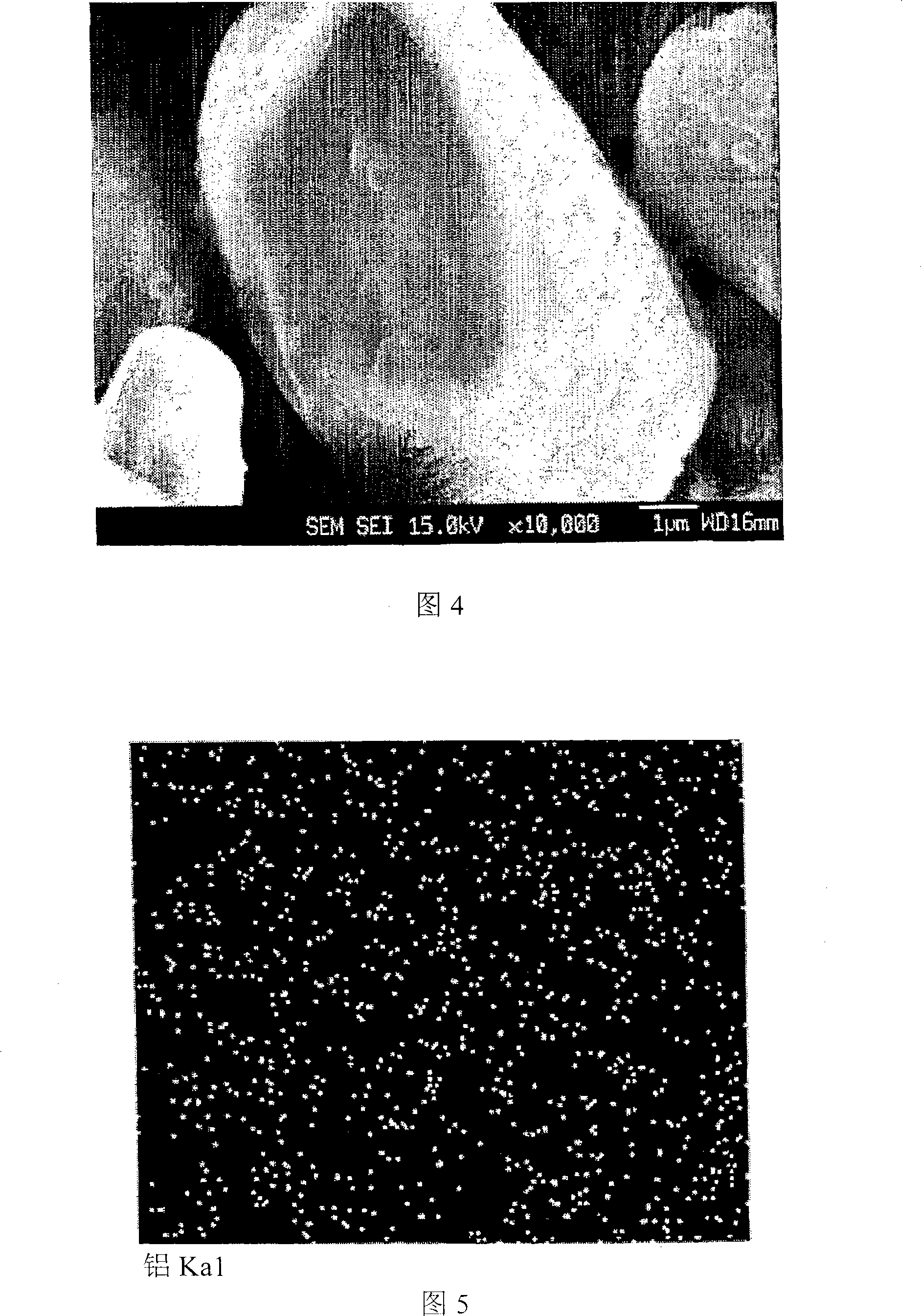
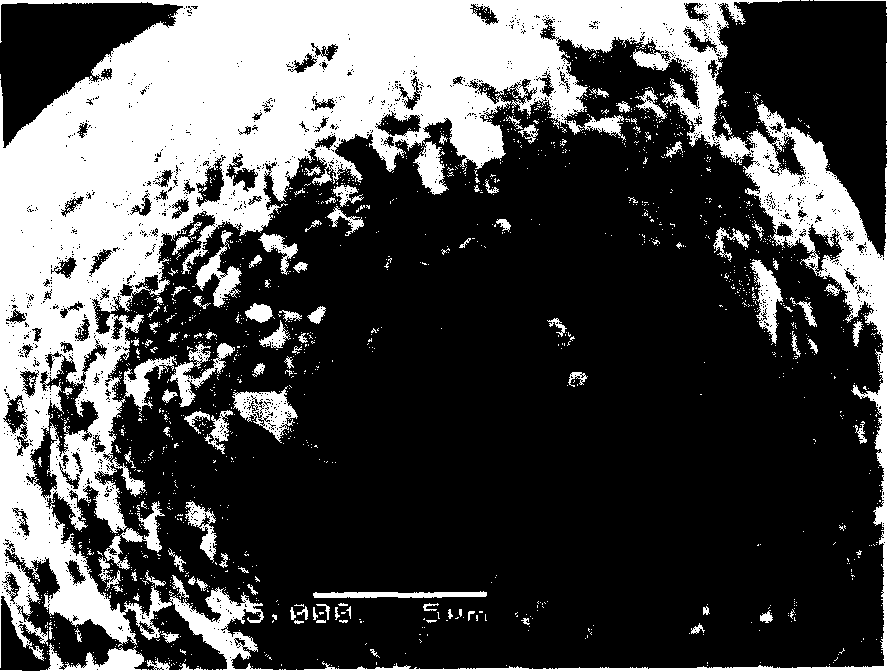
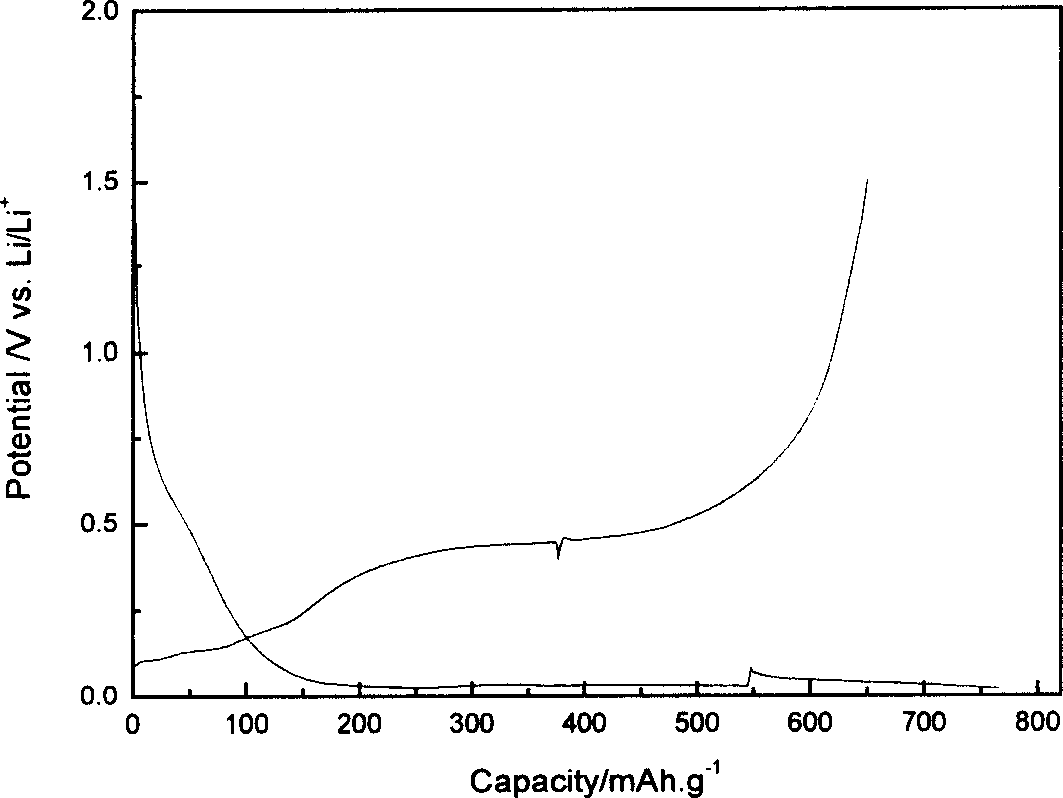
Cathode Active Material Coated With Fluorine Compound for Lithium Secondary Batteries and Method for Preparing the Same
InactiveUS20090087362A1Inhibition of performance deteriorationHigh voltageElectrode manufacturing processesLithium compoundsLithiumHigh rate
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Cathode active material coated with fluorine compound for lithium secondary batteries and method for preparing the same
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Silicon carbone compound negative polar material of lithium ion battery and its preparation method
ActiveCN1913200AHigh electrochemical reversible lithium storage capacityImprove cycle stabilityElectrode manufacturing processesSecondary cellsAmount of substanceSpecific volume
This invention discloses a silicon carbon compound negative material and its preparation method for Li ionic batteries, which takes silicon and carbon phase compound particles as the base of sphericity or its like covered by a carbon layer. The preparation method includes: crushing the carbon phase particles to be mixed with silicon phase particles and sized to become a compound particle matrix to be covered with the precursor of the organic pyrolyzed carbon then to be carbonized and crushed. Compared with the current technology, this invention takes the compound material of Si and C phase particles as the matrix covered by a compound negative material, in which, the reversible specific volume of which is greater than 450mAh / g, the first circulation coulomb efficiency is greater than 85% and the volume holding rate for 200 times is greater than 80% to greatly reduce the volume effect of the Si activated material when absorbing and discharging Li and improve the diffusion performance of Li in activated materials.
Owner:BTR NEW MATERIAL GRP CO LTD
Titanium-series cathode active material and preparation method thereof, titanium-series lithium ion power battery
ActiveCN101373829AIncrease capacityHigh bulk densityElectrode manufacturing processesLi-accumulatorsHigh rateLithium titanate
The invention discloses a titanium cathode active substance, a preparation method thereof and a titanium lithium ion power battery, and aims to solve the technical problem of enhancing the rate performance of a lithium ion power battery. The formula of the titanium cathode active substance is Li4Ti5O12 / Mx, wherein Li4Ti5O12 is spinel lithium titanate, M is a dopant such as a metal simple substance, a metal compound, a nonmetallic simple substance or a nonmetallic compound; the elements or the ions contained in the dopant enter the Li4Ti5O12 crystal lattice or are compounded with the Li4Ti5O12 crystal lattice; and the preparation method comprises the following steps: the precursor mixture of compound lithium titanate is prepared, and spray drying and heat treatment are performed. The cathode of the titanium lithium ion power battery adopts Li4Ti5O12 / Mx. Compared with the prior art, the titanium cathode active substance has the advantages of high capacity, high bulk density, high volume specific capacity, good high-rate performance, good product uniformity, good battery processability, low possibility of air bulking of the battery, and low cost.
Owner:BTR NEW MATERIAL GRP CO LTD
Method for preparing nanometer ferrous phosphate lithium /carbon composite material
InactiveCN101582498ARich sourcesLow priceElectrode manufacturing processesCapacitanceCarbon composites
The invention belongs to energy materials, particularly relating to a method for preparing nanometer ferrous phosphate lithium / carbon composite material. In the invention, ferrous source, lithium source, phosphorus source are mixed with a small quantity of doped metal salt and organic macromolecular polymer carbon source according to certain ratio followed by the steps of ball milling, parching and calcining. High temperature sintering is carried out on the above mixture in the atmosphere of non-oxidation gas to obtain nanometer lithium iron phosphate LiMxFe(1-x)PO4 / C coated with carbon and LiFe(1-x)NxPO4 / C material, and the particle sizes of which are remarkably reduced and are less than 100nm. When the material is applied to battery assemble, 0.2C multiplying power discharge capacity can reach above 160mAh / g at room temperature, 1C multiplying power discharge capacity can be 140-155mAh / g, and 5C multiplying power discharge capacity is 130-150mAh / g. the initial capacity is 120-140mAh / g under the large multiplying power of 10C, and remains more than 90% through thousands of cycles, demonstrating good multiplying power and cycle properties. The invention features low cost, simple production process and fine safety. The prepared nanometer ferrous phosphate lithium / carbon composite material can be widely applied into manufacturing of convenient and fast equipment, electric vehicles and the like.
Owner:NORTHEAST NORMAL UNIVERSITY
Preparation of multi-position doped lithium iron phosphate positive electrode material and application thereof
ActiveCN101339994AWide range of materialsIncrease base capacityElectrode manufacturing processesPhosphorus compoundsElectricityLithium iron phosphate
The invention discloses a preparation method of a multi-place doped lithium iron phosphate anode material and an application thereof, which belong to the technical field of the preparation of electrochemical power materials. The multi-place doped lithium iron phosphate anode material is expressed by the following formula: Li1-xAxFe1-yByP1-zCzO4Ddelta, wherein, at least two of x, y, x and delta can not be expressed zero at the same time. Multi-place doped anode material lithium iron phosphate powder which is used in a secondary lithium-ion battery and has good crystallization performance and even composition is prepared by adopting a solid phase method and a simple mixing and drying process; compared with the method doping in a certain crystal lattice place, the multi-place doped anode material lithium iron phosphate powder has wide doping material source, which can greatly improve the basic capacity and cycling electrical performance of matrix and is applied to a stable industrialized production and non-high-purity materials. The multi-place lithium iron phosphate of the invention is taken as the anode material and is usually used in the secondary lithium-ion battery and the secondary lithium-ion battery is taken as a power source.
Owner:甘肃大象能源科技有限公司
Graphite composite lithium ion battery anode material lithium iron phosphate and preparation method thereof
ActiveCN101562248AImprove electronic conductivityImprove tap densityElectrode manufacturing processesPower batteryChemical Linkage
The invention relates to a graphene composite lithium ion battery anode material lithium iron phosphate and a preparation method thereof. The composite material of lithium iron phosphate and graphene is connected by interface of chemical bonding. The invention also provides the method for preparing the graphene composite lithium ion battery anode material lithium iron phosphate in an in-situ symbiosis reaction mode, and the obtained anode material has high tap density and good magnifying performance, and is suitable to be used as a anode material of a lithium ion power battery.
Owner:龚思源
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com