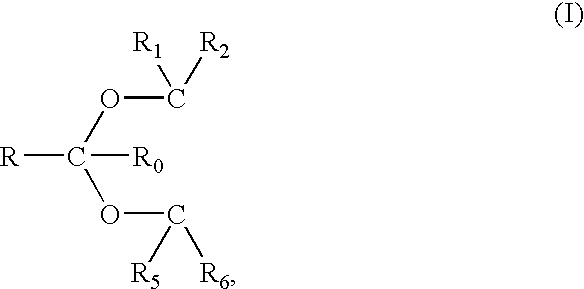Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
671 results about "Dioxolane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dioxolane is a heterocyclic acetal with the chemical formula (CH₂)₂O₂CH₂. It is related to tetrahydrofuran by interchange of one oxygen for a CH₂ group. The corresponding saturated 6-membered C₄O₂ rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-Dioxolane is used as a solvent and as a comonomer in polyacetals.
Separation of electrolytes
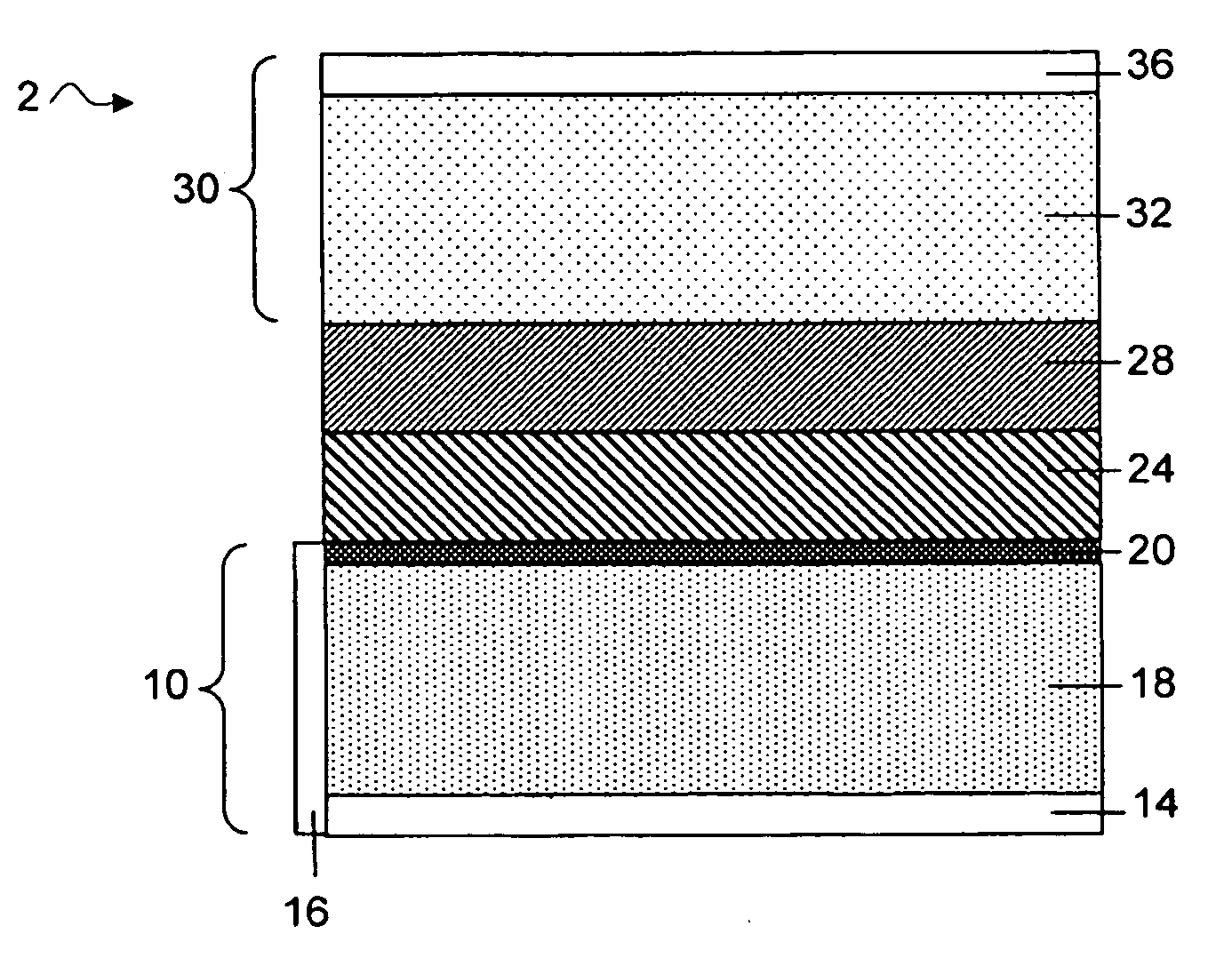
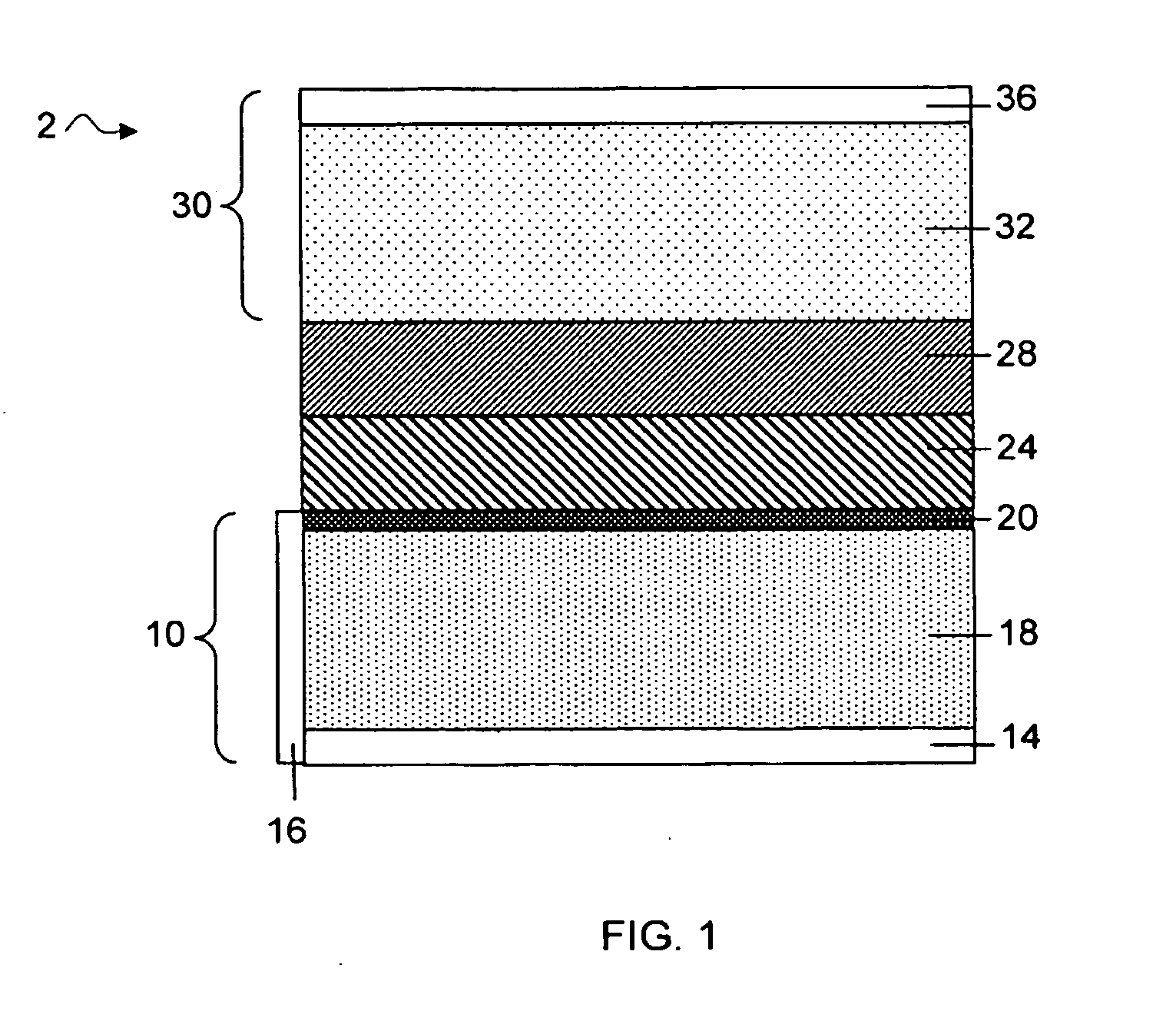
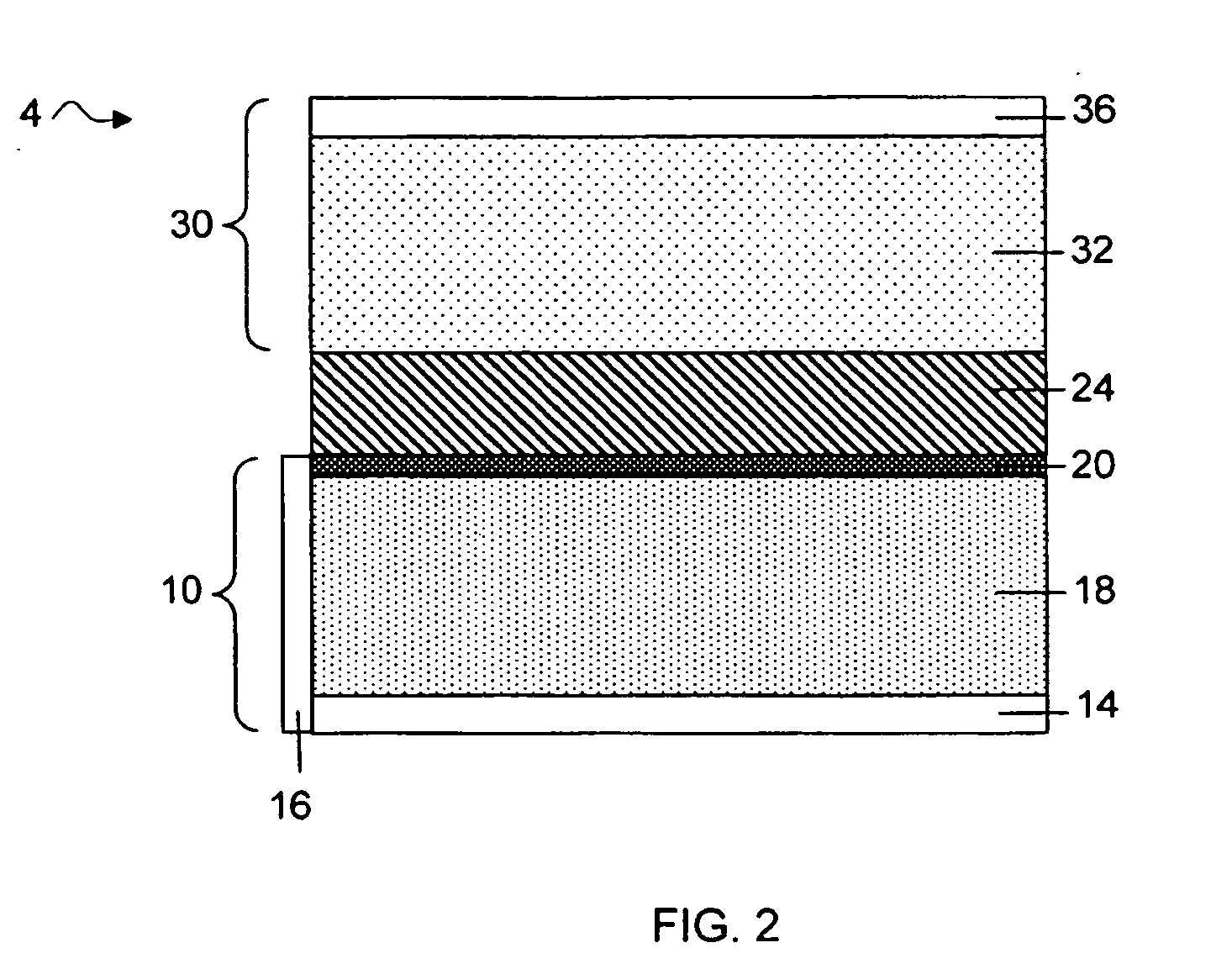
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Antifungal nail lacquer and method using same
InactiveUS6224887B1Effective preventionEffective treatmentBiocideCosmetic preparationsAntifungalLacquer
A nail lacquer effective for the treatment or prevention of fungal infections, such as, onychomycosis, includes fungicidally effective amount of ciclopirox, econazole, or other antifungal agent in a clear, stable, film-forming lacquer vehicle which includes a water-insoluble film-forming polymer; 2-n-nonyl-1,3-dioxolane or similar penetration enhancer; and volatile solvent. A plasticizer for the film-forming polymer which is also compatible with the other components may be included although the preferred penetration enhancers may also function as plasticizer. The composition, when applied to the nails provides a hard, clear, water-resistant film containing the antifungal agent. The film is resistant to multiple washings and is effective in the treatment of onychomycosis.
Owner:MACROCHEM CORP
Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof
A pharmaceutical composition comprising Compound 1, (R)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide, and at least one excipient selected from: a filler, a diluent, a disintegrant, a surfactant, a glidant and a lubricant, the composition being suitable for oral administration to a patient in need thereof to treat a CFTR mediated disease such as Cystic Fibrosis. Methods for treating a patient in need thereof include administering the pharmaceutical composition of Compound 1 are also disclosed.
Owner:VERTEX PHARMA INC
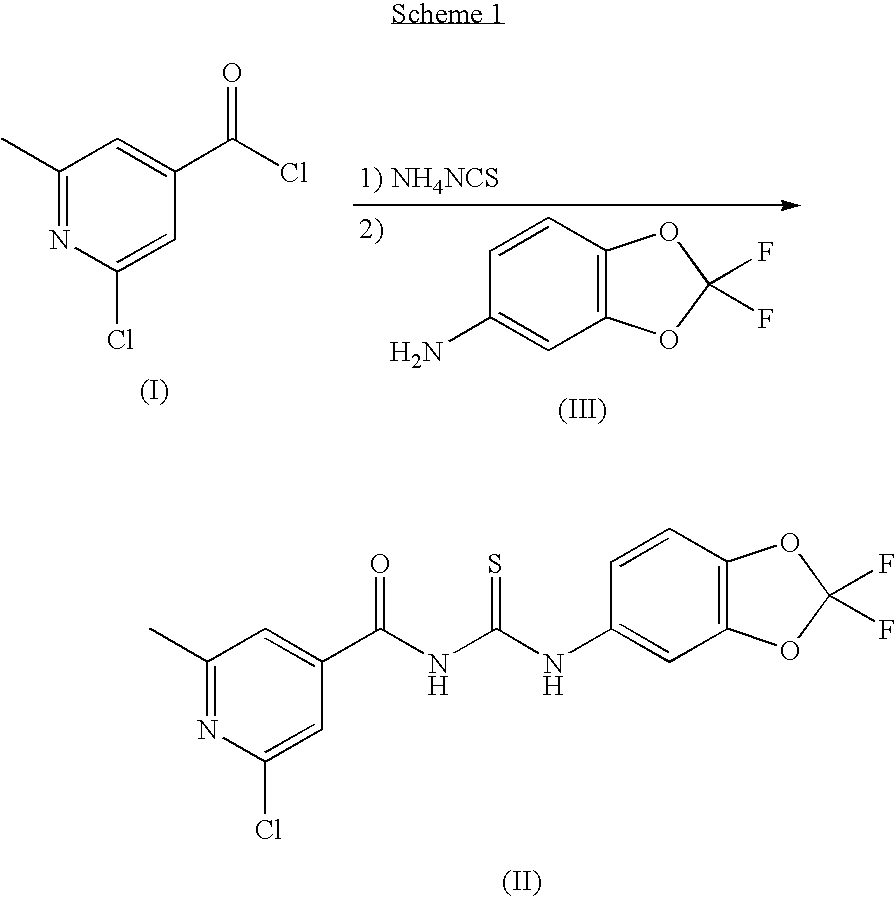
N4-acylcytosine-1,3-dioxolane nucleosides for treatment of viral infections
InactiveUS6908924B2Strong inhibitory activityBiocideOrganic active ingredientsImmunodeficiency virusHuman patient
The present invention is directed to a compound, method and composition of treating or preventing viral infections, in particular, human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections, in human patients or other animal hosts, comprising the administration of N4-acylcytosine-1,3-dioxolane and pharmaceutically acceptable salts, prodrugs, and other derivatives thereof.
Owner:GILEAD PHARMASSET LLC
Nonaqueous electrolyte material of fluorosulfonylimide lithium and application thereof
ActiveCN101882696AImprove solubilityNot easy to crystallizeOrganic chemistryCapacitor electrolytes/absorbentsDielectricPhosphate
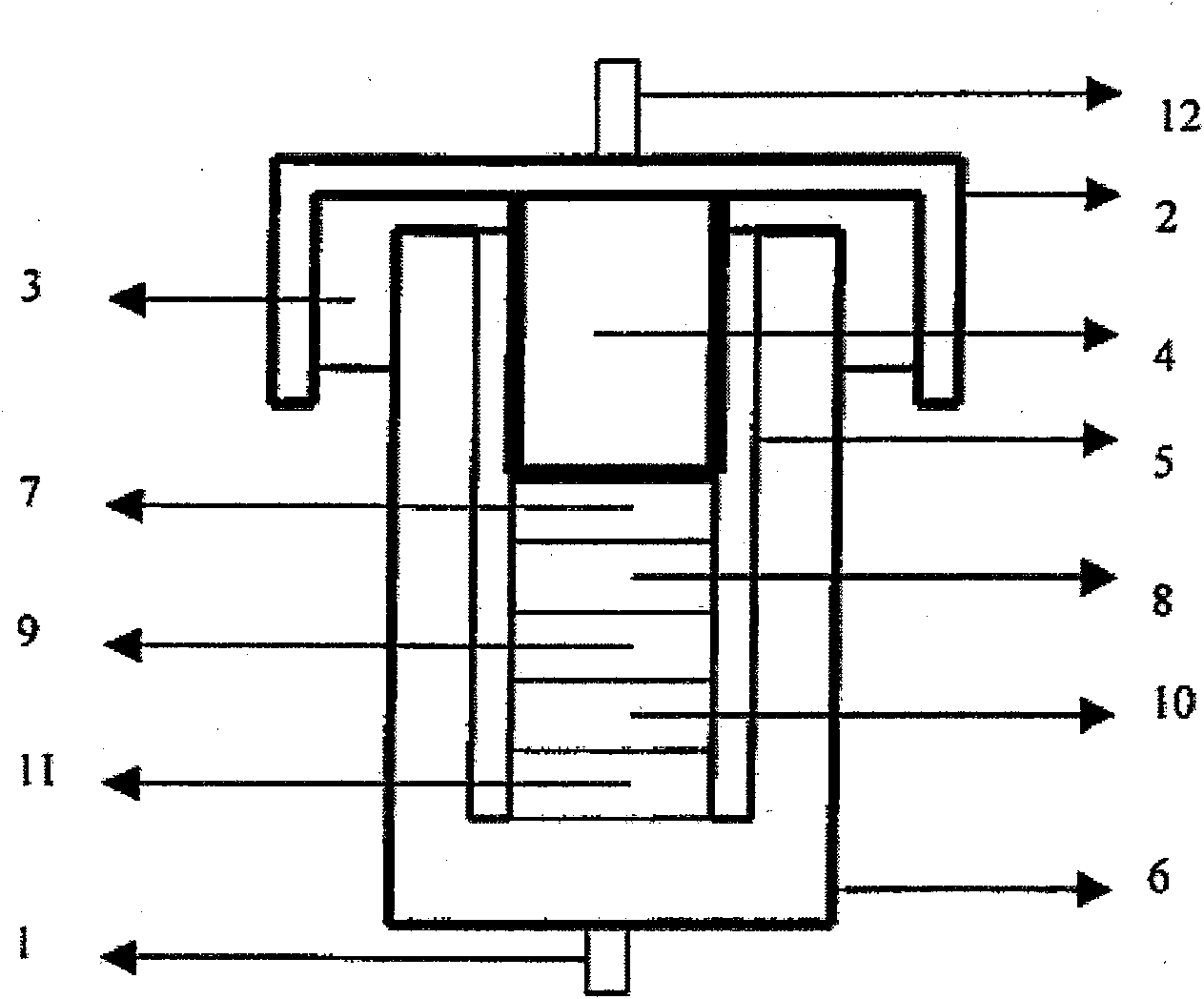
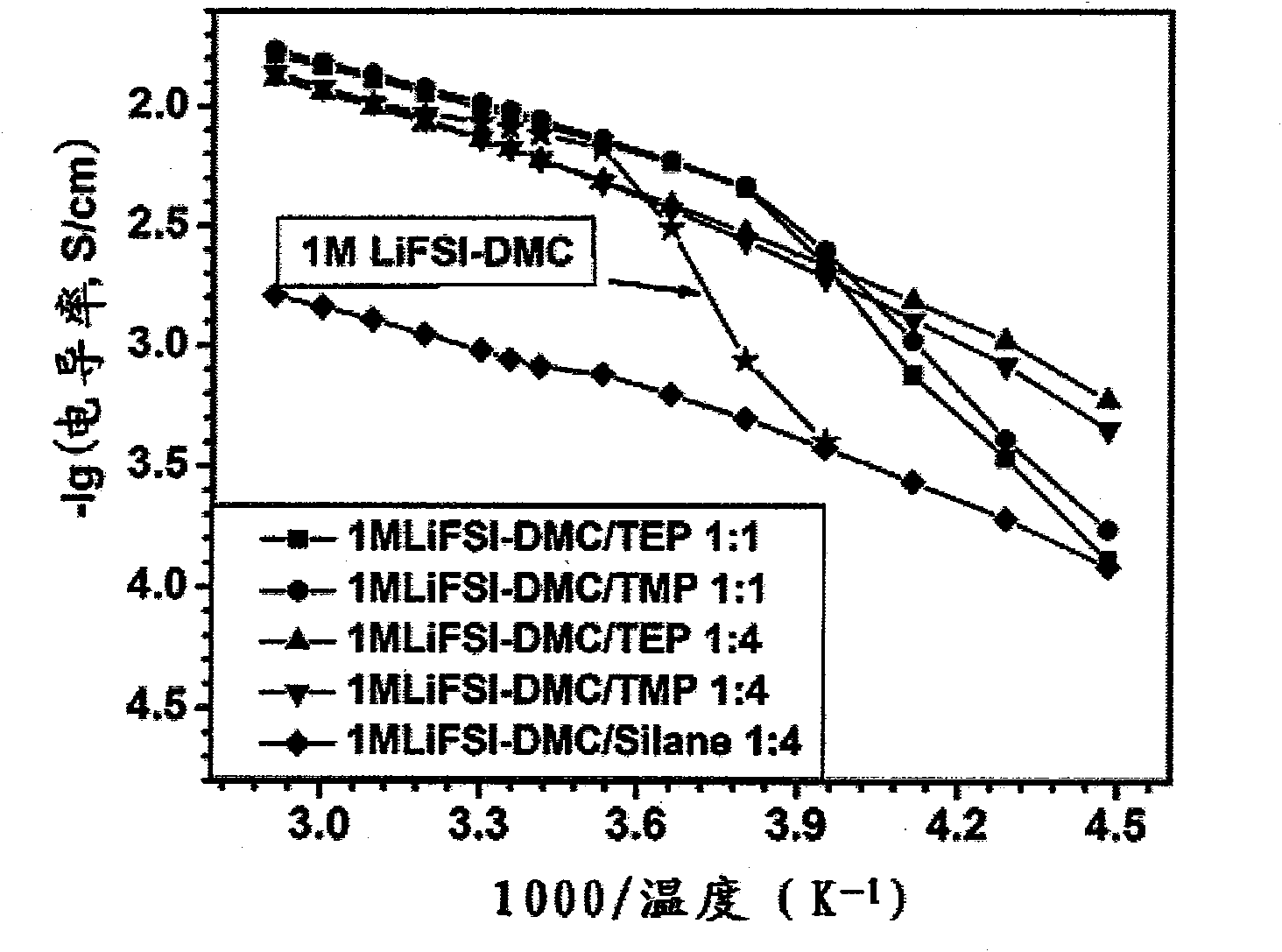
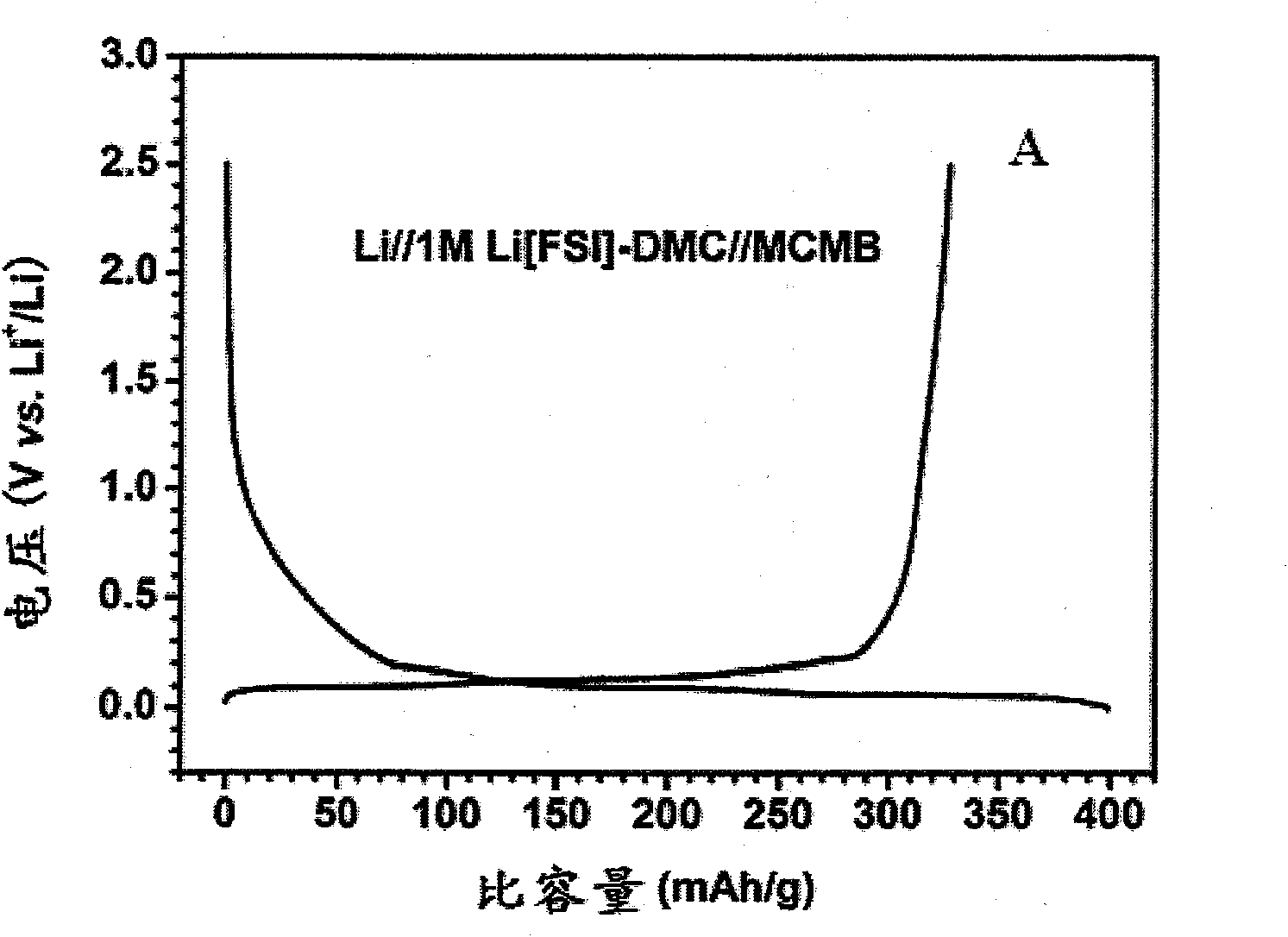
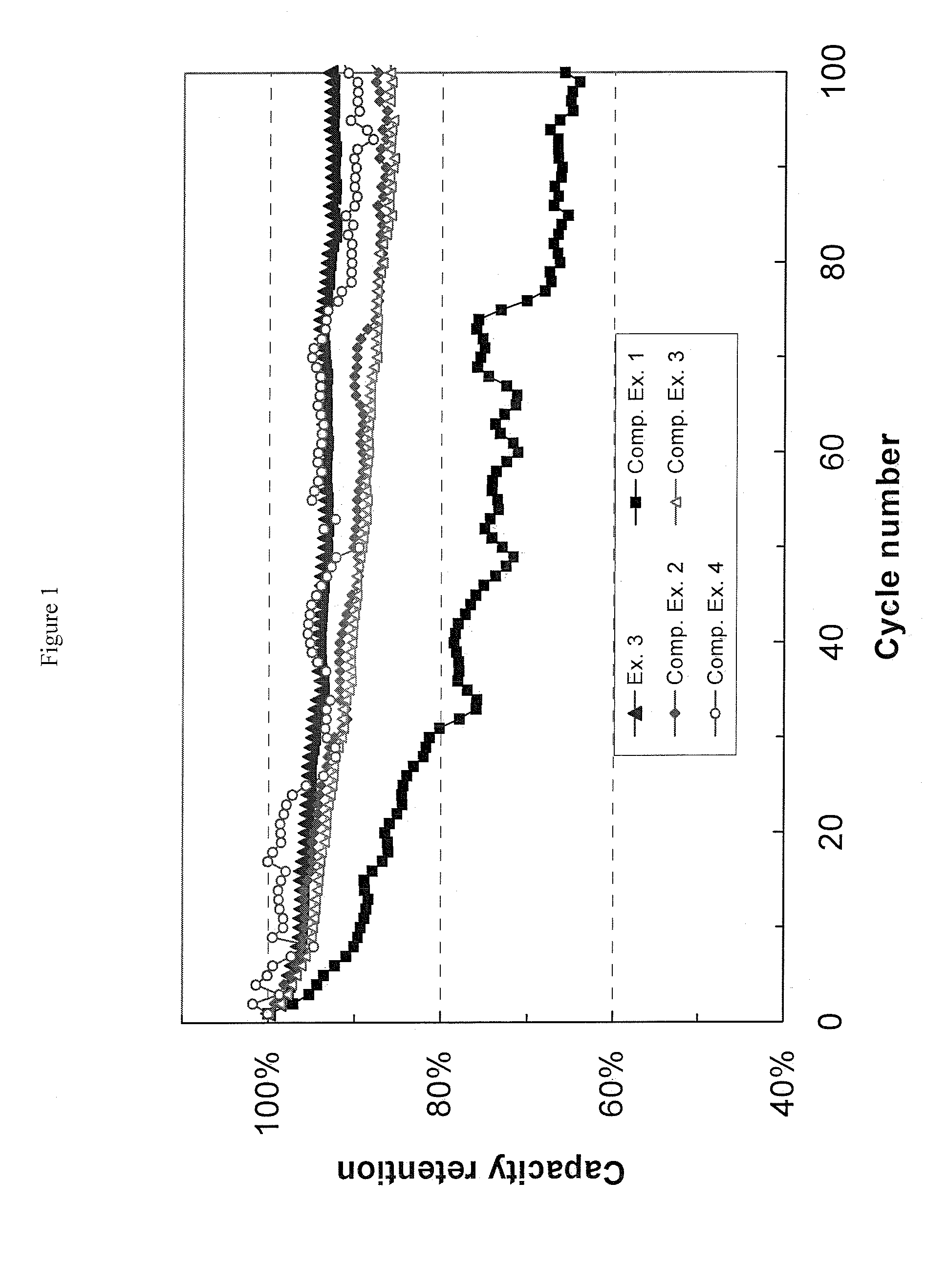
The invention provides a nonaqueous electrolyte material, which consists of fluorosulfonylimide lithium and organic solvent with dielectric constant less than 30, and the organic solvent is selected from one or more of chain-like carbonate solvent, phosphate solvent, siloxane solvent, boroxane solvent, acetate solvent, propionate solvent, butyrate solvent, CF3OCH2CH2OCF3 solvent, C2H5OCH2CH2OCH3 solvent, C2F5OCH2CH2OCF3 solvent, 1,3-dioxolane solvent and aliphatic nitrile solvent with more than two carbon atoms. The ionic conductivity of the nonaqueous electrolyte material is 0.01mS / cm to 18mS / cm, the lithium ion transference number is tLi plus equal to 0.2 to 0.8, and the applicable temperature range is 80DEG C below zero to 60DEG C below zero. The invention also provides an application of the nonaqueous electrolyte material in the preparation of lithium batteries and super capacitors. Furthermore, the invention provides a lithium battery and a super capacitor which contain the nonaqueous electrolyte material of fluorosulfonylimide lithium.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Asymmetric Gas Separation Membranes with Superior Capabilities for Gas Separation
InactiveUS20080143014A1Excellent permeation fluxHigh selectivityMembranesSemi-permeable membranesCellulose diacetatePolymer science
This invention relates to a method of making flat sheet asymmetric membranes, including cellulose diacetate / cellulose triacetate blended membranes, polyimide membranes, and polyimide / polyethersulfone blended membranes by formulating the polymer or the blended polymers dopes in a dual solvent mixture containing 1,3 dioxolane and a second solvent, such as N,N′-methylpyrrolidinone (NMP). The dopes are tailored to be closed to the point of phase separation with or without suitable non-solvent additives such as methanol, acetone, decane or a mixture of these non-solvents. The flat sheet asymmetric membranes are cast by the phase inversion processes using water as the coagulation bath and annealing bath. The dried membranes are coated with UV curable silicone rubber. The resulting asymmetric membranes exhibit excellent permeability and selectivity compared to the intrinsic dense film performances.
Owner:UOP LLC
Non-Aqueous Electrolytic Solutions And Electrochemical Cells Comprising The Same
ActiveUS20090017386A1Improve cycle lifeGood capacity retentionOrganic chemistryElectrolytic capacitorsLithium metalKetone
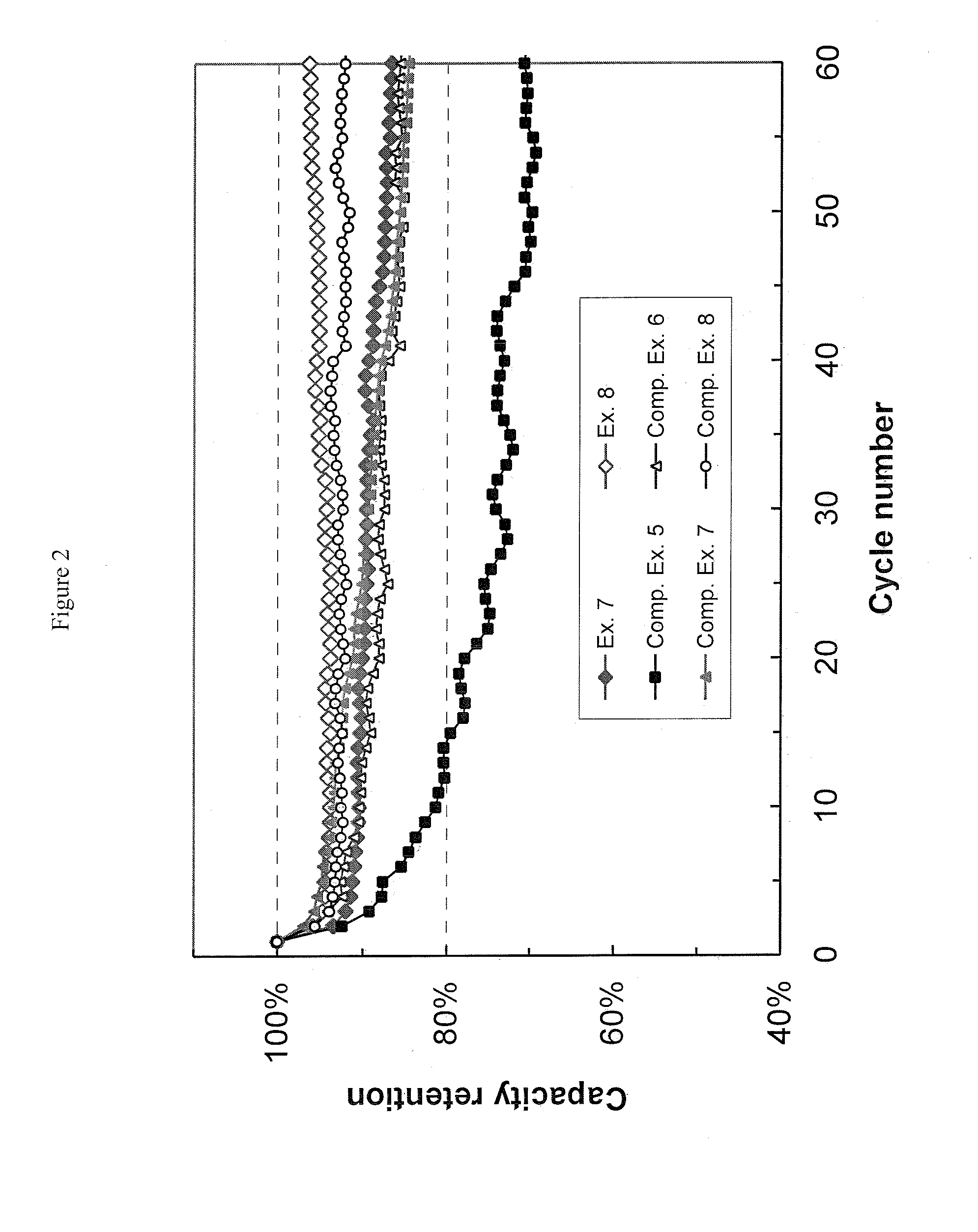
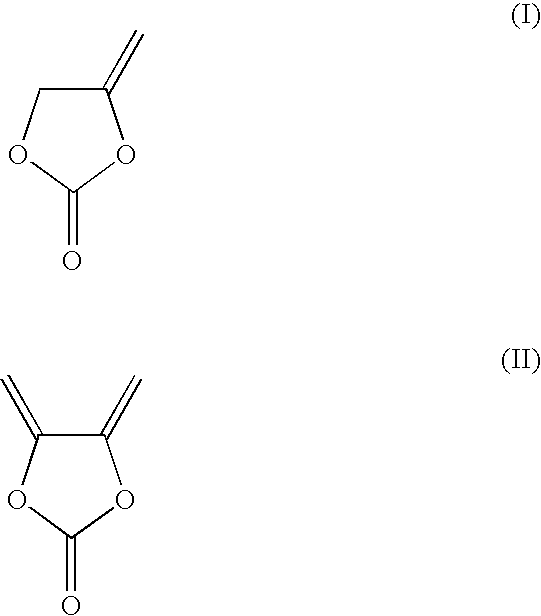
Non-aqueous electrolyte solutions capable of protecting negative electrode materials such as lithium metal and carbonaceous materials in energy storage electrochemical cells (e.g., lithium metal batteries, lithium ion batteries and supercapacitors) include an electrolyte salt, a non-aqueous electrolyte solvent mixture, an unsaturated organic compound 4-methylene-1,3-dioxolan-2-one or 4,5-dimethylene-1,3-dioxolan-2-one, and other optional additives. The 1,3-dioxolan-2-ones help to form a good solid electrolyte interface on the negative electrode surface.
Owner:SHENZHEN CAPCHEM TECH
Photoimageable coating composition and composite article thereof
InactiveUS20050266335A1Photosensitive materialsPhotomechanical coating apparatusResistPhotoacid generator
A photoimagable composition suitable for use as a negative photoresist comprising: (A) at least one epoxidized polyfunctional bisphenol A formaldehyde novolak resin; (B) at least one polycaprolactone polyol reactive diluent, wherein the amount of component (A) is from about 95% to about 75% by weight of the sum of (A) and (B) and the amount of component (B) is from about 5% to about 25% by weight of the sum of (A) and (B); (C) at least one photoacid generator in an amount from about 2.5 to about 12.5 parts per hundred parts of resin and reactive diluent, which initiates polymerization upon exposure to actinic radiation; and (D) a sufficient amount of solvent to dissolve (A), (B) and (C); wherein the solvent comprises 2-pentanone, 3-pentanone, and 1,3-dioxolane and mixtures thereof.
Owner:MICROCHEM CORP +1
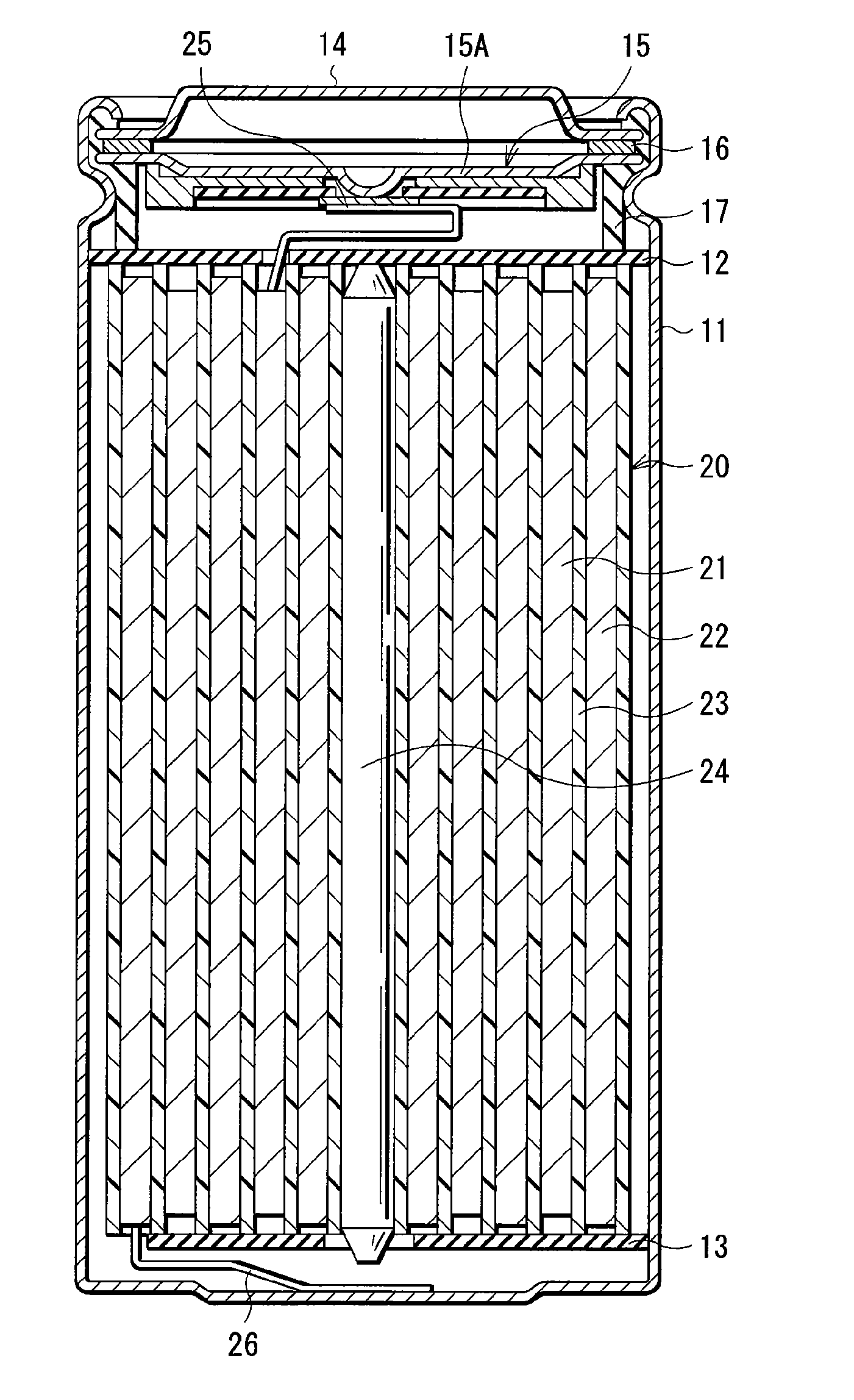
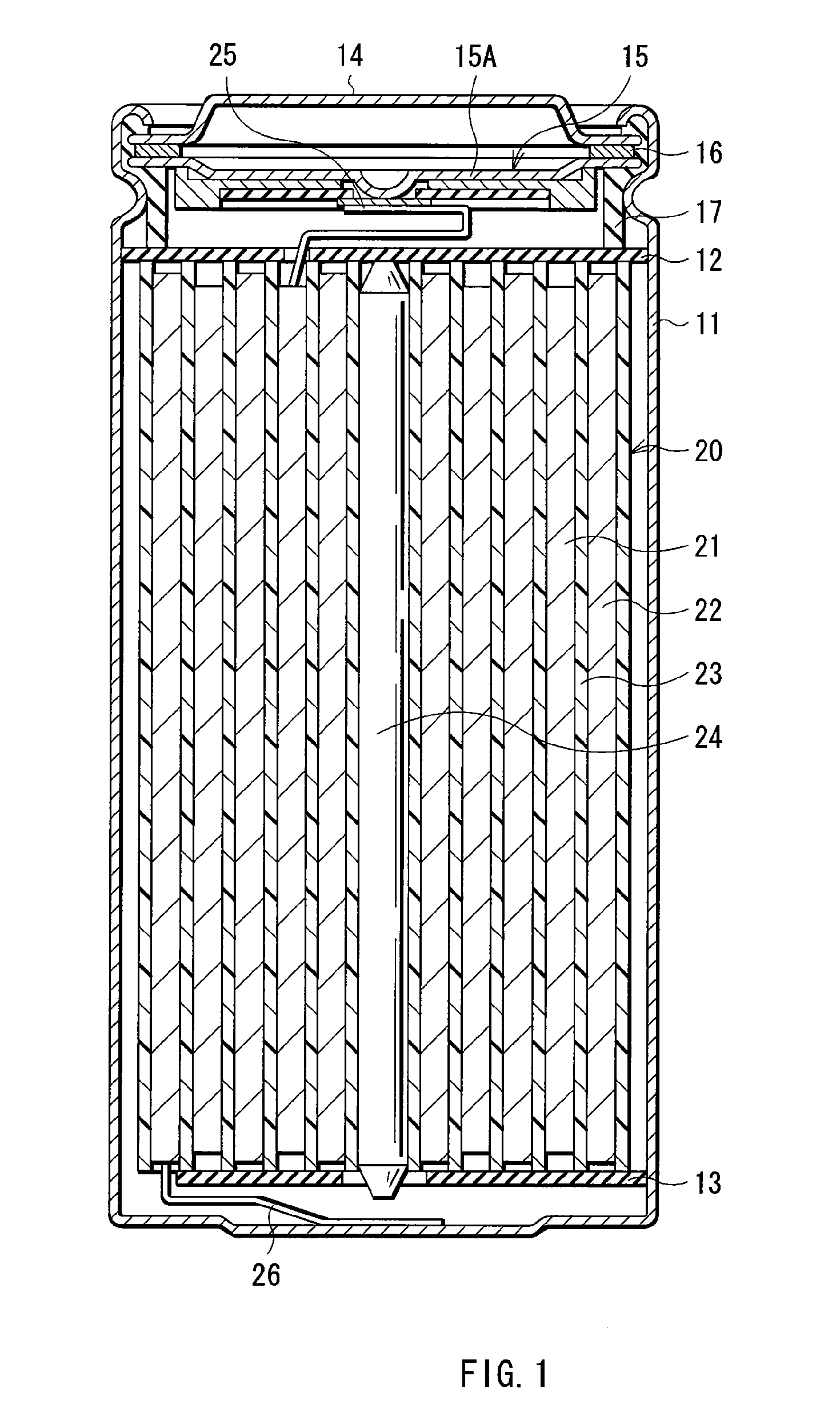
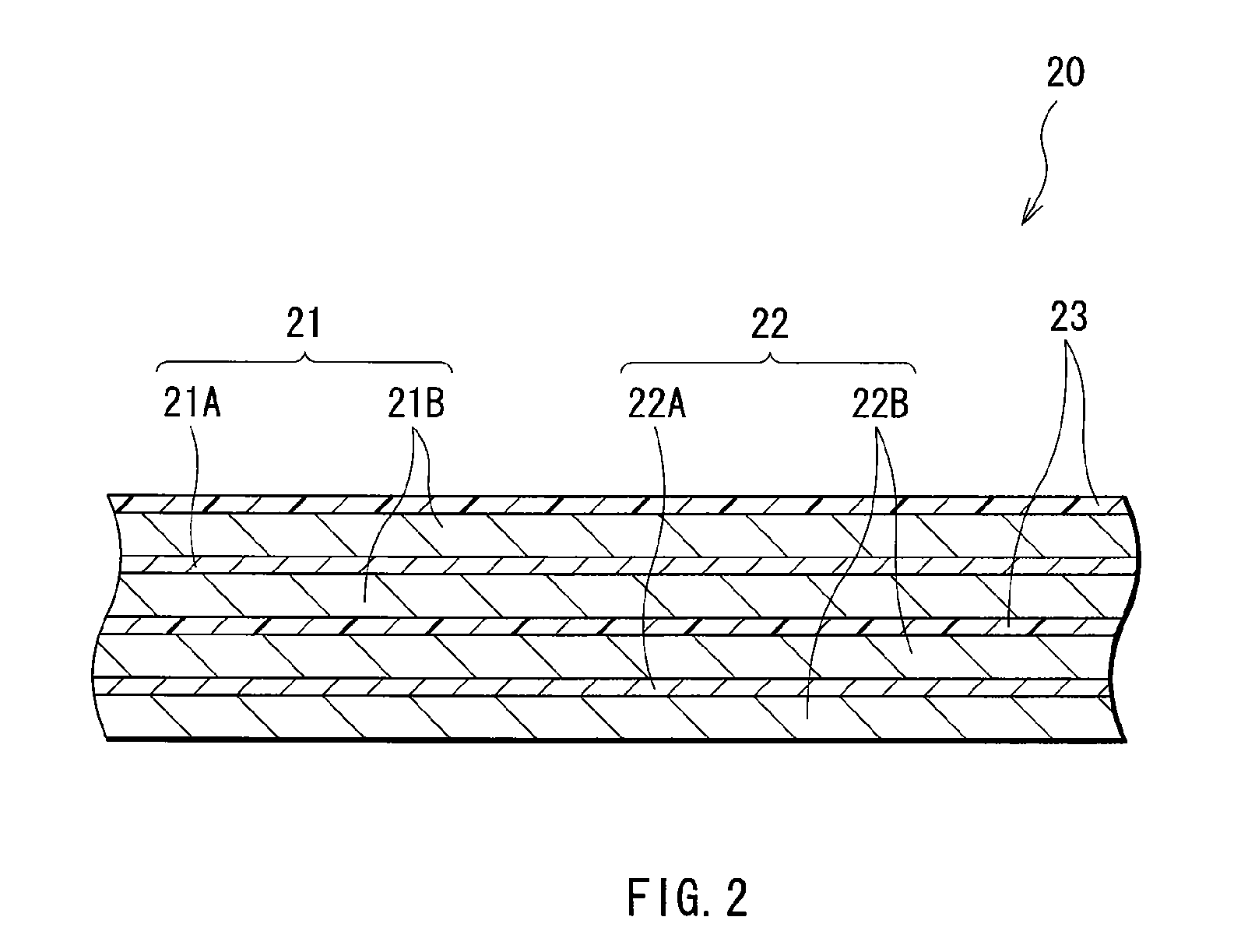
Electrolytic solution and battery
ActiveUS20060228626A1Efficient processAvoid reactionCell electrodesFinal product manufactureLithiumImide
An electrolytic solution and a battery capable of improving cycle characteristics are provided. A separator is impregnated with an electrolytic solution. The electrolytic solution includes a cyclic carbonate derivative having a halogen atom such as 4-fluoro-1,3-dioxolane-2-one or 4-chloro-1,3-dioxolane-2-one and a cyclic imide salt such as 1,1,2,2,3,3-hexafluoropropane-1,3-disulfonimide lithium. Thereby, the decomposition reaction of the electrolytic solution can be inhibited, and the cycle characteristics can be improved.
Owner:MURATA MFG CO LTD
Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide
The present invention relates to solid forms of (R)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide (Compound 1) in substantially crystalline form (Form A) or amorphous form, pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Tetrahydrofuro [3,4-D] dioxolane compounds for use in the treatment of viral infections and cancer
Owner:BIOCRYST PHARM INC
Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof
A pharmaceutical composition comprising Compound 1, (R)-1-(2,2-difluorobenzo[d][1,3]dioxo-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide, and at least one excipient selected from: a filler, a disintegrant, a surfactant, a glidant and a lubricant, the composition being suitable for oral administration to a patient in need thereof to treat a CFTR mediated disease such as Cystic Fibrosis. Methods for treating a patient in need thereof include administering the pharmaceutical composition of Compound 1 are also disclosed.
Owner:VERTEX PHARMA INC
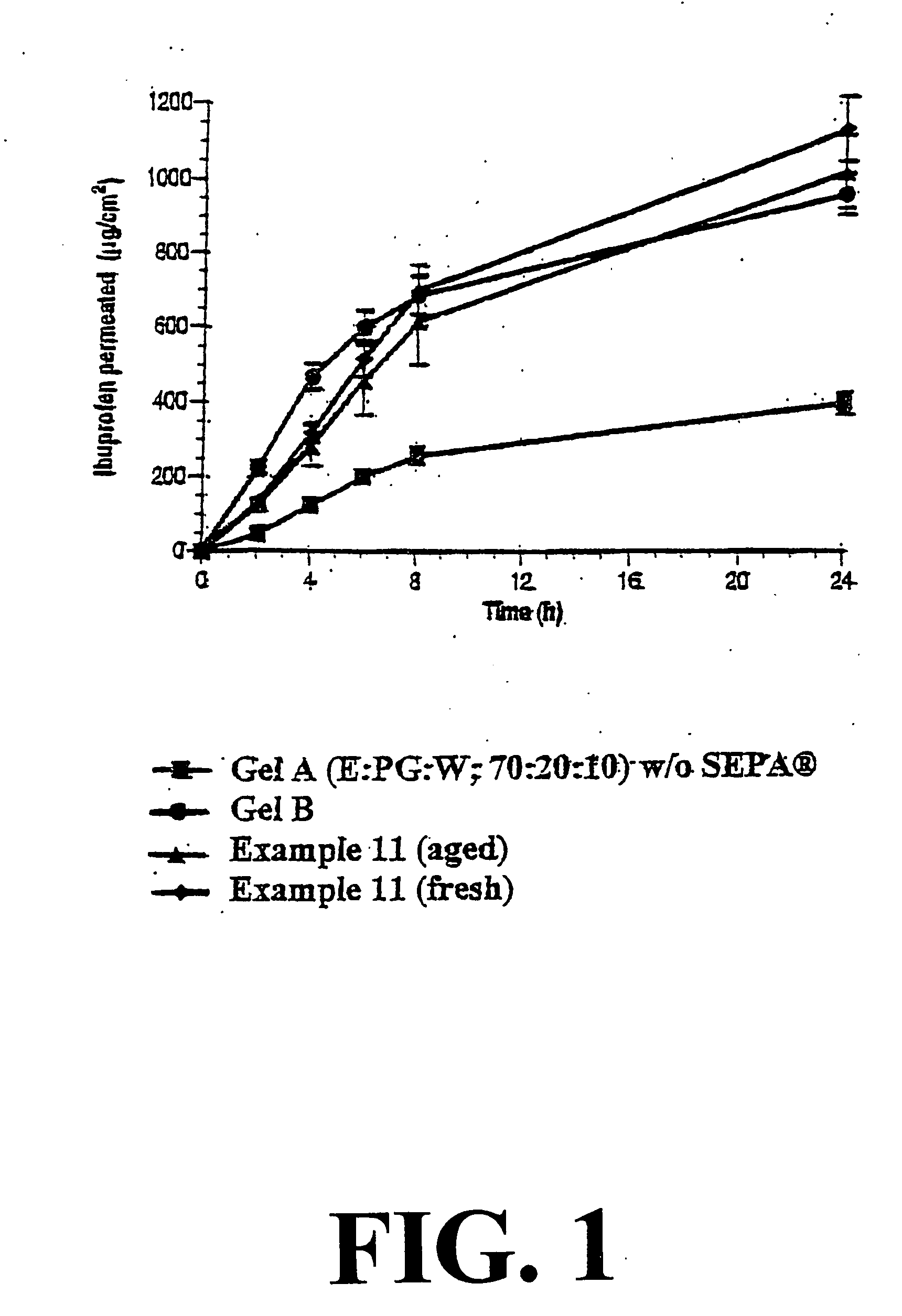
Non-steroidal antiinflammatory drug formulations for topical application to the skin
InactiveUS20030082226A1Improve performanceSignificant positive effectOrganic active ingredientsAntipyreticSkin penetrationAntiinflammatory drug
Topical alcoholic or aqueous alcoholic gels containing ibuprofen or other NSAIDs, such as, naproxen, in substantially neutral salt form, have enhanced penetration through skin and may provide rapid pain / inflammation relief by including in the formulation 2-n-nonyl-1,3-dioxolane or other hydrocarbyl derivative of 1,3-dioxolane-or 1,3-dioxane or acetal, as skin penetration enhancing compound. The amount of propylene glycol may be varied to adjust the initial flux of the NSAID through the skin, especially for ibuprofen, naproxen, and ketorolac.
Owner:SAMOUR CARLOS M +2
Herbicidal mixtures
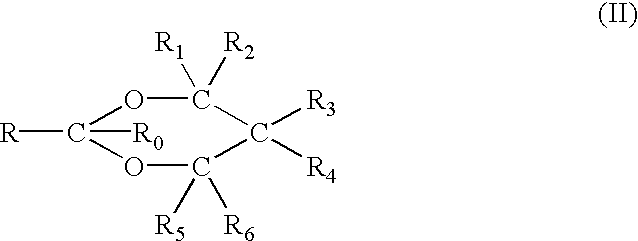
Disclosed is a herbicidal mixture comprising (a) at least one herbicide compound selected from the pyrimidines of Formula 1, including all geometric and stereoisomers, N-oxides, and salts thereof:whereinR1 is cyclopropyl, 4-Br-phenyl or 4-Cl-phenyl;X is Cl or Br;R2 is H, C1-C14 alkyl, C2-C14 alkoxyalkyl, C3-C14 alkoxyalkoxyalkyl, C2-C14 hydroxyalkyl or benzyl; and(b) at least one additional herbicide or herbicide safener compound selected from the group consisting of (b1) ACCase inhibitors, (b2) AHAS inhibitors, (b3) photosystem II inhibitors, (b4) photosystem I electron diverters, (b5) PPO inhibitors, (b6) EPSP synthase inhibitors, (b7) GS inhibitors, (b8) VLCFA inhibitors, (b9) auxin mimics, (b10) auxin transport inhibitors, (b11) other herbicides selected from the group consisting of flamprop-M-methyl, flamprop-M-isopropyl, difenzoquat, DSMA, MSMA, bromobutide, flurenol, cinmethylin, cumyluron, dazomet, dymron, methyldymron, etobenzanid, fosamine-ammonium, isoxaflutole, asulam, clomazone, mesotrione, metam, oxaziclomefone, oleic acid, pelargonic acid and pyributicarb, (b12) herbicide safeners selected from the group consisting of benoxacor, 1-bromo-4-[(chloromethyl)sulfonyl]benzene, cloquintocet-mexyl, cyometrinil, dichlormid, 2-(dichloromethyl)-2-methyl-1,3-dioxolane, fenchlorazole-ethyl, fenclorim, flurazole, fluxofenim, furilazole, isoxadifen-ethyl, mefenpyr-diethyl, methoxyphenone, naphthalic anhydride and oxabetrinil, and their salts. Also disclosed is a method for controlling the growth of undesired vegetation comprising contacting the vegetation or its environment with a herbicidally effective amount of a mixture of the invention (e.g., as a composition described herein).
Owner:ARMEL GREGORY RUSSELL +8
Battery
InactiveUS20060228625A1Improve featuresAvoid reactionOrganic electrolyte cellsActive material electrodesHalogenSuccinic anhydride
A battery capable of improving cycle characteristics is provided. A cathode and an anode are oppositely arranged with a separator in between. An electrolytic solution is impregnated in the separator. The electrolytic solution contains a derivative of cyclic carbonate having halogen atoms such as 4-fluoro-1,3-dioxolane-2-one and 4-chloro-1,3-dioxolane-2-one; and a cyclic acid anhydride such as succinic anhydride. The anode has an anode current collector and an anode active material layer which is provided on the anode current collect and is alloyed with the anode current collector at least at part of the interface with the anode current collector
Owner:MURATA MFG CO LTD
1,3,5-trisubstituted triazole derivative
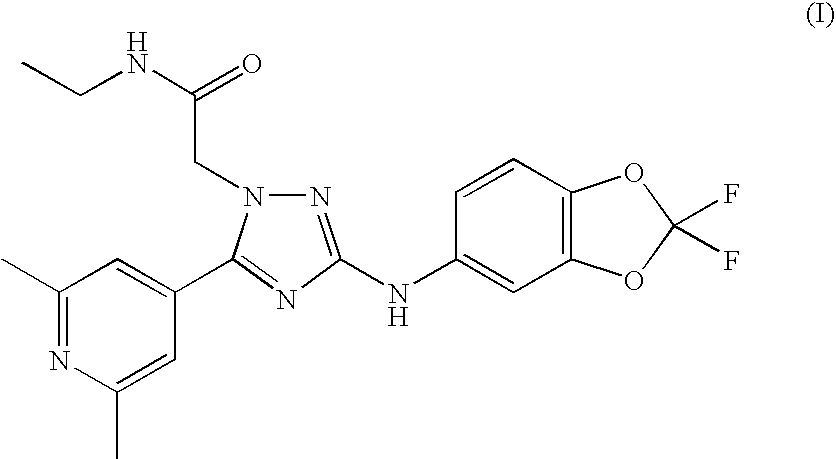
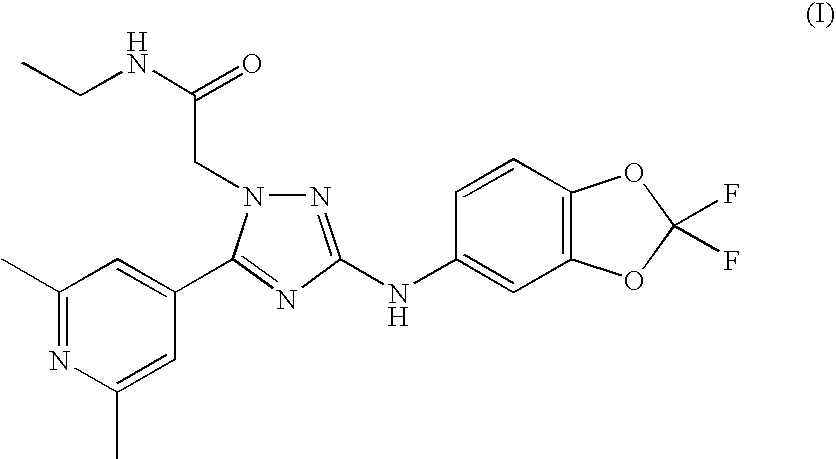
ActiveUS20100240707A1Good curative effectBiocideNervous disorderAllosteric modulatorTriazole derivatives
The present invention relates to 2-[3-(2,2-Difluoro-benzo[1,3]dioxol-5-ylamino)-5-(2,6-dimethyl-pyridin-4-yl)-[1,2,4]triazol-1-yl]-N-ethyl-acetamide and analogues or pharmaceutically acceptable salts thereof, processes for preparing them, pharmaceutical compositions containing them and their use in therapy.The invention particularly relates to a potent positive allosteric modulator of nicotinic acetylcholine receptors which have the capability of increasing the efficacy of nicotinic receptor agonists.
Owner:JANSSEN PHARMA NV
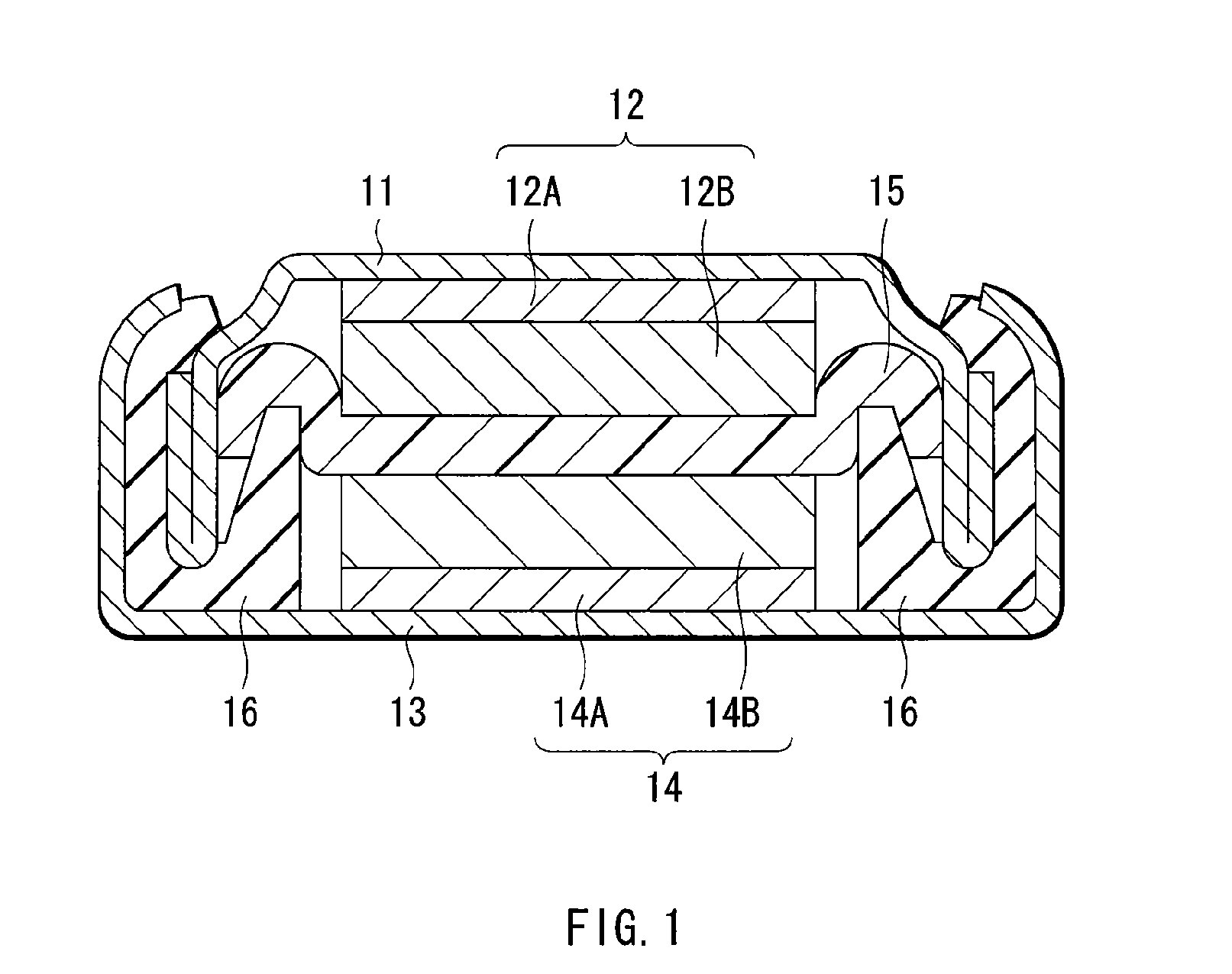
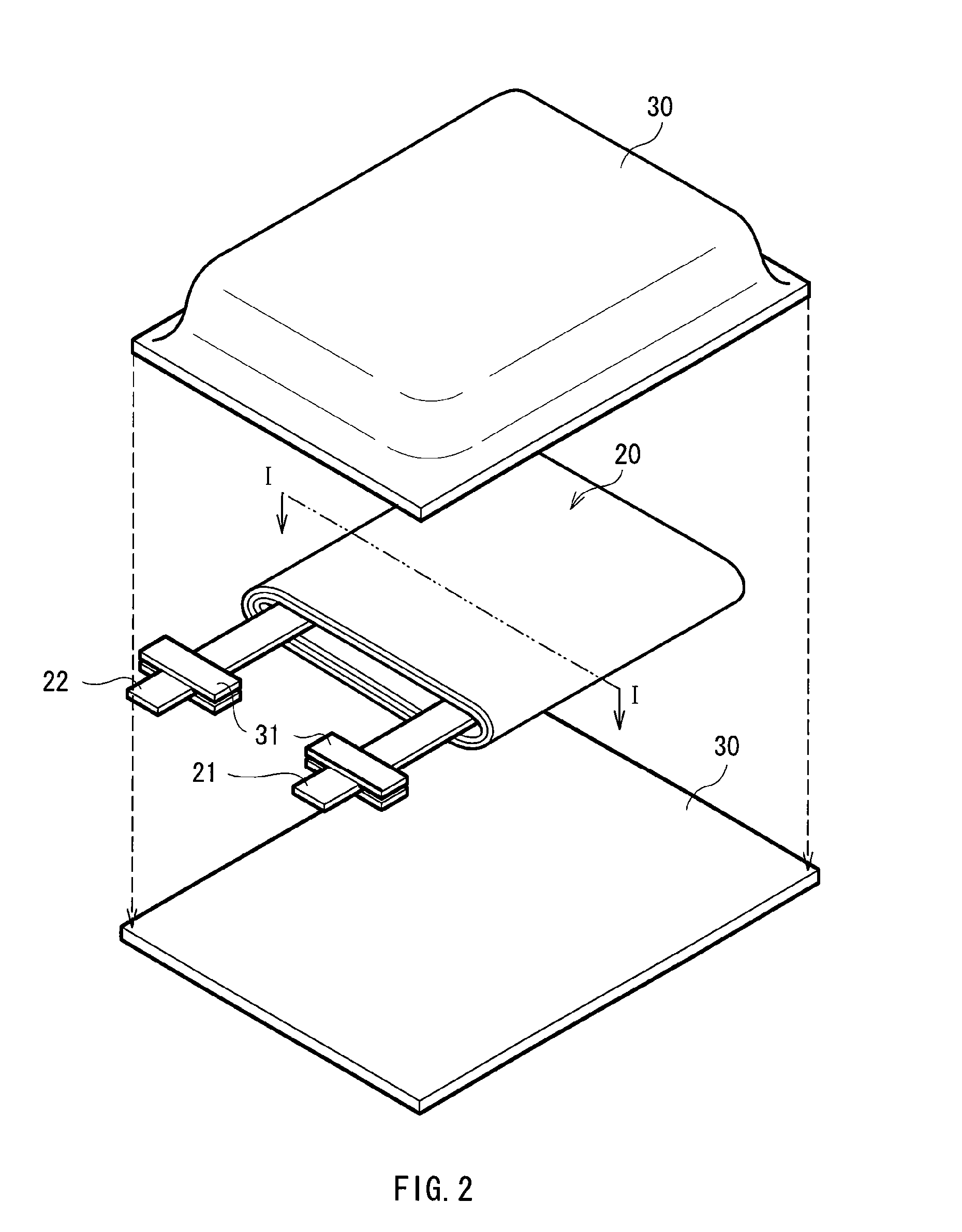
Lithium battery including storage stabilized dioxolane-containing electrolyte
InactiveUS6255021B1Reduced self dischargeEasy to storeOrganic electrolyte cellsLi-accumulatorsOrganic solventOxygen
A nonaqueous electrolyte battery having improved storage stability is disclosed. The battery includes a positive electrode; s negative electrode in which the active material is lithium or a compound capable of absorbing and desorbing lithium; and a nonaqueous electrolyte containing an organic solvent, at least 10 wt % of which is dioxolane, a solute and a storage stabilizing additive which is an oxygen acid ester, isoxazole, oxazole or oxazoline or a derivative thereof. The additive reduces the self-discharge rate of the battery during storage.
Owner:SANYO ELECTRIC CO LTD
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
The present invention relates to a substantially crystalline and free solid state form of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (Form I), pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Lithium cell
ActiveUS20090208849A1Reduce passive layer resistanceSlow build ratesPrimary cell maintainance/servicingElectrode carriers/collectorsSulfolaneAlloy
A primary cell having an anode comprising lithium or lithium alloy and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt preferably lithium iodide (LiI) dissolved in an organic solvent mixture. The solvent mixture preferably comprises dioxolane, dimethoxyethane and sulfolane. The electrolyte typically contains between about 100 and 2000 parts by weight water per million parts by weight (ppm) electrolyte therein. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:DURACELL U S OPERATIONS
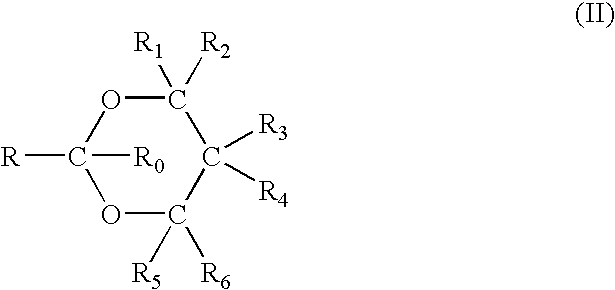
1,3-benzodioxole derivative
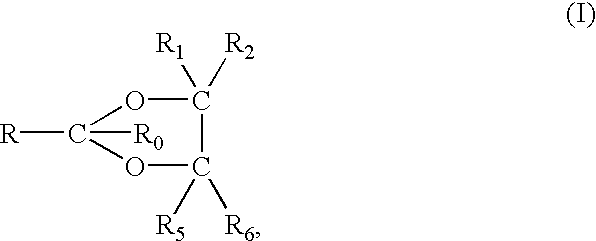
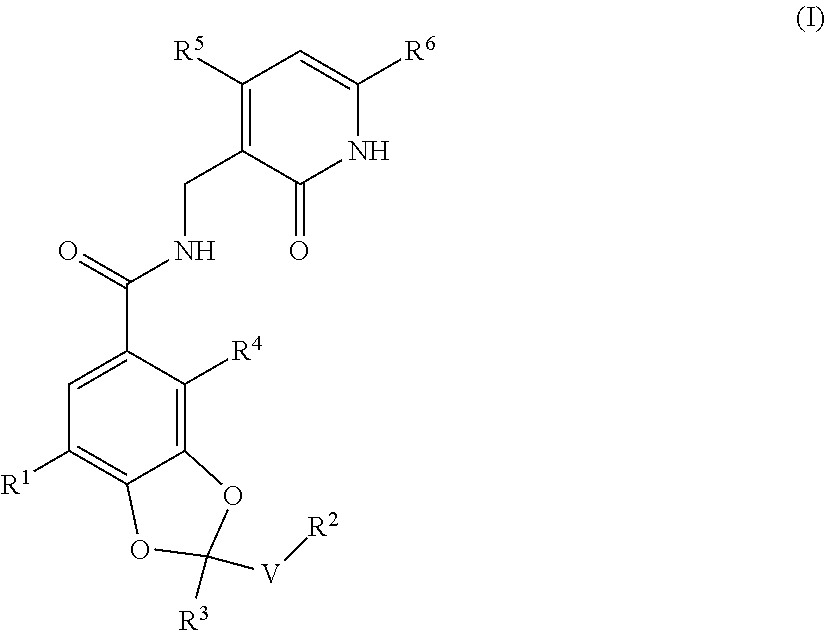
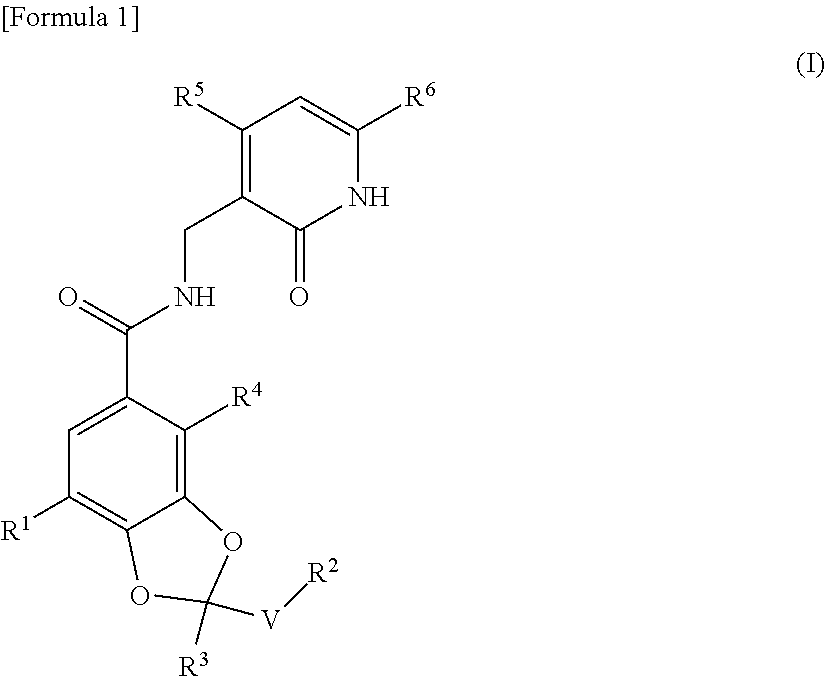
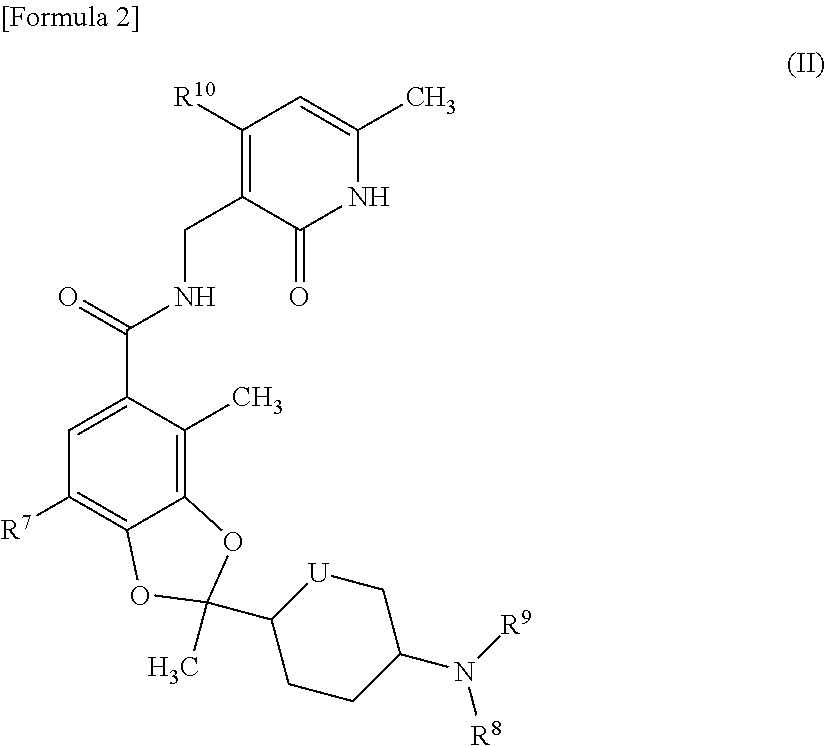
ActiveUS20170073335A1High activityInhibit cell growthOrganic active ingredientsOrganic chemistryBenzeneChemical structure
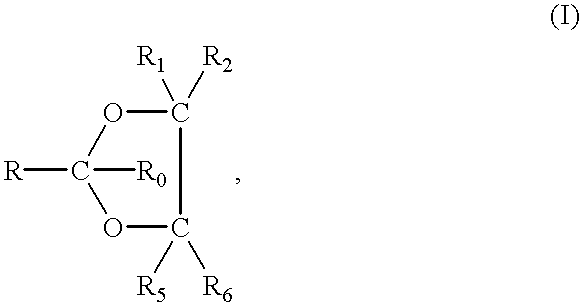
The present invention provides a compound having a particular chemical structure or a pharmacologically acceptable salt thereof which has an excellent inhibitory effect on EZH1 and / or EZH2 activity. The present invention provides a compound having a 1,3-benzodioxole structure represented by the general formula (I) or a pharmacologically acceptable salt thereof, or a pharmaceutical composition comprising the compound (wherein R1, R2, R3, R4, R5, R6, and V in the formula (I) are each as defined in the present specification).
Owner:DAIICHI SANKYO CO LTD
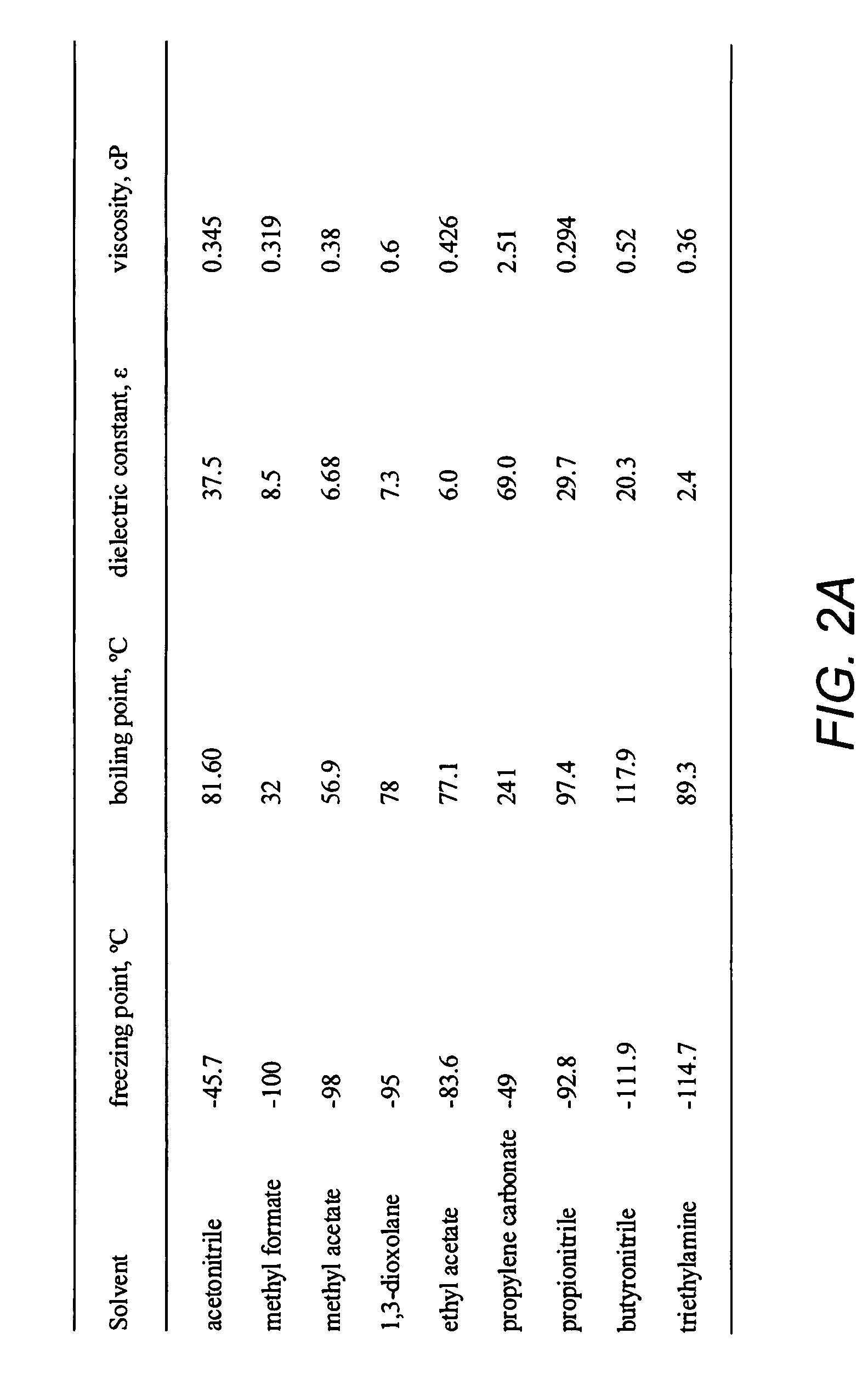
Low temperature double-layer capacitors
ActiveUS20080304207A1Low melting pointHybrid capacitor electrolytesClosuresTetrafluoroborateDevice form
Double-layer capacitors capable of operating at extremely low temperatures (e.g., as low as −75° C.) are disclosed. Electrolyte solutions combining a base solvent (e.g., acetonitrile) and a cosolvent are employed to lower the melting point of the base electrolyte. Example cosolvents include methyl formate, ethyl acetate, methyl acetate, propionitrile, butyronitrile, and 1,3-dioxolane. An optimized concentration (e.g., 0.10 M to 0.75 M) of salt, such as tetraethylammonium tetrafluoroborate, is disolved into the electrolyte solution. In some cases (e.g., 1,3-dioxolane cosolvent) additives, such as 2% by volume triethylamine, may be included in the solvent mixture to prevent polymerization of the solution. Conventional device form factors and structural elements (e.g., porous carbon electrodes and a polyethylene separator) may be employed.
Owner:CALIFORNIA INST OF TECH
Preparation method of organic silicon modified polyester wire enamel
InactiveCN102161860AImprove the disadvantages that the heat resistance performance is not good enoughGood storage stabilityPolyester coatingsPolyesterSilanes
The invention discloses a preparation method of organic silicon modified polyester wire enamel. Divalent alcohol, trihydric alcohol and dibasic acid are combined into polyester of low polymerization in a molar ratio of 1: (0.08-0.2): (0.95-1); dialkyl dichlorosilane or dialkyl dioxolane silane and alkyl trichlorosilane or alkyl trialkoxysilane are combined into organic silicon resin prepolymer solution; then the organic silicon resin prepolymer solution reacts with resin of low polymerization; and a solvent and a film forming assistant are added to blend into the organic silicon modified polyester wire enamel. The heat-resistant degree of the polyester wire enamel is effectively improved, and production process is simple and reliable; the organic silicon modified polyester wire enamel can be produced at normal pressure and common vacuum degree, and the storage stability of the product is good; and the solvent with small toxicity is used for replacing phenol solvents so as to lower product toxicity.
Owner:GUANGDONG HENGBAOCHANG ELECTRICAL TECH +1
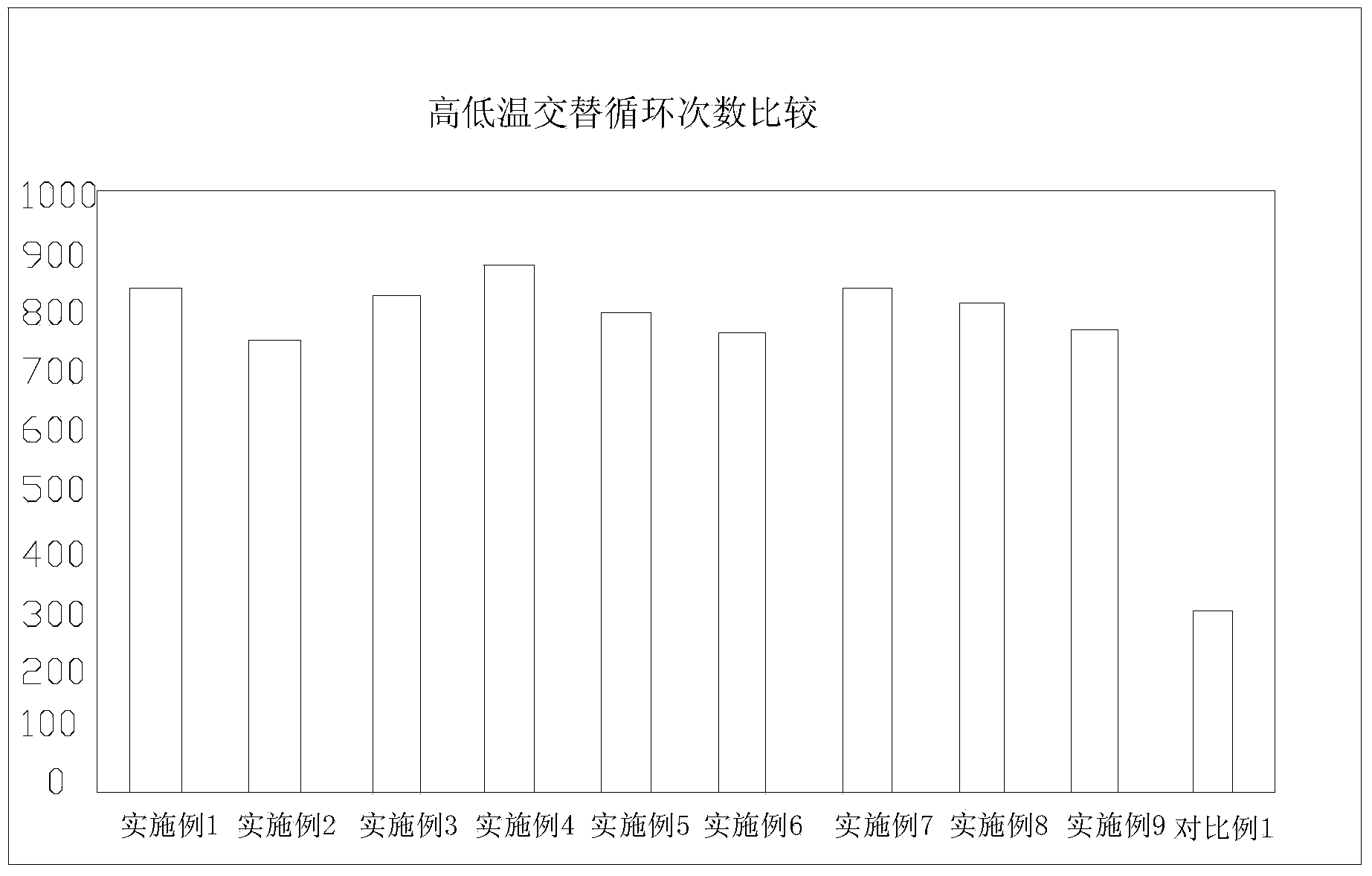
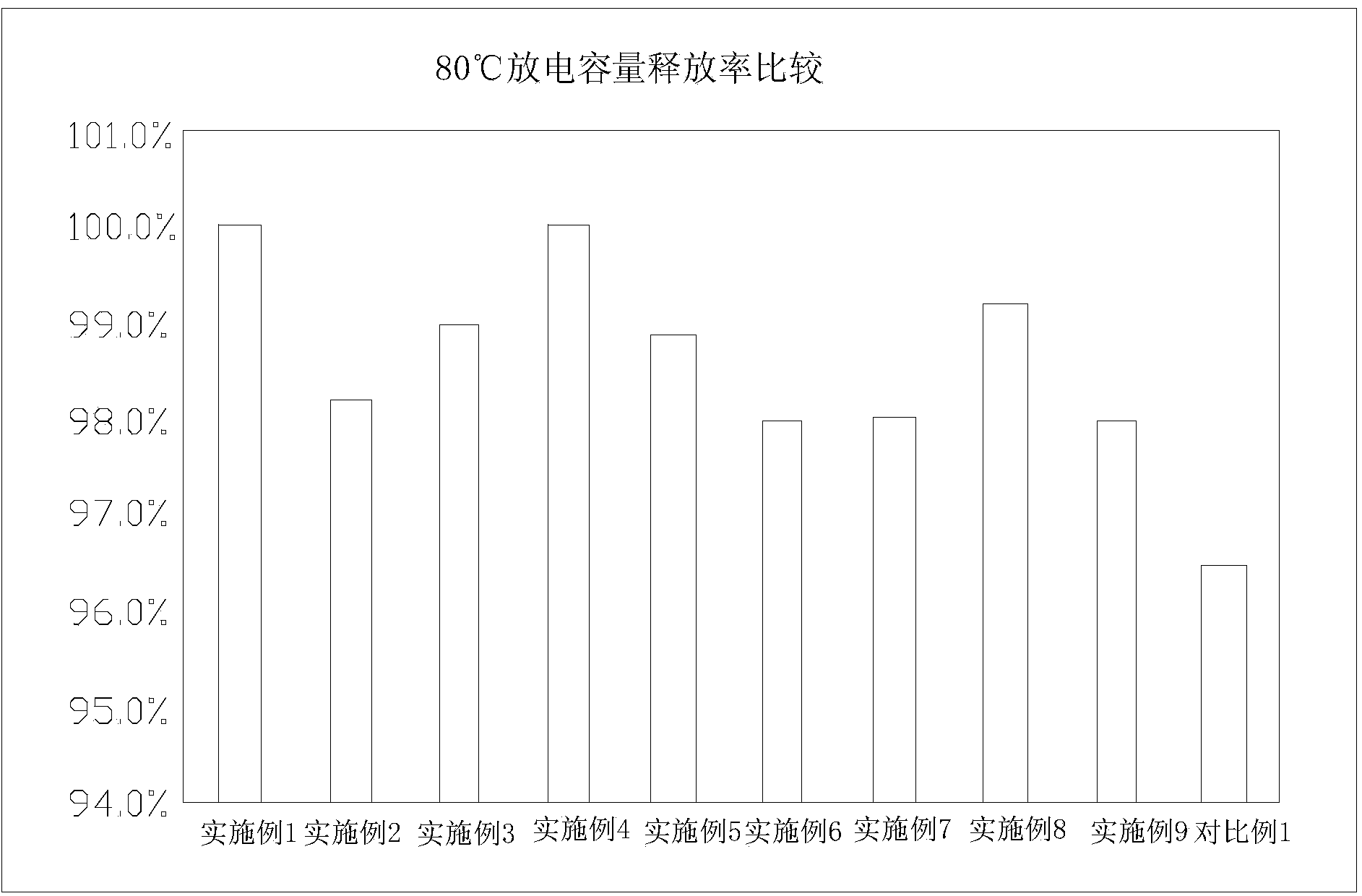
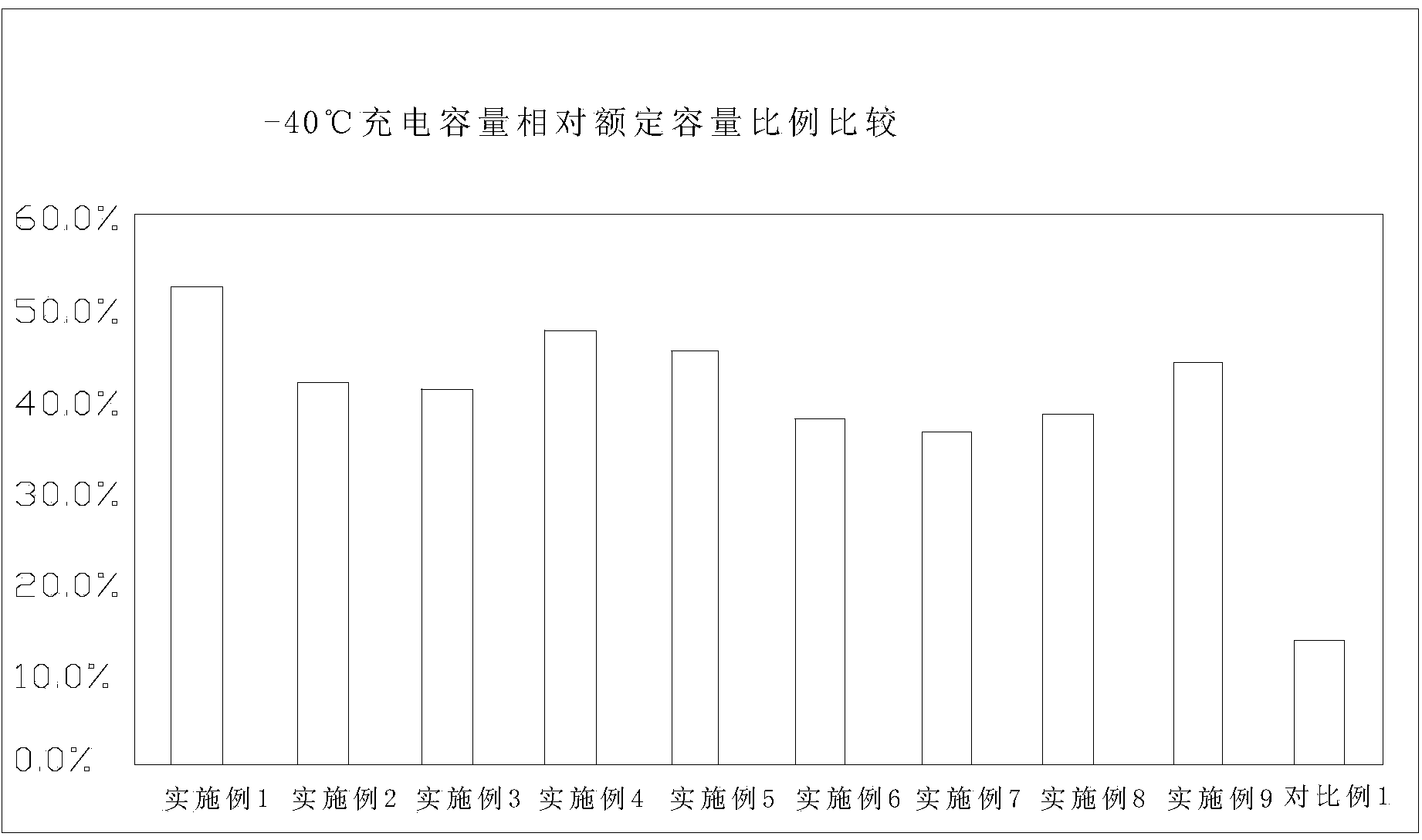
Lithium iron phosphate battery with lithium ion battery electrolyte suitable for ultralow-temperature charging and discharging
ActiveCN103367803AFacilitate quick migrationImprove ionic conductivitySecondary cellsMethyl carbonateEthyl butyrate
The invention relates to a lithium iron phosphate battery with lithium ion battery electrolyte suitable for ultralow-temperature charging and discharging. The lithium ion battery electrolyte comprises lithium salt, a multi-element organic solvent and additives, wherein the additives comprise a low-melting-point additive, a film forming additive and a high-temperature additive; the multi-element organic solvent contains at least three of ethylene carbonate, diethyl carbonate, dimethyl carbonate, methyl ethyl carbonate, propylene carbonate and butylene carbonate; the low-melting-point additive contains at least one of 4-methyl-1,3-dioxolane, methyl acetate, methyl propionate, methyl butyrate, ethyl butyrate, propyl butyrate and butyl acetate; the high-temperature additive is at least one of methyl ester, di-n-propyl carbonate and 1,3-propane sultone. The lithium iron phosphate battery with the electrolyte can charge and discharge at an ultralow temperature and in a high-temperature environment, and is stable in performance and long in recycling life.
Owner:HANGZHOU LIAO TECH
Process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols
The present invention regards a three-step process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols. The process comprises: (i) the reaction of pyrocatechin (1,2-dihydroxybenzene) with a dihalo or di-alkoxyalkane, with the formation of a benzo[1,3]dioxol derivative; (ii) 5-selective catalytic acylation of the benzo[1,3]dioxol, with formation of a 5-alkanoylbenzo[1,3]dioxol and its subsequent (iii) reduction to 5-(alpha-hydroxyalkyl) benzo[1,3]dioxol. Also described are new benzodioxols obtainable using the above-mentioned process. The process of the invention is industrially simple and has low environmental impact; it allows to obtain in high yields derivatives of considerable interest, in particular for the perfumery industry, and in the sector of insecticides.
Owner:ENDURA SPA
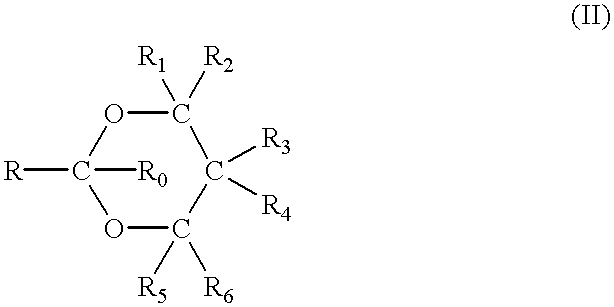
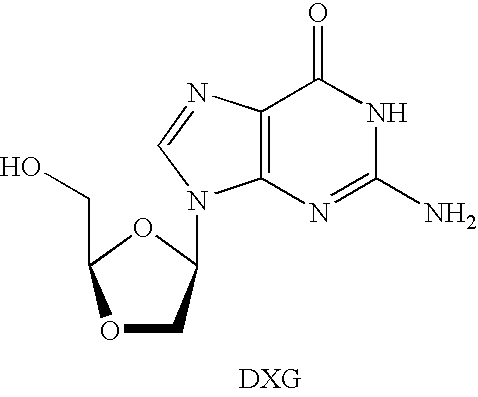
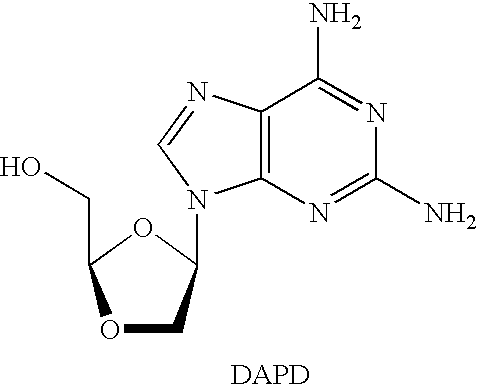
Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives
InactiveUS7560550B2High chemical yieldHigh stereoselectivitySilicon organic compoundsAntiviralsSilyleneSilanes
Glycosylation of 2,6-diaminopurines or silyl derivatives thereof with a compound of the structureis facilitated by the presence of an optionally silylated 1,3-dicarbonyl compound during glycosylation. Byproducts are minimized, while stereoselectivity may be altered by choice of Lewis acid and other reaction condictions.
Owner:RFS PHARMA
Fluorosulfonyl group-containing monomer and its polymer, and sulfonic acid group-containing polymer
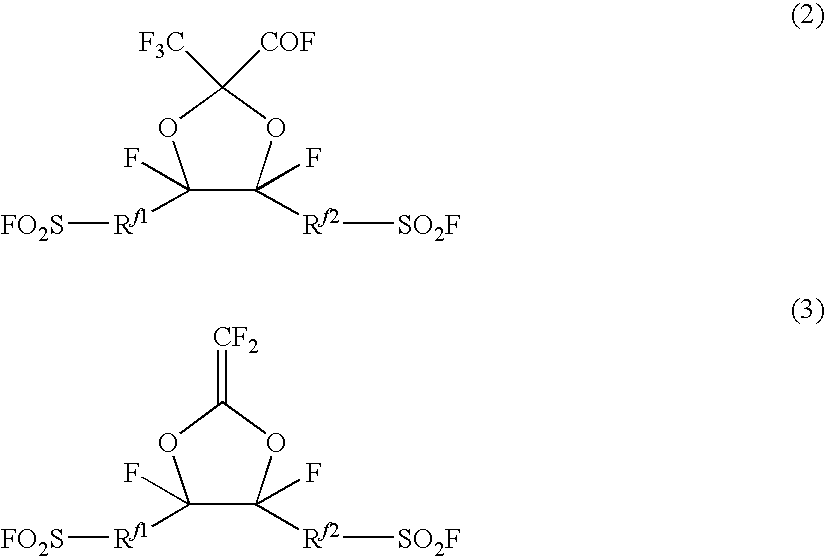
InactiveUS20090048424A1Easy to getHigh polymerization reactivityOrganic chemistryPolymer scienceOxygen atom
To provide a fluorosulfonyl group-containing monomer having a high polymerization reactivity and plural fluorosulfonyl groups. Further, to provide a fluorosulfonyl group-containing polymer and a sulfonic acid group-containing polymer, obtained by using the monomer.A perfluoro(2-methylene-1,3-dioxolane) derivative which is represented by the following formula (3) and which has two fluorosulfonyl groups, and its production process and its synthetic intermediate. A fluorosulfonyl group-containing polymer having monomer units represented by the following formula (3U) obtained by polymerizing the compound (3) by itself or with a comonomer, and a sulfonic acid group-containing polymer having the following units (5U) obtained by hydrolyzing a fluorosulfonyl group of the polymer. In the following formulae, each of Rf1 and Rf2 which are independent of each other, is a C1-8 perfluoroalkylene group which may have an etheric oxygen atom between carbon atoms.
Owner:ASAHI GLASS CO LTD
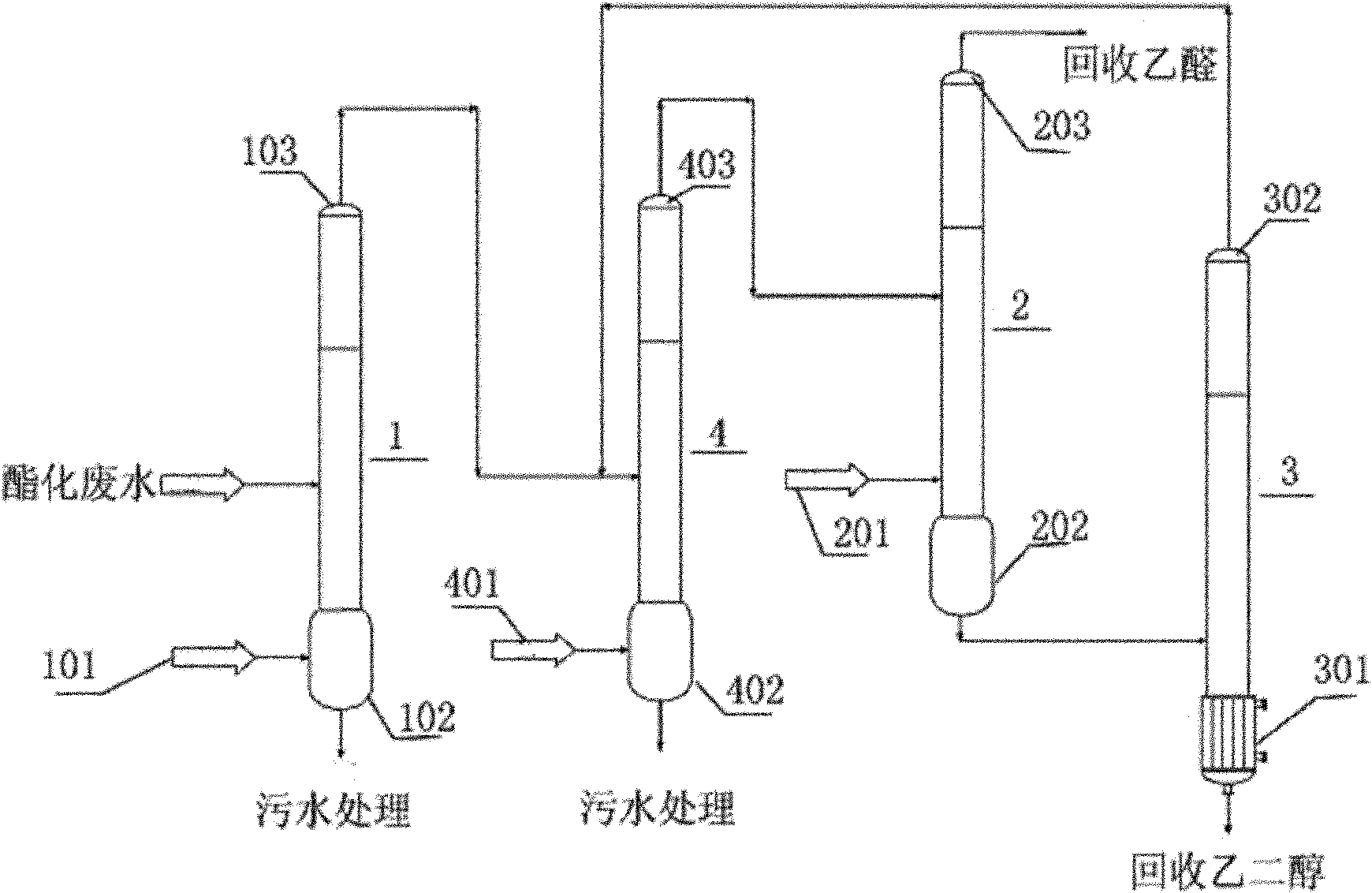
Method for recycling ethylene glycol and acetaldehyde from polyester waste water
ActiveCN102126926AReduce COD valueFix security issuesMultistage water/sewage treatmentWater/sewage treatment by degassingPolyesterDistillation
The invention discloses a method for recycling ethylene glycol and acetaldehyde from polyester waste water. The method is implemented by the following steps of: (1) introducing esterification waste water into a stripping tower for treating and directly introducing organic matter gas and water vapor collected from a tower top into an acetaldehyde rectifying tower; (2) treating in the acetaldehyde rectifying tower, collecting high-purity acetaldehyde from the tower top and introducing waste water collected from a tower bottom into an ethylene glycol distillation tower; and (3) treating in the ethylene glycol distillation tower, collecting a high-purity ethylene glycol solution from the tower bottom and introducing waste gas and water vapor produced on the tower top into the stripping tower for cyclic treatment. In the method, acetaldehyde and ethylene glycol are continuously collected, so that hydrolysis of byproduct of 2-methyl-1,3-dioxolane in the esterification waste water is continuously performed positively and negatively, acetaldehyde with the concentration of over 95 percent and ethylene glycol with the concentration of over 70 percent are obtained at the end of treatment, and the 2-methyl-1,3-dioxolane is fully hydrolyzed.
Owner:SHANGHAI JUYOU CHEM ENG +1
Process for preparation of 1,3-dioxolane-4-methanol compounds
PCT No. PCT / JP97 / 03165 Sec. 371 Date Feb. 8, 1999 Sec. 102(e) Date Feb. 8, 1999 PCT Filed Sep. 9, 1997 PCT Pub. No. WO98 / 11087 PCT Pub. Date Mar. 19, 1998A process for preparing easily and economically a 1,3-dioxolane-4-methanol compound in a racemic form or an optically active form with high purity and in high yield. The process comprises reacting an alkali metal or alkaline earth metal salt of an alcohol or a carboxylic acid with a halogenomethyl-1,3-dioxolane which is prepared by acetalizing a halogeno-1,2-propanediol of a formula (1) wherein X is a halogen atom, in an acid catalyst to conduct esterification or etherification, and then hydrolyzing the ester group and hydrogenolyzing the ether group to prepare a 1,3-dioxolane-4-methanol compound of a formula (5) wherein R1 and R2 are hydrogen atom, alkyl having 1 to 4 carbon atoms or phenyl, and R1 and R2 may form a cycloalkyl ring having 3 to 6 carbon atoms with the adjacent carbon atoms.
Owner:DAISO CO LTD
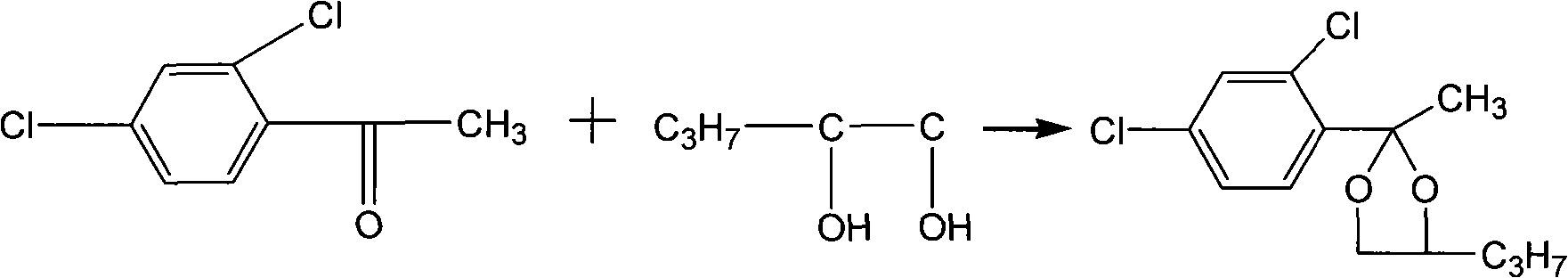
New method for producing propiconazole
ActiveCN101781290ASolve the problems of long reaction time, high water content and sticky materialsSimple processOrganic chemistryPropiconazoleBromine
The invention discloses a new method for producing propiconazole, comprising the following steps of: cyclizing 2,4-dichloroacetophenone and 1,2-pentanediol, brominating with bromine to generate 2-brooethyl-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolane, then condensing with 1,2,4-tolyltriazole sylvite to prepare a crude product, and desolventizing, washing and distilling the crude product in vacuum to obtain the propiconazole. When the brominating reaction is carried out after the cyclizing reaction is finished, the bromine is added for twice, firstly the bromine accounting for 1%-10% of the total weight of the bromine is added in a reaction system for brominating induction reaction, and then the residual bromine is added. The bromine is added for twice during brominating reaction, firstly the bromine accounting for 1%-10% of the total weight of the bromine is added for initiating the whole system, the brominating induction reaction guides the direction of the brominating reaction so that monobromides and dibromides generating ketone due to the repeated resolution of cyclized matter are reduced, and finally the method achieves the purpose of improving the yield of the brominating reaction; and the conversion rate of the bromides is high and can be greater than 97%.
Owner:利民化学有限责任公司
Ibuprofen salt emulsifiers and cream formulations containing same
Emulsions and creams containing ibuprofen effective for topical administration of the ibuprofen are provided without the presence of conventional O / W emulsifying agent. The emulsions and creams are formed by using the ibuprofen, in the form of its salt, as the O / W emulsifying agent. A minor amount of a W / O emulsifying agent may be included in the composition. Percutaneous delivery of ibuprofen is improved by using a skin penetration enhancing compound, such as 2-n-nonyl-1,3-dioxolane or decanaldimethyl acetal, as the oily phase of the emulsion.
Owner:MACROCHEM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
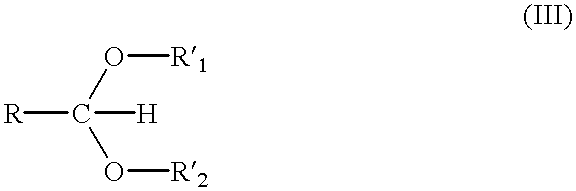
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00001.png)
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00002.png)
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00003.png)


![Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/92aba963-8965-4583-9cef-490ebeedc054/US08802868-20140812-D00001.png)
![Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/92aba963-8965-4583-9cef-490ebeedc054/US08802868-20140812-D00002.png)
![Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide Solid forms of (R)-1(2,2-difluorobenzo[D][1,3]dioxo1-5-yl)-N-(1-(2,3-dihydroxypropyl-6-fluoro-2-(1-hydroxy-2-methylpropan2-yl)-1H-Indol-5-yl)-Cyclopropanecarboxamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/92aba963-8965-4583-9cef-490ebeedc054/US08802868-20140812-D00003.png)
![Tetrahydrofuro [3,4-D] dioxolane compounds for use in the treatment of viral infections and cancer Tetrahydrofuro [3,4-D] dioxolane compounds for use in the treatment of viral infections and cancer](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3d019e20-9f9e-41d5-99b4-d31b4ff3c58e/US08242085-20120814-C00001.png)
![Tetrahydrofuro [3,4-D] dioxolane compounds for use in the treatment of viral infections and cancer Tetrahydrofuro [3,4-D] dioxolane compounds for use in the treatment of viral infections and cancer](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3d019e20-9f9e-41d5-99b4-d31b4ff3c58e/US08242085-20120814-C00002.png)
![Tetrahydrofuro [3,4-D] dioxolane compounds for use in the treatment of viral infections and cancer Tetrahydrofuro [3,4-D] dioxolane compounds for use in the treatment of viral infections and cancer](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3d019e20-9f9e-41d5-99b4-d31b4ff3c58e/US08242085-20120814-C00003.png)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00001.PNG)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00002.PNG)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00003.PNG)

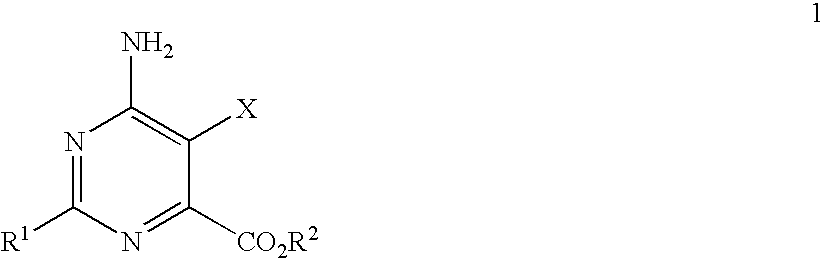

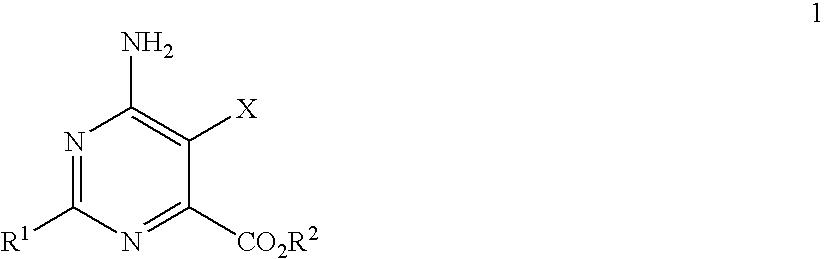



![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00001.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00002.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00003.png)


![Process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols Process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd81ecae-b732-4be7-8382-eea755718ca9/US06342613-20020129-C00001.png)
![Process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols Process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd81ecae-b732-4be7-8382-eea755718ca9/US06342613-20020129-C00002.png)
![Process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols Process for the synthesis of 5-(alpha-hydroxyalkyl) benzo[1,3]dioxols](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd81ecae-b732-4be7-8382-eea755718ca9/US06342613-20020129-C00003.png)
![Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d059af67-daa6-4b9f-8b4a-5e15884fccc5/US07560550-20090714-C00001.png)
![Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d059af67-daa6-4b9f-8b4a-5e15884fccc5/US07560550-20090714-C00002.png)
![Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives Method for the production of OH protected[4-(2.6-diamino-9H-purine-9-yl)-1.3-dioxolane-2-yl]methanol derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d059af67-daa6-4b9f-8b4a-5e15884fccc5/US07560550-20090714-C00003.png)