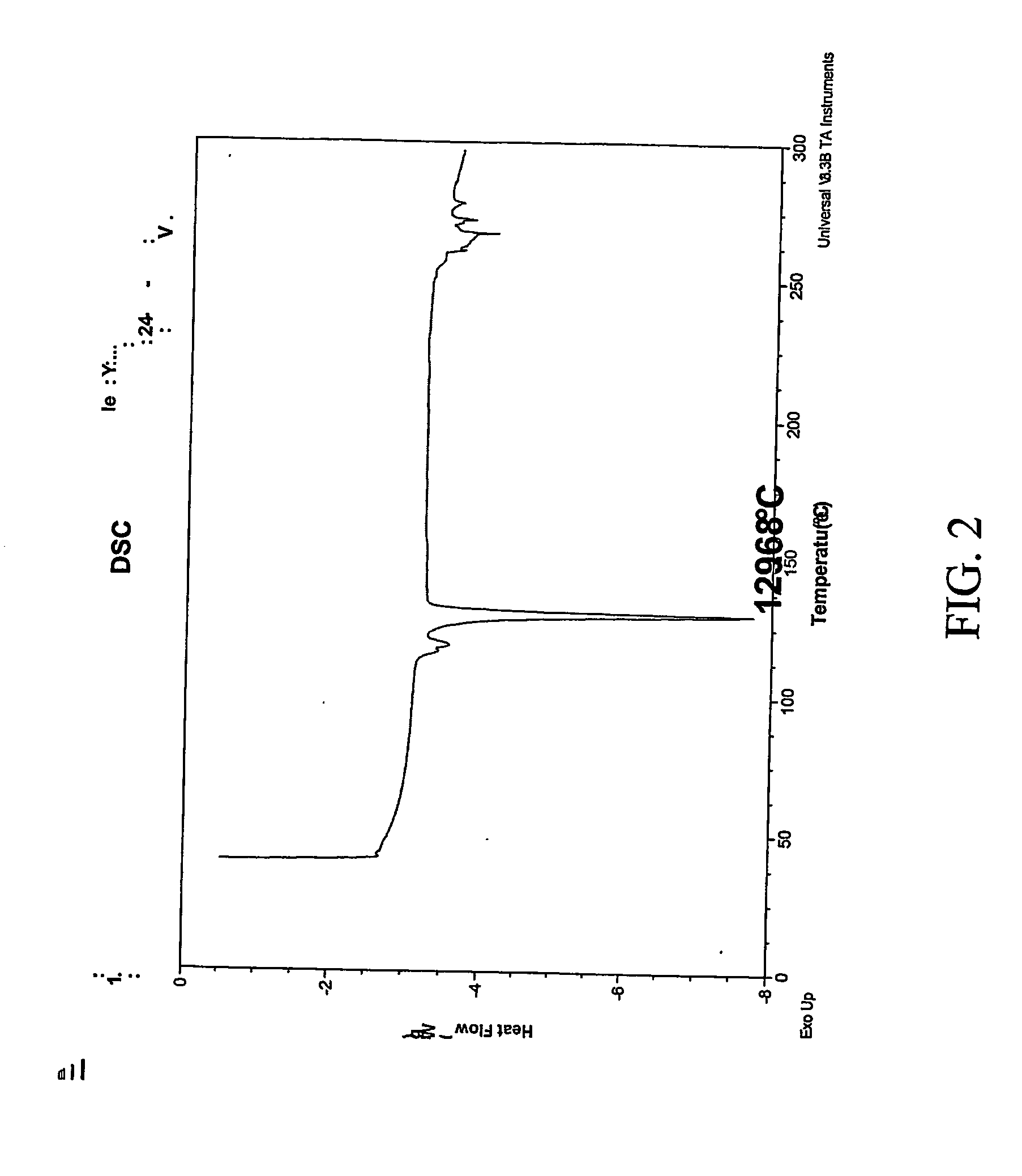
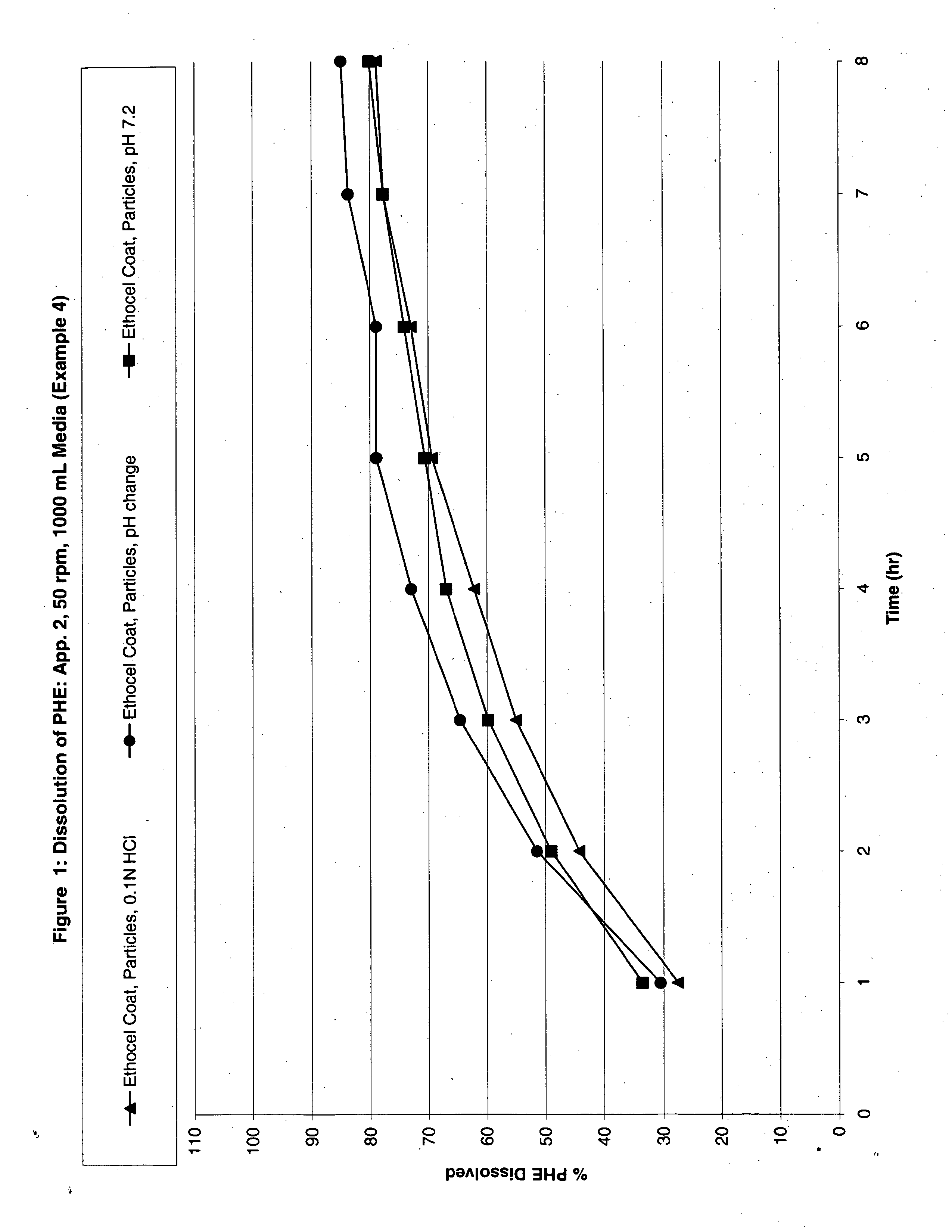
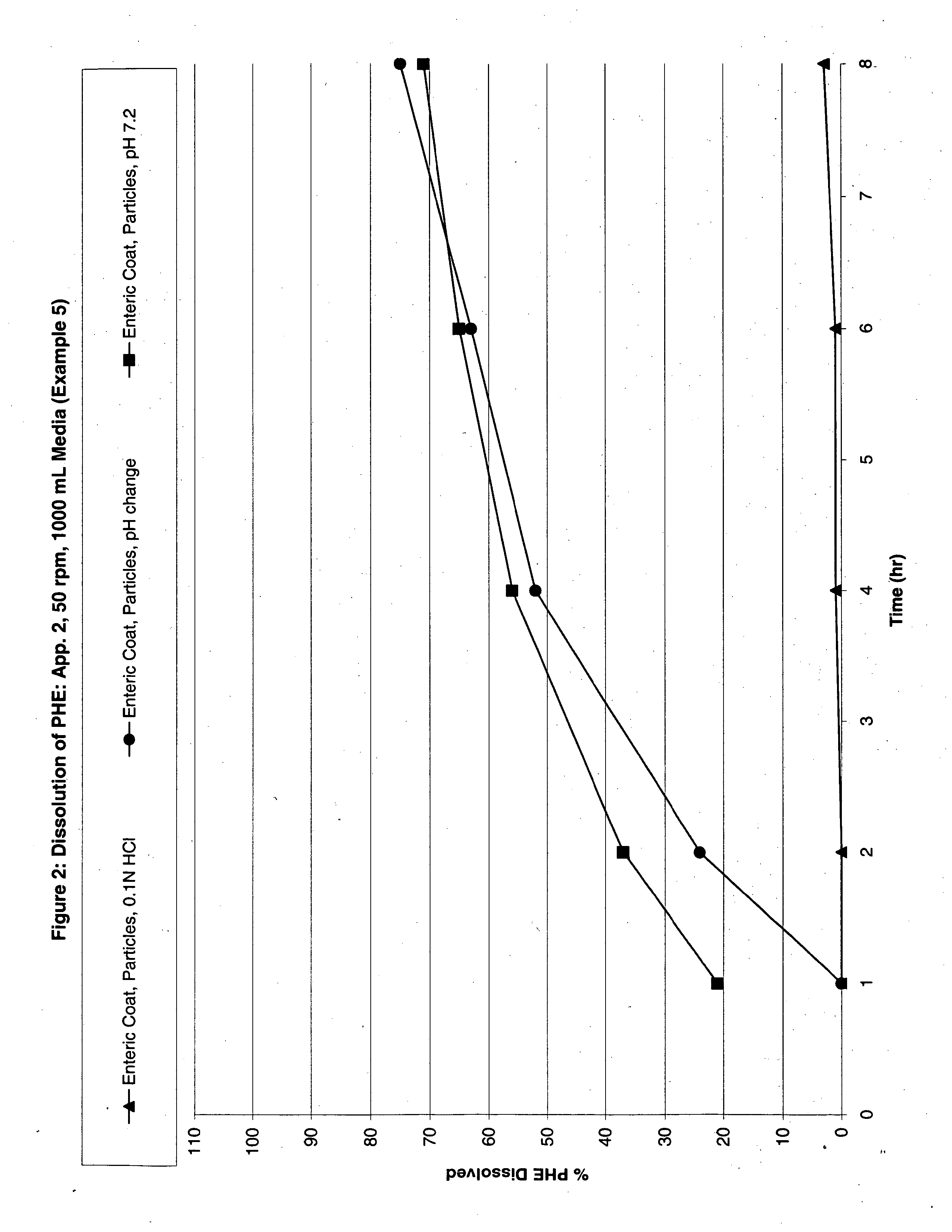
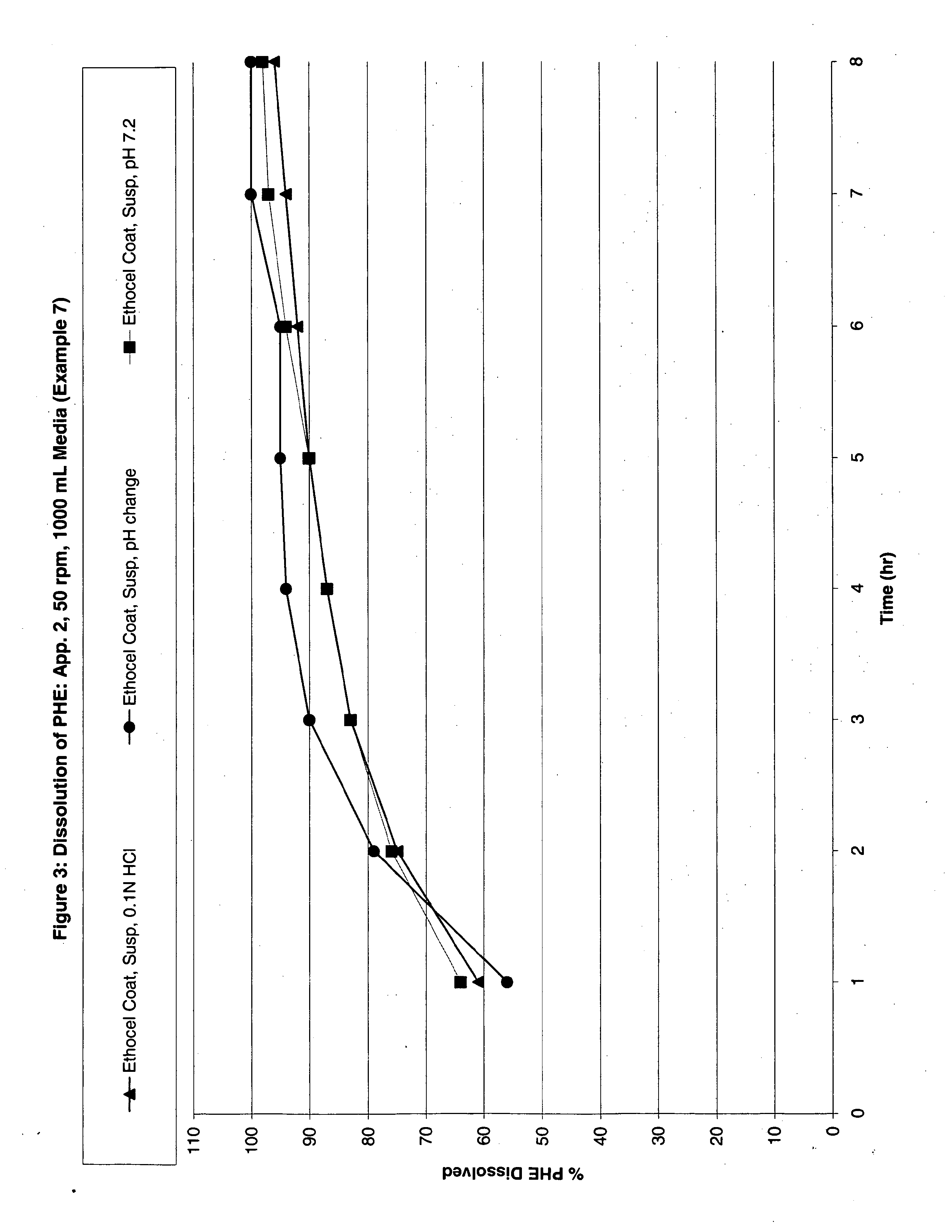
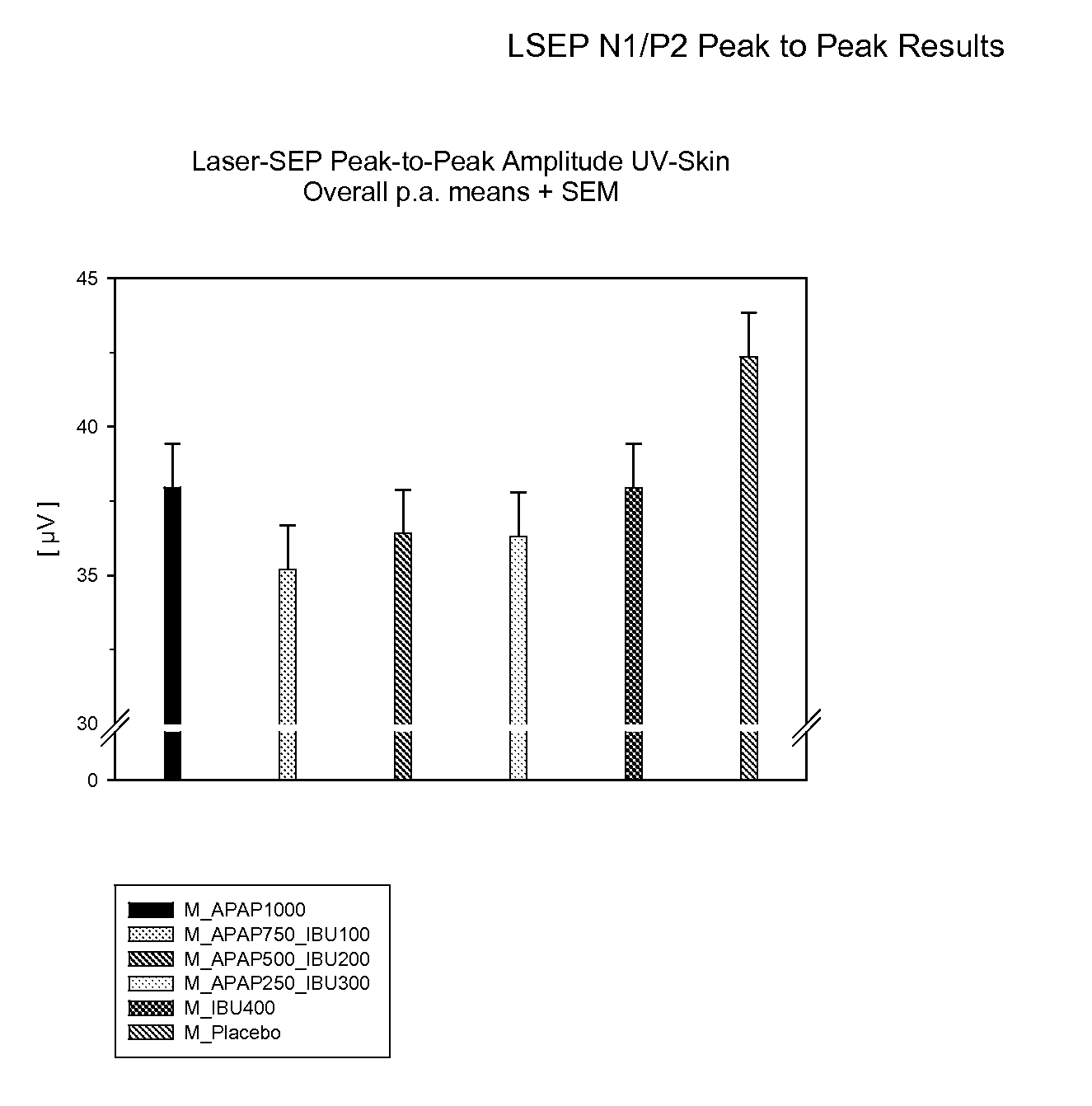
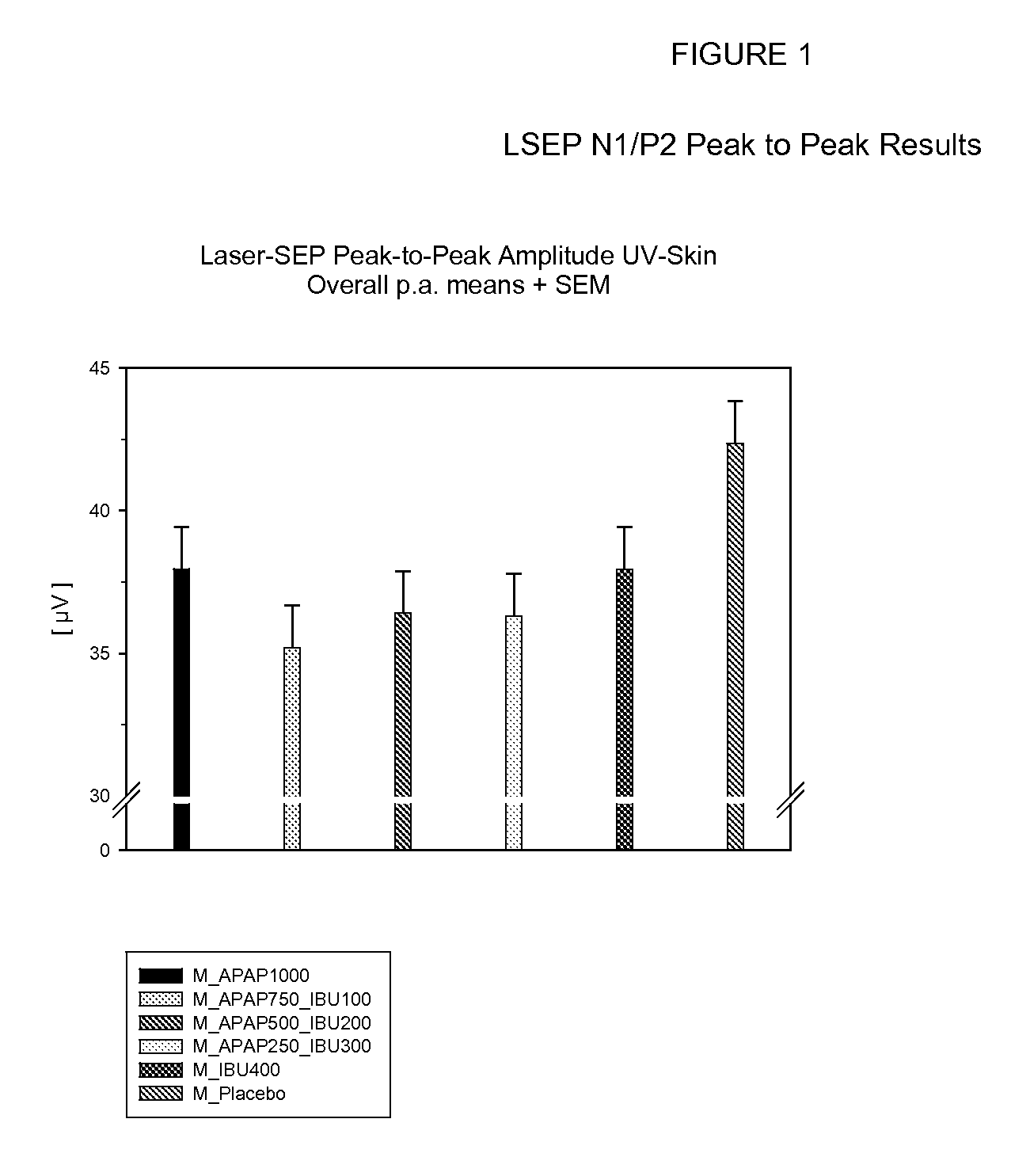
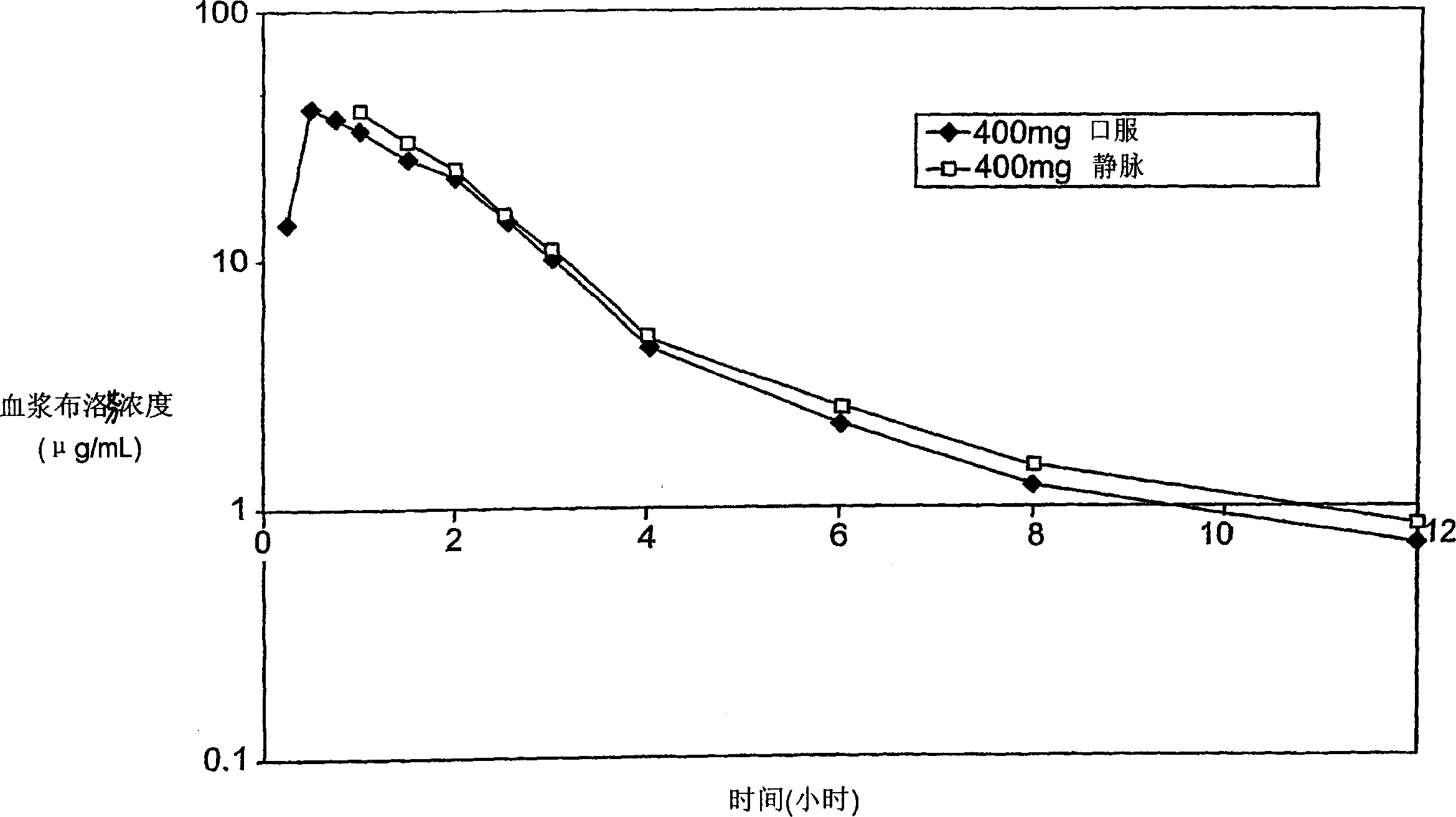
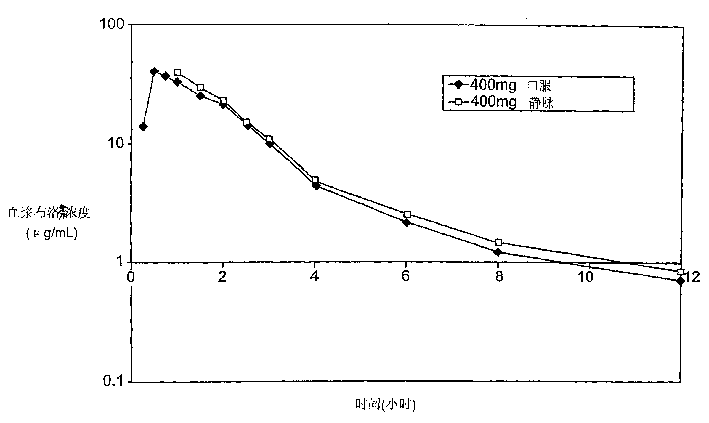
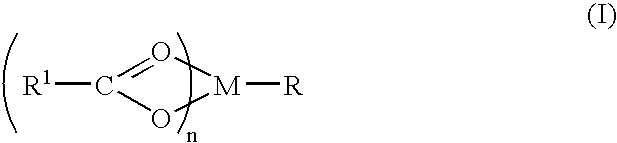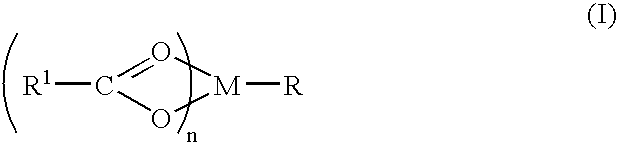Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
880 results about "Ibuprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
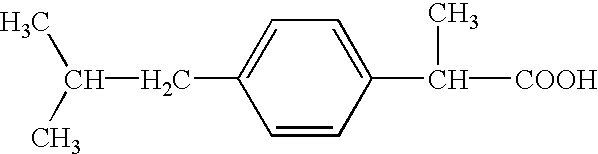
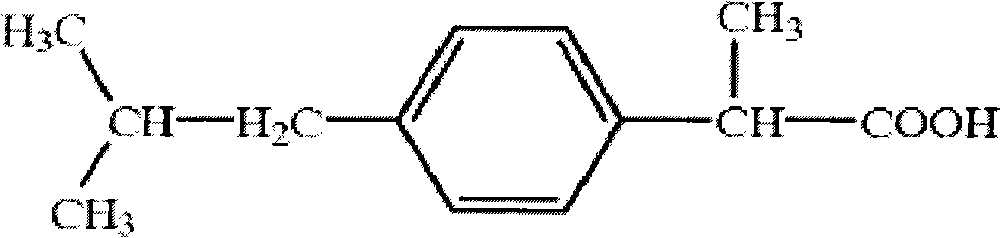
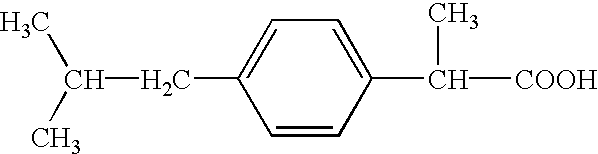
Ibuprofen is used to relieve pain from various conditions such as headache, dental pain, menstrual cramps, muscle aches, or arthritis. It is also used to reduce fever and to relieve minor aches and pain due to the common cold or flu.
Formulations of nonopioid and confined opioid analgesics
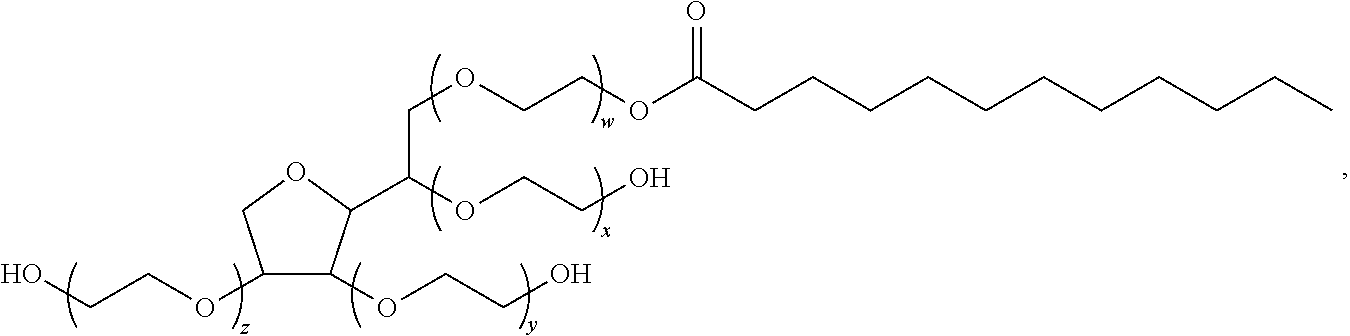
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
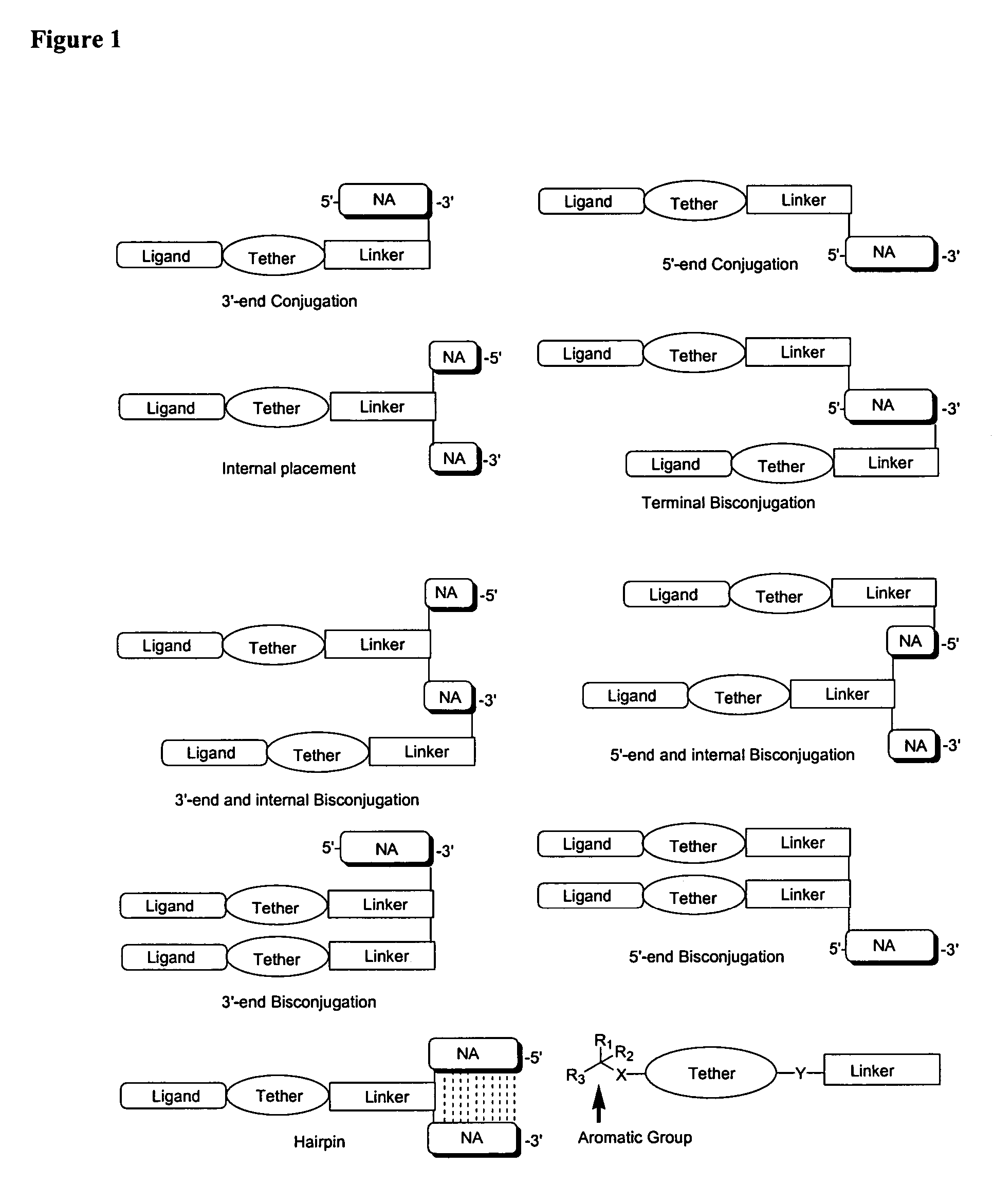
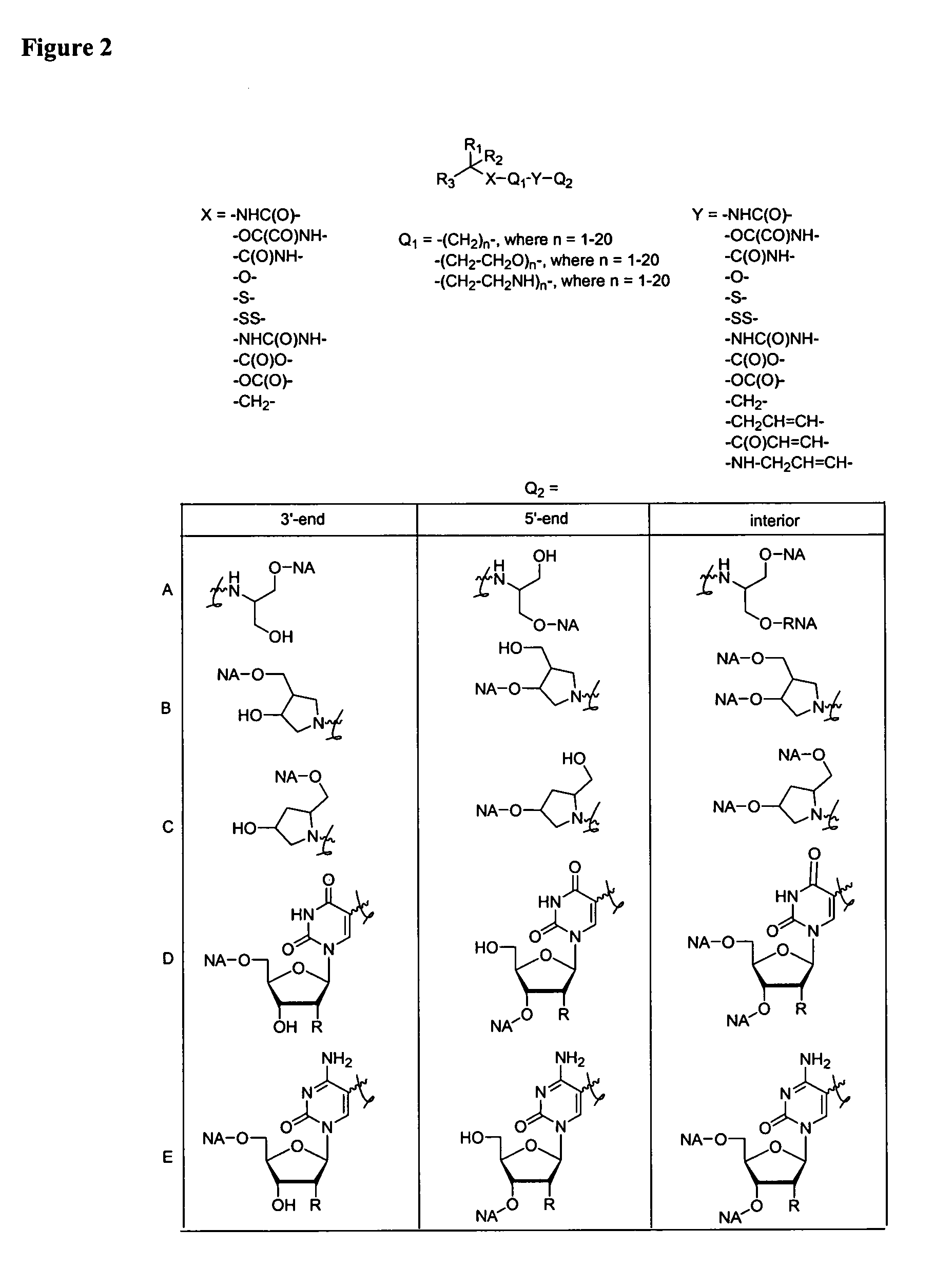
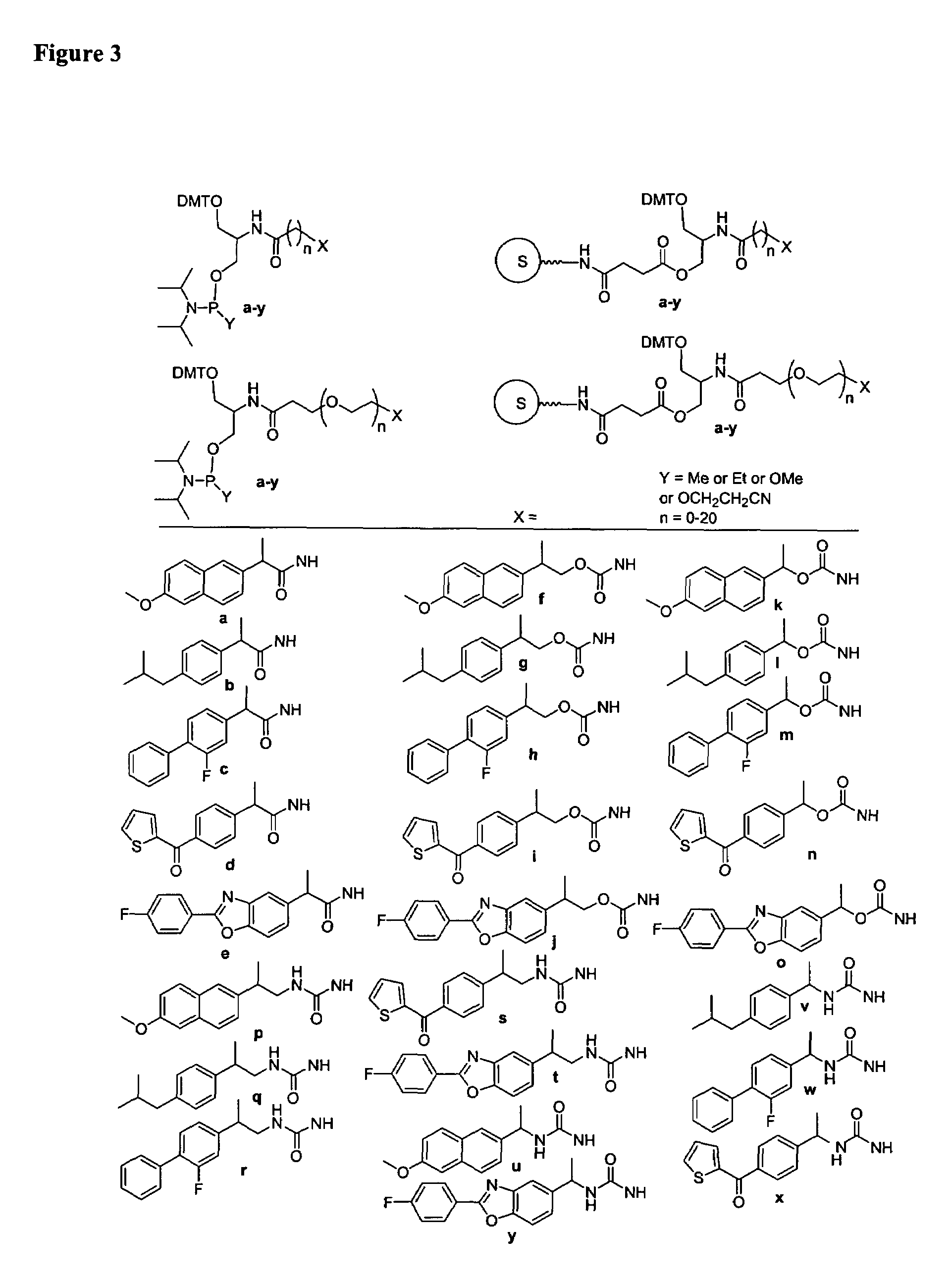
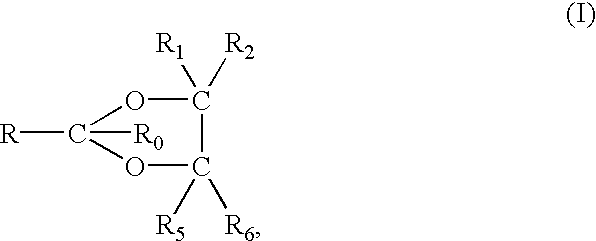
Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety
ActiveUS20060008822A1Improved pharmacokinetic propertiesAntibacterial agentsSenses disorderNucleotidePhosphate
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one aralkyl ligand. In certain embodiments, an aralkyl ligand is bound to only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, an aralkyl ligand is bound to both of the oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage. In a preferred embodiment, the aralkyl ligand is naproxen or ibuprofen. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one aralkyl ligand. In certain embodiments, the oligonucleotide comprises at least one modified sugar moiety. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage. In a preferred embodiment, the aralkyl ligand is naproxen or ibuprofen. The aralkyl ligand improves the pharmacokinetic properties of the oligonucleotide.
Owner:ALNYLAM PHARM INC
Sustained release matrix for high-dose insoluble drugs
Sustained release dosage forms of high dose insoluble drugs such as ibuprofen and methods for their manufacture are disclosed.
Owner:PENWEST PHARMA CO
Pharmaceutical formulations comprising ibuprofen, oxycodone, and 14-hydroxycodeinone
The present invention relates to pharmaceutical formulations of ibuprofen, oxycodone and 14-hydroxycodeinone and their use for the treatment of acute, moderate to severe pain.
Owner:FOREST LABORATORIES
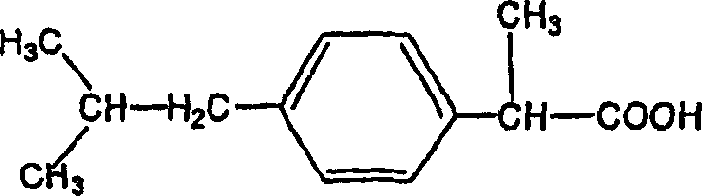
Pharmaceutical composition of 2-(4-isobutylphenyl) propionic acid
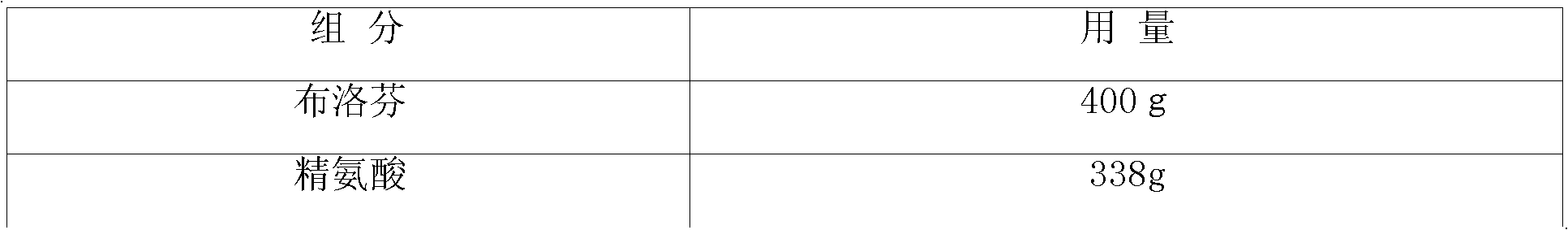
The present invention provides a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1, as well as a method of making the same. The present invention also provides a method of treating a condition chosen from pain, inflammation, fever, and / or other conditions alleviated by ibuprofen comprising administering a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1.
Owner:CUMBERLAND PHARM INC
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Modified release analgesic suspensions
A pharmaceutical dosage form comprising non-steroidal-anti-inflammatory drugs, in particular propionic acid derivatives such as ibuprofen, along with a second active ingredient having a shorter therapeutically effective plasma concentration duration, such as phenylephrine, and methods of administering the same are provided. This method provides improved therapeutic effect, in particular pain relief along with decongestant relief, over extended time periods.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety
ActiveUS7626014B2Improved pharmacokinetic propertiesAntibacterial agentsSenses disorderSingle strandDouble strand
The present invention provides single-stranded and double-stranded oligonucleotides comprising at least one aralkyl ligand that improves the pharmacokinetic properties of the oligonucleotides. The aralkyl ligands of the present invention include naproxen, ibuprofen and derivatives thereof. The present invention also provides methods for modulating gene expression using the modified oligonucleotide compounds and compositions comprising those modified oligonucleotides.
Owner:ALNYLAM PHARMA INC
Ibuprofen composition
The invention relates to an immediate release tablet capable of being chewed or disintegrated in the oral cavity, which comprises a plurality of particles in a matrix. The particles contain an active ingredient, such as ibuprofen, and a first water soluble acid having a solubility greater than about 10 g / 100 mL water at 20° C.; and the matrix contains a second water soluble acid having a solubility less than about 5 g / 100 mL water at 20° C.
Owner:MCNEIL PPC INC
Compositions for Preventing and Reducing Delayed Onset Muscle Soreness
InactiveUS20080317886A1Inhibit inflammationSpeed up recoveryBiocideSugar food ingredientsSucroseL glutamate
The present invention relates to the compositions that enhance post-exercise recovery processes to increase both strength and muscle mass, replace glycogen stores, and prevent inflammation, resulting in the prevention and / or reduction of delayed onset muscle soreness. Additionally, it provides a feeling of muscle relaxation as well as a feeling of mental tranquility immediately following exercise. The composition consists of any or all high-glycemic sugars and / or polysaccharides (e.g., sucrose, glucose, maltodextrin), all essential amino acids and beta-hydroxy-beta-methylbutyrate and can include other amino acids sources (e.g. whey protein), performance enhancing agents (e.g., caffeine, L-glutamate), anti-inflammatory agents (e.g., ginger, boswellia, curcumen), antioxidants (vitamin C, vitamin E, selenium, polyphenols,), insulin-mimicking agents (cinnamon, Banaba), analgesics (e.g. aspirin, ibuprofen, naproxen, acetaminophen), and to methods of treating humans and animals by administration of these novel compositions to humans and animals in need thereof.
Owner:SOUTHWEST IMMUNOLOGY
Non-steroidal antiinflammatory drug formulations for topical application to the skin
InactiveUS20030082226A1Improve performanceSignificant positive effectOrganic active ingredientsAntipyreticSkin penetrationAntiinflammatory drug
Topical alcoholic or aqueous alcoholic gels containing ibuprofen or other NSAIDs, such as, naproxen, in substantially neutral salt form, have enhanced penetration through skin and may provide rapid pain / inflammation relief by including in the formulation 2-n-nonyl-1,3-dioxolane or other hydrocarbyl derivative of 1,3-dioxolane-or 1,3-dioxane or acetal, as skin penetration enhancing compound. The amount of propylene glycol may be varied to adjust the initial flux of the NSAID through the skin, especially for ibuprofen, naproxen, and ketorolac.
Owner:SAMOUR CARLOS M +2
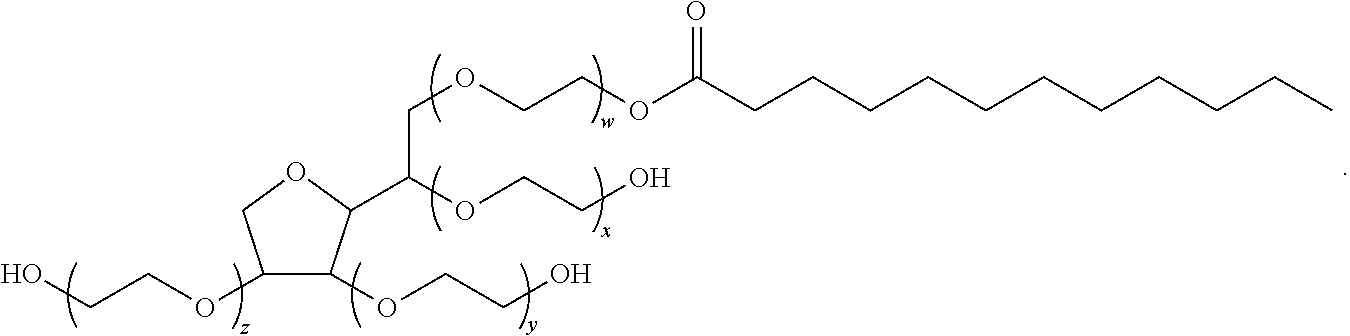
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Acetaminophen / ibuprofen combinations and method for their use
A pharmaceutical dosage form is provided comprising a non-steroidal anti-inflammatory agent and acetaminophen, and methods for their use. In one embodiment, the dosage form is comprised of ibuprofen and acetaminophen as the sole pharmaceutically effective agents, wherein the ibuprofen and acetaminophen are in a weight ratio of about 12 parts:about 88 parts.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
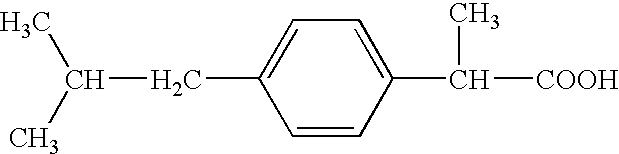
2-(4-isobutylphenyl) propionic acid medicinal composition
The present invention provides a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1, as well as a method of making the same. The present invention also provides a method of treating a condition chosen from pain, inflammation, fever, and / or other conditions alleviated by ibuprofen comprising administering a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1.
Owner:CUMBERLAND PHARM INC
Anti-Inflammatory and Antioxidant Conjugates Useful for Treating Metabolic Disorders
InactiveUS20100234452A1Improves anti-diabetic effectEliminate side effectsBiocideOrganic chemistryAntioxidantTriflusal
The present invention is directed to methods for treating metabolic disorders with compounds that are conjugates. The conjugates of the present invention are comprised of salicylic acid, triflusal, diflusinal, salsalate, IMD-0354, ibuprofen, diclofenac, licofelone, or HTB, and one or more antioxidants.
Owner:GENMEDICA THERAPEUTICS SL
Topical composition containing ibuprofen
The present invention generally relates to the transdermal delivery of various compositions. In some aspects, the transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising ibuprofen and / or an ibuprofen salt, a nitric oxide donor, and optionally, a hostile biophysical environment. In some cases, the composition may be stabilized using a stabilization polymer such as xanthan gum, KELTROL® BT and / or KELTROL® RD; propylene glycol; and a polysorbate surfactant such as Polysorbate 20, which unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
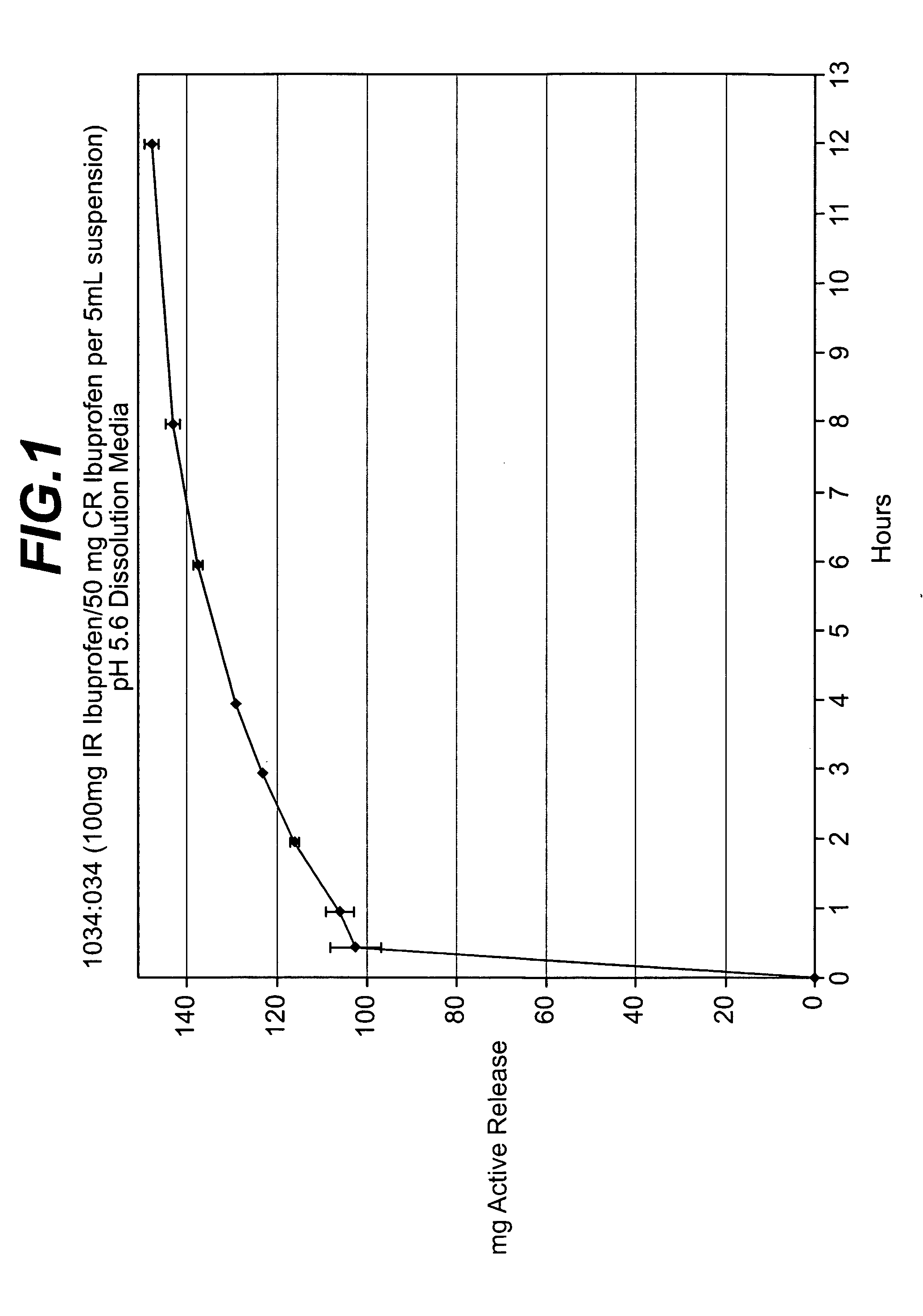
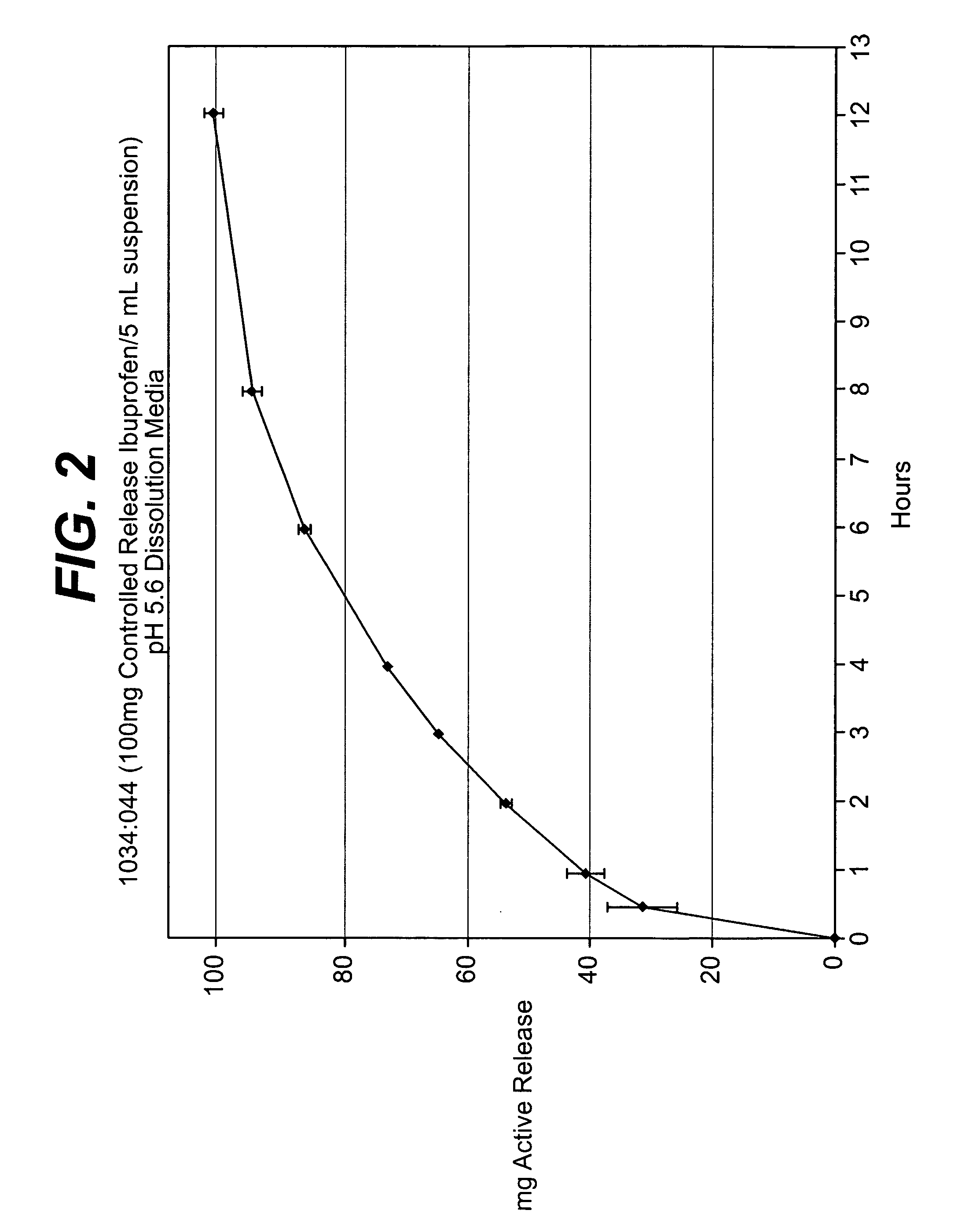
Controlled release analgesic suspensions
InactiveUS20050095300A1Maximum flexibilityPowder deliveryOrganic active ingredientsControlled releaseTreatment effect
A method of administering non-steroidal-anti-inflammatory drugs, in particular propionic acid derivatives such as ibuprofen, or acetaminophen via a liquid suspension is provided. This method provides improved therapeutic effect, in particular pain relief, over extended time periods.
Owner:MCNEIL PPC INC
Solubilized ibuprofen
ActiveUS20090175940A1Easy to produceIncreased blood levelsBiocideOrganic compound preparationSolubilityPotassium hydroxide
A process for producing a solubilized ibuprofen, preferably in the form of a granulate, the process comprising the steps of: providing a mixture comprising solid ibuprofen and a first base selected from the group consisting of sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, sodium glycinate, potassium glycinate and tribasic sodium and potassium phosphates and mixtures thereof, and reacting the ibuprofen and the first base in essentially dry state. The obtainable granulate and the pharmaceutical compositions and dosage forms that may be produced therefrom are distinguished by their high solubility and rapid disintegration and dissolution in aqueous media, by their good flow properties and compressibility, by rapidly achieving onset of analgesic effect.
Owner:LOSAN PHARMA GMBH
Combined nsaid and acetaminophen formulation and method
InactiveUS20090264530A1Reduce gastrointestinal irritationGood pain reliefBiocideNervous disorderCo administrationGastrointestinal irritation
The present invention is directed to co-administration of a non-steroidal anti-inflammatory agent (NSAID) and acetaminophen for the treatment of pain and inflammation with reduced gastrointestinal irritation. A pharmaceutical composition suitable for the co-administration contains a therapeutically effective amount of at least one non-steroidal anti-inflammatory agent, and a therapeutically effective amount of acetaminophen or a pharmaceutically acceptable salt thereof. A ratio of the non-steroidal anti-inflammatory agent to acetaminophen in the composition is within a range that provides greater pain relief and reduction of inflammation with less gastrointestinal irritation than that obtainable by the administration of the non-steroidal anti-inflammatory agent or acetaminophen alone. Examples of pharmaceutical compositions for co-administration of the agents are those containing ibuprofen in combination with the acetaminophen.
Owner:NICKELL ROBERT P
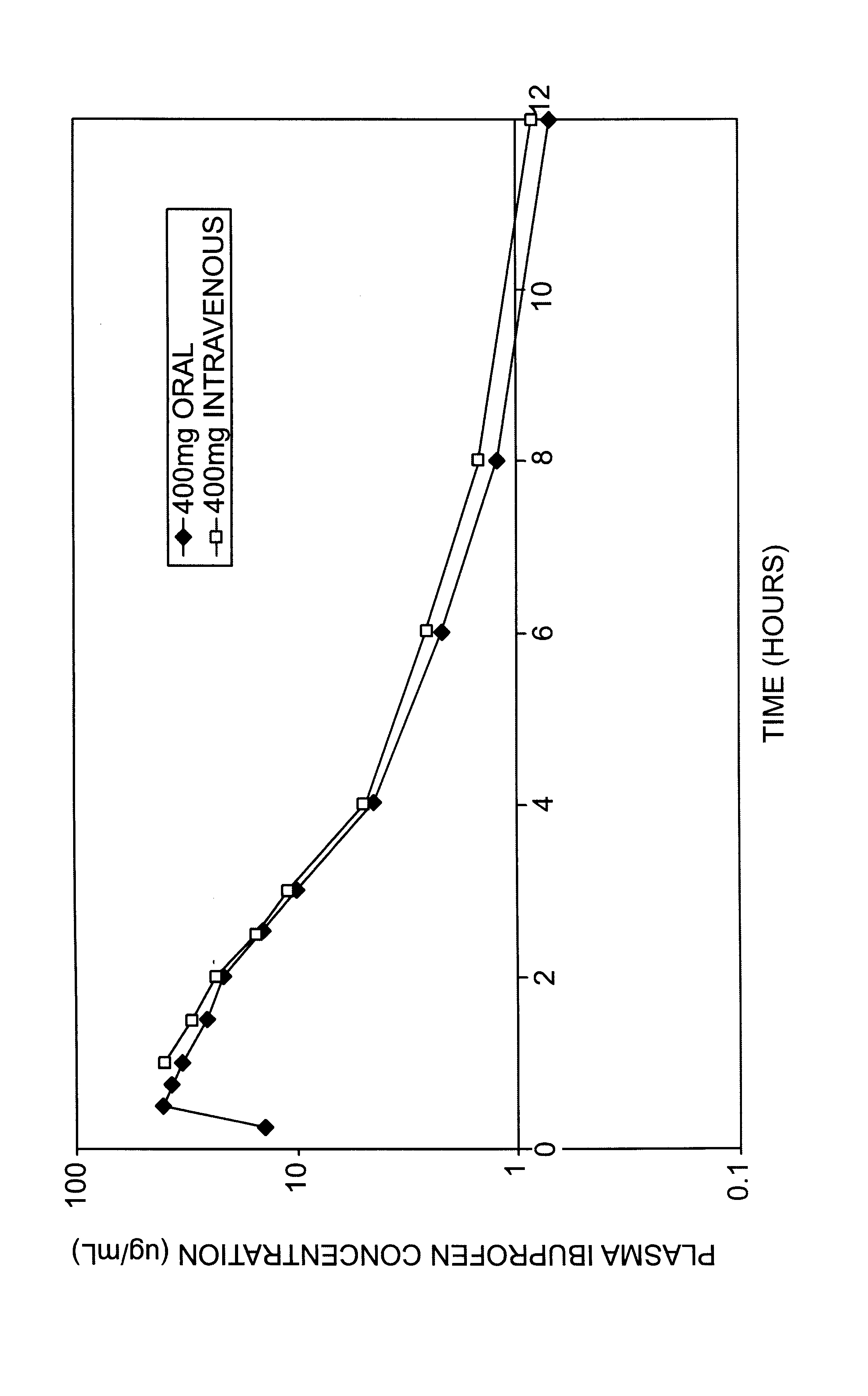
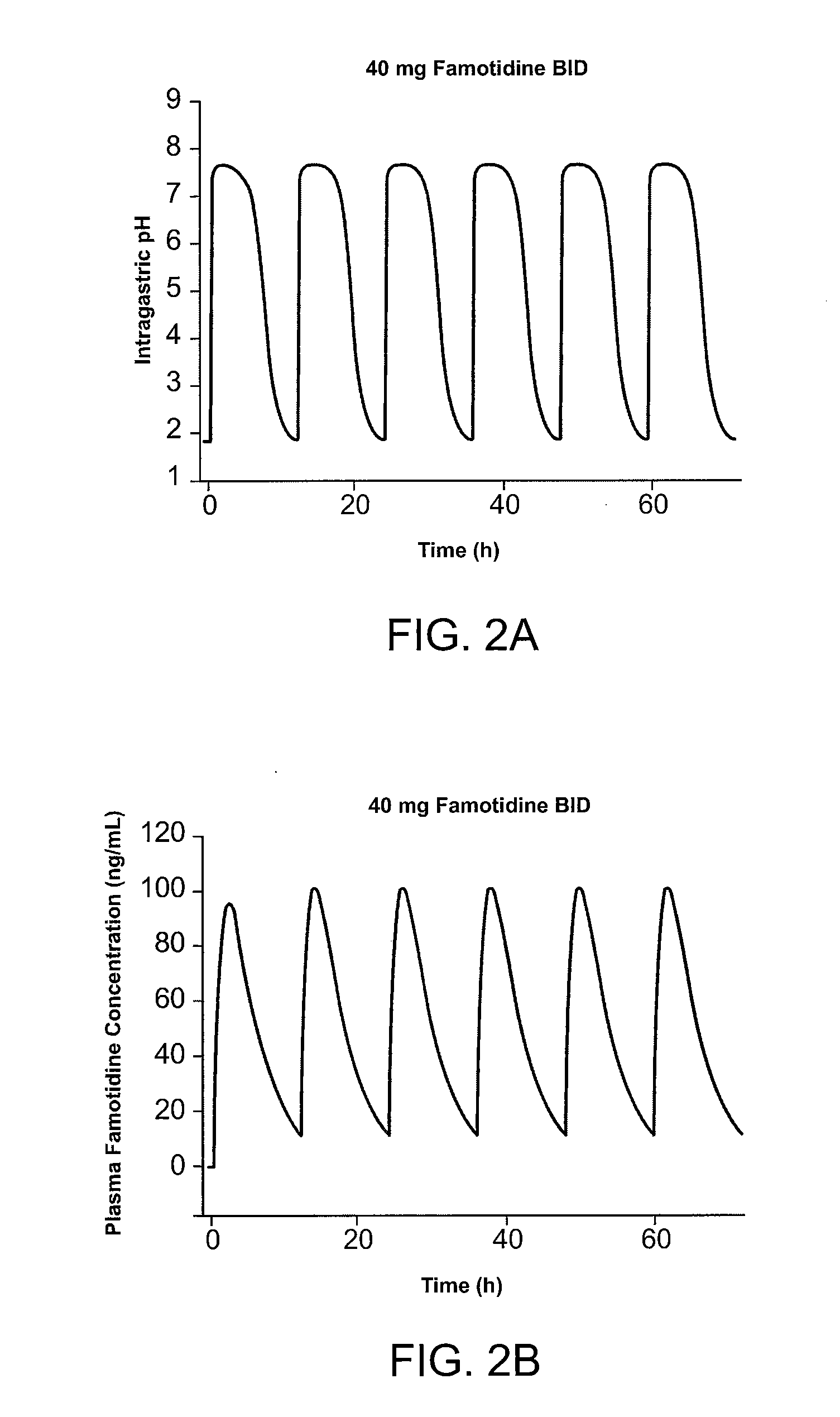
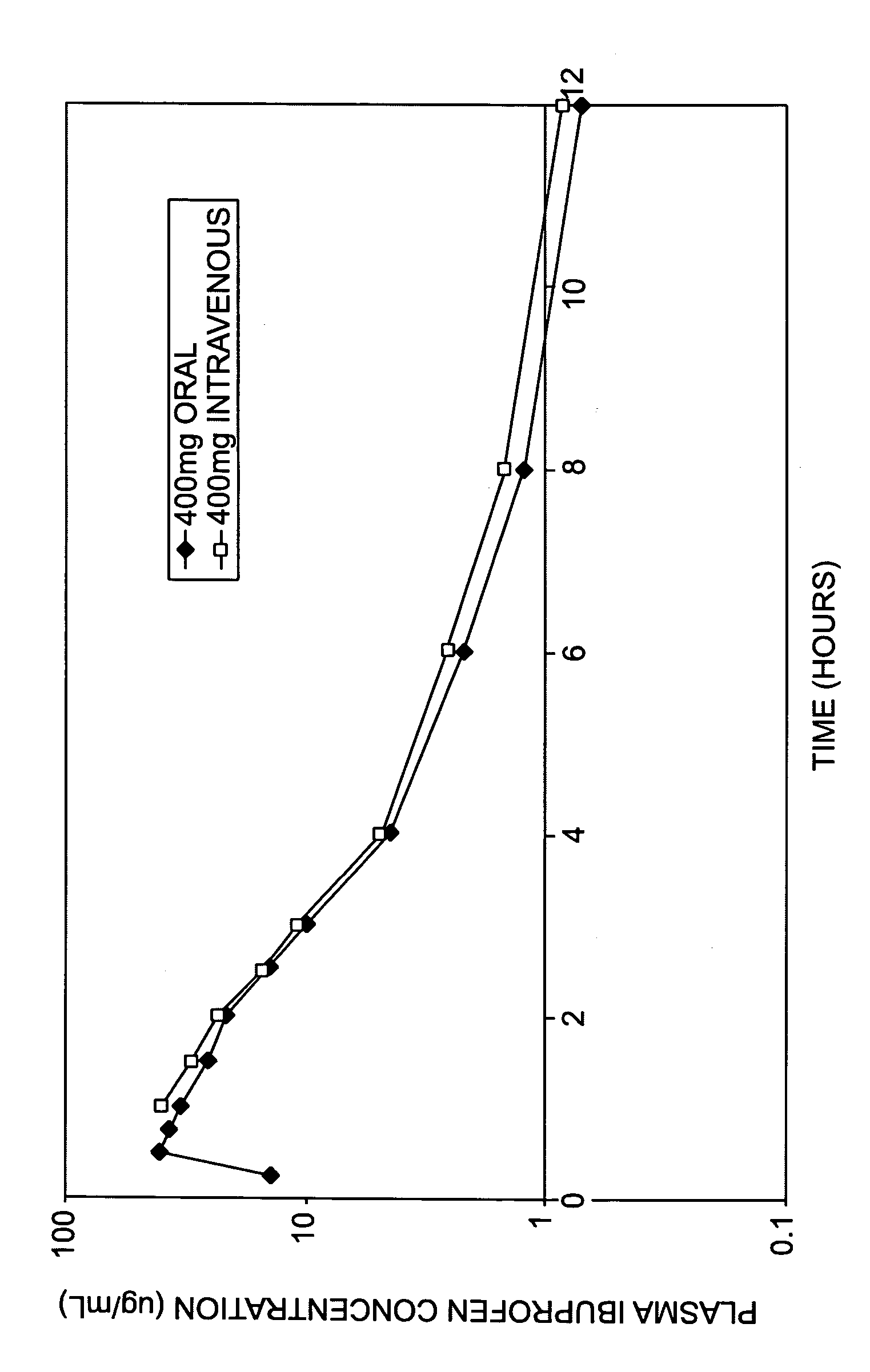
Methods and Medicaments for Administration of Ibuprofen
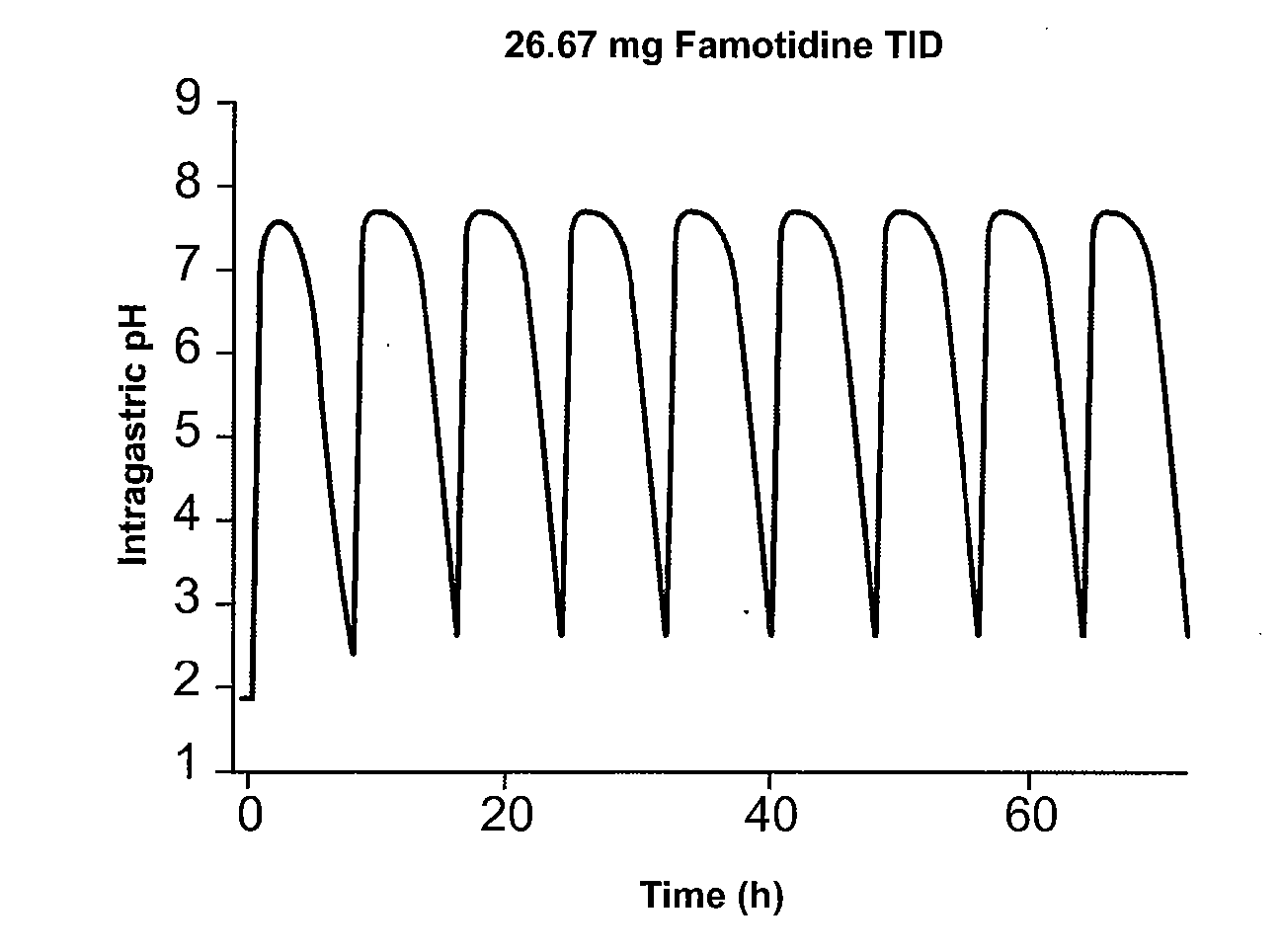
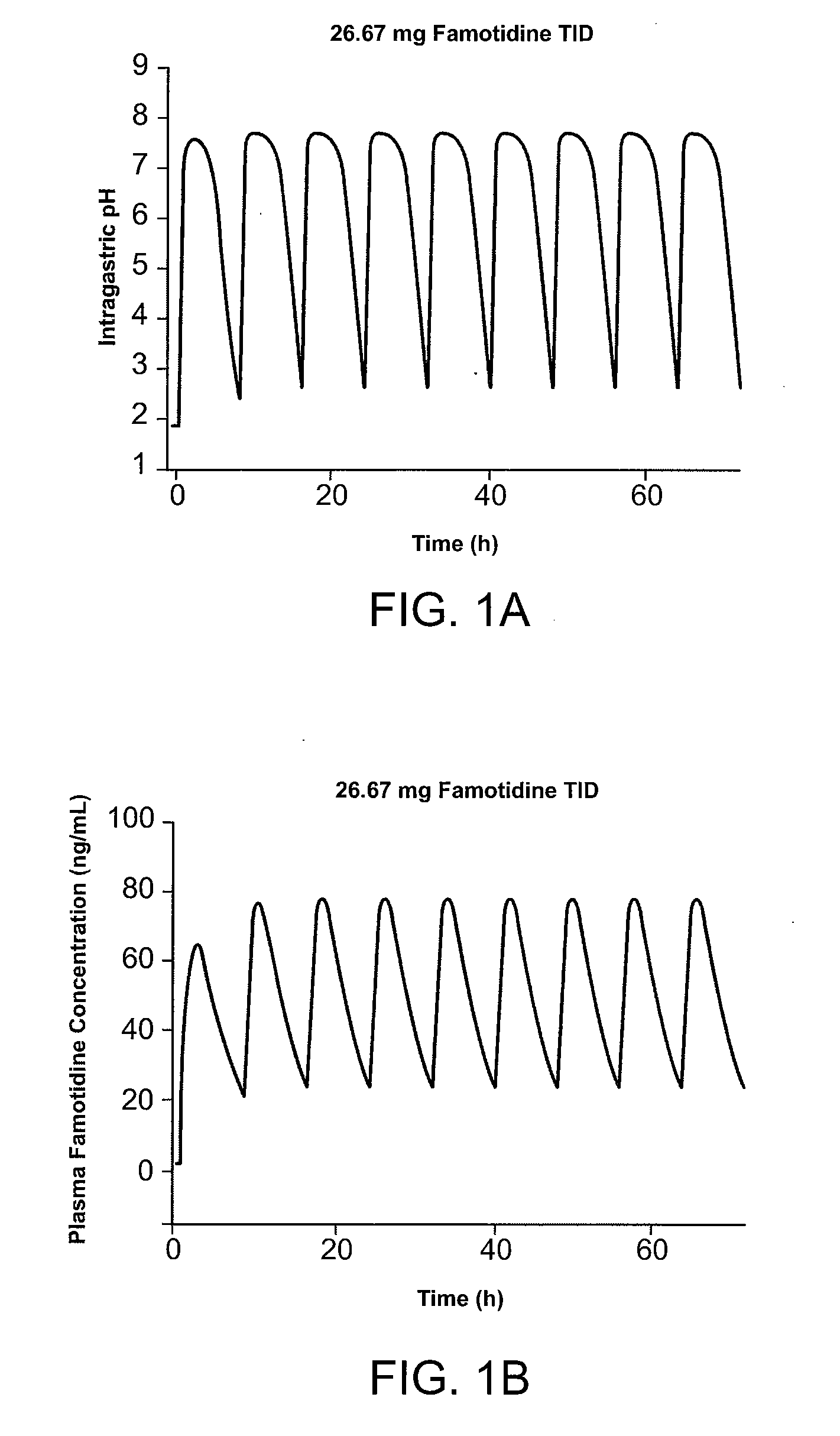
ActiveUS20080063706A1Reduce the possibilityReducing patient-to-patient variabilityBiocideAntipyreticFamotidineIbuprofen
A method for administration of ibuprofen to a subject in need of ibuprofen treatment is provided, in which an oral dosage form comprising a therapeutically effective amount of ibuprofen and a therapeutically effective amount of famotidine is administered three times per day.
Owner:HORIZON MEDICINES LLC
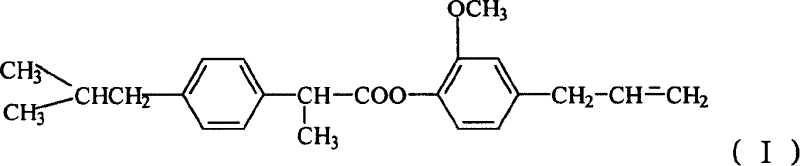
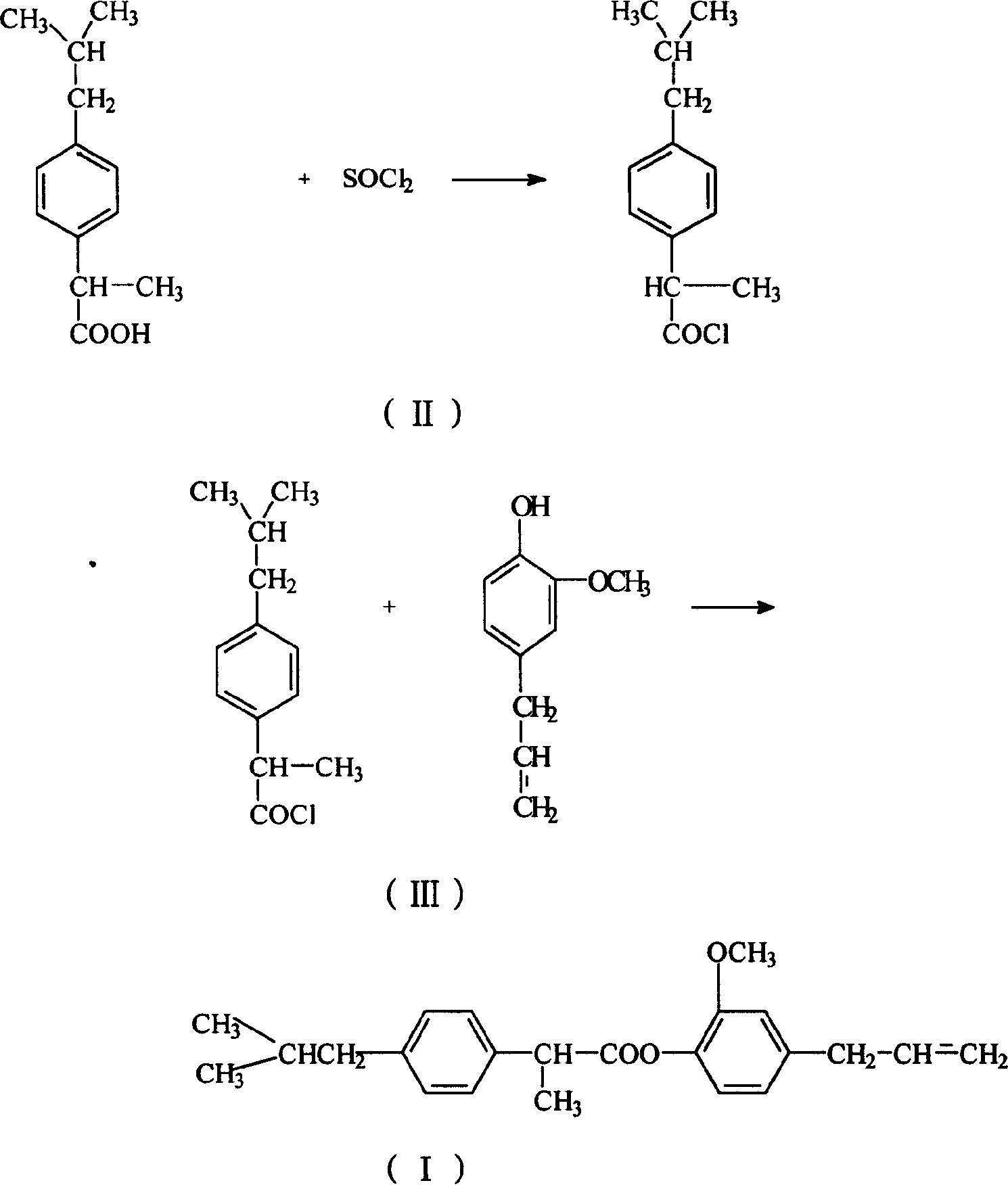
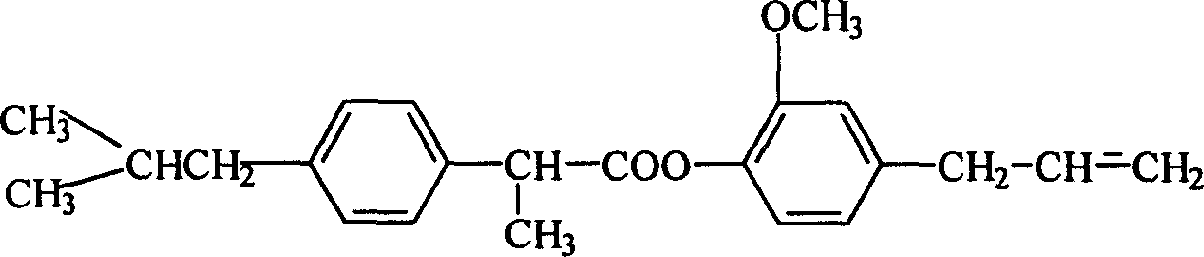
Eugenol ibuprofen ester medical compound and its preparation and preparation method
InactiveCN1597656AImprove stabilityExcellent pharmacological activityOrganic active ingredientsSenses disorderBenzeneEugenol
A medical eugenol brufen ester able to be used to prepare the medicines in the form of solid, liquid, injection, eyedrops, ointment, etc for treating viral infection is prepared frmo brufen through acylchlorinating or becoming acid anhydride, esterification in solvent, and recrystallizing.
Owner:SHENYANG PHARMA UNIVERSITY
Topical composition containing naproxen
The present invention generally relates to the transdermal delivery of various compositions. In some aspects, the transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising ibuprofen and / or an ibuprofen salt, a nitric oxide donor, and optionally, a hostile biophysical environment. In some cases, the composition may be stabilized using a stabilization polymer such as xanthan gum, KELTROL® BT and / or KELTROL® RD; propylene glycol; and a polysorbate surfactant such as Polysorbate 20, which unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Pharmaceutical composition of 2-(4-isobutylphenyl) propionic acid
The present invention provides a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1, as well as a method of making the same. The present invention also provides a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the solution has a pH of less than about 7.8, as well as a method of making the same. The present invention also provides a method of treating a condition chosen from pain, inflammation, fever, and / or other conditions alleviated by ibuprofen comprising administering a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1, and wherein the molar ratio of arginine to ibuprofen is selected to achieve a pH of less than about 7.8.
Owner:CUMBERLAND PHARM INC
Medicinal composition containing ibuprofen and arginine and preparation method and application thereof
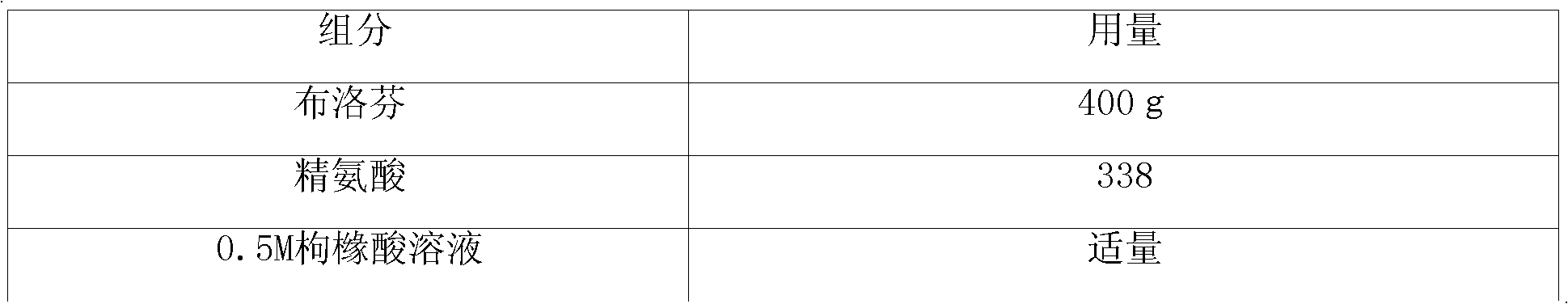
InactiveCN102100686AImprove solubilityGood analgesic effectOrganic active ingredientsAntipyreticArginineTreatment fever
The invention relates to a medicinal composition and a preparation method and application thereof. The medicinal composition is prepared from ibuprofen and arginine serving as medicinal active ingredients in a molar ratio of 1:1 and pharmaceutically acceptable auxiliary materials. The composition can be prepared into an oral preparation and an injection preparation for treating related symptoms such as fever, inflammation, pain and the like.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Injection containing ibuprofen and preparation method thereof
InactiveCN101810568AImprove solubilityImprove bioavailabilityOrganic active ingredientsAntipyreticArginineMedicine
The invention provides an injection containing ibuprofen and a preparation method thereof. The injection has the advantages of good stability, high safety and good treatment effect. The injection contains ibuprofen and arginine, wherein the molar ratio of the arginine to the ibuprofen is larger than or equal to 1 to 1.
Owner:FANTAI INST OF CHEM MEDICINES NANJING
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
InactiveUS7151084B2Nervous disorderPeptide/protein ingredientsMembrane TransportersPancreatic hormone
A complex is provided for the treatment of neurogenic conditions having the formula:where R1 isM is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium(II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium(II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium(II):transporter ligand.
Owner:MILLER LANDON C G
Pharmaceutical composition of 2-(4-isobutylphenyl) propionic acid
The present invention provides a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1, as well as a method of making the same. The present invention also provides a method of treating a condition chosen from pain, inflammation, fever, and / or other conditions alleviated by ibuprofen comprising administering a pharmaceutical composition comprising an aqueous solution of arginine and ibuprofen, wherein the molar ratio of arginine to ibuprofen is less than 1:1.
Owner:CUMBERLAND PHARM INC
Solid oral formulations for combination therapy
InactiveUS20080085314A1Reduce gastric acid secretionPromote gastric acid secretionBiocideDrug compositionsRanitidineOmeprazole
A first, solid oral pharmaceutical composition includes an extended release acetaminophen, a non-steroidal anti-inflammatory drug, such as naproxen or ibuprofen, and a third drug capable of reducing gastric acid secretion, such as ranitidine or omeprazole. A second, solid oral pharmaceutical composition includes a non-steroidal anti-inflammatory drug and an agent for reducing gastric acid secretion.
Owner:POLY MED
Ibuprofen amino acid salt injection and preparation thereof
InactiveCN101214235AFix stability issuesLess irritatingOrganic active ingredientsAntipyreticSodium bicarbonateHemolysis
The present invention discloses ibuprofen amino acid salt injection and a preparation method thereof. In the present invention, sodium bicarbonate is used as preparation auxiliary material, which ensures the prepared injection has stable quality, little stimulation, no hypersusceptibility effect, no hemolysis effect and stable curative effect and takes effect fast. The present invention is mainly applied to antipyresis and acesodyne under the circumstance which is not fit for oral medication or is not capable of the oral medication.
Owner:航天中心医院
Medicament carrying microballoons of hydrophobic nature medicament and method of preparing the same
InactiveCN101254307AIncrease loadGood sustained release effectPharmaceutical non-active ingredientsMicrospherePolymer solution
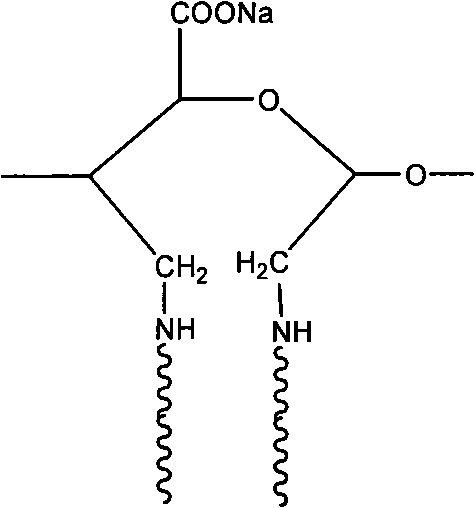
Drug-loaded microspheres of a hydrophobic drug and a preparation method thereof belong to the technology fields of the synthesis of gel microspheres and the drug embedding materials. An oxidized sodium alginate which is adopted by the invention is reacted with alkylamine under the function of a reducing agent, the obtained polymer solution after the modification is strongly stirred and dispersed in a NaCl / CaCl2 mixed solution to form gel microspheres; and the embedding release research is carried out to the hydrophobic drug of ibuprofen. The hydrophobic alkyl chain section is grafted on the sodium alginate frame by a simple chemical method, then the gen microspheres are prepared, the obtained amphoteric gel microspheres can greatly improve the embedding efficiency of the hydrophobic drug and have great sustained-release performance, thus expanding the application range of the sodium alginate in the drug-loaded field.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com