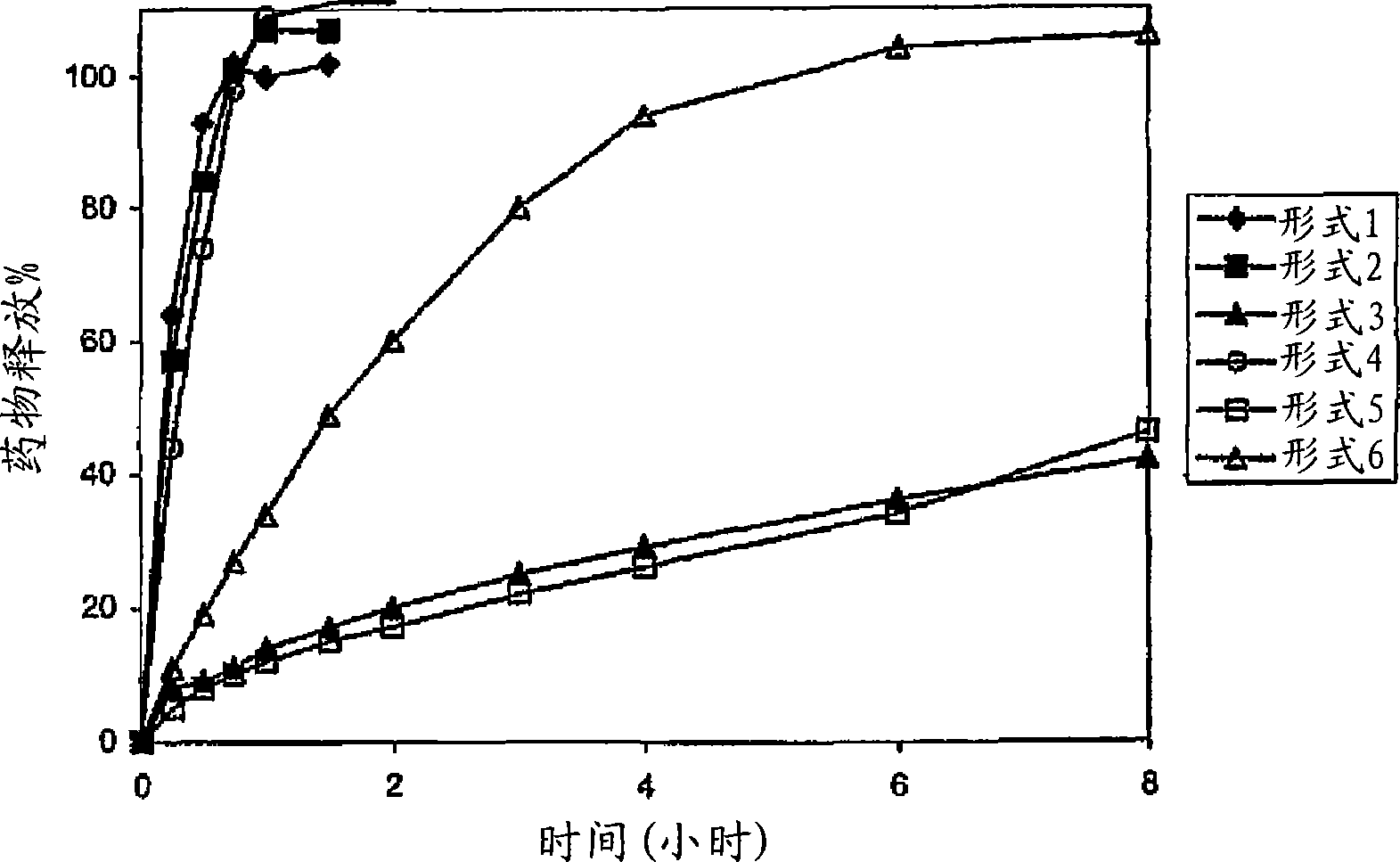
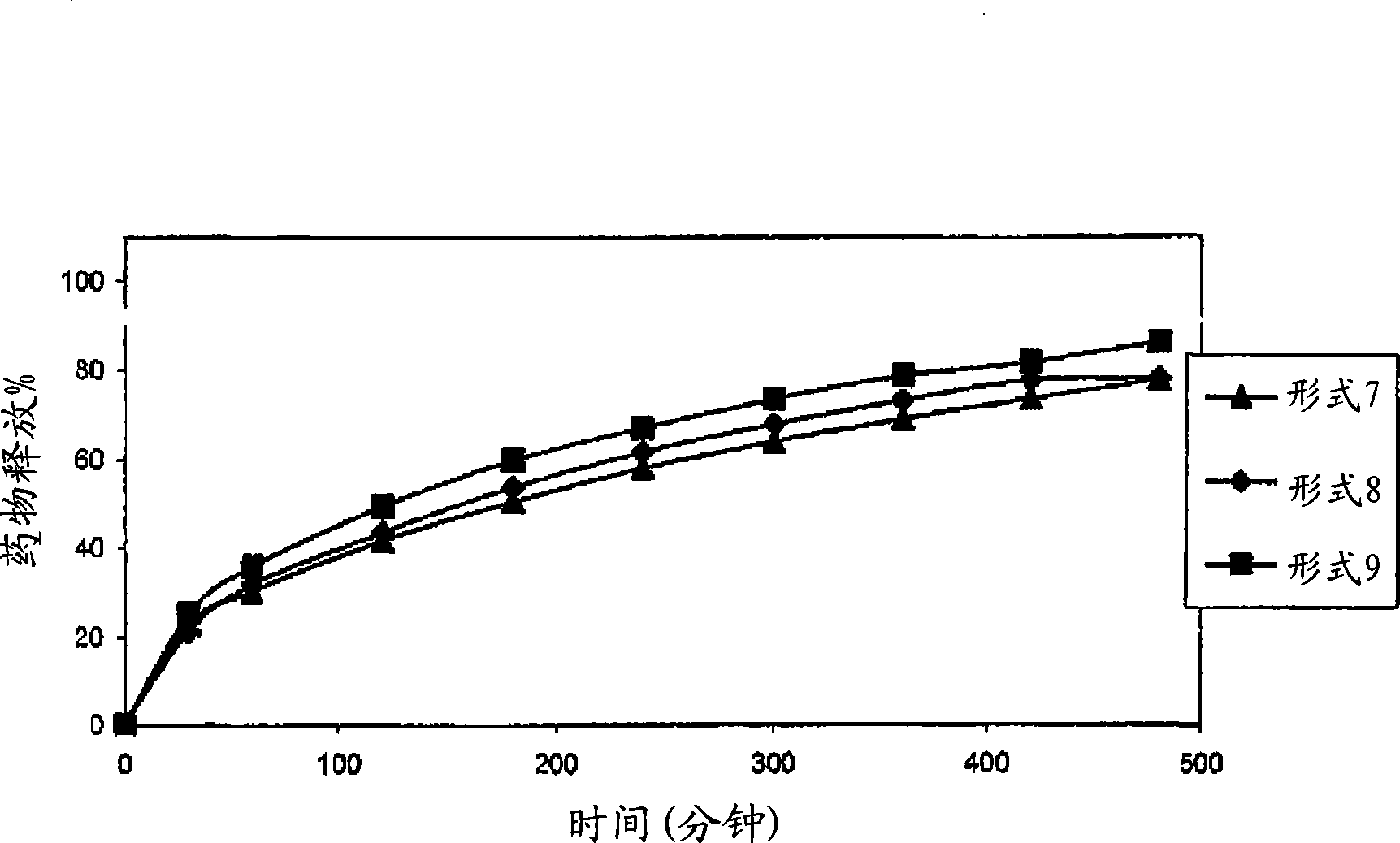
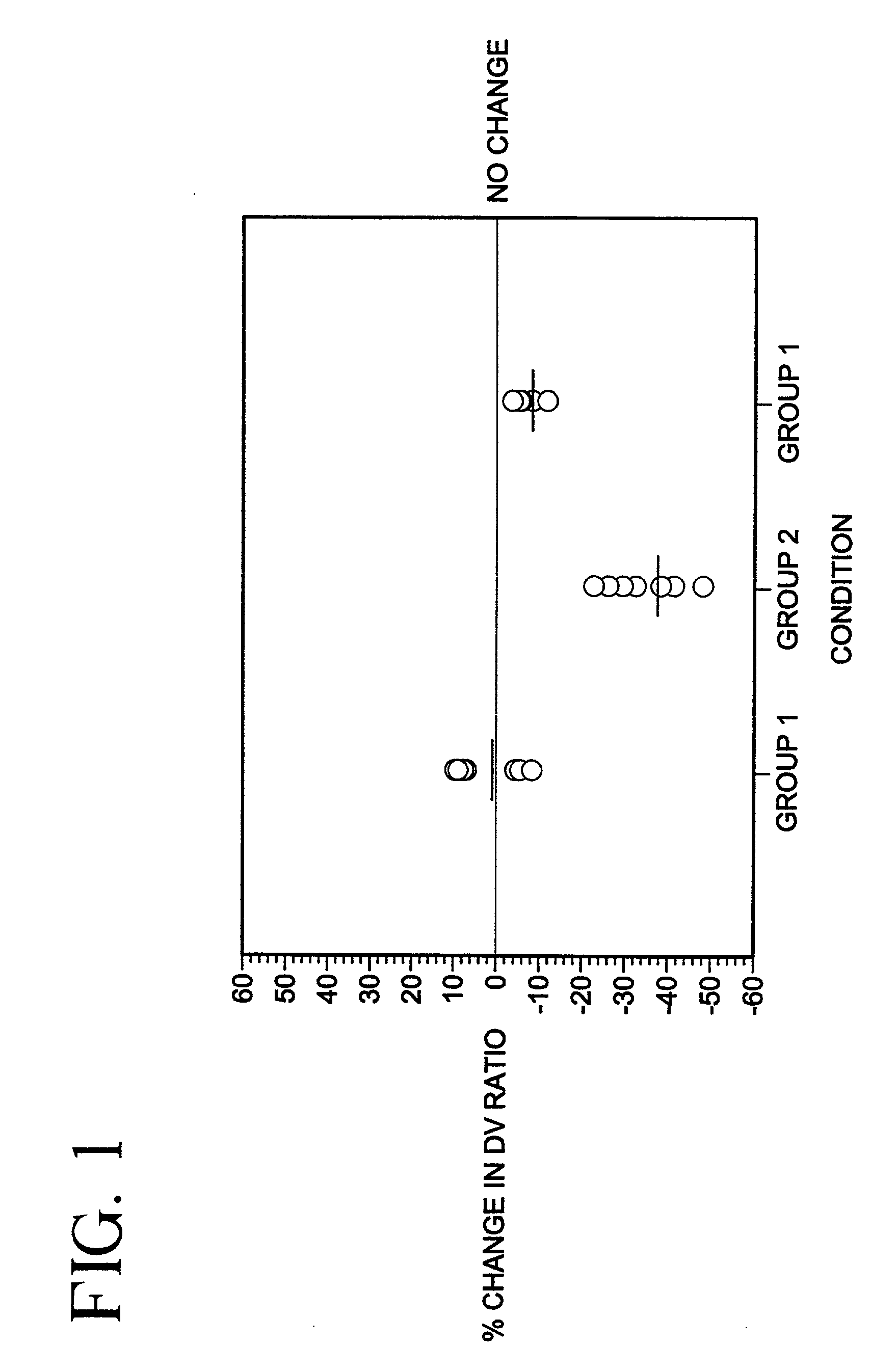
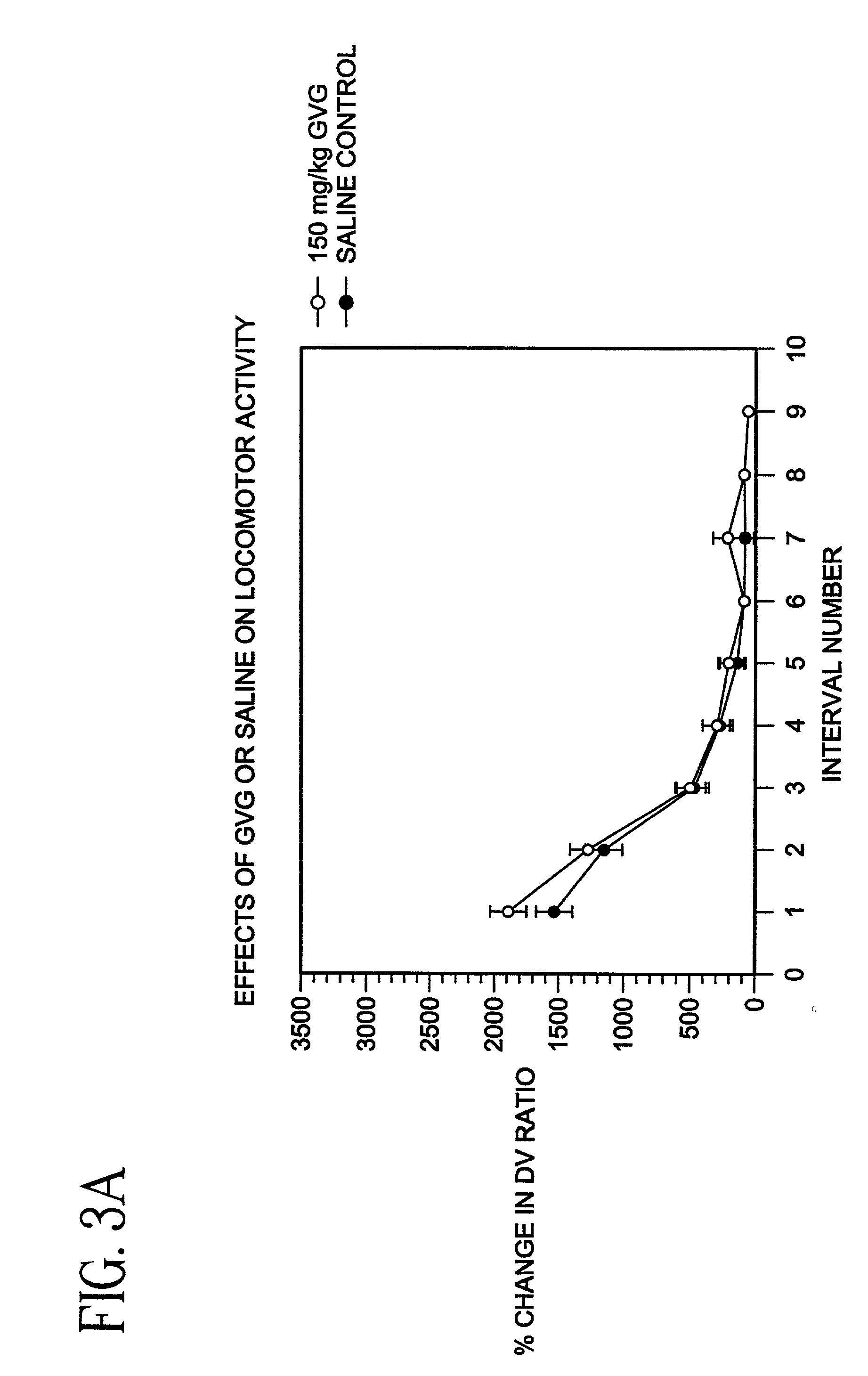
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
134 results about "Drugs of abuse" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
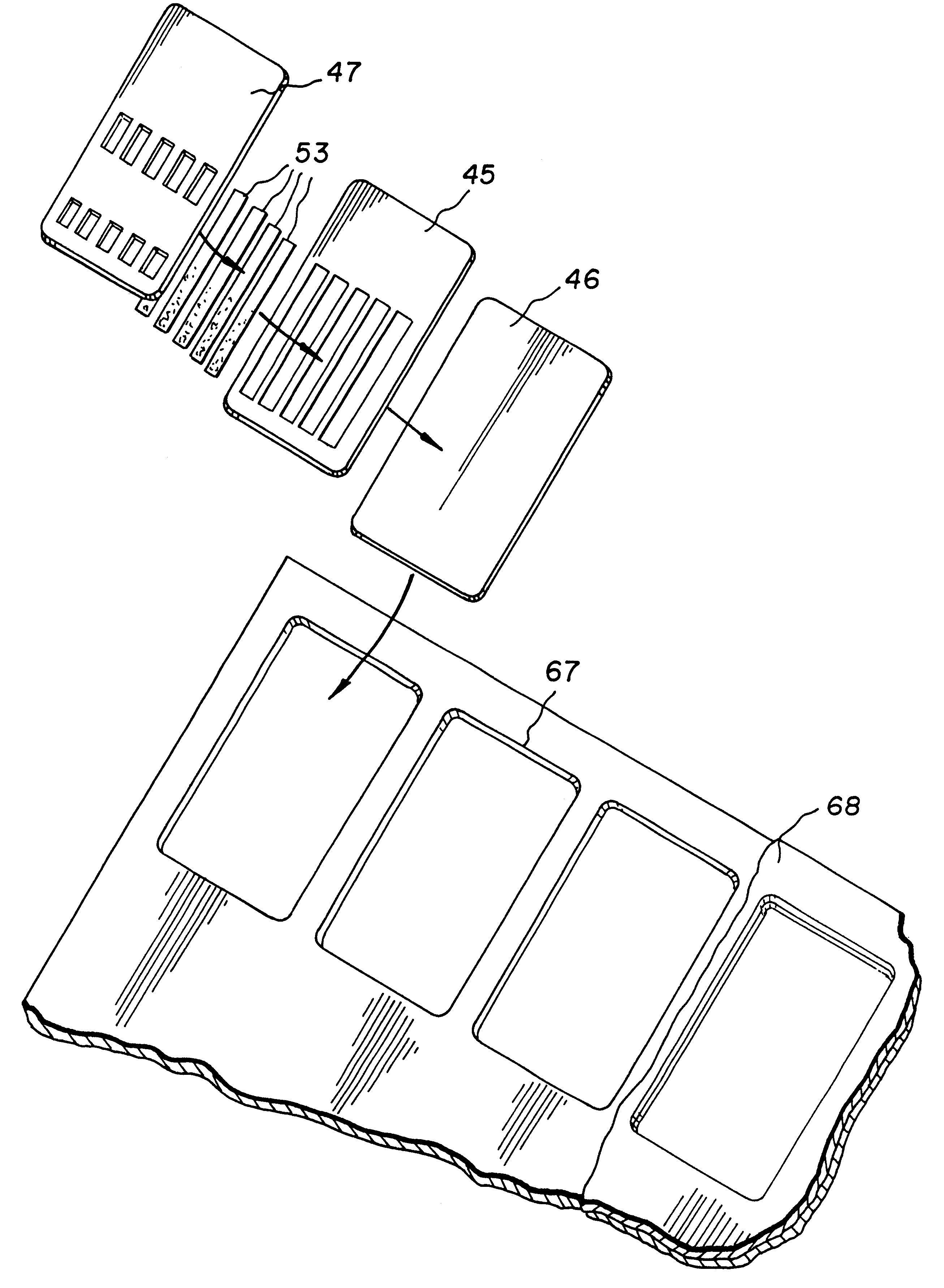
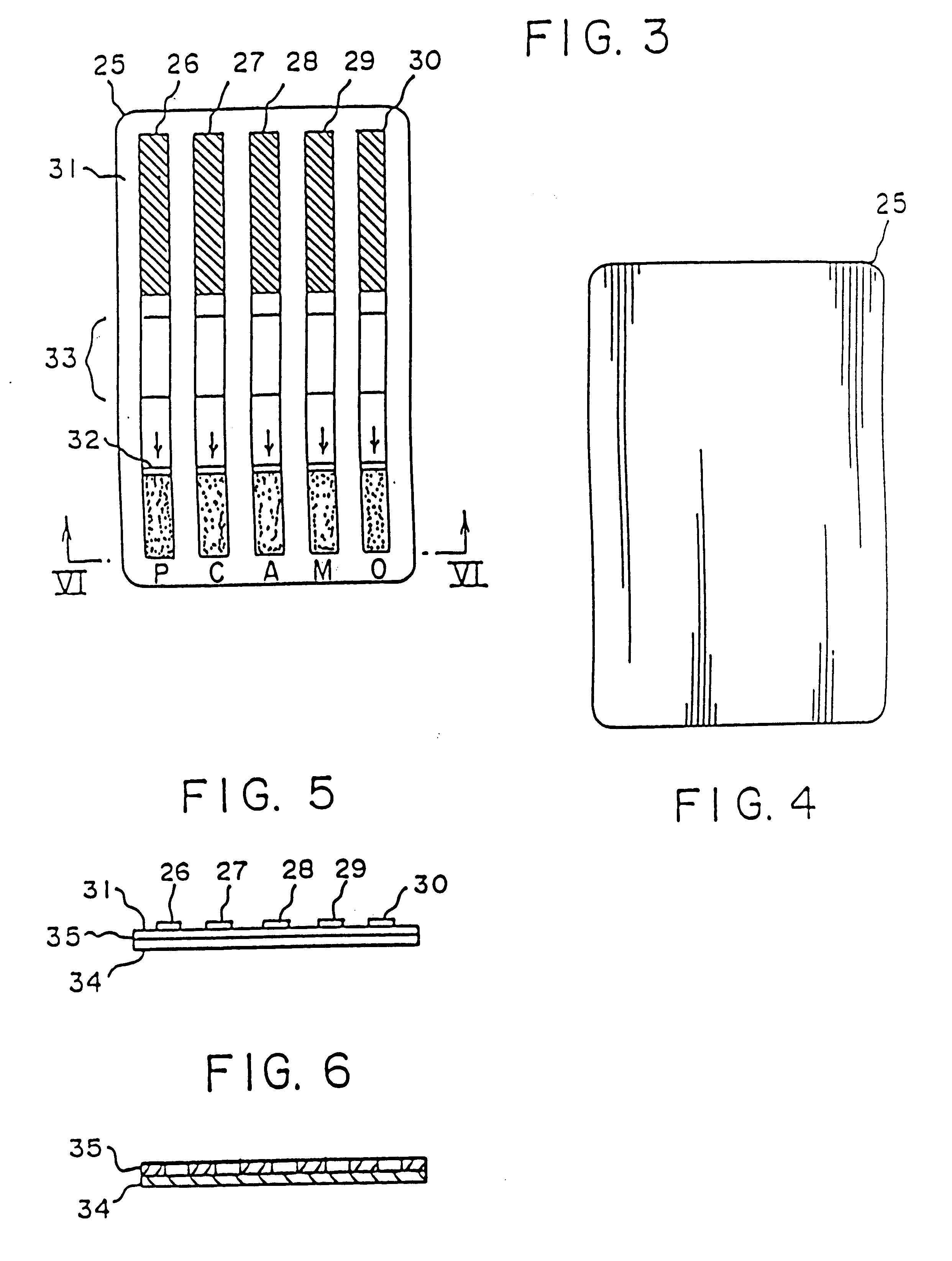
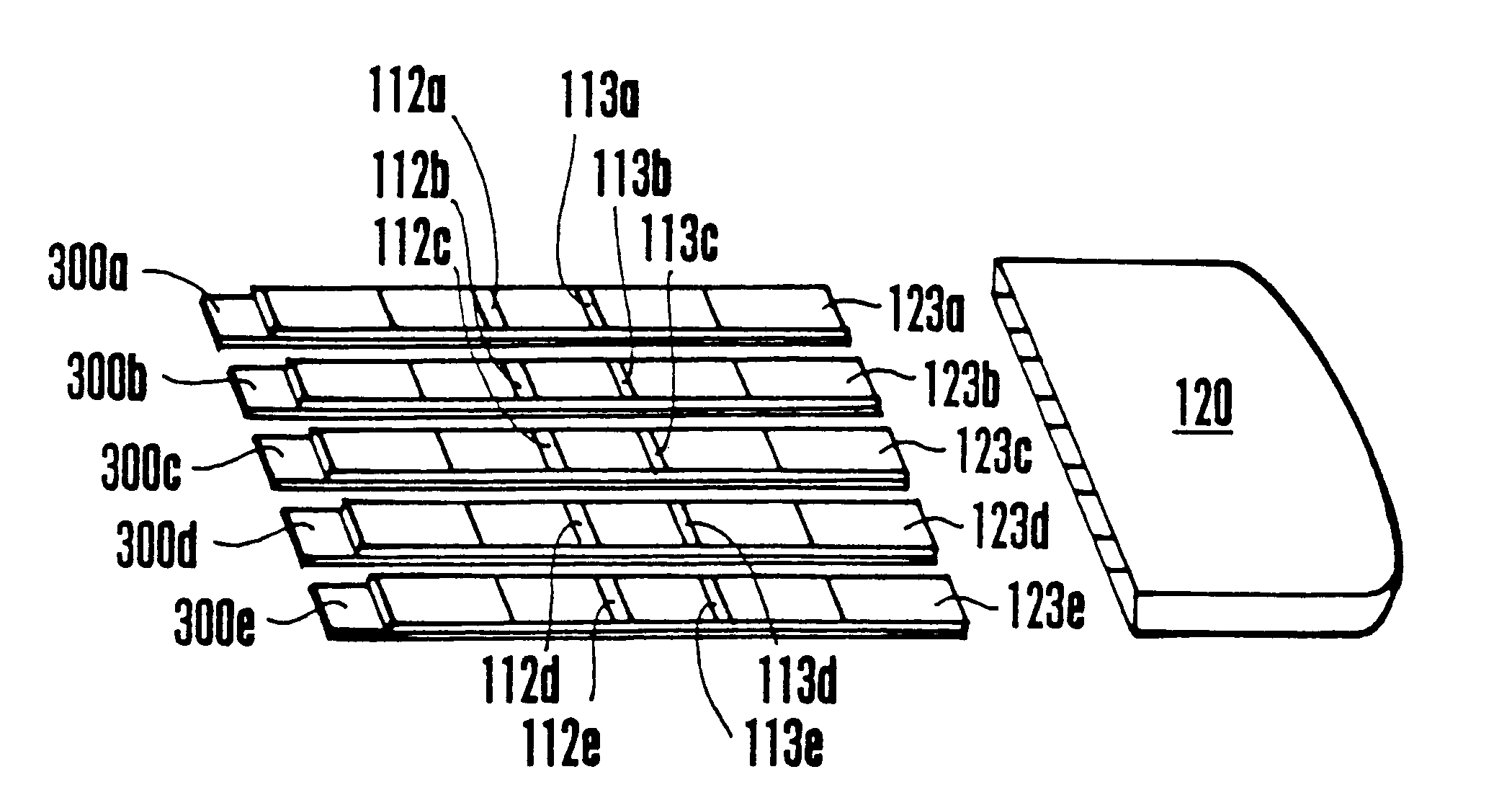
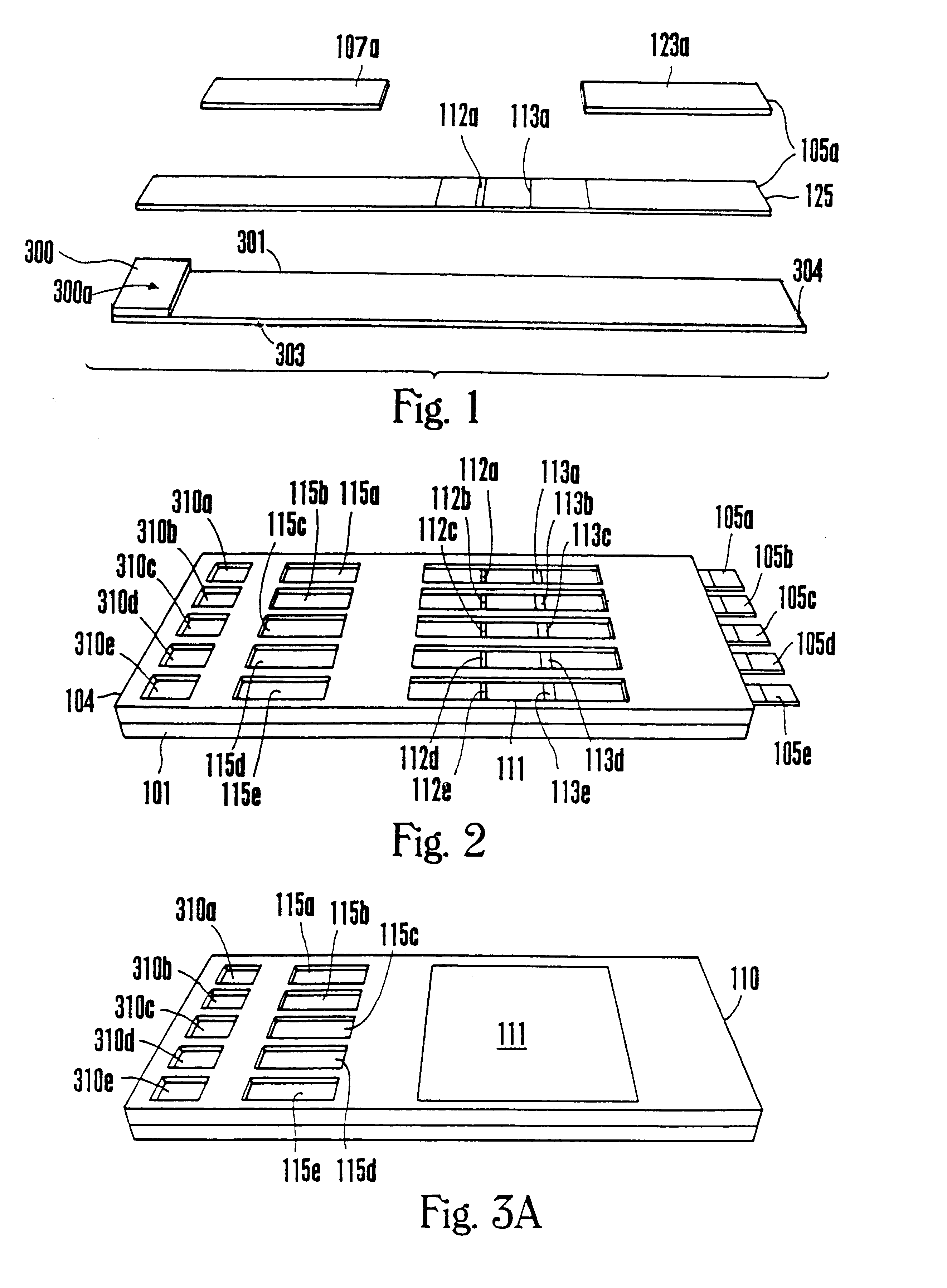
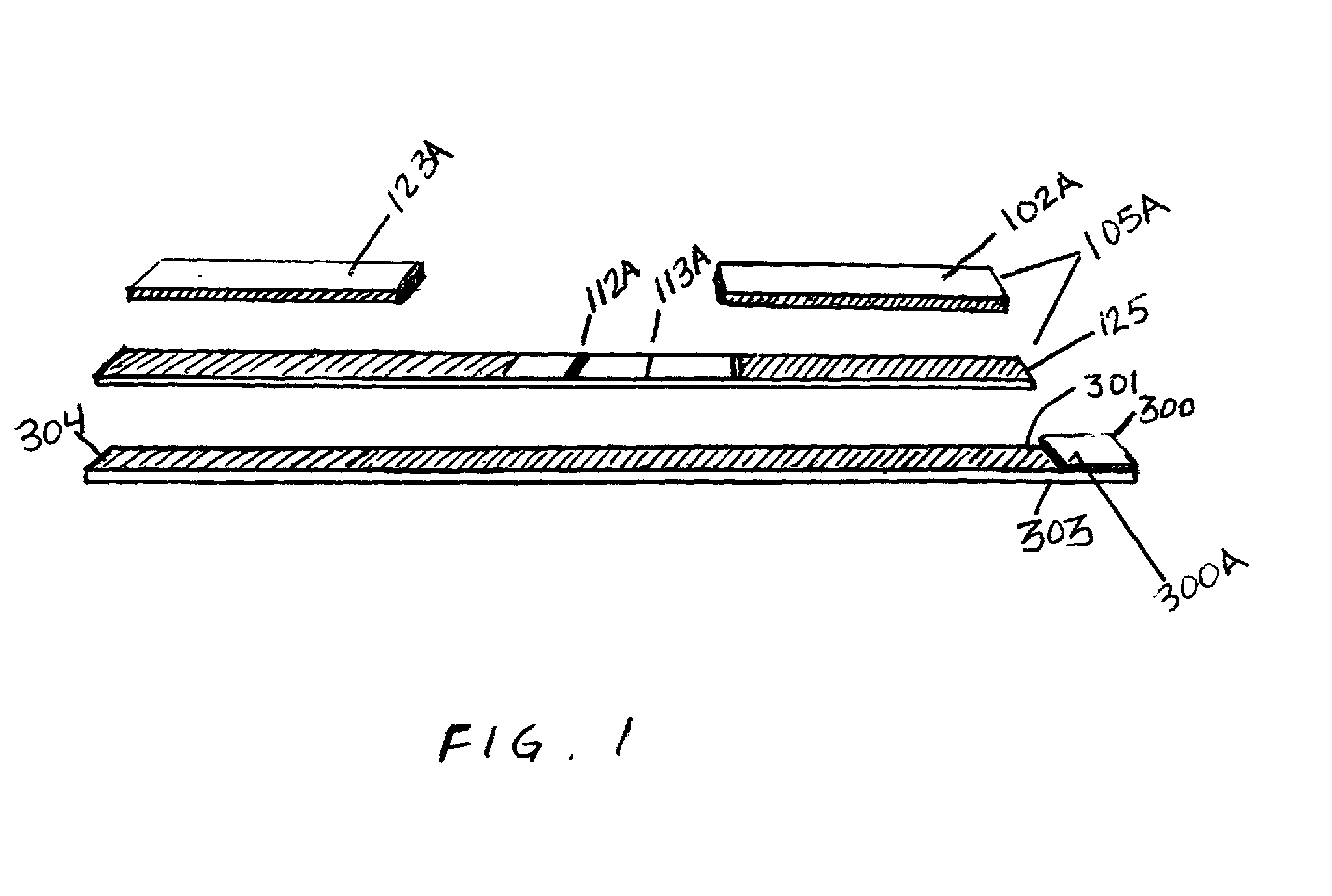
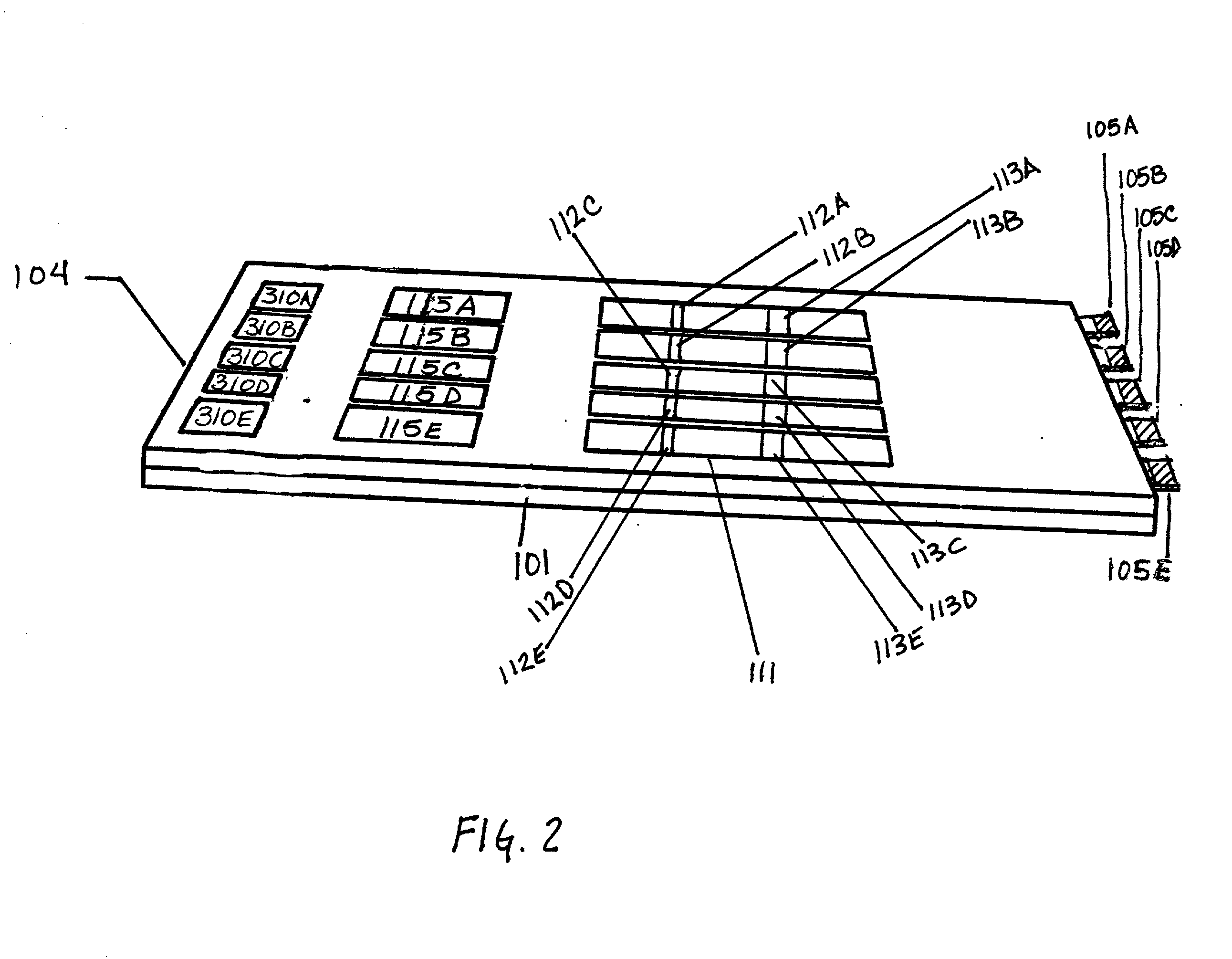
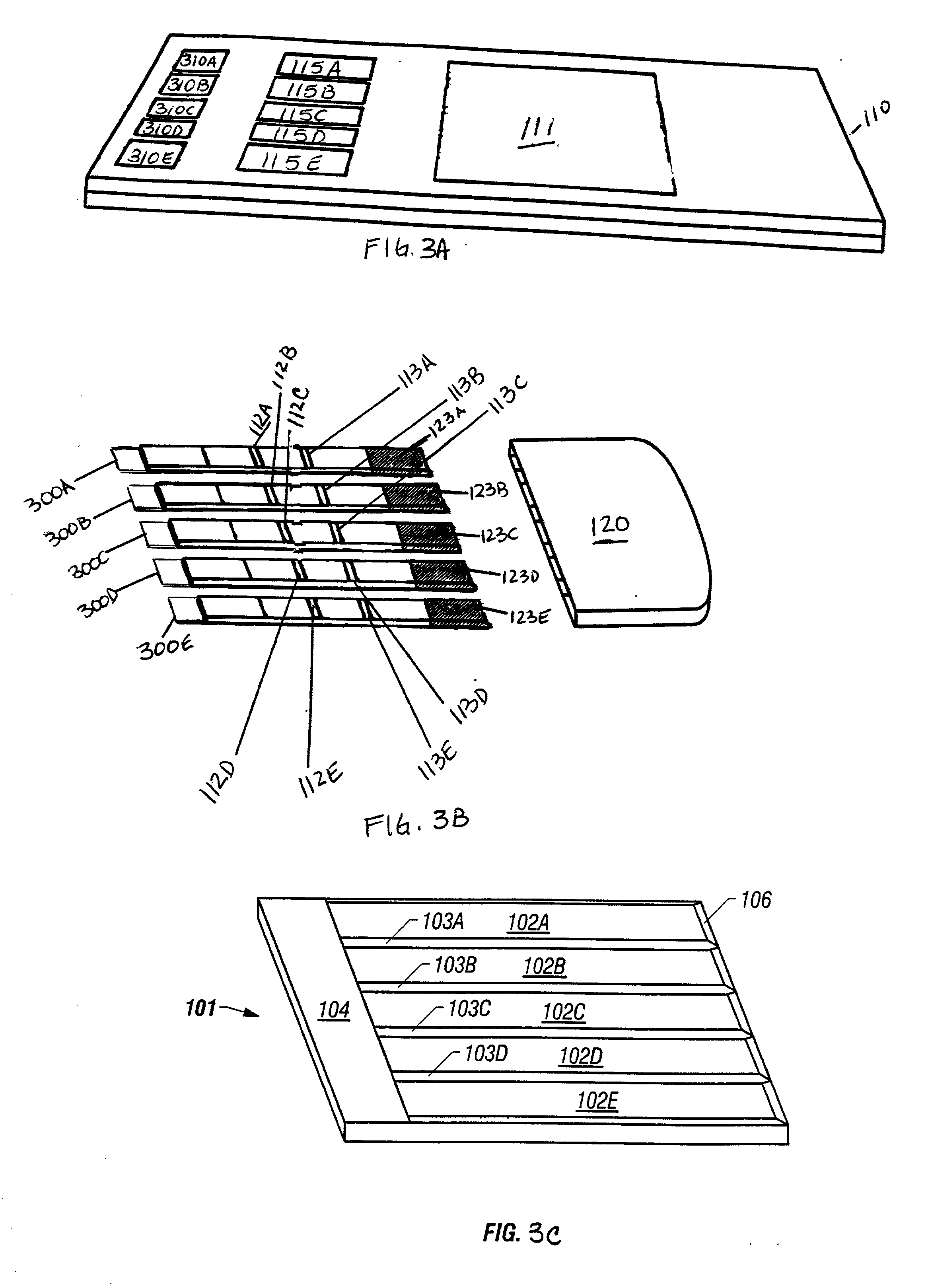
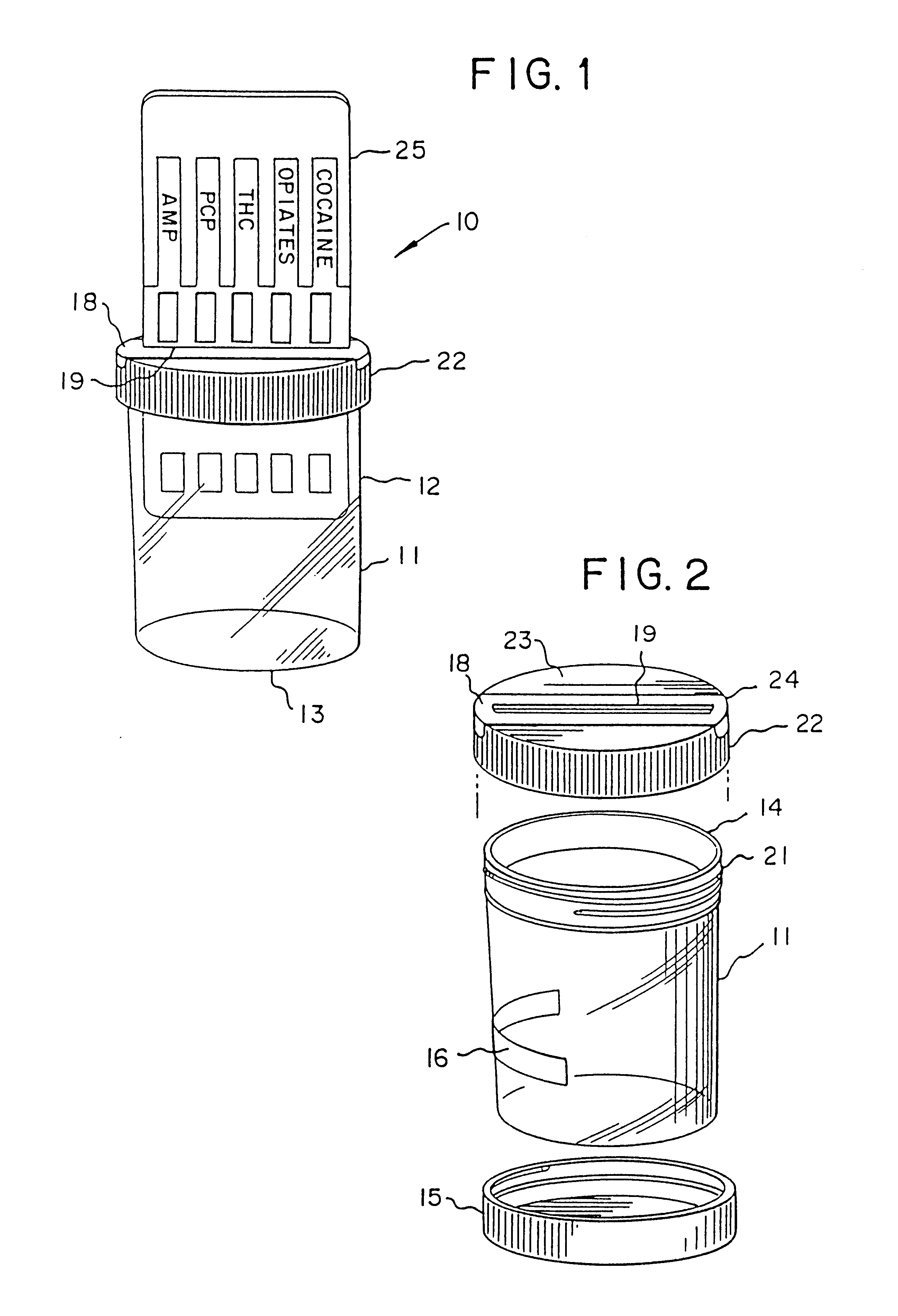
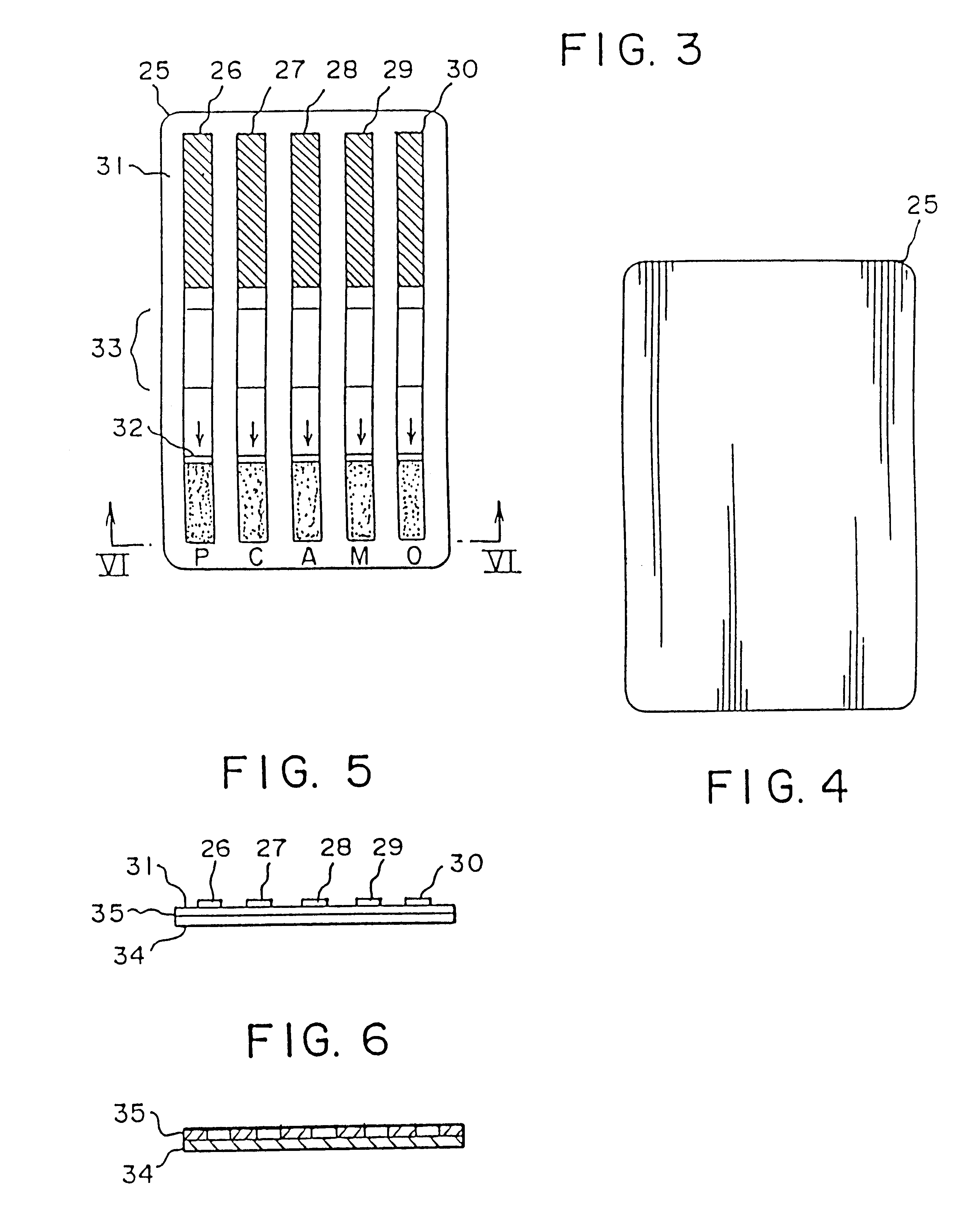
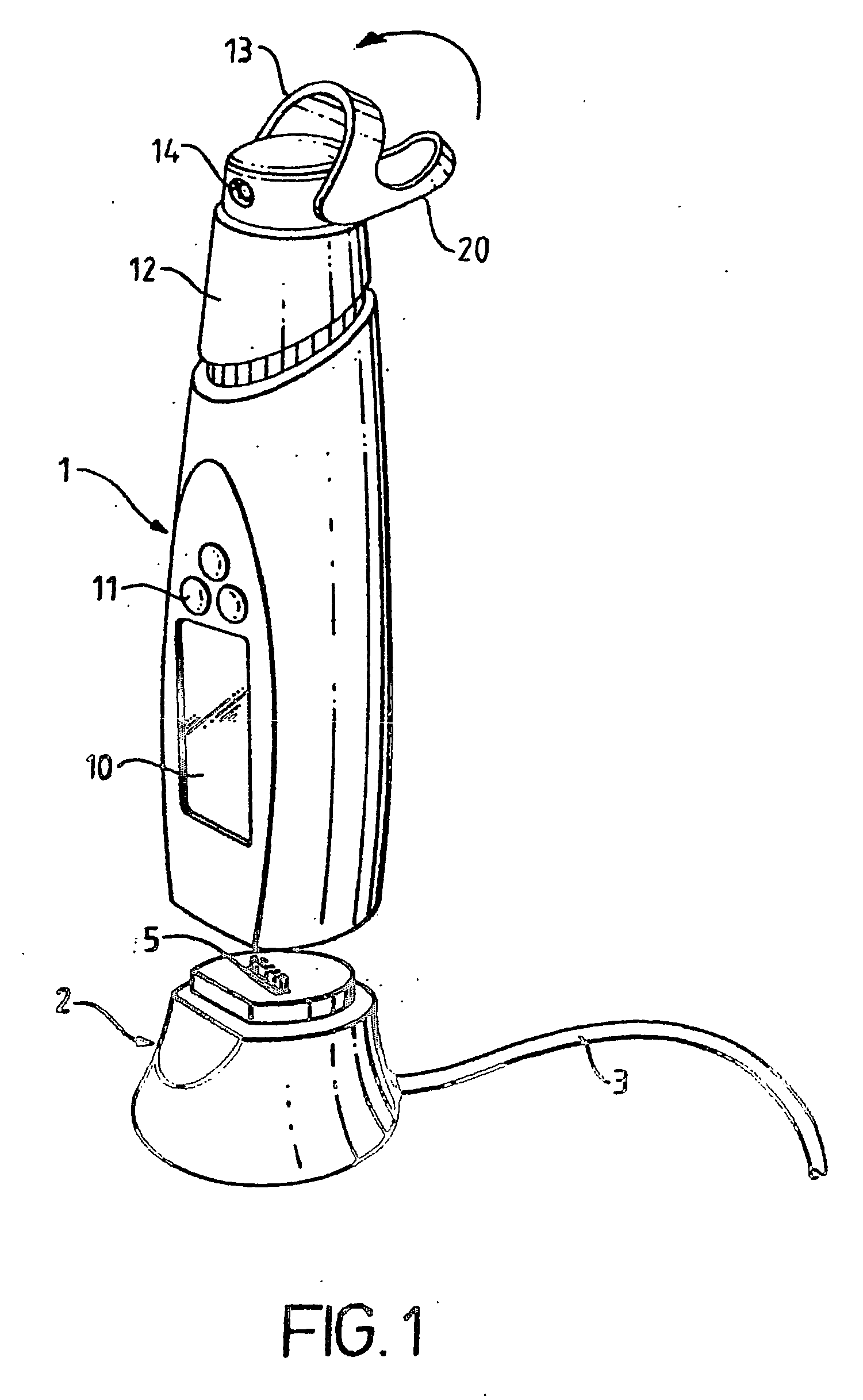
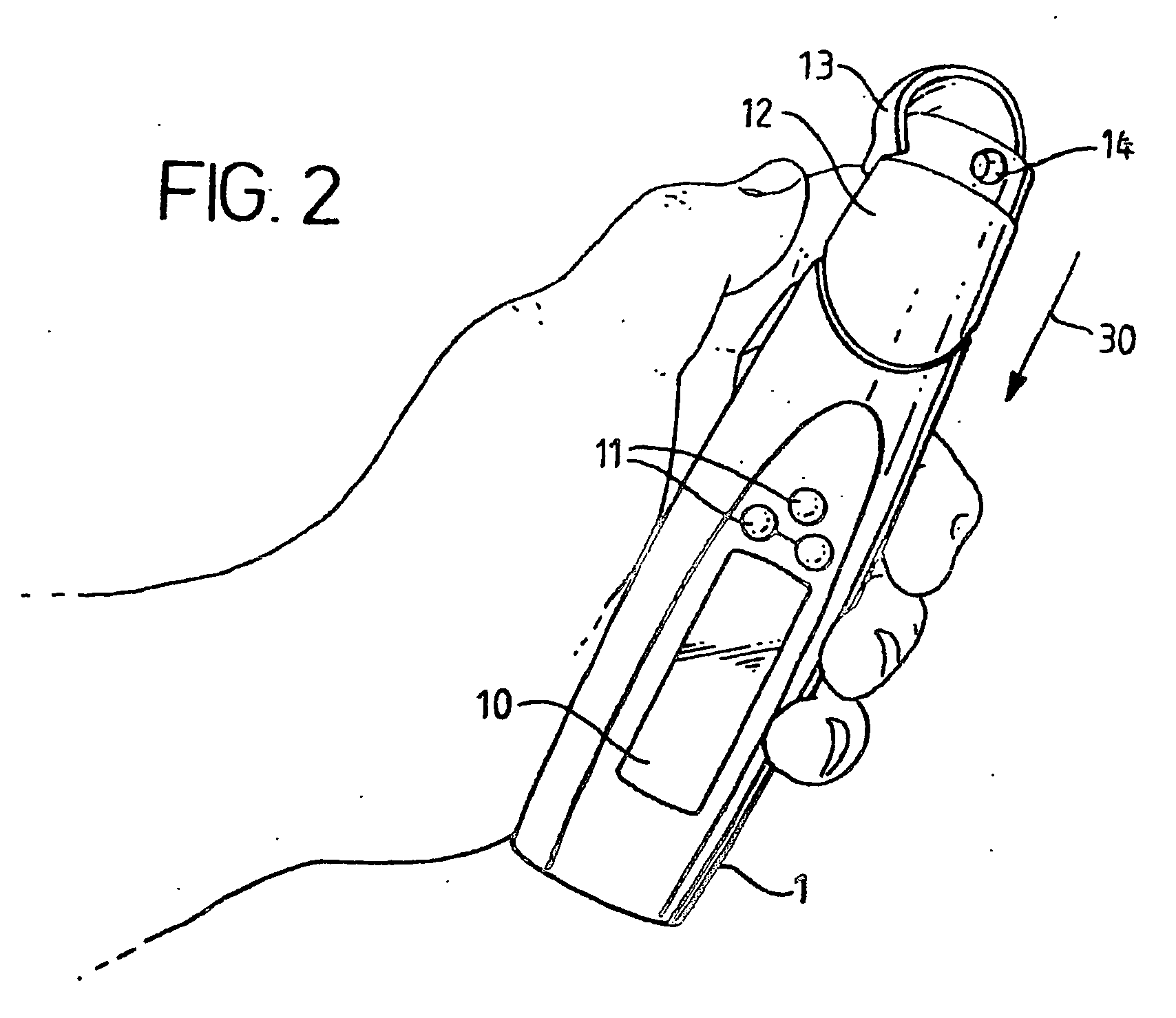
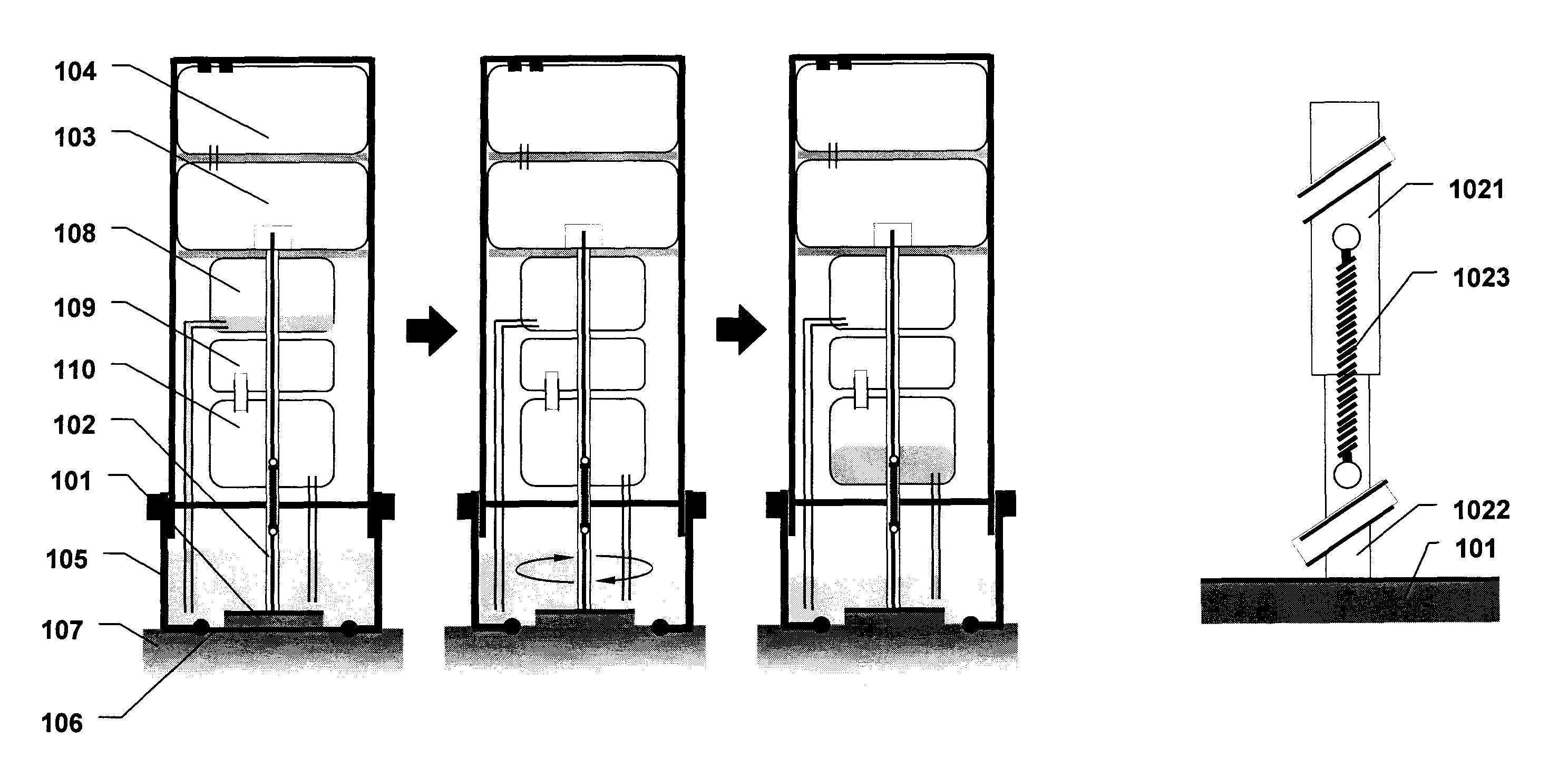
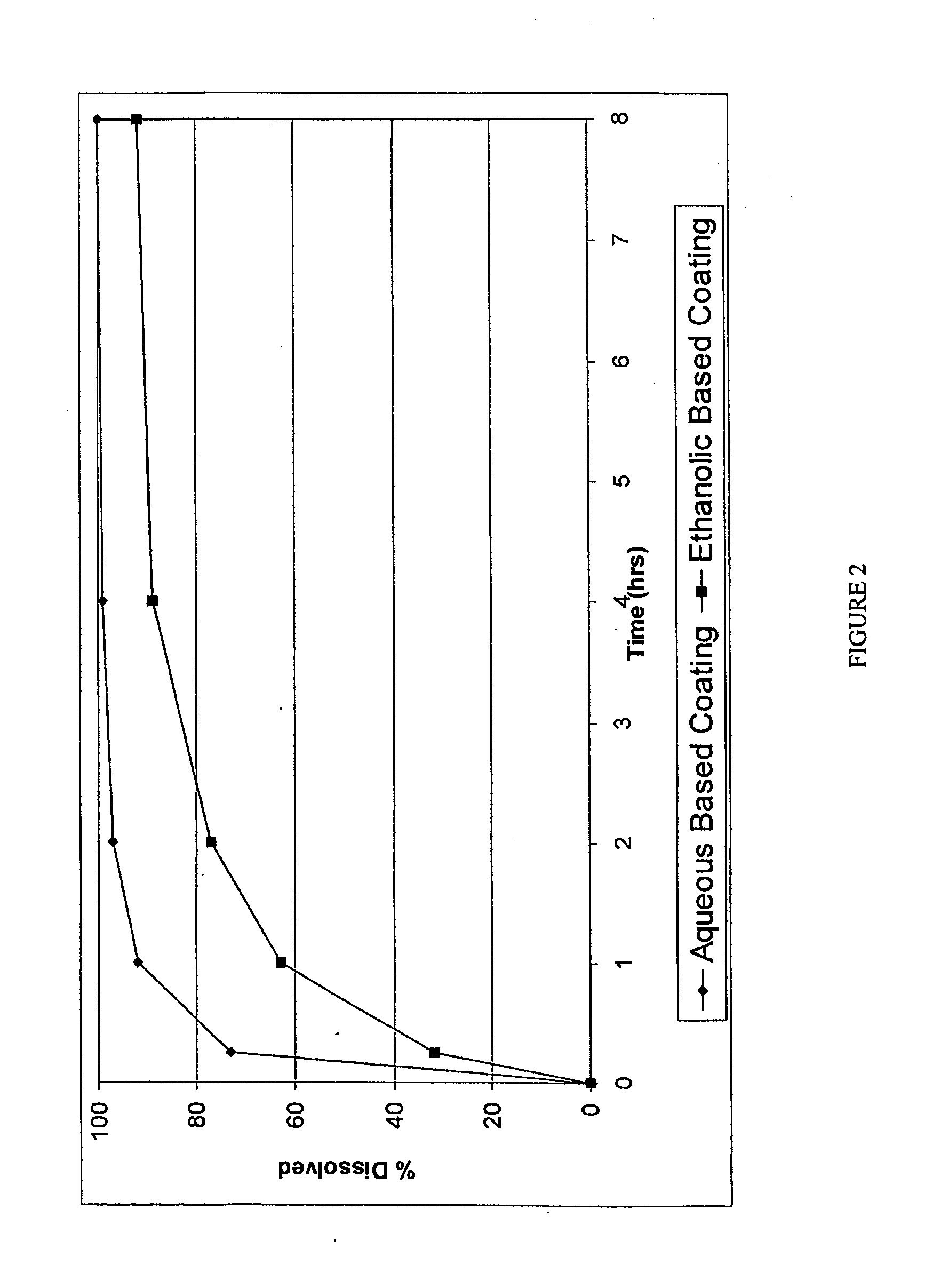
Device for the testing of fluid samples and process for making the device
InactiveUS6372515B1Simple processBioreactor/fermenter combinationsBiological substance pretreatmentsBusiness cardAnalyte
A test card for drugs of abuse has a thin flat member having the size and shape of a business card. A plurality of immunoassay test strips extend longitudinally from top to bottom at the card and are fastened side by side in parallel on one or both sides of the test card within the outline of the card. Each test strip is reactive to provide a visual indication in response to a particular drug of abuse. The test card thus provides for the simultaneous detection of multiple analytes. Processes are also disclosed for making the drug test card with test strips on one and both sides of the card.
Owner:HEALGEN SCI LLC
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition
Owner:ELAN PHRMA INT LTD
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Multiple analyte assay device with sample integrity monitoring system
InactiveUS6514769B2Avoid pollutionImprove securityAnalysis using chemical indicatorsSamplingMulti analyteSample integrity
An assay device, a fluid analyte sample separator device and methods for use of thereof for determining whether the integrity of a fluid analyte sample has been compromised and for contemporaneously assaying the sample for the presence or absence of multiple analytes, such as drugs of abuse. The device is composed of a housing having separate slots therein for insertion of one or more analyte test strips, one end of which protrudes from the housing, and one or more units of a sample integrity monitoring system. The device may be used in dipstick or cassette form. An analyte sample separator for division of sample and retention of uncontaminated sample for further testing is also provided. The analyte test strips and sample integrity monitoring system are replaceable, so that the panel of analytes and of sample condition parameters tested can be customized.
Owner:ASSURANCE BIOTECH
Abuse resistant drug formulation
A pharmaceutical composition may include a coated particulate which may include at least one active pharmaceutical ingredient, particularly one susceptible to abuse by an individual. The coated particles may include a fat / wax and have improved controlled release and / or crush resistance. Method of making these coated particulate and dosage forms therewith are also described.
Owner:CIMA LABS
Hapten-carrier conjugates and uses thereof
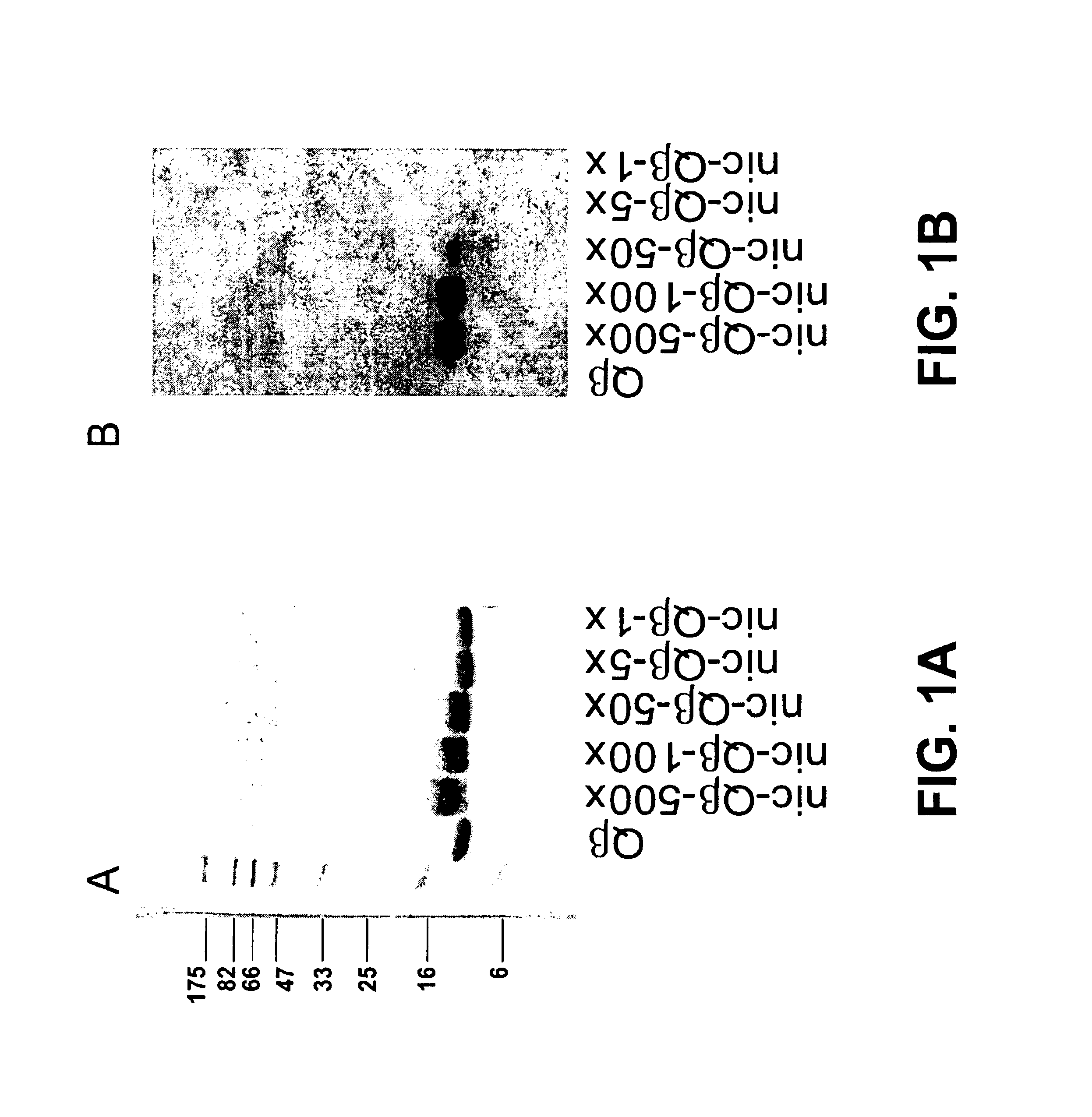
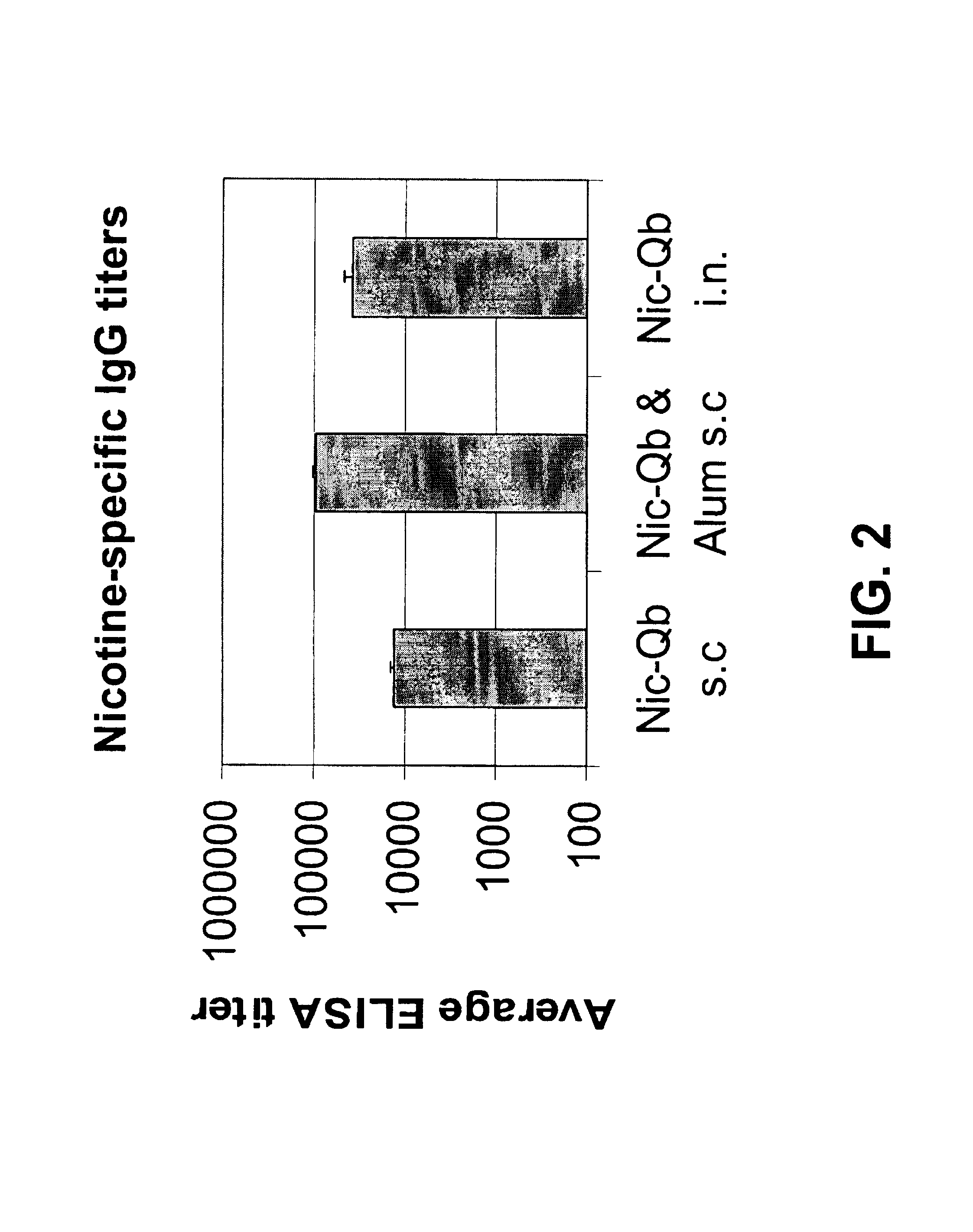
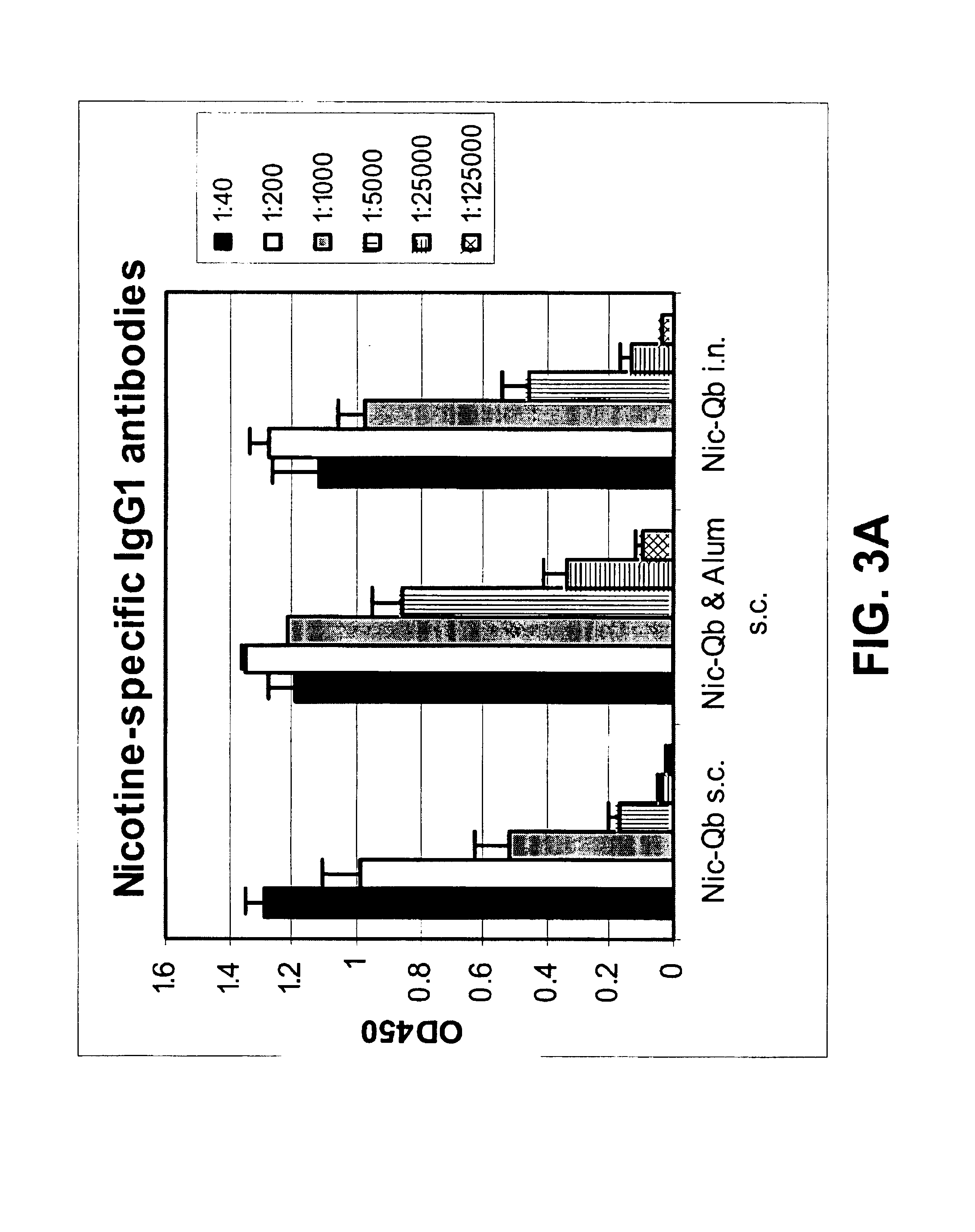
The present invention provides compositions comprising a conjugate of a hapten with a carrier in an ordered and repetitive array, and methods of making such compositions. The conjugates and compositions of the invention may comprise a variety of haptens, including hormones, toxins and drugs, especially drugs of addiction such as nicotine. Compositions and conjugates of the invention are useful for inducing immune responses against haptens, which can use useful in a variety of therapeutic, prophylactic and diagnostic regimens. In certain embodiments, immune responses generated using the conjugates, compositions and methods of the present invention are useful to prevent or treat addiction to drugs of abuse and the resultant diseases associated with drug addiction.
Owner:CYTOS BIOTECHNOLOGY AG
Abuse resistant drug formulation
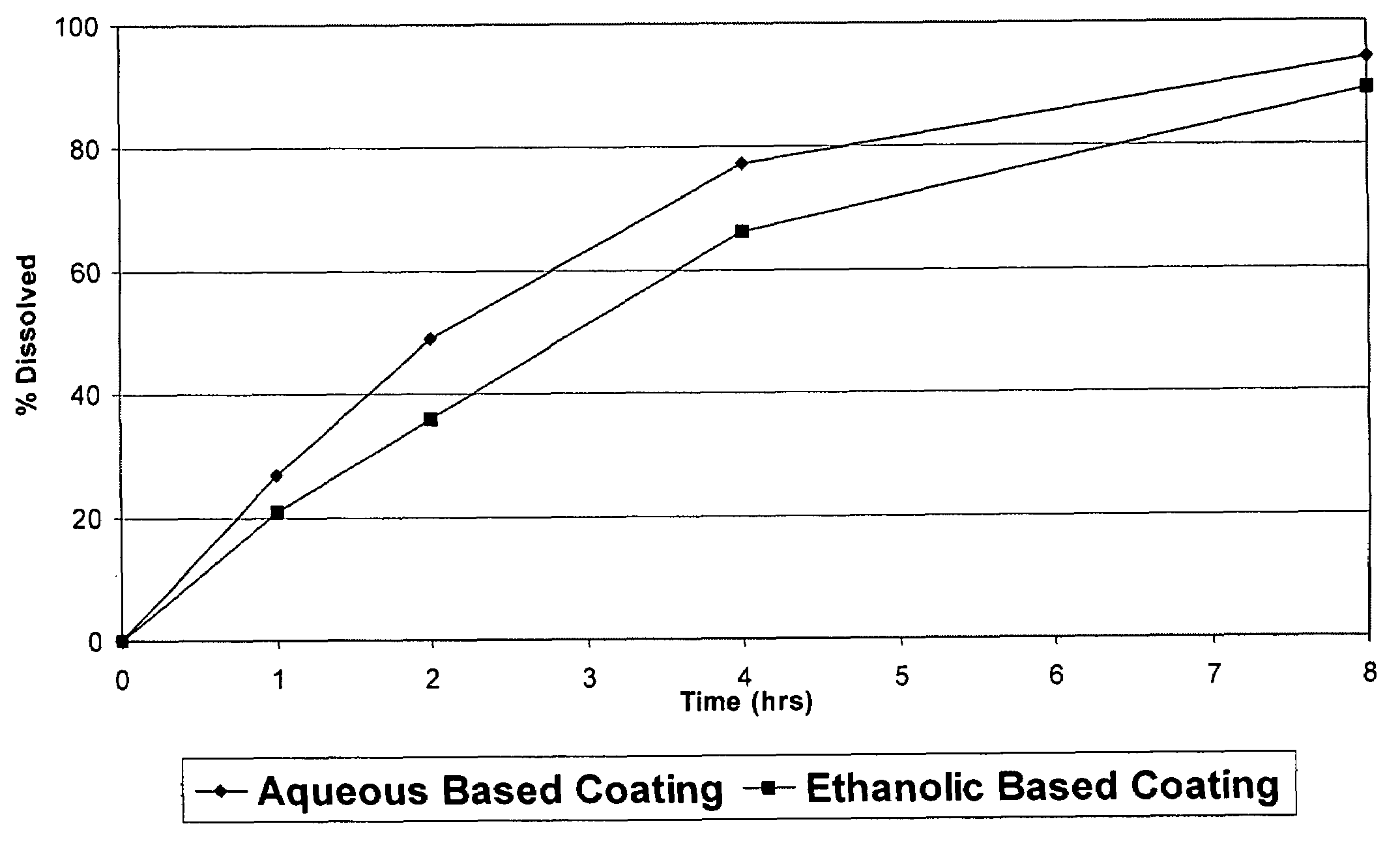
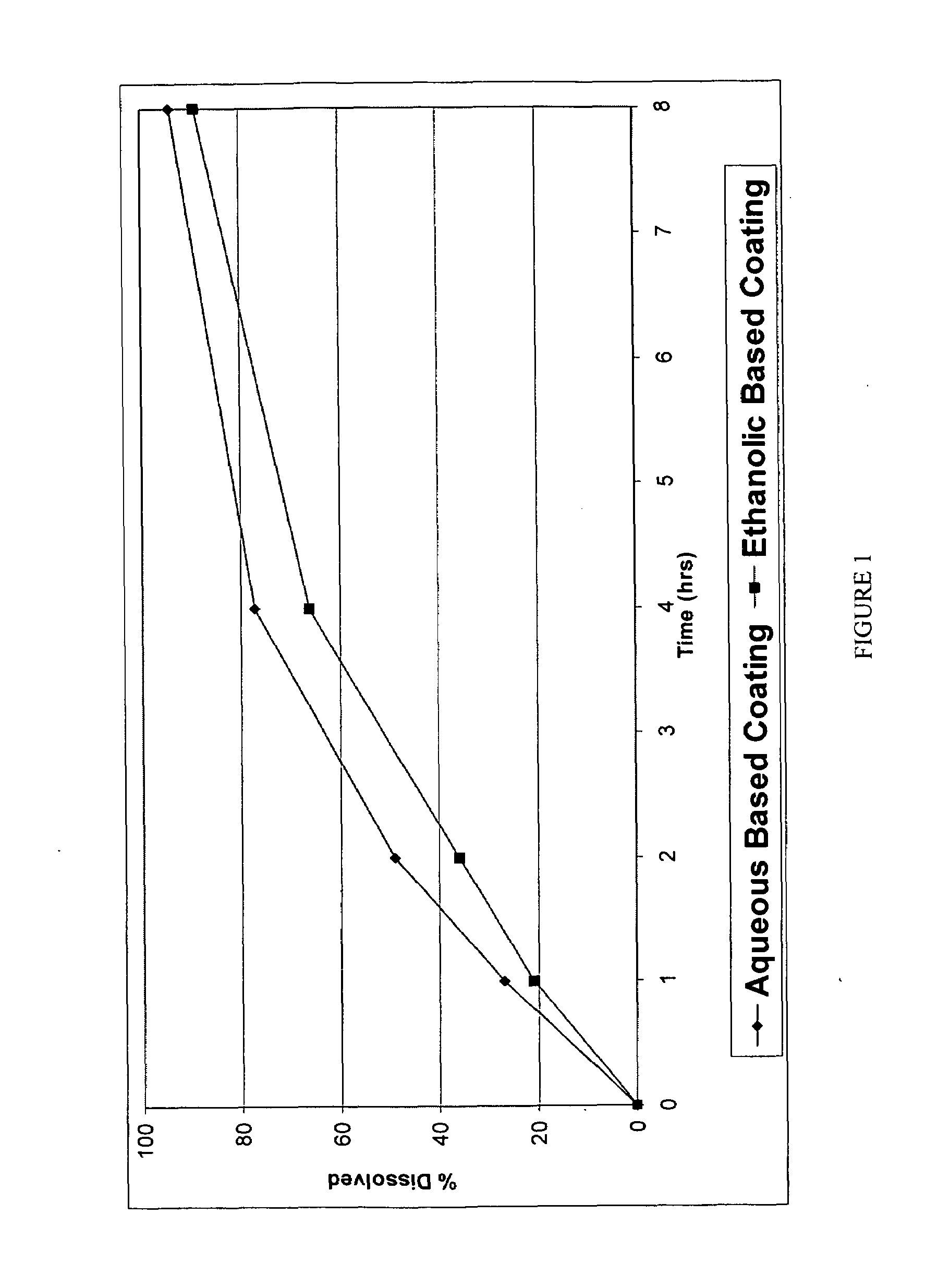
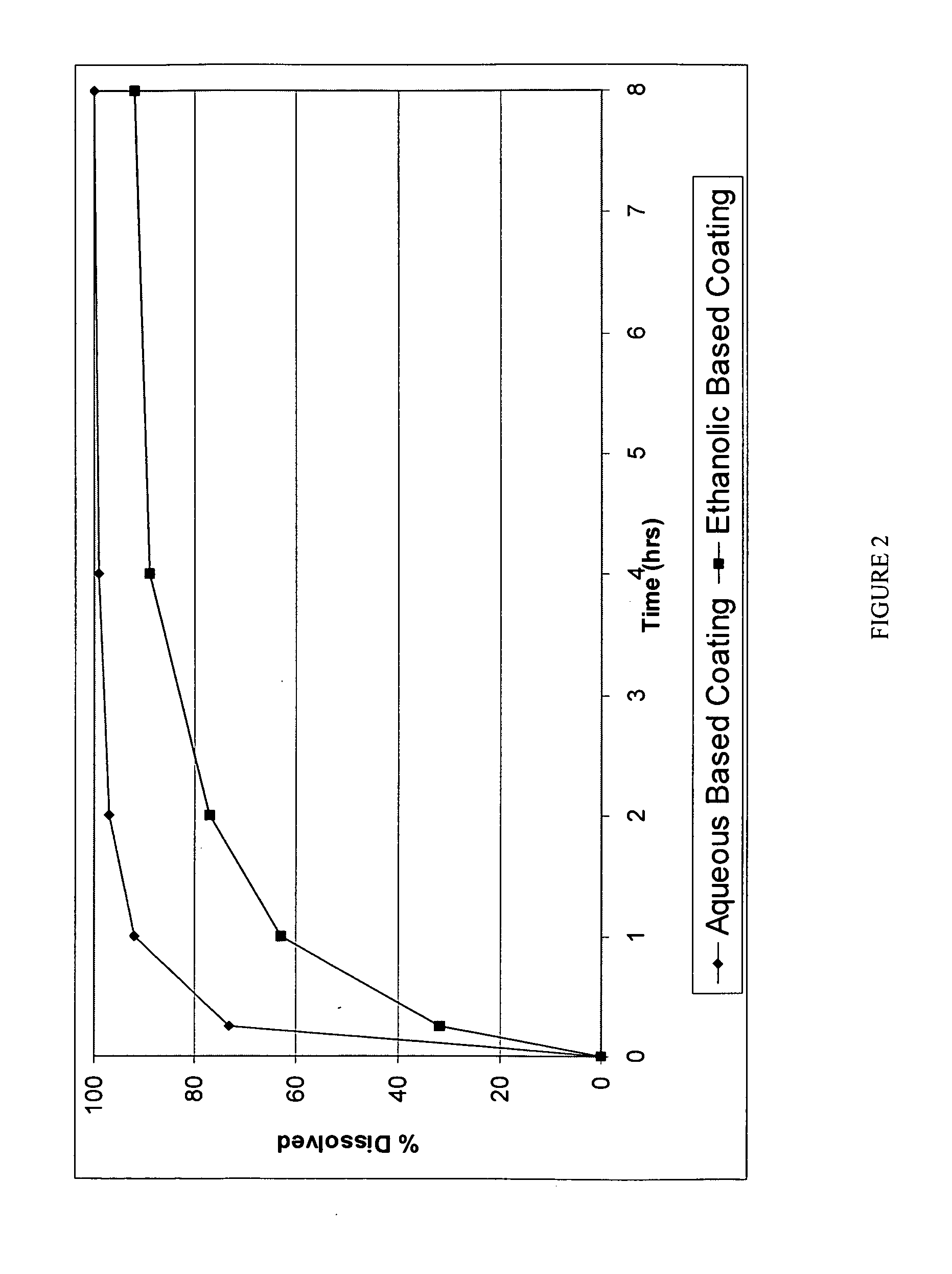
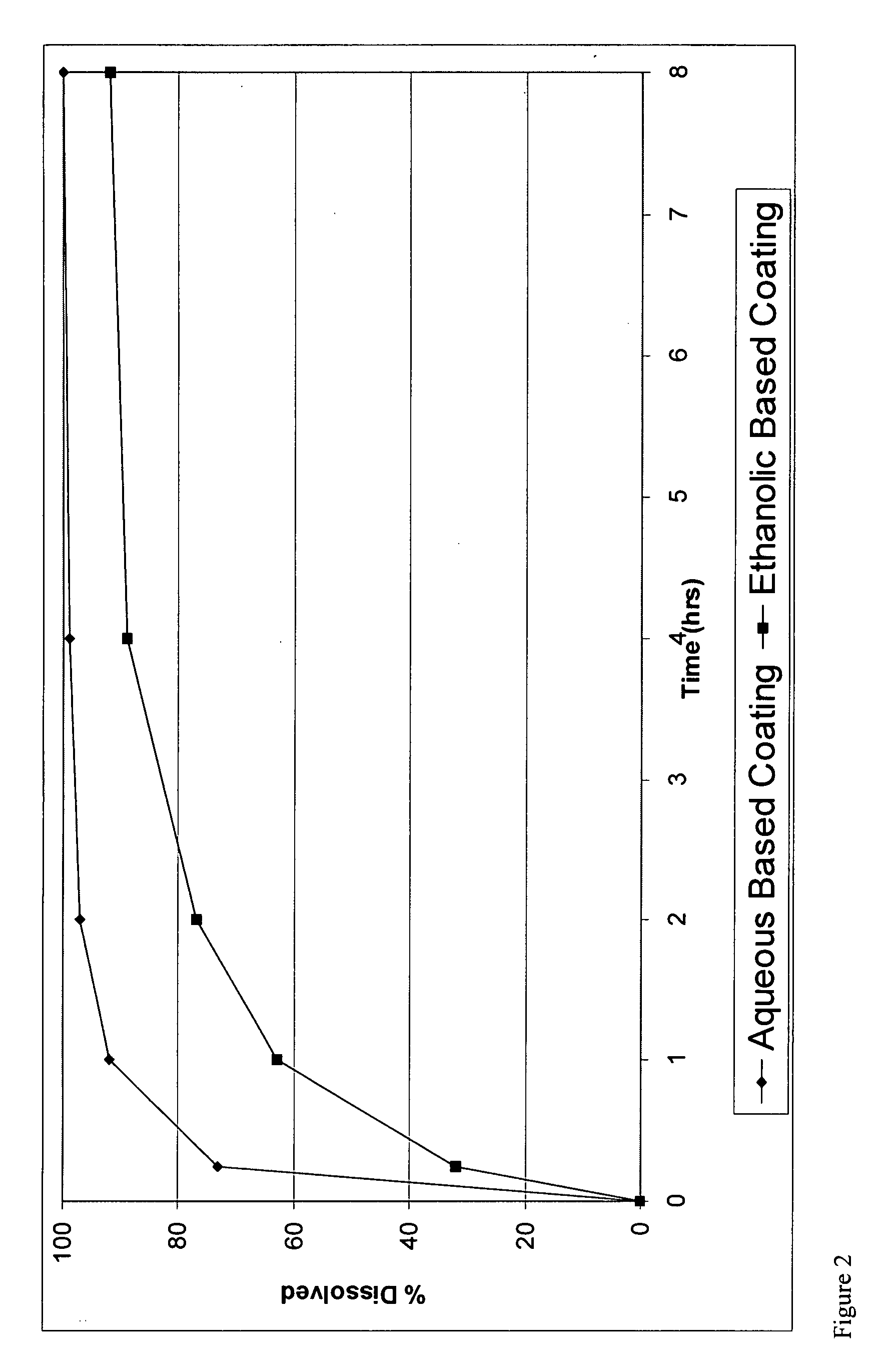
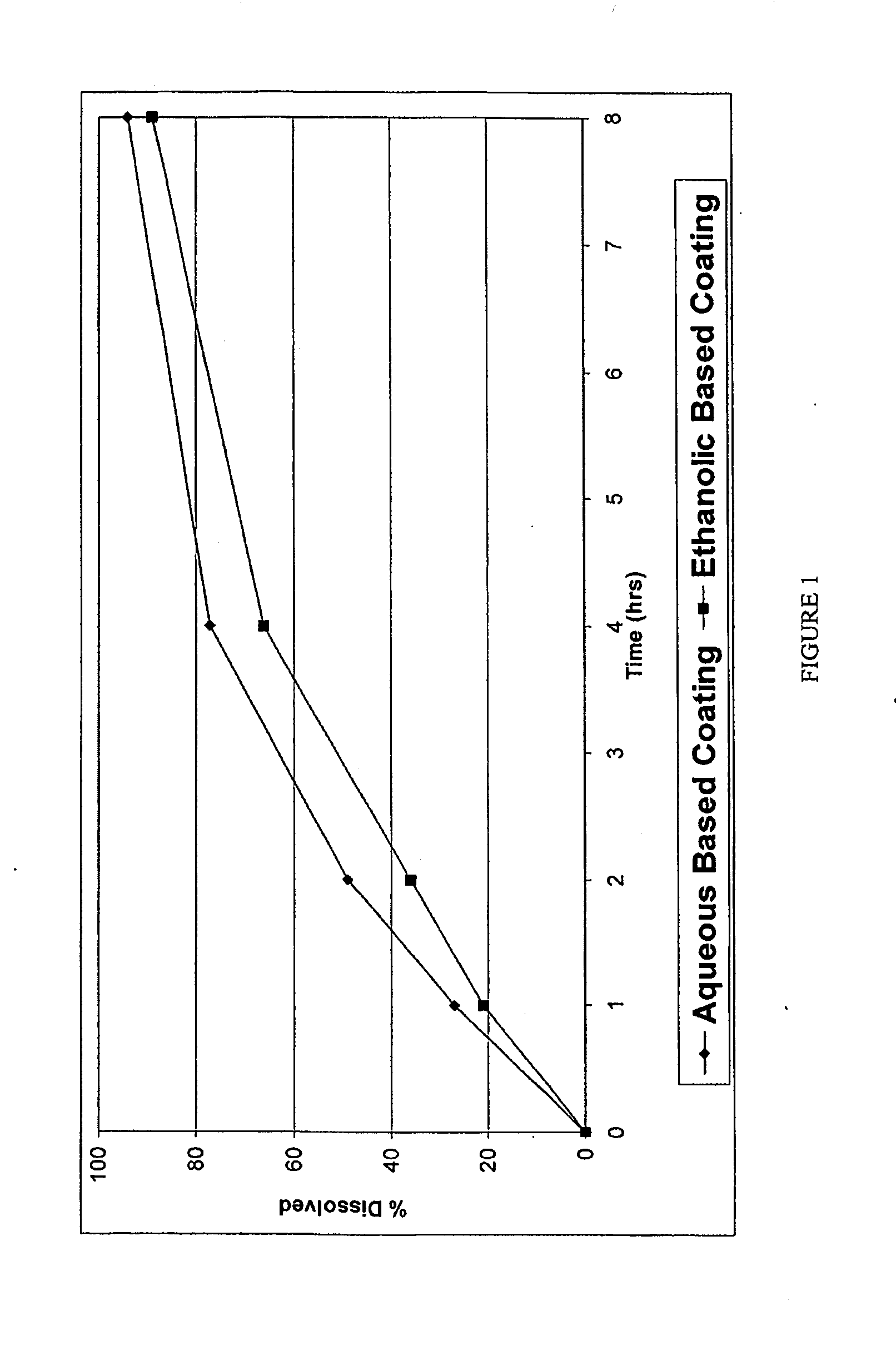
A pharmaceutical composition may include a granulate which may include at least one active pharmaceutical ingredient susceptible to abuse by an individual mixed with at least two materials, a first material that is substantially water insoluble and at least partially alcohol soluble and a second material that is substantially alcohol insoluble and at least partially water soluble, wherein the active pharmaceutical ingredient and the two materials are granulated in the presence of water and alcohol. The composition may also include a coating on the granulate exhibiting crush resistance which may have a material that is deposited on the granulate using an alcohol based solvent. The composition further comprises a second particle comprising a fat / wax. The present invention also includes a coated granulate, various dosage forms of the composition, as well as methods of production and tableting.
Owner:CIMA LABS +1
Multiple analyte assay device with sample integrity monitoring system
InactiveUS20020001854A1Avoid pollutionImprove securitySamplingBurette/pipette supportsAnalyteSample integrity
An assay device, a fluid analyte sample separator device and methods for use of thereof for determining whether the integrity of a fluid analyte sample has been compromised and for contemporaneously assaying the sample for the presence or absence of multiple analytes, such as drugs of abuse. The device is composed of a housing having separate slots therein for insertion of one or more analyte test strips, one end of which protrudes from the housing, and one or more units of a sample integrity monitoring system. The device may be used in dipstick or cassette form. An analyte sample separator for division of sample and retention of uncontaminated sample for further testing is also provided. The analyte test strips and sample integrity monitoring system are replaceable, so that the panel of analytes and of sample condition parameters tested can be customized.
Owner:ASSURANCE BIOTECH
Abuse-resistant pharmaceutical dosage form
InactiveUS20050191244A1Inhibition releaseUnnecessary stressOrganic active ingredientsNervous disorderAntagonistDosage form
A solid pharmaceutical dosage form that is safeguarded against abuse containing at least one active substance that could be subject to abuse and at least one antagonist for the active substance, which antagonist is spatially separate from the active substance. The active substance or substances is / are present in at least one subunit (a), and the at least one antagonist is present in at least one subunit (b), and the at least one antagonist in subunit (b) is to all intents and purposes not released in the body if the dosage form is correctly administered as prescribed.
Owner:GRUNENTHAL GMBH
Abuse Resistant and Extended Release Formulations and Method of Use Thereof
InactiveUS20090082466A1Reducing solvent extraction efficiencyReduce filtration efficiencyBiocidePharmaceutical non-active ingredientsOpioid abuseChemical toxicity
The present invention is in the field of oral, abuse resistant pharmaceutical compositions of opioids, extended release pharmaceutical compositions of opioids and extended release abuse resistant pharmaceutical compositions of opioids and the use thereof for the treatment of pain. The present invention is also directed to extended release pharmaceutical compositions and the use thereof for preventing or minimizing the risk of opioid abuse and / or opioid toxicity from either intentional or unintentional tampering. The present invention is further directed at a method of preventing or minimizing the risk of opioid abuse and / or opioid toxicity from either intentional or unintentional tampering.
Owner:RELMADA THERAPEUTICS
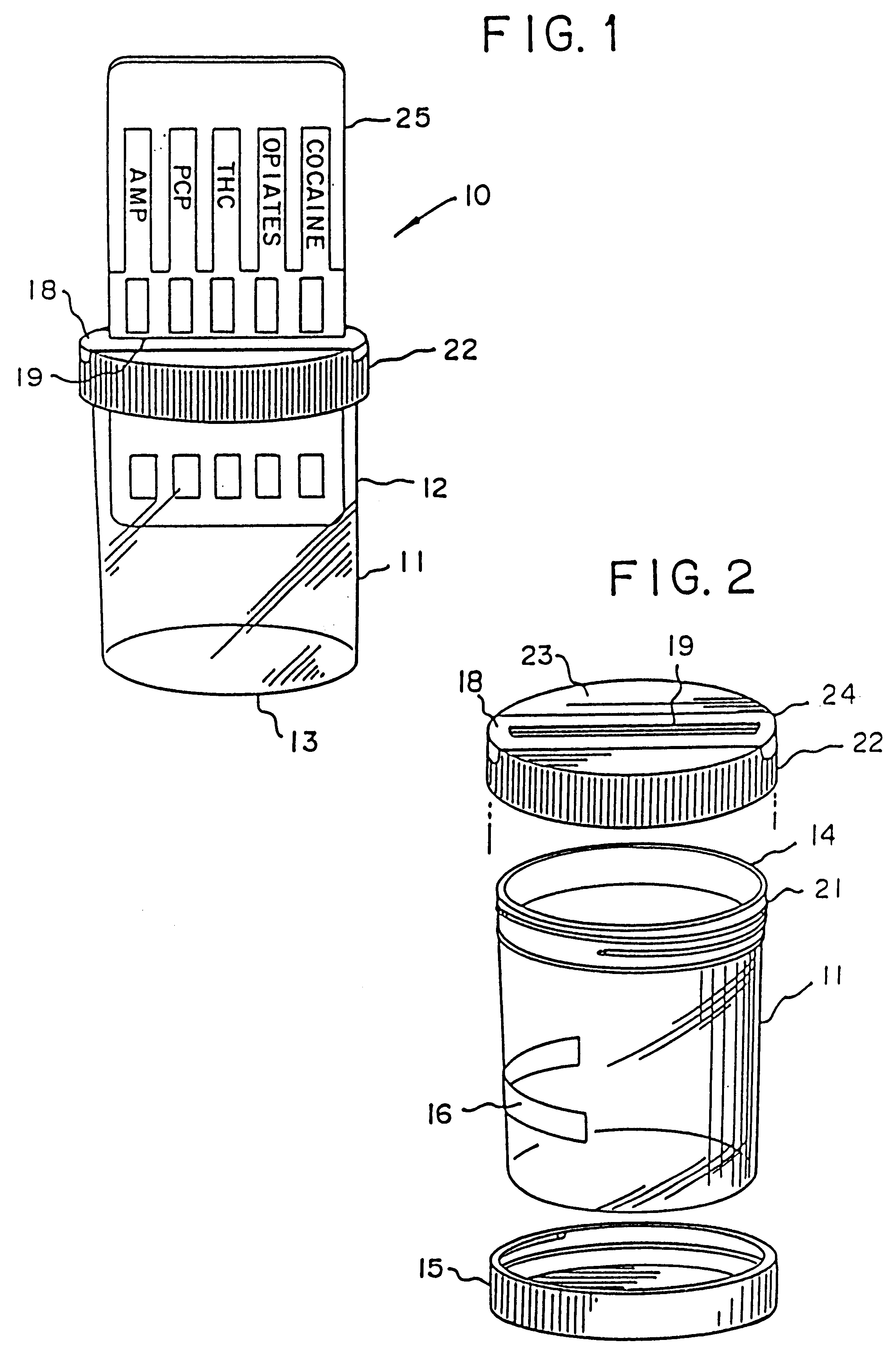
Device for the testing of body fluid samples
InactiveUS6406922B2Simplified and inexpensiveEliminate disadvantagesBioreactor/fermenter combinationsBiological substance pretreatmentsQuality controlBody fluid sample
Owner:HEALGEN SCI LLC
Methods for treating drug addiction and improving addiction-related behavior
ActiveUS8232315B2Improve behaviorAmeliorating and eliminating effectBiocideNervous disorderTherapy drug addictionAddiction
The invention is directed to a method of treating addiction to drugs of abuse in a subject, comprising administering a therapeutically effective amount of a cabamoyl compound, or pharmaceutically acceptable salt or ester thereof.
Owner:BIOPHARM
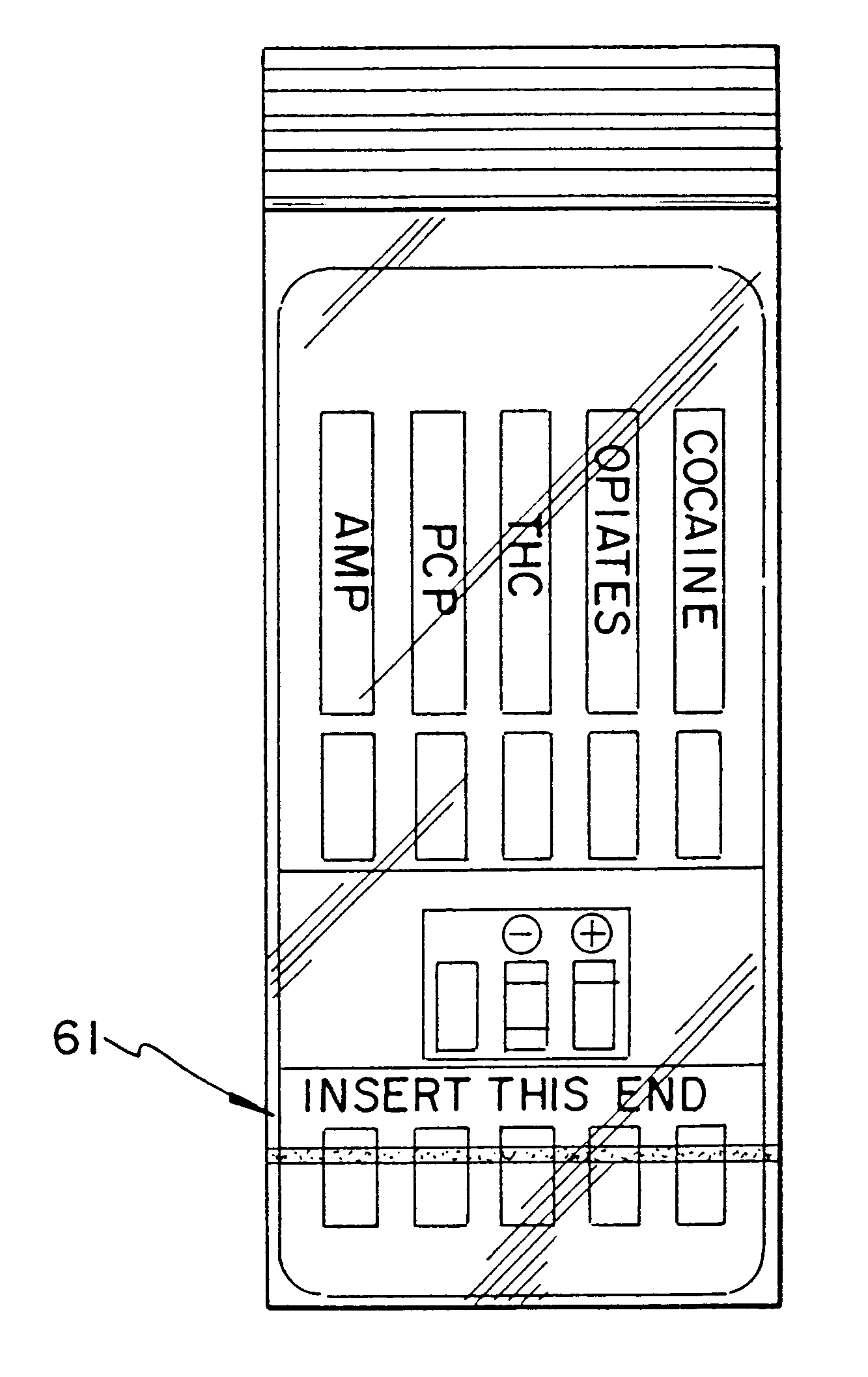
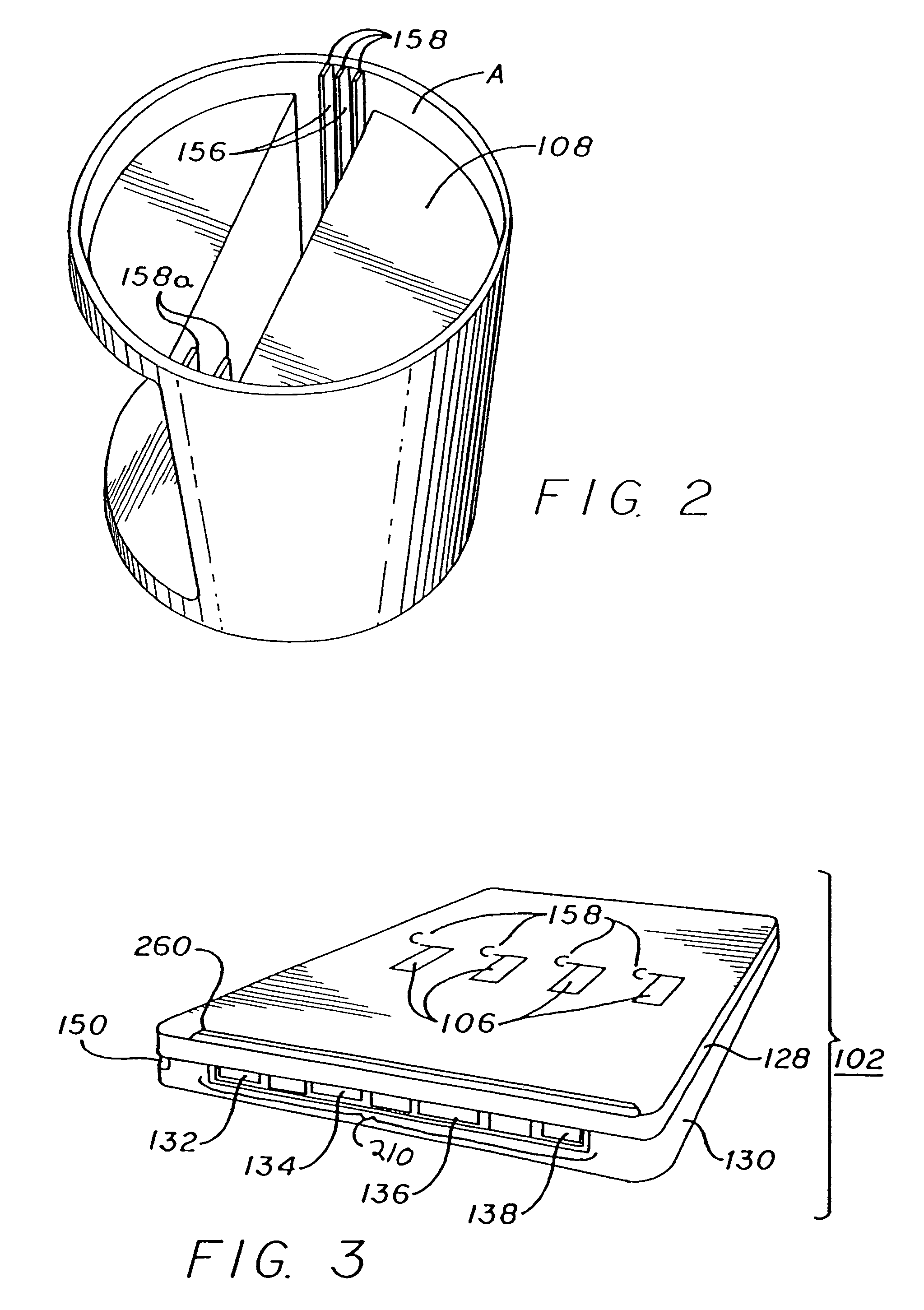
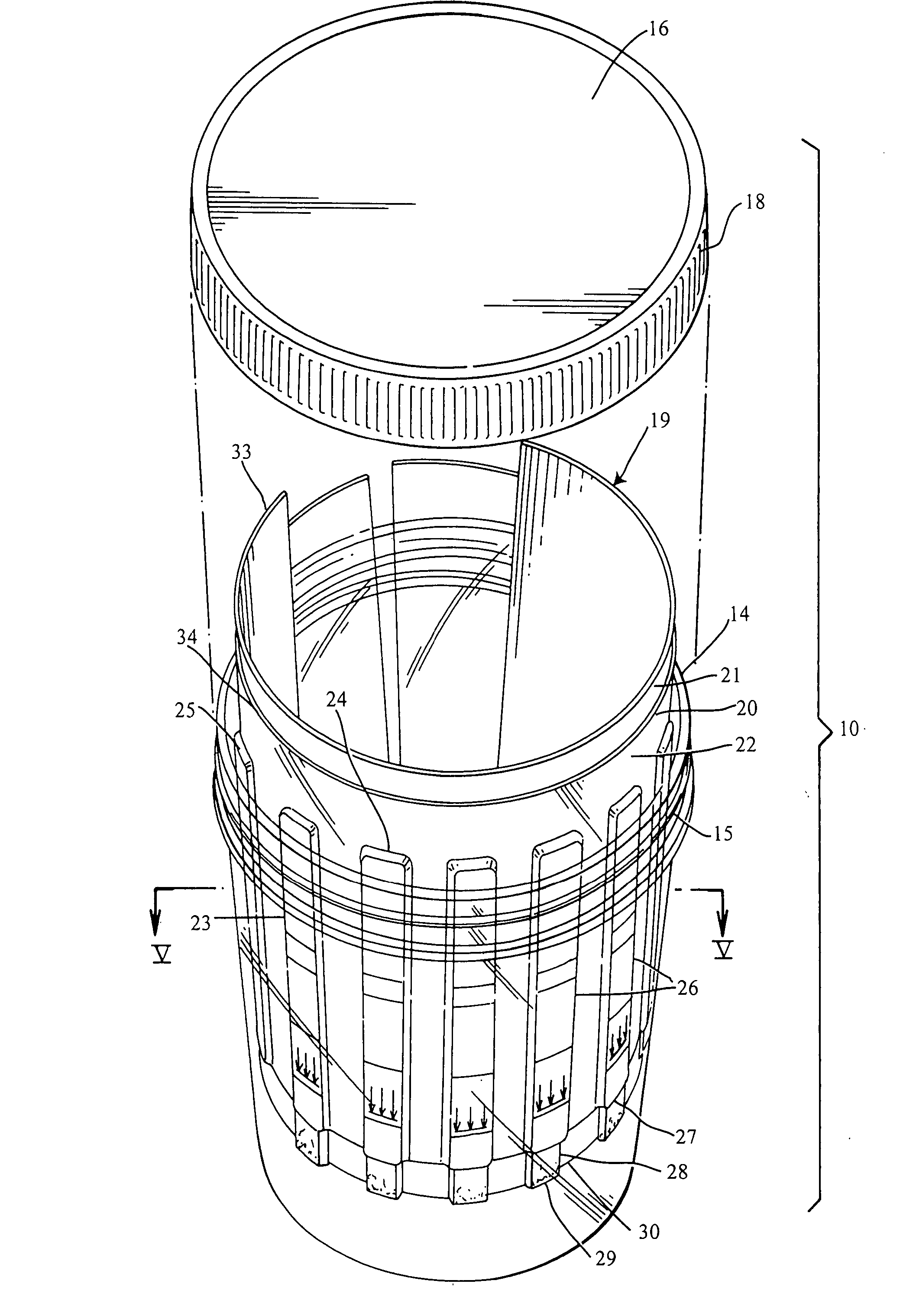
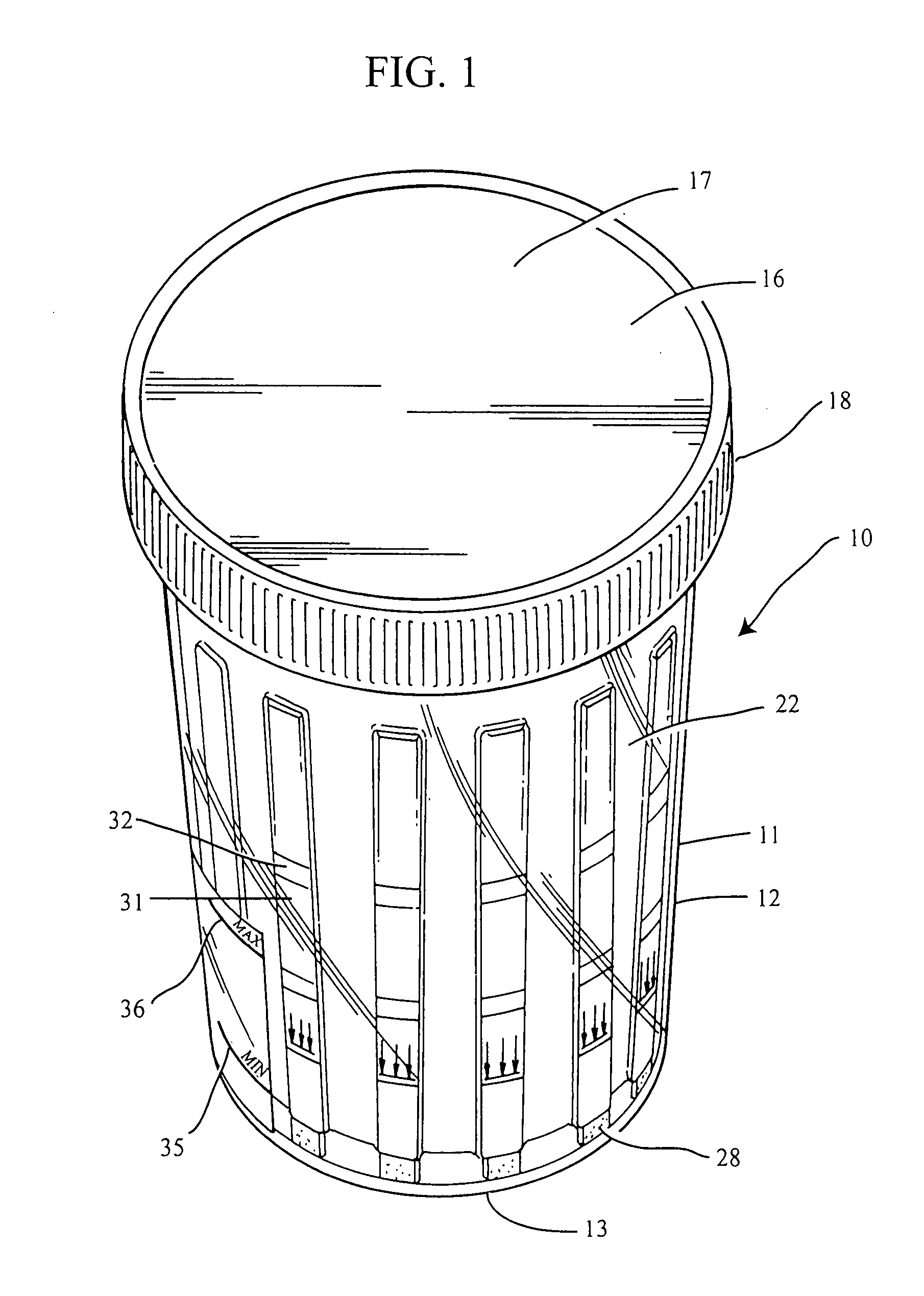
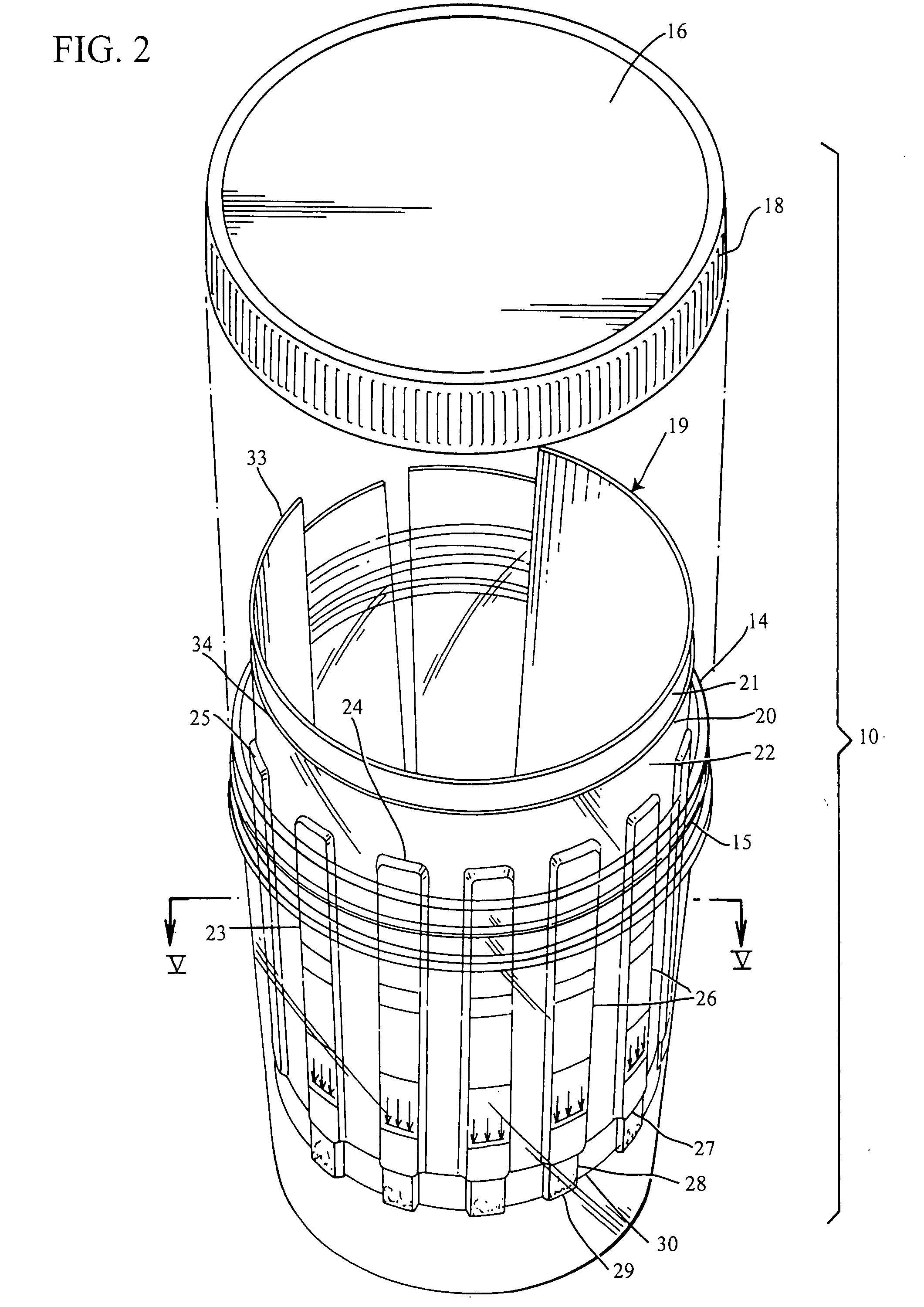
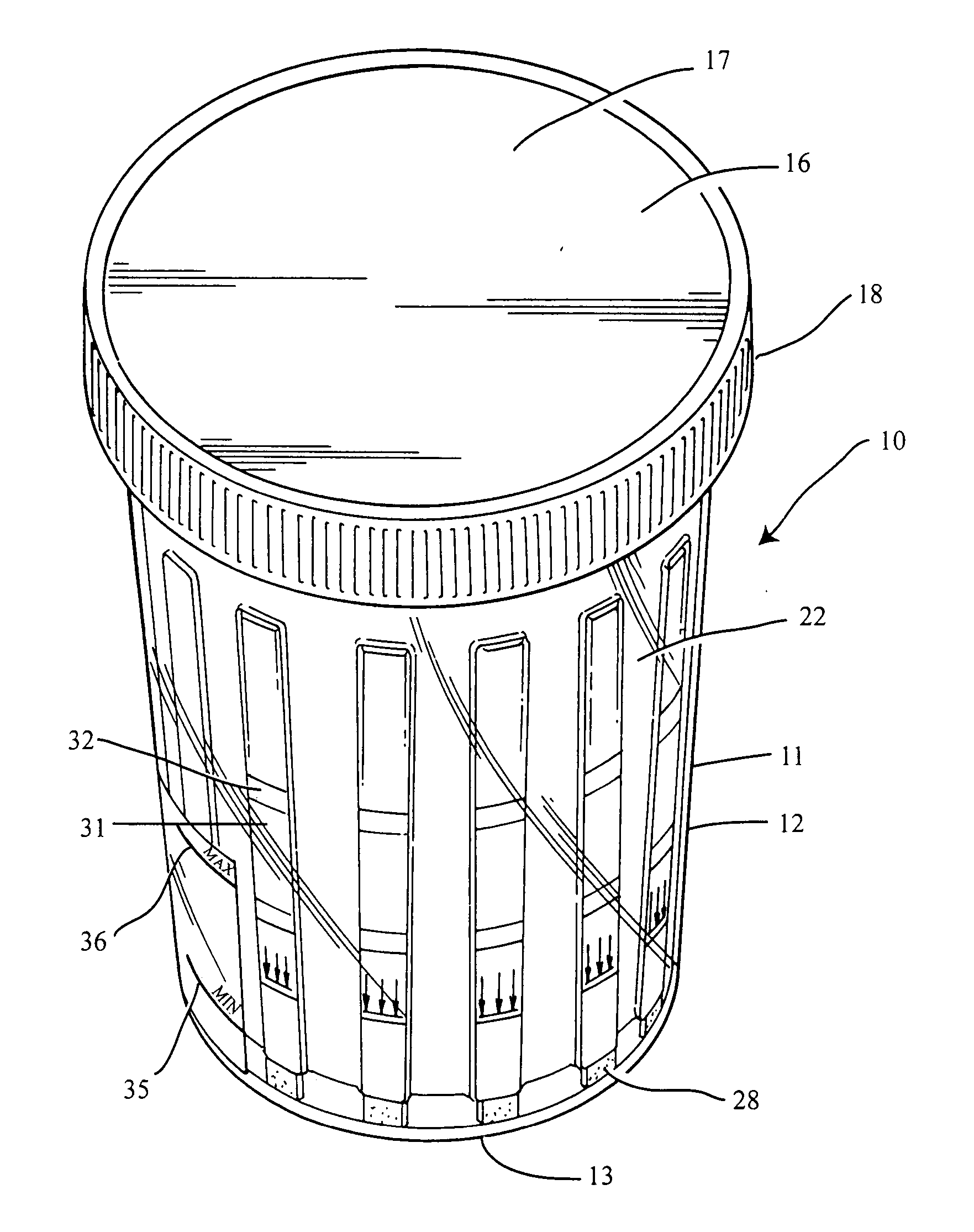
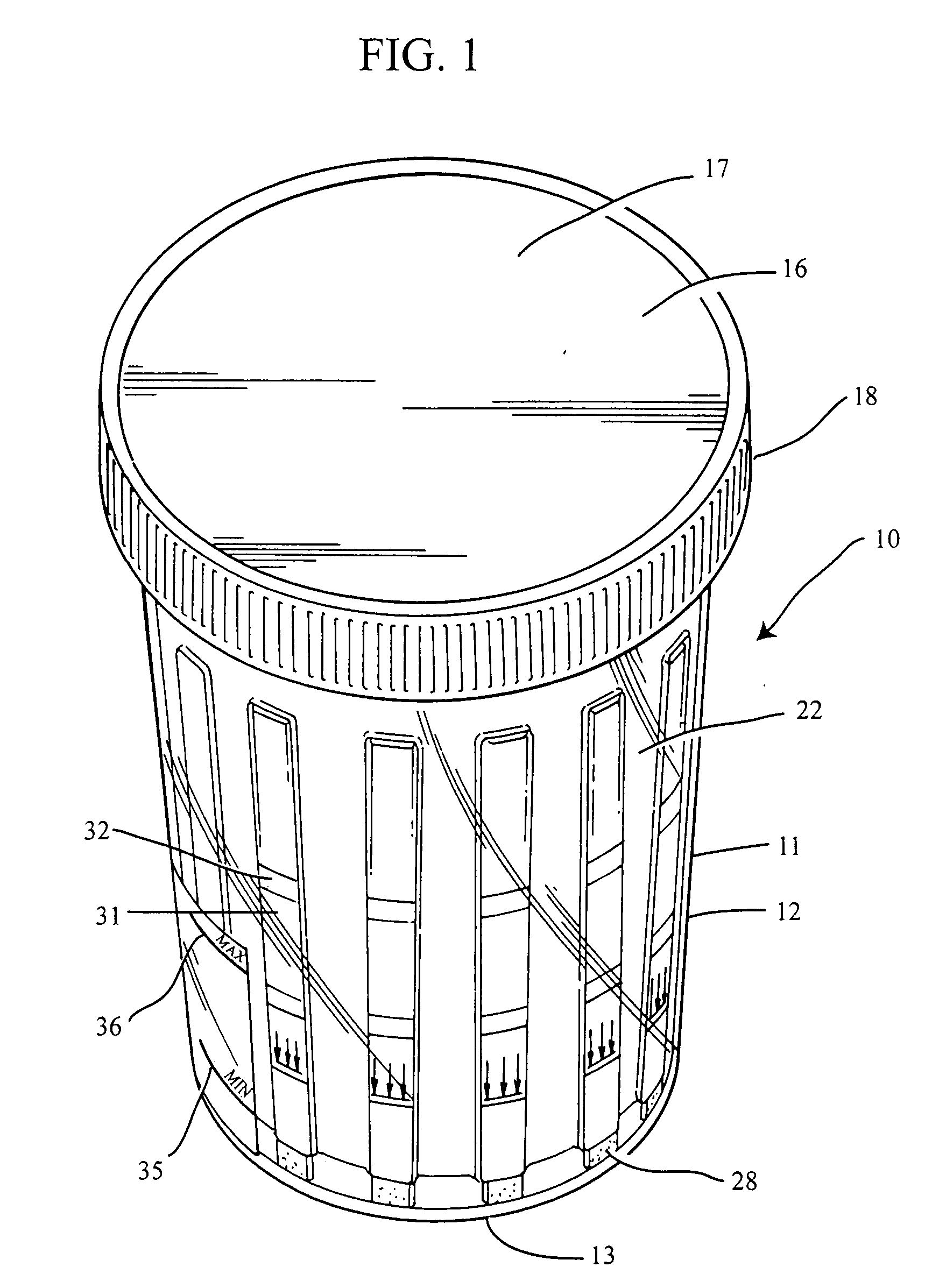
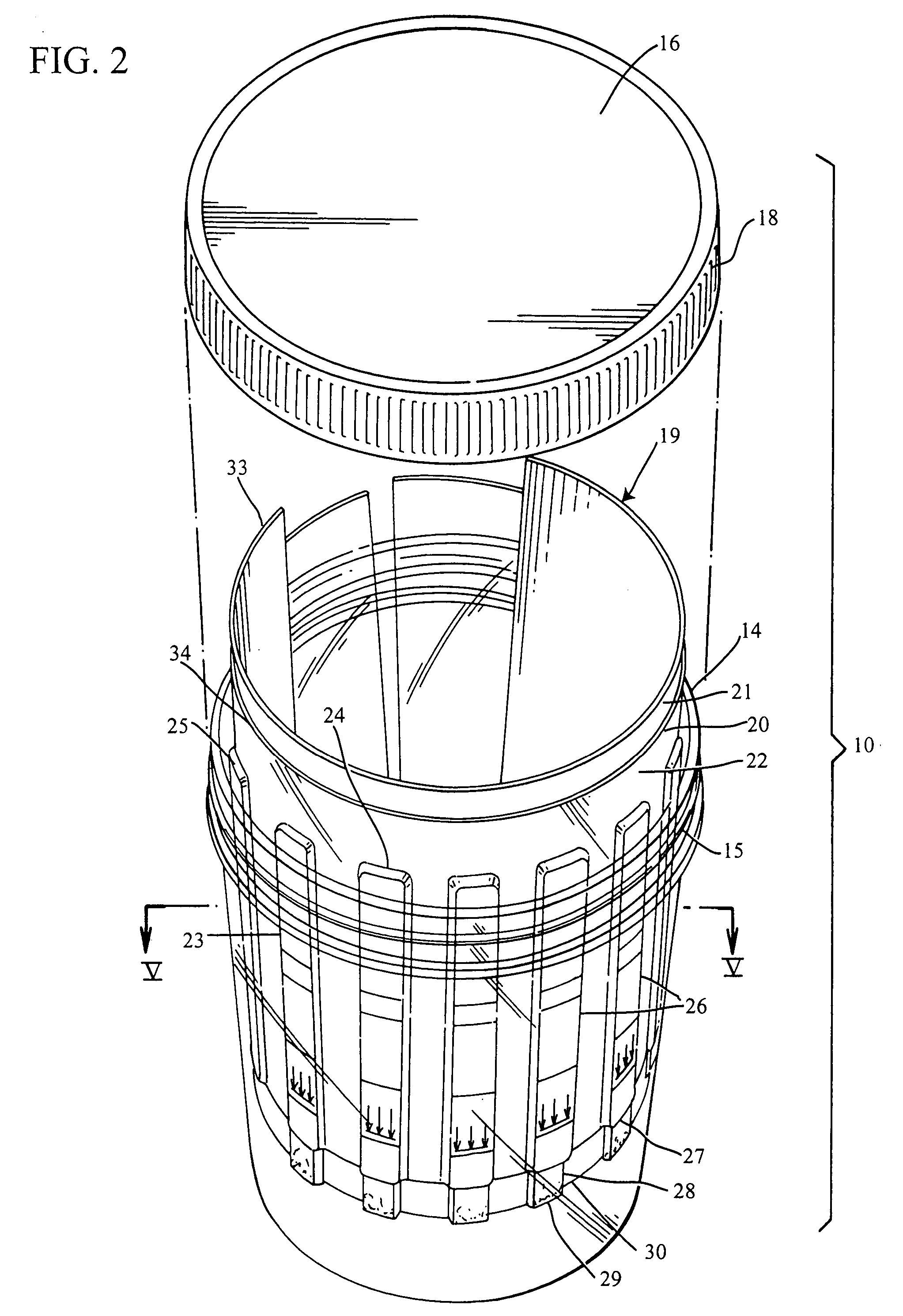
Slide-in cassette for a cup for testing of drugs of abuse
InactiveUS7244392B1Cut off available spaceEase of use and stabilityAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorMagnetic tapeHermetic seal
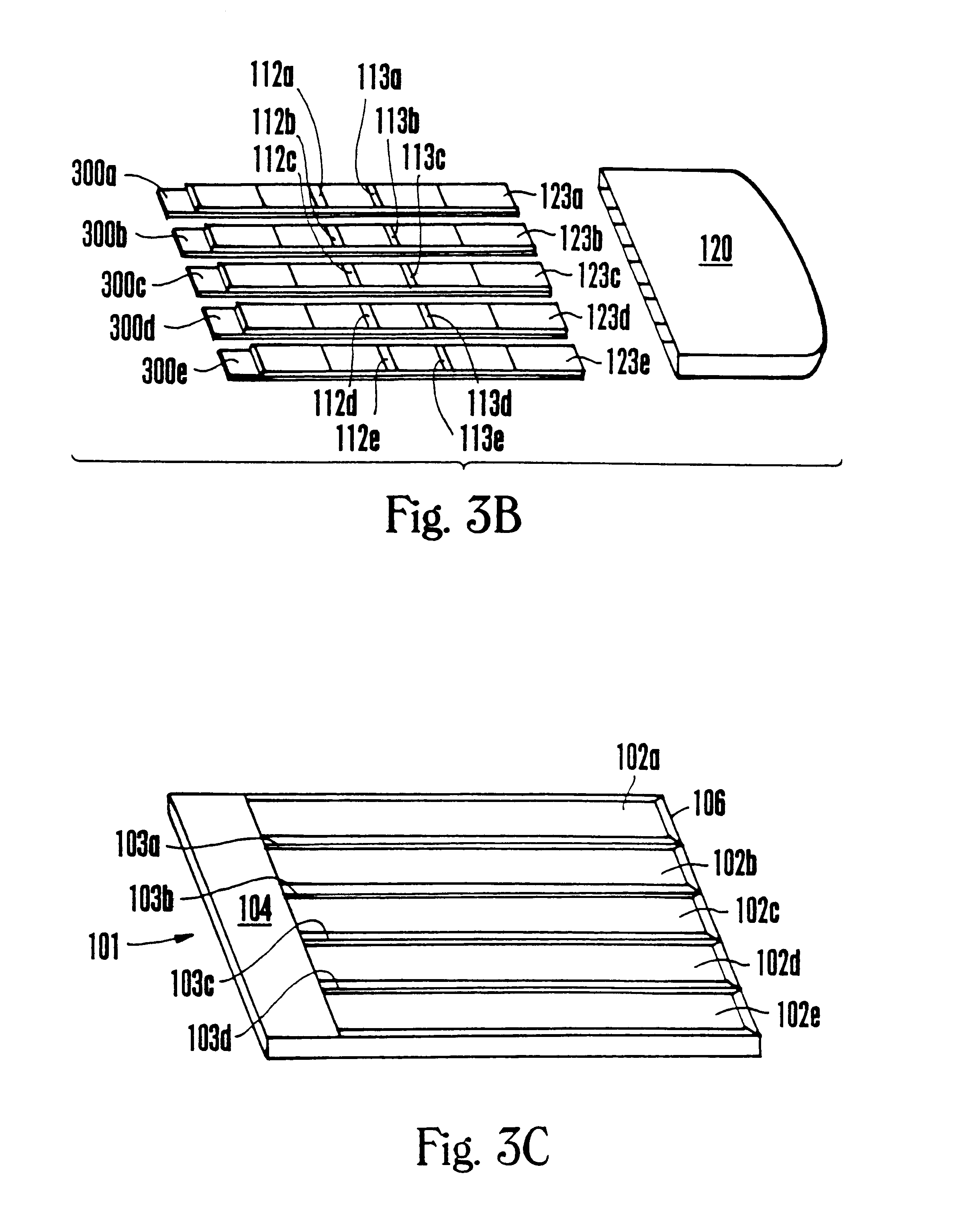
A specimen cup (100) has slide-in cassette (102) hermetically sealed in a chamber (104), with a outer partition being transparent. The cassette comprised chemical test strips (106) used to provide testing of drugs of abuse or other chemical or biological substances. The cassette is designed to draw urine up from the front bottom of the cup, thereby reduces the amount of urine required to perform the test. Further the cassette is designed to form isolated test channels through the use of strategically placed vertical and horizontal bars which are hermetically sealed. The cup further comprises a spill prevention flap or float (108) and an optionally enlarged sample collection portion (110) for its operation. The windows of the test cassette are covered with transparent fluid-resistant plate to prevent urine from accidentally spill onto the strips.
Owner:ABBOTT RAPID DIAGNOSTICS INT UNLTD
System, method and device for tissue-based diagnosis
InactiveUS20110212485A1Easy accessImprove throughputOrganic active ingredientsBioreactor/fermenter combinationsWhole bodyHazardous substance
The current invention provides devices, methods and systems involving application of energy and / or a liquefaction promoting medium to a tissue of interest to generate a liquefied sample comprising tissue constituents so as to provide for rapid tissue sampling, tissue decontamination as well as qualitative and / or quantitative detection of analytes that may be part of tissue constituents (e.g., several types of biomolecules, drugs, and microbes). In addition, the current invention provides specific compositions of the said liquefaction promoting medium so as to facilitate liquefaction, preserve liquefied tissue constituents, and enable delivery of molecules into tissues. Determination of tissue composition in the liquefied tissue sample can be used in a variety of applications, including diagnosis or prognosis of local as well as systemic diseases, evaluating bioavailability of therapeutics in different tissues following drug administration, forensic detection of drugs-of-abuse, evaluating changes in the tissue microenvironment following exposure to a harmful agent, and various other applications. The methods, devices and systems are used to deliver one or more drugs through or into the site of the tissue to be liquefied.
Owner:RGT UNIV OF CALIFORNIA
Abuse-Resistant Pharmaceutical Dosage Form
InactiveUS20100221322A1Inhibition releaseUnnecessary stressBiocideNervous disorderAntagonistDosage form
A solid pharmaceutical dosage form that is safeguarded against abuse containing at least one active substance that could be subject to abuse and at least one antagonist for the active substance, which antagonist is spatially separate from the active substance. The active substance or substances is / are present in at least one subunit (a), and the at least one antagonist is present in at least one subunit (b), and the at least one antagonist in subunit (b) is to all intents and purposes not released in the body if the dosage form is correctly administered as prescribed.
Owner:GRUNENTHAL GMBH
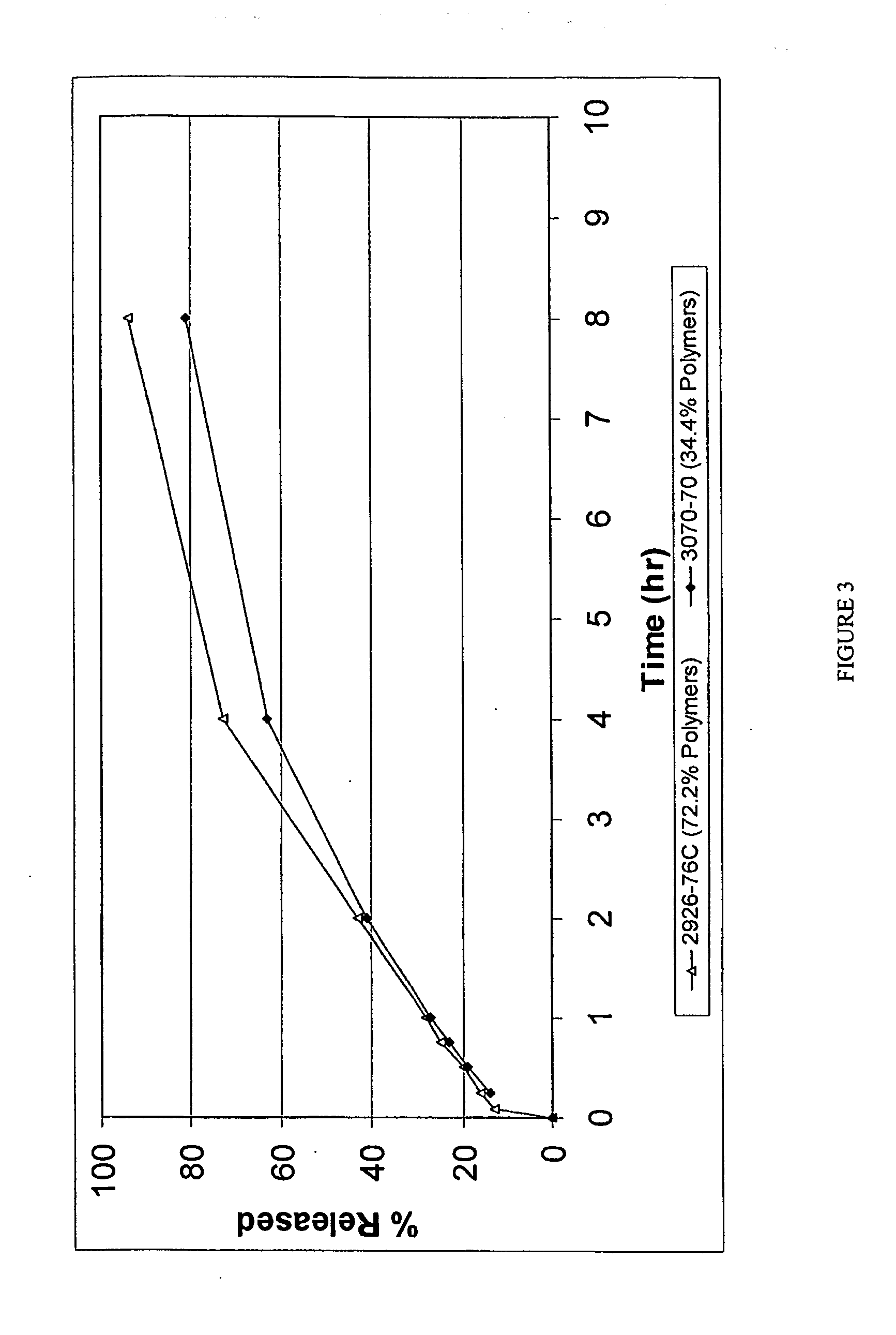
Dosage form with impeded abuse
InactiveUS8722086B2Prevent crushingPrevent the subsequent abuseOrganic active ingredientsPill deliveryWaxBreaking strength
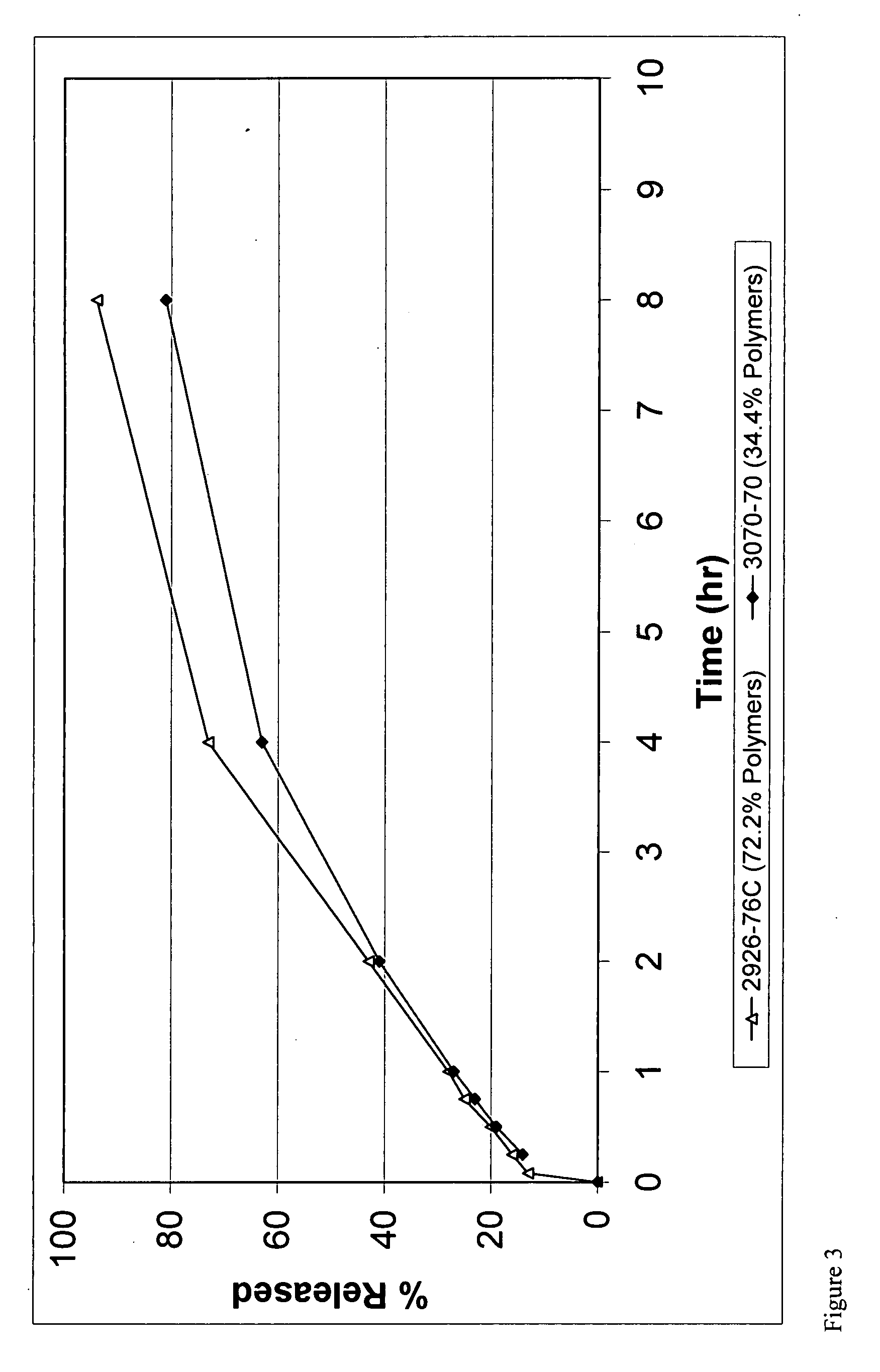
A multiparticulate dosage form formulated to make misuse more difficult containing least one active substance with potential for misuse (A), at least one synthetic or natural polymer (C), optionally at least one natural, semi-synthetic or synthetic wax (D), at least one disintegrant (E) and optionally one or more additional physiologically compatible excipients (B), wherein the individual particles of the dosage form display a breaking strength of at least 500 N and a release of active substance of at least 75% after 45 minutes measured according to Ph.Eur. in the paddle mixer with sinker in 600 ml of aqueous buffer solution with a pH value of 1.2 at 37° C. and 75 rpm.
Owner:GRUNENTHAL GMBH
Extraction method and apparatus for high-sensitivity body fluid testing device
ActiveUS20080166820A1Rapid and efficient mannerQuick and accurate testBioreactor/fermenter combinationsBiological substance pretreatmentsBottleSaliva
An extraction method and apparatus is provided for obtaining quick, safe and highly sensitive testing of any of a variety of body fluids including saliva, blood, urine or other fluids for drugs of abuse or other analytes. The apparatus includes a latchable extraction wand for obtaining body fluid samples from a subject which is adapted to maximize the portion of the body fluid sample that will go into a graduated bottle containing a buffer solution, and a testing device wherein the sample will be received and into which test strips can be inserted to determine levels of drugs of abuse or other analyte in the sample. In one of the methods of the invention, energy is imparted to the sample and buffer solution, such as by shaking, and this facilitates the reduction of sample viscosity, such as by promoting the breakdown of mucins when the sample is saliva.
Owner:HEALGEN SCI LLC
Abuse resistant drugs, method of use and method of making
An abuse resistant oral pharmaceutical composition, comprising: a barrier layer, comprising a first polymer; a diffusion layer, comprising a second polymer, substantially covering the barrier layer, wherein the diffusion layer is bonded to the barrier layer and comprises a drug that is substantially homogeneously distributed within the second polymer and diffuses from the diffusion layer within the gastrointestinal (GI) tract; and optionally an expansion layer comprising an expandable polymer, wherein the expansion layer is substantially covered by the barrier layer. Methods of making the same and methods of using the same are also provided.
Owner:OHEMO LIFE SCI INC
Abuse resistent pharmaceutical composition
InactiveUS20050271594A1Reduce abuseCapsule deliveryIn-vivo testing preparationsBULK ACTIVE INGREDIENTActive ingredient
An abuse-resistant pharmaceutical composition includes an oily substance, at least one active ingredient having abuse potential and a capsule. The active ingredient is mixed in the oily substance. The oily substance and the active ingredient are placed in the capsule. In one embodiment the active ingredient includes oxicodone.
Owner:MIKART
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Assay device and process for the testing of fluid samples
InactiveUS20070259442A1The process is simple and effectiveFacilitates screening procedureAnalysis using chemical indicatorsVaccination/ovulation diagnosticsMechanical engineeringTest strips
An assay device for testing of liquid samples for drugs of abuse has a transparent cylindrical container for retaining a liquid sample and a portion of the cylindrical wall is flat. A test strip holder is within the container and is flat so that its front surface corresponds to the flat portion of the container wall. Immunoassay test strips are in pockets on the front face of the strip holder and are visible through the container wall. Each test strip is enclosed in a transparent pocket which has a bottom opening through which the bottom portion of the test strip protrudes to contact the liquid sample within the container. The liquid then wicks upwardly through the test strip to react with reagents within the test strip.
Owner:AMERICAN BIO MEDICA CORP
Simulated ecological breeding method for Chinese soft-shelled turtles
The invention relates to a simulated ecological breeding method for Chinese soft-shelled turtles, which belongs to the technical field of soft-shelled turtle breeding, the steps are as follows: (1) environmental condition; (2) parent turtle breeding; (3) artificial reproduction and incubation; (4) breeding of turtles; the breeding method meets the growing physiological needs of the turtles to the largest extent in a loose and stable environment, the turtles bred outside cages fall ill less, drug abuse is avoided effectively, the turtles have higher value of commodity, the taste is like that of a wild turtle, and the simulated ecological breeding method has a shorter breeding period than ecological breeding.
Owner:惠州市财兴实业有限公司
Method and test strip of detecting oxidizing adulterant in urine
A single reagent system and a method to detect and measure oxidizing adulterants in bodily fluid being screened for drugs of abuse are disclosed. The system comprising a strip containing 0.05 to 0.2 micromole / 25 sq. mm. of a benzidine derivative and is used to detect sodium hypochlorite (bleach), chlorine, hydrogen peroxide, sodium bromide, sodium iodide, sodium nitrite, and pyridinium chlorochromate adulterants in urine, sweat, saliva, blood or other bodily fluids during screening for drugs of abuse.
Owner:BRANAN MEDICAL
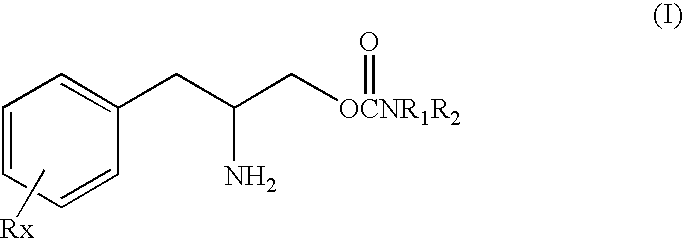
Dosage form and method for the delivery of drugs of abuse
A dosage form and method for the delivery of drugs, particularly drugs of abuse, characterized by resistance to solvent extraction, tampering, crushing, or grinding, and providing an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBOTTGMBH & CO
Treatment of addiction and addiction-related behavior
The present invention relates to the use of a composition that increases central nervous system GABA levels in a mammal, for the treatment of addiction to drugs of abuse and modification of behavior associated with addiction to drugs of abuse in said mammal.
Owner:BROOKHAVEN SCI ASSOCS
New abuse-resistant pharmaceutical composition for the treatment of opioid dependence
There is provided pharmaceutical compositions for the treatment of e.g. opioid dependency comprising microparticles of a pharmacologically-effective amount of buprenorphine, or a pharmaceutically-acceptable salt thereof, in associative admixture with particles comprising a weak acid, or particles comprising weakly-acidic buffer forming materials. The composition may further comprise a disintegrant and / or particles of a pharmacologically-effective amount of naloxone, or a pharmaceutically-acceptable salt thereof. The compositions are useful in the treatment of opioid dependency / addiction and / or pain.
Owner:OREXO AB
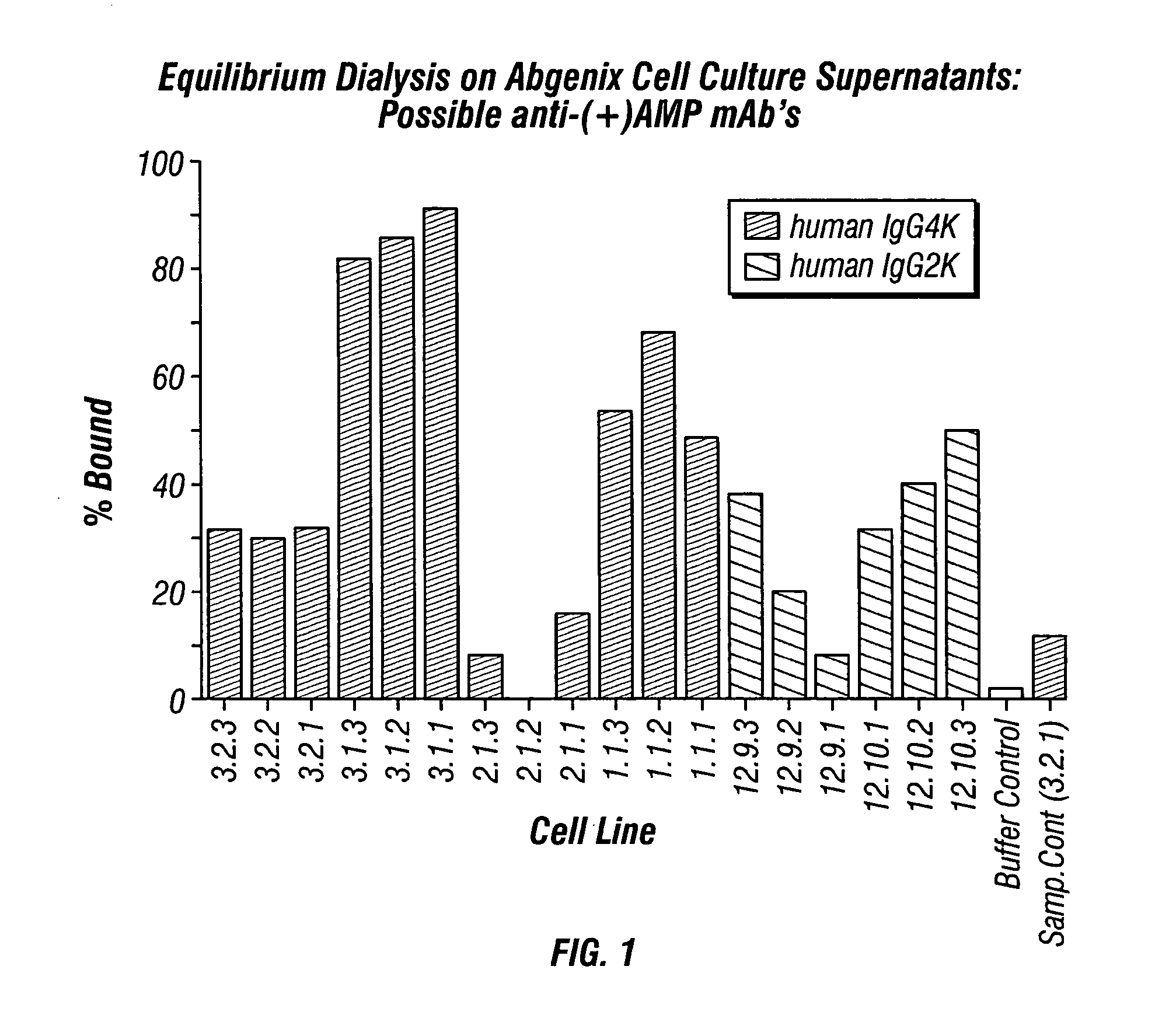
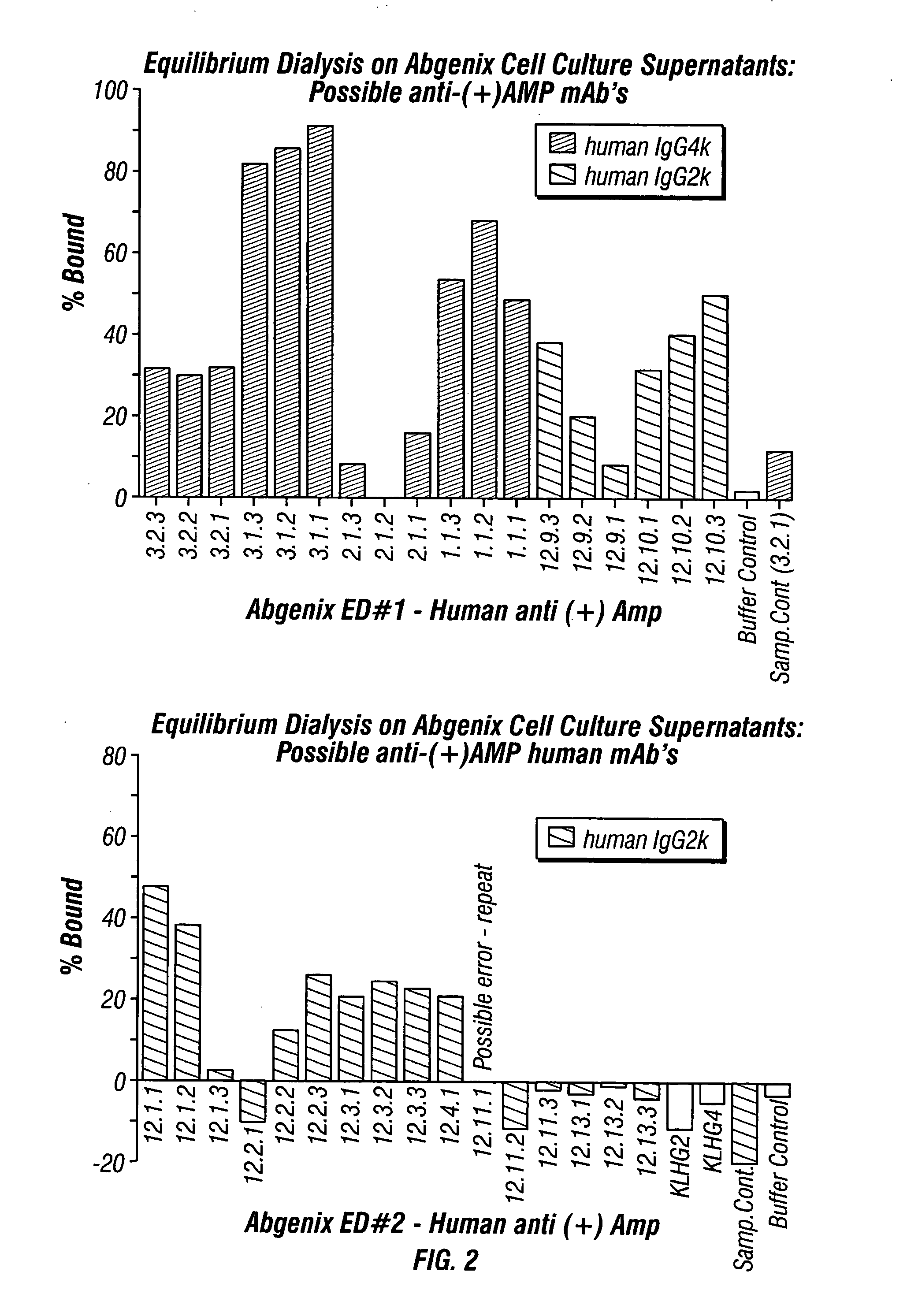
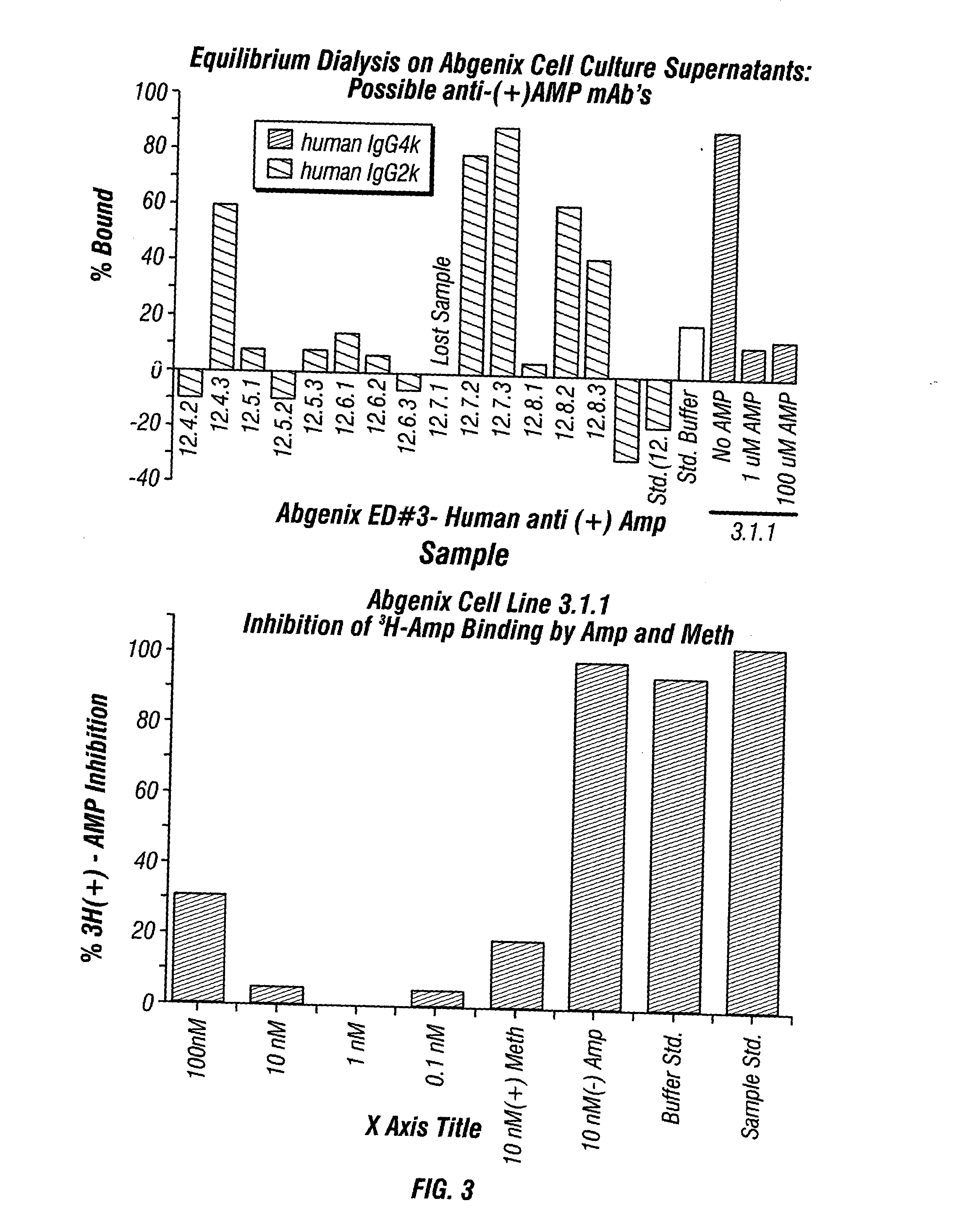
Antibodies against drugs of abuse
InactiveUS20050013809A1Reduce exposureAnimal cellsImmunoglobulins against animals/humansComplementarity determining regionHeavy chain
The present invention is related to antibodies directed to various drugs of abuse and uses of such antibodies. In preferred embodiments, the drugs of abuse are amphetamine, methamphetamine, or phencyclidine (PCP). In particular, in accordance with the present invention, there are provided fully man monoclonal antibodies directed to drugs of abuse. Nucelotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions and / or complementarity determining regions (CDR's), specifically from FR1 through FR3 or CDR1 through CDR3, are provided. Hybridomas or other cell lines expressing such immunoglobulin molecules and monoclonal antibodies are also provided.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
Abuse resistant drug formulation
A pharmaceutical composition may include a coated particulate which may include at least one active pharmaceutical ingredient, particularly one susceptible to abuse by an individual. The coated particles may include a fat / wax and have improved controlled release and / or crush resistance. Method of making these coated particulate and dosage forms therewith are also described.
Owner:CIMA LABS
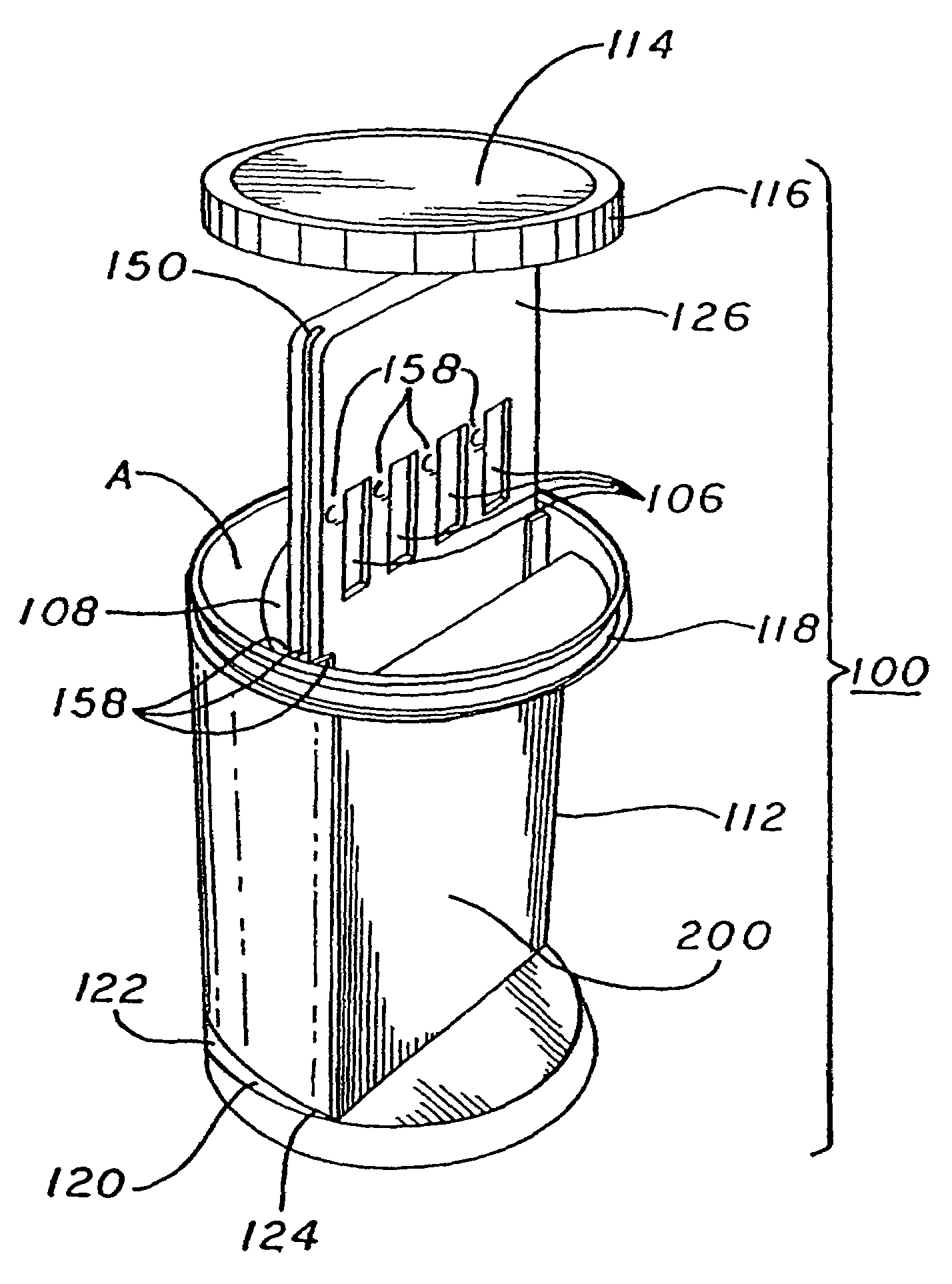
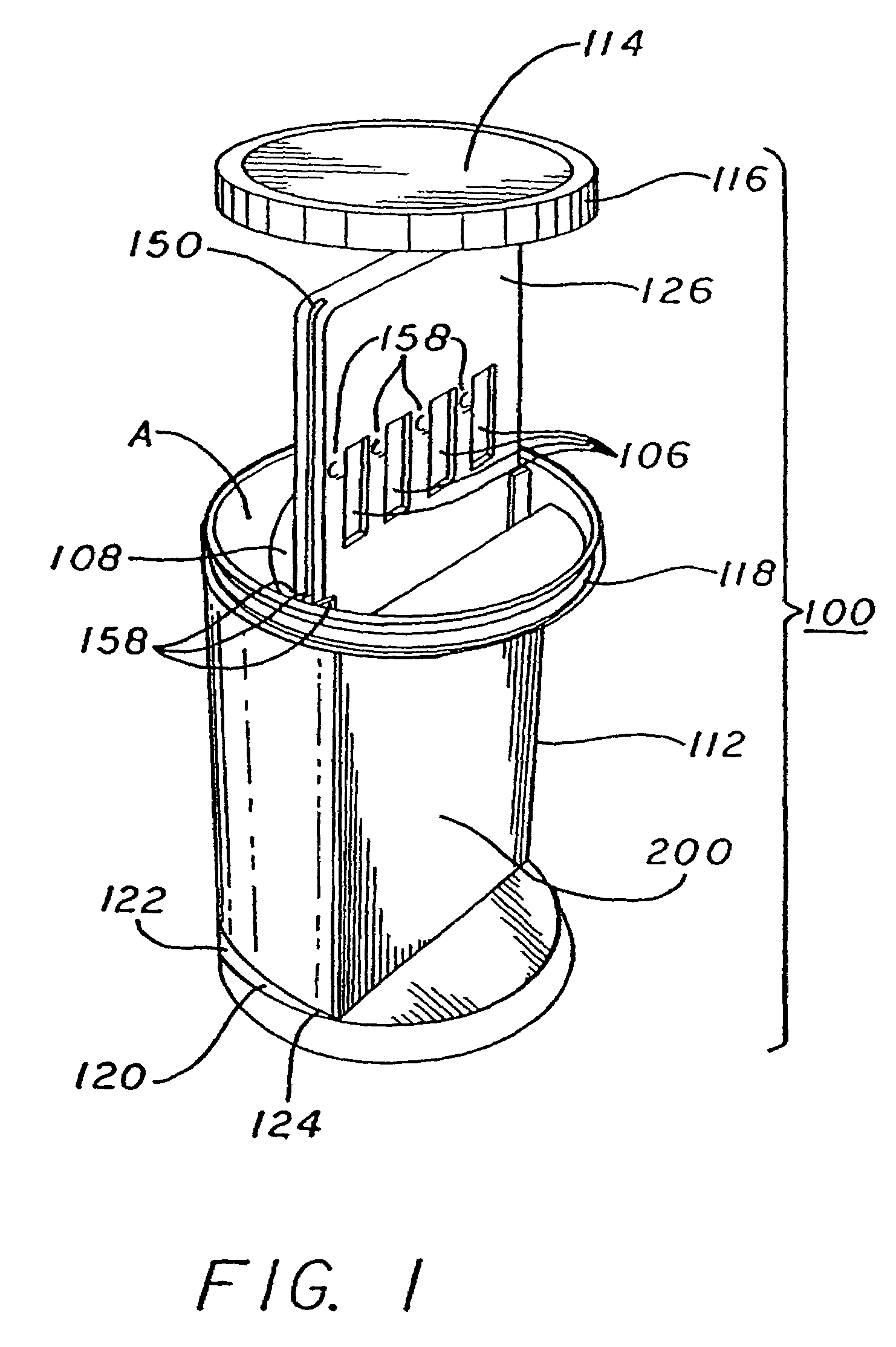
Assay device and process for the testing of fluid samples
ActiveUS20060127274A1The process is simple and effectiveFacilitates screening procedureAnalysis using chemical indicatorsMicrobiological testing/measurementEngineeringMechanical engineering
An assay device for testing of liquid samples for drugs of abuse has a transparent container for retaining a liquid sample. A backing member is within the container and is curved so that its front surface corresponds to the curvature of the container wall. Immunoassay test strips are on the front face of the backing and are visible through the container wall. Each test strip is enclosed in a transparent pocket which has a bottom opening through which the bottom portion of the test strip protrudes to contact the liquid sample within the container. The liquid then flows upwardly through the test strip to react with reagents within the test strip.
Owner:HEALGEN SCI LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com