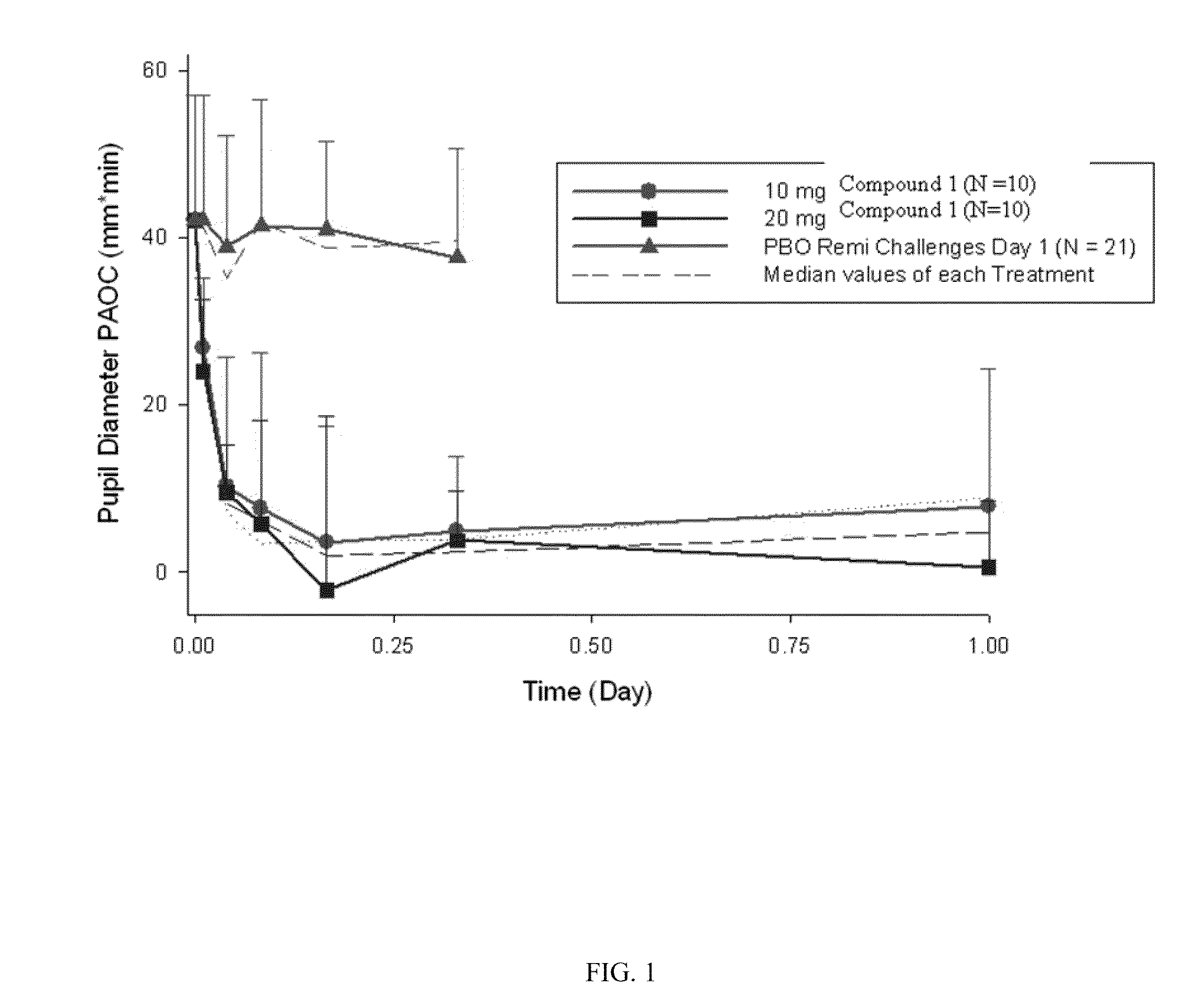
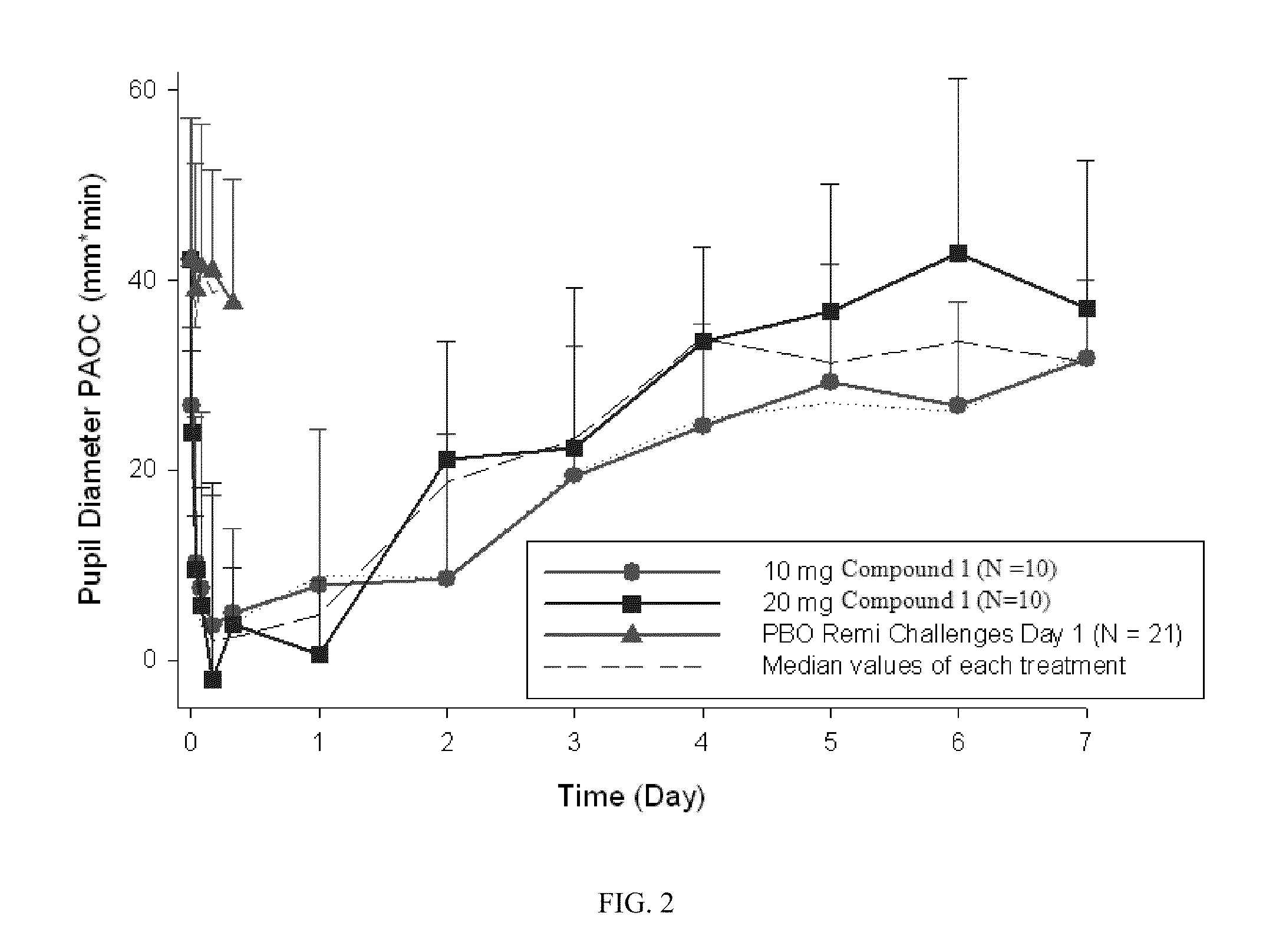
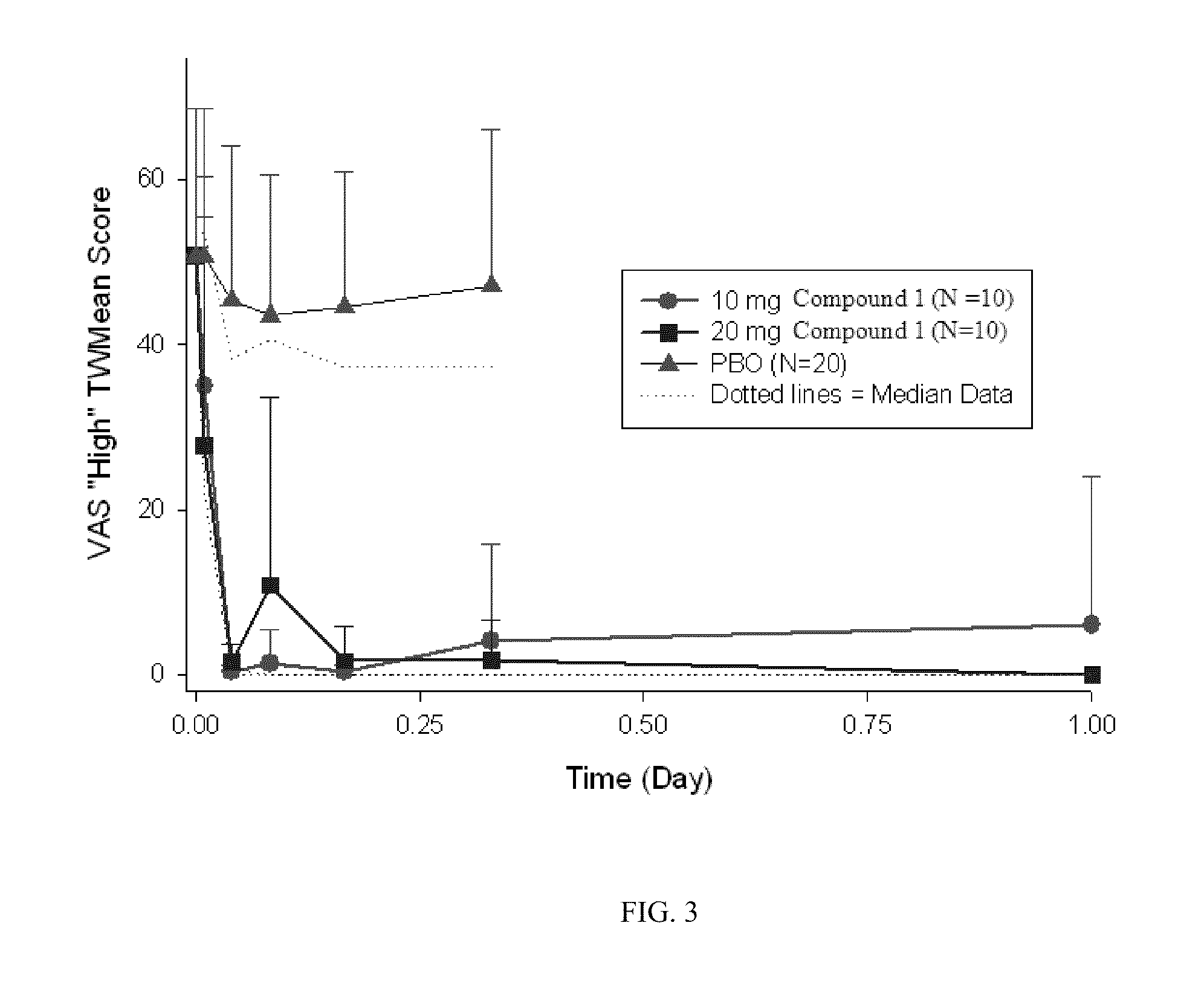
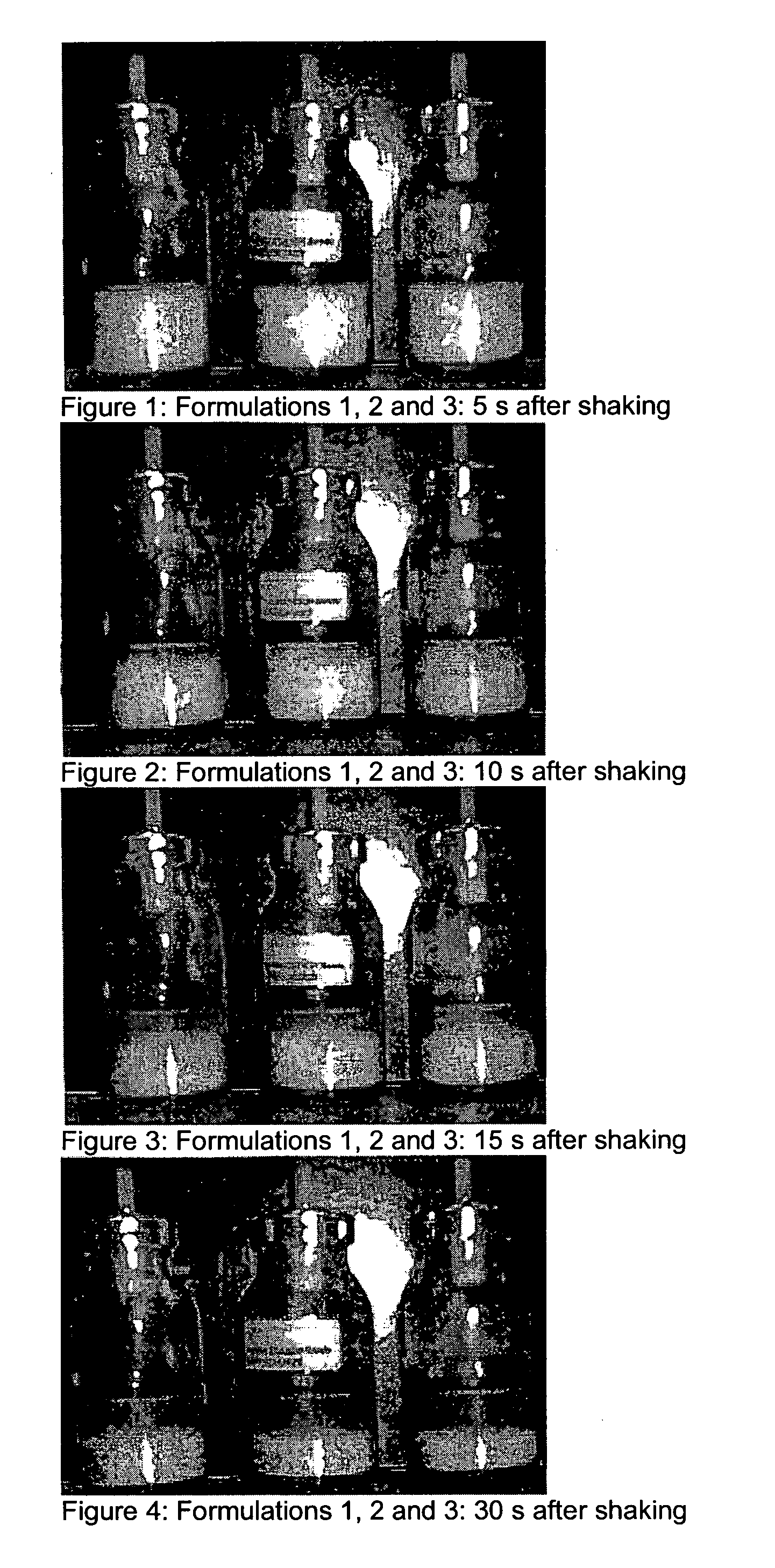
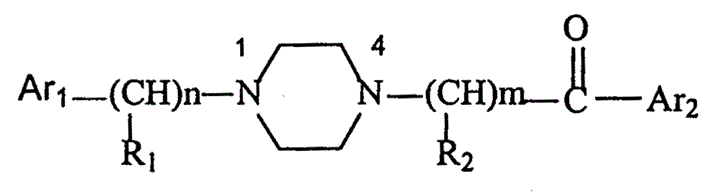Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
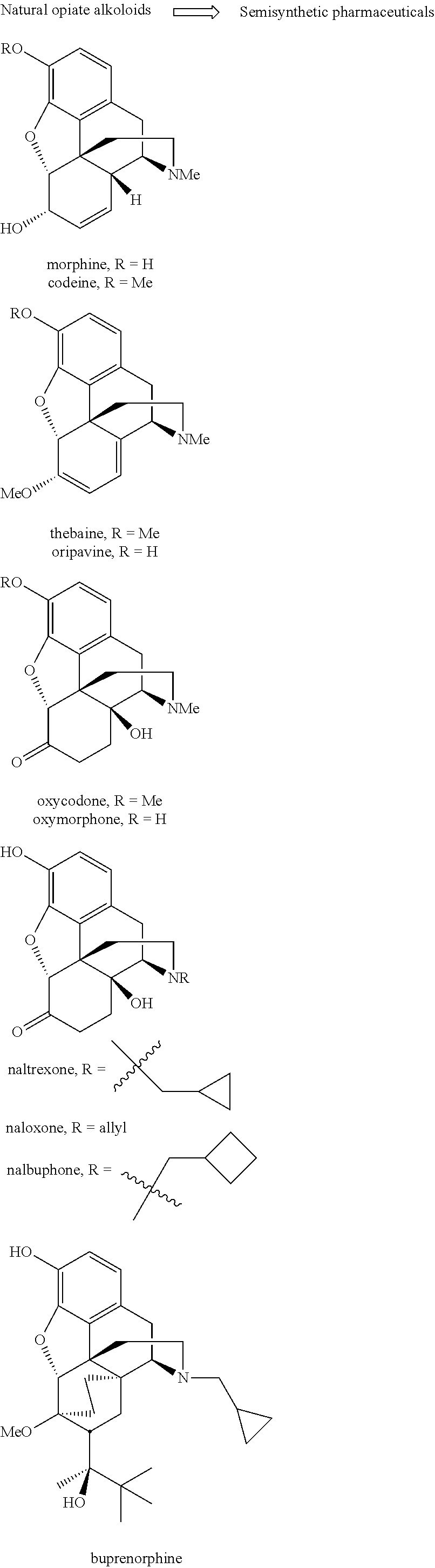
526 results about "Morphine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Morphine is used to help relieve moderate to severe pain.
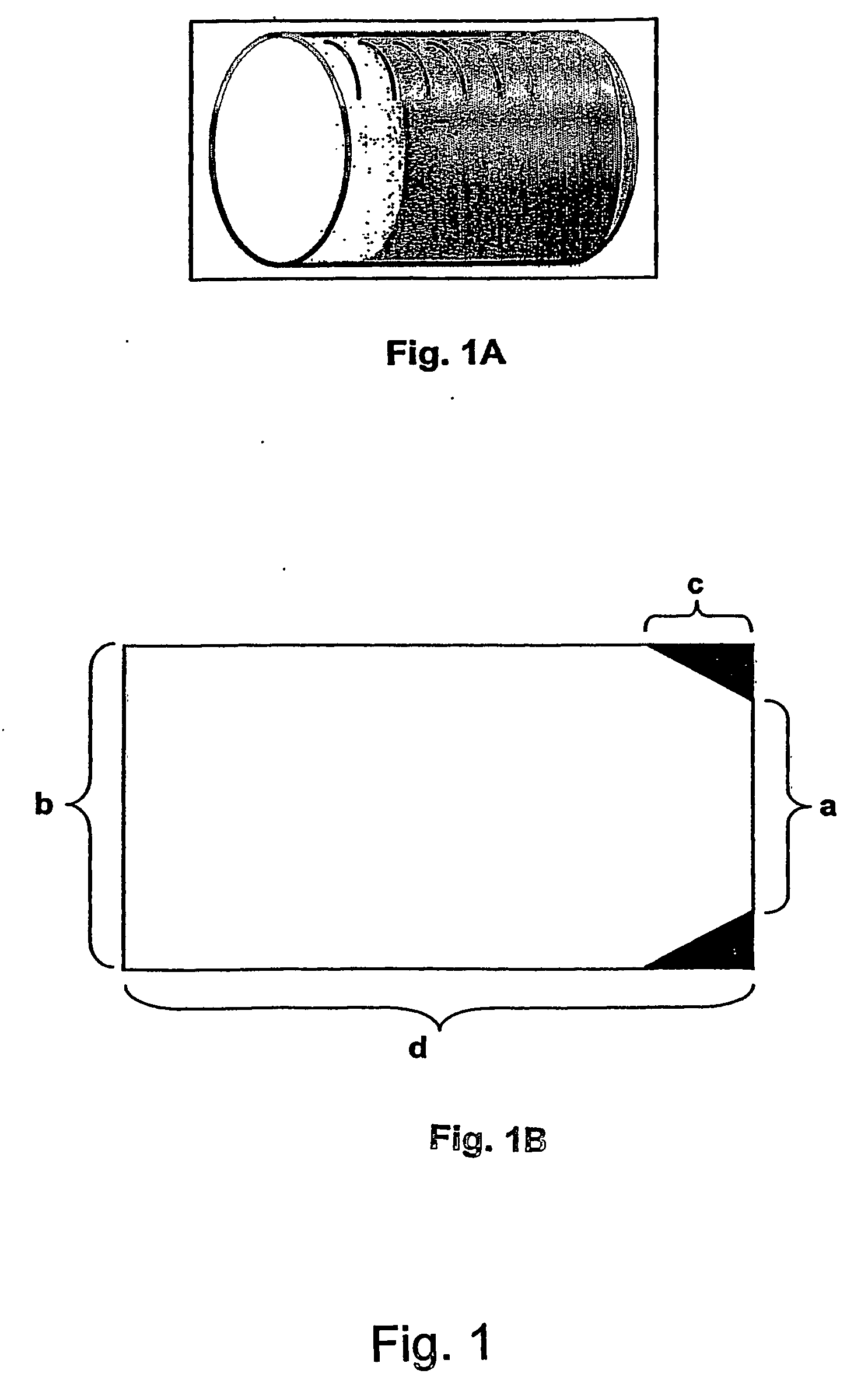
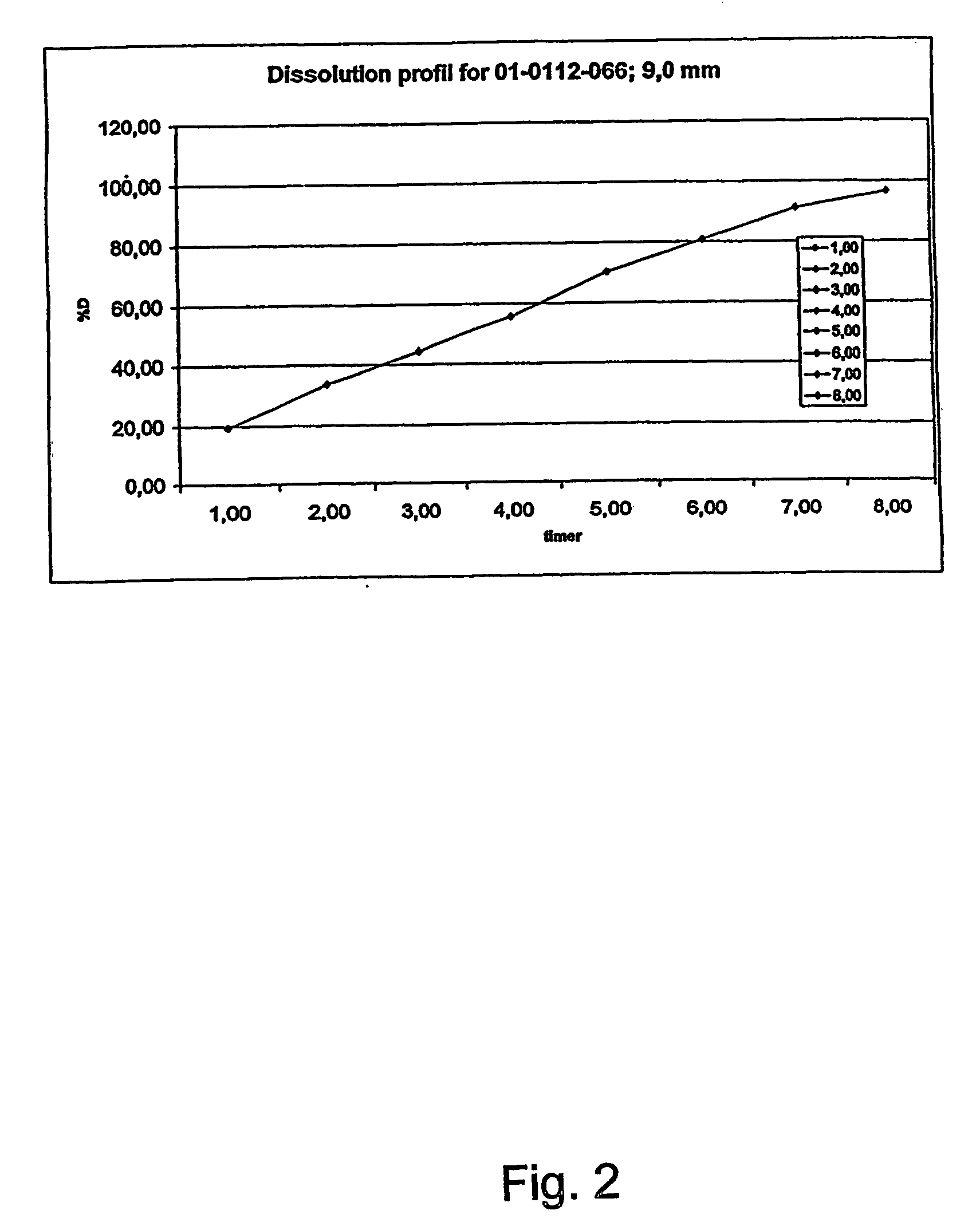
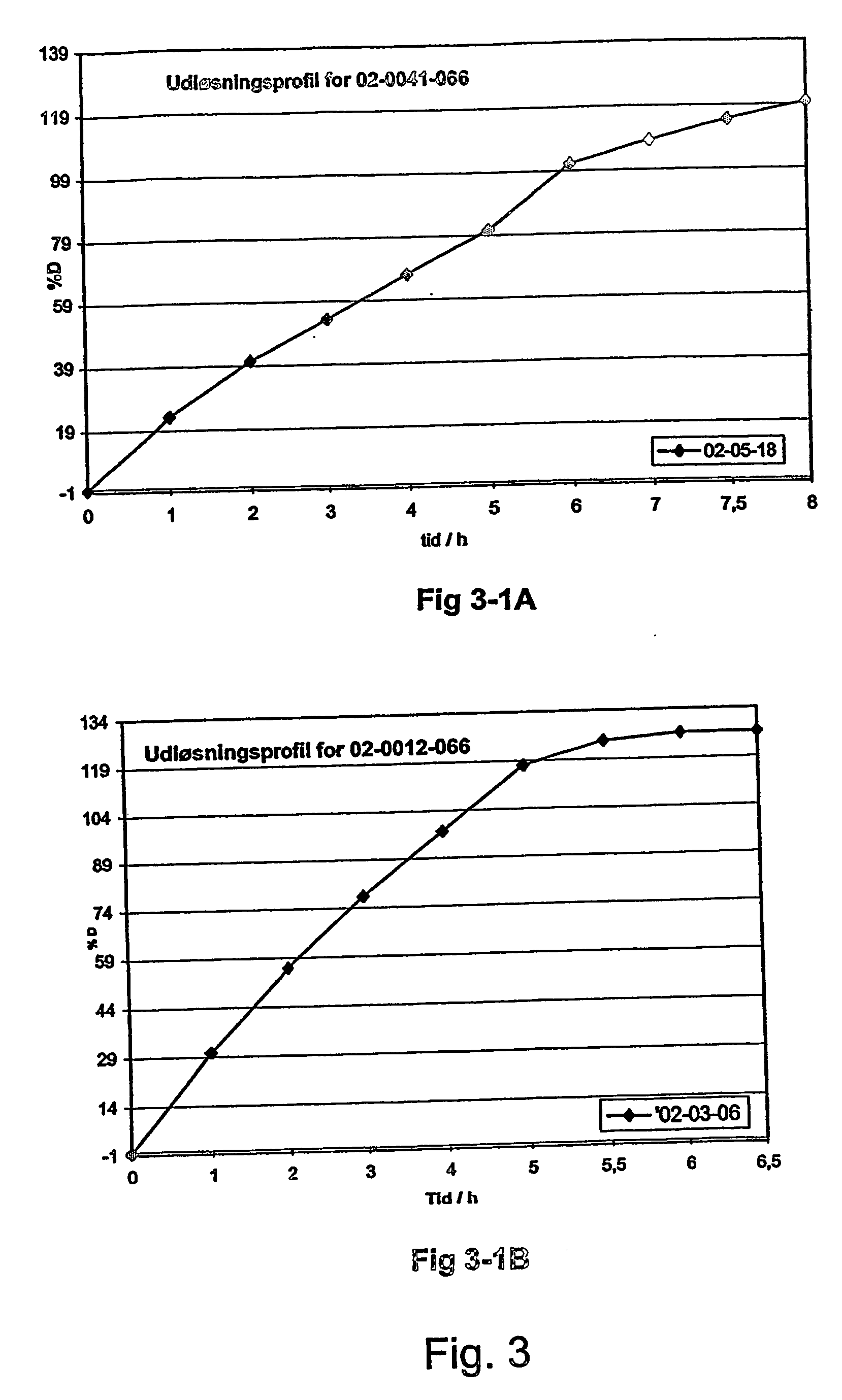
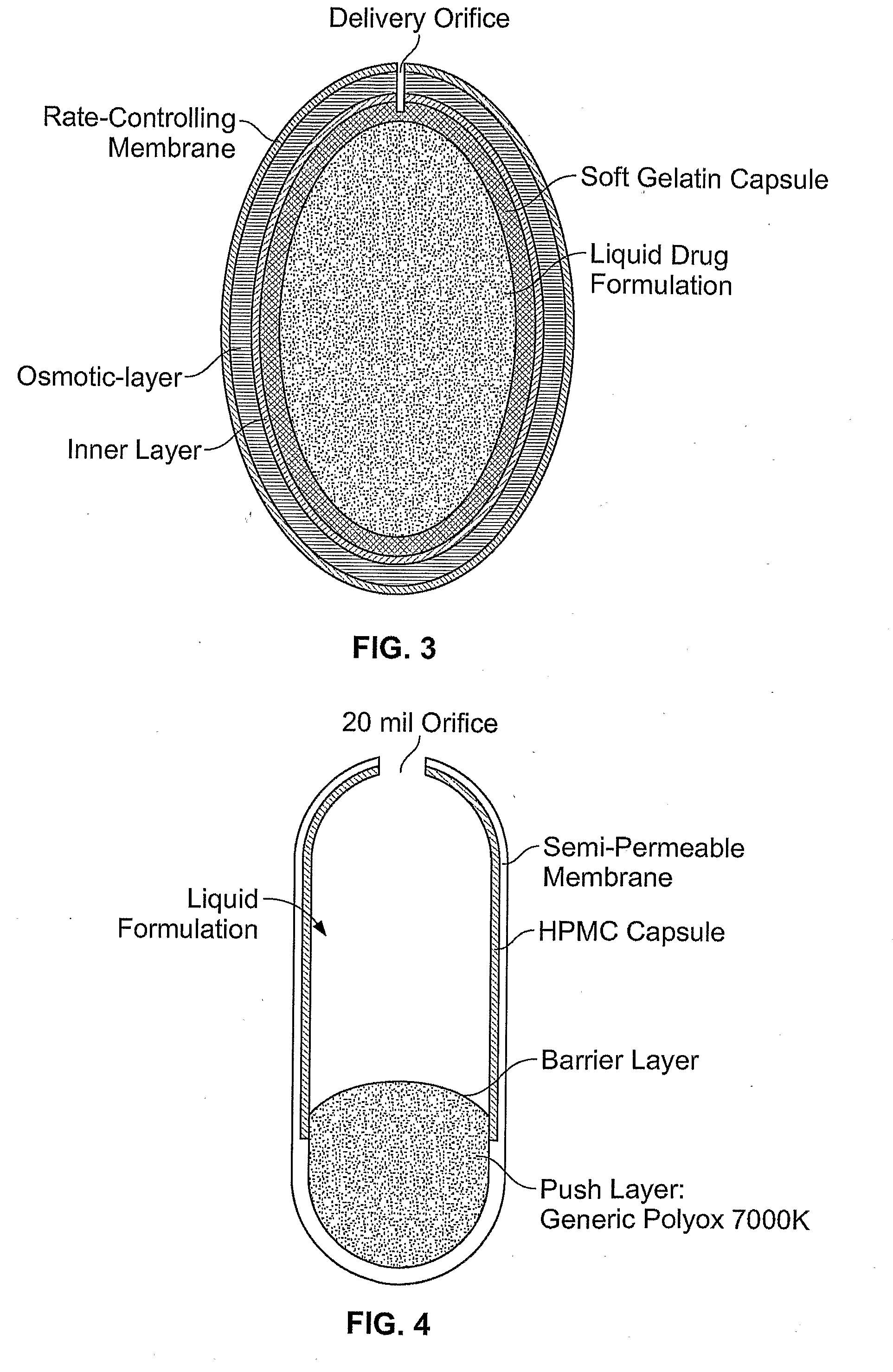
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
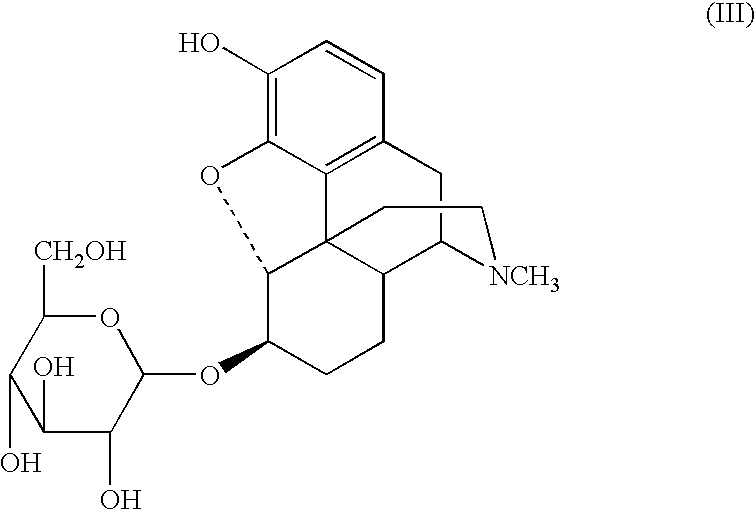
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
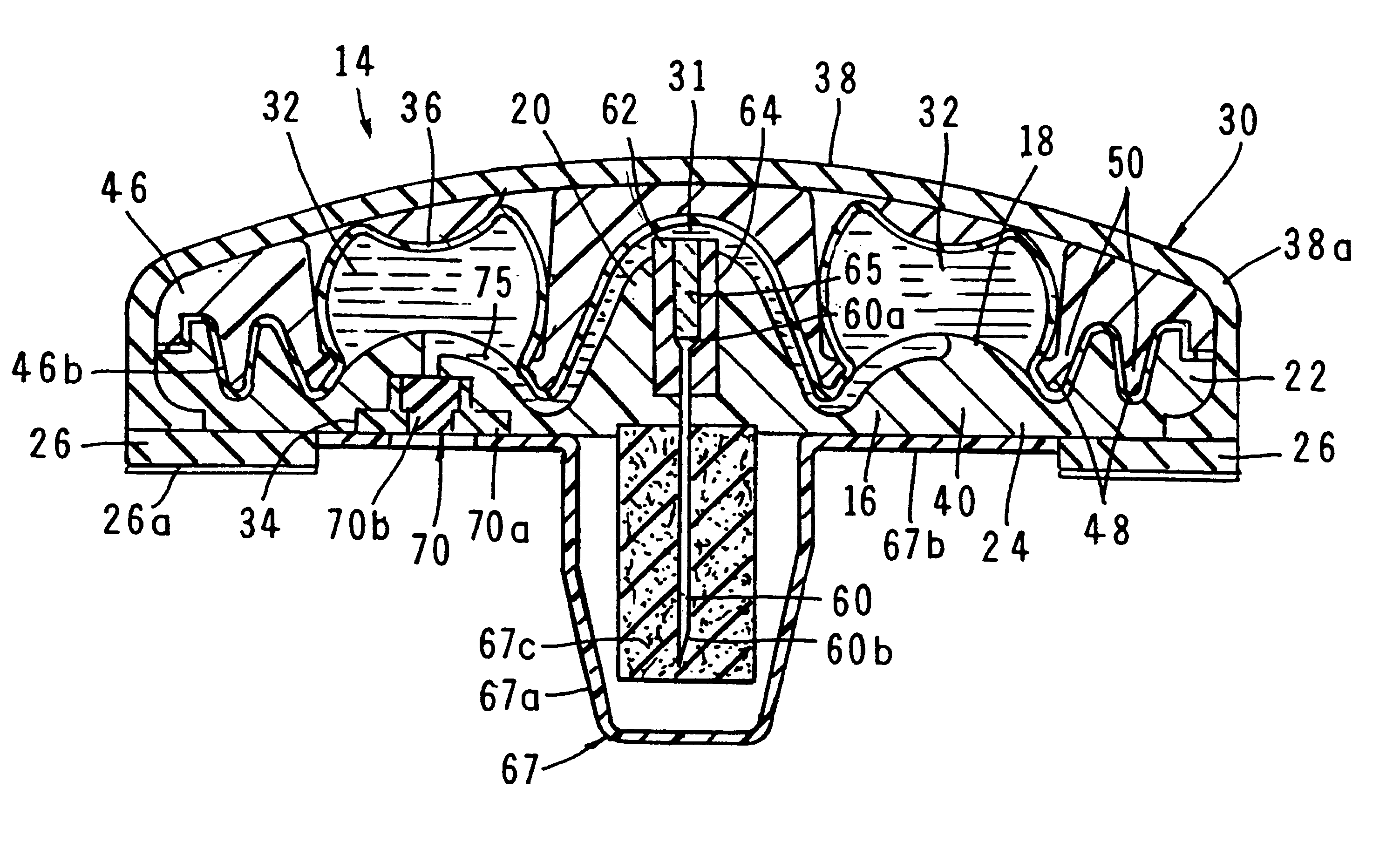
Multiple canopy
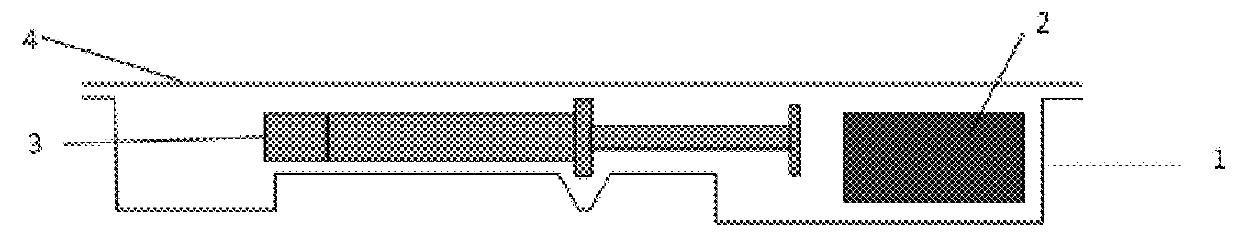
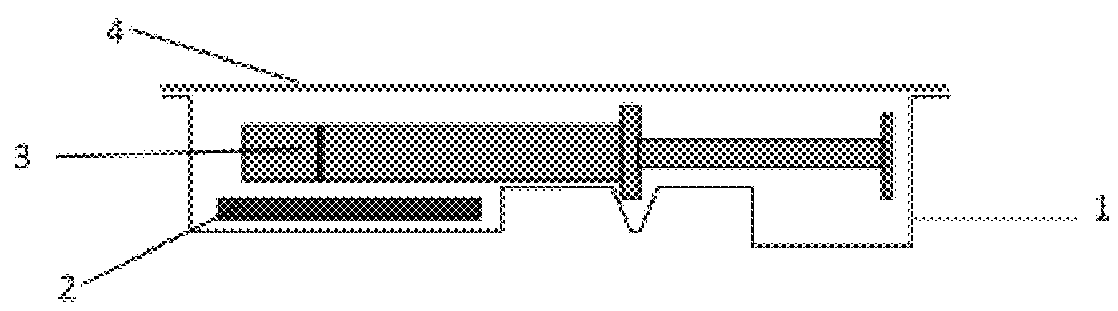
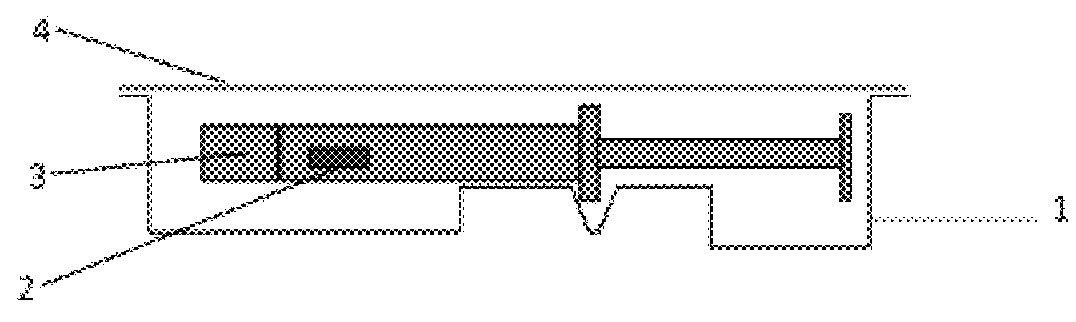
InactiveUS6537249B2Improve reliabilityReduce manufacturing costSurgeryMedical devicesStored energyMorphine
A fluid delivery device having a self-contained stored energy membrane for expelling fluids at a precisely controlled rate, which is of a compact, laminate construction. The device is of very low profile so that it can conveniently be used for the precise delivery of a small volume of pharmaceutical fluids, such as insulin, morphine and the like, into an ambulatory patient at precisely controlled rates over extended periods of time. The device includes strategically configured, multiple fluid chambers to achieve the maximum possible average percent of extension of the membrane and thereby assure adequate fluid delivery pressure.
Owner:PESCADERO BEACH HLDG
Sugar derivatives of hydromorphone, dihydromorphine and dihydroisomorphine,compositions thereof and uses for treating or preventing pain
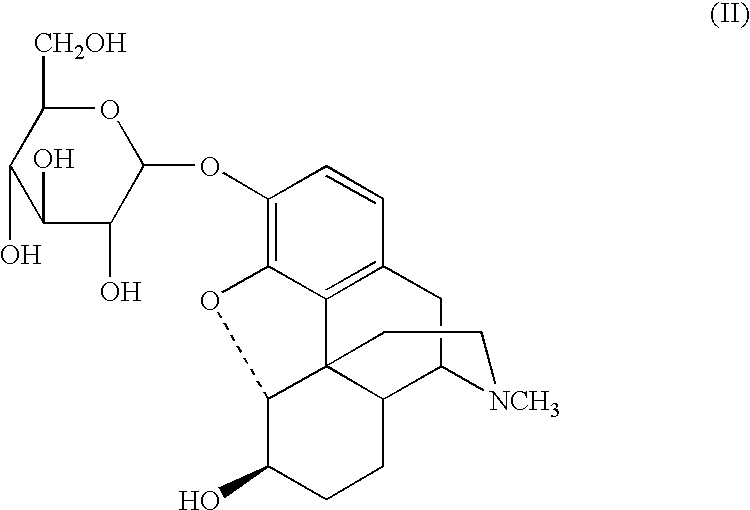
Glucoside and glucuronide derivatives of hydromorphone, dihydromorphine, and dihydroisomorphine and pharmaceutically acceptable salts thereof; pharmaceutical compositions comprising a glucoside or glucuronide derivative of hydromorphone, dihydromorphine, or dihydroisomorphine or a pharmaceutically acceptable salt thereof; and methods for treating or preventing pain in a patient comprising administering to a patient in need thereof a glucoside or glucuronide derivative of hydromorphone, dihydromorphine, or dihydroisomorphine or a pharmaceutically acceptable salt thereof are disclosed.
Owner:PURDUE PHARMA LP
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Morphine polymer release system
InactiveUS20080254123A1Affecting extent of drug bioavailabilityReduce frequencyBiocidePowder deliveryControlled releaseMedicine
A pharmaceutical composition for controlled release of an active substance is provided. The active substance is released into an aqueous medium by erosion of at least one surface of the composition. The composition comprises i) a matrix comprising a) polymer or a mixture of polymers, b) an active substance and, optionally, c) one or more pharmaceutically acceptable excipients, and ii) a coating.
Owner:EGALET LTD
Selective opioid compounds
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
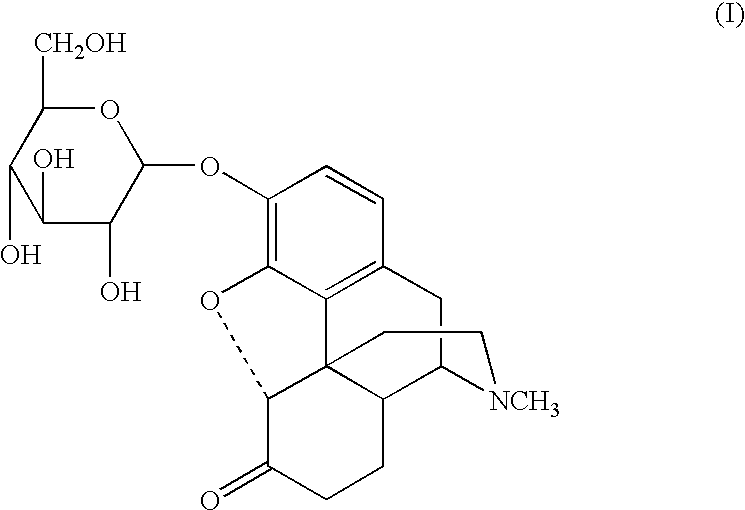
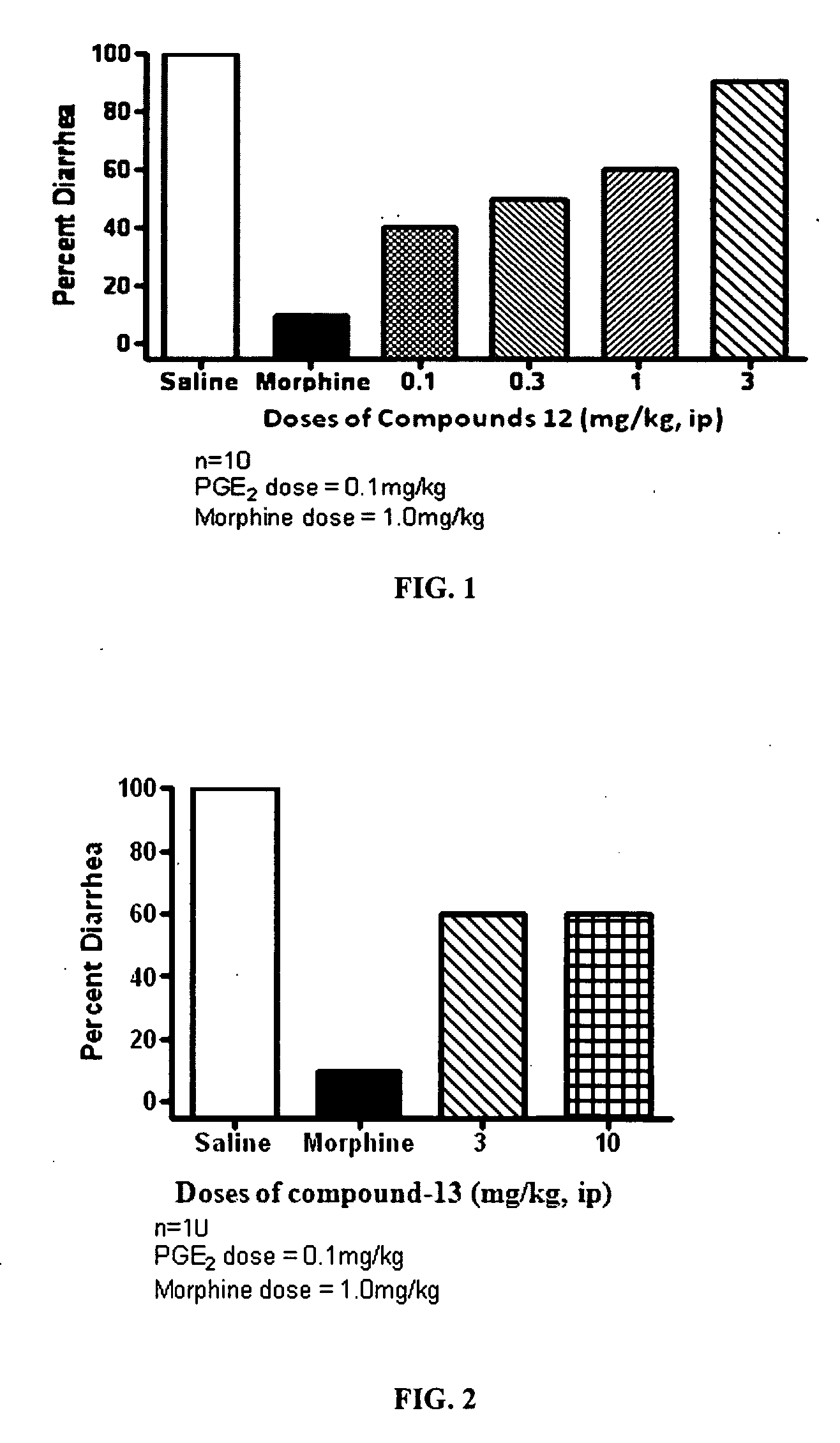
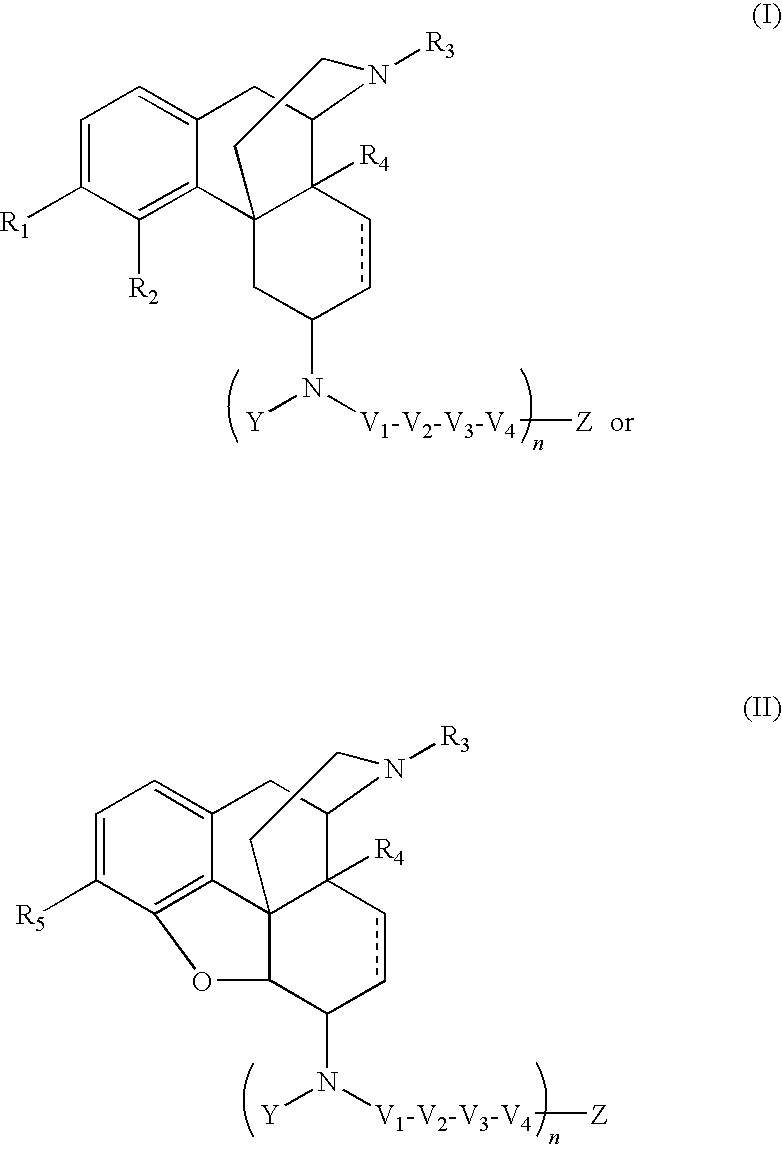
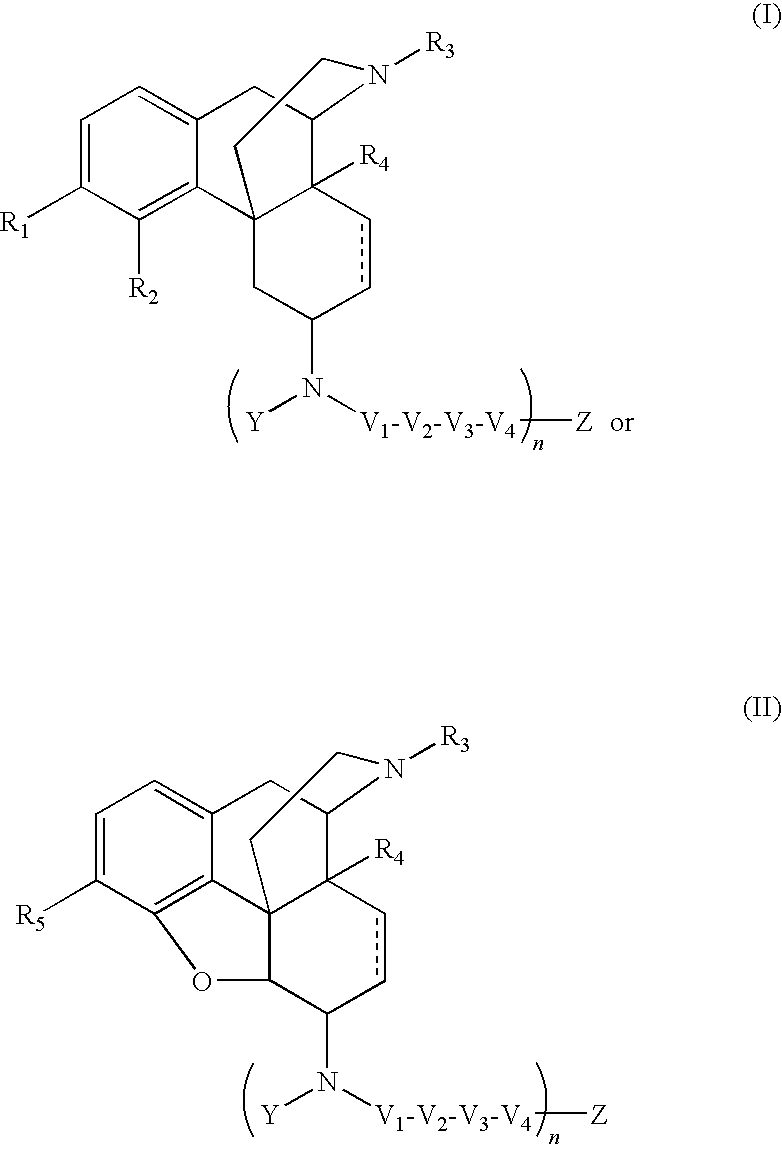
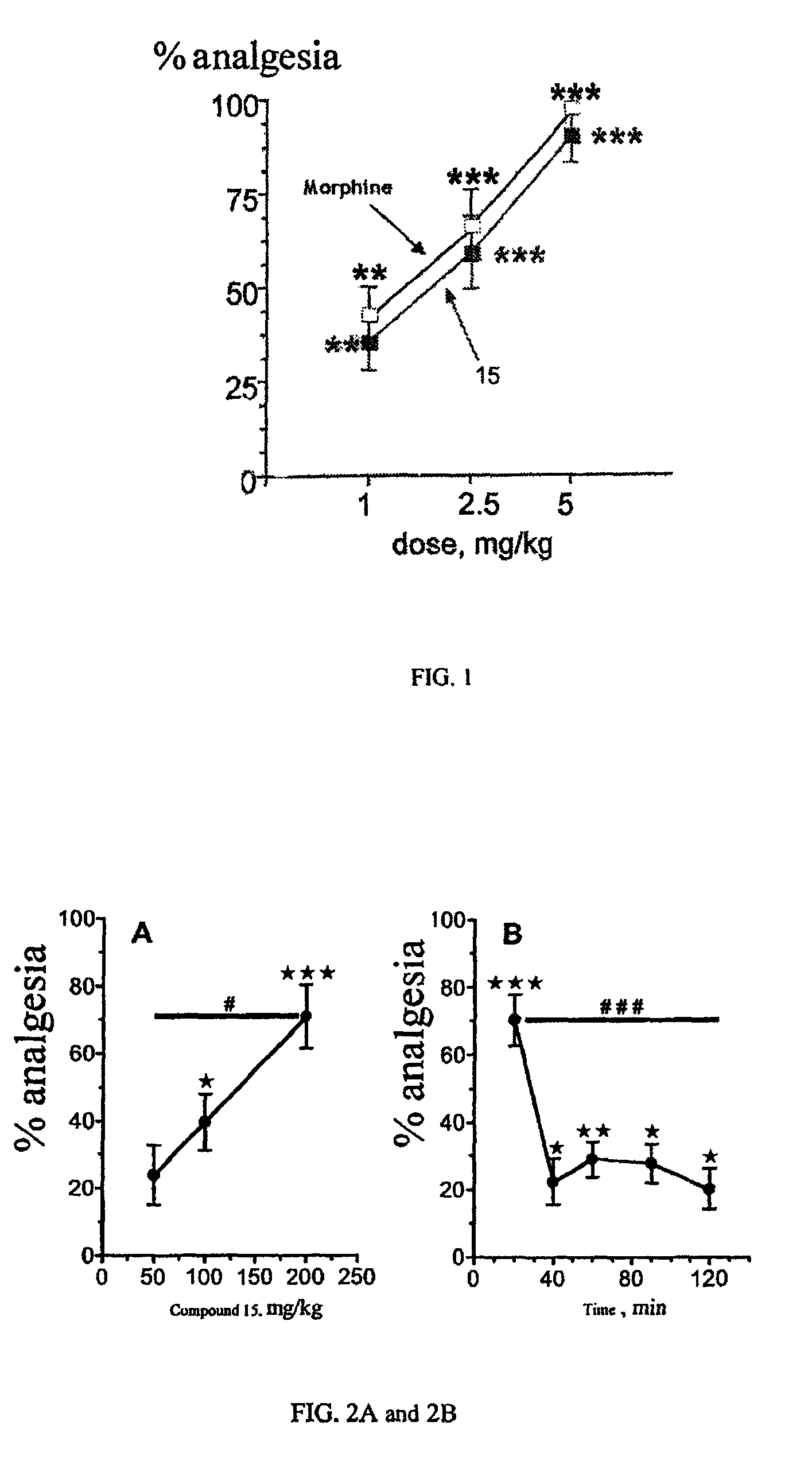
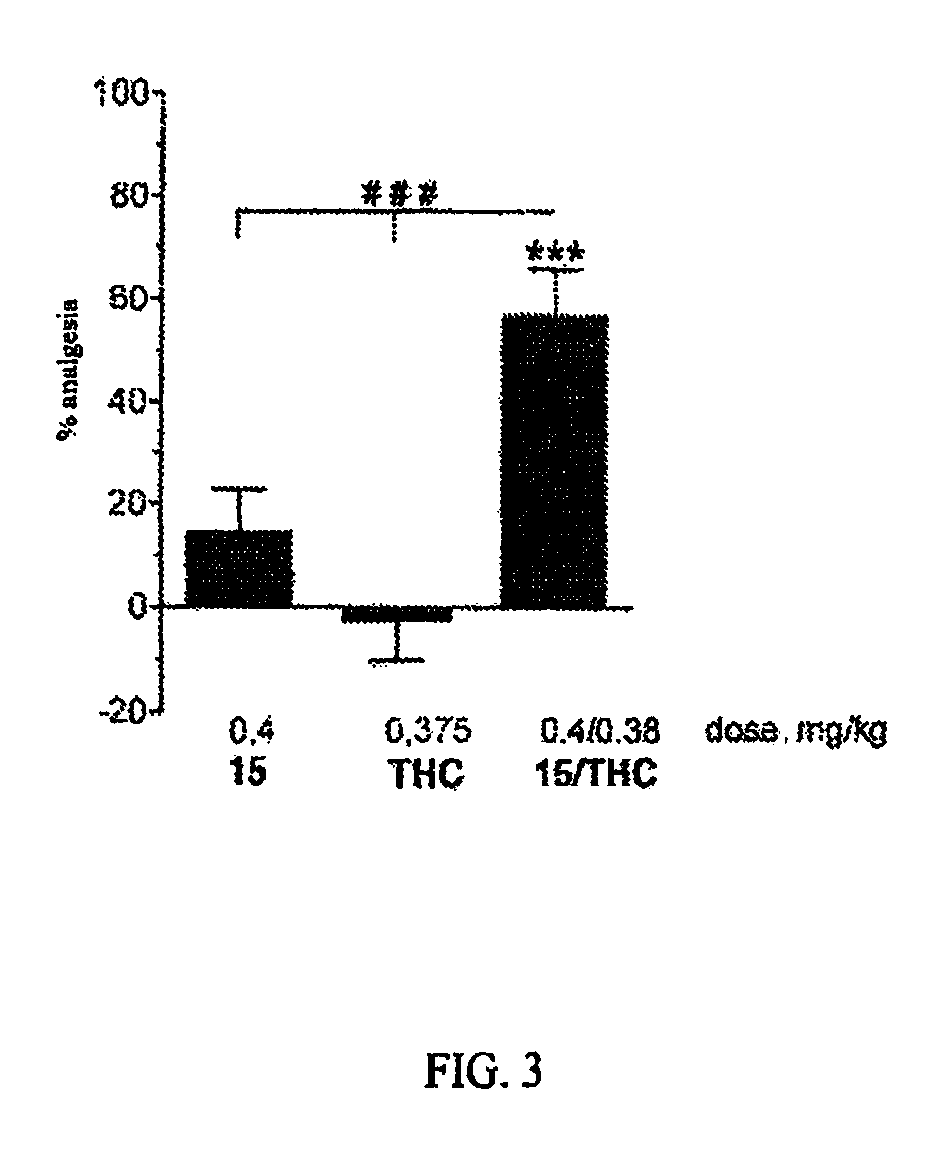
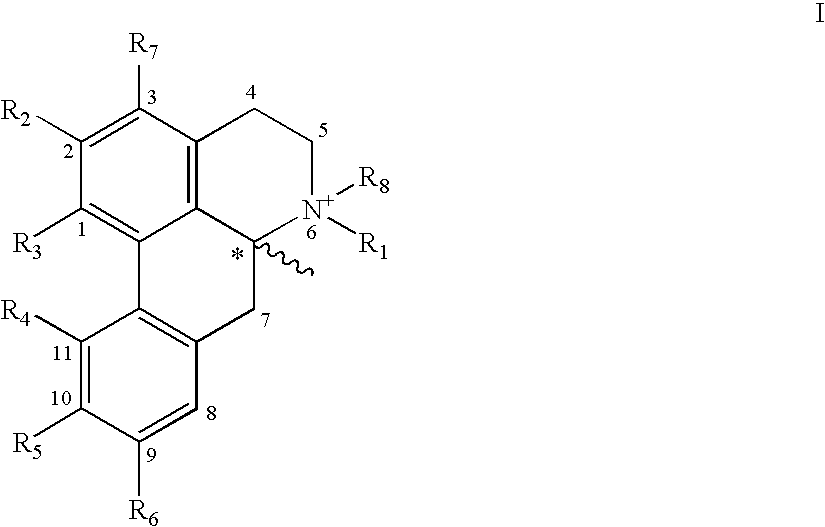
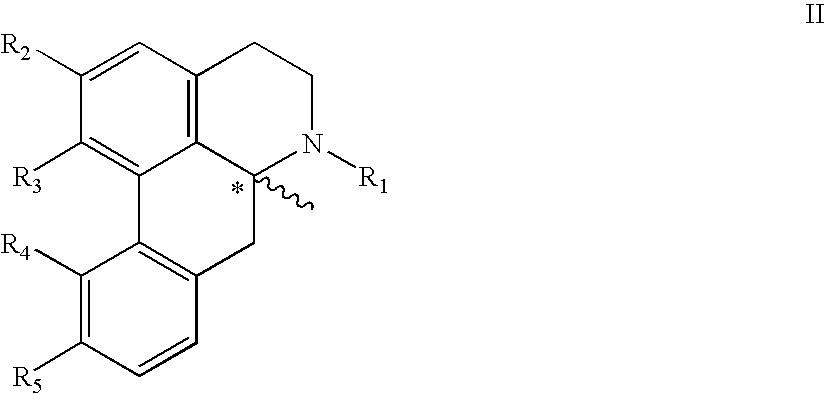
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Oxymorphone controlled release formulations
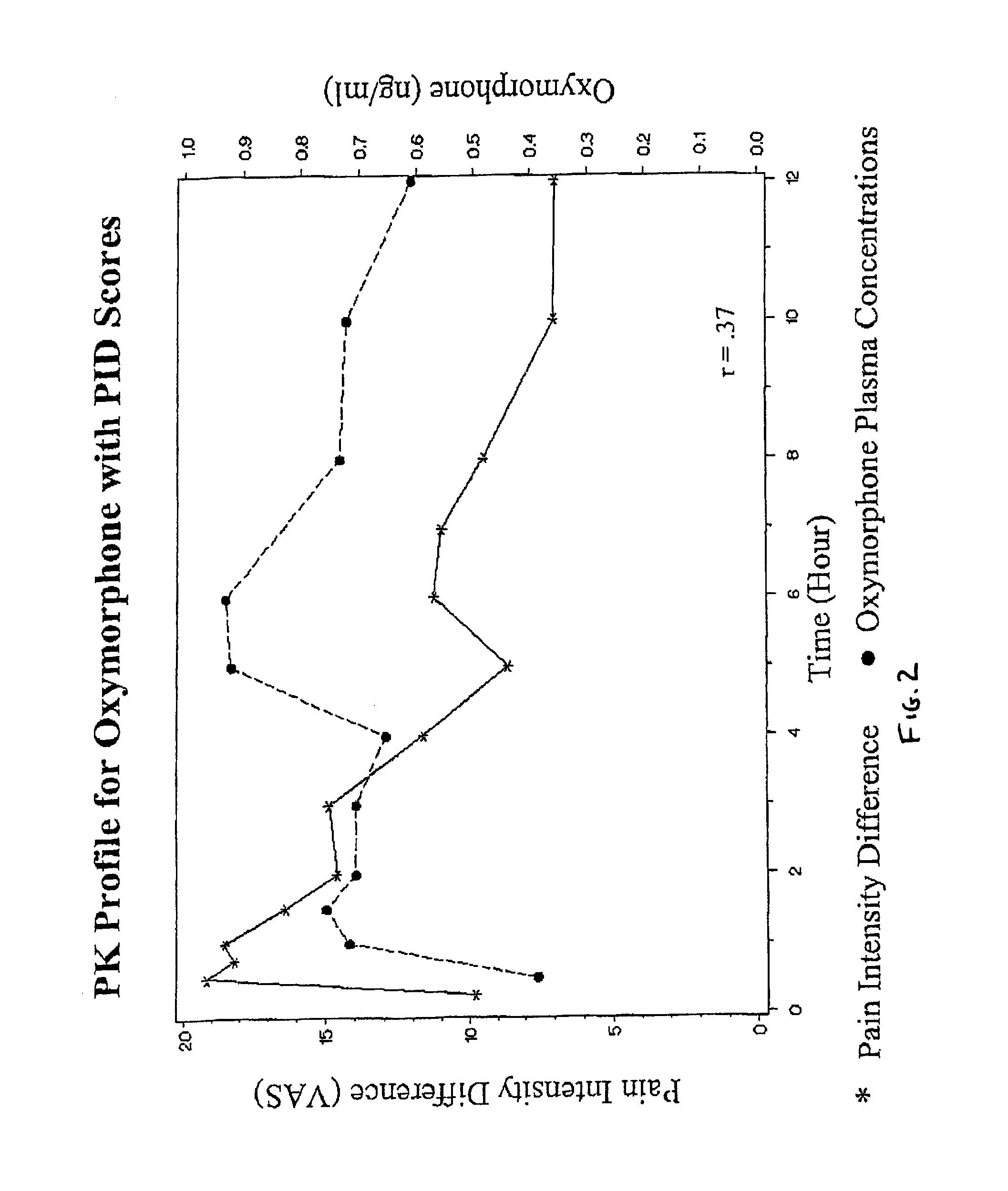
The invention pertains to a method of relieving pain by administering a controlled release pharmaceutical tablet containing oxymorphone which produces a mean minimum blood plasma level 12 to 24 hours after dosing, as well as the tablet producing the sustained pain relief.
Owner:ENDO PHARMA INC
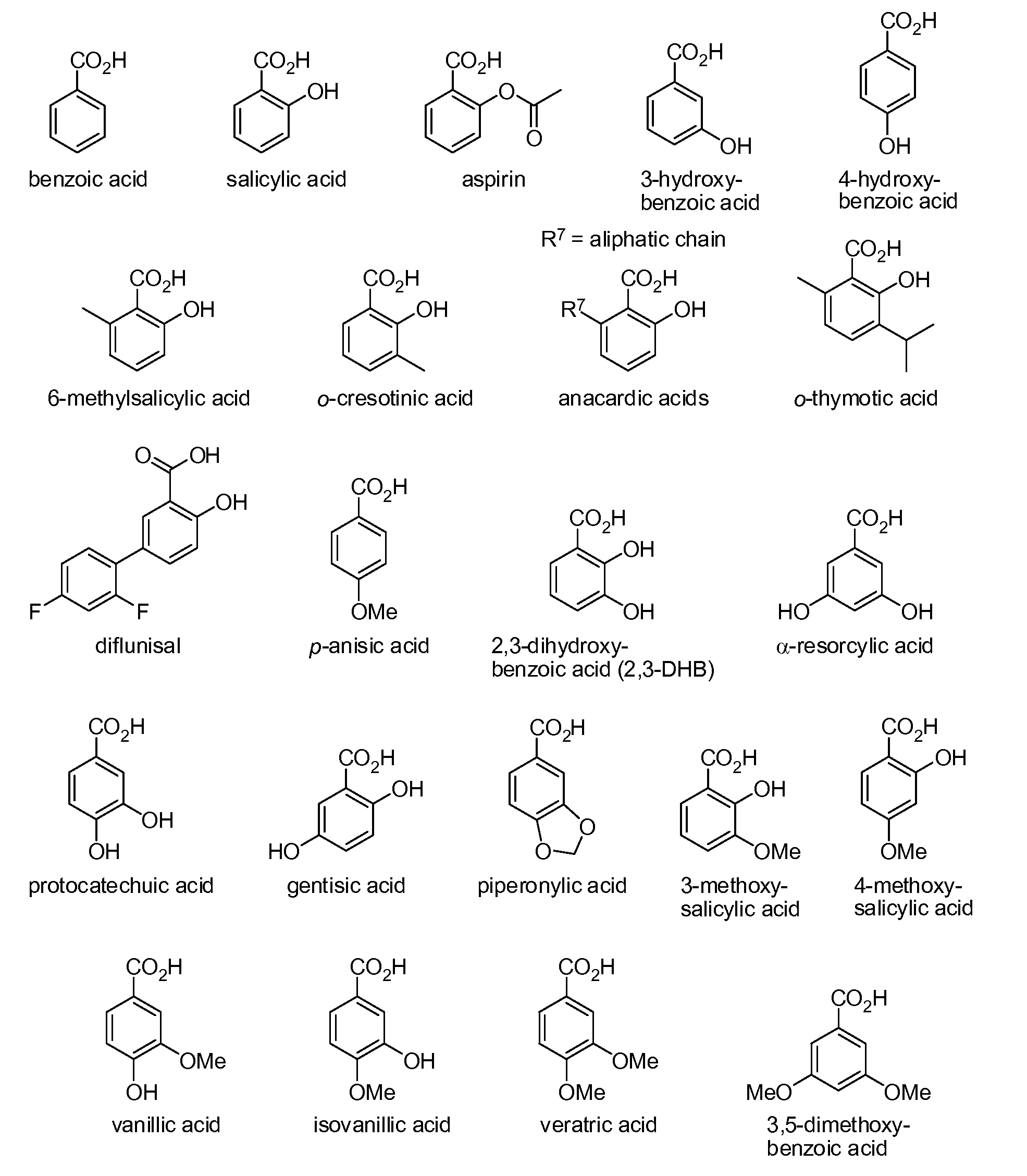
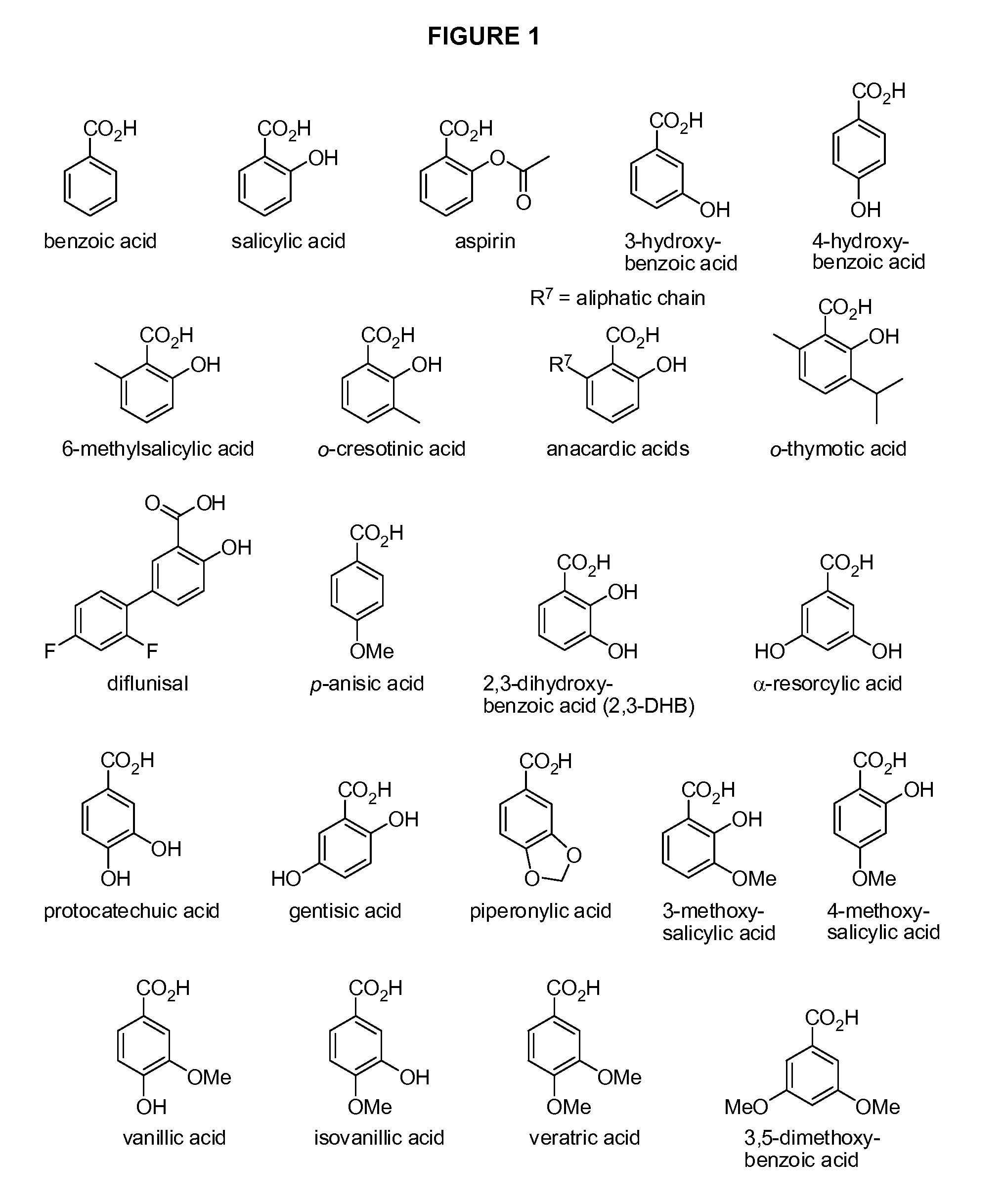
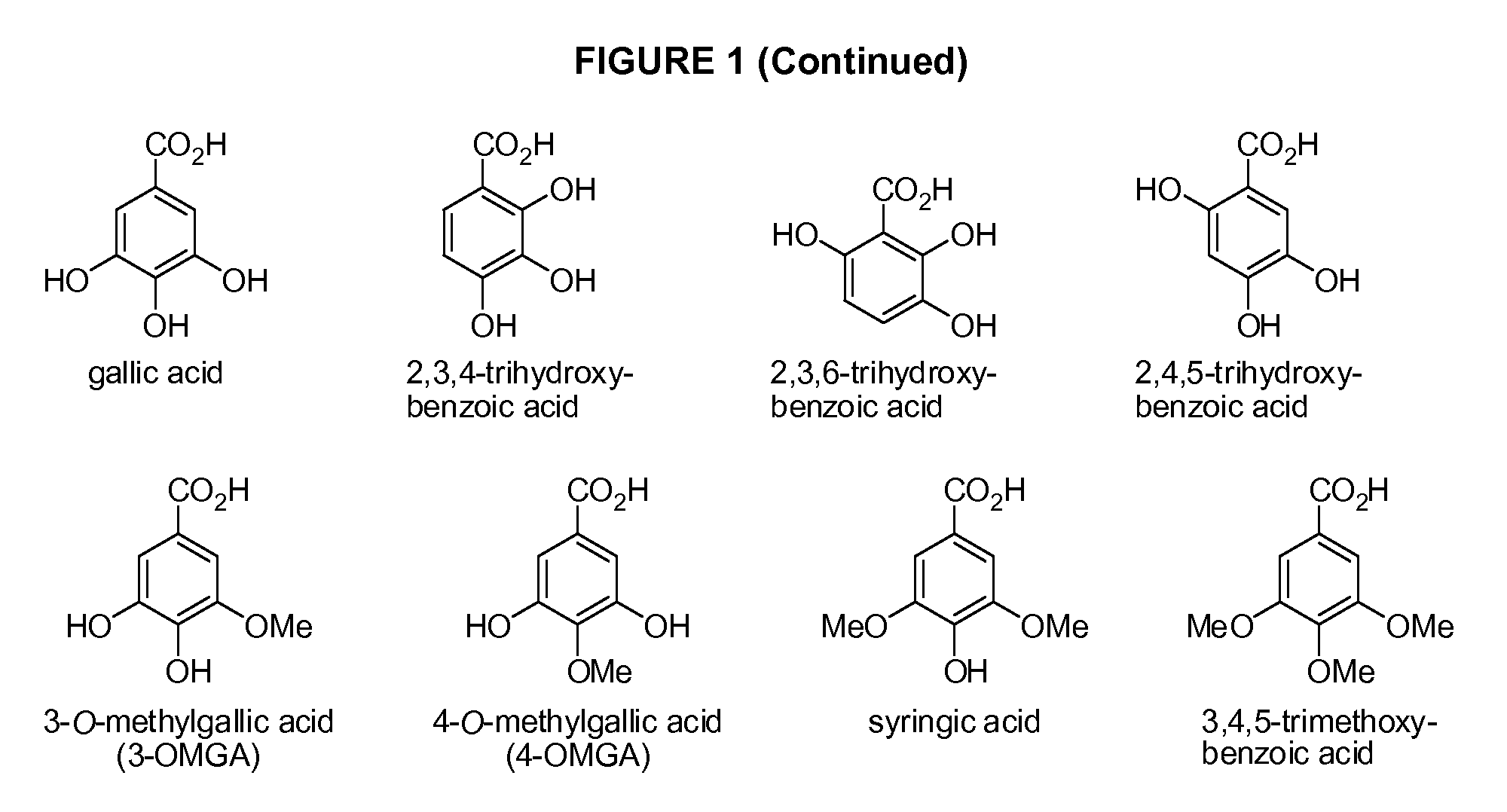
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydrocodone, prodrugs, methods of making and use thereof
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydrocodone (morphinan-6-one, 4,5-alpha-epoxy-3-methoxy-17-methyl) to form novel prodrugs / compositions of hydrocodone, including benzoates and heteroaryl carboxylic acids, which have a decreased potential for abuse of hydrocodone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
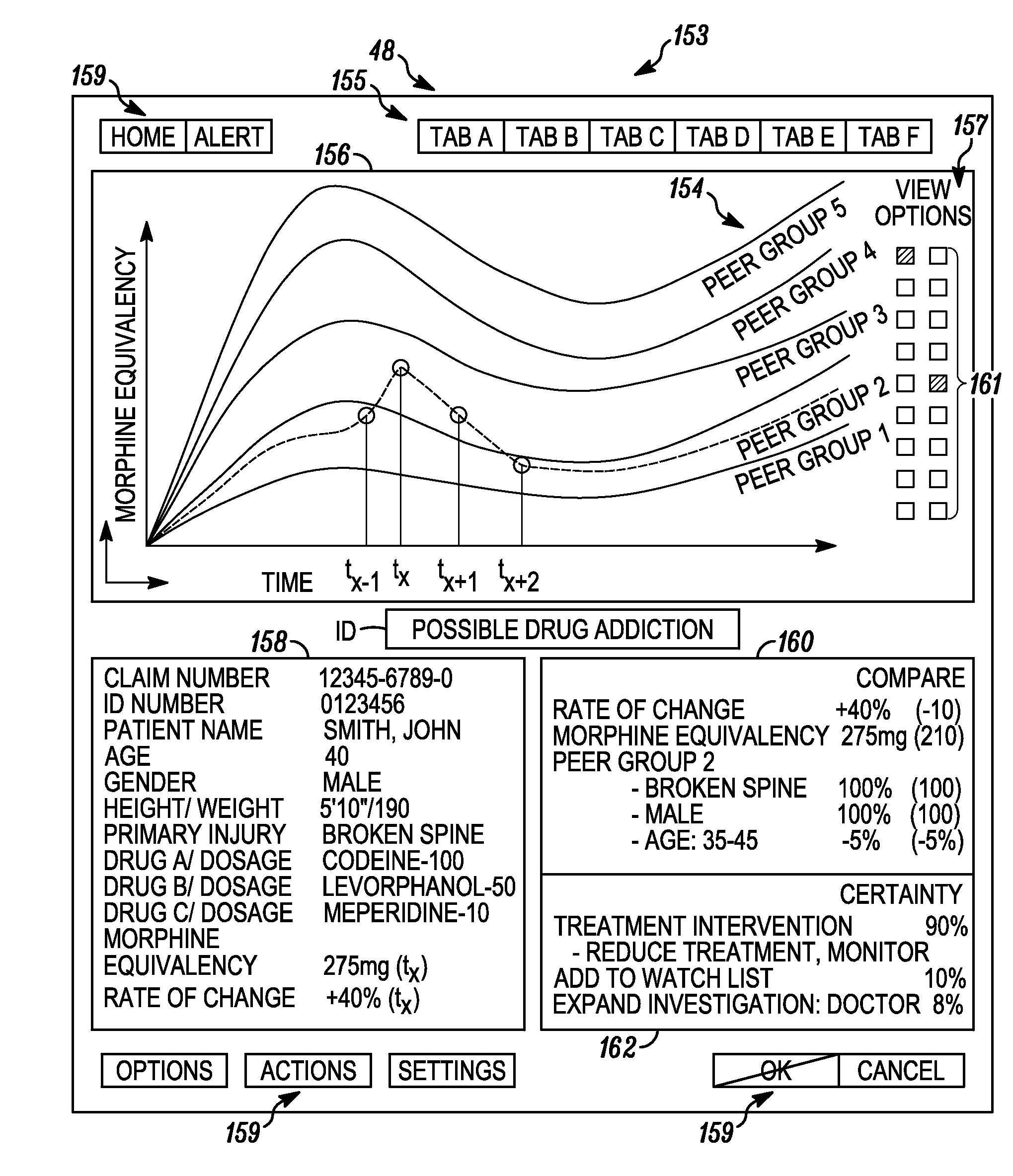
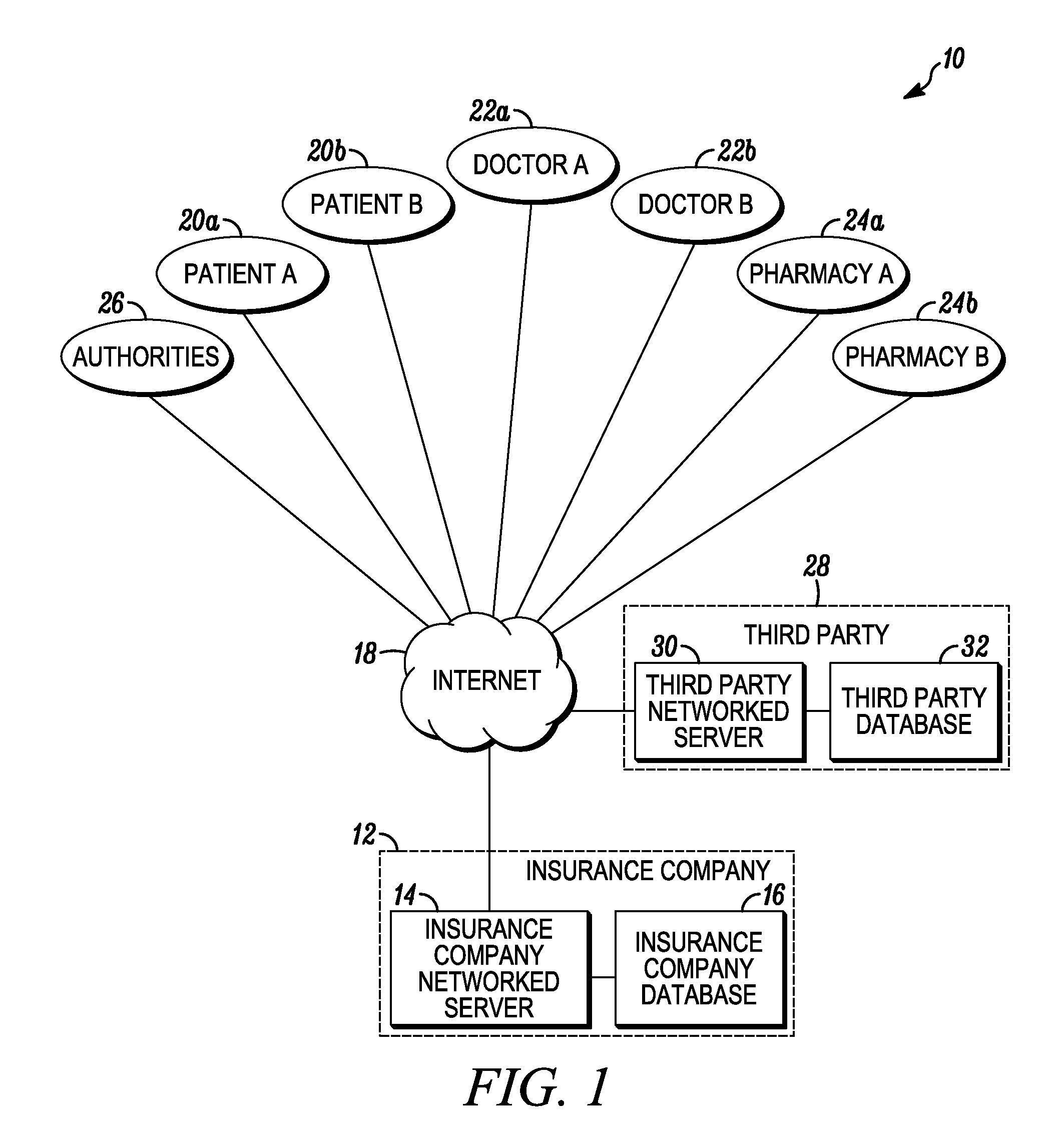
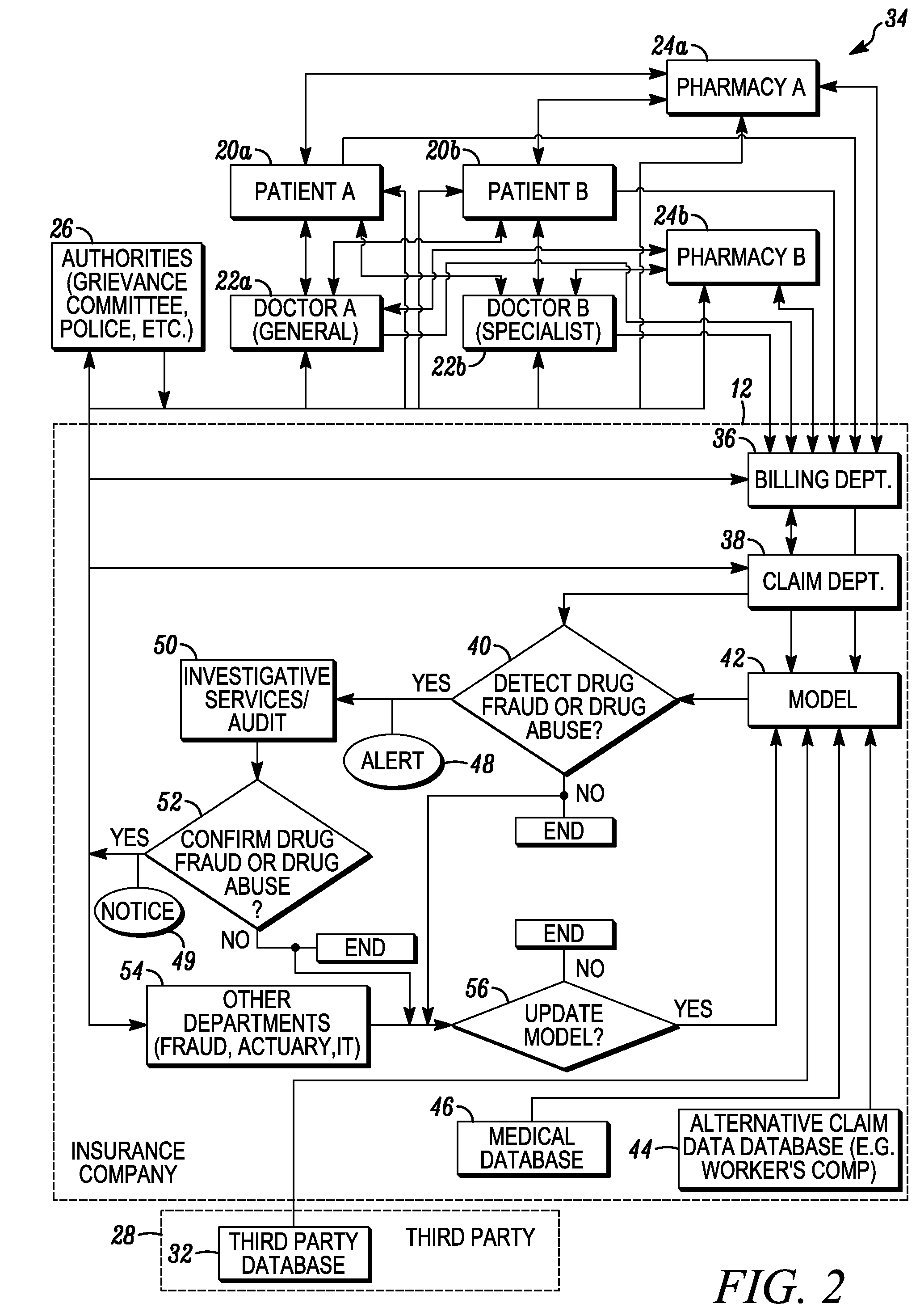
System and method for detecting drug fraud and abuse
A system and method for detecting improper drug use utilizes the morphine equivalency of a drug regimen of a patient having an injury to compare the morphine equivalency of the drug regimen of the patient to that of a peer group having the same diagnosis. The comparison may be conducted based on the aggregate morphine equivalency for respective periods of time following the injury or the initial treatment and based on the rate of change in the morphine equivalency for that or another period of time. Based on the results of the comparison, potential instances of drug fraud and / or drug abuse are identified and an alert is generated, and appropriate action is taken, where needed.
Owner:THE TRAVELERS INDEMNITY +1
Morphine polymer release system
InactiveUS8609143B2Affecting extent of drug bioavailabilityReduce frequencyBiocidePowder deliveryControlled releaseMorphine
A pharmaceutical composition for controlled release of an active substance is provided. The active substance is released into an aqueous medium by erosion of at least one surface of the composition. The composition comprises i) a matrix comprising a) polymer or a mixture of polymers, b) an active substance and, optionally, c) one or more pharmaceutically acceptable excipients, and ii) a coating.
Owner:EGALET LTD
6-Monoacetylmorphine derivatives useful in immunoassay
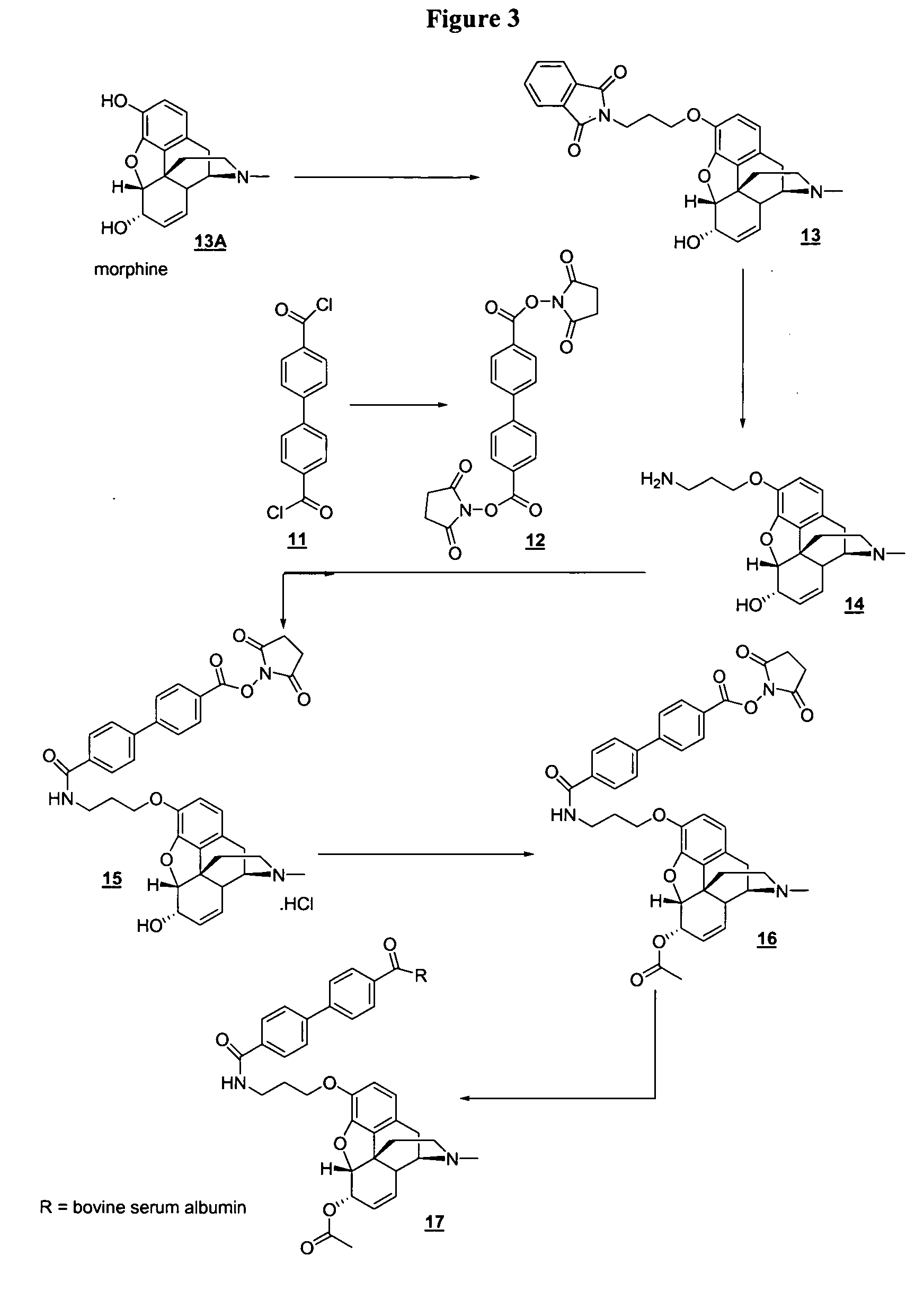
Analogs of 6-monoacetyl morphine (6-MAM) are described. These include analogs derivatized at either the C-3 position, the C-6 position, or the nor position of the molecule. These analogs allow for elaboration with linkers terminated by a functional group such as an activated ester, the functional groups being useful for attaching the molecule to other entities such as proteins, polysaccharides, and reporter groups.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Packaging system for oxygen-sensitive drugs
ActiveUS9248229B2Lower Level RequirementsOrganic active ingredientsFlexible coversPromethazineMorphine
Described herein are pharmaceutical packaging systems which prevent oxidative degradation of morphine, hydromorphone, promethazine and other oxygen-sensitive drugs, such systems including a syringe with an oxygen permeable tip cap, a hermetically sealed oxygen barrier blister packaging with very low permeability to oxygen and comprises ethylene vinyl, and an oxygen absorber.
Owner:FRESENIUS KABI DEUT GMBH
Morphinan derivatives for the treatment of drug overdose
ActiveUS20110136848A1Useful in treatmentEffective treatmentBiocideNervous disorderToxicityTherapy medication
Owner:ALKERMES PHARMA IRELAND LTD
Compositions for treating parkinson's disease
The present invention relates to improved treatment of diseases and disorders of the central nervous system by administration of apomorphine. In particular, the administration is via pulmonary inhalation. The invention provides the means for improving the treatment of a number of conditions, including Parkinson's Disease.
Owner:VECTURA LTD
Pharmaceutical product comprising the active substance diamorphine, and its use in a process for treating opiate addiction
The invention relates to pharmaceutical preparations that are used in a method for treating opiate addiction or opiate dependence, especially heroin dependence. The active substance used is preferably diamorphine and / or one of the pharmaceutically acceptable acid addition salts thereof. The invention also relates to a method for treating opiate dependence.
Owner:MATUSCH RUDOLF +4
Pharmaceutical formulation of apomorphine for buccal administration
InactiveUS20090023766A1Improve sexual functionIncrease libidoBiocideNervous disorderSexual functionDisease
The present invention provides a kit comprising, in separate compartments of a container, the following components (a) and (b): (a) a combination of apomorphine or a pharmaceutically acceptable acid addition salt thereof and a pharmaceutically acceptable excipient or carrier; and (b) a solution which comprises a diluent and a pH modifying agent; the components being presented such that they can be combined at the point of use into a formulation which is adjusted to a pH ranging from mildly acidic to alkaline and which is suitable for buccal administration. The formulation is useful in treating Parkinson's disease and in promoting sexual function.
Owner:AMARIN PHARMA IRELAND
Sublingual apomorphine
ActiveUS20110111011A1Alleviating dyskinesiaEffectively alleviatedPowder deliveryBiocideSexual functioningMorphine
The invention features sublingual formulations of apomorphine and apomorphine prodrugs, and methods of treating Parkinson's disease, sexual dysfunction, and depressive disorders therewith.
Owner:SUNOVION PHARMA INC
Methods of Reducing Alcohol-Induced Dose Dumping for Opioid Sustained Release Oral Dosage Forms
Disclosed are methods of sustained release administration of opioids, including but not limited to hydromorphone and oxycodone, that exhibit improved properties with respect to co-ingestion with aqueous alcohol.
Owner:ALZA CORP
Compositions and uses
According to the invention there is provided a method of treating and / or preventing the symptoms of Parkinson's disease comprising delivering apomorphine, optionally in combination with levodopa and / or a dopamine agonist that is not apomorphine, wherein apomorphine is administered by inhalation.
Owner:VECTURA LTD
Methods for One-Pot N-Demethylation/N-Functionalization of Morphine and Tropane Alkaloids
InactiveUS20110313163A1Safe and cost-effectiveReduce the temperatureOrganic chemistryMorphineTropane alkaloid
The present invention provides a method for the N-demethylation and N-functionalization of an N-methylated heterocycle such as a morphine alkaloid or tropane alkaloid. The method comprises reacting the heterocycle with an functionalization agent in the presence of a transition metal catalyst in air or in the presence of an oxidant.
Owner:BROCK UNIVERSITY
Conjoint administration of morphogens and ACE inhibitors in treatment of chronic renal failure
InactiveUS20050272649A1Preventing delaying needReducing necessary frequencyBiocidePeptide/protein ingredientsRenal disorderMorphine
The present invention provides reagents and methods for the treatment, and pharmaceuticals for use in the prevention and / or treatment, of chronic renal failure and other renal disorders in subjects (particularly mammalian subjects) renal replacement therapy. The methods involve the conjoint administration of ACE (Angiotensin-Converting Enzyme) inhibitors or Angiotensin II Receptor Antagonists (AIIRAs) with one or more OP / BMP family of proteins (morphogens, or inducers of morphogens, or agonists of the corresponding morphogen receptors, etc.). The invention also provides methods for implantation of renal cells induced with the conjoint administration of ACE inhibitors or AIIRAs with those morphogens.
Owner:BARNES JEWISH HOSPITAL +1
(+)-opioids and methods of use
The invention provides 4,5-epoxymorphinan or a derivative thereof, a morphinan or a derivative thereof, or a pharmaceutical salt or a prodrug thereof. The present invention also provides compositions comprising the same, and methods for using the same. In particular, the invention relates to TLR antagonistic opioids and methods for using the same.
Owner:UNIV OF COLORADO THE REGENTS OF
Remedies or preventives for urinary frequency or urinary incontinence and morphinan derivatives having nitrogen-containing heterocyclic group
InactiveUS20060040970A1Efficient processImprovement of side effectsBiocideOrganic chemistryAdditive ingredientMorphine
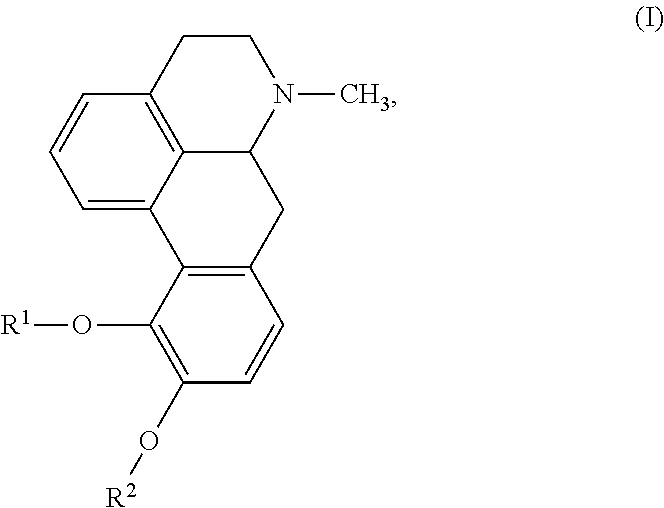
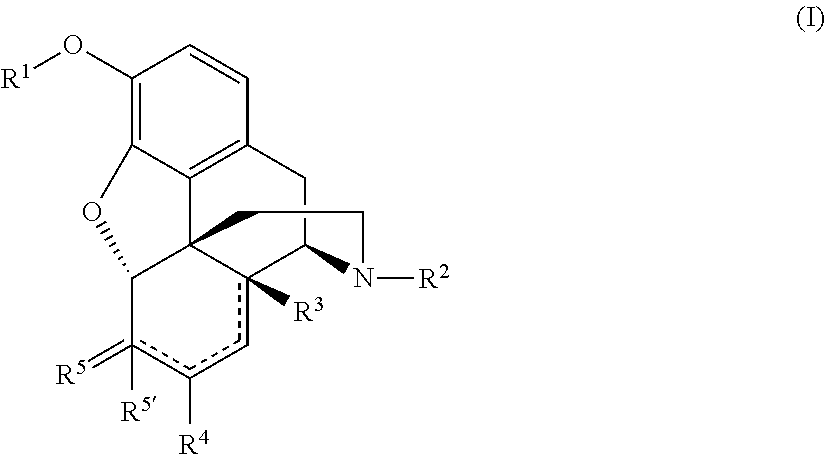
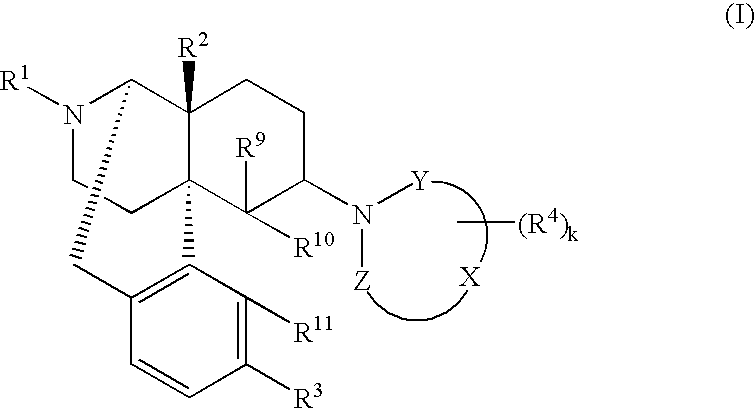
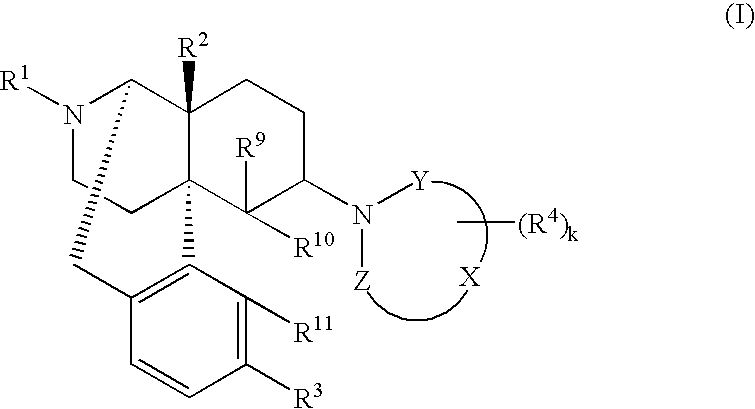
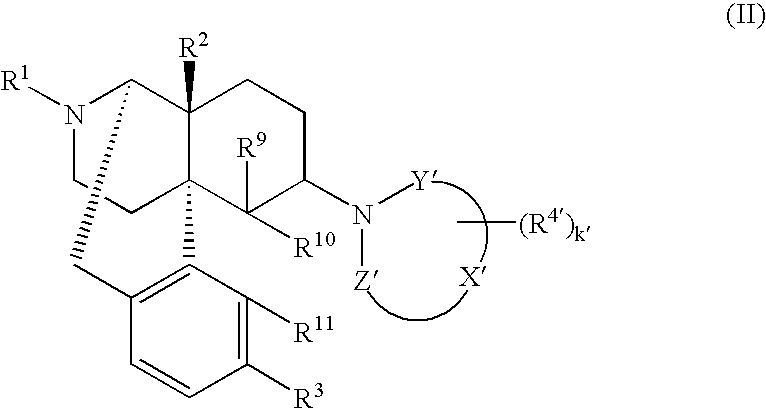
The invention provides a morphinan derivative of the Formula (I): (wherein R1 is methyl, cyclopropylmethyl or the like; R2 and R3 are hydroxy, methoxy, acetoxy or the like; both Y and Z are valence bonds, —C(═O)— or the like; X is C2-C5 carbon chain (one of the carbon atoms may be substituted by oxygen, sulfur or nitrogen) constituting a part of the ring structure, or the like; (R4)k is substituted or non-substituted benzene fused ring, carbonyl group or the like; R9 is hydrogen or the like; R10 and R11 are bound to represent —O—, or the like, and R6 is hydrogen or the like) or a pharmaceutically acceptable acid addition salt thereof. The invention also provides a therapeutic or prophylactic agent for urinary frequency or urinary incontinence, comprising as an effective ingredient the morphinan derivative or the pharmaceutically acceptable acid addition salt thereof; a method for therapy or prophylaxis of the diseases.
Owner:TORAY IND INC
Aminoacid derivatives containing a disulfanyl group in the form of mixed disulfanyl and aminopeptidase N inhibitors
The invention relates to novel compounds of formula (I): H2N—CH(R1)—CH2—S—S—CH2—CH(R2)—CONH—R5, wherein R1 is a hydrocarbon chain, phenyl or benzyl radical, methylene radical substituted by a 5 or 6 atom heterocycle; R2 is a phenyl or benzyl radical, a 5 or 6 atom aromatic heterocycle, methylene group substituted by a 5 or 6 atom heterocycle; R5 is a CH(R3)—COOR4 radical, wherein R3 is hydrogen, an OH or OR group, a saturated hydrocarbon group, a phenyl or benzyl radical and OR4 is hydrophile ester, or 5 or 6 membered heterocycle comprising several heteroatoms selected from a group consisting of nitrogen, sulphur and oxygen, with at least two nitrogene atoms, wherein said heterocycle is substitutable by an alkyl C1-C6, phenyl or benzyl radical. The use of the inventive compounds in the form of drugs, a pharmaceutical composition comprising said compounds, a pharmaceutically acceptable excipient, the use in conjunction of at least one type of cannabinoid derivative for potentiating the analgesic and antidepressant effect of the novel compounds of formula (I) and / or morphine or the derivatives thereof are also disclosed.
Owner:KOS THERAPEUTICS INC
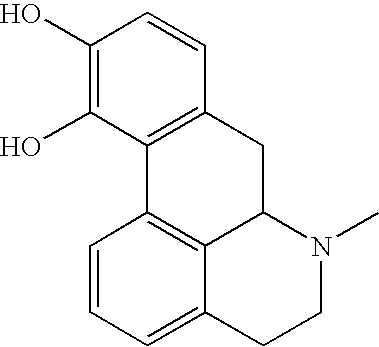
Apomorphine inhibitors of amyloid-beta (Abeta) fibril formation and their use in amyloidosis based disease
Described is a new class of small molecule inhibitors of amyloid beta protein (Abeta) aggregation, based on apomorphine. These molecules target the nucleation phase of Abeta self-assembly and interfere effectively with aggregation of Abeta 1-40 into amyloid fibrils in vitro as determined by transmission electron microscopy, Thioflavin T (ThT) fluorescence, and velocity sedimentation. Structure-activity studies using apomorphine analogues demonstrate that 10,11-dihydroxy substitutions of the D ring are preferred for the inhibitory effectiveness of these aporphines, and that methylation of these hydroxyl groups reduces their inhibitory potency. The ability of these small molecules to inhibit Abeta amyloid fibril formation appears to be linked to their ability to undergo auto-oxidation in solution, implicating an auto-oxidation product as the active Abeta inhibitor. Sedimentation velocity and electron microscopy studies demonstrate that apomorphine and analogues facilitate oligomerization of Abeta into short nonfibrillar soluble assemblies, but inhibit Abeta fibrillization.
Owner:CYTOKINE PHARMASCI
Dual opioid pain therapy
InactiveUS20090291975A1Eliminate side effectsGood analgesic effectBiocideDispersion deliveryPain therapySide effect
Provided are pharmaceutical compositions and methods for the alleviation of pain in a patient with optimal ratios of morphine and oxycodone that provide superior analgesic efficacy and lower incidence of adverse side effects compared to morphine and oxycodone alone. The pharmaceutical compositions comprise morphine and oxycodone, or pharmaceutically acceptable salts thereof, in ratios of about 3 to 2 to about 1 to 2, morphine to oxycodone by weight.
Owner:QRXPHARMA
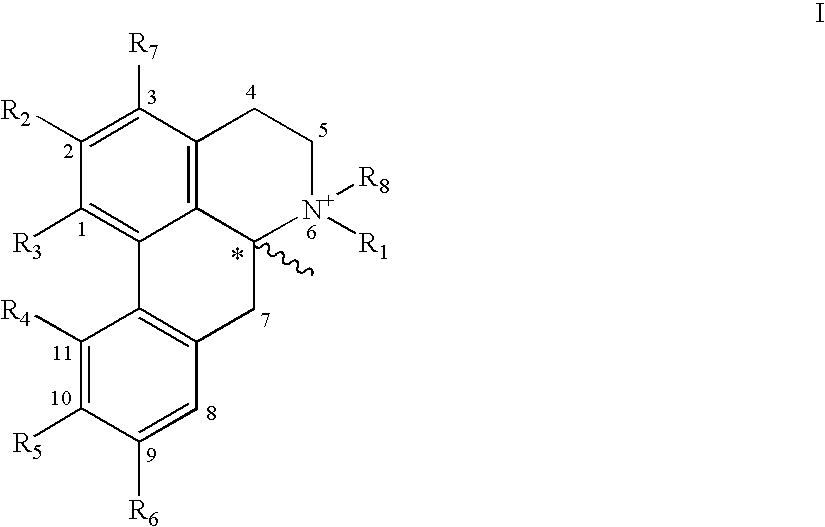
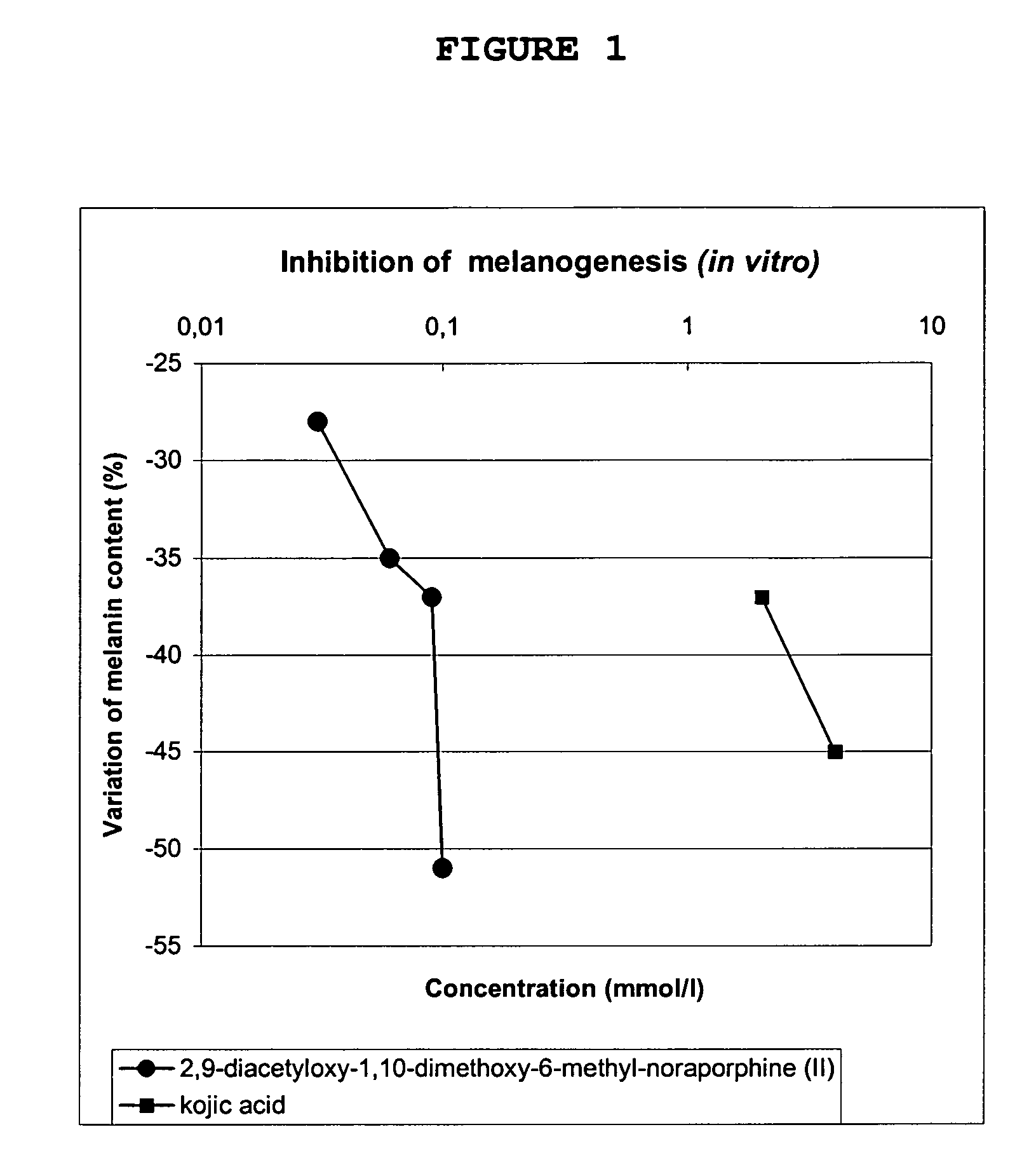
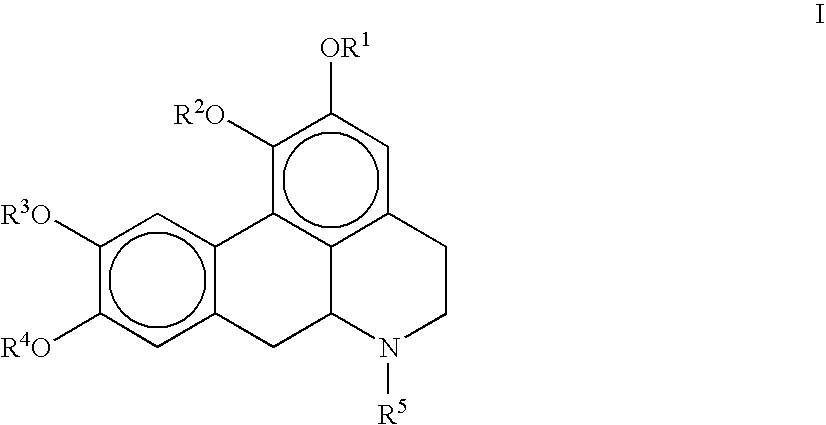
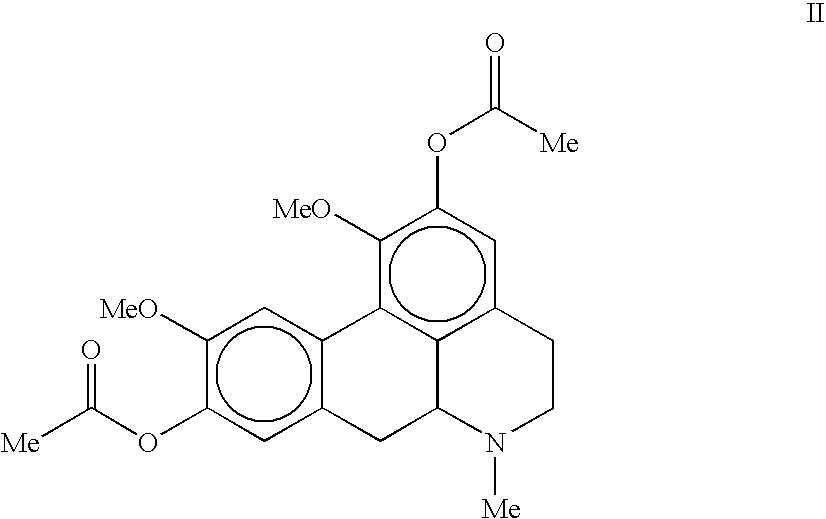
Molecules derived from noraporphine
The invention concerns novel molecules derived from noraporphine, as well as cosmetic and dermopharmaceutical compositions containing one or several of said derivatives, alone or combined with a plant extract, in particular glaucium flavum, and particularly preparations for reducing pigmentation, with anti-ageing effect or for slimming.
Owner:SEDERMA SA
Aralkylone pipeazine derivative and its application
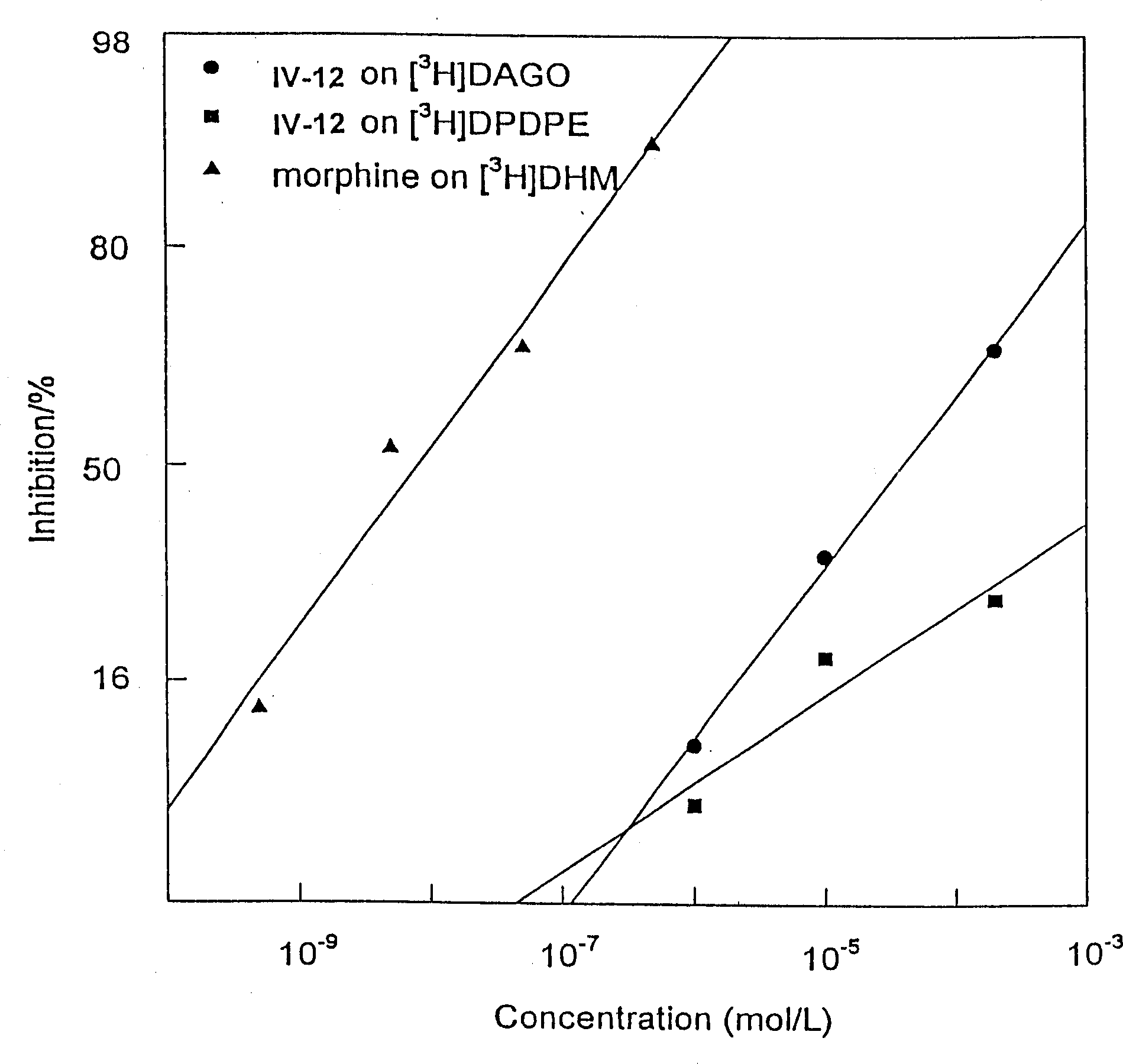
Owner:NHWA PHARMA CORPORATION +1
Chinese medicine gelsmium elegans total alkaloid for anticancer and analgesia, and medicinal composition containing it and preparing method thereof
The invention discloses an anticancer and antalgic Chinese medicinal Gelsmium elegans total alkaloid and drug combination containing it as well as its preparing method, where the Gelsmium elegans total alkaloid is extracted from the raw material Chinese medicinal Gelsmium elegans and the Gelsmium elegans total alkaloid and acceptable carrier or excipient in any pharmacy are made into drug combination whose drug form can be oral preparation and also injection, concretely speaking tablet, capsule, soft capsule, oral solution, injection solution, transfusion, powder injection and freeze-dried powder injection. The drug combination has good anticancer effect and high-quality ntalgic function and the drug combination's antalgic strength is inferior to morphia's and superior to other clinical antalgics'.
Owner:张晴龙
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com